
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 39
MIREX
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1990
This is a companion volume to Environmental Health Criteria 44: Mirex
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data Mirex : health and safety
guide.
(Health and safety guide ; no. 39)
1. Mirex - standards I. Series
ISBN 92 4 151039 0 (NLM Classification: WA 240)
ISSN 0259-7268
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Uses
2. SUMMARY AND EVALUATION
2.1. Human exposure to mirex
2.2. Kinetics and metabolism
2.3. Effects on experimental animals
2.4. Effects on human health
2.5. Effects on the environment
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.2. Recommendations
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Advice to physicians
4.1.2. Health surveillance advice
4.2. Safety in use
4.3. Explosion and fire hazards
4.3.1. Explosion hazards
4.3.2. Fire hazards
4.4. Storage
4.4.1. Leaking containers in store
4.5. Transport
4.6. Spillage and disposal
4.6.1. Spillage
4.6.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1. Hazards
5.2. Prevention
6. INTERNATIONAL CHEMICAL SAFETY CARD
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging, and transport
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) documents produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is an International Chemical Safety Card
which should be readily available, and should be clearly explained, to
all who could come into contact with the chemical. The section on
regulatory information has been extracted from the legal file of the
International Register of Potentially Toxic Chemicals (IRPTC) and from
other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Common name: Mirex
Chemical structure:
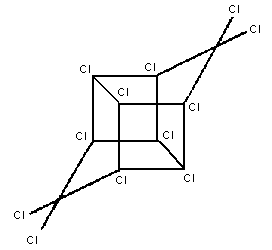 Molecular formula: C10Cl12
Common trade names: Dechlorane, Ferriamicide, GC 1283
Common synonyms: dodecachloropentacyclo[5.2.1.026039058]-
decanedodecachloro-octahydro-1,3,4-metheno-2H-
cyclo-buta [cd]pentalene
CAS chemical name: 1,1a,2,2,3,3a,4,5,5,5a,5b,6-
dodecachlorooctahydro-1,3,4-metheno-1H-
cyclobuta- [cd]pentalene
CAS registry number: 2385-85-5
Relative molecular mass: 545.5
1.2 Physical and Chemical Properties
Mirex is a white crystalline, odourless solid with a melting point of
485°C. It is soluble in several organic solvents including
tetrahydrofuran (30%), carbon disulfide (18%), chloroform (17%), and
benzene (12%), but is practically insoluble in water. It has a vapour
pressure of 3 × 10-7mmHg at 25°C.
Mirex is considered to be extremely stable. It does not react with
sulfuric, nitric, hydrochloric, or other common acids and is
unreactive with bases, chlorine, or ozone. Despite its stability,
reductive dechlorination of mirex can be brought about by reaction
with reduced iron porphyrin or more effectively by vitamin B12.
Slow partial decomposition will also result from exposure to
ultraviolet radiation (UVR) in hydrocarbon solvents or to gamma rays.
Photomirex (8-monohydro-mirex) is the major product of dechlorination
by UVR, and may represent the fate of most of the mirex in the
environment.
Mirex is quite resistant to pyrolysis; decomposition begins at 525°C,
and 98-99% combustion is accomplished at 700°C within 1 second.
Hexachlorobenzene is a major pyrolytic product with lesser amounts of
carbon monoxide, carbon dioxide, hydrogen chloride, chlorine, carbon
tetrachloride, and phosgene given off in the form of a vapour.
Technical grade preparations of mirex contain 95.19% mirex and 2.58%
chlordecone, the rest being unspecified. The term "mirex" is also
used to refer to bait comprising corncob grits, soya bean oil, and
mirex. Insect bait formulations for aerial application containing
0.3-0.5% mirex and fire ant formulations containing 0.075-0.3% mirex
have also been used in the USA.
1.3 Analytical Methods
Gas chromatography with electron capture detection is the analytical
method most commonly used for its determination.
1.4 Uses
Mirex is mainly used as a flame-retardant and as a stomach
insecticide, usually formulated into baits, for the control of ants,
especially fire ants and harvester ants. The USA appears to be the
main country in which mirex was used for pest control, but this use
was discontinued in 1978.
The same chemical substance is used, under the name Dechlorane, as a
fire retardant in plastics, rubbers, paints, etc. This application is
not restricted to the USA.
Recently, the use of mirex has become increasingly restricted or
prohibited in many countries (see, e.g., section 7.3).
2. SUMMARY AND EVALUATION
2.1 Human Exposure to Mirex
Food probably represents the major source of intake of mirex for the
general population, fish, wild game, and meat being the main sources.
Normally, such intake is below established residue tolerances. Mirex
may occur in breast milk, but levels are very low or below detection
limits.
No data are available regarding occupational exposure.
2.2 Kinetics and Metabolism
Following oral ingestion, mirex is only partly absorbed into the body
and the remainder, depending on the dose administered, is eliminated
unchanged in the faeces. Mirex can also be absorbed following
inhalation and via the skin.
It is a lipophilic compound and, as such, is stored in adipose tissue
to a greater extent than in any other tissue. Mirex is transferred
across the placenta to the fetus and is excreted with the milk.
Mirex does not appear to have been metabolized to any extent in any
animal species investigated. Its elimination from the body is slow
and, depending on the species, it has a half-life in the body of
several months.
It is one of the most stable pesticides in use today.
2.3 Effects on Experimental Animals
Mirex was moderately toxic in single-dose animal studies (oral LD50
values ranged from 365 to 3000 mg/kg body weight). Toxic effects
included neurological symptoms, especially tremors and convulsions.
The most sensitive effects of repeated exposure in experimental
animals are principally associated with the liver (liver hypertrophy
with morphological changes in the liver cells, and induction of
mixed-function oxidases). These effects have been observed with doses
as low as 1 mg/kg diet (0.05 mg/kg body weight per day), the lowest
dose tested.
In studies to investigate the toxicity of mirex in pregnant animals,
teratogenic effects were seen in rats given 6 mg/kg body weight per
day by gavage, and fetotoxic effects were seen in animals given
25 mg/kg diet. In addition, exposure of male mice to dietary levels
of about 2 mg/kg for 3 months resulted in impaired reproductive
performance.
Mirex was not generally active in short-term tests for genetic
activity. However, mirex is carcinogenic for both mice and rats.
2.4 Effects on Human Health
No data on effects on human beings were available to the Task Group.
2.5 Effects on the Environment
Mirex is one of the most stable and environmentally persistent
pesticides in use today. It is not biodegraded by microorganisms,
except occasionally under aerobic conditions, and hydrolysis is very
slow. Although general environmental levels are low, it is widespread
in the biotic and abiotic environment. Mirex is both accumulated and
biomagnified. It is strongly adsorbed on sediments and has a low
water solubility.
The delayed onset of toxic effects and mortality is typical of mirex
poisoning. The long-term toxicity of mirex is uniformly high. It is
toxic for a range of aquatic organisms, crustacea being particularly
sensitive. Mirex induces pervasive long-term physiological and
biological disorders in vertebrates.
Although no field data are available, the adverse effects of long-term
exposure to low levels of mirex, combined with its persistence,
suggest that the use of mirex presents a long-term environmental risk.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
1. No data on human health effects are available in connection with
occupational exposure to mirex. On the basis of findings in mice and
rats, this chemical should be considered, for practical purposes, as
being potentially carcinogenic for human beings.
2. For the same reason, reservations must remain about the safety of
this chemical in food, despite the relatively low residues so far
reported.
3. Effects on the organisms studied, as well as its persistence,
suggest that mirex presents a long-term hazard for the environment.
4. Taking into account these considerations, it is felt that the use
of this chemical for both agricultural and non-agricultural
applications should be discouraged, except where there is no adequate
alternative.
3.2 Recommendations
1. Surveillance should be maintained over any future production,
transport, and disposal of mirex and the nature and extent of both its
agricultural and non-agricultural use.
2. Comprehensive monitoring of levels of mirex in the environment
should be continued.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Mirex is an organochlorine insecticide. It is toxic and may be
hazardous for human beings if incorrectly or carelessly handled. It
is therefore essential that the correct precautions should be observed
during handling and use.
For details, see the International Chemical Safety Card (section 6).
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
Mirex is toxic by mouth, by skin contact (especially liquid
formulations), and by inhalation of dust from powder concentrates. It
acts as a stimulant of the central nervous system.
Following accidental ingestion or over-exposure, symptoms may include
headache, dizziness, nausea, vomiting, weakness in the legs, and
convulsions.
Organochlorines can cause respiratory depression. They also sensitize
the heart to endogenous catecholamines leading to ventricular
fibrillation and cardiac arrest in severe cases.
Respiratory depression may lead to metabolic acidosis and, if
necessary, blood gases should be checked. The use of an ECG monitor
is recommended if the symptoms are severe.
No cases of poisoning in man have been reported so far.
4.1.1.2 Medical advice
Medical treatment is largely symptomatic and supportive and directed
against convulsions and hypoxia. Because many liquid formulations
contain hydrocarbon solvent, vomiting should not be induced and
emetics are contraindicated. If swallowed, the stomach should be
emptied as soon as possible by careful gastric lavage (with a cuffed
endotracheal tube), avoiding aspiration into the lungs. This should
be followed by intragastric administration of 3-4 tablespoons of
activated charcoal and 30 g magnesium sulfate or sodium sulfate in a
30% aqueous solution. Oily purgatives are contraindicated. No fats,
oils, or milk should be given.
If convulsions occur, anti-convulsants should be given, e.g.,
diazepam, 10 mg slowly intravenously (children 1-5 mg), repeated as
necessary; or thiopental sodium, or hexobarbital sodium slowly
intravenously in a dose of 10 mg/kg body weight with a maximum total
dose of up to 750 mg for an adult. On account of their short action,
these barbiturates should always be followed by phenobarbital given
orally at 3 mg/kg body weight (up to 200 mg for an adult), or
phenobarbital sodium given intramuscularly at 3 mg/kg (also up to
200 mg for an adult).
Morphine and its derivatives, epinephrine and norepinephrine should
never be given.
An unobstructed airway must be maintained. Oxygen and/or artificial
respiration may be needed.
4.1.2 Health surveillance advice
A pre-employment and an annual general medical examination are advised
for regularly exposed workers. Special attention should be paid to
liver and kidney function.
4.2 Safety in Use
Handling liquid formulations: Wear protective neoprene or PVC
gloves, cotton overalls, rubber
boots, and face shield.
Handling powder formulations: Avoid raising a dust cloud. Wear
protective gloves and dust mask.
Follow the advice relating to
personal hygiene.
4.3 Explosion and Fire Hazards
4.3.1 Explosion hazards
The explosion hazard will depend on the solvent used in the
formulation, or on the characteristics of the dust.
4.3.2 Fire hazards
Liquid products containing organic solvents may be flammable.
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. With sufficient burning or external heat, mirex will
decompose, emitting toxic fumes. Fire-fighters should wear a
self-contained breathing apparatus, eye protection, and full
protective clothing.
Confine the use of water spray to the cooling of unaffected stock,
thus avoiding the accumulation of polluted run-off from the site.
4.4 Storage
Products should be stored in locked buildings, preferably dedicated to
insecticides.
Keep products out of reach of children and unauthorized personnel. Do
not store near foodstuffs or animal feed.
4.4.1 Leaking containers in store
Take precautions and use appropriate personal protection. Empty any
product remaining in damaged/leaking containers into a clean empty
drum, which should then be tightly closed and suitably labelled.
Sweep up spillage with sawdust, sand, or earth (moisten for powders),
and dispose of safely.
Emptied leaking liquid containers should be rinsed with at least
1 litre water per 20-litre drum. Swirl round to rinse the walls,
empty, and add the rinsings to the sawdust or earth. Do not re-use
containers for any other purpose. Puncture the container to prevent
re-use.
4.5 Transport
Comply with any local requirements regarding movement of hazardous
goods. Do not transport with foodstuffs or animal feed. Make sure
that containers are in good condition and labels undamaged before
dispatch.
4.6 Spillage and Disposal
4.6.1 Spillage
Before dealing with any spillage, precautions should be taken as
required and appropriate personal protection should be used.
Prevent liquid from spreading or contaminating other cargo and
vegetation, and avoid pollution of surface waters and ground water by
using the most suitable available material, e.g., earth or sand.
Absorb spilled liquid with sawdust, sand, or earth, sweep up and place
it in a closeable container for later transfer to a safe place for
disposal.
As soon as possible after the spillage and before re-use, cover all
contaminated areas with damp sawdust, sand, or earth. Sweep up and
place in a closeable container for later transfer to a safe place for
disposal. Care should be taken to avoid run-off into surface waters
or drains.
4.6.2 Disposal
Surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Mirex is not readily decomposed
chemically or biologically and is relatively persistent. Waste
material should be burned only in a proper incinerator designed for
organochlorine waste disposal (1000°C and 30-min residence time with
effluent gas scrubbing). If this is not possible, bury in an approved
dump or landfill where there is no risk of contamination of surface or
ground water. Comply with any local legislation regarding disposal of
toxic wastes.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1 Hazards
Mirex is one of the most stable of the organochlorine insecticides.
Although general environmental levels are low, it is widespread in the
biotic and abiotic environment. Mirex is both accumulated and
biomagnified. It is strongly adsorbed on sediments and has a low
water solubility.
Delayed onset of toxic effects and mortality is typical of mirex
poisoning. The long-term toxicity of mirex is uniformly high. Mirex
is toxic for a range of aquatic organisms, crustacea being
particularly sensitive.
Although no field data are available, the adverse effects of long-term
exposure to low levels of mirex, combined with its persistence,
suggest that the use of mirex presents a long-term environmental risk.
5.2 Prevention
Industrial discharges from manufacturing, formulation, and technical
applications should not be allowed to pollute the environment and
should be treated properly.
Any spillage or unused product should be prevented from spreading to
vegetation or waterways and should be treated and disposed of
properly.
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily available to all health workers concerned
with, and users of, mirex. It should be displayed at, or near,
entrances to areas where there is potential exposure to mirex, and on
processing equipment and containers. The card should be translated
into the appropriate language(s). All persons potentially exposed to
the chemical should also have the instructions on the chemical safety
card clearly explained.
Space is available on the card for insertion of the National
Occupational Exposure Limit, the address and telephone number of the
National Poison Control Centre, and for local trade names.
MIREX
CAS chemical name: 1,1a,2,2,3,3a,4,5,5,5a,5b,6-dodecachloroocta-hydro-1,3,4-metheno-1H-cyclobuta[cd]pentalene
CAS registry number: 2385-85-5
RTECS registry number: PC8225000
Molecular formula: C10Cl12
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Melting point (°C) 485 Mirex is a white crystalline, odourless solid; it is
Vapour pressure (mmHg at 25°C) 3 × 10-7 considered to be extremely stable and does not react
Relative molecular mass 545.5 with common acids, bases, chlorine, or ozone; slow,
Solubility: partial dechlorination by UV radiation yields
in water practically photomirex; it is quite resistant to pyrolysis,
insoluble hexachlorobenzene being a major pyrolysis product; it is a
in tetrahydrofuram 30% stomach insecticide with little contact activity;
in carbon disulfide 18% a major use is for ant control; it is also used as a
in chloroform 17% flame retardant under the name Dechlorane
in benzene 12%
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: Potential human
carcinogen; on repeated exposure
mirex may accumulate in the body
SKIN: Overexposure may cause Avoid skin contact; wear Remove contaminated clothing
poisoning protective clothing, PVC or immediately; wash skin with water
neoprene gloves, rubber boots and soap
EYES: Irritation, redness Wear face-shield or goggles Flush with clean water for 15 minutes;
if irritation persists, seek medical
attention
INHALATION: Dust may irritate Wear appropriate dust mask or
respirator
INGESTION: Unlikely Do not eat, drink, or smoke
occupational hazard during work; wash hands before
eating, drinking, or smoking
Accidental or intentional ingestion Obtain medical attention immediately; do
may cause poisoning not induce vomiting; keep at rest lying
face downwards; ensure clear airway;
fat, milk, or oil shoud not be given
ENVIRONMENT: Toxic for aquatic Do not spill on animal feed or
and terrestrial life; persistent in waterways
SPILLAGE STORAGE FIRE AND EXPLOSION
Take appropriate personal Products should be stored in Liquid products will burn and emulsifiable
precautions; prevent liquid locked buildings, preferably concentrates are miscible with water;
from spreading or contaminating dedicated to insecticides extinguish fires with alcohol-resistant foam,
other cargo, vegetation, or carbon dioxide, or powder; with sufficient
waterways, with a barrier of the burning or external heat, mirex will
most suitable available material, Keep products out of reach of decompose, emitting toxic fumes; the smoke
e.g., earth or sand children and unauthorized and fumes could be injurious through
personnel; do not store near inhalation, or absorption through the skin;
Absorb spilled liquid with foodstuffs or animal feed therefore, firefighters should wear
sawdust, sand, or earth; sweep up protective clothing and self-contained breathing
and place it in a closeable container apparatus; confine the use of water spray
for later safe disposal to the cooling of unaffected stock, thus
avoiding polluted run-off from the site
WASTE DISPOSAL NATIONAL INFORMATION
Mirex is not readily National Occupational Exposure UN No. 2762, 2995, 2996
decomposed chemically or biologically Limit:
and is relatively persistent;
waste material should be burned
in a proper incinerator designed
for organochlorine waste disposal; National Poison Control Centre:
if this is not possible, bury in
an approved dump or landfill where
there is no risk of contamination
of surface or ground water;
comply with any local legislation Local trade names:
regarding disposal of toxic wastes
Molecular formula: C10Cl12
Common trade names: Dechlorane, Ferriamicide, GC 1283
Common synonyms: dodecachloropentacyclo[5.2.1.026039058]-
decanedodecachloro-octahydro-1,3,4-metheno-2H-
cyclo-buta [cd]pentalene
CAS chemical name: 1,1a,2,2,3,3a,4,5,5,5a,5b,6-
dodecachlorooctahydro-1,3,4-metheno-1H-
cyclobuta- [cd]pentalene
CAS registry number: 2385-85-5
Relative molecular mass: 545.5
1.2 Physical and Chemical Properties
Mirex is a white crystalline, odourless solid with a melting point of
485°C. It is soluble in several organic solvents including
tetrahydrofuran (30%), carbon disulfide (18%), chloroform (17%), and
benzene (12%), but is practically insoluble in water. It has a vapour
pressure of 3 × 10-7mmHg at 25°C.
Mirex is considered to be extremely stable. It does not react with
sulfuric, nitric, hydrochloric, or other common acids and is
unreactive with bases, chlorine, or ozone. Despite its stability,
reductive dechlorination of mirex can be brought about by reaction
with reduced iron porphyrin or more effectively by vitamin B12.
Slow partial decomposition will also result from exposure to
ultraviolet radiation (UVR) in hydrocarbon solvents or to gamma rays.
Photomirex (8-monohydro-mirex) is the major product of dechlorination
by UVR, and may represent the fate of most of the mirex in the
environment.
Mirex is quite resistant to pyrolysis; decomposition begins at 525°C,
and 98-99% combustion is accomplished at 700°C within 1 second.
Hexachlorobenzene is a major pyrolytic product with lesser amounts of
carbon monoxide, carbon dioxide, hydrogen chloride, chlorine, carbon
tetrachloride, and phosgene given off in the form of a vapour.
Technical grade preparations of mirex contain 95.19% mirex and 2.58%
chlordecone, the rest being unspecified. The term "mirex" is also
used to refer to bait comprising corncob grits, soya bean oil, and
mirex. Insect bait formulations for aerial application containing
0.3-0.5% mirex and fire ant formulations containing 0.075-0.3% mirex
have also been used in the USA.
1.3 Analytical Methods
Gas chromatography with electron capture detection is the analytical
method most commonly used for its determination.
1.4 Uses
Mirex is mainly used as a flame-retardant and as a stomach
insecticide, usually formulated into baits, for the control of ants,
especially fire ants and harvester ants. The USA appears to be the
main country in which mirex was used for pest control, but this use
was discontinued in 1978.
The same chemical substance is used, under the name Dechlorane, as a
fire retardant in plastics, rubbers, paints, etc. This application is
not restricted to the USA.
Recently, the use of mirex has become increasingly restricted or
prohibited in many countries (see, e.g., section 7.3).
2. SUMMARY AND EVALUATION
2.1 Human Exposure to Mirex
Food probably represents the major source of intake of mirex for the
general population, fish, wild game, and meat being the main sources.
Normally, such intake is below established residue tolerances. Mirex
may occur in breast milk, but levels are very low or below detection
limits.
No data are available regarding occupational exposure.
2.2 Kinetics and Metabolism
Following oral ingestion, mirex is only partly absorbed into the body
and the remainder, depending on the dose administered, is eliminated
unchanged in the faeces. Mirex can also be absorbed following
inhalation and via the skin.
It is a lipophilic compound and, as such, is stored in adipose tissue
to a greater extent than in any other tissue. Mirex is transferred
across the placenta to the fetus and is excreted with the milk.
Mirex does not appear to have been metabolized to any extent in any
animal species investigated. Its elimination from the body is slow
and, depending on the species, it has a half-life in the body of
several months.
It is one of the most stable pesticides in use today.
2.3 Effects on Experimental Animals
Mirex was moderately toxic in single-dose animal studies (oral LD50
values ranged from 365 to 3000 mg/kg body weight). Toxic effects
included neurological symptoms, especially tremors and convulsions.
The most sensitive effects of repeated exposure in experimental
animals are principally associated with the liver (liver hypertrophy
with morphological changes in the liver cells, and induction of
mixed-function oxidases). These effects have been observed with doses
as low as 1 mg/kg diet (0.05 mg/kg body weight per day), the lowest
dose tested.
In studies to investigate the toxicity of mirex in pregnant animals,
teratogenic effects were seen in rats given 6 mg/kg body weight per
day by gavage, and fetotoxic effects were seen in animals given
25 mg/kg diet. In addition, exposure of male mice to dietary levels
of about 2 mg/kg for 3 months resulted in impaired reproductive
performance.
Mirex was not generally active in short-term tests for genetic
activity. However, mirex is carcinogenic for both mice and rats.
2.4 Effects on Human Health
No data on effects on human beings were available to the Task Group.
2.5 Effects on the Environment
Mirex is one of the most stable and environmentally persistent
pesticides in use today. It is not biodegraded by microorganisms,
except occasionally under aerobic conditions, and hydrolysis is very
slow. Although general environmental levels are low, it is widespread
in the biotic and abiotic environment. Mirex is both accumulated and
biomagnified. It is strongly adsorbed on sediments and has a low
water solubility.
The delayed onset of toxic effects and mortality is typical of mirex
poisoning. The long-term toxicity of mirex is uniformly high. It is
toxic for a range of aquatic organisms, crustacea being particularly
sensitive. Mirex induces pervasive long-term physiological and
biological disorders in vertebrates.
Although no field data are available, the adverse effects of long-term
exposure to low levels of mirex, combined with its persistence,
suggest that the use of mirex presents a long-term environmental risk.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
1. No data on human health effects are available in connection with
occupational exposure to mirex. On the basis of findings in mice and
rats, this chemical should be considered, for practical purposes, as
being potentially carcinogenic for human beings.
2. For the same reason, reservations must remain about the safety of
this chemical in food, despite the relatively low residues so far
reported.
3. Effects on the organisms studied, as well as its persistence,
suggest that mirex presents a long-term hazard for the environment.
4. Taking into account these considerations, it is felt that the use
of this chemical for both agricultural and non-agricultural
applications should be discouraged, except where there is no adequate
alternative.
3.2 Recommendations
1. Surveillance should be maintained over any future production,
transport, and disposal of mirex and the nature and extent of both its
agricultural and non-agricultural use.
2. Comprehensive monitoring of levels of mirex in the environment
should be continued.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Mirex is an organochlorine insecticide. It is toxic and may be
hazardous for human beings if incorrectly or carelessly handled. It
is therefore essential that the correct precautions should be observed
during handling and use.
For details, see the International Chemical Safety Card (section 6).
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
Mirex is toxic by mouth, by skin contact (especially liquid
formulations), and by inhalation of dust from powder concentrates. It
acts as a stimulant of the central nervous system.
Following accidental ingestion or over-exposure, symptoms may include
headache, dizziness, nausea, vomiting, weakness in the legs, and
convulsions.
Organochlorines can cause respiratory depression. They also sensitize
the heart to endogenous catecholamines leading to ventricular
fibrillation and cardiac arrest in severe cases.
Respiratory depression may lead to metabolic acidosis and, if
necessary, blood gases should be checked. The use of an ECG monitor
is recommended if the symptoms are severe.
No cases of poisoning in man have been reported so far.
4.1.1.2 Medical advice
Medical treatment is largely symptomatic and supportive and directed
against convulsions and hypoxia. Because many liquid formulations
contain hydrocarbon solvent, vomiting should not be induced and
emetics are contraindicated. If swallowed, the stomach should be
emptied as soon as possible by careful gastric lavage (with a cuffed
endotracheal tube), avoiding aspiration into the lungs. This should
be followed by intragastric administration of 3-4 tablespoons of
activated charcoal and 30 g magnesium sulfate or sodium sulfate in a
30% aqueous solution. Oily purgatives are contraindicated. No fats,
oils, or milk should be given.
If convulsions occur, anti-convulsants should be given, e.g.,
diazepam, 10 mg slowly intravenously (children 1-5 mg), repeated as
necessary; or thiopental sodium, or hexobarbital sodium slowly
intravenously in a dose of 10 mg/kg body weight with a maximum total
dose of up to 750 mg for an adult. On account of their short action,
these barbiturates should always be followed by phenobarbital given
orally at 3 mg/kg body weight (up to 200 mg for an adult), or
phenobarbital sodium given intramuscularly at 3 mg/kg (also up to
200 mg for an adult).
Morphine and its derivatives, epinephrine and norepinephrine should
never be given.
An unobstructed airway must be maintained. Oxygen and/or artificial
respiration may be needed.
4.1.2 Health surveillance advice
A pre-employment and an annual general medical examination are advised
for regularly exposed workers. Special attention should be paid to
liver and kidney function.
4.2 Safety in Use
Handling liquid formulations: Wear protective neoprene or PVC
gloves, cotton overalls, rubber
boots, and face shield.
Handling powder formulations: Avoid raising a dust cloud. Wear
protective gloves and dust mask.
Follow the advice relating to
personal hygiene.
4.3 Explosion and Fire Hazards
4.3.1 Explosion hazards
The explosion hazard will depend on the solvent used in the
formulation, or on the characteristics of the dust.
4.3.2 Fire hazards
Liquid products containing organic solvents may be flammable.
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. With sufficient burning or external heat, mirex will
decompose, emitting toxic fumes. Fire-fighters should wear a
self-contained breathing apparatus, eye protection, and full
protective clothing.
Confine the use of water spray to the cooling of unaffected stock,
thus avoiding the accumulation of polluted run-off from the site.
4.4 Storage
Products should be stored in locked buildings, preferably dedicated to
insecticides.
Keep products out of reach of children and unauthorized personnel. Do
not store near foodstuffs or animal feed.
4.4.1 Leaking containers in store
Take precautions and use appropriate personal protection. Empty any
product remaining in damaged/leaking containers into a clean empty
drum, which should then be tightly closed and suitably labelled.
Sweep up spillage with sawdust, sand, or earth (moisten for powders),
and dispose of safely.
Emptied leaking liquid containers should be rinsed with at least
1 litre water per 20-litre drum. Swirl round to rinse the walls,
empty, and add the rinsings to the sawdust or earth. Do not re-use
containers for any other purpose. Puncture the container to prevent
re-use.
4.5 Transport
Comply with any local requirements regarding movement of hazardous
goods. Do not transport with foodstuffs or animal feed. Make sure
that containers are in good condition and labels undamaged before
dispatch.
4.6 Spillage and Disposal
4.6.1 Spillage
Before dealing with any spillage, precautions should be taken as
required and appropriate personal protection should be used.
Prevent liquid from spreading or contaminating other cargo and
vegetation, and avoid pollution of surface waters and ground water by
using the most suitable available material, e.g., earth or sand.
Absorb spilled liquid with sawdust, sand, or earth, sweep up and place
it in a closeable container for later transfer to a safe place for
disposal.
As soon as possible after the spillage and before re-use, cover all
contaminated areas with damp sawdust, sand, or earth. Sweep up and
place in a closeable container for later transfer to a safe place for
disposal. Care should be taken to avoid run-off into surface waters
or drains.
4.6.2 Disposal
Surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Mirex is not readily decomposed
chemically or biologically and is relatively persistent. Waste
material should be burned only in a proper incinerator designed for
organochlorine waste disposal (1000°C and 30-min residence time with
effluent gas scrubbing). If this is not possible, bury in an approved
dump or landfill where there is no risk of contamination of surface or
ground water. Comply with any local legislation regarding disposal of
toxic wastes.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1 Hazards
Mirex is one of the most stable of the organochlorine insecticides.
Although general environmental levels are low, it is widespread in the
biotic and abiotic environment. Mirex is both accumulated and
biomagnified. It is strongly adsorbed on sediments and has a low
water solubility.
Delayed onset of toxic effects and mortality is typical of mirex
poisoning. The long-term toxicity of mirex is uniformly high. Mirex
is toxic for a range of aquatic organisms, crustacea being
particularly sensitive.
Although no field data are available, the adverse effects of long-term
exposure to low levels of mirex, combined with its persistence,
suggest that the use of mirex presents a long-term environmental risk.
5.2 Prevention
Industrial discharges from manufacturing, formulation, and technical
applications should not be allowed to pollute the environment and
should be treated properly.
Any spillage or unused product should be prevented from spreading to
vegetation or waterways and should be treated and disposed of
properly.
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily available to all health workers concerned
with, and users of, mirex. It should be displayed at, or near,
entrances to areas where there is potential exposure to mirex, and on
processing equipment and containers. The card should be translated
into the appropriate language(s). All persons potentially exposed to
the chemical should also have the instructions on the chemical safety
card clearly explained.
Space is available on the card for insertion of the National
Occupational Exposure Limit, the address and telephone number of the
National Poison Control Centre, and for local trade names.
MIREX
CAS chemical name: 1,1a,2,2,3,3a,4,5,5,5a,5b,6-dodecachloroocta-hydro-1,3,4-metheno-1H-cyclobuta[cd]pentalene
CAS registry number: 2385-85-5
RTECS registry number: PC8225000
Molecular formula: C10Cl12
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Melting point (°C) 485 Mirex is a white crystalline, odourless solid; it is
Vapour pressure (mmHg at 25°C) 3 × 10-7 considered to be extremely stable and does not react
Relative molecular mass 545.5 with common acids, bases, chlorine, or ozone; slow,
Solubility: partial dechlorination by UV radiation yields
in water practically photomirex; it is quite resistant to pyrolysis,
insoluble hexachlorobenzene being a major pyrolysis product; it is a
in tetrahydrofuram 30% stomach insecticide with little contact activity;
in carbon disulfide 18% a major use is for ant control; it is also used as a
in chloroform 17% flame retardant under the name Dechlorane
in benzene 12%
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: Potential human
carcinogen; on repeated exposure
mirex may accumulate in the body
SKIN: Overexposure may cause Avoid skin contact; wear Remove contaminated clothing
poisoning protective clothing, PVC or immediately; wash skin with water
neoprene gloves, rubber boots and soap
EYES: Irritation, redness Wear face-shield or goggles Flush with clean water for 15 minutes;
if irritation persists, seek medical
attention
INHALATION: Dust may irritate Wear appropriate dust mask or
respirator
INGESTION: Unlikely Do not eat, drink, or smoke
occupational hazard during work; wash hands before
eating, drinking, or smoking
Accidental or intentional ingestion Obtain medical attention immediately; do
may cause poisoning not induce vomiting; keep at rest lying
face downwards; ensure clear airway;
fat, milk, or oil shoud not be given
ENVIRONMENT: Toxic for aquatic Do not spill on animal feed or
and terrestrial life; persistent in waterways
SPILLAGE STORAGE FIRE AND EXPLOSION
Take appropriate personal Products should be stored in Liquid products will burn and emulsifiable
precautions; prevent liquid locked buildings, preferably concentrates are miscible with water;
from spreading or contaminating dedicated to insecticides extinguish fires with alcohol-resistant foam,
other cargo, vegetation, or carbon dioxide, or powder; with sufficient
waterways, with a barrier of the burning or external heat, mirex will
most suitable available material, Keep products out of reach of decompose, emitting toxic fumes; the smoke
e.g., earth or sand children and unauthorized and fumes could be injurious through
personnel; do not store near inhalation, or absorption through the skin;
Absorb spilled liquid with foodstuffs or animal feed therefore, firefighters should wear
sawdust, sand, or earth; sweep up protective clothing and self-contained breathing
and place it in a closeable container apparatus; confine the use of water spray
for later safe disposal to the cooling of unaffected stock, thus
avoiding polluted run-off from the site
WASTE DISPOSAL NATIONAL INFORMATION
Mirex is not readily National Occupational Exposure UN No. 2762, 2995, 2996
decomposed chemically or biologically Limit:
and is relatively persistent;
waste material should be burned
in a proper incinerator designed
for organochlorine waste disposal; National Poison Control Centre:
if this is not possible, bury in
an approved dump or landfill where
there is no risk of contamination
of surface or ground water;
comply with any local legislation Local trade names:
regarding disposal of toxic wastes
 7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. Its intention is to give the
reader a representative but non-exhaustive overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
7.1 Previous Evaluations by International Bodies
IARC (1979) evaluated the carcinogenic hazard resulting from exposure
to mirex and concluded that "there is sufficient evidence for its
carcinogenicity to mice and rats. In the absence of adequate data in
humans, it is reasonable, for practical purposes, to regard mirex as
if it presented a carcinogenic risk to humans".
An acceptable daily intake (ADI) for mirex has not been established by
FAO/WHO.
7.2 Exposure Limit Values
Some exposure limit values are given in the table on the opposite
page.
7.3 Specific Restrictions
Recently, the use of mirex has been increasingly restricted or
prohibited in many countries.
In the USA, all registered products containing mirex have been
cancelled. It has been banned in Ecuador and in various other
countries. In the German Democratic Republic, mirex is not permitted
in agricultural formulations.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD, USA Acceptable residue limit (ARL) 1981
ANIMAL - Specified animal products 0.1 mg/kg
FEED - General 0.01 mg/kg
FOOD Germany, Maximum residue limit (MRL) 1984
Federal - Plant (all) 0.01 mg/kg
Republic of
FOOD Animal Germany, Maximum residue limit (MRL) 1984
Federal - of animal origin (specified) 0.1 mg/kg
Republic of wet weight
0.1 mg/kg
lipid weight
- of animal origin (general) 0.01 mg/kg
wet weight
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classified mirex in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low
risk of poisoning in transport (mirex
liquid formulations >60%)
The label should be as follows:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. Its intention is to give the
reader a representative but non-exhaustive overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
7.1 Previous Evaluations by International Bodies
IARC (1979) evaluated the carcinogenic hazard resulting from exposure
to mirex and concluded that "there is sufficient evidence for its
carcinogenicity to mice and rats. In the absence of adequate data in
humans, it is reasonable, for practical purposes, to regard mirex as
if it presented a carcinogenic risk to humans".
An acceptable daily intake (ADI) for mirex has not been established by
FAO/WHO.
7.2 Exposure Limit Values
Some exposure limit values are given in the table on the opposite
page.
7.3 Specific Restrictions
Recently, the use of mirex has been increasingly restricted or
prohibited in many countries.
In the USA, all registered products containing mirex have been
cancelled. It has been banned in Ecuador and in various other
countries. In the German Democratic Republic, mirex is not permitted
in agricultural formulations.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD, USA Acceptable residue limit (ARL) 1981
ANIMAL - Specified animal products 0.1 mg/kg
FEED - General 0.01 mg/kg
FOOD Germany, Maximum residue limit (MRL) 1984
Federal - Plant (all) 0.01 mg/kg
Republic of
FOOD Animal Germany, Maximum residue limit (MRL) 1984
Federal - of animal origin (specified) 0.1 mg/kg
Republic of wet weight
0.1 mg/kg
lipid weight
- of animal origin (general) 0.01 mg/kg
wet weight
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classified mirex in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low
risk of poisoning in transport (mirex
liquid formulations >60%)
The label should be as follows:
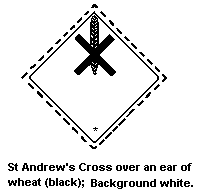 The bottom half of the label should bear the inscriptions:
Harmful, stow away from foodstuffs.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides,
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm, Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides,
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport, Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides, Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning, Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides,
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(unpublished document WHO/VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols. Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1984) Environmental Health Criteria 44: Mirex. Geneva, World
Health Organization.
WHO (1988) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1988-89, Geneva, World
Health Organization (unpublished document WHO/VBC/88.953).
WHO/FAO (1975-90) Data sheets on pesticides. Geneva, World Health
Organization (unpublished documents).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The bottom half of the label should bear the inscriptions:
Harmful, stow away from foodstuffs.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides,
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm, Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides,
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport, Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides, Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning, Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides,
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(unpublished document WHO/VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols. Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1984) Environmental Health Criteria 44: Mirex. Geneva, World
Health Organization.
WHO (1988) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1988-89, Geneva, World
Health Organization (unpublished document WHO/VBC/88.953).
WHO/FAO (1975-90) Data sheets on pesticides. Geneva, World Health
Organization (unpublished documents).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Lavenham, Lavenham Press Limited, British Crop Protection Council.
 Molecular formula: C10Cl12
Common trade names: Dechlorane, Ferriamicide, GC 1283
Common synonyms: dodecachloropentacyclo[5.2.1.026039058]-
decanedodecachloro-octahydro-1,3,4-metheno-2H-
cyclo-buta [cd]pentalene
CAS chemical name: 1,1a,2,2,3,3a,4,5,5,5a,5b,6-
dodecachlorooctahydro-1,3,4-metheno-1H-
cyclobuta- [cd]pentalene
CAS registry number:
Molecular formula: C10Cl12
Common trade names: Dechlorane, Ferriamicide, GC 1283
Common synonyms: dodecachloropentacyclo[5.2.1.026039058]-
decanedodecachloro-octahydro-1,3,4-metheno-2H-
cyclo-buta [cd]pentalene
CAS chemical name: 1,1a,2,2,3,3a,4,5,5,5a,5b,6-
dodecachlorooctahydro-1,3,4-metheno-1H-
cyclobuta- [cd]pentalene
CAS registry number:  7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. Its intention is to give the
reader a representative but non-exhaustive overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
7.1 Previous Evaluations by International Bodies
IARC (1979) evaluated the carcinogenic hazard resulting from exposure
to mirex and concluded that "there is sufficient evidence for its
carcinogenicity to mice and rats. In the absence of adequate data in
humans, it is reasonable, for practical purposes, to regard mirex as
if it presented a carcinogenic risk to humans".
An acceptable daily intake (ADI) for mirex has not been established by
FAO/WHO.
7.2 Exposure Limit Values
Some exposure limit values are given in the table on the opposite
page.
7.3 Specific Restrictions
Recently, the use of mirex has been increasingly restricted or
prohibited in many countries.
In the USA, all registered products containing mirex have been
cancelled. It has been banned in Ecuador and in various other
countries. In the German Democratic Republic, mirex is not permitted
in agricultural formulations.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD, USA Acceptable residue limit (ARL) 1981
ANIMAL - Specified animal products 0.1 mg/kg
FEED - General 0.01 mg/kg
FOOD Germany, Maximum residue limit (MRL) 1984
Federal - Plant (all) 0.01 mg/kg
Republic of
FOOD Animal Germany, Maximum residue limit (MRL) 1984
Federal - of animal origin (specified) 0.1 mg/kg
Republic of wet weight
0.1 mg/kg
lipid weight
- of animal origin (general) 0.01 mg/kg
wet weight
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classified mirex in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low
risk of poisoning in transport (mirex
liquid formulations >60%)
The label should be as follows:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. Its intention is to give the
reader a representative but non-exhaustive overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
7.1 Previous Evaluations by International Bodies
IARC (1979) evaluated the carcinogenic hazard resulting from exposure
to mirex and concluded that "there is sufficient evidence for its
carcinogenicity to mice and rats. In the absence of adequate data in
humans, it is reasonable, for practical purposes, to regard mirex as
if it presented a carcinogenic risk to humans".
An acceptable daily intake (ADI) for mirex has not been established by
FAO/WHO.
7.2 Exposure Limit Values
Some exposure limit values are given in the table on the opposite
page.
7.3 Specific Restrictions
Recently, the use of mirex has been increasingly restricted or
prohibited in many countries.
In the USA, all registered products containing mirex have been
cancelled. It has been banned in Ecuador and in various other
countries. In the German Democratic Republic, mirex is not permitted
in agricultural formulations.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD, USA Acceptable residue limit (ARL) 1981
ANIMAL - Specified animal products 0.1 mg/kg
FEED - General 0.01 mg/kg
FOOD Germany, Maximum residue limit (MRL) 1984
Federal - Plant (all) 0.01 mg/kg
Republic of
FOOD Animal Germany, Maximum residue limit (MRL) 1984
Federal - of animal origin (specified) 0.1 mg/kg
Republic of wet weight
0.1 mg/kg
lipid weight
- of animal origin (general) 0.01 mg/kg
wet weight
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classified mirex in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low
risk of poisoning in transport (mirex
liquid formulations >60%)
The label should be as follows:
 The bottom half of the label should bear the inscriptions:
Harmful, stow away from foodstuffs.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides,
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm, Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides,
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport, Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides, Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning, Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides,
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(unpublished document WHO/VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols. Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1984) Environmental Health Criteria 44: Mirex. Geneva, World
Health Organization.
WHO (1988) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1988-89, Geneva, World
Health Organization (unpublished document WHO/VBC/88.953).
WHO/FAO (1975-90) Data sheets on pesticides. Geneva, World Health
Organization (unpublished documents).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The bottom half of the label should bear the inscriptions:
Harmful, stow away from foodstuffs.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides,
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm, Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides,
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport, Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides, Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning, Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides,
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(unpublished document WHO/VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols. Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1984) Environmental Health Criteria 44: Mirex. Geneva, World
Health Organization.
WHO (1988) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1988-89, Geneva, World
Health Organization (unpublished document WHO/VBC/88.953).
WHO/FAO (1975-90) Data sheets on pesticides. Geneva, World Health
Organization (unpublished documents).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Lavenham, Lavenham Press Limited, British Crop Protection Council.
