
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 16
ETHYLENE OXIDE
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA
This is a companion volume to Environmental Health Criteria 55:
Ethylene Oxide
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
ISBN 92 4 154339 6
ISSN 0259 - 7268
(c) World Health Organization 1988
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Composition
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Human exposure to ethylene oxide
2.2. Fate in the environment
2.3. Uptake, metabolism, and excretion
2.4. Effects on organisms in the environment
2.5. Effects on animals
2.6. Effects on human beings
3. CONCLUSIONS AND RECOMMENDATIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and
protection, first aid
4.1.1. Advice to physicians
4.1.2. Health surveillance advice
4.2. Explosion and fire hazards
4.2.1. Explosion hazards
4.2.2. Fire hazards
4.2.3. Prevention
4.2.4. Fire-extinguishing agents
4.3. Storage
4.4. Transport
4.5. Spillage and disposal
4.5.1. Spillage
4.5.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. INTERNATIONAL CHEMICAL SAFETY CARD
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging, and transport
7.5. Waste disposal
7.6. Other measures
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) documents produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is an International Chemical Safety Card
which should be readily available, and should be clearly explained, to
all who could come into contact with the chemical. The section on
regulatory information has been extracted from the legal file of the
International Register of Potentially Toxic Chemicals (IRPTC) and from
other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Common name: Ethylene oxide
Chemical formula: C2H4O
Chemical structure: O
/ \
H - C - C - H
' '
H H
Common trade names: Anprolene, Melgas, Merpal, Sterigas P
(pure products); Carboxide, Cartox, Etox,
Oxyfume 20, Oxyfume 30, Sterigas 90/10,
Steroxide 20, T-gas (formulations with
carbon dioxide); Oxyfume 12, Sterigas
12/88, Steroxide 12/88 (formulations with
fluorocarbons); Etoxiat (formulation with
methyl formate)
Common synonyms: dihydrooxirene, dimethylene oxide,
1,2-epoxyethane, ethene oxide, oxane,
alpha,beta-oxido-ethane, oxirane
Abbreviations: EO, ETO, EtO
CAS registry number: 75-21-8
RTECS registry number: KX2450000
UN number: 1040 and 1041
Conversion factors: 1 ppm = 1.80 mg/m3 air; 1 mg/m3 = 0.55
ppm at 25°C and 101.3 kPa (760mmHg)
1.2 Physical and Chemical Properties
Ethylene oxide is a colourless gas at room temperature and normal
atmospheric pressure. It condenses to a liquid at 10°C. It has an
ethereal odour with reported odour thresholds of 470 mg/m3 for
perception and 900-1260 mg/m3 for recognition. It is highly
flammable and, in vapour form, is subject to explosive decomposition.
The liquid is stable to common detonating agents, but polymerization
may be violent after initiation by acids, bases, or heat.
Polymerization is catalysed by certain metal chlorides and hydroxides.
Both liquid and gaseous ethylene oxide are very reactive. It is
relatively stable in aqueous solution, or when diluted with carbon
dioxide or halocarbons. Some physical and chemical properties of
ethylene oxide are given on the International Chemical Safety Card on
pp. 20-23.
1.3 Composition
Common impurities found in ethylene oxide during the production
process involving the oxidation of ethene, include water, acetic acid,
acetaldehyde, and inorganic chlorides. Common impurities found in
ethylene oxide, produced by the chlorohydrin process, are vinyl
chloride, 1,2-dichloroethane, chloroethane, and ethylene chlorohydrin.
Ethylene oxide, sold for fumigation or sterilization purposes, is
often mixed with carbon dioxide (e.g., 10-30% ethylene oxide and
70-90% carbon dioxide) or fluorocarbons (e.g., 12% ethylene oxide and
88% fluorocarbons), as already indicated by the trade names in
section 1.1.
1.4 Production and Uses
The global production of ethylene oxide in 1985 was estimated to have
been greater than 5.5 million tonnes. The substance is produced in
many countries, the highest volume being produced in the USA.
Its major use is as an intermediate in the production of various
chemicals including: the antifreeze, ethylene glycol; polyethylene
terephthalate polyester for fibres, films, and bottles; non-ionic
surface active agents; glycol ethers; ethanolamines; and choline. A
small fraction of the total consumption (less than 1%) is used for the
fumigation and sterilization of foodstuffs and medical equipment.
2. SUMMARY AND EVALUATION
2.1 Human Exposure to Ethylene Oxide
Human exposure occurs mainly through inhalation in sterilization
facilities and in production plants. Residues in medical equipment
that has been sterilized with ethylene oxide and insufficiently
aerated can migrate into tissues and blood, producing primarily local
effects. Ingestion of ethylene oxide residues from most fumigated or
sterilized foodstuffs is unlikely, as they dissipate through
evaporation or reaction with food constituents. A major conversion
product in foodstuffs, pharmaceutical products, and medical equipment
is 2-chloroethanol, which is more persistent than ethylene oxide.
Levels of 2-chloroethanol as high as several g/kg have been measured
in food and levels of several hundred mg/kg in medical equipment.
Most ethylene oxide is used as a reactive intermediate in the chemical
plant in which it is produced. Because of the explosion hazard,
ethylene oxide is stored and handled in chemical process plants in
closed, automated systems. This equipment is often located outdoors,
and, except during maintenance, the probability of exposure of the
workers is minimal. Air samples collected in the processing areas of
chemical production plants have shown that ethylene oxide vapour
concentrations are now generally less than 4 mg/m3, with occasional
high peak values. Occupational exposure to ethylene oxide tends to be
much higher in health instrument manufacture and in hospital
sterilizationa areas than in the chemical processing industries.
Air levels of ethylene oxide near malfunctioning or improperly
designed equipment in industry may reach hundreds of mg/m3 for brief
periods. However, 8-h time-weighted average concentrations in the
breathing zone in hospital sterilization areas are generally less than
36 mg/m3. It should be emphasized that the exposure of hospital
workers to ethylene oxide tends to be of a short-term and intermittent
nature during loading and unloading, with the likelihood of exposure
to short-term (5-120 min) concentrations of about 100 mg/m3 and to
peak concentrations of up to 1800 mg/m3 following the opening of
sterilization chambers.
Ambient air levels to which members of the general population are
exposed at a distance from industrial point sources of emission have
been estimated to be below the limit of detection.
a Patients undergoing haemodialysis may also be exposed to
ethylene oxide from equipment sterilized with it. Cases of
anaphylaxis during dialysis have been ascribed to it.
2.2 Fate in the Environment
The main pathway of entry of ethylene oxide into the environment is
through evaporation or by venting gases during production, handling,
storage, transport, or use (e.g., sterilization procedures).
The removal of ethylene oxide from the atmosphere is slow, but may be
accelerated through washout by rain.
Ethylene oxide is highly soluble in water, but will evaporate to a
great extent. Degradation of ethylene oxide in neutral water is slow,
even in the presence of aerobic microorganisms, but more rapid under
acidic or basic conditions due to catalysis. Because of its limited
lipid solubility, it is unlikely that ethylene oxide and it conversion
products will bioaccumulate.
2.3 Uptake, Metabolism, and Excretion
Inhaled ethylene oxide is readily absorbed into the blood, distributed
throughout the body, and rapidly metabolized. The half-life in the
tissues of man and rodents is approximately 10 min; clearance from the
blood of dogs occurred with a half-life of 33 min. Marked nausea and
profuse vomiting following dermal exposure of man to aqueous solutions
of ethylene oxide indicate that absorption can occur through the skin.
Two metabolic pathways have been identified including hydrolysis to
1,2-ethanediol and conjugation with glutathione. Excretion is
primarily via the urine.
2.4 Effects on Organisms in the Environment
The toxicity of ethylene oxide for aquatic organisms is low.
Concentrations lethal for half the number of various aquatic organisms
in populations tested for 1-4 days were approximately 90 mg/litre
(LC50) or higher. 2-Chloroethanol, a degradation product in saline
water, is equally toxic, but, 1,2-ethanediol, a major degradation
product, is less toxic. Thus, the probable effects of ethylene oxide
on the aquatic environment are considered negligible. There are no
data concerning the toxicity of ethylene oxide for terrestrial
organisms.
2.5 Effects on Animals
Single oral doses of 330 mg/kg body weight (LD50) and exposure to
vapour concentrations of 2630 mg/m3 (LC50) for 4 h have been shown
to be lethal for half the number of exposed rats. The compound is
moderately toxic in acute exposures, according to the scale of Hodge &
Sterner.
Ethylene oxide directly alkylates proteins and DNA and is mutagenic in
microorganisms, plants, insects, mammalian cells in vitro, and mammals
in vivo, inducing both gene mutations and chromosomal abnormalities.
Sister chromatid exchanges and chromosomal aberrations have also been
observed in the blood lymphocytes of monkeys exposed by inhalation.
The potential of ethylene oxide to cause teratogenic or adverse
reproductive effects has been examined in four animal species (mouse,
rat, rabbit, and monkey) by two routes of administration. Results
from these studies show that ethylene oxide induces toxic effects on
the reproductive function in both males and females. The levels
needed to produce fetal effects approach or equal the dose needed to
produce maternal toxicity.
In experimental animal studies, it has been clearly demonstrated that
ethylene oxide is carcinogenic via different routes of exposure
(intragastric, subcutaneous injection, and inhalation). Ethylene
oxide induced forestomach carcinomas in rats following oral
administration and subcutaneous fibrosarcomas in mice following
subcutaneous injection. In two inhalation studies, confirmatory data
demonstrated dose-related increases in the incidences of leukaemia,
peritoneal mesothelioma, and cerebral glioma.
2.6 Effects on Human Beings
No ambient air monitoring data are available from which the effects of
ethylene oxide on the health of man and the environment can be
assessed. However, the risk of adverse health effects from exposure
to ethylene oxide in the ambient air, apart from point-source
emissions and accidental spillage, is likely to be negligible.
Case reports indicate that headache, nausea, vomiting, dyspnoea, and
respiratory tract irritation are common effects of acute inhalation
exposure to ethylene oxide. Respiratory tract irritation increases
with inhaled vapour concentration and may result in severe
life-threatening pulmonary disease after a latency period of several
hours. Cardiovascular collapse and renal failure have been attributed
to residues of ethylene oxide in medical equipment.a Case reports
and the results of animal studies indicate that sensorimotor
neuropathies may follow repeated exposure to concentrations of
ethylene oxide recognizable by its odour (approximately 900 mg/m3 or
more). Because of its high odour threshold (900-1260 mg/m3),
sensory recognition does not offer adequate warning of a health
hazard.
a Anaphylactic reactions have been reported during haemodialysis,
when using equipment sterilized with ethylene oxide.
Dermatological effects in man following skin contact with aqueous
ethylene oxide include erythema, oedema, and vesiculation, in that
order. The severity of the skin injury is related to concentration (a
50% solution (500 g/litre) being most hazardous) and duration of
contact. When liquid ethylene oxide vapourizes, it can result in a
freeze burn. On repeated exposure, ethylene oxide may cause allergic
contact dermatitis. Aqueous solutions of ethylene oxide and its
conversion products are irritating to the eyes and can produce corneal
injury. Ethylene oxide vapour or residues in medical equipment have
also been observed to produce irritant effects on the eyes and the
respiratory tract. The irritant effects on the eyes and skin are
often delayed. Cataracts have occurred following repeated exposure to
concentrations of the vapour recognizable by its odour (approximately
900 mg/m3).
In man, ethylene oxide may induce chromosomal aberrations and sister
chromatid exchanges in lymphocytes and micronuclei in erythroytes at
air concentrations that can be found in the work place. Tissue
distribution studies have provided evidence that ethylene oxide
reaches the gonads, supporting the findings of heritable mutations in
insects and rodents. Therefore, ethylene oxide may be considered a
potential human mutagen for both somatic and germ cells.
The results of animal studies suggest possible reproductive impairment
in human males, but are inadequate for assessing the fetal risk. Data
on the reproductive effects in human beings are insufficient. The
results of one study suggest an increase in spontaneous abortion rate
in pregnant women occupationally exposed to ethylene oxide at an 8-h
time-weighted average of 0.18-0.90 mg/m3, with peak concentrations
of up to 450 mg/m3. However, exposure data are limited and this
prevents the establishment of a relationship between abortion rates
and exposure levels.
Two epidemiological studies have shown an association between ethylene
oxide exposure and an excess risk of cancer, but both studies have
limitations. Airborne concentrations of ethylene oxide in the two
studies were reported to be time-weighted averages of 36±18 mg/m3
and 10-50 mg/m3, with occasional brief exposures in excess of the
odour threshold (900-1260 mg/m3). Although the evidence for the
carcinogenicity of ethylene oxide alone in man is inadequate, these
two studies indicate that exposure to ethylene oxide increases the
risk of malignancies.
Taking into account available data concerning the alkylating nature of
ethylene oxide, the demonstration of DNA adducts, the overwhelming
positive in vivo responses in mutagenic and clastogenic assays, the
reproducible positive carcinogenic findings in animals, and the
epidemiological findings suggesting an increase in the incidence of
human cancer, ethylene oxide should be considered as a probable human
carcinogen, and its levels in the environment should be kept as low as
feasible.
3. CONCLUSIONS AND RECOMMENDATIONS
The risks for the health of the general population from exposure to
ethylene oxide in the ambient air, apart from point-source emissions
and accidental spillage, are likely to be negligible.
Common effects of single inhalation exposures are headache, nausea,
vomiting, dyspnoea, and respiratory tract irritation, which may result
in lung oedema. Sensorimotor neuropathies and eye cataracts may
follow repeated exposure to a concentration of ethylene oxide
recognizable by its odour. Odour recognition does not offer adequate
warning of a health hazard. Ethylene oxide solutions in water are
irritating to the skin and eyes. The pure liquid can cause a freeze
burn. Absorption through the skin, even from dilute solutions, may
cause systemic effects. On repeated exposure of the skin, ethylene
oxide solutions may cause allergic contact dermatitis.
Taking into account all the available data, ethylene oxide should be
considered as a mutagen and a probable human carcinogen. It may pose
a reproductive hazard. Its levels in the environment should be kept
as low as possible.
From: Environmental Health Criteria 55: Ethylene Oxide (WHO, 1985).
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
The human health hazards associated with certain types of exposure to
ethylene oxide, together with preventive and protective measures and
first aid recommendations are listed on the International Chemical
Safety Card on pp. 20-23.
4.1.1 Advice to Physicians
No specific antidote is known. Treat symptomatically. If skin
sensitization has developed following exposure to ethylene oxide,
further exposure should not be allowed. Be aware of the possibility
of neuropathies.
4.1.2 Health Surveillance Advice
Human beings potentially exposed to ethylene oxide should undergo
periodic medical examination with particular emphasis given to the
pulmonary, haematological, neurological, and reproductive systems, and
to the eyes and skin. The physician should be aware of increased risk
of cancer and reproductive effects associated with exposure to
ethylene oxide.
Exposure to ethylene oxide should be checked regularly by general
and/or personal monitoring.
4.2 Explosion and Fire Hazards
4.2.1 Explosion hazards
Ethylene oxide-air mixtures containing 2.7% by volume or more of
ethylene oxide may be explosive (the flash point is -57°C and
autoignition occurs at 429°C) and can be ignited by sources of heat or
ignition. The vapour of ethylene oxide is subject to explosive
decomposition above 425°C. The vapour, which is heavier than air, may
travel along the ground and be ignited from a distance.
The liquid may polymerize violently after initiation by acids, bases,
or heat. The polymerization is catalysed by certain metal chlorides
and metal hydroxides. Ethylene oxide reacts violently with many
compounds, including oxidizing agents, acids, organic bases, ammonia,
amines, alcohols, mercaptans, alkane thiols, and bromoethane.
4.2.2 Fire hazards
Ethylene oxide is a flammable gas. In the vapour form, it will burn
without the presence of air or other oxidizing agents.
4.2.3 Prevention
Use closed systems, ventilation, explosion-proof electrical equipment
and lighting, and spark-proof hand tools. Do not use ethylene oxide
near sources of heat or ignition. Do not smoke. Do not use
compressed air for filling, discharging, or handling. Purge air from
unloading system before unloading. Avoid contact of the compound with
incompatible materials (section 4.2.1).
In case of fire, shut off the supply. If this is impossible and there
is no risk to the surroundings, let the fire burn itself out. Cool
fire-exposed containers by spraying with water from a safe distance.
Fire-fighters should be equipped with self-contained breathing
apparatus, eye protection, and full protective clothing.
4.2.4 Fire-extinguishing agents
Dilution of ethylene oxide with 23 volumes of water raises its flash
point, thereby reducing the possibility of ignition at ambient
temperature. Dry chemical powder can be used to extinguish small
fires. Alcohol resistant foam should be used on large fires.
However, in all cases of fire, the prime requirement before
extinguishing the fire should be to attempt to shut off the vapour
escape.
4.3 Storage
Ethylene oxide should be stored in a cool, well-labelled,
well-ventilated, fire-proof area, preferably away from other chemicals
and outdoors. The storage area should be surrounded by a retaining
wall or a sill. Keep away all sources of ignition and heat as well as
incompatible materials (section 4.2.1). Containers for ethylene oxide
can be made of stainless steel, aluminium 3003, zinc, nickel, copper,
teflon, ceramics, or glass. Storage vessels should contain a positive
pressure layer of inert nitrogen. A pressure vessel check programme
should be made. Storage pressure and temperature ranges are
240-410 kPa and 10-15°C, respectively. When long storage times are
anticipated, a lower temperature range is recommended to minimize
polymerization. Prolonged storage in small containers where there is
a high surface to volume ratio should be avoided, because the
polymerization rate is increased by the presence of any metal.
Mixtures of ethylene oxide and water should not be left dormant for
any length of time, and the temperature and pressure of vessels
containing these mixtures should be carefully monitored.
4.4 Transport
In case of accident, stop the engine. Remove all sources of ignition.
Keep bystanders at a distance, mark the roads and keep upwind.
Evacuate the area endangered by poison gas. Wear full protective
clothing and self-contained breathing apparatus. In case of spillage
or fire, use the methods advised in sections 4.5 and 4.2,
respectively. In case of poisoning, follow the advice in section 4.1.
Notify the police and fire brigade immediately.
4.5 Spillage and Disposal
4.5.1 Spillage
Remove all ignition sources and evacuate the danger area. Provide
optimum ventilation, which should be explosion-proof. Spilled liquid
should be washed away with water. Avoid run-off into drains or sewers
(explosion hazard). Ensure personal protection by use of
self-contained breathing apparatus and full protective clothing.
4.5.2 Disposal
Ethylene oxide can be disposed of by evaporation in a open area or by
burning, after ignition from a safe distance. Burning in an
incinerator can cause difficulties, unless a gas feed can be arranged,
as ethylene oxide boils at 10°C. It is soluble in water or alcohol,
and these solutions can be incinerated.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Ethylene oxide will evaporate from water. Degradation of ethylene
oxide in the atmosphere and in neutral water is slow, but it is more
rapid in acidic or basic solutions. Bioaccumulation of ethylene oxide
and its conversion products is unlikely.
The toxicity of ethylene oxide for aquatic organisms is low. The
effects of ethylene oxide on the aquatic environment are considered to
be negligible.
Contamination of soil, water, and the atmosphere can be avoided by
proper methods of storage, transport, handling, and waste disposal.
In cases of spillage, apply methods recommended in section 4.5.1.
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily available to all health workers concerned
with, and users of, ethylene oxide. It should be displayed at, or
near, entrances to areas where there is potential exposure to
ethylene oxide, and on processing equipment and containers. The card
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the instructions
on the chemical safety card clearly explained. Space is available on
the card for insertion of the National Occupational Exposure Limit,
the address and telephone number of the National Poison Control
Centre, and for local trade names.
ETHYLENE OXIDE
(EO, ETO, 1,2-epoxyethane, ethene oxide, oxirane)
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Relative molecular mass 44.05 Colourless gas with ethereal odour; the gas is heavier
Appearance colourless gas than air, may travel along the ground, and be ignited
Odour ethereal from a distance; it is subject to explosive decomposition
Odour perception threshold (mg/m3) 470 above 425°C; the liquid may polymerize violently
Melting point (°C) -111 after initiation by acids, bases, or heat;
Boiling point (°C) 10.4 polymerization is catalysed by metal chlorides and
Solubility in water (20°C) infinite metal hydroxides; ethylene oxide reacts violently with
Density (20°C) (g/ml) 0.87 many compounds, including oxidizing agents, acids,
Relative vapour density 1.5 organic bases, ammonia, amines, alcohols, mercaptans,
Vapour pressure (20°C) (kPa) 146 alkane thiols, and bromoethane; it can induce adverse
Flash point (°C) (open cup) -57°C effects at levels well below the odour threshold
Flammable (explosive) limits (vol %) 2.7-100
Autoignition (°C) 429
log n-octanol/water partition
coefficient -0.30
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: Irritation by solutions Wear clean protective impervious Remove contaminated clothing (not in case
in water; concentrated liquid gloves and boots and clean impermeable of frost bite) and shoes immediately; rinse
may cause freeze burn with body-covering clothing; do not wear with water for at least 15 minutes; treat
redness and pain leatherware for inhalation exposure; seek medical
attention
EYES: Irritation by vapour and Wear safety face shield or eye Rinse open eyes with plenty of water for
liquid; pure liquid may cause protection in combination with at least 15 minutes; treat for inhalation;
freeze burn with redness, pain, breathing protection seek medical attention
and blurred vision
INHALATION: Irritation of Use explosion-proof general or Remove victim to fresh air; keep victim
respiratory tract; dyspnoea; exhaust ventilation, and, for quiet and warm; if breathing has stopped,
effects on the central nervous non-routine activities, use apply artificial respiration; transport
system, such as headache, nausea, self-contained breathing to hospital: pulmonary oedema may occur
and vomiting apparatus after a latent period
INGESTION: Irritation of mouth, Do not eat, drink, or smoke during Rinse mouth; give plenty of water to drink;
throat, and stomach; see work do not induce vomiting; transport to
inhalation hospital
REPEATED EXPOSURE: Ethylene Exposure should be kept as low as
oxide should be considered feasible
as a probable human mutagen
and carcinogen; it may pose
a reproductive hazard; and may
cause allergic contact
dermatitis
ENVIRONMENT: Low toxicity Apply proper methods of storage,
for aquatic life transport, waste disposal, and
handling of spills (see below)
SPILLAGE STORAGE FIRE AND EXPLOSION
Remove all ignition sources; Store in well-labelled, cool, well- Flammable; vapour-air mixtures may be
evacuate area; provide optimum ventilated, fire-proof area, explosive; use closed systems, ventilation,
explosion-proof ventilation; preferably separated and outside; store and explosion-proof equipment; do not use
wash spilled liquid away with away from incompatible substances; compressed air for filling, discharging, or
water; ensure personal storage vessels should have a positive handling; no source of ignition or heat; in
protection by use of self- pressure layer of nitrogen case of fire, keep containers cool by
contained breathing apparatus spraying with water; extinguish fire with
and full protective clothing carbon dioxide, dry chemical, powder,
water, or foam
WASTE DISPOSAL
Evaporate in an open area; National Occupational Exposure Limit: UN: 1040 and 1041
burn after ignition from a
safe distance; incinerate National Poison Control Centre
after dissolution in water
or alcohol Local Trade Names:
 7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
An International Agency for Research on Cancer Working Group (IARC,
1985) evaluated the carcinogenicity of ethylene oxide and concluded
that:
"There is sufficient evidence for the carcinogenicity of ethylene
oxide to experimental animals; there is limited evidence for the
carcinogenicity to humans of exposures to ethylene oxide in
combination with other chemicals; there is inadequate evidence for the
carcinogenicity to humans of exposures to ethylene oxide alone. Taken
together, the data indicate that ethylene oxide is probably
carcinogenic to humans." This conclusion was confirmed in 1987.
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on ethylene oxide in 1965, 1968 and 1971.
It concluded that the data available were insufficient to establish an
acceptable daily intake (ADI) for man for either ethylene oxide or
ethylene chlorohydrin and other reaction products.
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pp. 26-28.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
7.3 Specific Restrictions
The European Community legislation prohibits the putting on the market
of cosmetics containing ethylene oxide.
The European Community legislation prohibits the putting on the market
and use of plant protection products containing ethylene oxide.
Nevertheless, the Member States may temporarily agree derogations for
some minor uses until 31 December, 1989.
In Czechoslovakia, as a suspected carcinogen, the use and handling of
ethylene oxide must be reported by the user to the authorities.
Requirements and restrictions on handling, labelling, packaging,
storage, and transport are listed (1985).
Denmark prohibits the manufacture, marketing, and importation of
foodstuffs or food ingredients treated with ethylene oxide (1986).
In the Federal Republic of Germany, handling of ethylene oxide is
prohibited or restricted for adolescents and pregnant and nursing
women (1980).
In Kenya, the compound is permitted for the fumigation of specified
food products, and maximum levels of use are listed (1982 (r)).
In the USSR, the pesticide ethylene oxide is prohibited in specified
food products (1983).
In the USA, in the occupational environment, an "action level" of
0.5 ppm (8-h time-weighted average) has been established as the level
above which employers must initiate certain compliance activities such
as periodic monitoring of employee exposure and medical surveillance
(1984).
In Canada, application is restricted to licenced commercial
applicators only and residues on food must be below 0.1 ppm (1987).
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
AIR Occupational Australia Threshold limit value (TLV) 1985 (r)
- Time-weighted average (TWA) 90 mg/m3
Czechoslovakia Maximum allowable concentration (MAC) 1985
- Time-weighted average (TWA) 1 mg/m3b
- Ceiling value 5 mg/m3 1985
Denmark Threshold limit value (TLV) 1985
- Ceiling value 1.8 mg/m3
Finland Maximum allowable concentration 1981
- Time-weighted average (TWA) 20 mg/m3 1981
- Short-term exposure limit (STEL) 40 mg/m3
(15-min time-weighted average)
Germany, Maximum work-site concentration withdrawn 1986 (r)
Federal (carcinogenicity)
Republic of
AIR Occupational Germany, Technical reference value 5 mg/m3 1986
Federal
Republic of
Hungary Maximum allowable concentration (MAC) 1985 (r)
- Time-weighted average (TWA) 1 mg/m3
- Short-term exposure limit (STEL) 1 mg/m3
(30-min time-weighted average)
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
Japan Maximum allowable concentration (MAC) 90 mg/m13 1982 (r)
Netherlands Maximum limit 1982 (r)
- Time-weighted average (TWA) 90 mg/m3
Romania Maximum permissible concentration 1985 (r)
- Time-weighted average (TWA) 30 mg/m3
- Ceiling value 60 mg/m3
Sweden Threshold limit value (TLV) 1985
- Time-weighted average (TWA) 9 mg/m3c
Sweden - Short-term exposure limit 18 mg/m3
(15-min time-weighted average)
AIR Occupational United Kingdom Threshold limit value (TLV) 10 mg/m3 1985 (r)
USA (OSHA) Permissible exposure limit (PEL) 1984
- Time-weighted average (TWA) 2 mg/m3
USA (ACGIH) Threshold limit value 1986
- Time-weighted average 2 mg/m3
USSR - Ceiling value 1 mg/m3 1977
AIR Ambient USSR Maximum allowable concentration (MAC) 1984
- Once per day 0.3 mg/m3
- Average per day 0.03 mg/m3
a TWA = time-weighted average over one working day (usually 8 h).
b Suspected carcinogenic potential for man.
c Carcinogenic; for new and renovated plants, a TWA of 1.8 mg/m3 is desired.
7.4 Labelling, Packaging, and Transport
The European Community legislation requires labelling as a dangerous
substance using the symbol(s):
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
An International Agency for Research on Cancer Working Group (IARC,
1985) evaluated the carcinogenicity of ethylene oxide and concluded
that:
"There is sufficient evidence for the carcinogenicity of ethylene
oxide to experimental animals; there is limited evidence for the
carcinogenicity to humans of exposures to ethylene oxide in
combination with other chemicals; there is inadequate evidence for the
carcinogenicity to humans of exposures to ethylene oxide alone. Taken
together, the data indicate that ethylene oxide is probably
carcinogenic to humans." This conclusion was confirmed in 1987.
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on ethylene oxide in 1965, 1968 and 1971.
It concluded that the data available were insufficient to establish an
acceptable daily intake (ADI) for man for either ethylene oxide or
ethylene chlorohydrin and other reaction products.
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pp. 26-28.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
7.3 Specific Restrictions
The European Community legislation prohibits the putting on the market
of cosmetics containing ethylene oxide.
The European Community legislation prohibits the putting on the market
and use of plant protection products containing ethylene oxide.
Nevertheless, the Member States may temporarily agree derogations for
some minor uses until 31 December, 1989.
In Czechoslovakia, as a suspected carcinogen, the use and handling of
ethylene oxide must be reported by the user to the authorities.
Requirements and restrictions on handling, labelling, packaging,
storage, and transport are listed (1985).
Denmark prohibits the manufacture, marketing, and importation of
foodstuffs or food ingredients treated with ethylene oxide (1986).
In the Federal Republic of Germany, handling of ethylene oxide is
prohibited or restricted for adolescents and pregnant and nursing
women (1980).
In Kenya, the compound is permitted for the fumigation of specified
food products, and maximum levels of use are listed (1982 (r)).
In the USSR, the pesticide ethylene oxide is prohibited in specified
food products (1983).
In the USA, in the occupational environment, an "action level" of
0.5 ppm (8-h time-weighted average) has been established as the level
above which employers must initiate certain compliance activities such
as periodic monitoring of employee exposure and medical surveillance
(1984).
In Canada, application is restricted to licenced commercial
applicators only and residues on food must be below 0.1 ppm (1987).
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
AIR Occupational Australia Threshold limit value (TLV) 1985 (r)
- Time-weighted average (TWA) 90 mg/m3
Czechoslovakia Maximum allowable concentration (MAC) 1985
- Time-weighted average (TWA) 1 mg/m3b
- Ceiling value 5 mg/m3 1985
Denmark Threshold limit value (TLV) 1985
- Ceiling value 1.8 mg/m3
Finland Maximum allowable concentration 1981
- Time-weighted average (TWA) 20 mg/m3 1981
- Short-term exposure limit (STEL) 40 mg/m3
(15-min time-weighted average)
Germany, Maximum work-site concentration withdrawn 1986 (r)
Federal (carcinogenicity)
Republic of
AIR Occupational Germany, Technical reference value 5 mg/m3 1986
Federal
Republic of
Hungary Maximum allowable concentration (MAC) 1985 (r)
- Time-weighted average (TWA) 1 mg/m3
- Short-term exposure limit (STEL) 1 mg/m3
(30-min time-weighted average)
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
Japan Maximum allowable concentration (MAC) 90 mg/m13 1982 (r)
Netherlands Maximum limit 1982 (r)
- Time-weighted average (TWA) 90 mg/m3
Romania Maximum permissible concentration 1985 (r)
- Time-weighted average (TWA) 30 mg/m3
- Ceiling value 60 mg/m3
Sweden Threshold limit value (TLV) 1985
- Time-weighted average (TWA) 9 mg/m3c
Sweden - Short-term exposure limit 18 mg/m3
(15-min time-weighted average)
AIR Occupational United Kingdom Threshold limit value (TLV) 10 mg/m3 1985 (r)
USA (OSHA) Permissible exposure limit (PEL) 1984
- Time-weighted average (TWA) 2 mg/m3
USA (ACGIH) Threshold limit value 1986
- Time-weighted average 2 mg/m3
USSR - Ceiling value 1 mg/m3 1977
AIR Ambient USSR Maximum allowable concentration (MAC) 1984
- Once per day 0.3 mg/m3
- Average per day 0.03 mg/m3
a TWA = time-weighted average over one working day (usually 8 h).
b Suspected carcinogenic potential for man.
c Carcinogenic; for new and renovated plants, a TWA of 1.8 mg/m3 is desired.
7.4 Labelling, Packaging, and Transport
The European Community legislation requires labelling as a dangerous
substance using the symbol(s):
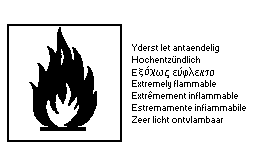
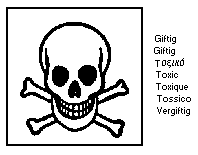 The label must read:
may cause cancer; may cause heritable genetic damage; extremely
flammable liquified gas; toxic by inhalation; irritating to
eyes, respiratory system and skin; not recommended for interior
use onlarge surface areas; keep container tightly closed, in a
cool, well-ventilated place; keep away from sources of ignition
-- no smoking; take precautionary measures against static
discharges; if you feel unwell, seek medical advice (show the
label where possible).
The European Community legislation on labelling of pesticide
preparations classifies ethylene oxide in Class I/a for the purpose of
determining the label for preparations containing ethylene oxide and
other active ingredients.
The International Maritime Organization classifies ethylene oxide as
a flammable, slightly poisonous (liquified) gas (Hazard Class 2(3,
6.1).
The recommended label is:
The label must read:
may cause cancer; may cause heritable genetic damage; extremely
flammable liquified gas; toxic by inhalation; irritating to
eyes, respiratory system and skin; not recommended for interior
use onlarge surface areas; keep container tightly closed, in a
cool, well-ventilated place; keep away from sources of ignition
-- no smoking; take precautionary measures against static
discharges; if you feel unwell, seek medical advice (show the
label where possible).
The European Community legislation on labelling of pesticide
preparations classifies ethylene oxide in Class I/a for the purpose of
determining the label for preparations containing ethylene oxide and
other active ingredients.
The International Maritime Organization classifies ethylene oxide as
a flammable, slightly poisonous (liquified) gas (Hazard Class 2(3,
6.1).
The recommended label is:
 In the USA, certain specified vessels carrying ethylene oxide in bulk
and bound for, or departing from, US ports must notify the captain of
the port at least 24 h in advance and must give him certain
information (1983 (r)).
7.5 Waste Disposal
In the Federal Republic of Germany, the emission into the air of
organic compounds in Class I, which includes ethylene oxide, must not
exceed (as the sum of all compounds in this class) a mass
concentration of 20 mg/m3 at a mass flow of greater than 0.1 kg/h.
If compounds of different classes are present, the mass concentration
must not exceed 30 mg/m3 (1982 (r)).
In the USA, any solid waste (except domestic) that contains ethylene
oxide must be listed as hazardous waste (subject to handling,
transport, treatment, storage and disposal regulation, and permit and
notification requirements) unless it is found that the waste cannot
pose a threat to human health or the environment when improperly
managed. If ethylene oxide is a commercial chemical product, it is
identified as "toxic waste" subject to handling, transport, treatment,
storage and disposal regulation and permit and notification
requirements (1980). An owner or operator of a hazardous waste
incinerator must achieve 99.99% destruction and removal efficiency for
this substance, if it is designated as a principal organic hazardous
constituent in its EPA permit (1981). Under the Comprehensive
Environmental Response, Compensation, and Liability Act of 1980
(CERCLA), unless in compliance with a specified permit or procedure,
owners/operators of vessels or on- or offshore facilities must notify
the US government (National Response Center) of any release of the
hazardous substance in or on navigable waters, adjoining shorelines,
the contiguous zone or beyond the contiguous zone or to any other
environmental media (air, land and ground water) in an amount equal to
or greater than 0.454 kilogram in any 24-h period (1985 (r)).
7.6 Other Measures
The European Community legislation concerning the major accident
hazards of certain industrial activities foresees that the
manufacturer must take all necessary measures to prevent accidents and
to limit their consequences for man and the environment, when
processing ethylene oxide or when storing it in quantities equal to,
or over, 50 tonnes.
Furthermore, when ethylene oxide is processed in quantities equal to,
or over, 50 tonnes, or is stored in quantities equal to, or over,
300 tonnes, notification has to be made to the competent authorities
including information on the substance, on the installation,
information on possible major accident situations, and emergency
plans.
BIBLIOGRAPHY
BRETHERICK, L. (1981) Hazards in the chemical laboratory, 3rd ed.
London, The Royal Society of Chemistry.
CEC (1984) Classification and labelling of dangerous substances.
Brussels, Commission of the European Communities.
DUTCH CHEMICAL INDUSTRY ASSOCIATION (1980) Handling chemicals
safely, 2nd ed. Dutch Association of Safety Experts, Dutch Safety
Institute.
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E (1976)
Clinical toxicology of commercial products, 5th ed. Baltimore,
Maryland, Williams and Wilkins Company.
HOMMEL, G. (1987) [Handbook of dangerous goods.] 2nd ed. Berlin,
Springer Verlag (in German).
IARC (1972 - present) IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC Data profile on individual chemical substances. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme (unpublished file).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH Current intelligence bulletins. Washington, DC, US
Department of Health, Education and Welfare.
US NIOSH (1976) A guide to industrial respirator protection.
Cincinnati, Ohio, US National Institute for Occupational Safety and
Health, pp. 76-189.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols, Washington DC, US Department of Health and Human
Services, US Departmnent of Labor (Publication No. DHSS(NIOSH)
01-123).
US NIOSH/OSHA (1985) Pocket guide to chemical hazards. Washington
DC, US Department of Health, Education and Welfare, US Department of
Labor (Publication No. 85.114).
WHO (1985) EHC No. 55: Ethylene oxide. Geneva, World Health
Organization, 78pp.
In the USA, certain specified vessels carrying ethylene oxide in bulk
and bound for, or departing from, US ports must notify the captain of
the port at least 24 h in advance and must give him certain
information (1983 (r)).
7.5 Waste Disposal
In the Federal Republic of Germany, the emission into the air of
organic compounds in Class I, which includes ethylene oxide, must not
exceed (as the sum of all compounds in this class) a mass
concentration of 20 mg/m3 at a mass flow of greater than 0.1 kg/h.
If compounds of different classes are present, the mass concentration
must not exceed 30 mg/m3 (1982 (r)).
In the USA, any solid waste (except domestic) that contains ethylene
oxide must be listed as hazardous waste (subject to handling,
transport, treatment, storage and disposal regulation, and permit and
notification requirements) unless it is found that the waste cannot
pose a threat to human health or the environment when improperly
managed. If ethylene oxide is a commercial chemical product, it is
identified as "toxic waste" subject to handling, transport, treatment,
storage and disposal regulation and permit and notification
requirements (1980). An owner or operator of a hazardous waste
incinerator must achieve 99.99% destruction and removal efficiency for
this substance, if it is designated as a principal organic hazardous
constituent in its EPA permit (1981). Under the Comprehensive
Environmental Response, Compensation, and Liability Act of 1980
(CERCLA), unless in compliance with a specified permit or procedure,
owners/operators of vessels or on- or offshore facilities must notify
the US government (National Response Center) of any release of the
hazardous substance in or on navigable waters, adjoining shorelines,
the contiguous zone or beyond the contiguous zone or to any other
environmental media (air, land and ground water) in an amount equal to
or greater than 0.454 kilogram in any 24-h period (1985 (r)).
7.6 Other Measures
The European Community legislation concerning the major accident
hazards of certain industrial activities foresees that the
manufacturer must take all necessary measures to prevent accidents and
to limit their consequences for man and the environment, when
processing ethylene oxide or when storing it in quantities equal to,
or over, 50 tonnes.
Furthermore, when ethylene oxide is processed in quantities equal to,
or over, 50 tonnes, or is stored in quantities equal to, or over,
300 tonnes, notification has to be made to the competent authorities
including information on the substance, on the installation,
information on possible major accident situations, and emergency
plans.
BIBLIOGRAPHY
BRETHERICK, L. (1981) Hazards in the chemical laboratory, 3rd ed.
London, The Royal Society of Chemistry.
CEC (1984) Classification and labelling of dangerous substances.
Brussels, Commission of the European Communities.
DUTCH CHEMICAL INDUSTRY ASSOCIATION (1980) Handling chemicals
safely, 2nd ed. Dutch Association of Safety Experts, Dutch Safety
Institute.
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E (1976)
Clinical toxicology of commercial products, 5th ed. Baltimore,
Maryland, Williams and Wilkins Company.
HOMMEL, G. (1987) [Handbook of dangerous goods.] 2nd ed. Berlin,
Springer Verlag (in German).
IARC (1972 - present) IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC Data profile on individual chemical substances. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme (unpublished file).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH Current intelligence bulletins. Washington, DC, US
Department of Health, Education and Welfare.
US NIOSH (1976) A guide to industrial respirator protection.
Cincinnati, Ohio, US National Institute for Occupational Safety and
Health, pp. 76-189.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols, Washington DC, US Department of Health and Human
Services, US Departmnent of Labor (Publication No. DHSS(NIOSH)
01-123).
US NIOSH/OSHA (1985) Pocket guide to chemical hazards. Washington
DC, US Department of Health, Education and Welfare, US Department of
Labor (Publication No. 85.114).
WHO (1985) EHC No. 55: Ethylene oxide. Geneva, World Health
Organization, 78pp.
 7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
An International Agency for Research on Cancer Working Group (IARC,
1985) evaluated the carcinogenicity of ethylene oxide and concluded
that:
"There is sufficient evidence for the carcinogenicity of ethylene
oxide to experimental animals; there is limited evidence for the
carcinogenicity to humans of exposures to ethylene oxide in
combination with other chemicals; there is inadequate evidence for the
carcinogenicity to humans of exposures to ethylene oxide alone. Taken
together, the data indicate that ethylene oxide is probably
carcinogenic to humans." This conclusion was confirmed in 1987.
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on ethylene oxide in 1965, 1968 and 1971.
It concluded that the data available were insufficient to establish an
acceptable daily intake (ADI) for man for either ethylene oxide or
ethylene chlorohydrin and other reaction products.
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pp. 26-28.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
7.3 Specific Restrictions
The European Community legislation prohibits the putting on the market
of cosmetics containing ethylene oxide.
The European Community legislation prohibits the putting on the market
and use of plant protection products containing ethylene oxide.
Nevertheless, the Member States may temporarily agree derogations for
some minor uses until 31 December, 1989.
In Czechoslovakia, as a suspected carcinogen, the use and handling of
ethylene oxide must be reported by the user to the authorities.
Requirements and restrictions on handling, labelling, packaging,
storage, and transport are listed (1985).
Denmark prohibits the manufacture, marketing, and importation of
foodstuffs or food ingredients treated with ethylene oxide (1986).
In the Federal Republic of Germany, handling of ethylene oxide is
prohibited or restricted for adolescents and pregnant and nursing
women (1980).
In Kenya, the compound is permitted for the fumigation of specified
food products, and maximum levels of use are listed (1982 (r)).
In the USSR, the pesticide ethylene oxide is prohibited in specified
food products (1983).
In the USA, in the occupational environment, an "action level" of
0.5 ppm (8-h time-weighted average) has been established as the level
above which employers must initiate certain compliance activities such
as periodic monitoring of employee exposure and medical surveillance
(1984).
In Canada, application is restricted to licenced commercial
applicators only and residues on food must be below 0.1 ppm (1987).
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
AIR Occupational Australia Threshold limit value (TLV) 1985 (r)
- Time-weighted average (TWA) 90 mg/m3
Czechoslovakia Maximum allowable concentration (MAC) 1985
- Time-weighted average (TWA) 1 mg/m3b
- Ceiling value 5 mg/m3 1985
Denmark Threshold limit value (TLV) 1985
- Ceiling value 1.8 mg/m3
Finland Maximum allowable concentration 1981
- Time-weighted average (TWA) 20 mg/m3 1981
- Short-term exposure limit (STEL) 40 mg/m3
(15-min time-weighted average)
Germany, Maximum work-site concentration withdrawn 1986 (r)
Federal (carcinogenicity)
Republic of
AIR Occupational Germany, Technical reference value 5 mg/m3 1986
Federal
Republic of
Hungary Maximum allowable concentration (MAC) 1985 (r)
- Time-weighted average (TWA) 1 mg/m3
- Short-term exposure limit (STEL) 1 mg/m3
(30-min time-weighted average)
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
Japan Maximum allowable concentration (MAC) 90 mg/m13 1982 (r)
Netherlands Maximum limit 1982 (r)
- Time-weighted average (TWA) 90 mg/m3
Romania Maximum permissible concentration 1985 (r)
- Time-weighted average (TWA) 30 mg/m3
- Ceiling value 60 mg/m3
Sweden Threshold limit value (TLV) 1985
- Time-weighted average (TWA) 9 mg/m3c
Sweden - Short-term exposure limit 18 mg/m3
(15-min time-weighted average)
AIR Occupational United Kingdom Threshold limit value (TLV) 10 mg/m3 1985 (r)
USA (OSHA) Permissible exposure limit (PEL) 1984
- Time-weighted average (TWA) 2 mg/m3
USA (ACGIH) Threshold limit value 1986
- Time-weighted average 2 mg/m3
USSR - Ceiling value 1 mg/m3 1977
AIR Ambient USSR Maximum allowable concentration (MAC) 1984
- Once per day 0.3 mg/m3
- Average per day 0.03 mg/m3
a TWA = time-weighted average over one working day (usually 8 h).
b Suspected carcinogenic potential for man.
c Carcinogenic; for new and renovated plants, a TWA of 1.8 mg/m3 is desired.
7.4 Labelling, Packaging, and Transport
The European Community legislation requires labelling as a dangerous
substance using the symbol(s):
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
An International Agency for Research on Cancer Working Group (IARC,
1985) evaluated the carcinogenicity of ethylene oxide and concluded
that:
"There is sufficient evidence for the carcinogenicity of ethylene
oxide to experimental animals; there is limited evidence for the
carcinogenicity to humans of exposures to ethylene oxide in
combination with other chemicals; there is inadequate evidence for the
carcinogenicity to humans of exposures to ethylene oxide alone. Taken
together, the data indicate that ethylene oxide is probably
carcinogenic to humans." This conclusion was confirmed in 1987.
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on ethylene oxide in 1965, 1968 and 1971.
It concluded that the data available were insufficient to establish an
acceptable daily intake (ADI) for man for either ethylene oxide or
ethylene chlorohydrin and other reaction products.
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pp. 26-28.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
7.3 Specific Restrictions
The European Community legislation prohibits the putting on the market
of cosmetics containing ethylene oxide.
The European Community legislation prohibits the putting on the market
and use of plant protection products containing ethylene oxide.
Nevertheless, the Member States may temporarily agree derogations for
some minor uses until 31 December, 1989.
In Czechoslovakia, as a suspected carcinogen, the use and handling of
ethylene oxide must be reported by the user to the authorities.
Requirements and restrictions on handling, labelling, packaging,
storage, and transport are listed (1985).
Denmark prohibits the manufacture, marketing, and importation of
foodstuffs or food ingredients treated with ethylene oxide (1986).
In the Federal Republic of Germany, handling of ethylene oxide is
prohibited or restricted for adolescents and pregnant and nursing
women (1980).
In Kenya, the compound is permitted for the fumigation of specified
food products, and maximum levels of use are listed (1982 (r)).
In the USSR, the pesticide ethylene oxide is prohibited in specified
food products (1983).
In the USA, in the occupational environment, an "action level" of
0.5 ppm (8-h time-weighted average) has been established as the level
above which employers must initiate certain compliance activities such
as periodic monitoring of employee exposure and medical surveillance
(1984).
In Canada, application is restricted to licenced commercial
applicators only and residues on food must be below 0.1 ppm (1987).
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
AIR Occupational Australia Threshold limit value (TLV) 1985 (r)
- Time-weighted average (TWA) 90 mg/m3
Czechoslovakia Maximum allowable concentration (MAC) 1985
- Time-weighted average (TWA) 1 mg/m3b
- Ceiling value 5 mg/m3 1985
Denmark Threshold limit value (TLV) 1985
- Ceiling value 1.8 mg/m3
Finland Maximum allowable concentration 1981
- Time-weighted average (TWA) 20 mg/m3 1981
- Short-term exposure limit (STEL) 40 mg/m3
(15-min time-weighted average)
Germany, Maximum work-site concentration withdrawn 1986 (r)
Federal (carcinogenicity)
Republic of
AIR Occupational Germany, Technical reference value 5 mg/m3 1986
Federal
Republic of
Hungary Maximum allowable concentration (MAC) 1985 (r)
- Time-weighted average (TWA) 1 mg/m3
- Short-term exposure limit (STEL) 1 mg/m3
(30-min time-weighted average)
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
Japan Maximum allowable concentration (MAC) 90 mg/m13 1982 (r)
Netherlands Maximum limit 1982 (r)
- Time-weighted average (TWA) 90 mg/m3
Romania Maximum permissible concentration 1985 (r)
- Time-weighted average (TWA) 30 mg/m3
- Ceiling value 60 mg/m3
Sweden Threshold limit value (TLV) 1985
- Time-weighted average (TWA) 9 mg/m3c
Sweden - Short-term exposure limit 18 mg/m3
(15-min time-weighted average)
AIR Occupational United Kingdom Threshold limit value (TLV) 10 mg/m3 1985 (r)
USA (OSHA) Permissible exposure limit (PEL) 1984
- Time-weighted average (TWA) 2 mg/m3
USA (ACGIH) Threshold limit value 1986
- Time-weighted average 2 mg/m3
USSR - Ceiling value 1 mg/m3 1977
AIR Ambient USSR Maximum allowable concentration (MAC) 1984
- Once per day 0.3 mg/m3
- Average per day 0.03 mg/m3
a TWA = time-weighted average over one working day (usually 8 h).
b Suspected carcinogenic potential for man.
c Carcinogenic; for new and renovated plants, a TWA of 1.8 mg/m3 is desired.
7.4 Labelling, Packaging, and Transport
The European Community legislation requires labelling as a dangerous
substance using the symbol(s):

 The label must read:
may cause cancer; may cause heritable genetic damage; extremely
flammable liquified gas; toxic by inhalation; irritating to
eyes, respiratory system and skin; not recommended for interior
use onlarge surface areas; keep container tightly closed, in a
cool, well-ventilated place; keep away from sources of ignition
-- no smoking; take precautionary measures against static
discharges; if you feel unwell, seek medical advice (show the
label where possible).
The European Community legislation on labelling of pesticide
preparations classifies ethylene oxide in Class I/a for the purpose of
determining the label for preparations containing ethylene oxide and
other active ingredients.
The International Maritime Organization classifies ethylene oxide as
a flammable, slightly poisonous (liquified) gas (Hazard Class 2(3,
6.1).
The recommended label is:
The label must read:
may cause cancer; may cause heritable genetic damage; extremely
flammable liquified gas; toxic by inhalation; irritating to
eyes, respiratory system and skin; not recommended for interior
use onlarge surface areas; keep container tightly closed, in a
cool, well-ventilated place; keep away from sources of ignition
-- no smoking; take precautionary measures against static
discharges; if you feel unwell, seek medical advice (show the
label where possible).
The European Community legislation on labelling of pesticide
preparations classifies ethylene oxide in Class I/a for the purpose of
determining the label for preparations containing ethylene oxide and
other active ingredients.
The International Maritime Organization classifies ethylene oxide as
a flammable, slightly poisonous (liquified) gas (Hazard Class 2(3,
6.1).
The recommended label is:
 In the USA, certain specified vessels carrying ethylene oxide in bulk
and bound for, or departing from, US ports must notify the captain of
the port at least 24 h in advance and must give him certain
information (1983 (r)).
7.5 Waste Disposal
In the Federal Republic of Germany, the emission into the air of
organic compounds in Class I, which includes ethylene oxide, must not
exceed (as the sum of all compounds in this class) a mass
concentration of 20 mg/m3 at a mass flow of greater than 0.1 kg/h.
If compounds of different classes are present, the mass concentration
must not exceed 30 mg/m3 (1982 (r)).
In the USA, any solid waste (except domestic) that contains ethylene
oxide must be listed as hazardous waste (subject to handling,
transport, treatment, storage and disposal regulation, and permit and
notification requirements) unless it is found that the waste cannot
pose a threat to human health or the environment when improperly
managed. If ethylene oxide is a commercial chemical product, it is
identified as "toxic waste" subject to handling, transport, treatment,
storage and disposal regulation and permit and notification
requirements (1980). An owner or operator of a hazardous waste
incinerator must achieve 99.99% destruction and removal efficiency for
this substance, if it is designated as a principal organic hazardous
constituent in its EPA permit (1981). Under the Comprehensive
Environmental Response, Compensation, and Liability Act of 1980
(CERCLA), unless in compliance with a specified permit or procedure,
owners/operators of vessels or on- or offshore facilities must notify
the US government (National Response Center) of any release of the
hazardous substance in or on navigable waters, adjoining shorelines,
the contiguous zone or beyond the contiguous zone or to any other
environmental media (air, land and ground water) in an amount equal to
or greater than 0.454 kilogram in any 24-h period (1985 (r)).
7.6 Other Measures
The European Community legislation concerning the major accident
hazards of certain industrial activities foresees that the
manufacturer must take all necessary measures to prevent accidents and
to limit their consequences for man and the environment, when
processing ethylene oxide or when storing it in quantities equal to,
or over, 50 tonnes.
Furthermore, when ethylene oxide is processed in quantities equal to,
or over, 50 tonnes, or is stored in quantities equal to, or over,
300 tonnes, notification has to be made to the competent authorities
including information on the substance, on the installation,
information on possible major accident situations, and emergency
plans.
BIBLIOGRAPHY
BRETHERICK, L. (1981) Hazards in the chemical laboratory, 3rd ed.
London, The Royal Society of Chemistry.
CEC (1984) Classification and labelling of dangerous substances.
Brussels, Commission of the European Communities.
DUTCH CHEMICAL INDUSTRY ASSOCIATION (1980) Handling chemicals
safely, 2nd ed. Dutch Association of Safety Experts, Dutch Safety
Institute.
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E (1976)
Clinical toxicology of commercial products, 5th ed. Baltimore,
Maryland, Williams and Wilkins Company.
HOMMEL, G. (1987) [Handbook of dangerous goods.] 2nd ed. Berlin,
Springer Verlag (in German).
IARC (1972 - present) IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC Data profile on individual chemical substances. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme (unpublished file).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH Current intelligence bulletins. Washington, DC, US
Department of Health, Education and Welfare.
US NIOSH (1976) A guide to industrial respirator protection.
Cincinnati, Ohio, US National Institute for Occupational Safety and
Health, pp. 76-189.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols, Washington DC, US Department of Health and Human
Services, US Departmnent of Labor (Publication No. DHSS(NIOSH)
01-123).
US NIOSH/OSHA (1985) Pocket guide to chemical hazards. Washington
DC, US Department of Health, Education and Welfare, US Department of
Labor (Publication No. 85.114).
WHO (1985) EHC No. 55: Ethylene oxide. Geneva, World Health
Organization, 78pp.
In the USA, certain specified vessels carrying ethylene oxide in bulk
and bound for, or departing from, US ports must notify the captain of
the port at least 24 h in advance and must give him certain
information (1983 (r)).
7.5 Waste Disposal
In the Federal Republic of Germany, the emission into the air of
organic compounds in Class I, which includes ethylene oxide, must not
exceed (as the sum of all compounds in this class) a mass
concentration of 20 mg/m3 at a mass flow of greater than 0.1 kg/h.
If compounds of different classes are present, the mass concentration
must not exceed 30 mg/m3 (1982 (r)).
In the USA, any solid waste (except domestic) that contains ethylene
oxide must be listed as hazardous waste (subject to handling,
transport, treatment, storage and disposal regulation, and permit and
notification requirements) unless it is found that the waste cannot
pose a threat to human health or the environment when improperly
managed. If ethylene oxide is a commercial chemical product, it is
identified as "toxic waste" subject to handling, transport, treatment,
storage and disposal regulation and permit and notification
requirements (1980). An owner or operator of a hazardous waste
incinerator must achieve 99.99% destruction and removal efficiency for
this substance, if it is designated as a principal organic hazardous
constituent in its EPA permit (1981). Under the Comprehensive
Environmental Response, Compensation, and Liability Act of 1980
(CERCLA), unless in compliance with a specified permit or procedure,
owners/operators of vessels or on- or offshore facilities must notify
the US government (National Response Center) of any release of the
hazardous substance in or on navigable waters, adjoining shorelines,
the contiguous zone or beyond the contiguous zone or to any other
environmental media (air, land and ground water) in an amount equal to
or greater than 0.454 kilogram in any 24-h period (1985 (r)).
7.6 Other Measures
The European Community legislation concerning the major accident
hazards of certain industrial activities foresees that the
manufacturer must take all necessary measures to prevent accidents and
to limit their consequences for man and the environment, when
processing ethylene oxide or when storing it in quantities equal to,
or over, 50 tonnes.
Furthermore, when ethylene oxide is processed in quantities equal to,
or over, 50 tonnes, or is stored in quantities equal to, or over,
300 tonnes, notification has to be made to the competent authorities
including information on the substance, on the installation,
information on possible major accident situations, and emergency
plans.
BIBLIOGRAPHY
BRETHERICK, L. (1981) Hazards in the chemical laboratory, 3rd ed.
London, The Royal Society of Chemistry.
CEC (1984) Classification and labelling of dangerous substances.
Brussels, Commission of the European Communities.
DUTCH CHEMICAL INDUSTRY ASSOCIATION (1980) Handling chemicals
safely, 2nd ed. Dutch Association of Safety Experts, Dutch Safety
Institute.
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E (1976)
Clinical toxicology of commercial products, 5th ed. Baltimore,
Maryland, Williams and Wilkins Company.
HOMMEL, G. (1987) [Handbook of dangerous goods.] 2nd ed. Berlin,
Springer Verlag (in German).
IARC (1972 - present) IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC Data profile on individual chemical substances. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme (unpublished file).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH Current intelligence bulletins. Washington, DC, US
Department of Health, Education and Welfare.
US NIOSH (1976) A guide to industrial respirator protection.
Cincinnati, Ohio, US National Institute for Occupational Safety and
Health, pp. 76-189.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols, Washington DC, US Department of Health and Human
Services, US Departmnent of Labor (Publication No. DHSS(NIOSH)
01-123).
US NIOSH/OSHA (1985) Pocket guide to chemical hazards. Washington
DC, US Department of Health, Education and Welfare, US Department of
Labor (Publication No. 85.114).
WHO (1985) EHC No. 55: Ethylene oxide. Geneva, World Health
Organization, 78pp.
