
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 65
BUTANOLS: FOUR ISOMERS
- 1-Butanol
- 2-Butanol
- tert-Butanol
- Isobutanol
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1987
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154265 9
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1987
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR BUTANOLS - FOUR ISOMERS:
1-BUTANOL, 2-BUTANOL, tert-BUTANOL, ISOBUTANOL
INTRODUCTION
1-BUTANOL
2-BUTANOL
tert-BUTANOL
ISOBUTANOL
REFERENCES
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
BUTANOLS - FOUR ISOMERS: 1-BUTANOL, 2-BUTANOL, tert-BUTANOL,
ISOBUTANOL
Members
Dr B.B. Chatterjee, Calcutta, India
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United Kingdom
(Rapporteur)
Dr R. Drew, Department of Clinical Pharmacology, Flinders
University of South Australia, Bedford Park, South Australia,
Australia (Chairman)
Dr M.-S. Galina Avilova, Institute of Occupational Hygiene and
Professional Diseases, Moscow, USSR
Dr A.A.E. Massoud, Department of Community, Environmental and
Occupational Medicine, Faculty of Medicine, Ain-Shams
University, Abbasia, Cairo, Egypt (Vice-Chairman)
Dr A.N. Mohammed, University of Calabar, Calabar, Nigeria
Dr C.P. Sadarangani, Bader Al Mulla and Brothers, Safat, Kuwait
Secretariat
Ms B. Bender, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Ms F. Ouane, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
Detailed data profiles and legal files can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR BUTANOLS - FOUR ISOMERS:
1-BUTANOL, 2-BUTANOL, tert-BUTANOL, ISOBUTANOL
A WHO Task Group on Environmental Health Criteria for Butanols
met in Geneva from 11 to 15 November 1985. Dr K.W. Jager opened
the meeting on behalf of the Director-General. The Task Group
reviewed and revised the draft criteria document and made an
evaluation of the health risks of exposure to butanols.
The first draft of this document was partially based on
information contained in Toxicology Data Sheets made available by
the Health, Safety and Environment Division of Shell Internationale
Petroleum Maatschappij B.V., and on information obtained by a
search of data bases by IRPTC. Additional information supplied by
IPCS Participating Institutions was added to the second draft.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously covered the costs of printing.
INTRODUCTION
The butanol isomers occur naturally as products of fermentation
and are also synthesized from petrochemicals. They are used widely
as solvents and intermediates in chemical industries. Human
exposure to high concentrations of the butanol isomers will be
primarily occupational while exposure to low concentrations will be
mainly through foods in which they occur naturally or as flavouring
agents. Apart from slight differences in the boiling point and
water solubility, the physical properties of the isomers are
similar.
With only minor differences between isomers, the toxicity for
aquatic organisms of all four butanols is low and none of the
compounds shows any capacity for bioaccumulation. Apart from
tert-butanol, all isomers are readily biodegradable and would be
expected to be fully oxidised by microorganisms within a few days.
The tert-butanol is metabolized more slowly and would be degraded
within a few weeks. The likely background concentrations of all
butanol isomers would not have any impact on the aquatic
environment.
In animals, the butanols are readily absorbed through the lungs
and gastrointestinal tract. 1-Butanol, 2-butanol, and isobutanol
are primarily metabolized by alcohol dehydrogenase and are rapidly
eliminated from the blood. tert-Butanol is not a substrate for
alcohol dehydrogenase and its elimination is slower than that of
the other isomers. On the basis of oral LD50 values in the rat,
the butanols can be classified as being slightly or practically
non-toxic. In large amounts, all isomers have the ability to
induce signs of alcoholic intoxication in both animals and man.
Data regarding other biological effects in man and animals cannot
be easily compared and symptoms and effects are covered in the
separate sections for each isomer.
On the basis of the available data, the Task Group did not
expect any adverse effects from occupational exposure under
conditions of good manufacturing practice.
The Task Group considered that the available data were
inadequate to give guidelines for the setting of occupational
exposure limits for any of the butanol isomers.
The effects of long-term exposure to low concentrations of the
butanols could not be judged because of lack of information. The
Task Group recommended that relevant studies should be conducted so
that this could be achieved.
ENVIRONMENTAL HEALTH CRITERIA
FOR
1-BUTANOL
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 1-BUTANOL
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.2 Terrestrial organisms
7.3 Microorganisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Acute toxicity
8.1.1.1 Signs of intoxication
8.2 Skin, eye, and respiratory tract irritation
8.2.1 Skin irritation
8.2.2 Eye irritation
8.2.3 Respiratory tract irritation
8.3 Repeated and continuous exposure
8.3.1 Inhalation studies
8.3.2 Other routes of administration
8.4 Mutagenicity
8.5 Carcinogenicity
8.6 Reproduction, embryotoxicity, and teratogenicity
8.7 Special studies
9. EFFECTS ON MAN
9.1 Toxicity
9.1.1 Eye irritation
9.1.2 Case reports of occupational exposure
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
10.1.2 Toxic effects
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
10.2.2 Toxic effects
10.3 Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY
1-Butanol is a flammable colourless liquid with a rancid sweet
odour. It has a boiling point of 118 °C, a water solubility of 77
g/litre and its 1-octanol/water partition coefficient is 0.88. Its
vapour is 2.6 times denser than air. It occurs naturally as a
product of fermentation of carbohydrates. 1-Butanol is also
synthesized from petrochemicals and is widely used as an organic
solvent and as an intermediate in the manufacture of other organic
chemicals. Human exposure is mainly occupational. Exposure of the
general population will be mainly through its natural occurrence in
foods and beverages, and its use as a flavouring agent. Exposure
may also result from industrial emissions. 1-Butanol is readily
biodegradeable and does not bioaccumulate. It is not directly
toxic for aquatic animals and practically non-toxic for algae.
However, some protozoa are slightly sensitive to 1-butanol and it
should be managed in the environment as a slightly toxic compound.
It poses an indirect hazard for the aquatic environment because it
is readily biodegraded and this may lead to oxygen depletion.
In animals, 1-butanol is readily absorbed through the skin,
lungs, and gastrointestinal tract. It is rapidly metabolized by
alcohol dehydrogenase to the corresponding acid, via the aldehyde,
and to carbon dioxide, which is the major metabolite. The rat oral
LD50 for 1-butanol ranges from 0.7 to 2.1 g/kg body weight. It is,
therefore, slightly toxic according to the classification of Hodge
& Sterner. It is markedly irritating to the eyes, and moderately
irritating to the skin. The primary effects from exposure to
vapour for short periods are various degrees of irritation of the
mucous membranes, and central nervous system depression. Its
potency for intoxication is approximately 6 times that of ethanol.
A variety of investigations have indicated the non-specific
membrane effects of 1-butanol. Effects of repeated inhalation
exposure in animals include pathological changes in the lungs,
degenerative lesions in the liver and kidneys, and narcosis.
However, it is not possible to determine a no-observed-adverse-
effect level on the basis of the animal studies available. 1-
Butanol has been found to be non-mutagenic. Adequate data are not
available on its carcinogenicity, teratogenicity, or effects on
reproduction.
The most likely acute effects of 1-butanol in man are alcoholic
intoxication and narcosis. Signs of excessive exposure may include
irritation of the eyes, nose, throat, and skin, headache, and
drowsiness. Vertigo has been reported under conditions of severe
and prolonged exposure to vapour mixtures of 1-butanol and
isobutanol. However, in this study, it was not possible to
attribute the vertigo to a single cause. It has been reported that
exposure to 1-butanol may affect hearing and also light adaptation
of the eye.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure: CH3-CH2-CH2-CH2OH
Chemical formula: C4H10O
Primary constituent: 1-butanol
Common synonyms: 1-butyl alcohol, butanol-1, normal-butyl
alcohol, 1-hydroxy butane, normal-propyl
carbinol, butyric alcohol, NBA, butan-1-
ol, butyl alcohol
CAS registry number: 71-36-3
2.2. Physical and Chemical Properties
Some physical and chemical properties of 1-butanol are listed
in Table 1.
Table 1. Physical and chemical properties of 1-butanol
-------------------------------------------------------------------
(at 20 °C and 101.3 kPa, unless otherwise stated)
Physical state colourless liquid
Odour rancid sweet
Odour threshold approximately 3.078 mg/m3
Relative molecular mass 74.12
Density (kg/m3) 809 - 811
Boiling point 118 °C
Freezing point -89 °C
Viscosity (mPa x s) 2.96
Vapour density (air = 1) 2.55
Vapour pressure (kPa) 0.56
Flashpoint (°C) 33
Autoignition temperature 345 °C
Explosion limits in air (v/v) lower = 1.4%
upper = 11.2%
Solubility (% weight) in water, 7.7; miscible with
ethyl alcohol, ether, and
other organic solvents
n-octanol/water partition coefficient 0.88
Conversion factors 1 ppm = 3.078 mg/m3
1 mg/m3 = 0.325 ppm
-------------------------------------------------------------------
2.3. Analytical Methods
NIOSH Method No. S66(321) has been recommended for the
determination of 1-butanol. It involves drawing a known volume of
air through charcoal to trap the organic vapours present
(recommended sample is 10 litres at a rate of 0.2 litre/min). The
analyte is desorbed with carbon disulfide containing 1% 2-propanol.
The sample is separated by injection into a gas chromatograph
equipped with a flame ionization detector and the area of the
resulting peak is determined and compared with standards (NIOSH,
1977).
A gas-chromatographic separation and determination method for
1-, sec-, and tert-butanols, with a sensitivity of 1 mg/m3 was
reported by Abbasov et al. (1971).
Testing methods for the butanols (ASTM D304-58) are described
in ASTM (1977).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
1-Butanol is used as an ingredient in perfumes and flavours
(Mellan, 1950), and for the extraction of: hop, lipid-free protein
from egg yolk (Meslar & White, 1978), natural flavouring materials
and vegetable oils, perfumes (Mellan, 1950), phenols, and
oligosaccharides from plant tissue (Sodini & Canella, 1977), and
as a solvent in removing pigments from moist curd leaf protein
concentrate (Bray & Humphries, 1978). 1-Butanol is also used as:
an extractant in the manufacture of antibiotics, hormones, and
vitamins (Mellan, 1950; Doolittle, 1954; Yamazaki & Kato, 1978),
and of rhenium (Gukosyan et al., 1979); a solvent for paints,
coatings, natural resins, gums, synthetic resins, dyes, alkaloids,
and camphor (Mellan, 1950; Doolittle, 1954); a cleanser for moulded
contact lenses (Mizatani et al., 1978); an intermediate in the
manufacture of butyl acetate, dibutyl phthalate, and dibutyl
sebacate (Mellan, 1950; Doolittle, 1954) as well as of the esters
of herbicides (e.g., 2,4-D, 2,4,5-T) (Monich, 1968). Other
miscellaneous applications of 1-butanol are as a swelling agent in
textiles, as a component of brake fluids, cleaning formulations,
degreasers (Monich, 1968; Sitanov et al., 1979), and repellents
(Zaikina et al., 1978); and as a component of ore floation agents
(Monich, 1968), of protective coatings for glass objects (Artigas
Gimenez et al., 1979) and of wood-treating systems (Amundsen et
al., 1979). Mixed with xylene, it is used to produce a glass
substitute that can be used for sunglasses, safety glasses, windows
for airplanes and others (Ferri, 1979). 1-Butanol is also used as
an additive to increase the fineness of ground cement (Tavlinova &
Dovyborova, 1979) and as a solvent in the purification of
polyolefins (Takeuchi et al., 1978). It may be liberated during
photographic processing operations.
A further use of 1-butanol is as a flavouring agent in butter,
cream, fruit, liquor, rum, and whiskey. Other foods in which it is
used include: beverages (12 mg/litre maximum), ice cream and ices
(7 mg/kg maximum), candy (34 mg/kg maximum), baked goods (32 mg/kg
maximum), cordials (1 mg/litre maximum), and cream (4 mg/kg
maximum) (Hall & Oser, 1965).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A high rate of degradation of 1-butanol has been found in a
wide range of test methods. The data in Table 2 suggest a high
proportion of the total oxygen required for its complete oxidation
is taken up within a few hours and degradation would be complete
within a few days. Its biodegradation in surface waters may
present a hazard in terms of oxygen depletion.
At a concentration of 20 mg/litre, butanol gives a strong
unpleasant odour to drinking-water. The odour threshold is 1
mg/litre (Nazarenko, 1969).
No data are available on distribution in soil, sediments, or
air.
Table 2. Biodegradation data for 1-butanol
------------------------------------------------------------------------
activated 36% of ThOD removed in 24 h by Gerhold & Malaney
sludge unadapted municipal sludge (1966)
44% of ThOD removed in 23 h by McKinney & Jeris
adapted sludge (1955)
biodegradation rate in adapted sludge Pitter (1976)
at 20 °C, 84.0 mg COD/g per h
5d BOD 68% of ThOD in fresh water Price et al. (1974)
45% of ThOD in synthetic sea water Price et al. (1974)
5d BOD 33% of ThOD (AFNOR Test) Dore et al. (1974)
66% of ThOD (APHA Test) Bridie et al. (1979b)
anaerobic degraded by acetate-enriched methane Chou et al. (1978a,b)
digestion culture after adaptation, 100% of ThOD
removed at 100 mg/1itre per day after
4 days of adaptation; 98% of ThOD
removed at 80 mg/1itre in anaerobic
upflow filters (hydraulic residence
time 2 - 10 days) after 52 days of
adaptation
---------------------------------------------------------------------------
ThOD = theoretical oxygen demand - the calculated amount of oxygen needed
for complete oxidation to water and
carbon dioxide.
COD = chemical oxygen demand - measures the chemically oxidizable
matter present.
BOD = biochemical oxygen demand - a simple bioassay measuring the
potential deoxygenating effect of
biologically oxidizable matter present
in an effluent.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
1-Butanol and other congeners occur naturally as a result of
carbohydrate fermentation in a number of alcoholic beverages
including beer (Bonte, 1979), grape brandies (Schreier et al.,
1979), apple brandies (Woidich et al., 1978), wine (Bikvaloi &
Pasztor, 1977; Bonte, 1978), and whisky (Pastel & Adam, 1978). It
has been detected in the volatiles of the following products: hops
(Tressl et al., 1978), jack fruit (Swords et al., 1978), heat-
treated milks (Juddou et al., 1978), muskmelon (Yabumoto et al.,
1978), cheese (Dumont & Adda, 1978), southern pea seed (Fisher et
al., 1979), and cooked rice (Yajima et al., 1978). 1-Butanol is
also formed during deep frying of corn oil, cottonseed oil,
trilinolein, and triolein (Chang et al., 1978). The production or,
in some cases, use of the following substances may result in
exposure to 1-butanol: artificial leather, butyl esters, rubber
cement, dyes, fruit essences, lacquers, motion picture and
photographic films, raincoats, perfumes, pyroxylin plastics, rayon,
safety glass, shellac varnish, and waterproofed cloth (Tabershaw et
al., 1944; Cogan & Grant, 1945; Sterner et al., 1949; Mellan, 1950;
Doolittle, 1954). It has also been detected by gas chromatographic
methods in waste gases obtained during the boiling and drying of
oil (Novokonskaya et al., 1978), and it is released from polyvinyl
chloride linoleum plasticized with poly(dibutyl maleate)
(Moshlakova et al., 1976) and from hardened parquet lacquer
(Dmitriev & Michahikin, 1979).
Whilst testing the air of mobile homes for the presence of
organic chemicals, 1-butanol was found with a frequency of 47%; the
mean concentration was 5 ppb and the range 0.07 - 26 ppb (Connor et
al., 1985).
An industrial emission study indicated that 616 tonnes of 1-
butanol were released into the air, over 1 year, in The Netherlands
(Anon, 1983).
6. KINETICS AND METABOLISM
1-Butanol is readily absorbed through the lungs, skin, and
intestinal tract (Sander, 1933; Theorell & Bonnichsen, 1951; Winer,
1958; Merritt & Tomkins, 1959; Wartburg et al., 1964) and is
primarily eliminated after metabolism by alcohol and aldehyde
dehydrogenases.
It has been shown that 1-butanol disappeared rapidly from the
blood of rats. After an oral dose of 2000 mg/kg body weight, the
maximum blood-alcohol concentration was 500 mg/litre after 2 h.
The concentration dropped to 150 mg/litre after 4 h and only 0.03%
of the dose was excreted in the urine after 8 h (Gaillard &
Derache, 1965).
In a study on rabbits, it was stated that aliphatic alcohols
appeared to be metabolized and eliminated from the body by:
(a) oxidation and elimination of the products (acids,
aldehydes, ketones, and carbon dioxide) in the urine and
expired air;
(b) conjugation as glucuronide or sulfate and elimination of
the products in the urine; and
(c) elimination of the unchanged alcohol in the expired air or
urine.
In the case of 1-butanol, though no specific numbers concerning
the expired air were given, it was found to oxidize to the
corresponding acid via the aldehyde, and to carbon dioxide (CO2);
1.8% of the alcohol was excreted conjugated with glucuronic acid
within 24 h (Kamil et al., 1953).
According to DiVincenzo & Hamilton (1979), rats dosed, by
gavage, with 450 mg 1-butanol/kg body weight excreted 83.3% of the
dose as carbon dioxide (CO2), at 24 h. Less than 1% was eliminated
in the faeces, 4.4% was excreted in the urine, and 12.3% remained
in the carcass. Similar excretion patterns were observed at 45 and
4.5 mg/kg body weight. About 75% of 1-butanol excreted in the
urine was in the form of o-sulfate (44%) or o-glucuronide (30%).
1-Butanol was absorbed through the skin of dogs at a rate of 8.8
µg/min per cm2; dogs exposed by inhalation to 1-butanol vapour at
53.9 mg/m3 (50 ppm) over 6 h absorbed about 55% of the inhaled
vapour. When administered orally to rats, 14C-labelled butanol was
found in the liver, kidneys, small intestine, and lungs, 1 h after
administration. A decrease in the radioactivity was observed in
the organs 4 h later. During the first 3 days, 95% of 14C was
excreted from the body; however, only 2.8% of 14C was eliminated in
the urine and faeces combined (Rumyanstev et al., 1975).
When administered intraperitoneally (ip) to rats in a single
dose, 1-butanol accumulated in the brain nuclei and liver nuclei
and, at a slower rate and reaching a lower maximum concentration,
in mitochondria (Mikheev et al., 1977).
Excretion of 1-butanol in the breath and urine of rabbits
following an oral dose of 2 ml/kg body weight was less than 0.5%
of the dose administered in each case (Patty, 1982). In a 1-month
study, 1-butanol, administered 5 times/week to mice at 0.1 - 0.5 of
the LD50, showed cumulative properties (Rumyanstev, 1976). The
elimination of 1-butanol from the perfusate of isolated rat liver
was a zero-order process above the concentration of 0.8 mmol and a
first-order process below this concentration (Auty & Branch, 1976).
In an in vitro study using rat liver slices, it was reported
that 1-butanol was oxidised by alcohol dehydrogenase. At the
concentration of 1-butanol tested (25 µl/500 mg liver per 2 ml
incubate), CO2 production was decreased by approximately 60% and
the lactate/pyruvate ratio in the medium was increased ten fold
(Forsander, 1967).
The in vitro metabolism of 1-butanol by rat hepatic microsomes
has been studied by Teschke et al. (1974) and Cederbaum et al.
(1978, 1979). The first authors showed that hepatic microsomes
catalysed the oxidation of 1-butanol to its aldehyde by means of a
reaction requiring molecular oxygen and NADPH. This reaction was
inhibited by carbon monoxide. A direct demonstration of the role
of hydrogen peroxide (H202) in the cytochrome P-450-mediated
pathway stems from the observation that reagent H2O2 added to
microsomal preparations stimulated the oxidation of butanol
(Cederbaum et al., 1978). Indirect evidence was provided by the
observation that azide, which prevents the decomposition of H2O2 by
catalase, actually stimulated the oxidation of 1-butanol.
Thiourea, a compound that reacts with hydroxyl radicals, inhibited
NADPH-dependent microsomal oxidation of 1-butanol to a similar
extent, in both the absence and presence of the catalase inhibitor
azide (Cederbaum et al., 1979). Achrem et al. (1978) showed that
the hydroxyl ion from butanol interacted with the Fe of haem in
cytochrome P-450.
Twelve human volunteers were exposed for 2 h to 1-butanol at
300 or 600 mg/m3 in inspired air during rest and during exercise
(50, 100, or 150 w) on a bicycle ergometer. At the highest dose
level, the difference between levels in inspired and expired air
indicated an uptake of 47% 1-butanol at rest, and 37, 40, and 41%
at 50, 100, and 150 w, respectively. After 30 min exposure to 300
or 600 mg/m3, the 1-butanol concentrations in the arterial blood
were 0.3 and 0.5 mg/litre, respectively. The combination of an
apparently high uptake and low concentrations in arterial blood is
probably because 1-butanol is dissolved in the water of the dead
space mucous membranes (Astrand et al., 1976).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Toxicity data for aquatic organisms are given in Table 3.
Table 3. Table of acute toxicity data for fresh-water organisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Fish
Fresh-water species
Creek chub 1900 - 2300 24-h LC50 Gillette et al. (1952)
(Semotitus
atromaculatus)
Golden orfe 1200 48-h LC50 Juhnke & Lüdemann
(Leuciscus idus (1978)
melanotus)
Goldfish 1900 24-h LC50 Bridié et al. (1979a)
(Carassius auratus)
Fathead minnow 1730 - 1910 96-h LC50 Mattson et al. (1976)
(Pimapheles promelas) Veith et al. (1981,
1983)
Bleak 2250 - 2400 96-h LC50 Linden et al. (1979)
(Alburnus alburnus) Bengtsson et al. (1984)
Amphibia
Tadpole 2820 threshold for Münch (1972)
( Rana sp.) narcosis
Invertebrates
Fresh-water species
Water flea 1880 24-h EC50 immobilization Bringmann & Kuehn
(Daphnia magna) (1982)
Harpacticoid copepod 1900 - 2300 96-h LC50 Mattson et al. (1976)
(Nitocra spinipes) Bengtsson et al. (1984)
Marine species
Brine shrimp 2950 24-h LC50 Price et al. (1974)
(Artemia salina) 2600 excystment Smith & Siegel (1975)
inhibited
------------------------------------------------------------------------------------------
Table 3. (contd.)
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Algae (fresh-water)
Green algae
(Scenedesmus 875 8-day no- total biomass Bringmann & Kuehn
quadricauda) observed- (1978a)
adverse-
effect level
Chlorella 8500 EC50 Jones (1971)
pyrenoidosa I chlorophyll
content
Blue-green algae
(Microcystis 100 8-day no- total biomass Bringmann & Kuehn
aeruginosa) observed- (1978a)
adverse-
effect level
------------------------------------------------------------------------------------------
LC50 data for 5 species of fish range from 1000 to 2400
mg/litre and for 3 species of aquatic invertebrates from 1880 to
2950 mg/litre. These concentrations are unlikely to be achieved in
the field except locally after accidental spills or through
effluence from industrial sites; even under these conditions, the
high levels of contamination would not last long. Fresh-water
algae are very resistant to the toxic effects of 1-butanol at
realistic exposures.
Hill et al. (1981) looked at effects of 1-butanol on goldfish
with a conditioned reflex of avoiding light followed by electric
shock. The fish were housed in a tank separated into 2
compartments by a metal plate with a hole big enough for the fish
to swim through. Training involved a 10-s light pulse followed by
a 20-s shock to one side of the tank. Experimental concentrations
of 1-butanol (2.5 - 15 mmol) were applied to the tanks and fish
were exposed to step-wise increases (2.5 mmol) in concentration
with approximately 1.5 h between each step (a 15 mmol concentration
of 1-butanol is 58% of the 24 h LC50 for this species). Two
responses were scored; "avoidance", defined as the fish leaving the
test side of the tank during the light stimulus and before the
shock, and "escape", defined as leaving during the 20-s shock.
With each step up to 10 mmol butanol, there was a transitory
reduction in avoidance. At 10 mmol or higher concentrations, there
was also a fall in escape response. Recovery to control scores
after first exposure to 10 mmol butanol was slow and incomplete
after 2 h. 1-Butanol at 15 mmol, achieved either step-wise or by
single application, led to a dramatic and non-recoverable fall in
both avoidance and escape to approximately 20% of control values.
Escape and avoidance success was correlated with brain levels of
butanol. Final concentrations of butanol in the fish brain were
75% of those expected, assuming complete equilibrium with tank
water. This compares with 90% for ethanol in the same species.
Measuring of brain butanol over a longer period indicated that
butanol was metabolized by the goldfish in a similar way to ethanol
(Hill et al., 1980). The concentrations of the alcohol that
produced effects in these studies are high compared with likely
exposure levels in natural waters.
7.2. Terrestrial Organisms
Seed germination in lettuce (Lactuca sativa) was inhibited by
50% at a concentration of 1-butanol of 390 mg/litre (Reynolds,
1977). Seed germination in cucumber (Cucumis sativus) was
inhibited at 2500 mg/litre (Smith & Siegal (1975). 1-Butanol had
an antisenescence effects on the leaves of oat seedlings (Avena
sativa). It both maintained chlorophyll levels and prevented
proteolysis in the dark (Satler & Thimann, 1980). There are no
relevant data on terrestrial animals; however, as for terrestrial
plants, significant exposure to butanol is unlikely.
7.3. Microorganisms
Some toxicity data for microorganisms are given in Table 4.
It would be highly unlikely that bacteria would be affected by
1-butanol in the field. Protozoans are more susceptible than
bacteria, but only transitory effects on protozoan populations are
likely from spills and effluent since the experimental no-observed-
adverse-effect levels are high.
1-Butanol at a concentration of 20 mg/litre in water reduced
nitrification; a concentration of 5 mg/litre was the no-observed-
adverse-effect level for nitrification (Nazarenko, 1969). 1-
Butanol does not bioaccumulate (Chiou et al., 1977).
Table 4. Toxicity data for microorganisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Protozoa
Uronema parduczi 8 20-h no-observed-adverse- total Bringmann &
(ciliate) effect level biomass Kuehn (1981)
Chilomonas paramaecium 28 48-h no-observed-adverse- total Bringmann &
(flagellate) effect level biomass Kuehn (1981)
Entosiphon sulcatum 55 72-h no-observed-adverse- total Bringmann &
(flagellate) effect level biomass Kuehn (1981)
------------------------------------------------------------------------------------------
Table 4. (contd.)
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Bacteria
Pseudomonas putida 650 16-h no-observed-adverse- total Bringmann &
effect level biomass Kuehn (1976)
Bacillus subtilis 1258 EC50 spore germination Yasuda-Yasaki
et al. (1978)
7400 no inhibition of Chou et al.
degradation by methane (1978)
culture on acetate
substrate
------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposure
8.1.1. Acute toxicity
Some acute toxicity data for experimental animals are given in
Table 5.
Table 5. Acute toxicity data for experimental animals
-------------------------------------------------------------------
Species Route LD50 LD100 Reference
(g/kg body (g/kg body
weight) weight)
-------------------------------------------------------------------
Rabbit dermal 5.3 - Patty (1982)
Rabbit dermal 4.2 - Egorov (1972)
Rabbit dermal - 7.5 Patty (1963)
Hamster oral 1.2 - Dubina & Maksimov (1976)
(0.6-2.3)a
Mouse oral 2.68 - Rumyanstev et al. (1979)
Rabbit oral 3.4 - Münch & Schwartze (1925)
Rabbit oral 3.5 - Münch (1972)
Rat oral 2.1 - Jenner et al. (1964)
Rat oral 0.8-2.0 - Purchase (1969)
Rat oral 0.7 - NIOSH (1977a)
Rat oral - 4.4 Smyth et al. (1951)
Mouse ip 0.1-0.3 - Maickel & McFadden (1979)
Rat ip 1.0 - Lendle (1928)
Rat ip 0.2 - Macht (1920)
Rat ip - 1.0 Browning (1965)
Cat iv - 0.24 Macht (1920)
Mouse sc - 5.0 Patty (1982)
-------------------------------------------------------------------
a 95% confidence limits for this study.
Rats survived inhalation exposure to 1-butanol at 24 624 mg/m3
(8000 ppm) for 4 h (Smyth et al., 1951). Mice did not show any
evidence of toxicity when exposed to 1-butanol at 5078.7 mg/m3 (650
ppm) for 7 h, but exposure to 20 314.8 mg/m3 (6600 ppm) produced
signs of marked central nervous system (CNS) depression (narcosis
after approximately 2 h) with lethality after 3 h (Patty, 1982).
Male Swiss mice (10 per group) were exposed by inhalation for
4 h to 1-butanol at 1446.7, 1686.7, 2597.8, or 2970.2 mg/m3 (470,
548, 844, or 965 ppm). Following this exposure, the animals were
tested in a "behavioural despair" swimming test. Compared with
controls, a dose-related decrease in the duration of immobility
measured over a 3-min period was observed (de Ceaurriz et al.,
1982).
8.1.1.1 Signs of intoxication
The acute toxicity of 1-butanol (Table 5) is moderate in
several animal species, and is mainly associated with effects on
the CNS. Injection (ip) of 1-butanol in rats induced behavioural
effects of intoxication (pronounced ataxia) that were virtually
identical to those of ethanol, but the intoxicating potency of
1-butanol was approximately 6 times higher (McCreery & Hunt, 1978).
Similarly, after ip administration in mice, 1-butanol was 6 times
more potent than ethanol in inducing narcosis (Browning, 1965).
The performance in a simple functional test of rats treated with a
non-toxic single oral dose of 1-butanol (0.0163 mol/kg body weight)
was studied by Wallgren (1960). Rat performance decreased soon
after treatment, but recovery was rapid (Wallgren, 1960). In
rabbits, the oral administration of 2.1 - 2.44 g 1-butanol/kg body
weight caused deep and rapid narcosis; in mice, the narcotic dose
by ip injection was 0.76 ml/kg body weight (compared with 4.5 for
ethanol) and 20.31 mg/m3 (6.6 ppm) by inhalation (Browning, 1965).
In conscious rabbits, the effects of 1-butanol on circulatory
variables was investigated in double blind studies. 1-Butanol did
not produce any significant effect at an intravenous (iv) dosage of
0.008 g/kg body weight. Doses of 0.1 g/kg body weight and
particularly of 0.33 g/kg resulted in a transitory decrease in
the heart rate and the systolic and especially diastolic blood
pressures. Butanol anaesthetized rats and mice at 15.7 and 15.3
mg/litre, respectively; the anaesthesia transiently lowered the
blood levels of erythrocytes and haemoglobin. The animals that
died during the treatment showed lung haemorrhages and hyperaemia
of other parenchymatous organs. The minimum concentration of
1-butanol disturbing conditioned reflexes was 65 mg/m3 (Rumyanstev
et al., 1979). In acute studies, when 1-butanol was administered
by the oral or ip route, post-mortem findings included marked
hyperaemia of the liver, congestion of several organs in animals
that died early, and degenerative signs becoming visible in the
liver and kidneys of the rats dying after 5 days. Haemorrhagic
areas in the lungs and blood changes were also noted. In the
kidney, hyperaemia and cloudy swelling with cast formation in the
cortex were seen, the only signs of necrosis were in the medulla
(Smyth & Smyth, 1928; Purchase, 1969; Maickel & McFadden, 1979).
In normothermic dogs, the mean lethal dose for 1-butanol,
administered iv, was 1.26 g/kg body weight and, at a constant
infusion rate, the blood alcohol level increased almost linearly
with time (McGregor et al., 1964).
8.2. Skin, Eye, and Respiratory Tract Irritation
8.2.1. Skin irritation
In a 24-h patch test, application of 405 or 500 mg 1-butanol to
the skin of rabbits resulted in moderate irritation (US DHEW,
1978).
8.2.2. Eye irritation
Instillation of 1.62 mg and 20 mg 1-butanol into rabbit eyes
resulted in severe irritation after 72 h and 24 h, respectively (US
DHEW, 1978).
1-Butanol is an irritant of mucous membranes, especially of the
eye and, in man, it causes an unusual form of keratitis (section
9.1.2).
Instillation of 0.005 ml undiluted 1-butanol or an excess of
40% solution in propylene glycol in the rabbit eye caused severe
corneal irritation. A 15% solution in propylene glycol caused
minor corneal injury (Patty, 1982).
8.2.3. Respiratory tract irritation
On the basis of the effects of 1-butanol on the respiratory
rate in male Swiss OF1 mice, de Ceaurriz et al. (1981) predicted
that exposure to a concentration of 40.01 mg/m3 (13 ppm) in air
would have only a minimal or no effect on man, a concentration of
390.9 mg/m3 (127 ppm) would be uncomfortable, but tolerable, and
3909 mg/m3 (1268 ppm) would be intolerable.
8.3. Repeated and Continuous Exposure
8.3.1. Inhalation studies
Inhalation studies on the effects of 1-butanol on experimental
animals are summarized in Table 6.
According to Rumyantsev et al. (1976), the no-observed-adverse-
effect level of 1-butanol can be set at 0.09 mg/m3 (0.03 ppm) for
both the rat and the mouse under conditions of long-term continuous
exposure.
When 3 groups of 3 guinea-pigs were exposed to 1-butanol in air
at 307.8 mg/litre (100 ppm), 4 h per day, for 64 days, the number
of red blood cells and relative and absolute lymphocyte counts were
decreased. Haemorrhagic areas were observed in the lungs of the
exposed animals. There were also early degenerative lesions in the
liver, as well as cortical and tubular degeneration in the kidneys
(Smyth & Smyth, 1928).
Five white mice were exposed to a concentration of 24.3 mg
1-butanol/litre of air (24 624 mg/m3) for a total exposure time of
130 h (number of h per day not specified). Although the exposed
animals were narcotized repeatedly, they gained in weight and
survived the exposures. Reversible fatty changes were observed in
the livers of the mice (Weese, 1928).
Table 6. Inhalation effects on experimental animals
---------------------------------------------------------------------------------------------------------
Species Dose Duration Effects Reference
---------------------------------------------------------------------------------------------------------
Mouse 24624 mg/m3 repeated exposure narcosis; no deaths; reversible fatty Weese (1928)
(8000 ppm) for several days infiltrations of the liver and the kidneys
Guinea- 307.8 mg/m3 64 days, 4 h/day degenerative lesions in liver, kidney, and lung Smyth & Smyth
pig (100 ppm) (1928)
Rat 218 mg/m3 5 h/day, 6 days/ during the first 2 months, a decrease in O2 Savelev et al.
(71 ppm) week for 6 months consumption and delay in the restoration of (1975)
normal body temperature after cooling; during
the next 4 months of long-term exposure, an
increase in O2 consumption and a return to
normal body temperature after cooling was noted
Rat and 6.8 and 4 months decreased sleeping time; stimulated blood Rumyanstev et
mouse 40.9 mg/m3 continuously cholinesterase; disturbances of reflexes and al. (1979)
(2.2 and neuromuscular sensitivity of the nervous
13.3 ppm) system; increased thyroid activity and
secretion of thyroxine; increases in
eosinophile leukocytes in blood after injection
of adreno-corticotrophin (ACTH)
Rat 0.09 and 92 days after 4 weeks at 21.8 mg/m3, the amount of RNA Baikov &
21.8 mg/m3 continuously and DNA in blood decreased; there was increased Khachaturyan
(0.03 and leukocyte luminescence, increased diastase (1973)
7.1 ppm) activity, decreased catalase activity,
increased penetration of butanol across blood-
tissue barriers in testis, spleen, and thyroid;
no effects were observed at 0.09 mg/m3
Mouse 13.6 and 30 days decreased sleeping time Kolesnikov
40.01 mg/m3 continuously (1975)
(2.1 and
13 ppm)
Rabbit -- prolonged mild bronchial irritation with some enlargement Browning (1965)
exposure of bronchial lymphnodes
---------------------------------------------------------------------------------------------------------
8.3.2. Other routes of administration
The neuropharmacological effects of 1-butanol were investigated
in 31 male Sprague Dawley rats weighing between 200 - 400 g.
1-Butanol was administered iv to the rats at concentrations of
6.7 - 8.1 mmol/kg body weight. Within 20 - 60 s of the iv
administration, the rats lost their righting reflexes.
Nonconvulsive epileptoid activity was noted in the
electroencephalographic tracing compared with the tracing prior to
the 1-butanol administration (Marcus et al., 1976).
DiVincenzo & Hamilton (1979) applied 1-14C-1-butanol to the
skin of 2 male beagle dogs and observed an absorption rate of 8.8
µg/min per cm2. Application of 42 - 55 ml/kg per day for 1 - 4
consecutive days to the skin of rabbits resulted in 100% mortality.
However, repeated applications of 20 ml/kg per day for 30 days over
a period of 6 weeks did not produce any fatalities (Patty, 1982).
According to Cater et al. (1977), the daily oral administration
of approximately 500 mg 1-butanol/kg body weight dissolved in corn
oil, for 4 days, did not affect the testicular tissues of rats.
1-Butanol caused a significant dose-dependent decrease in rat
liver contents of thiamine, riboflavin, pyrixodine, niacin, and
pantothenic acid, after daily oral administration of 1 or 2 ml/kg
body weight for 7 days (Shehata & Saad, 1978). Oral administration
of butanol (1 or 2 ml/kg of a 10% aqueous solution) for 7 days to
rats significantly increased the cerebral GABA levels in the
hemispheres (Saad, 1976).
The influence of 1-butanol on the metabolic status of some rat
organs and on the rabbit circulation was studied by Geppert et al.
(1976). Rats received 1-butanol im at a dose of 0.1 g/kg body
weight per day for 50 days. The metabolic status of some organs
was examined and compared with that in control rats that had
received NaCl solution (9 g/litre) in an equivalent volume (double
blind studies). The tissue levels of metabolites of the adenylic
acid-creatine phosphate system, glycogen, glucose, and lactate did
not differ significantly between the groups. In the liver, the
tissue levels of glycogen, free creatine, and total creatine were
significantly elevated in rats that had received 1-butanol.
A group of 30 male Wistar rats was exposed to 1-butanol in the
drinking-water (69 g/litre), which also contained sucrose (250
g/litre). A control group of equal size was used for comparison.
Electron microscopic studies that were carried out after 5, 9, and
13 weeks demonstrated that, within 5 weeks, 1-butanol at this
dose level gave rise to the formation of irregularly shaped
megamitochondria in liver cells. It was speculated that this was
an adaptive process (Wakabayashi et al., 1984). Ethanol and
1-propanol, both at 320 g/litre, produced similar effects under the
same test conditions.
8.4. Mutagenicity
1-Butanol was found not to be mutagenic in the Ames Salmonella/
microsome test (McCann et al., 1975). It inhibited the initiation
of a new cycle of DNA replication in E. coli but permitted the
completion of DNA replication initiated before the addition of
1-butanol to the medium (Patty, 1982). In spite of the fact that
the lymphocytes of alcoholic patients exhibit higher incidences of
exchange-type aberrations of the chromosome and the chromatid type
compared with controls, several alcohols tested including 1-butanol
do not produce any effects on the chromosomes of human lymphocytes
in culture (Obe et al., 1977). Obe & Ristow (1977) showed that
1-butanol does not affect sister chromatid exchange in Chinese
hamster cells in vitro. In the same cell system, ethanol was also
found to be inactive, but acetaldehyde induced sister chromatid
exchanges; butyric aldehyde was not tested.
1-Butanol was negative in a sister chromatid exchange test
using avian embryos (Bloom, 1981).
8.5. Carcinogenicity
Although two long-term studies on rats have been recorded by
the US National Cancer Institute, both of these studies were
inadequate, by present standards, for the assessment of the
carcinogenicity of the substance. No adequate data on
carcinogenicity are available.
8.6. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data on the effects of 1-butanol on reproduction,
embryotoxicity, and teratogenicity have yet been published. An
inhalation teratology study with 1-butanol is in progress in the
USA (US EPA, personal communication, 1985).
8.7. Special Studies
Various investigations have indicated that 1-butanol exerts
non-specific effects on biological membranes. Evidence of
reversible functional derangement of cell membranes by 1-butanol
was provided by Stark et al. (1983) and Shopsis & Sathe (1984).
The first group of authors was also able to demonstrate
cytotoxicity in cultured corneal endothelial and hepatoma cells.
The interaction of 1-butanol with rat liver microsome membranes
was studied by Birkett (1974) using a microsome-bound fluorescent
probe. 1-Butanol decreased the fluorescent binding to the
microsomal membrane, possibly because of a changed net charge on
the membrane. 1-Butanol increased the fluidity of Chinese hamster
cell plasma membranes (as measured by fluorescence polarization) at
concentrations that inhibited cell adhesion (Juliano & Gagalang,
1979). Moreover, 1-butanol reduced manganese binding to
phosphatidylserine or cardiolipin vesicles to the same extent
(Puskin & Martin, 1978). These authors also reported that
1-butanol increased cholestane mobility in phosphatidylserine
vesicles, thus indicating a more fluid bilayer.
1-Butanol inhibited several rat microsomal metabolic activities
in vitro including ethoxycumarin deethylation (Aitio, 1977) and
the activity of aldrin epoxidase (Wolff, 1978). A sex difference
in the spectral interaction of 1-butanol with liver microsomes from
adult mice has been reported by Van den Berg et al. (1979a). In
males, a profound reverse type I spectrum was elicited, whereas
only a small spectral change of irregular shape was apparent in
females. No sex difference was found in immature animals.
1-Butanol also interfered with both type II (aniline) and type I
(ethylmorphine) binding in mouse liver microsomes. The apparent
dissociation constant of 1-butanol for type I binding was 30 mmol
(Van den Berg et al., 1979b).
Prostaglandin biosynthesis requires the presence of a hydroxyl
radical. 1-Butanol was shown to be a hydroxyl radical scavenger
and, therefore, an inhibitor of the biosynthesis of prostaglandins.
A test system containing microsomes was prepared from bovine
vesicular glands. In the presence of epinephrine, incorporation of
14C-eicosa-8,11,14-trienoic acid into prostaglandins was 16.4% for
prostaglandin E and 23.4% for prostaglandin F. When 0.025 ml of
1-butanol was added to this incubation system, the amounts
incorporated were 7.8% and 9.1%, respectively (Panganamala et al.,
1976). In another study using isolated perfused rat lung, the
infusion of low concentrations of 1-butanol (0.002 - 0.2 mmol)
resulted in maximum release of prostaglandins into the venous
effluent at the lowest concentration tested. However, there was a
gradual decrease in the prostaglandin output as the concentration
of the alcohol increased (Thomas et al., 1980).
Adult male Swiss Cox mice (20 - 25 per group) were dosed orally
by intubation with 1-butanol in distilled water at levels of 0.5,
1.0, or 2.0 g/kg body weight in one single dose. This caused a
dose-related hypothermia and impairment of rotarod performance.
Repetitive doses, at 24 to 72-h intervals did not lead to the
development of tolerance in relation to these effects (Maickel &
Nash, 1985).
1-Butanol caused relaxation of the canine basilar artery,
whereas linear alcohols with fewer carbon atoms above a threshold
of 10-2 mol caused contraction (De Felice et al., 1976).
1-Butanol, applied to the lateral olfactory tract of the guinea-pig
as a dilute suspension (0.1 - 0.2 mmol), blocked the nerve impulse
(Hesketh et all., 1978). The compound also inhibited the
contraction of the CaCl2-depolarized guinea-pig ileum (Yashuda et
al., 1976) and prolonged frog miniature end-plate currents (Ashford
& Wann, 1979).
1-butanol can potentiate the toxicity of carbon tetrachloride
(Cornish & Adefuin, 1967) in Sprague Dawley rats.
9. EFFECTS ON MAN
9.1. Toxicity
The most important effects of 1-butanol inhalation are symptoms
of alcohol intoxication and narcosis (Smyth, 1956).
Following exposure to 1-butanol vapours, the signs of poisoning
in human beings, may include irritation of the nose, throat, and
eyes, the formation of translucent vacuoles in the superficial
layers of the cornea, headache, vertigo, and drowsiness. Defatting
of the skin leading to contact dermatitis involving the fingers and
hands may also occur, as with other solvents.
9.1.1. Eye irritation
Tabershaw et al. (1944) reported that exposure to levels of
more than 153.9 mg/m3 (50 ppm) resulted in irritation of the eyes.
However, results of a 10-year study revealed few or no complaints
of irritation among workers exposed to an average 1-butanol
concentration of 307.8 mg/m3 (100 ppm) (Sterner et al., 1949).
9.1.2. Case reports of occupational exposure
In a raincoat manufacturing plant, the solvent used for the
cementing process was 1-butanol, to which various amounts of
diacetone alcohol and denatured alcohol were added. Of the 35
employees working in the department, 28 were found to have from 10
to 1000 vacuoles in the corneal epithelium. The affected workers
complained of epiphora and burning and itching of the eyes.
Swelling of the eyelids and occasional redness of the eyes were
also observed. The symptoms were more severe on awakening in the
morning than during the day. When the patients were away from
work, the corneal changes were considerably improved and resolved
completely in 10 days (Cogan & Grant, 1945). The authors presumed
that these symptoms were caused by 1-butanol, but stated that the
other components might also be responsible for the symptomatology.
The physical condition of workers exposed to 1-butanol was
followed for 10 years. At the beginning of the study, when the
concentration of 1-butanol was 615.2 mg/m3 (200 ppm) or more,
corneal inflammation was occasionally observed. The symptoms
included a burning sensation that could continue for several days
after cessation of exposure, blurring of the vision, lachrymation,
and photophobia. These symptoms began in the middle of the working
week and became more severe towards the end of the week. In
addition, the mean erythrocyte count was slightly decreased. Later
in the study, after the average concentration was reduced to 307.6
mg/m3 (100 ppm), no systemic effects were observed. Complaints of
irritation of the eyes or disagreeable odour were rare at this
concentration (Sterner et al., 1949).
Velazquez et al. (1969) reported that prolonged exposure (3 -
11 years) to 1-butanol in a cellulose acetate ribbon factory had
caused hearing loss in 9 out of 11 exposed workers. Following this
finding, the 1-butanol level in the working atmosphere was found to
be 246.2 mg/m3 (80 ppm). However, this level may not be
representative of the past exposure.
Seitz (1972) reported 7 case histories, which occurred between
1965 and 1971, concerning workers who had been exposed to 1-butanol
and isobutanol in a non-ventilated photographic laboratory. They
handled the alcohols under intense and hot light without any
precautions. Exposure levels were not quantified but must have
been excessive, exposure time ranged from 1 1/2 months to 2 years.
Two workers had transient vertigo, 3 severe Meniere-like vertigo
with nausea, vomiting and/or headache. In one of these cases,
hearing was also perturbed. Two workers did not have any signs or
symptoms. For ACGIH (1980), the last two papers were the reason for
the reduction of the TLV from 307.8 to 153.9 mg/m3 (100 ppm to 50
ppm).
Several papers concerning clinical observations on workers
exposed to mixtures of solvents including 1-butanol have been
published (Kalekin & Brichenko, 1972; Petrova & Vishnevskii, 1972;
Sanatina, 1973; Shalaby et al., 1973; Kudrewicz Hubicka et al.,
1978; Zaikov & Bobey, 1978). Pathological observations included
effects on the central nervous system, liver, respiration, blood
composition, and complications during pregnancy. However, it is
not possible to judge whether these effects were due to exposure to
1-butanol.
Baikov & Khachaturyan (1973) recommended the maximum
permissible concentration of 1-butanol in ambient air to be set at
a level of about 0.09 mg/m3 (0.03 ppm), as a result of studies with
18 volunteers exposed to 1-butanol vapour at levels of between 0.3
and 15 mg/m3. Five concentrations of 1-butanol vapour were tested.
At 1.2 mg/m3, 1-butanol changed the light sensitivity of the dark-
adapted eye and the electrical activity in the brain. At 1 mg/m3,
these parameters were unaffected.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
General population levels of exposure to 1-butanol through food
and beverages are not available. Occupational levels of exposure
to 1-butanol are limited and inadequate.
10.1.2. Toxic effects
1-Butanol is readily absorbed through the skin, lungs, and
gastrointestinal tract. In animals, 1-butanol is rapidly
metabolized by alcohol dehydrogenase to the corresponding acid, via
the aldehyde, and to carbon dioxide, which is the major metabolite.
The rat oral LD50 for 1-butanol ranges from 0.7 to 2.1 g/kg body
weight; it is, therefore, slightly toxic according to the
classification of Hodge & Sterner. It is markedly irritating to
the eyes and moderately irritating to the skin. The primary
effects from exposure to vapour for short periods are various
levels of irritation of the mucous membranes and central nervous
system depression. Its potency for intoxication is approximately 6
times that of ethanol. A variety of investigations have indicated
non-specific membrane effects of 1-butanol. Effects of repeated
inhalation exposure in animals include pathological changes in the
lungs, degenerative lesions in the liver and kidneys, and narcosis.
However, from the animal studies available, it is not possible to
determine a no-observed-adverse-effect level. 1-Butanol has been
found to be non-mutagenic. No adequate data are available on
carcinogenicity, teratogenicity, or effects on reproduction.
In man, 1-butanol, in the liquid or vapour phase, can cause
moderate skin irritation and severe eye irritation manifested as
a burning sensation, lachrymation, blurring of vision, and
photophobia. Ingestion of the liquid or inhalation of the vapour
may result in headache, drowsiness, and narcosis. The occurrence
of vertigo under conditions of severe and prolonged exposure to
vapour mixtures of 1-butanol and isobutanol has been reported.
From this study, it was not possible to attribute the vertigo to a
single cause. The symptoms were reversible when exposure ceased.
The minimal information available suggests that occupational
human exposure to air concentrations below 307.8 mg/m3 (100 ppm) is
not associated with any adverse symptoms. However, studies on
human volunteers indicate that the light-sensitivity of dark-
adapted eyes and electrical activity of the brain may be influenced
by air concentrations as low as 0.092 mg/m3 (0.03 ppm).
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
No quantitative data on levels in the general environment are
available but, because 1-butanol is readily biodegradable,
substantial concentrations are only likely to occur locally in the
case of major spillages.
10.2.2. Toxic effects
At background concentrations likely to occur in the
environment, 1-butanol is not directly toxic for fish, amphibia, or
crustacea and is practically non-toxic for algae. Some protozoa
are slightly sensitive to 1-butanol.
1-Butanol should be managed in the environment as a slightly
toxic compound. It poses an indirect hazard for the aquatic
environment, because it is readily biodegradable, which may lead to
oxygen depletion.
10.3. Conclusions
1. On the available data, the Task Group was unable to make an
assessment of the health risks of 1-butanol for the general
population; however, it was considered unlikely to pose a
serious hazard under normal exposure conditions.
2. The Task Group was of the opinion that sufficient data were not
available to establish guidelines for setting occupational
exposure limits. There are reports of adverse effects
resulting from occupational overexposure to levels above 307.8
mg/m3 (100 ppm); therefore, and in line with good manufacturing
practice, exposure to 1-butanol should be minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of 1-butanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
1. The Task Group noted that, from the animal studies available,
it was not possible to determine a no-observed-adverse-effect
level. Relevant studies should be conducted so that this can
be achieved.
2. Information on residue and emission levels is desirable.
3. Epidemiological studies, including precise exposure data, would
assist in a better assessment of the occupational hazard of
1-butanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In 1974, the Council of Europe established an acceptable daily
intake (ADI) of 1 mg/kg body weight for butan-1-ol.
More recently (Council of Europe, 1981), specific limits of
30 mg butan-1-ol/kg in beverages and food have been established.
The Food Additives and Contaminants Committee (UK MAFF, 1978)
recommended that residues in food do not exceed 30 mg/kg and
required the results of a 90-day oral toxicity study in the rat
within two years.
Butan-1-ol was evaluated by the EEC Scientific Committee for
Food in 1980. The Committee agreed on the following evaluation:
The available toxicological data relate to metabolism
and short-term oral studies in rats. No long-term oral
studies are available. The Committee was therefore unable
to establish an ADI. Residues occur in food from use as
extraction and carrier solvent as well as from natural
occurrence, but adequate residue data are not available.
The Committee considers the use of this compound
temporarily acceptable as an extraction solvent provided
the residues are limited to 30 mg/kg food. The Committee
requires the provision of an adequate 90-day oral study in
rats as well as information on residue levels by 1983
(CEC, 1981).
At their 23rd meeting, the Joint FAO/WHO Expert Committee on
Food Additives (JECFA) reviewed the data on 1-butanol. They
concluded that:
"There was a lack of data on the effects of long-term
oral exposure to 1-butanol. There were some results of
studies on workers exposed for periods of up to 11 years
to known vapour concentrations, but these were inadequate
for setting an ADI for man. The evaluation of this
compound was not possible on the basis of the data
available. New specifications were prepared, but no
toxicological monograph" (WHO, 1980).
ENVIRONMENTAL HEALTH CRITERIA
FOR
2-BUTANOL
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 2-BUTANOL
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.2 Terrestrial organisms
7.3 Microorganisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Acute toxicity
8.1.2 Signs of intoxication
8.2 Skin and eye irritation
8.3 Short-term exposures
8.4 Long-term exposures
8.5 Reproduction, embryotoxicity, and teratogenicity
8.6 Mutagenicity
8.7 Carcinogenicity
8.8 Special studies
9. EFFECTS ON MAN
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
10.1.2 Toxic effects
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
10.2.2 Toxic effects
10.3 Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY
2-Butanol is a flammable colourless liquid with a
characteristic sweet odour. It has a boiling point of 98.5 °C, a
water solubility of 12.5%, and an n-octanol/water partition
coefficient of 0.61. Its vapour is 2.6 times denser than air.
2-Butanol occurs naturally as a product of fermentation of
carbohydrates. It is used for the extraction of fish meal to
produce fish protein concentrate, for the production of fruit
essences, and as a flavouring agent in food. Human exposure to
2-butanol is mainly occupational. The general population is
exposed through its natural occurrence in food and beverages and
its use as a flavouring agent. Exposure may also result through
industrial emissions.
2-Butanol is readily biodegradeable by bacteria and does not
bioaccumulate. It is not toxic for aquatic animals, algae,
protozoa, or bacteria. 2-Butanol should be managed in the
environment as a slightly toxic compound. It poses an indirect
hazard for the aquatic environment, because it is readily
biodegraded, which may lead to oxygen depletion.
In animals, 2-butanol is absorbed through the lungs and
gastrointestinal tract. No information is available regarding
dermal absorption. Approximately 97% of the dose of 2-butanol in
animals is converted by alcohol dehydrogenase to the corresponding
ketone, which is either excreted in the breath and urine or further
metabolized. The rat oral LD50 for 2-butanol is 6.5 g/kg body
weight; it is, therefore, practically non-toxic, according to the
classification of Hodge & Sterner. The acute toxic effects are
ataxia and narcosis. Its potency for intoxication is approximately
4 times that of ethanol. 2-Butanol is irritating to the eyes and
non-irritating to the skin. From the animal studies available, it
is not possible to determine a no-observed-adverse-effect level.
No adequate data are available on mutagenicity, carcinogenicity,
teratogenicity, or effects on reproduction.
In man, the most likely acute effect of 2-butanol is alcoholic
intoxication. No published data are available concerning other
effects in man.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure: OH
|
CH3-CH-CH2-CH3
Chemical formula: C4H10O
Primary constituent: 2-butanol
Common synonyms: sec-butyl alcohol, secondary butyl
alcohol, butylene hydrate, 2-hydroxy
butane, methyl ethyl carbinol,
butan-2-ol, sec-butanol, SBA,
2-hydroxybutane, CCS 301
CAS registry number: 78-92-2
2.2. Physical and Chemical Properties
Physical and chemical properties of 2-butanol are given in
Table 1.
Table 1. Physical and chemical properties of 2-butanol
-------------------------------------------------------------------
(at 20 °C and 101.3 kPa, unless otherwise stated)
Physical state colourless liquid
Odour characteristic sweet odour
Odour threshold approximately 7.69 mg/m3
(2.5 ppm)
Relative molecular mass 74.12
Density (kg/m3) 806 - 808
Boiling point (°C) initial 98.5 (min)
dry point 100.5 (max)
Freezing point (°C) -115
Viscosity (mPa x s) 3.54
Vapour density (air = 1) 2.55
Vapour pressure (kPa) 1.66
Flashpoint (°C) 23
Autoignition temperature (°C) 406
Explosion limits in air (%) (v/v) lower = 1.7
upper = 9.0
Solubility (% weight) in water, 12.5; miscible with
ethyl alcohol and ether
n-octanol/water partition 0.61
coefficient
Conversion factors 1 mg/m3 = 0.325 ppm
1 ppm = 3.078 mg/m3
-------------------------------------------------------------------
2.3. Analytical Methods
2-Butanol is usually determined quantitatively using gas
chromatography (Abbasov et al., 1971; Bartha et al., 1978; Beaud &
Ramuz, 1978).
NIOSH (1977b) Method No S53 (353) has been recommended. It
involves drawing a known volume of air through charcoal to trap the
organic vapours present (recommended sample is 10 litres at a rate
of 0.2 litre/min). The analyte is desorbed with carbon disulfide
containing 1% 2-propanol. The sample is separated by injection
into a gas chromatograph equipped with a flame ionization detector
and the area of the resulting peak is determined and compared with
standards.
Testing methods for the butanols (ASTM D304-58) are described
in ASTM (1977).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The principal use of 2-butanol is as a chemical intermediate
for conversion into methyl ethyl ketone, a solvent with a fairly
high boiling point (Monich, 1968).
2-Butanol is used for the extraction of fish meal to produce
fish protein concentrate. It is also used for the preparation of
fruit essence and as a flavouring agent in food (Federal Register,
1977). Very recently, 2-butanol has proved to be useful as a
debittering agent for protein hydrolysates (Latasidis & Sïpberg,
1978).
2-butanol is used, to some extent, as a solvent for lacquers,
enamels, vegetable oils, gums, and natural resins; it is also used
in hydraulic brake fluids, industrial cleaning compounds, polishes,
and penetrating oils, and in the preparation of ore-flotation
agents and perfumes (Patty, 1963).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A high rate of degradation of 2-butanol has been seen in a wide
range of test methods. The data in Table 2 suggest that a high
proportion of the total oxygen required for its complete oxidation
is used within a few hours, and degradation would be complete
within a few days. Its biodegradation in surface waters may
present a hazard in terms of oxygen depletion.
No data are available on distribution in soil, sediments, or
air.
Biodegradation data are given in Table 2.
Table 2. Some biodegradation data for 2-butanol
------------------------------------------------------------------------------
5d BOD 33% of ThOD (AFNOR) Dore et al. (1974)
83% of ThOD (APHA) Bridié et al. (1979)b
activated sludge 9.3% of ThOD removed in 24 h by Gerhold & Malaney
unadapted municipal sludge (1966)
58% of ThOD removed in 23 h by McKinny & Jeris (1955)
adapted sludge
biodegradation rate in adapted Pitter (1976)
sludge at 20 °C, 55.0 mg COD g
per h
anaerobic digestion degraded by acetate-enriched Chou et al. (1978)
methane culture after adaptation;
100% of ThOD removed at 342 mg/
litre after 14 days of adaptation
93% of ThOD removed at 110 mg/ Chou et al. (1977)
litre in anaerobic upflow filters
(hydraulic residence time 2 - 10
days) after 52 days of adaptation
bioaccumulation 2-butanol does not bioaccumulate Chiou et al. (1977)
------------------------------------------------------------------------------
ThOD = theoretical oxygen demand - the calculated amount of oxygen needed
for complete oxidation to water and carbon
dioxide.
COD = chemical oxygen demand - measures the chemically oxidizable matter
present.
BOD = biochemical oxygen demand - a simple bioassay measuring the potential
deoxygenating effect of biologically
oxidizable matter present in an effluent.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
A residue of between 10 and 70 mg 2-butanol/kg has been
reported to remain in the dry fish protein concentrate following
extraction with this solvent.
2-Butanol has been found in a number of alcoholic beverages
including beer (Bonte et al., 1978), wine (Bikvalvi & Pasztor,
1977; Bonte, 1978), apple brandies (Woidich et al., 1978), and
grape brandies (Bonte et al., 1978; Schreier et al., 1979);
2-butanol has also been detected in volatiles of cheese (Dumont &
Adda, 1978), southern pea seeds (Fischer et al., 1979), and virgin
oil (Olias Jimbnez et al., 1978).
An industrial emission study indicated that 33 tonnes of
2-butanol were released into the air of the Netherlands over a
period of 1 year (Anon, 1983).
6. KINETICS AND METABOLISM
In rabbits, 2-butanol was oxidized to methyl ethyl ketone,
which could be detected in the expired air, and also conjugated to
form 2-butyl glucuronide, which could be isolated from the urine
(Williams, 1969). 2,3-Butanediol and 3-hydroxy-2-butanone were the
main metabolites of 2-butanol found in the blood of rats given 2.2
ml 2-butanol/kg body weight (Dietz, 1980).
Male rabbits were given 2 ml 2-butanol/kg body weight orally,
and venous blood samples were analysed after 1, 2, 3, 4, 5, and
10 h. The concentration of 2-butanol peaked within an hour at
about 1 g/litre and disappeared to a trace after 10 h. Unchanged
2-butanol was excreted to the extent of 3.3% of the dose in the
breath and 2.6% in the urine. Methyl ethyl ketone, a metabolite,
was detected in the blood and reached a maximum level after 6 h; it
was excreted in amounts equivalent to 22.3% of the dose in the
breath and 4% in the urine (Saito, 1975).
Dietz et al. (1981) developed a pharmacokinetic model to
describe the biotransformation of 2-butanol and its metabolites
2-butanone, 3-hydroxy-2-butanone, and 2,3-butanediol (Fig. 1A).
Male Sprague Dawley rats were given 2-butanol (2.2 ml/kg body
weight, orally) after an overnight fast; blood concentrations of 2-
butanol and its metabolites were estimated at various times up to
30 h. Concentrations of 2-butanol reached a maximum (0.59 g/litre)
within 2 h and declined to less than 0.05 g/litre after 16 h. As
the blood concentration of 2-butanol fell, the concentrations of 2-
butanone, 3-hydroxy-2-butanone, and 2,3-butanediol rose to maximum
levels of 0.78, 0.04, and 0.21 g/litre at 8, 12, and 18 h,
respectively. Approximately 97% of the 2-butanol dose was
converted 2-butanone by alcohol dehydrogenase; the calculated
clearance constant for 2-butanol was 0.40 ml/min. In separate
studies, the individual metabolites were administered to rats in
order to calculate their clearance constants.
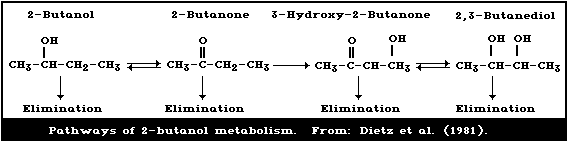 2-Butanol exhibited an apparent blood elimination half-life of
2.5 h in rats treated orally with 2.2 ml/kg body weight. With
decline in blood-alcohol concentration (maximum level 800 mg/litre,
1 h after administration), there was a rise in 2-butanone levels
with 430 mg/litre detected at 1 h and a maximum of 1050 mg/litre
detected 4 h after administration of the alcohol (Traiger &
Bruckner, 1976).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Some toxicity data for 2-butanol in aquatic organisms are given
in Table 3. Only two LC50 values are available for fish, and these
indicate low toxicity for these species. The toxicity of 2-butanol
for aquatic invertebrates is equally low.
Table 3. Toxicity data of 2-butanol for aquatic organisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Fish
Fresh-water species
Golden orfe 3520 48-h LC50 Juhnke & Lüdemann (1978)
(Leuciscus idus
melanotus)
Goldfish 4300 24-h LC50 Bridié et al. (1979a)
(Carassius auratus)
Invertebrates
Fresh-water species
Water flea 2300 24-h EC50 immobilization Bringmann & Kuehn (1982)
(Daphnia magna)
Marine species
Brine shrimp 3800 EC50 excystment Smith & Siegel (1975)
(Artemia salina)
Algae
Green algae
(Scenedesmus 95 8-day no- total biomass Bringmann & Kuehn
quadricauda) observed- (1978a)
adverse-
effect level
Chlorella 8900 EC50 Jones (1971)
pyrenoidosa chlorophyll
content
------------------------------------------------------------------------------------------
7.2. Terrestrial Organisms
An EC50 of 650 mg/litre was reported by Reynolds (1977) for
seed germination in lettuce (Lactuca sativa). Inhibition of seed
germination in cucumber (Cucumis sativus) was observed at 50 375 mg
2-butanol/litre (Smith & Siegel, 1975). There are no relevant data
for terrestrial animals, but, as in the case of terrestrial plants,
significant exposure to 2-butanol is unlikely.
7.3. Microorganisms
Some toxicity data for microorganisms are given in Table 4.
The toxicity of 2-butanol for both protozoa and bacteria is very
low.
Table 4. Toxicity of 2-butanol for microorganisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Protozoa
Uronema parduczi 1416 20-h no-observed- total Bringmann & Kuehn
(ciliate) adverse-effect level biomass (1981)
Chilomonas paramaecium 745 48-h no-observed- total Bringmann & Kuehn
(flagellate) adverse-effect level biomass (1981)
Entosiphon sulcatum 1282 72-h no-observed- total Bringmann & Kuehn
(flagellate) adverse-effect level biomass (1981)
Bacteria
Pseudomonas putida 500 16-h no-observed- total Bringmann & Kuehn
adverse-effect level biomass (1976)
Bacillus subtilis 1630 EC50 spore germination Yasuda-Yasaki et
al. (1978)
------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposure
8.1.1. Acute toxicity
Acute toxicity data for 2-butanol are given in Table 5.
2-Butanol shows low acute toxicity in rodents.
Table 5. Acute toxicity of 2-butanol
-------------------------------------------------------
Species Route of LD50 (g/kg Reference
administration body weight)
-------------------------------------------------------
Rat oral 6.5 US DHEW (1978)
Rabbit oral 4.9 Münch (1972)
Mouse intraperitoneal 0.8 US DHEW (1978)
-------------------------------------------------------
Six male albino rats were exposed to 2-butanol vapour at 48.5
mg/litre (16 000 ppm) for 4 h. Within 14 days, 5 of the 6 rats
died (Smyth et al., 1954).
8.1.2. Signs of intoxication
Signs of intoxication due to 2-butanol exposure include
restlessness, ataxia, prostration, and narcosis (Patty, 1963).
The effects of a moderate non-toxic oral dose of 2-butanol
(0.0163 mol/kg body weight) was studied in rats using a simple
functional test by Wallgren (1960). The intoxicating effect of
2-butanol, compared with that of ethanol (taken equal to 1), was
4.4 on an equimolar basis. Recovery after the treatment was slow.
The livers of mice that died 1 - 3 days following a single
intraperitoneal (ip) dose of 2-butanol showed an abnormal brownish
coloration in the peripheral areas, the livers of those that died
after 4 - 6 days were uniformally dark brown in colour (Maickel &
McFadden, 1979).
8.2. Skin and Eye Irritation
2-Butanol is practically not irritant to the skin of the
rabbit, but it is irritant to the rabbit eye (Smyth et al., 1954).
8.3. Short-Term Exposures
White mice (15 - 20 g) were repeatedly exposed to 2-butanol
vapour at 16.2 mg/litre (5330 ppm) for a total of 117 h. Although
the mice were narcotized, they survived.
Six groups of 2 mice each were exposed to 2-butanol vapour at
5 mg/litre (1650 ppm). After 420 min, no signs of intoxication
were observed. When increasing concentrations were used with
decreasing durations of exposure, ataxia, prostration, and deep
narcosis occurred. The time necessary to induce these symptoms
was inversely proportional to the level of exposure. At a
concentration of 10 mg/litre (3300 ppm), ataxia occurred in 51 -
100 min, prostration in 120 - 180 min, and narcosis in 300 min. At
a concentration of 60 mg/litre (19 800 ppm), these signs appeared
in 7 - 8 min, 12 - 20 min, and 40 min, respectively. No deaths
were observed in this study (Starrek, 1938, cited in Patty, 1982).
The neurophysiological effects induced by 2-butanol were
investigated in 31 male Sprague Dawley rats (200 - 400 g).
2-Butanol was administered intravenously (iv) to the rats at a
concentration of 8.1 mmoles/kg body weight. Within 20 - 60 seconds
of the iv administration, the rats lost righting reflexes. Some
changes were also noticed in the electroencephalographic tracings
compared with the tracings prior to the alcohol administration
(Marcus et al., 1976).
8.4. Long-Term Exposures
No long-term exposure studies are available.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data on reproduction, embryotoxicity, or
teratogenicity have yet been published. However, an inhalation
teratology study with 2-butanol is in progress in the USA (US EPA,
personal communication, 1985).
8.6. Mutagenicity
2-Butanol did not show any mutagenic activity in the yeast
Schizosaccharomyces pombe in both the presence and absence of
mouse liver microsomes (Abbondandolo et al., 1980).
8.7. Carcinogenicity
No carcinogenicity studies are available.
8.8. Special Studies
The effects of 2-butanol on cell survival were studied in the
yeast S. pombe and in V-79 Chinese hamster cells by Abbondandolo
et al. (1980). At 5% concentration, 2-butanol decreased the
survival of suspended yeast cells, but did not have any effect on
monolayer cultures of V-79 cells.
2-Butanol inhibited the contraction of the depolarized guinea-
pig ileum induced by calcium chloride (CaCl2) (Yashuda et al.,
1976).
2-Butanol can potentiate the toxicity of carbon tetrachloride
(CCl4) (Cornish & Adefuin, 1967). This potentiation may be due to
the metabolite 2,3-butanediol, which also has this effect (Traiger
& Bruckner, 1976; Dietz & Traiger, 1979).
Inhalation exposure of rats to 2-butanol (1539 mg/m3 (500 ppm)
for 5 days) resulted in a 47% increase in the cytochrome P-450
levels of kidney microsomes. A maximal increase of 33% in liver
microsomal cytochrome P-450 content was seen after inhalation of
2-butanol at 6156 mg/m3 (2000 ppm) for 3 days. This treatment led
to a 77% increase in the formation of the preneurotoxic metabolite
2-hexanol from 1-hexane by liver microsomes (Aarstad et al., in
press).
Rats receiving a single oral dose of 2-butanol at 2.2 ml/kg
body weight were sacrificed 16, 20, or 40 h after dosing. A 50 -
97% increase in microsomal acetanilide hydroxylase activity was
found. At 40 h, liver cells showed a marked proliferation of
smooth endoplasmic reticulum. This stimulation of the drug-
metabolizing system may explain, to a certain extent, the
potentiation of CCl4 hepatoxicity by 2-butanol (Traiger et al.,
1975).
9. EFFECTS ON MAN
Excessive exposure may result in headache, dizziness,
drowsiness, and narcosis (Muir, 1977). No adverse systemic effects
due to exposure to 2-butanol have been reported in man (Patty,
1982).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population to 2-butanol
through food and beverages, and occupational exposure levels are
not available.
10.1.2. Toxic effects
In animals, 2-butanol is absorbed through the lungs and
gastrointestinal tract. No information is available regarding
dermal absorption. Approximately 97% of the dose of 2-butanol in
animals is converted by alcohol dehydrogenase to the corresponding
ketone, which is either excreted in the breath and urine or further
metabolized. The rat acute oral LD50 for 2-butanol is 6.5 g/kg
body weight; it is, therefore, practically non-toxic according to
the classification of Hodge & Sterner. The toxic effects from
acute exposure are ataxia and narcosis. The potency of 2-butanol
for intoxication is approximately 4 times that of ethanol. It is
irritating to the eyes and non-irritating to the skin. From the
animal studies available, it is not possible to determine a no-
observed-adverse-effect level. No adequate data are available on
mutagenicity, carcinogenicity, teratogenicity, or effects on
reproduction.
In man, the most likely acute effect of 2-butanol is alcoholic
intoxication. No published data are available concerning other
effects on man.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
No quantitative data relating to levels of 2-butanol in the
general environment are available, but, because it is readily
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillage.
10.2.2. Toxic effects
At the background concentrations likely to occur in the
environment, 2-butanol is not toxic for aquatic animals, algae,
protozoa, or bacteria, and it should be managed in the environment
as a slightly toxic compound. It poses an indirect hazard for the
aquatic environment, because it is readily biodegradable, which may
lead to oxygen depletion.
10.3. Conclusions
1. The Task Group was unable to make an assessment of the health
risks of 2-butanol for the general population on the basis of
available data. However, it was considered that 2-butanol was
unlikely to pose a serious hazard, under normal exposure
conditions.
2. The Task Group was of the opinion that available data are not
sufficient to establish guidelines for setting occupational
exposure limits. In line with good manufacturing practice,
exposure to 2-butanol should be minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of 2-butanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
The Task Group recommended that:
1. As it was not possible to determine a no-observed-adverse-
effect level on the basis of available animal studies, relevant
studies should be conducted so that this could be achieved.
2. Information on residue and emission levels is desirable.
3. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
2-butanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Food Additives and Contaminants Committee (UK MAFF, 1978)
recommended that residues of butan-2-ol in food should not exceed
30 mg/kg and required the results of a 90-day oral toxicity study
in the rat within 2 years.
This compound could not be included in lists 1 or 2 by the
Council of Europe and it was included in list 3A (Council of
Europe, 1981).
At their 23rd meeting, the Joint FAO/WHO Expert Committee on
Food Additives (JECFA) reviewed the data on 2-butanol. They
concluded that "The evaluation of this compound was not possible on
the basis of the data available. New specifications were prepared,
but no toxicological monograph" (WHO, 1980).
ENVIRONMENTAL HEALTH CRITERIA
FOR
tert-BUTANOL
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR tert-BUTANOL
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.2 Terrestrial organisms
7.3 Microorganisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Acute toxicity
8.1.2 Signs of intoxication
8.2 Skin and eye irritation
8.3 Short-term exposures
8.4 Reproduction, embyrotoxicity, and teratogenicity
8.5 Mutagenicity
8.6 Carcinogenicity
8.7 Special studies
9. EFFECTS ON MAN
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
10.1.2 Toxic effects
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
10.2.2 Toxic effects
10.3 Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY
tert-Butanol is a colourless liquid or white crystalline solid
with a camphor-like odour. It has a melting point of 25 °C, a
boiling point of 81.5 - 83 °C, is freely soluble in water, and its
n-octanol/water partition coefficient is 0.37. Its vapour is 2.6
times denser than air. It is used primarily as a solvent, a
dehydrating agent, and as an intermediate in the manufacture of
other chemicals. It is also used as a denaturant for alcohols.
Human exposure will be mainly occupational. Data on exposure of
the general population are not available, but it may result from
industrial emissions. tert-Butanol is inherently biodegradable and
does not bioaccumulate. At ambient levels, it is not toxic for
fish, amphibia, crustacea, algae, or bacteria.
In animals, tert-butanol is absorbed through the lungs and
gastrointestinal tract; no information is available on dermal
absorption. tert-Butanol is not a substrate for alcohol
dehydrogenase and is slowly metabolized by mammals. Up to 24% of
the dose is eliminated in the urine as the glucuronide, and up to
10% of the dose can be excreted in the breath and urine as acetone
or carbon dioxide. The rat oral LD50 is 3.5 g/kg body weight; it
is, therefore, slightly toxic according to the classification of
Hodge & Sterner. The primary acute effects observed in animals are
signs of alcoholic intoxication. Its potency for intoxication is
approximately 1.5 times that of ethanol. Animal data regarding
skin and eye irritation are not available. tert-Butanol produces
physical dependance in animals and post-natal effects in offspring
exposed in utero. Data concerning the pathological effects of
repeated exposure of animals are not available. From the animal
studies available, it is not possible to determine a no-observed-
adverse-effect level. tert-Butanol has been found not to be
mutagenic. Adequate data are not available on carcinogenicity,
teratogenicity, or effects on reproduction.
In man, tert-butanol is a mild irritant to the skin. No other
effects on man have been reported, and there have been no reports
of poisonings.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure:
CH3
|
CH3 - C - CH3
|
OH
Chemical formula: C4H10O
Primary constituent: tert-butanol
Common synonyms: 2-methyl-2-propanol, tert-butyl
alcohol, tertiary butanol, t.
butanol, trimethyl carbinol, TBA,
TMA, t. butyl hydroxide, NCL-C55
367, trimethyl methanol
Cas registry number: 75-65-0
2.2. Physical and Chemical Properties
Some physical and chemical data for tert-butanol are given in
Table 1.
Table 1. Physical and chemical data for tert-butanol
-----------------------------------------------------------------------------
(at 20 °C and 101.3 kPa, unless otherwise stated)
Physical state solid (crystals)
Odour camphor-like
Odour threshold approximately 144.7 mg/m3(47 ppm)
Relative molecular mass 74.12
Density (kg/m3) 779 - 782 at 26 °C
Boiling point (°C) initial 81.5 (min.); dry point 83.0 (max.)
Melting point 25 °C
Viscosity (mPa x s) 3.3 at 30 °C
Vapour density (air = 1) 2.55
Vapour pressure (mm Hg) 31 (at 25 °C, 42; at 30 °C, 56)
Flashpoint (°C, TOC) 16
(°C, TCC) 4
Autoignition temperature 470 °C
Explosion limits in air (v/v %) lower 2.35; upper 8.0
Solubility soluble in water; miscible with ethyl
alcohol, ether; also soluble in ketones,
esters, aromatic and aliphatic hydrocarbons
n-octanol/water partition 0.37
coefficient
Conversion factors: 1 mg/m3 = 0.325 ppm
1 ppm = 3.078 mg/m3
-----------------------------------------------------------------------------
2.3. Analytical Methods
Testing methods for the butanols (ASTM D304-58) are described
in ASTM (1977).
It is known that several alcohols, including tert-butanol,
give colour reactions with aldehyde in the presence of sulfuric
acid (Patty, 1963). NIOSH (1977b) describes several methods.
AOAC has finalized the method for detecting tert-butanol in
distilled liquors (AOAC, 1975).
tert-Butanol has been determined in the air of industrial
premises by Abbasov et al. (1971) using a gas chromatographic
method and by Zamarakhina (1973) using a photometric method, and in
blood by Wood & Laverty (1976).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The primary use of tert-butanol is as a solvent. It is also
used as a dehydrating agent, in the extraction of drugs, in the
manufacture of perfumes (particularly in the preparation of
artificial musk), in the recrystallization of chemicals, and as a
chemical intermediate (e.g., in the manufacture of tert-butyl
chloride and in the manufacture of tert-butyl phenol). It is an
approved denaturant for ethyl alcohol and for several other
alcohols. Catalytic dehydration of tert-butanol is carried out to
obtain isobutylene, and it has been patented for use as a gasoline
antiknock agent.
Moreover, it is used in the purification of polyolefins, for
the separation of solids from coal liquids and as blowing agent for
the manufacture of imide group-containing foams from copolymers of
methacrylonitrile and methacrylic acid (Patty, 1963; Monich, 1968;
Sherman, 1978).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
No data are available on the distribution of tert-butanol in
soil, sediments, or air.
In short-term tests, there was little degradation but over a
longer period of about one month, most of the material was fully
degraded. Therefore, tert-butanol is inherently rather than
readily biodegradable. Some biodegradation data for tert-butanol
are given in Table 2.
tert-Butanol does not bioaccumulate (Chiou et al., 1977).
Table 2. Biodegradation data for tert-butanol
---------------------------------------------------------------------------
5d BOD 0% of ThOD (AFNOR) Dore et al. (1974)
1% of ThOD (APHA) Bridié et al. (1979b)
30d BOD 0% of ThOD (closed-bottle Gerike & Fischer (1979)
test, conventional)
0% of ThOD (closed-bottle Gerike & Fischer (1979)
test, preadaptation)
MITI test 0% of ThOD removed after Gerike & Fischer (1979)
14 days (BOD14: 7% of ThOD)
OECD screening 29% of ThOD removed after Gerike & Fischer (1979)
test 19 days; no pre-adaptation
Sturm test 32% of ThOD removed, but no Gerike & Fischer (1979)
production of CO2
AFNOR T90-302 80% of ThOD removed after Gerike & Fischer (1979)
test 28 days
93% of ThOD removed after Gerike & Fischer (1979)
42 days
Zahn-Wellens 96% of ThOD removed after Gerike & Fischer (1979)
test 6 days
Couple-units test 33% of ThOD removed after Gerike & Fischer (1979)
(conventional) 42 days adaptation
Square-wave 69% of ThOD removed after Gerike & Fischer (1979)
feeding 30 days adaptation
Activated 0.8% of ThOD removed in 24 h Gerhold & Malaney
sludge by unadapted municipal sludge (1966)
2% of ThOD removed in 23 h by McKinney & Jeris (1955)
adapted sludge
---------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------
98% of ThOD removed in 5 days Pitter (1976)
by adapted sludge;
biodegradation rate at 20 °C
0.03/h
Anaerobic 73% of ThOD removed at Chou et al. (1978)
digestion 400 mg/litre in anaerobic
up-flow filters (hydraulic
residence time 2 - 10 days)
after 52 days of adaptation
---------------------------------------------------------------------------
ThOD = theoretical oxygen demand - the calculated amount of oxygen
needed for complete oxidation to
water and carbon dioxide.
COD = chemical oxygen demand - measures the chemically oxidizable
matter present.
BOD = biochemical oxygen demand - a simple bioassay measuring the
potential deoxygenating effect of
biologically oxidizable matter
present in an effluent.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
An industrial emmission study indicated that 207 tonnes of
tert-butanol were released into the air in the Netherlands over a
period of 1 year (Anon, 1983).
No other data are available.
6. KINETICS AND METABOLISM
tert-Butanol is not a substrate for alcohol dehydrogenase
(Derache, 1970; Cederbaum et al., 1983) and is slowly metabolized
by mammals (Williams, 1969; Derache, 1970; Beaugé et al., 1981).
Possible routes of metabolism are direct conjugation of the
hydroxyl group with glucuronic acid and oxidation of one or more of
the alkyl substituents. Following treatment with tert-butanol,
24% of the dose was detected as the glucuronide conjugate in the
urine of rabbits (Kamil et al., 1953) and increased acetone
excretion has been observed in the breath and urine of rats treated
with tert-butanol (Baker et al., 1982; Yojay et al., 1982).
After a single oral dose of tert-butanol (25 mmol/kg), blood
concentrations in female Wistar rats declined slowly from 13.2 ±
0.5 mmol at 2 h to 11.4 ± 0.3 mmol at 20 h (Beaugé et al., 1981).
In rats maintained on a liquid diet containing 20 ml tert-
butanol/litre for 20 days, the blood level 30 min after withdrawal
of the diet of 20 mmol declined to 5 mmol in 8 h. By comparison,
blood levels of ethanol of 45 mmol (achieved by a liquid diet of
87 ml ethanol/litre for 20 days) were reduced to zero within 4.5 h
(Wood & Laverty, 1979). In female Sprague Dawley rats given single
oral doses of either ethanol (5.0 g/kg body weight) or tert-
butanol (1.2 g/kg body weight), the rates of elimination were 10.7
± 0.5 and 0.7 ± 0.1 mmol/kg per h, respectively (Thurman et al.,
1980).
Following an oral dose of 2 g tert-butanol/kg body weight to
rats, a maximum blood level of 1240 mg/litre (124 mg%) was reached
in 2 h; this decreased very slowly to 1200 mg/litre (120 mg%) after
4 h and to 1100 mg/litre (110 mg%) after 8 h; only about 1% of
the dose was excreted in the urine (Gaillard & Derache, 1965).
tert-Butanol was found in the blood of rabbits 70 h after oral
administration of 2 ml/kg body weight (Saito, 1975).
In Long-Evans rats treated with tert-butanol (1 g/kg body
weight, route not specified), the rate of disappearance of tert-
butanol from the blood was apparently of first order with a half
life of 9.1 h (Baker et al., 1982). Using 14C- and 13C-
tert-butanol, the same authors investigated the metabolism of
tert-butanol to form acetone. It was found that following
administration of tert-butanol (0.75 - 2 g/kg body weight),
approximately 0.5 - 9.5% of the dose was excreted as acetone in
the urine and breath. The total production of acetone varied
considerably between animals given the same dose, and, as a
result, no correlation between dose and acetone excretion could
be established. Evidence was also obtained indicating that carbon
dioxide (CO2) was a metabolic product of tert-butanol. The
conversion of tert-butanol (possibly via acetone) was not
quantified. Yojay et al. (1982) provided evidence that, in rats
treated intraperitoneally with tert-butanol at 1 or 2 mg/kg body
weight, blood levels of acetone were approximately proportional to
the dose of tert-butanol. In support of the in vivo metabolic
conversion of tert-butanol to acetone, Cederbaum & Cohen (1980)
and Cederbaum et al. (1983) demonstrated the metabolism of
tert-butanol to formaldehyde and acetone in an in vitro system
consisting of rat liver microsomes and a hydroxyl radical
generating system.
Investigations on the induction of tert-butanol metabolism
have been conducted by Baker et al. (1982), Thurman et al. (1980),
and McComb & Goldstein (1979). In rats, Baker et al. (1982) were
unable to demonstrate increased conversion of tert-butanol to
acetone in animals in which the hepatic mixed-function oxidase
activity had been induced by prior phenobarbital treatment.
Thurman et al. (1980) studied the effects on tert-butanol
elimination in rats of pre-treatment (oral) with 5.7% (w/v)
tert-butanol every 8 h, for either 1 or 2.5 days. After the
pre-treatment, animals were given tert-butanol to raise their blood
levels to between 1250 and 1500 mg/litre. The rates of elimination
were very similar, but there was a suggestion of slightly faster
elimination following pre-treatment. The investigators concluded
that, unlike ethanol pre-treatment, which induces its own
metabolism, tert-butanol pre-treatment had little or no effect on
the subsequent rate of tert-butanol elimination in the rat.
In contrast to the results obtained in rats, it appears that
tert-butanol elimination in mice can be substantially increased by
pre-treatment with tert-butanol. Male Swiss Webster mice were
given an ip loading dose of 6.8 mmol tert-butanol/kg body weight
and then exposed to various vapour concentrations of tert-butanol
for 24 h. It was found that 15 min after the 24-h inhalation
period, blood- tert-butanol levels were linearly related to the
vapour concentrations. Blood levels ranged from 3 to 14 mmol with
increasing vapour concentrations of 30 - 100 µmol/litre air (McComb
& Goldstein, 1979). It was also found that the rate of elimination
of blood- tert-butanol was significantly increased by inhalation.
In mice, after a single ip injection of 8.1 mmol tert-butanol/kg
body weight, initial blood levels of 8 mmol took 8 - 9 h for
elimination (blood- tert-butanol half-life was approximately 5 h).
However, after 3 days, inhalation at a vapour concentration to give
levels of 8 mmol/litre blood, tert-butanol disappeared within 3 h
of removal of mice from the inhalation chamber (half-life of tert-
butanol in blood was approximately 1.5 h) (McComb & Goldstein,
1979).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
The toxicity of tert-butanol has been tested in very few
organisms. However, in the few studies performed, the toxicity of
the compound has been very low.
Some toxicity data for aquatic organisms are given in Table 3.
Table 3. Toxicity of tert-butanol for aquatic organisms
-------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
-------------------------------------------------------------------
Fish (acute)
Fresh-water species
Creek chub 3000 - 6000 24-h LC50 Gillette
(Semotitus et al.
atromaculatus) (1952)
Goldfish > 5000 24-h LC50 Bridié
(Carassius et al.
auratus) (1979a)
Invertebrates (acute)
Marine species
Brine shrimp 7800 EC50 excystment Smith &
(Artemia salina) Siegel
(1975)
Algae
Fresh-water
Green algae
Chlorella 24 200 EC50 chlorophyll Jones
pyrenoidosa content (1971)
-------------------------------------------------------------------
7.2. Terrestrial Organisms
An EC50 of 90 800 mg/litre was reported for germination in
cucumber (Cucumis sativus) by Smith & Siegel (1975). There are
no relevant data for terrestrial animals. However, significant
terrestrial exposure to tert-butanol is unlikely for either plants
or animals.
7.3. Microorganisms
One study has indicated that Nitrosomonas (nitrifying
bacterium) shows a high tolerance for tert-butanol; tert-Butanol
inhibits nitrifying activity at 39 400 mg/litre (Blok, 1981).
There was no inhibition of degradation by methane culture on
acetate substrate at 7400 mg tert-butanol/litre (Chou et al.,
1978).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposure
8.1.1. Acute toxicity
Some acute toxicity data for animals are given in Table 4.
Table 4. Acute toxicity of tert-butanol in animals
------------------------------------------------------
Species Route of LD50 (g/kg Reference
administration body weight)
------------------------------------------------------
Rat oral 3.5 US DHEW (1979)
Rabbit oral 3.6 Münch (1972)
Mouse intravenous 1.5 Patty (1982)
Mouse intraperitoneal 0.9 US DHEW (1978)
------------------------------------------------------
8.1.2. Signs of intoxication
Animals exposed to the vapours of tert-butanol may manifest
the following signs of intoxication: restlessness, irritation of
mucous membranes, ataxia, prostration, and narcosis (Patty, 1963).
The narcotic potency of tert-butanol in rabbits is similar to
that of other butanols (Münch, 1972). After ip administration to
mice, the LD50 was > 1000 mg/kg body weight at 30 min and 441
mg/kg body weight at 7 days; gross post-mortem examination of the
livers showed an abnormal dark coloration (Maickel & McFadden,
1979). In rabbits, an oral dose of 1.3 g tert-butanol/kg body
weight was shown to be the minimum narcotic dose (Münch & Schwartz,
1925). The minimum oral lethal dose in the rabbit was 6.0 ml/kg
body weight (approximately 4.7 g/kg) (Patty, 1963). Injection (ip)
of tert-butanol in rats induced a spectrum of intoxication
virtually identical to that of ethanol, but its intoxicating
potency was approximately 1.5 times that of ethanol (McCreery &
Hunt, 1978).
Oral administration of a single dose of 24 mmol tert-
butanol/kg body weight caused a temporary disturbance in the fat
metabolism in the liver cells of female Wistar rats. The authors
suggest that this may be related to the stress induced by the
administration of tert-butanol, which is metabolized very slowly
(Beaugé et al., 1981).
8.2. Skin and Eye Irritation
No data are available on skin and eye irritation in animals.
8.3. Short-Term Exposures
Physical dependence following tert-butanol administration was
investigated using 12 random-bred male albino rats. A liquid diet
containing 20 ml tert-butanol/litre was given to the rats for
4 - 20 days. Docility and slight ataxia were observed in the rats
during this period. About 5 - 6 h after removal of the diet,
withdrawal-signs such as muscular rigidity, stiff curled tails,
abnormal gait, tremor, and irritability became apparant. Four rats
exhibited spontaneous forelimb convulsions. Audiogenic convulsions
were observed in 5 rats, and 3 of them died as a consequence. tert-
Butanol was still detectable in the blood of the rats, 8 h after
its withdrawal (Wood & Laverty, 1979).
In another study, 15 adult male Long Evans rats were exposed
to 4 different protocols using various concentrations of tert-
butanol in water as their only available fluid; 14 animals were
used as controls. At concentrations of 3.5 ml/litre, severe toxic
reactions were found, including anorexia, self mutilation, and
death. When animals consumed at least 3 g tert-butanol/kg body
weight per day for 90 days, withdrawal symptoms were observed,
independent of dosing conditions. Such an intake occurred only
when the concentration of tert-butanol was 3% or greater (Grant
& Samson, 1981). In female Sprague Dawley rats, tert-butanol
administered by gastric intubation, every 8 h, for up to 6 days,
was shown to produce physical dependence (Thurman et al., 1980).
An investigation was undertaken with 5 to 8-week-old male
Swiss-Webster mice weighing 22 - 30 g. A priming dose of 6.8 -
10.1 µmol tert-butanol/kg body weight (10% w/v in 0.9% saline) was
administered ip to groups of 24 mice. They were then exposed to
vapour concentrations of between 50 and 80 µmol tert-butanol/litre
air for 24 h per day; concentrations below or above these limits
either did not produce physical dependance or were initially too
toxic. Because it was found that continual exposure induced the
elimination of tert-butanol, exposure concentrations were
increased daily, in order to maintain a steady blood- tert-butanol
level of between 5 - 8.5 mmol. Exposure lasted for 1, 3, 6, or 9
days. Withdrawal signs were noted after removing the mice from the
inhalation exposure. The intensity of the withdrawal reaction
increased with the duration of inhalation and with the blood- tert-
butanol levels maintained during the intoxication period. The
withdrawal syndrome was qualitatively similar to that produced by
ethanol. tert-Butanol has been shown to be 4 - 5 times as potent
as ethanol in producing physical dependence. Thus, since it is
also 4 - 5 times more lipid soluble than ethanol, McComb &
Goldstein (1979) concluded that the dose of alcohol necessary to
induce physical dependence was inversely proportional to its
solubility.
In a study by Bellin & Edmonds (1976), physical dependence was
induced in 12 male Sprague Dawley rats (350 - 500 g) and 10 female
albino guinea-pigs (200 - 400 g) when given tert-butanol ip
(0.8 g/kg body weight as a 10% w/v solution) at 8-h intervals
for 4 days. Alteration of body temperature (decreased during
intoxication and increased during withdrawal) was more pronounced
in the rats than in the guinea-pigs. However, these differences
could be due to species or sex.
An impairment of avoidance behaviour was shown following short-
term ingestion of tert-butanol in mice (number not stated).
Experimental animals received a liquid diet containing tert-
butanol at 12.5 ml/litre for 7 days and during this time ingested
an average of 3.4 g tert-butanol/kg body weight, per day. One day
after cessation of this diet, the animals were exposed to the
avoidance training procedure. Avoidance was much less in the
treated group than in the controls (Snell & Harris, 1980).
Pregnant Swiss Webster mice (15 per group) were fed liquid
diets containing tert-butanol at concentrations of 0, 5.0, 7.5,
or 10 g/litre from day 6 to day 20 of gestation. In order to gain
insight into the effects of maternal nutrition and behaviour on
post-natal pup development, approximately half of the treated
maternal animals were replaced, within 24 h of parturition, with
untreated females, also recently delivered. tert-Butanol was
approximately 5 times more potent than the same dose of ethanol in
producing developmental delay in post-parturition physiological,
and psychomotor performance, scores. At the higher concentrations,
there were also significant postnatal maternal, nutritional, and
behavioural factors affecting lactation and/or nesting behaviour,
which influenced the development of pups exposed in utero (Daniel
& Evans, 1982).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data on reproduction, embryotoxicity, or
teratogenicity have yet been published. In contrast to ethanol,
tert-butanol at concentrations of 1000 - 4000 mg/litre did not
reduce the in vitro fertilizing capacity of mouse spermatozoa
(Anderson et al., 1982).
An inhalation teratology study is in progress in the USA (US
EPA, personal communication, 1985).
8.5. Mutagenicity
At a concentration of 1%, tert-butanol was classified among
the chemicals that had no apparent mutagenic effect on formation
of antibiotic-resistant mutants in Micrococcus aureus populations
(Clark, 1953). tert-Butanol was not mutagenic in Neurospora
crassa (Dickey et al., 1949). In short-term tests conducted by
the National Toxicology Program (USA), tert-butanol was negative
in the Ames test ( Salmonella, +/-activation), mouse lymphoma, and
in vitro cytogenetics assays (US EPA, personal communication,
1985).
8.6. Carcinogenicity
tert-Butanol is currently being evaluated for carcinogenicity
by the US National Cancer Institute using the standard bioassay
protocol. Groups composed of 50 male and 50 female B6C3F1 mice
were exposed for 2 days per week, from week 9 to 104, to 0, 5000,
10 000, or 20 000 mg tert-butanol/litre drinking-water. Groups
comprising 50 male and 50 female Fischer 344 rats were exposed at
the same rate to the same levels from weeks 7 to 104. The
treatment period has now been completed and histological
examinations are in progress (IARC, 1984).
An inhalation carcinogenicity study has been initiated in the
USA (US EPA, personal communication, 1985).
8.7. Special Studies
tert-Butanol was investigated for its ability to deplete the
cerebral calcium level. An ip dose of 2 g/kg body weight was
administered to male Sprague Dawley rats weighing between 150 and
225 g. The animals were sacrificed 30 min after the administration
of the alcohol. There was a significant decrease in cerebral
calcium contents compared with that in the control animals (35
mg/kg versus 55 mg/kg) (Ross, 1976).
In Sprague Dawley rats (190 - 250 g), ip administration of
aqueous tert-butanol (250 g/litre) was shown to increase carbon
tetrachloride-induced hepatotoxicity, as evaluated by serum
glutamate-pyruvate transaminase levels. However, there was no
depletion in hepatic glutathione or loss in body weight (Harris &
Anders, 1980). tert-Butanol can potentiate the toxicity of carbon
tetrachloride in Sprague Dawley rats (Cornish & Adefuin, 1967).
The inhibition of the synthesis of ornithine decarboxylase
(ODC) and tyrosine aminotransferase (TAT) by tert-butanol was
studied in partially-hepatectomized female rats (about 238 g) of
mixed strains. Immediately after partial hepatectomy, 15% (w/v)
aqueous tert-butanol (2.8 g/kg body weight) was given to 6 rats by
gastric intubation. In the liver, 4 h after partial hepatectomy,
the ODC activity was decreased to 22% and the TAT activity to about
52% of the activities in the control group. In the kidney, 4 h
after partial hepatectomy, the ODC activity was decreased to about
31% of the activity in the control group. In the brain, tert-
butanol did not induce any significant changes in the ODC activity
compared with that in the control group (Poso & Poso, 1980).
The presence of a hydroxy radical is needed for prostaglandin
biosynthesis. tert-Butanol was shown to be a hydroxyl radical
scavenger and, therefore, an inhibitor of prostaglandin
biosynthesis. In the incubation system of microsomes, prepared
from bovine vesicular glands containing epinephrine, the 14C
incorporation as a percentage of total labelled prostaglandins E
and F, respectively, was 16.4% and 23.4%. When 0.025 ml of tert-
butanol was added to this incubation system, the values were 15.5%
and 13.8%, respectively. Further increases in concentration
resulted in decreased incorporation (Panganamala et al., 1976).
In another study using isolated perfused rat lungs, the infusion of
low concentrations of tert-butanol (0.002 mmol - 0.2 mmol) resulted
in the continuous output of prostaglandins into the venous
effluent. However, there was a gradual decrease in the
prostaglandin output as the concentration of the alcohol increased
(Thomas et al., 1980).
Cation flux across membranes has been investigated in a number
of studies. tert-Butanol inhibited, in a dose-dependent manner,
the in vitro contraction of depolarized guinea-pig ileum induced
by calcium chloride (Yashuda et al., 1976) and at a 34 mmol
concentration impaired the response of rat brain cortex slices to
electrical stimulation, inhibiting the influx of sodium, but only
weakly affecting potassium afflux (Wallgren et al., 1974).
Wiesbrodt et al. (1973) studied the effects of tert-butanol on
the gastric mucosa of Heidenheim pouches in 4 unanaesthetized
dogs. They observed a decrease in the transmucosal potential and
an increase in the net flux of Na+ ion into the lumen of the pouch
at all concentrations tested (0.5 - 1.5 mol).
The effect of tert-butanol, at 1 mmol concentration, on
isolated rat mitochondria and lysosomes was studied by Sgaragli
et al. (1975). The alcohol did not affect lipid peroxidation and
membrane stability as evaluated by the release of total protein
acid phosphatase and glutamate dehydrogenase.
tert-Butanol decreased the in vitro Km of phosphorilase b for
glucose-1-phosphate with saturating glycogen concentrations and
markedly increased Vmax (Dreyfus et al., 1978). Akhtem et al.
(1978) studied the interaction between tert-butanol and cytochrome
P-450 from rat liver microsomes; they revealed spectral shifts
indicating the formation of alcohol-cytochrome P-450 complexes.
Sugiyama et al. (1976) found that tert-butanol greatly enhanced
the side chain cleavage activity of purified P-450.
A 15-min pre-session oral administration of tert-butanol
at 0.25 - 3 g/kg body weight to 4 trained male Long Evans rats
produced a dose-related decrease in fixed-ratio responding at
doses of 0.5 g/kg or more. tert-Butanol was 1.6 times as potent as
ethanol, in this respect. The 1 g/kg dose decreased responding by
about 20%, 15, 30, or 60 min after dosing (Witkin & Leander, 1982).
tert-Butanol produced a marked dose-dependent activation
of locomotor activity in short-sleep mice, selectively bred for
relative insensitivity to the hypnotic properties of ethanol. In
alcohol-sensitive long-sleep mice, the locomotor activity was
depressed in a dose-dependent fashion. Test doses were 0, 4, 5,
or 6 g/kg body weight, administered intravenously in 0.9% saline
(Dudek & Philips, 1983).
Twelve neonatal Long Evans rats were given tert-butanol at
doses of up to 2.69 g/kg body weight per day in a milk formula
through a cannula, from postnatal days 4 to 7 (corresponding to
the brain growth spurt). At this point, they were transferred to
a plain milk formula, for the next 11 days. After sacrifice on day
18, exposed animals showed significant decreases in absolute and
relative brain weights and lower DNA levels in hind-brain samples
in comparison with controls, indicating a certain degree of
microcephaly (Grant & Samson, 1982).
No effects were found in the basal synaptosomal membrane
fluidity or in the activity of Na+ + K+ ATPase in the brain of
Sprague Dawley rats given a single oral dose of 1.85 g tert-
butanol/kg body weight (Beaugé et al., 1984).
9. EFFECTS ON MAN
tert-Butanol is slightly irritant to the skin. When the
compound was applied to the skin of 5 human volunteers, no reaction
other than slight erythema and hyperaemia was observed (Oettel,
1936). However, Edwards & Edwards (1982), described an allergic
skin reaction to tert-butanol in a 58-year-old patient who used
skin screen containing tert-butanol. A patch test was positive
for tert-butanol. There are no other published reports of adverse
effects or poisonings in man.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population through food and
occupational exposure levels are not available.
10.1.2. Toxic effects
In animals, tert-butanol is absorbed through the lungs and
gastrointestinal tract; no information is available on dermal
absorption. tert-Butanol is not a substrate for alcohol
dehydrogenase and is slowly metabolized by mammals. Up to 24% of
the dose is eliminated in the urine as the glucuronide, and up to
10% of the dose can be excreted in the breath and urine as acetone
or carbon dioxide. The rat oral LD50 is 3.5 g/kg body weight; it
is, therefore, slightly toxic according to the classification of
Hodge & Sterner. The primary acute effects observed in animals are
signs of alcoholic intoxication. Its potency for intoxication is
approximately 1.5 times that of ethanol. Animal data regarding
skin and eye irritiation are not available. tert-Butanol produces
physical dependance in animals and post-natal effects in offspring
exposed in utero. Data on pathological effects of repeated
exposure of animals are not available. From the animal studies
available, it is not possible to determine a no-observed-adverse-
effect level. tert-Butanol has been found not to be mutagenic. No
adequate data are available on carcinogenicity, teratogenicity, or
effects on reproduction.
In man, tert-butanol is a mild irritant to the skin. There
have not been any reports of poisonings or any other effects in
man.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
No quantitative data relating to levels in the general
environment are available, but, because tert-butanol is inherently
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillage.
10.2.2. Toxic effects
tert-Butanol is inherently biodegradable and is not toxic for
fish, amphibia, crustacea, algae, or bacteria.
10.3. Conclusions
1. On the available data, the Task Group was unable to make an
assessment of the health risks from tert-butanol for the
general population. However, it was considered unlikely to
pose a serious hazard under normal exposure conditions.
2. The Task Group was of the opinion that available data are not
sufficient to establish guidelines for setting occupational
exposure limits. In line with good manufacturing practice,
exposure to tert-butanol should be minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of tert-butanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
1. The Task Group noted that, from the animal studies available,
it was not possible to determine a no-observed-adverse-effect
level. Relevant studies should be conducted so that this can
be achieved.
2. Information on residue and emission levels is desirable.
3. Epidemiological studies, including precise exposure data, would
assist in a better assessment of the occupational hazards from
tert-butanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Council of Europe has recently established (Council of
Europe, 1981) a specific limit of 30 mg tert-butanol/kg in candy
in confectionery. tert-Butanol was evaluated by the EEC
Scientific Committee for Food in 1980. The Committee agreed on the
following evaluation:
"The available data are insufficient to establish an
ADI. Biochemically, this solvent will behave like other
tertiary carbinols, which are generally not very reactive.
The residues in food are minimal and are not a hazard to
health. The Committee considers the use of this compound
temporarily acceptable as an extraction solvent provided
residues from use as an extraction solvent in food as
consumed do not exceed 10 mg/kg food. The provision of an
adequate 90-day feeding study in a rodent species is
required by 1983."
ENVIRONMENTAL HEALTH CRITERIA
FOR
ISOBUTANOL
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ISOBUTANOL
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.2 Terrestrial organisms
7.3 Microorganisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Acute toxicity
8.1.2 Signs of intoxication
8.2 Skin and eye irritation
8.3 Short-term exposures
8.4 Long-term exposures
8.5 Reproduction, embryotoxicity, and teratogenicity
8.6 Mutagenicity
8.7 Carcinogenicity
8.8 Special studies
9. EFFECTS ON MAN
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
10.1.2 Toxic effects
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
10.2.2 Toxic effects
10.3 Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY
Isobutanol (2 methyl propanol) is a inflammable colourless
liquid with a sweet odour similar to that of amyl alcohol. It
has a boiling point of 108 °C, a water solubility of 8.7%, and
its n-octanol/water partition coefficient is 0.83. Its vapour
is 2.6 times denser than air. It occurs naturally as a product of
fermentation and is synthesized from petrochemicals. It is used as
an organic solvent, as a plasticizer, in the manufacture of
isobutyl esters, in perfumes, and as a flavouring agent. Human
exposure is primarily occupational. Exposure of the general
population will mainly be from its natural occurrence in food and
its use as a flavouring agent, but may also result from industrial
emissions.
Isobutanol is readily biodegradable and does not bioaccumulate.
It is not directly toxic for fish, crustacea, amphibia, or algae.
Protozoa will tolerate levels of isobutanol likely to be found in
the environment. Isobutanol should be managed in the environment
as a slightly toxic compound. It poses an indirect hazard for the
aquatic environment, because it is readily biodegraded, which may
lead to oxygen depletion.
In animals, isobutanol is absorbed through the skin, lungs, and
gastrointestinal tract. It is metabolized by alcohol dehydrogenase
to isobutyric acid via the aldehyde and may enter the tricarboxylic
acid cycle. Small amounts of isobutanol are excreted unchanged
(< 0.5% of the dose), or as the glucuronide (< 5% of the dose)
in the urine. In rabbits, metabolites found in the urine include
acetaldehyde, acetic acid, isobutylaldehyde, and isovaleric acid.
Oral LD50 values (2.5 - 3.1 g/kg body weight) and the inhalation
LC50 (19.2 g/m3) in rats classifies isobutanol as slightly toxic
according to Hodge & Sterner. The acute toxic effects are
alcoholic intoxication and narcosis. Isobutanol is severely
irritating to the eyes and moderately irritating to the skin. A
group of rats given a solution of isobutanol (1 mol/litre) as their
sole drinking liquid for 4 months did not show any adverse effects
on the liver, while another group given a 2 mol/litre solution as
their sole drinking liquid for 2 months showed reductions in fat,
glycogen, RNA content, and overall size of the cells in the liver.
Continuous inhalation exposure of rats to 3 mg/m3 for 4 months
resulted in depression of leg withdrawal response to electrical
stimulation, and minor changes in formed elements of the blood and
serum enzymes. The estimated no-observed-adverse-effect level was
0.1 mg/m3. In a lifetime carcinogenicity study, groups of rats
received isobutanol subcutaneously (0.05 ml/kg, twice a week) or
orally (0.2 ml/kg body weight twice a week). The animals exhibited
toxic liver damage ranging from steatosis to cirrhosis. Animals
showing malignant tumours totalled 8 in the subcutaneous group,
3 in the oral group, and 0 in the control group. The majority of
treated animals also showed hyperplasia of blood-forming tissues.
From the animal studies available, it is not possible to
determine a no-observed-adverse-effect level for long-term
exposure. No adequate data are available to assess the
mutagenicity or teratogenicity of isobutanol or effects on
reproduction.
The only reported observations in man relate to the production
of vertigo under conditions of severe and prolonged exposure to
vapour mixtures of isobutanol and 1-butanol. Thus, it is not
possible to attribute the vertigo to a single cause.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure: CH3
\
CH-CH2OH
/
CH3
Chemical formula: C4H10O
Primary constituent: isobutanol
Common synonyms: isobutyl alcohol, isopropylcarbinol,
2-methyl-1-propanol, 2-methylpropyl
alcohol, 1-hydroxymethylpropane,
2-methylpropan-1-ol, 1-propanol,-2-methyl,
fermentation butyl alcohol
CAS registry number: 78-83-1
2.2. Physical and Chemical Properties
Physical and chemical properties of isobutanol are given in
Table 1.
Table 1. Physical and chemical properties of isobutanol
-----------------------------------------------------------------
(at 20 °C and 101.3 kPa, unless otherwise stated)
Physical state colourless liquid
Odour sweet, similar to that of amyl
alcohol, but weaker
Odour threshold approximately 4.6 mg/m3 (1.5 ppm)
Relative molecular mass 74.12
Density (kg/m3) 801-803
Boiling point (°C) 107.9
Freezing point (°C) -108
Viscosity (cP) 3.98
Vapour pressure (kPa) 1.17
12.2 at 25 °C
Vapour density (air = 1) 2.55
Flashpoint (°C) 27.8
Autoignition temperature (°C) 434
Explosion limits air (% v/v) lower = 1.7, upper = 10.9
Solubility (% weight) in water, 8.7; soluble in alcohol
and ether
n-octanol/water partition 0.83
coefficient
Conversion factors (25 °C) 1 mg/m3 = 0.324 ppm
1 ppm = 3.083 mg/m3
-----------------------------------------------------------------
2.3. Analytical Methods
Testing methods for the butanols (ASTM D304-58) are described
in ASTM (1977).
NIOSH (1977) Method No S64 (341) has been recommended. It
involves drawing a known volume of air through charcoal to trap the
organic vapours present (recommended sample is 10 litres at a rate
of 0.2 litre/min). The analyte is desorbed with carbon disulfide
containing 1% 2-propanol. The sample is separated by injection
into a gas chromatograph equipped with a flame ionization detector,
and the area of the resulting peak is determined and compared with
standards.
The Association for Official Analytical Chemists has published
an Official Final Action for the assay of isobutanol in spirits,
based on gas chromatographic analysis (AOAC methods, 1975).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The major use of isobutanol is in the manufacture of isobutyl
acetate, which is employed in the lacquer industry. Furthermore,
isobutanol is used as a solvent in paint and varnish removers and
in the manufacture of isobutyl esters, which serve as solvents,
plasticizers, flavourings, and perfumes. It is also used as a
flavouring agent in butter, cola, fruit, liquor, rum, and whisky
(Hall & Oser, 1965). The average maximum levels at which it is
used in the USA are listed in Table 2 (Hall & Oser, 1965).
Table 2. Average maximum use levels of isobutanol in the USA
-------------------------------------------------------------
Food in which used Approximate average maximum level (mg/kg)
-------------------------------------------------------------
Beverages 17
Ice cream, ices 7
Candy 30
Baked goods 24
-------------------------------------------------------------
Isobutanol is one of the three main alcohols in fusel oil, and
is present in large amounts in some alcoholic beverages (Hedlund
Kiessling, 1969).
Natural isobutanol is produced by the fermentation of
carbohydrates. Isobutanol is found in fruits: cherry (Postel et
al., 1975), raspberry and blackberry (McGlumphy, 1951; Nursten et
al., 1967; Broderick, 1976), grape (Bober, 1963), and apple
(Schreier et al., 1978). It also occurs in beverages: brandy
(Postel et al., 1975), coffee (Walter & Weidemann, 1969), cider
(Matthews et al., 1962; Kieser et al., 1964), and gin (Clutton &
Evans, 1978). Isobutanol has been identified in sundry other foods
including cheddar cheese (Liebich et al., 1970), and hop oil
(Lammers et al., 1968).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
No data are available on distribution in soil, sediments or
air.
Isobutanol is readily biodegradable (Table 3). It is degraded
in significant amounts within a few hours, and degradation would be
expected to be complete within a few days.
Table 3. Biodegradation of isobutanol
-------------------------------------------------------------------
5d BOD 64% of ThOD in fresh water Price et al. (1974)
46% of ThOD in synthetic Price et al. (1974)
seawater
5d BOD 16% of ThOD (APHA) Bridié et al. (1979a)
63% of ThOD after adaptation Bridié et al. (1979a)
(APHA)
Activated 32.5% of ThOD removed in 24 h by Gerhold & Malaney
sludge unadapted municipal sludge (1966)
44% of ThOD removed in 24 h by McKinney & Jeris
adapted sludge (1955)
-------------------------------------------------------------------
ThOD = theoretical oxygen demand - the calculated amount of
oxygen needed for complete
oxidation to water and carbon
dioxide.
COD = chemical oxygen demand - measures the chemically
oxidizable matter present.
BOD = biochemical oxygen demand - a simple bioassay measuring
the potential deoxygenating
effect of biologically
oxidizable matter present in
an effluent.
Nazarenko (1969) reports an oxygen requirement of approximately
1.4 mg to oxidize 1 mg of isobutanol. Isobutanol at a
concentration of 20 mg/litre inhibits nitrification in water
(Nazarenko, 1969).
Isobutanol does not bioaccumulate (Chiou et al., 1977).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
An industrial emission study indicated that 90 tonnes of
isobutanol were released into the air over 1 year in the
Netherlands (Anon, 1983). Isobutanol produced naturally during the
fermentation of carbohydrates can enter the environment by leaching
from industrial waste landfills. A concentration of 0.3 g/litre
was found to be leaching from a 1-year-old landfill (US EPA, 1980).
6. KINETICS AND METABOLISM
Isobutanol can be absorbed through the lungs and from the
gastrointestinal tract (Browning, 1965).
Isobutanol (2 ml/kg body weight) was given to male rabbits by
gavage, and blood levels of isobutanol were determined. After 1 h,
a maximum concentration of 0.5 g/litre was observed. After 6 h,
isobutanol was no longer detectable in the blood. Less than 0.5%
of the administered isobutanol was excreted unchanged in the breath
or urine within 40 h (Saito, 1975).
Isobutanol can be metabolized to isobutyric acid and then
proceed into the tricarboxylic acid cycle, possibly via succinate
(Fig. 1B) (Saito, 1975). The urinary metabolites resulting from
repeated ingestion of isobutanol were also studied by Saito (1975).
Isobutanol (2 ml/kg body weight) was administered by stomach tube
to male rabbits. The rabbits were then given water saturated with
isobutanol instead of pure water to drink and the urinary
metabolites were determined. The length of time over which the
urine was collected was not specified. The urinary metabolites
were acetaldehyde (80 mg), acetic acid (16 mg), isobutyraldehyde
(10 mg), isovaleric acid (128 mg), and unmetabolized isobutanol (40
mg). The origin of urinary isovaleric acid was not discussed by
the author. Saito (1975) has proposed the scheme shown in Fig. 1B
for the metabolism of isobutanol in rabbits.
2-Butanol exhibited an apparent blood elimination half-life of
2.5 h in rats treated orally with 2.2 ml/kg body weight. With
decline in blood-alcohol concentration (maximum level 800 mg/litre,
1 h after administration), there was a rise in 2-butanone levels
with 430 mg/litre detected at 1 h and a maximum of 1050 mg/litre
detected 4 h after administration of the alcohol (Traiger &
Bruckner, 1976).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Some toxicity data for 2-butanol in aquatic organisms are given
in Table 3. Only two LC50 values are available for fish, and these
indicate low toxicity for these species. The toxicity of 2-butanol
for aquatic invertebrates is equally low.
Table 3. Toxicity data of 2-butanol for aquatic organisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Fish
Fresh-water species
Golden orfe 3520 48-h LC50 Juhnke & Lüdemann (1978)
(Leuciscus idus
melanotus)
Goldfish 4300 24-h LC50 Bridié et al. (1979a)
(Carassius auratus)
Invertebrates
Fresh-water species
Water flea 2300 24-h EC50 immobilization Bringmann & Kuehn (1982)
(Daphnia magna)
Marine species
Brine shrimp 3800 EC50 excystment Smith & Siegel (1975)
(Artemia salina)
Algae
Green algae
(Scenedesmus 95 8-day no- total biomass Bringmann & Kuehn
quadricauda) observed- (1978a)
adverse-
effect level
Chlorella 8900 EC50 Jones (1971)
pyrenoidosa chlorophyll
content
------------------------------------------------------------------------------------------
7.2. Terrestrial Organisms
An EC50 of 650 mg/litre was reported by Reynolds (1977) for
seed germination in lettuce (Lactuca sativa). Inhibition of seed
germination in cucumber (Cucumis sativus) was observed at 50 375 mg
2-butanol/litre (Smith & Siegel, 1975). There are no relevant data
for terrestrial animals, but, as in the case of terrestrial plants,
significant exposure to 2-butanol is unlikely.
7.3. Microorganisms
Some toxicity data for microorganisms are given in Table 4.
The toxicity of 2-butanol for both protozoa and bacteria is very
low.
Table 4. Toxicity of 2-butanol for microorganisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Protozoa
Uronema parduczi 1416 20-h no-observed- total Bringmann & Kuehn
(ciliate) adverse-effect level biomass (1981)
Chilomonas paramaecium 745 48-h no-observed- total Bringmann & Kuehn
(flagellate) adverse-effect level biomass (1981)
Entosiphon sulcatum 1282 72-h no-observed- total Bringmann & Kuehn
(flagellate) adverse-effect level biomass (1981)
Bacteria
Pseudomonas putida 500 16-h no-observed- total Bringmann & Kuehn
adverse-effect level biomass (1976)
Bacillus subtilis 1630 EC50 spore germination Yasuda-Yasaki et
al. (1978)
------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposure
8.1.1. Acute toxicity
Acute toxicity data for 2-butanol are given in Table 5.
2-Butanol shows low acute toxicity in rodents.
Table 5. Acute toxicity of 2-butanol
-------------------------------------------------------
Species Route of LD50 (g/kg Reference
administration body weight)
-------------------------------------------------------
Rat oral 6.5 US DHEW (1978)
Rabbit oral 4.9 Münch (1972)
Mouse intraperitoneal 0.8 US DHEW (1978)
-------------------------------------------------------
Six male albino rats were exposed to 2-butanol vapour at 48.5
mg/litre (16 000 ppm) for 4 h. Within 14 days, 5 of the 6 rats
died (Smyth et al., 1954).
8.1.2. Signs of intoxication
Signs of intoxication due to 2-butanol exposure include
restlessness, ataxia, prostration, and narcosis (Patty, 1963).
The effects of a moderate non-toxic oral dose of 2-butanol
(0.0163 mol/kg body weight) was studied in rats using a simple
functional test by Wallgren (1960). The intoxicating effect of
2-butanol, compared with that of ethanol (taken equal to 1), was
4.4 on an equimolar basis. Recovery after the treatment was slow.
The livers of mice that died 1 - 3 days following a single
intraperitoneal (ip) dose of 2-butanol showed an abnormal brownish
coloration in the peripheral areas, the livers of those that died
after 4 - 6 days were uniformally dark brown in colour (Maickel &
McFadden, 1979).
8.2. Skin and Eye Irritation
2-Butanol is practically not irritant to the skin of the
rabbit, but it is irritant to the rabbit eye (Smyth et al., 1954).
8.3. Short-Term Exposures
White mice (15 - 20 g) were repeatedly exposed to 2-butanol
vapour at 16.2 mg/litre (5330 ppm) for a total of 117 h. Although
the mice were narcotized, they survived.
Six groups of 2 mice each were exposed to 2-butanol vapour at
5 mg/litre (1650 ppm). After 420 min, no signs of intoxication
were observed. When increasing concentrations were used with
decreasing durations of exposure, ataxia, prostration, and deep
narcosis occurred. The time necessary to induce these symptoms
was inversely proportional to the level of exposure. At a
concentration of 10 mg/litre (3300 ppm), ataxia occurred in 51 -
100 min, prostration in 120 - 180 min, and narcosis in 300 min. At
a concentration of 60 mg/litre (19 800 ppm), these signs appeared
in 7 - 8 min, 12 - 20 min, and 40 min, respectively. No deaths
were observed in this study (Starrek, 1938, cited in Patty, 1982).
The neurophysiological effects induced by 2-butanol were
investigated in 31 male Sprague Dawley rats (200 - 400 g).
2-Butanol was administered intravenously (iv) to the rats at a
concentration of 8.1 mmoles/kg body weight. Within 20 - 60 seconds
of the iv administration, the rats lost righting reflexes. Some
changes were also noticed in the electroencephalographic tracings
compared with the tracings prior to the alcohol administration
(Marcus et al., 1976).
8.4. Long-Term Exposures
No long-term exposure studies are available.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data on reproduction, embryotoxicity, or
teratogenicity have yet been published. However, an inhalation
teratology study with 2-butanol is in progress in the USA (US EPA,
personal communication, 1985).
8.6. Mutagenicity
2-Butanol did not show any mutagenic activity in the yeast
Schizosaccharomyces pombe in both the presence and absence of
mouse liver microsomes (Abbondandolo et al., 1980).
8.7. Carcinogenicity
No carcinogenicity studies are available.
8.8. Special Studies
The effects of 2-butanol on cell survival were studied in the
yeast S. pombe and in V-79 Chinese hamster cells by Abbondandolo
et al. (1980). At 5% concentration, 2-butanol decreased the
survival of suspended yeast cells, but did not have any effect on
monolayer cultures of V-79 cells.
2-Butanol inhibited the contraction of the depolarized guinea-
pig ileum induced by calcium chloride (CaCl2) (Yashuda et al.,
1976).
2-Butanol can potentiate the toxicity of carbon tetrachloride
(CCl4) (Cornish & Adefuin, 1967). This potentiation may be due to
the metabolite 2,3-butanediol, which also has this effect (Traiger
& Bruckner, 1976; Dietz & Traiger, 1979).
Inhalation exposure of rats to 2-butanol (1539 mg/m3 (500 ppm)
for 5 days) resulted in a 47% increase in the cytochrome P-450
levels of kidney microsomes. A maximal increase of 33% in liver
microsomal cytochrome P-450 content was seen after inhalation of
2-butanol at 6156 mg/m3 (2000 ppm) for 3 days. This treatment led
to a 77% increase in the formation of the preneurotoxic metabolite
2-hexanol from 1-hexane by liver microsomes (Aarstad et al., in
press).
Rats receiving a single oral dose of 2-butanol at 2.2 ml/kg
body weight were sacrificed 16, 20, or 40 h after dosing. A 50 -
97% increase in microsomal acetanilide hydroxylase activity was
found. At 40 h, liver cells showed a marked proliferation of
smooth endoplasmic reticulum. This stimulation of the drug-
metabolizing system may explain, to a certain extent, the
potentiation of CCl4 hepatoxicity by 2-butanol (Traiger et al.,
1975).
9. EFFECTS ON MAN
Excessive exposure may result in headache, dizziness,
drowsiness, and narcosis (Muir, 1977). No adverse systemic effects
due to exposure to 2-butanol have been reported in man (Patty,
1982).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population to 2-butanol
through food and beverages, and occupational exposure levels are
not available.
10.1.2. Toxic effects
In animals, 2-butanol is absorbed through the lungs and
gastrointestinal tract. No information is available regarding
dermal absorption. Approximately 97% of the dose of 2-butanol in
animals is converted by alcohol dehydrogenase to the corresponding
ketone, which is either excreted in the breath and urine or further
metabolized. The rat acute oral LD50 for 2-butanol is 6.5 g/kg
body weight; it is, therefore, practically non-toxic according to
the classification of Hodge & Sterner. The toxic effects from
acute exposure are ataxia and narcosis. The potency of 2-butanol
for intoxication is approximately 4 times that of ethanol. It is
irritating to the eyes and non-irritating to the skin. From the
animal studies available, it is not possible to determine a no-
observed-adverse-effect level. No adequate data are available on
mutagenicity, carcinogenicity, teratogenicity, or effects on
reproduction.
In man, the most likely acute effect of 2-butanol is alcoholic
intoxication. No published data are available concerning other
effects on man.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
No quantitative data relating to levels of 2-butanol in the
general environment are available, but, because it is readily
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillage.
10.2.2. Toxic effects
At the background concentrations likely to occur in the
environment, 2-butanol is not toxic for aquatic animals, algae,
protozoa, or bacteria, and it should be managed in the environment
as a slightly toxic compound. It poses an indirect hazard for the
aquatic environment, because it is readily biodegradable, which may
lead to oxygen depletion.
10.3. Conclusions
1. The Task Group was unable to make an assessment of the health
risks of 2-butanol for the general population on the basis of
available data. However, it was considered that 2-butanol was
unlikely to pose a serious hazard, under normal exposure
conditions.
2. The Task Group was of the opinion that available data are not
sufficient to establish guidelines for setting occupational
exposure limits. In line with good manufacturing practice,
exposure to 2-butanol should be minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of 2-butanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
The Task Group recommended that:
1. As it was not possible to determine a no-observed-adverse-
effect level on the basis of available animal studies, relevant
studies should be conducted so that this could be achieved.
2. Information on residue and emission levels is desirable.
3. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
2-butanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Food Additives and Contaminants Committee (UK MAFF, 1978)
recommended that residues of butan-2-ol in food should not exceed
30 mg/kg and required the results of a 90-day oral toxicity study
in the rat within 2 years.
This compound could not be included in lists 1 or 2 by the
Council of Europe and it was included in list 3A (Council of
Europe, 1981).
At their 23rd meeting, the Joint FAO/WHO Expert Committee on
Food Additives (JECFA) reviewed the data on 2-butanol. They
concluded that "The evaluation of this compound was not possible on
the basis of the data available. New specifications were prepared,
but no toxicological monograph" (WHO, 1980).
ENVIRONMENTAL HEALTH CRITERIA
FOR
tert-BUTANOL
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR tert-BUTANOL
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.2 Terrestrial organisms
7.3 Microorganisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Acute toxicity
8.1.2 Signs of intoxication
8.2 Skin and eye irritation
8.3 Short-term exposures
8.4 Reproduction, embyrotoxicity, and teratogenicity
8.5 Mutagenicity
8.6 Carcinogenicity
8.7 Special studies
9. EFFECTS ON MAN
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
10.1.2 Toxic effects
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
10.2.2 Toxic effects
10.3 Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY
tert-Butanol is a colourless liquid or white crystalline solid
with a camphor-like odour. It has a melting point of 25 °C, a
boiling point of 81.5 - 83 °C, is freely soluble in water, and its
n-octanol/water partition coefficient is 0.37. Its vapour is 2.6
times denser than air. It is used primarily as a solvent, a
dehydrating agent, and as an intermediate in the manufacture of
other chemicals. It is also used as a denaturant for alcohols.
Human exposure will be mainly occupational. Data on exposure of
the general population are not available, but it may result from
industrial emissions. tert-Butanol is inherently biodegradable and
does not bioaccumulate. At ambient levels, it is not toxic for
fish, amphibia, crustacea, algae, or bacteria.
In animals, tert-butanol is absorbed through the lungs and
gastrointestinal tract; no information is available on dermal
absorption. tert-Butanol is not a substrate for alcohol
dehydrogenase and is slowly metabolized by mammals. Up to 24% of
the dose is eliminated in the urine as the glucuronide, and up to
10% of the dose can be excreted in the breath and urine as acetone
or carbon dioxide. The rat oral LD50 is 3.5 g/kg body weight; it
is, therefore, slightly toxic according to the classification of
Hodge & Sterner. The primary acute effects observed in animals are
signs of alcoholic intoxication. Its potency for intoxication is
approximately 1.5 times that of ethanol. Animal data regarding
skin and eye irritation are not available. tert-Butanol produces
physical dependance in animals and post-natal effects in offspring
exposed in utero. Data concerning the pathological effects of
repeated exposure of animals are not available. From the animal
studies available, it is not possible to determine a no-observed-
adverse-effect level. tert-Butanol has been found not to be
mutagenic. Adequate data are not available on carcinogenicity,
teratogenicity, or effects on reproduction.
In man, tert-butanol is a mild irritant to the skin. No other
effects on man have been reported, and there have been no reports
of poisonings.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure:
CH3
|
CH3 - C - CH3
|
OH
Chemical formula: C4H10O
Primary constituent: tert-butanol
Common synonyms: 2-methyl-2-propanol, tert-butyl
alcohol, tertiary butanol, t.
butanol, trimethyl carbinol, TBA,
TMA, t. butyl hydroxide, NCL-C55
367, trimethyl methanol
Cas registry number: 75-65-0
2.2. Physical and Chemical Properties
Some physical and chemical data for tert-butanol are given in
Table 1.
Table 1. Physical and chemical data for tert-butanol
-----------------------------------------------------------------------------
(at 20 °C and 101.3 kPa, unless otherwise stated)
Physical state solid (crystals)
Odour camphor-like
Odour threshold approximately 144.7 mg/m3(47 ppm)
Relative molecular mass 74.12
Density (kg/m3) 779 - 782 at 26 °C
Boiling point (°C) initial 81.5 (min.); dry point 83.0 (max.)
Melting point 25 °C
Viscosity (mPa x s) 3.3 at 30 °C
Vapour density (air = 1) 2.55
Vapour pressure (mm Hg) 31 (at 25 °C, 42; at 30 °C, 56)
Flashpoint (°C, TOC) 16
(°C, TCC) 4
Autoignition temperature 470 °C
Explosion limits in air (v/v %) lower 2.35; upper 8.0
Solubility soluble in water; miscible with ethyl
alcohol, ether; also soluble in ketones,
esters, aromatic and aliphatic hydrocarbons
n-octanol/water partition 0.37
coefficient
Conversion factors: 1 mg/m3 = 0.325 ppm
1 ppm = 3.078 mg/m3
-----------------------------------------------------------------------------
2.3. Analytical Methods
Testing methods for the butanols (ASTM D304-58) are described
in ASTM (1977).
It is known that several alcohols, including tert-butanol,
give colour reactions with aldehyde in the presence of sulfuric
acid (Patty, 1963). NIOSH (1977b) describes several methods.
AOAC has finalized the method for detecting tert-butanol in
distilled liquors (AOAC, 1975).
tert-Butanol has been determined in the air of industrial
premises by Abbasov et al. (1971) using a gas chromatographic
method and by Zamarakhina (1973) using a photometric method, and in
blood by Wood & Laverty (1976).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The primary use of tert-butanol is as a solvent. It is also
used as a dehydrating agent, in the extraction of drugs, in the
manufacture of perfumes (particularly in the preparation of
artificial musk), in the recrystallization of chemicals, and as a
chemical intermediate (e.g., in the manufacture of tert-butyl
chloride and in the manufacture of tert-butyl phenol). It is an
approved denaturant for ethyl alcohol and for several other
alcohols. Catalytic dehydration of tert-butanol is carried out to
obtain isobutylene, and it has been patented for use as a gasoline
antiknock agent.
Moreover, it is used in the purification of polyolefins, for
the separation of solids from coal liquids and as blowing agent for
the manufacture of imide group-containing foams from copolymers of
methacrylonitrile and methacrylic acid (Patty, 1963; Monich, 1968;
Sherman, 1978).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
No data are available on the distribution of tert-butanol in
soil, sediments, or air.
In short-term tests, there was little degradation but over a
longer period of about one month, most of the material was fully
degraded. Therefore, tert-butanol is inherently rather than
readily biodegradable. Some biodegradation data for tert-butanol
are given in Table 2.
tert-Butanol does not bioaccumulate (Chiou et al., 1977).
Table 2. Biodegradation data for tert-butanol
---------------------------------------------------------------------------
5d BOD 0% of ThOD (AFNOR) Dore et al. (1974)
1% of ThOD (APHA) Bridié et al. (1979b)
30d BOD 0% of ThOD (closed-bottle Gerike & Fischer (1979)
test, conventional)
0% of ThOD (closed-bottle Gerike & Fischer (1979)
test, preadaptation)
MITI test 0% of ThOD removed after Gerike & Fischer (1979)
14 days (BOD14: 7% of ThOD)
OECD screening 29% of ThOD removed after Gerike & Fischer (1979)
test 19 days; no pre-adaptation
Sturm test 32% of ThOD removed, but no Gerike & Fischer (1979)
production of CO2
AFNOR T90-302 80% of ThOD removed after Gerike & Fischer (1979)
test 28 days
93% of ThOD removed after Gerike & Fischer (1979)
42 days
Zahn-Wellens 96% of ThOD removed after Gerike & Fischer (1979)
test 6 days
Couple-units test 33% of ThOD removed after Gerike & Fischer (1979)
(conventional) 42 days adaptation
Square-wave 69% of ThOD removed after Gerike & Fischer (1979)
feeding 30 days adaptation
Activated 0.8% of ThOD removed in 24 h Gerhold & Malaney
sludge by unadapted municipal sludge (1966)
2% of ThOD removed in 23 h by McKinney & Jeris (1955)
adapted sludge
---------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------
98% of ThOD removed in 5 days Pitter (1976)
by adapted sludge;
biodegradation rate at 20 °C
0.03/h
Anaerobic 73% of ThOD removed at Chou et al. (1978)
digestion 400 mg/litre in anaerobic
up-flow filters (hydraulic
residence time 2 - 10 days)
after 52 days of adaptation
---------------------------------------------------------------------------
ThOD = theoretical oxygen demand - the calculated amount of oxygen
needed for complete oxidation to
water and carbon dioxide.
COD = chemical oxygen demand - measures the chemically oxidizable
matter present.
BOD = biochemical oxygen demand - a simple bioassay measuring the
potential deoxygenating effect of
biologically oxidizable matter
present in an effluent.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
An industrial emmission study indicated that 207 tonnes of
tert-butanol were released into the air in the Netherlands over a
period of 1 year (Anon, 1983).
No other data are available.
6. KINETICS AND METABOLISM
tert-Butanol is not a substrate for alcohol dehydrogenase
(Derache, 1970; Cederbaum et al., 1983) and is slowly metabolized
by mammals (Williams, 1969; Derache, 1970; Beaugé et al., 1981).
Possible routes of metabolism are direct conjugation of the
hydroxyl group with glucuronic acid and oxidation of one or more of
the alkyl substituents. Following treatment with tert-butanol,
24% of the dose was detected as the glucuronide conjugate in the
urine of rabbits (Kamil et al., 1953) and increased acetone
excretion has been observed in the breath and urine of rats treated
with tert-butanol (Baker et al., 1982; Yojay et al., 1982).
After a single oral dose of tert-butanol (25 mmol/kg), blood
concentrations in female Wistar rats declined slowly from 13.2 ±
0.5 mmol at 2 h to 11.4 ± 0.3 mmol at 20 h (Beaugé et al., 1981).
In rats maintained on a liquid diet containing 20 ml tert-
butanol/litre for 20 days, the blood level 30 min after withdrawal
of the diet of 20 mmol declined to 5 mmol in 8 h. By comparison,
blood levels of ethanol of 45 mmol (achieved by a liquid diet of
87 ml ethanol/litre for 20 days) were reduced to zero within 4.5 h
(Wood & Laverty, 1979). In female Sprague Dawley rats given single
oral doses of either ethanol (5.0 g/kg body weight) or tert-
butanol (1.2 g/kg body weight), the rates of elimination were 10.7
± 0.5 and 0.7 ± 0.1 mmol/kg per h, respectively (Thurman et al.,
1980).
Following an oral dose of 2 g tert-butanol/kg body weight to
rats, a maximum blood level of 1240 mg/litre (124 mg%) was reached
in 2 h; this decreased very slowly to 1200 mg/litre (120 mg%) after
4 h and to 1100 mg/litre (110 mg%) after 8 h; only about 1% of
the dose was excreted in the urine (Gaillard & Derache, 1965).
tert-Butanol was found in the blood of rabbits 70 h after oral
administration of 2 ml/kg body weight (Saito, 1975).
In Long-Evans rats treated with tert-butanol (1 g/kg body
weight, route not specified), the rate of disappearance of tert-
butanol from the blood was apparently of first order with a half
life of 9.1 h (Baker et al., 1982). Using 14C- and 13C-
tert-butanol, the same authors investigated the metabolism of
tert-butanol to form acetone. It was found that following
administration of tert-butanol (0.75 - 2 g/kg body weight),
approximately 0.5 - 9.5% of the dose was excreted as acetone in
the urine and breath. The total production of acetone varied
considerably between animals given the same dose, and, as a
result, no correlation between dose and acetone excretion could
be established. Evidence was also obtained indicating that carbon
dioxide (CO2) was a metabolic product of tert-butanol. The
conversion of tert-butanol (possibly via acetone) was not
quantified. Yojay et al. (1982) provided evidence that, in rats
treated intraperitoneally with tert-butanol at 1 or 2 mg/kg body
weight, blood levels of acetone were approximately proportional to
the dose of tert-butanol. In support of the in vivo metabolic
conversion of tert-butanol to acetone, Cederbaum & Cohen (1980)
and Cederbaum et al. (1983) demonstrated the metabolism of
tert-butanol to formaldehyde and acetone in an in vitro system
consisting of rat liver microsomes and a hydroxyl radical
generating system.
Investigations on the induction of tert-butanol metabolism
have been conducted by Baker et al. (1982), Thurman et al. (1980),
and McComb & Goldstein (1979). In rats, Baker et al. (1982) were
unable to demonstrate increased conversion of tert-butanol to
acetone in animals in which the hepatic mixed-function oxidase
activity had been induced by prior phenobarbital treatment.
Thurman et al. (1980) studied the effects on tert-butanol
elimination in rats of pre-treatment (oral) with 5.7% (w/v)
tert-butanol every 8 h, for either 1 or 2.5 days. After the
pre-treatment, animals were given tert-butanol to raise their blood
levels to between 1250 and 1500 mg/litre. The rates of elimination
were very similar, but there was a suggestion of slightly faster
elimination following pre-treatment. The investigators concluded
that, unlike ethanol pre-treatment, which induces its own
metabolism, tert-butanol pre-treatment had little or no effect on
the subsequent rate of tert-butanol elimination in the rat.
In contrast to the results obtained in rats, it appears that
tert-butanol elimination in mice can be substantially increased by
pre-treatment with tert-butanol. Male Swiss Webster mice were
given an ip loading dose of 6.8 mmol tert-butanol/kg body weight
and then exposed to various vapour concentrations of tert-butanol
for 24 h. It was found that 15 min after the 24-h inhalation
period, blood- tert-butanol levels were linearly related to the
vapour concentrations. Blood levels ranged from 3 to 14 mmol with
increasing vapour concentrations of 30 - 100 µmol/litre air (McComb
& Goldstein, 1979). It was also found that the rate of elimination
of blood- tert-butanol was significantly increased by inhalation.
In mice, after a single ip injection of 8.1 mmol tert-butanol/kg
body weight, initial blood levels of 8 mmol took 8 - 9 h for
elimination (blood- tert-butanol half-life was approximately 5 h).
However, after 3 days, inhalation at a vapour concentration to give
levels of 8 mmol/litre blood, tert-butanol disappeared within 3 h
of removal of mice from the inhalation chamber (half-life of tert-
butanol in blood was approximately 1.5 h) (McComb & Goldstein,
1979).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
The toxicity of tert-butanol has been tested in very few
organisms. However, in the few studies performed, the toxicity of
the compound has been very low.
Some toxicity data for aquatic organisms are given in Table 3.
Table 3. Toxicity of tert-butanol for aquatic organisms
-------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
-------------------------------------------------------------------
Fish (acute)
Fresh-water species
Creek chub 3000 - 6000 24-h LC50 Gillette
(Semotitus et al.
atromaculatus) (1952)
Goldfish > 5000 24-h LC50 Bridié
(Carassius et al.
auratus) (1979a)
Invertebrates (acute)
Marine species
Brine shrimp 7800 EC50 excystment Smith &
(Artemia salina) Siegel
(1975)
Algae
Fresh-water
Green algae
Chlorella 24 200 EC50 chlorophyll Jones
pyrenoidosa content (1971)
-------------------------------------------------------------------
7.2. Terrestrial Organisms
An EC50 of 90 800 mg/litre was reported for germination in
cucumber (Cucumis sativus) by Smith & Siegel (1975). There are
no relevant data for terrestrial animals. However, significant
terrestrial exposure to tert-butanol is unlikely for either plants
or animals.
7.3. Microorganisms
One study has indicated that Nitrosomonas (nitrifying
bacterium) shows a high tolerance for tert-butanol; tert-Butanol
inhibits nitrifying activity at 39 400 mg/litre (Blok, 1981).
There was no inhibition of degradation by methane culture on
acetate substrate at 7400 mg tert-butanol/litre (Chou et al.,
1978).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposure
8.1.1. Acute toxicity
Some acute toxicity data for animals are given in Table 4.
Table 4. Acute toxicity of tert-butanol in animals
------------------------------------------------------
Species Route of LD50 (g/kg Reference
administration body weight)
------------------------------------------------------
Rat oral 3.5 US DHEW (1979)
Rabbit oral 3.6 Münch (1972)
Mouse intravenous 1.5 Patty (1982)
Mouse intraperitoneal 0.9 US DHEW (1978)
------------------------------------------------------
8.1.2. Signs of intoxication
Animals exposed to the vapours of tert-butanol may manifest
the following signs of intoxication: restlessness, irritation of
mucous membranes, ataxia, prostration, and narcosis (Patty, 1963).
The narcotic potency of tert-butanol in rabbits is similar to
that of other butanols (Münch, 1972). After ip administration to
mice, the LD50 was > 1000 mg/kg body weight at 30 min and 441
mg/kg body weight at 7 days; gross post-mortem examination of the
livers showed an abnormal dark coloration (Maickel & McFadden,
1979). In rabbits, an oral dose of 1.3 g tert-butanol/kg body
weight was shown to be the minimum narcotic dose (Münch & Schwartz,
1925). The minimum oral lethal dose in the rabbit was 6.0 ml/kg
body weight (approximately 4.7 g/kg) (Patty, 1963). Injection (ip)
of tert-butanol in rats induced a spectrum of intoxication
virtually identical to that of ethanol, but its intoxicating
potency was approximately 1.5 times that of ethanol (McCreery &
Hunt, 1978).
Oral administration of a single dose of 24 mmol tert-
butanol/kg body weight caused a temporary disturbance in the fat
metabolism in the liver cells of female Wistar rats. The authors
suggest that this may be related to the stress induced by the
administration of tert-butanol, which is metabolized very slowly
(Beaugé et al., 1981).
8.2. Skin and Eye Irritation
No data are available on skin and eye irritation in animals.
8.3. Short-Term Exposures
Physical dependence following tert-butanol administration was
investigated using 12 random-bred male albino rats. A liquid diet
containing 20 ml tert-butanol/litre was given to the rats for
4 - 20 days. Docility and slight ataxia were observed in the rats
during this period. About 5 - 6 h after removal of the diet,
withdrawal-signs such as muscular rigidity, stiff curled tails,
abnormal gait, tremor, and irritability became apparant. Four rats
exhibited spontaneous forelimb convulsions. Audiogenic convulsions
were observed in 5 rats, and 3 of them died as a consequence. tert-
Butanol was still detectable in the blood of the rats, 8 h after
its withdrawal (Wood & Laverty, 1979).
In another study, 15 adult male Long Evans rats were exposed
to 4 different protocols using various concentrations of tert-
butanol in water as their only available fluid; 14 animals were
used as controls. At concentrations of 3.5 ml/litre, severe toxic
reactions were found, including anorexia, self mutilation, and
death. When animals consumed at least 3 g tert-butanol/kg body
weight per day for 90 days, withdrawal symptoms were observed,
independent of dosing conditions. Such an intake occurred only
when the concentration of tert-butanol was 3% or greater (Grant
& Samson, 1981). In female Sprague Dawley rats, tert-butanol
administered by gastric intubation, every 8 h, for up to 6 days,
was shown to produce physical dependence (Thurman et al., 1980).
An investigation was undertaken with 5 to 8-week-old male
Swiss-Webster mice weighing 22 - 30 g. A priming dose of 6.8 -
10.1 µmol tert-butanol/kg body weight (10% w/v in 0.9% saline) was
administered ip to groups of 24 mice. They were then exposed to
vapour concentrations of between 50 and 80 µmol tert-butanol/litre
air for 24 h per day; concentrations below or above these limits
either did not produce physical dependance or were initially too
toxic. Because it was found that continual exposure induced the
elimination of tert-butanol, exposure concentrations were
increased daily, in order to maintain a steady blood- tert-butanol
level of between 5 - 8.5 mmol. Exposure lasted for 1, 3, 6, or 9
days. Withdrawal signs were noted after removing the mice from the
inhalation exposure. The intensity of the withdrawal reaction
increased with the duration of inhalation and with the blood- tert-
butanol levels maintained during the intoxication period. The
withdrawal syndrome was qualitatively similar to that produced by
ethanol. tert-Butanol has been shown to be 4 - 5 times as potent
as ethanol in producing physical dependence. Thus, since it is
also 4 - 5 times more lipid soluble than ethanol, McComb &
Goldstein (1979) concluded that the dose of alcohol necessary to
induce physical dependence was inversely proportional to its
solubility.
In a study by Bellin & Edmonds (1976), physical dependence was
induced in 12 male Sprague Dawley rats (350 - 500 g) and 10 female
albino guinea-pigs (200 - 400 g) when given tert-butanol ip
(0.8 g/kg body weight as a 10% w/v solution) at 8-h intervals
for 4 days. Alteration of body temperature (decreased during
intoxication and increased during withdrawal) was more pronounced
in the rats than in the guinea-pigs. However, these differences
could be due to species or sex.
An impairment of avoidance behaviour was shown following short-
term ingestion of tert-butanol in mice (number not stated).
Experimental animals received a liquid diet containing tert-
butanol at 12.5 ml/litre for 7 days and during this time ingested
an average of 3.4 g tert-butanol/kg body weight, per day. One day
after cessation of this diet, the animals were exposed to the
avoidance training procedure. Avoidance was much less in the
treated group than in the controls (Snell & Harris, 1980).
Pregnant Swiss Webster mice (15 per group) were fed liquid
diets containing tert-butanol at concentrations of 0, 5.0, 7.5,
or 10 g/litre from day 6 to day 20 of gestation. In order to gain
insight into the effects of maternal nutrition and behaviour on
post-natal pup development, approximately half of the treated
maternal animals were replaced, within 24 h of parturition, with
untreated females, also recently delivered. tert-Butanol was
approximately 5 times more potent than the same dose of ethanol in
producing developmental delay in post-parturition physiological,
and psychomotor performance, scores. At the higher concentrations,
there were also significant postnatal maternal, nutritional, and
behavioural factors affecting lactation and/or nesting behaviour,
which influenced the development of pups exposed in utero (Daniel
& Evans, 1982).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data on reproduction, embryotoxicity, or
teratogenicity have yet been published. In contrast to ethanol,
tert-butanol at concentrations of 1000 - 4000 mg/litre did not
reduce the in vitro fertilizing capacity of mouse spermatozoa
(Anderson et al., 1982).
An inhalation teratology study is in progress in the USA (US
EPA, personal communication, 1985).
8.5. Mutagenicity
At a concentration of 1%, tert-butanol was classified among
the chemicals that had no apparent mutagenic effect on formation
of antibiotic-resistant mutants in Micrococcus aureus populations
(Clark, 1953). tert-Butanol was not mutagenic in Neurospora
crassa (Dickey et al., 1949). In short-term tests conducted by
the National Toxicology Program (USA), tert-butanol was negative
in the Ames test ( Salmonella, +/-activation), mouse lymphoma, and
in vitro cytogenetics assays (US EPA, personal communication,
1985).
8.6. Carcinogenicity
tert-Butanol is currently being evaluated for carcinogenicity
by the US National Cancer Institute using the standard bioassay
protocol. Groups composed of 50 male and 50 female B6C3F1 mice
were exposed for 2 days per week, from week 9 to 104, to 0, 5000,
10 000, or 20 000 mg tert-butanol/litre drinking-water. Groups
comprising 50 male and 50 female Fischer 344 rats were exposed at
the same rate to the same levels from weeks 7 to 104. The
treatment period has now been completed and histological
examinations are in progress (IARC, 1984).
An inhalation carcinogenicity study has been initiated in the
USA (US EPA, personal communication, 1985).
8.7. Special Studies
tert-Butanol was investigated for its ability to deplete the
cerebral calcium level. An ip dose of 2 g/kg body weight was
administered to male Sprague Dawley rats weighing between 150 and
225 g. The animals were sacrificed 30 min after the administration
of the alcohol. There was a significant decrease in cerebral
calcium contents compared with that in the control animals (35
mg/kg versus 55 mg/kg) (Ross, 1976).
In Sprague Dawley rats (190 - 250 g), ip administration of
aqueous tert-butanol (250 g/litre) was shown to increase carbon
tetrachloride-induced hepatotoxicity, as evaluated by serum
glutamate-pyruvate transaminase levels. However, there was no
depletion in hepatic glutathione or loss in body weight (Harris &
Anders, 1980). tert-Butanol can potentiate the toxicity of carbon
tetrachloride in Sprague Dawley rats (Cornish & Adefuin, 1967).
The inhibition of the synthesis of ornithine decarboxylase
(ODC) and tyrosine aminotransferase (TAT) by tert-butanol was
studied in partially-hepatectomized female rats (about 238 g) of
mixed strains. Immediately after partial hepatectomy, 15% (w/v)
aqueous tert-butanol (2.8 g/kg body weight) was given to 6 rats by
gastric intubation. In the liver, 4 h after partial hepatectomy,
the ODC activity was decreased to 22% and the TAT activity to about
52% of the activities in the control group. In the kidney, 4 h
after partial hepatectomy, the ODC activity was decreased to about
31% of the activity in the control group. In the brain, tert-
butanol did not induce any significant changes in the ODC activity
compared with that in the control group (Poso & Poso, 1980).
The presence of a hydroxy radical is needed for prostaglandin
biosynthesis. tert-Butanol was shown to be a hydroxyl radical
scavenger and, therefore, an inhibitor of prostaglandin
biosynthesis. In the incubation system of microsomes, prepared
from bovine vesicular glands containing epinephrine, the 14C
incorporation as a percentage of total labelled prostaglandins E
and F, respectively, was 16.4% and 23.4%. When 0.025 ml of tert-
butanol was added to this incubation system, the values were 15.5%
and 13.8%, respectively. Further increases in concentration
resulted in decreased incorporation (Panganamala et al., 1976).
In another study using isolated perfused rat lungs, the infusion of
low concentrations of tert-butanol (0.002 mmol - 0.2 mmol) resulted
in the continuous output of prostaglandins into the venous
effluent. However, there was a gradual decrease in the
prostaglandin output as the concentration of the alcohol increased
(Thomas et al., 1980).
Cation flux across membranes has been investigated in a number
of studies. tert-Butanol inhibited, in a dose-dependent manner,
the in vitro contraction of depolarized guinea-pig ileum induced
by calcium chloride (Yashuda et al., 1976) and at a 34 mmol
concentration impaired the response of rat brain cortex slices to
electrical stimulation, inhibiting the influx of sodium, but only
weakly affecting potassium afflux (Wallgren et al., 1974).
Wiesbrodt et al. (1973) studied the effects of tert-butanol on
the gastric mucosa of Heidenheim pouches in 4 unanaesthetized
dogs. They observed a decrease in the transmucosal potential and
an increase in the net flux of Na+ ion into the lumen of the pouch
at all concentrations tested (0.5 - 1.5 mol).
The effect of tert-butanol, at 1 mmol concentration, on
isolated rat mitochondria and lysosomes was studied by Sgaragli
et al. (1975). The alcohol did not affect lipid peroxidation and
membrane stability as evaluated by the release of total protein
acid phosphatase and glutamate dehydrogenase.
tert-Butanol decreased the in vitro Km of phosphorilase b for
glucose-1-phosphate with saturating glycogen concentrations and
markedly increased Vmax (Dreyfus et al., 1978). Akhtem et al.
(1978) studied the interaction between tert-butanol and cytochrome
P-450 from rat liver microsomes; they revealed spectral shifts
indicating the formation of alcohol-cytochrome P-450 complexes.
Sugiyama et al. (1976) found that tert-butanol greatly enhanced
the side chain cleavage activity of purified P-450.
A 15-min pre-session oral administration of tert-butanol
at 0.25 - 3 g/kg body weight to 4 trained male Long Evans rats
produced a dose-related decrease in fixed-ratio responding at
doses of 0.5 g/kg or more. tert-Butanol was 1.6 times as potent as
ethanol, in this respect. The 1 g/kg dose decreased responding by
about 20%, 15, 30, or 60 min after dosing (Witkin & Leander, 1982).
tert-Butanol produced a marked dose-dependent activation
of locomotor activity in short-sleep mice, selectively bred for
relative insensitivity to the hypnotic properties of ethanol. In
alcohol-sensitive long-sleep mice, the locomotor activity was
depressed in a dose-dependent fashion. Test doses were 0, 4, 5,
or 6 g/kg body weight, administered intravenously in 0.9% saline
(Dudek & Philips, 1983).
Twelve neonatal Long Evans rats were given tert-butanol at
doses of up to 2.69 g/kg body weight per day in a milk formula
through a cannula, from postnatal days 4 to 7 (corresponding to
the brain growth spurt). At this point, they were transferred to
a plain milk formula, for the next 11 days. After sacrifice on day
18, exposed animals showed significant decreases in absolute and
relative brain weights and lower DNA levels in hind-brain samples
in comparison with controls, indicating a certain degree of
microcephaly (Grant & Samson, 1982).
No effects were found in the basal synaptosomal membrane
fluidity or in the activity of Na+ + K+ ATPase in the brain of
Sprague Dawley rats given a single oral dose of 1.85 g tert-
butanol/kg body weight (Beaugé et al., 1984).
9. EFFECTS ON MAN
tert-Butanol is slightly irritant to the skin. When the
compound was applied to the skin of 5 human volunteers, no reaction
other than slight erythema and hyperaemia was observed (Oettel,
1936). However, Edwards & Edwards (1982), described an allergic
skin reaction to tert-butanol in a 58-year-old patient who used
skin screen containing tert-butanol. A patch test was positive
for tert-butanol. There are no other published reports of adverse
effects or poisonings in man.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population through food and
occupational exposure levels are not available.
10.1.2. Toxic effects
In animals, tert-butanol is absorbed through the lungs and
gastrointestinal tract; no information is available on dermal
absorption. tert-Butanol is not a substrate for alcohol
dehydrogenase and is slowly metabolized by mammals. Up to 24% of
the dose is eliminated in the urine as the glucuronide, and up to
10% of the dose can be excreted in the breath and urine as acetone
or carbon dioxide. The rat oral LD50 is 3.5 g/kg body weight; it
is, therefore, slightly toxic according to the classification of
Hodge & Sterner. The primary acute effects observed in animals are
signs of alcoholic intoxication. Its potency for intoxication is
approximately 1.5 times that of ethanol. Animal data regarding
skin and eye irritiation are not available. tert-Butanol produces
physical dependance in animals and post-natal effects in offspring
exposed in utero. Data on pathological effects of repeated
exposure of animals are not available. From the animal studies
available, it is not possible to determine a no-observed-adverse-
effect level. tert-Butanol has been found not to be mutagenic. No
adequate data are available on carcinogenicity, teratogenicity, or
effects on reproduction.
In man, tert-butanol is a mild irritant to the skin. There
have not been any reports of poisonings or any other effects in
man.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
No quantitative data relating to levels in the general
environment are available, but, because tert-butanol is inherently
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillage.
10.2.2. Toxic effects
tert-Butanol is inherently biodegradable and is not toxic for
fish, amphibia, crustacea, algae, or bacteria.
10.3. Conclusions
1. On the available data, the Task Group was unable to make an
assessment of the health risks from tert-butanol for the
general population. However, it was considered unlikely to
pose a serious hazard under normal exposure conditions.
2. The Task Group was of the opinion that available data are not
sufficient to establish guidelines for setting occupational
exposure limits. In line with good manufacturing practice,
exposure to tert-butanol should be minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of tert-butanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
1. The Task Group noted that, from the animal studies available,
it was not possible to determine a no-observed-adverse-effect
level. Relevant studies should be conducted so that this can
be achieved.
2. Information on residue and emission levels is desirable.
3. Epidemiological studies, including precise exposure data, would
assist in a better assessment of the occupational hazards from
tert-butanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Council of Europe has recently established (Council of
Europe, 1981) a specific limit of 30 mg tert-butanol/kg in candy
in confectionery. tert-Butanol was evaluated by the EEC
Scientific Committee for Food in 1980. The Committee agreed on the
following evaluation:
"The available data are insufficient to establish an
ADI. Biochemically, this solvent will behave like other
tertiary carbinols, which are generally not very reactive.
The residues in food are minimal and are not a hazard to
health. The Committee considers the use of this compound
temporarily acceptable as an extraction solvent provided
residues from use as an extraction solvent in food as
consumed do not exceed 10 mg/kg food. The provision of an
adequate 90-day feeding study in a rodent species is
required by 1983."
ENVIRONMENTAL HEALTH CRITERIA
FOR
ISOBUTANOL
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ISOBUTANOL
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.2 Terrestrial organisms
7.3 Microorganisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Acute toxicity
8.1.2 Signs of intoxication
8.2 Skin and eye irritation
8.3 Short-term exposures
8.4 Long-term exposures
8.5 Reproduction, embryotoxicity, and teratogenicity
8.6 Mutagenicity
8.7 Carcinogenicity
8.8 Special studies
9. EFFECTS ON MAN
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
10.1.2 Toxic effects
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
10.2.2 Toxic effects
10.3 Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY
Isobutanol (2 methyl propanol) is a inflammable colourless
liquid with a sweet odour similar to that of amyl alcohol. It
has a boiling point of 108 °C, a water solubility of 8.7%, and
its n-octanol/water partition coefficient is 0.83. Its vapour
is 2.6 times denser than air. It occurs naturally as a product of
fermentation and is synthesized from petrochemicals. It is used as
an organic solvent, as a plasticizer, in the manufacture of
isobutyl esters, in perfumes, and as a flavouring agent. Human
exposure is primarily occupational. Exposure of the general
population will mainly be from its natural occurrence in food and
its use as a flavouring agent, but may also result from industrial
emissions.
Isobutanol is readily biodegradable and does not bioaccumulate.
It is not directly toxic for fish, crustacea, amphibia, or algae.
Protozoa will tolerate levels of isobutanol likely to be found in
the environment. Isobutanol should be managed in the environment
as a slightly toxic compound. It poses an indirect hazard for the
aquatic environment, because it is readily biodegraded, which may
lead to oxygen depletion.
In animals, isobutanol is absorbed through the skin, lungs, and
gastrointestinal tract. It is metabolized by alcohol dehydrogenase
to isobutyric acid via the aldehyde and may enter the tricarboxylic
acid cycle. Small amounts of isobutanol are excreted unchanged
(< 0.5% of the dose), or as the glucuronide (< 5% of the dose)
in the urine. In rabbits, metabolites found in the urine include
acetaldehyde, acetic acid, isobutylaldehyde, and isovaleric acid.
Oral LD50 values (2.5 - 3.1 g/kg body weight) and the inhalation
LC50 (19.2 g/m3) in rats classifies isobutanol as slightly toxic
according to Hodge & Sterner. The acute toxic effects are
alcoholic intoxication and narcosis. Isobutanol is severely
irritating to the eyes and moderately irritating to the skin. A
group of rats given a solution of isobutanol (1 mol/litre) as their
sole drinking liquid for 4 months did not show any adverse effects
on the liver, while another group given a 2 mol/litre solution as
their sole drinking liquid for 2 months showed reductions in fat,
glycogen, RNA content, and overall size of the cells in the liver.
Continuous inhalation exposure of rats to 3 mg/m3 for 4 months
resulted in depression of leg withdrawal response to electrical
stimulation, and minor changes in formed elements of the blood and
serum enzymes. The estimated no-observed-adverse-effect level was
0.1 mg/m3. In a lifetime carcinogenicity study, groups of rats
received isobutanol subcutaneously (0.05 ml/kg, twice a week) or
orally (0.2 ml/kg body weight twice a week). The animals exhibited
toxic liver damage ranging from steatosis to cirrhosis. Animals
showing malignant tumours totalled 8 in the subcutaneous group,
3 in the oral group, and 0 in the control group. The majority of
treated animals also showed hyperplasia of blood-forming tissues.
From the animal studies available, it is not possible to
determine a no-observed-adverse-effect level for long-term
exposure. No adequate data are available to assess the
mutagenicity or teratogenicity of isobutanol or effects on
reproduction.
The only reported observations in man relate to the production
of vertigo under conditions of severe and prolonged exposure to
vapour mixtures of isobutanol and 1-butanol. Thus, it is not
possible to attribute the vertigo to a single cause.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure: CH3
\
CH-CH2OH
/
CH3
Chemical formula: C4H10O
Primary constituent: isobutanol
Common synonyms: isobutyl alcohol, isopropylcarbinol,
2-methyl-1-propanol, 2-methylpropyl
alcohol, 1-hydroxymethylpropane,
2-methylpropan-1-ol, 1-propanol,-2-methyl,
fermentation butyl alcohol
CAS registry number: 78-83-1
2.2. Physical and Chemical Properties
Physical and chemical properties of isobutanol are given in
Table 1.
Table 1. Physical and chemical properties of isobutanol
-----------------------------------------------------------------
(at 20 °C and 101.3 kPa, unless otherwise stated)
Physical state colourless liquid
Odour sweet, similar to that of amyl
alcohol, but weaker
Odour threshold approximately 4.6 mg/m3 (1.5 ppm)
Relative molecular mass 74.12
Density (kg/m3) 801-803
Boiling point (°C) 107.9
Freezing point (°C) -108
Viscosity (cP) 3.98
Vapour pressure (kPa) 1.17
12.2 at 25 °C
Vapour density (air = 1) 2.55
Flashpoint (°C) 27.8
Autoignition temperature (°C) 434
Explosion limits air (% v/v) lower = 1.7, upper = 10.9
Solubility (% weight) in water, 8.7; soluble in alcohol
and ether
n-octanol/water partition 0.83
coefficient
Conversion factors (25 °C) 1 mg/m3 = 0.324 ppm
1 ppm = 3.083 mg/m3
-----------------------------------------------------------------
2.3. Analytical Methods
Testing methods for the butanols (ASTM D304-58) are described
in ASTM (1977).
NIOSH (1977) Method No S64 (341) has been recommended. It
involves drawing a known volume of air through charcoal to trap the
organic vapours present (recommended sample is 10 litres at a rate
of 0.2 litre/min). The analyte is desorbed with carbon disulfide
containing 1% 2-propanol. The sample is separated by injection
into a gas chromatograph equipped with a flame ionization detector,
and the area of the resulting peak is determined and compared with
standards.
The Association for Official Analytical Chemists has published
an Official Final Action for the assay of isobutanol in spirits,
based on gas chromatographic analysis (AOAC methods, 1975).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The major use of isobutanol is in the manufacture of isobutyl
acetate, which is employed in the lacquer industry. Furthermore,
isobutanol is used as a solvent in paint and varnish removers and
in the manufacture of isobutyl esters, which serve as solvents,
plasticizers, flavourings, and perfumes. It is also used as a
flavouring agent in butter, cola, fruit, liquor, rum, and whisky
(Hall & Oser, 1965). The average maximum levels at which it is
used in the USA are listed in Table 2 (Hall & Oser, 1965).
Table 2. Average maximum use levels of isobutanol in the USA
-------------------------------------------------------------
Food in which used Approximate average maximum level (mg/kg)
-------------------------------------------------------------
Beverages 17
Ice cream, ices 7
Candy 30
Baked goods 24
-------------------------------------------------------------
Isobutanol is one of the three main alcohols in fusel oil, and
is present in large amounts in some alcoholic beverages (Hedlund
Kiessling, 1969).
Natural isobutanol is produced by the fermentation of
carbohydrates. Isobutanol is found in fruits: cherry (Postel et
al., 1975), raspberry and blackberry (McGlumphy, 1951; Nursten et
al., 1967; Broderick, 1976), grape (Bober, 1963), and apple
(Schreier et al., 1978). It also occurs in beverages: brandy
(Postel et al., 1975), coffee (Walter & Weidemann, 1969), cider
(Matthews et al., 1962; Kieser et al., 1964), and gin (Clutton &
Evans, 1978). Isobutanol has been identified in sundry other foods
including cheddar cheese (Liebich et al., 1970), and hop oil
(Lammers et al., 1968).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
No data are available on distribution in soil, sediments or
air.
Isobutanol is readily biodegradable (Table 3). It is degraded
in significant amounts within a few hours, and degradation would be
expected to be complete within a few days.
Table 3. Biodegradation of isobutanol
-------------------------------------------------------------------
5d BOD 64% of ThOD in fresh water Price et al. (1974)
46% of ThOD in synthetic Price et al. (1974)
seawater
5d BOD 16% of ThOD (APHA) Bridié et al. (1979a)
63% of ThOD after adaptation Bridié et al. (1979a)
(APHA)
Activated 32.5% of ThOD removed in 24 h by Gerhold & Malaney
sludge unadapted municipal sludge (1966)
44% of ThOD removed in 24 h by McKinney & Jeris
adapted sludge (1955)
-------------------------------------------------------------------
ThOD = theoretical oxygen demand - the calculated amount of
oxygen needed for complete
oxidation to water and carbon
dioxide.
COD = chemical oxygen demand - measures the chemically
oxidizable matter present.
BOD = biochemical oxygen demand - a simple bioassay measuring
the potential deoxygenating
effect of biologically
oxidizable matter present in
an effluent.
Nazarenko (1969) reports an oxygen requirement of approximately
1.4 mg to oxidize 1 mg of isobutanol. Isobutanol at a
concentration of 20 mg/litre inhibits nitrification in water
(Nazarenko, 1969).
Isobutanol does not bioaccumulate (Chiou et al., 1977).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
An industrial emission study indicated that 90 tonnes of
isobutanol were released into the air over 1 year in the
Netherlands (Anon, 1983). Isobutanol produced naturally during the
fermentation of carbohydrates can enter the environment by leaching
from industrial waste landfills. A concentration of 0.3 g/litre
was found to be leaching from a 1-year-old landfill (US EPA, 1980).
6. KINETICS AND METABOLISM
Isobutanol can be absorbed through the lungs and from the
gastrointestinal tract (Browning, 1965).
Isobutanol (2 ml/kg body weight) was given to male rabbits by
gavage, and blood levels of isobutanol were determined. After 1 h,
a maximum concentration of 0.5 g/litre was observed. After 6 h,
isobutanol was no longer detectable in the blood. Less than 0.5%
of the administered isobutanol was excreted unchanged in the breath
or urine within 40 h (Saito, 1975).
Isobutanol can be metabolized to isobutyric acid and then
proceed into the tricarboxylic acid cycle, possibly via succinate
(Fig. 1B) (Saito, 1975). The urinary metabolites resulting from
repeated ingestion of isobutanol were also studied by Saito (1975).
Isobutanol (2 ml/kg body weight) was administered by stomach tube
to male rabbits. The rabbits were then given water saturated with
isobutanol instead of pure water to drink and the urinary
metabolites were determined. The length of time over which the
urine was collected was not specified. The urinary metabolites
were acetaldehyde (80 mg), acetic acid (16 mg), isobutyraldehyde
(10 mg), isovaleric acid (128 mg), and unmetabolized isobutanol (40
mg). The origin of urinary isovaleric acid was not discussed by
the author. Saito (1975) has proposed the scheme shown in Fig. 1B
for the metabolism of isobutanol in rabbits.
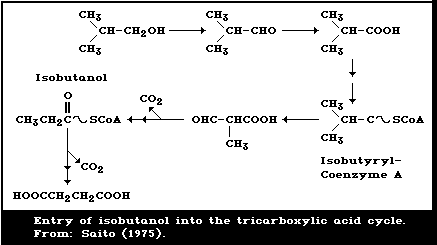 When Kamil et al. (1953) administered isobutanol (25 mmol
total) by stomach tube to Chinchilla rabbits, 4.4% of the applied
dose was excreted within 24 h, as the glucuronide. After
administration of 6 ml isobutanol to the stomach of rabbits (Kamil
et al., 1953), no aldehydes or ketones were observed in the expired
air within 6 h.
Gaillard & Derache (1965) reported that, following an oral dose
of 2 g/kg body weight to rats, 0.27% was excreted in the urine
within 8 h.
The oxidation of isobutanol and other lower alcohols by both
rat liver homogenates and by the perfusion in situ of rat liver
was studied by Hedlund & Kiessling (1969). The authors observed
that the alcohols were oxidized at rates that decreased in the
following order: 1-propanol, isobutanol, ethanol, and isoamyl
alcohol. Furthermore, from their studies with the alcohol
dehydrogenase (ADH) inhibitor pyrazole, these authors concluded
that all the alcohols studied must be oxidized by ADH in the same
manner as ethanol in an NAD-mediated oxidation.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Some data on the toxicity of isobutanol for aquatic organisms
are given in Table 4. The high lethal concentrations (1000 - 4000
mg/litre) for fish, amphibia, and crustacea indicate that, at
background levels, isobutanol would not be disruptive for an
aquatic ecosystem.
Table 4. Toxicity of isobutanol for aquatic organisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Fish
Fresh-water species
Bleak 1000-3000 96-h LC50 Lindén et al.
(Alburnus (1979)
alburnus)
Golden orfe 1520 48-h LC50 Juhnke & Lüdemann
(Leuciscus (1978)
idus melanotus)
Goldfish 2600 24-h LC50 Bridié et al.
(Carassius (1979a)
auratus)
Amphibia
Tadpole 4000 threshold for Münch (1972)
(Rana sp) narcosis
Invertebrates
Fresh-water species
Water flea 1250 24-h EC50 immobilization Bringmann & Kuehn
(Daphnia magna) (1982)
Marine species
Brine shrimp 1400 24-h LC50 Price et al. (1974)
(Artemia salina) 3800 EC50 excystment Smith & Siegel
(1975)
------------------------------------------------------------------------------------------
Table 4. (contd.)
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Algae
Green algae
Scenedesmus 350 8-day no-observed- total biomass Bringmann & Kuehn
quadricauda adverse-effect level (1978a)
Blue-green algae
(Microcystis 290 8-day no-observed- total biomass Bringmann & Kuehn
aeruginosa) adverse-effect level (1978a)
------------------------------------------------------------------------------------------
7.2. Terrestrial Organisms
Toxicity studies in plants indicate that germination will not
be affected by exposure to isobutanol at background levels. An
EC50 of 760 mg/litre was reported by Reynolds (1977) for seed
germination in lettuce (Lactuca sativa). Smith & Siegel (1975)
found an EC50 of 40 800 mg/litre for seed germination in cucumber
(Cucumis sativus).
There is no information on terrestrial animals, but exposure is
unlikely to be significant, except locally after spills.
7.3. Microorganisms
Some data on the toxicity of isobutanol for microorganisms are
given in Table 5. Available toxicity data on protozoa bacteria and
algae suggest tolerance to exposure to isobutanol. No-observed-
adverse-effect levels for various species ranged from 22 to 1180
mg/litre). Exposure to background levels of isobutanol should not
be disruptive for the ecosystem.
Table 5. Toxicity of isobutanol for microorganisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Protozoa
Chilomonas paramaecium 22 48-h no-observed- total biomass Bringmann &
(flagellate) adverse-effect level Kuehn (1981)
Uronema parduczi 169 20-h no-observed- total biomass Bringmann &
(ciliate) adverse-effect level Kuehn (1981)
Entosiphon sulcatum 296 72-h no-observed- total biomass Bringmann &
(flagellate) adverse-effect level Kuehn (1981)
Bacteria
Pseudomonas putida 280 16-h no-observed- total biomass Bringmann &
adverse-effect level Kuehn (1976)
Bacillus subtilis 1180 EC50 spore Yasuda-Yasaki
germination et al. (1978)
------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Acute toxicity
The oral EC50 for narcosis in rabbits was 19 mmol isobutanol/kg
body weight (Münch, 1972).
Acute toxicity data are given in Table 6.
Table 6. Acute toxicity of isobutanol
---------------------------------------------------------------------
Species Route of Parameter Results Reference
administration
---------------------------------------------------------------------
Guinea- inhalation LC50 19 900 mg/m3 Kushneva et al.
pig (1983)
Mouse inhalation LC50 15 500 mg/m3 Kushneva et al.
(1983)
Rabbit inhalation LC50 26 250 mg/m3 Kushneva et al.
(1983)
Rat inhalation LCLoa 8000 mg/m3 (4 h) US DHEW (1978)
Rat inhalation LC50 19 200 mg/m3 Kushneva et al.
(1983)
Cat intravenous LDLoa 0.018 g/kg US DHEW (1978)
Rabbit oral LDLoa 3.75 g/kg US DHEW (1978)
Rabbit oral LD50 41 mmol/kg Münch (1972)
Mouse oral LD50 3.5 g/kg Kushneva et al.
(1983)
Rat oral LD50 3.1 g/kg Kushneva et al.
(1983)
Rat oral LD50 2.46 g/kg US DHEW (1978)
Rabbit skin LD50 4.24 g/kg US DHEW (1978)
---------------------------------------------------------------------
a Lo = lowest.
After ip administration of isobutanol to mice, the LD50
was > 1000 mg/kg at 30 min and 544 mg/kg at 7 days. Gross post-
mortem examination of livers showed an abnormal dark coloration
(Maickel & McFadden, 1979).
Smyth et al. (1954) reported 100% survival of rats exposed for
2 h to saturated isobutanol vapour (approximately 49 248 mg/m3 or
16 000 ppm) in air, but observed 2 deaths in a group of 6 rats when
they were exposed for 4 h to a concentration of 24 624 mg/m3 (8000
ppm) in air.
8.1.2. Signs of intoxication
The toxic effects of isobutanol are mainly alcoholic
intoxication and narcosis. Stupor and loss of voluntary movements
in animals can be considered signs of intoxication by the oral
route (Münch, 1972). Rats and rabbits were exposed, by inhalation,
for a period of 4 h to various concentrations of isobutanol. At a
concentration of 15 700 mg/m3, there was irritation of the airways.
Three days later, symptoms included central nervous depression, a
decreased number of lymphocytes in bone marrow, a decreased blood-
lactate level, delay in elimination of bromophthalein from blood,
and morphological changes including dystrophia of hepatocytes and
olfactory neurons in the brain. After exposure to 8000 mg/m3,
symptoms were similar but less severe. An isobutanol concentration
of 1300 mg/m3 decreased bone marrow lymphocyte numbers. A
concentration of 100 mg/m3 only altered breathing frequency
(Kushneva et al., 1983).
8.2. Skin and Eye Irritation
Application of 500 mg isobutanol to the skin of rabbits for
24 h was moderately irritating. However, application of 2 mg to
the rabbit eye caused severe irritation (US DHEW, 1978).
8.3. Short-Term Exposures
Hillbom et al. (1974a,b) gave one group of 6 male Wister rats
a 1 mol/litre solution of isobutanol as their sole drinking liquid
for a period of 4 months. Another group of 5 rats of the same
species was given a 2 mol/litre solution of isobutanol as their
sole drinking liquid for 2 months. Both groups were provided an
ad libitum diet of ordinary laboratory food containing about 26%
of calories as protein, 55% as carbohydrate, and 19% as fat. At
the end of the study, all animals were decapitated, and their
livers examined. No adverse effects were detected in the group
given the 1 mol/litre solution, while the rats in the group given
the 2 mol/litre solution showed a decrease in fat, glycogen, RNA,
and overall size of the liver cells.
Oral administration of between 1/10 and 1/5 of the LD50 of
isobutanol for 6 days/week over 1 month, did not result in any
deaths in rats (Kushneva et al., 1983). Continuous exposure of
rats, by inhalation, to isobutanol at 3 mg/m3 over 4 months,
caused an increased threshold for leg withdrawal response to
electrical stimulation, and depression of haemoglobin content,
erythrocyte count, and activities of cholinesterase and catalase
in the blood. The activities of alanine-amino transferase and
aspartate amino transferase were elevated. At 0.5 mg/m3, numbers
of white cells and erythrocytes, haemogloglobin content, and
cholinesterase activity were all decreased. No effects were
observed at 0.1 mg/m3 (Tsulaya et al., 1978).
8.4. Long-Term Exposures
With the exception of a carcinogenicity study, no long-term
studies have been reported.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data are available on reproduction or
embryotoxicity and no conclusions can therefore be drawn.
8.6. Mutagenicity
The only reported study on mutagenicity is that of Hillscher et
al. (1969) who demonstrated an increased rate of reverse mutation
when Escherichia coli CA 274 was treated with 0.7% isobutanol,
without metabolic activation. This study in itself is inadequate
to assess the mutagenic potential of the compounds.
8.7. Carcinogenicity
Two groups of 19 and 24 male and female Wistar rats (10 weeks
old) were administered purified isobutanol. Group one received
0.2 ml/kg body weight, orally, twice weekly. Group two received
0.05 ml/kg, subcutaneously, twice weekly. Two control groups (25
rats each) received 1 ml of 0.9% sodium chloride twice weekly
(controls for group one were dosed orally; controls for group two
were dosed subcutaneously). All rats were dosed until spontaneous
death. Test rats presented liver carcinomas and sarcomas, spleen
sarcomas, stomach proventricular carcinomas, and myeoid leukaemia.
Tumours of these types did not occur in the control groups (Table
7). In the exposed animals, toxic liver damage was found, ranging
from steatosis and cell necrosis to fibrosis and cirrhosis. In
addition, most exposed animals had hyperplasia of blood-forming
tissues. The carcinogenic response was attributed to isobutanol
(Gibel et al., 1974, 1975). No statistical analysis of the data
was performed.
Table 7. Summary of tumours in experimental animalsa
-------------------------------------------------------------------
Agent Application Number Average Malignant Benign
(twice weekly) of survival tumour tumour
animals (days)
-------------------------------------------------------------------
Control oral: 1 ml/kg 25 643 0 3
(0.9% NaCl) body weight
solution)
subcutaneous: 25 643 0 2
1 ml/kg body
weight
Isobutanol oral: 0.2 ml/kg 19 495 3b 9
body weight
subcutaneous: 24 544 8c 3
0.05 ml/kg
body weight
-------------------------------------------------------------------
a Adapted from: Gibel et al. (1975).
b Proventriculus carcinoma and liver cell carcinoma; proventriculus
carcinoma and myeloid leukaemia; myeloid leukaemia.
c Proventriculus carcinoma (2); liver sarcoma (2); spleen sarcoma;
mesothelioma; retroperitoneal sarcoma (2).
8.8. Special Studies
In 8 guinea-pigs, serum enzyme (SGOT, SGPT, GLDH) activities
and triglyceride levels were measured 16 h after oral
administration of 33.5 mg isobutanol/kg olive oil. There was no
statistically-significant difference between treated animals and 8
olive-oil treated controls (Siegers et al., 1974).
Isobutanol can potentiate the hepatic toxicity of carbon
tetrachloride (Cornish & Adefuin, 1967).
9. EFFECTS ON MAN
Isobutanol may be absorbed through the lungs and the
gastrointestinal tract (Browning, 1965).
No data are available on the effects of isobutanol on the skin.
However, as with other defatting solvents, isobutanol may cause
erythematous skin lesions (Schwarz & Tulipan, 1939).
Immersion of the hands of 5 healthy male volunteers in a
1:1 (v/v) mixture of m-xylene and isobutanol, for 15 min at
room temperature, caused only a mild and sometimes barely
distinguishable erythema or a burning feeling. Isobutanol (in a
1:1 mixture with m-xylene) dehydrated the skin and decreased the
absorption of xylene. However, when water was added as 8% of the
mixture, this effect was reduced. The dermal effects of isobutanol
only and its absorption were not studied (Riihimaki, 1979).
Seitz (1972) reported 7 case histories, occurring between
1965 and 1971, of workers who had been exposed to 1-butanol and
isobutanol in a non-ventilated photographic laboratory. They
handled the alcohols under intense and hot light, without any
precautions. Exposure levels were not quantified, but must have
been excessive; exposure time ranged from 1 1/2 months to 2 years.
Two workers had transient vertigo, 3 severe Meniere-like vertigo
with nausea, vomiting and/or headache. In one of these cases,
hearing was also perturbed. Two workers did not present any signs
or symptoms.
Eye irritation, blurred vision, and transient corneal
vacuolization have been decribed in another excessive (no
measurements done) mixed exposure of workers to isobutanol and
butyl acetate. With adequate work room ventilation, it did not
recur. The author felt that the more irritant butyl acetate would
be the main contributor to this effect (Büsing, 1952).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population to isobutanol
through food and beverages are not available and occupational
exposure levels are limited and inadequate.
10.1.2. Toxic effects
In animals, isobutanol is absorbed through the skin, lungs,
and gastrointestinal tract. Isobutanol is metabolized by alcohol
dehydrogenase to isobutyric acid via the aldehyde and may enter the
tricarboxylic acid cycle. Small amounts of isobutanol are excreted
unchanged (< 0.5% of the dose), or as the glucuronide (< 5% of
the dose) in the urine. In rabbits, metabolites found in the urine
include acetaldehyde, acetic acid, isobutyraldehyde, and isovaleric
acid. Oral LD50 values (2.5 - 3.1 g/kg body weight) and inhalation
LC50 (19.2 g/m3) in rats classify isobutanol as slightly toxic
according to Hodge & Sterner. The acute toxic effects are
alcoholic intoxication and narcosis. Isobutanol is severely
irritating to the eyes and moderately irritating to the skin. A
group of rats given a 1 mol/litre solution of isobutanol as their
sole drinking liquid for 4 months did not show any adverse effects
in the liver; another group given a 2 mol/litre solution as their
sole drinking liquid for 2 months showed a reduction in fat,
glycogen, and RNA content, and in the overall size of the cells in
the liver. Continuous inhalation exposure of rats to 3 mg/m3 for
4 months resulted in depression of leg withdrawal response to
electrical stimulation, minor changes of formed elements of the
blood and serum enzymes. The estimated no-observed-adverse-effect
level was 0.1 mg/m3.
In a lifetime carcinogenicity study, groups of rats received
isobutanol subcutaneously (0.05 ml/kg body weight twice a week) or
orally (0.2 ml/kg body weight twice a week). The animals exhibited
toxic liver damage ranging from steatosis to cirrhosis. Numbers of
animals showing malignant tumours totalled 8 in the subcutaneous
group, 3 in the oral group, and 0 in the control group. The
majority of treated animals also showed hyperplasia of blood-
forming tissues.
Because of lack of mutagenicity studies, the Task Group could
not determine whether isobutanol was a genetically active compound.
The findings in the carcinogenicity study are a cause for concern.
Because of methodological inadequacies and the manner of reporting
the data, it was not possible to determine whether isobutanol
should be regarded as an animal carcinogen. Thus it is not
possible to extrapolate from this study to possible long-term
effects in man.
From the animal studies available, it is not possible to
determine a no-observed-adverse-effect level for long-term
exposure. No adequate data are available to assess mutagenicity
or teratogenicity of isobutanol or effects on reproduction.
Exposure of the general population to isobutanol through food
and beverages is unlikely to lead to acute toxic effects. The only
reported observations in man relate to the production of vertigo
under conditions of severe and prolonged exposure to vapour
mixtures of isobutanol and 1-butanol. From this study, it is
not possible to attribute the vertigo to a single cause.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
Little quantitative data relating to levels in the general
environment are available, but, because isobutanol is readily
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillages.
10.2.2. Toxic effects
At background concentrations likely to occur in the
environment, isobutanol is not directly toxic for fish, amphibia,
crustacea, or algae. Protozoa will be tolerant to levels of
isobutanol likely to be found in the environment.
Isobutanol should be managed in the environment as a slightly
toxic compound. It poses an indirect hazard to the aquatic
environment, because it is readily biodegradable, which may lead to
oxygen depletion.
10.3. Conclusions
1. On the basis of available data, the Task Group considered it
unlikely that isobutanol would pose a serious acute health risk
to the general population under normal exposure conditions.
However, the Task Group was unable to make an assessment of the
long-term health risk of isobutanol for the general population.
It was concluded that the results of the carcinogenicity study
need verification by a bioassay of modern standards.
2. The Task Group considered that the data available were
inadequate to set an occupational exposure limit. In line with
good manufacturing practice, exposure to isobutanol should be
minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of isobutanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
1. The Task Group noted that, from the animal studies available,
it is not possible to determine a no-observed-adverse-effect
level. Relevant studies should be conducted so that this can
be achieved.
2. The Task Group considered that adequate studies should be
conducted to assess the mutagenicity and carcinogenicity of
isobutanol.
3. Epidemiological studies, including precise exposure data, would
assist in a better assessment of the occupational hazard of
isobutanol.
4. Additional information on environmental pathways (notably
emission and leaching) and residues are desirable.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Council of Europe (1981) included isobutanol in the list
of flavouring substances that can be added to foodstuffs without
hazard to public health at a level of 25 mg/kg for beverages and
food.
At their 23rd meeting, the Joint FAO/WHO Expert Committee on
Food Additives (JECFA) reviewed the data on isobutanol. They
concluded that:
"The evaluation of this compound was not possible
owing to the paucity of toxicological data. New
specifications were prepared, but no toxicological
monograph" (WHO, 1980).
REFERENCES
AARSTAD, K., ZAHLSEN, K., & NILSEN, O.G. (in press) Inhalation of
butanols: changes in the cytochrome P-450 enzyme system. In:
Proceedings of the European Society of Toxicology, 1984 Annual
Meeting.
ABBASOV, A.Z., PANOV, V.N., & ALIEV, A.M. (1971) [Determination
of a mixture of butyl alcohols in the air of industrial premises by
means of a chromatographic method.] Gig. i Sanit., 36: 61-63
(in Russian).
ABBONDANDOLO, A., BONATTI, S., CORSI, C., CORTI, G., FIORIO, R.,
LEPORINI, L., MAZZACCARO, A., & NIEIR, R. (1980) The use of
organic solvents in mutagenicity testing. Mutat. Res., 79: 141-150.
ACGIH, (1980) Documentation of the threshold limit values, 4th
ed., Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
AITIO, A. (1977) Inhibition of ethoxycumarin deethylation by
organic solvents. Res. Commun. chem. Pathol. Pharmacol., 1:
773-776.
AKHREM, A.A., POPOVA, E.M., & METELITSA, O.I. (1978) Interaction
of aliphatic alcohols with cytochrome P-450 from rat liver
microsomes. Biokhimya (USSR), 43: 1485-1491.
AMUNDSEN, J., GOODWIN, R.J., & WETZEL, W.H. (1979) Water-soluble
pentachlorophenol and tetrachlorophenol wood-treating systems.
S. African, 78: 01,031 (3 January 1979).
ANDERSON, R.A., REDDY, J.M., JOYCE, C., WILLIS, B.R., VAN DER VEN,
H., & ZANEVELD, L.J.D. (1982) Inhibition of mouse sperm
capacitation by ethanol. Biol. Reprod., 27: 833-840.
ANON (1983) Selection of priority compounds: emissions. In:
Publikatiereeks Lucht, The Hague, The Netherlands, Ministry of
Housing, Physical Planning and Environment, Vol. 10.
AOAC METHODS (1975) In: Horwitz, W., ed. Official methods of
analysis of the Association of Official Analytical Chemists,
Washington DC, Association of Official Analytical Chemists.
ARTIGAS GIMENEZ, G., URDANGARAY ARGUELLES, V., GONZALES BLASQUEZ,
I., & ALONSO RODRIGUEZ, J. (1979) Protective coating for glass
objects. Fr. Demande, 2: 410, 115 (23 March 1979).
ASHFORD, M.L.J. & WANN, K.T. (1979) A comparison of the effects
of butanol and benzyl alcohol on the frog end-plate conductance.
J. Physiol., 295: 86-87.
ASTM (1977) Testing methods for butanols. Annual book of
standards. Part 29, Philadelphia, Pennsylvania, American Society
for Testing and Materials (ASTM D304-58).
ASTRAND, I., OVRUM, P., LINDQUIST, T., & HULTENGREN, M. (1976)
Exposure to butyl alcohol: uptake and distribution in man. Scand.
J. Work Environ. Health, 3: 165-175.
AUTY, R.M. & BRANCH, R.A. (1976) The elimination of ethyl,
n-propyl, n-butyl, and iso-amyl alcohols by the isolated perfused
rat liver. J. Pharm. exp. Ther., 197: 669-674.
BAIKOV, B.K. & KHACHATURYAN, M.Kh. (1973) [Hygienic assessment of
the reflex action on a body of small concentrations of butyl
alcohol in the atmosphere.] Gig. i Sanit., 12: 7-11 (in Russian).
BAKER, R.C., SORENSON, S.M., ELOG, S.R., & DEITRICH, R.A. (1979)
Acetone excretion following t-butanol treatment in rats. In:
Proceedings of the 3rd International Symposium on Alcohol and
Aldehyde Metabolizing Systems, Toronto (Abstract No. 44).
BAKER, R.C., SORENSEN, S.M., & DEITRICH, R.A. (1982) The in vivo
metabolism of tertiary butanol by adult rat. Alcohol. clin. exp.
Res., 6(2): 247-251.
BARTHA, T., GONCZIL, L., & MOLLO, A. (1973) The use of metabolic
product analysis with simplified gas-liquid chromatographic methods
in routine anaerobic laboratory analysis. Egeszesegtodamany, 22:
220-227.
BEAUD, P. & RAMUZ, A. (1978) Gas-liquid chromatography of
simultaneous determination of higher alcohols and ethyl acetate in
spirits. Mitt. Geb. Lebensmittelunters. Hyg., 69: 423-30.
BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1981)
Liver lipid disposal following t-butanol administration to rats.
Chem.-biol. Interact., 38: 45-51.
BEAUGE, F., FLEURET, C., BARIN, F., & NORDMANN, R. (1984) Brain
membrane disordering after acute in vivo administration of ethanol,
isopropanol, or t-butanol in rats. Biochem. Pharmacol., 33:
3591-3593.
BELLIN, S.I. & EDMONDS, H.L., Jr (1976) The use of tert-butanol
in alcohol dependence studies. Proc. West. Pharmacol. Soc., 19:
351-354.
BENGTSSON, B.E., RENBERG, L., & TARKPEA, M. (1984) Molecular
structure and aquatic toxicity: an example with C1-C13 aliphatic
alcohols. Chemosphere, 13(5/6): 613-622.
BIKFALVI, I. & PASZTOR, L. (1977) Study on the components
distillates of wine using gas chromatography. Szeszipar, 25:
96-100.
BIRKETT, D.J. (1974) Interaction of some drugs, metal ions, and
alcohols with rat liver microsomes as studied with a fluorescent
probe. Clin. exp. Pharmacol. Physiol., 1: 415-427.
BLOK, J. (1981) [A simple toxicity test using nitrifying
bacteria.] H2 O, 14(11): 242-245 (in Dutch).
BLOOM, S.E. (1981) Detection of sister chromatid exchanges in
vivo using avian embryos. In: Cytogenetic assays of environmental
mutagens, T.C.H.S.U. ed: Allenheld, Osmun, Totowa, New Jersey.
BOBER, A. & HADDAWAY, L.W. (1963) Gas chromatographic
identification of alcoholic beverages. J. gas Chromatogr., 1: 8-13.
BONTE, W. (1978) Congener content of wine and similar beverages.
Blutalkohol, 15: 392-404.
BONTE, W. (1979) Congener substances in German and foreign
beers. Blutalkohol, 16: 108-124.
BONTE, W., DECKER, J., & BUSSE, J. (1978) Congener content of
high-proof alcoholic beverages. Blutalkohol, 16: 108-124.
BRAY, W.J. & HUMPHRIES, C. (1978) Solvent fractionation of leaf
juice to prepare green and white protein products. J. Sci. Food
Agric., 29: 839-846.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRINGMANN, G. & KUEHN, R. (1976) [Comparative findings concerning
the harmful effect of water pollutants on bacteria (Pseudomonas
putida) and blue-green algae (Microcystis aeruginosa).] GWF-
Wasser-Abwasser, 117(9): 410-413 (in German).
BRINGMANN, G. & KUEHN, R. (1977) Results of the damaging effect
of water pollutants on Daphnia magna. Z. Wasser Abwasser Forsch.,
10: 161-166.
BRINGMANN, G. & KUEHN, R. (1978a) [Limit values for the harmful
effect of water pollutants on blue-green algae (Microcystis
aeruginosa) and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Vom Wasser, 50: 45-60 (in German).
BRINGMANN, G. & KUEHN, R. (1978b) Testing of substances for their
toxicity threshold: model organisms Microcystis (Diplocystis)
aeruginosa and Scenedesmus quadriconda. Mitt. Int. Ver. Theor.
Angew. Limnol., 21: 275-284.
BRINGMANN, G. & KUEHN, R. (1981) [Comparison of the effect of
harmful substances on flagellates, on ciliates, on holozoic
bacteriophagic protozoa and on saprozoic protozoa.] GWF-Wasser-
Abwasser, 122(7): 308-312 (in German).
BRINGMANN, G. & KUEHN, R. (1982) [Findings concerning the
harmful effect of water pollutants on Daphnia magna in an advanced
standardized test procedure.] Z. Wasser Abwasser Forsch., 15(1):
1-6 (in German).
BRODERICK, J.J. (1976) Raspberry: a case history. Int. Flavours
Food Add., 7: 27-30.
BROWNING, E. (1965) Toxicology and metabolism of industrial
solvents, Amsterdam, Elsevier, p. 349.
BUSING, K.H. (1952) [Eye damage caused by butylacetate and
isobutylalcohol in a cable factory.] Zent. Arbeitsmed.
Arbeitsschutz, 2: 13-14 (in German).
CATER, B.R., COOK, M.W., GANGOLLI, S.D., & GRASSO, P. (1977)
Studies on dibutyl phthalate-induced testicular atrophy in the rat:
effect on zinc metabolism. Toxicol. appl. Pharmacol., 41: 609-614.
CEC (1981) Reports of the Scientific Committe for Food. XI.
Series, Luxembourg, Commission of the European Communities.
CEDERBAUM, A.I. & COHEN, G. (1980) Oxidative demethylation of
t-butyl alcohol by rat liver microsomes. Biochem. Biophys. Res.
Commun., 97(2): 730-736.
CEDERBAUM, A.I., DICKER, A.I., & COHEN, G. (1978) Effect of
hydroxyl radical scavanger on microsomal oxidation of alcohols and
on associated microsomal reaction. Biochemistry, 17: 3058-3064.
CEDERBAUM, A.I., DICHER, E., RUBIN, E., & COHEN, G. (1979)
Effect of thiourea on microsomal oxidation of alcohols and
associated microsomal functions. Biochemistry, 18: 1187-1191.
CEDERBAUM, A.I., QURESHI, A., & COHEN, G. (1983) Production of
formaldehyde and acetone by hydroxyl-radical generating systems
during the metabolism of tertiary butylalcohols. Biochem.
Pharmacol., 32(23): 3517-3524.
CHANG, T., LEWIS, J., & GLAZKO, A.J. (1967) Effects of ethanol
and other alcohols on the transport of amino acids and glucose by
everted sacs of rat small intestine. Biochim. Biophys. Acta, 135:
1000-1007.
CHANG, S.S., PETERSON, K.J., & HO, C. (1978) Chemical reactions
involved in the deep-fat frying of foods. J. Am. Oil. Chem. Soc.,
55: 718-727.
CHIOU, C.T., FREED, V.H., SCHMEDDING, D.W., & KOHNERT, R.L. (1977)
Partition coefficient and bioaccumulation of selected organic
chemicals. Environ. Sci. Technol., 11(5): 475-478.
CHOU, W.L., SPEECE, R.E., SIDDIQIE, R.H., & MCKEON, K. (1978a)
The effect of petrochemical structure on methane fermentation
toxicity. Prog. Water Technol., 10(5/6): 545-558.
CHOU, W.L., SPEECE, R.E., & SIDDIQIE, R.H. (1978b) Acclimation
and degradation of petrochemical wastewater components by methane
fermentation. In: Scott, C.D., ed. Proceedings of the First
Symposium on Biotechnology, Energy, Production, and Conservation,
New York, Interscience Publishers, Vol. 8, pp. 391-414.
CLARK, J.B. (1953) The mutagenic action of various chemicals on
Micrococcus aureus. Proc. Oklahoma Acad. Sci., 34: 114-118.
CLEGG, D.J. (1964) The hen egg in toxicity and teratogenicity
studies. Food Cosmetol. Toxicol., 2: 717-727.
CLUTTON, D.W. & EVANS, M.B. (1978) The flavour constituents of
gin. J. Chromatogr., 167: 409-419.
COGAN, D.G. & GRANT, W.M. (1945) An unusual type of keratitis
associated with exposure to n-butyl alcohol (butanol). Arch.
Ophthalmol., 33: 106-108.
CONNOR, T.H., THEISS, J.C., HANNA, H.A., MONTEITH, D.K., &
MATHEY, T.S. (1985) Genotoxicity of organic chemicals
frequently found in the air of mobile homes. Toxicol. Lett.,
25: 33-40.
CORNISH, H.H. & ADEFUIN, J. (1967) Potentiation of carbon
tetrachloride toxicity by aliphatic alcohols. Arch. environ.
Health, 14, 447-449.
COUNCIL OF EUROPE (1981) Flavouring substances and natural
sources of flavourings, 3rd ed., Strasbourg.
DANIEL, M.A. & EVANS, M.A. (1982) Quantitative comparison of
maternal ethanol and maternal tertiary butanol diet on postnatal
developments. J. Pharmacol. exp. Ther., 222(2): 294-300.
DE CEAURRIZ, J.G., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DE CEAURRIZ, J.C., DESILES, J.P., BONNET, P., MARIGNAC, B.,
MULLER, J., & GUENIER, J.P. (1982) Concentration-dependent
behavioural changes in mice following short-term inhalation
exposure to various industrial solvents. Toxicol. appl. Pharmacol.,
67: 383-389.
DE FELICE, A., WILSON, W., & AMBRE, J. (1976) Vasoactive effects
of methanol and sodium formate on isolated canine basilar artery.
Toxicol. appl. Pharmacol., 36: 515-601.
DERACHE, R. (1970) Toxicology, pharmacology, and metabolism of
higher alcohols. In: Tremalières, J., ed. International
encyclopedia of pharmacology and therapeutics. XX. Alcohols and
derivatives, Oxford, Pergamon Press, Vol. 2.
DICKEY, F.H., CLELAND, G.H., & LOTZ, C. (1949) The role of
organic perioxides in the induction of mutations. Proc. Natl Acad.
Sci. (USA), 35: 581.
DIETZ, F.K. (1980) The role of 2-butanol and 2-butanone
metabolism in the potentiation of carbon-tetrachloride induced
hepatotoxicity. Diss. Abstr. Int. B, 41(1): 150.
DIETZ, F.K. & TRAIGER, G.J. (1979) Potentiation of CCl4
hepatotoxicity in rats by a metabolite of 2-butanol: 2,3-
butanediol. Toxicology, 14: 209-215.
DIETZ, F.K., RODRIGUEZ-GIAXOLA, M., TRAIGER, G.J., STELLA, V.J., &
HIMMELSTEIN, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9(5): 553-576.
DI VINCENZO, G.D. & HAMILTON, M.L. (1979) Fate of n-butanol in
rats after oral administration and its uptake by dogs after
inhalation or skin application. Toxicol. appl. Pharmacol., 48:
317-325.
DMITRIEV, M.T. & LISHCHIKHIN, V.A. (1979) [Determination of
toxic substances given off by polymeric materials under
experimental conditions.] Gig. i Sanit., 6: 45-48 (in Russian).
DOOLITTLE, A.K. (1954) The technology of solvents and
plasticizers, New York, John Wiley and Sons, pp. 644-645.
DORE, M., BRUNET, N., & LEGUBE, B. (1974) Participation de
différents composés organiques à la valeur des critères globaux de
pollution. Trib. Cebedeau, 28(374): 3-11.
DREYFUS, M., VANDENBUNDER, R., & BUCH, H. (1978) Stabilization of
a phosphorilase b-active conformation by hydrophobic solvents.
FEBS Lett., 95: 185-189.
DUBINA, O.N. & MAKSIMOV, G.G. (1976) [Testing the use of golden
hamsters in toxicological research.] Gig. Tr. Ohkhr. Zdorov'ya Rab.
Neft. Neftekhim. Prom-sti, 9: 100-103 (in Russian).
DUDEK, B.C. & PHILIPS, T.J. (1983) Locomotor stimulant and
intoxicant proportion of methanol, ethanol, tert-butyl alcohol, and
pentobarbital in long-sleep and short-sleep mice. Subst. Alcohol
Actions/misuse, 4: 31-36.
DUMONT, J.P. & ADDA, J. (1978) Occurrence of sesquiterpones in
mountain cheese volatiles. J. agric. food Chem., 26: 364-367.
EDWARDS, E.K., Jr & EDWARDS, E.K. (1982) Allergic reaction to
tertiary butyl alcohol in a suncreen. Cutis, 29: 476-478.
EGOROV, Y.L. (1972) Dependance of dermal toxicity of alcohols on
solubility index: oil/water. Toksikol. Gig. Prod. Neftekhim Yarosl.,
98: 102.
FEDERAL REGISTER (1977) 6 May (42188, 23148).
FERRI, S.S. (1979) Scratch-resistant coating for lenses, etc.
Braz. Pedido PI, 78 05,443 (20 March 1979).
FISHER, G.S., LEGENDRE, M.G., LOVGREN, N.V., SCHULLER, W.H., &
WELLS, J.A. (1979) Volatile constituents of southern pea seed.
J. agric. food Chem., 27: 7-11.
FLATH, R.A. & TAKAHASHI, J.R. (1978) Volatile constituents of
prickly pear. J. agric. food Chem., 26: 835-837.
FLATH, R.A., FORREY, R.R., JOHN, J.O., & CHAN, B.G. (1978)
Volatile components of corn silk (Zea mays): possible Heliothis
zea (Boddie) attractants. J. agric. food Chem., 26: 1290-1293.
FORSANDER, O. (1967) Influence of some aliphatic alcohols on the
metabolism of rat liver slices. Biochem. J., 105: 93-97.
GAILLARD, D. & DERACHE, R. (1965) Métabolisation de différents
alcools présents dans les boissons alcooliques chez le rat. Trav.
Soc. Pharmacol. Montpellier, 25: 51-62.
GEPPERT, E. VON, STURZ, J., HAASE, W., & ISSELHARD, W. (1976)
[Effect of n-butanol on the metabolic status of certain rat organs
and on the circulation of the rabbit.] Arzneim. Forsch., 26:
1333-1337 (in German).
GERARDE, H.W. & AHLSTROM, D.B. (1966) The aspiration hazard and
toxicity of a homologous series of alcohols. Arch. environ. Health,
13: 457-461.
GERHOLD, R.M. & MALANEY, G.W. (1966) Structural determinants in
the oxidation of aliphatic compounds by activated sludge. J. Water
Pollut. Control Fed., 38(4): 562-579.
GERIKE, P. & FISCHER, W.K. (1979) A correlation study of
biodegradability determinations with various chemicals in various
tests. Ecotoxicol. environ. Saf., 3: 159-173.
GIBEL, W., LOHS, K.H., WILDNER, G.P., & SCHRAMM, T. (1974)
[Experimental research on the carcinogenic effect of higher
alcohols, using 3-methyl-1-butanol, 1-propanol and 2-methyl-1-
propanol as examples.] Z. exp. Chir., 7: 235-239 (in German).
GIBEL, W., LOHS, K.H., & WILDNER, G.P. (1975) [Experimental
research on the carcinogenic effect of solvents, using
propanol-1,2- methylpropanol-1 and 3-methyl- butanol-1 as
examples.] Arch. Geschwulstforsch., 45(1): 19-24 (in German).
GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952) Appraisal of
a chemical waste problem by fish toxicity tests. Sewage ind. Wastes,
24(11): 1397-1401.
GRANT, K.A. & SAMSON, H.H. (1981) Development of physical
dependence on t-butanol in rats: an examination using schedule-
induced drinking. Pharmacol. Biochem. Behav., 14: 633-637.
GRANT, K.A. & SAMSON, H.H. (1982) Ethanol and tertiairy butanol
induced microcephaly in the neonatal rat: comparison of brain
growth parameters. Neurobehav. Toxicol. Teratol., 4: 315-321.
GUKASYAN, ZH.G., BARYSHEVA, K.F., SAAKYAN, O.A., & ARUSTAMYAN, R.K.
(1979) Nauch. Soobshch. N.-i.Proekt.In-t Tsvet.Metallur-
gii.Armniprotsvetmet, 21: 18-21.
HALL, R.L. & OSER, B.L. (1965) Recent progress in the
consideration of flavouring ingredients under the food additives
amendement. III. Gras substances. Food Technol., 151.
HARRIS, R.N. & ANDERS, M.W. (1980) Effect of fasting, diethyl
maleate, and alcohols on carbon tetrachloride-induced
hepatotoxicity. Toxicol. appl. Pharmacol., 56(2): 191-198.
HEDLUND, S.G. & KIESSLING, K.H. (1969) The physiological
mechanism involved in hangover. I. The oxidation of some lower
aliphatic fusel alcohols and aldehydes in rat liver and their
effect on the mitochondrial oxidation of various substrates.
Acta pharmacol. toxicol., 27: 381-396.
HESKETH, T.R., KEIGHTLEY, C.A., METCALFE, J.C., & RICHARDS, C.D.
(1978) Long-chain alcohols (C10-C12) can block nerve impulse.
J. Physiol., 278: 5-6.
HILL, M.W., NEALE, E., & BANEHAM, A.D. (1981) Acute tolerance to
the effects of n-butanol and n-hexanol in goldfish. J. comp.
Physiol., 142: 61-65.
HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974a) Effects
of chronic ingestion of some lower aliphatic alcohols in rats.
Res. Commun. chem. Pathol. Pharmacol., 9(1): 177-180.
HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974b) Effects
of chronic ingestion of some lower aliphatic alcohols in rats.
Jpn. J. Stud. Alcohol, 9(2): 101-108.
HILSCHER, H., GEISSLER, E., & GIBEL, W. (1969) [Research on the
toxicity and mutagenicity of certain fusel oil components in
E. coli.] Acta biol. med. Germ., 23: 843-852 (in German).
HODGE, H.C. & STERNER, J.H. (1943) Tabulation of toxicity
classes. Am. Ind. Hyg. Assoc. Q., 10: 93-96.
IARC (1984) Information bulletin on the survey of chemicals
being tested for carcinogenicity, Lyons, International Agency
for Research on Cancer, Vol. 11.
ILO (1977) Occupational exposure limits for airborne toxic
substances, Geneva, International Labour Office (Occupational
Safety and Health Series No. 37).
JADDOU, H.A., PAVEY, J.A., & MANNING, D.J. (1978) Chemical
analysis of flavor volatiles in heat-treated milks. J. dairy
Res., 45: 391-403.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L., & FITZHUGH,
D.G. (1964) Food flavourings and compounds of related structure.
I. Acute oral toxicity. Food Cosmet. Toxicol., 2: 327.
JONES, H.R. (1971) Environmental control in the organic and
petrochemical industries, Park Ridge, New Jersey, Noyes Data.
JUHNKE, I. & LUEDEMANN, D. (1978) [Results of the testing of 200
chemical compounds for acute toxicity in fish by the orfe test.]
Z. Wasser-Abwasser-Forsch., 11(5): 161-164 (in German).
JULIANO, R.L. & GAGALANG, E. (1979) The effect of membrane-
fluidizing agents on the adhesion of CHO cells. J. cell Physiol.,
98: 483-490.
KABAYASHI, H., MIYOSHI, Y., & KITAMURA, K. (1977) Effects of
various alcohols on the intramuscular absorption of isonicotinamide
in the rat. Chem. pharm. Bull., 25(11): 3078-3080.
KALEKIN, R.M. & BRICHENKO, V.S. (1972) [Toxic effect of products
from the production of butyl alcohols on the central nervous
system.] Nauch. Tr. Irkutsk. Med. Inst., 115: 22-25 (in Russian).
KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) Studies in
detoxication. The metabolism of aliphatic alcohols. The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochem. J., 53:
129-136.
KIESER, M.E., POLLARD, A., STEVENS, P.M., & TUCKNOTT, O.G. (1964)
Determination of 2-phenylethanol in cider. Nature (Lond.), 204: 887.
KOLESNIKOV, P.A. (1975) [Adaptation to butyl alcohol.] Gig i
Sanit., (5): 104-105 (in Russian).
KUDREWICZ-HUBICKA, Z., KOLACZKOWSKA, M., BORZEMSKA, & WESOLOWSKA, A.
(1978) [Serum antitrypsin activity and glycoprotein level in
workers exposed to organic solvents.] Pol. Tyg. Lek., 33, 395-397
(in Polish).
KUSHNEVA, V.S., KOLOSKOVA, G.A., KOLTUNOVA, J.G., & KIRILENKO, V.T.
(1983) Experimental data to hygienic reglementation of
isobutylalcohol in the working zone. Gig. Tr. Prof. Zabol., 1:
46-47.
LALASIDIS, G. & SJOBERG, L.B. (1978) Two new methods of
debittering protein hydrolysates and a fraction of hydrolysates
with exceptionally high content of essential amino acids. J. agric.
food Chem., 26: 742-749.
LAMMENS, H. & VERZELE, M. (1968) Aroma of hops. II. The
composition of hop oil. J. Inst. Brew., 74: 341-346.
LENDLE, L. (1928) [Investigations on the speed at which
homologous and isomeric monovalent alcohols produce narcosis.]
Naunyn-Schmiedeberg's Arch. exp. Pathol. Pharmakol., 129: 85
(in German).
LIEBICH, H.M., DOUGLAS, D.R., BAYER, E., & ZLATKIS, A. (1970)
Volatile flavour components of Cheddar cheese. J. chromatogr. Sci.,
8: 355-359.
LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROEM, G. (1979)
The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the bleak (Alburnus alburnus)
and the harpaticoid (Nitocra spinipes). Chemosphere, 8(11/12):
843-851.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975) Detection
of carcinogens as mutagens in the Salmonella/microsome test: assay
of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72: 5735-5739.
MCCOMB, J.A. & GOLDSTEIN, D.B. (1979) Quantitative comparison of
physical dependence on tertiary butanol and ethanol in mice:
correlation with lipid solubility. J. Pharmacol. exp. Ther.,
208(1): 113-117.
MCCREERY, N.J. & HUNT, W.A. (1978) Physico-chemical correlates of
alcohol intoxication. Neuropharmacology, 17: 451-461.
MCGLUMPHY, J.H. (1951) Fruit flavours. Food Technol., 5: 353-355.
MCGREGOR, D.C., SCHONBAUM, E., & BIGELOW, W.G. (1964) Acute
toxicity studies on ethanol, propanol, and butanol. Can. J. Physiol.
Pharmacol., 42: 689-696.
MACHT, D.I. (1920) A toxicological study of some alcohols, with
special reference to isomers. J. Pharmacol. exp. Ther., 16: 1-10.
MCKEE, J.E. & WOLF, H.W. (1963) Water quality criteria, 2nd ed.,
California State Quality Control Board, pp. 148-149.
MCKINNEY, R.E. & JERIS, J.S. (1955) Metabolism of low molecular
weight alcohols by activated sludge. Sewage ind. Wastes, 27(6):
728-735.
MCLAUGHIN, J., Jr, MARLIAC, J.P., VERRET, M.J., MUTCHLER, M.K., &
FITZHUGH, O.C. (1964) Toxicity of fourteen volatile chemicals as
measured by the chick embryo method. Am. Ind. Hyg. Assoc. J., 25:
282-284.
MAICKEL, R.P. & MCFADDEN, D.P. (1979) Acute toxicology of butyl
nitrites and butyl alcohols. Res. Commun. chem. Pathol. Pharmacol.,
26: 75-83.
MAICKEL, R.P. & NASH, J.F., Jr (1985) Differing effects of short-
chain alcohols on body temperature and coordinated muscular
activity in mice. Neuropharmacology, 24(1): 83-89.
MARCUS, R.J., WINTERS, W.D., & HULTIN, E. (1976) Neuro-
pharmacological effects induced by butanol, 4-hydroxy butyrate,
4-mercaptobutyric acid, thiolactone, tetrahydrofuran, pyrrolidine,
2-deoxy-d-glucose, and related substances in the rat.
Neuropharmacology, 15(1): 29-38.
MARKOVA, ET AL. (1962) J. food Sci., 27: 353.
MATTHEWS, J.S., SUGISAWA, H., & MACGREGOR, D.R. (1962) Flavour
spectrum of apple-wine volatiles. J. food Sci., 27: 355-362.
MATTSON, V.R., ARTHUR, J.W., & WALBRIDGE, C.T. (1976) Acute
toxicity of selected organic compounds to fathead minnows,
Duluth, Minnesota, US EPA Environmental Research Laboratory
(EPA No. 600/3-76-097).
MELLAN, I. (1950) Industrial solvents, New York, Van Nostrand
Reinhold Company, pp. 482-488.
MERRITT, A.D. & TOMKINS, G.M. (1959) Reversible oxidation of
cyclic secondary alcohols by liver alcohol dehydrogenase.
J. biol. Chem., 234: 2778.
MESLAR, H.W. & WHITE, H.B., III (1978) Preparation of lipid-free
protein extracts of egg yolk. Anal. Biochem., 91: 75-81.
MIKHEEV, M.I., FROLOVA, A.D., & LYUBLINA, E.I. (1977)
[Physicochemical properties and toxokinetics of some
representatives of a homologous series of alcohols.] Nek. Vopr.
Eksperim. Prom. Toksikol., 11-17 (in Russian).
MIZUTANI, Y., MIWA, Y., & MORIGUCHI, J. (1978) Jpn Kokai 78:
41,359, 14 Apr. 1978.
MONICH, J.A. (1968) Alcohols: their chemistry, properties, and
manufacture, New York, Amsterdam, London, Chapman and Reinhold.
MOREL, C. & CAVIGNEAUX, A. (1975) Alcohol isobutylique: fiche
toxicologique. Cah. notes doc., 80: 411-413.
MOSHLAKOVA, L.A., SOLDATCHENKOVA, T.P., & DRUZHININA, V.A. (1976)
Preliminary data of hygienic-chemical studies on the identification
of volatile substances released from poly(vinyl chloride) linoleum
plasticized with poly(dibutyl maleate). Gig. Aspecty Okhr.
Okruzhayushchei Sredy, 65-69.
MUIR, G.D., ed. (1977) Hazards in the chemical laboratory, 2nd
ed., London, The Chemical Society, p. 167.
MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters: narcotic
and lethal potencies to tadpoles and to rabbits. Ind. Med., 41(4):
31-33.
MUNCH, J.C. & SCHWARTZE, E.W. (1925) Narcotic and toxic potency
of aliphatic alcohols upon rabbits. J. lab. clin. Med., 10: 985-996.
NAZARENKO, I.V. (1969) [Maximum allowable concentrations for
butyl and isobutyl alcohol in drinking-water.] Sanit. ochrana
vodojemov at zagraznen. stochn. vodami. M, Medgiz, 4: 65-75
(in Russian).
NIOSH (1977a) Registry of toxic effects of chemical substances,
Rockville, Maryland, National Institute of Occupational Safety and
Health.
NIOSH (1977b) Manual of analytical methods, 2nd ed., Rockville,
Maryland, National Institute of Occupational Safety and Health,
Vol. 2.
NOVOKOVSKAYA, M.I., KLYUKVINA, T.D., KIRILLOVA, N.N., &
SHAPOSHNIKOV, YU.K. (1978) Composition of gaseous discharges
from drying oil production. Prom. Sanit. Ochistka Gazov, 5:
21-22.
NURSTEN, H.E. & WILLIAMS, A.A. (1967) Fruit aromas survey of
components identified. Chem. Ind., 12: 486-497.
OBE, G. & RISTOW, M.J. (1977) Acetaldehyde but not ethanol,
induces sister chromatid exchanges in Chinese hamster cell in
vitro. Mutat. Res., 55: 211-213.
OBE, G., RISTOW, M.J., & HERMA, J. (1977) Chromosomal damage by
alcohol in vitro and in vivo. Adv. exp. Med. Biol., 85a: 47-70.
OETTEL, H. (1936) [Effects of organic fluids on the skin.]
Arch. exp. Pathol. Pharmakol., 183: 641-696 (in German).
OLIAS JIMENEZ, J.M., DOBARGANES GARCIA, M.C., GUTIERREZ ROSALES, F.,
& GUTIERREZ GONZALES-QUIJANO, R. (1978) Volatile components in
the aroma of virgin olive oil. II. Identification sensorial
analysis of the chromatographic elements. Grasas Aceites (Seville),
29: 211-218.
PANGANAMALA, R.V., SHARMA, H.M., HEIKKILA, R.E., GEER, J.C., &
CORNWELL, D.G. (1976) Role of hydroxyl radical scavengers
dimethyl sulfoxide, alcohols, and methional in the inhibition of
prostaglandin biosynthesis. Prostaglandins, 11(4): 599-607.
PATTY, F.A., ed. (1963) Industrial hygiene and toxicology, 2nd
ed., New York, London, Sydney, John Wiley and Sons, Interscience
Publishers, Vol. 2, pp. 1441-1450.
PATTY, F.A. (1982) Industrial hygiene and toxicology, 3rd ed.,
New York, Chichester, Brisbane, Toronto, Singapore, Wiley-
Interscience, Vol. IIC, pp. 4571-4578.
PETROVA, N.I. & VISHEVSKII, A.A. (1972) [Course of pregnancy and
deliveries in women working in the organosilicon varnish and enamel
industries.] Nauch. Tr. Irkutsh. Med. Inst., 115: 102-106
(in Russian).
PITTER, P. (1976) Determination of biological degradability of
organic substances. Water Res., 10: 231-235.
POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic alcohols of
the stimulated activity of ornithine decarboxylase and tyrosine
aminotransferase occurring in regenerating rat liver. Biochem.
Pharmacol., 29(20): 2799-2803.
POSTEL, W. & ADAM, L. (1978) Gas chromatographic characterization
of whiskey. III. Irish whiskey. Brannt Wein Wirtschafft, 118:
404-407.
POSTEL, W., DRAWERT, F., & ADAM, L. (1975) [Flavourings in
brandies.] [Flavourings, International Symposium], 99-111
(in German).
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46(1): 63-77.
PURCHASE, F.H. (1969) Studies on Kaffircorn malting and brewing.
XII. The acute toxicity of some fusel oil found in Bantu beer.
S. Afr. med. J., 53: 795-462
PUSKIN, J.S. & MARTIN, T. (1978) Effects of anesthetics on
divalent cation binding and fluidity of phosphatidylserine
vescicles. Mol. Pharmacol., 14: 454-462.
REYNOLDS, T. (1977) Comparative effects of aliphatic compounds on
inhibition of lettuce fruit germination. Ann. Bot., 41(173):
637-648.
RIIHIMAKI, V. (1979) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand. J. Work
environ. Health, 5: 143-150.
ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. NY Acad. Sci., 273: 280-394.
RUMYANSTEV, A.P., GEER, V.G., OSTROUMOVA, N.A., SPIRIN, B.A., &
SHAKHIDZHANYAN, L.G. (1975) [Cumulative properties of butyl
alcohol.] Gig. i Sanit., 10: 112-113 (in Russian).
RUMYANSTEV, A.P., OSTROUMOVA, N.A., KUSTOVA, S.A., LOBANOVA, I.UA.,
TIUNOVA, L.V., DHERNIKOVA, V.V., & KOLESHNIKOV, P.A. (1976)
[Sanitary-toxicological features of butyl alcohol under conditions
of prolonged inhalation route entry.] Gig. i Sanit., 11: 12-15
(in Russian).
RUMYANSTEV, A.P., LOBANOVA, I.YA., TIUNOVA, L.V., & CHERNIKOVA, V.V.
(1979) [Toxicology of butyl alcohol.] Khim. Prom.-st. Ser.
Toksikol. Sanit. Khim. Plastmass, 2: 24-26 (in Russian).
SAAD, S.F. (1976) Effects of aliphatic alcohols on gamma-
aminobutyric acid levels in the cerebral hemispheres of rats. IRCS
med.-sci. Libr. Compend., 4: 499.
SAITO, M. (1975) [Studies on the metabolism of lower alcohols.]
Nichidai Igaku Zasshi, 34(8-9): 569-585 (in Japanese).
SAKAGAMI, Y., YOKOYAMA, H., KITANAKA, E., & IOLU, M. (1977) The
influence of phtalates on chick embryos. Osaka-furitsu Koshu
Eisei Kenkyusho Kenkyu Hokoku, Yakuji Shido Hen, 11: 15-20.
SANATINA, K.G. (1973) [Electrophysiological changes in the
peripheral neuromuscular function of persons exposed to toluene and
butyl alcohol vapors.] In: Gurghinas, S.V., ed. Vopr. Epidemiol.
Gig. Litov. SSR, Mater. Nauch. Konf. Ozdorevleniyu Vneshn. Sredy,
Nauchno-Issled., Institute of Epidemiology, Microbiology Gig.
Volnius, USSR, pp. 183-184 (in Russian).
SANDER, F. (1933) Cited by von Oettingen, W.F., Washington DC, US
Public Health Service (Public Health Bulletin No. 281) (1943).
SATLER, S.D. & THIMANN, K.V. (1980) The influence of aliphatic
alcohols on leaf senescences. Plant Physiol., 66: 395-399.
SAVELEV, A.I., BABANOV, A.G., SKOBEI, N.A., & TROITSKAYA, I.A.
(1975) [Adaptation reactions of white rats after prolonged
administration of small concentrations of butyl alcohol.] In:
Zaikina, M.G., ed. [Pathophysiology of the cardiovascular system,]
Yaroslav, Yaroslav Medical Institute, pp. 59-62, 76-80 (in Russian).
SCARAGLI, G.P., RIZZOTTI CONTI, M., BENCINI, R., DELLA CORTE, L., &
GIOTTI, A. (1975) [Toxicity of food additives. II. Membrane
damage produced by monocyclic compounds in the mitochondria and
lysosomes of rat's liver. Correlation between structure and action.]
Boll. Soc. Ital. Biol. Sper., 51: 1702-1706 (in Italian).
SCHREIER, P., DRAWERT, F., & SCHMID, M. (1978) Changes in the
composition of neutral volatile components during the production of
apple brandy. J. Sci. Food Agric., 29: 728-736.
SCHREIER, P., DRAWERT, F., & WINKLER, F. (1979) Composition of
neutral volatile constituents in grape brandies. J. agric. food
Chem., 27: 365-372.
SCHWARTZ, L. & TULIPAN, L. (1939) A textbook of occupational
diseases of the skin, Philadelphia, Pennsylvania, Lea & Febiger,
p. 717.
SEITZ, B., (1972) Vertiges graves apparus après manipulation de
butanol et d'isobutanol. A propos de trois cas. Arch. Mal. prof.
Méd. Trav. sécur. soc., 33: 393-395.
SERAFINI CESSI, S. (1975) Effects of alcohols on protein
synthetic activity in subcellular fractions from brain and liver of
rat. Arch. Sci. Biol., 59: 127-137.
SHALABY, E.S., DANASORY, M.El., & MASSOUD, A.A.E. (1973) Toxic
effects of fat solvents used in paints on liver, blood, and lung.
J. Egypt. Med. Assoc., 54: 340-347.
SHEHATA, M. & SAAD, S. (1978) The effect of aliphatic alcohols on
certain vitamins of the B-complex group in the liver of the rat.
Pol. J. Pharmacol. Pharm., 30: 35-39.
SHERMAN, P.D., Jr (1978) The butyl alcohols. In: Kirk-Othmer
Encyclopaedia of Chemical Technology, 3rd ed., Vol. 4, pp. 338-345.
SHOPSIS, C. & SATHE, S. (1984) Uridine uptake inhibition as a
cytotoxicity test: correlations with the Draize test. Toxicology,
29: 195-206.
SIEGERS, C.P., STRUBELT, O., & BREINING, H. (1974) The acute
hepatotoxicity of alcoholic beverages and some of their congeners
in guinea-pigs. Pharmacology, 12: 296-302.
SITANOV, V.S., BANDIK, K.A., ENAKAEVA, V.G., & BARANOVA, R.K.
(1979) Composition for removing grease and oil from textiles.
Otkrytiya, Izobert., Prom. Obraztsy, Tovaznye Znaky, 38: 90.
SMITH, C.W. & SIEGEL, S.M. (1975) Differential permeation of
Artemia cysts and cucumber seeds by alcohols. J. Histochem.
Cytochem., 23(1): 80-83.
SMUSIN, Y.S. & CHENSTOVA, T.F. (1973) Zdravookhr. Kaz., 4: 51-52.
SMYTH, H.F., Jr (1956) Hygienic standards for daily inhalation.
Cummings Memorial Lecture. Am. Ind. Hyg. Assoc., 17: 129-185.
SMYTH, H.F. & SMYTH, H.F., Jr (1928) Inhalation experiments with
certain lacquer solvents. J. ind. Hyg., 10: 261-271.
SMYTH, H.F., Jr, CARPENTER, C.P., & WEIL, C.S. (1951) Range-
finding toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4:
119-122.
SMYTH, H.F., Jr, CARPENTER, C.P., WEIL, C.S., & POZZANI, U.C.
(1954) Range-finding toxicity data: list V. Arch. ind. Hyg. occup.
Med., 10: 61-68.
SNELL, D. & HARRIS, R.A. (1980) Impairment of avoidance behaviour
following short-term ingestion of ethanol, tertiary-butanol, or
pentobarbital in mice. Psychopharmacology, 69(1): 53-57, 1980.
SODINI, G. & CANELLA, M. (1977) Extraction of phenols and
oligosaccharides from plant tissues (Span 445,653 1 Jun 1977).
STARK, D.M., SHOPSIS, C., BORENFREUND, E., & WALBERG, J. (1983)
Alternative approaches to the Draize assay-chemotaxis, cytology,
differentiation, and membrane transport studies. In: Goldberg, A.,
ed. Product safety and evaluation, New York, Mary Ann Liebert,
pp. 180-203.
STERNER, J.H., CROUCH, H.C., BROCKMYRE, H.F., & CUSACK, M. (1949)
A ten-year study of butyl alcohol exposure. Am. Ind. Hyg. Assoc. Q.,
10: 53-59.
SUGIYAMA, T., MIURA, R., & YAMANO, T. (1976) Purification and
properties of cytochrome P-450 from adrenocortical mithocondria and
its interaction with adrenodoxin. Adv. exp. Med. Biol., 74: 290-302.
SWORDS, G., BOBBIO, P.A., & HUNTER, G.L.K. (1978) Volatile
constituents of jack fruit. J. food Sci., 43: 639-640.
TABERSHAW, I.R., FAHY, J.P., & SKINNER, J.B. (1944) Industrial
exposure to butanol. J. ind. Hyg. Toxicol., 26: 328-330.
TAKEUCHI, H., KATADA, M., TAKAHASHI, M., KAWAMATA, S., & KOHARI, H.
(1978) Purification of polyolefins. Tokkyo Koho, 79: 126,291
1 Oct 1979.
TAVLINOVA, T.I. & DOVYBOROVA, L.N. (1979) Effect of aliphatic
alcohols on the crystal formation of hydrates of clinker minerals.
Isv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol., 22: 972-975.
TESCHKE, R., HAMASURA, Y., & LIEBER, C.S. (1974) NADPH-dependent
oxidation of methanol, ethanol, propanol, and butanol by hepatic
microsomes. Biochem. biophys. Res. Commun., 60: 851-857.
THEORELL, H. & BONNICHSEN, R. (1951) Studies on liver alcohol
dehydrogenase I. Equilibria and initial reaction velocities.
Acta. chem. Scand., 5: 1105-1126.
THOMAS, M., BOURA, A.L.A., & VIJAYAKUMAR, R. (1980) Prostaglandin
release by aliphatic alcohols from the rat isolated lung. Clin. exp.
Pharmacol. Physiol., 7(4): 373-381.
THURMAN, R.G., WINN, K., & URQUHART, B. (1980) Rat brain cyclic
AMP levels and withdrawal behaviour following treatment with
t-butanol. Adv. exp. Med. Biol., 126: 271-281.
TRAIGER, G.J. & BRUCKNER, J.V. (1976) The participation of 2
butanone in 2-butanol-induced potentiation of carbon tetrachloride
hepatotoxicity. J. Pharmacol. exp. Ther., 196: 493-500.
TRAIGER, G.J., BRUCKNER, J.V., & COOKE, P.H. (1975) Effect of
2-butanol and 2-butanone on rat hepatic ultrastructure and
microsomal drug metabolising enzyme activity. Toxicol. appl.
Pharmacol., 33(1): 132.
TRESSL, H., FRIESE, L., FENDESACK, F., & KOEPPLER, H. (1978)
Studies of the volatile composition of hops during storage.
J. agric. food Chem., 26: 1426-1430.
TSULAYA, V.R., MORENKOVA, N.V., VOLOKHOVA, L.E., & VORONIN, V.M.
(1978) [Description of the biological properties of small
concentrations of isobutyl alcohol.] Gig. i Sanit., 5: 6-9
(in Russian).
UK MAFF (1978) Food additives and contaminants. Committee
report on the review of solvents in food, London, United Kingdom
Ministry of Agriculture, Fisheries and Food.
US DHEW (1951) Survey of compounds which have been tested for
carcinogenic activity, 2nd ed., Washington DC, US Department of
Health, Education, and Welfare (Prepared by Hartwell, J.L. for the
US DHEW).
US DHEW (1978) Registry of toxic effects of chemicals, Washington
DC, US Department of Health, Education and Welfare.
US EPA (1980) Hazard information review, Washington DC, US
Environmental Protection Agency, p. 10.
VAN DEN BERG, A.P., NOORDHOEK, J., & KOOPMAN-KOOL, E. (1979a) The
relation between the sex-dependency of type I binding of
ethylmorphine and the 1-butanol-induced spectral change in mouse
liver microsomes. Biochem. Pharmacol., 28: 31-36.
VAN DEN BERG, A.P., NOORDHOEK, J., & KOOPMAN-KOOL, E. (1979b) The
use of competitive inhibition of substrate binding to cytochrome
P-450 in the determination of spectral dissociation constants for
substrates with multiple types of binding, as illustrated with
1-butanol. Biochem. Pharmacol., 28: 37-41.
VEITH, G.D., CALL, D.J., & BROOKE, L.T. (1981) Estimating the
acute toxicity of narcotic industrial chemicals to fathead
minnows, Philadelphia, Pennsylvania, American Society of Testing
and Materials (ASTM Special Technical Publication No. 802).
VELASQUEZ (1969) Audiologic impairment due to n-butyl alcohol
exposition. In: Proceedings of the International Congress on
Occupational Health, Tokyo, 1969.
VERSCHUEREN, K. (1977) Handbook of environmental data of organic
chemicals, New York, Van Nostrand Reinhold Company.
WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., & IIJIMA, M.
(1984) Induction of metamitochondria in the rat liver by n-propyl
alcohol and n-butyl alcohol. Acta pathol. Jpn., 34(3): 471-480.
WALLGREN, H. (1960) Relative intoxicating effects on rats of
ethyl, propyl, and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
WALLGREN, H., NIKANDER, P., BOGUSLAWSKY, P.V., & LINKOLA, J.
(1974) Effects of ethanol, tert-butanol, and clomethiazole on
net movements of sodium and potassium in electrically stimulated
cerebral tissue. Acta physiol. Scand., 91: 83-93.
WALTER, W. & WEIDEMANN, H.L. (1969) Coffee flavour compounds.
Z. Ernaehrungswiss, 9: 123-147.
WARTBURG, J.P. VON, BETHANE, J.L., & VALLEE, B.L. (1964) Human
liver alcohol dehydrogenase: kinetic and physiochemical properties.
Biochemistry, 3: 1775-1782.
WEESE, H. (1928) [Comparative studies on the efficacy and
toxicity of the vapours of low aliphatic alcohols.] Arch. exp.
Pathol. Pharmakol., 135: 118-130 (in German).
WEISBRODT, N.W., KIENZLE, M., & COOKE, A.R. (1973) Comparative
effects of aliphatic alcohols on the gastric mucosa. Proc. Soc. Exp.
Biol. Med., 142: 450-454.
WHO (1980) Twenty-third Report of the Joint FAO/WHO Expert
Committee on Food Additives, Geneva, World Health Organization
(WHO Technical Report Series 648).
WILLIAMS, R.T. (1969) Detoxication mechanisms, 2nd ed., London,
Chapman & Hall Ltd., p. 66.
WINER, A.D. (1958) A note of the substrate specificity of horse
liver alcohol dehydrogenase. Acta. chem. Scand., 12: 965.
WITKIN, J.M. & LEANDER, J.D. (1982) Effects of orally
administered ethanol and tert-butanol on fixed-ratio responding of
rats. Subst. Alcohol Actions/Misuse, 3: 275-279.
WOIDICH, H., PFANNHAUSER, W., & EBERHARDT, R. (1978) Results of
gas chromatographic-mass spectrographic studies of the volatile
components of applie brandies. Mitt. Hoeheren Bundeslehr-
Versuchsanst. Wein-Obstbau, Klosterneuberg, 28: 56-63.
WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.-Excerpta
Med., 440: 196-199.
WOOD, J.M. & LAVERTY, R. (1979) Physical dependence following
prolonged ethanol or t-butanol administration to rats. Pharmacol.
Biochem. Behav., 10: 113-119.
WOOD, J.N. & LAVERTY, R. (1976) Alcohol withdrawal syndrome
following prolonged 5-butanol administration to rats. Proc. Univ.
Otago Med. Sch., 54: 86-87.
YABUMOTO, K., YAMAGUCHI, M., & JENNINGS, W.G. (1978) Production
of volatile compounds by musk melon Cucumis melo. Food Chem.,
3: 7-16.
YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., & HABU, T.
(1978) Volatile flavor components of cooked rice. Agric. biol.
Chem., 42: 1229-1223.
YAMAZAKI, Y. & KATO, K. (1978) Penicillins or cephalosporins.
Jpn. Kokai Tokkio Koho, 78, 107, 484, 19 Sep. 1978.
YASHUDA, Y., CHABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
YASUDA-YASAKI, Y., NAMIKE-KANIE, S., & HACHISAKU, Y. (1978)
inhibition of germination of Bacillus subtilis spores by alcohols.
Spores, 7: 13-16.
YOJAY, L., YOJAY, R., & MARDONES, J. (1982) Acetone blood levels
after t-butanol administration in rats. IRCS Med. Sci., 10: 215.
ZAIKINA, E.I., TEREKHOVA, A.I., CHUDOV, L.N., SHATENSHTEIN, A.I.,
PETROV, E.S., SHCHERBAK, V.P., ZAKOMYRDIN, A.A., SIMETSKII, W.A., &
SOKHADZE, L.A. (1978) Repellent composition. Otkrytiya, Izobret.,
Prom. Obraztsy, Tovarnye, Znaky, 55: 18.
ZAIKOV, KH. & BOBEV, G. (1978) Chemical damages in the furniture
industry and morbidity with temporary loss of working capacity.
Khig. Zdraveopaz., 21: 141-147.
ZAMARAKHINA, L.E. (1973) [Determination of tert-butyl alcohol in
the air of industrial premises.] Gig. i Sanit., 38: 72-73
(in Russian)
When Kamil et al. (1953) administered isobutanol (25 mmol
total) by stomach tube to Chinchilla rabbits, 4.4% of the applied
dose was excreted within 24 h, as the glucuronide. After
administration of 6 ml isobutanol to the stomach of rabbits (Kamil
et al., 1953), no aldehydes or ketones were observed in the expired
air within 6 h.
Gaillard & Derache (1965) reported that, following an oral dose
of 2 g/kg body weight to rats, 0.27% was excreted in the urine
within 8 h.
The oxidation of isobutanol and other lower alcohols by both
rat liver homogenates and by the perfusion in situ of rat liver
was studied by Hedlund & Kiessling (1969). The authors observed
that the alcohols were oxidized at rates that decreased in the
following order: 1-propanol, isobutanol, ethanol, and isoamyl
alcohol. Furthermore, from their studies with the alcohol
dehydrogenase (ADH) inhibitor pyrazole, these authors concluded
that all the alcohols studied must be oxidized by ADH in the same
manner as ethanol in an NAD-mediated oxidation.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Some data on the toxicity of isobutanol for aquatic organisms
are given in Table 4. The high lethal concentrations (1000 - 4000
mg/litre) for fish, amphibia, and crustacea indicate that, at
background levels, isobutanol would not be disruptive for an
aquatic ecosystem.
Table 4. Toxicity of isobutanol for aquatic organisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Fish
Fresh-water species
Bleak 1000-3000 96-h LC50 Lindén et al.
(Alburnus (1979)
alburnus)
Golden orfe 1520 48-h LC50 Juhnke & Lüdemann
(Leuciscus (1978)
idus melanotus)
Goldfish 2600 24-h LC50 Bridié et al.
(Carassius (1979a)
auratus)
Amphibia
Tadpole 4000 threshold for Münch (1972)
(Rana sp) narcosis
Invertebrates
Fresh-water species
Water flea 1250 24-h EC50 immobilization Bringmann & Kuehn
(Daphnia magna) (1982)
Marine species
Brine shrimp 1400 24-h LC50 Price et al. (1974)
(Artemia salina) 3800 EC50 excystment Smith & Siegel
(1975)
------------------------------------------------------------------------------------------
Table 4. (contd.)
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Algae
Green algae
Scenedesmus 350 8-day no-observed- total biomass Bringmann & Kuehn
quadricauda adverse-effect level (1978a)
Blue-green algae
(Microcystis 290 8-day no-observed- total biomass Bringmann & Kuehn
aeruginosa) adverse-effect level (1978a)
------------------------------------------------------------------------------------------
7.2. Terrestrial Organisms
Toxicity studies in plants indicate that germination will not
be affected by exposure to isobutanol at background levels. An
EC50 of 760 mg/litre was reported by Reynolds (1977) for seed
germination in lettuce (Lactuca sativa). Smith & Siegel (1975)
found an EC50 of 40 800 mg/litre for seed germination in cucumber
(Cucumis sativus).
There is no information on terrestrial animals, but exposure is
unlikely to be significant, except locally after spills.
7.3. Microorganisms
Some data on the toxicity of isobutanol for microorganisms are
given in Table 5. Available toxicity data on protozoa bacteria and
algae suggest tolerance to exposure to isobutanol. No-observed-
adverse-effect levels for various species ranged from 22 to 1180
mg/litre). Exposure to background levels of isobutanol should not
be disruptive for the ecosystem.
Table 5. Toxicity of isobutanol for microorganisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Protozoa
Chilomonas paramaecium 22 48-h no-observed- total biomass Bringmann &
(flagellate) adverse-effect level Kuehn (1981)
Uronema parduczi 169 20-h no-observed- total biomass Bringmann &
(ciliate) adverse-effect level Kuehn (1981)
Entosiphon sulcatum 296 72-h no-observed- total biomass Bringmann &
(flagellate) adverse-effect level Kuehn (1981)
Bacteria
Pseudomonas putida 280 16-h no-observed- total biomass Bringmann &
adverse-effect level Kuehn (1976)
Bacillus subtilis 1180 EC50 spore Yasuda-Yasaki
germination et al. (1978)
------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Acute toxicity
The oral EC50 for narcosis in rabbits was 19 mmol isobutanol/kg
body weight (Münch, 1972).
Acute toxicity data are given in Table 6.
Table 6. Acute toxicity of isobutanol
---------------------------------------------------------------------
Species Route of Parameter Results Reference
administration
---------------------------------------------------------------------
Guinea- inhalation LC50 19 900 mg/m3 Kushneva et al.
pig (1983)
Mouse inhalation LC50 15 500 mg/m3 Kushneva et al.
(1983)
Rabbit inhalation LC50 26 250 mg/m3 Kushneva et al.
(1983)
Rat inhalation LCLoa 8000 mg/m3 (4 h) US DHEW (1978)
Rat inhalation LC50 19 200 mg/m3 Kushneva et al.
(1983)
Cat intravenous LDLoa 0.018 g/kg US DHEW (1978)
Rabbit oral LDLoa 3.75 g/kg US DHEW (1978)
Rabbit oral LD50 41 mmol/kg Münch (1972)
Mouse oral LD50 3.5 g/kg Kushneva et al.
(1983)
Rat oral LD50 3.1 g/kg Kushneva et al.
(1983)
Rat oral LD50 2.46 g/kg US DHEW (1978)
Rabbit skin LD50 4.24 g/kg US DHEW (1978)
---------------------------------------------------------------------
a Lo = lowest.
After ip administration of isobutanol to mice, the LD50
was > 1000 mg/kg at 30 min and 544 mg/kg at 7 days. Gross post-
mortem examination of livers showed an abnormal dark coloration
(Maickel & McFadden, 1979).
Smyth et al. (1954) reported 100% survival of rats exposed for
2 h to saturated isobutanol vapour (approximately 49 248 mg/m3 or
16 000 ppm) in air, but observed 2 deaths in a group of 6 rats when
they were exposed for 4 h to a concentration of 24 624 mg/m3 (8000
ppm) in air.
8.1.2. Signs of intoxication
The toxic effects of isobutanol are mainly alcoholic
intoxication and narcosis. Stupor and loss of voluntary movements
in animals can be considered signs of intoxication by the oral
route (Münch, 1972). Rats and rabbits were exposed, by inhalation,
for a period of 4 h to various concentrations of isobutanol. At a
concentration of 15 700 mg/m3, there was irritation of the airways.
Three days later, symptoms included central nervous depression, a
decreased number of lymphocytes in bone marrow, a decreased blood-
lactate level, delay in elimination of bromophthalein from blood,
and morphological changes including dystrophia of hepatocytes and
olfactory neurons in the brain. After exposure to 8000 mg/m3,
symptoms were similar but less severe. An isobutanol concentration
of 1300 mg/m3 decreased bone marrow lymphocyte numbers. A
concentration of 100 mg/m3 only altered breathing frequency
(Kushneva et al., 1983).
8.2. Skin and Eye Irritation
Application of 500 mg isobutanol to the skin of rabbits for
24 h was moderately irritating. However, application of 2 mg to
the rabbit eye caused severe irritation (US DHEW, 1978).
8.3. Short-Term Exposures
Hillbom et al. (1974a,b) gave one group of 6 male Wister rats
a 1 mol/litre solution of isobutanol as their sole drinking liquid
for a period of 4 months. Another group of 5 rats of the same
species was given a 2 mol/litre solution of isobutanol as their
sole drinking liquid for 2 months. Both groups were provided an
ad libitum diet of ordinary laboratory food containing about 26%
of calories as protein, 55% as carbohydrate, and 19% as fat. At
the end of the study, all animals were decapitated, and their
livers examined. No adverse effects were detected in the group
given the 1 mol/litre solution, while the rats in the group given
the 2 mol/litre solution showed a decrease in fat, glycogen, RNA,
and overall size of the liver cells.
Oral administration of between 1/10 and 1/5 of the LD50 of
isobutanol for 6 days/week over 1 month, did not result in any
deaths in rats (Kushneva et al., 1983). Continuous exposure of
rats, by inhalation, to isobutanol at 3 mg/m3 over 4 months,
caused an increased threshold for leg withdrawal response to
electrical stimulation, and depression of haemoglobin content,
erythrocyte count, and activities of cholinesterase and catalase
in the blood. The activities of alanine-amino transferase and
aspartate amino transferase were elevated. At 0.5 mg/m3, numbers
of white cells and erythrocytes, haemogloglobin content, and
cholinesterase activity were all decreased. No effects were
observed at 0.1 mg/m3 (Tsulaya et al., 1978).
8.4. Long-Term Exposures
With the exception of a carcinogenicity study, no long-term
studies have been reported.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data are available on reproduction or
embryotoxicity and no conclusions can therefore be drawn.
8.6. Mutagenicity
The only reported study on mutagenicity is that of Hillscher et
al. (1969) who demonstrated an increased rate of reverse mutation
when Escherichia coli CA 274 was treated with 0.7% isobutanol,
without metabolic activation. This study in itself is inadequate
to assess the mutagenic potential of the compounds.
8.7. Carcinogenicity
Two groups of 19 and 24 male and female Wistar rats (10 weeks
old) were administered purified isobutanol. Group one received
0.2 ml/kg body weight, orally, twice weekly. Group two received
0.05 ml/kg, subcutaneously, twice weekly. Two control groups (25
rats each) received 1 ml of 0.9% sodium chloride twice weekly
(controls for group one were dosed orally; controls for group two
were dosed subcutaneously). All rats were dosed until spontaneous
death. Test rats presented liver carcinomas and sarcomas, spleen
sarcomas, stomach proventricular carcinomas, and myeoid leukaemia.
Tumours of these types did not occur in the control groups (Table
7). In the exposed animals, toxic liver damage was found, ranging
from steatosis and cell necrosis to fibrosis and cirrhosis. In
addition, most exposed animals had hyperplasia of blood-forming
tissues. The carcinogenic response was attributed to isobutanol
(Gibel et al., 1974, 1975). No statistical analysis of the data
was performed.
Table 7. Summary of tumours in experimental animalsa
-------------------------------------------------------------------
Agent Application Number Average Malignant Benign
(twice weekly) of survival tumour tumour
animals (days)
-------------------------------------------------------------------
Control oral: 1 ml/kg 25 643 0 3
(0.9% NaCl) body weight
solution)
subcutaneous: 25 643 0 2
1 ml/kg body
weight
Isobutanol oral: 0.2 ml/kg 19 495 3b 9
body weight
subcutaneous: 24 544 8c 3
0.05 ml/kg
body weight
-------------------------------------------------------------------
a Adapted from: Gibel et al. (1975).
b Proventriculus carcinoma and liver cell carcinoma; proventriculus
carcinoma and myeloid leukaemia; myeloid leukaemia.
c Proventriculus carcinoma (2); liver sarcoma (2); spleen sarcoma;
mesothelioma; retroperitoneal sarcoma (2).
8.8. Special Studies
In 8 guinea-pigs, serum enzyme (SGOT, SGPT, GLDH) activities
and triglyceride levels were measured 16 h after oral
administration of 33.5 mg isobutanol/kg olive oil. There was no
statistically-significant difference between treated animals and 8
olive-oil treated controls (Siegers et al., 1974).
Isobutanol can potentiate the hepatic toxicity of carbon
tetrachloride (Cornish & Adefuin, 1967).
9. EFFECTS ON MAN
Isobutanol may be absorbed through the lungs and the
gastrointestinal tract (Browning, 1965).
No data are available on the effects of isobutanol on the skin.
However, as with other defatting solvents, isobutanol may cause
erythematous skin lesions (Schwarz & Tulipan, 1939).
Immersion of the hands of 5 healthy male volunteers in a
1:1 (v/v) mixture of m-xylene and isobutanol, for 15 min at
room temperature, caused only a mild and sometimes barely
distinguishable erythema or a burning feeling. Isobutanol (in a
1:1 mixture with m-xylene) dehydrated the skin and decreased the
absorption of xylene. However, when water was added as 8% of the
mixture, this effect was reduced. The dermal effects of isobutanol
only and its absorption were not studied (Riihimaki, 1979).
Seitz (1972) reported 7 case histories, occurring between
1965 and 1971, of workers who had been exposed to 1-butanol and
isobutanol in a non-ventilated photographic laboratory. They
handled the alcohols under intense and hot light, without any
precautions. Exposure levels were not quantified, but must have
been excessive; exposure time ranged from 1 1/2 months to 2 years.
Two workers had transient vertigo, 3 severe Meniere-like vertigo
with nausea, vomiting and/or headache. In one of these cases,
hearing was also perturbed. Two workers did not present any signs
or symptoms.
Eye irritation, blurred vision, and transient corneal
vacuolization have been decribed in another excessive (no
measurements done) mixed exposure of workers to isobutanol and
butyl acetate. With adequate work room ventilation, it did not
recur. The author felt that the more irritant butyl acetate would
be the main contributor to this effect (Büsing, 1952).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population to isobutanol
through food and beverages are not available and occupational
exposure levels are limited and inadequate.
10.1.2. Toxic effects
In animals, isobutanol is absorbed through the skin, lungs,
and gastrointestinal tract. Isobutanol is metabolized by alcohol
dehydrogenase to isobutyric acid via the aldehyde and may enter the
tricarboxylic acid cycle. Small amounts of isobutanol are excreted
unchanged (< 0.5% of the dose), or as the glucuronide (< 5% of
the dose) in the urine. In rabbits, metabolites found in the urine
include acetaldehyde, acetic acid, isobutyraldehyde, and isovaleric
acid. Oral LD50 values (2.5 - 3.1 g/kg body weight) and inhalation
LC50 (19.2 g/m3) in rats classify isobutanol as slightly toxic
according to Hodge & Sterner. The acute toxic effects are
alcoholic intoxication and narcosis. Isobutanol is severely
irritating to the eyes and moderately irritating to the skin. A
group of rats given a 1 mol/litre solution of isobutanol as their
sole drinking liquid for 4 months did not show any adverse effects
in the liver; another group given a 2 mol/litre solution as their
sole drinking liquid for 2 months showed a reduction in fat,
glycogen, and RNA content, and in the overall size of the cells in
the liver. Continuous inhalation exposure of rats to 3 mg/m3 for
4 months resulted in depression of leg withdrawal response to
electrical stimulation, minor changes of formed elements of the
blood and serum enzymes. The estimated no-observed-adverse-effect
level was 0.1 mg/m3.
In a lifetime carcinogenicity study, groups of rats received
isobutanol subcutaneously (0.05 ml/kg body weight twice a week) or
orally (0.2 ml/kg body weight twice a week). The animals exhibited
toxic liver damage ranging from steatosis to cirrhosis. Numbers of
animals showing malignant tumours totalled 8 in the subcutaneous
group, 3 in the oral group, and 0 in the control group. The
majority of treated animals also showed hyperplasia of blood-
forming tissues.
Because of lack of mutagenicity studies, the Task Group could
not determine whether isobutanol was a genetically active compound.
The findings in the carcinogenicity study are a cause for concern.
Because of methodological inadequacies and the manner of reporting
the data, it was not possible to determine whether isobutanol
should be regarded as an animal carcinogen. Thus it is not
possible to extrapolate from this study to possible long-term
effects in man.
From the animal studies available, it is not possible to
determine a no-observed-adverse-effect level for long-term
exposure. No adequate data are available to assess mutagenicity
or teratogenicity of isobutanol or effects on reproduction.
Exposure of the general population to isobutanol through food
and beverages is unlikely to lead to acute toxic effects. The only
reported observations in man relate to the production of vertigo
under conditions of severe and prolonged exposure to vapour
mixtures of isobutanol and 1-butanol. From this study, it is
not possible to attribute the vertigo to a single cause.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
Little quantitative data relating to levels in the general
environment are available, but, because isobutanol is readily
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillages.
10.2.2. Toxic effects
At background concentrations likely to occur in the
environment, isobutanol is not directly toxic for fish, amphibia,
crustacea, or algae. Protozoa will be tolerant to levels of
isobutanol likely to be found in the environment.
Isobutanol should be managed in the environment as a slightly
toxic compound. It poses an indirect hazard to the aquatic
environment, because it is readily biodegradable, which may lead to
oxygen depletion.
10.3. Conclusions
1. On the basis of available data, the Task Group considered it
unlikely that isobutanol would pose a serious acute health risk
to the general population under normal exposure conditions.
However, the Task Group was unable to make an assessment of the
long-term health risk of isobutanol for the general population.
It was concluded that the results of the carcinogenicity study
need verification by a bioassay of modern standards.
2. The Task Group considered that the data available were
inadequate to set an occupational exposure limit. In line with
good manufacturing practice, exposure to isobutanol should be
minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of isobutanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
1. The Task Group noted that, from the animal studies available,
it is not possible to determine a no-observed-adverse-effect
level. Relevant studies should be conducted so that this can
be achieved.
2. The Task Group considered that adequate studies should be
conducted to assess the mutagenicity and carcinogenicity of
isobutanol.
3. Epidemiological studies, including precise exposure data, would
assist in a better assessment of the occupational hazard of
isobutanol.
4. Additional information on environmental pathways (notably
emission and leaching) and residues are desirable.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Council of Europe (1981) included isobutanol in the list
of flavouring substances that can be added to foodstuffs without
hazard to public health at a level of 25 mg/kg for beverages and
food.
At their 23rd meeting, the Joint FAO/WHO Expert Committee on
Food Additives (JECFA) reviewed the data on isobutanol. They
concluded that:
"The evaluation of this compound was not possible
owing to the paucity of toxicological data. New
specifications were prepared, but no toxicological
monograph" (WHO, 1980).
REFERENCES
AARSTAD, K., ZAHLSEN, K., & NILSEN, O.G. (in press) Inhalation of
butanols: changes in the cytochrome P-450 enzyme system. In:
Proceedings of the European Society of Toxicology, 1984 Annual
Meeting.
ABBASOV, A.Z., PANOV, V.N., & ALIEV, A.M. (1971) [Determination
of a mixture of butyl alcohols in the air of industrial premises by
means of a chromatographic method.] Gig. i Sanit., 36: 61-63
(in Russian).
ABBONDANDOLO, A., BONATTI, S., CORSI, C., CORTI, G., FIORIO, R.,
LEPORINI, L., MAZZACCARO, A., & NIEIR, R. (1980) The use of
organic solvents in mutagenicity testing. Mutat. Res., 79: 141-150.
ACGIH, (1980) Documentation of the threshold limit values, 4th
ed., Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
AITIO, A. (1977) Inhibition of ethoxycumarin deethylation by
organic solvents. Res. Commun. chem. Pathol. Pharmacol., 1:
773-776.
AKHREM, A.A., POPOVA, E.M., & METELITSA, O.I. (1978) Interaction
of aliphatic alcohols with cytochrome P-450 from rat liver
microsomes. Biokhimya (USSR), 43: 1485-1491.
AMUNDSEN, J., GOODWIN, R.J., & WETZEL, W.H. (1979) Water-soluble
pentachlorophenol and tetrachlorophenol wood-treating systems.
S. African, 78: 01,031 (3 January 1979).
ANDERSON, R.A., REDDY, J.M., JOYCE, C., WILLIS, B.R., VAN DER VEN,
H., & ZANEVELD, L.J.D. (1982) Inhibition of mouse sperm
capacitation by ethanol. Biol. Reprod., 27: 833-840.
ANON (1983) Selection of priority compounds: emissions. In:
Publikatiereeks Lucht, The Hague, The Netherlands, Ministry of
Housing, Physical Planning and Environment, Vol. 10.
AOAC METHODS (1975) In: Horwitz, W., ed. Official methods of
analysis of the Association of Official Analytical Chemists,
Washington DC, Association of Official Analytical Chemists.
ARTIGAS GIMENEZ, G., URDANGARAY ARGUELLES, V., GONZALES BLASQUEZ,
I., & ALONSO RODRIGUEZ, J. (1979) Protective coating for glass
objects. Fr. Demande, 2: 410, 115 (23 March 1979).
ASHFORD, M.L.J. & WANN, K.T. (1979) A comparison of the effects
of butanol and benzyl alcohol on the frog end-plate conductance.
J. Physiol., 295: 86-87.
ASTM (1977) Testing methods for butanols. Annual book of
standards. Part 29, Philadelphia, Pennsylvania, American Society
for Testing and Materials (ASTM D304-58).
ASTRAND, I., OVRUM, P., LINDQUIST, T., & HULTENGREN, M. (1976)
Exposure to butyl alcohol: uptake and distribution in man. Scand.
J. Work Environ. Health, 3: 165-175.
AUTY, R.M. & BRANCH, R.A. (1976) The elimination of ethyl,
n-propyl, n-butyl, and iso-amyl alcohols by the isolated perfused
rat liver. J. Pharm. exp. Ther., 197: 669-674.
BAIKOV, B.K. & KHACHATURYAN, M.Kh. (1973) [Hygienic assessment of
the reflex action on a body of small concentrations of butyl
alcohol in the atmosphere.] Gig. i Sanit., 12: 7-11 (in Russian).
BAKER, R.C., SORENSON, S.M., ELOG, S.R., & DEITRICH, R.A. (1979)
Acetone excretion following t-butanol treatment in rats. In:
Proceedings of the 3rd International Symposium on Alcohol and
Aldehyde Metabolizing Systems, Toronto (Abstract No. 44).
BAKER, R.C., SORENSEN, S.M., & DEITRICH, R.A. (1982) The in vivo
metabolism of tertiary butanol by adult rat. Alcohol. clin. exp.
Res., 6(2): 247-251.
BARTHA, T., GONCZIL, L., & MOLLO, A. (1973) The use of metabolic
product analysis with simplified gas-liquid chromatographic methods
in routine anaerobic laboratory analysis. Egeszesegtodamany, 22:
220-227.
BEAUD, P. & RAMUZ, A. (1978) Gas-liquid chromatography of
simultaneous determination of higher alcohols and ethyl acetate in
spirits. Mitt. Geb. Lebensmittelunters. Hyg., 69: 423-30.
BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1981)
Liver lipid disposal following t-butanol administration to rats.
Chem.-biol. Interact., 38: 45-51.
BEAUGE, F., FLEURET, C., BARIN, F., & NORDMANN, R. (1984) Brain
membrane disordering after acute in vivo administration of ethanol,
isopropanol, or t-butanol in rats. Biochem. Pharmacol., 33:
3591-3593.
BELLIN, S.I. & EDMONDS, H.L., Jr (1976) The use of tert-butanol
in alcohol dependence studies. Proc. West. Pharmacol. Soc., 19:
351-354.
BENGTSSON, B.E., RENBERG, L., & TARKPEA, M. (1984) Molecular
structure and aquatic toxicity: an example with C1-C13 aliphatic
alcohols. Chemosphere, 13(5/6): 613-622.
BIKFALVI, I. & PASZTOR, L. (1977) Study on the components
distillates of wine using gas chromatography. Szeszipar, 25:
96-100.
BIRKETT, D.J. (1974) Interaction of some drugs, metal ions, and
alcohols with rat liver microsomes as studied with a fluorescent
probe. Clin. exp. Pharmacol. Physiol., 1: 415-427.
BLOK, J. (1981) [A simple toxicity test using nitrifying
bacteria.] H2 O, 14(11): 242-245 (in Dutch).
BLOOM, S.E. (1981) Detection of sister chromatid exchanges in
vivo using avian embryos. In: Cytogenetic assays of environmental
mutagens, T.C.H.S.U. ed: Allenheld, Osmun, Totowa, New Jersey.
BOBER, A. & HADDAWAY, L.W. (1963) Gas chromatographic
identification of alcoholic beverages. J. gas Chromatogr., 1: 8-13.
BONTE, W. (1978) Congener content of wine and similar beverages.
Blutalkohol, 15: 392-404.
BONTE, W. (1979) Congener substances in German and foreign
beers. Blutalkohol, 16: 108-124.
BONTE, W., DECKER, J., & BUSSE, J. (1978) Congener content of
high-proof alcoholic beverages. Blutalkohol, 16: 108-124.
BRAY, W.J. & HUMPHRIES, C. (1978) Solvent fractionation of leaf
juice to prepare green and white protein products. J. Sci. Food
Agric., 29: 839-846.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRINGMANN, G. & KUEHN, R. (1976) [Comparative findings concerning
the harmful effect of water pollutants on bacteria (Pseudomonas
putida) and blue-green algae (Microcystis aeruginosa).] GWF-
Wasser-Abwasser, 117(9): 410-413 (in German).
BRINGMANN, G. & KUEHN, R. (1977) Results of the damaging effect
of water pollutants on Daphnia magna. Z. Wasser Abwasser Forsch.,
10: 161-166.
BRINGMANN, G. & KUEHN, R. (1978a) [Limit values for the harmful
effect of water pollutants on blue-green algae (Microcystis
aeruginosa) and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Vom Wasser, 50: 45-60 (in German).
BRINGMANN, G. & KUEHN, R. (1978b) Testing of substances for their
toxicity threshold: model organisms Microcystis (Diplocystis)
aeruginosa and Scenedesmus quadriconda. Mitt. Int. Ver. Theor.
Angew. Limnol., 21: 275-284.
BRINGMANN, G. & KUEHN, R. (1981) [Comparison of the effect of
harmful substances on flagellates, on ciliates, on holozoic
bacteriophagic protozoa and on saprozoic protozoa.] GWF-Wasser-
Abwasser, 122(7): 308-312 (in German).
BRINGMANN, G. & KUEHN, R. (1982) [Findings concerning the
harmful effect of water pollutants on Daphnia magna in an advanced
standardized test procedure.] Z. Wasser Abwasser Forsch., 15(1):
1-6 (in German).
BRODERICK, J.J. (1976) Raspberry: a case history. Int. Flavours
Food Add., 7: 27-30.
BROWNING, E. (1965) Toxicology and metabolism of industrial
solvents, Amsterdam, Elsevier, p. 349.
BUSING, K.H. (1952) [Eye damage caused by butylacetate and
isobutylalcohol in a cable factory.] Zent. Arbeitsmed.
Arbeitsschutz, 2: 13-14 (in German).
CATER, B.R., COOK, M.W., GANGOLLI, S.D., & GRASSO, P. (1977)
Studies on dibutyl phthalate-induced testicular atrophy in the rat:
effect on zinc metabolism. Toxicol. appl. Pharmacol., 41: 609-614.
CEC (1981) Reports of the Scientific Committe for Food. XI.
Series, Luxembourg, Commission of the European Communities.
CEDERBAUM, A.I. & COHEN, G. (1980) Oxidative demethylation of
t-butyl alcohol by rat liver microsomes. Biochem. Biophys. Res.
Commun., 97(2): 730-736.
CEDERBAUM, A.I., DICKER, A.I., & COHEN, G. (1978) Effect of
hydroxyl radical scavanger on microsomal oxidation of alcohols and
on associated microsomal reaction. Biochemistry, 17: 3058-3064.
CEDERBAUM, A.I., DICHER, E., RUBIN, E., & COHEN, G. (1979)
Effect of thiourea on microsomal oxidation of alcohols and
associated microsomal functions. Biochemistry, 18: 1187-1191.
CEDERBAUM, A.I., QURESHI, A., & COHEN, G. (1983) Production of
formaldehyde and acetone by hydroxyl-radical generating systems
during the metabolism of tertiary butylalcohols. Biochem.
Pharmacol., 32(23): 3517-3524.
CHANG, T., LEWIS, J., & GLAZKO, A.J. (1967) Effects of ethanol
and other alcohols on the transport of amino acids and glucose by
everted sacs of rat small intestine. Biochim. Biophys. Acta, 135:
1000-1007.
CHANG, S.S., PETERSON, K.J., & HO, C. (1978) Chemical reactions
involved in the deep-fat frying of foods. J. Am. Oil. Chem. Soc.,
55: 718-727.
CHIOU, C.T., FREED, V.H., SCHMEDDING, D.W., & KOHNERT, R.L. (1977)
Partition coefficient and bioaccumulation of selected organic
chemicals. Environ. Sci. Technol., 11(5): 475-478.
CHOU, W.L., SPEECE, R.E., SIDDIQIE, R.H., & MCKEON, K. (1978a)
The effect of petrochemical structure on methane fermentation
toxicity. Prog. Water Technol., 10(5/6): 545-558.
CHOU, W.L., SPEECE, R.E., & SIDDIQIE, R.H. (1978b) Acclimation
and degradation of petrochemical wastewater components by methane
fermentation. In: Scott, C.D., ed. Proceedings of the First
Symposium on Biotechnology, Energy, Production, and Conservation,
New York, Interscience Publishers, Vol. 8, pp. 391-414.
CLARK, J.B. (1953) The mutagenic action of various chemicals on
Micrococcus aureus. Proc. Oklahoma Acad. Sci., 34: 114-118.
CLEGG, D.J. (1964) The hen egg in toxicity and teratogenicity
studies. Food Cosmetol. Toxicol., 2: 717-727.
CLUTTON, D.W. & EVANS, M.B. (1978) The flavour constituents of
gin. J. Chromatogr., 167: 409-419.
COGAN, D.G. & GRANT, W.M. (1945) An unusual type of keratitis
associated with exposure to n-butyl alcohol (butanol). Arch.
Ophthalmol., 33: 106-108.
CONNOR, T.H., THEISS, J.C., HANNA, H.A., MONTEITH, D.K., &
MATHEY, T.S. (1985) Genotoxicity of organic chemicals
frequently found in the air of mobile homes. Toxicol. Lett.,
25: 33-40.
CORNISH, H.H. & ADEFUIN, J. (1967) Potentiation of carbon
tetrachloride toxicity by aliphatic alcohols. Arch. environ.
Health, 14, 447-449.
COUNCIL OF EUROPE (1981) Flavouring substances and natural
sources of flavourings, 3rd ed., Strasbourg.
DANIEL, M.A. & EVANS, M.A. (1982) Quantitative comparison of
maternal ethanol and maternal tertiary butanol diet on postnatal
developments. J. Pharmacol. exp. Ther., 222(2): 294-300.
DE CEAURRIZ, J.G., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DE CEAURRIZ, J.C., DESILES, J.P., BONNET, P., MARIGNAC, B.,
MULLER, J., & GUENIER, J.P. (1982) Concentration-dependent
behavioural changes in mice following short-term inhalation
exposure to various industrial solvents. Toxicol. appl. Pharmacol.,
67: 383-389.
DE FELICE, A., WILSON, W., & AMBRE, J. (1976) Vasoactive effects
of methanol and sodium formate on isolated canine basilar artery.
Toxicol. appl. Pharmacol., 36: 515-601.
DERACHE, R. (1970) Toxicology, pharmacology, and metabolism of
higher alcohols. In: Tremalières, J., ed. International
encyclopedia of pharmacology and therapeutics. XX. Alcohols and
derivatives, Oxford, Pergamon Press, Vol. 2.
DICKEY, F.H., CLELAND, G.H., & LOTZ, C. (1949) The role of
organic perioxides in the induction of mutations. Proc. Natl Acad.
Sci. (USA), 35: 581.
DIETZ, F.K. (1980) The role of 2-butanol and 2-butanone
metabolism in the potentiation of carbon-tetrachloride induced
hepatotoxicity. Diss. Abstr. Int. B, 41(1): 150.
DIETZ, F.K. & TRAIGER, G.J. (1979) Potentiation of CCl4
hepatotoxicity in rats by a metabolite of 2-butanol: 2,3-
butanediol. Toxicology, 14: 209-215.
DIETZ, F.K., RODRIGUEZ-GIAXOLA, M., TRAIGER, G.J., STELLA, V.J., &
HIMMELSTEIN, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9(5): 553-576.
DI VINCENZO, G.D. & HAMILTON, M.L. (1979) Fate of n-butanol in
rats after oral administration and its uptake by dogs after
inhalation or skin application. Toxicol. appl. Pharmacol., 48:
317-325.
DMITRIEV, M.T. & LISHCHIKHIN, V.A. (1979) [Determination of
toxic substances given off by polymeric materials under
experimental conditions.] Gig. i Sanit., 6: 45-48 (in Russian).
DOOLITTLE, A.K. (1954) The technology of solvents and
plasticizers, New York, John Wiley and Sons, pp. 644-645.
DORE, M., BRUNET, N., & LEGUBE, B. (1974) Participation de
différents composés organiques à la valeur des critères globaux de
pollution. Trib. Cebedeau, 28(374): 3-11.
DREYFUS, M., VANDENBUNDER, R., & BUCH, H. (1978) Stabilization of
a phosphorilase b-active conformation by hydrophobic solvents.
FEBS Lett., 95: 185-189.
DUBINA, O.N. & MAKSIMOV, G.G. (1976) [Testing the use of golden
hamsters in toxicological research.] Gig. Tr. Ohkhr. Zdorov'ya Rab.
Neft. Neftekhim. Prom-sti, 9: 100-103 (in Russian).
DUDEK, B.C. & PHILIPS, T.J. (1983) Locomotor stimulant and
intoxicant proportion of methanol, ethanol, tert-butyl alcohol, and
pentobarbital in long-sleep and short-sleep mice. Subst. Alcohol
Actions/misuse, 4: 31-36.
DUMONT, J.P. & ADDA, J. (1978) Occurrence of sesquiterpones in
mountain cheese volatiles. J. agric. food Chem., 26: 364-367.
EDWARDS, E.K., Jr & EDWARDS, E.K. (1982) Allergic reaction to
tertiary butyl alcohol in a suncreen. Cutis, 29: 476-478.
EGOROV, Y.L. (1972) Dependance of dermal toxicity of alcohols on
solubility index: oil/water. Toksikol. Gig. Prod. Neftekhim Yarosl.,
98: 102.
FEDERAL REGISTER (1977) 6 May (42188, 23148).
FERRI, S.S. (1979) Scratch-resistant coating for lenses, etc.
Braz. Pedido PI, 78 05,443 (20 March 1979).
FISHER, G.S., LEGENDRE, M.G., LOVGREN, N.V., SCHULLER, W.H., &
WELLS, J.A. (1979) Volatile constituents of southern pea seed.
J. agric. food Chem., 27: 7-11.
FLATH, R.A. & TAKAHASHI, J.R. (1978) Volatile constituents of
prickly pear. J. agric. food Chem., 26: 835-837.
FLATH, R.A., FORREY, R.R., JOHN, J.O., & CHAN, B.G. (1978)
Volatile components of corn silk (Zea mays): possible Heliothis
zea (Boddie) attractants. J. agric. food Chem., 26: 1290-1293.
FORSANDER, O. (1967) Influence of some aliphatic alcohols on the
metabolism of rat liver slices. Biochem. J., 105: 93-97.
GAILLARD, D. & DERACHE, R. (1965) Métabolisation de différents
alcools présents dans les boissons alcooliques chez le rat. Trav.
Soc. Pharmacol. Montpellier, 25: 51-62.
GEPPERT, E. VON, STURZ, J., HAASE, W., & ISSELHARD, W. (1976)
[Effect of n-butanol on the metabolic status of certain rat organs
and on the circulation of the rabbit.] Arzneim. Forsch., 26:
1333-1337 (in German).
GERARDE, H.W. & AHLSTROM, D.B. (1966) The aspiration hazard and
toxicity of a homologous series of alcohols. Arch. environ. Health,
13: 457-461.
GERHOLD, R.M. & MALANEY, G.W. (1966) Structural determinants in
the oxidation of aliphatic compounds by activated sludge. J. Water
Pollut. Control Fed., 38(4): 562-579.
GERIKE, P. & FISCHER, W.K. (1979) A correlation study of
biodegradability determinations with various chemicals in various
tests. Ecotoxicol. environ. Saf., 3: 159-173.
GIBEL, W., LOHS, K.H., WILDNER, G.P., & SCHRAMM, T. (1974)
[Experimental research on the carcinogenic effect of higher
alcohols, using 3-methyl-1-butanol, 1-propanol and 2-methyl-1-
propanol as examples.] Z. exp. Chir., 7: 235-239 (in German).
GIBEL, W., LOHS, K.H., & WILDNER, G.P. (1975) [Experimental
research on the carcinogenic effect of solvents, using
propanol-1,2- methylpropanol-1 and 3-methyl- butanol-1 as
examples.] Arch. Geschwulstforsch., 45(1): 19-24 (in German).
GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952) Appraisal of
a chemical waste problem by fish toxicity tests. Sewage ind. Wastes,
24(11): 1397-1401.
GRANT, K.A. & SAMSON, H.H. (1981) Development of physical
dependence on t-butanol in rats: an examination using schedule-
induced drinking. Pharmacol. Biochem. Behav., 14: 633-637.
GRANT, K.A. & SAMSON, H.H. (1982) Ethanol and tertiairy butanol
induced microcephaly in the neonatal rat: comparison of brain
growth parameters. Neurobehav. Toxicol. Teratol., 4: 315-321.
GUKASYAN, ZH.G., BARYSHEVA, K.F., SAAKYAN, O.A., & ARUSTAMYAN, R.K.
(1979) Nauch. Soobshch. N.-i.Proekt.In-t Tsvet.Metallur-
gii.Armniprotsvetmet, 21: 18-21.
HALL, R.L. & OSER, B.L. (1965) Recent progress in the
consideration of flavouring ingredients under the food additives
amendement. III. Gras substances. Food Technol., 151.
HARRIS, R.N. & ANDERS, M.W. (1980) Effect of fasting, diethyl
maleate, and alcohols on carbon tetrachloride-induced
hepatotoxicity. Toxicol. appl. Pharmacol., 56(2): 191-198.
HEDLUND, S.G. & KIESSLING, K.H. (1969) The physiological
mechanism involved in hangover. I. The oxidation of some lower
aliphatic fusel alcohols and aldehydes in rat liver and their
effect on the mitochondrial oxidation of various substrates.
Acta pharmacol. toxicol., 27: 381-396.
HESKETH, T.R., KEIGHTLEY, C.A., METCALFE, J.C., & RICHARDS, C.D.
(1978) Long-chain alcohols (C10-C12) can block nerve impulse.
J. Physiol., 278: 5-6.
HILL, M.W., NEALE, E., & BANEHAM, A.D. (1981) Acute tolerance to
the effects of n-butanol and n-hexanol in goldfish. J. comp.
Physiol., 142: 61-65.
HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974a) Effects
of chronic ingestion of some lower aliphatic alcohols in rats.
Res. Commun. chem. Pathol. Pharmacol., 9(1): 177-180.
HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974b) Effects
of chronic ingestion of some lower aliphatic alcohols in rats.
Jpn. J. Stud. Alcohol, 9(2): 101-108.
HILSCHER, H., GEISSLER, E., & GIBEL, W. (1969) [Research on the
toxicity and mutagenicity of certain fusel oil components in
E. coli.] Acta biol. med. Germ., 23: 843-852 (in German).
HODGE, H.C. & STERNER, J.H. (1943) Tabulation of toxicity
classes. Am. Ind. Hyg. Assoc. Q., 10: 93-96.
IARC (1984) Information bulletin on the survey of chemicals
being tested for carcinogenicity, Lyons, International Agency
for Research on Cancer, Vol. 11.
ILO (1977) Occupational exposure limits for airborne toxic
substances, Geneva, International Labour Office (Occupational
Safety and Health Series No. 37).
JADDOU, H.A., PAVEY, J.A., & MANNING, D.J. (1978) Chemical
analysis of flavor volatiles in heat-treated milks. J. dairy
Res., 45: 391-403.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L., & FITZHUGH,
D.G. (1964) Food flavourings and compounds of related structure.
I. Acute oral toxicity. Food Cosmet. Toxicol., 2: 327.
JONES, H.R. (1971) Environmental control in the organic and
petrochemical industries, Park Ridge, New Jersey, Noyes Data.
JUHNKE, I. & LUEDEMANN, D. (1978) [Results of the testing of 200
chemical compounds for acute toxicity in fish by the orfe test.]
Z. Wasser-Abwasser-Forsch., 11(5): 161-164 (in German).
JULIANO, R.L. & GAGALANG, E. (1979) The effect of membrane-
fluidizing agents on the adhesion of CHO cells. J. cell Physiol.,
98: 483-490.
KABAYASHI, H., MIYOSHI, Y., & KITAMURA, K. (1977) Effects of
various alcohols on the intramuscular absorption of isonicotinamide
in the rat. Chem. pharm. Bull., 25(11): 3078-3080.
KALEKIN, R.M. & BRICHENKO, V.S. (1972) [Toxic effect of products
from the production of butyl alcohols on the central nervous
system.] Nauch. Tr. Irkutsk. Med. Inst., 115: 22-25 (in Russian).
KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) Studies in
detoxication. The metabolism of aliphatic alcohols. The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochem. J., 53:
129-136.
KIESER, M.E., POLLARD, A., STEVENS, P.M., & TUCKNOTT, O.G. (1964)
Determination of 2-phenylethanol in cider. Nature (Lond.), 204: 887.
KOLESNIKOV, P.A. (1975) [Adaptation to butyl alcohol.] Gig i
Sanit., (5): 104-105 (in Russian).
KUDREWICZ-HUBICKA, Z., KOLACZKOWSKA, M., BORZEMSKA, & WESOLOWSKA, A.
(1978) [Serum antitrypsin activity and glycoprotein level in
workers exposed to organic solvents.] Pol. Tyg. Lek., 33, 395-397
(in Polish).
KUSHNEVA, V.S., KOLOSKOVA, G.A., KOLTUNOVA, J.G., & KIRILENKO, V.T.
(1983) Experimental data to hygienic reglementation of
isobutylalcohol in the working zone. Gig. Tr. Prof. Zabol., 1:
46-47.
LALASIDIS, G. & SJOBERG, L.B. (1978) Two new methods of
debittering protein hydrolysates and a fraction of hydrolysates
with exceptionally high content of essential amino acids. J. agric.
food Chem., 26: 742-749.
LAMMENS, H. & VERZELE, M. (1968) Aroma of hops. II. The
composition of hop oil. J. Inst. Brew., 74: 341-346.
LENDLE, L. (1928) [Investigations on the speed at which
homologous and isomeric monovalent alcohols produce narcosis.]
Naunyn-Schmiedeberg's Arch. exp. Pathol. Pharmakol., 129: 85
(in German).
LIEBICH, H.M., DOUGLAS, D.R., BAYER, E., & ZLATKIS, A. (1970)
Volatile flavour components of Cheddar cheese. J. chromatogr. Sci.,
8: 355-359.
LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROEM, G. (1979)
The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the bleak (Alburnus alburnus)
and the harpaticoid (Nitocra spinipes). Chemosphere, 8(11/12):
843-851.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975) Detection
of carcinogens as mutagens in the Salmonella/microsome test: assay
of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72: 5735-5739.
MCCOMB, J.A. & GOLDSTEIN, D.B. (1979) Quantitative comparison of
physical dependence on tertiary butanol and ethanol in mice:
correlation with lipid solubility. J. Pharmacol. exp. Ther.,
208(1): 113-117.
MCCREERY, N.J. & HUNT, W.A. (1978) Physico-chemical correlates of
alcohol intoxication. Neuropharmacology, 17: 451-461.
MCGLUMPHY, J.H. (1951) Fruit flavours. Food Technol., 5: 353-355.
MCGREGOR, D.C., SCHONBAUM, E., & BIGELOW, W.G. (1964) Acute
toxicity studies on ethanol, propanol, and butanol. Can. J. Physiol.
Pharmacol., 42: 689-696.
MACHT, D.I. (1920) A toxicological study of some alcohols, with
special reference to isomers. J. Pharmacol. exp. Ther., 16: 1-10.
MCKEE, J.E. & WOLF, H.W. (1963) Water quality criteria, 2nd ed.,
California State Quality Control Board, pp. 148-149.
MCKINNEY, R.E. & JERIS, J.S. (1955) Metabolism of low molecular
weight alcohols by activated sludge. Sewage ind. Wastes, 27(6):
728-735.
MCLAUGHIN, J., Jr, MARLIAC, J.P., VERRET, M.J., MUTCHLER, M.K., &
FITZHUGH, O.C. (1964) Toxicity of fourteen volatile chemicals as
measured by the chick embryo method. Am. Ind. Hyg. Assoc. J., 25:
282-284.
MAICKEL, R.P. & MCFADDEN, D.P. (1979) Acute toxicology of butyl
nitrites and butyl alcohols. Res. Commun. chem. Pathol. Pharmacol.,
26: 75-83.
MAICKEL, R.P. & NASH, J.F., Jr (1985) Differing effects of short-
chain alcohols on body temperature and coordinated muscular
activity in mice. Neuropharmacology, 24(1): 83-89.
MARCUS, R.J., WINTERS, W.D., & HULTIN, E. (1976) Neuro-
pharmacological effects induced by butanol, 4-hydroxy butyrate,
4-mercaptobutyric acid, thiolactone, tetrahydrofuran, pyrrolidine,
2-deoxy-d-glucose, and related substances in the rat.
Neuropharmacology, 15(1): 29-38.
MARKOVA, ET AL. (1962) J. food Sci., 27: 353.
MATTHEWS, J.S., SUGISAWA, H., & MACGREGOR, D.R. (1962) Flavour
spectrum of apple-wine volatiles. J. food Sci., 27: 355-362.
MATTSON, V.R., ARTHUR, J.W., & WALBRIDGE, C.T. (1976) Acute
toxicity of selected organic compounds to fathead minnows,
Duluth, Minnesota, US EPA Environmental Research Laboratory
(EPA No. 600/3-76-097).
MELLAN, I. (1950) Industrial solvents, New York, Van Nostrand
Reinhold Company, pp. 482-488.
MERRITT, A.D. & TOMKINS, G.M. (1959) Reversible oxidation of
cyclic secondary alcohols by liver alcohol dehydrogenase.
J. biol. Chem., 234: 2778.
MESLAR, H.W. & WHITE, H.B., III (1978) Preparation of lipid-free
protein extracts of egg yolk. Anal. Biochem., 91: 75-81.
MIKHEEV, M.I., FROLOVA, A.D., & LYUBLINA, E.I. (1977)
[Physicochemical properties and toxokinetics of some
representatives of a homologous series of alcohols.] Nek. Vopr.
Eksperim. Prom. Toksikol., 11-17 (in Russian).
MIZUTANI, Y., MIWA, Y., & MORIGUCHI, J. (1978) Jpn Kokai 78:
41,359, 14 Apr. 1978.
MONICH, J.A. (1968) Alcohols: their chemistry, properties, and
manufacture, New York, Amsterdam, London, Chapman and Reinhold.
MOREL, C. & CAVIGNEAUX, A. (1975) Alcohol isobutylique: fiche
toxicologique. Cah. notes doc., 80: 411-413.
MOSHLAKOVA, L.A., SOLDATCHENKOVA, T.P., & DRUZHININA, V.A. (1976)
Preliminary data of hygienic-chemical studies on the identification
of volatile substances released from poly(vinyl chloride) linoleum
plasticized with poly(dibutyl maleate). Gig. Aspecty Okhr.
Okruzhayushchei Sredy, 65-69.
MUIR, G.D., ed. (1977) Hazards in the chemical laboratory, 2nd
ed., London, The Chemical Society, p. 167.
MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters: narcotic
and lethal potencies to tadpoles and to rabbits. Ind. Med., 41(4):
31-33.
MUNCH, J.C. & SCHWARTZE, E.W. (1925) Narcotic and toxic potency
of aliphatic alcohols upon rabbits. J. lab. clin. Med., 10: 985-996.
NAZARENKO, I.V. (1969) [Maximum allowable concentrations for
butyl and isobutyl alcohol in drinking-water.] Sanit. ochrana
vodojemov at zagraznen. stochn. vodami. M, Medgiz, 4: 65-75
(in Russian).
NIOSH (1977a) Registry of toxic effects of chemical substances,
Rockville, Maryland, National Institute of Occupational Safety and
Health.
NIOSH (1977b) Manual of analytical methods, 2nd ed., Rockville,
Maryland, National Institute of Occupational Safety and Health,
Vol. 2.
NOVOKOVSKAYA, M.I., KLYUKVINA, T.D., KIRILLOVA, N.N., &
SHAPOSHNIKOV, YU.K. (1978) Composition of gaseous discharges
from drying oil production. Prom. Sanit. Ochistka Gazov, 5:
21-22.
NURSTEN, H.E. & WILLIAMS, A.A. (1967) Fruit aromas survey of
components identified. Chem. Ind., 12: 486-497.
OBE, G. & RISTOW, M.J. (1977) Acetaldehyde but not ethanol,
induces sister chromatid exchanges in Chinese hamster cell in
vitro. Mutat. Res., 55: 211-213.
OBE, G., RISTOW, M.J., & HERMA, J. (1977) Chromosomal damage by
alcohol in vitro and in vivo. Adv. exp. Med. Biol., 85a: 47-70.
OETTEL, H. (1936) [Effects of organic fluids on the skin.]
Arch. exp. Pathol. Pharmakol., 183: 641-696 (in German).
OLIAS JIMENEZ, J.M., DOBARGANES GARCIA, M.C., GUTIERREZ ROSALES, F.,
& GUTIERREZ GONZALES-QUIJANO, R. (1978) Volatile components in
the aroma of virgin olive oil. II. Identification sensorial
analysis of the chromatographic elements. Grasas Aceites (Seville),
29: 211-218.
PANGANAMALA, R.V., SHARMA, H.M., HEIKKILA, R.E., GEER, J.C., &
CORNWELL, D.G. (1976) Role of hydroxyl radical scavengers
dimethyl sulfoxide, alcohols, and methional in the inhibition of
prostaglandin biosynthesis. Prostaglandins, 11(4): 599-607.
PATTY, F.A., ed. (1963) Industrial hygiene and toxicology, 2nd
ed., New York, London, Sydney, John Wiley and Sons, Interscience
Publishers, Vol. 2, pp. 1441-1450.
PATTY, F.A. (1982) Industrial hygiene and toxicology, 3rd ed.,
New York, Chichester, Brisbane, Toronto, Singapore, Wiley-
Interscience, Vol. IIC, pp. 4571-4578.
PETROVA, N.I. & VISHEVSKII, A.A. (1972) [Course of pregnancy and
deliveries in women working in the organosilicon varnish and enamel
industries.] Nauch. Tr. Irkutsh. Med. Inst., 115: 102-106
(in Russian).
PITTER, P. (1976) Determination of biological degradability of
organic substances. Water Res., 10: 231-235.
POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic alcohols of
the stimulated activity of ornithine decarboxylase and tyrosine
aminotransferase occurring in regenerating rat liver. Biochem.
Pharmacol., 29(20): 2799-2803.
POSTEL, W. & ADAM, L. (1978) Gas chromatographic characterization
of whiskey. III. Irish whiskey. Brannt Wein Wirtschafft, 118:
404-407.
POSTEL, W., DRAWERT, F., & ADAM, L. (1975) [Flavourings in
brandies.] [Flavourings, International Symposium], 99-111
(in German).
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46(1): 63-77.
PURCHASE, F.H. (1969) Studies on Kaffircorn malting and brewing.
XII. The acute toxicity of some fusel oil found in Bantu beer.
S. Afr. med. J., 53: 795-462
PUSKIN, J.S. & MARTIN, T. (1978) Effects of anesthetics on
divalent cation binding and fluidity of phosphatidylserine
vescicles. Mol. Pharmacol., 14: 454-462.
REYNOLDS, T. (1977) Comparative effects of aliphatic compounds on
inhibition of lettuce fruit germination. Ann. Bot., 41(173):
637-648.
RIIHIMAKI, V. (1979) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand. J. Work
environ. Health, 5: 143-150.
ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. NY Acad. Sci., 273: 280-394.
RUMYANSTEV, A.P., GEER, V.G., OSTROUMOVA, N.A., SPIRIN, B.A., &
SHAKHIDZHANYAN, L.G. (1975) [Cumulative properties of butyl
alcohol.] Gig. i Sanit., 10: 112-113 (in Russian).
RUMYANSTEV, A.P., OSTROUMOVA, N.A., KUSTOVA, S.A., LOBANOVA, I.UA.,
TIUNOVA, L.V., DHERNIKOVA, V.V., & KOLESHNIKOV, P.A. (1976)
[Sanitary-toxicological features of butyl alcohol under conditions
of prolonged inhalation route entry.] Gig. i Sanit., 11: 12-15
(in Russian).
RUMYANSTEV, A.P., LOBANOVA, I.YA., TIUNOVA, L.V., & CHERNIKOVA, V.V.
(1979) [Toxicology of butyl alcohol.] Khim. Prom.-st. Ser.
Toksikol. Sanit. Khim. Plastmass, 2: 24-26 (in Russian).
SAAD, S.F. (1976) Effects of aliphatic alcohols on gamma-
aminobutyric acid levels in the cerebral hemispheres of rats. IRCS
med.-sci. Libr. Compend., 4: 499.
SAITO, M. (1975) [Studies on the metabolism of lower alcohols.]
Nichidai Igaku Zasshi, 34(8-9): 569-585 (in Japanese).
SAKAGAMI, Y., YOKOYAMA, H., KITANAKA, E., & IOLU, M. (1977) The
influence of phtalates on chick embryos. Osaka-furitsu Koshu
Eisei Kenkyusho Kenkyu Hokoku, Yakuji Shido Hen, 11: 15-20.
SANATINA, K.G. (1973) [Electrophysiological changes in the
peripheral neuromuscular function of persons exposed to toluene and
butyl alcohol vapors.] In: Gurghinas, S.V., ed. Vopr. Epidemiol.
Gig. Litov. SSR, Mater. Nauch. Konf. Ozdorevleniyu Vneshn. Sredy,
Nauchno-Issled., Institute of Epidemiology, Microbiology Gig.
Volnius, USSR, pp. 183-184 (in Russian).
SANDER, F. (1933) Cited by von Oettingen, W.F., Washington DC, US
Public Health Service (Public Health Bulletin No. 281) (1943).
SATLER, S.D. & THIMANN, K.V. (1980) The influence of aliphatic
alcohols on leaf senescences. Plant Physiol., 66: 395-399.
SAVELEV, A.I., BABANOV, A.G., SKOBEI, N.A., & TROITSKAYA, I.A.
(1975) [Adaptation reactions of white rats after prolonged
administration of small concentrations of butyl alcohol.] In:
Zaikina, M.G., ed. [Pathophysiology of the cardiovascular system,]
Yaroslav, Yaroslav Medical Institute, pp. 59-62, 76-80 (in Russian).
SCARAGLI, G.P., RIZZOTTI CONTI, M., BENCINI, R., DELLA CORTE, L., &
GIOTTI, A. (1975) [Toxicity of food additives. II. Membrane
damage produced by monocyclic compounds in the mitochondria and
lysosomes of rat's liver. Correlation between structure and action.]
Boll. Soc. Ital. Biol. Sper., 51: 1702-1706 (in Italian).
SCHREIER, P., DRAWERT, F., & SCHMID, M. (1978) Changes in the
composition of neutral volatile components during the production of
apple brandy. J. Sci. Food Agric., 29: 728-736.
SCHREIER, P., DRAWERT, F., & WINKLER, F. (1979) Composition of
neutral volatile constituents in grape brandies. J. agric. food
Chem., 27: 365-372.
SCHWARTZ, L. & TULIPAN, L. (1939) A textbook of occupational
diseases of the skin, Philadelphia, Pennsylvania, Lea & Febiger,
p. 717.
SEITZ, B., (1972) Vertiges graves apparus après manipulation de
butanol et d'isobutanol. A propos de trois cas. Arch. Mal. prof.
Méd. Trav. sécur. soc., 33: 393-395.
SERAFINI CESSI, S. (1975) Effects of alcohols on protein
synthetic activity in subcellular fractions from brain and liver of
rat. Arch. Sci. Biol., 59: 127-137.
SHALABY, E.S., DANASORY, M.El., & MASSOUD, A.A.E. (1973) Toxic
effects of fat solvents used in paints on liver, blood, and lung.
J. Egypt. Med. Assoc., 54: 340-347.
SHEHATA, M. & SAAD, S. (1978) The effect of aliphatic alcohols on
certain vitamins of the B-complex group in the liver of the rat.
Pol. J. Pharmacol. Pharm., 30: 35-39.
SHERMAN, P.D., Jr (1978) The butyl alcohols. In: Kirk-Othmer
Encyclopaedia of Chemical Technology, 3rd ed., Vol. 4, pp. 338-345.
SHOPSIS, C. & SATHE, S. (1984) Uridine uptake inhibition as a
cytotoxicity test: correlations with the Draize test. Toxicology,
29: 195-206.
SIEGERS, C.P., STRUBELT, O., & BREINING, H. (1974) The acute
hepatotoxicity of alcoholic beverages and some of their congeners
in guinea-pigs. Pharmacology, 12: 296-302.
SITANOV, V.S., BANDIK, K.A., ENAKAEVA, V.G., & BARANOVA, R.K.
(1979) Composition for removing grease and oil from textiles.
Otkrytiya, Izobert., Prom. Obraztsy, Tovaznye Znaky, 38: 90.
SMITH, C.W. & SIEGEL, S.M. (1975) Differential permeation of
Artemia cysts and cucumber seeds by alcohols. J. Histochem.
Cytochem., 23(1): 80-83.
SMUSIN, Y.S. & CHENSTOVA, T.F. (1973) Zdravookhr. Kaz., 4: 51-52.
SMYTH, H.F., Jr (1956) Hygienic standards for daily inhalation.
Cummings Memorial Lecture. Am. Ind. Hyg. Assoc., 17: 129-185.
SMYTH, H.F. & SMYTH, H.F., Jr (1928) Inhalation experiments with
certain lacquer solvents. J. ind. Hyg., 10: 261-271.
SMYTH, H.F., Jr, CARPENTER, C.P., & WEIL, C.S. (1951) Range-
finding toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4:
119-122.
SMYTH, H.F., Jr, CARPENTER, C.P., WEIL, C.S., & POZZANI, U.C.
(1954) Range-finding toxicity data: list V. Arch. ind. Hyg. occup.
Med., 10: 61-68.
SNELL, D. & HARRIS, R.A. (1980) Impairment of avoidance behaviour
following short-term ingestion of ethanol, tertiary-butanol, or
pentobarbital in mice. Psychopharmacology, 69(1): 53-57, 1980.
SODINI, G. & CANELLA, M. (1977) Extraction of phenols and
oligosaccharides from plant tissues (Span 445,653 1 Jun 1977).
STARK, D.M., SHOPSIS, C., BORENFREUND, E., & WALBERG, J. (1983)
Alternative approaches to the Draize assay-chemotaxis, cytology,
differentiation, and membrane transport studies. In: Goldberg, A.,
ed. Product safety and evaluation, New York, Mary Ann Liebert,
pp. 180-203.
STERNER, J.H., CROUCH, H.C., BROCKMYRE, H.F., & CUSACK, M. (1949)
A ten-year study of butyl alcohol exposure. Am. Ind. Hyg. Assoc. Q.,
10: 53-59.
SUGIYAMA, T., MIURA, R., & YAMANO, T. (1976) Purification and
properties of cytochrome P-450 from adrenocortical mithocondria and
its interaction with adrenodoxin. Adv. exp. Med. Biol., 74: 290-302.
SWORDS, G., BOBBIO, P.A., & HUNTER, G.L.K. (1978) Volatile
constituents of jack fruit. J. food Sci., 43: 639-640.
TABERSHAW, I.R., FAHY, J.P., & SKINNER, J.B. (1944) Industrial
exposure to butanol. J. ind. Hyg. Toxicol., 26: 328-330.
TAKEUCHI, H., KATADA, M., TAKAHASHI, M., KAWAMATA, S., & KOHARI, H.
(1978) Purification of polyolefins. Tokkyo Koho, 79: 126,291
1 Oct 1979.
TAVLINOVA, T.I. & DOVYBOROVA, L.N. (1979) Effect of aliphatic
alcohols on the crystal formation of hydrates of clinker minerals.
Isv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol., 22: 972-975.
TESCHKE, R., HAMASURA, Y., & LIEBER, C.S. (1974) NADPH-dependent
oxidation of methanol, ethanol, propanol, and butanol by hepatic
microsomes. Biochem. biophys. Res. Commun., 60: 851-857.
THEORELL, H. & BONNICHSEN, R. (1951) Studies on liver alcohol
dehydrogenase I. Equilibria and initial reaction velocities.
Acta. chem. Scand., 5: 1105-1126.
THOMAS, M., BOURA, A.L.A., & VIJAYAKUMAR, R. (1980) Prostaglandin
release by aliphatic alcohols from the rat isolated lung. Clin. exp.
Pharmacol. Physiol., 7(4): 373-381.
THURMAN, R.G., WINN, K., & URQUHART, B. (1980) Rat brain cyclic
AMP levels and withdrawal behaviour following treatment with
t-butanol. Adv. exp. Med. Biol., 126: 271-281.
TRAIGER, G.J. & BRUCKNER, J.V. (1976) The participation of 2
butanone in 2-butanol-induced potentiation of carbon tetrachloride
hepatotoxicity. J. Pharmacol. exp. Ther., 196: 493-500.
TRAIGER, G.J., BRUCKNER, J.V., & COOKE, P.H. (1975) Effect of
2-butanol and 2-butanone on rat hepatic ultrastructure and
microsomal drug metabolising enzyme activity. Toxicol. appl.
Pharmacol., 33(1): 132.
TRESSL, H., FRIESE, L., FENDESACK, F., & KOEPPLER, H. (1978)
Studies of the volatile composition of hops during storage.
J. agric. food Chem., 26: 1426-1430.
TSULAYA, V.R., MORENKOVA, N.V., VOLOKHOVA, L.E., & VORONIN, V.M.
(1978) [Description of the biological properties of small
concentrations of isobutyl alcohol.] Gig. i Sanit., 5: 6-9
(in Russian).
UK MAFF (1978) Food additives and contaminants. Committee
report on the review of solvents in food, London, United Kingdom
Ministry of Agriculture, Fisheries and Food.
US DHEW (1951) Survey of compounds which have been tested for
carcinogenic activity, 2nd ed., Washington DC, US Department of
Health, Education, and Welfare (Prepared by Hartwell, J.L. for the
US DHEW).
US DHEW (1978) Registry of toxic effects of chemicals, Washington
DC, US Department of Health, Education and Welfare.
US EPA (1980) Hazard information review, Washington DC, US
Environmental Protection Agency, p. 10.
VAN DEN BERG, A.P., NOORDHOEK, J., & KOOPMAN-KOOL, E. (1979a) The
relation between the sex-dependency of type I binding of
ethylmorphine and the 1-butanol-induced spectral change in mouse
liver microsomes. Biochem. Pharmacol., 28: 31-36.
VAN DEN BERG, A.P., NOORDHOEK, J., & KOOPMAN-KOOL, E. (1979b) The
use of competitive inhibition of substrate binding to cytochrome
P-450 in the determination of spectral dissociation constants for
substrates with multiple types of binding, as illustrated with
1-butanol. Biochem. Pharmacol., 28: 37-41.
VEITH, G.D., CALL, D.J., & BROOKE, L.T. (1981) Estimating the
acute toxicity of narcotic industrial chemicals to fathead
minnows, Philadelphia, Pennsylvania, American Society of Testing
and Materials (ASTM Special Technical Publication No. 802).
VELASQUEZ (1969) Audiologic impairment due to n-butyl alcohol
exposition. In: Proceedings of the International Congress on
Occupational Health, Tokyo, 1969.
VERSCHUEREN, K. (1977) Handbook of environmental data of organic
chemicals, New York, Van Nostrand Reinhold Company.
WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., & IIJIMA, M.
(1984) Induction of metamitochondria in the rat liver by n-propyl
alcohol and n-butyl alcohol. Acta pathol. Jpn., 34(3): 471-480.
WALLGREN, H. (1960) Relative intoxicating effects on rats of
ethyl, propyl, and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
WALLGREN, H., NIKANDER, P., BOGUSLAWSKY, P.V., & LINKOLA, J.
(1974) Effects of ethanol, tert-butanol, and clomethiazole on
net movements of sodium and potassium in electrically stimulated
cerebral tissue. Acta physiol. Scand., 91: 83-93.
WALTER, W. & WEIDEMANN, H.L. (1969) Coffee flavour compounds.
Z. Ernaehrungswiss, 9: 123-147.
WARTBURG, J.P. VON, BETHANE, J.L., & VALLEE, B.L. (1964) Human
liver alcohol dehydrogenase: kinetic and physiochemical properties.
Biochemistry, 3: 1775-1782.
WEESE, H. (1928) [Comparative studies on the efficacy and
toxicity of the vapours of low aliphatic alcohols.] Arch. exp.
Pathol. Pharmakol., 135: 118-130 (in German).
WEISBRODT, N.W., KIENZLE, M., & COOKE, A.R. (1973) Comparative
effects of aliphatic alcohols on the gastric mucosa. Proc. Soc. Exp.
Biol. Med., 142: 450-454.
WHO (1980) Twenty-third Report of the Joint FAO/WHO Expert
Committee on Food Additives, Geneva, World Health Organization
(WHO Technical Report Series 648).
WILLIAMS, R.T. (1969) Detoxication mechanisms, 2nd ed., London,
Chapman & Hall Ltd., p. 66.
WINER, A.D. (1958) A note of the substrate specificity of horse
liver alcohol dehydrogenase. Acta. chem. Scand., 12: 965.
WITKIN, J.M. & LEANDER, J.D. (1982) Effects of orally
administered ethanol and tert-butanol on fixed-ratio responding of
rats. Subst. Alcohol Actions/Misuse, 3: 275-279.
WOIDICH, H., PFANNHAUSER, W., & EBERHARDT, R. (1978) Results of
gas chromatographic-mass spectrographic studies of the volatile
components of applie brandies. Mitt. Hoeheren Bundeslehr-
Versuchsanst. Wein-Obstbau, Klosterneuberg, 28: 56-63.
WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.-Excerpta
Med., 440: 196-199.
WOOD, J.M. & LAVERTY, R. (1979) Physical dependence following
prolonged ethanol or t-butanol administration to rats. Pharmacol.
Biochem. Behav., 10: 113-119.
WOOD, J.N. & LAVERTY, R. (1976) Alcohol withdrawal syndrome
following prolonged 5-butanol administration to rats. Proc. Univ.
Otago Med. Sch., 54: 86-87.
YABUMOTO, K., YAMAGUCHI, M., & JENNINGS, W.G. (1978) Production
of volatile compounds by musk melon Cucumis melo. Food Chem.,
3: 7-16.
YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., & HABU, T.
(1978) Volatile flavor components of cooked rice. Agric. biol.
Chem., 42: 1229-1223.
YAMAZAKI, Y. & KATO, K. (1978) Penicillins or cephalosporins.
Jpn. Kokai Tokkio Koho, 78, 107, 484, 19 Sep. 1978.
YASHUDA, Y., CHABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
YASUDA-YASAKI, Y., NAMIKE-KANIE, S., & HACHISAKU, Y. (1978)
inhibition of germination of Bacillus subtilis spores by alcohols.
Spores, 7: 13-16.
YOJAY, L., YOJAY, R., & MARDONES, J. (1982) Acetone blood levels
after t-butanol administration in rats. IRCS Med. Sci., 10: 215.
ZAIKINA, E.I., TEREKHOVA, A.I., CHUDOV, L.N., SHATENSHTEIN, A.I.,
PETROV, E.S., SHCHERBAK, V.P., ZAKOMYRDIN, A.A., SIMETSKII, W.A., &
SOKHADZE, L.A. (1978) Repellent composition. Otkrytiya, Izobret.,
Prom. Obraztsy, Tovarnye, Znaky, 55: 18.
ZAIKOV, KH. & BOBEV, G. (1978) Chemical damages in the furniture
industry and morbidity with temporary loss of working capacity.
Khig. Zdraveopaz., 21: 141-147.
ZAMARAKHINA, L.E. (1973) [Determination of tert-butyl alcohol in
the air of industrial premises.] Gig. i Sanit., 38: 72-73
(in Russian)
 2-Butanol exhibited an apparent blood elimination half-life of
2.5 h in rats treated orally with 2.2 ml/kg body weight. With
decline in blood-alcohol concentration (maximum level 800 mg/litre,
1 h after administration), there was a rise in 2-butanone levels
with 430 mg/litre detected at 1 h and a maximum of 1050 mg/litre
detected 4 h after administration of the alcohol (Traiger &
Bruckner, 1976).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Some toxicity data for 2-butanol in aquatic organisms are given
in Table 3. Only two LC50 values are available for fish, and these
indicate low toxicity for these species. The toxicity of 2-butanol
for aquatic invertebrates is equally low.
Table 3. Toxicity data of 2-butanol for aquatic organisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Fish
Fresh-water species
Golden orfe 3520 48-h LC50 Juhnke & Lüdemann (1978)
(Leuciscus idus
melanotus)
Goldfish 4300 24-h LC50 Bridié et al. (1979a)
(Carassius auratus)
Invertebrates
Fresh-water species
Water flea 2300 24-h EC50 immobilization Bringmann & Kuehn (1982)
(Daphnia magna)
Marine species
Brine shrimp 3800 EC50 excystment Smith & Siegel (1975)
(Artemia salina)
Algae
Green algae
(Scenedesmus 95 8-day no- total biomass Bringmann & Kuehn
quadricauda) observed- (1978a)
adverse-
effect level
Chlorella 8900 EC50 Jones (1971)
pyrenoidosa chlorophyll
content
------------------------------------------------------------------------------------------
7.2. Terrestrial Organisms
An EC50 of 650 mg/litre was reported by Reynolds (1977) for
seed germination in lettuce (Lactuca sativa). Inhibition of seed
germination in cucumber (Cucumis sativus) was observed at 50 375 mg
2-butanol/litre (Smith & Siegel, 1975). There are no relevant data
for terrestrial animals, but, as in the case of terrestrial plants,
significant exposure to 2-butanol is unlikely.
7.3. Microorganisms
Some toxicity data for microorganisms are given in Table 4.
The toxicity of 2-butanol for both protozoa and bacteria is very
low.
Table 4. Toxicity of 2-butanol for microorganisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Protozoa
Uronema parduczi 1416 20-h no-observed- total Bringmann & Kuehn
(ciliate) adverse-effect level biomass (1981)
Chilomonas paramaecium 745 48-h no-observed- total Bringmann & Kuehn
(flagellate) adverse-effect level biomass (1981)
Entosiphon sulcatum 1282 72-h no-observed- total Bringmann & Kuehn
(flagellate) adverse-effect level biomass (1981)
Bacteria
Pseudomonas putida 500 16-h no-observed- total Bringmann & Kuehn
adverse-effect level biomass (1976)
Bacillus subtilis 1630 EC50 spore germination Yasuda-Yasaki et
al. (1978)
------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposure
8.1.1. Acute toxicity
Acute toxicity data for 2-butanol are given in Table 5.
2-Butanol shows low acute toxicity in rodents.
Table 5. Acute toxicity of 2-butanol
-------------------------------------------------------
Species Route of LD50 (g/kg Reference
administration body weight)
-------------------------------------------------------
Rat oral 6.5 US DHEW (1978)
Rabbit oral 4.9 Münch (1972)
Mouse intraperitoneal 0.8 US DHEW (1978)
-------------------------------------------------------
Six male albino rats were exposed to 2-butanol vapour at 48.5
mg/litre (16 000 ppm) for 4 h. Within 14 days, 5 of the 6 rats
died (Smyth et al., 1954).
8.1.2. Signs of intoxication
Signs of intoxication due to 2-butanol exposure include
restlessness, ataxia, prostration, and narcosis (Patty, 1963).
The effects of a moderate non-toxic oral dose of 2-butanol
(0.0163 mol/kg body weight) was studied in rats using a simple
functional test by Wallgren (1960). The intoxicating effect of
2-butanol, compared with that of ethanol (taken equal to 1), was
4.4 on an equimolar basis. Recovery after the treatment was slow.
The livers of mice that died 1 - 3 days following a single
intraperitoneal (ip) dose of 2-butanol showed an abnormal brownish
coloration in the peripheral areas, the livers of those that died
after 4 - 6 days were uniformally dark brown in colour (Maickel &
McFadden, 1979).
8.2. Skin and Eye Irritation
2-Butanol is practically not irritant to the skin of the
rabbit, but it is irritant to the rabbit eye (Smyth et al., 1954).
8.3. Short-Term Exposures
White mice (15 - 20 g) were repeatedly exposed to 2-butanol
vapour at 16.2 mg/litre (5330 ppm) for a total of 117 h. Although
the mice were narcotized, they survived.
Six groups of 2 mice each were exposed to 2-butanol vapour at
5 mg/litre (1650 ppm). After 420 min, no signs of intoxication
were observed. When increasing concentrations were used with
decreasing durations of exposure, ataxia, prostration, and deep
narcosis occurred. The time necessary to induce these symptoms
was inversely proportional to the level of exposure. At a
concentration of 10 mg/litre (3300 ppm), ataxia occurred in 51 -
100 min, prostration in 120 - 180 min, and narcosis in 300 min. At
a concentration of 60 mg/litre (19 800 ppm), these signs appeared
in 7 - 8 min, 12 - 20 min, and 40 min, respectively. No deaths
were observed in this study (Starrek, 1938, cited in Patty, 1982).
The neurophysiological effects induced by 2-butanol were
investigated in 31 male Sprague Dawley rats (200 - 400 g).
2-Butanol was administered intravenously (iv) to the rats at a
concentration of 8.1 mmoles/kg body weight. Within 20 - 60 seconds
of the iv administration, the rats lost righting reflexes. Some
changes were also noticed in the electroencephalographic tracings
compared with the tracings prior to the alcohol administration
(Marcus et al., 1976).
8.4. Long-Term Exposures
No long-term exposure studies are available.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data on reproduction, embryotoxicity, or
teratogenicity have yet been published. However, an inhalation
teratology study with 2-butanol is in progress in the USA (US EPA,
personal communication, 1985).
8.6. Mutagenicity
2-Butanol did not show any mutagenic activity in the yeast
Schizosaccharomyces pombe in both the presence and absence of
mouse liver microsomes (Abbondandolo et al., 1980).
8.7. Carcinogenicity
No carcinogenicity studies are available.
8.8. Special Studies
The effects of 2-butanol on cell survival were studied in the
yeast S. pombe and in V-79 Chinese hamster cells by Abbondandolo
et al. (1980). At 5% concentration, 2-butanol decreased the
survival of suspended yeast cells, but did not have any effect on
monolayer cultures of V-79 cells.
2-Butanol inhibited the contraction of the depolarized guinea-
pig ileum induced by calcium chloride (CaCl2) (Yashuda et al.,
1976).
2-Butanol can potentiate the toxicity of carbon tetrachloride
(CCl4) (Cornish & Adefuin, 1967). This potentiation may be due to
the metabolite 2,3-butanediol, which also has this effect (Traiger
& Bruckner, 1976; Dietz & Traiger, 1979).
Inhalation exposure of rats to 2-butanol (1539 mg/m3 (500 ppm)
for 5 days) resulted in a 47% increase in the cytochrome P-450
levels of kidney microsomes. A maximal increase of 33% in liver
microsomal cytochrome P-450 content was seen after inhalation of
2-butanol at 6156 mg/m3 (2000 ppm) for 3 days. This treatment led
to a 77% increase in the formation of the preneurotoxic metabolite
2-hexanol from 1-hexane by liver microsomes (Aarstad et al., in
press).
Rats receiving a single oral dose of 2-butanol at 2.2 ml/kg
body weight were sacrificed 16, 20, or 40 h after dosing. A 50 -
97% increase in microsomal acetanilide hydroxylase activity was
found. At 40 h, liver cells showed a marked proliferation of
smooth endoplasmic reticulum. This stimulation of the drug-
metabolizing system may explain, to a certain extent, the
potentiation of CCl4 hepatoxicity by 2-butanol (Traiger et al.,
1975).
9. EFFECTS ON MAN
Excessive exposure may result in headache, dizziness,
drowsiness, and narcosis (Muir, 1977). No adverse systemic effects
due to exposure to 2-butanol have been reported in man (Patty,
1982).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population to 2-butanol
through food and beverages, and occupational exposure levels are
not available.
10.1.2. Toxic effects
In animals, 2-butanol is absorbed through the lungs and
gastrointestinal tract. No information is available regarding
dermal absorption. Approximately 97% of the dose of 2-butanol in
animals is converted by alcohol dehydrogenase to the corresponding
ketone, which is either excreted in the breath and urine or further
metabolized. The rat acute oral LD50 for 2-butanol is 6.5 g/kg
body weight; it is, therefore, practically non-toxic according to
the classification of Hodge & Sterner. The toxic effects from
acute exposure are ataxia and narcosis. The potency of 2-butanol
for intoxication is approximately 4 times that of ethanol. It is
irritating to the eyes and non-irritating to the skin. From the
animal studies available, it is not possible to determine a no-
observed-adverse-effect level. No adequate data are available on
mutagenicity, carcinogenicity, teratogenicity, or effects on
reproduction.
In man, the most likely acute effect of 2-butanol is alcoholic
intoxication. No published data are available concerning other
effects on man.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
No quantitative data relating to levels of 2-butanol in the
general environment are available, but, because it is readily
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillage.
10.2.2. Toxic effects
At the background concentrations likely to occur in the
environment, 2-butanol is not toxic for aquatic animals, algae,
protozoa, or bacteria, and it should be managed in the environment
as a slightly toxic compound. It poses an indirect hazard for the
aquatic environment, because it is readily biodegradable, which may
lead to oxygen depletion.
10.3. Conclusions
1. The Task Group was unable to make an assessment of the health
risks of 2-butanol for the general population on the basis of
available data. However, it was considered that 2-butanol was
unlikely to pose a serious hazard, under normal exposure
conditions.
2. The Task Group was of the opinion that available data are not
sufficient to establish guidelines for setting occupational
exposure limits. In line with good manufacturing practice,
exposure to 2-butanol should be minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of 2-butanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
The Task Group recommended that:
1. As it was not possible to determine a no-observed-adverse-
effect level on the basis of available animal studies, relevant
studies should be conducted so that this could be achieved.
2. Information on residue and emission levels is desirable.
3. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
2-butanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Food Additives and Contaminants Committee (UK MAFF, 1978)
recommended that residues of butan-2-ol in food should not exceed
30 mg/kg and required the results of a 90-day oral toxicity study
in the rat within 2 years.
This compound could not be included in lists 1 or 2 by the
Council of Europe and it was included in list 3A (Council of
Europe, 1981).
At their 23rd meeting, the Joint FAO/WHO Expert Committee on
Food Additives (JECFA) reviewed the data on 2-butanol. They
concluded that "The evaluation of this compound was not possible on
the basis of the data available. New specifications were prepared,
but no toxicological monograph" (WHO, 1980).
ENVIRONMENTAL HEALTH CRITERIA
FOR
tert-BUTANOL
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR tert-BUTANOL
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.2 Terrestrial organisms
7.3 Microorganisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Acute toxicity
8.1.2 Signs of intoxication
8.2 Skin and eye irritation
8.3 Short-term exposures
8.4 Reproduction, embyrotoxicity, and teratogenicity
8.5 Mutagenicity
8.6 Carcinogenicity
8.7 Special studies
9. EFFECTS ON MAN
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
10.1.2 Toxic effects
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
10.2.2 Toxic effects
10.3 Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY
tert-Butanol is a colourless liquid or white crystalline solid
with a camphor-like odour. It has a melting point of 25 °C, a
boiling point of 81.5 - 83 °C, is freely soluble in water, and its
n-octanol/water partition coefficient is 0.37. Its vapour is 2.6
times denser than air. It is used primarily as a solvent, a
dehydrating agent, and as an intermediate in the manufacture of
other chemicals. It is also used as a denaturant for alcohols.
Human exposure will be mainly occupational. Data on exposure of
the general population are not available, but it may result from
industrial emissions. tert-Butanol is inherently biodegradable and
does not bioaccumulate. At ambient levels, it is not toxic for
fish, amphibia, crustacea, algae, or bacteria.
In animals, tert-butanol is absorbed through the lungs and
gastrointestinal tract; no information is available on dermal
absorption. tert-Butanol is not a substrate for alcohol
dehydrogenase and is slowly metabolized by mammals. Up to 24% of
the dose is eliminated in the urine as the glucuronide, and up to
10% of the dose can be excreted in the breath and urine as acetone
or carbon dioxide. The rat oral LD50 is 3.5 g/kg body weight; it
is, therefore, slightly toxic according to the classification of
Hodge & Sterner. The primary acute effects observed in animals are
signs of alcoholic intoxication. Its potency for intoxication is
approximately 1.5 times that of ethanol. Animal data regarding
skin and eye irritation are not available. tert-Butanol produces
physical dependance in animals and post-natal effects in offspring
exposed in utero. Data concerning the pathological effects of
repeated exposure of animals are not available. From the animal
studies available, it is not possible to determine a no-observed-
adverse-effect level. tert-Butanol has been found not to be
mutagenic. Adequate data are not available on carcinogenicity,
teratogenicity, or effects on reproduction.
In man, tert-butanol is a mild irritant to the skin. No other
effects on man have been reported, and there have been no reports
of poisonings.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure:
CH3
|
CH3 - C - CH3
|
OH
Chemical formula: C4H10O
Primary constituent: tert-butanol
Common synonyms: 2-methyl-2-propanol, tert-butyl
alcohol, tertiary butanol, t.
butanol, trimethyl carbinol, TBA,
TMA, t. butyl hydroxide, NCL-C55
367, trimethyl methanol
Cas registry number:
2-Butanol exhibited an apparent blood elimination half-life of
2.5 h in rats treated orally with 2.2 ml/kg body weight. With
decline in blood-alcohol concentration (maximum level 800 mg/litre,
1 h after administration), there was a rise in 2-butanone levels
with 430 mg/litre detected at 1 h and a maximum of 1050 mg/litre
detected 4 h after administration of the alcohol (Traiger &
Bruckner, 1976).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Some toxicity data for 2-butanol in aquatic organisms are given
in Table 3. Only two LC50 values are available for fish, and these
indicate low toxicity for these species. The toxicity of 2-butanol
for aquatic invertebrates is equally low.
Table 3. Toxicity data of 2-butanol for aquatic organisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Fish
Fresh-water species
Golden orfe 3520 48-h LC50 Juhnke & Lüdemann (1978)
(Leuciscus idus
melanotus)
Goldfish 4300 24-h LC50 Bridié et al. (1979a)
(Carassius auratus)
Invertebrates
Fresh-water species
Water flea 2300 24-h EC50 immobilization Bringmann & Kuehn (1982)
(Daphnia magna)
Marine species
Brine shrimp 3800 EC50 excystment Smith & Siegel (1975)
(Artemia salina)
Algae
Green algae
(Scenedesmus 95 8-day no- total biomass Bringmann & Kuehn
quadricauda) observed- (1978a)
adverse-
effect level
Chlorella 8900 EC50 Jones (1971)
pyrenoidosa chlorophyll
content
------------------------------------------------------------------------------------------
7.2. Terrestrial Organisms
An EC50 of 650 mg/litre was reported by Reynolds (1977) for
seed germination in lettuce (Lactuca sativa). Inhibition of seed
germination in cucumber (Cucumis sativus) was observed at 50 375 mg
2-butanol/litre (Smith & Siegel, 1975). There are no relevant data
for terrestrial animals, but, as in the case of terrestrial plants,
significant exposure to 2-butanol is unlikely.
7.3. Microorganisms
Some toxicity data for microorganisms are given in Table 4.
The toxicity of 2-butanol for both protozoa and bacteria is very
low.
Table 4. Toxicity of 2-butanol for microorganisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Protozoa
Uronema parduczi 1416 20-h no-observed- total Bringmann & Kuehn
(ciliate) adverse-effect level biomass (1981)
Chilomonas paramaecium 745 48-h no-observed- total Bringmann & Kuehn
(flagellate) adverse-effect level biomass (1981)
Entosiphon sulcatum 1282 72-h no-observed- total Bringmann & Kuehn
(flagellate) adverse-effect level biomass (1981)
Bacteria
Pseudomonas putida 500 16-h no-observed- total Bringmann & Kuehn
adverse-effect level biomass (1976)
Bacillus subtilis 1630 EC50 spore germination Yasuda-Yasaki et
al. (1978)
------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposure
8.1.1. Acute toxicity
Acute toxicity data for 2-butanol are given in Table 5.
2-Butanol shows low acute toxicity in rodents.
Table 5. Acute toxicity of 2-butanol
-------------------------------------------------------
Species Route of LD50 (g/kg Reference
administration body weight)
-------------------------------------------------------
Rat oral 6.5 US DHEW (1978)
Rabbit oral 4.9 Münch (1972)
Mouse intraperitoneal 0.8 US DHEW (1978)
-------------------------------------------------------
Six male albino rats were exposed to 2-butanol vapour at 48.5
mg/litre (16 000 ppm) for 4 h. Within 14 days, 5 of the 6 rats
died (Smyth et al., 1954).
8.1.2. Signs of intoxication
Signs of intoxication due to 2-butanol exposure include
restlessness, ataxia, prostration, and narcosis (Patty, 1963).
The effects of a moderate non-toxic oral dose of 2-butanol
(0.0163 mol/kg body weight) was studied in rats using a simple
functional test by Wallgren (1960). The intoxicating effect of
2-butanol, compared with that of ethanol (taken equal to 1), was
4.4 on an equimolar basis. Recovery after the treatment was slow.
The livers of mice that died 1 - 3 days following a single
intraperitoneal (ip) dose of 2-butanol showed an abnormal brownish
coloration in the peripheral areas, the livers of those that died
after 4 - 6 days were uniformally dark brown in colour (Maickel &
McFadden, 1979).
8.2. Skin and Eye Irritation
2-Butanol is practically not irritant to the skin of the
rabbit, but it is irritant to the rabbit eye (Smyth et al., 1954).
8.3. Short-Term Exposures
White mice (15 - 20 g) were repeatedly exposed to 2-butanol
vapour at 16.2 mg/litre (5330 ppm) for a total of 117 h. Although
the mice were narcotized, they survived.
Six groups of 2 mice each were exposed to 2-butanol vapour at
5 mg/litre (1650 ppm). After 420 min, no signs of intoxication
were observed. When increasing concentrations were used with
decreasing durations of exposure, ataxia, prostration, and deep
narcosis occurred. The time necessary to induce these symptoms
was inversely proportional to the level of exposure. At a
concentration of 10 mg/litre (3300 ppm), ataxia occurred in 51 -
100 min, prostration in 120 - 180 min, and narcosis in 300 min. At
a concentration of 60 mg/litre (19 800 ppm), these signs appeared
in 7 - 8 min, 12 - 20 min, and 40 min, respectively. No deaths
were observed in this study (Starrek, 1938, cited in Patty, 1982).
The neurophysiological effects induced by 2-butanol were
investigated in 31 male Sprague Dawley rats (200 - 400 g).
2-Butanol was administered intravenously (iv) to the rats at a
concentration of 8.1 mmoles/kg body weight. Within 20 - 60 seconds
of the iv administration, the rats lost righting reflexes. Some
changes were also noticed in the electroencephalographic tracings
compared with the tracings prior to the alcohol administration
(Marcus et al., 1976).
8.4. Long-Term Exposures
No long-term exposure studies are available.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data on reproduction, embryotoxicity, or
teratogenicity have yet been published. However, an inhalation
teratology study with 2-butanol is in progress in the USA (US EPA,
personal communication, 1985).
8.6. Mutagenicity
2-Butanol did not show any mutagenic activity in the yeast
Schizosaccharomyces pombe in both the presence and absence of
mouse liver microsomes (Abbondandolo et al., 1980).
8.7. Carcinogenicity
No carcinogenicity studies are available.
8.8. Special Studies
The effects of 2-butanol on cell survival were studied in the
yeast S. pombe and in V-79 Chinese hamster cells by Abbondandolo
et al. (1980). At 5% concentration, 2-butanol decreased the
survival of suspended yeast cells, but did not have any effect on
monolayer cultures of V-79 cells.
2-Butanol inhibited the contraction of the depolarized guinea-
pig ileum induced by calcium chloride (CaCl2) (Yashuda et al.,
1976).
2-Butanol can potentiate the toxicity of carbon tetrachloride
(CCl4) (Cornish & Adefuin, 1967). This potentiation may be due to
the metabolite 2,3-butanediol, which also has this effect (Traiger
& Bruckner, 1976; Dietz & Traiger, 1979).
Inhalation exposure of rats to 2-butanol (1539 mg/m3 (500 ppm)
for 5 days) resulted in a 47% increase in the cytochrome P-450
levels of kidney microsomes. A maximal increase of 33% in liver
microsomal cytochrome P-450 content was seen after inhalation of
2-butanol at 6156 mg/m3 (2000 ppm) for 3 days. This treatment led
to a 77% increase in the formation of the preneurotoxic metabolite
2-hexanol from 1-hexane by liver microsomes (Aarstad et al., in
press).
Rats receiving a single oral dose of 2-butanol at 2.2 ml/kg
body weight were sacrificed 16, 20, or 40 h after dosing. A 50 -
97% increase in microsomal acetanilide hydroxylase activity was
found. At 40 h, liver cells showed a marked proliferation of
smooth endoplasmic reticulum. This stimulation of the drug-
metabolizing system may explain, to a certain extent, the
potentiation of CCl4 hepatoxicity by 2-butanol (Traiger et al.,
1975).
9. EFFECTS ON MAN
Excessive exposure may result in headache, dizziness,
drowsiness, and narcosis (Muir, 1977). No adverse systemic effects
due to exposure to 2-butanol have been reported in man (Patty,
1982).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population to 2-butanol
through food and beverages, and occupational exposure levels are
not available.
10.1.2. Toxic effects
In animals, 2-butanol is absorbed through the lungs and
gastrointestinal tract. No information is available regarding
dermal absorption. Approximately 97% of the dose of 2-butanol in
animals is converted by alcohol dehydrogenase to the corresponding
ketone, which is either excreted in the breath and urine or further
metabolized. The rat acute oral LD50 for 2-butanol is 6.5 g/kg
body weight; it is, therefore, practically non-toxic according to
the classification of Hodge & Sterner. The toxic effects from
acute exposure are ataxia and narcosis. The potency of 2-butanol
for intoxication is approximately 4 times that of ethanol. It is
irritating to the eyes and non-irritating to the skin. From the
animal studies available, it is not possible to determine a no-
observed-adverse-effect level. No adequate data are available on
mutagenicity, carcinogenicity, teratogenicity, or effects on
reproduction.
In man, the most likely acute effect of 2-butanol is alcoholic
intoxication. No published data are available concerning other
effects on man.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
No quantitative data relating to levels of 2-butanol in the
general environment are available, but, because it is readily
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillage.
10.2.2. Toxic effects
At the background concentrations likely to occur in the
environment, 2-butanol is not toxic for aquatic animals, algae,
protozoa, or bacteria, and it should be managed in the environment
as a slightly toxic compound. It poses an indirect hazard for the
aquatic environment, because it is readily biodegradable, which may
lead to oxygen depletion.
10.3. Conclusions
1. The Task Group was unable to make an assessment of the health
risks of 2-butanol for the general population on the basis of
available data. However, it was considered that 2-butanol was
unlikely to pose a serious hazard, under normal exposure
conditions.
2. The Task Group was of the opinion that available data are not
sufficient to establish guidelines for setting occupational
exposure limits. In line with good manufacturing practice,
exposure to 2-butanol should be minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of 2-butanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
The Task Group recommended that:
1. As it was not possible to determine a no-observed-adverse-
effect level on the basis of available animal studies, relevant
studies should be conducted so that this could be achieved.
2. Information on residue and emission levels is desirable.
3. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
2-butanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Food Additives and Contaminants Committee (UK MAFF, 1978)
recommended that residues of butan-2-ol in food should not exceed
30 mg/kg and required the results of a 90-day oral toxicity study
in the rat within 2 years.
This compound could not be included in lists 1 or 2 by the
Council of Europe and it was included in list 3A (Council of
Europe, 1981).
At their 23rd meeting, the Joint FAO/WHO Expert Committee on
Food Additives (JECFA) reviewed the data on 2-butanol. They
concluded that "The evaluation of this compound was not possible on
the basis of the data available. New specifications were prepared,
but no toxicological monograph" (WHO, 1980).
ENVIRONMENTAL HEALTH CRITERIA
FOR
tert-BUTANOL
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR tert-BUTANOL
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.2 Terrestrial organisms
7.3 Microorganisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Acute toxicity
8.1.2 Signs of intoxication
8.2 Skin and eye irritation
8.3 Short-term exposures
8.4 Reproduction, embyrotoxicity, and teratogenicity
8.5 Mutagenicity
8.6 Carcinogenicity
8.7 Special studies
9. EFFECTS ON MAN
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
10.1.2 Toxic effects
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
10.2.2 Toxic effects
10.3 Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY
tert-Butanol is a colourless liquid or white crystalline solid
with a camphor-like odour. It has a melting point of 25 °C, a
boiling point of 81.5 - 83 °C, is freely soluble in water, and its
n-octanol/water partition coefficient is 0.37. Its vapour is 2.6
times denser than air. It is used primarily as a solvent, a
dehydrating agent, and as an intermediate in the manufacture of
other chemicals. It is also used as a denaturant for alcohols.
Human exposure will be mainly occupational. Data on exposure of
the general population are not available, but it may result from
industrial emissions. tert-Butanol is inherently biodegradable and
does not bioaccumulate. At ambient levels, it is not toxic for
fish, amphibia, crustacea, algae, or bacteria.
In animals, tert-butanol is absorbed through the lungs and
gastrointestinal tract; no information is available on dermal
absorption. tert-Butanol is not a substrate for alcohol
dehydrogenase and is slowly metabolized by mammals. Up to 24% of
the dose is eliminated in the urine as the glucuronide, and up to
10% of the dose can be excreted in the breath and urine as acetone
or carbon dioxide. The rat oral LD50 is 3.5 g/kg body weight; it
is, therefore, slightly toxic according to the classification of
Hodge & Sterner. The primary acute effects observed in animals are
signs of alcoholic intoxication. Its potency for intoxication is
approximately 1.5 times that of ethanol. Animal data regarding
skin and eye irritation are not available. tert-Butanol produces
physical dependance in animals and post-natal effects in offspring
exposed in utero. Data concerning the pathological effects of
repeated exposure of animals are not available. From the animal
studies available, it is not possible to determine a no-observed-
adverse-effect level. tert-Butanol has been found not to be
mutagenic. Adequate data are not available on carcinogenicity,
teratogenicity, or effects on reproduction.
In man, tert-butanol is a mild irritant to the skin. No other
effects on man have been reported, and there have been no reports
of poisonings.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure:
CH3
|
CH3 - C - CH3
|
OH
Chemical formula: C4H10O
Primary constituent: tert-butanol
Common synonyms: 2-methyl-2-propanol, tert-butyl
alcohol, tertiary butanol, t.
butanol, trimethyl carbinol, TBA,
TMA, t. butyl hydroxide, NCL-C55
367, trimethyl methanol
Cas registry number:  When Kamil et al. (1953) administered isobutanol (25 mmol
total) by stomach tube to Chinchilla rabbits, 4.4% of the applied
dose was excreted within 24 h, as the glucuronide. After
administration of 6 ml isobutanol to the stomach of rabbits (Kamil
et al., 1953), no aldehydes or ketones were observed in the expired
air within 6 h.
Gaillard & Derache (1965) reported that, following an oral dose
of 2 g/kg body weight to rats, 0.27% was excreted in the urine
within 8 h.
The oxidation of isobutanol and other lower alcohols by both
rat liver homogenates and by the perfusion in situ of rat liver
was studied by Hedlund & Kiessling (1969). The authors observed
that the alcohols were oxidized at rates that decreased in the
following order: 1-propanol, isobutanol, ethanol, and isoamyl
alcohol. Furthermore, from their studies with the alcohol
dehydrogenase (ADH) inhibitor pyrazole, these authors concluded
that all the alcohols studied must be oxidized by ADH in the same
manner as ethanol in an NAD-mediated oxidation.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Some data on the toxicity of isobutanol for aquatic organisms
are given in Table 4. The high lethal concentrations (1000 - 4000
mg/litre) for fish, amphibia, and crustacea indicate that, at
background levels, isobutanol would not be disruptive for an
aquatic ecosystem.
Table 4. Toxicity of isobutanol for aquatic organisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Fish
Fresh-water species
Bleak 1000-3000 96-h LC50 Lindén et al.
(Alburnus (1979)
alburnus)
Golden orfe 1520 48-h LC50 Juhnke & Lüdemann
(Leuciscus (1978)
idus melanotus)
Goldfish 2600 24-h LC50 Bridié et al.
(Carassius (1979a)
auratus)
Amphibia
Tadpole 4000 threshold for Münch (1972)
(Rana sp) narcosis
Invertebrates
Fresh-water species
Water flea 1250 24-h EC50 immobilization Bringmann & Kuehn
(Daphnia magna) (1982)
Marine species
Brine shrimp 1400 24-h LC50 Price et al. (1974)
(Artemia salina) 3800 EC50 excystment Smith & Siegel
(1975)
------------------------------------------------------------------------------------------
Table 4. (contd.)
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Algae
Green algae
Scenedesmus 350 8-day no-observed- total biomass Bringmann & Kuehn
quadricauda adverse-effect level (1978a)
Blue-green algae
(Microcystis 290 8-day no-observed- total biomass Bringmann & Kuehn
aeruginosa) adverse-effect level (1978a)
------------------------------------------------------------------------------------------
7.2. Terrestrial Organisms
Toxicity studies in plants indicate that germination will not
be affected by exposure to isobutanol at background levels. An
EC50 of 760 mg/litre was reported by Reynolds (1977) for seed
germination in lettuce (Lactuca sativa). Smith & Siegel (1975)
found an EC50 of 40 800 mg/litre for seed germination in cucumber
(Cucumis sativus).
There is no information on terrestrial animals, but exposure is
unlikely to be significant, except locally after spills.
7.3. Microorganisms
Some data on the toxicity of isobutanol for microorganisms are
given in Table 5. Available toxicity data on protozoa bacteria and
algae suggest tolerance to exposure to isobutanol. No-observed-
adverse-effect levels for various species ranged from 22 to 1180
mg/litre). Exposure to background levels of isobutanol should not
be disruptive for the ecosystem.
Table 5. Toxicity of isobutanol for microorganisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Protozoa
Chilomonas paramaecium 22 48-h no-observed- total biomass Bringmann &
(flagellate) adverse-effect level Kuehn (1981)
Uronema parduczi 169 20-h no-observed- total biomass Bringmann &
(ciliate) adverse-effect level Kuehn (1981)
Entosiphon sulcatum 296 72-h no-observed- total biomass Bringmann &
(flagellate) adverse-effect level Kuehn (1981)
Bacteria
Pseudomonas putida 280 16-h no-observed- total biomass Bringmann &
adverse-effect level Kuehn (1976)
Bacillus subtilis 1180 EC50 spore Yasuda-Yasaki
germination et al. (1978)
------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Acute toxicity
The oral EC50 for narcosis in rabbits was 19 mmol isobutanol/kg
body weight (Münch, 1972).
Acute toxicity data are given in Table 6.
Table 6. Acute toxicity of isobutanol
---------------------------------------------------------------------
Species Route of Parameter Results Reference
administration
---------------------------------------------------------------------
Guinea- inhalation LC50 19 900 mg/m3 Kushneva et al.
pig (1983)
Mouse inhalation LC50 15 500 mg/m3 Kushneva et al.
(1983)
Rabbit inhalation LC50 26 250 mg/m3 Kushneva et al.
(1983)
Rat inhalation LCLoa 8000 mg/m3 (4 h) US DHEW (1978)
Rat inhalation LC50 19 200 mg/m3 Kushneva et al.
(1983)
Cat intravenous LDLoa 0.018 g/kg US DHEW (1978)
Rabbit oral LDLoa 3.75 g/kg US DHEW (1978)
Rabbit oral LD50 41 mmol/kg Münch (1972)
Mouse oral LD50 3.5 g/kg Kushneva et al.
(1983)
Rat oral LD50 3.1 g/kg Kushneva et al.
(1983)
Rat oral LD50 2.46 g/kg US DHEW (1978)
Rabbit skin LD50 4.24 g/kg US DHEW (1978)
---------------------------------------------------------------------
a Lo = lowest.
After ip administration of isobutanol to mice, the LD50
was > 1000 mg/kg at 30 min and 544 mg/kg at 7 days. Gross post-
mortem examination of livers showed an abnormal dark coloration
(Maickel & McFadden, 1979).
Smyth et al. (1954) reported 100% survival of rats exposed for
2 h to saturated isobutanol vapour (approximately 49 248 mg/m3 or
16 000 ppm) in air, but observed 2 deaths in a group of 6 rats when
they were exposed for 4 h to a concentration of 24 624 mg/m3 (8000
ppm) in air.
8.1.2. Signs of intoxication
The toxic effects of isobutanol are mainly alcoholic
intoxication and narcosis. Stupor and loss of voluntary movements
in animals can be considered signs of intoxication by the oral
route (Münch, 1972). Rats and rabbits were exposed, by inhalation,
for a period of 4 h to various concentrations of isobutanol. At a
concentration of 15 700 mg/m3, there was irritation of the airways.
Three days later, symptoms included central nervous depression, a
decreased number of lymphocytes in bone marrow, a decreased blood-
lactate level, delay in elimination of bromophthalein from blood,
and morphological changes including dystrophia of hepatocytes and
olfactory neurons in the brain. After exposure to 8000 mg/m3,
symptoms were similar but less severe. An isobutanol concentration
of 1300 mg/m3 decreased bone marrow lymphocyte numbers. A
concentration of 100 mg/m3 only altered breathing frequency
(Kushneva et al., 1983).
8.2. Skin and Eye Irritation
Application of 500 mg isobutanol to the skin of rabbits for
24 h was moderately irritating. However, application of 2 mg to
the rabbit eye caused severe irritation (US DHEW, 1978).
8.3. Short-Term Exposures
Hillbom et al. (1974a,b) gave one group of 6 male Wister rats
a 1 mol/litre solution of isobutanol as their sole drinking liquid
for a period of 4 months. Another group of 5 rats of the same
species was given a 2 mol/litre solution of isobutanol as their
sole drinking liquid for 2 months. Both groups were provided an
ad libitum diet of ordinary laboratory food containing about 26%
of calories as protein, 55% as carbohydrate, and 19% as fat. At
the end of the study, all animals were decapitated, and their
livers examined. No adverse effects were detected in the group
given the 1 mol/litre solution, while the rats in the group given
the 2 mol/litre solution showed a decrease in fat, glycogen, RNA,
and overall size of the liver cells.
Oral administration of between 1/10 and 1/5 of the LD50 of
isobutanol for 6 days/week over 1 month, did not result in any
deaths in rats (Kushneva et al., 1983). Continuous exposure of
rats, by inhalation, to isobutanol at 3 mg/m3 over 4 months,
caused an increased threshold for leg withdrawal response to
electrical stimulation, and depression of haemoglobin content,
erythrocyte count, and activities of cholinesterase and catalase
in the blood. The activities of alanine-amino transferase and
aspartate amino transferase were elevated. At 0.5 mg/m3, numbers
of white cells and erythrocytes, haemogloglobin content, and
cholinesterase activity were all decreased. No effects were
observed at 0.1 mg/m3 (Tsulaya et al., 1978).
8.4. Long-Term Exposures
With the exception of a carcinogenicity study, no long-term
studies have been reported.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data are available on reproduction or
embryotoxicity and no conclusions can therefore be drawn.
8.6. Mutagenicity
The only reported study on mutagenicity is that of Hillscher et
al. (1969) who demonstrated an increased rate of reverse mutation
when Escherichia coli CA 274 was treated with 0.7% isobutanol,
without metabolic activation. This study in itself is inadequate
to assess the mutagenic potential of the compounds.
8.7. Carcinogenicity
Two groups of 19 and 24 male and female Wistar rats (10 weeks
old) were administered purified isobutanol. Group one received
0.2 ml/kg body weight, orally, twice weekly. Group two received
0.05 ml/kg, subcutaneously, twice weekly. Two control groups (25
rats each) received 1 ml of 0.9% sodium chloride twice weekly
(controls for group one were dosed orally; controls for group two
were dosed subcutaneously). All rats were dosed until spontaneous
death. Test rats presented liver carcinomas and sarcomas, spleen
sarcomas, stomach proventricular carcinomas, and myeoid leukaemia.
Tumours of these types did not occur in the control groups (Table
7). In the exposed animals, toxic liver damage was found, ranging
from steatosis and cell necrosis to fibrosis and cirrhosis. In
addition, most exposed animals had hyperplasia of blood-forming
tissues. The carcinogenic response was attributed to isobutanol
(Gibel et al., 1974, 1975). No statistical analysis of the data
was performed.
Table 7. Summary of tumours in experimental animalsa
-------------------------------------------------------------------
Agent Application Number Average Malignant Benign
(twice weekly) of survival tumour tumour
animals (days)
-------------------------------------------------------------------
Control oral: 1 ml/kg 25 643 0 3
(0.9% NaCl) body weight
solution)
subcutaneous: 25 643 0 2
1 ml/kg body
weight
Isobutanol oral: 0.2 ml/kg 19 495 3b 9
body weight
subcutaneous: 24 544 8c 3
0.05 ml/kg
body weight
-------------------------------------------------------------------
a Adapted from: Gibel et al. (1975).
b Proventriculus carcinoma and liver cell carcinoma; proventriculus
carcinoma and myeloid leukaemia; myeloid leukaemia.
c Proventriculus carcinoma (2); liver sarcoma (2); spleen sarcoma;
mesothelioma; retroperitoneal sarcoma (2).
8.8. Special Studies
In 8 guinea-pigs, serum enzyme (SGOT, SGPT, GLDH) activities
and triglyceride levels were measured 16 h after oral
administration of 33.5 mg isobutanol/kg olive oil. There was no
statistically-significant difference between treated animals and 8
olive-oil treated controls (Siegers et al., 1974).
Isobutanol can potentiate the hepatic toxicity of carbon
tetrachloride (Cornish & Adefuin, 1967).
9. EFFECTS ON MAN
Isobutanol may be absorbed through the lungs and the
gastrointestinal tract (Browning, 1965).
No data are available on the effects of isobutanol on the skin.
However, as with other defatting solvents, isobutanol may cause
erythematous skin lesions (Schwarz & Tulipan, 1939).
Immersion of the hands of 5 healthy male volunteers in a
1:1 (v/v) mixture of m-xylene and isobutanol, for 15 min at
room temperature, caused only a mild and sometimes barely
distinguishable erythema or a burning feeling. Isobutanol (in a
1:1 mixture with m-xylene) dehydrated the skin and decreased the
absorption of xylene. However, when water was added as 8% of the
mixture, this effect was reduced. The dermal effects of isobutanol
only and its absorption were not studied (Riihimaki, 1979).
Seitz (1972) reported 7 case histories, occurring between
1965 and 1971, of workers who had been exposed to 1-butanol and
isobutanol in a non-ventilated photographic laboratory. They
handled the alcohols under intense and hot light, without any
precautions. Exposure levels were not quantified, but must have
been excessive; exposure time ranged from 1 1/2 months to 2 years.
Two workers had transient vertigo, 3 severe Meniere-like vertigo
with nausea, vomiting and/or headache. In one of these cases,
hearing was also perturbed. Two workers did not present any signs
or symptoms.
Eye irritation, blurred vision, and transient corneal
vacuolization have been decribed in another excessive (no
measurements done) mixed exposure of workers to isobutanol and
butyl acetate. With adequate work room ventilation, it did not
recur. The author felt that the more irritant butyl acetate would
be the main contributor to this effect (Büsing, 1952).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population to isobutanol
through food and beverages are not available and occupational
exposure levels are limited and inadequate.
10.1.2. Toxic effects
In animals, isobutanol is absorbed through the skin, lungs,
and gastrointestinal tract. Isobutanol is metabolized by alcohol
dehydrogenase to isobutyric acid via the aldehyde and may enter the
tricarboxylic acid cycle. Small amounts of isobutanol are excreted
unchanged (< 0.5% of the dose), or as the glucuronide (< 5% of
the dose) in the urine. In rabbits, metabolites found in the urine
include acetaldehyde, acetic acid, isobutyraldehyde, and isovaleric
acid. Oral LD50 values (2.5 - 3.1 g/kg body weight) and inhalation
LC50 (19.2 g/m3) in rats classify isobutanol as slightly toxic
according to Hodge & Sterner. The acute toxic effects are
alcoholic intoxication and narcosis. Isobutanol is severely
irritating to the eyes and moderately irritating to the skin. A
group of rats given a 1 mol/litre solution of isobutanol as their
sole drinking liquid for 4 months did not show any adverse effects
in the liver; another group given a 2 mol/litre solution as their
sole drinking liquid for 2 months showed a reduction in fat,
glycogen, and RNA content, and in the overall size of the cells in
the liver. Continuous inhalation exposure of rats to 3 mg/m3 for
4 months resulted in depression of leg withdrawal response to
electrical stimulation, minor changes of formed elements of the
blood and serum enzymes. The estimated no-observed-adverse-effect
level was 0.1 mg/m3.
In a lifetime carcinogenicity study, groups of rats received
isobutanol subcutaneously (0.05 ml/kg body weight twice a week) or
orally (0.2 ml/kg body weight twice a week). The animals exhibited
toxic liver damage ranging from steatosis to cirrhosis. Numbers of
animals showing malignant tumours totalled 8 in the subcutaneous
group, 3 in the oral group, and 0 in the control group. The
majority of treated animals also showed hyperplasia of blood-
forming tissues.
Because of lack of mutagenicity studies, the Task Group could
not determine whether isobutanol was a genetically active compound.
The findings in the carcinogenicity study are a cause for concern.
Because of methodological inadequacies and the manner of reporting
the data, it was not possible to determine whether isobutanol
should be regarded as an animal carcinogen. Thus it is not
possible to extrapolate from this study to possible long-term
effects in man.
From the animal studies available, it is not possible to
determine a no-observed-adverse-effect level for long-term
exposure. No adequate data are available to assess mutagenicity
or teratogenicity of isobutanol or effects on reproduction.
Exposure of the general population to isobutanol through food
and beverages is unlikely to lead to acute toxic effects. The only
reported observations in man relate to the production of vertigo
under conditions of severe and prolonged exposure to vapour
mixtures of isobutanol and 1-butanol. From this study, it is
not possible to attribute the vertigo to a single cause.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
Little quantitative data relating to levels in the general
environment are available, but, because isobutanol is readily
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillages.
10.2.2. Toxic effects
At background concentrations likely to occur in the
environment, isobutanol is not directly toxic for fish, amphibia,
crustacea, or algae. Protozoa will be tolerant to levels of
isobutanol likely to be found in the environment.
Isobutanol should be managed in the environment as a slightly
toxic compound. It poses an indirect hazard to the aquatic
environment, because it is readily biodegradable, which may lead to
oxygen depletion.
10.3. Conclusions
1. On the basis of available data, the Task Group considered it
unlikely that isobutanol would pose a serious acute health risk
to the general population under normal exposure conditions.
However, the Task Group was unable to make an assessment of the
long-term health risk of isobutanol for the general population.
It was concluded that the results of the carcinogenicity study
need verification by a bioassay of modern standards.
2. The Task Group considered that the data available were
inadequate to set an occupational exposure limit. In line with
good manufacturing practice, exposure to isobutanol should be
minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of isobutanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
1. The Task Group noted that, from the animal studies available,
it is not possible to determine a no-observed-adverse-effect
level. Relevant studies should be conducted so that this can
be achieved.
2. The Task Group considered that adequate studies should be
conducted to assess the mutagenicity and carcinogenicity of
isobutanol.
3. Epidemiological studies, including precise exposure data, would
assist in a better assessment of the occupational hazard of
isobutanol.
4. Additional information on environmental pathways (notably
emission and leaching) and residues are desirable.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Council of Europe (1981) included isobutanol in the list
of flavouring substances that can be added to foodstuffs without
hazard to public health at a level of 25 mg/kg for beverages and
food.
At their 23rd meeting, the Joint FAO/WHO Expert Committee on
Food Additives (JECFA) reviewed the data on isobutanol. They
concluded that:
"The evaluation of this compound was not possible
owing to the paucity of toxicological data. New
specifications were prepared, but no toxicological
monograph" (WHO, 1980).
REFERENCES
AARSTAD, K., ZAHLSEN, K., & NILSEN, O.G. (in press) Inhalation of
butanols: changes in the cytochrome P-450 enzyme system. In:
Proceedings of the European Society of Toxicology, 1984 Annual
Meeting.
ABBASOV, A.Z., PANOV, V.N., & ALIEV, A.M. (1971) [Determination
of a mixture of butyl alcohols in the air of industrial premises by
means of a chromatographic method.] Gig. i Sanit., 36: 61-63
(in Russian).
ABBONDANDOLO, A., BONATTI, S., CORSI, C., CORTI, G., FIORIO, R.,
LEPORINI, L., MAZZACCARO, A., & NIEIR, R. (1980) The use of
organic solvents in mutagenicity testing. Mutat. Res., 79: 141-150.
ACGIH, (1980) Documentation of the threshold limit values, 4th
ed., Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
AITIO, A. (1977) Inhibition of ethoxycumarin deethylation by
organic solvents. Res. Commun. chem. Pathol. Pharmacol., 1:
773-776.
AKHREM, A.A., POPOVA, E.M., & METELITSA, O.I. (1978) Interaction
of aliphatic alcohols with cytochrome P-450 from rat liver
microsomes. Biokhimya (USSR), 43: 1485-1491.
AMUNDSEN, J., GOODWIN, R.J., & WETZEL, W.H. (1979) Water-soluble
pentachlorophenol and tetrachlorophenol wood-treating systems.
S. African, 78: 01,031 (3 January 1979).
ANDERSON, R.A., REDDY, J.M., JOYCE, C., WILLIS, B.R., VAN DER VEN,
H., & ZANEVELD, L.J.D. (1982) Inhibition of mouse sperm
capacitation by ethanol. Biol. Reprod., 27: 833-840.
ANON (1983) Selection of priority compounds: emissions. In:
Publikatiereeks Lucht, The Hague, The Netherlands, Ministry of
Housing, Physical Planning and Environment, Vol. 10.
AOAC METHODS (1975) In: Horwitz, W., ed. Official methods of
analysis of the Association of Official Analytical Chemists,
Washington DC, Association of Official Analytical Chemists.
ARTIGAS GIMENEZ, G., URDANGARAY ARGUELLES, V., GONZALES BLASQUEZ,
I., & ALONSO RODRIGUEZ, J. (1979) Protective coating for glass
objects. Fr. Demande, 2: 410, 115 (23 March 1979).
ASHFORD, M.L.J. & WANN, K.T. (1979) A comparison of the effects
of butanol and benzyl alcohol on the frog end-plate conductance.
J. Physiol., 295: 86-87.
ASTM (1977) Testing methods for butanols. Annual book of
standards. Part 29, Philadelphia, Pennsylvania, American Society
for Testing and Materials (ASTM D304-58).
ASTRAND, I., OVRUM, P., LINDQUIST, T., & HULTENGREN, M. (1976)
Exposure to butyl alcohol: uptake and distribution in man. Scand.
J. Work Environ. Health, 3: 165-175.
AUTY, R.M. & BRANCH, R.A. (1976) The elimination of ethyl,
n-propyl, n-butyl, and iso-amyl alcohols by the isolated perfused
rat liver. J. Pharm. exp. Ther., 197: 669-674.
BAIKOV, B.K. & KHACHATURYAN, M.Kh. (1973) [Hygienic assessment of
the reflex action on a body of small concentrations of butyl
alcohol in the atmosphere.] Gig. i Sanit., 12: 7-11 (in Russian).
BAKER, R.C., SORENSON, S.M., ELOG, S.R., & DEITRICH, R.A. (1979)
Acetone excretion following t-butanol treatment in rats. In:
Proceedings of the 3rd International Symposium on Alcohol and
Aldehyde Metabolizing Systems, Toronto (Abstract No. 44).
BAKER, R.C., SORENSEN, S.M., & DEITRICH, R.A. (1982) The in vivo
metabolism of tertiary butanol by adult rat. Alcohol. clin. exp.
Res., 6(2): 247-251.
BARTHA, T., GONCZIL, L., & MOLLO, A. (1973) The use of metabolic
product analysis with simplified gas-liquid chromatographic methods
in routine anaerobic laboratory analysis. Egeszesegtodamany, 22:
220-227.
BEAUD, P. & RAMUZ, A. (1978) Gas-liquid chromatography of
simultaneous determination of higher alcohols and ethyl acetate in
spirits. Mitt. Geb. Lebensmittelunters. Hyg., 69: 423-30.
BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1981)
Liver lipid disposal following t-butanol administration to rats.
Chem.-biol. Interact., 38: 45-51.
BEAUGE, F., FLEURET, C., BARIN, F., & NORDMANN, R. (1984) Brain
membrane disordering after acute in vivo administration of ethanol,
isopropanol, or t-butanol in rats. Biochem. Pharmacol., 33:
3591-3593.
BELLIN, S.I. & EDMONDS, H.L., Jr (1976) The use of tert-butanol
in alcohol dependence studies. Proc. West. Pharmacol. Soc., 19:
351-354.
BENGTSSON, B.E., RENBERG, L., & TARKPEA, M. (1984) Molecular
structure and aquatic toxicity: an example with C1-C13 aliphatic
alcohols. Chemosphere, 13(5/6): 613-622.
BIKFALVI, I. & PASZTOR, L. (1977) Study on the components
distillates of wine using gas chromatography. Szeszipar, 25:
96-100.
BIRKETT, D.J. (1974) Interaction of some drugs, metal ions, and
alcohols with rat liver microsomes as studied with a fluorescent
probe. Clin. exp. Pharmacol. Physiol., 1: 415-427.
BLOK, J. (1981) [A simple toxicity test using nitrifying
bacteria.] H2 O, 14(11): 242-245 (in Dutch).
BLOOM, S.E. (1981) Detection of sister chromatid exchanges in
vivo using avian embryos. In: Cytogenetic assays of environmental
mutagens, T.C.H.S.U. ed: Allenheld, Osmun, Totowa, New Jersey.
BOBER, A. & HADDAWAY, L.W. (1963) Gas chromatographic
identification of alcoholic beverages. J. gas Chromatogr., 1: 8-13.
BONTE, W. (1978) Congener content of wine and similar beverages.
Blutalkohol, 15: 392-404.
BONTE, W. (1979) Congener substances in German and foreign
beers. Blutalkohol, 16: 108-124.
BONTE, W., DECKER, J., & BUSSE, J. (1978) Congener content of
high-proof alcoholic beverages. Blutalkohol, 16: 108-124.
BRAY, W.J. & HUMPHRIES, C. (1978) Solvent fractionation of leaf
juice to prepare green and white protein products. J. Sci. Food
Agric., 29: 839-846.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRINGMANN, G. & KUEHN, R. (1976) [Comparative findings concerning
the harmful effect of water pollutants on bacteria (Pseudomonas
putida) and blue-green algae (Microcystis aeruginosa).] GWF-
Wasser-Abwasser, 117(9): 410-413 (in German).
BRINGMANN, G. & KUEHN, R. (1977) Results of the damaging effect
of water pollutants on Daphnia magna. Z. Wasser Abwasser Forsch.,
10: 161-166.
BRINGMANN, G. & KUEHN, R. (1978a) [Limit values for the harmful
effect of water pollutants on blue-green algae (Microcystis
aeruginosa) and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Vom Wasser, 50: 45-60 (in German).
BRINGMANN, G. & KUEHN, R. (1978b) Testing of substances for their
toxicity threshold: model organisms Microcystis (Diplocystis)
aeruginosa and Scenedesmus quadriconda. Mitt. Int. Ver. Theor.
Angew. Limnol., 21: 275-284.
BRINGMANN, G. & KUEHN, R. (1981) [Comparison of the effect of
harmful substances on flagellates, on ciliates, on holozoic
bacteriophagic protozoa and on saprozoic protozoa.] GWF-Wasser-
Abwasser, 122(7): 308-312 (in German).
BRINGMANN, G. & KUEHN, R. (1982) [Findings concerning the
harmful effect of water pollutants on Daphnia magna in an advanced
standardized test procedure.] Z. Wasser Abwasser Forsch., 15(1):
1-6 (in German).
BRODERICK, J.J. (1976) Raspberry: a case history. Int. Flavours
Food Add., 7: 27-30.
BROWNING, E. (1965) Toxicology and metabolism of industrial
solvents, Amsterdam, Elsevier, p. 349.
BUSING, K.H. (1952) [Eye damage caused by butylacetate and
isobutylalcohol in a cable factory.] Zent. Arbeitsmed.
Arbeitsschutz, 2: 13-14 (in German).
CATER, B.R., COOK, M.W., GANGOLLI, S.D., & GRASSO, P. (1977)
Studies on dibutyl phthalate-induced testicular atrophy in the rat:
effect on zinc metabolism. Toxicol. appl. Pharmacol., 41: 609-614.
CEC (1981) Reports of the Scientific Committe for Food. XI.
Series, Luxembourg, Commission of the European Communities.
CEDERBAUM, A.I. & COHEN, G. (1980) Oxidative demethylation of
t-butyl alcohol by rat liver microsomes. Biochem. Biophys. Res.
Commun., 97(2): 730-736.
CEDERBAUM, A.I., DICKER, A.I., & COHEN, G. (1978) Effect of
hydroxyl radical scavanger on microsomal oxidation of alcohols and
on associated microsomal reaction. Biochemistry, 17: 3058-3064.
CEDERBAUM, A.I., DICHER, E., RUBIN, E., & COHEN, G. (1979)
Effect of thiourea on microsomal oxidation of alcohols and
associated microsomal functions. Biochemistry, 18: 1187-1191.
CEDERBAUM, A.I., QURESHI, A., & COHEN, G. (1983) Production of
formaldehyde and acetone by hydroxyl-radical generating systems
during the metabolism of tertiary butylalcohols. Biochem.
Pharmacol., 32(23): 3517-3524.
CHANG, T., LEWIS, J., & GLAZKO, A.J. (1967) Effects of ethanol
and other alcohols on the transport of amino acids and glucose by
everted sacs of rat small intestine. Biochim. Biophys. Acta, 135:
1000-1007.
CHANG, S.S., PETERSON, K.J., & HO, C. (1978) Chemical reactions
involved in the deep-fat frying of foods. J. Am. Oil. Chem. Soc.,
55: 718-727.
CHIOU, C.T., FREED, V.H., SCHMEDDING, D.W., & KOHNERT, R.L. (1977)
Partition coefficient and bioaccumulation of selected organic
chemicals. Environ. Sci. Technol., 11(5): 475-478.
CHOU, W.L., SPEECE, R.E., SIDDIQIE, R.H., & MCKEON, K. (1978a)
The effect of petrochemical structure on methane fermentation
toxicity. Prog. Water Technol., 10(5/6): 545-558.
CHOU, W.L., SPEECE, R.E., & SIDDIQIE, R.H. (1978b) Acclimation
and degradation of petrochemical wastewater components by methane
fermentation. In: Scott, C.D., ed. Proceedings of the First
Symposium on Biotechnology, Energy, Production, and Conservation,
New York, Interscience Publishers, Vol. 8, pp. 391-414.
CLARK, J.B. (1953) The mutagenic action of various chemicals on
Micrococcus aureus. Proc. Oklahoma Acad. Sci., 34: 114-118.
CLEGG, D.J. (1964) The hen egg in toxicity and teratogenicity
studies. Food Cosmetol. Toxicol., 2: 717-727.
CLUTTON, D.W. & EVANS, M.B. (1978) The flavour constituents of
gin. J. Chromatogr., 167: 409-419.
COGAN, D.G. & GRANT, W.M. (1945) An unusual type of keratitis
associated with exposure to n-butyl alcohol (butanol). Arch.
Ophthalmol., 33: 106-108.
CONNOR, T.H., THEISS, J.C., HANNA, H.A., MONTEITH, D.K., &
MATHEY, T.S. (1985) Genotoxicity of organic chemicals
frequently found in the air of mobile homes. Toxicol. Lett.,
25: 33-40.
CORNISH, H.H. & ADEFUIN, J. (1967) Potentiation of carbon
tetrachloride toxicity by aliphatic alcohols. Arch. environ.
Health, 14, 447-449.
COUNCIL OF EUROPE (1981) Flavouring substances and natural
sources of flavourings, 3rd ed., Strasbourg.
DANIEL, M.A. & EVANS, M.A. (1982) Quantitative comparison of
maternal ethanol and maternal tertiary butanol diet on postnatal
developments. J. Pharmacol. exp. Ther., 222(2): 294-300.
DE CEAURRIZ, J.G., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DE CEAURRIZ, J.C., DESILES, J.P., BONNET, P., MARIGNAC, B.,
MULLER, J., & GUENIER, J.P. (1982) Concentration-dependent
behavioural changes in mice following short-term inhalation
exposure to various industrial solvents. Toxicol. appl. Pharmacol.,
67: 383-389.
DE FELICE, A., WILSON, W., & AMBRE, J. (1976) Vasoactive effects
of methanol and sodium formate on isolated canine basilar artery.
Toxicol. appl. Pharmacol., 36: 515-601.
DERACHE, R. (1970) Toxicology, pharmacology, and metabolism of
higher alcohols. In: Tremalières, J., ed. International
encyclopedia of pharmacology and therapeutics. XX. Alcohols and
derivatives, Oxford, Pergamon Press, Vol. 2.
DICKEY, F.H., CLELAND, G.H., & LOTZ, C. (1949) The role of
organic perioxides in the induction of mutations. Proc. Natl Acad.
Sci. (USA), 35: 581.
DIETZ, F.K. (1980) The role of 2-butanol and 2-butanone
metabolism in the potentiation of carbon-tetrachloride induced
hepatotoxicity. Diss. Abstr. Int. B, 41(1): 150.
DIETZ, F.K. & TRAIGER, G.J. (1979) Potentiation of CCl4
hepatotoxicity in rats by a metabolite of 2-butanol: 2,3-
butanediol. Toxicology, 14: 209-215.
DIETZ, F.K., RODRIGUEZ-GIAXOLA, M., TRAIGER, G.J., STELLA, V.J., &
HIMMELSTEIN, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9(5): 553-576.
DI VINCENZO, G.D. & HAMILTON, M.L. (1979) Fate of n-butanol in
rats after oral administration and its uptake by dogs after
inhalation or skin application. Toxicol. appl. Pharmacol., 48:
317-325.
DMITRIEV, M.T. & LISHCHIKHIN, V.A. (1979) [Determination of
toxic substances given off by polymeric materials under
experimental conditions.] Gig. i Sanit., 6: 45-48 (in Russian).
DOOLITTLE, A.K. (1954) The technology of solvents and
plasticizers, New York, John Wiley and Sons, pp. 644-645.
DORE, M., BRUNET, N., & LEGUBE, B. (1974) Participation de
différents composés organiques à la valeur des critères globaux de
pollution. Trib. Cebedeau, 28(374): 3-11.
DREYFUS, M., VANDENBUNDER, R., & BUCH, H. (1978) Stabilization of
a phosphorilase b-active conformation by hydrophobic solvents.
FEBS Lett., 95: 185-189.
DUBINA, O.N. & MAKSIMOV, G.G. (1976) [Testing the use of golden
hamsters in toxicological research.] Gig. Tr. Ohkhr. Zdorov'ya Rab.
Neft. Neftekhim. Prom-sti, 9: 100-103 (in Russian).
DUDEK, B.C. & PHILIPS, T.J. (1983) Locomotor stimulant and
intoxicant proportion of methanol, ethanol, tert-butyl alcohol, and
pentobarbital in long-sleep and short-sleep mice. Subst. Alcohol
Actions/misuse, 4: 31-36.
DUMONT, J.P. & ADDA, J. (1978) Occurrence of sesquiterpones in
mountain cheese volatiles. J. agric. food Chem., 26: 364-367.
EDWARDS, E.K., Jr & EDWARDS, E.K. (1982) Allergic reaction to
tertiary butyl alcohol in a suncreen. Cutis, 29: 476-478.
EGOROV, Y.L. (1972) Dependance of dermal toxicity of alcohols on
solubility index: oil/water. Toksikol. Gig. Prod. Neftekhim Yarosl.,
98: 102.
FEDERAL REGISTER (1977) 6 May (42188, 23148).
FERRI, S.S. (1979) Scratch-resistant coating for lenses, etc.
Braz. Pedido PI, 78 05,443 (20 March 1979).
FISHER, G.S., LEGENDRE, M.G., LOVGREN, N.V., SCHULLER, W.H., &
WELLS, J.A. (1979) Volatile constituents of southern pea seed.
J. agric. food Chem., 27: 7-11.
FLATH, R.A. & TAKAHASHI, J.R. (1978) Volatile constituents of
prickly pear. J. agric. food Chem., 26: 835-837.
FLATH, R.A., FORREY, R.R., JOHN, J.O., & CHAN, B.G. (1978)
Volatile components of corn silk (Zea mays): possible Heliothis
zea (Boddie) attractants. J. agric. food Chem., 26: 1290-1293.
FORSANDER, O. (1967) Influence of some aliphatic alcohols on the
metabolism of rat liver slices. Biochem. J., 105: 93-97.
GAILLARD, D. & DERACHE, R. (1965) Métabolisation de différents
alcools présents dans les boissons alcooliques chez le rat. Trav.
Soc. Pharmacol. Montpellier, 25: 51-62.
GEPPERT, E. VON, STURZ, J., HAASE, W., & ISSELHARD, W. (1976)
[Effect of n-butanol on the metabolic status of certain rat organs
and on the circulation of the rabbit.] Arzneim. Forsch., 26:
1333-1337 (in German).
GERARDE, H.W. & AHLSTROM, D.B. (1966) The aspiration hazard and
toxicity of a homologous series of alcohols. Arch. environ. Health,
13: 457-461.
GERHOLD, R.M. & MALANEY, G.W. (1966) Structural determinants in
the oxidation of aliphatic compounds by activated sludge. J. Water
Pollut. Control Fed., 38(4): 562-579.
GERIKE, P. & FISCHER, W.K. (1979) A correlation study of
biodegradability determinations with various chemicals in various
tests. Ecotoxicol. environ. Saf., 3: 159-173.
GIBEL, W., LOHS, K.H., WILDNER, G.P., & SCHRAMM, T. (1974)
[Experimental research on the carcinogenic effect of higher
alcohols, using 3-methyl-1-butanol, 1-propanol and 2-methyl-1-
propanol as examples.] Z. exp. Chir., 7: 235-239 (in German).
GIBEL, W., LOHS, K.H., & WILDNER, G.P. (1975) [Experimental
research on the carcinogenic effect of solvents, using
propanol-1,2- methylpropanol-1 and 3-methyl- butanol-1 as
examples.] Arch. Geschwulstforsch., 45(1): 19-24 (in German).
GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952) Appraisal of
a chemical waste problem by fish toxicity tests. Sewage ind. Wastes,
24(11): 1397-1401.
GRANT, K.A. & SAMSON, H.H. (1981) Development of physical
dependence on t-butanol in rats: an examination using schedule-
induced drinking. Pharmacol. Biochem. Behav., 14: 633-637.
GRANT, K.A. & SAMSON, H.H. (1982) Ethanol and tertiairy butanol
induced microcephaly in the neonatal rat: comparison of brain
growth parameters. Neurobehav. Toxicol. Teratol., 4: 315-321.
GUKASYAN, ZH.G., BARYSHEVA, K.F., SAAKYAN, O.A., & ARUSTAMYAN, R.K.
(1979) Nauch. Soobshch. N.-i.Proekt.In-t Tsvet.Metallur-
gii.Armniprotsvetmet, 21: 18-21.
HALL, R.L. & OSER, B.L. (1965) Recent progress in the
consideration of flavouring ingredients under the food additives
amendement. III. Gras substances. Food Technol., 151.
HARRIS, R.N. & ANDERS, M.W. (1980) Effect of fasting, diethyl
maleate, and alcohols on carbon tetrachloride-induced
hepatotoxicity. Toxicol. appl. Pharmacol., 56(2): 191-198.
HEDLUND, S.G. & KIESSLING, K.H. (1969) The physiological
mechanism involved in hangover. I. The oxidation of some lower
aliphatic fusel alcohols and aldehydes in rat liver and their
effect on the mitochondrial oxidation of various substrates.
Acta pharmacol. toxicol., 27: 381-396.
HESKETH, T.R., KEIGHTLEY, C.A., METCALFE, J.C., & RICHARDS, C.D.
(1978) Long-chain alcohols (C10-C12) can block nerve impulse.
J. Physiol., 278: 5-6.
HILL, M.W., NEALE, E., & BANEHAM, A.D. (1981) Acute tolerance to
the effects of n-butanol and n-hexanol in goldfish. J. comp.
Physiol., 142: 61-65.
HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974a) Effects
of chronic ingestion of some lower aliphatic alcohols in rats.
Res. Commun. chem. Pathol. Pharmacol., 9(1): 177-180.
HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974b) Effects
of chronic ingestion of some lower aliphatic alcohols in rats.
Jpn. J. Stud. Alcohol, 9(2): 101-108.
HILSCHER, H., GEISSLER, E., & GIBEL, W. (1969) [Research on the
toxicity and mutagenicity of certain fusel oil components in
E. coli.] Acta biol. med. Germ., 23: 843-852 (in German).
HODGE, H.C. & STERNER, J.H. (1943) Tabulation of toxicity
classes. Am. Ind. Hyg. Assoc. Q., 10: 93-96.
IARC (1984) Information bulletin on the survey of chemicals
being tested for carcinogenicity, Lyons, International Agency
for Research on Cancer, Vol. 11.
ILO (1977) Occupational exposure limits for airborne toxic
substances, Geneva, International Labour Office (Occupational
Safety and Health Series No. 37).
JADDOU, H.A., PAVEY, J.A., & MANNING, D.J. (1978) Chemical
analysis of flavor volatiles in heat-treated milks. J. dairy
Res., 45: 391-403.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L., & FITZHUGH,
D.G. (1964) Food flavourings and compounds of related structure.
I. Acute oral toxicity. Food Cosmet. Toxicol., 2: 327.
JONES, H.R. (1971) Environmental control in the organic and
petrochemical industries, Park Ridge, New Jersey, Noyes Data.
JUHNKE, I. & LUEDEMANN, D. (1978) [Results of the testing of 200
chemical compounds for acute toxicity in fish by the orfe test.]
Z. Wasser-Abwasser-Forsch., 11(5): 161-164 (in German).
JULIANO, R.L. & GAGALANG, E. (1979) The effect of membrane-
fluidizing agents on the adhesion of CHO cells. J. cell Physiol.,
98: 483-490.
KABAYASHI, H., MIYOSHI, Y., & KITAMURA, K. (1977) Effects of
various alcohols on the intramuscular absorption of isonicotinamide
in the rat. Chem. pharm. Bull., 25(11): 3078-3080.
KALEKIN, R.M. & BRICHENKO, V.S. (1972) [Toxic effect of products
from the production of butyl alcohols on the central nervous
system.] Nauch. Tr. Irkutsk. Med. Inst., 115: 22-25 (in Russian).
KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) Studies in
detoxication. The metabolism of aliphatic alcohols. The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochem. J., 53:
129-136.
KIESER, M.E., POLLARD, A., STEVENS, P.M., & TUCKNOTT, O.G. (1964)
Determination of 2-phenylethanol in cider. Nature (Lond.), 204: 887.
KOLESNIKOV, P.A. (1975) [Adaptation to butyl alcohol.] Gig i
Sanit., (5): 104-105 (in Russian).
KUDREWICZ-HUBICKA, Z., KOLACZKOWSKA, M., BORZEMSKA, & WESOLOWSKA, A.
(1978) [Serum antitrypsin activity and glycoprotein level in
workers exposed to organic solvents.] Pol. Tyg. Lek., 33, 395-397
(in Polish).
KUSHNEVA, V.S., KOLOSKOVA, G.A., KOLTUNOVA, J.G., & KIRILENKO, V.T.
(1983) Experimental data to hygienic reglementation of
isobutylalcohol in the working zone. Gig. Tr. Prof. Zabol., 1:
46-47.
LALASIDIS, G. & SJOBERG, L.B. (1978) Two new methods of
debittering protein hydrolysates and a fraction of hydrolysates
with exceptionally high content of essential amino acids. J. agric.
food Chem., 26: 742-749.
LAMMENS, H. & VERZELE, M. (1968) Aroma of hops. II. The
composition of hop oil. J. Inst. Brew., 74: 341-346.
LENDLE, L. (1928) [Investigations on the speed at which
homologous and isomeric monovalent alcohols produce narcosis.]
Naunyn-Schmiedeberg's Arch. exp. Pathol. Pharmakol., 129: 85
(in German).
LIEBICH, H.M., DOUGLAS, D.R., BAYER, E., & ZLATKIS, A. (1970)
Volatile flavour components of Cheddar cheese. J. chromatogr. Sci.,
8: 355-359.
LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROEM, G. (1979)
The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the bleak (Alburnus alburnus)
and the harpaticoid (Nitocra spinipes). Chemosphere, 8(11/12):
843-851.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975) Detection
of carcinogens as mutagens in the Salmonella/microsome test: assay
of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72: 5735-5739.
MCCOMB, J.A. & GOLDSTEIN, D.B. (1979) Quantitative comparison of
physical dependence on tertiary butanol and ethanol in mice:
correlation with lipid solubility. J. Pharmacol. exp. Ther.,
208(1): 113-117.
MCCREERY, N.J. & HUNT, W.A. (1978) Physico-chemical correlates of
alcohol intoxication. Neuropharmacology, 17: 451-461.
MCGLUMPHY, J.H. (1951) Fruit flavours. Food Technol., 5: 353-355.
MCGREGOR, D.C., SCHONBAUM, E., & BIGELOW, W.G. (1964) Acute
toxicity studies on ethanol, propanol, and butanol. Can. J. Physiol.
Pharmacol., 42: 689-696.
MACHT, D.I. (1920) A toxicological study of some alcohols, with
special reference to isomers. J. Pharmacol. exp. Ther., 16: 1-10.
MCKEE, J.E. & WOLF, H.W. (1963) Water quality criteria, 2nd ed.,
California State Quality Control Board, pp. 148-149.
MCKINNEY, R.E. & JERIS, J.S. (1955) Metabolism of low molecular
weight alcohols by activated sludge. Sewage ind. Wastes, 27(6):
728-735.
MCLAUGHIN, J., Jr, MARLIAC, J.P., VERRET, M.J., MUTCHLER, M.K., &
FITZHUGH, O.C. (1964) Toxicity of fourteen volatile chemicals as
measured by the chick embryo method. Am. Ind. Hyg. Assoc. J., 25:
282-284.
MAICKEL, R.P. & MCFADDEN, D.P. (1979) Acute toxicology of butyl
nitrites and butyl alcohols. Res. Commun. chem. Pathol. Pharmacol.,
26: 75-83.
MAICKEL, R.P. & NASH, J.F., Jr (1985) Differing effects of short-
chain alcohols on body temperature and coordinated muscular
activity in mice. Neuropharmacology, 24(1): 83-89.
MARCUS, R.J., WINTERS, W.D., & HULTIN, E. (1976) Neuro-
pharmacological effects induced by butanol, 4-hydroxy butyrate,
4-mercaptobutyric acid, thiolactone, tetrahydrofuran, pyrrolidine,
2-deoxy-d-glucose, and related substances in the rat.
Neuropharmacology, 15(1): 29-38.
MARKOVA, ET AL. (1962) J. food Sci., 27: 353.
MATTHEWS, J.S., SUGISAWA, H., & MACGREGOR, D.R. (1962) Flavour
spectrum of apple-wine volatiles. J. food Sci., 27: 355-362.
MATTSON, V.R., ARTHUR, J.W., & WALBRIDGE, C.T. (1976) Acute
toxicity of selected organic compounds to fathead minnows,
Duluth, Minnesota, US EPA Environmental Research Laboratory
(EPA No. 600/3-76-097).
MELLAN, I. (1950) Industrial solvents, New York, Van Nostrand
Reinhold Company, pp. 482-488.
MERRITT, A.D. & TOMKINS, G.M. (1959) Reversible oxidation of
cyclic secondary alcohols by liver alcohol dehydrogenase.
J. biol. Chem., 234: 2778.
MESLAR, H.W. & WHITE, H.B., III (1978) Preparation of lipid-free
protein extracts of egg yolk. Anal. Biochem., 91: 75-81.
MIKHEEV, M.I., FROLOVA, A.D., & LYUBLINA, E.I. (1977)
[Physicochemical properties and toxokinetics of some
representatives of a homologous series of alcohols.] Nek. Vopr.
Eksperim. Prom. Toksikol., 11-17 (in Russian).
MIZUTANI, Y., MIWA, Y., & MORIGUCHI, J. (1978) Jpn Kokai 78:
41,359, 14 Apr. 1978.
MONICH, J.A. (1968) Alcohols: their chemistry, properties, and
manufacture, New York, Amsterdam, London, Chapman and Reinhold.
MOREL, C. & CAVIGNEAUX, A. (1975) Alcohol isobutylique: fiche
toxicologique. Cah. notes doc., 80: 411-413.
MOSHLAKOVA, L.A., SOLDATCHENKOVA, T.P., & DRUZHININA, V.A. (1976)
Preliminary data of hygienic-chemical studies on the identification
of volatile substances released from poly(vinyl chloride) linoleum
plasticized with poly(dibutyl maleate). Gig. Aspecty Okhr.
Okruzhayushchei Sredy, 65-69.
MUIR, G.D., ed. (1977) Hazards in the chemical laboratory, 2nd
ed., London, The Chemical Society, p. 167.
MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters: narcotic
and lethal potencies to tadpoles and to rabbits. Ind. Med., 41(4):
31-33.
MUNCH, J.C. & SCHWARTZE, E.W. (1925) Narcotic and toxic potency
of aliphatic alcohols upon rabbits. J. lab. clin. Med., 10: 985-996.
NAZARENKO, I.V. (1969) [Maximum allowable concentrations for
butyl and isobutyl alcohol in drinking-water.] Sanit. ochrana
vodojemov at zagraznen. stochn. vodami. M, Medgiz, 4: 65-75
(in Russian).
NIOSH (1977a) Registry of toxic effects of chemical substances,
Rockville, Maryland, National Institute of Occupational Safety and
Health.
NIOSH (1977b) Manual of analytical methods, 2nd ed., Rockville,
Maryland, National Institute of Occupational Safety and Health,
Vol. 2.
NOVOKOVSKAYA, M.I., KLYUKVINA, T.D., KIRILLOVA, N.N., &
SHAPOSHNIKOV, YU.K. (1978) Composition of gaseous discharges
from drying oil production. Prom. Sanit. Ochistka Gazov, 5:
21-22.
NURSTEN, H.E. & WILLIAMS, A.A. (1967) Fruit aromas survey of
components identified. Chem. Ind., 12: 486-497.
OBE, G. & RISTOW, M.J. (1977) Acetaldehyde but not ethanol,
induces sister chromatid exchanges in Chinese hamster cell in
vitro. Mutat. Res., 55: 211-213.
OBE, G., RISTOW, M.J., & HERMA, J. (1977) Chromosomal damage by
alcohol in vitro and in vivo. Adv. exp. Med. Biol., 85a: 47-70.
OETTEL, H. (1936) [Effects of organic fluids on the skin.]
Arch. exp. Pathol. Pharmakol., 183: 641-696 (in German).
OLIAS JIMENEZ, J.M., DOBARGANES GARCIA, M.C., GUTIERREZ ROSALES, F.,
& GUTIERREZ GONZALES-QUIJANO, R. (1978) Volatile components in
the aroma of virgin olive oil. II. Identification sensorial
analysis of the chromatographic elements. Grasas Aceites (Seville),
29: 211-218.
PANGANAMALA, R.V., SHARMA, H.M., HEIKKILA, R.E., GEER, J.C., &
CORNWELL, D.G. (1976) Role of hydroxyl radical scavengers
dimethyl sulfoxide, alcohols, and methional in the inhibition of
prostaglandin biosynthesis. Prostaglandins, 11(4): 599-607.
PATTY, F.A., ed. (1963) Industrial hygiene and toxicology, 2nd
ed., New York, London, Sydney, John Wiley and Sons, Interscience
Publishers, Vol. 2, pp. 1441-1450.
PATTY, F.A. (1982) Industrial hygiene and toxicology, 3rd ed.,
New York, Chichester, Brisbane, Toronto, Singapore, Wiley-
Interscience, Vol. IIC, pp. 4571-4578.
PETROVA, N.I. & VISHEVSKII, A.A. (1972) [Course of pregnancy and
deliveries in women working in the organosilicon varnish and enamel
industries.] Nauch. Tr. Irkutsh. Med. Inst., 115: 102-106
(in Russian).
PITTER, P. (1976) Determination of biological degradability of
organic substances. Water Res., 10: 231-235.
POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic alcohols of
the stimulated activity of ornithine decarboxylase and tyrosine
aminotransferase occurring in regenerating rat liver. Biochem.
Pharmacol., 29(20): 2799-2803.
POSTEL, W. & ADAM, L. (1978) Gas chromatographic characterization
of whiskey. III. Irish whiskey. Brannt Wein Wirtschafft, 118:
404-407.
POSTEL, W., DRAWERT, F., & ADAM, L. (1975) [Flavourings in
brandies.] [Flavourings, International Symposium], 99-111
(in German).
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46(1): 63-77.
PURCHASE, F.H. (1969) Studies on Kaffircorn malting and brewing.
XII. The acute toxicity of some fusel oil found in Bantu beer.
S. Afr. med. J., 53: 795-462
PUSKIN, J.S. & MARTIN, T. (1978) Effects of anesthetics on
divalent cation binding and fluidity of phosphatidylserine
vescicles. Mol. Pharmacol., 14: 454-462.
REYNOLDS, T. (1977) Comparative effects of aliphatic compounds on
inhibition of lettuce fruit germination. Ann. Bot., 41(173):
637-648.
RIIHIMAKI, V. (1979) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand. J. Work
environ. Health, 5: 143-150.
ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. NY Acad. Sci., 273: 280-394.
RUMYANSTEV, A.P., GEER, V.G., OSTROUMOVA, N.A., SPIRIN, B.A., &
SHAKHIDZHANYAN, L.G. (1975) [Cumulative properties of butyl
alcohol.] Gig. i Sanit., 10: 112-113 (in Russian).
RUMYANSTEV, A.P., OSTROUMOVA, N.A., KUSTOVA, S.A., LOBANOVA, I.UA.,
TIUNOVA, L.V., DHERNIKOVA, V.V., & KOLESHNIKOV, P.A. (1976)
[Sanitary-toxicological features of butyl alcohol under conditions
of prolonged inhalation route entry.] Gig. i Sanit., 11: 12-15
(in Russian).
RUMYANSTEV, A.P., LOBANOVA, I.YA., TIUNOVA, L.V., & CHERNIKOVA, V.V.
(1979) [Toxicology of butyl alcohol.] Khim. Prom.-st. Ser.
Toksikol. Sanit. Khim. Plastmass, 2: 24-26 (in Russian).
SAAD, S.F. (1976) Effects of aliphatic alcohols on gamma-
aminobutyric acid levels in the cerebral hemispheres of rats. IRCS
med.-sci. Libr. Compend., 4: 499.
SAITO, M. (1975) [Studies on the metabolism of lower alcohols.]
Nichidai Igaku Zasshi, 34(8-9): 569-585 (in Japanese).
SAKAGAMI, Y., YOKOYAMA, H., KITANAKA, E., & IOLU, M. (1977) The
influence of phtalates on chick embryos. Osaka-furitsu Koshu
Eisei Kenkyusho Kenkyu Hokoku, Yakuji Shido Hen, 11: 15-20.
SANATINA, K.G. (1973) [Electrophysiological changes in the
peripheral neuromuscular function of persons exposed to toluene and
butyl alcohol vapors.] In: Gurghinas, S.V., ed. Vopr. Epidemiol.
Gig. Litov. SSR, Mater. Nauch. Konf. Ozdorevleniyu Vneshn. Sredy,
Nauchno-Issled., Institute of Epidemiology, Microbiology Gig.
Volnius, USSR, pp. 183-184 (in Russian).
SANDER, F. (1933) Cited by von Oettingen, W.F., Washington DC, US
Public Health Service (Public Health Bulletin No. 281) (1943).
SATLER, S.D. & THIMANN, K.V. (1980) The influence of aliphatic
alcohols on leaf senescences. Plant Physiol., 66: 395-399.
SAVELEV, A.I., BABANOV, A.G., SKOBEI, N.A., & TROITSKAYA, I.A.
(1975) [Adaptation reactions of white rats after prolonged
administration of small concentrations of butyl alcohol.] In:
Zaikina, M.G., ed. [Pathophysiology of the cardiovascular system,]
Yaroslav, Yaroslav Medical Institute, pp. 59-62, 76-80 (in Russian).
SCARAGLI, G.P., RIZZOTTI CONTI, M., BENCINI, R., DELLA CORTE, L., &
GIOTTI, A. (1975) [Toxicity of food additives. II. Membrane
damage produced by monocyclic compounds in the mitochondria and
lysosomes of rat's liver. Correlation between structure and action.]
Boll. Soc. Ital. Biol. Sper., 51: 1702-1706 (in Italian).
SCHREIER, P., DRAWERT, F., & SCHMID, M. (1978) Changes in the
composition of neutral volatile components during the production of
apple brandy. J. Sci. Food Agric., 29: 728-736.
SCHREIER, P., DRAWERT, F., & WINKLER, F. (1979) Composition of
neutral volatile constituents in grape brandies. J. agric. food
Chem., 27: 365-372.
SCHWARTZ, L. & TULIPAN, L. (1939) A textbook of occupational
diseases of the skin, Philadelphia, Pennsylvania, Lea & Febiger,
p. 717.
SEITZ, B., (1972) Vertiges graves apparus après manipulation de
butanol et d'isobutanol. A propos de trois cas. Arch. Mal. prof.
Méd. Trav. sécur. soc., 33: 393-395.
SERAFINI CESSI, S. (1975) Effects of alcohols on protein
synthetic activity in subcellular fractions from brain and liver of
rat. Arch. Sci. Biol., 59: 127-137.
SHALABY, E.S., DANASORY, M.El., & MASSOUD, A.A.E. (1973) Toxic
effects of fat solvents used in paints on liver, blood, and lung.
J. Egypt. Med. Assoc., 54: 340-347.
SHEHATA, M. & SAAD, S. (1978) The effect of aliphatic alcohols on
certain vitamins of the B-complex group in the liver of the rat.
Pol. J. Pharmacol. Pharm., 30: 35-39.
SHERMAN, P.D., Jr (1978) The butyl alcohols. In: Kirk-Othmer
Encyclopaedia of Chemical Technology, 3rd ed., Vol. 4, pp. 338-345.
SHOPSIS, C. & SATHE, S. (1984) Uridine uptake inhibition as a
cytotoxicity test: correlations with the Draize test. Toxicology,
29: 195-206.
SIEGERS, C.P., STRUBELT, O., & BREINING, H. (1974) The acute
hepatotoxicity of alcoholic beverages and some of their congeners
in guinea-pigs. Pharmacology, 12: 296-302.
SITANOV, V.S., BANDIK, K.A., ENAKAEVA, V.G., & BARANOVA, R.K.
(1979) Composition for removing grease and oil from textiles.
Otkrytiya, Izobert., Prom. Obraztsy, Tovaznye Znaky, 38: 90.
SMITH, C.W. & SIEGEL, S.M. (1975) Differential permeation of
Artemia cysts and cucumber seeds by alcohols. J. Histochem.
Cytochem., 23(1): 80-83.
SMUSIN, Y.S. & CHENSTOVA, T.F. (1973) Zdravookhr. Kaz., 4: 51-52.
SMYTH, H.F., Jr (1956) Hygienic standards for daily inhalation.
Cummings Memorial Lecture. Am. Ind. Hyg. Assoc., 17: 129-185.
SMYTH, H.F. & SMYTH, H.F., Jr (1928) Inhalation experiments with
certain lacquer solvents. J. ind. Hyg., 10: 261-271.
SMYTH, H.F., Jr, CARPENTER, C.P., & WEIL, C.S. (1951) Range-
finding toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4:
119-122.
SMYTH, H.F., Jr, CARPENTER, C.P., WEIL, C.S., & POZZANI, U.C.
(1954) Range-finding toxicity data: list V. Arch. ind. Hyg. occup.
Med., 10: 61-68.
SNELL, D. & HARRIS, R.A. (1980) Impairment of avoidance behaviour
following short-term ingestion of ethanol, tertiary-butanol, or
pentobarbital in mice. Psychopharmacology, 69(1): 53-57, 1980.
SODINI, G. & CANELLA, M. (1977) Extraction of phenols and
oligosaccharides from plant tissues (Span 445,653 1 Jun 1977).
STARK, D.M., SHOPSIS, C., BORENFREUND, E., & WALBERG, J. (1983)
Alternative approaches to the Draize assay-chemotaxis, cytology,
differentiation, and membrane transport studies. In: Goldberg, A.,
ed. Product safety and evaluation, New York, Mary Ann Liebert,
pp. 180-203.
STERNER, J.H., CROUCH, H.C., BROCKMYRE, H.F., & CUSACK, M. (1949)
A ten-year study of butyl alcohol exposure. Am. Ind. Hyg. Assoc. Q.,
10: 53-59.
SUGIYAMA, T., MIURA, R., & YAMANO, T. (1976) Purification and
properties of cytochrome P-450 from adrenocortical mithocondria and
its interaction with adrenodoxin. Adv. exp. Med. Biol., 74: 290-302.
SWORDS, G., BOBBIO, P.A., & HUNTER, G.L.K. (1978) Volatile
constituents of jack fruit. J. food Sci., 43: 639-640.
TABERSHAW, I.R., FAHY, J.P., & SKINNER, J.B. (1944) Industrial
exposure to butanol. J. ind. Hyg. Toxicol., 26: 328-330.
TAKEUCHI, H., KATADA, M., TAKAHASHI, M., KAWAMATA, S., & KOHARI, H.
(1978) Purification of polyolefins. Tokkyo Koho, 79: 126,291
1 Oct 1979.
TAVLINOVA, T.I. & DOVYBOROVA, L.N. (1979) Effect of aliphatic
alcohols on the crystal formation of hydrates of clinker minerals.
Isv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol., 22: 972-975.
TESCHKE, R., HAMASURA, Y., & LIEBER, C.S. (1974) NADPH-dependent
oxidation of methanol, ethanol, propanol, and butanol by hepatic
microsomes. Biochem. biophys. Res. Commun., 60: 851-857.
THEORELL, H. & BONNICHSEN, R. (1951) Studies on liver alcohol
dehydrogenase I. Equilibria and initial reaction velocities.
Acta. chem. Scand., 5: 1105-1126.
THOMAS, M., BOURA, A.L.A., & VIJAYAKUMAR, R. (1980) Prostaglandin
release by aliphatic alcohols from the rat isolated lung. Clin. exp.
Pharmacol. Physiol., 7(4): 373-381.
THURMAN, R.G., WINN, K., & URQUHART, B. (1980) Rat brain cyclic
AMP levels and withdrawal behaviour following treatment with
t-butanol. Adv. exp. Med. Biol., 126: 271-281.
TRAIGER, G.J. & BRUCKNER, J.V. (1976) The participation of 2
butanone in 2-butanol-induced potentiation of carbon tetrachloride
hepatotoxicity. J. Pharmacol. exp. Ther., 196: 493-500.
TRAIGER, G.J., BRUCKNER, J.V., & COOKE, P.H. (1975) Effect of
2-butanol and 2-butanone on rat hepatic ultrastructure and
microsomal drug metabolising enzyme activity. Toxicol. appl.
Pharmacol., 33(1): 132.
TRESSL, H., FRIESE, L., FENDESACK, F., & KOEPPLER, H. (1978)
Studies of the volatile composition of hops during storage.
J. agric. food Chem., 26: 1426-1430.
TSULAYA, V.R., MORENKOVA, N.V., VOLOKHOVA, L.E., & VORONIN, V.M.
(1978) [Description of the biological properties of small
concentrations of isobutyl alcohol.] Gig. i Sanit., 5: 6-9
(in Russian).
UK MAFF (1978) Food additives and contaminants. Committee
report on the review of solvents in food, London, United Kingdom
Ministry of Agriculture, Fisheries and Food.
US DHEW (1951) Survey of compounds which have been tested for
carcinogenic activity, 2nd ed., Washington DC, US Department of
Health, Education, and Welfare (Prepared by Hartwell, J.L. for the
US DHEW).
US DHEW (1978) Registry of toxic effects of chemicals, Washington
DC, US Department of Health, Education and Welfare.
US EPA (1980) Hazard information review, Washington DC, US
Environmental Protection Agency, p. 10.
VAN DEN BERG, A.P., NOORDHOEK, J., & KOOPMAN-KOOL, E. (1979a) The
relation between the sex-dependency of type I binding of
ethylmorphine and the 1-butanol-induced spectral change in mouse
liver microsomes. Biochem. Pharmacol., 28: 31-36.
VAN DEN BERG, A.P., NOORDHOEK, J., & KOOPMAN-KOOL, E. (1979b) The
use of competitive inhibition of substrate binding to cytochrome
P-450 in the determination of spectral dissociation constants for
substrates with multiple types of binding, as illustrated with
1-butanol. Biochem. Pharmacol., 28: 37-41.
VEITH, G.D., CALL, D.J., & BROOKE, L.T. (1981) Estimating the
acute toxicity of narcotic industrial chemicals to fathead
minnows, Philadelphia, Pennsylvania, American Society of Testing
and Materials (ASTM Special Technical Publication No. 802).
VELASQUEZ (1969) Audiologic impairment due to n-butyl alcohol
exposition. In: Proceedings of the International Congress on
Occupational Health, Tokyo, 1969.
VERSCHUEREN, K. (1977) Handbook of environmental data of organic
chemicals, New York, Van Nostrand Reinhold Company.
WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., & IIJIMA, M.
(1984) Induction of metamitochondria in the rat liver by n-propyl
alcohol and n-butyl alcohol. Acta pathol. Jpn., 34(3): 471-480.
WALLGREN, H. (1960) Relative intoxicating effects on rats of
ethyl, propyl, and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
WALLGREN, H., NIKANDER, P., BOGUSLAWSKY, P.V., & LINKOLA, J.
(1974) Effects of ethanol, tert-butanol, and clomethiazole on
net movements of sodium and potassium in electrically stimulated
cerebral tissue. Acta physiol. Scand., 91: 83-93.
WALTER, W. & WEIDEMANN, H.L. (1969) Coffee flavour compounds.
Z. Ernaehrungswiss, 9: 123-147.
WARTBURG, J.P. VON, BETHANE, J.L., & VALLEE, B.L. (1964) Human
liver alcohol dehydrogenase: kinetic and physiochemical properties.
Biochemistry, 3: 1775-1782.
WEESE, H. (1928) [Comparative studies on the efficacy and
toxicity of the vapours of low aliphatic alcohols.] Arch. exp.
Pathol. Pharmakol., 135: 118-130 (in German).
WEISBRODT, N.W., KIENZLE, M., & COOKE, A.R. (1973) Comparative
effects of aliphatic alcohols on the gastric mucosa. Proc. Soc. Exp.
Biol. Med., 142: 450-454.
WHO (1980) Twenty-third Report of the Joint FAO/WHO Expert
Committee on Food Additives, Geneva, World Health Organization
(WHO Technical Report Series 648).
WILLIAMS, R.T. (1969) Detoxication mechanisms, 2nd ed., London,
Chapman & Hall Ltd., p. 66.
WINER, A.D. (1958) A note of the substrate specificity of horse
liver alcohol dehydrogenase. Acta. chem. Scand., 12: 965.
WITKIN, J.M. & LEANDER, J.D. (1982) Effects of orally
administered ethanol and tert-butanol on fixed-ratio responding of
rats. Subst. Alcohol Actions/Misuse, 3: 275-279.
WOIDICH, H., PFANNHAUSER, W., & EBERHARDT, R. (1978) Results of
gas chromatographic-mass spectrographic studies of the volatile
components of applie brandies. Mitt. Hoeheren Bundeslehr-
Versuchsanst. Wein-Obstbau, Klosterneuberg, 28: 56-63.
WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.-Excerpta
Med., 440: 196-199.
WOOD, J.M. & LAVERTY, R. (1979) Physical dependence following
prolonged ethanol or t-butanol administration to rats. Pharmacol.
Biochem. Behav., 10: 113-119.
WOOD, J.N. & LAVERTY, R. (1976) Alcohol withdrawal syndrome
following prolonged 5-butanol administration to rats. Proc. Univ.
Otago Med. Sch., 54: 86-87.
YABUMOTO, K., YAMAGUCHI, M., & JENNINGS, W.G. (1978) Production
of volatile compounds by musk melon Cucumis melo. Food Chem.,
3: 7-16.
YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., & HABU, T.
(1978) Volatile flavor components of cooked rice. Agric. biol.
Chem., 42: 1229-1223.
YAMAZAKI, Y. & KATO, K. (1978) Penicillins or cephalosporins.
Jpn. Kokai Tokkio Koho, 78, 107, 484, 19 Sep. 1978.
YASHUDA, Y., CHABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
YASUDA-YASAKI, Y., NAMIKE-KANIE, S., & HACHISAKU, Y. (1978)
inhibition of germination of Bacillus subtilis spores by alcohols.
Spores, 7: 13-16.
YOJAY, L., YOJAY, R., & MARDONES, J. (1982) Acetone blood levels
after t-butanol administration in rats. IRCS Med. Sci., 10: 215.
ZAIKINA, E.I., TEREKHOVA, A.I., CHUDOV, L.N., SHATENSHTEIN, A.I.,
PETROV, E.S., SHCHERBAK, V.P., ZAKOMYRDIN, A.A., SIMETSKII, W.A., &
SOKHADZE, L.A. (1978) Repellent composition. Otkrytiya, Izobret.,
Prom. Obraztsy, Tovarnye, Znaky, 55: 18.
ZAIKOV, KH. & BOBEV, G. (1978) Chemical damages in the furniture
industry and morbidity with temporary loss of working capacity.
Khig. Zdraveopaz., 21: 141-147.
ZAMARAKHINA, L.E. (1973) [Determination of tert-butyl alcohol in
the air of industrial premises.] Gig. i Sanit., 38: 72-73
(in Russian)
When Kamil et al. (1953) administered isobutanol (25 mmol
total) by stomach tube to Chinchilla rabbits, 4.4% of the applied
dose was excreted within 24 h, as the glucuronide. After
administration of 6 ml isobutanol to the stomach of rabbits (Kamil
et al., 1953), no aldehydes or ketones were observed in the expired
air within 6 h.
Gaillard & Derache (1965) reported that, following an oral dose
of 2 g/kg body weight to rats, 0.27% was excreted in the urine
within 8 h.
The oxidation of isobutanol and other lower alcohols by both
rat liver homogenates and by the perfusion in situ of rat liver
was studied by Hedlund & Kiessling (1969). The authors observed
that the alcohols were oxidized at rates that decreased in the
following order: 1-propanol, isobutanol, ethanol, and isoamyl
alcohol. Furthermore, from their studies with the alcohol
dehydrogenase (ADH) inhibitor pyrazole, these authors concluded
that all the alcohols studied must be oxidized by ADH in the same
manner as ethanol in an NAD-mediated oxidation.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Some data on the toxicity of isobutanol for aquatic organisms
are given in Table 4. The high lethal concentrations (1000 - 4000
mg/litre) for fish, amphibia, and crustacea indicate that, at
background levels, isobutanol would not be disruptive for an
aquatic ecosystem.
Table 4. Toxicity of isobutanol for aquatic organisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Fish
Fresh-water species
Bleak 1000-3000 96-h LC50 Lindén et al.
(Alburnus (1979)
alburnus)
Golden orfe 1520 48-h LC50 Juhnke & Lüdemann
(Leuciscus (1978)
idus melanotus)
Goldfish 2600 24-h LC50 Bridié et al.
(Carassius (1979a)
auratus)
Amphibia
Tadpole 4000 threshold for Münch (1972)
(Rana sp) narcosis
Invertebrates
Fresh-water species
Water flea 1250 24-h EC50 immobilization Bringmann & Kuehn
(Daphnia magna) (1982)
Marine species
Brine shrimp 1400 24-h LC50 Price et al. (1974)
(Artemia salina) 3800 EC50 excystment Smith & Siegel
(1975)
------------------------------------------------------------------------------------------
Table 4. (contd.)
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Algae
Green algae
Scenedesmus 350 8-day no-observed- total biomass Bringmann & Kuehn
quadricauda adverse-effect level (1978a)
Blue-green algae
(Microcystis 290 8-day no-observed- total biomass Bringmann & Kuehn
aeruginosa) adverse-effect level (1978a)
------------------------------------------------------------------------------------------
7.2. Terrestrial Organisms
Toxicity studies in plants indicate that germination will not
be affected by exposure to isobutanol at background levels. An
EC50 of 760 mg/litre was reported by Reynolds (1977) for seed
germination in lettuce (Lactuca sativa). Smith & Siegel (1975)
found an EC50 of 40 800 mg/litre for seed germination in cucumber
(Cucumis sativus).
There is no information on terrestrial animals, but exposure is
unlikely to be significant, except locally after spills.
7.3. Microorganisms
Some data on the toxicity of isobutanol for microorganisms are
given in Table 5. Available toxicity data on protozoa bacteria and
algae suggest tolerance to exposure to isobutanol. No-observed-
adverse-effect levels for various species ranged from 22 to 1180
mg/litre). Exposure to background levels of isobutanol should not
be disruptive for the ecosystem.
Table 5. Toxicity of isobutanol for microorganisms
------------------------------------------------------------------------------------------
Species Concentration Parameter Comments Reference
(mg/litre)
------------------------------------------------------------------------------------------
Protozoa
Chilomonas paramaecium 22 48-h no-observed- total biomass Bringmann &
(flagellate) adverse-effect level Kuehn (1981)
Uronema parduczi 169 20-h no-observed- total biomass Bringmann &
(ciliate) adverse-effect level Kuehn (1981)
Entosiphon sulcatum 296 72-h no-observed- total biomass Bringmann &
(flagellate) adverse-effect level Kuehn (1981)
Bacteria
Pseudomonas putida 280 16-h no-observed- total biomass Bringmann &
adverse-effect level Kuehn (1976)
Bacillus subtilis 1180 EC50 spore Yasuda-Yasaki
germination et al. (1978)
------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Acute toxicity
The oral EC50 for narcosis in rabbits was 19 mmol isobutanol/kg
body weight (Münch, 1972).
Acute toxicity data are given in Table 6.
Table 6. Acute toxicity of isobutanol
---------------------------------------------------------------------
Species Route of Parameter Results Reference
administration
---------------------------------------------------------------------
Guinea- inhalation LC50 19 900 mg/m3 Kushneva et al.
pig (1983)
Mouse inhalation LC50 15 500 mg/m3 Kushneva et al.
(1983)
Rabbit inhalation LC50 26 250 mg/m3 Kushneva et al.
(1983)
Rat inhalation LCLoa 8000 mg/m3 (4 h) US DHEW (1978)
Rat inhalation LC50 19 200 mg/m3 Kushneva et al.
(1983)
Cat intravenous LDLoa 0.018 g/kg US DHEW (1978)
Rabbit oral LDLoa 3.75 g/kg US DHEW (1978)
Rabbit oral LD50 41 mmol/kg Münch (1972)
Mouse oral LD50 3.5 g/kg Kushneva et al.
(1983)
Rat oral LD50 3.1 g/kg Kushneva et al.
(1983)
Rat oral LD50 2.46 g/kg US DHEW (1978)
Rabbit skin LD50 4.24 g/kg US DHEW (1978)
---------------------------------------------------------------------
a Lo = lowest.
After ip administration of isobutanol to mice, the LD50
was > 1000 mg/kg at 30 min and 544 mg/kg at 7 days. Gross post-
mortem examination of livers showed an abnormal dark coloration
(Maickel & McFadden, 1979).
Smyth et al. (1954) reported 100% survival of rats exposed for
2 h to saturated isobutanol vapour (approximately 49 248 mg/m3 or
16 000 ppm) in air, but observed 2 deaths in a group of 6 rats when
they were exposed for 4 h to a concentration of 24 624 mg/m3 (8000
ppm) in air.
8.1.2. Signs of intoxication
The toxic effects of isobutanol are mainly alcoholic
intoxication and narcosis. Stupor and loss of voluntary movements
in animals can be considered signs of intoxication by the oral
route (Münch, 1972). Rats and rabbits were exposed, by inhalation,
for a period of 4 h to various concentrations of isobutanol. At a
concentration of 15 700 mg/m3, there was irritation of the airways.
Three days later, symptoms included central nervous depression, a
decreased number of lymphocytes in bone marrow, a decreased blood-
lactate level, delay in elimination of bromophthalein from blood,
and morphological changes including dystrophia of hepatocytes and
olfactory neurons in the brain. After exposure to 8000 mg/m3,
symptoms were similar but less severe. An isobutanol concentration
of 1300 mg/m3 decreased bone marrow lymphocyte numbers. A
concentration of 100 mg/m3 only altered breathing frequency
(Kushneva et al., 1983).
8.2. Skin and Eye Irritation
Application of 500 mg isobutanol to the skin of rabbits for
24 h was moderately irritating. However, application of 2 mg to
the rabbit eye caused severe irritation (US DHEW, 1978).
8.3. Short-Term Exposures
Hillbom et al. (1974a,b) gave one group of 6 male Wister rats
a 1 mol/litre solution of isobutanol as their sole drinking liquid
for a period of 4 months. Another group of 5 rats of the same
species was given a 2 mol/litre solution of isobutanol as their
sole drinking liquid for 2 months. Both groups were provided an
ad libitum diet of ordinary laboratory food containing about 26%
of calories as protein, 55% as carbohydrate, and 19% as fat. At
the end of the study, all animals were decapitated, and their
livers examined. No adverse effects were detected in the group
given the 1 mol/litre solution, while the rats in the group given
the 2 mol/litre solution showed a decrease in fat, glycogen, RNA,
and overall size of the liver cells.
Oral administration of between 1/10 and 1/5 of the LD50 of
isobutanol for 6 days/week over 1 month, did not result in any
deaths in rats (Kushneva et al., 1983). Continuous exposure of
rats, by inhalation, to isobutanol at 3 mg/m3 over 4 months,
caused an increased threshold for leg withdrawal response to
electrical stimulation, and depression of haemoglobin content,
erythrocyte count, and activities of cholinesterase and catalase
in the blood. The activities of alanine-amino transferase and
aspartate amino transferase were elevated. At 0.5 mg/m3, numbers
of white cells and erythrocytes, haemogloglobin content, and
cholinesterase activity were all decreased. No effects were
observed at 0.1 mg/m3 (Tsulaya et al., 1978).
8.4. Long-Term Exposures
With the exception of a carcinogenicity study, no long-term
studies have been reported.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
No relevant data are available on reproduction or
embryotoxicity and no conclusions can therefore be drawn.
8.6. Mutagenicity
The only reported study on mutagenicity is that of Hillscher et
al. (1969) who demonstrated an increased rate of reverse mutation
when Escherichia coli CA 274 was treated with 0.7% isobutanol,
without metabolic activation. This study in itself is inadequate
to assess the mutagenic potential of the compounds.
8.7. Carcinogenicity
Two groups of 19 and 24 male and female Wistar rats (10 weeks
old) were administered purified isobutanol. Group one received
0.2 ml/kg body weight, orally, twice weekly. Group two received
0.05 ml/kg, subcutaneously, twice weekly. Two control groups (25
rats each) received 1 ml of 0.9% sodium chloride twice weekly
(controls for group one were dosed orally; controls for group two
were dosed subcutaneously). All rats were dosed until spontaneous
death. Test rats presented liver carcinomas and sarcomas, spleen
sarcomas, stomach proventricular carcinomas, and myeoid leukaemia.
Tumours of these types did not occur in the control groups (Table
7). In the exposed animals, toxic liver damage was found, ranging
from steatosis and cell necrosis to fibrosis and cirrhosis. In
addition, most exposed animals had hyperplasia of blood-forming
tissues. The carcinogenic response was attributed to isobutanol
(Gibel et al., 1974, 1975). No statistical analysis of the data
was performed.
Table 7. Summary of tumours in experimental animalsa
-------------------------------------------------------------------
Agent Application Number Average Malignant Benign
(twice weekly) of survival tumour tumour
animals (days)
-------------------------------------------------------------------
Control oral: 1 ml/kg 25 643 0 3
(0.9% NaCl) body weight
solution)
subcutaneous: 25 643 0 2
1 ml/kg body
weight
Isobutanol oral: 0.2 ml/kg 19 495 3b 9
body weight
subcutaneous: 24 544 8c 3
0.05 ml/kg
body weight
-------------------------------------------------------------------
a Adapted from: Gibel et al. (1975).
b Proventriculus carcinoma and liver cell carcinoma; proventriculus
carcinoma and myeloid leukaemia; myeloid leukaemia.
c Proventriculus carcinoma (2); liver sarcoma (2); spleen sarcoma;
mesothelioma; retroperitoneal sarcoma (2).
8.8. Special Studies
In 8 guinea-pigs, serum enzyme (SGOT, SGPT, GLDH) activities
and triglyceride levels were measured 16 h after oral
administration of 33.5 mg isobutanol/kg olive oil. There was no
statistically-significant difference between treated animals and 8
olive-oil treated controls (Siegers et al., 1974).
Isobutanol can potentiate the hepatic toxicity of carbon
tetrachloride (Cornish & Adefuin, 1967).
9. EFFECTS ON MAN
Isobutanol may be absorbed through the lungs and the
gastrointestinal tract (Browning, 1965).
No data are available on the effects of isobutanol on the skin.
However, as with other defatting solvents, isobutanol may cause
erythematous skin lesions (Schwarz & Tulipan, 1939).
Immersion of the hands of 5 healthy male volunteers in a
1:1 (v/v) mixture of m-xylene and isobutanol, for 15 min at
room temperature, caused only a mild and sometimes barely
distinguishable erythema or a burning feeling. Isobutanol (in a
1:1 mixture with m-xylene) dehydrated the skin and decreased the
absorption of xylene. However, when water was added as 8% of the
mixture, this effect was reduced. The dermal effects of isobutanol
only and its absorption were not studied (Riihimaki, 1979).
Seitz (1972) reported 7 case histories, occurring between
1965 and 1971, of workers who had been exposed to 1-butanol and
isobutanol in a non-ventilated photographic laboratory. They
handled the alcohols under intense and hot light, without any
precautions. Exposure levels were not quantified, but must have
been excessive; exposure time ranged from 1 1/2 months to 2 years.
Two workers had transient vertigo, 3 severe Meniere-like vertigo
with nausea, vomiting and/or headache. In one of these cases,
hearing was also perturbed. Two workers did not present any signs
or symptoms.
Eye irritation, blurred vision, and transient corneal
vacuolization have been decribed in another excessive (no
measurements done) mixed exposure of workers to isobutanol and
butyl acetate. With adequate work room ventilation, it did not
recur. The author felt that the more irritant butyl acetate would
be the main contributor to this effect (Büsing, 1952).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure levels
Levels of exposure of the general population to isobutanol
through food and beverages are not available and occupational
exposure levels are limited and inadequate.
10.1.2. Toxic effects
In animals, isobutanol is absorbed through the skin, lungs,
and gastrointestinal tract. Isobutanol is metabolized by alcohol
dehydrogenase to isobutyric acid via the aldehyde and may enter the
tricarboxylic acid cycle. Small amounts of isobutanol are excreted
unchanged (< 0.5% of the dose), or as the glucuronide (< 5% of
the dose) in the urine. In rabbits, metabolites found in the urine
include acetaldehyde, acetic acid, isobutyraldehyde, and isovaleric
acid. Oral LD50 values (2.5 - 3.1 g/kg body weight) and inhalation
LC50 (19.2 g/m3) in rats classify isobutanol as slightly toxic
according to Hodge & Sterner. The acute toxic effects are
alcoholic intoxication and narcosis. Isobutanol is severely
irritating to the eyes and moderately irritating to the skin. A
group of rats given a 1 mol/litre solution of isobutanol as their
sole drinking liquid for 4 months did not show any adverse effects
in the liver; another group given a 2 mol/litre solution as their
sole drinking liquid for 2 months showed a reduction in fat,
glycogen, and RNA content, and in the overall size of the cells in
the liver. Continuous inhalation exposure of rats to 3 mg/m3 for
4 months resulted in depression of leg withdrawal response to
electrical stimulation, minor changes of formed elements of the
blood and serum enzymes. The estimated no-observed-adverse-effect
level was 0.1 mg/m3.
In a lifetime carcinogenicity study, groups of rats received
isobutanol subcutaneously (0.05 ml/kg body weight twice a week) or
orally (0.2 ml/kg body weight twice a week). The animals exhibited
toxic liver damage ranging from steatosis to cirrhosis. Numbers of
animals showing malignant tumours totalled 8 in the subcutaneous
group, 3 in the oral group, and 0 in the control group. The
majority of treated animals also showed hyperplasia of blood-
forming tissues.
Because of lack of mutagenicity studies, the Task Group could
not determine whether isobutanol was a genetically active compound.
The findings in the carcinogenicity study are a cause for concern.
Because of methodological inadequacies and the manner of reporting
the data, it was not possible to determine whether isobutanol
should be regarded as an animal carcinogen. Thus it is not
possible to extrapolate from this study to possible long-term
effects in man.
From the animal studies available, it is not possible to
determine a no-observed-adverse-effect level for long-term
exposure. No adequate data are available to assess mutagenicity
or teratogenicity of isobutanol or effects on reproduction.
Exposure of the general population to isobutanol through food
and beverages is unlikely to lead to acute toxic effects. The only
reported observations in man relate to the production of vertigo
under conditions of severe and prolonged exposure to vapour
mixtures of isobutanol and 1-butanol. From this study, it is
not possible to attribute the vertigo to a single cause.
10.2. Evaluation of Effects on the Environment
10.2.1. Exposure levels
Little quantitative data relating to levels in the general
environment are available, but, because isobutanol is readily
biodegradable, substantial concentrations are only likely to occur
locally in the case of major spillages.
10.2.2. Toxic effects
At background concentrations likely to occur in the
environment, isobutanol is not directly toxic for fish, amphibia,
crustacea, or algae. Protozoa will be tolerant to levels of
isobutanol likely to be found in the environment.
Isobutanol should be managed in the environment as a slightly
toxic compound. It poses an indirect hazard to the aquatic
environment, because it is readily biodegradable, which may lead to
oxygen depletion.
10.3. Conclusions
1. On the basis of available data, the Task Group considered it
unlikely that isobutanol would pose a serious acute health risk
to the general population under normal exposure conditions.
However, the Task Group was unable to make an assessment of the
long-term health risk of isobutanol for the general population.
It was concluded that the results of the carcinogenicity study
need verification by a bioassay of modern standards.
2. The Task Group considered that the data available were
inadequate to set an occupational exposure limit. In line with
good manufacturing practice, exposure to isobutanol should be
minimized.
3. The ecotoxicological data available indicate that the impact of
background concentrations of isobutanol on the aquatic
environment can be expected to be minimal.
11. RECOMMENDATIONS
1. The Task Group noted that, from the animal studies available,
it is not possible to determine a no-observed-adverse-effect
level. Relevant studies should be conducted so that this can
be achieved.
2. The Task Group considered that adequate studies should be
conducted to assess the mutagenicity and carcinogenicity of
isobutanol.
3. Epidemiological studies, including precise exposure data, would
assist in a better assessment of the occupational hazard of
isobutanol.
4. Additional information on environmental pathways (notably
emission and leaching) and residues are desirable.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Council of Europe (1981) included isobutanol in the list
of flavouring substances that can be added to foodstuffs without
hazard to public health at a level of 25 mg/kg for beverages and
food.
At their 23rd meeting, the Joint FAO/WHO Expert Committee on
Food Additives (JECFA) reviewed the data on isobutanol. They
concluded that:
"The evaluation of this compound was not possible
owing to the paucity of toxicological data. New
specifications were prepared, but no toxicological
monograph" (WHO, 1980).
REFERENCES
AARSTAD, K., ZAHLSEN, K., & NILSEN, O.G. (in press) Inhalation of
butanols: changes in the cytochrome P-450 enzyme system. In:
Proceedings of the European Society of Toxicology, 1984 Annual
Meeting.
ABBASOV, A.Z., PANOV, V.N., & ALIEV, A.M. (1971) [Determination
of a mixture of butyl alcohols in the air of industrial premises by
means of a chromatographic method.] Gig. i Sanit., 36: 61-63
(in Russian).
ABBONDANDOLO, A., BONATTI, S., CORSI, C., CORTI, G., FIORIO, R.,
LEPORINI, L., MAZZACCARO, A., & NIEIR, R. (1980) The use of
organic solvents in mutagenicity testing. Mutat. Res., 79: 141-150.
ACGIH, (1980) Documentation of the threshold limit values, 4th
ed., Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
AITIO, A. (1977) Inhibition of ethoxycumarin deethylation by
organic solvents. Res. Commun. chem. Pathol. Pharmacol., 1:
773-776.
AKHREM, A.A., POPOVA, E.M., & METELITSA, O.I. (1978) Interaction
of aliphatic alcohols with cytochrome P-450 from rat liver
microsomes. Biokhimya (USSR), 43: 1485-1491.
AMUNDSEN, J., GOODWIN, R.J., & WETZEL, W.H. (1979) Water-soluble
pentachlorophenol and tetrachlorophenol wood-treating systems.
S. African, 78: 01,031 (3 January 1979).
ANDERSON, R.A., REDDY, J.M., JOYCE, C., WILLIS, B.R., VAN DER VEN,
H., & ZANEVELD, L.J.D. (1982) Inhibition of mouse sperm
capacitation by ethanol. Biol. Reprod., 27: 833-840.
ANON (1983) Selection of priority compounds: emissions. In:
Publikatiereeks Lucht, The Hague, The Netherlands, Ministry of
Housing, Physical Planning and Environment, Vol. 10.
AOAC METHODS (1975) In: Horwitz, W., ed. Official methods of
analysis of the Association of Official Analytical Chemists,
Washington DC, Association of Official Analytical Chemists.
ARTIGAS GIMENEZ, G., URDANGARAY ARGUELLES, V., GONZALES BLASQUEZ,
I., & ALONSO RODRIGUEZ, J. (1979) Protective coating for glass
objects. Fr. Demande, 2: 410, 115 (23 March 1979).
ASHFORD, M.L.J. & WANN, K.T. (1979) A comparison of the effects
of butanol and benzyl alcohol on the frog end-plate conductance.
J. Physiol., 295: 86-87.
ASTM (1977) Testing methods for butanols. Annual book of
standards. Part 29, Philadelphia, Pennsylvania, American Society
for Testing and Materials (ASTM D304-58).
ASTRAND, I., OVRUM, P., LINDQUIST, T., & HULTENGREN, M. (1976)
Exposure to butyl alcohol: uptake and distribution in man. Scand.
J. Work Environ. Health, 3: 165-175.
AUTY, R.M. & BRANCH, R.A. (1976) The elimination of ethyl,
n-propyl, n-butyl, and iso-amyl alcohols by the isolated perfused
rat liver. J. Pharm. exp. Ther., 197: 669-674.
BAIKOV, B.K. & KHACHATURYAN, M.Kh. (1973) [Hygienic assessment of
the reflex action on a body of small concentrations of butyl
alcohol in the atmosphere.] Gig. i Sanit., 12: 7-11 (in Russian).
BAKER, R.C., SORENSON, S.M., ELOG, S.R., & DEITRICH, R.A. (1979)
Acetone excretion following t-butanol treatment in rats. In:
Proceedings of the 3rd International Symposium on Alcohol and
Aldehyde Metabolizing Systems, Toronto (Abstract No. 44).
BAKER, R.C., SORENSEN, S.M., & DEITRICH, R.A. (1982) The in vivo
metabolism of tertiary butanol by adult rat. Alcohol. clin. exp.
Res., 6(2): 247-251.
BARTHA, T., GONCZIL, L., & MOLLO, A. (1973) The use of metabolic
product analysis with simplified gas-liquid chromatographic methods
in routine anaerobic laboratory analysis. Egeszesegtodamany, 22:
220-227.
BEAUD, P. & RAMUZ, A. (1978) Gas-liquid chromatography of
simultaneous determination of higher alcohols and ethyl acetate in
spirits. Mitt. Geb. Lebensmittelunters. Hyg., 69: 423-30.
BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1981)
Liver lipid disposal following t-butanol administration to rats.
Chem.-biol. Interact., 38: 45-51.
BEAUGE, F., FLEURET, C., BARIN, F., & NORDMANN, R. (1984) Brain
membrane disordering after acute in vivo administration of ethanol,
isopropanol, or t-butanol in rats. Biochem. Pharmacol., 33:
3591-3593.
BELLIN, S.I. & EDMONDS, H.L., Jr (1976) The use of tert-butanol
in alcohol dependence studies. Proc. West. Pharmacol. Soc., 19:
351-354.
BENGTSSON, B.E., RENBERG, L., & TARKPEA, M. (1984) Molecular
structure and aquatic toxicity: an example with C1-C13 aliphatic
alcohols. Chemosphere, 13(5/6): 613-622.
BIKFALVI, I. & PASZTOR, L. (1977) Study on the components
distillates of wine using gas chromatography. Szeszipar, 25:
96-100.
BIRKETT, D.J. (1974) Interaction of some drugs, metal ions, and
alcohols with rat liver microsomes as studied with a fluorescent
probe. Clin. exp. Pharmacol. Physiol., 1: 415-427.
BLOK, J. (1981) [A simple toxicity test using nitrifying
bacteria.] H2 O, 14(11): 242-245 (in Dutch).
BLOOM, S.E. (1981) Detection of sister chromatid exchanges in
vivo using avian embryos. In: Cytogenetic assays of environmental
mutagens, T.C.H.S.U. ed: Allenheld, Osmun, Totowa, New Jersey.
BOBER, A. & HADDAWAY, L.W. (1963) Gas chromatographic
identification of alcoholic beverages. J. gas Chromatogr., 1: 8-13.
BONTE, W. (1978) Congener content of wine and similar beverages.
Blutalkohol, 15: 392-404.
BONTE, W. (1979) Congener substances in German and foreign
beers. Blutalkohol, 16: 108-124.
BONTE, W., DECKER, J., & BUSSE, J. (1978) Congener content of
high-proof alcoholic beverages. Blutalkohol, 16: 108-124.
BRAY, W.J. & HUMPHRIES, C. (1978) Solvent fractionation of leaf
juice to prepare green and white protein products. J. Sci. Food
Agric., 29: 839-846.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRINGMANN, G. & KUEHN, R. (1976) [Comparative findings concerning
the harmful effect of water pollutants on bacteria (Pseudomonas
putida) and blue-green algae (Microcystis aeruginosa).] GWF-
Wasser-Abwasser, 117(9): 410-413 (in German).
BRINGMANN, G. & KUEHN, R. (1977) Results of the damaging effect
of water pollutants on Daphnia magna. Z. Wasser Abwasser Forsch.,
10: 161-166.
BRINGMANN, G. & KUEHN, R. (1978a) [Limit values for the harmful
effect of water pollutants on blue-green algae (Microcystis
aeruginosa) and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Vom Wasser, 50: 45-60 (in German).
BRINGMANN, G. & KUEHN, R. (1978b) Testing of substances for their
toxicity threshold: model organisms Microcystis (Diplocystis)
aeruginosa and Scenedesmus quadriconda. Mitt. Int. Ver. Theor.
Angew. Limnol., 21: 275-284.
BRINGMANN, G. & KUEHN, R. (1981) [Comparison of the effect of
harmful substances on flagellates, on ciliates, on holozoic
bacteriophagic protozoa and on saprozoic protozoa.] GWF-Wasser-
Abwasser, 122(7): 308-312 (in German).
BRINGMANN, G. & KUEHN, R. (1982) [Findings concerning the
harmful effect of water pollutants on Daphnia magna in an advanced
standardized test procedure.] Z. Wasser Abwasser Forsch., 15(1):
1-6 (in German).
BRODERICK, J.J. (1976) Raspberry: a case history. Int. Flavours
Food Add., 7: 27-30.
BROWNING, E. (1965) Toxicology and metabolism of industrial
solvents, Amsterdam, Elsevier, p. 349.
BUSING, K.H. (1952) [Eye damage caused by butylacetate and
isobutylalcohol in a cable factory.] Zent. Arbeitsmed.
Arbeitsschutz, 2: 13-14 (in German).
CATER, B.R., COOK, M.W., GANGOLLI, S.D., & GRASSO, P. (1977)
Studies on dibutyl phthalate-induced testicular atrophy in the rat:
effect on zinc metabolism. Toxicol. appl. Pharmacol., 41: 609-614.
CEC (1981) Reports of the Scientific Committe for Food. XI.
Series, Luxembourg, Commission of the European Communities.
CEDERBAUM, A.I. & COHEN, G. (1980) Oxidative demethylation of
t-butyl alcohol by rat liver microsomes. Biochem. Biophys. Res.
Commun., 97(2): 730-736.
CEDERBAUM, A.I., DICKER, A.I., & COHEN, G. (1978) Effect of
hydroxyl radical scavanger on microsomal oxidation of alcohols and
on associated microsomal reaction. Biochemistry, 17: 3058-3064.
CEDERBAUM, A.I., DICHER, E., RUBIN, E., & COHEN, G. (1979)
Effect of thiourea on microsomal oxidation of alcohols and
associated microsomal functions. Biochemistry, 18: 1187-1191.
CEDERBAUM, A.I., QURESHI, A., & COHEN, G. (1983) Production of
formaldehyde and acetone by hydroxyl-radical generating systems
during the metabolism of tertiary butylalcohols. Biochem.
Pharmacol., 32(23): 3517-3524.
CHANG, T., LEWIS, J., & GLAZKO, A.J. (1967) Effects of ethanol
and other alcohols on the transport of amino acids and glucose by
everted sacs of rat small intestine. Biochim. Biophys. Acta, 135:
1000-1007.
CHANG, S.S., PETERSON, K.J., & HO, C. (1978) Chemical reactions
involved in the deep-fat frying of foods. J. Am. Oil. Chem. Soc.,
55: 718-727.
CHIOU, C.T., FREED, V.H., SCHMEDDING, D.W., & KOHNERT, R.L. (1977)
Partition coefficient and bioaccumulation of selected organic
chemicals. Environ. Sci. Technol., 11(5): 475-478.
CHOU, W.L., SPEECE, R.E., SIDDIQIE, R.H., & MCKEON, K. (1978a)
The effect of petrochemical structure on methane fermentation
toxicity. Prog. Water Technol., 10(5/6): 545-558.
CHOU, W.L., SPEECE, R.E., & SIDDIQIE, R.H. (1978b) Acclimation
and degradation of petrochemical wastewater components by methane
fermentation. In: Scott, C.D., ed. Proceedings of the First
Symposium on Biotechnology, Energy, Production, and Conservation,
New York, Interscience Publishers, Vol. 8, pp. 391-414.
CLARK, J.B. (1953) The mutagenic action of various chemicals on
Micrococcus aureus. Proc. Oklahoma Acad. Sci., 34: 114-118.
CLEGG, D.J. (1964) The hen egg in toxicity and teratogenicity
studies. Food Cosmetol. Toxicol., 2: 717-727.
CLUTTON, D.W. & EVANS, M.B. (1978) The flavour constituents of
gin. J. Chromatogr., 167: 409-419.
COGAN, D.G. & GRANT, W.M. (1945) An unusual type of keratitis
associated with exposure to n-butyl alcohol (butanol). Arch.
Ophthalmol., 33: 106-108.
CONNOR, T.H., THEISS, J.C., HANNA, H.A., MONTEITH, D.K., &
MATHEY, T.S. (1985) Genotoxicity of organic chemicals
frequently found in the air of mobile homes. Toxicol. Lett.,
25: 33-40.
CORNISH, H.H. & ADEFUIN, J. (1967) Potentiation of carbon
tetrachloride toxicity by aliphatic alcohols. Arch. environ.
Health, 14, 447-449.
COUNCIL OF EUROPE (1981) Flavouring substances and natural
sources of flavourings, 3rd ed., Strasbourg.
DANIEL, M.A. & EVANS, M.A. (1982) Quantitative comparison of
maternal ethanol and maternal tertiary butanol diet on postnatal
developments. J. Pharmacol. exp. Ther., 222(2): 294-300.
DE CEAURRIZ, J.G., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DE CEAURRIZ, J.C., DESILES, J.P., BONNET, P., MARIGNAC, B.,
MULLER, J., & GUENIER, J.P. (1982) Concentration-dependent
behavioural changes in mice following short-term inhalation
exposure to various industrial solvents. Toxicol. appl. Pharmacol.,
67: 383-389.
DE FELICE, A., WILSON, W., & AMBRE, J. (1976) Vasoactive effects
of methanol and sodium formate on isolated canine basilar artery.
Toxicol. appl. Pharmacol., 36: 515-601.
DERACHE, R. (1970) Toxicology, pharmacology, and metabolism of
higher alcohols. In: Tremalières, J., ed. International
encyclopedia of pharmacology and therapeutics. XX. Alcohols and
derivatives, Oxford, Pergamon Press, Vol. 2.
DICKEY, F.H., CLELAND, G.H., & LOTZ, C. (1949) The role of
organic perioxides in the induction of mutations. Proc. Natl Acad.
Sci. (USA), 35: 581.
DIETZ, F.K. (1980) The role of 2-butanol and 2-butanone
metabolism in the potentiation of carbon-tetrachloride induced
hepatotoxicity. Diss. Abstr. Int. B, 41(1): 150.
DIETZ, F.K. & TRAIGER, G.J. (1979) Potentiation of CCl4
hepatotoxicity in rats by a metabolite of 2-butanol: 2,3-
butanediol. Toxicology, 14: 209-215.
DIETZ, F.K., RODRIGUEZ-GIAXOLA, M., TRAIGER, G.J., STELLA, V.J., &
HIMMELSTEIN, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9(5): 553-576.
DI VINCENZO, G.D. & HAMILTON, M.L. (1979) Fate of n-butanol in
rats after oral administration and its uptake by dogs after
inhalation or skin application. Toxicol. appl. Pharmacol., 48:
317-325.
DMITRIEV, M.T. & LISHCHIKHIN, V.A. (1979) [Determination of
toxic substances given off by polymeric materials under
experimental conditions.] Gig. i Sanit., 6: 45-48 (in Russian).
DOOLITTLE, A.K. (1954) The technology of solvents and
plasticizers, New York, John Wiley and Sons, pp. 644-645.
DORE, M., BRUNET, N., & LEGUBE, B. (1974) Participation de
différents composés organiques à la valeur des critères globaux de
pollution. Trib. Cebedeau, 28(374): 3-11.
DREYFUS, M., VANDENBUNDER, R., & BUCH, H. (1978) Stabilization of
a phosphorilase b-active conformation by hydrophobic solvents.
FEBS Lett., 95: 185-189.
DUBINA, O.N. & MAKSIMOV, G.G. (1976) [Testing the use of golden
hamsters in toxicological research.] Gig. Tr. Ohkhr. Zdorov'ya Rab.
Neft. Neftekhim. Prom-sti, 9: 100-103 (in Russian).
DUDEK, B.C. & PHILIPS, T.J. (1983) Locomotor stimulant and
intoxicant proportion of methanol, ethanol, tert-butyl alcohol, and
pentobarbital in long-sleep and short-sleep mice. Subst. Alcohol
Actions/misuse, 4: 31-36.
DUMONT, J.P. & ADDA, J. (1978) Occurrence of sesquiterpones in
mountain cheese volatiles. J. agric. food Chem., 26: 364-367.
EDWARDS, E.K., Jr & EDWARDS, E.K. (1982) Allergic reaction to
tertiary butyl alcohol in a suncreen. Cutis, 29: 476-478.
EGOROV, Y.L. (1972) Dependance of dermal toxicity of alcohols on
solubility index: oil/water. Toksikol. Gig. Prod. Neftekhim Yarosl.,
98: 102.
FEDERAL REGISTER (1977) 6 May (42188, 23148).
FERRI, S.S. (1979) Scratch-resistant coating for lenses, etc.
Braz. Pedido PI, 78 05,443 (20 March 1979).
FISHER, G.S., LEGENDRE, M.G., LOVGREN, N.V., SCHULLER, W.H., &
WELLS, J.A. (1979) Volatile constituents of southern pea seed.
J. agric. food Chem., 27: 7-11.
FLATH, R.A. & TAKAHASHI, J.R. (1978) Volatile constituents of
prickly pear. J. agric. food Chem., 26: 835-837.
FLATH, R.A., FORREY, R.R., JOHN, J.O., & CHAN, B.G. (1978)
Volatile components of corn silk (Zea mays): possible Heliothis
zea (Boddie) attractants. J. agric. food Chem., 26: 1290-1293.
FORSANDER, O. (1967) Influence of some aliphatic alcohols on the
metabolism of rat liver slices. Biochem. J., 105: 93-97.
GAILLARD, D. & DERACHE, R. (1965) Métabolisation de différents
alcools présents dans les boissons alcooliques chez le rat. Trav.
Soc. Pharmacol. Montpellier, 25: 51-62.
GEPPERT, E. VON, STURZ, J., HAASE, W., & ISSELHARD, W. (1976)
[Effect of n-butanol on the metabolic status of certain rat organs
and on the circulation of the rabbit.] Arzneim. Forsch., 26:
1333-1337 (in German).
GERARDE, H.W. & AHLSTROM, D.B. (1966) The aspiration hazard and
toxicity of a homologous series of alcohols. Arch. environ. Health,
13: 457-461.
GERHOLD, R.M. & MALANEY, G.W. (1966) Structural determinants in
the oxidation of aliphatic compounds by activated sludge. J. Water
Pollut. Control Fed., 38(4): 562-579.
GERIKE, P. & FISCHER, W.K. (1979) A correlation study of
biodegradability determinations with various chemicals in various
tests. Ecotoxicol. environ. Saf., 3: 159-173.
GIBEL, W., LOHS, K.H., WILDNER, G.P., & SCHRAMM, T. (1974)
[Experimental research on the carcinogenic effect of higher
alcohols, using 3-methyl-1-butanol, 1-propanol and 2-methyl-1-
propanol as examples.] Z. exp. Chir., 7: 235-239 (in German).
GIBEL, W., LOHS, K.H., & WILDNER, G.P. (1975) [Experimental
research on the carcinogenic effect of solvents, using
propanol-1,2- methylpropanol-1 and 3-methyl- butanol-1 as
examples.] Arch. Geschwulstforsch., 45(1): 19-24 (in German).
GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952) Appraisal of
a chemical waste problem by fish toxicity tests. Sewage ind. Wastes,
24(11): 1397-1401.
GRANT, K.A. & SAMSON, H.H. (1981) Development of physical
dependence on t-butanol in rats: an examination using schedule-
induced drinking. Pharmacol. Biochem. Behav., 14: 633-637.
GRANT, K.A. & SAMSON, H.H. (1982) Ethanol and tertiairy butanol
induced microcephaly in the neonatal rat: comparison of brain
growth parameters. Neurobehav. Toxicol. Teratol., 4: 315-321.
GUKASYAN, ZH.G., BARYSHEVA, K.F., SAAKYAN, O.A., & ARUSTAMYAN, R.K.
(1979) Nauch. Soobshch. N.-i.Proekt.In-t Tsvet.Metallur-
gii.Armniprotsvetmet, 21: 18-21.
HALL, R.L. & OSER, B.L. (1965) Recent progress in the
consideration of flavouring ingredients under the food additives
amendement. III. Gras substances. Food Technol., 151.
HARRIS, R.N. & ANDERS, M.W. (1980) Effect of fasting, diethyl
maleate, and alcohols on carbon tetrachloride-induced
hepatotoxicity. Toxicol. appl. Pharmacol., 56(2): 191-198.
HEDLUND, S.G. & KIESSLING, K.H. (1969) The physiological
mechanism involved in hangover. I. The oxidation of some lower
aliphatic fusel alcohols and aldehydes in rat liver and their
effect on the mitochondrial oxidation of various substrates.
Acta pharmacol. toxicol., 27: 381-396.
HESKETH, T.R., KEIGHTLEY, C.A., METCALFE, J.C., & RICHARDS, C.D.
(1978) Long-chain alcohols (C10-C12) can block nerve impulse.
J. Physiol., 278: 5-6.
HILL, M.W., NEALE, E., & BANEHAM, A.D. (1981) Acute tolerance to
the effects of n-butanol and n-hexanol in goldfish. J. comp.
Physiol., 142: 61-65.
HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974a) Effects
of chronic ingestion of some lower aliphatic alcohols in rats.
Res. Commun. chem. Pathol. Pharmacol., 9(1): 177-180.
HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974b) Effects
of chronic ingestion of some lower aliphatic alcohols in rats.
Jpn. J. Stud. Alcohol, 9(2): 101-108.
HILSCHER, H., GEISSLER, E., & GIBEL, W. (1969) [Research on the
toxicity and mutagenicity of certain fusel oil components in
E. coli.] Acta biol. med. Germ., 23: 843-852 (in German).
HODGE, H.C. & STERNER, J.H. (1943) Tabulation of toxicity
classes. Am. Ind. Hyg. Assoc. Q., 10: 93-96.
IARC (1984) Information bulletin on the survey of chemicals
being tested for carcinogenicity, Lyons, International Agency
for Research on Cancer, Vol. 11.
ILO (1977) Occupational exposure limits for airborne toxic
substances, Geneva, International Labour Office (Occupational
Safety and Health Series No. 37).
JADDOU, H.A., PAVEY, J.A., & MANNING, D.J. (1978) Chemical
analysis of flavor volatiles in heat-treated milks. J. dairy
Res., 45: 391-403.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L., & FITZHUGH,
D.G. (1964) Food flavourings and compounds of related structure.
I. Acute oral toxicity. Food Cosmet. Toxicol., 2: 327.
JONES, H.R. (1971) Environmental control in the organic and
petrochemical industries, Park Ridge, New Jersey, Noyes Data.
JUHNKE, I. & LUEDEMANN, D. (1978) [Results of the testing of 200
chemical compounds for acute toxicity in fish by the orfe test.]
Z. Wasser-Abwasser-Forsch., 11(5): 161-164 (in German).
JULIANO, R.L. & GAGALANG, E. (1979) The effect of membrane-
fluidizing agents on the adhesion of CHO cells. J. cell Physiol.,
98: 483-490.
KABAYASHI, H., MIYOSHI, Y., & KITAMURA, K. (1977) Effects of
various alcohols on the intramuscular absorption of isonicotinamide
in the rat. Chem. pharm. Bull., 25(11): 3078-3080.
KALEKIN, R.M. & BRICHENKO, V.S. (1972) [Toxic effect of products
from the production of butyl alcohols on the central nervous
system.] Nauch. Tr. Irkutsk. Med. Inst., 115: 22-25 (in Russian).
KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) Studies in
detoxication. The metabolism of aliphatic alcohols. The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochem. J., 53:
129-136.
KIESER, M.E., POLLARD, A., STEVENS, P.M., & TUCKNOTT, O.G. (1964)
Determination of 2-phenylethanol in cider. Nature (Lond.), 204: 887.
KOLESNIKOV, P.A. (1975) [Adaptation to butyl alcohol.] Gig i
Sanit., (5): 104-105 (in Russian).
KUDREWICZ-HUBICKA, Z., KOLACZKOWSKA, M., BORZEMSKA, & WESOLOWSKA, A.
(1978) [Serum antitrypsin activity and glycoprotein level in
workers exposed to organic solvents.] Pol. Tyg. Lek., 33, 395-397
(in Polish).
KUSHNEVA, V.S., KOLOSKOVA, G.A., KOLTUNOVA, J.G., & KIRILENKO, V.T.
(1983) Experimental data to hygienic reglementation of
isobutylalcohol in the working zone. Gig. Tr. Prof. Zabol., 1:
46-47.
LALASIDIS, G. & SJOBERG, L.B. (1978) Two new methods of
debittering protein hydrolysates and a fraction of hydrolysates
with exceptionally high content of essential amino acids. J. agric.
food Chem., 26: 742-749.
LAMMENS, H. & VERZELE, M. (1968) Aroma of hops. II. The
composition of hop oil. J. Inst. Brew., 74: 341-346.
LENDLE, L. (1928) [Investigations on the speed at which
homologous and isomeric monovalent alcohols produce narcosis.]
Naunyn-Schmiedeberg's Arch. exp. Pathol. Pharmakol., 129: 85
(in German).
LIEBICH, H.M., DOUGLAS, D.R., BAYER, E., & ZLATKIS, A. (1970)
Volatile flavour components of Cheddar cheese. J. chromatogr. Sci.,
8: 355-359.
LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROEM, G. (1979)
The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the bleak (Alburnus alburnus)
and the harpaticoid (Nitocra spinipes). Chemosphere, 8(11/12):
843-851.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975) Detection
of carcinogens as mutagens in the Salmonella/microsome test: assay
of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72: 5735-5739.
MCCOMB, J.A. & GOLDSTEIN, D.B. (1979) Quantitative comparison of
physical dependence on tertiary butanol and ethanol in mice:
correlation with lipid solubility. J. Pharmacol. exp. Ther.,
208(1): 113-117.
MCCREERY, N.J. & HUNT, W.A. (1978) Physico-chemical correlates of
alcohol intoxication. Neuropharmacology, 17: 451-461.
MCGLUMPHY, J.H. (1951) Fruit flavours. Food Technol., 5: 353-355.
MCGREGOR, D.C., SCHONBAUM, E., & BIGELOW, W.G. (1964) Acute
toxicity studies on ethanol, propanol, and butanol. Can. J. Physiol.
Pharmacol., 42: 689-696.
MACHT, D.I. (1920) A toxicological study of some alcohols, with
special reference to isomers. J. Pharmacol. exp. Ther., 16: 1-10.
MCKEE, J.E. & WOLF, H.W. (1963) Water quality criteria, 2nd ed.,
California State Quality Control Board, pp. 148-149.
MCKINNEY, R.E. & JERIS, J.S. (1955) Metabolism of low molecular
weight alcohols by activated sludge. Sewage ind. Wastes, 27(6):
728-735.
MCLAUGHIN, J., Jr, MARLIAC, J.P., VERRET, M.J., MUTCHLER, M.K., &
FITZHUGH, O.C. (1964) Toxicity of fourteen volatile chemicals as
measured by the chick embryo method. Am. Ind. Hyg. Assoc. J., 25:
282-284.
MAICKEL, R.P. & MCFADDEN, D.P. (1979) Acute toxicology of butyl
nitrites and butyl alcohols. Res. Commun. chem. Pathol. Pharmacol.,
26: 75-83.
MAICKEL, R.P. & NASH, J.F., Jr (1985) Differing effects of short-
chain alcohols on body temperature and coordinated muscular
activity in mice. Neuropharmacology, 24(1): 83-89.
MARCUS, R.J., WINTERS, W.D., & HULTIN, E. (1976) Neuro-
pharmacological effects induced by butanol, 4-hydroxy butyrate,
4-mercaptobutyric acid, thiolactone, tetrahydrofuran, pyrrolidine,
2-deoxy-d-glucose, and related substances in the rat.
Neuropharmacology, 15(1): 29-38.
MARKOVA, ET AL. (1962) J. food Sci., 27: 353.
MATTHEWS, J.S., SUGISAWA, H., & MACGREGOR, D.R. (1962) Flavour
spectrum of apple-wine volatiles. J. food Sci., 27: 355-362.
MATTSON, V.R., ARTHUR, J.W., & WALBRIDGE, C.T. (1976) Acute
toxicity of selected organic compounds to fathead minnows,
Duluth, Minnesota, US EPA Environmental Research Laboratory
(EPA No. 600/3-76-097).
MELLAN, I. (1950) Industrial solvents, New York, Van Nostrand
Reinhold Company, pp. 482-488.
MERRITT, A.D. & TOMKINS, G.M. (1959) Reversible oxidation of
cyclic secondary alcohols by liver alcohol dehydrogenase.
J. biol. Chem., 234: 2778.
MESLAR, H.W. & WHITE, H.B., III (1978) Preparation of lipid-free
protein extracts of egg yolk. Anal. Biochem., 91: 75-81.
MIKHEEV, M.I., FROLOVA, A.D., & LYUBLINA, E.I. (1977)
[Physicochemical properties and toxokinetics of some
representatives of a homologous series of alcohols.] Nek. Vopr.
Eksperim. Prom. Toksikol., 11-17 (in Russian).
MIZUTANI, Y., MIWA, Y., & MORIGUCHI, J. (1978) Jpn Kokai 78:
41,359, 14 Apr. 1978.
MONICH, J.A. (1968) Alcohols: their chemistry, properties, and
manufacture, New York, Amsterdam, London, Chapman and Reinhold.
MOREL, C. & CAVIGNEAUX, A. (1975) Alcohol isobutylique: fiche
toxicologique. Cah. notes doc., 80: 411-413.
MOSHLAKOVA, L.A., SOLDATCHENKOVA, T.P., & DRUZHININA, V.A. (1976)
Preliminary data of hygienic-chemical studies on the identification
of volatile substances released from poly(vinyl chloride) linoleum
plasticized with poly(dibutyl maleate). Gig. Aspecty Okhr.
Okruzhayushchei Sredy, 65-69.
MUIR, G.D., ed. (1977) Hazards in the chemical laboratory, 2nd
ed., London, The Chemical Society, p. 167.
MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters: narcotic
and lethal potencies to tadpoles and to rabbits. Ind. Med., 41(4):
31-33.
MUNCH, J.C. & SCHWARTZE, E.W. (1925) Narcotic and toxic potency
of aliphatic alcohols upon rabbits. J. lab. clin. Med., 10: 985-996.
NAZARENKO, I.V. (1969) [Maximum allowable concentrations for
butyl and isobutyl alcohol in drinking-water.] Sanit. ochrana
vodojemov at zagraznen. stochn. vodami. M, Medgiz, 4: 65-75
(in Russian).
NIOSH (1977a) Registry of toxic effects of chemical substances,
Rockville, Maryland, National Institute of Occupational Safety and
Health.
NIOSH (1977b) Manual of analytical methods, 2nd ed., Rockville,
Maryland, National Institute of Occupational Safety and Health,
Vol. 2.
NOVOKOVSKAYA, M.I., KLYUKVINA, T.D., KIRILLOVA, N.N., &
SHAPOSHNIKOV, YU.K. (1978) Composition of gaseous discharges
from drying oil production. Prom. Sanit. Ochistka Gazov, 5:
21-22.
NURSTEN, H.E. & WILLIAMS, A.A. (1967) Fruit aromas survey of
components identified. Chem. Ind., 12: 486-497.
OBE, G. & RISTOW, M.J. (1977) Acetaldehyde but not ethanol,
induces sister chromatid exchanges in Chinese hamster cell in
vitro. Mutat. Res., 55: 211-213.
OBE, G., RISTOW, M.J., & HERMA, J. (1977) Chromosomal damage by
alcohol in vitro and in vivo. Adv. exp. Med. Biol., 85a: 47-70.
OETTEL, H. (1936) [Effects of organic fluids on the skin.]
Arch. exp. Pathol. Pharmakol., 183: 641-696 (in German).
OLIAS JIMENEZ, J.M., DOBARGANES GARCIA, M.C., GUTIERREZ ROSALES, F.,
& GUTIERREZ GONZALES-QUIJANO, R. (1978) Volatile components in
the aroma of virgin olive oil. II. Identification sensorial
analysis of the chromatographic elements. Grasas Aceites (Seville),
29: 211-218.
PANGANAMALA, R.V., SHARMA, H.M., HEIKKILA, R.E., GEER, J.C., &
CORNWELL, D.G. (1976) Role of hydroxyl radical scavengers
dimethyl sulfoxide, alcohols, and methional in the inhibition of
prostaglandin biosynthesis. Prostaglandins, 11(4): 599-607.
PATTY, F.A., ed. (1963) Industrial hygiene and toxicology, 2nd
ed., New York, London, Sydney, John Wiley and Sons, Interscience
Publishers, Vol. 2, pp. 1441-1450.
PATTY, F.A. (1982) Industrial hygiene and toxicology, 3rd ed.,
New York, Chichester, Brisbane, Toronto, Singapore, Wiley-
Interscience, Vol. IIC, pp. 4571-4578.
PETROVA, N.I. & VISHEVSKII, A.A. (1972) [Course of pregnancy and
deliveries in women working in the organosilicon varnish and enamel
industries.] Nauch. Tr. Irkutsh. Med. Inst., 115: 102-106
(in Russian).
PITTER, P. (1976) Determination of biological degradability of
organic substances. Water Res., 10: 231-235.
POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic alcohols of
the stimulated activity of ornithine decarboxylase and tyrosine
aminotransferase occurring in regenerating rat liver. Biochem.
Pharmacol., 29(20): 2799-2803.
POSTEL, W. & ADAM, L. (1978) Gas chromatographic characterization
of whiskey. III. Irish whiskey. Brannt Wein Wirtschafft, 118:
404-407.
POSTEL, W., DRAWERT, F., & ADAM, L. (1975) [Flavourings in
brandies.] [Flavourings, International Symposium], 99-111
(in German).
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46(1): 63-77.
PURCHASE, F.H. (1969) Studies on Kaffircorn malting and brewing.
XII. The acute toxicity of some fusel oil found in Bantu beer.
S. Afr. med. J., 53: 795-462
PUSKIN, J.S. & MARTIN, T. (1978) Effects of anesthetics on
divalent cation binding and fluidity of phosphatidylserine
vescicles. Mol. Pharmacol., 14: 454-462.
REYNOLDS, T. (1977) Comparative effects of aliphatic compounds on
inhibition of lettuce fruit germination. Ann. Bot., 41(173):
637-648.
RIIHIMAKI, V. (1979) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand. J. Work
environ. Health, 5: 143-150.
ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. NY Acad. Sci., 273: 280-394.
RUMYANSTEV, A.P., GEER, V.G., OSTROUMOVA, N.A., SPIRIN, B.A., &
SHAKHIDZHANYAN, L.G. (1975) [Cumulative properties of butyl
alcohol.] Gig. i Sanit., 10: 112-113 (in Russian).
RUMYANSTEV, A.P., OSTROUMOVA, N.A., KUSTOVA, S.A., LOBANOVA, I.UA.,
TIUNOVA, L.V., DHERNIKOVA, V.V., & KOLESHNIKOV, P.A. (1976)
[Sanitary-toxicological features of butyl alcohol under conditions
of prolonged inhalation route entry.] Gig. i Sanit., 11: 12-15
(in Russian).
RUMYANSTEV, A.P., LOBANOVA, I.YA., TIUNOVA, L.V., & CHERNIKOVA, V.V.
(1979) [Toxicology of butyl alcohol.] Khim. Prom.-st. Ser.
Toksikol. Sanit. Khim. Plastmass, 2: 24-26 (in Russian).
SAAD, S.F. (1976) Effects of aliphatic alcohols on gamma-
aminobutyric acid levels in the cerebral hemispheres of rats. IRCS
med.-sci. Libr. Compend., 4: 499.
SAITO, M. (1975) [Studies on the metabolism of lower alcohols.]
Nichidai Igaku Zasshi, 34(8-9): 569-585 (in Japanese).
SAKAGAMI, Y., YOKOYAMA, H., KITANAKA, E., & IOLU, M. (1977) The
influence of phtalates on chick embryos. Osaka-furitsu Koshu
Eisei Kenkyusho Kenkyu Hokoku, Yakuji Shido Hen, 11: 15-20.
SANATINA, K.G. (1973) [Electrophysiological changes in the
peripheral neuromuscular function of persons exposed to toluene and
butyl alcohol vapors.] In: Gurghinas, S.V., ed. Vopr. Epidemiol.
Gig. Litov. SSR, Mater. Nauch. Konf. Ozdorevleniyu Vneshn. Sredy,
Nauchno-Issled., Institute of Epidemiology, Microbiology Gig.
Volnius, USSR, pp. 183-184 (in Russian).
SANDER, F. (1933) Cited by von Oettingen, W.F., Washington DC, US
Public Health Service (Public Health Bulletin No. 281) (1943).
SATLER, S.D. & THIMANN, K.V. (1980) The influence of aliphatic
alcohols on leaf senescences. Plant Physiol., 66: 395-399.
SAVELEV, A.I., BABANOV, A.G., SKOBEI, N.A., & TROITSKAYA, I.A.
(1975) [Adaptation reactions of white rats after prolonged
administration of small concentrations of butyl alcohol.] In:
Zaikina, M.G., ed. [Pathophysiology of the cardiovascular system,]
Yaroslav, Yaroslav Medical Institute, pp. 59-62, 76-80 (in Russian).
SCARAGLI, G.P., RIZZOTTI CONTI, M., BENCINI, R., DELLA CORTE, L., &
GIOTTI, A. (1975) [Toxicity of food additives. II. Membrane
damage produced by monocyclic compounds in the mitochondria and
lysosomes of rat's liver. Correlation between structure and action.]
Boll. Soc. Ital. Biol. Sper., 51: 1702-1706 (in Italian).
SCHREIER, P., DRAWERT, F., & SCHMID, M. (1978) Changes in the
composition of neutral volatile components during the production of
apple brandy. J. Sci. Food Agric., 29: 728-736.
SCHREIER, P., DRAWERT, F., & WINKLER, F. (1979) Composition of
neutral volatile constituents in grape brandies. J. agric. food
Chem., 27: 365-372.
SCHWARTZ, L. & TULIPAN, L. (1939) A textbook of occupational
diseases of the skin, Philadelphia, Pennsylvania, Lea & Febiger,
p. 717.
SEITZ, B., (1972) Vertiges graves apparus après manipulation de
butanol et d'isobutanol. A propos de trois cas. Arch. Mal. prof.
Méd. Trav. sécur. soc., 33: 393-395.
SERAFINI CESSI, S. (1975) Effects of alcohols on protein
synthetic activity in subcellular fractions from brain and liver of
rat. Arch. Sci. Biol., 59: 127-137.
SHALABY, E.S., DANASORY, M.El., & MASSOUD, A.A.E. (1973) Toxic
effects of fat solvents used in paints on liver, blood, and lung.
J. Egypt. Med. Assoc., 54: 340-347.
SHEHATA, M. & SAAD, S. (1978) The effect of aliphatic alcohols on
certain vitamins of the B-complex group in the liver of the rat.
Pol. J. Pharmacol. Pharm., 30: 35-39.
SHERMAN, P.D., Jr (1978) The butyl alcohols. In: Kirk-Othmer
Encyclopaedia of Chemical Technology, 3rd ed., Vol. 4, pp. 338-345.
SHOPSIS, C. & SATHE, S. (1984) Uridine uptake inhibition as a
cytotoxicity test: correlations with the Draize test. Toxicology,
29: 195-206.
SIEGERS, C.P., STRUBELT, O., & BREINING, H. (1974) The acute
hepatotoxicity of alcoholic beverages and some of their congeners
in guinea-pigs. Pharmacology, 12: 296-302.
SITANOV, V.S., BANDIK, K.A., ENAKAEVA, V.G., & BARANOVA, R.K.
(1979) Composition for removing grease and oil from textiles.
Otkrytiya, Izobert., Prom. Obraztsy, Tovaznye Znaky, 38: 90.
SMITH, C.W. & SIEGEL, S.M. (1975) Differential permeation of
Artemia cysts and cucumber seeds by alcohols. J. Histochem.
Cytochem., 23(1): 80-83.
SMUSIN, Y.S. & CHENSTOVA, T.F. (1973) Zdravookhr. Kaz., 4: 51-52.
SMYTH, H.F., Jr (1956) Hygienic standards for daily inhalation.
Cummings Memorial Lecture. Am. Ind. Hyg. Assoc., 17: 129-185.
SMYTH, H.F. & SMYTH, H.F., Jr (1928) Inhalation experiments with
certain lacquer solvents. J. ind. Hyg., 10: 261-271.
SMYTH, H.F., Jr, CARPENTER, C.P., & WEIL, C.S. (1951) Range-
finding toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4:
119-122.
SMYTH, H.F., Jr, CARPENTER, C.P., WEIL, C.S., & POZZANI, U.C.
(1954) Range-finding toxicity data: list V. Arch. ind. Hyg. occup.
Med., 10: 61-68.
SNELL, D. & HARRIS, R.A. (1980) Impairment of avoidance behaviour
following short-term ingestion of ethanol, tertiary-butanol, or
pentobarbital in mice. Psychopharmacology, 69(1): 53-57, 1980.
SODINI, G. & CANELLA, M. (1977) Extraction of phenols and
oligosaccharides from plant tissues (Span 445,653 1 Jun 1977).
STARK, D.M., SHOPSIS, C., BORENFREUND, E., & WALBERG, J. (1983)
Alternative approaches to the Draize assay-chemotaxis, cytology,
differentiation, and membrane transport studies. In: Goldberg, A.,
ed. Product safety and evaluation, New York, Mary Ann Liebert,
pp. 180-203.
STERNER, J.H., CROUCH, H.C., BROCKMYRE, H.F., & CUSACK, M. (1949)
A ten-year study of butyl alcohol exposure. Am. Ind. Hyg. Assoc. Q.,
10: 53-59.
SUGIYAMA, T., MIURA, R., & YAMANO, T. (1976) Purification and
properties of cytochrome P-450 from adrenocortical mithocondria and
its interaction with adrenodoxin. Adv. exp. Med. Biol., 74: 290-302.
SWORDS, G., BOBBIO, P.A., & HUNTER, G.L.K. (1978) Volatile
constituents of jack fruit. J. food Sci., 43: 639-640.
TABERSHAW, I.R., FAHY, J.P., & SKINNER, J.B. (1944) Industrial
exposure to butanol. J. ind. Hyg. Toxicol., 26: 328-330.
TAKEUCHI, H., KATADA, M., TAKAHASHI, M., KAWAMATA, S., & KOHARI, H.
(1978) Purification of polyolefins. Tokkyo Koho, 79: 126,291
1 Oct 1979.
TAVLINOVA, T.I. & DOVYBOROVA, L.N. (1979) Effect of aliphatic
alcohols on the crystal formation of hydrates of clinker minerals.
Isv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol., 22: 972-975.
TESCHKE, R., HAMASURA, Y., & LIEBER, C.S. (1974) NADPH-dependent
oxidation of methanol, ethanol, propanol, and butanol by hepatic
microsomes. Biochem. biophys. Res. Commun., 60: 851-857.
THEORELL, H. & BONNICHSEN, R. (1951) Studies on liver alcohol
dehydrogenase I. Equilibria and initial reaction velocities.
Acta. chem. Scand., 5: 1105-1126.
THOMAS, M., BOURA, A.L.A., & VIJAYAKUMAR, R. (1980) Prostaglandin
release by aliphatic alcohols from the rat isolated lung. Clin. exp.
Pharmacol. Physiol., 7(4): 373-381.
THURMAN, R.G., WINN, K., & URQUHART, B. (1980) Rat brain cyclic
AMP levels and withdrawal behaviour following treatment with
t-butanol. Adv. exp. Med. Biol., 126: 271-281.
TRAIGER, G.J. & BRUCKNER, J.V. (1976) The participation of 2
butanone in 2-butanol-induced potentiation of carbon tetrachloride
hepatotoxicity. J. Pharmacol. exp. Ther., 196: 493-500.
TRAIGER, G.J., BRUCKNER, J.V., & COOKE, P.H. (1975) Effect of
2-butanol and 2-butanone on rat hepatic ultrastructure and
microsomal drug metabolising enzyme activity. Toxicol. appl.
Pharmacol., 33(1): 132.
TRESSL, H., FRIESE, L., FENDESACK, F., & KOEPPLER, H. (1978)
Studies of the volatile composition of hops during storage.
J. agric. food Chem., 26: 1426-1430.
TSULAYA, V.R., MORENKOVA, N.V., VOLOKHOVA, L.E., & VORONIN, V.M.
(1978) [Description of the biological properties of small
concentrations of isobutyl alcohol.] Gig. i Sanit., 5: 6-9
(in Russian).
UK MAFF (1978) Food additives and contaminants. Committee
report on the review of solvents in food, London, United Kingdom
Ministry of Agriculture, Fisheries and Food.
US DHEW (1951) Survey of compounds which have been tested for
carcinogenic activity, 2nd ed., Washington DC, US Department of
Health, Education, and Welfare (Prepared by Hartwell, J.L. for the
US DHEW).
US DHEW (1978) Registry of toxic effects of chemicals, Washington
DC, US Department of Health, Education and Welfare.
US EPA (1980) Hazard information review, Washington DC, US
Environmental Protection Agency, p. 10.
VAN DEN BERG, A.P., NOORDHOEK, J., & KOOPMAN-KOOL, E. (1979a) The
relation between the sex-dependency of type I binding of
ethylmorphine and the 1-butanol-induced spectral change in mouse
liver microsomes. Biochem. Pharmacol., 28: 31-36.
VAN DEN BERG, A.P., NOORDHOEK, J., & KOOPMAN-KOOL, E. (1979b) The
use of competitive inhibition of substrate binding to cytochrome
P-450 in the determination of spectral dissociation constants for
substrates with multiple types of binding, as illustrated with
1-butanol. Biochem. Pharmacol., 28: 37-41.
VEITH, G.D., CALL, D.J., & BROOKE, L.T. (1981) Estimating the
acute toxicity of narcotic industrial chemicals to fathead
minnows, Philadelphia, Pennsylvania, American Society of Testing
and Materials (ASTM Special Technical Publication No. 802).
VELASQUEZ (1969) Audiologic impairment due to n-butyl alcohol
exposition. In: Proceedings of the International Congress on
Occupational Health, Tokyo, 1969.
VERSCHUEREN, K. (1977) Handbook of environmental data of organic
chemicals, New York, Van Nostrand Reinhold Company.
WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., & IIJIMA, M.
(1984) Induction of metamitochondria in the rat liver by n-propyl
alcohol and n-butyl alcohol. Acta pathol. Jpn., 34(3): 471-480.
WALLGREN, H. (1960) Relative intoxicating effects on rats of
ethyl, propyl, and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
WALLGREN, H., NIKANDER, P., BOGUSLAWSKY, P.V., & LINKOLA, J.
(1974) Effects of ethanol, tert-butanol, and clomethiazole on
net movements of sodium and potassium in electrically stimulated
cerebral tissue. Acta physiol. Scand., 91: 83-93.
WALTER, W. & WEIDEMANN, H.L. (1969) Coffee flavour compounds.
Z. Ernaehrungswiss, 9: 123-147.
WARTBURG, J.P. VON, BETHANE, J.L., & VALLEE, B.L. (1964) Human
liver alcohol dehydrogenase: kinetic and physiochemical properties.
Biochemistry, 3: 1775-1782.
WEESE, H. (1928) [Comparative studies on the efficacy and
toxicity of the vapours of low aliphatic alcohols.] Arch. exp.
Pathol. Pharmakol., 135: 118-130 (in German).
WEISBRODT, N.W., KIENZLE, M., & COOKE, A.R. (1973) Comparative
effects of aliphatic alcohols on the gastric mucosa. Proc. Soc. Exp.
Biol. Med., 142: 450-454.
WHO (1980) Twenty-third Report of the Joint FAO/WHO Expert
Committee on Food Additives, Geneva, World Health Organization
(WHO Technical Report Series 648).
WILLIAMS, R.T. (1969) Detoxication mechanisms, 2nd ed., London,
Chapman & Hall Ltd., p. 66.
WINER, A.D. (1958) A note of the substrate specificity of horse
liver alcohol dehydrogenase. Acta. chem. Scand., 12: 965.
WITKIN, J.M. & LEANDER, J.D. (1982) Effects of orally
administered ethanol and tert-butanol on fixed-ratio responding of
rats. Subst. Alcohol Actions/Misuse, 3: 275-279.
WOIDICH, H., PFANNHAUSER, W., & EBERHARDT, R. (1978) Results of
gas chromatographic-mass spectrographic studies of the volatile
components of applie brandies. Mitt. Hoeheren Bundeslehr-
Versuchsanst. Wein-Obstbau, Klosterneuberg, 28: 56-63.
WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.-Excerpta
Med., 440: 196-199.
WOOD, J.M. & LAVERTY, R. (1979) Physical dependence following
prolonged ethanol or t-butanol administration to rats. Pharmacol.
Biochem. Behav., 10: 113-119.
WOOD, J.N. & LAVERTY, R. (1976) Alcohol withdrawal syndrome
following prolonged 5-butanol administration to rats. Proc. Univ.
Otago Med. Sch., 54: 86-87.
YABUMOTO, K., YAMAGUCHI, M., & JENNINGS, W.G. (1978) Production
of volatile compounds by musk melon Cucumis melo. Food Chem.,
3: 7-16.
YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., & HABU, T.
(1978) Volatile flavor components of cooked rice. Agric. biol.
Chem., 42: 1229-1223.
YAMAZAKI, Y. & KATO, K. (1978) Penicillins or cephalosporins.
Jpn. Kokai Tokkio Koho, 78, 107, 484, 19 Sep. 1978.
YASHUDA, Y., CHABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
YASUDA-YASAKI, Y., NAMIKE-KANIE, S., & HACHISAKU, Y. (1978)
inhibition of germination of Bacillus subtilis spores by alcohols.
Spores, 7: 13-16.
YOJAY, L., YOJAY, R., & MARDONES, J. (1982) Acetone blood levels
after t-butanol administration in rats. IRCS Med. Sci., 10: 215.
ZAIKINA, E.I., TEREKHOVA, A.I., CHUDOV, L.N., SHATENSHTEIN, A.I.,
PETROV, E.S., SHCHERBAK, V.P., ZAKOMYRDIN, A.A., SIMETSKII, W.A., &
SOKHADZE, L.A. (1978) Repellent composition. Otkrytiya, Izobret.,
Prom. Obraztsy, Tovarnye, Znaky, 55: 18.
ZAIKOV, KH. & BOBEV, G. (1978) Chemical damages in the furniture
industry and morbidity with temporary loss of working capacity.
Khig. Zdraveopaz., 21: 141-147.
ZAMARAKHINA, L.E. (1973) [Determination of tert-butyl alcohol in
the air of industrial premises.] Gig. i Sanit., 38: 72-73
(in Russian)
