
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 61
CHROMIUM
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1988
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154261 6
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1988
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CHROMIUM
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Analytical methods
1.1.2. Sources of chromium, environmental levels and exposure
1.1.3. Metabolism
1.1.4. Effects on experimental animals
1.1.5. Effects on human beings
1.1.5.1 Clinical and epidemiological studies
1.1.6. Evaluation of risks for human health
1.2. Recommendations for further research
1.2.1. Analytical methods
1.2.2. Sources of chromium intake
1.2.3. Studies on health effects
1.2.4. Interaction with other environmental factors
2. PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and chemical properties
2.2. Analytical methods
2.2.1. Sampling
2.2.2. Analytical methods
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1. Natural occurrence
3.1.1. Rocks
3.1.2. Soils
3.1.3. Water
3.1.4. Air
3.1.5. Plants and wildlife
3.1.6. Environmental contamination from natural sources
3.2. Production, consumption, and uses
3.3. Waste disposal
3.4. Miscellaneous sources of pollution
3.5. Environmental transport and distribution
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental levels
4.1.1. Air
4.1.2. Water
4.1.3. Food
4.2. General population exposure
4.2.1. Food and water
4.2.2. Other exposures
4.3. Occupational exposure
4.3.1. Inhalation exposure
4.3.2. Dermal exposure
5. KINETICS AND METABOLISM
5.1. Absorption
5.1.1. Absorption through inhalation
5.1.1.1 Animal studies
5.1.1.2 Human data
5.1.2. Absorption from the gastrointestinal tract
5.1.2.1 Animal studies
5.1.2.2 Human data
5.2. Distribution, retention, excretion
5.2.1. Animal studies
5.2.2. Human data
5.2.2.1 Concentration in tissues, blood, urine,
and hair including possible biological
indicators of exposure
5.2.2.2 Dynamic aspects of metabolism
and the influence of pathological states
5.3. Influence of chemical form
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Microorganisms
6.2. Plants
6.3. Aquatic organisms
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Nutritional effects of chromium
7.1.1. Effects of deficiency on glucose metabolism
7.1.2. Effects of deficiency on lipid metabolism
7.1.3. Effects of deficiency on life span, growth, and reproduction
7.1.4. Other effects of deficiency
7.1.5. Mechanism of action of chromium as an essential nutrient
7.1.5.1 Enzymes, nucleic acids, and thyroid
7.1.5.2 Interaction of chromium with insulin
7.1.6. Chromium nutritional requirements of animals
7.2. Toxicity studies
7.2.1. Effects on experimental animals
7.2.1.1 Carcinogenicity
7.2.1.2 Genotoxicity
7.2.1.3 Developmental toxicity and other
reproductive effects
7.2.1.4 Cytotoxicity and micromolecular syntheses
7.2.1.5 Fibrogenicity
7.2.2. Observations in farm animals
8. EFFECTS ON MAN
8.1. Nutritional role of chromium
8.1.1. Biological measurements and their interpretation
8.1.2. Chromium deficiency
8.1.2.1 Adults
8.1.2.2 Malnourished children
8.1.2.3 Patients on total parenteral alimentation
8.1.2.4 Epidemiological studies
8.1.3. Mode of action
8.2. Acute toxic effects
8.3. Chronic toxic effects
8.3.1. Effects on skin and mucous membranes
8.3.1.1 Primary irritation of the skin
and mucous membranes
8.3.1.2 Allergic contact dermatoses
8.3.2. Effects on the lung
8.3.2.1 Bronchial irritation and sensitization
8.3.3. Effects on the kidney
8.3.4. Effects on the liver
8.3.5. Effects on the gastrointestinal tract
8.3.6. Effects on the circulatory system
8.3.7. Teratogenicity
8.3.8. Mutagenicity and other short-term tests
8.3.9. Carcinogenicity
8.3.9.1 Lung cancer
8.3.9.2 Cancer in organs other than lungs
8.3.9.3 Relative risk between cancer risk
and chromium compound
9. EVALUATION OF HEALTH RISKS FOR MAN
9.1. Occupational exposure
9.1.1. Effects other than cancer
9.1.1.1 Respiratory tract
9.1.1.2 Skin
9.1.1.3 Kidney
9.1.1.4 Other organs and systems
9.1.2. Teratogenicity
9.2. General population
REFERENCES
WHO TASK GROUP ON CHROMIUM
Members
Professor Chen Bingheng, Department of Environmental Health,
Shanghai Medical University, Shanghai, China
Dr H.N.B. Gopalan, University of Nairobi, Department of Botany,
Nairobi, Kenya
Professor C.R. Krishna Murti, Integrated Environmental
Programme on Heavy Metals, Department of Environment,
Government of India, New Delhi, India (Vice-Chairman)
Professor Aly Massoud, Department of Community, Environmental
and Occupational Medicine, Faculty of Medicine, Ain Shams
University, Cairo, Egypt
Dr W. Mertz, Human Nutrition Research Center, US Department of
Agriculture, Beltsville, Maryland, USA (Chairman)
Professor I.V. Sanotsky, Department of Toxicology, Institute
of Industrial Hygiene and Occupational Diseases, Academy
of Medical Sciences of the USSR, Moscow, USSR
Professor W. Stöber, Fraunhofer Institute for Toxicology and
Aerosol Research, Hanover, Federal Republic of Germany
Secretariat
Dr J. Parizek, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)
Dr R.F. Hertel, Fraunhofer Institute for Toxicology and
Aerosol Research, Hanover, Federal Republic of Germany
(Temporary Adviser) (Rapporteur)
Dr T. Ng, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
Mr J.D. Wilbourn, Unit of Carcinogen Identification and
Evaluation, International Agency for Research on Cancer,
Lyons, France
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR CHROMIUM
A WHO Task Group on Environmental Health Criteria for Chromium
met in Geneva from 24 to 27 March 1986. Dr J. Parizek opened the
meeting on behalf of the Director-General. The Task Group reviewed
and revised the draft criteria document and made an evaluation of
the health risks of exposure to chromium.
The initial draft was prepared by the INSTITUTE FOR GENERAL AND
COMMUNITY HYGIENE, MOSCOW. The second draft criteria document was
prepared by DR W. MERTZ, HUMAN NUTRITION RESEARCH CENTER, US
DEPARTMENT OF AGRICULTURE, USA, PROFESSOR ANNA BAETGER, JOHN
HOPKINS UNIVERSITY, BALTIMORE, USA (deceased), and Dr R.F. HERTEL,
FRAUNHOFER INSTITUTE OF TOXICOLOGY AND AEROSOL RESEARCH, Federal
Republic of Germany.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the cost of printing.
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Analytical methods
Many analytical methods are available for the determination of
chromium at trace levels, often in the 0.001 mg/kg range. Among
these are flameless atomic absorption spectrometry, atomic emission
spectrometry with various excitation sources (the inductively-
coupled plasma torch is particularly advantageous), gas
chromatography, destructive or non-destructive neutron activation
analysis, and mass spectrometry using double-isotope dilution.
Depending on the particular sample under examination as well as the
analytical technique selected for the determination, wet or dry
ashing procedures may be necessary to destroy the organic/inorganic
matrix and minimize interelemental effects.
Determination of very low chromium concentrations in
"unexposed" biological material (animal and human tissues, blood,
urine, food, as well as water and air) is extremely difficult and
many problems still have to be solved. An accurate assessment of
human exposure and nutritional chromium requirements depends on
reliable analytical results. Chromium concentrations in blood,
urine, and some low-chromium foods are close to or less than 1
µg/kg, which is near the detection limit of even the most sensitive
analytical methods. Thus, agreement as to "normal" levels of
chromium among analytical investigators has been poor, and results
of interlaboratory comparisons have differed widely, usually by one
order of magnitude. Only in recent years has agreement been reached
that "normal" chromium concentrations in unexposed blood and urine
are in the range of 0.1 - 0.5 µg/litre. In this concentration
range, it is not only the sensitivity of the final determination
step that is limiting. The preceding steps of sample collection,
preparation, and digestion are equally important. Contamination,
easily introduced through cutting instruments and dust during
collection, must be carefully controlled. Digestion procedures are
of the greatest importance. Too rigorous treatment by heat or
certain acids can cause a loss of chromium. Few biological
standard reference materials, certified for chromium, are available
and almost all of the older, and most of the recent, published data
were not checked using certified standards. For this reason,
quantitative data concerning chromium concentrations in the range
of < 1 - 100 µg/kg in biological materials must be considered
uncertain, and caution must be used in interpreting their health-
related significance.
Differential analysis for chromium species is of great
scientific and public health concern, in view of the substantial
differences in the biological availability and in the toxicity of
hexavalent chromium (Cr VI) compared with trivalent (Cr III).
Though methods based on solvent extraction, with or without prior
oxidation, differentiate between these two oxidation states, few
analytical data contain this important information.
The understanding of the chemical and physical principles of
chromium determination is increasing, and existing methods are
being improved and new methods developed. However, at present,
analysis for chromium is a sophisticated procedure requiring the
full attention of a highly trained analytical chemist.
1.1.2. Sources of chromium, environmental levels and exposure
Chromium occurs ubiquitously in nature (< 0.1 µg/m3 in air).
Natural levels in uncontaminated waters range from fractions of 1
µg to a few µg/litre.
The concentration of chromium in rocks varies from an average
of 5 mg/kg (granitic rocks) to 1800 mg/kg (ultramafic/basic and
serpentine rocks). The earth's most important deposits are either
in the elemental or the trivalent oxidation state.
In most soils, chromium occurs in low concentrations (2 - 60
mg/kg), but values of up to 4 g/kg have been reported in some
uncontaminated soils. Only a fraction of this chromium is
available to plants. It is not known whether chromium is an
essential nutrient for plants, but all plants contain the element
(up to 0.19 mg/kg on a wet weight basis).
Almost all the hexavalent chromium in the environment arises
from human activities. It is derived from the industrial oxidation
of mined chromium deposits and possibly from the combustion of
fossil fuels, wood, paper, etc. In this oxidation state, chromium
is relatively stable in air and pure water, but it is reduced to
the trivalent state, when it comes into contact with organic matter
in biota, soil, and water. There is an environmental cycle for
chromium, from rocks and soils to water, biota, air, and back to
the soil. However, a substantial amount (estimated at 6.7 x 106 kg
per year) is diverted from this cycle by discharge into streams,
and by runoff and dumping into the sea. The ultimate repository is
ocean sediment.
Chromium compounds are used in ferrochrome production,
electroplating, pigment production, and tanning. These industries,
the burning of fossil fuels, and waste incineration are sources of
chromium in air and water. Most of the liquid effluent from the
chromium industries is trapped and disposed of in land fills and
sewage sludges, the chromium being in the form of the insoluble
trivalent hydroxide.
In chromium ore mines, the concentration of chromium in dust
ranges from 1.3 to 16.9 mg/m3. During the production of refined
ferrochromium, the air in the work-place may contain large amounts
of dust (0.03 - 3.2 mg/m3). In chromium plating factories,
concentrations of 1 µg/m3 up to 1.4 mg/m3 have been measured. In
Portland cement from 9 European countries, the contents of chromium
(VI), extractable with sodium sulfate, varied from 1 to 83 g/kg
cement.
Today, it is generally accepted that only the zero-, di-, tri-,
and hexavalent oxidation states have biological importance. The
effects of the last 2 oxidation states are so fundamentally
different that they must always be considered separately. The
trivalent form is an essential nutrient for man, in amounts of 50 -
200 µg/day.
1.1.3. Metabolism
The kinetics of chromium depend on its oxidation state and the
chemical and physical form within the oxidation state. Most of the
daily chromium intake (50 - 200 µg) is ingested with food and is in
the trivalent form. About 0.5 - 3% of the total intake of
trivalent chromium is absorbed in the body. It is possible, but it
has not yet been proved, that chromium in the form of some
complexes, such as a dinicotinic-acid-complex, glucose tolerance
factor, is better available for absorption. The gastrointestinal
absorption of 3 - 6% of the total intake of hexavalent chromium has
been reported. Once absorbed, chromium is almost entirely excreted
with the urine; the daily urinary-chromium loss of 0.5 - 1.5 µg is
approximately equal to the amount absorbed from the average diet.
However, dermal losses, losses by desquamation of intestinal cells
and by perspiration have not been quantified. Ingested or injected
chromium leaves the blood rapidly. Blood-chromium levels do not
reflect the overall chromium content of tissues, except after a
glucose load, which induces an immediate increase in the plasma-
and urine-chromium levels of chromium-sufficient subjects.
Trivalent chromium inhaled from the air is trapped in the lung
tissues, if in the form of small particles within the respirable
range. The chromium concentrations in lungs increases with age.
Larger particles (greater than 5 µm), regardless of oxidation
state, are moved to the larynx by ciliary action and become part of
the dietary intake.
The intestinal absorption of hexavalent chromium is 3 - 5 times
greater than that of trivalent forms; however, some of it is
reduced by gastric juice. Soluble chromates are rapidly absorbed
through the epithelium of the alveoli and bronchi and cleared into
the circulation, where part is preferentially accumulated by the
red cells and part is excreted by the kidneys. With the exception
of the lungs, tissue levels of chromium decline with age.
1.1.4. Effects on experimental animal
Doses of hexavalent chromium greater than 10 mg/kg diet affect
mainly the gastrointestinal tract, kidneys, and probably the
haematopoetic system. When a similar dose is introduced
parenterally, the principal effect is on the kidney, mainly in the
proximal convoluted tubules, without evidence of glomerular damage.
Toxic effects from trivalent chromium have been reported only
following parenteral administration. Dietary toxicity has not been
reported, even in studies on cats administered amounts of up to 1
g/day for 1 - 3 months. When intravenously injected in mice, the
LD50 of chromium carbonyl was 30 mg/kg body weight; this represents
a 10 000-fold excess over the therapeutic dose required to cure
signs of chromium deficiency.
Many studies on experimental animals have been conducted with
chromium compounds in efforts to reproduce cancer similar to that
found in man, when exposed to chromium.
Most tests have involved subcutaneous, intramuscular, or
intrapleural injection. In addition, several hexavalent chromium
compounds have been administered to rats by intrabronchial
implantation or intratracheal instillation. Relatively insoluble
compounds, calcium chromate, strontium chromate, and certain forms
of zinc chromate produced bronchogenic carcinomas; lead chromate,
and barium chromate produced weak responses. Intratracheal
instillation of soluble sodium dichromate and dissolved calcium
chromate produced bronchogenic tumours. Injection of lead
chromate, lead chromate oxide, and cobalt-chromium alloy resulted
in the production of local sarcomas. Thus, there is sufficient
evidence that certain hexavalent chromium compounds are
carcinogenic for experimental animals. No increased tumour
incidence was observed when trivalent compounds were given orally;
however, the doses administered were low.
Hexavalent chromium has been reported to cause various forms of
genetic damage in short-term mutagenicity tests, including damage
to DNA, and misincoporation of nucleotides in DNA transcription.
It was mutagenic in bacteria in the absence of an exogenous
metabolic activation system, and in fungi. Hexavalent chromium was
also mutagenic in mammalian cells in vitro and in vivo.
Hexavalent chromium caused chromosomal abererations and sister
chromatid exchanges in mamalian cells in vitro. A few positive
results in in vitro assays for mammalian cell chromosomal
aberrations and sister chromatid exchanges were obtained only with
very high doses and could be explained by nonspecific toxic
effects. It induced formation of micronuclei in mice in vivo.
Potassium dichromate induced dominant lethal mutations in mice
treated in vivo.
Trivalent chromium is genetically active only in in vitro
tests, where it can have a direct interaction with DNA, e.g., in
experiments using purified DNA or tests to measure decreased
fidelity of DNA synthesis in vitro. Reduction of chromium (VI)
within the cell nucleus and the formation of chromium (III)
complexes suggests that chromium (III) would be the ultimate
mutagenic form of chromium. Trivalent chromium was present in RNAs
from all sources examined and probably contributes to the stability
of the structure. Injected chromium trichloride (CrCl3)
accumulated in the cell nucleus (up to 20% of cellular chromium
content). It enhanced RNA synthesis in mice and in regenerating
rat liver, suggesting that chromium (III) is involved directly in
RNA synthesis. On the other hand, chromium (VI) inhibited RNA
synthesis and DNA replication in several systems.
1.1.5. Effects on human beings
Studies on man and experimental animals have established the
essential role of trivalent chromium for the maintenance of normal
glucose metabolism. Chromium deficiency has been demonstrated in
malnourished children, in two patients on total parenteral
nutrition, and in middle-aged subjects, the basic disturbance being
an impairment of the action of circulating insulin.
1.1.5.1. Clinical and epidemiological studies
In adult human subjects, the lethal oral dose is 50 - 70 mg
soluble chromates/kg body weight. The most important clinical
features produced following this route of entry are liver and
kidney necrosis and poisoning of blood-forming organs.
Hexavalent chromium causes marked irritation of the respiratory
tract. Ulceration and perforation of the nasal septum have
occurred frequently in workers employed in the chromate producing
and hexavalent chromium-using industries. In addition to
inhalation, direct contact of the nasal septum with contaminated
hands contributes to nasal exposure. Cancer of the septum has not
been reported. Rhinitis, bronchospasm, and pneumonia may result
from exposure to hexavalent compounds together with impairment of
pneumodynamics during respiration.
Chromate compounds, mainly sodium and potassium chromate and
dichromate, cause irritation of the skin and ulcers may develop at
the site of skin damage. Exposure to trivalent chromium does not
produce such effects. Certain persons manifest allergic skin
reactions to hexavalent and possibly trivalent chromium. Skin
reactions through dermal exposure to chromium are often described,
chromate being the most common contact allergen. However, cancer
of the skin due to chromium exposure has not been reported.
Chronic effects of exposure to chromium (excessive industrial
exposure of the skin to hexavalent chromium, when associated with
damaged skin or inhalation of airborne chromium (VI) or mixed dust)
occur in the lung, liver, kidney, gastrointestinal tract, and
ciculatory system. Teratogenic risks from chromium exposure have
not been reported for human subjects; a mutagenic potency is shown
for potassium dichromate and therefore cannot be excluded for
chromates in the chromate-using industries.
The results of epidemiological studies in various countries
have demonstrated that men working in chromate-production plants
before 1950 had a very high rate of bronchogenic carcinoma,
compared with control populations. Because of the long period
between initial exposure and the detection of cancer and the lack
of data on the extent and type of exposure, the dose-response
relationship has not been quantified. However, the few data
available indicate that, before the danger of cancer was
recognized, the exposure levels in such plants were very high.
Recent data show clearly that, though the risk of cancer in workers
in modern plants has been greatly reduced, it still remains a
problem.
Some epidemiological data suggest that an excess of lung cancer
has also occurred in the chromate-pigment industry. A few cases of
cancer involving the upper respiratory tract have been reported,
but cancer has not been convincingly demonstrated in other body
tissues. The specific compounds responsible for the cancers have
not been identified. Both hexavalent and trivalent compounds were
present in the old plants. However, on the basis of experimental
animal studies, it is currently assumed that the slowly soluble,
hexavalent chemicals, such as calcium and zinc chromate are
responsible for the cancers. This is based on the theory that
these compounds remain in contact with the tissues for long periods
of time (depot effect).
Trivalent chromium is not considered to be carcinogenic for the
following reasons: (a) there was no evidence of excess cancer in
studies in two industries where only trivalent compounds were
present; (b) results of experimental animal and mutagenicity
studies with trivalent chromium, were negative; and (c) because of
the chemical and biological characteristics of the trivalent state,
i.e., non-oxidizing, non-irritating, and probably unable to
penetrate cell membranes.
1.1.6. Evaluation of risks for human health
Chromium in the form of trivalent compounds is an essential
nutrient. The daily human intake of chromium varies considerably
between regions. Typical values range from 50 to 200 µg/day. Such
intakes do not represent a toxicity problem, and they coincide with
the calculated human requirements. Not enough data are available
for a quantitative assessment of the risk of chromium deficiency in
different populations.
Evidence from studies on experimental animals shows that
hexavalent chromium compounds, especially those of low solubility
can induce lung cancer. Mutagenicity and related studies have
shown convincingly that hexavalent chromium is genetically active.
On the other hand, trivalent chromium compounds are inactive in
most test systems, except in systems where they can directly
interact with DNA.
Both oxidation states, when injected at high levels
parenterally in animals, are teratogenic, with the hexavalent form
accumulating in the embryos to a much greater extent than the
trivalent.
A number of effects can result from occupational exposure to
airborne chromium, including irritative lesions of the skin and
upper respiratory tract, allergic reactions, and cancers of the
respiratory tract. The data on other effects in, e.g., the
gastrointestinal, cardiovascular, and urogenital systems are
insufficient for evaluation.
Epidemiological studies have shown that workers engaged in the
production of chromate salts and chromate pigments are at increased
risk of developing bronchial carcinoma. No detailed data on dose-
response relationships are available. Although a suspicion of
increased lung cancer risks in chromium-plating workers has been
raised, the available data are inconclusive and so are data for
other industrial processes where chromium compounds are used rather
than produced. There is insufficient evidence to implicate
chromium as a causative agent of cancer in any organ other than the
lung. The frequency of sister chromatid exchanges in the
lymphocytes of workers in chromium-plating factories was higher
than in control groups.
The general population living in the vicinity of ferro-alloy
plants and exposed to ambient air concentrations of up to 1 µg/m3
did not show increased lung cancer mortality.
The results of many studies suggest that exposure to chromium
through inhalation and skin contact can pose health problems for
the general population, but no data on dose-response relationships
are available. Thus, there is no reason, at present, to be
concerned that chromium in the air presents a health problem,
except under conditions of industrial exposure.
1.2. Recommendations for Further Research
1.2.1. Analytical methods
Data from the determination of chromium should not be accepted
unless proper quality assurance procedures have been used,
including the analysis of a certified reference material of similar
composition. There is a great need for the preparation and
certification of additional standards, especially of blood, serum,
or plasma, urine containing only physiological chromium
concentrations, hair, and foods.
All analyses related to the environmental role of chromium
should differentiate between hexavalent and trivalent forms and
these values should be reported separately. While the
differentiation between hexavalent and trivalent chromium can be
accomplished by established methods, the definition of the exact
chemical species of the trivalent and hexavalent forms in air,
water, food, and tissues will require much further research.
Further development of analytical instrumentation and
preanalytical processing techniques to extend the detection limit
by one order of magnitude is recommended. The need for
interlaboratory comparison persists to improve existing methods and
to validate new procedures.
1.2.2. Sources of chromium intake
More data are needed on the chemical and physical properties of
airborne chromium, such as the oxidation state, particle size, and
solubility. These are important determinants of biological and
toxic action. Existing information on the chromium contents of
foodstuffs is unreliable and incomplete and more composition data
are needed for a valid assessment of the human chromium requirement
and the supplies available in different regions of the world to
meet these requirements. Diagnostic procedures to detect marginal
deficiency and marginal overexposure in man must be developed and
the long-term effects of both these imbalances must be defined.
Finally, not enough is known about the fate of chromium in
landfills, sewage sludges, and aquatic environments. Further
studies are needed to investigate environmental factors that
influence the mobilization, migration, and bioavailability of
chromium in the biosphere.
1.2.3. Studies on health effects
Prospective studies on the health of industrial workers,
combined with the determination of the composition and
environmental levels of the chromium compounds to which they were
exposed, are needed to determine the specific chemical or chemicals
responsible for cancer, and the dose-response relationship between
hexavalent chromium and bronchogenic carcinoma. Smoking histories
should be recorded and, when possible, information on exposure to
ionizing radiation and other chemical carcinogens should be
obtained in order to evaluate possible synergistic relationships.
More studies should be carried out on the chrome-using industries.
Preventive measures include searching for more specific biochemical
indicators of chromium exposure and early effects.
Epidemiological studies are needed to assess the incidence and
severity of chromium deficiency. The relation of chromium status
to cardiovascular diseases needs further investigation,
particularly in areas with protein-energy malnutrition.
1.2.4. Interaction with other environmental factors
The interaction of other pollutants in the atmosphere with
chromium, particularly with respect to particle size, adsorption at
the particle surface, etc., require further studies.
Interactions between trivalent chromium in the diet and other
dietary constituents are poorly understood and should be
investigated.
2. PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and Chemical Properties
Chromium (atomic number 24, relative atomic mass 51.996) occurs
in each of the oxidation states from -2 to +6, but only the 0
(elemental), +2, +3, and +6 states are common. Divalent chromium
is unstable in most compounds, as it is easily oxidized to the
trivalent form by air. Only the trivalent and hexavalent oxidation
states are important for human health. In the context of this
publication, it is of great importance to realize that these two
oxidation states have very different properties and biological
effects on living organisms, including man. Therefore, they must
always be examined separately: a valid generalization of the
biological effects of chromium as an element cannot be made.
This discussion will concentrate only on the aspects of
chromium chemistry that are of concern for health.
The relation between the hexavalent and trivalent states of
chromium is described by the equation:
Cr2O72- + 14H+ + 6e -> 2 Cr(III) + 7H2O + 1.33v.
The difference electric potential between these 2 states reflects
the strong oxidizing properties of hexavalent chromium and the
substantial energy needed to oxidize the trivalent to the
hexavalent form. For practical purposes, it can be stated that
this oxidation never occurs in biological systems. The reduction
of hexavalent chromium occurs spontaneously in the organism, unless
present in an insoluble form. A gradual reduction of hexavalent
chromium to the trivalent state is demonstrated by the colour
change of the conventional chromate cleaning solution in the
laboratory from bright orange to green, in the presence of organic
matter. In blood, chromate is reduced to the trivalent state, once
it has penetrated the red cell membrane and becomes bound to the
haemoglobin and other constituents of the cell and therefore unable
to leave the cell again. The rapid reduction of injected
51chromium-labelled chromate in the rat has been demonstrated by
Feldman (1968). Although a compound CrF6 is well known, the stable
forms of hexavalent chromium are almost always bound to oxygen
(e.g., CrO4-2, Cr2O7-2). The trivalent form exists in coordination
compounds, but never as the free ion. As a rule, its coordination
number is 6, the complexes being generally octahedral.
A large number of complexes and chelates of chromium have been
investigated, ranging from simple hexa- or tetra-aquo complexes to
those with organic acids, vitamins, amino acids and others. The
rate of ligand exchange of chromium complexes is slow in comparison
with other transition elements, with the exception of the even
slower rate of cobalt complexes; most of the Cr(III)-complexes are
kinetically stable in solutions. This property adds to the relative
inertness of trivalent compounds, in addition to the
electrochemical stability of the trivalent state. However, at near
neutral or alkaline pH, the milieu of the animal organism, the
simple chromium compounds to which the organism is exposed in the
environment or through supplementation, rapidly become insoluble,
because hydroxyl ions replace the coordinated water molecules from
the metal and form bridges, linking the chromium atoms into very
large, insoluble complexes. Coordination of trivalent chromium to
biological ligands is the prerequisite for its solubility at
physiological pH and therefore for its biological function and for
its availability for intestinal absorption. The coordination
chemistry and the specific biochemical reactions have been reviewed
by Cotton & Wilkinson (1966) and Mertz (1969), respectively. The
physical and chemical properties of chromium and some chromium-
compounds are summarized in Table 1.
Common chromium compounds
Poorly soluble "sandwich complexes" of metallic chromium
(oxidation state = O) are known, e.g., Cr(C6H6)2; these have little
practical application. Divalent compounds, such as chromium (II)
chloride (CrCl2) are used as strong reducing agents in the
laboratory, but have little industrial use. Of the many hundreds
of trivalent chromium compounds known, chromic oxide (Cr2O3 x
nH2O), is used as a pigment in paints and as a faecal marker in
digestive studies. It dissolves in acids and forms the hexa-aquo
or tetra-aquo complex, e.g.,
Cr2O3 x 9H2O + 6HCl -> 2 [Cr(H2O)6] Cl3
(colour: violet)
or
2 [Cr(H2O)4Cl2] Cl + 4H2O
(colour: dark green).
Chromium chloride ([Cr(H2O)6]Cl3 or [Cr(H2O)4Cl2]Cl) is used in
basic solution for leather tanning. The fluoride is used
industrially in printing and dyeing, and chromium sulfates and
nitrates are used as colouring and printing dyes.
One of the numerous organic complexes of chromium, a
dinicotinatoglutathionato-chromium complex has been isolated from
yeast. It is postulated as the physiologically active form in the
animal organism, but its exact structure is not known (Toepfer et
al., 1977).
Table 1. Physical and chemical properties of chromium and some selected chromium compounds
--------------------------------------------------------------------------------------------------------
Name Chemical Relative Specific Melting Boiling Colour Solubility CAS registry
symbol molecular gravity point point in water number
mass (g/cm3) (°C) (°C) (weight %)
--------------------------------------------------------------------------------------------------------
Chromium Cr 51.996 7.19 1857 2672 steel- insoluble 7440-47-3
grey
Chromium (III)- Cr2O3 151.99 5.21 2266 4000 green insoluble 1308-38-9
oxide
Chromium (VI)- CrO3 99.99 2.70 196 decompo- red 62.41 1333-82-0
oxide sition
Potassium- K2CrO4 194.20 2.732 968.3 decompo- yellow 39.96 7789-00-6
chromate (VI) sition
Potassium- K2Cr2O7 294.19 2.676 398 decompo- red 11.7 7778-50-9
dichromate (VI) sition
Calcium- CaCrO4 192.09 1025 decompo- yellow 3.5 13765-19-0
chromate (VI) x 2H2O sition
dihydrate
Calcium- CaCr2O4 208.07 4.8 2090 - olive- insoluble
chromium (III)- green
oxide
--------------------------------------------------------------------------------------------------------
For vapour pressure at 20°C, no data.
The earth's most important deposits of chromium are in either
the elemental or the trivalent oxidation state. Hexavalent
compounds of chromium in the biosphere are predominantly man-made,
and experience with hexavalent chromium is relatively short.
Chromates and dichromates are produced from chromite ore by
roasting in the presence of soda ash. From these, chromium (VI)
oxide, (CrO3), is precipitated out by the addition of sulfuric
acid. Sodium and potassium dichromates are widely used
industrially as sources of other chromium compounds, particularly
of chromium (VI) oxide, and these processes are a major source of
hexavalent chromium pollution (US EPA, 1978).
2.2. Analytical Methods
Methods for the determination of chromium in biological and
environmental samples are developing rapidly, as shown by the fact
that chromium concentrations in the blood and urine of unexposed
subjects, reported as normal, have been revised downwards by 2
orders of magnitude, in only 15 years (Versieck et al., 1978).
This development is not only due to the increasing powers of
detection and specificity of more recent methods, but also to the
better methods of contamination control that have become available.
For these reasons, all data concerning chromium levels in blood and
urine (particularly the early results), should be interpreted with
caution following scrutiny of all experimental details. On the
other hand, analytical results concerning the much higher chromium
levels in foodstuffs and human tissue have not changed as much and
can be accepted with more confidence. However, all interpretations
of chromium data should take into account the need for caution
expressed in section 2.2.2.
2.2.1. Sampling
As chromium is present in biological materials in very low
concentrations, care must be taken to avoid contamination. The
collection of dust from air samples may introduce contamination
from the chromium in the filters; blood or tissue samples may
become highly contaminated by the chromium in needles, knives,
blenders, and other instruments (Behne & Brätter, 1979). Water
samples may extract chromium from containers. Finally, reagents
used in sample dissolution, separation, chelation, acid digestion,
and other reactions, may contribute significant amounts of
chromium. Thus, it is necessary to control for these influences by
simultaneously performing a blank analysis, i.e., by carrying out
the whole analysis, including sampling, preparation, and digestion,
using all reagents, excluding a sample (Davis & Grossman, 1971).
Conversely, chromium in low concentrations may be adsorbed on
the surface of containers during long periods of storage. This
aspect has not yet been sufficiently investigated (Shendrikar &
West, 1974). Procedures for the sampling of different materials
for chromium determination have been reviewed by Beyermann (1962),
Brown et al. (1970), Murrman et al. (1971), Versieck & Speecke
(1972), Skogerboe (1974), Johnson (1974), and US DHEW (1975). All
suggest strictest contamination control (clean rooms or laminar
flow facilities).
2.2.2. Analytical methods
The voluminous literature on analysis for chromium was reviewed
by US EPA (1978). A discussion on analytical methods must
distinguish between two categories: (a) methods for measuring
large, potentially toxic concentrations of chromium as a
contaminant, and (b) methods of analysis for chromium as an
essential nutrient. The first category requires reliable
determinations of chromium at the µg/kg level; the second requires
greater sensitivity, e.g., to determine accurately the chromium
level in urine at several hundred ng/litre.
The sensitivity of instrumental analysis for the determination
of chromium does not present any problems for concentrations in the
mg/kg range, and a number of techniques can furnish satisfactory
precision and accuracy (Table 2). On the other hand, the
sensitivity of instrumentation for the determination of chromium in
the ng or µg/kg range is severely limited, and no one method is
entirely satisfactory, at present (Seeling et al., 1979). The
biologically active concentrations are near the detection limits of
the most sensitive methods, such as neutron activation analysis or
flameless atomic absorption spectrometry. In an inter-laboratory
comparison, there was poor agreement between the analytical results
obtained by well-established, experienced analytical laboratories
in several countries (Parr, 1977). Some of the results are
presented in the Table 3. It is of paramount importance for the
interpretation of all published analytical data on chromium to
realize the great variation in reported results, even for high
concentrations. These results indicate the following conclusions:
1. No one analytical method can be expected to produce
"true" results of absolute chromium concentrations, unless
the analyses are controlled by the use of a certified
reference material with a matrix composition similar to
that of the material to be analysed.
2. No valid comparisons can be made on the basis of
analytical results obtained by different laboratories,
unless the same reference materials have been used by
both, or samples have been exchanged.
3. There is a great need for certified Standard
Reference Materials with many different matrix
compositions. Six such standards of biological materials
have been certified for chromium content (tomato leaves,
pine needles, citrus leaves, oyster tissue, unexposed
bovine serum, and brewer's yeast). In addition, seven
standard reference materials of environmental samples are
available (coal, fly ash, water, sediment, urban
particulate, etc.) and more than 180 industrial samples of
various steels and metal alloys. These are available from
the National Bureau of Standards, Washington DC, USA. New
reference specimens of blood and urine have been produced
for the quality control of heavy metals in industrial
medicine and toxicology (Müller-Wiegand et al., 1983).
The assigned values were determined by reference
laboratories of the "Deutsche Gesellschaft für
Arbeitsmedizin; the control blood and urine preparations
are offered by Behringwerke AG, D-3550 Marburg, Federal
Republic of Germany.
4. In inter-laboratory quality assurance studies, it is
preferable to use the methodology developed in the
WHO/UNEP project on biological monitoring for lead and
cadmium (Vahter, 1982).
In 1983, the German DIN-Committee AAS adopted a method for the
determination of the chromium content of water and sewage (by means
of the flame AAS) (Kempf & Sonneborn, 1976); inductively coupled
plasma emission spectrometry is recommended with regard to serial
analyses (Kempf & Sonneborn, 1981).
Two special problems in the analysis for chromium may add to,
or subtract from, the true concentrations, i.e., contamination and
possible loss through volatilization or formation of refractory
compounds during sample preparation. Contamination is a serious
problem when low concentrations in blood or urine are measured.
Dust in laboratories may contain a chromium level of 700 mg/kg
(Mertz, 1969), approximately 6 orders of magnitude higher than the
concentration in urine of 0.2 - 0.7 µg/litre (Guthrie et al.,
1979). In other words, contamination of a one-ml urine sample by
only 1 µg of dust will increase the apparent chromium concentration
two-fold. Special precautions, such as those proposed by Tölg
(1974), must be taken to control this problem. The second problem,
that of potential loss during sample preparation, has been
discussed by Wolf & Greene (1976). There is evidence from several
studies that certain methods of sample preparation, such as heating
or acid digestion in open systems, may lead to the loss of
detectable amounts of chromium (Kotz et al., 1972). A typical
example, in which identical samples were determined by the
identical method, by the same analyst, in the same laboratory is
presented in Table 4.
Table 2. Instrumental methods for the determination of chromiuma
--------------------------------------------------------------------------------------------------------
Analytical Relevant Detection Interfering substance Selectivity
method applications limit
--------------------------------------------------------------------------------------------------------
Atomic fresh and saline 2 µg/litreb interfering substances all of the extracted
absorption water, industrial present in the original chromium is measured,
spectroscopy waste fluids, sample are usually not but only hexavalent
(flame) dust, and sediments extracted into the chromium is extracted
biological solids organic solvent from the original sample,
and liquids, alloys unless oxidative
pretreatment is used
Atomic biological solids 0.005 µg/ no interfering sub- total chromium is
absorption and fluids; tissue, litreb substances reported determined
(electrothermal) blood, urine; for samples of urinen,
industrial waste and bloodo; less than
waters 10% interference ob-
served for Na+, K+,
Ca2+, Mg3+, Cl-,
F-, SO4-3, and PO4-3
in certain industrial
waste watersp
Emission a wide variety of 4 µg/litrel no interfering total chromium is
spectroscopy biological and substances reported determined
(inductively- environmental
coupled plasma samples
source)
Emission a wide variety of 0.5 ngc total chromium is
spectroscopy environmental determined
(arc) samples
--------------------------------------------------------------------------------------------------------
Table 2. (contd.)
--------------------------------------------------------------------------------------------------------
Analytical Relevant Detection Interfering substance Selectivity
method applications limit
--------------------------------------------------------------------------------------------------------
Spectrophotometry natural water and 3 µg/litred iron, vanadium, and after chelation, only
industrial waste mercury may interfere the hexavalent chromium
solutions having in solution is determined
5 - 400 mg hexa-
valent chromium/
litre can be
analysed; higher
concentrations
must be reduced
by dilution
X-ray fluorescence atmospheric 2 - 10 µg/g the particle size of total chromium is
particulates, (liver)e; the sample and the determined
geological 1.5 µg/g matrix may influence
materials (coal)f the observed measure-
ments
Neutron activation air pollution depends on interference may arise total chromium is
analysis particulates, activation from gamma ray activity measured
fresh and saline procedure; from other elements,
waters, biological typical especially Na-24, Cl-38,
liquids and solids, limit: K-42, and Mn-56; P-32
sediments, metals, 10 ngm may also cause inter-
foods ference
Gas chromatography blood, serum, 0.03 pgg excess chelating agent only chromium that is
(electron capture natural water or other electron- chelated and extracted
detection) samples capturing constituents is measured; other
in the sample may lead electro-negative substances
to erroneous results may elute from the column
and be detected at the
same time as the chromium
chelate
Stable isotope all biological not expected high precision and accuracy
dilution mass materialsk,q (1%) complete sample
spectrometry digestion and exchange of
endogenous chromium with
added stable isotope
--------------------------------------------------------------------------------------------------------
Table 2. (contd.)
--------------------------------------------------------------------------------------------------------
Analytical Relevant Detection Interfering substance Selectivity
method applications limit
--------------------------------------------------------------------------------------------------------
Gas chromatography blood, serum, ~1 ngh no interference only chromium that is
(atomic biological material reported chelated and extracted is
spectroscopic detected; atomic
detection) spectroscopic methods
of detection are
inherently more selective
for chromium in complex
samples
Gas chromatography blood, plasma, 0.5 pgi no interference only chromium that is
(mass serum reported chelated and extracted
spectrometric can be detected
detection)
Chemiluminescence fresh, natural 30 ng/ Co(II), Fe(II), and only trivalent chromium
waters; dissolved litrek Fe(III) interfere but ion is measured
biological material may be compensated for
by running a blankc
--------------------------------------------------------------------------------------------------------
a Modified from: US EPA (1978).
b From: Welz (1983).
c From: Seeley & Skogerboe (1974).
d From: American Public Health Association, American Water Works Association,
and Water Pollution Control Federation (1971).
e From: Kemp et al. (1974).
f From: Kuhn (1973).
g From: Savory et al. (1969).
h From: Wolf (1976).
i From: Wolf et al. (1972).
k From: Seitz et al. (1972).
l From: Welz (1980).
m From: Keller (1980).
n From: Schaller et al. (1973).
o From: Environmental Instrumentation Group (1973).
p From: Morrow & McElhaney (1974).
q From: Veillon et al. (1979).
Table 3. Results for 3 IAEA intercomparison studiesa
-----------------------------------------------------------
Laboratory Method Number of Laboratory SD (%)
code codeb determinations mean
-----------------------------------------------------------
A. Simulated air filter
(true chromium concentration: 1.85 µg/filter)
a 7 2 1.3 3
b 3 1 1.6 7
c 2 4 1.78 5
d 2 10 1.85 6
e 2 6 1.86 52
f 2 2 2.00 30
g 2 3 2.07 10
h 2 6 2.07 9
i 2 6 2.07 6
j 7 10 2.16 10
k 5 6 2.83 40
l 7 2 3.00 -c
m 3 5 3.17 4
n 7 1 4.20 8
o 2 5 6.14 6
p 2 1 7.50 22
B. Water
(true chromium concentration: 11.1 µg/kg)
a 3 4 1.85 18
b 3 5 3.80 12
c 7 6 4.16 15
d 7 1 4.50 11
e 7 6 4.77 6
f 7 2 5.25 -
g 2 5 5.51 3
h 2 5 5.84 4
i 7 1 6.00 -
j 2 3 6.08 4
k 7 2 6.50 -
l 7 2 6.85 17
m 2 2 7.00 -
n 7 2 7.30 -
o 7 3 8.67 3
p 7 2 8.90 20
q 3 1 9.00 20
r 7 5 9.60 12
s 7 6 9.92 10
t 2 5 10.8 11
u 2 4 11.3 11
v 7 3 11.5 45
w 5 2 18.0 30
x 7 1 73.0 14
-----------------------------------------------------------
Table 3. (contd.)
-----------------------------------------------------------
Laboratory Method Number of Laboratory SD (%)
code codeb determinations mean
-----------------------------------------------------------
C. Bovine liver (SRM 1577)
(certified chromium concentration: 88 ± 12 µg/kg)
a 2 -c 5 -c
b 1 -c 51 13
c 1 -c 140 -c
d 2 -c 150 13
e 1 -c 150 33
f 1 -c 160 24
g 1 -c 240 53
h 2 -c 490 39
i 1 -c 540 64
j 2 -c 1300 -c
k 2 -c 1600 25
-----------------------------------------------------------
a From: Parr (1977).
b Method code:
1. Destructive activation analysis.
2. Nondestructive activation analysis.
3. Emission spectroscopy.
5. Spark source mass spectrometry.
7. Atomic absorption, unspecified.
c Information not given.
At present, there is no explanation of the reason why "losses"
of almost 90% were associated with the direct graphite furnace
ashing, compared with oxygen plasma ashing in the case of molasses,
but not of refined sugar. Canfield & Doisy (1976) and Tuman et al.
(1978) suggested that the loss of chromium in biological samples,
such as urine, yeast extracts, or synthetic glucose tolerance
factor (GTF) preparations represented the biologically active GTF
fraction of the chromium. They correlated this "volatile" fraction
in yeast extracts with the antidiabetic activity of the extract in
genetically diabetic mice, and the amount of "volatile" chromium in
the urine of human subjects with the efficiency of the glucose
metabolism of these subjects. This hypothesis of "volatile"
chromium has been confirmed by some investigators (Behne et al.,
1976; Koirtyohann & Hopkins, 1976; Shapcott et al., 1977;
McClendon, 1978), and contradicted by others (Jones et al., 1975;
Rook & Wolf, 1977). While the question of the "volatility" of
chromium, under various conditions, remains unanswered, it is
obvious that chromium determination presents many problems, the
most pressing of which is the selection and control of sample
digestion (Wolf & Greene, 1976).
Table 4. Apparent chromium content depending on the method of
ashinga
-----------------------------------------------------------------
Type of sugar Number Chromium content + SEM (µg/kg)
of Oxygen Muffle Graphite
samples plasma furnace furnace ashing
ashing ashing (1000 °C)
(150 °C) (450 °C) (direct
analysis)
-----------------------------------------------------------------
molasses 3 266 ± 50 129 ± 54 29 ± 5
sugar (unrefined) 8 162 ± 36 88 ± 20 37 ± 13
sugar (brown) 5 64 ± 5 53 ± 8 31 ± 2
sugar (refined) 7 20 ± 3 25 ± 3 < 10
-----------------------------------------------------------------
a From: Wolf et al. (1974).
Finally, it is important in any study of the environmental
effects of chromium, to distinguish analytically between the
trivalent and hexavalent forms. This can be accomplished by
dithiocarbamate chelation and solvent extraction (for example, with
methyl isobutylketone) prior to oxidation. Only the hexavalent
chromium remains after this process, and thus it is possible to
differentiate between the oxidation states (Feldman et al., 1967;
Cresser & Hargitt, 1976; Bergmann & Hardt, 1979; Joschi & Neeb,
1980). When determining chromium in biomaterial, the samples are
usually ashed with strong acids to destroy the organic components.
The relationship between the acids used and the behaviour of
chromium were investigated by Hara (1982) who showed that the
oxidation state of chromium was apt to change (hexavalent to
trivalent), because of the reducing action of each acid and the
conditions under which they were used.
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1. Natural Occurrence
Chromium is ubiquitous in nature; it can be detected in all
matter in concentrations ranging from less than 0.1 µg/m3 in air to
4 g/kg in soils. Naturally occurring chromium is almost always
present in the trivalent state: hexavalent chromium in the
environment is almost totally derived from human activities.
Merian (1984) has compiled the global sources of chromium in
the environment. Total input (100%) consists of inputs by:
volcanic emissions (less than 1%); the biological cycle (30%)
including extraction from soil by plants (15%) and weathering of
rocks and soils (15%); and man-made emissions (70%) including those
from general ore and metal production (3%), from metal use (60%),
and from coal burning and other combustion processes (7%).
3.1.1. Rocks
Almost all the sources of chromium in the earth's crust are in
the trivalent state, the most important mineral deposit being in
the form of chromite (FeOCr2O3) which, however, is rarely pure.
Living matter does not produce the energy necessary to oxidize
trivalent to hexavalent chromium in the organism, therefore, it can
be stated that nearly all hexavalent chromium in the environment is
produced by human activities. The industrial use of chromium and
the oxidation to the hexavalent state on an industrial scale did
not begin until 1816. Thus, man's experience with this form is
very short.
The concentration of chromium in rocks varies from an average
of 5 mg/kg (range of 2 - 60 mg/kg) in granitic rocks, to an average
1800 mg/kg (range, 1100 - 3400 mg/kg) in ultrabasic and serpentine
rocks (US NAS, 1974b).
Chromium deposits in the hexavalent oxidation state (crocoite
PbCrO4), were described by Lomonossow, in the year 1763 (Hintze,
1930), who found it in the Ural Mountains. Being a rare mineral,
chromium is found in the oxidized zones of lead deposits in regions
in which lead veins have traversed rocks containing chromite. It
may be associated with pyromorphite, cerussite, and wulfenite.
Notable localities are: Dundas, Tasmania; Beresovsk near
Sverdlovsk, Ural Mountains (Aleksandrov & Kainov, 1975), and
Rezbanya, Rumania. It is found in small quantities in the Vulture
district, Arizona, USA (Dana, 1971) and in the German Democratic
Republic in Callenberg, Saxony (Rohde et al., 1978).
Chromium concentrations in igneous rocks are positively
correlated with concentrations of silica, magnesium, and nickel.
Of agricultural importance, is the high chromium concentration in
sedimentary rocks, where the element is present in phosphorites.
This material is used as a phosphate fertilizer in agriculture and
is a significant source of chromium for agricultural soils.
Chromium-containing rocks and ores are found in all regions of
the world, but the major sources of the world's chromium supplies
are the ultra basic rocks of South Africa, Turkey, the USSR and
Zimbabwe (US NAS, 1974b). While underlying undisturbed rocks
contribute little chromium to the vegetation directly, the chromium
content is strongly correlated with that of the overlying soils.
Chromium can also be found in coal (5 - 10 mg/kg) (Merian,
1984).
3.1.2. Soils
The weathering of rocks produces chromium complexes that are
almost exclusively in the trivalent state. In most soils, chromium
occurs in low concentrations; an average of 863 soil samples from
the USA contained 53 mg/kg (Shacklette et al., 1970). The highest
concentrations, as high as 3.5 g/kg (Swaine & Mitchell, 1960), are
always found in serpentine soils. In a small area in Maryland,
USA, with soil infertility, the chromium concentration (as Cr2O3)
was as high as 27.4 g/kg (Robinson & Edington, 1935). Conversely,
low chromium concentrations (10 - 40 mg/kg) have been detected in
soils derived from granite or sandstone (Swaine & Mitchell, 1960).
Only a fraction of the chromium in soil is available to the plant;
thus, it is important to determine "available" soil-chromium. A
rough approximation of this available chromium fraction can be made
by extracting soil with acids or chelating agents and by measuring
the chromium in the extract. Though the amount of extractable
chromium is not identical with that truly available to the plant,
it is a much better measure of availability than the total
chromium. In the study of Swaine & Mitchell (1960), the amount of
chromium extracted from the soil with acetic acid varied much less
than the total soil content, and was not correlated with the latter
(Table 5).
The comparisons in this Table indicate that the amount of
chromium available to the plant is relatively independent of the
total concentration. The complex principles determining the
availability of chromium for plants are poorly understood.
Table 5. Total versus extractable chromium in different
Scottish soilsa
----------------------------------------------------------
Soil Total chromium Extractable chromium
derived from: (mg/kg) (mg/kg)
----------------------------------------------------------
Granite 20, 40, 20 0.15, 0.1, 0.11
Serpentine, 3500, 2000, 3000 0.31, 0.24, 0.63
ultrabasic
----------------------------------------------------------
a From: Swaine & Mitchell (1960).
3.1.3. Water
It is now generally agreed that, except in areas with
substantial chromium deposits, high chromium levels in water arise
from industrial sources (US NAS, 1974b).
With the exception of areas bearing chromium deposits or in
highly industrialized areas, most surface waters contain very low
levels of chromium. The chromium content in surface water in the
Tia-ding county, Shanghai, ranged from 0 to 80 µg/litre (256
samples). According to the Yang-Pu water works, which is the
biggest water works in Shanghai, the chromium levels in well water
are below 50 µg/litre. Between 1980 and 1982, chromium was not
detectable in the Yellow River. There is no information concerning
the analytical methods used (Chen Bingheng, personal communication
to the Task Group, 1986). Kopp & Kroner (1968) detected chromium
in only 25% of surface water samples from sources in the USA, with
a range of 1 - 112 µg/litre, and a mean concentration of 9.7
µg/litre. The remaining 75% contained less than 1 µg/litre, the
detection limit. Another survey of 15 rivers in the USA revealed
levels ranging from 0.7 to 84 µg/litre, the majority of samples
containing between 1 and 10 µg/litre (Durum & Haffty, 1963). On
the other hand, chromium contents in natural water of up to 215
µg/litre were reported by Novakova et al. (1974). Although modern
methods of water treatment remove much of the naturally present
chromium, it should be noted that chlorinated drinking-water
usually contains traces of hexavalent chromium. The mean level in
the drinking-water supplies in 100 cities in the USA was only 0.43
µg/litre, with a range from barely detectable to 35 µg/litre
(Durfor & Becker, 1964).
Sea water contains less than 1 µg chromium/litre (US NAS,
1974b), but the exact chemical forms in which chromium is present
in the ocean, and surface water are not known. Theoretically,
chromium can persist in the hexavalent state in water with a low
organic matter content. In the trivalent form, chromium will form
insoluble compounds at the natural pH of water, unless protected by
complex formation. The exact distribution between the trivalent
and hexavalent state is unknown.
3.1.4. Air
Chromium occurs in the air of non-industrialized areas in
concentrations of less than 0.1 µg/m3. The natural sources of air-
chromium are forest fires and, perhaps, volcanic eruptions (section
3.5). Man-made sources include all types of combustion and
emissions by the chromium industry (section 4.1.1). The chemical
forms of chromium in the air are not known, but it should be
assumed that part of the air-chromium exists in the hexavalent
form, especially that derived from high-temperature combustion.
Chromium trioxide (CrO3) may be the most important compound in the
air (Sullivan, 1969).
3.1.5. Plants and wildlife
It is not known whether chromium is an essential nutrient for
plants, but all plants contain the element in concentrations
detectable by modern methods.
Chromium concentrations in food plants growing on normal soils
range from not detectable to 0.19 mg/kg wet weight (Schoeder et
al., 1962). In addition, chromium of vegetable origin has a
relatively low biological activity (Toepfer et al., 1973).
Much higher concentrations have been reported in plants growing
on chromium deposits. For example, ash analysis showed a chromium
level of 0.34% in New Zealand lichen and 0.3% in Yugoslav Allysium
markgrafi (US NAS, 1974b). Growing on a serpentine soil (chromium
concentration 62 000 mg/kg in old mine tailings), the plant
chromium concentrations (on the basis of ash analysis) ranged from
700 mg/kg in Phormium colensoi and Liliacae to 5400 mg/kg in
Gentiana corymbifera (Lyon et al., 1970). Not all plants tolerate
high concentrations of available soil-chromium; chlorosis of citrus
trees has been observed in high-chromium areas and in laboratory
experiments. Plants grown in the vicinity of chromium-emitting
industries or those fertilized by sewage sludge are exposed to
substantial amounts of chromium. The chromium contents of plants
growing were determined by Taylor et al. (1975) near cooling
towers, where chromates were present as corrosion inhibitors. It
was shown that chromium levels in grasses, trees, and litter,
decreased with increasing distance from the towers. No information
was given as to whether the variations in chromium concentrations
were the result of surface contamination or of true absorption by
the roots of the plant.
The atmospheric deposition of metals and their retention in
ecosystems were studied by Mayer (1983). He measured mean annual
deposition rates in a beech and spruce forest ecosystem in the
Solling (Federal Republic of Germany) in 1974-78 and found that
chromium deposition in the forest canopy was in the range of 13.5 -
15.1 mg/m2 per year; the deposition on the soil below the forest
canopy ranged from 1.6 to 2.3 mg/m2 per year. Thus, up to 80% of
the metals from the atmosphere were retained in the canopy, and 30
- 50% of chromium remained in the noncycling parts of forest
biomass (bark and wood).
Sewage sludge can contain chromium levels as high as 9000
mg/kg. Application of sewage sludge to soils, which increased the
chromium levels from 36.1 to 61 mg/kg on a dry weight basis,
increased the contents of chromium in plants growing in the soil
from, e.g., 2.6 to 4.1 mg/kg in fodder rape (Andersson & Nilsson,
1972). However, most of the increased uptake in plants is retained
in the roots, and only a small fraction appears in the edible part
(Cary et al., 1977). Other elements within the sludge, e.g.,
cadmium or nickel, pose a greater problem for human health (Chaney,
1973).
Of particular importance is the chromium concentration in the
forage of meat animals. Kirchgessner et al. (1960) found strong
seasonal variations in the chromium levels in 3 different kinds of
grasses; the highest level found was 590 µg/kg dry weight in hay.
Higher levels of chromium in vegetation not used for human
consumption may account for the generally higher chromium contents
in the organs of wild animals, compared with man (Schroeder, 1966).
Schroeder (1970) determined the chromium concentrations in
different organs and muscles of wild animals and found that they
ranged from 0.04 to 0.48 mg/kg on a wet weight basis. Chromium
concentrations in the hair of several wild-animal species,
collected by Huckabee et al. (1972), ranged from 640 mg/kg in a
pronghorn antelope living in Lemhi Range, Idaho, USA, to about 0.6
mg/kg in a coyote, sampled in Jackson Hole, Wyoming, USA.
3.1.6. Environmental contamination from natural sources
No data have been found that indicate any significant
contamination of the environment from natural sources, though major
catastrophic events, such as large forest fires or volcanic
eruptions, could conceivably contribute to the concentration of
chromium in air. Water supplies originating in areas with chromium
deposits may contain elevated chromium concentrations (section
4.1.2). However, none of these natural sources contributes enough
chromium to pose a hazard for human or animal health.
3.2. Production, Consumption, and Uses
The world's mining production of chromium ore was approximately
9.73 million tonnes (gross weight) in 1980; it fell to 9 million
during 1981 (Thomson, 1982), but rose again to 11 million tonnes in
1985. Exact data on the yield of elemental chromium are not
available, but may range around half of the gross weight of the
mined ore.
The major uses and amounts of chromium used in the USA in 1968
in thousands of tonnes were: transportation, 77; construction
products, 105; machinery and equipment, 72; home appliances and
equipment, 25; refractory products, 68; plating of metals, 20;
pigment and paints, 15; leather goods, 10; and other uses, 66;
giving a total of 458 thousand tonnes.
The principal uses of chromium are in the metallurgical
processing of ferrochromium and other metallurgical products,
chiefly stainless steel, and, to a much lesser extent, in the
refractory processing of chrome bricks and chemical processing to
make chromic acid and chromates.
Chromates are used for the oxidation of various organic
materials, in the purification of chemicals, in inorganic
oxidation, and in the production of pigments. A large percentage
of chromic acid is used for plating. Dichromate is converted to
chromic sulfate for tanning. Fungicides and wood preservatives
consume an estimated 1.3 million kg of chromium annually.
Chromates are used as rust and corrosion inhibitors, for example,
in diesel engines. Because chromite has a high melting point and
is chemically inert, it is used in the manufacture of bricks for
lining metallurgical furnaces.
In 1981, the demand for chromium was at its lowest level since
1975. However, on the basis of 1978 figures, the demand for
chromium is expected to increase at an annual rate of about 3.2%,
up to 1990. While the level of stainless steel production will
continue to be the principal influence affecting markets for
chromite and ferrochrome, other factors could have a significant
impact on future trends, e.g., purchases for government stockpiles
(in 1981, the USA had a stockpile of 1.48 million tonnes, and
France and Japan announced the build up of stockpiles) or the
development of new alloys and steels (Thomson, 1982).
3.3. Waste Disposal
Substantial amounts of chromium enter sewage-treatment plants
in major cities. Klein et al. (1974) estimated a total daily
chromium burden for New York city treatment plants of 676 kg, of
which 43% came from electroplating, 9% from other industries, 9%
from runoff, 11% from unknown sources, and 28% from residential
homes. This waste from one city alone (amounting to 2.4 x 105
kg/year), if untreated, would add a significant burden to the
ocean, in comparison with the estimated global natural chromium
mobilization by weathering of 3.6 x 107 kg/year (Bertine &
Goldberg, 1971). The high chromium discharge from homes is
difficult to explain; it has been suggested that this could arise
from the corrosion of stainless steel or the use of waste disposal
units in domestic sinks. The contribution from excreta, estimated
at 100 µg chromium/day per person, should not exceed 1 kg/day for
the 10 million people in the New York area.
The concentration of chromium in the waste-water received at
the New York treatment plants varied between 40 and 500 µg/litre;
this range is probably representative of the chromium discharge in
major cities. The removal of chromium from the waste-water was
studied by Brown et al. (1973). Primary sewage treatment removed
only 27%, secondary treatment using a trickling-filter method
removed 38%; the most effective secondary treatment method
(activated sludge) removed 78%. In another study of a treatment
plant (Chen et al., 1974), the primary effluent contained 300
µg/litre and the secondary effluent, after the activated sewage
sludge and sedimentation process, 60 µg/litre (80% removal). The
final discharge from the plant, a mixture of primary and secondary
effluent and digested sludge had quite a high chromium content of
200 µg/litre. This level is substantially higher than the natural
chromium content of surface water and represents a significant
source of contamination.
Waste-waters from chromium industries contain very high levels
of chromium, ranging from 40 mg/litre (leather industry) to 50 000
mg/litre (chromium plating) (Cheremisinoff & Habib, 1972). These
levels must be reduced by precipitation before the waste-water can
be discharged. The steps include reduction of hexavalent to
trivalent chromium at an acidic pH, followed by precipitation of
the hydroxides at pH 9.5 (Ottinger et al., 1973). The precipitates
containing chromium and other metals are then collected in settling
ponds and disposed of by landfill, incineration, or dumping in the
ocean (US EPA, 1980). If the last procedure is used, the waste-
water treatment itself will contribute to the contamination of the
oceans.
Landfill and sewage sludge operations are, in turn, potential
sources of contamination of soil and groundwater by chromium.
However, at alkaline pH values, chromium hydroxides are insoluble
and leaching by any but very acidic water should be minimal.
Pohland (1975) did not detect any measurable concentrations of
chromium in the leachate from a simulated landfill.
Similary, the chromium in sewage sludge is very poorly soluble.
Berrow & Webber (1972) found a mean concentration of only 22
mg/litre (range, < 0.9 - 170 mg/litre) in samples of 42 sludges
extracted with 2.5% acetic acid. This represented 0.7 - 8.5% of
the chromium concentration in the original sludge. As acetic acid
is a good complexing and extracting agent for chromium, the
reported levels of extractable chromium are probably much greater
than those resulting from extraction with water at near neutral pH.
However, sludge application to land does increase the chromium
content of the soil (LeRiche, 1968). The application of 66
tonnes/hectare each year, for 19 years, resulted in an increase in
the acetic acid-soluble chromium in the soil from 0.9 to 2.6 mg/kg,
7 years after sludge application was discontinued. This
extractable chromium is presumably available to the plant. The
final link in the cycle of the soil-chromium derived from sewage
sludge is not well known. Undoubtedly, some will be removed by the
growth of vegetation (section 3.1.5). The rate of migration into
ground water depends on the properties of the soil and climatic
conditions. Thus, it is not surprising that, in one study
(LeRiche, 1968), a very slow rate of disappearance was reported
(reduction of extractable chromium from 2.8 to 2.6 mg/kg in 8
years), whereas in another, there was a very rapid rate of
disappearance (reduction of total chromium from 118 to 30 mg/kg, in
3 years) (Page, 1974).
3.4. Miscellaneous Sources of Pollution
As discussed earlier, waste-waters from residential areas in
New York carried approximately 200 kg of chromium daily to the
treatment plants. Of this amount, only 1 kg can be accounted for
by the human excreta of 10 million persons. If a water use of 200
litres per person and a (high) chromium content of 10 µg/litre is
assumed, this concentration would account only for an additional 20
kg. The origin of the rest is unknown (section 3.3). It should be
pointed out that analytical accuracy is difficult to achieve in
chromium analysis (section 2.2.2) and will affect the results of
all "balance" calculations.
It is evident that the chief source of air pollution with
chromium is ferrochromium refining. Appreciable, but far smaller
emissions, come from refractory operations and inadvertent sources.
The lowest emissions come from the chemical processes in the
production of dichromate and other chrome chemicals. Combustion of
coal and oil, and cement production, large-scale, spray-painting
operations (e.g., ships and planes) and glass plants constitute
other major sources of chromium emissions.
3.5. Environmental Transport and Distribution
Industrial effluents containing chromium, some of which is in
the hexavalent form, are emitted into streams and the air. Whether
the chromium remains hexavalent until it reaches the ocean depends
on the amount of organic matter present in the water. If it is
present in large quantities, the hexavalent chromium may be reduced
by, and the trivalent chromium adsorbed on, the particulate matter.
If it is not adsorbed, the trivalent chromium will form large,
polynucleate complexes that are no longer soluble. These may
remain in colloidal suspension and be transported to the ocean as
such, or they may precipitate and become part of the stream
sediment. Similar processes occur in the oceans: hexavalent
chromium is reduced and settles on the ocean bed. It is replaced
by an estimated 6.7 x 106 kg of chromium from rivers (Bowen, 1966).
In a study of the oxidation state of chromium in ocean water, Fukai
(1967) detected an increased proportion of the trivalent form with
increasing depth.
Chromium is emitted into the air, not only by the chromium
industries, but also by every combustion process, including forest
fires. The oxidation state of chromium emissions is not well
defined quantitatively, but it can be assumed that the heat of
combustion may oxidize an unknown proportion of the element to the
hexavalent state. While suspended in the air, this state is
probably stable, until it settles down and comes into contact with
organic matter, which will eventually reduce it to the trivalent
form. Living plants and animals absorb the hexavalent form in
preference to the trivalent, but once absorbed, it is reduced to
the stable, trivalent state.
The transport of chromium in the environment is summarized in
Fig. 1. It should be noted that there is a complete cycle from
rocks or soil to plants, animals, and man, and back to soil. Only
part of the chromium is diverted to a second pathway leading to the
repository, the ocean floor. This part consists of chromium from
rocks and soil carried by water (concentrations, a few µg/litre)
and animal and human excreta, a small part of which may find their
way into water (e.g., runoff from sewage sludge). Another cycle
consists of airborne chromium from natural sources, such as fires,
and from the chromate industry. This cycle also contains some
hexavalent chromium, with byproducts going into the water and air.
Part of the air-chromium completes the cycle by settling on the
land, but a very significant portion goes into the repository, the
ocean, where it ends up as sediment on the ocean floor.
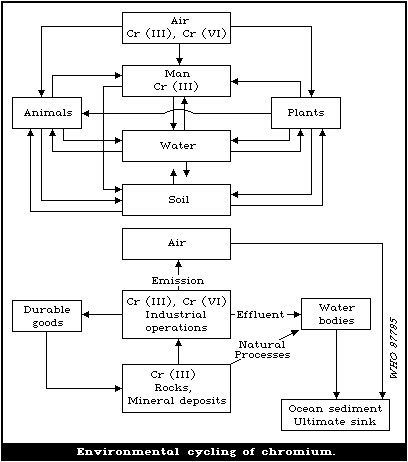 4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental Levels
4.1.1. Air
Chromium concentrations in air vary with location. Background
levels determined at the South Pole ranged from 2.5 to 10 pg/m3 and
are believed to be due to the weathering of crustal material (US
NAS, 1974a). Data collected by the US National Air Sampling
Network in 1964 gave the national average concentration for
chromium in the ambient air as 0.015 µg/m3, varying from non-
measurable levels to a maximum of 0.35 µg/m3. Chromium
concentrations in most non-urban areas and even in many urban areas
were below detection levels. Yearly average concentrations for
cities in the USA varied from 0.009 to 0.102 µg/m3. Concentrations
ranging from 0.017 to 0.087 µg/m3 have been reported for Osaka,
Japan (US EPA, 1978). The chromium content of the air in the
vicinity of industrial plants may be higher. In 1973, the reported
chromium concentrations ranged from 1 to 100 mg/m3 for coal-fired
power plants, from 100 to 1000 mg/m3 for cement plants, from 10 to
100 mg/m3 for iron and steel industries, and from 100 to 1000 mg/m3
for municipal incinerators (US EPA, 1978). Ferrochromium plants
have the highest emission rates (Radian Corporation, 1983).
However, modern chromium chemical plants contribute very little to
pollution today, because of the installation of collecting
equipment that returns the material for reuse. Drift from cooling-
towers contributes to atmospheric pollution, when chromium is used
as a corrosion inhibitor (section 3.1.5). Little information
exists on the particle size distribution of chromium in the air.
The mass median diameter in a study in the United Kingdom was
1.5 µm (Cawse, 1974).
The chemical form of chromium in air depends on the source.
Chromium from metallurgical production is usually in the trivalent
or zero state. During chromate production, chromate dusts can be
emitted. Aerosols containing chromic acid can be produced during
the chrome-plating process; chromate is also the form found in air
contaminated by cooling-tower drift.
4.1.2. Water
Except for regions with substantial chromium deposits, the
natural content of chromium in surface waters and drinking-water is
very low, most of the samples containing between 1 and 10 µg/litre
(US NAS, 1974a). Substantially higher concentrations are almost
always the result of human activities, reflecting pollution from
industrial activities or sewage waste (Perlmutter & Lieber, 1970).
Thus, the chromium concentration in untreated surface water
supplies reflects the extent of the industrial activity in an area
(Table 6).
Table 6. Chromium in water supplies
-----------------------------------------------------------------------
Country Chromium Range Reference
concentration (µg/litre)
(µg/litre)
-----------------------------------------------------------------------
Bulgaria
flat district -a 7 - 8 Novakova et al.
hilly district - 60 - 215 (1974)
Canada
Great Lakes 1 0.2 - 19 Weiler & Chawla
(1969)
Ottawa River 0.01 - Durum & Haffty
(1963);
Merrit (1971)
China
Yellow River undetectable - Chen Bingheng
Tia-Ding country - 0 - 80 (personal
surface water communication, 1986)
Germany, Federal
Republic of
Rhine River 18 DeGroot & Allersma
(1973)
Poland
Wisla - 31 - 112 Pasternak (1973)
USA
Illinois River 21 5 - 38 Mathis & Cummings
(1973)
Lake Tahoe < 0.07 - Bond et al. (1973)
Mississipi River 3 - 20 Bond et al. (1973)
New York area, 1250 - Lieber et al. (1964)
contaminated stream
-----------------------------------------------------------------------
a No data available.
Drinking-water from 100 public water supplies in the USA had a
median chromium content of 0.43 µg/litre, ranging from non-
detectable concentrations to 35 µg/litre. In the Federal Republic
of Germany, levels in about 90% of drinking-water samples from 1062
public water supplies were below 0.5 µg/litre; in 1.4% of the water
samples, levels exceeded the prescribed limit value of 50 µg/litre
(Kempf & Sonneborn, 1981).
Both trivalent and hexavalent forms of chromium occur in water.
National and international drinking-water standards reject
drinking-water containing hexavalent chromium concentrations of
more than 50 µg/litre. Such high concentrations occur naturally,
only in areas with substantial chromium deposits (Novakova et al.,
1974); in all other regions they would be caused by industrial
wastes.
4.1.3. Food
The available food data (Schroeder et al., 1962; Schlettwein-
Gsell & Mommsen-Straub, 1971; Toepfer et al., 1973; Kumpulainen et
al., 1979) indicate a range of the chromium concentrations in
different foodstuffs of 5 -250 mg/kg (Table 7). Highly refined
foods, such as sugar and flour of low extraction, contain the
lowest levels. Very high concentrations have been reported in
pepper (Schroeder et al., 1962) and brewer's yeast.
Table 7. Ranges of chromium concentrations in some
food groupsa
---------------------------------------------------
Food Chromium content
(µg/kg of wet weight)
Mean Range
---------------------------------------------------
Flour, refined < 20
Bran 50
Meat (beef, pork, chicken) 10 - 60
Fish, fresh < 10 - 10
Vegetables 5 - 30
Nuts 140
Whole Milk 10
Cheeses 10 - 130
Sugar, refined < 20
Egg yolk 200
---------------------------------------------------
a From: Koivistoinen (1980).
4.2. General Population Exposure
4.2.1. Food and water
The chromium intake from diet and water varies considerably
between regions (Table 8). However, these variations should be
interpreted with caution because not all the analyses have been
controlled by the use of standard reference material or proper
quality assurance procedures, and discrepancies in methods cannot
be completely discounted.
Table 8. Chromium intake from diet and water
--------------------------------------------------------------------
Region Chromium Remarks Reference
intake
(µg/day)
(range)
--------------------------------------------------------------------
Canada 189 - Canada, National
(136 - Health and Welfare
282) (1980)
Germany, Federal 62 DAa; 4 subjects, Schelenz (1977)
Republic of (11 - 1 week
195)
Japan 723 urban adults Murakami et al.
(202 - (1965)
1710)
943 rural adultsb Murakami et al.
(> 180 - (1965)
1190)
New Zealand 81 ± 32 DAa; 11 women, Guthrie (1973)
(39 - 190) self-selected
diets
United Kingdom (80 - 100) Facer, J.L.
(1983)c
USA 52 DAa Levine et al.
(5 - 115) (1968)
USA 78 ± 42 DA; 28 diets, Kumpulainen
(25 - 224) complete et al.
(controlled (1979)
by SRM)
USSR
Tatar (88 - 126) childrend Goncharov (1968)
--------------------------------------------------------------------
a DA = Direct analysis of composite diets as consumed.
b Analysis of composite of cooked servings for one complete day
collected from 10 families in different localities.
c Personal communication to Dr M. Mercier, IPCS (United Kingdom
Ministry of Agriculture, Fisheries and Food, London).
d Analysis of diets in kindergartens.
Most reported chromium intakes range from 50 to 200 µg/day.
However, a comparison of the chromium levels reported by different
investigators reveals substantial differences, some of which may be
due to the influence of the location where the foods were grown.
Only one study (Kumpulainen et al., 1979) was controlled by the use
of standard reference materials. The data should therefore be
treated as preliminary. Furthermore, data concerning total
chromium concentrations do not include information on the species
of chromium in the food and their biological availability. In an
attempt to estimate the biologically available chromium in food,
Toepfer et al. (1973) measured the effects of extracts from foods
on the potentiation of insulin action in epididymal fat tissue in
vitro. No correlation was found between the insulin potentiation
and the total chromium extracted from the foods by acid hydrolysis,
but a significant correlation ( P = 0.01) appeared between the
ethanol-extractable amount of chromium and biological activity.
The highest concentrations of ethanol-extractable chromium were
found in brewer's yeast, black pepper, calf liver, cheese, and
wheatgerm.
4.2.2. Other exposures
Since chromium compounds are increasingly present in products
used in daily life, chromium eczemas are often observed in the
general population. Polak et al. (1973) surveyed the most
important chromium-containing materials or objects: chromium ore,
baths, colours, lubricating oils, anti-corrosive agents, wood
preservation salts, cement, cleaning materials, textiles, and
leather tanned with chromium. According to Polak et al. (1973),
people who work with material containing mere traces of chromium
salts are more at risk than workers who come into contact with high
concentrations of chromium salts. Some less frequently occurring
cases include sensitization by tattooing (especially green and
light-blue)( Tazelaar, 1970), artificial dentures made of chromium-
containing steel, metal pins used for internal fixation of broken
bones, and bullets retained in the body (Langard & Hensten-
Pettersen, 1981).
4.3. Occupational Exposure
4.3.1. Inhalation exposure
In chromium ore mines, the concentration of dust in the air in
different work-places ranged from 1.3 to 16.9 mg/m3. In the
crushing and sorting factory, it varied from 6.1 to 148 mg/m3. The
chromium content in settled dust (calculated as Cr2O3) varied from
3.6 to 48%. During the period 1955-69, levels of trivalent
chromium in the dust in different work-places in ferro-alloy
factories ranged from 16 to 42%, while the concentrations of dust
in the air varied from 14 to 38 mg/m3 (Pokrovskaja & Shabynina,
1974).
In the past, the production of refined ferrochromium led to
high concentrations of dust in the air of the work-place (10 - 30
mg/m3) (Velichkovsky & Pokrovskaja, 1973). The concentrations of
hexavalent chromium after implementation of a number of sanitation
and hygienic measures were 0.03 - 0.06 mg/m3 (Velichkovsky &
Pokrovskaja, 1973).
In the manufacture of chromates, the oxidation state,
solubility, and composition of air-borne material varied in
different areas of the plant. Exposure in the ore-crushing area
was to trivalent, insoluble particulates; in the leaching area,
exposure was to tri- and hexavalent, soluble and insoluble
particulates and droplets; at the dry end of the process, the
workers were exposed to the very soluble hexavalent chromates in
particulate form, and to the insoluble residue after leaching
(Velichkovsky & Pokrovskaja, 1973). In plants using calcium in the
roasting process, the residue that is recycled contains, among
other products, calcium chromate, currently believed, on the basis
of animal studies, to be at least partly responsible for the
carcinogenicity of chromium.
Chromium plating of metal surfaces was accompanied by the
release of hexavalent chromium into the air in work premises, in
concentrations ranging from 0.04 to 0.4 mg/m3 (Yunisova &
Pavlovskaja, 1975). In one electroplating factory, the
concentration of chromic acid vapours in the air varied from 0.1 to
1.4 mg/m3 (Gomes, 1972). In the vicinity of 3 different baths in a
Swedish chromium plating factory, chromium concentrations ranged
from 20 to 46 µg chromium (VI)/m3, while, at another factory, the
exposure levels near all baths were below 1 µg/m3 (Lindberg et al.,
1985).
Occupational exposure to chromium during welding has been
analysed and the results published by several authors (Stern,
1981). Welding of metals using chromium and nickel electrodes
require high temperatures that melt both the material welded and
the electrode, producing a complex mixture of gases, oxides, and
other compounds, the chemistry of which is determined by the
technology, materials, and welding parameters used in each case
(Lautner et al., 1978). Hexavalent chromium compounds were found in
the respiratory zone of the welder at concentrations ranging from
3.8 to 6.6 µg/m3 (Migai, 1975). For the welding industry as a
whole, the average exposure arising from welding is not homogeneous
but depends on the type and conditions of the welding process
(Stern, 1982).
In a cement-producing factory, the concentration of hexavalent
chromium in the air in the work-place varied from 0.0047 to 0.008
mg/m3. The presence of chromium was explained by the fact that the
lining of the kilns was composed of chrome-magnesium bricks
containing 17 - 28% chromium compounds (Retnev, 1960). Forty-two
types of American cement were analysed for total chromium content
and particularly for hexavalent chromium. It was found that
hexavalent chromium was present in 18 out of 42 samples in
concentrations varying from 0.1 to 5.4 g/kg cement, while the total
chromium content ranged from 5 to 124 g/kg (Perone et al., 1974).
Analysis of 59 samples of Portland cement from 9 European countries
showed that the contents of hexavalent chromium extractable with
sodium sulfate varied from 1 to 83 g/kg of cement, while the total
chromium contents ranged from 35 to 173 g/kg (Fregert & Gruvberger,
1972).
4.3.2. Dermal exposure
Occupational dermal exposure can result in percutaneous
absorption and in harmful effects on the skin (section 8.3.1),
though the percutaneous absorption of chromium (III) sulfate has
been questioned by Aitio et al. (1984).
Chromium, especially chromate, is the most common contact
allergen and of great importance in occupational contact dermatitis
(Thormann et al., 1979).
Chromium eczema occurred most frequently in building labourers
followed by painters, galvanizers, machine drillers, metal-workers,
graphic artists, and workers in the timber, chemical, leather, and
textile industries (Polak et al., 1973). This is likely to reflect
the exposure to chromium compounds from a large number of every-day
products (section 4.2.2). The skin exposure to cement may be of
particular importance as building labourers belong to the most
affected group.
5. KINETICS AND METABOLISM
5.1. Absorption
5.1.1. Absorption through inhalation
5.1.1.1. Animal studies
Few animal studies have been performed to determine the
absorption of chromium compounds via inhalation. In one early
study, mice and rats were exposed to chromium particulates in an
inhalation chamber for various periods of time. The concentrations
of soluble chromium (CrO3) in air were between 1 and 2 mg/m3 for
the mice and 2 and 3 mg/m3 for the rats. The concentrations of
soluble versus insoluble chromium in the lung tissue of the mice
varied greatly. The soluble chromium concentrations ranged from
4.3 to 10.7 µg/kg dry tissue, after 100 weeks of exposure (Baetjer
et al., 1959a).
The amount of chromium that is absorbed through inhalation
depends on the size of the particles and droplets, on their
solubility in body fluids, and on their reaction with the
respiratory mucosa. Particles greater than 5 µm in diameter
(aerodynamic size) are deposited on the mucosal surface of the
nasal membrane, trachea, and bronchi and are carried by the action
of the cilia to the pharynx, where they are swallowed. Smaller
particles and droplets, especially those below 2 mm in size,
penetrate to the alveoli. Particles and droplets of soluble
compounds, such as hexavalent chromium compounds, are rapidly
absorbed in the blood. Insoluble particles, such as chromite, are
taken up by macrophages and slowly cleared. Soluble materials that
react with the constituents of the lung tissue, such as soluble
trivalent compounds, are also cleared slowly. Baetjer et al.
(1959b) were the first to describe the differences in the clearance
rates of soluble chromates and chromic chloride, when injected
intratracheally into the lungs of animals. The hexavalent chromate
was more rapidly transported from the lungs to other tissues than
the trivalent chromic chloride. Ten minutes after injection, only
15% chromium (IV) remained in the lung compared with 70% chromium
(III). After 60 days, the corresponding figures were 1.7% and 13%
(Baetjer et al., 1959b). Hexavalent chromium is taken up by the
red blood cells in much larger quantities than trivalent chromium.
This finding has been confirmed by Wiegand et al. (1984b)
performing intratracheal instillation (Na251CrO4) studies on
anaesthetized rabbits, as shown in Fig. 2. Confirmation of the
macrophage uptake of insoluble chromate was obtained by exposing
hamsters to 0.5 - 1 mg chromic oxide dust/m3 for 4 h. The median
diameter of the particles was 0.17 µm. Over 90% of the oxide was
found in the macrophages (Sanders et al., 1971).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental Levels
4.1.1. Air
Chromium concentrations in air vary with location. Background
levels determined at the South Pole ranged from 2.5 to 10 pg/m3 and
are believed to be due to the weathering of crustal material (US
NAS, 1974a). Data collected by the US National Air Sampling
Network in 1964 gave the national average concentration for
chromium in the ambient air as 0.015 µg/m3, varying from non-
measurable levels to a maximum of 0.35 µg/m3. Chromium
concentrations in most non-urban areas and even in many urban areas
were below detection levels. Yearly average concentrations for
cities in the USA varied from 0.009 to 0.102 µg/m3. Concentrations
ranging from 0.017 to 0.087 µg/m3 have been reported for Osaka,
Japan (US EPA, 1978). The chromium content of the air in the
vicinity of industrial plants may be higher. In 1973, the reported
chromium concentrations ranged from 1 to 100 mg/m3 for coal-fired
power plants, from 100 to 1000 mg/m3 for cement plants, from 10 to
100 mg/m3 for iron and steel industries, and from 100 to 1000 mg/m3
for municipal incinerators (US EPA, 1978). Ferrochromium plants
have the highest emission rates (Radian Corporation, 1983).
However, modern chromium chemical plants contribute very little to
pollution today, because of the installation of collecting
equipment that returns the material for reuse. Drift from cooling-
towers contributes to atmospheric pollution, when chromium is used
as a corrosion inhibitor (section 3.1.5). Little information
exists on the particle size distribution of chromium in the air.
The mass median diameter in a study in the United Kingdom was
1.5 µm (Cawse, 1974).
The chemical form of chromium in air depends on the source.
Chromium from metallurgical production is usually in the trivalent
or zero state. During chromate production, chromate dusts can be
emitted. Aerosols containing chromic acid can be produced during
the chrome-plating process; chromate is also the form found in air
contaminated by cooling-tower drift.
4.1.2. Water
Except for regions with substantial chromium deposits, the
natural content of chromium in surface waters and drinking-water is
very low, most of the samples containing between 1 and 10 µg/litre
(US NAS, 1974a). Substantially higher concentrations are almost
always the result of human activities, reflecting pollution from
industrial activities or sewage waste (Perlmutter & Lieber, 1970).
Thus, the chromium concentration in untreated surface water
supplies reflects the extent of the industrial activity in an area
(Table 6).
Table 6. Chromium in water supplies
-----------------------------------------------------------------------
Country Chromium Range Reference
concentration (µg/litre)
(µg/litre)
-----------------------------------------------------------------------
Bulgaria
flat district -a 7 - 8 Novakova et al.
hilly district - 60 - 215 (1974)
Canada
Great Lakes 1 0.2 - 19 Weiler & Chawla
(1969)
Ottawa River 0.01 - Durum & Haffty
(1963);
Merrit (1971)
China
Yellow River undetectable - Chen Bingheng
Tia-Ding country - 0 - 80 (personal
surface water communication, 1986)
Germany, Federal
Republic of
Rhine River 18 DeGroot & Allersma
(1973)
Poland
Wisla - 31 - 112 Pasternak (1973)
USA
Illinois River 21 5 - 38 Mathis & Cummings
(1973)
Lake Tahoe < 0.07 - Bond et al. (1973)
Mississipi River 3 - 20 Bond et al. (1973)
New York area, 1250 - Lieber et al. (1964)
contaminated stream
-----------------------------------------------------------------------
a No data available.
Drinking-water from 100 public water supplies in the USA had a
median chromium content of 0.43 µg/litre, ranging from non-
detectable concentrations to 35 µg/litre. In the Federal Republic
of Germany, levels in about 90% of drinking-water samples from 1062
public water supplies were below 0.5 µg/litre; in 1.4% of the water
samples, levels exceeded the prescribed limit value of 50 µg/litre
(Kempf & Sonneborn, 1981).
Both trivalent and hexavalent forms of chromium occur in water.
National and international drinking-water standards reject
drinking-water containing hexavalent chromium concentrations of
more than 50 µg/litre. Such high concentrations occur naturally,
only in areas with substantial chromium deposits (Novakova et al.,
1974); in all other regions they would be caused by industrial
wastes.
4.1.3. Food
The available food data (Schroeder et al., 1962; Schlettwein-
Gsell & Mommsen-Straub, 1971; Toepfer et al., 1973; Kumpulainen et
al., 1979) indicate a range of the chromium concentrations in
different foodstuffs of 5 -250 mg/kg (Table 7). Highly refined
foods, such as sugar and flour of low extraction, contain the
lowest levels. Very high concentrations have been reported in
pepper (Schroeder et al., 1962) and brewer's yeast.
Table 7. Ranges of chromium concentrations in some
food groupsa
---------------------------------------------------
Food Chromium content
(µg/kg of wet weight)
Mean Range
---------------------------------------------------
Flour, refined < 20
Bran 50
Meat (beef, pork, chicken) 10 - 60
Fish, fresh < 10 - 10
Vegetables 5 - 30
Nuts 140
Whole Milk 10
Cheeses 10 - 130
Sugar, refined < 20
Egg yolk 200
---------------------------------------------------
a From: Koivistoinen (1980).
4.2. General Population Exposure
4.2.1. Food and water
The chromium intake from diet and water varies considerably
between regions (Table 8). However, these variations should be
interpreted with caution because not all the analyses have been
controlled by the use of standard reference material or proper
quality assurance procedures, and discrepancies in methods cannot
be completely discounted.
Table 8. Chromium intake from diet and water
--------------------------------------------------------------------
Region Chromium Remarks Reference
intake
(µg/day)
(range)
--------------------------------------------------------------------
Canada 189 - Canada, National
(136 - Health and Welfare
282) (1980)
Germany, Federal 62 DAa; 4 subjects, Schelenz (1977)
Republic of (11 - 1 week
195)
Japan 723 urban adults Murakami et al.
(202 - (1965)
1710)
943 rural adultsb Murakami et al.
(> 180 - (1965)
1190)
New Zealand 81 ± 32 DAa; 11 women, Guthrie (1973)
(39 - 190) self-selected
diets
United Kingdom (80 - 100) Facer, J.L.
(1983)c
USA 52 DAa Levine et al.
(5 - 115) (1968)
USA 78 ± 42 DA; 28 diets, Kumpulainen
(25 - 224) complete et al.
(controlled (1979)
by SRM)
USSR
Tatar (88 - 126) childrend Goncharov (1968)
--------------------------------------------------------------------
a DA = Direct analysis of composite diets as consumed.
b Analysis of composite of cooked servings for one complete day
collected from 10 families in different localities.
c Personal communication to Dr M. Mercier, IPCS (United Kingdom
Ministry of Agriculture, Fisheries and Food, London).
d Analysis of diets in kindergartens.
Most reported chromium intakes range from 50 to 200 µg/day.
However, a comparison of the chromium levels reported by different
investigators reveals substantial differences, some of which may be
due to the influence of the location where the foods were grown.
Only one study (Kumpulainen et al., 1979) was controlled by the use
of standard reference materials. The data should therefore be
treated as preliminary. Furthermore, data concerning total
chromium concentrations do not include information on the species
of chromium in the food and their biological availability. In an
attempt to estimate the biologically available chromium in food,
Toepfer et al. (1973) measured the effects of extracts from foods
on the potentiation of insulin action in epididymal fat tissue in
vitro. No correlation was found between the insulin potentiation
and the total chromium extracted from the foods by acid hydrolysis,
but a significant correlation ( P = 0.01) appeared between the
ethanol-extractable amount of chromium and biological activity.
The highest concentrations of ethanol-extractable chromium were
found in brewer's yeast, black pepper, calf liver, cheese, and
wheatgerm.
4.2.2. Other exposures
Since chromium compounds are increasingly present in products
used in daily life, chromium eczemas are often observed in the
general population. Polak et al. (1973) surveyed the most
important chromium-containing materials or objects: chromium ore,
baths, colours, lubricating oils, anti-corrosive agents, wood
preservation salts, cement, cleaning materials, textiles, and
leather tanned with chromium. According to Polak et al. (1973),
people who work with material containing mere traces of chromium
salts are more at risk than workers who come into contact with high
concentrations of chromium salts. Some less frequently occurring
cases include sensitization by tattooing (especially green and
light-blue)( Tazelaar, 1970), artificial dentures made of chromium-
containing steel, metal pins used for internal fixation of broken
bones, and bullets retained in the body (Langard & Hensten-
Pettersen, 1981).
4.3. Occupational Exposure
4.3.1. Inhalation exposure
In chromium ore mines, the concentration of dust in the air in
different work-places ranged from 1.3 to 16.9 mg/m3. In the
crushing and sorting factory, it varied from 6.1 to 148 mg/m3. The
chromium content in settled dust (calculated as Cr2O3) varied from
3.6 to 48%. During the period 1955-69, levels of trivalent
chromium in the dust in different work-places in ferro-alloy
factories ranged from 16 to 42%, while the concentrations of dust
in the air varied from 14 to 38 mg/m3 (Pokrovskaja & Shabynina,
1974).
In the past, the production of refined ferrochromium led to
high concentrations of dust in the air of the work-place (10 - 30
mg/m3) (Velichkovsky & Pokrovskaja, 1973). The concentrations of
hexavalent chromium after implementation of a number of sanitation
and hygienic measures were 0.03 - 0.06 mg/m3 (Velichkovsky &
Pokrovskaja, 1973).
In the manufacture of chromates, the oxidation state,
solubility, and composition of air-borne material varied in
different areas of the plant. Exposure in the ore-crushing area
was to trivalent, insoluble particulates; in the leaching area,
exposure was to tri- and hexavalent, soluble and insoluble
particulates and droplets; at the dry end of the process, the
workers were exposed to the very soluble hexavalent chromates in
particulate form, and to the insoluble residue after leaching
(Velichkovsky & Pokrovskaja, 1973). In plants using calcium in the
roasting process, the residue that is recycled contains, among
other products, calcium chromate, currently believed, on the basis
of animal studies, to be at least partly responsible for the
carcinogenicity of chromium.
Chromium plating of metal surfaces was accompanied by the
release of hexavalent chromium into the air in work premises, in
concentrations ranging from 0.04 to 0.4 mg/m3 (Yunisova &
Pavlovskaja, 1975). In one electroplating factory, the
concentration of chromic acid vapours in the air varied from 0.1 to
1.4 mg/m3 (Gomes, 1972). In the vicinity of 3 different baths in a
Swedish chromium plating factory, chromium concentrations ranged
from 20 to 46 µg chromium (VI)/m3, while, at another factory, the
exposure levels near all baths were below 1 µg/m3 (Lindberg et al.,
1985).
Occupational exposure to chromium during welding has been
analysed and the results published by several authors (Stern,
1981). Welding of metals using chromium and nickel electrodes
require high temperatures that melt both the material welded and
the electrode, producing a complex mixture of gases, oxides, and
other compounds, the chemistry of which is determined by the
technology, materials, and welding parameters used in each case
(Lautner et al., 1978). Hexavalent chromium compounds were found in
the respiratory zone of the welder at concentrations ranging from
3.8 to 6.6 µg/m3 (Migai, 1975). For the welding industry as a
whole, the average exposure arising from welding is not homogeneous
but depends on the type and conditions of the welding process
(Stern, 1982).
In a cement-producing factory, the concentration of hexavalent
chromium in the air in the work-place varied from 0.0047 to 0.008
mg/m3. The presence of chromium was explained by the fact that the
lining of the kilns was composed of chrome-magnesium bricks
containing 17 - 28% chromium compounds (Retnev, 1960). Forty-two
types of American cement were analysed for total chromium content
and particularly for hexavalent chromium. It was found that
hexavalent chromium was present in 18 out of 42 samples in
concentrations varying from 0.1 to 5.4 g/kg cement, while the total
chromium content ranged from 5 to 124 g/kg (Perone et al., 1974).
Analysis of 59 samples of Portland cement from 9 European countries
showed that the contents of hexavalent chromium extractable with
sodium sulfate varied from 1 to 83 g/kg of cement, while the total
chromium contents ranged from 35 to 173 g/kg (Fregert & Gruvberger,
1972).
4.3.2. Dermal exposure
Occupational dermal exposure can result in percutaneous
absorption and in harmful effects on the skin (section 8.3.1),
though the percutaneous absorption of chromium (III) sulfate has
been questioned by Aitio et al. (1984).
Chromium, especially chromate, is the most common contact
allergen and of great importance in occupational contact dermatitis
(Thormann et al., 1979).
Chromium eczema occurred most frequently in building labourers
followed by painters, galvanizers, machine drillers, metal-workers,
graphic artists, and workers in the timber, chemical, leather, and
textile industries (Polak et al., 1973). This is likely to reflect
the exposure to chromium compounds from a large number of every-day
products (section 4.2.2). The skin exposure to cement may be of
particular importance as building labourers belong to the most
affected group.
5. KINETICS AND METABOLISM
5.1. Absorption
5.1.1. Absorption through inhalation
5.1.1.1. Animal studies
Few animal studies have been performed to determine the
absorption of chromium compounds via inhalation. In one early
study, mice and rats were exposed to chromium particulates in an
inhalation chamber for various periods of time. The concentrations
of soluble chromium (CrO3) in air were between 1 and 2 mg/m3 for
the mice and 2 and 3 mg/m3 for the rats. The concentrations of
soluble versus insoluble chromium in the lung tissue of the mice
varied greatly. The soluble chromium concentrations ranged from
4.3 to 10.7 µg/kg dry tissue, after 100 weeks of exposure (Baetjer
et al., 1959a).
The amount of chromium that is absorbed through inhalation
depends on the size of the particles and droplets, on their
solubility in body fluids, and on their reaction with the
respiratory mucosa. Particles greater than 5 µm in diameter
(aerodynamic size) are deposited on the mucosal surface of the
nasal membrane, trachea, and bronchi and are carried by the action
of the cilia to the pharynx, where they are swallowed. Smaller
particles and droplets, especially those below 2 mm in size,
penetrate to the alveoli. Particles and droplets of soluble
compounds, such as hexavalent chromium compounds, are rapidly
absorbed in the blood. Insoluble particles, such as chromite, are
taken up by macrophages and slowly cleared. Soluble materials that
react with the constituents of the lung tissue, such as soluble
trivalent compounds, are also cleared slowly. Baetjer et al.
(1959b) were the first to describe the differences in the clearance
rates of soluble chromates and chromic chloride, when injected
intratracheally into the lungs of animals. The hexavalent chromate
was more rapidly transported from the lungs to other tissues than
the trivalent chromic chloride. Ten minutes after injection, only
15% chromium (IV) remained in the lung compared with 70% chromium
(III). After 60 days, the corresponding figures were 1.7% and 13%
(Baetjer et al., 1959b). Hexavalent chromium is taken up by the
red blood cells in much larger quantities than trivalent chromium.
This finding has been confirmed by Wiegand et al. (1984b)
performing intratracheal instillation (Na251CrO4) studies on
anaesthetized rabbits, as shown in Fig. 2. Confirmation of the
macrophage uptake of insoluble chromate was obtained by exposing
hamsters to 0.5 - 1 mg chromic oxide dust/m3 for 4 h. The median
diameter of the particles was 0.17 µm. Over 90% of the oxide was
found in the macrophages (Sanders et al., 1971).
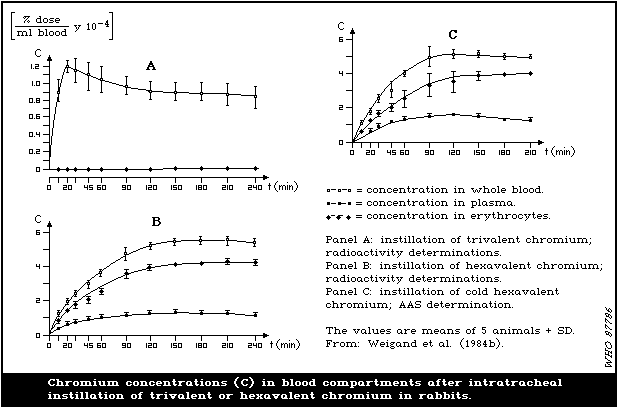 5.1.1.2. Human data
A mean chromium concentration of 0.22 mg/kg wet weight was
found in the lung tissue of subjects from various locations in the
USA, but there was no correlation between chromium levels in the
lungs and those in the air (Schroeder et al., 1962).
A Committee of the National Research Council (US NAS, 1974a)
concluded: "It is unlikely that the intake from air under ordinary
conditions contributes significantly to the total intake of
available chromium; the intake from the air is calculated to be
less than 1 µg/day; but excessive exposure to airborne chromium
does result in some increased intake".
5.1.2. Absorption from the gastrointestinal tract
The absorption of ingested chromium compounds can be estimated
by measuring the amount of chromium excreted in the urine, as
almost all of intravenously injected chromium is excreted via the
urine and only 2% is found in the faeces. Although a potential loss
of endogenous chromium via the skin and its annexa has not yet been
measured and quantified, it can be stated that this organ, as well
as the gastrointestinal tract are of minor importance in the
excretion of endogenous chromium. The gastrointestinal tract is,
of course, the major organ for the excretion of exogenous chromium.
When considering the gastrointestinal absorption of chromium,
it is essential to recognize the substantial differences in the
efficiency of absorption of trivalent and hexavalent compounds.
These differences exist in both man and animals. Many trivalent
chromium compounds are so poorly absorbed that they have been used
as faecal markers in man and animals. The absorption of hexavalent
chromium, administered orally, was higher in all species examined,
but did not exceed 5% of the dose (Donaldson & Barreras, 1966). No
physiological regulation has yet been established for chromium
absorption.
5.1.2.1. Animal studies
The gastrointestinal absorption of chromate in rats has been
reported to be between 3 and 6% of a tracer dose (MacKenzie et al.,
1958; Byerrum, 1961). As in man, trivalent chromium compounds are
less well absorbed in the rat, with reported efficiencies ranging
from less than 0.5% (Visek et al., 1953) to 3% (Mertz et al.,
1965a). Within the category of trivalent compounds, there are
moderate differences in absorption, depending on the chemical form.
Binding of the chromium ion to suitable ligands, such as certain
organic acids, stabilizes the metal against precipitation in the
alkaline milieu of the intestines and increases absorption
efficiency by a factor of 3 - 5 times, compared with that for
chromium chloride. This has been shown for certain chelating
agents (Chen et al., 1973), a yet unidentified small peptide
complex isolated from yeast (Votava et al., 1973), and synthetic
glucose tolerance factor (GTF), a dinicotinic-acid-glutathione-
chromium complex (Mertz et al., 1974). Nothing is known about the
interaction of chromium with the flora of the gastrointestinal
tract. Absorption of chromium chloride by ruminant species is
similar to that in rats, with a mean efficiency of 0.76% (Anke et
al., 1971); laying hens have been found to absorb almost 15% of a
tracer dose of the element (Hennig et al., 1971).
5.1.2.2. Human studies
Donaldson & Barreras (1966) studied the gastrointestinal
absorption of hexavalent chromium by administering trace doses of
Na251CrO4 orally to 6 volunteer patients, who were hospitalized,
and by measuring the amount of radioactivity in the faeces and
urine. The mean urinary excretion, expressing the absorption
efficiency, was 2.1 ± 1.5% of the dose given. Administration by
jejunal infusion in 4 volunteers increased these values, suggesting
reduction of the chromate to trivalent compounds by the acid
content of the gastric juice. The same authors reported a mean
absorption efficiency of only 0.5 ± 0.3% for trivalent chromium,
administered as CrCl3 x 6H2O, with a range of 0.1 - 1.2%.
On the basis of the chromium content in diets (60 µg) and
chromium excretion (0.22 µg) in healthy subjects, Anderson et al.
(1983) calculated a minimum chromium absorption of about 0.4%.
Increasing intake by supplementation with chromium (chromic
chloride tablets, furnishing 200 µg chromium/day) led to an
excretion of 0.99 µg, equivalent to 0.4% of the intake.
Aitio et al. (1984) investigated the intake and urinary
excretion of chromium (III) in leather tanning workers. The
environmental concentrations were recorded as low, but chromium was
present in air in the form of large droplets that were not
collected by the standard air measurement technique. It was assumed
that the large droplets were cleared by the upper respiratory tract
and swallowed, and that the chromium in the droplets was absorbed
from the gastrointestinal tract. A calculation showed that this
would explain the urinary excretion levels. No absorption of
chromium through the skin was detected.
In a recent study, the minimum chromium absorption calculated
on the basis of urinary-chromium excretion was about 0.4%.
Increasing intake 5-fold, by chromium supplementation, led to a
nearly 5-fold increase in chromium excretion, suggesting that the
extent of absorption of supplemental inorganic chromium was similar
to that from normal dietary sources (Anderson et al., 1983a).
A similar absorption for trivalent chromium of 0.69% was
reported by Doisy et al. (1968) in healthy human subjects,
regardless of age. However, a group of 14 insulin-requiring
diabetic patients absorbed 4 times as much of the chromium dose as
the non-diabetic or maturity-onset diabetic subjects, as shown by
elevated levels of 51chromium in blood plasma and urine (Doisy et
al., 1971).
5.2. Distribution, Retention, Excretion
5.2.1. Animal studies
Most animal studies on chromium metabolism have been performed
on rats. From the site of intestinal absorption, chromium is taken
up by plasma-protein fractions. Small, physiological doses of
51chromium have been shown to bind almost entirely to the iron-
binding protein, transferrin (Hopkins & Schwarz, 1964). On the
other hand, inhaled chromium (Glaser et al., 1984) was bound to
albumin rather than to transferrin. With larger quantities of
trivalent chromium, non-specific binding to other proteins also
occurred, but not to the red blood cells. Visek et al. (1953)
measured the effects of the different chemical forms of chromium on
tissue distribution and found that soluble, chelated forms, such as
acetate or citrate complexes, were cleared quite rapidly, in
contrast with colloidal or protein-binding forms (chromite, chromic
chloride), which have a great affinity for the reticulo-endothelial
system (bone marrow, liver, spleen), and clear more slowly. The
blood clearance of hexavalent chromium, such as chromate, was slow,
because of irreversible binding within the red blood cells. Tissue
distribution of 51chromium, administered in nanogram doses to rats
was studied by Hopkins (1965). As in the preceeding studies, the
element accumulated in bone, spleen, testes, and epididymis; much
less was retained in the lungs, brain, heart, and pancreas. This
obvious difference in chromium distribution between man and rats is
unexplained.
As in man, trivalent 51chromium in the rat was rapidly cleared
from the blood, after absorption, and was retained by the tissues
(Mertz et al., 1965a). These tissue stores, labelled with
51chromium chloride, administered orally or intravenously, were not
immediately available for specific physiological functions. For
example, 51chromium, administered as CrCl3 x 6H2O to pregnant rats,
was not transported into the embryos (Mertz et al., 1969), nor did
any 51chromium appear in the blood in response to glucose or
insulin injections (Mertz & Roginski, 1971). The fact that fetal
chromium concentrations are low, when the pregnant rats are fed a
low-chromium Torula yeast diet, and increase when a high-chromium
natural stock ration is fed, indicates that placental transport and
possibly, the acute chromium response depend on a special form of
chromium, which is different from chromium chloride. It is
possible, but has not yet been proved, that this form is the
dinicotinic acid-glutathione-chromium complex, known as glucose
tolerance factor. Yeast extracts containing this factor labelled
with 51chromium have been shown to cross the placenta (Mertz et
al., 1969) and, in preliminary studies, to furnish chromium for the
acute chromium response (Mertz & Roginski, 1971).
With reference to interactions between chromium and other trace
elements, competition with iron by way of their common carrier
(transferrin) has been suggested in rats (Hopkins & Schwarz, 1964)
and in human beings (Sargent et al., 1979). Goncharov (1968)
reported a close interaction between chromium and dietary iodine.
In iodine-deficient white rats, addition of chromium to the diets
in amounts supplying from 0.6 to 600 µg/animal per day stimulated
thyroid function, as indicated by morphological and functional
changes. Conversely, chromium, in all but the lowest dose,
decreased thyroid function in animals receiving adequate iodine
levels. This relation is in agreement with epidemiological data
from the USSR (Goncharov, 1968).
5.2.2. Human data
5.2.2.1. Concentration in tissues, blood, urine, and hair
including possible biological indicators of exposure
(a) Tissues
The most comprehensive survey of tissue-chromium concentrations
is that of Schroeder et al. (1962), who carried out a
spectrographic analyses on 20 - 39 samples for each autopsy tissue,
all of which had been carefully collected to avoid extraneous
contamination. The following results were obtained (mean values in
mg/kg ash) for a group of subjects who had died between the ages of
30 and 40 years: lung, 15.6; aorta, 9.1; pancreas, 6.5; heart, 3.8;
testes, 3.1; kidney, 2.1; liver, 1.8; spleen, 1.7. In all tissues,
except for the lungs there was a rapid decline in chromium
concentrations from time of birth to the age of 10 years, followed
by a more gradual decrease to the age of 80 years. It cannot be
stated with certainty whether the decline is an expression of a
physiological mechanism or of a dietary deficiency. The lungs lost
their initially high chromium levels (85.2 mg/kg ash) up to the age
of 20 years (6.8 mg/kg); subsequently, the concentrations increased
to between 20 and 38 mg/kg. This discrepancy demonstrates that the
chromium in lungs is not in equilibrium with the general pool. The
decline of chromium in the aorta, quite pronounced in subjects in
the USA, was much less dramatic in the aortas from subjects of
other countries (Schroeder et al., 1970).
Mancuso & Hueper (1951), as well as Baetjer et al. (1959b),
found concentrations of chromium in the lungs of former chromate
workers that were several orders of magnitude higher than those in
control subjects. In a study on 16 chromate workers, including 11
with lung cancer, Baetjer et al. (1959b), using a colorimetric
method, observed a median concentration of water- and acid-soluble
chromium in the lung of 70 mg/kg dry weight and a median
concentration of acid-insoluble chromium of 17 mg/kg. The chromium
concentration did not differ between cancer cases and non-cancer
cases. The tissue specimens were obtained 0 - 23 years after the
termination of occupational exposure, which had lasted 1.5 - 42
years. With the use of emission spectrometry and atomic absorption
spectrophotometry, Hyodo et al. (1980) found a chromium content of
3.6 mg/kg wet weight in the lung in a male smoker with lung cancer,
who died 10 years after employment for 30 years in a chromate-
producing plant. The concentration in other tissues ranged from
0.05 (bone marrow) to 1.5 mg/kg (suprarenal gland). In five
unexposed controls, lung concentrations ranged from 0.09 to 0.88
mg/kg; concentrations in other tissues ranged from 0.003 (kidney)
to 0.156 mg/kg (suprarenal gland). The ratio of hexavalent to
total chromium in the lungs was 29% in the worker and 22.7 ± 10.6%
(mean ± SD) in the controls.
Brune et al. (1980) gave tissue concentrations for chromium and
other metals in the lung, liver, and kidney of 20 deceased copper
smelter workers, who had retired 0 - 19 years prior to death, and
of a control group (8 subjects). Tissue analysis was carried out
using neutron activation analysis as well as atomic absorption
spectrophotometry, and included a comparison with certified
reference samples (National Bureau Standards, bovine liver). In
the controls, the concentrations ranged from below detection (0.003
mg/kg wet weight) to 0.07 mg/kg in kidneys and 0.11 mg/kg in liver.
There was no marked difference between the chromium contents of
these 2 tissues and those in the exposed workers. On the other
hand, the lung concentrations were between 3 and 4 times higher in
the workers than in the controls (median levels, 0.29 and 0.08
mg/kg, respectively).
Chromium determinations were carried out on lung and kidney
samples of 45 autopsies from the Northern Bavaria area (Federal
Republic of Germany) (Zober et al., 1984). The analyses were
carried out using electrothermal AAS after wet oxidative digestion.
Median values of 0.097 mg/kg wet weight (range, 0.0006 - 1.230
mg/kg) in lung tissue and 0.0096 mg/kg wet weight (range, 0.0002 -
0.690 mg/kg) in kidney were found.
Very limited data are available on chromium levels in tissues
other than those referred to above. Shmitova (1978) estimated
chromium levels in fetal and placental tissues (abortive material),
derived on the 12th week of pregnancy, and found 92.8 and 30 µg
Cr/kg tissue, respectively.
It can be concluded that chromium may be retained in the lungs,
several years after the termination of occupational exposure.
However, it is not known whether this observation has any
biological relevance to the appearance of lung cancer. In this
context, it is of interest to note that chromium is retained in the
human lung for a relatively long period, even without occupational
exposure.
(b) Blood
The values reported by various investigators for chromium
concentrations in the blood of unexposed human beings range from
0.2 to 70 µg/litre in serum and plasma and 5 to 54 µg/litre in red
blood cells (US EPA, 1978). Insufficient evidence is available to
state whether the blood values in the normal population are
influenced by concentrations in the ambient air. The most
extensive determination of blood-chromium levels in chromate
workers was made by Mancuso (1951). Blood values during exposure
varied from 5 to 170 µg/litre and from 10 to 140 µg/litre, 74 days
after work ended. No decrease in blood-chromium levels was found,
even after 74 days without exposure.
Because of a selective affinity of hexavalent chromium for the
erythrocyte, substantially increased environmental exposure to
chromates is reflected in an increased ratio of the hexavalent
chromium level in red blood cells to that in plasma. Baetjer et
al. (1959a) found the following concentrations in 3 exposed
chromate workers: blood cells, 30, 54, and 140 µg/litre; plasma, 0,
20, and 17 µg/litre, respectively. In the absence of exposure to
chromate, the chromium concentrations in erythrocytes and plasma,
as serum, were nearly identical (Paixao & Yoe, 1959). Chromium
(VI) is incorporated into human red blood cells and remains there
over a long period of time (Wiegand et al., 1985), since the
approximate lifetime of human red cells is about 100 days. Exposure
to 2 mg trivalent chromium/day, for 3 months, resulted in an
increased concentration (0.2 mg/kg) in the red cells of 5 human
males, compared with 0.11 mg/kg in 5 controls without supplement
(Schroeder et al., 1962). However, later studies did not show any
increase in chromium concentrations in red cells, following
exposure to trivalent chromium (Beyersmann et al., 1984; Wiegand et
al., 1985). Monitoring of red blood cell-chromium may be a useful
indicator of exposure to hexavalent, but not to trivalent,
chromium.
Most of the later studies show that the true chromium
concentration in the plasma or serum of healthy subjects is of the
order of 1 µg/litre or less (Guthrie et al., 1978; Versieck et al.,
1978). Seeling et al. (1979) determined chromium in serum and
plasma using flameless AAS. This study was supported by measuring
a standard reference material (National Bureau of Standards, 1569
brewer's yeast, reference data: 2.12 ± 0.05 mg/kg, measured data:
2.3 ± 0.2 mg/kg). The chromium levels in serum ranged from 0.7 to
2.2 µg/litre and in plasma from 1 to 1.5 µg/litre (central
parameters of a long normal distribution).
Pooled serum samples of 6 healthy Finnish volunteers were
studied by Kumpulainen et al. (1983). A mean value of 0.11 ± 0.05
µg chromium/litre (range, 0.06 - 0.20) was found. Nomiyama et al.
(1980b) analysed 20 blood samples of Japanese subjects, using
direct flameless ASS, and reported a value of 2.9 ± 1.7 µg
chromium/litre whole blood.
Zober et al. (1984) analysed the blood of 45 autopsied subjects
from the Northern Bavaria area (Federal Republic of Germany). A
median chromium concentration of 2.8 µg/litre of postmortem blood
(range, 0.20 - 24 µg/litre) was found, the value was not influenced
by age or sex.
A reference material for chromium, bovine serum (RM 8419) is
available from the National Bureau of Standards, Washington DC,
USA.
(c) Urine
Chromium concentrations in the urine of non-occupationally
exposed subjects have been reported to range from 1.8 to 11
µg/litre (Imbus et al., 1963). Except for exposed persons and
juvenile diabetic patients, the reported values for daily chromium
excretion in urine (Table 9) do not differ as much as those for
blood. In later studies, such as that of Guthrie et al. (1979), a
method of flameless atomic absorption was used in which it was
possible to correct for spectral interference (Zander et al.,
1977). Such interference was difficult to eliminate with earlier
methods (Guthrie et al., 1978). Urine samples from 189 Japanese
volunteers, aged 10 - 80 years, in 4 pollution-free areas, were
analysed for chromium by Nomiyama et al. (1980a), using direct
flameless AAS. The average level was 0.4 ± 0.37 µg/litre (X ± SE)
or 0.47± 0.42 mg/kg creatinine. Urinary chromium tended to be
higher in males than in females and to decrease with age, but the
differences were not significant. Using electrothermal AAS, the
urinary excretion of various metals in non-occupationally exposed
adults was measured by Schaller & Zober (1982). For smokers, a
median value of 1.6 µg chromium/litre was found (non-smoker: 1.4 µg
chromium/litre). Commercially available urine samples were used
for quality control (Angerer et al., 1981). Although a "normal"
level of chromium excretion for healthy, unexposed persons cannot
yet be established with certainty, such a level may be less than 1
µg/day.
Several investigators have measured urinary-chromium excretion
in exposed workers. In a study on chromate workers, the urine
values ranged from 5 to 380 µg chromium/litre during exposure and
from 10 to 54 µg chromium/litre, 74 days after the end of exposure
(Mancuso, 1951). No relationship was evident between the urine
levels and the weighted average number of years of exposure. In
every case where urine values were recorded, both during, and 74
days after the end of, exposure, the chromium concentrations
decreased with time away from exposure.
A study of 12 workers in a galvanizing plant in the Federal
Republic of Germany showed an average concentration of chromium in
urine of 9.5 µg/litre (range, 1.4 - 24.6 µg/litre) compared with a
value of 1.8 ± 1.1 µg/litre in 60 unexposed workers (Schaller et
al., 1972). Gylseth et al. (1977) investigated a group of 14
welders exposed to about 0.05 mg chromium/m3 air, and reported a
urinary chromium concentration of approximately 40 mg/litre. At
the same exposure level, Tola et al. (1977) found a concentration
of 30 mg chromium/kg of creatinine in the urine. In both of these
studies, there was a correlation between recent exposure to
airborne chromium and chromium concentrations in urine. In the
study by Tola et al. (1977), it was shown that the water-soluble
fraction of airborne chromium was better correlated with the
concentration excreted in the urine than with total chromium. It
was also shown that the water-soluble fraction consisted mainly of
hexavalent chromium.
Table 9. Daily chromium excretion in urine
---------------------------------------------------------------------------
Subjects Number Excretion Range Reference
(µg/day) (µg/day)
(mean ± SD)
---------------------------------------------------------------------------
Adult males 2 0.72a 0.58 - 0.86 Schroeder et al.
(1962)
Adult males, fed 3 31.0a 20.8 - 46.5 Schroeder et al.
2 mg trivalent, (1962)
chromium/day for
3 months
Normal adults 16 13 ± 6 4 - 24 Voelkl (1971)
Chromate workerb 16 000
Normal adults 60 1.6 ± 1.1 Schaller et al.
(1972)
Galvano-technical 12 9.7 ± 6.6 1.4 - 24.6 Schaller et al.
workers (1972)
Normal young 20 8.4 ± 5.2 Hambidge (1974)
adults
Normal children, 18 5.5 ± 2.9 Hambidge (1974)
8 years old
Insulin-dependent 7 19.2 ± 18.9 Hambidge (1974)
diabetic children,
11 years old
Young women 9 7.2 ± 1.2 5.9 - 10.0 Mitman et al.
(1975)
Adult males 12 0.8 ± 0.4 0.4 - 1.8 Guthrie et al.
(1979)
---------------------------------------------------------------------------
Table 9. (contd.)
---------------------------------------------------------------------------
Subjects Number Excretion Range Reference
(µg/day) (µg/day)
(mean ± SD)
---------------------------------------------------------------------------
Adult males 91 0.48 ± 0.41a 0.42 - 0.53 Nomiyama et al.
(1980b)
Adult females 98 0.34 ± 0.31a 0.22 - 0.43 Nomiyama et al.
(1980b)
Adult males 48 0.20 ± 0.01a 0.05 - 0.58 Anderson et al.
(1982b)
Adult females 28
Adult males 27 0.17 ± 0.10 Anderson et al.
(1983a)
Adult females 15 0.20 ± 0.12 Anderson et al.
(1983a)
Adult males and 299 0.80 ± 0.6a 0.4 - 2.1 Fang (1983)
females
Normal adults 10 0.11 ± 0.05a 0.06 - 0.20 Kumpulainen et al.
(1983)
Normal adultsc 10 4.9 0.10 - 14.2 Zober et al.
(1984)
---------------------------------------------------------------------------
a Data calculated as µg/litre urine.
b Tanner, suffering from an acute ulceric gasteroenterocolitis.
c Samples taken post-mortem from autopsies. Original values are related
to mass (kg) instead of volume (litre).
Lindberg & Vesterberg (1983a) measured airborne, and urinary-
chromium levels among platers. Concentrations of chromium in urine
of < 5 µg/litre occurred when the time-weighted average values of
exposure were about or below 2 µg/m3 air. Severe damage to the
nasal septum and effects on lung function have not been found at
levels lower than this. It was shown that post-shift urinary-
chromium determinations could be used to monitor exposure in this
occupational group.
The urinary-chromium excretion and chromium clearance in 22
welders, who had been exposed to airborne hexavalent chromium (5 -
150 µg/m3) during 2 - 40 years (mean working time, 18.9 years) were
measured by Mutti et al. (1979). The method used was flameless
atomic absorption spectrometry. A highly significant correlation
was detected between the ratio of urinary-chromium to creatinine
and the airborne chromium concentration in the workplace, with
excretions ranging from 5.3 ± 3.7 to 33.3 ± 6.9 mg chromium/kg
creatinine in slightly exposed and heavily exposed welders,
respectively. The authors also reported an increase in chromium
clearance with increasing body burden of chromium, which indicates
that high urinary-chromium excretion may be caused by previous high
exposure as well as by current exposure.
Baseline data for chromium excretion in unexposed subjects in
the report by Mutti et al. (1979) are approximately 10 times higher
than the values recently proposed and generally accepted as normal.
However, there is reason (section 2.2.2) to accept relative
differences in analytical results in studies by one author using
one method, even if the absolute values reported may be questioned.
To test whether chromium excretion is also associated with
exercise-induced increases in glucose utilization, the urinary
chromium excretion, serum glucose, insulin, and glucagon of 9 male
runners (23 - 46 years old) were evaluated by Anderson et al.
(1982a). The mean urinary-chromium concentration was increased
nearly 5-fold, 2 h after running; excretion of sodium, potassium,
and calcium was unchanged. These data demonstrate an increase in
chromium excretion with exercise-induced increase in glucose
utilization.
(d) Milk
The chromium concentration was determined in 261 samples of
breast milk collected by manual expression from 45 American women.
Chromium levels were measured in whole, liquid milk by graphite-
furnace AAS, using the method of standard additions. The mean
chromium content of the breast milk samples was 0.30 µg/litre. The
range of individual values was 0.06 - 1.56 µg/litre and did not
change significantly with duration of lactation (Casey & Hambidge,
1984). Kumpulainen et al. (1983) analysed frozen samples of pooled
breast milk taken from women in different stages of lactation and
obtained from the Milk Bank of the Children's Hospital of Helsinki.
The mean chromium content ± SD was 0.49 ± 0.067 µg/litre (range,
0.37 - 0.57 µg/litre).
(e) Hair
Hair-chromium concentrations in children during the first 6
months of life were significantly higher than at any other age
(Hambidge & Baum, 1972); they declined from an initial value of
1493 µg/kg to an average of 412 µg/kg at 2 - 3 years. Hambidge
(1971) compared the chromium concentration in the hair of 15
newborn babies and that of their mothers: in only one case was the
chromium level in the mother's hair higher than that in the newborn
baby. Chromium levels were significantly lower than those
mentioned above in 50 Turkish women and their newborn babies (203
and 119 µg/kg, respectively) and only 12 newborn babies were found
to have higher concentrations than their mothers, suggesting
suboptimal chromium status (Gürson, 1977). Hair appears to reflect
the nutritional chromium status of groups. The hair-chromium level
is significantly lower in parous women than in nulliparae (Hambidge
& Rodgerson, 1969; Mahalko & Bennion, 1976) and in diabetic
children compared with normal controls (Hambidge et al., 1968). It
is low in adult-onset diabetic adults (Benjanuratra & Bennion,
1975). These findings are in agreement with the expected changes
in chromium balance during pregnancy and in diabetes.
5.2.2.2. Dynamic aspects of metabolism and the influence of
pathological states
Once chromium is absorbed into the organism, it clears rapidly
from the blood stream and is excreted or taken up by the tissues.
In a clinical study, Sargent et al. (1979) detected a 4-
compartment-type clearance from blood, with mean half-times of 13
min, 6.3 h, 1.9 days, and 8.3 days. However, the disappearance
from 3 tissue compartments was much slower, with half-times of
0.56, 12.7, and 192 days. The half-times for blood 3-compartment
clearance in rats (Hopkins, 1965) were calculated to be 0.56, 5.33,
and 57 h (Withey, 1983).
Whether any organ is specifically responsible for the storage
and release of the "metabolically responsive" chromium is not
known. The "metabolically responsive" chromium in blood is defined
as the fraction that increases acutely in response to an elevation
of blood-glucose or blood-insulin levels. It is believed that this
chromium increment interacts with the increased insulin secreted in
response to a glucose load, to facilitate the action of the hormone
on the insulin receptors of the insulin-sensitive cells.
In young, healthy subjects, but not in elderly subjects and
diabetic patients, an oral glucose load or the injection of insulin
results in a sudden increase in serum- or plasma-chromium
(Glinsmann et al., 1966; Levine et al., 1968; Hambidge, 1971; Behne
& Diel, 1972; Liu & Morris, 1978). This increase may appear 30
min, or as late as 120 min, after the challenge; much of the
chromium responsible for the increase is subsequently lost in the
urine. Lack of this increase, also termed "relative chromium
response", is often associated with impaired glucose tolerance,
indicative of chromium deficiency. It should be noted that, when
the glucose load was given intravenously (Pekarek et al., 1975) to
healthy volunteers, the serum-chromium level decreased rapidly,
while the blood-glucose level increased. Supplementation with
chromium chloride or high-chromium yeast extracts for several weeks
resulted in the reappearance of the relative chromium response and
improvement of glucose tolerance (Glinsmann et al., 1966; Liu &
Morris, 1978). These findings support the conclusion that the
"relative chromium response" measured during a glucose or insulin
tolerance test may serve as an indicator of the adequacy of
metabolically responsive chromium.
Much of the chromium increment secreted into the blood stream
in response to glucose or insulin is subsequently lost in the
urine. Hambidge (1971) observed greatly increased urinary-chromium
excretion in 2 diabetic children after insulin therapy had begun,
compared with the excretion in the same children before insulin
treatment. It is not known whether the increased urinary loss of
chromium is compensated for by an increase in absorption efficiency
from the intestines. Hambidge et al. (1968) reported significantly
lower chromium concentrations in the hair of diabetic children
compared with normal children, and Morgan (1972) found that
the chromium contents in the livers of 31 diabetic adults at
autopsy were lower than those in 24 control livers from non-
diabetic persons (8.57 versus 12.7 mg/kg ash; P = 0.05).
On the other hand, Doisy et al. (1971) demonstrated greatly
increased intestinal absorption, together with elevated chromium
excretion, in 14 insulin-dependent diabetic patients administered
51CrCl3 x 6H2O, orally. It is not known whether the greater
absorption efficiency is adequate to compensate for the increased
urinary losses; the decreased chromium concentrations in hair and
liver, discussed above, suggest that a negative balance may prevail.
Several other pathological conditions affect chromium
metabolism. Sargent et al. (1979) detected significantly less
retention of intravenously administered 51chromium in 11 patients
with haemochromatosis compared with 5 normal controls. This may be
related to the high saturation with iron of transferrin, which is
also the carrier protein for newly absorbed chromium.
Chronic ischaemic heart disease also affects chromium
metabolism. Neiko & Del'va (1978) observed a significantly
increased urinary-chromium loss, greater by a factor of 1.5 - 1.6
than that of normal controls, in 65 heart patients. The urinary-,
and to a lesser extent, the faecal-chromium loss increased
progressively with increasing severity of signs and symptoms and
resulted in a negative chromium balance in the post-infarct state.
The balance became positive again on discharge from the hospital
after medical treatment.
Acute infections also appear to influence chromium metabolism.
Pekarek et al. (1975) measured glucose tolerance, and insulin and
chromium levels in human volunteers, before and after infection
with the benign sandfly fever virus. Impaired glucose tolerance and
a significantly increased insulin response to a glucose load,
observed at the height of the infection, were accompanied by very
significantly depressed serum-chromium levels (0.5 µg/litre,
compared with 1.4 µg/litre before infection; P < 0.05) In
contrast with the sharp decline in serum-chromium following the
intravenous injection of glucose in the healthy state observed by
these authors, the depressed serum-chromium levels declined very
little during the glucose tolerance test at the height of
infection. The mechanism of the changes in chromium metabolism in
heart disease and sandfly fever is not clear. Of great potential
importance is the unanswered question of whether the observations
described here reflect an increased chromium requirement in the
patients or a normal reaction to various forms of stress.
The information on the dynamic aspects of chromium metabolism
in animals is limited and should be considered in connexion with
the more detailed studies on human subjects. Diabetes, induced in
rats by the injection of streptozotocin, affected the tissue
distribution of injected 51CrCl3. Sixteen days after injection of
streptozotocin, 51CrCl3 was injected into 5 diabetic rats and 6
normal controls and the 51Cr content measured 5 days later. The
serum of the diabetic rats contained more than 3 times the 51Cr
activity found in the controls (0.24 versus 0.07% of the injected
dose P < 0.01). Significant differences were also detected in the
distribution of 51Cr in the subcellular fractions of the liver; in
the diabetic tissue, 51Cr activity was higher in the nuclear and
supernatant fractions ( P < 0.01) and lower in mitochondria and
microsomes ( P < 0.05). The mechanism responsible for these
changes is not known (Mathur & Doisy, 1972).
5.3. Influence of Chemical Form
The diverse biological effects of chromium on living organisms
cannot be understood without knowledge of the chemical and physical
forms in which the element is present. As stated earlier, the
metallic state (zero valence) is biologically inert, the trivalent
state represents the essential element chromium, and the hexavalent
state is of concern to the toxicologist. Compounds of trivalent
chromium are poorly absorbed, whereas those of the hexavalent state
easily penetrate physiological barriers, such as cell membranes.
Hexavalent chromium compounds are easily reduced by living matter,
but oxidation of trivalent to hexavalent chromium does not occur in
the organism.
The physical form of hexavalent compounds (such as particle
size) and chemical properties (such as solubility) determine
metabolic pathways after inhalation and, therefore, health effects.
There is an equally strong influence of the chemical form of
trivalent chromium on metabolism and health effects. When chromium
is bound to water or small anions (e.g., CrCl3 x 6H20), it
precipitates in the neutral or alkaline milieu of the body fluids.
When it is bound to ligands, such as organic acids, the element is
light in solution and is available for intestinal absorption. The
forms in which trivalent chromium occurs in nature are not really
known. Plants probably contain chromium complexes with organic
acids.
The biological availability of chromium compounds in foods is
of great nutritional importance, but is poorly defined. One
compound or a group of closely related compounds, glucose tolerance
factor, has been isolated from yeast and shown to be more active
than chromium chloride in genetically diabetic mice and in the in
vitro potentiation of the action of insulin on rat epididymal fat
tissue (section 7.1.5.2). It has been identified as a dinicotinic-
acid glutathione complex, but the exact stereochemical structure is
not yet known (Toepfer et al., 1977). It has been postulated, but
not proved, that this factor is the active form of chromium within
the organism.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The environmental effects of chromium as a pollutant have been
reviewed by US EPA (1978), Anderson (1982), and EIFAC (1983).
Various effects have been reported, but, because of the presence of
other chemicals, it remains doubtful whether chromium alone is
responsible for the effects observed. Data are available on
microorganisms, plants, and aquatic organisms.
6.1. Microorganisms
Most microorganisms (protozoa, protophyta, fungi, algae,
bacteria) are able to absorb chromium. The active uptake of
chromate by the sulfate transport system has been shown in
Neurospora crassa (Roberts & Marzluf, 1971). No distinction has
been made between ab- and adsorption in other studies (e.g., algae)
(Calow & Fletcher, 1972), and it has not yet been shown that
chromium is an essential element for microorganisms. In general,
toxicity for most microorganisms occurs in the range of 0.05 - 5 mg
chromium/kg of medium. The internal concentration of chromium
depends on the species. In most groups of microorganisms, it
ranges between the levels of 0.6 mg dry weight present in one litre
of sample of microplankton from Monterey Bay, California, USA, and
21.4 mg/litre phytoplankton collected in the Pacific Ocean (Martin
& Knauer, 1973).
Trivalent chromium is less toxic than hexavalent. The main
features are inhibition of growth (at concentrations greater than
0.5 mg/litre in Chlorella cultures) (Nollendorf et al., 1972) and
inhibition of various metabolic processes, such as photosynthesis
or protein synthesis (US EPA, 1978).
The toxicity of chromium for soil bacterial isolates was
studied by measuring the turbidity of liquid cultures supplemented
with hexavalent chromium and trivalent chromium. Gram-negative
bacteria were more affected by hexavalent chromium (1 - 12 mg/kg)
than gram-positive bacteria. Toxicity due to trivalent chromium
was not observed at similar levels. The toxicity of low levels of
hexavalent chromium (1 mg/kg) indicates that soil microbial
transformations, such as nitrification, may be affected (Ross et
al., 1981).
6.2. Plants
Although chromium is present in all plants, it has not been
proved to be an essential element for plants. Most substances,
including chromium, can be absorbed through either the root or the
leaf surface. Several factors affect the availability of chromium
for the plant (Black, 1968), including the pH of the soil,
interactions with other minerals or organic chelating compounds,
and carbon dioxide and oxygen concentrations.
Little chromium is translocated from the site of absorption;
however, the chelated form is transported throughout the plant
(Verfaillie, 1974).
Chromium in high concentrations can be toxic for plants, but
Yopp et al. (1974) stated that there was no specific pattern of
chromium intoxication.
During the smelting of chromite, considerable quantities of
waste are produced, which contain soluble chromates. When combined
with a high pH, Gemmell (1973) showed an inhibition of germination
and growth in white mustard plants (Sinapis alba) growing on waste
heaps. Covering the waste with a 25-to 30-cm layer of granular-
free-draining subsoil followed with layers of soil, peat, or sewage
sludge was shown to be the best revegetation technique (Gemmell,
1974).
The main feature of chromium intoxication is chlorosis, which
is similar to iron deficiency (Hewitt, 1953).
Soybeans, treated in nutrient culture containing 0 - 5 mg
hexavalent chromium/litre showed decreased uptake of calcium,
potassium, phosphorus, iron, and manganese (Turner & Rust, 1971).
Death of plants occurred within 3 days of treatment with 30 or 60
mg chromium/litre.
A reduction in leaf dry weight occurred after treatment with
0.01 mg hexavalent chromium/litre (Rediske et al., 1955). Chromium
affects the carbohydrate metabolism, and the leaf chlorophyll
concentration decreased with increasing hexavalent chromium
concentration (0.01 - 1 mg hexavalent chromium/litre) (Rediske,
1956). Hexavalent chromium appears to be more toxic than trivalent
chromium (Hewitt, 1953; Stanley, 1974; Verfaillie, 1974). At
present, no data are available concerning the mechanism of action
or the dose-dependent pattern of chromium intoxication.
6.3. Aquatic Organisms
More studies have been performed with aquatic species than with
free-living (non-parasitic) animals. Depending on the species,
chromium can be less toxic for fish in warm water, but marked
decreases in toxicity are found with increasing pH or water
hardness; changes in salinity have little if any effect on its
toxicity. Chromium can make fish more susceptible to infection;
high concentrations can damage and/or accumulate in various fish
tissues and in invertebrates such as snails and worms.
Reproduction of Daphnia was affected by exposure to 0.01 mg
hexavalent chromium/litre (EIFAC, 1983). Numerous other factors
influence the availability of chromium and, therefore, its
toxicity. These include the presence of other minerals and organic
pollutants, and the temperature of the environment; this has been
shown in mice (Nomiyama et al., 1980a).
Hexavalent chromium is accumulated by aquatic species by
passive diffusion (US EPA, 1978). Ecological factors, in the
abiotic and living environment, are involved in this process, which
varies according to the sensitivity of different species. The
physiological state and activity of the fish also affect
accumulation (Reichenbach-Klinke, 1977, 1980). Kittelberger (1973)
analysed the organs and tissues of the roach (Leuciscus rutilus)
from the river Rhine and found that concentrations of chromium in
the spleen, bronchi, and intestine (between 30 and 37.5 mg/kg) were
10 - 30 times higher than those in the heart, skin, muscle, and
scales.
LC50s are listed in Table 10 for hexavalent and trivalent
chromium compounds in the aquatic environment.
Table 10. The toxicity of chromium for fresh-water organisms
(expressed as 50% mortality)a
-----------------------------------------------------------------------
Compound Category Exposure Toxicity range Most
(mg/litre) sensitive
species
-----------------------------------------------------------------------
hexavalent invertebrate acute 0.067 - 59.9 scud
chromium long-termb - -
vertebrate acute 17.6 - 249 fathead minnow
long-term 0.265 - 2.0 rainbow trout
trivalent invertebrate acute 2.0 - 64.0 cladoceran
chromium long-term 0.066 cladoceran
vertebrate acute 33.0 - 71.9 guppy
long-term 1.0 fathead minnow
-----------------------------------------------------------------------
a From: US EPA (1980).
b No data available.
In general, invertebrate species, such as polychaete worms,
insects, and crustaceans are more sensitive to the toxic effects of
chromium than vertebrates, such as some fish (Mathis & Cummings,
1973). The lethal chromium level for several aquatic and
nonaquatic invertebrates has been reported to be 0.05 mg/litre (US
NAS/NAE, 1972).
EIFAC (1983) reviewed the literature on the occurrence and
effects of chromium in fresh water and proposed tentative water-
quality criteria that distinguish between salmonid and non-salmonid
waters. To protect salmonid waters, the mean aqueous concentration
of "soluble" chromium should not exceed 0.025 mg chromium/litre,
and the 95 percentile should not exceed 0.1 mg chromium/litre.
However, more stringent values may be necessary in very soft, acid
waters, and less stringent values in alkaline waters.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Nutritional Effects of Chromium
The criteria for an essential nutrient have been defined in
different ways by different authors, but all definitions postulate
that a reduction in the total daily intake of the nutrient below a
certain level must consistently induce signs of deficiency, and
that the supplementation of the daily intake above this level must
prevent and cure the deficiency signs. Chromium deficiency has
been produced experimentally in mice, rats, and squirrel monkeys,
and the full reversal of the deficiency signs by the oral
administration of chromium has been demonstrated in rats (Mertz,
1969). For these reasons, chromium must be considered an essential
micro-nutrient. It is physiologically active in the trivalent
oxidation state at concentrations of approximately 100 µg/kg diet.
7.1.1. Effects of deficiency on glucose metabolism
Semipurified rations, containing Torula yeast as the source of
protein, were fed to groups of 10 male Sprague Dawley rats at
weaning, and intravenous glucose tolerance tests were performed by
injecting 1250 mg glucose/kg body weight and measuring the
subsequent decline in blood-glucose levels. The rate of glucose
disappearance can be calculated from the straight line plot of log
increment glucose (excess of glucose at any time (t) over fasting
glucose), versus time. The disappearance constant k is expressed
as % decline of the increment glucose per min; it is a measure of
the efficiency of glucose utilization. In weaning rats, the rate
constant was found to decline, within 3 weeks or less, from an
average of 4%/min to 2.6%/min in animals fed the Torula yeast diet,
but not in animals administered a diet in which 4 or 8% of Torula
yeast was replaced by an equal amount of brewer's yeast. This
observation suggested that brewer's yeast, but not Torula yeast,
contained an unknown substance necessary for the maintenance of
normal glucose tolerance. Because the only known effect of the
substance was that on glucose tolerance, it was named "Glucose
Tolerance Factor" (GTF) (Mertz & Schwarz, 1959). GTF was extracted
from brewer's yeast and pork kidney powder, concentrated and
purified, and its active ingredient was identified as trivalent
chromium (Schwarz & Mertz, 1959). Chromium in the form of most
common complexes (except for very stable ones) cured the impairment
of glucose tolerance in deficient rats, either as one oral dose of
200 µg/kg body weight, or as an intravenous (iv) injection of 2.5
µg/kg body weight. Chromium in the diet also prevented the
impairment of glucose tolerance.
In order to produce more pronounced deficiencies of chromium
and other trace elements, Schroeder et al. (1963) constructed a
special animal house on a mountain top in Vermont, USA, far removed
from traffic and industry. The interior was specially coated with
organic resins to reduce any metallic exposure. Air was introduced
through special filters, and strict practices were enforced to
avoid the introduction of dust and dirt. This will be referred to
as the "controlled environment". Under these conditions, a more
severe chromium deficiency resulted in very low glucose removal
rates of 1.12%/min in 6 rats and of 0.18%/min in 4 female breeder
rats (482 days old), after 11 days on a chromium-deficient diet.
The removal rate in 4 control rats receiving the same diet with a
chromium supplement improved from 0.51 to 1.39% during the same
period (Mertz et al., 1965b). This strong impairment of glucose
tolerance in rats kept in the "controlled environment" was
reflected in their fasting blood-glucose levels, compared with
those of chromium-supplemented controls: 1370 ± 68 versus 1170 ± 17
mg/litre in males and 1390 ± 68 versus 960 ± 65 mg/litre in
females. More than half of 185 deficient rats excreted more than
0.25% glucose in the urine, whereas glycosuria was found in only 9
of the chromium-supplemented controls (Schroeder, 1966). A
significant reduction in the intravenous glucose removal rate was
also observed in 8 rats in plastic cages, fed an EDTA-washed low-
protein diet (7% casein), compared with 8 controls receiving 50 µg
chromium as the chloride, by stomach tube, daily for 2 weeks (2.1
versus 3.6%/min; P < 0.01). However, chromium supplements did not
improve the near-normal removal rates in rats receiving a 20%
casein diet (Mickail et al., 1976). Significantly lower plasma-
glucose levels, due to supplementation with chromium, were reported
in rats fed the chromium-deficient Torula yeast diet (Whanger &
Weswig, 1975). In another series of studies, only a slight
reduction in blood-glucose (from 1240 to 1180 µg/litre) was found
in 10 rats receiving a diet supplemented with chromium (10 µg/kg)
(Djahanshiri, 1976).
Impaired glucose tolerance in squirrel monkeys, fed a
commercial laboratory chow, was shown to respond to chromium
supplementation. Of 9 monkeys with an impaired glucose removal
rate (1.38%/min), 8 responded after 22 weeks of supplementation of
their drinking-water (chromium acetate, 10 mg/litre) with a
normalization of their glucose tolerance (average removal rate, N =
9, 2.33 ± 0.3%/min) (Davidson & Blackwell, 1968). There was no
effect on food consumption, growth rate, or serum-insulin
concentrations. The chromium content of the commercial chow was
stated to be 3.3 mg/kg, a very high concentration. In view of the
uncertainties of methods of analysis for chromium (section 2.2), it
is not possible to interpret the results as indicative of a very
high chromium requirement of the squirrel monkey or of an unusually
poor bioavailability of the chromium in that particular ration. A
marginal chromium deficiency may have existed in mice fed a bread
and milk diet, as daily administration of 10 µg chromium for 16
days to 8 months produced a 10 - 30% decline in blood-glucose
levels (Vakhrusheva, 1960). However, this effect was not specific
for chromium, as it was also observed when manganese was
administered.
The glucose tolerance of guinea-pigs did not differ
significantly between groups fed diets containing chromium at
0.125, 0.625, or 50 mg/kg, even though the animals fed the 2 higher
levels exhibited a lower mortality rate during pregnancy (Preston
et al., 1976).
Although the administration of synthetic glucose tolerance
factor (a chromium-dinicotinic acid-glutathione complex) to 6 pigs
did not affect glucose tolerance tests, it resulted in a
significant increase in the hypoglycaemic effect of insulin
injected at 0.1 U/kg body weight (Steele et al., 1977a).
Turkey poults, fed a practical ration containing chromium
levels of 5 mg/kg, responded to chromium supplementation (20 mg/kg
diet) with a significant increase in liver glycogen and in glycogen
formation, following a fast, and with a significant increase in
glycogen synthetase (EC 2.4.1.11) activity in the liver (Rosebrough
& Steele, 1981).
It can be concluded that the impairment of glucose tolerance in
rats fed a low-chromium Torula yeast diet is due to chromium
deficiency. The effects of chromium in squirrel monkeys, pigs, and
turkeys, though statistically significant, are somewhat difficult
to interpret, because of the reported high chromium content of the
basal diet. No evidence for chromium deficiency has yet been
obtained through glucose tolerance tests on other animal species.
7.1.2. Effects of deficiency on lipid metabolism
Though trivalent chromium in high doses (2.5 mg/kg body weight)
has been shown to increase the synthesis of fatty acids and
cholesterol in the liver (Curran, 1954), lower, physiological doses
appear to decrease serum-cholesterol concentrations in rats.
Schroeder & Balassa (1965) found an average level of 927 mg
cholesterol/litre serum in 24- to 26-month-old male rats, kept in a
controlled environment and administered chromium in the drinking-
water at a concentration of 5 mg/litre, compared with an average of
1229 mg/litre in controls not receiving chromium ( P < 0.01). The
effects in female rats were ambigous, one study producing the
expected reduction in cholesterol due to chromium, another showing
an elevation in the supplemented female rats. Schroeder's
observations of a cholesterol-reducing effect of chromium in male
rats were confirmed by Staub et al. (1969) and by Whanger & Weswig
(1975) but were contradicted by results of a third study in which
there were not any significant effects of chromium on sucrose-
induced triglyceridaemia and cholesterolaemia (Bruckdorfer et al.,
1971). Perhaps more significant than the effect on circulating
cholesterol is the direct effect of chromium on the occurrence of
aortic plaques. Schroeder & Balassa (1965) observed 6 plaques in 54
male and female chromium-deficient rats, but only one plaque in 48
animals receiving 5 mg chromium/litre in drinking-water. These
results are in agreement with those from a subsequent report of the
protective effect of the natural chromium content of water (60 -
215 µg/litre) against atherosclerosis in cholesterol-fed rabbits
(Novakova et al., 1974). Abraham et al. (1980) extended these
observations by demonstrating that daily chromium injections (20 µg
K2CrO4) reversed the established atherosclerosis in the aorta of 11
cholesterol-fed rabbits, compared with 12 controls. The mean
plaque area was reduced from 95% to 63%, the total aortic
cholesterol from 729 mg to 458 mg, and the atheromatous lesions, as
measured by technetium incorporation from 285 000 cpm to 114 000
cpm, all differences being statistically significant. These
results are reinforced by observations in man discussed in section
8.1.
7.1.3. Effects of deficiency on life span, growth, and
reproduction
The mortality of male, but not of female mice, raised in a
"controlled environment" (section 7.1.1) was reduced by trivalent
chromium administered as acetate in the drinking-water at a
concentration of 5 mg/litre (Schroeder et al., 1964). The survival
rates at 12 months were 92.6% and 68.8% ( P < 0.0001) in
supplemented and deficient animals, respectively. Similary, male,
but not female, rats receiving a chromium concentration of 5
mg/litre in the drinking-water had longer life spans than deficient
controls. The mean age of the last surviving 10% of animals was
1249 days, compared with 1141 days in the deficient animals ( P <
0.01). Survival of male rats fed a low-chromium (< 100 µg/kg),
low-protein ration and subjected to a controlled acute haemorrhage
was significantly less than that of chromium-supplemented rats, in
2 studies (67 versus 92%; P < 0.05 and 27 versus 60%; P < 0.01,
respectively) (Mertz & Roginski, 1969).
In Schroeder's study, growth rates in treated mice and rats of
both sexes raised in a "controlled environment", were higher after
6 and 12 months, with highly significant differences in body weight
ranging from 9 to 17% ( P < 0.005) compared with the controls.
Again, the effects of chromium supplementation were greater in
males than in females (Schroeder et al., 1964, 1965).
Similar results were reported by Djahanschiri (1976), who
studied a total of 2750 rats of a special inbred strain (Hk51) fed
a basal diet (0.15 mg chromium/kg diet) and chromium supplements
ranging from 10 to 500 mg/kg diet. At 12 weeks, the average
weights of the chromium-supplemented animals, regardless of dose
level, were significantly higher (by 6% in the males and 3% in the
females) than those of the animals on the basal diet. The same
author reported a progressive diminution in both the milk
production of lactating rats and weight gain in 3 consecutive
generations fed the low-chromium diet, compared with rats receiving
chromium supplementation. Increased mortality was reported in
pregnant guinea-pigs fed a low-chromium diet (125 µg/kg diet)
compared with animals receiving a chromium supplement of either 625
µg/kg or 50 mg/kg (Preston et al., 1976).
When rats raised on a low-chromium Torula yeast diet (< 100
g/kg) mated with those on a normal diet, they were able to
impregnate the females at a 100% conception rate only up to the age
of 4 months. After this age, the conception rate declined to 25%,
25%, and 0%, at the age of 7, 8, and 9 months, respectively. This
decline was accompanied by a significant ( P < 0.01) decrease in
the sperm count in the chromium-deficient males to approximately
half of the count in supplemented controls at the age of 8 months
(Anderson & Polansky, 1981).
7.1.4. Other effects of deficiency
Male weanling rats, fed a 10% soya protein ration with a
chromium content of less than 100 µg/kg, developed a visible
opacity of the cornea in one or both eyes. In several studies, the
incidence of this effect ranged from 10 to 15% in deficient rats.
No opacities developed in control animals receiving 2 mg
chromium/kg diet (Roginski & Mertz, 1967).
Chromium deficiency has been shown to reduce the physical
performance of rats under stress. Ten male rats raised on a
chromium-deficient diet (150 µg/kg diet) swam for an average of 250
min, until exhaustion, in contrast with 10 rats receiving a
supplement of 10 mg chromium/kg diet, which were exhausted only
after 320 min (Djahanschiri, 1976).
7.1.5. Mechanism of action of chromium as an essential nutrient
7.1.5.1. Enzymes, nucleic acids, and thyroid
Chromium is present in nucleic acids in very high
concentrations, but the function of these is not clear at present
(Mertz, 1969). However, recent work suggests a biological function
of chromium in nucleic acid metabolism (Okada et al., 1984).
Ribonucleic acid synthesis in mouse liver was significantly
increased by as little as 1 µmol trivalent chromium, in the
presence of DNA or chromatin (Okada et al., 1981). These effects
were also present when the DNA or chromatin were first complexed
with chromium prior to incubation. However, prior complexation of
RNA polymerase with chromium depressed activity. These effects
were obtained in vitro with a concentration (52 µg/litre) that is
similar to physiological levels. Goncharov (1968) presented data
suggesting that chromium is involved in the function of the thyroid
gland. These findings have been supported by Lifschitz et al.
(1980).
An oligopeptide with a relative molecular mass of 1480, which
was crystallized from liver tissue, had a specific affinity for
chromium (Wu, 1981).
7.1.5.2. Interaction of chromium with insulin
The interaction of chromium with insulin has been extensively
studied and can therefore be presented in some detail, but this
does not imply that this is the only, or the most important,
function of chromium.
The effects of chromium in vitro, and probably in vivo, depend
on the presence of endogenous or exogenous insulin, no effects
having been demonstrated in in vitro systems that did not either
depend on, or contain, insulin. Chromium deficiency causes an
impaired response to added insulin in rat epididymal fat tissue,
and, when glucose uptake or glucose oxidation or utilization for
lipid synthesis is measured, the dose-effect curve is flat.
Addition of suitable chromium compounds significantly increases the
slope of the curve (Fig. 3). This demonstrates the true
potentiation of the insulin action and indicates that chromium
alone does not act as an insulin-like substance (Mertz et al.,
1961; Mertz & Roginski, 1971; Mertz, 1981). Chromium was also
shown to stimulate the transport of D-galactose into epididymal fat
cells. This suggests cell transport, the first step of sugar
metabolism, as a major site of action for chromium (Mertz &
Roginski, 1963). Insulin-potentiating effects have also been
observed on the swelling of liver mitochondria (Campbell & Mertz,
1963) and on glucose utilization in isolated rat lens (Farkas &
Roberson, 1965).
5.1.1.2. Human data
A mean chromium concentration of 0.22 mg/kg wet weight was
found in the lung tissue of subjects from various locations in the
USA, but there was no correlation between chromium levels in the
lungs and those in the air (Schroeder et al., 1962).
A Committee of the National Research Council (US NAS, 1974a)
concluded: "It is unlikely that the intake from air under ordinary
conditions contributes significantly to the total intake of
available chromium; the intake from the air is calculated to be
less than 1 µg/day; but excessive exposure to airborne chromium
does result in some increased intake".
5.1.2. Absorption from the gastrointestinal tract
The absorption of ingested chromium compounds can be estimated
by measuring the amount of chromium excreted in the urine, as
almost all of intravenously injected chromium is excreted via the
urine and only 2% is found in the faeces. Although a potential loss
of endogenous chromium via the skin and its annexa has not yet been
measured and quantified, it can be stated that this organ, as well
as the gastrointestinal tract are of minor importance in the
excretion of endogenous chromium. The gastrointestinal tract is,
of course, the major organ for the excretion of exogenous chromium.
When considering the gastrointestinal absorption of chromium,
it is essential to recognize the substantial differences in the
efficiency of absorption of trivalent and hexavalent compounds.
These differences exist in both man and animals. Many trivalent
chromium compounds are so poorly absorbed that they have been used
as faecal markers in man and animals. The absorption of hexavalent
chromium, administered orally, was higher in all species examined,
but did not exceed 5% of the dose (Donaldson & Barreras, 1966). No
physiological regulation has yet been established for chromium
absorption.
5.1.2.1. Animal studies
The gastrointestinal absorption of chromate in rats has been
reported to be between 3 and 6% of a tracer dose (MacKenzie et al.,
1958; Byerrum, 1961). As in man, trivalent chromium compounds are
less well absorbed in the rat, with reported efficiencies ranging
from less than 0.5% (Visek et al., 1953) to 3% (Mertz et al.,
1965a). Within the category of trivalent compounds, there are
moderate differences in absorption, depending on the chemical form.
Binding of the chromium ion to suitable ligands, such as certain
organic acids, stabilizes the metal against precipitation in the
alkaline milieu of the intestines and increases absorption
efficiency by a factor of 3 - 5 times, compared with that for
chromium chloride. This has been shown for certain chelating
agents (Chen et al., 1973), a yet unidentified small peptide
complex isolated from yeast (Votava et al., 1973), and synthetic
glucose tolerance factor (GTF), a dinicotinic-acid-glutathione-
chromium complex (Mertz et al., 1974). Nothing is known about the
interaction of chromium with the flora of the gastrointestinal
tract. Absorption of chromium chloride by ruminant species is
similar to that in rats, with a mean efficiency of 0.76% (Anke et
al., 1971); laying hens have been found to absorb almost 15% of a
tracer dose of the element (Hennig et al., 1971).
5.1.2.2. Human studies
Donaldson & Barreras (1966) studied the gastrointestinal
absorption of hexavalent chromium by administering trace doses of
Na251CrO4 orally to 6 volunteer patients, who were hospitalized,
and by measuring the amount of radioactivity in the faeces and
urine. The mean urinary excretion, expressing the absorption
efficiency, was 2.1 ± 1.5% of the dose given. Administration by
jejunal infusion in 4 volunteers increased these values, suggesting
reduction of the chromate to trivalent compounds by the acid
content of the gastric juice. The same authors reported a mean
absorption efficiency of only 0.5 ± 0.3% for trivalent chromium,
administered as CrCl3 x 6H2O, with a range of 0.1 - 1.2%.
On the basis of the chromium content in diets (60 µg) and
chromium excretion (0.22 µg) in healthy subjects, Anderson et al.
(1983) calculated a minimum chromium absorption of about 0.4%.
Increasing intake by supplementation with chromium (chromic
chloride tablets, furnishing 200 µg chromium/day) led to an
excretion of 0.99 µg, equivalent to 0.4% of the intake.
Aitio et al. (1984) investigated the intake and urinary
excretion of chromium (III) in leather tanning workers. The
environmental concentrations were recorded as low, but chromium was
present in air in the form of large droplets that were not
collected by the standard air measurement technique. It was assumed
that the large droplets were cleared by the upper respiratory tract
and swallowed, and that the chromium in the droplets was absorbed
from the gastrointestinal tract. A calculation showed that this
would explain the urinary excretion levels. No absorption of
chromium through the skin was detected.
In a recent study, the minimum chromium absorption calculated
on the basis of urinary-chromium excretion was about 0.4%.
Increasing intake 5-fold, by chromium supplementation, led to a
nearly 5-fold increase in chromium excretion, suggesting that the
extent of absorption of supplemental inorganic chromium was similar
to that from normal dietary sources (Anderson et al., 1983a).
A similar absorption for trivalent chromium of 0.69% was
reported by Doisy et al. (1968) in healthy human subjects,
regardless of age. However, a group of 14 insulin-requiring
diabetic patients absorbed 4 times as much of the chromium dose as
the non-diabetic or maturity-onset diabetic subjects, as shown by
elevated levels of 51chromium in blood plasma and urine (Doisy et
al., 1971).
5.2. Distribution, Retention, Excretion
5.2.1. Animal studies
Most animal studies on chromium metabolism have been performed
on rats. From the site of intestinal absorption, chromium is taken
up by plasma-protein fractions. Small, physiological doses of
51chromium have been shown to bind almost entirely to the iron-
binding protein, transferrin (Hopkins & Schwarz, 1964). On the
other hand, inhaled chromium (Glaser et al., 1984) was bound to
albumin rather than to transferrin. With larger quantities of
trivalent chromium, non-specific binding to other proteins also
occurred, but not to the red blood cells. Visek et al. (1953)
measured the effects of the different chemical forms of chromium on
tissue distribution and found that soluble, chelated forms, such as
acetate or citrate complexes, were cleared quite rapidly, in
contrast with colloidal or protein-binding forms (chromite, chromic
chloride), which have a great affinity for the reticulo-endothelial
system (bone marrow, liver, spleen), and clear more slowly. The
blood clearance of hexavalent chromium, such as chromate, was slow,
because of irreversible binding within the red blood cells. Tissue
distribution of 51chromium, administered in nanogram doses to rats
was studied by Hopkins (1965). As in the preceeding studies, the
element accumulated in bone, spleen, testes, and epididymis; much
less was retained in the lungs, brain, heart, and pancreas. This
obvious difference in chromium distribution between man and rats is
unexplained.
As in man, trivalent 51chromium in the rat was rapidly cleared
from the blood, after absorption, and was retained by the tissues
(Mertz et al., 1965a). These tissue stores, labelled with
51chromium chloride, administered orally or intravenously, were not
immediately available for specific physiological functions. For
example, 51chromium, administered as CrCl3 x 6H2O to pregnant rats,
was not transported into the embryos (Mertz et al., 1969), nor did
any 51chromium appear in the blood in response to glucose or
insulin injections (Mertz & Roginski, 1971). The fact that fetal
chromium concentrations are low, when the pregnant rats are fed a
low-chromium Torula yeast diet, and increase when a high-chromium
natural stock ration is fed, indicates that placental transport and
possibly, the acute chromium response depend on a special form of
chromium, which is different from chromium chloride. It is
possible, but has not yet been proved, that this form is the
dinicotinic acid-glutathione-chromium complex, known as glucose
tolerance factor. Yeast extracts containing this factor labelled
with 51chromium have been shown to cross the placenta (Mertz et
al., 1969) and, in preliminary studies, to furnish chromium for the
acute chromium response (Mertz & Roginski, 1971).
With reference to interactions between chromium and other trace
elements, competition with iron by way of their common carrier
(transferrin) has been suggested in rats (Hopkins & Schwarz, 1964)
and in human beings (Sargent et al., 1979). Goncharov (1968)
reported a close interaction between chromium and dietary iodine.
In iodine-deficient white rats, addition of chromium to the diets
in amounts supplying from 0.6 to 600 µg/animal per day stimulated
thyroid function, as indicated by morphological and functional
changes. Conversely, chromium, in all but the lowest dose,
decreased thyroid function in animals receiving adequate iodine
levels. This relation is in agreement with epidemiological data
from the USSR (Goncharov, 1968).
5.2.2. Human data
5.2.2.1. Concentration in tissues, blood, urine, and hair
including possible biological indicators of exposure
(a) Tissues
The most comprehensive survey of tissue-chromium concentrations
is that of Schroeder et al. (1962), who carried out a
spectrographic analyses on 20 - 39 samples for each autopsy tissue,
all of which had been carefully collected to avoid extraneous
contamination. The following results were obtained (mean values in
mg/kg ash) for a group of subjects who had died between the ages of
30 and 40 years: lung, 15.6; aorta, 9.1; pancreas, 6.5; heart, 3.8;
testes, 3.1; kidney, 2.1; liver, 1.8; spleen, 1.7. In all tissues,
except for the lungs there was a rapid decline in chromium
concentrations from time of birth to the age of 10 years, followed
by a more gradual decrease to the age of 80 years. It cannot be
stated with certainty whether the decline is an expression of a
physiological mechanism or of a dietary deficiency. The lungs lost
their initially high chromium levels (85.2 mg/kg ash) up to the age
of 20 years (6.8 mg/kg); subsequently, the concentrations increased
to between 20 and 38 mg/kg. This discrepancy demonstrates that the
chromium in lungs is not in equilibrium with the general pool. The
decline of chromium in the aorta, quite pronounced in subjects in
the USA, was much less dramatic in the aortas from subjects of
other countries (Schroeder et al., 1970).
Mancuso & Hueper (1951), as well as Baetjer et al. (1959b),
found concentrations of chromium in the lungs of former chromate
workers that were several orders of magnitude higher than those in
control subjects. In a study on 16 chromate workers, including 11
with lung cancer, Baetjer et al. (1959b), using a colorimetric
method, observed a median concentration of water- and acid-soluble
chromium in the lung of 70 mg/kg dry weight and a median
concentration of acid-insoluble chromium of 17 mg/kg. The chromium
concentration did not differ between cancer cases and non-cancer
cases. The tissue specimens were obtained 0 - 23 years after the
termination of occupational exposure, which had lasted 1.5 - 42
years. With the use of emission spectrometry and atomic absorption
spectrophotometry, Hyodo et al. (1980) found a chromium content of
3.6 mg/kg wet weight in the lung in a male smoker with lung cancer,
who died 10 years after employment for 30 years in a chromate-
producing plant. The concentration in other tissues ranged from
0.05 (bone marrow) to 1.5 mg/kg (suprarenal gland). In five
unexposed controls, lung concentrations ranged from 0.09 to 0.88
mg/kg; concentrations in other tissues ranged from 0.003 (kidney)
to 0.156 mg/kg (suprarenal gland). The ratio of hexavalent to
total chromium in the lungs was 29% in the worker and 22.7 ± 10.6%
(mean ± SD) in the controls.
Brune et al. (1980) gave tissue concentrations for chromium and
other metals in the lung, liver, and kidney of 20 deceased copper
smelter workers, who had retired 0 - 19 years prior to death, and
of a control group (8 subjects). Tissue analysis was carried out
using neutron activation analysis as well as atomic absorption
spectrophotometry, and included a comparison with certified
reference samples (National Bureau Standards, bovine liver). In
the controls, the concentrations ranged from below detection (0.003
mg/kg wet weight) to 0.07 mg/kg in kidneys and 0.11 mg/kg in liver.
There was no marked difference between the chromium contents of
these 2 tissues and those in the exposed workers. On the other
hand, the lung concentrations were between 3 and 4 times higher in
the workers than in the controls (median levels, 0.29 and 0.08
mg/kg, respectively).
Chromium determinations were carried out on lung and kidney
samples of 45 autopsies from the Northern Bavaria area (Federal
Republic of Germany) (Zober et al., 1984). The analyses were
carried out using electrothermal AAS after wet oxidative digestion.
Median values of 0.097 mg/kg wet weight (range, 0.0006 - 1.230
mg/kg) in lung tissue and 0.0096 mg/kg wet weight (range, 0.0002 -
0.690 mg/kg) in kidney were found.
Very limited data are available on chromium levels in tissues
other than those referred to above. Shmitova (1978) estimated
chromium levels in fetal and placental tissues (abortive material),
derived on the 12th week of pregnancy, and found 92.8 and 30 µg
Cr/kg tissue, respectively.
It can be concluded that chromium may be retained in the lungs,
several years after the termination of occupational exposure.
However, it is not known whether this observation has any
biological relevance to the appearance of lung cancer. In this
context, it is of interest to note that chromium is retained in the
human lung for a relatively long period, even without occupational
exposure.
(b) Blood
The values reported by various investigators for chromium
concentrations in the blood of unexposed human beings range from
0.2 to 70 µg/litre in serum and plasma and 5 to 54 µg/litre in red
blood cells (US EPA, 1978). Insufficient evidence is available to
state whether the blood values in the normal population are
influenced by concentrations in the ambient air. The most
extensive determination of blood-chromium levels in chromate
workers was made by Mancuso (1951). Blood values during exposure
varied from 5 to 170 µg/litre and from 10 to 140 µg/litre, 74 days
after work ended. No decrease in blood-chromium levels was found,
even after 74 days without exposure.
Because of a selective affinity of hexavalent chromium for the
erythrocyte, substantially increased environmental exposure to
chromates is reflected in an increased ratio of the hexavalent
chromium level in red blood cells to that in plasma. Baetjer et
al. (1959a) found the following concentrations in 3 exposed
chromate workers: blood cells, 30, 54, and 140 µg/litre; plasma, 0,
20, and 17 µg/litre, respectively. In the absence of exposure to
chromate, the chromium concentrations in erythrocytes and plasma,
as serum, were nearly identical (Paixao & Yoe, 1959). Chromium
(VI) is incorporated into human red blood cells and remains there
over a long period of time (Wiegand et al., 1985), since the
approximate lifetime of human red cells is about 100 days. Exposure
to 2 mg trivalent chromium/day, for 3 months, resulted in an
increased concentration (0.2 mg/kg) in the red cells of 5 human
males, compared with 0.11 mg/kg in 5 controls without supplement
(Schroeder et al., 1962). However, later studies did not show any
increase in chromium concentrations in red cells, following
exposure to trivalent chromium (Beyersmann et al., 1984; Wiegand et
al., 1985). Monitoring of red blood cell-chromium may be a useful
indicator of exposure to hexavalent, but not to trivalent,
chromium.
Most of the later studies show that the true chromium
concentration in the plasma or serum of healthy subjects is of the
order of 1 µg/litre or less (Guthrie et al., 1978; Versieck et al.,
1978). Seeling et al. (1979) determined chromium in serum and
plasma using flameless AAS. This study was supported by measuring
a standard reference material (National Bureau of Standards, 1569
brewer's yeast, reference data: 2.12 ± 0.05 mg/kg, measured data:
2.3 ± 0.2 mg/kg). The chromium levels in serum ranged from 0.7 to
2.2 µg/litre and in plasma from 1 to 1.5 µg/litre (central
parameters of a long normal distribution).
Pooled serum samples of 6 healthy Finnish volunteers were
studied by Kumpulainen et al. (1983). A mean value of 0.11 ± 0.05
µg chromium/litre (range, 0.06 - 0.20) was found. Nomiyama et al.
(1980b) analysed 20 blood samples of Japanese subjects, using
direct flameless ASS, and reported a value of 2.9 ± 1.7 µg
chromium/litre whole blood.
Zober et al. (1984) analysed the blood of 45 autopsied subjects
from the Northern Bavaria area (Federal Republic of Germany). A
median chromium concentration of 2.8 µg/litre of postmortem blood
(range, 0.20 - 24 µg/litre) was found, the value was not influenced
by age or sex.
A reference material for chromium, bovine serum (RM 8419) is
available from the National Bureau of Standards, Washington DC,
USA.
(c) Urine
Chromium concentrations in the urine of non-occupationally
exposed subjects have been reported to range from 1.8 to 11
µg/litre (Imbus et al., 1963). Except for exposed persons and
juvenile diabetic patients, the reported values for daily chromium
excretion in urine (Table 9) do not differ as much as those for
blood. In later studies, such as that of Guthrie et al. (1979), a
method of flameless atomic absorption was used in which it was
possible to correct for spectral interference (Zander et al.,
1977). Such interference was difficult to eliminate with earlier
methods (Guthrie et al., 1978). Urine samples from 189 Japanese
volunteers, aged 10 - 80 years, in 4 pollution-free areas, were
analysed for chromium by Nomiyama et al. (1980a), using direct
flameless AAS. The average level was 0.4 ± 0.37 µg/litre (X ± SE)
or 0.47± 0.42 mg/kg creatinine. Urinary chromium tended to be
higher in males than in females and to decrease with age, but the
differences were not significant. Using electrothermal AAS, the
urinary excretion of various metals in non-occupationally exposed
adults was measured by Schaller & Zober (1982). For smokers, a
median value of 1.6 µg chromium/litre was found (non-smoker: 1.4 µg
chromium/litre). Commercially available urine samples were used
for quality control (Angerer et al., 1981). Although a "normal"
level of chromium excretion for healthy, unexposed persons cannot
yet be established with certainty, such a level may be less than 1
µg/day.
Several investigators have measured urinary-chromium excretion
in exposed workers. In a study on chromate workers, the urine
values ranged from 5 to 380 µg chromium/litre during exposure and
from 10 to 54 µg chromium/litre, 74 days after the end of exposure
(Mancuso, 1951). No relationship was evident between the urine
levels and the weighted average number of years of exposure. In
every case where urine values were recorded, both during, and 74
days after the end of, exposure, the chromium concentrations
decreased with time away from exposure.
A study of 12 workers in a galvanizing plant in the Federal
Republic of Germany showed an average concentration of chromium in
urine of 9.5 µg/litre (range, 1.4 - 24.6 µg/litre) compared with a
value of 1.8 ± 1.1 µg/litre in 60 unexposed workers (Schaller et
al., 1972). Gylseth et al. (1977) investigated a group of 14
welders exposed to about 0.05 mg chromium/m3 air, and reported a
urinary chromium concentration of approximately 40 mg/litre. At
the same exposure level, Tola et al. (1977) found a concentration
of 30 mg chromium/kg of creatinine in the urine. In both of these
studies, there was a correlation between recent exposure to
airborne chromium and chromium concentrations in urine. In the
study by Tola et al. (1977), it was shown that the water-soluble
fraction of airborne chromium was better correlated with the
concentration excreted in the urine than with total chromium. It
was also shown that the water-soluble fraction consisted mainly of
hexavalent chromium.
Table 9. Daily chromium excretion in urine
---------------------------------------------------------------------------
Subjects Number Excretion Range Reference
(µg/day) (µg/day)
(mean ± SD)
---------------------------------------------------------------------------
Adult males 2 0.72a 0.58 - 0.86 Schroeder et al.
(1962)
Adult males, fed 3 31.0a 20.8 - 46.5 Schroeder et al.
2 mg trivalent, (1962)
chromium/day for
3 months
Normal adults 16 13 ± 6 4 - 24 Voelkl (1971)
Chromate workerb 16 000
Normal adults 60 1.6 ± 1.1 Schaller et al.
(1972)
Galvano-technical 12 9.7 ± 6.6 1.4 - 24.6 Schaller et al.
workers (1972)
Normal young 20 8.4 ± 5.2 Hambidge (1974)
adults
Normal children, 18 5.5 ± 2.9 Hambidge (1974)
8 years old
Insulin-dependent 7 19.2 ± 18.9 Hambidge (1974)
diabetic children,
11 years old
Young women 9 7.2 ± 1.2 5.9 - 10.0 Mitman et al.
(1975)
Adult males 12 0.8 ± 0.4 0.4 - 1.8 Guthrie et al.
(1979)
---------------------------------------------------------------------------
Table 9. (contd.)
---------------------------------------------------------------------------
Subjects Number Excretion Range Reference
(µg/day) (µg/day)
(mean ± SD)
---------------------------------------------------------------------------
Adult males 91 0.48 ± 0.41a 0.42 - 0.53 Nomiyama et al.
(1980b)
Adult females 98 0.34 ± 0.31a 0.22 - 0.43 Nomiyama et al.
(1980b)
Adult males 48 0.20 ± 0.01a 0.05 - 0.58 Anderson et al.
(1982b)
Adult females 28
Adult males 27 0.17 ± 0.10 Anderson et al.
(1983a)
Adult females 15 0.20 ± 0.12 Anderson et al.
(1983a)
Adult males and 299 0.80 ± 0.6a 0.4 - 2.1 Fang (1983)
females
Normal adults 10 0.11 ± 0.05a 0.06 - 0.20 Kumpulainen et al.
(1983)
Normal adultsc 10 4.9 0.10 - 14.2 Zober et al.
(1984)
---------------------------------------------------------------------------
a Data calculated as µg/litre urine.
b Tanner, suffering from an acute ulceric gasteroenterocolitis.
c Samples taken post-mortem from autopsies. Original values are related
to mass (kg) instead of volume (litre).
Lindberg & Vesterberg (1983a) measured airborne, and urinary-
chromium levels among platers. Concentrations of chromium in urine
of < 5 µg/litre occurred when the time-weighted average values of
exposure were about or below 2 µg/m3 air. Severe damage to the
nasal septum and effects on lung function have not been found at
levels lower than this. It was shown that post-shift urinary-
chromium determinations could be used to monitor exposure in this
occupational group.
The urinary-chromium excretion and chromium clearance in 22
welders, who had been exposed to airborne hexavalent chromium (5 -
150 µg/m3) during 2 - 40 years (mean working time, 18.9 years) were
measured by Mutti et al. (1979). The method used was flameless
atomic absorption spectrometry. A highly significant correlation
was detected between the ratio of urinary-chromium to creatinine
and the airborne chromium concentration in the workplace, with
excretions ranging from 5.3 ± 3.7 to 33.3 ± 6.9 mg chromium/kg
creatinine in slightly exposed and heavily exposed welders,
respectively. The authors also reported an increase in chromium
clearance with increasing body burden of chromium, which indicates
that high urinary-chromium excretion may be caused by previous high
exposure as well as by current exposure.
Baseline data for chromium excretion in unexposed subjects in
the report by Mutti et al. (1979) are approximately 10 times higher
than the values recently proposed and generally accepted as normal.
However, there is reason (section 2.2.2) to accept relative
differences in analytical results in studies by one author using
one method, even if the absolute values reported may be questioned.
To test whether chromium excretion is also associated with
exercise-induced increases in glucose utilization, the urinary
chromium excretion, serum glucose, insulin, and glucagon of 9 male
runners (23 - 46 years old) were evaluated by Anderson et al.
(1982a). The mean urinary-chromium concentration was increased
nearly 5-fold, 2 h after running; excretion of sodium, potassium,
and calcium was unchanged. These data demonstrate an increase in
chromium excretion with exercise-induced increase in glucose
utilization.
(d) Milk
The chromium concentration was determined in 261 samples of
breast milk collected by manual expression from 45 American women.
Chromium levels were measured in whole, liquid milk by graphite-
furnace AAS, using the method of standard additions. The mean
chromium content of the breast milk samples was 0.30 µg/litre. The
range of individual values was 0.06 - 1.56 µg/litre and did not
change significantly with duration of lactation (Casey & Hambidge,
1984). Kumpulainen et al. (1983) analysed frozen samples of pooled
breast milk taken from women in different stages of lactation and
obtained from the Milk Bank of the Children's Hospital of Helsinki.
The mean chromium content ± SD was 0.49 ± 0.067 µg/litre (range,
0.37 - 0.57 µg/litre).
(e) Hair
Hair-chromium concentrations in children during the first 6
months of life were significantly higher than at any other age
(Hambidge & Baum, 1972); they declined from an initial value of
1493 µg/kg to an average of 412 µg/kg at 2 - 3 years. Hambidge
(1971) compared the chromium concentration in the hair of 15
newborn babies and that of their mothers: in only one case was the
chromium level in the mother's hair higher than that in the newborn
baby. Chromium levels were significantly lower than those
mentioned above in 50 Turkish women and their newborn babies (203
and 119 µg/kg, respectively) and only 12 newborn babies were found
to have higher concentrations than their mothers, suggesting
suboptimal chromium status (Gürson, 1977). Hair appears to reflect
the nutritional chromium status of groups. The hair-chromium level
is significantly lower in parous women than in nulliparae (Hambidge
& Rodgerson, 1969; Mahalko & Bennion, 1976) and in diabetic
children compared with normal controls (Hambidge et al., 1968). It
is low in adult-onset diabetic adults (Benjanuratra & Bennion,
1975). These findings are in agreement with the expected changes
in chromium balance during pregnancy and in diabetes.
5.2.2.2. Dynamic aspects of metabolism and the influence of
pathological states
Once chromium is absorbed into the organism, it clears rapidly
from the blood stream and is excreted or taken up by the tissues.
In a clinical study, Sargent et al. (1979) detected a 4-
compartment-type clearance from blood, with mean half-times of 13
min, 6.3 h, 1.9 days, and 8.3 days. However, the disappearance
from 3 tissue compartments was much slower, with half-times of
0.56, 12.7, and 192 days. The half-times for blood 3-compartment
clearance in rats (Hopkins, 1965) were calculated to be 0.56, 5.33,
and 57 h (Withey, 1983).
Whether any organ is specifically responsible for the storage
and release of the "metabolically responsive" chromium is not
known. The "metabolically responsive" chromium in blood is defined
as the fraction that increases acutely in response to an elevation
of blood-glucose or blood-insulin levels. It is believed that this
chromium increment interacts with the increased insulin secreted in
response to a glucose load, to facilitate the action of the hormone
on the insulin receptors of the insulin-sensitive cells.
In young, healthy subjects, but not in elderly subjects and
diabetic patients, an oral glucose load or the injection of insulin
results in a sudden increase in serum- or plasma-chromium
(Glinsmann et al., 1966; Levine et al., 1968; Hambidge, 1971; Behne
& Diel, 1972; Liu & Morris, 1978). This increase may appear 30
min, or as late as 120 min, after the challenge; much of the
chromium responsible for the increase is subsequently lost in the
urine. Lack of this increase, also termed "relative chromium
response", is often associated with impaired glucose tolerance,
indicative of chromium deficiency. It should be noted that, when
the glucose load was given intravenously (Pekarek et al., 1975) to
healthy volunteers, the serum-chromium level decreased rapidly,
while the blood-glucose level increased. Supplementation with
chromium chloride or high-chromium yeast extracts for several weeks
resulted in the reappearance of the relative chromium response and
improvement of glucose tolerance (Glinsmann et al., 1966; Liu &
Morris, 1978). These findings support the conclusion that the
"relative chromium response" measured during a glucose or insulin
tolerance test may serve as an indicator of the adequacy of
metabolically responsive chromium.
Much of the chromium increment secreted into the blood stream
in response to glucose or insulin is subsequently lost in the
urine. Hambidge (1971) observed greatly increased urinary-chromium
excretion in 2 diabetic children after insulin therapy had begun,
compared with the excretion in the same children before insulin
treatment. It is not known whether the increased urinary loss of
chromium is compensated for by an increase in absorption efficiency
from the intestines. Hambidge et al. (1968) reported significantly
lower chromium concentrations in the hair of diabetic children
compared with normal children, and Morgan (1972) found that
the chromium contents in the livers of 31 diabetic adults at
autopsy were lower than those in 24 control livers from non-
diabetic persons (8.57 versus 12.7 mg/kg ash; P = 0.05).
On the other hand, Doisy et al. (1971) demonstrated greatly
increased intestinal absorption, together with elevated chromium
excretion, in 14 insulin-dependent diabetic patients administered
51CrCl3 x 6H2O, orally. It is not known whether the greater
absorption efficiency is adequate to compensate for the increased
urinary losses; the decreased chromium concentrations in hair and
liver, discussed above, suggest that a negative balance may prevail.
Several other pathological conditions affect chromium
metabolism. Sargent et al. (1979) detected significantly less
retention of intravenously administered 51chromium in 11 patients
with haemochromatosis compared with 5 normal controls. This may be
related to the high saturation with iron of transferrin, which is
also the carrier protein for newly absorbed chromium.
Chronic ischaemic heart disease also affects chromium
metabolism. Neiko & Del'va (1978) observed a significantly
increased urinary-chromium loss, greater by a factor of 1.5 - 1.6
than that of normal controls, in 65 heart patients. The urinary-,
and to a lesser extent, the faecal-chromium loss increased
progressively with increasing severity of signs and symptoms and
resulted in a negative chromium balance in the post-infarct state.
The balance became positive again on discharge from the hospital
after medical treatment.
Acute infections also appear to influence chromium metabolism.
Pekarek et al. (1975) measured glucose tolerance, and insulin and
chromium levels in human volunteers, before and after infection
with the benign sandfly fever virus. Impaired glucose tolerance and
a significantly increased insulin response to a glucose load,
observed at the height of the infection, were accompanied by very
significantly depressed serum-chromium levels (0.5 µg/litre,
compared with 1.4 µg/litre before infection; P < 0.05) In
contrast with the sharp decline in serum-chromium following the
intravenous injection of glucose in the healthy state observed by
these authors, the depressed serum-chromium levels declined very
little during the glucose tolerance test at the height of
infection. The mechanism of the changes in chromium metabolism in
heart disease and sandfly fever is not clear. Of great potential
importance is the unanswered question of whether the observations
described here reflect an increased chromium requirement in the
patients or a normal reaction to various forms of stress.
The information on the dynamic aspects of chromium metabolism
in animals is limited and should be considered in connexion with
the more detailed studies on human subjects. Diabetes, induced in
rats by the injection of streptozotocin, affected the tissue
distribution of injected 51CrCl3. Sixteen days after injection of
streptozotocin, 51CrCl3 was injected into 5 diabetic rats and 6
normal controls and the 51Cr content measured 5 days later. The
serum of the diabetic rats contained more than 3 times the 51Cr
activity found in the controls (0.24 versus 0.07% of the injected
dose P < 0.01). Significant differences were also detected in the
distribution of 51Cr in the subcellular fractions of the liver; in
the diabetic tissue, 51Cr activity was higher in the nuclear and
supernatant fractions ( P < 0.01) and lower in mitochondria and
microsomes ( P < 0.05). The mechanism responsible for these
changes is not known (Mathur & Doisy, 1972).
5.3. Influence of Chemical Form
The diverse biological effects of chromium on living organisms
cannot be understood without knowledge of the chemical and physical
forms in which the element is present. As stated earlier, the
metallic state (zero valence) is biologically inert, the trivalent
state represents the essential element chromium, and the hexavalent
state is of concern to the toxicologist. Compounds of trivalent
chromium are poorly absorbed, whereas those of the hexavalent state
easily penetrate physiological barriers, such as cell membranes.
Hexavalent chromium compounds are easily reduced by living matter,
but oxidation of trivalent to hexavalent chromium does not occur in
the organism.
The physical form of hexavalent compounds (such as particle
size) and chemical properties (such as solubility) determine
metabolic pathways after inhalation and, therefore, health effects.
There is an equally strong influence of the chemical form of
trivalent chromium on metabolism and health effects. When chromium
is bound to water or small anions (e.g., CrCl3 x 6H20), it
precipitates in the neutral or alkaline milieu of the body fluids.
When it is bound to ligands, such as organic acids, the element is
light in solution and is available for intestinal absorption. The
forms in which trivalent chromium occurs in nature are not really
known. Plants probably contain chromium complexes with organic
acids.
The biological availability of chromium compounds in foods is
of great nutritional importance, but is poorly defined. One
compound or a group of closely related compounds, glucose tolerance
factor, has been isolated from yeast and shown to be more active
than chromium chloride in genetically diabetic mice and in the in
vitro potentiation of the action of insulin on rat epididymal fat
tissue (section 7.1.5.2). It has been identified as a dinicotinic-
acid glutathione complex, but the exact stereochemical structure is
not yet known (Toepfer et al., 1977). It has been postulated, but
not proved, that this factor is the active form of chromium within
the organism.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The environmental effects of chromium as a pollutant have been
reviewed by US EPA (1978), Anderson (1982), and EIFAC (1983).
Various effects have been reported, but, because of the presence of
other chemicals, it remains doubtful whether chromium alone is
responsible for the effects observed. Data are available on
microorganisms, plants, and aquatic organisms.
6.1. Microorganisms
Most microorganisms (protozoa, protophyta, fungi, algae,
bacteria) are able to absorb chromium. The active uptake of
chromate by the sulfate transport system has been shown in
Neurospora crassa (Roberts & Marzluf, 1971). No distinction has
been made between ab- and adsorption in other studies (e.g., algae)
(Calow & Fletcher, 1972), and it has not yet been shown that
chromium is an essential element for microorganisms. In general,
toxicity for most microorganisms occurs in the range of 0.05 - 5 mg
chromium/kg of medium. The internal concentration of chromium
depends on the species. In most groups of microorganisms, it
ranges between the levels of 0.6 mg dry weight present in one litre
of sample of microplankton from Monterey Bay, California, USA, and
21.4 mg/litre phytoplankton collected in the Pacific Ocean (Martin
& Knauer, 1973).
Trivalent chromium is less toxic than hexavalent. The main
features are inhibition of growth (at concentrations greater than
0.5 mg/litre in Chlorella cultures) (Nollendorf et al., 1972) and
inhibition of various metabolic processes, such as photosynthesis
or protein synthesis (US EPA, 1978).
The toxicity of chromium for soil bacterial isolates was
studied by measuring the turbidity of liquid cultures supplemented
with hexavalent chromium and trivalent chromium. Gram-negative
bacteria were more affected by hexavalent chromium (1 - 12 mg/kg)
than gram-positive bacteria. Toxicity due to trivalent chromium
was not observed at similar levels. The toxicity of low levels of
hexavalent chromium (1 mg/kg) indicates that soil microbial
transformations, such as nitrification, may be affected (Ross et
al., 1981).
6.2. Plants
Although chromium is present in all plants, it has not been
proved to be an essential element for plants. Most substances,
including chromium, can be absorbed through either the root or the
leaf surface. Several factors affect the availability of chromium
for the plant (Black, 1968), including the pH of the soil,
interactions with other minerals or organic chelating compounds,
and carbon dioxide and oxygen concentrations.
Little chromium is translocated from the site of absorption;
however, the chelated form is transported throughout the plant
(Verfaillie, 1974).
Chromium in high concentrations can be toxic for plants, but
Yopp et al. (1974) stated that there was no specific pattern of
chromium intoxication.
During the smelting of chromite, considerable quantities of
waste are produced, which contain soluble chromates. When combined
with a high pH, Gemmell (1973) showed an inhibition of germination
and growth in white mustard plants (Sinapis alba) growing on waste
heaps. Covering the waste with a 25-to 30-cm layer of granular-
free-draining subsoil followed with layers of soil, peat, or sewage
sludge was shown to be the best revegetation technique (Gemmell,
1974).
The main feature of chromium intoxication is chlorosis, which
is similar to iron deficiency (Hewitt, 1953).
Soybeans, treated in nutrient culture containing 0 - 5 mg
hexavalent chromium/litre showed decreased uptake of calcium,
potassium, phosphorus, iron, and manganese (Turner & Rust, 1971).
Death of plants occurred within 3 days of treatment with 30 or 60
mg chromium/litre.
A reduction in leaf dry weight occurred after treatment with
0.01 mg hexavalent chromium/litre (Rediske et al., 1955). Chromium
affects the carbohydrate metabolism, and the leaf chlorophyll
concentration decreased with increasing hexavalent chromium
concentration (0.01 - 1 mg hexavalent chromium/litre) (Rediske,
1956). Hexavalent chromium appears to be more toxic than trivalent
chromium (Hewitt, 1953; Stanley, 1974; Verfaillie, 1974). At
present, no data are available concerning the mechanism of action
or the dose-dependent pattern of chromium intoxication.
6.3. Aquatic Organisms
More studies have been performed with aquatic species than with
free-living (non-parasitic) animals. Depending on the species,
chromium can be less toxic for fish in warm water, but marked
decreases in toxicity are found with increasing pH or water
hardness; changes in salinity have little if any effect on its
toxicity. Chromium can make fish more susceptible to infection;
high concentrations can damage and/or accumulate in various fish
tissues and in invertebrates such as snails and worms.
Reproduction of Daphnia was affected by exposure to 0.01 mg
hexavalent chromium/litre (EIFAC, 1983). Numerous other factors
influence the availability of chromium and, therefore, its
toxicity. These include the presence of other minerals and organic
pollutants, and the temperature of the environment; this has been
shown in mice (Nomiyama et al., 1980a).
Hexavalent chromium is accumulated by aquatic species by
passive diffusion (US EPA, 1978). Ecological factors, in the
abiotic and living environment, are involved in this process, which
varies according to the sensitivity of different species. The
physiological state and activity of the fish also affect
accumulation (Reichenbach-Klinke, 1977, 1980). Kittelberger (1973)
analysed the organs and tissues of the roach (Leuciscus rutilus)
from the river Rhine and found that concentrations of chromium in
the spleen, bronchi, and intestine (between 30 and 37.5 mg/kg) were
10 - 30 times higher than those in the heart, skin, muscle, and
scales.
LC50s are listed in Table 10 for hexavalent and trivalent
chromium compounds in the aquatic environment.
Table 10. The toxicity of chromium for fresh-water organisms
(expressed as 50% mortality)a
-----------------------------------------------------------------------
Compound Category Exposure Toxicity range Most
(mg/litre) sensitive
species
-----------------------------------------------------------------------
hexavalent invertebrate acute 0.067 - 59.9 scud
chromium long-termb - -
vertebrate acute 17.6 - 249 fathead minnow
long-term 0.265 - 2.0 rainbow trout
trivalent invertebrate acute 2.0 - 64.0 cladoceran
chromium long-term 0.066 cladoceran
vertebrate acute 33.0 - 71.9 guppy
long-term 1.0 fathead minnow
-----------------------------------------------------------------------
a From: US EPA (1980).
b No data available.
In general, invertebrate species, such as polychaete worms,
insects, and crustaceans are more sensitive to the toxic effects of
chromium than vertebrates, such as some fish (Mathis & Cummings,
1973). The lethal chromium level for several aquatic and
nonaquatic invertebrates has been reported to be 0.05 mg/litre (US
NAS/NAE, 1972).
EIFAC (1983) reviewed the literature on the occurrence and
effects of chromium in fresh water and proposed tentative water-
quality criteria that distinguish between salmonid and non-salmonid
waters. To protect salmonid waters, the mean aqueous concentration
of "soluble" chromium should not exceed 0.025 mg chromium/litre,
and the 95 percentile should not exceed 0.1 mg chromium/litre.
However, more stringent values may be necessary in very soft, acid
waters, and less stringent values in alkaline waters.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Nutritional Effects of Chromium
The criteria for an essential nutrient have been defined in
different ways by different authors, but all definitions postulate
that a reduction in the total daily intake of the nutrient below a
certain level must consistently induce signs of deficiency, and
that the supplementation of the daily intake above this level must
prevent and cure the deficiency signs. Chromium deficiency has
been produced experimentally in mice, rats, and squirrel monkeys,
and the full reversal of the deficiency signs by the oral
administration of chromium has been demonstrated in rats (Mertz,
1969). For these reasons, chromium must be considered an essential
micro-nutrient. It is physiologically active in the trivalent
oxidation state at concentrations of approximately 100 µg/kg diet.
7.1.1. Effects of deficiency on glucose metabolism
Semipurified rations, containing Torula yeast as the source of
protein, were fed to groups of 10 male Sprague Dawley rats at
weaning, and intravenous glucose tolerance tests were performed by
injecting 1250 mg glucose/kg body weight and measuring the
subsequent decline in blood-glucose levels. The rate of glucose
disappearance can be calculated from the straight line plot of log
increment glucose (excess of glucose at any time (t) over fasting
glucose), versus time. The disappearance constant k is expressed
as % decline of the increment glucose per min; it is a measure of
the efficiency of glucose utilization. In weaning rats, the rate
constant was found to decline, within 3 weeks or less, from an
average of 4%/min to 2.6%/min in animals fed the Torula yeast diet,
but not in animals administered a diet in which 4 or 8% of Torula
yeast was replaced by an equal amount of brewer's yeast. This
observation suggested that brewer's yeast, but not Torula yeast,
contained an unknown substance necessary for the maintenance of
normal glucose tolerance. Because the only known effect of the
substance was that on glucose tolerance, it was named "Glucose
Tolerance Factor" (GTF) (Mertz & Schwarz, 1959). GTF was extracted
from brewer's yeast and pork kidney powder, concentrated and
purified, and its active ingredient was identified as trivalent
chromium (Schwarz & Mertz, 1959). Chromium in the form of most
common complexes (except for very stable ones) cured the impairment
of glucose tolerance in deficient rats, either as one oral dose of
200 µg/kg body weight, or as an intravenous (iv) injection of 2.5
µg/kg body weight. Chromium in the diet also prevented the
impairment of glucose tolerance.
In order to produce more pronounced deficiencies of chromium
and other trace elements, Schroeder et al. (1963) constructed a
special animal house on a mountain top in Vermont, USA, far removed
from traffic and industry. The interior was specially coated with
organic resins to reduce any metallic exposure. Air was introduced
through special filters, and strict practices were enforced to
avoid the introduction of dust and dirt. This will be referred to
as the "controlled environment". Under these conditions, a more
severe chromium deficiency resulted in very low glucose removal
rates of 1.12%/min in 6 rats and of 0.18%/min in 4 female breeder
rats (482 days old), after 11 days on a chromium-deficient diet.
The removal rate in 4 control rats receiving the same diet with a
chromium supplement improved from 0.51 to 1.39% during the same
period (Mertz et al., 1965b). This strong impairment of glucose
tolerance in rats kept in the "controlled environment" was
reflected in their fasting blood-glucose levels, compared with
those of chromium-supplemented controls: 1370 ± 68 versus 1170 ± 17
mg/litre in males and 1390 ± 68 versus 960 ± 65 mg/litre in
females. More than half of 185 deficient rats excreted more than
0.25% glucose in the urine, whereas glycosuria was found in only 9
of the chromium-supplemented controls (Schroeder, 1966). A
significant reduction in the intravenous glucose removal rate was
also observed in 8 rats in plastic cages, fed an EDTA-washed low-
protein diet (7% casein), compared with 8 controls receiving 50 µg
chromium as the chloride, by stomach tube, daily for 2 weeks (2.1
versus 3.6%/min; P < 0.01). However, chromium supplements did not
improve the near-normal removal rates in rats receiving a 20%
casein diet (Mickail et al., 1976). Significantly lower plasma-
glucose levels, due to supplementation with chromium, were reported
in rats fed the chromium-deficient Torula yeast diet (Whanger &
Weswig, 1975). In another series of studies, only a slight
reduction in blood-glucose (from 1240 to 1180 µg/litre) was found
in 10 rats receiving a diet supplemented with chromium (10 µg/kg)
(Djahanshiri, 1976).
Impaired glucose tolerance in squirrel monkeys, fed a
commercial laboratory chow, was shown to respond to chromium
supplementation. Of 9 monkeys with an impaired glucose removal
rate (1.38%/min), 8 responded after 22 weeks of supplementation of
their drinking-water (chromium acetate, 10 mg/litre) with a
normalization of their glucose tolerance (average removal rate, N =
9, 2.33 ± 0.3%/min) (Davidson & Blackwell, 1968). There was no
effect on food consumption, growth rate, or serum-insulin
concentrations. The chromium content of the commercial chow was
stated to be 3.3 mg/kg, a very high concentration. In view of the
uncertainties of methods of analysis for chromium (section 2.2), it
is not possible to interpret the results as indicative of a very
high chromium requirement of the squirrel monkey or of an unusually
poor bioavailability of the chromium in that particular ration. A
marginal chromium deficiency may have existed in mice fed a bread
and milk diet, as daily administration of 10 µg chromium for 16
days to 8 months produced a 10 - 30% decline in blood-glucose
levels (Vakhrusheva, 1960). However, this effect was not specific
for chromium, as it was also observed when manganese was
administered.
The glucose tolerance of guinea-pigs did not differ
significantly between groups fed diets containing chromium at
0.125, 0.625, or 50 mg/kg, even though the animals fed the 2 higher
levels exhibited a lower mortality rate during pregnancy (Preston
et al., 1976).
Although the administration of synthetic glucose tolerance
factor (a chromium-dinicotinic acid-glutathione complex) to 6 pigs
did not affect glucose tolerance tests, it resulted in a
significant increase in the hypoglycaemic effect of insulin
injected at 0.1 U/kg body weight (Steele et al., 1977a).
Turkey poults, fed a practical ration containing chromium
levels of 5 mg/kg, responded to chromium supplementation (20 mg/kg
diet) with a significant increase in liver glycogen and in glycogen
formation, following a fast, and with a significant increase in
glycogen synthetase (EC 2.4.1.11) activity in the liver (Rosebrough
& Steele, 1981).
It can be concluded that the impairment of glucose tolerance in
rats fed a low-chromium Torula yeast diet is due to chromium
deficiency. The effects of chromium in squirrel monkeys, pigs, and
turkeys, though statistically significant, are somewhat difficult
to interpret, because of the reported high chromium content of the
basal diet. No evidence for chromium deficiency has yet been
obtained through glucose tolerance tests on other animal species.
7.1.2. Effects of deficiency on lipid metabolism
Though trivalent chromium in high doses (2.5 mg/kg body weight)
has been shown to increase the synthesis of fatty acids and
cholesterol in the liver (Curran, 1954), lower, physiological doses
appear to decrease serum-cholesterol concentrations in rats.
Schroeder & Balassa (1965) found an average level of 927 mg
cholesterol/litre serum in 24- to 26-month-old male rats, kept in a
controlled environment and administered chromium in the drinking-
water at a concentration of 5 mg/litre, compared with an average of
1229 mg/litre in controls not receiving chromium ( P < 0.01). The
effects in female rats were ambigous, one study producing the
expected reduction in cholesterol due to chromium, another showing
an elevation in the supplemented female rats. Schroeder's
observations of a cholesterol-reducing effect of chromium in male
rats were confirmed by Staub et al. (1969) and by Whanger & Weswig
(1975) but were contradicted by results of a third study in which
there were not any significant effects of chromium on sucrose-
induced triglyceridaemia and cholesterolaemia (Bruckdorfer et al.,
1971). Perhaps more significant than the effect on circulating
cholesterol is the direct effect of chromium on the occurrence of
aortic plaques. Schroeder & Balassa (1965) observed 6 plaques in 54
male and female chromium-deficient rats, but only one plaque in 48
animals receiving 5 mg chromium/litre in drinking-water. These
results are in agreement with those from a subsequent report of the
protective effect of the natural chromium content of water (60 -
215 µg/litre) against atherosclerosis in cholesterol-fed rabbits
(Novakova et al., 1974). Abraham et al. (1980) extended these
observations by demonstrating that daily chromium injections (20 µg
K2CrO4) reversed the established atherosclerosis in the aorta of 11
cholesterol-fed rabbits, compared with 12 controls. The mean
plaque area was reduced from 95% to 63%, the total aortic
cholesterol from 729 mg to 458 mg, and the atheromatous lesions, as
measured by technetium incorporation from 285 000 cpm to 114 000
cpm, all differences being statistically significant. These
results are reinforced by observations in man discussed in section
8.1.
7.1.3. Effects of deficiency on life span, growth, and
reproduction
The mortality of male, but not of female mice, raised in a
"controlled environment" (section 7.1.1) was reduced by trivalent
chromium administered as acetate in the drinking-water at a
concentration of 5 mg/litre (Schroeder et al., 1964). The survival
rates at 12 months were 92.6% and 68.8% ( P < 0.0001) in
supplemented and deficient animals, respectively. Similary, male,
but not female, rats receiving a chromium concentration of 5
mg/litre in the drinking-water had longer life spans than deficient
controls. The mean age of the last surviving 10% of animals was
1249 days, compared with 1141 days in the deficient animals ( P <
0.01). Survival of male rats fed a low-chromium (< 100 µg/kg),
low-protein ration and subjected to a controlled acute haemorrhage
was significantly less than that of chromium-supplemented rats, in
2 studies (67 versus 92%; P < 0.05 and 27 versus 60%; P < 0.01,
respectively) (Mertz & Roginski, 1969).
In Schroeder's study, growth rates in treated mice and rats of
both sexes raised in a "controlled environment", were higher after
6 and 12 months, with highly significant differences in body weight
ranging from 9 to 17% ( P < 0.005) compared with the controls.
Again, the effects of chromium supplementation were greater in
males than in females (Schroeder et al., 1964, 1965).
Similar results were reported by Djahanschiri (1976), who
studied a total of 2750 rats of a special inbred strain (Hk51) fed
a basal diet (0.15 mg chromium/kg diet) and chromium supplements
ranging from 10 to 500 mg/kg diet. At 12 weeks, the average
weights of the chromium-supplemented animals, regardless of dose
level, were significantly higher (by 6% in the males and 3% in the
females) than those of the animals on the basal diet. The same
author reported a progressive diminution in both the milk
production of lactating rats and weight gain in 3 consecutive
generations fed the low-chromium diet, compared with rats receiving
chromium supplementation. Increased mortality was reported in
pregnant guinea-pigs fed a low-chromium diet (125 µg/kg diet)
compared with animals receiving a chromium supplement of either 625
µg/kg or 50 mg/kg (Preston et al., 1976).
When rats raised on a low-chromium Torula yeast diet (< 100
g/kg) mated with those on a normal diet, they were able to
impregnate the females at a 100% conception rate only up to the age
of 4 months. After this age, the conception rate declined to 25%,
25%, and 0%, at the age of 7, 8, and 9 months, respectively. This
decline was accompanied by a significant ( P < 0.01) decrease in
the sperm count in the chromium-deficient males to approximately
half of the count in supplemented controls at the age of 8 months
(Anderson & Polansky, 1981).
7.1.4. Other effects of deficiency
Male weanling rats, fed a 10% soya protein ration with a
chromium content of less than 100 µg/kg, developed a visible
opacity of the cornea in one or both eyes. In several studies, the
incidence of this effect ranged from 10 to 15% in deficient rats.
No opacities developed in control animals receiving 2 mg
chromium/kg diet (Roginski & Mertz, 1967).
Chromium deficiency has been shown to reduce the physical
performance of rats under stress. Ten male rats raised on a
chromium-deficient diet (150 µg/kg diet) swam for an average of 250
min, until exhaustion, in contrast with 10 rats receiving a
supplement of 10 mg chromium/kg diet, which were exhausted only
after 320 min (Djahanschiri, 1976).
7.1.5. Mechanism of action of chromium as an essential nutrient
7.1.5.1. Enzymes, nucleic acids, and thyroid
Chromium is present in nucleic acids in very high
concentrations, but the function of these is not clear at present
(Mertz, 1969). However, recent work suggests a biological function
of chromium in nucleic acid metabolism (Okada et al., 1984).
Ribonucleic acid synthesis in mouse liver was significantly
increased by as little as 1 µmol trivalent chromium, in the
presence of DNA or chromatin (Okada et al., 1981). These effects
were also present when the DNA or chromatin were first complexed
with chromium prior to incubation. However, prior complexation of
RNA polymerase with chromium depressed activity. These effects
were obtained in vitro with a concentration (52 µg/litre) that is
similar to physiological levels. Goncharov (1968) presented data
suggesting that chromium is involved in the function of the thyroid
gland. These findings have been supported by Lifschitz et al.
(1980).
An oligopeptide with a relative molecular mass of 1480, which
was crystallized from liver tissue, had a specific affinity for
chromium (Wu, 1981).
7.1.5.2. Interaction of chromium with insulin
The interaction of chromium with insulin has been extensively
studied and can therefore be presented in some detail, but this
does not imply that this is the only, or the most important,
function of chromium.
The effects of chromium in vitro, and probably in vivo, depend
on the presence of endogenous or exogenous insulin, no effects
having been demonstrated in in vitro systems that did not either
depend on, or contain, insulin. Chromium deficiency causes an
impaired response to added insulin in rat epididymal fat tissue,
and, when glucose uptake or glucose oxidation or utilization for
lipid synthesis is measured, the dose-effect curve is flat.
Addition of suitable chromium compounds significantly increases the
slope of the curve (Fig. 3). This demonstrates the true
potentiation of the insulin action and indicates that chromium
alone does not act as an insulin-like substance (Mertz et al.,
1961; Mertz & Roginski, 1971; Mertz, 1981). Chromium was also
shown to stimulate the transport of D-galactose into epididymal fat
cells. This suggests cell transport, the first step of sugar
metabolism, as a major site of action for chromium (Mertz &
Roginski, 1963). Insulin-potentiating effects have also been
observed on the swelling of liver mitochondria (Campbell & Mertz,
1963) and on glucose utilization in isolated rat lens (Farkas &
Roberson, 1965).
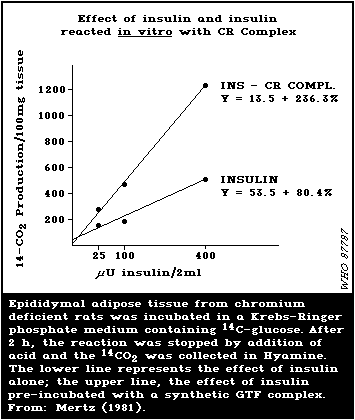 Stimulation of the effects of insulin has been observed in a
glucose-independent, but insulin-responsive, system. Significantly
more alpha-amino isobutyric acid (a non-metabolizable amino acid
analogue) was incorporated into the heart and liver tissue of
chromium-supplemented male rats than in the tissues of chromium-
deficient controls, in response to the in vivo injection of the
labelled analogue and insulin (Roginski & Mertz, 1969).
These observations suggest a peripheral action of chromium to
facilitate the action of insulin; no evidence has been produced
indicating that chromium plays any role in the production, storage,
or release of insulin by the pancreas. Thus, the primary result of
chromium deficiency is a diminution in the effectiveness of
insulin. The resulting metabolic impairment may be compensated for
by increased insulin production in some cases, resulting in
elevated concentrations of the hormone, but not enough data exist
from experimental animal studies to assess the action of the
element on insulin metabolism. More information is available for
human subjects and this is discussed in section 8.1. The
interaction between chromium, insulin, and receptor sites of liver
mitochondrial membranes was studied using polarographic techniques.
The results formed the basis for the hypothesis that chromium may
facilitate bond formation between the intra-chain disulfide of
insulin and sulfur-containing groups of the receptors, by
participating in a ternary complex (Christian et al., 1963).
This hypothesis is consistent with results of studies on rats
fed, either a low-chromium Torula yeast diet or a brewer's yeast
diet known to be adequate in chromium. While the insulin-binding
capacity of hepatocytes was not significantly different, the
insulin affinity of the cells was significantly greater ( P < 0.01)
for the chromium-adequate rats than for the deficient Torula yeast
rats (Steele et al., 1977b).
7.1.6. Chromium nutritional requirements of animals
In the preceding sections, studies were evaluated in which the
effects of chromium supplementation were determined in animals that
were at least marginally chromium deficient. In other studies, the
effects of chromium were investigated in animal systems in which
the existence of chromium deficiency was either not ascertained or
not investigated. Before these studies are described and
interpreted for the determination of chromium nutritional
requirements of animals, it is helpful to consider them against the
background of Venchikov's (1974) model. This model is generally
applicable to trace element effects defining 3 zones of action, the
zone of biological action, that of pharmacodynamic action, and that
of toxicity (Fig. 4). The biological zone, in response to
supplements with low amounts of an element, represents the
correction of a deficiency and the resulting level of biological
activity is that of optimal function. Increasing the amount of
supplement further may lead to a certain depression, followed by a
zone of new, increased activity, in which the element no longer
acts as an essential nutrient, but as a drug. Still greater
supplements, beyond the homeostatic control capability of the
organism produce toxic effects and death. Because all the studies
described subsequently involved amounts of chromium supplements
that were higher than the levels normally needed to correct a
deficiency, it is possible, according to Venchikov's definition,
that the observed effects might be pharmacological.
Stimulation of the effects of insulin has been observed in a
glucose-independent, but insulin-responsive, system. Significantly
more alpha-amino isobutyric acid (a non-metabolizable amino acid
analogue) was incorporated into the heart and liver tissue of
chromium-supplemented male rats than in the tissues of chromium-
deficient controls, in response to the in vivo injection of the
labelled analogue and insulin (Roginski & Mertz, 1969).
These observations suggest a peripheral action of chromium to
facilitate the action of insulin; no evidence has been produced
indicating that chromium plays any role in the production, storage,
or release of insulin by the pancreas. Thus, the primary result of
chromium deficiency is a diminution in the effectiveness of
insulin. The resulting metabolic impairment may be compensated for
by increased insulin production in some cases, resulting in
elevated concentrations of the hormone, but not enough data exist
from experimental animal studies to assess the action of the
element on insulin metabolism. More information is available for
human subjects and this is discussed in section 8.1. The
interaction between chromium, insulin, and receptor sites of liver
mitochondrial membranes was studied using polarographic techniques.
The results formed the basis for the hypothesis that chromium may
facilitate bond formation between the intra-chain disulfide of
insulin and sulfur-containing groups of the receptors, by
participating in a ternary complex (Christian et al., 1963).
This hypothesis is consistent with results of studies on rats
fed, either a low-chromium Torula yeast diet or a brewer's yeast
diet known to be adequate in chromium. While the insulin-binding
capacity of hepatocytes was not significantly different, the
insulin affinity of the cells was significantly greater ( P < 0.01)
for the chromium-adequate rats than for the deficient Torula yeast
rats (Steele et al., 1977b).
7.1.6. Chromium nutritional requirements of animals
In the preceding sections, studies were evaluated in which the
effects of chromium supplementation were determined in animals that
were at least marginally chromium deficient. In other studies, the
effects of chromium were investigated in animal systems in which
the existence of chromium deficiency was either not ascertained or
not investigated. Before these studies are described and
interpreted for the determination of chromium nutritional
requirements of animals, it is helpful to consider them against the
background of Venchikov's (1974) model. This model is generally
applicable to trace element effects defining 3 zones of action, the
zone of biological action, that of pharmacodynamic action, and that
of toxicity (Fig. 4). The biological zone, in response to
supplements with low amounts of an element, represents the
correction of a deficiency and the resulting level of biological
activity is that of optimal function. Increasing the amount of
supplement further may lead to a certain depression, followed by a
zone of new, increased activity, in which the element no longer
acts as an essential nutrient, but as a drug. Still greater
supplements, beyond the homeostatic control capability of the
organism produce toxic effects and death. Because all the studies
described subsequently involved amounts of chromium supplements
that were higher than the levels normally needed to correct a
deficiency, it is possible, according to Venchikov's definition,
that the observed effects might be pharmacological.
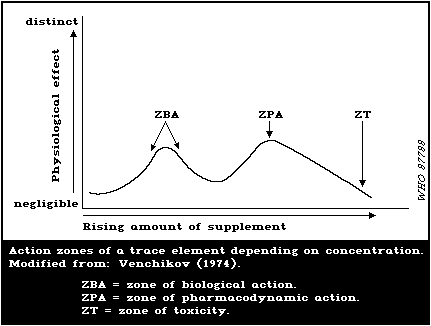 Tuman & Doisy (1977) studied the effects of yeast concentrates
of high chromium (glucose tolerance factor) content and of
synthetic chromium complexes with GTF activity (Tuman et al., 1978)
in mice, raised on a presumably complete commercial stock diet.
Six animals were used for each test, either genetically diabetic
mice or their control litter mates of the C57Bl-KSI strain.
Injections of either 5 mg of the GTF-containing yeast extracts or
0.1 mg of the synthetic chromium complex, acutely reduced the
elevated plasma-glucose levels in the diabetic and the non-fasting
normal mice by 10 - 38% of the initial values ( P < 0.01) and the
plasma-triglycerides by 26 - 56% ( P < 0.01), compared with control
mice injected with saline. Injection of insulin into diabetic mice
produced only an 11 - 18% decrease in plasma-glucose levels,
whereas injection of the GTF-containing extract together with
insulin reduced plasma-glucose levels by 39 - 51% and plasma-
triglyceride levels by 76% (Table 11).
The results suggest either a much higher increase in the
chromium requirement of the genetically diabetic mice or their
inability to use chromium in the diet.
Table 11. Acute effects of GTF and exogenous insulin on non-
fasting plasma-glucose and plasma-triglyceride (TG) concentrations
in 19-week-old genetically diabetic micea
------------------------------------------------------------------
Treatment Plasma- deltaGlucose Plasma- deltaTG
glucoseb triglyceridesb
(mg/litre) (mg/litre)
------------------------------------------------------------------
saline 11 120 ± 3960 ± 160(5)c
490(5)c
GTF 9320 ± -180 (16%) 2790 ± 170(5)d -117 (30%)
280(5)d
insulin 9840 ± -128 (12%) 3020 ± 400(5) - 94 (24%)
410(5)
insulin 7060 ± -406 (37%) 940 ± 220(5)e -302 (76%)
and GTF 840(5)e
------------------------------------------------------------------
a Modified from: Tuman & Doisy (1977).
b Glucose and triglyceride values represent mean ± SEM for 6 mice in
each treatment group. Dose of GTF was 5 mg (WL-10-AT) administered
intraperitoneally, 12 h prior to collection of blood. Lente insulin
(0.1 U per mouse) was administered subcutaneously, 12 h prior to
collection of blood. Data were treated by analysis of variance to
detect differences between the various treatment groups; independent
orthogonal comparisons were performed in the following groups ( P value
indicates level of significance for each comparison).
c Saline versus all other treatments, P < 0.005.
d GTF versus insulin, P < 0.05.
e GTF and insulin alone versus combined GTF and insulin, P < 0.005.
Thus, GTF and insulin > GTF = insulin > saline.
Steele & Rosebrough (1979) reported a significant stimulation
of the growth rate of one-week-old turkey poults (both sexes) by
supplementation of a practical ration with 20 mg chromium (as
chloride)/kg. The weight gains within the 2-week study were 235 g
and 267 g for the 60 controls and 60 supplemented turkeys,
respectively ( P < 0.001). As the practical ration contained
ground yellow corn and soybean meal, limestone, and dicalcium
phosphate, chromium deficiency would appear unlikely. The amount
of the chromium supplement (20 mg/kg diet) is quite high, and
further studies are needed to decide whether the observed effects
were of a pharmaco-dynamic nature or truly nutritional, i.e.,
correcting a deficiency. A similar interpretation should be
applied to a report of improved egg quality in the laying hen
(Jensen et al., 1978).
The quantitative aspects of the effects of chromium on animals
can be summarized as follows: normal rats fed semi-purified, semi-
synthetic rations, with Torula yeast or individual proteins and
sucrose or starch as the source of carbohydrates, develop mild
signs of deficiency at a dietary level of 100 - 150 µg chromium/kg.
To prevent deficiency, most authors used very high supplements of
several mg/kg diet and did not determine the biological
availability of the chromium complexes used for the
supplementation. Diets containing raw ingredients and supplying
chromium levels between 0.5 and 1 mg/kg do not induce signs of
deficiency and probably meet the requirement of the rat. A very
tentative estimate of the dietary chromium need of the rat and
probably the mouse would be approximately 0.5 mg/kg diet. This
estimate should be interpreted with caution, because of the lack of
knowledge concerning the biological availability of chromium and of
its interaction with dietary constituents.
7.2. Toxicity Studies
The toxicology of chromium compounds has been reviewed by the
US National Academy of Science (US NAS, 1974a), Langard & Norseth
(1979), the International Agency for Research on Cancer (IARC,
1980), Langard (1980a, 1982), and Burrows (1983).
In discussing toxicological problems, it is important to
differentiate between the various oxidation states of chromium and
its compounds. Trivalent chromium, when administered to animals in
food or water, does not appear to induce any harmful effects, even
when given in large doses (US NAS, 1974a) (section 7.2.1). Acute
and chronic toxic effects of chromium are mainly caused by
hexavalent compounds. Since it has been shown that both industrial
trivalent chromium compounds as well as reagent-grade trivalent
chromium compounds can be contaminated by hexavalent chromium
(Petrilli & DeFlora, 1978a; Levis & Majone, 1979), the evaluation
of experimental studies becomes difficult, especially when the
purity of the chemical compounds used is not known.
Discrimination between the biological effects, caused by
hexavalent chromium and trivalent chromium is difficult, because,
after penetration of membranes in tissues, hexavalent chromium is
immediately reduced to trivalent chromium (Gray & Sterling, 1950;
US NAS, 1974a), and it is not evident whether the observed
phenomena are caused by this reduction or even by the trapping of
trivalent chromium by ligands after uptake in the cells. Another
problem in evaluating the data is associated with the route of
administration. Hexavalent chromium, introduced by the oral route,
is partly reduced to trivalent chromium by acidic gastric juice
(Donaldson & Barreras, 1966; DeFlora & Boido, 1980); thus, the
effects or lack of effects observed may be caused mainly by
trivalent chromium and not by the hexavalent chromium, actually
administered.
7.2.1. Effects on experimental animals
Many local effects on human beings have been reported (section
8.3), but only a few studies have verified these effects in
experimental animals. A comprehensive survey of hexavalent
chromium-induced effects is given in Table 12 (US NAS, 1974a). For
most studies, details were not given of the length of exposure,
number of treated animals and controls, etc. Diagnoses were stated
without presenting all the original data. Thus, in this section,
some papers will be discussed that refer to the most prominent
local and systemic effects to support and clarify the effects shown
in human beings.
It is evident that the toxicity of hexavalent chromium in
animals varies with the route of entry into the body. Low
concentrations of hexavalent chromium may be tolerated, when
administered in the feed or drinking-water, the extent of
absorption being a factor of importance. For example, rats
tolerated hexavalent chromium in drinking-water at 25 mg/litre, for
1 year, and dogs did not show any effects from chromium
administered as potassium chromate at 0.45 - 11.2 mg/litre over a
4-year period (US NAS, 1974a). However, oral exposure of both male
and female rabbits to sodium dichromate (0.1% solution, 0.2 - 5
mg/kg body weight, for up to 545 days) resulted in significant
morphological changes in the gonads, including atrophy of the
epithelium and dystrophic alterations of the Sertoli and Leydig
cells in the testes, and sclerotic and atrophic changes in ovaries
(Kucher, 1966).
Larger doses of hexavalent chromium are highly toxic and may
cause death, especially when injected iv, subcutaneously (sc), or
intragastrically. The LD50 of chromium compounds was determined
for several experimental animal species. The LD50 of potassium
dichromate (hexavalent chromium), administered orally (stomach
tube) to rats, was 177 mg/kg body weight in males and 149 mg/kg
body weight in females (Hertel, 1982). When injected iv in mice
(sex not given), the LD50 of chromium carbonyl was 30 mg/kg body
weight (IARC, 1980).
Performing a life-time inhalation study on the rat, Glaser et
al. (1984) found an LC50 for Na2Cr2O7 of 28.1 mg/m3 (range, 16.7 -
47.3 mg/m3). Assuming a deposition rate in the lung of 30% of the
dose administered, the LC50 dose was 1 mg/kg body weight in male
and 1.2 mg/kg in female rats.
Table 12. Effects of hexavalent chromium in animalsa
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit, inhalation chromates 1 - 50 mg/m3 14 h/day for pathological Lukanin (1930)
cat 1 - 8 months changes
in the lungs
Rabbit inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day none Lehmann (1914)
as dichromate for 5 days
Cat inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day bronchitis, Lehmann (1914)
as dichromate for 5 days pneumonia
perforation of
nasal septum
Mouse inhalation mixed dust 1.5 mg/m3 4 h/day, no tumours Baetjer et al.
containing as CrO3 5 days/week, (1959a);
chromates for 1 year Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 16 - 27 mg/m3 1/2 h/day tumours in Baetjer et al.
containing as CrO3 intermit- some strains (1959a);
chromates tently Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 7 mg/m3 37 h over increased Baetjer et al.
containing as CrO3 10 days tumour rate (1959a);
chromates Steffee &
Baetjer (1965)
Rat inhalation mixed dust 7 mg/m3 37 h over barely Baetjer et al.
containing as CrO3 10 days toleratedb (1959a);
chromates Steffee &
Baetjer (1965)
Rabbit, inhalation mixed dust 5 mg/m3 4 h/day, none marked Baetjer et al.
guinea- containing as CrO3 5 days/week, (1959a);
pig chromates for 1 year Steffee &
Baetjer (1965)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rat, inhalation hexacarbonyl 1.6 mg/m3 4 months, anaemia; lipid Roschina (1976)
rabbit 4 h, 5 days and/or protein
a week dystrophia
in liver
and kidneys
Rat, inhalation hexacarbonyl 0.16 mg/m3 4 months, anaemia; no Roschina (1976)
rabbit 4 h, 5 days biochemical
a week or morphological
effects
Rat inhalation hexacarbonyl 35 mg/m3 30 min 100% death Roschina (1976)
Rat inhalation dichromates 0.006 - 0.2 28 days/ increase in Glaser et al.
mg/m3 90 days lung-macrophages (1985)
23 h/day
7 days/week lymphocytes
immunoglobulin,
reduced Fe2O3
lung clearance
Rat intratra- dichromates 5 per week up to 30 toleratedc Steinhoff
cheal in- 0.01 - 0.25 months et al. (1983)
stillation mg/kg
Rat intratra- dichromates 1 per week up to 30 tolerated; Steinhoff
cheal in- 0.05 - 1.25 months 1.25 mg/kg et al. (1983)
stillation mg/kg harmful
Rat oral potassium 500 mg/litre daily maximal Gross & Heller
chromate in non-toxic (1946)
drinking-water concentration
Rat, oral zinc chromate 10 g/kg daily maximal Gross & Heller
mouse in feed non-toxic (1946)
(mature) concentration
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit oral sodium di- 0.2 - 5.0 mg/kg 545 days morphological Kucher (1966)
chromate changes in
gonads (testes:
atrophy of
epithelium,
dystrophic
alterations of
Sertoli &
Leydiz alls.
Ovaries:
sclerotic and
atrophic
changes)
Rat oral zinc chromate 1.2 g/kg daily maximal Gross & Heller
(young) in feed non-toxic (1946)
concentration
Rat oral potassium 1.2 g/kg daily maximal Gross & Heller
(young) chromate non-toxic (1946)
in feed concentration
Dog, oral monochromate 1.9 - 5.5 mg 29 - 685 none harmful Lehmann (1914)
cat, or dichromates chromium/kg days
rabbit body weight per
day 1 mg chrom-
ium equivalent
to 2.83 mg
K2Cr2O7
or 3.8 mg
K2CrO4
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog oral potassium 1 - 2 g as daily fatal in 3 Brard (1935)
dichromate chromium months anaemia
Dog stomach potassium 1 - 10 g as - rapidly fatald Brard (1935)
tube dichromate chromium
Monkey subcutan- potassium 0.02 - 0.7 g in - fatald Hunter & Roberts
eous dichromate 2% solution (1933)
Dog subcutan- potassium 210 mg as - rapidly fatal Brard (1935)
eous dichromate chromium
Guinea- subcutan- potassium 10 mg - lethald Ophüls (1911a)
pig eous dichromate Ophüls (1911b)
Rabbit subcutan- potassium 1.5 cc of 1% - 80% fatald Hasegawa (1938)
eous dichromate solution/kg body
weight
Rabbit subcutan- potassium 20 mg - lethald Ohta (1940)
eous dichromate
Rabbit subcutan- potassium 0.5 - 1 cc of - nephritisd Ohta (1940)
eous dichromate 0.5% solution/kg
body weight
Rabbit, subcutan- sodium 0.1 - 0.3 g as - rapid deathd Priestley
guinea- eous or chromate CrO3 fall in blood (1877)
pig intravenous pressure
Rabbit intra- potassium 0.7 cc of 2% - lethald Mazgon (1932)
venous solution/kg 8-10 days after
body weight injection
Dog intra- potassium 10 grains - instant death Gmelin (1826)
venous chromate
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog intra- potassium 1 grain - none marked Gmelin (1826)
venous chromate
Dog intra- potassium 210 mg as - rapidly fatal Brard (1935)
venous dichromate chromium
Dog intra- potassium 3 mg/100 cc 2 doses marked renal Hepler & Simonds
venous dichromate blood per dose damage (1946); Simonds
& Hepler (1945)
---------------------------------------------------------------------------------------------------------
a Modifed from: US NAS (1974a).
b Pathological changes in experimental and control rats, 101 weeks after exposure.
c The same weekly dose distributed over 5 days was clearly better tolerated than a single weekly
administration.
d Renal damage.
A local corrosive action of hexavalent chromium on the skin,
similar to that seen in man, was described by Samitz & Epstein
(1962), who induced chrome ulcers in guinea-pigs at 4 trauma sites,
with daily exposure to 0.34 MK2Cr2O7 solution for 3 days. Mosinger
& Fiorentini (1954) showed the same effects using potassium
chromates.
Following parenteral administration, the most common systemic
effects of chromium were parenchymatous changes in the liver and
kidney (Mosinger & Fiorentini, 1954). Later studies showed
selective damage in the renal proximal convoluted tubules, without
evidence of glomerular damage, as demonstrated after one single sc
injection of potassium dichromate of 10 mg/kg body weight (Schubert
et al., 1970) or after one single intraperitoneal (ip) injection of
sodium chromate of 10 or 20 mg/kg body weight (Evan & Dail, 1974).
Effects have also been found in fish (Strik et al., 1975). After 32
days of continuous exposure to 0.1, 1, or 10 mg hexavalent chromium
(as potassium dichromate)/litre, the fish Rutilus rutilus developed
lysis of the intestinal epithelium with haemorrhages as well as
hypertrophy and hyperplasia of the gill epithelium.
Franchini et al. (1978) found an increase in urinary protein,
lysozyme, glucose, and beta-glucuronidase in rats after a single sc
injection of potassium dichromate at 15 mg/kg body weight. After
sc injection (3 mg/kg body weight), every other day for 2 - 8
weeks, the authors observed a correlation between the chromium
contents of the renal cortex and chromium clearance.
Five-week-old male Wistar rats of the strain TNO-W-74 were
continuously exposed in inhalation chambers to submicron aerosols
of sodium dichromate at concentrations ranging from 25 (low level)
to 200 µg chromium/m3 (high level) (Glaser et al., 1985). Exposure
for 28 days to 25 or 50 µg chromium/m3 resulted in "activated"
alveolar macrophages with stimulated phagocytic activity, and
significantly elevated antibody responses to injected sheep red
blood cells. After 90 days of low-level exposure, there was a more
pronounced effect on the activation of the alveolar macrophages,
with increased phagocytic activity. However, inhibited phagocytic
function of the alveolar macrophages was seen at the high
hexavalent chromium exposure level (200 µg/m3). In rats exposed to
this chromium aerosol concentration for 42 days, the lung clearance
of inert iron oxide was significantly reduced. The humoral immune
system was still stimulated at a low chromium aerosol concentration
of 100 µg/m3, but significantly depressed at 200 µg chromium/m3.
Exposure of rats, through inhalation, to chromium carbide or
chromium boride dust at very high levels (300 - 350 mg/m3 for each
substance) for 3 months (2 h/day) resulted in effects on the
vascular system of the lungs, e.g., endothelial hyperplasia.
Bronchitis and a decrease in the blood-haemoglobin concentration
were also observed (Roschina, 1964). The effects of chromium boride
were more pronounced than those of chromium carbide.
Steinhoff et al. (1983) performed an intracheal injection study
on rats (930 rats, 30 months, 0.05 - 1.25 mg Na2Cr2O7/kg body
weight per week; 1.25 mg CaCrO4/kg body weight per week). Half of
the rats were intratracheally injected once a week and the other
half received the same weekly dose distributed over 5 injections
per week. In rats receiving doses 5 times a week, there were some
significant changes in levels of total plasma-protein and
-cholesterol, in some haematological variables, in organ weights,
and in survival times in females. Only male rats receiving 1.25 mg
sodium dichromate/kg body weight, once a week, showed a sharp
reduction in body weight, female rats being less affected. Rats
receiving calcium chromate in the same dose showed reduced body
weight but to a lesser extent. The weight of lung and trachea was
increased by both substances in all doses.
Marked histopathological changes (congestion, fairly large
areas of focal necrosis, bile duct proliferation) described after
long-term exposure of rabbits to hexavalent chromium (ip injection
of 2 mg chromium/kg body weight per day for 6 weeks) (Tandon et
al., 1978), as well as the increase in hepatic metallothionein and
decrease in cytochrome P-450 levels after ip injection of 400 µmol
chromium/kg per day (type of chromium compound not mentioned)
(Eaton et al., 1980) need further confirmation. The finding of an
accumulation of hexavalent chromium in the reticuloendothelial
system including bone marrow (Baetjer et al., 1959b; Langard, 1977)
may be of importance for a disturbed blood picture.
Merkurieva et al. (1980a,b) studied the effects of potassium
dichromate in the drinking-water on the activities of different
enzymes in rats. Exposure included daily doses of 0.0005, 0.005,
0.05, or 0.5 mg/kg body weight for up to 6 months. After 20 days
of exposure at the highest dose level, enzyme activities increased
by 15 - 28% in liver microsomes (inosine-5-diphosphatase),
lysosomes (beta-D-galactosidase), and cytosol (lactate dehydrogenase
(EC 1.1.1.27)). For some of these enzymes, as well as for
acetylesterase (EC 3.1.1.6), increases in activity of up to 54%
were found in the gonads, kidneys, seminal fluid, and serum. At a
dose of 0.05 mg/kg, the only statistically significant finding was
a 54% increase in the activity of acetylesterase in the gonads.
After a 6-month exposure at this dose level, there was a 70%
increase in lactate dehydrogenase activity in the seminal fluid as
well as a 50% increase in free beta-galactosidase in the liver. There
were no changes in enzyme activities at the two lowest dose levels.
Painting of rat skin with an aqueous solution of potassium
dichromate (0.5%), daily, for 20 days, resulted in a local
inflammatory reaction, an increased level of hexose glyco-proteins
in the skin and serum, and an elevated concentration of serotonin
in the skin and liver (Merkurieva et al., 1982). Most of these
effects were also seen earlier in the exposure period, though they
were not as pronounced. Ten days after the start of exposure, a
nearly 3-fold increase in the serum-acetylcholine concentration
occurred together with decreased acetylcholinesterase activity.
Thus, the data of Merkurieva et al. show systemic effects following
both oral and dermal exposure to hexavalent chromium.
Cats fed chromic phosphate or oxydicarbonate at 50 - 1000 mg/day
for 80 days did not exhibit signs of illness or tissue damage.
Similarly, toxic reactions were not observed in rats administered
drinking-water containing 25 mg trivalent chromium/litre, for 1
year, or 5 mg trivalent chromium/litre throughout their lifetime
(US NAS, 1974a). The toxicity of trivalent chromium is so low that
even by parenteral administration, a chromic acetate level of 2.29
g/kg body weight or a chromic chloride level of 0.8 g/kg body
weight is required to kill mice. Even very large doses given
intragastrically were not fatal for dogs. Brard (1935) reported
that 10 or 15 g of chromium as chromic chloride proved fatal in one
dog (US NAS, 1974a). Some fatal doses of trivalent chromium
compounds reported in the literature are listed in Table 13.
Rats exposed through inhalation to chromic oxide (trivalent
chromium) at 42 mg/m3 or to chromic phosphate at 43 mg/m3 (5 h/day
for 5 days/week) for 4 months developed chronic irritation of the
bronchus and lung parenchyma, and dystrophic changes in the liver
and kidney (Blokin & Trop, 1977).
Inhalation exposure of rats (number not given) to dusts
containing 36 or 50% chromite for 4 months (2 h/day), at
concentrations of 375 - 400 mg/m3, resulted in thickening of the
walls of pulmonary vessels and bronchi (Roschina, 1959). The high
exposure levels in these studies make it difficult to evaluate the
effects.
Inhalation studies have also been performed with chromium
carbonyl, where chromium is in the 0 oxidation state (Roschina,
1976). Twelve rabbits and 48 rats were exposed for 4 months (4
h/day, 6 days/week) at a concentration of 1.6 or 0.16 mg/m3. At
both exposure levels, there was loss of body weight (25 and 12%,
respectively) as well as anaemia and leukocytosis. In the higher
exposure group, the animals showed an elevated gamma-globulin level
in serum and an increased transaminase activity. The contents of
cholesterol and SH-groups were reduced, and there was a decrease in
cholinesterase (EC 3.1.1.7) activity. Lipid and/or protein
dystrophy were noted in several organs, e.g., in the liver and
kidneys. No such effects were detected in the animals in the low-
exposure group.
Table 13. Fatal doses of trivalent chromium in animalsa
---------------------------------------------------------------------------------------------
Animal Number of Routeb Compound Chromium Effect Reference
animals dose (g/kg)
---------------------------------------------------------------------------------------------
Dog 2 sc chromic chloride 0.8 fatal Brard (1935)
Rabbit 1 sc chromic chloride 0.52 fatal Brard (1935)
Rat 38 iv chrome alum 0.01 - 0.018 LD50 Mertz et al.
chromium-hexaurea (1965a)
chloride
Mouse -c iv chromic chloride 0.8 MLDd Windholz et al. (1960)
Mouse -c iv chromic acetate 2.29 MLD Windholz et al. (1960)
Mouse -c iv chromic chloride 0.4 MLD Schroeder (1970)
Mouse -c iv trivalent chromium ? 0.25 - 2.3 MLD Windholz et al. (1960)
Mouse -c iv chromic sulfate 0.247 MLD Windholz et al. (1976)
Mouse -c iv chromic sulfate 0.085 MLD Schroeder (1970)
Mouse -c iv chromium carbonyl 0.03 LD50 Schroeder (1970)
---------------------------------------------------------------------------------------------
a Modified from: US NAS (1974a).
b sc = subcutaneous; iv = intravenous.
c No figures given.
d Minimum lethal dose.
7.2.1.1. Carcinogenicity
Various types of chromium chemicals, methods of administration,
and species of animals have been studied (IARC, 1980a).
Ideally, carcinogenicity should be tested with the methods
recommended by IARC (1980b), but, in many of the early studies,
this was not done. Carcinomas of the lung have been reported in
animals as a result of the administration of chromium chemicals.
Hueper (1958) found 2 squamous cell carcinomas and one
carcinosarcoma in 25 rats following intra-pleural injection of
chromite ore roast. After intrapleural implantation of strontium
chromate lasting 27 months, Hueper (1961) found tumours (type
unspecified) in 17/28 rats. Laskin et al. (1970) and Levy & Venitt
(1975) produced a number of bronchogenic carcinomas by implanting
pellets of cholesterol mixed with various chromium compounds
encased in a wire mesh cage in the bronchi of rats. Calcium and
zinc potassium chromate produced a number of bronchogenic
carcinomas, but soluble chromates and trivalent chromium chemicals
failed to produce cancer.
Using the same technique, Levy & Martin (1983) tested 21
different chromium-containing materials (pigments, intermediates,
and residues from the bichromate-producing industry, relatively
pure crystalline compounds) in 2250 random-bred rats and found that
chromates, described as sparingly soluble, were carcinogenic in the
rat lung. These materials included strontium and calcium chromate
and, to a far lesser extent, certain forms of zinc chromate.
Barium and lead chromate evoked only a very weak carcinogenic
response compared with strontium and calcium chromate. In the
study of Laskin et al. (1970), it was shown that chromium trioxide
produced hepatocellular carcinomas in 2/100 rats (controls, 0/24).
After inhalation of 13 mg calcium chromate/m3 (5 h/day, 5
days/week, for lifetime), Nettesheim et al. (1971) found 14 lung
adenomas in 136 treated mice and 5 in 136 untreated controls, but
no carcinomas. Steffee & Baetjer (1965), performing inhalation
studies on rats, mice, guinea-pigs, and rabbits (inhalation of
mixed chromate dust, corresponding to 3 - 4 mg CrO3/m3, 4 - 5
h/day, 4 days/week, for lifetime, or 50 months, respectively) could
only find 3 alveologenic adenomas in 50 treated guinea-pigs.
Laskin (1972) and Laskin et al. (1970) found 1 squamous cell
carcinoma of the lung, 1 of the larynx and 1 "peritruncal tumor" in
rats (inhalation of calcium chromate, 2 mg/m3, 589 exposures of 5 h
over 891 days) and 1 squamous cell carcinoma and 1 papilloma of the
larynx in hamsters. The number of treated animals was not
specified in either paper. Steinhoff et al. (1983) performed
intratracheal instillations of chromates in rats for 30 months with
one treatment/week and the same weekly dose distributed over 5
treatments/week (Table 14). In 880 exposed rats, 28 adenomas of
alveolar-bronchiolar origin (benign) and 12 malignant tumours (3
adenocarcinomas and 9 squamous cell carcinomas) were found. All
lung tumours developed very late and were only detected at the end
of the lifetime study, often in lungs with callosities. The
tumours were tiny and none of them caused the animal to die.
Sodium dichromate was not carcinogenic after exposure on 5
days/week. With calcium chromate, the carcinogenic effect was more
pronounced after treatment once per week, than after treatment 5
times per week.
Table 14. Incidence of benign and malignant lung tumours among
880 rats intracheally injected with Na dichromate and Ca chromatea
----------------------------------------------------------------
Dose Number of Incidence of lung tumours
(mg/kg) injections benign malignant
per week
----------------------------------------------------------------
Na2Cr2O7 x 2H20 0.25 5 none none
Na2Cr2O7 x 2H20 1.25 1 12 8
CaCrO4 0.25 5 5 1
CaCrO4 1.25 1 11 3
----------------------------------------------------------------
a From: Steinhoff et al. (1983).
Hueper (1961) reported unspecified tumours at implantation
sites in 12/34 rats, following intramuscular (im) implantation of
sintered calcium chromate (25 mg). No tumours were observed in 32
control animals. The same author found implantation-site tumours in
15/33 animals treated with strontium chromate compared with no
tumours in 32 control animals. Single sc injections of 30 mg lead
chromate (Maltoni 1974, 1976) resulted in injection-site sarcomas
in 26/40 treated rats. After administration of the same amount of
lead chromate oxide, injection-site sarcomas were found in 27/40
rats; no sarcomas were found in 60 vehicle controls. Following 9
im injections of 8 mg lead chromate, Furst et al. (1976) found
injection-site sarcomas in 31/47 treated rats, and renal carcinomas
in 3/24 treated rats; no sarcomas were found in 0/22 vehicle
controls. Heath et al. (1971) found injection-site sarcomas and
other tumours in 7/74 treated rats after im injection of 28 mg
cobalt-chromium alloy.
Administration of low levels of trivalent chromium acetate (5
mg/litre in the drinking-water) to mice and rats for the lifetime
did not result in increased tumour incidences compared with
controls (Schroeder et al., 1964, 1965).
Trivalent chromium oxide incorporated in bread at
concentrations of 10, 20, or 50 g/kg and fed to BD rats did not
increase tumour incidence compared with controls (Ivankovic &
Preussmann, 1975). Hexavalent chromium compounds have not been
tested by oral administration.
IARC (1980a) summarized the data of all available references
and concluded as follows: "There is sufficient evidence for the
carcinogenicity of calcium chromate and some relatively insoluble
chromium (VI) compounds (sintered calcium chromate, lead chromate,
strontium chromate, sintered chromium trioxide and zinc chromate)
in rats. There is limited evidence for the carcinogenicity of lead
chromate (VI) oxide and cobalt-chromium alloy in rats. The data
were inadequate for the evaluation of the carcinogenicity of other
chromium (VI) compounds and of chromium (III) compounds".
(a) Interaction with other factors
The possibility that chromium may act synergistically with
other agents in the production of cancer has been tested in several
studies. Nettesheim et al. (1970) pretreated one group of mice
with 100 R whole-body radiation and infected another group with PR8
influenza virus. These mice and control mice were exposed through
inhalation to chromium oxide dust for 6 - 18 months. No measurable
effects of either of the pretreatments were found on the incidence
of tumours. In a similar study with inhalation of calcium chromate
(CaCrO4, 5 h/day, 5 days per week for the lifetime), pre-exposure
to X-rays did not affect the tumour rate, but PR8 influenza
infection reduced tumour incidence (Nettesheim et al., 1971).
Steffee & Baetjer (1965) also found that the infection of rats with
PR8 influenza virus associated with intratracheal injection of
chromates did not lead to the production of tumours. Chromite ore
injected iv did not affect the development of tumours induced by iv
injection of 20-methyl-cholanthrene (Shimkin & Leiter, 1940).
Thus, at present, there are not sufficient data to suggest that
chromium acts synergistically with virus infections, ionizing
radiation, or other chemical carcinogens to produce cancers.
7.2.1.2. Genotoxicity
The mutagenicity of chromium compounds was reviewed by IARC
(1980a), Petrilli & DeFlora (1980), Levis & Bianchi (1982), and
Baker (1984). Present knowledge, in the light of recent findings,
is summarized in Table 15 (hexavalent chromium) and Table 16
(trivalent chromium).
When evaluating the results of chromium mutagenicity tests, it
is necessary to take into consideration several properties of the
tested compound (oxidation state, solubility, ability to penetrate
cell membranes, intracellular stability, and reactivity with
cellular components). Most of the in vitro mutagenicity
experiments have been performed with chromium chemicals, following
the introduction of the reverse mutation test by Ames (1973).
Bacterial strains of Salmonella typhimurium (Petrilli & DeFlora,
1977) containing mutants that require histidine for growth, and
Escherichia coli (Venitt & Levy, 1974) with mutants requiring
tryptophan for growth have been used. In each case, the bacterial
cultures have been incubated with the test chromium compounds and
the number of revertant colonies scored. The chromium compounds
studied included both hexavalent and trivalent compounds; a survey
of the results is given in Table 17 (DeFlora, 1981). Zhurkov
(1981) performed an Ames test to study the mutagenic activity of
potassium dichromate (K2Cr2O7) with S. typhimurium TA 1535 and TA
1538. A medium degree of mutagenic activity without metabolic
activation was shown by the strain TA 1535.
Only hexavalent chromium compounds have shown mutagenic
effects. Using the Salmonella /mammalian microsome tests of Ames
et al. (1973), DeFlora (1978) and Löfroth (1978) both showed a
decrease in the mutagenicity of hexavalent chromium in the presence
of an NADPH-generating system, suggesting reduction of mutagenic
hexavalent chromium to trivalent chromium. In this chromate
metabolism, cytochrome P-450 functions as the reductase (Garcia &
Wetterhahn Jennette, 1981). In recent in vitro studies, Wiegand et
al. (1984b) showed that glutathione could reduce hexavalent
chromium to trivalent chromium, without any further cofactors or
metabolizing enzymes. In rats, the most efficient tissue in
decreasing hexavalent chromium mutagenicity was the liver, followed
by the suprarenal glands, kidney, stomach, and lung (Petrilli &
DeFlora, 1980). These studies suggest a possible way of
intracellular detoxification of hexavalent chromium in low doses.
Schoental (1975) suggested that hexavalent chromium caused the
formation of epoxyaldehydes having mutagenic potential. Venitt &
Levy (1974) assumed that chromates belong to the transition-
inducing class of mutagens. They cause both frameshift and base-
pair substitutions (Petrilli & DeFlora, 1980). The hypothesis of
Kazantzis & Lilly (1979), that chromates cause guanine-cytosine to
adenine-thymine transitions in the subsequent round of DNA-
replication, needs further confirmation.
Table 15. Genotoxic activity of chromium (VI) compoundsa
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
DNA degradation CaCrO4 - Casto et al. (1976)
K2Cr2O7 - Bianchi et al. (1983)
PbCrO4 - Douglas et al. (1980)
K2CrO4 + Whiting et al. (1979)
Decreased fidelity PbCrO4, + (1) Levis & Majone (1981)
of DNA synthesis K2Cr2O7
CrO3 + Sirover & Loeb (1976)
K2Cr2O7 + Bianchi et al. (1983)
Microbial DNA K2CrO4, + Yagi & NishioKa (1977)
repair K2Cr2O7
CrO3, K2CrO4, + Kanematsu et al. (1980)
K2Cr2O7
K2CrO4, + Nishioka (1975)
K2Cr2O7
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
CrO3, + Gentile et al. (1981)
K2Cr2O7,
Na2Cr2O7, + Gentile et al. (1981)
(NH4)2Cr2O7
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Microbial gene K2Cr2O7 + (2) Bonatti et al. (1976)
mutation CrO3 + (2) Fukunaga et al. (1982)
Na2Cr2O7, +/-(3) Petrilli & De Flora
CrO3, +/-(3) (1978a,b)
ZnCrO4xZn(OH)2 +/-(3)
K2Cr2O7 + Zhurkov (1981)
Na2Cr2O7 +/-(3) DeFlora (1978)
chromate (4) +/-(3) Löfroth (1978)
dichromate +/-(3)
K2Cr2O7 - Kanematsu et al. (1980)
K2CrO4, + Löfroth & Ames (1978)
K2Cr2O7 +
K2Cr2O7 + Nishioka (1975)
K2CrO4, CaCrO4 + DeFlora (1981)
(NH4)2CrO4,
CrO3,
Na2CrO4, + Venitt & Levy (1974)
K2CrO4
CaCrO4
K2Cr2O7 + Bianchi et al. (1983)
Na2Cr2O7,
ZnCrO4
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
K2CrO4, CaCrO4 + Petrilli & DeFlora
CrO3 (1977)
Na2Cr2O7
K2CrO4 + Green et al. (1976)
PbCrO4, CrO3 + Nestmann et al. (1979)
Mammalian cell K2Cr2O7 + Bianchi et al. (1983)
gene mutation K2Cr2O7, + Newbold et al. (1979)
ZnCrO4 +
PbCrO4 -
K2Cr2O7 + (5) Bonatti et al. (1976)
K2Cr2O7 + Pashin et al. (1982)
NaeCr2O + Pashin & Kozachenko
(NHy)2Cr2O7 + (1981)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell K2CrO4, + Umeda & Nishimura (1979)
chromosomal K2Cr2O7 +
mutation K2Cr2O7 + Bigaliev et al. (1978)
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7 +
Na2CrO4, + Majone & Levis (1979)
K2CrO4 +
Na2Cr2O7, Levis & Majone (1979)
K2Cr2O7
CrO3, CaCrO4
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CrO3 + Kaneko (1976)
Na2Cr2O7 + Sarto et al. (1980)
K2Cr2O7 +
CrCl3 +
CrO3 + Tsuda & Kato (1977)
K2Cr2O7 + Raffetto (1977)
K2Cr2O7 + Stella et al. (1982)
Na2Cr2O7, + Majone & Levis (1979)
K2Cr2O7 +
K2CrO4 + Wild (1978)
Na2Cr2O7 + (7) Krishnaja & Rige (1982)
Mammalian cell CrO3, K2CrO4, + Ohno et al. (1982)
SCE K2Cr2O7 +
K2Cr2O7 + Bianchi et al. (1983)
K2CrO4,
Na2CrO4 + Levis & Majone (1979)
CrO3, +
K2Cr2O7, + Majone & Levis (1979)
Na2Cr2O7 +
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CaCrO4, CrO3, + Gomez-Arroyo et al.
K2Cr2O7 + (1981)
K2Cr2O7 + Majone & Rensi (1979)
CrO3 + (6) Stella et al. (1982)
K2Cr2O4, + Elias et al. (1983)
Na2CrO4, +
Na2Cr2O7 +
K2Cr2O7,
K2CrO4 + McRae et al. (1979)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell CaCrO4, K2CrO4 + (8) Casto et al. (1979)
transformation ZnCrO4 + (9)
Na2CrO4,
CaCrO4 + Casto et al. (1976)
+ Di Paolo & Casto (1979)
K2Cr2O7 + Bianchi et al. (1983)
K2Cr2O7 + Tsuda & Kato (1977)
CaCrO4 + Fradkin et al. (1975)
K2Cr2O7 + Raffetto (1977)
---------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) As industrial pigments (Cr-yellow-orange; Zn-yellow; Mo-orange).
(2) Gene conversion.
(3) No mutagenicity in the presence of mammalian microsomal activation
system (S-9 mix).
(4) No compounds given.
(5) Forward mutation was observed in 7/480 054 colonies (controls 0/84
546). A significant increase can be stated if comparison is made
with the historical spontaneous mutation rate, i.e., no mutants in
106 colonies.
(6) Occupatinal exposure.
(7) Induction of chromosomal aberrations in gill cells of the fish
Boleophthalmus dussumieri (Goby).
(8) 2 times control levels.
(9) 4 times control levels.
The results summarized in Table 17 show that the chromate ion
(hexavalent chromium) induces different kinds of genetic damage,
even at low doses, whereas the chromosome-damaging capacity of
trivalent chromium was only observed when it was tested in very
high doses.
In assays with whole-cell systems, trivalent chromium is
inactive, unless there is a direct interaction with DNA, e.g., in
studies in which purified DNA was exposed to trivalent chromium,
where modifications of the physical and chemical properties, as
well as decreased fidelity of DNA synthesis, were produced (Levis &
Bianchi, 1982). According to Warren et al. (1981), it appeared
that trivalent chromium in a proper ligand environment could have
considerable genetic toxicity. However, in recent studies, Bianchi
et al. (1984) found that trivalent chromium (CrCl3, 10-3 to 10-5 M)
was neither cytotoxic nor mutagenic in permeabilized hamster
fibroblasts (BHK were incubated for 30 min in Ea or BSS made
hypertonic with 4.2% NaCl).
Table 16. Genotoxic activity of chromium (III) compoundsa
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
DNA degradation CrCl3 + Bianchi et al.
(1983)
Decreased fidelity Cr2O3 + Levis & Majone
of DNA synthesis (1981)
Microbial DNA cis-[Cr(bipy)2ox] + Warren et al. (1981)
repair I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 + Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O + Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O + Warren et al. (1981)
trans-[Cr(en)2 + Warren et al. (1981)
(SCN)2]SCN
CrCl3, Cr2O3, - Yagi & Nishioka
Cr(OH)3 (1977)
K2Cr2(SO4)4 - Yagi & Nishioka
(1977)
K2Cr2(SO4)4 - Kanematsu et al.
(1980)
Cr2(SO4)3 - Kanematsu et al.
(1980)
CrCl3 - Nishioka (1975)
Cr(NO3)3, + De Flora (1981)
Cr(CH3COO)3
CrCl3 - Nakamuro et al.
(1978)
CrCl3 + Gentile et al.
(1981)
CrCl3, KCr(SO4)2, - Gentile et al.
(1981)
Cr2(SO4)3 - Gentile et al.
(1981)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Microbial gene CrCl3, Cr(NO3)3 - Löfroth & Ames
mutation (1978)
Cr(CIO4)3 - Löfroth & Ames
(1978)
CrCl3, Cr(NO3)3 - Venitt & Levy (1974)
Cr2SO4, - Venitt & Levy (1974)
K2SO4 x 2H2O
Cr(CH3COO)3 + Nakamuro et al.
(1978)
Cr(NO3)3 + Nakamuro et al.
(1978)
CrCl3 - Nakamuro et al.
(1978)
CrK(SO4)2 x 12H2O - Petrilli & De Flora
(1977)
CrCl3 x 6H2O - Petrilli & De Flora
(1978a)
Cr(NO3)3 x 9H2O - Petrilli & De Flora
(1978b)
Microbial gene cis-[Cr(bipy)2ox] + Warren et al. (1981)
mutation I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 - Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O - Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O - Warren et al. (1981)
trans-[Cr(en)2 - Warren et al. (1981)
(SCN)2]SCN
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
gene mutation (1983)
Cr(CH3COO)3 - Newbold et al.
(1979)
Mammalian cell Cr2(SO4)3 - Umeda & Nishimura
chromosomal (1979)
mutation
Cr(CH3COO)3 + Nakamuro et al.
(1978)
CrCl3, Cr(NO3)3 - Nakamuro et al.
(1978)
Cr(NO3)3, + Levis & Majone
(1979)
KCr(SO4)2, + Levis & Majone
(1979)
Cr(CH3COO)3 + Levis & Majone
(1979)
CrCl3 + Kaneko (1976)
CrCl3 - Sarto et al. (1980)
Cr2(SO4)3, CrCl3 - Tsuda & Kato (1977)
CrCl3 + Raffetto (1977)
CrCl3 - Stella et al. (1982)
CrCl3 - (1) Bianchi et al.
(1984)
Mammalian cell CrCl3, Cr2O3 + Elias et al. (1983)
SCE
Cr2O3 + Levis & Majone
(1981)
Cr(NO3)3, - Levis & Majone
KCr(SO4)2 (1979)
CrCl3, Cr(CH3COO)3 - Levis & Majone
(1979)
CrCl3 + (2) Ohno et al. (1982)
Cr2(SO4)3 - Ohno et al. (1982)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
SCE (contd). (1983)
CrCl3 - Majone & Rensi
(1979)
Mammalian cell CrCl3 - Bianchi et al.
transformation (1983)
---------------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) Thymidine uptake, DNA replication, damage and repair and SCE were
tested in permeabilized hamster fibroblasts.
(2) Weakly positive.
Levis & Buttignol (1977) and Levis et al. (1978) raised the
question of whether trivalent chromium could be an active mutagenic
agent within a cell. Others, such as Bianchi et al. (1980),
supported this theory, suggesting that trivalent chromium is bound
to genetic material after reduction of the hexavalent form by an
NADPH-dependent microsomal enzyme system (Norseth, 1978; Jennette,
1979). The studies of Sirover & Loeb (1976), showing a 30% error
in DNA synthesis after application of 0.64 mM trivalent chromium
compared with 16 mM of hexavalent chromium, support this theory.
Pashin et al. (1982) demonstrated the induction of dominant
lethal mutations in mice. They treated male mice with a single
injection of potassium dichromate (0.5 - 20 mg/kg body weight, ip)
or with daily injections (1 and 2 mg/kg, ip, for 21 days). The
single injections (0.5 - 2 mg/kg) did not induce any effects;
repeated or higher dosages of potassium dichromate induced a
statistically significant decrease in the survival of embryos from
cells treated at the early spermatid and late spermatocyte stages.
Karyological alterations in mammalian cells were induced after
exposure to concentrations of hexavalent chromium that were more
than 100 times lower than the concentration of trivalent chromium
causing the same changes (Majone & Rensi, 1979).
Analysing the effects of fume particles from stainless steel
welding on sister chromatid exchanges and chromosome aberrations in
cultured Chinese hamster cells, Koshi (1979) found an increase in
cytogenic effects with increasing fume doses, due to the dissolved
hexavalent chromium. Hexavalent chromium and stainless steel
welding fume particles containing chromium compounds produced
positive results in the "mammalian spot test", a somatic tissue
assay (Knudsen, 1980).
Table 17. Mutagenicity of chromium compoundsa
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
L. Hexavalent chromium compounds
1) Sodium dichromate (Merck) - +w +w +w + 50 - 250 4.4 decrease toxic and mutagenic
Na2Cr2O7 x 2H2O Cr6+ was converted
into inactive Cr3+
2) Potassium chromate (BDH) - +w +w +w + 80 - 410 2.9 decrease by reducing
K2CrO4 chemicals (ascorbic
acid, sodium
3) Calcium chromate (BDH) - +w +w +w + 60 - 290 3.2 decrease sulfite) or
CaCrO4 metabolites (NADH,
NADPH, GSH), by
4) Ammonium chromate - +w +w +w + 50 - 320 3.7 decrease human gastric juice
(Carlo Erba) and erythrocyte
(NH4)2CrO4 lysates, by S9 mix
containing human
liver S9 fractions
5) Chromium trioxide or - +w +w +w + 40 - 220 5.1 decrease or rat tissue S9
chromic acid (Merck) fractions (in order
CrO3 of efficiency:
liver>suprarenal
glands>kidney>
stomach>lung);
pretreatment of
rats (Aroclor(R)
1254), ether,
ethanol) influenced
the efficiency of
metabolic systems;
conversely, Cr6+
mutagenicity was not
affected by human
serum or plasma nor
by S9 mix containing
other rat (spleen,
colon, bladder,
striated muscle) S9
fractions
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
6) Zinc yellow (Montedison) - +w +w +w + 90 - 590 1.4 decrease the mutagenic effects
ZnCrO4 x Zn(OH)2 + of Cr6+ compounds and
10% CrO3 benzo( a)pyrene were
less than additive
7) Chromyl chloride (BDH) - +w +w +w + 100 - 430 1.8 decrease liquid volatile
Cl2CrO2 compound; vapours
were also toxic and
mutagenic
8) Chromium orange or basic - - - - +b 2 mg the three pigments
lead (spot test) containing PbCrO4
PbCrO4 x PbO were scarcely (L8),
chromate (Montedison) very scarcely (L9),
or totally (L10)
insoluble in water;
they were assayed
by directly spotting
these compounds at
the centre of plates
9) Molybdenum orange or - - - - +b 2 mg
lead solfomolybdo- (spot test)
chromat (Montedison)
PbCrO4 72-77%
PbSO4 4-6%
Al2O3 2%
PbMoO4 12-14%
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
10) Chromium yellow or - - - - -b
lead solfochromate
(Montedison)
PbCrO4 41-85%
PbSO4 4-45%
SiO2 0.1-3%
Al2O3 2-6%
11) Chromium - - - - - -2.3x103 - - hexacoordinated
hexacarbonyl (BDH) compound; formally,
Cr(CO)6 its oxidation state
is 0; insoluble in
water, dissolved in
ether
M. Trivalent chromium compounds
1) Chromic chloride - - - - - -3x104 - - inactive Cr3+
(BDH) CrCl3 x 6H2O compounds could be
converted into toxic
and mutagenic Cr6+
only in the presence
of oxidizing chemicals
(potassium
permanganate), while a
variety of metabolic
systems were
ineffective
2) Chromic nitrate (Riedel- - - - - - -2x104 - -
de Haen)
Cr(NO3)3 x 9H2O
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
3) Chromic potassium - - - - - -1.6x104 -
sulfate (BDH)
CrK(SO4)2 x 12H2O
4) Chromic acetate (BDH) - - - - - -7x104 - -
Cr(CH3COO)3
5) Neochromium (Montedison) - - - - - -5x104 - -
Cr(OH)SO4 56-58%
Na2SO4 23-24%
H2O 18-21%
6) Chromium alum (Montedison) - - - - - -4.3x104 - -
Cr2(SO4)3 37-39%
K2SO4 16-18%
H2O 43-37%
7) Chromite (Montedison) - - - - + 2 mg - Contaminated with Cr6+
Cr2O3 44-46% (spot test) traces ( sim-diphenyl-
Fe2O3 29-30% carbazide reagent);
Al2O3 15-16% scarcely soluble in
SiO3 0.5-3% water; assayed by
CoO 0.5-2% directly spotting the
powder at the centre
of plates
--------------------------------------------------------------------------------------------------------
Table 17 (contd.)
Note:
Sensitivity of Salmonella strains: Range of activity:
In the assays carried out in this study, This column indicates the lower and
the numbers of spontaneous revertants of the upper limits (in nmol per plate)
Salmonella tester strains fell within the of the mutagenic response, as in-
following ranges: ferred from dose-response curves
TA 1535: 10-25, both with and without S9 mix; with the most sensitive bacterial
TA 1537: 3-18, both with and without S9 mix; strain and under the most favourable
TA 1538: 10-25 without S9 mix; 15-35 with S9 mix; metabolic situation (i.e., with or
TA 98: 20-35 without S9 mix; 25-40 with S9 mix; without S9 mix) for each positive
TA 100: 150-200 without S9 mix; 140-180 with S9 mix compound; for negative compounds,
the maximum dose tested is indicated
The key for the interpretation of symbols in the
column "Reverted strains" is the following: Mutagenic potency:
+ clearly positive result indicated by a dose-
related and reproducible increase of his+ the values presented have
revertants over controls (at least a 3-fold been calculated by dividing the number
increase); of revertants in excess of controls
+w weak positivity indicated by an increase of by the corresponding amount of compounds
revertants 2 - 3 times in controls; (in nmoles); the number of net revertants
± reproducible but less than 2-fold increase was determinated at the top level of the
of revertants; linear part of dose-response curves, which
No symbol: no conclusive experiment carried out were drawn as indicated under the previous
with the corresponding strain. sub-heading.
Effects of S9 mix
The effect of S9 mix containing
liver S9 fractions from
Arochlor(R) pretreated rats on
the mutagenicity of test
compounds is reported
--------------------------------------------------------------------------------------------------------
a From: DeFlora (1981).
b L8, L9, and L10, as well as PbCrO4, were positive in the plate test when
dissolved in 0.5 N NAOH.
Embryonic fibroblast cell cultures showed significant
chromosome aberrations, when potassium dichromate was added to the
medium (Tsuda & Kato, 1976). When the potassium dichromate was
reduced with sodium disulfite (Na2SO3), no significant aberrations
occurred, even with 100 times the chromium concentrations.
At present, it is widely accepted that hexavalent chromium is
genetically active, because of its ability to cross the membranes
and enter the cells. If reduction of hexavalent chromium takes
place outside the cell (or even outside the cell nucleus, e.g., in
mitochondria or microsomes) its genetic activity is suppressed; if
the reduction takes place inside the nucleus (near, or at, the
target DNA molecules) alterations in DNA can occur, depending on
the oxidation power of hexavalent chromium or the formation of
trivalent chromium complexes with nucleophilic sites of the DNA;
thus, trivalent chromium could be the ultimate mutagenic form of
chromium (Levis & Bianchi, 1982). More recent investigations
(Wiegand et al., 1984) have shown that hexavalent chromium may
enter the body unreduced via the lung and be partly deposited in
erythrocytes over a prolonged period of time. From a DNA-repair
test in bacteria, DeFlora et al. (1984) concluded that hexavalent
chromium causes irritation in nucleotide pools and, due to its
oxidizing power, induces single-strand breaks in DNA, while
trivalent chromium induces DNA-protein and DNA-DNA cross-links and
decreases the fidelity of DNA replication.
7.2.1.3. Developmental toxicity and other reproductive effects
The teratogenicity of hexavalent chromium as chromium trioxide
was tested in golden hamsters (Gale, 1974, 1978, 1982; Gale &
Bunch, 1979) and that of trivalent chromium as chromic-chloride in
ICR mice (Iijima et al., 1975; Matsumoto et al., 1976). Injections
of hexavalent chromium into the fertilized chick egg increased
embryolethality and produced malformations in the survivors (Gilani
& Marano, 1979).
The golden hamsters were treated with a single iv injection of
chromium trioxide (5, 7.5, 10, or 15 mg/kg body weight) on the 8th
day of gestation (Gale, 1978).
Dose-response relationships were demonstrated between the rate
of absorption and the rate of malformations including delayed
ossification of the skeletal system. At 5 mg/kg body weight, 4% of
the fetuses showed external abnormalities (oedema, exencephaly),
and 34% showed cleft palate (controls: 2%), which increased to 85%
with the higher doses of 7.5 or 10 mg/kg body weight. Thirty-one
percent of fetuses were retarded following treatment with 7.5 mg/kg
body weight and 49% following 10 mg/kg body weight. The dose of 15
mg/kg body weight was lethal for 75% of the dams. Gale & Bunch
(1979) injected a single dose of 8 mg CrO3/kg body weight on day 7,
8, 9, 10, or 11 of gestation in female golden hamsters (Table 18).
The treated females were adversely affected (body weight loss,
tubular necrosis of the kidneys). In a later study, Gale (1982)
injected 8 mg CrO3/kg iv in hamsters on day 8 of gestation. An
increased incidence of cleft palate was seen in strains LVG, LSH,
and MHA, but no effects were reported in strains CB, LHC, and PD4.
ICR mice were injected sc with chromic chloride (Iijima et al.,
1975; Matsumoto et al., 1976). In the first series (animals
administered 9.8 or 19.5 mg/kg body weight, every other day, from
the 0th to 16th day of gestation), according to the authors, a
slight increase in the rate of malformations could not be excluded.
In the second series, with ip injections of 9.8 - 24.4 mg/kg body
weight, on the 8th day of gestation, the authors reported a dose-
dependent increase in the frequency of exencephaly, anencephaly,
and the occurrence of rib fusion.
Danielsson et al. (1982) studied the rate of embryonic or fetal
uptake of trivalent chromium and hexavalent chromium in early and
late gestational mice (Table 19) and found placental passage of
chromium. Hexavalent chromium appeared in the fetus in high enough
concentrations for a direct effect on embryonic structures to be
likely, trivalent chromium may act on placental structures.
Gilani & Marano (1979) injected chromium trioxide into 840
embryonating chicken eggs (air sack) at doses ranging from 0.002 to
0.05 mg per egg, on days 0, 1, 2, 3, and 4 of incubation (0.1 ml
physiol. saline injections per control egg). All embryos were
examined on day 8. The following malformations were observed in
the 720 eggs affected: short and twisted limbs, microphthalamia,
exencephaly, short and twisted neck, everted viscera, oedema, and
reduced body size. However, the incidence of abnormalities in the
120 controls was reported to be "low".
Inhalation studies were conducted by Glaser et al. (1984) on
Wistar rats (TNO-W-74 SPF) to study the effects of sodium
dichromate on reproduction and teratogenicity in 3 generations.
Whole-body exposures to sodium dichromate aerosols (0.2 mg
chromium/m3) were performed with an exposure time of 130 days per
generation. No effects on reproduction were found. All
teratogenicity tests were negative, and there was no increase in
fetal chromium content. However, from generation to generation,
there was an increase in immunosuppression and hyperplasia of
organs (especially in the lungs) and changes in haematological
variables.
Table 18. Embryotoxicity following maternal exposure to CrO3 on different days
of gestation in hamstersa
-------------------------------------------------------------------------------------
Findings in live fetuses
Day of No. of No. of No. of Frequency External Internal Cleft
gestation females implantation living resorptions defects defects palate
treated sites fetuses No. %b No. %b No. %b No. %b
-------------------------------------------------------------------------------------
Treated CrO3 8 mg/kg
7 6 64 10 54 84 1 10 1c 10 5 50
8 6 88 54 14 20 8d 15 4e 7 17 31
9 6 77 67 10 13 18f 27 0 0 25 37
10 6 77 77 0 0 0 0 0 0 0 0
11 6 68 67 1 1 0 0 0 0 0 0
Controls H2O 5 ml/kg
7 3 35 33 2 6 0 0 0 0 0 0
8 3 44 41 3 7 0 0 0 0 0 0
9 3 37 36 1 3 0 0 0 0 0 0
10 2 20 19 1 5 0 0 0 0 0 0
11 3 34 33 1 3 0 0 0 0 0 0
-------------------------------------------------------------------------------------
a From: Gale & Bunch (1979).
b Underlined %, significantly different statistically from controls.
b Right hindlimb with two digits.
c Right kidney absent.
d Oedema (7), tail short (1).
e Small kidneys (3), right kidney absent (1).
f Oedema (17), omphalocele (1).
Table 19. Comparison of concentrations of trivalent and hexavalent
chromium in fetuses, placentas, and maternal organsa,b
------------------------------------------------------------------
Day 13 Day 16
Hexavalent Trivalent Hexavalent Trivalent
chromium chromium chromium chromium
------------------------------------------------------------------
Fetus 0.3 ± 0.01 0.03 ± 0.002 0.5 ± 0.04 0.1 ± 0.01
Placenta - - 3.0 ± 0.2 2.4 ± 0.2
Liver 29.4 ± 2.2 30.8 ± 6.5 33.9 ± 3.9 27.7 ± 3.5
Kidney 38.1 ± 1.9 5.3 ± 0.7 36.0 ± 4.3 8.7 ± 2.3
Serum 2.6 ± 0.2 9.3 ± 0.9 2.8 ± 0.4 11.0 ± 1.6
------------------------------------------------------------------
a From: Danielsson et al. (1982).
b Trivalent chromium (51CrCl3) and hexavalent chromium
(Na251Cr2O7) were injected iv on day 13 and day 16 of gestation
at doses of 10 mg chromium/kg body weight. The pregnant animals
were autopsied after 1 h. Concentrations are expressed in
mg/litre or g ± SEM (N = 4).
7.2.1.4. Cytotoxicity and micromolecular syntheses
Wacker & Vallee (1959) reported that trivalent chromium was
present in RNA from all sources examined; they hypothesized that
chromium might contribute to the stabilization of the structure.
Administration of trivalent chromium to mice (ip injection of
CrCl3 at 5 or 0.5 mg chromium/kg body weight; P < 0.01) caused
accumulation of chromium in the cell nucleus, which amounted to
about 20% of the accumulated chromium content of the liver cell,
and also enhanced RNA synthesis. Hexavalent chromium inhibited RNA
synthesis (K2CrO4 at 5 or 0.5 mg chromium/kg body weight;
P < 0.01) (Okada et al., 1983). In further studies, Okada et al.
(1984) pretreated rats with CrCl3 (ip injection of 5 mg chromium/kg
body weight) and then partially hepatectomized the rats. Chromium
accumulated in the regenerating liver, and hepatic RNA synthesis
was accelerated compared with that of partially hepatectomiced
controls. These findings suggest a direct participation of
chromium in RNA synthesis.
Potassium dichromate enhanced the passive uptake of ribo-and
desoxyribonucleosides (Levis et al., 1978; Bianchi et al., 1979),
caused an inhibition of DNA replication to BHK fibroblasts, and
reduced the colony-forming ability of BHK cells (doses: 10-4 - 20-7
mol). Hexavalent chromium (potassium dichromate with a
concentration of 5 x 10-7 mol/litre) reduced by half the
proliferation of NHIK 3025 cells originating from a human cervix
carcinoma (White et al., 1979).
The addition of calcium chromate to BHK21 cell cultures altered
their growth characteristics. Whereas these cells normally grow as
elongated fibroblast cells in parallel orientation, the chromium-
exposed cells grew as shortened fibroblasts with enlarged nuclei
and granular cytoplasm, randomly orientated. When grown in
carboxymethyl cellulose, the chromate-exposed cells underwent many
divisions in contrast to the 1 or 2 divisions of normal cells.
These alterations in growth properties persisted, even after
transfer to a normal medium (Fradkin et al., 1975).
Cultures of HeLa and rat embryonic cells were incubated with
various hexavalent and trivalent chromium compounds for 3 days and
the 50% inhibitory dose (ID50) for cell growth was determined. The
ID50 for sodium and potassium chromate and dichromate and calcium
chromate varied from 0.34 to 0.78 µg/ml of the medium. The ID50
for the trivalent compounds varied over a wider range: chromic
oxalate, 4; acetate, 52; nitrate, 720; chloride, 1030; and bromide,
1500 mg/litre. Similar results were found in rat embryonic cell
cultures (Susa et al., 1977).
7.2.1.5. Fibrogenicity
Because of the well-known fibrogenic effects of silica and
asbestos minerals, studies were initiated to determine whether
chromite and various other dusts exhibited similar properties. A
single intratracheal injection in rats of 40 mg chromite particles
(33% chromium, 0.7% SiO2, 99.9% of the particles being smaller than
5 µm) suspended in 1 ml Ringer's solution caused a moderate
cellular reaction that reached a peak after 2 months and then
decreased. After 8 months (end of observation), the lung weight
was within normal limits. Only an increase in fibrils that was not
significant was observed. The tissue reaction in regional lymph
nodes was minimal (Swensson, 1977).
Chromite FeO(CrAl)2O3 (10 mg), ground to an average particle
diameter of 1 µm, was injected into the pleural cavities of 25
mice. The animals were killed at intervals ranging from 2 weeks to
18 months after injection. Chromite produced the least reaction of
all the 15 dusts tested. This material attracted very few cells
and the eventual production of collagen was minimal (Davies, 1972).
7.2.2. Observations in farm animals
Systemic poisoning of farm animals with chromates is rare and
is mainly suspected to be a consequence of accidental industrial
contamination of drinking-water. The acute lethal dose for mature
cattle is approximately 600 - 800 mg/kg body weight; chronic
poisoning in young calves was produced by a daily dose of 30 - 40
mg/kg body weight for one month (NZ Department of Agriculture,
1954) with a clinical picture of profuse scouring, severe
dehydration, low blood pressure, changes in the alimentary tract,
including congestion and inflammation, and the rumen and abomasum
showing ulceration near perforation.
High chromium levels were found in the blood and liver of
calves that had died from poisoning. The minimum tissue levels
causing poisoning were 30 mg/kg in the liver and 4 mg/litre in
whole blood (Harrison & Staples, 1955). According to Romoser et al.
(1961), a level of 100 mg hexavalent chromium (as sodium
chromate)/kg did not affect growing chicks.
8. EFFECTS ON MAN
8.1. Nutritional Role of Chromium
The physiological role of chromium and the need for small
amounts of dietary chromium are indicated by:
(a) chromium-responsive cases of impaired glucose
metabolism in malnourished children and middle-aged
persons;
(b) 2 cases of proved chromium deficiency in patients fed
exclusively by parenteral alimentation; and
(c) epidemiological studies suggesting a link between
chromium deficiency and risk factors for cardiovascular
diseases.
8.1.1. Biological measurements and their interpretation
Biochemical methods for the diagnosis of the human nutritional
chromium status are still at the developmental stage. Blood- or
urine-chromium concentrations are not indicative of chromium
status. Several studies suggest the validity of the "relative
chromium response" in plasma or serum to the ingestion of 50 - 100
g of glucose within 30 - 120 min; the response also appears in the
urine. Lack of this response suggests chromium deficiency,
especially when combined with impaired glucose tolerance in the
presence of normal or elevated plasma-insulin levels.
8.1.2. Chromium deficiency
8.1.2.1. Adults
Chromium deficiency is diagnosed mainly through therapeutic
trials in which an impaired function is restored by supplementation
of the diet with small amounts of chromium close to the estimated
human requirement. Glinsmann & Mertz (1966) studied 4 male
diabetic patients for several months to 1 year, under the strictly
controlled conditions of a metabolic ward. The subjects, who were
fed identical portions of a diet prepared before the start of the
study, were allowed a measured amount of physical activity and were
stabilized under these conditions before the beginning of the
control periods. Three of the 4 patients responded to a chromium
level of 180 - 1000 µg/day as CrCl3 x 6H2O with a significant
improvement in oral glucose tolerance; the fourth showed a very
slight lowering of the glycaemic curve, which was not statistically
significant. A representative example is given in Table 20.
Table 20. Effect of chromium on mean glucose tolerancea,b
------------------------------------------------------------------------
Supplementation Days Number Mean blood glucose concentration
of ---------------------------------
tests Fast- Min after glucose load
ing 30 60 90 120
------------------------------------------------------------------------
None 0 - 32 10 840 2170 2290 1950 1560
60 µg, 3 x per day 33 - 74 12 850 2240 2380 2090 1620
60 µg, 3 x per day 75 - 119 13 840 2100 2130 1860 1410
60 µg, 3 x per day 120 - 140 5 840 2030 2010c 1750 1120c
None 180 - 194 1 960 2070 2900 2930 2370
60 µg, 3 x per day 195 - 211 5 830 1960 1930c 1750 1180c
None 256 - 313 10 870 2200 2450 2240 1660
1 mg, 3 x per day 337 - 340 1 680 1480 1620 1510 960
------------------------------------------------------------------------
a Modified from: Glinsmann & Mertz (1966).
b A 46-year-old man with maturity-onset diabetes, controlled on diet;
oral glucose tolerance test consisted of constant noon meal and 100 g
glucose.
c Mean values significantly different from control ( P < 0.025).
The condition of one of 2 diabetic out-patients, observed over
several months, was improved by chromium supplementation, the
condition of the other was not. Short-term treatment of 7 diabetic
patients for 1 - 7 days with 1 mg chromium did not affect any
variables of glucose metabolism, nor did chromium supplementation
change the normal glucose tolerance tests of 10 healthy, young
volunteers.
Glinsmann's positive results were not confirmed in a double-
blind crossover study on 4 normal volunteers and 10 diabetic
persons, with a crossover after 16 weeks. No effects of chromium
were detected on glucose tolerance or on fasting or postprandial
blood-glucose levels (Sherman et al., 1968). Schroeder et al.
(1970), while reporting that 2 - 10 mg chromium/day "almost always"
restored the impaired glucose tolerance in older, non-diabetic
subjects to normal, found only erratic and mild improvement in 4
out of 12 diabetic out-patients. On the basis of these
observations, Mertz (1969) concluded that chromium should not be
considered a therapeutic agent for diabetic patients.
On the other hand, it was reported in a later study on chromium
metabolism that chromium administration (500 µg/day, for 2 weeks)
produced a marked improvement in glucose utilization in both
juvenile and maturity-onset diabetic patients. Five of the
patients were followed for more than 1 year after exposure and were
found to maintain their improved glucose metabolism (Nath, 1976).
Of 10 non-diabetic elderly subjects with impaired glucose
tolerance, 4 responded to 150 µg chromium/day with complete
normalization of oral glucose tolerance tests. The remaining 6
subjects were not affected by the supplements (Levine et al.,
1968). Significant effects of a GTF-chromium-containing yeast
preparation were reported by Doisy et al. (1976) in 6 out of 12
elderly subjects with impaired glucose tolerance
Table 21. Effect of GTF supplementation on glucose tolerance tests in
elderly subjects with impaired tolerancea,b
-------------------------------------------------------------------------
Mean plasma-glucose levels Cholesterol Triglyceride
(mg/litre) (mg/litre) (mg/litre)
0 h 1 h 2 h
-------------------------------------------------------------------------
Before GTF 1060 ± 40c 2010 ± 70 1780 ± 80 2450 ± 90 1210 ± 80
11 tests
After GTF 990 ± 40 1620 ± 110 1320 ± 50 2050 ± 100 1120 ± 120
9 tests
Signifi- NS < 0.01 < 0.001 < 0.01 NS
canced
-------------------------------------------------------------------------
Mean serum-insulin levels
(microunits/ml)
0 h 1 h 2 h
Before GTF 24 ± 6 78 ± 17 118 ± 17
after GTF 26 ± 12 70 ± 8 83 ± 8
-------------------------------------------------------------------------
a From: Doisy et al. (1976).
b Supplement: 4 g yeast per min/day (Yeastamin Powder 95, Staley Co.,
Chicago, Illinois, USA). GTT: 100 g oral load.
c Mean ± SEM.
d Using paired t test, difference is significant.
In addition to the improvement in glucose tolerance in the
presence of a reduced insulin response, plasma-cholesterol
concentrations were significantly reduced. Several other
individual cases are reported in the publication of Doisy et al.
(1976), all of whom showed a lower serum-insulin response to a
glucose tolerance test during chromium supplementation.
Liu & Morris (1978) performed a similar study on the effects of
a yeast-chromium supplementation in 27 women, 40 - 75 years old, of
whom 15 had a normal and 12 an abnormal glucose tolerance test. In
11 of the normal subjects (73%), the integrated insulin response
was significantly reduced from 436 to 335 µU/ml ( P < 0.025) during
supplementation, without a statistically significant change in
glucose tolerance test. Significant reductions in the total glucose
response (10 650 - 9820 mg/litre; P < 0.001) and in the total
insulin response to glucose were observed in the subjects with
impaired glucose tolerance (1288 - 831 µU/ml; P < 0.001). The
glucose tolerance improved in 7 out of 12 women, but all 12 showed
a reduction in plasma-insulin.
The effects of supplementation of the diet with 200 µg
chromium/day (as chloride) on the glucose tolerance of 76 normal
adult volunteers was investigated in a double-blind cross over
study, with each period lasting 3 months. All 18 subjects entering
the study with impaired glucose tolerance (serum-glucose equal to
or greater than 1 g/litre, 90 min after a glucose load), showed
significant improvement during chromium supplements, but not with a
placebo, with an average decline in the 90-min serum-glucose levels
of 180 mg/litre ( P < 0.01) (Anderson et al., 1983b).
In another placebo-controlled study, 24 elderly volunteers were
given a daily supplement of either chromium-rich brewer's yeast or
chromium-deficient Torula yeast (9 g/day, for 8 weeks). The
glucose tolerance of the subjects given brewer's yeast
significantly improved with a concomitant decrease in plasma-
insulin levels, and a significant decline in plasma-cholesterol as
well as total plasma-lipids. No significant changes were observed
in the control group given the chromium-poor Torula yeast
(Offenbacher & Pi-Sunyer, 1980).
Additional, preliminary studies suggest a specific effect of
brewer's yeast on individual lipoprotein cholesterol fractions.
Twenty normal human subjects responded to daily supplementation
with 18 g of brewer's yeast over a 8-week period with a significant
reduction in low-density lipoprotein-cholesterol ( P < 0.01) and
with a simultaneous increase of 14% in the high-density lipoprotein
( P < 0.01) (Nash et al., 1979).
Similar results were observed in a 6-week supplementation trial
involving 8 physician volunteers, each of whom received 7 g/day of
brewer's yeast. Low-density lipoprotein-cholesterol declined by
nearly 18% ( P < 0.01), whereas the high-density cholesterol
fraction increased by 17.6%, resulting in a significant
( P < 0.001) decrease in the LDL/HDL ratio of 28% (Riales, 1979).
These studies suggest that the primary effect of the
supplementation must have been an increase in the effectiveness of
the insulin, because glucose tolerance was maintained or improved
in spite of lower insulin responses. It is important to note that
while the reduction in insulin levels occurred in all cases, an
improvement in glucose tolerance occurred in only a fraction. This
suggests two possible interpretations of all the results discussed
here, including those showing improvement in glucose tolerance in
only some of the subjects or no improvement at all. Lack of a
response to chromium could suggest that the subjects were not
chromium deficient and that their impaired glucose metabolism was
the result of other causes. Second, they may have responded with a
reduction in circulating insulin levels, as did all the subjects
with impaired glucose tolerance in whom the insulin response was
measured. Unfortunately, these measurements were not performed in
the earlier studies.
8.1.2.2. Malnourished children
Chromium deficiency has been implicated as a factor
contributing to the impairment of glucose tolerance in children
with protein calorie malnutrition in 3 countries, Jordan, Nigeria,
and Turkey. Hopkins et al. (1968) measured intravenous glucose
tolerance in 10 malnourished infants from the mountainous area of
Jordan and 9 equally malnourished infants from the valley area.
They found a severely depressed glucose removal rate of 0.7%/min in
the first group and a normal rate in the second, 3.8%/min (Table
22). The protein intake of both groups prior to hospitalization
had been identical; it was supplied in the form of a milk powder
with a chromium content of 18 µg/litre, when reconstituted.
However, analysis of 10 water samples revealed 3 times as much
chromium in the samples from the valley as in those from the
mountainous area (0.5 versus 1.6 µg/litre). This suggested the
possibility of chromium being a major determinant in the glucose
metabolism of these children, and the effects of supplementation
were measured in 6 children from the hills, with a severely
impaired tolerance test. The morning after oral administration of
250 µg chromium, as CrCl3 x 6H2O, the glucose removal rate had
markedly improved to 2.9%/min from a pretreatment level of
0.6%/min. Similar results were obtained in Nigeria (Table 22);
these tests also included control children who did not receive
chromium.
The immediate effects of chromium on these children were
remarkable and different from the lag phase always present in the
studies on adults. Equally important, was the short duration of
the effect of one dose demonstrated in a Jordanian child observed
for a longer period of time. These observations suggest that a
marked depletion in chromium stores must have existed in the
children.
Table 22. Significance of the effect of chromium on the impaired glucose
tolerances of malnourished infantsa
---------------------------------------------------------------------------
Subjects Number Glucose Significance
of removal of
infants rate difference
(%/min)
---------------------------------------------------------------------------
Jordanian infants from hill area with
0.5 µg chromium/litre in drinking-water 10 0.7
Jordanian infants from valley with
1.6 µg chromium/litre in drinking-water 9 3.8 P < 0.001
Jordanian infants;
initial glucose tolerance test 6 0.6 P < 0.001
after chromium treatment 6 2.9
Nigerian infants;
initial glucose tolerance test 6 1.2
after chromium treatment 6 2.9 P < 0.05
Non-treated infants:
Initial glucose tolerance test 5 1.9 not signi-
Repeated glucose tolerance test 5 2.1 ficant
---------------------------------------------------------------------------
a From: Hopkins et al. (1968).
Similar results were reported from a study in the Istanbul area
of Turkey (Gürson & Saner, 1971). Fourteen malnourished children
with an impaired glucose removal rate of 1.71%/min were given 250
µg of chromium (CrCl3 x 6H2O), and a second test was performed
after 15 h. The removal rate increased to 3.91%/min ( P < 0.001).
This average increase was due to a pronounced increase in 9 of the
children; no change was observed in the remaining 5. No
improvement in glucose tolerance occurred in 5 control children
receiving identical hospital treatment, except for the
administration of the chromium. The children receiving the
chromium supplementation grew significantly more than the control
children during the following 30 days, with the greatest growth
rates found in the 9 children who had responded to administration
of chromium with an improvement in glucose tolerance.
The fact that chromium deficiency interacts with protein-
calorie malnutrition only in certain geographical areas was
confirmed by Carter et al. (1968), who gave chromium
supplementation (250 µg/day as CrCl3 x 6H2O) without effects on
glucose tolerance in 9 Egyptian children tested. Plasma-chromium
levels were in the normal range, and chromium levels in
representative foods were high, ranging from 200 to 390 µg/kg.
These findings suggest that the chromium status of these subjects
was normal, which would explain the lack of an effect.
8.1.2.3. Patients on total parenteral alimentation
A female patient, maintained on total parenteral alimentation
since the age of 35 years, developed an unexpected weight loss of
15% after 5 years combined with peripheral neuropathy, impaired
intravenous glucose tolerance, in spite of normal insulin response,
and a decreased respiratory quotient of 0.66. Insulin treatment
(45 U/day) was ineffective. A strongly negative chromium balance
and low plasma- and hair-chromium levels suggested that the daily
estimated chromium intake from the infusion fluids of 5.3 µg had
not met the patient's requirement and resulted in chromium
deficiency. The insulin infusions were stopped and replaced by the
daily infusion of 250 µg chromium (as CrCl3 x 6H2O) for 2 weeks.
The result was normalization of glucose tolerance and respiratory
quotient, disappearance of signs and symptoms of neuropathy, return
to the previous normal body weight, in spite of reduced caloric
intake, disappearance of the requirement for exogenous insulin, and
a positive chromium balance. The nitrogen balance, which had been
erratic and often negative, became consistently positive following
chromium supplementation. Reduction of the daily supplement to 20
µg chromium proved to be sufficient to maintain the patient in a
state of well-being (Jeejeebhoy et al., 1977).
This case is the best demonstrated example of chromium
deficiency in a human subject. In addition to confirming the well-
known signs of chromium deficiency reported earlier, such as
insulin resistance and its consequences, it was the first case to
demonstrate the occurrence of peripheral neuropathy and disturbance
of nitrogen balance as a result of deficiency. These disturbances
require further study. A second case of chromium deficiency as a
consequence of long-term total parenteral alimentation was reported
by Freund et al. (1979). Signs and symptoms were similar to those
of the first case, except that central encephalopathy was present
instead of peripheral neural disorder. Supplementation with 150 µg
of chromium/day resulted in normalization of the deficiency signs.
Chromium at that time was not routinely added to intravenous
solutions, therefore it is possible that more cases of deficiency
may exist in patients on long-term infusion treatment. As an
outcome of this study, an Expert Panel of the American Medical
Association (AMA, 1979) suggested that the stable adult patient on
total parenteral alimentation should receive 10 - 15 µg chromium
daily.
8.1.2.4. Epidemiological studies
Two epidemiological studies suggest a link between chromium
status and cardiovascular diseases (Schroeder et al., 1970; Punsar
et al., 1975). Lack of chromium is associated with impaired
glucose tolerance, elevation of circulating cholesterol, and aortic
plaques. All of these variables are recognized risk factors for
cardiovascular disease. It has also been suggested that the
elevated insulin levels, often present in chromium-deficient human
subjects (Liu & Morris, 1978) represent a substantial risk for the
development of cardiovascular disease (Strout, 1977).
Schroeder et al. (1970) have summarized their studies of
chromium concentrations in normal and diseased tissues,
particularly in aortas (Table 23). The total body burden of
chromium, excluding the lungs, was lower in the USA, where there is
a higher incidence of cardiovascular disease, compared with other
regions, 1.72 - 1.86 mg/kg ash weight in the USA compared with 7.7,
9.6, and 2.7 mg in Africa, the Far East, and the Near East,
respectively. The only significant difference in tissue-chromium
concentrations in deaths from heart disease compared with deaths
from accidental causes was in the aorta (Table 23). Concentrations
in aortas from Africa, the Far and Near East were generally higher
and did not show such a difference.
Table 23. Chromium in aortasa
----------------------------------------------------------
Location Chromium (mg/kg dry weight)
Accident P Heart disease
----------------------------------------------------------
Africa 0.072 (5)b NS 0.116 (2)
Far East 0.97 (8) NS 0.246 (5)
Middle East 0.36 (8) NS 0.216 (3)
USA (Nine cities) 0.26 (103) 0.005 0.052 (13)
USA (San Francisco) 0.228 (10) 0.005 0.048 (15)
----------------------------------------------------------
a From: Schroeder et al. (1970).
b The number in parenthesis denotes the number of cases
observed.
The second epidemiological study was part of a cohort study
conducted in Finland beginning in 1959 (Punsar et al., 1975). A
total of 327 drinking-water samples were analysed for 22
characteristics including pH, anions, bulk minerals, and several
trace elements including chromium. The study included 504 and 622
men in the Western and Eastern areas of Finland, respectively,
whose domicile had not changed since 1959.
The coronary heart disease mortality rate is much higher in the
east of Finland than in the west. As seen in Table 24, the
chromium levels in drinking-water were much lower in the east than
in the west, whereas the opposite was true for copper. The metal
concentrations in the drinking-water of those with heart disease
and others only differed slightly (Table 24), but, according to the
authors, the data suggest that low chromium levels may play a role
in the high heart disease death rate of eastern Finland.
Table 24. Concentrations of copper and chromium in the
drinking-water of men who died between 1969 and 1973, and
of those who were alive in 1973a
-------------------------------------------------------------
Alive
Deaths (Clinical status in 1969)
CHDb Other CHDb Other No
heart heart
disease disease
-------------------------------------------------------------
West N = 19 N = 23 N = 63 N = 31 N = 288
Copper X 22.15c 20.17 20.05 20.94 20.15
(µg/litre) SD 3.92 4.69 4.69 5.12 4.74
Chromium X 7.52 9.38 9.45 9.74 8.45
(µg/litre) SD 2.06 3.42 4.01 4.44 3.31
East N = 23 N = 26 N = 129 N = 44 N = 279
Copper X 41.59 41.24 42.67 39.50 36.32
(µg/litre) SD 29.91 30.43 33.65 28.74 28.66
Chromium X 2.20 2.09 2.04d 2.49 3.01
(µg/litre) SD 2.17 2.41 2.08 2.17 2.37
-------------------------------------------------------------
a From: Punsar et al. (1975).
b CHD = coronary heart disease.
c P < 0.05.
d P < 0.001.
Note: Levels of significance refer to differences observed
when the concentrations were compared with those in the
"no heart disease" category.
Newman et al. (1978) measured serum-chromium concentrations in
32 patients, 18 male and 14 female, aged 25 - 65 years, in whom
coronary artery cine-arteriography was performed for medical
reasons. Serum-chromium was significantly lower in the 15 subjects
with coronary artery disease than in the 17 subjects without
involvement (2.51 versus 6.09 µg/litre; P < 0.01), whereas serum-
cholesterol, weight index, and systolic or diastolic blood pressure
did not differ among the groups. Serum triglycerides were higher
in the group with coronary artery disease (1.84 versus 1.12
g/litre; P < 0.05). No subject with a serum-chromium
concentration of more than 6 µg/litre exhibited coronary artery
involve- ment, and by regression analysis, serum-chromium was
judged to explain 17% of the total variance of the disease.
These results were independently confirmed in a second study in
which plasma chromium concentrations of 23 healthy persons were
more than 8 times greater than those of 67 patients with coronary
artery disease, as confirmed by cine-angiography (Simonoff et al.,
1984).
8.1.3. Mode of Action
As in experimental animals, the best known action of chromium
is the potentiation of insulin. This is shown by the
supplementation studies of Doisy et al. (1976) and Liu & Morris
(1978) in which a reduction in insulin levels in response to
glucose was observed, together with an improvement in glucose
tolerance. Direct proof, for example from in vitro studies with
human biopsy tissue, has not yet been obtained. Furthermore, it is
not known whether the potentiation of insulin is responsible for
all the effects observed during chromium supplementation, including
the reduction of circulating cholesterol and the growth stimulation
observed in malnourished children and during parenteral nutrition.
In the healthy, chromium-sufficient human subject, chromium
levels in the bloodstream increases acutely following a glucose
load or an injection of insulin. Glinsmann et al. (1966) observed
acute increases in 5 young, healthy volunteers during a glucose
tolerance test, but not when the same subjects were given an
equivalent volume of water. Maturity-onset diabetic patients did
not exhibit this chromium response initially, but did show a rise
in plasma-chromium, after several weeks of chromium
supplementation. These observations were confirmed by Levine et
al. (1968), Hambidge (1971), Behne & Diel (1972), and Liu & Morris
(1978), who saw the acute chromium rise in some, but not all,
subjects. In the study of Liu & Morris (1978), which was the most
detailed, the relative chromium response (CrR) was measured by
neutron activation analysis in 15 women with normal glucose balance
and in 12 with impaired tolerance.
(1-h serum-chromium)
CrR = ------------------------ x 100
(fasting serum-chromium)
Initially, the normal group had a mild positive response (CrR =
107), compared with a negative response (CrR = 81) in the impaired
subjects. Following three months of supplementation with a
chromium-rich yeast extract, the CrR of the normal subjects
increased to 140, and that of the impaired group became positive
with CrR = 149. As discussed in section 8.1.2, these changes were
accompanied by a significant decrease in the insulin response to
glucose and an improvement in glucose tolerance. The results of
these studies are in apparent contrast with those of Davidson &
Burt (1973), who found a significant decline in plasma-chromium
levels following a glucose load in normal, non-pregnant women (mean
age 26 years), but a mild to moderate increase in all but 1 of 10
pregnant women (mean age 20 years). As the time of the acute rise
varied between women, statistical analysis at any one time did not
produce any significant results. Hambidge (1971) and Behne & Diel
(1972) demonstrated an acute plasma-chromium response following
injection of insulin; it can therefore be assumed that the effect
of glucose on the chromium response is associated with
hyperinsulinaemia. The acute "relative chromium response" is
believed to reflect the chromium status of human subjects. It does
not appear when stores from which the chromium increment must come
are depleted. Depleted stores can be replenished by
supplementation, with a resulting reappearance of the response
shown in the two previously discussed studies. As it has been
shown that chromium levels in urine increase following a glucose
load (Wolf et al. 1974; Gürson & Saner, 1978), it is likely that a
fraction or all of the chromium increment is subsequently excreted.
8.2. Acute Toxic Effects
In adults, the lethal oral dose of soluble chromates is
considered to be between 50 mg/kg body weight (RTECS, 1978) and 70
mg/kg body weight (Deichmann & Gerarde, 1969). The clinical
features of toxicity are vomiting, diarrhoea, haemorrhagic
diathesis, and blood loss into the gastrointestinal tract causing
cardiovascular shock. If the patient survives about 8 days, the
outstanding effects are liver necrosis (Brieger, 1920), tubular
necrosis of kidneys, and poisoning of blood-forming organs
(Langard, 1980). A 14-year-old boy, who had ingested about 1.5 g
of potassium dichromate, died 8 days later. Kaufmann et al. (1970)
also reported enlargement and oedema in the boy's brain.
8.3. Chronic Toxic Effects
The chronic effects of trivalent and hexavalent chromium have
been reviewed by Langard & Norseth (1979). The most important
features are changes in the skin and mucous membranes, and allergic
dermal and broncho-pulmonary effects. Important systemic effects
occur in the kidneys, liver, gastrointestinal tract, and
circulatory system.
Mancuso (1951) studied several biological variables in persons
occupationally exposed to chromate and related them to clinical
signs and symptoms. He found occasional leukocytosis or
leukopenia, monocytosis, eosinophilia, reduced haemoglobin
concentrations and prolonged bleeding times, but none of them were
consistent enough to serve as reliable diagnostic tests.
Mild normochronic anaemia was reported by Myslyaeva (1965) in
94 subjects exposed to chromium compounds with 68 of them showing
signs of chronic toxicity. Though the report of Baetjer et al.
(1959b) was based only on 3 cases, it indicated that chromate
attached preferentially to red blood cells. Koutras et al. (1964)
reported the inhibition of the red blood cell enzyme gluthathione
reductase by chromate concentrations of 5 - 25 mg/kg body weight.
However, the lowest effective dose is far in excess of levels
found, even in heavily exposed persons. Thus, all attempts made so
far have failed to identify a specific sensitive biochemical test
for the assessment of chronic chromium toxicity.
Long-term surveillance of pregnant women working at a plant
producing dichromates and those living in its immediate vicinity
(Shmitova, 1980) revealed increased chromium levels in the blood
and urine, compared with those in the control group. At 32 weeks
of pregnancy, the blood concentrations of chromium were,
respectively, 2.5 mg/litre in the women workers, 1.2 mg/litre in
the women living near the plant, and 0.19 mg/litre in the controls;
the levels in the urine were 1, 0.47, and 0.045 mg/litre,
respectively. Umbilical venous blood contained 1.8, 0.66, and 0.37
mg chromium/litre, respectively. The placental chromium contents
in the surveyed women were 162, 179, and 40 µg/kg, respectively,
and the breast-milk levels were 119, 20, and 31 µg/litre, which
means that chromium is capable of transplacental entry into breast
milk and into the fetal and infant organism. Women working at the
bichromate plant exhibited a high incidence of obstetric pathology
and geotoses. Chromium levels were estimated in fetal and
placental tissues (abortive material) derived on the 12th week of
pregnancy from women workers of the dichromate facility and those
not exposed to chromium. In the test group, chromium levels were
1140 µg/kg in the fetal tissues and 135 µg/kg in the placental
tissue, whereas, in the controls, they were 928 and 30 µg/kg,
respectively. No teratogenic effects have been revealed (Shmitova,
1980).
8.3.1. Effects on skin and mucous membranes
Several different types of effects on skin and mucous membranes
may result from exposure to chromium chemicals, and the main
features are:
(a) primary irritation: ulcers (corrosive reactions),
scarred and non-ulcerative contact dermatitis;
(b) allergic contact dermatitis: eczematous and
noneczematous.
A general survey of the nature and cause of contact dermatitis
is given by Fregert (1981).
8.3.1.1. Primary irritation of the skin and mucous membranes
(a) Skin
Skin irritation, manifested by a sharp hyperaemia as well as by
vesicular, papular, and rash pattern, may result from contact with
chromates. However, the major problems in the primary irritation
dermatoses are ulcers, often called chrome holes or chrome sores.
Ulceration is likely to occur among workers who have contact with
high concentrations of chromic acid, sodium or potassium dichromate
or chromate, or ammonium dichromate. It does not result from
contact with trivalent chromium compounds. An ulcer may develop if
the chromium compound, either a dust or a liquid, comes into
contact with any break in the skin such as an abrasion, scratch,
puncture, or a laceration. Favoured sites for ulcer development
are the nailroot areas, the creases over the knuckles, finger webs,
the backs of the hands, and the forearms (Bloomfield & Blum, 1928).
Primary irritation by chromium can be attributed to hexavalent
chromium, as the salts of trivalent chromium are not considered
capable of producing such effects (Fregert, 1981). However,
prolonged exposure to trivalent chromium salts has been reported to
cause skin lesions, less marked than those following exposure to
hexavalent chromium salts (Domaseva, 1971). Findings in 164
patients with eczema showed that skin tests on people who had
contact with trivalent chromium did not give positive results with
dichromate (Valer & Racz, 1971).
Ordinarily, a chrome sore, if not deep, persists for about 3
weeks after exposure is discontinued. There is no evidence that
the ulcers undergo malignant transformation. Furthermore, the
presence of chromium ulcers did not influence the development of
sensitization to chromates (Edmundson, 1951), evidenced by patch
testing with a 0.5% solution of K2Cr2O7.
(b) Nasal septal mucosa
Perforation of the nasal septum was one of the main chronic
effects in workers who had contact with chromates and chromic acid
(Bloomfield & Blum, 1928; Langard & Norseth, 1979). In a group of
100 persons constantly in contact with chromium compounds, 44 had
superficial ulceration of the anterior nasal septal mucosa.
Twenty-one people had profound ulcers and 5 had perforations of
various sizes in the cartilaginous part of the nasal septum.
Perforation of the nasal septum and cicatricial alterations were
observed in people with 3 or more years of service, whereas
ulcerous lesions arose in 2 years of work with chromium compounds.
An additional consequence of the effect on the nasal mucous
membranes is a loss of the senses of smell and taste (Seeber et
al., 1976). In workers with profound ulcerous lesions of the nasal
septal mucosa, strains of white staphylococcus with elevated
resistance to chromium are sometimes isolated (Cherevaty et al.,
1965).
Pokrovskaya et al. (1976) studied 561 workers engaged in the
open excavation of chromium ores (chromium content was 62% in the
form of a complex compound of magnesium chromite and other chromium
ores). Almost half of the workers had worked there for more than 5
years, and about 40% had been working there for 6 - 15 years.
Changes in the nasal septal mucosa, typical of the effect of
chromium compounds, were found in 8.2% of the workers. The longer
the service record, the greater the prevalence of these lesions.
After 5, 11, and 15 years of work, the prevalence was 2.3%, 8.3%,
and 11.2%, respectively.
The prevalence of nasal septum perforation in chromium plating
workers was very high (24%) in the early 1950s in China (Yang,
1956). With the introduction of control measures, the prevalence
dropped so that, in 1982, only 2 cases of nasal septum perforations
were found among 393 chromium-exposed workers (Peng, 1982).
One hundred and four subjects (85 male, 19 female) exposed to
chrome plating and 19 unexposed controls were studied by Lindberg &
Hedenstierna (1983) with regard to changes in the nasal septum.
Exposed subjects were employed at 13 different companies and
included all who were working on the day of study at the particular
company. The median exposure time was 4.5 years (range, 0.1 - 36
years). Forty-three subjects were exposed almost exclusively to
chromic acid and constituted both a "low exposure" group (8-h mean
below 2 µg/m3, N = 22) and a "high exposure" group (2 µg/m3 or
more, N = 21). Their median exposure time was 2.5 years (range,
0.2 - 23.6 years). The other 61 subjects were exposed to a mixture
of chromic acid (0.2 - 1.7 µg/m3) and other pollutants, such as
nitric, hydrochloric, and boric acids, as well as caustic soda,
nickel, and copper salts. The last group was included to disclose
any additive or synergistic effects of chromic acid and other
pollutants and was studied with regard to lung function only. The
breakdown of subjects into various groups is shown in Table 25.
Nasal septal ulceration and perforation were seen in 10 out of 14
subjects exposed to peak levels of 20 - 46 µg chromic acid/m3 or
more; no one in the control group showed signs of atrophy,
ulcerations, or perforations.
8.3.1.2. Allergic contact dermatoses
Chromium is a very important skin sensitizer. Sensitization
is reported to require about 6 - 9 months, but can occur in less
than 3 months (Pirilä & Kilpio, 1949). Genetic factors appear to
contribute to the appearance of chrome sensitivity within the
Jewish population (Wahba & Cohen, 1979).
Apart from the primary irritation and ulceration effects,
direct contact with small amounts of the chromates may cause
allergic dermatosis (dermatitis and eczema). As this allergic
reaction is cell-mediated, i.e., antibody formation is intra-
cellular and not humoral, delayed allergic reactions are rarely
observed.
Fregert et al. (1969) reported that the skin-patch test for
chromium was positive in 8 - 15% of all patients suffering from
eczema. The dermatitis caused by chromium is described as a
diffuse erythematous type with severe cases progressing to an
exudative stage. Persons sensitive to chromium react to patch and
intracutaneous tests with nonirritant concentrations of potassium
dichromate (K2Cr2O7). Opinion is divided about the role of the
oxidation state of chromium as a causal agent in allergic contact
dermatoses (Morris, 1958; Fregert & Rorsman, 1964; Kogan, 1971).
In a review, Polak et al. (1973) showed that hexavalent chromium,
which penetrates the skin by a direct effect of a hapten, is
reduced to the trivalent state, chiefly by sulfhydryl groups of
aminoacids. It then conjugates with proteins thus forming the full
antigen that initiates sensitization.
Guinea-pigs sensitized with either trivalent chromium chloride
or hexavalent potassium dichromate are capable of reacting in vivo
and in vitro to challenges with both chromium salts. This double
reactivity is also found after repeated stimulation with only one
of these chromium compounds. Since it is not possible to select
lymphocytes directed specifically against a chromium determinant of
a particular valence, it is concluded that, by sensitization with
chromium salts of different valences, a common determinant or
closely related determinants are formed. It is suggested that this
determinant is formed by chromium in the trivalent form
(Siegenthaler et al., 1983).
Two to three years after medical treatment, a follow-up study
on 555 patients with contact dermatitis was completed by means of
questionnaires (Fregert, 1975). The eczema had healed in one-
quarter of the patients, one-half had periodic symptoms, and one-
quarter had permanent symptoms. The prognosis for persons who
changed their work or stopped working was the same as that for
those who continued eczema-inducing work.
From 48 positively tested patients (0.5% potassium dichromate),
38 persons, i.e., 79%, still had a positive patch test after 4 - 7
years. In 72% of the cases, a history of occupational exposure to
chromates could be proved (Thormann et al., 1979).
A fairly rapid transformation of dermatitis into eczema is a
characteristic feature of chromium-induced skin lesions. Kogan
(1971) recognized the following important properties: (a) absence
of adaptation, which makes it necessary to discontinue chromium
handling until the slightest clinical manifestations have
disappeared; (b) a persistent chronic development of an eczematous
process; (c) a long-term latent period; and (d) absence of a direct
correlation between the incidence of sensibilization in the exposed
workers and the chromium concentration to which they have been
exposed.
Table 25. Series of exposed subjects and referencesa
--------------------------------------------------------------------------------------------------------
Exposed subjects
Mixed exposure to
Low exposure to High exposure to chromic acid
chromic acid chromic acid (< 2 µg/m3) and other
(< 2 µg Cr6+/m3) (> 2 µg Cr6+/m3) acids and metallic salts References
----------------- ------------------ ----------------------- -----------------
Nb Age (years)c N Age (years) N Age (years) N Age (years)
--------------------------------------------------------------------------------------------------
Men
Non-smokers 10 35.8 ± 16.7 6 33.4 ± 14.1 12 33.6 ± 12.2 52 34.2 ± 13.2
Smokers 6 30.8 ± 9.7 16 30.2 ± 11.8 36 42.8 ± 13.1 67 30.5 ± 8.9
Women
Non-smokers 3 30.7(17-46) 0 9 48.3 ± 13.0
Smokers 2 44.5(29-60) 1 51.2 4 31.0(19-43)
--------------------------------------------------------------------------------------------------
a From: Lindberg & Hedenstierna (1983).
b When N < 5, the range is given instead of SD.
c X ± SD.
Patients suffering from chromium-induced allergic skin
dermatoses had a tendency to develop cross-correlated hyper-
sensitivity to other metals, in particular, to cobalt and nickel
(Rostenberg & Perkins, 1951; Geiser et al., 1960; Clark & Kunitsch,
1972; Kogan et al., 1972).
Increased sensitivity to chromium is proved by skin reaction
tests. In a number of cases, additional immuno-haematological
bioassays are made to confirm the specificity of these tests. A
method for studying the basophil (mast-cells) degranulation in vivo,
at the site of positive compress skin tests, was suggested by Kogan
(1968). The method was used as a basis for proving the specificity
of cutaneous tests for chromium and other sensitising metals of the
chromium group (cobalt and nickel) (Kogan, 1968; Kogan et al.,
1972).
Photosensitivity is sometimes observed in patients with chronic
dermatitis. Wahlberg & Wennerstein (1977) investigated patients
allergic to chromium, with or without clinically observed light
sensitivity, by a standardized photopatch test procedure. A
significantly more intense reaction was observed in 48% of the
cases in the UV-irradiated sites (4/5 minimal erythema dose)
compared with the non-irradiated control sites.
No cases of cancer of the skin have been reported to result
from exposure to any form of chromium (Fregert, 1981).
8.3.2. Effects on the lung
Airborne chromium trioxide is rapidly absorbed in the broncho-
pulmonary tract causing corrosive reactions (Borghetti et al.,
1977). The absorption of chromium aerosols may depend on the
physical and chemical properties of the particles (Taylor et al.,
1965), as well as on the activity of alveolar macrophages, and the
lymphatic drainage (Sanders et al. 1971).
Both Jindrichova (1978) and Keskinen et al. (1980) reported
that welders using chromium-containing electrodes suffered from
bronchitis. Zober (1982) studied arc welders who had used filler
metals containing chromium and nickel for some years. Compared
with controls, the welders reported more previous ailments of the
respiratory system, mainly in the form of acute bronchitis. In
X-rays of welders, more roundish shadows were found, probably
indicative of benign accumulations of ferrous dust. The greater
incidence of significant findings was clearly apparent in welders
who smoked (probably a combined effect of tobacco smoke and welding
fumes). Reggiani et al. (1973) studied 101 electroplaters by means
of spirometric tests and found an obstructive disease pattern in
13% of them. After inhalation of acetylcholine, a bronchospastic
reaction occurred in 23% of the workers. In another spirometry
study (Bovet et al., 1977) on 44 chromium electroplaters, an
obstructive respiratory syndrome was found among the workers with a
high urinary-chromium content. The authors concluded that the
influence of tobacco smoking on the spirometric tests was much less
than the influence of chromium.
Studying lung function and generalized obstructive lung
diseases in electrofurnace workers in a ferrochromium and
ferrosilicon-producing plant, Langard (1980b) found a reduced
forced vital capacity (FVC) and an increased prevalence of
obstructive lung diseases. Unable to document a connection between
the presence of chromates and increased lung diseases, the author
suggested that the effects were due to high levels of total dust,
especially amorphous silica dust.
Lindberg & Hedenstierna (1983) studied lung function in more
than 100 subjects exposed to chrome plating and in 119 car
mechanics as controls (no car painters or welders). In the exposed
subjects, FVC and the forced expired volume in one second (FEV1)
were both reduced by 0.2 litre, and the forced mid-expiratory flow
was reduced by 0.4 litre/second. The authors concluded that an 8-h
mean exposure level of more than 2 µg/m3 might cause a transient
decrease in lung function.
Pneumoconiosis was diagnosed in Germany (Letterer et al., 1944)
and again in chromite miners in South Africa (Sluis-Cremer & du
Toit, 1968). However, American medical officers could not confirm
this feature when examining 897 workers in chromate-producing
plants in the USA (US PHS, 1953). It should be noted that chromium
dusts had a poor fibrogenic potency in experimental animal studies
(section 7.2.1.5). Davies (1974) X-rayed workers employed in the
manufacture of ferro-chrome and found results similar to miliary
lung tuberculosis. The workers were in the immediate area of the
furnace and were exposed to dust-particles, 90% of which were less
than 2 µm in diameter. The dust deposited on girders consisted of
95% SiO2 and 2.4% Cr2O3. The author did not find any symptoms or
signs of tuberculosis, but there was sufficient evidence to
indicate an inhalation hazard in the ferro-chrome industry, thus
underlining earlier observations of Princi et al. (1962).
8.3.2.1. Bronchial irritation and sensitization
Chromium irritates mucous membranes, as has been seen with
exposure to airborne chromium trioxide fume (Meyers, 1950) or mixed
dust (Card, 1935; Broch, 1949; Williams, 1969). The first symptom
was an irritating cough, 4 - 8 h after exposure. Chromium causes
sensitization that can result in asthmatic attacks. These can last
for 24 - 36 h, without treatment (Langard & Norseth, 1979). An
attack may recur on later exposure, even when this exposure is to a
much lower concentration (US NAS, 1974a).
A double-blind experimental exposure study on an asthmatic
patient, who had been exposed to chromium and nickel, showed that
chromium was the main cause of the asthma (Novey et al., 1983).
Keskinen et al. (1980) pretreated their patients, stainless steel
welders, with both disodium cromoglycate and betamethasone and
found an inhibition of this reaction. Placebo medication failed to
produce this inhibition.
8.3.3. Effects on the kidney
In the early part of this century, chromates and chromic acid
were occasionally used as therapeutic chemicals for some skin
lesions. Fatal cases of acute nephritis occurred with albumin,
hyaline, and granular casts and red cells appearing in the urine,
accompanied by oliguria. Autopsies revealed tubular necrosis, but
not glomerular damage (Kaufman et al., 1970). Acute toxic
gastroenteritis developed, followed by arterial hypotonia, in
suicide cases a few hours after swallowing 1.5 - 10 g potassium
dichromate. Within 2 - 4 days, all patients developed signs of
acute renal insufficiency with oliguria, anuria, and hyperhydration
and also evidence of acute toxic hepatitis (Luzhnikov et al.,
1976). Kidney damage has also occurred in industry, as a result of
accidental exposure of the skin to large amounts of chromate,
especially when associated with extensive skin damage (Luzhnikov et
al., 1976).
The mortality from kidney diseases among workers in the
chromium industry does not appear to have increased. In one study,
no deaths from nephritis and uraemia were found in 4 chromate-
producing plants; in only 1 plant was the mortality rate above that
for the controls (Machle & Gregorius, 1948). Hayes et al. (1979)
reported that 4 deaths from nephritis and nephrosis had occurred
between 1945 and 1977 in workers in a chromate-producing plant,
compared with 4.95 cases in the control population. Satoh et al.
(1981) found one case of nephritis and nephrosis in a group of
chromate workers compared with an expected number of 2.18.
Some authors (Franchini et al., 1975; Borghetti et al., 1977;
Gylseth et al., 1977; Tola et al., 1977) have concluded that
urinary excretion and renal clearance of diffusible chromium can be
used as biological indicators for evaluating the extent of exposure
to airborne chromium and the body burden. However, a dose-effect
relationship could not be established between duration of exposure
and either the level of chromium excretion (Tandon et al., 1977) or
early nephrotoxic indicators (Mutti et al., 1979). A dose-
response relationship for hexavalent chromium effects on the kidney
was described by Mutti et al. (1979), measuring beta-glucuronidase,
protein, and lysozyme in the urine. Abnormal patterns were not
observed in 39 stainless-steel welders during 4.5 ± 3.2 years of
exposure. In another group of workers employed in welding armoured
steel with special electrodes for 1 ± 0.4 years, 22% showed an
increase in beta-glucoronidase and 10% in protein in the urine. In a
study on 24 workers in the chromium-plating industry who were
exposed for 8.9 ± 7.3 years, the authors found increased urinary
levels of beta-glucoronidase in 37% of the workers, an increase in
protein in 17%, and an elevated level of lysozyme in 4%. Urinary
beta2-microglobulin was also studied by Lindberg & Vesterberg (1983b)
in chromeplaters (24 males, mean age 36 years, working in 4
different factories). The exposure was measured using personal air
samplers. The 8-h mean value ranged between 2 and 20 µg hexavalent
chromium/m3 and averaged 6 µg/m3. Most of the workers showed
symptoms of airway irritation, 2 of them had ulcerated nasal septum
and another 2, complete perforation. Significantly higher urine
beta2-microglobulin levels (0.23 mg/litre; range, 0.04 - 1.24 mg/litre;
SD, 0.27) were found in this group of workers in comparison with a
reference group of workers of a comparable age (mean age, 38 years;
mean urinary beta2-microglobulin level, 0.15 mg/litre; range, 0.06 -
0.19 mg/litre; SD, 0.08). A relationship was observed (Table 26)
between the range of exposure and prevalence of urinary beta2-micro-
globulin levels exceeding 0.3 mg/litre. The 2 workers with
perforated nasal septum were in the highest exposure group. A
difference in urinary beta2-microglobulin levels was not found between
a group of workers employed several years earlier in an old
chromeplating plant (mean, 0.25 mg/litre) and a group of workers of
comparable age (mean, 0.29 mg/litre), but it should be noted that
the mean age of both these groups exceeded 52 years.
Table 26. beta2-microglobulin U-beta2 levels in urine of workers
related to present exposure levelsa
-------------------------------------------------------------------------
Plant Exposure Number Age Persons with an "elevated" U-beta2
No. range of (mean) (U-beta2 > 0.30 mg/litre) range
(µg/m3) workers concentration (mg/litre)
No. Ages
-------------------------------------------------------------------------
1 11 - 20 5 39 3 21, 37, 58 0.23 - 1.30
2 - 3 14 - 8 13 37 2 20, 21 0.04 - 0.44
4 2 - 3 6 6 - - 0.06 - 0.18
-------------------------------------------------------------------------
a From: Lindberg & Vesterberg (1983b).
8.3.4. Effects on the liver
Statistics on diseases of the liver are seldom published
separately; they are usually included in the group of diseases of
the digestive system. The chromate industry was reported to have
the same rate in this group of diseases as many other industries
(US PHS, 1953). The Federal Security Agency in the USA
investigated 14 chromate workers reported to have "enlarged livers"
but found that these cases were not related to the time spent in
the industry. Acute hepatitis with jaundice was described in one
worker employed in a chromium-plating plant.
In an epidemiological study, Satoh et al. (1981) reported a low
rate of cirrhosis of the liver in chromium workers and liver
function test results that did not differ significantly from those
of the controls.
However, following ingestion of high doses of potassium
dichromate, liver necrosis (Brieger, 1920) and congested liver
(Kaufman et al., 1970) with loss of its architecture have been
described. There are also case studies of liver function and
histology in electroplaters (Pascale et al., 1952) and chromate
chemical plant workers (Etmanova, 1965), but the results are
difficult to interpret.
8.3.5. Effects on the gastrointestinal tract
The following symptoms and signs were reported in workers
engaged in the production of chromium salts (Sterekhova et al.,
1978): hyperchlorhydria, elevated pepsin and pepsinogen level,
oedema, hyperaemia and erosion of the mucosa, polyposis,
dyskinesia, and gastritis. However, Satoh et al. (1981) reported
that the incidence of peptic ulcer in chromate workers was below
the expected rate, and another study showed that the mortality rate
for diseases of the digestive system was lower in chromate workers
than in the control population (Hayes et al., 1979).
The gastric juice has been shown to play a role in the
detoxification of ingested hexavalent chromium by reducing it to
trivalent chromium, which is poorly absorbed and eliminated with
the faeces (Donaldson & Barreras, 1966; DeFlora & Boido, 1980).
8.3.6. Effects on the circulatory system
A clinical study was performed by Kleiner et al. (1970) on
myocardial function in more than 200 workers in the potassium
chromate industry, suffering from pulmonary or gastrointestinal
diseases, attributed by the authors to chromium poisoning. A
control group of 70 healthy individuals was included. Pathological
changes in the ECG and in other tests of heart function indicated
disturbance of right heart function, which the authors considered
secondary to the pulmonary pathology. The full paper was not
available and, consequently, the data could not be evaluated in
detail. It should be noted that no unexpected findings in
cardiovascular mortality were cited in the epidemiological studies
described in section 7.3.9.
8.3.7. Teratogenicity
The only human data available concerning this topic were
reported in an abstract (Morton & Elwood, 1974). The authors did
not find any correlation between frequency of malformations in the
central nervous system and the chromium content in water samples
collected in South Wales. Furthermore, Suzuki et al. (1979) did
not find any indication of accumulation of chromium in fetal
tissues.
8.3.8. Mutagenicity and other short-term tests
Few reports exist dealing with chromium-induced mutagenicity in
human subjects, and only hexavalent chromium causes any effects.
Bigaliev et al. (1978) found a 3 - 8% increase in chromosomal
aberrations of peripheral blood leukocytes in workers handling
chromium-compounds, compared with 2% in unexposed controls.
When classified according to the Papanicolaou system,
cytological samples of the sputum of 116 workers in the chromate-
production industry did not show any stages of tumour development
(Maltoni, 1976). However, intermediate stage lesions found in 30
workers were described as atypical adenomatous proliferation,
squamous-cell, and basal-cell dysplasia.
Studying cultured lymphocytes obtained from workers
occupationally exposed to CrO3, Sarto et al. (1982) found an
increased frequency of sister chromatid exchanges compared with
controls, which was correlated with urinary-chromium levels and
enhanced by smoking habits.
8.3.9. Carcinogenicity
8.3.9.1. Lung cancer
(a) Epidemiological studies in chromate-producing plants
The first recognition that cancer of the respiratory tract
might be related to chromium exposures resulted from the reporting
in 1932 of 2 cases of bronchogenic carcinoma that had occurred in
an old German chromate chemical plant (Lehmann, 1932). Gross &
Kölsch (1943) reported 10 deaths from lung cancer in small plants
producing the chromates of lead and zinc. By 1947, 52 such cases
had been reported from this industry and 10 cases from a chrome
pigment plant, also in the Federal Republic of Germany (Baetjer,
1950a).
The first epidemiological study was reported in the USA in
1948. The mortality data were based on the records of the group
life insurance policies of 6 chromate-producing plants in the USA
compared with similar data from a non-chromium industry or from a
life insurance company. The study reported that 21.8% of all
deaths between 1938 and 1947 in the chromate plants were due to
cancer of the respiratory tract compared with 1.4% in the control
group. The crude death rate for cancer of the bronchi and lungs
was 28 times that for the control groups (Machle & Gregorius,
1948). In one of these plants (Mancuso & Hueper, 1951), the cancer
death rate was 15 times that of the general population in the
county where the plant was located. The US PHS (1953) also studied
the respiratory cancer risk in these 6 plants and estimated a
relative risk of 28.9 using the average cancer mortality rates for
US males between 1940 and 1948 as a comparison.
In addition to these studies, where the cause of death was
taken from insurance or death certificate records, a case-control
study, using only cases diagnosed by autopsy or biopsy, was
conducted by Baetjer (1950b). Two hospitals near the largest
chromate plant in the USA were asked to supply the histories of all
cases diagnosed in 1924-46 as cancer of the lung bronchi, and to
confirm histopathologically. A group of non-cancer cases matched
individually by age, sex, and date of admission was selected as
controls. Of the 290 confirmed cancer cases, 3.26% had been
employed in the chromate plant, whereas none of the 900 control
cases was found to have had chromate exposure. The percentage of
chromate workers in the lung cancer series was 28.6 times the
percentage of chromate workers among the employed male population
of the city. The plant involved in this study was rebuilt in 1950,
following the discovery of the cancer problems. A new study of
this plant was made by Hayes et al. (1979) to determine whether the
risk of cancer had been eliminated. The investigation covered 438
deaths among 2101 males, who were initially employed between 1945
and 1974 for more than 90 days, and who died between 1945 and 1977.
The SMRa for malignant neoplasms of the trachea bronchi and lungs
was 202. Cancer deaths in the city in which the plant was located
were used to calculate the expected number of cases. No cases of
respiratory cancer occurred in the plant between 1960 and 1977
among workers employed only in the new plant. Because of the long
latent period, further follow-up is necessary.
Increased lung cancer rates among chromate workers in the USA
have been confirmed by Taylor (1966), Enterline (1974), and Mancuso
(1975), who also showed that the risks were especially high during
the early years of the study of the cohorts, e.g., before 1950.
Studies conducted in other countries have shown similar results.
In a 1956 study on workers in chromium-producing plants in the
United Kingdom, the rate of lung cancer mortality was 3.6 times the
rate for England and Wales (Bidstrup & Case, 1956). In a follow-up
study on 2715 men who had worked at the 3 chromate-producing
factories in the United Kingdom from 1948 to 1977, Alderson et al.
(1981) found 116 deaths from lung cancer (expected, 48). Since
modification of the one plant that is still in operation, the
relative risk of lung cancer has decreased from over 3 to about
1.8.
Two epidemiological studies have been conducted in the Japanese
chromate industry. In one plant, 5 lung cancer deaths occurred
between 1960 and 1973 in 136 workers who had been exposed for more
than 9 years; the SMR was 1510 (Watanabe & Fukuchi, 1975). In
another plant, the mortality rate for respiratory cancer was
determined for 896 males who worked in the plant from 1918 to 1975
and were followed to 1978; the SMR was 920 (expected deaths based
on 1975 age-cause-specific mortality rates for Japanese males)
(Satoh et al., 1981).
In the Federal Republic of Germany, a study was conducted in 2
chromate-producing plants (Korallus et al., 1982). Comparing the
SMR for lung cancer during the period 1948-79 for these 2 groups, a
clearly decreasing tendency was observed. In the first plant, for
1948-52, the SMR was 512 and, for 1978-79, it was 98; for the other
plant, the corresponding figures were 1905 and 150, respectively.
The newest data indicate a decreasing risk approaching the
frequency in unexposed people (Korallus & Loenhoff, 1981). This is
a result of occupational hygienic and technological measures,
especially of a change in manufacture namely the "on line"
--------------------------------------------------------------------
a The SMR (standardized mortality ratio) is the ratio of
observed to expected deaths times 100.
processing of the chrome ore, which has minimized the contents of
the carcinogenic hexavalent chromium compounds within the process
(Korallus et al., 1982).
Bittersohl (1972) described the results of a study on 30 000
employees of a large chemical unit for the period 1921-70 in the
German Democratic Republic. In particular, 588 malignancies in men
and 170 in women were evaluated for the period 1957-70. In 1971,
108 new malignancies in men and 29 in women came to light. In a
chromate factory, the rate of all cancers was far above average.
The factory manufactured catalysts through the reaction of chromic
acid and iron (III) oxide and nitric acid. The airborne
concentrations of chromium were not reported in detail, but it was
stated that short-term exposures above 400 µg/m3 occurred. The
incidence of malignant neoplasms in employees in the chromate
factory was 852 per 10 000 employees. In "unexposed" personnel,
the incidence of malignant neoplasms was 84 per 10 000 employees.
Approximately 86% of all those with malignant neoplasms were
smokers, and 78% of those without malignant neoplasms were also
smokers, indicating that smoking is not likely to be a confounding
factor.
In the USSR, a high incidence of cancer of the lungs was
reported for men engaged in the production of chrome salts. The
ratio of lung cancer in the plant to that in the control population
was 6.4 for ages 50 - 59 (748 observed, 116 expected) and 15.7 for
ages 60 - 69 (2657 observed, 170 expected) (Tyushnyakova et al.,
1974).
A survey was conducted in China in 1982 to investigate the
problem of lung cancer among chromate-producing workers. It
covered 7 cities and included 2184 male and 798 female workers who
had worked for at least one year in the industry. The preliminary
report revealed that 11 of the 101 deaths among male workers were
due to lung cancer, but none of the 13 deaths among female workers
was due to this cause.a
(b) Epidemiological studies - chrome pigment industry
The first epidemiological study, conducted in 1973, covered 3
small chrome pigment-manufacturing plants in the USA. The
percentage of lung cancer deaths was tentatively reported to be
about 3 times that in unexposed workers (Equitable Environmental
Health Inc., 1976).
Lung cancer mortality was studied among 1152 men working at 3
chromate pigment factories in the United Kingom from the 1930s or
1940s until 1981 (Davies, 1979, 1984). Among workers at factory A,
which produced both zinc and lead chromates, entrants to the
factory between 1932-45 with high and medium exposures had excess
---------------------------------------------------------------------------
a Report of an Investigation Team for Cancer in Chromate
Workers (1983).
risks of lung cancer (SMR = 223). A similar excess was seen in
1946-54 entrants, but the excess risk fell just short of
statistical significance. Highly significant excess risks of lung
cancer were seen among 1947-64 entrants that had high or medium
exposures at factory B, which also produced lead and zinc
chromates. Excesses were more severe in workers employed for more
than 10 years. In factory C, which produced only lead chromate, no
excess of lung cancer was found. However, the number of workers
and the power of the study were small.
Twenty-four male workers, who had been employed in a small
Norwegian company for more than 3 years between 1948 and 1972, were
identified and followed up by Langard & Norseth (1975), because
their principal exposure was to zinc chromate dust (measured
routinely with readings occasionally up to 0.5 mg hexavalent
chromium/m3). By December 1980, 6 cases of lung cancer had been
diagnosed, giving a relative risk of 44 compared with that of the
general population in the country. The authors stated that 5 out of
the 6 patients were smokers and only one had been exposed to
chromates other than zinc chromates (Langard & Vigander, 1983).
Sheffet et al. (1982) undertook a detailed mortality study on
workers employed in a pigment plant in Newark, where lead and zinc
chromates were used. Observed deaths from each cause among 1296
white and 650 non-white males employed between 1940 and 1969, were
compared with expected deaths, as computed from cause-, age-, and
time-specific standard death rates for the USA. A statistically-
significant relative risk of 1.6 for lung cancer among white
males, employed for 10 years or more, was found. A relative risk
of 1.9 was noted for individuals employed for at least 2 years, who
were at least moderately exposed to chromates.
An epidemiological study covering several centres in Europe was
conducted by Frentzel-Beyme (1983). This study was designed to
quantify the mortality from cancer and other diseases among workers
in European factories producing chromate pigments (3 German, 2
Dutch factories). Observed deaths in factories were compared with
expected deaths calculated on the basis of mortality figures for
the region in which a given factory was located. The overall
mortality did not deviate from the expected rates. Lung cancer
rates were always in excess of expected numbers, but only in one
cohort was the excess statistically significant. The pattern of
duration of exposure indicates that the lung-cancer risk was not
clearly correlated with length of employment.
A slight excess of lung cancer risk in chrome pigment (zinc
chromate) spray painters from 2 maintenance bases in the USA was
reported by Dalager et al. (1980). Among the 202 deaths
identified, 21 were due to respiratory cancer (11.4 expected;
comparison population: proportionate mortality, US males). No
statistically significant excess of lung cancer was found in a
group of automobile painters in which 226 deaths, including 22
cancer cases, were analysed using proportionate mortality for areas
in which the plants were located as a comparison (Chiazze et al.,
1980).
(c) Epidemiological studies - ferro-chrome industry
Langard et al. (1980) reported that 7 cases of bronchogenic
carcinoma occurred among 976 workers, who started work before
January 1, 1960. The expected number was 3.1 using national rates,
1.8 using local rates, and < 1 when using an international
reference rate for comparison. The difference was not significant
for the national and local rates, but was significant for the
international rate.
A study in Sweden did not reveal any significant excess of
respiratory cancer in ferro-chrome workers (Axelsson et al., 1980).
The incidence of cancer among 1932 workers employed for at least
one year between 1930 and 1975 was compared with the expected
number based on the National Cancer Registry data for the county in
which the plant was located. Five cases of respiratory cancer were
found against an expected 7.2 cases.
An excessive rate of lung cancer was reported for a ferro-
chrome plant in Russia (Pokrovskaya & Shabynina, 1973). The number
of deaths from cancer between 1955 and 1969 was higher than that in
the town in which the plant was located. The authors considered
that both hexavalent and trivalent chromium exposures were
responsible for the excess, since similar excessive rates were
found in a nearby ore-crushing plant, where only trivalent chromium
was present. The number of cases and the rates involved in these
studies were not given.
Hexavalent chromium is produced in some types of welding. In
section 7.2.1.2, it was mentioned that welding fume particles were
positive in the mammalian spot test, indicating a mutagenic and
possible carcinogenic potential. The incidence of lung cancer was
determined by Sjögren (1980), in a cohort of 234 welders, who had
welded stainless steel for more than 5 years between 1950 and 1965,
and were followed to 1977. Three welders died from lung cancer in
comparison with an expected number of 0.68 in the general
population ( P = 0.03).
Comparing lung cancer mortality in 2 parishes with ferro-alloy
industries to mortality in other parishes of similar size without
such industries from the same county, Axelson & Rylander (1980)
were unable to detect an increased incidence of death due to lung
cancer mortality. The concentration of chromium in the ambient air
of the most polluted areas ranged from 0.1 to 0.4 µg/m3. It should
be noted that several possible confounding factors were not under
control, e.g., migration, occupational exposure, and smoking
habits.
(d) Epidemiological studies - chromium-plating workers
Epidemiological studies on workers in the chromium-plating and
related industries were reviewed by Hayes (1982). The relevant
data from these studies are presented in Table 27. According to
Hayes, the epidemiological evidence is not sufficient to determine
the risk of cancer associated with industrial exposure to chromic
acid and, if an excess risk exists, it is probably lower than that
typically described for employment in the chromium-chemical
producing industry.
In a more recent paper, Franchini et al. (1983) conducted a
retrospective cohort study in 9 chromium-plating plants to examine
the mortality of workers employed for at least one year during the
period January 1951 to December 1981. The study group totalled 178
individuals; vital status ascertainment was 97% complete. The
total number of deaths was close to the expected figure (15
observed, 15.2 expected), whereas death from lung cancer exceeded
the expected number (3 observed, 0.7 expected; P = 0.03). The
increased mortality from lung cancer among chromium-platers seemed
to be related to exposure intensity.
8.3.9.2. Cancer in organs other than lungs
A few cancers of the upper respiratory tract and oral region,
but no excessive rates have been reported to occur in the chromate-
producing industry. The cancers have involved the buccal cavity,
pharynx, and oesophagus. Cancers of the nasal septum have not been
documented, with the exception of one case in Italy (Vigliani &
Zurlo, 1955).
In a joint Danish-Finnish-Swedish case-reference investigation,
initiated in 1977, the connection between nasal and sinonasal
cancer and various occupational exposures was studied. All new
cases of nasal and sinonasal cancer were collected from the
national cancer registers (Finland and Sweden) or from the hospitals
(Denmark). The results showed associations between nasal and
sinonasal cancer and exposure to chromium, welding, flame-cutting,
and soldering, hardwood or mixed wood dust, and softwood dust alone
(13.4) (Hernberg et al., 1983).
In 5 plants in the USA, during the early years of extremely
high exposures, the rate of cancer of the digestive tract varied
from 0 to 3.04/1000 compared with a rate of 0.59/1000 for the
controls (Machle & Gregorius, 1948). The mortality rates from
stomach cancer have been reported for chromate-producing plants in
Japan (SMR = 90) (Satoh et al., 1981) the USA (SMR = 40) (Hayes et
al., 1979). No significant differences were reported between the
incidence of stomach cancer in a Russian plant (Pokrovskaya &
Shabynina, 1973) and the incidence in the surrounding area.
Studying cancer mortality in a pigment plant using lead and
zinc chromates, Sheffet et al. (1982) found an increased (but not
statistically risk of stomach (SMR = 170) and pancreas (SMR = 200)
cancer among the total cohort (1946 employees).
Table 27. Summary of epidemiological studies on respiratory cancer in
workers employed in the chromium plating and related industriesa
------------------------------------------------------------------------------------------------
Study population Follow-up Respiratory cancer Comparison population
Number Estimated
relative risk
------------------------------------------------------------------------------------------------
Chromium plating
1056 workers employed Deaths in former and 24 1.8b Unexposed workers in
> 3 months in 54 plants current (followed 2 plants and in 2 non-
in the United Kingdom years) workers, plating industries
about 80% traced
About 5000 workers 49 1.4c Mortality analysis,
employed since 1945 in method not specified
1 plating factory in
the United Kingdom
889 workers exposed > 6 Reports from management 0 < 1b Tokyo mortality
months in Tokyo chromium of plating firms and rates
plating plants, 1970-76 follow-up of retired
workers; 19 total deaths
reported
Related industries
1292 deaths among US Union death benefit 62 1.1b Proportionate
metal polishers and claims mortality, US males
platers, union members,
1951-69
238 deaths among employees Pension, insurance and 39 2.1c Proportionate
for 10 years in one US benefit records mortality, US
plant for metal die
casting, finishing, and
electroplating, 1974-78.
------------------------------------------------------------------------------------------------
a Adapted from: Hayes (1982).
b Not statistically significant.
c P < 0.05, calculated using an assumption that the observed number is distributed as a
Poisson random variable.
8.3.9.3. Relationship between cancer risk and type of chromium
compound
As the exposures are often poorly defined, it is sometimes not
certain whether workers have been exposed to hexavalent chromium,
trivalent chromium, or a combination of the two. Some authors have
concluded that there is no increased risk of cancer mortality due
to trivalent chromium compounds (Korallus et al., 1974 a,b,c;
Axelsson et al., 1980; Langard et al., 1980; Norseth, 1980),
whereas others do not exclude that trivalent chromium compounds
have a carcinogenic capacity (Essing et al., 1971; Mancuso, 1975;
Zober 1979).
Summing up the data from case reports as well as
epidemiological studies in chromate-producing, chromate-pigment,
chromium-plating, and ferrochromium industries, the IARC Working
Group on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans (IARC, 1980) came to the conclusion that "there is
sufficient evidence of respiratory carcinogenicity in men
occupationally exposed during chromate production. Data on lung
cancer risk in other chromium-associated occupations and for cancer
at other sites are insufficient. The epidemiological data do not
allow an evaluation of the relative contributions to carcinogenic
risk of metallic chromium, trivalent chromium and hexavalent
chromium or of soluble versus insoluble chromium compounds".
9. EVALUATION OF HEALTH RISKS FOR MAN
Low levels of chromium are omnipresent in the environment.
Under normal conditions, human exposure to chromium does not
represent a toxicological risk, but it should be pointed out that
too low an intake of chromium may lead to deficiency. Airborne
concentrations of chromium, predominantly as trivalent chromium,
are usually below 0.1 µg/m3 and there are many places where the
concentration is below the detection limit. Concentrations in
river water are typically in the range of 1 - 10 µg/litre and do
not constitute a health threat. Drinking-water from municipal
water supplies does not contribute more than a few micrograms of
chromium to the daily human intake, but untreated water in certain
areas may be contaminated by runoff or effluents from industrial
sources and may contribute significant amounts of chromium. The
oceans contain less than 1 µg/litre. The daily human intake
through food varies considerably between regions. Typical values
range from 50 to 200 µg/day. They do not represent a toxicity
problem.
However, significant exposures exist in the occupational field.
In the past, chromium-ore mining generated chromium-containing
dusts, levels of which ranged up to 20 mg/m3 in different work-
places. Other exposures ranged up to 150 mg/m3 and consisted of
dust containing as much as 48% chromium (Cr2O3). In production
plants, hexavalent chromium can occur in the airborne state.
However, implementation of protective measures at ferrochromium
production sites reduced the airborne hexavalent chromium to levels
of 30 - 60 µg/m3.
The health effects of the two common oxidation states of
chromium are so fundamentally different that they must be
considered separately. In the form of trivalent compounds,
chromium is an essential nutrient and is relatively non-toxic for
man and other mammalian species. The hexavalent form is man-made
by oxidation of naturally-occurring trivalent chromium minerals and
is widely used. Compounds of hexavalent chromium penetrate
biological membranes easily and can thus interact with essential
constituents of the cells, including genetic material which they
can damage through oxidation and complexation with resulting
trivalent species. On the other hand, oxidation of trivalent
chromium has not been demonstrated in the living organism, and for
practical purposes, the reduction of hexavalent chromium to
trivalent chromium in lungs and other animal tissues is
irreversible.
9.1. Occupational Exposure
A number of effects can result from occupational exposure to
airborne chromium including irritative lesions of the skin and
upper respiratory tract, allergic reactions, and cancers of the
respiratory tract. The data on other effects, e.g., in the
gastrointestinal, cardiovascular, and urogenital systems are
insufficient for evaluation.
Epidemiological studies have shown that workers engaged in the
production of chromate salts and chromate pigments experience an
increased risk of developing bronchial carcinoma. No detailed data
on dose-response relationships are available from epidemiological
studies. Although a suspicion of increased lung cancer risks in
chromium plating workers has been raised, the available data are
inconclusive as are data for other industrial processes where
exposure to chromium occurs. There is insufficient evidence on the
role of chromium as a cause of cancer in any organ other than the
lung. Evidence from studies on laboratory animals shows that
hexavalent chromium compounds, especially those of low solubility,
can induce lung cancer.
In the lymphocytes of workers in chromium-plating factories,
the frequency of sister chromatid exchanges was higher in exposed
than in control groups.
Mutagenicity and related studies have convincingly shown that
hexavalent chromium is genetically active. Hexavalent chromium can
cross cellular membranes and is then reduced to trivalent chromium,
which can cause DNA cross-links and increase the infertility of DNA
implication. Trivalent chromium compounds have been shown to be
genetically inactive in most test systems, except in systems where
they can directly interact with DNA.
9.1.1. Effects other than cancer
9.1.1.1. Respiratory tract
It has been reported that the threshold for acute irritative
effects in the upper respiratory tract is 25 µg/m3 for the most
sensitive individuals. Long-term exposure to doses over 1 µg
chromic acid/m3 can cause nasal irritation, atrophy of the nasal
mucosa, and ulceration of perforation of the nasal septum.
Bronchial asthma was previously attributed to exposure to
chromium compounds, but scientific data are too scarce to draw
conclusions.
9.1.1.2. Skin
Skin rashes, ulcers, sores, and eczema have been reported among
occupationally exposed workers. Both trivalent and hexavalent
chromium compounds can give rise to sensitization of skin,
especially under certain environmental conditions, such as those
encountered in the cement industry, where the high incidence of
chromium-induced skin lesions can be attributed to the alkaline
exposure conditions.
Eczematous dermatitis, which manifests first as a diffuse
erythematous type, progresses in severe cases to an exudative
stage and is associated in 8 - 15% of patients with sensitivity to
chromium, as revealed by skin-patch tests. Although opinion is
divided on the oxidation state of chromium responsible for inducing
sensitization, there is evidence of hexavalent chromium penetrating
the skin as a hapten to be reduced to the trivalent state and
conjugated with proteins and transformed into the full antigen
that initiates the sensitization reaction, involving presumably
only a cell-mediated immune response. It should be noted that
patients suffering from chromium-induced skin allergy tend to
become hypersensitive to cobalt and nickel. Furthermore, 48% of
cases of skin allergy induced by chromium, with or without
clinically observed light sensitivity, showed a significantly more
intense reaction by a standardized photopatch test procedure, when
they were exposed to a 4/5 minimal erythema dose of irradiation.
9.1.1.3. Kidney
After high-dose, short-term oral ingestion of chromium, acute
nephritis and tubular necrosis were observed. A few
epidemiological studies on workers in chrome-plating industries
include data on diseases of the kidney, most of them without giving
exact exposure levels. A recent study related increased urinary-beta2
microglobulin levels to exposure ranges between 2 and 20 µg/m3.
The dose-response relationship observed in this study needs
confirmation on a larger number of exposed workers.
9.1.1.4 Other organs and systems
There are no conclusive data to evaluate the effects of
chromium compounds on the liver or gastrointestinal and circulatory
systems.
9.1.2. Teratogenicity
Both oxidation states, when injected at high levels
parenterally into animals, are teratogenic, with the hexavalent
form accumulating in the embryos to much higher concentrations than
the trivalent. The Task Group was not aware of any report
indicating teratogenicity in human populations.
9.2. General Population
Persons living in the vicinity of ferro-alloy plants, exposed
to an ambient air concentration of up to 1 µg/m3, did not show
increased lung cancer mortality.
The results of many studies suggest that exposure to chromium
through inhalation and skin contact can pose health problems for
the general population. Very little information is available on
the health effects of chromium ingested through untreated drinking-
water, though, in a single study, a correlation was observed
between frequency of malformation in the central nervous system and
the chromium content of water samples (Morton & Elwood, 1974). In
order to assess the nature of the magnitude of these problems,
there appears to be a need for more general population studies on
the effects of inhaled, absorbed, or ingested chromium on
respiratory, cardiovascular, and renal functions, and on the skin.
Studies on animals and man have established trivalent chromium
as an essential micronutrient that interacts with insulin and
enhances the physiological effects of the hormone. Supplementation
trials in a number of countries including Finland, Jordan, Nigeria,
Sweden, Turkey, and the USA have shown that segments of the
population could improve their glucose metabolism and, in some
instances, fat metabolism, by ingesting trivalent chromium
compounds. These observations suggest that some populations are at
risk of chromium deficiency. Children suffering from protein-
energy malnutrition may be at special risk.
As chromium concentrations in the body fluids of unexposed
persons are generally less than 1 µg/litre, analysis for chromium
requires the strictest quality control, including measures to
exclude sample contamination. Even with adequate analytical
methods, it is not possible, as yet, to diagnose chromium
deficiency in individuals by chemical or biochemical methods alone.
Therefore, a quantitative assessment of the prevalence of chromium
deficiency in human populations is not yet possible.
REFERENCES
ABRAHAM, A.S., SONNENBLICK, M., EINI, M., SHEMESH, O., & BATT,
A.P. (1980) The effect of chromium on established athero-
sclerotic plaques in rabbits. Am. J. clin. Nutr., 33:
2294-2298.
AITIO, A., JARVISALO, J., KULUNEN, M., TOSSAVAINEN, A., &
VAITTINEN, P. (1984) Urinary excretion of chromium as an
indicator of exposure to trivalent chromium sulfate in leather
tanning. Int. Arch. occup. environ. Health, 54: 241-249.
ALDERSON, M.R., RATTAN, N.S., & BIDSTRUP, L. (1981) Health
of workmen in the chromate-producing industry in Britain. Br.
J. ind. Med., 38: 117-124.
ALEKSANDROV, A.I. & KAINOV, V.I. (1975) [Crocoite from the
Pervomaisk-Zverevo deposit at the Central Urals.] Tr. Sverdl.
Gorn. Inst., 106: 152-155 (in Russian).
AMA (1979) Guidelines for essential trace element
preparations for parenteral use. J. Am. Med. Assoc., 241:
2051-2054.
AMERICAN PUBLIC HEALTH ASSOCIATION, AMERICAN WATER WORKS
ASSOCIATION, AND WATER POLLUTION CONTROL FEDERATION (1971)
117A. Hexavalent chromium. In: Standard methods for the
examination of water and wastewater, 13th ed., Washington DC,
American Public Health Association, pp. 156-157.
AMES, B.N., DURSTON, E., YAMASAKI, E., & LEE, F.D. (1973)
Carcinogens as mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci. (USA), 70: 2281-2285.
ANDERSON, F.E. (1960) Cement and oil dermatitis. The part
played by chrome sensitivity. Br. J. Derm., 72: 108-117.
ANDERSON, N. (1982) The environmental impact of chromium.
Final draft March 1982, Maine, Department of Environmental
Protection, Bureau of Air Quality Control, 126 pp.
ANDERSON, R.A. & POLANSKY, M.M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Tr. El. Res., 3: 1-5.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., & REAMER, D.C. (1982a) Effect of exercise
(running) on serum glucose, insulin, glucagon, and chromium
excretion. Diabetes, 31: 212-216.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., VEILLON, C., & GLINSMANN, W. (1982b)
Urinary chromium excretion of human subjects: effects of
chromium supplementation and glucose loading. Am. J. clin.
Nutr., 36: 1184-1193.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., PATTERSON, K.Y.,
VEILLON, C., & GLINSMANN, W. (1983) Effects of chromium
supplementation on urinary Cr excretion of human subjects and
correlation of Cr excretion with selected clinical parameters.
J. Nutr., 113: 276-281.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
MERTZ, W., & GLINSMANN, W. (1983b) Chromium supplementation
of human subjects: effects on glucose, insulin, and lipid
variables. Metabolism, 32: 894-899.
ANDERSSON, A. & NILSSON, K.O. (1972) Enrichment of trace
elements from sewage sludge fertilizer in soils and plants.
Ambio, 1: 176-179.
ANGERER, J., SCHALLER, K.-H., & HEINRICH, R. (1981)
[Commercially-available blood and urine samples for quality
control in toxicology.] Arbeitsmed. Sozialmed. Praeventivmed.,
16: 125-127 (in German).
ANIMAL RESEARCH DIVISION OF THE NEW ZEALAND DEPARTMENT OF
AGRICULTURE (1954) Annual report 1953-54, Wellington, R.E.
Owen Government Printer, 40 pp.
ANKE, M., DIETTRICH, M., WICKE, G., PFLUG, D., & SCHUELER, D.
(1971) Absorption, excretion, and distribution of 51chromium
given by mouth to lactating ruminants. Arch. Tierern., 21:
599-607.
AXELSSON, G. & RYLANDER, R. (1980) Environmental chromium
dust and lung cancer mortality. Environ. Res., 23: 469-476.
AXELSSON, G., RYLANDER, R., & SCHMIDT, A. (1980) Mortality
and incidence of tumours among ferrochromium workers. Br. J.
ind. Med., 37: 121-127.
BAER, H. (1920) [Dermatitis resulting from the use of a
beard trainer made partly of artificial leather.] Münch. med.
Wschr., 67: 874 (in German).
BAETJER, A.M. (1950a) Pulmonary carcinoma in chromate
workers. I. A review of the literature and report of cases.
Arch. ind. Hyg. occup. Med., 2: 487-504.
BAETJER, A.M. (1950b) Pulmonary carcinoma in chromate
workers. II. Incidence on basis of hospital records. Arch.
ind. Hyg. occup. Med., 2: 505-516.
BAETJER, A.M., LOWNEY, J.F., STEFFEE, H., & BUDACZ, V.
(1959a) Effect of chromium on incidence of lung tumours in
mice and rats. Am. Med. Assoc. Arch. Ind. Health, 20: 124-135.
BAETJER, A.M., DAMRON, C., & BUDACZ, V. (1959b) The
distribution and retention of chromium in man and animals. Am.
Med. Assoc. Arch. Ind. Health, 20: 136-150.
BAKER, R.S.V. (1984) Evaluation of metals in in vitro
assays: interpretation of data and possible mechanisms of
action. Toxicol. environ. Chem., 7: 191-212.
BEHNE, D. & BRATTER, P. (1979) [Problems of trace element
analysis in medicine.] In: Gladtke, E., Heimann, G., & Eckert,
I., ed. [Trace elements], Stuttgart, Thieme, pp. 42-52 (in
German).
BEHNE, D. & DIEHL, F. (1972) Relations between carbohydrate
and trace element metabolism investigated by neutron
activation analysis. In: Nuclear Activation Techniques in the
Life Sciences. Proceedings of a Symposium, Bled, Yugoslavia,
Vienna, International Atomic Energy Agency, pp. 407-414.
BEHNE, D., BRATTER, P., GESSNER, H., HUBE, G., MERTZ, W., &
ROSICK, U. (1976) Problems in determination of chromium in
biological materials: comparison of flameless atomic
absorption spectrometry and neutron activation analysis. Z.
anal. Chem., 278: 269-272.
BEHRBOHM, P. (1957) [Allergic contact dermatitis caused by
anticorrosives containing chromium.] Berufsdermatosen, 5:
271-283 (in German).
BENJANUVATRA, N.K. & BENNION, M. (1975) Hair chromium
concentration of Thai subjects with and without diabetes
mellitus. Nutr. Rep. Int., 12: 325-330.
BERGMANN, H. & HARDT, K. (1979) Analysis of dissolved Cr(VI)
in water by APDC-MIBK extraction and atomic absorption
spectrometry. Z. anal. Chem., 297: 381-383.
BERING, F. & ZITZKE, E. (1935) [Occupational skin diseases,]
Leipzig, Voss (in German).
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric., 23: 93-100.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BERTRAND, D. & DEWOLF, A. (1968) Requirement for the trace
element chromium in the growth of potatoes. C R Ser. D., 266:
1494-1495.
BEYERMANN, K. (1962) The analytical behaviour of very small
amounts of chromium. Part I. Z. anal. Chem., 190: 4-33. Part
II. Z. anal. Chem., 191: 345-369.
BEYERSMANN, D., KOSTER, A., BUTTNER, B., & FLESSEL, P.
(1984) Model reactions of chromium compounds with mammalian
and bacterial cells. Toxicol. environ. Chem., 8: 279-286.
BIANCHI, V., DAL TOSO, R., DEBETTO, P., LEVIS, A.G., LUCIANI,
S., MAJONE, F., & TAMINO, G. (1980) Mechanisms of chromium
toxicity in mamalian cell cultures. Toxicology, 17: 219-224.
BIANCHI, V., LEVIS, A.G., & SAGGIORO, D. (1979) Differential
cytotoxic activity of potassium dichromate on nucleoside
uptake in BHK fibroblasts. Chem.-biol.Interact., 24: 137-151.
BIANCHI, V., CELOTTI, L., LANFRANCHI, G., MAJONE, F., MARIN,
G., MONTALDI, G., SPONZA, G., TAMINO, G., VENIER, P.,
ZANTEDESCHI, A., & LEVIS, A.G. (1983) Genetic effects of
chromium compounds. Mutat. Res., 117: 279-300.
BIANCHI, V., ZANTEDESCHI, A., MONTALDI, A., & MAJONE, F.
(1984) Trivalent chromium is neither cytotoxic nor mutagenic
in permeabilized hamster fibroblasts. Toxicol. Lett., 23:
51-59.
BIDSTRUP, P.L. & CASE, R.A.M. (1956) Carcinoma of the lung
in workmen in the bichromates-producing industry in Great
Britain. Br. J. ind. Med., 13: 260-264.
BIGALIEV, A.B., ELEMESOVA, M.S., TUREBAEV, M.N., & BIGALIEVA,
R.K. (1978) [Cytogenetic study of the mutagenic activity of
industrial substances.] Zdravookhr. Kaz., 8: 48-50 (in
Russian).
BITTERSOHL, G. (1972) [Health and the working environment-
Epidemiology of carcinomatosis in the chemical industry.] In:
Proceedings of the 5th Merseburger Symposium, 17-18 November
(in German).
BLACK, C.A. (1968) Soil-plant relationships, New York, John
Wiley and Sons, 792 pp.
BLOKIN, V.S. & TROP, F.S. (1977) [Dynamics of the morpho-
logical changes in animals subjected to inhalational dust
treatment using chromium oxide and trisubstituted chromium
phosphate.] In: Dubinin, N.P., ed. [Genetic effects of the
pollution of the environment,] Moscow, Nauka, pp. 173-176 (in
Russian).
BLOOMFIELD, J.J. & BLUM, W. (1928) Health hazards in
chromium plating. Public Health Rep., 43: 2330-2347.
BONATTI, S., MEINI, M., & ABBONDANDOLO, A. (1976) Genetic
effects of potassium dichromate. Mutat. Res., 38: 147-150.
BOND, R.G., STRAUB, C.P., & PROBER, R., ed. (1973) Handbook
of environmental control. III. Water supply and treatment,
Cleveland, Ohio, CRC Press, pp. 763-764.
BONNEVIE, P. (1939) [Etiology and pathogenesis of eczemas,]
Copenhagen, Busck, Leipzig, Barth (in German).
BORGHETTI, A., MUTTI, A., CAVATORTA, A., FALZOI, M., CIGALA,
F., & FRANCHINI, I. (1977) Indices rénaux d'exposition aiguë
et d'imprégnation chronique par le chrome. Med. Lav., 68(5):
355-363.
BORY, R. & LECOCQ, I. (1953) Eczéma par cuivre chromé. Arch.
Mal. prof., 14: 144.
BOSTROM, G. (1936) [Hypersensitivity test for chromium
allergy.] Am. Derm. Vener., 17: 631-632 (in German).
BOVET, P., LOB, M., & GRANDJEAN, M. (1977) Spirometric
alterations in workers in the chromium electroplating
industry. Int. Arch. occup. environ. Health, 40: 25-32.
BOWEN, H.J.M. (1966) Trace elements in biochemistry, New
York, London, Academic Press, 241 pp.
BRARD, D. (1935) Toxicologie du chrome, Paris, Herrmann &
Co., 80 pp.
BRIEGER, H. (1920) [Clinical aspects of acute chromate
poisoning.] Z. exp. Path. Ther., 21: 393-408 (in German).
BROCH, C. (1949) [Bronchial asthma caused by chromic oxide.]
Nord. Med., 41: 996-997 (in Norwegian).
BROWN, E., SKOUGSTAD, M.W., & FISHMAN, M.J. (1970) Methods
for collection and analysis of water samples for dissolved
minerals and gases. In: Techniques of water resources
investigation of the United States geological survey,
Washington DC, US Department Interior, 160 pp.
BROWN, H.G., HENSLEY, C.P., MCKINNEY, G.L., & ROBINSON, J.L.
(1973) Efficiency of heavy metals removal in municipal sewage
treatment plants. Environ. Lett., 5: 103-114.
BRUCKDORFER, K.R., KHAN, I.H., & YUDKIN, J. (1971) Chromium
and sucrose-induced hyperglyceridemia. Proc. Nutr. Soc., 30:
74A.
BRUNE, D., NORDBERG, G., & WESTER, P.O. (1980) Distribution
of 23 elements in the kidney, liver, and lungs of workers from
a smeltery and refinery in north Sweden exposed to a number of
elements and of a control group. Sci. total Environ., 16:
13-35.
BURCKHARD, W. (1962) [Occupational skin diseases.] In:
Jadassohn, W., ed. [Handbook of skin and venereal diseases,]
Berlin, Springer, Vol. II(1), pp. 368-474.
BURROWS, D., ed. (1983) Chromium: metabolism and toxicity,
Boca Raton, Florida, CRC Press, 172 pp.
BYERRUM, R.U. (1961) Some studies on the chronic toxicity of
cadmium and hexavalent chromium in drinking-water. Eng. Bull.
Purdue Univ. Serv., 106: 1-8.
CALNAN, C.D. & HARMAN, R.R.M. (1961) Studies in contact
dermatitis. XIII. Diesel coolant chromate dermatitis. Trans.
St. John's Hosp. Derm. Soc., 46: 13.
CALOW, P. & FLETCHER, C.R. (1972) A new radiotracer
technique involving 14C and 51Cr for estimating the
assimilation efficiencies of aquatic, primary consumers.
Oecologia (Berlin), 9: 155-170.
CAMPBELL, W.J. & MERTZ, W. (1963) Interaction of insulin and
chromium (III) on mitochondrial swelling. Am. J. Physiol.,
204: 1028-1030.
CANADA, NATIONAL HEALTH AND WELFARE (1980) Chromium
drinking-water criteria review. In: Guidelines for Canadian
drinking-water quality, 1978. Supporting documentation,
Ottawa, Canada, National Health and Welfare, pp. 255-268.
CANFIELD, W.K. & DOISY, R.J. (1976) Chromium and diabetes in
the aged. In: Hsu, J.M., Davis, R.L., & Neithammer, R.W., ed.
The biomedical role of trace elements in aging, St. Peters-
burg, Florida, Eckert College, pp. 119-128.
CARD, W.I. (1935) A case of asthma sensitivity to chromates.
Lancet, 2: 1348-1349.
CARTER, J.P., KATTAB, A., ABD-EL-HADI, K., DAVIS, J., EL
GHOLMY, A., & PATWARDHAN, V.N. (1968) Chromium (III) in
hypoglycemia and in impaired glucose utilization in
kwashiorkor. Am. J. clin. Nutr., 21: 195-202.
CARY, F.E., ALLWAY, W.H., & OLSON, O.E. (1977) Control of
chromium concentrations in food plants. I. Absorption and
translocations of chromium by plants. J. agric. food Chem.,
25: 300-304. II. Chemistry of chromium in soils and its
availability to plants. J. agric. food Chem., 25: 305-309.
CASEY, C.E. & HAMBIDGE, K.M. (1984) Chromium in human milk
from American mothers. Br. J. Nutr., 52: 73-77.
CASTO, B.C., PIECZYNSKI, W.J., NELSON, R.L., & DI PAOLO, J.
(1976) In vitro transformation and enhancement of viral
transformation with metals. Proc. Am. Assoc. Cancer Res., 17:
12-20.
CASTO, B.C., MEYERS, J., & DIPAOLO, J.A. (1979) Enhancement
of viral transformation for evaluation of the carcinogenic or
mutagenic potential of inorganic metal salts. Cancer Res., 39:
193-198.
CAWSE, P.A. (1974) A survey of atmospheric trace elements in
the UK, 1972-1973, Harwell, England, Atomic Energy Research
Establishment, 95 pp.
CHANEY, R.L. (1973) Crop and food chain effects of toxic
elements in sludges and effluents. In: Recycling Municipal
Sludges and Effluents on Land. Proceedings of a Joint
Committee, National Association of State Universities and Land
Grant Colleges, Washington DC, July 9-13, 1973, pp. 129-141.
CHEN, N.S.G., TSAI, A., & DYER, I.A. (1973) Effect of
chelating agents on chromium absorption in rats. J. Nutr.,
103: 1182-1186.
CHEN, K.Y., YOUNG, C.S., JAN, T.K., & ROHATGI, N. (1974)
Trace metals in waste water effluents. J. Water Pollut.
Control Fed., 46: 2663-2675.
CHEREMISINOFF, P.N. & HABIB, Y.H. (1972) Cadmium, chromium,
lead, mercury: a plenary account for water pollution. Part I.
Occurrence, toxicity, and detection. Water Sewage Works, 119:
73-86.
CHEREVATY, V.S., EREMEEVA, E.A., & SMAGULOV, S.K. (1965) [On
the nature of nasal microflora in the workers of a chromium
compound plant.] In: Proceedings of 4th Scientific Session of
Aktubinsk State Medical Institute, Alma-Ata, Kazakhstan,
pp. 337-342 (in Russian).
CHIAZZE, L., FERENCE, L.D., & WOLF, P.H. (1980) Mortality
among automobile assembly workers. I. Spray painters. J.
occup. Med., 22: 520-526.
CHRISTIAN, G.D., KNOBLOCK, E.G., PURDY, W.C., & MERTZ, W.
(1963) A polarographic study of chromium-insulin-
mitochondrial interaction. Biochem. Biophys. Acta, 66: 420-423.
CLARK, R. & KUNITSCH, G. (1972) [Statistical studies of
cross allergy in metal salts.] Berufsdermatosen, 20(5):
222-238 (in German).
COLLINS, R.J., FROMM, P.O., & COLLINGS, W.D. (1961) Chromium
excretion in the dog. Am. J. Physiol., 201(5): 795-798.
CONDE-SALAZAR, L., MARTO-CASTANO, A., & ARROYO VICENTE, M.
(1980) Determination of chrome in rubber gloves. Contact
Dermatit., 6: 237-238.
COTTON, F.A. & WILKINSON, G. (1966) Advanced inorganic
chemistry, New York, Interscience, 1136 pp.
CRESSER, M.S. & HARGITT, R. (1976) The determination of
chromium (III) and chromium (VI) by total anion exchange and
atomic absorption spectrometry. Anal. Chim. Acta, 81: 196-198.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
DALAGER, N.A., MASON, T.J., FRAUMENI, J.F., HOOVER, R., &
PAYNE, W.W. (1980) Cancer mortality among workers exposed to
zinc chromate paints. J. occup. Med., 22: 25-29.
DANA, P. (1971) Crocoite-PbCrO4 In: Hurlbut, C.S., ed.
Dana's manual of mineralogy, 18th ed., New York, J. Wiley and
Sons Inc., pp. 346-347.
DANIELSSON, B.R.G., HASSOUN, E., & DENCKER, L. (1982)
Embryotoxicity of chromium: distribution in pregnant mice and
effects on embryonic cells in vitro. Arch. Toxicol., 51:
233-245.
DAVIDSON, I.W.F. & BLACKWELL, W.L. (1968) Changes in
carbohydrate metabolism of squirrel monkeys with chromium
dietary supplementation. Proc. Soc. Exp. Biol. Med., 127:
66-70.
DAVIDSON, I.W.F. & BURT, R.L. (1973) Physiological changes
in plasma chromium of normal and pregnant women: effect of
glucose load. Am. J. Obstet. Gynecol., 116: 601-608.
DAVIES, J.C.A. (1974) Inhalation hazards in the manufacture
of silicon alloys. Cent. Afr. J. Med., 20: 140-143.
DAVIES, J.M.G. (1972) The fibrogenic effects of mineral
dusts injected into the pleural cavity of mice. Br. J. exp.
Pathol., 53: 190-201.
DAVIES, J.M.G. (1979) Lung cancer mortality of workers in
chromate pigment manufacture: an epidemiological survey. J.
Oil. Col. Chem. Assoc., 62: 157-163.
DAVIES. J.M.G. (1984) Lung cancer mortality among workers
making lead chromate and zinc chromate pigments at three
English factories. Br. J. Ind. Med., 41: 158-169.
DAVIS, M.H. & GROSSMANN, V.B. (1971) A sensitive method for
determination of chromium in biological materials. Anal.
Biochem., 44: 339-344.
DEFLORA, S. (1978) Metabolic deactivation of mutagens in
the Salmonella microsome test. Nature (Lond.), 271: 455-456.
DEFLORA, S. (1981) Study of 106 organic and inorganic
compounds in the Salmonella microsome test. Carcinogenesis, 2:
283-298.
DEFLORA, S. & BOIDO, V. (1980) Effect of human gastric
juice on the mutagenicity of chemicals. Mutat. Res., 77:
307-315.
DEFLORA, S., COPPOLA, R., CAMOIRANO, A., BATTAGLIA, M.A., &
BENNICELLI, C. (1980) Mutagenicity of chromyl chloride and
its vapours. Carcinogenesis, 1(7): 583-587.
DEFLORA, S., BIANCHI, V., & LEVIS, A.G. (1984) Distinctive
mechanisms for interaction of hexavalent and trivalent
chromium with DNA? Toxicol. environ. Chem., 8: 287-294.
DE GROOT, A.J. & ALLERSMA, E. (1973) Field observations on
the transport of heavy metals in sediments. In: Proceedings of
the Conference on Heavy Metals in the Aquatic Environment,
Nashville, Tennessee, December, Oxford, Pergamon Press,
pp. 1-16.
DEICHMANN, W.B. & GERARDE, H.W. (1969) Toxicology of drugs
and chemicals, 4th ed., New York, London, Academic Press,
167 pp.
DI PAOLO, J.A. & CASTO, B.C. (1979) Quantitative studies of
in vitro morphological transformation of Syrian hamster cells
by inorganic metal salts. Cancer Res., 32: 1008-1013.
DJAHANSHIRI, H. (1976) [Nutritional physiology problems
concerning the trace element chromiun in the rat. V. Effects
on overall metabolic performande.] Z. Tierphysiol. Tierernähr.
Futtermittelkd., 37: 298-315 (in German).
DOBROLYUBSKII, O.K. (1959) [Changes in the biochemical
process in grapes under the influence of a microfertilizer
which contains chromium.] Biochemistry (USSR), 24: 577-582 (in
Russian).
DOISY, R.J., STREETEN, D.H.P., LEVINE, R.A., & CHODOS, R.B.
(1968) Effects and metabolism of chromium in normals, elderly
subjects, and diabetics. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
Univerisity of Missouri, Vol. 2, 75 pp.
DOISY, R.J., STREETEN D.H.P., SOUMA, M.L., KALAFER, M.E.,
REKANT, S.L., & DALAKOS, T.G. (1971) Metabolism of
51chromium in human subjects. In: Mertz, W. & Cornatzer, W.E.,
ed. Newer trace elements in nutrition, New York, Marcel
Dekker, pp. 155-169.
DOISY, R.J., STREETEN, D.H.P., FREIBERG, J.M., & SCHNEIDER,
A.J. (1976) Chromium metabolism in man and biochemical
effects. In: Prasad, A.S., ed. Trace elements in human health
and disease, New York, London, Academic Press, Vol. 2, pp.
79-104.
DOMASEVA, T.V. (1971) [On the effect produced by chromium
compounds on the skin.] In: [Occupational skin diseases,]
Leningrad, Leningrad Institute of Sanitation and Hygiene,
Vol. 93, pp. 168-173 (in Russian).
DONALDSON, R.M., Jr & BARRERAS, R.F. (1966) Intestinal
absorption of trace quantities of chromium. J. lab. clin.
Med., 68: 484-493.
DOUGLAS, G.R., BELL, R.D.K., GRANT, C.E., WYTSMA, J.M., &
BORA, K.C. (1980) Effect of lead chromate on chromosome
aberration, sister-chromatid exchange, and DNA damage in
mammalian cells in vitro. Mutat. Res., 77: 157-163.
DURFOR, C.N. & BECKER, E. (1964) Public water supplies of
the 100 largest cities in the United States, 1962, Washington
DC, US Geological Survey, 364 pp (Water Supply Paper No. 1812).
DURUM, W.H. & HAFFTY, J. (1963) Implications of the
minor-element content of some major streams in the world.
Geochim. Cosmochim Acta, 27: 1-11.
EATON, D.L., STACEY, N.H., WONG, K.L., & KLAASSEN, C.D.
(1980) Dose-response effects of various metal ions on rat
liver metallothionein, glutathione, heme oxygenase, and
cytochrome P-450. Toxicol. appl. Pharmacol., 55: 393-402.
EDMUNDSON, W.F. (1951) Chrome ulcers of the skin and nasal
septum and their relation to patch testing. J. Invest. Derm.,
17: 17-19.
EIFAC (1983) Water quality criteria for european freshwater
fish. Report on chromium and freshwater fish, European Inland
Fisheries Advisory Commission, Working Party on Water Quality
Criteria for European Freshwater Fish, 31 pp (EIFAC Technical
Paper No. 43).
ELIAS, Z., SCHNEIDER, O., AUBRY, F., DANIERE, M.C., & POIROT,
O. (1983) Sister chromatid exchanges in chinese hamster V79
cells treated with the trivalent chromium compounds chromic
chloride and chromic oxide. Carcinogenesis, 3: 605-611.
ENTERLINE, P.E. (1974) Respiratory cancer among chromate
workers. J. occup. Med., 16: 523-526.
ENVIRONMENTAL INSTRUMENTATION GROUP (1973) Instrumentation
for environmental monitoring: biomedical. IV, Berkeley,
California, Lawrence Berkeley Laboratory.
EQUITABLE ENVIRONMENTAL HEALTH, INC. (1976) An epidemiologic
study of workers in lead chromate plants: Final report
(prepared for the Dry Color Manufacturer's Association,
Berkeley California).
ESSING, H.G., SZADKOWSKI, D., & VALENTIN, H. (1971)
[Significance of the valence gradations of chromium compounds
in occupational medicine examinations.] Med. Sachverständige,
67: 35-39 (in German).
ETMANOVA, Z.J. (1965) [On the functional state of the liver
in workers of Aktubinsk chromium compound plant.] Gig. Tr.
prof. Zabol., 10: 55-56 (in Russian).
EVAN, A.P. & DAIL, W.G. (1974) The effect of sodium chromate
on the proximal tubules of the rat kidney. Lab. Invest.,
30(6): 704-715.
FANG, Y. (1983) [The normal value of chromium in urine for
299 unexposed persons in Yanjian city.] J. Yanbian Med.
College, 6 (1): 35-37 (in Chinese).
FARKAS, T.G. & ROBERSON, S.L. (1965) The effect of Cr(III)
on the glucose utilization of isolated lenses. Exp. Eye Res.,
4: 124-126.
FELDMAN, F.J. (1968) The state of chromium in biological
materials. Fed. Proc., 27: 482.
FELDMAN, F.J., KNOBLOCK, E.C., & PURDY, W.C. (1967) The
determination of chromium in biological materials by atomic
absorption spectroscopy. Anal. Chim. Acta, 38: 489-497.
FRADKIN, A., JANOFF, A., LANE, B.P., & KUSCHNER, M. (1975)
In vitro transformation of BHK21 cells grown in the presence
of calcium chromate. Cancer Res., 35: 1058-1061.
FRANCHINI, I., MUTTI, A., GARDINI, F., & BORGHETTI, A.
(1975) [Excretion and renal elimination of chromium compared
to degree and length of occupational exposure.] In: Roche, L.
& Trager, J., ed. Rein et Toxique. Réunion de la Société
Francaise de Toxicologie (Compte rendu), Paris, Groupement
Francais Des Centre Anti-Poisons, pp. 271-281.
FRANCHINI, I., MUTTI, A., CAVATORTA, A., CORRADI, A., COSI,
A., OLIVETTI, A., & BORGHETTI, A. (1978) Nephrotoxicity of
chromium. Control Nephrol., 10: 98-110.
FRANCHINI, J., MAGNANI, F., & MUTTI, A. (1983) Mortality
experience among chrome plating workers. Scand. J. Work
environ. Health, 9: 247-252.
FREGERT, S. (1975) Occupational dermatitis in a 10-year
material. Contact Dermatit., 1: 96-107.
FREGERT, S. (1981) Manual of contact dermatitis, 2nd ed.,
Copenhagen, Munksgaard, 139 pp.
FREGERT, S. & GRUVBERGER, B. (1972) Chemical properties of
cement. Berufsdermatosen, 20(5): 238-245.
FREGERT, S. & GRUVBERGER, B. (1979) Chromium in industrial
leather gloves. Contact Dermatit., 5: 189.
FREGERT, S. & RORSMAN, H. (1964) Allergy to trivalent
chromium. Arch. Dermatol., 90: 4-6.
FREGERT, S., HJORTH, N., MAGNUSSON, B., BANDMANN, H.-J.,
CALNAN, C.D., CRONIN, E., MALTEN, K., MENEGHINI, C.L., PIRILA,
V., & WILKINSON, D.S. (1969) Epidemiology of contact
dermatitis. Trans. St. John's Hosp. Dermatol. Soc., 55: 17-35.
FRENTZEL-BEYME, R. (1983) Lung cancer mortality of workers
employed in chromate pigment factories. J. Cancer Res. clin.
Oncol., 105: 183-188.
FREUND, H., ATAMIAN, S., & FISCHER, J.E. (1979) Chromium
deficiency during total parenteral nutrition. J. Am. Med.
Assoc., 241: 496-498.
FSA (1953) Health workers in chromate producing industry. A
study, Washington DC, Federal Security Agency, 131 pp (US PHS
Publication No. 192).
FUKAI, R. (1967) Valency state of chromium in seawater.
Nature (Lond.), 213: 901.
FUKUNAGA, M., KURACHI, Y., & MIZUGUCHI, Y. (1982) Action of
some metal ions on yeast chromosomes. Chem. pharm. Bull., 30:
3017-3019.
FURST, A., SCHLAUDER, M., & SASMORE, D.P. (1976) Tumour-
igenic activity of lead chromate. Cancer Res., 36: 1779-1783.
GALE, T.F. (1974) Effects of chromium on the hamster embryo.
Teratology, 9: A-17.
GALE, T.F. (1978) Embryotoxic effects of chromium trioxide
in hamsters. Environ. Res., 16: 101-109.
GALE, T.F. (1982) The embryotoxic response to maternal
chromium trioxide exposure in different strains of hamsters.
Environ. Res., 29: 196-203.
GALE, T.F. & BUNCH, J.D. (1979) The effect of the time of
administration of chromium trioxide on the embryotoxic
response in hamsters. Teratology, 19: 81-86.
GARCIA, J.D. & WETTERHAHN JENNETTE, K. (1981) Electron-
transport cytochrome P-450 system is involved in the
microsomal metabolism of the carcinogen chromate. J. inorg.
Biochem., 14: 281-295.
GEISER, J.D., JEANNERET, J.P., & DELACRETAZ, J. (1960) Eczema
due to cement and sensitization to cobalt. Dermatologica
(Basel), 121: 1-7.
GEMMELL, R.P. (1973) Revegetation of derelict land polluted
by a chromate smelter. Part 1. Chemical factors causing
substrate toxicity in chromate smelter waste. Environ.
Pollut., 5: 181-197.
GEMMELL, R.P. (1974) Revegetation of derelict land polluted
by a chromate smelter. Part 2. Techniques of revegetation of
chromate smelter waste. Environ. Pollut., 6: 31-37.
GENTILE, J.M., HYDE, K., & SCHUBERT, J. (1981) Chromium
genotoxicity as influenced by complexation and rate effects.
Toxicol. Lett., 7: 439-448.
GERAUER, A. (1954) [On the growing importance of
epicutaneous allergy to chromium.] Z. Haut-Geschlechtskr., 17:
97-102 (in German).
GILANI, S.H. & MARANO, M. (1979) Chromium poisoning and
chick embryogenesis. Environ. Res., 29: 427-431.
GERICKE, S. (1943) Studies on the effect of the trace
element chromium on the growth of plants. Bodenkd.
Pflanzenernaehr., 33: 114-128.
GLASER, U., HOCHRAINER, D., KLOPPEL, H., KORDEL, W., & KUHNEN,
H. (1984) [Inhalation studies with Wistar rats and patho-
physiological effects of chromium,] Berlin, 156 pp (Report to
Umweltbundesamt, D-1 UFOPLAN F+E 10606007/2) (in German).
GLASER, U., HOCHRAINER, D., KLOPPEL, H., & KUHNEN, H. (1985)
Low-level chromium VI inhalation effects on alveolar macro-
phages and immune functions in Wistar rats. Arch. Toxicol.,
57: 250-256.
GLINSMANN, W.H., FELDMAN, F.J., & MERTZ, W. (1966) Plasma
chromium after glucose administration. Science, 152: 1243.
GLINSMANN, W.H. & MERTZ, W. (1966) Effect of trivalent
chromium on glucose tolerance. Metabolism, 15: 510-520.
GMELIN, C.G. (1826) Experiments on the effects of baryta,
strontia, chrome, molybdenum, tungsten, tellurium, titanium,
osmium, platinum, iridium, rhodium, palladium, nickel, cobalt,
uranium, cerium, iron, and manganese, on the animal system.
Edinburgh med. Surg. J., 26: 131-139.
GOMES, E.R. (1972) Incidence of chromium-induced lesions
among electroplating workers in Brazil. Ind. Med., 41(12):
21-25.
GOMEZ-ARROYO, S., ALTAMIRANO, M., & VILLALOBOS-PIETRINI, R.
(1981) Sister-chromatid exchanges induced by some chromium
compounds in human lymphocytes in vitro. Mutat. Res., 90:
425-431.
GONCHAROV, A.T. (1968) [Role of chromium in the nutrition of
animals and man.] Vopr. Pitaniya, 27: 59-66 (in Russian).
GONCHAROV, A.T. (1978) [Maximum physiological dose of
chromium in food for human consumption.] Kazanskij med. Z.,
59(2): 90-93 (in Russian).
GRAY, S.J. & STERLING, K. (1950) The tagging of red cells
and plasma proteins with radioactive chromium. J. clin.
Invest., 29: 1604-1613.
GREBE, W.-H., KASTNER, H., KIPPENBERGER, C., KRAUSS, U.,
KRUSZONA, M., SCHMIDT, H., LIEBRUCKS, M., RUMBERGER, M., &
WETTIG, E. (1975) [Investigation on supply and demand for
mineral raw materials. VII. Chromium,] Berlin, Hannover,
Bundesanstalt für Gewissenschaften und Rohstoffe/Deutsches
Institut für Wirtschaftsforschung, 147 pp.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38: 33-42.
GROSS, W.G. & HELLER, V.G. (1946) Chromates in animal
nutrition. J. ind. Hyg. Toxicol., 28: 52-56.
GROSS, E. & KOLSCH, F. (1943) [Lung cancer in the chromate
dye industry.] Gewerbepathol. Gewerbehyg., 12: 164-170 (in
German).
GURSON, C.T. (1977) The metabolic significance of dietary
chromium. In: Draper, H.H., ed. Advances in nutritional
research, New York, Plenum Publishing Company, Vol. 1,
pp. 23-53.
GURSON, C.T. & SANER, G. (1971) Effect of chromium on
glucose utilization in marasmic protein-calorie malnutrition.
Am. J. clin. Nutr., 24: 1313-1319.
GURSON, C.T. & SANER, G. (1973) Effect of chromium
supplementation on growth in marasmic protein-calorie
malnutrition. Am. J. clin. Nutr., 26: 988-991.
GURSON, C.T. & SANER, G. (1978) The effect of glucose
loading on urinary excretion of chromium in normal adults,
individuals from diabetic families, and in diabetics. Am. J.
clin. Nutr., 31: 1158-1161.
GUTHRIE, B.E. (1973) Daily dietary intakes of zinc, copper,
manganese, chromium, and cadmium by some New Zealand women.
Proc. Univ. Otago Med. School., 51: 47-49.
GUTHRIE, B.E., WOLF, W.R., & VEILLON, C. (1978) Background
correction and related problems in the determination of
chromium in urine by graphite furnace atomic absorption
spectrometry. Anal. Chem., 50: 1900-1902.
GUTHRIE, B.E., WOLF, W.R., VEILLON, C., & MERTZ, W. (1979)
Chromium in urine. In: Hemphill, D.D., ed. Trace substances in
environmental health, Columbia, Missouri, University of
Missouri, Vol. XII, pp. 490-492.
GUY, W.B. (1954) Dermatologic problems in the railroad
industry resulting from conversion to Diesel power. Am. Med.
Assoc. Arch. Derm. Syph., 70: 289-292.
GYLSETH, B., GUNDERSEN, N., & LANGORD, S. (1977) Evaluation
of chromium exposure based on a simplified method for urinary
chromium determination. Scand. J. Work environ. Health, 3:
28-31.
HAMBIDGE, K.M. (1971) Chromium nutrition in the mother and
in the growing child. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 169-194.
HAMBIDGE, K.M. (1974) Chromium nutrition in man. Am. J.
clin. Nutr., 27: 505-514.
HAMBIDGE, K.M. & BAUM, J.D. (1972) Hair chromium concen-
trations of human newborn and changes during infancy. Am. J.
clin. Nutr., 25: 376-379.
HAMBIDGE, K.M. & RODGERSON, D.O. (1969) A comparison of the
hair chromium levels of nulliparous and parous women. Am. J.
Obstet. Gynecol., 103: 320-321.
HAMBIDGE, K.M., RODGERSON, D.O., & O'BRIEN, D. (1968)
Concentration of chromium in the hair of normal children and
children with juvenile mellitus. Diabetes, 17: 517-519.
HARA, N. (1982) [The change of valence of chromium by acid
treatment.] Sangyo Igaku, 24: 658-662 (in Japanese).
HARRISON, D.L. & STAPLES, E.L.J. (1955) Zinc chromate
poisoning in calves. N.Z. vet. J., 3: 63-73.
HASEGAWA, M. (1938) Studies on the detexicating hormone of
the liver (Yakriton). Toholu J. exp. Med., 32: 163-176.
HAYES, R.B. (1982) Carcinogenic effects of chromium, In:
Langard, S., ed. Biological and environmental aspects of
chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 221-247.
HAYES, R.B., LILIENFELD, A.M., & SNELL, L.M. (1979)
Mortality in chromium chemical production workers: a
prospective study. Int. J. Epidemiol., 8: 365-374.
HEATH, J.C., FREEMAN, M.A.R., & SWANSON, S.A.V. (1971)
Carcinogenic properties of wear particles from prostheses made
in cobalt-chromium alloy. Lancet, 1: 564-566.
HENNIG, A., ANKE, M., DIETRICH, M., HARTMANN, G., & HOFFMANN,
G. (1971) Absorption and distribution of 51chromium after
oral administration to laying hens as a function of time.
Arch. Tierern., 21: 609-615.
HEPLER, O.E. & SIMONDS, J.P. (1946) Experimental nephro-
pathies. IV. Glycosuria in dogs poisoned with uranyl nitrate,
mercury bichloride and potassium dichromate. Arch. Path., 41:
42-49.
HERNBERG, S., WESTERHOLM, P., SCHULTZ-LARSEN, K., DEGERTH,
R., KUOSMA, E., ENGLUND, A., ENGZELL, U., SAND HANSEN, H., &
MUTANEN, P. (1983) Nasal and sinonasal cancer: connection
with occupational exposures in Denmark, Finland, and Sweden.
Scand. J. Work environ. Health, 9: 315-326.
HERTEL, R.F. (1982) [Chromium as a problem in physiology,
epidemiology and biological monitoring.] Staub-Reinhalt. Luft,
42: 135-137 (in German).
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand
cultures. J. exp. Bot., 4: 59-64.
HILT, G. (1954) La dermite du chrome hexavalent dans le
cadre des dermites eczémateuses par sensibilisation aux
métaux. Dermatologica (Basel), 109: 143-174.
HINTZE, C. (1930) [Handbook of mineralogy,] Berlin, Leipzig,
De Gruyter, Vol. 1. III/2, pp. 4012-4030.
HOPKINS, L.L., Jr (1965) Distribution in the rat of physio-
logical amounts of injected 51Cr(III) with time. Am. J.
Physiol., 209: 731-735.
HOPKINS, L.L., Jr & SCHWARZ, K. (1964) Chromium (III)
binding to serum proteins, specifically siderophidin. Biochem.
Biophys. Acta, 90: 484-491.
HOPKINS, L.L., Jr, RANSOME-KUTI, O., & MAJAJ, A.S. (1968)
Improvement of impaired carbohydrate metabolism by chromium
(III) in malnourished infants. Am. J. clin. Nutr., 21: 203-211.
HUCKABEE, J.W., CARTAN, F.O., & KENNINGTON, G.S. (1972)
Environmental influence on trace elements in hair of 15
species of mammals, Oak Ridge, Tennessee, Oak Ridge National
Laboratory, 36 pp (ORNL-TM-3747).
HUEPER, W.C. (1958) Experimental studies in metal
cancerogenesis. X. Cancerigenic effects of chromite ore roast
deposited in muscle tissue and pleural cavity of rats. Arch.
ind. Health, 18: 284-291.
HUEPER, W.C. (1961) Environmental carcinogenisis and cancer.
Cancer Res., 21: 842-857.
HUNTER, W.C. & ROBERTS, J.M. (1933) Experimental study of
the effects of potassium bichromate in the monkey's kidney.
Am. J. Pathol., 9: 133-147.
HYODO, K., SUZUKI, S., FURUYA, N., & MESHIZUKA, K. (1980) An
analysis of chromium, copper, and zinc in organs of a chromate
worker. Int. Arch. occup. environ. Health, 46: 141-150.
IARC (1980) Some metals and metallic compounds, Lyons,
International Agency for Research on Cancer, pp. 205-323 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 23).
IIJIMA, S., MATSUMOTO, N., LU, C.C., & KATSUNUMA, H. (1975)
Placental transfer of CrCl3 and its effects on fetal growth
and development and development in mice. Teratology, 12: 198.
IMBUS, H.R., CHOLAK, J., MILLER, L.H., & STERLING, T. (1963)
Boron, cadmium, chromium, and nickel in blood and urine. Arch.
environ. Health, 6: 112-121.
IMREH, ST. & RADULESCU, D. (1982) Cytogenic effects of
chromium in vivo and in vitro. Mutat. Res., 97: 192-193.
IVANKOVIC, S. & PREUSSMANN, R. (1975) Absence of toxic and
carcinogenic effects after administration of high doses of
chromium oxide pigment in subacute and long-term feeding
experiments in rats. Food Cosmet. Toxicol., 13: 347-351.
JAEGER, H. & PELLONI, E. (1950) Tests épicutanés aux
bichromates, positifs dans l'eczéma au ciment. Dermatologica
(Basel), 100: 207-216.
JEEJEEBHOY, K.N., CHU, R.G., MARLISS, E.B., GREENBERG, G.R., &
BRUCE-ROBERTSON, A. (1977) Chromium deficiency, glucose
intolerance, and neuropathy reversed by chromium supplement-
ation, in a patient receiving long-term total parenteral
nutrition. Am. J. clin. Nutr., 30: 531-538.
JENNETTE, K.W. (1979) Chromate metabolism in liver micro-
some. Biol. Trace Elem. Res., 1: 55-62.
JENSEN, L.S., MAURICE, D.V., & MURRAY, M.W. (1978) Evidence
for a new biological function of chromium. Fed. Proc., 37: 404.
JINDRICHOVA, J. (1978) [Chromium damage in arc welders.] Z.
Ges. Hyg., 24: 86-89 (in German).
JOHNSON, J.L. (1974) Characterization of air-borne partic-
ulates. Microchem. J., 19:168-178.
JONES, G.B., BUCKLEY, R.A., & CHANDLER, C.S. (1975) The
volability of chromium from brewer's yeast during assay. Anal.
Chim. Acta, 80: 389-392.
JOSCHI, A.P. & NEEB, R. (1980) Gas chromatographic elemental
analysis via di(trifluorethyl)dithiocarbamatochelates. Z.
anal. Chem., 303: 389-393.
KAPLAN, I. & ZELIGMAN, I. (1962) Occupational dermatitis in
railroad workers. Am. Med. Assoc. Arch. Derm., 85: 135-142.
KAUFMANN, D.B., DINICOLA, W., & MCINTOSH, R. (1970) Acute
potassium dichromate poisoning. Am. J. dis. Child, 119:
374-376.
KANEKO, T. (1976) Chromosome damage in cultured human
leucocytes induced by chromium chloride and chromium trioxide.
Jpn. J. ind. Health, 18: 136-137.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec assay and
mutagenicity studies on metal compounds. Mutat. Res., 77:
109-116.
KAZANTZIS, G. & LILLY, L. (1979) Mutagenic and carcinogenic
effects of metals. In: Friedberg, L., Nordberg, G.F., & Vouk,
V.B., ed. Handbook on the toxicology of metals, Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 237-273.
KELLER, C. (1980) [Activation analysis.] In: Bartholomé, E.,
Biekert, E., Hellmann, H., Ley, H., Weigert, M., & Weise, E.,
ed. [Ullmann's encyclopaedia of technical chemistry,] 4th ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
Vol. 5, pp. 685-707 (in German).
KEMP, K., JENSEN, F.P., MOELLER, J.T., & HANSEN, G. (1974)
Trace multi-element analysis of biological tissue by proton-
induced X-ray fluorescence spectroscope, Roskilde, Denmark,
Danish Atomic Energy Commission, 22 pp (Report Riso-M-1732).
KEMPF, T. & SONNEBORN, M. (1976) [Determination of Pb, Cd,
Cr, and Zn according to the drinking-water regulation Federal
Republic of Germany.] In: Aurand, K., Hässelbarth, U., Müller,
G., Schumacher, W., & Steuer, W., ed. [The drinking-water
regulation,] Berlin, Erich Schmidt Verlag, pp. 119-125 (in
German).
KEMPF, T. & SONNEBORN, M. (1981) [Chemical composition of
drinking-waters in various regions of the Federal Republic of
Germany.] Vom Wasser, 57: 83-94 (in German).
KESKINEN, H., KALLIOMAKI, P.-L., & ALANKO, K. (1980) Occupa-
tional asthma due to stainless steel welding fumes. Clin.
Allerg., 10: 151-159.
KIRCHGESSNER, M., MERZ, G., & OELSCHLAEGER, W. (1960)
[Effect of vegetation state on content of macro and micro
elements of three species of grass.] Arch. Tierern., 10:
414-427 (in German).
KITTELBERGER, F. (1973) [Contribution to residue analysis in
fish.] Tierärztl. Prax., 1: 465-479.
KLEIN, L.A., LANG, M., NASH, N., & KIRSCHNER, S.L. (1974)
Sources of metals in New York City wastewater. J. Water
Pollut. Control Fed., 46: 2653-2662.
KLEINER, A.M., STOLBUN, B.M., LIKHACHEVA, E.L., & BELJAEVA,
L.N. (1970) [Some indices of the functional state of the
myocardium and hemodynamics in chronic intoxication with
chromium compounds.] Gig. Tr. prof. Zabol., 12: 7-10 (in
Russian).
KNUDSEN, I. (1980) The mammalian spot test and its use for
the testing of potential carcinogenicity of welding fume
particles and hexavalent chromium. Acta pharmacol. toxicol.,
47: 66-70.
KOGAN, V.Y. (1968) [The basophil degranulation as an index
of skin sensitization to cobalt compounds.] In: Belenky, G.B.,
ed. [Modern aspects of clinical and experimental dermato-
venerology. Works of the clinic of skin and venerological
diseases,] Moscow, Medizina, pp. 100-104 (in Russian).
KOGAN, V.Y. (1971) [Chromium as a specific cause for
occupation dermatoses.] In: Babayants, R.S., ed. [Topical
questions of dermato-venerology. First Medical Institute,
All-Union Conference, Moscow, I.A. Sechenov, pp. 172-176
(in Russian).
KOGAN, V.Y, ARUTYUNYAN, K.E., & KOCHINYAN, Y.E. (1972) [A
comparative characteristic of chromium, nickel, and cobalt
salts as a causative factor in allergic contact dermatosis.]
In: [Clinical Pathology of Chemical Aetiology. Materials of
the 1st All-Union Conference, Kiev, USSR, 24-25 October,
1972,] Kiev, pp. 144 and 146 (in Russian).
KOHLER, H. (1951) [Constitutional examinations of subjects
with skin diseases in the offset department of a printing
works (Doctoral thesis, Leipzig.)] Berufsdermatosen, 4: 93 (in
German).
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace
metals during the ashing of biological materials. Analyst,
101: 870-875.
KOIVISTOINEN, P., ed. (1980) Mineral element composition of
Finnish foods. Acta agric. Scand., Suppl. 22: 171
pp.
KOPP, J.E. & KRONER, R.C. (1968) Trace metals in waters of
the United States: a five-year summary of trace metals in
rivers and lakes of the United States (October 1 1962 -
September 30, 1967), Cincinnati, Ohio, US Department of the
Interior, 32 pp.
KORALLUS, U. & LOENHOFF, N. (1981) [Industrial medical and
epidemiological experience with the manufacture and processing
of chromates.] Arbeitsmed. Sozialmed. Praeventivmed., 16:
285-289 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974a)
[Trivalent chromium compounds. Results of a study in
occupational medicine. I. General; Technology; Preliminary
study.] Arbeitsmed. Sozialmed. Praeventivmed., 9: 51-54 (in
German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974b)
[Trivalent chromium compounds: results of a study in
occupational medicine. II. Disease status analysis.]
Arbeitsmed. Sozialmed. Praeventivmed., 9: 76-79 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974c)
[Trivalent chromium compounds: Results of a study in
occupational medicine. III. Clinical studies.] Arbeitsmed.
Sozialmed. Praeventivmed., 9: 248-252 (in German).
KORALLUS, U., LANGE, H.-J., NEISS, A., WUESTEFELD, E., &
ZWINGERS, T. (1982) [Correlations between reorganization
measures and bronchial carcinoma mortality in the chromate-
manufacturing industry.] Arbeitsmed. Sozialmed. Praevent-
ivmed., 7: 159-167 (in German).
KOSHI, K. (1979) Effects of fume particles from stainless
steel welding on sister chromatid exchanges and chromosome
aberrations in cultured Chinese hamster cells. Ind. Health.,
17: 39-49.
KOTZ, L., KAISER, G., TSCHOPEL, P., & TOLG, G. (1972)
[Clarification of biological matrices for determining very low
contents of trace elements when limited amounts are weighed
with nitric acid under pressure in a teflon vessel.] Z. anal.
Chem., 260: 207-209 (in German).
KOUTRAS, G.A., HATTORY, M., SCHNEIDER, A.S., EBAUGH, F.G., &
VALENTINE, W.N. (1964) Studies on chromated erythrocytes:
effect of sodium chromate on erythrocyte glutathione reduc-
tase. J. clin. Invest., 43: 323-331.
KRISHNAJA, A.P. & REGE, M.S. (1982) Induction of chromosomal
aberrations in fish Boleophthalmus dussumieri after exposure
in vivo to mitomycin C and heavy metals mercury, selenium, and
chromium. Mutat. Res., 102: 71-82.
KROEPFLI, P. & SCHUPPLI, R. (1955) [Detergent eczema.]
Dermatologica (Basel), 110: 1-7 (in German).
KRUGER, H. & DORN, H. (1956) [Clinical observations on
detergent eczema in association with chromium and nickel
allergy.] Z. Haut-Geschlechtskr., 20: 307-314 (in German).
KUCHER, I.M. (1966) In: [Proceedings of the 5th Scientific
Session of the Aktiubinsk Medical Institute, Alma-Ata,
Kazakhstan,] pp. 24-26 (in Russian).
KUHN, J.K. (1973) Trace elements in whole coal determined by
X-ray fluorescence. Norelco Rep., 20(3): 7-10.
KUMPULAINEN, J.T., WOLF, W.R., VEILLON, C., & MERTZ, W.
(1979) Determination of chromium in selected United States
diets. J. agric. food Chem., 27: 490-494.
KUMPULAINEN, J., LEHTO, J., KOIVISTOINEN, P., UUSITUPA, M., &
VUORI, E. (1983) Determination of chromium in human milk
serum and urine by electrothermal atomic absorption spectro-
metry without preliminary ashing. Sci. total Environ., 31:
71-80.
KURYLOWICZ, B. & GASIOROWSKI, S. (1958) [Agrochemical study
on calcinated phosphate-containing admixtures of chromium
compounds.] Przemysl Chem., 37: 797-800 (in Polish).
LANGARD, S. (1977) The fate of chromium after intravenous
administration of Na251 CrO4 and CrCl3 x 6H2 O to the rat,
Guildford, England, University of Surrey, 66 pp (M.Sc.
Toxicology Thesis).
LANGARD, S. (1980a) Chromium. In: Waldron, H.A., ed. Metals
in the environment, London, New York, Academic Press,
pp. 111-132.
LANGARD, S. (1980b) A survey of respiratory symptoms and
lung function in ferrochromium and ferrosilicon workers. Int.
Arch. occup. environ. Health, 46: 1-9.
LANGARD, S., ed. (1982) Biological and environmental aspects
of chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, 285 pp.
LANGARD, S. & HENSTEN-PETTERSEN, A. (1981) Chromium
toxicology. In: Williams, D.F., ed. Systemic aspects of
biocompatibility, Boca Raton, Florida, CRC Press, Vol. 1, pp.
144-161.
LANGARD, S. & NORSETH, T. (1975) A short study of bronchial
carcinomas in workers producing chromate pigments. Br. J. ind.
Med., 32: 62-65.
LANGARD, S. & NORSETH, T. (1979) Chromium. In: Friberg, L.,
Nordberg, G.F., & Vouk, V.B., ed. Handbook on the toxicology
of metals, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 383-397.
LANGARD, S. & STERN, R.M. (1985) Nickel in welding fumes: a
cancer hazard to welders? In: Sunderman, F.W.J., Aitio, A.,
Berlin, A., Bishop, C., Buringh, E., Davis, W., Gounar, M.,
Jacquignon, P.C., Mastromatteo, E., Rigaut, J.P., Rosenfeld,
C., Saracci, R., & Sors, A., ed. Nickel in the human
environment, Lyons, International Agency for Research on
Cancer, pp. 95-103 (IARC Scientific Publications No. 53).
LANGARD, S. & VIGANDER, T. (1983) Occurrence of lung cancer
in workers producing chromium pigments. Br. J. ind. Med., 40:
71-74.
LANGARD, S., GUNDERSEN, N., TSALEV, D.L., & GYLSETH, B.
(1978) Whole blood chromium level and chromium excretion in
the rat after zinc chromate inhalation. Acta pharmacol.
toxicol., 42: 142-149.
LANGARD, S., ANDERSEN, A., & GYLSETH, B. (1980) Incidence of
cancer among ferrosilicon workers. Br. J. ind. Med., 37:
114-120.
LASKIN, S. (1972) Research in environmental sciences,
Washington DC, Institute of Environmental Medicine, pp. 92-97.
LASKIN, S., KUSCHNER, M., & DREW, R.T. (1970) Studies in
pulmonary carcinogenesis. In: Hanna, M.G., Jr, Nettesheim, P.,
& Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 321-351 (US Atomic Energy
Commission Symposium Series No. 18).
LAUTNER, G.M., CARVER, J.C., & KONZEN, R.B. (1978)
Measurement of chromium (VI) and chromium (III) in stainless
steel welding fumes with electron spectroscopy for chemical
analysis and neutron activation analysis. Am. Ind. Hyg. Assoc.
J., 39: 651-660.
LEHMANN, K.B. (1914) [Historical and critical account of our
present knowledge of the effect of small doses of chromate.]
In: Gesamtgebiet gewerbehygiene, Berlin, Springer Verlag, pp.
1-119 (in German).
LEHMANN, K.B. (1932) [Does the occurrence of lung cancer in
chromium workers give grounds for any particular anxiety?]
Zentralbl. Gewerbehyg., 9: 168-170 (in German).
LERICHE, H.H. (1968) Metal contamination of soil in the
Woburn market-garden experiment resulting from the application
of sewage sludge. J. agric. Sci. Camb., 71: 205-208.
LETTERER, E., NEIDHARDT, K., & KLETT, H. (1944) [Chromate
lung cancer and chromate pneumoconiosis. A clinical, morbid
anatomical and industrial-hygiene-related study.] Arch.
Gewerbepath. Gewerbehyg., 12: 323-361 (in German).
LEVIN, H.M., BRUNNER, M.J., & RATTNER, H. (1959)
Lithographer's dermatitis. J. Am. Med. Assoc., 169: 566-569.
LEVINE, R.A., STREETEN, D.H.P., & DOISY, R.J. (1968) Effects
of oral chromium supplementation on the glucose tolerance of
elderly human subjects. Metabolism, 17: 114-125.
LEVIS, A.G. & BIANCHI, V. (1982) Mutagenic and cytogenetic
effects of chromium compounds. In: Langard, S., ed. Biological
and environmental aspects of chromium, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 171-208.
LEVIS, A.G. & BUTTIGNOL, M. (1977) Effects of potassium
dichromate on DNA Synthesis in hamster fibroblast. Br. J.
Cancer, 35: 496-499.
LEVIS, A.G. & MAJONE, F. (1979) Cytotoxic and caryogenic
effects of soluble chromium compounds on mammalian cell
cultures. Br. J. Cancer, 40: 523-533.
LEVIS, A.G. & MAJONE, F. (1981) Cytologic and clastogenic
effects of soluble and insoluble compounds containing
hexavalent and trivalent chromium. Br. J. Cancer, 44: 219-235.
LEVIS, A.G., BUTTIGNOL, M., BIANCHI, V., & SPONZA, G. (1978)
Effects of potassium dichromate on nucleic acid and protein
synthesis and on precursor uptake in BHK fibroblasts. Cancer
Res., 38: 110-116.
LEVY, L.S. & MARTIN, P.A. (1983) The effects of a range of
chromium-containing materials on rat lung, Birmingham,
England, University of Aston, Department of Environmental and
Occupational Health, p. 224 and suppl (Prepared for Dry Color
Manufacturers Association, Alexandria, Virginia, USA).
LEVY, L.S. & VENITT, S. (1975) Carcinogenic and mutagenic
activity of chromium-containing materials. Br. J. Cancer, 32:
254-255.
LIEBER, M., PERLMUTTER, N.M., & FRAUENTHAL, H.L. (1964)
Cadmium and hexavalent chromium in Nassau County ground
water. J. Am. Water Works Assoc., 56: 739-747.
LIFSCHITZ, M.L., WALLACH, S., PEABODY, R.A., VERCH, R.L., &
AGRAWAL, R. (1980) Radiochromium distribution in thyroid
and parathyroid deficiency. Am. J. clin. Nutr., 33: 57-62.
LINDBERG, E. & HEDENSTIERNA, G. (1983) Chrome plating.
Symptoms, findings in the upper airways, and effects on lung
function. Arch. environ. Health, 38: 367-374.
LINDBERG, E. & VESTERBERG, O. (1983a) Monitoring exposure to
chromic acid in chromeplating by measuring chromium in urine.
Scand. J. Work environ. Health, 9: 333-340.
LINDBERG, E. & VESTERBERG, O. (1983b) Urinary excretion of
proteins in chromplaters, previously exposed chromplaters, and
referents. Scand. J. Work. environ. Health, 9: 505-510.
LINDBERG, E., EKHOLM, U. & ULFVARSON, U. (1985) Extent and
conditions of exposure in the Swedish chrome plating industry.
Int. Arch. Occup. Environ. Health, 56: 197-205.
LIU, V.J.K. & MORRIS, J.S. (1978) Relative chromium response
as an indicator of chromium status. Am. J. clin. Nutr., 31:
972-976.
LOFROTH, G. (1978) The mutagenicity of hexavalent chromium
is decreased by microsomal metabolism. Naturwissenschaften,
65: 207-208.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LUKANIN, W.P. (1930) [The pathology of chromate pneumo-
coniosis.] Arch. Hyg. Bakt., 104: 166-174 (in German).
LUZHNIKOV, E.A., SHIMANKO, I.I., KOSTOMAROVA, L.G., MALYGINA,
S.I., & TRAKHTENGARTS, M.I. (1976) [Acute poisoning with
chromium compounds.] Terapeut. Arkh., 48(7): 121-125 (in
Russian).
LYON, G.L., BROOKS, R.R., PETERSON, P.J., & BUTLER, G.W.
(1970) Some trace elements in plants from serpentine soils.
N.Z. J. Sci., 13: 133-139.
MACHLE, W. & GREGORIUS, F. (1948) Cancer of the respiratory
system in the United States chromate-producing industry.
Public Health Rep., 63: 1114-1127.
MCCLENDON, L.T. (1978) Determination of chromium in
biological matrices by neutron activation: application to
standard reference materials. J. radioanal. Chem., 42: 85-91.
MACKENZIE, R.D., BYERRUM, R.U., DECKER, C.F., HOPPERT, C.A., &
LANGHAM, R.F. (1958) Chronic toxicity studies. II. Hexa-
valent and trivalent chromium administered in drinking-water
to rats. Am. Med. Assoc. Arch. ind. Health, 18: 232-234.
MCRAE, W.D., WHITING, R.F., & STICH, H.F. (1979) Sister-
chromatid exchanges induced in cultured mammalian cells by
chromate. Chem.-biol. Interact., 26: 281-286.
MAHALKO, J.R. & BENNION, M. (1976) The effect of parity and
time between pregnancies on maternal hair chromium concentra-
tion. Am. J. clin. Nutr., 29: 1069-1072.
MAJONE, F. & LEVIS, A.G. (1979) Chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster cells treated in
vitro with hexavalent chromium compounds. Mutat. Res., 67:
231-238.
MAJONE, F. & RENSI, D. (1979) Mitotic alterations, chromo-
some aberrations, and sister-chromatid exchanges induced by
hexavalent and trivalent chromium on mammalian cells in vitro.
Caryologia, 32: 379-392.
MALTONI, C. (1974) Occupational carcinogenesis. Excerpta
Med. Int. Congr. Ser., 322: 19-26.
MALTONI, C. (1976) Precursor lesions in exposed populations
as indicators of occupational cancer risk. Ann. NY Acad. Sci.,
271: 444-447.
MANCUSO, T.F. (1951) Occupational cancer and other health
hazards in a chromate plant: a medical appraisal. II. Clinical
and toxicologic aspects. Ind. Med. Surg., 20: 393-407.
MANCUSO, T.F. (1975) Consideration of chromium as an
industrial carcinogen. In: Hutchinson, T.C., ed. Proceedings
of the International Conference on Heavy Metals in the
Environment, Toronto, Canada, October 27-31, 1975, Toronto,
Institute for Environmental Studies, pp. 343-356.
MANCUSO, T.F. & HUEPER, W.C. (1951) Occupational cancer and
other health hazards in a chromate plant: a medical appraisal.
I. Lung cancer in chromate workers. Ind. Med. Surg., 20:
358-363.
MARTIN, J.H. & KNAUER, G.A. (1973) The elemental composition
of plankton. Geochim. Cosmochim. Acta, 3: 1639-1653.
MATHIS, B.J. & CUMMINGS, T.F. (1973) Selected metals in
sediments, water, and biota in the Illinois river. J. Water
Pollut. Control Fed., 45: 1573-1583.
MATHUR, R.K. & DOISY, R.J. (1972) Effect of diabetes and
diet on the distribution of tracer doses of chromium in rats.
Proc. Soc. Exp. Biol. Med., 139: 836-838.
MATSUMOTO, N., IIJIMA, S., & KATSUNUMA, H. (1976) Placental
transfer of chromic chloride and its teratogenic potential in
embryonic mice. J. toxicol. Sci., 2: 1-13.
MAYER, R. (1983) Interaction of forest canopies with atmo-
spheric constituents: aluminium and heavy metals. In: Ulrich,
B. & Pankrath, J., ed. Effects of accumulation of air
pollutants in forest ecosystems, Dordrecht, Boston, London,
D. Reidel, pp. 47-55.
MAZGON, R. (1932) [Therapeutic effect of common salt on
experimental chromium nephritis in the rabbit.] Z. Gesamte
exp. Med., 81: 195-207 (in German).
MERIAN, E. (1984) Introduction on environmental chemistry
and global cycles of arsenic, beryllium, cadmium, chromium,
cobald, nickel, selenium, and their derivatives. Toxicol.
environ. Chem., 8: 9-38.
MERKURIEVA, R.V., KRASOVSKY, G.N., BURMANTOVA, N.P.,
SHATERNIKOVA, I.L., VARSHAVSKAYA, S.P., VASIUKOVICH, L.YA.,
KOGANOVA, Z.I., BUSHINSKAYA, L.I., KONSTANTINOVA, I.N.,
DOLINSKAYA, S.I., ASTAKHOVA, L.F., & VOTYAKOV, A.V. (1980a)
[Changes in various enzyme systems resulting from exposure to
some chemical substances.] Gig. i. Sanit., N1: 25-28 (in
Russian).
MERKURIEVA, R.V., KRASOVSKY, G.N., VOTYAROV, A.V., BURMANTOVA,
N.P., MUCHAMBETOVA, L.H., & KONSTANTINOVA, I.N. (1980b)
[Enzyme disarrangement of various subcellular structures under
the influence of the general toxic and gonadotoxic effects of
environmental chemical factors.] Zdravookhranenie belorus.,
N2: 28-29 (in Russian).
MERKURIEVA, R.V., KOGANOVA, Z.I., GABDULLINA, M.H., TSAPKOVA,
N.N., DUKHATEROV, O.N., & DOLINSKAYA, S.I. (1982) [A
comparative study of metabolic reactions at various routes of
chromium6+ administration in the organism of laboratory
animals.] Gig. i Sanit., N8: 75-78 (in Russian).
MERRITT, W.F. (1971) Identification and measurement of trace
elements in fresh water by neutron activation. In: Proceedings
of the International Symposium on Identification and Measure-
ment of Environmental Pollution, 358-362.
MERTZ, W. (1969) Chromium occurrence and function in
biological systems. Physiol. Rev., 49: 163-239.
MERTZ, W. (1981) The interaction between chromium and
insulin. Adv. Physiol. Sci., 12: 101-105.
MERTZ, W. & ROGINSKI, E.E. (1963) The effect of trivalent
chromium in galactose entry in rat epidymal fat tissue. J.
biol. Chem., 238: 868-872.
MERTZ, W. & ROGINSKI, E.E. (1969) Effects of chromium (III)
supplementation on growth and survival under stress in rats
fed low protein diets. J. Nutr., 97: 531-536.
MERTZ, W. & ROGINSKI, E.E. (1971) Chromium metabolsm: the
glucose tolerance factor. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 123-153.
MERTZ, W. & SCHWARZ, K. (1959) Relation of glucose tolerance
factor to impaired glucose tolerance in rats on stock diets.
Am. J. Physiol., 196: 614-618.
MERTZ, W., ROGINSKI, E.E., & SCHWARZ, K. (1961) Effect of
trivalent chromium complexes on glucose uptake by epididymal
fat tissue of rats. J. biol. Chem., 236: 318-322.
MERTZ, W., ROGINSKI, E.E., & REBA, R.C. (1965a) Biological
activity and fate of intravenous chromium (III) in the rat.
Am. J. Physiol., 209: 489-494.
MERTZ, W., ROGINSKI, E.E., & SCHROEDER, H.A. (1965b) Some
aspects of glucose metabolism of chromium-deficient rats
raised in a strictly controlled environment. J. Nutr., 86:
107-112.
MERTZ, W., ROGINSKI, E.E., FELDMAN, R.J., & THURMAN, D.E.
(1969) Dependence of chromium transfer into the rat embryo on
the chemical form. J. Nutr., 99: 363-367.
MERTZ, W., TOEPFER, E.W., ROGINSKI, E.E., & POLANSKY, M.M.
(1974) Present knowledge of the role of chromium. Fed. Proc.,
33: 2275-2280.
MEYERS, J.B. (1950) Acute pulmonary complications following
inhalations of chromic acid mist. Arch. ind. Hyg. occup. Med.,
2: 742-747.
MICKAIL, T.H., HABIB, Y.A., GABRIAL, G.N., & MORCOS, S.R.
(1976) The effect of chromium III on the intravenous glucose
tolerance of rats given diets of defficient protein values. Z.
Ernährungswiss., 15: 182-187.
MIESCHER, G., AMREIN, H.P., & LEDER, M. (1955) [Chromate
hypersensitivity and cement eczema.] Dermatologica (Basel),
110: 266-283 (in German).
MIGAI, K.V. (1975) [Hygiene and safety of labor in electro
welding works in shipbuilding,] Leningrad, pp. 27-35 (in
Russian).
MITMAN, F.W., WOLF, W.R., KELSAY, J.L., & PRATHER, E.S.
(1975) Urinary chromium levels of nine young women eating
freely chosen diets. J. Nutr., 105: 64-68.
MORGAN, J.M. (1972) Hepatic chromium content in diabetic
subjects. Metabolism, 21: 313-316.
MORRIS, G.E. (1955) Chromate dermatitis from chrome glue and
other aspects of the chrome problem. Am. Med. Assoc. Arch.
Ind. Health, 11: 368-371.
MORRIS, G.E. (1956) The manufacture of leather. Its chemical
and dermatological aspects. Ann. Meet. Am. Acad. Derm. Syph.
(Chicago).
MORRIS, G.E. (1958) Chrome dermatitis. A study of the
chemistry of shoe leather with particular reference to basic
chromic sulfate. Am. Med. Assoc. Arch. Derm., 78: 612-618.
MORRIS, G.E. (1959) [The basic form of chromic sulfate.
Results of epicutaneous testing of a chromium-III salt.]
Berufsdermatosen, 7: 126-131 (in German).
MORROW, R.W. & MCELHANEY, R.J. (1974) Determination of
chromium in industrial effluent water by flameless atomic
absorption spectroscopy. At. Absorpt. Newsl., 13(2): 45-46.
MORTON, M.S. & ELWOOD, P.C. (1974) CNS malformations and
trace elements in water. Teratology, 10: 318.
MOSINGER, M. & FIORENTINI, H. (1954) Sur la pathologie due
aux chromates: Premières recherches expérimentales. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 15: 187-199.
MULLER-WIEGAND, B., STEUER, D., & HEESSELS, P. (1983) Blood
and urine samples for quality control of heavy metals. Chem.
Toxicol. Clin. Chem. Met. Proc. Int. Conf., 2nd: 73-76.
MURAKAMI, Y., SUZUKI, Y., YAMAGATA, T., & YAMAGATA, N. (1965)
Cr and Mn in Japanese diet. J. Radiat. Res., 6: 105-110.
MURRMAN, R.P., WINTERS, R.W., & MARTIN, T.G. (1971)
Determination of trace elements in soils and clay minerals by
resonnance neutron activation analysis. Soil. Sci. Soc. Am.
Proc., 35: 647-652.
MUTTI, A., CAVATORTA, A., PEDRONI, C., BORGHI, A., GIAROLI,
C., & FRANCHINI, I. (1979) The role of chromium accumulation
in the relationship between airborne and urinary chromium in
welders. Int. Arch. occup. environ. Health, 43: 123-133.
MYSLYAEVA, A.V. (1965) [Changes in the peripheral blood in
workers of a chromium compound plant.] In: [Proceedings of
the 4th Scientific Conference of Aktubinsk State Medical
Institute, Alma-Ata,] Kazakhstan, Alma-Ata, Aktubinsk State
Medical Institute, pp. 331-332 (in Russian).
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., & KURATA, H. (1978)
Comparative studies of chromosomal aberrations and muta-
genicity of trivalent and hexavalent chromium. Mutat. Res.,
58: 175-181.
NASH, D.T., ELWOOD, I.G., & STREETEN, D.H.P. (1979) The
effect of brewer's yeast containing chromium-rich glucose
tolerance factor on serum lipids. In: Proceedings of the 5th
International Symposium on Atherosclerosis, Houston, Texas.
NATER, J.P. (1963) Possible causes of chromate eczema.
Dermatologica (Basel), 126: 160-166.
NATH, R. (1976) Assessment of chromium metabolism in
maturity onset and juvenile diabetics using chromium: 51Cr and
therapeutic response of chromium administration. Excerpta Med.
Int. Congr. Ser., 400: 194.
NEIKO, E.M. & DELVA, IU.V. (1978) [Chromium balance in
chronic ischemic heart disease.] Klinicheskaia Meditsina
(Mosk.), 56(4): 97-99 (in Russian).
NESTMANN, E.R., MATULA, T.I., DOUGLAS, G.R., BORA, K.C., &
KOWBEL, D.J. (1979) Detection of the mutagenic activity of
lead chromate using a battery of microbial tests. Mutat. Res.,
66: 357-365.
NETTESHEIM, P., HANNA, M.G., Jr., DOHERTY, D.G., NEWELL, R.F.,
& HELLMANN, A. (1970) Effects of chronic exposure to
artificial smog and chromium oxide dust on the incidence of
lung tumours in mice. In: Hanna, M.G., Jr, Nettesheim, P., &
Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 305-320 (US Atomic Energy
Commission Symposium Series No. 18).
NETTESHEIM, P., HANNA, M.G., DOHERTY, D.G., NEWELL, R.F., &
HELLMAN, A. (1971) Effect of calcium chromate dust,
influenza virus, and 100R whole-body X-radiation on lung
tumour incidence in mice. J. Natl Cancer Inst., 47: 1129-1144.
NEWBOLD, R.F., AMOS, J., & CONNELL, J.R. (1979) The
cytotoxic, mutagenic, and clastogenic effects of chromium-
containing compounds on mammalian cells in culture. Mutat.
Res., 67: 55-63.
NEWMAN, H.A.I., LEIGHTON, R.F., LANESE, R.R., & FREELAND N.A.
(1978) Serum chromium and angiographically-determined
coronary artery disease. Clin. Chem., 24: 541-544.
NISHIOKA, H. (1975) Mutagenic activities of metal compounds
in bacteria. Mutat. Res., 31: 185-189.
NOLLENDORF, A., PAKALNE, E., & UPITIS, V. (1972) [Little
known trace elements in Chlorella culture.] Latv. PSR Zinat.
Akad. Vestis (Latvian SSR), 7: 33-43 (in Russian).
NOMIYAMA, H., YOTORIYAMA, M., & NOMIYAMA, K. (1980b) Normal
chromium levels in urine and blood of Japanese subjects
determined by direct flameless atomic absorption spectrophoto-
metry, and valency of chromium in urine after exposure to
hexavalent chromium. Am. Ind. Hyg. Assoc. J., 41: 98-102.
NOMIYAMA, K., MATSUI, K., & NOMIYAMA, H. (1980a) Environ-
mental temperature: a factor modifying the acute toxicity of
organic solvents, heavy metals, and agricultural chemicals.
Toxicol. Lett., 6: 67-70.
NORSETH, T. (1978) Health effects of nickel and chromium.
In: Elvira di Ferrante, E., ed. Trace metals: exposure and
health effects, Oxford, New York, Toronto, Sydney, Paris,
Frankfurt, Pergamon Press, pp. 135-146.
NORSETH, T. (1980) Cancer hazards caused by nickel and
chromium exposure. J. Toxicol. environ. Health, 6: 1218-1227.
NOVAKOVA, S., MAUTNER, G., & DINOEVA, A. (1974) [Content of
hexavalent chromium in water supplies and its effect on the
development of experimental atherosclerosis in warm-blooded
animals.] Gig. i Sanit., 5: 78-80 (in Russian).
NOVEY, H.S., HABIB, M., WELLS, J.A. (1983) Asthma and JgE
antibodies induced by chromium and nickel salts. J. Allergy
clin. Immunol., 72: 407-412.
OFFENBACHER, E.G. & PI-SUNYER, F.X. (1980) Benefical effect
of chromium-rich yeast on glucose tolerance and blood lipids
in elderly subjects. Diabetes, 29: 919-925.
OHNO, H., HANAOKA, F., & YAMADA, M. (1982) Inducibility of
sister-chromatid exchanges by heavy-metal ions. Mutat. Res.,
104: 141-145.
OHTA, F. (1940) Studies on the detoxicating hormone of the
liver (yakriton). Contribution to the usage of yakriton
against experimental chromate nephritis. Tohoku J. exp. Med.,
39: 37-46.
OKADA, S., OHBA, H., & TANIYAMA, M. (1981) Alterations in
ribonucleic acid synthesis by chromium (III). J. inorg.
Biochem., 15: 223-231.
OKADA, S., TRUKADA, H., & OHBA, H. (1983) Enhancement of
ribonucleic acid synthesis by chromium (III) in mouse liver.
J. inorg. Biochem., 19: 95-103
OKADA, S., TSUKADA, H., & OHBA, H. (1984) Enhancement of
nucleolar RNA synthesis by chromium (III) in regenerating rat
liver. J. inorg. Biochem., 21, 113-124.
OPHULS, W. (1911a) Experimental nephritis in guinea-pigs by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9: 11-13.
OPHULS, W. (1911b) Experimental nephritis in rabbits by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9:13.
OTTINGER, R.S., BLUMENTHAL, J.L., DALPORTO, D.F., GURBER,
G.I., SANTY, M.J., & SHIH, C.C. (1973) Recommended methods
of reduction, neutralization, recovery or disposal of
hazardous waste, Cincinnati, Ohio, US Environmental Protection
Agency, Vol. 6, pp. 143-162 (EPA 670/2-73-053-F).
PAGE, A.L. (1974) Fate and effects of trace elements in
sewage sludge when applied to agricultural lands, Cincinnati,
Ohio, US Environmental Protection Agency (EPA 670/2-74-005).
PAIXAO, L.M. & YOE, J.H. (1959) Spectochemical determination
of magnesium, chromium, nickel, copper, and zinc in human
plasma and red cells. Clin. Chim. Acta, 4: 507-514.
PARR, R.M. (1977) Problems of chromium analysis in
biological materials: an international perspective with
special reference to results for analytical quality control
samples. J. radioanal. Chem., 39: 421-433.
PASCALE, L.R., WALDSTEIN, S.S., ENGBRING, G., DUBIN, A., &
SZANTO, P.B. (1952) Chromium intoxication with special
reference to hepatic injury. J. Am. Med. Assoc., 149:
1385-1389.
PASHIN, Y.V. & KOZACHENKO, V.J. (1981) [Mutagenic activity
of chromium compounds.] Gig. i. Sanit., 5: 46-49 (in Russian).
PASHIN, Y.V., ZACEPILOVA, T.A., & KOZACHENKO, V.I. (1982)
Induction of dominant lethal mutations in male mice by
potassium dichromate. Mutat. Res., 103: 345-347.
PASTERNAK, K. (1973) The spreading of heavy metals in
flowing waters in the region of occurrence of natural deposits
of the zinc and lead industry. Acta hydrobiol., 15: 145-166.
PEKAREK, R.S., HAUER, E.C., RAYFIELD, E.J., WANNEMACHER, R.W.,
& BIESEL, W.R. (1975) Relationship between serum chromium
concentrations and glucose utilization in normal and infected
subjects. Diabetes, 24: 350-353.
PENG, H.Z. (1982) [The investigation of nasal disease
morbidity of chromium exposed workers.] J. Shan-Xie Med.
College, 3: 19-21 (in Chinese).
PERLMUTTER, N.M. & LIEBER, M. (1970) Dispersal of plating
wastes and sewage contaminants in ground water and surface
water, Washington DC, US Government Printing Office, 67 pp
(Geological Survey Water Supply Paper 1879-G).
PERONE, V.B., MOFFITT, A.A., POSSICK, P.A., KEY, M.M.,
DANZINGER, S.J., & GELLIN, G.A. (1974) The chromium, cobalt,
and nickel contents of American cement and their relationship
to cement dermatitis. Am. Ind. Hyg. Assoc. J., 35: 301-306.
PETRILLI, F.L. & DE FLORA, S. (1977) Toxicity and mutagenicity of
hexavalent chromium on Salmonella typhimurium. Appl. environ.
Microbiol., 33: 805-809.
PETRILLI, F.L. & DE FLORA, S. (1978a) Oxidation of inactive
trivalent chromium to the mutagenic hexavalent form. Mutat.
Res., 58: 167-173.
PETRILLI, F.L. & DE FLORA, S. (1978b) Metabolic deactivation
of hexavalent chromium mutagenicity. Mutat. Res., 54: 139-147.
PETRILLI, F.L. & DE FLORA, S. (1980) Mutagenicity of
chromium compounds. In: Chromates Symposium 80, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 76-99.
PETRILLI, F.L. & DE FLORA, S. (1982) Interpretations on
chromium mutagenicity and carcinogenicity. In: Sorsa, M. &
Vainmio, H., ed. Mutagens in our environment, New York, Alan
R. Liss, pp. 453-464.
PIERCE, J.V. & SCHEEL, L.D. (1965) Toxicity of alloys of
chromium. Arch. environ. Health, 10: 870-876.
PIRILA, V. & KILPIO, O. (1949) On dermatoses caused by
bichromates. Acta derm. venerol., 29: 550-563.
POHLAND, F.G. (1975) Sanitary landfill stabilization with
leachate recycle and residual treatment, Cincinnati, Ohio, US
Environmental Protection Agency, 106 pp (EPA 600/2-75-043).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1973) [On carcinogenous
hazards in the product of chromium ferroalloys.] Gig. Tr.
prof. Zabol., 10: 23-26 (in Russian).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1974) [Epidemiological
data on malignant neoplasms in workers who are in contact with
different chromium compounds.] In: Shabad, L.M., Velich-
kovskii, B.T. & Kogan, F.M., ed. [Occupational cancer (a
collection of scientific works),] Moscow, Academic Academy of
Medical Science, pp. 64-71 (in Russian).
POKROVSKAYA, L.V., TJUSHUYAKOVA, A.V., GORODNOVA, N.V.,
ZALOMOVA, E.N., & ANDREEVA, T.D. (1976) [Dust factor and
occupational pathology in workers engaged in the open
excavation of chromium ores.] In: [Occupational diseases of
dust etiology (collection of works),] Vol. 3, pp. 38-43 (in
Russian).
POLAK, L., TURK, J.L., & FREY, J.R. (1973) Studies on
contact hypersensitivity to chromium compounds. In: Kallos,
P., Waksman, B.H., & DeWeck, A., ed. Progress in allergy,
Basel, Karger, Vol. 17, pp. 145-226.
PRESTON, A.M., DOWDY, R.P., PRESTON, M.A., & FREEMAN, J.N.
(1976) Effects of dietary chromium on glucose and serum
cholesterol in guinea-pigs. J. Nutr., 106: 1391-1397.
PRIESTLEY, J. (1877) Observations on the physiological
action of chromium. J. Anat. Physiol., 11: 285-301.
PRINCI, F., MILLER, L.H., DAVIS, A., & CHOLAK, J. (1962)
Pulmonary disease of ferrochromium workers. J. occup. Med., 4:
301-310.
PUNSAR, S., ERAMETSA, O., KARVONEN, M.I., RYHANEN, A., HILSKA,
P., & WORNAMO, H. (1975) Coronary heart disease and
drinking-water. A search in two Finnish male cohorts for
epidemiologic evidence of a water factor. J. chron. Dis., 28:
259-287.
RABEAU, H. & UKRAINCZYK, V. (1939) Dermites des
blanchisseuses. Rôle du chrome et du chlore. Ann. Derm. Syph.,
10: 656-680.
RADIAN CORPORATION (1983) Estimates of population exposure
to ambient chromium emissions, Research Triangle Park, North
Carolina, US Environmental Protection Agency (Final Report to
K.L. Blanchard) (EPA Contract No. 68-02-3818).
RAFFETTO, G. (1977) Direct interaction with cellular targets
as the mechanism for chromium carcinogenesis. Tumori, 63:
503-512.
RAFFETTO, G., PARODI, S., DE FERRARI, M., PARODI, C., TROIANO,
R., & BRAMBILLA, G. (1978) Direct interaction with cellular
targets as the mechanism for chromium carcinogensis. Tumori,
73: 503-512.
REDISKE, J.H. (1956) Chromium toxicity in plants. In:
Biology research: annual report, 1955, Richland, Washington,
Hamford Atomic Products Operations, pp. 48-52 (Report
HW-41500).
REDISKE, J.H., CLINE, J.F., & SELDERS, A.A. (1955) Chromium
toxicity in plants. In: Biology research: annual report, 1954,
Richland, Washington, Hamford Atomic Products Operations,
pp. 32-39 (Report HW-35917).
REGGIANI, A., LOTTI, M., DE ROSA, E., & SAIA, B. (1973)
[Impairments of respiratory functions in subjects exposed to
chromium. Note 1. Spirographic changes.] Lav. Umano, 25: 23-27
(in Italian).
REICHENBACH-KLINKE, H.H. (1977) [To the ecology of the
accumulation of heavy metals in fishes.] In: Reichenbach-
Klinke, H.H., ed. [Contributions to fish ecology: fish and the
environment,] Stuttgart, New York, Gustav Fischer, pp. 7-11
(in German).
REICHENBACH-KLINKE, H.H. (1980) [Fish diseases and harm
suffered by fish,] 2nd ed., Stuttgart, New York, Gustav
Fischer, 474 pp (in German).
RETNEV, V.M. (1960) [On the effect produced by chromium
compounds contained in cement dust on the development of
bronchial asthma.] Gig. Tr. prof. Zabol., 7: 29-32 (in
Russian).
RIALES, R. (1979) Influence of brewer's yeast on lipoprotein
cholesterol concentrations. A preliminary report. In:
Shapcott, D. & Hubert, I., ed. Chromium in nutrition and
metabolism. Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 199-212.
ROBERTS, K.R. & MARZLUF, G.A. (1971) The specific inter-
action of chromate with the dual sulfate permease systems of
Neurospora crassa. Arch. Biochem. Biophys., 142: 651-659.
ROBINSON, W.D. & EDINGTON, G. (1935) Chemical studies of
infertile soils derived from rocks high in magnesium and
generally high in chromium and nickel, Washington DC, US
Department of Agriculture, pp. 1-29 (US Department of
Agriculture Technical Bulletin No. 471).
ROGINSKI, E.E. & MERTZ, W. (1967) An eye lesion in rats fed
low-chromium rations. J. Nutr., 93: 249-251.
ROGINSKI, E.E. & MERTZ, W. (1969) Effects of chromium (III)
supplementation on glucose and amino acid metabolism in rats
fed a low protein diet. J. Nutr., 97: 525-530.
ROHDE, G., TISCHENDORF, G., LEONHARDT, J., & DAMASCHUN, F.
(1978) [Study of crocoite occurrence in Callenberg (Saxony)
(Germany).] Z. angew. Geol., 24: 168-173 (in German).
ROMOSER, G.L., DUDLEY, W.A., MACHLIN, L.J., & LOVELESS, L.
(1961) Toxicity of vanadium and chromium for the growing
chick. Poultry Sci., 40: 1171-1173.
ROOK, H.L. & WOLF, W.R. (1977) The quantitative
determination of volatile trace elements in NBS biological
standard reference materials. In: Hemphill, D.D., ed.
Proceedings of the Eleventh Annual Conference on Trace
Substances in Environmental Health, Columbia, Missouri, the
Curators of the University of Missouri, pp. 324-333.
ROSCHINA, T.A. (1959) [Experimental study on the effects of
magnezite chromium and chromite magnezite dust exposure in the
production of refrectories.] Gig. Tr. prof. Zabol., 4: 28-32
(in Russian).
ROSCHINA, T.A. (1964) [A comparative evaluation of the
effects of carbide dust and chromium boride on the organism.]
Gig. i Sanit., 8: 25-28 (in Russian).
ROSCHINA, T.A. (1976) [The toxicity of chrominum (sic)
hexacarbonyl.] Gig. Tr. Prof. Zabol., 2: 38-42 (in Russian).
ROSEBROUGH, R.W. & STEELE, N.C. (1981) Effect of
supplemental dietary chromium or nicotinic acid on
carbohydrate metabolism during basal, starvation, and
refeeding periods in poults. Poultry Sci., 60: 407-417.
ROSS, D.S., SJOGREN, R.E., & BARTLETT, R.J. (1981) Behaviour
of chromium in soils. IV. Toxicity to microorganisms. J.
environ. Qual., 10: 145-148.
ROSTENBERG, A., Jr & PERKINS, A.J. (1951) Nickel and cobalt
dermatitis. J. Allerg., 22: 466-474.
RTECS (1978) Registry of toxic effects of chemical substances,
Washington DC, National Institute for Occupational Safety and
Health, pp. 450.
SAMITZ, M.H. (1955) Some dermatologic aspects of the
chromate problem. Arch. ind. Health, 11: 361-367.
SAMITZ, M.H. (1956) Studies of chromate dermatitis. Arch.
ind. Health, 14: 269-271.
SAMITZ, M.H. & EPSTEIN, E. (1962) Experimental cutaneous
chrome ulcers in guinea-pigs. Arch. environ. Health, 5:
463-468.
SAMITZ, M.H. & GROSS, S. (1960) Extraction by sweat of
chromium from chrome tanned leathers. J. occup. Med., 2: 12-14.
SAMITZ, M.H. & GROSS, S. (1961) Effects of hexavalent and
trivalent chromium compounds on the skin. Am. Med. Assoc.
Arch. Dermatol., 84: 404-409.
SAMITZ, M.H., KATZ, S., & GROSS, S. (1960) Nature of the
chromium extracted from leather by sweat. J. occup. Med., 2:
435-436.
SANDERS, C.L., JACKSON, T.A., ADEE, R.R., POWERS, G.J., &
WEHNER, A.P. (1971) Distribution of inhaled metal oxide
particles in pulmonary alveoli. Arch. intern. Med., 127:
1085-1089.
SARGENT, T., III, LIM, T.H., & JENSON, R.L. (1979) Reduced
chromium retention in patients with hemochromatosis, a
possible basis of hemochromatotic diabetes. Metabolism, 28:
70-79.
SARTO, F., LEVIS, A.G., & PAULON, C. (1980) Clastogenic
activity of hexavalent and trivalent chromium in cultured
human lymphocytes. Caryologia, 33: 239-250.
SARTO, F., COMINATO, J., BIANCHI, V., & LEVIS, A.G. (1982)
Increased incidence of chromosomal aberrations and sister
chromatid exchanges in workers exposed to chromic acid (CrO3)
in electroplating factories. Carcinogenesis, 3: 1011-1016.
SASSI, C. (1956) [Occupational disease in a chromate
factory.] Med. Lavoro, 47: 314-326 (in Italian).
SATOH, K., FUKUDA, Y., TORII, K., & KATSUNO, N. (1981)
Epidemiological study of workers engaged in the manufacture of
chromium compounds. J. occup. Med., 23: 835-838.
SAVORY, J., MUSHAK, P., & SUNDERMAN, F.W. Jr (1969) Gas
Chromatographic determination of chromium in serum. J.
chromatogr. Sci., 7: 674-679.
SCHALLER, K.H. & ZOBER, A. (1982) [Renal excretion of
toxicologically relevant metals in occupationally non-exposed
individuals.] Arztl. Lab., 28: 209-214 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1972) [Quantitative chromium determination in urine by
flameless atomic absorption spectrometry.] Z. klin. Chem.
klin. Biochem., 10: 434-437 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1973) The quantitative determination of chromium in urine by
flameless atomic absorption spectroscopy. At. Absorpt.
Newsl., 12(6): 147-150.
SCHELENZ, R. (1977) Dietary intake of 25 elements by man
estimated by neutron activation analysis. J. radioanal. Chem.,
37: 539-548.
SCHLETTWEIN-GSELL, D. & MOMMSEN-STRAUB, S. (1971) [Review
trace elements in foods. III. Chromium.] Int. J. Vitam. Nutr.
Res., 41: 116-125 and (1973) Suppl. 13, 188 pp (in German).
SCHOENTAL, R. (1975) Chromium carcinogenesis formation of
epoxyaldehydes and tanning. Br. J. Cancer, 32: 403-404.
SCHROEDER, H.A. (1966) Chromium deficiency in rats: a
syndrome simulating diabetes mellities with retarded growth.
J. Nutr., 88: 439-445.
SCHROEDER, H.A. (1970) Chromium, Washington DC, American
Petroleum Institute, 28 pp (Air Quality Monograph No. 70-15).
SCHROEDER, H.A. & BALASSA, J.J. (1965) Influence of
chromium, cadmium, and lead on rat aortic lipids and
circulating cholesterol. Am. J. Physiol., 209: 433-437.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1962)
Abnormal trace metals in man: chromium. J. chron. Dis., 15:
941-964.
SCHROEDER, H.A., VINTON, W.H., Jr, & BALASSA, J.J. (1963)
Effects of chromium, cadmium, and other trace metals on the
growth and survival of mice. J. Nutr., 80: 39-47.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1964)
Chromium, lead, cadmium, nickel, and titanium in mice: effect
on mortality, tumours, and tissue levels. J. Nutr., 83:
239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1965)
Chromium, cadmium, and lead in rats: effects on life span,
tumours, and tissue levels. J. Nutr., 86: 51-66.
SCHROEDER, H.A., NASON, A.P., & TIPTON, I.H. (1970) Chromium
deficiency as a factor in atherosclerosis. J. chron. Dis., 23:
123-142.
SCHROEDER, K.H. (1955) [Nickel eczema in a blueline (ozalid)
machine operator.] Berufsdermatosen, 3: 170-171 (in German).
SCHUBERT, G.E., GEBHARD, K., & HOENLEIN, F. (1970) [Kidney
damage following injection of serotonin and potassium
dichromate for chronic sodium overloading or restriction.]
Virchows Arch. Abt. A Path. Anat., 351: 68-82 (in German).
SCHULTHEISS, E. (1959) [Rubber and eczema,], Aulendorf i.
Württemberg, Cantor, 213 pp (in German).
SCHWARZ, K. & MERTZ, W. (1959) Chromium (III) and the
glucose tolerance factor. Arch. Biochem. Biophys., 85: 292-295.
SCHWARTZ, L. & DUNN, J.E. (1942) Dermatitis occurring among
operators of air conditioning equipment. Ind. Med. Surg., 11:
375.
SCHWARTZ, L., TULIPAN, L., & PECK, S. (1947) Occupational
diseases of the skin, 2nd ed., Philadelphia, Pennsylvania, Lea
& Febiger.
SEEBER, H., FIKENTSCHER, R., & ROSEBURG, B. (1976)
[Disorders of smell and taste in chromium dye workers.] Z.
Gesamte Hyg. Grenzgeb., 22: 820-822 (in German).
SEELEY, J.L. & SKOGERBOE, R.K. (1974) Combined sampling-
analysis method for the determination of trace elements in
atmospheric particulates. Anal. Chem., 46: 415-421.
SEELING, W., GRUENERT, A., KIENLE, K.H., OPFERKUCH, R., &
SWOBODNIK, M. (1979) [Determination of chromium in human
serum and plasma by flameless atomic absorption spectro-
photometry.] Z. anal. Chem., 299: 368-374 (in German).
SEITZ, W.R., SUYDAM, W.W., & HERCULES, D.M. (1972)
Determination of trace amounts of chromium (III) using
chemiluminescence analysis. Anal. Chem., 44(6): 957-963.
SHACKLETTE, H.T., SAUER, H.I., & MIESCH, A.T. (1970)
Geochemical environments and cardiovascular mortality rates in
Georgia, Washington DC, US Governmental Printing Office, 39 pp
(US Geological Survey Professional Paper No. 574-C).
SHAPCOTT, D., KHOURI, K., DEMERS, P.P., VOBECKY, J., &
VOBECKY, J. (1977) The measurement of volatile chromium in
biological materials. Clin. Biochem, 10: 178-180.
SHEFFET, A., THIND, J., MILLER, A.M., & LOURIA, D.B. (1982)
Cancer mortality in a pigment plant utilizing lead and zinc
chromates. Arch. environ. Health, 37: 44-52.
SHENDRIKAR, A.D. & WEST, P.A. (1974) A study of adsorption
characteristics of traces of chromium (III) and (VI) on
selected surfaces. Anal. Chim. Acta, 72: 91-96.
SHERMAN, L., GLENNON, J.A., BRECK, W.J., KLOMBERG, G.H., &
GORDON, E.S. (1968) Failure of trivalent chromium to improve
hyperglycemia in diabetes mellitus. Metabolism, 17: 439-442.
SHIMKIN, M.M. & LEITER, J. (1940) Induced pulmonary tumours
in mice. III. Role of chromic irritation in the production of
pulmonary tumours in strain A mice. J. Natl Cancer Inst., 1:
241-254.
SHMITOVA, L.A. (1978) In: [The impact of job-related factors
on specific function of the female organism,] Svedlovsk, pp.
105-108 (in Russian).
SHMITOVA, L.A. (1980) Gig. Tr. prof. zabol., 2:
33-35 (in Russian).
SIDI, E. & LONGUEVILLE, R. (1953) Dermites au ciment. Rôle
des sels de chrome. Arch. Mal. prof., 14: 41.
SIEGENTHALER, U., LAINE, A., POLAK, L. (1983) Studies on
contact sensitivity to chromium in the guinea-pig. The role
of valence in the formation of the antigenic determinant. J.
Invest. Dermatol., 80: 44-47.
SIMONOFF, M., LLABADOR, Y. HAMON, C., PEERS; A.M., & SIMONOFF,
G.N. (1984) Low plasma chromium in patients with coronary
artery and heart diseases. Biol. Trace Element Res., 6:
431-439.
SIMONDS, J.P. & HEPLER, O.E. (1945) Experimental nephro-
pathies. I. A method of producing controlled selective injury
of renal units by means of chemical agents. Arch. Path., 39:
103-108.
SIROVER, M.A. & LOEB, L.A. (1976) Infidelity of DNA
synthesis in vitro: screening for potential metal mutagens or
carcinogens. Science, 194: 1434-1436.
SJOGREN, B. (1980) A retrospective cohort study of mortality
among stainless steel welders. Scand. J. Work. environ.
Health, 6: 197-200.
SKOGERBOE, R.K. (1974) Monitoring trace metal particulates:
an evaluation of the sampling and analysis problems. In:
Instrumentation for monitoring air quality, Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp.
125-136.
SLUIS-CREMER, G.K. & DU TOIT, R.S. (1968) Pneumoconiosis in
chromite miners in South Africa. Br. J. ind. Med., 25: 63-67.
SPIER, H.W. (1956) [Wood preservatives and chromate eczema.]
Desinfekt. Gesundheitswes., 4: 58 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil ( Myriophyllum spicatum L.). Arch.
environ. Contam. Toxicol., 2: 331-341.
STAUB, H.W., REUSSNER, G., & THIESSEN, R., Jr (1969) Serum
cholesterol reduction by chromium in hypercholesteremic rats.
Science, 166: 746-747.
STEELE, N.C. & ROSEBROUGH, R.W. (1979) Trivalent chromium
and nicotinic acid supplementation for the turkey poult.
Poultry Sci., 58: 983-984.
STEELE, N.G., ALTHEN, T.G., & FROBISH, L.T. (1977a) Bio-
logical activity of glucose tolerance factor in swine. J.
anim. Sci., 45: 1341-1345.
STEELE, N.C., PELURA, I., & FROBISH, L.T. (1977b) Effect of
dietary protein source on glucose utilization and hepatic
insulin-receptor characteristics of the rat. Nutr. Rep. Int.,
15: 429-435.
STELLA, M., MENTALDI, A., ROSSI, R., ROSSI, G., & LEVIS, A.G.
(1982) Clastogenic effects of chromium on human lymphocytes
in vitro and in vivo. Mutat. Res., 101: 151-164.
STEFFEE, C.H. & BAETJER, A.M. (1965) Histopathologic effects
of chromate chemicals. Report of studies in rabbits, guinea-
pigs, rats, and mice. Arch. environ. Health, 11: 66-75.
STEINHOFF, D., GAD, S.C., HATFIELD, G.K., & MOHR, U. (1983)
Testing sodium dichromate and soluble calcium chromate for
carcinogenicity in rats. Report to the Industrial Health
Foundation, Wuppertal, Bayer AG Institute of Toxicology,
126 pp.
STEREKHOVA, N.P., ZELENEVA, N.I., SOLOMINA, S.N.,
TYUSHNYAKOVA, N.V., MYASNIKOVA, A.G., FOKINA, G.P., & YARINA,
A.L. (1978) [Gastric pathology in workers engaged in the
production of chromium salts.] Gig. Tr. prof. Zabol., 3: 19-23
(in Russian).
STERN, R.M. (1981) Process-dependent risk of delayed health
effects for welders. Environ. Health Perspect., 41: 235-253.
STERN, R.M. (1982) Chromium compounds: production and
occupational exposure. In: Langard, S., ed. Biological and
environmental aspects of chromium, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 5-47.
STRIK, J.J.T.W.A., DE IONGH, H.H., VAN RIJN VAN ALKEMADE,
J.W.A., & WIUTE, T.P. (1975) Toxicity of chromium (VI) in
fish, with special reference to organoweights, liver and
plasma enzyme activities, blood parameters, and histological
alterations. In: Koeman, S.H. & Strik, J.J.T.W.A., ed.
Sublethal effects of toxic chemicals on aquatic animals,
Amsterdam, New York, Oxford, Elsevier Science Publishers,
pp. 31-41.
STROUT, R.W. (1977) The relationship of abnormal circulating
insulin levels to atherosclerosis. Atherosclerosis, 27: 1-13.
SULLIVAN, R.J. (1969) Air pollution aspects of chromium and
its compounds, Raleigh, North Carolina, US Department of
Health, Education and Welfare, 75 pp (National Air Pollution
Control Administration Publication No. APTD 69-34).
SUSA, N., FURUKAWA, YO., & TSUBAKI, S. (1977) Comparative
cytotoxic effects of hexavalent and trivalent chromium
compounds on cultured cells. Jap. J. vet. Sci., 39: 325-336.
SUZUKI, K., ESASHI, T., SUZUE, R., NISHIMURA, H., & MATSUMOTO,
H. (1979) Tissue concentrations of cadmium, lead, and
chromium in Japanese ranging from the fetus to elder subjects.
Toxicol. Lett., 4: 481-484.
SWAINE, D.J. & MITCHELL, R.L. (1960) Trace element
distribution in soil profiles. J. soil Sci., 11 (2): 347-368.
SWENSSON, A. (1977) [Experimental research on the
fibrogenetic effect of chromite.] Arbete och Haelsa, 2: 1-14
(in Swedish).
TABOR, E.C. & WARREN, W.V. (1958) Distribution of certain
metals in the atmosphere of some American cities. Arch. ind.
Hyg., 17: 145-151.
TAMARO, M., BANFI, E., VENTURINI, S., & MONTI-BRAGADIN, C.
(1975) [Hexavalent chromium compounds are mutagens for
bacteria.] In: Proceedings of the XVII Congress Naz. Soc. It.
Microbiol., Padova, pp. 411-415 (in Italian).
TANDON, S.K., SAXENA, D.K., GAUR, J.S., & CHANDRA, S.W.
(1978) Comparative toxicity of trivalent and hexavalent
chromium. Environ. Res., 15: 90-99.
TANDON, S.K., BEHARI, J.R., & KACHRU, D.N. (1979) Distri-
bution of chromium in poisoned rats. Toxicology, 13: 29-34.
TANDON, S.K., MATHUR, A.K., & GAUR, J.S. (1977) Urinary
excretion of chromium and nickel among electroplaters and
pigment industry workers. Int. Arch. occup. environ. Health,
40: 71-76.
TATARU, C., MARINSECU, A., CAPUSAN, I., RIPAN, R., & LITEANU,
C. (1957) [Skin damage during the industrial recovery of
potassium dichromate.] Berufsdermatosen, 5: 283-295 (in
German).
TAYLOR, A.E., GUYTON, M.O., & BISHOP, V.S. (1965)
Permeability of the alveolar membrane to solutes. Circ. Res.,
16: 353-362.
TAYLOR, F.G., Jr, MANN, L.K., DAHLMANN, R.C., & MILLER, F.L.
(1975) Environmental effects of chromium and zinc in
cooling-water drift. In: Cooling tower environment, 1974,
Washington DC, US Energy Research and Development
Administration, pp. 408-426 (Report Conf. No. 740302).
TAYLOR, F.H. (1966) The relationship of mortality and
duration of employment as reflected by a cohort of chromate
workers. Am. J. public Health, 56: 218-229.
TAZELAAR, D.J. (1970) Hypersensitivity to chromium in a
light-blue Tattoo. Dermatologica (Basel), 141: 282-287.
THOMSON, A.G. (1982) Chromite, Mining Annual Review,
pp. 70-71.
THORMANN, J., JESPERSEN, N.B., & JOENSEN, H.D. (1979)
Persistence of contact allergy to chromium. Contact Dermatit.,
5: 261-264.
TOEPFER, E.W., MERTZ, W., ROGINSKI, E.E., & POLANSKY, M.M.
(1973) Chromium in foods in relation to biological activity.
J. agric. food Chem., 21: 69-73.
TOEPFER, E.W., MERTZ, W., POLANSKY, M.M., ROGINSKI, E.E., &
WOLF, W.R. (1977) Preparation of chromium-containing
material of glucose tolerance factor activity from brewer's
yeast extracts and by synthesis. J. agric. food Chem., 25:
162-166.
TOLA, S., KILPIO, J., VIRTAMO, M., & HAAPA, K. (1977)
Urinary chromium as an indicator of the exposure of welders to
chromium. Scand. J. Work environ. Health, 3: 192-202.
TOLG, G. (1974) Recent problems and limitations in the
analytical characterization of high-purity material. Talanta,
21: 327-345.
TSUDA, H. & KATO, K. (1976) Potassium dichromate: induced
chromosome aberrations and its control with sodium sulfite in
hamster embryonic cells in vitro. Gann, 67: 469-470.
TSUDA, H. & KATO, K. (1977) Chromosomal aberrations and
morphological transformation in hamster embryonic cells
treated with potassium dichromate in vitro. Mutat. Res., 46:
87-94.
TUMAN, R.W. & DOISY, R.J. (1977) Metabolic effects of the
glucose tolerance factor (GTF) in normal and genetically
diabetic mice. Diabetes, 26: 820-826.
TUMAN, R.W., BILBO, J.T., & DOISY, R.J. (1978) Comparison
and effects of natural and synthetic glucose tolerance factor
in normal and genetically diabetic mice. Diabetes, 27: 49-56.
TURNER, M.A. & RUST, R.H. (1971) Effects of chromium on
growth and mineral nutrition of soybeans. Soil Sci. Soc. Am.
Proc., 35: 755-758.
TYUSHNYAKOVA, N.V., ZISLIN, D.M., SHABYNINA, N.K., & BELJAEVA,
L.N. (1974) [Criteria of occupational cancer in the
production of chromium salts and some ideas concerning its
pathogenesis.] In: Occupational cancer (collection of
scientific works), Sverdlovsk, pp. 56-63 (in Russian).
UMEDA, M. & NISHIMURA, M. (1979) Inducibility of chromosomal
aberrations by metal compounds in cultured mammalian cells.
Mutat. Res., 67: 221-229.
URCHS, O. (1953) [Occupational skin diseases,] Cantor,
Aulendorf i. Württemberg, 108 pp.
US DHEW (1975) Criteria for a recommended standard.
Occupational exposure to chromium (VI), Washington DC, US
Department of Health, Education and Welfare, 200 pp (US DHEW
Publication No. NIOSH 76-129).
US DHEW (1977) Occupational diseases: a guide to their
recognition, revised ed., Washington DC, US Department of
Health, Education and Welfare, pp. 352-355 (Stock No.
017-033-00266-5).
US EPA (1978) Reviews of the environmental effects of
pollutants. III. Chromium, Washington DC, US Environmental
Protection Agency, 285 pp (ORNL/EIS-80; EPA 600/1-78-023).
US EPA (1980) Ambient water quality criteria for chromium,
Washington DC, US Environmental Protection Agency, pp. A1-C48.
US NAS (1974a) Chromium, Washington DC, US National Academy
of Sciences, 155 pp.
US NAS (1974b) Geochemistry and the environment. I. The
relation of selected trace elements to health and disease,
Washington DC, US National Academy of Sciences, 113 pp.
US NAS/NAE (1972) Water quality criteria 1972, Washington
DC, US Environmental Protection Agency, US National Academy of
Sciences, US National Academy of Engineering, 533 pp.
US PHS (1953) Health of workers in chromate producing
industry, Washington DC, US Department of Health, Education
and Welfare, US Public Health Service, 131 pp (Publication No.
192).
VAKHRUSHEVA, V.A. (1960) [Effect of Mn and Cr as trace
elements on the level of blood sugar in white mice.] Tr.
Izhevskogo Otd. Fiziol. Obschchestva, 1: 184-190 (in Russian).
VALER, M. & RACZ, J. (1971) Investigations concerning the
sensitizing effect of trivalent chromium salts. I. Correlation
between concentration and skin penetration of trivalent
chromium and between the number of positive results obtained
in patch tests. J. ind. Med., 41(12): 302-317.
VEILLON, C., WOLF, W.R., GUTHRIE, B.E. (1979) Determination
of chromium in biological materials by stable isotope
dilution. Anal. Chem., 51: 1022-1024.
VELICHKOVSKY, B.T. & POKROVSKAJA, L.V. (1973) [Labour
hygiene in a modern production of multy-tonnage ferrolloys.]
In: [Proceedings of the National Symposium on Labor Hygiene
and Prophylaxis of Occupational Diseases in the Production of
Ferroalloys,] Sverdlovsk, pp. 3-8 (in Russian).
VENITT, S. & LEVY, L.S. (1974) Mutagenicity of chromates in
bacteria and its relevance to chromate carcinogenesis. Nature
(Lond.), 250: 493-494.
VENCGUJIV, A.J. (1974) Zones of display of biological and
pharmacological action of trace elements. In: Hoekstra, W.G.,
Gantherl, H.E., & Mertz, W., ed. Trace elements metabolism in
animals. II, Baltimore, London, Tokyo, University Park Press,
pp. 295-310.
VERFAILLIE, G.R.M. (1974) Kinetics of chromium absorption by
intact rice plants. In: Comparative studies of food and
environmental contamination, Vienna, International Atomic
Energy Agency, pp. 315-331.
VERSIECK, J.M.J. & SPEECKE, A.B.H. (1972) Contaminations
induced by collection of liver biopsies and human blood. In:
Nuclear Activation Techniques in the Life Sciences.
Proceedings of a Symposium, Bled, Yugoslavia, Vienna
International Atomic Energy Agency, pp. 39-49.
VERSIECK, I., HOSTE, J., BARBIER, F., STEYAERT, H., DERUDDER,
I., & MICHELS, H. (1978) Determination of chromium and
cobalt in human serum by neutron activation analysis. Clin.
Chem., 24: 303-308.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experience of the
occupational health department with some industrial threshold
limit values for industrial poisons.] Arch. Gewerbepath.
Gewerbehyg., 13: 528-534 (in German).
VISEK, W.J., WHITNEY, I.B., KUHN, U.S.G., & COMAR, C.L.
(1953) Metabolism of 51Cr by animals as influenced by
chemical state. Proc. Soc. Exp. Biol. Med., 84: 610-615.
VOELKL, A. (1971) [The daily excretion of chromium in normal
persons.] Zentralbl. Arbeitsmed., 21: 122 (in German).
VOTAVA, H.J., HAHN, C.J., & EVAN, G.W. (1973) Isolation and
partial purification of a 51Cr complex from brewer's yeast.
Biochem. Biophys. Res. Comm., 55: 312-319.
WACKER, W.E.C. & VALLEE, B.L. (1959) Nucleic acids and
metals. I. Chromium mangenese, nickel, iron, and other metals
in ribonucleic acid from diverse biological sources. J. biol.
Chem., 234: 3257-3262.
WAHBA, A. & COHEN, T. (1979) Chrome sensitivity in Israel.
Contact Dermatit., 5: 101-107.
WAHLBERG, J.E. & WENNERSTEN, G. (1977) Light sensitivity and
chromium dermatitis. Br. J. Dermatol., 97: 411-416.
WALSH, E.N. (1953) Chromate hazards in industry. J. Am. Med.
Assoc., 153: 1305-1308.
WARREN, G., SCHULTZ, P., BANCROFT, D., BENNETT, K., ABBOTT,
E.H., & ROGERS, S. (1981) Mutagenicity of a series of
hexacoordinate chromium (III) compounds. Mutat. Res., 90:
111-118.
WATANABE, S. & FUKUCHI, Y. (1975) An epidemiological survey
on lung cancer in workers of a chromate-producing industry in
Hokkaido, Japan (Presented at: The XVIII International Congress
on Occupational Health, Brighton, England, 14-19 September.
WEILER, R.R. & CHAWLA, V.K. (1969) Dissolved mineral and
quality of Great Lakes waters. In: Proceedings of the 12th
Conference on Great Lakes Res., 801.
WELZ, B. (1980) [Use of AAS and ICP. Relative advantage of
each method.] Chem. Techn., 9: 161-170. (in German).
WELZ, B. (1983) [Atomic absorption spectrometry,] 3rd ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
527 pp (in German).
WENDELBERGER, J. & WEITGASSER, H. (1956) [The role of alkali
and degreasing agents in the development of contact eczemas in
electroplasters.] Berufsdermatosen, 4: 16-21 (in German).
WHANGER, P.D. & WESWIG, P.H. (1975) Effects of selenium,
chromium, antioxidants on growth, eye cataracts, plasma
cholesterol, and blood glucose in selenium-deficient, vitamin
E-supplemented rats. Nutr. Rep. Int., 12: 345-348.
WHITE, L.R., JAKOBSEN, K., & 0STGAAD, K. (1979) Comparative
toxicity studies of chromium rich welding fumes and chromium
on an established human cell line. Environ. Res., 20: 366-374.
WHITING, R.F., STICH, H.F., & KOROPATNICK, D.J. (1979) DNA
damage and DNA repair in cultured human cells exposed to
chromate. Chem.-biol. Interact., 26: 267-280.
WHO (1983) EHC 27: Guidelines on studies in environmental
e pidemiology, Geneva, World Health Organization, 351 pp.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984a)
Disposition of intratracheally administered chromium (III) and
chromium (VI) in rabbits. Toxicol. Lett., 22: 273-276.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984b) The
reduction of chromium (VI) to chromium (III) by glutathione:
an intracellular redox pathway in the metabolism of the
carcinogen chromate. Toxicology, 33: 341-348.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1985)
[Determination of chromium in human red blood cells. Basis for
a concept of biological monitoring.] Arbeitsmed. Sozialmed.
Praeventivmed., 20: 1-4 (in German).
WILD, D. (1978) Cytogenetic effects in the mouse of 17
chemical mutagens and carcinogens evaluated by the micronuclei
test. Mutat. Res., 56: 319-327.
WILLIAMS, C.D. (1969) Asthma related to chromium compounds.
Report of two cases and review of the literature on chromate
diseases. NC med. J., 30: 482-491.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1960) The Merck index. An encyclopedia of chemicals and
drugs, 7th ed., Rahway, New Jersey, Merck and Co., 139 pp.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) The Merck index. An encyclopedia of chemicals and
drugs, 9th ed., Rahway, New Jersey, Merck and Co., 1313 pp.
WINSTON, J.R. & WALSH, E.N. (1951) Chromate dermatitis in
railroad employees working with diesel locomotives. J. Am.
Med. Assoc., 147: 1133-1134.
WITHEY, J. (1983) Memorandum addressed to Si Duk Lee, dated
March 4, 1983, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office.
WOLF, W.R. (1976) Coupled gas chromatography/atomic
absorption spectrometry for the nanogram determination of
chromium. Anal. Chem., 48: 1717-1720.
WOLF, W.R. & GREENE, F.E. (1976) Preparation of biological
materials for chromium analysis. In: Accuracy in trace
analysis: sampling, sample handling, and analysis,
Gaithersburg, Maryland, National Bureau of Standards, pp.
605-610 (Special Publication No. 422).
WOLF, W.R., MERTZ, W., & MASIRONI, R. (1974) Determination
of chromium in refined and unrefined sugars by oxygen plasma
ashing flameless atomic absorption. J. agric. food Chem., 22:
1037-1042.
WOLF, W.R., TAYLOR, M.L., HUGHES, B.M., TIERNAN, T.O., &
SIEVERS, R.E. (1972) Determination of chromium and beryllium
at the picogram level by gas chromatography/mass spectrometry.
Anal. Chem., 44: 616-618.
WU, G.-Y. (1981) [The research on the purification and the
chemical structure of the specific chromium binding
materials.] Jpn. J. Ind. Health, 33: 505 (in Chinese).
YAGI, T. & NISHIOKA, H. (1977) DNA damage and its degrada-
tion by metal compounds. Sci. Eng. Rev. Doshisha Univ., 18:
63-70.
YANG, G.L. (1956) [An investigation of occupational disease
among chromium plating workers.] Acta Acad. Med. primae
Shanghai, 2: 36-46 (in Chinese).
YOPP, J.H., SCHMID, W.E., & HOLST, R.W. (1974) Determination
of maximum permissible levels of selected chemicals that exert
toxic effects on plants of economic importance in Illinois,
Chicago, Illinois, Institute for Environmental Quality,
pp. 89-96.
YUNISOVA, H.K. & PAVLOVSKAJA, G.S. (1975) The effect of
regimen of chrome plating on the properties of chromine.
Protect. Metal., 2, 248-250.
ZANDER, A.T., O'HAVER, T.C., & KELIHER, P.N. (1977)
Correction for interferences of spectral origin with continuum
source, echelle wavelength modulated atomic absorption
spectrometry. Anal. Chem., 49: 838-842.
ZHURKOV, U.S. (1981) [Approaches to the regulation of
environmental chemical pollutants with mutagenic activity.]
In: Sidorenko, G.I.M., ed. [Medical problems of environmental
protection,] Moscow, USSR Academy of Medical Sciences,
pp. 88-95 (in Russian).
ZOBER, A. (1979) [On the problems of evaluating bronchial
carcinoma after exposition to chromium compounds.] Int. Arch.
occup. environ. Health, 43(2): 107-121 (in German).
ZOBER, A. (1982) [Possible dangers to the respiratory tract
from welding fumer: methods of approach in an industrial
health care context and results.] Schweissen & Schneiden,
34(2): 77-81 (in German).
ZOBER, A., KICK, K., SCHALLER, K.H., SCHELLMANN, B., &
VALENTIN, H. (1984) ["Normal values" of chromium and nickel
in human lung-, kidney-, blood-, and urine samples.] Zbl.
Bakt. Hyg. I. Abt. Orig. B, 179: 80-95 (in German).
1969). For these reasons, chromium must be considered an essential
micro-nutrient. It is physiologically active in the trivalent
oxidation state at concentrations of approximately 100 µg/kg diet.
7.1.1. Effects of deficiency on glucose metabolism
Semipurified rations, containing Torula yeast as the source of
protein, were fed to groups of 10 male Sprague Dawley rats at
weaning, and intravenous glucose tolerance tests were performed by
injecting 1250 mg glucose/kg body weight and measuring the
subsequent decline in blood-glucose levels. The rate of glucose
disappearance can be calculated from the straight line plot of log
increment glucose (excess of glucose at any time (t) over fasting
glucose), versus time. The disappearance constant k is expressed
as % decline of the increment glucose per min; it is a measure of
the efficiency of glucose utilization. In weaning rats, the rate
constant was found to decline, within 3 weeks or less, from an
average of 4%/min to 2.6%/min in animals fed the Torula yeast diet,
but not in animals administered a diet in which 4 or 8% of Torula
yeast was replaced by an equal amount of brewer's yeast. This
observation suggested that brewer's yeast, but not Torula yeast,
contained an unknown substance necessary for the maintenance of
normal glucose tolerance. Because the only known effect of the
substance was that on glucose tolerance, it was named "Glucose
Tolerance Factor" (GTF) (Mertz & Schwarz, 1959). GTF was extracted
from brewer's yeast and pork kidney powder, concentrated and
purified, and its active ingredient was identified as trivalent
chromium (Schwarz & Mertz, 1959). Chromium in the form of most
common complexes (except for very stable ones) cured the impairment
of glucose tolerance in deficient rats, either as one oral dose of
200 µg/kg body weight, or as an intravenous (iv) injection of 2.5
µg/kg body weight. Chromium in the diet also prevented the
impairment of glucose tolerance.
In order to produce more pronounced deficiencies of chromium
and other trace elements, Schroeder et al. (1963) constructed a
special animal house on a mountain top in Vermont, USA, far removed
from traffic and industry. The interior was specially coated with
organic resins to reduce any metallic exposure. Air was introduced
through special filters, and strict practices were enforced to
avoid the introduction of dust and dirt. This will be referred to
as the "controlled environment". Under these conditions, a more
severe chromium deficiency resulted in very low glucose removal
rates of 1.12%/min in 6 rats and of 0.18%/min in 4 female breeder
rats (482 days old), after 11 days on a chromium-deficient diet.
The removal rate in 4 control rats receiving the same diet with a
chromium supplement improved from 0.51 to 1.39% during the same
period (Mertz et al., 1965b). This strong impairment of glucose
tolerance in rats kept in the "controlled environment" was
reflected in their fasting blood-glucose levels, compared with
those of chromium-supplemented controls: 1370 ± 68 versus 1170 ± 17
mg/litre in males and 1390 ± 68 versus 960 ± 65 mg/litre in
females. More than half of 185 deficient rats excreted more than
0.25% glucose in the urine, whereas glycosuria was found in only 9
of the chromium-supplemented controls (Schroeder, 1966). A
significant reduction in the intravenous glucose removal rate was
also observed in 8 rats in plastic cages, fed an EDTA-washed low-
protein diet (7% casein), compared with 8 controls receiving 50 µg
chromium as the chloride, by stomach tube, daily for 2 weeks (2.1
versus 3.6%/min; P < 0.01). However, chromium supplements did not
improve the near-normal removal rates in rats receiving a 20%
casein diet (Mickail et al., 1976). Significantly lower plasma-
glucose levels, due to supplementation with chromium, were reported
in rats fed the chromium-deficient Torula yeast diet (Whanger &
Weswig, 1975). In another series of studies, only a slight
reduction in blood-glucose (from 1240 to 1180 µg/litre) was found
in 10 rats receiving a diet supplemented with chromium (10 µg/kg)
(Djahanshiri, 1976).
Impaired glucose tolerance in squirrel monkeys, fed a
commercial laboratory chow, was shown to respond to chromium
supplementation. Of 9 monkeys with an impaired glucose removal
rate (1.38%/min), 8 responded after 22 weeks of supplementation of
their drinking-water (chromium acetate, 10 mg/litre) with a
normalization of their glucose tolerance (average removal rate, N =
9, 2.33 ± 0.3%/min) (Davidson & Blackwell, 1968). There was no
effect on food consumption, growth rate, or serum-insulin
concentrations. The chromium content of the commercial chow was
stated to be 3.3 mg/kg, a very high concentration. In view of the
uncertainties of methods of analysis for chromium (section 2.2), it
is not possible to interpret the results as indicative of a very
high chromium requirement of the squirrel monkey or of an unusually
poor bioavailability of the chromium in that particular ration. A
marginal chromium deficiency may have existed in mice fed a bread
and milk diet, as daily administration of 10 µg chromium for 16
days to 8 months produced a 10 - 30% decline in blood-glucose
levels (Vakhrusheva, 1960). However, this effect was not specific
for chromium, as it was also observed when manganese was
administered.
The glucose tolerance of guinea-pigs did not differ
significantly between groups fed diets containing chromium at
0.125, 0.625, or 50 mg/kg, even though the animals fed the 2 higher
levels exhibited a lower mortality rate during pregnancy (Preston
et al., 1976).
Although the administration of synthetic glucose tolerance
factor (a chromium-dinicotinic acid-glutathione complex) to 6 pigs
did not affect glucose tolerance tests, it resulted in a
significant increase in the hypoglycaemic effect of insulin
injected at 0.1 U/kg body weight (Steele et al., 1977a).
Turkey poults, fed a practical ration containing chromium
levels of 5 mg/kg, responded to chromium supplementation (20 mg/kg
diet) with a significant increase in liver glycogen and in glycogen
formation, following a fast, and with a significant increase in
glycogen synthetase (EC 2.4.1.11) activity in the liver (Rosebrough
& Steele, 1981).
It can be concluded that the impairment of glucose tolerance in
rats fed a low-chromium Torula yeast diet is due to chromium
deficiency. The effects of chromium in squirrel monkeys, pigs, and
turkeys, though statistically significant, are somewhat difficult
to interpret, because of the reported high chromium content of the
basal diet. No evidence for chromium deficiency has yet been
obtained through glucose tolerance tests on other animal species.
7.1.2. Effects of deficiency on lipid metabolism
Though trivalent chromium in high doses (2.5 mg/kg body weight)
has been shown to increase the synthesis of fatty acids and
cholesterol in the liver (Curran, 1954), lower, physiological doses
appear to decrease serum-cholesterol concentrations in rats.
Schroeder & Balassa (1965) found an average level of 927 mg
cholesterol/litre serum in 24- to 26-month-old male rats, kept in a
controlled environment and administered chromium in the drinking-
water at a concentration of 5 mg/litre, compared with an average of
1229 mg/litre in controls not receiving chromium ( P < 0.01). The
effects in female rats were ambigous, one study producing the
expected reduction in cholesterol due to chromium, another showing
an elevation in the supplemented female rats. Schroeder's
observations of a cholesterol-reducing effect of chromium in male
rats were confirmed by Staub et al. (1969) and by Whanger & Weswig
(1975) but were contradicted by results of a third study in which
there were not any significant effects of chromium on sucrose-
induced triglyceridaemia and cholesterolaemia (Bruckdorfer et al.,
1971). Perhaps more significant than the effect on circulating
cholesterol is the direct effect of chromium on the occurrence of
aortic plaques. Schroeder & Balassa (1965) observed 6 plaques in 54
male and female chromium-deficient rats, but only one plaque in 48
animals receiving 5 mg chromium/litre in drinking-water. These
results are in agreement with those from a subsequent report of the
protective effect of the natural chromium content of water (60 -
215 µg/litre) against atherosclerosis in cholesterol-fed rabbits
(Novakova et al., 1974). Abraham et al. (1980) extended these
observations by demonstrating that daily chromium injections (20 µg
K2CrO4) reversed the established atherosclerosis in the aorta of 11
cholesterol-fed rabbits, compared with 12 controls. The mean
plaque area was reduced from 95% to 63%, the total aortic
cholesterol from 729 mg to 458 mg, and the atheromatous lesions, as
measured by technetium incorporation from 285 000 cpm to 114 000
cpm, all differences being statistically significant. These
results are reinforced by observations in man discussed in section
8.1.
7.1.3. Effects of deficiency on life span, growth, and
reproduction
The mortality of male, but not of female mice, raised in a
"controlled environment" (section 7.1.1) was reduced by trivalent
chromium administered as acetate in the drinking-water at a
concentration of 5 mg/litre (Schroeder et al., 1964). The survival
rates at 12 months were 92.6% and 68.8% ( P < 0.0001) in
supplemented and deficient animals, respectively. Similary, male,
but not female, rats receiving a chromium concentration of 5
mg/litre in the drinking-water had longer life spans than deficient
controls. The mean age of the last surviving 10% of animals was
1249 days, compared with 1141 days in the deficient animals ( P <
0.01). Survival of male rats fed a low-chromium (< 100 µg/kg),
low-protein ration and subjected to a controlled acute haemorrhage
was significantly less than that of chromium-supplemented rats, in
2 studies (67 versus 92%; P < 0.05 and 27 versus 60%; P < 0.01,
respectively) (Mertz & Roginski, 1969).
In Schroeder's study, growth rates in treated mice and rats of
both sexes raised in a "controlled environment", were higher after
6 and 12 months, with highly significant differences in body weight
ranging from 9 to 17% ( P < 0.005) compared with the controls.
Again, the effects of chromium supplementation were greater in
males than in females (Schroeder et al., 1964, 1965).
Similar results were reported by Djahanschiri (1976), who
studied a total of 2750 rats of a special inbred strain (Hk51) fed
a basal diet (0.15 mg chromium/kg diet) and chromium supplements
ranging from 10 to 500 mg/kg diet. At 12 weeks, the average
weights of the chromium-supplemented animals, regardless of dose
level, were significantly higher (by 6% in the males and 3% in the
females) than those of the animals on the basal diet. The same
author reported a progressive diminution in both the milk
production of lactating rats and weight gain in 3 consecutive
generations fed the low-chromium diet, compared with rats receiving
chromium supplementation. Increased mortality was reported in
pregnant guinea-pigs fed a low-chromium diet (125 µg/kg diet)
compared with animals receiving a chromium supplement of either 625
µg/kg or 50 mg/kg (Preston et al., 1976).
When rats raised on a low-chromium Torula yeast diet (< 100
g/kg) mated with those on a normal diet, they were able to
impregnate the females at a 100% conception rate only up to the age
of 4 months. After this age, the conception rate declined to 25%,
25%, and 0%, at the age of 7, 8, and 9 months, respectively. This
decline was accompanied by a significant ( P < 0.01) decrease in
the sperm count in the chromium-deficient males to approximately
half of the count in supplemented controls at the age of 8 months
(Anderson & Polansky, 1981).
7.1.4. Other effects of deficiency
Male weanling rats, fed a 10% soya protein ration with a
chromium content of less than 100 µg/kg, developed a visible
opacity of the cornea in one or both eyes. In several studies, the
incidence of this effect ranged from 10 to 15% in deficient rats.
No opacities developed in control animals receiving 2 mg
chromium/kg diet (Roginski & Mertz, 1967).
Chromium deficiency has been shown to reduce the physical
performance of rats under stress. Ten male rats raised on a
chromium-deficient diet (150 µg/kg diet) swam for an average of 250
min, until exhaustion, in contrast with 10 rats receiving a
supplement of 10 mg chromium/kg diet, which were exhausted only
after 320 min (Djahanschiri, 1976).
7.1.5. Mechanism of action of chromium as an essential nutrient
7.1.5.1. Enzymes, nucleic acids, and thyroid
Chromium is present in nucleic acids in very high
concentrations, but the function of these is not clear at present
(Mertz, 1969). However, recent work suggests a biological function
of chromium in nucleic acid metabolism (Okada et al., 1984).
Ribonucleic acid synthesis in mouse liver was significantly
increased by as little as 1 µmol trivalent chromium, in the
presence of DNA or chromatin (Okada et al., 1981). These effects
were also present when the DNA or chromatin were first complexed
with chromium prior to incubation. However, prior complexation of
RNA polymerase with chromium depressed activity. These effects
were obtained in vitro with a concentration (52 µg/litre) that is
similar to physiological levels. Goncharov (1968) presented data
suggesting that chromium is involved in the function of the thyroid
gland. These findings have been supported by Lifschitz et al.
(1980).
An oligopeptide with a relative molecular mass of 1480, which
was crystallized from liver tissue, had a specific affinity for
chromium (Wu, 1981).
7.1.5.2. Interaction of chromium with insulin
The interaction of chromium with insulin has been extensively
studied and can therefore be presented in some detail, but this
does not imply that this is the only, or the most important,
function of chromium.
The effects of chromium in vitro, and probably in vivo, depend
on the presence of endogenous or exogenous insulin, no effects
having been demonstrated in in vitro systems that did not either
depend on, or contain, insulin. Chromium deficiency causes an
impaired response to added insulin in rat epididymal fat tissue,
and, when glucose uptake or glucose oxidation or utilization for
lipid synthesis is measured, the dose-effect curve is flat.
Addition of suitable chromium compounds significantly increases the
slope of the curve (Fig. 3). This demonstrates the true
potentiation of the insulin action and indicates that chromium
alone does not act as an insulin-like substance (Mertz et al.,
1961; Mertz & Roginski, 1971; Mertz, 1981). Chromium was also
shown to stimulate the transport of D-galactose into epididymal fat
cells. This suggests cell transport, the first step of sugar
metabolism, as a major site of action for chromium (Mertz &
Roginski, 1963). Insulin-potentiating effects have also been
observed on the swelling of liver mitochondria (Campbell & Mertz,
1963) and on glucose utilization in isolated rat lens (Farkas &
Roberson, 1965).
Fig. 3
Stimulation of the effects of insulin has been observed in a
glucose-independent, but insulin-responsive, system. Significantly
more alpha-amino isobutyric acid (a non-metabolizable amino acid
analogue) was incorporated into the heart and liver tissue of
chromium-supplemented male rats than in the tissues of chromium-
deficient controls, in response to the in vivo injection of the
labelled analogue and insulin (Roginski & Mertz, 1969).
These observations suggest a peripheral action of chromium to
facilitate the action of insulin; no evidence has been produced
indicating that chromium plays any role in the production, storage,
or release of insulin by the pancreas. Thus, the primary result of
chromium deficiency is a diminution in the effectiveness of
insulin. The resulting metabolic impairment may be compensated for
by increased insulin production in some cases, resulting in
elevated concentrations of the hormone, but not enough data exist
from experimental animal studies to assess the action of the
element on insulin metabolism. More information is available for
human subjects and this is discussed in section 8.1. The
interaction between chromium, insulin, and receptor sites of liver
mitochondrial membranes was studied using polarographic techniques.
The results formed the basis for the hypothesis that chromium may
facilitate bond formation between the intra-chain disulfide of
insulin and sulfur-containing groups of the receptors, by
participating in a ternary complex (Christian et al., 1963).
This hypothesis is consistent with results of studies on rats
fed, either a low-chromium Torula yeast diet or a brewer's yeast
diet known to be adequate in chromium. While the insulin-binding
capacity of hepatocytes was not significantly different, the
insulin affinity of the cells was significantly greater ( P < 0.01)
for the chromium-adequate rats than for the deficient Torula yeast
rats (Steele et al., 1977b).
7.1.6. Chromium nutritional requirements of animals
In the preceding sections, studies were evaluated in which the
effects of chromium supplementation were determined in animals that
were at least marginally chromium deficient. In other studies, the
effects of chromium were investigated in animal systems in which
the existence of chromium deficiency was either not ascertained or
not investigated. Before these studies are described and
interpreted for the determination of chromium nutritional
requirements of animals, it is helpful to consider them against the
background of Venchikov's (1974) model. This model is generally
applicable to trace element effects defining 3 zones of action, the
zone of biological action, that of pharmacodynamic action, and that
of toxicity (Fig. 4). The biological zone, in response to
supplements with low amounts of an element, represents the
correction of a deficiency and the resulting level of biological
activity is that of optimal function. Increasing the amount of
supplement further may lead to a certain depression, followed by a
zone of new, increased activity, in which the element no longer
acts as an essential nutrient, but as a drug. Still greater
supplements, beyond the homeostatic control capability of the
organism produce toxic effects and death. Because all the studies
described subsequently involved amounts of chromium supplements
that were higher than the levels normally needed to correct a
deficiency, it is possible, according to Venchikov's definition,
that the observed effects might be pharmacological.
Fig. 4
Tuman & Doisy (1977) studied the effects of yeast concentrates
of high chromium (glucose tolerance factor) content and of
synthetic chromium complexes with GTF activity (Tuman et al., 1978)
in mice, raised on a presumably complete commercial stock diet.
Six animals were used for each test, either genetically diabetic
mice or their control litter mates of the C57Bl-KSI strain.
Injections of either 5 mg of the GTF-containing yeast extracts or
0.1 mg of the synthetic chromium complex, acutely reduced the
elevated plasma-glucose levels in the diabetic and the non-fasting
normal mice by 10 - 38% of the initial values ( P < 0.01) and the
plasma-triglycerides by 26 - 56% ( P < 0.01), compared with control
mice injected with saline. Injection of insulin into diabetic mice
produced only an 11 - 18% decrease in plasma-glucose levels,
whereas injection of the GTF-containing extract together with
insulin reduced plasma-glucose levels by 39 - 51% and plasma-
triglyceride levels by 76% (Table 11).
The results suggest either a much higher increase in the
chromium requirement of the genetically diabetic mice or their
inability to use chromium in the diet.
Table 11. Acute effects of GTF and exogenous insulin on non-
fasting plasma-glucose and plasma-triglyceride (TG) concentrations
in 19-week-old genetically diabetic micea
------------------------------------------------------------------
Treatment Plasma- deltaGlucose Plasma- deltaTG
glucoseb triglyceridesb
(mg/litre) (mg/litre)
------------------------------------------------------------------
saline 11 120 ± 3960 ± 160(5)c
490(5)c
GTF 9320 ± -180 (16%) 2790 ± 170(5)d -117 (30%)
280(5)d
insulin 9840 ± -128 (12%) 3020 ± 400(5) - 94 (24%)
410(5)
insulin 7060 ± -406 (37%) 940 ± 220(5)e -302 (76%)
and GTF 840(5)e
------------------------------------------------------------------
a Modified from: Tuman & Doisy (1977).
b Glucose and triglyceride values represent mean ± SEM for 6 mice in
each treatment group. Dose of GTF was 5 mg (WL-10-AT) administered
intraperitoneally, 12 h prior to collection of blood. Lente insulin
(0.1 U per mouse) was administered subcutaneously, 12 h prior to
collection of blood. Data were treated by analysis of variance to
detect differences between the various treatment groups; independent
orthogonal comparisons were performed in the following groups ( P value
indicates level of significance for each comparison).
c Saline versus all other treatments, P < 0.005.
d GTF versus insulin, P < 0.05.
e GTF and insulin alone versus combined GTF and insulin, P < 0.005.
Thus, GTF and insulin > GTF = insulin > saline.
Steele & Rosebrough (1979) reported a significant stimulation
of the growth rate of one-week-old turkey poults (both sexes) by
supplementation of a practical ration with 20 mg chromium (as
chloride)/kg. The weight gains within the 2-week study were 235 g
and 267 g for the 60 controls and 60 supplemented turkeys,
respectively ( P < 0.001). As the practical ration contained
ground yellow corn and soybean meal, limestone, and dicalcium
phosphate, chromium deficiency would appear unlikely. The amount
of the chromium supplement (20 mg/kg diet) is quite high, and
further studies are needed to decide whether the observed effects
were of a pharmaco-dynamic nature or truly nutritional, i.e.,
correcting a deficiency. A similar interpretation should be
applied to a report of improved egg quality in the laying hen
(Jensen et al., 1978).
The quantitative aspects of the effects of chromium on animals
can be summarized as follows: normal rats fed semi-purified, semi-
synthetic rations, with Torula yeast or individual proteins and
sucrose or starch as the source of carbohydrates, develop mild
signs of deficiency at a dietary level of 100 - 150 µg chromium/kg.
To prevent deficiency, most authors used very high supplements of
several mg/kg diet and did not determine the biological
availability of the chromium complexes used for the
supplementation. Diets containing raw ingredients and supplying
chromium levels between 0.5 and 1 mg/kg do not induce signs of
deficiency and probably meet the requirement of the rat. A very
tentative estimate of the dietary chromium need of the rat and
probably the mouse would be approximately 0.5 mg/kg diet. This
estimate should be interpreted with caution, because of the lack of
knowledge concerning the biological availability of chromium and of
its interaction with dietary constituents.
7.2. Toxicity Studies
The toxicology of chromium compounds has been reviewed by the
US National Academy of Science (US NAS, 1974a), Langard & Norseth
(1979), the International Agency for Research on Cancer (IARC,
1980), Langard (1980a, 1982), and Burrows (1983).
In discussing toxicological problems, it is important to
differentiate between the various oxidation states of chromium and
its compounds. Trivalent chromium, when administered to animals in
food or water, does not appear to induce any harmful effects, even
when given in large doses (US NAS, 1974a) (section 7.2.1). Acute
and chronic toxic effects of chromium are mainly caused by
hexavalent compounds. Since it has been shown that both industrial
trivalent chromium compounds as well as reagent-grade trivalent
chromium compounds can be contaminated by hexavalent chromium
(Petrilli & DeFlora, 1978a; Levis & Majone, 1979), the evaluation
of experimental studies becomes difficult, especially when the
purity of the chemical compounds used is not known.
Discrimination between the biological effects, caused by
hexavalent chromium and trivalent chromium is difficult, because,
after penetration of membranes in tissues, hexavalent chromium is
immediately reduced to trivalent chromium (Gray & Sterling, 1950;
US NAS, 1974a), and it is not evident whether the observed
phenomena are caused by this reduction or even by the trapping of
trivalent chromium by ligands after uptake in the cells. Another
problem in evaluating the data is associated with the route of
administration. Hexavalent chromium, introduced by the oral route,
is partly reduced to trivalent chromium by acidic gastric juice
(Donaldson & Barreras, 1966; DeFlora & Boido, 1980); thus, the
effects or lack of effects observed may be caused mainly by
trivalent chromium and not by the hexavalent chromium, actually
administered.
7.2.1. Effects on experimental animals
Many local effects on human beings have been reported (section
8.3), but only a few studies have verified these effects in
experimental animals. A comprehensive survey of hexavalent
chromium-induced effects is given in Table 12 (US NAS, 1974a). For
most studies, details were not given of the length of exposure,
number of treated animals and controls, etc. Diagnoses were stated
without presenting all the original data. Thus, in this section,
some papers will be discussed that refer to the most prominent
local and systemic effects to support and clarify the effects shown
in human beings.
It is evident that the toxicity of hexavalent chromium in
animals varies with the route of entry into the body. Low
concentrations of hexavalent chromium may be tolerated, when
administered in the feed or drinking-water, the extent of
absorption being a factor of importance. For example, rats
tolerated hexavalent chromium in drinking-water at 25 mg/litre, for
1 year, and dogs did not show any effects from chromium
administered as potassium chromate at 0.45 - 11.2 mg/litre over a
4-year period (US NAS, 1974a). However, oral exposure of both male
and female rabbits to sodium dichromate (0.1% solution, 0.2 - 5
mg/kg body weight, for up to 545 days) resulted in significant
morphological changes in the gonads, including atrophy of the
epithelium and dystrophic alterations of the Sertoli and Leydig
cells in the testes, and sclerotic and atrophic changes in ovaries
(Kucher, 1966).
Larger doses of hexavalent chromium are highly toxic and may
cause death, especially when injected iv, subcutaneously (sc), or
intragastrically. The LD50 of chromium compounds was determined
for several experimental animal species. The LD50 of potassium
dichromate (hexavalent chromium), administered orally (stomach
tube) to rats, was 177 mg/kg body weight in males and 149 mg/kg
body weight in females (Hertel, 1982). When injected iv in mice
(sex not given), the LD50 of chromium carbonyl was 30 mg/kg body
weight (IARC, 1980).
Performing a life-time inhalation study on the rat, Glaser et
al. (1984) found an LC50 for Na2Cr2O7 of 28.1 mg/m3 (range, 16.7 -
47.3 mg/m3). Assuming a deposition rate in the lung of 30% of the
dose administered, the LC50 dose was 1 mg/kg body weight in male
and 1.2 mg/kg in female rats.
A local corrosive action of hexavalent chromium on the skin,
similar to that seen in man, was described by Samitz & Epstein
(1962), who induced chrome ulcers in guinea-pigs at 4 trauma sites,
with daily exposure to 0.34 MK2Cr2O7 solution for 3 days. Mosinger
& Fiorentini (1954) showed the same effects using potassium
chromates.
Table 12. Effects of hexavalent chromium in animalsa
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit, inhalation chromates 1 - 50 mg/m3 14 h/day for pathological Lukanin (1930)
cat 1 - 8 months changes
in the lungs
Rabbit inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day none Lehmann (1914)
as dichromate for 5 days
Cat inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day bronchitis, Lehmann (1914)
as dichromate for 5 days pneumonia
perforation of
nasal septum
Mouse inhalation mixed dust 1.5 mg/m3 4 h/day, no tumours Baetjer et al.
containing as CrO3 5 days/week, (1959a);
chromates for 1 year Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 16 - 27 mg/m3 1/2 h/day tumours in Baetjer et al.
containing as CrO3 intermit- some strains (1959a);
chromates tently Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 7 mg/m3 37 h over increased Baetjer et al.
containing as CrO3 10 days tumour rate (1959a);
chromates Steffee &
Baetjer (1965)
Rat inhalation mixed dust 7 mg/m3 37 h over barely Baetjer et al.
containing as CrO3 10 days toleratedb (1959a);
chromates Steffee &
Baetjer (1965)
Rabbit, inhalation mixed dust 5 mg/m3 4 h/day, none marked Baetjer et al.
guinea- containing as CrO3 5 days/week, (1959a);
pig chromates for 1 year Steffee &
Baetjer (1965)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rat, inhalation hexacarbonyl 1.6 mg/m3 4 months, anaemia; lipid Roschina (1976)
rabbit 4 h, 5 days and/or protein
a week dystrophia
in liver
and kidneys
Rat, inhalation hexacarbonyl 0.16 mg/m3 4 months, anaemia; no Roschina (1976)
rabbit 4 h, 5 days biochemical
a week or morphological
effects
Rat inhalation hexacarbonyl 35 mg/m3 30 min 100% death Roschina (1976)
Rat inhalation dichromates 0.006 - 0.2 28 days/ increase in Glaser et al.
mg/m3 90 days lung-macrophages (1985)
23 h/day
7 days/week lymphocytes
immunoglobulin,
reduced Fe2O3
lung clearance
Rat intratra- dichromates 5 per week up to 30 toleratedc Steinhoff
cheal in- 0.01 - 0.25 months et al. (1983)
stillation mg/kg
Rat intratra- dichromates 1 per week up to 30 tolerated; Steinhoff
cheal in- 0.05 - 1.25 months 1.25 mg/kg et al. (1983)
stillation mg/kg harmful
Rat oral potassium 500 mg/litre daily maximal Gross & Heller
chromate in non-toxic (1946)
drinking-water concentration
Rat, oral zinc chromate 10 g/kg daily maximal Gross & Heller
mouse in feed non-toxic (1946)
(mature) concentration
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit oral sodium di- 0.2 - 5.0 mg/kg 545 days morphological Kucher (1966)
chromate changes in
gonads (testes:
atrophy of
epithelium,
dystrophic
alterations of
Sertoli &
Leydiz alls.
Ovaries:
sclerotic and
atrophic
changes)
Rat oral zinc chromate 1.2 g/kg daily maximal Gross & Heller
(young) in feed non-toxic (1946)
concentration
Rat oral potassium 1.2 g/kg daily maximal Gross & Heller
(young) chromate non-toxic (1946)
in feed concentration
Dog, oral monochromate 1.9 - 5.5 mg 29 - 685 none harmful Lehmann (1914)
cat, or dichromates chromium/kg days
rabbit body weight per
day 1 mg chrom-
ium equivalent
to 2.83 mg
K2Cr2O7
or 3.8 mg
K2CrO4
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog oral potassium 1 - 2 g as daily fatal in 3 Brard (1935)
dichromate chromium months anaemia
Dog stomach potassium 1 - 10 g as - rapidly fatald Brard (1935)
tube dichromate chromium
Monkey subcutan- potassium 0.02 - 0.7 g in - fatald Hunter & Roberts
eous dichromate 2% solution (1933)
Dog subcutan- potassium 210 mg as - rapidly fatal Brard (1935)
eous dichromate chromium
Guinea- subcutan- potassium 10 mg - lethald Ophüls (1911a)
pig eous dichromate Ophüls (1911b)
Rabbit subcutan- potassium 1.5 cc of 1% - 80% fatald Hasegawa (1938)
eous dichromate solution/kg body
weight
Rabbit subcutan- potassium 20 mg - lethald Ohta (1940)
eous dichromate
Rabbit subcutan- potassium 0.5 - 1 cc of - nephritisd Ohta (1940)
eous dichromate 0.5% solution/kg
body weight
Rabbit, subcutan- sodium 0.1 - 0.3 g as - rapid deathd Priestley
guinea- eous or chromate CrO3 fall in blood (1877)
pig intravenous pressure
Rabbit intra- potassium 0.7 cc of 2% - lethald Mazgon (1932)
venous solution/kg 8-10 days after
body weight injection
Dog intra- potassium 10 grains - instant death Gmelin (1826)
venous chromate
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog intra- potassium 1 grain - none marked Gmelin (1826)
venous chromate
Dog intra- potassium 210 mg as - rapidly fatal Brard (1935)
venous dichromate chromium
Dog intra- potassium 3 mg/100 cc 2 doses marked renal Hepler & Simonds
venous dichromate blood per dose damage (1946); Simonds
& Hepler (1945)
---------------------------------------------------------------------------------------------------------
a Modifed from: US NAS (1974a).
b Pathological changes in experimental and control rats, 101 weeks after exposure.
c The same weekly dose distributed over 5 days was clearly better tolerated than a single weekly
administration.
d Renal damage.
Following parenteral administration, the most common systemic
effects of chromium were parenchymatous changes in the liver and
kidney (Mosinger & Fiorentini, 1954). Later studies showed
selective damage in the renal proximal convoluted tubules, without
evidence of glomerular damage, as demonstrated after one single sc
injection of potassium dichromate of 10 mg/kg body weight (Schubert
et al., 1970) or after one single intraperitoneal (ip) injection of
sodium chromate of 10 or 20 mg/kg body weight (Evan & Dail, 1974).
Effects have also been found in fish (Strik et al., 1975). After 32
days of continuous exposure to 0.1, 1, or 10 mg hexavalent chromium
(as potassium dichromate)/litre, the fish Rutilus rutilus developed
lysis of the intestinal epithelium with haemorrhages as well as
hypertrophy and hyperplasia of the gill epithelium.
Franchini et al. (1978) found an increase in urinary protein,
lysozyme, glucose, and beta-glucuronidase in rats after a single sc
injection of potassium dichromate at 15 mg/kg body weight. After
sc injection (3 mg/kg body weight), every other day for 2 - 8
weeks, the authors observed a correlation between the chromium
contents of the renal cortex and chromium clearance.
Five-week-old male Wistar rats of the strain TNO-W-74 were
continuously exposed in inhalation chambers to submicron aerosols
of sodium dichromate at concentrations ranging from 25 (low level)
to 200 µg chromium/m3 (high level) (Glaser et al., 1985). Exposure
for 28 days to 25 or 50 µg chromium/m3 resulted in "activated"
alveolar macrophages with stimulated phagocytic activity, and
significantly elevated antibody responses to injected sheep red
blood cells. After 90 days of low-level exposure, there was a more
pronounced effect on the activation of the alveolar macrophages,
with increased phagocytic activity. However, inhibited phagocytic
function of the alveolar macrophages was seen at the high
hexavalent chromium exposure level (200 µg/m3). In rats exposed to
this chromium aerosol concentration for 42 days, the lung clearance
of inert iron oxide was significantly reduced. The humoral immune
system was still stimulated at a low chromium aerosol concentration
of 100 µg/m3, but significantly depressed at 200 µg chromium/m3.
Exposure of rats, through inhalation, to chromium carbide or
chromium boride dust at very high levels (300 - 350 mg/m3 for each
substance) for 3 months (2 h/day) resulted in effects on the
vascular system of the lungs, e.g., endothelial hyperplasia.
Bronchitis and a decrease in the blood-haemoglobin concentration
were also observed (Roschina, 1964). The effects of chromium boride
were more pronounced than those of chromium carbide.
Steinhoff et al. (1983) performed an intracheal injection study
on rats (930 rats, 30 months, 0.05 - 1.25 mg Na2Cr2O7/kg body
weight per week; 1.25 mg CaCrO4/kg body weight per week). Half of
the rats were intratracheally injected once a week and the other
half received the same weekly dose distributed over 5 injections
per week. In rats receiving doses 5 times a week, there were some
significant changes in levels of total plasma-protein and
-cholesterol, in some haematological variables, in organ weights,
and in survival times in females. Only male rats receiving 1.25 mg
sodium dichromate/kg body weight, once a week, showed a sharp
reduction in body weight, female rats being less affected. Rats
receiving calcium chromate in the same dose showed reduced body
weight but to a lesser extent. The weight of lung and trachea was
increased by both substances in all doses.
Marked histopathological changes (congestion, fairly large
areas of focal necrosis, bile duct proliferation) described after
long-term exposure of rabbits to hexavalent chromium (ip injection
of 2 mg chromium/kg body weight per day for 6 weeks) (Tandon et
al., 1978), as well as the increase in hepatic metallothionein and
decrease in cytochrome P-450 levels after ip injection of 400 µmol
chromium/kg per day (type of chromium compound not mentioned)
(Eaton et al., 1980) need further confirmation. The finding of an
accumulation of hexavalent chromium in the reticuloendothelial
system including bone marrow (Baetjer et al., 1959b; Langard, 1977)
may be of importance for a disturbed blood picture.
Merkurieva et al. (1980a,b) studied the effects of potassium
dichromate in the drinking-water on the activities of different
enzymes in rats. Exposure included daily doses of 0.0005, 0.005,
0.05, or 0.5 mg/kg body weight for up to 6 months. After 20 days
of exposure at the highest dose level, enzyme activities increased
by 15 - 28% in liver microsomes (inosine-5-diphosphatase),
lysosomes (beta-D-galactosidase), and cytosol (lactate dehydrogenase
(EC 1.1.1.27)). For some of these enzymes, as well as for
acetylesterase (EC 3.1.1.6), increases in activity of up to 54%
were found in the gonads, kidneys, seminal fluid, and serum. At a
dose of 0.05 mg/kg, the only statistically significant finding was
a 54% increase in the activity of acetylesterase in the gonads.
After a 6-month exposure at this dose level, there was a 70%
increase in lactate dehydrogenase activity in the seminal fluid as
well as a 50% increase in free beta-galactosidase in the liver. There
were no changes in enzyme activities at the two lowest dose levels.
Painting of rat skin with an aqueous solution of potassium
dichromate (0.5%), daily, for 20 days, resulted in a local
inflammatory reaction, an increased level of hexose glyco-proteins
in the skin and serum, and an elevated concentration of serotonin
in the skin and liver (Merkurieva et al., 1982). Most of these
effects were also seen earlier in the exposure period, though they
were not as pronounced. Ten days after the start of exposure, a
nearly 3-fold increase in the serum-acetylcholine concentration
occurred together with decreased acetylcholinesterase activity.
Thus, the data of Merkurieva et al. show systemic effects following
both oral and dermal exposure to hexavalent chromium.
Cats fed chromic phosphate or oxydicarbonate at 50 - 1000 mg/day
for 80 days did not exhibit signs of illness or tissue damage.
Similarly, toxic reactions were not observed in rats administered
drinking-water containing 25 mg trivalent chromium/litre, for 1
year, or 5 mg trivalent chromium/litre throughout their lifetime
(US NAS, 1974a). The toxicity of trivalent chromium is so low that
even by parenteral administration, a chromic acetate level of 2.29
g/kg body weight or a chromic chloride level of 0.8 g/kg body
weight is required to kill mice. Even very large doses given
intragastrically were not fatal for dogs. Brard (1935) reported
that 10 or 15 g of chromium as chromic chloride proved fatal in one
dog (US NAS, 1974a). Some fatal doses of trivalent chromium
compounds reported in the literature are listed in Table 13.
Rats exposed through inhalation to chromic oxide (trivalent
chromium) at 42 mg/m3 or to chromic phosphate at 43 mg/m3 (5 h/day
for 5 days/week) for 4 months developed chronic irritation of the
bronchus and lung parenchyma, and dystrophic changes in the liver
and kidney (Blokin & Trop, 1977).
Inhalation exposure of rats (number not given) to dusts
containing 36 or 50% chromite for 4 months (2 h/day), at
concentrations of 375 - 400 mg/m3, resulted in thickening of the
walls of pulmonary vessels and bronchi (Roschina, 1959). The high
exposure levels in these studies make it difficult to evaluate the
effects.
Inhalation studies have also been performed with chromium
carbonyl, where chromium is in the 0 oxidation state (Roschina,
1976). Twelve rabbits and 48 rats were exposed for 4 months (4
h/day, 6 days/week) at a concentration of 1.6 or 0.16 mg/m3. At
both exposure levels, there was loss of body weight (25 and 12%,
respectively) as well as anaemia and leukocytosis. In the higher
exposure group, the animals showed an elevated gamma-globulin level
in serum and an increased transaminase activity. The contents of
cholesterol and SH-groups were reduced, and there was a decrease in
cholinesterase (EC 3.1.1.7) activity. Lipid and/or protein
dystrophy were noted in several organs, e.g., in the liver and
kidneys. No such effects were detected in the animals in the low-
exposure group.
Table 13. Fatal doses of trivalent chromium in animalsa
---------------------------------------------------------------------------------------------
Animal Number of Routeb Compound Chromium Effect Reference
animals dose (g/kg)
---------------------------------------------------------------------------------------------
Dog 2 sc chromic chloride 0.8 fatal Brard (1935)
Rabbit 1 sc chromic chloride 0.52 fatal Brard (1935)
Rat 38 iv chrome alum 0.01 - 0.018 LD50 Mertz et al.
chromium-hexaurea (1965a)
chloride
Mouse -c iv chromic chloride 0.8 MLDd Windholz et al. (1960)
Mouse -c iv chromic acetate 2.29 MLD Windholz et al. (1960)
Mouse -c iv chromic chloride 0.4 MLD Schroeder (1970)
Mouse -c iv trivalent chromium ? 0.25 - 2.3 MLD Windholz et al. (1960)
Mouse -c iv chromic sulfate 0.247 MLD Windholz et al. (1976)
Mouse -c iv chromic sulfate 0.085 MLD Schroeder (1970)
Mouse -c iv chromium carbonyl 0.03 LD50 Schroeder (1970)
---------------------------------------------------------------------------------------------
a Modified from: US NAS (1974a).
b sc = subcutaneous; iv = intravenous.
c No figures given.
d Minimum lethal dose.
7.2.1.1. Carcinogenicity
Various types of chromium chemicals, methods of administration,
and species of animals have been studied (IARC, 1980a).
Ideally, carcinogenicity should be tested with the methods
recommended by IARC (1980b), but, in many of the early studies,
this was not done. Carcinomas of the lung have been reported in
animals as a result of the administration of chromium chemicals.
Hueper (1958) found 2 squamous cell carcinomas and one
carcinosarcoma in 25 rats following intra-pleural injection of
chromite ore roast. After intrapleural implantation of strontium
chromate lasting 27 months, Hueper (1961) found tumours (type
unspecified) in 17/28 rats. Laskin et al. (1970) and Levy & Venitt
(1975) produced a number of bronchogenic carcinomas by implanting
pellets of cholesterol mixed with various chromium compounds
encased in a wire mesh cage in the bronchi of rats. Calcium and
zinc potassium chromate produced a number of bronchogenic
carcinomas, but soluble chromates and trivalent chromium chemicals
failed to produce cancer.
Using the same technique, Levy & Martin (1983) tested 21
different chromium-containing materials (pigments, intermediates,
and residues from the bichromate-producing industry, relatively
pure crystalline compounds) in 2250 random-bred rats and found that
chromates, described as sparingly soluble, were carcinogenic in the
rat lung. These materials included strontium and calcium chromate
and, to a far lesser extent, certain forms of zinc chromate.
Barium and lead chromate evoked only a very weak carcinogenic
response compared with strontium and calcium chromate. In the
study of Laskin et al. (1970), it was shown that chromium trioxide
produced hepatocellular carcinomas in 2/100 rats (controls, 0/24).
After inhalation of 13 mg calcium chromate/m3 (5 h/day, 5
days/week, for lifetime), Nettesheim et al. (1971) found 14 lung
adenomas in 136 treated mice and 5 in 136 untreated controls, but
no carcinomas. Steffee & Baetjer (1965), performing inhalation
studies on rats, mice, guinea-pigs, and rabbits (inhalation of
mixed chromate dust, corresponding to 3 - 4 mg CrO3/m3, 4 - 5
h/day, 4 days/week, for lifetime, or 50 months, respectively) could
only find 3 alveologenic adenomas in 50 treated guinea-pigs.
Laskin (1972) and Laskin et al. (1970) found 1 squamous cell
carcinoma of the lung, 1 of the larynx and 1 "peritruncal tumor" in
rats (inhalation of calcium chromate, 2 mg/m3, 589 exposures of 5 h
over 891 days) and 1 squamous cell carcinoma and 1 papilloma of the
larynx in hamsters. The number of treated animals was not
specified in either paper. Steinhoff et al. (1983) performed
intratracheal instillations of chromates in rats for 30 months with
one treatment/week and the same weekly dose distributed over 5
treatments/week (Table 14). In 880 exposed rats, 28 adenomas of
alveolar-bronchiolar origin (benign) and 12 malignant tumours (3
adenocarcinomas and 9 squamous cell carcinomas) were found. All
lung tumours developed very late and were only detected at the end
of the lifetime study, often in lungs with callosities. The
tumours were tiny and none of them caused the animal to die.
Sodium dichromate was not carcinogenic after exposure on 5
days/week. With calcium chromate, the carcinogenic effect was more
pronounced after treatment once per week, than after treatment 5
times per week.
Table 14. Incidence of benign and malignant lung tumours among
880 rats intracheally injected with Na dichromate and Ca chromatea
----------------------------------------------------------------
Dose Number of Incidence of lung tumours
(mg/kg) injections benign malignant
per week
----------------------------------------------------------------
Na2Cr2O7 x 2H20 0.25 5 none none
Na2Cr2O7 x 2H20 1.25 1 12 8
CaCrO4 0.25 5 5 1
CaCrO4 1.25 1 11 3
----------------------------------------------------------------
a From: Steinhoff et al. (1983).
Hueper (1961) reported unspecified tumours at implantation
sites in 12/34 rats, following intramuscular (im) implantation of
sintered calcium chromate (25 mg). No tumours were observed in 32
control animals. The same author found implantation-site tumours in
15/33 animals treated with strontium chromate compared with no
tumours in 32 control animals. Single sc injections of 30 mg lead
chromate (Maltoni 1974, 1976) resulted in injection-site sarcomas
in 26/40 treated rats. After administration of the same amount of
lead chromate oxide, injection-site sarcomas were found in 27/40
rats; no sarcomas were found in 60 vehicle controls. Following 9
im injections of 8 mg lead chromate, Furst et al. (1976) found
injection-site sarcomas in 31/47 treated rats, and renal carcinomas
in 3/24 treated rats; no sarcomas were found in 0/22 vehicle
controls. Heath et al. (1971) found injection-site sarcomas and
other tumours in 7/74 treated rats after im injection of 28 mg
cobalt-chromium alloy.
Administration of low levels of trivalent chromium acetate (5
mg/litre in the drinking-water) to mice and rats for the lifetime
did not result in increased tumour incidences compared with
controls (Schroeder et al., 1964, 1965).
Trivalent chromium oxide incorporated in bread at
concentrations of 10, 20, or 50 g/kg and fed to BD rats did not
increase tumour incidence compared with controls (Ivankovic &
Preussmann, 1975). Hexavalent chromium compounds have not been
tested by oral administration.
IARC (1980a) summarized the data of all available references
and concluded as follows: "There is sufficient evidence for the
carcinogenicity of calcium chromate and some relatively insoluble
chromium (VI) compounds (sintered calcium chromate, lead chromate,
strontium chromate, sintered chromium trioxide and zinc chromate)
in rats. There is limited evidence for the carcinogenicity of lead
chromate (VI) oxide and cobalt-chromium alloy in rats. The data
were inadequate for the evaluation of the carcinogenicity of other
chromium (VI) compounds and of chromium (III) compounds".
(a) Interaction with other factors
The possibility that chromium may act synergistically with
other agents in the production of cancer has been tested in several
studies. Nettesheim et al. (1970) pretreated one group of mice
with 100 R whole-body radiation and infected another group with PR8
influenza virus. These mice and control mice were exposed through
inhalation to chromium oxide dust for 6 - 18 months. No measurable
effects of either of the pretreatments were found on the incidence
of tumours. In a similar study with inhalation of calcium chromate
(CaCrO4, 5 h/day, 5 days per week for the lifetime), pre-exposure
to X-rays did not affect the tumour rate, but PR8 influenza
infection reduced tumour incidence (Nettesheim et al., 1971).
Steffee & Baetjer (1965) also found that the infection of rats with
PR8 influenza virus associated with intratracheal injection of
chromates did not lead to the production of tumours. Chromite ore
injected iv did not affect the development of tumours induced by iv
injection of 20-methyl-cholanthrene (Shimkin & Leiter, 1940).
Thus, at present, there are not sufficient data to suggest that
chromium acts synergistically with virus infections, ionizing
radiation, or other chemical carcinogens to produce cancers.
7.2.1.2. Genotoxicity
The mutagenicity of chromium compounds was reviewed by IARC
(1980a), Petrilli & DeFlora (1980), Levis & Bianchi (1982), and
Baker (1984). Present knowledge, in the light of recent findings,
is summarized in Table 15 (hexavalent chromium) and Table 16
(trivalent chromium).
When evaluating the results of chromium mutagenicity tests, it
is necessary to take into consideration several properties of the
tested compound (oxidation state, solubility, ability to penetrate
cell membranes, intracellular stability, and reactivity with
cellular components). Most of the in vitro mutagenicity
experiments have been performed with chromium chemicals, following
the introduction of the reverse mutation test by Ames (1973).
Bacterial strains of Salmonella typhimurium (Petrilli & DeFlora,
1977) containing mutants that require histidine for growth, and
Escherichia coli (Venitt & Levy, 1974) with mutants requiring
tryptophan for growth have been used. In each case, the bacterial
cultures have been incubated with the test chromium compounds and
the number of revertant colonies scored. The chromium compounds
studied included both hexavalent and trivalent compounds; a survey
of the results is given in Table 17 (DeFlora, 1981). Zhurkov
(1981) performed an Ames test to study the mutagenic activity of
potassium dichromate (K2Cr2O7) with S. typhimurium TA 1535 and TA
1538. A medium degree of mutagenic activity without metabolic
activation was shown by the strain TA 1535.
Only hexavalent chromium compounds have shown mutagenic
effects. Using the Salmonella /mammalian microsome tests of Ames
et al. (1973), DeFlora (1978) and Löfroth (1978) both showed a
decrease in the mutagenicity of hexavalent chromium in the presence
of an NADPH-generating system, suggesting reduction of mutagenic
hexavalent chromium to trivalent chromium. In this chromate
metabolism, cytochrome P-450 functions as the reductase (Garcia &
Wetterhahn Jennette, 1981). In recent in vitro studies, Wiegand et
al. (1984b) showed that glutathione could reduce hexavalent
chromium to trivalent chromium, without any further cofactors or
metabolizing enzymes. In rats, the most efficient tissue in
decreasing hexavalent chromium mutagenicity was the liver, followed
by the suprarenal glands, kidney, stomach, and lung (Petrilli &
DeFlora, 1980). These studies suggest a possible way of
intracellular detoxification of hexavalent chromium in low doses.
Schoental (1975) suggested that hexavalent chromium caused the
formation of epoxyaldehydes having mutagenic potential. Venitt &
Levy (1974) assumed that chromates belong to the transition-
inducing class of mutagens. They cause both frameshift and base-
pair substitutions (Petrilli & DeFlora, 1980). The hypothesis of
Kazantzis & Lilly (1979), that chromates cause guanine-cytosine to
adenine-thymine transitions in the subsequent round of DNA-
replication, needs further confirmation.
Table 15. Genotoxic activity of chromium (VI) compoundsa
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
DNA degradation CaCrO4 - Casto et al. (1976)
K2Cr2O7 - Bianchi et al. (1983)
PbCrO4 - Douglas et al. (1980)
K2CrO4 + Whiting et al. (1979)
Decreased fidelity PbCrO4, + (1) Levis & Majone (1981)
of DNA synthesis K2Cr2O7
CrO3 + Sirover & Loeb (1976)
K2Cr2O7 + Bianchi et al. (1983)
Microbial DNA K2CrO4, + Yagi & NishioKa (1977)
repair K2Cr2O7
CrO3, K2CrO4, + Kanematsu et al. (1980)
K2Cr2O7
K2CrO4, + Nishioka (1975)
K2Cr2O7
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
CrO3, + Gentile et al. (1981)
K2Cr2O7,
Na2Cr2O7, + Gentile et al. (1981)
(NH4)2Cr2O7
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Microbial gene K2Cr2O7 + (2) Bonatti et al. (1976)
mutation CrO3 + (2) Fukunaga et al. (1982)
Na2Cr2O7, +/-(3) Petrilli & De Flora
CrO3, +/-(3) (1978a,b)
ZnCrO4xZn(OH)2 +/-(3)
K2Cr2O7 + Zhurkov (1981)
Na2Cr2O7 +/-(3) DeFlora (1978)
chromate (4) +/-(3) Löfroth (1978)
dichromate +/-(3)
K2Cr2O7 - Kanematsu et al. (1980)
K2CrO4, + Löfroth & Ames (1978)
K2Cr2O7 +
K2Cr2O7 + Nishioka (1975)
K2CrO4, CaCrO4 + DeFlora (1981)
(NH4)2CrO4,
CrO3,
Na2CrO4, + Venitt & Levy (1974)
K2CrO4
CaCrO4
K2Cr2O7 + Bianchi et al. (1983)
Na2Cr2O7,
ZnCrO4
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
K2CrO4, CaCrO4 + Petrilli & DeFlora
CrO3 (1977)
Na2Cr2O7
K2CrO4 + Green et al. (1976)
PbCrO4, CrO3 + Nestmann et al. (1979)
Mammalian cell K2Cr2O7 + Bianchi et al. (1983)
gene mutation K2Cr2O7, + Newbold et al. (1979)
ZnCrO4 +
PbCrO4 -
K2Cr2O7 + (5) Bonatti et al. (1976)
K2Cr2O7 + Pashin et al. (1982)
NaeCr2O + Pashin & Kozachenko
(NHy)2Cr2O7 + (1981)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell K2CrO4, + Umeda & Nishimura (1979)
chromosomal K2Cr2O7 +
mutation K2Cr2O7 + Bigaliev et al. (1978)
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7 +
Na2CrO4, + Majone & Levis (1979)
K2CrO4 +
Na2Cr2O7, Levis & Majone (1979)
K2Cr2O7
CrO3, CaCrO4
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CrO3 + Kaneko (1976)
Na2Cr2O7 + Sarto et al. (1980)
K2Cr2O7 +
CrCl3 +
CrO3 + Tsuda & Kato (1977)
K2Cr2O7 + Raffetto (1977)
K2Cr2O7 + Stella et al. (1982)
Na2Cr2O7, + Majone & Levis (1979)
K2Cr2O7 +
K2CrO4 + Wild (1978)
Na2Cr2O7 + (7) Krishnaja & Rige (1982)
Mammalian cell CrO3, K2CrO4, + Ohno et al. (1982)
SCE K2Cr2O7 +
K2Cr2O7 + Bianchi et al. (1983)
K2CrO4,
Na2CrO4 + Levis & Majone (1979)
CrO3, +
K2Cr2O7, + Majone & Levis (1979)
Na2Cr2O7 +
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CaCrO4, CrO3, + Gomez-Arroyo et al.
K2Cr2O7 + (1981)
K2Cr2O7 + Majone & Rensi (1979)
CrO3 + (6) Stella et al. (1982)
K2Cr2O4, + Elias et al. (1983)
Na2CrO4, +
Na2Cr2O7 +
K2Cr2O7,
K2CrO4 + McRae et al. (1979)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell CaCrO4, K2CrO4 + (8) Casto et al. (1979)
transformation ZnCrO4 + (9)
Na2CrO4,
CaCrO4 + Casto et al. (1976)
+ Di Paolo & Casto (1979)
K2Cr2O7 + Bianchi et al. (1983)
K2Cr2O7 + Tsuda & Kato (1977)
CaCrO4 + Fradkin et al. (1975)
K2Cr2O7 + Raffetto (1977)
---------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) As industrial pigments (Cr-yellow-orange; Zn-yellow; Mo-orange).
(2) Gene conversion.
(3) No mutagenicity in the presence of mammalian microsomal activation
system (S-9 mix).
(4) No compounds given.
(5) Forward mutation was observed in 7/480 054 colonies (controls 0/84
546). A significant increase can be stated if comparison is made
with the historical spontaneous mutation rate, i.e., no mutants in
106 colonies.
(6) Occupatinal exposure.
(7) Induction of chromosomal aberrations in gill cells of the fish
Boleophthalmus dussumieri (Goby).
(8) 2 times control levels.
(9) 4 times control levels.
The results summarized in Table 17 show that the chromate ion
(hexavalent chromium) induces different kinds of genetic damage,
even at low doses, whereas the chromosome-damaging capacity of
trivalent chromium was only observed when it was tested in very
high doses.
In assays with whole-cell systems, trivalent chromium is
inactive, unless there is a direct interaction with DNA, e.g., in
studies in which purified DNA was exposed to trivalent chromium,
where modifications of the physical and chemical properties, as
well as decreased fidelity of DNA synthesis, were produced (Levis &
Bianchi, 1982). According to Warren et al. (1981), it appeared
that trivalent chromium in a proper ligand environment could have
considerable genetic toxicity. However, in recent studies, Bianchi
et al. (1984) found that trivalent chromium (CrCl3, 10-3 to 10-5 M)
was neither cytotoxic nor mutagenic in permeabilized hamster
fibroblasts (BHK were incubated for 30 min in Ea or BSS made
hypertonic with 4.2% NaCl).
Table 16. Genotoxic activity of chromium (III) compoundsa
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
DNA degradation CrCl3 + Bianchi et al.
(1983)
Decreased fidelity Cr2O3 + Levis & Majone
of DNA synthesis (1981)
Microbial DNA cis-[Cr(bipy)2ox] + Warren et al. (1981)
repair I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 + Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O + Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O + Warren et al. (1981)
trans-[Cr(en)2 + Warren et al. (1981)
(SCN)2]SCN
CrCl3, Cr2O3, - Yagi & Nishioka
Cr(OH)3 (1977)
K2Cr2(SO4)4 - Yagi & Nishioka
(1977)
K2Cr2(SO4)4 - Kanematsu et al.
(1980)
Cr2(SO4)3 - Kanematsu et al.
(1980)
CrCl3 - Nishioka (1975)
Cr(NO3)3, + De Flora (1981)
Cr(CH3COO)3
CrCl3 - Nakamuro et al.
(1978)
CrCl3 + Gentile et al.
(1981)
CrCl3, KCr(SO4)2, - Gentile et al.
(1981)
Cr2(SO4)3 - Gentile et al.
(1981)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Microbial gene CrCl3, Cr(NO3)3 - Löfroth & Ames
mutation (1978)
Cr(CIO4)3 - Löfroth & Ames
(1978)
CrCl3, Cr(NO3)3 - Venitt & Levy (1974)
Cr2SO4, - Venitt & Levy (1974)
K2SO4 x 2H2O
Cr(CH3COO)3 + Nakamuro et al.
(1978)
Cr(NO3)3 + Nakamuro et al.
(1978)
CrCl3 - Nakamuro et al.
(1978)
CrK(SO4)2 x 12H2O - Petrilli & De Flora
(1977)
CrCl3 x 6H2O - Petrilli & De Flora
(1978a)
Cr(NO3)3 x 9H2O - Petrilli & De Flora
(1978b)
Microbial gene cis-[Cr(bipy)2ox] + Warren et al. (1981)
mutation I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 - Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O - Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O - Warren et al. (1981)
trans-[Cr(en)2 - Warren et al. (1981)
(SCN)2]SCN
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
gene mutation (1983)
Cr(CH3COO)3 - Newbold et al.
(1979)
Mammalian cell Cr2(SO4)3 - Umeda & Nishimura
chromosomal (1979)
mutation
Cr(CH3COO)3 + Nakamuro et al.
(1978)
CrCl3, Cr(NO3)3 - Nakamuro et al.
(1978)
Cr(NO3)3, + Levis & Majone
(1979)
KCr(SO4)2, + Levis & Majone
(1979)
Cr(CH3COO)3 + Levis & Majone
(1979)
CrCl3 + Kaneko (1976)
CrCl3 - Sarto et al. (1980)
Cr2(SO4)3, CrCl3 - Tsuda & Kato (1977)
CrCl3 + Raffetto (1977)
CrCl3 - Stella et al. (1982)
CrCl3 - (1) Bianchi et al.
(1984)
Mammalian cell CrCl3, Cr2O3 + Elias et al. (1983)
SCE
Cr2O3 + Levis & Majone
(1981)
Cr(NO3)3, - Levis & Majone
KCr(SO4)2 (1979)
CrCl3, Cr(CH3COO)3 - Levis & Majone
(1979)
CrCl3 + (2) Ohno et al. (1982)
Cr2(SO4)3 - Ohno et al. (1982)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
SCE (contd). (1983)
CrCl3 - Majone & Rensi
(1979)
Mammalian cell CrCl3 - Bianchi et al.
transformation (1983)
---------------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) Thymidine uptake, DNA replication, damage and repair and SCE were
tested in permeabilized hamster fibroblasts.
(2) Weakly positive.
Levis & Buttignol (1977) and Levis et al. (1978) raised the
question of whether trivalent chromium could be an active mutagenic
agent within a cell. Others, such as Bianchi et al. (1980),
supported this theory, suggesting that trivalent chromium is bound
to genetic material after reduction of the hexavalent form by an
NADPH-dependent microsomal enzyme system (Norseth, 1978; Jennette,
1979). The studies of Sirover & Loeb (1976), showing a 30% error
in DNA synthesis after application of 0.64 mM trivalent chromium
compared with 16 mM of hexavalent chromium, support this theory.
Pashin et al. (1982) demonstrated the induction of dominant
lethal mutations in mice. They treated male mice with a single
injection of potassium dichromate (0.5 - 20 mg/kg body weight, ip)
or with daily injections (1 and 2 mg/kg, ip, for 21 days). The
single injections (0.5 - 2 mg/kg) did not induce any effects;
repeated or higher dosages of potassium dichromate induced a
statistically significant decrease in the survival of embryos from
cells treated at the early spermatid and late spermatocyte stages.
Karyological alterations in mammalian cells were induced after
exposure to concentrations of hexavalent chromium that were more
than 100 times lower than the concentration of trivalent chromium
causing the same changes (Majone & Rensi, 1979).
Analysing the effects of fume particles from stainless steel
welding on sister chromatid exchanges and chromosome aberrations in
cultured Chinese hamster cells, Koshi (1979) found an increase in
cytogenic effects with increasing fume doses, due to the dissolved
hexavalent chromium. Hexavalent chromium and stainless steel
welding fume particles containing chromium compounds produced
positive results in the "mammalian spot test", a somatic tissue
assay (Knudsen, 1980).
Table 17. Mutagenicity of chromium compoundsa
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
L. Hexavalent chromium compounds
1) Sodium dichromate (Merck) - +w +w +w + 50 - 250 4.4 decrease toxic and mutagenic
Na2Cr2O7 x 2H2O Cr6+ was converted
into inactive Cr3+
2) Potassium chromate (BDH) - +w +w +w + 80 - 410 2.9 decrease by reducing
K2CrO4 chemicals (ascorbic
acid, sodium
3) Calcium chromate (BDH) - +w +w +w + 60 - 290 3.2 decrease sulfite) or
CaCrO4 metabolites (NADH,
NADPH, GSH), by
4) Ammonium chromate - +w +w +w + 50 - 320 3.7 decrease human gastric juice
(Carlo Erba) and erythrocyte
(NH4)2CrO4 lysates, by S9 mix
containing human
liver S9 fractions
5) Chromium trioxide or - +w +w +w + 40 - 220 5.1 decrease or rat tissue S9
chromic acid (Merck) fractions (in order
CrO3 of efficiency:
liver>suprarenal
glands>kidney>
stomach>lung);
pretreatment of
rats (Aroclor(R)
1254), ether,
ethanol) influenced
the efficiency of
metabolic systems;
conversely, Cr6+
mutagenicity was not
affected by human
serum or plasma nor
by S9 mix containing
other rat (spleen,
colon, bladder,
striated muscle) S9
fractions
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
6) Zinc yellow (Montedison) - +w +w +w + 90 - 590 1.4 decrease the mutagenic effects
ZnCrO4 x Zn(OH)2 + of Cr6+ compounds and
10% CrO3 benzo( a)pyrene were
less than additive
7) Chromyl chloride (BDH) - +w +w +w + 100 - 430 1.8 decrease liquid volatile
Cl2CrO2 compound; vapours
were also toxic and
mutagenic
8) Chromium orange or basic - - - - +b 2 mg the three pigments
lead (spot test) containing PbCrO4
PbCrO4 x PbO were scarcely (L8),
chromate (Montedison) very scarcely (L9),
or totally (L10)
insoluble in water;
they were assayed
by directly spotting
these compounds at
the centre of plates
9) Molybdenum orange or - - - - +b 2 mg
lead solfomolybdo- (spot test)
chromat (Montedison)
PbCrO4 72-77%
PbSO4 4-6%
Al2O3 2%
PbMoO4 12-14%
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
10) Chromium yellow or - - - - -b
lead solfochromate
(Montedison)
PbCrO4 41-85%
PbSO4 4-45%
SiO2 0.1-3%
Al2O3 2-6%
11) Chromium - - - - - -2.3x103 - - hexacoordinated
hexacarbonyl (BDH) compound; formally,
Cr(CO)6 its oxidation state
is 0; insoluble in
water, dissolved in
ether
M. Trivalent chromium compounds
1) Chromic chloride - - - - - -3x104 - - inactive Cr3+
(BDH) CrCl3 x 6H2O compounds could be
converted into toxic
and mutagenic Cr6+
only in the presence
of oxidizing chemicals
(potassium
permanganate), while a
variety of metabolic
systems were
ineffective
2) Chromic nitrate (Riedel- - - - - - -2x104 - -
de Haen)
Cr(NO3)3 x 9H2O
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
3) Chromic potassium - - - - - -1.6x104 -
sulfate (BDH)
CrK(SO4)2 x 12H2O
4) Chromic acetate (BDH) - - - - - -7x104 - -
Cr(CH3COO)3
5) Neochromium (Montedison) - - - - - -5x104 - -
Cr(OH)SO4 56-58%
Na2SO4 23-24%
H2O 18-21%
6) Chromium alum (Montedison) - - - - - -4.3x104 - -
Cr2(SO4)3 37-39%
K2SO4 16-18%
H2O 43-37%
7) Chromite (Montedison) - - - - + 2 mg - Contaminated with Cr6+
Cr2O3 44-46% (spot test) traces ( sim-diphenyl-
Fe2O3 29-30% carbazide reagent);
Al2O3 15-16% scarcely soluble in
SiO3 0.5-3% water; assayed by
CoO 0.5-2% directly spotting the
powder at the centre
of plates
--------------------------------------------------------------------------------------------------------
Table 17 (contd.)
Note:
Sensitivity of Salmonella strains: Range of activity:
In the assays carried out in this study, This column indicates the lower and
the numbers of spontaneous revertants of the upper limits (in nmol per plate)
Salmonella tester strains fell within the of the mutagenic response, as in-
following ranges: ferred from dose-response curves
TA 1535: 10-25, both with and without S9 mix; with the most sensitive bacterial
TA 1537: 3-18, both with and without S9 mix; strain and under the most favourable
TA 1538: 10-25 without S9 mix; 15-35 with S9 mix; metabolic situation (i.e., with or
TA 98: 20-35 without S9 mix; 25-40 with S9 mix; without S9 mix) for each positive
TA 100: 150-200 without S9 mix; 140-180 with S9 mix compound; for negative compounds,
the maximum dose tested is indicated
The key for the interpretation of symbols in the
column "Reverted strains" is the following: Mutagenic potency:
+ clearly positive result indicated by a dose-
related and reproducible increase of his+ the values presented have
revertants over controls (at least a 3-fold been calculated by dividing the number
increase); of revertants in excess of controls
+w weak positivity indicated by an increase of by the corresponding amount of compounds
revertants 2 - 3 times in controls; (in nmoles); the number of net revertants
± reproducible but less than 2-fold increase was determinated at the top level of the
of revertants; linear part of dose-response curves, which
No symbol: no conclusive experiment carried out were drawn as indicated under the previous
with the corresponding strain. sub-heading.
Effects of S9 mix
The effect of S9 mix containing
liver S9 fractions from
Arochlor(R) pretreated rats on
the mutagenicity of test
compounds is reported
--------------------------------------------------------------------------------------------------------
a From: DeFlora (1981).
b L8, L9, and L10, as well as PbCrO4, were positive in the plate test when
dissolved in 0.5 N NAOH.
Embryonic fibroblast cell cultures showed significant
chromosome aberrations, when potassium dichromate was added to the
medium (Tsuda & Kato, 1976). When the potassium dichromate was
reduced with sodium disulfite (Na2SO3), no significant aberrations
occurred, even with 100 times the chromium concentrations.
At present, it is widely accepted that hexavalent chromium is
genetically active, because of its ability to cross the membranes
and enter the cells. If reduction of hexavalent chromium takes
place outside the cell (or even outside the cell nucleus, e.g., in
mitochondria or microsomes) its genetic activity is suppressed; if
the reduction takes place inside the nucleus (near, or at, the
target DNA molecules) alterations in DNA can occur, depending on
the oxidation power of hexavalent chromium or the formation of
trivalent chromium complexes with nucleophilic sites of the DNA;
thus, trivalent chromium could be the ultimate mutagenic form of
chromium (Levis & Bianchi, 1982). More recent investigations
(Wiegand et al., 1984) have shown that hexavalent chromium may
enter the body unreduced via the lung and be partly deposited in
erythrocytes over a prolonged period of time. From a DNA-repair
test in bacteria, DeFlora et al. (1984) concluded that hexavalent
chromium causes irritation in nucleotide pools and, due to its
oxidizing power, induces single-strand breaks in DNA, while
trivalent chromium induces DNA-protein and DNA-DNA cross-links and
decreases the fidelity of DNA replication.
7.2.1.3. Developmental toxicity and other reproductive effects
The teratogenicity of hexavalent chromium as chromium trioxide
was tested in golden hamsters (Gale, 1974, 1978, 1982; Gale &
Bunch, 1979) and that of trivalent chromium as chromic-chloride in
ICR mice (Iijima et al., 1975; Matsumoto et al., 1976). Injections
of hexavalent chromium into the fertilized chick egg increased
embryolethality and produced malformations in the survivors (Gilani
& Marano, 1979).
The golden hamsters were treated with a single iv injection of
chromium trioxide (5, 7.5, 10, or 15 mg/kg body weight) on the 8th
day of gestation (Gale, 1978).
Dose-response relationships were demonstrated between the rate
of absorption and the rate of malformations including delayed
ossification of the skeletal system. At 5 mg/kg body weight, 4% of
the fetuses showed external abnormalities (oedema, exencephaly),
and 34% showed cleft palate (controls: 2%), which increased to 85%
with the higher doses of 7.5 or 10 mg/kg body weight. Thirty-one
percent of fetuses were retarded following treatment with 7.5 mg/kg
body weight and 49% following 10 mg/kg body weight. The dose of 15
mg/kg body weight was lethal for 75% of the dams. Gale & Bunch
(1979) injected a single dose of 8 mg CrO3/kg body weight on day 7,
8, 9, 10, or 11 of gestation in female golden hamsters (Table 18).
The treated females were adversely affected (body weight loss,
tubular necrosis of the kidneys). In a later study, Gale (1982)
injected 8 mg CrO3/kg iv in hamsters on day 8 of gestation. An
increased incidence of cleft palate was seen in strains LVG, LSH,
and MHA, but no effects were reported in strains CB, LHC, and PD4.
ICR mice were injected sc with chromic chloride (Iijima et al.,
1975; Matsumoto et al., 1976). In the first series (animals
administered 9.8 or 19.5 mg/kg body weight, every other day, from
the 0th to 16th day of gestation), according to the authors, a
slight increase in the rate of malformations could not be excluded.
In the second series, with ip injections of 9.8 - 24.4 mg/kg body
weight, on the 8th day of gestation, the authors reported a dose-
dependent increase in the frequency of exencephaly, anencephaly,
and the occurrence of rib fusion.
Danielsson et al. (1982) studied the rate of embryonic or fetal
uptake of trivalent chromium and hexavalent chromium in early and
late gestational mice (Table 19) and found placental passage of
chromium. Hexavalent chromium appeared in the fetus in high enough
concentrations for a direct effect on embryonic structures to be
likely, trivalent chromium may act on placental structures.
Gilani & Marano (1979) injected chromium trioxide into 840
embryonating chicken eggs (air sack) at doses ranging from 0.002 to
0.05 mg per egg, on days 0, 1, 2, 3, and 4 of incubation (0.1 ml
physiol. saline injections per control egg). All embryos were
examined on day 8. The following malformations were observed in
the 720 eggs affected: short and twisted limbs, microphthalamia,
exencephaly, short and twisted neck, everted viscera, oedema, and
reduced body size. However, the incidence of abnormalities in the
120 controls was reported to be "low".
Inhalation studies were conducted by Glaser et al. (1984) on
Wistar rats (TNO-W-74 SPF) to study the effects of sodium
dichromate on reproduction and teratogenicity in 3 generations.
Whole-body exposures to sodium dichromate aerosols (0.2 mg
chromium/m3) were performed with an exposure time of 130 days per
generation. No effects on reproduction were found. All
teratogenicity tests were negative, and there was no increase in
fetal chromium content. However, from generation to generation,
there was an increase in immunosuppression and hyperplasia of
organs (especially in the lungs) and changes in haematological
variables.
Table 18. Embryotoxicity following maternal exposure to CrO3 on different days
of gestation in hamstersa
-------------------------------------------------------------------------------------
Findings in live fetuses
Day of No. of No. of No. of Frequency External Internal Cleft
gestation females implantation living resorptions defects defects palate
treated sites fetuses No. %b No. %b No. %b No. %b
-------------------------------------------------------------------------------------
Treated CrO3 8 mg/kg
7 6 64 10 54 84 1 10 1c 10 5 50
8 6 88 54 14 20 8d 15 4e 7 17 31
9 6 77 67 10 13 18f 27 0 0 25 37
10 6 77 77 0 0 0 0 0 0 0 0
11 6 68 67 1 1 0 0 0 0 0 0
Controls H2O 5 ml/kg
7 3 35 33 2 6 0 0 0 0 0 0
8 3 44 41 3 7 0 0 0 0 0 0
9 3 37 36 1 3 0 0 0 0 0 0
10 2 20 19 1 5 0 0 0 0 0 0
11 3 34 33 1 3 0 0 0 0 0 0
-------------------------------------------------------------------------------------
a From: Gale & Bunch (1979).
b Underlined %, significantly different statistically from controls.
b Right hindlimb with two digits.
c Right kidney absent.
d Oedema (7), tail short (1).
e Small kidneys (3), right kidney absent (1).
f Oedema (17), omphalocele (1).
Table 19. Comparison of concentrations of trivalent and hexavalent
chromium in fetuses, placentas, and maternal organsa,b
------------------------------------------------------------------
Day 13 Day 16
Hexavalent Trivalent Hexavalent Trivalent
chromium chromium chromium chromium
------------------------------------------------------------------
Fetus 0.3 ± 0.01 0.03 ± 0.002 0.5 ± 0.04 0.1 ± 0.01
Placenta - - 3.0 ± 0.2 2.4 ± 0.2
Liver 29.4 ± 2.2 30.8 ± 6.5 33.9 ± 3.9 27.7 ± 3.5
Kidney 38.1 ± 1.9 5.3 ± 0.7 36.0 ± 4.3 8.7 ± 2.3
Serum 2.6 ± 0.2 9.3 ± 0.9 2.8 ± 0.4 11.0 ± 1.6
------------------------------------------------------------------
a From: Danielsson et al. (1982).
b Trivalent chromium (51CrCl3) and hexavalent chromium
(Na251Cr2O7) were injected iv on day 13 and day 16 of gestation
at doses of 10 mg chromium/kg body weight. The pregnant animals
were autopsied after 1 h. Concentrations are expressed in
mg/litre or g ± SEM (N = 4).
7.2.1.4. Cytotoxicity and micromolecular syntheses
Wacker & Vallee (1959) reported that trivalent chromium was
present in RNA from all sources examined; they hypothesized that
chromium might contribute to the stabilization of the structure.
Administration of trivalent chromium to mice (ip injection of
CrCl3 at 5 or 0.5 mg chromium/kg body weight; P < 0.01) caused
accumulation of chromium in the cell nucleus, which amounted to
about 20% of the accumulated chromium content of the liver cell,
and also enhanced RNA synthesis. Hexavalent chromium inhibited RNA
synthesis (K2CrO4 at 5 or 0.5 mg chromium/kg body weight;
P < 0.01) (Okada et al., 1983). In further studies, Okada et al.
(1984) pretreated rats with CrCl3 (ip injection of 5 mg chromium/kg
body weight) and then partially hepatectomized the rats. Chromium
accumulated in the regenerating liver, and hepatic RNA synthesis
was accelerated compared with that of partially hepatectomiced
controls. These findings suggest a direct participation of
chromium in RNA synthesis.
Potassium dichromate enhanced the passive uptake of ribo-and
desoxyribonucleosides (Levis et al., 1978; Bianchi et al., 1979),
caused an inhibition of DNA replication to BHK fibroblasts, and
reduced the colony-forming ability of BHK cells (doses: 10-4 - 20-7
mol). Hexavalent chromium (potassium dichromate with a
concentration of 5 x 10-7 mol/litre) reduced by half the
proliferation of NHIK 3025 cells originating from a human cervix
carcinoma (White et al., 1979).
The addition of calcium chromate to BHK21 cell cultures altered
their growth characteristics. Whereas these cells normally grow as
elongated fibroblast cells in parallel orientation, the chromium-
exposed cells grew as shortened fibroblasts with enlarged nuclei
and granular cytoplasm, randomly orientated. When grown in
carboxymethyl cellulose, the chromate-exposed cells underwent many
divisions in contrast to the 1 or 2 divisions of normal cells.
These alterations in growth properties persisted, even after
transfer to a normal medium (Fradkin et al., 1975).
Cultures of HeLa and rat embryonic cells were incubated with
various hexavalent and trivalent chromium compounds for 3 days and
the 50% inhibitory dose (ID50) for cell growth was determined. The
ID50 for sodium and potassium chromate and dichromate and calcium
chromate varied from 0.34 to 0.78 µg/ml of the medium. The ID50
for the trivalent compounds varied over a wider range: chromic
oxalate, 4; acetate, 52; nitrate, 720; chloride, 1030; and bromide,
1500 mg/litre. Similar results were found in rat embryonic cell
cultures (Susa et al., 1977).
7.2.1.5. Fibrogenicity
Because of the well-known fibrogenic effects of silica and
asbestos minerals, studies were initiated to determine whether
chromite and various other dusts exhibited similar properties. A
single intratracheal injection in rats of 40 mg chromite particles
(33% chromium, 0.7% SiO2, 99.9% of the particles being smaller than
5 µm) suspended in 1 ml Ringer's solution caused a moderate
cellular reaction that reached a peak after 2 months and then
decreased. After 8 months (end of observation), the lung weight
was within normal limits. Only an increase in fibrils that was not
significant was observed. The tissue reaction in regional lymph
nodes was minimal (Swensson, 1977).
Chromite FeO(CrAl)2O3 (10 mg), ground to an average particle
diameter of 1 µm, was injected into the pleural cavities of 25
mice. The animals were killed at intervals ranging from 2 weeks to
18 months after injection. Chromite produced the least reaction of
all the 15 dusts tested. This material attracted very few cells
and the eventual production of collagen was minimal (Davies, 1972).
7.2.2. Observations in farm animals
Systemic poisoning of farm animals with chromates is rare and
is mainly suspected to be a consequence of accidental industrial
contamination of drinking-water. The acute lethal dose for mature
cattle is approximately 600 - 800 mg/kg body weight; chronic
poisoning in young calves was produced by a daily dose of 30 - 40
mg/kg body weight for one month (NZ Department of Agriculture,
1954) with a clinical picture of profuse scouring, severe
dehydration, low blood pressure, changes in the alimentary tract,
including congestion and inflammation, and the rumen and abomasum
showing ulceration near perforation.
High chromium levels were found in the blood and liver of
calves that had died from poisoning. The minimum tissue levels
causing poisoning were 30 mg/kg in the liver and 4 mg/litre in
whole blood (Harrison & Staples, 1955). According to Romoser et al.
(1961), a level of 100 mg hexavalent chromium (as sodium
chromate)/kg did not affect growing chicks.
8. EFFECTS ON MAN
8.1. Nutritional Role of Chromium
The physiological role of chromium and the need for small
amounts of dietary chromium are indicated by:
(a) chromium-responsive cases of impaired glucose
metabolism in malnourished children and middle-aged
persons;
(b) 2 cases of proved chromium deficiency in patients fed
exclusively by parenteral alimentation; and
(c) epidemiological studies suggesting a link between
chromium deficiency and risk factors for cardiovascular
diseases.
8.1.1. Biological measurements and their interpretation
Biochemical methods for the diagnosis of the human nutritional
chromium status are still at the developmental stage. Blood- or
urine-chromium concentrations are not indicative of chromium
status. Several studies suggest the validity of the "relative
chromium response" in plasma or serum to the ingestion of 50 - 100
g of glucose within 30 - 120 min; the response also appears in the
urine. Lack of this response suggests chromium deficiency,
especially when combined with impaired glucose tolerance in the
presence of normal or elevated plasma-insulin levels.
8.1.2. Chromium deficiency
8.1.2.1. Adults
Chromium deficiency is diagnosed mainly through therapeutic
trials in which an impaired function is restored by supplementation
of the diet with small amounts of chromium close to the estimated
human requirement. Glinsmann & Mertz (1966) studied 4 male
diabetic patients for several months to 1 year, under the strictly
controlled conditions of a metabolic ward. The subjects, who were
fed identical portions of a diet prepared before the start of the
study, were allowed a measured amount of physical activity and were
stabilized under these conditions before the beginning of the
control periods. Three of the 4 patients responded to a chromium
level of 180 - 1000 µg/day as CrCl3 x 6H2O with a significant
improvement in oral glucose tolerance; the fourth showed a very
slight lowering of the glycaemic curve, which was not statistically
significant. A representative example is given in Table 20.
Table 20. Effect of chromium on mean glucose tolerancea,b
------------------------------------------------------------------------
Supplementation Days Number Mean blood glucose concentration
of ---------------------------------
tests Fast- Min after glucose load
ing 30 60 90 120
------------------------------------------------------------------------
None 0 - 32 10 840 2170 2290 1950 1560
60 µg, 3 x per day 33 - 74 12 850 2240 2380 2090 1620
60 µg, 3 x per day 75 - 119 13 840 2100 2130 1860 1410
60 µg, 3 x per day 120 - 140 5 840 2030 2010c 1750 1120c
None 180 - 194 1 960 2070 2900 2930 2370
60 µg, 3 x per day 195 - 211 5 830 1960 1930c 1750 1180c
None 256 - 313 10 870 2200 2450 2240 1660
1 mg, 3 x per day 337 - 340 1 680 1480 1620 1510 960
------------------------------------------------------------------------
a Modified from: Glinsmann & Mertz (1966).
b A 46-year-old man with maturity-onset diabetes, controlled on diet;
oral glucose tolerance test consisted of constant noon meal and 100 g
glucose.
c Mean values significantly different from control ( P < 0.025).
The condition of one of 2 diabetic out-patients, observed over
several months, was improved by chromium supplementation, the
condition of the other was not. Short-term treatment of 7 diabetic
patients for 1 - 7 days with 1 mg chromium did not affect any
variables of glucose metabolism, nor did chromium supplementation
change the normal glucose tolerance tests of 10 healthy, young
volunteers.
Glinsmann's positive results were not confirmed in a double-
blind crossover study on 4 normal volunteers and 10 diabetic
persons, with a crossover after 16 weeks. No effects of chromium
were detected on glucose tolerance or on fasting or postprandial
blood-glucose levels (Sherman et al., 1968). Schroeder et al.
(1970), while reporting that 2 - 10 mg chromium/day "almost always"
restored the impaired glucose tolerance in older, non-diabetic
subjects to normal, found only erratic and mild improvement in 4
out of 12 diabetic out-patients. On the basis of these
observations, Mertz (1969) concluded that chromium should not be
considered a therapeutic agent for diabetic patients.
On the other hand, it was reported in a later study on chromium
metabolism that chromium administration (500 µg/day, for 2 weeks)
produced a marked improvement in glucose utilization in both
juvenile and maturity-onset diabetic patients. Five of the
patients were followed for more than 1 year after exposure and were
found to maintain their improved glucose metabolism (Nath, 1976).
Of 10 non-diabetic elderly subjects with impaired glucose
tolerance, 4 responded to 150 µg chromium/day with complete
normalization of oral glucose tolerance tests. The remaining 6
subjects were not affected by the supplements (Levine et al.,
1968). Significant effects of a GTF-chromium-containing yeast
preparation were reported by Doisy et al. (1976) in 6 out of 12
elderly subjects with impaired glucose tolerance
Table 21. Effect of GTF supplementation on glucose tolerance tests in
elderly subjects with impaired tolerancea,b
-------------------------------------------------------------------------
Mean plasma-glucose levels Cholesterol Triglyceride
(mg/litre) (mg/litre) (mg/litre)
0 h 1 h 2 h
-------------------------------------------------------------------------
Before GTF 1060 ± 40c 2010 ± 70 1780 ± 80 2450 ± 90 1210 ± 80
11 tests
After GTF 990 ± 40 1620 ± 110 1320 ± 50 2050 ± 100 1120 ± 120
9 tests
Signifi- NS < 0.01 < 0.001 < 0.01 NS
canced
-------------------------------------------------------------------------
Mean serum-insulin levels
(microunits/ml)
0 h 1 h 2 h
Before GTF 24 ± 6 78 ± 17 118 ± 17
after GTF 26 ± 12 70 ± 8 83 ± 8
-------------------------------------------------------------------------
a From: Doisy et al. (1976).
b Supplement: 4 g yeast per min/day (Yeastamin Powder 95, Staley Co.,
Chicago, Illinois, USA). GTT: 100 g oral load.
c Mean ± SEM.
d Using paired t test, difference is significant.
In addition to the improvement in glucose tolerance in the
presence of a reduced insulin response, plasma-cholesterol
concentrations were significantly reduced. Several other
individual cases are reported in the publication of Doisy et al.
(1976), all of whom showed a lower serum-insulin response to a
glucose tolerance test during chromium supplementation.
Liu & Morris (1978) performed a similar study on the effects of
a yeast-chromium supplementation in 27 women, 40 - 75 years old, of
whom 15 had a normal and 12 an abnormal glucose tolerance test. In
11 of the normal subjects (73%), the integrated insulin response
was significantly reduced from 436 to 335 µU/ml ( P < 0.025) during
supplementation, without a statistically significant change in
glucose tolerance test. Significant reductions in the total glucose
response (10 650 - 9820 mg/litre; P < 0.001) and in the total
insulin response to glucose were observed in the subjects with
impaired glucose tolerance (1288 - 831 µU/ml; P < 0.001). The
glucose tolerance improved in 7 out of 12 women, but all 12 showed
a reduction in plasma-insulin.
The effects of supplementation of the diet with 200 µg
chromium/day (as chloride) on the glucose tolerance of 76 normal
adult volunteers was investigated in a double-blind cross over
study, with each period lasting 3 months. All 18 subjects entering
the study with impaired glucose tolerance (serum-glucose equal to
or greater than 1 g/litre, 90 min after a glucose load), showed
significant improvement during chromium supplements, but not with a
placebo, with an average decline in the 90-min serum-glucose levels
of 180 mg/litre ( P < 0.01) (Anderson et al., 1983b).
In another placebo-controlled study, 24 elderly volunteers were
given a daily supplement of either chromium-rich brewer's yeast or
chromium-deficient Torula yeast (9 g/day, for 8 weeks). The
glucose tolerance of the subjects given brewer's yeast
significantly improved with a concomitant decrease in plasma-
insulin levels, and a significant decline in plasma-cholesterol as
well as total plasma-lipids. No significant changes were observed
in the control group given the chromium-poor Torula yeast
(Offenbacher & Pi-Sunyer, 1980).
Additional, preliminary studies suggest a specific effect of
brewer's yeast on individual lipoprotein cholesterol fractions.
Twenty normal human subjects responded to daily supplementation
with 18 g of brewer's yeast over a 8-week period with a significant
reduction in low-density lipoprotein-cholesterol ( P < 0.01) and
with a simultaneous increase of 14% in the high-density lipoprotein
( P < 0.01) (Nash et al., 1979).
Similar results were observed in a 6-week supplementation trial
involving 8 physician volunteers, each of whom received 7 g/day of
brewer's yeast. Low-density lipoprotein-cholesterol declined by
nearly 18% ( P < 0.01), whereas the high-density cholesterol
fraction increased by 17.6%, resulting in a significant
( P < 0.001) decrease in the LDL/HDL ratio of 28% (Riales, 1979).
These studies suggest that the primary effect of the
supplementation must have been an increase in the effectiveness of
the insulin, because glucose tolerance was maintained or improved
in spite of lower insulin responses. It is important to note that
while the reduction in insulin levels occurred in all cases, an
improvement in glucose tolerance occurred in only a fraction. This
suggests two possible interpretations of all the results discussed
here, including those showing improvement in glucose tolerance in
only some of the subjects or no improvement at all. Lack of a
response to chromium could suggest that the subjects were not
chromium deficient and that their impaired glucose metabolism was
the result of other causes. Second, they may have responded with a
reduction in circulating insulin levels, as did all the subjects
with impaired glucose tolerance in whom the insulin response was
measured. Unfortunately, these measurements were not performed in
the earlier studies.
8.1.2.2. Malnourished children
Chromium deficiency has been implicated as a factor
contributing to the impairment of glucose tolerance in children
with protein calorie malnutrition in 3 countries, Jordan, Nigeria,
and Turkey. Hopkins et al. (1968) measured intravenous glucose
tolerance in 10 malnourished infants from the mountainous area of
Jordan and 9 equally malnourished infants from the valley area.
They found a severely depressed glucose removal rate of 0.7%/min in
the first group and a normal rate in the second, 3.8%/min (Table
22). The protein intake of both groups prior to hospitalization
had been identical; it was supplied in the form of a milk powder
with a chromium content of 18 µg/litre, when reconstituted.
However, analysis of 10 water samples revealed 3 times as much
chromium in the samples from the valley as in those from the
mountainous area (0.5 versus 1.6 µg/litre). This suggested the
possibility of chromium being a major determinant in the glucose
metabolism of these children, and the effects of supplementation
were measured in 6 children from the hills, with a severely
impaired tolerance test. The morning after oral administration of
250 µg chromium, as CrCl3 x 6H2O, the glucose removal rate had
markedly improved to 2.9%/min from a pretreatment level of
0.6%/min. Similar results were obtained in Nigeria (Table 22);
these tests also included control children who did not receive
chromium.
The immediate effects of chromium on these children were
remarkable and different from the lag phase always present in the
studies on adults. Equally important, was the short duration of
the effect of one dose demonstrated in a Jordanian child observed
for a longer period of time. These observations suggest that a
marked depletion in chromium stores must have existed in the
children.
Table 22. Significance of the effect of chromium on the impaired glucose
tolerances of malnourished infantsa
---------------------------------------------------------------------------
Subjects Number Glucose Significance
of removal of
infants rate difference
(%/min)
---------------------------------------------------------------------------
Jordanian infants from hill area with
0.5 µg chromium/litre in drinking-water 10 0.7
Jordanian infants from valley with
1.6 µg chromium/litre in drinking-water 9 3.8 P < 0.001
Jordanian infants;
initial glucose tolerance test 6 0.6 P < 0.001
after chromium treatment 6 2.9
Nigerian infants;
initial glucose tolerance test 6 1.2
after chromium treatment 6 2.9 P < 0.05
Non-treated infants:
Initial glucose tolerance test 5 1.9 not signi-
Repeated glucose tolerance test 5 2.1 ficant
---------------------------------------------------------------------------
a From: Hopkins et al. (1968).
Similar results were reported from a study in the Istanbul area
of Turkey (Gürson & Saner, 1971). Fourteen malnourished children
with an impaired glucose removal rate of 1.71%/min were given 250
µg of chromium (CrCl3 x 6H2O), and a second test was performed
after 15 h. The removal rate increased to 3.91%/min ( P < 0.001).
This average increase was due to a pronounced increase in 9 of the
children; no change was observed in the remaining 5. No
improvement in glucose tolerance occurred in 5 control children
receiving identical hospital treatment, except for the
administration of the chromium. The children receiving the
chromium supplementation grew significantly more than the control
children during the following 30 days, with the greatest growth
rates found in the 9 children who had responded to administration
of chromium with an improvement in glucose tolerance.
The fact that chromium deficiency interacts with protein-
calorie malnutrition only in certain geographical areas was
confirmed by Carter et al. (1968), who gave chromium
supplementation (250 µg/day as CrCl3 x 6H2O) without effects on
glucose tolerance in 9 Egyptian children tested. Plasma-chromium
levels were in the normal range, and chromium levels in
representative foods were high, ranging from 200 to 390 µg/kg.
These findings suggest that the chromium status of these subjects
was normal, which would explain the lack of an effect.
8.1.2.3. Patients on total parenteral alimentation
A female patient, maintained on total parenteral alimentation
since the age of 35 years, developed an unexpected weight loss of
15% after 5 years combined with peripheral neuropathy, impaired
intravenous glucose tolerance, in spite of normal insulin response,
and a decreased respiratory quotient of 0.66. Insulin treatment
(45 U/day) was ineffective. A strongly negative chromium balance
and low plasma- and hair-chromium levels suggested that the daily
estimated chromium intake from the infusion fluids of 5.3 µg had
not met the patient's requirement and resulted in chromium
deficiency. The insulin infusions were stopped and replaced by the
daily infusion of 250 µg chromium (as CrCl3 x 6H2O) for 2 weeks.
The result was normalization of glucose tolerance and respiratory
quotient, disappearance of signs and symptoms of neuropathy, return
to the previous normal body weight, in spite of reduced caloric
intake, disappearance of the requirement for exogenous insulin, and
a positive chromium balance. The nitrogen balance, which had been
erratic and often negative, became consistently positive following
chromium supplementation. Reduction of the daily supplement to 20
µg chromium proved to be sufficient to maintain the patient in a
state of well-being (Jeejeebhoy et al., 1977).
This case is the best demonstrated example of chromium
deficiency in a human subject. In addition to confirming the well-
known signs of chromium deficiency reported earlier, such as
insulin resistance and its consequences, it was the first case to
demonstrate the occurrence of peripheral neuropathy and disturbance
of nitrogen balance as a result of deficiency. These disturbances
require further study. A second case of chromium deficiency as a
consequence of long-term total parenteral alimentation was reported
by Freund et al. (1979). Signs and symptoms were similar to those
of the first case, except that central encephalopathy was present
instead of peripheral neural disorder. Supplementation with 150 µg
of chromium/day resulted in normalization of the deficiency signs.
Chromium at that time was not routinely added to intravenous
solutions, therefore it is possible that more cases of deficiency
may exist in patients on long-term infusion treatment. As an
outcome of this study, an Expert Panel of the American Medical
Association (AMA, 1979) suggested that the stable adult patient on
total parenteral alimentation should receive 10 - 15 µg chromium
daily.
8.1.2.4. Epidemiological studies
Two epidemiological studies suggest a link between chromium
status and cardiovascular diseases (Schroeder et al., 1970; Punsar
et al., 1975). Lack of chromium is associated with impaired
glucose tolerance, elevation of circulating cholesterol, and aortic
plaques. All of these variables are recognized risk factors for
cardiovascular disease. It has also been suggested that the
elevated insulin levels, often present in chromium-deficient human
subjects (Liu & Morris, 1978) represent a substantial risk for the
development of cardiovascular disease (Strout, 1977).
Schroeder et al. (1970) have summarized their studies of
chromium concentrations in normal and diseased tissues,
particularly in aortas (Table 23). The total body burden of
chromium, excluding the lungs, was lower in the USA, where there is
a higher incidence of cardiovascular disease, compared with other
regions, 1.72 - 1.86 mg/kg ash weight in the USA compared with 7.7,
9.6, and 2.7 mg in Africa, the Far East, and the Near East,
respectively. The only significant difference in tissue-chromium
concentrations in deaths from heart disease compared with deaths
from accidental causes was in the aorta (Table 23). Concentrations
in aortas from Africa, the Far and Near East were generally higher
and did not show such a difference.
Table 23. Chromium in aortasa
----------------------------------------------------------
Location Chromium (mg/kg dry weight)
Accident P Heart disease
----------------------------------------------------------
Africa 0.072 (5)b NS 0.116 (2)
Far East 0.97 (8) NS 0.246 (5)
Middle East 0.36 (8) NS 0.216 (3)
USA (Nine cities) 0.26 (103) 0.005 0.052 (13)
USA (San Francisco) 0.228 (10) 0.005 0.048 (15)
----------------------------------------------------------
a From: Schroeder et al. (1970).
b The number in parenthesis denotes the number of cases
observed.
The second epidemiological study was part of a cohort study
conducted in Finland beginning in 1959 (Punsar et al., 1975). A
total of 327 drinking-water samples were analysed for 22
characteristics including pH, anions, bulk minerals, and several
trace elements including chromium. The study included 504 and 622
men in the Western and Eastern areas of Finland, respectively,
whose domicile had not changed since 1959.
The coronary heart disease mortality rate is much higher in the
east of Finland than in the west. As seen in Table 24, the
chromium levels in drinking-water were much lower in the east than
in the west, whereas the opposite was true for copper. The metal
concentrations in the drinking-water of those with heart disease
and others only differed slightly (Table 24), but, according to the
authors, the data suggest that low chromium levels may play a role
in the high heart disease death rate of eastern Finland.
Table 24. Concentrations of copper and chromium in the
drinking-water of men who died between 1969 and 1973, and
of those who were alive in 1973a
-------------------------------------------------------------
Alive
Deaths (Clinical status in 1969)
CHDb Other CHDb Other No
heart heart
disease disease
-------------------------------------------------------------
West N = 19 N = 23 N = 63 N = 31 N = 288
Copper X 22.15c 20.17 20.05 20.94 20.15
(µg/litre) SD 3.92 4.69 4.69 5.12 4.74
Chromium X 7.52 9.38 9.45 9.74 8.45
(µg/litre) SD 2.06 3.42 4.01 4.44 3.31
East N = 23 N = 26 N = 129 N = 44 N = 279
Copper X 41.59 41.24 42.67 39.50 36.32
(µg/litre) SD 29.91 30.43 33.65 28.74 28.66
Chromium X 2.20 2.09 2.04d 2.49 3.01
(µg/litre) SD 2.17 2.41 2.08 2.17 2.37
-------------------------------------------------------------
a From: Punsar et al. (1975).
b CHD = coronary heart disease.
c P < 0.05.
d P < 0.001.
Note: Levels of significance refer to differences observed
when the concentrations were compared with those in the
"no heart disease" category.
Newman et al. (1978) measured serum-chromium concentrations in
32 patients, 18 male and 14 female, aged 25 - 65 years, in whom
coronary artery cine-arteriography was performed for medical
reasons. Serum-chromium was significantly lower in the 15 subjects
with coronary artery disease than in the 17 subjects without
involvement (2.51 versus 6.09 µg/litre; P < 0.01), whereas serum-
cholesterol, weight index, and systolic or diastolic blood pressure
did not differ among the groups. Serum triglycerides were higher
in the group with coronary artery disease (1.84 versus 1.12
g/litre; P < 0.05). No subject with a serum-chromium
concentration of more than 6 µg/litre exhibited coronary artery
involve- ment, and by regression analysis, serum-chromium was
judged to explain 17% of the total variance of the disease.
These results were independently confirmed in a second study in
which plasma chromium concentrations of 23 healthy persons were
more than 8 times greater than those of 67 patients with coronary
artery disease, as confirmed by cine-angiography (Simonoff et al.,
1984).
8.1.3. Mode of Action
As in experimental animals, the best known action of chromium
is the potentiation of insulin. This is shown by the
supplementation studies of Doisy et al. (1976) and Liu & Morris
(1978) in which a reduction in insulin levels in response to
glucose was observed, together with an improvement in glucose
tolerance. Direct proof, for example from in vitro studies with
human biopsy tissue, has not yet been obtained. Furthermore, it is
not known whether the potentiation of insulin is responsible for
all the effects observed during chromium supplementation, including
the reduction of circulating cholesterol and the growth stimulation
observed in malnourished children and during parenteral nutrition.
In the healthy, chromium-sufficient human subject, chromium
levels in the bloodstream increases acutely following a glucose
load or an injection of insulin. Glinsmann et al. (1966) observed
acute increases in 5 young, healthy volunteers during a glucose
tolerance test, but not when the same subjects were given an
equivalent volume of water. Maturity-onset diabetic patients did
not exhibit this chromium response initially, but did show a rise
in plasma-chromium, after several weeks of chromium
supplementation. These observations were confirmed by Levine et
al. (1968), Hambidge (1971), Behne & Diel (1972), and Liu & Morris
(1978), who saw the acute chromium rise in some, but not all,
subjects. In the study of Liu & Morris (1978), which was the most
detailed, the relative chromium response (CrR) was measured by
neutron activation analysis in 15 women with normal glucose balance
and in 12 with impaired tolerance.
(1-h serum-chromium)
CrR = ------------------------ x 100
(fasting serum-chromium)
Initially, the normal group had a mild positive response (CrR =
107), compared with a negative response (CrR = 81) in the impaired
subjects. Following three months of supplementation with a
chromium-rich yeast extract, the CrR of the normal subjects
increased to 140, and that of the impaired group became positive
with CrR = 149. As discussed in section 8.1.2, these changes were
accompanied by a significant decrease in the insulin response to
glucose and an improvement in glucose tolerance. The results of
these studies are in apparent contrast with those of Davidson &
Burt (1973), who found a significant decline in plasma-chromium
levels following a glucose load in normal, non-pregnant women (mean
age 26 years), but a mild to moderate increase in all but 1 of 10
pregnant women (mean age 20 years). As the time of the acute rise
varied between women, statistical analysis at any one time did not
produce any significant results. Hambidge (1971) and Behne & Diel
(1972) demonstrated an acute plasma-chromium response following
injection of insulin; it can therefore be assumed that the effect
of glucose on the chromium response is associated with
hyperinsulinaemia. The acute "relative chromium response" is
believed to reflect the chromium status of human subjects. It does
not appear when stores from which the chromium increment must come
are depleted. Depleted stores can be replenished by
supplementation, with a resulting reappearance of the response
shown in the two previously discussed studies. As it has been
shown that chromium levels in urine increase following a glucose
load (Wolf et al. 1974; Gürson & Saner, 1978), it is likely that a
fraction or all of the chromium increment is subsequently excreted.
8.2. Acute Toxic Effects
In adults, the lethal oral dose of soluble chromates is
considered to be between 50 mg/kg body weight (RTECS, 1978) and 70
mg/kg body weight (Deichmann & Gerarde, 1969). The clinical
features of toxicity are vomiting, diarrhoea, haemorrhagic
diathesis, and blood loss into the gastrointestinal tract causing
cardiovascular shock. If the patient survives about 8 days, the
outstanding effects are liver necrosis (Brieger, 1920), tubular
necrosis of kidneys, and poisoning of blood-forming organs
(Langard, 1980). A 14-year-old boy, who had ingested about 1.5 g
of potassium dichromate, died 8 days later. Kaufmann et al. (1970)
also reported enlargement and oedema in the boy's brain.
8.3. Chronic Toxic Effects
The chronic effects of trivalent and hexavalent chromium have
been reviewed by Langard & Norseth (1979). The most important
features are changes in the skin and mucous membranes, and allergic
dermal and broncho-pulmonary effects. Important systemic effects
occur in the kidneys, liver, gastrointestinal tract, and
circulatory system.
Mancuso (1951) studied several biological variables in persons
occupationally exposed to chromate and related them to clinical
signs and symptoms. He found occasional leukocytosis or
leukopenia, monocytosis, eosinophilia, reduced haemoglobin
concentrations and prolonged bleeding times, but none of them were
consistent enough to serve as reliable diagnostic tests.
Mild normochronic anaemia was reported by Myslyaeva (1965) in
94 subjects exposed to chromium compounds with 68 of them showing
signs of chronic toxicity. Though the report of Baetjer et al.
(1959b) was based only on 3 cases, it indicated that chromate
attached preferentially to red blood cells. Koutras et al. (1964)
reported the inhibition of the red blood cell enzyme gluthathione
reductase by chromate concentrations of 5 - 25 mg/kg body weight.
However, the lowest effective dose is far in excess of levels
found, even in heavily exposed persons. Thus, all attempts made so
far have failed to identify a specific sensitive biochemical test
for the assessment of chronic chromium toxicity.
Long-term surveillance of pregnant women working at a plant
producing dichromates and those living in its immediate vicinity
(Shmitova, 1980) revealed increased chromium levels in the blood
and urine, compared with those in the control group. At 32 weeks
of pregnancy, the blood concentrations of chromium were,
respectively, 2.5 mg/litre in the women workers, 1.2 mg/litre in
the women living near the plant, and 0.19 mg/litre in the controls;
the levels in the urine were 1, 0.47, and 0.045 mg/litre,
respectively. Umbilical venous blood contained 1.8, 0.66, and 0.37
mg chromium/litre, respectively. The placental chromium contents
in the surveyed women were 162, 179, and 40 µg/kg, respectively,
and the breast-milk levels were 119, 20, and 31 µg/litre, which
means that chromium is capable of transplacental entry into breast
milk and into the fetal and infant organism. Women working at the
bichromate plant exhibited a high incidence of obstetric pathology
and geotoses. Chromium levels were estimated in fetal and
placental tissues (abortive material) derived on the 12th week of
pregnancy from women workers of the dichromate facility and those
not exposed to chromium. In the test group, chromium levels were
1140 µg/kg in the fetal tissues and 135 µg/kg in the placental
tissue, whereas, in the controls, they were 928 and 30 µg/kg,
respectively. No teratogenic effects have been revealed (Shmitova,
1980).
8.3.1. Effects on skin and mucous membranes
Several different types of effects on skin and mucous membranes
may result from exposure to chromium chemicals, and the main
features are:
(a) primary irritation: ulcers (corrosive reactions),
scarred and non-ulcerative contact dermatitis;
(b) allergic contact dermatitis: eczematous and
noneczematous.
A general survey of the nature and cause of contact dermatitis
is given by Fregert (1981).
8.3.1.1. Primary irritation of the skin and mucous membranes
(a) Skin
Skin irritation, manifested by a sharp hyperaemia as well as by
vesicular, papular, and rash pattern, may result from contact with
chromates. However, the major problems in the primary irritation
dermatoses are ulcers, often called chrome holes or chrome sores.
Ulceration is likely to occur among workers who have contact with
high concentrations of chromic acid, sodium or potassium dichromate
or chromate, or ammonium dichromate. It does not result from
contact with trivalent chromium compounds. An ulcer may develop if
the chromium compound, either a dust or a liquid, comes into
contact with any break in the skin such as an abrasion, scratch,
puncture, or a laceration. Favoured sites for ulcer development
are the nailroot areas, the creases over the knuckles, finger webs,
the backs of the hands, and the forearms (Bloomfield & Blum, 1928).
Primary irritation by chromium can be attributed to hexavalent
chromium, as the salts of trivalent chromium are not considered
capable of producing such effects (Fregert, 1981). However,
prolonged exposure to trivalent chromium salts has been reported to
cause skin lesions, less marked than those following exposure to
hexavalent chromium salts (Domaseva, 1971). Findings in 164
patients with eczema showed that skin tests on people who had
contact with trivalent chromium did not give positive results with
dichromate (Valer & Racz, 1971).
Ordinarily, a chrome sore, if not deep, persists for about 3
weeks after exposure is discontinued. There is no evidence that
the ulcers undergo malignant transformation. Furthermore, the
presence of chromium ulcers did not influence the development of
sensitization to chromates (Edmundson, 1951), evidenced by patch
testing with a 0.5% solution of K2Cr2O7.
(b) Nasal septal mucosa
Perforation of the nasal septum was one of the main chronic
effects in workers who had contact with chromates and chromic acid
(Bloomfield & Blum, 1928; Langard & Norseth, 1979). In a group of
100 persons constantly in contact with chromium compounds, 44 had
superficial ulceration of the anterior nasal septal mucosa.
Twenty-one people had profound ulcers and 5 had perforations of
various sizes in the cartilaginous part of the nasal septum.
Perforation of the nasal septum and cicatricial alterations were
observed in people with 3 or more years of service, whereas
ulcerous lesions arose in 2 years of work with chromium compounds.
An additional consequence of the effect on the nasal mucous
membranes is a loss of the senses of smell and taste (Seeber et
al., 1976). In workers with profound ulcerous lesions of the nasal
septal mucosa, strains of white staphylococcus with elevated
resistance to chromium are sometimes isolated (Cherevaty et al.,
1965).
Pokrovskaya et al. (1976) studied 561 workers engaged in the
open excavation of chromium ores (chromium content was 62% in the
form of a complex compound of magnesium chromite and other chromium
ores). Almost half of the workers had worked there for more than 5
years, and about 40% had been working there for 6 - 15 years.
Changes in the nasal septal mucosa, typical of the effect of
chromium compounds, were found in 8.2% of the workers. The longer
the service record, the greater the prevalence of these lesions.
After 5, 11, and 15 years of work, the prevalence was 2.3%, 8.3%,
and 11.2%, respectively.
The prevalence of nasal septum perforation in chromium plating
workers was very high (24%) in the early 1950s in China (Yang,
1956). With the introduction of control measures, the prevalence
dropped so that, in 1982, only 2 cases of nasal septum perforations
were found among 393 chromium-exposed workers (Peng, 1982).
One hundred and four subjects (85 male, 19 female) exposed to
chrome plating and 19 unexposed controls were studied by Lindberg &
Hedenstierna (1983) with regard to changes in the nasal septum.
Exposed subjects were employed at 13 different companies and
included all who were working on the day of study at the particular
company. The median exposure time was 4.5 years (range, 0.1 - 36
years). Forty-three subjects were exposed almost exclusively to
chromic acid and constituted both a "low exposure" group (8-h mean
below 2 µg/m3, N = 22) and a "high exposure" group (2 µg/m3 or
more, N = 21). Their median exposure time was 2.5 years (range,
0.2 - 23.6 years). The other 61 subjects were exposed to a mixture
of chromic acid (0.2 - 1.7 µg/m3) and other pollutants, such as
nitric, hydrochloric, and boric acids, as well as caustic soda,
nickel, and copper salts. The last group was included to disclose
any additive or synergistic effects of chromic acid and other
pollutants and was studied with regard to lung function only. The
breakdown of subjects into various groups is shown in Table 25.
Nasal septal ulceration and perforation were seen in 10 out of 14
subjects exposed to peak levels of 20 - 46 µg chromic acid/m3 or
more; no one in the control group showed signs of atrophy,
ulcerations, or perforations.
8.3.1.2. Allergic contact dermatoses
Chromium is a very important skin sensitizer. Sensitization
is reported to require about 6 - 9 months, but can occur in less
than 3 months (Pirilä & Kilpio, 1949). Genetic factors appear to
contribute to the appearance of chrome sensitivity within the
Jewish population (Wahba & Cohen, 1979).
Apart from the primary irritation and ulceration effects,
direct contact with small amounts of the chromates may cause
allergic dermatosis (dermatitis and eczema). As this allergic
reaction is cell-mediated, i.e., antibody formation is intra-
cellular and not humoral, delayed allergic reactions are rarely
observed.
Fregert et al. (1969) reported that the skin-patch test for
chromium was positive in 8 - 15% of all patients suffering from
eczema. The dermatitis caused by chromium is described as a
diffuse erythematous type with severe cases progressing to an
exudative stage. Persons sensitive to chromium react to patch and
intracutaneous tests with nonirritant concentrations of potassium
dichromate (K2Cr2O7). Opinion is divided about the role of the
oxidation state of chromium as a causal agent in allergic contact
dermatoses (Morris, 1958; Fregert & Rorsman, 1964; Kogan, 1971).
In a review, Polak et al. (1973) showed that hexavalent chromium,
which penetrates the skin by a direct effect of a hapten, is
reduced to the trivalent state, chiefly by sulfhydryl groups of
aminoacids. It then conjugates with proteins thus forming the full
antigen that initiates sensitization.
Guinea-pigs sensitized with either trivalent chromium chloride
or hexavalent potassium dichromate are capable of reacting in vivo
and in vitro to challenges with both chromium salts. This double
reactivity is also found after repeated stimulation with only one
of these chromium compounds. Since it is not possible to select
lymphocytes directed specifically against a chromium determinant of
a particular valence, it is concluded that, by sensitization with
chromium salts of different valences, a common determinant or
closely related determinants are formed. It is suggested that this
determinant is formed by chromium in the trivalent form
(Siegenthaler et al., 1983).
Two to three years after medical treatment, a follow-up study
on 555 patients with contact dermatitis was completed by means of
questionnaires (Fregert, 1975). The eczema had healed in one-
quarter of the patients, one-half had periodic symptoms, and one-
quarter had permanent symptoms. The prognosis for persons who
changed their work or stopped working was the same as that for
those who continued eczema-inducing work.
From 48 positively tested patients (0.5% potassium dichromate),
38 persons, i.e., 79%, still had a positive patch test after 4 - 7
years. In 72% of the cases, a history of occupational exposure to
chromates could be proved (Thormann et al., 1979).
A fairly rapid transformation of dermatitis into eczema is a
characteristic feature of chromium-induced skin lesions. Kogan
(1971) recognized the following important properties: (a) absence
of adaptation, which makes it necessary to discontinue chromium
handling until the slightest clinical manifestations have
disappeared; (b) a persistent chronic development of an eczematous
process; (c) a long-term latent period; and (d) absence of a direct
correlation between the incidence of sensibilization in the exposed
workers and the chromium concentration to which they have been
exposed.
Table 25. Series of exposed subjects and referencesa
--------------------------------------------------------------------------------------------------------
Exposed subjects
Mixed exposure to
Low exposure to High exposure to chromic acid
chromic acid chromic acid (< 2 µg/m3) and other
(< 2 µg Cr6+/m3) (> 2 µg Cr6+/m3) acids and metallic salts References
----------------- ------------------ ----------------------- -----------------
Nb Age (years)c N Age (years) N Age (years) N Age (years)
--------------------------------------------------------------------------------------------------
Men
Non-smokers 10 35.8 ± 16.7 6 33.4 ± 14.1 12 33.6 ± 12.2 52 34.2 ± 13.2
Smokers 6 30.8 ± 9.7 16 30.2 ± 11.8 36 42.8 ± 13.1 67 30.5 ± 8.9
Women
Non-smokers 3 30.7(17-46) 0 9 48.3 ± 13.0
Smokers 2 44.5(29-60) 1 51.2 4 31.0(19-43)
--------------------------------------------------------------------------------------------------
a From: Lindberg & Hedenstierna (1983).
b When N < 5, the range is given instead of SD.
c X ± SD.
Patients suffering from chromium-induced allergic skin
dermatoses had a tendency to develop cross-correlated hyper-
sensitivity to other metals, in particular, to cobalt and nickel
(Rostenberg & Perkins, 1951; Geiser et al., 1960; Clark & Kunitsch,
1972; Kogan et al., 1972).
Increased sensitivity to chromium is proved by skin reaction
tests. In a number of cases, additional immuno-haematological
bioassays are made to confirm the specificity of these tests. A
method for studying the basophil (mast-cells) degranulation in vivo,
at the site of positive compress skin tests, was suggested by Kogan
(1968). The method was used as a basis for proving the specificity
of cutaneous tests for chromium and other sensitising metals of the
chromium group (cobalt and nickel) (Kogan, 1968; Kogan et al.,
1972).
Photosensitivity is sometimes observed in patients with chronic
dermatitis. Wahlberg & Wennerstein (1977) investigated patients
allergic to chromium, with or without clinically observed light
sensitivity, by a standardized photopatch test procedure. A
significantly more intense reaction was observed in 48% of the
cases in the UV-irradiated sites (4/5 minimal erythema dose)
compared with the non-irradiated control sites.
No cases of cancer of the skin have been reported to result
from exposure to any form of chromium (Fregert, 1981).
8.3.2. Effects on the lung
Airborne chromium trioxide is rapidly absorbed in the broncho-
pulmonary tract causing corrosive reactions (Borghetti et al.,
1977). The absorption of chromium aerosols may depend on the
physical and chemical properties of the particles (Taylor et al.,
1965), as well as on the activity of alveolar macrophages, and the
lymphatic drainage (Sanders et al. 1971).
Both Jindrichova (1978) and Keskinen et al. (1980) reported
that welders using chromium-containing electrodes suffered from
bronchitis. Zober (1982) studied arc welders who had used filler
metals containing chromium and nickel for some years. Compared
with controls, the welders reported more previous ailments of the
respiratory system, mainly in the form of acute bronchitis. In
X-rays of welders, more roundish shadows were found, probably
indicative of benign accumulations of ferrous dust. The greater
incidence of significant findings was clearly apparent in welders
who smoked (probably a combined effect of tobacco smoke and welding
fumes). Reggiani et al. (1973) studied 101 electroplaters by means
of spirometric tests and found an obstructive disease pattern in
13% of them. After inhalation of acetylcholine, a bronchospastic
reaction occurred in 23% of the workers. In another spirometry
study (Bovet et al., 1977) on 44 chromium electroplaters, an
obstructive respiratory syndrome was found among the workers with a
high urinary-chromium content. The authors concluded that the
influence of tobacco smoking on the spirometric tests was much less
than the influence of chromium.
Studying lung function and generalized obstructive lung
diseases in electrofurnace workers in a ferrochromium and
ferrosilicon-producing plant, Langard (1980b) found a reduced
forced vital capacity (FVC) and an increased prevalence of
obstructive lung diseases. Unable to document a connection between
the presence of chromates and increased lung diseases, the author
suggested that the effects were due to high levels of total dust,
especially amorphous silica dust.
Lindberg & Hedenstierna (1983) studied lung function in more
than 100 subjects exposed to chrome plating and in 119 car
mechanics as controls (no car painters or welders). In the exposed
subjects, FVC and the forced expired volume in one second (FEV1)
were both reduced by 0.2 litre, and the forced mid-expiratory flow
was reduced by 0.4 litre/second. The authors concluded that an 8-h
mean exposure level of more than 2 µg/m3 might cause a transient
decrease in lung function.
Pneumoconiosis was diagnosed in Germany (Letterer et al., 1944)
and again in chromite miners in South Africa (Sluis-Cremer & du
Toit, 1968). However, American medical officers could not confirm
this feature when examining 897 workers in chromate-producing
plants in the USA (US PHS, 1953). It should be noted that chromium
dusts had a poor fibrogenic potency in experimental animal studies
(section 7.2.1.5). Davies (1974) X-rayed workers employed in the
manufacture of ferro-chrome and found results similar to miliary
lung tuberculosis. The workers were in the immediate area of the
furnace and were exposed to dust-particles, 90% of which were less
than 2 µm in diameter. The dust deposited on girders consisted of
95% SiO2 and 2.4% Cr2O3. The author did not find any symptoms or
signs of tuberculosis, but there was sufficient evidence to
indicate an inhalation hazard in the ferro-chrome industry, thus
underlining earlier observations of Princi et al. (1962).
8.3.2.1. Bronchial irritation and sensitization
Chromium irritates mucous membranes, as has been seen with
exposure to airborne chromium trioxide fume (Meyers, 1950) or mixed
dust (Card, 1935; Broch, 1949; Williams, 1969). The first symptom
was an irritating cough, 4 - 8 h after exposure. Chromium causes
sensitization that can result in asthmatic attacks. These can last
for 24 - 36 h, without treatment (Langard & Norseth, 1979). An
attack may recur on later exposure, even when this exposure is to a
much lower concentration (US NAS, 1974a).
A double-blind experimental exposure study on an asthmatic
patient, who had been exposed to chromium and nickel, showed that
chromium was the main cause of the asthma (Novey et al., 1983).
Keskinen et al. (1980) pretreated their patients, stainless steel
welders, with both disodium cromoglycate and betamethasone and
found an inhibition of this reaction. Placebo medication failed to
produce this inhibition.
8.3.3. Effects on the kidney
In the early part of this century, chromates and chromic acid
were occasionally used as therapeutic chemicals for some skin
lesions. Fatal cases of acute nephritis occurred with albumin,
hyaline, and granular casts and red cells appearing in the urine,
accompanied by oliguria. Autopsies revealed tubular necrosis, but
not glomerular damage (Kaufman et al., 1970). Acute toxic
gastroenteritis developed, followed by arterial hypotonia, in
suicide cases a few hours after swallowing 1.5 - 10 g potassium
dichromate. Within 2 - 4 days, all patients developed signs of
acute renal insufficiency with oliguria, anuria, and hyperhydration
and also evidence of acute toxic hepatitis (Luzhnikov et al.,
1976). Kidney damage has also occurred in industry, as a result of
accidental exposure of the skin to large amounts of chromate,
especially when associated with extensive skin damage (Luzhnikov et
al., 1976).
The mortality from kidney diseases among workers in the
chromium industry does not appear to have increased. In one study,
no deaths from nephritis and uraemia were found in 4 chromate-
producing plants; in only 1 plant was the mortality rate above that
for the controls (Machle & Gregorius, 1948). Hayes et al. (1979)
reported that 4 deaths from nephritis and nephrosis had occurred
between 1945 and 1977 in workers in a chromate-producing plant,
compared with 4.95 cases in the control population. Satoh et al.
(1981) found one case of nephritis and nephrosis in a group of
chromate workers compared with an expected number of 2.18.
Some authors (Franchini et al., 1975; Borghetti et al., 1977;
Gylseth et al., 1977; Tola et al., 1977) have concluded that
urinary excretion and renal clearance of diffusible chromium can be
used as biological indicators for evaluating the extent of exposure
to airborne chromium and the body burden. However, a dose-effect
relationship could not be established between duration of exposure
and either the level of chromium excretion (Tandon et al., 1977) or
early nephrotoxic indicators (Mutti et al., 1979). A dose-
response relationship for hexavalent chromium effects on the kidney
was described by Mutti et al. (1979), measuring beta-glucuronidase,
protein, and lysozyme in the urine. Abnormal patterns were not
observed in 39 stainless-steel welders during 4.5 ± 3.2 years of
exposure. In another group of workers employed in welding armoured
steel with special electrodes for 1 ± 0.4 years, 22% showed an
increase in beta-glucoronidase and 10% in protein in the urine. In a
study on 24 workers in the chromium-plating industry who were
exposed for 8.9 ± 7.3 years, the authors found increased urinary
levels of beta-glucoronidase in 37% of the workers, an increase in
protein in 17%, and an elevated level of lysozyme in 4%. Urinary
beta2-microglobulin was also studied by Lindberg & Vesterberg (1983b)
in chromeplaters (24 males, mean age 36 years, working in 4
different factories). The exposure was measured using personal air
samplers. The 8-h mean value ranged between 2 and 20 µg hexavalent
chromium/m3 and averaged 6 µg/m3. Most of the workers showed
symptoms of airway irritation, 2 of them had ulcerated nasal septum
and another 2, complete perforation. Significantly higher urine
beta2-microglobulin levels (0.23 mg/litre; range, 0.04 - 1.24 mg/litre;
SD, 0.27) were found in this group of workers in comparison with a
reference group of workers of a comparable age (mean age, 38 years;
mean urinary beta2-microglobulin level, 0.15 mg/litre; range, 0.06 -
0.19 mg/litre; SD, 0.08). A relationship was observed (Table 26)
between the range of exposure and prevalence of urinary beta2-micro-
globulin levels exceeding 0.3 mg/litre. The 2 workers with
perforated nasal septum were in the highest exposure group. A
difference in urinary beta2-microglobulin levels was not found between
a group of workers employed several years earlier in an old
chromeplating plant (mean, 0.25 mg/litre) and a group of workers of
comparable age (mean, 0.29 mg/litre), but it should be noted that
the mean age of both these groups exceeded 52 years.
Table 26. beta2-microglobulin U-beta2 levels in urine of workers
related to present exposure levelsa
-------------------------------------------------------------------------
Plant Exposure Number Age Persons with an "elevated" U-beta2
No. range of (mean) (U-beta2 > 0.30 mg/litre) range
(µg/m3) workers concentration (mg/litre)
No. Ages
-------------------------------------------------------------------------
1 11 - 20 5 39 3 21, 37, 58 0.23 - 1.30
2 - 3 14 - 8 13 37 2 20, 21 0.04 - 0.44
4 2 - 3 6 6 - - 0.06 - 0.18
-------------------------------------------------------------------------
a From: Lindberg & Vesterberg (1983b).
8.3.4. Effects on the liver
Statistics on diseases of the liver are seldom published
separately; they are usually included in the group of diseases of
the digestive system. The chromate industry was reported to have
the same rate in this group of diseases as many other industries
(US PHS, 1953). The Federal Security Agency in the USA
investigated 14 chromate workers reported to have "enlarged livers"
but found that these cases were not related to the time spent in
the industry. Acute hepatitis with jaundice was described in one
worker employed in a chromium-plating plant.
In an epidemiological study, Satoh et al. (1981) reported a low
rate of cirrhosis of the liver in chromium workers and liver
function test results that did not differ significantly from those
of the controls.
However, following ingestion of high doses of potassium
dichromate, liver necrosis (Brieger, 1920) and congested liver
(Kaufman et al., 1970) with loss of its architecture have been
described. There are also case studies of liver function and
histology in electroplaters (Pascale et al., 1952) and chromate
chemical plant workers (Etmanova, 1965), but the results are
difficult to interpret.
8.3.5. Effects on the gastrointestinal tract
The following symptoms and signs were reported in workers
engaged in the production of chromium salts (Sterekhova et al.,
1978): hyperchlorhydria, elevated pepsin and pepsinogen level,
oedema, hyperaemia and erosion of the mucosa, polyposis,
dyskinesia, and gastritis. However, Satoh et al. (1981) reported
that the incidence of peptic ulcer in chromate workers was below
the expected rate, and another study showed that the mortality rate
for diseases of the digestive system was lower in chromate workers
than in the control population (Hayes et al., 1979).
The gastric juice has been shown to play a role in the
detoxification of ingested hexavalent chromium by reducing it to
trivalent chromium, which is poorly absorbed and eliminated with
the faeces (Donaldson & Barreras, 1966; DeFlora & Boido, 1980).
8.3.6. Effects on the circulatory system
A clinical study was performed by Kleiner et al. (1970) on
myocardial function in more than 200 workers in the potassium
chromate industry, suffering from pulmonary or gastrointestinal
diseases, attributed by the authors to chromium poisoning. A
control group of 70 healthy individuals was included. Pathological
changes in the ECG and in other tests of heart function indicated
disturbance of right heart function, which the authors considered
secondary to the pulmonary pathology. The full paper was not
available and, consequently, the data could not be evaluated in
detail. It should be noted that no unexpected findings in
cardiovascular mortality were cited in the epidemiological studies
described in section 7.3.9.
8.3.7. Teratogenicity
The only human data available concerning this topic were
reported in an abstract (Morton & Elwood, 1974). The authors did
not find any correlation between frequency of malformations in the
central nervous system and the chromium content in water samples
collected in South Wales. Furthermore, Suzuki et al. (1979) did
not find any indication of accumulation of chromium in fetal
tissues.
8.3.8. Mutagenicity and other short-term tests
Few reports exist dealing with chromium-induced mutagenicity in
human subjects, and only hexavalent chromium causes any effects.
Bigaliev et al. (1978) found a 3 - 8% increase in chromosomal
aberrations of peripheral blood leukocytes in workers handling
chromium-compounds, compared with 2% in unexposed controls.
When classified according to the Papanicolaou system,
cytological samples of the sputum of 116 workers in the chromate-
production industry did not show any stages of tumour development
(Maltoni, 1976). However, intermediate stage lesions found in 30
workers were described as atypical adenomatous proliferation,
squamous-cell, and basal-cell dysplasia.
Studying cultured lymphocytes obtained from workers
occupationally exposed to CrO3, Sarto et al. (1982) found an
increased frequency of sister chromatid exchanges compared with
controls, which was correlated with urinary-chromium levels and
enhanced by smoking habits.
8.3.9. Carcinogenicity
8.3.9.1. Lung cancer
(a) Epidemiological studies in chromate-producing plants
The first recognition that cancer of the respiratory tract
might be related to chromium exposures resulted from the reporting
in 1932 of 2 cases of bronchogenic carcinoma that had occurred in
an old German chromate chemical plant (Lehmann, 1932). Gross &
Kölsch (1943) reported 10 deaths from lung cancer in small plants
producing the chromates of lead and zinc. By 1947, 52 such cases
had been reported from this industry and 10 cases from a chrome
pigment plant, also in the Federal Republic of Germany (Baetjer,
1950a).
The first epidemiological study was reported in the USA in
1948. The mortality data were based on the records of the group
life insurance policies of 6 chromate-producing plants in the USA
compared with similar data from a non-chromium industry or from a
life insurance company. The study reported that 21.8% of all
deaths between 1938 and 1947 in the chromate plants were due to
cancer of the respiratory tract compared with 1.4% in the control
group. The crude death rate for cancer of the bronchi and lungs
was 28 times that for the control groups (Machle & Gregorius,
1948). In one of these plants (Mancuso & Hueper, 1951), the cancer
death rate was 15 times that of the general population in the
county where the plant was located. The US PHS (1953) also studied
the respiratory cancer risk in these 6 plants and estimated a
relative risk of 28.9 using the average cancer mortality rates for
US males between 1940 and 1948 as a comparison.
In addition to these studies, where the cause of death was
taken from insurance or death certificate records, a case-control
study, using only cases diagnosed by autopsy or biopsy, was
conducted by Baetjer (1950b). Two hospitals near the largest
chromate plant in the USA were asked to supply the histories of all
cases diagnosed in 1924-46 as cancer of the lung bronchi, and to
confirm histopathologically. A group of non-cancer cases matched
individually by age, sex, and date of admission was selected as
controls. Of the 290 confirmed cancer cases, 3.26% had been
employed in the chromate plant, whereas none of the 900 control
cases was found to have had chromate exposure. The percentage of
chromate workers in the lung cancer series was 28.6 times the
percentage of chromate workers among the employed male population
of the city. The plant involved in this study was rebuilt in 1950,
following the discovery of the cancer problems. A new study of
this plant was made by Hayes et al. (1979) to determine whether the
risk of cancer had been eliminated. The investigation covered 438
deaths among 2101 males, who were initially employed between 1945
and 1974 for more than 90 days, and who died between 1945 and 1977.
The SMRa for malignant neoplasms of the trachea bronchi and lungs
was 202. Cancer deaths in the city in which the plant was located
were used to calculate the expected number of cases. No cases of
respiratory cancer occurred in the plant between 1960 and 1977
among workers employed only in the new plant. Because of the long
latent period, further follow-up is necessary.
Increased lung cancer rates among chromate workers in the USA
have been confirmed by Taylor (1966), Enterline (1974), and Mancuso
(1975), who also showed that the risks were especially high during
the early years of the study of the cohorts, e.g., before 1950.
Studies conducted in other countries have shown similar results.
In a 1956 study on workers in chromium-producing plants in the
United Kingdom, the rate of lung cancer mortality was 3.6 times the
rate for England and Wales (Bidstrup & Case, 1956). In a follow-up
study on 2715 men who had worked at the 3 chromate-producing
factories in the United Kingdom from 1948 to 1977, Alderson et al.
(1981) found 116 deaths from lung cancer (expected, 48). Since
modification of the one plant that is still in operation, the
relative risk of lung cancer has decreased from over 3 to about
1.8.
Two epidemiological studies have been conducted in the Japanese
chromate industry. In one plant, 5 lung cancer deaths occurred
between 1960 and 1973 in 136 workers who had been exposed for more
than 9 years; the SMR was 1510 (Watanabe & Fukuchi, 1975). In
another plant, the mortality rate for respiratory cancer was
determined for 896 males who worked in the plant from 1918 to 1975
and were followed to 1978; the SMR was 920 (expected deaths based
on 1975 age-cause-specific mortality rates for Japanese males)
(Satoh et al., 1981).
In the Federal Republic of Germany, a study was conducted in 2
chromate-producing plants (Korallus et al., 1982). Comparing the
SMR for lung cancer during the period 1948-79 for these 2 groups, a
clearly decreasing tendency was observed. In the first plant, for
1948-52, the SMR was 512 and, for 1978-79, it was 98; for the other
plant, the corresponding figures were 1905 and 150, respectively.
The newest data indicate a decreasing risk approaching the
frequency in unexposed people (Korallus & Loenhoff, 1981). This is
a result of occupational hygienic and technological measures,
especially of a change in manufacture namely the "on line"
--------------------------------------------------------------------
a The SMR (standardized mortality ratio) is the ratio of
observed to expected deaths times 100.
processing of the chrome ore, which has minimized the contents of
the carcinogenic hexavalent chromium compounds within the process
(Korallus et al., 1982).
Bittersohl (1972) described the results of a study on 30 000
employees of a large chemical unit for the period 1921-70 in the
German Democratic Republic. In particular, 588 malignancies in men
and 170 in women were evaluated for the period 1957-70. In 1971,
108 new malignancies in men and 29 in women came to light. In a
chromate factory, the rate of all cancers was far above average.
The factory manufactured catalysts through the reaction of chromic
acid and iron (III) oxide and nitric acid. The airborne
concentrations of chromium were not reported in detail, but it was
stated that short-term exposures above 400 µg/m3 occurred. The
incidence of malignant neoplasms in employees in the chromate
factory was 852 per 10 000 employees. In "unexposed" personnel,
the incidence of malignant neoplasms was 84 per 10 000 employees.
Approximately 86% of all those with malignant neoplasms were
smokers, and 78% of those without malignant neoplasms were also
smokers, indicating that smoking is not likely to be a confounding
factor.
In the USSR, a high incidence of cancer of the lungs was
reported for men engaged in the production of chrome salts. The
ratio of lung cancer in the plant to that in the control population
was 6.4 for ages 50 - 59 (748 observed, 116 expected) and 15.7 for
ages 60 - 69 (2657 observed, 170 expected) (Tyushnyakova et al.,
1974).
A survey was conducted in China in 1982 to investigate the
problem of lung cancer among chromate-producing workers. It
covered 7 cities and included 2184 male and 798 female workers who
had worked for at least one year in the industry. The preliminary
report revealed that 11 of the 101 deaths among male workers were
due to lung cancer, but none of the 13 deaths among female workers
was due to this cause.a
(b) Epidemiological studies - chrome pigment industry
The first epidemiological study, conducted in 1973, covered 3
small chrome pigment-manufacturing plants in the USA. The
percentage of lung cancer deaths was tentatively reported to be
about 3 times that in unexposed workers (Equitable Environmental
Health Inc., 1976).
Lung cancer mortality was studied among 1152 men working at 3
chromate pigment factories in the United Kingom from the 1930s or
1940s until 1981 (Davies, 1979, 1984). Among workers at factory A,
which produced both zinc and lead chromates, entrants to the
factory between 1932-45 with high and medium exposures had excess
---------------------------------------------------------------------------
a Report of an Investigation Team for Cancer in Chromate
Workers (1983).
risks of lung cancer (SMR = 223). A similar excess was seen in
1946-54 entrants, but the excess risk fell just short of
statistical significance. Highly significant excess risks of lung
cancer were seen among 1947-64 entrants that had high or medium
exposures at factory B, which also produced lead and zinc
chromates. Excesses were more severe in workers employed for more
than 10 years. In factory C, which produced only lead chromate, no
excess of lung cancer was found. However, the number of workers
and the power of the study were small.
Twenty-four male workers, who had been employed in a small
Norwegian company for more than 3 years between 1948 and 1972, were
identified and followed up by Langard & Norseth (1975), because
their principal exposure was to zinc chromate dust (measured
routinely with readings occasionally up to 0.5 mg hexavalent
chromium/m3). By December 1980, 6 cases of lung cancer had been
diagnosed, giving a relative risk of 44 compared with that of the
general population in the country. The authors stated that 5 out of
the 6 patients were smokers and only one had been exposed to
chromates other than zinc chromates (Langard & Vigander, 1983).
Sheffet et al. (1982) undertook a detailed mortality study on
workers employed in a pigment plant in Newark, where lead and zinc
chromates were used. Observed deaths from each cause among 1296
white and 650 non-white males employed between 1940 and 1969, were
compared with expected deaths, as computed from cause-, age-, and
time-specific standard death rates for the USA. A statistically-
significant relative risk of 1.6 for lung cancer among white
males, employed for 10 years or more, was found. A relative risk
of 1.9 was noted for individuals employed for at least 2 years, who
were at least moderately exposed to chromates.
An epidemiological study covering several centres in Europe was
conducted by Frentzel-Beyme (1983). This study was designed to
quantify the mortality from cancer and other diseases among workers
in European factories producing chromate pigments (3 German, 2
Dutch factories). Observed deaths in factories were compared with
expected deaths calculated on the basis of mortality figures for
the region in which a given factory was located. The overall
mortality did not deviate from the expected rates. Lung cancer
rates were always in excess of expected numbers, but only in one
cohort was the excess statistically significant. The pattern of
duration of exposure indicates that the lung-cancer risk was not
clearly correlated with length of employment.
A slight excess of lung cancer risk in chrome pigment (zinc
chromate) spray painters from 2 maintenance bases in the USA was
reported by Dalager et al. (1980). Among the 202 deaths
identified, 21 were due to respiratory cancer (11.4 expected;
comparison population: proportionate mortality, US males). No
statistically significant excess of lung cancer was found in a
group of automobile painters in which 226 deaths, including 22
cancer cases, were analysed using proportionate mortality for areas
in which the plants were located as a comparison (Chiazze et al.,
1980).
(c) Epidemiological studies - ferro-chrome industry
Langard et al. (1980) reported that 7 cases of bronchogenic
carcinoma occurred among 976 workers, who started work before
January 1, 1960. The expected number was 3.1 using national rates,
1.8 using local rates, and < 1 when using an international
reference rate for comparison. The difference was not significant
for the national and local rates, but was significant for the
international rate.
A study in Sweden did not reveal any significant excess of
respiratory cancer in ferro-chrome workers (Axelsson et al., 1980).
The incidence of cancer among 1932 workers employed for at least
one year between 1930 and 1975 was compared with the expected
number based on the National Cancer Registry data for the county in
which the plant was located. Five cases of respiratory cancer were
found against an expected 7.2 cases.
An excessive rate of lung cancer was reported for a ferro-
chrome plant in Russia (Pokrovskaya & Shabynina, 1973). The number
of deaths from cancer between 1955 and 1969 was higher than that in
the town in which the plant was located. The authors considered
that both hexavalent and trivalent chromium exposures were
responsible for the excess, since similar excessive rates were
found in a nearby ore-crushing plant, where only trivalent chromium
was present. The number of cases and the rates involved in these
studies were not given.
Hexavalent chromium is produced in some types of welding. In
section 7.2.1.2, it was mentioned that welding fume particles were
positive in the mammalian spot test, indicating a mutagenic and
possible carcinogenic potential. The incidence of lung cancer was
determined by Sjögren (1980), in a cohort of 234 welders, who had
welded stainless steel for more than 5 years between 1950 and 1965,
and were followed to 1977. Three welders died from lung cancer in
comparison with an expected number of 0.68 in the general
population ( P = 0.03).
Comparing lung cancer mortality in 2 parishes with ferro-alloy
industries to mortality in other parishes of similar size without
such industries from the same county, Axelson & Rylander (1980)
were unable to detect an increased incidence of death due to lung
cancer mortality. The concentration of chromium in the ambient air
of the most polluted areas ranged from 0.1 to 0.4 µg/m3. It should
be noted that several possible confounding factors were not under
control, e.g., migration, occupational exposure, and smoking
habits.
(d) Epidemiological studies - chromium-plating workers
Epidemiological studies on workers in the chromium-plating and
related industries were reviewed by Hayes (1982). The relevant
data from these studies are presented in Table 27. According to
Hayes, the epidemiological evidence is not sufficient to determine
the risk of cancer associated with industrial exposure to chromic
acid and, if an excess risk exists, it is probably lower than that
typically described for employment in the chromium-chemical
producing industry.
In a more recent paper, Franchini et al. (1983) conducted a
retrospective cohort study in 9 chromium-plating plants to examine
the mortality of workers employed for at least one year during the
period January 1951 to December 1981. The study group totalled 178
individuals; vital status ascertainment was 97% complete. The
total number of deaths was close to the expected figure (15
observed, 15.2 expected), whereas death from lung cancer exceeded
the expected number (3 observed, 0.7 expected; P = 0.03). The
increased mortality from lung cancer among chromium-platers seemed
to be related to exposure intensity.
8.3.9.2. Cancer in organs other than lungs
A few cancers of the upper respiratory tract and oral region,
but no excessive rates have been reported to occur in the chromate-
producing industry. The cancers have involved the buccal cavity,
pharynx, and oesophagus. Cancers of the nasal septum have not been
documented, with the exception of one case in Italy (Vigliani &
Zurlo, 1955).
In a joint Danish-Finnish-Swedish case-reference investigation,
initiated in 1977, the connection between nasal and sinonasal
cancer and various occupational exposures was studied. All new
cases of nasal and sinonasal cancer were collected from the
national cancer registers (Finland and Sweden) or from the hospitals
(Denmark). The results showed associations between nasal and
sinonasal cancer and exposure to chromium, welding, flame-cutting,
and soldering, hardwood or mixed wood dust, and softwood dust alone
(13.4) (Hernberg et al., 1983).
In 5 plants in the USA, during the early years of extremely
high exposures, the rate of cancer of the digestive tract varied
from 0 to 3.04/1000 compared with a rate of 0.59/1000 for the
controls (Machle & Gregorius, 1948). The mortality rates from
stomach cancer have been reported for chromate-producing plants in
Japan (SMR = 90) (Satoh et al., 1981) the USA (SMR = 40) (Hayes et
al., 1979). No significant differences were reported between the
incidence of stomach cancer in a Russian plant (Pokrovskaya &
Shabynina, 1973) and the incidence in the surrounding area.
Studying cancer mortality in a pigment plant using lead and
zinc chromates, Sheffet et al. (1982) found an increased (but not
statistically risk of stomach (SMR = 170) and pancreas (SMR = 200)
cancer among the total cohort (1946 employees).
Table 27. Summary of epidemiological studies on respiratory cancer in
workers employed in the chromium plating and related industriesa
------------------------------------------------------------------------------------------------
Study population Follow-up Respiratory cancer Comparison population
Number Estimated
relative risk
------------------------------------------------------------------------------------------------
Chromium plating
1056 workers employed Deaths in former and 24 1.8b Unexposed workers in
> 3 months in 54 plants current (followed 2 plants and in 2 non-
in the United Kingdom years) workers, plating industries
about 80% traced
About 5000 workers 49 1.4c Mortality analysis,
employed since 1945 in method not specified
1 plating factory in
the United Kingdom
889 workers exposed > 6 Reports from management 0 < 1b Tokyo mortality
months in Tokyo chromium of plating firms and rates
plating plants, 1970-76 follow-up of retired
workers; 19 total deaths
reported
Related industries
1292 deaths among US Union death benefit 62 1.1b Proportionate
metal polishers and claims mortality, US males
platers, union members,
1951-69
238 deaths among employees Pension, insurance and 39 2.1c Proportionate
for 10 years in one US benefit records mortality, US
plant for metal die
casting, finishing, and
electroplating, 1974-78.
------------------------------------------------------------------------------------------------
a Adapted from: Hayes (1982).
b Not statistically significant.
c P < 0.05, calculated using an assumption that the observed number is distributed as a
Poisson random variable.
8.3.9.3. Relationship between cancer risk and type of chromium
compound
As the exposures are often poorly defined, it is sometimes not
certain whether workers have been exposed to hexavalent chromium,
trivalent chromium, or a combination of the two. Some authors have
concluded that there is no increased risk of cancer mortality due
to trivalent chromium compounds (Korallus et al., 1974 a,b,c;
Axelsson et al., 1980; Langard et al., 1980; Norseth, 1980),
whereas others do not exclude that trivalent chromium compounds
have a carcinogenic capacity (Essing et al., 1971; Mancuso, 1975;
Zober 1979).
Summing up the data from case reports as well as
epidemiological studies in chromate-producing, chromate-pigment,
chromium-plating, and ferrochromium industries, the IARC Working
Group on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans (IARC, 1980) came to the conclusion that "there is
sufficient evidence of respiratory carcinogenicity in men
occupationally exposed during chromate production. Data on lung
cancer risk in other chromium-associated occupations and for cancer
at other sites are insufficient. The epidemiological data do not
allow an evaluation of the relative contributions to carcinogenic
risk of metallic chromium, trivalent chromium and hexavalent
chromium or of soluble versus insoluble chromium compounds".
9. EVALUATION OF HEALTH RISKS FOR MAN
Low levels of chromium are omnipresent in the environment.
Under normal conditions, human exposure to chromium does not
represent a toxicological risk, but it should be pointed out that
too low an intake of chromium may lead to deficiency. Airborne
concentrations of chromium, predominantly as trivalent chromium,
are usually below 0.1 µg/m3 and there are many places where the
concentration is below the detection limit. Concentrations in
river water are typically in the range of 1 - 10 µg/litre and do
not constitute a health threat. Drinking-water from municipal
water supplies does not contribute more than a few micrograms of
chromium to the daily human intake, but untreated water in certain
areas may be contaminated by runoff or effluents from industrial
sources and may contribute significant amounts of chromium. The
oceans contain less than 1 µg/litre. The daily human intake
through food varies considerably between regions. Typical values
range from 50 to 200 µg/day. They do not represent a toxicity
problem.
However, significant exposures exist in the occupational field.
In the past, chromium-ore mining generated chromium-containing
dusts, levels of which ranged up to 20 mg/m3 in different work-
places. Other exposures ranged up to 150 mg/m3 and consisted of
dust containing as much as 48% chromium (Cr2O3). In production
plants, hexavalent chromium can occur in the airborne state.
However, implementation of protective measures at ferrochromium
production sites reduced the airborne hexavalent chromium to levels
of 30 - 60 µg/m3.
The health effects of the two common oxidation states of
chromium are so fundamentally different that they must be
considered separately. In the form of trivalent compounds,
chromium is an essential nutrient and is relatively non-toxic for
man and other mammalian species. The hexavalent form is man-made
by oxidation of naturally-occurring trivalent chromium minerals and
is widely used. Compounds of hexavalent chromium penetrate
biological membranes easily and can thus interact with essential
constituents of the cells, including genetic material which they
can damage through oxidation and complexation with resulting
trivalent species. On the other hand, oxidation of trivalent
chromium has not been demonstrated in the living organism, and for
practical purposes, the reduction of hexavalent chromium to
trivalent chromium in lungs and other animal tissues is
irreversible.
9.1. Occupational Exposure
A number of effects can result from occupational exposure to
airborne chromium including irritative lesions of the skin and
upper respiratory tract, allergic reactions, and cancers of the
respiratory tract. The data on other effects, e.g., in the
gastrointestinal, cardiovascular, and urogenital systems are
insufficient for evaluation.
Epidemiological studies have shown that workers engaged in the
production of chromate salts and chromate pigments experience an
increased risk of developing bronchial carcinoma. No detailed data
on dose-response relationships are available from epidemiological
studies. Although a suspicion of increased lung cancer risks in
chromium plating workers has been raised, the available data are
inconclusive as are data for other industrial processes where
exposure to chromium occurs. There is insufficient evidence on the
role of chromium as a cause of cancer in any organ other than the
lung. Evidence from studies on laboratory animals shows that
hexavalent chromium compounds, especially those of low solubility,
can induce lung cancer.
In the lymphocytes of workers in chromium-plating factories,
the frequency of sister chromatid exchanges was higher in exposed
than in control groups.
Mutagenicity and related studies have convincingly shown that
hexavalent chromium is genetically active. Hexavalent chromium can
cross cellular membranes and is then reduced to trivalent chromium,
which can cause DNA cross-links and increase the infertility of DNA
implication. Trivalent chromium compounds have been shown to be
genetically inactive in most test systems, except in systems where
they can directly interact with DNA.
9.1.1. Effects other than cancer
9.1.1.1. Respiratory tract
It has been reported that the threshold for acute irritative
effects in the upper respiratory tract is 25 µg/m3 for the most
sensitive individuals. Long-term exposure to doses over 1 µg
chromic acid/m3 can cause nasal irritation, atrophy of the nasal
mucosa, and ulceration of perforation of the nasal septum.
Bronchial asthma was previously attributed to exposure to
chromium compounds, but scientific data are too scarce to draw
conclusions.
9.1.1.2. Skin
Skin rashes, ulcers, sores, and eczema have been reported among
occupationally exposed workers. Both trivalent and hexavalent
chromium compounds can give rise to sensitization of skin,
especially under certain environmental conditions, such as those
encountered in the cement industry, where the high incidence of
chromium-induced skin lesions can be attributed to the alkaline
exposure conditions.
Eczematous dermatitis, which manifests first as a diffuse
erythematous type, progresses in severe cases to an exudative
stage and is associated in 8 - 15% of patients with sensitivity to
chromium, as revealed by skin-patch tests. Although opinion is
divided on the oxidation state of chromium responsible for inducing
sensitization, there is evidence of hexavalent chromium penetrating
the skin as a hapten to be reduced to the trivalent state and
conjugated with proteins and transformed into the full antigen
that initiates the sensitization reaction, involving presumably
only a cell-mediated immune response. It should be noted that
patients suffering from chromium-induced skin allergy tend to
become hypersensitive to cobalt and nickel. Furthermore, 48% of
cases of skin allergy induced by chromium, with or without
clinically observed light sensitivity, showed a significantly more
intense reaction by a standardized photopatch test procedure, when
they were exposed to a 4/5 minimal erythema dose of irradiation.
9.1.1.3. Kidney
After high-dose, short-term oral ingestion of chromium, acute
nephritis and tubular necrosis were observed. A few
epidemiological studies on workers in chrome-plating industries
include data on diseases of the kidney, most of them without giving
exact exposure levels. A recent study related increased urinary-beta2
microglobulin levels to exposure ranges between 2 and 20 µg/m3.
The dose-response relationship observed in this study needs
confirmation on a larger number of exposed workers.
9.1.1.4 Other organs and systems
There are no conclusive data to evaluate the effects of
chromium compounds on the liver or gastrointestinal and circulatory
systems.
9.1.2. Teratogenicity
Both oxidation states, when injected at high levels
parenterally into animals, are teratogenic, with the hexavalent
form accumulating in the embryos to much higher concentrations than
the trivalent. The Task Group was not aware of any report
indicating teratogenicity in human populations.
9.2. General Population
Persons living in the vicinity of ferro-alloy plants, exposed
to an ambient air concentration of up to 1 µg/m3, did not show
increased lung cancer mortality.
The results of many studies suggest that exposure to chromium
through inhalation and skin contact can pose health problems for
the general population. Very little information is available on
the health effects of chromium ingested through untreated drinking-
water, though, in a single study, a correlation was observed
between frequency of malformation in the central nervous system and
the chromium content of water samples (Morton & Elwood, 1974). In
order to assess the nature of the magnitude of these problems,
there appears to be a need for more general population studies on
the effects of inhaled, absorbed, or ingested chromium on
respiratory, cardiovascular, and renal functions, and on the skin.
Studies on animals and man have established trivalent chromium
as an essential micronutrient that interacts with insulin and
enhances the physiological effects of the hormone. Supplementation
trials in a number of countries including Finland, Jordan, Nigeria,
Sweden, Turkey, and the USA have shown that segments of the
population could improve their glucose metabolism and, in some
instances, fat metabolism, by ingesting trivalent chromium
compounds. These observations suggest that some populations are at
risk of chromium deficiency. Children suffering from protein-
energy malnutrition may be at special risk.
As chromium concentrations in the body fluids of unexposed
persons are generally less than 1 µg/litre, analysis for chromium
requires the strictest quality control, including measures to
exclude sample contamination. Even with adequate analytical
methods, it is not possible, as yet, to diagnose chromium
deficiency in individuals by chemical or biochemical methods alone.
Therefore, a quantitative assessment of the prevalence of chromium
deficiency in human populations is not yet possible.
REFERENCES
ABRAHAM, A.S., SONNENBLICK, M., EINI, M., SHEMESH, O., & BATT,
A.P. (1980) The effect of chromium on established athero-
sclerotic plaques in rabbits. Am. J. clin. Nutr., 33:
2294-2298.
AITIO, A., JARVISALO, J., KULUNEN, M., TOSSAVAINEN, A., &
VAITTINEN, P. (1984) Urinary excretion of chromium as an
indicator of exposure to trivalent chromium sulfate in leather
tanning. Int. Arch. occup. environ. Health, 54: 241-249.
ALDERSON, M.R., RATTAN, N.S., & BIDSTRUP, L. (1981) Health
of workmen in the chromate-producing industry in Britain. Br.
J. ind. Med., 38: 117-124.
ALEKSANDROV, A.I. & KAINOV, V.I. (1975) [Crocoite from the
Pervomaisk-Zverevo deposit at the Central Urals.] Tr. Sverdl.
Gorn. Inst., 106: 152-155 (in Russian).
AMA (1979) Guidelines for essential trace element
preparations for parenteral use. J. Am. Med. Assoc., 241:
2051-2054.
AMERICAN PUBLIC HEALTH ASSOCIATION, AMERICAN WATER WORKS
ASSOCIATION, AND WATER POLLUTION CONTROL FEDERATION (1971)
117A. Hexavalent chromium. In: Standard methods for the
examination of water and wastewater, 13th ed., Washington DC,
American Public Health Association, pp. 156-157.
AMES, B.N., DURSTON, E., YAMASAKI, E., & LEE, F.D. (1973)
Carcinogens as mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci. (USA), 70: 2281-2285.
ANDERSON, F.E. (1960) Cement and oil dermatitis. The part
played by chrome sensitivity. Br. J. Derm., 72: 108-117.
ANDERSON, N. (1982) The environmental impact of chromium.
Final draft March 1982, Maine, Department of Environmental
Protection, Bureau of Air Quality Control, 126 pp.
ANDERSON, R.A. & POLANSKY, M.M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Tr. El. Res., 3: 1-5.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., & REAMER, D.C. (1982a) Effect of exercise
(running) on serum glucose, insulin, glucagon, and chromium
excretion. Diabetes, 31: 212-216.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., VEILLON, C., & GLINSMANN, W. (1982b)
Urinary chromium excretion of human subjects: effects of
chromium supplementation and glucose loading. Am. J. clin.
Nutr., 36: 1184-1193.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., PATTERSON, K.Y.,
VEILLON, C., & GLINSMANN, W. (1983) Effects of chromium
supplementation on urinary Cr excretion of human subjects and
correlation of Cr excretion with selected clinical parameters.
J. Nutr., 113: 276-281.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
MERTZ, W., & GLINSMANN, W. (1983b) Chromium supplementation
of human subjects: effects on glucose, insulin, and lipid
variables. Metabolism, 32: 894-899.
ANDERSSON, A. & NILSSON, K.O. (1972) Enrichment of trace
elements from sewage sludge fertilizer in soils and plants.
Ambio, 1: 176-179.
ANGERER, J., SCHALLER, K.-H., & HEINRICH, R. (1981)
[Commercially-available blood and urine samples for quality
control in toxicology.] Arbeitsmed. Sozialmed. Praeventivmed.,
16: 125-127 (in German).
ANIMAL RESEARCH DIVISION OF THE NEW ZEALAND DEPARTMENT OF
AGRICULTURE (1954) Annual report 1953-54, Wellington, R.E.
Owen Government Printer, 40 pp.
ANKE, M., DIETTRICH, M., WICKE, G., PFLUG, D., & SCHUELER, D.
(1971) Absorption, excretion, and distribution of 51chromium
given by mouth to lactating ruminants. Arch. Tierern., 21:
599-607.
AXELSSON, G. & RYLANDER, R. (1980) Environmental chromium
dust and lung cancer mortality. Environ. Res., 23: 469-476.
AXELSSON, G., RYLANDER, R., & SCHMIDT, A. (1980) Mortality
and incidence of tumours among ferrochromium workers. Br. J.
ind. Med., 37: 121-127.
BAER, H. (1920) [Dermatitis resulting from the use of a
beard trainer made partly of artificial leather.] Münch. med.
Wschr., 67: 874 (in German).
BAETJER, A.M. (1950a) Pulmonary carcinoma in chromate
workers. I. A review of the literature and report of cases.
Arch. ind. Hyg. occup. Med., 2: 487-504.
BAETJER, A.M. (1950b) Pulmonary carcinoma in chromate
workers. II. Incidence on basis of hospital records. Arch.
ind. Hyg. occup. Med., 2: 505-516.
BAETJER, A.M., LOWNEY, J.F., STEFFEE, H., & BUDACZ, V.
(1959a) Effect of chromium on incidence of lung tumours in
mice and rats. Am. Med. Assoc. Arch. Ind. Health, 20: 124-135.
BAETJER, A.M., DAMRON, C., & BUDACZ, V. (1959b) The
distribution and retention of chromium in man and animals. Am.
Med. Assoc. Arch. Ind. Health, 20: 136-150.
BAKER, R.S.V. (1984) Evaluation of metals in in vitro
assays: interpretation of data and possible mechanisms of
action. Toxicol. environ. Chem., 7: 191-212.
BEHNE, D. & BRATTER, P. (1979) [Problems of trace element
analysis in medicine.] In: Gladtke, E., Heimann, G., & Eckert,
I., ed. [Trace elements], Stuttgart, Thieme, pp. 42-52 (in
German).
BEHNE, D. & DIEHL, F. (1972) Relations between carbohydrate
and trace element metabolism investigated by neutron
activation analysis. In: Nuclear Activation Techniques in the
Life Sciences. Proceedings of a Symposium, Bled, Yugoslavia,
Vienna, International Atomic Energy Agency, pp. 407-414.
BEHNE, D., BRATTER, P., GESSNER, H., HUBE, G., MERTZ, W., &
ROSICK, U. (1976) Problems in determination of chromium in
biological materials: comparison of flameless atomic
absorption spectrometry and neutron activation analysis. Z.
anal. Chem., 278: 269-272.
BEHRBOHM, P. (1957) [Allergic contact dermatitis caused by
anticorrosives containing chromium.] Berufsdermatosen, 5:
271-283 (in German).
BENJANUVATRA, N.K. & BENNION, M. (1975) Hair chromium
concentration of Thai subjects with and without diabetes
mellitus. Nutr. Rep. Int., 12: 325-330.
BERGMANN, H. & HARDT, K. (1979) Analysis of dissolved Cr(VI)
in water by APDC-MIBK extraction and atomic absorption
spectrometry. Z. anal. Chem., 297: 381-383.
BERING, F. & ZITZKE, E. (1935) [Occupational skin diseases,]
Leipzig, Voss (in German).
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric., 23: 93-100.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BERTRAND, D. & DEWOLF, A. (1968) Requirement for the trace
element chromium in the growth of potatoes. C R Ser. D., 266:
1494-1495.
BEYERMANN, K. (1962) The analytical behaviour of very small
amounts of chromium. Part I. Z. anal. Chem., 190: 4-33. Part
II. Z. anal. Chem., 191: 345-369.
BEYERSMANN, D., KOSTER, A., BUTTNER, B., & FLESSEL, P.
(1984) Model reactions of chromium compounds with mammalian
and bacterial cells. Toxicol. environ. Chem., 8: 279-286.
BIANCHI, V., DAL TOSO, R., DEBETTO, P., LEVIS, A.G., LUCIANI,
S., MAJONE, F., & TAMINO, G. (1980) Mechanisms of chromium
toxicity in mamalian cell cultures. Toxicology, 17: 219-224.
BIANCHI, V., LEVIS, A.G., & SAGGIORO, D. (1979) Differential
cytotoxic activity of potassium dichromate on nucleoside
uptake in BHK fibroblasts. Chem.-biol.Interact., 24: 137-151.
BIANCHI, V., CELOTTI, L., LANFRANCHI, G., MAJONE, F., MARIN,
G., MONTALDI, G., SPONZA, G., TAMINO, G., VENIER, P.,
ZANTEDESCHI, A., & LEVIS, A.G. (1983) Genetic effects of
chromium compounds. Mutat. Res., 117: 279-300.
BIANCHI, V., ZANTEDESCHI, A., MONTALDI, A., & MAJONE, F.
(1984) Trivalent chromium is neither cytotoxic nor mutagenic
in permeabilized hamster fibroblasts. Toxicol. Lett., 23:
51-59.
BIDSTRUP, P.L. & CASE, R.A.M. (1956) Carcinoma of the lung
in workmen in the bichromates-producing industry in Great
Britain. Br. J. ind. Med., 13: 260-264.
BIGALIEV, A.B., ELEMESOVA, M.S., TUREBAEV, M.N., & BIGALIEVA,
R.K. (1978) [Cytogenetic study of the mutagenic activity of
industrial substances.] Zdravookhr. Kaz., 8: 48-50 (in
Russian).
BITTERSOHL, G. (1972) [Health and the working environment-
Epidemiology of carcinomatosis in the chemical industry.] In:
Proceedings of the 5th Merseburger Symposium, 17-18 November
(in German).
BLACK, C.A. (1968) Soil-plant relationships, New York, John
Wiley and Sons, 792 pp.
BLOKIN, V.S. & TROP, F.S. (1977) [Dynamics of the morpho-
logical changes in animals subjected to inhalational dust
treatment using chromium oxide and trisubstituted chromium
phosphate.] In: Dubinin, N.P., ed. [Genetic effects of the
pollution of the environment,] Moscow, Nauka, pp. 173-176 (in
Russian).
BLOOMFIELD, J.J. & BLUM, W. (1928) Health hazards in
chromium plating. Public Health Rep., 43: 2330-2347.
BONATTI, S., MEINI, M., & ABBONDANDOLO, A. (1976) Genetic
effects of potassium dichromate. Mutat. Res., 38: 147-150.
BOND, R.G., STRAUB, C.P., & PROBER, R., ed. (1973) Handbook
of environmental control. III. Water supply and treatment,
Cleveland, Ohio, CRC Press, pp. 763-764.
BONNEVIE, P. (1939) [Etiology and pathogenesis of eczemas,]
Copenhagen, Busck, Leipzig, Barth (in German).
BORGHETTI, A., MUTTI, A., CAVATORTA, A., FALZOI, M., CIGALA,
F., & FRANCHINI, I. (1977) Indices rénaux d'exposition aiguë
et d'imprégnation chronique par le chrome. Med. Lav., 68(5):
355-363.
BORY, R. & LECOCQ, I. (1953) Eczéma par cuivre chromé. Arch.
Mal. prof., 14: 144.
BOSTROM, G. (1936) [Hypersensitivity test for chromium
allergy.] Am. Derm. Vener., 17: 631-632 (in German).
BOVET, P., LOB, M., & GRANDJEAN, M. (1977) Spirometric
alterations in workers in the chromium electroplating
industry. Int. Arch. occup. environ. Health, 40: 25-32.
BOWEN, H.J.M. (1966) Trace elements in biochemistry, New
York, London, Academic Press, 241 pp.
BRARD, D. (1935) Toxicologie du chrome, Paris, Herrmann &
Co., 80 pp.
BRIEGER, H. (1920) [Clinical aspects of acute chromate
poisoning.] Z. exp. Path. Ther., 21: 393-408 (in German).
BROCH, C. (1949) [Bronchial asthma caused by chromic oxide.]
Nord. Med., 41: 996-997 (in Norwegian).
BROWN, E., SKOUGSTAD, M.W., & FISHMAN, M.J. (1970) Methods
for collection and analysis of water samples for dissolved
minerals and gases. In: Techniques of water resources
investigation of the United States geological survey,
Washington DC, US Department Interior, 160 pp.
BROWN, H.G., HENSLEY, C.P., MCKINNEY, G.L., & ROBINSON, J.L.
(1973) Efficiency of heavy metals removal in municipal sewage
treatment plants. Environ. Lett., 5: 103-114.
BRUCKDORFER, K.R., KHAN, I.H., & YUDKIN, J. (1971) Chromium
and sucrose-induced hyperglyceridemia. Proc. Nutr. Soc., 30:
74A.
BRUNE, D., NORDBERG, G., & WESTER, P.O. (1980) Distribution
of 23 elements in the kidney, liver, and lungs of workers from
a smeltery and refinery in north Sweden exposed to a number of
elements and of a control group. Sci. total Environ., 16:
13-35.
BURCKHARD, W. (1962) [Occupational skin diseases.] In:
Jadassohn, W., ed. [Handbook of skin and venereal diseases,]
Berlin, Springer, Vol. II(1), pp. 368-474.
BURROWS, D., ed. (1983) Chromium: metabolism and toxicity,
Boca Raton, Florida, CRC Press, 172 pp.
BYERRUM, R.U. (1961) Some studies on the chronic toxicity of
cadmium and hexavalent chromium in drinking-water. Eng. Bull.
Purdue Univ. Serv., 106: 1-8.
CALNAN, C.D. & HARMAN, R.R.M. (1961) Studies in contact
dermatitis. XIII. Diesel coolant chromate dermatitis. Trans.
St. John's Hosp. Derm. Soc., 46: 13.
CALOW, P. & FLETCHER, C.R. (1972) A new radiotracer
technique involving 14C and 51Cr for estimating the
assimilation efficiencies of aquatic, primary consumers.
Oecologia (Berlin), 9: 155-170.
CAMPBELL, W.J. & MERTZ, W. (1963) Interaction of insulin and
chromium (III) on mitochondrial swelling. Am. J. Physiol.,
204: 1028-1030.
CANADA, NATIONAL HEALTH AND WELFARE (1980) Chromium
drinking-water criteria review. In: Guidelines for Canadian
drinking-water quality, 1978. Supporting documentation,
Ottawa, Canada, National Health and Welfare, pp. 255-268.
CANFIELD, W.K. & DOISY, R.J. (1976) Chromium and diabetes in
the aged. In: Hsu, J.M., Davis, R.L., & Neithammer, R.W., ed.
The biomedical role of trace elements in aging, St. Peters-
burg, Florida, Eckert College, pp. 119-128.
CARD, W.I. (1935) A case of asthma sensitivity to chromates.
Lancet, 2: 1348-1349.
CARTER, J.P., KATTAB, A., ABD-EL-HADI, K., DAVIS, J., EL
GHOLMY, A., & PATWARDHAN, V.N. (1968) Chromium (III) in
hypoglycemia and in impaired glucose utilization in
kwashiorkor. Am. J. clin. Nutr., 21: 195-202.
CARY, F.E., ALLWAY, W.H., & OLSON, O.E. (1977) Control of
chromium concentrations in food plants. I. Absorption and
translocations of chromium by plants. J. agric. food Chem.,
25: 300-304. II. Chemistry of chromium in soils and its
availability to plants. J. agric. food Chem., 25: 305-309.
CASEY, C.E. & HAMBIDGE, K.M. (1984) Chromium in human milk
from American mothers. Br. J. Nutr., 52: 73-77.
CASTO, B.C., PIECZYNSKI, W.J., NELSON, R.L., & DI PAOLO, J.
(1976) In vitro transformation and enhancement of viral
transformation with metals. Proc. Am. Assoc. Cancer Res., 17:
12-20.
CASTO, B.C., MEYERS, J., & DIPAOLO, J.A. (1979) Enhancement
of viral transformation for evaluation of the carcinogenic or
mutagenic potential of inorganic metal salts. Cancer Res., 39:
193-198.
CAWSE, P.A. (1974) A survey of atmospheric trace elements in
the UK, 1972-1973, Harwell, England, Atomic Energy Research
Establishment, 95 pp.
CHANEY, R.L. (1973) Crop and food chain effects of toxic
elements in sludges and effluents. In: Recycling Municipal
Sludges and Effluents on Land. Proceedings of a Joint
Committee, National Association of State Universities and Land
Grant Colleges, Washington DC, July 9-13, 1973, pp. 129-141.
CHEN, N.S.G., TSAI, A., & DYER, I.A. (1973) Effect of
chelating agents on chromium absorption in rats. J. Nutr.,
103: 1182-1186.
CHEN, K.Y., YOUNG, C.S., JAN, T.K., & ROHATGI, N. (1974)
Trace metals in waste water effluents. J. Water Pollut.
Control Fed., 46: 2663-2675.
CHEREMISINOFF, P.N. & HABIB, Y.H. (1972) Cadmium, chromium,
lead, mercury: a plenary account for water pollution. Part I.
Occurrence, toxicity, and detection. Water Sewage Works, 119:
73-86.
CHEREVATY, V.S., EREMEEVA, E.A., & SMAGULOV, S.K. (1965) [On
the nature of nasal microflora in the workers of a chromium
compound plant.] In: Proceedings of 4th Scientific Session of
Aktubinsk State Medical Institute, Alma-Ata, Kazakhstan,
pp. 337-342 (in Russian).
CHIAZZE, L., FERENCE, L.D., & WOLF, P.H. (1980) Mortality
among automobile assembly workers. I. Spray painters. J.
occup. Med., 22: 520-526.
CHRISTIAN, G.D., KNOBLOCK, E.G., PURDY, W.C., & MERTZ, W.
(1963) A polarographic study of chromium-insulin-
mitochondrial interaction. Biochem. Biophys. Acta, 66: 420-423.
CLARK, R. & KUNITSCH, G. (1972) [Statistical studies of
cross allergy in metal salts.] Berufsdermatosen, 20(5):
222-238 (in German).
COLLINS, R.J., FROMM, P.O., & COLLINGS, W.D. (1961) Chromium
excretion in the dog. Am. J. Physiol., 201(5): 795-798.
CONDE-SALAZAR, L., MARTO-CASTANO, A., & ARROYO VICENTE, M.
(1980) Determination of chrome in rubber gloves. Contact
Dermatit., 6: 237-238.
COTTON, F.A. & WILKINSON, G. (1966) Advanced inorganic
chemistry, New York, Interscience, 1136 pp.
CRESSER, M.S. & HARGITT, R. (1976) The determination of
chromium (III) and chromium (VI) by total anion exchange and
atomic absorption spectrometry. Anal. Chim. Acta, 81: 196-198.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
DALAGER, N.A., MASON, T.J., FRAUMENI, J.F., HOOVER, R., &
PAYNE, W.W. (1980) Cancer mortality among workers exposed to
zinc chromate paints. J. occup. Med., 22: 25-29.
DANA, P. (1971) Crocoite-PbCrO4 In: Hurlbut, C.S., ed.
Dana's manual of mineralogy, 18th ed., New York, J. Wiley and
Sons Inc., pp. 346-347.
DANIELSSON, B.R.G., HASSOUN, E., & DENCKER, L. (1982)
Embryotoxicity of chromium: distribution in pregnant mice and
effects on embryonic cells in vitro. Arch. Toxicol., 51:
233-245.
DAVIDSON, I.W.F. & BLACKWELL, W.L. (1968) Changes in
carbohydrate metabolism of squirrel monkeys with chromium
dietary supplementation. Proc. Soc. Exp. Biol. Med., 127:
66-70.
DAVIDSON, I.W.F. & BURT, R.L. (1973) Physiological changes
in plasma chromium of normal and pregnant women: effect of
glucose load. Am. J. Obstet. Gynecol., 116: 601-608.
DAVIES, J.C.A. (1974) Inhalation hazards in the manufacture
of silicon alloys. Cent. Afr. J. Med., 20: 140-143.
DAVIES, J.M.G. (1972) The fibrogenic effects of mineral
dusts injected into the pleural cavity of mice. Br. J. exp.
Pathol., 53: 190-201.
DAVIES, J.M.G. (1979) Lung cancer mortality of workers in
chromate pigment manufacture: an epidemiological survey. J.
Oil. Col. Chem. Assoc., 62: 157-163.
DAVIES. J.M.G. (1984) Lung cancer mortality among workers
making lead chromate and zinc chromate pigments at three
English factories. Br. J. Ind. Med., 41: 158-169.
DAVIS, M.H. & GROSSMANN, V.B. (1971) A sensitive method for
determination of chromium in biological materials. Anal.
Biochem., 44: 339-344.
DEFLORA, S. (1978) Metabolic deactivation of mutagens in
the Salmonella microsome test. Nature (Lond.), 271: 455-456.
DEFLORA, S. (1981) Study of 106 organic and inorganic
compounds in the Salmonella microsome test. Carcinogenesis, 2:
283-298.
DEFLORA, S. & BOIDO, V. (1980) Effect of human gastric
juice on the mutagenicity of chemicals. Mutat. Res., 77:
307-315.
DEFLORA, S., COPPOLA, R., CAMOIRANO, A., BATTAGLIA, M.A., &
BENNICELLI, C. (1980) Mutagenicity of chromyl chloride and
its vapours. Carcinogenesis, 1(7): 583-587.
DEFLORA, S., BIANCHI, V., & LEVIS, A.G. (1984) Distinctive
mechanisms for interaction of hexavalent and trivalent
chromium with DNA? Toxicol. environ. Chem., 8: 287-294.
DE GROOT, A.J. & ALLERSMA, E. (1973) Field observations on
the transport of heavy metals in sediments. In: Proceedings of
the Conference on Heavy Metals in the Aquatic Environment,
Nashville, Tennessee, December, Oxford, Pergamon Press,
pp. 1-16.
DEICHMANN, W.B. & GERARDE, H.W. (1969) Toxicology of drugs
and chemicals, 4th ed., New York, London, Academic Press,
167 pp.
DI PAOLO, J.A. & CASTO, B.C. (1979) Quantitative studies of
in vitro morphological transformation of Syrian hamster cells
by inorganic metal salts. Cancer Res., 32: 1008-1013.
DJAHANSHIRI, H. (1976) [Nutritional physiology problems
concerning the trace element chromiun in the rat. V. Effects
on overall metabolic performande.] Z. Tierphysiol. Tierernähr.
Futtermittelkd., 37: 298-315 (in German).
DOBROLYUBSKII, O.K. (1959) [Changes in the biochemical
process in grapes under the influence of a microfertilizer
which contains chromium.] Biochemistry (USSR), 24: 577-582 (in
Russian).
DOISY, R.J., STREETEN, D.H.P., LEVINE, R.A., & CHODOS, R.B.
(1968) Effects and metabolism of chromium in normals, elderly
subjects, and diabetics. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
Univerisity of Missouri, Vol. 2, 75 pp.
DOISY, R.J., STREETEN D.H.P., SOUMA, M.L., KALAFER, M.E.,
REKANT, S.L., & DALAKOS, T.G. (1971) Metabolism of
51chromium in human subjects. In: Mertz, W. & Cornatzer, W.E.,
ed. Newer trace elements in nutrition, New York, Marcel
Dekker, pp. 155-169.
DOISY, R.J., STREETEN, D.H.P., FREIBERG, J.M., & SCHNEIDER,
A.J. (1976) Chromium metabolism in man and biochemical
effects. In: Prasad, A.S., ed. Trace elements in human health
and disease, New York, London, Academic Press, Vol. 2, pp.
79-104.
DOMASEVA, T.V. (1971) [On the effect produced by chromium
compounds on the skin.] In: [Occupational skin diseases,]
Leningrad, Leningrad Institute of Sanitation and Hygiene,
Vol. 93, pp. 168-173 (in Russian).
DONALDSON, R.M., Jr & BARRERAS, R.F. (1966) Intestinal
absorption of trace quantities of chromium. J. lab. clin.
Med., 68: 484-493.
DOUGLAS, G.R., BELL, R.D.K., GRANT, C.E., WYTSMA, J.M., &
BORA, K.C. (1980) Effect of lead chromate on chromosome
aberration, sister-chromatid exchange, and DNA damage in
mammalian cells in vitro. Mutat. Res., 77: 157-163.
DURFOR, C.N. & BECKER, E. (1964) Public water supplies of
the 100 largest cities in the United States, 1962, Washington
DC, US Geological Survey, 364 pp (Water Supply Paper No. 1812).
DURUM, W.H. & HAFFTY, J. (1963) Implications of the
minor-element content of some major streams in the world.
Geochim. Cosmochim Acta, 27: 1-11.
EATON, D.L., STACEY, N.H., WONG, K.L., & KLAASSEN, C.D.
(1980) Dose-response effects of various metal ions on rat
liver metallothionein, glutathione, heme oxygenase, and
cytochrome P-450. Toxicol. appl. Pharmacol., 55: 393-402.
EDMUNDSON, W.F. (1951) Chrome ulcers of the skin and nasal
septum and their relation to patch testing. J. Invest. Derm.,
17: 17-19.
EIFAC (1983) Water quality criteria for european freshwater
fish. Report on chromium and freshwater fish, European Inland
Fisheries Advisory Commission, Working Party on Water Quality
Criteria for European Freshwater Fish, 31 pp (EIFAC Technical
Paper No. 43).
ELIAS, Z., SCHNEIDER, O., AUBRY, F., DANIERE, M.C., & POIROT,
O. (1983) Sister chromatid exchanges in chinese hamster V79
cells treated with the trivalent chromium compounds chromic
chloride and chromic oxide. Carcinogenesis, 3: 605-611.
ENTERLINE, P.E. (1974) Respiratory cancer among chromate
workers. J. occup. Med., 16: 523-526.
ENVIRONMENTAL INSTRUMENTATION GROUP (1973) Instrumentation
for environmental monitoring: biomedical. IV, Berkeley,
California, Lawrence Berkeley Laboratory.
EQUITABLE ENVIRONMENTAL HEALTH, INC. (1976) An epidemiologic
study of workers in lead chromate plants: Final report
(prepared for the Dry Color Manufacturer's Association,
Berkeley California).
ESSING, H.G., SZADKOWSKI, D., & VALENTIN, H. (1971)
[Significance of the valence gradations of chromium compounds
in occupational medicine examinations.] Med. Sachverständige,
67: 35-39 (in German).
ETMANOVA, Z.J. (1965) [On the functional state of the liver
in workers of Aktubinsk chromium compound plant.] Gig. Tr.
prof. Zabol., 10: 55-56 (in Russian).
EVAN, A.P. & DAIL, W.G. (1974) The effect of sodium chromate
on the proximal tubules of the rat kidney. Lab. Invest.,
30(6): 704-715.
FANG, Y. (1983) [The normal value of chromium in urine for
299 unexposed persons in Yanjian city.] J. Yanbian Med.
College, 6 (1): 35-37 (in Chinese).
FARKAS, T.G. & ROBERSON, S.L. (1965) The effect of Cr(III)
on the glucose utilization of isolated lenses. Exp. Eye Res.,
4: 124-126.
FELDMAN, F.J. (1968) The state of chromium in biological
materials. Fed. Proc., 27: 482.
FELDMAN, F.J., KNOBLOCK, E.C., & PURDY, W.C. (1967) The
determination of chromium in biological materials by atomic
absorption spectroscopy. Anal. Chim. Acta, 38: 489-497.
FRADKIN, A., JANOFF, A., LANE, B.P., & KUSCHNER, M. (1975)
In vitro transformation of BHK21 cells grown in the presence
of calcium chromate. Cancer Res., 35: 1058-1061.
FRANCHINI, I., MUTTI, A., GARDINI, F., & BORGHETTI, A.
(1975) [Excretion and renal elimination of chromium compared
to degree and length of occupational exposure.] In: Roche, L.
& Trager, J., ed. Rein et Toxique. Réunion de la Société
Francaise de Toxicologie (Compte rendu), Paris, Groupement
Francais Des Centre Anti-Poisons, pp. 271-281.
FRANCHINI, I., MUTTI, A., CAVATORTA, A., CORRADI, A., COSI,
A., OLIVETTI, A., & BORGHETTI, A. (1978) Nephrotoxicity of
chromium. Control Nephrol., 10: 98-110.
FRANCHINI, J., MAGNANI, F., & MUTTI, A. (1983) Mortality
experience among chrome plating workers. Scand. J. Work
environ. Health, 9: 247-252.
FREGERT, S. (1975) Occupational dermatitis in a 10-year
material. Contact Dermatit., 1: 96-107.
FREGERT, S. (1981) Manual of contact dermatitis, 2nd ed.,
Copenhagen, Munksgaard, 139 pp.
FREGERT, S. & GRUVBERGER, B. (1972) Chemical properties of
cement. Berufsdermatosen, 20(5): 238-245.
FREGERT, S. & GRUVBERGER, B. (1979) Chromium in industrial
leather gloves. Contact Dermatit., 5: 189.
FREGERT, S. & RORSMAN, H. (1964) Allergy to trivalent
chromium. Arch. Dermatol., 90: 4-6.
FREGERT, S., HJORTH, N., MAGNUSSON, B., BANDMANN, H.-J.,
CALNAN, C.D., CRONIN, E., MALTEN, K., MENEGHINI, C.L., PIRILA,
V., & WILKINSON, D.S. (1969) Epidemiology of contact
dermatitis. Trans. St. John's Hosp. Dermatol. Soc., 55: 17-35.
FRENTZEL-BEYME, R. (1983) Lung cancer mortality of workers
employed in chromate pigment factories. J. Cancer Res. clin.
Oncol., 105: 183-188.
FREUND, H., ATAMIAN, S., & FISCHER, J.E. (1979) Chromium
deficiency during total parenteral nutrition. J. Am. Med.
Assoc., 241: 496-498.
FSA (1953) Health workers in chromate producing industry. A
study, Washington DC, Federal Security Agency, 131 pp (US PHS
Publication No. 192).
FUKAI, R. (1967) Valency state of chromium in seawater.
Nature (Lond.), 213: 901.
FUKUNAGA, M., KURACHI, Y., & MIZUGUCHI, Y. (1982) Action of
some metal ions on yeast chromosomes. Chem. pharm. Bull., 30:
3017-3019.
FURST, A., SCHLAUDER, M., & SASMORE, D.P. (1976) Tumour-
igenic activity of lead chromate. Cancer Res., 36: 1779-1783.
GALE, T.F. (1974) Effects of chromium on the hamster embryo.
Teratology, 9: A-17.
GALE, T.F. (1978) Embryotoxic effects of chromium trioxide
in hamsters. Environ. Res., 16: 101-109.
GALE, T.F. (1982) The embryotoxic response to maternal
chromium trioxide exposure in different strains of hamsters.
Environ. Res., 29: 196-203.
GALE, T.F. & BUNCH, J.D. (1979) The effect of the time of
administration of chromium trioxide on the embryotoxic
response in hamsters. Teratology, 19: 81-86.
GARCIA, J.D. & WETTERHAHN JENNETTE, K. (1981) Electron-
transport cytochrome P-450 system is involved in the
microsomal metabolism of the carcinogen chromate. J. inorg.
Biochem., 14: 281-295.
GEISER, J.D., JEANNERET, J.P., & DELACRETAZ, J. (1960) Eczema
due to cement and sensitization to cobalt. Dermatologica
(Basel), 121: 1-7.
GEMMELL, R.P. (1973) Revegetation of derelict land polluted
by a chromate smelter. Part 1. Chemical factors causing
substrate toxicity in chromate smelter waste. Environ.
Pollut., 5: 181-197.
GEMMELL, R.P. (1974) Revegetation of derelict land polluted
by a chromate smelter. Part 2. Techniques of revegetation of
chromate smelter waste. Environ. Pollut., 6: 31-37.
GENTILE, J.M., HYDE, K., & SCHUBERT, J. (1981) Chromium
genotoxicity as influenced by complexation and rate effects.
Toxicol. Lett., 7: 439-448.
GERAUER, A. (1954) [On the growing importance of
epicutaneous allergy to chromium.] Z. Haut-Geschlechtskr., 17:
97-102 (in German).
GILANI, S.H. & MARANO, M. (1979) Chromium poisoning and
chick embryogenesis. Environ. Res., 29: 427-431.
GERICKE, S. (1943) Studies on the effect of the trace
element chromium on the growth of plants. Bodenkd.
Pflanzenernaehr., 33: 114-128.
GLASER, U., HOCHRAINER, D., KLOPPEL, H., KORDEL, W., & KUHNEN,
H. (1984) [Inhalation studies with Wistar rats and patho-
physiological effects of chromium,] Berlin, 156 pp (Report to
Umweltbundesamt, D-1 UFOPLAN F+E 10606007/2) (in German).
GLASER, U., HOCHRAINER, D., KLOPPEL, H., & KUHNEN, H. (1985)
Low-level chromium VI inhalation effects on alveolar macro-
phages and immune functions in Wistar rats. Arch. Toxicol.,
57: 250-256.
GLINSMANN, W.H., FELDMAN, F.J., & MERTZ, W. (1966) Plasma
chromium after glucose administration. Science, 152: 1243.
GLINSMANN, W.H. & MERTZ, W. (1966) Effect of trivalent
chromium on glucose tolerance. Metabolism, 15: 510-520.
GMELIN, C.G. (1826) Experiments on the effects of baryta,
strontia, chrome, molybdenum, tungsten, tellurium, titanium,
osmium, platinum, iridium, rhodium, palladium, nickel, cobalt,
uranium, cerium, iron, and manganese, on the animal system.
Edinburgh med. Surg. J., 26: 131-139.
GOMES, E.R. (1972) Incidence of chromium-induced lesions
among electroplating workers in Brazil. Ind. Med., 41(12):
21-25.
GOMEZ-ARROYO, S., ALTAMIRANO, M., & VILLALOBOS-PIETRINI, R.
(1981) Sister-chromatid exchanges induced by some chromium
compounds in human lymphocytes in vitro. Mutat. Res., 90:
425-431.
GONCHAROV, A.T. (1968) [Role of chromium in the nutrition of
animals and man.] Vopr. Pitaniya, 27: 59-66 (in Russian).
GONCHAROV, A.T. (1978) [Maximum physiological dose of
chromium in food for human consumption.] Kazanskij med. Z.,
59(2): 90-93 (in Russian).
GRAY, S.J. & STERLING, K. (1950) The tagging of red cells
and plasma proteins with radioactive chromium. J. clin.
Invest., 29: 1604-1613.
GREBE, W.-H., KASTNER, H., KIPPENBERGER, C., KRAUSS, U.,
KRUSZONA, M., SCHMIDT, H., LIEBRUCKS, M., RUMBERGER, M., &
WETTIG, E. (1975) [Investigation on supply and demand for
mineral raw materials. VII. Chromium,] Berlin, Hannover,
Bundesanstalt für Gewissenschaften und Rohstoffe/Deutsches
Institut für Wirtschaftsforschung, 147 pp.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38: 33-42.
GROSS, W.G. & HELLER, V.G. (1946) Chromates in animal
nutrition. J. ind. Hyg. Toxicol., 28: 52-56.
GROSS, E. & KOLSCH, F. (1943) [Lung cancer in the chromate
dye industry.] Gewerbepathol. Gewerbehyg., 12: 164-170 (in
German).
GURSON, C.T. (1977) The metabolic significance of dietary
chromium. In: Draper, H.H., ed. Advances in nutritional
research, New York, Plenum Publishing Company, Vol. 1,
pp. 23-53.
GURSON, C.T. & SANER, G. (1971) Effect of chromium on
glucose utilization in marasmic protein-calorie malnutrition.
Am. J. clin. Nutr., 24: 1313-1319.
GURSON, C.T. & SANER, G. (1973) Effect of chromium
supplementation on growth in marasmic protein-calorie
malnutrition. Am. J. clin. Nutr., 26: 988-991.
GURSON, C.T. & SANER, G. (1978) The effect of glucose
loading on urinary excretion of chromium in normal adults,
individuals from diabetic families, and in diabetics. Am. J.
clin. Nutr., 31: 1158-1161.
GUTHRIE, B.E. (1973) Daily dietary intakes of zinc, copper,
manganese, chromium, and cadmium by some New Zealand women.
Proc. Univ. Otago Med. School., 51: 47-49.
GUTHRIE, B.E., WOLF, W.R., & VEILLON, C. (1978) Background
correction and related problems in the determination of
chromium in urine by graphite furnace atomic absorption
spectrometry. Anal. Chem., 50: 1900-1902.
GUTHRIE, B.E., WOLF, W.R., VEILLON, C., & MERTZ, W. (1979)
Chromium in urine. In: Hemphill, D.D., ed. Trace substances in
environmental health, Columbia, Missouri, University of
Missouri, Vol. XII, pp. 490-492.
GUY, W.B. (1954) Dermatologic problems in the railroad
industry resulting from conversion to Diesel power. Am. Med.
Assoc. Arch. Derm. Syph., 70: 289-292.
GYLSETH, B., GUNDERSEN, N., & LANGORD, S. (1977) Evaluation
of chromium exposure based on a simplified method for urinary
chromium determination. Scand. J. Work environ. Health, 3:
28-31.
HAMBIDGE, K.M. (1971) Chromium nutrition in the mother and
in the growing child. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 169-194.
HAMBIDGE, K.M. (1974) Chromium nutrition in man. Am. J.
clin. Nutr., 27: 505-514.
HAMBIDGE, K.M. & BAUM, J.D. (1972) Hair chromium concen-
trations of human newborn and changes during infancy. Am. J.
clin. Nutr., 25: 376-379.
HAMBIDGE, K.M. & RODGERSON, D.O. (1969) A comparison of the
hair chromium levels of nulliparous and parous women. Am. J.
Obstet. Gynecol., 103: 320-321.
HAMBIDGE, K.M., RODGERSON, D.O., & O'BRIEN, D. (1968)
Concentration of chromium in the hair of normal children and
children with juvenile mellitus. Diabetes, 17: 517-519.
HARA, N. (1982) [The change of valence of chromium by acid
treatment.] Sangyo Igaku, 24: 658-662 (in Japanese).
HARRISON, D.L. & STAPLES, E.L.J. (1955) Zinc chromate
poisoning in calves. N.Z. vet. J., 3: 63-73.
HASEGAWA, M. (1938) Studies on the detexicating hormone of
the liver (Yakriton). Toholu J. exp. Med., 32: 163-176.
HAYES, R.B. (1982) Carcinogenic effects of chromium, In:
Langard, S., ed. Biological and environmental aspects of
chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 221-247.
HAYES, R.B., LILIENFELD, A.M., & SNELL, L.M. (1979)
Mortality in chromium chemical production workers: a
prospective study. Int. J. Epidemiol., 8: 365-374.
HEATH, J.C., FREEMAN, M.A.R., & SWANSON, S.A.V. (1971)
Carcinogenic properties of wear particles from prostheses made
in cobalt-chromium alloy. Lancet, 1: 564-566.
HENNIG, A., ANKE, M., DIETRICH, M., HARTMANN, G., & HOFFMANN,
G. (1971) Absorption and distribution of 51chromium after
oral administration to laying hens as a function of time.
Arch. Tierern., 21: 609-615.
HEPLER, O.E. & SIMONDS, J.P. (1946) Experimental nephro-
pathies. IV. Glycosuria in dogs poisoned with uranyl nitrate,
mercury bichloride and potassium dichromate. Arch. Path., 41:
42-49.
HERNBERG, S., WESTERHOLM, P., SCHULTZ-LARSEN, K., DEGERTH,
R., KUOSMA, E., ENGLUND, A., ENGZELL, U., SAND HANSEN, H., &
MUTANEN, P. (1983) Nasal and sinonasal cancer: connection
with occupational exposures in Denmark, Finland, and Sweden.
Scand. J. Work environ. Health, 9: 315-326.
HERTEL, R.F. (1982) [Chromium as a problem in physiology,
epidemiology and biological monitoring.] Staub-Reinhalt. Luft,
42: 135-137 (in German).
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand
cultures. J. exp. Bot., 4: 59-64.
HILT, G. (1954) La dermite du chrome hexavalent dans le
cadre des dermites eczémateuses par sensibilisation aux
métaux. Dermatologica (Basel), 109: 143-174.
HINTZE, C. (1930) [Handbook of mineralogy,] Berlin, Leipzig,
De Gruyter, Vol. 1. III/2, pp. 4012-4030.
HOPKINS, L.L., Jr (1965) Distribution in the rat of physio-
logical amounts of injected 51Cr(III) with time. Am. J.
Physiol., 209: 731-735.
HOPKINS, L.L., Jr & SCHWARZ, K. (1964) Chromium (III)
binding to serum proteins, specifically siderophidin. Biochem.
Biophys. Acta, 90: 484-491.
HOPKINS, L.L., Jr, RANSOME-KUTI, O., & MAJAJ, A.S. (1968)
Improvement of impaired carbohydrate metabolism by chromium
(III) in malnourished infants. Am. J. clin. Nutr., 21: 203-211.
HUCKABEE, J.W., CARTAN, F.O., & KENNINGTON, G.S. (1972)
Environmental influence on trace elements in hair of 15
species of mammals, Oak Ridge, Tennessee, Oak Ridge National
Laboratory, 36 pp (ORNL-TM-3747).
HUEPER, W.C. (1958) Experimental studies in metal
cancerogenesis. X. Cancerigenic effects of chromite ore roast
deposited in muscle tissue and pleural cavity of rats. Arch.
ind. Health, 18: 284-291.
HUEPER, W.C. (1961) Environmental carcinogenisis and cancer.
Cancer Res., 21: 842-857.
HUNTER, W.C. & ROBERTS, J.M. (1933) Experimental study of
the effects of potassium bichromate in the monkey's kidney.
Am. J. Pathol., 9: 133-147.
HYODO, K., SUZUKI, S., FURUYA, N., & MESHIZUKA, K. (1980) An
analysis of chromium, copper, and zinc in organs of a chromate
worker. Int. Arch. occup. environ. Health, 46: 141-150.
IARC (1980) Some metals and metallic compounds, Lyons,
International Agency for Research on Cancer, pp. 205-323 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 23).
IIJIMA, S., MATSUMOTO, N., LU, C.C., & KATSUNUMA, H. (1975)
Placental transfer of CrCl3 and its effects on fetal growth
and development and development in mice. Teratology, 12: 198.
IMBUS, H.R., CHOLAK, J., MILLER, L.H., & STERLING, T. (1963)
Boron, cadmium, chromium, and nickel in blood and urine. Arch.
environ. Health, 6: 112-121.
IMREH, ST. & RADULESCU, D. (1982) Cytogenic effects of
chromium in vivo and in vitro. Mutat. Res., 97: 192-193.
IVANKOVIC, S. & PREUSSMANN, R. (1975) Absence of toxic and
carcinogenic effects after administration of high doses of
chromium oxide pigment in subacute and long-term feeding
experiments in rats. Food Cosmet. Toxicol., 13: 347-351.
JAEGER, H. & PELLONI, E. (1950) Tests épicutanés aux
bichromates, positifs dans l'eczéma au ciment. Dermatologica
(Basel), 100: 207-216.
JEEJEEBHOY, K.N., CHU, R.G., MARLISS, E.B., GREENBERG, G.R., &
BRUCE-ROBERTSON, A. (1977) Chromium deficiency, glucose
intolerance, and neuropathy reversed by chromium supplement-
ation, in a patient receiving long-term total parenteral
nutrition. Am. J. clin. Nutr., 30: 531-538.
JENNETTE, K.W. (1979) Chromate metabolism in liver micro-
some. Biol. Trace Elem. Res., 1: 55-62.
JENSEN, L.S., MAURICE, D.V., & MURRAY, M.W. (1978) Evidence
for a new biological function of chromium. Fed. Proc., 37: 404.
JINDRICHOVA, J. (1978) [Chromium damage in arc welders.] Z.
Ges. Hyg., 24: 86-89 (in German).
JOHNSON, J.L. (1974) Characterization of air-borne partic-
ulates. Microchem. J., 19:168-178.
JONES, G.B., BUCKLEY, R.A., & CHANDLER, C.S. (1975) The
volability of chromium from brewer's yeast during assay. Anal.
Chim. Acta, 80: 389-392.
JOSCHI, A.P. & NEEB, R. (1980) Gas chromatographic elemental
analysis via di(trifluorethyl)dithiocarbamatochelates. Z.
anal. Chem., 303: 389-393.
KAPLAN, I. & ZELIGMAN, I. (1962) Occupational dermatitis in
railroad workers. Am. Med. Assoc. Arch. Derm., 85: 135-142.
KAUFMANN, D.B., DINICOLA, W., & MCINTOSH, R. (1970) Acute
potassium dichromate poisoning. Am. J. dis. Child, 119:
374-376.
KANEKO, T. (1976) Chromosome damage in cultured human
leucocytes induced by chromium chloride and chromium trioxide.
Jpn. J. ind. Health, 18: 136-137.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec assay and
mutagenicity studies on metal compounds. Mutat. Res., 77:
109-116.
KAZANTZIS, G. & LILLY, L. (1979) Mutagenic and carcinogenic
effects of metals. In: Friedberg, L., Nordberg, G.F., & Vouk,
V.B., ed. Handbook on the toxicology of metals, Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 237-273.
KELLER, C. (1980) [Activation analysis.] In: Bartholomé, E.,
Biekert, E., Hellmann, H., Ley, H., Weigert, M., & Weise, E.,
ed. [Ullmann's encyclopaedia of technical chemistry,] 4th ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
Vol. 5, pp. 685-707 (in German).
KEMP, K., JENSEN, F.P., MOELLER, J.T., & HANSEN, G. (1974)
Trace multi-element analysis of biological tissue by proton-
induced X-ray fluorescence spectroscope, Roskilde, Denmark,
Danish Atomic Energy Commission, 22 pp (Report Riso-M-1732).
KEMPF, T. & SONNEBORN, M. (1976) [Determination of Pb, Cd,
Cr, and Zn according to the drinking-water regulation Federal
Republic of Germany.] In: Aurand, K., Hässelbarth, U., Müller,
G., Schumacher, W., & Steuer, W., ed. [The drinking-water
regulation,] Berlin, Erich Schmidt Verlag, pp. 119-125 (in
German).
KEMPF, T. & SONNEBORN, M. (1981) [Chemical composition of
drinking-waters in various regions of the Federal Republic of
Germany.] Vom Wasser, 57: 83-94 (in German).
KESKINEN, H., KALLIOMAKI, P.-L., & ALANKO, K. (1980) Occupa-
tional asthma due to stainless steel welding fumes. Clin.
Allerg., 10: 151-159.
KIRCHGESSNER, M., MERZ, G., & OELSCHLAEGER, W. (1960)
[Effect of vegetation state on content of macro and micro
elements of three species of grass.] Arch. Tierern., 10:
414-427 (in German).
KITTELBERGER, F. (1973) [Contribution to residue analysis in
fish.] Tierärztl. Prax., 1: 465-479.
KLEIN, L.A., LANG, M., NASH, N., & KIRSCHNER, S.L. (1974)
Sources of metals in New York City wastewater. J. Water
Pollut. Control Fed., 46: 2653-2662.
KLEINER, A.M., STOLBUN, B.M., LIKHACHEVA, E.L., & BELJAEVA,
L.N. (1970) [Some indices of the functional state of the
myocardium and hemodynamics in chronic intoxication with
chromium compounds.] Gig. Tr. prof. Zabol., 12: 7-10 (in
Russian).
KNUDSEN, I. (1980) The mammalian spot test and its use for
the testing of potential carcinogenicity of welding fume
particles and hexavalent chromium. Acta pharmacol. toxicol.,
47: 66-70.
KOGAN, V.Y. (1968) [The basophil degranulation as an index
of skin sensitization to cobalt compounds.] In: Belenky, G.B.,
ed. [Modern aspects of clinical and experimental dermato-
venerology. Works of the clinic of skin and venerological
diseases,] Moscow, Medizina, pp. 100-104 (in Russian).
KOGAN, V.Y. (1971) [Chromium as a specific cause for
occupation dermatoses.] In: Babayants, R.S., ed. [Topical
questions of dermato-venerology. First Medical Institute,
All-Union Conference, Moscow, I.A. Sechenov, pp. 172-176
(in Russian).
KOGAN, V.Y, ARUTYUNYAN, K.E., & KOCHINYAN, Y.E. (1972) [A
comparative characteristic of chromium, nickel, and cobalt
salts as a causative factor in allergic contact dermatosis.]
In: [Clinical Pathology of Chemical Aetiology. Materials of
the 1st All-Union Conference, Kiev, USSR, 24-25 October,
1972,] Kiev, pp. 144 and 146 (in Russian).
KOHLER, H. (1951) [Constitutional examinations of subjects
with skin diseases in the offset department of a printing
works (Doctoral thesis, Leipzig.)] Berufsdermatosen, 4: 93 (in
German).
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace
metals during the ashing of biological materials. Analyst,
101: 870-875.
KOIVISTOINEN, P., ed. (1980) Mineral element composition of
Finnish foods. Acta agric. Scand., Suppl. 22: 171
pp.
KOPP, J.E. & KRONER, R.C. (1968) Trace metals in waters of
the United States: a five-year summary of trace metals in
rivers and lakes of the United States (October 1 1962 -
September 30, 1967), Cincinnati, Ohio, US Department of the
Interior, 32 pp.
KORALLUS, U. & LOENHOFF, N. (1981) [Industrial medical and
epidemiological experience with the manufacture and processing
of chromates.] Arbeitsmed. Sozialmed. Praeventivmed., 16:
285-289 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974a)
[Trivalent chromium compounds. Results of a study in
occupational medicine. I. General; Technology; Preliminary
study.] Arbeitsmed. Sozialmed. Praeventivmed., 9: 51-54 (in
German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974b)
[Trivalent chromium compounds: results of a study in
occupational medicine. II. Disease status analysis.]
Arbeitsmed. Sozialmed. Praeventivmed., 9: 76-79 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974c)
[Trivalent chromium compounds: Results of a study in
occupational medicine. III. Clinical studies.] Arbeitsmed.
Sozialmed. Praeventivmed., 9: 248-252 (in German).
KORALLUS, U., LANGE, H.-J., NEISS, A., WUESTEFELD, E., &
ZWINGERS, T. (1982) [Correlations between reorganization
measures and bronchial carcinoma mortality in the chromate-
manufacturing industry.] Arbeitsmed. Sozialmed. Praevent-
ivmed., 7: 159-167 (in German).
KOSHI, K. (1979) Effects of fume particles from stainless
steel welding on sister chromatid exchanges and chromosome
aberrations in cultured Chinese hamster cells. Ind. Health.,
17: 39-49.
KOTZ, L., KAISER, G., TSCHOPEL, P., & TOLG, G. (1972)
[Clarification of biological matrices for determining very low
contents of trace elements when limited amounts are weighed
with nitric acid under pressure in a teflon vessel.] Z. anal.
Chem., 260: 207-209 (in German).
KOUTRAS, G.A., HATTORY, M., SCHNEIDER, A.S., EBAUGH, F.G., &
VALENTINE, W.N. (1964) Studies on chromated erythrocytes:
effect of sodium chromate on erythrocyte glutathione reduc-
tase. J. clin. Invest., 43: 323-331.
KRISHNAJA, A.P. & REGE, M.S. (1982) Induction of chromosomal
aberrations in fish Boleophthalmus dussumieri after exposure
in vivo to mitomycin C and heavy metals mercury, selenium, and
chromium. Mutat. Res., 102: 71-82.
KROEPFLI, P. & SCHUPPLI, R. (1955) [Detergent eczema.]
Dermatologica (Basel), 110: 1-7 (in German).
KRUGER, H. & DORN, H. (1956) [Clinical observations on
detergent eczema in association with chromium and nickel
allergy.] Z. Haut-Geschlechtskr., 20: 307-314 (in German).
KUCHER, I.M. (1966) In: [Proceedings of the 5th Scientific
Session of the Aktiubinsk Medical Institute, Alma-Ata,
Kazakhstan,] pp. 24-26 (in Russian).
KUHN, J.K. (1973) Trace elements in whole coal determined by
X-ray fluorescence. Norelco Rep., 20(3): 7-10.
KUMPULAINEN, J.T., WOLF, W.R., VEILLON, C., & MERTZ, W.
(1979) Determination of chromium in selected United States
diets. J. agric. food Chem., 27: 490-494.
KUMPULAINEN, J., LEHTO, J., KOIVISTOINEN, P., UUSITUPA, M., &
VUORI, E. (1983) Determination of chromium in human milk
serum and urine by electrothermal atomic absorption spectro-
metry without preliminary ashing. Sci. total Environ., 31:
71-80.
KURYLOWICZ, B. & GASIOROWSKI, S. (1958) [Agrochemical study
on calcinated phosphate-containing admixtures of chromium
compounds.] Przemysl Chem., 37: 797-800 (in Polish).
LANGARD, S. (1977) The fate of chromium after intravenous
administration of Na251 CrO4 and CrCl3 x 6H2 O to the rat,
Guildford, England, University of Surrey, 66 pp (M.Sc.
Toxicology Thesis).
LANGARD, S. (1980a) Chromium. In: Waldron, H.A., ed. Metals
in the environment, London, New York, Academic Press,
pp. 111-132.
LANGARD, S. (1980b) A survey of respiratory symptoms and
lung function in ferrochromium and ferrosilicon workers. Int.
Arch. occup. environ. Health, 46: 1-9.
LANGARD, S., ed. (1982) Biological and environmental aspects
of chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, 285 pp.
LANGARD, S. & HENSTEN-PETTERSEN, A. (1981) Chromium
toxicology. In: Williams, D.F., ed. Systemic aspects of
biocompatibility, Boca Raton, Florida, CRC Press, Vol. 1, pp.
144-161.
LANGARD, S. & NORSETH, T. (1975) A short study of bronchial
carcinomas in workers producing chromate pigments. Br. J. ind.
Med., 32: 62-65.
LANGARD, S. & NORSETH, T. (1979) Chromium. In: Friberg, L.,
Nordberg, G.F., & Vouk, V.B., ed. Handbook on the toxicology
of metals, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 383-397.
LANGARD, S. & STERN, R.M. (1985) Nickel in welding fumes: a
cancer hazard to welders? In: Sunderman, F.W.J., Aitio, A.,
Berlin, A., Bishop, C., Buringh, E., Davis, W., Gounar, M.,
Jacquignon, P.C., Mastromatteo, E., Rigaut, J.P., Rosenfeld,
C., Saracci, R., & Sors, A., ed. Nickel in the human
environment, Lyons, International Agency for Research on
Cancer, pp. 95-103 (IARC Scientific Publications No. 53).
LANGARD, S. & VIGANDER, T. (1983) Occurrence of lung cancer
in workers producing chromium pigments. Br. J. ind. Med., 40:
71-74.
LANGARD, S., GUNDERSEN, N., TSALEV, D.L., & GYLSETH, B.
(1978) Whole blood chromium level and chromium excretion in
the rat after zinc chromate inhalation. Acta pharmacol.
toxicol., 42: 142-149.
LANGARD, S., ANDERSEN, A., & GYLSETH, B. (1980) Incidence of
cancer among ferrosilicon workers. Br. J. ind. Med., 37:
114-120.
LASKIN, S. (1972) Research in environmental sciences,
Washington DC, Institute of Environmental Medicine, pp. 92-97.
LASKIN, S., KUSCHNER, M., & DREW, R.T. (1970) Studies in
pulmonary carcinogenesis. In: Hanna, M.G., Jr, Nettesheim, P.,
& Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 321-351 (US Atomic Energy
Commission Symposium Series No. 18).
LAUTNER, G.M., CARVER, J.C., & KONZEN, R.B. (1978)
Measurement of chromium (VI) and chromium (III) in stainless
steel welding fumes with electron spectroscopy for chemical
analysis and neutron activation analysis. Am. Ind. Hyg. Assoc.
J., 39: 651-660.
LEHMANN, K.B. (1914) [Historical and critical account of our
present knowledge of the effect of small doses of chromate.]
In: Gesamtgebiet gewerbehygiene, Berlin, Springer Verlag, pp.
1-119 (in German).
LEHMANN, K.B. (1932) [Does the occurrence of lung cancer in
chromium workers give grounds for any particular anxiety?]
Zentralbl. Gewerbehyg., 9: 168-170 (in German).
LERICHE, H.H. (1968) Metal contamination of soil in the
Woburn market-garden experiment resulting from the application
of sewage sludge. J. agric. Sci. Camb., 71: 205-208.
LETTERER, E., NEIDHARDT, K., & KLETT, H. (1944) [Chromate
lung cancer and chromate pneumoconiosis. A clinical, morbid
anatomical and industrial-hygiene-related study.] Arch.
Gewerbepath. Gewerbehyg., 12: 323-361 (in German).
LEVIN, H.M., BRUNNER, M.J., & RATTNER, H. (1959)
Lithographer's dermatitis. J. Am. Med. Assoc., 169: 566-569.
LEVINE, R.A., STREETEN, D.H.P., & DOISY, R.J. (1968) Effects
of oral chromium supplementation on the glucose tolerance of
elderly human subjects. Metabolism, 17: 114-125.
LEVIS, A.G. & BIANCHI, V. (1982) Mutagenic and cytogenetic
effects of chromium compounds. In: Langard, S., ed. Biological
and environmental aspects of chromium, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 171-208.
LEVIS, A.G. & BUTTIGNOL, M. (1977) Effects of potassium
dichromate on DNA Synthesis in hamster fibroblast. Br. J.
Cancer, 35: 496-499.
LEVIS, A.G. & MAJONE, F. (1979) Cytotoxic and caryogenic
effects of soluble chromium compounds on mammalian cell
cultures. Br. J. Cancer, 40: 523-533.
LEVIS, A.G. & MAJONE, F. (1981) Cytologic and clastogenic
effects of soluble and insoluble compounds containing
hexavalent and trivalent chromium. Br. J. Cancer, 44: 219-235.
LEVIS, A.G., BUTTIGNOL, M., BIANCHI, V., & SPONZA, G. (1978)
Effects of potassium dichromate on nucleic acid and protein
synthesis and on precursor uptake in BHK fibroblasts. Cancer
Res., 38: 110-116.
LEVY, L.S. & MARTIN, P.A. (1983) The effects of a range of
chromium-containing materials on rat lung, Birmingham,
England, University of Aston, Department of Environmental and
Occupational Health, p. 224 and suppl (Prepared for Dry Color
Manufacturers Association, Alexandria, Virginia, USA).
LEVY, L.S. & VENITT, S. (1975) Carcinogenic and mutagenic
activity of chromium-containing materials. Br. J. Cancer, 32:
254-255.
LIEBER, M., PERLMUTTER, N.M., & FRAUENTHAL, H.L. (1964)
Cadmium and hexavalent chromium in Nassau County ground
water. J. Am. Water Works Assoc., 56: 739-747.
LIFSCHITZ, M.L., WALLACH, S., PEABODY, R.A., VERCH, R.L., &
AGRAWAL, R. (1980) Radiochromium distribution in thyroid
and parathyroid deficiency. Am. J. clin. Nutr., 33: 57-62.
LINDBERG, E. & HEDENSTIERNA, G. (1983) Chrome plating.
Symptoms, findings in the upper airways, and effects on lung
function. Arch. environ. Health, 38: 367-374.
LINDBERG, E. & VESTERBERG, O. (1983a) Monitoring exposure to
chromic acid in chromeplating by measuring chromium in urine.
Scand. J. Work environ. Health, 9: 333-340.
LINDBERG, E. & VESTERBERG, O. (1983b) Urinary excretion of
proteins in chromplaters, previously exposed chromplaters, and
referents. Scand. J. Work. environ. Health, 9: 505-510.
LINDBERG, E., EKHOLM, U. & ULFVARSON, U. (1985) Extent and
conditions of exposure in the Swedish chrome plating industry.
Int. Arch. Occup. Environ. Health, 56: 197-205.
LIU, V.J.K. & MORRIS, J.S. (1978) Relative chromium response
as an indicator of chromium status. Am. J. clin. Nutr., 31:
972-976.
LOFROTH, G. (1978) The mutagenicity of hexavalent chromium
is decreased by microsomal metabolism. Naturwissenschaften,
65: 207-208.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LUKANIN, W.P. (1930) [The pathology of chromate pneumo-
coniosis.] Arch. Hyg. Bakt., 104: 166-174 (in German).
LUZHNIKOV, E.A., SHIMANKO, I.I., KOSTOMAROVA, L.G., MALYGINA,
S.I., & TRAKHTENGARTS, M.I. (1976) [Acute poisoning with
chromium compounds.] Terapeut. Arkh., 48(7): 121-125 (in
Russian).
LYON, G.L., BROOKS, R.R., PETERSON, P.J., & BUTLER, G.W.
(1970) Some trace elements in plants from serpentine soils.
N.Z. J. Sci., 13: 133-139.
MACHLE, W. & GREGORIUS, F. (1948) Cancer of the respiratory
system in the United States chromate-producing industry.
Public Health Rep., 63: 1114-1127.
MCCLENDON, L.T. (1978) Determination of chromium in
biological matrices by neutron activation: application to
standard reference materials. J. radioanal. Chem., 42: 85-91.
MACKENZIE, R.D., BYERRUM, R.U., DECKER, C.F., HOPPERT, C.A., &
LANGHAM, R.F. (1958) Chronic toxicity studies. II. Hexa-
valent and trivalent chromium administered in drinking-water
to rats. Am. Med. Assoc. Arch. ind. Health, 18: 232-234.
MCRAE, W.D., WHITING, R.F., & STICH, H.F. (1979) Sister-
chromatid exchanges induced in cultured mammalian cells by
chromate. Chem.-biol. Interact., 26: 281-286.
MAHALKO, J.R. & BENNION, M. (1976) The effect of parity and
time between pregnancies on maternal hair chromium concentra-
tion. Am. J. clin. Nutr., 29: 1069-1072.
MAJONE, F. & LEVIS, A.G. (1979) Chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster cells treated in
vitro with hexavalent chromium compounds. Mutat. Res., 67:
231-238.
MAJONE, F. & RENSI, D. (1979) Mitotic alterations, chromo-
some aberrations, and sister-chromatid exchanges induced by
hexavalent and trivalent chromium on mammalian cells in vitro.
Caryologia, 32: 379-392.
MALTONI, C. (1974) Occupational carcinogenesis. Excerpta
Med. Int. Congr. Ser., 322: 19-26.
MALTONI, C. (1976) Precursor lesions in exposed populations
as indicators of occupational cancer risk. Ann. NY Acad. Sci.,
271: 444-447.
MANCUSO, T.F. (1951) Occupational cancer and other health
hazards in a chromate plant: a medical appraisal. II. Clinical
and toxicologic aspects. Ind. Med. Surg., 20: 393-407.
MANCUSO, T.F. (1975) Consideration of chromium as an
industrial carcinogen. In: Hutchinson, T.C., ed. Proceedings
of the International Conference on Heavy Metals in the
Environment, Toronto, Canada, October 27-31, 1975, Toronto,
Institute for Environmental Studies, pp. 343-356.
MANCUSO, T.F. & HUEPER, W.C. (1951) Occupational cancer and
other health hazards in a chromate plant: a medical appraisal.
I. Lung cancer in chromate workers. Ind. Med. Surg., 20:
358-363.
MARTIN, J.H. & KNAUER, G.A. (1973) The elemental composition
of plankton. Geochim. Cosmochim. Acta, 3: 1639-1653.
MATHIS, B.J. & CUMMINGS, T.F. (1973) Selected metals in
sediments, water, and biota in the Illinois river. J. Water
Pollut. Control Fed., 45: 1573-1583.
MATHUR, R.K. & DOISY, R.J. (1972) Effect of diabetes and
diet on the distribution of tracer doses of chromium in rats.
Proc. Soc. Exp. Biol. Med., 139: 836-838.
MATSUMOTO, N., IIJIMA, S., & KATSUNUMA, H. (1976) Placental
transfer of chromic chloride and its teratogenic potential in
embryonic mice. J. toxicol. Sci., 2: 1-13.
MAYER, R. (1983) Interaction of forest canopies with atmo-
spheric constituents: aluminium and heavy metals. In: Ulrich,
B. & Pankrath, J., ed. Effects of accumulation of air
pollutants in forest ecosystems, Dordrecht, Boston, London,
D. Reidel, pp. 47-55.
MAZGON, R. (1932) [Therapeutic effect of common salt on
experimental chromium nephritis in the rabbit.] Z. Gesamte
exp. Med., 81: 195-207 (in German).
MERIAN, E. (1984) Introduction on environmental chemistry
and global cycles of arsenic, beryllium, cadmium, chromium,
cobald, nickel, selenium, and their derivatives. Toxicol.
environ. Chem., 8: 9-38.
MERKURIEVA, R.V., KRASOVSKY, G.N., BURMANTOVA, N.P.,
SHATERNIKOVA, I.L., VARSHAVSKAYA, S.P., VASIUKOVICH, L.YA.,
KOGANOVA, Z.I., BUSHINSKAYA, L.I., KONSTANTINOVA, I.N.,
DOLINSKAYA, S.I., ASTAKHOVA, L.F., & VOTYAKOV, A.V. (1980a)
[Changes in various enzyme systems resulting from exposure to
some chemical substances.] Gig. i. Sanit., N1: 25-28 (in
Russian).
MERKURIEVA, R.V., KRASOVSKY, G.N., VOTYAROV, A.V., BURMANTOVA,
N.P., MUCHAMBETOVA, L.H., & KONSTANTINOVA, I.N. (1980b)
[Enzyme disarrangement of various subcellular structures under
the influence of the general toxic and gonadotoxic effects of
environmental chemical factors.] Zdravookhranenie belorus.,
N2: 28-29 (in Russian).
MERKURIEVA, R.V., KOGANOVA, Z.I., GABDULLINA, M.H., TSAPKOVA,
N.N., DUKHATEROV, O.N., & DOLINSKAYA, S.I. (1982) [A
comparative study of metabolic reactions at various routes of
chromium6+ administration in the organism of laboratory
animals.] Gig. i Sanit., N8: 75-78 (in Russian).
MERRITT, W.F. (1971) Identification and measurement of trace
elements in fresh water by neutron activation. In: Proceedings
of the International Symposium on Identification and Measure-
ment of Environmental Pollution, 358-362.
MERTZ, W. (1969) Chromium occurrence and function in
biological systems. Physiol. Rev., 49: 163-239.
MERTZ, W. (1981) The interaction between chromium and
insulin. Adv. Physiol. Sci., 12: 101-105.
MERTZ, W. & ROGINSKI, E.E. (1963) The effect of trivalent
chromium in galactose entry in rat epidymal fat tissue. J.
biol. Chem., 238: 868-872.
MERTZ, W. & ROGINSKI, E.E. (1969) Effects of chromium (III)
supplementation on growth and survival under stress in rats
fed low protein diets. J. Nutr., 97: 531-536.
MERTZ, W. & ROGINSKI, E.E. (1971) Chromium metabolsm: the
glucose tolerance factor. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 123-153.
MERTZ, W. & SCHWARZ, K. (1959) Relation of glucose tolerance
factor to impaired glucose tolerance in rats on stock diets.
Am. J. Physiol., 196: 614-618.
MERTZ, W., ROGINSKI, E.E., & SCHWARZ, K. (1961) Effect of
trivalent chromium complexes on glucose uptake by epididymal
fat tissue of rats. J. biol. Chem., 236: 318-322.
MERTZ, W., ROGINSKI, E.E., & REBA, R.C. (1965a) Biological
activity and fate of intravenous chromium (III) in the rat.
Am. J. Physiol., 209: 489-494.
MERTZ, W., ROGINSKI, E.E., & SCHROEDER, H.A. (1965b) Some
aspects of glucose metabolism of chromium-deficient rats
raised in a strictly controlled environment. J. Nutr., 86:
107-112.
MERTZ, W., ROGINSKI, E.E., FELDMAN, R.J., & THURMAN, D.E.
(1969) Dependence of chromium transfer into the rat embryo on
the chemical form. J. Nutr., 99: 363-367.
MERTZ, W., TOEPFER, E.W., ROGINSKI, E.E., & POLANSKY, M.M.
(1974) Present knowledge of the role of chromium. Fed. Proc.,
33: 2275-2280.
MEYERS, J.B. (1950) Acute pulmonary complications following
inhalations of chromic acid mist. Arch. ind. Hyg. occup. Med.,
2: 742-747.
MICKAIL, T.H., HABIB, Y.A., GABRIAL, G.N., & MORCOS, S.R.
(1976) The effect of chromium III on the intravenous glucose
tolerance of rats given diets of defficient protein values. Z.
Ernährungswiss., 15: 182-187.
MIESCHER, G., AMREIN, H.P., & LEDER, M. (1955) [Chromate
hypersensitivity and cement eczema.] Dermatologica (Basel),
110: 266-283 (in German).
MIGAI, K.V. (1975) [Hygiene and safety of labor in electro
welding works in shipbuilding,] Leningrad, pp. 27-35 (in
Russian).
MITMAN, F.W., WOLF, W.R., KELSAY, J.L., & PRATHER, E.S.
(1975) Urinary chromium levels of nine young women eating
freely chosen diets. J. Nutr., 105: 64-68.
MORGAN, J.M. (1972) Hepatic chromium content in diabetic
subjects. Metabolism, 21: 313-316.
MORRIS, G.E. (1955) Chromate dermatitis from chrome glue and
other aspects of the chrome problem. Am. Med. Assoc. Arch.
Ind. Health, 11: 368-371.
MORRIS, G.E. (1956) The manufacture of leather. Its chemical
and dermatological aspects. Ann. Meet. Am. Acad. Derm. Syph.
(Chicago).
MORRIS, G.E. (1958) Chrome dermatitis. A study of the
chemistry of shoe leather with particular reference to basic
chromic sulfate. Am. Med. Assoc. Arch. Derm., 78: 612-618.
MORRIS, G.E. (1959) [The basic form of chromic sulfate.
Results of epicutaneous testing of a chromium-III salt.]
Berufsdermatosen, 7: 126-131 (in German).
MORROW, R.W. & MCELHANEY, R.J. (1974) Determination of
chromium in industrial effluent water by flameless atomic
absorption spectroscopy. At. Absorpt. Newsl., 13(2): 45-46.
MORTON, M.S. & ELWOOD, P.C. (1974) CNS malformations and
trace elements in water. Teratology, 10: 318.
MOSINGER, M. & FIORENTINI, H. (1954) Sur la pathologie due
aux chromates: Premières recherches expérimentales. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 15: 187-199.
MULLER-WIEGAND, B., STEUER, D., & HEESSELS, P. (1983) Blood
and urine samples for quality control of heavy metals. Chem.
Toxicol. Clin. Chem. Met. Proc. Int. Conf., 2nd: 73-76.
MURAKAMI, Y., SUZUKI, Y., YAMAGATA, T., & YAMAGATA, N. (1965)
Cr and Mn in Japanese diet. J. Radiat. Res., 6: 105-110.
MURRMAN, R.P., WINTERS, R.W., & MARTIN, T.G. (1971)
Determination of trace elements in soils and clay minerals by
resonnance neutron activation analysis. Soil. Sci. Soc. Am.
Proc., 35: 647-652.
MUTTI, A., CAVATORTA, A., PEDRONI, C., BORGHI, A., GIAROLI,
C., & FRANCHINI, I. (1979) The role of chromium accumulation
in the relationship between airborne and urinary chromium in
welders. Int. Arch. occup. environ. Health, 43: 123-133.
MYSLYAEVA, A.V. (1965) [Changes in the peripheral blood in
workers of a chromium compound plant.] In: [Proceedings of
the 4th Scientific Conference of Aktubinsk State Medical
Institute, Alma-Ata,] Kazakhstan, Alma-Ata, Aktubinsk State
Medical Institute, pp. 331-332 (in Russian).
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., & KURATA, H. (1978)
Comparative studies of chromosomal aberrations and muta-
genicity of trivalent and hexavalent chromium. Mutat. Res.,
58: 175-181.
NASH, D.T., ELWOOD, I.G., & STREETEN, D.H.P. (1979) The
effect of brewer's yeast containing chromium-rich glucose
tolerance factor on serum lipids. In: Proceedings of the 5th
International Symposium on Atherosclerosis, Houston, Texas.
NATER, J.P. (1963) Possible causes of chromate eczema.
Dermatologica (Basel), 126: 160-166.
NATH, R. (1976) Assessment of chromium metabolism in
maturity onset and juvenile diabetics using chromium: 51Cr and
therapeutic response of chromium administration. Excerpta Med.
Int. Congr. Ser., 400: 194.
NEIKO, E.M. & DELVA, IU.V. (1978) [Chromium balance in
chronic ischemic heart disease.] Klinicheskaia Meditsina
(Mosk.), 56(4): 97-99 (in Russian).
NESTMANN, E.R., MATULA, T.I., DOUGLAS, G.R., BORA, K.C., &
KOWBEL, D.J. (1979) Detection of the mutagenic activity of
lead chromate using a battery of microbial tests. Mutat. Res.,
66: 357-365.
NETTESHEIM, P., HANNA, M.G., Jr., DOHERTY, D.G., NEWELL, R.F.,
& HELLMANN, A. (1970) Effects of chronic exposure to
artificial smog and chromium oxide dust on the incidence of
lung tumours in mice. In: Hanna, M.G., Jr, Nettesheim, P., &
Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 305-320 (US Atomic Energy
Commission Symposium Series No. 18).
NETTESHEIM, P., HANNA, M.G., DOHERTY, D.G., NEWELL, R.F., &
HELLMAN, A. (1971) Effect of calcium chromate dust,
influenza virus, and 100R whole-body X-radiation on lung
tumour incidence in mice. J. Natl Cancer Inst., 47: 1129-1144.
NEWBOLD, R.F., AMOS, J., & CONNELL, J.R. (1979) The
cytotoxic, mutagenic, and clastogenic effects of chromium-
containing compounds on mammalian cells in culture. Mutat.
Res., 67: 55-63.
NEWMAN, H.A.I., LEIGHTON, R.F., LANESE, R.R., & FREELAND N.A.
(1978) Serum chromium and angiographically-determined
coronary artery disease. Clin. Chem., 24: 541-544.
NISHIOKA, H. (1975) Mutagenic activities of metal compounds
in bacteria. Mutat. Res., 31: 185-189.
NOLLENDORF, A., PAKALNE, E., & UPITIS, V. (1972) [Little
known trace elements in Chlorella culture.] Latv. PSR Zinat.
Akad. Vestis (Latvian SSR), 7: 33-43 (in Russian).
NOMIYAMA, H., YOTORIYAMA, M., & NOMIYAMA, K. (1980b) Normal
chromium levels in urine and blood of Japanese subjects
determined by direct flameless atomic absorption spectrophoto-
metry, and valency of chromium in urine after exposure to
hexavalent chromium. Am. Ind. Hyg. Assoc. J., 41: 98-102.
NOMIYAMA, K., MATSUI, K., & NOMIYAMA, H. (1980a) Environ-
mental temperature: a factor modifying the acute toxicity of
organic solvents, heavy metals, and agricultural chemicals.
Toxicol. Lett., 6: 67-70.
NORSETH, T. (1978) Health effects of nickel and chromium.
In: Elvira di Ferrante, E., ed. Trace metals: exposure and
health effects, Oxford, New York, Toronto, Sydney, Paris,
Frankfurt, Pergamon Press, pp. 135-146.
NORSETH, T. (1980) Cancer hazards caused by nickel and
chromium exposure. J. Toxicol. environ. Health, 6: 1218-1227.
NOVAKOVA, S., MAUTNER, G., & DINOEVA, A. (1974) [Content of
hexavalent chromium in water supplies and its effect on the
development of experimental atherosclerosis in warm-blooded
animals.] Gig. i Sanit., 5: 78-80 (in Russian).
NOVEY, H.S., HABIB, M., WELLS, J.A. (1983) Asthma and JgE
antibodies induced by chromium and nickel salts. J. Allergy
clin. Immunol., 72: 407-412.
OFFENBACHER, E.G. & PI-SUNYER, F.X. (1980) Benefical effect
of chromium-rich yeast on glucose tolerance and blood lipids
in elderly subjects. Diabetes, 29: 919-925.
OHNO, H., HANAOKA, F., & YAMADA, M. (1982) Inducibility of
sister-chromatid exchanges by heavy-metal ions. Mutat. Res.,
104: 141-145.
OHTA, F. (1940) Studies on the detoxicating hormone of the
liver (yakriton). Contribution to the usage of yakriton
against experimental chromate nephritis. Tohoku J. exp. Med.,
39: 37-46.
OKADA, S., OHBA, H., & TANIYAMA, M. (1981) Alterations in
ribonucleic acid synthesis by chromium (III). J. inorg.
Biochem., 15: 223-231.
OKADA, S., TRUKADA, H., & OHBA, H. (1983) Enhancement of
ribonucleic acid synthesis by chromium (III) in mouse liver.
J. inorg. Biochem., 19: 95-103
OKADA, S., TSUKADA, H., & OHBA, H. (1984) Enhancement of
nucleolar RNA synthesis by chromium (III) in regenerating rat
liver. J. inorg. Biochem., 21, 113-124.
OPHULS, W. (1911a) Experimental nephritis in guinea-pigs by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9: 11-13.
OPHULS, W. (1911b) Experimental nephritis in rabbits by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9:13.
OTTINGER, R.S., BLUMENTHAL, J.L., DALPORTO, D.F., GURBER,
G.I., SANTY, M.J., & SHIH, C.C. (1973) Recommended methods
of reduction, neutralization, recovery or disposal of
hazardous waste, Cincinnati, Ohio, US Environmental Protection
Agency, Vol. 6, pp. 143-162 (EPA 670/2-73-053-F).
PAGE, A.L. (1974) Fate and effects of trace elements in
sewage sludge when applied to agricultural lands, Cincinnati,
Ohio, US Environmental Protection Agency (EPA 670/2-74-005).
PAIXAO, L.M. & YOE, J.H. (1959) Spectochemical determination
of magnesium, chromium, nickel, copper, and zinc in human
plasma and red cells. Clin. Chim. Acta, 4: 507-514.
PARR, R.M. (1977) Problems of chromium analysis in
biological materials: an international perspective with
special reference to results for analytical quality control
samples. J. radioanal. Chem., 39: 421-433.
PASCALE, L.R., WALDSTEIN, S.S., ENGBRING, G., DUBIN, A., &
SZANTO, P.B. (1952) Chromium intoxication with special
reference to hepatic injury. J. Am. Med. Assoc., 149:
1385-1389.
PASHIN, Y.V. & KOZACHENKO, V.J. (1981) [Mutagenic activity
of chromium compounds.] Gig. i. Sanit., 5: 46-49 (in Russian).
PASHIN, Y.V., ZACEPILOVA, T.A., & KOZACHENKO, V.I. (1982)
Induction of dominant lethal mutations in male mice by
potassium dichromate. Mutat. Res., 103: 345-347.
PASTERNAK, K. (1973) The spreading of heavy metals in
flowing waters in the region of occurrence of natural deposits
of the zinc and lead industry. Acta hydrobiol., 15: 145-166.
PEKAREK, R.S., HAUER, E.C., RAYFIELD, E.J., WANNEMACHER, R.W.,
& BIESEL, W.R. (1975) Relationship between serum chromium
concentrations and glucose utilization in normal and infected
subjects. Diabetes, 24: 350-353.
PENG, H.Z. (1982) [The investigation of nasal disease
morbidity of chromium exposed workers.] J. Shan-Xie Med.
College, 3: 19-21 (in Chinese).
PERLMUTTER, N.M. & LIEBER, M. (1970) Dispersal of plating
wastes and sewage contaminants in ground water and surface
water, Washington DC, US Government Printing Office, 67 pp
(Geological Survey Water Supply Paper 1879-G).
PERONE, V.B., MOFFITT, A.A., POSSICK, P.A., KEY, M.M.,
DANZINGER, S.J., & GELLIN, G.A. (1974) The chromium, cobalt,
and nickel contents of American cement and their relationship
to cement dermatitis. Am. Ind. Hyg. Assoc. J., 35: 301-306.
PETRILLI, F.L. & DE FLORA, S. (1977) Toxicity and mutagenicity of
hexavalent chromium on Salmonella typhimurium. Appl. environ.
Microbiol., 33: 805-809.
PETRILLI, F.L. & DE FLORA, S. (1978a) Oxidation of inactive
trivalent chromium to the mutagenic hexavalent form. Mutat.
Res., 58: 167-173.
PETRILLI, F.L. & DE FLORA, S. (1978b) Metabolic deactivation
of hexavalent chromium mutagenicity. Mutat. Res., 54: 139-147.
PETRILLI, F.L. & DE FLORA, S. (1980) Mutagenicity of
chromium compounds. In: Chromates Symposium 80, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 76-99.
PETRILLI, F.L. & DE FLORA, S. (1982) Interpretations on
chromium mutagenicity and carcinogenicity. In: Sorsa, M. &
Vainmio, H., ed. Mutagens in our environment, New York, Alan
R. Liss, pp. 453-464.
PIERCE, J.V. & SCHEEL, L.D. (1965) Toxicity of alloys of
chromium. Arch. environ. Health, 10: 870-876.
PIRILA, V. & KILPIO, O. (1949) On dermatoses caused by
bichromates. Acta derm. venerol., 29: 550-563.
POHLAND, F.G. (1975) Sanitary landfill stabilization with
leachate recycle and residual treatment, Cincinnati, Ohio, US
Environmental Protection Agency, 106 pp (EPA 600/2-75-043).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1973) [On carcinogenous
hazards in the product of chromium ferroalloys.] Gig. Tr.
prof. Zabol., 10: 23-26 (in Russian).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1974) [Epidemiological
data on malignant neoplasms in workers who are in contact with
different chromium compounds.] In: Shabad, L.M., Velich-
kovskii, B.T. & Kogan, F.M., ed. [Occupational cancer (a
collection of scientific works),] Moscow, Academic Academy of
Medical Science, pp. 64-71 (in Russian).
POKROVSKAYA, L.V., TJUSHUYAKOVA, A.V., GORODNOVA, N.V.,
ZALOMOVA, E.N., & ANDREEVA, T.D. (1976) [Dust factor and
occupational pathology in workers engaged in the open
excavation of chromium ores.] In: [Occupational diseases of
dust etiology (collection of works),] Vol. 3, pp. 38-43 (in
Russian).
POLAK, L., TURK, J.L., & FREY, J.R. (1973) Studies on
contact hypersensitivity to chromium compounds. In: Kallos,
P., Waksman, B.H., & DeWeck, A., ed. Progress in allergy,
Basel, Karger, Vol. 17, pp. 145-226.
PRESTON, A.M., DOWDY, R.P., PRESTON, M.A., & FREEMAN, J.N.
(1976) Effects of dietary chromium on glucose and serum
cholesterol in guinea-pigs. J. Nutr., 106: 1391-1397.
PRIESTLEY, J. (1877) Observations on the physiological
action of chromium. J. Anat. Physiol., 11: 285-301.
PRINCI, F., MILLER, L.H., DAVIS, A., & CHOLAK, J. (1962)
Pulmonary disease of ferrochromium workers. J. occup. Med., 4:
301-310.
PUNSAR, S., ERAMETSA, O., KARVONEN, M.I., RYHANEN, A., HILSKA,
P., & WORNAMO, H. (1975) Coronary heart disease and
drinking-water. A search in two Finnish male cohorts for
epidemiologic evidence of a water factor. J. chron. Dis., 28:
259-287.
RABEAU, H. & UKRAINCZYK, V. (1939) Dermites des
blanchisseuses. Rôle du chrome et du chlore. Ann. Derm. Syph.,
10: 656-680.
RADIAN CORPORATION (1983) Estimates of population exposure
to ambient chromium emissions, Research Triangle Park, North
Carolina, US Environmental Protection Agency (Final Report to
K.L. Blanchard) (EPA Contract No. 68-02-3818).
RAFFETTO, G. (1977) Direct interaction with cellular targets
as the mechanism for chromium carcinogenesis. Tumori, 63:
503-512.
RAFFETTO, G., PARODI, S., DE FERRARI, M., PARODI, C., TROIANO,
R., & BRAMBILLA, G. (1978) Direct interaction with cellular
targets as the mechanism for chromium carcinogensis. Tumori,
73: 503-512.
REDISKE, J.H. (1956) Chromium toxicity in plants. In:
Biology research: annual report, 1955, Richland, Washington,
Hamford Atomic Products Operations, pp. 48-52 (Report
HW-41500).
REDISKE, J.H., CLINE, J.F., & SELDERS, A.A. (1955) Chromium
toxicity in plants. In: Biology research: annual report, 1954,
Richland, Washington, Hamford Atomic Products Operations,
pp. 32-39 (Report HW-35917).
REGGIANI, A., LOTTI, M., DE ROSA, E., & SAIA, B. (1973)
[Impairments of respiratory functions in subjects exposed to
chromium. Note 1. Spirographic changes.] Lav. Umano, 25: 23-27
(in Italian).
REICHENBACH-KLINKE, H.H. (1977) [To the ecology of the
accumulation of heavy metals in fishes.] In: Reichenbach-
Klinke, H.H., ed. [Contributions to fish ecology: fish and the
environment,] Stuttgart, New York, Gustav Fischer, pp. 7-11
(in German).
REICHENBACH-KLINKE, H.H. (1980) [Fish diseases and harm
suffered by fish,] 2nd ed., Stuttgart, New York, Gustav
Fischer, 474 pp (in German).
RETNEV, V.M. (1960) [On the effect produced by chromium
compounds contained in cement dust on the development of
bronchial asthma.] Gig. Tr. prof. Zabol., 7: 29-32 (in
Russian).
RIALES, R. (1979) Influence of brewer's yeast on lipoprotein
cholesterol concentrations. A preliminary report. In:
Shapcott, D. & Hubert, I., ed. Chromium in nutrition and
metabolism. Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 199-212.
ROBERTS, K.R. & MARZLUF, G.A. (1971) The specific inter-
action of chromate with the dual sulfate permease systems of
Neurospora crassa. Arch. Biochem. Biophys., 142: 651-659.
ROBINSON, W.D. & EDINGTON, G. (1935) Chemical studies of
infertile soils derived from rocks high in magnesium and
generally high in chromium and nickel, Washington DC, US
Department of Agriculture, pp. 1-29 (US Department of
Agriculture Technical Bulletin No. 471).
ROGINSKI, E.E. & MERTZ, W. (1967) An eye lesion in rats fed
low-chromium rations. J. Nutr., 93: 249-251.
ROGINSKI, E.E. & MERTZ, W. (1969) Effects of chromium (III)
supplementation on glucose and amino acid metabolism in rats
fed a low protein diet. J. Nutr., 97: 525-530.
ROHDE, G., TISCHENDORF, G., LEONHARDT, J., & DAMASCHUN, F.
(1978) [Study of crocoite occurrence in Callenberg (Saxony)
(Germany).] Z. angew. Geol., 24: 168-173 (in German).
ROMOSER, G.L., DUDLEY, W.A., MACHLIN, L.J., & LOVELESS, L.
(1961) Toxicity of vanadium and chromium for the growing
chick. Poultry Sci., 40: 1171-1173.
ROOK, H.L. & WOLF, W.R. (1977) The quantitative
determination of volatile trace elements in NBS biological
standard reference materials. In: Hemphill, D.D., ed.
Proceedings of the Eleventh Annual Conference on Trace
Substances in Environmental Health, Columbia, Missouri, the
Curators of the University of Missouri, pp. 324-333.
ROSCHINA, T.A. (1959) [Experimental study on the effects of
magnezite chromium and chromite magnezite dust exposure in the
production of refrectories.] Gig. Tr. prof. Zabol., 4: 28-32
(in Russian).
ROSCHINA, T.A. (1964) [A comparative evaluation of the
effects of carbide dust and chromium boride on the organism.]
Gig. i Sanit., 8: 25-28 (in Russian).
ROSCHINA, T.A. (1976) [The toxicity of chrominum (sic)
hexacarbonyl.] Gig. Tr. Prof. Zabol., 2: 38-42 (in Russian).
ROSEBROUGH, R.W. & STEELE, N.C. (1981) Effect of
supplemental dietary chromium or nicotinic acid on
carbohydrate metabolism during basal, starvation, and
refeeding periods in poults. Poultry Sci., 60: 407-417.
ROSS, D.S., SJOGREN, R.E., & BARTLETT, R.J. (1981) Behaviour
of chromium in soils. IV. Toxicity to microorganisms. J.
environ. Qual., 10: 145-148.
ROSTENBERG, A., Jr & PERKINS, A.J. (1951) Nickel and cobalt
dermatitis. J. Allerg., 22: 466-474.
RTECS (1978) Registry of toxic effects of chemical substances,
Washington DC, National Institute for Occupational Safety and
Health, pp. 450.
SAMITZ, M.H. (1955) Some dermatologic aspects of the
chromate problem. Arch. ind. Health, 11: 361-367.
SAMITZ, M.H. (1956) Studies of chromate dermatitis. Arch.
ind. Health, 14: 269-271.
SAMITZ, M.H. & EPSTEIN, E. (1962) Experimental cutaneous
chrome ulcers in guinea-pigs. Arch. environ. Health, 5:
463-468.
SAMITZ, M.H. & GROSS, S. (1960) Extraction by sweat of
chromium from chrome tanned leathers. J. occup. Med., 2: 12-14.
SAMITZ, M.H. & GROSS, S. (1961) Effects of hexavalent and
trivalent chromium compounds on the skin. Am. Med. Assoc.
Arch. Dermatol., 84: 404-409.
SAMITZ, M.H., KATZ, S., & GROSS, S. (1960) Nature of the
chromium extracted from leather by sweat. J. occup. Med., 2:
435-436.
SANDERS, C.L., JACKSON, T.A., ADEE, R.R., POWERS, G.J., &
WEHNER, A.P. (1971) Distribution of inhaled metal oxide
particles in pulmonary alveoli. Arch. intern. Med., 127:
1085-1089.
SARGENT, T., III, LIM, T.H., & JENSON, R.L. (1979) Reduced
chromium retention in patients with hemochromatosis, a
possible basis of hemochromatotic diabetes. Metabolism, 28:
70-79.
SARTO, F., LEVIS, A.G., & PAULON, C. (1980) Clastogenic
activity of hexavalent and trivalent chromium in cultured
human lymphocytes. Caryologia, 33: 239-250.
SARTO, F., COMINATO, J., BIANCHI, V., & LEVIS, A.G. (1982)
Increased incidence of chromosomal aberrations and sister
chromatid exchanges in workers exposed to chromic acid (CrO3)
in electroplating factories. Carcinogenesis, 3: 1011-1016.
SASSI, C. (1956) [Occupational disease in a chromate
factory.] Med. Lavoro, 47: 314-326 (in Italian).
SATOH, K., FUKUDA, Y., TORII, K., & KATSUNO, N. (1981)
Epidemiological study of workers engaged in the manufacture of
chromium compounds. J. occup. Med., 23: 835-838.
SAVORY, J., MUSHAK, P., & SUNDERMAN, F.W. Jr (1969) Gas
Chromatographic determination of chromium in serum. J.
chromatogr. Sci., 7: 674-679.
SCHALLER, K.H. & ZOBER, A. (1982) [Renal excretion of
toxicologically relevant metals in occupationally non-exposed
individuals.] Arztl. Lab., 28: 209-214 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1972) [Quantitative chromium determination in urine by
flameless atomic absorption spectrometry.] Z. klin. Chem.
klin. Biochem., 10: 434-437 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1973) The quantitative determination of chromium in urine by
flameless atomic absorption spectroscopy. At. Absorpt.
Newsl., 12(6): 147-150.
SCHELENZ, R. (1977) Dietary intake of 25 elements by man
estimated by neutron activation analysis. J. radioanal. Chem.,
37: 539-548.
SCHLETTWEIN-GSELL, D. & MOMMSEN-STRAUB, S. (1971) [Review
trace elements in foods. III. Chromium.] Int. J. Vitam. Nutr.
Res., 41: 116-125 and (1973) Suppl. 13, 188 pp (in German).
SCHOENTAL, R. (1975) Chromium carcinogenesis formation of
epoxyaldehydes and tanning. Br. J. Cancer, 32: 403-404.
SCHROEDER, H.A. (1966) Chromium deficiency in rats: a
syndrome simulating diabetes mellities with retarded growth.
J. Nutr., 88: 439-445.
SCHROEDER, H.A. (1970) Chromium, Washington DC, American
Petroleum Institute, 28 pp (Air Quality Monograph No. 70-15).
SCHROEDER, H.A. & BALASSA, J.J. (1965) Influence of
chromium, cadmium, and lead on rat aortic lipids and
circulating cholesterol. Am. J. Physiol., 209: 433-437.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1962)
Abnormal trace metals in man: chromium. J. chron. Dis., 15:
941-964.
SCHROEDER, H.A., VINTON, W.H., Jr, & BALASSA, J.J. (1963)
Effects of chromium, cadmium, and other trace metals on the
growth and survival of mice. J. Nutr., 80: 39-47.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1964)
Chromium, lead, cadmium, nickel, and titanium in mice: effect
on mortality, tumours, and tissue levels. J. Nutr., 83:
239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1965)
Chromium, cadmium, and lead in rats: effects on life span,
tumours, and tissue levels. J. Nutr., 86: 51-66.
SCHROEDER, H.A., NASON, A.P., & TIPTON, I.H. (1970) Chromium
deficiency as a factor in atherosclerosis. J. chron. Dis., 23:
123-142.
SCHROEDER, K.H. (1955) [Nickel eczema in a blueline (ozalid)
machine operator.] Berufsdermatosen, 3: 170-171 (in German).
SCHUBERT, G.E., GEBHARD, K., & HOENLEIN, F. (1970) [Kidney
damage following injection of serotonin and potassium
dichromate for chronic sodium overloading or restriction.]
Virchows Arch. Abt. A Path. Anat., 351: 68-82 (in German).
SCHULTHEISS, E. (1959) [Rubber and eczema,], Aulendorf i.
Württemberg, Cantor, 213 pp (in German).
SCHWARZ, K. & MERTZ, W. (1959) Chromium (III) and the
glucose tolerance factor. Arch. Biochem. Biophys., 85: 292-295.
SCHWARTZ, L. & DUNN, J.E. (1942) Dermatitis occurring among
operators of air conditioning equipment. Ind. Med. Surg., 11:
375.
SCHWARTZ, L., TULIPAN, L., & PECK, S. (1947) Occupational
diseases of the skin, 2nd ed., Philadelphia, Pennsylvania, Lea
& Febiger.
SEEBER, H., FIKENTSCHER, R., & ROSEBURG, B. (1976)
[Disorders of smell and taste in chromium dye workers.] Z.
Gesamte Hyg. Grenzgeb., 22: 820-822 (in German).
SEELEY, J.L. & SKOGERBOE, R.K. (1974) Combined sampling-
analysis method for the determination of trace elements in
atmospheric particulates. Anal. Chem., 46: 415-421.
SEELING, W., GRUENERT, A., KIENLE, K.H., OPFERKUCH, R., &
SWOBODNIK, M. (1979) [Determination of chromium in human
serum and plasma by flameless atomic absorption spectro-
photometry.] Z. anal. Chem., 299: 368-374 (in German).
SEITZ, W.R., SUYDAM, W.W., & HERCULES, D.M. (1972)
Determination of trace amounts of chromium (III) using
chemiluminescence analysis. Anal. Chem., 44(6): 957-963.
SHACKLETTE, H.T., SAUER, H.I., & MIESCH, A.T. (1970)
Geochemical environments and cardiovascular mortality rates in
Georgia, Washington DC, US Governmental Printing Office, 39 pp
(US Geological Survey Professional Paper No. 574-C).
SHAPCOTT, D., KHOURI, K., DEMERS, P.P., VOBECKY, J., &
VOBECKY, J. (1977) The measurement of volatile chromium in
biological materials. Clin. Biochem, 10: 178-180.
SHEFFET, A., THIND, J., MILLER, A.M., & LOURIA, D.B. (1982)
Cancer mortality in a pigment plant utilizing lead and zinc
chromates. Arch. environ. Health, 37: 44-52.
SHENDRIKAR, A.D. & WEST, P.A. (1974) A study of adsorption
characteristics of traces of chromium (III) and (VI) on
selected surfaces. Anal. Chim. Acta, 72: 91-96.
SHERMAN, L., GLENNON, J.A., BRECK, W.J., KLOMBERG, G.H., &
GORDON, E.S. (1968) Failure of trivalent chromium to improve
hyperglycemia in diabetes mellitus. Metabolism, 17: 439-442.
SHIMKIN, M.M. & LEITER, J. (1940) Induced pulmonary tumours
in mice. III. Role of chromic irritation in the production of
pulmonary tumours in strain A mice. J. Natl Cancer Inst., 1:
241-254.
SHMITOVA, L.A. (1978) In: [The impact of job-related factors
on specific function of the female organism,] Svedlovsk, pp.
105-108 (in Russian).
SHMITOVA, L.A. (1980) Gig. Tr. prof. zabol., 2:
33-35 (in Russian).
SIDI, E. & LONGUEVILLE, R. (1953) Dermites au ciment. Rôle
des sels de chrome. Arch. Mal. prof., 14: 41.
SIEGENTHALER, U., LAINE, A., POLAK, L. (1983) Studies on
contact sensitivity to chromium in the guinea-pig. The role
of valence in the formation of the antigenic determinant. J.
Invest. Dermatol., 80: 44-47.
SIMONOFF, M., LLABADOR, Y. HAMON, C., PEERS; A.M., & SIMONOFF,
G.N. (1984) Low plasma chromium in patients with coronary
artery and heart diseases. Biol. Trace Element Res., 6:
431-439.
SIMONDS, J.P. & HEPLER, O.E. (1945) Experimental nephro-
pathies. I. A method of producing controlled selective injury
of renal units by means of chemical agents. Arch. Path., 39:
103-108.
SIROVER, M.A. & LOEB, L.A. (1976) Infidelity of DNA
synthesis in vitro: screening for potential metal mutagens or
carcinogens. Science, 194: 1434-1436.
SJOGREN, B. (1980) A retrospective cohort study of mortality
among stainless steel welders. Scand. J. Work. environ.
Health, 6: 197-200.
SKOGERBOE, R.K. (1974) Monitoring trace metal particulates:
an evaluation of the sampling and analysis problems. In:
Instrumentation for monitoring air quality, Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp.
125-136.
SLUIS-CREMER, G.K. & DU TOIT, R.S. (1968) Pneumoconiosis in
chromite miners in South Africa. Br. J. ind. Med., 25: 63-67.
SPIER, H.W. (1956) [Wood preservatives and chromate eczema.]
Desinfekt. Gesundheitswes., 4: 58 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil ( Myriophyllum spicatum L.). Arch.
environ. Contam. Toxicol., 2: 331-341.
STAUB, H.W., REUSSNER, G., & THIESSEN, R., Jr (1969) Serum
cholesterol reduction by chromium in hypercholesteremic rats.
Science, 166: 746-747.
STEELE, N.C. & ROSEBROUGH, R.W. (1979) Trivalent chromium
and nicotinic acid supplementation for the turkey poult.
Poultry Sci., 58: 983-984.
STEELE, N.G., ALTHEN, T.G., & FROBISH, L.T. (1977a) Bio-
logical activity of glucose tolerance factor in swine. J.
anim. Sci., 45: 1341-1345.
STEELE, N.C., PELURA, I., & FROBISH, L.T. (1977b) Effect of
dietary protein source on glucose utilization and hepatic
insulin-receptor characteristics of the rat. Nutr. Rep. Int.,
15: 429-435.
STELLA, M., MENTALDI, A., ROSSI, R., ROSSI, G., & LEVIS, A.G.
(1982) Clastogenic effects of chromium on human lymphocytes
in vitro and in vivo. Mutat. Res., 101: 151-164.
STEFFEE, C.H. & BAETJER, A.M. (1965) Histopathologic effects
of chromate chemicals. Report of studies in rabbits, guinea-
pigs, rats, and mice. Arch. environ. Health, 11: 66-75.
STEINHOFF, D., GAD, S.C., HATFIELD, G.K., & MOHR, U. (1983)
Testing sodium dichromate and soluble calcium chromate for
carcinogenicity in rats. Report to the Industrial Health
Foundation, Wuppertal, Bayer AG Institute of Toxicology,
126 pp.
STEREKHOVA, N.P., ZELENEVA, N.I., SOLOMINA, S.N.,
TYUSHNYAKOVA, N.V., MYASNIKOVA, A.G., FOKINA, G.P., & YARINA,
A.L. (1978) [Gastric pathology in workers engaged in the
production of chromium salts.] Gig. Tr. prof. Zabol., 3: 19-23
(in Russian).
STERN, R.M. (1981) Process-dependent risk of delayed health
effects for welders. Environ. Health Perspect., 41: 235-253.
STERN, R.M. (1982) Chromium compounds: production and
occupational exposure. In: Langard, S., ed. Biological and
environmental aspects of chromium, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 5-47.
STRIK, J.J.T.W.A., DE IONGH, H.H., VAN RIJN VAN ALKEMADE,
J.W.A., & WIUTE, T.P. (1975) Toxicity of chromium (VI) in
fish, with special reference to organoweights, liver and
plasma enzyme activities, blood parameters, and histological
alterations. In: Koeman, S.H. & Strik, J.J.T.W.A., ed.
Sublethal effects of toxic chemicals on aquatic animals,
Amsterdam, New York, Oxford, Elsevier Science Publishers,
pp. 31-41.
STROUT, R.W. (1977) The relationship of abnormal circulating
insulin levels to atherosclerosis. Atherosclerosis, 27: 1-13.
SULLIVAN, R.J. (1969) Air pollution aspects of chromium and
its compounds, Raleigh, North Carolina, US Department of
Health, Education and Welfare, 75 pp (National Air Pollution
Control Administration Publication No. APTD 69-34).
SUSA, N., FURUKAWA, YO., & TSUBAKI, S. (1977) Comparative
cytotoxic effects of hexavalent and trivalent chromium
compounds on cultured cells. Jap. J. vet. Sci., 39: 325-336.
SUZUKI, K., ESASHI, T., SUZUE, R., NISHIMURA, H., & MATSUMOTO,
H. (1979) Tissue concentrations of cadmium, lead, and
chromium in Japanese ranging from the fetus to elder subjects.
Toxicol. Lett., 4: 481-484.
SWAINE, D.J. & MITCHELL, R.L. (1960) Trace element
distribution in soil profiles. J. soil Sci., 11 (2): 347-368.
SWENSSON, A. (1977) [Experimental research on the
fibrogenetic effect of chromite.] Arbete och Haelsa, 2: 1-14
(in Swedish).
TABOR, E.C. & WARREN, W.V. (1958) Distribution of certain
metals in the atmosphere of some American cities. Arch. ind.
Hyg., 17: 145-151.
TAMARO, M., BANFI, E., VENTURINI, S., & MONTI-BRAGADIN, C.
(1975) [Hexavalent chromium compounds are mutagens for
bacteria.] In: Proceedings of the XVII Congress Naz. Soc. It.
Microbiol., Padova, pp. 411-415 (in Italian).
TANDON, S.K., SAXENA, D.K., GAUR, J.S., & CHANDRA, S.W.
(1978) Comparative toxicity of trivalent and hexavalent
chromium. Environ. Res., 15: 90-99.
TANDON, S.K., BEHARI, J.R., & KACHRU, D.N. (1979) Distri-
bution of chromium in poisoned rats. Toxicology, 13: 29-34.
TANDON, S.K., MATHUR, A.K., & GAUR, J.S. (1977) Urinary
excretion of chromium and nickel among electroplaters and
pigment industry workers. Int. Arch. occup. environ. Health,
40: 71-76.
TATARU, C., MARINSECU, A., CAPUSAN, I., RIPAN, R., & LITEANU,
C. (1957) [Skin damage during the industrial recovery of
potassium dichromate.] Berufsdermatosen, 5: 283-295 (in
German).
TAYLOR, A.E., GUYTON, M.O., & BISHOP, V.S. (1965)
Permeability of the alveolar membrane to solutes. Circ. Res.,
16: 353-362.
TAYLOR, F.G., Jr, MANN, L.K., DAHLMANN, R.C., & MILLER, F.L.
(1975) Environmental effects of chromium and zinc in
cooling-water drift. In: Cooling tower environment, 1974,
Washington DC, US Energy Research and Development
Administration, pp. 408-426 (Report Conf. No. 740302).
TAYLOR, F.H. (1966) The relationship of mortality and
duration of employment as reflected by a cohort of chromate
workers. Am. J. public Health, 56: 218-229.
TAZELAAR, D.J. (1970) Hypersensitivity to chromium in a
light-blue Tattoo. Dermatologica (Basel), 141: 282-287.
THOMSON, A.G. (1982) Chromite, Mining Annual Review,
pp. 70-71.
THORMANN, J., JESPERSEN, N.B., & JOENSEN, H.D. (1979)
Persistence of contact allergy to chromium. Contact Dermatit.,
5: 261-264.
TOEPFER, E.W., MERTZ, W., ROGINSKI, E.E., & POLANSKY, M.M.
(1973) Chromium in foods in relation to biological activity.
J. agric. food Chem., 21: 69-73.
TOEPFER, E.W., MERTZ, W., POLANSKY, M.M., ROGINSKI, E.E., &
WOLF, W.R. (1977) Preparation of chromium-containing
material of glucose tolerance factor activity from brewer's
yeast extracts and by synthesis. J. agric. food Chem., 25:
162-166.
TOLA, S., KILPIO, J., VIRTAMO, M., & HAAPA, K. (1977)
Urinary chromium as an indicator of the exposure of welders to
chromium. Scand. J. Work environ. Health, 3: 192-202.
TOLG, G. (1974) Recent problems and limitations in the
analytical characterization of high-purity material. Talanta,
21: 327-345.
TSUDA, H. & KATO, K. (1976) Potassium dichromate: induced
chromosome aberrations and its control with sodium sulfite in
hamster embryonic cells in vitro. Gann, 67: 469-470.
TSUDA, H. & KATO, K. (1977) Chromosomal aberrations and
morphological transformation in hamster embryonic cells
treated with potassium dichromate in vitro. Mutat. Res., 46:
87-94.
TUMAN, R.W. & DOISY, R.J. (1977) Metabolic effects of the
glucose tolerance factor (GTF) in normal and genetically
diabetic mice. Diabetes, 26: 820-826.
TUMAN, R.W., BILBO, J.T., & DOISY, R.J. (1978) Comparison
and effects of natural and synthetic glucose tolerance factor
in normal and genetically diabetic mice. Diabetes, 27: 49-56.
TURNER, M.A. & RUST, R.H. (1971) Effects of chromium on
growth and mineral nutrition of soybeans. Soil Sci. Soc. Am.
Proc., 35: 755-758.
TYUSHNYAKOVA, N.V., ZISLIN, D.M., SHABYNINA, N.K., & BELJAEVA,
L.N. (1974) [Criteria of occupational cancer in the
production of chromium salts and some ideas concerning its
pathogenesis.] In: Occupational cancer (collection of
scientific works), Sverdlovsk, pp. 56-63 (in Russian).
UMEDA, M. & NISHIMURA, M. (1979) Inducibility of chromosomal
aberrations by metal compounds in cultured mammalian cells.
Mutat. Res., 67: 221-229.
URCHS, O. (1953) [Occupational skin diseases,] Cantor,
Aulendorf i. Württemberg, 108 pp.
US DHEW (1975) Criteria for a recommended standard.
Occupational exposure to chromium (VI), Washington DC, US
Department of Health, Education and Welfare, 200 pp (US DHEW
Publication No. NIOSH 76-129).
US DHEW (1977) Occupational diseases: a guide to their
recognition, revised ed., Washington DC, US Department of
Health, Education and Welfare, pp. 352-355 (Stock No.
017-033-00266-5).
US EPA (1978) Reviews of the environmental effects of
pollutants. III. Chromium, Washington DC, US Environmental
Protection Agency, 285 pp (ORNL/EIS-80; EPA 600/1-78-023).
US EPA (1980) Ambient water quality criteria for chromium,
Washington DC, US Environmental Protection Agency, pp. A1-C48.
US NAS (1974a) Chromium, Washington DC, US National Academy
of Sciences, 155 pp.
US NAS (1974b) Geochemistry and the environment. I. The
relation of selected trace elements to health and disease,
Washington DC, US National Academy of Sciences, 113 pp.
US NAS/NAE (1972) Water quality criteria 1972, Washington
DC, US Environmental Protection Agency, US National Academy of
Sciences, US National Academy of Engineering, 533 pp.
US PHS (1953) Health of workers in chromate producing
industry, Washington DC, US Department of Health, Education
and Welfare, US Public Health Service, 131 pp (Publication No.
192).
VAKHRUSHEVA, V.A. (1960) [Effect of Mn and Cr as trace
elements on the level of blood sugar in white mice.] Tr.
Izhevskogo Otd. Fiziol. Obschchestva, 1: 184-190 (in Russian).
VALER, M. & RACZ, J. (1971) Investigations concerning the
sensitizing effect of trivalent chromium salts. I. Correlation
between concentration and skin penetration of trivalent
chromium and between the number of positive results obtained
in patch tests. J. ind. Med., 41(12): 302-317.
VEILLON, C., WOLF, W.R., GUTHRIE, B.E. (1979) Determination
of chromium in biological materials by stable isotope
dilution. Anal. Chem., 51: 1022-1024.
VELICHKOVSKY, B.T. & POKROVSKAJA, L.V. (1973) [Labour
hygiene in a modern production of multy-tonnage ferrolloys.]
In: [Proceedings of the National Symposium on Labor Hygiene
and Prophylaxis of Occupational Diseases in the Production of
Ferroalloys,] Sverdlovsk, pp. 3-8 (in Russian).
VENITT, S. & LEVY, L.S. (1974) Mutagenicity of chromates in
bacteria and its relevance to chromate carcinogenesis. Nature
(Lond.), 250: 493-494.
VENCGUJIV, A.J. (1974) Zones of display of biological and
pharmacological action of trace elements. In: Hoekstra, W.G.,
Gantherl, H.E., & Mertz, W., ed. Trace elements metabolism in
animals. II, Baltimore, London, Tokyo, University Park Press,
pp. 295-310.
VERFAILLIE, G.R.M. (1974) Kinetics of chromium absorption by
intact rice plants. In: Comparative studies of food and
environmental contamination, Vienna, International Atomic
Energy Agency, pp. 315-331.
VERSIECK, J.M.J. & SPEECKE, A.B.H. (1972) Contaminations
induced by collection of liver biopsies and human blood. In:
Nuclear Activation Techniques in the Life Sciences.
Proceedings of a Symposium, Bled, Yugoslavia, Vienna
International Atomic Energy Agency, pp. 39-49.
VERSIECK, I., HOSTE, J., BARBIER, F., STEYAERT, H., DERUDDER,
I., & MICHELS, H. (1978) Determination of chromium and
cobalt in human serum by neutron activation analysis. Clin.
Chem., 24: 303-308.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experience of the
occupational health department with some industrial threshold
limit values for industrial poisons.] Arch. Gewerbepath.
Gewerbehyg., 13: 528-534 (in German).
VISEK, W.J., WHITNEY, I.B., KUHN, U.S.G., & COMAR, C.L.
(1953) Metabolism of 51Cr by animals as influenced by
chemical state. Proc. Soc. Exp. Biol. Med., 84: 610-615.
VOELKL, A. (1971) [The daily excretion of chromium in normal
persons.] Zentralbl. Arbeitsmed., 21: 122 (in German).
VOTAVA, H.J., HAHN, C.J., & EVAN, G.W. (1973) Isolation and
partial purification of a 51Cr complex from brewer's yeast.
Biochem. Biophys. Res. Comm., 55: 312-319.
WACKER, W.E.C. & VALLEE, B.L. (1959) Nucleic acids and
metals. I. Chromium mangenese, nickel, iron, and other metals
in ribonucleic acid from diverse biological sources. J. biol.
Chem., 234: 3257-3262.
WAHBA, A. & COHEN, T. (1979) Chrome sensitivity in Israel.
Contact Dermatit., 5: 101-107.
WAHLBERG, J.E. & WENNERSTEN, G. (1977) Light sensitivity and
chromium dermatitis. Br. J. Dermatol., 97: 411-416.
WALSH, E.N. (1953) Chromate hazards in industry. J. Am. Med.
Assoc., 153: 1305-1308.
WARREN, G., SCHULTZ, P., BANCROFT, D., BENNETT, K., ABBOTT,
E.H., & ROGERS, S. (1981) Mutagenicity of a series of
hexacoordinate chromium (III) compounds. Mutat. Res., 90:
111-118.
WATANABE, S. & FUKUCHI, Y. (1975) An epidemiological survey
on lung cancer in workers of a chromate-producing industry in
Hokkaido, Japan (Presented at: The XVIII International Congress
on Occupational Health, Brighton, England, 14-19 September.
WEILER, R.R. & CHAWLA, V.K. (1969) Dissolved mineral and
quality of Great Lakes waters. In: Proceedings of the 12th
Conference on Great Lakes Res., 801.
WELZ, B. (1980) [Use of AAS and ICP. Relative advantage of
each method.] Chem. Techn., 9: 161-170. (in German).
WELZ, B. (1983) [Atomic absorption spectrometry,] 3rd ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
527 pp (in German).
WENDELBERGER, J. & WEITGASSER, H. (1956) [The role of alkali
and degreasing agents in the development of contact eczemas in
electroplasters.] Berufsdermatosen, 4: 16-21 (in German).
WHANGER, P.D. & WESWIG, P.H. (1975) Effects of selenium,
chromium, antioxidants on growth, eye cataracts, plasma
cholesterol, and blood glucose in selenium-deficient, vitamin
E-supplemented rats. Nutr. Rep. Int., 12: 345-348.
WHITE, L.R., JAKOBSEN, K., & 0STGAAD, K. (1979) Comparative
toxicity studies of chromium rich welding fumes and chromium
on an established human cell line. Environ. Res., 20: 366-374.
WHITING, R.F., STICH, H.F., & KOROPATNICK, D.J. (1979) DNA
damage and DNA repair in cultured human cells exposed to
chromate. Chem.-biol. Interact., 26: 267-280.
WHO (1983) EHC 27: Guidelines on studies in environmental
e pidemiology, Geneva, World Health Organization, 351 pp.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984a)
Disposition of intratracheally administered chromium (III) and
chromium (VI) in rabbits. Toxicol. Lett., 22: 273-276.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984b) The
reduction of chromium (VI) to chromium (III) by glutathione:
an intracellular redox pathway in the metabolism of the
carcinogen chromate. Toxicology, 33: 341-348.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1985)
[Determination of chromium in human red blood cells. Basis for
a concept of biological monitoring.] Arbeitsmed. Sozialmed.
Praeventivmed., 20: 1-4 (in German).
WILD, D. (1978) Cytogenetic effects in the mouse of 17
chemical mutagens and carcinogens evaluated by the micronuclei
test. Mutat. Res., 56: 319-327.
WILLIAMS, C.D. (1969) Asthma related to chromium compounds.
Report of two cases and review of the literature on chromate
diseases. NC med. J., 30: 482-491.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1960) The Merck index. An encyclopedia of chemicals and
drugs, 7th ed., Rahway, New Jersey, Merck and Co., 139 pp.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) The Merck index. An encyclopedia of chemicals and
drugs, 9th ed., Rahway, New Jersey, Merck and Co., 1313 pp.
WINSTON, J.R. & WALSH, E.N. (1951) Chromate dermatitis in
railroad employees working with diesel locomotives. J. Am.
Med. Assoc., 147: 1133-1134.
WITHEY, J. (1983) Memorandum addressed to Si Duk Lee, dated
March 4, 1983, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office.
WOLF, W.R. (1976) Coupled gas chromatography/atomic
absorption spectrometry for the nanogram determination of
chromium. Anal. Chem., 48: 1717-1720.
WOLF, W.R. & GREENE, F.E. (1976) Preparation of biological
materials for chromium analysis. In: Accuracy in trace
analysis: sampling, sample handling, and analysis,
Gaithersburg, Maryland, National Bureau of Standards, pp.
605-610 (Special Publication No. 422).
WOLF, W.R., MERTZ, W., & MASIRONI, R. (1974) Determination
of chromium in refined and unrefined sugars by oxygen plasma
ashing flameless atomic absorption. J. agric. food Chem., 22:
1037-1042.
WOLF, W.R., TAYLOR, M.L., HUGHES, B.M., TIERNAN, T.O., &
SIEVERS, R.E. (1972) Determination of chromium and beryllium
at the picogram level by gas chromatography/mass spectrometry.
Anal. Chem., 44: 616-618.
WU, G.-Y. (1981) [The research on the purification and the
chemical structure of the specific chromium binding
materials.] Jpn. J. Ind. Health, 33: 505 (in Chinese).
YAGI, T. & NISHIOKA, H. (1977) DNA damage and its degrada-
tion by metal compounds. Sci. Eng. Rev. Doshisha Univ., 18:
63-70.
YANG, G.L. (1956) [An investigation of occupational disease
among chromium plating workers.] Acta Acad. Med. primae
Shanghai, 2: 36-46 (in Chinese).
YOPP, J.H., SCHMID, W.E., & HOLST, R.W. (1974) Determination
of maximum permissible levels of selected chemicals that exert
toxic effects on plants of economic importance in Illinois,
Chicago, Illinois, Institute for Environmental Quality,
pp. 89-96.
YUNISOVA, H.K. & PAVLOVSKAJA, G.S. (1975) The effect of
regimen of chrome plating on the properties of chromine.
Protect. Metal., 2, 248-250.
ZANDER, A.T., O'HAVER, T.C., & KELIHER, P.N. (1977)
Correction for interferences of spectral origin with continuum
source, echelle wavelength modulated atomic absorption
spectrometry. Anal. Chem., 49: 838-842.
ZHURKOV, U.S. (1981) [Approaches to the regulation of
environmental chemical pollutants with mutagenic activity.]
In: Sidorenko, G.I.M., ed. [Medical problems of environmental
protection,] Moscow, USSR Academy of Medical Sciences,
pp. 88-95 (in Russian).
ZOBER, A. (1979) [On the problems of evaluating bronchial
carcinoma after exposition to chromium compounds.] Int. Arch.
occup. environ. Health, 43(2): 107-121 (in German).
ZOBER, A. (1982) [Possible dangers to the respiratory tract
from welding fumer: methods of approach in an industrial
health care context and results.] Schweissen & Schneiden,
34(2): 77-81 (in German).
ZOBER, A., KICK, K., SCHALLER, K.H., SCHELLMANN, B., &
VALENTIN, H. (1984) ["Normal values" of chromium and nickel
in human lung-, kidney-, blood-, and urine samples.] Zbl.
Bakt. Hyg. I. Abt. Orig. B, 179: 80-95 (in German).
Tuman & Doisy (1977) studied the effects of yeast concentrates
of high chromium (glucose tolerance factor) content and of
synthetic chromium complexes with GTF activity (Tuman et al., 1978)
in mice, raised on a presumably complete commercial stock diet.
Six animals were used for each test, either genetically diabetic
mice or their control litter mates of the C57Bl-KSI strain.
Injections of either 5 mg of the GTF-containing yeast extracts or
0.1 mg of the synthetic chromium complex, acutely reduced the
elevated plasma-glucose levels in the diabetic and the non-fasting
normal mice by 10 - 38% of the initial values ( P < 0.01) and the
plasma-triglycerides by 26 - 56% ( P < 0.01), compared with control
mice injected with saline. Injection of insulin into diabetic mice
produced only an 11 - 18% decrease in plasma-glucose levels,
whereas injection of the GTF-containing extract together with
insulin reduced plasma-glucose levels by 39 - 51% and plasma-
triglyceride levels by 76% (Table 11).
The results suggest either a much higher increase in the
chromium requirement of the genetically diabetic mice or their
inability to use chromium in the diet.
Table 11. Acute effects of GTF and exogenous insulin on non-
fasting plasma-glucose and plasma-triglyceride (TG) concentrations
in 19-week-old genetically diabetic micea
------------------------------------------------------------------
Treatment Plasma- deltaGlucose Plasma- deltaTG
glucoseb triglyceridesb
(mg/litre) (mg/litre)
------------------------------------------------------------------
saline 11 120 ± 3960 ± 160(5)c
490(5)c
GTF 9320 ± -180 (16%) 2790 ± 170(5)d -117 (30%)
280(5)d
insulin 9840 ± -128 (12%) 3020 ± 400(5) - 94 (24%)
410(5)
insulin 7060 ± -406 (37%) 940 ± 220(5)e -302 (76%)
and GTF 840(5)e
------------------------------------------------------------------
a Modified from: Tuman & Doisy (1977).
b Glucose and triglyceride values represent mean ± SEM for 6 mice in
each treatment group. Dose of GTF was 5 mg (WL-10-AT) administered
intraperitoneally, 12 h prior to collection of blood. Lente insulin
(0.1 U per mouse) was administered subcutaneously, 12 h prior to
collection of blood. Data were treated by analysis of variance to
detect differences between the various treatment groups; independent
orthogonal comparisons were performed in the following groups ( P value
indicates level of significance for each comparison).
c Saline versus all other treatments, P < 0.005.
d GTF versus insulin, P < 0.05.
e GTF and insulin alone versus combined GTF and insulin, P < 0.005.
Thus, GTF and insulin > GTF = insulin > saline.
Steele & Rosebrough (1979) reported a significant stimulation
of the growth rate of one-week-old turkey poults (both sexes) by
supplementation of a practical ration with 20 mg chromium (as
chloride)/kg. The weight gains within the 2-week study were 235 g
and 267 g for the 60 controls and 60 supplemented turkeys,
respectively ( P < 0.001). As the practical ration contained
ground yellow corn and soybean meal, limestone, and dicalcium
phosphate, chromium deficiency would appear unlikely. The amount
of the chromium supplement (20 mg/kg diet) is quite high, and
further studies are needed to decide whether the observed effects
were of a pharmaco-dynamic nature or truly nutritional, i.e.,
correcting a deficiency. A similar interpretation should be
applied to a report of improved egg quality in the laying hen
(Jensen et al., 1978).
The quantitative aspects of the effects of chromium on animals
can be summarized as follows: normal rats fed semi-purified, semi-
synthetic rations, with Torula yeast or individual proteins and
sucrose or starch as the source of carbohydrates, develop mild
signs of deficiency at a dietary level of 100 - 150 µg chromium/kg.
To prevent deficiency, most authors used very high supplements of
several mg/kg diet and did not determine the biological
availability of the chromium complexes used for the
supplementation. Diets containing raw ingredients and supplying
chromium levels between 0.5 and 1 mg/kg do not induce signs of
deficiency and probably meet the requirement of the rat. A very
tentative estimate of the dietary chromium need of the rat and
probably the mouse would be approximately 0.5 mg/kg diet. This
estimate should be interpreted with caution, because of the lack of
knowledge concerning the biological availability of chromium and of
its interaction with dietary constituents.
7.2. Toxicity Studies
The toxicology of chromium compounds has been reviewed by the
US National Academy of Science (US NAS, 1974a), Langard & Norseth
(1979), the International Agency for Research on Cancer (IARC,
1980), Langard (1980a, 1982), and Burrows (1983).
In discussing toxicological problems, it is important to
differentiate between the various oxidation states of chromium and
its compounds. Trivalent chromium, when administered to animals in
food or water, does not appear to induce any harmful effects, even
when given in large doses (US NAS, 1974a) (section 7.2.1). Acute
and chronic toxic effects of chromium are mainly caused by
hexavalent compounds. Since it has been shown that both industrial
trivalent chromium compounds as well as reagent-grade trivalent
chromium compounds can be contaminated by hexavalent chromium
(Petrilli & DeFlora, 1978a; Levis & Majone, 1979), the evaluation
of experimental studies becomes difficult, especially when the
purity of the chemical compounds used is not known.
Discrimination between the biological effects, caused by
hexavalent chromium and trivalent chromium is difficult, because,
after penetration of membranes in tissues, hexavalent chromium is
immediately reduced to trivalent chromium (Gray & Sterling, 1950;
US NAS, 1974a), and it is not evident whether the observed
phenomena are caused by this reduction or even by the trapping of
trivalent chromium by ligands after uptake in the cells. Another
problem in evaluating the data is associated with the route of
administration. Hexavalent chromium, introduced by the oral route,
is partly reduced to trivalent chromium by acidic gastric juice
(Donaldson & Barreras, 1966; DeFlora & Boido, 1980); thus, the
effects or lack of effects observed may be caused mainly by
trivalent chromium and not by the hexavalent chromium, actually
administered.
7.2.1. Effects on experimental animals
Many local effects on human beings have been reported (section
8.3), but only a few studies have verified these effects in
experimental animals. A comprehensive survey of hexavalent
chromium-induced effects is given in Table 12 (US NAS, 1974a). For
most studies, details were not given of the length of exposure,
number of treated animals and controls, etc. Diagnoses were stated
without presenting all the original data. Thus, in this section,
some papers will be discussed that refer to the most prominent
local and systemic effects to support and clarify the effects shown
in human beings.
It is evident that the toxicity of hexavalent chromium in
animals varies with the route of entry into the body. Low
concentrations of hexavalent chromium may be tolerated, when
administered in the feed or drinking-water, the extent of
absorption being a factor of importance. For example, rats
tolerated hexavalent chromium in drinking-water at 25 mg/litre, for
1 year, and dogs did not show any effects from chromium
administered as potassium chromate at 0.45 - 11.2 mg/litre over a
4-year period (US NAS, 1974a). However, oral exposure of both male
and female rabbits to sodium dichromate (0.1% solution, 0.2 - 5
mg/kg body weight, for up to 545 days) resulted in significant
morphological changes in the gonads, including atrophy of the
epithelium and dystrophic alterations of the Sertoli and Leydig
cells in the testes, and sclerotic and atrophic changes in ovaries
(Kucher, 1966).
Larger doses of hexavalent chromium are highly toxic and may
cause death, especially when injected iv, subcutaneously (sc), or
intragastrically. The LD50 of chromium compounds was determined
for several experimental animal species. The LD50 of potassium
dichromate (hexavalent chromium), administered orally (stomach
tube) to rats, was 177 mg/kg body weight in males and 149 mg/kg
body weight in females (Hertel, 1982). When injected iv in mice
(sex not given), the LD50 of chromium carbonyl was 30 mg/kg body
weight (IARC, 1980).
Performing a life-time inhalation study on the rat, Glaser et
al. (1984) found an LC50 for Na2Cr2O7 of 28.1 mg/m3 (range, 16.7 -
47.3 mg/m3). Assuming a deposition rate in the lung of 30% of the
dose administered, the LC50 dose was 1 mg/kg body weight in male
and 1.2 mg/kg in female rats.
Table 12. Effects of hexavalent chromium in animalsa
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit, inhalation chromates 1 - 50 mg/m3 14 h/day for pathological Lukanin (1930)
cat 1 - 8 months changes
in the lungs
Rabbit inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day none Lehmann (1914)
as dichromate for 5 days
Cat inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day bronchitis, Lehmann (1914)
as dichromate for 5 days pneumonia
perforation of
nasal septum
Mouse inhalation mixed dust 1.5 mg/m3 4 h/day, no tumours Baetjer et al.
containing as CrO3 5 days/week, (1959a);
chromates for 1 year Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 16 - 27 mg/m3 1/2 h/day tumours in Baetjer et al.
containing as CrO3 intermit- some strains (1959a);
chromates tently Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 7 mg/m3 37 h over increased Baetjer et al.
containing as CrO3 10 days tumour rate (1959a);
chromates Steffee &
Baetjer (1965)
Rat inhalation mixed dust 7 mg/m3 37 h over barely Baetjer et al.
containing as CrO3 10 days toleratedb (1959a);
chromates Steffee &
Baetjer (1965)
Rabbit, inhalation mixed dust 5 mg/m3 4 h/day, none marked Baetjer et al.
guinea- containing as CrO3 5 days/week, (1959a);
pig chromates for 1 year Steffee &
Baetjer (1965)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rat, inhalation hexacarbonyl 1.6 mg/m3 4 months, anaemia; lipid Roschina (1976)
rabbit 4 h, 5 days and/or protein
a week dystrophia
in liver
and kidneys
Rat, inhalation hexacarbonyl 0.16 mg/m3 4 months, anaemia; no Roschina (1976)
rabbit 4 h, 5 days biochemical
a week or morphological
effects
Rat inhalation hexacarbonyl 35 mg/m3 30 min 100% death Roschina (1976)
Rat inhalation dichromates 0.006 - 0.2 28 days/ increase in Glaser et al.
mg/m3 90 days lung-macrophages (1985)
23 h/day
7 days/week lymphocytes
immunoglobulin,
reduced Fe2O3
lung clearance
Rat intratra- dichromates 5 per week up to 30 toleratedc Steinhoff
cheal in- 0.01 - 0.25 months et al. (1983)
stillation mg/kg
Rat intratra- dichromates 1 per week up to 30 tolerated; Steinhoff
cheal in- 0.05 - 1.25 months 1.25 mg/kg et al. (1983)
stillation mg/kg harmful
Rat oral potassium 500 mg/litre daily maximal Gross & Heller
chromate in non-toxic (1946)
drinking-water concentration
Rat, oral zinc chromate 10 g/kg daily maximal Gross & Heller
mouse in feed non-toxic (1946)
(mature) concentration
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit oral sodium di- 0.2 - 5.0 mg/kg 545 days morphological Kucher (1966)
chromate changes in
gonads (testes:
atrophy of
epithelium,
dystrophic
alterations of
Sertoli &
Leydiz alls.
Ovaries:
sclerotic and
atrophic
changes)
Rat oral zinc chromate 1.2 g/kg daily maximal Gross & Heller
(young) in feed non-toxic (1946)
concentration
Rat oral potassium 1.2 g/kg daily maximal Gross & Heller
(young) chromate non-toxic (1946)
in feed concentration
Dog, oral monochromate 1.9 - 5.5 mg 29 - 685 none harmful Lehmann (1914)
cat, or dichromates chromium/kg days
rabbit body weight per
day 1 mg chrom-
ium equivalent
to 2.83 mg
K2Cr2O7
or 3.8 mg
K2CrO4
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog oral potassium 1 - 2 g as daily fatal in 3 Brard (1935)
dichromate chromium months anaemia
Dog stomach potassium 1 - 10 g as - rapidly fatald Brard (1935)
tube dichromate chromium
Monkey subcutan- potassium 0.02 - 0.7 g in - fatald Hunter & Roberts
eous dichromate 2% solution (1933)
Dog subcutan- potassium 210 mg as - rapidly fatal Brard (1935)
eous dichromate chromium
Guinea- subcutan- potassium 10 mg - lethald Ophüls (1911a)
pig eous dichromate Ophüls (1911b)
Rabbit subcutan- potassium 1.5 cc of 1% - 80% fatald Hasegawa (1938)
eous dichromate solution/kg body
weight
Rabbit subcutan- potassium 20 mg - lethald Ohta (1940)
eous dichromate
Rabbit subcutan- potassium 0.5 - 1 cc of - nephritisd Ohta (1940)
eous dichromate 0.5% solution/kg
body weight
Rabbit, subcutan- sodium 0.1 - 0.3 g as - rapid deathd Priestley
guinea- eous or chromate CrO3 fall in blood (1877)
pig intravenous pressure
Rabbit intra- potassium 0.7 cc of 2% - lethald Mazgon (1932)
venous solution/kg 8-10 days after
body weight injection
Dog intra- potassium 10 grains - instant death Gmelin (1826)
venous chromate
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog intra- potassium 1 grain - none marked Gmelin (1826)
venous chromate
Dog intra- potassium 210 mg as - rapidly fatal Brard (1935)
venous dichromate chromium
Dog intra- potassium 3 mg/100 cc 2 doses marked renal Hepler & Simonds
venous dichromate blood per dose damage (1946); Simonds
& Hepler (1945)
---------------------------------------------------------------------------------------------------------
a Modifed from: US NAS (1974a).
b Pathological changes in experimental and control rats, 101 weeks after exposure.
c The same weekly dose distributed over 5 days was clearly better tolerated than a single weekly
administration.
d Renal damage.
A local corrosive action of hexavalent chromium on the skin,
similar to that seen in man, was described by Samitz & Epstein
(1962), who induced chrome ulcers in guinea-pigs at 4 trauma sites,
with daily exposure to 0.34 MK2Cr2O7 solution for 3 days. Mosinger
& Fiorentini (1954) showed the same effects using potassium
chromates.
Following parenteral administration, the most common systemic
effects of chromium were parenchymatous changes in the liver and
kidney (Mosinger & Fiorentini, 1954). Later studies showed
selective damage in the renal proximal convoluted tubules, without
evidence of glomerular damage, as demonstrated after one single sc
injection of potassium dichromate of 10 mg/kg body weight (Schubert
et al., 1970) or after one single intraperitoneal (ip) injection of
sodium chromate of 10 or 20 mg/kg body weight (Evan & Dail, 1974).
Effects have also been found in fish (Strik et al., 1975). After 32
days of continuous exposure to 0.1, 1, or 10 mg hexavalent chromium
(as potassium dichromate)/litre, the fish Rutilus rutilus developed
lysis of the intestinal epithelium with haemorrhages as well as
hypertrophy and hyperplasia of the gill epithelium.
Franchini et al. (1978) found an increase in urinary protein,
lysozyme, glucose, and beta-glucuronidase in rats after a single sc
injection of potassium dichromate at 15 mg/kg body weight. After
sc injection (3 mg/kg body weight), every other day for 2 - 8
weeks, the authors observed a correlation between the chromium
contents of the renal cortex and chromium clearance.
Five-week-old male Wistar rats of the strain TNO-W-74 were
continuously exposed in inhalation chambers to submicron aerosols
of sodium dichromate at concentrations ranging from 25 (low level)
to 200 µg chromium/m3 (high level) (Glaser et al., 1985). Exposure
for 28 days to 25 or 50 µg chromium/m3 resulted in "activated"
alveolar macrophages with stimulated phagocytic activity, and
significantly elevated antibody responses to injected sheep red
blood cells. After 90 days of low-level exposure, there was a more
pronounced effect on the activation of the alveolar macrophages,
with increased phagocytic activity. However, inhibited phagocytic
function of the alveolar macrophages was seen at the high
hexavalent chromium exposure level (200 µg/m3). In rats exposed to
this chromium aerosol concentration for 42 days, the lung clearance
of inert iron oxide was significantly reduced. The humoral immune
system was still stimulated at a low chromium aerosol concentration
of 100 µg/m3, but significantly depressed at 200 µg chromium/m3.
Exposure of rats, through inhalation, to chromium carbide or
chromium boride dust at very high levels (300 - 350 mg/m3 for each
substance) for 3 months (2 h/day) resulted in effects on the
vascular system of the lungs, e.g., endothelial hyperplasia.
Bronchitis and a decrease in the blood-haemoglobin concentration
were also observed (Roschina, 1964). The effects of chromium boride
were more pronounced than those of chromium carbide.
Steinhoff et al. (1983) performed an intracheal injection study
on rats (930 rats, 30 months, 0.05 - 1.25 mg Na2Cr2O7/kg body
weight per week; 1.25 mg CaCrO4/kg body weight per week). Half of
the rats were intratracheally injected once a week and the other
half received the same weekly dose distributed over 5 injections
per week. In rats receiving doses 5 times a week, there were some
significant changes in levels of total plasma-protein and
-cholesterol, in some haematological variables, in organ weights,
and in survival times in females. Only male rats receiving 1.25 mg
sodium dichromate/kg body weight, once a week, showed a sharp
reduction in body weight, female rats being less affected. Rats
receiving calcium chromate in the same dose showed reduced body
weight but to a lesser extent. The weight of lung and trachea was
increased by both substances in all doses.
Marked histopathological changes (congestion, fairly large
areas of focal necrosis, bile duct proliferation) described after
long-term exposure of rabbits to hexavalent chromium (ip injection
of 2 mg chromium/kg body weight per day for 6 weeks) (Tandon et
al., 1978), as well as the increase in hepatic metallothionein and
decrease in cytochrome P-450 levels after ip injection of 400 µmol
chromium/kg per day (type of chromium compound not mentioned)
(Eaton et al., 1980) need further confirmation. The finding of an
accumulation of hexavalent chromium in the reticuloendothelial
system including bone marrow (Baetjer et al., 1959b; Langard, 1977)
may be of importance for a disturbed blood picture.
Merkurieva et al. (1980a,b) studied the effects of potassium
dichromate in the drinking-water on the activities of different
enzymes in rats. Exposure included daily doses of 0.0005, 0.005,
0.05, or 0.5 mg/kg body weight for up to 6 months. After 20 days
of exposure at the highest dose level, enzyme activities increased
by 15 - 28% in liver microsomes (inosine-5-diphosphatase),
lysosomes (beta-D-galactosidase), and cytosol (lactate dehydrogenase
(EC 1.1.1.27)). For some of these enzymes, as well as for
acetylesterase (EC 3.1.1.6), increases in activity of up to 54%
were found in the gonads, kidneys, seminal fluid, and serum. At a
dose of 0.05 mg/kg, the only statistically significant finding was
a 54% increase in the activity of acetylesterase in the gonads.
After a 6-month exposure at this dose level, there was a 70%
increase in lactate dehydrogenase activity in the seminal fluid as
well as a 50% increase in free beta-galactosidase in the liver. There
were no changes in enzyme activities at the two lowest dose levels.
Painting of rat skin with an aqueous solution of potassium
dichromate (0.5%), daily, for 20 days, resulted in a local
inflammatory reaction, an increased level of hexose glyco-proteins
in the skin and serum, and an elevated concentration of serotonin
in the skin and liver (Merkurieva et al., 1982). Most of these
effects were also seen earlier in the exposure period, though they
were not as pronounced. Ten days after the start of exposure, a
nearly 3-fold increase in the serum-acetylcholine concentration
occurred together with decreased acetylcholinesterase activity.
Thus, the data of Merkurieva et al. show systemic effects following
both oral and dermal exposure to hexavalent chromium.
Cats fed chromic phosphate or oxydicarbonate at 50 - 1000 mg/day
for 80 days did not exhibit signs of illness or tissue damage.
Similarly, toxic reactions were not observed in rats administered
drinking-water containing 25 mg trivalent chromium/litre, for 1
year, or 5 mg trivalent chromium/litre throughout their lifetime
(US NAS, 1974a). The toxicity of trivalent chromium is so low that
even by parenteral administration, a chromic acetate level of 2.29
g/kg body weight or a chromic chloride level of 0.8 g/kg body
weight is required to kill mice. Even very large doses given
intragastrically were not fatal for dogs. Brard (1935) reported
that 10 or 15 g of chromium as chromic chloride proved fatal in one
dog (US NAS, 1974a). Some fatal doses of trivalent chromium
compounds reported in the literature are listed in Table 13.
Rats exposed through inhalation to chromic oxide (trivalent
chromium) at 42 mg/m3 or to chromic phosphate at 43 mg/m3 (5 h/day
for 5 days/week) for 4 months developed chronic irritation of the
bronchus and lung parenchyma, and dystrophic changes in the liver
and kidney (Blokin & Trop, 1977).
Inhalation exposure of rats (number not given) to dusts
containing 36 or 50% chromite for 4 months (2 h/day), at
concentrations of 375 - 400 mg/m3, resulted in thickening of the
walls of pulmonary vessels and bronchi (Roschina, 1959). The high
exposure levels in these studies make it difficult to evaluate the
effects.
Inhalation studies have also been performed with chromium
carbonyl, where chromium is in the 0 oxidation state (Roschina,
1976). Twelve rabbits and 48 rats were exposed for 4 months (4
h/day, 6 days/week) at a concentration of 1.6 or 0.16 mg/m3. At
both exposure levels, there was loss of body weight (25 and 12%,
respectively) as well as anaemia and leukocytosis. In the higher
exposure group, the animals showed an elevated gamma-globulin level
in serum and an increased transaminase activity. The contents of
cholesterol and SH-groups were reduced, and there was a decrease in
cholinesterase (EC 3.1.1.7) activity. Lipid and/or protein
dystrophy were noted in several organs, e.g., in the liver and
kidneys. No such effects were detected in the animals in the low-
exposure group.
Table 13. Fatal doses of trivalent chromium in animalsa
---------------------------------------------------------------------------------------------
Animal Number of Routeb Compound Chromium Effect Reference
animals dose (g/kg)
---------------------------------------------------------------------------------------------
Dog 2 sc chromic chloride 0.8 fatal Brard (1935)
Rabbit 1 sc chromic chloride 0.52 fatal Brard (1935)
Rat 38 iv chrome alum 0.01 - 0.018 LD50 Mertz et al.
chromium-hexaurea (1965a)
chloride
Mouse -c iv chromic chloride 0.8 MLDd Windholz et al. (1960)
Mouse -c iv chromic acetate 2.29 MLD Windholz et al. (1960)
Mouse -c iv chromic chloride 0.4 MLD Schroeder (1970)
Mouse -c iv trivalent chromium ? 0.25 - 2.3 MLD Windholz et al. (1960)
Mouse -c iv chromic sulfate 0.247 MLD Windholz et al. (1976)
Mouse -c iv chromic sulfate 0.085 MLD Schroeder (1970)
Mouse -c iv chromium carbonyl 0.03 LD50 Schroeder (1970)
---------------------------------------------------------------------------------------------
a Modified from: US NAS (1974a).
b sc = subcutaneous; iv = intravenous.
c No figures given.
d Minimum lethal dose.
7.2.1.1. Carcinogenicity
Various types of chromium chemicals, methods of administration,
and species of animals have been studied (IARC, 1980a).
Ideally, carcinogenicity should be tested with the methods
recommended by IARC (1980b), but, in many of the early studies,
this was not done. Carcinomas of the lung have been reported in
animals as a result of the administration of chromium chemicals.
Hueper (1958) found 2 squamous cell carcinomas and one
carcinosarcoma in 25 rats following intra-pleural injection of
chromite ore roast. After intrapleural implantation of strontium
chromate lasting 27 months, Hueper (1961) found tumours (type
unspecified) in 17/28 rats. Laskin et al. (1970) and Levy & Venitt
(1975) produced a number of bronchogenic carcinomas by implanting
pellets of cholesterol mixed with various chromium compounds
encased in a wire mesh cage in the bronchi of rats. Calcium and
zinc potassium chromate produced a number of bronchogenic
carcinomas, but soluble chromates and trivalent chromium chemicals
failed to produce cancer.
Using the same technique, Levy & Martin (1983) tested 21
different chromium-containing materials (pigments, intermediates,
and residues from the bichromate-producing industry, relatively
pure crystalline compounds) in 2250 random-bred rats and found that
chromates, described as sparingly soluble, were carcinogenic in the
rat lung. These materials included strontium and calcium chromate
and, to a far lesser extent, certain forms of zinc chromate.
Barium and lead chromate evoked only a very weak carcinogenic
response compared with strontium and calcium chromate. In the
study of Laskin et al. (1970), it was shown that chromium trioxide
produced hepatocellular carcinomas in 2/100 rats (controls, 0/24).
After inhalation of 13 mg calcium chromate/m3 (5 h/day, 5
days/week, for lifetime), Nettesheim et al. (1971) found 14 lung
adenomas in 136 treated mice and 5 in 136 untreated controls, but
no carcinomas. Steffee & Baetjer (1965), performing inhalation
studies on rats, mice, guinea-pigs, and rabbits (inhalation of
mixed chromate dust, corresponding to 3 - 4 mg CrO3/m3, 4 - 5
h/day, 4 days/week, for lifetime, or 50 months, respectively) could
only find 3 alveologenic adenomas in 50 treated guinea-pigs.
Laskin (1972) and Laskin et al. (1970) found 1 squamous cell
carcinoma of the lung, 1 of the larynx and 1 "peritruncal tumor" in
rats (inhalation of calcium chromate, 2 mg/m3, 589 exposures of 5 h
over 891 days) and 1 squamous cell carcinoma and 1 papilloma of the
larynx in hamsters. The number of treated animals was not
specified in either paper. Steinhoff et al. (1983) performed
intratracheal instillations of chromates in rats for 30 months with
one treatment/week and the same weekly dose distributed over 5
treatments/week (Table 14). In 880 exposed rats, 28 adenomas of
alveolar-bronchiolar origin (benign) and 12 malignant tumours (3
adenocarcinomas and 9 squamous cell carcinomas) were found. All
lung tumours developed very late and were only detected at the end
of the lifetime study, often in lungs with callosities. The
tumours were tiny and none of them caused the animal to die.
Sodium dichromate was not carcinogenic after exposure on 5
days/week. With calcium chromate, the carcinogenic effect was more
pronounced after treatment once per week, than after treatment 5
times per week.
Table 14. Incidence of benign and malignant lung tumours among
880 rats intracheally injected with Na dichromate and Ca chromatea
----------------------------------------------------------------
Dose Number of Incidence of lung tumours
(mg/kg) injections benign malignant
per week
----------------------------------------------------------------
Na2Cr2O7 x 2H20 0.25 5 none none
Na2Cr2O7 x 2H20 1.25 1 12 8
CaCrO4 0.25 5 5 1
CaCrO4 1.25 1 11 3
----------------------------------------------------------------
a From: Steinhoff et al. (1983).
Hueper (1961) reported unspecified tumours at implantation
sites in 12/34 rats, following intramuscular (im) implantation of
sintered calcium chromate (25 mg). No tumours were observed in 32
control animals. The same author found implantation-site tumours in
15/33 animals treated with strontium chromate compared with no
tumours in 32 control animals. Single sc injections of 30 mg lead
chromate (Maltoni 1974, 1976) resulted in injection-site sarcomas
in 26/40 treated rats. After administration of the same amount of
lead chromate oxide, injection-site sarcomas were found in 27/40
rats; no sarcomas were found in 60 vehicle controls. Following 9
im injections of 8 mg lead chromate, Furst et al. (1976) found
injection-site sarcomas in 31/47 treated rats, and renal carcinomas
in 3/24 treated rats; no sarcomas were found in 0/22 vehicle
controls. Heath et al. (1971) found injection-site sarcomas and
other tumours in 7/74 treated rats after im injection of 28 mg
cobalt-chromium alloy.
Administration of low levels of trivalent chromium acetate (5
mg/litre in the drinking-water) to mice and rats for the lifetime
did not result in increased tumour incidences compared with
controls (Schroeder et al., 1964, 1965).
Trivalent chromium oxide incorporated in bread at
concentrations of 10, 20, or 50 g/kg and fed to BD rats did not
increase tumour incidence compared with controls (Ivankovic &
Preussmann, 1975). Hexavalent chromium compounds have not been
tested by oral administration.
IARC (1980a) summarized the data of all available references
and concluded as follows: "There is sufficient evidence for the
carcinogenicity of calcium chromate and some relatively insoluble
chromium (VI) compounds (sintered calcium chromate, lead chromate,
strontium chromate, sintered chromium trioxide and zinc chromate)
in rats. There is limited evidence for the carcinogenicity of lead
chromate (VI) oxide and cobalt-chromium alloy in rats. The data
were inadequate for the evaluation of the carcinogenicity of other
chromium (VI) compounds and of chromium (III) compounds".
(a) Interaction with other factors
The possibility that chromium may act synergistically with
other agents in the production of cancer has been tested in several
studies. Nettesheim et al. (1970) pretreated one group of mice
with 100 R whole-body radiation and infected another group with PR8
influenza virus. These mice and control mice were exposed through
inhalation to chromium oxide dust for 6 - 18 months. No measurable
effects of either of the pretreatments were found on the incidence
of tumours. In a similar study with inhalation of calcium chromate
(CaCrO4, 5 h/day, 5 days per week for the lifetime), pre-exposure
to X-rays did not affect the tumour rate, but PR8 influenza
infection reduced tumour incidence (Nettesheim et al., 1971).
Steffee & Baetjer (1965) also found that the infection of rats with
PR8 influenza virus associated with intratracheal injection of
chromates did not lead to the production of tumours. Chromite ore
injected iv did not affect the development of tumours induced by iv
injection of 20-methyl-cholanthrene (Shimkin & Leiter, 1940).
Thus, at present, there are not sufficient data to suggest that
chromium acts synergistically with virus infections, ionizing
radiation, or other chemical carcinogens to produce cancers.
7.2.1.2. Genotoxicity
The mutagenicity of chromium compounds was reviewed by IARC
(1980a), Petrilli & DeFlora (1980), Levis & Bianchi (1982), and
Baker (1984). Present knowledge, in the light of recent findings,
is summarized in Table 15 (hexavalent chromium) and Table 16
(trivalent chromium).
When evaluating the results of chromium mutagenicity tests, it
is necessary to take into consideration several properties of the
tested compound (oxidation state, solubility, ability to penetrate
cell membranes, intracellular stability, and reactivity with
cellular components). Most of the in vitro mutagenicity
experiments have been performed with chromium chemicals, following
the introduction of the reverse mutation test by Ames (1973).
Bacterial strains of Salmonella typhimurium (Petrilli & DeFlora,
1977) containing mutants that require histidine for growth, and
Escherichia coli (Venitt & Levy, 1974) with mutants requiring
tryptophan for growth have been used. In each case, the bacterial
cultures have been incubated with the test chromium compounds and
the number of revertant colonies scored. The chromium compounds
studied included both hexavalent and trivalent compounds; a survey
of the results is given in Table 17 (DeFlora, 1981). Zhurkov
(1981) performed an Ames test to study the mutagenic activity of
potassium dichromate (K2Cr2O7) with S. typhimurium TA 1535 and TA
1538. A medium degree of mutagenic activity without metabolic
activation was shown by the strain TA 1535.
Only hexavalent chromium compounds have shown mutagenic
effects. Using the Salmonella /mammalian microsome tests of Ames
et al. (1973), DeFlora (1978) and Löfroth (1978) both showed a
decrease in the mutagenicity of hexavalent chromium in the presence
of an NADPH-generating system, suggesting reduction of mutagenic
hexavalent chromium to trivalent chromium. In this chromate
metabolism, cytochrome P-450 functions as the reductase (Garcia &
Wetterhahn Jennette, 1981). In recent in vitro studies, Wiegand et
al. (1984b) showed that glutathione could reduce hexavalent
chromium to trivalent chromium, without any further cofactors or
metabolizing enzymes. In rats, the most efficient tissue in
decreasing hexavalent chromium mutagenicity was the liver, followed
by the suprarenal glands, kidney, stomach, and lung (Petrilli &
DeFlora, 1980). These studies suggest a possible way of
intracellular detoxification of hexavalent chromium in low doses.
Schoental (1975) suggested that hexavalent chromium caused the
formation of epoxyaldehydes having mutagenic potential. Venitt &
Levy (1974) assumed that chromates belong to the transition-
inducing class of mutagens. They cause both frameshift and base-
pair substitutions (Petrilli & DeFlora, 1980). The hypothesis of
Kazantzis & Lilly (1979), that chromates cause guanine-cytosine to
adenine-thymine transitions in the subsequent round of DNA-
replication, needs further confirmation.
Table 15. Genotoxic activity of chromium (VI) compoundsa
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
DNA degradation CaCrO4 - Casto et al. (1976)
K2Cr2O7 - Bianchi et al. (1983)
PbCrO4 - Douglas et al. (1980)
K2CrO4 + Whiting et al. (1979)
Decreased fidelity PbCrO4, + (1) Levis & Majone (1981)
of DNA synthesis K2Cr2O7
CrO3 + Sirover & Loeb (1976)
K2Cr2O7 + Bianchi et al. (1983)
Microbial DNA K2CrO4, + Yagi & NishioKa (1977)
repair K2Cr2O7
CrO3, K2CrO4, + Kanematsu et al. (1980)
K2Cr2O7
K2CrO4, + Nishioka (1975)
K2Cr2O7
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
CrO3, + Gentile et al. (1981)
K2Cr2O7,
Na2Cr2O7, + Gentile et al. (1981)
(NH4)2Cr2O7
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Microbial gene K2Cr2O7 + (2) Bonatti et al. (1976)
mutation CrO3 + (2) Fukunaga et al. (1982)
Na2Cr2O7, +/-(3) Petrilli & De Flora
CrO3, +/-(3) (1978a,b)
ZnCrO4xZn(OH)2 +/-(3)
K2Cr2O7 + Zhurkov (1981)
Na2Cr2O7 +/-(3) DeFlora (1978)
chromate (4) +/-(3) Löfroth (1978)
dichromate +/-(3)
K2Cr2O7 - Kanematsu et al. (1980)
K2CrO4, + Löfroth & Ames (1978)
K2Cr2O7 +
K2Cr2O7 + Nishioka (1975)
K2CrO4, CaCrO4 + DeFlora (1981)
(NH4)2CrO4,
CrO3,
Na2CrO4, + Venitt & Levy (1974)
K2CrO4
CaCrO4
K2Cr2O7 + Bianchi et al. (1983)
Na2Cr2O7,
ZnCrO4
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
K2CrO4, CaCrO4 + Petrilli & DeFlora
CrO3 (1977)
Na2Cr2O7
K2CrO4 + Green et al. (1976)
PbCrO4, CrO3 + Nestmann et al. (1979)
Mammalian cell K2Cr2O7 + Bianchi et al. (1983)
gene mutation K2Cr2O7, + Newbold et al. (1979)
ZnCrO4 +
PbCrO4 -
K2Cr2O7 + (5) Bonatti et al. (1976)
K2Cr2O7 + Pashin et al. (1982)
NaeCr2O + Pashin & Kozachenko
(NHy)2Cr2O7 + (1981)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell K2CrO4, + Umeda & Nishimura (1979)
chromosomal K2Cr2O7 +
mutation K2Cr2O7 + Bigaliev et al. (1978)
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7 +
Na2CrO4, + Majone & Levis (1979)
K2CrO4 +
Na2Cr2O7, Levis & Majone (1979)
K2Cr2O7
CrO3, CaCrO4
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CrO3 + Kaneko (1976)
Na2Cr2O7 + Sarto et al. (1980)
K2Cr2O7 +
CrCl3 +
CrO3 + Tsuda & Kato (1977)
K2Cr2O7 + Raffetto (1977)
K2Cr2O7 + Stella et al. (1982)
Na2Cr2O7, + Majone & Levis (1979)
K2Cr2O7 +
K2CrO4 + Wild (1978)
Na2Cr2O7 + (7) Krishnaja & Rige (1982)
Mammalian cell CrO3, K2CrO4, + Ohno et al. (1982)
SCE K2Cr2O7 +
K2Cr2O7 + Bianchi et al. (1983)
K2CrO4,
Na2CrO4 + Levis & Majone (1979)
CrO3, +
K2Cr2O7, + Majone & Levis (1979)
Na2Cr2O7 +
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CaCrO4, CrO3, + Gomez-Arroyo et al.
K2Cr2O7 + (1981)
K2Cr2O7 + Majone & Rensi (1979)
CrO3 + (6) Stella et al. (1982)
K2Cr2O4, + Elias et al. (1983)
Na2CrO4, +
Na2Cr2O7 +
K2Cr2O7,
K2CrO4 + McRae et al. (1979)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell CaCrO4, K2CrO4 + (8) Casto et al. (1979)
transformation ZnCrO4 + (9)
Na2CrO4,
CaCrO4 + Casto et al. (1976)
+ Di Paolo & Casto (1979)
K2Cr2O7 + Bianchi et al. (1983)
K2Cr2O7 + Tsuda & Kato (1977)
CaCrO4 + Fradkin et al. (1975)
K2Cr2O7 + Raffetto (1977)
---------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) As industrial pigments (Cr-yellow-orange; Zn-yellow; Mo-orange).
(2) Gene conversion.
(3) No mutagenicity in the presence of mammalian microsomal activation
system (S-9 mix).
(4) No compounds given.
(5) Forward mutation was observed in 7/480 054 colonies (controls 0/84
546). A significant increase can be stated if comparison is made
with the historical spontaneous mutation rate, i.e., no mutants in
106 colonies.
(6) Occupatinal exposure.
(7) Induction of chromosomal aberrations in gill cells of the fish
Boleophthalmus dussumieri (Goby).
(8) 2 times control levels.
(9) 4 times control levels.
The results summarized in Table 17 show that the chromate ion
(hexavalent chromium) induces different kinds of genetic damage,
even at low doses, whereas the chromosome-damaging capacity of
trivalent chromium was only observed when it was tested in very
high doses.
In assays with whole-cell systems, trivalent chromium is
inactive, unless there is a direct interaction with DNA, e.g., in
studies in which purified DNA was exposed to trivalent chromium,
where modifications of the physical and chemical properties, as
well as decreased fidelity of DNA synthesis, were produced (Levis &
Bianchi, 1982). According to Warren et al. (1981), it appeared
that trivalent chromium in a proper ligand environment could have
considerable genetic toxicity. However, in recent studies, Bianchi
et al. (1984) found that trivalent chromium (CrCl3, 10-3 to 10-5 M)
was neither cytotoxic nor mutagenic in permeabilized hamster
fibroblasts (BHK were incubated for 30 min in Ea or BSS made
hypertonic with 4.2% NaCl).
Table 16. Genotoxic activity of chromium (III) compoundsa
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
DNA degradation CrCl3 + Bianchi et al.
(1983)
Decreased fidelity Cr2O3 + Levis & Majone
of DNA synthesis (1981)
Microbial DNA cis-[Cr(bipy)2ox] + Warren et al. (1981)
repair I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 + Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O + Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O + Warren et al. (1981)
trans-[Cr(en)2 + Warren et al. (1981)
(SCN)2]SCN
CrCl3, Cr2O3, - Yagi & Nishioka
Cr(OH)3 (1977)
K2Cr2(SO4)4 - Yagi & Nishioka
(1977)
K2Cr2(SO4)4 - Kanematsu et al.
(1980)
Cr2(SO4)3 - Kanematsu et al.
(1980)
CrCl3 - Nishioka (1975)
Cr(NO3)3, + De Flora (1981)
Cr(CH3COO)3
CrCl3 - Nakamuro et al.
(1978)
CrCl3 + Gentile et al.
(1981)
CrCl3, KCr(SO4)2, - Gentile et al.
(1981)
Cr2(SO4)3 - Gentile et al.
(1981)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Microbial gene CrCl3, Cr(NO3)3 - Löfroth & Ames
mutation (1978)
Cr(CIO4)3 - Löfroth & Ames
(1978)
CrCl3, Cr(NO3)3 - Venitt & Levy (1974)
Cr2SO4, - Venitt & Levy (1974)
K2SO4 x 2H2O
Cr(CH3COO)3 + Nakamuro et al.
(1978)
Cr(NO3)3 + Nakamuro et al.
(1978)
CrCl3 - Nakamuro et al.
(1978)
CrK(SO4)2 x 12H2O - Petrilli & De Flora
(1977)
CrCl3 x 6H2O - Petrilli & De Flora
(1978a)
Cr(NO3)3 x 9H2O - Petrilli & De Flora
(1978b)
Microbial gene cis-[Cr(bipy)2ox] + Warren et al. (1981)
mutation I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 - Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O - Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O - Warren et al. (1981)
trans-[Cr(en)2 - Warren et al. (1981)
(SCN)2]SCN
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
gene mutation (1983)
Cr(CH3COO)3 - Newbold et al.
(1979)
Mammalian cell Cr2(SO4)3 - Umeda & Nishimura
chromosomal (1979)
mutation
Cr(CH3COO)3 + Nakamuro et al.
(1978)
CrCl3, Cr(NO3)3 - Nakamuro et al.
(1978)
Cr(NO3)3, + Levis & Majone
(1979)
KCr(SO4)2, + Levis & Majone
(1979)
Cr(CH3COO)3 + Levis & Majone
(1979)
CrCl3 + Kaneko (1976)
CrCl3 - Sarto et al. (1980)
Cr2(SO4)3, CrCl3 - Tsuda & Kato (1977)
CrCl3 + Raffetto (1977)
CrCl3 - Stella et al. (1982)
CrCl3 - (1) Bianchi et al.
(1984)
Mammalian cell CrCl3, Cr2O3 + Elias et al. (1983)
SCE
Cr2O3 + Levis & Majone
(1981)
Cr(NO3)3, - Levis & Majone
KCr(SO4)2 (1979)
CrCl3, Cr(CH3COO)3 - Levis & Majone
(1979)
CrCl3 + (2) Ohno et al. (1982)
Cr2(SO4)3 - Ohno et al. (1982)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
SCE (contd). (1983)
CrCl3 - Majone & Rensi
(1979)
Mammalian cell CrCl3 - Bianchi et al.
transformation (1983)
---------------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) Thymidine uptake, DNA replication, damage and repair and SCE were
tested in permeabilized hamster fibroblasts.
(2) Weakly positive.
Levis & Buttignol (1977) and Levis et al. (1978) raised the
question of whether trivalent chromium could be an active mutagenic
agent within a cell. Others, such as Bianchi et al. (1980),
supported this theory, suggesting that trivalent chromium is bound
to genetic material after reduction of the hexavalent form by an
NADPH-dependent microsomal enzyme system (Norseth, 1978; Jennette,
1979). The studies of Sirover & Loeb (1976), showing a 30% error
in DNA synthesis after application of 0.64 mM trivalent chromium
compared with 16 mM of hexavalent chromium, support this theory.
Pashin et al. (1982) demonstrated the induction of dominant
lethal mutations in mice. They treated male mice with a single
injection of potassium dichromate (0.5 - 20 mg/kg body weight, ip)
or with daily injections (1 and 2 mg/kg, ip, for 21 days). The
single injections (0.5 - 2 mg/kg) did not induce any effects;
repeated or higher dosages of potassium dichromate induced a
statistically significant decrease in the survival of embryos from
cells treated at the early spermatid and late spermatocyte stages.
Karyological alterations in mammalian cells were induced after
exposure to concentrations of hexavalent chromium that were more
than 100 times lower than the concentration of trivalent chromium
causing the same changes (Majone & Rensi, 1979).
Analysing the effects of fume particles from stainless steel
welding on sister chromatid exchanges and chromosome aberrations in
cultured Chinese hamster cells, Koshi (1979) found an increase in
cytogenic effects with increasing fume doses, due to the dissolved
hexavalent chromium. Hexavalent chromium and stainless steel
welding fume particles containing chromium compounds produced
positive results in the "mammalian spot test", a somatic tissue
assay (Knudsen, 1980).
Table 17. Mutagenicity of chromium compoundsa
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
L. Hexavalent chromium compounds
1) Sodium dichromate (Merck) - +w +w +w + 50 - 250 4.4 decrease toxic and mutagenic
Na2Cr2O7 x 2H2O Cr6+ was converted
into inactive Cr3+
2) Potassium chromate (BDH) - +w +w +w + 80 - 410 2.9 decrease by reducing
K2CrO4 chemicals (ascorbic
acid, sodium
3) Calcium chromate (BDH) - +w +w +w + 60 - 290 3.2 decrease sulfite) or
CaCrO4 metabolites (NADH,
NADPH, GSH), by
4) Ammonium chromate - +w +w +w + 50 - 320 3.7 decrease human gastric juice
(Carlo Erba) and erythrocyte
(NH4)2CrO4 lysates, by S9 mix
containing human
liver S9 fractions
5) Chromium trioxide or - +w +w +w + 40 - 220 5.1 decrease or rat tissue S9
chromic acid (Merck) fractions (in order
CrO3 of efficiency:
liver>suprarenal
glands>kidney>
stomach>lung);
pretreatment of
rats (Aroclor(R)
1254), ether,
ethanol) influenced
the efficiency of
metabolic systems;
conversely, Cr6+
mutagenicity was not
affected by human
serum or plasma nor
by S9 mix containing
other rat (spleen,
colon, bladder,
striated muscle) S9
fractions
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
6) Zinc yellow (Montedison) - +w +w +w + 90 - 590 1.4 decrease the mutagenic effects
ZnCrO4 x Zn(OH)2 + of Cr6+ compounds and
10% CrO3 benzo( a)pyrene were
less than additive
7) Chromyl chloride (BDH) - +w +w +w + 100 - 430 1.8 decrease liquid volatile
Cl2CrO2 compound; vapours
were also toxic and
mutagenic
8) Chromium orange or basic - - - - +b 2 mg the three pigments
lead (spot test) containing PbCrO4
PbCrO4 x PbO were scarcely (L8),
chromate (Montedison) very scarcely (L9),
or totally (L10)
insoluble in water;
they were assayed
by directly spotting
these compounds at
the centre of plates
9) Molybdenum orange or - - - - +b 2 mg
lead solfomolybdo- (spot test)
chromat (Montedison)
PbCrO4 72-77%
PbSO4 4-6%
Al2O3 2%
PbMoO4 12-14%
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
10) Chromium yellow or - - - - -b
lead solfochromate
(Montedison)
PbCrO4 41-85%
PbSO4 4-45%
SiO2 0.1-3%
Al2O3 2-6%
11) Chromium - - - - - -2.3x103 - - hexacoordinated
hexacarbonyl (BDH) compound; formally,
Cr(CO)6 its oxidation state
is 0; insoluble in
water, dissolved in
ether
M. Trivalent chromium compounds
1) Chromic chloride - - - - - -3x104 - - inactive Cr3+
(BDH) CrCl3 x 6H2O compounds could be
converted into toxic
and mutagenic Cr6+
only in the presence
of oxidizing chemicals
(potassium
permanganate), while a
variety of metabolic
systems were
ineffective
2) Chromic nitrate (Riedel- - - - - - -2x104 - -
de Haen)
Cr(NO3)3 x 9H2O
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
3) Chromic potassium - - - - - -1.6x104 -
sulfate (BDH)
CrK(SO4)2 x 12H2O
4) Chromic acetate (BDH) - - - - - -7x104 - -
Cr(CH3COO)3
5) Neochromium (Montedison) - - - - - -5x104 - -
Cr(OH)SO4 56-58%
Na2SO4 23-24%
H2O 18-21%
6) Chromium alum (Montedison) - - - - - -4.3x104 - -
Cr2(SO4)3 37-39%
K2SO4 16-18%
H2O 43-37%
7) Chromite (Montedison) - - - - + 2 mg - Contaminated with Cr6+
Cr2O3 44-46% (spot test) traces ( sim-diphenyl-
Fe2O3 29-30% carbazide reagent);
Al2O3 15-16% scarcely soluble in
SiO3 0.5-3% water; assayed by
CoO 0.5-2% directly spotting the
powder at the centre
of plates
--------------------------------------------------------------------------------------------------------
Table 17 (contd.)
Note:
Sensitivity of Salmonella strains: Range of activity:
In the assays carried out in this study, This column indicates the lower and
the numbers of spontaneous revertants of the upper limits (in nmol per plate)
Salmonella tester strains fell within the of the mutagenic response, as in-
following ranges: ferred from dose-response curves
TA 1535: 10-25, both with and without S9 mix; with the most sensitive bacterial
TA 1537: 3-18, both with and without S9 mix; strain and under the most favourable
TA 1538: 10-25 without S9 mix; 15-35 with S9 mix; metabolic situation (i.e., with or
TA 98: 20-35 without S9 mix; 25-40 with S9 mix; without S9 mix) for each positive
TA 100: 150-200 without S9 mix; 140-180 with S9 mix compound; for negative compounds,
the maximum dose tested is indicated
The key for the interpretation of symbols in the
column "Reverted strains" is the following: Mutagenic potency:
+ clearly positive result indicated by a dose-
related and reproducible increase of his+ the values presented have
revertants over controls (at least a 3-fold been calculated by dividing the number
increase); of revertants in excess of controls
+w weak positivity indicated by an increase of by the corresponding amount of compounds
revertants 2 - 3 times in controls; (in nmoles); the number of net revertants
± reproducible but less than 2-fold increase was determinated at the top level of the
of revertants; linear part of dose-response curves, which
No symbol: no conclusive experiment carried out were drawn as indicated under the previous
with the corresponding strain. sub-heading.
Effects of S9 mix
The effect of S9 mix containing
liver S9 fractions from
Arochlor(R) pretreated rats on
the mutagenicity of test
compounds is reported
--------------------------------------------------------------------------------------------------------
a From: DeFlora (1981).
b L8, L9, and L10, as well as PbCrO4, were positive in the plate test when
dissolved in 0.5 N NAOH.
Embryonic fibroblast cell cultures showed significant
chromosome aberrations, when potassium dichromate was added to the
medium (Tsuda & Kato, 1976). When the potassium dichromate was
reduced with sodium disulfite (Na2SO3), no significant aberrations
occurred, even with 100 times the chromium concentrations.
At present, it is widely accepted that hexavalent chromium is
genetically active, because of its ability to cross the membranes
and enter the cells. If reduction of hexavalent chromium takes
place outside the cell (or even outside the cell nucleus, e.g., in
mitochondria or microsomes) its genetic activity is suppressed; if
the reduction takes place inside the nucleus (near, or at, the
target DNA molecules) alterations in DNA can occur, depending on
the oxidation power of hexavalent chromium or the formation of
trivalent chromium complexes with nucleophilic sites of the DNA;
thus, trivalent chromium could be the ultimate mutagenic form of
chromium (Levis & Bianchi, 1982). More recent investigations
(Wiegand et al., 1984) have shown that hexavalent chromium may
enter the body unreduced via the lung and be partly deposited in
erythrocytes over a prolonged period of time. From a DNA-repair
test in bacteria, DeFlora et al. (1984) concluded that hexavalent
chromium causes irritation in nucleotide pools and, due to its
oxidizing power, induces single-strand breaks in DNA, while
trivalent chromium induces DNA-protein and DNA-DNA cross-links and
decreases the fidelity of DNA replication.
7.2.1.3. Developmental toxicity and other reproductive effects
The teratogenicity of hexavalent chromium as chromium trioxide
was tested in golden hamsters (Gale, 1974, 1978, 1982; Gale &
Bunch, 1979) and that of trivalent chromium as chromic-chloride in
ICR mice (Iijima et al., 1975; Matsumoto et al., 1976). Injections
of hexavalent chromium into the fertilized chick egg increased
embryolethality and produced malformations in the survivors (Gilani
& Marano, 1979).
The golden hamsters were treated with a single iv injection of
chromium trioxide (5, 7.5, 10, or 15 mg/kg body weight) on the 8th
day of gestation (Gale, 1978).
Dose-response relationships were demonstrated between the rate
of absorption and the rate of malformations including delayed
ossification of the skeletal system. At 5 mg/kg body weight, 4% of
the fetuses showed external abnormalities (oedema, exencephaly),
and 34% showed cleft palate (controls: 2%), which increased to 85%
with the higher doses of 7.5 or 10 mg/kg body weight. Thirty-one
percent of fetuses were retarded following treatment with 7.5 mg/kg
body weight and 49% following 10 mg/kg body weight. The dose of 15
mg/kg body weight was lethal for 75% of the dams. Gale & Bunch
(1979) injected a single dose of 8 mg CrO3/kg body weight on day 7,
8, 9, 10, or 11 of gestation in female golden hamsters (Table 18).
The treated females were adversely affected (body weight loss,
tubular necrosis of the kidneys). In a later study, Gale (1982)
injected 8 mg CrO3/kg iv in hamsters on day 8 of gestation. An
increased incidence of cleft palate was seen in strains LVG, LSH,
and MHA, but no effects were reported in strains CB, LHC, and PD4.
ICR mice were injected sc with chromic chloride (Iijima et al.,
1975; Matsumoto et al., 1976). In the first series (animals
administered 9.8 or 19.5 mg/kg body weight, every other day, from
the 0th to 16th day of gestation), according to the authors, a
slight increase in the rate of malformations could not be excluded.
In the second series, with ip injections of 9.8 - 24.4 mg/kg body
weight, on the 8th day of gestation, the authors reported a dose-
dependent increase in the frequency of exencephaly, anencephaly,
and the occurrence of rib fusion.
Danielsson et al. (1982) studied the rate of embryonic or fetal
uptake of trivalent chromium and hexavalent chromium in early and
late gestational mice (Table 19) and found placental passage of
chromium. Hexavalent chromium appeared in the fetus in high enough
concentrations for a direct effect on embryonic structures to be
likely, trivalent chromium may act on placental structures.
Gilani & Marano (1979) injected chromium trioxide into 840
embryonating chicken eggs (air sack) at doses ranging from 0.002 to
0.05 mg per egg, on days 0, 1, 2, 3, and 4 of incubation (0.1 ml
physiol. saline injections per control egg). All embryos were
examined on day 8. The following malformations were observed in
the 720 eggs affected: short and twisted limbs, microphthalamia,
exencephaly, short and twisted neck, everted viscera, oedema, and
reduced body size. However, the incidence of abnormalities in the
120 controls was reported to be "low".
Inhalation studies were conducted by Glaser et al. (1984) on
Wistar rats (TNO-W-74 SPF) to study the effects of sodium
dichromate on reproduction and teratogenicity in 3 generations.
Whole-body exposures to sodium dichromate aerosols (0.2 mg
chromium/m3) were performed with an exposure time of 130 days per
generation. No effects on reproduction were found. All
teratogenicity tests were negative, and there was no increase in
fetal chromium content. However, from generation to generation,
there was an increase in immunosuppression and hyperplasia of
organs (especially in the lungs) and changes in haematological
variables.
Table 18. Embryotoxicity following maternal exposure to CrO3 on different days
of gestation in hamstersa
-------------------------------------------------------------------------------------
Findings in live fetuses
Day of No. of No. of No. of Frequency External Internal Cleft
gestation females implantation living resorptions defects defects palate
treated sites fetuses No. %b No. %b No. %b No. %b
-------------------------------------------------------------------------------------
Treated CrO3 8 mg/kg
7 6 64 10 54 84 1 10 1c 10 5 50
8 6 88 54 14 20 8d 15 4e 7 17 31
9 6 77 67 10 13 18f 27 0 0 25 37
10 6 77 77 0 0 0 0 0 0 0 0
11 6 68 67 1 1 0 0 0 0 0 0
Controls H2O 5 ml/kg
7 3 35 33 2 6 0 0 0 0 0 0
8 3 44 41 3 7 0 0 0 0 0 0
9 3 37 36 1 3 0 0 0 0 0 0
10 2 20 19 1 5 0 0 0 0 0 0
11 3 34 33 1 3 0 0 0 0 0 0
-------------------------------------------------------------------------------------
a From: Gale & Bunch (1979).
b Underlined %, significantly different statistically from controls.
b Right hindlimb with two digits.
c Right kidney absent.
d Oedema (7), tail short (1).
e Small kidneys (3), right kidney absent (1).
f Oedema (17), omphalocele (1).
Table 19. Comparison of concentrations of trivalent and hexavalent
chromium in fetuses, placentas, and maternal organsa,b
------------------------------------------------------------------
Day 13 Day 16
Hexavalent Trivalent Hexavalent Trivalent
chromium chromium chromium chromium
------------------------------------------------------------------
Fetus 0.3 ± 0.01 0.03 ± 0.002 0.5 ± 0.04 0.1 ± 0.01
Placenta - - 3.0 ± 0.2 2.4 ± 0.2
Liver 29.4 ± 2.2 30.8 ± 6.5 33.9 ± 3.9 27.7 ± 3.5
Kidney 38.1 ± 1.9 5.3 ± 0.7 36.0 ± 4.3 8.7 ± 2.3
Serum 2.6 ± 0.2 9.3 ± 0.9 2.8 ± 0.4 11.0 ± 1.6
------------------------------------------------------------------
a From: Danielsson et al. (1982).
b Trivalent chromium (51CrCl3) and hexavalent chromium
(Na251Cr2O7) were injected iv on day 13 and day 16 of gestation
at doses of 10 mg chromium/kg body weight. The pregnant animals
were autopsied after 1 h. Concentrations are expressed in
mg/litre or g ± SEM (N = 4).
7.2.1.4. Cytotoxicity and micromolecular syntheses
Wacker & Vallee (1959) reported that trivalent chromium was
present in RNA from all sources examined; they hypothesized that
chromium might contribute to the stabilization of the structure.
Administration of trivalent chromium to mice (ip injection of
CrCl3 at 5 or 0.5 mg chromium/kg body weight; P < 0.01) caused
accumulation of chromium in the cell nucleus, which amounted to
about 20% of the accumulated chromium content of the liver cell,
and also enhanced RNA synthesis. Hexavalent chromium inhibited RNA
synthesis (K2CrO4 at 5 or 0.5 mg chromium/kg body weight;
P < 0.01) (Okada et al., 1983). In further studies, Okada et al.
(1984) pretreated rats with CrCl3 (ip injection of 5 mg chromium/kg
body weight) and then partially hepatectomized the rats. Chromium
accumulated in the regenerating liver, and hepatic RNA synthesis
was accelerated compared with that of partially hepatectomiced
controls. These findings suggest a direct participation of
chromium in RNA synthesis.
Potassium dichromate enhanced the passive uptake of ribo-and
desoxyribonucleosides (Levis et al., 1978; Bianchi et al., 1979),
caused an inhibition of DNA replication to BHK fibroblasts, and
reduced the colony-forming ability of BHK cells (doses: 10-4 - 20-7
mol). Hexavalent chromium (potassium dichromate with a
concentration of 5 x 10-7 mol/litre) reduced by half the
proliferation of NHIK 3025 cells originating from a human cervix
carcinoma (White et al., 1979).
The addition of calcium chromate to BHK21 cell cultures altered
their growth characteristics. Whereas these cells normally grow as
elongated fibroblast cells in parallel orientation, the chromium-
exposed cells grew as shortened fibroblasts with enlarged nuclei
and granular cytoplasm, randomly orientated. When grown in
carboxymethyl cellulose, the chromate-exposed cells underwent many
divisions in contrast to the 1 or 2 divisions of normal cells.
These alterations in growth properties persisted, even after
transfer to a normal medium (Fradkin et al., 1975).
Cultures of HeLa and rat embryonic cells were incubated with
various hexavalent and trivalent chromium compounds for 3 days and
the 50% inhibitory dose (ID50) for cell growth was determined. The
ID50 for sodium and potassium chromate and dichromate and calcium
chromate varied from 0.34 to 0.78 µg/ml of the medium. The ID50
for the trivalent compounds varied over a wider range: chromic
oxalate, 4; acetate, 52; nitrate, 720; chloride, 1030; and bromide,
1500 mg/litre. Similar results were found in rat embryonic cell
cultures (Susa et al., 1977).
7.2.1.5. Fibrogenicity
Because of the well-known fibrogenic effects of silica and
asbestos minerals, studies were initiated to determine whether
chromite and various other dusts exhibited similar properties. A
single intratracheal injection in rats of 40 mg chromite particles
(33% chromium, 0.7% SiO2, 99.9% of the particles being smaller than
5 µm) suspended in 1 ml Ringer's solution caused a moderate
cellular reaction that reached a peak after 2 months and then
decreased. After 8 months (end of observation), the lung weight
was within normal limits. Only an increase in fibrils that was not
significant was observed. The tissue reaction in regional lymph
nodes was minimal (Swensson, 1977).
Chromite FeO(CrAl)2O3 (10 mg), ground to an average particle
diameter of 1 µm, was injected into the pleural cavities of 25
mice. The animals were killed at intervals ranging from 2 weeks to
18 months after injection. Chromite produced the least reaction of
all the 15 dusts tested. This material attracted very few cells
and the eventual production of collagen was minimal (Davies, 1972).
7.2.2. Observations in farm animals
Systemic poisoning of farm animals with chromates is rare and
is mainly suspected to be a consequence of accidental industrial
contamination of drinking-water. The acute lethal dose for mature
cattle is approximately 600 - 800 mg/kg body weight; chronic
poisoning in young calves was produced by a daily dose of 30 - 40
mg/kg body weight for one month (NZ Department of Agriculture,
1954) with a clinical picture of profuse scouring, severe
dehydration, low blood pressure, changes in the alimentary tract,
including congestion and inflammation, and the rumen and abomasum
showing ulceration near perforation.
High chromium levels were found in the blood and liver of
calves that had died from poisoning. The minimum tissue levels
causing poisoning were 30 mg/kg in the liver and 4 mg/litre in
whole blood (Harrison & Staples, 1955). According to Romoser et al.
(1961), a level of 100 mg hexavalent chromium (as sodium
chromate)/kg did not affect growing chicks.
8. EFFECTS ON MAN
8.1. Nutritional Role of Chromium
The physiological role of chromium and the need for small
amounts of dietary chromium are indicated by:
(a) chromium-responsive cases of impaired glucose
metabolism in malnourished children and middle-aged
persons;
(b) 2 cases of proved chromium deficiency in patients fed
exclusively by parenteral alimentation; and
(c) epidemiological studies suggesting a link between
chromium deficiency and risk factors for cardiovascular
diseases.
8.1.1. Biological measurements and their interpretation
Biochemical methods for the diagnosis of the human nutritional
chromium status are still at the developmental stage. Blood- or
urine-chromium concentrations are not indicative of chromium
status. Several studies suggest the validity of the "relative
chromium response" in plasma or serum to the ingestion of 50 - 100
g of glucose within 30 - 120 min; the response also appears in the
urine. Lack of this response suggests chromium deficiency,
especially when combined with impaired glucose tolerance in the
presence of normal or elevated plasma-insulin levels.
8.1.2. Chromium deficiency
8.1.2.1. Adults
Chromium deficiency is diagnosed mainly through therapeutic
trials in which an impaired function is restored by supplementation
of the diet with small amounts of chromium close to the estimated
human requirement. Glinsmann & Mertz (1966) studied 4 male
diabetic patients for several months to 1 year, under the strictly
controlled conditions of a metabolic ward. The subjects, who were
fed identical portions of a diet prepared before the start of the
study, were allowed a measured amount of physical activity and were
stabilized under these conditions before the beginning of the
control periods. Three of the 4 patients responded to a chromium
level of 180 - 1000 µg/day as CrCl3 x 6H2O with a significant
improvement in oral glucose tolerance; the fourth showed a very
slight lowering of the glycaemic curve, which was not statistically
significant. A representative example is given in Table 20.
Table 20. Effect of chromium on mean glucose tolerancea,b
------------------------------------------------------------------------
Supplementation Days Number Mean blood glucose concentration
of ---------------------------------
tests Fast- Min after glucose load
ing 30 60 90 120
------------------------------------------------------------------------
None 0 - 32 10 840 2170 2290 1950 1560
60 µg, 3 x per day 33 - 74 12 850 2240 2380 2090 1620
60 µg, 3 x per day 75 - 119 13 840 2100 2130 1860 1410
60 µg, 3 x per day 120 - 140 5 840 2030 2010c 1750 1120c
None 180 - 194 1 960 2070 2900 2930 2370
60 µg, 3 x per day 195 - 211 5 830 1960 1930c 1750 1180c
None 256 - 313 10 870 2200 2450 2240 1660
1 mg, 3 x per day 337 - 340 1 680 1480 1620 1510 960
------------------------------------------------------------------------
a Modified from: Glinsmann & Mertz (1966).
b A 46-year-old man with maturity-onset diabetes, controlled on diet;
oral glucose tolerance test consisted of constant noon meal and 100 g
glucose.
c Mean values significantly different from control ( P < 0.025).
The condition of one of 2 diabetic out-patients, observed over
several months, was improved by chromium supplementation, the
condition of the other was not. Short-term treatment of 7 diabetic
patients for 1 - 7 days with 1 mg chromium did not affect any
variables of glucose metabolism, nor did chromium supplementation
change the normal glucose tolerance tests of 10 healthy, young
volunteers.
Glinsmann's positive results were not confirmed in a double-
blind crossover study on 4 normal volunteers and 10 diabetic
persons, with a crossover after 16 weeks. No effects of chromium
were detected on glucose tolerance or on fasting or postprandial
blood-glucose levels (Sherman et al., 1968). Schroeder et al.
(1970), while reporting that 2 - 10 mg chromium/day "almost always"
restored the impaired glucose tolerance in older, non-diabetic
subjects to normal, found only erratic and mild improvement in 4
out of 12 diabetic out-patients. On the basis of these
observations, Mertz (1969) concluded that chromium should not be
considered a therapeutic agent for diabetic patients.
On the other hand, it was reported in a later study on chromium
metabolism that chromium administration (500 µg/day, for 2 weeks)
produced a marked improvement in glucose utilization in both
juvenile and maturity-onset diabetic patients. Five of the
patients were followed for more than 1 year after exposure and were
found to maintain their improved glucose metabolism (Nath, 1976).
Of 10 non-diabetic elderly subjects with impaired glucose
tolerance, 4 responded to 150 µg chromium/day with complete
normalization of oral glucose tolerance tests. The remaining 6
subjects were not affected by the supplements (Levine et al.,
1968). Significant effects of a GTF-chromium-containing yeast
preparation were reported by Doisy et al. (1976) in 6 out of 12
elderly subjects with impaired glucose tolerance
Table 21. Effect of GTF supplementation on glucose tolerance tests in
elderly subjects with impaired tolerancea,b
-------------------------------------------------------------------------
Mean plasma-glucose levels Cholesterol Triglyceride
(mg/litre) (mg/litre) (mg/litre)
0 h 1 h 2 h
-------------------------------------------------------------------------
Before GTF 1060 ± 40c 2010 ± 70 1780 ± 80 2450 ± 90 1210 ± 80
11 tests
After GTF 990 ± 40 1620 ± 110 1320 ± 50 2050 ± 100 1120 ± 120
9 tests
Signifi- NS < 0.01 < 0.001 < 0.01 NS
canced
-------------------------------------------------------------------------
Mean serum-insulin levels
(microunits/ml)
0 h 1 h 2 h
Before GTF 24 ± 6 78 ± 17 118 ± 17
after GTF 26 ± 12 70 ± 8 83 ± 8
-------------------------------------------------------------------------
a From: Doisy et al. (1976).
b Supplement: 4 g yeast per min/day (Yeastamin Powder 95, Staley Co.,
Chicago, Illinois, USA). GTT: 100 g oral load.
c Mean ± SEM.
d Using paired t test, difference is significant.
In addition to the improvement in glucose tolerance in the
presence of a reduced insulin response, plasma-cholesterol
concentrations were significantly reduced. Several other
individual cases are reported in the publication of Doisy et al.
(1976), all of whom showed a lower serum-insulin response to a
glucose tolerance test during chromium supplementation.
Liu & Morris (1978) performed a similar study on the effects of
a yeast-chromium supplementation in 27 women, 40 - 75 years old, of
whom 15 had a normal and 12 an abnormal glucose tolerance test. In
11 of the normal subjects (73%), the integrated insulin response
was significantly reduced from 436 to 335 µU/ml ( P < 0.025) during
supplementation, without a statistically significant change in
glucose tolerance test. Significant reductions in the total glucose
response (10 650 - 9820 mg/litre; P < 0.001) and in the total
insulin response to glucose were observed in the subjects with
impaired glucose tolerance (1288 - 831 µU/ml; P < 0.001). The
glucose tolerance improved in 7 out of 12 women, but all 12 showed
a reduction in plasma-insulin.
The effects of supplementation of the diet with 200 µg
chromium/day (as chloride) on the glucose tolerance of 76 normal
adult volunteers was investigated in a double-blind cross over
study, with each period lasting 3 months. All 18 subjects entering
the study with impaired glucose tolerance (serum-glucose equal to
or greater than 1 g/litre, 90 min after a glucose load), showed
significant improvement during chromium supplements, but not with a
placebo, with an average decline in the 90-min serum-glucose levels
of 180 mg/litre ( P < 0.01) (Anderson et al., 1983b).
In another placebo-controlled study, 24 elderly volunteers were
given a daily supplement of either chromium-rich brewer's yeast or
chromium-deficient Torula yeast (9 g/day, for 8 weeks). The
glucose tolerance of the subjects given brewer's yeast
significantly improved with a concomitant decrease in plasma-
insulin levels, and a significant decline in plasma-cholesterol as
well as total plasma-lipids. No significant changes were observed
in the control group given the chromium-poor Torula yeast
(Offenbacher & Pi-Sunyer, 1980).
Additional, preliminary studies suggest a specific effect of
brewer's yeast on individual lipoprotein cholesterol fractions.
Twenty normal human subjects responded to daily supplementation
with 18 g of brewer's yeast over a 8-week period with a significant
reduction in low-density lipoprotein-cholesterol ( P < 0.01) and
with a simultaneous increase of 14% in the high-density lipoprotein
( P < 0.01) (Nash et al., 1979).
Similar results were observed in a 6-week supplementation trial
involving 8 physician volunteers, each of whom received 7 g/day of
brewer's yeast. Low-density lipoprotein-cholesterol declined by
nearly 18% ( P < 0.01), whereas the high-density cholesterol
fraction increased by 17.6%, resulting in a significant
( P < 0.001) decrease in the LDL/HDL ratio of 28% (Riales, 1979).
These studies suggest that the primary effect of the
supplementation must have been an increase in the effectiveness of
the insulin, because glucose tolerance was maintained or improved
in spite of lower insulin responses. It is important to note that
while the reduction in insulin levels occurred in all cases, an
improvement in glucose tolerance occurred in only a fraction. This
suggests two possible interpretations of all the results discussed
here, including those showing improvement in glucose tolerance in
only some of the subjects or no improvement at all. Lack of a
response to chromium could suggest that the subjects were not
chromium deficient and that their impaired glucose metabolism was
the result of other causes. Second, they may have responded with a
reduction in circulating insulin levels, as did all the subjects
with impaired glucose tolerance in whom the insulin response was
measured. Unfortunately, these measurements were not performed in
the earlier studies.
8.1.2.2. Malnourished children
Chromium deficiency has been implicated as a factor
contributing to the impairment of glucose tolerance in children
with protein calorie malnutrition in 3 countries, Jordan, Nigeria,
and Turkey. Hopkins et al. (1968) measured intravenous glucose
tolerance in 10 malnourished infants from the mountainous area of
Jordan and 9 equally malnourished infants from the valley area.
They found a severely depressed glucose removal rate of 0.7%/min in
the first group and a normal rate in the second, 3.8%/min (Table
22). The protein intake of both groups prior to hospitalization
had been identical; it was supplied in the form of a milk powder
with a chromium content of 18 µg/litre, when reconstituted.
However, analysis of 10 water samples revealed 3 times as much
chromium in the samples from the valley as in those from the
mountainous area (0.5 versus 1.6 µg/litre). This suggested the
possibility of chromium being a major determinant in the glucose
metabolism of these children, and the effects of supplementation
were measured in 6 children from the hills, with a severely
impaired tolerance test. The morning after oral administration of
250 µg chromium, as CrCl3 x 6H2O, the glucose removal rate had
markedly improved to 2.9%/min from a pretreatment level of
0.6%/min. Similar results were obtained in Nigeria (Table 22);
these tests also included control children who did not receive
chromium.
The immediate effects of chromium on these children were
remarkable and different from the lag phase always present in the
studies on adults. Equally important, was the short duration of
the effect of one dose demonstrated in a Jordanian child observed
for a longer period of time. These observations suggest that a
marked depletion in chromium stores must have existed in the
children.
Table 22. Significance of the effect of chromium on the impaired glucose
tolerances of malnourished infantsa
---------------------------------------------------------------------------
Subjects Number Glucose Significance
of removal of
infants rate difference
(%/min)
---------------------------------------------------------------------------
Jordanian infants from hill area with
0.5 µg chromium/litre in drinking-water 10 0.7
Jordanian infants from valley with
1.6 µg chromium/litre in drinking-water 9 3.8 P < 0.001
Jordanian infants;
initial glucose tolerance test 6 0.6 P < 0.001
after chromium treatment 6 2.9
Nigerian infants;
initial glucose tolerance test 6 1.2
after chromium treatment 6 2.9 P < 0.05
Non-treated infants:
Initial glucose tolerance test 5 1.9 not signi-
Repeated glucose tolerance test 5 2.1 ficant
---------------------------------------------------------------------------
a From: Hopkins et al. (1968).
Similar results were reported from a study in the Istanbul area
of Turkey (Gürson & Saner, 1971). Fourteen malnourished children
with an impaired glucose removal rate of 1.71%/min were given 250
µg of chromium (CrCl3 x 6H2O), and a second test was performed
after 15 h. The removal rate increased to 3.91%/min ( P < 0.001).
This average increase was due to a pronounced increase in 9 of the
children; no change was observed in the remaining 5. No
improvement in glucose tolerance occurred in 5 control children
receiving identical hospital treatment, except for the
administration of the chromium. The children receiving the
chromium supplementation grew significantly more than the control
children during the following 30 days, with the greatest growth
rates found in the 9 children who had responded to administration
of chromium with an improvement in glucose tolerance.
The fact that chromium deficiency interacts with protein-
calorie malnutrition only in certain geographical areas was
confirmed by Carter et al. (1968), who gave chromium
supplementation (250 µg/day as CrCl3 x 6H2O) without effects on
glucose tolerance in 9 Egyptian children tested. Plasma-chromium
levels were in the normal range, and chromium levels in
representative foods were high, ranging from 200 to 390 µg/kg.
These findings suggest that the chromium status of these subjects
was normal, which would explain the lack of an effect.
8.1.2.3. Patients on total parenteral alimentation
A female patient, maintained on total parenteral alimentation
since the age of 35 years, developed an unexpected weight loss of
15% after 5 years combined with peripheral neuropathy, impaired
intravenous glucose tolerance, in spite of normal insulin response,
and a decreased respiratory quotient of 0.66. Insulin treatment
(45 U/day) was ineffective. A strongly negative chromium balance
and low plasma- and hair-chromium levels suggested that the daily
estimated chromium intake from the infusion fluids of 5.3 µg had
not met the patient's requirement and resulted in chromium
deficiency. The insulin infusions were stopped and replaced by the
daily infusion of 250 µg chromium (as CrCl3 x 6H2O) for 2 weeks.
The result was normalization of glucose tolerance and respiratory
quotient, disappearance of signs and symptoms of neuropathy, return
to the previous normal body weight, in spite of reduced caloric
intake, disappearance of the requirement for exogenous insulin, and
a positive chromium balance. The nitrogen balance, which had been
erratic and often negative, became consistently positive following
chromium supplementation. Reduction of the daily supplement to 20
µg chromium proved to be sufficient to maintain the patient in a
state of well-being (Jeejeebhoy et al., 1977).
This case is the best demonstrated example of chromium
deficiency in a human subject. In addition to confirming the well-
known signs of chromium deficiency reported earlier, such as
insulin resistance and its consequences, it was the first case to
demonstrate the occurrence of peripheral neuropathy and disturbance
of nitrogen balance as a result of deficiency. These disturbances
require further study. A second case of chromium deficiency as a
consequence of long-term total parenteral alimentation was reported
by Freund et al. (1979). Signs and symptoms were similar to those
of the first case, except that central encephalopathy was present
instead of peripheral neural disorder. Supplementation with 150 µg
of chromium/day resulted in normalization of the deficiency signs.
Chromium at that time was not routinely added to intravenous
solutions, therefore it is possible that more cases of deficiency
may exist in patients on long-term infusion treatment. As an
outcome of this study, an Expert Panel of the American Medical
Association (AMA, 1979) suggested that the stable adult patient on
total parenteral alimentation should receive 10 - 15 µg chromium
daily.
8.1.2.4. Epidemiological studies
Two epidemiological studies suggest a link between chromium
status and cardiovascular diseases (Schroeder et al., 1970; Punsar
et al., 1975). Lack of chromium is associated with impaired
glucose tolerance, elevation of circulating cholesterol, and aortic
plaques. All of these variables are recognized risk factors for
cardiovascular disease. It has also been suggested that the
elevated insulin levels, often present in chromium-deficient human
subjects (Liu & Morris, 1978) represent a substantial risk for the
development of cardiovascular disease (Strout, 1977).
Schroeder et al. (1970) have summarized their studies of
chromium concentrations in normal and diseased tissues,
particularly in aortas (Table 23). The total body burden of
chromium, excluding the lungs, was lower in the USA, where there is
a higher incidence of cardiovascular disease, compared with other
regions, 1.72 - 1.86 mg/kg ash weight in the USA compared with 7.7,
9.6, and 2.7 mg in Africa, the Far East, and the Near East,
respectively. The only significant difference in tissue-chromium
concentrations in deaths from heart disease compared with deaths
from accidental causes was in the aorta (Table 23). Concentrations
in aortas from Africa, the Far and Near East were generally higher
and did not show such a difference.
Table 23. Chromium in aortasa
----------------------------------------------------------
Location Chromium (mg/kg dry weight)
Accident P Heart disease
----------------------------------------------------------
Africa 0.072 (5)b NS 0.116 (2)
Far East 0.97 (8) NS 0.246 (5)
Middle East 0.36 (8) NS 0.216 (3)
USA (Nine cities) 0.26 (103) 0.005 0.052 (13)
USA (San Francisco) 0.228 (10) 0.005 0.048 (15)
----------------------------------------------------------
a From: Schroeder et al. (1970).
b The number in parenthesis denotes the number of cases
observed.
The second epidemiological study was part of a cohort study
conducted in Finland beginning in 1959 (Punsar et al., 1975). A
total of 327 drinking-water samples were analysed for 22
characteristics including pH, anions, bulk minerals, and several
trace elements including chromium. The study included 504 and 622
men in the Western and Eastern areas of Finland, respectively,
whose domicile had not changed since 1959.
The coronary heart disease mortality rate is much higher in the
east of Finland than in the west. As seen in Table 24, the
chromium levels in drinking-water were much lower in the east than
in the west, whereas the opposite was true for copper. The metal
concentrations in the drinking-water of those with heart disease
and others only differed slightly (Table 24), but, according to the
authors, the data suggest that low chromium levels may play a role
in the high heart disease death rate of eastern Finland.
Table 24. Concentrations of copper and chromium in the
drinking-water of men who died between 1969 and 1973, and
of those who were alive in 1973a
-------------------------------------------------------------
Alive
Deaths (Clinical status in 1969)
CHDb Other CHDb Other No
heart heart
disease disease
-------------------------------------------------------------
West N = 19 N = 23 N = 63 N = 31 N = 288
Copper X 22.15c 20.17 20.05 20.94 20.15
(µg/litre) SD 3.92 4.69 4.69 5.12 4.74
Chromium X 7.52 9.38 9.45 9.74 8.45
(µg/litre) SD 2.06 3.42 4.01 4.44 3.31
East N = 23 N = 26 N = 129 N = 44 N = 279
Copper X 41.59 41.24 42.67 39.50 36.32
(µg/litre) SD 29.91 30.43 33.65 28.74 28.66
Chromium X 2.20 2.09 2.04d 2.49 3.01
(µg/litre) SD 2.17 2.41 2.08 2.17 2.37
-------------------------------------------------------------
a From: Punsar et al. (1975).
b CHD = coronary heart disease.
c P < 0.05.
d P < 0.001.
Note: Levels of significance refer to differences observed
when the concentrations were compared with those in the
"no heart disease" category.
Newman et al. (1978) measured serum-chromium concentrations in
32 patients, 18 male and 14 female, aged 25 - 65 years, in whom
coronary artery cine-arteriography was performed for medical
reasons. Serum-chromium was significantly lower in the 15 subjects
with coronary artery disease than in the 17 subjects without
involvement (2.51 versus 6.09 µg/litre; P < 0.01), whereas serum-
cholesterol, weight index, and systolic or diastolic blood pressure
did not differ among the groups. Serum triglycerides were higher
in the group with coronary artery disease (1.84 versus 1.12
g/litre; P < 0.05). No subject with a serum-chromium
concentration of more than 6 µg/litre exhibited coronary artery
involve- ment, and by regression analysis, serum-chromium was
judged to explain 17% of the total variance of the disease.
These results were independently confirmed in a second study in
which plasma chromium concentrations of 23 healthy persons were
more than 8 times greater than those of 67 patients with coronary
artery disease, as confirmed by cine-angiography (Simonoff et al.,
1984).
8.1.3. Mode of Action
As in experimental animals, the best known action of chromium
is the potentiation of insulin. This is shown by the
supplementation studies of Doisy et al. (1976) and Liu & Morris
(1978) in which a reduction in insulin levels in response to
glucose was observed, together with an improvement in glucose
tolerance. Direct proof, for example from in vitro studies with
human biopsy tissue, has not yet been obtained. Furthermore, it is
not known whether the potentiation of insulin is responsible for
all the effects observed during chromium supplementation, including
the reduction of circulating cholesterol and the growth stimulation
observed in malnourished children and during parenteral nutrition.
In the healthy, chromium-sufficient human subject, chromium
levels in the bloodstream increases acutely following a glucose
load or an injection of insulin. Glinsmann et al. (1966) observed
acute increases in 5 young, healthy volunteers during a glucose
tolerance test, but not when the same subjects were given an
equivalent volume of water. Maturity-onset diabetic patients did
not exhibit this chromium response initially, but did show a rise
in plasma-chromium, after several weeks of chromium
supplementation. These observations were confirmed by Levine et
al. (1968), Hambidge (1971), Behne & Diel (1972), and Liu & Morris
(1978), who saw the acute chromium rise in some, but not all,
subjects. In the study of Liu & Morris (1978), which was the most
detailed, the relative chromium response (CrR) was measured by
neutron activation analysis in 15 women with normal glucose balance
and in 12 with impaired tolerance.
(1-h serum-chromium)
CrR = ------------------------ x 100
(fasting serum-chromium)
Initially, the normal group had a mild positive response (CrR =
107), compared with a negative response (CrR = 81) in the impaired
subjects. Following three months of supplementation with a
chromium-rich yeast extract, the CrR of the normal subjects
increased to 140, and that of the impaired group became positive
with CrR = 149. As discussed in section 8.1.2, these changes were
accompanied by a significant decrease in the insulin response to
glucose and an improvement in glucose tolerance. The results of
these studies are in apparent contrast with those of Davidson &
Burt (1973), who found a significant decline in plasma-chromium
levels following a glucose load in normal, non-pregnant women (mean
age 26 years), but a mild to moderate increase in all but 1 of 10
pregnant women (mean age 20 years). As the time of the acute rise
varied between women, statistical analysis at any one time did not
produce any significant results. Hambidge (1971) and Behne & Diel
(1972) demonstrated an acute plasma-chromium response following
injection of insulin; it can therefore be assumed that the effect
of glucose on the chromium response is associated with
hyperinsulinaemia. The acute "relative chromium response" is
believed to reflect the chromium status of human subjects. It does
not appear when stores from which the chromium increment must come
are depleted. Depleted stores can be replenished by
supplementation, with a resulting reappearance of the response
shown in the two previously discussed studies. As it has been
shown that chromium levels in urine increase following a glucose
load (Wolf et al. 1974; Gürson & Saner, 1978), it is likely that a
fraction or all of the chromium increment is subsequently excreted.
8.2. Acute Toxic Effects
In adults, the lethal oral dose of soluble chromates is
considered to be between 50 mg/kg body weight (RTECS, 1978) and 70
mg/kg body weight (Deichmann & Gerarde, 1969). The clinical
features of toxicity are vomiting, diarrhoea, haemorrhagic
diathesis, and blood loss into the gastrointestinal tract causing
cardiovascular shock. If the patient survives about 8 days, the
outstanding effects are liver necrosis (Brieger, 1920), tubular
necrosis of kidneys, and poisoning of blood-forming organs
(Langard, 1980). A 14-year-old boy, who had ingested about 1.5 g
of potassium dichromate, died 8 days later. Kaufmann et al. (1970)
also reported enlargement and oedema in the boy's brain.
8.3. Chronic Toxic Effects
The chronic effects of trivalent and hexavalent chromium have
been reviewed by Langard & Norseth (1979). The most important
features are changes in the skin and mucous membranes, and allergic
dermal and broncho-pulmonary effects. Important systemic effects
occur in the kidneys, liver, gastrointestinal tract, and
circulatory system.
Mancuso (1951) studied several biological variables in persons
occupationally exposed to chromate and related them to clinical
signs and symptoms. He found occasional leukocytosis or
leukopenia, monocytosis, eosinophilia, reduced haemoglobin
concentrations and prolonged bleeding times, but none of them were
consistent enough to serve as reliable diagnostic tests.
Mild normochronic anaemia was reported by Myslyaeva (1965) in
94 subjects exposed to chromium compounds with 68 of them showing
signs of chronic toxicity. Though the report of Baetjer et al.
(1959b) was based only on 3 cases, it indicated that chromate
attached preferentially to red blood cells. Koutras et al. (1964)
reported the inhibition of the red blood cell enzyme gluthathione
reductase by chromate concentrations of 5 - 25 mg/kg body weight.
However, the lowest effective dose is far in excess of levels
found, even in heavily exposed persons. Thus, all attempts made so
far have failed to identify a specific sensitive biochemical test
for the assessment of chronic chromium toxicity.
Long-term surveillance of pregnant women working at a plant
producing dichromates and those living in its immediate vicinity
(Shmitova, 1980) revealed increased chromium levels in the blood
and urine, compared with those in the control group. At 32 weeks
of pregnancy, the blood concentrations of chromium were,
respectively, 2.5 mg/litre in the women workers, 1.2 mg/litre in
the women living near the plant, and 0.19 mg/litre in the controls;
the levels in the urine were 1, 0.47, and 0.045 mg/litre,
respectively. Umbilical venous blood contained 1.8, 0.66, and 0.37
mg chromium/litre, respectively. The placental chromium contents
in the surveyed women were 162, 179, and 40 µg/kg, respectively,
and the breast-milk levels were 119, 20, and 31 µg/litre, which
means that chromium is capable of transplacental entry into breast
milk and into the fetal and infant organism. Women working at the
bichromate plant exhibited a high incidence of obstetric pathology
and geotoses. Chromium levels were estimated in fetal and
placental tissues (abortive material) derived on the 12th week of
pregnancy from women workers of the dichromate facility and those
not exposed to chromium. In the test group, chromium levels were
1140 µg/kg in the fetal tissues and 135 µg/kg in the placental
tissue, whereas, in the controls, they were 928 and 30 µg/kg,
respectively. No teratogenic effects have been revealed (Shmitova,
1980).
8.3.1. Effects on skin and mucous membranes
Several different types of effects on skin and mucous membranes
may result from exposure to chromium chemicals, and the main
features are:
(a) primary irritation: ulcers (corrosive reactions),
scarred and non-ulcerative contact dermatitis;
(b) allergic contact dermatitis: eczematous and
noneczematous.
A general survey of the nature and cause of contact dermatitis
is given by Fregert (1981).
8.3.1.1. Primary irritation of the skin and mucous membranes
(a) Skin
Skin irritation, manifested by a sharp hyperaemia as well as by
vesicular, papular, and rash pattern, may result from contact with
chromates. However, the major problems in the primary irritation
dermatoses are ulcers, often called chrome holes or chrome sores.
Ulceration is likely to occur among workers who have contact with
high concentrations of chromic acid, sodium or potassium dichromate
or chromate, or ammonium dichromate. It does not result from
contact with trivalent chromium compounds. An ulcer may develop if
the chromium compound, either a dust or a liquid, comes into
contact with any break in the skin such as an abrasion, scratch,
puncture, or a laceration. Favoured sites for ulcer development
are the nailroot areas, the creases over the knuckles, finger webs,
the backs of the hands, and the forearms (Bloomfield & Blum, 1928).
Primary irritation by chromium can be attributed to hexavalent
chromium, as the salts of trivalent chromium are not considered
capable of producing such effects (Fregert, 1981). However,
prolonged exposure to trivalent chromium salts has been reported to
cause skin lesions, less marked than those following exposure to
hexavalent chromium salts (Domaseva, 1971). Findings in 164
patients with eczema showed that skin tests on people who had
contact with trivalent chromium did not give positive results with
dichromate (Valer & Racz, 1971).
Ordinarily, a chrome sore, if not deep, persists for about 3
weeks after exposure is discontinued. There is no evidence that
the ulcers undergo malignant transformation. Furthermore, the
presence of chromium ulcers did not influence the development of
sensitization to chromates (Edmundson, 1951), evidenced by patch
testing with a 0.5% solution of K2Cr2O7.
(b) Nasal septal mucosa
Perforation of the nasal septum was one of the main chronic
effects in workers who had contact with chromates and chromic acid
(Bloomfield & Blum, 1928; Langard & Norseth, 1979). In a group of
100 persons constantly in contact with chromium compounds, 44 had
superficial ulceration of the anterior nasal septal mucosa.
Twenty-one people had profound ulcers and 5 had perforations of
various sizes in the cartilaginous part of the nasal septum.
Perforation of the nasal septum and cicatricial alterations were
observed in people with 3 or more years of service, whereas
ulcerous lesions arose in 2 years of work with chromium compounds.
An additional consequence of the effect on the nasal mucous
membranes is a loss of the senses of smell and taste (Seeber et
al., 1976). In workers with profound ulcerous lesions of the nasal
septal mucosa, strains of white staphylococcus with elevated
resistance to chromium are sometimes isolated (Cherevaty et al.,
1965).
Pokrovskaya et al. (1976) studied 561 workers engaged in the
open excavation of chromium ores (chromium content was 62% in the
form of a complex compound of magnesium chromite and other chromium
ores). Almost half of the workers had worked there for more than 5
years, and about 40% had been working there for 6 - 15 years.
Changes in the nasal septal mucosa, typical of the effect of
chromium compounds, were found in 8.2% of the workers. The longer
the service record, the greater the prevalence of these lesions.
After 5, 11, and 15 years of work, the prevalence was 2.3%, 8.3%,
and 11.2%, respectively.
The prevalence of nasal septum perforation in chromium plating
workers was very high (24%) in the early 1950s in China (Yang,
1956). With the introduction of control measures, the prevalence
dropped so that, in 1982, only 2 cases of nasal septum perforations
were found among 393 chromium-exposed workers (Peng, 1982).
One hundred and four subjects (85 male, 19 female) exposed to
chrome plating and 19 unexposed controls were studied by Lindberg &
Hedenstierna (1983) with regard to changes in the nasal septum.
Exposed subjects were employed at 13 different companies and
included all who were working on the day of study at the particular
company. The median exposure time was 4.5 years (range, 0.1 - 36
years). Forty-three subjects were exposed almost exclusively to
chromic acid and constituted both a "low exposure" group (8-h mean
below 2 µg/m3, N = 22) and a "high exposure" group (2 µg/m3 or
more, N = 21). Their median exposure time was 2.5 years (range,
0.2 - 23.6 years). The other 61 subjects were exposed to a mixture
of chromic acid (0.2 - 1.7 µg/m3) and other pollutants, such as
nitric, hydrochloric, and boric acids, as well as caustic soda,
nickel, and copper salts. The last group was included to disclose
any additive or synergistic effects of chromic acid and other
pollutants and was studied with regard to lung function only. The
breakdown of subjects into various groups is shown in Table 25.
Nasal septal ulceration and perforation were seen in 10 out of 14
subjects exposed to peak levels of 20 - 46 µg chromic acid/m3 or
more; no one in the control group showed signs of atrophy,
ulcerations, or perforations.
8.3.1.2. Allergic contact dermatoses
Chromium is a very important skin sensitizer. Sensitization
is reported to require about 6 - 9 months, but can occur in less
than 3 months (Pirilä & Kilpio, 1949). Genetic factors appear to
contribute to the appearance of chrome sensitivity within the
Jewish population (Wahba & Cohen, 1979).
Apart from the primary irritation and ulceration effects,
direct contact with small amounts of the chromates may cause
allergic dermatosis (dermatitis and eczema). As this allergic
reaction is cell-mediated, i.e., antibody formation is intra-
cellular and not humoral, delayed allergic reactions are rarely
observed.
Fregert et al. (1969) reported that the skin-patch test for
chromium was positive in 8 - 15% of all patients suffering from
eczema. The dermatitis caused by chromium is described as a
diffuse erythematous type with severe cases progressing to an
exudative stage. Persons sensitive to chromium react to patch and
intracutaneous tests with nonirritant concentrations of potassium
dichromate (K2Cr2O7). Opinion is divided about the role of the
oxidation state of chromium as a causal agent in allergic contact
dermatoses (Morris, 1958; Fregert & Rorsman, 1964; Kogan, 1971).
In a review, Polak et al. (1973) showed that hexavalent chromium,
which penetrates the skin by a direct effect of a hapten, is
reduced to the trivalent state, chiefly by sulfhydryl groups of
aminoacids. It then conjugates with proteins thus forming the full
antigen that initiates sensitization.
Guinea-pigs sensitized with either trivalent chromium chloride
or hexavalent potassium dichromate are capable of reacting in vivo
and in vitro to challenges with both chromium salts. This double
reactivity is also found after repeated stimulation with only one
of these chromium compounds. Since it is not possible to select
lymphocytes directed specifically against a chromium determinant of
a particular valence, it is concluded that, by sensitization with
chromium salts of different valences, a common determinant or
closely related determinants are formed. It is suggested that this
determinant is formed by chromium in the trivalent form
(Siegenthaler et al., 1983).
Two to three years after medical treatment, a follow-up study
on 555 patients with contact dermatitis was completed by means of
questionnaires (Fregert, 1975). The eczema had healed in one-
quarter of the patients, one-half had periodic symptoms, and one-
quarter had permanent symptoms. The prognosis for persons who
changed their work or stopped working was the same as that for
those who continued eczema-inducing work.
From 48 positively tested patients (0.5% potassium dichromate),
38 persons, i.e., 79%, still had a positive patch test after 4 - 7
years. In 72% of the cases, a history of occupational exposure to
chromates could be proved (Thormann et al., 1979).
A fairly rapid transformation of dermatitis into eczema is a
characteristic feature of chromium-induced skin lesions. Kogan
(1971) recognized the following important properties: (a) absence
of adaptation, which makes it necessary to discontinue chromium
handling until the slightest clinical manifestations have
disappeared; (b) a persistent chronic development of an eczematous
process; (c) a long-term latent period; and (d) absence of a direct
correlation between the incidence of sensibilization in the exposed
workers and the chromium concentration to which they have been
exposed.
Table 25. Series of exposed subjects and referencesa
--------------------------------------------------------------------------------------------------------
Exposed subjects
Mixed exposure to
Low exposure to High exposure to chromic acid
chromic acid chromic acid (< 2 µg/m3) and other
(< 2 µg Cr6+/m3) (> 2 µg Cr6+/m3) acids and metallic salts References
----------------- ------------------ ----------------------- -----------------
Nb Age (years)c N Age (years) N Age (years) N Age (years)
--------------------------------------------------------------------------------------------------
Men
Non-smokers 10 35.8 ± 16.7 6 33.4 ± 14.1 12 33.6 ± 12.2 52 34.2 ± 13.2
Smokers 6 30.8 ± 9.7 16 30.2 ± 11.8 36 42.8 ± 13.1 67 30.5 ± 8.9
Women
Non-smokers 3 30.7(17-46) 0 9 48.3 ± 13.0
Smokers 2 44.5(29-60) 1 51.2 4 31.0(19-43)
--------------------------------------------------------------------------------------------------
a From: Lindberg & Hedenstierna (1983).
b When N < 5, the range is given instead of SD.
c X ± SD.
Patients suffering from chromium-induced allergic skin
dermatoses had a tendency to develop cross-correlated hyper-
sensitivity to other metals, in particular, to cobalt and nickel
(Rostenberg & Perkins, 1951; Geiser et al., 1960; Clark & Kunitsch,
1972; Kogan et al., 1972).
Increased sensitivity to chromium is proved by skin reaction
tests. In a number of cases, additional immuno-haematological
bioassays are made to confirm the specificity of these tests. A
method for studying the basophil (mast-cells) degranulation in vivo,
at the site of positive compress skin tests, was suggested by Kogan
(1968). The method was used as a basis for proving the specificity
of cutaneous tests for chromium and other sensitising metals of the
chromium group (cobalt and nickel) (Kogan, 1968; Kogan et al.,
1972).
Photosensitivity is sometimes observed in patients with chronic
dermatitis. Wahlberg & Wennerstein (1977) investigated patients
allergic to chromium, with or without clinically observed light
sensitivity, by a standardized photopatch test procedure. A
significantly more intense reaction was observed in 48% of the
cases in the UV-irradiated sites (4/5 minimal erythema dose)
compared with the non-irradiated control sites.
No cases of cancer of the skin have been reported to result
from exposure to any form of chromium (Fregert, 1981).
8.3.2. Effects on the lung
Airborne chromium trioxide is rapidly absorbed in the broncho-
pulmonary tract causing corrosive reactions (Borghetti et al.,
1977). The absorption of chromium aerosols may depend on the
physical and chemical properties of the particles (Taylor et al.,
1965), as well as on the activity of alveolar macrophages, and the
lymphatic drainage (Sanders et al. 1971).
Both Jindrichova (1978) and Keskinen et al. (1980) reported
that welders using chromium-containing electrodes suffered from
bronchitis. Zober (1982) studied arc welders who had used filler
metals containing chromium and nickel for some years. Compared
with controls, the welders reported more previous ailments of the
respiratory system, mainly in the form of acute bronchitis. In
X-rays of welders, more roundish shadows were found, probably
indicative of benign accumulations of ferrous dust. The greater
incidence of significant findings was clearly apparent in welders
who smoked (probably a combined effect of tobacco smoke and welding
fumes). Reggiani et al. (1973) studied 101 electroplaters by means
of spirometric tests and found an obstructive disease pattern in
13% of them. After inhalation of acetylcholine, a bronchospastic
reaction occurred in 23% of the workers. In another spirometry
study (Bovet et al., 1977) on 44 chromium electroplaters, an
obstructive respiratory syndrome was found among the workers with a
high urinary-chromium content. The authors concluded that the
influence of tobacco smoking on the spirometric tests was much less
than the influence of chromium.
Studying lung function and generalized obstructive lung
diseases in electrofurnace workers in a ferrochromium and
ferrosilicon-producing plant, Langard (1980b) found a reduced
forced vital capacity (FVC) and an increased prevalence of
obstructive lung diseases. Unable to document a connection between
the presence of chromates and increased lung diseases, the author
suggested that the effects were due to high levels of total dust,
especially amorphous silica dust.
Lindberg & Hedenstierna (1983) studied lung function in more
than 100 subjects exposed to chrome plating and in 119 car
mechanics as controls (no car painters or welders). In the exposed
subjects, FVC and the forced expired volume in one second (FEV1)
were both reduced by 0.2 litre, and the forced mid-expiratory flow
was reduced by 0.4 litre/second. The authors concluded that an 8-h
mean exposure level of more than 2 µg/m3 might cause a transient
decrease in lung function.
Pneumoconiosis was diagnosed in Germany (Letterer et al., 1944)
and again in chromite miners in South Africa (Sluis-Cremer & du
Toit, 1968). However, American medical officers could not confirm
this feature when examining 897 workers in chromate-producing
plants in the USA (US PHS, 1953). It should be noted that chromium
dusts had a poor fibrogenic potency in experimental animal studies
(section 7.2.1.5). Davies (1974) X-rayed workers employed in the
manufacture of ferro-chrome and found results similar to miliary
lung tuberculosis. The workers were in the immediate area of the
furnace and were exposed to dust-particles, 90% of which were less
than 2 µm in diameter. The dust deposited on girders consisted of
95% SiO2 and 2.4% Cr2O3. The author did not find any symptoms or
signs of tuberculosis, but there was sufficient evidence to
indicate an inhalation hazard in the ferro-chrome industry, thus
underlining earlier observations of Princi et al. (1962).
8.3.2.1. Bronchial irritation and sensitization
Chromium irritates mucous membranes, as has been seen with
exposure to airborne chromium trioxide fume (Meyers, 1950) or mixed
dust (Card, 1935; Broch, 1949; Williams, 1969). The first symptom
was an irritating cough, 4 - 8 h after exposure. Chromium causes
sensitization that can result in asthmatic attacks. These can last
for 24 - 36 h, without treatment (Langard & Norseth, 1979). An
attack may recur on later exposure, even when this exposure is to a
much lower concentration (US NAS, 1974a).
A double-blind experimental exposure study on an asthmatic
patient, who had been exposed to chromium and nickel, showed that
chromium was the main cause of the asthma (Novey et al., 1983).
Keskinen et al. (1980) pretreated their patients, stainless steel
welders, with both disodium cromoglycate and betamethasone and
found an inhibition of this reaction. Placebo medication failed to
produce this inhibition.
8.3.3. Effects on the kidney
In the early part of this century, chromates and chromic acid
were occasionally used as therapeutic chemicals for some skin
lesions. Fatal cases of acute nephritis occurred with albumin,
hyaline, and granular casts and red cells appearing in the urine,
accompanied by oliguria. Autopsies revealed tubular necrosis, but
not glomerular damage (Kaufman et al., 1970). Acute toxic
gastroenteritis developed, followed by arterial hypotonia, in
suicide cases a few hours after swallowing 1.5 - 10 g potassium
dichromate. Within 2 - 4 days, all patients developed signs of
acute renal insufficiency with oliguria, anuria, and hyperhydration
and also evidence of acute toxic hepatitis (Luzhnikov et al.,
1976). Kidney damage has also occurred in industry, as a result of
accidental exposure of the skin to large amounts of chromate,
especially when associated with extensive skin damage (Luzhnikov et
al., 1976).
The mortality from kidney diseases among workers in the
chromium industry does not appear to have increased. In one study,
no deaths from nephritis and uraemia were found in 4 chromate-
producing plants; in only 1 plant was the mortality rate above that
for the controls (Machle & Gregorius, 1948). Hayes et al. (1979)
reported that 4 deaths from nephritis and nephrosis had occurred
between 1945 and 1977 in workers in a chromate-producing plant,
compared with 4.95 cases in the control population. Satoh et al.
(1981) found one case of nephritis and nephrosis in a group of
chromate workers compared with an expected number of 2.18.
Some authors (Franchini et al., 1975; Borghetti et al., 1977;
Gylseth et al., 1977; Tola et al., 1977) have concluded that
urinary excretion and renal clearance of diffusible chromium can be
used as biological indicators for evaluating the extent of exposure
to airborne chromium and the body burden. However, a dose-effect
relationship could not be established between duration of exposure
and either the level of chromium excretion (Tandon et al., 1977) or
early nephrotoxic indicators (Mutti et al., 1979). A dose-
response relationship for hexavalent chromium effects on the kidney
was described by Mutti et al. (1979), measuring beta-glucuronidase,
protein, and lysozyme in the urine. Abnormal patterns were not
observed in 39 stainless-steel welders during 4.5 ± 3.2 years of
exposure. In another group of workers employed in welding armoured
steel with special electrodes for 1 ± 0.4 years, 22% showed an
increase in beta-glucoronidase and 10% in protein in the urine. In a
study on 24 workers in the chromium-plating industry who were
exposed for 8.9 ± 7.3 years, the authors found increased urinary
levels of beta-glucoronidase in 37% of the workers, an increase in
protein in 17%, and an elevated level of lysozyme in 4%. Urinary
beta2-microglobulin was also studied by Lindberg & Vesterberg (1983b)
in chromeplaters (24 males, mean age 36 years, working in 4
different factories). The exposure was measured using personal air
samplers. The 8-h mean value ranged between 2 and 20 µg hexavalent
chromium/m3 and averaged 6 µg/m3. Most of the workers showed
symptoms of airway irritation, 2 of them had ulcerated nasal septum
and another 2, complete perforation. Significantly higher urine
beta2-microglobulin levels (0.23 mg/litre; range, 0.04 - 1.24 mg/litre;
SD, 0.27) were found in this group of workers in comparison with a
reference group of workers of a comparable age (mean age, 38 years;
mean urinary beta2-microglobulin level, 0.15 mg/litre; range, 0.06 -
0.19 mg/litre; SD, 0.08). A relationship was observed (Table 26)
between the range of exposure and prevalence of urinary beta2-micro-
globulin levels exceeding 0.3 mg/litre. The 2 workers with
perforated nasal septum were in the highest exposure group. A
difference in urinary beta2-microglobulin levels was not found between
a group of workers employed several years earlier in an old
chromeplating plant (mean, 0.25 mg/litre) and a group of workers of
comparable age (mean, 0.29 mg/litre), but it should be noted that
the mean age of both these groups exceeded 52 years.
Table 26. beta2-microglobulin U-beta2 levels in urine of workers
related to present exposure levelsa
-------------------------------------------------------------------------
Plant Exposure Number Age Persons with an "elevated" U-beta2
No. range of (mean) (U-beta2 > 0.30 mg/litre) range
(µg/m3) workers concentration (mg/litre)
No. Ages
-------------------------------------------------------------------------
1 11 - 20 5 39 3 21, 37, 58 0.23 - 1.30
2 - 3 14 - 8 13 37 2 20, 21 0.04 - 0.44
4 2 - 3 6 6 - - 0.06 - 0.18
-------------------------------------------------------------------------
a From: Lindberg & Vesterberg (1983b).
8.3.4. Effects on the liver
Statistics on diseases of the liver are seldom published
separately; they are usually included in the group of diseases of
the digestive system. The chromate industry was reported to have
the same rate in this group of diseases as many other industries
(US PHS, 1953). The Federal Security Agency in the USA
investigated 14 chromate workers reported to have "enlarged livers"
but found that these cases were not related to the time spent in
the industry. Acute hepatitis with jaundice was described in one
worker employed in a chromium-plating plant.
In an epidemiological study, Satoh et al. (1981) reported a low
rate of cirrhosis of the liver in chromium workers and liver
function test results that did not differ significantly from those
of the controls.
However, following ingestion of high doses of potassium
dichromate, liver necrosis (Brieger, 1920) and congested liver
(Kaufman et al., 1970) with loss of its architecture have been
described. There are also case studies of liver function and
histology in electroplaters (Pascale et al., 1952) and chromate
chemical plant workers (Etmanova, 1965), but the results are
difficult to interpret.
8.3.5. Effects on the gastrointestinal tract
The following symptoms and signs were reported in workers
engaged in the production of chromium salts (Sterekhova et al.,
1978): hyperchlorhydria, elevated pepsin and pepsinogen level,
oedema, hyperaemia and erosion of the mucosa, polyposis,
dyskinesia, and gastritis. However, Satoh et al. (1981) reported
that the incidence of peptic ulcer in chromate workers was below
the expected rate, and another study showed that the mortality rate
for diseases of the digestive system was lower in chromate workers
than in the control population (Hayes et al., 1979).
The gastric juice has been shown to play a role in the
detoxification of ingested hexavalent chromium by reducing it to
trivalent chromium, which is poorly absorbed and eliminated with
the faeces (Donaldson & Barreras, 1966; DeFlora & Boido, 1980).
8.3.6. Effects on the circulatory system
A clinical study was performed by Kleiner et al. (1970) on
myocardial function in more than 200 workers in the potassium
chromate industry, suffering from pulmonary or gastrointestinal
diseases, attributed by the authors to chromium poisoning. A
control group of 70 healthy individuals was included. Pathological
changes in the ECG and in other tests of heart function indicated
disturbance of right heart function, which the authors considered
secondary to the pulmonary pathology. The full paper was not
available and, consequently, the data could not be evaluated in
detail. It should be noted that no unexpected findings in
cardiovascular mortality were cited in the epidemiological studies
described in section 7.3.9.
8.3.7. Teratogenicity
The only human data available concerning this topic were
reported in an abstract (Morton & Elwood, 1974). The authors did
not find any correlation between frequency of malformations in the
central nervous system and the chromium content in water samples
collected in South Wales. Furthermore, Suzuki et al. (1979) did
not find any indication of accumulation of chromium in fetal
tissues.
8.3.8. Mutagenicity and other short-term tests
Few reports exist dealing with chromium-induced mutagenicity in
human subjects, and only hexavalent chromium causes any effects.
Bigaliev et al. (1978) found a 3 - 8% increase in chromosomal
aberrations of peripheral blood leukocytes in workers handling
chromium-compounds, compared with 2% in unexposed controls.
When classified according to the Papanicolaou system,
cytological samples of the sputum of 116 workers in the chromate-
production industry did not show any stages of tumour development
(Maltoni, 1976). However, intermediate stage lesions found in 30
workers were described as atypical adenomatous proliferation,
squamous-cell, and basal-cell dysplasia.
Studying cultured lymphocytes obtained from workers
occupationally exposed to CrO3, Sarto et al. (1982) found an
increased frequency of sister chromatid exchanges compared with
controls, which was correlated with urinary-chromium levels and
enhanced by smoking habits.
8.3.9. Carcinogenicity
8.3.9.1. Lung cancer
(a) Epidemiological studies in chromate-producing plants
The first recognition that cancer of the respiratory tract
might be related to chromium exposures resulted from the reporting
in 1932 of 2 cases of bronchogenic carcinoma that had occurred in
an old German chromate chemical plant (Lehmann, 1932). Gross &
Kölsch (1943) reported 10 deaths from lung cancer in small plants
producing the chromates of lead and zinc. By 1947, 52 such cases
had been reported from this industry and 10 cases from a chrome
pigment plant, also in the Federal Republic of Germany (Baetjer,
1950a).
The first epidemiological study was reported in the USA in
1948. The mortality data were based on the records of the group
life insurance policies of 6 chromate-producing plants in the USA
compared with similar data from a non-chromium industry or from a
life insurance company. The study reported that 21.8% of all
deaths between 1938 and 1947 in the chromate plants were due to
cancer of the respiratory tract compared with 1.4% in the control
group. The crude death rate for cancer of the bronchi and lungs
was 28 times that for the control groups (Machle & Gregorius,
1948). In one of these plants (Mancuso & Hueper, 1951), the cancer
death rate was 15 times that of the general population in the
county where the plant was located. The US PHS (1953) also studied
the respiratory cancer risk in these 6 plants and estimated a
relative risk of 28.9 using the average cancer mortality rates for
US males between 1940 and 1948 as a comparison.
In addition to these studies, where the cause of death was
taken from insurance or death certificate records, a case-control
study, using only cases diagnosed by autopsy or biopsy, was
conducted by Baetjer (1950b). Two hospitals near the largest
chromate plant in the USA were asked to supply the histories of all
cases diagnosed in 1924-46 as cancer of the lung bronchi, and to
confirm histopathologically. A group of non-cancer cases matched
individually by age, sex, and date of admission was selected as
controls. Of the 290 confirmed cancer cases, 3.26% had been
employed in the chromate plant, whereas none of the 900 control
cases was found to have had chromate exposure. The percentage of
chromate workers in the lung cancer series was 28.6 times the
percentage of chromate workers among the employed male population
of the city. The plant involved in this study was rebuilt in 1950,
following the discovery of the cancer problems. A new study of
this plant was made by Hayes et al. (1979) to determine whether the
risk of cancer had been eliminated. The investigation covered 438
deaths among 2101 males, who were initially employed between 1945
and 1974 for more than 90 days, and who died between 1945 and 1977.
The SMRa for malignant neoplasms of the trachea bronchi and lungs
was 202. Cancer deaths in the city in which the plant was located
were used to calculate the expected number of cases. No cases of
respiratory cancer occurred in the plant between 1960 and 1977
among workers employed only in the new plant. Because of the long
latent period, further follow-up is necessary.
Increased lung cancer rates among chromate workers in the USA
have been confirmed by Taylor (1966), Enterline (1974), and Mancuso
(1975), who also showed that the risks were especially high during
the early years of the study of the cohorts, e.g., before 1950.
Studies conducted in other countries have shown similar results.
In a 1956 study on workers in chromium-producing plants in the
United Kingdom, the rate of lung cancer mortality was 3.6 times the
rate for England and Wales (Bidstrup & Case, 1956). In a follow-up
study on 2715 men who had worked at the 3 chromate-producing
factories in the United Kingdom from 1948 to 1977, Alderson et al.
(1981) found 116 deaths from lung cancer (expected, 48). Since
modification of the one plant that is still in operation, the
relative risk of lung cancer has decreased from over 3 to about
1.8.
Two epidemiological studies have been conducted in the Japanese
chromate industry. In one plant, 5 lung cancer deaths occurred
between 1960 and 1973 in 136 workers who had been exposed for more
than 9 years; the SMR was 1510 (Watanabe & Fukuchi, 1975). In
another plant, the mortality rate for respiratory cancer was
determined for 896 males who worked in the plant from 1918 to 1975
and were followed to 1978; the SMR was 920 (expected deaths based
on 1975 age-cause-specific mortality rates for Japanese males)
(Satoh et al., 1981).
In the Federal Republic of Germany, a study was conducted in 2
chromate-producing plants (Korallus et al., 1982). Comparing the
SMR for lung cancer during the period 1948-79 for these 2 groups, a
clearly decreasing tendency was observed. In the first plant, for
1948-52, the SMR was 512 and, for 1978-79, it was 98; for the other
plant, the corresponding figures were 1905 and 150, respectively.
The newest data indicate a decreasing risk approaching the
frequency in unexposed people (Korallus & Loenhoff, 1981). This is
a result of occupational hygienic and technological measures,
especially of a change in manufacture namely the "on line"
--------------------------------------------------------------------
a The SMR (standardized mortality ratio) is the ratio of
observed to expected deaths times 100.
processing of the chrome ore, which has minimized the contents of
the carcinogenic hexavalent chromium compounds within the process
(Korallus et al., 1982).
Bittersohl (1972) described the results of a study on 30 000
employees of a large chemical unit for the period 1921-70 in the
German Democratic Republic. In particular, 588 malignancies in men
and 170 in women were evaluated for the period 1957-70. In 1971,
108 new malignancies in men and 29 in women came to light. In a
chromate factory, the rate of all cancers was far above average.
The factory manufactured catalysts through the reaction of chromic
acid and iron (III) oxide and nitric acid. The airborne
concentrations of chromium were not reported in detail, but it was
stated that short-term exposures above 400 µg/m3 occurred. The
incidence of malignant neoplasms in employees in the chromate
factory was 852 per 10 000 employees. In "unexposed" personnel,
the incidence of malignant neoplasms was 84 per 10 000 employees.
Approximately 86% of all those with malignant neoplasms were
smokers, and 78% of those without malignant neoplasms were also
smokers, indicating that smoking is not likely to be a confounding
factor.
In the USSR, a high incidence of cancer of the lungs was
reported for men engaged in the production of chrome salts. The
ratio of lung cancer in the plant to that in the control population
was 6.4 for ages 50 - 59 (748 observed, 116 expected) and 15.7 for
ages 60 - 69 (2657 observed, 170 expected) (Tyushnyakova et al.,
1974).
A survey was conducted in China in 1982 to investigate the
problem of lung cancer among chromate-producing workers. It
covered 7 cities and included 2184 male and 798 female workers who
had worked for at least one year in the industry. The preliminary
report revealed that 11 of the 101 deaths among male workers were
due to lung cancer, but none of the 13 deaths among female workers
was due to this cause.a
(b) Epidemiological studies - chrome pigment industry
The first epidemiological study, conducted in 1973, covered 3
small chrome pigment-manufacturing plants in the USA. The
percentage of lung cancer deaths was tentatively reported to be
about 3 times that in unexposed workers (Equitable Environmental
Health Inc., 1976).
Lung cancer mortality was studied among 1152 men working at 3
chromate pigment factories in the United Kingom from the 1930s or
1940s until 1981 (Davies, 1979, 1984). Among workers at factory A,
which produced both zinc and lead chromates, entrants to the
factory between 1932-45 with high and medium exposures had excess
---------------------------------------------------------------------------
a Report of an Investigation Team for Cancer in Chromate
Workers (1983).
risks of lung cancer (SMR = 223). A similar excess was seen in
1946-54 entrants, but the excess risk fell just short of
statistical significance. Highly significant excess risks of lung
cancer were seen among 1947-64 entrants that had high or medium
exposures at factory B, which also produced lead and zinc
chromates. Excesses were more severe in workers employed for more
than 10 years. In factory C, which produced only lead chromate, no
excess of lung cancer was found. However, the number of workers
and the power of the study were small.
Twenty-four male workers, who had been employed in a small
Norwegian company for more than 3 years between 1948 and 1972, were
identified and followed up by Langard & Norseth (1975), because
their principal exposure was to zinc chromate dust (measured
routinely with readings occasionally up to 0.5 mg hexavalent
chromium/m3). By December 1980, 6 cases of lung cancer had been
diagnosed, giving a relative risk of 44 compared with that of the
general population in the country. The authors stated that 5 out of
the 6 patients were smokers and only one had been exposed to
chromates other than zinc chromates (Langard & Vigander, 1983).
Sheffet et al. (1982) undertook a detailed mortality study on
workers employed in a pigment plant in Newark, where lead and zinc
chromates were used. Observed deaths from each cause among 1296
white and 650 non-white males employed between 1940 and 1969, were
compared with expected deaths, as computed from cause-, age-, and
time-specific standard death rates for the USA. A statistically-
significant relative risk of 1.6 for lung cancer among white
males, employed for 10 years or more, was found. A relative risk
of 1.9 was noted for individuals employed for at least 2 years, who
were at least moderately exposed to chromates.
An epidemiological study covering several centres in Europe was
conducted by Frentzel-Beyme (1983). This study was designed to
quantify the mortality from cancer and other diseases among workers
in European factories producing chromate pigments (3 German, 2
Dutch factories). Observed deaths in factories were compared with
expected deaths calculated on the basis of mortality figures for
the region in which a given factory was located. The overall
mortality did not deviate from the expected rates. Lung cancer
rates were always in excess of expected numbers, but only in one
cohort was the excess statistically significant. The pattern of
duration of exposure indicates that the lung-cancer risk was not
clearly correlated with length of employment.
A slight excess of lung cancer risk in chrome pigment (zinc
chromate) spray painters from 2 maintenance bases in the USA was
reported by Dalager et al. (1980). Among the 202 deaths
identified, 21 were due to respiratory cancer (11.4 expected;
comparison population: proportionate mortality, US males). No
statistically significant excess of lung cancer was found in a
group of automobile painters in which 226 deaths, including 22
cancer cases, were analysed using proportionate mortality for areas
in which the plants were located as a comparison (Chiazze et al.,
1980).
(c) Epidemiological studies - ferro-chrome industry
Langard et al. (1980) reported that 7 cases of bronchogenic
carcinoma occurred among 976 workers, who started work before
January 1, 1960. The expected number was 3.1 using national rates,
1.8 using local rates, and < 1 when using an international
reference rate for comparison. The difference was not significant
for the national and local rates, but was significant for the
international rate.
A study in Sweden did not reveal any significant excess of
respiratory cancer in ferro-chrome workers (Axelsson et al., 1980).
The incidence of cancer among 1932 workers employed for at least
one year between 1930 and 1975 was compared with the expected
number based on the National Cancer Registry data for the county in
which the plant was located. Five cases of respiratory cancer were
found against an expected 7.2 cases.
An excessive rate of lung cancer was reported for a ferro-
chrome plant in Russia (Pokrovskaya & Shabynina, 1973). The number
of deaths from cancer between 1955 and 1969 was higher than that in
the town in which the plant was located. The authors considered
that both hexavalent and trivalent chromium exposures were
responsible for the excess, since similar excessive rates were
found in a nearby ore-crushing plant, where only trivalent chromium
was present. The number of cases and the rates involved in these
studies were not given.
Hexavalent chromium is produced in some types of welding. In
section 7.2.1.2, it was mentioned that welding fume particles were
positive in the mammalian spot test, indicating a mutagenic and
possible carcinogenic potential. The incidence of lung cancer was
determined by Sjögren (1980), in a cohort of 234 welders, who had
welded stainless steel for more than 5 years between 1950 and 1965,
and were followed to 1977. Three welders died from lung cancer in
comparison with an expected number of 0.68 in the general
population ( P = 0.03).
Comparing lung cancer mortality in 2 parishes with ferro-alloy
industries to mortality in other parishes of similar size without
such industries from the same county, Axelson & Rylander (1980)
were unable to detect an increased incidence of death due to lung
cancer mortality. The concentration of chromium in the ambient air
of the most polluted areas ranged from 0.1 to 0.4 µg/m3. It should
be noted that several possible confounding factors were not under
control, e.g., migration, occupational exposure, and smoking
habits.
(d) Epidemiological studies - chromium-plating workers
Epidemiological studies on workers in the chromium-plating and
related industries were reviewed by Hayes (1982). The relevant
data from these studies are presented in Table 27. According to
Hayes, the epidemiological evidence is not sufficient to determine
the risk of cancer associated with industrial exposure to chromic
acid and, if an excess risk exists, it is probably lower than that
typically described for employment in the chromium-chemical
producing industry.
In a more recent paper, Franchini et al. (1983) conducted a
retrospective cohort study in 9 chromium-plating plants to examine
the mortality of workers employed for at least one year during the
period January 1951 to December 1981. The study group totalled 178
individuals; vital status ascertainment was 97% complete. The
total number of deaths was close to the expected figure (15
observed, 15.2 expected), whereas death from lung cancer exceeded
the expected number (3 observed, 0.7 expected; P = 0.03). The
increased mortality from lung cancer among chromium-platers seemed
to be related to exposure intensity.
8.3.9.2. Cancer in organs other than lungs
A few cancers of the upper respiratory tract and oral region,
but no excessive rates have been reported to occur in the chromate-
producing industry. The cancers have involved the buccal cavity,
pharynx, and oesophagus. Cancers of the nasal septum have not been
documented, with the exception of one case in Italy (Vigliani &
Zurlo, 1955).
In a joint Danish-Finnish-Swedish case-reference investigation,
initiated in 1977, the connection between nasal and sinonasal
cancer and various occupational exposures was studied. All new
cases of nasal and sinonasal cancer were collected from the
national cancer registers (Finland and Sweden) or from the hospitals
(Denmark). The results showed associations between nasal and
sinonasal cancer and exposure to chromium, welding, flame-cutting,
and soldering, hardwood or mixed wood dust, and softwood dust alone
(13.4) (Hernberg et al., 1983).
In 5 plants in the USA, during the early years of extremely
high exposures, the rate of cancer of the digestive tract varied
from 0 to 3.04/1000 compared with a rate of 0.59/1000 for the
controls (Machle & Gregorius, 1948). The mortality rates from
stomach cancer have been reported for chromate-producing plants in
Japan (SMR = 90) (Satoh et al., 1981) the USA (SMR = 40) (Hayes et
al., 1979). No significant differences were reported between the
incidence of stomach cancer in a Russian plant (Pokrovskaya &
Shabynina, 1973) and the incidence in the surrounding area.
Studying cancer mortality in a pigment plant using lead and
zinc chromates, Sheffet et al. (1982) found an increased (but not
statistically risk of stomach (SMR = 170) and pancreas (SMR = 200)
cancer among the total cohort (1946 employees).
Table 27. Summary of epidemiological studies on respiratory cancer in
workers employed in the chromium plating and related industriesa
------------------------------------------------------------------------------------------------
Study population Follow-up Respiratory cancer Comparison population
Number Estimated
relative risk
------------------------------------------------------------------------------------------------
Chromium plating
1056 workers employed Deaths in former and 24 1.8b Unexposed workers in
> 3 months in 54 plants current (followed 2 plants and in 2 non-
in the United Kingdom years) workers, plating industries
about 80% traced
About 5000 workers 49 1.4c Mortality analysis,
employed since 1945 in method not specified
1 plating factory in
the United Kingdom
889 workers exposed > 6 Reports from management 0 < 1b Tokyo mortality
months in Tokyo chromium of plating firms and rates
plating plants, 1970-76 follow-up of retired
workers; 19 total deaths
reported
Related industries
1292 deaths among US Union death benefit 62 1.1b Proportionate
metal polishers and claims mortality, US males
platers, union members,
1951-69
238 deaths among employees Pension, insurance and 39 2.1c Proportionate
for 10 years in one US benefit records mortality, US
plant for metal die
casting, finishing, and
electroplating, 1974-78.
------------------------------------------------------------------------------------------------
a Adapted from: Hayes (1982).
b Not statistically significant.
c P < 0.05, calculated using an assumption that the observed number is distributed as a
Poisson random variable.
8.3.9.3. Relationship between cancer risk and type of chromium
compound
As the exposures are often poorly defined, it is sometimes not
certain whether workers have been exposed to hexavalent chromium,
trivalent chromium, or a combination of the two. Some authors have
concluded that there is no increased risk of cancer mortality due
to trivalent chromium compounds (Korallus et al., 1974 a,b,c;
Axelsson et al., 1980; Langard et al., 1980; Norseth, 1980),
whereas others do not exclude that trivalent chromium compounds
have a carcinogenic capacity (Essing et al., 1971; Mancuso, 1975;
Zober 1979).
Summing up the data from case reports as well as
epidemiological studies in chromate-producing, chromate-pigment,
chromium-plating, and ferrochromium industries, the IARC Working
Group on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans (IARC, 1980) came to the conclusion that "there is
sufficient evidence of respiratory carcinogenicity in men
occupationally exposed during chromate production. Data on lung
cancer risk in other chromium-associated occupations and for cancer
at other sites are insufficient. The epidemiological data do not
allow an evaluation of the relative contributions to carcinogenic
risk of metallic chromium, trivalent chromium and hexavalent
chromium or of soluble versus insoluble chromium compounds".
9. EVALUATION OF HEALTH RISKS FOR MAN
Low levels of chromium are omnipresent in the environment.
Under normal conditions, human exposure to chromium does not
represent a toxicological risk, but it should be pointed out that
too low an intake of chromium may lead to deficiency. Airborne
concentrations of chromium, predominantly as trivalent chromium,
are usually below 0.1 µg/m3 and there are many places where the
concentration is below the detection limit. Concentrations in
river water are typically in the range of 1 - 10 µg/litre and do
not constitute a health threat. Drinking-water from municipal
water supplies does not contribute more than a few micrograms of
chromium to the daily human intake, but untreated water in certain
areas may be contaminated by runoff or effluents from industrial
sources and may contribute significant amounts of chromium. The
oceans contain less than 1 µg/litre. The daily human intake
through food varies considerably between regions. Typical values
range from 50 to 200 µg/day. They do not represent a toxicity
problem.
However, significant exposures exist in the occupational field.
In the past, chromium-ore mining generated chromium-containing
dusts, levels of which ranged up to 20 mg/m3 in different work-
places. Other exposures ranged up to 150 mg/m3 and consisted of
dust containing as much as 48% chromium (Cr2O3). In production
plants, hexavalent chromium can occur in the airborne state.
However, implementation of protective measures at ferrochromium
production sites reduced the airborne hexavalent chromium to levels
of 30 - 60 µg/m3.
The health effects of the two common oxidation states of
chromium are so fundamentally different that they must be
considered separately. In the form of trivalent compounds,
chromium is an essential nutrient and is relatively non-toxic for
man and other mammalian species. The hexavalent form is man-made
by oxidation of naturally-occurring trivalent chromium minerals and
is widely used. Compounds of hexavalent chromium penetrate
biological membranes easily and can thus interact with essential
constituents of the cells, including genetic material which they
can damage through oxidation and complexation with resulting
trivalent species. On the other hand, oxidation of trivalent
chromium has not been demonstrated in the living organism, and for
practical purposes, the reduction of hexavalent chromium to
trivalent chromium in lungs and other animal tissues is
irreversible.
9.1. Occupational Exposure
A number of effects can result from occupational exposure to
airborne chromium including irritative lesions of the skin and
upper respiratory tract, allergic reactions, and cancers of the
respiratory tract. The data on other effects, e.g., in the
gastrointestinal, cardiovascular, and urogenital systems are
insufficient for evaluation.
Epidemiological studies have shown that workers engaged in the
production of chromate salts and chromate pigments experience an
increased risk of developing bronchial carcinoma. No detailed data
on dose-response relationships are available from epidemiological
studies. Although a suspicion of increased lung cancer risks in
chromium plating workers has been raised, the available data are
inconclusive as are data for other industrial processes where
exposure to chromium occurs. There is insufficient evidence on the
role of chromium as a cause of cancer in any organ other than the
lung. Evidence from studies on laboratory animals shows that
hexavalent chromium compounds, especially those of low solubility,
can induce lung cancer.
In the lymphocytes of workers in chromium-plating factories,
the frequency of sister chromatid exchanges was higher in exposed
than in control groups.
Mutagenicity and related studies have convincingly shown that
hexavalent chromium is genetically active. Hexavalent chromium can
cross cellular membranes and is then reduced to trivalent chromium,
which can cause DNA cross-links and increase the infertility of DNA
implication. Trivalent chromium compounds have been shown to be
genetically inactive in most test systems, except in systems where
they can directly interact with DNA.
9.1.1. Effects other than cancer
9.1.1.1. Respiratory tract
It has been reported that the threshold for acute irritative
effects in the upper respiratory tract is 25 µg/m3 for the most
sensitive individuals. Long-term exposure to doses over 1 µg
chromic acid/m3 can cause nasal irritation, atrophy of the nasal
mucosa, and ulceration of perforation of the nasal septum.
Bronchial asthma was previously attributed to exposure to
chromium compounds, but scientific data are too scarce to draw
conclusions.
9.1.1.2. Skin
Skin rashes, ulcers, sores, and eczema have been reported among
occupationally exposed workers. Both trivalent and hexavalent
chromium compounds can give rise to sensitization of skin,
especially under certain environmental conditions, such as those
encountered in the cement industry, where the high incidence of
chromium-induced skin lesions can be attributed to the alkaline
exposure conditions.
Eczematous dermatitis, which manifests first as a diffuse
erythematous type, progresses in severe cases to an exudative
stage and is associated in 8 - 15% of patients with sensitivity to
chromium, as revealed by skin-patch tests. Although opinion is
divided on the oxidation state of chromium responsible for inducing
sensitization, there is evidence of hexavalent chromium penetrating
the skin as a hapten to be reduced to the trivalent state and
conjugated with proteins and transformed into the full antigen
that initiates the sensitization reaction, involving presumably
only a cell-mediated immune response. It should be noted that
patients suffering from chromium-induced skin allergy tend to
become hypersensitive to cobalt and nickel. Furthermore, 48% of
cases of skin allergy induced by chromium, with or without
clinically observed light sensitivity, showed a significantly more
intense reaction by a standardized photopatch test procedure, when
they were exposed to a 4/5 minimal erythema dose of irradiation.
9.1.1.3. Kidney
After high-dose, short-term oral ingestion of chromium, acute
nephritis and tubular necrosis were observed. A few
epidemiological studies on workers in chrome-plating industries
include data on diseases of the kidney, most of them without giving
exact exposure levels. A recent study related increased urinary-beta2
microglobulin levels to exposure ranges between 2 and 20 µg/m3.
The dose-response relationship observed in this study needs
confirmation on a larger number of exposed workers.
9.1.1.4 Other organs and systems
There are no conclusive data to evaluate the effects of
chromium compounds on the liver or gastrointestinal and circulatory
systems.
9.1.2. Teratogenicity
Both oxidation states, when injected at high levels
parenterally into animals, are teratogenic, with the hexavalent
form accumulating in the embryos to much higher concentrations than
the trivalent. The Task Group was not aware of any report
indicating teratogenicity in human populations.
9.2. General Population
Persons living in the vicinity of ferro-alloy plants, exposed
to an ambient air concentration of up to 1 µg/m3, did not show
increased lung cancer mortality.
The results of many studies suggest that exposure to chromium
through inhalation and skin contact can pose health problems for
the general population. Very little information is available on
the health effects of chromium ingested through untreated drinking-
water, though, in a single study, a correlation was observed
between frequency of malformation in the central nervous system and
the chromium content of water samples (Morton & Elwood, 1974). In
order to assess the nature of the magnitude of these problems,
there appears to be a need for more general population studies on
the effects of inhaled, absorbed, or ingested chromium on
respiratory, cardiovascular, and renal functions, and on the skin.
Studies on animals and man have established trivalent chromium
as an essential micronutrient that interacts with insulin and
enhances the physiological effects of the hormone. Supplementation
trials in a number of countries including Finland, Jordan, Nigeria,
Sweden, Turkey, and the USA have shown that segments of the
population could improve their glucose metabolism and, in some
instances, fat metabolism, by ingesting trivalent chromium
compounds. These observations suggest that some populations are at
risk of chromium deficiency. Children suffering from protein-
energy malnutrition may be at special risk.
As chromium concentrations in the body fluids of unexposed
persons are generally less than 1 µg/litre, analysis for chromium
requires the strictest quality control, including measures to
exclude sample contamination. Even with adequate analytical
methods, it is not possible, as yet, to diagnose chromium
deficiency in individuals by chemical or biochemical methods alone.
Therefore, a quantitative assessment of the prevalence of chromium
deficiency in human populations is not yet possible.
REFERENCES
ABRAHAM, A.S., SONNENBLICK, M., EINI, M., SHEMESH, O., & BATT,
A.P. (1980) The effect of chromium on established athero-
sclerotic plaques in rabbits. Am. J. clin. Nutr., 33:
2294-2298.
AITIO, A., JARVISALO, J., KULUNEN, M., TOSSAVAINEN, A., &
VAITTINEN, P. (1984) Urinary excretion of chromium as an
indicator of exposure to trivalent chromium sulfate in leather
tanning. Int. Arch. occup. environ. Health, 54: 241-249.
ALDERSON, M.R., RATTAN, N.S., & BIDSTRUP, L. (1981) Health
of workmen in the chromate-producing industry in Britain. Br.
J. ind. Med., 38: 117-124.
ALEKSANDROV, A.I. & KAINOV, V.I. (1975) [Crocoite from the
Pervomaisk-Zverevo deposit at the Central Urals.] Tr. Sverdl.
Gorn. Inst., 106: 152-155 (in Russian).
AMA (1979) Guidelines for essential trace element
preparations for parenteral use. J. Am. Med. Assoc., 241:
2051-2054.
AMERICAN PUBLIC HEALTH ASSOCIATION, AMERICAN WATER WORKS
ASSOCIATION, AND WATER POLLUTION CONTROL FEDERATION (1971)
117A. Hexavalent chromium. In: Standard methods for the
examination of water and wastewater, 13th ed., Washington DC,
American Public Health Association, pp. 156-157.
AMES, B.N., DURSTON, E., YAMASAKI, E., & LEE, F.D. (1973)
Carcinogens as mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci. (USA), 70: 2281-2285.
ANDERSON, F.E. (1960) Cement and oil dermatitis. The part
played by chrome sensitivity. Br. J. Derm., 72: 108-117.
ANDERSON, N. (1982) The environmental impact of chromium.
Final draft March 1982, Maine, Department of Environmental
Protection, Bureau of Air Quality Control, 126 pp.
ANDERSON, R.A. & POLANSKY, M.M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Tr. El. Res., 3: 1-5.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., & REAMER, D.C. (1982a) Effect of exercise
(running) on serum glucose, insulin, glucagon, and chromium
excretion. Diabetes, 31: 212-216.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., VEILLON, C., & GLINSMANN, W. (1982b)
Urinary chromium excretion of human subjects: effects of
chromium supplementation and glucose loading. Am. J. clin.
Nutr., 36: 1184-1193.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., PATTERSON, K.Y.,
VEILLON, C., & GLINSMANN, W. (1983) Effects of chromium
supplementation on urinary Cr excretion of human subjects and
correlation of Cr excretion with selected clinical parameters.
J. Nutr., 113: 276-281.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
MERTZ, W., & GLINSMANN, W. (1983b) Chromium supplementation
of human subjects: effects on glucose, insulin, and lipid
variables. Metabolism, 32: 894-899.
ANDERSSON, A. & NILSSON, K.O. (1972) Enrichment of trace
elements from sewage sludge fertilizer in soils and plants.
Ambio, 1: 176-179.
ANGERER, J., SCHALLER, K.-H., & HEINRICH, R. (1981)
[Commercially-available blood and urine samples for quality
control in toxicology.] Arbeitsmed. Sozialmed. Praeventivmed.,
16: 125-127 (in German).
ANIMAL RESEARCH DIVISION OF THE NEW ZEALAND DEPARTMENT OF
AGRICULTURE (1954) Annual report 1953-54, Wellington, R.E.
Owen Government Printer, 40 pp.
ANKE, M., DIETTRICH, M., WICKE, G., PFLUG, D., & SCHUELER, D.
(1971) Absorption, excretion, and distribution of 51chromium
given by mouth to lactating ruminants. Arch. Tierern., 21:
599-607.
AXELSSON, G. & RYLANDER, R. (1980) Environmental chromium
dust and lung cancer mortality. Environ. Res., 23: 469-476.
AXELSSON, G., RYLANDER, R., & SCHMIDT, A. (1980) Mortality
and incidence of tumours among ferrochromium workers. Br. J.
ind. Med., 37: 121-127.
BAER, H. (1920) [Dermatitis resulting from the use of a
beard trainer made partly of artificial leather.] Münch. med.
Wschr., 67: 874 (in German).
BAETJER, A.M. (1950a) Pulmonary carcinoma in chromate
workers. I. A review of the literature and report of cases.
Arch. ind. Hyg. occup. Med., 2: 487-504.
BAETJER, A.M. (1950b) Pulmonary carcinoma in chromate
workers. II. Incidence on basis of hospital records. Arch.
ind. Hyg. occup. Med., 2: 505-516.
BAETJER, A.M., LOWNEY, J.F., STEFFEE, H., & BUDACZ, V.
(1959a) Effect of chromium on incidence of lung tumours in
mice and rats. Am. Med. Assoc. Arch. Ind. Health, 20: 124-135.
BAETJER, A.M., DAMRON, C., & BUDACZ, V. (1959b) The
distribution and retention of chromium in man and animals. Am.
Med. Assoc. Arch. Ind. Health, 20: 136-150.
BAKER, R.S.V. (1984) Evaluation of metals in in vitro
assays: interpretation of data and possible mechanisms of
action. Toxicol. environ. Chem., 7: 191-212.
BEHNE, D. & BRATTER, P. (1979) [Problems of trace element
analysis in medicine.] In: Gladtke, E., Heimann, G., & Eckert,
I., ed. [Trace elements], Stuttgart, Thieme, pp. 42-52 (in
German).
BEHNE, D. & DIEHL, F. (1972) Relations between carbohydrate
and trace element metabolism investigated by neutron
activation analysis. In: Nuclear Activation Techniques in the
Life Sciences. Proceedings of a Symposium, Bled, Yugoslavia,
Vienna, International Atomic Energy Agency, pp. 407-414.
BEHNE, D., BRATTER, P., GESSNER, H., HUBE, G., MERTZ, W., &
ROSICK, U. (1976) Problems in determination of chromium in
biological materials: comparison of flameless atomic
absorption spectrometry and neutron activation analysis. Z.
anal. Chem., 278: 269-272.
BEHRBOHM, P. (1957) [Allergic contact dermatitis caused by
anticorrosives containing chromium.] Berufsdermatosen, 5:
271-283 (in German).
BENJANUVATRA, N.K. & BENNION, M. (1975) Hair chromium
concentration of Thai subjects with and without diabetes
mellitus. Nutr. Rep. Int., 12: 325-330.
BERGMANN, H. & HARDT, K. (1979) Analysis of dissolved Cr(VI)
in water by APDC-MIBK extraction and atomic absorption
spectrometry. Z. anal. Chem., 297: 381-383.
BERING, F. & ZITZKE, E. (1935) [Occupational skin diseases,]
Leipzig, Voss (in German).
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric., 23: 93-100.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BERTRAND, D. & DEWOLF, A. (1968) Requirement for the trace
element chromium in the growth of potatoes. C R Ser. D., 266:
1494-1495.
BEYERMANN, K. (1962) The analytical behaviour of very small
amounts of chromium. Part I. Z. anal. Chem., 190: 4-33. Part
II. Z. anal. Chem., 191: 345-369.
BEYERSMANN, D., KOSTER, A., BUTTNER, B., & FLESSEL, P.
(1984) Model reactions of chromium compounds with mammalian
and bacterial cells. Toxicol. environ. Chem., 8: 279-286.
BIANCHI, V., DAL TOSO, R., DEBETTO, P., LEVIS, A.G., LUCIANI,
S., MAJONE, F., & TAMINO, G. (1980) Mechanisms of chromium
toxicity in mamalian cell cultures. Toxicology, 17: 219-224.
BIANCHI, V., LEVIS, A.G., & SAGGIORO, D. (1979) Differential
cytotoxic activity of potassium dichromate on nucleoside
uptake in BHK fibroblasts. Chem.-biol.Interact., 24: 137-151.
BIANCHI, V., CELOTTI, L., LANFRANCHI, G., MAJONE, F., MARIN,
G., MONTALDI, G., SPONZA, G., TAMINO, G., VENIER, P.,
ZANTEDESCHI, A., & LEVIS, A.G. (1983) Genetic effects of
chromium compounds. Mutat. Res., 117: 279-300.
BIANCHI, V., ZANTEDESCHI, A., MONTALDI, A., & MAJONE, F.
(1984) Trivalent chromium is neither cytotoxic nor mutagenic
in permeabilized hamster fibroblasts. Toxicol. Lett., 23:
51-59.
BIDSTRUP, P.L. & CASE, R.A.M. (1956) Carcinoma of the lung
in workmen in the bichromates-producing industry in Great
Britain. Br. J. ind. Med., 13: 260-264.
BIGALIEV, A.B., ELEMESOVA, M.S., TUREBAEV, M.N., & BIGALIEVA,
R.K. (1978) [Cytogenetic study of the mutagenic activity of
industrial substances.] Zdravookhr. Kaz., 8: 48-50 (in
Russian).
BITTERSOHL, G. (1972) [Health and the working environment-
Epidemiology of carcinomatosis in the chemical industry.] In:
Proceedings of the 5th Merseburger Symposium, 17-18 November
(in German).
BLACK, C.A. (1968) Soil-plant relationships, New York, John
Wiley and Sons, 792 pp.
BLOKIN, V.S. & TROP, F.S. (1977) [Dynamics of the morpho-
logical changes in animals subjected to inhalational dust
treatment using chromium oxide and trisubstituted chromium
phosphate.] In: Dubinin, N.P., ed. [Genetic effects of the
pollution of the environment,] Moscow, Nauka, pp. 173-176 (in
Russian).
BLOOMFIELD, J.J. & BLUM, W. (1928) Health hazards in
chromium plating. Public Health Rep., 43: 2330-2347.
BONATTI, S., MEINI, M., & ABBONDANDOLO, A. (1976) Genetic
effects of potassium dichromate. Mutat. Res., 38: 147-150.
BOND, R.G., STRAUB, C.P., & PROBER, R., ed. (1973) Handbook
of environmental control. III. Water supply and treatment,
Cleveland, Ohio, CRC Press, pp. 763-764.
BONNEVIE, P. (1939) [Etiology and pathogenesis of eczemas,]
Copenhagen, Busck, Leipzig, Barth (in German).
BORGHETTI, A., MUTTI, A., CAVATORTA, A., FALZOI, M., CIGALA,
F., & FRANCHINI, I. (1977) Indices rénaux d'exposition aiguë
et d'imprégnation chronique par le chrome. Med. Lav., 68(5):
355-363.
BORY, R. & LECOCQ, I. (1953) Eczéma par cuivre chromé. Arch.
Mal. prof., 14: 144.
BOSTROM, G. (1936) [Hypersensitivity test for chromium
allergy.] Am. Derm. Vener., 17: 631-632 (in German).
BOVET, P., LOB, M., & GRANDJEAN, M. (1977) Spirometric
alterations in workers in the chromium electroplating
industry. Int. Arch. occup. environ. Health, 40: 25-32.
BOWEN, H.J.M. (1966) Trace elements in biochemistry, New
York, London, Academic Press, 241 pp.
BRARD, D. (1935) Toxicologie du chrome, Paris, Herrmann &
Co., 80 pp.
BRIEGER, H. (1920) [Clinical aspects of acute chromate
poisoning.] Z. exp. Path. Ther., 21: 393-408 (in German).
BROCH, C. (1949) [Bronchial asthma caused by chromic oxide.]
Nord. Med., 41: 996-997 (in Norwegian).
BROWN, E., SKOUGSTAD, M.W., & FISHMAN, M.J. (1970) Methods
for collection and analysis of water samples for dissolved
minerals and gases. In: Techniques of water resources
investigation of the United States geological survey,
Washington DC, US Department Interior, 160 pp.
BROWN, H.G., HENSLEY, C.P., MCKINNEY, G.L., & ROBINSON, J.L.
(1973) Efficiency of heavy metals removal in municipal sewage
treatment plants. Environ. Lett., 5: 103-114.
BRUCKDORFER, K.R., KHAN, I.H., & YUDKIN, J. (1971) Chromium
and sucrose-induced hyperglyceridemia. Proc. Nutr. Soc., 30:
74A.
BRUNE, D., NORDBERG, G., & WESTER, P.O. (1980) Distribution
of 23 elements in the kidney, liver, and lungs of workers from
a smeltery and refinery in north Sweden exposed to a number of
elements and of a control group. Sci. total Environ., 16:
13-35.
BURCKHARD, W. (1962) [Occupational skin diseases.] In:
Jadassohn, W., ed. [Handbook of skin and venereal diseases,]
Berlin, Springer, Vol. II(1), pp. 368-474.
BURROWS, D., ed. (1983) Chromium: metabolism and toxicity,
Boca Raton, Florida, CRC Press, 172 pp.
BYERRUM, R.U. (1961) Some studies on the chronic toxicity of
cadmium and hexavalent chromium in drinking-water. Eng. Bull.
Purdue Univ. Serv., 106: 1-8.
CALNAN, C.D. & HARMAN, R.R.M. (1961) Studies in contact
dermatitis. XIII. Diesel coolant chromate dermatitis. Trans.
St. John's Hosp. Derm. Soc., 46: 13.
CALOW, P. & FLETCHER, C.R. (1972) A new radiotracer
technique involving 14C and 51Cr for estimating the
assimilation efficiencies of aquatic, primary consumers.
Oecologia (Berlin), 9: 155-170.
CAMPBELL, W.J. & MERTZ, W. (1963) Interaction of insulin and
chromium (III) on mitochondrial swelling. Am. J. Physiol.,
204: 1028-1030.
CANADA, NATIONAL HEALTH AND WELFARE (1980) Chromium
drinking-water criteria review. In: Guidelines for Canadian
drinking-water quality, 1978. Supporting documentation,
Ottawa, Canada, National Health and Welfare, pp. 255-268.
CANFIELD, W.K. & DOISY, R.J. (1976) Chromium and diabetes in
the aged. In: Hsu, J.M., Davis, R.L., & Neithammer, R.W., ed.
The biomedical role of trace elements in aging, St. Peters-
burg, Florida, Eckert College, pp. 119-128.
CARD, W.I. (1935) A case of asthma sensitivity to chromates.
Lancet, 2: 1348-1349.
CARTER, J.P., KATTAB, A., ABD-EL-HADI, K., DAVIS, J., EL
GHOLMY, A., & PATWARDHAN, V.N. (1968) Chromium (III) in
hypoglycemia and in impaired glucose utilization in
kwashiorkor. Am. J. clin. Nutr., 21: 195-202.
CARY, F.E., ALLWAY, W.H., & OLSON, O.E. (1977) Control of
chromium concentrations in food plants. I. Absorption and
translocations of chromium by plants. J. agric. food Chem.,
25: 300-304. II. Chemistry of chromium in soils and its
availability to plants. J. agric. food Chem., 25: 305-309.
CASEY, C.E. & HAMBIDGE, K.M. (1984) Chromium in human milk
from American mothers. Br. J. Nutr., 52: 73-77.
CASTO, B.C., PIECZYNSKI, W.J., NELSON, R.L., & DI PAOLO, J.
(1976) In vitro transformation and enhancement of viral
transformation with metals. Proc. Am. Assoc. Cancer Res., 17:
12-20.
CASTO, B.C., MEYERS, J., & DIPAOLO, J.A. (1979) Enhancement
of viral transformation for evaluation of the carcinogenic or
mutagenic potential of inorganic metal salts. Cancer Res., 39:
193-198.
CAWSE, P.A. (1974) A survey of atmospheric trace elements in
the UK, 1972-1973, Harwell, England, Atomic Energy Research
Establishment, 95 pp.
CHANEY, R.L. (1973) Crop and food chain effects of toxic
elements in sludges and effluents. In: Recycling Municipal
Sludges and Effluents on Land. Proceedings of a Joint
Committee, National Association of State Universities and Land
Grant Colleges, Washington DC, July 9-13, 1973, pp. 129-141.
CHEN, N.S.G., TSAI, A., & DYER, I.A. (1973) Effect of
chelating agents on chromium absorption in rats. J. Nutr.,
103: 1182-1186.
CHEN, K.Y., YOUNG, C.S., JAN, T.K., & ROHATGI, N. (1974)
Trace metals in waste water effluents. J. Water Pollut.
Control Fed., 46: 2663-2675.
CHEREMISINOFF, P.N. & HABIB, Y.H. (1972) Cadmium, chromium,
lead, mercury: a plenary account for water pollution. Part I.
Occurrence, toxicity, and detection. Water Sewage Works, 119:
73-86.
CHEREVATY, V.S., EREMEEVA, E.A., & SMAGULOV, S.K. (1965) [On
the nature of nasal microflora in the workers of a chromium
compound plant.] In: Proceedings of 4th Scientific Session of
Aktubinsk State Medical Institute, Alma-Ata, Kazakhstan,
pp. 337-342 (in Russian).
CHIAZZE, L., FERENCE, L.D., & WOLF, P.H. (1980) Mortality
among automobile assembly workers. I. Spray painters. J.
occup. Med., 22: 520-526.
CHRISTIAN, G.D., KNOBLOCK, E.G., PURDY, W.C., & MERTZ, W.
(1963) A polarographic study of chromium-insulin-
mitochondrial interaction. Biochem. Biophys. Acta, 66: 420-423.
CLARK, R. & KUNITSCH, G. (1972) [Statistical studies of
cross allergy in metal salts.] Berufsdermatosen, 20(5):
222-238 (in German).
COLLINS, R.J., FROMM, P.O., & COLLINGS, W.D. (1961) Chromium
excretion in the dog. Am. J. Physiol., 201(5): 795-798.
CONDE-SALAZAR, L., MARTO-CASTANO, A., & ARROYO VICENTE, M.
(1980) Determination of chrome in rubber gloves. Contact
Dermatit., 6: 237-238.
COTTON, F.A. & WILKINSON, G. (1966) Advanced inorganic
chemistry, New York, Interscience, 1136 pp.
CRESSER, M.S. & HARGITT, R. (1976) The determination of
chromium (III) and chromium (VI) by total anion exchange and
atomic absorption spectrometry. Anal. Chim. Acta, 81: 196-198.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
DALAGER, N.A., MASON, T.J., FRAUMENI, J.F., HOOVER, R., &
PAYNE, W.W. (1980) Cancer mortality among workers exposed to
zinc chromate paints. J. occup. Med., 22: 25-29.
DANA, P. (1971) Crocoite-PbCrO4 In: Hurlbut, C.S., ed.
Dana's manual of mineralogy, 18th ed., New York, J. Wiley and
Sons Inc., pp. 346-347.
DANIELSSON, B.R.G., HASSOUN, E., & DENCKER, L. (1982)
Embryotoxicity of chromium: distribution in pregnant mice and
effects on embryonic cells in vitro. Arch. Toxicol., 51:
233-245.
DAVIDSON, I.W.F. & BLACKWELL, W.L. (1968) Changes in
carbohydrate metabolism of squirrel monkeys with chromium
dietary supplementation. Proc. Soc. Exp. Biol. Med., 127:
66-70.
DAVIDSON, I.W.F. & BURT, R.L. (1973) Physiological changes
in plasma chromium of normal and pregnant women: effect of
glucose load. Am. J. Obstet. Gynecol., 116: 601-608.
DAVIES, J.C.A. (1974) Inhalation hazards in the manufacture
of silicon alloys. Cent. Afr. J. Med., 20: 140-143.
DAVIES, J.M.G. (1972) The fibrogenic effects of mineral
dusts injected into the pleural cavity of mice. Br. J. exp.
Pathol., 53: 190-201.
DAVIES, J.M.G. (1979) Lung cancer mortality of workers in
chromate pigment manufacture: an epidemiological survey. J.
Oil. Col. Chem. Assoc., 62: 157-163.
DAVIES. J.M.G. (1984) Lung cancer mortality among workers
making lead chromate and zinc chromate pigments at three
English factories. Br. J. Ind. Med., 41: 158-169.
DAVIS, M.H. & GROSSMANN, V.B. (1971) A sensitive method for
determination of chromium in biological materials. Anal.
Biochem., 44: 339-344.
DEFLORA, S. (1978) Metabolic deactivation of mutagens in
the Salmonella microsome test. Nature (Lond.), 271: 455-456.
DEFLORA, S. (1981) Study of 106 organic and inorganic
compounds in the Salmonella microsome test. Carcinogenesis, 2:
283-298.
DEFLORA, S. & BOIDO, V. (1980) Effect of human gastric
juice on the mutagenicity of chemicals. Mutat. Res., 77:
307-315.
DEFLORA, S., COPPOLA, R., CAMOIRANO, A., BATTAGLIA, M.A., &
BENNICELLI, C. (1980) Mutagenicity of chromyl chloride and
its vapours. Carcinogenesis, 1(7): 583-587.
DEFLORA, S., BIANCHI, V., & LEVIS, A.G. (1984) Distinctive
mechanisms for interaction of hexavalent and trivalent
chromium with DNA? Toxicol. environ. Chem., 8: 287-294.
DE GROOT, A.J. & ALLERSMA, E. (1973) Field observations on
the transport of heavy metals in sediments. In: Proceedings of
the Conference on Heavy Metals in the Aquatic Environment,
Nashville, Tennessee, December, Oxford, Pergamon Press,
pp. 1-16.
DEICHMANN, W.B. & GERARDE, H.W. (1969) Toxicology of drugs
and chemicals, 4th ed., New York, London, Academic Press,
167 pp.
DI PAOLO, J.A. & CASTO, B.C. (1979) Quantitative studies of
in vitro morphological transformation of Syrian hamster cells
by inorganic metal salts. Cancer Res., 32: 1008-1013.
DJAHANSHIRI, H. (1976) [Nutritional physiology problems
concerning the trace element chromiun in the rat. V. Effects
on overall metabolic performande.] Z. Tierphysiol. Tierernähr.
Futtermittelkd., 37: 298-315 (in German).
DOBROLYUBSKII, O.K. (1959) [Changes in the biochemical
process in grapes under the influence of a microfertilizer
which contains chromium.] Biochemistry (USSR), 24: 577-582 (in
Russian).
DOISY, R.J., STREETEN, D.H.P., LEVINE, R.A., & CHODOS, R.B.
(1968) Effects and metabolism of chromium in normals, elderly
subjects, and diabetics. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
Univerisity of Missouri, Vol. 2, 75 pp.
DOISY, R.J., STREETEN D.H.P., SOUMA, M.L., KALAFER, M.E.,
REKANT, S.L., & DALAKOS, T.G. (1971) Metabolism of
51chromium in human subjects. In: Mertz, W. & Cornatzer, W.E.,
ed. Newer trace elements in nutrition, New York, Marcel
Dekker, pp. 155-169.
DOISY, R.J., STREETEN, D.H.P., FREIBERG, J.M., & SCHNEIDER,
A.J. (1976) Chromium metabolism in man and biochemical
effects. In: Prasad, A.S., ed. Trace elements in human health
and disease, New York, London, Academic Press, Vol. 2, pp.
79-104.
DOMASEVA, T.V. (1971) [On the effect produced by chromium
compounds on the skin.] In: [Occupational skin diseases,]
Leningrad, Leningrad Institute of Sanitation and Hygiene,
Vol. 93, pp. 168-173 (in Russian).
DONALDSON, R.M., Jr & BARRERAS, R.F. (1966) Intestinal
absorption of trace quantities of chromium. J. lab. clin.
Med., 68: 484-493.
DOUGLAS, G.R., BELL, R.D.K., GRANT, C.E., WYTSMA, J.M., &
BORA, K.C. (1980) Effect of lead chromate on chromosome
aberration, sister-chromatid exchange, and DNA damage in
mammalian cells in vitro. Mutat. Res., 77: 157-163.
DURFOR, C.N. & BECKER, E. (1964) Public water supplies of
the 100 largest cities in the United States, 1962, Washington
DC, US Geological Survey, 364 pp (Water Supply Paper No. 1812).
DURUM, W.H. & HAFFTY, J. (1963) Implications of the
minor-element content of some major streams in the world.
Geochim. Cosmochim Acta, 27: 1-11.
EATON, D.L., STACEY, N.H., WONG, K.L., & KLAASSEN, C.D.
(1980) Dose-response effects of various metal ions on rat
liver metallothionein, glutathione, heme oxygenase, and
cytochrome P-450. Toxicol. appl. Pharmacol., 55: 393-402.
EDMUNDSON, W.F. (1951) Chrome ulcers of the skin and nasal
septum and their relation to patch testing. J. Invest. Derm.,
17: 17-19.
EIFAC (1983) Water quality criteria for european freshwater
fish. Report on chromium and freshwater fish, European Inland
Fisheries Advisory Commission, Working Party on Water Quality
Criteria for European Freshwater Fish, 31 pp (EIFAC Technical
Paper No. 43).
ELIAS, Z., SCHNEIDER, O., AUBRY, F., DANIERE, M.C., & POIROT,
O. (1983) Sister chromatid exchanges in chinese hamster V79
cells treated with the trivalent chromium compounds chromic
chloride and chromic oxide. Carcinogenesis, 3: 605-611.
ENTERLINE, P.E. (1974) Respiratory cancer among chromate
workers. J. occup. Med., 16: 523-526.
ENVIRONMENTAL INSTRUMENTATION GROUP (1973) Instrumentation
for environmental monitoring: biomedical. IV, Berkeley,
California, Lawrence Berkeley Laboratory.
EQUITABLE ENVIRONMENTAL HEALTH, INC. (1976) An epidemiologic
study of workers in lead chromate plants: Final report
(prepared for the Dry Color Manufacturer's Association,
Berkeley California).
ESSING, H.G., SZADKOWSKI, D., & VALENTIN, H. (1971)
[Significance of the valence gradations of chromium compounds
in occupational medicine examinations.] Med. Sachverständige,
67: 35-39 (in German).
ETMANOVA, Z.J. (1965) [On the functional state of the liver
in workers of Aktubinsk chromium compound plant.] Gig. Tr.
prof. Zabol., 10: 55-56 (in Russian).
EVAN, A.P. & DAIL, W.G. (1974) The effect of sodium chromate
on the proximal tubules of the rat kidney. Lab. Invest.,
30(6): 704-715.
FANG, Y. (1983) [The normal value of chromium in urine for
299 unexposed persons in Yanjian city.] J. Yanbian Med.
College, 6 (1): 35-37 (in Chinese).
FARKAS, T.G. & ROBERSON, S.L. (1965) The effect of Cr(III)
on the glucose utilization of isolated lenses. Exp. Eye Res.,
4: 124-126.
FELDMAN, F.J. (1968) The state of chromium in biological
materials. Fed. Proc., 27: 482.
FELDMAN, F.J., KNOBLOCK, E.C., & PURDY, W.C. (1967) The
determination of chromium in biological materials by atomic
absorption spectroscopy. Anal. Chim. Acta, 38: 489-497.
FRADKIN, A., JANOFF, A., LANE, B.P., & KUSCHNER, M. (1975)
In vitro transformation of BHK21 cells grown in the presence
of calcium chromate. Cancer Res., 35: 1058-1061.
FRANCHINI, I., MUTTI, A., GARDINI, F., & BORGHETTI, A.
(1975) [Excretion and renal elimination of chromium compared
to degree and length of occupational exposure.] In: Roche, L.
& Trager, J., ed. Rein et Toxique. Réunion de la Société
Francaise de Toxicologie (Compte rendu), Paris, Groupement
Francais Des Centre Anti-Poisons, pp. 271-281.
FRANCHINI, I., MUTTI, A., CAVATORTA, A., CORRADI, A., COSI,
A., OLIVETTI, A., & BORGHETTI, A. (1978) Nephrotoxicity of
chromium. Control Nephrol., 10: 98-110.
FRANCHINI, J., MAGNANI, F., & MUTTI, A. (1983) Mortality
experience among chrome plating workers. Scand. J. Work
environ. Health, 9: 247-252.
FREGERT, S. (1975) Occupational dermatitis in a 10-year
material. Contact Dermatit., 1: 96-107.
FREGERT, S. (1981) Manual of contact dermatitis, 2nd ed.,
Copenhagen, Munksgaard, 139 pp.
FREGERT, S. & GRUVBERGER, B. (1972) Chemical properties of
cement. Berufsdermatosen, 20(5): 238-245.
FREGERT, S. & GRUVBERGER, B. (1979) Chromium in industrial
leather gloves. Contact Dermatit., 5: 189.
FREGERT, S. & RORSMAN, H. (1964) Allergy to trivalent
chromium. Arch. Dermatol., 90: 4-6.
FREGERT, S., HJORTH, N., MAGNUSSON, B., BANDMANN, H.-J.,
CALNAN, C.D., CRONIN, E., MALTEN, K., MENEGHINI, C.L., PIRILA,
V., & WILKINSON, D.S. (1969) Epidemiology of contact
dermatitis. Trans. St. John's Hosp. Dermatol. Soc., 55: 17-35.
FRENTZEL-BEYME, R. (1983) Lung cancer mortality of workers
employed in chromate pigment factories. J. Cancer Res. clin.
Oncol., 105: 183-188.
FREUND, H., ATAMIAN, S., & FISCHER, J.E. (1979) Chromium
deficiency during total parenteral nutrition. J. Am. Med.
Assoc., 241: 496-498.
FSA (1953) Health workers in chromate producing industry. A
study, Washington DC, Federal Security Agency, 131 pp (US PHS
Publication No. 192).
FUKAI, R. (1967) Valency state of chromium in seawater.
Nature (Lond.), 213: 901.
FUKUNAGA, M., KURACHI, Y., & MIZUGUCHI, Y. (1982) Action of
some metal ions on yeast chromosomes. Chem. pharm. Bull., 30:
3017-3019.
FURST, A., SCHLAUDER, M., & SASMORE, D.P. (1976) Tumour-
igenic activity of lead chromate. Cancer Res., 36: 1779-1783.
GALE, T.F. (1974) Effects of chromium on the hamster embryo.
Teratology, 9: A-17.
GALE, T.F. (1978) Embryotoxic effects of chromium trioxide
in hamsters. Environ. Res., 16: 101-109.
GALE, T.F. (1982) The embryotoxic response to maternal
chromium trioxide exposure in different strains of hamsters.
Environ. Res., 29: 196-203.
GALE, T.F. & BUNCH, J.D. (1979) The effect of the time of
administration of chromium trioxide on the embryotoxic
response in hamsters. Teratology, 19: 81-86.
GARCIA, J.D. & WETTERHAHN JENNETTE, K. (1981) Electron-
transport cytochrome P-450 system is involved in the
microsomal metabolism of the carcinogen chromate. J. inorg.
Biochem., 14: 281-295.
GEISER, J.D., JEANNERET, J.P., & DELACRETAZ, J. (1960) Eczema
due to cement and sensitization to cobalt. Dermatologica
(Basel), 121: 1-7.
GEMMELL, R.P. (1973) Revegetation of derelict land polluted
by a chromate smelter. Part 1. Chemical factors causing
substrate toxicity in chromate smelter waste. Environ.
Pollut., 5: 181-197.
GEMMELL, R.P. (1974) Revegetation of derelict land polluted
by a chromate smelter. Part 2. Techniques of revegetation of
chromate smelter waste. Environ. Pollut., 6: 31-37.
GENTILE, J.M., HYDE, K., & SCHUBERT, J. (1981) Chromium
genotoxicity as influenced by complexation and rate effects.
Toxicol. Lett., 7: 439-448.
GERAUER, A. (1954) [On the growing importance of
epicutaneous allergy to chromium.] Z. Haut-Geschlechtskr., 17:
97-102 (in German).
GILANI, S.H. & MARANO, M. (1979) Chromium poisoning and
chick embryogenesis. Environ. Res., 29: 427-431.
GERICKE, S. (1943) Studies on the effect of the trace
element chromium on the growth of plants. Bodenkd.
Pflanzenernaehr., 33: 114-128.
GLASER, U., HOCHRAINER, D., KLOPPEL, H., KORDEL, W., & KUHNEN,
H. (1984) [Inhalation studies with Wistar rats and patho-
physiological effects of chromium,] Berlin, 156 pp (Report to
Umweltbundesamt, D-1 UFOPLAN F+E 10606007/2) (in German).
GLASER, U., HOCHRAINER, D., KLOPPEL, H., & KUHNEN, H. (1985)
Low-level chromium VI inhalation effects on alveolar macro-
phages and immune functions in Wistar rats. Arch. Toxicol.,
57: 250-256.
GLINSMANN, W.H., FELDMAN, F.J., & MERTZ, W. (1966) Plasma
chromium after glucose administration. Science, 152: 1243.
GLINSMANN, W.H. & MERTZ, W. (1966) Effect of trivalent
chromium on glucose tolerance. Metabolism, 15: 510-520.
GMELIN, C.G. (1826) Experiments on the effects of baryta,
strontia, chrome, molybdenum, tungsten, tellurium, titanium,
osmium, platinum, iridium, rhodium, palladium, nickel, cobalt,
uranium, cerium, iron, and manganese, on the animal system.
Edinburgh med. Surg. J., 26: 131-139.
GOMES, E.R. (1972) Incidence of chromium-induced lesions
among electroplating workers in Brazil. Ind. Med., 41(12):
21-25.
GOMEZ-ARROYO, S., ALTAMIRANO, M., & VILLALOBOS-PIETRINI, R.
(1981) Sister-chromatid exchanges induced by some chromium
compounds in human lymphocytes in vitro. Mutat. Res., 90:
425-431.
GONCHAROV, A.T. (1968) [Role of chromium in the nutrition of
animals and man.] Vopr. Pitaniya, 27: 59-66 (in Russian).
GONCHAROV, A.T. (1978) [Maximum physiological dose of
chromium in food for human consumption.] Kazanskij med. Z.,
59(2): 90-93 (in Russian).
GRAY, S.J. & STERLING, K. (1950) The tagging of red cells
and plasma proteins with radioactive chromium. J. clin.
Invest., 29: 1604-1613.
GREBE, W.-H., KASTNER, H., KIPPENBERGER, C., KRAUSS, U.,
KRUSZONA, M., SCHMIDT, H., LIEBRUCKS, M., RUMBERGER, M., &
WETTIG, E. (1975) [Investigation on supply and demand for
mineral raw materials. VII. Chromium,] Berlin, Hannover,
Bundesanstalt für Gewissenschaften und Rohstoffe/Deutsches
Institut für Wirtschaftsforschung, 147 pp.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38: 33-42.
GROSS, W.G. & HELLER, V.G. (1946) Chromates in animal
nutrition. J. ind. Hyg. Toxicol., 28: 52-56.
GROSS, E. & KOLSCH, F. (1943) [Lung cancer in the chromate
dye industry.] Gewerbepathol. Gewerbehyg., 12: 164-170 (in
German).
GURSON, C.T. (1977) The metabolic significance of dietary
chromium. In: Draper, H.H., ed. Advances in nutritional
research, New York, Plenum Publishing Company, Vol. 1,
pp. 23-53.
GURSON, C.T. & SANER, G. (1971) Effect of chromium on
glucose utilization in marasmic protein-calorie malnutrition.
Am. J. clin. Nutr., 24: 1313-1319.
GURSON, C.T. & SANER, G. (1973) Effect of chromium
supplementation on growth in marasmic protein-calorie
malnutrition. Am. J. clin. Nutr., 26: 988-991.
GURSON, C.T. & SANER, G. (1978) The effect of glucose
loading on urinary excretion of chromium in normal adults,
individuals from diabetic families, and in diabetics. Am. J.
clin. Nutr., 31: 1158-1161.
GUTHRIE, B.E. (1973) Daily dietary intakes of zinc, copper,
manganese, chromium, and cadmium by some New Zealand women.
Proc. Univ. Otago Med. School., 51: 47-49.
GUTHRIE, B.E., WOLF, W.R., & VEILLON, C. (1978) Background
correction and related problems in the determination of
chromium in urine by graphite furnace atomic absorption
spectrometry. Anal. Chem., 50: 1900-1902.
GUTHRIE, B.E., WOLF, W.R., VEILLON, C., & MERTZ, W. (1979)
Chromium in urine. In: Hemphill, D.D., ed. Trace substances in
environmental health, Columbia, Missouri, University of
Missouri, Vol. XII, pp. 490-492.
GUY, W.B. (1954) Dermatologic problems in the railroad
industry resulting from conversion to Diesel power. Am. Med.
Assoc. Arch. Derm. Syph., 70: 289-292.
GYLSETH, B., GUNDERSEN, N., & LANGORD, S. (1977) Evaluation
of chromium exposure based on a simplified method for urinary
chromium determination. Scand. J. Work environ. Health, 3:
28-31.
HAMBIDGE, K.M. (1971) Chromium nutrition in the mother and
in the growing child. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 169-194.
HAMBIDGE, K.M. (1974) Chromium nutrition in man. Am. J.
clin. Nutr., 27: 505-514.
HAMBIDGE, K.M. & BAUM, J.D. (1972) Hair chromium concen-
trations of human newborn and changes during infancy. Am. J.
clin. Nutr., 25: 376-379.
HAMBIDGE, K.M. & RODGERSON, D.O. (1969) A comparison of the
hair chromium levels of nulliparous and parous women. Am. J.
Obstet. Gynecol., 103: 320-321.
HAMBIDGE, K.M., RODGERSON, D.O., & O'BRIEN, D. (1968)
Concentration of chromium in the hair of normal children and
children with juvenile mellitus. Diabetes, 17: 517-519.
HARA, N. (1982) [The change of valence of chromium by acid
treatment.] Sangyo Igaku, 24: 658-662 (in Japanese).
HARRISON, D.L. & STAPLES, E.L.J. (1955) Zinc chromate
poisoning in calves. N.Z. vet. J., 3: 63-73.
HASEGAWA, M. (1938) Studies on the detexicating hormone of
the liver (Yakriton). Toholu J. exp. Med., 32: 163-176.
HAYES, R.B. (1982) Carcinogenic effects of chromium, In:
Langard, S., ed. Biological and environmental aspects of
chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 221-247.
HAYES, R.B., LILIENFELD, A.M., & SNELL, L.M. (1979)
Mortality in chromium chemical production workers: a
prospective study. Int. J. Epidemiol., 8: 365-374.
HEATH, J.C., FREEMAN, M.A.R., & SWANSON, S.A.V. (1971)
Carcinogenic properties of wear particles from prostheses made
in cobalt-chromium alloy. Lancet, 1: 564-566.
HENNIG, A., ANKE, M., DIETRICH, M., HARTMANN, G., & HOFFMANN,
G. (1971) Absorption and distribution of 51chromium after
oral administration to laying hens as a function of time.
Arch. Tierern., 21: 609-615.
HEPLER, O.E. & SIMONDS, J.P. (1946) Experimental nephro-
pathies. IV. Glycosuria in dogs poisoned with uranyl nitrate,
mercury bichloride and potassium dichromate. Arch. Path., 41:
42-49.
HERNBERG, S., WESTERHOLM, P., SCHULTZ-LARSEN, K., DEGERTH,
R., KUOSMA, E., ENGLUND, A., ENGZELL, U., SAND HANSEN, H., &
MUTANEN, P. (1983) Nasal and sinonasal cancer: connection
with occupational exposures in Denmark, Finland, and Sweden.
Scand. J. Work environ. Health, 9: 315-326.
HERTEL, R.F. (1982) [Chromium as a problem in physiology,
epidemiology and biological monitoring.] Staub-Reinhalt. Luft,
42: 135-137 (in German).
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand
cultures. J. exp. Bot., 4: 59-64.
HILT, G. (1954) La dermite du chrome hexavalent dans le
cadre des dermites eczémateuses par sensibilisation aux
métaux. Dermatologica (Basel), 109: 143-174.
HINTZE, C. (1930) [Handbook of mineralogy,] Berlin, Leipzig,
De Gruyter, Vol. 1. III/2, pp. 4012-4030.
HOPKINS, L.L., Jr (1965) Distribution in the rat of physio-
logical amounts of injected 51Cr(III) with time. Am. J.
Physiol., 209: 731-735.
HOPKINS, L.L., Jr & SCHWARZ, K. (1964) Chromium (III)
binding to serum proteins, specifically siderophidin. Biochem.
Biophys. Acta, 90: 484-491.
HOPKINS, L.L., Jr, RANSOME-KUTI, O., & MAJAJ, A.S. (1968)
Improvement of impaired carbohydrate metabolism by chromium
(III) in malnourished infants. Am. J. clin. Nutr., 21: 203-211.
HUCKABEE, J.W., CARTAN, F.O., & KENNINGTON, G.S. (1972)
Environmental influence on trace elements in hair of 15
species of mammals, Oak Ridge, Tennessee, Oak Ridge National
Laboratory, 36 pp (ORNL-TM-3747).
HUEPER, W.C. (1958) Experimental studies in metal
cancerogenesis. X. Cancerigenic effects of chromite ore roast
deposited in muscle tissue and pleural cavity of rats. Arch.
ind. Health, 18: 284-291.
HUEPER, W.C. (1961) Environmental carcinogenisis and cancer.
Cancer Res., 21: 842-857.
HUNTER, W.C. & ROBERTS, J.M. (1933) Experimental study of
the effects of potassium bichromate in the monkey's kidney.
Am. J. Pathol., 9: 133-147.
HYODO, K., SUZUKI, S., FURUYA, N., & MESHIZUKA, K. (1980) An
analysis of chromium, copper, and zinc in organs of a chromate
worker. Int. Arch. occup. environ. Health, 46: 141-150.
IARC (1980) Some metals and metallic compounds, Lyons,
International Agency for Research on Cancer, pp. 205-323 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 23).
IIJIMA, S., MATSUMOTO, N., LU, C.C., & KATSUNUMA, H. (1975)
Placental transfer of CrCl3 and its effects on fetal growth
and development and development in mice. Teratology, 12: 198.
IMBUS, H.R., CHOLAK, J., MILLER, L.H., & STERLING, T. (1963)
Boron, cadmium, chromium, and nickel in blood and urine. Arch.
environ. Health, 6: 112-121.
IMREH, ST. & RADULESCU, D. (1982) Cytogenic effects of
chromium in vivo and in vitro. Mutat. Res., 97: 192-193.
IVANKOVIC, S. & PREUSSMANN, R. (1975) Absence of toxic and
carcinogenic effects after administration of high doses of
chromium oxide pigment in subacute and long-term feeding
experiments in rats. Food Cosmet. Toxicol., 13: 347-351.
JAEGER, H. & PELLONI, E. (1950) Tests épicutanés aux
bichromates, positifs dans l'eczéma au ciment. Dermatologica
(Basel), 100: 207-216.
JEEJEEBHOY, K.N., CHU, R.G., MARLISS, E.B., GREENBERG, G.R., &
BRUCE-ROBERTSON, A. (1977) Chromium deficiency, glucose
intolerance, and neuropathy reversed by chromium supplement-
ation, in a patient receiving long-term total parenteral
nutrition. Am. J. clin. Nutr., 30: 531-538.
JENNETTE, K.W. (1979) Chromate metabolism in liver micro-
some. Biol. Trace Elem. Res., 1: 55-62.
JENSEN, L.S., MAURICE, D.V., & MURRAY, M.W. (1978) Evidence
for a new biological function of chromium. Fed. Proc., 37: 404.
JINDRICHOVA, J. (1978) [Chromium damage in arc welders.] Z.
Ges. Hyg., 24: 86-89 (in German).
JOHNSON, J.L. (1974) Characterization of air-borne partic-
ulates. Microchem. J., 19:168-178.
JONES, G.B., BUCKLEY, R.A., & CHANDLER, C.S. (1975) The
volability of chromium from brewer's yeast during assay. Anal.
Chim. Acta, 80: 389-392.
JOSCHI, A.P. & NEEB, R. (1980) Gas chromatographic elemental
analysis via di(trifluorethyl)dithiocarbamatochelates. Z.
anal. Chem., 303: 389-393.
KAPLAN, I. & ZELIGMAN, I. (1962) Occupational dermatitis in
railroad workers. Am. Med. Assoc. Arch. Derm., 85: 135-142.
KAUFMANN, D.B., DINICOLA, W., & MCINTOSH, R. (1970) Acute
potassium dichromate poisoning. Am. J. dis. Child, 119:
374-376.
KANEKO, T. (1976) Chromosome damage in cultured human
leucocytes induced by chromium chloride and chromium trioxide.
Jpn. J. ind. Health, 18: 136-137.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec assay and
mutagenicity studies on metal compounds. Mutat. Res., 77:
109-116.
KAZANTZIS, G. & LILLY, L. (1979) Mutagenic and carcinogenic
effects of metals. In: Friedberg, L., Nordberg, G.F., & Vouk,
V.B., ed. Handbook on the toxicology of metals, Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 237-273.
KELLER, C. (1980) [Activation analysis.] In: Bartholomé, E.,
Biekert, E., Hellmann, H., Ley, H., Weigert, M., & Weise, E.,
ed. [Ullmann's encyclopaedia of technical chemistry,] 4th ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
Vol. 5, pp. 685-707 (in German).
KEMP, K., JENSEN, F.P., MOELLER, J.T., & HANSEN, G. (1974)
Trace multi-element analysis of biological tissue by proton-
induced X-ray fluorescence spectroscope, Roskilde, Denmark,
Danish Atomic Energy Commission, 22 pp (Report Riso-M-1732).
KEMPF, T. & SONNEBORN, M. (1976) [Determination of Pb, Cd,
Cr, and Zn according to the drinking-water regulation Federal
Republic of Germany.] In: Aurand, K., Hässelbarth, U., Müller,
G., Schumacher, W., & Steuer, W., ed. [The drinking-water
regulation,] Berlin, Erich Schmidt Verlag, pp. 119-125 (in
German).
KEMPF, T. & SONNEBORN, M. (1981) [Chemical composition of
drinking-waters in various regions of the Federal Republic of
Germany.] Vom Wasser, 57: 83-94 (in German).
KESKINEN, H., KALLIOMAKI, P.-L., & ALANKO, K. (1980) Occupa-
tional asthma due to stainless steel welding fumes. Clin.
Allerg., 10: 151-159.
KIRCHGESSNER, M., MERZ, G., & OELSCHLAEGER, W. (1960)
[Effect of vegetation state on content of macro and micro
elements of three species of grass.] Arch. Tierern., 10:
414-427 (in German).
KITTELBERGER, F. (1973) [Contribution to residue analysis in
fish.] Tierärztl. Prax., 1: 465-479.
KLEIN, L.A., LANG, M., NASH, N., & KIRSCHNER, S.L. (1974)
Sources of metals in New York City wastewater. J. Water
Pollut. Control Fed., 46: 2653-2662.
KLEINER, A.M., STOLBUN, B.M., LIKHACHEVA, E.L., & BELJAEVA,
L.N. (1970) [Some indices of the functional state of the
myocardium and hemodynamics in chronic intoxication with
chromium compounds.] Gig. Tr. prof. Zabol., 12: 7-10 (in
Russian).
KNUDSEN, I. (1980) The mammalian spot test and its use for
the testing of potential carcinogenicity of welding fume
particles and hexavalent chromium. Acta pharmacol. toxicol.,
47: 66-70.
KOGAN, V.Y. (1968) [The basophil degranulation as an index
of skin sensitization to cobalt compounds.] In: Belenky, G.B.,
ed. [Modern aspects of clinical and experimental dermato-
venerology. Works of the clinic of skin and venerological
diseases,] Moscow, Medizina, pp. 100-104 (in Russian).
KOGAN, V.Y. (1971) [Chromium as a specific cause for
occupation dermatoses.] In: Babayants, R.S., ed. [Topical
questions of dermato-venerology. First Medical Institute,
All-Union Conference, Moscow, I.A. Sechenov, pp. 172-176
(in Russian).
KOGAN, V.Y, ARUTYUNYAN, K.E., & KOCHINYAN, Y.E. (1972) [A
comparative characteristic of chromium, nickel, and cobalt
salts as a causative factor in allergic contact dermatosis.]
In: [Clinical Pathology of Chemical Aetiology. Materials of
the 1st All-Union Conference, Kiev, USSR, 24-25 October,
1972,] Kiev, pp. 144 and 146 (in Russian).
KOHLER, H. (1951) [Constitutional examinations of subjects
with skin diseases in the offset department of a printing
works (Doctoral thesis, Leipzig.)] Berufsdermatosen, 4: 93 (in
German).
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace
metals during the ashing of biological materials. Analyst,
101: 870-875.
KOIVISTOINEN, P., ed. (1980) Mineral element composition of
Finnish foods. Acta agric. Scand., Suppl. 22: 171
pp.
KOPP, J.E. & KRONER, R.C. (1968) Trace metals in waters of
the United States: a five-year summary of trace metals in
rivers and lakes of the United States (October 1 1962 -
September 30, 1967), Cincinnati, Ohio, US Department of the
Interior, 32 pp.
KORALLUS, U. & LOENHOFF, N. (1981) [Industrial medical and
epidemiological experience with the manufacture and processing
of chromates.] Arbeitsmed. Sozialmed. Praeventivmed., 16:
285-289 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974a)
[Trivalent chromium compounds. Results of a study in
occupational medicine. I. General; Technology; Preliminary
study.] Arbeitsmed. Sozialmed. Praeventivmed., 9: 51-54 (in
German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974b)
[Trivalent chromium compounds: results of a study in
occupational medicine. II. Disease status analysis.]
Arbeitsmed. Sozialmed. Praeventivmed., 9: 76-79 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974c)
[Trivalent chromium compounds: Results of a study in
occupational medicine. III. Clinical studies.] Arbeitsmed.
Sozialmed. Praeventivmed., 9: 248-252 (in German).
KORALLUS, U., LANGE, H.-J., NEISS, A., WUESTEFELD, E., &
ZWINGERS, T. (1982) [Correlations between reorganization
measures and bronchial carcinoma mortality in the chromate-
manufacturing industry.] Arbeitsmed. Sozialmed. Praevent-
ivmed., 7: 159-167 (in German).
KOSHI, K. (1979) Effects of fume particles from stainless
steel welding on sister chromatid exchanges and chromosome
aberrations in cultured Chinese hamster cells. Ind. Health.,
17: 39-49.
KOTZ, L., KAISER, G., TSCHOPEL, P., & TOLG, G. (1972)
[Clarification of biological matrices for determining very low
contents of trace elements when limited amounts are weighed
with nitric acid under pressure in a teflon vessel.] Z. anal.
Chem., 260: 207-209 (in German).
KOUTRAS, G.A., HATTORY, M., SCHNEIDER, A.S., EBAUGH, F.G., &
VALENTINE, W.N. (1964) Studies on chromated erythrocytes:
effect of sodium chromate on erythrocyte glutathione reduc-
tase. J. clin. Invest., 43: 323-331.
KRISHNAJA, A.P. & REGE, M.S. (1982) Induction of chromosomal
aberrations in fish Boleophthalmus dussumieri after exposure
in vivo to mitomycin C and heavy metals mercury, selenium, and
chromium. Mutat. Res., 102: 71-82.
KROEPFLI, P. & SCHUPPLI, R. (1955) [Detergent eczema.]
Dermatologica (Basel), 110: 1-7 (in German).
KRUGER, H. & DORN, H. (1956) [Clinical observations on
detergent eczema in association with chromium and nickel
allergy.] Z. Haut-Geschlechtskr., 20: 307-314 (in German).
KUCHER, I.M. (1966) In: [Proceedings of the 5th Scientific
Session of the Aktiubinsk Medical Institute, Alma-Ata,
Kazakhstan,] pp. 24-26 (in Russian).
KUHN, J.K. (1973) Trace elements in whole coal determined by
X-ray fluorescence. Norelco Rep., 20(3): 7-10.
KUMPULAINEN, J.T., WOLF, W.R., VEILLON, C., & MERTZ, W.
(1979) Determination of chromium in selected United States
diets. J. agric. food Chem., 27: 490-494.
KUMPULAINEN, J., LEHTO, J., KOIVISTOINEN, P., UUSITUPA, M., &
VUORI, E. (1983) Determination of chromium in human milk
serum and urine by electrothermal atomic absorption spectro-
metry without preliminary ashing. Sci. total Environ., 31:
71-80.
KURYLOWICZ, B. & GASIOROWSKI, S. (1958) [Agrochemical study
on calcinated phosphate-containing admixtures of chromium
compounds.] Przemysl Chem., 37: 797-800 (in Polish).
LANGARD, S. (1977) The fate of chromium after intravenous
administration of Na251 CrO4 and CrCl3 x 6H2 O to the rat,
Guildford, England, University of Surrey, 66 pp (M.Sc.
Toxicology Thesis).
LANGARD, S. (1980a) Chromium. In: Waldron, H.A., ed. Metals
in the environment, London, New York, Academic Press,
pp. 111-132.
LANGARD, S. (1980b) A survey of respiratory symptoms and
lung function in ferrochromium and ferrosilicon workers. Int.
Arch. occup. environ. Health, 46: 1-9.
LANGARD, S., ed. (1982) Biological and environmental aspects
of chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, 285 pp.
LANGARD, S. & HENSTEN-PETTERSEN, A. (1981) Chromium
toxicology. In: Williams, D.F., ed. Systemic aspects of
biocompatibility, Boca Raton, Florida, CRC Press, Vol. 1, pp.
144-161.
LANGARD, S. & NORSETH, T. (1975) A short study of bronchial
carcinomas in workers producing chromate pigments. Br. J. ind.
Med., 32: 62-65.
LANGARD, S. & NORSETH, T. (1979) Chromium. In: Friberg, L.,
Nordberg, G.F., & Vouk, V.B., ed. Handbook on the toxicology
of metals, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 383-397.
LANGARD, S. & STERN, R.M. (1985) Nickel in welding fumes: a
cancer hazard to welders? In: Sunderman, F.W.J., Aitio, A.,
Berlin, A., Bishop, C., Buringh, E., Davis, W., Gounar, M.,
Jacquignon, P.C., Mastromatteo, E., Rigaut, J.P., Rosenfeld,
C., Saracci, R., & Sors, A., ed. Nickel in the human
environment, Lyons, International Agency for Research on
Cancer, pp. 95-103 (IARC Scientific Publications No. 53).
LANGARD, S. & VIGANDER, T. (1983) Occurrence of lung cancer
in workers producing chromium pigments. Br. J. ind. Med., 40:
71-74.
LANGARD, S., GUNDERSEN, N., TSALEV, D.L., & GYLSETH, B.
(1978) Whole blood chromium level and chromium excretion in
the rat after zinc chromate inhalation. Acta pharmacol.
toxicol., 42: 142-149.
LANGARD, S., ANDERSEN, A., & GYLSETH, B. (1980) Incidence of
cancer among ferrosilicon workers. Br. J. ind. Med., 37:
114-120.
LASKIN, S. (1972) Research in environmental sciences,
Washington DC, Institute of Environmental Medicine, pp. 92-97.
LASKIN, S., KUSCHNER, M., & DREW, R.T. (1970) Studies in
pulmonary carcinogenesis. In: Hanna, M.G., Jr, Nettesheim, P.,
& Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 321-351 (US Atomic Energy
Commission Symposium Series No. 18).
LAUTNER, G.M., CARVER, J.C., & KONZEN, R.B. (1978)
Measurement of chromium (VI) and chromium (III) in stainless
steel welding fumes with electron spectroscopy for chemical
analysis and neutron activation analysis. Am. Ind. Hyg. Assoc.
J., 39: 651-660.
LEHMANN, K.B. (1914) [Historical and critical account of our
present knowledge of the effect of small doses of chromate.]
In: Gesamtgebiet gewerbehygiene, Berlin, Springer Verlag, pp.
1-119 (in German).
LEHMANN, K.B. (1932) [Does the occurrence of lung cancer in
chromium workers give grounds for any particular anxiety?]
Zentralbl. Gewerbehyg., 9: 168-170 (in German).
LERICHE, H.H. (1968) Metal contamination of soil in the
Woburn market-garden experiment resulting from the application
of sewage sludge. J. agric. Sci. Camb., 71: 205-208.
LETTERER, E., NEIDHARDT, K., & KLETT, H. (1944) [Chromate
lung cancer and chromate pneumoconiosis. A clinical, morbid
anatomical and industrial-hygiene-related study.] Arch.
Gewerbepath. Gewerbehyg., 12: 323-361 (in German).
LEVIN, H.M., BRUNNER, M.J., & RATTNER, H. (1959)
Lithographer's dermatitis. J. Am. Med. Assoc., 169: 566-569.
LEVINE, R.A., STREETEN, D.H.P., & DOISY, R.J. (1968) Effects
of oral chromium supplementation on the glucose tolerance of
elderly human subjects. Metabolism, 17: 114-125.
LEVIS, A.G. & BIANCHI, V. (1982) Mutagenic and cytogenetic
effects of chromium compounds. In: Langard, S., ed. Biological
and environmental aspects of chromium, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 171-208.
LEVIS, A.G. & BUTTIGNOL, M. (1977) Effects of potassium
dichromate on DNA Synthesis in hamster fibroblast. Br. J.
Cancer, 35: 496-499.
LEVIS, A.G. & MAJONE, F. (1979) Cytotoxic and caryogenic
effects of soluble chromium compounds on mammalian cell
cultures. Br. J. Cancer, 40: 523-533.
LEVIS, A.G. & MAJONE, F. (1981) Cytologic and clastogenic
effects of soluble and insoluble compounds containing
hexavalent and trivalent chromium. Br. J. Cancer, 44: 219-235.
LEVIS, A.G., BUTTIGNOL, M., BIANCHI, V., & SPONZA, G. (1978)
Effects of potassium dichromate on nucleic acid and protein
synthesis and on precursor uptake in BHK fibroblasts. Cancer
Res., 38: 110-116.
LEVY, L.S. & MARTIN, P.A. (1983) The effects of a range of
chromium-containing materials on rat lung, Birmingham,
England, University of Aston, Department of Environmental and
Occupational Health, p. 224 and suppl (Prepared for Dry Color
Manufacturers Association, Alexandria, Virginia, USA).
LEVY, L.S. & VENITT, S. (1975) Carcinogenic and mutagenic
activity of chromium-containing materials. Br. J. Cancer, 32:
254-255.
LIEBER, M., PERLMUTTER, N.M., & FRAUENTHAL, H.L. (1964)
Cadmium and hexavalent chromium in Nassau County ground
water. J. Am. Water Works Assoc., 56: 739-747.
LIFSCHITZ, M.L., WALLACH, S., PEABODY, R.A., VERCH, R.L., &
AGRAWAL, R. (1980) Radiochromium distribution in thyroid
and parathyroid deficiency. Am. J. clin. Nutr., 33: 57-62.
LINDBERG, E. & HEDENSTIERNA, G. (1983) Chrome plating.
Symptoms, findings in the upper airways, and effects on lung
function. Arch. environ. Health, 38: 367-374.
LINDBERG, E. & VESTERBERG, O. (1983a) Monitoring exposure to
chromic acid in chromeplating by measuring chromium in urine.
Scand. J. Work environ. Health, 9: 333-340.
LINDBERG, E. & VESTERBERG, O. (1983b) Urinary excretion of
proteins in chromplaters, previously exposed chromplaters, and
referents. Scand. J. Work. environ. Health, 9: 505-510.
LINDBERG, E., EKHOLM, U. & ULFVARSON, U. (1985) Extent and
conditions of exposure in the Swedish chrome plating industry.
Int. Arch. Occup. Environ. Health, 56: 197-205.
LIU, V.J.K. & MORRIS, J.S. (1978) Relative chromium response
as an indicator of chromium status. Am. J. clin. Nutr., 31:
972-976.
LOFROTH, G. (1978) The mutagenicity of hexavalent chromium
is decreased by microsomal metabolism. Naturwissenschaften,
65: 207-208.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LUKANIN, W.P. (1930) [The pathology of chromate pneumo-
coniosis.] Arch. Hyg. Bakt., 104: 166-174 (in German).
LUZHNIKOV, E.A., SHIMANKO, I.I., KOSTOMAROVA, L.G., MALYGINA,
S.I., & TRAKHTENGARTS, M.I. (1976) [Acute poisoning with
chromium compounds.] Terapeut. Arkh., 48(7): 121-125 (in
Russian).
LYON, G.L., BROOKS, R.R., PETERSON, P.J., & BUTLER, G.W.
(1970) Some trace elements in plants from serpentine soils.
N.Z. J. Sci., 13: 133-139.
MACHLE, W. & GREGORIUS, F. (1948) Cancer of the respiratory
system in the United States chromate-producing industry.
Public Health Rep., 63: 1114-1127.
MCCLENDON, L.T. (1978) Determination of chromium in
biological matrices by neutron activation: application to
standard reference materials. J. radioanal. Chem., 42: 85-91.
MACKENZIE, R.D., BYERRUM, R.U., DECKER, C.F., HOPPERT, C.A., &
LANGHAM, R.F. (1958) Chronic toxicity studies. II. Hexa-
valent and trivalent chromium administered in drinking-water
to rats. Am. Med. Assoc. Arch. ind. Health, 18: 232-234.
MCRAE, W.D., WHITING, R.F., & STICH, H.F. (1979) Sister-
chromatid exchanges induced in cultured mammalian cells by
chromate. Chem.-biol. Interact., 26: 281-286.
MAHALKO, J.R. & BENNION, M. (1976) The effect of parity and
time between pregnancies on maternal hair chromium concentra-
tion. Am. J. clin. Nutr., 29: 1069-1072.
MAJONE, F. & LEVIS, A.G. (1979) Chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster cells treated in
vitro with hexavalent chromium compounds. Mutat. Res., 67:
231-238.
MAJONE, F. & RENSI, D. (1979) Mitotic alterations, chromo-
some aberrations, and sister-chromatid exchanges induced by
hexavalent and trivalent chromium on mammalian cells in vitro.
Caryologia, 32: 379-392.
MALTONI, C. (1974) Occupational carcinogenesis. Excerpta
Med. Int. Congr. Ser., 322: 19-26.
MALTONI, C. (1976) Precursor lesions in exposed populations
as indicators of occupational cancer risk. Ann. NY Acad. Sci.,
271: 444-447.
MANCUSO, T.F. (1951) Occupational cancer and other health
hazards in a chromate plant: a medical appraisal. II. Clinical
and toxicologic aspects. Ind. Med. Surg., 20: 393-407.
MANCUSO, T.F. (1975) Consideration of chromium as an
industrial carcinogen. In: Hutchinson, T.C., ed. Proceedings
of the International Conference on Heavy Metals in the
Environment, Toronto, Canada, October 27-31, 1975, Toronto,
Institute for Environmental Studies, pp. 343-356.
MANCUSO, T.F. & HUEPER, W.C. (1951) Occupational cancer and
other health hazards in a chromate plant: a medical appraisal.
I. Lung cancer in chromate workers. Ind. Med. Surg., 20:
358-363.
MARTIN, J.H. & KNAUER, G.A. (1973) The elemental composition
of plankton. Geochim. Cosmochim. Acta, 3: 1639-1653.
MATHIS, B.J. & CUMMINGS, T.F. (1973) Selected metals in
sediments, water, and biota in the Illinois river. J. Water
Pollut. Control Fed., 45: 1573-1583.
MATHUR, R.K. & DOISY, R.J. (1972) Effect of diabetes and
diet on the distribution of tracer doses of chromium in rats.
Proc. Soc. Exp. Biol. Med., 139: 836-838.
MATSUMOTO, N., IIJIMA, S., & KATSUNUMA, H. (1976) Placental
transfer of chromic chloride and its teratogenic potential in
embryonic mice. J. toxicol. Sci., 2: 1-13.
MAYER, R. (1983) Interaction of forest canopies with atmo-
spheric constituents: aluminium and heavy metals. In: Ulrich,
B. & Pankrath, J., ed. Effects of accumulation of air
pollutants in forest ecosystems, Dordrecht, Boston, London,
D. Reidel, pp. 47-55.
MAZGON, R. (1932) [Therapeutic effect of common salt on
experimental chromium nephritis in the rabbit.] Z. Gesamte
exp. Med., 81: 195-207 (in German).
MERIAN, E. (1984) Introduction on environmental chemistry
and global cycles of arsenic, beryllium, cadmium, chromium,
cobald, nickel, selenium, and their derivatives. Toxicol.
environ. Chem., 8: 9-38.
MERKURIEVA, R.V., KRASOVSKY, G.N., BURMANTOVA, N.P.,
SHATERNIKOVA, I.L., VARSHAVSKAYA, S.P., VASIUKOVICH, L.YA.,
KOGANOVA, Z.I., BUSHINSKAYA, L.I., KONSTANTINOVA, I.N.,
DOLINSKAYA, S.I., ASTAKHOVA, L.F., & VOTYAKOV, A.V. (1980a)
[Changes in various enzyme systems resulting from exposure to
some chemical substances.] Gig. i. Sanit., N1: 25-28 (in
Russian).
MERKURIEVA, R.V., KRASOVSKY, G.N., VOTYAROV, A.V., BURMANTOVA,
N.P., MUCHAMBETOVA, L.H., & KONSTANTINOVA, I.N. (1980b)
[Enzyme disarrangement of various subcellular structures under
the influence of the general toxic and gonadotoxic effects of
environmental chemical factors.] Zdravookhranenie belorus.,
N2: 28-29 (in Russian).
MERKURIEVA, R.V., KOGANOVA, Z.I., GABDULLINA, M.H., TSAPKOVA,
N.N., DUKHATEROV, O.N., & DOLINSKAYA, S.I. (1982) [A
comparative study of metabolic reactions at various routes of
chromium6+ administration in the organism of laboratory
animals.] Gig. i Sanit., N8: 75-78 (in Russian).
MERRITT, W.F. (1971) Identification and measurement of trace
elements in fresh water by neutron activation. In: Proceedings
of the International Symposium on Identification and Measure-
ment of Environmental Pollution, 358-362.
MERTZ, W. (1969) Chromium occurrence and function in
biological systems. Physiol. Rev., 49: 163-239.
MERTZ, W. (1981) The interaction between chromium and
insulin. Adv. Physiol. Sci., 12: 101-105.
MERTZ, W. & ROGINSKI, E.E. (1963) The effect of trivalent
chromium in galactose entry in rat epidymal fat tissue. J.
biol. Chem., 238: 868-872.
MERTZ, W. & ROGINSKI, E.E. (1969) Effects of chromium (III)
supplementation on growth and survival under stress in rats
fed low protein diets. J. Nutr., 97: 531-536.
MERTZ, W. & ROGINSKI, E.E. (1971) Chromium metabolsm: the
glucose tolerance factor. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 123-153.
MERTZ, W. & SCHWARZ, K. (1959) Relation of glucose tolerance
factor to impaired glucose tolerance in rats on stock diets.
Am. J. Physiol., 196: 614-618.
MERTZ, W., ROGINSKI, E.E., & SCHWARZ, K. (1961) Effect of
trivalent chromium complexes on glucose uptake by epididymal
fat tissue of rats. J. biol. Chem., 236: 318-322.
MERTZ, W., ROGINSKI, E.E., & REBA, R.C. (1965a) Biological
activity and fate of intravenous chromium (III) in the rat.
Am. J. Physiol., 209: 489-494.
MERTZ, W., ROGINSKI, E.E., & SCHROEDER, H.A. (1965b) Some
aspects of glucose metabolism of chromium-deficient rats
raised in a strictly controlled environment. J. Nutr., 86:
107-112.
MERTZ, W., ROGINSKI, E.E., FELDMAN, R.J., & THURMAN, D.E.
(1969) Dependence of chromium transfer into the rat embryo on
the chemical form. J. Nutr., 99: 363-367.
MERTZ, W., TOEPFER, E.W., ROGINSKI, E.E., & POLANSKY, M.M.
(1974) Present knowledge of the role of chromium. Fed. Proc.,
33: 2275-2280.
MEYERS, J.B. (1950) Acute pulmonary complications following
inhalations of chromic acid mist. Arch. ind. Hyg. occup. Med.,
2: 742-747.
MICKAIL, T.H., HABIB, Y.A., GABRIAL, G.N., & MORCOS, S.R.
(1976) The effect of chromium III on the intravenous glucose
tolerance of rats given diets of defficient protein values. Z.
Ernährungswiss., 15: 182-187.
MIESCHER, G., AMREIN, H.P., & LEDER, M. (1955) [Chromate
hypersensitivity and cement eczema.] Dermatologica (Basel),
110: 266-283 (in German).
MIGAI, K.V. (1975) [Hygiene and safety of labor in electro
welding works in shipbuilding,] Leningrad, pp. 27-35 (in
Russian).
MITMAN, F.W., WOLF, W.R., KELSAY, J.L., & PRATHER, E.S.
(1975) Urinary chromium levels of nine young women eating
freely chosen diets. J. Nutr., 105: 64-68.
MORGAN, J.M. (1972) Hepatic chromium content in diabetic
subjects. Metabolism, 21: 313-316.
MORRIS, G.E. (1955) Chromate dermatitis from chrome glue and
other aspects of the chrome problem. Am. Med. Assoc. Arch.
Ind. Health, 11: 368-371.
MORRIS, G.E. (1956) The manufacture of leather. Its chemical
and dermatological aspects. Ann. Meet. Am. Acad. Derm. Syph.
(Chicago).
MORRIS, G.E. (1958) Chrome dermatitis. A study of the
chemistry of shoe leather with particular reference to basic
chromic sulfate. Am. Med. Assoc. Arch. Derm., 78: 612-618.
MORRIS, G.E. (1959) [The basic form of chromic sulfate.
Results of epicutaneous testing of a chromium-III salt.]
Berufsdermatosen, 7: 126-131 (in German).
MORROW, R.W. & MCELHANEY, R.J. (1974) Determination of
chromium in industrial effluent water by flameless atomic
absorption spectroscopy. At. Absorpt. Newsl., 13(2): 45-46.
MORTON, M.S. & ELWOOD, P.C. (1974) CNS malformations and
trace elements in water. Teratology, 10: 318.
MOSINGER, M. & FIORENTINI, H. (1954) Sur la pathologie due
aux chromates: Premières recherches expérimentales. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 15: 187-199.
MULLER-WIEGAND, B., STEUER, D., & HEESSELS, P. (1983) Blood
and urine samples for quality control of heavy metals. Chem.
Toxicol. Clin. Chem. Met. Proc. Int. Conf., 2nd: 73-76.
MURAKAMI, Y., SUZUKI, Y., YAMAGATA, T., & YAMAGATA, N. (1965)
Cr and Mn in Japanese diet. J. Radiat. Res., 6: 105-110.
MURRMAN, R.P., WINTERS, R.W., & MARTIN, T.G. (1971)
Determination of trace elements in soils and clay minerals by
resonnance neutron activation analysis. Soil. Sci. Soc. Am.
Proc., 35: 647-652.
MUTTI, A., CAVATORTA, A., PEDRONI, C., BORGHI, A., GIAROLI,
C., & FRANCHINI, I. (1979) The role of chromium accumulation
in the relationship between airborne and urinary chromium in
welders. Int. Arch. occup. environ. Health, 43: 123-133.
MYSLYAEVA, A.V. (1965) [Changes in the peripheral blood in
workers of a chromium compound plant.] In: [Proceedings of
the 4th Scientific Conference of Aktubinsk State Medical
Institute, Alma-Ata,] Kazakhstan, Alma-Ata, Aktubinsk State
Medical Institute, pp. 331-332 (in Russian).
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., & KURATA, H. (1978)
Comparative studies of chromosomal aberrations and muta-
genicity of trivalent and hexavalent chromium. Mutat. Res.,
58: 175-181.
NASH, D.T., ELWOOD, I.G., & STREETEN, D.H.P. (1979) The
effect of brewer's yeast containing chromium-rich glucose
tolerance factor on serum lipids. In: Proceedings of the 5th
International Symposium on Atherosclerosis, Houston, Texas.
NATER, J.P. (1963) Possible causes of chromate eczema.
Dermatologica (Basel), 126: 160-166.
NATH, R. (1976) Assessment of chromium metabolism in
maturity onset and juvenile diabetics using chromium: 51Cr and
therapeutic response of chromium administration. Excerpta Med.
Int. Congr. Ser., 400: 194.
NEIKO, E.M. & DELVA, IU.V. (1978) [Chromium balance in
chronic ischemic heart disease.] Klinicheskaia Meditsina
(Mosk.), 56(4): 97-99 (in Russian).
NESTMANN, E.R., MATULA, T.I., DOUGLAS, G.R., BORA, K.C., &
KOWBEL, D.J. (1979) Detection of the mutagenic activity of
lead chromate using a battery of microbial tests. Mutat. Res.,
66: 357-365.
NETTESHEIM, P., HANNA, M.G., Jr., DOHERTY, D.G., NEWELL, R.F.,
& HELLMANN, A. (1970) Effects of chronic exposure to
artificial smog and chromium oxide dust on the incidence of
lung tumours in mice. In: Hanna, M.G., Jr, Nettesheim, P., &
Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 305-320 (US Atomic Energy
Commission Symposium Series No. 18).
NETTESHEIM, P., HANNA, M.G., DOHERTY, D.G., NEWELL, R.F., &
HELLMAN, A. (1971) Effect of calcium chromate dust,
influenza virus, and 100R whole-body X-radiation on lung
tumour incidence in mice. J. Natl Cancer Inst., 47: 1129-1144.
NEWBOLD, R.F., AMOS, J., & CONNELL, J.R. (1979) The
cytotoxic, mutagenic, and clastogenic effects of chromium-
containing compounds on mammalian cells in culture. Mutat.
Res., 67: 55-63.
NEWMAN, H.A.I., LEIGHTON, R.F., LANESE, R.R., & FREELAND N.A.
(1978) Serum chromium and angiographically-determined
coronary artery disease. Clin. Chem., 24: 541-544.
NISHIOKA, H. (1975) Mutagenic activities of metal compounds
in bacteria. Mutat. Res., 31: 185-189.
NOLLENDORF, A., PAKALNE, E., & UPITIS, V. (1972) [Little
known trace elements in Chlorella culture.] Latv. PSR Zinat.
Akad. Vestis (Latvian SSR), 7: 33-43 (in Russian).
NOMIYAMA, H., YOTORIYAMA, M., & NOMIYAMA, K. (1980b) Normal
chromium levels in urine and blood of Japanese subjects
determined by direct flameless atomic absorption spectrophoto-
metry, and valency of chromium in urine after exposure to
hexavalent chromium. Am. Ind. Hyg. Assoc. J., 41: 98-102.
NOMIYAMA, K., MATSUI, K., & NOMIYAMA, H. (1980a) Environ-
mental temperature: a factor modifying the acute toxicity of
organic solvents, heavy metals, and agricultural chemicals.
Toxicol. Lett., 6: 67-70.
NORSETH, T. (1978) Health effects of nickel and chromium.
In: Elvira di Ferrante, E., ed. Trace metals: exposure and
health effects, Oxford, New York, Toronto, Sydney, Paris,
Frankfurt, Pergamon Press, pp. 135-146.
NORSETH, T. (1980) Cancer hazards caused by nickel and
chromium exposure. J. Toxicol. environ. Health, 6: 1218-1227.
NOVAKOVA, S., MAUTNER, G., & DINOEVA, A. (1974) [Content of
hexavalent chromium in water supplies and its effect on the
development of experimental atherosclerosis in warm-blooded
animals.] Gig. i Sanit., 5: 78-80 (in Russian).
NOVEY, H.S., HABIB, M., WELLS, J.A. (1983) Asthma and JgE
antibodies induced by chromium and nickel salts. J. Allergy
clin. Immunol., 72: 407-412.
OFFENBACHER, E.G. & PI-SUNYER, F.X. (1980) Benefical effect
of chromium-rich yeast on glucose tolerance and blood lipids
in elderly subjects. Diabetes, 29: 919-925.
OHNO, H., HANAOKA, F., & YAMADA, M. (1982) Inducibility of
sister-chromatid exchanges by heavy-metal ions. Mutat. Res.,
104: 141-145.
OHTA, F. (1940) Studies on the detoxicating hormone of the
liver (yakriton). Contribution to the usage of yakriton
against experimental chromate nephritis. Tohoku J. exp. Med.,
39: 37-46.
OKADA, S., OHBA, H., & TANIYAMA, M. (1981) Alterations in
ribonucleic acid synthesis by chromium (III). J. inorg.
Biochem., 15: 223-231.
OKADA, S., TRUKADA, H., & OHBA, H. (1983) Enhancement of
ribonucleic acid synthesis by chromium (III) in mouse liver.
J. inorg. Biochem., 19: 95-103
OKADA, S., TSUKADA, H., & OHBA, H. (1984) Enhancement of
nucleolar RNA synthesis by chromium (III) in regenerating rat
liver. J. inorg. Biochem., 21, 113-124.
OPHULS, W. (1911a) Experimental nephritis in guinea-pigs by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9: 11-13.
OPHULS, W. (1911b) Experimental nephritis in rabbits by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9:13.
OTTINGER, R.S., BLUMENTHAL, J.L., DALPORTO, D.F., GURBER,
G.I., SANTY, M.J., & SHIH, C.C. (1973) Recommended methods
of reduction, neutralization, recovery or disposal of
hazardous waste, Cincinnati, Ohio, US Environmental Protection
Agency, Vol. 6, pp. 143-162 (EPA 670/2-73-053-F).
PAGE, A.L. (1974) Fate and effects of trace elements in
sewage sludge when applied to agricultural lands, Cincinnati,
Ohio, US Environmental Protection Agency (EPA 670/2-74-005).
PAIXAO, L.M. & YOE, J.H. (1959) Spectochemical determination
of magnesium, chromium, nickel, copper, and zinc in human
plasma and red cells. Clin. Chim. Acta, 4: 507-514.
PARR, R.M. (1977) Problems of chromium analysis in
biological materials: an international perspective with
special reference to results for analytical quality control
samples. J. radioanal. Chem., 39: 421-433.
PASCALE, L.R., WALDSTEIN, S.S., ENGBRING, G., DUBIN, A., &
SZANTO, P.B. (1952) Chromium intoxication with special
reference to hepatic injury. J. Am. Med. Assoc., 149:
1385-1389.
PASHIN, Y.V. & KOZACHENKO, V.J. (1981) [Mutagenic activity
of chromium compounds.] Gig. i. Sanit., 5: 46-49 (in Russian).
PASHIN, Y.V., ZACEPILOVA, T.A., & KOZACHENKO, V.I. (1982)
Induction of dominant lethal mutations in male mice by
potassium dichromate. Mutat. Res., 103: 345-347.
PASTERNAK, K. (1973) The spreading of heavy metals in
flowing waters in the region of occurrence of natural deposits
of the zinc and lead industry. Acta hydrobiol., 15: 145-166.
PEKAREK, R.S., HAUER, E.C., RAYFIELD, E.J., WANNEMACHER, R.W.,
& BIESEL, W.R. (1975) Relationship between serum chromium
concentrations and glucose utilization in normal and infected
subjects. Diabetes, 24: 350-353.
PENG, H.Z. (1982) [The investigation of nasal disease
morbidity of chromium exposed workers.] J. Shan-Xie Med.
College, 3: 19-21 (in Chinese).
PERLMUTTER, N.M. & LIEBER, M. (1970) Dispersal of plating
wastes and sewage contaminants in ground water and surface
water, Washington DC, US Government Printing Office, 67 pp
(Geological Survey Water Supply Paper 1879-G).
PERONE, V.B., MOFFITT, A.A., POSSICK, P.A., KEY, M.M.,
DANZINGER, S.J., & GELLIN, G.A. (1974) The chromium, cobalt,
and nickel contents of American cement and their relationship
to cement dermatitis. Am. Ind. Hyg. Assoc. J., 35: 301-306.
PETRILLI, F.L. & DE FLORA, S. (1977) Toxicity and mutagenicity of
hexavalent chromium on Salmonella typhimurium. Appl. environ.
Microbiol., 33: 805-809.
PETRILLI, F.L. & DE FLORA, S. (1978a) Oxidation of inactive
trivalent chromium to the mutagenic hexavalent form. Mutat.
Res., 58: 167-173.
PETRILLI, F.L. & DE FLORA, S. (1978b) Metabolic deactivation
of hexavalent chromium mutagenicity. Mutat. Res., 54: 139-147.
PETRILLI, F.L. & DE FLORA, S. (1980) Mutagenicity of
chromium compounds. In: Chromates Symposium 80, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 76-99.
PETRILLI, F.L. & DE FLORA, S. (1982) Interpretations on
chromium mutagenicity and carcinogenicity. In: Sorsa, M. &
Vainmio, H., ed. Mutagens in our environment, New York, Alan
R. Liss, pp. 453-464.
PIERCE, J.V. & SCHEEL, L.D. (1965) Toxicity of alloys of
chromium. Arch. environ. Health, 10: 870-876.
PIRILA, V. & KILPIO, O. (1949) On dermatoses caused by
bichromates. Acta derm. venerol., 29: 550-563.
POHLAND, F.G. (1975) Sanitary landfill stabilization with
leachate recycle and residual treatment, Cincinnati, Ohio, US
Environmental Protection Agency, 106 pp (EPA 600/2-75-043).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1973) [On carcinogenous
hazards in the product of chromium ferroalloys.] Gig. Tr.
prof. Zabol., 10: 23-26 (in Russian).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1974) [Epidemiological
data on malignant neoplasms in workers who are in contact with
different chromium compounds.] In: Shabad, L.M., Velich-
kovskii, B.T. & Kogan, F.M., ed. [Occupational cancer (a
collection of scientific works),] Moscow, Academic Academy of
Medical Science, pp. 64-71 (in Russian).
POKROVSKAYA, L.V., TJUSHUYAKOVA, A.V., GORODNOVA, N.V.,
ZALOMOVA, E.N., & ANDREEVA, T.D. (1976) [Dust factor and
occupational pathology in workers engaged in the open
excavation of chromium ores.] In: [Occupational diseases of
dust etiology (collection of works),] Vol. 3, pp. 38-43 (in
Russian).
POLAK, L., TURK, J.L., & FREY, J.R. (1973) Studies on
contact hypersensitivity to chromium compounds. In: Kallos,
P., Waksman, B.H., & DeWeck, A., ed. Progress in allergy,
Basel, Karger, Vol. 17, pp. 145-226.
PRESTON, A.M., DOWDY, R.P., PRESTON, M.A., & FREEMAN, J.N.
(1976) Effects of dietary chromium on glucose and serum
cholesterol in guinea-pigs. J. Nutr., 106: 1391-1397.
PRIESTLEY, J. (1877) Observations on the physiological
action of chromium. J. Anat. Physiol., 11: 285-301.
PRINCI, F., MILLER, L.H., DAVIS, A., & CHOLAK, J. (1962)
Pulmonary disease of ferrochromium workers. J. occup. Med., 4:
301-310.
PUNSAR, S., ERAMETSA, O., KARVONEN, M.I., RYHANEN, A., HILSKA,
P., & WORNAMO, H. (1975) Coronary heart disease and
drinking-water. A search in two Finnish male cohorts for
epidemiologic evidence of a water factor. J. chron. Dis., 28:
259-287.
RABEAU, H. & UKRAINCZYK, V. (1939) Dermites des
blanchisseuses. Rôle du chrome et du chlore. Ann. Derm. Syph.,
10: 656-680.
RADIAN CORPORATION (1983) Estimates of population exposure
to ambient chromium emissions, Research Triangle Park, North
Carolina, US Environmental Protection Agency (Final Report to
K.L. Blanchard) (EPA Contract No. 68-02-3818).
RAFFETTO, G. (1977) Direct interaction with cellular targets
as the mechanism for chromium carcinogenesis. Tumori, 63:
503-512.
RAFFETTO, G., PARODI, S., DE FERRARI, M., PARODI, C., TROIANO,
R., & BRAMBILLA, G. (1978) Direct interaction with cellular
targets as the mechanism for chromium carcinogensis. Tumori,
73: 503-512.
REDISKE, J.H. (1956) Chromium toxicity in plants. In:
Biology research: annual report, 1955, Richland, Washington,
Hamford Atomic Products Operations, pp. 48-52 (Report
HW-41500).
REDISKE, J.H., CLINE, J.F., & SELDERS, A.A. (1955) Chromium
toxicity in plants. In: Biology research: annual report, 1954,
Richland, Washington, Hamford Atomic Products Operations,
pp. 32-39 (Report HW-35917).
REGGIANI, A., LOTTI, M., DE ROSA, E., & SAIA, B. (1973)
[Impairments of respiratory functions in subjects exposed to
chromium. Note 1. Spirographic changes.] Lav. Umano, 25: 23-27
(in Italian).
REICHENBACH-KLINKE, H.H. (1977) [To the ecology of the
accumulation of heavy metals in fishes.] In: Reichenbach-
Klinke, H.H., ed. [Contributions to fish ecology: fish and the
environment,] Stuttgart, New York, Gustav Fischer, pp. 7-11
(in German).
REICHENBACH-KLINKE, H.H. (1980) [Fish diseases and harm
suffered by fish,] 2nd ed., Stuttgart, New York, Gustav
Fischer, 474 pp (in German).
RETNEV, V.M. (1960) [On the effect produced by chromium
compounds contained in cement dust on the development of
bronchial asthma.] Gig. Tr. prof. Zabol., 7: 29-32 (in
Russian).
RIALES, R. (1979) Influence of brewer's yeast on lipoprotein
cholesterol concentrations. A preliminary report. In:
Shapcott, D. & Hubert, I., ed. Chromium in nutrition and
metabolism. Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 199-212.
ROBERTS, K.R. & MARZLUF, G.A. (1971) The specific inter-
action of chromate with the dual sulfate permease systems of
Neurospora crassa. Arch. Biochem. Biophys., 142: 651-659.
ROBINSON, W.D. & EDINGTON, G. (1935) Chemical studies of
infertile soils derived from rocks high in magnesium and
generally high in chromium and nickel, Washington DC, US
Department of Agriculture, pp. 1-29 (US Department of
Agriculture Technical Bulletin No. 471).
ROGINSKI, E.E. & MERTZ, W. (1967) An eye lesion in rats fed
low-chromium rations. J. Nutr., 93: 249-251.
ROGINSKI, E.E. & MERTZ, W. (1969) Effects of chromium (III)
supplementation on glucose and amino acid metabolism in rats
fed a low protein diet. J. Nutr., 97: 525-530.
ROHDE, G., TISCHENDORF, G., LEONHARDT, J., & DAMASCHUN, F.
(1978) [Study of crocoite occurrence in Callenberg (Saxony)
(Germany).] Z. angew. Geol., 24: 168-173 (in German).
ROMOSER, G.L., DUDLEY, W.A., MACHLIN, L.J., & LOVELESS, L.
(1961) Toxicity of vanadium and chromium for the growing
chick. Poultry Sci., 40: 1171-1173.
ROOK, H.L. & WOLF, W.R. (1977) The quantitative
determination of volatile trace elements in NBS biological
standard reference materials. In: Hemphill, D.D., ed.
Proceedings of the Eleventh Annual Conference on Trace
Substances in Environmental Health, Columbia, Missouri, the
Curators of the University of Missouri, pp. 324-333.
ROSCHINA, T.A. (1959) [Experimental study on the effects of
magnezite chromium and chromite magnezite dust exposure in the
production of refrectories.] Gig. Tr. prof. Zabol., 4: 28-32
(in Russian).
ROSCHINA, T.A. (1964) [A comparative evaluation of the
effects of carbide dust and chromium boride on the organism.]
Gig. i Sanit., 8: 25-28 (in Russian).
ROSCHINA, T.A. (1976) [The toxicity of chrominum (sic)
hexacarbonyl.] Gig. Tr. Prof. Zabol., 2: 38-42 (in Russian).
ROSEBROUGH, R.W. & STEELE, N.C. (1981) Effect of
supplemental dietary chromium or nicotinic acid on
carbohydrate metabolism during basal, starvation, and
refeeding periods in poults. Poultry Sci., 60: 407-417.
ROSS, D.S., SJOGREN, R.E., & BARTLETT, R.J. (1981) Behaviour
of chromium in soils. IV. Toxicity to microorganisms. J.
environ. Qual., 10: 145-148.
ROSTENBERG, A., Jr & PERKINS, A.J. (1951) Nickel and cobalt
dermatitis. J. Allerg., 22: 466-474.
RTECS (1978) Registry of toxic effects of chemical substances,
Washington DC, National Institute for Occupational Safety and
Health, pp. 450.
SAMITZ, M.H. (1955) Some dermatologic aspects of the
chromate problem. Arch. ind. Health, 11: 361-367.
SAMITZ, M.H. (1956) Studies of chromate dermatitis. Arch.
ind. Health, 14: 269-271.
SAMITZ, M.H. & EPSTEIN, E. (1962) Experimental cutaneous
chrome ulcers in guinea-pigs. Arch. environ. Health, 5:
463-468.
SAMITZ, M.H. & GROSS, S. (1960) Extraction by sweat of
chromium from chrome tanned leathers. J. occup. Med., 2: 12-14.
SAMITZ, M.H. & GROSS, S. (1961) Effects of hexavalent and
trivalent chromium compounds on the skin. Am. Med. Assoc.
Arch. Dermatol., 84: 404-409.
SAMITZ, M.H., KATZ, S., & GROSS, S. (1960) Nature of the
chromium extracted from leather by sweat. J. occup. Med., 2:
435-436.
SANDERS, C.L., JACKSON, T.A., ADEE, R.R., POWERS, G.J., &
WEHNER, A.P. (1971) Distribution of inhaled metal oxide
particles in pulmonary alveoli. Arch. intern. Med., 127:
1085-1089.
SARGENT, T., III, LIM, T.H., & JENSON, R.L. (1979) Reduced
chromium retention in patients with hemochromatosis, a
possible basis of hemochromatotic diabetes. Metabolism, 28:
70-79.
SARTO, F., LEVIS, A.G., & PAULON, C. (1980) Clastogenic
activity of hexavalent and trivalent chromium in cultured
human lymphocytes. Caryologia, 33: 239-250.
SARTO, F., COMINATO, J., BIANCHI, V., & LEVIS, A.G. (1982)
Increased incidence of chromosomal aberrations and sister
chromatid exchanges in workers exposed to chromic acid (CrO3)
in electroplating factories. Carcinogenesis, 3: 1011-1016.
SASSI, C. (1956) [Occupational disease in a chromate
factory.] Med. Lavoro, 47: 314-326 (in Italian).
SATOH, K., FUKUDA, Y., TORII, K., & KATSUNO, N. (1981)
Epidemiological study of workers engaged in the manufacture of
chromium compounds. J. occup. Med., 23: 835-838.
SAVORY, J., MUSHAK, P., & SUNDERMAN, F.W. Jr (1969) Gas
Chromatographic determination of chromium in serum. J.
chromatogr. Sci., 7: 674-679.
SCHALLER, K.H. & ZOBER, A. (1982) [Renal excretion of
toxicologically relevant metals in occupationally non-exposed
individuals.] Arztl. Lab., 28: 209-214 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1972) [Quantitative chromium determination in urine by
flameless atomic absorption spectrometry.] Z. klin. Chem.
klin. Biochem., 10: 434-437 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1973) The quantitative determination of chromium in urine by
flameless atomic absorption spectroscopy. At. Absorpt.
Newsl., 12(6): 147-150.
SCHELENZ, R. (1977) Dietary intake of 25 elements by man
estimated by neutron activation analysis. J. radioanal. Chem.,
37: 539-548.
SCHLETTWEIN-GSELL, D. & MOMMSEN-STRAUB, S. (1971) [Review
trace elements in foods. III. Chromium.] Int. J. Vitam. Nutr.
Res., 41: 116-125 and (1973) Suppl. 13, 188 pp (in German).
SCHOENTAL, R. (1975) Chromium carcinogenesis formation of
epoxyaldehydes and tanning. Br. J. Cancer, 32: 403-404.
SCHROEDER, H.A. (1966) Chromium deficiency in rats: a
syndrome simulating diabetes mellities with retarded growth.
J. Nutr., 88: 439-445.
SCHROEDER, H.A. (1970) Chromium, Washington DC, American
Petroleum Institute, 28 pp (Air Quality Monograph No. 70-15).
SCHROEDER, H.A. & BALASSA, J.J. (1965) Influence of
chromium, cadmium, and lead on rat aortic lipids and
circulating cholesterol. Am. J. Physiol., 209: 433-437.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1962)
Abnormal trace metals in man: chromium. J. chron. Dis., 15:
941-964.
SCHROEDER, H.A., VINTON, W.H., Jr, & BALASSA, J.J. (1963)
Effects of chromium, cadmium, and other trace metals on the
growth and survival of mice. J. Nutr., 80: 39-47.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1964)
Chromium, lead, cadmium, nickel, and titanium in mice: effect
on mortality, tumours, and tissue levels. J. Nutr., 83:
239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1965)
Chromium, cadmium, and lead in rats: effects on life span,
tumours, and tissue levels. J. Nutr., 86: 51-66.
SCHROEDER, H.A., NASON, A.P., & TIPTON, I.H. (1970) Chromium
deficiency as a factor in atherosclerosis. J. chron. Dis., 23:
123-142.
SCHROEDER, K.H. (1955) [Nickel eczema in a blueline (ozalid)
machine operator.] Berufsdermatosen, 3: 170-171 (in German).
SCHUBERT, G.E., GEBHARD, K., & HOENLEIN, F. (1970) [Kidney
damage following injection of serotonin and potassium
dichromate for chronic sodium overloading or restriction.]
Virchows Arch. Abt. A Path. Anat., 351: 68-82 (in German).
SCHULTHEISS, E. (1959) [Rubber and eczema,], Aulendorf i.
Württemberg, Cantor, 213 pp (in German).
SCHWARZ, K. & MERTZ, W. (1959) Chromium (III) and the
glucose tolerance factor. Arch. Biochem. Biophys., 85: 292-295.
SCHWARTZ, L. & DUNN, J.E. (1942) Dermatitis occurring among
operators of air conditioning equipment. Ind. Med. Surg., 11:
375.
SCHWARTZ, L., TULIPAN, L., & PECK, S. (1947) Occupational
diseases of the skin, 2nd ed., Philadelphia, Pennsylvania, Lea
& Febiger.
SEEBER, H., FIKENTSCHER, R., & ROSEBURG, B. (1976)
[Disorders of smell and taste in chromium dye workers.] Z.
Gesamte Hyg. Grenzgeb., 22: 820-822 (in German).
SEELEY, J.L. & SKOGERBOE, R.K. (1974) Combined sampling-
analysis method for the determination of trace elements in
atmospheric particulates. Anal. Chem., 46: 415-421.
SEELING, W., GRUENERT, A., KIENLE, K.H., OPFERKUCH, R., &
SWOBODNIK, M. (1979) [Determination of chromium in human
serum and plasma by flameless atomic absorption spectro-
photometry.] Z. anal. Chem., 299: 368-374 (in German).
SEITZ, W.R., SUYDAM, W.W., & HERCULES, D.M. (1972)
Determination of trace amounts of chromium (III) using
chemiluminescence analysis. Anal. Chem., 44(6): 957-963.
SHACKLETTE, H.T., SAUER, H.I., & MIESCH, A.T. (1970)
Geochemical environments and cardiovascular mortality rates in
Georgia, Washington DC, US Governmental Printing Office, 39 pp
(US Geological Survey Professional Paper No. 574-C).
SHAPCOTT, D., KHOURI, K., DEMERS, P.P., VOBECKY, J., &
VOBECKY, J. (1977) The measurement of volatile chromium in
biological materials. Clin. Biochem, 10: 178-180.
SHEFFET, A., THIND, J., MILLER, A.M., & LOURIA, D.B. (1982)
Cancer mortality in a pigment plant utilizing lead and zinc
chromates. Arch. environ. Health, 37: 44-52.
SHENDRIKAR, A.D. & WEST, P.A. (1974) A study of adsorption
characteristics of traces of chromium (III) and (VI) on
selected surfaces. Anal. Chim. Acta, 72: 91-96.
SHERMAN, L., GLENNON, J.A., BRECK, W.J., KLOMBERG, G.H., &
GORDON, E.S. (1968) Failure of trivalent chromium to improve
hyperglycemia in diabetes mellitus. Metabolism, 17: 439-442.
SHIMKIN, M.M. & LEITER, J. (1940) Induced pulmonary tumours
in mice. III. Role of chromic irritation in the production of
pulmonary tumours in strain A mice. J. Natl Cancer Inst., 1:
241-254.
SHMITOVA, L.A. (1978) In: [The impact of job-related factors
on specific function of the female organism,] Svedlovsk, pp.
105-108 (in Russian).
SHMITOVA, L.A. (1980) Gig. Tr. prof. zabol., 2:
33-35 (in Russian).
SIDI, E. & LONGUEVILLE, R. (1953) Dermites au ciment. Rôle
des sels de chrome. Arch. Mal. prof., 14: 41.
SIEGENTHALER, U., LAINE, A., POLAK, L. (1983) Studies on
contact sensitivity to chromium in the guinea-pig. The role
of valence in the formation of the antigenic determinant. J.
Invest. Dermatol., 80: 44-47.
SIMONOFF, M., LLABADOR, Y. HAMON, C., PEERS; A.M., & SIMONOFF,
G.N. (1984) Low plasma chromium in patients with coronary
artery and heart diseases. Biol. Trace Element Res., 6:
431-439.
SIMONDS, J.P. & HEPLER, O.E. (1945) Experimental nephro-
pathies. I. A method of producing controlled selective injury
of renal units by means of chemical agents. Arch. Path., 39:
103-108.
SIROVER, M.A. & LOEB, L.A. (1976) Infidelity of DNA
synthesis in vitro: screening for potential metal mutagens or
carcinogens. Science, 194: 1434-1436.
SJOGREN, B. (1980) A retrospective cohort study of mortality
among stainless steel welders. Scand. J. Work. environ.
Health, 6: 197-200.
SKOGERBOE, R.K. (1974) Monitoring trace metal particulates:
an evaluation of the sampling and analysis problems. In:
Instrumentation for monitoring air quality, Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp.
125-136.
SLUIS-CREMER, G.K. & DU TOIT, R.S. (1968) Pneumoconiosis in
chromite miners in South Africa. Br. J. ind. Med., 25: 63-67.
SPIER, H.W. (1956) [Wood preservatives and chromate eczema.]
Desinfekt. Gesundheitswes., 4: 58 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil ( Myriophyllum spicatum L.). Arch.
environ. Contam. Toxicol., 2: 331-341.
STAUB, H.W., REUSSNER, G., & THIESSEN, R., Jr (1969) Serum
cholesterol reduction by chromium in hypercholesteremic rats.
Science, 166: 746-747.
STEELE, N.C. & ROSEBROUGH, R.W. (1979) Trivalent chromium
and nicotinic acid supplementation for the turkey poult.
Poultry Sci., 58: 983-984.
STEELE, N.G., ALTHEN, T.G., & FROBISH, L.T. (1977a) Bio-
logical activity of glucose tolerance factor in swine. J.
anim. Sci., 45: 1341-1345.
STEELE, N.C., PELURA, I., & FROBISH, L.T. (1977b) Effect of
dietary protein source on glucose utilization and hepatic
insulin-receptor characteristics of the rat. Nutr. Rep. Int.,
15: 429-435.
STELLA, M., MENTALDI, A., ROSSI, R., ROSSI, G., & LEVIS, A.G.
(1982) Clastogenic effects of chromium on human lymphocytes
in vitro and in vivo. Mutat. Res., 101: 151-164.
STEFFEE, C.H. & BAETJER, A.M. (1965) Histopathologic effects
of chromate chemicals. Report of studies in rabbits, guinea-
pigs, rats, and mice. Arch. environ. Health, 11: 66-75.
STEINHOFF, D., GAD, S.C., HATFIELD, G.K., & MOHR, U. (1983)
Testing sodium dichromate and soluble calcium chromate for
carcinogenicity in rats. Report to the Industrial Health
Foundation, Wuppertal, Bayer AG Institute of Toxicology,
126 pp.
STEREKHOVA, N.P., ZELENEVA, N.I., SOLOMINA, S.N.,
TYUSHNYAKOVA, N.V., MYASNIKOVA, A.G., FOKINA, G.P., & YARINA,
A.L. (1978) [Gastric pathology in workers engaged in the
production of chromium salts.] Gig. Tr. prof. Zabol., 3: 19-23
(in Russian).
STERN, R.M. (1981) Process-dependent risk of delayed health
effects for welders. Environ. Health Perspect., 41: 235-253.
STERN, R.M. (1982) Chromium compounds: production and
occupational exposure. In: Langard, S., ed. Biological and
environmental aspects of chromium, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 5-47.
STRIK, J.J.T.W.A., DE IONGH, H.H., VAN RIJN VAN ALKEMADE,
J.W.A., & WIUTE, T.P. (1975) Toxicity of chromium (VI) in
fish, with special reference to organoweights, liver and
plasma enzyme activities, blood parameters, and histological
alterations. In: Koeman, S.H. & Strik, J.J.T.W.A., ed.
Sublethal effects of toxic chemicals on aquatic animals,
Amsterdam, New York, Oxford, Elsevier Science Publishers,
pp. 31-41.
STROUT, R.W. (1977) The relationship of abnormal circulating
insulin levels to atherosclerosis. Atherosclerosis, 27: 1-13.
SULLIVAN, R.J. (1969) Air pollution aspects of chromium and
its compounds, Raleigh, North Carolina, US Department of
Health, Education and Welfare, 75 pp (National Air Pollution
Control Administration Publication No. APTD 69-34).
SUSA, N., FURUKAWA, YO., & TSUBAKI, S. (1977) Comparative
cytotoxic effects of hexavalent and trivalent chromium
compounds on cultured cells. Jap. J. vet. Sci., 39: 325-336.
SUZUKI, K., ESASHI, T., SUZUE, R., NISHIMURA, H., & MATSUMOTO,
H. (1979) Tissue concentrations of cadmium, lead, and
chromium in Japanese ranging from the fetus to elder subjects.
Toxicol. Lett., 4: 481-484.
SWAINE, D.J. & MITCHELL, R.L. (1960) Trace element
distribution in soil profiles. J. soil Sci., 11 (2): 347-368.
SWENSSON, A. (1977) [Experimental research on the
fibrogenetic effect of chromite.] Arbete och Haelsa, 2: 1-14
(in Swedish).
TABOR, E.C. & WARREN, W.V. (1958) Distribution of certain
metals in the atmosphere of some American cities. Arch. ind.
Hyg., 17: 145-151.
TAMARO, M., BANFI, E., VENTURINI, S., & MONTI-BRAGADIN, C.
(1975) [Hexavalent chromium compounds are mutagens for
bacteria.] In: Proceedings of the XVII Congress Naz. Soc. It.
Microbiol., Padova, pp. 411-415 (in Italian).
TANDON, S.K., SAXENA, D.K., GAUR, J.S., & CHANDRA, S.W.
(1978) Comparative toxicity of trivalent and hexavalent
chromium. Environ. Res., 15: 90-99.
TANDON, S.K., BEHARI, J.R., & KACHRU, D.N. (1979) Distri-
bution of chromium in poisoned rats. Toxicology, 13: 29-34.
TANDON, S.K., MATHUR, A.K., & GAUR, J.S. (1977) Urinary
excretion of chromium and nickel among electroplaters and
pigment industry workers. Int. Arch. occup. environ. Health,
40: 71-76.
TATARU, C., MARINSECU, A., CAPUSAN, I., RIPAN, R., & LITEANU,
C. (1957) [Skin damage during the industrial recovery of
potassium dichromate.] Berufsdermatosen, 5: 283-295 (in
German).
TAYLOR, A.E., GUYTON, M.O., & BISHOP, V.S. (1965)
Permeability of the alveolar membrane to solutes. Circ. Res.,
16: 353-362.
TAYLOR, F.G., Jr, MANN, L.K., DAHLMANN, R.C., & MILLER, F.L.
(1975) Environmental effects of chromium and zinc in
cooling-water drift. In: Cooling tower environment, 1974,
Washington DC, US Energy Research and Development
Administration, pp. 408-426 (Report Conf. No. 740302).
TAYLOR, F.H. (1966) The relationship of mortality and
duration of employment as reflected by a cohort of chromate
workers. Am. J. public Health, 56: 218-229.
TAZELAAR, D.J. (1970) Hypersensitivity to chromium in a
light-blue Tattoo. Dermatologica (Basel), 141: 282-287.
THOMSON, A.G. (1982) Chromite, Mining Annual Review,
pp. 70-71.
THORMANN, J., JESPERSEN, N.B., & JOENSEN, H.D. (1979)
Persistence of contact allergy to chromium. Contact Dermatit.,
5: 261-264.
TOEPFER, E.W., MERTZ, W., ROGINSKI, E.E., & POLANSKY, M.M.
(1973) Chromium in foods in relation to biological activity.
J. agric. food Chem., 21: 69-73.
TOEPFER, E.W., MERTZ, W., POLANSKY, M.M., ROGINSKI, E.E., &
WOLF, W.R. (1977) Preparation of chromium-containing
material of glucose tolerance factor activity from brewer's
yeast extracts and by synthesis. J. agric. food Chem., 25:
162-166.
TOLA, S., KILPIO, J., VIRTAMO, M., & HAAPA, K. (1977)
Urinary chromium as an indicator of the exposure of welders to
chromium. Scand. J. Work environ. Health, 3: 192-202.
TOLG, G. (1974) Recent problems and limitations in the
analytical characterization of high-purity material. Talanta,
21: 327-345.
TSUDA, H. & KATO, K. (1976) Potassium dichromate: induced
chromosome aberrations and its control with sodium sulfite in
hamster embryonic cells in vitro. Gann, 67: 469-470.
TSUDA, H. & KATO, K. (1977) Chromosomal aberrations and
morphological transformation in hamster embryonic cells
treated with potassium dichromate in vitro. Mutat. Res., 46:
87-94.
TUMAN, R.W. & DOISY, R.J. (1977) Metabolic effects of the
glucose tolerance factor (GTF) in normal and genetically
diabetic mice. Diabetes, 26: 820-826.
TUMAN, R.W., BILBO, J.T., & DOISY, R.J. (1978) Comparison
and effects of natural and synthetic glucose tolerance factor
in normal and genetically diabetic mice. Diabetes, 27: 49-56.
TURNER, M.A. & RUST, R.H. (1971) Effects of chromium on
growth and mineral nutrition of soybeans. Soil Sci. Soc. Am.
Proc., 35: 755-758.
TYUSHNYAKOVA, N.V., ZISLIN, D.M., SHABYNINA, N.K., & BELJAEVA,
L.N. (1974) [Criteria of occupational cancer in the
production of chromium salts and some ideas concerning its
pathogenesis.] In: Occupational cancer (collection of
scientific works), Sverdlovsk, pp. 56-63 (in Russian).
UMEDA, M. & NISHIMURA, M. (1979) Inducibility of chromosomal
aberrations by metal compounds in cultured mammalian cells.
Mutat. Res., 67: 221-229.
URCHS, O. (1953) [Occupational skin diseases,] Cantor,
Aulendorf i. Württemberg, 108 pp.
US DHEW (1975) Criteria for a recommended standard.
Occupational exposure to chromium (VI), Washington DC, US
Department of Health, Education and Welfare, 200 pp (US DHEW
Publication No. NIOSH 76-129).
US DHEW (1977) Occupational diseases: a guide to their
recognition, revised ed., Washington DC, US Department of
Health, Education and Welfare, pp. 352-355 (Stock No.
017-033-00266-5).
US EPA (1978) Reviews of the environmental effects of
pollutants. III. Chromium, Washington DC, US Environmental
Protection Agency, 285 pp (ORNL/EIS-80; EPA 600/1-78-023).
US EPA (1980) Ambient water quality criteria for chromium,
Washington DC, US Environmental Protection Agency, pp. A1-C48.
US NAS (1974a) Chromium, Washington DC, US National Academy
of Sciences, 155 pp.
US NAS (1974b) Geochemistry and the environment. I. The
relation of selected trace elements to health and disease,
Washington DC, US National Academy of Sciences, 113 pp.
US NAS/NAE (1972) Water quality criteria 1972, Washington
DC, US Environmental Protection Agency, US National Academy of
Sciences, US National Academy of Engineering, 533 pp.
US PHS (1953) Health of workers in chromate producing
industry, Washington DC, US Department of Health, Education
and Welfare, US Public Health Service, 131 pp (Publication No.
192).
VAKHRUSHEVA, V.A. (1960) [Effect of Mn and Cr as trace
elements on the level of blood sugar in white mice.] Tr.
Izhevskogo Otd. Fiziol. Obschchestva, 1: 184-190 (in Russian).
VALER, M. & RACZ, J. (1971) Investigations concerning the
sensitizing effect of trivalent chromium salts. I. Correlation
between concentration and skin penetration of trivalent
chromium and between the number of positive results obtained
in patch tests. J. ind. Med., 41(12): 302-317.
VEILLON, C., WOLF, W.R., GUTHRIE, B.E. (1979) Determination
of chromium in biological materials by stable isotope
dilution. Anal. Chem., 51: 1022-1024.
VELICHKOVSKY, B.T. & POKROVSKAJA, L.V. (1973) [Labour
hygiene in a modern production of multy-tonnage ferrolloys.]
In: [Proceedings of the National Symposium on Labor Hygiene
and Prophylaxis of Occupational Diseases in the Production of
Ferroalloys,] Sverdlovsk, pp. 3-8 (in Russian).
VENITT, S. & LEVY, L.S. (1974) Mutagenicity of chromates in
bacteria and its relevance to chromate carcinogenesis. Nature
(Lond.), 250: 493-494.
VENCGUJIV, A.J. (1974) Zones of display of biological and
pharmacological action of trace elements. In: Hoekstra, W.G.,
Gantherl, H.E., & Mertz, W., ed. Trace elements metabolism in
animals. II, Baltimore, London, Tokyo, University Park Press,
pp. 295-310.
VERFAILLIE, G.R.M. (1974) Kinetics of chromium absorption by
intact rice plants. In: Comparative studies of food and
environmental contamination, Vienna, International Atomic
Energy Agency, pp. 315-331.
VERSIECK, J.M.J. & SPEECKE, A.B.H. (1972) Contaminations
induced by collection of liver biopsies and human blood. In:
Nuclear Activation Techniques in the Life Sciences.
Proceedings of a Symposium, Bled, Yugoslavia, Vienna
International Atomic Energy Agency, pp. 39-49.
VERSIECK, I., HOSTE, J., BARBIER, F., STEYAERT, H., DERUDDER,
I., & MICHELS, H. (1978) Determination of chromium and
cobalt in human serum by neutron activation analysis. Clin.
Chem., 24: 303-308.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experience of the
occupational health department with some industrial threshold
limit values for industrial poisons.] Arch. Gewerbepath.
Gewerbehyg., 13: 528-534 (in German).
VISEK, W.J., WHITNEY, I.B., KUHN, U.S.G., & COMAR, C.L.
(1953) Metabolism of 51Cr by animals as influenced by
chemical state. Proc. Soc. Exp. Biol. Med., 84: 610-615.
VOELKL, A. (1971) [The daily excretion of chromium in normal
persons.] Zentralbl. Arbeitsmed., 21: 122 (in German).
VOTAVA, H.J., HAHN, C.J., & EVAN, G.W. (1973) Isolation and
partial purification of a 51Cr complex from brewer's yeast.
Biochem. Biophys. Res. Comm., 55: 312-319.
WACKER, W.E.C. & VALLEE, B.L. (1959) Nucleic acids and
metals. I. Chromium mangenese, nickel, iron, and other metals
in ribonucleic acid from diverse biological sources. J. biol.
Chem., 234: 3257-3262.
WAHBA, A. & COHEN, T. (1979) Chrome sensitivity in Israel.
Contact Dermatit., 5: 101-107.
WAHLBERG, J.E. & WENNERSTEN, G. (1977) Light sensitivity and
chromium dermatitis. Br. J. Dermatol., 97: 411-416.
WALSH, E.N. (1953) Chromate hazards in industry. J. Am. Med.
Assoc., 153: 1305-1308.
WARREN, G., SCHULTZ, P., BANCROFT, D., BENNETT, K., ABBOTT,
E.H., & ROGERS, S. (1981) Mutagenicity of a series of
hexacoordinate chromium (III) compounds. Mutat. Res., 90:
111-118.
WATANABE, S. & FUKUCHI, Y. (1975) An epidemiological survey
on lung cancer in workers of a chromate-producing industry in
Hokkaido, Japan (Presented at: The XVIII International Congress
on Occupational Health, Brighton, England, 14-19 September.
WEILER, R.R. & CHAWLA, V.K. (1969) Dissolved mineral and
quality of Great Lakes waters. In: Proceedings of the 12th
Conference on Great Lakes Res., 801.
WELZ, B. (1980) [Use of AAS and ICP. Relative advantage of
each method.] Chem. Techn., 9: 161-170. (in German).
WELZ, B. (1983) [Atomic absorption spectrometry,] 3rd ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
527 pp (in German).
WENDELBERGER, J. & WEITGASSER, H. (1956) [The role of alkali
and degreasing agents in the development of contact eczemas in
electroplasters.] Berufsdermatosen, 4: 16-21 (in German).
WHANGER, P.D. & WESWIG, P.H. (1975) Effects of selenium,
chromium, antioxidants on growth, eye cataracts, plasma
cholesterol, and blood glucose in selenium-deficient, vitamin
E-supplemented rats. Nutr. Rep. Int., 12: 345-348.
WHITE, L.R., JAKOBSEN, K., & 0STGAAD, K. (1979) Comparative
toxicity studies of chromium rich welding fumes and chromium
on an established human cell line. Environ. Res., 20: 366-374.
WHITING, R.F., STICH, H.F., & KOROPATNICK, D.J. (1979) DNA
damage and DNA repair in cultured human cells exposed to
chromate. Chem.-biol. Interact., 26: 267-280.
WHO (1983) EHC 27: Guidelines on studies in environmental
e pidemiology, Geneva, World Health Organization, 351 pp.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984a)
Disposition of intratracheally administered chromium (III) and
chromium (VI) in rabbits. Toxicol. Lett., 22: 273-276.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984b) The
reduction of chromium (VI) to chromium (III) by glutathione:
an intracellular redox pathway in the metabolism of the
carcinogen chromate. Toxicology, 33: 341-348.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1985)
[Determination of chromium in human red blood cells. Basis for
a concept of biological monitoring.] Arbeitsmed. Sozialmed.
Praeventivmed., 20: 1-4 (in German).
WILD, D. (1978) Cytogenetic effects in the mouse of 17
chemical mutagens and carcinogens evaluated by the micronuclei
test. Mutat. Res., 56: 319-327.
WILLIAMS, C.D. (1969) Asthma related to chromium compounds.
Report of two cases and review of the literature on chromate
diseases. NC med. J., 30: 482-491.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1960) The Merck index. An encyclopedia of chemicals and
drugs, 7th ed., Rahway, New Jersey, Merck and Co., 139 pp.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) The Merck index. An encyclopedia of chemicals and
drugs, 9th ed., Rahway, New Jersey, Merck and Co., 1313 pp.
WINSTON, J.R. & WALSH, E.N. (1951) Chromate dermatitis in
railroad employees working with diesel locomotives. J. Am.
Med. Assoc., 147: 1133-1134.
WITHEY, J. (1983) Memorandum addressed to Si Duk Lee, dated
March 4, 1983, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office.
WOLF, W.R. (1976) Coupled gas chromatography/atomic
absorption spectrometry for the nanogram determination of
chromium. Anal. Chem., 48: 1717-1720.
WOLF, W.R. & GREENE, F.E. (1976) Preparation of biological
materials for chromium analysis. In: Accuracy in trace
analysis: sampling, sample handling, and analysis,
Gaithersburg, Maryland, National Bureau of Standards, pp.
605-610 (Special Publication No. 422).
WOLF, W.R., MERTZ, W., & MASIRONI, R. (1974) Determination
of chromium in refined and unrefined sugars by oxygen plasma
ashing flameless atomic absorption. J. agric. food Chem., 22:
1037-1042.
WOLF, W.R., TAYLOR, M.L., HUGHES, B.M., TIERNAN, T.O., &
SIEVERS, R.E. (1972) Determination of chromium and beryllium
at the picogram level by gas chromatography/mass spectrometry.
Anal. Chem., 44: 616-618.
WU, G.-Y. (1981) [The research on the purification and the
chemical structure of the specific chromium binding
materials.] Jpn. J. Ind. Health, 33: 505 (in Chinese).
YAGI, T. & NISHIOKA, H. (1977) DNA damage and its degrada-
tion by metal compounds. Sci. Eng. Rev. Doshisha Univ., 18:
63-70.
YANG, G.L. (1956) [An investigation of occupational disease
among chromium plating workers.] Acta Acad. Med. primae
Shanghai, 2: 36-46 (in Chinese).
YOPP, J.H., SCHMID, W.E., & HOLST, R.W. (1974) Determination
of maximum permissible levels of selected chemicals that exert
toxic effects on plants of economic importance in Illinois,
Chicago, Illinois, Institute for Environmental Quality,
pp. 89-96.
YUNISOVA, H.K. & PAVLOVSKAJA, G.S. (1975) The effect of
regimen of chrome plating on the properties of chromine.
Protect. Metal., 2, 248-250.
ZANDER, A.T., O'HAVER, T.C., & KELIHER, P.N. (1977)
Correction for interferences of spectral origin with continuum
source, echelle wavelength modulated atomic absorption
spectrometry. Anal. Chem., 49: 838-842.
ZHURKOV, U.S. (1981) [Approaches to the regulation of
environmental chemical pollutants with mutagenic activity.]
In: Sidorenko, G.I.M., ed. [Medical problems of environmental
protection,] Moscow, USSR Academy of Medical Sciences,
pp. 88-95 (in Russian).
ZOBER, A. (1979) [On the problems of evaluating bronchial
carcinoma after exposition to chromium compounds.] Int. Arch.
occup. environ. Health, 43(2): 107-121 (in German).
ZOBER, A. (1982) [Possible dangers to the respiratory tract
from welding fumer: methods of approach in an industrial
health care context and results.] Schweissen & Schneiden,
34(2): 77-81 (in German).
ZOBER, A., KICK, K., SCHALLER, K.H., SCHELLMANN, B., &
VALENTIN, H. (1984) ["Normal values" of chromium and nickel
in human lung-, kidney-, blood-, and urine samples.] Zbl.
Bakt. Hyg. I. Abt. Orig. B, 179: 80-95 (in German).
1969). For these reasons, chromium must be considered an essential
micro-nutrient. It is physiologically active in the trivalent
oxidation state at concentrations of approximately 100 µg/kg diet.
7.1.1. Effects of deficiency on glucose metabolism
Semipurified rations, containing Torula yeast as the source of
protein, were fed to groups of 10 male Sprague Dawley rats at
weaning, and intravenous glucose tolerance tests were performed by
injecting 1250 mg glucose/kg body weight and measuring the
subsequent decline in blood-glucose levels. The rate of glucose
disappearance can be calculated from the straight line plot of log
increment glucose (excess of glucose at any time (t) over fasting
glucose), versus time. The disappearance constant k is expressed
as % decline of the increment glucose per min; it is a measure of
the efficiency of glucose utilization. In weaning rats, the rate
constant was found to decline, within 3 weeks or less, from an
average of 4%/min to 2.6%/min in animals fed the Torula yeast diet,
but not in animals administered a diet in which 4 or 8% of Torula
yeast was replaced by an equal amount of brewer's yeast. This
observation suggested that brewer's yeast, but not Torula yeast,
contained an unknown substance necessary for the maintenance of
normal glucose tolerance. Because the only known effect of the
substance was that on glucose tolerance, it was named "Glucose
Tolerance Factor" (GTF) (Mertz & Schwarz, 1959). GTF was extracted
from brewer's yeast and pork kidney powder, concentrated and
purified, and its active ingredient was identified as trivalent
chromium (Schwarz & Mertz, 1959). Chromium in the form of most
common complexes (except for very stable ones) cured the impairment
of glucose tolerance in deficient rats, either as one oral dose of
200 µg/kg body weight, or as an intravenous (iv) injection of 2.5
µg/kg body weight. Chromium in the diet also prevented the
impairment of glucose tolerance.
In order to produce more pronounced deficiencies of chromium
and other trace elements, Schroeder et al. (1963) constructed a
special animal house on a mountain top in Vermont, USA, far removed
from traffic and industry. The interior was specially coated with
organic resins to reduce any metallic exposure. Air was introduced
through special filters, and strict practices were enforced to
avoid the introduction of dust and dirt. This will be referred to
as the "controlled environment". Under these conditions, a more
severe chromium deficiency resulted in very low glucose removal
rates of 1.12%/min in 6 rats and of 0.18%/min in 4 female breeder
rats (482 days old), after 11 days on a chromium-deficient diet.
The removal rate in 4 control rats receiving the same diet with a
chromium supplement improved from 0.51 to 1.39% during the same
period (Mertz et al., 1965b). This strong impairment of glucose
tolerance in rats kept in the "controlled environment" was
reflected in their fasting blood-glucose levels, compared with
those of chromium-supplemented controls: 1370 ± 68 versus 1170 ± 17
mg/litre in males and 1390 ± 68 versus 960 ± 65 mg/litre in
females. More than half of 185 deficient rats excreted more than
0.25% glucose in the urine, whereas glycosuria was found in only 9
of the chromium-supplemented controls (Schroeder, 1966). A
significant reduction in the intravenous glucose removal rate was
also observed in 8 rats in plastic cages, fed an EDTA-washed low-
protein diet (7% casein), compared with 8 controls receiving 50 µg
chromium as the chloride, by stomach tube, daily for 2 weeks (2.1
versus 3.6%/min; P < 0.01). However, chromium supplements did not
improve the near-normal removal rates in rats receiving a 20%
casein diet (Mickail et al., 1976). Significantly lower plasma-
glucose levels, due to supplementation with chromium, were reported
in rats fed the chromium-deficient Torula yeast diet (Whanger &
Weswig, 1975). In another series of studies, only a slight
reduction in blood-glucose (from 1240 to 1180 µg/litre) was found
in 10 rats receiving a diet supplemented with chromium (10 µg/kg)
(Djahanshiri, 1976).
Impaired glucose tolerance in squirrel monkeys, fed a
commercial laboratory chow, was shown to respond to chromium
supplementation. Of 9 monkeys with an impaired glucose removal
rate (1.38%/min), 8 responded after 22 weeks of supplementation of
their drinking-water (chromium acetate, 10 mg/litre) with a
normalization of their glucose tolerance (average removal rate, N =
9, 2.33 ± 0.3%/min) (Davidson & Blackwell, 1968). There was no
effect on food consumption, growth rate, or serum-insulin
concentrations. The chromium content of the commercial chow was
stated to be 3.3 mg/kg, a very high concentration. In view of the
uncertainties of methods of analysis for chromium (section 2.2), it
is not possible to interpret the results as indicative of a very
high chromium requirement of the squirrel monkey or of an unusually
poor bioavailability of the chromium in that particular ration. A
marginal chromium deficiency may have existed in mice fed a bread
and milk diet, as daily administration of 10 µg chromium for 16
days to 8 months produced a 10 - 30% decline in blood-glucose
levels (Vakhrusheva, 1960). However, this effect was not specific
for chromium, as it was also observed when manganese was
administered.
The glucose tolerance of guinea-pigs did not differ
significantly between groups fed diets containing chromium at
0.125, 0.625, or 50 mg/kg, even though the animals fed the 2 higher
levels exhibited a lower mortality rate during pregnancy (Preston
et al., 1976).
Although the administration of synthetic glucose tolerance
factor (a chromium-dinicotinic acid-glutathione complex) to 6 pigs
did not affect glucose tolerance tests, it resulted in a
significant increase in the hypoglycaemic effect of insulin
injected at 0.1 U/kg body weight (Steele et al., 1977a).
Turkey poults, fed a practical ration containing chromium
levels of 5 mg/kg, responded to chromium supplementation (20 mg/kg
diet) with a significant increase in liver glycogen and in glycogen
formation, following a fast, and with a significant increase in
glycogen synthetase (EC 2.4.1.11) activity in the liver (Rosebrough
& Steele, 1981).
It can be concluded that the impairment of glucose tolerance in
rats fed a low-chromium Torula yeast diet is due to chromium
deficiency. The effects of chromium in squirrel monkeys, pigs, and
turkeys, though statistically significant, are somewhat difficult
to interpret, because of the reported high chromium content of the
basal diet. No evidence for chromium deficiency has yet been
obtained through glucose tolerance tests on other animal species.
7.1.2. Effects of deficiency on lipid metabolism
Though trivalent chromium in high doses (2.5 mg/kg body weight)
has been shown to increase the synthesis of fatty acids and
cholesterol in the liver (Curran, 1954), lower, physiological doses
appear to decrease serum-cholesterol concentrations in rats.
Schroeder & Balassa (1965) found an average level of 927 mg
cholesterol/litre serum in 24- to 26-month-old male rats, kept in a
controlled environment and administered chromium in the drinking-
water at a concentration of 5 mg/litre, compared with an average of
1229 mg/litre in controls not receiving chromium ( P < 0.01). The
effects in female rats were ambigous, one study producing the
expected reduction in cholesterol due to chromium, another showing
an elevation in the supplemented female rats. Schroeder's
observations of a cholesterol-reducing effect of chromium in male
rats were confirmed by Staub et al. (1969) and by Whanger & Weswig
(1975) but were contradicted by results of a third study in which
there were not any significant effects of chromium on sucrose-
induced triglyceridaemia and cholesterolaemia (Bruckdorfer et al.,
1971). Perhaps more significant than the effect on circulating
cholesterol is the direct effect of chromium on the occurrence of
aortic plaques. Schroeder & Balassa (1965) observed 6 plaques in 54
male and female chromium-deficient rats, but only one plaque in 48
animals receiving 5 mg chromium/litre in drinking-water. These
results are in agreement with those from a subsequent report of the
protective effect of the natural chromium content of water (60 -
215 µg/litre) against atherosclerosis in cholesterol-fed rabbits
(Novakova et al., 1974). Abraham et al. (1980) extended these
observations by demonstrating that daily chromium injections (20 µg
K2CrO4) reversed the established atherosclerosis in the aorta of 11
cholesterol-fed rabbits, compared with 12 controls. The mean
plaque area was reduced from 95% to 63%, the total aortic
cholesterol from 729 mg to 458 mg, and the atheromatous lesions, as
measured by technetium incorporation from 285 000 cpm to 114 000
cpm, all differences being statistically significant. These
results are reinforced by observations in man discussed in section
8.1.
7.1.3. Effects of deficiency on life span, growth, and
reproduction
The mortality of male, but not of female mice, raised in a
"controlled environment" (section 7.1.1) was reduced by trivalent
chromium administered as acetate in the drinking-water at a
concentration of 5 mg/litre (Schroeder et al., 1964). The survival
rates at 12 months were 92.6% and 68.8% ( P < 0.0001) in
supplemented and deficient animals, respectively. Similary, male,
but not female, rats receiving a chromium concentration of 5
mg/litre in the drinking-water had longer life spans than deficient
controls. The mean age of the last surviving 10% of animals was
1249 days, compared with 1141 days in the deficient animals ( P <
0.01). Survival of male rats fed a low-chromium (< 100 µg/kg),
low-protein ration and subjected to a controlled acute haemorrhage
was significantly less than that of chromium-supplemented rats, in
2 studies (67 versus 92%; P < 0.05 and 27 versus 60%; P < 0.01,
respectively) (Mertz & Roginski, 1969).
In Schroeder's study, growth rates in treated mice and rats of
both sexes raised in a "controlled environment", were higher after
6 and 12 months, with highly significant differences in body weight
ranging from 9 to 17% ( P < 0.005) compared with the controls.
Again, the effects of chromium supplementation were greater in
males than in females (Schroeder et al., 1964, 1965).
Similar results were reported by Djahanschiri (1976), who
studied a total of 2750 rats of a special inbred strain (Hk51) fed
a basal diet (0.15 mg chromium/kg diet) and chromium supplements
ranging from 10 to 500 mg/kg diet. At 12 weeks, the average
weights of the chromium-supplemented animals, regardless of dose
level, were significantly higher (by 6% in the males and 3% in the
females) than those of the animals on the basal diet. The same
author reported a progressive diminution in both the milk
production of lactating rats and weight gain in 3 consecutive
generations fed the low-chromium diet, compared with rats receiving
chromium supplementation. Increased mortality was reported in
pregnant guinea-pigs fed a low-chromium diet (125 µg/kg diet)
compared with animals receiving a chromium supplement of either 625
µg/kg or 50 mg/kg (Preston et al., 1976).
When rats raised on a low-chromium Torula yeast diet (< 100
g/kg) mated with those on a normal diet, they were able to
impregnate the females at a 100% conception rate only up to the age
of 4 months. After this age, the conception rate declined to 25%,
25%, and 0%, at the age of 7, 8, and 9 months, respectively. This
decline was accompanied by a significant ( P < 0.01) decrease in
the sperm count in the chromium-deficient males to approximately
half of the count in supplemented controls at the age of 8 months
(Anderson & Polansky, 1981).
7.1.4. Other effects of deficiency
Male weanling rats, fed a 10% soya protein ration with a
chromium content of less than 100 µg/kg, developed a visible
opacity of the cornea in one or both eyes. In several studies, the
incidence of this effect ranged from 10 to 15% in deficient rats.
No opacities developed in control animals receiving 2 mg
chromium/kg diet (Roginski & Mertz, 1967).
Chromium deficiency has been shown to reduce the physical
performance of rats under stress. Ten male rats raised on a
chromium-deficient diet (150 µg/kg diet) swam for an average of 250
min, until exhaustion, in contrast with 10 rats receiving a
supplement of 10 mg chromium/kg diet, which were exhausted only
after 320 min (Djahanschiri, 1976).
7.1.5. Mechanism of action of chromium as an essential nutrient
7.1.5.1. Enzymes, nucleic acids, and thyroid
Chromium is present in nucleic acids in very high
concentrations, but the function of these is not clear at present
(Mertz, 1969). However, recent work suggests a biological function
of chromium in nucleic acid metabolism (Okada et al., 1984).
Ribonucleic acid synthesis in mouse liver was significantly
increased by as little as 1 µmol trivalent chromium, in the
presence of DNA or chromatin (Okada et al., 1981). These effects
were also present when the DNA or chromatin were first complexed
with chromium prior to incubation. However, prior complexation of
RNA polymerase with chromium depressed activity. These effects
were obtained in vitro with a concentration (52 µg/litre) that is
similar to physiological levels. Goncharov (1968) presented data
suggesting that chromium is involved in the function of the thyroid
gland. These findings have been supported by Lifschitz et al.
(1980).
An oligopeptide with a relative molecular mass of 1480, which
was crystallized from liver tissue, had a specific affinity for
chromium (Wu, 1981).
7.1.5.2. Interaction of chromium with insulin
The interaction of chromium with insulin has been extensively
studied and can therefore be presented in some detail, but this
does not imply that this is the only, or the most important,
function of chromium.
The effects of chromium in vitro, and probably in vivo, depend
on the presence of endogenous or exogenous insulin, no effects
having been demonstrated in in vitro systems that did not either
depend on, or contain, insulin. Chromium deficiency causes an
impaired response to added insulin in rat epididymal fat tissue,
and, when glucose uptake or glucose oxidation or utilization for
lipid synthesis is measured, the dose-effect curve is flat.
Addition of suitable chromium compounds significantly increases the
slope of the curve (Fig. 3). This demonstrates the true
potentiation of the insulin action and indicates that chromium
alone does not act as an insulin-like substance (Mertz et al.,
1961; Mertz & Roginski, 1971; Mertz, 1981). Chromium was also
shown to stimulate the transport of D-galactose into epididymal fat
cells. This suggests cell transport, the first step of sugar
metabolism, as a major site of action for chromium (Mertz &
Roginski, 1963). Insulin-potentiating effects have also been
observed on the swelling of liver mitochondria (Campbell & Mertz,
1963) and on glucose utilization in isolated rat lens (Farkas &
Roberson, 1965).
Fig. 3
Stimulation of the effects of insulin has been observed in a
glucose-independent, but insulin-responsive, system. Significantly
more alpha-amino isobutyric acid (a non-metabolizable amino acid
analogue) was incorporated into the heart and liver tissue of
chromium-supplemented male rats than in the tissues of chromium-
deficient controls, in response to the in vivo injection of the
labelled analogue and insulin (Roginski & Mertz, 1969).
These observations suggest a peripheral action of chromium to
facilitate the action of insulin; no evidence has been produced
indicating that chromium plays any role in the production, storage,
or release of insulin by the pancreas. Thus, the primary result of
chromium deficiency is a diminution in the effectiveness of
insulin. The resulting metabolic impairment may be compensated for
by increased insulin production in some cases, resulting in
elevated concentrations of the hormone, but not enough data exist
from experimental animal studies to assess the action of the
element on insulin metabolism. More information is available for
human subjects and this is discussed in section 8.1. The
interaction between chromium, insulin, and receptor sites of liver
mitochondrial membranes was studied using polarographic techniques.
The results formed the basis for the hypothesis that chromium may
facilitate bond formation between the intra-chain disulfide of
insulin and sulfur-containing groups of the receptors, by
participating in a ternary complex (Christian et al., 1963).
This hypothesis is consistent with results of studies on rats
fed, either a low-chromium Torula yeast diet or a brewer's yeast
diet known to be adequate in chromium. While the insulin-binding
capacity of hepatocytes was not significantly different, the
insulin affinity of the cells was significantly greater ( P < 0.01)
for the chromium-adequate rats than for the deficient Torula yeast
rats (Steele et al., 1977b).
7.1.6. Chromium nutritional requirements of animals
In the preceding sections, studies were evaluated in which the
effects of chromium supplementation were determined in animals that
were at least marginally chromium deficient. In other studies, the
effects of chromium were investigated in animal systems in which
the existence of chromium deficiency was either not ascertained or
not investigated. Before these studies are described and
interpreted for the determination of chromium nutritional
requirements of animals, it is helpful to consider them against the
background of Venchikov's (1974) model. This model is generally
applicable to trace element effects defining 3 zones of action, the
zone of biological action, that of pharmacodynamic action, and that
of toxicity (Fig. 4). The biological zone, in response to
supplements with low amounts of an element, represents the
correction of a deficiency and the resulting level of biological
activity is that of optimal function. Increasing the amount of
supplement further may lead to a certain depression, followed by a
zone of new, increased activity, in which the element no longer
acts as an essential nutrient, but as a drug. Still greater
supplements, beyond the homeostatic control capability of the
organism produce toxic effects and death. Because all the studies
described subsequently involved amounts of chromium supplements
that were higher than the levels normally needed to correct a
deficiency, it is possible, according to Venchikov's definition,
that the observed effects might be pharmacological.
Fig. 4
Tuman & Doisy (1977) studied the effects of yeast concentrates
of high chromium (glucose tolerance factor) content and of
synthetic chromium complexes with GTF activity (Tuman et al., 1978)
in mice, raised on a presumably complete commercial stock diet.
Six animals were used for each test, either genetically diabetic
mice or their control litter mates of the C57Bl-KSI strain.
Injections of either 5 mg of the GTF-containing yeast extracts or
0.1 mg of the synthetic chromium complex, acutely reduced the
elevated plasma-glucose levels in the diabetic and the non-fasting
normal mice by 10 - 38% of the initial values ( P < 0.01) and the
plasma-triglycerides by 26 - 56% ( P < 0.01), compared with control
mice injected with saline. Injection of insulin into diabetic mice
produced only an 11 - 18% decrease in plasma-glucose levels,
whereas injection of the GTF-containing extract together with
insulin reduced plasma-glucose levels by 39 - 51% and plasma-
triglyceride levels by 76% (Table 11).
The results suggest either a much higher increase in the
chromium requirement of the genetically diabetic mice or their
inability to use chromium in the diet.
Table 11. Acute effects of GTF and exogenous insulin on non-
fasting plasma-glucose and plasma-triglyceride (TG) concentrations
in 19-week-old genetically diabetic micea
------------------------------------------------------------------
Treatment Plasma- deltaGlucose Plasma- deltaTG
glucoseb triglyceridesb
(mg/litre) (mg/litre)
------------------------------------------------------------------
saline 11 120 ± 3960 ± 160(5)c
490(5)c
GTF 9320 ± -180 (16%) 2790 ± 170(5)d -117 (30%)
280(5)d
insulin 9840 ± -128 (12%) 3020 ± 400(5) - 94 (24%)
410(5)
insulin 7060 ± -406 (37%) 940 ± 220(5)e -302 (76%)
and GTF 840(5)e
------------------------------------------------------------------
a Modified from: Tuman & Doisy (1977).
b Glucose and triglyceride values represent mean ± SEM for 6 mice in
each treatment group. Dose of GTF was 5 mg (WL-10-AT) administered
intraperitoneally, 12 h prior to collection of blood. Lente insulin
(0.1 U per mouse) was administered subcutaneously, 12 h prior to
collection of blood. Data were treated by analysis of variance to
detect differences between the various treatment groups; independent
orthogonal comparisons were performed in the following groups ( P value
indicates level of significance for each comparison).
c Saline versus all other treatments, P < 0.005.
d GTF versus insulin, P < 0.05.
e GTF and insulin alone versus combined GTF and insulin, P < 0.005.
Thus, GTF and insulin > GTF = insulin > saline.
Steele & Rosebrough (1979) reported a significant stimulation
of the growth rate of one-week-old turkey poults (both sexes) by
supplementation of a practical ration with 20 mg chromium (as
chloride)/kg. The weight gains within the 2-week study were 235 g
and 267 g for the 60 controls and 60 supplemented turkeys,
respectively ( P < 0.001). As the practical ration contained
ground yellow corn and soybean meal, limestone, and dicalcium
phosphate, chromium deficiency would appear unlikely. The amount
of the chromium supplement (20 mg/kg diet) is quite high, and
further studies are needed to decide whether the observed effects
were of a pharmaco-dynamic nature or truly nutritional, i.e.,
correcting a deficiency. A similar interpretation should be
applied to a report of improved egg quality in the laying hen
(Jensen et al., 1978).
The quantitative aspects of the effects of chromium on animals
can be summarized as follows: normal rats fed semi-purified, semi-
synthetic rations, with Torula yeast or individual proteins and
sucrose or starch as the source of carbohydrates, develop mild
signs of deficiency at a dietary level of 100 - 150 µg chromium/kg.
To prevent deficiency, most authors used very high supplements of
several mg/kg diet and did not determine the biological
availability of the chromium complexes used for the
supplementation. Diets containing raw ingredients and supplying
chromium levels between 0.5 and 1 mg/kg do not induce signs of
deficiency and probably meet the requirement of the rat. A very
tentative estimate of the dietary chromium need of the rat and
probably the mouse would be approximately 0.5 mg/kg diet. This
estimate should be interpreted with caution, because of the lack of
knowledge concerning the biological availability of chromium and of
its interaction with dietary constituents.
7.2. Toxicity Studies
The toxicology of chromium compounds has been reviewed by the
US National Academy of Science (US NAS, 1974a), Langard & Norseth
(1979), the International Agency for Research on Cancer (IARC,
1980), Langard (1980a, 1982), and Burrows (1983).
In discussing toxicological problems, it is important to
differentiate between the various oxidation states of chromium and
its compounds. Trivalent chromium, when administered to animals in
food or water, does not appear to induce any harmful effects, even
when given in large doses (US NAS, 1974a) (section 7.2.1). Acute
and chronic toxic effects of chromium are mainly caused by
hexavalent compounds. Since it has been shown that both industrial
trivalent chromium compounds as well as reagent-grade trivalent
chromium compounds can be contaminated by hexavalent chromium
(Petrilli & DeFlora, 1978a; Levis & Majone, 1979), the evaluation
of experimental studies becomes difficult, especially when the
purity of the chemical compounds used is not known.
Discrimination between the biological effects, caused by
hexavalent chromium and trivalent chromium is difficult, because,
after penetration of membranes in tissues, hexavalent chromium is
immediately reduced to trivalent chromium (Gray & Sterling, 1950;
US NAS, 1974a), and it is not evident whether the observed
phenomena are caused by this reduction or even by the trapping of
trivalent chromium by ligands after uptake in the cells. Another
problem in evaluating the data is associated with the route of
administration. Hexavalent chromium, introduced by the oral route,
is partly reduced to trivalent chromium by acidic gastric juice
(Donaldson & Barreras, 1966; DeFlora & Boido, 1980); thus, the
effects or lack of effects observed may be caused mainly by
trivalent chromium and not by the hexavalent chromium, actually
administered.
7.2.1. Effects on experimental animals
Many local effects on human beings have been reported (section
8.3), but only a few studies have verified these effects in
experimental animals. A comprehensive survey of hexavalent
chromium-induced effects is given in Table 12 (US NAS, 1974a). For
most studies, details were not given of the length of exposure,
number of treated animals and controls, etc. Diagnoses were stated
without presenting all the original data. Thus, in this section,
some papers will be discussed that refer to the most prominent
local and systemic effects to support and clarify the effects shown
in human beings.
It is evident that the toxicity of hexavalent chromium in
animals varies with the route of entry into the body. Low
concentrations of hexavalent chromium may be tolerated, when
administered in the feed or drinking-water, the extent of
absorption being a factor of importance. For example, rats
tolerated hexavalent chromium in drinking-water at 25 mg/litre, for
1 year, and dogs did not show any effects from chromium
administered as potassium chromate at 0.45 - 11.2 mg/litre over a
4-year period (US NAS, 1974a). However, oral exposure of both male
and female rabbits to sodium dichromate (0.1% solution, 0.2 - 5
mg/kg body weight, for up to 545 days) resulted in significant
morphological changes in the gonads, including atrophy of the
epithelium and dystrophic alterations of the Sertoli and Leydig
cells in the testes, and sclerotic and atrophic changes in ovaries
(Kucher, 1966).
Larger doses of hexavalent chromium are highly toxic and may
cause death, especially when injected iv, subcutaneously (sc), or
intragastrically. The LD50 of chromium compounds was determined
for several experimental animal species. The LD50 of potassium
dichromate (hexavalent chromium), administered orally (stomach
tube) to rats, was 177 mg/kg body weight in males and 149 mg/kg
body weight in females (Hertel, 1982). When injected iv in mice
(sex not given), the LD50 of chromium carbonyl was 30 mg/kg body
weight (IARC, 1980).
Performing a life-time inhalation study on the rat, Glaser et
al. (1984) found an LC50 for Na2Cr2O7 of 28.1 mg/m3 (range, 16.7 -
47.3 mg/m3). Assuming a deposition rate in the lung of 30% of the
dose administered, the LC50 dose was 1 mg/kg body weight in male
and 1.2 mg/kg in female rats.
A local corrosive action of hexavalent chromium on the skin,
similar to that seen in man, was described by Samitz & Epstein
(1962), who induced chrome ulcers in guinea-pigs at 4 trauma sites,
with daily exposure to 0.34 MK2Cr2O7 solution for 3 days. Mosinger
& Fiorentini (1954) showed the same effects using potassium
chromates.
Table 12. Effects of hexavalent chromium in animalsa
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit, inhalation chromates 1 - 50 mg/m3 14 h/day for pathological Lukanin (1930)
cat 1 - 8 months changes
in the lungs
Rabbit inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day none Lehmann (1914)
as dichromate for 5 days
Cat inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day bronchitis, Lehmann (1914)
as dichromate for 5 days pneumonia
perforation of
nasal septum
Mouse inhalation mixed dust 1.5 mg/m3 4 h/day, no tumours Baetjer et al.
containing as CrO3 5 days/week, (1959a);
chromates for 1 year Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 16 - 27 mg/m3 1/2 h/day tumours in Baetjer et al.
containing as CrO3 intermit- some strains (1959a);
chromates tently Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 7 mg/m3 37 h over increased Baetjer et al.
containing as CrO3 10 days tumour rate (1959a);
chromates Steffee &
Baetjer (1965)
Rat inhalation mixed dust 7 mg/m3 37 h over barely Baetjer et al.
containing as CrO3 10 days toleratedb (1959a);
chromates Steffee &
Baetjer (1965)
Rabbit, inhalation mixed dust 5 mg/m3 4 h/day, none marked Baetjer et al.
guinea- containing as CrO3 5 days/week, (1959a);
pig chromates for 1 year Steffee &
Baetjer (1965)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rat, inhalation hexacarbonyl 1.6 mg/m3 4 months, anaemia; lipid Roschina (1976)
rabbit 4 h, 5 days and/or protein
a week dystrophia
in liver
and kidneys
Rat, inhalation hexacarbonyl 0.16 mg/m3 4 months, anaemia; no Roschina (1976)
rabbit 4 h, 5 days biochemical
a week or morphological
effects
Rat inhalation hexacarbonyl 35 mg/m3 30 min 100% death Roschina (1976)
Rat inhalation dichromates 0.006 - 0.2 28 days/ increase in Glaser et al.
mg/m3 90 days lung-macrophages (1985)
23 h/day
7 days/week lymphocytes
immunoglobulin,
reduced Fe2O3
lung clearance
Rat intratra- dichromates 5 per week up to 30 toleratedc Steinhoff
cheal in- 0.01 - 0.25 months et al. (1983)
stillation mg/kg
Rat intratra- dichromates 1 per week up to 30 tolerated; Steinhoff
cheal in- 0.05 - 1.25 months 1.25 mg/kg et al. (1983)
stillation mg/kg harmful
Rat oral potassium 500 mg/litre daily maximal Gross & Heller
chromate in non-toxic (1946)
drinking-water concentration
Rat, oral zinc chromate 10 g/kg daily maximal Gross & Heller
mouse in feed non-toxic (1946)
(mature) concentration
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit oral sodium di- 0.2 - 5.0 mg/kg 545 days morphological Kucher (1966)
chromate changes in
gonads (testes:
atrophy of
epithelium,
dystrophic
alterations of
Sertoli &
Leydiz alls.
Ovaries:
sclerotic and
atrophic
changes)
Rat oral zinc chromate 1.2 g/kg daily maximal Gross & Heller
(young) in feed non-toxic (1946)
concentration
Rat oral potassium 1.2 g/kg daily maximal Gross & Heller
(young) chromate non-toxic (1946)
in feed concentration
Dog, oral monochromate 1.9 - 5.5 mg 29 - 685 none harmful Lehmann (1914)
cat, or dichromates chromium/kg days
rabbit body weight per
day 1 mg chrom-
ium equivalent
to 2.83 mg
K2Cr2O7
or 3.8 mg
K2CrO4
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog oral potassium 1 - 2 g as daily fatal in 3 Brard (1935)
dichromate chromium months anaemia
Dog stomach potassium 1 - 10 g as - rapidly fatald Brard (1935)
tube dichromate chromium
Monkey subcutan- potassium 0.02 - 0.7 g in - fatald Hunter & Roberts
eous dichromate 2% solution (1933)
Dog subcutan- potassium 210 mg as - rapidly fatal Brard (1935)
eous dichromate chromium
Guinea- subcutan- potassium 10 mg - lethald Ophüls (1911a)
pig eous dichromate Ophüls (1911b)
Rabbit subcutan- potassium 1.5 cc of 1% - 80% fatald Hasegawa (1938)
eous dichromate solution/kg body
weight
Rabbit subcutan- potassium 20 mg - lethald Ohta (1940)
eous dichromate
Rabbit subcutan- potassium 0.5 - 1 cc of - nephritisd Ohta (1940)
eous dichromate 0.5% solution/kg
body weight
Rabbit, subcutan- sodium 0.1 - 0.3 g as - rapid deathd Priestley
guinea- eous or chromate CrO3 fall in blood (1877)
pig intravenous pressure
Rabbit intra- potassium 0.7 cc of 2% - lethald Mazgon (1932)
venous solution/kg 8-10 days after
body weight injection
Dog intra- potassium 10 grains - instant death Gmelin (1826)
venous chromate
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog intra- potassium 1 grain - none marked Gmelin (1826)
venous chromate
Dog intra- potassium 210 mg as - rapidly fatal Brard (1935)
venous dichromate chromium
Dog intra- potassium 3 mg/100 cc 2 doses marked renal Hepler & Simonds
venous dichromate blood per dose damage (1946); Simonds
& Hepler (1945)
---------------------------------------------------------------------------------------------------------
a Modifed from: US NAS (1974a).
b Pathological changes in experimental and control rats, 101 weeks after exposure.
c The same weekly dose distributed over 5 days was clearly better tolerated than a single weekly
administration.
d Renal damage.
Following parenteral administration, the most common systemic
effects of chromium were parenchymatous changes in the liver and
kidney (Mosinger & Fiorentini, 1954). Later studies showed
selective damage in the renal proximal convoluted tubules, without
evidence of glomerular damage, as demonstrated after one single sc
injection of potassium dichromate of 10 mg/kg body weight (Schubert
et al., 1970) or after one single intraperitoneal (ip) injection of
sodium chromate of 10 or 20 mg/kg body weight (Evan & Dail, 1974).
Effects have also been found in fish (Strik et al., 1975). After 32
days of continuous exposure to 0.1, 1, or 10 mg hexavalent chromium
(as potassium dichromate)/litre, the fish Rutilus rutilus developed
lysis of the intestinal epithelium with haemorrhages as well as
hypertrophy and hyperplasia of the gill epithelium.
Franchini et al. (1978) found an increase in urinary protein,
lysozyme, glucose, and beta-glucuronidase in rats after a single sc
injection of potassium dichromate at 15 mg/kg body weight. After
sc injection (3 mg/kg body weight), every other day for 2 - 8
weeks, the authors observed a correlation between the chromium
contents of the renal cortex and chromium clearance.
Five-week-old male Wistar rats of the strain TNO-W-74 were
continuously exposed in inhalation chambers to submicron aerosols
of sodium dichromate at concentrations ranging from 25 (low level)
to 200 µg chromium/m3 (high level) (Glaser et al., 1985). Exposure
for 28 days to 25 or 50 µg chromium/m3 resulted in "activated"
alveolar macrophages with stimulated phagocytic activity, and
significantly elevated antibody responses to injected sheep red
blood cells. After 90 days of low-level exposure, there was a more
pronounced effect on the activation of the alveolar macrophages,
with increased phagocytic activity. However, inhibited phagocytic
function of the alveolar macrophages was seen at the high
hexavalent chromium exposure level (200 µg/m3). In rats exposed to
this chromium aerosol concentration for 42 days, the lung clearance
of inert iron oxide was significantly reduced. The humoral immune
system was still stimulated at a low chromium aerosol concentration
of 100 µg/m3, but significantly depressed at 200 µg chromium/m3.
Exposure of rats, through inhalation, to chromium carbide or
chromium boride dust at very high levels (300 - 350 mg/m3 for each
substance) for 3 months (2 h/day) resulted in effects on the
vascular system of the lungs, e.g., endothelial hyperplasia.
Bronchitis and a decrease in the blood-haemoglobin concentration
were also observed (Roschina, 1964). The effects of chromium boride
were more pronounced than those of chromium carbide.
Steinhoff et al. (1983) performed an intracheal injection study
on rats (930 rats, 30 months, 0.05 - 1.25 mg Na2Cr2O7/kg body
weight per week; 1.25 mg CaCrO4/kg body weight per week). Half of
the rats were intratracheally injected once a week and the other
half received the same weekly dose distributed over 5 injections
per week. In rats receiving doses 5 times a week, there were some
significant changes in levels of total plasma-protein and
-cholesterol, in some haematological variables, in organ weights,
and in survival times in females. Only male rats receiving 1.25 mg
sodium dichromate/kg body weight, once a week, showed a sharp
reduction in body weight, female rats being less affected. Rats
receiving calcium chromate in the same dose showed reduced body
weight but to a lesser extent. The weight of lung and trachea was
increased by both substances in all doses.
Marked histopathological changes (congestion, fairly large
areas of focal necrosis, bile duct proliferation) described after
long-term exposure of rabbits to hexavalent chromium (ip injection
of 2 mg chromium/kg body weight per day for 6 weeks) (Tandon et
al., 1978), as well as the increase in hepatic metallothionein and
decrease in cytochrome P-450 levels after ip injection of 400 µmol
chromium/kg per day (type of chromium compound not mentioned)
(Eaton et al., 1980) need further confirmation. The finding of an
accumulation of hexavalent chromium in the reticuloendothelial
system including bone marrow (Baetjer et al., 1959b; Langard, 1977)
may be of importance for a disturbed blood picture.
Merkurieva et al. (1980a,b) studied the effects of potassium
dichromate in the drinking-water on the activities of different
enzymes in rats. Exposure included daily doses of 0.0005, 0.005,
0.05, or 0.5 mg/kg body weight for up to 6 months. After 20 days
of exposure at the highest dose level, enzyme activities increased
by 15 - 28% in liver microsomes (inosine-5-diphosphatase),
lysosomes (beta-D-galactosidase), and cytosol (lactate dehydrogenase
(EC 1.1.1.27)). For some of these enzymes, as well as for
acetylesterase (EC 3.1.1.6), increases in activity of up to 54%
were found in the gonads, kidneys, seminal fluid, and serum. At a
dose of 0.05 mg/kg, the only statistically significant finding was
a 54% increase in the activity of acetylesterase in the gonads.
After a 6-month exposure at this dose level, there was a 70%
increase in lactate dehydrogenase activity in the seminal fluid as
well as a 50% increase in free beta-galactosidase in the liver. There
were no changes in enzyme activities at the two lowest dose levels.
Painting of rat skin with an aqueous solution of potassium
dichromate (0.5%), daily, for 20 days, resulted in a local
inflammatory reaction, an increased level of hexose glyco-proteins
in the skin and serum, and an elevated concentration of serotonin
in the skin and liver (Merkurieva et al., 1982). Most of these
effects were also seen earlier in the exposure period, though they
were not as pronounced. Ten days after the start of exposure, a
nearly 3-fold increase in the serum-acetylcholine concentration
occurred together with decreased acetylcholinesterase activity.
Thus, the data of Merkurieva et al. show systemic effects following
both oral and dermal exposure to hexavalent chromium.
Cats fed chromic phosphate or oxydicarbonate at 50 - 1000 mg/day
for 80 days did not exhibit signs of illness or tissue damage.
Similarly, toxic reactions were not observed in rats administered
drinking-water containing 25 mg trivalent chromium/litre, for 1
year, or 5 mg trivalent chromium/litre throughout their lifetime
(US NAS, 1974a). The toxicity of trivalent chromium is so low that
even by parenteral administration, a chromic acetate level of 2.29
g/kg body weight or a chromic chloride level of 0.8 g/kg body
weight is required to kill mice. Even very large doses given
intragastrically were not fatal for dogs. Brard (1935) reported
that 10 or 15 g of chromium as chromic chloride proved fatal in one
dog (US NAS, 1974a). Some fatal doses of trivalent chromium
compounds reported in the literature are listed in Table 13.
Rats exposed through inhalation to chromic oxide (trivalent
chromium) at 42 mg/m3 or to chromic phosphate at 43 mg/m3 (5 h/day
for 5 days/week) for 4 months developed chronic irritation of the
bronchus and lung parenchyma, and dystrophic changes in the liver
and kidney (Blokin & Trop, 1977).
Inhalation exposure of rats (number not given) to dusts
containing 36 or 50% chromite for 4 months (2 h/day), at
concentrations of 375 - 400 mg/m3, resulted in thickening of the
walls of pulmonary vessels and bronchi (Roschina, 1959). The high
exposure levels in these studies make it difficult to evaluate the
effects.
Inhalation studies have also been performed with chromium
carbonyl, where chromium is in the 0 oxidation state (Roschina,
1976). Twelve rabbits and 48 rats were exposed for 4 months (4
h/day, 6 days/week) at a concentration of 1.6 or 0.16 mg/m3. At
both exposure levels, there was loss of body weight (25 and 12%,
respectively) as well as anaemia and leukocytosis. In the higher
exposure group, the animals showed an elevated gamma-globulin level
in serum and an increased transaminase activity. The contents of
cholesterol and SH-groups were reduced, and there was a decrease in
cholinesterase (EC 3.1.1.7) activity. Lipid and/or protein
dystrophy were noted in several organs, e.g., in the liver and
kidneys. No such effects were detected in the animals in the low-
exposure group.
Table 13. Fatal doses of trivalent chromium in animalsa
---------------------------------------------------------------------------------------------
Animal Number of Routeb Compound Chromium Effect Reference
animals dose (g/kg)
---------------------------------------------------------------------------------------------
Dog 2 sc chromic chloride 0.8 fatal Brard (1935)
Rabbit 1 sc chromic chloride 0.52 fatal Brard (1935)
Rat 38 iv chrome alum 0.01 - 0.018 LD50 Mertz et al.
chromium-hexaurea (1965a)
chloride
Mouse -c iv chromic chloride 0.8 MLDd Windholz et al. (1960)
Mouse -c iv chromic acetate 2.29 MLD Windholz et al. (1960)
Mouse -c iv chromic chloride 0.4 MLD Schroeder (1970)
Mouse -c iv trivalent chromium ? 0.25 - 2.3 MLD Windholz et al. (1960)
Mouse -c iv chromic sulfate 0.247 MLD Windholz et al. (1976)
Mouse -c iv chromic sulfate 0.085 MLD Schroeder (1970)
Mouse -c iv chromium carbonyl 0.03 LD50 Schroeder (1970)
---------------------------------------------------------------------------------------------
a Modified from: US NAS (1974a).
b sc = subcutaneous; iv = intravenous.
c No figures given.
d Minimum lethal dose.
7.2.1.1. Carcinogenicity
Various types of chromium chemicals, methods of administration,
and species of animals have been studied (IARC, 1980a).
Ideally, carcinogenicity should be tested with the methods
recommended by IARC (1980b), but, in many of the early studies,
this was not done. Carcinomas of the lung have been reported in
animals as a result of the administration of chromium chemicals.
Hueper (1958) found 2 squamous cell carcinomas and one
carcinosarcoma in 25 rats following intra-pleural injection of
chromite ore roast. After intrapleural implantation of strontium
chromate lasting 27 months, Hueper (1961) found tumours (type
unspecified) in 17/28 rats. Laskin et al. (1970) and Levy & Venitt
(1975) produced a number of bronchogenic carcinomas by implanting
pellets of cholesterol mixed with various chromium compounds
encased in a wire mesh cage in the bronchi of rats. Calcium and
zinc potassium chromate produced a number of bronchogenic
carcinomas, but soluble chromates and trivalent chromium chemicals
failed to produce cancer.
Using the same technique, Levy & Martin (1983) tested 21
different chromium-containing materials (pigments, intermediates,
and residues from the bichromate-producing industry, relatively
pure crystalline compounds) in 2250 random-bred rats and found that
chromates, described as sparingly soluble, were carcinogenic in the
rat lung. These materials included strontium and calcium chromate
and, to a far lesser extent, certain forms of zinc chromate.
Barium and lead chromate evoked only a very weak carcinogenic
response compared with strontium and calcium chromate. In the
study of Laskin et al. (1970), it was shown that chromium trioxide
produced hepatocellular carcinomas in 2/100 rats (controls, 0/24).
After inhalation of 13 mg calcium chromate/m3 (5 h/day, 5
days/week, for lifetime), Nettesheim et al. (1971) found 14 lung
adenomas in 136 treated mice and 5 in 136 untreated controls, but
no carcinomas. Steffee & Baetjer (1965), performing inhalation
studies on rats, mice, guinea-pigs, and rabbits (inhalation of
mixed chromate dust, corresponding to 3 - 4 mg CrO3/m3, 4 - 5
h/day, 4 days/week, for lifetime, or 50 months, respectively) could
only find 3 alveologenic adenomas in 50 treated guinea-pigs.
Laskin (1972) and Laskin et al. (1970) found 1 squamous cell
carcinoma of the lung, 1 of the larynx and 1 "peritruncal tumor" in
rats (inhalation of calcium chromate, 2 mg/m3, 589 exposures of 5 h
over 891 days) and 1 squamous cell carcinoma and 1 papilloma of the
larynx in hamsters. The number of treated animals was not
specified in either paper. Steinhoff et al. (1983) performed
intratracheal instillations of chromates in rats for 30 months with
one treatment/week and the same weekly dose distributed over 5
treatments/week (Table 14). In 880 exposed rats, 28 adenomas of
alveolar-bronchiolar origin (benign) and 12 malignant tumours (3
adenocarcinomas and 9 squamous cell carcinomas) were found. All
lung tumours developed very late and were only detected at the end
of the lifetime study, often in lungs with callosities. The
tumours were tiny and none of them caused the animal to die.
Sodium dichromate was not carcinogenic after exposure on 5
days/week. With calcium chromate, the carcinogenic effect was more
pronounced after treatment once per week, than after treatment 5
times per week.
Table 14. Incidence of benign and malignant lung tumours among
880 rats intracheally injected with Na dichromate and Ca chromatea
----------------------------------------------------------------
Dose Number of Incidence of lung tumours
(mg/kg) injections benign malignant
per week
----------------------------------------------------------------
Na2Cr2O7 x 2H20 0.25 5 none none
Na2Cr2O7 x 2H20 1.25 1 12 8
CaCrO4 0.25 5 5 1
CaCrO4 1.25 1 11 3
----------------------------------------------------------------
a From: Steinhoff et al. (1983).
Hueper (1961) reported unspecified tumours at implantation
sites in 12/34 rats, following intramuscular (im) implantation of
sintered calcium chromate (25 mg). No tumours were observed in 32
control animals. The same author found implantation-site tumours in
15/33 animals treated with strontium chromate compared with no
tumours in 32 control animals. Single sc injections of 30 mg lead
chromate (Maltoni 1974, 1976) resulted in injection-site sarcomas
in 26/40 treated rats. After administration of the same amount of
lead chromate oxide, injection-site sarcomas were found in 27/40
rats; no sarcomas were found in 60 vehicle controls. Following 9
im injections of 8 mg lead chromate, Furst et al. (1976) found
injection-site sarcomas in 31/47 treated rats, and renal carcinomas
in 3/24 treated rats; no sarcomas were found in 0/22 vehicle
controls. Heath et al. (1971) found injection-site sarcomas and
other tumours in 7/74 treated rats after im injection of 28 mg
cobalt-chromium alloy.
Administration of low levels of trivalent chromium acetate (5
mg/litre in the drinking-water) to mice and rats for the lifetime
did not result in increased tumour incidences compared with
controls (Schroeder et al., 1964, 1965).
Trivalent chromium oxide incorporated in bread at
concentrations of 10, 20, or 50 g/kg and fed to BD rats did not
increase tumour incidence compared with controls (Ivankovic &
Preussmann, 1975). Hexavalent chromium compounds have not been
tested by oral administration.
IARC (1980a) summarized the data of all available references
and concluded as follows: "There is sufficient evidence for the
carcinogenicity of calcium chromate and some relatively insoluble
chromium (VI) compounds (sintered calcium chromate, lead chromate,
strontium chromate, sintered chromium trioxide and zinc chromate)
in rats. There is limited evidence for the carcinogenicity of lead
chromate (VI) oxide and cobalt-chromium alloy in rats. The data
were inadequate for the evaluation of the carcinogenicity of other
chromium (VI) compounds and of chromium (III) compounds".
(a) Interaction with other factors
The possibility that chromium may act synergistically with
other agents in the production of cancer has been tested in several
studies. Nettesheim et al. (1970) pretreated one group of mice
with 100 R whole-body radiation and infected another group with PR8
influenza virus. These mice and control mice were exposed through
inhalation to chromium oxide dust for 6 - 18 months. No measurable
effects of either of the pretreatments were found on the incidence
of tumours. In a similar study with inhalation of calcium chromate
(CaCrO4, 5 h/day, 5 days per week for the lifetime), pre-exposure
to X-rays did not affect the tumour rate, but PR8 influenza
infection reduced tumour incidence (Nettesheim et al., 1971).
Steffee & Baetjer (1965) also found that the infection of rats with
PR8 influenza virus associated with intratracheal injection of
chromates did not lead to the production of tumours. Chromite ore
injected iv did not affect the development of tumours induced by iv
injection of 20-methyl-cholanthrene (Shimkin & Leiter, 1940).
Thus, at present, there are not sufficient data to suggest that
chromium acts synergistically with virus infections, ionizing
radiation, or other chemical carcinogens to produce cancers.
7.2.1.2. Genotoxicity
The mutagenicity of chromium compounds was reviewed by IARC
(1980a), Petrilli & DeFlora (1980), Levis & Bianchi (1982), and
Baker (1984). Present knowledge, in the light of recent findings,
is summarized in Table 15 (hexavalent chromium) and Table 16
(trivalent chromium).
When evaluating the results of chromium mutagenicity tests, it
is necessary to take into consideration several properties of the
tested compound (oxidation state, solubility, ability to penetrate
cell membranes, intracellular stability, and reactivity with
cellular components). Most of the in vitro mutagenicity
experiments have been performed with chromium chemicals, following
the introduction of the reverse mutation test by Ames (1973).
Bacterial strains of Salmonella typhimurium (Petrilli & DeFlora,
1977) containing mutants that require histidine for growth, and
Escherichia coli (Venitt & Levy, 1974) with mutants requiring
tryptophan for growth have been used. In each case, the bacterial
cultures have been incubated with the test chromium compounds and
the number of revertant colonies scored. The chromium compounds
studied included both hexavalent and trivalent compounds; a survey
of the results is given in Table 17 (DeFlora, 1981). Zhurkov
(1981) performed an Ames test to study the mutagenic activity of
potassium dichromate (K2Cr2O7) with S. typhimurium TA 1535 and TA
1538. A medium degree of mutagenic activity without metabolic
activation was shown by the strain TA 1535.
Only hexavalent chromium compounds have shown mutagenic
effects. Using the Salmonella /mammalian microsome tests of Ames
et al. (1973), DeFlora (1978) and Löfroth (1978) both showed a
decrease in the mutagenicity of hexavalent chromium in the presence
of an NADPH-generating system, suggesting reduction of mutagenic
hexavalent chromium to trivalent chromium. In this chromate
metabolism, cytochrome P-450 functions as the reductase (Garcia &
Wetterhahn Jennette, 1981). In recent in vitro studies, Wiegand et
al. (1984b) showed that glutathione could reduce hexavalent
chromium to trivalent chromium, without any further cofactors or
metabolizing enzymes. In rats, the most efficient tissue in
decreasing hexavalent chromium mutagenicity was the liver, followed
by the suprarenal glands, kidney, stomach, and lung (Petrilli &
DeFlora, 1980). These studies suggest a possible way of
intracellular detoxification of hexavalent chromium in low doses.
Schoental (1975) suggested that hexavalent chromium caused the
formation of epoxyaldehydes having mutagenic potential. Venitt &
Levy (1974) assumed that chromates belong to the transition-
inducing class of mutagens. They cause both frameshift and base-
pair substitutions (Petrilli & DeFlora, 1980). The hypothesis of
Kazantzis & Lilly (1979), that chromates cause guanine-cytosine to
adenine-thymine transitions in the subsequent round of DNA-
replication, needs further confirmation.
Table 15. Genotoxic activity of chromium (VI) compoundsa
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
DNA degradation CaCrO4 - Casto et al. (1976)
K2Cr2O7 - Bianchi et al. (1983)
PbCrO4 - Douglas et al. (1980)
K2CrO4 + Whiting et al. (1979)
Decreased fidelity PbCrO4, + (1) Levis & Majone (1981)
of DNA synthesis K2Cr2O7
CrO3 + Sirover & Loeb (1976)
K2Cr2O7 + Bianchi et al. (1983)
Microbial DNA K2CrO4, + Yagi & NishioKa (1977)
repair K2Cr2O7
CrO3, K2CrO4, + Kanematsu et al. (1980)
K2Cr2O7
K2CrO4, + Nishioka (1975)
K2Cr2O7
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
CrO3, + Gentile et al. (1981)
K2Cr2O7,
Na2Cr2O7, + Gentile et al. (1981)
(NH4)2Cr2O7
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Microbial gene K2Cr2O7 + (2) Bonatti et al. (1976)
mutation CrO3 + (2) Fukunaga et al. (1982)
Na2Cr2O7, +/-(3) Petrilli & De Flora
CrO3, +/-(3) (1978a,b)
ZnCrO4xZn(OH)2 +/-(3)
K2Cr2O7 + Zhurkov (1981)
Na2Cr2O7 +/-(3) DeFlora (1978)
chromate (4) +/-(3) Löfroth (1978)
dichromate +/-(3)
K2Cr2O7 - Kanematsu et al. (1980)
K2CrO4, + Löfroth & Ames (1978)
K2Cr2O7 +
K2Cr2O7 + Nishioka (1975)
K2CrO4, CaCrO4 + DeFlora (1981)
(NH4)2CrO4,
CrO3,
Na2CrO4, + Venitt & Levy (1974)
K2CrO4
CaCrO4
K2Cr2O7 + Bianchi et al. (1983)
Na2Cr2O7,
ZnCrO4
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
K2CrO4, CaCrO4 + Petrilli & DeFlora
CrO3 (1977)
Na2Cr2O7
K2CrO4 + Green et al. (1976)
PbCrO4, CrO3 + Nestmann et al. (1979)
Mammalian cell K2Cr2O7 + Bianchi et al. (1983)
gene mutation K2Cr2O7, + Newbold et al. (1979)
ZnCrO4 +
PbCrO4 -
K2Cr2O7 + (5) Bonatti et al. (1976)
K2Cr2O7 + Pashin et al. (1982)
NaeCr2O + Pashin & Kozachenko
(NHy)2Cr2O7 + (1981)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell K2CrO4, + Umeda & Nishimura (1979)
chromosomal K2Cr2O7 +
mutation K2Cr2O7 + Bigaliev et al. (1978)
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7 +
Na2CrO4, + Majone & Levis (1979)
K2CrO4 +
Na2Cr2O7, Levis & Majone (1979)
K2Cr2O7
CrO3, CaCrO4
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CrO3 + Kaneko (1976)
Na2Cr2O7 + Sarto et al. (1980)
K2Cr2O7 +
CrCl3 +
CrO3 + Tsuda & Kato (1977)
K2Cr2O7 + Raffetto (1977)
K2Cr2O7 + Stella et al. (1982)
Na2Cr2O7, + Majone & Levis (1979)
K2Cr2O7 +
K2CrO4 + Wild (1978)
Na2Cr2O7 + (7) Krishnaja & Rige (1982)
Mammalian cell CrO3, K2CrO4, + Ohno et al. (1982)
SCE K2Cr2O7 +
K2Cr2O7 + Bianchi et al. (1983)
K2CrO4,
Na2CrO4 + Levis & Majone (1979)
CrO3, +
K2Cr2O7, + Majone & Levis (1979)
Na2Cr2O7 +
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CaCrO4, CrO3, + Gomez-Arroyo et al.
K2Cr2O7 + (1981)
K2Cr2O7 + Majone & Rensi (1979)
CrO3 + (6) Stella et al. (1982)
K2Cr2O4, + Elias et al. (1983)
Na2CrO4, +
Na2Cr2O7 +
K2Cr2O7,
K2CrO4 + McRae et al. (1979)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell CaCrO4, K2CrO4 + (8) Casto et al. (1979)
transformation ZnCrO4 + (9)
Na2CrO4,
CaCrO4 + Casto et al. (1976)
+ Di Paolo & Casto (1979)
K2Cr2O7 + Bianchi et al. (1983)
K2Cr2O7 + Tsuda & Kato (1977)
CaCrO4 + Fradkin et al. (1975)
K2Cr2O7 + Raffetto (1977)
---------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) As industrial pigments (Cr-yellow-orange; Zn-yellow; Mo-orange).
(2) Gene conversion.
(3) No mutagenicity in the presence of mammalian microsomal activation
system (S-9 mix).
(4) No compounds given.
(5) Forward mutation was observed in 7/480 054 colonies (controls 0/84
546). A significant increase can be stated if comparison is made
with the historical spontaneous mutation rate, i.e., no mutants in
106 colonies.
(6) Occupatinal exposure.
(7) Induction of chromosomal aberrations in gill cells of the fish
Boleophthalmus dussumieri (Goby).
(8) 2 times control levels.
(9) 4 times control levels.
The results summarized in Table 17 show that the chromate ion
(hexavalent chromium) induces different kinds of genetic damage,
even at low doses, whereas the chromosome-damaging capacity of
trivalent chromium was only observed when it was tested in very
high doses.
In assays with whole-cell systems, trivalent chromium is
inactive, unless there is a direct interaction with DNA, e.g., in
studies in which purified DNA was exposed to trivalent chromium,
where modifications of the physical and chemical properties, as
well as decreased fidelity of DNA synthesis, were produced (Levis &
Bianchi, 1982). According to Warren et al. (1981), it appeared
that trivalent chromium in a proper ligand environment could have
considerable genetic toxicity. However, in recent studies, Bianchi
et al. (1984) found that trivalent chromium (CrCl3, 10-3 to 10-5 M)
was neither cytotoxic nor mutagenic in permeabilized hamster
fibroblasts (BHK were incubated for 30 min in Ea or BSS made
hypertonic with 4.2% NaCl).
Table 16. Genotoxic activity of chromium (III) compoundsa
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
DNA degradation CrCl3 + Bianchi et al.
(1983)
Decreased fidelity Cr2O3 + Levis & Majone
of DNA synthesis (1981)
Microbial DNA cis-[Cr(bipy)2ox] + Warren et al. (1981)
repair I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 + Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O + Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O + Warren et al. (1981)
trans-[Cr(en)2 + Warren et al. (1981)
(SCN)2]SCN
CrCl3, Cr2O3, - Yagi & Nishioka
Cr(OH)3 (1977)
K2Cr2(SO4)4 - Yagi & Nishioka
(1977)
K2Cr2(SO4)4 - Kanematsu et al.
(1980)
Cr2(SO4)3 - Kanematsu et al.
(1980)
CrCl3 - Nishioka (1975)
Cr(NO3)3, + De Flora (1981)
Cr(CH3COO)3
CrCl3 - Nakamuro et al.
(1978)
CrCl3 + Gentile et al.
(1981)
CrCl3, KCr(SO4)2, - Gentile et al.
(1981)
Cr2(SO4)3 - Gentile et al.
(1981)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Microbial gene CrCl3, Cr(NO3)3 - Löfroth & Ames
mutation (1978)
Cr(CIO4)3 - Löfroth & Ames
(1978)
CrCl3, Cr(NO3)3 - Venitt & Levy (1974)
Cr2SO4, - Venitt & Levy (1974)
K2SO4 x 2H2O
Cr(CH3COO)3 + Nakamuro et al.
(1978)
Cr(NO3)3 + Nakamuro et al.
(1978)
CrCl3 - Nakamuro et al.
(1978)
CrK(SO4)2 x 12H2O - Petrilli & De Flora
(1977)
CrCl3 x 6H2O - Petrilli & De Flora
(1978a)
Cr(NO3)3 x 9H2O - Petrilli & De Flora
(1978b)
Microbial gene cis-[Cr(bipy)2ox] + Warren et al. (1981)
mutation I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 - Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O - Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O - Warren et al. (1981)
trans-[Cr(en)2 - Warren et al. (1981)
(SCN)2]SCN
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
gene mutation (1983)
Cr(CH3COO)3 - Newbold et al.
(1979)
Mammalian cell Cr2(SO4)3 - Umeda & Nishimura
chromosomal (1979)
mutation
Cr(CH3COO)3 + Nakamuro et al.
(1978)
CrCl3, Cr(NO3)3 - Nakamuro et al.
(1978)
Cr(NO3)3, + Levis & Majone
(1979)
KCr(SO4)2, + Levis & Majone
(1979)
Cr(CH3COO)3 + Levis & Majone
(1979)
CrCl3 + Kaneko (1976)
CrCl3 - Sarto et al. (1980)
Cr2(SO4)3, CrCl3 - Tsuda & Kato (1977)
CrCl3 + Raffetto (1977)
CrCl3 - Stella et al. (1982)
CrCl3 - (1) Bianchi et al.
(1984)
Mammalian cell CrCl3, Cr2O3 + Elias et al. (1983)
SCE
Cr2O3 + Levis & Majone
(1981)
Cr(NO3)3, - Levis & Majone
KCr(SO4)2 (1979)
CrCl3, Cr(CH3COO)3 - Levis & Majone
(1979)
CrCl3 + (2) Ohno et al. (1982)
Cr2(SO4)3 - Ohno et al. (1982)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
SCE (contd). (1983)
CrCl3 - Majone & Rensi
(1979)
Mammalian cell CrCl3 - Bianchi et al.
transformation (1983)
---------------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) Thymidine uptake, DNA replication, damage and repair and SCE were
tested in permeabilized hamster fibroblasts.
(2) Weakly positive.
Levis & Buttignol (1977) and Levis et al. (1978) raised the
question of whether trivalent chromium could be an active mutagenic
agent within a cell. Others, such as Bianchi et al. (1980),
supported this theory, suggesting that trivalent chromium is bound
to genetic material after reduction of the hexavalent form by an
NADPH-dependent microsomal enzyme system (Norseth, 1978; Jennette,
1979). The studies of Sirover & Loeb (1976), showing a 30% error
in DNA synthesis after application of 0.64 mM trivalent chromium
compared with 16 mM of hexavalent chromium, support this theory.
Pashin et al. (1982) demonstrated the induction of dominant
lethal mutations in mice. They treated male mice with a single
injection of potassium dichromate (0.5 - 20 mg/kg body weight, ip)
or with daily injections (1 and 2 mg/kg, ip, for 21 days). The
single injections (0.5 - 2 mg/kg) did not induce any effects;
repeated or higher dosages of potassium dichromate induced a
statistically significant decrease in the survival of embryos from
cells treated at the early spermatid and late spermatocyte stages.
Karyological alterations in mammalian cells were induced after
exposure to concentrations of hexavalent chromium that were more
than 100 times lower than the concentration of trivalent chromium
causing the same changes (Majone & Rensi, 1979).
Analysing the effects of fume particles from stainless steel
welding on sister chromatid exchanges and chromosome aberrations in
cultured Chinese hamster cells, Koshi (1979) found an increase in
cytogenic effects with increasing fume doses, due to the dissolved
hexavalent chromium. Hexavalent chromium and stainless steel
welding fume particles containing chromium compounds produced
positive results in the "mammalian spot test", a somatic tissue
assay (Knudsen, 1980).
Table 17. Mutagenicity of chromium compoundsa
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
L. Hexavalent chromium compounds
1) Sodium dichromate (Merck) - +w +w +w + 50 - 250 4.4 decrease toxic and mutagenic
Na2Cr2O7 x 2H2O Cr6+ was converted
into inactive Cr3+
2) Potassium chromate (BDH) - +w +w +w + 80 - 410 2.9 decrease by reducing
K2CrO4 chemicals (ascorbic
acid, sodium
3) Calcium chromate (BDH) - +w +w +w + 60 - 290 3.2 decrease sulfite) or
CaCrO4 metabolites (NADH,
NADPH, GSH), by
4) Ammonium chromate - +w +w +w + 50 - 320 3.7 decrease human gastric juice
(Carlo Erba) and erythrocyte
(NH4)2CrO4 lysates, by S9 mix
containing human
liver S9 fractions
5) Chromium trioxide or - +w +w +w + 40 - 220 5.1 decrease or rat tissue S9
chromic acid (Merck) fractions (in order
CrO3 of efficiency:
liver>suprarenal
glands>kidney>
stomach>lung);
pretreatment of
rats (Aroclor(R)
1254), ether,
ethanol) influenced
the efficiency of
metabolic systems;
conversely, Cr6+
mutagenicity was not
affected by human
serum or plasma nor
by S9 mix containing
other rat (spleen,
colon, bladder,
striated muscle) S9
fractions
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
6) Zinc yellow (Montedison) - +w +w +w + 90 - 590 1.4 decrease the mutagenic effects
ZnCrO4 x Zn(OH)2 + of Cr6+ compounds and
10% CrO3 benzo( a)pyrene were
less than additive
7) Chromyl chloride (BDH) - +w +w +w + 100 - 430 1.8 decrease liquid volatile
Cl2CrO2 compound; vapours
were also toxic and
mutagenic
8) Chromium orange or basic - - - - +b 2 mg the three pigments
lead (spot test) containing PbCrO4
PbCrO4 x PbO were scarcely (L8),
chromate (Montedison) very scarcely (L9),
or totally (L10)
insoluble in water;
they were assayed
by directly spotting
these compounds at
the centre of plates
9) Molybdenum orange or - - - - +b 2 mg
lead solfomolybdo- (spot test)
chromat (Montedison)
PbCrO4 72-77%
PbSO4 4-6%
Al2O3 2%
PbMoO4 12-14%
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
10) Chromium yellow or - - - - -b
lead solfochromate
(Montedison)
PbCrO4 41-85%
PbSO4 4-45%
SiO2 0.1-3%
Al2O3 2-6%
11) Chromium - - - - - -2.3x103 - - hexacoordinated
hexacarbonyl (BDH) compound; formally,
Cr(CO)6 its oxidation state
is 0; insoluble in
water, dissolved in
ether
M. Trivalent chromium compounds
1) Chromic chloride - - - - - -3x104 - - inactive Cr3+
(BDH) CrCl3 x 6H2O compounds could be
converted into toxic
and mutagenic Cr6+
only in the presence
of oxidizing chemicals
(potassium
permanganate), while a
variety of metabolic
systems were
ineffective
2) Chromic nitrate (Riedel- - - - - - -2x104 - -
de Haen)
Cr(NO3)3 x 9H2O
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
3) Chromic potassium - - - - - -1.6x104 -
sulfate (BDH)
CrK(SO4)2 x 12H2O
4) Chromic acetate (BDH) - - - - - -7x104 - -
Cr(CH3COO)3
5) Neochromium (Montedison) - - - - - -5x104 - -
Cr(OH)SO4 56-58%
Na2SO4 23-24%
H2O 18-21%
6) Chromium alum (Montedison) - - - - - -4.3x104 - -
Cr2(SO4)3 37-39%
K2SO4 16-18%
H2O 43-37%
7) Chromite (Montedison) - - - - + 2 mg - Contaminated with Cr6+
Cr2O3 44-46% (spot test) traces ( sim-diphenyl-
Fe2O3 29-30% carbazide reagent);
Al2O3 15-16% scarcely soluble in
SiO3 0.5-3% water; assayed by
CoO 0.5-2% directly spotting the
powder at the centre
of plates
--------------------------------------------------------------------------------------------------------
Table 17 (contd.)
Note:
Sensitivity of Salmonella strains: Range of activity:
In the assays carried out in this study, This column indicates the lower and
the numbers of spontaneous revertants of the upper limits (in nmol per plate)
Salmonella tester strains fell within the of the mutagenic response, as in-
following ranges: ferred from dose-response curves
TA 1535: 10-25, both with and without S9 mix; with the most sensitive bacterial
TA 1537: 3-18, both with and without S9 mix; strain and under the most favourable
TA 1538: 10-25 without S9 mix; 15-35 with S9 mix; metabolic situation (i.e., with or
TA 98: 20-35 without S9 mix; 25-40 with S9 mix; without S9 mix) for each positive
TA 100: 150-200 without S9 mix; 140-180 with S9 mix compound; for negative compounds,
the maximum dose tested is indicated
The key for the interpretation of symbols in the
column "Reverted strains" is the following: Mutagenic potency:
+ clearly positive result indicated by a dose-
related and reproducible increase of his+ the values presented have
revertants over controls (at least a 3-fold been calculated by dividing the number
increase); of revertants in excess of controls
+w weak positivity indicated by an increase of by the corresponding amount of compounds
revertants 2 - 3 times in controls; (in nmoles); the number of net revertants
± reproducible but less than 2-fold increase was determinated at the top level of the
of revertants; linear part of dose-response curves, which
No symbol: no conclusive experiment carried out were drawn as indicated under the previous
with the corresponding strain. sub-heading.
Effects of S9 mix
The effect of S9 mix containing
liver S9 fractions from
Arochlor(R) pretreated rats on
the mutagenicity of test
compounds is reported
--------------------------------------------------------------------------------------------------------
a From: DeFlora (1981).
b L8, L9, and L10, as well as PbCrO4, were positive in the plate test when
dissolved in 0.5 N NAOH.
Embryonic fibroblast cell cultures showed significant
chromosome aberrations, when potassium dichromate was added to the
medium (Tsuda & Kato, 1976). When the potassium dichromate was
reduced with sodium disulfite (Na2SO3), no significant aberrations
occurred, even with 100 times the chromium concentrations.
At present, it is widely accepted that hexavalent chromium is
genetically active, because of its ability to cross the membranes
and enter the cells. If reduction of hexavalent chromium takes
place outside the cell (or even outside the cell nucleus, e.g., in
mitochondria or microsomes) its genetic activity is suppressed; if
the reduction takes place inside the nucleus (near, or at, the
target DNA molecules) alterations in DNA can occur, depending on
the oxidation power of hexavalent chromium or the formation of
trivalent chromium complexes with nucleophilic sites of the DNA;
thus, trivalent chromium could be the ultimate mutagenic form of
chromium (Levis & Bianchi, 1982). More recent investigations
(Wiegand et al., 1984) have shown that hexavalent chromium may
enter the body unreduced via the lung and be partly deposited in
erythrocytes over a prolonged period of time. From a DNA-repair
test in bacteria, DeFlora et al. (1984) concluded that hexavalent
chromium causes irritation in nucleotide pools and, due to its
oxidizing power, induces single-strand breaks in DNA, while
trivalent chromium induces DNA-protein and DNA-DNA cross-links and
decreases the fidelity of DNA replication.
7.2.1.3. Developmental toxicity and other reproductive effects
The teratogenicity of hexavalent chromium as chromium trioxide
was tested in golden hamsters (Gale, 1974, 1978, 1982; Gale &
Bunch, 1979) and that of trivalent chromium as chromic-chloride in
ICR mice (Iijima et al., 1975; Matsumoto et al., 1976). Injections
of hexavalent chromium into the fertilized chick egg increased
embryolethality and produced malformations in the survivors (Gilani
& Marano, 1979).
The golden hamsters were treated with a single iv injection of
chromium trioxide (5, 7.5, 10, or 15 mg/kg body weight) on the 8th
day of gestation (Gale, 1978).
Dose-response relationships were demonstrated between the rate
of absorption and the rate of malformations including delayed
ossification of the skeletal system. At 5 mg/kg body weight, 4% of
the fetuses showed external abnormalities (oedema, exencephaly),
and 34% showed cleft palate (controls: 2%), which increased to 85%
with the higher doses of 7.5 or 10 mg/kg body weight. Thirty-one
percent of fetuses were retarded following treatment with 7.5 mg/kg
body weight and 49% following 10 mg/kg body weight. The dose of 15
mg/kg body weight was lethal for 75% of the dams. Gale & Bunch
(1979) injected a single dose of 8 mg CrO3/kg body weight on day 7,
8, 9, 10, or 11 of gestation in female golden hamsters (Table 18).
The treated females were adversely affected (body weight loss,
tubular necrosis of the kidneys). In a later study, Gale (1982)
injected 8 mg CrO3/kg iv in hamsters on day 8 of gestation. An
increased incidence of cleft palate was seen in strains LVG, LSH,
and MHA, but no effects were reported in strains CB, LHC, and PD4.
ICR mice were injected sc with chromic chloride (Iijima et al.,
1975; Matsumoto et al., 1976). In the first series (animals
administered 9.8 or 19.5 mg/kg body weight, every other day, from
the 0th to 16th day of gestation), according to the authors, a
slight increase in the rate of malformations could not be excluded.
In the second series, with ip injections of 9.8 - 24.4 mg/kg body
weight, on the 8th day of gestation, the authors reported a dose-
dependent increase in the frequency of exencephaly, anencephaly,
and the occurrence of rib fusion.
Danielsson et al. (1982) studied the rate of embryonic or fetal
uptake of trivalent chromium and hexavalent chromium in early and
late gestational mice (Table 19) and found placental passage of
chromium. Hexavalent chromium appeared in the fetus in high enough
concentrations for a direct effect on embryonic structures to be
likely, trivalent chromium may act on placental structures.
Gilani & Marano (1979) injected chromium trioxide into 840
embryonating chicken eggs (air sack) at doses ranging from 0.002 to
0.05 mg per egg, on days 0, 1, 2, 3, and 4 of incubation (0.1 ml
physiol. saline injections per control egg). All embryos were
examined on day 8. The following malformations were observed in
the 720 eggs affected: short and twisted limbs, microphthalamia,
exencephaly, short and twisted neck, everted viscera, oedema, and
reduced body size. However, the incidence of abnormalities in the
120 controls was reported to be "low".
Inhalation studies were conducted by Glaser et al. (1984) on
Wistar rats (TNO-W-74 SPF) to study the effects of sodium
dichromate on reproduction and teratogenicity in 3 generations.
Whole-body exposures to sodium dichromate aerosols (0.2 mg
chromium/m3) were performed with an exposure time of 130 days per
generation. No effects on reproduction were found. All
teratogenicity tests were negative, and there was no increase in
fetal chromium content. However, from generation to generation,
there was an increase in immunosuppression and hyperplasia of
organs (especially in the lungs) and changes in haematological
variables.
Table 18. Embryotoxicity following maternal exposure to CrO3 on different days
of gestation in hamstersa
-------------------------------------------------------------------------------------
Findings in live fetuses
Day of No. of No. of No. of Frequency External Internal Cleft
gestation females implantation living resorptions defects defects palate
treated sites fetuses No. %b No. %b No. %b No. %b
-------------------------------------------------------------------------------------
Treated CrO3 8 mg/kg
7 6 64 10 54 84 1 10 1c 10 5 50
8 6 88 54 14 20 8d 15 4e 7 17 31
9 6 77 67 10 13 18f 27 0 0 25 37
10 6 77 77 0 0 0 0 0 0 0 0
11 6 68 67 1 1 0 0 0 0 0 0
Controls H2O 5 ml/kg
7 3 35 33 2 6 0 0 0 0 0 0
8 3 44 41 3 7 0 0 0 0 0 0
9 3 37 36 1 3 0 0 0 0 0 0
10 2 20 19 1 5 0 0 0 0 0 0
11 3 34 33 1 3 0 0 0 0 0 0
-------------------------------------------------------------------------------------
a From: Gale & Bunch (1979).
b Underlined %, significantly different statistically from controls.
b Right hindlimb with two digits.
c Right kidney absent.
d Oedema (7), tail short (1).
e Small kidneys (3), right kidney absent (1).
f Oedema (17), omphalocele (1).
Table 19. Comparison of concentrations of trivalent and hexavalent
chromium in fetuses, placentas, and maternal organsa,b
------------------------------------------------------------------
Day 13 Day 16
Hexavalent Trivalent Hexavalent Trivalent
chromium chromium chromium chromium
------------------------------------------------------------------
Fetus 0.3 ± 0.01 0.03 ± 0.002 0.5 ± 0.04 0.1 ± 0.01
Placenta - - 3.0 ± 0.2 2.4 ± 0.2
Liver 29.4 ± 2.2 30.8 ± 6.5 33.9 ± 3.9 27.7 ± 3.5
Kidney 38.1 ± 1.9 5.3 ± 0.7 36.0 ± 4.3 8.7 ± 2.3
Serum 2.6 ± 0.2 9.3 ± 0.9 2.8 ± 0.4 11.0 ± 1.6
------------------------------------------------------------------
a From: Danielsson et al. (1982).
b Trivalent chromium (51CrCl3) and hexavalent chromium
(Na251Cr2O7) were injected iv on day 13 and day 16 of gestation
at doses of 10 mg chromium/kg body weight. The pregnant animals
were autopsied after 1 h. Concentrations are expressed in
mg/litre or g ± SEM (N = 4).
7.2.1.4. Cytotoxicity and micromolecular syntheses
Wacker & Vallee (1959) reported that trivalent chromium was
present in RNA from all sources examined; they hypothesized that
chromium might contribute to the stabilization of the structure.
Administration of trivalent chromium to mice (ip injection of
CrCl3 at 5 or 0.5 mg chromium/kg body weight; P < 0.01) caused
accumulation of chromium in the cell nucleus, which amounted to
about 20% of the accumulated chromium content of the liver cell,
and also enhanced RNA synthesis. Hexavalent chromium inhibited RNA
synthesis (K2CrO4 at 5 or 0.5 mg chromium/kg body weight;
P < 0.01) (Okada et al., 1983). In further studies, Okada et al.
(1984) pretreated rats with CrCl3 (ip injection of 5 mg chromium/kg
body weight) and then partially hepatectomized the rats. Chromium
accumulated in the regenerating liver, and hepatic RNA synthesis
was accelerated compared with that of partially hepatectomiced
controls. These findings suggest a direct participation of
chromium in RNA synthesis.
Potassium dichromate enhanced the passive uptake of ribo-and
desoxyribonucleosides (Levis et al., 1978; Bianchi et al., 1979),
caused an inhibition of DNA replication to BHK fibroblasts, and
reduced the colony-forming ability of BHK cells (doses: 10-4 - 20-7
mol). Hexavalent chromium (potassium dichromate with a
concentration of 5 x 10-7 mol/litre) reduced by half the
proliferation of NHIK 3025 cells originating from a human cervix
carcinoma (White et al., 1979).
The addition of calcium chromate to BHK21 cell cultures altered
their growth characteristics. Whereas these cells normally grow as
elongated fibroblast cells in parallel orientation, the chromium-
exposed cells grew as shortened fibroblasts with enlarged nuclei
and granular cytoplasm, randomly orientated. When grown in
carboxymethyl cellulose, the chromate-exposed cells underwent many
divisions in contrast to the 1 or 2 divisions of normal cells.
These alterations in growth properties persisted, even after
transfer to a normal medium (Fradkin et al., 1975).
Cultures of HeLa and rat embryonic cells were incubated with
various hexavalent and trivalent chromium compounds for 3 days and
the 50% inhibitory dose (ID50) for cell growth was determined. The
ID50 for sodium and potassium chromate and dichromate and calcium
chromate varied from 0.34 to 0.78 µg/ml of the medium. The ID50
for the trivalent compounds varied over a wider range: chromic
oxalate, 4; acetate, 52; nitrate, 720; chloride, 1030; and bromide,
1500 mg/litre. Similar results were found in rat embryonic cell
cultures (Susa et al., 1977).
7.2.1.5. Fibrogenicity
Because of the well-known fibrogenic effects of silica and
asbestos minerals, studies were initiated to determine whether
chromite and various other dusts exhibited similar properties. A
single intratracheal injection in rats of 40 mg chromite particles
(33% chromium, 0.7% SiO2, 99.9% of the particles being smaller than
5 µm) suspended in 1 ml Ringer's solution caused a moderate
cellular reaction that reached a peak after 2 months and then
decreased. After 8 months (end of observation), the lung weight
was within normal limits. Only an increase in fibrils that was not
significant was observed. The tissue reaction in regional lymph
nodes was minimal (Swensson, 1977).
Chromite FeO(CrAl)2O3 (10 mg), ground to an average particle
diameter of 1 µm, was injected into the pleural cavities of 25
mice. The animals were killed at intervals ranging from 2 weeks to
18 months after injection. Chromite produced the least reaction of
all the 15 dusts tested. This material attracted very few cells
and the eventual production of collagen was minimal (Davies, 1972).
7.2.2. Observations in farm animals
Systemic poisoning of farm animals with chromates is rare and
is mainly suspected to be a consequence of accidental industrial
contamination of drinking-water. The acute lethal dose for mature
cattle is approximately 600 - 800 mg/kg body weight; chronic
poisoning in young calves was produced by a daily dose of 30 - 40
mg/kg body weight for one month (NZ Department of Agriculture,
1954) with a clinical picture of profuse scouring, severe
dehydration, low blood pressure, changes in the alimentary tract,
including congestion and inflammation, and the rumen and abomasum
showing ulceration near perforation.
High chromium levels were found in the blood and liver of
calves that had died from poisoning. The minimum tissue levels
causing poisoning were 30 mg/kg in the liver and 4 mg/litre in
whole blood (Harrison & Staples, 1955). According to Romoser et al.
(1961), a level of 100 mg hexavalent chromium (as sodium
chromate)/kg did not affect growing chicks.
8. EFFECTS ON MAN
8.1. Nutritional Role of Chromium
The physiological role of chromium and the need for small
amounts of dietary chromium are indicated by:
(a) chromium-responsive cases of impaired glucose
metabolism in malnourished children and middle-aged
persons;
(b) 2 cases of proved chromium deficiency in patients fed
exclusively by parenteral alimentation; and
(c) epidemiological studies suggesting a link between
chromium deficiency and risk factors for cardiovascular
diseases.
8.1.1. Biological measurements and their interpretation
Biochemical methods for the diagnosis of the human nutritional
chromium status are still at the developmental stage. Blood- or
urine-chromium concentrations are not indicative of chromium
status. Several studies suggest the validity of the "relative
chromium response" in plasma or serum to the ingestion of 50 - 100
g of glucose within 30 - 120 min; the response also appears in the
urine. Lack of this response suggests chromium deficiency,
especially when combined with impaired glucose tolerance in the
presence of normal or elevated plasma-insulin levels.
8.1.2. Chromium deficiency
8.1.2.1. Adults
Chromium deficiency is diagnosed mainly through therapeutic
trials in which an impaired function is restored by supplementation
of the diet with small amounts of chromium close to the estimated
human requirement. Glinsmann & Mertz (1966) studied 4 male
diabetic patients for several months to 1 year, under the strictly
controlled conditions of a metabolic ward. The subjects, who were
fed identical portions of a diet prepared before the start of the
study, were allowed a measured amount of physical activity and were
stabilized under these conditions before the beginning of the
control periods. Three of the 4 patients responded to a chromium
level of 180 - 1000 µg/day as CrCl3 x 6H2O with a significant
improvement in oral glucose tolerance; the fourth showed a very
slight lowering of the glycaemic curve, which was not statistically
significant. A representative example is given in Table 20.
Table 20. Effect of chromium on mean glucose tolerancea,b
------------------------------------------------------------------------
Supplementation Days Number Mean blood glucose concentration
of ---------------------------------
tests Fast- Min after glucose load
ing 30 60 90 120
------------------------------------------------------------------------
None 0 - 32 10 840 2170 2290 1950 1560
60 µg, 3 x per day 33 - 74 12 850 2240 2380 2090 1620
60 µg, 3 x per day 75 - 119 13 840 2100 2130 1860 1410
60 µg, 3 x per day 120 - 140 5 840 2030 2010c 1750 1120c
None 180 - 194 1 960 2070 2900 2930 2370
60 µg, 3 x per day 195 - 211 5 830 1960 1930c 1750 1180c
None 256 - 313 10 870 2200 2450 2240 1660
1 mg, 3 x per day 337 - 340 1 680 1480 1620 1510 960
------------------------------------------------------------------------
a Modified from: Glinsmann & Mertz (1966).
b A 46-year-old man with maturity-onset diabetes, controlled on diet;
oral glucose tolerance test consisted of constant noon meal and 100 g
glucose.
c Mean values significantly different from control ( P < 0.025).
The condition of one of 2 diabetic out-patients, observed over
several months, was improved by chromium supplementation, the
condition of the other was not. Short-term treatment of 7 diabetic
patients for 1 - 7 days with 1 mg chromium did not affect any
variables of glucose metabolism, nor did chromium supplementation
change the normal glucose tolerance tests of 10 healthy, young
volunteers.
Glinsmann's positive results were not confirmed in a double-
blind crossover study on 4 normal volunteers and 10 diabetic
persons, with a crossover after 16 weeks. No effects of chromium
were detected on glucose tolerance or on fasting or postprandial
blood-glucose levels (Sherman et al., 1968). Schroeder et al.
(1970), while reporting that 2 - 10 mg chromium/day "almost always"
restored the impaired glucose tolerance in older, non-diabetic
subjects to normal, found only erratic and mild improvement in 4
out of 12 diabetic out-patients. On the basis of these
observations, Mertz (1969) concluded that chromium should not be
considered a therapeutic agent for diabetic patients.
On the other hand, it was reported in a later study on chromium
metabolism that chromium administration (500 µg/day, for 2 weeks)
produced a marked improvement in glucose utilization in both
juvenile and maturity-onset diabetic patients. Five of the
patients were followed for more than 1 year after exposure and were
found to maintain their improved glucose metabolism (Nath, 1976).
Of 10 non-diabetic elderly subjects with impaired glucose
tolerance, 4 responded to 150 µg chromium/day with complete
normalization of oral glucose tolerance tests. The remaining 6
subjects were not affected by the supplements (Levine et al.,
1968). Significant effects of a GTF-chromium-containing yeast
preparation were reported by Doisy et al. (1976) in 6 out of 12
elderly subjects with impaired glucose tolerance
Table 21. Effect of GTF supplementation on glucose tolerance tests in
elderly subjects with impaired tolerancea,b
-------------------------------------------------------------------------
Mean plasma-glucose levels Cholesterol Triglyceride
(mg/litre) (mg/litre) (mg/litre)
0 h 1 h 2 h
-------------------------------------------------------------------------
Before GTF 1060 ± 40c 2010 ± 70 1780 ± 80 2450 ± 90 1210 ± 80
11 tests
After GTF 990 ± 40 1620 ± 110 1320 ± 50 2050 ± 100 1120 ± 120
9 tests
Signifi- NS < 0.01 < 0.001 < 0.01 NS
canced
-------------------------------------------------------------------------
Mean serum-insulin levels
(microunits/ml)
0 h 1 h 2 h
Before GTF 24 ± 6 78 ± 17 118 ± 17
after GTF 26 ± 12 70 ± 8 83 ± 8
-------------------------------------------------------------------------
a From: Doisy et al. (1976).
b Supplement: 4 g yeast per min/day (Yeastamin Powder 95, Staley Co.,
Chicago, Illinois, USA). GTT: 100 g oral load.
c Mean ± SEM.
d Using paired t test, difference is significant.
In addition to the improvement in glucose tolerance in the
presence of a reduced insulin response, plasma-cholesterol
concentrations were significantly reduced. Several other
individual cases are reported in the publication of Doisy et al.
(1976), all of whom showed a lower serum-insulin response to a
glucose tolerance test during chromium supplementation.
Liu & Morris (1978) performed a similar study on the effects of
a yeast-chromium supplementation in 27 women, 40 - 75 years old, of
whom 15 had a normal and 12 an abnormal glucose tolerance test. In
11 of the normal subjects (73%), the integrated insulin response
was significantly reduced from 436 to 335 µU/ml ( P < 0.025) during
supplementation, without a statistically significant change in
glucose tolerance test. Significant reductions in the total glucose
response (10 650 - 9820 mg/litre; P < 0.001) and in the total
insulin response to glucose were observed in the subjects with
impaired glucose tolerance (1288 - 831 µU/ml; P < 0.001). The
glucose tolerance improved in 7 out of 12 women, but all 12 showed
a reduction in plasma-insulin.
The effects of supplementation of the diet with 200 µg
chromium/day (as chloride) on the glucose tolerance of 76 normal
adult volunteers was investigated in a double-blind cross over
study, with each period lasting 3 months. All 18 subjects entering
the study with impaired glucose tolerance (serum-glucose equal to
or greater than 1 g/litre, 90 min after a glucose load), showed
significant improvement during chromium supplements, but not with a
placebo, with an average decline in the 90-min serum-glucose levels
of 180 mg/litre ( P < 0.01) (Anderson et al., 1983b).
In another placebo-controlled study, 24 elderly volunteers were
given a daily supplement of either chromium-rich brewer's yeast or
chromium-deficient Torula yeast (9 g/day, for 8 weeks). The
glucose tolerance of the subjects given brewer's yeast
significantly improved with a concomitant decrease in plasma-
insulin levels, and a significant decline in plasma-cholesterol as
well as total plasma-lipids. No significant changes were observed
in the control group given the chromium-poor Torula yeast
(Offenbacher & Pi-Sunyer, 1980).
Additional, preliminary studies suggest a specific effect of
brewer's yeast on individual lipoprotein cholesterol fractions.
Twenty normal human subjects responded to daily supplementation
with 18 g of brewer's yeast over a 8-week period with a significant
reduction in low-density lipoprotein-cholesterol ( P < 0.01) and
with a simultaneous increase of 14% in the high-density lipoprotein
( P < 0.01) (Nash et al., 1979).
Similar results were observed in a 6-week supplementation trial
involving 8 physician volunteers, each of whom received 7 g/day of
brewer's yeast. Low-density lipoprotein-cholesterol declined by
nearly 18% ( P < 0.01), whereas the high-density cholesterol
fraction increased by 17.6%, resulting in a significant
( P < 0.001) decrease in the LDL/HDL ratio of 28% (Riales, 1979).
These studies suggest that the primary effect of the
supplementation must have been an increase in the effectiveness of
the insulin, because glucose tolerance was maintained or improved
in spite of lower insulin responses. It is important to note that
while the reduction in insulin levels occurred in all cases, an
improvement in glucose tolerance occurred in only a fraction. This
suggests two possible interpretations of all the results discussed
here, including those showing improvement in glucose tolerance in
only some of the subjects or no improvement at all. Lack of a
response to chromium could suggest that the subjects were not
chromium deficient and that their impaired glucose metabolism was
the result of other causes. Second, they may have responded with a
reduction in circulating insulin levels, as did all the subjects
with impaired glucose tolerance in whom the insulin response was
measured. Unfortunately, these measurements were not performed in
the earlier studies.
8.1.2.2. Malnourished children
Chromium deficiency has been implicated as a factor
contributing to the impairment of glucose tolerance in children
with protein calorie malnutrition in 3 countries, Jordan, Nigeria,
and Turkey. Hopkins et al. (1968) measured intravenous glucose
tolerance in 10 malnourished infants from the mountainous area of
Jordan and 9 equally malnourished infants from the valley area.
They found a severely depressed glucose removal rate of 0.7%/min in
the first group and a normal rate in the second, 3.8%/min (Table
22). The protein intake of both groups prior to hospitalization
had been identical; it was supplied in the form of a milk powder
with a chromium content of 18 µg/litre, when reconstituted.
However, analysis of 10 water samples revealed 3 times as much
chromium in the samples from the valley as in those from the
mountainous area (0.5 versus 1.6 µg/litre). This suggested the
possibility of chromium being a major determinant in the glucose
metabolism of these children, and the effects of supplementation
were measured in 6 children from the hills, with a severely
impaired tolerance test. The morning after oral administration of
250 µg chromium, as CrCl3 x 6H2O, the glucose removal rate had
markedly improved to 2.9%/min from a pretreatment level of
0.6%/min. Similar results were obtained in Nigeria (Table 22);
these tests also included control children who did not receive
chromium.
The immediate effects of chromium on these children were
remarkable and different from the lag phase always present in the
studies on adults. Equally important, was the short duration of
the effect of one dose demonstrated in a Jordanian child observed
for a longer period of time. These observations suggest that a
marked depletion in chromium stores must have existed in the
children.
Table 22. Significance of the effect of chromium on the impaired glucose
tolerances of malnourished infantsa
---------------------------------------------------------------------------
Subjects Number Glucose Significance
of removal of
infants rate difference
(%/min)
---------------------------------------------------------------------------
Jordanian infants from hill area with
0.5 µg chromium/litre in drinking-water 10 0.7
Jordanian infants from valley with
1.6 µg chromium/litre in drinking-water 9 3.8 P < 0.001
Jordanian infants;
initial glucose tolerance test 6 0.6 P < 0.001
after chromium treatment 6 2.9
Nigerian infants;
initial glucose tolerance test 6 1.2
after chromium treatment 6 2.9 P < 0.05
Non-treated infants:
Initial glucose tolerance test 5 1.9 not signi-
Repeated glucose tolerance test 5 2.1 ficant
---------------------------------------------------------------------------
a From: Hopkins et al. (1968).
Similar results were reported from a study in the Istanbul area
of Turkey (Gürson & Saner, 1971). Fourteen malnourished children
with an impaired glucose removal rate of 1.71%/min were given 250
µg of chromium (CrCl3 x 6H2O), and a second test was performed
after 15 h. The removal rate increased to 3.91%/min ( P < 0.001).
This average increase was due to a pronounced increase in 9 of the
children; no change was observed in the remaining 5. No
improvement in glucose tolerance occurred in 5 control children
receiving identical hospital treatment, except for the
administration of the chromium. The children receiving the
chromium supplementation grew significantly more than the control
children during the following 30 days, with the greatest growth
rates found in the 9 children who had responded to administration
of chromium with an improvement in glucose tolerance.
The fact that chromium deficiency interacts with protein-
calorie malnutrition only in certain geographical areas was
confirmed by Carter et al. (1968), who gave chromium
supplementation (250 µg/day as CrCl3 x 6H2O) without effects on
glucose tolerance in 9 Egyptian children tested. Plasma-chromium
levels were in the normal range, and chromium levels in
representative foods were high, ranging from 200 to 390 µg/kg.
These findings suggest that the chromium status of these subjects
was normal, which would explain the lack of an effect.
8.1.2.3. Patients on total parenteral alimentation
A female patient, maintained on total parenteral alimentation
since the age of 35 years, developed an unexpected weight loss of
15% after 5 years combined with peripheral neuropathy, impaired
intravenous glucose tolerance, in spite of normal insulin response,
and a decreased respiratory quotient of 0.66. Insulin treatment
(45 U/day) was ineffective. A strongly negative chromium balance
and low plasma- and hair-chromium levels suggested that the daily
estimated chromium intake from the infusion fluids of 5.3 µg had
not met the patient's requirement and resulted in chromium
deficiency. The insulin infusions were stopped and replaced by the
daily infusion of 250 µg chromium (as CrCl3 x 6H2O) for 2 weeks.
The result was normalization of glucose tolerance and respiratory
quotient, disappearance of signs and symptoms of neuropathy, return
to the previous normal body weight, in spite of reduced caloric
intake, disappearance of the requirement for exogenous insulin, and
a positive chromium balance. The nitrogen balance, which had been
erratic and often negative, became consistently positive following
chromium supplementation. Reduction of the daily supplement to 20
µg chromium proved to be sufficient to maintain the patient in a
state of well-being (Jeejeebhoy et al., 1977).
This case is the best demonstrated example of chromium
deficiency in a human subject. In addition to confirming the well-
known signs of chromium deficiency reported earlier, such as
insulin resistance and its consequences, it was the first case to
demonstrate the occurrence of peripheral neuropathy and disturbance
of nitrogen balance as a result of deficiency. These disturbances
require further study. A second case of chromium deficiency as a
consequence of long-term total parenteral alimentation was reported
by Freund et al. (1979). Signs and symptoms were similar to those
of the first case, except that central encephalopathy was present
instead of peripheral neural disorder. Supplementation with 150 µg
of chromium/day resulted in normalization of the deficiency signs.
Chromium at that time was not routinely added to intravenous
solutions, therefore it is possible that more cases of deficiency
may exist in patients on long-term infusion treatment. As an
outcome of this study, an Expert Panel of the American Medical
Association (AMA, 1979) suggested that the stable adult patient on
total parenteral alimentation should receive 10 - 15 µg chromium
daily.
8.1.2.4. Epidemiological studies
Two epidemiological studies suggest a link between chromium
status and cardiovascular diseases (Schroeder et al., 1970; Punsar
et al., 1975). Lack of chromium is associated with impaired
glucose tolerance, elevation of circulating cholesterol, and aortic
plaques. All of these variables are recognized risk factors for
cardiovascular disease. It has also been suggested that the
elevated insulin levels, often present in chromium-deficient human
subjects (Liu & Morris, 1978) represent a substantial risk for the
development of cardiovascular disease (Strout, 1977).
Schroeder et al. (1970) have summarized their studies of
chromium concentrations in normal and diseased tissues,
particularly in aortas (Table 23). The total body burden of
chromium, excluding the lungs, was lower in the USA, where there is
a higher incidence of cardiovascular disease, compared with other
regions, 1.72 - 1.86 mg/kg ash weight in the USA compared with 7.7,
9.6, and 2.7 mg in Africa, the Far East, and the Near East,
respectively. The only significant difference in tissue-chromium
concentrations in deaths from heart disease compared with deaths
from accidental causes was in the aorta (Table 23). Concentrations
in aortas from Africa, the Far and Near East were generally higher
and did not show such a difference.
Table 23. Chromium in aortasa
----------------------------------------------------------
Location Chromium (mg/kg dry weight)
Accident P Heart disease
----------------------------------------------------------
Africa 0.072 (5)b NS 0.116 (2)
Far East 0.97 (8) NS 0.246 (5)
Middle East 0.36 (8) NS 0.216 (3)
USA (Nine cities) 0.26 (103) 0.005 0.052 (13)
USA (San Francisco) 0.228 (10) 0.005 0.048 (15)
----------------------------------------------------------
a From: Schroeder et al. (1970).
b The number in parenthesis denotes the number of cases
observed.
The second epidemiological study was part of a cohort study
conducted in Finland beginning in 1959 (Punsar et al., 1975). A
total of 327 drinking-water samples were analysed for 22
characteristics including pH, anions, bulk minerals, and several
trace elements including chromium. The study included 504 and 622
men in the Western and Eastern areas of Finland, respectively,
whose domicile had not changed since 1959.
The coronary heart disease mortality rate is much higher in the
east of Finland than in the west. As seen in Table 24, the
chromium levels in drinking-water were much lower in the east than
in the west, whereas the opposite was true for copper. The metal
concentrations in the drinking-water of those with heart disease
and others only differed slightly (Table 24), but, according to the
authors, the data suggest that low chromium levels may play a role
in the high heart disease death rate of eastern Finland.
Table 24. Concentrations of copper and chromium in the
drinking-water of men who died between 1969 and 1973, and
of those who were alive in 1973a
-------------------------------------------------------------
Alive
Deaths (Clinical status in 1969)
CHDb Other CHDb Other No
heart heart
disease disease
-------------------------------------------------------------
West N = 19 N = 23 N = 63 N = 31 N = 288
Copper X 22.15c 20.17 20.05 20.94 20.15
(µg/litre) SD 3.92 4.69 4.69 5.12 4.74
Chromium X 7.52 9.38 9.45 9.74 8.45
(µg/litre) SD 2.06 3.42 4.01 4.44 3.31
East N = 23 N = 26 N = 129 N = 44 N = 279
Copper X 41.59 41.24 42.67 39.50 36.32
(µg/litre) SD 29.91 30.43 33.65 28.74 28.66
Chromium X 2.20 2.09 2.04d 2.49 3.01
(µg/litre) SD 2.17 2.41 2.08 2.17 2.37
-------------------------------------------------------------
a From: Punsar et al. (1975).
b CHD = coronary heart disease.
c P < 0.05.
d P < 0.001.
Note: Levels of significance refer to differences observed
when the concentrations were compared with those in the
"no heart disease" category.
Newman et al. (1978) measured serum-chromium concentrations in
32 patients, 18 male and 14 female, aged 25 - 65 years, in whom
coronary artery cine-arteriography was performed for medical
reasons. Serum-chromium was significantly lower in the 15 subjects
with coronary artery disease than in the 17 subjects without
involvement (2.51 versus 6.09 µg/litre; P < 0.01), whereas serum-
cholesterol, weight index, and systolic or diastolic blood pressure
did not differ among the groups. Serum triglycerides were higher
in the group with coronary artery disease (1.84 versus 1.12
g/litre; P < 0.05). No subject with a serum-chromium
concentration of more than 6 µg/litre exhibited coronary artery
involve- ment, and by regression analysis, serum-chromium was
judged to explain 17% of the total variance of the disease.
These results were independently confirmed in a second study in
which plasma chromium concentrations of 23 healthy persons were
more than 8 times greater than those of 67 patients with coronary
artery disease, as confirmed by cine-angiography (Simonoff et al.,
1984).
8.1.3. Mode of Action
As in experimental animals, the best known action of chromium
is the potentiation of insulin. This is shown by the
supplementation studies of Doisy et al. (1976) and Liu & Morris
(1978) in which a reduction in insulin levels in response to
glucose was observed, together with an improvement in glucose
tolerance. Direct proof, for example from in vitro studies with
human biopsy tissue, has not yet been obtained. Furthermore, it is
not known whether the potentiation of insulin is responsible for
all the effects observed during chromium supplementation, including
the reduction of circulating cholesterol and the growth stimulation
observed in malnourished children and during parenteral nutrition.
In the healthy, chromium-sufficient human subject, chromium
levels in the bloodstream increases acutely following a glucose
load or an injection of insulin. Glinsmann et al. (1966) observed
acute increases in 5 young, healthy volunteers during a glucose
tolerance test, but not when the same subjects were given an
equivalent volume of water. Maturity-onset diabetic patients did
not exhibit this chromium response initially, but did show a rise
in plasma-chromium, after several weeks of chromium
supplementation. These observations were confirmed by Levine et
al. (1968), Hambidge (1971), Behne & Diel (1972), and Liu & Morris
(1978), who saw the acute chromium rise in some, but not all,
subjects. In the study of Liu & Morris (1978), which was the most
detailed, the relative chromium response (CrR) was measured by
neutron activation analysis in 15 women with normal glucose balance
and in 12 with impaired tolerance.
(1-h serum-chromium)
CrR = ------------------------ x 100
(fasting serum-chromium)
Initially, the normal group had a mild positive response (CrR =
107), compared with a negative response (CrR = 81) in the impaired
subjects. Following three months of supplementation with a
chromium-rich yeast extract, the CrR of the normal subjects
increased to 140, and that of the impaired group became positive
with CrR = 149. As discussed in section 8.1.2, these changes were
accompanied by a significant decrease in the insulin response to
glucose and an improvement in glucose tolerance. The results of
these studies are in apparent contrast with those of Davidson &
Burt (1973), who found a significant decline in plasma-chromium
levels following a glucose load in normal, non-pregnant women (mean
age 26 years), but a mild to moderate increase in all but 1 of 10
pregnant women (mean age 20 years). As the time of the acute rise
varied between women, statistical analysis at any one time did not
produce any significant results. Hambidge (1971) and Behne & Diel
(1972) demonstrated an acute plasma-chromium response following
injection of insulin; it can therefore be assumed that the effect
of glucose on the chromium response is associated with
hyperinsulinaemia. The acute "relative chromium response" is
believed to reflect the chromium status of human subjects. It does
not appear when stores from which the chromium increment must come
are depleted. Depleted stores can be replenished by
supplementation, with a resulting reappearance of the response
shown in the two previously discussed studies. As it has been
shown that chromium levels in urine increase following a glucose
load (Wolf et al. 1974; Gürson & Saner, 1978), it is likely that a
fraction or all of the chromium increment is subsequently excreted.
8.2. Acute Toxic Effects
In adults, the lethal oral dose of soluble chromates is
considered to be between 50 mg/kg body weight (RTECS, 1978) and 70
mg/kg body weight (Deichmann & Gerarde, 1969). The clinical
features of toxicity are vomiting, diarrhoea, haemorrhagic
diathesis, and blood loss into the gastrointestinal tract causing
cardiovascular shock. If the patient survives about 8 days, the
outstanding effects are liver necrosis (Brieger, 1920), tubular
necrosis of kidneys, and poisoning of blood-forming organs
(Langard, 1980). A 14-year-old boy, who had ingested about 1.5 g
of potassium dichromate, died 8 days later. Kaufmann et al. (1970)
also reported enlargement and oedema in the boy's brain.
8.3. Chronic Toxic Effects
The chronic effects of trivalent and hexavalent chromium have
been reviewed by Langard & Norseth (1979). The most important
features are changes in the skin and mucous membranes, and allergic
dermal and broncho-pulmonary effects. Important systemic effects
occur in the kidneys, liver, gastrointestinal tract, and
circulatory system.
Mancuso (1951) studied several biological variables in persons
occupationally exposed to chromate and related them to clinical
signs and symptoms. He found occasional leukocytosis or
leukopenia, monocytosis, eosinophilia, reduced haemoglobin
concentrations and prolonged bleeding times, but none of them were
consistent enough to serve as reliable diagnostic tests.
Mild normochronic anaemia was reported by Myslyaeva (1965) in
94 subjects exposed to chromium compounds with 68 of them showing
signs of chronic toxicity. Though the report of Baetjer et al.
(1959b) was based only on 3 cases, it indicated that chromate
attached preferentially to red blood cells. Koutras et al. (1964)
reported the inhibition of the red blood cell enzyme gluthathione
reductase by chromate concentrations of 5 - 25 mg/kg body weight.
However, the lowest effective dose is far in excess of levels
found, even in heavily exposed persons. Thus, all attempts made so
far have failed to identify a specific sensitive biochemical test
for the assessment of chronic chromium toxicity.
Long-term surveillance of pregnant women working at a plant
producing dichromates and those living in its immediate vicinity
(Shmitova, 1980) revealed increased chromium levels in the blood
and urine, compared with those in the control group. At 32 weeks
of pregnancy, the blood concentrations of chromium were,
respectively, 2.5 mg/litre in the women workers, 1.2 mg/litre in
the women living near the plant, and 0.19 mg/litre in the controls;
the levels in the urine were 1, 0.47, and 0.045 mg/litre,
respectively. Umbilical venous blood contained 1.8, 0.66, and 0.37
mg chromium/litre, respectively. The placental chromium contents
in the surveyed women were 162, 179, and 40 µg/kg, respectively,
and the breast-milk levels were 119, 20, and 31 µg/litre, which
means that chromium is capable of transplacental entry into breast
milk and into the fetal and infant organism. Women working at the
bichromate plant exhibited a high incidence of obstetric pathology
and geotoses. Chromium levels were estimated in fetal and
placental tissues (abortive material) derived on the 12th week of
pregnancy from women workers of the dichromate facility and those
not exposed to chromium. In the test group, chromium levels were
1140 µg/kg in the fetal tissues and 135 µg/kg in the placental
tissue, whereas, in the controls, they were 928 and 30 µg/kg,
respectively. No teratogenic effects have been revealed (Shmitova,
1980).
8.3.1. Effects on skin and mucous membranes
Several different types of effects on skin and mucous membranes
may result from exposure to chromium chemicals, and the main
features are:
(a) primary irritation: ulcers (corrosive reactions),
scarred and non-ulcerative contact dermatitis;
(b) allergic contact dermatitis: eczematous and
noneczematous.
A general survey of the nature and cause of contact dermatitis
is given by Fregert (1981).
8.3.1.1. Primary irritation of the skin and mucous membranes
(a) Skin
Skin irritation, manifested by a sharp hyperaemia as well as by
vesicular, papular, and rash pattern, may result from contact with
chromates. However, the major problems in the primary irritation
dermatoses are ulcers, often called chrome holes or chrome sores.
Ulceration is likely to occur among workers who have contact with
high concentrations of chromic acid, sodium or potassium dichromate
or chromate, or ammonium dichromate. It does not result from
contact with trivalent chromium compounds. An ulcer may develop if
the chromium compound, either a dust or a liquid, comes into
contact with any break in the skin such as an abrasion, scratch,
puncture, or a laceration. Favoured sites for ulcer development
are the nailroot areas, the creases over the knuckles, finger webs,
the backs of the hands, and the forearms (Bloomfield & Blum, 1928).
Primary irritation by chromium can be attributed to hexavalent
chromium, as the salts of trivalent chromium are not considered
capable of producing such effects (Fregert, 1981). However,
prolonged exposure to trivalent chromium salts has been reported to
cause skin lesions, less marked than those following exposure to
hexavalent chromium salts (Domaseva, 1971). Findings in 164
patients with eczema showed that skin tests on people who had
contact with trivalent chromium did not give positive results with
dichromate (Valer & Racz, 1971).
Ordinarily, a chrome sore, if not deep, persists for about 3
weeks after exposure is discontinued. There is no evidence that
the ulcers undergo malignant transformation. Furthermore, the
presence of chromium ulcers did not influence the development of
sensitization to chromates (Edmundson, 1951), evidenced by patch
testing with a 0.5% solution of K2Cr2O7.
(b) Nasal septal mucosa
Perforation of the nasal septum was one of the main chronic
effects in workers who had contact with chromates and chromic acid
(Bloomfield & Blum, 1928; Langard & Norseth, 1979). In a group of
100 persons constantly in contact with chromium compounds, 44 had
superficial ulceration of the anterior nasal septal mucosa.
Twenty-one people had profound ulcers and 5 had perforations of
various sizes in the cartilaginous part of the nasal septum.
Perforation of the nasal septum and cicatricial alterations were
observed in people with 3 or more years of service, whereas
ulcerous lesions arose in 2 years of work with chromium compounds.
An additional consequence of the effect on the nasal mucous
membranes is a loss of the senses of smell and taste (Seeber et
al., 1976). In workers with profound ulcerous lesions of the nasal
septal mucosa, strains of white staphylococcus with elevated
resistance to chromium are sometimes isolated (Cherevaty et al.,
1965).
Pokrovskaya et al. (1976) studied 561 workers engaged in the
open excavation of chromium ores (chromium content was 62% in the
form of a complex compound of magnesium chromite and other chromium
ores). Almost half of the workers had worked there for more than 5
years, and about 40% had been working there for 6 - 15 years.
Changes in the nasal septal mucosa, typical of the effect of
chromium compounds, were found in 8.2% of the workers. The longer
the service record, the greater the prevalence of these lesions.
After 5, 11, and 15 years of work, the prevalence was 2.3%, 8.3%,
and 11.2%, respectively.
The prevalence of nasal septum perforation in chromium plating
workers was very high (24%) in the early 1950s in China (Yang,
1956). With the introduction of control measures, the prevalence
dropped so that, in 1982, only 2 cases of nasal septum perforations
were found among 393 chromium-exposed workers (Peng, 1982).
One hundred and four subjects (85 male, 19 female) exposed to
chrome plating and 19 unexposed controls were studied by Lindberg &
Hedenstierna (1983) with regard to changes in the nasal septum.
Exposed subjects were employed at 13 different companies and
included all who were working on the day of study at the particular
company. The median exposure time was 4.5 years (range, 0.1 - 36
years). Forty-three subjects were exposed almost exclusively to
chromic acid and constituted both a "low exposure" group (8-h mean
below 2 µg/m3, N = 22) and a "high exposure" group (2 µg/m3 or
more, N = 21). Their median exposure time was 2.5 years (range,
0.2 - 23.6 years). The other 61 subjects were exposed to a mixture
of chromic acid (0.2 - 1.7 µg/m3) and other pollutants, such as
nitric, hydrochloric, and boric acids, as well as caustic soda,
nickel, and copper salts. The last group was included to disclose
any additive or synergistic effects of chromic acid and other
pollutants and was studied with regard to lung function only. The
breakdown of subjects into various groups is shown in Table 25.
Nasal septal ulceration and perforation were seen in 10 out of 14
subjects exposed to peak levels of 20 - 46 µg chromic acid/m3 or
more; no one in the control group showed signs of atrophy,
ulcerations, or perforations.
8.3.1.2. Allergic contact dermatoses
Chromium is a very important skin sensitizer. Sensitization
is reported to require about 6 - 9 months, but can occur in less
than 3 months (Pirilä & Kilpio, 1949). Genetic factors appear to
contribute to the appearance of chrome sensitivity within the
Jewish population (Wahba & Cohen, 1979).
Apart from the primary irritation and ulceration effects,
direct contact with small amounts of the chromates may cause
allergic dermatosis (dermatitis and eczema). As this allergic
reaction is cell-mediated, i.e., antibody formation is intra-
cellular and not humoral, delayed allergic reactions are rarely
observed.
Fregert et al. (1969) reported that the skin-patch test for
chromium was positive in 8 - 15% of all patients suffering from
eczema. The dermatitis caused by chromium is described as a
diffuse erythematous type with severe cases progressing to an
exudative stage. Persons sensitive to chromium react to patch and
intracutaneous tests with nonirritant concentrations of potassium
dichromate (K2Cr2O7). Opinion is divided about the role of the
oxidation state of chromium as a causal agent in allergic contact
dermatoses (Morris, 1958; Fregert & Rorsman, 1964; Kogan, 1971).
In a review, Polak et al. (1973) showed that hexavalent chromium,
which penetrates the skin by a direct effect of a hapten, is
reduced to the trivalent state, chiefly by sulfhydryl groups of
aminoacids. It then conjugates with proteins thus forming the full
antigen that initiates sensitization.
Guinea-pigs sensitized with either trivalent chromium chloride
or hexavalent potassium dichromate are capable of reacting in vivo
and in vitro to challenges with both chromium salts. This double
reactivity is also found after repeated stimulation with only one
of these chromium compounds. Since it is not possible to select
lymphocytes directed specifically against a chromium determinant of
a particular valence, it is concluded that, by sensitization with
chromium salts of different valences, a common determinant or
closely related determinants are formed. It is suggested that this
determinant is formed by chromium in the trivalent form
(Siegenthaler et al., 1983).
Two to three years after medical treatment, a follow-up study
on 555 patients with contact dermatitis was completed by means of
questionnaires (Fregert, 1975). The eczema had healed in one-
quarter of the patients, one-half had periodic symptoms, and one-
quarter had permanent symptoms. The prognosis for persons who
changed their work or stopped working was the same as that for
those who continued eczema-inducing work.
From 48 positively tested patients (0.5% potassium dichromate),
38 persons, i.e., 79%, still had a positive patch test after 4 - 7
years. In 72% of the cases, a history of occupational exposure to
chromates could be proved (Thormann et al., 1979).
A fairly rapid transformation of dermatitis into eczema is a
characteristic feature of chromium-induced skin lesions. Kogan
(1971) recognized the following important properties: (a) absence
of adaptation, which makes it necessary to discontinue chromium
handling until the slightest clinical manifestations have
disappeared; (b) a persistent chronic development of an eczematous
process; (c) a long-term latent period; and (d) absence of a direct
correlation between the incidence of sensibilization in the exposed
workers and the chromium concentration to which they have been
exposed.
Table 25. Series of exposed subjects and referencesa
--------------------------------------------------------------------------------------------------------
Exposed subjects
Mixed exposure to
Low exposure to High exposure to chromic acid
chromic acid chromic acid (< 2 µg/m3) and other
(< 2 µg Cr6+/m3) (> 2 µg Cr6+/m3) acids and metallic salts References
----------------- ------------------ ----------------------- -----------------
Nb Age (years)c N Age (years) N Age (years) N Age (years)
--------------------------------------------------------------------------------------------------
Men
Non-smokers 10 35.8 ± 16.7 6 33.4 ± 14.1 12 33.6 ± 12.2 52 34.2 ± 13.2
Smokers 6 30.8 ± 9.7 16 30.2 ± 11.8 36 42.8 ± 13.1 67 30.5 ± 8.9
Women
Non-smokers 3 30.7(17-46) 0 9 48.3 ± 13.0
Smokers 2 44.5(29-60) 1 51.2 4 31.0(19-43)
--------------------------------------------------------------------------------------------------
a From: Lindberg & Hedenstierna (1983).
b When N < 5, the range is given instead of SD.
c X ± SD.
Patients suffering from chromium-induced allergic skin
dermatoses had a tendency to develop cross-correlated hyper-
sensitivity to other metals, in particular, to cobalt and nickel
(Rostenberg & Perkins, 1951; Geiser et al., 1960; Clark & Kunitsch,
1972; Kogan et al., 1972).
Increased sensitivity to chromium is proved by skin reaction
tests. In a number of cases, additional immuno-haematological
bioassays are made to confirm the specificity of these tests. A
method for studying the basophil (mast-cells) degranulation in vivo,
at the site of positive compress skin tests, was suggested by Kogan
(1968). The method was used as a basis for proving the specificity
of cutaneous tests for chromium and other sensitising metals of the
chromium group (cobalt and nickel) (Kogan, 1968; Kogan et al.,
1972).
Photosensitivity is sometimes observed in patients with chronic
dermatitis. Wahlberg & Wennerstein (1977) investigated patients
allergic to chromium, with or without clinically observed light
sensitivity, by a standardized photopatch test procedure. A
significantly more intense reaction was observed in 48% of the
cases in the UV-irradiated sites (4/5 minimal erythema dose)
compared with the non-irradiated control sites.
No cases of cancer of the skin have been reported to result
from exposure to any form of chromium (Fregert, 1981).
8.3.2. Effects on the lung
Airborne chromium trioxide is rapidly absorbed in the broncho-
pulmonary tract causing corrosive reactions (Borghetti et al.,
1977). The absorption of chromium aerosols may depend on the
physical and chemical properties of the particles (Taylor et al.,
1965), as well as on the activity of alveolar macrophages, and the
lymphatic drainage (Sanders et al. 1971).
Both Jindrichova (1978) and Keskinen et al. (1980) reported
that welders using chromium-containing electrodes suffered from
bronchitis. Zober (1982) studied arc welders who had used filler
metals containing chromium and nickel for some years. Compared
with controls, the welders reported more previous ailments of the
respiratory system, mainly in the form of acute bronchitis. In
X-rays of welders, more roundish shadows were found, probably
indicative of benign accumulations of ferrous dust. The greater
incidence of significant findings was clearly apparent in welders
who smoked (probably a combined effect of tobacco smoke and welding
fumes). Reggiani et al. (1973) studied 101 electroplaters by means
of spirometric tests and found an obstructive disease pattern in
13% of them. After inhalation of acetylcholine, a bronchospastic
reaction occurred in 23% of the workers. In another spirometry
study (Bovet et al., 1977) on 44 chromium electroplaters, an
obstructive respiratory syndrome was found among the workers with a
high urinary-chromium content. The authors concluded that the
influence of tobacco smoking on the spirometric tests was much less
than the influence of chromium.
Studying lung function and generalized obstructive lung
diseases in electrofurnace workers in a ferrochromium and
ferrosilicon-producing plant, Langard (1980b) found a reduced
forced vital capacity (FVC) and an increased prevalence of
obstructive lung diseases. Unable to document a connection between
the presence of chromates and increased lung diseases, the author
suggested that the effects were due to high levels of total dust,
especially amorphous silica dust.
Lindberg & Hedenstierna (1983) studied lung function in more
than 100 subjects exposed to chrome plating and in 119 car
mechanics as controls (no car painters or welders). In the exposed
subjects, FVC and the forced expired volume in one second (FEV1)
were both reduced by 0.2 litre, and the forced mid-expiratory flow
was reduced by 0.4 litre/second. The authors concluded that an 8-h
mean exposure level of more than 2 µg/m3 might cause a transient
decrease in lung function.
Pneumoconiosis was diagnosed in Germany (Letterer et al., 1944)
and again in chromite miners in South Africa (Sluis-Cremer & du
Toit, 1968). However, American medical officers could not confirm
this feature when examining 897 workers in chromate-producing
plants in the USA (US PHS, 1953). It should be noted that chromium
dusts had a poor fibrogenic potency in experimental animal studies
(section 7.2.1.5). Davies (1974) X-rayed workers employed in the
manufacture of ferro-chrome and found results similar to miliary
lung tuberculosis. The workers were in the immediate area of the
furnace and were exposed to dust-particles, 90% of which were less
than 2 µm in diameter. The dust deposited on girders consisted of
95% SiO2 and 2.4% Cr2O3. The author did not find any symptoms or
signs of tuberculosis, but there was sufficient evidence to
indicate an inhalation hazard in the ferro-chrome industry, thus
underlining earlier observations of Princi et al. (1962).
8.3.2.1. Bronchial irritation and sensitization
Chromium irritates mucous membranes, as has been seen with
exposure to airborne chromium trioxide fume (Meyers, 1950) or mixed
dust (Card, 1935; Broch, 1949; Williams, 1969). The first symptom
was an irritating cough, 4 - 8 h after exposure. Chromium causes
sensitization that can result in asthmatic attacks. These can last
for 24 - 36 h, without treatment (Langard & Norseth, 1979). An
attack may recur on later exposure, even when this exposure is to a
much lower concentration (US NAS, 1974a).
A double-blind experimental exposure study on an asthmatic
patient, who had been exposed to chromium and nickel, showed that
chromium was the main cause of the asthma (Novey et al., 1983).
Keskinen et al. (1980) pretreated their patients, stainless steel
welders, with both disodium cromoglycate and betamethasone and
found an inhibition of this reaction. Placebo medication failed to
produce this inhibition.
8.3.3. Effects on the kidney
In the early part of this century, chromates and chromic acid
were occasionally used as therapeutic chemicals for some skin
lesions. Fatal cases of acute nephritis occurred with albumin,
hyaline, and granular casts and red cells appearing in the urine,
accompanied by oliguria. Autopsies revealed tubular necrosis, but
not glomerular damage (Kaufman et al., 1970). Acute toxic
gastroenteritis developed, followed by arterial hypotonia, in
suicide cases a few hours after swallowing 1.5 - 10 g potassium
dichromate. Within 2 - 4 days, all patients developed signs of
acute renal insufficiency with oliguria, anuria, and hyperhydration
and also evidence of acute toxic hepatitis (Luzhnikov et al.,
1976). Kidney damage has also occurred in industry, as a result of
accidental exposure of the skin to large amounts of chromate,
especially when associated with extensive skin damage (Luzhnikov et
al., 1976).
The mortality from kidney diseases among workers in the
chromium industry does not appear to have increased. In one study,
no deaths from nephritis and uraemia were found in 4 chromate-
producing plants; in only 1 plant was the mortality rate above that
for the controls (Machle & Gregorius, 1948). Hayes et al. (1979)
reported that 4 deaths from nephritis and nephrosis had occurred
between 1945 and 1977 in workers in a chromate-producing plant,
compared with 4.95 cases in the control population. Satoh et al.
(1981) found one case of nephritis and nephrosis in a group of
chromate workers compared with an expected number of 2.18.
Some authors (Franchini et al., 1975; Borghetti et al., 1977;
Gylseth et al., 1977; Tola et al., 1977) have concluded that
urinary excretion and renal clearance of diffusible chromium can be
used as biological indicators for evaluating the extent of exposure
to airborne chromium and the body burden. However, a dose-effect
relationship could not be established between duration of exposure
and either the level of chromium excretion (Tandon et al., 1977) or
early nephrotoxic indicators (Mutti et al., 1979). A dose-
response relationship for hexavalent chromium effects on the kidney
was described by Mutti et al. (1979), measuring beta-glucuronidase,
protein, and lysozyme in the urine. Abnormal patterns were not
observed in 39 stainless-steel welders during 4.5 ± 3.2 years of
exposure. In another group of workers employed in welding armoured
steel with special electrodes for 1 ± 0.4 years, 22% showed an
increase in beta-glucoronidase and 10% in protein in the urine. In a
study on 24 workers in the chromium-plating industry who were
exposed for 8.9 ± 7.3 years, the authors found increased urinary
levels of beta-glucoronidase in 37% of the workers, an increase in
protein in 17%, and an elevated level of lysozyme in 4%. Urinary
beta2-microglobulin was also studied by Lindberg & Vesterberg (1983b)
in chromeplaters (24 males, mean age 36 years, working in 4
different factories). The exposure was measured using personal air
samplers. The 8-h mean value ranged between 2 and 20 µg hexavalent
chromium/m3 and averaged 6 µg/m3. Most of the workers showed
symptoms of airway irritation, 2 of them had ulcerated nasal septum
and another 2, complete perforation. Significantly higher urine
beta2-microglobulin levels (0.23 mg/litre; range, 0.04 - 1.24 mg/litre;
SD, 0.27) were found in this group of workers in comparison with a
reference group of workers of a comparable age (mean age, 38 years;
mean urinary beta2-microglobulin level, 0.15 mg/litre; range, 0.06 -
0.19 mg/litre; SD, 0.08). A relationship was observed (Table 26)
between the range of exposure and prevalence of urinary beta2-micro-
globulin levels exceeding 0.3 mg/litre. The 2 workers with
perforated nasal septum were in the highest exposure group. A
difference in urinary beta2-microglobulin levels was not found between
a group of workers employed several years earlier in an old
chromeplating plant (mean, 0.25 mg/litre) and a group of workers of
comparable age (mean, 0.29 mg/litre), but it should be noted that
the mean age of both these groups exceeded 52 years.
Table 26. beta2-microglobulin U-beta2 levels in urine of workers
related to present exposure levelsa
-------------------------------------------------------------------------
Plant Exposure Number Age Persons with an "elevated" U-beta2
No. range of (mean) (U-beta2 > 0.30 mg/litre) range
(µg/m3) workers concentration (mg/litre)
No. Ages
-------------------------------------------------------------------------
1 11 - 20 5 39 3 21, 37, 58 0.23 - 1.30
2 - 3 14 - 8 13 37 2 20, 21 0.04 - 0.44
4 2 - 3 6 6 - - 0.06 - 0.18
-------------------------------------------------------------------------
a From: Lindberg & Vesterberg (1983b).
8.3.4. Effects on the liver
Statistics on diseases of the liver are seldom published
separately; they are usually included in the group of diseases of
the digestive system. The chromate industry was reported to have
the same rate in this group of diseases as many other industries
(US PHS, 1953). The Federal Security Agency in the USA
investigated 14 chromate workers reported to have "enlarged livers"
but found that these cases were not related to the time spent in
the industry. Acute hepatitis with jaundice was described in one
worker employed in a chromium-plating plant.
In an epidemiological study, Satoh et al. (1981) reported a low
rate of cirrhosis of the liver in chromium workers and liver
function test results that did not differ significantly from those
of the controls.
However, following ingestion of high doses of potassium
dichromate, liver necrosis (Brieger, 1920) and congested liver
(Kaufman et al., 1970) with loss of its architecture have been
described. There are also case studies of liver function and
histology in electroplaters (Pascale et al., 1952) and chromate
chemical plant workers (Etmanova, 1965), but the results are
difficult to interpret.
8.3.5. Effects on the gastrointestinal tract
The following symptoms and signs were reported in workers
engaged in the production of chromium salts (Sterekhova et al.,
1978): hyperchlorhydria, elevated pepsin and pepsinogen level,
oedema, hyperaemia and erosion of the mucosa, polyposis,
dyskinesia, and gastritis. However, Satoh et al. (1981) reported
that the incidence of peptic ulcer in chromate workers was below
the expected rate, and another study showed that the mortality rate
for diseases of the digestive system was lower in chromate workers
than in the control population (Hayes et al., 1979).
The gastric juice has been shown to play a role in the
detoxification of ingested hexavalent chromium by reducing it to
trivalent chromium, which is poorly absorbed and eliminated with
the faeces (Donaldson & Barreras, 1966; DeFlora & Boido, 1980).
8.3.6. Effects on the circulatory system
A clinical study was performed by Kleiner et al. (1970) on
myocardial function in more than 200 workers in the potassium
chromate industry, suffering from pulmonary or gastrointestinal
diseases, attributed by the authors to chromium poisoning. A
control group of 70 healthy individuals was included. Pathological
changes in the ECG and in other tests of heart function indicated
disturbance of right heart function, which the authors considered
secondary to the pulmonary pathology. The full paper was not
available and, consequently, the data could not be evaluated in
detail. It should be noted that no unexpected findings in
cardiovascular mortality were cited in the epidemiological studies
described in section 7.3.9.
8.3.7. Teratogenicity
The only human data available concerning this topic were
reported in an abstract (Morton & Elwood, 1974). The authors did
not find any correlation between frequency of malformations in the
central nervous system and the chromium content in water samples
collected in South Wales. Furthermore, Suzuki et al. (1979) did
not find any indication of accumulation of chromium in fetal
tissues.
8.3.8. Mutagenicity and other short-term tests
Few reports exist dealing with chromium-induced mutagenicity in
human subjects, and only hexavalent chromium causes any effects.
Bigaliev et al. (1978) found a 3 - 8% increase in chromosomal
aberrations of peripheral blood leukocytes in workers handling
chromium-compounds, compared with 2% in unexposed controls.
When classified according to the Papanicolaou system,
cytological samples of the sputum of 116 workers in the chromate-
production industry did not show any stages of tumour development
(Maltoni, 1976). However, intermediate stage lesions found in 30
workers were described as atypical adenomatous proliferation,
squamous-cell, and basal-cell dysplasia.
Studying cultured lymphocytes obtained from workers
occupationally exposed to CrO3, Sarto et al. (1982) found an
increased frequency of sister chromatid exchanges compared with
controls, which was correlated with urinary-chromium levels and
enhanced by smoking habits.
8.3.9. Carcinogenicity
8.3.9.1. Lung cancer
(a) Epidemiological studies in chromate-producing plants
The first recognition that cancer of the respiratory tract
might be related to chromium exposures resulted from the reporting
in 1932 of 2 cases of bronchogenic carcinoma that had occurred in
an old German chromate chemical plant (Lehmann, 1932). Gross &
Kölsch (1943) reported 10 deaths from lung cancer in small plants
producing the chromates of lead and zinc. By 1947, 52 such cases
had been reported from this industry and 10 cases from a chrome
pigment plant, also in the Federal Republic of Germany (Baetjer,
1950a).
The first epidemiological study was reported in the USA in
1948. The mortality data were based on the records of the group
life insurance policies of 6 chromate-producing plants in the USA
compared with similar data from a non-chromium industry or from a
life insurance company. The study reported that 21.8% of all
deaths between 1938 and 1947 in the chromate plants were due to
cancer of the respiratory tract compared with 1.4% in the control
group. The crude death rate for cancer of the bronchi and lungs
was 28 times that for the control groups (Machle & Gregorius,
1948). In one of these plants (Mancuso & Hueper, 1951), the cancer
death rate was 15 times that of the general population in the
county where the plant was located. The US PHS (1953) also studied
the respiratory cancer risk in these 6 plants and estimated a
relative risk of 28.9 using the average cancer mortality rates for
US males between 1940 and 1948 as a comparison.
In addition to these studies, where the cause of death was
taken from insurance or death certificate records, a case-control
study, using only cases diagnosed by autopsy or biopsy, was
conducted by Baetjer (1950b). Two hospitals near the largest
chromate plant in the USA were asked to supply the histories of all
cases diagnosed in 1924-46 as cancer of the lung bronchi, and to
confirm histopathologically. A group of non-cancer cases matched
individually by age, sex, and date of admission was selected as
controls. Of the 290 confirmed cancer cases, 3.26% had been
employed in the chromate plant, whereas none of the 900 control
cases was found to have had chromate exposure. The percentage of
chromate workers in the lung cancer series was 28.6 times the
percentage of chromate workers among the employed male population
of the city. The plant involved in this study was rebuilt in 1950,
following the discovery of the cancer problems. A new study of
this plant was made by Hayes et al. (1979) to determine whether the
risk of cancer had been eliminated. The investigation covered 438
deaths among 2101 males, who were initially employed between 1945
and 1974 for more than 90 days, and who died between 1945 and 1977.
The SMRa for malignant neoplasms of the trachea bronchi and lungs
was 202. Cancer deaths in the city in which the plant was located
were used to calculate the expected number of cases. No cases of
respiratory cancer occurred in the plant between 1960 and 1977
among workers employed only in the new plant. Because of the long
latent period, further follow-up is necessary.
Increased lung cancer rates among chromate workers in the USA
have been confirmed by Taylor (1966), Enterline (1974), and Mancuso
(1975), who also showed that the risks were especially high during
the early years of the study of the cohorts, e.g., before 1950.
Studies conducted in other countries have shown similar results.
In a 1956 study on workers in chromium-producing plants in the
United Kingdom, the rate of lung cancer mortality was 3.6 times the
rate for England and Wales (Bidstrup & Case, 1956). In a follow-up
study on 2715 men who had worked at the 3 chromate-producing
factories in the United Kingdom from 1948 to 1977, Alderson et al.
(1981) found 116 deaths from lung cancer (expected, 48). Since
modification of the one plant that is still in operation, the
relative risk of lung cancer has decreased from over 3 to about
1.8.
Two epidemiological studies have been conducted in the Japanese
chromate industry. In one plant, 5 lung cancer deaths occurred
between 1960 and 1973 in 136 workers who had been exposed for more
than 9 years; the SMR was 1510 (Watanabe & Fukuchi, 1975). In
another plant, the mortality rate for respiratory cancer was
determined for 896 males who worked in the plant from 1918 to 1975
and were followed to 1978; the SMR was 920 (expected deaths based
on 1975 age-cause-specific mortality rates for Japanese males)
(Satoh et al., 1981).
In the Federal Republic of Germany, a study was conducted in 2
chromate-producing plants (Korallus et al., 1982). Comparing the
SMR for lung cancer during the period 1948-79 for these 2 groups, a
clearly decreasing tendency was observed. In the first plant, for
1948-52, the SMR was 512 and, for 1978-79, it was 98; for the other
plant, the corresponding figures were 1905 and 150, respectively.
The newest data indicate a decreasing risk approaching the
frequency in unexposed people (Korallus & Loenhoff, 1981). This is
a result of occupational hygienic and technological measures,
especially of a change in manufacture namely the "on line"
--------------------------------------------------------------------
a The SMR (standardized mortality ratio) is the ratio of
observed to expected deaths times 100.
processing of the chrome ore, which has minimized the contents of
the carcinogenic hexavalent chromium compounds within the process
(Korallus et al., 1982).
Bittersohl (1972) described the results of a study on 30 000
employees of a large chemical unit for the period 1921-70 in the
German Democratic Republic. In particular, 588 malignancies in men
and 170 in women were evaluated for the period 1957-70. In 1971,
108 new malignancies in men and 29 in women came to light. In a
chromate factory, the rate of all cancers was far above average.
The factory manufactured catalysts through the reaction of chromic
acid and iron (III) oxide and nitric acid. The airborne
concentrations of chromium were not reported in detail, but it was
stated that short-term exposures above 400 µg/m3 occurred. The
incidence of malignant neoplasms in employees in the chromate
factory was 852 per 10 000 employees. In "unexposed" personnel,
the incidence of malignant neoplasms was 84 per 10 000 employees.
Approximately 86% of all those with malignant neoplasms were
smokers, and 78% of those without malignant neoplasms were also
smokers, indicating that smoking is not likely to be a confounding
factor.
In the USSR, a high incidence of cancer of the lungs was
reported for men engaged in the production of chrome salts. The
ratio of lung cancer in the plant to that in the control population
was 6.4 for ages 50 - 59 (748 observed, 116 expected) and 15.7 for
ages 60 - 69 (2657 observed, 170 expected) (Tyushnyakova et al.,
1974).
A survey was conducted in China in 1982 to investigate the
problem of lung cancer among chromate-producing workers. It
covered 7 cities and included 2184 male and 798 female workers who
had worked for at least one year in the industry. The preliminary
report revealed that 11 of the 101 deaths among male workers were
due to lung cancer, but none of the 13 deaths among female workers
was due to this cause.a
(b) Epidemiological studies - chrome pigment industry
The first epidemiological study, conducted in 1973, covered 3
small chrome pigment-manufacturing plants in the USA. The
percentage of lung cancer deaths was tentatively reported to be
about 3 times that in unexposed workers (Equitable Environmental
Health Inc., 1976).
Lung cancer mortality was studied among 1152 men working at 3
chromate pigment factories in the United Kingom from the 1930s or
1940s until 1981 (Davies, 1979, 1984). Among workers at factory A,
which produced both zinc and lead chromates, entrants to the
factory between 1932-45 with high and medium exposures had excess
---------------------------------------------------------------------------
a Report of an Investigation Team for Cancer in Chromate
Workers (1983).
risks of lung cancer (SMR = 223). A similar excess was seen in
1946-54 entrants, but the excess risk fell just short of
statistical significance. Highly significant excess risks of lung
cancer were seen among 1947-64 entrants that had high or medium
exposures at factory B, which also produced lead and zinc
chromates. Excesses were more severe in workers employed for more
than 10 years. In factory C, which produced only lead chromate, no
excess of lung cancer was found. However, the number of workers
and the power of the study were small.
Twenty-four male workers, who had been employed in a small
Norwegian company for more than 3 years between 1948 and 1972, were
identified and followed up by Langard & Norseth (1975), because
their principal exposure was to zinc chromate dust (measured
routinely with readings occasionally up to 0.5 mg hexavalent
chromium/m3). By December 1980, 6 cases of lung cancer had been
diagnosed, giving a relative risk of 44 compared with that of the
general population in the country. The authors stated that 5 out of
the 6 patients were smokers and only one had been exposed to
chromates other than zinc chromates (Langard & Vigander, 1983).
Sheffet et al. (1982) undertook a detailed mortality study on
workers employed in a pigment plant in Newark, where lead and zinc
chromates were used. Observed deaths from each cause among 1296
white and 650 non-white males employed between 1940 and 1969, were
compared with expected deaths, as computed from cause-, age-, and
time-specific standard death rates for the USA. A statistically-
significant relative risk of 1.6 for lung cancer among white
males, employed for 10 years or more, was found. A relative risk
of 1.9 was noted for individuals employed for at least 2 years, who
were at least moderately exposed to chromates.
An epidemiological study covering several centres in Europe was
conducted by Frentzel-Beyme (1983). This study was designed to
quantify the mortality from cancer and other diseases among workers
in European factories producing chromate pigments (3 German, 2
Dutch factories). Observed deaths in factories were compared with
expected deaths calculated on the basis of mortality figures for
the region in which a given factory was located. The overall
mortality did not deviate from the expected rates. Lung cancer
rates were always in excess of expected numbers, but only in one
cohort was the excess statistically significant. The pattern of
duration of exposure indicates that the lung-cancer risk was not
clearly correlated with length of employment.
A slight excess of lung cancer risk in chrome pigment (zinc
chromate) spray painters from 2 maintenance bases in the USA was
reported by Dalager et al. (1980). Among the 202 deaths
identified, 21 were due to respiratory cancer (11.4 expected;
comparison population: proportionate mortality, US males). No
statistically significant excess of lung cancer was found in a
group of automobile painters in which 226 deaths, including 22
cancer cases, were analysed using proportionate mortality for areas
in which the plants were located as a comparison (Chiazze et al.,
1980).
(c) Epidemiological studies - ferro-chrome industry
Langard et al. (1980) reported that 7 cases of bronchogenic
carcinoma occurred among 976 workers, who started work before
January 1, 1960. The expected number was 3.1 using national rates,
1.8 using local rates, and < 1 when using an international
reference rate for comparison. The difference was not significant
for the national and local rates, but was significant for the
international rate.
A study in Sweden did not reveal any significant excess of
respiratory cancer in ferro-chrome workers (Axelsson et al., 1980).
The incidence of cancer among 1932 workers employed for at least
one year between 1930 and 1975 was compared with the expected
number based on the National Cancer Registry data for the county in
which the plant was located. Five cases of respiratory cancer were
found against an expected 7.2 cases.
An excessive rate of lung cancer was reported for a ferro-
chrome plant in Russia (Pokrovskaya & Shabynina, 1973). The number
of deaths from cancer between 1955 and 1969 was higher than that in
the town in which the plant was located. The authors considered
that both hexavalent and trivalent chromium exposures were
responsible for the excess, since similar excessive rates were
found in a nearby ore-crushing plant, where only trivalent chromium
was present. The number of cases and the rates involved in these
studies were not given.
Hexavalent chromium is produced in some types of welding. In
section 7.2.1.2, it was mentioned that welding fume particles were
positive in the mammalian spot test, indicating a mutagenic and
possible carcinogenic potential. The incidence of lung cancer was
determined by Sjögren (1980), in a cohort of 234 welders, who had
welded stainless steel for more than 5 years between 1950 and 1965,
and were followed to 1977. Three welders died from lung cancer in
comparison with an expected number of 0.68 in the general
population ( P = 0.03).
Comparing lung cancer mortality in 2 parishes with ferro-alloy
industries to mortality in other parishes of similar size without
such industries from the same county, Axelson & Rylander (1980)
were unable to detect an increased incidence of death due to lung
cancer mortality. The concentration of chromium in the ambient air
of the most polluted areas ranged from 0.1 to 0.4 µg/m3. It should
be noted that several possible confounding factors were not under
control, e.g., migration, occupational exposure, and smoking
habits.
(d) Epidemiological studies - chromium-plating workers
Epidemiological studies on workers in the chromium-plating and
related industries were reviewed by Hayes (1982). The relevant
data from these studies are presented in Table 27. According to
Hayes, the epidemiological evidence is not sufficient to determine
the risk of cancer associated with industrial exposure to chromic
acid and, if an excess risk exists, it is probably lower than that
typically described for employment in the chromium-chemical
producing industry.
In a more recent paper, Franchini et al. (1983) conducted a
retrospective cohort study in 9 chromium-plating plants to examine
the mortality of workers employed for at least one year during the
period January 1951 to December 1981. The study group totalled 178
individuals; vital status ascertainment was 97% complete. The
total number of deaths was close to the expected figure (15
observed, 15.2 expected), whereas death from lung cancer exceeded
the expected number (3 observed, 0.7 expected; P = 0.03). The
increased mortality from lung cancer among chromium-platers seemed
to be related to exposure intensity.
8.3.9.2. Cancer in organs other than lungs
A few cancers of the upper respiratory tract and oral region,
but no excessive rates have been reported to occur in the chromate-
producing industry. The cancers have involved the buccal cavity,
pharynx, and oesophagus. Cancers of the nasal septum have not been
documented, with the exception of one case in Italy (Vigliani &
Zurlo, 1955).
In a joint Danish-Finnish-Swedish case-reference investigation,
initiated in 1977, the connection between nasal and sinonasal
cancer and various occupational exposures was studied. All new
cases of nasal and sinonasal cancer were collected from the
national cancer registers (Finland and Sweden) or from the hospitals
(Denmark). The results showed associations between nasal and
sinonasal cancer and exposure to chromium, welding, flame-cutting,
and soldering, hardwood or mixed wood dust, and softwood dust alone
(13.4) (Hernberg et al., 1983).
In 5 plants in the USA, during the early years of extremely
high exposures, the rate of cancer of the digestive tract varied
from 0 to 3.04/1000 compared with a rate of 0.59/1000 for the
controls (Machle & Gregorius, 1948). The mortality rates from
stomach cancer have been reported for chromate-producing plants in
Japan (SMR = 90) (Satoh et al., 1981) the USA (SMR = 40) (Hayes et
al., 1979). No significant differences were reported between the
incidence of stomach cancer in a Russian plant (Pokrovskaya &
Shabynina, 1973) and the incidence in the surrounding area.
Studying cancer mortality in a pigment plant using lead and
zinc chromates, Sheffet et al. (1982) found an increased (but not
statistically risk of stomach (SMR = 170) and pancreas (SMR = 200)
cancer among the total cohort (1946 employees).
Table 27. Summary of epidemiological studies on respiratory cancer in
workers employed in the chromium plating and related industriesa
------------------------------------------------------------------------------------------------
Study population Follow-up Respiratory cancer Comparison population
Number Estimated
relative risk
------------------------------------------------------------------------------------------------
Chromium plating
1056 workers employed Deaths in former and 24 1.8b Unexposed workers in
> 3 months in 54 plants current (followed 2 plants and in 2 non-
in the United Kingdom years) workers, plating industries
about 80% traced
About 5000 workers 49 1.4c Mortality analysis,
employed since 1945 in method not specified
1 plating factory in
the United Kingdom
889 workers exposed > 6 Reports from management 0 < 1b Tokyo mortality
months in Tokyo chromium of plating firms and rates
plating plants, 1970-76 follow-up of retired
workers; 19 total deaths
reported
Related industries
1292 deaths among US Union death benefit 62 1.1b Proportionate
metal polishers and claims mortality, US males
platers, union members,
1951-69
238 deaths among employees Pension, insurance and 39 2.1c Proportionate
for 10 years in one US benefit records mortality, US
plant for metal die
casting, finishing, and
electroplating, 1974-78.
------------------------------------------------------------------------------------------------
a Adapted from: Hayes (1982).
b Not statistically significant.
c P < 0.05, calculated using an assumption that the observed number is distributed as a
Poisson random variable.
8.3.9.3. Relationship between cancer risk and type of chromium
compound
As the exposures are often poorly defined, it is sometimes not
certain whether workers have been exposed to hexavalent chromium,
trivalent chromium, or a combination of the two. Some authors have
concluded that there is no increased risk of cancer mortality due
to trivalent chromium compounds (Korallus et al., 1974 a,b,c;
Axelsson et al., 1980; Langard et al., 1980; Norseth, 1980),
whereas others do not exclude that trivalent chromium compounds
have a carcinogenic capacity (Essing et al., 1971; Mancuso, 1975;
Zober 1979).
Summing up the data from case reports as well as
epidemiological studies in chromate-producing, chromate-pigment,
chromium-plating, and ferrochromium industries, the IARC Working
Group on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans (IARC, 1980) came to the conclusion that "there is
sufficient evidence of respiratory carcinogenicity in men
occupationally exposed during chromate production. Data on lung
cancer risk in other chromium-associated occupations and for cancer
at other sites are insufficient. The epidemiological data do not
allow an evaluation of the relative contributions to carcinogenic
risk of metallic chromium, trivalent chromium and hexavalent
chromium or of soluble versus insoluble chromium compounds".
9. EVALUATION OF HEALTH RISKS FOR MAN
Low levels of chromium are omnipresent in the environment.
Under normal conditions, human exposure to chromium does not
represent a toxicological risk, but it should be pointed out that
too low an intake of chromium may lead to deficiency. Airborne
concentrations of chromium, predominantly as trivalent chromium,
are usually below 0.1 µg/m3 and there are many places where the
concentration is below the detection limit. Concentrations in
river water are typically in the range of 1 - 10 µg/litre and do
not constitute a health threat. Drinking-water from municipal
water supplies does not contribute more than a few micrograms of
chromium to the daily human intake, but untreated water in certain
areas may be contaminated by runoff or effluents from industrial
sources and may contribute significant amounts of chromium. The
oceans contain less than 1 µg/litre. The daily human intake
through food varies considerably between regions. Typical values
range from 50 to 200 µg/day. They do not represent a toxicity
problem.
However, significant exposures exist in the occupational field.
In the past, chromium-ore mining generated chromium-containing
dusts, levels of which ranged up to 20 mg/m3 in different work-
places. Other exposures ranged up to 150 mg/m3 and consisted of
dust containing as much as 48% chromium (Cr2O3). In production
plants, hexavalent chromium can occur in the airborne state.
However, implementation of protective measures at ferrochromium
production sites reduced the airborne hexavalent chromium to levels
of 30 - 60 µg/m3.
The health effects of the two common oxidation states of
chromium are so fundamentally different that they must be
considered separately. In the form of trivalent compounds,
chromium is an essential nutrient and is relatively non-toxic for
man and other mammalian species. The hexavalent form is man-made
by oxidation of naturally-occurring trivalent chromium minerals and
is widely used. Compounds of hexavalent chromium penetrate
biological membranes easily and can thus interact with essential
constituents of the cells, including genetic material which they
can damage through oxidation and complexation with resulting
trivalent species. On the other hand, oxidation of trivalent
chromium has not been demonstrated in the living organism, and for
practical purposes, the reduction of hexavalent chromium to
trivalent chromium in lungs and other animal tissues is
irreversible.
9.1. Occupational Exposure
A number of effects can result from occupational exposure to
airborne chromium including irritative lesions of the skin and
upper respiratory tract, allergic reactions, and cancers of the
respiratory tract. The data on other effects, e.g., in the
gastrointestinal, cardiovascular, and urogenital systems are
insufficient for evaluation.
Epidemiological studies have shown that workers engaged in the
production of chromate salts and chromate pigments experience an
increased risk of developing bronchial carcinoma. No detailed data
on dose-response relationships are available from epidemiological
studies. Although a suspicion of increased lung cancer risks in
chromium plating workers has been raised, the available data are
inconclusive as are data for other industrial processes where
exposure to chromium occurs. There is insufficient evidence on the
role of chromium as a cause of cancer in any organ other than the
lung. Evidence from studies on laboratory animals shows that
hexavalent chromium compounds, especially those of low solubility,
can induce lung cancer.
In the lymphocytes of workers in chromium-plating factories,
the frequency of sister chromatid exchanges was higher in exposed
than in control groups.
Mutagenicity and related studies have convincingly shown that
hexavalent chromium is genetically active. Hexavalent chromium can
cross cellular membranes and is then reduced to trivalent chromium,
which can cause DNA cross-links and increase the infertility of DNA
implication. Trivalent chromium compounds have been shown to be
genetically inactive in most test systems, except in systems where
they can directly interact with DNA.
9.1.1. Effects other than cancer
9.1.1.1. Respiratory tract
It has been reported that the threshold for acute irritative
effects in the upper respiratory tract is 25 µg/m3 for the most
sensitive individuals. Long-term exposure to doses over 1 µg
chromic acid/m3 can cause nasal irritation, atrophy of the nasal
mucosa, and ulceration of perforation of the nasal septum.
Bronchial asthma was previously attributed to exposure to
chromium compounds, but scientific data are too scarce to draw
conclusions.
9.1.1.2. Skin
Skin rashes, ulcers, sores, and eczema have been reported among
occupationally exposed workers. Both trivalent and hexavalent
chromium compounds can give rise to sensitization of skin,
especially under certain environmental conditions, such as those
encountered in the cement industry, where the high incidence of
chromium-induced skin lesions can be attributed to the alkaline
exposure conditions.
Eczematous dermatitis, which manifests first as a diffuse
erythematous type, progresses in severe cases to an exudative
stage and is associated in 8 - 15% of patients with sensitivity to
chromium, as revealed by skin-patch tests. Although opinion is
divided on the oxidation state of chromium responsible for inducing
sensitization, there is evidence of hexavalent chromium penetrating
the skin as a hapten to be reduced to the trivalent state and
conjugated with proteins and transformed into the full antigen
that initiates the sensitization reaction, involving presumably
only a cell-mediated immune response. It should be noted that
patients suffering from chromium-induced skin allergy tend to
become hypersensitive to cobalt and nickel. Furthermore, 48% of
cases of skin allergy induced by chromium, with or without
clinically observed light sensitivity, showed a significantly more
intense reaction by a standardized photopatch test procedure, when
they were exposed to a 4/5 minimal erythema dose of irradiation.
9.1.1.3. Kidney
After high-dose, short-term oral ingestion of chromium, acute
nephritis and tubular necrosis were observed. A few
epidemiological studies on workers in chrome-plating industries
include data on diseases of the kidney, most of them without giving
exact exposure levels. A recent study related increased urinary-beta2
microglobulin levels to exposure ranges between 2 and 20 µg/m3.
The dose-response relationship observed in this study needs
confirmation on a larger number of exposed workers.
9.1.1.4 Other organs and systems
There are no conclusive data to evaluate the effects of
chromium compounds on the liver or gastrointestinal and circulatory
systems.
9.1.2. Teratogenicity
Both oxidation states, when injected at high levels
parenterally into animals, are teratogenic, with the hexavalent
form accumulating in the embryos to much higher concentrations than
the trivalent. The Task Group was not aware of any report
indicating teratogenicity in human populations.
9.2. General Population
Persons living in the vicinity of ferro-alloy plants, exposed
to an ambient air concentration of up to 1 µg/m3, did not show
increased lung cancer mortality.
The results of many studies suggest that exposure to chromium
through inhalation and skin contact can pose health problems for
the general population. Very little information is available on
the health effects of chromium ingested through untreated drinking-
water, though, in a single study, a correlation was observed
between frequency of malformation in the central nervous system and
the chromium content of water samples (Morton & Elwood, 1974). In
order to assess the nature of the magnitude of these problems,
there appears to be a need for more general population studies on
the effects of inhaled, absorbed, or ingested chromium on
respiratory, cardiovascular, and renal functions, and on the skin.
Studies on animals and man have established trivalent chromium
as an essential micronutrient that interacts with insulin and
enhances the physiological effects of the hormone. Supplementation
trials in a number of countries including Finland, Jordan, Nigeria,
Sweden, Turkey, and the USA have shown that segments of the
population could improve their glucose metabolism and, in some
instances, fat metabolism, by ingesting trivalent chromium
compounds. These observations suggest that some populations are at
risk of chromium deficiency. Children suffering from protein-
energy malnutrition may be at special risk.
As chromium concentrations in the body fluids of unexposed
persons are generally less than 1 µg/litre, analysis for chromium
requires the strictest quality control, including measures to
exclude sample contamination. Even with adequate analytical
methods, it is not possible, as yet, to diagnose chromium
deficiency in individuals by chemical or biochemical methods alone.
Therefore, a quantitative assessment of the prevalence of chromium
deficiency in human populations is not yet possible.
REFERENCES
ABRAHAM, A.S., SONNENBLICK, M., EINI, M., SHEMESH, O., & BATT,
A.P. (1980) The effect of chromium on established athero-
sclerotic plaques in rabbits. Am. J. clin. Nutr., 33:
2294-2298.
AITIO, A., JARVISALO, J., KULUNEN, M., TOSSAVAINEN, A., &
VAITTINEN, P. (1984) Urinary excretion of chromium as an
indicator of exposure to trivalent chromium sulfate in leather
tanning. Int. Arch. occup. environ. Health, 54: 241-249.
ALDERSON, M.R., RATTAN, N.S., & BIDSTRUP, L. (1981) Health
of workmen in the chromate-producing industry in Britain. Br.
J. ind. Med., 38: 117-124.
ALEKSANDROV, A.I. & KAINOV, V.I. (1975) [Crocoite from the
Pervomaisk-Zverevo deposit at the Central Urals.] Tr. Sverdl.
Gorn. Inst., 106: 152-155 (in Russian).
AMA (1979) Guidelines for essential trace element
preparations for parenteral use. J. Am. Med. Assoc., 241:
2051-2054.
AMERICAN PUBLIC HEALTH ASSOCIATION, AMERICAN WATER WORKS
ASSOCIATION, AND WATER POLLUTION CONTROL FEDERATION (1971)
117A. Hexavalent chromium. In: Standard methods for the
examination of water and wastewater, 13th ed., Washington DC,
American Public Health Association, pp. 156-157.
AMES, B.N., DURSTON, E., YAMASAKI, E., & LEE, F.D. (1973)
Carcinogens as mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci. (USA), 70: 2281-2285.
ANDERSON, F.E. (1960) Cement and oil dermatitis. The part
played by chrome sensitivity. Br. J. Derm., 72: 108-117.
ANDERSON, N. (1982) The environmental impact of chromium.
Final draft March 1982, Maine, Department of Environmental
Protection, Bureau of Air Quality Control, 126 pp.
ANDERSON, R.A. & POLANSKY, M.M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Tr. El. Res., 3: 1-5.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., & REAMER, D.C. (1982a) Effect of exercise
(running) on serum glucose, insulin, glucagon, and chromium
excretion. Diabetes, 31: 212-216.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., VEILLON, C., & GLINSMANN, W. (1982b)
Urinary chromium excretion of human subjects: effects of
chromium supplementation and glucose loading. Am. J. clin.
Nutr., 36: 1184-1193.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., PATTERSON, K.Y.,
VEILLON, C., & GLINSMANN, W. (1983) Effects of chromium
supplementation on urinary Cr excretion of human subjects and
correlation of Cr excretion with selected clinical parameters.
J. Nutr., 113: 276-281.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
MERTZ, W., & GLINSMANN, W. (1983b) Chromium supplementation
of human subjects: effects on glucose, insulin, and lipid
variables. Metabolism, 32: 894-899.
ANDERSSON, A. & NILSSON, K.O. (1972) Enrichment of trace
elements from sewage sludge fertilizer in soils and plants.
Ambio, 1: 176-179.
ANGERER, J., SCHALLER, K.-H., & HEINRICH, R. (1981)
[Commercially-available blood and urine samples for quality
control in toxicology.] Arbeitsmed. Sozialmed. Praeventivmed.,
16: 125-127 (in German).
ANIMAL RESEARCH DIVISION OF THE NEW ZEALAND DEPARTMENT OF
AGRICULTURE (1954) Annual report 1953-54, Wellington, R.E.
Owen Government Printer, 40 pp.
ANKE, M., DIETTRICH, M., WICKE, G., PFLUG, D., & SCHUELER, D.
(1971) Absorption, excretion, and distribution of 51chromium
given by mouth to lactating ruminants. Arch. Tierern., 21:
599-607.
AXELSSON, G. & RYLANDER, R. (1980) Environmental chromium
dust and lung cancer mortality. Environ. Res., 23: 469-476.
AXELSSON, G., RYLANDER, R., & SCHMIDT, A. (1980) Mortality
and incidence of tumours among ferrochromium workers. Br. J.
ind. Med., 37: 121-127.
BAER, H. (1920) [Dermatitis resulting from the use of a
beard trainer made partly of artificial leather.] Münch. med.
Wschr., 67: 874 (in German).
BAETJER, A.M. (1950a) Pulmonary carcinoma in chromate
workers. I. A review of the literature and report of cases.
Arch. ind. Hyg. occup. Med., 2: 487-504.
BAETJER, A.M. (1950b) Pulmonary carcinoma in chromate
workers. II. Incidence on basis of hospital records. Arch.
ind. Hyg. occup. Med., 2: 505-516.
BAETJER, A.M., LOWNEY, J.F., STEFFEE, H., & BUDACZ, V.
(1959a) Effect of chromium on incidence of lung tumours in
mice and rats. Am. Med. Assoc. Arch. Ind. Health, 20: 124-135.
BAETJER, A.M., DAMRON, C., & BUDACZ, V. (1959b) The
distribution and retention of chromium in man and animals. Am.
Med. Assoc. Arch. Ind. Health, 20: 136-150.
BAKER, R.S.V. (1984) Evaluation of metals in in vitro
assays: interpretation of data and possible mechanisms of
action. Toxicol. environ. Chem., 7: 191-212.
BEHNE, D. & BRATTER, P. (1979) [Problems of trace element
analysis in medicine.] In: Gladtke, E., Heimann, G., & Eckert,
I., ed. [Trace elements], Stuttgart, Thieme, pp. 42-52 (in
German).
BEHNE, D. & DIEHL, F. (1972) Relations between carbohydrate
and trace element metabolism investigated by neutron
activation analysis. In: Nuclear Activation Techniques in the
Life Sciences. Proceedings of a Symposium, Bled, Yugoslavia,
Vienna, International Atomic Energy Agency, pp. 407-414.
BEHNE, D., BRATTER, P., GESSNER, H., HUBE, G., MERTZ, W., &
ROSICK, U. (1976) Problems in determination of chromium in
biological materials: comparison of flameless atomic
absorption spectrometry and neutron activation analysis. Z.
anal. Chem., 278: 269-272.
BEHRBOHM, P. (1957) [Allergic contact dermatitis caused by
anticorrosives containing chromium.] Berufsdermatosen, 5:
271-283 (in German).
BENJANUVATRA, N.K. & BENNION, M. (1975) Hair chromium
concentration of Thai subjects with and without diabetes
mellitus. Nutr. Rep. Int., 12: 325-330.
BERGMANN, H. & HARDT, K. (1979) Analysis of dissolved Cr(VI)
in water by APDC-MIBK extraction and atomic absorption
spectrometry. Z. anal. Chem., 297: 381-383.
BERING, F. & ZITZKE, E. (1935) [Occupational skin diseases,]
Leipzig, Voss (in German).
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric., 23: 93-100.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BERTRAND, D. & DEWOLF, A. (1968) Requirement for the trace
element chromium in the growth of potatoes. C R Ser. D., 266:
1494-1495.
BEYERMANN, K. (1962) The analytical behaviour of very small
amounts of chromium. Part I. Z. anal. Chem., 190: 4-33. Part
II. Z. anal. Chem., 191: 345-369.
BEYERSMANN, D., KOSTER, A., BUTTNER, B., & FLESSEL, P.
(1984) Model reactions of chromium compounds with mammalian
and bacterial cells. Toxicol. environ. Chem., 8: 279-286.
BIANCHI, V., DAL TOSO, R., DEBETTO, P., LEVIS, A.G., LUCIANI,
S., MAJONE, F., & TAMINO, G. (1980) Mechanisms of chromium
toxicity in mamalian cell cultures. Toxicology, 17: 219-224.
BIANCHI, V., LEVIS, A.G., & SAGGIORO, D. (1979) Differential
cytotoxic activity of potassium dichromate on nucleoside
uptake in BHK fibroblasts. Chem.-biol.Interact., 24: 137-151.
BIANCHI, V., CELOTTI, L., LANFRANCHI, G., MAJONE, F., MARIN,
G., MONTALDI, G., SPONZA, G., TAMINO, G., VENIER, P.,
ZANTEDESCHI, A., & LEVIS, A.G. (1983) Genetic effects of
chromium compounds. Mutat. Res., 117: 279-300.
BIANCHI, V., ZANTEDESCHI, A., MONTALDI, A., & MAJONE, F.
(1984) Trivalent chromium is neither cytotoxic nor mutagenic
in permeabilized hamster fibroblasts. Toxicol. Lett., 23:
51-59.
BIDSTRUP, P.L. & CASE, R.A.M. (1956) Carcinoma of the lung
in workmen in the bichromates-producing industry in Great
Britain. Br. J. ind. Med., 13: 260-264.
BIGALIEV, A.B., ELEMESOVA, M.S., TUREBAEV, M.N., & BIGALIEVA,
R.K. (1978) [Cytogenetic study of the mutagenic activity of
industrial substances.] Zdravookhr. Kaz., 8: 48-50 (in
Russian).
BITTERSOHL, G. (1972) [Health and the working environment-
Epidemiology of carcinomatosis in the chemical industry.] In:
Proceedings of the 5th Merseburger Symposium, 17-18 November
(in German).
BLACK, C.A. (1968) Soil-plant relationships, New York, John
Wiley and Sons, 792 pp.
BLOKIN, V.S. & TROP, F.S. (1977) [Dynamics of the morpho-
logical changes in animals subjected to inhalational dust
treatment using chromium oxide and trisubstituted chromium
phosphate.] In: Dubinin, N.P., ed. [Genetic effects of the
pollution of the environment,] Moscow, Nauka, pp. 173-176 (in
Russian).
BLOOMFIELD, J.J. & BLUM, W. (1928) Health hazards in
chromium plating. Public Health Rep., 43: 2330-2347.
BONATTI, S., MEINI, M., & ABBONDANDOLO, A. (1976) Genetic
effects of potassium dichromate. Mutat. Res., 38: 147-150.
BOND, R.G., STRAUB, C.P., & PROBER, R., ed. (1973) Handbook
of environmental control. III. Water supply and treatment,
Cleveland, Ohio, CRC Press, pp. 763-764.
BONNEVIE, P. (1939) [Etiology and pathogenesis of eczemas,]
Copenhagen, Busck, Leipzig, Barth (in German).
BORGHETTI, A., MUTTI, A., CAVATORTA, A., FALZOI, M., CIGALA,
F., & FRANCHINI, I. (1977) Indices rénaux d'exposition aiguë
et d'imprégnation chronique par le chrome. Med. Lav., 68(5):
355-363.
BORY, R. & LECOCQ, I. (1953) Eczéma par cuivre chromé. Arch.
Mal. prof., 14: 144.
BOSTROM, G. (1936) [Hypersensitivity test for chromium
allergy.] Am. Derm. Vener., 17: 631-632 (in German).
BOVET, P., LOB, M., & GRANDJEAN, M. (1977) Spirometric
alterations in workers in the chromium electroplating
industry. Int. Arch. occup. environ. Health, 40: 25-32.
BOWEN, H.J.M. (1966) Trace elements in biochemistry, New
York, London, Academic Press, 241 pp.
BRARD, D. (1935) Toxicologie du chrome, Paris, Herrmann &
Co., 80 pp.
BRIEGER, H. (1920) [Clinical aspects of acute chromate
poisoning.] Z. exp. Path. Ther., 21: 393-408 (in German).
BROCH, C. (1949) [Bronchial asthma caused by chromic oxide.]
Nord. Med., 41: 996-997 (in Norwegian).
BROWN, E., SKOUGSTAD, M.W., & FISHMAN, M.J. (1970) Methods
for collection and analysis of water samples for dissolved
minerals and gases. In: Techniques of water resources
investigation of the United States geological survey,
Washington DC, US Department Interior, 160 pp.
BROWN, H.G., HENSLEY, C.P., MCKINNEY, G.L., & ROBINSON, J.L.
(1973) Efficiency of heavy metals removal in municipal sewage
treatment plants. Environ. Lett., 5: 103-114.
BRUCKDORFER, K.R., KHAN, I.H., & YUDKIN, J. (1971) Chromium
and sucrose-induced hyperglyceridemia. Proc. Nutr. Soc., 30:
74A.
BRUNE, D., NORDBERG, G., & WESTER, P.O. (1980) Distribution
of 23 elements in the kidney, liver, and lungs of workers from
a smeltery and refinery in north Sweden exposed to a number of
elements and of a control group. Sci. total Environ., 16:
13-35.
BURCKHARD, W. (1962) [Occupational skin diseases.] In:
Jadassohn, W., ed. [Handbook of skin and venereal diseases,]
Berlin, Springer, Vol. II(1), pp. 368-474.
BURROWS, D., ed. (1983) Chromium: metabolism and toxicity,
Boca Raton, Florida, CRC Press, 172 pp.
BYERRUM, R.U. (1961) Some studies on the chronic toxicity of
cadmium and hexavalent chromium in drinking-water. Eng. Bull.
Purdue Univ. Serv., 106: 1-8.
CALNAN, C.D. & HARMAN, R.R.M. (1961) Studies in contact
dermatitis. XIII. Diesel coolant chromate dermatitis. Trans.
St. John's Hosp. Derm. Soc., 46: 13.
CALOW, P. & FLETCHER, C.R. (1972) A new radiotracer
technique involving 14C and 51Cr for estimating the
assimilation efficiencies of aquatic, primary consumers.
Oecologia (Berlin), 9: 155-170.
CAMPBELL, W.J. & MERTZ, W. (1963) Interaction of insulin and
chromium (III) on mitochondrial swelling. Am. J. Physiol.,
204: 1028-1030.
CANADA, NATIONAL HEALTH AND WELFARE (1980) Chromium
drinking-water criteria review. In: Guidelines for Canadian
drinking-water quality, 1978. Supporting documentation,
Ottawa, Canada, National Health and Welfare, pp. 255-268.
CANFIELD, W.K. & DOISY, R.J. (1976) Chromium and diabetes in
the aged. In: Hsu, J.M., Davis, R.L., & Neithammer, R.W., ed.
The biomedical role of trace elements in aging, St. Peters-
burg, Florida, Eckert College, pp. 119-128.
CARD, W.I. (1935) A case of asthma sensitivity to chromates.
Lancet, 2: 1348-1349.
CARTER, J.P., KATTAB, A., ABD-EL-HADI, K., DAVIS, J., EL
GHOLMY, A., & PATWARDHAN, V.N. (1968) Chromium (III) in
hypoglycemia and in impaired glucose utilization in
kwashiorkor. Am. J. clin. Nutr., 21: 195-202.
CARY, F.E., ALLWAY, W.H., & OLSON, O.E. (1977) Control of
chromium concentrations in food plants. I. Absorption and
translocations of chromium by plants. J. agric. food Chem.,
25: 300-304. II. Chemistry of chromium in soils and its
availability to plants. J. agric. food Chem., 25: 305-309.
CASEY, C.E. & HAMBIDGE, K.M. (1984) Chromium in human milk
from American mothers. Br. J. Nutr., 52: 73-77.
CASTO, B.C., PIECZYNSKI, W.J., NELSON, R.L., & DI PAOLO, J.
(1976) In vitro transformation and enhancement of viral
transformation with metals. Proc. Am. Assoc. Cancer Res., 17:
12-20.
CASTO, B.C., MEYERS, J., & DIPAOLO, J.A. (1979) Enhancement
of viral transformation for evaluation of the carcinogenic or
mutagenic potential of inorganic metal salts. Cancer Res., 39:
193-198.
CAWSE, P.A. (1974) A survey of atmospheric trace elements in
the UK, 1972-1973, Harwell, England, Atomic Energy Research
Establishment, 95 pp.
CHANEY, R.L. (1973) Crop and food chain effects of toxic
elements in sludges and effluents. In: Recycling Municipal
Sludges and Effluents on Land. Proceedings of a Joint
Committee, National Association of State Universities and Land
Grant Colleges, Washington DC, July 9-13, 1973, pp. 129-141.
CHEN, N.S.G., TSAI, A., & DYER, I.A. (1973) Effect of
chelating agents on chromium absorption in rats. J. Nutr.,
103: 1182-1186.
CHEN, K.Y., YOUNG, C.S., JAN, T.K., & ROHATGI, N. (1974)
Trace metals in waste water effluents. J. Water Pollut.
Control Fed., 46: 2663-2675.
CHEREMISINOFF, P.N. & HABIB, Y.H. (1972) Cadmium, chromium,
lead, mercury: a plenary account for water pollution. Part I.
Occurrence, toxicity, and detection. Water Sewage Works, 119:
73-86.
CHEREVATY, V.S., EREMEEVA, E.A., & SMAGULOV, S.K. (1965) [On
the nature of nasal microflora in the workers of a chromium
compound plant.] In: Proceedings of 4th Scientific Session of
Aktubinsk State Medical Institute, Alma-Ata, Kazakhstan,
pp. 337-342 (in Russian).
CHIAZZE, L., FERENCE, L.D., & WOLF, P.H. (1980) Mortality
among automobile assembly workers. I. Spray painters. J.
occup. Med., 22: 520-526.
CHRISTIAN, G.D., KNOBLOCK, E.G., PURDY, W.C., & MERTZ, W.
(1963) A polarographic study of chromium-insulin-
mitochondrial interaction. Biochem. Biophys. Acta, 66: 420-423.
CLARK, R. & KUNITSCH, G. (1972) [Statistical studies of
cross allergy in metal salts.] Berufsdermatosen, 20(5):
222-238 (in German).
COLLINS, R.J., FROMM, P.O., & COLLINGS, W.D. (1961) Chromium
excretion in the dog. Am. J. Physiol., 201(5): 795-798.
CONDE-SALAZAR, L., MARTO-CASTANO, A., & ARROYO VICENTE, M.
(1980) Determination of chrome in rubber gloves. Contact
Dermatit., 6: 237-238.
COTTON, F.A. & WILKINSON, G. (1966) Advanced inorganic
chemistry, New York, Interscience, 1136 pp.
CRESSER, M.S. & HARGITT, R. (1976) The determination of
chromium (III) and chromium (VI) by total anion exchange and
atomic absorption spectrometry. Anal. Chim. Acta, 81: 196-198.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
DALAGER, N.A., MASON, T.J., FRAUMENI, J.F., HOOVER, R., &
PAYNE, W.W. (1980) Cancer mortality among workers exposed to
zinc chromate paints. J. occup. Med., 22: 25-29.
DANA, P. (1971) Crocoite-PbCrO4 In: Hurlbut, C.S., ed.
Dana's manual of mineralogy, 18th ed., New York, J. Wiley and
Sons Inc., pp. 346-347.
DANIELSSON, B.R.G., HASSOUN, E., & DENCKER, L. (1982)
Embryotoxicity of chromium: distribution in pregnant mice and
effects on embryonic cells in vitro. Arch. Toxicol., 51:
233-245.
DAVIDSON, I.W.F. & BLACKWELL, W.L. (1968) Changes in
carbohydrate metabolism of squirrel monkeys with chromium
dietary supplementation. Proc. Soc. Exp. Biol. Med., 127:
66-70.
DAVIDSON, I.W.F. & BURT, R.L. (1973) Physiological changes
in plasma chromium of normal and pregnant women: effect of
glucose load. Am. J. Obstet. Gynecol., 116: 601-608.
DAVIES, J.C.A. (1974) Inhalation hazards in the manufacture
of silicon alloys. Cent. Afr. J. Med., 20: 140-143.
DAVIES, J.M.G. (1972) The fibrogenic effects of mineral
dusts injected into the pleural cavity of mice. Br. J. exp.
Pathol., 53: 190-201.
DAVIES, J.M.G. (1979) Lung cancer mortality of workers in
chromate pigment manufacture: an epidemiological survey. J.
Oil. Col. Chem. Assoc., 62: 157-163.
DAVIES. J.M.G. (1984) Lung cancer mortality among workers
making lead chromate and zinc chromate pigments at three
English factories. Br. J. Ind. Med., 41: 158-169.
DAVIS, M.H. & GROSSMANN, V.B. (1971) A sensitive method for
determination of chromium in biological materials. Anal.
Biochem., 44: 339-344.
DEFLORA, S. (1978) Metabolic deactivation of mutagens in
the Salmonella microsome test. Nature (Lond.), 271: 455-456.
DEFLORA, S. (1981) Study of 106 organic and inorganic
compounds in the Salmonella microsome test. Carcinogenesis, 2:
283-298.
DEFLORA, S. & BOIDO, V. (1980) Effect of human gastric
juice on the mutagenicity of chemicals. Mutat. Res., 77:
307-315.
DEFLORA, S., COPPOLA, R., CAMOIRANO, A., BATTAGLIA, M.A., &
BENNICELLI, C. (1980) Mutagenicity of chromyl chloride and
its vapours. Carcinogenesis, 1(7): 583-587.
DEFLORA, S., BIANCHI, V., & LEVIS, A.G. (1984) Distinctive
mechanisms for interaction of hexavalent and trivalent
chromium with DNA? Toxicol. environ. Chem., 8: 287-294.
DE GROOT, A.J. & ALLERSMA, E. (1973) Field observations on
the transport of heavy metals in sediments. In: Proceedings of
the Conference on Heavy Metals in the Aquatic Environment,
Nashville, Tennessee, December, Oxford, Pergamon Press,
pp. 1-16.
DEICHMANN, W.B. & GERARDE, H.W. (1969) Toxicology of drugs
and chemicals, 4th ed., New York, London, Academic Press,
167 pp.
DI PAOLO, J.A. & CASTO, B.C. (1979) Quantitative studies of
in vitro morphological transformation of Syrian hamster cells
by inorganic metal salts. Cancer Res., 32: 1008-1013.
DJAHANSHIRI, H. (1976) [Nutritional physiology problems
concerning the trace element chromiun in the rat. V. Effects
on overall metabolic performande.] Z. Tierphysiol. Tierernähr.
Futtermittelkd., 37: 298-315 (in German).
DOBROLYUBSKII, O.K. (1959) [Changes in the biochemical
process in grapes under the influence of a microfertilizer
which contains chromium.] Biochemistry (USSR), 24: 577-582 (in
Russian).
DOISY, R.J., STREETEN, D.H.P., LEVINE, R.A., & CHODOS, R.B.
(1968) Effects and metabolism of chromium in normals, elderly
subjects, and diabetics. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
Univerisity of Missouri, Vol. 2, 75 pp.
DOISY, R.J., STREETEN D.H.P., SOUMA, M.L., KALAFER, M.E.,
REKANT, S.L., & DALAKOS, T.G. (1971) Metabolism of
51chromium in human subjects. In: Mertz, W. & Cornatzer, W.E.,
ed. Newer trace elements in nutrition, New York, Marcel
Dekker, pp. 155-169.
DOISY, R.J., STREETEN, D.H.P., FREIBERG, J.M., & SCHNEIDER,
A.J. (1976) Chromium metabolism in man and biochemical
effects. In: Prasad, A.S., ed. Trace elements in human health
and disease, New York, London, Academic Press, Vol. 2, pp.
79-104.
DOMASEVA, T.V. (1971) [On the effect produced by chromium
compounds on the skin.] In: [Occupational skin diseases,]
Leningrad, Leningrad Institute of Sanitation and Hygiene,
Vol. 93, pp. 168-173 (in Russian).
DONALDSON, R.M., Jr & BARRERAS, R.F. (1966) Intestinal
absorption of trace quantities of chromium. J. lab. clin.
Med., 68: 484-493.
DOUGLAS, G.R., BELL, R.D.K., GRANT, C.E., WYTSMA, J.M., &
BORA, K.C. (1980) Effect of lead chromate on chromosome
aberration, sister-chromatid exchange, and DNA damage in
mammalian cells in vitro. Mutat. Res., 77: 157-163.
DURFOR, C.N. & BECKER, E. (1964) Public water supplies of
the 100 largest cities in the United States, 1962, Washington
DC, US Geological Survey, 364 pp (Water Supply Paper No. 1812).
DURUM, W.H. & HAFFTY, J. (1963) Implications of the
minor-element content of some major streams in the world.
Geochim. Cosmochim Acta, 27: 1-11.
EATON, D.L., STACEY, N.H., WONG, K.L., & KLAASSEN, C.D.
(1980) Dose-response effects of various metal ions on rat
liver metallothionein, glutathione, heme oxygenase, and
cytochrome P-450. Toxicol. appl. Pharmacol., 55: 393-402.
EDMUNDSON, W.F. (1951) Chrome ulcers of the skin and nasal
septum and their relation to patch testing. J. Invest. Derm.,
17: 17-19.
EIFAC (1983) Water quality criteria for european freshwater
fish. Report on chromium and freshwater fish, European Inland
Fisheries Advisory Commission, Working Party on Water Quality
Criteria for European Freshwater Fish, 31 pp (EIFAC Technical
Paper No. 43).
ELIAS, Z., SCHNEIDER, O., AUBRY, F., DANIERE, M.C., & POIROT,
O. (1983) Sister chromatid exchanges in chinese hamster V79
cells treated with the trivalent chromium compounds chromic
chloride and chromic oxide. Carcinogenesis, 3: 605-611.
ENTERLINE, P.E. (1974) Respiratory cancer among chromate
workers. J. occup. Med., 16: 523-526.
ENVIRONMENTAL INSTRUMENTATION GROUP (1973) Instrumentation
for environmental monitoring: biomedical. IV, Berkeley,
California, Lawrence Berkeley Laboratory.
EQUITABLE ENVIRONMENTAL HEALTH, INC. (1976) An epidemiologic
study of workers in lead chromate plants: Final report
(prepared for the Dry Color Manufacturer's Association,
Berkeley California).
ESSING, H.G., SZADKOWSKI, D., & VALENTIN, H. (1971)
[Significance of the valence gradations of chromium compounds
in occupational medicine examinations.] Med. Sachverständige,
67: 35-39 (in German).
ETMANOVA, Z.J. (1965) [On the functional state of the liver
in workers of Aktubinsk chromium compound plant.] Gig. Tr.
prof. Zabol., 10: 55-56 (in Russian).
EVAN, A.P. & DAIL, W.G. (1974) The effect of sodium chromate
on the proximal tubules of the rat kidney. Lab. Invest.,
30(6): 704-715.
FANG, Y. (1983) [The normal value of chromium in urine for
299 unexposed persons in Yanjian city.] J. Yanbian Med.
College, 6 (1): 35-37 (in Chinese).
FARKAS, T.G. & ROBERSON, S.L. (1965) The effect of Cr(III)
on the glucose utilization of isolated lenses. Exp. Eye Res.,
4: 124-126.
FELDMAN, F.J. (1968) The state of chromium in biological
materials. Fed. Proc., 27: 482.
FELDMAN, F.J., KNOBLOCK, E.C., & PURDY, W.C. (1967) The
determination of chromium in biological materials by atomic
absorption spectroscopy. Anal. Chim. Acta, 38: 489-497.
FRADKIN, A., JANOFF, A., LANE, B.P., & KUSCHNER, M. (1975)
In vitro transformation of BHK21 cells grown in the presence
of calcium chromate. Cancer Res., 35: 1058-1061.
FRANCHINI, I., MUTTI, A., GARDINI, F., & BORGHETTI, A.
(1975) [Excretion and renal elimination of chromium compared
to degree and length of occupational exposure.] In: Roche, L.
& Trager, J., ed. Rein et Toxique. Réunion de la Société
Francaise de Toxicologie (Compte rendu), Paris, Groupement
Francais Des Centre Anti-Poisons, pp. 271-281.
FRANCHINI, I., MUTTI, A., CAVATORTA, A., CORRADI, A., COSI,
A., OLIVETTI, A., & BORGHETTI, A. (1978) Nephrotoxicity of
chromium. Control Nephrol., 10: 98-110.
FRANCHINI, J., MAGNANI, F., & MUTTI, A. (1983) Mortality
experience among chrome plating workers. Scand. J. Work
environ. Health, 9: 247-252.
FREGERT, S. (1975) Occupational dermatitis in a 10-year
material. Contact Dermatit., 1: 96-107.
FREGERT, S. (1981) Manual of contact dermatitis, 2nd ed.,
Copenhagen, Munksgaard, 139 pp.
FREGERT, S. & GRUVBERGER, B. (1972) Chemical properties of
cement. Berufsdermatosen, 20(5): 238-245.
FREGERT, S. & GRUVBERGER, B. (1979) Chromium in industrial
leather gloves. Contact Dermatit., 5: 189.
FREGERT, S. & RORSMAN, H. (1964) Allergy to trivalent
chromium. Arch. Dermatol., 90: 4-6.
FREGERT, S., HJORTH, N., MAGNUSSON, B., BANDMANN, H.-J.,
CALNAN, C.D., CRONIN, E., MALTEN, K., MENEGHINI, C.L., PIRILA,
V., & WILKINSON, D.S. (1969) Epidemiology of contact
dermatitis. Trans. St. John's Hosp. Dermatol. Soc., 55: 17-35.
FRENTZEL-BEYME, R. (1983) Lung cancer mortality of workers
employed in chromate pigment factories. J. Cancer Res. clin.
Oncol., 105: 183-188.
FREUND, H., ATAMIAN, S., & FISCHER, J.E. (1979) Chromium
deficiency during total parenteral nutrition. J. Am. Med.
Assoc., 241: 496-498.
FSA (1953) Health workers in chromate producing industry. A
study, Washington DC, Federal Security Agency, 131 pp (US PHS
Publication No. 192).
FUKAI, R. (1967) Valency state of chromium in seawater.
Nature (Lond.), 213: 901.
FUKUNAGA, M., KURACHI, Y., & MIZUGUCHI, Y. (1982) Action of
some metal ions on yeast chromosomes. Chem. pharm. Bull., 30:
3017-3019.
FURST, A., SCHLAUDER, M., & SASMORE, D.P. (1976) Tumour-
igenic activity of lead chromate. Cancer Res., 36: 1779-1783.
GALE, T.F. (1974) Effects of chromium on the hamster embryo.
Teratology, 9: A-17.
GALE, T.F. (1978) Embryotoxic effects of chromium trioxide
in hamsters. Environ. Res., 16: 101-109.
GALE, T.F. (1982) The embryotoxic response to maternal
chromium trioxide exposure in different strains of hamsters.
Environ. Res., 29: 196-203.
GALE, T.F. & BUNCH, J.D. (1979) The effect of the time of
administration of chromium trioxide on the embryotoxic
response in hamsters. Teratology, 19: 81-86.
GARCIA, J.D. & WETTERHAHN JENNETTE, K. (1981) Electron-
transport cytochrome P-450 system is involved in the
microsomal metabolism of the carcinogen chromate. J. inorg.
Biochem., 14: 281-295.
GEISER, J.D., JEANNERET, J.P., & DELACRETAZ, J. (1960) Eczema
due to cement and sensitization to cobalt. Dermatologica
(Basel), 121: 1-7.
GEMMELL, R.P. (1973) Revegetation of derelict land polluted
by a chromate smelter. Part 1. Chemical factors causing
substrate toxicity in chromate smelter waste. Environ.
Pollut., 5: 181-197.
GEMMELL, R.P. (1974) Revegetation of derelict land polluted
by a chromate smelter. Part 2. Techniques of revegetation of
chromate smelter waste. Environ. Pollut., 6: 31-37.
GENTILE, J.M., HYDE, K., & SCHUBERT, J. (1981) Chromium
genotoxicity as influenced by complexation and rate effects.
Toxicol. Lett., 7: 439-448.
GERAUER, A. (1954) [On the growing importance of
epicutaneous allergy to chromium.] Z. Haut-Geschlechtskr., 17:
97-102 (in German).
GILANI, S.H. & MARANO, M. (1979) Chromium poisoning and
chick embryogenesis. Environ. Res., 29: 427-431.
GERICKE, S. (1943) Studies on the effect of the trace
element chromium on the growth of plants. Bodenkd.
Pflanzenernaehr., 33: 114-128.
GLASER, U., HOCHRAINER, D., KLOPPEL, H., KORDEL, W., & KUHNEN,
H. (1984) [Inhalation studies with Wistar rats and patho-
physiological effects of chromium,] Berlin, 156 pp (Report to
Umweltbundesamt, D-1 UFOPLAN F+E 10606007/2) (in German).
GLASER, U., HOCHRAINER, D., KLOPPEL, H., & KUHNEN, H. (1985)
Low-level chromium VI inhalation effects on alveolar macro-
phages and immune functions in Wistar rats. Arch. Toxicol.,
57: 250-256.
GLINSMANN, W.H., FELDMAN, F.J., & MERTZ, W. (1966) Plasma
chromium after glucose administration. Science, 152: 1243.
GLINSMANN, W.H. & MERTZ, W. (1966) Effect of trivalent
chromium on glucose tolerance. Metabolism, 15: 510-520.
GMELIN, C.G. (1826) Experiments on the effects of baryta,
strontia, chrome, molybdenum, tungsten, tellurium, titanium,
osmium, platinum, iridium, rhodium, palladium, nickel, cobalt,
uranium, cerium, iron, and manganese, on the animal system.
Edinburgh med. Surg. J., 26: 131-139.
GOMES, E.R. (1972) Incidence of chromium-induced lesions
among electroplating workers in Brazil. Ind. Med., 41(12):
21-25.
GOMEZ-ARROYO, S., ALTAMIRANO, M., & VILLALOBOS-PIETRINI, R.
(1981) Sister-chromatid exchanges induced by some chromium
compounds in human lymphocytes in vitro. Mutat. Res., 90:
425-431.
GONCHAROV, A.T. (1968) [Role of chromium in the nutrition of
animals and man.] Vopr. Pitaniya, 27: 59-66 (in Russian).
GONCHAROV, A.T. (1978) [Maximum physiological dose of
chromium in food for human consumption.] Kazanskij med. Z.,
59(2): 90-93 (in Russian).
GRAY, S.J. & STERLING, K. (1950) The tagging of red cells
and plasma proteins with radioactive chromium. J. clin.
Invest., 29: 1604-1613.
GREBE, W.-H., KASTNER, H., KIPPENBERGER, C., KRAUSS, U.,
KRUSZONA, M., SCHMIDT, H., LIEBRUCKS, M., RUMBERGER, M., &
WETTIG, E. (1975) [Investigation on supply and demand for
mineral raw materials. VII. Chromium,] Berlin, Hannover,
Bundesanstalt für Gewissenschaften und Rohstoffe/Deutsches
Institut für Wirtschaftsforschung, 147 pp.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38: 33-42.
GROSS, W.G. & HELLER, V.G. (1946) Chromates in animal
nutrition. J. ind. Hyg. Toxicol., 28: 52-56.
GROSS, E. & KOLSCH, F. (1943) [Lung cancer in the chromate
dye industry.] Gewerbepathol. Gewerbehyg., 12: 164-170 (in
German).
GURSON, C.T. (1977) The metabolic significance of dietary
chromium. In: Draper, H.H., ed. Advances in nutritional
research, New York, Plenum Publishing Company, Vol. 1,
pp. 23-53.
GURSON, C.T. & SANER, G. (1971) Effect of chromium on
glucose utilization in marasmic protein-calorie malnutrition.
Am. J. clin. Nutr., 24: 1313-1319.
GURSON, C.T. & SANER, G. (1973) Effect of chromium
supplementation on growth in marasmic protein-calorie
malnutrition. Am. J. clin. Nutr., 26: 988-991.
GURSON, C.T. & SANER, G. (1978) The effect of glucose
loading on urinary excretion of chromium in normal adults,
individuals from diabetic families, and in diabetics. Am. J.
clin. Nutr., 31: 1158-1161.
GUTHRIE, B.E. (1973) Daily dietary intakes of zinc, copper,
manganese, chromium, and cadmium by some New Zealand women.
Proc. Univ. Otago Med. School., 51: 47-49.
GUTHRIE, B.E., WOLF, W.R., & VEILLON, C. (1978) Background
correction and related problems in the determination of
chromium in urine by graphite furnace atomic absorption
spectrometry. Anal. Chem., 50: 1900-1902.
GUTHRIE, B.E., WOLF, W.R., VEILLON, C., & MERTZ, W. (1979)
Chromium in urine. In: Hemphill, D.D., ed. Trace substances in
environmental health, Columbia, Missouri, University of
Missouri, Vol. XII, pp. 490-492.
GUY, W.B. (1954) Dermatologic problems in the railroad
industry resulting from conversion to Diesel power. Am. Med.
Assoc. Arch. Derm. Syph., 70: 289-292.
GYLSETH, B., GUNDERSEN, N., & LANGORD, S. (1977) Evaluation
of chromium exposure based on a simplified method for urinary
chromium determination. Scand. J. Work environ. Health, 3:
28-31.
HAMBIDGE, K.M. (1971) Chromium nutrition in the mother and
in the growing child. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 169-194.
HAMBIDGE, K.M. (1974) Chromium nutrition in man. Am. J.
clin. Nutr., 27: 505-514.
HAMBIDGE, K.M. & BAUM, J.D. (1972) Hair chromium concen-
trations of human newborn and changes during infancy. Am. J.
clin. Nutr., 25: 376-379.
HAMBIDGE, K.M. & RODGERSON, D.O. (1969) A comparison of the
hair chromium levels of nulliparous and parous women. Am. J.
Obstet. Gynecol., 103: 320-321.
HAMBIDGE, K.M., RODGERSON, D.O., & O'BRIEN, D. (1968)
Concentration of chromium in the hair of normal children and
children with juvenile mellitus. Diabetes, 17: 517-519.
HARA, N. (1982) [The change of valence of chromium by acid
treatment.] Sangyo Igaku, 24: 658-662 (in Japanese).
HARRISON, D.L. & STAPLES, E.L.J. (1955) Zinc chromate
poisoning in calves. N.Z. vet. J., 3: 63-73.
HASEGAWA, M. (1938) Studies on the detexicating hormone of
the liver (Yakriton). Toholu J. exp. Med., 32: 163-176.
HAYES, R.B. (1982) Carcinogenic effects of chromium, In:
Langard, S., ed. Biological and environmental aspects of
chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 221-247.
HAYES, R.B., LILIENFELD, A.M., & SNELL, L.M. (1979)
Mortality in chromium chemical production workers: a
prospective study. Int. J. Epidemiol., 8: 365-374.
HEATH, J.C., FREEMAN, M.A.R., & SWANSON, S.A.V. (1971)
Carcinogenic properties of wear particles from prostheses made
in cobalt-chromium alloy. Lancet, 1: 564-566.
HENNIG, A., ANKE, M., DIETRICH, M., HARTMANN, G., & HOFFMANN,
G. (1971) Absorption and distribution of 51chromium after
oral administration to laying hens as a function of time.
Arch. Tierern., 21: 609-615.
HEPLER, O.E. & SIMONDS, J.P. (1946) Experimental nephro-
pathies. IV. Glycosuria in dogs poisoned with uranyl nitrate,
mercury bichloride and potassium dichromate. Arch. Path., 41:
42-49.
HERNBERG, S., WESTERHOLM, P., SCHULTZ-LARSEN, K., DEGERTH,
R., KUOSMA, E., ENGLUND, A., ENGZELL, U., SAND HANSEN, H., &
MUTANEN, P. (1983) Nasal and sinonasal cancer: connection
with occupational exposures in Denmark, Finland, and Sweden.
Scand. J. Work environ. Health, 9: 315-326.
HERTEL, R.F. (1982) [Chromium as a problem in physiology,
epidemiology and biological monitoring.] Staub-Reinhalt. Luft,
42: 135-137 (in German).
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand
cultures. J. exp. Bot., 4: 59-64.
HILT, G. (1954) La dermite du chrome hexavalent dans le
cadre des dermites eczémateuses par sensibilisation aux
métaux. Dermatologica (Basel), 109: 143-174.
HINTZE, C. (1930) [Handbook of mineralogy,] Berlin, Leipzig,
De Gruyter, Vol. 1. III/2, pp. 4012-4030.
HOPKINS, L.L., Jr (1965) Distribution in the rat of physio-
logical amounts of injected 51Cr(III) with time. Am. J.
Physiol., 209: 731-735.
HOPKINS, L.L., Jr & SCHWARZ, K. (1964) Chromium (III)
binding to serum proteins, specifically siderophidin. Biochem.
Biophys. Acta, 90: 484-491.
HOPKINS, L.L., Jr, RANSOME-KUTI, O., & MAJAJ, A.S. (1968)
Improvement of impaired carbohydrate metabolism by chromium
(III) in malnourished infants. Am. J. clin. Nutr., 21: 203-211.
HUCKABEE, J.W., CARTAN, F.O., & KENNINGTON, G.S. (1972)
Environmental influence on trace elements in hair of 15
species of mammals, Oak Ridge, Tennessee, Oak Ridge National
Laboratory, 36 pp (ORNL-TM-3747).
HUEPER, W.C. (1958) Experimental studies in metal
cancerogenesis. X. Cancerigenic effects of chromite ore roast
deposited in muscle tissue and pleural cavity of rats. Arch.
ind. Health, 18: 284-291.
HUEPER, W.C. (1961) Environmental carcinogenisis and cancer.
Cancer Res., 21: 842-857.
HUNTER, W.C. & ROBERTS, J.M. (1933) Experimental study of
the effects of potassium bichromate in the monkey's kidney.
Am. J. Pathol., 9: 133-147.
HYODO, K., SUZUKI, S., FURUYA, N., & MESHIZUKA, K. (1980) An
analysis of chromium, copper, and zinc in organs of a chromate
worker. Int. Arch. occup. environ. Health, 46: 141-150.
IARC (1980) Some metals and metallic compounds, Lyons,
International Agency for Research on Cancer, pp. 205-323 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 23).
IIJIMA, S., MATSUMOTO, N., LU, C.C., & KATSUNUMA, H. (1975)
Placental transfer of CrCl3 and its effects on fetal growth
and development and development in mice. Teratology, 12: 198.
IMBUS, H.R., CHOLAK, J., MILLER, L.H., & STERLING, T. (1963)
Boron, cadmium, chromium, and nickel in blood and urine. Arch.
environ. Health, 6: 112-121.
IMREH, ST. & RADULESCU, D. (1982) Cytogenic effects of
chromium in vivo and in vitro. Mutat. Res., 97: 192-193.
IVANKOVIC, S. & PREUSSMANN, R. (1975) Absence of toxic and
carcinogenic effects after administration of high doses of
chromium oxide pigment in subacute and long-term feeding
experiments in rats. Food Cosmet. Toxicol., 13: 347-351.
JAEGER, H. & PELLONI, E. (1950) Tests épicutanés aux
bichromates, positifs dans l'eczéma au ciment. Dermatologica
(Basel), 100: 207-216.
JEEJEEBHOY, K.N., CHU, R.G., MARLISS, E.B., GREENBERG, G.R., &
BRUCE-ROBERTSON, A. (1977) Chromium deficiency, glucose
intolerance, and neuropathy reversed by chromium supplement-
ation, in a patient receiving long-term total parenteral
nutrition. Am. J. clin. Nutr., 30: 531-538.
JENNETTE, K.W. (1979) Chromate metabolism in liver micro-
some. Biol. Trace Elem. Res., 1: 55-62.
JENSEN, L.S., MAURICE, D.V., & MURRAY, M.W. (1978) Evidence
for a new biological function of chromium. Fed. Proc., 37: 404.
JINDRICHOVA, J. (1978) [Chromium damage in arc welders.] Z.
Ges. Hyg., 24: 86-89 (in German).
JOHNSON, J.L. (1974) Characterization of air-borne partic-
ulates. Microchem. J., 19:168-178.
JONES, G.B., BUCKLEY, R.A., & CHANDLER, C.S. (1975) The
volability of chromium from brewer's yeast during assay. Anal.
Chim. Acta, 80: 389-392.
JOSCHI, A.P. & NEEB, R. (1980) Gas chromatographic elemental
analysis via di(trifluorethyl)dithiocarbamatochelates. Z.
anal. Chem., 303: 389-393.
KAPLAN, I. & ZELIGMAN, I. (1962) Occupational dermatitis in
railroad workers. Am. Med. Assoc. Arch. Derm., 85: 135-142.
KAUFMANN, D.B., DINICOLA, W., & MCINTOSH, R. (1970) Acute
potassium dichromate poisoning. Am. J. dis. Child, 119:
374-376.
KANEKO, T. (1976) Chromosome damage in cultured human
leucocytes induced by chromium chloride and chromium trioxide.
Jpn. J. ind. Health, 18: 136-137.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec assay and
mutagenicity studies on metal compounds. Mutat. Res., 77:
109-116.
KAZANTZIS, G. & LILLY, L. (1979) Mutagenic and carcinogenic
effects of metals. In: Friedberg, L., Nordberg, G.F., & Vouk,
V.B., ed. Handbook on the toxicology of metals, Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 237-273.
KELLER, C. (1980) [Activation analysis.] In: Bartholomé, E.,
Biekert, E., Hellmann, H., Ley, H., Weigert, M., & Weise, E.,
ed. [Ullmann's encyclopaedia of technical chemistry,] 4th ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
Vol. 5, pp. 685-707 (in German).
KEMP, K., JENSEN, F.P., MOELLER, J.T., & HANSEN, G. (1974)
Trace multi-element analysis of biological tissue by proton-
induced X-ray fluorescence spectroscope, Roskilde, Denmark,
Danish Atomic Energy Commission, 22 pp (Report Riso-M-1732).
KEMPF, T. & SONNEBORN, M. (1976) [Determination of Pb, Cd,
Cr, and Zn according to the drinking-water regulation Federal
Republic of Germany.] In: Aurand, K., Hässelbarth, U., Müller,
G., Schumacher, W., & Steuer, W., ed. [The drinking-water
regulation,] Berlin, Erich Schmidt Verlag, pp. 119-125 (in
German).
KEMPF, T. & SONNEBORN, M. (1981) [Chemical composition of
drinking-waters in various regions of the Federal Republic of
Germany.] Vom Wasser, 57: 83-94 (in German).
KESKINEN, H., KALLIOMAKI, P.-L., & ALANKO, K. (1980) Occupa-
tional asthma due to stainless steel welding fumes. Clin.
Allerg., 10: 151-159.
KIRCHGESSNER, M., MERZ, G., & OELSCHLAEGER, W. (1960)
[Effect of vegetation state on content of macro and micro
elements of three species of grass.] Arch. Tierern., 10:
414-427 (in German).
KITTELBERGER, F. (1973) [Contribution to residue analysis in
fish.] Tierärztl. Prax., 1: 465-479.
KLEIN, L.A., LANG, M., NASH, N., & KIRSCHNER, S.L. (1974)
Sources of metals in New York City wastewater. J. Water
Pollut. Control Fed., 46: 2653-2662.
KLEINER, A.M., STOLBUN, B.M., LIKHACHEVA, E.L., & BELJAEVA,
L.N. (1970) [Some indices of the functional state of the
myocardium and hemodynamics in chronic intoxication with
chromium compounds.] Gig. Tr. prof. Zabol., 12: 7-10 (in
Russian).
KNUDSEN, I. (1980) The mammalian spot test and its use for
the testing of potential carcinogenicity of welding fume
particles and hexavalent chromium. Acta pharmacol. toxicol.,
47: 66-70.
KOGAN, V.Y. (1968) [The basophil degranulation as an index
of skin sensitization to cobalt compounds.] In: Belenky, G.B.,
ed. [Modern aspects of clinical and experimental dermato-
venerology. Works of the clinic of skin and venerological
diseases,] Moscow, Medizina, pp. 100-104 (in Russian).
KOGAN, V.Y. (1971) [Chromium as a specific cause for
occupation dermatoses.] In: Babayants, R.S., ed. [Topical
questions of dermato-venerology. First Medical Institute,
All-Union Conference, Moscow, I.A. Sechenov, pp. 172-176
(in Russian).
KOGAN, V.Y, ARUTYUNYAN, K.E., & KOCHINYAN, Y.E. (1972) [A
comparative characteristic of chromium, nickel, and cobalt
salts as a causative factor in allergic contact dermatosis.]
In: [Clinical Pathology of Chemical Aetiology. Materials of
the 1st All-Union Conference, Kiev, USSR, 24-25 October,
1972,] Kiev, pp. 144 and 146 (in Russian).
KOHLER, H. (1951) [Constitutional examinations of subjects
with skin diseases in the offset department of a printing
works (Doctoral thesis, Leipzig.)] Berufsdermatosen, 4: 93 (in
German).
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace
metals during the ashing of biological materials. Analyst,
101: 870-875.
KOIVISTOINEN, P., ed. (1980) Mineral element composition of
Finnish foods. Acta agric. Scand., Suppl. 22: 171
pp.
KOPP, J.E. & KRONER, R.C. (1968) Trace metals in waters of
the United States: a five-year summary of trace metals in
rivers and lakes of the United States (October 1 1962 -
September 30, 1967), Cincinnati, Ohio, US Department of the
Interior, 32 pp.
KORALLUS, U. & LOENHOFF, N. (1981) [Industrial medical and
epidemiological experience with the manufacture and processing
of chromates.] Arbeitsmed. Sozialmed. Praeventivmed., 16:
285-289 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974a)
[Trivalent chromium compounds. Results of a study in
occupational medicine. I. General; Technology; Preliminary
study.] Arbeitsmed. Sozialmed. Praeventivmed., 9: 51-54 (in
German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974b)
[Trivalent chromium compounds: results of a study in
occupational medicine. II. Disease status analysis.]
Arbeitsmed. Sozialmed. Praeventivmed., 9: 76-79 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974c)
[Trivalent chromium compounds: Results of a study in
occupational medicine. III. Clinical studies.] Arbeitsmed.
Sozialmed. Praeventivmed., 9: 248-252 (in German).
KORALLUS, U., LANGE, H.-J., NEISS, A., WUESTEFELD, E., &
ZWINGERS, T. (1982) [Correlations between reorganization
measures and bronchial carcinoma mortality in the chromate-
manufacturing industry.] Arbeitsmed. Sozialmed. Praevent-
ivmed., 7: 159-167 (in German).
KOSHI, K. (1979) Effects of fume particles from stainless
steel welding on sister chromatid exchanges and chromosome
aberrations in cultured Chinese hamster cells. Ind. Health.,
17: 39-49.
KOTZ, L., KAISER, G., TSCHOPEL, P., & TOLG, G. (1972)
[Clarification of biological matrices for determining very low
contents of trace elements when limited amounts are weighed
with nitric acid under pressure in a teflon vessel.] Z. anal.
Chem., 260: 207-209 (in German).
KOUTRAS, G.A., HATTORY, M., SCHNEIDER, A.S., EBAUGH, F.G., &
VALENTINE, W.N. (1964) Studies on chromated erythrocytes:
effect of sodium chromate on erythrocyte glutathione reduc-
tase. J. clin. Invest., 43: 323-331.
KRISHNAJA, A.P. & REGE, M.S. (1982) Induction of chromosomal
aberrations in fish Boleophthalmus dussumieri after exposure
in vivo to mitomycin C and heavy metals mercury, selenium, and
chromium. Mutat. Res., 102: 71-82.
KROEPFLI, P. & SCHUPPLI, R. (1955) [Detergent eczema.]
Dermatologica (Basel), 110: 1-7 (in German).
KRUGER, H. & DORN, H. (1956) [Clinical observations on
detergent eczema in association with chromium and nickel
allergy.] Z. Haut-Geschlechtskr., 20: 307-314 (in German).
KUCHER, I.M. (1966) In: [Proceedings of the 5th Scientific
Session of the Aktiubinsk Medical Institute, Alma-Ata,
Kazakhstan,] pp. 24-26 (in Russian).
KUHN, J.K. (1973) Trace elements in whole coal determined by
X-ray fluorescence. Norelco Rep., 20(3): 7-10.
KUMPULAINEN, J.T., WOLF, W.R., VEILLON, C., & MERTZ, W.
(1979) Determination of chromium in selected United States
diets. J. agric. food Chem., 27: 490-494.
KUMPULAINEN, J., LEHTO, J., KOIVISTOINEN, P., UUSITUPA, M., &
VUORI, E. (1983) Determination of chromium in human milk
serum and urine by electrothermal atomic absorption spectro-
metry without preliminary ashing. Sci. total Environ., 31:
71-80.
KURYLOWICZ, B. & GASIOROWSKI, S. (1958) [Agrochemical study
on calcinated phosphate-containing admixtures of chromium
compounds.] Przemysl Chem., 37: 797-800 (in Polish).
LANGARD, S. (1977) The fate of chromium after intravenous
administration of Na251 CrO4 and CrCl3 x 6H2 O to the rat,
Guildford, England, University of Surrey, 66 pp (M.Sc.
Toxicology Thesis).
LANGARD, S. (1980a) Chromium. In: Waldron, H.A., ed. Metals
in the environment, London, New York, Academic Press,
pp. 111-132.
LANGARD, S. (1980b) A survey of respiratory symptoms and
lung function in ferrochromium and ferrosilicon workers. Int.
Arch. occup. environ. Health, 46: 1-9.
LANGARD, S., ed. (1982) Biological and environmental aspects
of chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, 285 pp.
LANGARD, S. & HENSTEN-PETTERSEN, A. (1981) Chromium
toxicology. In: Williams, D.F., ed. Systemic aspects of
biocompatibility, Boca Raton, Florida, CRC Press, Vol. 1, pp.
144-161.
LANGARD, S. & NORSETH, T. (1975) A short study of bronchial
carcinomas in workers producing chromate pigments. Br. J. ind.
Med., 32: 62-65.
LANGARD, S. & NORSETH, T. (1979) Chromium. In: Friberg, L.,
Nordberg, G.F., & Vouk, V.B., ed. Handbook on the toxicology
of metals, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 383-397.
LANGARD, S. & STERN, R.M. (1985) Nickel in welding fumes: a
cancer hazard to welders? In: Sunderman, F.W.J., Aitio, A.,
Berlin, A., Bishop, C., Buringh, E., Davis, W., Gounar, M.,
Jacquignon, P.C., Mastromatteo, E., Rigaut, J.P., Rosenfeld,
C., Saracci, R., & Sors, A., ed. Nickel in the human
environment, Lyons, International Agency for Research on
Cancer, pp. 95-103 (IARC Scientific Publications No. 53).
LANGARD, S. & VIGANDER, T. (1983) Occurrence of lung cancer
in workers producing chromium pigments. Br. J. ind. Med., 40:
71-74.
LANGARD, S., GUNDERSEN, N., TSALEV, D.L., & GYLSETH, B.
(1978) Whole blood chromium level and chromium excretion in
the rat after zinc chromate inhalation. Acta pharmacol.
toxicol., 42: 142-149.
LANGARD, S., ANDERSEN, A., & GYLSETH, B. (1980) Incidence of
cancer among ferrosilicon workers. Br. J. ind. Med., 37:
114-120.
LASKIN, S. (1972) Research in environmental sciences,
Washington DC, Institute of Environmental Medicine, pp. 92-97.
LASKIN, S., KUSCHNER, M., & DREW, R.T. (1970) Studies in
pulmonary carcinogenesis. In: Hanna, M.G., Jr, Nettesheim, P.,
& Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 321-351 (US Atomic Energy
Commission Symposium Series No. 18).
LAUTNER, G.M., CARVER, J.C., & KONZEN, R.B. (1978)
Measurement of chromium (VI) and chromium (III) in stainless
steel welding fumes with electron spectroscopy for chemical
analysis and neutron activation analysis. Am. Ind. Hyg. Assoc.
J., 39: 651-660.
LEHMANN, K.B. (1914) [Historical and critical account of our
present knowledge of the effect of small doses of chromate.]
In: Gesamtgebiet gewerbehygiene, Berlin, Springer Verlag, pp.
1-119 (in German).
LEHMANN, K.B. (1932) [Does the occurrence of lung cancer in
chromium workers give grounds for any particular anxiety?]
Zentralbl. Gewerbehyg., 9: 168-170 (in German).
LERICHE, H.H. (1968) Metal contamination of soil in the
Woburn market-garden experiment resulting from the application
of sewage sludge. J. agric. Sci. Camb., 71: 205-208.
LETTERER, E., NEIDHARDT, K., & KLETT, H. (1944) [Chromate
lung cancer and chromate pneumoconiosis. A clinical, morbid
anatomical and industrial-hygiene-related study.] Arch.
Gewerbepath. Gewerbehyg., 12: 323-361 (in German).
LEVIN, H.M., BRUNNER, M.J., & RATTNER, H. (1959)
Lithographer's dermatitis. J. Am. Med. Assoc., 169: 566-569.
LEVINE, R.A., STREETEN, D.H.P., & DOISY, R.J. (1968) Effects
of oral chromium supplementation on the glucose tolerance of
elderly human subjects. Metabolism, 17: 114-125.
LEVIS, A.G. & BIANCHI, V. (1982) Mutagenic and cytogenetic
effects of chromium compounds. In: Langard, S., ed. Biological
and environmental aspects of chromium, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 171-208.
LEVIS, A.G. & BUTTIGNOL, M. (1977) Effects of potassium
dichromate on DNA Synthesis in hamster fibroblast. Br. J.
Cancer, 35: 496-499.
LEVIS, A.G. & MAJONE, F. (1979) Cytotoxic and caryogenic
effects of soluble chromium compounds on mammalian cell
cultures. Br. J. Cancer, 40: 523-533.
LEVIS, A.G. & MAJONE, F. (1981) Cytologic and clastogenic
effects of soluble and insoluble compounds containing
hexavalent and trivalent chromium. Br. J. Cancer, 44: 219-235.
LEVIS, A.G., BUTTIGNOL, M., BIANCHI, V., & SPONZA, G. (1978)
Effects of potassium dichromate on nucleic acid and protein
synthesis and on precursor uptake in BHK fibroblasts. Cancer
Res., 38: 110-116.
LEVY, L.S. & MARTIN, P.A. (1983) The effects of a range of
chromium-containing materials on rat lung, Birmingham,
England, University of Aston, Department of Environmental and
Occupational Health, p. 224 and suppl (Prepared for Dry Color
Manufacturers Association, Alexandria, Virginia, USA).
LEVY, L.S. & VENITT, S. (1975) Carcinogenic and mutagenic
activity of chromium-containing materials. Br. J. Cancer, 32:
254-255.
LIEBER, M., PERLMUTTER, N.M., & FRAUENTHAL, H.L. (1964)
Cadmium and hexavalent chromium in Nassau County ground
water. J. Am. Water Works Assoc., 56: 739-747.
LIFSCHITZ, M.L., WALLACH, S., PEABODY, R.A., VERCH, R.L., &
AGRAWAL, R. (1980) Radiochromium distribution in thyroid
and parathyroid deficiency. Am. J. clin. Nutr., 33: 57-62.
LINDBERG, E. & HEDENSTIERNA, G. (1983) Chrome plating.
Symptoms, findings in the upper airways, and effects on lung
function. Arch. environ. Health, 38: 367-374.
LINDBERG, E. & VESTERBERG, O. (1983a) Monitoring exposure to
chromic acid in chromeplating by measuring chromium in urine.
Scand. J. Work environ. Health, 9: 333-340.
LINDBERG, E. & VESTERBERG, O. (1983b) Urinary excretion of
proteins in chromplaters, previously exposed chromplaters, and
referents. Scand. J. Work. environ. Health, 9: 505-510.
LINDBERG, E., EKHOLM, U. & ULFVARSON, U. (1985) Extent and
conditions of exposure in the Swedish chrome plating industry.
Int. Arch. Occup. Environ. Health, 56: 197-205.
LIU, V.J.K. & MORRIS, J.S. (1978) Relative chromium response
as an indicator of chromium status. Am. J. clin. Nutr., 31:
972-976.
LOFROTH, G. (1978) The mutagenicity of hexavalent chromium
is decreased by microsomal metabolism. Naturwissenschaften,
65: 207-208.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LUKANIN, W.P. (1930) [The pathology of chromate pneumo-
coniosis.] Arch. Hyg. Bakt., 104: 166-174 (in German).
LUZHNIKOV, E.A., SHIMANKO, I.I., KOSTOMAROVA, L.G., MALYGINA,
S.I., & TRAKHTENGARTS, M.I. (1976) [Acute poisoning with
chromium compounds.] Terapeut. Arkh., 48(7): 121-125 (in
Russian).
LYON, G.L., BROOKS, R.R., PETERSON, P.J., & BUTLER, G.W.
(1970) Some trace elements in plants from serpentine soils.
N.Z. J. Sci., 13: 133-139.
MACHLE, W. & GREGORIUS, F. (1948) Cancer of the respiratory
system in the United States chromate-producing industry.
Public Health Rep., 63: 1114-1127.
MCCLENDON, L.T. (1978) Determination of chromium in
biological matrices by neutron activation: application to
standard reference materials. J. radioanal. Chem., 42: 85-91.
MACKENZIE, R.D., BYERRUM, R.U., DECKER, C.F., HOPPERT, C.A., &
LANGHAM, R.F. (1958) Chronic toxicity studies. II. Hexa-
valent and trivalent chromium administered in drinking-water
to rats. Am. Med. Assoc. Arch. ind. Health, 18: 232-234.
MCRAE, W.D., WHITING, R.F., & STICH, H.F. (1979) Sister-
chromatid exchanges induced in cultured mammalian cells by
chromate. Chem.-biol. Interact., 26: 281-286.
MAHALKO, J.R. & BENNION, M. (1976) The effect of parity and
time between pregnancies on maternal hair chromium concentra-
tion. Am. J. clin. Nutr., 29: 1069-1072.
MAJONE, F. & LEVIS, A.G. (1979) Chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster cells treated in
vitro with hexavalent chromium compounds. Mutat. Res., 67:
231-238.
MAJONE, F. & RENSI, D. (1979) Mitotic alterations, chromo-
some aberrations, and sister-chromatid exchanges induced by
hexavalent and trivalent chromium on mammalian cells in vitro.
Caryologia, 32: 379-392.
MALTONI, C. (1974) Occupational carcinogenesis. Excerpta
Med. Int. Congr. Ser., 322: 19-26.
MALTONI, C. (1976) Precursor lesions in exposed populations
as indicators of occupational cancer risk. Ann. NY Acad. Sci.,
271: 444-447.
MANCUSO, T.F. (1951) Occupational cancer and other health
hazards in a chromate plant: a medical appraisal. II. Clinical
and toxicologic aspects. Ind. Med. Surg., 20: 393-407.
MANCUSO, T.F. (1975) Consideration of chromium as an
industrial carcinogen. In: Hutchinson, T.C., ed. Proceedings
of the International Conference on Heavy Metals in the
Environment, Toronto, Canada, October 27-31, 1975, Toronto,
Institute for Environmental Studies, pp. 343-356.
MANCUSO, T.F. & HUEPER, W.C. (1951) Occupational cancer and
other health hazards in a chromate plant: a medical appraisal.
I. Lung cancer in chromate workers. Ind. Med. Surg., 20:
358-363.
MARTIN, J.H. & KNAUER, G.A. (1973) The elemental composition
of plankton. Geochim. Cosmochim. Acta, 3: 1639-1653.
MATHIS, B.J. & CUMMINGS, T.F. (1973) Selected metals in
sediments, water, and biota in the Illinois river. J. Water
Pollut. Control Fed., 45: 1573-1583.
MATHUR, R.K. & DOISY, R.J. (1972) Effect of diabetes and
diet on the distribution of tracer doses of chromium in rats.
Proc. Soc. Exp. Biol. Med., 139: 836-838.
MATSUMOTO, N., IIJIMA, S., & KATSUNUMA, H. (1976) Placental
transfer of chromic chloride and its teratogenic potential in
embryonic mice. J. toxicol. Sci., 2: 1-13.
MAYER, R. (1983) Interaction of forest canopies with atmo-
spheric constituents: aluminium and heavy metals. In: Ulrich,
B. & Pankrath, J., ed. Effects of accumulation of air
pollutants in forest ecosystems, Dordrecht, Boston, London,
D. Reidel, pp. 47-55.
MAZGON, R. (1932) [Therapeutic effect of common salt on
experimental chromium nephritis in the rabbit.] Z. Gesamte
exp. Med., 81: 195-207 (in German).
MERIAN, E. (1984) Introduction on environmental chemistry
and global cycles of arsenic, beryllium, cadmium, chromium,
cobald, nickel, selenium, and their derivatives. Toxicol.
environ. Chem., 8: 9-38.
MERKURIEVA, R.V., KRASOVSKY, G.N., BURMANTOVA, N.P.,
SHATERNIKOVA, I.L., VARSHAVSKAYA, S.P., VASIUKOVICH, L.YA.,
KOGANOVA, Z.I., BUSHINSKAYA, L.I., KONSTANTINOVA, I.N.,
DOLINSKAYA, S.I., ASTAKHOVA, L.F., & VOTYAKOV, A.V. (1980a)
[Changes in various enzyme systems resulting from exposure to
some chemical substances.] Gig. i. Sanit., N1: 25-28 (in
Russian).
MERKURIEVA, R.V., KRASOVSKY, G.N., VOTYAROV, A.V., BURMANTOVA,
N.P., MUCHAMBETOVA, L.H., & KONSTANTINOVA, I.N. (1980b)
[Enzyme disarrangement of various subcellular structures under
the influence of the general toxic and gonadotoxic effects of
environmental chemical factors.] Zdravookhranenie belorus.,
N2: 28-29 (in Russian).
MERKURIEVA, R.V., KOGANOVA, Z.I., GABDULLINA, M.H., TSAPKOVA,
N.N., DUKHATEROV, O.N., & DOLINSKAYA, S.I. (1982) [A
comparative study of metabolic reactions at various routes of
chromium6+ administration in the organism of laboratory
animals.] Gig. i Sanit., N8: 75-78 (in Russian).
MERRITT, W.F. (1971) Identification and measurement of trace
elements in fresh water by neutron activation. In: Proceedings
of the International Symposium on Identification and Measure-
ment of Environmental Pollution, 358-362.
MERTZ, W. (1969) Chromium occurrence and function in
biological systems. Physiol. Rev., 49: 163-239.
MERTZ, W. (1981) The interaction between chromium and
insulin. Adv. Physiol. Sci., 12: 101-105.
MERTZ, W. & ROGINSKI, E.E. (1963) The effect of trivalent
chromium in galactose entry in rat epidymal fat tissue. J.
biol. Chem., 238: 868-872.
MERTZ, W. & ROGINSKI, E.E. (1969) Effects of chromium (III)
supplementation on growth and survival under stress in rats
fed low protein diets. J. Nutr., 97: 531-536.
MERTZ, W. & ROGINSKI, E.E. (1971) Chromium metabolsm: the
glucose tolerance factor. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 123-153.
MERTZ, W. & SCHWARZ, K. (1959) Relation of glucose tolerance
factor to impaired glucose tolerance in rats on stock diets.
Am. J. Physiol., 196: 614-618.
MERTZ, W., ROGINSKI, E.E., & SCHWARZ, K. (1961) Effect of
trivalent chromium complexes on glucose uptake by epididymal
fat tissue of rats. J. biol. Chem., 236: 318-322.
MERTZ, W., ROGINSKI, E.E., & REBA, R.C. (1965a) Biological
activity and fate of intravenous chromium (III) in the rat.
Am. J. Physiol., 209: 489-494.
MERTZ, W., ROGINSKI, E.E., & SCHROEDER, H.A. (1965b) Some
aspects of glucose metabolism of chromium-deficient rats
raised in a strictly controlled environment. J. Nutr., 86:
107-112.
MERTZ, W., ROGINSKI, E.E., FELDMAN, R.J., & THURMAN, D.E.
(1969) Dependence of chromium transfer into the rat embryo on
the chemical form. J. Nutr., 99: 363-367.
MERTZ, W., TOEPFER, E.W., ROGINSKI, E.E., & POLANSKY, M.M.
(1974) Present knowledge of the role of chromium. Fed. Proc.,
33: 2275-2280.
MEYERS, J.B. (1950) Acute pulmonary complications following
inhalations of chromic acid mist. Arch. ind. Hyg. occup. Med.,
2: 742-747.
MICKAIL, T.H., HABIB, Y.A., GABRIAL, G.N., & MORCOS, S.R.
(1976) The effect of chromium III on the intravenous glucose
tolerance of rats given diets of defficient protein values. Z.
Ernährungswiss., 15: 182-187.
MIESCHER, G., AMREIN, H.P., & LEDER, M. (1955) [Chromate
hypersensitivity and cement eczema.] Dermatologica (Basel),
110: 266-283 (in German).
MIGAI, K.V. (1975) [Hygiene and safety of labor in electro
welding works in shipbuilding,] Leningrad, pp. 27-35 (in
Russian).
MITMAN, F.W., WOLF, W.R., KELSAY, J.L., & PRATHER, E.S.
(1975) Urinary chromium levels of nine young women eating
freely chosen diets. J. Nutr., 105: 64-68.
MORGAN, J.M. (1972) Hepatic chromium content in diabetic
subjects. Metabolism, 21: 313-316.
MORRIS, G.E. (1955) Chromate dermatitis from chrome glue and
other aspects of the chrome problem. Am. Med. Assoc. Arch.
Ind. Health, 11: 368-371.
MORRIS, G.E. (1956) The manufacture of leather. Its chemical
and dermatological aspects. Ann. Meet. Am. Acad. Derm. Syph.
(Chicago).
MORRIS, G.E. (1958) Chrome dermatitis. A study of the
chemistry of shoe leather with particular reference to basic
chromic sulfate. Am. Med. Assoc. Arch. Derm., 78: 612-618.
MORRIS, G.E. (1959) [The basic form of chromic sulfate.
Results of epicutaneous testing of a chromium-III salt.]
Berufsdermatosen, 7: 126-131 (in German).
MORROW, R.W. & MCELHANEY, R.J. (1974) Determination of
chromium in industrial effluent water by flameless atomic
absorption spectroscopy. At. Absorpt. Newsl., 13(2): 45-46.
MORTON, M.S. & ELWOOD, P.C. (1974) CNS malformations and
trace elements in water. Teratology, 10: 318.
MOSINGER, M. & FIORENTINI, H. (1954) Sur la pathologie due
aux chromates: Premières recherches expérimentales. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 15: 187-199.
MULLER-WIEGAND, B., STEUER, D., & HEESSELS, P. (1983) Blood
and urine samples for quality control of heavy metals. Chem.
Toxicol. Clin. Chem. Met. Proc. Int. Conf., 2nd: 73-76.
MURAKAMI, Y., SUZUKI, Y., YAMAGATA, T., & YAMAGATA, N. (1965)
Cr and Mn in Japanese diet. J. Radiat. Res., 6: 105-110.
MURRMAN, R.P., WINTERS, R.W., & MARTIN, T.G. (1971)
Determination of trace elements in soils and clay minerals by
resonnance neutron activation analysis. Soil. Sci. Soc. Am.
Proc., 35: 647-652.
MUTTI, A., CAVATORTA, A., PEDRONI, C., BORGHI, A., GIAROLI,
C., & FRANCHINI, I. (1979) The role of chromium accumulation
in the relationship between airborne and urinary chromium in
welders. Int. Arch. occup. environ. Health, 43: 123-133.
MYSLYAEVA, A.V. (1965) [Changes in the peripheral blood in
workers of a chromium compound plant.] In: [Proceedings of
the 4th Scientific Conference of Aktubinsk State Medical
Institute, Alma-Ata,] Kazakhstan, Alma-Ata, Aktubinsk State
Medical Institute, pp. 331-332 (in Russian).
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., & KURATA, H. (1978)
Comparative studies of chromosomal aberrations and muta-
genicity of trivalent and hexavalent chromium. Mutat. Res.,
58: 175-181.
NASH, D.T., ELWOOD, I.G., & STREETEN, D.H.P. (1979) The
effect of brewer's yeast containing chromium-rich glucose
tolerance factor on serum lipids. In: Proceedings of the 5th
International Symposium on Atherosclerosis, Houston, Texas.
NATER, J.P. (1963) Possible causes of chromate eczema.
Dermatologica (Basel), 126: 160-166.
NATH, R. (1976) Assessment of chromium metabolism in
maturity onset and juvenile diabetics using chromium: 51Cr and
therapeutic response of chromium administration. Excerpta Med.
Int. Congr. Ser., 400: 194.
NEIKO, E.M. & DELVA, IU.V. (1978) [Chromium balance in
chronic ischemic heart disease.] Klinicheskaia Meditsina
(Mosk.), 56(4): 97-99 (in Russian).
NESTMANN, E.R., MATULA, T.I., DOUGLAS, G.R., BORA, K.C., &
KOWBEL, D.J. (1979) Detection of the mutagenic activity of
lead chromate using a battery of microbial tests. Mutat. Res.,
66: 357-365.
NETTESHEIM, P., HANNA, M.G., Jr., DOHERTY, D.G., NEWELL, R.F.,
& HELLMANN, A. (1970) Effects of chronic exposure to
artificial smog and chromium oxide dust on the incidence of
lung tumours in mice. In: Hanna, M.G., Jr, Nettesheim, P., &
Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 305-320 (US Atomic Energy
Commission Symposium Series No. 18).
NETTESHEIM, P., HANNA, M.G., DOHERTY, D.G., NEWELL, R.F., &
HELLMAN, A. (1971) Effect of calcium chromate dust,
influenza virus, and 100R whole-body X-radiation on lung
tumour incidence in mice. J. Natl Cancer Inst., 47: 1129-1144.
NEWBOLD, R.F., AMOS, J., & CONNELL, J.R. (1979) The
cytotoxic, mutagenic, and clastogenic effects of chromium-
containing compounds on mammalian cells in culture. Mutat.
Res., 67: 55-63.
NEWMAN, H.A.I., LEIGHTON, R.F., LANESE, R.R., & FREELAND N.A.
(1978) Serum chromium and angiographically-determined
coronary artery disease. Clin. Chem., 24: 541-544.
NISHIOKA, H. (1975) Mutagenic activities of metal compounds
in bacteria. Mutat. Res., 31: 185-189.
NOLLENDORF, A., PAKALNE, E., & UPITIS, V. (1972) [Little
known trace elements in Chlorella culture.] Latv. PSR Zinat.
Akad. Vestis (Latvian SSR), 7: 33-43 (in Russian).
NOMIYAMA, H., YOTORIYAMA, M., & NOMIYAMA, K. (1980b) Normal
chromium levels in urine and blood of Japanese subjects
determined by direct flameless atomic absorption spectrophoto-
metry, and valency of chromium in urine after exposure to
hexavalent chromium. Am. Ind. Hyg. Assoc. J., 41: 98-102.
NOMIYAMA, K., MATSUI, K., & NOMIYAMA, H. (1980a) Environ-
mental temperature: a factor modifying the acute toxicity of
organic solvents, heavy metals, and agricultural chemicals.
Toxicol. Lett., 6: 67-70.
NORSETH, T. (1978) Health effects of nickel and chromium.
In: Elvira di Ferrante, E., ed. Trace metals: exposure and
health effects, Oxford, New York, Toronto, Sydney, Paris,
Frankfurt, Pergamon Press, pp. 135-146.
NORSETH, T. (1980) Cancer hazards caused by nickel and
chromium exposure. J. Toxicol. environ. Health, 6: 1218-1227.
NOVAKOVA, S., MAUTNER, G., & DINOEVA, A. (1974) [Content of
hexavalent chromium in water supplies and its effect on the
development of experimental atherosclerosis in warm-blooded
animals.] Gig. i Sanit., 5: 78-80 (in Russian).
NOVEY, H.S., HABIB, M., WELLS, J.A. (1983) Asthma and JgE
antibodies induced by chromium and nickel salts. J. Allergy
clin. Immunol., 72: 407-412.
OFFENBACHER, E.G. & PI-SUNYER, F.X. (1980) Benefical effect
of chromium-rich yeast on glucose tolerance and blood lipids
in elderly subjects. Diabetes, 29: 919-925.
OHNO, H., HANAOKA, F., & YAMADA, M. (1982) Inducibility of
sister-chromatid exchanges by heavy-metal ions. Mutat. Res.,
104: 141-145.
OHTA, F. (1940) Studies on the detoxicating hormone of the
liver (yakriton). Contribution to the usage of yakriton
against experimental chromate nephritis. Tohoku J. exp. Med.,
39: 37-46.
OKADA, S., OHBA, H., & TANIYAMA, M. (1981) Alterations in
ribonucleic acid synthesis by chromium (III). J. inorg.
Biochem., 15: 223-231.
OKADA, S., TRUKADA, H., & OHBA, H. (1983) Enhancement of
ribonucleic acid synthesis by chromium (III) in mouse liver.
J. inorg. Biochem., 19: 95-103
OKADA, S., TSUKADA, H., & OHBA, H. (1984) Enhancement of
nucleolar RNA synthesis by chromium (III) in regenerating rat
liver. J. inorg. Biochem., 21, 113-124.
OPHULS, W. (1911a) Experimental nephritis in guinea-pigs by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9: 11-13.
OPHULS, W. (1911b) Experimental nephritis in rabbits by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9:13.
OTTINGER, R.S., BLUMENTHAL, J.L., DALPORTO, D.F., GURBER,
G.I., SANTY, M.J., & SHIH, C.C. (1973) Recommended methods
of reduction, neutralization, recovery or disposal of
hazardous waste, Cincinnati, Ohio, US Environmental Protection
Agency, Vol. 6, pp. 143-162 (EPA 670/2-73-053-F).
PAGE, A.L. (1974) Fate and effects of trace elements in
sewage sludge when applied to agricultural lands, Cincinnati,
Ohio, US Environmental Protection Agency (EPA 670/2-74-005).
PAIXAO, L.M. & YOE, J.H. (1959) Spectochemical determination
of magnesium, chromium, nickel, copper, and zinc in human
plasma and red cells. Clin. Chim. Acta, 4: 507-514.
PARR, R.M. (1977) Problems of chromium analysis in
biological materials: an international perspective with
special reference to results for analytical quality control
samples. J. radioanal. Chem., 39: 421-433.
PASCALE, L.R., WALDSTEIN, S.S., ENGBRING, G., DUBIN, A., &
SZANTO, P.B. (1952) Chromium intoxication with special
reference to hepatic injury. J. Am. Med. Assoc., 149:
1385-1389.
PASHIN, Y.V. & KOZACHENKO, V.J. (1981) [Mutagenic activity
of chromium compounds.] Gig. i. Sanit., 5: 46-49 (in Russian).
PASHIN, Y.V., ZACEPILOVA, T.A., & KOZACHENKO, V.I. (1982)
Induction of dominant lethal mutations in male mice by
potassium dichromate. Mutat. Res., 103: 345-347.
PASTERNAK, K. (1973) The spreading of heavy metals in
flowing waters in the region of occurrence of natural deposits
of the zinc and lead industry. Acta hydrobiol., 15: 145-166.
PEKAREK, R.S., HAUER, E.C., RAYFIELD, E.J., WANNEMACHER, R.W.,
& BIESEL, W.R. (1975) Relationship between serum chromium
concentrations and glucose utilization in normal and infected
subjects. Diabetes, 24: 350-353.
PENG, H.Z. (1982) [The investigation of nasal disease
morbidity of chromium exposed workers.] J. Shan-Xie Med.
College, 3: 19-21 (in Chinese).
PERLMUTTER, N.M. & LIEBER, M. (1970) Dispersal of plating
wastes and sewage contaminants in ground water and surface
water, Washington DC, US Government Printing Office, 67 pp
(Geological Survey Water Supply Paper 1879-G).
PERONE, V.B., MOFFITT, A.A., POSSICK, P.A., KEY, M.M.,
DANZINGER, S.J., & GELLIN, G.A. (1974) The chromium, cobalt,
and nickel contents of American cement and their relationship
to cement dermatitis. Am. Ind. Hyg. Assoc. J., 35: 301-306.
PETRILLI, F.L. & DE FLORA, S. (1977) Toxicity and mutagenicity of
hexavalent chromium on Salmonella typhimurium. Appl. environ.
Microbiol., 33: 805-809.
PETRILLI, F.L. & DE FLORA, S. (1978a) Oxidation of inactive
trivalent chromium to the mutagenic hexavalent form. Mutat.
Res., 58: 167-173.
PETRILLI, F.L. & DE FLORA, S. (1978b) Metabolic deactivation
of hexavalent chromium mutagenicity. Mutat. Res., 54: 139-147.
PETRILLI, F.L. & DE FLORA, S. (1980) Mutagenicity of
chromium compounds. In: Chromates Symposium 80, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 76-99.
PETRILLI, F.L. & DE FLORA, S. (1982) Interpretations on
chromium mutagenicity and carcinogenicity. In: Sorsa, M. &
Vainmio, H., ed. Mutagens in our environment, New York, Alan
R. Liss, pp. 453-464.
PIERCE, J.V. & SCHEEL, L.D. (1965) Toxicity of alloys of
chromium. Arch. environ. Health, 10: 870-876.
PIRILA, V. & KILPIO, O. (1949) On dermatoses caused by
bichromates. Acta derm. venerol., 29: 550-563.
POHLAND, F.G. (1975) Sanitary landfill stabilization with
leachate recycle and residual treatment, Cincinnati, Ohio, US
Environmental Protection Agency, 106 pp (EPA 600/2-75-043).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1973) [On carcinogenous
hazards in the product of chromium ferroalloys.] Gig. Tr.
prof. Zabol., 10: 23-26 (in Russian).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1974) [Epidemiological
data on malignant neoplasms in workers who are in contact with
different chromium compounds.] In: Shabad, L.M., Velich-
kovskii, B.T. & Kogan, F.M., ed. [Occupational cancer (a
collection of scientific works),] Moscow, Academic Academy of
Medical Science, pp. 64-71 (in Russian).
POKROVSKAYA, L.V., TJUSHUYAKOVA, A.V., GORODNOVA, N.V.,
ZALOMOVA, E.N., & ANDREEVA, T.D. (1976) [Dust factor and
occupational pathology in workers engaged in the open
excavation of chromium ores.] In: [Occupational diseases of
dust etiology (collection of works),] Vol. 3, pp. 38-43 (in
Russian).
POLAK, L., TURK, J.L., & FREY, J.R. (1973) Studies on
contact hypersensitivity to chromium compounds. In: Kallos,
P., Waksman, B.H., & DeWeck, A., ed. Progress in allergy,
Basel, Karger, Vol. 17, pp. 145-226.
PRESTON, A.M., DOWDY, R.P., PRESTON, M.A., & FREEMAN, J.N.
(1976) Effects of dietary chromium on glucose and serum
cholesterol in guinea-pigs. J. Nutr., 106: 1391-1397.
PRIESTLEY, J. (1877) Observations on the physiological
action of chromium. J. Anat. Physiol., 11: 285-301.
PRINCI, F., MILLER, L.H., DAVIS, A., & CHOLAK, J. (1962)
Pulmonary disease of ferrochromium workers. J. occup. Med., 4:
301-310.
PUNSAR, S., ERAMETSA, O., KARVONEN, M.I., RYHANEN, A., HILSKA,
P., & WORNAMO, H. (1975) Coronary heart disease and
drinking-water. A search in two Finnish male cohorts for
epidemiologic evidence of a water factor. J. chron. Dis., 28:
259-287.
RABEAU, H. & UKRAINCZYK, V. (1939) Dermites des
blanchisseuses. Rôle du chrome et du chlore. Ann. Derm. Syph.,
10: 656-680.
RADIAN CORPORATION (1983) Estimates of population exposure
to ambient chromium emissions, Research Triangle Park, North
Carolina, US Environmental Protection Agency (Final Report to
K.L. Blanchard) (EPA Contract No. 68-02-3818).
RAFFETTO, G. (1977) Direct interaction with cellular targets
as the mechanism for chromium carcinogenesis. Tumori, 63:
503-512.
RAFFETTO, G., PARODI, S., DE FERRARI, M., PARODI, C., TROIANO,
R., & BRAMBILLA, G. (1978) Direct interaction with cellular
targets as the mechanism for chromium carcinogensis. Tumori,
73: 503-512.
REDISKE, J.H. (1956) Chromium toxicity in plants. In:
Biology research: annual report, 1955, Richland, Washington,
Hamford Atomic Products Operations, pp. 48-52 (Report
HW-41500).
REDISKE, J.H., CLINE, J.F., & SELDERS, A.A. (1955) Chromium
toxicity in plants. In: Biology research: annual report, 1954,
Richland, Washington, Hamford Atomic Products Operations,
pp. 32-39 (Report HW-35917).
REGGIANI, A., LOTTI, M., DE ROSA, E., & SAIA, B. (1973)
[Impairments of respiratory functions in subjects exposed to
chromium. Note 1. Spirographic changes.] Lav. Umano, 25: 23-27
(in Italian).
REICHENBACH-KLINKE, H.H. (1977) [To the ecology of the
accumulation of heavy metals in fishes.] In: Reichenbach-
Klinke, H.H., ed. [Contributions to fish ecology: fish and the
environment,] Stuttgart, New York, Gustav Fischer, pp. 7-11
(in German).
REICHENBACH-KLINKE, H.H. (1980) [Fish diseases and harm
suffered by fish,] 2nd ed., Stuttgart, New York, Gustav
Fischer, 474 pp (in German).
RETNEV, V.M. (1960) [On the effect produced by chromium
compounds contained in cement dust on the development of
bronchial asthma.] Gig. Tr. prof. Zabol., 7: 29-32 (in
Russian).
RIALES, R. (1979) Influence of brewer's yeast on lipoprotein
cholesterol concentrations. A preliminary report. In:
Shapcott, D. & Hubert, I., ed. Chromium in nutrition and
metabolism. Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 199-212.
ROBERTS, K.R. & MARZLUF, G.A. (1971) The specific inter-
action of chromate with the dual sulfate permease systems of
Neurospora crassa. Arch. Biochem. Biophys., 142: 651-659.
ROBINSON, W.D. & EDINGTON, G. (1935) Chemical studies of
infertile soils derived from rocks high in magnesium and
generally high in chromium and nickel, Washington DC, US
Department of Agriculture, pp. 1-29 (US Department of
Agriculture Technical Bulletin No. 471).
ROGINSKI, E.E. & MERTZ, W. (1967) An eye lesion in rats fed
low-chromium rations. J. Nutr., 93: 249-251.
ROGINSKI, E.E. & MERTZ, W. (1969) Effects of chromium (III)
supplementation on glucose and amino acid metabolism in rats
fed a low protein diet. J. Nutr., 97: 525-530.
ROHDE, G., TISCHENDORF, G., LEONHARDT, J., & DAMASCHUN, F.
(1978) [Study of crocoite occurrence in Callenberg (Saxony)
(Germany).] Z. angew. Geol., 24: 168-173 (in German).
ROMOSER, G.L., DUDLEY, W.A., MACHLIN, L.J., & LOVELESS, L.
(1961) Toxicity of vanadium and chromium for the growing
chick. Poultry Sci., 40: 1171-1173.
ROOK, H.L. & WOLF, W.R. (1977) The quantitative
determination of volatile trace elements in NBS biological
standard reference materials. In: Hemphill, D.D., ed.
Proceedings of the Eleventh Annual Conference on Trace
Substances in Environmental Health, Columbia, Missouri, the
Curators of the University of Missouri, pp. 324-333.
ROSCHINA, T.A. (1959) [Experimental study on the effects of
magnezite chromium and chromite magnezite dust exposure in the
production of refrectories.] Gig. Tr. prof. Zabol., 4: 28-32
(in Russian).
ROSCHINA, T.A. (1964) [A comparative evaluation of the
effects of carbide dust and chromium boride on the organism.]
Gig. i Sanit., 8: 25-28 (in Russian).
ROSCHINA, T.A. (1976) [The toxicity of chrominum (sic)
hexacarbonyl.] Gig. Tr. Prof. Zabol., 2: 38-42 (in Russian).
ROSEBROUGH, R.W. & STEELE, N.C. (1981) Effect of
supplemental dietary chromium or nicotinic acid on
carbohydrate metabolism during basal, starvation, and
refeeding periods in poults. Poultry Sci., 60: 407-417.
ROSS, D.S., SJOGREN, R.E., & BARTLETT, R.J. (1981) Behaviour
of chromium in soils. IV. Toxicity to microorganisms. J.
environ. Qual., 10: 145-148.
ROSTENBERG, A., Jr & PERKINS, A.J. (1951) Nickel and cobalt
dermatitis. J. Allerg., 22: 466-474.
RTECS (1978) Registry of toxic effects of chemical substances,
Washington DC, National Institute for Occupational Safety and
Health, pp. 450.
SAMITZ, M.H. (1955) Some dermatologic aspects of the
chromate problem. Arch. ind. Health, 11: 361-367.
SAMITZ, M.H. (1956) Studies of chromate dermatitis. Arch.
ind. Health, 14: 269-271.
SAMITZ, M.H. & EPSTEIN, E. (1962) Experimental cutaneous
chrome ulcers in guinea-pigs. Arch. environ. Health, 5:
463-468.
SAMITZ, M.H. & GROSS, S. (1960) Extraction by sweat of
chromium from chrome tanned leathers. J. occup. Med., 2: 12-14.
SAMITZ, M.H. & GROSS, S. (1961) Effects of hexavalent and
trivalent chromium compounds on the skin. Am. Med. Assoc.
Arch. Dermatol., 84: 404-409.
SAMITZ, M.H., KATZ, S., & GROSS, S. (1960) Nature of the
chromium extracted from leather by sweat. J. occup. Med., 2:
435-436.
SANDERS, C.L., JACKSON, T.A., ADEE, R.R., POWERS, G.J., &
WEHNER, A.P. (1971) Distribution of inhaled metal oxide
particles in pulmonary alveoli. Arch. intern. Med., 127:
1085-1089.
SARGENT, T., III, LIM, T.H., & JENSON, R.L. (1979) Reduced
chromium retention in patients with hemochromatosis, a
possible basis of hemochromatotic diabetes. Metabolism, 28:
70-79.
SARTO, F., LEVIS, A.G., & PAULON, C. (1980) Clastogenic
activity of hexavalent and trivalent chromium in cultured
human lymphocytes. Caryologia, 33: 239-250.
SARTO, F., COMINATO, J., BIANCHI, V., & LEVIS, A.G. (1982)
Increased incidence of chromosomal aberrations and sister
chromatid exchanges in workers exposed to chromic acid (CrO3)
in electroplating factories. Carcinogenesis, 3: 1011-1016.
SASSI, C. (1956) [Occupational disease in a chromate
factory.] Med. Lavoro, 47: 314-326 (in Italian).
SATOH, K., FUKUDA, Y., TORII, K., & KATSUNO, N. (1981)
Epidemiological study of workers engaged in the manufacture of
chromium compounds. J. occup. Med., 23: 835-838.
SAVORY, J., MUSHAK, P., & SUNDERMAN, F.W. Jr (1969) Gas
Chromatographic determination of chromium in serum. J.
chromatogr. Sci., 7: 674-679.
SCHALLER, K.H. & ZOBER, A. (1982) [Renal excretion of
toxicologically relevant metals in occupationally non-exposed
individuals.] Arztl. Lab., 28: 209-214 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1972) [Quantitative chromium determination in urine by
flameless atomic absorption spectrometry.] Z. klin. Chem.
klin. Biochem., 10: 434-437 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1973) The quantitative determination of chromium in urine by
flameless atomic absorption spectroscopy. At. Absorpt.
Newsl., 12(6): 147-150.
SCHELENZ, R. (1977) Dietary intake of 25 elements by man
estimated by neutron activation analysis. J. radioanal. Chem.,
37: 539-548.
SCHLETTWEIN-GSELL, D. & MOMMSEN-STRAUB, S. (1971) [Review
trace elements in foods. III. Chromium.] Int. J. Vitam. Nutr.
Res., 41: 116-125 and (1973) Suppl. 13, 188 pp (in German).
SCHOENTAL, R. (1975) Chromium carcinogenesis formation of
epoxyaldehydes and tanning. Br. J. Cancer, 32: 403-404.
SCHROEDER, H.A. (1966) Chromium deficiency in rats: a
syndrome simulating diabetes mellities with retarded growth.
J. Nutr., 88: 439-445.
SCHROEDER, H.A. (1970) Chromium, Washington DC, American
Petroleum Institute, 28 pp (Air Quality Monograph No. 70-15).
SCHROEDER, H.A. & BALASSA, J.J. (1965) Influence of
chromium, cadmium, and lead on rat aortic lipids and
circulating cholesterol. Am. J. Physiol., 209: 433-437.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1962)
Abnormal trace metals in man: chromium. J. chron. Dis., 15:
941-964.
SCHROEDER, H.A., VINTON, W.H., Jr, & BALASSA, J.J. (1963)
Effects of chromium, cadmium, and other trace metals on the
growth and survival of mice. J. Nutr., 80: 39-47.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1964)
Chromium, lead, cadmium, nickel, and titanium in mice: effect
on mortality, tumours, and tissue levels. J. Nutr., 83:
239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1965)
Chromium, cadmium, and lead in rats: effects on life span,
tumours, and tissue levels. J. Nutr., 86: 51-66.
SCHROEDER, H.A., NASON, A.P., & TIPTON, I.H. (1970) Chromium
deficiency as a factor in atherosclerosis. J. chron. Dis., 23:
123-142.
SCHROEDER, K.H. (1955) [Nickel eczema in a blueline (ozalid)
machine operator.] Berufsdermatosen, 3: 170-171 (in German).
SCHUBERT, G.E., GEBHARD, K., & HOENLEIN, F. (1970) [Kidney
damage following injection of serotonin and potassium
dichromate for chronic sodium overloading or restriction.]
Virchows Arch. Abt. A Path. Anat., 351: 68-82 (in German).
SCHULTHEISS, E. (1959) [Rubber and eczema,], Aulendorf i.
Württemberg, Cantor, 213 pp (in German).
SCHWARZ, K. & MERTZ, W. (1959) Chromium (III) and the
glucose tolerance factor. Arch. Biochem. Biophys., 85: 292-295.
SCHWARTZ, L. & DUNN, J.E. (1942) Dermatitis occurring among
operators of air conditioning equipment. Ind. Med. Surg., 11:
375.
SCHWARTZ, L., TULIPAN, L., & PECK, S. (1947) Occupational
diseases of the skin, 2nd ed., Philadelphia, Pennsylvania, Lea
& Febiger.
SEEBER, H., FIKENTSCHER, R., & ROSEBURG, B. (1976)
[Disorders of smell and taste in chromium dye workers.] Z.
Gesamte Hyg. Grenzgeb., 22: 820-822 (in German).
SEELEY, J.L. & SKOGERBOE, R.K. (1974) Combined sampling-
analysis method for the determination of trace elements in
atmospheric particulates. Anal. Chem., 46: 415-421.
SEELING, W., GRUENERT, A., KIENLE, K.H., OPFERKUCH, R., &
SWOBODNIK, M. (1979) [Determination of chromium in human
serum and plasma by flameless atomic absorption spectro-
photometry.] Z. anal. Chem., 299: 368-374 (in German).
SEITZ, W.R., SUYDAM, W.W., & HERCULES, D.M. (1972)
Determination of trace amounts of chromium (III) using
chemiluminescence analysis. Anal. Chem., 44(6): 957-963.
SHACKLETTE, H.T., SAUER, H.I., & MIESCH, A.T. (1970)
Geochemical environments and cardiovascular mortality rates in
Georgia, Washington DC, US Governmental Printing Office, 39 pp
(US Geological Survey Professional Paper No. 574-C).
SHAPCOTT, D., KHOURI, K., DEMERS, P.P., VOBECKY, J., &
VOBECKY, J. (1977) The measurement of volatile chromium in
biological materials. Clin. Biochem, 10: 178-180.
SHEFFET, A., THIND, J., MILLER, A.M., & LOURIA, D.B. (1982)
Cancer mortality in a pigment plant utilizing lead and zinc
chromates. Arch. environ. Health, 37: 44-52.
SHENDRIKAR, A.D. & WEST, P.A. (1974) A study of adsorption
characteristics of traces of chromium (III) and (VI) on
selected surfaces. Anal. Chim. Acta, 72: 91-96.
SHERMAN, L., GLENNON, J.A., BRECK, W.J., KLOMBERG, G.H., &
GORDON, E.S. (1968) Failure of trivalent chromium to improve
hyperglycemia in diabetes mellitus. Metabolism, 17: 439-442.
SHIMKIN, M.M. & LEITER, J. (1940) Induced pulmonary tumours
in mice. III. Role of chromic irritation in the production of
pulmonary tumours in strain A mice. J. Natl Cancer Inst., 1:
241-254.
SHMITOVA, L.A. (1978) In: [The impact of job-related factors
on specific function of the female organism,] Svedlovsk, pp.
105-108 (in Russian).
SHMITOVA, L.A. (1980) Gig. Tr. prof. zabol., 2:
33-35 (in Russian).
SIDI, E. & LONGUEVILLE, R. (1953) Dermites au ciment. Rôle
des sels de chrome. Arch. Mal. prof., 14: 41.
SIEGENTHALER, U., LAINE, A., POLAK, L. (1983) Studies on
contact sensitivity to chromium in the guinea-pig. The role
of valence in the formation of the antigenic determinant. J.
Invest. Dermatol., 80: 44-47.
SIMONOFF, M., LLABADOR, Y. HAMON, C., PEERS; A.M., & SIMONOFF,
G.N. (1984) Low plasma chromium in patients with coronary
artery and heart diseases. Biol. Trace Element Res., 6:
431-439.
SIMONDS, J.P. & HEPLER, O.E. (1945) Experimental nephro-
pathies. I. A method of producing controlled selective injury
of renal units by means of chemical agents. Arch. Path., 39:
103-108.
SIROVER, M.A. & LOEB, L.A. (1976) Infidelity of DNA
synthesis in vitro: screening for potential metal mutagens or
carcinogens. Science, 194: 1434-1436.
SJOGREN, B. (1980) A retrospective cohort study of mortality
among stainless steel welders. Scand. J. Work. environ.
Health, 6: 197-200.
SKOGERBOE, R.K. (1974) Monitoring trace metal particulates:
an evaluation of the sampling and analysis problems. In:
Instrumentation for monitoring air quality, Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp.
125-136.
SLUIS-CREMER, G.K. & DU TOIT, R.S. (1968) Pneumoconiosis in
chromite miners in South Africa. Br. J. ind. Med., 25: 63-67.
SPIER, H.W. (1956) [Wood preservatives and chromate eczema.]
Desinfekt. Gesundheitswes., 4: 58 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil ( Myriophyllum spicatum L.). Arch.
environ. Contam. Toxicol., 2: 331-341.
STAUB, H.W., REUSSNER, G., & THIESSEN, R., Jr (1969) Serum
cholesterol reduction by chromium in hypercholesteremic rats.
Science, 166: 746-747.
STEELE, N.C. & ROSEBROUGH, R.W. (1979) Trivalent chromium
and nicotinic acid supplementation for the turkey poult.
Poultry Sci., 58: 983-984.
STEELE, N.G., ALTHEN, T.G., & FROBISH, L.T. (1977a) Bio-
logical activity of glucose tolerance factor in swine. J.
anim. Sci., 45: 1341-1345.
STEELE, N.C., PELURA, I., & FROBISH, L.T. (1977b) Effect of
dietary protein source on glucose utilization and hepatic
insulin-receptor characteristics of the rat. Nutr. Rep. Int.,
15: 429-435.
STELLA, M., MENTALDI, A., ROSSI, R., ROSSI, G., & LEVIS, A.G.
(1982) Clastogenic effects of chromium on human lymphocytes
in vitro and in vivo. Mutat. Res., 101: 151-164.
STEFFEE, C.H. & BAETJER, A.M. (1965) Histopathologic effects
of chromate chemicals. Report of studies in rabbits, guinea-
pigs, rats, and mice. Arch. environ. Health, 11: 66-75.
STEINHOFF, D., GAD, S.C., HATFIELD, G.K., & MOHR, U. (1983)
Testing sodium dichromate and soluble calcium chromate for
carcinogenicity in rats. Report to the Industrial Health
Foundation, Wuppertal, Bayer AG Institute of Toxicology,
126 pp.
STEREKHOVA, N.P., ZELENEVA, N.I., SOLOMINA, S.N.,
TYUSHNYAKOVA, N.V., MYASNIKOVA, A.G., FOKINA, G.P., & YARINA,
A.L. (1978) [Gastric pathology in workers engaged in the
production of chromium salts.] Gig. Tr. prof. Zabol., 3: 19-23
(in Russian).
STERN, R.M. (1981) Process-dependent risk of delayed health
effects for welders. Environ. Health Perspect., 41: 235-253.
STERN, R.M. (1982) Chromium compounds: production and
occupational exposure. In: Langard, S., ed. Biological and
environmental aspects of chromium, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 5-47.
STRIK, J.J.T.W.A., DE IONGH, H.H., VAN RIJN VAN ALKEMADE,
J.W.A., & WIUTE, T.P. (1975) Toxicity of chromium (VI) in
fish, with special reference to organoweights, liver and
plasma enzyme activities, blood parameters, and histological
alterations. In: Koeman, S.H. & Strik, J.J.T.W.A., ed.
Sublethal effects of toxic chemicals on aquatic animals,
Amsterdam, New York, Oxford, Elsevier Science Publishers,
pp. 31-41.
STROUT, R.W. (1977) The relationship of abnormal circulating
insulin levels to atherosclerosis. Atherosclerosis, 27: 1-13.
SULLIVAN, R.J. (1969) Air pollution aspects of chromium and
its compounds, Raleigh, North Carolina, US Department of
Health, Education and Welfare, 75 pp (National Air Pollution
Control Administration Publication No. APTD 69-34).
SUSA, N., FURUKAWA, YO., & TSUBAKI, S. (1977) Comparative
cytotoxic effects of hexavalent and trivalent chromium
compounds on cultured cells. Jap. J. vet. Sci., 39: 325-336.
SUZUKI, K., ESASHI, T., SUZUE, R., NISHIMURA, H., & MATSUMOTO,
H. (1979) Tissue concentrations of cadmium, lead, and
chromium in Japanese ranging from the fetus to elder subjects.
Toxicol. Lett., 4: 481-484.
SWAINE, D.J. & MITCHELL, R.L. (1960) Trace element
distribution in soil profiles. J. soil Sci., 11 (2): 347-368.
SWENSSON, A. (1977) [Experimental research on the
fibrogenetic effect of chromite.] Arbete och Haelsa, 2: 1-14
(in Swedish).
TABOR, E.C. & WARREN, W.V. (1958) Distribution of certain
metals in the atmosphere of some American cities. Arch. ind.
Hyg., 17: 145-151.
TAMARO, M., BANFI, E., VENTURINI, S., & MONTI-BRAGADIN, C.
(1975) [Hexavalent chromium compounds are mutagens for
bacteria.] In: Proceedings of the XVII Congress Naz. Soc. It.
Microbiol., Padova, pp. 411-415 (in Italian).
TANDON, S.K., SAXENA, D.K., GAUR, J.S., & CHANDRA, S.W.
(1978) Comparative toxicity of trivalent and hexavalent
chromium. Environ. Res., 15: 90-99.
TANDON, S.K., BEHARI, J.R., & KACHRU, D.N. (1979) Distri-
bution of chromium in poisoned rats. Toxicology, 13: 29-34.
TANDON, S.K., MATHUR, A.K., & GAUR, J.S. (1977) Urinary
excretion of chromium and nickel among electroplaters and
pigment industry workers. Int. Arch. occup. environ. Health,
40: 71-76.
TATARU, C., MARINSECU, A., CAPUSAN, I., RIPAN, R., & LITEANU,
C. (1957) [Skin damage during the industrial recovery of
potassium dichromate.] Berufsdermatosen, 5: 283-295 (in
German).
TAYLOR, A.E., GUYTON, M.O., & BISHOP, V.S. (1965)
Permeability of the alveolar membrane to solutes. Circ. Res.,
16: 353-362.
TAYLOR, F.G., Jr, MANN, L.K., DAHLMANN, R.C., & MILLER, F.L.
(1975) Environmental effects of chromium and zinc in
cooling-water drift. In: Cooling tower environment, 1974,
Washington DC, US Energy Research and Development
Administration, pp. 408-426 (Report Conf. No. 740302).
TAYLOR, F.H. (1966) The relationship of mortality and
duration of employment as reflected by a cohort of chromate
workers. Am. J. public Health, 56: 218-229.
TAZELAAR, D.J. (1970) Hypersensitivity to chromium in a
light-blue Tattoo. Dermatologica (Basel), 141: 282-287.
THOMSON, A.G. (1982) Chromite, Mining Annual Review,
pp. 70-71.
THORMANN, J., JESPERSEN, N.B., & JOENSEN, H.D. (1979)
Persistence of contact allergy to chromium. Contact Dermatit.,
5: 261-264.
TOEPFER, E.W., MERTZ, W., ROGINSKI, E.E., & POLANSKY, M.M.
(1973) Chromium in foods in relation to biological activity.
J. agric. food Chem., 21: 69-73.
TOEPFER, E.W., MERTZ, W., POLANSKY, M.M., ROGINSKI, E.E., &
WOLF, W.R. (1977) Preparation of chromium-containing
material of glucose tolerance factor activity from brewer's
yeast extracts and by synthesis. J. agric. food Chem., 25:
162-166.
TOLA, S., KILPIO, J., VIRTAMO, M., & HAAPA, K. (1977)
Urinary chromium as an indicator of the exposure of welders to
chromium. Scand. J. Work environ. Health, 3: 192-202.
TOLG, G. (1974) Recent problems and limitations in the
analytical characterization of high-purity material. Talanta,
21: 327-345.
TSUDA, H. & KATO, K. (1976) Potassium dichromate: induced
chromosome aberrations and its control with sodium sulfite in
hamster embryonic cells in vitro. Gann, 67: 469-470.
TSUDA, H. & KATO, K. (1977) Chromosomal aberrations and
morphological transformation in hamster embryonic cells
treated with potassium dichromate in vitro. Mutat. Res., 46:
87-94.
TUMAN, R.W. & DOISY, R.J. (1977) Metabolic effects of the
glucose tolerance factor (GTF) in normal and genetically
diabetic mice. Diabetes, 26: 820-826.
TUMAN, R.W., BILBO, J.T., & DOISY, R.J. (1978) Comparison
and effects of natural and synthetic glucose tolerance factor
in normal and genetically diabetic mice. Diabetes, 27: 49-56.
TURNER, M.A. & RUST, R.H. (1971) Effects of chromium on
growth and mineral nutrition of soybeans. Soil Sci. Soc. Am.
Proc., 35: 755-758.
TYUSHNYAKOVA, N.V., ZISLIN, D.M., SHABYNINA, N.K., & BELJAEVA,
L.N. (1974) [Criteria of occupational cancer in the
production of chromium salts and some ideas concerning its
pathogenesis.] In: Occupational cancer (collection of
scientific works), Sverdlovsk, pp. 56-63 (in Russian).
UMEDA, M. & NISHIMURA, M. (1979) Inducibility of chromosomal
aberrations by metal compounds in cultured mammalian cells.
Mutat. Res., 67: 221-229.
URCHS, O. (1953) [Occupational skin diseases,] Cantor,
Aulendorf i. Württemberg, 108 pp.
US DHEW (1975) Criteria for a recommended standard.
Occupational exposure to chromium (VI), Washington DC, US
Department of Health, Education and Welfare, 200 pp (US DHEW
Publication No. NIOSH 76-129).
US DHEW (1977) Occupational diseases: a guide to their
recognition, revised ed., Washington DC, US Department of
Health, Education and Welfare, pp. 352-355 (Stock No.
017-033-00266-5).
US EPA (1978) Reviews of the environmental effects of
pollutants. III. Chromium, Washington DC, US Environmental
Protection Agency, 285 pp (ORNL/EIS-80; EPA 600/1-78-023).
US EPA (1980) Ambient water quality criteria for chromium,
Washington DC, US Environmental Protection Agency, pp. A1-C48.
US NAS (1974a) Chromium, Washington DC, US National Academy
of Sciences, 155 pp.
US NAS (1974b) Geochemistry and the environment. I. The
relation of selected trace elements to health and disease,
Washington DC, US National Academy of Sciences, 113 pp.
US NAS/NAE (1972) Water quality criteria 1972, Washington
DC, US Environmental Protection Agency, US National Academy of
Sciences, US National Academy of Engineering, 533 pp.
US PHS (1953) Health of workers in chromate producing
industry, Washington DC, US Department of Health, Education
and Welfare, US Public Health Service, 131 pp (Publication No.
192).
VAKHRUSHEVA, V.A. (1960) [Effect of Mn and Cr as trace
elements on the level of blood sugar in white mice.] Tr.
Izhevskogo Otd. Fiziol. Obschchestva, 1: 184-190 (in Russian).
VALER, M. & RACZ, J. (1971) Investigations concerning the
sensitizing effect of trivalent chromium salts. I. Correlation
between concentration and skin penetration of trivalent
chromium and between the number of positive results obtained
in patch tests. J. ind. Med., 41(12): 302-317.
VEILLON, C., WOLF, W.R., GUTHRIE, B.E. (1979) Determination
of chromium in biological materials by stable isotope
dilution. Anal. Chem., 51: 1022-1024.
VELICHKOVSKY, B.T. & POKROVSKAJA, L.V. (1973) [Labour
hygiene in a modern production of multy-tonnage ferrolloys.]
In: [Proceedings of the National Symposium on Labor Hygiene
and Prophylaxis of Occupational Diseases in the Production of
Ferroalloys,] Sverdlovsk, pp. 3-8 (in Russian).
VENITT, S. & LEVY, L.S. (1974) Mutagenicity of chromates in
bacteria and its relevance to chromate carcinogenesis. Nature
(Lond.), 250: 493-494.
VENCGUJIV, A.J. (1974) Zones of display of biological and
pharmacological action of trace elements. In: Hoekstra, W.G.,
Gantherl, H.E., & Mertz, W., ed. Trace elements metabolism in
animals. II, Baltimore, London, Tokyo, University Park Press,
pp. 295-310.
VERFAILLIE, G.R.M. (1974) Kinetics of chromium absorption by
intact rice plants. In: Comparative studies of food and
environmental contamination, Vienna, International Atomic
Energy Agency, pp. 315-331.
VERSIECK, J.M.J. & SPEECKE, A.B.H. (1972) Contaminations
induced by collection of liver biopsies and human blood. In:
Nuclear Activation Techniques in the Life Sciences.
Proceedings of a Symposium, Bled, Yugoslavia, Vienna
International Atomic Energy Agency, pp. 39-49.
VERSIECK, I., HOSTE, J., BARBIER, F., STEYAERT, H., DERUDDER,
I., & MICHELS, H. (1978) Determination of chromium and
cobalt in human serum by neutron activation analysis. Clin.
Chem., 24: 303-308.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experience of the
occupational health department with some industrial threshold
limit values for industrial poisons.] Arch. Gewerbepath.
Gewerbehyg., 13: 528-534 (in German).
VISEK, W.J., WHITNEY, I.B., KUHN, U.S.G., & COMAR, C.L.
(1953) Metabolism of 51Cr by animals as influenced by
chemical state. Proc. Soc. Exp. Biol. Med., 84: 610-615.
VOELKL, A. (1971) [The daily excretion of chromium in normal
persons.] Zentralbl. Arbeitsmed., 21: 122 (in German).
VOTAVA, H.J., HAHN, C.J., & EVAN, G.W. (1973) Isolation and
partial purification of a 51Cr complex from brewer's yeast.
Biochem. Biophys. Res. Comm., 55: 312-319.
WACKER, W.E.C. & VALLEE, B.L. (1959) Nucleic acids and
metals. I. Chromium mangenese, nickel, iron, and other metals
in ribonucleic acid from diverse biological sources. J. biol.
Chem., 234: 3257-3262.
WAHBA, A. & COHEN, T. (1979) Chrome sensitivity in Israel.
Contact Dermatit., 5: 101-107.
WAHLBERG, J.E. & WENNERSTEN, G. (1977) Light sensitivity and
chromium dermatitis. Br. J. Dermatol., 97: 411-416.
WALSH, E.N. (1953) Chromate hazards in industry. J. Am. Med.
Assoc., 153: 1305-1308.
WARREN, G., SCHULTZ, P., BANCROFT, D., BENNETT, K., ABBOTT,
E.H., & ROGERS, S. (1981) Mutagenicity of a series of
hexacoordinate chromium (III) compounds. Mutat. Res., 90:
111-118.
WATANABE, S. & FUKUCHI, Y. (1975) An epidemiological survey
on lung cancer in workers of a chromate-producing industry in
Hokkaido, Japan (Presented at: The XVIII International Congress
on Occupational Health, Brighton, England, 14-19 September.
WEILER, R.R. & CHAWLA, V.K. (1969) Dissolved mineral and
quality of Great Lakes waters. In: Proceedings of the 12th
Conference on Great Lakes Res., 801.
WELZ, B. (1980) [Use of AAS and ICP. Relative advantage of
each method.] Chem. Techn., 9: 161-170. (in German).
WELZ, B. (1983) [Atomic absorption spectrometry,] 3rd ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
527 pp (in German).
WENDELBERGER, J. & WEITGASSER, H. (1956) [The role of alkali
and degreasing agents in the development of contact eczemas in
electroplasters.] Berufsdermatosen, 4: 16-21 (in German).
WHANGER, P.D. & WESWIG, P.H. (1975) Effects of selenium,
chromium, antioxidants on growth, eye cataracts, plasma
cholesterol, and blood glucose in selenium-deficient, vitamin
E-supplemented rats. Nutr. Rep. Int., 12: 345-348.
WHITE, L.R., JAKOBSEN, K., & 0STGAAD, K. (1979) Comparative
toxicity studies of chromium rich welding fumes and chromium
on an established human cell line. Environ. Res., 20: 366-374.
WHITING, R.F., STICH, H.F., & KOROPATNICK, D.J. (1979) DNA
damage and DNA repair in cultured human cells exposed to
chromate. Chem.-biol. Interact., 26: 267-280.
WHO (1983) EHC 27: Guidelines on studies in environmental
e pidemiology, Geneva, World Health Organization, 351 pp.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984a)
Disposition of intratracheally administered chromium (III) and
chromium (VI) in rabbits. Toxicol. Lett., 22: 273-276.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984b) The
reduction of chromium (VI) to chromium (III) by glutathione:
an intracellular redox pathway in the metabolism of the
carcinogen chromate. Toxicology, 33: 341-348.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1985)
[Determination of chromium in human red blood cells. Basis for
a concept of biological monitoring.] Arbeitsmed. Sozialmed.
Praeventivmed., 20: 1-4 (in German).
WILD, D. (1978) Cytogenetic effects in the mouse of 17
chemical mutagens and carcinogens evaluated by the micronuclei
test. Mutat. Res., 56: 319-327.
WILLIAMS, C.D. (1969) Asthma related to chromium compounds.
Report of two cases and review of the literature on chromate
diseases. NC med. J., 30: 482-491.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1960) The Merck index. An encyclopedia of chemicals and
drugs, 7th ed., Rahway, New Jersey, Merck and Co., 139 pp.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) The Merck index. An encyclopedia of chemicals and
drugs, 9th ed., Rahway, New Jersey, Merck and Co., 1313 pp.
WINSTON, J.R. & WALSH, E.N. (1951) Chromate dermatitis in
railroad employees working with diesel locomotives. J. Am.
Med. Assoc., 147: 1133-1134.
WITHEY, J. (1983) Memorandum addressed to Si Duk Lee, dated
March 4, 1983, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office.
WOLF, W.R. (1976) Coupled gas chromatography/atomic
absorption spectrometry for the nanogram determination of
chromium. Anal. Chem., 48: 1717-1720.
WOLF, W.R. & GREENE, F.E. (1976) Preparation of biological
materials for chromium analysis. In: Accuracy in trace
analysis: sampling, sample handling, and analysis,
Gaithersburg, Maryland, National Bureau of Standards, pp.
605-610 (Special Publication No. 422).
WOLF, W.R., MERTZ, W., & MASIRONI, R. (1974) Determination
of chromium in refined and unrefined sugars by oxygen plasma
ashing flameless atomic absorption. J. agric. food Chem., 22:
1037-1042.
WOLF, W.R., TAYLOR, M.L., HUGHES, B.M., TIERNAN, T.O., &
SIEVERS, R.E. (1972) Determination of chromium and beryllium
at the picogram level by gas chromatography/mass spectrometry.
Anal. Chem., 44: 616-618.
WU, G.-Y. (1981) [The research on the purification and the
chemical structure of the specific chromium binding
materials.] Jpn. J. Ind. Health, 33: 505 (in Chinese).
YAGI, T. & NISHIOKA, H. (1977) DNA damage and its degrada-
tion by metal compounds. Sci. Eng. Rev. Doshisha Univ., 18:
63-70.
YANG, G.L. (1956) [An investigation of occupational disease
among chromium plating workers.] Acta Acad. Med. primae
Shanghai, 2: 36-46 (in Chinese).
YOPP, J.H., SCHMID, W.E., & HOLST, R.W. (1974) Determination
of maximum permissible levels of selected chemicals that exert
toxic effects on plants of economic importance in Illinois,
Chicago, Illinois, Institute for Environmental Quality,
pp. 89-96.
YUNISOVA, H.K. & PAVLOVSKAJA, G.S. (1975) The effect of
regimen of chrome plating on the properties of chromine.
Protect. Metal., 2, 248-250.
ZANDER, A.T., O'HAVER, T.C., & KELIHER, P.N. (1977)
Correction for interferences of spectral origin with continuum
source, echelle wavelength modulated atomic absorption
spectrometry. Anal. Chem., 49: 838-842.
ZHURKOV, U.S. (1981) [Approaches to the regulation of
environmental chemical pollutants with mutagenic activity.]
In: Sidorenko, G.I.M., ed. [Medical problems of environmental
protection,] Moscow, USSR Academy of Medical Sciences,
pp. 88-95 (in Russian).
ZOBER, A. (1979) [On the problems of evaluating bronchial
carcinoma after exposition to chromium compounds.] Int. Arch.
occup. environ. Health, 43(2): 107-121 (in German).
ZOBER, A. (1982) [Possible dangers to the respiratory tract
from welding fumer: methods of approach in an industrial
health care context and results.] Schweissen & Schneiden,
34(2): 77-81 (in German).
ZOBER, A., KICK, K., SCHALLER, K.H., SCHELLMANN, B., &
VALENTIN, H. (1984) ["Normal values" of chromium and nickel
in human lung-, kidney-, blood-, and urine samples.] Zbl.
Bakt. Hyg. I. Abt. Orig. B, 179: 80-95 (in German).
 4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental Levels
4.1.1. Air
Chromium concentrations in air vary with location. Background
levels determined at the South Pole ranged from 2.5 to 10 pg/m3 and
are believed to be due to the weathering of crustal material (US
NAS, 1974a). Data collected by the US National Air Sampling
Network in 1964 gave the national average concentration for
chromium in the ambient air as 0.015 µg/m3, varying from non-
measurable levels to a maximum of 0.35 µg/m3. Chromium
concentrations in most non-urban areas and even in many urban areas
were below detection levels. Yearly average concentrations for
cities in the USA varied from 0.009 to 0.102 µg/m3. Concentrations
ranging from 0.017 to 0.087 µg/m3 have been reported for Osaka,
Japan (US EPA, 1978). The chromium content of the air in the
vicinity of industrial plants may be higher. In 1973, the reported
chromium concentrations ranged from 1 to 100 mg/m3 for coal-fired
power plants, from 100 to 1000 mg/m3 for cement plants, from 10 to
100 mg/m3 for iron and steel industries, and from 100 to 1000 mg/m3
for municipal incinerators (US EPA, 1978). Ferrochromium plants
have the highest emission rates (Radian Corporation, 1983).
However, modern chromium chemical plants contribute very little to
pollution today, because of the installation of collecting
equipment that returns the material for reuse. Drift from cooling-
towers contributes to atmospheric pollution, when chromium is used
as a corrosion inhibitor (section 3.1.5). Little information
exists on the particle size distribution of chromium in the air.
The mass median diameter in a study in the United Kingdom was
1.5 µm (Cawse, 1974).
The chemical form of chromium in air depends on the source.
Chromium from metallurgical production is usually in the trivalent
or zero state. During chromate production, chromate dusts can be
emitted. Aerosols containing chromic acid can be produced during
the chrome-plating process; chromate is also the form found in air
contaminated by cooling-tower drift.
4.1.2. Water
Except for regions with substantial chromium deposits, the
natural content of chromium in surface waters and drinking-water is
very low, most of the samples containing between 1 and 10 µg/litre
(US NAS, 1974a). Substantially higher concentrations are almost
always the result of human activities, reflecting pollution from
industrial activities or sewage waste (Perlmutter & Lieber, 1970).
Thus, the chromium concentration in untreated surface water
supplies reflects the extent of the industrial activity in an area
(Table 6).
Table 6. Chromium in water supplies
-----------------------------------------------------------------------
Country Chromium Range Reference
concentration (µg/litre)
(µg/litre)
-----------------------------------------------------------------------
Bulgaria
flat district -a 7 - 8 Novakova et al.
hilly district - 60 - 215 (1974)
Canada
Great Lakes 1 0.2 - 19 Weiler & Chawla
(1969)
Ottawa River 0.01 - Durum & Haffty
(1963);
Merrit (1971)
China
Yellow River undetectable - Chen Bingheng
Tia-Ding country - 0 - 80 (personal
surface water communication, 1986)
Germany, Federal
Republic of
Rhine River 18 DeGroot & Allersma
(1973)
Poland
Wisla - 31 - 112 Pasternak (1973)
USA
Illinois River 21 5 - 38 Mathis & Cummings
(1973)
Lake Tahoe < 0.07 - Bond et al. (1973)
Mississipi River 3 - 20 Bond et al. (1973)
New York area, 1250 - Lieber et al. (1964)
contaminated stream
-----------------------------------------------------------------------
a No data available.
Drinking-water from 100 public water supplies in the USA had a
median chromium content of 0.43 µg/litre, ranging from non-
detectable concentrations to 35 µg/litre. In the Federal Republic
of Germany, levels in about 90% of drinking-water samples from 1062
public water supplies were below 0.5 µg/litre; in 1.4% of the water
samples, levels exceeded the prescribed limit value of 50 µg/litre
(Kempf & Sonneborn, 1981).
Both trivalent and hexavalent forms of chromium occur in water.
National and international drinking-water standards reject
drinking-water containing hexavalent chromium concentrations of
more than 50 µg/litre. Such high concentrations occur naturally,
only in areas with substantial chromium deposits (Novakova et al.,
1974); in all other regions they would be caused by industrial
wastes.
4.1.3. Food
The available food data (Schroeder et al., 1962; Schlettwein-
Gsell & Mommsen-Straub, 1971; Toepfer et al., 1973; Kumpulainen et
al., 1979) indicate a range of the chromium concentrations in
different foodstuffs of 5 -250 mg/kg (Table 7). Highly refined
foods, such as sugar and flour of low extraction, contain the
lowest levels. Very high concentrations have been reported in
pepper (Schroeder et al., 1962) and brewer's yeast.
Table 7. Ranges of chromium concentrations in some
food groupsa
---------------------------------------------------
Food Chromium content
(µg/kg of wet weight)
Mean Range
---------------------------------------------------
Flour, refined < 20
Bran 50
Meat (beef, pork, chicken) 10 - 60
Fish, fresh < 10 - 10
Vegetables 5 - 30
Nuts 140
Whole Milk 10
Cheeses 10 - 130
Sugar, refined < 20
Egg yolk 200
---------------------------------------------------
a From: Koivistoinen (1980).
4.2. General Population Exposure
4.2.1. Food and water
The chromium intake from diet and water varies considerably
between regions (Table 8). However, these variations should be
interpreted with caution because not all the analyses have been
controlled by the use of standard reference material or proper
quality assurance procedures, and discrepancies in methods cannot
be completely discounted.
Table 8. Chromium intake from diet and water
--------------------------------------------------------------------
Region Chromium Remarks Reference
intake
(µg/day)
(range)
--------------------------------------------------------------------
Canada 189 - Canada, National
(136 - Health and Welfare
282) (1980)
Germany, Federal 62 DAa; 4 subjects, Schelenz (1977)
Republic of (11 - 1 week
195)
Japan 723 urban adults Murakami et al.
(202 - (1965)
1710)
943 rural adultsb Murakami et al.
(> 180 - (1965)
1190)
New Zealand 81 ± 32 DAa; 11 women, Guthrie (1973)
(39 - 190) self-selected
diets
United Kingdom (80 - 100) Facer, J.L.
(1983)c
USA 52 DAa Levine et al.
(5 - 115) (1968)
USA 78 ± 42 DA; 28 diets, Kumpulainen
(25 - 224) complete et al.
(controlled (1979)
by SRM)
USSR
Tatar (88 - 126) childrend Goncharov (1968)
--------------------------------------------------------------------
a DA = Direct analysis of composite diets as consumed.
b Analysis of composite of cooked servings for one complete day
collected from 10 families in different localities.
c Personal communication to Dr M. Mercier, IPCS (United Kingdom
Ministry of Agriculture, Fisheries and Food, London).
d Analysis of diets in kindergartens.
Most reported chromium intakes range from 50 to 200 µg/day.
However, a comparison of the chromium levels reported by different
investigators reveals substantial differences, some of which may be
due to the influence of the location where the foods were grown.
Only one study (Kumpulainen et al., 1979) was controlled by the use
of standard reference materials. The data should therefore be
treated as preliminary. Furthermore, data concerning total
chromium concentrations do not include information on the species
of chromium in the food and their biological availability. In an
attempt to estimate the biologically available chromium in food,
Toepfer et al. (1973) measured the effects of extracts from foods
on the potentiation of insulin action in epididymal fat tissue in
vitro. No correlation was found between the insulin potentiation
and the total chromium extracted from the foods by acid hydrolysis,
but a significant correlation ( P = 0.01) appeared between the
ethanol-extractable amount of chromium and biological activity.
The highest concentrations of ethanol-extractable chromium were
found in brewer's yeast, black pepper, calf liver, cheese, and
wheatgerm.
4.2.2. Other exposures
Since chromium compounds are increasingly present in products
used in daily life, chromium eczemas are often observed in the
general population. Polak et al. (1973) surveyed the most
important chromium-containing materials or objects: chromium ore,
baths, colours, lubricating oils, anti-corrosive agents, wood
preservation salts, cement, cleaning materials, textiles, and
leather tanned with chromium. According to Polak et al. (1973),
people who work with material containing mere traces of chromium
salts are more at risk than workers who come into contact with high
concentrations of chromium salts. Some less frequently occurring
cases include sensitization by tattooing (especially green and
light-blue)( Tazelaar, 1970), artificial dentures made of chromium-
containing steel, metal pins used for internal fixation of broken
bones, and bullets retained in the body (Langard & Hensten-
Pettersen, 1981).
4.3. Occupational Exposure
4.3.1. Inhalation exposure
In chromium ore mines, the concentration of dust in the air in
different work-places ranged from 1.3 to 16.9 mg/m3. In the
crushing and sorting factory, it varied from 6.1 to 148 mg/m3. The
chromium content in settled dust (calculated as Cr2O3) varied from
3.6 to 48%. During the period 1955-69, levels of trivalent
chromium in the dust in different work-places in ferro-alloy
factories ranged from 16 to 42%, while the concentrations of dust
in the air varied from 14 to 38 mg/m3 (Pokrovskaja & Shabynina,
1974).
In the past, the production of refined ferrochromium led to
high concentrations of dust in the air of the work-place (10 - 30
mg/m3) (Velichkovsky & Pokrovskaja, 1973). The concentrations of
hexavalent chromium after implementation of a number of sanitation
and hygienic measures were 0.03 - 0.06 mg/m3 (Velichkovsky &
Pokrovskaja, 1973).
In the manufacture of chromates, the oxidation state,
solubility, and composition of air-borne material varied in
different areas of the plant. Exposure in the ore-crushing area
was to trivalent, insoluble particulates; in the leaching area,
exposure was to tri- and hexavalent, soluble and insoluble
particulates and droplets; at the dry end of the process, the
workers were exposed to the very soluble hexavalent chromates in
particulate form, and to the insoluble residue after leaching
(Velichkovsky & Pokrovskaja, 1973). In plants using calcium in the
roasting process, the residue that is recycled contains, among
other products, calcium chromate, currently believed, on the basis
of animal studies, to be at least partly responsible for the
carcinogenicity of chromium.
Chromium plating of metal surfaces was accompanied by the
release of hexavalent chromium into the air in work premises, in
concentrations ranging from 0.04 to 0.4 mg/m3 (Yunisova &
Pavlovskaja, 1975). In one electroplating factory, the
concentration of chromic acid vapours in the air varied from 0.1 to
1.4 mg/m3 (Gomes, 1972). In the vicinity of 3 different baths in a
Swedish chromium plating factory, chromium concentrations ranged
from 20 to 46 µg chromium (VI)/m3, while, at another factory, the
exposure levels near all baths were below 1 µg/m3 (Lindberg et al.,
1985).
Occupational exposure to chromium during welding has been
analysed and the results published by several authors (Stern,
1981). Welding of metals using chromium and nickel electrodes
require high temperatures that melt both the material welded and
the electrode, producing a complex mixture of gases, oxides, and
other compounds, the chemistry of which is determined by the
technology, materials, and welding parameters used in each case
(Lautner et al., 1978). Hexavalent chromium compounds were found in
the respiratory zone of the welder at concentrations ranging from
3.8 to 6.6 µg/m3 (Migai, 1975). For the welding industry as a
whole, the average exposure arising from welding is not homogeneous
but depends on the type and conditions of the welding process
(Stern, 1982).
In a cement-producing factory, the concentration of hexavalent
chromium in the air in the work-place varied from 0.0047 to 0.008
mg/m3. The presence of chromium was explained by the fact that the
lining of the kilns was composed of chrome-magnesium bricks
containing 17 - 28% chromium compounds (Retnev, 1960). Forty-two
types of American cement were analysed for total chromium content
and particularly for hexavalent chromium. It was found that
hexavalent chromium was present in 18 out of 42 samples in
concentrations varying from 0.1 to 5.4 g/kg cement, while the total
chromium content ranged from 5 to 124 g/kg (Perone et al., 1974).
Analysis of 59 samples of Portland cement from 9 European countries
showed that the contents of hexavalent chromium extractable with
sodium sulfate varied from 1 to 83 g/kg of cement, while the total
chromium contents ranged from 35 to 173 g/kg (Fregert & Gruvberger,
1972).
4.3.2. Dermal exposure
Occupational dermal exposure can result in percutaneous
absorption and in harmful effects on the skin (section 8.3.1),
though the percutaneous absorption of chromium (III) sulfate has
been questioned by Aitio et al. (1984).
Chromium, especially chromate, is the most common contact
allergen and of great importance in occupational contact dermatitis
(Thormann et al., 1979).
Chromium eczema occurred most frequently in building labourers
followed by painters, galvanizers, machine drillers, metal-workers,
graphic artists, and workers in the timber, chemical, leather, and
textile industries (Polak et al., 1973). This is likely to reflect
the exposure to chromium compounds from a large number of every-day
products (section 4.2.2). The skin exposure to cement may be of
particular importance as building labourers belong to the most
affected group.
5. KINETICS AND METABOLISM
5.1. Absorption
5.1.1. Absorption through inhalation
5.1.1.1. Animal studies
Few animal studies have been performed to determine the
absorption of chromium compounds via inhalation. In one early
study, mice and rats were exposed to chromium particulates in an
inhalation chamber for various periods of time. The concentrations
of soluble chromium (CrO3) in air were between 1 and 2 mg/m3 for
the mice and 2 and 3 mg/m3 for the rats. The concentrations of
soluble versus insoluble chromium in the lung tissue of the mice
varied greatly. The soluble chromium concentrations ranged from
4.3 to 10.7 µg/kg dry tissue, after 100 weeks of exposure (Baetjer
et al., 1959a).
The amount of chromium that is absorbed through inhalation
depends on the size of the particles and droplets, on their
solubility in body fluids, and on their reaction with the
respiratory mucosa. Particles greater than 5 µm in diameter
(aerodynamic size) are deposited on the mucosal surface of the
nasal membrane, trachea, and bronchi and are carried by the action
of the cilia to the pharynx, where they are swallowed. Smaller
particles and droplets, especially those below 2 mm in size,
penetrate to the alveoli. Particles and droplets of soluble
compounds, such as hexavalent chromium compounds, are rapidly
absorbed in the blood. Insoluble particles, such as chromite, are
taken up by macrophages and slowly cleared. Soluble materials that
react with the constituents of the lung tissue, such as soluble
trivalent compounds, are also cleared slowly. Baetjer et al.
(1959b) were the first to describe the differences in the clearance
rates of soluble chromates and chromic chloride, when injected
intratracheally into the lungs of animals. The hexavalent chromate
was more rapidly transported from the lungs to other tissues than
the trivalent chromic chloride. Ten minutes after injection, only
15% chromium (IV) remained in the lung compared with 70% chromium
(III). After 60 days, the corresponding figures were 1.7% and 13%
(Baetjer et al., 1959b). Hexavalent chromium is taken up by the
red blood cells in much larger quantities than trivalent chromium.
This finding has been confirmed by Wiegand et al. (1984b)
performing intratracheal instillation (Na251CrO4) studies on
anaesthetized rabbits, as shown in Fig. 2. Confirmation of the
macrophage uptake of insoluble chromate was obtained by exposing
hamsters to 0.5 - 1 mg chromic oxide dust/m3 for 4 h. The median
diameter of the particles was 0.17 µm. Over 90% of the oxide was
found in the macrophages (Sanders et al., 1971).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental Levels
4.1.1. Air
Chromium concentrations in air vary with location. Background
levels determined at the South Pole ranged from 2.5 to 10 pg/m3 and
are believed to be due to the weathering of crustal material (US
NAS, 1974a). Data collected by the US National Air Sampling
Network in 1964 gave the national average concentration for
chromium in the ambient air as 0.015 µg/m3, varying from non-
measurable levels to a maximum of 0.35 µg/m3. Chromium
concentrations in most non-urban areas and even in many urban areas
were below detection levels. Yearly average concentrations for
cities in the USA varied from 0.009 to 0.102 µg/m3. Concentrations
ranging from 0.017 to 0.087 µg/m3 have been reported for Osaka,
Japan (US EPA, 1978). The chromium content of the air in the
vicinity of industrial plants may be higher. In 1973, the reported
chromium concentrations ranged from 1 to 100 mg/m3 for coal-fired
power plants, from 100 to 1000 mg/m3 for cement plants, from 10 to
100 mg/m3 for iron and steel industries, and from 100 to 1000 mg/m3
for municipal incinerators (US EPA, 1978). Ferrochromium plants
have the highest emission rates (Radian Corporation, 1983).
However, modern chromium chemical plants contribute very little to
pollution today, because of the installation of collecting
equipment that returns the material for reuse. Drift from cooling-
towers contributes to atmospheric pollution, when chromium is used
as a corrosion inhibitor (section 3.1.5). Little information
exists on the particle size distribution of chromium in the air.
The mass median diameter in a study in the United Kingdom was
1.5 µm (Cawse, 1974).
The chemical form of chromium in air depends on the source.
Chromium from metallurgical production is usually in the trivalent
or zero state. During chromate production, chromate dusts can be
emitted. Aerosols containing chromic acid can be produced during
the chrome-plating process; chromate is also the form found in air
contaminated by cooling-tower drift.
4.1.2. Water
Except for regions with substantial chromium deposits, the
natural content of chromium in surface waters and drinking-water is
very low, most of the samples containing between 1 and 10 µg/litre
(US NAS, 1974a). Substantially higher concentrations are almost
always the result of human activities, reflecting pollution from
industrial activities or sewage waste (Perlmutter & Lieber, 1970).
Thus, the chromium concentration in untreated surface water
supplies reflects the extent of the industrial activity in an area
(Table 6).
Table 6. Chromium in water supplies
-----------------------------------------------------------------------
Country Chromium Range Reference
concentration (µg/litre)
(µg/litre)
-----------------------------------------------------------------------
Bulgaria
flat district -a 7 - 8 Novakova et al.
hilly district - 60 - 215 (1974)
Canada
Great Lakes 1 0.2 - 19 Weiler & Chawla
(1969)
Ottawa River 0.01 - Durum & Haffty
(1963);
Merrit (1971)
China
Yellow River undetectable - Chen Bingheng
Tia-Ding country - 0 - 80 (personal
surface water communication, 1986)
Germany, Federal
Republic of
Rhine River 18 DeGroot & Allersma
(1973)
Poland
Wisla - 31 - 112 Pasternak (1973)
USA
Illinois River 21 5 - 38 Mathis & Cummings
(1973)
Lake Tahoe < 0.07 - Bond et al. (1973)
Mississipi River 3 - 20 Bond et al. (1973)
New York area, 1250 - Lieber et al. (1964)
contaminated stream
-----------------------------------------------------------------------
a No data available.
Drinking-water from 100 public water supplies in the USA had a
median chromium content of 0.43 µg/litre, ranging from non-
detectable concentrations to 35 µg/litre. In the Federal Republic
of Germany, levels in about 90% of drinking-water samples from 1062
public water supplies were below 0.5 µg/litre; in 1.4% of the water
samples, levels exceeded the prescribed limit value of 50 µg/litre
(Kempf & Sonneborn, 1981).
Both trivalent and hexavalent forms of chromium occur in water.
National and international drinking-water standards reject
drinking-water containing hexavalent chromium concentrations of
more than 50 µg/litre. Such high concentrations occur naturally,
only in areas with substantial chromium deposits (Novakova et al.,
1974); in all other regions they would be caused by industrial
wastes.
4.1.3. Food
The available food data (Schroeder et al., 1962; Schlettwein-
Gsell & Mommsen-Straub, 1971; Toepfer et al., 1973; Kumpulainen et
al., 1979) indicate a range of the chromium concentrations in
different foodstuffs of 5 -250 mg/kg (Table 7). Highly refined
foods, such as sugar and flour of low extraction, contain the
lowest levels. Very high concentrations have been reported in
pepper (Schroeder et al., 1962) and brewer's yeast.
Table 7. Ranges of chromium concentrations in some
food groupsa
---------------------------------------------------
Food Chromium content
(µg/kg of wet weight)
Mean Range
---------------------------------------------------
Flour, refined < 20
Bran 50
Meat (beef, pork, chicken) 10 - 60
Fish, fresh < 10 - 10
Vegetables 5 - 30
Nuts 140
Whole Milk 10
Cheeses 10 - 130
Sugar, refined < 20
Egg yolk 200
---------------------------------------------------
a From: Koivistoinen (1980).
4.2. General Population Exposure
4.2.1. Food and water
The chromium intake from diet and water varies considerably
between regions (Table 8). However, these variations should be
interpreted with caution because not all the analyses have been
controlled by the use of standard reference material or proper
quality assurance procedures, and discrepancies in methods cannot
be completely discounted.
Table 8. Chromium intake from diet and water
--------------------------------------------------------------------
Region Chromium Remarks Reference
intake
(µg/day)
(range)
--------------------------------------------------------------------
Canada 189 - Canada, National
(136 - Health and Welfare
282) (1980)
Germany, Federal 62 DAa; 4 subjects, Schelenz (1977)
Republic of (11 - 1 week
195)
Japan 723 urban adults Murakami et al.
(202 - (1965)
1710)
943 rural adultsb Murakami et al.
(> 180 - (1965)
1190)
New Zealand 81 ± 32 DAa; 11 women, Guthrie (1973)
(39 - 190) self-selected
diets
United Kingdom (80 - 100) Facer, J.L.
(1983)c
USA 52 DAa Levine et al.
(5 - 115) (1968)
USA 78 ± 42 DA; 28 diets, Kumpulainen
(25 - 224) complete et al.
(controlled (1979)
by SRM)
USSR
Tatar (88 - 126) childrend Goncharov (1968)
--------------------------------------------------------------------
a DA = Direct analysis of composite diets as consumed.
b Analysis of composite of cooked servings for one complete day
collected from 10 families in different localities.
c Personal communication to Dr M. Mercier, IPCS (United Kingdom
Ministry of Agriculture, Fisheries and Food, London).
d Analysis of diets in kindergartens.
Most reported chromium intakes range from 50 to 200 µg/day.
However, a comparison of the chromium levels reported by different
investigators reveals substantial differences, some of which may be
due to the influence of the location where the foods were grown.
Only one study (Kumpulainen et al., 1979) was controlled by the use
of standard reference materials. The data should therefore be
treated as preliminary. Furthermore, data concerning total
chromium concentrations do not include information on the species
of chromium in the food and their biological availability. In an
attempt to estimate the biologically available chromium in food,
Toepfer et al. (1973) measured the effects of extracts from foods
on the potentiation of insulin action in epididymal fat tissue in
vitro. No correlation was found between the insulin potentiation
and the total chromium extracted from the foods by acid hydrolysis,
but a significant correlation ( P = 0.01) appeared between the
ethanol-extractable amount of chromium and biological activity.
The highest concentrations of ethanol-extractable chromium were
found in brewer's yeast, black pepper, calf liver, cheese, and
wheatgerm.
4.2.2. Other exposures
Since chromium compounds are increasingly present in products
used in daily life, chromium eczemas are often observed in the
general population. Polak et al. (1973) surveyed the most
important chromium-containing materials or objects: chromium ore,
baths, colours, lubricating oils, anti-corrosive agents, wood
preservation salts, cement, cleaning materials, textiles, and
leather tanned with chromium. According to Polak et al. (1973),
people who work with material containing mere traces of chromium
salts are more at risk than workers who come into contact with high
concentrations of chromium salts. Some less frequently occurring
cases include sensitization by tattooing (especially green and
light-blue)( Tazelaar, 1970), artificial dentures made of chromium-
containing steel, metal pins used for internal fixation of broken
bones, and bullets retained in the body (Langard & Hensten-
Pettersen, 1981).
4.3. Occupational Exposure
4.3.1. Inhalation exposure
In chromium ore mines, the concentration of dust in the air in
different work-places ranged from 1.3 to 16.9 mg/m3. In the
crushing and sorting factory, it varied from 6.1 to 148 mg/m3. The
chromium content in settled dust (calculated as Cr2O3) varied from
3.6 to 48%. During the period 1955-69, levels of trivalent
chromium in the dust in different work-places in ferro-alloy
factories ranged from 16 to 42%, while the concentrations of dust
in the air varied from 14 to 38 mg/m3 (Pokrovskaja & Shabynina,
1974).
In the past, the production of refined ferrochromium led to
high concentrations of dust in the air of the work-place (10 - 30
mg/m3) (Velichkovsky & Pokrovskaja, 1973). The concentrations of
hexavalent chromium after implementation of a number of sanitation
and hygienic measures were 0.03 - 0.06 mg/m3 (Velichkovsky &
Pokrovskaja, 1973).
In the manufacture of chromates, the oxidation state,
solubility, and composition of air-borne material varied in
different areas of the plant. Exposure in the ore-crushing area
was to trivalent, insoluble particulates; in the leaching area,
exposure was to tri- and hexavalent, soluble and insoluble
particulates and droplets; at the dry end of the process, the
workers were exposed to the very soluble hexavalent chromates in
particulate form, and to the insoluble residue after leaching
(Velichkovsky & Pokrovskaja, 1973). In plants using calcium in the
roasting process, the residue that is recycled contains, among
other products, calcium chromate, currently believed, on the basis
of animal studies, to be at least partly responsible for the
carcinogenicity of chromium.
Chromium plating of metal surfaces was accompanied by the
release of hexavalent chromium into the air in work premises, in
concentrations ranging from 0.04 to 0.4 mg/m3 (Yunisova &
Pavlovskaja, 1975). In one electroplating factory, the
concentration of chromic acid vapours in the air varied from 0.1 to
1.4 mg/m3 (Gomes, 1972). In the vicinity of 3 different baths in a
Swedish chromium plating factory, chromium concentrations ranged
from 20 to 46 µg chromium (VI)/m3, while, at another factory, the
exposure levels near all baths were below 1 µg/m3 (Lindberg et al.,
1985).
Occupational exposure to chromium during welding has been
analysed and the results published by several authors (Stern,
1981). Welding of metals using chromium and nickel electrodes
require high temperatures that melt both the material welded and
the electrode, producing a complex mixture of gases, oxides, and
other compounds, the chemistry of which is determined by the
technology, materials, and welding parameters used in each case
(Lautner et al., 1978). Hexavalent chromium compounds were found in
the respiratory zone of the welder at concentrations ranging from
3.8 to 6.6 µg/m3 (Migai, 1975). For the welding industry as a
whole, the average exposure arising from welding is not homogeneous
but depends on the type and conditions of the welding process
(Stern, 1982).
In a cement-producing factory, the concentration of hexavalent
chromium in the air in the work-place varied from 0.0047 to 0.008
mg/m3. The presence of chromium was explained by the fact that the
lining of the kilns was composed of chrome-magnesium bricks
containing 17 - 28% chromium compounds (Retnev, 1960). Forty-two
types of American cement were analysed for total chromium content
and particularly for hexavalent chromium. It was found that
hexavalent chromium was present in 18 out of 42 samples in
concentrations varying from 0.1 to 5.4 g/kg cement, while the total
chromium content ranged from 5 to 124 g/kg (Perone et al., 1974).
Analysis of 59 samples of Portland cement from 9 European countries
showed that the contents of hexavalent chromium extractable with
sodium sulfate varied from 1 to 83 g/kg of cement, while the total
chromium contents ranged from 35 to 173 g/kg (Fregert & Gruvberger,
1972).
4.3.2. Dermal exposure
Occupational dermal exposure can result in percutaneous
absorption and in harmful effects on the skin (section 8.3.1),
though the percutaneous absorption of chromium (III) sulfate has
been questioned by Aitio et al. (1984).
Chromium, especially chromate, is the most common contact
allergen and of great importance in occupational contact dermatitis
(Thormann et al., 1979).
Chromium eczema occurred most frequently in building labourers
followed by painters, galvanizers, machine drillers, metal-workers,
graphic artists, and workers in the timber, chemical, leather, and
textile industries (Polak et al., 1973). This is likely to reflect
the exposure to chromium compounds from a large number of every-day
products (section 4.2.2). The skin exposure to cement may be of
particular importance as building labourers belong to the most
affected group.
5. KINETICS AND METABOLISM
5.1. Absorption
5.1.1. Absorption through inhalation
5.1.1.1. Animal studies
Few animal studies have been performed to determine the
absorption of chromium compounds via inhalation. In one early
study, mice and rats were exposed to chromium particulates in an
inhalation chamber for various periods of time. The concentrations
of soluble chromium (CrO3) in air were between 1 and 2 mg/m3 for
the mice and 2 and 3 mg/m3 for the rats. The concentrations of
soluble versus insoluble chromium in the lung tissue of the mice
varied greatly. The soluble chromium concentrations ranged from
4.3 to 10.7 µg/kg dry tissue, after 100 weeks of exposure (Baetjer
et al., 1959a).
The amount of chromium that is absorbed through inhalation
depends on the size of the particles and droplets, on their
solubility in body fluids, and on their reaction with the
respiratory mucosa. Particles greater than 5 µm in diameter
(aerodynamic size) are deposited on the mucosal surface of the
nasal membrane, trachea, and bronchi and are carried by the action
of the cilia to the pharynx, where they are swallowed. Smaller
particles and droplets, especially those below 2 mm in size,
penetrate to the alveoli. Particles and droplets of soluble
compounds, such as hexavalent chromium compounds, are rapidly
absorbed in the blood. Insoluble particles, such as chromite, are
taken up by macrophages and slowly cleared. Soluble materials that
react with the constituents of the lung tissue, such as soluble
trivalent compounds, are also cleared slowly. Baetjer et al.
(1959b) were the first to describe the differences in the clearance
rates of soluble chromates and chromic chloride, when injected
intratracheally into the lungs of animals. The hexavalent chromate
was more rapidly transported from the lungs to other tissues than
the trivalent chromic chloride. Ten minutes after injection, only
15% chromium (IV) remained in the lung compared with 70% chromium
(III). After 60 days, the corresponding figures were 1.7% and 13%
(Baetjer et al., 1959b). Hexavalent chromium is taken up by the
red blood cells in much larger quantities than trivalent chromium.
This finding has been confirmed by Wiegand et al. (1984b)
performing intratracheal instillation (Na251CrO4) studies on
anaesthetized rabbits, as shown in Fig. 2. Confirmation of the
macrophage uptake of insoluble chromate was obtained by exposing
hamsters to 0.5 - 1 mg chromic oxide dust/m3 for 4 h. The median
diameter of the particles was 0.17 µm. Over 90% of the oxide was
found in the macrophages (Sanders et al., 1971).
 5.1.1.2. Human data
A mean chromium concentration of 0.22 mg/kg wet weight was
found in the lung tissue of subjects from various locations in the
USA, but there was no correlation between chromium levels in the
lungs and those in the air (Schroeder et al., 1962).
A Committee of the National Research Council (US NAS, 1974a)
concluded: "It is unlikely that the intake from air under ordinary
conditions contributes significantly to the total intake of
available chromium; the intake from the air is calculated to be
less than 1 µg/day; but excessive exposure to airborne chromium
does result in some increased intake".
5.1.2. Absorption from the gastrointestinal tract
The absorption of ingested chromium compounds can be estimated
by measuring the amount of chromium excreted in the urine, as
almost all of intravenously injected chromium is excreted via the
urine and only 2% is found in the faeces. Although a potential loss
of endogenous chromium via the skin and its annexa has not yet been
measured and quantified, it can be stated that this organ, as well
as the gastrointestinal tract are of minor importance in the
excretion of endogenous chromium. The gastrointestinal tract is,
of course, the major organ for the excretion of exogenous chromium.
When considering the gastrointestinal absorption of chromium,
it is essential to recognize the substantial differences in the
efficiency of absorption of trivalent and hexavalent compounds.
These differences exist in both man and animals. Many trivalent
chromium compounds are so poorly absorbed that they have been used
as faecal markers in man and animals. The absorption of hexavalent
chromium, administered orally, was higher in all species examined,
but did not exceed 5% of the dose (Donaldson & Barreras, 1966). No
physiological regulation has yet been established for chromium
absorption.
5.1.2.1. Animal studies
The gastrointestinal absorption of chromate in rats has been
reported to be between 3 and 6% of a tracer dose (MacKenzie et al.,
1958; Byerrum, 1961). As in man, trivalent chromium compounds are
less well absorbed in the rat, with reported efficiencies ranging
from less than 0.5% (Visek et al., 1953) to 3% (Mertz et al.,
1965a). Within the category of trivalent compounds, there are
moderate differences in absorption, depending on the chemical form.
Binding of the chromium ion to suitable ligands, such as certain
organic acids, stabilizes the metal against precipitation in the
alkaline milieu of the intestines and increases absorption
efficiency by a factor of 3 - 5 times, compared with that for
chromium chloride. This has been shown for certain chelating
agents (Chen et al., 1973), a yet unidentified small peptide
complex isolated from yeast (Votava et al., 1973), and synthetic
glucose tolerance factor (GTF), a dinicotinic-acid-glutathione-
chromium complex (Mertz et al., 1974). Nothing is known about the
interaction of chromium with the flora of the gastrointestinal
tract. Absorption of chromium chloride by ruminant species is
similar to that in rats, with a mean efficiency of 0.76% (Anke et
al., 1971); laying hens have been found to absorb almost 15% of a
tracer dose of the element (Hennig et al., 1971).
5.1.2.2. Human studies
Donaldson & Barreras (1966) studied the gastrointestinal
absorption of hexavalent chromium by administering trace doses of
Na251CrO4 orally to 6 volunteer patients, who were hospitalized,
and by measuring the amount of radioactivity in the faeces and
urine. The mean urinary excretion, expressing the absorption
efficiency, was 2.1 ± 1.5% of the dose given. Administration by
jejunal infusion in 4 volunteers increased these values, suggesting
reduction of the chromate to trivalent compounds by the acid
content of the gastric juice. The same authors reported a mean
absorption efficiency of only 0.5 ± 0.3% for trivalent chromium,
administered as CrCl3 x 6H2O, with a range of 0.1 - 1.2%.
On the basis of the chromium content in diets (60 µg) and
chromium excretion (0.22 µg) in healthy subjects, Anderson et al.
(1983) calculated a minimum chromium absorption of about 0.4%.
Increasing intake by supplementation with chromium (chromic
chloride tablets, furnishing 200 µg chromium/day) led to an
excretion of 0.99 µg, equivalent to 0.4% of the intake.
Aitio et al. (1984) investigated the intake and urinary
excretion of chromium (III) in leather tanning workers. The
environmental concentrations were recorded as low, but chromium was
present in air in the form of large droplets that were not
collected by the standard air measurement technique. It was assumed
that the large droplets were cleared by the upper respiratory tract
and swallowed, and that the chromium in the droplets was absorbed
from the gastrointestinal tract. A calculation showed that this
would explain the urinary excretion levels. No absorption of
chromium through the skin was detected.
In a recent study, the minimum chromium absorption calculated
on the basis of urinary-chromium excretion was about 0.4%.
Increasing intake 5-fold, by chromium supplementation, led to a
nearly 5-fold increase in chromium excretion, suggesting that the
extent of absorption of supplemental inorganic chromium was similar
to that from normal dietary sources (Anderson et al., 1983a).
A similar absorption for trivalent chromium of 0.69% was
reported by Doisy et al. (1968) in healthy human subjects,
regardless of age. However, a group of 14 insulin-requiring
diabetic patients absorbed 4 times as much of the chromium dose as
the non-diabetic or maturity-onset diabetic subjects, as shown by
elevated levels of 51chromium in blood plasma and urine (Doisy et
al., 1971).
5.2. Distribution, Retention, Excretion
5.2.1. Animal studies
Most animal studies on chromium metabolism have been performed
on rats. From the site of intestinal absorption, chromium is taken
up by plasma-protein fractions. Small, physiological doses of
51chromium have been shown to bind almost entirely to the iron-
binding protein, transferrin (Hopkins & Schwarz, 1964). On the
other hand, inhaled chromium (Glaser et al., 1984) was bound to
albumin rather than to transferrin. With larger quantities of
trivalent chromium, non-specific binding to other proteins also
occurred, but not to the red blood cells. Visek et al. (1953)
measured the effects of the different chemical forms of chromium on
tissue distribution and found that soluble, chelated forms, such as
acetate or citrate complexes, were cleared quite rapidly, in
contrast with colloidal or protein-binding forms (chromite, chromic
chloride), which have a great affinity for the reticulo-endothelial
system (bone marrow, liver, spleen), and clear more slowly. The
blood clearance of hexavalent chromium, such as chromate, was slow,
because of irreversible binding within the red blood cells. Tissue
distribution of 51chromium, administered in nanogram doses to rats
was studied by Hopkins (1965). As in the preceeding studies, the
element accumulated in bone, spleen, testes, and epididymis; much
less was retained in the lungs, brain, heart, and pancreas. This
obvious difference in chromium distribution between man and rats is
unexplained.
As in man, trivalent 51chromium in the rat was rapidly cleared
from the blood, after absorption, and was retained by the tissues
(Mertz et al., 1965a). These tissue stores, labelled with
51chromium chloride, administered orally or intravenously, were not
immediately available for specific physiological functions. For
example, 51chromium, administered as CrCl3 x 6H2O to pregnant rats,
was not transported into the embryos (Mertz et al., 1969), nor did
any 51chromium appear in the blood in response to glucose or
insulin injections (Mertz & Roginski, 1971). The fact that fetal
chromium concentrations are low, when the pregnant rats are fed a
low-chromium Torula yeast diet, and increase when a high-chromium
natural stock ration is fed, indicates that placental transport and
possibly, the acute chromium response depend on a special form of
chromium, which is different from chromium chloride. It is
possible, but has not yet been proved, that this form is the
dinicotinic acid-glutathione-chromium complex, known as glucose
tolerance factor. Yeast extracts containing this factor labelled
with 51chromium have been shown to cross the placenta (Mertz et
al., 1969) and, in preliminary studies, to furnish chromium for the
acute chromium response (Mertz & Roginski, 1971).
With reference to interactions between chromium and other trace
elements, competition with iron by way of their common carrier
(transferrin) has been suggested in rats (Hopkins & Schwarz, 1964)
and in human beings (Sargent et al., 1979). Goncharov (1968)
reported a close interaction between chromium and dietary iodine.
In iodine-deficient white rats, addition of chromium to the diets
in amounts supplying from 0.6 to 600 µg/animal per day stimulated
thyroid function, as indicated by morphological and functional
changes. Conversely, chromium, in all but the lowest dose,
decreased thyroid function in animals receiving adequate iodine
levels. This relation is in agreement with epidemiological data
from the USSR (Goncharov, 1968).
5.2.2. Human data
5.2.2.1. Concentration in tissues, blood, urine, and hair
including possible biological indicators of exposure
(a) Tissues
The most comprehensive survey of tissue-chromium concentrations
is that of Schroeder et al. (1962), who carried out a
spectrographic analyses on 20 - 39 samples for each autopsy tissue,
all of which had been carefully collected to avoid extraneous
contamination. The following results were obtained (mean values in
mg/kg ash) for a group of subjects who had died between the ages of
30 and 40 years: lung, 15.6; aorta, 9.1; pancreas, 6.5; heart, 3.8;
testes, 3.1; kidney, 2.1; liver, 1.8; spleen, 1.7. In all tissues,
except for the lungs there was a rapid decline in chromium
concentrations from time of birth to the age of 10 years, followed
by a more gradual decrease to the age of 80 years. It cannot be
stated with certainty whether the decline is an expression of a
physiological mechanism or of a dietary deficiency. The lungs lost
their initially high chromium levels (85.2 mg/kg ash) up to the age
of 20 years (6.8 mg/kg); subsequently, the concentrations increased
to between 20 and 38 mg/kg. This discrepancy demonstrates that the
chromium in lungs is not in equilibrium with the general pool. The
decline of chromium in the aorta, quite pronounced in subjects in
the USA, was much less dramatic in the aortas from subjects of
other countries (Schroeder et al., 1970).
Mancuso & Hueper (1951), as well as Baetjer et al. (1959b),
found concentrations of chromium in the lungs of former chromate
workers that were several orders of magnitude higher than those in
control subjects. In a study on 16 chromate workers, including 11
with lung cancer, Baetjer et al. (1959b), using a colorimetric
method, observed a median concentration of water- and acid-soluble
chromium in the lung of 70 mg/kg dry weight and a median
concentration of acid-insoluble chromium of 17 mg/kg. The chromium
concentration did not differ between cancer cases and non-cancer
cases. The tissue specimens were obtained 0 - 23 years after the
termination of occupational exposure, which had lasted 1.5 - 42
years. With the use of emission spectrometry and atomic absorption
spectrophotometry, Hyodo et al. (1980) found a chromium content of
3.6 mg/kg wet weight in the lung in a male smoker with lung cancer,
who died 10 years after employment for 30 years in a chromate-
producing plant. The concentration in other tissues ranged from
0.05 (bone marrow) to 1.5 mg/kg (suprarenal gland). In five
unexposed controls, lung concentrations ranged from 0.09 to 0.88
mg/kg; concentrations in other tissues ranged from 0.003 (kidney)
to 0.156 mg/kg (suprarenal gland). The ratio of hexavalent to
total chromium in the lungs was 29% in the worker and 22.7 ± 10.6%
(mean ± SD) in the controls.
Brune et al. (1980) gave tissue concentrations for chromium and
other metals in the lung, liver, and kidney of 20 deceased copper
smelter workers, who had retired 0 - 19 years prior to death, and
of a control group (8 subjects). Tissue analysis was carried out
using neutron activation analysis as well as atomic absorption
spectrophotometry, and included a comparison with certified
reference samples (National Bureau Standards, bovine liver). In
the controls, the concentrations ranged from below detection (0.003
mg/kg wet weight) to 0.07 mg/kg in kidneys and 0.11 mg/kg in liver.
There was no marked difference between the chromium contents of
these 2 tissues and those in the exposed workers. On the other
hand, the lung concentrations were between 3 and 4 times higher in
the workers than in the controls (median levels, 0.29 and 0.08
mg/kg, respectively).
Chromium determinations were carried out on lung and kidney
samples of 45 autopsies from the Northern Bavaria area (Federal
Republic of Germany) (Zober et al., 1984). The analyses were
carried out using electrothermal AAS after wet oxidative digestion.
Median values of 0.097 mg/kg wet weight (range, 0.0006 - 1.230
mg/kg) in lung tissue and 0.0096 mg/kg wet weight (range, 0.0002 -
0.690 mg/kg) in kidney were found.
Very limited data are available on chromium levels in tissues
other than those referred to above. Shmitova (1978) estimated
chromium levels in fetal and placental tissues (abortive material),
derived on the 12th week of pregnancy, and found 92.8 and 30 µg
Cr/kg tissue, respectively.
It can be concluded that chromium may be retained in the lungs,
several years after the termination of occupational exposure.
However, it is not known whether this observation has any
biological relevance to the appearance of lung cancer. In this
context, it is of interest to note that chromium is retained in the
human lung for a relatively long period, even without occupational
exposure.
(b) Blood
The values reported by various investigators for chromium
concentrations in the blood of unexposed human beings range from
0.2 to 70 µg/litre in serum and plasma and 5 to 54 µg/litre in red
blood cells (US EPA, 1978). Insufficient evidence is available to
state whether the blood values in the normal population are
influenced by concentrations in the ambient air. The most
extensive determination of blood-chromium levels in chromate
workers was made by Mancuso (1951). Blood values during exposure
varied from 5 to 170 µg/litre and from 10 to 140 µg/litre, 74 days
after work ended. No decrease in blood-chromium levels was found,
even after 74 days without exposure.
Because of a selective affinity of hexavalent chromium for the
erythrocyte, substantially increased environmental exposure to
chromates is reflected in an increased ratio of the hexavalent
chromium level in red blood cells to that in plasma. Baetjer et
al. (1959a) found the following concentrations in 3 exposed
chromate workers: blood cells, 30, 54, and 140 µg/litre; plasma, 0,
20, and 17 µg/litre, respectively. In the absence of exposure to
chromate, the chromium concentrations in erythrocytes and plasma,
as serum, were nearly identical (Paixao & Yoe, 1959). Chromium
(VI) is incorporated into human red blood cells and remains there
over a long period of time (Wiegand et al., 1985), since the
approximate lifetime of human red cells is about 100 days. Exposure
to 2 mg trivalent chromium/day, for 3 months, resulted in an
increased concentration (0.2 mg/kg) in the red cells of 5 human
males, compared with 0.11 mg/kg in 5 controls without supplement
(Schroeder et al., 1962). However, later studies did not show any
increase in chromium concentrations in red cells, following
exposure to trivalent chromium (Beyersmann et al., 1984; Wiegand et
al., 1985). Monitoring of red blood cell-chromium may be a useful
indicator of exposure to hexavalent, but not to trivalent,
chromium.
Most of the later studies show that the true chromium
concentration in the plasma or serum of healthy subjects is of the
order of 1 µg/litre or less (Guthrie et al., 1978; Versieck et al.,
1978). Seeling et al. (1979) determined chromium in serum and
plasma using flameless AAS. This study was supported by measuring
a standard reference material (National Bureau of Standards, 1569
brewer's yeast, reference data: 2.12 ± 0.05 mg/kg, measured data:
2.3 ± 0.2 mg/kg). The chromium levels in serum ranged from 0.7 to
2.2 µg/litre and in plasma from 1 to 1.5 µg/litre (central
parameters of a long normal distribution).
Pooled serum samples of 6 healthy Finnish volunteers were
studied by Kumpulainen et al. (1983). A mean value of 0.11 ± 0.05
µg chromium/litre (range, 0.06 - 0.20) was found. Nomiyama et al.
(1980b) analysed 20 blood samples of Japanese subjects, using
direct flameless ASS, and reported a value of 2.9 ± 1.7 µg
chromium/litre whole blood.
Zober et al. (1984) analysed the blood of 45 autopsied subjects
from the Northern Bavaria area (Federal Republic of Germany). A
median chromium concentration of 2.8 µg/litre of postmortem blood
(range, 0.20 - 24 µg/litre) was found, the value was not influenced
by age or sex.
A reference material for chromium, bovine serum (RM 8419) is
available from the National Bureau of Standards, Washington DC,
USA.
(c) Urine
Chromium concentrations in the urine of non-occupationally
exposed subjects have been reported to range from 1.8 to 11
µg/litre (Imbus et al., 1963). Except for exposed persons and
juvenile diabetic patients, the reported values for daily chromium
excretion in urine (Table 9) do not differ as much as those for
blood. In later studies, such as that of Guthrie et al. (1979), a
method of flameless atomic absorption was used in which it was
possible to correct for spectral interference (Zander et al.,
1977). Such interference was difficult to eliminate with earlier
methods (Guthrie et al., 1978). Urine samples from 189 Japanese
volunteers, aged 10 - 80 years, in 4 pollution-free areas, were
analysed for chromium by Nomiyama et al. (1980a), using direct
flameless AAS. The average level was 0.4 ± 0.37 µg/litre (X ± SE)
or 0.47± 0.42 mg/kg creatinine. Urinary chromium tended to be
higher in males than in females and to decrease with age, but the
differences were not significant. Using electrothermal AAS, the
urinary excretion of various metals in non-occupationally exposed
adults was measured by Schaller & Zober (1982). For smokers, a
median value of 1.6 µg chromium/litre was found (non-smoker: 1.4 µg
chromium/litre). Commercially available urine samples were used
for quality control (Angerer et al., 1981). Although a "normal"
level of chromium excretion for healthy, unexposed persons cannot
yet be established with certainty, such a level may be less than 1
µg/day.
Several investigators have measured urinary-chromium excretion
in exposed workers. In a study on chromate workers, the urine
values ranged from 5 to 380 µg chromium/litre during exposure and
from 10 to 54 µg chromium/litre, 74 days after the end of exposure
(Mancuso, 1951). No relationship was evident between the urine
levels and the weighted average number of years of exposure. In
every case where urine values were recorded, both during, and 74
days after the end of, exposure, the chromium concentrations
decreased with time away from exposure.
A study of 12 workers in a galvanizing plant in the Federal
Republic of Germany showed an average concentration of chromium in
urine of 9.5 µg/litre (range, 1.4 - 24.6 µg/litre) compared with a
value of 1.8 ± 1.1 µg/litre in 60 unexposed workers (Schaller et
al., 1972). Gylseth et al. (1977) investigated a group of 14
welders exposed to about 0.05 mg chromium/m3 air, and reported a
urinary chromium concentration of approximately 40 mg/litre. At
the same exposure level, Tola et al. (1977) found a concentration
of 30 mg chromium/kg of creatinine in the urine. In both of these
studies, there was a correlation between recent exposure to
airborne chromium and chromium concentrations in urine. In the
study by Tola et al. (1977), it was shown that the water-soluble
fraction of airborne chromium was better correlated with the
concentration excreted in the urine than with total chromium. It
was also shown that the water-soluble fraction consisted mainly of
hexavalent chromium.
Table 9. Daily chromium excretion in urine
---------------------------------------------------------------------------
Subjects Number Excretion Range Reference
(µg/day) (µg/day)
(mean ± SD)
---------------------------------------------------------------------------
Adult males 2 0.72a 0.58 - 0.86 Schroeder et al.
(1962)
Adult males, fed 3 31.0a 20.8 - 46.5 Schroeder et al.
2 mg trivalent, (1962)
chromium/day for
3 months
Normal adults 16 13 ± 6 4 - 24 Voelkl (1971)
Chromate workerb 16 000
Normal adults 60 1.6 ± 1.1 Schaller et al.
(1972)
Galvano-technical 12 9.7 ± 6.6 1.4 - 24.6 Schaller et al.
workers (1972)
Normal young 20 8.4 ± 5.2 Hambidge (1974)
adults
Normal children, 18 5.5 ± 2.9 Hambidge (1974)
8 years old
Insulin-dependent 7 19.2 ± 18.9 Hambidge (1974)
diabetic children,
11 years old
Young women 9 7.2 ± 1.2 5.9 - 10.0 Mitman et al.
(1975)
Adult males 12 0.8 ± 0.4 0.4 - 1.8 Guthrie et al.
(1979)
---------------------------------------------------------------------------
Table 9. (contd.)
---------------------------------------------------------------------------
Subjects Number Excretion Range Reference
(µg/day) (µg/day)
(mean ± SD)
---------------------------------------------------------------------------
Adult males 91 0.48 ± 0.41a 0.42 - 0.53 Nomiyama et al.
(1980b)
Adult females 98 0.34 ± 0.31a 0.22 - 0.43 Nomiyama et al.
(1980b)
Adult males 48 0.20 ± 0.01a 0.05 - 0.58 Anderson et al.
(1982b)
Adult females 28
Adult males 27 0.17 ± 0.10 Anderson et al.
(1983a)
Adult females 15 0.20 ± 0.12 Anderson et al.
(1983a)
Adult males and 299 0.80 ± 0.6a 0.4 - 2.1 Fang (1983)
females
Normal adults 10 0.11 ± 0.05a 0.06 - 0.20 Kumpulainen et al.
(1983)
Normal adultsc 10 4.9 0.10 - 14.2 Zober et al.
(1984)
---------------------------------------------------------------------------
a Data calculated as µg/litre urine.
b Tanner, suffering from an acute ulceric gasteroenterocolitis.
c Samples taken post-mortem from autopsies. Original values are related
to mass (kg) instead of volume (litre).
Lindberg & Vesterberg (1983a) measured airborne, and urinary-
chromium levels among platers. Concentrations of chromium in urine
of < 5 µg/litre occurred when the time-weighted average values of
exposure were about or below 2 µg/m3 air. Severe damage to the
nasal septum and effects on lung function have not been found at
levels lower than this. It was shown that post-shift urinary-
chromium determinations could be used to monitor exposure in this
occupational group.
The urinary-chromium excretion and chromium clearance in 22
welders, who had been exposed to airborne hexavalent chromium (5 -
150 µg/m3) during 2 - 40 years (mean working time, 18.9 years) were
measured by Mutti et al. (1979). The method used was flameless
atomic absorption spectrometry. A highly significant correlation
was detected between the ratio of urinary-chromium to creatinine
and the airborne chromium concentration in the workplace, with
excretions ranging from 5.3 ± 3.7 to 33.3 ± 6.9 mg chromium/kg
creatinine in slightly exposed and heavily exposed welders,
respectively. The authors also reported an increase in chromium
clearance with increasing body burden of chromium, which indicates
that high urinary-chromium excretion may be caused by previous high
exposure as well as by current exposure.
Baseline data for chromium excretion in unexposed subjects in
the report by Mutti et al. (1979) are approximately 10 times higher
than the values recently proposed and generally accepted as normal.
However, there is reason (section 2.2.2) to accept relative
differences in analytical results in studies by one author using
one method, even if the absolute values reported may be questioned.
To test whether chromium excretion is also associated with
exercise-induced increases in glucose utilization, the urinary
chromium excretion, serum glucose, insulin, and glucagon of 9 male
runners (23 - 46 years old) were evaluated by Anderson et al.
(1982a). The mean urinary-chromium concentration was increased
nearly 5-fold, 2 h after running; excretion of sodium, potassium,
and calcium was unchanged. These data demonstrate an increase in
chromium excretion with exercise-induced increase in glucose
utilization.
(d) Milk
The chromium concentration was determined in 261 samples of
breast milk collected by manual expression from 45 American women.
Chromium levels were measured in whole, liquid milk by graphite-
furnace AAS, using the method of standard additions. The mean
chromium content of the breast milk samples was 0.30 µg/litre. The
range of individual values was 0.06 - 1.56 µg/litre and did not
change significantly with duration of lactation (Casey & Hambidge,
1984). Kumpulainen et al. (1983) analysed frozen samples of pooled
breast milk taken from women in different stages of lactation and
obtained from the Milk Bank of the Children's Hospital of Helsinki.
The mean chromium content ± SD was 0.49 ± 0.067 µg/litre (range,
0.37 - 0.57 µg/litre).
(e) Hair
Hair-chromium concentrations in children during the first 6
months of life were significantly higher than at any other age
(Hambidge & Baum, 1972); they declined from an initial value of
1493 µg/kg to an average of 412 µg/kg at 2 - 3 years. Hambidge
(1971) compared the chromium concentration in the hair of 15
newborn babies and that of their mothers: in only one case was the
chromium level in the mother's hair higher than that in the newborn
baby. Chromium levels were significantly lower than those
mentioned above in 50 Turkish women and their newborn babies (203
and 119 µg/kg, respectively) and only 12 newborn babies were found
to have higher concentrations than their mothers, suggesting
suboptimal chromium status (Gürson, 1977). Hair appears to reflect
the nutritional chromium status of groups. The hair-chromium level
is significantly lower in parous women than in nulliparae (Hambidge
& Rodgerson, 1969; Mahalko & Bennion, 1976) and in diabetic
children compared with normal controls (Hambidge et al., 1968). It
is low in adult-onset diabetic adults (Benjanuratra & Bennion,
1975). These findings are in agreement with the expected changes
in chromium balance during pregnancy and in diabetes.
5.2.2.2. Dynamic aspects of metabolism and the influence of
pathological states
Once chromium is absorbed into the organism, it clears rapidly
from the blood stream and is excreted or taken up by the tissues.
In a clinical study, Sargent et al. (1979) detected a 4-
compartment-type clearance from blood, with mean half-times of 13
min, 6.3 h, 1.9 days, and 8.3 days. However, the disappearance
from 3 tissue compartments was much slower, with half-times of
0.56, 12.7, and 192 days. The half-times for blood 3-compartment
clearance in rats (Hopkins, 1965) were calculated to be 0.56, 5.33,
and 57 h (Withey, 1983).
Whether any organ is specifically responsible for the storage
and release of the "metabolically responsive" chromium is not
known. The "metabolically responsive" chromium in blood is defined
as the fraction that increases acutely in response to an elevation
of blood-glucose or blood-insulin levels. It is believed that this
chromium increment interacts with the increased insulin secreted in
response to a glucose load, to facilitate the action of the hormone
on the insulin receptors of the insulin-sensitive cells.
In young, healthy subjects, but not in elderly subjects and
diabetic patients, an oral glucose load or the injection of insulin
results in a sudden increase in serum- or plasma-chromium
(Glinsmann et al., 1966; Levine et al., 1968; Hambidge, 1971; Behne
& Diel, 1972; Liu & Morris, 1978). This increase may appear 30
min, or as late as 120 min, after the challenge; much of the
chromium responsible for the increase is subsequently lost in the
urine. Lack of this increase, also termed "relative chromium
response", is often associated with impaired glucose tolerance,
indicative of chromium deficiency. It should be noted that, when
the glucose load was given intravenously (Pekarek et al., 1975) to
healthy volunteers, the serum-chromium level decreased rapidly,
while the blood-glucose level increased. Supplementation with
chromium chloride or high-chromium yeast extracts for several weeks
resulted in the reappearance of the relative chromium response and
improvement of glucose tolerance (Glinsmann et al., 1966; Liu &
Morris, 1978). These findings support the conclusion that the
"relative chromium response" measured during a glucose or insulin
tolerance test may serve as an indicator of the adequacy of
metabolically responsive chromium.
Much of the chromium increment secreted into the blood stream
in response to glucose or insulin is subsequently lost in the
urine. Hambidge (1971) observed greatly increased urinary-chromium
excretion in 2 diabetic children after insulin therapy had begun,
compared with the excretion in the same children before insulin
treatment. It is not known whether the increased urinary loss of
chromium is compensated for by an increase in absorption efficiency
from the intestines. Hambidge et al. (1968) reported significantly
lower chromium concentrations in the hair of diabetic children
compared with normal children, and Morgan (1972) found that
the chromium contents in the livers of 31 diabetic adults at
autopsy were lower than those in 24 control livers from non-
diabetic persons (8.57 versus 12.7 mg/kg ash; P = 0.05).
On the other hand, Doisy et al. (1971) demonstrated greatly
increased intestinal absorption, together with elevated chromium
excretion, in 14 insulin-dependent diabetic patients administered
51CrCl3 x 6H2O, orally. It is not known whether the greater
absorption efficiency is adequate to compensate for the increased
urinary losses; the decreased chromium concentrations in hair and
liver, discussed above, suggest that a negative balance may prevail.
Several other pathological conditions affect chromium
metabolism. Sargent et al. (1979) detected significantly less
retention of intravenously administered 51chromium in 11 patients
with haemochromatosis compared with 5 normal controls. This may be
related to the high saturation with iron of transferrin, which is
also the carrier protein for newly absorbed chromium.
Chronic ischaemic heart disease also affects chromium
metabolism. Neiko & Del'va (1978) observed a significantly
increased urinary-chromium loss, greater by a factor of 1.5 - 1.6
than that of normal controls, in 65 heart patients. The urinary-,
and to a lesser extent, the faecal-chromium loss increased
progressively with increasing severity of signs and symptoms and
resulted in a negative chromium balance in the post-infarct state.
The balance became positive again on discharge from the hospital
after medical treatment.
Acute infections also appear to influence chromium metabolism.
Pekarek et al. (1975) measured glucose tolerance, and insulin and
chromium levels in human volunteers, before and after infection
with the benign sandfly fever virus. Impaired glucose tolerance and
a significantly increased insulin response to a glucose load,
observed at the height of the infection, were accompanied by very
significantly depressed serum-chromium levels (0.5 µg/litre,
compared with 1.4 µg/litre before infection; P < 0.05) In
contrast with the sharp decline in serum-chromium following the
intravenous injection of glucose in the healthy state observed by
these authors, the depressed serum-chromium levels declined very
little during the glucose tolerance test at the height of
infection. The mechanism of the changes in chromium metabolism in
heart disease and sandfly fever is not clear. Of great potential
importance is the unanswered question of whether the observations
described here reflect an increased chromium requirement in the
patients or a normal reaction to various forms of stress.
The information on the dynamic aspects of chromium metabolism
in animals is limited and should be considered in connexion with
the more detailed studies on human subjects. Diabetes, induced in
rats by the injection of streptozotocin, affected the tissue
distribution of injected 51CrCl3. Sixteen days after injection of
streptozotocin, 51CrCl3 was injected into 5 diabetic rats and 6
normal controls and the 51Cr content measured 5 days later. The
serum of the diabetic rats contained more than 3 times the 51Cr
activity found in the controls (0.24 versus 0.07% of the injected
dose P < 0.01). Significant differences were also detected in the
distribution of 51Cr in the subcellular fractions of the liver; in
the diabetic tissue, 51Cr activity was higher in the nuclear and
supernatant fractions ( P < 0.01) and lower in mitochondria and
microsomes ( P < 0.05). The mechanism responsible for these
changes is not known (Mathur & Doisy, 1972).
5.3. Influence of Chemical Form
The diverse biological effects of chromium on living organisms
cannot be understood without knowledge of the chemical and physical
forms in which the element is present. As stated earlier, the
metallic state (zero valence) is biologically inert, the trivalent
state represents the essential element chromium, and the hexavalent
state is of concern to the toxicologist. Compounds of trivalent
chromium are poorly absorbed, whereas those of the hexavalent state
easily penetrate physiological barriers, such as cell membranes.
Hexavalent chromium compounds are easily reduced by living matter,
but oxidation of trivalent to hexavalent chromium does not occur in
the organism.
The physical form of hexavalent compounds (such as particle
size) and chemical properties (such as solubility) determine
metabolic pathways after inhalation and, therefore, health effects.
There is an equally strong influence of the chemical form of
trivalent chromium on metabolism and health effects. When chromium
is bound to water or small anions (e.g., CrCl3 x 6H20), it
precipitates in the neutral or alkaline milieu of the body fluids.
When it is bound to ligands, such as organic acids, the element is
light in solution and is available for intestinal absorption. The
forms in which trivalent chromium occurs in nature are not really
known. Plants probably contain chromium complexes with organic
acids.
The biological availability of chromium compounds in foods is
of great nutritional importance, but is poorly defined. One
compound or a group of closely related compounds, glucose tolerance
factor, has been isolated from yeast and shown to be more active
than chromium chloride in genetically diabetic mice and in the in
vitro potentiation of the action of insulin on rat epididymal fat
tissue (section 7.1.5.2). It has been identified as a dinicotinic-
acid glutathione complex, but the exact stereochemical structure is
not yet known (Toepfer et al., 1977). It has been postulated, but
not proved, that this factor is the active form of chromium within
the organism.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The environmental effects of chromium as a pollutant have been
reviewed by US EPA (1978), Anderson (1982), and EIFAC (1983).
Various effects have been reported, but, because of the presence of
other chemicals, it remains doubtful whether chromium alone is
responsible for the effects observed. Data are available on
microorganisms, plants, and aquatic organisms.
6.1. Microorganisms
Most microorganisms (protozoa, protophyta, fungi, algae,
bacteria) are able to absorb chromium. The active uptake of
chromate by the sulfate transport system has been shown in
Neurospora crassa (Roberts & Marzluf, 1971). No distinction has
been made between ab- and adsorption in other studies (e.g., algae)
(Calow & Fletcher, 1972), and it has not yet been shown that
chromium is an essential element for microorganisms. In general,
toxicity for most microorganisms occurs in the range of 0.05 - 5 mg
chromium/kg of medium. The internal concentration of chromium
depends on the species. In most groups of microorganisms, it
ranges between the levels of 0.6 mg dry weight present in one litre
of sample of microplankton from Monterey Bay, California, USA, and
21.4 mg/litre phytoplankton collected in the Pacific Ocean (Martin
& Knauer, 1973).
Trivalent chromium is less toxic than hexavalent. The main
features are inhibition of growth (at concentrations greater than
0.5 mg/litre in Chlorella cultures) (Nollendorf et al., 1972) and
inhibition of various metabolic processes, such as photosynthesis
or protein synthesis (US EPA, 1978).
The toxicity of chromium for soil bacterial isolates was
studied by measuring the turbidity of liquid cultures supplemented
with hexavalent chromium and trivalent chromium. Gram-negative
bacteria were more affected by hexavalent chromium (1 - 12 mg/kg)
than gram-positive bacteria. Toxicity due to trivalent chromium
was not observed at similar levels. The toxicity of low levels of
hexavalent chromium (1 mg/kg) indicates that soil microbial
transformations, such as nitrification, may be affected (Ross et
al., 1981).
6.2. Plants
Although chromium is present in all plants, it has not been
proved to be an essential element for plants. Most substances,
including chromium, can be absorbed through either the root or the
leaf surface. Several factors affect the availability of chromium
for the plant (Black, 1968), including the pH of the soil,
interactions with other minerals or organic chelating compounds,
and carbon dioxide and oxygen concentrations.
Little chromium is translocated from the site of absorption;
however, the chelated form is transported throughout the plant
(Verfaillie, 1974).
Chromium in high concentrations can be toxic for plants, but
Yopp et al. (1974) stated that there was no specific pattern of
chromium intoxication.
During the smelting of chromite, considerable quantities of
waste are produced, which contain soluble chromates. When combined
with a high pH, Gemmell (1973) showed an inhibition of germination
and growth in white mustard plants (Sinapis alba) growing on waste
heaps. Covering the waste with a 25-to 30-cm layer of granular-
free-draining subsoil followed with layers of soil, peat, or sewage
sludge was shown to be the best revegetation technique (Gemmell,
1974).
The main feature of chromium intoxication is chlorosis, which
is similar to iron deficiency (Hewitt, 1953).
Soybeans, treated in nutrient culture containing 0 - 5 mg
hexavalent chromium/litre showed decreased uptake of calcium,
potassium, phosphorus, iron, and manganese (Turner & Rust, 1971).
Death of plants occurred within 3 days of treatment with 30 or 60
mg chromium/litre.
A reduction in leaf dry weight occurred after treatment with
0.01 mg hexavalent chromium/litre (Rediske et al., 1955). Chromium
affects the carbohydrate metabolism, and the leaf chlorophyll
concentration decreased with increasing hexavalent chromium
concentration (0.01 - 1 mg hexavalent chromium/litre) (Rediske,
1956). Hexavalent chromium appears to be more toxic than trivalent
chromium (Hewitt, 1953; Stanley, 1974; Verfaillie, 1974). At
present, no data are available concerning the mechanism of action
or the dose-dependent pattern of chromium intoxication.
6.3. Aquatic Organisms
More studies have been performed with aquatic species than with
free-living (non-parasitic) animals. Depending on the species,
chromium can be less toxic for fish in warm water, but marked
decreases in toxicity are found with increasing pH or water
hardness; changes in salinity have little if any effect on its
toxicity. Chromium can make fish more susceptible to infection;
high concentrations can damage and/or accumulate in various fish
tissues and in invertebrates such as snails and worms.
Reproduction of Daphnia was affected by exposure to 0.01 mg
hexavalent chromium/litre (EIFAC, 1983). Numerous other factors
influence the availability of chromium and, therefore, its
toxicity. These include the presence of other minerals and organic
pollutants, and the temperature of the environment; this has been
shown in mice (Nomiyama et al., 1980a).
Hexavalent chromium is accumulated by aquatic species by
passive diffusion (US EPA, 1978). Ecological factors, in the
abiotic and living environment, are involved in this process, which
varies according to the sensitivity of different species. The
physiological state and activity of the fish also affect
accumulation (Reichenbach-Klinke, 1977, 1980). Kittelberger (1973)
analysed the organs and tissues of the roach (Leuciscus rutilus)
from the river Rhine and found that concentrations of chromium in
the spleen, bronchi, and intestine (between 30 and 37.5 mg/kg) were
10 - 30 times higher than those in the heart, skin, muscle, and
scales.
LC50s are listed in Table 10 for hexavalent and trivalent
chromium compounds in the aquatic environment.
Table 10. The toxicity of chromium for fresh-water organisms
(expressed as 50% mortality)a
-----------------------------------------------------------------------
Compound Category Exposure Toxicity range Most
(mg/litre) sensitive
species
-----------------------------------------------------------------------
hexavalent invertebrate acute 0.067 - 59.9 scud
chromium long-termb - -
vertebrate acute 17.6 - 249 fathead minnow
long-term 0.265 - 2.0 rainbow trout
trivalent invertebrate acute 2.0 - 64.0 cladoceran
chromium long-term 0.066 cladoceran
vertebrate acute 33.0 - 71.9 guppy
long-term 1.0 fathead minnow
-----------------------------------------------------------------------
a From: US EPA (1980).
b No data available.
In general, invertebrate species, such as polychaete worms,
insects, and crustaceans are more sensitive to the toxic effects of
chromium than vertebrates, such as some fish (Mathis & Cummings,
1973). The lethal chromium level for several aquatic and
nonaquatic invertebrates has been reported to be 0.05 mg/litre (US
NAS/NAE, 1972).
EIFAC (1983) reviewed the literature on the occurrence and
effects of chromium in fresh water and proposed tentative water-
quality criteria that distinguish between salmonid and non-salmonid
waters. To protect salmonid waters, the mean aqueous concentration
of "soluble" chromium should not exceed 0.025 mg chromium/litre,
and the 95 percentile should not exceed 0.1 mg chromium/litre.
However, more stringent values may be necessary in very soft, acid
waters, and less stringent values in alkaline waters.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Nutritional Effects of Chromium
The criteria for an essential nutrient have been defined in
different ways by different authors, but all definitions postulate
that a reduction in the total daily intake of the nutrient below a
certain level must consistently induce signs of deficiency, and
that the supplementation of the daily intake above this level must
prevent and cure the deficiency signs. Chromium deficiency has
been produced experimentally in mice, rats, and squirrel monkeys,
and the full reversal of the deficiency signs by the oral
administration of chromium has been demonstrated in rats (Mertz,
1969). For these reasons, chromium must be considered an essential
micro-nutrient. It is physiologically active in the trivalent
oxidation state at concentrations of approximately 100 µg/kg diet.
7.1.1. Effects of deficiency on glucose metabolism
Semipurified rations, containing Torula yeast as the source of
protein, were fed to groups of 10 male Sprague Dawley rats at
weaning, and intravenous glucose tolerance tests were performed by
injecting 1250 mg glucose/kg body weight and measuring the
subsequent decline in blood-glucose levels. The rate of glucose
disappearance can be calculated from the straight line plot of log
increment glucose (excess of glucose at any time (t) over fasting
glucose), versus time. The disappearance constant k is expressed
as % decline of the increment glucose per min; it is a measure of
the efficiency of glucose utilization. In weaning rats, the rate
constant was found to decline, within 3 weeks or less, from an
average of 4%/min to 2.6%/min in animals fed the Torula yeast diet,
but not in animals administered a diet in which 4 or 8% of Torula
yeast was replaced by an equal amount of brewer's yeast. This
observation suggested that brewer's yeast, but not Torula yeast,
contained an unknown substance necessary for the maintenance of
normal glucose tolerance. Because the only known effect of the
substance was that on glucose tolerance, it was named "Glucose
Tolerance Factor" (GTF) (Mertz & Schwarz, 1959). GTF was extracted
from brewer's yeast and pork kidney powder, concentrated and
purified, and its active ingredient was identified as trivalent
chromium (Schwarz & Mertz, 1959). Chromium in the form of most
common complexes (except for very stable ones) cured the impairment
of glucose tolerance in deficient rats, either as one oral dose of
200 µg/kg body weight, or as an intravenous (iv) injection of 2.5
µg/kg body weight. Chromium in the diet also prevented the
impairment of glucose tolerance.
In order to produce more pronounced deficiencies of chromium
and other trace elements, Schroeder et al. (1963) constructed a
special animal house on a mountain top in Vermont, USA, far removed
from traffic and industry. The interior was specially coated with
organic resins to reduce any metallic exposure. Air was introduced
through special filters, and strict practices were enforced to
avoid the introduction of dust and dirt. This will be referred to
as the "controlled environment". Under these conditions, a more
severe chromium deficiency resulted in very low glucose removal
rates of 1.12%/min in 6 rats and of 0.18%/min in 4 female breeder
rats (482 days old), after 11 days on a chromium-deficient diet.
The removal rate in 4 control rats receiving the same diet with a
chromium supplement improved from 0.51 to 1.39% during the same
period (Mertz et al., 1965b). This strong impairment of glucose
tolerance in rats kept in the "controlled environment" was
reflected in their fasting blood-glucose levels, compared with
those of chromium-supplemented controls: 1370 ± 68 versus 1170 ± 17
mg/litre in males and 1390 ± 68 versus 960 ± 65 mg/litre in
females. More than half of 185 deficient rats excreted more than
0.25% glucose in the urine, whereas glycosuria was found in only 9
of the chromium-supplemented controls (Schroeder, 1966). A
significant reduction in the intravenous glucose removal rate was
also observed in 8 rats in plastic cages, fed an EDTA-washed low-
protein diet (7% casein), compared with 8 controls receiving 50 µg
chromium as the chloride, by stomach tube, daily for 2 weeks (2.1
versus 3.6%/min; P < 0.01). However, chromium supplements did not
improve the near-normal removal rates in rats receiving a 20%
casein diet (Mickail et al., 1976). Significantly lower plasma-
glucose levels, due to supplementation with chromium, were reported
in rats fed the chromium-deficient Torula yeast diet (Whanger &
Weswig, 1975). In another series of studies, only a slight
reduction in blood-glucose (from 1240 to 1180 µg/litre) was found
in 10 rats receiving a diet supplemented with chromium (10 µg/kg)
(Djahanshiri, 1976).
Impaired glucose tolerance in squirrel monkeys, fed a
commercial laboratory chow, was shown to respond to chromium
supplementation. Of 9 monkeys with an impaired glucose removal
rate (1.38%/min), 8 responded after 22 weeks of supplementation of
their drinking-water (chromium acetate, 10 mg/litre) with a
normalization of their glucose tolerance (average removal rate, N =
9, 2.33 ± 0.3%/min) (Davidson & Blackwell, 1968). There was no
effect on food consumption, growth rate, or serum-insulin
concentrations. The chromium content of the commercial chow was
stated to be 3.3 mg/kg, a very high concentration. In view of the
uncertainties of methods of analysis for chromium (section 2.2), it
is not possible to interpret the results as indicative of a very
high chromium requirement of the squirrel monkey or of an unusually
poor bioavailability of the chromium in that particular ration. A
marginal chromium deficiency may have existed in mice fed a bread
and milk diet, as daily administration of 10 µg chromium for 16
days to 8 months produced a 10 - 30% decline in blood-glucose
levels (Vakhrusheva, 1960). However, this effect was not specific
for chromium, as it was also observed when manganese was
administered.
The glucose tolerance of guinea-pigs did not differ
significantly between groups fed diets containing chromium at
0.125, 0.625, or 50 mg/kg, even though the animals fed the 2 higher
levels exhibited a lower mortality rate during pregnancy (Preston
et al., 1976).
Although the administration of synthetic glucose tolerance
factor (a chromium-dinicotinic acid-glutathione complex) to 6 pigs
did not affect glucose tolerance tests, it resulted in a
significant increase in the hypoglycaemic effect of insulin
injected at 0.1 U/kg body weight (Steele et al., 1977a).
Turkey poults, fed a practical ration containing chromium
levels of 5 mg/kg, responded to chromium supplementation (20 mg/kg
diet) with a significant increase in liver glycogen and in glycogen
formation, following a fast, and with a significant increase in
glycogen synthetase (EC 2.4.1.11) activity in the liver (Rosebrough
& Steele, 1981).
It can be concluded that the impairment of glucose tolerance in
rats fed a low-chromium Torula yeast diet is due to chromium
deficiency. The effects of chromium in squirrel monkeys, pigs, and
turkeys, though statistically significant, are somewhat difficult
to interpret, because of the reported high chromium content of the
basal diet. No evidence for chromium deficiency has yet been
obtained through glucose tolerance tests on other animal species.
7.1.2. Effects of deficiency on lipid metabolism
Though trivalent chromium in high doses (2.5 mg/kg body weight)
has been shown to increase the synthesis of fatty acids and
cholesterol in the liver (Curran, 1954), lower, physiological doses
appear to decrease serum-cholesterol concentrations in rats.
Schroeder & Balassa (1965) found an average level of 927 mg
cholesterol/litre serum in 24- to 26-month-old male rats, kept in a
controlled environment and administered chromium in the drinking-
water at a concentration of 5 mg/litre, compared with an average of
1229 mg/litre in controls not receiving chromium ( P < 0.01). The
effects in female rats were ambigous, one study producing the
expected reduction in cholesterol due to chromium, another showing
an elevation in the supplemented female rats. Schroeder's
observations of a cholesterol-reducing effect of chromium in male
rats were confirmed by Staub et al. (1969) and by Whanger & Weswig
(1975) but were contradicted by results of a third study in which
there were not any significant effects of chromium on sucrose-
induced triglyceridaemia and cholesterolaemia (Bruckdorfer et al.,
1971). Perhaps more significant than the effect on circulating
cholesterol is the direct effect of chromium on the occurrence of
aortic plaques. Schroeder & Balassa (1965) observed 6 plaques in 54
male and female chromium-deficient rats, but only one plaque in 48
animals receiving 5 mg chromium/litre in drinking-water. These
results are in agreement with those from a subsequent report of the
protective effect of the natural chromium content of water (60 -
215 µg/litre) against atherosclerosis in cholesterol-fed rabbits
(Novakova et al., 1974). Abraham et al. (1980) extended these
observations by demonstrating that daily chromium injections (20 µg
K2CrO4) reversed the established atherosclerosis in the aorta of 11
cholesterol-fed rabbits, compared with 12 controls. The mean
plaque area was reduced from 95% to 63%, the total aortic
cholesterol from 729 mg to 458 mg, and the atheromatous lesions, as
measured by technetium incorporation from 285 000 cpm to 114 000
cpm, all differences being statistically significant. These
results are reinforced by observations in man discussed in section
8.1.
7.1.3. Effects of deficiency on life span, growth, and
reproduction
The mortality of male, but not of female mice, raised in a
"controlled environment" (section 7.1.1) was reduced by trivalent
chromium administered as acetate in the drinking-water at a
concentration of 5 mg/litre (Schroeder et al., 1964). The survival
rates at 12 months were 92.6% and 68.8% ( P < 0.0001) in
supplemented and deficient animals, respectively. Similary, male,
but not female, rats receiving a chromium concentration of 5
mg/litre in the drinking-water had longer life spans than deficient
controls. The mean age of the last surviving 10% of animals was
1249 days, compared with 1141 days in the deficient animals ( P <
0.01). Survival of male rats fed a low-chromium (< 100 µg/kg),
low-protein ration and subjected to a controlled acute haemorrhage
was significantly less than that of chromium-supplemented rats, in
2 studies (67 versus 92%; P < 0.05 and 27 versus 60%; P < 0.01,
respectively) (Mertz & Roginski, 1969).
In Schroeder's study, growth rates in treated mice and rats of
both sexes raised in a "controlled environment", were higher after
6 and 12 months, with highly significant differences in body weight
ranging from 9 to 17% ( P < 0.005) compared with the controls.
Again, the effects of chromium supplementation were greater in
males than in females (Schroeder et al., 1964, 1965).
Similar results were reported by Djahanschiri (1976), who
studied a total of 2750 rats of a special inbred strain (Hk51) fed
a basal diet (0.15 mg chromium/kg diet) and chromium supplements
ranging from 10 to 500 mg/kg diet. At 12 weeks, the average
weights of the chromium-supplemented animals, regardless of dose
level, were significantly higher (by 6% in the males and 3% in the
females) than those of the animals on the basal diet. The same
author reported a progressive diminution in both the milk
production of lactating rats and weight gain in 3 consecutive
generations fed the low-chromium diet, compared with rats receiving
chromium supplementation. Increased mortality was reported in
pregnant guinea-pigs fed a low-chromium diet (125 µg/kg diet)
compared with animals receiving a chromium supplement of either 625
µg/kg or 50 mg/kg (Preston et al., 1976).
When rats raised on a low-chromium Torula yeast diet (< 100
g/kg) mated with those on a normal diet, they were able to
impregnate the females at a 100% conception rate only up to the age
of 4 months. After this age, the conception rate declined to 25%,
25%, and 0%, at the age of 7, 8, and 9 months, respectively. This
decline was accompanied by a significant ( P < 0.01) decrease in
the sperm count in the chromium-deficient males to approximately
half of the count in supplemented controls at the age of 8 months
(Anderson & Polansky, 1981).
7.1.4. Other effects of deficiency
Male weanling rats, fed a 10% soya protein ration with a
chromium content of less than 100 µg/kg, developed a visible
opacity of the cornea in one or both eyes. In several studies, the
incidence of this effect ranged from 10 to 15% in deficient rats.
No opacities developed in control animals receiving 2 mg
chromium/kg diet (Roginski & Mertz, 1967).
Chromium deficiency has been shown to reduce the physical
performance of rats under stress. Ten male rats raised on a
chromium-deficient diet (150 µg/kg diet) swam for an average of 250
min, until exhaustion, in contrast with 10 rats receiving a
supplement of 10 mg chromium/kg diet, which were exhausted only
after 320 min (Djahanschiri, 1976).
7.1.5. Mechanism of action of chromium as an essential nutrient
7.1.5.1. Enzymes, nucleic acids, and thyroid
Chromium is present in nucleic acids in very high
concentrations, but the function of these is not clear at present
(Mertz, 1969). However, recent work suggests a biological function
of chromium in nucleic acid metabolism (Okada et al., 1984).
Ribonucleic acid synthesis in mouse liver was significantly
increased by as little as 1 µmol trivalent chromium, in the
presence of DNA or chromatin (Okada et al., 1981). These effects
were also present when the DNA or chromatin were first complexed
with chromium prior to incubation. However, prior complexation of
RNA polymerase with chromium depressed activity. These effects
were obtained in vitro with a concentration (52 µg/litre) that is
similar to physiological levels. Goncharov (1968) presented data
suggesting that chromium is involved in the function of the thyroid
gland. These findings have been supported by Lifschitz et al.
(1980).
An oligopeptide with a relative molecular mass of 1480, which
was crystallized from liver tissue, had a specific affinity for
chromium (Wu, 1981).
7.1.5.2. Interaction of chromium with insulin
The interaction of chromium with insulin has been extensively
studied and can therefore be presented in some detail, but this
does not imply that this is the only, or the most important,
function of chromium.
The effects of chromium in vitro, and probably in vivo, depend
on the presence of endogenous or exogenous insulin, no effects
having been demonstrated in in vitro systems that did not either
depend on, or contain, insulin. Chromium deficiency causes an
impaired response to added insulin in rat epididymal fat tissue,
and, when glucose uptake or glucose oxidation or utilization for
lipid synthesis is measured, the dose-effect curve is flat.
Addition of suitable chromium compounds significantly increases the
slope of the curve (Fig. 3). This demonstrates the true
potentiation of the insulin action and indicates that chromium
alone does not act as an insulin-like substance (Mertz et al.,
1961; Mertz & Roginski, 1971; Mertz, 1981). Chromium was also
shown to stimulate the transport of D-galactose into epididymal fat
cells. This suggests cell transport, the first step of sugar
metabolism, as a major site of action for chromium (Mertz &
Roginski, 1963). Insulin-potentiating effects have also been
observed on the swelling of liver mitochondria (Campbell & Mertz,
1963) and on glucose utilization in isolated rat lens (Farkas &
Roberson, 1965).
5.1.1.2. Human data
A mean chromium concentration of 0.22 mg/kg wet weight was
found in the lung tissue of subjects from various locations in the
USA, but there was no correlation between chromium levels in the
lungs and those in the air (Schroeder et al., 1962).
A Committee of the National Research Council (US NAS, 1974a)
concluded: "It is unlikely that the intake from air under ordinary
conditions contributes significantly to the total intake of
available chromium; the intake from the air is calculated to be
less than 1 µg/day; but excessive exposure to airborne chromium
does result in some increased intake".
5.1.2. Absorption from the gastrointestinal tract
The absorption of ingested chromium compounds can be estimated
by measuring the amount of chromium excreted in the urine, as
almost all of intravenously injected chromium is excreted via the
urine and only 2% is found in the faeces. Although a potential loss
of endogenous chromium via the skin and its annexa has not yet been
measured and quantified, it can be stated that this organ, as well
as the gastrointestinal tract are of minor importance in the
excretion of endogenous chromium. The gastrointestinal tract is,
of course, the major organ for the excretion of exogenous chromium.
When considering the gastrointestinal absorption of chromium,
it is essential to recognize the substantial differences in the
efficiency of absorption of trivalent and hexavalent compounds.
These differences exist in both man and animals. Many trivalent
chromium compounds are so poorly absorbed that they have been used
as faecal markers in man and animals. The absorption of hexavalent
chromium, administered orally, was higher in all species examined,
but did not exceed 5% of the dose (Donaldson & Barreras, 1966). No
physiological regulation has yet been established for chromium
absorption.
5.1.2.1. Animal studies
The gastrointestinal absorption of chromate in rats has been
reported to be between 3 and 6% of a tracer dose (MacKenzie et al.,
1958; Byerrum, 1961). As in man, trivalent chromium compounds are
less well absorbed in the rat, with reported efficiencies ranging
from less than 0.5% (Visek et al., 1953) to 3% (Mertz et al.,
1965a). Within the category of trivalent compounds, there are
moderate differences in absorption, depending on the chemical form.
Binding of the chromium ion to suitable ligands, such as certain
organic acids, stabilizes the metal against precipitation in the
alkaline milieu of the intestines and increases absorption
efficiency by a factor of 3 - 5 times, compared with that for
chromium chloride. This has been shown for certain chelating
agents (Chen et al., 1973), a yet unidentified small peptide
complex isolated from yeast (Votava et al., 1973), and synthetic
glucose tolerance factor (GTF), a dinicotinic-acid-glutathione-
chromium complex (Mertz et al., 1974). Nothing is known about the
interaction of chromium with the flora of the gastrointestinal
tract. Absorption of chromium chloride by ruminant species is
similar to that in rats, with a mean efficiency of 0.76% (Anke et
al., 1971); laying hens have been found to absorb almost 15% of a
tracer dose of the element (Hennig et al., 1971).
5.1.2.2. Human studies
Donaldson & Barreras (1966) studied the gastrointestinal
absorption of hexavalent chromium by administering trace doses of
Na251CrO4 orally to 6 volunteer patients, who were hospitalized,
and by measuring the amount of radioactivity in the faeces and
urine. The mean urinary excretion, expressing the absorption
efficiency, was 2.1 ± 1.5% of the dose given. Administration by
jejunal infusion in 4 volunteers increased these values, suggesting
reduction of the chromate to trivalent compounds by the acid
content of the gastric juice. The same authors reported a mean
absorption efficiency of only 0.5 ± 0.3% for trivalent chromium,
administered as CrCl3 x 6H2O, with a range of 0.1 - 1.2%.
On the basis of the chromium content in diets (60 µg) and
chromium excretion (0.22 µg) in healthy subjects, Anderson et al.
(1983) calculated a minimum chromium absorption of about 0.4%.
Increasing intake by supplementation with chromium (chromic
chloride tablets, furnishing 200 µg chromium/day) led to an
excretion of 0.99 µg, equivalent to 0.4% of the intake.
Aitio et al. (1984) investigated the intake and urinary
excretion of chromium (III) in leather tanning workers. The
environmental concentrations were recorded as low, but chromium was
present in air in the form of large droplets that were not
collected by the standard air measurement technique. It was assumed
that the large droplets were cleared by the upper respiratory tract
and swallowed, and that the chromium in the droplets was absorbed
from the gastrointestinal tract. A calculation showed that this
would explain the urinary excretion levels. No absorption of
chromium through the skin was detected.
In a recent study, the minimum chromium absorption calculated
on the basis of urinary-chromium excretion was about 0.4%.
Increasing intake 5-fold, by chromium supplementation, led to a
nearly 5-fold increase in chromium excretion, suggesting that the
extent of absorption of supplemental inorganic chromium was similar
to that from normal dietary sources (Anderson et al., 1983a).
A similar absorption for trivalent chromium of 0.69% was
reported by Doisy et al. (1968) in healthy human subjects,
regardless of age. However, a group of 14 insulin-requiring
diabetic patients absorbed 4 times as much of the chromium dose as
the non-diabetic or maturity-onset diabetic subjects, as shown by
elevated levels of 51chromium in blood plasma and urine (Doisy et
al., 1971).
5.2. Distribution, Retention, Excretion
5.2.1. Animal studies
Most animal studies on chromium metabolism have been performed
on rats. From the site of intestinal absorption, chromium is taken
up by plasma-protein fractions. Small, physiological doses of
51chromium have been shown to bind almost entirely to the iron-
binding protein, transferrin (Hopkins & Schwarz, 1964). On the
other hand, inhaled chromium (Glaser et al., 1984) was bound to
albumin rather than to transferrin. With larger quantities of
trivalent chromium, non-specific binding to other proteins also
occurred, but not to the red blood cells. Visek et al. (1953)
measured the effects of the different chemical forms of chromium on
tissue distribution and found that soluble, chelated forms, such as
acetate or citrate complexes, were cleared quite rapidly, in
contrast with colloidal or protein-binding forms (chromite, chromic
chloride), which have a great affinity for the reticulo-endothelial
system (bone marrow, liver, spleen), and clear more slowly. The
blood clearance of hexavalent chromium, such as chromate, was slow,
because of irreversible binding within the red blood cells. Tissue
distribution of 51chromium, administered in nanogram doses to rats
was studied by Hopkins (1965). As in the preceeding studies, the
element accumulated in bone, spleen, testes, and epididymis; much
less was retained in the lungs, brain, heart, and pancreas. This
obvious difference in chromium distribution between man and rats is
unexplained.
As in man, trivalent 51chromium in the rat was rapidly cleared
from the blood, after absorption, and was retained by the tissues
(Mertz et al., 1965a). These tissue stores, labelled with
51chromium chloride, administered orally or intravenously, were not
immediately available for specific physiological functions. For
example, 51chromium, administered as CrCl3 x 6H2O to pregnant rats,
was not transported into the embryos (Mertz et al., 1969), nor did
any 51chromium appear in the blood in response to glucose or
insulin injections (Mertz & Roginski, 1971). The fact that fetal
chromium concentrations are low, when the pregnant rats are fed a
low-chromium Torula yeast diet, and increase when a high-chromium
natural stock ration is fed, indicates that placental transport and
possibly, the acute chromium response depend on a special form of
chromium, which is different from chromium chloride. It is
possible, but has not yet been proved, that this form is the
dinicotinic acid-glutathione-chromium complex, known as glucose
tolerance factor. Yeast extracts containing this factor labelled
with 51chromium have been shown to cross the placenta (Mertz et
al., 1969) and, in preliminary studies, to furnish chromium for the
acute chromium response (Mertz & Roginski, 1971).
With reference to interactions between chromium and other trace
elements, competition with iron by way of their common carrier
(transferrin) has been suggested in rats (Hopkins & Schwarz, 1964)
and in human beings (Sargent et al., 1979). Goncharov (1968)
reported a close interaction between chromium and dietary iodine.
In iodine-deficient white rats, addition of chromium to the diets
in amounts supplying from 0.6 to 600 µg/animal per day stimulated
thyroid function, as indicated by morphological and functional
changes. Conversely, chromium, in all but the lowest dose,
decreased thyroid function in animals receiving adequate iodine
levels. This relation is in agreement with epidemiological data
from the USSR (Goncharov, 1968).
5.2.2. Human data
5.2.2.1. Concentration in tissues, blood, urine, and hair
including possible biological indicators of exposure
(a) Tissues
The most comprehensive survey of tissue-chromium concentrations
is that of Schroeder et al. (1962), who carried out a
spectrographic analyses on 20 - 39 samples for each autopsy tissue,
all of which had been carefully collected to avoid extraneous
contamination. The following results were obtained (mean values in
mg/kg ash) for a group of subjects who had died between the ages of
30 and 40 years: lung, 15.6; aorta, 9.1; pancreas, 6.5; heart, 3.8;
testes, 3.1; kidney, 2.1; liver, 1.8; spleen, 1.7. In all tissues,
except for the lungs there was a rapid decline in chromium
concentrations from time of birth to the age of 10 years, followed
by a more gradual decrease to the age of 80 years. It cannot be
stated with certainty whether the decline is an expression of a
physiological mechanism or of a dietary deficiency. The lungs lost
their initially high chromium levels (85.2 mg/kg ash) up to the age
of 20 years (6.8 mg/kg); subsequently, the concentrations increased
to between 20 and 38 mg/kg. This discrepancy demonstrates that the
chromium in lungs is not in equilibrium with the general pool. The
decline of chromium in the aorta, quite pronounced in subjects in
the USA, was much less dramatic in the aortas from subjects of
other countries (Schroeder et al., 1970).
Mancuso & Hueper (1951), as well as Baetjer et al. (1959b),
found concentrations of chromium in the lungs of former chromate
workers that were several orders of magnitude higher than those in
control subjects. In a study on 16 chromate workers, including 11
with lung cancer, Baetjer et al. (1959b), using a colorimetric
method, observed a median concentration of water- and acid-soluble
chromium in the lung of 70 mg/kg dry weight and a median
concentration of acid-insoluble chromium of 17 mg/kg. The chromium
concentration did not differ between cancer cases and non-cancer
cases. The tissue specimens were obtained 0 - 23 years after the
termination of occupational exposure, which had lasted 1.5 - 42
years. With the use of emission spectrometry and atomic absorption
spectrophotometry, Hyodo et al. (1980) found a chromium content of
3.6 mg/kg wet weight in the lung in a male smoker with lung cancer,
who died 10 years after employment for 30 years in a chromate-
producing plant. The concentration in other tissues ranged from
0.05 (bone marrow) to 1.5 mg/kg (suprarenal gland). In five
unexposed controls, lung concentrations ranged from 0.09 to 0.88
mg/kg; concentrations in other tissues ranged from 0.003 (kidney)
to 0.156 mg/kg (suprarenal gland). The ratio of hexavalent to
total chromium in the lungs was 29% in the worker and 22.7 ± 10.6%
(mean ± SD) in the controls.
Brune et al. (1980) gave tissue concentrations for chromium and
other metals in the lung, liver, and kidney of 20 deceased copper
smelter workers, who had retired 0 - 19 years prior to death, and
of a control group (8 subjects). Tissue analysis was carried out
using neutron activation analysis as well as atomic absorption
spectrophotometry, and included a comparison with certified
reference samples (National Bureau Standards, bovine liver). In
the controls, the concentrations ranged from below detection (0.003
mg/kg wet weight) to 0.07 mg/kg in kidneys and 0.11 mg/kg in liver.
There was no marked difference between the chromium contents of
these 2 tissues and those in the exposed workers. On the other
hand, the lung concentrations were between 3 and 4 times higher in
the workers than in the controls (median levels, 0.29 and 0.08
mg/kg, respectively).
Chromium determinations were carried out on lung and kidney
samples of 45 autopsies from the Northern Bavaria area (Federal
Republic of Germany) (Zober et al., 1984). The analyses were
carried out using electrothermal AAS after wet oxidative digestion.
Median values of 0.097 mg/kg wet weight (range, 0.0006 - 1.230
mg/kg) in lung tissue and 0.0096 mg/kg wet weight (range, 0.0002 -
0.690 mg/kg) in kidney were found.
Very limited data are available on chromium levels in tissues
other than those referred to above. Shmitova (1978) estimated
chromium levels in fetal and placental tissues (abortive material),
derived on the 12th week of pregnancy, and found 92.8 and 30 µg
Cr/kg tissue, respectively.
It can be concluded that chromium may be retained in the lungs,
several years after the termination of occupational exposure.
However, it is not known whether this observation has any
biological relevance to the appearance of lung cancer. In this
context, it is of interest to note that chromium is retained in the
human lung for a relatively long period, even without occupational
exposure.
(b) Blood
The values reported by various investigators for chromium
concentrations in the blood of unexposed human beings range from
0.2 to 70 µg/litre in serum and plasma and 5 to 54 µg/litre in red
blood cells (US EPA, 1978). Insufficient evidence is available to
state whether the blood values in the normal population are
influenced by concentrations in the ambient air. The most
extensive determination of blood-chromium levels in chromate
workers was made by Mancuso (1951). Blood values during exposure
varied from 5 to 170 µg/litre and from 10 to 140 µg/litre, 74 days
after work ended. No decrease in blood-chromium levels was found,
even after 74 days without exposure.
Because of a selective affinity of hexavalent chromium for the
erythrocyte, substantially increased environmental exposure to
chromates is reflected in an increased ratio of the hexavalent
chromium level in red blood cells to that in plasma. Baetjer et
al. (1959a) found the following concentrations in 3 exposed
chromate workers: blood cells, 30, 54, and 140 µg/litre; plasma, 0,
20, and 17 µg/litre, respectively. In the absence of exposure to
chromate, the chromium concentrations in erythrocytes and plasma,
as serum, were nearly identical (Paixao & Yoe, 1959). Chromium
(VI) is incorporated into human red blood cells and remains there
over a long period of time (Wiegand et al., 1985), since the
approximate lifetime of human red cells is about 100 days. Exposure
to 2 mg trivalent chromium/day, for 3 months, resulted in an
increased concentration (0.2 mg/kg) in the red cells of 5 human
males, compared with 0.11 mg/kg in 5 controls without supplement
(Schroeder et al., 1962). However, later studies did not show any
increase in chromium concentrations in red cells, following
exposure to trivalent chromium (Beyersmann et al., 1984; Wiegand et
al., 1985). Monitoring of red blood cell-chromium may be a useful
indicator of exposure to hexavalent, but not to trivalent,
chromium.
Most of the later studies show that the true chromium
concentration in the plasma or serum of healthy subjects is of the
order of 1 µg/litre or less (Guthrie et al., 1978; Versieck et al.,
1978). Seeling et al. (1979) determined chromium in serum and
plasma using flameless AAS. This study was supported by measuring
a standard reference material (National Bureau of Standards, 1569
brewer's yeast, reference data: 2.12 ± 0.05 mg/kg, measured data:
2.3 ± 0.2 mg/kg). The chromium levels in serum ranged from 0.7 to
2.2 µg/litre and in plasma from 1 to 1.5 µg/litre (central
parameters of a long normal distribution).
Pooled serum samples of 6 healthy Finnish volunteers were
studied by Kumpulainen et al. (1983). A mean value of 0.11 ± 0.05
µg chromium/litre (range, 0.06 - 0.20) was found. Nomiyama et al.
(1980b) analysed 20 blood samples of Japanese subjects, using
direct flameless ASS, and reported a value of 2.9 ± 1.7 µg
chromium/litre whole blood.
Zober et al. (1984) analysed the blood of 45 autopsied subjects
from the Northern Bavaria area (Federal Republic of Germany). A
median chromium concentration of 2.8 µg/litre of postmortem blood
(range, 0.20 - 24 µg/litre) was found, the value was not influenced
by age or sex.
A reference material for chromium, bovine serum (RM 8419) is
available from the National Bureau of Standards, Washington DC,
USA.
(c) Urine
Chromium concentrations in the urine of non-occupationally
exposed subjects have been reported to range from 1.8 to 11
µg/litre (Imbus et al., 1963). Except for exposed persons and
juvenile diabetic patients, the reported values for daily chromium
excretion in urine (Table 9) do not differ as much as those for
blood. In later studies, such as that of Guthrie et al. (1979), a
method of flameless atomic absorption was used in which it was
possible to correct for spectral interference (Zander et al.,
1977). Such interference was difficult to eliminate with earlier
methods (Guthrie et al., 1978). Urine samples from 189 Japanese
volunteers, aged 10 - 80 years, in 4 pollution-free areas, were
analysed for chromium by Nomiyama et al. (1980a), using direct
flameless AAS. The average level was 0.4 ± 0.37 µg/litre (X ± SE)
or 0.47± 0.42 mg/kg creatinine. Urinary chromium tended to be
higher in males than in females and to decrease with age, but the
differences were not significant. Using electrothermal AAS, the
urinary excretion of various metals in non-occupationally exposed
adults was measured by Schaller & Zober (1982). For smokers, a
median value of 1.6 µg chromium/litre was found (non-smoker: 1.4 µg
chromium/litre). Commercially available urine samples were used
for quality control (Angerer et al., 1981). Although a "normal"
level of chromium excretion for healthy, unexposed persons cannot
yet be established with certainty, such a level may be less than 1
µg/day.
Several investigators have measured urinary-chromium excretion
in exposed workers. In a study on chromate workers, the urine
values ranged from 5 to 380 µg chromium/litre during exposure and
from 10 to 54 µg chromium/litre, 74 days after the end of exposure
(Mancuso, 1951). No relationship was evident between the urine
levels and the weighted average number of years of exposure. In
every case where urine values were recorded, both during, and 74
days after the end of, exposure, the chromium concentrations
decreased with time away from exposure.
A study of 12 workers in a galvanizing plant in the Federal
Republic of Germany showed an average concentration of chromium in
urine of 9.5 µg/litre (range, 1.4 - 24.6 µg/litre) compared with a
value of 1.8 ± 1.1 µg/litre in 60 unexposed workers (Schaller et
al., 1972). Gylseth et al. (1977) investigated a group of 14
welders exposed to about 0.05 mg chromium/m3 air, and reported a
urinary chromium concentration of approximately 40 mg/litre. At
the same exposure level, Tola et al. (1977) found a concentration
of 30 mg chromium/kg of creatinine in the urine. In both of these
studies, there was a correlation between recent exposure to
airborne chromium and chromium concentrations in urine. In the
study by Tola et al. (1977), it was shown that the water-soluble
fraction of airborne chromium was better correlated with the
concentration excreted in the urine than with total chromium. It
was also shown that the water-soluble fraction consisted mainly of
hexavalent chromium.
Table 9. Daily chromium excretion in urine
---------------------------------------------------------------------------
Subjects Number Excretion Range Reference
(µg/day) (µg/day)
(mean ± SD)
---------------------------------------------------------------------------
Adult males 2 0.72a 0.58 - 0.86 Schroeder et al.
(1962)
Adult males, fed 3 31.0a 20.8 - 46.5 Schroeder et al.
2 mg trivalent, (1962)
chromium/day for
3 months
Normal adults 16 13 ± 6 4 - 24 Voelkl (1971)
Chromate workerb 16 000
Normal adults 60 1.6 ± 1.1 Schaller et al.
(1972)
Galvano-technical 12 9.7 ± 6.6 1.4 - 24.6 Schaller et al.
workers (1972)
Normal young 20 8.4 ± 5.2 Hambidge (1974)
adults
Normal children, 18 5.5 ± 2.9 Hambidge (1974)
8 years old
Insulin-dependent 7 19.2 ± 18.9 Hambidge (1974)
diabetic children,
11 years old
Young women 9 7.2 ± 1.2 5.9 - 10.0 Mitman et al.
(1975)
Adult males 12 0.8 ± 0.4 0.4 - 1.8 Guthrie et al.
(1979)
---------------------------------------------------------------------------
Table 9. (contd.)
---------------------------------------------------------------------------
Subjects Number Excretion Range Reference
(µg/day) (µg/day)
(mean ± SD)
---------------------------------------------------------------------------
Adult males 91 0.48 ± 0.41a 0.42 - 0.53 Nomiyama et al.
(1980b)
Adult females 98 0.34 ± 0.31a 0.22 - 0.43 Nomiyama et al.
(1980b)
Adult males 48 0.20 ± 0.01a 0.05 - 0.58 Anderson et al.
(1982b)
Adult females 28
Adult males 27 0.17 ± 0.10 Anderson et al.
(1983a)
Adult females 15 0.20 ± 0.12 Anderson et al.
(1983a)
Adult males and 299 0.80 ± 0.6a 0.4 - 2.1 Fang (1983)
females
Normal adults 10 0.11 ± 0.05a 0.06 - 0.20 Kumpulainen et al.
(1983)
Normal adultsc 10 4.9 0.10 - 14.2 Zober et al.
(1984)
---------------------------------------------------------------------------
a Data calculated as µg/litre urine.
b Tanner, suffering from an acute ulceric gasteroenterocolitis.
c Samples taken post-mortem from autopsies. Original values are related
to mass (kg) instead of volume (litre).
Lindberg & Vesterberg (1983a) measured airborne, and urinary-
chromium levels among platers. Concentrations of chromium in urine
of < 5 µg/litre occurred when the time-weighted average values of
exposure were about or below 2 µg/m3 air. Severe damage to the
nasal septum and effects on lung function have not been found at
levels lower than this. It was shown that post-shift urinary-
chromium determinations could be used to monitor exposure in this
occupational group.
The urinary-chromium excretion and chromium clearance in 22
welders, who had been exposed to airborne hexavalent chromium (5 -
150 µg/m3) during 2 - 40 years (mean working time, 18.9 years) were
measured by Mutti et al. (1979). The method used was flameless
atomic absorption spectrometry. A highly significant correlation
was detected between the ratio of urinary-chromium to creatinine
and the airborne chromium concentration in the workplace, with
excretions ranging from 5.3 ± 3.7 to 33.3 ± 6.9 mg chromium/kg
creatinine in slightly exposed and heavily exposed welders,
respectively. The authors also reported an increase in chromium
clearance with increasing body burden of chromium, which indicates
that high urinary-chromium excretion may be caused by previous high
exposure as well as by current exposure.
Baseline data for chromium excretion in unexposed subjects in
the report by Mutti et al. (1979) are approximately 10 times higher
than the values recently proposed and generally accepted as normal.
However, there is reason (section 2.2.2) to accept relative
differences in analytical results in studies by one author using
one method, even if the absolute values reported may be questioned.
To test whether chromium excretion is also associated with
exercise-induced increases in glucose utilization, the urinary
chromium excretion, serum glucose, insulin, and glucagon of 9 male
runners (23 - 46 years old) were evaluated by Anderson et al.
(1982a). The mean urinary-chromium concentration was increased
nearly 5-fold, 2 h after running; excretion of sodium, potassium,
and calcium was unchanged. These data demonstrate an increase in
chromium excretion with exercise-induced increase in glucose
utilization.
(d) Milk
The chromium concentration was determined in 261 samples of
breast milk collected by manual expression from 45 American women.
Chromium levels were measured in whole, liquid milk by graphite-
furnace AAS, using the method of standard additions. The mean
chromium content of the breast milk samples was 0.30 µg/litre. The
range of individual values was 0.06 - 1.56 µg/litre and did not
change significantly with duration of lactation (Casey & Hambidge,
1984). Kumpulainen et al. (1983) analysed frozen samples of pooled
breast milk taken from women in different stages of lactation and
obtained from the Milk Bank of the Children's Hospital of Helsinki.
The mean chromium content ± SD was 0.49 ± 0.067 µg/litre (range,
0.37 - 0.57 µg/litre).
(e) Hair
Hair-chromium concentrations in children during the first 6
months of life were significantly higher than at any other age
(Hambidge & Baum, 1972); they declined from an initial value of
1493 µg/kg to an average of 412 µg/kg at 2 - 3 years. Hambidge
(1971) compared the chromium concentration in the hair of 15
newborn babies and that of their mothers: in only one case was the
chromium level in the mother's hair higher than that in the newborn
baby. Chromium levels were significantly lower than those
mentioned above in 50 Turkish women and their newborn babies (203
and 119 µg/kg, respectively) and only 12 newborn babies were found
to have higher concentrations than their mothers, suggesting
suboptimal chromium status (Gürson, 1977). Hair appears to reflect
the nutritional chromium status of groups. The hair-chromium level
is significantly lower in parous women than in nulliparae (Hambidge
& Rodgerson, 1969; Mahalko & Bennion, 1976) and in diabetic
children compared with normal controls (Hambidge et al., 1968). It
is low in adult-onset diabetic adults (Benjanuratra & Bennion,
1975). These findings are in agreement with the expected changes
in chromium balance during pregnancy and in diabetes.
5.2.2.2. Dynamic aspects of metabolism and the influence of
pathological states
Once chromium is absorbed into the organism, it clears rapidly
from the blood stream and is excreted or taken up by the tissues.
In a clinical study, Sargent et al. (1979) detected a 4-
compartment-type clearance from blood, with mean half-times of 13
min, 6.3 h, 1.9 days, and 8.3 days. However, the disappearance
from 3 tissue compartments was much slower, with half-times of
0.56, 12.7, and 192 days. The half-times for blood 3-compartment
clearance in rats (Hopkins, 1965) were calculated to be 0.56, 5.33,
and 57 h (Withey, 1983).
Whether any organ is specifically responsible for the storage
and release of the "metabolically responsive" chromium is not
known. The "metabolically responsive" chromium in blood is defined
as the fraction that increases acutely in response to an elevation
of blood-glucose or blood-insulin levels. It is believed that this
chromium increment interacts with the increased insulin secreted in
response to a glucose load, to facilitate the action of the hormone
on the insulin receptors of the insulin-sensitive cells.
In young, healthy subjects, but not in elderly subjects and
diabetic patients, an oral glucose load or the injection of insulin
results in a sudden increase in serum- or plasma-chromium
(Glinsmann et al., 1966; Levine et al., 1968; Hambidge, 1971; Behne
& Diel, 1972; Liu & Morris, 1978). This increase may appear 30
min, or as late as 120 min, after the challenge; much of the
chromium responsible for the increase is subsequently lost in the
urine. Lack of this increase, also termed "relative chromium
response", is often associated with impaired glucose tolerance,
indicative of chromium deficiency. It should be noted that, when
the glucose load was given intravenously (Pekarek et al., 1975) to
healthy volunteers, the serum-chromium level decreased rapidly,
while the blood-glucose level increased. Supplementation with
chromium chloride or high-chromium yeast extracts for several weeks
resulted in the reappearance of the relative chromium response and
improvement of glucose tolerance (Glinsmann et al., 1966; Liu &
Morris, 1978). These findings support the conclusion that the
"relative chromium response" measured during a glucose or insulin
tolerance test may serve as an indicator of the adequacy of
metabolically responsive chromium.
Much of the chromium increment secreted into the blood stream
in response to glucose or insulin is subsequently lost in the
urine. Hambidge (1971) observed greatly increased urinary-chromium
excretion in 2 diabetic children after insulin therapy had begun,
compared with the excretion in the same children before insulin
treatment. It is not known whether the increased urinary loss of
chromium is compensated for by an increase in absorption efficiency
from the intestines. Hambidge et al. (1968) reported significantly
lower chromium concentrations in the hair of diabetic children
compared with normal children, and Morgan (1972) found that
the chromium contents in the livers of 31 diabetic adults at
autopsy were lower than those in 24 control livers from non-
diabetic persons (8.57 versus 12.7 mg/kg ash; P = 0.05).
On the other hand, Doisy et al. (1971) demonstrated greatly
increased intestinal absorption, together with elevated chromium
excretion, in 14 insulin-dependent diabetic patients administered
51CrCl3 x 6H2O, orally. It is not known whether the greater
absorption efficiency is adequate to compensate for the increased
urinary losses; the decreased chromium concentrations in hair and
liver, discussed above, suggest that a negative balance may prevail.
Several other pathological conditions affect chromium
metabolism. Sargent et al. (1979) detected significantly less
retention of intravenously administered 51chromium in 11 patients
with haemochromatosis compared with 5 normal controls. This may be
related to the high saturation with iron of transferrin, which is
also the carrier protein for newly absorbed chromium.
Chronic ischaemic heart disease also affects chromium
metabolism. Neiko & Del'va (1978) observed a significantly
increased urinary-chromium loss, greater by a factor of 1.5 - 1.6
than that of normal controls, in 65 heart patients. The urinary-,
and to a lesser extent, the faecal-chromium loss increased
progressively with increasing severity of signs and symptoms and
resulted in a negative chromium balance in the post-infarct state.
The balance became positive again on discharge from the hospital
after medical treatment.
Acute infections also appear to influence chromium metabolism.
Pekarek et al. (1975) measured glucose tolerance, and insulin and
chromium levels in human volunteers, before and after infection
with the benign sandfly fever virus. Impaired glucose tolerance and
a significantly increased insulin response to a glucose load,
observed at the height of the infection, were accompanied by very
significantly depressed serum-chromium levels (0.5 µg/litre,
compared with 1.4 µg/litre before infection; P < 0.05) In
contrast with the sharp decline in serum-chromium following the
intravenous injection of glucose in the healthy state observed by
these authors, the depressed serum-chromium levels declined very
little during the glucose tolerance test at the height of
infection. The mechanism of the changes in chromium metabolism in
heart disease and sandfly fever is not clear. Of great potential
importance is the unanswered question of whether the observations
described here reflect an increased chromium requirement in the
patients or a normal reaction to various forms of stress.
The information on the dynamic aspects of chromium metabolism
in animals is limited and should be considered in connexion with
the more detailed studies on human subjects. Diabetes, induced in
rats by the injection of streptozotocin, affected the tissue
distribution of injected 51CrCl3. Sixteen days after injection of
streptozotocin, 51CrCl3 was injected into 5 diabetic rats and 6
normal controls and the 51Cr content measured 5 days later. The
serum of the diabetic rats contained more than 3 times the 51Cr
activity found in the controls (0.24 versus 0.07% of the injected
dose P < 0.01). Significant differences were also detected in the
distribution of 51Cr in the subcellular fractions of the liver; in
the diabetic tissue, 51Cr activity was higher in the nuclear and
supernatant fractions ( P < 0.01) and lower in mitochondria and
microsomes ( P < 0.05). The mechanism responsible for these
changes is not known (Mathur & Doisy, 1972).
5.3. Influence of Chemical Form
The diverse biological effects of chromium on living organisms
cannot be understood without knowledge of the chemical and physical
forms in which the element is present. As stated earlier, the
metallic state (zero valence) is biologically inert, the trivalent
state represents the essential element chromium, and the hexavalent
state is of concern to the toxicologist. Compounds of trivalent
chromium are poorly absorbed, whereas those of the hexavalent state
easily penetrate physiological barriers, such as cell membranes.
Hexavalent chromium compounds are easily reduced by living matter,
but oxidation of trivalent to hexavalent chromium does not occur in
the organism.
The physical form of hexavalent compounds (such as particle
size) and chemical properties (such as solubility) determine
metabolic pathways after inhalation and, therefore, health effects.
There is an equally strong influence of the chemical form of
trivalent chromium on metabolism and health effects. When chromium
is bound to water or small anions (e.g., CrCl3 x 6H20), it
precipitates in the neutral or alkaline milieu of the body fluids.
When it is bound to ligands, such as organic acids, the element is
light in solution and is available for intestinal absorption. The
forms in which trivalent chromium occurs in nature are not really
known. Plants probably contain chromium complexes with organic
acids.
The biological availability of chromium compounds in foods is
of great nutritional importance, but is poorly defined. One
compound or a group of closely related compounds, glucose tolerance
factor, has been isolated from yeast and shown to be more active
than chromium chloride in genetically diabetic mice and in the in
vitro potentiation of the action of insulin on rat epididymal fat
tissue (section 7.1.5.2). It has been identified as a dinicotinic-
acid glutathione complex, but the exact stereochemical structure is
not yet known (Toepfer et al., 1977). It has been postulated, but
not proved, that this factor is the active form of chromium within
the organism.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The environmental effects of chromium as a pollutant have been
reviewed by US EPA (1978), Anderson (1982), and EIFAC (1983).
Various effects have been reported, but, because of the presence of
other chemicals, it remains doubtful whether chromium alone is
responsible for the effects observed. Data are available on
microorganisms, plants, and aquatic organisms.
6.1. Microorganisms
Most microorganisms (protozoa, protophyta, fungi, algae,
bacteria) are able to absorb chromium. The active uptake of
chromate by the sulfate transport system has been shown in
Neurospora crassa (Roberts & Marzluf, 1971). No distinction has
been made between ab- and adsorption in other studies (e.g., algae)
(Calow & Fletcher, 1972), and it has not yet been shown that
chromium is an essential element for microorganisms. In general,
toxicity for most microorganisms occurs in the range of 0.05 - 5 mg
chromium/kg of medium. The internal concentration of chromium
depends on the species. In most groups of microorganisms, it
ranges between the levels of 0.6 mg dry weight present in one litre
of sample of microplankton from Monterey Bay, California, USA, and
21.4 mg/litre phytoplankton collected in the Pacific Ocean (Martin
& Knauer, 1973).
Trivalent chromium is less toxic than hexavalent. The main
features are inhibition of growth (at concentrations greater than
0.5 mg/litre in Chlorella cultures) (Nollendorf et al., 1972) and
inhibition of various metabolic processes, such as photosynthesis
or protein synthesis (US EPA, 1978).
The toxicity of chromium for soil bacterial isolates was
studied by measuring the turbidity of liquid cultures supplemented
with hexavalent chromium and trivalent chromium. Gram-negative
bacteria were more affected by hexavalent chromium (1 - 12 mg/kg)
than gram-positive bacteria. Toxicity due to trivalent chromium
was not observed at similar levels. The toxicity of low levels of
hexavalent chromium (1 mg/kg) indicates that soil microbial
transformations, such as nitrification, may be affected (Ross et
al., 1981).
6.2. Plants
Although chromium is present in all plants, it has not been
proved to be an essential element for plants. Most substances,
including chromium, can be absorbed through either the root or the
leaf surface. Several factors affect the availability of chromium
for the plant (Black, 1968), including the pH of the soil,
interactions with other minerals or organic chelating compounds,
and carbon dioxide and oxygen concentrations.
Little chromium is translocated from the site of absorption;
however, the chelated form is transported throughout the plant
(Verfaillie, 1974).
Chromium in high concentrations can be toxic for plants, but
Yopp et al. (1974) stated that there was no specific pattern of
chromium intoxication.
During the smelting of chromite, considerable quantities of
waste are produced, which contain soluble chromates. When combined
with a high pH, Gemmell (1973) showed an inhibition of germination
and growth in white mustard plants (Sinapis alba) growing on waste
heaps. Covering the waste with a 25-to 30-cm layer of granular-
free-draining subsoil followed with layers of soil, peat, or sewage
sludge was shown to be the best revegetation technique (Gemmell,
1974).
The main feature of chromium intoxication is chlorosis, which
is similar to iron deficiency (Hewitt, 1953).
Soybeans, treated in nutrient culture containing 0 - 5 mg
hexavalent chromium/litre showed decreased uptake of calcium,
potassium, phosphorus, iron, and manganese (Turner & Rust, 1971).
Death of plants occurred within 3 days of treatment with 30 or 60
mg chromium/litre.
A reduction in leaf dry weight occurred after treatment with
0.01 mg hexavalent chromium/litre (Rediske et al., 1955). Chromium
affects the carbohydrate metabolism, and the leaf chlorophyll
concentration decreased with increasing hexavalent chromium
concentration (0.01 - 1 mg hexavalent chromium/litre) (Rediske,
1956). Hexavalent chromium appears to be more toxic than trivalent
chromium (Hewitt, 1953; Stanley, 1974; Verfaillie, 1974). At
present, no data are available concerning the mechanism of action
or the dose-dependent pattern of chromium intoxication.
6.3. Aquatic Organisms
More studies have been performed with aquatic species than with
free-living (non-parasitic) animals. Depending on the species,
chromium can be less toxic for fish in warm water, but marked
decreases in toxicity are found with increasing pH or water
hardness; changes in salinity have little if any effect on its
toxicity. Chromium can make fish more susceptible to infection;
high concentrations can damage and/or accumulate in various fish
tissues and in invertebrates such as snails and worms.
Reproduction of Daphnia was affected by exposure to 0.01 mg
hexavalent chromium/litre (EIFAC, 1983). Numerous other factors
influence the availability of chromium and, therefore, its
toxicity. These include the presence of other minerals and organic
pollutants, and the temperature of the environment; this has been
shown in mice (Nomiyama et al., 1980a).
Hexavalent chromium is accumulated by aquatic species by
passive diffusion (US EPA, 1978). Ecological factors, in the
abiotic and living environment, are involved in this process, which
varies according to the sensitivity of different species. The
physiological state and activity of the fish also affect
accumulation (Reichenbach-Klinke, 1977, 1980). Kittelberger (1973)
analysed the organs and tissues of the roach (Leuciscus rutilus)
from the river Rhine and found that concentrations of chromium in
the spleen, bronchi, and intestine (between 30 and 37.5 mg/kg) were
10 - 30 times higher than those in the heart, skin, muscle, and
scales.
LC50s are listed in Table 10 for hexavalent and trivalent
chromium compounds in the aquatic environment.
Table 10. The toxicity of chromium for fresh-water organisms
(expressed as 50% mortality)a
-----------------------------------------------------------------------
Compound Category Exposure Toxicity range Most
(mg/litre) sensitive
species
-----------------------------------------------------------------------
hexavalent invertebrate acute 0.067 - 59.9 scud
chromium long-termb - -
vertebrate acute 17.6 - 249 fathead minnow
long-term 0.265 - 2.0 rainbow trout
trivalent invertebrate acute 2.0 - 64.0 cladoceran
chromium long-term 0.066 cladoceran
vertebrate acute 33.0 - 71.9 guppy
long-term 1.0 fathead minnow
-----------------------------------------------------------------------
a From: US EPA (1980).
b No data available.
In general, invertebrate species, such as polychaete worms,
insects, and crustaceans are more sensitive to the toxic effects of
chromium than vertebrates, such as some fish (Mathis & Cummings,
1973). The lethal chromium level for several aquatic and
nonaquatic invertebrates has been reported to be 0.05 mg/litre (US
NAS/NAE, 1972).
EIFAC (1983) reviewed the literature on the occurrence and
effects of chromium in fresh water and proposed tentative water-
quality criteria that distinguish between salmonid and non-salmonid
waters. To protect salmonid waters, the mean aqueous concentration
of "soluble" chromium should not exceed 0.025 mg chromium/litre,
and the 95 percentile should not exceed 0.1 mg chromium/litre.
However, more stringent values may be necessary in very soft, acid
waters, and less stringent values in alkaline waters.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Nutritional Effects of Chromium
The criteria for an essential nutrient have been defined in
different ways by different authors, but all definitions postulate
that a reduction in the total daily intake of the nutrient below a
certain level must consistently induce signs of deficiency, and
that the supplementation of the daily intake above this level must
prevent and cure the deficiency signs. Chromium deficiency has
been produced experimentally in mice, rats, and squirrel monkeys,
and the full reversal of the deficiency signs by the oral
administration of chromium has been demonstrated in rats (Mertz,
1969). For these reasons, chromium must be considered an essential
micro-nutrient. It is physiologically active in the trivalent
oxidation state at concentrations of approximately 100 µg/kg diet.
7.1.1. Effects of deficiency on glucose metabolism
Semipurified rations, containing Torula yeast as the source of
protein, were fed to groups of 10 male Sprague Dawley rats at
weaning, and intravenous glucose tolerance tests were performed by
injecting 1250 mg glucose/kg body weight and measuring the
subsequent decline in blood-glucose levels. The rate of glucose
disappearance can be calculated from the straight line plot of log
increment glucose (excess of glucose at any time (t) over fasting
glucose), versus time. The disappearance constant k is expressed
as % decline of the increment glucose per min; it is a measure of
the efficiency of glucose utilization. In weaning rats, the rate
constant was found to decline, within 3 weeks or less, from an
average of 4%/min to 2.6%/min in animals fed the Torula yeast diet,
but not in animals administered a diet in which 4 or 8% of Torula
yeast was replaced by an equal amount of brewer's yeast. This
observation suggested that brewer's yeast, but not Torula yeast,
contained an unknown substance necessary for the maintenance of
normal glucose tolerance. Because the only known effect of the
substance was that on glucose tolerance, it was named "Glucose
Tolerance Factor" (GTF) (Mertz & Schwarz, 1959). GTF was extracted
from brewer's yeast and pork kidney powder, concentrated and
purified, and its active ingredient was identified as trivalent
chromium (Schwarz & Mertz, 1959). Chromium in the form of most
common complexes (except for very stable ones) cured the impairment
of glucose tolerance in deficient rats, either as one oral dose of
200 µg/kg body weight, or as an intravenous (iv) injection of 2.5
µg/kg body weight. Chromium in the diet also prevented the
impairment of glucose tolerance.
In order to produce more pronounced deficiencies of chromium
and other trace elements, Schroeder et al. (1963) constructed a
special animal house on a mountain top in Vermont, USA, far removed
from traffic and industry. The interior was specially coated with
organic resins to reduce any metallic exposure. Air was introduced
through special filters, and strict practices were enforced to
avoid the introduction of dust and dirt. This will be referred to
as the "controlled environment". Under these conditions, a more
severe chromium deficiency resulted in very low glucose removal
rates of 1.12%/min in 6 rats and of 0.18%/min in 4 female breeder
rats (482 days old), after 11 days on a chromium-deficient diet.
The removal rate in 4 control rats receiving the same diet with a
chromium supplement improved from 0.51 to 1.39% during the same
period (Mertz et al., 1965b). This strong impairment of glucose
tolerance in rats kept in the "controlled environment" was
reflected in their fasting blood-glucose levels, compared with
those of chromium-supplemented controls: 1370 ± 68 versus 1170 ± 17
mg/litre in males and 1390 ± 68 versus 960 ± 65 mg/litre in
females. More than half of 185 deficient rats excreted more than
0.25% glucose in the urine, whereas glycosuria was found in only 9
of the chromium-supplemented controls (Schroeder, 1966). A
significant reduction in the intravenous glucose removal rate was
also observed in 8 rats in plastic cages, fed an EDTA-washed low-
protein diet (7% casein), compared with 8 controls receiving 50 µg
chromium as the chloride, by stomach tube, daily for 2 weeks (2.1
versus 3.6%/min; P < 0.01). However, chromium supplements did not
improve the near-normal removal rates in rats receiving a 20%
casein diet (Mickail et al., 1976). Significantly lower plasma-
glucose levels, due to supplementation with chromium, were reported
in rats fed the chromium-deficient Torula yeast diet (Whanger &
Weswig, 1975). In another series of studies, only a slight
reduction in blood-glucose (from 1240 to 1180 µg/litre) was found
in 10 rats receiving a diet supplemented with chromium (10 µg/kg)
(Djahanshiri, 1976).
Impaired glucose tolerance in squirrel monkeys, fed a
commercial laboratory chow, was shown to respond to chromium
supplementation. Of 9 monkeys with an impaired glucose removal
rate (1.38%/min), 8 responded after 22 weeks of supplementation of
their drinking-water (chromium acetate, 10 mg/litre) with a
normalization of their glucose tolerance (average removal rate, N =
9, 2.33 ± 0.3%/min) (Davidson & Blackwell, 1968). There was no
effect on food consumption, growth rate, or serum-insulin
concentrations. The chromium content of the commercial chow was
stated to be 3.3 mg/kg, a very high concentration. In view of the
uncertainties of methods of analysis for chromium (section 2.2), it
is not possible to interpret the results as indicative of a very
high chromium requirement of the squirrel monkey or of an unusually
poor bioavailability of the chromium in that particular ration. A
marginal chromium deficiency may have existed in mice fed a bread
and milk diet, as daily administration of 10 µg chromium for 16
days to 8 months produced a 10 - 30% decline in blood-glucose
levels (Vakhrusheva, 1960). However, this effect was not specific
for chromium, as it was also observed when manganese was
administered.
The glucose tolerance of guinea-pigs did not differ
significantly between groups fed diets containing chromium at
0.125, 0.625, or 50 mg/kg, even though the animals fed the 2 higher
levels exhibited a lower mortality rate during pregnancy (Preston
et al., 1976).
Although the administration of synthetic glucose tolerance
factor (a chromium-dinicotinic acid-glutathione complex) to 6 pigs
did not affect glucose tolerance tests, it resulted in a
significant increase in the hypoglycaemic effect of insulin
injected at 0.1 U/kg body weight (Steele et al., 1977a).
Turkey poults, fed a practical ration containing chromium
levels of 5 mg/kg, responded to chromium supplementation (20 mg/kg
diet) with a significant increase in liver glycogen and in glycogen
formation, following a fast, and with a significant increase in
glycogen synthetase (EC 2.4.1.11) activity in the liver (Rosebrough
& Steele, 1981).
It can be concluded that the impairment of glucose tolerance in
rats fed a low-chromium Torula yeast diet is due to chromium
deficiency. The effects of chromium in squirrel monkeys, pigs, and
turkeys, though statistically significant, are somewhat difficult
to interpret, because of the reported high chromium content of the
basal diet. No evidence for chromium deficiency has yet been
obtained through glucose tolerance tests on other animal species.
7.1.2. Effects of deficiency on lipid metabolism
Though trivalent chromium in high doses (2.5 mg/kg body weight)
has been shown to increase the synthesis of fatty acids and
cholesterol in the liver (Curran, 1954), lower, physiological doses
appear to decrease serum-cholesterol concentrations in rats.
Schroeder & Balassa (1965) found an average level of 927 mg
cholesterol/litre serum in 24- to 26-month-old male rats, kept in a
controlled environment and administered chromium in the drinking-
water at a concentration of 5 mg/litre, compared with an average of
1229 mg/litre in controls not receiving chromium ( P < 0.01). The
effects in female rats were ambigous, one study producing the
expected reduction in cholesterol due to chromium, another showing
an elevation in the supplemented female rats. Schroeder's
observations of a cholesterol-reducing effect of chromium in male
rats were confirmed by Staub et al. (1969) and by Whanger & Weswig
(1975) but were contradicted by results of a third study in which
there were not any significant effects of chromium on sucrose-
induced triglyceridaemia and cholesterolaemia (Bruckdorfer et al.,
1971). Perhaps more significant than the effect on circulating
cholesterol is the direct effect of chromium on the occurrence of
aortic plaques. Schroeder & Balassa (1965) observed 6 plaques in 54
male and female chromium-deficient rats, but only one plaque in 48
animals receiving 5 mg chromium/litre in drinking-water. These
results are in agreement with those from a subsequent report of the
protective effect of the natural chromium content of water (60 -
215 µg/litre) against atherosclerosis in cholesterol-fed rabbits
(Novakova et al., 1974). Abraham et al. (1980) extended these
observations by demonstrating that daily chromium injections (20 µg
K2CrO4) reversed the established atherosclerosis in the aorta of 11
cholesterol-fed rabbits, compared with 12 controls. The mean
plaque area was reduced from 95% to 63%, the total aortic
cholesterol from 729 mg to 458 mg, and the atheromatous lesions, as
measured by technetium incorporation from 285 000 cpm to 114 000
cpm, all differences being statistically significant. These
results are reinforced by observations in man discussed in section
8.1.
7.1.3. Effects of deficiency on life span, growth, and
reproduction
The mortality of male, but not of female mice, raised in a
"controlled environment" (section 7.1.1) was reduced by trivalent
chromium administered as acetate in the drinking-water at a
concentration of 5 mg/litre (Schroeder et al., 1964). The survival
rates at 12 months were 92.6% and 68.8% ( P < 0.0001) in
supplemented and deficient animals, respectively. Similary, male,
but not female, rats receiving a chromium concentration of 5
mg/litre in the drinking-water had longer life spans than deficient
controls. The mean age of the last surviving 10% of animals was
1249 days, compared with 1141 days in the deficient animals ( P <
0.01). Survival of male rats fed a low-chromium (< 100 µg/kg),
low-protein ration and subjected to a controlled acute haemorrhage
was significantly less than that of chromium-supplemented rats, in
2 studies (67 versus 92%; P < 0.05 and 27 versus 60%; P < 0.01,
respectively) (Mertz & Roginski, 1969).
In Schroeder's study, growth rates in treated mice and rats of
both sexes raised in a "controlled environment", were higher after
6 and 12 months, with highly significant differences in body weight
ranging from 9 to 17% ( P < 0.005) compared with the controls.
Again, the effects of chromium supplementation were greater in
males than in females (Schroeder et al., 1964, 1965).
Similar results were reported by Djahanschiri (1976), who
studied a total of 2750 rats of a special inbred strain (Hk51) fed
a basal diet (0.15 mg chromium/kg diet) and chromium supplements
ranging from 10 to 500 mg/kg diet. At 12 weeks, the average
weights of the chromium-supplemented animals, regardless of dose
level, were significantly higher (by 6% in the males and 3% in the
females) than those of the animals on the basal diet. The same
author reported a progressive diminution in both the milk
production of lactating rats and weight gain in 3 consecutive
generations fed the low-chromium diet, compared with rats receiving
chromium supplementation. Increased mortality was reported in
pregnant guinea-pigs fed a low-chromium diet (125 µg/kg diet)
compared with animals receiving a chromium supplement of either 625
µg/kg or 50 mg/kg (Preston et al., 1976).
When rats raised on a low-chromium Torula yeast diet (< 100
g/kg) mated with those on a normal diet, they were able to
impregnate the females at a 100% conception rate only up to the age
of 4 months. After this age, the conception rate declined to 25%,
25%, and 0%, at the age of 7, 8, and 9 months, respectively. This
decline was accompanied by a significant ( P < 0.01) decrease in
the sperm count in the chromium-deficient males to approximately
half of the count in supplemented controls at the age of 8 months
(Anderson & Polansky, 1981).
7.1.4. Other effects of deficiency
Male weanling rats, fed a 10% soya protein ration with a
chromium content of less than 100 µg/kg, developed a visible
opacity of the cornea in one or both eyes. In several studies, the
incidence of this effect ranged from 10 to 15% in deficient rats.
No opacities developed in control animals receiving 2 mg
chromium/kg diet (Roginski & Mertz, 1967).
Chromium deficiency has been shown to reduce the physical
performance of rats under stress. Ten male rats raised on a
chromium-deficient diet (150 µg/kg diet) swam for an average of 250
min, until exhaustion, in contrast with 10 rats receiving a
supplement of 10 mg chromium/kg diet, which were exhausted only
after 320 min (Djahanschiri, 1976).
7.1.5. Mechanism of action of chromium as an essential nutrient
7.1.5.1. Enzymes, nucleic acids, and thyroid
Chromium is present in nucleic acids in very high
concentrations, but the function of these is not clear at present
(Mertz, 1969). However, recent work suggests a biological function
of chromium in nucleic acid metabolism (Okada et al., 1984).
Ribonucleic acid synthesis in mouse liver was significantly
increased by as little as 1 µmol trivalent chromium, in the
presence of DNA or chromatin (Okada et al., 1981). These effects
were also present when the DNA or chromatin were first complexed
with chromium prior to incubation. However, prior complexation of
RNA polymerase with chromium depressed activity. These effects
were obtained in vitro with a concentration (52 µg/litre) that is
similar to physiological levels. Goncharov (1968) presented data
suggesting that chromium is involved in the function of the thyroid
gland. These findings have been supported by Lifschitz et al.
(1980).
An oligopeptide with a relative molecular mass of 1480, which
was crystallized from liver tissue, had a specific affinity for
chromium (Wu, 1981).
7.1.5.2. Interaction of chromium with insulin
The interaction of chromium with insulin has been extensively
studied and can therefore be presented in some detail, but this
does not imply that this is the only, or the most important,
function of chromium.
The effects of chromium in vitro, and probably in vivo, depend
on the presence of endogenous or exogenous insulin, no effects
having been demonstrated in in vitro systems that did not either
depend on, or contain, insulin. Chromium deficiency causes an
impaired response to added insulin in rat epididymal fat tissue,
and, when glucose uptake or glucose oxidation or utilization for
lipid synthesis is measured, the dose-effect curve is flat.
Addition of suitable chromium compounds significantly increases the
slope of the curve (Fig. 3). This demonstrates the true
potentiation of the insulin action and indicates that chromium
alone does not act as an insulin-like substance (Mertz et al.,
1961; Mertz & Roginski, 1971; Mertz, 1981). Chromium was also
shown to stimulate the transport of D-galactose into epididymal fat
cells. This suggests cell transport, the first step of sugar
metabolism, as a major site of action for chromium (Mertz &
Roginski, 1963). Insulin-potentiating effects have also been
observed on the swelling of liver mitochondria (Campbell & Mertz,
1963) and on glucose utilization in isolated rat lens (Farkas &
Roberson, 1965).
 Stimulation of the effects of insulin has been observed in a
glucose-independent, but insulin-responsive, system. Significantly
more alpha-amino isobutyric acid (a non-metabolizable amino acid
analogue) was incorporated into the heart and liver tissue of
chromium-supplemented male rats than in the tissues of chromium-
deficient controls, in response to the in vivo injection of the
labelled analogue and insulin (Roginski & Mertz, 1969).
These observations suggest a peripheral action of chromium to
facilitate the action of insulin; no evidence has been produced
indicating that chromium plays any role in the production, storage,
or release of insulin by the pancreas. Thus, the primary result of
chromium deficiency is a diminution in the effectiveness of
insulin. The resulting metabolic impairment may be compensated for
by increased insulin production in some cases, resulting in
elevated concentrations of the hormone, but not enough data exist
from experimental animal studies to assess the action of the
element on insulin metabolism. More information is available for
human subjects and this is discussed in section 8.1. The
interaction between chromium, insulin, and receptor sites of liver
mitochondrial membranes was studied using polarographic techniques.
The results formed the basis for the hypothesis that chromium may
facilitate bond formation between the intra-chain disulfide of
insulin and sulfur-containing groups of the receptors, by
participating in a ternary complex (Christian et al., 1963).
This hypothesis is consistent with results of studies on rats
fed, either a low-chromium Torula yeast diet or a brewer's yeast
diet known to be adequate in chromium. While the insulin-binding
capacity of hepatocytes was not significantly different, the
insulin affinity of the cells was significantly greater ( P < 0.01)
for the chromium-adequate rats than for the deficient Torula yeast
rats (Steele et al., 1977b).
7.1.6. Chromium nutritional requirements of animals
In the preceding sections, studies were evaluated in which the
effects of chromium supplementation were determined in animals that
were at least marginally chromium deficient. In other studies, the
effects of chromium were investigated in animal systems in which
the existence of chromium deficiency was either not ascertained or
not investigated. Before these studies are described and
interpreted for the determination of chromium nutritional
requirements of animals, it is helpful to consider them against the
background of Venchikov's (1974) model. This model is generally
applicable to trace element effects defining 3 zones of action, the
zone of biological action, that of pharmacodynamic action, and that
of toxicity (Fig. 4). The biological zone, in response to
supplements with low amounts of an element, represents the
correction of a deficiency and the resulting level of biological
activity is that of optimal function. Increasing the amount of
supplement further may lead to a certain depression, followed by a
zone of new, increased activity, in which the element no longer
acts as an essential nutrient, but as a drug. Still greater
supplements, beyond the homeostatic control capability of the
organism produce toxic effects and death. Because all the studies
described subsequently involved amounts of chromium supplements
that were higher than the levels normally needed to correct a
deficiency, it is possible, according to Venchikov's definition,
that the observed effects might be pharmacological.
Stimulation of the effects of insulin has been observed in a
glucose-independent, but insulin-responsive, system. Significantly
more alpha-amino isobutyric acid (a non-metabolizable amino acid
analogue) was incorporated into the heart and liver tissue of
chromium-supplemented male rats than in the tissues of chromium-
deficient controls, in response to the in vivo injection of the
labelled analogue and insulin (Roginski & Mertz, 1969).
These observations suggest a peripheral action of chromium to
facilitate the action of insulin; no evidence has been produced
indicating that chromium plays any role in the production, storage,
or release of insulin by the pancreas. Thus, the primary result of
chromium deficiency is a diminution in the effectiveness of
insulin. The resulting metabolic impairment may be compensated for
by increased insulin production in some cases, resulting in
elevated concentrations of the hormone, but not enough data exist
from experimental animal studies to assess the action of the
element on insulin metabolism. More information is available for
human subjects and this is discussed in section 8.1. The
interaction between chromium, insulin, and receptor sites of liver
mitochondrial membranes was studied using polarographic techniques.
The results formed the basis for the hypothesis that chromium may
facilitate bond formation between the intra-chain disulfide of
insulin and sulfur-containing groups of the receptors, by
participating in a ternary complex (Christian et al., 1963).
This hypothesis is consistent with results of studies on rats
fed, either a low-chromium Torula yeast diet or a brewer's yeast
diet known to be adequate in chromium. While the insulin-binding
capacity of hepatocytes was not significantly different, the
insulin affinity of the cells was significantly greater ( P < 0.01)
for the chromium-adequate rats than for the deficient Torula yeast
rats (Steele et al., 1977b).
7.1.6. Chromium nutritional requirements of animals
In the preceding sections, studies were evaluated in which the
effects of chromium supplementation were determined in animals that
were at least marginally chromium deficient. In other studies, the
effects of chromium were investigated in animal systems in which
the existence of chromium deficiency was either not ascertained or
not investigated. Before these studies are described and
interpreted for the determination of chromium nutritional
requirements of animals, it is helpful to consider them against the
background of Venchikov's (1974) model. This model is generally
applicable to trace element effects defining 3 zones of action, the
zone of biological action, that of pharmacodynamic action, and that
of toxicity (Fig. 4). The biological zone, in response to
supplements with low amounts of an element, represents the
correction of a deficiency and the resulting level of biological
activity is that of optimal function. Increasing the amount of
supplement further may lead to a certain depression, followed by a
zone of new, increased activity, in which the element no longer
acts as an essential nutrient, but as a drug. Still greater
supplements, beyond the homeostatic control capability of the
organism produce toxic effects and death. Because all the studies
described subsequently involved amounts of chromium supplements
that were higher than the levels normally needed to correct a
deficiency, it is possible, according to Venchikov's definition,
that the observed effects might be pharmacological.
 Tuman & Doisy (1977) studied the effects of yeast concentrates
of high chromium (glucose tolerance factor) content and of
synthetic chromium complexes with GTF activity (Tuman et al., 1978)
in mice, raised on a presumably complete commercial stock diet.
Six animals were used for each test, either genetically diabetic
mice or their control litter mates of the C57Bl-KSI strain.
Injections of either 5 mg of the GTF-containing yeast extracts or
0.1 mg of the synthetic chromium complex, acutely reduced the
elevated plasma-glucose levels in the diabetic and the non-fasting
normal mice by 10 - 38% of the initial values ( P < 0.01) and the
plasma-triglycerides by 26 - 56% ( P < 0.01), compared with control
mice injected with saline. Injection of insulin into diabetic mice
produced only an 11 - 18% decrease in plasma-glucose levels,
whereas injection of the GTF-containing extract together with
insulin reduced plasma-glucose levels by 39 - 51% and plasma-
triglyceride levels by 76% (Table 11).
The results suggest either a much higher increase in the
chromium requirement of the genetically diabetic mice or their
inability to use chromium in the diet.
Table 11. Acute effects of GTF and exogenous insulin on non-
fasting plasma-glucose and plasma-triglyceride (TG) concentrations
in 19-week-old genetically diabetic micea
------------------------------------------------------------------
Treatment Plasma- deltaGlucose Plasma- deltaTG
glucoseb triglyceridesb
(mg/litre) (mg/litre)
------------------------------------------------------------------
saline 11 120 ± 3960 ± 160(5)c
490(5)c
GTF 9320 ± -180 (16%) 2790 ± 170(5)d -117 (30%)
280(5)d
insulin 9840 ± -128 (12%) 3020 ± 400(5) - 94 (24%)
410(5)
insulin 7060 ± -406 (37%) 940 ± 220(5)e -302 (76%)
and GTF 840(5)e
------------------------------------------------------------------
a Modified from: Tuman & Doisy (1977).
b Glucose and triglyceride values represent mean ± SEM for 6 mice in
each treatment group. Dose of GTF was 5 mg (WL-10-AT) administered
intraperitoneally, 12 h prior to collection of blood. Lente insulin
(0.1 U per mouse) was administered subcutaneously, 12 h prior to
collection of blood. Data were treated by analysis of variance to
detect differences between the various treatment groups; independent
orthogonal comparisons were performed in the following groups ( P value
indicates level of significance for each comparison).
c Saline versus all other treatments, P < 0.005.
d GTF versus insulin, P < 0.05.
e GTF and insulin alone versus combined GTF and insulin, P < 0.005.
Thus, GTF and insulin > GTF = insulin > saline.
Steele & Rosebrough (1979) reported a significant stimulation
of the growth rate of one-week-old turkey poults (both sexes) by
supplementation of a practical ration with 20 mg chromium (as
chloride)/kg. The weight gains within the 2-week study were 235 g
and 267 g for the 60 controls and 60 supplemented turkeys,
respectively ( P < 0.001). As the practical ration contained
ground yellow corn and soybean meal, limestone, and dicalcium
phosphate, chromium deficiency would appear unlikely. The amount
of the chromium supplement (20 mg/kg diet) is quite high, and
further studies are needed to decide whether the observed effects
were of a pharmaco-dynamic nature or truly nutritional, i.e.,
correcting a deficiency. A similar interpretation should be
applied to a report of improved egg quality in the laying hen
(Jensen et al., 1978).
The quantitative aspects of the effects of chromium on animals
can be summarized as follows: normal rats fed semi-purified, semi-
synthetic rations, with Torula yeast or individual proteins and
sucrose or starch as the source of carbohydrates, develop mild
signs of deficiency at a dietary level of 100 - 150 µg chromium/kg.
To prevent deficiency, most authors used very high supplements of
several mg/kg diet and did not determine the biological
availability of the chromium complexes used for the
supplementation. Diets containing raw ingredients and supplying
chromium levels between 0.5 and 1 mg/kg do not induce signs of
deficiency and probably meet the requirement of the rat. A very
tentative estimate of the dietary chromium need of the rat and
probably the mouse would be approximately 0.5 mg/kg diet. This
estimate should be interpreted with caution, because of the lack of
knowledge concerning the biological availability of chromium and of
its interaction with dietary constituents.
7.2. Toxicity Studies
The toxicology of chromium compounds has been reviewed by the
US National Academy of Science (US NAS, 1974a), Langard & Norseth
(1979), the International Agency for Research on Cancer (IARC,
1980), Langard (1980a, 1982), and Burrows (1983).
In discussing toxicological problems, it is important to
differentiate between the various oxidation states of chromium and
its compounds. Trivalent chromium, when administered to animals in
food or water, does not appear to induce any harmful effects, even
when given in large doses (US NAS, 1974a) (section 7.2.1). Acute
and chronic toxic effects of chromium are mainly caused by
hexavalent compounds. Since it has been shown that both industrial
trivalent chromium compounds as well as reagent-grade trivalent
chromium compounds can be contaminated by hexavalent chromium
(Petrilli & DeFlora, 1978a; Levis & Majone, 1979), the evaluation
of experimental studies becomes difficult, especially when the
purity of the chemical compounds used is not known.
Discrimination between the biological effects, caused by
hexavalent chromium and trivalent chromium is difficult, because,
after penetration of membranes in tissues, hexavalent chromium is
immediately reduced to trivalent chromium (Gray & Sterling, 1950;
US NAS, 1974a), and it is not evident whether the observed
phenomena are caused by this reduction or even by the trapping of
trivalent chromium by ligands after uptake in the cells. Another
problem in evaluating the data is associated with the route of
administration. Hexavalent chromium, introduced by the oral route,
is partly reduced to trivalent chromium by acidic gastric juice
(Donaldson & Barreras, 1966; DeFlora & Boido, 1980); thus, the
effects or lack of effects observed may be caused mainly by
trivalent chromium and not by the hexavalent chromium, actually
administered.
7.2.1. Effects on experimental animals
Many local effects on human beings have been reported (section
8.3), but only a few studies have verified these effects in
experimental animals. A comprehensive survey of hexavalent
chromium-induced effects is given in Table 12 (US NAS, 1974a). For
most studies, details were not given of the length of exposure,
number of treated animals and controls, etc. Diagnoses were stated
without presenting all the original data. Thus, in this section,
some papers will be discussed that refer to the most prominent
local and systemic effects to support and clarify the effects shown
in human beings.
It is evident that the toxicity of hexavalent chromium in
animals varies with the route of entry into the body. Low
concentrations of hexavalent chromium may be tolerated, when
administered in the feed or drinking-water, the extent of
absorption being a factor of importance. For example, rats
tolerated hexavalent chromium in drinking-water at 25 mg/litre, for
1 year, and dogs did not show any effects from chromium
administered as potassium chromate at 0.45 - 11.2 mg/litre over a
4-year period (US NAS, 1974a). However, oral exposure of both male
and female rabbits to sodium dichromate (0.1% solution, 0.2 - 5
mg/kg body weight, for up to 545 days) resulted in significant
morphological changes in the gonads, including atrophy of the
epithelium and dystrophic alterations of the Sertoli and Leydig
cells in the testes, and sclerotic and atrophic changes in ovaries
(Kucher, 1966).
Larger doses of hexavalent chromium are highly toxic and may
cause death, especially when injected iv, subcutaneously (sc), or
intragastrically. The LD50 of chromium compounds was determined
for several experimental animal species. The LD50 of potassium
dichromate (hexavalent chromium), administered orally (stomach
tube) to rats, was 177 mg/kg body weight in males and 149 mg/kg
body weight in females (Hertel, 1982). When injected iv in mice
(sex not given), the LD50 of chromium carbonyl was 30 mg/kg body
weight (IARC, 1980).
Performing a life-time inhalation study on the rat, Glaser et
al. (1984) found an LC50 for Na2Cr2O7 of 28.1 mg/m3 (range, 16.7 -
47.3 mg/m3). Assuming a deposition rate in the lung of 30% of the
dose administered, the LC50 dose was 1 mg/kg body weight in male
and 1.2 mg/kg in female rats.
Table 12. Effects of hexavalent chromium in animalsa
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit, inhalation chromates 1 - 50 mg/m3 14 h/day for pathological Lukanin (1930)
cat 1 - 8 months changes
in the lungs
Rabbit inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day none Lehmann (1914)
as dichromate for 5 days
Cat inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day bronchitis, Lehmann (1914)
as dichromate for 5 days pneumonia
perforation of
nasal septum
Mouse inhalation mixed dust 1.5 mg/m3 4 h/day, no tumours Baetjer et al.
containing as CrO3 5 days/week, (1959a);
chromates for 1 year Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 16 - 27 mg/m3 1/2 h/day tumours in Baetjer et al.
containing as CrO3 intermit- some strains (1959a);
chromates tently Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 7 mg/m3 37 h over increased Baetjer et al.
containing as CrO3 10 days tumour rate (1959a);
chromates Steffee &
Baetjer (1965)
Rat inhalation mixed dust 7 mg/m3 37 h over barely Baetjer et al.
containing as CrO3 10 days toleratedb (1959a);
chromates Steffee &
Baetjer (1965)
Rabbit, inhalation mixed dust 5 mg/m3 4 h/day, none marked Baetjer et al.
guinea- containing as CrO3 5 days/week, (1959a);
pig chromates for 1 year Steffee &
Baetjer (1965)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rat, inhalation hexacarbonyl 1.6 mg/m3 4 months, anaemia; lipid Roschina (1976)
rabbit 4 h, 5 days and/or protein
a week dystrophia
in liver
and kidneys
Rat, inhalation hexacarbonyl 0.16 mg/m3 4 months, anaemia; no Roschina (1976)
rabbit 4 h, 5 days biochemical
a week or morphological
effects
Rat inhalation hexacarbonyl 35 mg/m3 30 min 100% death Roschina (1976)
Rat inhalation dichromates 0.006 - 0.2 28 days/ increase in Glaser et al.
mg/m3 90 days lung-macrophages (1985)
23 h/day
7 days/week lymphocytes
immunoglobulin,
reduced Fe2O3
lung clearance
Rat intratra- dichromates 5 per week up to 30 toleratedc Steinhoff
cheal in- 0.01 - 0.25 months et al. (1983)
stillation mg/kg
Rat intratra- dichromates 1 per week up to 30 tolerated; Steinhoff
cheal in- 0.05 - 1.25 months 1.25 mg/kg et al. (1983)
stillation mg/kg harmful
Rat oral potassium 500 mg/litre daily maximal Gross & Heller
chromate in non-toxic (1946)
drinking-water concentration
Rat, oral zinc chromate 10 g/kg daily maximal Gross & Heller
mouse in feed non-toxic (1946)
(mature) concentration
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit oral sodium di- 0.2 - 5.0 mg/kg 545 days morphological Kucher (1966)
chromate changes in
gonads (testes:
atrophy of
epithelium,
dystrophic
alterations of
Sertoli &
Leydiz alls.
Ovaries:
sclerotic and
atrophic
changes)
Rat oral zinc chromate 1.2 g/kg daily maximal Gross & Heller
(young) in feed non-toxic (1946)
concentration
Rat oral potassium 1.2 g/kg daily maximal Gross & Heller
(young) chromate non-toxic (1946)
in feed concentration
Dog, oral monochromate 1.9 - 5.5 mg 29 - 685 none harmful Lehmann (1914)
cat, or dichromates chromium/kg days
rabbit body weight per
day 1 mg chrom-
ium equivalent
to 2.83 mg
K2Cr2O7
or 3.8 mg
K2CrO4
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog oral potassium 1 - 2 g as daily fatal in 3 Brard (1935)
dichromate chromium months anaemia
Dog stomach potassium 1 - 10 g as - rapidly fatald Brard (1935)
tube dichromate chromium
Monkey subcutan- potassium 0.02 - 0.7 g in - fatald Hunter & Roberts
eous dichromate 2% solution (1933)
Dog subcutan- potassium 210 mg as - rapidly fatal Brard (1935)
eous dichromate chromium
Guinea- subcutan- potassium 10 mg - lethald Ophüls (1911a)
pig eous dichromate Ophüls (1911b)
Rabbit subcutan- potassium 1.5 cc of 1% - 80% fatald Hasegawa (1938)
eous dichromate solution/kg body
weight
Rabbit subcutan- potassium 20 mg - lethald Ohta (1940)
eous dichromate
Rabbit subcutan- potassium 0.5 - 1 cc of - nephritisd Ohta (1940)
eous dichromate 0.5% solution/kg
body weight
Rabbit, subcutan- sodium 0.1 - 0.3 g as - rapid deathd Priestley
guinea- eous or chromate CrO3 fall in blood (1877)
pig intravenous pressure
Rabbit intra- potassium 0.7 cc of 2% - lethald Mazgon (1932)
venous solution/kg 8-10 days after
body weight injection
Dog intra- potassium 10 grains - instant death Gmelin (1826)
venous chromate
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog intra- potassium 1 grain - none marked Gmelin (1826)
venous chromate
Dog intra- potassium 210 mg as - rapidly fatal Brard (1935)
venous dichromate chromium
Dog intra- potassium 3 mg/100 cc 2 doses marked renal Hepler & Simonds
venous dichromate blood per dose damage (1946); Simonds
& Hepler (1945)
---------------------------------------------------------------------------------------------------------
a Modifed from: US NAS (1974a).
b Pathological changes in experimental and control rats, 101 weeks after exposure.
c The same weekly dose distributed over 5 days was clearly better tolerated than a single weekly
administration.
d Renal damage.
A local corrosive action of hexavalent chromium on the skin,
similar to that seen in man, was described by Samitz & Epstein
(1962), who induced chrome ulcers in guinea-pigs at 4 trauma sites,
with daily exposure to 0.34 MK2Cr2O7 solution for 3 days. Mosinger
& Fiorentini (1954) showed the same effects using potassium
chromates.
Following parenteral administration, the most common systemic
effects of chromium were parenchymatous changes in the liver and
kidney (Mosinger & Fiorentini, 1954). Later studies showed
selective damage in the renal proximal convoluted tubules, without
evidence of glomerular damage, as demonstrated after one single sc
injection of potassium dichromate of 10 mg/kg body weight (Schubert
et al., 1970) or after one single intraperitoneal (ip) injection of
sodium chromate of 10 or 20 mg/kg body weight (Evan & Dail, 1974).
Effects have also been found in fish (Strik et al., 1975). After 32
days of continuous exposure to 0.1, 1, or 10 mg hexavalent chromium
(as potassium dichromate)/litre, the fish Rutilus rutilus developed
lysis of the intestinal epithelium with haemorrhages as well as
hypertrophy and hyperplasia of the gill epithelium.
Franchini et al. (1978) found an increase in urinary protein,
lysozyme, glucose, and beta-glucuronidase in rats after a single sc
injection of potassium dichromate at 15 mg/kg body weight. After
sc injection (3 mg/kg body weight), every other day for 2 - 8
weeks, the authors observed a correlation between the chromium
contents of the renal cortex and chromium clearance.
Five-week-old male Wistar rats of the strain TNO-W-74 were
continuously exposed in inhalation chambers to submicron aerosols
of sodium dichromate at concentrations ranging from 25 (low level)
to 200 µg chromium/m3 (high level) (Glaser et al., 1985). Exposure
for 28 days to 25 or 50 µg chromium/m3 resulted in "activated"
alveolar macrophages with stimulated phagocytic activity, and
significantly elevated antibody responses to injected sheep red
blood cells. After 90 days of low-level exposure, there was a more
pronounced effect on the activation of the alveolar macrophages,
with increased phagocytic activity. However, inhibited phagocytic
function of the alveolar macrophages was seen at the high
hexavalent chromium exposure level (200 µg/m3). In rats exposed to
this chromium aerosol concentration for 42 days, the lung clearance
of inert iron oxide was significantly reduced. The humoral immune
system was still stimulated at a low chromium aerosol concentration
of 100 µg/m3, but significantly depressed at 200 µg chromium/m3.
Exposure of rats, through inhalation, to chromium carbide or
chromium boride dust at very high levels (300 - 350 mg/m3 for each
substance) for 3 months (2 h/day) resulted in effects on the
vascular system of the lungs, e.g., endothelial hyperplasia.
Bronchitis and a decrease in the blood-haemoglobin concentration
were also observed (Roschina, 1964). The effects of chromium boride
were more pronounced than those of chromium carbide.
Steinhoff et al. (1983) performed an intracheal injection study
on rats (930 rats, 30 months, 0.05 - 1.25 mg Na2Cr2O7/kg body
weight per week; 1.25 mg CaCrO4/kg body weight per week). Half of
the rats were intratracheally injected once a week and the other
half received the same weekly dose distributed over 5 injections
per week. In rats receiving doses 5 times a week, there were some
significant changes in levels of total plasma-protein and
-cholesterol, in some haematological variables, in organ weights,
and in survival times in females. Only male rats receiving 1.25 mg
sodium dichromate/kg body weight, once a week, showed a sharp
reduction in body weight, female rats being less affected. Rats
receiving calcium chromate in the same dose showed reduced body
weight but to a lesser extent. The weight of lung and trachea was
increased by both substances in all doses.
Marked histopathological changes (congestion, fairly large
areas of focal necrosis, bile duct proliferation) described after
long-term exposure of rabbits to hexavalent chromium (ip injection
of 2 mg chromium/kg body weight per day for 6 weeks) (Tandon et
al., 1978), as well as the increase in hepatic metallothionein and
decrease in cytochrome P-450 levels after ip injection of 400 µmol
chromium/kg per day (type of chromium compound not mentioned)
(Eaton et al., 1980) need further confirmation. The finding of an
accumulation of hexavalent chromium in the reticuloendothelial
system including bone marrow (Baetjer et al., 1959b; Langard, 1977)
may be of importance for a disturbed blood picture.
Merkurieva et al. (1980a,b) studied the effects of potassium
dichromate in the drinking-water on the activities of different
enzymes in rats. Exposure included daily doses of 0.0005, 0.005,
0.05, or 0.5 mg/kg body weight for up to 6 months. After 20 days
of exposure at the highest dose level, enzyme activities increased
by 15 - 28% in liver microsomes (inosine-5-diphosphatase),
lysosomes (beta-D-galactosidase), and cytosol (lactate dehydrogenase
(EC 1.1.1.27)). For some of these enzymes, as well as for
acetylesterase (EC 3.1.1.6), increases in activity of up to 54%
were found in the gonads, kidneys, seminal fluid, and serum. At a
dose of 0.05 mg/kg, the only statistically significant finding was
a 54% increase in the activity of acetylesterase in the gonads.
After a 6-month exposure at this dose level, there was a 70%
increase in lactate dehydrogenase activity in the seminal fluid as
well as a 50% increase in free beta-galactosidase in the liver. There
were no changes in enzyme activities at the two lowest dose levels.
Painting of rat skin with an aqueous solution of potassium
dichromate (0.5%), daily, for 20 days, resulted in a local
inflammatory reaction, an increased level of hexose glyco-proteins
in the skin and serum, and an elevated concentration of serotonin
in the skin and liver (Merkurieva et al., 1982). Most of these
effects were also seen earlier in the exposure period, though they
were not as pronounced. Ten days after the start of exposure, a
nearly 3-fold increase in the serum-acetylcholine concentration
occurred together with decreased acetylcholinesterase activity.
Thus, the data of Merkurieva et al. show systemic effects following
both oral and dermal exposure to hexavalent chromium.
Cats fed chromic phosphate or oxydicarbonate at 50 - 1000 mg/day
for 80 days did not exhibit signs of illness or tissue damage.
Similarly, toxic reactions were not observed in rats administered
drinking-water containing 25 mg trivalent chromium/litre, for 1
year, or 5 mg trivalent chromium/litre throughout their lifetime
(US NAS, 1974a). The toxicity of trivalent chromium is so low that
even by parenteral administration, a chromic acetate level of 2.29
g/kg body weight or a chromic chloride level of 0.8 g/kg body
weight is required to kill mice. Even very large doses given
intragastrically were not fatal for dogs. Brard (1935) reported
that 10 or 15 g of chromium as chromic chloride proved fatal in one
dog (US NAS, 1974a). Some fatal doses of trivalent chromium
compounds reported in the literature are listed in Table 13.
Rats exposed through inhalation to chromic oxide (trivalent
chromium) at 42 mg/m3 or to chromic phosphate at 43 mg/m3 (5 h/day
for 5 days/week) for 4 months developed chronic irritation of the
bronchus and lung parenchyma, and dystrophic changes in the liver
and kidney (Blokin & Trop, 1977).
Inhalation exposure of rats (number not given) to dusts
containing 36 or 50% chromite for 4 months (2 h/day), at
concentrations of 375 - 400 mg/m3, resulted in thickening of the
walls of pulmonary vessels and bronchi (Roschina, 1959). The high
exposure levels in these studies make it difficult to evaluate the
effects.
Inhalation studies have also been performed with chromium
carbonyl, where chromium is in the 0 oxidation state (Roschina,
1976). Twelve rabbits and 48 rats were exposed for 4 months (4
h/day, 6 days/week) at a concentration of 1.6 or 0.16 mg/m3. At
both exposure levels, there was loss of body weight (25 and 12%,
respectively) as well as anaemia and leukocytosis. In the higher
exposure group, the animals showed an elevated gamma-globulin level
in serum and an increased transaminase activity. The contents of
cholesterol and SH-groups were reduced, and there was a decrease in
cholinesterase (EC 3.1.1.7) activity. Lipid and/or protein
dystrophy were noted in several organs, e.g., in the liver and
kidneys. No such effects were detected in the animals in the low-
exposure group.
Table 13. Fatal doses of trivalent chromium in animalsa
---------------------------------------------------------------------------------------------
Animal Number of Routeb Compound Chromium Effect Reference
animals dose (g/kg)
---------------------------------------------------------------------------------------------
Dog 2 sc chromic chloride 0.8 fatal Brard (1935)
Rabbit 1 sc chromic chloride 0.52 fatal Brard (1935)
Rat 38 iv chrome alum 0.01 - 0.018 LD50 Mertz et al.
chromium-hexaurea (1965a)
chloride
Mouse -c iv chromic chloride 0.8 MLDd Windholz et al. (1960)
Mouse -c iv chromic acetate 2.29 MLD Windholz et al. (1960)
Mouse -c iv chromic chloride 0.4 MLD Schroeder (1970)
Mouse -c iv trivalent chromium ? 0.25 - 2.3 MLD Windholz et al. (1960)
Mouse -c iv chromic sulfate 0.247 MLD Windholz et al. (1976)
Mouse -c iv chromic sulfate 0.085 MLD Schroeder (1970)
Mouse -c iv chromium carbonyl 0.03 LD50 Schroeder (1970)
---------------------------------------------------------------------------------------------
a Modified from: US NAS (1974a).
b sc = subcutaneous; iv = intravenous.
c No figures given.
d Minimum lethal dose.
7.2.1.1. Carcinogenicity
Various types of chromium chemicals, methods of administration,
and species of animals have been studied (IARC, 1980a).
Ideally, carcinogenicity should be tested with the methods
recommended by IARC (1980b), but, in many of the early studies,
this was not done. Carcinomas of the lung have been reported in
animals as a result of the administration of chromium chemicals.
Hueper (1958) found 2 squamous cell carcinomas and one
carcinosarcoma in 25 rats following intra-pleural injection of
chromite ore roast. After intrapleural implantation of strontium
chromate lasting 27 months, Hueper (1961) found tumours (type
unspecified) in 17/28 rats. Laskin et al. (1970) and Levy & Venitt
(1975) produced a number of bronchogenic carcinomas by implanting
pellets of cholesterol mixed with various chromium compounds
encased in a wire mesh cage in the bronchi of rats. Calcium and
zinc potassium chromate produced a number of bronchogenic
carcinomas, but soluble chromates and trivalent chromium chemicals
failed to produce cancer.
Using the same technique, Levy & Martin (1983) tested 21
different chromium-containing materials (pigments, intermediates,
and residues from the bichromate-producing industry, relatively
pure crystalline compounds) in 2250 random-bred rats and found that
chromates, described as sparingly soluble, were carcinogenic in the
rat lung. These materials included strontium and calcium chromate
and, to a far lesser extent, certain forms of zinc chromate.
Barium and lead chromate evoked only a very weak carcinogenic
response compared with strontium and calcium chromate. In the
study of Laskin et al. (1970), it was shown that chromium trioxide
produced hepatocellular carcinomas in 2/100 rats (controls, 0/24).
After inhalation of 13 mg calcium chromate/m3 (5 h/day, 5
days/week, for lifetime), Nettesheim et al. (1971) found 14 lung
adenomas in 136 treated mice and 5 in 136 untreated controls, but
no carcinomas. Steffee & Baetjer (1965), performing inhalation
studies on rats, mice, guinea-pigs, and rabbits (inhalation of
mixed chromate dust, corresponding to 3 - 4 mg CrO3/m3, 4 - 5
h/day, 4 days/week, for lifetime, or 50 months, respectively) could
only find 3 alveologenic adenomas in 50 treated guinea-pigs.
Laskin (1972) and Laskin et al. (1970) found 1 squamous cell
carcinoma of the lung, 1 of the larynx and 1 "peritruncal tumor" in
rats (inhalation of calcium chromate, 2 mg/m3, 589 exposures of 5 h
over 891 days) and 1 squamous cell carcinoma and 1 papilloma of the
larynx in hamsters. The number of treated animals was not
specified in either paper. Steinhoff et al. (1983) performed
intratracheal instillations of chromates in rats for 30 months with
one treatment/week and the same weekly dose distributed over 5
treatments/week (Table 14). In 880 exposed rats, 28 adenomas of
alveolar-bronchiolar origin (benign) and 12 malignant tumours (3
adenocarcinomas and 9 squamous cell carcinomas) were found. All
lung tumours developed very late and were only detected at the end
of the lifetime study, often in lungs with callosities. The
tumours were tiny and none of them caused the animal to die.
Sodium dichromate was not carcinogenic after exposure on 5
days/week. With calcium chromate, the carcinogenic effect was more
pronounced after treatment once per week, than after treatment 5
times per week.
Table 14. Incidence of benign and malignant lung tumours among
880 rats intracheally injected with Na dichromate and Ca chromatea
----------------------------------------------------------------
Dose Number of Incidence of lung tumours
(mg/kg) injections benign malignant
per week
----------------------------------------------------------------
Na2Cr2O7 x 2H20 0.25 5 none none
Na2Cr2O7 x 2H20 1.25 1 12 8
CaCrO4 0.25 5 5 1
CaCrO4 1.25 1 11 3
----------------------------------------------------------------
a From: Steinhoff et al. (1983).
Hueper (1961) reported unspecified tumours at implantation
sites in 12/34 rats, following intramuscular (im) implantation of
sintered calcium chromate (25 mg). No tumours were observed in 32
control animals. The same author found implantation-site tumours in
15/33 animals treated with strontium chromate compared with no
tumours in 32 control animals. Single sc injections of 30 mg lead
chromate (Maltoni 1974, 1976) resulted in injection-site sarcomas
in 26/40 treated rats. After administration of the same amount of
lead chromate oxide, injection-site sarcomas were found in 27/40
rats; no sarcomas were found in 60 vehicle controls. Following 9
im injections of 8 mg lead chromate, Furst et al. (1976) found
injection-site sarcomas in 31/47 treated rats, and renal carcinomas
in 3/24 treated rats; no sarcomas were found in 0/22 vehicle
controls. Heath et al. (1971) found injection-site sarcomas and
other tumours in 7/74 treated rats after im injection of 28 mg
cobalt-chromium alloy.
Administration of low levels of trivalent chromium acetate (5
mg/litre in the drinking-water) to mice and rats for the lifetime
did not result in increased tumour incidences compared with
controls (Schroeder et al., 1964, 1965).
Trivalent chromium oxide incorporated in bread at
concentrations of 10, 20, or 50 g/kg and fed to BD rats did not
increase tumour incidence compared with controls (Ivankovic &
Preussmann, 1975). Hexavalent chromium compounds have not been
tested by oral administration.
IARC (1980a) summarized the data of all available references
and concluded as follows: "There is sufficient evidence for the
carcinogenicity of calcium chromate and some relatively insoluble
chromium (VI) compounds (sintered calcium chromate, lead chromate,
strontium chromate, sintered chromium trioxide and zinc chromate)
in rats. There is limited evidence for the carcinogenicity of lead
chromate (VI) oxide and cobalt-chromium alloy in rats. The data
were inadequate for the evaluation of the carcinogenicity of other
chromium (VI) compounds and of chromium (III) compounds".
(a) Interaction with other factors
The possibility that chromium may act synergistically with
other agents in the production of cancer has been tested in several
studies. Nettesheim et al. (1970) pretreated one group of mice
with 100 R whole-body radiation and infected another group with PR8
influenza virus. These mice and control mice were exposed through
inhalation to chromium oxide dust for 6 - 18 months. No measurable
effects of either of the pretreatments were found on the incidence
of tumours. In a similar study with inhalation of calcium chromate
(CaCrO4, 5 h/day, 5 days per week for the lifetime), pre-exposure
to X-rays did not affect the tumour rate, but PR8 influenza
infection reduced tumour incidence (Nettesheim et al., 1971).
Steffee & Baetjer (1965) also found that the infection of rats with
PR8 influenza virus associated with intratracheal injection of
chromates did not lead to the production of tumours. Chromite ore
injected iv did not affect the development of tumours induced by iv
injection of 20-methyl-cholanthrene (Shimkin & Leiter, 1940).
Thus, at present, there are not sufficient data to suggest that
chromium acts synergistically with virus infections, ionizing
radiation, or other chemical carcinogens to produce cancers.
7.2.1.2. Genotoxicity
The mutagenicity of chromium compounds was reviewed by IARC
(1980a), Petrilli & DeFlora (1980), Levis & Bianchi (1982), and
Baker (1984). Present knowledge, in the light of recent findings,
is summarized in Table 15 (hexavalent chromium) and Table 16
(trivalent chromium).
When evaluating the results of chromium mutagenicity tests, it
is necessary to take into consideration several properties of the
tested compound (oxidation state, solubility, ability to penetrate
cell membranes, intracellular stability, and reactivity with
cellular components). Most of the in vitro mutagenicity
experiments have been performed with chromium chemicals, following
the introduction of the reverse mutation test by Ames (1973).
Bacterial strains of Salmonella typhimurium (Petrilli & DeFlora,
1977) containing mutants that require histidine for growth, and
Escherichia coli (Venitt & Levy, 1974) with mutants requiring
tryptophan for growth have been used. In each case, the bacterial
cultures have been incubated with the test chromium compounds and
the number of revertant colonies scored. The chromium compounds
studied included both hexavalent and trivalent compounds; a survey
of the results is given in Table 17 (DeFlora, 1981). Zhurkov
(1981) performed an Ames test to study the mutagenic activity of
potassium dichromate (K2Cr2O7) with S. typhimurium TA 1535 and TA
1538. A medium degree of mutagenic activity without metabolic
activation was shown by the strain TA 1535.
Only hexavalent chromium compounds have shown mutagenic
effects. Using the Salmonella /mammalian microsome tests of Ames
et al. (1973), DeFlora (1978) and Löfroth (1978) both showed a
decrease in the mutagenicity of hexavalent chromium in the presence
of an NADPH-generating system, suggesting reduction of mutagenic
hexavalent chromium to trivalent chromium. In this chromate
metabolism, cytochrome P-450 functions as the reductase (Garcia &
Wetterhahn Jennette, 1981). In recent in vitro studies, Wiegand et
al. (1984b) showed that glutathione could reduce hexavalent
chromium to trivalent chromium, without any further cofactors or
metabolizing enzymes. In rats, the most efficient tissue in
decreasing hexavalent chromium mutagenicity was the liver, followed
by the suprarenal glands, kidney, stomach, and lung (Petrilli &
DeFlora, 1980). These studies suggest a possible way of
intracellular detoxification of hexavalent chromium in low doses.
Schoental (1975) suggested that hexavalent chromium caused the
formation of epoxyaldehydes having mutagenic potential. Venitt &
Levy (1974) assumed that chromates belong to the transition-
inducing class of mutagens. They cause both frameshift and base-
pair substitutions (Petrilli & DeFlora, 1980). The hypothesis of
Kazantzis & Lilly (1979), that chromates cause guanine-cytosine to
adenine-thymine transitions in the subsequent round of DNA-
replication, needs further confirmation.
Table 15. Genotoxic activity of chromium (VI) compoundsa
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
DNA degradation CaCrO4 - Casto et al. (1976)
K2Cr2O7 - Bianchi et al. (1983)
PbCrO4 - Douglas et al. (1980)
K2CrO4 + Whiting et al. (1979)
Decreased fidelity PbCrO4, + (1) Levis & Majone (1981)
of DNA synthesis K2Cr2O7
CrO3 + Sirover & Loeb (1976)
K2Cr2O7 + Bianchi et al. (1983)
Microbial DNA K2CrO4, + Yagi & NishioKa (1977)
repair K2Cr2O7
CrO3, K2CrO4, + Kanematsu et al. (1980)
K2Cr2O7
K2CrO4, + Nishioka (1975)
K2Cr2O7
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
CrO3, + Gentile et al. (1981)
K2Cr2O7,
Na2Cr2O7, + Gentile et al. (1981)
(NH4)2Cr2O7
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Microbial gene K2Cr2O7 + (2) Bonatti et al. (1976)
mutation CrO3 + (2) Fukunaga et al. (1982)
Na2Cr2O7, +/-(3) Petrilli & De Flora
CrO3, +/-(3) (1978a,b)
ZnCrO4xZn(OH)2 +/-(3)
K2Cr2O7 + Zhurkov (1981)
Na2Cr2O7 +/-(3) DeFlora (1978)
chromate (4) +/-(3) Löfroth (1978)
dichromate +/-(3)
K2Cr2O7 - Kanematsu et al. (1980)
K2CrO4, + Löfroth & Ames (1978)
K2Cr2O7 +
K2Cr2O7 + Nishioka (1975)
K2CrO4, CaCrO4 + DeFlora (1981)
(NH4)2CrO4,
CrO3,
Na2CrO4, + Venitt & Levy (1974)
K2CrO4
CaCrO4
K2Cr2O7 + Bianchi et al. (1983)
Na2Cr2O7,
ZnCrO4
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
K2CrO4, CaCrO4 + Petrilli & DeFlora
CrO3 (1977)
Na2Cr2O7
K2CrO4 + Green et al. (1976)
PbCrO4, CrO3 + Nestmann et al. (1979)
Mammalian cell K2Cr2O7 + Bianchi et al. (1983)
gene mutation K2Cr2O7, + Newbold et al. (1979)
ZnCrO4 +
PbCrO4 -
K2Cr2O7 + (5) Bonatti et al. (1976)
K2Cr2O7 + Pashin et al. (1982)
NaeCr2O + Pashin & Kozachenko
(NHy)2Cr2O7 + (1981)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell K2CrO4, + Umeda & Nishimura (1979)
chromosomal K2Cr2O7 +
mutation K2Cr2O7 + Bigaliev et al. (1978)
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7 +
Na2CrO4, + Majone & Levis (1979)
K2CrO4 +
Na2Cr2O7, Levis & Majone (1979)
K2Cr2O7
CrO3, CaCrO4
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CrO3 + Kaneko (1976)
Na2Cr2O7 + Sarto et al. (1980)
K2Cr2O7 +
CrCl3 +
CrO3 + Tsuda & Kato (1977)
K2Cr2O7 + Raffetto (1977)
K2Cr2O7 + Stella et al. (1982)
Na2Cr2O7, + Majone & Levis (1979)
K2Cr2O7 +
K2CrO4 + Wild (1978)
Na2Cr2O7 + (7) Krishnaja & Rige (1982)
Mammalian cell CrO3, K2CrO4, + Ohno et al. (1982)
SCE K2Cr2O7 +
K2Cr2O7 + Bianchi et al. (1983)
K2CrO4,
Na2CrO4 + Levis & Majone (1979)
CrO3, +
K2Cr2O7, + Majone & Levis (1979)
Na2Cr2O7 +
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CaCrO4, CrO3, + Gomez-Arroyo et al.
K2Cr2O7 + (1981)
K2Cr2O7 + Majone & Rensi (1979)
CrO3 + (6) Stella et al. (1982)
K2Cr2O4, + Elias et al. (1983)
Na2CrO4, +
Na2Cr2O7 +
K2Cr2O7,
K2CrO4 + McRae et al. (1979)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell CaCrO4, K2CrO4 + (8) Casto et al. (1979)
transformation ZnCrO4 + (9)
Na2CrO4,
CaCrO4 + Casto et al. (1976)
+ Di Paolo & Casto (1979)
K2Cr2O7 + Bianchi et al. (1983)
K2Cr2O7 + Tsuda & Kato (1977)
CaCrO4 + Fradkin et al. (1975)
K2Cr2O7 + Raffetto (1977)
---------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) As industrial pigments (Cr-yellow-orange; Zn-yellow; Mo-orange).
(2) Gene conversion.
(3) No mutagenicity in the presence of mammalian microsomal activation
system (S-9 mix).
(4) No compounds given.
(5) Forward mutation was observed in 7/480 054 colonies (controls 0/84
546). A significant increase can be stated if comparison is made
with the historical spontaneous mutation rate, i.e., no mutants in
106 colonies.
(6) Occupatinal exposure.
(7) Induction of chromosomal aberrations in gill cells of the fish
Boleophthalmus dussumieri (Goby).
(8) 2 times control levels.
(9) 4 times control levels.
The results summarized in Table 17 show that the chromate ion
(hexavalent chromium) induces different kinds of genetic damage,
even at low doses, whereas the chromosome-damaging capacity of
trivalent chromium was only observed when it was tested in very
high doses.
In assays with whole-cell systems, trivalent chromium is
inactive, unless there is a direct interaction with DNA, e.g., in
studies in which purified DNA was exposed to trivalent chromium,
where modifications of the physical and chemical properties, as
well as decreased fidelity of DNA synthesis, were produced (Levis &
Bianchi, 1982). According to Warren et al. (1981), it appeared
that trivalent chromium in a proper ligand environment could have
considerable genetic toxicity. However, in recent studies, Bianchi
et al. (1984) found that trivalent chromium (CrCl3, 10-3 to 10-5 M)
was neither cytotoxic nor mutagenic in permeabilized hamster
fibroblasts (BHK were incubated for 30 min in Ea or BSS made
hypertonic with 4.2% NaCl).
Table 16. Genotoxic activity of chromium (III) compoundsa
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
DNA degradation CrCl3 + Bianchi et al.
(1983)
Decreased fidelity Cr2O3 + Levis & Majone
of DNA synthesis (1981)
Microbial DNA cis-[Cr(bipy)2ox] + Warren et al. (1981)
repair I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 + Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O + Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O + Warren et al. (1981)
trans-[Cr(en)2 + Warren et al. (1981)
(SCN)2]SCN
CrCl3, Cr2O3, - Yagi & Nishioka
Cr(OH)3 (1977)
K2Cr2(SO4)4 - Yagi & Nishioka
(1977)
K2Cr2(SO4)4 - Kanematsu et al.
(1980)
Cr2(SO4)3 - Kanematsu et al.
(1980)
CrCl3 - Nishioka (1975)
Cr(NO3)3, + De Flora (1981)
Cr(CH3COO)3
CrCl3 - Nakamuro et al.
(1978)
CrCl3 + Gentile et al.
(1981)
CrCl3, KCr(SO4)2, - Gentile et al.
(1981)
Cr2(SO4)3 - Gentile et al.
(1981)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Microbial gene CrCl3, Cr(NO3)3 - Löfroth & Ames
mutation (1978)
Cr(CIO4)3 - Löfroth & Ames
(1978)
CrCl3, Cr(NO3)3 - Venitt & Levy (1974)
Cr2SO4, - Venitt & Levy (1974)
K2SO4 x 2H2O
Cr(CH3COO)3 + Nakamuro et al.
(1978)
Cr(NO3)3 + Nakamuro et al.
(1978)
CrCl3 - Nakamuro et al.
(1978)
CrK(SO4)2 x 12H2O - Petrilli & De Flora
(1977)
CrCl3 x 6H2O - Petrilli & De Flora
(1978a)
Cr(NO3)3 x 9H2O - Petrilli & De Flora
(1978b)
Microbial gene cis-[Cr(bipy)2ox] + Warren et al. (1981)
mutation I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 - Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O - Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O - Warren et al. (1981)
trans-[Cr(en)2 - Warren et al. (1981)
(SCN)2]SCN
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
gene mutation (1983)
Cr(CH3COO)3 - Newbold et al.
(1979)
Mammalian cell Cr2(SO4)3 - Umeda & Nishimura
chromosomal (1979)
mutation
Cr(CH3COO)3 + Nakamuro et al.
(1978)
CrCl3, Cr(NO3)3 - Nakamuro et al.
(1978)
Cr(NO3)3, + Levis & Majone
(1979)
KCr(SO4)2, + Levis & Majone
(1979)
Cr(CH3COO)3 + Levis & Majone
(1979)
CrCl3 + Kaneko (1976)
CrCl3 - Sarto et al. (1980)
Cr2(SO4)3, CrCl3 - Tsuda & Kato (1977)
CrCl3 + Raffetto (1977)
CrCl3 - Stella et al. (1982)
CrCl3 - (1) Bianchi et al.
(1984)
Mammalian cell CrCl3, Cr2O3 + Elias et al. (1983)
SCE
Cr2O3 + Levis & Majone
(1981)
Cr(NO3)3, - Levis & Majone
KCr(SO4)2 (1979)
CrCl3, Cr(CH3COO)3 - Levis & Majone
(1979)
CrCl3 + (2) Ohno et al. (1982)
Cr2(SO4)3 - Ohno et al. (1982)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
SCE (contd). (1983)
CrCl3 - Majone & Rensi
(1979)
Mammalian cell CrCl3 - Bianchi et al.
transformation (1983)
---------------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) Thymidine uptake, DNA replication, damage and repair and SCE were
tested in permeabilized hamster fibroblasts.
(2) Weakly positive.
Levis & Buttignol (1977) and Levis et al. (1978) raised the
question of whether trivalent chromium could be an active mutagenic
agent within a cell. Others, such as Bianchi et al. (1980),
supported this theory, suggesting that trivalent chromium is bound
to genetic material after reduction of the hexavalent form by an
NADPH-dependent microsomal enzyme system (Norseth, 1978; Jennette,
1979). The studies of Sirover & Loeb (1976), showing a 30% error
in DNA synthesis after application of 0.64 mM trivalent chromium
compared with 16 mM of hexavalent chromium, support this theory.
Pashin et al. (1982) demonstrated the induction of dominant
lethal mutations in mice. They treated male mice with a single
injection of potassium dichromate (0.5 - 20 mg/kg body weight, ip)
or with daily injections (1 and 2 mg/kg, ip, for 21 days). The
single injections (0.5 - 2 mg/kg) did not induce any effects;
repeated or higher dosages of potassium dichromate induced a
statistically significant decrease in the survival of embryos from
cells treated at the early spermatid and late spermatocyte stages.
Karyological alterations in mammalian cells were induced after
exposure to concentrations of hexavalent chromium that were more
than 100 times lower than the concentration of trivalent chromium
causing the same changes (Majone & Rensi, 1979).
Analysing the effects of fume particles from stainless steel
welding on sister chromatid exchanges and chromosome aberrations in
cultured Chinese hamster cells, Koshi (1979) found an increase in
cytogenic effects with increasing fume doses, due to the dissolved
hexavalent chromium. Hexavalent chromium and stainless steel
welding fume particles containing chromium compounds produced
positive results in the "mammalian spot test", a somatic tissue
assay (Knudsen, 1980).
Table 17. Mutagenicity of chromium compoundsa
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
L. Hexavalent chromium compounds
1) Sodium dichromate (Merck) - +w +w +w + 50 - 250 4.4 decrease toxic and mutagenic
Na2Cr2O7 x 2H2O Cr6+ was converted
into inactive Cr3+
2) Potassium chromate (BDH) - +w +w +w + 80 - 410 2.9 decrease by reducing
K2CrO4 chemicals (ascorbic
acid, sodium
3) Calcium chromate (BDH) - +w +w +w + 60 - 290 3.2 decrease sulfite) or
CaCrO4 metabolites (NADH,
NADPH, GSH), by
4) Ammonium chromate - +w +w +w + 50 - 320 3.7 decrease human gastric juice
(Carlo Erba) and erythrocyte
(NH4)2CrO4 lysates, by S9 mix
containing human
liver S9 fractions
5) Chromium trioxide or - +w +w +w + 40 - 220 5.1 decrease or rat tissue S9
chromic acid (Merck) fractions (in order
CrO3 of efficiency:
liver>suprarenal
glands>kidney>
stomach>lung);
pretreatment of
rats (Aroclor(R)
1254), ether,
ethanol) influenced
the efficiency of
metabolic systems;
conversely, Cr6+
mutagenicity was not
affected by human
serum or plasma nor
by S9 mix containing
other rat (spleen,
colon, bladder,
striated muscle) S9
fractions
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
6) Zinc yellow (Montedison) - +w +w +w + 90 - 590 1.4 decrease the mutagenic effects
ZnCrO4 x Zn(OH)2 + of Cr6+ compounds and
10% CrO3 benzo( a)pyrene were
less than additive
7) Chromyl chloride (BDH) - +w +w +w + 100 - 430 1.8 decrease liquid volatile
Cl2CrO2 compound; vapours
were also toxic and
mutagenic
8) Chromium orange or basic - - - - +b 2 mg the three pigments
lead (spot test) containing PbCrO4
PbCrO4 x PbO were scarcely (L8),
chromate (Montedison) very scarcely (L9),
or totally (L10)
insoluble in water;
they were assayed
by directly spotting
these compounds at
the centre of plates
9) Molybdenum orange or - - - - +b 2 mg
lead solfomolybdo- (spot test)
chromat (Montedison)
PbCrO4 72-77%
PbSO4 4-6%
Al2O3 2%
PbMoO4 12-14%
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
10) Chromium yellow or - - - - -b
lead solfochromate
(Montedison)
PbCrO4 41-85%
PbSO4 4-45%
SiO2 0.1-3%
Al2O3 2-6%
11) Chromium - - - - - -2.3x103 - - hexacoordinated
hexacarbonyl (BDH) compound; formally,
Cr(CO)6 its oxidation state
is 0; insoluble in
water, dissolved in
ether
M. Trivalent chromium compounds
1) Chromic chloride - - - - - -3x104 - - inactive Cr3+
(BDH) CrCl3 x 6H2O compounds could be
converted into toxic
and mutagenic Cr6+
only in the presence
of oxidizing chemicals
(potassium
permanganate), while a
variety of metabolic
systems were
ineffective
2) Chromic nitrate (Riedel- - - - - - -2x104 - -
de Haen)
Cr(NO3)3 x 9H2O
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
3) Chromic potassium - - - - - -1.6x104 -
sulfate (BDH)
CrK(SO4)2 x 12H2O
4) Chromic acetate (BDH) - - - - - -7x104 - -
Cr(CH3COO)3
5) Neochromium (Montedison) - - - - - -5x104 - -
Cr(OH)SO4 56-58%
Na2SO4 23-24%
H2O 18-21%
6) Chromium alum (Montedison) - - - - - -4.3x104 - -
Cr2(SO4)3 37-39%
K2SO4 16-18%
H2O 43-37%
7) Chromite (Montedison) - - - - + 2 mg - Contaminated with Cr6+
Cr2O3 44-46% (spot test) traces ( sim-diphenyl-
Fe2O3 29-30% carbazide reagent);
Al2O3 15-16% scarcely soluble in
SiO3 0.5-3% water; assayed by
CoO 0.5-2% directly spotting the
powder at the centre
of plates
--------------------------------------------------------------------------------------------------------
Table 17 (contd.)
Note:
Sensitivity of Salmonella strains: Range of activity:
In the assays carried out in this study, This column indicates the lower and
the numbers of spontaneous revertants of the upper limits (in nmol per plate)
Salmonella tester strains fell within the of the mutagenic response, as in-
following ranges: ferred from dose-response curves
TA 1535: 10-25, both with and without S9 mix; with the most sensitive bacterial
TA 1537: 3-18, both with and without S9 mix; strain and under the most favourable
TA 1538: 10-25 without S9 mix; 15-35 with S9 mix; metabolic situation (i.e., with or
TA 98: 20-35 without S9 mix; 25-40 with S9 mix; without S9 mix) for each positive
TA 100: 150-200 without S9 mix; 140-180 with S9 mix compound; for negative compounds,
the maximum dose tested is indicated
The key for the interpretation of symbols in the
column "Reverted strains" is the following: Mutagenic potency:
+ clearly positive result indicated by a dose-
related and reproducible increase of his+ the values presented have
revertants over controls (at least a 3-fold been calculated by dividing the number
increase); of revertants in excess of controls
+w weak positivity indicated by an increase of by the corresponding amount of compounds
revertants 2 - 3 times in controls; (in nmoles); the number of net revertants
± reproducible but less than 2-fold increase was determinated at the top level of the
of revertants; linear part of dose-response curves, which
No symbol: no conclusive experiment carried out were drawn as indicated under the previous
with the corresponding strain. sub-heading.
Effects of S9 mix
The effect of S9 mix containing
liver S9 fractions from
Arochlor(R) pretreated rats on
the mutagenicity of test
compounds is reported
--------------------------------------------------------------------------------------------------------
a From: DeFlora (1981).
b L8, L9, and L10, as well as PbCrO4, were positive in the plate test when
dissolved in 0.5 N NAOH.
Embryonic fibroblast cell cultures showed significant
chromosome aberrations, when potassium dichromate was added to the
medium (Tsuda & Kato, 1976). When the potassium dichromate was
reduced with sodium disulfite (Na2SO3), no significant aberrations
occurred, even with 100 times the chromium concentrations.
At present, it is widely accepted that hexavalent chromium is
genetically active, because of its ability to cross the membranes
and enter the cells. If reduction of hexavalent chromium takes
place outside the cell (or even outside the cell nucleus, e.g., in
mitochondria or microsomes) its genetic activity is suppressed; if
the reduction takes place inside the nucleus (near, or at, the
target DNA molecules) alterations in DNA can occur, depending on
the oxidation power of hexavalent chromium or the formation of
trivalent chromium complexes with nucleophilic sites of the DNA;
thus, trivalent chromium could be the ultimate mutagenic form of
chromium (Levis & Bianchi, 1982). More recent investigations
(Wiegand et al., 1984) have shown that hexavalent chromium may
enter the body unreduced via the lung and be partly deposited in
erythrocytes over a prolonged period of time. From a DNA-repair
test in bacteria, DeFlora et al. (1984) concluded that hexavalent
chromium causes irritation in nucleotide pools and, due to its
oxidizing power, induces single-strand breaks in DNA, while
trivalent chromium induces DNA-protein and DNA-DNA cross-links and
decreases the fidelity of DNA replication.
7.2.1.3. Developmental toxicity and other reproductive effects
The teratogenicity of hexavalent chromium as chromium trioxide
was tested in golden hamsters (Gale, 1974, 1978, 1982; Gale &
Bunch, 1979) and that of trivalent chromium as chromic-chloride in
ICR mice (Iijima et al., 1975; Matsumoto et al., 1976). Injections
of hexavalent chromium into the fertilized chick egg increased
embryolethality and produced malformations in the survivors (Gilani
& Marano, 1979).
The golden hamsters were treated with a single iv injection of
chromium trioxide (5, 7.5, 10, or 15 mg/kg body weight) on the 8th
day of gestation (Gale, 1978).
Dose-response relationships were demonstrated between the rate
of absorption and the rate of malformations including delayed
ossification of the skeletal system. At 5 mg/kg body weight, 4% of
the fetuses showed external abnormalities (oedema, exencephaly),
and 34% showed cleft palate (controls: 2%), which increased to 85%
with the higher doses of 7.5 or 10 mg/kg body weight. Thirty-one
percent of fetuses were retarded following treatment with 7.5 mg/kg
body weight and 49% following 10 mg/kg body weight. The dose of 15
mg/kg body weight was lethal for 75% of the dams. Gale & Bunch
(1979) injected a single dose of 8 mg CrO3/kg body weight on day 7,
8, 9, 10, or 11 of gestation in female golden hamsters (Table 18).
The treated females were adversely affected (body weight loss,
tubular necrosis of the kidneys). In a later study, Gale (1982)
injected 8 mg CrO3/kg iv in hamsters on day 8 of gestation. An
increased incidence of cleft palate was seen in strains LVG, LSH,
and MHA, but no effects were reported in strains CB, LHC, and PD4.
ICR mice were injected sc with chromic chloride (Iijima et al.,
1975; Matsumoto et al., 1976). In the first series (animals
administered 9.8 or 19.5 mg/kg body weight, every other day, from
the 0th to 16th day of gestation), according to the authors, a
slight increase in the rate of malformations could not be excluded.
In the second series, with ip injections of 9.8 - 24.4 mg/kg body
weight, on the 8th day of gestation, the authors reported a dose-
dependent increase in the frequency of exencephaly, anencephaly,
and the occurrence of rib fusion.
Danielsson et al. (1982) studied the rate of embryonic or fetal
uptake of trivalent chromium and hexavalent chromium in early and
late gestational mice (Table 19) and found placental passage of
chromium. Hexavalent chromium appeared in the fetus in high enough
concentrations for a direct effect on embryonic structures to be
likely, trivalent chromium may act on placental structures.
Gilani & Marano (1979) injected chromium trioxide into 840
embryonating chicken eggs (air sack) at doses ranging from 0.002 to
0.05 mg per egg, on days 0, 1, 2, 3, and 4 of incubation (0.1 ml
physiol. saline injections per control egg). All embryos were
examined on day 8. The following malformations were observed in
the 720 eggs affected: short and twisted limbs, microphthalamia,
exencephaly, short and twisted neck, everted viscera, oedema, and
reduced body size. However, the incidence of abnormalities in the
120 controls was reported to be "low".
Inhalation studies were conducted by Glaser et al. (1984) on
Wistar rats (TNO-W-74 SPF) to study the effects of sodium
dichromate on reproduction and teratogenicity in 3 generations.
Whole-body exposures to sodium dichromate aerosols (0.2 mg
chromium/m3) were performed with an exposure time of 130 days per
generation. No effects on reproduction were found. All
teratogenicity tests were negative, and there was no increase in
fetal chromium content. However, from generation to generation,
there was an increase in immunosuppression and hyperplasia of
organs (especially in the lungs) and changes in haematological
variables.
Table 18. Embryotoxicity following maternal exposure to CrO3 on different days
of gestation in hamstersa
-------------------------------------------------------------------------------------
Findings in live fetuses
Day of No. of No. of No. of Frequency External Internal Cleft
gestation females implantation living resorptions defects defects palate
treated sites fetuses No. %b No. %b No. %b No. %b
-------------------------------------------------------------------------------------
Treated CrO3 8 mg/kg
7 6 64 10 54 84 1 10 1c 10 5 50
8 6 88 54 14 20 8d 15 4e 7 17 31
9 6 77 67 10 13 18f 27 0 0 25 37
10 6 77 77 0 0 0 0 0 0 0 0
11 6 68 67 1 1 0 0 0 0 0 0
Controls H2O 5 ml/kg
7 3 35 33 2 6 0 0 0 0 0 0
8 3 44 41 3 7 0 0 0 0 0 0
9 3 37 36 1 3 0 0 0 0 0 0
10 2 20 19 1 5 0 0 0 0 0 0
11 3 34 33 1 3 0 0 0 0 0 0
-------------------------------------------------------------------------------------
a From: Gale & Bunch (1979).
b Underlined %, significantly different statistically from controls.
b Right hindlimb with two digits.
c Right kidney absent.
d Oedema (7), tail short (1).
e Small kidneys (3), right kidney absent (1).
f Oedema (17), omphalocele (1).
Table 19. Comparison of concentrations of trivalent and hexavalent
chromium in fetuses, placentas, and maternal organsa,b
------------------------------------------------------------------
Day 13 Day 16
Hexavalent Trivalent Hexavalent Trivalent
chromium chromium chromium chromium
------------------------------------------------------------------
Fetus 0.3 ± 0.01 0.03 ± 0.002 0.5 ± 0.04 0.1 ± 0.01
Placenta - - 3.0 ± 0.2 2.4 ± 0.2
Liver 29.4 ± 2.2 30.8 ± 6.5 33.9 ± 3.9 27.7 ± 3.5
Kidney 38.1 ± 1.9 5.3 ± 0.7 36.0 ± 4.3 8.7 ± 2.3
Serum 2.6 ± 0.2 9.3 ± 0.9 2.8 ± 0.4 11.0 ± 1.6
------------------------------------------------------------------
a From: Danielsson et al. (1982).
b Trivalent chromium (51CrCl3) and hexavalent chromium
(Na251Cr2O7) were injected iv on day 13 and day 16 of gestation
at doses of 10 mg chromium/kg body weight. The pregnant animals
were autopsied after 1 h. Concentrations are expressed in
mg/litre or g ± SEM (N = 4).
7.2.1.4. Cytotoxicity and micromolecular syntheses
Wacker & Vallee (1959) reported that trivalent chromium was
present in RNA from all sources examined; they hypothesized that
chromium might contribute to the stabilization of the structure.
Administration of trivalent chromium to mice (ip injection of
CrCl3 at 5 or 0.5 mg chromium/kg body weight; P < 0.01) caused
accumulation of chromium in the cell nucleus, which amounted to
about 20% of the accumulated chromium content of the liver cell,
and also enhanced RNA synthesis. Hexavalent chromium inhibited RNA
synthesis (K2CrO4 at 5 or 0.5 mg chromium/kg body weight;
P < 0.01) (Okada et al., 1983). In further studies, Okada et al.
(1984) pretreated rats with CrCl3 (ip injection of 5 mg chromium/kg
body weight) and then partially hepatectomized the rats. Chromium
accumulated in the regenerating liver, and hepatic RNA synthesis
was accelerated compared with that of partially hepatectomiced
controls. These findings suggest a direct participation of
chromium in RNA synthesis.
Potassium dichromate enhanced the passive uptake of ribo-and
desoxyribonucleosides (Levis et al., 1978; Bianchi et al., 1979),
caused an inhibition of DNA replication to BHK fibroblasts, and
reduced the colony-forming ability of BHK cells (doses: 10-4 - 20-7
mol). Hexavalent chromium (potassium dichromate with a
concentration of 5 x 10-7 mol/litre) reduced by half the
proliferation of NHIK 3025 cells originating from a human cervix
carcinoma (White et al., 1979).
The addition of calcium chromate to BHK21 cell cultures altered
their growth characteristics. Whereas these cells normally grow as
elongated fibroblast cells in parallel orientation, the chromium-
exposed cells grew as shortened fibroblasts with enlarged nuclei
and granular cytoplasm, randomly orientated. When grown in
carboxymethyl cellulose, the chromate-exposed cells underwent many
divisions in contrast to the 1 or 2 divisions of normal cells.
These alterations in growth properties persisted, even after
transfer to a normal medium (Fradkin et al., 1975).
Cultures of HeLa and rat embryonic cells were incubated with
various hexavalent and trivalent chromium compounds for 3 days and
the 50% inhibitory dose (ID50) for cell growth was determined. The
ID50 for sodium and potassium chromate and dichromate and calcium
chromate varied from 0.34 to 0.78 µg/ml of the medium. The ID50
for the trivalent compounds varied over a wider range: chromic
oxalate, 4; acetate, 52; nitrate, 720; chloride, 1030; and bromide,
1500 mg/litre. Similar results were found in rat embryonic cell
cultures (Susa et al., 1977).
7.2.1.5. Fibrogenicity
Because of the well-known fibrogenic effects of silica and
asbestos minerals, studies were initiated to determine whether
chromite and various other dusts exhibited similar properties. A
single intratracheal injection in rats of 40 mg chromite particles
(33% chromium, 0.7% SiO2, 99.9% of the particles being smaller than
5 µm) suspended in 1 ml Ringer's solution caused a moderate
cellular reaction that reached a peak after 2 months and then
decreased. After 8 months (end of observation), the lung weight
was within normal limits. Only an increase in fibrils that was not
significant was observed. The tissue reaction in regional lymph
nodes was minimal (Swensson, 1977).
Chromite FeO(CrAl)2O3 (10 mg), ground to an average particle
diameter of 1 µm, was injected into the pleural cavities of 25
mice. The animals were killed at intervals ranging from 2 weeks to
18 months after injection. Chromite produced the least reaction of
all the 15 dusts tested. This material attracted very few cells
and the eventual production of collagen was minimal (Davies, 1972).
7.2.2. Observations in farm animals
Systemic poisoning of farm animals with chromates is rare and
is mainly suspected to be a consequence of accidental industrial
contamination of drinking-water. The acute lethal dose for mature
cattle is approximately 600 - 800 mg/kg body weight; chronic
poisoning in young calves was produced by a daily dose of 30 - 40
mg/kg body weight for one month (NZ Department of Agriculture,
1954) with a clinical picture of profuse scouring, severe
dehydration, low blood pressure, changes in the alimentary tract,
including congestion and inflammation, and the rumen and abomasum
showing ulceration near perforation.
High chromium levels were found in the blood and liver of
calves that had died from poisoning. The minimum tissue levels
causing poisoning were 30 mg/kg in the liver and 4 mg/litre in
whole blood (Harrison & Staples, 1955). According to Romoser et al.
(1961), a level of 100 mg hexavalent chromium (as sodium
chromate)/kg did not affect growing chicks.
8. EFFECTS ON MAN
8.1. Nutritional Role of Chromium
The physiological role of chromium and the need for small
amounts of dietary chromium are indicated by:
(a) chromium-responsive cases of impaired glucose
metabolism in malnourished children and middle-aged
persons;
(b) 2 cases of proved chromium deficiency in patients fed
exclusively by parenteral alimentation; and
(c) epidemiological studies suggesting a link between
chromium deficiency and risk factors for cardiovascular
diseases.
8.1.1. Biological measurements and their interpretation
Biochemical methods for the diagnosis of the human nutritional
chromium status are still at the developmental stage. Blood- or
urine-chromium concentrations are not indicative of chromium
status. Several studies suggest the validity of the "relative
chromium response" in plasma or serum to the ingestion of 50 - 100
g of glucose within 30 - 120 min; the response also appears in the
urine. Lack of this response suggests chromium deficiency,
especially when combined with impaired glucose tolerance in the
presence of normal or elevated plasma-insulin levels.
8.1.2. Chromium deficiency
8.1.2.1. Adults
Chromium deficiency is diagnosed mainly through therapeutic
trials in which an impaired function is restored by supplementation
of the diet with small amounts of chromium close to the estimated
human requirement. Glinsmann & Mertz (1966) studied 4 male
diabetic patients for several months to 1 year, under the strictly
controlled conditions of a metabolic ward. The subjects, who were
fed identical portions of a diet prepared before the start of the
study, were allowed a measured amount of physical activity and were
stabilized under these conditions before the beginning of the
control periods. Three of the 4 patients responded to a chromium
level of 180 - 1000 µg/day as CrCl3 x 6H2O with a significant
improvement in oral glucose tolerance; the fourth showed a very
slight lowering of the glycaemic curve, which was not statistically
significant. A representative example is given in Table 20.
Table 20. Effect of chromium on mean glucose tolerancea,b
------------------------------------------------------------------------
Supplementation Days Number Mean blood glucose concentration
of ---------------------------------
tests Fast- Min after glucose load
ing 30 60 90 120
------------------------------------------------------------------------
None 0 - 32 10 840 2170 2290 1950 1560
60 µg, 3 x per day 33 - 74 12 850 2240 2380 2090 1620
60 µg, 3 x per day 75 - 119 13 840 2100 2130 1860 1410
60 µg, 3 x per day 120 - 140 5 840 2030 2010c 1750 1120c
None 180 - 194 1 960 2070 2900 2930 2370
60 µg, 3 x per day 195 - 211 5 830 1960 1930c 1750 1180c
None 256 - 313 10 870 2200 2450 2240 1660
1 mg, 3 x per day 337 - 340 1 680 1480 1620 1510 960
------------------------------------------------------------------------
a Modified from: Glinsmann & Mertz (1966).
b A 46-year-old man with maturity-onset diabetes, controlled on diet;
oral glucose tolerance test consisted of constant noon meal and 100 g
glucose.
c Mean values significantly different from control ( P < 0.025).
The condition of one of 2 diabetic out-patients, observed over
several months, was improved by chromium supplementation, the
condition of the other was not. Short-term treatment of 7 diabetic
patients for 1 - 7 days with 1 mg chromium did not affect any
variables of glucose metabolism, nor did chromium supplementation
change the normal glucose tolerance tests of 10 healthy, young
volunteers.
Glinsmann's positive results were not confirmed in a double-
blind crossover study on 4 normal volunteers and 10 diabetic
persons, with a crossover after 16 weeks. No effects of chromium
were detected on glucose tolerance or on fasting or postprandial
blood-glucose levels (Sherman et al., 1968). Schroeder et al.
(1970), while reporting that 2 - 10 mg chromium/day "almost always"
restored the impaired glucose tolerance in older, non-diabetic
subjects to normal, found only erratic and mild improvement in 4
out of 12 diabetic out-patients. On the basis of these
observations, Mertz (1969) concluded that chromium should not be
considered a therapeutic agent for diabetic patients.
On the other hand, it was reported in a later study on chromium
metabolism that chromium administration (500 µg/day, for 2 weeks)
produced a marked improvement in glucose utilization in both
juvenile and maturity-onset diabetic patients. Five of the
patients were followed for more than 1 year after exposure and were
found to maintain their improved glucose metabolism (Nath, 1976).
Of 10 non-diabetic elderly subjects with impaired glucose
tolerance, 4 responded to 150 µg chromium/day with complete
normalization of oral glucose tolerance tests. The remaining 6
subjects were not affected by the supplements (Levine et al.,
1968). Significant effects of a GTF-chromium-containing yeast
preparation were reported by Doisy et al. (1976) in 6 out of 12
elderly subjects with impaired glucose tolerance
Table 21. Effect of GTF supplementation on glucose tolerance tests in
elderly subjects with impaired tolerancea,b
-------------------------------------------------------------------------
Mean plasma-glucose levels Cholesterol Triglyceride
(mg/litre) (mg/litre) (mg/litre)
0 h 1 h 2 h
-------------------------------------------------------------------------
Before GTF 1060 ± 40c 2010 ± 70 1780 ± 80 2450 ± 90 1210 ± 80
11 tests
After GTF 990 ± 40 1620 ± 110 1320 ± 50 2050 ± 100 1120 ± 120
9 tests
Signifi- NS < 0.01 < 0.001 < 0.01 NS
canced
-------------------------------------------------------------------------
Mean serum-insulin levels
(microunits/ml)
0 h 1 h 2 h
Before GTF 24 ± 6 78 ± 17 118 ± 17
after GTF 26 ± 12 70 ± 8 83 ± 8
-------------------------------------------------------------------------
a From: Doisy et al. (1976).
b Supplement: 4 g yeast per min/day (Yeastamin Powder 95, Staley Co.,
Chicago, Illinois, USA). GTT: 100 g oral load.
c Mean ± SEM.
d Using paired t test, difference is significant.
In addition to the improvement in glucose tolerance in the
presence of a reduced insulin response, plasma-cholesterol
concentrations were significantly reduced. Several other
individual cases are reported in the publication of Doisy et al.
(1976), all of whom showed a lower serum-insulin response to a
glucose tolerance test during chromium supplementation.
Liu & Morris (1978) performed a similar study on the effects of
a yeast-chromium supplementation in 27 women, 40 - 75 years old, of
whom 15 had a normal and 12 an abnormal glucose tolerance test. In
11 of the normal subjects (73%), the integrated insulin response
was significantly reduced from 436 to 335 µU/ml ( P < 0.025) during
supplementation, without a statistically significant change in
glucose tolerance test. Significant reductions in the total glucose
response (10 650 - 9820 mg/litre; P < 0.001) and in the total
insulin response to glucose were observed in the subjects with
impaired glucose tolerance (1288 - 831 µU/ml; P < 0.001). The
glucose tolerance improved in 7 out of 12 women, but all 12 showed
a reduction in plasma-insulin.
The effects of supplementation of the diet with 200 µg
chromium/day (as chloride) on the glucose tolerance of 76 normal
adult volunteers was investigated in a double-blind cross over
study, with each period lasting 3 months. All 18 subjects entering
the study with impaired glucose tolerance (serum-glucose equal to
or greater than 1 g/litre, 90 min after a glucose load), showed
significant improvement during chromium supplements, but not with a
placebo, with an average decline in the 90-min serum-glucose levels
of 180 mg/litre ( P < 0.01) (Anderson et al., 1983b).
In another placebo-controlled study, 24 elderly volunteers were
given a daily supplement of either chromium-rich brewer's yeast or
chromium-deficient Torula yeast (9 g/day, for 8 weeks). The
glucose tolerance of the subjects given brewer's yeast
significantly improved with a concomitant decrease in plasma-
insulin levels, and a significant decline in plasma-cholesterol as
well as total plasma-lipids. No significant changes were observed
in the control group given the chromium-poor Torula yeast
(Offenbacher & Pi-Sunyer, 1980).
Additional, preliminary studies suggest a specific effect of
brewer's yeast on individual lipoprotein cholesterol fractions.
Twenty normal human subjects responded to daily supplementation
with 18 g of brewer's yeast over a 8-week period with a significant
reduction in low-density lipoprotein-cholesterol ( P < 0.01) and
with a simultaneous increase of 14% in the high-density lipoprotein
( P < 0.01) (Nash et al., 1979).
Similar results were observed in a 6-week supplementation trial
involving 8 physician volunteers, each of whom received 7 g/day of
brewer's yeast. Low-density lipoprotein-cholesterol declined by
nearly 18% ( P < 0.01), whereas the high-density cholesterol
fraction increased by 17.6%, resulting in a significant
( P < 0.001) decrease in the LDL/HDL ratio of 28% (Riales, 1979).
These studies suggest that the primary effect of the
supplementation must have been an increase in the effectiveness of
the insulin, because glucose tolerance was maintained or improved
in spite of lower insulin responses. It is important to note that
while the reduction in insulin levels occurred in all cases, an
improvement in glucose tolerance occurred in only a fraction. This
suggests two possible interpretations of all the results discussed
here, including those showing improvement in glucose tolerance in
only some of the subjects or no improvement at all. Lack of a
response to chromium could suggest that the subjects were not
chromium deficient and that their impaired glucose metabolism was
the result of other causes. Second, they may have responded with a
reduction in circulating insulin levels, as did all the subjects
with impaired glucose tolerance in whom the insulin response was
measured. Unfortunately, these measurements were not performed in
the earlier studies.
8.1.2.2. Malnourished children
Chromium deficiency has been implicated as a factor
contributing to the impairment of glucose tolerance in children
with protein calorie malnutrition in 3 countries, Jordan, Nigeria,
and Turkey. Hopkins et al. (1968) measured intravenous glucose
tolerance in 10 malnourished infants from the mountainous area of
Jordan and 9 equally malnourished infants from the valley area.
They found a severely depressed glucose removal rate of 0.7%/min in
the first group and a normal rate in the second, 3.8%/min (Table
22). The protein intake of both groups prior to hospitalization
had been identical; it was supplied in the form of a milk powder
with a chromium content of 18 µg/litre, when reconstituted.
However, analysis of 10 water samples revealed 3 times as much
chromium in the samples from the valley as in those from the
mountainous area (0.5 versus 1.6 µg/litre). This suggested the
possibility of chromium being a major determinant in the glucose
metabolism of these children, and the effects of supplementation
were measured in 6 children from the hills, with a severely
impaired tolerance test. The morning after oral administration of
250 µg chromium, as CrCl3 x 6H2O, the glucose removal rate had
markedly improved to 2.9%/min from a pretreatment level of
0.6%/min. Similar results were obtained in Nigeria (Table 22);
these tests also included control children who did not receive
chromium.
The immediate effects of chromium on these children were
remarkable and different from the lag phase always present in the
studies on adults. Equally important, was the short duration of
the effect of one dose demonstrated in a Jordanian child observed
for a longer period of time. These observations suggest that a
marked depletion in chromium stores must have existed in the
children.
Table 22. Significance of the effect of chromium on the impaired glucose
tolerances of malnourished infantsa
---------------------------------------------------------------------------
Subjects Number Glucose Significance
of removal of
infants rate difference
(%/min)
---------------------------------------------------------------------------
Jordanian infants from hill area with
0.5 µg chromium/litre in drinking-water 10 0.7
Jordanian infants from valley with
1.6 µg chromium/litre in drinking-water 9 3.8 P < 0.001
Jordanian infants;
initial glucose tolerance test 6 0.6 P < 0.001
after chromium treatment 6 2.9
Nigerian infants;
initial glucose tolerance test 6 1.2
after chromium treatment 6 2.9 P < 0.05
Non-treated infants:
Initial glucose tolerance test 5 1.9 not signi-
Repeated glucose tolerance test 5 2.1 ficant
---------------------------------------------------------------------------
a From: Hopkins et al. (1968).
Similar results were reported from a study in the Istanbul area
of Turkey (Gürson & Saner, 1971). Fourteen malnourished children
with an impaired glucose removal rate of 1.71%/min were given 250
µg of chromium (CrCl3 x 6H2O), and a second test was performed
after 15 h. The removal rate increased to 3.91%/min ( P < 0.001).
This average increase was due to a pronounced increase in 9 of the
children; no change was observed in the remaining 5. No
improvement in glucose tolerance occurred in 5 control children
receiving identical hospital treatment, except for the
administration of the chromium. The children receiving the
chromium supplementation grew significantly more than the control
children during the following 30 days, with the greatest growth
rates found in the 9 children who had responded to administration
of chromium with an improvement in glucose tolerance.
The fact that chromium deficiency interacts with protein-
calorie malnutrition only in certain geographical areas was
confirmed by Carter et al. (1968), who gave chromium
supplementation (250 µg/day as CrCl3 x 6H2O) without effects on
glucose tolerance in 9 Egyptian children tested. Plasma-chromium
levels were in the normal range, and chromium levels in
representative foods were high, ranging from 200 to 390 µg/kg.
These findings suggest that the chromium status of these subjects
was normal, which would explain the lack of an effect.
8.1.2.3. Patients on total parenteral alimentation
A female patient, maintained on total parenteral alimentation
since the age of 35 years, developed an unexpected weight loss of
15% after 5 years combined with peripheral neuropathy, impaired
intravenous glucose tolerance, in spite of normal insulin response,
and a decreased respiratory quotient of 0.66. Insulin treatment
(45 U/day) was ineffective. A strongly negative chromium balance
and low plasma- and hair-chromium levels suggested that the daily
estimated chromium intake from the infusion fluids of 5.3 µg had
not met the patient's requirement and resulted in chromium
deficiency. The insulin infusions were stopped and replaced by the
daily infusion of 250 µg chromium (as CrCl3 x 6H2O) for 2 weeks.
The result was normalization of glucose tolerance and respiratory
quotient, disappearance of signs and symptoms of neuropathy, return
to the previous normal body weight, in spite of reduced caloric
intake, disappearance of the requirement for exogenous insulin, and
a positive chromium balance. The nitrogen balance, which had been
erratic and often negative, became consistently positive following
chromium supplementation. Reduction of the daily supplement to 20
µg chromium proved to be sufficient to maintain the patient in a
state of well-being (Jeejeebhoy et al., 1977).
This case is the best demonstrated example of chromium
deficiency in a human subject. In addition to confirming the well-
known signs of chromium deficiency reported earlier, such as
insulin resistance and its consequences, it was the first case to
demonstrate the occurrence of peripheral neuropathy and disturbance
of nitrogen balance as a result of deficiency. These disturbances
require further study. A second case of chromium deficiency as a
consequence of long-term total parenteral alimentation was reported
by Freund et al. (1979). Signs and symptoms were similar to those
of the first case, except that central encephalopathy was present
instead of peripheral neural disorder. Supplementation with 150 µg
of chromium/day resulted in normalization of the deficiency signs.
Chromium at that time was not routinely added to intravenous
solutions, therefore it is possible that more cases of deficiency
may exist in patients on long-term infusion treatment. As an
outcome of this study, an Expert Panel of the American Medical
Association (AMA, 1979) suggested that the stable adult patient on
total parenteral alimentation should receive 10 - 15 µg chromium
daily.
8.1.2.4. Epidemiological studies
Two epidemiological studies suggest a link between chromium
status and cardiovascular diseases (Schroeder et al., 1970; Punsar
et al., 1975). Lack of chromium is associated with impaired
glucose tolerance, elevation of circulating cholesterol, and aortic
plaques. All of these variables are recognized risk factors for
cardiovascular disease. It has also been suggested that the
elevated insulin levels, often present in chromium-deficient human
subjects (Liu & Morris, 1978) represent a substantial risk for the
development of cardiovascular disease (Strout, 1977).
Schroeder et al. (1970) have summarized their studies of
chromium concentrations in normal and diseased tissues,
particularly in aortas (Table 23). The total body burden of
chromium, excluding the lungs, was lower in the USA, where there is
a higher incidence of cardiovascular disease, compared with other
regions, 1.72 - 1.86 mg/kg ash weight in the USA compared with 7.7,
9.6, and 2.7 mg in Africa, the Far East, and the Near East,
respectively. The only significant difference in tissue-chromium
concentrations in deaths from heart disease compared with deaths
from accidental causes was in the aorta (Table 23). Concentrations
in aortas from Africa, the Far and Near East were generally higher
and did not show such a difference.
Table 23. Chromium in aortasa
----------------------------------------------------------
Location Chromium (mg/kg dry weight)
Accident P Heart disease
----------------------------------------------------------
Africa 0.072 (5)b NS 0.116 (2)
Far East 0.97 (8) NS 0.246 (5)
Middle East 0.36 (8) NS 0.216 (3)
USA (Nine cities) 0.26 (103) 0.005 0.052 (13)
USA (San Francisco) 0.228 (10) 0.005 0.048 (15)
----------------------------------------------------------
a From: Schroeder et al. (1970).
b The number in parenthesis denotes the number of cases
observed.
The second epidemiological study was part of a cohort study
conducted in Finland beginning in 1959 (Punsar et al., 1975). A
total of 327 drinking-water samples were analysed for 22
characteristics including pH, anions, bulk minerals, and several
trace elements including chromium. The study included 504 and 622
men in the Western and Eastern areas of Finland, respectively,
whose domicile had not changed since 1959.
The coronary heart disease mortality rate is much higher in the
east of Finland than in the west. As seen in Table 24, the
chromium levels in drinking-water were much lower in the east than
in the west, whereas the opposite was true for copper. The metal
concentrations in the drinking-water of those with heart disease
and others only differed slightly (Table 24), but, according to the
authors, the data suggest that low chromium levels may play a role
in the high heart disease death rate of eastern Finland.
Table 24. Concentrations of copper and chromium in the
drinking-water of men who died between 1969 and 1973, and
of those who were alive in 1973a
-------------------------------------------------------------
Alive
Deaths (Clinical status in 1969)
CHDb Other CHDb Other No
heart heart
disease disease
-------------------------------------------------------------
West N = 19 N = 23 N = 63 N = 31 N = 288
Copper X 22.15c 20.17 20.05 20.94 20.15
(µg/litre) SD 3.92 4.69 4.69 5.12 4.74
Chromium X 7.52 9.38 9.45 9.74 8.45
(µg/litre) SD 2.06 3.42 4.01 4.44 3.31
East N = 23 N = 26 N = 129 N = 44 N = 279
Copper X 41.59 41.24 42.67 39.50 36.32
(µg/litre) SD 29.91 30.43 33.65 28.74 28.66
Chromium X 2.20 2.09 2.04d 2.49 3.01
(µg/litre) SD 2.17 2.41 2.08 2.17 2.37
-------------------------------------------------------------
a From: Punsar et al. (1975).
b CHD = coronary heart disease.
c P < 0.05.
d P < 0.001.
Note: Levels of significance refer to differences observed
when the concentrations were compared with those in the
"no heart disease" category.
Newman et al. (1978) measured serum-chromium concentrations in
32 patients, 18 male and 14 female, aged 25 - 65 years, in whom
coronary artery cine-arteriography was performed for medical
reasons. Serum-chromium was significantly lower in the 15 subjects
with coronary artery disease than in the 17 subjects without
involvement (2.51 versus 6.09 µg/litre; P < 0.01), whereas serum-
cholesterol, weight index, and systolic or diastolic blood pressure
did not differ among the groups. Serum triglycerides were higher
in the group with coronary artery disease (1.84 versus 1.12
g/litre; P < 0.05). No subject with a serum-chromium
concentration of more than 6 µg/litre exhibited coronary artery
involve- ment, and by regression analysis, serum-chromium was
judged to explain 17% of the total variance of the disease.
These results were independently confirmed in a second study in
which plasma chromium concentrations of 23 healthy persons were
more than 8 times greater than those of 67 patients with coronary
artery disease, as confirmed by cine-angiography (Simonoff et al.,
1984).
8.1.3. Mode of Action
As in experimental animals, the best known action of chromium
is the potentiation of insulin. This is shown by the
supplementation studies of Doisy et al. (1976) and Liu & Morris
(1978) in which a reduction in insulin levels in response to
glucose was observed, together with an improvement in glucose
tolerance. Direct proof, for example from in vitro studies with
human biopsy tissue, has not yet been obtained. Furthermore, it is
not known whether the potentiation of insulin is responsible for
all the effects observed during chromium supplementation, including
the reduction of circulating cholesterol and the growth stimulation
observed in malnourished children and during parenteral nutrition.
In the healthy, chromium-sufficient human subject, chromium
levels in the bloodstream increases acutely following a glucose
load or an injection of insulin. Glinsmann et al. (1966) observed
acute increases in 5 young, healthy volunteers during a glucose
tolerance test, but not when the same subjects were given an
equivalent volume of water. Maturity-onset diabetic patients did
not exhibit this chromium response initially, but did show a rise
in plasma-chromium, after several weeks of chromium
supplementation. These observations were confirmed by Levine et
al. (1968), Hambidge (1971), Behne & Diel (1972), and Liu & Morris
(1978), who saw the acute chromium rise in some, but not all,
subjects. In the study of Liu & Morris (1978), which was the most
detailed, the relative chromium response (CrR) was measured by
neutron activation analysis in 15 women with normal glucose balance
and in 12 with impaired tolerance.
(1-h serum-chromium)
CrR = ------------------------ x 100
(fasting serum-chromium)
Initially, the normal group had a mild positive response (CrR =
107), compared with a negative response (CrR = 81) in the impaired
subjects. Following three months of supplementation with a
chromium-rich yeast extract, the CrR of the normal subjects
increased to 140, and that of the impaired group became positive
with CrR = 149. As discussed in section 8.1.2, these changes were
accompanied by a significant decrease in the insulin response to
glucose and an improvement in glucose tolerance. The results of
these studies are in apparent contrast with those of Davidson &
Burt (1973), who found a significant decline in plasma-chromium
levels following a glucose load in normal, non-pregnant women (mean
age 26 years), but a mild to moderate increase in all but 1 of 10
pregnant women (mean age 20 years). As the time of the acute rise
varied between women, statistical analysis at any one time did not
produce any significant results. Hambidge (1971) and Behne & Diel
(1972) demonstrated an acute plasma-chromium response following
injection of insulin; it can therefore be assumed that the effect
of glucose on the chromium response is associated with
hyperinsulinaemia. The acute "relative chromium response" is
believed to reflect the chromium status of human subjects. It does
not appear when stores from which the chromium increment must come
are depleted. Depleted stores can be replenished by
supplementation, with a resulting reappearance of the response
shown in the two previously discussed studies. As it has been
shown that chromium levels in urine increase following a glucose
load (Wolf et al. 1974; Gürson & Saner, 1978), it is likely that a
fraction or all of the chromium increment is subsequently excreted.
8.2. Acute Toxic Effects
In adults, the lethal oral dose of soluble chromates is
considered to be between 50 mg/kg body weight (RTECS, 1978) and 70
mg/kg body weight (Deichmann & Gerarde, 1969). The clinical
features of toxicity are vomiting, diarrhoea, haemorrhagic
diathesis, and blood loss into the gastrointestinal tract causing
cardiovascular shock. If the patient survives about 8 days, the
outstanding effects are liver necrosis (Brieger, 1920), tubular
necrosis of kidneys, and poisoning of blood-forming organs
(Langard, 1980). A 14-year-old boy, who had ingested about 1.5 g
of potassium dichromate, died 8 days later. Kaufmann et al. (1970)
also reported enlargement and oedema in the boy's brain.
8.3. Chronic Toxic Effects
The chronic effects of trivalent and hexavalent chromium have
been reviewed by Langard & Norseth (1979). The most important
features are changes in the skin and mucous membranes, and allergic
dermal and broncho-pulmonary effects. Important systemic effects
occur in the kidneys, liver, gastrointestinal tract, and
circulatory system.
Mancuso (1951) studied several biological variables in persons
occupationally exposed to chromate and related them to clinical
signs and symptoms. He found occasional leukocytosis or
leukopenia, monocytosis, eosinophilia, reduced haemoglobin
concentrations and prolonged bleeding times, but none of them were
consistent enough to serve as reliable diagnostic tests.
Mild normochronic anaemia was reported by Myslyaeva (1965) in
94 subjects exposed to chromium compounds with 68 of them showing
signs of chronic toxicity. Though the report of Baetjer et al.
(1959b) was based only on 3 cases, it indicated that chromate
attached preferentially to red blood cells. Koutras et al. (1964)
reported the inhibition of the red blood cell enzyme gluthathione
reductase by chromate concentrations of 5 - 25 mg/kg body weight.
However, the lowest effective dose is far in excess of levels
found, even in heavily exposed persons. Thus, all attempts made so
far have failed to identify a specific sensitive biochemical test
for the assessment of chronic chromium toxicity.
Long-term surveillance of pregnant women working at a plant
producing dichromates and those living in its immediate vicinity
(Shmitova, 1980) revealed increased chromium levels in the blood
and urine, compared with those in the control group. At 32 weeks
of pregnancy, the blood concentrations of chromium were,
respectively, 2.5 mg/litre in the women workers, 1.2 mg/litre in
the women living near the plant, and 0.19 mg/litre in the controls;
the levels in the urine were 1, 0.47, and 0.045 mg/litre,
respectively. Umbilical venous blood contained 1.8, 0.66, and 0.37
mg chromium/litre, respectively. The placental chromium contents
in the surveyed women were 162, 179, and 40 µg/kg, respectively,
and the breast-milk levels were 119, 20, and 31 µg/litre, which
means that chromium is capable of transplacental entry into breast
milk and into the fetal and infant organism. Women working at the
bichromate plant exhibited a high incidence of obstetric pathology
and geotoses. Chromium levels were estimated in fetal and
placental tissues (abortive material) derived on the 12th week of
pregnancy from women workers of the dichromate facility and those
not exposed to chromium. In the test group, chromium levels were
1140 µg/kg in the fetal tissues and 135 µg/kg in the placental
tissue, whereas, in the controls, they were 928 and 30 µg/kg,
respectively. No teratogenic effects have been revealed (Shmitova,
1980).
8.3.1. Effects on skin and mucous membranes
Several different types of effects on skin and mucous membranes
may result from exposure to chromium chemicals, and the main
features are:
(a) primary irritation: ulcers (corrosive reactions),
scarred and non-ulcerative contact dermatitis;
(b) allergic contact dermatitis: eczematous and
noneczematous.
A general survey of the nature and cause of contact dermatitis
is given by Fregert (1981).
8.3.1.1. Primary irritation of the skin and mucous membranes
(a) Skin
Skin irritation, manifested by a sharp hyperaemia as well as by
vesicular, papular, and rash pattern, may result from contact with
chromates. However, the major problems in the primary irritation
dermatoses are ulcers, often called chrome holes or chrome sores.
Ulceration is likely to occur among workers who have contact with
high concentrations of chromic acid, sodium or potassium dichromate
or chromate, or ammonium dichromate. It does not result from
contact with trivalent chromium compounds. An ulcer may develop if
the chromium compound, either a dust or a liquid, comes into
contact with any break in the skin such as an abrasion, scratch,
puncture, or a laceration. Favoured sites for ulcer development
are the nailroot areas, the creases over the knuckles, finger webs,
the backs of the hands, and the forearms (Bloomfield & Blum, 1928).
Primary irritation by chromium can be attributed to hexavalent
chromium, as the salts of trivalent chromium are not considered
capable of producing such effects (Fregert, 1981). However,
prolonged exposure to trivalent chromium salts has been reported to
cause skin lesions, less marked than those following exposure to
hexavalent chromium salts (Domaseva, 1971). Findings in 164
patients with eczema showed that skin tests on people who had
contact with trivalent chromium did not give positive results with
dichromate (Valer & Racz, 1971).
Ordinarily, a chrome sore, if not deep, persists for about 3
weeks after exposure is discontinued. There is no evidence that
the ulcers undergo malignant transformation. Furthermore, the
presence of chromium ulcers did not influence the development of
sensitization to chromates (Edmundson, 1951), evidenced by patch
testing with a 0.5% solution of K2Cr2O7.
(b) Nasal septal mucosa
Perforation of the nasal septum was one of the main chronic
effects in workers who had contact with chromates and chromic acid
(Bloomfield & Blum, 1928; Langard & Norseth, 1979). In a group of
100 persons constantly in contact with chromium compounds, 44 had
superficial ulceration of the anterior nasal septal mucosa.
Twenty-one people had profound ulcers and 5 had perforations of
various sizes in the cartilaginous part of the nasal septum.
Perforation of the nasal septum and cicatricial alterations were
observed in people with 3 or more years of service, whereas
ulcerous lesions arose in 2 years of work with chromium compounds.
An additional consequence of the effect on the nasal mucous
membranes is a loss of the senses of smell and taste (Seeber et
al., 1976). In workers with profound ulcerous lesions of the nasal
septal mucosa, strains of white staphylococcus with elevated
resistance to chromium are sometimes isolated (Cherevaty et al.,
1965).
Pokrovskaya et al. (1976) studied 561 workers engaged in the
open excavation of chromium ores (chromium content was 62% in the
form of a complex compound of magnesium chromite and other chromium
ores). Almost half of the workers had worked there for more than 5
years, and about 40% had been working there for 6 - 15 years.
Changes in the nasal septal mucosa, typical of the effect of
chromium compounds, were found in 8.2% of the workers. The longer
the service record, the greater the prevalence of these lesions.
After 5, 11, and 15 years of work, the prevalence was 2.3%, 8.3%,
and 11.2%, respectively.
The prevalence of nasal septum perforation in chromium plating
workers was very high (24%) in the early 1950s in China (Yang,
1956). With the introduction of control measures, the prevalence
dropped so that, in 1982, only 2 cases of nasal septum perforations
were found among 393 chromium-exposed workers (Peng, 1982).
One hundred and four subjects (85 male, 19 female) exposed to
chrome plating and 19 unexposed controls were studied by Lindberg &
Hedenstierna (1983) with regard to changes in the nasal septum.
Exposed subjects were employed at 13 different companies and
included all who were working on the day of study at the particular
company. The median exposure time was 4.5 years (range, 0.1 - 36
years). Forty-three subjects were exposed almost exclusively to
chromic acid and constituted both a "low exposure" group (8-h mean
below 2 µg/m3, N = 22) and a "high exposure" group (2 µg/m3 or
more, N = 21). Their median exposure time was 2.5 years (range,
0.2 - 23.6 years). The other 61 subjects were exposed to a mixture
of chromic acid (0.2 - 1.7 µg/m3) and other pollutants, such as
nitric, hydrochloric, and boric acids, as well as caustic soda,
nickel, and copper salts. The last group was included to disclose
any additive or synergistic effects of chromic acid and other
pollutants and was studied with regard to lung function only. The
breakdown of subjects into various groups is shown in Table 25.
Nasal septal ulceration and perforation were seen in 10 out of 14
subjects exposed to peak levels of 20 - 46 µg chromic acid/m3 or
more; no one in the control group showed signs of atrophy,
ulcerations, or perforations.
8.3.1.2. Allergic contact dermatoses
Chromium is a very important skin sensitizer. Sensitization
is reported to require about 6 - 9 months, but can occur in less
than 3 months (Pirilä & Kilpio, 1949). Genetic factors appear to
contribute to the appearance of chrome sensitivity within the
Jewish population (Wahba & Cohen, 1979).
Apart from the primary irritation and ulceration effects,
direct contact with small amounts of the chromates may cause
allergic dermatosis (dermatitis and eczema). As this allergic
reaction is cell-mediated, i.e., antibody formation is intra-
cellular and not humoral, delayed allergic reactions are rarely
observed.
Fregert et al. (1969) reported that the skin-patch test for
chromium was positive in 8 - 15% of all patients suffering from
eczema. The dermatitis caused by chromium is described as a
diffuse erythematous type with severe cases progressing to an
exudative stage. Persons sensitive to chromium react to patch and
intracutaneous tests with nonirritant concentrations of potassium
dichromate (K2Cr2O7). Opinion is divided about the role of the
oxidation state of chromium as a causal agent in allergic contact
dermatoses (Morris, 1958; Fregert & Rorsman, 1964; Kogan, 1971).
In a review, Polak et al. (1973) showed that hexavalent chromium,
which penetrates the skin by a direct effect of a hapten, is
reduced to the trivalent state, chiefly by sulfhydryl groups of
aminoacids. It then conjugates with proteins thus forming the full
antigen that initiates sensitization.
Guinea-pigs sensitized with either trivalent chromium chloride
or hexavalent potassium dichromate are capable of reacting in vivo
and in vitro to challenges with both chromium salts. This double
reactivity is also found after repeated stimulation with only one
of these chromium compounds. Since it is not possible to select
lymphocytes directed specifically against a chromium determinant of
a particular valence, it is concluded that, by sensitization with
chromium salts of different valences, a common determinant or
closely related determinants are formed. It is suggested that this
determinant is formed by chromium in the trivalent form
(Siegenthaler et al., 1983).
Two to three years after medical treatment, a follow-up study
on 555 patients with contact dermatitis was completed by means of
questionnaires (Fregert, 1975). The eczema had healed in one-
quarter of the patients, one-half had periodic symptoms, and one-
quarter had permanent symptoms. The prognosis for persons who
changed their work or stopped working was the same as that for
those who continued eczema-inducing work.
From 48 positively tested patients (0.5% potassium dichromate),
38 persons, i.e., 79%, still had a positive patch test after 4 - 7
years. In 72% of the cases, a history of occupational exposure to
chromates could be proved (Thormann et al., 1979).
A fairly rapid transformation of dermatitis into eczema is a
characteristic feature of chromium-induced skin lesions. Kogan
(1971) recognized the following important properties: (a) absence
of adaptation, which makes it necessary to discontinue chromium
handling until the slightest clinical manifestations have
disappeared; (b) a persistent chronic development of an eczematous
process; (c) a long-term latent period; and (d) absence of a direct
correlation between the incidence of sensibilization in the exposed
workers and the chromium concentration to which they have been
exposed.
Table 25. Series of exposed subjects and referencesa
--------------------------------------------------------------------------------------------------------
Exposed subjects
Mixed exposure to
Low exposure to High exposure to chromic acid
chromic acid chromic acid (< 2 µg/m3) and other
(< 2 µg Cr6+/m3) (> 2 µg Cr6+/m3) acids and metallic salts References
----------------- ------------------ ----------------------- -----------------
Nb Age (years)c N Age (years) N Age (years) N Age (years)
--------------------------------------------------------------------------------------------------
Men
Non-smokers 10 35.8 ± 16.7 6 33.4 ± 14.1 12 33.6 ± 12.2 52 34.2 ± 13.2
Smokers 6 30.8 ± 9.7 16 30.2 ± 11.8 36 42.8 ± 13.1 67 30.5 ± 8.9
Women
Non-smokers 3 30.7(17-46) 0 9 48.3 ± 13.0
Smokers 2 44.5(29-60) 1 51.2 4 31.0(19-43)
--------------------------------------------------------------------------------------------------
a From: Lindberg & Hedenstierna (1983).
b When N < 5, the range is given instead of SD.
c X ± SD.
Patients suffering from chromium-induced allergic skin
dermatoses had a tendency to develop cross-correlated hyper-
sensitivity to other metals, in particular, to cobalt and nickel
(Rostenberg & Perkins, 1951; Geiser et al., 1960; Clark & Kunitsch,
1972; Kogan et al., 1972).
Increased sensitivity to chromium is proved by skin reaction
tests. In a number of cases, additional immuno-haematological
bioassays are made to confirm the specificity of these tests. A
method for studying the basophil (mast-cells) degranulation in vivo,
at the site of positive compress skin tests, was suggested by Kogan
(1968). The method was used as a basis for proving the specificity
of cutaneous tests for chromium and other sensitising metals of the
chromium group (cobalt and nickel) (Kogan, 1968; Kogan et al.,
1972).
Photosensitivity is sometimes observed in patients with chronic
dermatitis. Wahlberg & Wennerstein (1977) investigated patients
allergic to chromium, with or without clinically observed light
sensitivity, by a standardized photopatch test procedure. A
significantly more intense reaction was observed in 48% of the
cases in the UV-irradiated sites (4/5 minimal erythema dose)
compared with the non-irradiated control sites.
No cases of cancer of the skin have been reported to result
from exposure to any form of chromium (Fregert, 1981).
8.3.2. Effects on the lung
Airborne chromium trioxide is rapidly absorbed in the broncho-
pulmonary tract causing corrosive reactions (Borghetti et al.,
1977). The absorption of chromium aerosols may depend on the
physical and chemical properties of the particles (Taylor et al.,
1965), as well as on the activity of alveolar macrophages, and the
lymphatic drainage (Sanders et al. 1971).
Both Jindrichova (1978) and Keskinen et al. (1980) reported
that welders using chromium-containing electrodes suffered from
bronchitis. Zober (1982) studied arc welders who had used filler
metals containing chromium and nickel for some years. Compared
with controls, the welders reported more previous ailments of the
respiratory system, mainly in the form of acute bronchitis. In
X-rays of welders, more roundish shadows were found, probably
indicative of benign accumulations of ferrous dust. The greater
incidence of significant findings was clearly apparent in welders
who smoked (probably a combined effect of tobacco smoke and welding
fumes). Reggiani et al. (1973) studied 101 electroplaters by means
of spirometric tests and found an obstructive disease pattern in
13% of them. After inhalation of acetylcholine, a bronchospastic
reaction occurred in 23% of the workers. In another spirometry
study (Bovet et al., 1977) on 44 chromium electroplaters, an
obstructive respiratory syndrome was found among the workers with a
high urinary-chromium content. The authors concluded that the
influence of tobacco smoking on the spirometric tests was much less
than the influence of chromium.
Studying lung function and generalized obstructive lung
diseases in electrofurnace workers in a ferrochromium and
ferrosilicon-producing plant, Langard (1980b) found a reduced
forced vital capacity (FVC) and an increased prevalence of
obstructive lung diseases. Unable to document a connection between
the presence of chromates and increased lung diseases, the author
suggested that the effects were due to high levels of total dust,
especially amorphous silica dust.
Lindberg & Hedenstierna (1983) studied lung function in more
than 100 subjects exposed to chrome plating and in 119 car
mechanics as controls (no car painters or welders). In the exposed
subjects, FVC and the forced expired volume in one second (FEV1)
were both reduced by 0.2 litre, and the forced mid-expiratory flow
was reduced by 0.4 litre/second. The authors concluded that an 8-h
mean exposure level of more than 2 µg/m3 might cause a transient
decrease in lung function.
Pneumoconiosis was diagnosed in Germany (Letterer et al., 1944)
and again in chromite miners in South Africa (Sluis-Cremer & du
Toit, 1968). However, American medical officers could not confirm
this feature when examining 897 workers in chromate-producing
plants in the USA (US PHS, 1953). It should be noted that chromium
dusts had a poor fibrogenic potency in experimental animal studies
(section 7.2.1.5). Davies (1974) X-rayed workers employed in the
manufacture of ferro-chrome and found results similar to miliary
lung tuberculosis. The workers were in the immediate area of the
furnace and were exposed to dust-particles, 90% of which were less
than 2 µm in diameter. The dust deposited on girders consisted of
95% SiO2 and 2.4% Cr2O3. The author did not find any symptoms or
signs of tuberculosis, but there was sufficient evidence to
indicate an inhalation hazard in the ferro-chrome industry, thus
underlining earlier observations of Princi et al. (1962).
8.3.2.1. Bronchial irritation and sensitization
Chromium irritates mucous membranes, as has been seen with
exposure to airborne chromium trioxide fume (Meyers, 1950) or mixed
dust (Card, 1935; Broch, 1949; Williams, 1969). The first symptom
was an irritating cough, 4 - 8 h after exposure. Chromium causes
sensitization that can result in asthmatic attacks. These can last
for 24 - 36 h, without treatment (Langard & Norseth, 1979). An
attack may recur on later exposure, even when this exposure is to a
much lower concentration (US NAS, 1974a).
A double-blind experimental exposure study on an asthmatic
patient, who had been exposed to chromium and nickel, showed that
chromium was the main cause of the asthma (Novey et al., 1983).
Keskinen et al. (1980) pretreated their patients, stainless steel
welders, with both disodium cromoglycate and betamethasone and
found an inhibition of this reaction. Placebo medication failed to
produce this inhibition.
8.3.3. Effects on the kidney
In the early part of this century, chromates and chromic acid
were occasionally used as therapeutic chemicals for some skin
lesions. Fatal cases of acute nephritis occurred with albumin,
hyaline, and granular casts and red cells appearing in the urine,
accompanied by oliguria. Autopsies revealed tubular necrosis, but
not glomerular damage (Kaufman et al., 1970). Acute toxic
gastroenteritis developed, followed by arterial hypotonia, in
suicide cases a few hours after swallowing 1.5 - 10 g potassium
dichromate. Within 2 - 4 days, all patients developed signs of
acute renal insufficiency with oliguria, anuria, and hyperhydration
and also evidence of acute toxic hepatitis (Luzhnikov et al.,
1976). Kidney damage has also occurred in industry, as a result of
accidental exposure of the skin to large amounts of chromate,
especially when associated with extensive skin damage (Luzhnikov et
al., 1976).
The mortality from kidney diseases among workers in the
chromium industry does not appear to have increased. In one study,
no deaths from nephritis and uraemia were found in 4 chromate-
producing plants; in only 1 plant was the mortality rate above that
for the controls (Machle & Gregorius, 1948). Hayes et al. (1979)
reported that 4 deaths from nephritis and nephrosis had occurred
between 1945 and 1977 in workers in a chromate-producing plant,
compared with 4.95 cases in the control population. Satoh et al.
(1981) found one case of nephritis and nephrosis in a group of
chromate workers compared with an expected number of 2.18.
Some authors (Franchini et al., 1975; Borghetti et al., 1977;
Gylseth et al., 1977; Tola et al., 1977) have concluded that
urinary excretion and renal clearance of diffusible chromium can be
used as biological indicators for evaluating the extent of exposure
to airborne chromium and the body burden. However, a dose-effect
relationship could not be established between duration of exposure
and either the level of chromium excretion (Tandon et al., 1977) or
early nephrotoxic indicators (Mutti et al., 1979). A dose-
response relationship for hexavalent chromium effects on the kidney
was described by Mutti et al. (1979), measuring beta-glucuronidase,
protein, and lysozyme in the urine. Abnormal patterns were not
observed in 39 stainless-steel welders during 4.5 ± 3.2 years of
exposure. In another group of workers employed in welding armoured
steel with special electrodes for 1 ± 0.4 years, 22% showed an
increase in beta-glucoronidase and 10% in protein in the urine. In a
study on 24 workers in the chromium-plating industry who were
exposed for 8.9 ± 7.3 years, the authors found increased urinary
levels of beta-glucoronidase in 37% of the workers, an increase in
protein in 17%, and an elevated level of lysozyme in 4%. Urinary
beta2-microglobulin was also studied by Lindberg & Vesterberg (1983b)
in chromeplaters (24 males, mean age 36 years, working in 4
different factories). The exposure was measured using personal air
samplers. The 8-h mean value ranged between 2 and 20 µg hexavalent
chromium/m3 and averaged 6 µg/m3. Most of the workers showed
symptoms of airway irritation, 2 of them had ulcerated nasal septum
and another 2, complete perforation. Significantly higher urine
beta2-microglobulin levels (0.23 mg/litre; range, 0.04 - 1.24 mg/litre;
SD, 0.27) were found in this group of workers in comparison with a
reference group of workers of a comparable age (mean age, 38 years;
mean urinary beta2-microglobulin level, 0.15 mg/litre; range, 0.06 -
0.19 mg/litre; SD, 0.08). A relationship was observed (Table 26)
between the range of exposure and prevalence of urinary beta2-micro-
globulin levels exceeding 0.3 mg/litre. The 2 workers with
perforated nasal septum were in the highest exposure group. A
difference in urinary beta2-microglobulin levels was not found between
a group of workers employed several years earlier in an old
chromeplating plant (mean, 0.25 mg/litre) and a group of workers of
comparable age (mean, 0.29 mg/litre), but it should be noted that
the mean age of both these groups exceeded 52 years.
Table 26. beta2-microglobulin U-beta2 levels in urine of workers
related to present exposure levelsa
-------------------------------------------------------------------------
Plant Exposure Number Age Persons with an "elevated" U-beta2
No. range of (mean) (U-beta2 > 0.30 mg/litre) range
(µg/m3) workers concentration (mg/litre)
No. Ages
-------------------------------------------------------------------------
1 11 - 20 5 39 3 21, 37, 58 0.23 - 1.30
2 - 3 14 - 8 13 37 2 20, 21 0.04 - 0.44
4 2 - 3 6 6 - - 0.06 - 0.18
-------------------------------------------------------------------------
a From: Lindberg & Vesterberg (1983b).
8.3.4. Effects on the liver
Statistics on diseases of the liver are seldom published
separately; they are usually included in the group of diseases of
the digestive system. The chromate industry was reported to have
the same rate in this group of diseases as many other industries
(US PHS, 1953). The Federal Security Agency in the USA
investigated 14 chromate workers reported to have "enlarged livers"
but found that these cases were not related to the time spent in
the industry. Acute hepatitis with jaundice was described in one
worker employed in a chromium-plating plant.
In an epidemiological study, Satoh et al. (1981) reported a low
rate of cirrhosis of the liver in chromium workers and liver
function test results that did not differ significantly from those
of the controls.
However, following ingestion of high doses of potassium
dichromate, liver necrosis (Brieger, 1920) and congested liver
(Kaufman et al., 1970) with loss of its architecture have been
described. There are also case studies of liver function and
histology in electroplaters (Pascale et al., 1952) and chromate
chemical plant workers (Etmanova, 1965), but the results are
difficult to interpret.
8.3.5. Effects on the gastrointestinal tract
The following symptoms and signs were reported in workers
engaged in the production of chromium salts (Sterekhova et al.,
1978): hyperchlorhydria, elevated pepsin and pepsinogen level,
oedema, hyperaemia and erosion of the mucosa, polyposis,
dyskinesia, and gastritis. However, Satoh et al. (1981) reported
that the incidence of peptic ulcer in chromate workers was below
the expected rate, and another study showed that the mortality rate
for diseases of the digestive system was lower in chromate workers
than in the control population (Hayes et al., 1979).
The gastric juice has been shown to play a role in the
detoxification of ingested hexavalent chromium by reducing it to
trivalent chromium, which is poorly absorbed and eliminated with
the faeces (Donaldson & Barreras, 1966; DeFlora & Boido, 1980).
8.3.6. Effects on the circulatory system
A clinical study was performed by Kleiner et al. (1970) on
myocardial function in more than 200 workers in the potassium
chromate industry, suffering from pulmonary or gastrointestinal
diseases, attributed by the authors to chromium poisoning. A
control group of 70 healthy individuals was included. Pathological
changes in the ECG and in other tests of heart function indicated
disturbance of right heart function, which the authors considered
secondary to the pulmonary pathology. The full paper was not
available and, consequently, the data could not be evaluated in
detail. It should be noted that no unexpected findings in
cardiovascular mortality were cited in the epidemiological studies
described in section 7.3.9.
8.3.7. Teratogenicity
The only human data available concerning this topic were
reported in an abstract (Morton & Elwood, 1974). The authors did
not find any correlation between frequency of malformations in the
central nervous system and the chromium content in water samples
collected in South Wales. Furthermore, Suzuki et al. (1979) did
not find any indication of accumulation of chromium in fetal
tissues.
8.3.8. Mutagenicity and other short-term tests
Few reports exist dealing with chromium-induced mutagenicity in
human subjects, and only hexavalent chromium causes any effects.
Bigaliev et al. (1978) found a 3 - 8% increase in chromosomal
aberrations of peripheral blood leukocytes in workers handling
chromium-compounds, compared with 2% in unexposed controls.
When classified according to the Papanicolaou system,
cytological samples of the sputum of 116 workers in the chromate-
production industry did not show any stages of tumour development
(Maltoni, 1976). However, intermediate stage lesions found in 30
workers were described as atypical adenomatous proliferation,
squamous-cell, and basal-cell dysplasia.
Studying cultured lymphocytes obtained from workers
occupationally exposed to CrO3, Sarto et al. (1982) found an
increased frequency of sister chromatid exchanges compared with
controls, which was correlated with urinary-chromium levels and
enhanced by smoking habits.
8.3.9. Carcinogenicity
8.3.9.1. Lung cancer
(a) Epidemiological studies in chromate-producing plants
The first recognition that cancer of the respiratory tract
might be related to chromium exposures resulted from the reporting
in 1932 of 2 cases of bronchogenic carcinoma that had occurred in
an old German chromate chemical plant (Lehmann, 1932). Gross &
Kölsch (1943) reported 10 deaths from lung cancer in small plants
producing the chromates of lead and zinc. By 1947, 52 such cases
had been reported from this industry and 10 cases from a chrome
pigment plant, also in the Federal Republic of Germany (Baetjer,
1950a).
The first epidemiological study was reported in the USA in
1948. The mortality data were based on the records of the group
life insurance policies of 6 chromate-producing plants in the USA
compared with similar data from a non-chromium industry or from a
life insurance company. The study reported that 21.8% of all
deaths between 1938 and 1947 in the chromate plants were due to
cancer of the respiratory tract compared with 1.4% in the control
group. The crude death rate for cancer of the bronchi and lungs
was 28 times that for the control groups (Machle & Gregorius,
1948). In one of these plants (Mancuso & Hueper, 1951), the cancer
death rate was 15 times that of the general population in the
county where the plant was located. The US PHS (1953) also studied
the respiratory cancer risk in these 6 plants and estimated a
relative risk of 28.9 using the average cancer mortality rates for
US males between 1940 and 1948 as a comparison.
In addition to these studies, where the cause of death was
taken from insurance or death certificate records, a case-control
study, using only cases diagnosed by autopsy or biopsy, was
conducted by Baetjer (1950b). Two hospitals near the largest
chromate plant in the USA were asked to supply the histories of all
cases diagnosed in 1924-46 as cancer of the lung bronchi, and to
confirm histopathologically. A group of non-cancer cases matched
individually by age, sex, and date of admission was selected as
controls. Of the 290 confirmed cancer cases, 3.26% had been
employed in the chromate plant, whereas none of the 900 control
cases was found to have had chromate exposure. The percentage of
chromate workers in the lung cancer series was 28.6 times the
percentage of chromate workers among the employed male population
of the city. The plant involved in this study was rebuilt in 1950,
following the discovery of the cancer problems. A new study of
this plant was made by Hayes et al. (1979) to determine whether the
risk of cancer had been eliminated. The investigation covered 438
deaths among 2101 males, who were initially employed between 1945
and 1974 for more than 90 days, and who died between 1945 and 1977.
The SMRa for malignant neoplasms of the trachea bronchi and lungs
was 202. Cancer deaths in the city in which the plant was located
were used to calculate the expected number of cases. No cases of
respiratory cancer occurred in the plant between 1960 and 1977
among workers employed only in the new plant. Because of the long
latent period, further follow-up is necessary.
Increased lung cancer rates among chromate workers in the USA
have been confirmed by Taylor (1966), Enterline (1974), and Mancuso
(1975), who also showed that the risks were especially high during
the early years of the study of the cohorts, e.g., before 1950.
Studies conducted in other countries have shown similar results.
In a 1956 study on workers in chromium-producing plants in the
United Kingdom, the rate of lung cancer mortality was 3.6 times the
rate for England and Wales (Bidstrup & Case, 1956). In a follow-up
study on 2715 men who had worked at the 3 chromate-producing
factories in the United Kingdom from 1948 to 1977, Alderson et al.
(1981) found 116 deaths from lung cancer (expected, 48). Since
modification of the one plant that is still in operation, the
relative risk of lung cancer has decreased from over 3 to about
1.8.
Two epidemiological studies have been conducted in the Japanese
chromate industry. In one plant, 5 lung cancer deaths occurred
between 1960 and 1973 in 136 workers who had been exposed for more
than 9 years; the SMR was 1510 (Watanabe & Fukuchi, 1975). In
another plant, the mortality rate for respiratory cancer was
determined for 896 males who worked in the plant from 1918 to 1975
and were followed to 1978; the SMR was 920 (expected deaths based
on 1975 age-cause-specific mortality rates for Japanese males)
(Satoh et al., 1981).
In the Federal Republic of Germany, a study was conducted in 2
chromate-producing plants (Korallus et al., 1982). Comparing the
SMR for lung cancer during the period 1948-79 for these 2 groups, a
clearly decreasing tendency was observed. In the first plant, for
1948-52, the SMR was 512 and, for 1978-79, it was 98; for the other
plant, the corresponding figures were 1905 and 150, respectively.
The newest data indicate a decreasing risk approaching the
frequency in unexposed people (Korallus & Loenhoff, 1981). This is
a result of occupational hygienic and technological measures,
especially of a change in manufacture namely the "on line"
--------------------------------------------------------------------
a The SMR (standardized mortality ratio) is the ratio of
observed to expected deaths times 100.
processing of the chrome ore, which has minimized the contents of
the carcinogenic hexavalent chromium compounds within the process
(Korallus et al., 1982).
Bittersohl (1972) described the results of a study on 30 000
employees of a large chemical unit for the period 1921-70 in the
German Democratic Republic. In particular, 588 malignancies in men
and 170 in women were evaluated for the period 1957-70. In 1971,
108 new malignancies in men and 29 in women came to light. In a
chromate factory, the rate of all cancers was far above average.
The factory manufactured catalysts through the reaction of chromic
acid and iron (III) oxide and nitric acid. The airborne
concentrations of chromium were not reported in detail, but it was
stated that short-term exposures above 400 µg/m3 occurred. The
incidence of malignant neoplasms in employees in the chromate
factory was 852 per 10 000 employees. In "unexposed" personnel,
the incidence of malignant neoplasms was 84 per 10 000 employees.
Approximately 86% of all those with malignant neoplasms were
smokers, and 78% of those without malignant neoplasms were also
smokers, indicating that smoking is not likely to be a confounding
factor.
In the USSR, a high incidence of cancer of the lungs was
reported for men engaged in the production of chrome salts. The
ratio of lung cancer in the plant to that in the control population
was 6.4 for ages 50 - 59 (748 observed, 116 expected) and 15.7 for
ages 60 - 69 (2657 observed, 170 expected) (Tyushnyakova et al.,
1974).
A survey was conducted in China in 1982 to investigate the
problem of lung cancer among chromate-producing workers. It
covered 7 cities and included 2184 male and 798 female workers who
had worked for at least one year in the industry. The preliminary
report revealed that 11 of the 101 deaths among male workers were
due to lung cancer, but none of the 13 deaths among female workers
was due to this cause.a
(b) Epidemiological studies - chrome pigment industry
The first epidemiological study, conducted in 1973, covered 3
small chrome pigment-manufacturing plants in the USA. The
percentage of lung cancer deaths was tentatively reported to be
about 3 times that in unexposed workers (Equitable Environmental
Health Inc., 1976).
Lung cancer mortality was studied among 1152 men working at 3
chromate pigment factories in the United Kingom from the 1930s or
1940s until 1981 (Davies, 1979, 1984). Among workers at factory A,
which produced both zinc and lead chromates, entrants to the
factory between 1932-45 with high and medium exposures had excess
---------------------------------------------------------------------------
a Report of an Investigation Team for Cancer in Chromate
Workers (1983).
risks of lung cancer (SMR = 223). A similar excess was seen in
1946-54 entrants, but the excess risk fell just short of
statistical significance. Highly significant excess risks of lung
cancer were seen among 1947-64 entrants that had high or medium
exposures at factory B, which also produced lead and zinc
chromates. Excesses were more severe in workers employed for more
than 10 years. In factory C, which produced only lead chromate, no
excess of lung cancer was found. However, the number of workers
and the power of the study were small.
Twenty-four male workers, who had been employed in a small
Norwegian company for more than 3 years between 1948 and 1972, were
identified and followed up by Langard & Norseth (1975), because
their principal exposure was to zinc chromate dust (measured
routinely with readings occasionally up to 0.5 mg hexavalent
chromium/m3). By December 1980, 6 cases of lung cancer had been
diagnosed, giving a relative risk of 44 compared with that of the
general population in the country. The authors stated that 5 out of
the 6 patients were smokers and only one had been exposed to
chromates other than zinc chromates (Langard & Vigander, 1983).
Sheffet et al. (1982) undertook a detailed mortality study on
workers employed in a pigment plant in Newark, where lead and zinc
chromates were used. Observed deaths from each cause among 1296
white and 650 non-white males employed between 1940 and 1969, were
compared with expected deaths, as computed from cause-, age-, and
time-specific standard death rates for the USA. A statistically-
significant relative risk of 1.6 for lung cancer among white
males, employed for 10 years or more, was found. A relative risk
of 1.9 was noted for individuals employed for at least 2 years, who
were at least moderately exposed to chromates.
An epidemiological study covering several centres in Europe was
conducted by Frentzel-Beyme (1983). This study was designed to
quantify the mortality from cancer and other diseases among workers
in European factories producing chromate pigments (3 German, 2
Dutch factories). Observed deaths in factories were compared with
expected deaths calculated on the basis of mortality figures for
the region in which a given factory was located. The overall
mortality did not deviate from the expected rates. Lung cancer
rates were always in excess of expected numbers, but only in one
cohort was the excess statistically significant. The pattern of
duration of exposure indicates that the lung-cancer risk was not
clearly correlated with length of employment.
A slight excess of lung cancer risk in chrome pigment (zinc
chromate) spray painters from 2 maintenance bases in the USA was
reported by Dalager et al. (1980). Among the 202 deaths
identified, 21 were due to respiratory cancer (11.4 expected;
comparison population: proportionate mortality, US males). No
statistically significant excess of lung cancer was found in a
group of automobile painters in which 226 deaths, including 22
cancer cases, were analysed using proportionate mortality for areas
in which the plants were located as a comparison (Chiazze et al.,
1980).
(c) Epidemiological studies - ferro-chrome industry
Langard et al. (1980) reported that 7 cases of bronchogenic
carcinoma occurred among 976 workers, who started work before
January 1, 1960. The expected number was 3.1 using national rates,
1.8 using local rates, and < 1 when using an international
reference rate for comparison. The difference was not significant
for the national and local rates, but was significant for the
international rate.
A study in Sweden did not reveal any significant excess of
respiratory cancer in ferro-chrome workers (Axelsson et al., 1980).
The incidence of cancer among 1932 workers employed for at least
one year between 1930 and 1975 was compared with the expected
number based on the National Cancer Registry data for the county in
which the plant was located. Five cases of respiratory cancer were
found against an expected 7.2 cases.
An excessive rate of lung cancer was reported for a ferro-
chrome plant in Russia (Pokrovskaya & Shabynina, 1973). The number
of deaths from cancer between 1955 and 1969 was higher than that in
the town in which the plant was located. The authors considered
that both hexavalent and trivalent chromium exposures were
responsible for the excess, since similar excessive rates were
found in a nearby ore-crushing plant, where only trivalent chromium
was present. The number of cases and the rates involved in these
studies were not given.
Hexavalent chromium is produced in some types of welding. In
section 7.2.1.2, it was mentioned that welding fume particles were
positive in the mammalian spot test, indicating a mutagenic and
possible carcinogenic potential. The incidence of lung cancer was
determined by Sjögren (1980), in a cohort of 234 welders, who had
welded stainless steel for more than 5 years between 1950 and 1965,
and were followed to 1977. Three welders died from lung cancer in
comparison with an expected number of 0.68 in the general
population ( P = 0.03).
Comparing lung cancer mortality in 2 parishes with ferro-alloy
industries to mortality in other parishes of similar size without
such industries from the same county, Axelson & Rylander (1980)
were unable to detect an increased incidence of death due to lung
cancer mortality. The concentration of chromium in the ambient air
of the most polluted areas ranged from 0.1 to 0.4 µg/m3. It should
be noted that several possible confounding factors were not under
control, e.g., migration, occupational exposure, and smoking
habits.
(d) Epidemiological studies - chromium-plating workers
Epidemiological studies on workers in the chromium-plating and
related industries were reviewed by Hayes (1982). The relevant
data from these studies are presented in Table 27. According to
Hayes, the epidemiological evidence is not sufficient to determine
the risk of cancer associated with industrial exposure to chromic
acid and, if an excess risk exists, it is probably lower than that
typically described for employment in the chromium-chemical
producing industry.
In a more recent paper, Franchini et al. (1983) conducted a
retrospective cohort study in 9 chromium-plating plants to examine
the mortality of workers employed for at least one year during the
period January 1951 to December 1981. The study group totalled 178
individuals; vital status ascertainment was 97% complete. The
total number of deaths was close to the expected figure (15
observed, 15.2 expected), whereas death from lung cancer exceeded
the expected number (3 observed, 0.7 expected; P = 0.03). The
increased mortality from lung cancer among chromium-platers seemed
to be related to exposure intensity.
8.3.9.2. Cancer in organs other than lungs
A few cancers of the upper respiratory tract and oral region,
but no excessive rates have been reported to occur in the chromate-
producing industry. The cancers have involved the buccal cavity,
pharynx, and oesophagus. Cancers of the nasal septum have not been
documented, with the exception of one case in Italy (Vigliani &
Zurlo, 1955).
In a joint Danish-Finnish-Swedish case-reference investigation,
initiated in 1977, the connection between nasal and sinonasal
cancer and various occupational exposures was studied. All new
cases of nasal and sinonasal cancer were collected from the
national cancer registers (Finland and Sweden) or from the hospitals
(Denmark). The results showed associations between nasal and
sinonasal cancer and exposure to chromium, welding, flame-cutting,
and soldering, hardwood or mixed wood dust, and softwood dust alone
(13.4) (Hernberg et al., 1983).
In 5 plants in the USA, during the early years of extremely
high exposures, the rate of cancer of the digestive tract varied
from 0 to 3.04/1000 compared with a rate of 0.59/1000 for the
controls (Machle & Gregorius, 1948). The mortality rates from
stomach cancer have been reported for chromate-producing plants in
Japan (SMR = 90) (Satoh et al., 1981) the USA (SMR = 40) (Hayes et
al., 1979). No significant differences were reported between the
incidence of stomach cancer in a Russian plant (Pokrovskaya &
Shabynina, 1973) and the incidence in the surrounding area.
Studying cancer mortality in a pigment plant using lead and
zinc chromates, Sheffet et al. (1982) found an increased (but not
statistically risk of stomach (SMR = 170) and pancreas (SMR = 200)
cancer among the total cohort (1946 employees).
Table 27. Summary of epidemiological studies on respiratory cancer in
workers employed in the chromium plating and related industriesa
------------------------------------------------------------------------------------------------
Study population Follow-up Respiratory cancer Comparison population
Number Estimated
relative risk
------------------------------------------------------------------------------------------------
Chromium plating
1056 workers employed Deaths in former and 24 1.8b Unexposed workers in
> 3 months in 54 plants current (followed 2 plants and in 2 non-
in the United Kingdom years) workers, plating industries
about 80% traced
About 5000 workers 49 1.4c Mortality analysis,
employed since 1945 in method not specified
1 plating factory in
the United Kingdom
889 workers exposed > 6 Reports from management 0 < 1b Tokyo mortality
months in Tokyo chromium of plating firms and rates
plating plants, 1970-76 follow-up of retired
workers; 19 total deaths
reported
Related industries
1292 deaths among US Union death benefit 62 1.1b Proportionate
metal polishers and claims mortality, US males
platers, union members,
1951-69
238 deaths among employees Pension, insurance and 39 2.1c Proportionate
for 10 years in one US benefit records mortality, US
plant for metal die
casting, finishing, and
electroplating, 1974-78.
------------------------------------------------------------------------------------------------
a Adapted from: Hayes (1982).
b Not statistically significant.
c P < 0.05, calculated using an assumption that the observed number is distributed as a
Poisson random variable.
8.3.9.3. Relationship between cancer risk and type of chromium
compound
As the exposures are often poorly defined, it is sometimes not
certain whether workers have been exposed to hexavalent chromium,
trivalent chromium, or a combination of the two. Some authors have
concluded that there is no increased risk of cancer mortality due
to trivalent chromium compounds (Korallus et al., 1974 a,b,c;
Axelsson et al., 1980; Langard et al., 1980; Norseth, 1980),
whereas others do not exclude that trivalent chromium compounds
have a carcinogenic capacity (Essing et al., 1971; Mancuso, 1975;
Zober 1979).
Summing up the data from case reports as well as
epidemiological studies in chromate-producing, chromate-pigment,
chromium-plating, and ferrochromium industries, the IARC Working
Group on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans (IARC, 1980) came to the conclusion that "there is
sufficient evidence of respiratory carcinogenicity in men
occupationally exposed during chromate production. Data on lung
cancer risk in other chromium-associated occupations and for cancer
at other sites are insufficient. The epidemiological data do not
allow an evaluation of the relative contributions to carcinogenic
risk of metallic chromium, trivalent chromium and hexavalent
chromium or of soluble versus insoluble chromium compounds".
9. EVALUATION OF HEALTH RISKS FOR MAN
Low levels of chromium are omnipresent in the environment.
Under normal conditions, human exposure to chromium does not
represent a toxicological risk, but it should be pointed out that
too low an intake of chromium may lead to deficiency. Airborne
concentrations of chromium, predominantly as trivalent chromium,
are usually below 0.1 µg/m3 and there are many places where the
concentration is below the detection limit. Concentrations in
river water are typically in the range of 1 - 10 µg/litre and do
not constitute a health threat. Drinking-water from municipal
water supplies does not contribute more than a few micrograms of
chromium to the daily human intake, but untreated water in certain
areas may be contaminated by runoff or effluents from industrial
sources and may contribute significant amounts of chromium. The
oceans contain less than 1 µg/litre. The daily human intake
through food varies considerably between regions. Typical values
range from 50 to 200 µg/day. They do not represent a toxicity
problem.
However, significant exposures exist in the occupational field.
In the past, chromium-ore mining generated chromium-containing
dusts, levels of which ranged up to 20 mg/m3 in different work-
places. Other exposures ranged up to 150 mg/m3 and consisted of
dust containing as much as 48% chromium (Cr2O3). In production
plants, hexavalent chromium can occur in the airborne state.
However, implementation of protective measures at ferrochromium
production sites reduced the airborne hexavalent chromium to levels
of 30 - 60 µg/m3.
The health effects of the two common oxidation states of
chromium are so fundamentally different that they must be
considered separately. In the form of trivalent compounds,
chromium is an essential nutrient and is relatively non-toxic for
man and other mammalian species. The hexavalent form is man-made
by oxidation of naturally-occurring trivalent chromium minerals and
is widely used. Compounds of hexavalent chromium penetrate
biological membranes easily and can thus interact with essential
constituents of the cells, including genetic material which they
can damage through oxidation and complexation with resulting
trivalent species. On the other hand, oxidation of trivalent
chromium has not been demonstrated in the living organism, and for
practical purposes, the reduction of hexavalent chromium to
trivalent chromium in lungs and other animal tissues is
irreversible.
9.1. Occupational Exposure
A number of effects can result from occupational exposure to
airborne chromium including irritative lesions of the skin and
upper respiratory tract, allergic reactions, and cancers of the
respiratory tract. The data on other effects, e.g., in the
gastrointestinal, cardiovascular, and urogenital systems are
insufficient for evaluation.
Epidemiological studies have shown that workers engaged in the
production of chromate salts and chromate pigments experience an
increased risk of developing bronchial carcinoma. No detailed data
on dose-response relationships are available from epidemiological
studies. Although a suspicion of increased lung cancer risks in
chromium plating workers has been raised, the available data are
inconclusive as are data for other industrial processes where
exposure to chromium occurs. There is insufficient evidence on the
role of chromium as a cause of cancer in any organ other than the
lung. Evidence from studies on laboratory animals shows that
hexavalent chromium compounds, especially those of low solubility,
can induce lung cancer.
In the lymphocytes of workers in chromium-plating factories,
the frequency of sister chromatid exchanges was higher in exposed
than in control groups.
Mutagenicity and related studies have convincingly shown that
hexavalent chromium is genetically active. Hexavalent chromium can
cross cellular membranes and is then reduced to trivalent chromium,
which can cause DNA cross-links and increase the infertility of DNA
implication. Trivalent chromium compounds have been shown to be
genetically inactive in most test systems, except in systems where
they can directly interact with DNA.
9.1.1. Effects other than cancer
9.1.1.1. Respiratory tract
It has been reported that the threshold for acute irritative
effects in the upper respiratory tract is 25 µg/m3 for the most
sensitive individuals. Long-term exposure to doses over 1 µg
chromic acid/m3 can cause nasal irritation, atrophy of the nasal
mucosa, and ulceration of perforation of the nasal septum.
Bronchial asthma was previously attributed to exposure to
chromium compounds, but scientific data are too scarce to draw
conclusions.
9.1.1.2. Skin
Skin rashes, ulcers, sores, and eczema have been reported among
occupationally exposed workers. Both trivalent and hexavalent
chromium compounds can give rise to sensitization of skin,
especially under certain environmental conditions, such as those
encountered in the cement industry, where the high incidence of
chromium-induced skin lesions can be attributed to the alkaline
exposure conditions.
Eczematous dermatitis, which manifests first as a diffuse
erythematous type, progresses in severe cases to an exudative
stage and is associated in 8 - 15% of patients with sensitivity to
chromium, as revealed by skin-patch tests. Although opinion is
divided on the oxidation state of chromium responsible for inducing
sensitization, there is evidence of hexavalent chromium penetrating
the skin as a hapten to be reduced to the trivalent state and
conjugated with proteins and transformed into the full antigen
that initiates the sensitization reaction, involving presumably
only a cell-mediated immune response. It should be noted that
patients suffering from chromium-induced skin allergy tend to
become hypersensitive to cobalt and nickel. Furthermore, 48% of
cases of skin allergy induced by chromium, with or without
clinically observed light sensitivity, showed a significantly more
intense reaction by a standardized photopatch test procedure, when
they were exposed to a 4/5 minimal erythema dose of irradiation.
9.1.1.3. Kidney
After high-dose, short-term oral ingestion of chromium, acute
nephritis and tubular necrosis were observed. A few
epidemiological studies on workers in chrome-plating industries
include data on diseases of the kidney, most of them without giving
exact exposure levels. A recent study related increased urinary-beta2
microglobulin levels to exposure ranges between 2 and 20 µg/m3.
The dose-response relationship observed in this study needs
confirmation on a larger number of exposed workers.
9.1.1.4 Other organs and systems
There are no conclusive data to evaluate the effects of
chromium compounds on the liver or gastrointestinal and circulatory
systems.
9.1.2. Teratogenicity
Both oxidation states, when injected at high levels
parenterally into animals, are teratogenic, with the hexavalent
form accumulating in the embryos to much higher concentrations than
the trivalent. The Task Group was not aware of any report
indicating teratogenicity in human populations.
9.2. General Population
Persons living in the vicinity of ferro-alloy plants, exposed
to an ambient air concentration of up to 1 µg/m3, did not show
increased lung cancer mortality.
The results of many studies suggest that exposure to chromium
through inhalation and skin contact can pose health problems for
the general population. Very little information is available on
the health effects of chromium ingested through untreated drinking-
water, though, in a single study, a correlation was observed
between frequency of malformation in the central nervous system and
the chromium content of water samples (Morton & Elwood, 1974). In
order to assess the nature of the magnitude of these problems,
there appears to be a need for more general population studies on
the effects of inhaled, absorbed, or ingested chromium on
respiratory, cardiovascular, and renal functions, and on the skin.
Studies on animals and man have established trivalent chromium
as an essential micronutrient that interacts with insulin and
enhances the physiological effects of the hormone. Supplementation
trials in a number of countries including Finland, Jordan, Nigeria,
Sweden, Turkey, and the USA have shown that segments of the
population could improve their glucose metabolism and, in some
instances, fat metabolism, by ingesting trivalent chromium
compounds. These observations suggest that some populations are at
risk of chromium deficiency. Children suffering from protein-
energy malnutrition may be at special risk.
As chromium concentrations in the body fluids of unexposed
persons are generally less than 1 µg/litre, analysis for chromium
requires the strictest quality control, including measures to
exclude sample contamination. Even with adequate analytical
methods, it is not possible, as yet, to diagnose chromium
deficiency in individuals by chemical or biochemical methods alone.
Therefore, a quantitative assessment of the prevalence of chromium
deficiency in human populations is not yet possible.
REFERENCES
ABRAHAM, A.S., SONNENBLICK, M., EINI, M., SHEMESH, O., & BATT,
A.P. (1980) The effect of chromium on established athero-
sclerotic plaques in rabbits. Am. J. clin. Nutr., 33:
2294-2298.
AITIO, A., JARVISALO, J., KULUNEN, M., TOSSAVAINEN, A., &
VAITTINEN, P. (1984) Urinary excretion of chromium as an
indicator of exposure to trivalent chromium sulfate in leather
tanning. Int. Arch. occup. environ. Health, 54: 241-249.
ALDERSON, M.R., RATTAN, N.S., & BIDSTRUP, L. (1981) Health
of workmen in the chromate-producing industry in Britain. Br.
J. ind. Med., 38: 117-124.
ALEKSANDROV, A.I. & KAINOV, V.I. (1975) [Crocoite from the
Pervomaisk-Zverevo deposit at the Central Urals.] Tr. Sverdl.
Gorn. Inst., 106: 152-155 (in Russian).
AMA (1979) Guidelines for essential trace element
preparations for parenteral use. J. Am. Med. Assoc., 241:
2051-2054.
AMERICAN PUBLIC HEALTH ASSOCIATION, AMERICAN WATER WORKS
ASSOCIATION, AND WATER POLLUTION CONTROL FEDERATION (1971)
117A. Hexavalent chromium. In: Standard methods for the
examination of water and wastewater, 13th ed., Washington DC,
American Public Health Association, pp. 156-157.
AMES, B.N., DURSTON, E., YAMASAKI, E., & LEE, F.D. (1973)
Carcinogens as mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci. (USA), 70: 2281-2285.
ANDERSON, F.E. (1960) Cement and oil dermatitis. The part
played by chrome sensitivity. Br. J. Derm., 72: 108-117.
ANDERSON, N. (1982) The environmental impact of chromium.
Final draft March 1982, Maine, Department of Environmental
Protection, Bureau of Air Quality Control, 126 pp.
ANDERSON, R.A. & POLANSKY, M.M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Tr. El. Res., 3: 1-5.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., & REAMER, D.C. (1982a) Effect of exercise
(running) on serum glucose, insulin, glucagon, and chromium
excretion. Diabetes, 31: 212-216.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., VEILLON, C., & GLINSMANN, W. (1982b)
Urinary chromium excretion of human subjects: effects of
chromium supplementation and glucose loading. Am. J. clin.
Nutr., 36: 1184-1193.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., PATTERSON, K.Y.,
VEILLON, C., & GLINSMANN, W. (1983) Effects of chromium
supplementation on urinary Cr excretion of human subjects and
correlation of Cr excretion with selected clinical parameters.
J. Nutr., 113: 276-281.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
MERTZ, W., & GLINSMANN, W. (1983b) Chromium supplementation
of human subjects: effects on glucose, insulin, and lipid
variables. Metabolism, 32: 894-899.
ANDERSSON, A. & NILSSON, K.O. (1972) Enrichment of trace
elements from sewage sludge fertilizer in soils and plants.
Ambio, 1: 176-179.
ANGERER, J., SCHALLER, K.-H., & HEINRICH, R. (1981)
[Commercially-available blood and urine samples for quality
control in toxicology.] Arbeitsmed. Sozialmed. Praeventivmed.,
16: 125-127 (in German).
ANIMAL RESEARCH DIVISION OF THE NEW ZEALAND DEPARTMENT OF
AGRICULTURE (1954) Annual report 1953-54, Wellington, R.E.
Owen Government Printer, 40 pp.
ANKE, M., DIETTRICH, M., WICKE, G., PFLUG, D., & SCHUELER, D.
(1971) Absorption, excretion, and distribution of 51chromium
given by mouth to lactating ruminants. Arch. Tierern., 21:
599-607.
AXELSSON, G. & RYLANDER, R. (1980) Environmental chromium
dust and lung cancer mortality. Environ. Res., 23: 469-476.
AXELSSON, G., RYLANDER, R., & SCHMIDT, A. (1980) Mortality
and incidence of tumours among ferrochromium workers. Br. J.
ind. Med., 37: 121-127.
BAER, H. (1920) [Dermatitis resulting from the use of a
beard trainer made partly of artificial leather.] Münch. med.
Wschr., 67: 874 (in German).
BAETJER, A.M. (1950a) Pulmonary carcinoma in chromate
workers. I. A review of the literature and report of cases.
Arch. ind. Hyg. occup. Med., 2: 487-504.
BAETJER, A.M. (1950b) Pulmonary carcinoma in chromate
workers. II. Incidence on basis of hospital records. Arch.
ind. Hyg. occup. Med., 2: 505-516.
BAETJER, A.M., LOWNEY, J.F., STEFFEE, H., & BUDACZ, V.
(1959a) Effect of chromium on incidence of lung tumours in
mice and rats. Am. Med. Assoc. Arch. Ind. Health, 20: 124-135.
BAETJER, A.M., DAMRON, C., & BUDACZ, V. (1959b) The
distribution and retention of chromium in man and animals. Am.
Med. Assoc. Arch. Ind. Health, 20: 136-150.
BAKER, R.S.V. (1984) Evaluation of metals in in vitro
assays: interpretation of data and possible mechanisms of
action. Toxicol. environ. Chem., 7: 191-212.
BEHNE, D. & BRATTER, P. (1979) [Problems of trace element
analysis in medicine.] In: Gladtke, E., Heimann, G., & Eckert,
I., ed. [Trace elements], Stuttgart, Thieme, pp. 42-52 (in
German).
BEHNE, D. & DIEHL, F. (1972) Relations between carbohydrate
and trace element metabolism investigated by neutron
activation analysis. In: Nuclear Activation Techniques in the
Life Sciences. Proceedings of a Symposium, Bled, Yugoslavia,
Vienna, International Atomic Energy Agency, pp. 407-414.
BEHNE, D., BRATTER, P., GESSNER, H., HUBE, G., MERTZ, W., &
ROSICK, U. (1976) Problems in determination of chromium in
biological materials: comparison of flameless atomic
absorption spectrometry and neutron activation analysis. Z.
anal. Chem., 278: 269-272.
BEHRBOHM, P. (1957) [Allergic contact dermatitis caused by
anticorrosives containing chromium.] Berufsdermatosen, 5:
271-283 (in German).
BENJANUVATRA, N.K. & BENNION, M. (1975) Hair chromium
concentration of Thai subjects with and without diabetes
mellitus. Nutr. Rep. Int., 12: 325-330.
BERGMANN, H. & HARDT, K. (1979) Analysis of dissolved Cr(VI)
in water by APDC-MIBK extraction and atomic absorption
spectrometry. Z. anal. Chem., 297: 381-383.
BERING, F. & ZITZKE, E. (1935) [Occupational skin diseases,]
Leipzig, Voss (in German).
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric., 23: 93-100.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BERTRAND, D. & DEWOLF, A. (1968) Requirement for the trace
element chromium in the growth of potatoes. C R Ser. D., 266:
1494-1495.
BEYERMANN, K. (1962) The analytical behaviour of very small
amounts of chromium. Part I. Z. anal. Chem., 190: 4-33. Part
II. Z. anal. Chem., 191: 345-369.
BEYERSMANN, D., KOSTER, A., BUTTNER, B., & FLESSEL, P.
(1984) Model reactions of chromium compounds with mammalian
and bacterial cells. Toxicol. environ. Chem., 8: 279-286.
BIANCHI, V., DAL TOSO, R., DEBETTO, P., LEVIS, A.G., LUCIANI,
S., MAJONE, F., & TAMINO, G. (1980) Mechanisms of chromium
toxicity in mamalian cell cultures. Toxicology, 17: 219-224.
BIANCHI, V., LEVIS, A.G., & SAGGIORO, D. (1979) Differential
cytotoxic activity of potassium dichromate on nucleoside
uptake in BHK fibroblasts. Chem.-biol.Interact., 24: 137-151.
BIANCHI, V., CELOTTI, L., LANFRANCHI, G., MAJONE, F., MARIN,
G., MONTALDI, G., SPONZA, G., TAMINO, G., VENIER, P.,
ZANTEDESCHI, A., & LEVIS, A.G. (1983) Genetic effects of
chromium compounds. Mutat. Res., 117: 279-300.
BIANCHI, V., ZANTEDESCHI, A., MONTALDI, A., & MAJONE, F.
(1984) Trivalent chromium is neither cytotoxic nor mutagenic
in permeabilized hamster fibroblasts. Toxicol. Lett., 23:
51-59.
BIDSTRUP, P.L. & CASE, R.A.M. (1956) Carcinoma of the lung
in workmen in the bichromates-producing industry in Great
Britain. Br. J. ind. Med., 13: 260-264.
BIGALIEV, A.B., ELEMESOVA, M.S., TUREBAEV, M.N., & BIGALIEVA,
R.K. (1978) [Cytogenetic study of the mutagenic activity of
industrial substances.] Zdravookhr. Kaz., 8: 48-50 (in
Russian).
BITTERSOHL, G. (1972) [Health and the working environment-
Epidemiology of carcinomatosis in the chemical industry.] In:
Proceedings of the 5th Merseburger Symposium, 17-18 November
(in German).
BLACK, C.A. (1968) Soil-plant relationships, New York, John
Wiley and Sons, 792 pp.
BLOKIN, V.S. & TROP, F.S. (1977) [Dynamics of the morpho-
logical changes in animals subjected to inhalational dust
treatment using chromium oxide and trisubstituted chromium
phosphate.] In: Dubinin, N.P., ed. [Genetic effects of the
pollution of the environment,] Moscow, Nauka, pp. 173-176 (in
Russian).
BLOOMFIELD, J.J. & BLUM, W. (1928) Health hazards in
chromium plating. Public Health Rep., 43: 2330-2347.
BONATTI, S., MEINI, M., & ABBONDANDOLO, A. (1976) Genetic
effects of potassium dichromate. Mutat. Res., 38: 147-150.
BOND, R.G., STRAUB, C.P., & PROBER, R., ed. (1973) Handbook
of environmental control. III. Water supply and treatment,
Cleveland, Ohio, CRC Press, pp. 763-764.
BONNEVIE, P. (1939) [Etiology and pathogenesis of eczemas,]
Copenhagen, Busck, Leipzig, Barth (in German).
BORGHETTI, A., MUTTI, A., CAVATORTA, A., FALZOI, M., CIGALA,
F., & FRANCHINI, I. (1977) Indices rénaux d'exposition aiguë
et d'imprégnation chronique par le chrome. Med. Lav., 68(5):
355-363.
BORY, R. & LECOCQ, I. (1953) Eczéma par cuivre chromé. Arch.
Mal. prof., 14: 144.
BOSTROM, G. (1936) [Hypersensitivity test for chromium
allergy.] Am. Derm. Vener., 17: 631-632 (in German).
BOVET, P., LOB, M., & GRANDJEAN, M. (1977) Spirometric
alterations in workers in the chromium electroplating
industry. Int. Arch. occup. environ. Health, 40: 25-32.
BOWEN, H.J.M. (1966) Trace elements in biochemistry, New
York, London, Academic Press, 241 pp.
BRARD, D. (1935) Toxicologie du chrome, Paris, Herrmann &
Co., 80 pp.
BRIEGER, H. (1920) [Clinical aspects of acute chromate
poisoning.] Z. exp. Path. Ther., 21: 393-408 (in German).
BROCH, C. (1949) [Bronchial asthma caused by chromic oxide.]
Nord. Med., 41: 996-997 (in Norwegian).
BROWN, E., SKOUGSTAD, M.W., & FISHMAN, M.J. (1970) Methods
for collection and analysis of water samples for dissolved
minerals and gases. In: Techniques of water resources
investigation of the United States geological survey,
Washington DC, US Department Interior, 160 pp.
BROWN, H.G., HENSLEY, C.P., MCKINNEY, G.L., & ROBINSON, J.L.
(1973) Efficiency of heavy metals removal in municipal sewage
treatment plants. Environ. Lett., 5: 103-114.
BRUCKDORFER, K.R., KHAN, I.H., & YUDKIN, J. (1971) Chromium
and sucrose-induced hyperglyceridemia. Proc. Nutr. Soc., 30:
74A.
BRUNE, D., NORDBERG, G., & WESTER, P.O. (1980) Distribution
of 23 elements in the kidney, liver, and lungs of workers from
a smeltery and refinery in north Sweden exposed to a number of
elements and of a control group. Sci. total Environ., 16:
13-35.
BURCKHARD, W. (1962) [Occupational skin diseases.] In:
Jadassohn, W., ed. [Handbook of skin and venereal diseases,]
Berlin, Springer, Vol. II(1), pp. 368-474.
BURROWS, D., ed. (1983) Chromium: metabolism and toxicity,
Boca Raton, Florida, CRC Press, 172 pp.
BYERRUM, R.U. (1961) Some studies on the chronic toxicity of
cadmium and hexavalent chromium in drinking-water. Eng. Bull.
Purdue Univ. Serv., 106: 1-8.
CALNAN, C.D. & HARMAN, R.R.M. (1961) Studies in contact
dermatitis. XIII. Diesel coolant chromate dermatitis. Trans.
St. John's Hosp. Derm. Soc., 46: 13.
CALOW, P. & FLETCHER, C.R. (1972) A new radiotracer
technique involving 14C and 51Cr for estimating the
assimilation efficiencies of aquatic, primary consumers.
Oecologia (Berlin), 9: 155-170.
CAMPBELL, W.J. & MERTZ, W. (1963) Interaction of insulin and
chromium (III) on mitochondrial swelling. Am. J. Physiol.,
204: 1028-1030.
CANADA, NATIONAL HEALTH AND WELFARE (1980) Chromium
drinking-water criteria review. In: Guidelines for Canadian
drinking-water quality, 1978. Supporting documentation,
Ottawa, Canada, National Health and Welfare, pp. 255-268.
CANFIELD, W.K. & DOISY, R.J. (1976) Chromium and diabetes in
the aged. In: Hsu, J.M., Davis, R.L., & Neithammer, R.W., ed.
The biomedical role of trace elements in aging, St. Peters-
burg, Florida, Eckert College, pp. 119-128.
CARD, W.I. (1935) A case of asthma sensitivity to chromates.
Lancet, 2: 1348-1349.
CARTER, J.P., KATTAB, A., ABD-EL-HADI, K., DAVIS, J., EL
GHOLMY, A., & PATWARDHAN, V.N. (1968) Chromium (III) in
hypoglycemia and in impaired glucose utilization in
kwashiorkor. Am. J. clin. Nutr., 21: 195-202.
CARY, F.E., ALLWAY, W.H., & OLSON, O.E. (1977) Control of
chromium concentrations in food plants. I. Absorption and
translocations of chromium by plants. J. agric. food Chem.,
25: 300-304. II. Chemistry of chromium in soils and its
availability to plants. J. agric. food Chem., 25: 305-309.
CASEY, C.E. & HAMBIDGE, K.M. (1984) Chromium in human milk
from American mothers. Br. J. Nutr., 52: 73-77.
CASTO, B.C., PIECZYNSKI, W.J., NELSON, R.L., & DI PAOLO, J.
(1976) In vitro transformation and enhancement of viral
transformation with metals. Proc. Am. Assoc. Cancer Res., 17:
12-20.
CASTO, B.C., MEYERS, J., & DIPAOLO, J.A. (1979) Enhancement
of viral transformation for evaluation of the carcinogenic or
mutagenic potential of inorganic metal salts. Cancer Res., 39:
193-198.
CAWSE, P.A. (1974) A survey of atmospheric trace elements in
the UK, 1972-1973, Harwell, England, Atomic Energy Research
Establishment, 95 pp.
CHANEY, R.L. (1973) Crop and food chain effects of toxic
elements in sludges and effluents. In: Recycling Municipal
Sludges and Effluents on Land. Proceedings of a Joint
Committee, National Association of State Universities and Land
Grant Colleges, Washington DC, July 9-13, 1973, pp. 129-141.
CHEN, N.S.G., TSAI, A., & DYER, I.A. (1973) Effect of
chelating agents on chromium absorption in rats. J. Nutr.,
103: 1182-1186.
CHEN, K.Y., YOUNG, C.S., JAN, T.K., & ROHATGI, N. (1974)
Trace metals in waste water effluents. J. Water Pollut.
Control Fed., 46: 2663-2675.
CHEREMISINOFF, P.N. & HABIB, Y.H. (1972) Cadmium, chromium,
lead, mercury: a plenary account for water pollution. Part I.
Occurrence, toxicity, and detection. Water Sewage Works, 119:
73-86.
CHEREVATY, V.S., EREMEEVA, E.A., & SMAGULOV, S.K. (1965) [On
the nature of nasal microflora in the workers of a chromium
compound plant.] In: Proceedings of 4th Scientific Session of
Aktubinsk State Medical Institute, Alma-Ata, Kazakhstan,
pp. 337-342 (in Russian).
CHIAZZE, L., FERENCE, L.D., & WOLF, P.H. (1980) Mortality
among automobile assembly workers. I. Spray painters. J.
occup. Med., 22: 520-526.
CHRISTIAN, G.D., KNOBLOCK, E.G., PURDY, W.C., & MERTZ, W.
(1963) A polarographic study of chromium-insulin-
mitochondrial interaction. Biochem. Biophys. Acta, 66: 420-423.
CLARK, R. & KUNITSCH, G. (1972) [Statistical studies of
cross allergy in metal salts.] Berufsdermatosen, 20(5):
222-238 (in German).
COLLINS, R.J., FROMM, P.O., & COLLINGS, W.D. (1961) Chromium
excretion in the dog. Am. J. Physiol., 201(5): 795-798.
CONDE-SALAZAR, L., MARTO-CASTANO, A., & ARROYO VICENTE, M.
(1980) Determination of chrome in rubber gloves. Contact
Dermatit., 6: 237-238.
COTTON, F.A. & WILKINSON, G. (1966) Advanced inorganic
chemistry, New York, Interscience, 1136 pp.
CRESSER, M.S. & HARGITT, R. (1976) The determination of
chromium (III) and chromium (VI) by total anion exchange and
atomic absorption spectrometry. Anal. Chim. Acta, 81: 196-198.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
DALAGER, N.A., MASON, T.J., FRAUMENI, J.F., HOOVER, R., &
PAYNE, W.W. (1980) Cancer mortality among workers exposed to
zinc chromate paints. J. occup. Med., 22: 25-29.
DANA, P. (1971) Crocoite-PbCrO4 In: Hurlbut, C.S., ed.
Dana's manual of mineralogy, 18th ed., New York, J. Wiley and
Sons Inc., pp. 346-347.
DANIELSSON, B.R.G., HASSOUN, E., & DENCKER, L. (1982)
Embryotoxicity of chromium: distribution in pregnant mice and
effects on embryonic cells in vitro. Arch. Toxicol., 51:
233-245.
DAVIDSON, I.W.F. & BLACKWELL, W.L. (1968) Changes in
carbohydrate metabolism of squirrel monkeys with chromium
dietary supplementation. Proc. Soc. Exp. Biol. Med., 127:
66-70.
DAVIDSON, I.W.F. & BURT, R.L. (1973) Physiological changes
in plasma chromium of normal and pregnant women: effect of
glucose load. Am. J. Obstet. Gynecol., 116: 601-608.
DAVIES, J.C.A. (1974) Inhalation hazards in the manufacture
of silicon alloys. Cent. Afr. J. Med., 20: 140-143.
DAVIES, J.M.G. (1972) The fibrogenic effects of mineral
dusts injected into the pleural cavity of mice. Br. J. exp.
Pathol., 53: 190-201.
DAVIES, J.M.G. (1979) Lung cancer mortality of workers in
chromate pigment manufacture: an epidemiological survey. J.
Oil. Col. Chem. Assoc., 62: 157-163.
DAVIES. J.M.G. (1984) Lung cancer mortality among workers
making lead chromate and zinc chromate pigments at three
English factories. Br. J. Ind. Med., 41: 158-169.
DAVIS, M.H. & GROSSMANN, V.B. (1971) A sensitive method for
determination of chromium in biological materials. Anal.
Biochem., 44: 339-344.
DEFLORA, S. (1978) Metabolic deactivation of mutagens in
the Salmonella microsome test. Nature (Lond.), 271: 455-456.
DEFLORA, S. (1981) Study of 106 organic and inorganic
compounds in the Salmonella microsome test. Carcinogenesis, 2:
283-298.
DEFLORA, S. & BOIDO, V. (1980) Effect of human gastric
juice on the mutagenicity of chemicals. Mutat. Res., 77:
307-315.
DEFLORA, S., COPPOLA, R., CAMOIRANO, A., BATTAGLIA, M.A., &
BENNICELLI, C. (1980) Mutagenicity of chromyl chloride and
its vapours. Carcinogenesis, 1(7): 583-587.
DEFLORA, S., BIANCHI, V., & LEVIS, A.G. (1984) Distinctive
mechanisms for interaction of hexavalent and trivalent
chromium with DNA? Toxicol. environ. Chem., 8: 287-294.
DE GROOT, A.J. & ALLERSMA, E. (1973) Field observations on
the transport of heavy metals in sediments. In: Proceedings of
the Conference on Heavy Metals in the Aquatic Environment,
Nashville, Tennessee, December, Oxford, Pergamon Press,
pp. 1-16.
DEICHMANN, W.B. & GERARDE, H.W. (1969) Toxicology of drugs
and chemicals, 4th ed., New York, London, Academic Press,
167 pp.
DI PAOLO, J.A. & CASTO, B.C. (1979) Quantitative studies of
in vitro morphological transformation of Syrian hamster cells
by inorganic metal salts. Cancer Res., 32: 1008-1013.
DJAHANSHIRI, H. (1976) [Nutritional physiology problems
concerning the trace element chromiun in the rat. V. Effects
on overall metabolic performande.] Z. Tierphysiol. Tierernähr.
Futtermittelkd., 37: 298-315 (in German).
DOBROLYUBSKII, O.K. (1959) [Changes in the biochemical
process in grapes under the influence of a microfertilizer
which contains chromium.] Biochemistry (USSR), 24: 577-582 (in
Russian).
DOISY, R.J., STREETEN, D.H.P., LEVINE, R.A., & CHODOS, R.B.
(1968) Effects and metabolism of chromium in normals, elderly
subjects, and diabetics. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
Univerisity of Missouri, Vol. 2, 75 pp.
DOISY, R.J., STREETEN D.H.P., SOUMA, M.L., KALAFER, M.E.,
REKANT, S.L., & DALAKOS, T.G. (1971) Metabolism of
51chromium in human subjects. In: Mertz, W. & Cornatzer, W.E.,
ed. Newer trace elements in nutrition, New York, Marcel
Dekker, pp. 155-169.
DOISY, R.J., STREETEN, D.H.P., FREIBERG, J.M., & SCHNEIDER,
A.J. (1976) Chromium metabolism in man and biochemical
effects. In: Prasad, A.S., ed. Trace elements in human health
and disease, New York, London, Academic Press, Vol. 2, pp.
79-104.
DOMASEVA, T.V. (1971) [On the effect produced by chromium
compounds on the skin.] In: [Occupational skin diseases,]
Leningrad, Leningrad Institute of Sanitation and Hygiene,
Vol. 93, pp. 168-173 (in Russian).
DONALDSON, R.M., Jr & BARRERAS, R.F. (1966) Intestinal
absorption of trace quantities of chromium. J. lab. clin.
Med., 68: 484-493.
DOUGLAS, G.R., BELL, R.D.K., GRANT, C.E., WYTSMA, J.M., &
BORA, K.C. (1980) Effect of lead chromate on chromosome
aberration, sister-chromatid exchange, and DNA damage in
mammalian cells in vitro. Mutat. Res., 77: 157-163.
DURFOR, C.N. & BECKER, E. (1964) Public water supplies of
the 100 largest cities in the United States, 1962, Washington
DC, US Geological Survey, 364 pp (Water Supply Paper No. 1812).
DURUM, W.H. & HAFFTY, J. (1963) Implications of the
minor-element content of some major streams in the world.
Geochim. Cosmochim Acta, 27: 1-11.
EATON, D.L., STACEY, N.H., WONG, K.L., & KLAASSEN, C.D.
(1980) Dose-response effects of various metal ions on rat
liver metallothionein, glutathione, heme oxygenase, and
cytochrome P-450. Toxicol. appl. Pharmacol., 55: 393-402.
EDMUNDSON, W.F. (1951) Chrome ulcers of the skin and nasal
septum and their relation to patch testing. J. Invest. Derm.,
17: 17-19.
EIFAC (1983) Water quality criteria for european freshwater
fish. Report on chromium and freshwater fish, European Inland
Fisheries Advisory Commission, Working Party on Water Quality
Criteria for European Freshwater Fish, 31 pp (EIFAC Technical
Paper No. 43).
ELIAS, Z., SCHNEIDER, O., AUBRY, F., DANIERE, M.C., & POIROT,
O. (1983) Sister chromatid exchanges in chinese hamster V79
cells treated with the trivalent chromium compounds chromic
chloride and chromic oxide. Carcinogenesis, 3: 605-611.
ENTERLINE, P.E. (1974) Respiratory cancer among chromate
workers. J. occup. Med., 16: 523-526.
ENVIRONMENTAL INSTRUMENTATION GROUP (1973) Instrumentation
for environmental monitoring: biomedical. IV, Berkeley,
California, Lawrence Berkeley Laboratory.
EQUITABLE ENVIRONMENTAL HEALTH, INC. (1976) An epidemiologic
study of workers in lead chromate plants: Final report
(prepared for the Dry Color Manufacturer's Association,
Berkeley California).
ESSING, H.G., SZADKOWSKI, D., & VALENTIN, H. (1971)
[Significance of the valence gradations of chromium compounds
in occupational medicine examinations.] Med. Sachverständige,
67: 35-39 (in German).
ETMANOVA, Z.J. (1965) [On the functional state of the liver
in workers of Aktubinsk chromium compound plant.] Gig. Tr.
prof. Zabol., 10: 55-56 (in Russian).
EVAN, A.P. & DAIL, W.G. (1974) The effect of sodium chromate
on the proximal tubules of the rat kidney. Lab. Invest.,
30(6): 704-715.
FANG, Y. (1983) [The normal value of chromium in urine for
299 unexposed persons in Yanjian city.] J. Yanbian Med.
College, 6 (1): 35-37 (in Chinese).
FARKAS, T.G. & ROBERSON, S.L. (1965) The effect of Cr(III)
on the glucose utilization of isolated lenses. Exp. Eye Res.,
4: 124-126.
FELDMAN, F.J. (1968) The state of chromium in biological
materials. Fed. Proc., 27: 482.
FELDMAN, F.J., KNOBLOCK, E.C., & PURDY, W.C. (1967) The
determination of chromium in biological materials by atomic
absorption spectroscopy. Anal. Chim. Acta, 38: 489-497.
FRADKIN, A., JANOFF, A., LANE, B.P., & KUSCHNER, M. (1975)
In vitro transformation of BHK21 cells grown in the presence
of calcium chromate. Cancer Res., 35: 1058-1061.
FRANCHINI, I., MUTTI, A., GARDINI, F., & BORGHETTI, A.
(1975) [Excretion and renal elimination of chromium compared
to degree and length of occupational exposure.] In: Roche, L.
& Trager, J., ed. Rein et Toxique. Réunion de la Société
Francaise de Toxicologie (Compte rendu), Paris, Groupement
Francais Des Centre Anti-Poisons, pp. 271-281.
FRANCHINI, I., MUTTI, A., CAVATORTA, A., CORRADI, A., COSI,
A., OLIVETTI, A., & BORGHETTI, A. (1978) Nephrotoxicity of
chromium. Control Nephrol., 10: 98-110.
FRANCHINI, J., MAGNANI, F., & MUTTI, A. (1983) Mortality
experience among chrome plating workers. Scand. J. Work
environ. Health, 9: 247-252.
FREGERT, S. (1975) Occupational dermatitis in a 10-year
material. Contact Dermatit., 1: 96-107.
FREGERT, S. (1981) Manual of contact dermatitis, 2nd ed.,
Copenhagen, Munksgaard, 139 pp.
FREGERT, S. & GRUVBERGER, B. (1972) Chemical properties of
cement. Berufsdermatosen, 20(5): 238-245.
FREGERT, S. & GRUVBERGER, B. (1979) Chromium in industrial
leather gloves. Contact Dermatit., 5: 189.
FREGERT, S. & RORSMAN, H. (1964) Allergy to trivalent
chromium. Arch. Dermatol., 90: 4-6.
FREGERT, S., HJORTH, N., MAGNUSSON, B., BANDMANN, H.-J.,
CALNAN, C.D., CRONIN, E., MALTEN, K., MENEGHINI, C.L., PIRILA,
V., & WILKINSON, D.S. (1969) Epidemiology of contact
dermatitis. Trans. St. John's Hosp. Dermatol. Soc., 55: 17-35.
FRENTZEL-BEYME, R. (1983) Lung cancer mortality of workers
employed in chromate pigment factories. J. Cancer Res. clin.
Oncol., 105: 183-188.
FREUND, H., ATAMIAN, S., & FISCHER, J.E. (1979) Chromium
deficiency during total parenteral nutrition. J. Am. Med.
Assoc., 241: 496-498.
FSA (1953) Health workers in chromate producing industry. A
study, Washington DC, Federal Security Agency, 131 pp (US PHS
Publication No. 192).
FUKAI, R. (1967) Valency state of chromium in seawater.
Nature (Lond.), 213: 901.
FUKUNAGA, M., KURACHI, Y., & MIZUGUCHI, Y. (1982) Action of
some metal ions on yeast chromosomes. Chem. pharm. Bull., 30:
3017-3019.
FURST, A., SCHLAUDER, M., & SASMORE, D.P. (1976) Tumour-
igenic activity of lead chromate. Cancer Res., 36: 1779-1783.
GALE, T.F. (1974) Effects of chromium on the hamster embryo.
Teratology, 9: A-17.
GALE, T.F. (1978) Embryotoxic effects of chromium trioxide
in hamsters. Environ. Res., 16: 101-109.
GALE, T.F. (1982) The embryotoxic response to maternal
chromium trioxide exposure in different strains of hamsters.
Environ. Res., 29: 196-203.
GALE, T.F. & BUNCH, J.D. (1979) The effect of the time of
administration of chromium trioxide on the embryotoxic
response in hamsters. Teratology, 19: 81-86.
GARCIA, J.D. & WETTERHAHN JENNETTE, K. (1981) Electron-
transport cytochrome P-450 system is involved in the
microsomal metabolism of the carcinogen chromate. J. inorg.
Biochem., 14: 281-295.
GEISER, J.D., JEANNERET, J.P., & DELACRETAZ, J. (1960) Eczema
due to cement and sensitization to cobalt. Dermatologica
(Basel), 121: 1-7.
GEMMELL, R.P. (1973) Revegetation of derelict land polluted
by a chromate smelter. Part 1. Chemical factors causing
substrate toxicity in chromate smelter waste. Environ.
Pollut., 5: 181-197.
GEMMELL, R.P. (1974) Revegetation of derelict land polluted
by a chromate smelter. Part 2. Techniques of revegetation of
chromate smelter waste. Environ. Pollut., 6: 31-37.
GENTILE, J.M., HYDE, K., & SCHUBERT, J. (1981) Chromium
genotoxicity as influenced by complexation and rate effects.
Toxicol. Lett., 7: 439-448.
GERAUER, A. (1954) [On the growing importance of
epicutaneous allergy to chromium.] Z. Haut-Geschlechtskr., 17:
97-102 (in German).
GILANI, S.H. & MARANO, M. (1979) Chromium poisoning and
chick embryogenesis. Environ. Res., 29: 427-431.
GERICKE, S. (1943) Studies on the effect of the trace
element chromium on the growth of plants. Bodenkd.
Pflanzenernaehr., 33: 114-128.
GLASER, U., HOCHRAINER, D., KLOPPEL, H., KORDEL, W., & KUHNEN,
H. (1984) [Inhalation studies with Wistar rats and patho-
physiological effects of chromium,] Berlin, 156 pp (Report to
Umweltbundesamt, D-1 UFOPLAN F+E 10606007/2) (in German).
GLASER, U., HOCHRAINER, D., KLOPPEL, H., & KUHNEN, H. (1985)
Low-level chromium VI inhalation effects on alveolar macro-
phages and immune functions in Wistar rats. Arch. Toxicol.,
57: 250-256.
GLINSMANN, W.H., FELDMAN, F.J., & MERTZ, W. (1966) Plasma
chromium after glucose administration. Science, 152: 1243.
GLINSMANN, W.H. & MERTZ, W. (1966) Effect of trivalent
chromium on glucose tolerance. Metabolism, 15: 510-520.
GMELIN, C.G. (1826) Experiments on the effects of baryta,
strontia, chrome, molybdenum, tungsten, tellurium, titanium,
osmium, platinum, iridium, rhodium, palladium, nickel, cobalt,
uranium, cerium, iron, and manganese, on the animal system.
Edinburgh med. Surg. J., 26: 131-139.
GOMES, E.R. (1972) Incidence of chromium-induced lesions
among electroplating workers in Brazil. Ind. Med., 41(12):
21-25.
GOMEZ-ARROYO, S., ALTAMIRANO, M., & VILLALOBOS-PIETRINI, R.
(1981) Sister-chromatid exchanges induced by some chromium
compounds in human lymphocytes in vitro. Mutat. Res., 90:
425-431.
GONCHAROV, A.T. (1968) [Role of chromium in the nutrition of
animals and man.] Vopr. Pitaniya, 27: 59-66 (in Russian).
GONCHAROV, A.T. (1978) [Maximum physiological dose of
chromium in food for human consumption.] Kazanskij med. Z.,
59(2): 90-93 (in Russian).
GRAY, S.J. & STERLING, K. (1950) The tagging of red cells
and plasma proteins with radioactive chromium. J. clin.
Invest., 29: 1604-1613.
GREBE, W.-H., KASTNER, H., KIPPENBERGER, C., KRAUSS, U.,
KRUSZONA, M., SCHMIDT, H., LIEBRUCKS, M., RUMBERGER, M., &
WETTIG, E. (1975) [Investigation on supply and demand for
mineral raw materials. VII. Chromium,] Berlin, Hannover,
Bundesanstalt für Gewissenschaften und Rohstoffe/Deutsches
Institut für Wirtschaftsforschung, 147 pp.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38: 33-42.
GROSS, W.G. & HELLER, V.G. (1946) Chromates in animal
nutrition. J. ind. Hyg. Toxicol., 28: 52-56.
GROSS, E. & KOLSCH, F. (1943) [Lung cancer in the chromate
dye industry.] Gewerbepathol. Gewerbehyg., 12: 164-170 (in
German).
GURSON, C.T. (1977) The metabolic significance of dietary
chromium. In: Draper, H.H., ed. Advances in nutritional
research, New York, Plenum Publishing Company, Vol. 1,
pp. 23-53.
GURSON, C.T. & SANER, G. (1971) Effect of chromium on
glucose utilization in marasmic protein-calorie malnutrition.
Am. J. clin. Nutr., 24: 1313-1319.
GURSON, C.T. & SANER, G. (1973) Effect of chromium
supplementation on growth in marasmic protein-calorie
malnutrition. Am. J. clin. Nutr., 26: 988-991.
GURSON, C.T. & SANER, G. (1978) The effect of glucose
loading on urinary excretion of chromium in normal adults,
individuals from diabetic families, and in diabetics. Am. J.
clin. Nutr., 31: 1158-1161.
GUTHRIE, B.E. (1973) Daily dietary intakes of zinc, copper,
manganese, chromium, and cadmium by some New Zealand women.
Proc. Univ. Otago Med. School., 51: 47-49.
GUTHRIE, B.E., WOLF, W.R., & VEILLON, C. (1978) Background
correction and related problems in the determination of
chromium in urine by graphite furnace atomic absorption
spectrometry. Anal. Chem., 50: 1900-1902.
GUTHRIE, B.E., WOLF, W.R., VEILLON, C., & MERTZ, W. (1979)
Chromium in urine. In: Hemphill, D.D., ed. Trace substances in
environmental health, Columbia, Missouri, University of
Missouri, Vol. XII, pp. 490-492.
GUY, W.B. (1954) Dermatologic problems in the railroad
industry resulting from conversion to Diesel power. Am. Med.
Assoc. Arch. Derm. Syph., 70: 289-292.
GYLSETH, B., GUNDERSEN, N., & LANGORD, S. (1977) Evaluation
of chromium exposure based on a simplified method for urinary
chromium determination. Scand. J. Work environ. Health, 3:
28-31.
HAMBIDGE, K.M. (1971) Chromium nutrition in the mother and
in the growing child. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 169-194.
HAMBIDGE, K.M. (1974) Chromium nutrition in man. Am. J.
clin. Nutr., 27: 505-514.
HAMBIDGE, K.M. & BAUM, J.D. (1972) Hair chromium concen-
trations of human newborn and changes during infancy. Am. J.
clin. Nutr., 25: 376-379.
HAMBIDGE, K.M. & RODGERSON, D.O. (1969) A comparison of the
hair chromium levels of nulliparous and parous women. Am. J.
Obstet. Gynecol., 103: 320-321.
HAMBIDGE, K.M., RODGERSON, D.O., & O'BRIEN, D. (1968)
Concentration of chromium in the hair of normal children and
children with juvenile mellitus. Diabetes, 17: 517-519.
HARA, N. (1982) [The change of valence of chromium by acid
treatment.] Sangyo Igaku, 24: 658-662 (in Japanese).
HARRISON, D.L. & STAPLES, E.L.J. (1955) Zinc chromate
poisoning in calves. N.Z. vet. J., 3: 63-73.
HASEGAWA, M. (1938) Studies on the detexicating hormone of
the liver (Yakriton). Toholu J. exp. Med., 32: 163-176.
HAYES, R.B. (1982) Carcinogenic effects of chromium, In:
Langard, S., ed. Biological and environmental aspects of
chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 221-247.
HAYES, R.B., LILIENFELD, A.M., & SNELL, L.M. (1979)
Mortality in chromium chemical production workers: a
prospective study. Int. J. Epidemiol., 8: 365-374.
HEATH, J.C., FREEMAN, M.A.R., & SWANSON, S.A.V. (1971)
Carcinogenic properties of wear particles from prostheses made
in cobalt-chromium alloy. Lancet, 1: 564-566.
HENNIG, A., ANKE, M., DIETRICH, M., HARTMANN, G., & HOFFMANN,
G. (1971) Absorption and distribution of 51chromium after
oral administration to laying hens as a function of time.
Arch. Tierern., 21: 609-615.
HEPLER, O.E. & SIMONDS, J.P. (1946) Experimental nephro-
pathies. IV. Glycosuria in dogs poisoned with uranyl nitrate,
mercury bichloride and potassium dichromate. Arch. Path., 41:
42-49.
HERNBERG, S., WESTERHOLM, P., SCHULTZ-LARSEN, K., DEGERTH,
R., KUOSMA, E., ENGLUND, A., ENGZELL, U., SAND HANSEN, H., &
MUTANEN, P. (1983) Nasal and sinonasal cancer: connection
with occupational exposures in Denmark, Finland, and Sweden.
Scand. J. Work environ. Health, 9: 315-326.
HERTEL, R.F. (1982) [Chromium as a problem in physiology,
epidemiology and biological monitoring.] Staub-Reinhalt. Luft,
42: 135-137 (in German).
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand
cultures. J. exp. Bot., 4: 59-64.
HILT, G. (1954) La dermite du chrome hexavalent dans le
cadre des dermites eczémateuses par sensibilisation aux
métaux. Dermatologica (Basel), 109: 143-174.
HINTZE, C. (1930) [Handbook of mineralogy,] Berlin, Leipzig,
De Gruyter, Vol. 1. III/2, pp. 4012-4030.
HOPKINS, L.L., Jr (1965) Distribution in the rat of physio-
logical amounts of injected 51Cr(III) with time. Am. J.
Physiol., 209: 731-735.
HOPKINS, L.L., Jr & SCHWARZ, K. (1964) Chromium (III)
binding to serum proteins, specifically siderophidin. Biochem.
Biophys. Acta, 90: 484-491.
HOPKINS, L.L., Jr, RANSOME-KUTI, O., & MAJAJ, A.S. (1968)
Improvement of impaired carbohydrate metabolism by chromium
(III) in malnourished infants. Am. J. clin. Nutr., 21: 203-211.
HUCKABEE, J.W., CARTAN, F.O., & KENNINGTON, G.S. (1972)
Environmental influence on trace elements in hair of 15
species of mammals, Oak Ridge, Tennessee, Oak Ridge National
Laboratory, 36 pp (ORNL-TM-3747).
HUEPER, W.C. (1958) Experimental studies in metal
cancerogenesis. X. Cancerigenic effects of chromite ore roast
deposited in muscle tissue and pleural cavity of rats. Arch.
ind. Health, 18: 284-291.
HUEPER, W.C. (1961) Environmental carcinogenisis and cancer.
Cancer Res., 21: 842-857.
HUNTER, W.C. & ROBERTS, J.M. (1933) Experimental study of
the effects of potassium bichromate in the monkey's kidney.
Am. J. Pathol., 9: 133-147.
HYODO, K., SUZUKI, S., FURUYA, N., & MESHIZUKA, K. (1980) An
analysis of chromium, copper, and zinc in organs of a chromate
worker. Int. Arch. occup. environ. Health, 46: 141-150.
IARC (1980) Some metals and metallic compounds, Lyons,
International Agency for Research on Cancer, pp. 205-323 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 23).
IIJIMA, S., MATSUMOTO, N., LU, C.C., & KATSUNUMA, H. (1975)
Placental transfer of CrCl3 and its effects on fetal growth
and development and development in mice. Teratology, 12: 198.
IMBUS, H.R., CHOLAK, J., MILLER, L.H., & STERLING, T. (1963)
Boron, cadmium, chromium, and nickel in blood and urine. Arch.
environ. Health, 6: 112-121.
IMREH, ST. & RADULESCU, D. (1982) Cytogenic effects of
chromium in vivo and in vitro. Mutat. Res., 97: 192-193.
IVANKOVIC, S. & PREUSSMANN, R. (1975) Absence of toxic and
carcinogenic effects after administration of high doses of
chromium oxide pigment in subacute and long-term feeding
experiments in rats. Food Cosmet. Toxicol., 13: 347-351.
JAEGER, H. & PELLONI, E. (1950) Tests épicutanés aux
bichromates, positifs dans l'eczéma au ciment. Dermatologica
(Basel), 100: 207-216.
JEEJEEBHOY, K.N., CHU, R.G., MARLISS, E.B., GREENBERG, G.R., &
BRUCE-ROBERTSON, A. (1977) Chromium deficiency, glucose
intolerance, and neuropathy reversed by chromium supplement-
ation, in a patient receiving long-term total parenteral
nutrition. Am. J. clin. Nutr., 30: 531-538.
JENNETTE, K.W. (1979) Chromate metabolism in liver micro-
some. Biol. Trace Elem. Res., 1: 55-62.
JENSEN, L.S., MAURICE, D.V., & MURRAY, M.W. (1978) Evidence
for a new biological function of chromium. Fed. Proc., 37: 404.
JINDRICHOVA, J. (1978) [Chromium damage in arc welders.] Z.
Ges. Hyg., 24: 86-89 (in German).
JOHNSON, J.L. (1974) Characterization of air-borne partic-
ulates. Microchem. J., 19:168-178.
JONES, G.B., BUCKLEY, R.A., & CHANDLER, C.S. (1975) The
volability of chromium from brewer's yeast during assay. Anal.
Chim. Acta, 80: 389-392.
JOSCHI, A.P. & NEEB, R. (1980) Gas chromatographic elemental
analysis via di(trifluorethyl)dithiocarbamatochelates. Z.
anal. Chem., 303: 389-393.
KAPLAN, I. & ZELIGMAN, I. (1962) Occupational dermatitis in
railroad workers. Am. Med. Assoc. Arch. Derm., 85: 135-142.
KAUFMANN, D.B., DINICOLA, W., & MCINTOSH, R. (1970) Acute
potassium dichromate poisoning. Am. J. dis. Child, 119:
374-376.
KANEKO, T. (1976) Chromosome damage in cultured human
leucocytes induced by chromium chloride and chromium trioxide.
Jpn. J. ind. Health, 18: 136-137.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec assay and
mutagenicity studies on metal compounds. Mutat. Res., 77:
109-116.
KAZANTZIS, G. & LILLY, L. (1979) Mutagenic and carcinogenic
effects of metals. In: Friedberg, L., Nordberg, G.F., & Vouk,
V.B., ed. Handbook on the toxicology of metals, Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 237-273.
KELLER, C. (1980) [Activation analysis.] In: Bartholomé, E.,
Biekert, E., Hellmann, H., Ley, H., Weigert, M., & Weise, E.,
ed. [Ullmann's encyclopaedia of technical chemistry,] 4th ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
Vol. 5, pp. 685-707 (in German).
KEMP, K., JENSEN, F.P., MOELLER, J.T., & HANSEN, G. (1974)
Trace multi-element analysis of biological tissue by proton-
induced X-ray fluorescence spectroscope, Roskilde, Denmark,
Danish Atomic Energy Commission, 22 pp (Report Riso-M-1732).
KEMPF, T. & SONNEBORN, M. (1976) [Determination of Pb, Cd,
Cr, and Zn according to the drinking-water regulation Federal
Republic of Germany.] In: Aurand, K., Hässelbarth, U., Müller,
G., Schumacher, W., & Steuer, W., ed. [The drinking-water
regulation,] Berlin, Erich Schmidt Verlag, pp. 119-125 (in
German).
KEMPF, T. & SONNEBORN, M. (1981) [Chemical composition of
drinking-waters in various regions of the Federal Republic of
Germany.] Vom Wasser, 57: 83-94 (in German).
KESKINEN, H., KALLIOMAKI, P.-L., & ALANKO, K. (1980) Occupa-
tional asthma due to stainless steel welding fumes. Clin.
Allerg., 10: 151-159.
KIRCHGESSNER, M., MERZ, G., & OELSCHLAEGER, W. (1960)
[Effect of vegetation state on content of macro and micro
elements of three species of grass.] Arch. Tierern., 10:
414-427 (in German).
KITTELBERGER, F. (1973) [Contribution to residue analysis in
fish.] Tierärztl. Prax., 1: 465-479.
KLEIN, L.A., LANG, M., NASH, N., & KIRSCHNER, S.L. (1974)
Sources of metals in New York City wastewater. J. Water
Pollut. Control Fed., 46: 2653-2662.
KLEINER, A.M., STOLBUN, B.M., LIKHACHEVA, E.L., & BELJAEVA,
L.N. (1970) [Some indices of the functional state of the
myocardium and hemodynamics in chronic intoxication with
chromium compounds.] Gig. Tr. prof. Zabol., 12: 7-10 (in
Russian).
KNUDSEN, I. (1980) The mammalian spot test and its use for
the testing of potential carcinogenicity of welding fume
particles and hexavalent chromium. Acta pharmacol. toxicol.,
47: 66-70.
KOGAN, V.Y. (1968) [The basophil degranulation as an index
of skin sensitization to cobalt compounds.] In: Belenky, G.B.,
ed. [Modern aspects of clinical and experimental dermato-
venerology. Works of the clinic of skin and venerological
diseases,] Moscow, Medizina, pp. 100-104 (in Russian).
KOGAN, V.Y. (1971) [Chromium as a specific cause for
occupation dermatoses.] In: Babayants, R.S., ed. [Topical
questions of dermato-venerology. First Medical Institute,
All-Union Conference, Moscow, I.A. Sechenov, pp. 172-176
(in Russian).
KOGAN, V.Y, ARUTYUNYAN, K.E., & KOCHINYAN, Y.E. (1972) [A
comparative characteristic of chromium, nickel, and cobalt
salts as a causative factor in allergic contact dermatosis.]
In: [Clinical Pathology of Chemical Aetiology. Materials of
the 1st All-Union Conference, Kiev, USSR, 24-25 October,
1972,] Kiev, pp. 144 and 146 (in Russian).
KOHLER, H. (1951) [Constitutional examinations of subjects
with skin diseases in the offset department of a printing
works (Doctoral thesis, Leipzig.)] Berufsdermatosen, 4: 93 (in
German).
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace
metals during the ashing of biological materials. Analyst,
101: 870-875.
KOIVISTOINEN, P., ed. (1980) Mineral element composition of
Finnish foods. Acta agric. Scand., Suppl. 22: 171
pp.
KOPP, J.E. & KRONER, R.C. (1968) Trace metals in waters of
the United States: a five-year summary of trace metals in
rivers and lakes of the United States (October 1 1962 -
September 30, 1967), Cincinnati, Ohio, US Department of the
Interior, 32 pp.
KORALLUS, U. & LOENHOFF, N. (1981) [Industrial medical and
epidemiological experience with the manufacture and processing
of chromates.] Arbeitsmed. Sozialmed. Praeventivmed., 16:
285-289 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974a)
[Trivalent chromium compounds. Results of a study in
occupational medicine. I. General; Technology; Preliminary
study.] Arbeitsmed. Sozialmed. Praeventivmed., 9: 51-54 (in
German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974b)
[Trivalent chromium compounds: results of a study in
occupational medicine. II. Disease status analysis.]
Arbeitsmed. Sozialmed. Praeventivmed., 9: 76-79 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974c)
[Trivalent chromium compounds: Results of a study in
occupational medicine. III. Clinical studies.] Arbeitsmed.
Sozialmed. Praeventivmed., 9: 248-252 (in German).
KORALLUS, U., LANGE, H.-J., NEISS, A., WUESTEFELD, E., &
ZWINGERS, T. (1982) [Correlations between reorganization
measures and bronchial carcinoma mortality in the chromate-
manufacturing industry.] Arbeitsmed. Sozialmed. Praevent-
ivmed., 7: 159-167 (in German).
KOSHI, K. (1979) Effects of fume particles from stainless
steel welding on sister chromatid exchanges and chromosome
aberrations in cultured Chinese hamster cells. Ind. Health.,
17: 39-49.
KOTZ, L., KAISER, G., TSCHOPEL, P., & TOLG, G. (1972)
[Clarification of biological matrices for determining very low
contents of trace elements when limited amounts are weighed
with nitric acid under pressure in a teflon vessel.] Z. anal.
Chem., 260: 207-209 (in German).
KOUTRAS, G.A., HATTORY, M., SCHNEIDER, A.S., EBAUGH, F.G., &
VALENTINE, W.N. (1964) Studies on chromated erythrocytes:
effect of sodium chromate on erythrocyte glutathione reduc-
tase. J. clin. Invest., 43: 323-331.
KRISHNAJA, A.P. & REGE, M.S. (1982) Induction of chromosomal
aberrations in fish Boleophthalmus dussumieri after exposure
in vivo to mitomycin C and heavy metals mercury, selenium, and
chromium. Mutat. Res., 102: 71-82.
KROEPFLI, P. & SCHUPPLI, R. (1955) [Detergent eczema.]
Dermatologica (Basel), 110: 1-7 (in German).
KRUGER, H. & DORN, H. (1956) [Clinical observations on
detergent eczema in association with chromium and nickel
allergy.] Z. Haut-Geschlechtskr., 20: 307-314 (in German).
KUCHER, I.M. (1966) In: [Proceedings of the 5th Scientific
Session of the Aktiubinsk Medical Institute, Alma-Ata,
Kazakhstan,] pp. 24-26 (in Russian).
KUHN, J.K. (1973) Trace elements in whole coal determined by
X-ray fluorescence. Norelco Rep., 20(3): 7-10.
KUMPULAINEN, J.T., WOLF, W.R., VEILLON, C., & MERTZ, W.
(1979) Determination of chromium in selected United States
diets. J. agric. food Chem., 27: 490-494.
KUMPULAINEN, J., LEHTO, J., KOIVISTOINEN, P., UUSITUPA, M., &
VUORI, E. (1983) Determination of chromium in human milk
serum and urine by electrothermal atomic absorption spectro-
metry without preliminary ashing. Sci. total Environ., 31:
71-80.
KURYLOWICZ, B. & GASIOROWSKI, S. (1958) [Agrochemical study
on calcinated phosphate-containing admixtures of chromium
compounds.] Przemysl Chem., 37: 797-800 (in Polish).
LANGARD, S. (1977) The fate of chromium after intravenous
administration of Na251 CrO4 and CrCl3 x 6H2 O to the rat,
Guildford, England, University of Surrey, 66 pp (M.Sc.
Toxicology Thesis).
LANGARD, S. (1980a) Chromium. In: Waldron, H.A., ed. Metals
in the environment, London, New York, Academic Press,
pp. 111-132.
LANGARD, S. (1980b) A survey of respiratory symptoms and
lung function in ferrochromium and ferrosilicon workers. Int.
Arch. occup. environ. Health, 46: 1-9.
LANGARD, S., ed. (1982) Biological and environmental aspects
of chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, 285 pp.
LANGARD, S. & HENSTEN-PETTERSEN, A. (1981) Chromium
toxicology. In: Williams, D.F., ed. Systemic aspects of
biocompatibility, Boca Raton, Florida, CRC Press, Vol. 1, pp.
144-161.
LANGARD, S. & NORSETH, T. (1975) A short study of bronchial
carcinomas in workers producing chromate pigments. Br. J. ind.
Med., 32: 62-65.
LANGARD, S. & NORSETH, T. (1979) Chromium. In: Friberg, L.,
Nordberg, G.F., & Vouk, V.B., ed. Handbook on the toxicology
of metals, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 383-397.
LANGARD, S. & STERN, R.M. (1985) Nickel in welding fumes: a
cancer hazard to welders? In: Sunderman, F.W.J., Aitio, A.,
Berlin, A., Bishop, C., Buringh, E., Davis, W., Gounar, M.,
Jacquignon, P.C., Mastromatteo, E., Rigaut, J.P., Rosenfeld,
C., Saracci, R., & Sors, A., ed. Nickel in the human
environment, Lyons, International Agency for Research on
Cancer, pp. 95-103 (IARC Scientific Publications No. 53).
LANGARD, S. & VIGANDER, T. (1983) Occurrence of lung cancer
in workers producing chromium pigments. Br. J. ind. Med., 40:
71-74.
LANGARD, S., GUNDERSEN, N., TSALEV, D.L., & GYLSETH, B.
(1978) Whole blood chromium level and chromium excretion in
the rat after zinc chromate inhalation. Acta pharmacol.
toxicol., 42: 142-149.
LANGARD, S., ANDERSEN, A., & GYLSETH, B. (1980) Incidence of
cancer among ferrosilicon workers. Br. J. ind. Med., 37:
114-120.
LASKIN, S. (1972) Research in environmental sciences,
Washington DC, Institute of Environmental Medicine, pp. 92-97.
LASKIN, S., KUSCHNER, M., & DREW, R.T. (1970) Studies in
pulmonary carcinogenesis. In: Hanna, M.G., Jr, Nettesheim, P.,
& Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 321-351 (US Atomic Energy
Commission Symposium Series No. 18).
LAUTNER, G.M., CARVER, J.C., & KONZEN, R.B. (1978)
Measurement of chromium (VI) and chromium (III) in stainless
steel welding fumes with electron spectroscopy for chemical
analysis and neutron activation analysis. Am. Ind. Hyg. Assoc.
J., 39: 651-660.
LEHMANN, K.B. (1914) [Historical and critical account of our
present knowledge of the effect of small doses of chromate.]
In: Gesamtgebiet gewerbehygiene, Berlin, Springer Verlag, pp.
1-119 (in German).
LEHMANN, K.B. (1932) [Does the occurrence of lung cancer in
chromium workers give grounds for any particular anxiety?]
Zentralbl. Gewerbehyg., 9: 168-170 (in German).
LERICHE, H.H. (1968) Metal contamination of soil in the
Woburn market-garden experiment resulting from the application
of sewage sludge. J. agric. Sci. Camb., 71: 205-208.
LETTERER, E., NEIDHARDT, K., & KLETT, H. (1944) [Chromate
lung cancer and chromate pneumoconiosis. A clinical, morbid
anatomical and industrial-hygiene-related study.] Arch.
Gewerbepath. Gewerbehyg., 12: 323-361 (in German).
LEVIN, H.M., BRUNNER, M.J., & RATTNER, H. (1959)
Lithographer's dermatitis. J. Am. Med. Assoc., 169: 566-569.
LEVINE, R.A., STREETEN, D.H.P., & DOISY, R.J. (1968) Effects
of oral chromium supplementation on the glucose tolerance of
elderly human subjects. Metabolism, 17: 114-125.
LEVIS, A.G. & BIANCHI, V. (1982) Mutagenic and cytogenetic
effects of chromium compounds. In: Langard, S., ed. Biological
and environmental aspects of chromium, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 171-208.
LEVIS, A.G. & BUTTIGNOL, M. (1977) Effects of potassium
dichromate on DNA Synthesis in hamster fibroblast. Br. J.
Cancer, 35: 496-499.
LEVIS, A.G. & MAJONE, F. (1979) Cytotoxic and caryogenic
effects of soluble chromium compounds on mammalian cell
cultures. Br. J. Cancer, 40: 523-533.
LEVIS, A.G. & MAJONE, F. (1981) Cytologic and clastogenic
effects of soluble and insoluble compounds containing
hexavalent and trivalent chromium. Br. J. Cancer, 44: 219-235.
LEVIS, A.G., BUTTIGNOL, M., BIANCHI, V., & SPONZA, G. (1978)
Effects of potassium dichromate on nucleic acid and protein
synthesis and on precursor uptake in BHK fibroblasts. Cancer
Res., 38: 110-116.
LEVY, L.S. & MARTIN, P.A. (1983) The effects of a range of
chromium-containing materials on rat lung, Birmingham,
England, University of Aston, Department of Environmental and
Occupational Health, p. 224 and suppl (Prepared for Dry Color
Manufacturers Association, Alexandria, Virginia, USA).
LEVY, L.S. & VENITT, S. (1975) Carcinogenic and mutagenic
activity of chromium-containing materials. Br. J. Cancer, 32:
254-255.
LIEBER, M., PERLMUTTER, N.M., & FRAUENTHAL, H.L. (1964)
Cadmium and hexavalent chromium in Nassau County ground
water. J. Am. Water Works Assoc., 56: 739-747.
LIFSCHITZ, M.L., WALLACH, S., PEABODY, R.A., VERCH, R.L., &
AGRAWAL, R. (1980) Radiochromium distribution in thyroid
and parathyroid deficiency. Am. J. clin. Nutr., 33: 57-62.
LINDBERG, E. & HEDENSTIERNA, G. (1983) Chrome plating.
Symptoms, findings in the upper airways, and effects on lung
function. Arch. environ. Health, 38: 367-374.
LINDBERG, E. & VESTERBERG, O. (1983a) Monitoring exposure to
chromic acid in chromeplating by measuring chromium in urine.
Scand. J. Work environ. Health, 9: 333-340.
LINDBERG, E. & VESTERBERG, O. (1983b) Urinary excretion of
proteins in chromplaters, previously exposed chromplaters, and
referents. Scand. J. Work. environ. Health, 9: 505-510.
LINDBERG, E., EKHOLM, U. & ULFVARSON, U. (1985) Extent and
conditions of exposure in the Swedish chrome plating industry.
Int. Arch. Occup. Environ. Health, 56: 197-205.
LIU, V.J.K. & MORRIS, J.S. (1978) Relative chromium response
as an indicator of chromium status. Am. J. clin. Nutr., 31:
972-976.
LOFROTH, G. (1978) The mutagenicity of hexavalent chromium
is decreased by microsomal metabolism. Naturwissenschaften,
65: 207-208.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LUKANIN, W.P. (1930) [The pathology of chromate pneumo-
coniosis.] Arch. Hyg. Bakt., 104: 166-174 (in German).
LUZHNIKOV, E.A., SHIMANKO, I.I., KOSTOMAROVA, L.G., MALYGINA,
S.I., & TRAKHTENGARTS, M.I. (1976) [Acute poisoning with
chromium compounds.] Terapeut. Arkh., 48(7): 121-125 (in
Russian).
LYON, G.L., BROOKS, R.R., PETERSON, P.J., & BUTLER, G.W.
(1970) Some trace elements in plants from serpentine soils.
N.Z. J. Sci., 13: 133-139.
MACHLE, W. & GREGORIUS, F. (1948) Cancer of the respiratory
system in the United States chromate-producing industry.
Public Health Rep., 63: 1114-1127.
MCCLENDON, L.T. (1978) Determination of chromium in
biological matrices by neutron activation: application to
standard reference materials. J. radioanal. Chem., 42: 85-91.
MACKENZIE, R.D., BYERRUM, R.U., DECKER, C.F., HOPPERT, C.A., &
LANGHAM, R.F. (1958) Chronic toxicity studies. II. Hexa-
valent and trivalent chromium administered in drinking-water
to rats. Am. Med. Assoc. Arch. ind. Health, 18: 232-234.
MCRAE, W.D., WHITING, R.F., & STICH, H.F. (1979) Sister-
chromatid exchanges induced in cultured mammalian cells by
chromate. Chem.-biol. Interact., 26: 281-286.
MAHALKO, J.R. & BENNION, M. (1976) The effect of parity and
time between pregnancies on maternal hair chromium concentra-
tion. Am. J. clin. Nutr., 29: 1069-1072.
MAJONE, F. & LEVIS, A.G. (1979) Chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster cells treated in
vitro with hexavalent chromium compounds. Mutat. Res., 67:
231-238.
MAJONE, F. & RENSI, D. (1979) Mitotic alterations, chromo-
some aberrations, and sister-chromatid exchanges induced by
hexavalent and trivalent chromium on mammalian cells in vitro.
Caryologia, 32: 379-392.
MALTONI, C. (1974) Occupational carcinogenesis. Excerpta
Med. Int. Congr. Ser., 322: 19-26.
MALTONI, C. (1976) Precursor lesions in exposed populations
as indicators of occupational cancer risk. Ann. NY Acad. Sci.,
271: 444-447.
MANCUSO, T.F. (1951) Occupational cancer and other health
hazards in a chromate plant: a medical appraisal. II. Clinical
and toxicologic aspects. Ind. Med. Surg., 20: 393-407.
MANCUSO, T.F. (1975) Consideration of chromium as an
industrial carcinogen. In: Hutchinson, T.C., ed. Proceedings
of the International Conference on Heavy Metals in the
Environment, Toronto, Canada, October 27-31, 1975, Toronto,
Institute for Environmental Studies, pp. 343-356.
MANCUSO, T.F. & HUEPER, W.C. (1951) Occupational cancer and
other health hazards in a chromate plant: a medical appraisal.
I. Lung cancer in chromate workers. Ind. Med. Surg., 20:
358-363.
MARTIN, J.H. & KNAUER, G.A. (1973) The elemental composition
of plankton. Geochim. Cosmochim. Acta, 3: 1639-1653.
MATHIS, B.J. & CUMMINGS, T.F. (1973) Selected metals in
sediments, water, and biota in the Illinois river. J. Water
Pollut. Control Fed., 45: 1573-1583.
MATHUR, R.K. & DOISY, R.J. (1972) Effect of diabetes and
diet on the distribution of tracer doses of chromium in rats.
Proc. Soc. Exp. Biol. Med., 139: 836-838.
MATSUMOTO, N., IIJIMA, S., & KATSUNUMA, H. (1976) Placental
transfer of chromic chloride and its teratogenic potential in
embryonic mice. J. toxicol. Sci., 2: 1-13.
MAYER, R. (1983) Interaction of forest canopies with atmo-
spheric constituents: aluminium and heavy metals. In: Ulrich,
B. & Pankrath, J., ed. Effects of accumulation of air
pollutants in forest ecosystems, Dordrecht, Boston, London,
D. Reidel, pp. 47-55.
MAZGON, R. (1932) [Therapeutic effect of common salt on
experimental chromium nephritis in the rabbit.] Z. Gesamte
exp. Med., 81: 195-207 (in German).
MERIAN, E. (1984) Introduction on environmental chemistry
and global cycles of arsenic, beryllium, cadmium, chromium,
cobald, nickel, selenium, and their derivatives. Toxicol.
environ. Chem., 8: 9-38.
MERKURIEVA, R.V., KRASOVSKY, G.N., BURMANTOVA, N.P.,
SHATERNIKOVA, I.L., VARSHAVSKAYA, S.P., VASIUKOVICH, L.YA.,
KOGANOVA, Z.I., BUSHINSKAYA, L.I., KONSTANTINOVA, I.N.,
DOLINSKAYA, S.I., ASTAKHOVA, L.F., & VOTYAKOV, A.V. (1980a)
[Changes in various enzyme systems resulting from exposure to
some chemical substances.] Gig. i. Sanit., N1: 25-28 (in
Russian).
MERKURIEVA, R.V., KRASOVSKY, G.N., VOTYAROV, A.V., BURMANTOVA,
N.P., MUCHAMBETOVA, L.H., & KONSTANTINOVA, I.N. (1980b)
[Enzyme disarrangement of various subcellular structures under
the influence of the general toxic and gonadotoxic effects of
environmental chemical factors.] Zdravookhranenie belorus.,
N2: 28-29 (in Russian).
MERKURIEVA, R.V., KOGANOVA, Z.I., GABDULLINA, M.H., TSAPKOVA,
N.N., DUKHATEROV, O.N., & DOLINSKAYA, S.I. (1982) [A
comparative study of metabolic reactions at various routes of
chromium6+ administration in the organism of laboratory
animals.] Gig. i Sanit., N8: 75-78 (in Russian).
MERRITT, W.F. (1971) Identification and measurement of trace
elements in fresh water by neutron activation. In: Proceedings
of the International Symposium on Identification and Measure-
ment of Environmental Pollution, 358-362.
MERTZ, W. (1969) Chromium occurrence and function in
biological systems. Physiol. Rev., 49: 163-239.
MERTZ, W. (1981) The interaction between chromium and
insulin. Adv. Physiol. Sci., 12: 101-105.
MERTZ, W. & ROGINSKI, E.E. (1963) The effect of trivalent
chromium in galactose entry in rat epidymal fat tissue. J.
biol. Chem., 238: 868-872.
MERTZ, W. & ROGINSKI, E.E. (1969) Effects of chromium (III)
supplementation on growth and survival under stress in rats
fed low protein diets. J. Nutr., 97: 531-536.
MERTZ, W. & ROGINSKI, E.E. (1971) Chromium metabolsm: the
glucose tolerance factor. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 123-153.
MERTZ, W. & SCHWARZ, K. (1959) Relation of glucose tolerance
factor to impaired glucose tolerance in rats on stock diets.
Am. J. Physiol., 196: 614-618.
MERTZ, W., ROGINSKI, E.E., & SCHWARZ, K. (1961) Effect of
trivalent chromium complexes on glucose uptake by epididymal
fat tissue of rats. J. biol. Chem., 236: 318-322.
MERTZ, W., ROGINSKI, E.E., & REBA, R.C. (1965a) Biological
activity and fate of intravenous chromium (III) in the rat.
Am. J. Physiol., 209: 489-494.
MERTZ, W., ROGINSKI, E.E., & SCHROEDER, H.A. (1965b) Some
aspects of glucose metabolism of chromium-deficient rats
raised in a strictly controlled environment. J. Nutr., 86:
107-112.
MERTZ, W., ROGINSKI, E.E., FELDMAN, R.J., & THURMAN, D.E.
(1969) Dependence of chromium transfer into the rat embryo on
the chemical form. J. Nutr., 99: 363-367.
MERTZ, W., TOEPFER, E.W., ROGINSKI, E.E., & POLANSKY, M.M.
(1974) Present knowledge of the role of chromium. Fed. Proc.,
33: 2275-2280.
MEYERS, J.B. (1950) Acute pulmonary complications following
inhalations of chromic acid mist. Arch. ind. Hyg. occup. Med.,
2: 742-747.
MICKAIL, T.H., HABIB, Y.A., GABRIAL, G.N., & MORCOS, S.R.
(1976) The effect of chromium III on the intravenous glucose
tolerance of rats given diets of defficient protein values. Z.
Ernährungswiss., 15: 182-187.
MIESCHER, G., AMREIN, H.P., & LEDER, M. (1955) [Chromate
hypersensitivity and cement eczema.] Dermatologica (Basel),
110: 266-283 (in German).
MIGAI, K.V. (1975) [Hygiene and safety of labor in electro
welding works in shipbuilding,] Leningrad, pp. 27-35 (in
Russian).
MITMAN, F.W., WOLF, W.R., KELSAY, J.L., & PRATHER, E.S.
(1975) Urinary chromium levels of nine young women eating
freely chosen diets. J. Nutr., 105: 64-68.
MORGAN, J.M. (1972) Hepatic chromium content in diabetic
subjects. Metabolism, 21: 313-316.
MORRIS, G.E. (1955) Chromate dermatitis from chrome glue and
other aspects of the chrome problem. Am. Med. Assoc. Arch.
Ind. Health, 11: 368-371.
MORRIS, G.E. (1956) The manufacture of leather. Its chemical
and dermatological aspects. Ann. Meet. Am. Acad. Derm. Syph.
(Chicago).
MORRIS, G.E. (1958) Chrome dermatitis. A study of the
chemistry of shoe leather with particular reference to basic
chromic sulfate. Am. Med. Assoc. Arch. Derm., 78: 612-618.
MORRIS, G.E. (1959) [The basic form of chromic sulfate.
Results of epicutaneous testing of a chromium-III salt.]
Berufsdermatosen, 7: 126-131 (in German).
MORROW, R.W. & MCELHANEY, R.J. (1974) Determination of
chromium in industrial effluent water by flameless atomic
absorption spectroscopy. At. Absorpt. Newsl., 13(2): 45-46.
MORTON, M.S. & ELWOOD, P.C. (1974) CNS malformations and
trace elements in water. Teratology, 10: 318.
MOSINGER, M. & FIORENTINI, H. (1954) Sur la pathologie due
aux chromates: Premières recherches expérimentales. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 15: 187-199.
MULLER-WIEGAND, B., STEUER, D., & HEESSELS, P. (1983) Blood
and urine samples for quality control of heavy metals. Chem.
Toxicol. Clin. Chem. Met. Proc. Int. Conf., 2nd: 73-76.
MURAKAMI, Y., SUZUKI, Y., YAMAGATA, T., & YAMAGATA, N. (1965)
Cr and Mn in Japanese diet. J. Radiat. Res., 6: 105-110.
MURRMAN, R.P., WINTERS, R.W., & MARTIN, T.G. (1971)
Determination of trace elements in soils and clay minerals by
resonnance neutron activation analysis. Soil. Sci. Soc. Am.
Proc., 35: 647-652.
MUTTI, A., CAVATORTA, A., PEDRONI, C., BORGHI, A., GIAROLI,
C., & FRANCHINI, I. (1979) The role of chromium accumulation
in the relationship between airborne and urinary chromium in
welders. Int. Arch. occup. environ. Health, 43: 123-133.
MYSLYAEVA, A.V. (1965) [Changes in the peripheral blood in
workers of a chromium compound plant.] In: [Proceedings of
the 4th Scientific Conference of Aktubinsk State Medical
Institute, Alma-Ata,] Kazakhstan, Alma-Ata, Aktubinsk State
Medical Institute, pp. 331-332 (in Russian).
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., & KURATA, H. (1978)
Comparative studies of chromosomal aberrations and muta-
genicity of trivalent and hexavalent chromium. Mutat. Res.,
58: 175-181.
NASH, D.T., ELWOOD, I.G., & STREETEN, D.H.P. (1979) The
effect of brewer's yeast containing chromium-rich glucose
tolerance factor on serum lipids. In: Proceedings of the 5th
International Symposium on Atherosclerosis, Houston, Texas.
NATER, J.P. (1963) Possible causes of chromate eczema.
Dermatologica (Basel), 126: 160-166.
NATH, R. (1976) Assessment of chromium metabolism in
maturity onset and juvenile diabetics using chromium: 51Cr and
therapeutic response of chromium administration. Excerpta Med.
Int. Congr. Ser., 400: 194.
NEIKO, E.M. & DELVA, IU.V. (1978) [Chromium balance in
chronic ischemic heart disease.] Klinicheskaia Meditsina
(Mosk.), 56(4): 97-99 (in Russian).
NESTMANN, E.R., MATULA, T.I., DOUGLAS, G.R., BORA, K.C., &
KOWBEL, D.J. (1979) Detection of the mutagenic activity of
lead chromate using a battery of microbial tests. Mutat. Res.,
66: 357-365.
NETTESHEIM, P., HANNA, M.G., Jr., DOHERTY, D.G., NEWELL, R.F.,
& HELLMANN, A. (1970) Effects of chronic exposure to
artificial smog and chromium oxide dust on the incidence of
lung tumours in mice. In: Hanna, M.G., Jr, Nettesheim, P., &
Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 305-320 (US Atomic Energy
Commission Symposium Series No. 18).
NETTESHEIM, P., HANNA, M.G., DOHERTY, D.G., NEWELL, R.F., &
HELLMAN, A. (1971) Effect of calcium chromate dust,
influenza virus, and 100R whole-body X-radiation on lung
tumour incidence in mice. J. Natl Cancer Inst., 47: 1129-1144.
NEWBOLD, R.F., AMOS, J., & CONNELL, J.R. (1979) The
cytotoxic, mutagenic, and clastogenic effects of chromium-
containing compounds on mammalian cells in culture. Mutat.
Res., 67: 55-63.
NEWMAN, H.A.I., LEIGHTON, R.F., LANESE, R.R., & FREELAND N.A.
(1978) Serum chromium and angiographically-determined
coronary artery disease. Clin. Chem., 24: 541-544.
NISHIOKA, H. (1975) Mutagenic activities of metal compounds
in bacteria. Mutat. Res., 31: 185-189.
NOLLENDORF, A., PAKALNE, E., & UPITIS, V. (1972) [Little
known trace elements in Chlorella culture.] Latv. PSR Zinat.
Akad. Vestis (Latvian SSR), 7: 33-43 (in Russian).
NOMIYAMA, H., YOTORIYAMA, M., & NOMIYAMA, K. (1980b) Normal
chromium levels in urine and blood of Japanese subjects
determined by direct flameless atomic absorption spectrophoto-
metry, and valency of chromium in urine after exposure to
hexavalent chromium. Am. Ind. Hyg. Assoc. J., 41: 98-102.
NOMIYAMA, K., MATSUI, K., & NOMIYAMA, H. (1980a) Environ-
mental temperature: a factor modifying the acute toxicity of
organic solvents, heavy metals, and agricultural chemicals.
Toxicol. Lett., 6: 67-70.
NORSETH, T. (1978) Health effects of nickel and chromium.
In: Elvira di Ferrante, E., ed. Trace metals: exposure and
health effects, Oxford, New York, Toronto, Sydney, Paris,
Frankfurt, Pergamon Press, pp. 135-146.
NORSETH, T. (1980) Cancer hazards caused by nickel and
chromium exposure. J. Toxicol. environ. Health, 6: 1218-1227.
NOVAKOVA, S., MAUTNER, G., & DINOEVA, A. (1974) [Content of
hexavalent chromium in water supplies and its effect on the
development of experimental atherosclerosis in warm-blooded
animals.] Gig. i Sanit., 5: 78-80 (in Russian).
NOVEY, H.S., HABIB, M., WELLS, J.A. (1983) Asthma and JgE
antibodies induced by chromium and nickel salts. J. Allergy
clin. Immunol., 72: 407-412.
OFFENBACHER, E.G. & PI-SUNYER, F.X. (1980) Benefical effect
of chromium-rich yeast on glucose tolerance and blood lipids
in elderly subjects. Diabetes, 29: 919-925.
OHNO, H., HANAOKA, F., & YAMADA, M. (1982) Inducibility of
sister-chromatid exchanges by heavy-metal ions. Mutat. Res.,
104: 141-145.
OHTA, F. (1940) Studies on the detoxicating hormone of the
liver (yakriton). Contribution to the usage of yakriton
against experimental chromate nephritis. Tohoku J. exp. Med.,
39: 37-46.
OKADA, S., OHBA, H., & TANIYAMA, M. (1981) Alterations in
ribonucleic acid synthesis by chromium (III). J. inorg.
Biochem., 15: 223-231.
OKADA, S., TRUKADA, H., & OHBA, H. (1983) Enhancement of
ribonucleic acid synthesis by chromium (III) in mouse liver.
J. inorg. Biochem., 19: 95-103
OKADA, S., TSUKADA, H., & OHBA, H. (1984) Enhancement of
nucleolar RNA synthesis by chromium (III) in regenerating rat
liver. J. inorg. Biochem., 21, 113-124.
OPHULS, W. (1911a) Experimental nephritis in guinea-pigs by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9: 11-13.
OPHULS, W. (1911b) Experimental nephritis in rabbits by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9:13.
OTTINGER, R.S., BLUMENTHAL, J.L., DALPORTO, D.F., GURBER,
G.I., SANTY, M.J., & SHIH, C.C. (1973) Recommended methods
of reduction, neutralization, recovery or disposal of
hazardous waste, Cincinnati, Ohio, US Environmental Protection
Agency, Vol. 6, pp. 143-162 (EPA 670/2-73-053-F).
PAGE, A.L. (1974) Fate and effects of trace elements in
sewage sludge when applied to agricultural lands, Cincinnati,
Ohio, US Environmental Protection Agency (EPA 670/2-74-005).
PAIXAO, L.M. & YOE, J.H. (1959) Spectochemical determination
of magnesium, chromium, nickel, copper, and zinc in human
plasma and red cells. Clin. Chim. Acta, 4: 507-514.
PARR, R.M. (1977) Problems of chromium analysis in
biological materials: an international perspective with
special reference to results for analytical quality control
samples. J. radioanal. Chem., 39: 421-433.
PASCALE, L.R., WALDSTEIN, S.S., ENGBRING, G., DUBIN, A., &
SZANTO, P.B. (1952) Chromium intoxication with special
reference to hepatic injury. J. Am. Med. Assoc., 149:
1385-1389.
PASHIN, Y.V. & KOZACHENKO, V.J. (1981) [Mutagenic activity
of chromium compounds.] Gig. i. Sanit., 5: 46-49 (in Russian).
PASHIN, Y.V., ZACEPILOVA, T.A., & KOZACHENKO, V.I. (1982)
Induction of dominant lethal mutations in male mice by
potassium dichromate. Mutat. Res., 103: 345-347.
PASTERNAK, K. (1973) The spreading of heavy metals in
flowing waters in the region of occurrence of natural deposits
of the zinc and lead industry. Acta hydrobiol., 15: 145-166.
PEKAREK, R.S., HAUER, E.C., RAYFIELD, E.J., WANNEMACHER, R.W.,
& BIESEL, W.R. (1975) Relationship between serum chromium
concentrations and glucose utilization in normal and infected
subjects. Diabetes, 24: 350-353.
PENG, H.Z. (1982) [The investigation of nasal disease
morbidity of chromium exposed workers.] J. Shan-Xie Med.
College, 3: 19-21 (in Chinese).
PERLMUTTER, N.M. & LIEBER, M. (1970) Dispersal of plating
wastes and sewage contaminants in ground water and surface
water, Washington DC, US Government Printing Office, 67 pp
(Geological Survey Water Supply Paper 1879-G).
PERONE, V.B., MOFFITT, A.A., POSSICK, P.A., KEY, M.M.,
DANZINGER, S.J., & GELLIN, G.A. (1974) The chromium, cobalt,
and nickel contents of American cement and their relationship
to cement dermatitis. Am. Ind. Hyg. Assoc. J., 35: 301-306.
PETRILLI, F.L. & DE FLORA, S. (1977) Toxicity and mutagenicity of
hexavalent chromium on Salmonella typhimurium. Appl. environ.
Microbiol., 33: 805-809.
PETRILLI, F.L. & DE FLORA, S. (1978a) Oxidation of inactive
trivalent chromium to the mutagenic hexavalent form. Mutat.
Res., 58: 167-173.
PETRILLI, F.L. & DE FLORA, S. (1978b) Metabolic deactivation
of hexavalent chromium mutagenicity. Mutat. Res., 54: 139-147.
PETRILLI, F.L. & DE FLORA, S. (1980) Mutagenicity of
chromium compounds. In: Chromates Symposium 80, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 76-99.
PETRILLI, F.L. & DE FLORA, S. (1982) Interpretations on
chromium mutagenicity and carcinogenicity. In: Sorsa, M. &
Vainmio, H., ed. Mutagens in our environment, New York, Alan
R. Liss, pp. 453-464.
PIERCE, J.V. & SCHEEL, L.D. (1965) Toxicity of alloys of
chromium. Arch. environ. Health, 10: 870-876.
PIRILA, V. & KILPIO, O. (1949) On dermatoses caused by
bichromates. Acta derm. venerol., 29: 550-563.
POHLAND, F.G. (1975) Sanitary landfill stabilization with
leachate recycle and residual treatment, Cincinnati, Ohio, US
Environmental Protection Agency, 106 pp (EPA 600/2-75-043).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1973) [On carcinogenous
hazards in the product of chromium ferroalloys.] Gig. Tr.
prof. Zabol., 10: 23-26 (in Russian).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1974) [Epidemiological
data on malignant neoplasms in workers who are in contact with
different chromium compounds.] In: Shabad, L.M., Velich-
kovskii, B.T. & Kogan, F.M., ed. [Occupational cancer (a
collection of scientific works),] Moscow, Academic Academy of
Medical Science, pp. 64-71 (in Russian).
POKROVSKAYA, L.V., TJUSHUYAKOVA, A.V., GORODNOVA, N.V.,
ZALOMOVA, E.N., & ANDREEVA, T.D. (1976) [Dust factor and
occupational pathology in workers engaged in the open
excavation of chromium ores.] In: [Occupational diseases of
dust etiology (collection of works),] Vol. 3, pp. 38-43 (in
Russian).
POLAK, L., TURK, J.L., & FREY, J.R. (1973) Studies on
contact hypersensitivity to chromium compounds. In: Kallos,
P., Waksman, B.H., & DeWeck, A., ed. Progress in allergy,
Basel, Karger, Vol. 17, pp. 145-226.
PRESTON, A.M., DOWDY, R.P., PRESTON, M.A., & FREEMAN, J.N.
(1976) Effects of dietary chromium on glucose and serum
cholesterol in guinea-pigs. J. Nutr., 106: 1391-1397.
PRIESTLEY, J. (1877) Observations on the physiological
action of chromium. J. Anat. Physiol., 11: 285-301.
PRINCI, F., MILLER, L.H., DAVIS, A., & CHOLAK, J. (1962)
Pulmonary disease of ferrochromium workers. J. occup. Med., 4:
301-310.
PUNSAR, S., ERAMETSA, O., KARVONEN, M.I., RYHANEN, A., HILSKA,
P., & WORNAMO, H. (1975) Coronary heart disease and
drinking-water. A search in two Finnish male cohorts for
epidemiologic evidence of a water factor. J. chron. Dis., 28:
259-287.
RABEAU, H. & UKRAINCZYK, V. (1939) Dermites des
blanchisseuses. Rôle du chrome et du chlore. Ann. Derm. Syph.,
10: 656-680.
RADIAN CORPORATION (1983) Estimates of population exposure
to ambient chromium emissions, Research Triangle Park, North
Carolina, US Environmental Protection Agency (Final Report to
K.L. Blanchard) (EPA Contract No. 68-02-3818).
RAFFETTO, G. (1977) Direct interaction with cellular targets
as the mechanism for chromium carcinogenesis. Tumori, 63:
503-512.
RAFFETTO, G., PARODI, S., DE FERRARI, M., PARODI, C., TROIANO,
R., & BRAMBILLA, G. (1978) Direct interaction with cellular
targets as the mechanism for chromium carcinogensis. Tumori,
73: 503-512.
REDISKE, J.H. (1956) Chromium toxicity in plants. In:
Biology research: annual report, 1955, Richland, Washington,
Hamford Atomic Products Operations, pp. 48-52 (Report
HW-41500).
REDISKE, J.H., CLINE, J.F., & SELDERS, A.A. (1955) Chromium
toxicity in plants. In: Biology research: annual report, 1954,
Richland, Washington, Hamford Atomic Products Operations,
pp. 32-39 (Report HW-35917).
REGGIANI, A., LOTTI, M., DE ROSA, E., & SAIA, B. (1973)
[Impairments of respiratory functions in subjects exposed to
chromium. Note 1. Spirographic changes.] Lav. Umano, 25: 23-27
(in Italian).
REICHENBACH-KLINKE, H.H. (1977) [To the ecology of the
accumulation of heavy metals in fishes.] In: Reichenbach-
Klinke, H.H., ed. [Contributions to fish ecology: fish and the
environment,] Stuttgart, New York, Gustav Fischer, pp. 7-11
(in German).
REICHENBACH-KLINKE, H.H. (1980) [Fish diseases and harm
suffered by fish,] 2nd ed., Stuttgart, New York, Gustav
Fischer, 474 pp (in German).
RETNEV, V.M. (1960) [On the effect produced by chromium
compounds contained in cement dust on the development of
bronchial asthma.] Gig. Tr. prof. Zabol., 7: 29-32 (in
Russian).
RIALES, R. (1979) Influence of brewer's yeast on lipoprotein
cholesterol concentrations. A preliminary report. In:
Shapcott, D. & Hubert, I., ed. Chromium in nutrition and
metabolism. Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 199-212.
ROBERTS, K.R. & MARZLUF, G.A. (1971) The specific inter-
action of chromate with the dual sulfate permease systems of
Neurospora crassa. Arch. Biochem. Biophys., 142: 651-659.
ROBINSON, W.D. & EDINGTON, G. (1935) Chemical studies of
infertile soils derived from rocks high in magnesium and
generally high in chromium and nickel, Washington DC, US
Department of Agriculture, pp. 1-29 (US Department of
Agriculture Technical Bulletin No. 471).
ROGINSKI, E.E. & MERTZ, W. (1967) An eye lesion in rats fed
low-chromium rations. J. Nutr., 93: 249-251.
ROGINSKI, E.E. & MERTZ, W. (1969) Effects of chromium (III)
supplementation on glucose and amino acid metabolism in rats
fed a low protein diet. J. Nutr., 97: 525-530.
ROHDE, G., TISCHENDORF, G., LEONHARDT, J., & DAMASCHUN, F.
(1978) [Study of crocoite occurrence in Callenberg (Saxony)
(Germany).] Z. angew. Geol., 24: 168-173 (in German).
ROMOSER, G.L., DUDLEY, W.A., MACHLIN, L.J., & LOVELESS, L.
(1961) Toxicity of vanadium and chromium for the growing
chick. Poultry Sci., 40: 1171-1173.
ROOK, H.L. & WOLF, W.R. (1977) The quantitative
determination of volatile trace elements in NBS biological
standard reference materials. In: Hemphill, D.D., ed.
Proceedings of the Eleventh Annual Conference on Trace
Substances in Environmental Health, Columbia, Missouri, the
Curators of the University of Missouri, pp. 324-333.
ROSCHINA, T.A. (1959) [Experimental study on the effects of
magnezite chromium and chromite magnezite dust exposure in the
production of refrectories.] Gig. Tr. prof. Zabol., 4: 28-32
(in Russian).
ROSCHINA, T.A. (1964) [A comparative evaluation of the
effects of carbide dust and chromium boride on the organism.]
Gig. i Sanit., 8: 25-28 (in Russian).
ROSCHINA, T.A. (1976) [The toxicity of chrominum (sic)
hexacarbonyl.] Gig. Tr. Prof. Zabol., 2: 38-42 (in Russian).
ROSEBROUGH, R.W. & STEELE, N.C. (1981) Effect of
supplemental dietary chromium or nicotinic acid on
carbohydrate metabolism during basal, starvation, and
refeeding periods in poults. Poultry Sci., 60: 407-417.
ROSS, D.S., SJOGREN, R.E., & BARTLETT, R.J. (1981) Behaviour
of chromium in soils. IV. Toxicity to microorganisms. J.
environ. Qual., 10: 145-148.
ROSTENBERG, A., Jr & PERKINS, A.J. (1951) Nickel and cobalt
dermatitis. J. Allerg., 22: 466-474.
RTECS (1978) Registry of toxic effects of chemical substances,
Washington DC, National Institute for Occupational Safety and
Health, pp. 450.
SAMITZ, M.H. (1955) Some dermatologic aspects of the
chromate problem. Arch. ind. Health, 11: 361-367.
SAMITZ, M.H. (1956) Studies of chromate dermatitis. Arch.
ind. Health, 14: 269-271.
SAMITZ, M.H. & EPSTEIN, E. (1962) Experimental cutaneous
chrome ulcers in guinea-pigs. Arch. environ. Health, 5:
463-468.
SAMITZ, M.H. & GROSS, S. (1960) Extraction by sweat of
chromium from chrome tanned leathers. J. occup. Med., 2: 12-14.
SAMITZ, M.H. & GROSS, S. (1961) Effects of hexavalent and
trivalent chromium compounds on the skin. Am. Med. Assoc.
Arch. Dermatol., 84: 404-409.
SAMITZ, M.H., KATZ, S., & GROSS, S. (1960) Nature of the
chromium extracted from leather by sweat. J. occup. Med., 2:
435-436.
SANDERS, C.L., JACKSON, T.A., ADEE, R.R., POWERS, G.J., &
WEHNER, A.P. (1971) Distribution of inhaled metal oxide
particles in pulmonary alveoli. Arch. intern. Med., 127:
1085-1089.
SARGENT, T., III, LIM, T.H., & JENSON, R.L. (1979) Reduced
chromium retention in patients with hemochromatosis, a
possible basis of hemochromatotic diabetes. Metabolism, 28:
70-79.
SARTO, F., LEVIS, A.G., & PAULON, C. (1980) Clastogenic
activity of hexavalent and trivalent chromium in cultured
human lymphocytes. Caryologia, 33: 239-250.
SARTO, F., COMINATO, J., BIANCHI, V., & LEVIS, A.G. (1982)
Increased incidence of chromosomal aberrations and sister
chromatid exchanges in workers exposed to chromic acid (CrO3)
in electroplating factories. Carcinogenesis, 3: 1011-1016.
SASSI, C. (1956) [Occupational disease in a chromate
factory.] Med. Lavoro, 47: 314-326 (in Italian).
SATOH, K., FUKUDA, Y., TORII, K., & KATSUNO, N. (1981)
Epidemiological study of workers engaged in the manufacture of
chromium compounds. J. occup. Med., 23: 835-838.
SAVORY, J., MUSHAK, P., & SUNDERMAN, F.W. Jr (1969) Gas
Chromatographic determination of chromium in serum. J.
chromatogr. Sci., 7: 674-679.
SCHALLER, K.H. & ZOBER, A. (1982) [Renal excretion of
toxicologically relevant metals in occupationally non-exposed
individuals.] Arztl. Lab., 28: 209-214 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1972) [Quantitative chromium determination in urine by
flameless atomic absorption spectrometry.] Z. klin. Chem.
klin. Biochem., 10: 434-437 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1973) The quantitative determination of chromium in urine by
flameless atomic absorption spectroscopy. At. Absorpt.
Newsl., 12(6): 147-150.
SCHELENZ, R. (1977) Dietary intake of 25 elements by man
estimated by neutron activation analysis. J. radioanal. Chem.,
37: 539-548.
SCHLETTWEIN-GSELL, D. & MOMMSEN-STRAUB, S. (1971) [Review
trace elements in foods. III. Chromium.] Int. J. Vitam. Nutr.
Res., 41: 116-125 and (1973) Suppl. 13, 188 pp (in German).
SCHOENTAL, R. (1975) Chromium carcinogenesis formation of
epoxyaldehydes and tanning. Br. J. Cancer, 32: 403-404.
SCHROEDER, H.A. (1966) Chromium deficiency in rats: a
syndrome simulating diabetes mellities with retarded growth.
J. Nutr., 88: 439-445.
SCHROEDER, H.A. (1970) Chromium, Washington DC, American
Petroleum Institute, 28 pp (Air Quality Monograph No. 70-15).
SCHROEDER, H.A. & BALASSA, J.J. (1965) Influence of
chromium, cadmium, and lead on rat aortic lipids and
circulating cholesterol. Am. J. Physiol., 209: 433-437.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1962)
Abnormal trace metals in man: chromium. J. chron. Dis., 15:
941-964.
SCHROEDER, H.A., VINTON, W.H., Jr, & BALASSA, J.J. (1963)
Effects of chromium, cadmium, and other trace metals on the
growth and survival of mice. J. Nutr., 80: 39-47.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1964)
Chromium, lead, cadmium, nickel, and titanium in mice: effect
on mortality, tumours, and tissue levels. J. Nutr., 83:
239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1965)
Chromium, cadmium, and lead in rats: effects on life span,
tumours, and tissue levels. J. Nutr., 86: 51-66.
SCHROEDER, H.A., NASON, A.P., & TIPTON, I.H. (1970) Chromium
deficiency as a factor in atherosclerosis. J. chron. Dis., 23:
123-142.
SCHROEDER, K.H. (1955) [Nickel eczema in a blueline (ozalid)
machine operator.] Berufsdermatosen, 3: 170-171 (in German).
SCHUBERT, G.E., GEBHARD, K., & HOENLEIN, F. (1970) [Kidney
damage following injection of serotonin and potassium
dichromate for chronic sodium overloading or restriction.]
Virchows Arch. Abt. A Path. Anat., 351: 68-82 (in German).
SCHULTHEISS, E. (1959) [Rubber and eczema,], Aulendorf i.
Württemberg, Cantor, 213 pp (in German).
SCHWARZ, K. & MERTZ, W. (1959) Chromium (III) and the
glucose tolerance factor. Arch. Biochem. Biophys., 85: 292-295.
SCHWARTZ, L. & DUNN, J.E. (1942) Dermatitis occurring among
operators of air conditioning equipment. Ind. Med. Surg., 11:
375.
SCHWARTZ, L., TULIPAN, L., & PECK, S. (1947) Occupational
diseases of the skin, 2nd ed., Philadelphia, Pennsylvania, Lea
& Febiger.
SEEBER, H., FIKENTSCHER, R., & ROSEBURG, B. (1976)
[Disorders of smell and taste in chromium dye workers.] Z.
Gesamte Hyg. Grenzgeb., 22: 820-822 (in German).
SEELEY, J.L. & SKOGERBOE, R.K. (1974) Combined sampling-
analysis method for the determination of trace elements in
atmospheric particulates. Anal. Chem., 46: 415-421.
SEELING, W., GRUENERT, A., KIENLE, K.H., OPFERKUCH, R., &
SWOBODNIK, M. (1979) [Determination of chromium in human
serum and plasma by flameless atomic absorption spectro-
photometry.] Z. anal. Chem., 299: 368-374 (in German).
SEITZ, W.R., SUYDAM, W.W., & HERCULES, D.M. (1972)
Determination of trace amounts of chromium (III) using
chemiluminescence analysis. Anal. Chem., 44(6): 957-963.
SHACKLETTE, H.T., SAUER, H.I., & MIESCH, A.T. (1970)
Geochemical environments and cardiovascular mortality rates in
Georgia, Washington DC, US Governmental Printing Office, 39 pp
(US Geological Survey Professional Paper No. 574-C).
SHAPCOTT, D., KHOURI, K., DEMERS, P.P., VOBECKY, J., &
VOBECKY, J. (1977) The measurement of volatile chromium in
biological materials. Clin. Biochem, 10: 178-180.
SHEFFET, A., THIND, J., MILLER, A.M., & LOURIA, D.B. (1982)
Cancer mortality in a pigment plant utilizing lead and zinc
chromates. Arch. environ. Health, 37: 44-52.
SHENDRIKAR, A.D. & WEST, P.A. (1974) A study of adsorption
characteristics of traces of chromium (III) and (VI) on
selected surfaces. Anal. Chim. Acta, 72: 91-96.
SHERMAN, L., GLENNON, J.A., BRECK, W.J., KLOMBERG, G.H., &
GORDON, E.S. (1968) Failure of trivalent chromium to improve
hyperglycemia in diabetes mellitus. Metabolism, 17: 439-442.
SHIMKIN, M.M. & LEITER, J. (1940) Induced pulmonary tumours
in mice. III. Role of chromic irritation in the production of
pulmonary tumours in strain A mice. J. Natl Cancer Inst., 1:
241-254.
SHMITOVA, L.A. (1978) In: [The impact of job-related factors
on specific function of the female organism,] Svedlovsk, pp.
105-108 (in Russian).
SHMITOVA, L.A. (1980) Gig. Tr. prof. zabol., 2:
33-35 (in Russian).
SIDI, E. & LONGUEVILLE, R. (1953) Dermites au ciment. Rôle
des sels de chrome. Arch. Mal. prof., 14: 41.
SIEGENTHALER, U., LAINE, A., POLAK, L. (1983) Studies on
contact sensitivity to chromium in the guinea-pig. The role
of valence in the formation of the antigenic determinant. J.
Invest. Dermatol., 80: 44-47.
SIMONOFF, M., LLABADOR, Y. HAMON, C., PEERS; A.M., & SIMONOFF,
G.N. (1984) Low plasma chromium in patients with coronary
artery and heart diseases. Biol. Trace Element Res., 6:
431-439.
SIMONDS, J.P. & HEPLER, O.E. (1945) Experimental nephro-
pathies. I. A method of producing controlled selective injury
of renal units by means of chemical agents. Arch. Path., 39:
103-108.
SIROVER, M.A. & LOEB, L.A. (1976) Infidelity of DNA
synthesis in vitro: screening for potential metal mutagens or
carcinogens. Science, 194: 1434-1436.
SJOGREN, B. (1980) A retrospective cohort study of mortality
among stainless steel welders. Scand. J. Work. environ.
Health, 6: 197-200.
SKOGERBOE, R.K. (1974) Monitoring trace metal particulates:
an evaluation of the sampling and analysis problems. In:
Instrumentation for monitoring air quality, Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp.
125-136.
SLUIS-CREMER, G.K. & DU TOIT, R.S. (1968) Pneumoconiosis in
chromite miners in South Africa. Br. J. ind. Med., 25: 63-67.
SPIER, H.W. (1956) [Wood preservatives and chromate eczema.]
Desinfekt. Gesundheitswes., 4: 58 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil ( Myriophyllum spicatum L.). Arch.
environ. Contam. Toxicol., 2: 331-341.
STAUB, H.W., REUSSNER, G., & THIESSEN, R., Jr (1969) Serum
cholesterol reduction by chromium in hypercholesteremic rats.
Science, 166: 746-747.
STEELE, N.C. & ROSEBROUGH, R.W. (1979) Trivalent chromium
and nicotinic acid supplementation for the turkey poult.
Poultry Sci., 58: 983-984.
STEELE, N.G., ALTHEN, T.G., & FROBISH, L.T. (1977a) Bio-
logical activity of glucose tolerance factor in swine. J.
anim. Sci., 45: 1341-1345.
STEELE, N.C., PELURA, I., & FROBISH, L.T. (1977b) Effect of
dietary protein source on glucose utilization and hepatic
insulin-receptor characteristics of the rat. Nutr. Rep. Int.,
15: 429-435.
STELLA, M., MENTALDI, A., ROSSI, R., ROSSI, G., & LEVIS, A.G.
(1982) Clastogenic effects of chromium on human lymphocytes
in vitro and in vivo. Mutat. Res., 101: 151-164.
STEFFEE, C.H. & BAETJER, A.M. (1965) Histopathologic effects
of chromate chemicals. Report of studies in rabbits, guinea-
pigs, rats, and mice. Arch. environ. Health, 11: 66-75.
STEINHOFF, D., GAD, S.C., HATFIELD, G.K., & MOHR, U. (1983)
Testing sodium dichromate and soluble calcium chromate for
carcinogenicity in rats. Report to the Industrial Health
Foundation, Wuppertal, Bayer AG Institute of Toxicology,
126 pp.
STEREKHOVA, N.P., ZELENEVA, N.I., SOLOMINA, S.N.,
TYUSHNYAKOVA, N.V., MYASNIKOVA, A.G., FOKINA, G.P., & YARINA,
A.L. (1978) [Gastric pathology in workers engaged in the
production of chromium salts.] Gig. Tr. prof. Zabol., 3: 19-23
(in Russian).
STERN, R.M. (1981) Process-dependent risk of delayed health
effects for welders. Environ. Health Perspect., 41: 235-253.
STERN, R.M. (1982) Chromium compounds: production and
occupational exposure. In: Langard, S., ed. Biological and
environmental aspects of chromium, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 5-47.
STRIK, J.J.T.W.A., DE IONGH, H.H., VAN RIJN VAN ALKEMADE,
J.W.A., & WIUTE, T.P. (1975) Toxicity of chromium (VI) in
fish, with special reference to organoweights, liver and
plasma enzyme activities, blood parameters, and histological
alterations. In: Koeman, S.H. & Strik, J.J.T.W.A., ed.
Sublethal effects of toxic chemicals on aquatic animals,
Amsterdam, New York, Oxford, Elsevier Science Publishers,
pp. 31-41.
STROUT, R.W. (1977) The relationship of abnormal circulating
insulin levels to atherosclerosis. Atherosclerosis, 27: 1-13.
SULLIVAN, R.J. (1969) Air pollution aspects of chromium and
its compounds, Raleigh, North Carolina, US Department of
Health, Education and Welfare, 75 pp (National Air Pollution
Control Administration Publication No. APTD 69-34).
SUSA, N., FURUKAWA, YO., & TSUBAKI, S. (1977) Comparative
cytotoxic effects of hexavalent and trivalent chromium
compounds on cultured cells. Jap. J. vet. Sci., 39: 325-336.
SUZUKI, K., ESASHI, T., SUZUE, R., NISHIMURA, H., & MATSUMOTO,
H. (1979) Tissue concentrations of cadmium, lead, and
chromium in Japanese ranging from the fetus to elder subjects.
Toxicol. Lett., 4: 481-484.
SWAINE, D.J. & MITCHELL, R.L. (1960) Trace element
distribution in soil profiles. J. soil Sci., 11 (2): 347-368.
SWENSSON, A. (1977) [Experimental research on the
fibrogenetic effect of chromite.] Arbete och Haelsa, 2: 1-14
(in Swedish).
TABOR, E.C. & WARREN, W.V. (1958) Distribution of certain
metals in the atmosphere of some American cities. Arch. ind.
Hyg., 17: 145-151.
TAMARO, M., BANFI, E., VENTURINI, S., & MONTI-BRAGADIN, C.
(1975) [Hexavalent chromium compounds are mutagens for
bacteria.] In: Proceedings of the XVII Congress Naz. Soc. It.
Microbiol., Padova, pp. 411-415 (in Italian).
TANDON, S.K., SAXENA, D.K., GAUR, J.S., & CHANDRA, S.W.
(1978) Comparative toxicity of trivalent and hexavalent
chromium. Environ. Res., 15: 90-99.
TANDON, S.K., BEHARI, J.R., & KACHRU, D.N. (1979) Distri-
bution of chromium in poisoned rats. Toxicology, 13: 29-34.
TANDON, S.K., MATHUR, A.K., & GAUR, J.S. (1977) Urinary
excretion of chromium and nickel among electroplaters and
pigment industry workers. Int. Arch. occup. environ. Health,
40: 71-76.
TATARU, C., MARINSECU, A., CAPUSAN, I., RIPAN, R., & LITEANU,
C. (1957) [Skin damage during the industrial recovery of
potassium dichromate.] Berufsdermatosen, 5: 283-295 (in
German).
TAYLOR, A.E., GUYTON, M.O., & BISHOP, V.S. (1965)
Permeability of the alveolar membrane to solutes. Circ. Res.,
16: 353-362.
TAYLOR, F.G., Jr, MANN, L.K., DAHLMANN, R.C., & MILLER, F.L.
(1975) Environmental effects of chromium and zinc in
cooling-water drift. In: Cooling tower environment, 1974,
Washington DC, US Energy Research and Development
Administration, pp. 408-426 (Report Conf. No. 740302).
TAYLOR, F.H. (1966) The relationship of mortality and
duration of employment as reflected by a cohort of chromate
workers. Am. J. public Health, 56: 218-229.
TAZELAAR, D.J. (1970) Hypersensitivity to chromium in a
light-blue Tattoo. Dermatologica (Basel), 141: 282-287.
THOMSON, A.G. (1982) Chromite, Mining Annual Review,
pp. 70-71.
THORMANN, J., JESPERSEN, N.B., & JOENSEN, H.D. (1979)
Persistence of contact allergy to chromium. Contact Dermatit.,
5: 261-264.
TOEPFER, E.W., MERTZ, W., ROGINSKI, E.E., & POLANSKY, M.M.
(1973) Chromium in foods in relation to biological activity.
J. agric. food Chem., 21: 69-73.
TOEPFER, E.W., MERTZ, W., POLANSKY, M.M., ROGINSKI, E.E., &
WOLF, W.R. (1977) Preparation of chromium-containing
material of glucose tolerance factor activity from brewer's
yeast extracts and by synthesis. J. agric. food Chem., 25:
162-166.
TOLA, S., KILPIO, J., VIRTAMO, M., & HAAPA, K. (1977)
Urinary chromium as an indicator of the exposure of welders to
chromium. Scand. J. Work environ. Health, 3: 192-202.
TOLG, G. (1974) Recent problems and limitations in the
analytical characterization of high-purity material. Talanta,
21: 327-345.
TSUDA, H. & KATO, K. (1976) Potassium dichromate: induced
chromosome aberrations and its control with sodium sulfite in
hamster embryonic cells in vitro. Gann, 67: 469-470.
TSUDA, H. & KATO, K. (1977) Chromosomal aberrations and
morphological transformation in hamster embryonic cells
treated with potassium dichromate in vitro. Mutat. Res., 46:
87-94.
TUMAN, R.W. & DOISY, R.J. (1977) Metabolic effects of the
glucose tolerance factor (GTF) in normal and genetically
diabetic mice. Diabetes, 26: 820-826.
TUMAN, R.W., BILBO, J.T., & DOISY, R.J. (1978) Comparison
and effects of natural and synthetic glucose tolerance factor
in normal and genetically diabetic mice. Diabetes, 27: 49-56.
TURNER, M.A. & RUST, R.H. (1971) Effects of chromium on
growth and mineral nutrition of soybeans. Soil Sci. Soc. Am.
Proc., 35: 755-758.
TYUSHNYAKOVA, N.V., ZISLIN, D.M., SHABYNINA, N.K., & BELJAEVA,
L.N. (1974) [Criteria of occupational cancer in the
production of chromium salts and some ideas concerning its
pathogenesis.] In: Occupational cancer (collection of
scientific works), Sverdlovsk, pp. 56-63 (in Russian).
UMEDA, M. & NISHIMURA, M. (1979) Inducibility of chromosomal
aberrations by metal compounds in cultured mammalian cells.
Mutat. Res., 67: 221-229.
URCHS, O. (1953) [Occupational skin diseases,] Cantor,
Aulendorf i. Württemberg, 108 pp.
US DHEW (1975) Criteria for a recommended standard.
Occupational exposure to chromium (VI), Washington DC, US
Department of Health, Education and Welfare, 200 pp (US DHEW
Publication No. NIOSH 76-129).
US DHEW (1977) Occupational diseases: a guide to their
recognition, revised ed., Washington DC, US Department of
Health, Education and Welfare, pp. 352-355 (Stock No.
017-033-00266-5).
US EPA (1978) Reviews of the environmental effects of
pollutants. III. Chromium, Washington DC, US Environmental
Protection Agency, 285 pp (ORNL/EIS-80; EPA 600/1-78-023).
US EPA (1980) Ambient water quality criteria for chromium,
Washington DC, US Environmental Protection Agency, pp. A1-C48.
US NAS (1974a) Chromium, Washington DC, US National Academy
of Sciences, 155 pp.
US NAS (1974b) Geochemistry and the environment. I. The
relation of selected trace elements to health and disease,
Washington DC, US National Academy of Sciences, 113 pp.
US NAS/NAE (1972) Water quality criteria 1972, Washington
DC, US Environmental Protection Agency, US National Academy of
Sciences, US National Academy of Engineering, 533 pp.
US PHS (1953) Health of workers in chromate producing
industry, Washington DC, US Department of Health, Education
and Welfare, US Public Health Service, 131 pp (Publication No.
192).
VAKHRUSHEVA, V.A. (1960) [Effect of Mn and Cr as trace
elements on the level of blood sugar in white mice.] Tr.
Izhevskogo Otd. Fiziol. Obschchestva, 1: 184-190 (in Russian).
VALER, M. & RACZ, J. (1971) Investigations concerning the
sensitizing effect of trivalent chromium salts. I. Correlation
between concentration and skin penetration of trivalent
chromium and between the number of positive results obtained
in patch tests. J. ind. Med., 41(12): 302-317.
VEILLON, C., WOLF, W.R., GUTHRIE, B.E. (1979) Determination
of chromium in biological materials by stable isotope
dilution. Anal. Chem., 51: 1022-1024.
VELICHKOVSKY, B.T. & POKROVSKAJA, L.V. (1973) [Labour
hygiene in a modern production of multy-tonnage ferrolloys.]
In: [Proceedings of the National Symposium on Labor Hygiene
and Prophylaxis of Occupational Diseases in the Production of
Ferroalloys,] Sverdlovsk, pp. 3-8 (in Russian).
VENITT, S. & LEVY, L.S. (1974) Mutagenicity of chromates in
bacteria and its relevance to chromate carcinogenesis. Nature
(Lond.), 250: 493-494.
VENCGUJIV, A.J. (1974) Zones of display of biological and
pharmacological action of trace elements. In: Hoekstra, W.G.,
Gantherl, H.E., & Mertz, W., ed. Trace elements metabolism in
animals. II, Baltimore, London, Tokyo, University Park Press,
pp. 295-310.
VERFAILLIE, G.R.M. (1974) Kinetics of chromium absorption by
intact rice plants. In: Comparative studies of food and
environmental contamination, Vienna, International Atomic
Energy Agency, pp. 315-331.
VERSIECK, J.M.J. & SPEECKE, A.B.H. (1972) Contaminations
induced by collection of liver biopsies and human blood. In:
Nuclear Activation Techniques in the Life Sciences.
Proceedings of a Symposium, Bled, Yugoslavia, Vienna
International Atomic Energy Agency, pp. 39-49.
VERSIECK, I., HOSTE, J., BARBIER, F., STEYAERT, H., DERUDDER,
I., & MICHELS, H. (1978) Determination of chromium and
cobalt in human serum by neutron activation analysis. Clin.
Chem., 24: 303-308.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experience of the
occupational health department with some industrial threshold
limit values for industrial poisons.] Arch. Gewerbepath.
Gewerbehyg., 13: 528-534 (in German).
VISEK, W.J., WHITNEY, I.B., KUHN, U.S.G., & COMAR, C.L.
(1953) Metabolism of 51Cr by animals as influenced by
chemical state. Proc. Soc. Exp. Biol. Med., 84: 610-615.
VOELKL, A. (1971) [The daily excretion of chromium in normal
persons.] Zentralbl. Arbeitsmed., 21: 122 (in German).
VOTAVA, H.J., HAHN, C.J., & EVAN, G.W. (1973) Isolation and
partial purification of a 51Cr complex from brewer's yeast.
Biochem. Biophys. Res. Comm., 55: 312-319.
WACKER, W.E.C. & VALLEE, B.L. (1959) Nucleic acids and
metals. I. Chromium mangenese, nickel, iron, and other metals
in ribonucleic acid from diverse biological sources. J. biol.
Chem., 234: 3257-3262.
WAHBA, A. & COHEN, T. (1979) Chrome sensitivity in Israel.
Contact Dermatit., 5: 101-107.
WAHLBERG, J.E. & WENNERSTEN, G. (1977) Light sensitivity and
chromium dermatitis. Br. J. Dermatol., 97: 411-416.
WALSH, E.N. (1953) Chromate hazards in industry. J. Am. Med.
Assoc., 153: 1305-1308.
WARREN, G., SCHULTZ, P., BANCROFT, D., BENNETT, K., ABBOTT,
E.H., & ROGERS, S. (1981) Mutagenicity of a series of
hexacoordinate chromium (III) compounds. Mutat. Res., 90:
111-118.
WATANABE, S. & FUKUCHI, Y. (1975) An epidemiological survey
on lung cancer in workers of a chromate-producing industry in
Hokkaido, Japan (Presented at: The XVIII International Congress
on Occupational Health, Brighton, England, 14-19 September.
WEILER, R.R. & CHAWLA, V.K. (1969) Dissolved mineral and
quality of Great Lakes waters. In: Proceedings of the 12th
Conference on Great Lakes Res., 801.
WELZ, B. (1980) [Use of AAS and ICP. Relative advantage of
each method.] Chem. Techn., 9: 161-170. (in German).
WELZ, B. (1983) [Atomic absorption spectrometry,] 3rd ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
527 pp (in German).
WENDELBERGER, J. & WEITGASSER, H. (1956) [The role of alkali
and degreasing agents in the development of contact eczemas in
electroplasters.] Berufsdermatosen, 4: 16-21 (in German).
WHANGER, P.D. & WESWIG, P.H. (1975) Effects of selenium,
chromium, antioxidants on growth, eye cataracts, plasma
cholesterol, and blood glucose in selenium-deficient, vitamin
E-supplemented rats. Nutr. Rep. Int., 12: 345-348.
WHITE, L.R., JAKOBSEN, K., & 0STGAAD, K. (1979) Comparative
toxicity studies of chromium rich welding fumes and chromium
on an established human cell line. Environ. Res., 20: 366-374.
WHITING, R.F., STICH, H.F., & KOROPATNICK, D.J. (1979) DNA
damage and DNA repair in cultured human cells exposed to
chromate. Chem.-biol. Interact., 26: 267-280.
WHO (1983) EHC 27: Guidelines on studies in environmental
e pidemiology, Geneva, World Health Organization, 351 pp.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984a)
Disposition of intratracheally administered chromium (III) and
chromium (VI) in rabbits. Toxicol. Lett., 22: 273-276.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984b) The
reduction of chromium (VI) to chromium (III) by glutathione:
an intracellular redox pathway in the metabolism of the
carcinogen chromate. Toxicology, 33: 341-348.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1985)
[Determination of chromium in human red blood cells. Basis for
a concept of biological monitoring.] Arbeitsmed. Sozialmed.
Praeventivmed., 20: 1-4 (in German).
WILD, D. (1978) Cytogenetic effects in the mouse of 17
chemical mutagens and carcinogens evaluated by the micronuclei
test. Mutat. Res., 56: 319-327.
WILLIAMS, C.D. (1969) Asthma related to chromium compounds.
Report of two cases and review of the literature on chromate
diseases. NC med. J., 30: 482-491.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1960) The Merck index. An encyclopedia of chemicals and
drugs, 7th ed., Rahway, New Jersey, Merck and Co., 139 pp.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) The Merck index. An encyclopedia of chemicals and
drugs, 9th ed., Rahway, New Jersey, Merck and Co., 1313 pp.
WINSTON, J.R. & WALSH, E.N. (1951) Chromate dermatitis in
railroad employees working with diesel locomotives. J. Am.
Med. Assoc., 147: 1133-1134.
WITHEY, J. (1983) Memorandum addressed to Si Duk Lee, dated
March 4, 1983, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office.
WOLF, W.R. (1976) Coupled gas chromatography/atomic
absorption spectrometry for the nanogram determination of
chromium. Anal. Chem., 48: 1717-1720.
WOLF, W.R. & GREENE, F.E. (1976) Preparation of biological
materials for chromium analysis. In: Accuracy in trace
analysis: sampling, sample handling, and analysis,
Gaithersburg, Maryland, National Bureau of Standards, pp.
605-610 (Special Publication No. 422).
WOLF, W.R., MERTZ, W., & MASIRONI, R. (1974) Determination
of chromium in refined and unrefined sugars by oxygen plasma
ashing flameless atomic absorption. J. agric. food Chem., 22:
1037-1042.
WOLF, W.R., TAYLOR, M.L., HUGHES, B.M., TIERNAN, T.O., &
SIEVERS, R.E. (1972) Determination of chromium and beryllium
at the picogram level by gas chromatography/mass spectrometry.
Anal. Chem., 44: 616-618.
WU, G.-Y. (1981) [The research on the purification and the
chemical structure of the specific chromium binding
materials.] Jpn. J. Ind. Health, 33: 505 (in Chinese).
YAGI, T. & NISHIOKA, H. (1977) DNA damage and its degrada-
tion by metal compounds. Sci. Eng. Rev. Doshisha Univ., 18:
63-70.
YANG, G.L. (1956) [An investigation of occupational disease
among chromium plating workers.] Acta Acad. Med. primae
Shanghai, 2: 36-46 (in Chinese).
YOPP, J.H., SCHMID, W.E., & HOLST, R.W. (1974) Determination
of maximum permissible levels of selected chemicals that exert
toxic effects on plants of economic importance in Illinois,
Chicago, Illinois, Institute for Environmental Quality,
pp. 89-96.
YUNISOVA, H.K. & PAVLOVSKAJA, G.S. (1975) The effect of
regimen of chrome plating on the properties of chromine.
Protect. Metal., 2, 248-250.
ZANDER, A.T., O'HAVER, T.C., & KELIHER, P.N. (1977)
Correction for interferences of spectral origin with continuum
source, echelle wavelength modulated atomic absorption
spectrometry. Anal. Chem., 49: 838-842.
ZHURKOV, U.S. (1981) [Approaches to the regulation of
environmental chemical pollutants with mutagenic activity.]
In: Sidorenko, G.I.M., ed. [Medical problems of environmental
protection,] Moscow, USSR Academy of Medical Sciences,
pp. 88-95 (in Russian).
ZOBER, A. (1979) [On the problems of evaluating bronchial
carcinoma after exposition to chromium compounds.] Int. Arch.
occup. environ. Health, 43(2): 107-121 (in German).
ZOBER, A. (1982) [Possible dangers to the respiratory tract
from welding fumer: methods of approach in an industrial
health care context and results.] Schweissen & Schneiden,
34(2): 77-81 (in German).
ZOBER, A., KICK, K., SCHALLER, K.H., SCHELLMANN, B., &
VALENTIN, H. (1984) ["Normal values" of chromium and nickel
in human lung-, kidney-, blood-, and urine samples.] Zbl.
Bakt. Hyg. I. Abt. Orig. B, 179: 80-95 (in German).
1969). For these reasons, chromium must be considered an essential
micro-nutrient. It is physiologically active in the trivalent
oxidation state at concentrations of approximately 100 µg/kg diet.
7.1.1. Effects of deficiency on glucose metabolism
Semipurified rations, containing Torula yeast as the source of
protein, were fed to groups of 10 male Sprague Dawley rats at
weaning, and intravenous glucose tolerance tests were performed by
injecting 1250 mg glucose/kg body weight and measuring the
subsequent decline in blood-glucose levels. The rate of glucose
disappearance can be calculated from the straight line plot of log
increment glucose (excess of glucose at any time (t) over fasting
glucose), versus time. The disappearance constant k is expressed
as % decline of the increment glucose per min; it is a measure of
the efficiency of glucose utilization. In weaning rats, the rate
constant was found to decline, within 3 weeks or less, from an
average of 4%/min to 2.6%/min in animals fed the Torula yeast diet,
but not in animals administered a diet in which 4 or 8% of Torula
yeast was replaced by an equal amount of brewer's yeast. This
observation suggested that brewer's yeast, but not Torula yeast,
contained an unknown substance necessary for the maintenance of
normal glucose tolerance. Because the only known effect of the
substance was that on glucose tolerance, it was named "Glucose
Tolerance Factor" (GTF) (Mertz & Schwarz, 1959). GTF was extracted
from brewer's yeast and pork kidney powder, concentrated and
purified, and its active ingredient was identified as trivalent
chromium (Schwarz & Mertz, 1959). Chromium in the form of most
common complexes (except for very stable ones) cured the impairment
of glucose tolerance in deficient rats, either as one oral dose of
200 µg/kg body weight, or as an intravenous (iv) injection of 2.5
µg/kg body weight. Chromium in the diet also prevented the
impairment of glucose tolerance.
In order to produce more pronounced deficiencies of chromium
and other trace elements, Schroeder et al. (1963) constructed a
special animal house on a mountain top in Vermont, USA, far removed
from traffic and industry. The interior was specially coated with
organic resins to reduce any metallic exposure. Air was introduced
through special filters, and strict practices were enforced to
avoid the introduction of dust and dirt. This will be referred to
as the "controlled environment". Under these conditions, a more
severe chromium deficiency resulted in very low glucose removal
rates of 1.12%/min in 6 rats and of 0.18%/min in 4 female breeder
rats (482 days old), after 11 days on a chromium-deficient diet.
The removal rate in 4 control rats receiving the same diet with a
chromium supplement improved from 0.51 to 1.39% during the same
period (Mertz et al., 1965b). This strong impairment of glucose
tolerance in rats kept in the "controlled environment" was
reflected in their fasting blood-glucose levels, compared with
those of chromium-supplemented controls: 1370 ± 68 versus 1170 ± 17
mg/litre in males and 1390 ± 68 versus 960 ± 65 mg/litre in
females. More than half of 185 deficient rats excreted more than
0.25% glucose in the urine, whereas glycosuria was found in only 9
of the chromium-supplemented controls (Schroeder, 1966). A
significant reduction in the intravenous glucose removal rate was
also observed in 8 rats in plastic cages, fed an EDTA-washed low-
protein diet (7% casein), compared with 8 controls receiving 50 µg
chromium as the chloride, by stomach tube, daily for 2 weeks (2.1
versus 3.6%/min; P < 0.01). However, chromium supplements did not
improve the near-normal removal rates in rats receiving a 20%
casein diet (Mickail et al., 1976). Significantly lower plasma-
glucose levels, due to supplementation with chromium, were reported
in rats fed the chromium-deficient Torula yeast diet (Whanger &
Weswig, 1975). In another series of studies, only a slight
reduction in blood-glucose (from 1240 to 1180 µg/litre) was found
in 10 rats receiving a diet supplemented with chromium (10 µg/kg)
(Djahanshiri, 1976).
Impaired glucose tolerance in squirrel monkeys, fed a
commercial laboratory chow, was shown to respond to chromium
supplementation. Of 9 monkeys with an impaired glucose removal
rate (1.38%/min), 8 responded after 22 weeks of supplementation of
their drinking-water (chromium acetate, 10 mg/litre) with a
normalization of their glucose tolerance (average removal rate, N =
9, 2.33 ± 0.3%/min) (Davidson & Blackwell, 1968). There was no
effect on food consumption, growth rate, or serum-insulin
concentrations. The chromium content of the commercial chow was
stated to be 3.3 mg/kg, a very high concentration. In view of the
uncertainties of methods of analysis for chromium (section 2.2), it
is not possible to interpret the results as indicative of a very
high chromium requirement of the squirrel monkey or of an unusually
poor bioavailability of the chromium in that particular ration. A
marginal chromium deficiency may have existed in mice fed a bread
and milk diet, as daily administration of 10 µg chromium for 16
days to 8 months produced a 10 - 30% decline in blood-glucose
levels (Vakhrusheva, 1960). However, this effect was not specific
for chromium, as it was also observed when manganese was
administered.
The glucose tolerance of guinea-pigs did not differ
significantly between groups fed diets containing chromium at
0.125, 0.625, or 50 mg/kg, even though the animals fed the 2 higher
levels exhibited a lower mortality rate during pregnancy (Preston
et al., 1976).
Although the administration of synthetic glucose tolerance
factor (a chromium-dinicotinic acid-glutathione complex) to 6 pigs
did not affect glucose tolerance tests, it resulted in a
significant increase in the hypoglycaemic effect of insulin
injected at 0.1 U/kg body weight (Steele et al., 1977a).
Turkey poults, fed a practical ration containing chromium
levels of 5 mg/kg, responded to chromium supplementation (20 mg/kg
diet) with a significant increase in liver glycogen and in glycogen
formation, following a fast, and with a significant increase in
glycogen synthetase (EC 2.4.1.11) activity in the liver (Rosebrough
& Steele, 1981).
It can be concluded that the impairment of glucose tolerance in
rats fed a low-chromium Torula yeast diet is due to chromium
deficiency. The effects of chromium in squirrel monkeys, pigs, and
turkeys, though statistically significant, are somewhat difficult
to interpret, because of the reported high chromium content of the
basal diet. No evidence for chromium deficiency has yet been
obtained through glucose tolerance tests on other animal species.
7.1.2. Effects of deficiency on lipid metabolism
Though trivalent chromium in high doses (2.5 mg/kg body weight)
has been shown to increase the synthesis of fatty acids and
cholesterol in the liver (Curran, 1954), lower, physiological doses
appear to decrease serum-cholesterol concentrations in rats.
Schroeder & Balassa (1965) found an average level of 927 mg
cholesterol/litre serum in 24- to 26-month-old male rats, kept in a
controlled environment and administered chromium in the drinking-
water at a concentration of 5 mg/litre, compared with an average of
1229 mg/litre in controls not receiving chromium ( P < 0.01). The
effects in female rats were ambigous, one study producing the
expected reduction in cholesterol due to chromium, another showing
an elevation in the supplemented female rats. Schroeder's
observations of a cholesterol-reducing effect of chromium in male
rats were confirmed by Staub et al. (1969) and by Whanger & Weswig
(1975) but were contradicted by results of a third study in which
there were not any significant effects of chromium on sucrose-
induced triglyceridaemia and cholesterolaemia (Bruckdorfer et al.,
1971). Perhaps more significant than the effect on circulating
cholesterol is the direct effect of chromium on the occurrence of
aortic plaques. Schroeder & Balassa (1965) observed 6 plaques in 54
male and female chromium-deficient rats, but only one plaque in 48
animals receiving 5 mg chromium/litre in drinking-water. These
results are in agreement with those from a subsequent report of the
protective effect of the natural chromium content of water (60 -
215 µg/litre) against atherosclerosis in cholesterol-fed rabbits
(Novakova et al., 1974). Abraham et al. (1980) extended these
observations by demonstrating that daily chromium injections (20 µg
K2CrO4) reversed the established atherosclerosis in the aorta of 11
cholesterol-fed rabbits, compared with 12 controls. The mean
plaque area was reduced from 95% to 63%, the total aortic
cholesterol from 729 mg to 458 mg, and the atheromatous lesions, as
measured by technetium incorporation from 285 000 cpm to 114 000
cpm, all differences being statistically significant. These
results are reinforced by observations in man discussed in section
8.1.
7.1.3. Effects of deficiency on life span, growth, and
reproduction
The mortality of male, but not of female mice, raised in a
"controlled environment" (section 7.1.1) was reduced by trivalent
chromium administered as acetate in the drinking-water at a
concentration of 5 mg/litre (Schroeder et al., 1964). The survival
rates at 12 months were 92.6% and 68.8% ( P < 0.0001) in
supplemented and deficient animals, respectively. Similary, male,
but not female, rats receiving a chromium concentration of 5
mg/litre in the drinking-water had longer life spans than deficient
controls. The mean age of the last surviving 10% of animals was
1249 days, compared with 1141 days in the deficient animals ( P <
0.01). Survival of male rats fed a low-chromium (< 100 µg/kg),
low-protein ration and subjected to a controlled acute haemorrhage
was significantly less than that of chromium-supplemented rats, in
2 studies (67 versus 92%; P < 0.05 and 27 versus 60%; P < 0.01,
respectively) (Mertz & Roginski, 1969).
In Schroeder's study, growth rates in treated mice and rats of
both sexes raised in a "controlled environment", were higher after
6 and 12 months, with highly significant differences in body weight
ranging from 9 to 17% ( P < 0.005) compared with the controls.
Again, the effects of chromium supplementation were greater in
males than in females (Schroeder et al., 1964, 1965).
Similar results were reported by Djahanschiri (1976), who
studied a total of 2750 rats of a special inbred strain (Hk51) fed
a basal diet (0.15 mg chromium/kg diet) and chromium supplements
ranging from 10 to 500 mg/kg diet. At 12 weeks, the average
weights of the chromium-supplemented animals, regardless of dose
level, were significantly higher (by 6% in the males and 3% in the
females) than those of the animals on the basal diet. The same
author reported a progressive diminution in both the milk
production of lactating rats and weight gain in 3 consecutive
generations fed the low-chromium diet, compared with rats receiving
chromium supplementation. Increased mortality was reported in
pregnant guinea-pigs fed a low-chromium diet (125 µg/kg diet)
compared with animals receiving a chromium supplement of either 625
µg/kg or 50 mg/kg (Preston et al., 1976).
When rats raised on a low-chromium Torula yeast diet (< 100
g/kg) mated with those on a normal diet, they were able to
impregnate the females at a 100% conception rate only up to the age
of 4 months. After this age, the conception rate declined to 25%,
25%, and 0%, at the age of 7, 8, and 9 months, respectively. This
decline was accompanied by a significant ( P < 0.01) decrease in
the sperm count in the chromium-deficient males to approximately
half of the count in supplemented controls at the age of 8 months
(Anderson & Polansky, 1981).
7.1.4. Other effects of deficiency
Male weanling rats, fed a 10% soya protein ration with a
chromium content of less than 100 µg/kg, developed a visible
opacity of the cornea in one or both eyes. In several studies, the
incidence of this effect ranged from 10 to 15% in deficient rats.
No opacities developed in control animals receiving 2 mg
chromium/kg diet (Roginski & Mertz, 1967).
Chromium deficiency has been shown to reduce the physical
performance of rats under stress. Ten male rats raised on a
chromium-deficient diet (150 µg/kg diet) swam for an average of 250
min, until exhaustion, in contrast with 10 rats receiving a
supplement of 10 mg chromium/kg diet, which were exhausted only
after 320 min (Djahanschiri, 1976).
7.1.5. Mechanism of action of chromium as an essential nutrient
7.1.5.1. Enzymes, nucleic acids, and thyroid
Chromium is present in nucleic acids in very high
concentrations, but the function of these is not clear at present
(Mertz, 1969). However, recent work suggests a biological function
of chromium in nucleic acid metabolism (Okada et al., 1984).
Ribonucleic acid synthesis in mouse liver was significantly
increased by as little as 1 µmol trivalent chromium, in the
presence of DNA or chromatin (Okada et al., 1981). These effects
were also present when the DNA or chromatin were first complexed
with chromium prior to incubation. However, prior complexation of
RNA polymerase with chromium depressed activity. These effects
were obtained in vitro with a concentration (52 µg/litre) that is
similar to physiological levels. Goncharov (1968) presented data
suggesting that chromium is involved in the function of the thyroid
gland. These findings have been supported by Lifschitz et al.
(1980).
An oligopeptide with a relative molecular mass of 1480, which
was crystallized from liver tissue, had a specific affinity for
chromium (Wu, 1981).
7.1.5.2. Interaction of chromium with insulin
The interaction of chromium with insulin has been extensively
studied and can therefore be presented in some detail, but this
does not imply that this is the only, or the most important,
function of chromium.
The effects of chromium in vitro, and probably in vivo, depend
on the presence of endogenous or exogenous insulin, no effects
having been demonstrated in in vitro systems that did not either
depend on, or contain, insulin. Chromium deficiency causes an
impaired response to added insulin in rat epididymal fat tissue,
and, when glucose uptake or glucose oxidation or utilization for
lipid synthesis is measured, the dose-effect curve is flat.
Addition of suitable chromium compounds significantly increases the
slope of the curve (Fig. 3). This demonstrates the true
potentiation of the insulin action and indicates that chromium
alone does not act as an insulin-like substance (Mertz et al.,
1961; Mertz & Roginski, 1971; Mertz, 1981). Chromium was also
shown to stimulate the transport of D-galactose into epididymal fat
cells. This suggests cell transport, the first step of sugar
metabolism, as a major site of action for chromium (Mertz &
Roginski, 1963). Insulin-potentiating effects have also been
observed on the swelling of liver mitochondria (Campbell & Mertz,
1963) and on glucose utilization in isolated rat lens (Farkas &
Roberson, 1965).
Fig. 3
Stimulation of the effects of insulin has been observed in a
glucose-independent, but insulin-responsive, system. Significantly
more alpha-amino isobutyric acid (a non-metabolizable amino acid
analogue) was incorporated into the heart and liver tissue of
chromium-supplemented male rats than in the tissues of chromium-
deficient controls, in response to the in vivo injection of the
labelled analogue and insulin (Roginski & Mertz, 1969).
These observations suggest a peripheral action of chromium to
facilitate the action of insulin; no evidence has been produced
indicating that chromium plays any role in the production, storage,
or release of insulin by the pancreas. Thus, the primary result of
chromium deficiency is a diminution in the effectiveness of
insulin. The resulting metabolic impairment may be compensated for
by increased insulin production in some cases, resulting in
elevated concentrations of the hormone, but not enough data exist
from experimental animal studies to assess the action of the
element on insulin metabolism. More information is available for
human subjects and this is discussed in section 8.1. The
interaction between chromium, insulin, and receptor sites of liver
mitochondrial membranes was studied using polarographic techniques.
The results formed the basis for the hypothesis that chromium may
facilitate bond formation between the intra-chain disulfide of
insulin and sulfur-containing groups of the receptors, by
participating in a ternary complex (Christian et al., 1963).
This hypothesis is consistent with results of studies on rats
fed, either a low-chromium Torula yeast diet or a brewer's yeast
diet known to be adequate in chromium. While the insulin-binding
capacity of hepatocytes was not significantly different, the
insulin affinity of the cells was significantly greater ( P < 0.01)
for the chromium-adequate rats than for the deficient Torula yeast
rats (Steele et al., 1977b).
7.1.6. Chromium nutritional requirements of animals
In the preceding sections, studies were evaluated in which the
effects of chromium supplementation were determined in animals that
were at least marginally chromium deficient. In other studies, the
effects of chromium were investigated in animal systems in which
the existence of chromium deficiency was either not ascertained or
not investigated. Before these studies are described and
interpreted for the determination of chromium nutritional
requirements of animals, it is helpful to consider them against the
background of Venchikov's (1974) model. This model is generally
applicable to trace element effects defining 3 zones of action, the
zone of biological action, that of pharmacodynamic action, and that
of toxicity (Fig. 4). The biological zone, in response to
supplements with low amounts of an element, represents the
correction of a deficiency and the resulting level of biological
activity is that of optimal function. Increasing the amount of
supplement further may lead to a certain depression, followed by a
zone of new, increased activity, in which the element no longer
acts as an essential nutrient, but as a drug. Still greater
supplements, beyond the homeostatic control capability of the
organism produce toxic effects and death. Because all the studies
described subsequently involved amounts of chromium supplements
that were higher than the levels normally needed to correct a
deficiency, it is possible, according to Venchikov's definition,
that the observed effects might be pharmacological.
Fig. 4
Tuman & Doisy (1977) studied the effects of yeast concentrates
of high chromium (glucose tolerance factor) content and of
synthetic chromium complexes with GTF activity (Tuman et al., 1978)
in mice, raised on a presumably complete commercial stock diet.
Six animals were used for each test, either genetically diabetic
mice or their control litter mates of the C57Bl-KSI strain.
Injections of either 5 mg of the GTF-containing yeast extracts or
0.1 mg of the synthetic chromium complex, acutely reduced the
elevated plasma-glucose levels in the diabetic and the non-fasting
normal mice by 10 - 38% of the initial values ( P < 0.01) and the
plasma-triglycerides by 26 - 56% ( P < 0.01), compared with control
mice injected with saline. Injection of insulin into diabetic mice
produced only an 11 - 18% decrease in plasma-glucose levels,
whereas injection of the GTF-containing extract together with
insulin reduced plasma-glucose levels by 39 - 51% and plasma-
triglyceride levels by 76% (Table 11).
The results suggest either a much higher increase in the
chromium requirement of the genetically diabetic mice or their
inability to use chromium in the diet.
Table 11. Acute effects of GTF and exogenous insulin on non-
fasting plasma-glucose and plasma-triglyceride (TG) concentrations
in 19-week-old genetically diabetic micea
------------------------------------------------------------------
Treatment Plasma- deltaGlucose Plasma- deltaTG
glucoseb triglyceridesb
(mg/litre) (mg/litre)
------------------------------------------------------------------
saline 11 120 ± 3960 ± 160(5)c
490(5)c
GTF 9320 ± -180 (16%) 2790 ± 170(5)d -117 (30%)
280(5)d
insulin 9840 ± -128 (12%) 3020 ± 400(5) - 94 (24%)
410(5)
insulin 7060 ± -406 (37%) 940 ± 220(5)e -302 (76%)
and GTF 840(5)e
------------------------------------------------------------------
a Modified from: Tuman & Doisy (1977).
b Glucose and triglyceride values represent mean ± SEM for 6 mice in
each treatment group. Dose of GTF was 5 mg (WL-10-AT) administered
intraperitoneally, 12 h prior to collection of blood. Lente insulin
(0.1 U per mouse) was administered subcutaneously, 12 h prior to
collection of blood. Data were treated by analysis of variance to
detect differences between the various treatment groups; independent
orthogonal comparisons were performed in the following groups ( P value
indicates level of significance for each comparison).
c Saline versus all other treatments, P < 0.005.
d GTF versus insulin, P < 0.05.
e GTF and insulin alone versus combined GTF and insulin, P < 0.005.
Thus, GTF and insulin > GTF = insulin > saline.
Steele & Rosebrough (1979) reported a significant stimulation
of the growth rate of one-week-old turkey poults (both sexes) by
supplementation of a practical ration with 20 mg chromium (as
chloride)/kg. The weight gains within the 2-week study were 235 g
and 267 g for the 60 controls and 60 supplemented turkeys,
respectively ( P < 0.001). As the practical ration contained
ground yellow corn and soybean meal, limestone, and dicalcium
phosphate, chromium deficiency would appear unlikely. The amount
of the chromium supplement (20 mg/kg diet) is quite high, and
further studies are needed to decide whether the observed effects
were of a pharmaco-dynamic nature or truly nutritional, i.e.,
correcting a deficiency. A similar interpretation should be
applied to a report of improved egg quality in the laying hen
(Jensen et al., 1978).
The quantitative aspects of the effects of chromium on animals
can be summarized as follows: normal rats fed semi-purified, semi-
synthetic rations, with Torula yeast or individual proteins and
sucrose or starch as the source of carbohydrates, develop mild
signs of deficiency at a dietary level of 100 - 150 µg chromium/kg.
To prevent deficiency, most authors used very high supplements of
several mg/kg diet and did not determine the biological
availability of the chromium complexes used for the
supplementation. Diets containing raw ingredients and supplying
chromium levels between 0.5 and 1 mg/kg do not induce signs of
deficiency and probably meet the requirement of the rat. A very
tentative estimate of the dietary chromium need of the rat and
probably the mouse would be approximately 0.5 mg/kg diet. This
estimate should be interpreted with caution, because of the lack of
knowledge concerning the biological availability of chromium and of
its interaction with dietary constituents.
7.2. Toxicity Studies
The toxicology of chromium compounds has been reviewed by the
US National Academy of Science (US NAS, 1974a), Langard & Norseth
(1979), the International Agency for Research on Cancer (IARC,
1980), Langard (1980a, 1982), and Burrows (1983).
In discussing toxicological problems, it is important to
differentiate between the various oxidation states of chromium and
its compounds. Trivalent chromium, when administered to animals in
food or water, does not appear to induce any harmful effects, even
when given in large doses (US NAS, 1974a) (section 7.2.1). Acute
and chronic toxic effects of chromium are mainly caused by
hexavalent compounds. Since it has been shown that both industrial
trivalent chromium compounds as well as reagent-grade trivalent
chromium compounds can be contaminated by hexavalent chromium
(Petrilli & DeFlora, 1978a; Levis & Majone, 1979), the evaluation
of experimental studies becomes difficult, especially when the
purity of the chemical compounds used is not known.
Discrimination between the biological effects, caused by
hexavalent chromium and trivalent chromium is difficult, because,
after penetration of membranes in tissues, hexavalent chromium is
immediately reduced to trivalent chromium (Gray & Sterling, 1950;
US NAS, 1974a), and it is not evident whether the observed
phenomena are caused by this reduction or even by the trapping of
trivalent chromium by ligands after uptake in the cells. Another
problem in evaluating the data is associated with the route of
administration. Hexavalent chromium, introduced by the oral route,
is partly reduced to trivalent chromium by acidic gastric juice
(Donaldson & Barreras, 1966; DeFlora & Boido, 1980); thus, the
effects or lack of effects observed may be caused mainly by
trivalent chromium and not by the hexavalent chromium, actually
administered.
7.2.1. Effects on experimental animals
Many local effects on human beings have been reported (section
8.3), but only a few studies have verified these effects in
experimental animals. A comprehensive survey of hexavalent
chromium-induced effects is given in Table 12 (US NAS, 1974a). For
most studies, details were not given of the length of exposure,
number of treated animals and controls, etc. Diagnoses were stated
without presenting all the original data. Thus, in this section,
some papers will be discussed that refer to the most prominent
local and systemic effects to support and clarify the effects shown
in human beings.
It is evident that the toxicity of hexavalent chromium in
animals varies with the route of entry into the body. Low
concentrations of hexavalent chromium may be tolerated, when
administered in the feed or drinking-water, the extent of
absorption being a factor of importance. For example, rats
tolerated hexavalent chromium in drinking-water at 25 mg/litre, for
1 year, and dogs did not show any effects from chromium
administered as potassium chromate at 0.45 - 11.2 mg/litre over a
4-year period (US NAS, 1974a). However, oral exposure of both male
and female rabbits to sodium dichromate (0.1% solution, 0.2 - 5
mg/kg body weight, for up to 545 days) resulted in significant
morphological changes in the gonads, including atrophy of the
epithelium and dystrophic alterations of the Sertoli and Leydig
cells in the testes, and sclerotic and atrophic changes in ovaries
(Kucher, 1966).
Larger doses of hexavalent chromium are highly toxic and may
cause death, especially when injected iv, subcutaneously (sc), or
intragastrically. The LD50 of chromium compounds was determined
for several experimental animal species. The LD50 of potassium
dichromate (hexavalent chromium), administered orally (stomach
tube) to rats, was 177 mg/kg body weight in males and 149 mg/kg
body weight in females (Hertel, 1982). When injected iv in mice
(sex not given), the LD50 of chromium carbonyl was 30 mg/kg body
weight (IARC, 1980).
Performing a life-time inhalation study on the rat, Glaser et
al. (1984) found an LC50 for Na2Cr2O7 of 28.1 mg/m3 (range, 16.7 -
47.3 mg/m3). Assuming a deposition rate in the lung of 30% of the
dose administered, the LC50 dose was 1 mg/kg body weight in male
and 1.2 mg/kg in female rats.
A local corrosive action of hexavalent chromium on the skin,
similar to that seen in man, was described by Samitz & Epstein
(1962), who induced chrome ulcers in guinea-pigs at 4 trauma sites,
with daily exposure to 0.34 MK2Cr2O7 solution for 3 days. Mosinger
& Fiorentini (1954) showed the same effects using potassium
chromates.
Table 12. Effects of hexavalent chromium in animalsa
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit, inhalation chromates 1 - 50 mg/m3 14 h/day for pathological Lukanin (1930)
cat 1 - 8 months changes
in the lungs
Rabbit inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day none Lehmann (1914)
as dichromate for 5 days
Cat inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day bronchitis, Lehmann (1914)
as dichromate for 5 days pneumonia
perforation of
nasal septum
Mouse inhalation mixed dust 1.5 mg/m3 4 h/day, no tumours Baetjer et al.
containing as CrO3 5 days/week, (1959a);
chromates for 1 year Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 16 - 27 mg/m3 1/2 h/day tumours in Baetjer et al.
containing as CrO3 intermit- some strains (1959a);
chromates tently Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 7 mg/m3 37 h over increased Baetjer et al.
containing as CrO3 10 days tumour rate (1959a);
chromates Steffee &
Baetjer (1965)
Rat inhalation mixed dust 7 mg/m3 37 h over barely Baetjer et al.
containing as CrO3 10 days toleratedb (1959a);
chromates Steffee &
Baetjer (1965)
Rabbit, inhalation mixed dust 5 mg/m3 4 h/day, none marked Baetjer et al.
guinea- containing as CrO3 5 days/week, (1959a);
pig chromates for 1 year Steffee &
Baetjer (1965)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rat, inhalation hexacarbonyl 1.6 mg/m3 4 months, anaemia; lipid Roschina (1976)
rabbit 4 h, 5 days and/or protein
a week dystrophia
in liver
and kidneys
Rat, inhalation hexacarbonyl 0.16 mg/m3 4 months, anaemia; no Roschina (1976)
rabbit 4 h, 5 days biochemical
a week or morphological
effects
Rat inhalation hexacarbonyl 35 mg/m3 30 min 100% death Roschina (1976)
Rat inhalation dichromates 0.006 - 0.2 28 days/ increase in Glaser et al.
mg/m3 90 days lung-macrophages (1985)
23 h/day
7 days/week lymphocytes
immunoglobulin,
reduced Fe2O3
lung clearance
Rat intratra- dichromates 5 per week up to 30 toleratedc Steinhoff
cheal in- 0.01 - 0.25 months et al. (1983)
stillation mg/kg
Rat intratra- dichromates 1 per week up to 30 tolerated; Steinhoff
cheal in- 0.05 - 1.25 months 1.25 mg/kg et al. (1983)
stillation mg/kg harmful
Rat oral potassium 500 mg/litre daily maximal Gross & Heller
chromate in non-toxic (1946)
drinking-water concentration
Rat, oral zinc chromate 10 g/kg daily maximal Gross & Heller
mouse in feed non-toxic (1946)
(mature) concentration
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit oral sodium di- 0.2 - 5.0 mg/kg 545 days morphological Kucher (1966)
chromate changes in
gonads (testes:
atrophy of
epithelium,
dystrophic
alterations of
Sertoli &
Leydiz alls.
Ovaries:
sclerotic and
atrophic
changes)
Rat oral zinc chromate 1.2 g/kg daily maximal Gross & Heller
(young) in feed non-toxic (1946)
concentration
Rat oral potassium 1.2 g/kg daily maximal Gross & Heller
(young) chromate non-toxic (1946)
in feed concentration
Dog, oral monochromate 1.9 - 5.5 mg 29 - 685 none harmful Lehmann (1914)
cat, or dichromates chromium/kg days
rabbit body weight per
day 1 mg chrom-
ium equivalent
to 2.83 mg
K2Cr2O7
or 3.8 mg
K2CrO4
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog oral potassium 1 - 2 g as daily fatal in 3 Brard (1935)
dichromate chromium months anaemia
Dog stomach potassium 1 - 10 g as - rapidly fatald Brard (1935)
tube dichromate chromium
Monkey subcutan- potassium 0.02 - 0.7 g in - fatald Hunter & Roberts
eous dichromate 2% solution (1933)
Dog subcutan- potassium 210 mg as - rapidly fatal Brard (1935)
eous dichromate chromium
Guinea- subcutan- potassium 10 mg - lethald Ophüls (1911a)
pig eous dichromate Ophüls (1911b)
Rabbit subcutan- potassium 1.5 cc of 1% - 80% fatald Hasegawa (1938)
eous dichromate solution/kg body
weight
Rabbit subcutan- potassium 20 mg - lethald Ohta (1940)
eous dichromate
Rabbit subcutan- potassium 0.5 - 1 cc of - nephritisd Ohta (1940)
eous dichromate 0.5% solution/kg
body weight
Rabbit, subcutan- sodium 0.1 - 0.3 g as - rapid deathd Priestley
guinea- eous or chromate CrO3 fall in blood (1877)
pig intravenous pressure
Rabbit intra- potassium 0.7 cc of 2% - lethald Mazgon (1932)
venous solution/kg 8-10 days after
body weight injection
Dog intra- potassium 10 grains - instant death Gmelin (1826)
venous chromate
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog intra- potassium 1 grain - none marked Gmelin (1826)
venous chromate
Dog intra- potassium 210 mg as - rapidly fatal Brard (1935)
venous dichromate chromium
Dog intra- potassium 3 mg/100 cc 2 doses marked renal Hepler & Simonds
venous dichromate blood per dose damage (1946); Simonds
& Hepler (1945)
---------------------------------------------------------------------------------------------------------
a Modifed from: US NAS (1974a).
b Pathological changes in experimental and control rats, 101 weeks after exposure.
c The same weekly dose distributed over 5 days was clearly better tolerated than a single weekly
administration.
d Renal damage.
Following parenteral administration, the most common systemic
effects of chromium were parenchymatous changes in the liver and
kidney (Mosinger & Fiorentini, 1954). Later studies showed
selective damage in the renal proximal convoluted tubules, without
evidence of glomerular damage, as demonstrated after one single sc
injection of potassium dichromate of 10 mg/kg body weight (Schubert
et al., 1970) or after one single intraperitoneal (ip) injection of
sodium chromate of 10 or 20 mg/kg body weight (Evan & Dail, 1974).
Effects have also been found in fish (Strik et al., 1975). After 32
days of continuous exposure to 0.1, 1, or 10 mg hexavalent chromium
(as potassium dichromate)/litre, the fish Rutilus rutilus developed
lysis of the intestinal epithelium with haemorrhages as well as
hypertrophy and hyperplasia of the gill epithelium.
Franchini et al. (1978) found an increase in urinary protein,
lysozyme, glucose, and beta-glucuronidase in rats after a single sc
injection of potassium dichromate at 15 mg/kg body weight. After
sc injection (3 mg/kg body weight), every other day for 2 - 8
weeks, the authors observed a correlation between the chromium
contents of the renal cortex and chromium clearance.
Five-week-old male Wistar rats of the strain TNO-W-74 were
continuously exposed in inhalation chambers to submicron aerosols
of sodium dichromate at concentrations ranging from 25 (low level)
to 200 µg chromium/m3 (high level) (Glaser et al., 1985). Exposure
for 28 days to 25 or 50 µg chromium/m3 resulted in "activated"
alveolar macrophages with stimulated phagocytic activity, and
significantly elevated antibody responses to injected sheep red
blood cells. After 90 days of low-level exposure, there was a more
pronounced effect on the activation of the alveolar macrophages,
with increased phagocytic activity. However, inhibited phagocytic
function of the alveolar macrophages was seen at the high
hexavalent chromium exposure level (200 µg/m3). In rats exposed to
this chromium aerosol concentration for 42 days, the lung clearance
of inert iron oxide was significantly reduced. The humoral immune
system was still stimulated at a low chromium aerosol concentration
of 100 µg/m3, but significantly depressed at 200 µg chromium/m3.
Exposure of rats, through inhalation, to chromium carbide or
chromium boride dust at very high levels (300 - 350 mg/m3 for each
substance) for 3 months (2 h/day) resulted in effects on the
vascular system of the lungs, e.g., endothelial hyperplasia.
Bronchitis and a decrease in the blood-haemoglobin concentration
were also observed (Roschina, 1964). The effects of chromium boride
were more pronounced than those of chromium carbide.
Steinhoff et al. (1983) performed an intracheal injection study
on rats (930 rats, 30 months, 0.05 - 1.25 mg Na2Cr2O7/kg body
weight per week; 1.25 mg CaCrO4/kg body weight per week). Half of
the rats were intratracheally injected once a week and the other
half received the same weekly dose distributed over 5 injections
per week. In rats receiving doses 5 times a week, there were some
significant changes in levels of total plasma-protein and
-cholesterol, in some haematological variables, in organ weights,
and in survival times in females. Only male rats receiving 1.25 mg
sodium dichromate/kg body weight, once a week, showed a sharp
reduction in body weight, female rats being less affected. Rats
receiving calcium chromate in the same dose showed reduced body
weight but to a lesser extent. The weight of lung and trachea was
increased by both substances in all doses.
Marked histopathological changes (congestion, fairly large
areas of focal necrosis, bile duct proliferation) described after
long-term exposure of rabbits to hexavalent chromium (ip injection
of 2 mg chromium/kg body weight per day for 6 weeks) (Tandon et
al., 1978), as well as the increase in hepatic metallothionein and
decrease in cytochrome P-450 levels after ip injection of 400 µmol
chromium/kg per day (type of chromium compound not mentioned)
(Eaton et al., 1980) need further confirmation. The finding of an
accumulation of hexavalent chromium in the reticuloendothelial
system including bone marrow (Baetjer et al., 1959b; Langard, 1977)
may be of importance for a disturbed blood picture.
Merkurieva et al. (1980a,b) studied the effects of potassium
dichromate in the drinking-water on the activities of different
enzymes in rats. Exposure included daily doses of 0.0005, 0.005,
0.05, or 0.5 mg/kg body weight for up to 6 months. After 20 days
of exposure at the highest dose level, enzyme activities increased
by 15 - 28% in liver microsomes (inosine-5-diphosphatase),
lysosomes (beta-D-galactosidase), and cytosol (lactate dehydrogenase
(EC 1.1.1.27)). For some of these enzymes, as well as for
acetylesterase (EC 3.1.1.6), increases in activity of up to 54%
were found in the gonads, kidneys, seminal fluid, and serum. At a
dose of 0.05 mg/kg, the only statistically significant finding was
a 54% increase in the activity of acetylesterase in the gonads.
After a 6-month exposure at this dose level, there was a 70%
increase in lactate dehydrogenase activity in the seminal fluid as
well as a 50% increase in free beta-galactosidase in the liver. There
were no changes in enzyme activities at the two lowest dose levels.
Painting of rat skin with an aqueous solution of potassium
dichromate (0.5%), daily, for 20 days, resulted in a local
inflammatory reaction, an increased level of hexose glyco-proteins
in the skin and serum, and an elevated concentration of serotonin
in the skin and liver (Merkurieva et al., 1982). Most of these
effects were also seen earlier in the exposure period, though they
were not as pronounced. Ten days after the start of exposure, a
nearly 3-fold increase in the serum-acetylcholine concentration
occurred together with decreased acetylcholinesterase activity.
Thus, the data of Merkurieva et al. show systemic effects following
both oral and dermal exposure to hexavalent chromium.
Cats fed chromic phosphate or oxydicarbonate at 50 - 1000 mg/day
for 80 days did not exhibit signs of illness or tissue damage.
Similarly, toxic reactions were not observed in rats administered
drinking-water containing 25 mg trivalent chromium/litre, for 1
year, or 5 mg trivalent chromium/litre throughout their lifetime
(US NAS, 1974a). The toxicity of trivalent chromium is so low that
even by parenteral administration, a chromic acetate level of 2.29
g/kg body weight or a chromic chloride level of 0.8 g/kg body
weight is required to kill mice. Even very large doses given
intragastrically were not fatal for dogs. Brard (1935) reported
that 10 or 15 g of chromium as chromic chloride proved fatal in one
dog (US NAS, 1974a). Some fatal doses of trivalent chromium
compounds reported in the literature are listed in Table 13.
Rats exposed through inhalation to chromic oxide (trivalent
chromium) at 42 mg/m3 or to chromic phosphate at 43 mg/m3 (5 h/day
for 5 days/week) for 4 months developed chronic irritation of the
bronchus and lung parenchyma, and dystrophic changes in the liver
and kidney (Blokin & Trop, 1977).
Inhalation exposure of rats (number not given) to dusts
containing 36 or 50% chromite for 4 months (2 h/day), at
concentrations of 375 - 400 mg/m3, resulted in thickening of the
walls of pulmonary vessels and bronchi (Roschina, 1959). The high
exposure levels in these studies make it difficult to evaluate the
effects.
Inhalation studies have also been performed with chromium
carbonyl, where chromium is in the 0 oxidation state (Roschina,
1976). Twelve rabbits and 48 rats were exposed for 4 months (4
h/day, 6 days/week) at a concentration of 1.6 or 0.16 mg/m3. At
both exposure levels, there was loss of body weight (25 and 12%,
respectively) as well as anaemia and leukocytosis. In the higher
exposure group, the animals showed an elevated gamma-globulin level
in serum and an increased transaminase activity. The contents of
cholesterol and SH-groups were reduced, and there was a decrease in
cholinesterase (EC 3.1.1.7) activity. Lipid and/or protein
dystrophy were noted in several organs, e.g., in the liver and
kidneys. No such effects were detected in the animals in the low-
exposure group.
Table 13. Fatal doses of trivalent chromium in animalsa
---------------------------------------------------------------------------------------------
Animal Number of Routeb Compound Chromium Effect Reference
animals dose (g/kg)
---------------------------------------------------------------------------------------------
Dog 2 sc chromic chloride 0.8 fatal Brard (1935)
Rabbit 1 sc chromic chloride 0.52 fatal Brard (1935)
Rat 38 iv chrome alum 0.01 - 0.018 LD50 Mertz et al.
chromium-hexaurea (1965a)
chloride
Mouse -c iv chromic chloride 0.8 MLDd Windholz et al. (1960)
Mouse -c iv chromic acetate 2.29 MLD Windholz et al. (1960)
Mouse -c iv chromic chloride 0.4 MLD Schroeder (1970)
Mouse -c iv trivalent chromium ? 0.25 - 2.3 MLD Windholz et al. (1960)
Mouse -c iv chromic sulfate 0.247 MLD Windholz et al. (1976)
Mouse -c iv chromic sulfate 0.085 MLD Schroeder (1970)
Mouse -c iv chromium carbonyl 0.03 LD50 Schroeder (1970)
---------------------------------------------------------------------------------------------
a Modified from: US NAS (1974a).
b sc = subcutaneous; iv = intravenous.
c No figures given.
d Minimum lethal dose.
7.2.1.1. Carcinogenicity
Various types of chromium chemicals, methods of administration,
and species of animals have been studied (IARC, 1980a).
Ideally, carcinogenicity should be tested with the methods
recommended by IARC (1980b), but, in many of the early studies,
this was not done. Carcinomas of the lung have been reported in
animals as a result of the administration of chromium chemicals.
Hueper (1958) found 2 squamous cell carcinomas and one
carcinosarcoma in 25 rats following intra-pleural injection of
chromite ore roast. After intrapleural implantation of strontium
chromate lasting 27 months, Hueper (1961) found tumours (type
unspecified) in 17/28 rats. Laskin et al. (1970) and Levy & Venitt
(1975) produced a number of bronchogenic carcinomas by implanting
pellets of cholesterol mixed with various chromium compounds
encased in a wire mesh cage in the bronchi of rats. Calcium and
zinc potassium chromate produced a number of bronchogenic
carcinomas, but soluble chromates and trivalent chromium chemicals
failed to produce cancer.
Using the same technique, Levy & Martin (1983) tested 21
different chromium-containing materials (pigments, intermediates,
and residues from the bichromate-producing industry, relatively
pure crystalline compounds) in 2250 random-bred rats and found that
chromates, described as sparingly soluble, were carcinogenic in the
rat lung. These materials included strontium and calcium chromate
and, to a far lesser extent, certain forms of zinc chromate.
Barium and lead chromate evoked only a very weak carcinogenic
response compared with strontium and calcium chromate. In the
study of Laskin et al. (1970), it was shown that chromium trioxide
produced hepatocellular carcinomas in 2/100 rats (controls, 0/24).
After inhalation of 13 mg calcium chromate/m3 (5 h/day, 5
days/week, for lifetime), Nettesheim et al. (1971) found 14 lung
adenomas in 136 treated mice and 5 in 136 untreated controls, but
no carcinomas. Steffee & Baetjer (1965), performing inhalation
studies on rats, mice, guinea-pigs, and rabbits (inhalation of
mixed chromate dust, corresponding to 3 - 4 mg CrO3/m3, 4 - 5
h/day, 4 days/week, for lifetime, or 50 months, respectively) could
only find 3 alveologenic adenomas in 50 treated guinea-pigs.
Laskin (1972) and Laskin et al. (1970) found 1 squamous cell
carcinoma of the lung, 1 of the larynx and 1 "peritruncal tumor" in
rats (inhalation of calcium chromate, 2 mg/m3, 589 exposures of 5 h
over 891 days) and 1 squamous cell carcinoma and 1 papilloma of the
larynx in hamsters. The number of treated animals was not
specified in either paper. Steinhoff et al. (1983) performed
intratracheal instillations of chromates in rats for 30 months with
one treatment/week and the same weekly dose distributed over 5
treatments/week (Table 14). In 880 exposed rats, 28 adenomas of
alveolar-bronchiolar origin (benign) and 12 malignant tumours (3
adenocarcinomas and 9 squamous cell carcinomas) were found. All
lung tumours developed very late and were only detected at the end
of the lifetime study, often in lungs with callosities. The
tumours were tiny and none of them caused the animal to die.
Sodium dichromate was not carcinogenic after exposure on 5
days/week. With calcium chromate, the carcinogenic effect was more
pronounced after treatment once per week, than after treatment 5
times per week.
Table 14. Incidence of benign and malignant lung tumours among
880 rats intracheally injected with Na dichromate and Ca chromatea
----------------------------------------------------------------
Dose Number of Incidence of lung tumours
(mg/kg) injections benign malignant
per week
----------------------------------------------------------------
Na2Cr2O7 x 2H20 0.25 5 none none
Na2Cr2O7 x 2H20 1.25 1 12 8
CaCrO4 0.25 5 5 1
CaCrO4 1.25 1 11 3
----------------------------------------------------------------
a From: Steinhoff et al. (1983).
Hueper (1961) reported unspecified tumours at implantation
sites in 12/34 rats, following intramuscular (im) implantation of
sintered calcium chromate (25 mg). No tumours were observed in 32
control animals. The same author found implantation-site tumours in
15/33 animals treated with strontium chromate compared with no
tumours in 32 control animals. Single sc injections of 30 mg lead
chromate (Maltoni 1974, 1976) resulted in injection-site sarcomas
in 26/40 treated rats. After administration of the same amount of
lead chromate oxide, injection-site sarcomas were found in 27/40
rats; no sarcomas were found in 60 vehicle controls. Following 9
im injections of 8 mg lead chromate, Furst et al. (1976) found
injection-site sarcomas in 31/47 treated rats, and renal carcinomas
in 3/24 treated rats; no sarcomas were found in 0/22 vehicle
controls. Heath et al. (1971) found injection-site sarcomas and
other tumours in 7/74 treated rats after im injection of 28 mg
cobalt-chromium alloy.
Administration of low levels of trivalent chromium acetate (5
mg/litre in the drinking-water) to mice and rats for the lifetime
did not result in increased tumour incidences compared with
controls (Schroeder et al., 1964, 1965).
Trivalent chromium oxide incorporated in bread at
concentrations of 10, 20, or 50 g/kg and fed to BD rats did not
increase tumour incidence compared with controls (Ivankovic &
Preussmann, 1975). Hexavalent chromium compounds have not been
tested by oral administration.
IARC (1980a) summarized the data of all available references
and concluded as follows: "There is sufficient evidence for the
carcinogenicity of calcium chromate and some relatively insoluble
chromium (VI) compounds (sintered calcium chromate, lead chromate,
strontium chromate, sintered chromium trioxide and zinc chromate)
in rats. There is limited evidence for the carcinogenicity of lead
chromate (VI) oxide and cobalt-chromium alloy in rats. The data
were inadequate for the evaluation of the carcinogenicity of other
chromium (VI) compounds and of chromium (III) compounds".
(a) Interaction with other factors
The possibility that chromium may act synergistically with
other agents in the production of cancer has been tested in several
studies. Nettesheim et al. (1970) pretreated one group of mice
with 100 R whole-body radiation and infected another group with PR8
influenza virus. These mice and control mice were exposed through
inhalation to chromium oxide dust for 6 - 18 months. No measurable
effects of either of the pretreatments were found on the incidence
of tumours. In a similar study with inhalation of calcium chromate
(CaCrO4, 5 h/day, 5 days per week for the lifetime), pre-exposure
to X-rays did not affect the tumour rate, but PR8 influenza
infection reduced tumour incidence (Nettesheim et al., 1971).
Steffee & Baetjer (1965) also found that the infection of rats with
PR8 influenza virus associated with intratracheal injection of
chromates did not lead to the production of tumours. Chromite ore
injected iv did not affect the development of tumours induced by iv
injection of 20-methyl-cholanthrene (Shimkin & Leiter, 1940).
Thus, at present, there are not sufficient data to suggest that
chromium acts synergistically with virus infections, ionizing
radiation, or other chemical carcinogens to produce cancers.
7.2.1.2. Genotoxicity
The mutagenicity of chromium compounds was reviewed by IARC
(1980a), Petrilli & DeFlora (1980), Levis & Bianchi (1982), and
Baker (1984). Present knowledge, in the light of recent findings,
is summarized in Table 15 (hexavalent chromium) and Table 16
(trivalent chromium).
When evaluating the results of chromium mutagenicity tests, it
is necessary to take into consideration several properties of the
tested compound (oxidation state, solubility, ability to penetrate
cell membranes, intracellular stability, and reactivity with
cellular components). Most of the in vitro mutagenicity
experiments have been performed with chromium chemicals, following
the introduction of the reverse mutation test by Ames (1973).
Bacterial strains of Salmonella typhimurium (Petrilli & DeFlora,
1977) containing mutants that require histidine for growth, and
Escherichia coli (Venitt & Levy, 1974) with mutants requiring
tryptophan for growth have been used. In each case, the bacterial
cultures have been incubated with the test chromium compounds and
the number of revertant colonies scored. The chromium compounds
studied included both hexavalent and trivalent compounds; a survey
of the results is given in Table 17 (DeFlora, 1981). Zhurkov
(1981) performed an Ames test to study the mutagenic activity of
potassium dichromate (K2Cr2O7) with S. typhimurium TA 1535 and TA
1538. A medium degree of mutagenic activity without metabolic
activation was shown by the strain TA 1535.
Only hexavalent chromium compounds have shown mutagenic
effects. Using the Salmonella /mammalian microsome tests of Ames
et al. (1973), DeFlora (1978) and Löfroth (1978) both showed a
decrease in the mutagenicity of hexavalent chromium in the presence
of an NADPH-generating system, suggesting reduction of mutagenic
hexavalent chromium to trivalent chromium. In this chromate
metabolism, cytochrome P-450 functions as the reductase (Garcia &
Wetterhahn Jennette, 1981). In recent in vitro studies, Wiegand et
al. (1984b) showed that glutathione could reduce hexavalent
chromium to trivalent chromium, without any further cofactors or
metabolizing enzymes. In rats, the most efficient tissue in
decreasing hexavalent chromium mutagenicity was the liver, followed
by the suprarenal glands, kidney, stomach, and lung (Petrilli &
DeFlora, 1980). These studies suggest a possible way of
intracellular detoxification of hexavalent chromium in low doses.
Schoental (1975) suggested that hexavalent chromium caused the
formation of epoxyaldehydes having mutagenic potential. Venitt &
Levy (1974) assumed that chromates belong to the transition-
inducing class of mutagens. They cause both frameshift and base-
pair substitutions (Petrilli & DeFlora, 1980). The hypothesis of
Kazantzis & Lilly (1979), that chromates cause guanine-cytosine to
adenine-thymine transitions in the subsequent round of DNA-
replication, needs further confirmation.
Table 15. Genotoxic activity of chromium (VI) compoundsa
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
DNA degradation CaCrO4 - Casto et al. (1976)
K2Cr2O7 - Bianchi et al. (1983)
PbCrO4 - Douglas et al. (1980)
K2CrO4 + Whiting et al. (1979)
Decreased fidelity PbCrO4, + (1) Levis & Majone (1981)
of DNA synthesis K2Cr2O7
CrO3 + Sirover & Loeb (1976)
K2Cr2O7 + Bianchi et al. (1983)
Microbial DNA K2CrO4, + Yagi & NishioKa (1977)
repair K2Cr2O7
CrO3, K2CrO4, + Kanematsu et al. (1980)
K2Cr2O7
K2CrO4, + Nishioka (1975)
K2Cr2O7
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
CrO3, + Gentile et al. (1981)
K2Cr2O7,
Na2Cr2O7, + Gentile et al. (1981)
(NH4)2Cr2O7
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Microbial gene K2Cr2O7 + (2) Bonatti et al. (1976)
mutation CrO3 + (2) Fukunaga et al. (1982)
Na2Cr2O7, +/-(3) Petrilli & De Flora
CrO3, +/-(3) (1978a,b)
ZnCrO4xZn(OH)2 +/-(3)
K2Cr2O7 + Zhurkov (1981)
Na2Cr2O7 +/-(3) DeFlora (1978)
chromate (4) +/-(3) Löfroth (1978)
dichromate +/-(3)
K2Cr2O7 - Kanematsu et al. (1980)
K2CrO4, + Löfroth & Ames (1978)
K2Cr2O7 +
K2Cr2O7 + Nishioka (1975)
K2CrO4, CaCrO4 + DeFlora (1981)
(NH4)2CrO4,
CrO3,
Na2CrO4, + Venitt & Levy (1974)
K2CrO4
CaCrO4
K2Cr2O7 + Bianchi et al. (1983)
Na2Cr2O7,
ZnCrO4
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
K2CrO4, CaCrO4 + Petrilli & DeFlora
CrO3 (1977)
Na2Cr2O7
K2CrO4 + Green et al. (1976)
PbCrO4, CrO3 + Nestmann et al. (1979)
Mammalian cell K2Cr2O7 + Bianchi et al. (1983)
gene mutation K2Cr2O7, + Newbold et al. (1979)
ZnCrO4 +
PbCrO4 -
K2Cr2O7 + (5) Bonatti et al. (1976)
K2Cr2O7 + Pashin et al. (1982)
NaeCr2O + Pashin & Kozachenko
(NHy)2Cr2O7 + (1981)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell K2CrO4, + Umeda & Nishimura (1979)
chromosomal K2Cr2O7 +
mutation K2Cr2O7 + Bigaliev et al. (1978)
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7 +
Na2CrO4, + Majone & Levis (1979)
K2CrO4 +
Na2Cr2O7, Levis & Majone (1979)
K2Cr2O7
CrO3, CaCrO4
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CrO3 + Kaneko (1976)
Na2Cr2O7 + Sarto et al. (1980)
K2Cr2O7 +
CrCl3 +
CrO3 + Tsuda & Kato (1977)
K2Cr2O7 + Raffetto (1977)
K2Cr2O7 + Stella et al. (1982)
Na2Cr2O7, + Majone & Levis (1979)
K2Cr2O7 +
K2CrO4 + Wild (1978)
Na2Cr2O7 + (7) Krishnaja & Rige (1982)
Mammalian cell CrO3, K2CrO4, + Ohno et al. (1982)
SCE K2Cr2O7 +
K2Cr2O7 + Bianchi et al. (1983)
K2CrO4,
Na2CrO4 + Levis & Majone (1979)
CrO3, +
K2Cr2O7, + Majone & Levis (1979)
Na2Cr2O7 +
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CaCrO4, CrO3, + Gomez-Arroyo et al.
K2Cr2O7 + (1981)
K2Cr2O7 + Majone & Rensi (1979)
CrO3 + (6) Stella et al. (1982)
K2Cr2O4, + Elias et al. (1983)
Na2CrO4, +
Na2Cr2O7 +
K2Cr2O7,
K2CrO4 + McRae et al. (1979)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell CaCrO4, K2CrO4 + (8) Casto et al. (1979)
transformation ZnCrO4 + (9)
Na2CrO4,
CaCrO4 + Casto et al. (1976)
+ Di Paolo & Casto (1979)
K2Cr2O7 + Bianchi et al. (1983)
K2Cr2O7 + Tsuda & Kato (1977)
CaCrO4 + Fradkin et al. (1975)
K2Cr2O7 + Raffetto (1977)
---------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) As industrial pigments (Cr-yellow-orange; Zn-yellow; Mo-orange).
(2) Gene conversion.
(3) No mutagenicity in the presence of mammalian microsomal activation
system (S-9 mix).
(4) No compounds given.
(5) Forward mutation was observed in 7/480 054 colonies (controls 0/84
546). A significant increase can be stated if comparison is made
with the historical spontaneous mutation rate, i.e., no mutants in
106 colonies.
(6) Occupatinal exposure.
(7) Induction of chromosomal aberrations in gill cells of the fish
Boleophthalmus dussumieri (Goby).
(8) 2 times control levels.
(9) 4 times control levels.
The results summarized in Table 17 show that the chromate ion
(hexavalent chromium) induces different kinds of genetic damage,
even at low doses, whereas the chromosome-damaging capacity of
trivalent chromium was only observed when it was tested in very
high doses.
In assays with whole-cell systems, trivalent chromium is
inactive, unless there is a direct interaction with DNA, e.g., in
studies in which purified DNA was exposed to trivalent chromium,
where modifications of the physical and chemical properties, as
well as decreased fidelity of DNA synthesis, were produced (Levis &
Bianchi, 1982). According to Warren et al. (1981), it appeared
that trivalent chromium in a proper ligand environment could have
considerable genetic toxicity. However, in recent studies, Bianchi
et al. (1984) found that trivalent chromium (CrCl3, 10-3 to 10-5 M)
was neither cytotoxic nor mutagenic in permeabilized hamster
fibroblasts (BHK were incubated for 30 min in Ea or BSS made
hypertonic with 4.2% NaCl).
Table 16. Genotoxic activity of chromium (III) compoundsa
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
DNA degradation CrCl3 + Bianchi et al.
(1983)
Decreased fidelity Cr2O3 + Levis & Majone
of DNA synthesis (1981)
Microbial DNA cis-[Cr(bipy)2ox] + Warren et al. (1981)
repair I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 + Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O + Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O + Warren et al. (1981)
trans-[Cr(en)2 + Warren et al. (1981)
(SCN)2]SCN
CrCl3, Cr2O3, - Yagi & Nishioka
Cr(OH)3 (1977)
K2Cr2(SO4)4 - Yagi & Nishioka
(1977)
K2Cr2(SO4)4 - Kanematsu et al.
(1980)
Cr2(SO4)3 - Kanematsu et al.
(1980)
CrCl3 - Nishioka (1975)
Cr(NO3)3, + De Flora (1981)
Cr(CH3COO)3
CrCl3 - Nakamuro et al.
(1978)
CrCl3 + Gentile et al.
(1981)
CrCl3, KCr(SO4)2, - Gentile et al.
(1981)
Cr2(SO4)3 - Gentile et al.
(1981)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Microbial gene CrCl3, Cr(NO3)3 - Löfroth & Ames
mutation (1978)
Cr(CIO4)3 - Löfroth & Ames
(1978)
CrCl3, Cr(NO3)3 - Venitt & Levy (1974)
Cr2SO4, - Venitt & Levy (1974)
K2SO4 x 2H2O
Cr(CH3COO)3 + Nakamuro et al.
(1978)
Cr(NO3)3 + Nakamuro et al.
(1978)
CrCl3 - Nakamuro et al.
(1978)
CrK(SO4)2 x 12H2O - Petrilli & De Flora
(1977)
CrCl3 x 6H2O - Petrilli & De Flora
(1978a)
Cr(NO3)3 x 9H2O - Petrilli & De Flora
(1978b)
Microbial gene cis-[Cr(bipy)2ox] + Warren et al. (1981)
mutation I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 - Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O - Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O - Warren et al. (1981)
trans-[Cr(en)2 - Warren et al. (1981)
(SCN)2]SCN
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
gene mutation (1983)
Cr(CH3COO)3 - Newbold et al.
(1979)
Mammalian cell Cr2(SO4)3 - Umeda & Nishimura
chromosomal (1979)
mutation
Cr(CH3COO)3 + Nakamuro et al.
(1978)
CrCl3, Cr(NO3)3 - Nakamuro et al.
(1978)
Cr(NO3)3, + Levis & Majone
(1979)
KCr(SO4)2, + Levis & Majone
(1979)
Cr(CH3COO)3 + Levis & Majone
(1979)
CrCl3 + Kaneko (1976)
CrCl3 - Sarto et al. (1980)
Cr2(SO4)3, CrCl3 - Tsuda & Kato (1977)
CrCl3 + Raffetto (1977)
CrCl3 - Stella et al. (1982)
CrCl3 - (1) Bianchi et al.
(1984)
Mammalian cell CrCl3, Cr2O3 + Elias et al. (1983)
SCE
Cr2O3 + Levis & Majone
(1981)
Cr(NO3)3, - Levis & Majone
KCr(SO4)2 (1979)
CrCl3, Cr(CH3COO)3 - Levis & Majone
(1979)
CrCl3 + (2) Ohno et al. (1982)
Cr2(SO4)3 - Ohno et al. (1982)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
SCE (contd). (1983)
CrCl3 - Majone & Rensi
(1979)
Mammalian cell CrCl3 - Bianchi et al.
transformation (1983)
---------------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) Thymidine uptake, DNA replication, damage and repair and SCE were
tested in permeabilized hamster fibroblasts.
(2) Weakly positive.
Levis & Buttignol (1977) and Levis et al. (1978) raised the
question of whether trivalent chromium could be an active mutagenic
agent within a cell. Others, such as Bianchi et al. (1980),
supported this theory, suggesting that trivalent chromium is bound
to genetic material after reduction of the hexavalent form by an
NADPH-dependent microsomal enzyme system (Norseth, 1978; Jennette,
1979). The studies of Sirover & Loeb (1976), showing a 30% error
in DNA synthesis after application of 0.64 mM trivalent chromium
compared with 16 mM of hexavalent chromium, support this theory.
Pashin et al. (1982) demonstrated the induction of dominant
lethal mutations in mice. They treated male mice with a single
injection of potassium dichromate (0.5 - 20 mg/kg body weight, ip)
or with daily injections (1 and 2 mg/kg, ip, for 21 days). The
single injections (0.5 - 2 mg/kg) did not induce any effects;
repeated or higher dosages of potassium dichromate induced a
statistically significant decrease in the survival of embryos from
cells treated at the early spermatid and late spermatocyte stages.
Karyological alterations in mammalian cells were induced after
exposure to concentrations of hexavalent chromium that were more
than 100 times lower than the concentration of trivalent chromium
causing the same changes (Majone & Rensi, 1979).
Analysing the effects of fume particles from stainless steel
welding on sister chromatid exchanges and chromosome aberrations in
cultured Chinese hamster cells, Koshi (1979) found an increase in
cytogenic effects with increasing fume doses, due to the dissolved
hexavalent chromium. Hexavalent chromium and stainless steel
welding fume particles containing chromium compounds produced
positive results in the "mammalian spot test", a somatic tissue
assay (Knudsen, 1980).
Table 17. Mutagenicity of chromium compoundsa
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
L. Hexavalent chromium compounds
1) Sodium dichromate (Merck) - +w +w +w + 50 - 250 4.4 decrease toxic and mutagenic
Na2Cr2O7 x 2H2O Cr6+ was converted
into inactive Cr3+
2) Potassium chromate (BDH) - +w +w +w + 80 - 410 2.9 decrease by reducing
K2CrO4 chemicals (ascorbic
acid, sodium
3) Calcium chromate (BDH) - +w +w +w + 60 - 290 3.2 decrease sulfite) or
CaCrO4 metabolites (NADH,
NADPH, GSH), by
4) Ammonium chromate - +w +w +w + 50 - 320 3.7 decrease human gastric juice
(Carlo Erba) and erythrocyte
(NH4)2CrO4 lysates, by S9 mix
containing human
liver S9 fractions
5) Chromium trioxide or - +w +w +w + 40 - 220 5.1 decrease or rat tissue S9
chromic acid (Merck) fractions (in order
CrO3 of efficiency:
liver>suprarenal
glands>kidney>
stomach>lung);
pretreatment of
rats (Aroclor(R)
1254), ether,
ethanol) influenced
the efficiency of
metabolic systems;
conversely, Cr6+
mutagenicity was not
affected by human
serum or plasma nor
by S9 mix containing
other rat (spleen,
colon, bladder,
striated muscle) S9
fractions
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
6) Zinc yellow (Montedison) - +w +w +w + 90 - 590 1.4 decrease the mutagenic effects
ZnCrO4 x Zn(OH)2 + of Cr6+ compounds and
10% CrO3 benzo( a)pyrene were
less than additive
7) Chromyl chloride (BDH) - +w +w +w + 100 - 430 1.8 decrease liquid volatile
Cl2CrO2 compound; vapours
were also toxic and
mutagenic
8) Chromium orange or basic - - - - +b 2 mg the three pigments
lead (spot test) containing PbCrO4
PbCrO4 x PbO were scarcely (L8),
chromate (Montedison) very scarcely (L9),
or totally (L10)
insoluble in water;
they were assayed
by directly spotting
these compounds at
the centre of plates
9) Molybdenum orange or - - - - +b 2 mg
lead solfomolybdo- (spot test)
chromat (Montedison)
PbCrO4 72-77%
PbSO4 4-6%
Al2O3 2%
PbMoO4 12-14%
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
10) Chromium yellow or - - - - -b
lead solfochromate
(Montedison)
PbCrO4 41-85%
PbSO4 4-45%
SiO2 0.1-3%
Al2O3 2-6%
11) Chromium - - - - - -2.3x103 - - hexacoordinated
hexacarbonyl (BDH) compound; formally,
Cr(CO)6 its oxidation state
is 0; insoluble in
water, dissolved in
ether
M. Trivalent chromium compounds
1) Chromic chloride - - - - - -3x104 - - inactive Cr3+
(BDH) CrCl3 x 6H2O compounds could be
converted into toxic
and mutagenic Cr6+
only in the presence
of oxidizing chemicals
(potassium
permanganate), while a
variety of metabolic
systems were
ineffective
2) Chromic nitrate (Riedel- - - - - - -2x104 - -
de Haen)
Cr(NO3)3 x 9H2O
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
3) Chromic potassium - - - - - -1.6x104 -
sulfate (BDH)
CrK(SO4)2 x 12H2O
4) Chromic acetate (BDH) - - - - - -7x104 - -
Cr(CH3COO)3
5) Neochromium (Montedison) - - - - - -5x104 - -
Cr(OH)SO4 56-58%
Na2SO4 23-24%
H2O 18-21%
6) Chromium alum (Montedison) - - - - - -4.3x104 - -
Cr2(SO4)3 37-39%
K2SO4 16-18%
H2O 43-37%
7) Chromite (Montedison) - - - - + 2 mg - Contaminated with Cr6+
Cr2O3 44-46% (spot test) traces ( sim-diphenyl-
Fe2O3 29-30% carbazide reagent);
Al2O3 15-16% scarcely soluble in
SiO3 0.5-3% water; assayed by
CoO 0.5-2% directly spotting the
powder at the centre
of plates
--------------------------------------------------------------------------------------------------------
Table 17 (contd.)
Note:
Sensitivity of Salmonella strains: Range of activity:
In the assays carried out in this study, This column indicates the lower and
the numbers of spontaneous revertants of the upper limits (in nmol per plate)
Salmonella tester strains fell within the of the mutagenic response, as in-
following ranges: ferred from dose-response curves
TA 1535: 10-25, both with and without S9 mix; with the most sensitive bacterial
TA 1537: 3-18, both with and without S9 mix; strain and under the most favourable
TA 1538: 10-25 without S9 mix; 15-35 with S9 mix; metabolic situation (i.e., with or
TA 98: 20-35 without S9 mix; 25-40 with S9 mix; without S9 mix) for each positive
TA 100: 150-200 without S9 mix; 140-180 with S9 mix compound; for negative compounds,
the maximum dose tested is indicated
The key for the interpretation of symbols in the
column "Reverted strains" is the following: Mutagenic potency:
+ clearly positive result indicated by a dose-
related and reproducible increase of his+ the values presented have
revertants over controls (at least a 3-fold been calculated by dividing the number
increase); of revertants in excess of controls
+w weak positivity indicated by an increase of by the corresponding amount of compounds
revertants 2 - 3 times in controls; (in nmoles); the number of net revertants
± reproducible but less than 2-fold increase was determinated at the top level of the
of revertants; linear part of dose-response curves, which
No symbol: no conclusive experiment carried out were drawn as indicated under the previous
with the corresponding strain. sub-heading.
Effects of S9 mix
The effect of S9 mix containing
liver S9 fractions from
Arochlor(R) pretreated rats on
the mutagenicity of test
compounds is reported
--------------------------------------------------------------------------------------------------------
a From: DeFlora (1981).
b L8, L9, and L10, as well as PbCrO4, were positive in the plate test when
dissolved in 0.5 N NAOH.
Embryonic fibroblast cell cultures showed significant
chromosome aberrations, when potassium dichromate was added to the
medium (Tsuda & Kato, 1976). When the potassium dichromate was
reduced with sodium disulfite (Na2SO3), no significant aberrations
occurred, even with 100 times the chromium concentrations.
At present, it is widely accepted that hexavalent chromium is
genetically active, because of its ability to cross the membranes
and enter the cells. If reduction of hexavalent chromium takes
place outside the cell (or even outside the cell nucleus, e.g., in
mitochondria or microsomes) its genetic activity is suppressed; if
the reduction takes place inside the nucleus (near, or at, the
target DNA molecules) alterations in DNA can occur, depending on
the oxidation power of hexavalent chromium or the formation of
trivalent chromium complexes with nucleophilic sites of the DNA;
thus, trivalent chromium could be the ultimate mutagenic form of
chromium (Levis & Bianchi, 1982). More recent investigations
(Wiegand et al., 1984) have shown that hexavalent chromium may
enter the body unreduced via the lung and be partly deposited in
erythrocytes over a prolonged period of time. From a DNA-repair
test in bacteria, DeFlora et al. (1984) concluded that hexavalent
chromium causes irritation in nucleotide pools and, due to its
oxidizing power, induces single-strand breaks in DNA, while
trivalent chromium induces DNA-protein and DNA-DNA cross-links and
decreases the fidelity of DNA replication.
7.2.1.3. Developmental toxicity and other reproductive effects
The teratogenicity of hexavalent chromium as chromium trioxide
was tested in golden hamsters (Gale, 1974, 1978, 1982; Gale &
Bunch, 1979) and that of trivalent chromium as chromic-chloride in
ICR mice (Iijima et al., 1975; Matsumoto et al., 1976). Injections
of hexavalent chromium into the fertilized chick egg increased
embryolethality and produced malformations in the survivors (Gilani
& Marano, 1979).
The golden hamsters were treated with a single iv injection of
chromium trioxide (5, 7.5, 10, or 15 mg/kg body weight) on the 8th
day of gestation (Gale, 1978).
Dose-response relationships were demonstrated between the rate
of absorption and the rate of malformations including delayed
ossification of the skeletal system. At 5 mg/kg body weight, 4% of
the fetuses showed external abnormalities (oedema, exencephaly),
and 34% showed cleft palate (controls: 2%), which increased to 85%
with the higher doses of 7.5 or 10 mg/kg body weight. Thirty-one
percent of fetuses were retarded following treatment with 7.5 mg/kg
body weight and 49% following 10 mg/kg body weight. The dose of 15
mg/kg body weight was lethal for 75% of the dams. Gale & Bunch
(1979) injected a single dose of 8 mg CrO3/kg body weight on day 7,
8, 9, 10, or 11 of gestation in female golden hamsters (Table 18).
The treated females were adversely affected (body weight loss,
tubular necrosis of the kidneys). In a later study, Gale (1982)
injected 8 mg CrO3/kg iv in hamsters on day 8 of gestation. An
increased incidence of cleft palate was seen in strains LVG, LSH,
and MHA, but no effects were reported in strains CB, LHC, and PD4.
ICR mice were injected sc with chromic chloride (Iijima et al.,
1975; Matsumoto et al., 1976). In the first series (animals
administered 9.8 or 19.5 mg/kg body weight, every other day, from
the 0th to 16th day of gestation), according to the authors, a
slight increase in the rate of malformations could not be excluded.
In the second series, with ip injections of 9.8 - 24.4 mg/kg body
weight, on the 8th day of gestation, the authors reported a dose-
dependent increase in the frequency of exencephaly, anencephaly,
and the occurrence of rib fusion.
Danielsson et al. (1982) studied the rate of embryonic or fetal
uptake of trivalent chromium and hexavalent chromium in early and
late gestational mice (Table 19) and found placental passage of
chromium. Hexavalent chromium appeared in the fetus in high enough
concentrations for a direct effect on embryonic structures to be
likely, trivalent chromium may act on placental structures.
Gilani & Marano (1979) injected chromium trioxide into 840
embryonating chicken eggs (air sack) at doses ranging from 0.002 to
0.05 mg per egg, on days 0, 1, 2, 3, and 4 of incubation (0.1 ml
physiol. saline injections per control egg). All embryos were
examined on day 8. The following malformations were observed in
the 720 eggs affected: short and twisted limbs, microphthalamia,
exencephaly, short and twisted neck, everted viscera, oedema, and
reduced body size. However, the incidence of abnormalities in the
120 controls was reported to be "low".
Inhalation studies were conducted by Glaser et al. (1984) on
Wistar rats (TNO-W-74 SPF) to study the effects of sodium
dichromate on reproduction and teratogenicity in 3 generations.
Whole-body exposures to sodium dichromate aerosols (0.2 mg
chromium/m3) were performed with an exposure time of 130 days per
generation. No effects on reproduction were found. All
teratogenicity tests were negative, and there was no increase in
fetal chromium content. However, from generation to generation,
there was an increase in immunosuppression and hyperplasia of
organs (especially in the lungs) and changes in haematological
variables.
Table 18. Embryotoxicity following maternal exposure to CrO3 on different days
of gestation in hamstersa
-------------------------------------------------------------------------------------
Findings in live fetuses
Day of No. of No. of No. of Frequency External Internal Cleft
gestation females implantation living resorptions defects defects palate
treated sites fetuses No. %b No. %b No. %b No. %b
-------------------------------------------------------------------------------------
Treated CrO3 8 mg/kg
7 6 64 10 54 84 1 10 1c 10 5 50
8 6 88 54 14 20 8d 15 4e 7 17 31
9 6 77 67 10 13 18f 27 0 0 25 37
10 6 77 77 0 0 0 0 0 0 0 0
11 6 68 67 1 1 0 0 0 0 0 0
Controls H2O 5 ml/kg
7 3 35 33 2 6 0 0 0 0 0 0
8 3 44 41 3 7 0 0 0 0 0 0
9 3 37 36 1 3 0 0 0 0 0 0
10 2 20 19 1 5 0 0 0 0 0 0
11 3 34 33 1 3 0 0 0 0 0 0
-------------------------------------------------------------------------------------
a From: Gale & Bunch (1979).
b Underlined %, significantly different statistically from controls.
b Right hindlimb with two digits.
c Right kidney absent.
d Oedema (7), tail short (1).
e Small kidneys (3), right kidney absent (1).
f Oedema (17), omphalocele (1).
Table 19. Comparison of concentrations of trivalent and hexavalent
chromium in fetuses, placentas, and maternal organsa,b
------------------------------------------------------------------
Day 13 Day 16
Hexavalent Trivalent Hexavalent Trivalent
chromium chromium chromium chromium
------------------------------------------------------------------
Fetus 0.3 ± 0.01 0.03 ± 0.002 0.5 ± 0.04 0.1 ± 0.01
Placenta - - 3.0 ± 0.2 2.4 ± 0.2
Liver 29.4 ± 2.2 30.8 ± 6.5 33.9 ± 3.9 27.7 ± 3.5
Kidney 38.1 ± 1.9 5.3 ± 0.7 36.0 ± 4.3 8.7 ± 2.3
Serum 2.6 ± 0.2 9.3 ± 0.9 2.8 ± 0.4 11.0 ± 1.6
------------------------------------------------------------------
a From: Danielsson et al. (1982).
b Trivalent chromium (51CrCl3) and hexavalent chromium
(Na251Cr2O7) were injected iv on day 13 and day 16 of gestation
at doses of 10 mg chromium/kg body weight. The pregnant animals
were autopsied after 1 h. Concentrations are expressed in
mg/litre or g ± SEM (N = 4).
7.2.1.4. Cytotoxicity and micromolecular syntheses
Wacker & Vallee (1959) reported that trivalent chromium was
present in RNA from all sources examined; they hypothesized that
chromium might contribute to the stabilization of the structure.
Administration of trivalent chromium to mice (ip injection of
CrCl3 at 5 or 0.5 mg chromium/kg body weight; P < 0.01) caused
accumulation of chromium in the cell nucleus, which amounted to
about 20% of the accumulated chromium content of the liver cell,
and also enhanced RNA synthesis. Hexavalent chromium inhibited RNA
synthesis (K2CrO4 at 5 or 0.5 mg chromium/kg body weight;
P < 0.01) (Okada et al., 1983). In further studies, Okada et al.
(1984) pretreated rats with CrCl3 (ip injection of 5 mg chromium/kg
body weight) and then partially hepatectomized the rats. Chromium
accumulated in the regenerating liver, and hepatic RNA synthesis
was accelerated compared with that of partially hepatectomiced
controls. These findings suggest a direct participation of
chromium in RNA synthesis.
Potassium dichromate enhanced the passive uptake of ribo-and
desoxyribonucleosides (Levis et al., 1978; Bianchi et al., 1979),
caused an inhibition of DNA replication to BHK fibroblasts, and
reduced the colony-forming ability of BHK cells (doses: 10-4 - 20-7
mol). Hexavalent chromium (potassium dichromate with a
concentration of 5 x 10-7 mol/litre) reduced by half the
proliferation of NHIK 3025 cells originating from a human cervix
carcinoma (White et al., 1979).
The addition of calcium chromate to BHK21 cell cultures altered
their growth characteristics. Whereas these cells normally grow as
elongated fibroblast cells in parallel orientation, the chromium-
exposed cells grew as shortened fibroblasts with enlarged nuclei
and granular cytoplasm, randomly orientated. When grown in
carboxymethyl cellulose, the chromate-exposed cells underwent many
divisions in contrast to the 1 or 2 divisions of normal cells.
These alterations in growth properties persisted, even after
transfer to a normal medium (Fradkin et al., 1975).
Cultures of HeLa and rat embryonic cells were incubated with
various hexavalent and trivalent chromium compounds for 3 days and
the 50% inhibitory dose (ID50) for cell growth was determined. The
ID50 for sodium and potassium chromate and dichromate and calcium
chromate varied from 0.34 to 0.78 µg/ml of the medium. The ID50
for the trivalent compounds varied over a wider range: chromic
oxalate, 4; acetate, 52; nitrate, 720; chloride, 1030; and bromide,
1500 mg/litre. Similar results were found in rat embryonic cell
cultures (Susa et al., 1977).
7.2.1.5. Fibrogenicity
Because of the well-known fibrogenic effects of silica and
asbestos minerals, studies were initiated to determine whether
chromite and various other dusts exhibited similar properties. A
single intratracheal injection in rats of 40 mg chromite particles
(33% chromium, 0.7% SiO2, 99.9% of the particles being smaller than
5 µm) suspended in 1 ml Ringer's solution caused a moderate
cellular reaction that reached a peak after 2 months and then
decreased. After 8 months (end of observation), the lung weight
was within normal limits. Only an increase in fibrils that was not
significant was observed. The tissue reaction in regional lymph
nodes was minimal (Swensson, 1977).
Chromite FeO(CrAl)2O3 (10 mg), ground to an average particle
diameter of 1 µm, was injected into the pleural cavities of 25
mice. The animals were killed at intervals ranging from 2 weeks to
18 months after injection. Chromite produced the least reaction of
all the 15 dusts tested. This material attracted very few cells
and the eventual production of collagen was minimal (Davies, 1972).
7.2.2. Observations in farm animals
Systemic poisoning of farm animals with chromates is rare and
is mainly suspected to be a consequence of accidental industrial
contamination of drinking-water. The acute lethal dose for mature
cattle is approximately 600 - 800 mg/kg body weight; chronic
poisoning in young calves was produced by a daily dose of 30 - 40
mg/kg body weight for one month (NZ Department of Agriculture,
1954) with a clinical picture of profuse scouring, severe
dehydration, low blood pressure, changes in the alimentary tract,
including congestion and inflammation, and the rumen and abomasum
showing ulceration near perforation.
High chromium levels were found in the blood and liver of
calves that had died from poisoning. The minimum tissue levels
causing poisoning were 30 mg/kg in the liver and 4 mg/litre in
whole blood (Harrison & Staples, 1955). According to Romoser et al.
(1961), a level of 100 mg hexavalent chromium (as sodium
chromate)/kg did not affect growing chicks.
8. EFFECTS ON MAN
8.1. Nutritional Role of Chromium
The physiological role of chromium and the need for small
amounts of dietary chromium are indicated by:
(a) chromium-responsive cases of impaired glucose
metabolism in malnourished children and middle-aged
persons;
(b) 2 cases of proved chromium deficiency in patients fed
exclusively by parenteral alimentation; and
(c) epidemiological studies suggesting a link between
chromium deficiency and risk factors for cardiovascular
diseases.
8.1.1. Biological measurements and their interpretation
Biochemical methods for the diagnosis of the human nutritional
chromium status are still at the developmental stage. Blood- or
urine-chromium concentrations are not indicative of chromium
status. Several studies suggest the validity of the "relative
chromium response" in plasma or serum to the ingestion of 50 - 100
g of glucose within 30 - 120 min; the response also appears in the
urine. Lack of this response suggests chromium deficiency,
especially when combined with impaired glucose tolerance in the
presence of normal or elevated plasma-insulin levels.
8.1.2. Chromium deficiency
8.1.2.1. Adults
Chromium deficiency is diagnosed mainly through therapeutic
trials in which an impaired function is restored by supplementation
of the diet with small amounts of chromium close to the estimated
human requirement. Glinsmann & Mertz (1966) studied 4 male
diabetic patients for several months to 1 year, under the strictly
controlled conditions of a metabolic ward. The subjects, who were
fed identical portions of a diet prepared before the start of the
study, were allowed a measured amount of physical activity and were
stabilized under these conditions before the beginning of the
control periods. Three of the 4 patients responded to a chromium
level of 180 - 1000 µg/day as CrCl3 x 6H2O with a significant
improvement in oral glucose tolerance; the fourth showed a very
slight lowering of the glycaemic curve, which was not statistically
significant. A representative example is given in Table 20.
Table 20. Effect of chromium on mean glucose tolerancea,b
------------------------------------------------------------------------
Supplementation Days Number Mean blood glucose concentration
of ---------------------------------
tests Fast- Min after glucose load
ing 30 60 90 120
------------------------------------------------------------------------
None 0 - 32 10 840 2170 2290 1950 1560
60 µg, 3 x per day 33 - 74 12 850 2240 2380 2090 1620
60 µg, 3 x per day 75 - 119 13 840 2100 2130 1860 1410
60 µg, 3 x per day 120 - 140 5 840 2030 2010c 1750 1120c
None 180 - 194 1 960 2070 2900 2930 2370
60 µg, 3 x per day 195 - 211 5 830 1960 1930c 1750 1180c
None 256 - 313 10 870 2200 2450 2240 1660
1 mg, 3 x per day 337 - 340 1 680 1480 1620 1510 960
------------------------------------------------------------------------
a Modified from: Glinsmann & Mertz (1966).
b A 46-year-old man with maturity-onset diabetes, controlled on diet;
oral glucose tolerance test consisted of constant noon meal and 100 g
glucose.
c Mean values significantly different from control ( P < 0.025).
The condition of one of 2 diabetic out-patients, observed over
several months, was improved by chromium supplementation, the
condition of the other was not. Short-term treatment of 7 diabetic
patients for 1 - 7 days with 1 mg chromium did not affect any
variables of glucose metabolism, nor did chromium supplementation
change the normal glucose tolerance tests of 10 healthy, young
volunteers.
Glinsmann's positive results were not confirmed in a double-
blind crossover study on 4 normal volunteers and 10 diabetic
persons, with a crossover after 16 weeks. No effects of chromium
were detected on glucose tolerance or on fasting or postprandial
blood-glucose levels (Sherman et al., 1968). Schroeder et al.
(1970), while reporting that 2 - 10 mg chromium/day "almost always"
restored the impaired glucose tolerance in older, non-diabetic
subjects to normal, found only erratic and mild improvement in 4
out of 12 diabetic out-patients. On the basis of these
observations, Mertz (1969) concluded that chromium should not be
considered a therapeutic agent for diabetic patients.
On the other hand, it was reported in a later study on chromium
metabolism that chromium administration (500 µg/day, for 2 weeks)
produced a marked improvement in glucose utilization in both
juvenile and maturity-onset diabetic patients. Five of the
patients were followed for more than 1 year after exposure and were
found to maintain their improved glucose metabolism (Nath, 1976).
Of 10 non-diabetic elderly subjects with impaired glucose
tolerance, 4 responded to 150 µg chromium/day with complete
normalization of oral glucose tolerance tests. The remaining 6
subjects were not affected by the supplements (Levine et al.,
1968). Significant effects of a GTF-chromium-containing yeast
preparation were reported by Doisy et al. (1976) in 6 out of 12
elderly subjects with impaired glucose tolerance
Table 21. Effect of GTF supplementation on glucose tolerance tests in
elderly subjects with impaired tolerancea,b
-------------------------------------------------------------------------
Mean plasma-glucose levels Cholesterol Triglyceride
(mg/litre) (mg/litre) (mg/litre)
0 h 1 h 2 h
-------------------------------------------------------------------------
Before GTF 1060 ± 40c 2010 ± 70 1780 ± 80 2450 ± 90 1210 ± 80
11 tests
After GTF 990 ± 40 1620 ± 110 1320 ± 50 2050 ± 100 1120 ± 120
9 tests
Signifi- NS < 0.01 < 0.001 < 0.01 NS
canced
-------------------------------------------------------------------------
Mean serum-insulin levels
(microunits/ml)
0 h 1 h 2 h
Before GTF 24 ± 6 78 ± 17 118 ± 17
after GTF 26 ± 12 70 ± 8 83 ± 8
-------------------------------------------------------------------------
a From: Doisy et al. (1976).
b Supplement: 4 g yeast per min/day (Yeastamin Powder 95, Staley Co.,
Chicago, Illinois, USA). GTT: 100 g oral load.
c Mean ± SEM.
d Using paired t test, difference is significant.
In addition to the improvement in glucose tolerance in the
presence of a reduced insulin response, plasma-cholesterol
concentrations were significantly reduced. Several other
individual cases are reported in the publication of Doisy et al.
(1976), all of whom showed a lower serum-insulin response to a
glucose tolerance test during chromium supplementation.
Liu & Morris (1978) performed a similar study on the effects of
a yeast-chromium supplementation in 27 women, 40 - 75 years old, of
whom 15 had a normal and 12 an abnormal glucose tolerance test. In
11 of the normal subjects (73%), the integrated insulin response
was significantly reduced from 436 to 335 µU/ml ( P < 0.025) during
supplementation, without a statistically significant change in
glucose tolerance test. Significant reductions in the total glucose
response (10 650 - 9820 mg/litre; P < 0.001) and in the total
insulin response to glucose were observed in the subjects with
impaired glucose tolerance (1288 - 831 µU/ml; P < 0.001). The
glucose tolerance improved in 7 out of 12 women, but all 12 showed
a reduction in plasma-insulin.
The effects of supplementation of the diet with 200 µg
chromium/day (as chloride) on the glucose tolerance of 76 normal
adult volunteers was investigated in a double-blind cross over
study, with each period lasting 3 months. All 18 subjects entering
the study with impaired glucose tolerance (serum-glucose equal to
or greater than 1 g/litre, 90 min after a glucose load), showed
significant improvement during chromium supplements, but not with a
placebo, with an average decline in the 90-min serum-glucose levels
of 180 mg/litre ( P < 0.01) (Anderson et al., 1983b).
In another placebo-controlled study, 24 elderly volunteers were
given a daily supplement of either chromium-rich brewer's yeast or
chromium-deficient Torula yeast (9 g/day, for 8 weeks). The
glucose tolerance of the subjects given brewer's yeast
significantly improved with a concomitant decrease in plasma-
insulin levels, and a significant decline in plasma-cholesterol as
well as total plasma-lipids. No significant changes were observed
in the control group given the chromium-poor Torula yeast
(Offenbacher & Pi-Sunyer, 1980).
Additional, preliminary studies suggest a specific effect of
brewer's yeast on individual lipoprotein cholesterol fractions.
Twenty normal human subjects responded to daily supplementation
with 18 g of brewer's yeast over a 8-week period with a significant
reduction in low-density lipoprotein-cholesterol ( P < 0.01) and
with a simultaneous increase of 14% in the high-density lipoprotein
( P < 0.01) (Nash et al., 1979).
Similar results were observed in a 6-week supplementation trial
involving 8 physician volunteers, each of whom received 7 g/day of
brewer's yeast. Low-density lipoprotein-cholesterol declined by
nearly 18% ( P < 0.01), whereas the high-density cholesterol
fraction increased by 17.6%, resulting in a significant
( P < 0.001) decrease in the LDL/HDL ratio of 28% (Riales, 1979).
These studies suggest that the primary effect of the
supplementation must have been an increase in the effectiveness of
the insulin, because glucose tolerance was maintained or improved
in spite of lower insulin responses. It is important to note that
while the reduction in insulin levels occurred in all cases, an
improvement in glucose tolerance occurred in only a fraction. This
suggests two possible interpretations of all the results discussed
here, including those showing improvement in glucose tolerance in
only some of the subjects or no improvement at all. Lack of a
response to chromium could suggest that the subjects were not
chromium deficient and that their impaired glucose metabolism was
the result of other causes. Second, they may have responded with a
reduction in circulating insulin levels, as did all the subjects
with impaired glucose tolerance in whom the insulin response was
measured. Unfortunately, these measurements were not performed in
the earlier studies.
8.1.2.2. Malnourished children
Chromium deficiency has been implicated as a factor
contributing to the impairment of glucose tolerance in children
with protein calorie malnutrition in 3 countries, Jordan, Nigeria,
and Turkey. Hopkins et al. (1968) measured intravenous glucose
tolerance in 10 malnourished infants from the mountainous area of
Jordan and 9 equally malnourished infants from the valley area.
They found a severely depressed glucose removal rate of 0.7%/min in
the first group and a normal rate in the second, 3.8%/min (Table
22). The protein intake of both groups prior to hospitalization
had been identical; it was supplied in the form of a milk powder
with a chromium content of 18 µg/litre, when reconstituted.
However, analysis of 10 water samples revealed 3 times as much
chromium in the samples from the valley as in those from the
mountainous area (0.5 versus 1.6 µg/litre). This suggested the
possibility of chromium being a major determinant in the glucose
metabolism of these children, and the effects of supplementation
were measured in 6 children from the hills, with a severely
impaired tolerance test. The morning after oral administration of
250 µg chromium, as CrCl3 x 6H2O, the glucose removal rate had
markedly improved to 2.9%/min from a pretreatment level of
0.6%/min. Similar results were obtained in Nigeria (Table 22);
these tests also included control children who did not receive
chromium.
The immediate effects of chromium on these children were
remarkable and different from the lag phase always present in the
studies on adults. Equally important, was the short duration of
the effect of one dose demonstrated in a Jordanian child observed
for a longer period of time. These observations suggest that a
marked depletion in chromium stores must have existed in the
children.
Table 22. Significance of the effect of chromium on the impaired glucose
tolerances of malnourished infantsa
---------------------------------------------------------------------------
Subjects Number Glucose Significance
of removal of
infants rate difference
(%/min)
---------------------------------------------------------------------------
Jordanian infants from hill area with
0.5 µg chromium/litre in drinking-water 10 0.7
Jordanian infants from valley with
1.6 µg chromium/litre in drinking-water 9 3.8 P < 0.001
Jordanian infants;
initial glucose tolerance test 6 0.6 P < 0.001
after chromium treatment 6 2.9
Nigerian infants;
initial glucose tolerance test 6 1.2
after chromium treatment 6 2.9 P < 0.05
Non-treated infants:
Initial glucose tolerance test 5 1.9 not signi-
Repeated glucose tolerance test 5 2.1 ficant
---------------------------------------------------------------------------
a From: Hopkins et al. (1968).
Similar results were reported from a study in the Istanbul area
of Turkey (Gürson & Saner, 1971). Fourteen malnourished children
with an impaired glucose removal rate of 1.71%/min were given 250
µg of chromium (CrCl3 x 6H2O), and a second test was performed
after 15 h. The removal rate increased to 3.91%/min ( P < 0.001).
This average increase was due to a pronounced increase in 9 of the
children; no change was observed in the remaining 5. No
improvement in glucose tolerance occurred in 5 control children
receiving identical hospital treatment, except for the
administration of the chromium. The children receiving the
chromium supplementation grew significantly more than the control
children during the following 30 days, with the greatest growth
rates found in the 9 children who had responded to administration
of chromium with an improvement in glucose tolerance.
The fact that chromium deficiency interacts with protein-
calorie malnutrition only in certain geographical areas was
confirmed by Carter et al. (1968), who gave chromium
supplementation (250 µg/day as CrCl3 x 6H2O) without effects on
glucose tolerance in 9 Egyptian children tested. Plasma-chromium
levels were in the normal range, and chromium levels in
representative foods were high, ranging from 200 to 390 µg/kg.
These findings suggest that the chromium status of these subjects
was normal, which would explain the lack of an effect.
8.1.2.3. Patients on total parenteral alimentation
A female patient, maintained on total parenteral alimentation
since the age of 35 years, developed an unexpected weight loss of
15% after 5 years combined with peripheral neuropathy, impaired
intravenous glucose tolerance, in spite of normal insulin response,
and a decreased respiratory quotient of 0.66. Insulin treatment
(45 U/day) was ineffective. A strongly negative chromium balance
and low plasma- and hair-chromium levels suggested that the daily
estimated chromium intake from the infusion fluids of 5.3 µg had
not met the patient's requirement and resulted in chromium
deficiency. The insulin infusions were stopped and replaced by the
daily infusion of 250 µg chromium (as CrCl3 x 6H2O) for 2 weeks.
The result was normalization of glucose tolerance and respiratory
quotient, disappearance of signs and symptoms of neuropathy, return
to the previous normal body weight, in spite of reduced caloric
intake, disappearance of the requirement for exogenous insulin, and
a positive chromium balance. The nitrogen balance, which had been
erratic and often negative, became consistently positive following
chromium supplementation. Reduction of the daily supplement to 20
µg chromium proved to be sufficient to maintain the patient in a
state of well-being (Jeejeebhoy et al., 1977).
This case is the best demonstrated example of chromium
deficiency in a human subject. In addition to confirming the well-
known signs of chromium deficiency reported earlier, such as
insulin resistance and its consequences, it was the first case to
demonstrate the occurrence of peripheral neuropathy and disturbance
of nitrogen balance as a result of deficiency. These disturbances
require further study. A second case of chromium deficiency as a
consequence of long-term total parenteral alimentation was reported
by Freund et al. (1979). Signs and symptoms were similar to those
of the first case, except that central encephalopathy was present
instead of peripheral neural disorder. Supplementation with 150 µg
of chromium/day resulted in normalization of the deficiency signs.
Chromium at that time was not routinely added to intravenous
solutions, therefore it is possible that more cases of deficiency
may exist in patients on long-term infusion treatment. As an
outcome of this study, an Expert Panel of the American Medical
Association (AMA, 1979) suggested that the stable adult patient on
total parenteral alimentation should receive 10 - 15 µg chromium
daily.
8.1.2.4. Epidemiological studies
Two epidemiological studies suggest a link between chromium
status and cardiovascular diseases (Schroeder et al., 1970; Punsar
et al., 1975). Lack of chromium is associated with impaired
glucose tolerance, elevation of circulating cholesterol, and aortic
plaques. All of these variables are recognized risk factors for
cardiovascular disease. It has also been suggested that the
elevated insulin levels, often present in chromium-deficient human
subjects (Liu & Morris, 1978) represent a substantial risk for the
development of cardiovascular disease (Strout, 1977).
Schroeder et al. (1970) have summarized their studies of
chromium concentrations in normal and diseased tissues,
particularly in aortas (Table 23). The total body burden of
chromium, excluding the lungs, was lower in the USA, where there is
a higher incidence of cardiovascular disease, compared with other
regions, 1.72 - 1.86 mg/kg ash weight in the USA compared with 7.7,
9.6, and 2.7 mg in Africa, the Far East, and the Near East,
respectively. The only significant difference in tissue-chromium
concentrations in deaths from heart disease compared with deaths
from accidental causes was in the aorta (Table 23). Concentrations
in aortas from Africa, the Far and Near East were generally higher
and did not show such a difference.
Table 23. Chromium in aortasa
----------------------------------------------------------
Location Chromium (mg/kg dry weight)
Accident P Heart disease
----------------------------------------------------------
Africa 0.072 (5)b NS 0.116 (2)
Far East 0.97 (8) NS 0.246 (5)
Middle East 0.36 (8) NS 0.216 (3)
USA (Nine cities) 0.26 (103) 0.005 0.052 (13)
USA (San Francisco) 0.228 (10) 0.005 0.048 (15)
----------------------------------------------------------
a From: Schroeder et al. (1970).
b The number in parenthesis denotes the number of cases
observed.
The second epidemiological study was part of a cohort study
conducted in Finland beginning in 1959 (Punsar et al., 1975). A
total of 327 drinking-water samples were analysed for 22
characteristics including pH, anions, bulk minerals, and several
trace elements including chromium. The study included 504 and 622
men in the Western and Eastern areas of Finland, respectively,
whose domicile had not changed since 1959.
The coronary heart disease mortality rate is much higher in the
east of Finland than in the west. As seen in Table 24, the
chromium levels in drinking-water were much lower in the east than
in the west, whereas the opposite was true for copper. The metal
concentrations in the drinking-water of those with heart disease
and others only differed slightly (Table 24), but, according to the
authors, the data suggest that low chromium levels may play a role
in the high heart disease death rate of eastern Finland.
Table 24. Concentrations of copper and chromium in the
drinking-water of men who died between 1969 and 1973, and
of those who were alive in 1973a
-------------------------------------------------------------
Alive
Deaths (Clinical status in 1969)
CHDb Other CHDb Other No
heart heart
disease disease
-------------------------------------------------------------
West N = 19 N = 23 N = 63 N = 31 N = 288
Copper X 22.15c 20.17 20.05 20.94 20.15
(µg/litre) SD 3.92 4.69 4.69 5.12 4.74
Chromium X 7.52 9.38 9.45 9.74 8.45
(µg/litre) SD 2.06 3.42 4.01 4.44 3.31
East N = 23 N = 26 N = 129 N = 44 N = 279
Copper X 41.59 41.24 42.67 39.50 36.32
(µg/litre) SD 29.91 30.43 33.65 28.74 28.66
Chromium X 2.20 2.09 2.04d 2.49 3.01
(µg/litre) SD 2.17 2.41 2.08 2.17 2.37
-------------------------------------------------------------
a From: Punsar et al. (1975).
b CHD = coronary heart disease.
c P < 0.05.
d P < 0.001.
Note: Levels of significance refer to differences observed
when the concentrations were compared with those in the
"no heart disease" category.
Newman et al. (1978) measured serum-chromium concentrations in
32 patients, 18 male and 14 female, aged 25 - 65 years, in whom
coronary artery cine-arteriography was performed for medical
reasons. Serum-chromium was significantly lower in the 15 subjects
with coronary artery disease than in the 17 subjects without
involvement (2.51 versus 6.09 µg/litre; P < 0.01), whereas serum-
cholesterol, weight index, and systolic or diastolic blood pressure
did not differ among the groups. Serum triglycerides were higher
in the group with coronary artery disease (1.84 versus 1.12
g/litre; P < 0.05). No subject with a serum-chromium
concentration of more than 6 µg/litre exhibited coronary artery
involve- ment, and by regression analysis, serum-chromium was
judged to explain 17% of the total variance of the disease.
These results were independently confirmed in a second study in
which plasma chromium concentrations of 23 healthy persons were
more than 8 times greater than those of 67 patients with coronary
artery disease, as confirmed by cine-angiography (Simonoff et al.,
1984).
8.1.3. Mode of Action
As in experimental animals, the best known action of chromium
is the potentiation of insulin. This is shown by the
supplementation studies of Doisy et al. (1976) and Liu & Morris
(1978) in which a reduction in insulin levels in response to
glucose was observed, together with an improvement in glucose
tolerance. Direct proof, for example from in vitro studies with
human biopsy tissue, has not yet been obtained. Furthermore, it is
not known whether the potentiation of insulin is responsible for
all the effects observed during chromium supplementation, including
the reduction of circulating cholesterol and the growth stimulation
observed in malnourished children and during parenteral nutrition.
In the healthy, chromium-sufficient human subject, chromium
levels in the bloodstream increases acutely following a glucose
load or an injection of insulin. Glinsmann et al. (1966) observed
acute increases in 5 young, healthy volunteers during a glucose
tolerance test, but not when the same subjects were given an
equivalent volume of water. Maturity-onset diabetic patients did
not exhibit this chromium response initially, but did show a rise
in plasma-chromium, after several weeks of chromium
supplementation. These observations were confirmed by Levine et
al. (1968), Hambidge (1971), Behne & Diel (1972), and Liu & Morris
(1978), who saw the acute chromium rise in some, but not all,
subjects. In the study of Liu & Morris (1978), which was the most
detailed, the relative chromium response (CrR) was measured by
neutron activation analysis in 15 women with normal glucose balance
and in 12 with impaired tolerance.
(1-h serum-chromium)
CrR = ------------------------ x 100
(fasting serum-chromium)
Initially, the normal group had a mild positive response (CrR =
107), compared with a negative response (CrR = 81) in the impaired
subjects. Following three months of supplementation with a
chromium-rich yeast extract, the CrR of the normal subjects
increased to 140, and that of the impaired group became positive
with CrR = 149. As discussed in section 8.1.2, these changes were
accompanied by a significant decrease in the insulin response to
glucose and an improvement in glucose tolerance. The results of
these studies are in apparent contrast with those of Davidson &
Burt (1973), who found a significant decline in plasma-chromium
levels following a glucose load in normal, non-pregnant women (mean
age 26 years), but a mild to moderate increase in all but 1 of 10
pregnant women (mean age 20 years). As the time of the acute rise
varied between women, statistical analysis at any one time did not
produce any significant results. Hambidge (1971) and Behne & Diel
(1972) demonstrated an acute plasma-chromium response following
injection of insulin; it can therefore be assumed that the effect
of glucose on the chromium response is associated with
hyperinsulinaemia. The acute "relative chromium response" is
believed to reflect the chromium status of human subjects. It does
not appear when stores from which the chromium increment must come
are depleted. Depleted stores can be replenished by
supplementation, with a resulting reappearance of the response
shown in the two previously discussed studies. As it has been
shown that chromium levels in urine increase following a glucose
load (Wolf et al. 1974; Gürson & Saner, 1978), it is likely that a
fraction or all of the chromium increment is subsequently excreted.
8.2. Acute Toxic Effects
In adults, the lethal oral dose of soluble chromates is
considered to be between 50 mg/kg body weight (RTECS, 1978) and 70
mg/kg body weight (Deichmann & Gerarde, 1969). The clinical
features of toxicity are vomiting, diarrhoea, haemorrhagic
diathesis, and blood loss into the gastrointestinal tract causing
cardiovascular shock. If the patient survives about 8 days, the
outstanding effects are liver necrosis (Brieger, 1920), tubular
necrosis of kidneys, and poisoning of blood-forming organs
(Langard, 1980). A 14-year-old boy, who had ingested about 1.5 g
of potassium dichromate, died 8 days later. Kaufmann et al. (1970)
also reported enlargement and oedema in the boy's brain.
8.3. Chronic Toxic Effects
The chronic effects of trivalent and hexavalent chromium have
been reviewed by Langard & Norseth (1979). The most important
features are changes in the skin and mucous membranes, and allergic
dermal and broncho-pulmonary effects. Important systemic effects
occur in the kidneys, liver, gastrointestinal tract, and
circulatory system.
Mancuso (1951) studied several biological variables in persons
occupationally exposed to chromate and related them to clinical
signs and symptoms. He found occasional leukocytosis or
leukopenia, monocytosis, eosinophilia, reduced haemoglobin
concentrations and prolonged bleeding times, but none of them were
consistent enough to serve as reliable diagnostic tests.
Mild normochronic anaemia was reported by Myslyaeva (1965) in
94 subjects exposed to chromium compounds with 68 of them showing
signs of chronic toxicity. Though the report of Baetjer et al.
(1959b) was based only on 3 cases, it indicated that chromate
attached preferentially to red blood cells. Koutras et al. (1964)
reported the inhibition of the red blood cell enzyme gluthathione
reductase by chromate concentrations of 5 - 25 mg/kg body weight.
However, the lowest effective dose is far in excess of levels
found, even in heavily exposed persons. Thus, all attempts made so
far have failed to identify a specific sensitive biochemical test
for the assessment of chronic chromium toxicity.
Long-term surveillance of pregnant women working at a plant
producing dichromates and those living in its immediate vicinity
(Shmitova, 1980) revealed increased chromium levels in the blood
and urine, compared with those in the control group. At 32 weeks
of pregnancy, the blood concentrations of chromium were,
respectively, 2.5 mg/litre in the women workers, 1.2 mg/litre in
the women living near the plant, and 0.19 mg/litre in the controls;
the levels in the urine were 1, 0.47, and 0.045 mg/litre,
respectively. Umbilical venous blood contained 1.8, 0.66, and 0.37
mg chromium/litre, respectively. The placental chromium contents
in the surveyed women were 162, 179, and 40 µg/kg, respectively,
and the breast-milk levels were 119, 20, and 31 µg/litre, which
means that chromium is capable of transplacental entry into breast
milk and into the fetal and infant organism. Women working at the
bichromate plant exhibited a high incidence of obstetric pathology
and geotoses. Chromium levels were estimated in fetal and
placental tissues (abortive material) derived on the 12th week of
pregnancy from women workers of the dichromate facility and those
not exposed to chromium. In the test group, chromium levels were
1140 µg/kg in the fetal tissues and 135 µg/kg in the placental
tissue, whereas, in the controls, they were 928 and 30 µg/kg,
respectively. No teratogenic effects have been revealed (Shmitova,
1980).
8.3.1. Effects on skin and mucous membranes
Several different types of effects on skin and mucous membranes
may result from exposure to chromium chemicals, and the main
features are:
(a) primary irritation: ulcers (corrosive reactions),
scarred and non-ulcerative contact dermatitis;
(b) allergic contact dermatitis: eczematous and
noneczematous.
A general survey of the nature and cause of contact dermatitis
is given by Fregert (1981).
8.3.1.1. Primary irritation of the skin and mucous membranes
(a) Skin
Skin irritation, manifested by a sharp hyperaemia as well as by
vesicular, papular, and rash pattern, may result from contact with
chromates. However, the major problems in the primary irritation
dermatoses are ulcers, often called chrome holes or chrome sores.
Ulceration is likely to occur among workers who have contact with
high concentrations of chromic acid, sodium or potassium dichromate
or chromate, or ammonium dichromate. It does not result from
contact with trivalent chromium compounds. An ulcer may develop if
the chromium compound, either a dust or a liquid, comes into
contact with any break in the skin such as an abrasion, scratch,
puncture, or a laceration. Favoured sites for ulcer development
are the nailroot areas, the creases over the knuckles, finger webs,
the backs of the hands, and the forearms (Bloomfield & Blum, 1928).
Primary irritation by chromium can be attributed to hexavalent
chromium, as the salts of trivalent chromium are not considered
capable of producing such effects (Fregert, 1981). However,
prolonged exposure to trivalent chromium salts has been reported to
cause skin lesions, less marked than those following exposure to
hexavalent chromium salts (Domaseva, 1971). Findings in 164
patients with eczema showed that skin tests on people who had
contact with trivalent chromium did not give positive results with
dichromate (Valer & Racz, 1971).
Ordinarily, a chrome sore, if not deep, persists for about 3
weeks after exposure is discontinued. There is no evidence that
the ulcers undergo malignant transformation. Furthermore, the
presence of chromium ulcers did not influence the development of
sensitization to chromates (Edmundson, 1951), evidenced by patch
testing with a 0.5% solution of K2Cr2O7.
(b) Nasal septal mucosa
Perforation of the nasal septum was one of the main chronic
effects in workers who had contact with chromates and chromic acid
(Bloomfield & Blum, 1928; Langard & Norseth, 1979). In a group of
100 persons constantly in contact with chromium compounds, 44 had
superficial ulceration of the anterior nasal septal mucosa.
Twenty-one people had profound ulcers and 5 had perforations of
various sizes in the cartilaginous part of the nasal septum.
Perforation of the nasal septum and cicatricial alterations were
observed in people with 3 or more years of service, whereas
ulcerous lesions arose in 2 years of work with chromium compounds.
An additional consequence of the effect on the nasal mucous
membranes is a loss of the senses of smell and taste (Seeber et
al., 1976). In workers with profound ulcerous lesions of the nasal
septal mucosa, strains of white staphylococcus with elevated
resistance to chromium are sometimes isolated (Cherevaty et al.,
1965).
Pokrovskaya et al. (1976) studied 561 workers engaged in the
open excavation of chromium ores (chromium content was 62% in the
form of a complex compound of magnesium chromite and other chromium
ores). Almost half of the workers had worked there for more than 5
years, and about 40% had been working there for 6 - 15 years.
Changes in the nasal septal mucosa, typical of the effect of
chromium compounds, were found in 8.2% of the workers. The longer
the service record, the greater the prevalence of these lesions.
After 5, 11, and 15 years of work, the prevalence was 2.3%, 8.3%,
and 11.2%, respectively.
The prevalence of nasal septum perforation in chromium plating
workers was very high (24%) in the early 1950s in China (Yang,
1956). With the introduction of control measures, the prevalence
dropped so that, in 1982, only 2 cases of nasal septum perforations
were found among 393 chromium-exposed workers (Peng, 1982).
One hundred and four subjects (85 male, 19 female) exposed to
chrome plating and 19 unexposed controls were studied by Lindberg &
Hedenstierna (1983) with regard to changes in the nasal septum.
Exposed subjects were employed at 13 different companies and
included all who were working on the day of study at the particular
company. The median exposure time was 4.5 years (range, 0.1 - 36
years). Forty-three subjects were exposed almost exclusively to
chromic acid and constituted both a "low exposure" group (8-h mean
below 2 µg/m3, N = 22) and a "high exposure" group (2 µg/m3 or
more, N = 21). Their median exposure time was 2.5 years (range,
0.2 - 23.6 years). The other 61 subjects were exposed to a mixture
of chromic acid (0.2 - 1.7 µg/m3) and other pollutants, such as
nitric, hydrochloric, and boric acids, as well as caustic soda,
nickel, and copper salts. The last group was included to disclose
any additive or synergistic effects of chromic acid and other
pollutants and was studied with regard to lung function only. The
breakdown of subjects into various groups is shown in Table 25.
Nasal septal ulceration and perforation were seen in 10 out of 14
subjects exposed to peak levels of 20 - 46 µg chromic acid/m3 or
more; no one in the control group showed signs of atrophy,
ulcerations, or perforations.
8.3.1.2. Allergic contact dermatoses
Chromium is a very important skin sensitizer. Sensitization
is reported to require about 6 - 9 months, but can occur in less
than 3 months (Pirilä & Kilpio, 1949). Genetic factors appear to
contribute to the appearance of chrome sensitivity within the
Jewish population (Wahba & Cohen, 1979).
Apart from the primary irritation and ulceration effects,
direct contact with small amounts of the chromates may cause
allergic dermatosis (dermatitis and eczema). As this allergic
reaction is cell-mediated, i.e., antibody formation is intra-
cellular and not humoral, delayed allergic reactions are rarely
observed.
Fregert et al. (1969) reported that the skin-patch test for
chromium was positive in 8 - 15% of all patients suffering from
eczema. The dermatitis caused by chromium is described as a
diffuse erythematous type with severe cases progressing to an
exudative stage. Persons sensitive to chromium react to patch and
intracutaneous tests with nonirritant concentrations of potassium
dichromate (K2Cr2O7). Opinion is divided about the role of the
oxidation state of chromium as a causal agent in allergic contact
dermatoses (Morris, 1958; Fregert & Rorsman, 1964; Kogan, 1971).
In a review, Polak et al. (1973) showed that hexavalent chromium,
which penetrates the skin by a direct effect of a hapten, is
reduced to the trivalent state, chiefly by sulfhydryl groups of
aminoacids. It then conjugates with proteins thus forming the full
antigen that initiates sensitization.
Guinea-pigs sensitized with either trivalent chromium chloride
or hexavalent potassium dichromate are capable of reacting in vivo
and in vitro to challenges with both chromium salts. This double
reactivity is also found after repeated stimulation with only one
of these chromium compounds. Since it is not possible to select
lymphocytes directed specifically against a chromium determinant of
a particular valence, it is concluded that, by sensitization with
chromium salts of different valences, a common determinant or
closely related determinants are formed. It is suggested that this
determinant is formed by chromium in the trivalent form
(Siegenthaler et al., 1983).
Two to three years after medical treatment, a follow-up study
on 555 patients with contact dermatitis was completed by means of
questionnaires (Fregert, 1975). The eczema had healed in one-
quarter of the patients, one-half had periodic symptoms, and one-
quarter had permanent symptoms. The prognosis for persons who
changed their work or stopped working was the same as that for
those who continued eczema-inducing work.
From 48 positively tested patients (0.5% potassium dichromate),
38 persons, i.e., 79%, still had a positive patch test after 4 - 7
years. In 72% of the cases, a history of occupational exposure to
chromates could be proved (Thormann et al., 1979).
A fairly rapid transformation of dermatitis into eczema is a
characteristic feature of chromium-induced skin lesions. Kogan
(1971) recognized the following important properties: (a) absence
of adaptation, which makes it necessary to discontinue chromium
handling until the slightest clinical manifestations have
disappeared; (b) a persistent chronic development of an eczematous
process; (c) a long-term latent period; and (d) absence of a direct
correlation between the incidence of sensibilization in the exposed
workers and the chromium concentration to which they have been
exposed.
Table 25. Series of exposed subjects and referencesa
--------------------------------------------------------------------------------------------------------
Exposed subjects
Mixed exposure to
Low exposure to High exposure to chromic acid
chromic acid chromic acid (< 2 µg/m3) and other
(< 2 µg Cr6+/m3) (> 2 µg Cr6+/m3) acids and metallic salts References
----------------- ------------------ ----------------------- -----------------
Nb Age (years)c N Age (years) N Age (years) N Age (years)
--------------------------------------------------------------------------------------------------
Men
Non-smokers 10 35.8 ± 16.7 6 33.4 ± 14.1 12 33.6 ± 12.2 52 34.2 ± 13.2
Smokers 6 30.8 ± 9.7 16 30.2 ± 11.8 36 42.8 ± 13.1 67 30.5 ± 8.9
Women
Non-smokers 3 30.7(17-46) 0 9 48.3 ± 13.0
Smokers 2 44.5(29-60) 1 51.2 4 31.0(19-43)
--------------------------------------------------------------------------------------------------
a From: Lindberg & Hedenstierna (1983).
b When N < 5, the range is given instead of SD.
c X ± SD.
Patients suffering from chromium-induced allergic skin
dermatoses had a tendency to develop cross-correlated hyper-
sensitivity to other metals, in particular, to cobalt and nickel
(Rostenberg & Perkins, 1951; Geiser et al., 1960; Clark & Kunitsch,
1972; Kogan et al., 1972).
Increased sensitivity to chromium is proved by skin reaction
tests. In a number of cases, additional immuno-haematological
bioassays are made to confirm the specificity of these tests. A
method for studying the basophil (mast-cells) degranulation in vivo,
at the site of positive compress skin tests, was suggested by Kogan
(1968). The method was used as a basis for proving the specificity
of cutaneous tests for chromium and other sensitising metals of the
chromium group (cobalt and nickel) (Kogan, 1968; Kogan et al.,
1972).
Photosensitivity is sometimes observed in patients with chronic
dermatitis. Wahlberg & Wennerstein (1977) investigated patients
allergic to chromium, with or without clinically observed light
sensitivity, by a standardized photopatch test procedure. A
significantly more intense reaction was observed in 48% of the
cases in the UV-irradiated sites (4/5 minimal erythema dose)
compared with the non-irradiated control sites.
No cases of cancer of the skin have been reported to result
from exposure to any form of chromium (Fregert, 1981).
8.3.2. Effects on the lung
Airborne chromium trioxide is rapidly absorbed in the broncho-
pulmonary tract causing corrosive reactions (Borghetti et al.,
1977). The absorption of chromium aerosols may depend on the
physical and chemical properties of the particles (Taylor et al.,
1965), as well as on the activity of alveolar macrophages, and the
lymphatic drainage (Sanders et al. 1971).
Both Jindrichova (1978) and Keskinen et al. (1980) reported
that welders using chromium-containing electrodes suffered from
bronchitis. Zober (1982) studied arc welders who had used filler
metals containing chromium and nickel for some years. Compared
with controls, the welders reported more previous ailments of the
respiratory system, mainly in the form of acute bronchitis. In
X-rays of welders, more roundish shadows were found, probably
indicative of benign accumulations of ferrous dust. The greater
incidence of significant findings was clearly apparent in welders
who smoked (probably a combined effect of tobacco smoke and welding
fumes). Reggiani et al. (1973) studied 101 electroplaters by means
of spirometric tests and found an obstructive disease pattern in
13% of them. After inhalation of acetylcholine, a bronchospastic
reaction occurred in 23% of the workers. In another spirometry
study (Bovet et al., 1977) on 44 chromium electroplaters, an
obstructive respiratory syndrome was found among the workers with a
high urinary-chromium content. The authors concluded that the
influence of tobacco smoking on the spirometric tests was much less
than the influence of chromium.
Studying lung function and generalized obstructive lung
diseases in electrofurnace workers in a ferrochromium and
ferrosilicon-producing plant, Langard (1980b) found a reduced
forced vital capacity (FVC) and an increased prevalence of
obstructive lung diseases. Unable to document a connection between
the presence of chromates and increased lung diseases, the author
suggested that the effects were due to high levels of total dust,
especially amorphous silica dust.
Lindberg & Hedenstierna (1983) studied lung function in more
than 100 subjects exposed to chrome plating and in 119 car
mechanics as controls (no car painters or welders). In the exposed
subjects, FVC and the forced expired volume in one second (FEV1)
were both reduced by 0.2 litre, and the forced mid-expiratory flow
was reduced by 0.4 litre/second. The authors concluded that an 8-h
mean exposure level of more than 2 µg/m3 might cause a transient
decrease in lung function.
Pneumoconiosis was diagnosed in Germany (Letterer et al., 1944)
and again in chromite miners in South Africa (Sluis-Cremer & du
Toit, 1968). However, American medical officers could not confirm
this feature when examining 897 workers in chromate-producing
plants in the USA (US PHS, 1953). It should be noted that chromium
dusts had a poor fibrogenic potency in experimental animal studies
(section 7.2.1.5). Davies (1974) X-rayed workers employed in the
manufacture of ferro-chrome and found results similar to miliary
lung tuberculosis. The workers were in the immediate area of the
furnace and were exposed to dust-particles, 90% of which were less
than 2 µm in diameter. The dust deposited on girders consisted of
95% SiO2 and 2.4% Cr2O3. The author did not find any symptoms or
signs of tuberculosis, but there was sufficient evidence to
indicate an inhalation hazard in the ferro-chrome industry, thus
underlining earlier observations of Princi et al. (1962).
8.3.2.1. Bronchial irritation and sensitization
Chromium irritates mucous membranes, as has been seen with
exposure to airborne chromium trioxide fume (Meyers, 1950) or mixed
dust (Card, 1935; Broch, 1949; Williams, 1969). The first symptom
was an irritating cough, 4 - 8 h after exposure. Chromium causes
sensitization that can result in asthmatic attacks. These can last
for 24 - 36 h, without treatment (Langard & Norseth, 1979). An
attack may recur on later exposure, even when this exposure is to a
much lower concentration (US NAS, 1974a).
A double-blind experimental exposure study on an asthmatic
patient, who had been exposed to chromium and nickel, showed that
chromium was the main cause of the asthma (Novey et al., 1983).
Keskinen et al. (1980) pretreated their patients, stainless steel
welders, with both disodium cromoglycate and betamethasone and
found an inhibition of this reaction. Placebo medication failed to
produce this inhibition.
8.3.3. Effects on the kidney
In the early part of this century, chromates and chromic acid
were occasionally used as therapeutic chemicals for some skin
lesions. Fatal cases of acute nephritis occurred with albumin,
hyaline, and granular casts and red cells appearing in the urine,
accompanied by oliguria. Autopsies revealed tubular necrosis, but
not glomerular damage (Kaufman et al., 1970). Acute toxic
gastroenteritis developed, followed by arterial hypotonia, in
suicide cases a few hours after swallowing 1.5 - 10 g potassium
dichromate. Within 2 - 4 days, all patients developed signs of
acute renal insufficiency with oliguria, anuria, and hyperhydration
and also evidence of acute toxic hepatitis (Luzhnikov et al.,
1976). Kidney damage has also occurred in industry, as a result of
accidental exposure of the skin to large amounts of chromate,
especially when associated with extensive skin damage (Luzhnikov et
al., 1976).
The mortality from kidney diseases among workers in the
chromium industry does not appear to have increased. In one study,
no deaths from nephritis and uraemia were found in 4 chromate-
producing plants; in only 1 plant was the mortality rate above that
for the controls (Machle & Gregorius, 1948). Hayes et al. (1979)
reported that 4 deaths from nephritis and nephrosis had occurred
between 1945 and 1977 in workers in a chromate-producing plant,
compared with 4.95 cases in the control population. Satoh et al.
(1981) found one case of nephritis and nephrosis in a group of
chromate workers compared with an expected number of 2.18.
Some authors (Franchini et al., 1975; Borghetti et al., 1977;
Gylseth et al., 1977; Tola et al., 1977) have concluded that
urinary excretion and renal clearance of diffusible chromium can be
used as biological indicators for evaluating the extent of exposure
to airborne chromium and the body burden. However, a dose-effect
relationship could not be established between duration of exposure
and either the level of chromium excretion (Tandon et al., 1977) or
early nephrotoxic indicators (Mutti et al., 1979). A dose-
response relationship for hexavalent chromium effects on the kidney
was described by Mutti et al. (1979), measuring beta-glucuronidase,
protein, and lysozyme in the urine. Abnormal patterns were not
observed in 39 stainless-steel welders during 4.5 ± 3.2 years of
exposure. In another group of workers employed in welding armoured
steel with special electrodes for 1 ± 0.4 years, 22% showed an
increase in beta-glucoronidase and 10% in protein in the urine. In a
study on 24 workers in the chromium-plating industry who were
exposed for 8.9 ± 7.3 years, the authors found increased urinary
levels of beta-glucoronidase in 37% of the workers, an increase in
protein in 17%, and an elevated level of lysozyme in 4%. Urinary
beta2-microglobulin was also studied by Lindberg & Vesterberg (1983b)
in chromeplaters (24 males, mean age 36 years, working in 4
different factories). The exposure was measured using personal air
samplers. The 8-h mean value ranged between 2 and 20 µg hexavalent
chromium/m3 and averaged 6 µg/m3. Most of the workers showed
symptoms of airway irritation, 2 of them had ulcerated nasal septum
and another 2, complete perforation. Significantly higher urine
beta2-microglobulin levels (0.23 mg/litre; range, 0.04 - 1.24 mg/litre;
SD, 0.27) were found in this group of workers in comparison with a
reference group of workers of a comparable age (mean age, 38 years;
mean urinary beta2-microglobulin level, 0.15 mg/litre; range, 0.06 -
0.19 mg/litre; SD, 0.08). A relationship was observed (Table 26)
between the range of exposure and prevalence of urinary beta2-micro-
globulin levels exceeding 0.3 mg/litre. The 2 workers with
perforated nasal septum were in the highest exposure group. A
difference in urinary beta2-microglobulin levels was not found between
a group of workers employed several years earlier in an old
chromeplating plant (mean, 0.25 mg/litre) and a group of workers of
comparable age (mean, 0.29 mg/litre), but it should be noted that
the mean age of both these groups exceeded 52 years.
Table 26. beta2-microglobulin U-beta2 levels in urine of workers
related to present exposure levelsa
-------------------------------------------------------------------------
Plant Exposure Number Age Persons with an "elevated" U-beta2
No. range of (mean) (U-beta2 > 0.30 mg/litre) range
(µg/m3) workers concentration (mg/litre)
No. Ages
-------------------------------------------------------------------------
1 11 - 20 5 39 3 21, 37, 58 0.23 - 1.30
2 - 3 14 - 8 13 37 2 20, 21 0.04 - 0.44
4 2 - 3 6 6 - - 0.06 - 0.18
-------------------------------------------------------------------------
a From: Lindberg & Vesterberg (1983b).
8.3.4. Effects on the liver
Statistics on diseases of the liver are seldom published
separately; they are usually included in the group of diseases of
the digestive system. The chromate industry was reported to have
the same rate in this group of diseases as many other industries
(US PHS, 1953). The Federal Security Agency in the USA
investigated 14 chromate workers reported to have "enlarged livers"
but found that these cases were not related to the time spent in
the industry. Acute hepatitis with jaundice was described in one
worker employed in a chromium-plating plant.
In an epidemiological study, Satoh et al. (1981) reported a low
rate of cirrhosis of the liver in chromium workers and liver
function test results that did not differ significantly from those
of the controls.
However, following ingestion of high doses of potassium
dichromate, liver necrosis (Brieger, 1920) and congested liver
(Kaufman et al., 1970) with loss of its architecture have been
described. There are also case studies of liver function and
histology in electroplaters (Pascale et al., 1952) and chromate
chemical plant workers (Etmanova, 1965), but the results are
difficult to interpret.
8.3.5. Effects on the gastrointestinal tract
The following symptoms and signs were reported in workers
engaged in the production of chromium salts (Sterekhova et al.,
1978): hyperchlorhydria, elevated pepsin and pepsinogen level,
oedema, hyperaemia and erosion of the mucosa, polyposis,
dyskinesia, and gastritis. However, Satoh et al. (1981) reported
that the incidence of peptic ulcer in chromate workers was below
the expected rate, and another study showed that the mortality rate
for diseases of the digestive system was lower in chromate workers
than in the control population (Hayes et al., 1979).
The gastric juice has been shown to play a role in the
detoxification of ingested hexavalent chromium by reducing it to
trivalent chromium, which is poorly absorbed and eliminated with
the faeces (Donaldson & Barreras, 1966; DeFlora & Boido, 1980).
8.3.6. Effects on the circulatory system
A clinical study was performed by Kleiner et al. (1970) on
myocardial function in more than 200 workers in the potassium
chromate industry, suffering from pulmonary or gastrointestinal
diseases, attributed by the authors to chromium poisoning. A
control group of 70 healthy individuals was included. Pathological
changes in the ECG and in other tests of heart function indicated
disturbance of right heart function, which the authors considered
secondary to the pulmonary pathology. The full paper was not
available and, consequently, the data could not be evaluated in
detail. It should be noted that no unexpected findings in
cardiovascular mortality were cited in the epidemiological studies
described in section 7.3.9.
8.3.7. Teratogenicity
The only human data available concerning this topic were
reported in an abstract (Morton & Elwood, 1974). The authors did
not find any correlation between frequency of malformations in the
central nervous system and the chromium content in water samples
collected in South Wales. Furthermore, Suzuki et al. (1979) did
not find any indication of accumulation of chromium in fetal
tissues.
8.3.8. Mutagenicity and other short-term tests
Few reports exist dealing with chromium-induced mutagenicity in
human subjects, and only hexavalent chromium causes any effects.
Bigaliev et al. (1978) found a 3 - 8% increase in chromosomal
aberrations of peripheral blood leukocytes in workers handling
chromium-compounds, compared with 2% in unexposed controls.
When classified according to the Papanicolaou system,
cytological samples of the sputum of 116 workers in the chromate-
production industry did not show any stages of tumour development
(Maltoni, 1976). However, intermediate stage lesions found in 30
workers were described as atypical adenomatous proliferation,
squamous-cell, and basal-cell dysplasia.
Studying cultured lymphocytes obtained from workers
occupationally exposed to CrO3, Sarto et al. (1982) found an
increased frequency of sister chromatid exchanges compared with
controls, which was correlated with urinary-chromium levels and
enhanced by smoking habits.
8.3.9. Carcinogenicity
8.3.9.1. Lung cancer
(a) Epidemiological studies in chromate-producing plants
The first recognition that cancer of the respiratory tract
might be related to chromium exposures resulted from the reporting
in 1932 of 2 cases of bronchogenic carcinoma that had occurred in
an old German chromate chemical plant (Lehmann, 1932). Gross &
Kölsch (1943) reported 10 deaths from lung cancer in small plants
producing the chromates of lead and zinc. By 1947, 52 such cases
had been reported from this industry and 10 cases from a chrome
pigment plant, also in the Federal Republic of Germany (Baetjer,
1950a).
The first epidemiological study was reported in the USA in
1948. The mortality data were based on the records of the group
life insurance policies of 6 chromate-producing plants in the USA
compared with similar data from a non-chromium industry or from a
life insurance company. The study reported that 21.8% of all
deaths between 1938 and 1947 in the chromate plants were due to
cancer of the respiratory tract compared with 1.4% in the control
group. The crude death rate for cancer of the bronchi and lungs
was 28 times that for the control groups (Machle & Gregorius,
1948). In one of these plants (Mancuso & Hueper, 1951), the cancer
death rate was 15 times that of the general population in the
county where the plant was located. The US PHS (1953) also studied
the respiratory cancer risk in these 6 plants and estimated a
relative risk of 28.9 using the average cancer mortality rates for
US males between 1940 and 1948 as a comparison.
In addition to these studies, where the cause of death was
taken from insurance or death certificate records, a case-control
study, using only cases diagnosed by autopsy or biopsy, was
conducted by Baetjer (1950b). Two hospitals near the largest
chromate plant in the USA were asked to supply the histories of all
cases diagnosed in 1924-46 as cancer of the lung bronchi, and to
confirm histopathologically. A group of non-cancer cases matched
individually by age, sex, and date of admission was selected as
controls. Of the 290 confirmed cancer cases, 3.26% had been
employed in the chromate plant, whereas none of the 900 control
cases was found to have had chromate exposure. The percentage of
chromate workers in the lung cancer series was 28.6 times the
percentage of chromate workers among the employed male population
of the city. The plant involved in this study was rebuilt in 1950,
following the discovery of the cancer problems. A new study of
this plant was made by Hayes et al. (1979) to determine whether the
risk of cancer had been eliminated. The investigation covered 438
deaths among 2101 males, who were initially employed between 1945
and 1974 for more than 90 days, and who died between 1945 and 1977.
The SMRa for malignant neoplasms of the trachea bronchi and lungs
was 202. Cancer deaths in the city in which the plant was located
were used to calculate the expected number of cases. No cases of
respiratory cancer occurred in the plant between 1960 and 1977
among workers employed only in the new plant. Because of the long
latent period, further follow-up is necessary.
Increased lung cancer rates among chromate workers in the USA
have been confirmed by Taylor (1966), Enterline (1974), and Mancuso
(1975), who also showed that the risks were especially high during
the early years of the study of the cohorts, e.g., before 1950.
Studies conducted in other countries have shown similar results.
In a 1956 study on workers in chromium-producing plants in the
United Kingdom, the rate of lung cancer mortality was 3.6 times the
rate for England and Wales (Bidstrup & Case, 1956). In a follow-up
study on 2715 men who had worked at the 3 chromate-producing
factories in the United Kingdom from 1948 to 1977, Alderson et al.
(1981) found 116 deaths from lung cancer (expected, 48). Since
modification of the one plant that is still in operation, the
relative risk of lung cancer has decreased from over 3 to about
1.8.
Two epidemiological studies have been conducted in the Japanese
chromate industry. In one plant, 5 lung cancer deaths occurred
between 1960 and 1973 in 136 workers who had been exposed for more
than 9 years; the SMR was 1510 (Watanabe & Fukuchi, 1975). In
another plant, the mortality rate for respiratory cancer was
determined for 896 males who worked in the plant from 1918 to 1975
and were followed to 1978; the SMR was 920 (expected deaths based
on 1975 age-cause-specific mortality rates for Japanese males)
(Satoh et al., 1981).
In the Federal Republic of Germany, a study was conducted in 2
chromate-producing plants (Korallus et al., 1982). Comparing the
SMR for lung cancer during the period 1948-79 for these 2 groups, a
clearly decreasing tendency was observed. In the first plant, for
1948-52, the SMR was 512 and, for 1978-79, it was 98; for the other
plant, the corresponding figures were 1905 and 150, respectively.
The newest data indicate a decreasing risk approaching the
frequency in unexposed people (Korallus & Loenhoff, 1981). This is
a result of occupational hygienic and technological measures,
especially of a change in manufacture namely the "on line"
--------------------------------------------------------------------
a The SMR (standardized mortality ratio) is the ratio of
observed to expected deaths times 100.
processing of the chrome ore, which has minimized the contents of
the carcinogenic hexavalent chromium compounds within the process
(Korallus et al., 1982).
Bittersohl (1972) described the results of a study on 30 000
employees of a large chemical unit for the period 1921-70 in the
German Democratic Republic. In particular, 588 malignancies in men
and 170 in women were evaluated for the period 1957-70. In 1971,
108 new malignancies in men and 29 in women came to light. In a
chromate factory, the rate of all cancers was far above average.
The factory manufactured catalysts through the reaction of chromic
acid and iron (III) oxide and nitric acid. The airborne
concentrations of chromium were not reported in detail, but it was
stated that short-term exposures above 400 µg/m3 occurred. The
incidence of malignant neoplasms in employees in the chromate
factory was 852 per 10 000 employees. In "unexposed" personnel,
the incidence of malignant neoplasms was 84 per 10 000 employees.
Approximately 86% of all those with malignant neoplasms were
smokers, and 78% of those without malignant neoplasms were also
smokers, indicating that smoking is not likely to be a confounding
factor.
In the USSR, a high incidence of cancer of the lungs was
reported for men engaged in the production of chrome salts. The
ratio of lung cancer in the plant to that in the control population
was 6.4 for ages 50 - 59 (748 observed, 116 expected) and 15.7 for
ages 60 - 69 (2657 observed, 170 expected) (Tyushnyakova et al.,
1974).
A survey was conducted in China in 1982 to investigate the
problem of lung cancer among chromate-producing workers. It
covered 7 cities and included 2184 male and 798 female workers who
had worked for at least one year in the industry. The preliminary
report revealed that 11 of the 101 deaths among male workers were
due to lung cancer, but none of the 13 deaths among female workers
was due to this cause.a
(b) Epidemiological studies - chrome pigment industry
The first epidemiological study, conducted in 1973, covered 3
small chrome pigment-manufacturing plants in the USA. The
percentage of lung cancer deaths was tentatively reported to be
about 3 times that in unexposed workers (Equitable Environmental
Health Inc., 1976).
Lung cancer mortality was studied among 1152 men working at 3
chromate pigment factories in the United Kingom from the 1930s or
1940s until 1981 (Davies, 1979, 1984). Among workers at factory A,
which produced both zinc and lead chromates, entrants to the
factory between 1932-45 with high and medium exposures had excess
---------------------------------------------------------------------------
a Report of an Investigation Team for Cancer in Chromate
Workers (1983).
risks of lung cancer (SMR = 223). A similar excess was seen in
1946-54 entrants, but the excess risk fell just short of
statistical significance. Highly significant excess risks of lung
cancer were seen among 1947-64 entrants that had high or medium
exposures at factory B, which also produced lead and zinc
chromates. Excesses were more severe in workers employed for more
than 10 years. In factory C, which produced only lead chromate, no
excess of lung cancer was found. However, the number of workers
and the power of the study were small.
Twenty-four male workers, who had been employed in a small
Norwegian company for more than 3 years between 1948 and 1972, were
identified and followed up by Langard & Norseth (1975), because
their principal exposure was to zinc chromate dust (measured
routinely with readings occasionally up to 0.5 mg hexavalent
chromium/m3). By December 1980, 6 cases of lung cancer had been
diagnosed, giving a relative risk of 44 compared with that of the
general population in the country. The authors stated that 5 out of
the 6 patients were smokers and only one had been exposed to
chromates other than zinc chromates (Langard & Vigander, 1983).
Sheffet et al. (1982) undertook a detailed mortality study on
workers employed in a pigment plant in Newark, where lead and zinc
chromates were used. Observed deaths from each cause among 1296
white and 650 non-white males employed between 1940 and 1969, were
compared with expected deaths, as computed from cause-, age-, and
time-specific standard death rates for the USA. A statistically-
significant relative risk of 1.6 for lung cancer among white
males, employed for 10 years or more, was found. A relative risk
of 1.9 was noted for individuals employed for at least 2 years, who
were at least moderately exposed to chromates.
An epidemiological study covering several centres in Europe was
conducted by Frentzel-Beyme (1983). This study was designed to
quantify the mortality from cancer and other diseases among workers
in European factories producing chromate pigments (3 German, 2
Dutch factories). Observed deaths in factories were compared with
expected deaths calculated on the basis of mortality figures for
the region in which a given factory was located. The overall
mortality did not deviate from the expected rates. Lung cancer
rates were always in excess of expected numbers, but only in one
cohort was the excess statistically significant. The pattern of
duration of exposure indicates that the lung-cancer risk was not
clearly correlated with length of employment.
A slight excess of lung cancer risk in chrome pigment (zinc
chromate) spray painters from 2 maintenance bases in the USA was
reported by Dalager et al. (1980). Among the 202 deaths
identified, 21 were due to respiratory cancer (11.4 expected;
comparison population: proportionate mortality, US males). No
statistically significant excess of lung cancer was found in a
group of automobile painters in which 226 deaths, including 22
cancer cases, were analysed using proportionate mortality for areas
in which the plants were located as a comparison (Chiazze et al.,
1980).
(c) Epidemiological studies - ferro-chrome industry
Langard et al. (1980) reported that 7 cases of bronchogenic
carcinoma occurred among 976 workers, who started work before
January 1, 1960. The expected number was 3.1 using national rates,
1.8 using local rates, and < 1 when using an international
reference rate for comparison. The difference was not significant
for the national and local rates, but was significant for the
international rate.
A study in Sweden did not reveal any significant excess of
respiratory cancer in ferro-chrome workers (Axelsson et al., 1980).
The incidence of cancer among 1932 workers employed for at least
one year between 1930 and 1975 was compared with the expected
number based on the National Cancer Registry data for the county in
which the plant was located. Five cases of respiratory cancer were
found against an expected 7.2 cases.
An excessive rate of lung cancer was reported for a ferro-
chrome plant in Russia (Pokrovskaya & Shabynina, 1973). The number
of deaths from cancer between 1955 and 1969 was higher than that in
the town in which the plant was located. The authors considered
that both hexavalent and trivalent chromium exposures were
responsible for the excess, since similar excessive rates were
found in a nearby ore-crushing plant, where only trivalent chromium
was present. The number of cases and the rates involved in these
studies were not given.
Hexavalent chromium is produced in some types of welding. In
section 7.2.1.2, it was mentioned that welding fume particles were
positive in the mammalian spot test, indicating a mutagenic and
possible carcinogenic potential. The incidence of lung cancer was
determined by Sjögren (1980), in a cohort of 234 welders, who had
welded stainless steel for more than 5 years between 1950 and 1965,
and were followed to 1977. Three welders died from lung cancer in
comparison with an expected number of 0.68 in the general
population ( P = 0.03).
Comparing lung cancer mortality in 2 parishes with ferro-alloy
industries to mortality in other parishes of similar size without
such industries from the same county, Axelson & Rylander (1980)
were unable to detect an increased incidence of death due to lung
cancer mortality. The concentration of chromium in the ambient air
of the most polluted areas ranged from 0.1 to 0.4 µg/m3. It should
be noted that several possible confounding factors were not under
control, e.g., migration, occupational exposure, and smoking
habits.
(d) Epidemiological studies - chromium-plating workers
Epidemiological studies on workers in the chromium-plating and
related industries were reviewed by Hayes (1982). The relevant
data from these studies are presented in Table 27. According to
Hayes, the epidemiological evidence is not sufficient to determine
the risk of cancer associated with industrial exposure to chromic
acid and, if an excess risk exists, it is probably lower than that
typically described for employment in the chromium-chemical
producing industry.
In a more recent paper, Franchini et al. (1983) conducted a
retrospective cohort study in 9 chromium-plating plants to examine
the mortality of workers employed for at least one year during the
period January 1951 to December 1981. The study group totalled 178
individuals; vital status ascertainment was 97% complete. The
total number of deaths was close to the expected figure (15
observed, 15.2 expected), whereas death from lung cancer exceeded
the expected number (3 observed, 0.7 expected; P = 0.03). The
increased mortality from lung cancer among chromium-platers seemed
to be related to exposure intensity.
8.3.9.2. Cancer in organs other than lungs
A few cancers of the upper respiratory tract and oral region,
but no excessive rates have been reported to occur in the chromate-
producing industry. The cancers have involved the buccal cavity,
pharynx, and oesophagus. Cancers of the nasal septum have not been
documented, with the exception of one case in Italy (Vigliani &
Zurlo, 1955).
In a joint Danish-Finnish-Swedish case-reference investigation,
initiated in 1977, the connection between nasal and sinonasal
cancer and various occupational exposures was studied. All new
cases of nasal and sinonasal cancer were collected from the
national cancer registers (Finland and Sweden) or from the hospitals
(Denmark). The results showed associations between nasal and
sinonasal cancer and exposure to chromium, welding, flame-cutting,
and soldering, hardwood or mixed wood dust, and softwood dust alone
(13.4) (Hernberg et al., 1983).
In 5 plants in the USA, during the early years of extremely
high exposures, the rate of cancer of the digestive tract varied
from 0 to 3.04/1000 compared with a rate of 0.59/1000 for the
controls (Machle & Gregorius, 1948). The mortality rates from
stomach cancer have been reported for chromate-producing plants in
Japan (SMR = 90) (Satoh et al., 1981) the USA (SMR = 40) (Hayes et
al., 1979). No significant differences were reported between the
incidence of stomach cancer in a Russian plant (Pokrovskaya &
Shabynina, 1973) and the incidence in the surrounding area.
Studying cancer mortality in a pigment plant using lead and
zinc chromates, Sheffet et al. (1982) found an increased (but not
statistically risk of stomach (SMR = 170) and pancreas (SMR = 200)
cancer among the total cohort (1946 employees).
Table 27. Summary of epidemiological studies on respiratory cancer in
workers employed in the chromium plating and related industriesa
------------------------------------------------------------------------------------------------
Study population Follow-up Respiratory cancer Comparison population
Number Estimated
relative risk
------------------------------------------------------------------------------------------------
Chromium plating
1056 workers employed Deaths in former and 24 1.8b Unexposed workers in
> 3 months in 54 plants current (followed 2 plants and in 2 non-
in the United Kingdom years) workers, plating industries
about 80% traced
About 5000 workers 49 1.4c Mortality analysis,
employed since 1945 in method not specified
1 plating factory in
the United Kingdom
889 workers exposed > 6 Reports from management 0 < 1b Tokyo mortality
months in Tokyo chromium of plating firms and rates
plating plants, 1970-76 follow-up of retired
workers; 19 total deaths
reported
Related industries
1292 deaths among US Union death benefit 62 1.1b Proportionate
metal polishers and claims mortality, US males
platers, union members,
1951-69
238 deaths among employees Pension, insurance and 39 2.1c Proportionate
for 10 years in one US benefit records mortality, US
plant for metal die
casting, finishing, and
electroplating, 1974-78.
------------------------------------------------------------------------------------------------
a Adapted from: Hayes (1982).
b Not statistically significant.
c P < 0.05, calculated using an assumption that the observed number is distributed as a
Poisson random variable.
8.3.9.3. Relationship between cancer risk and type of chromium
compound
As the exposures are often poorly defined, it is sometimes not
certain whether workers have been exposed to hexavalent chromium,
trivalent chromium, or a combination of the two. Some authors have
concluded that there is no increased risk of cancer mortality due
to trivalent chromium compounds (Korallus et al., 1974 a,b,c;
Axelsson et al., 1980; Langard et al., 1980; Norseth, 1980),
whereas others do not exclude that trivalent chromium compounds
have a carcinogenic capacity (Essing et al., 1971; Mancuso, 1975;
Zober 1979).
Summing up the data from case reports as well as
epidemiological studies in chromate-producing, chromate-pigment,
chromium-plating, and ferrochromium industries, the IARC Working
Group on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans (IARC, 1980) came to the conclusion that "there is
sufficient evidence of respiratory carcinogenicity in men
occupationally exposed during chromate production. Data on lung
cancer risk in other chromium-associated occupations and for cancer
at other sites are insufficient. The epidemiological data do not
allow an evaluation of the relative contributions to carcinogenic
risk of metallic chromium, trivalent chromium and hexavalent
chromium or of soluble versus insoluble chromium compounds".
9. EVALUATION OF HEALTH RISKS FOR MAN
Low levels of chromium are omnipresent in the environment.
Under normal conditions, human exposure to chromium does not
represent a toxicological risk, but it should be pointed out that
too low an intake of chromium may lead to deficiency. Airborne
concentrations of chromium, predominantly as trivalent chromium,
are usually below 0.1 µg/m3 and there are many places where the
concentration is below the detection limit. Concentrations in
river water are typically in the range of 1 - 10 µg/litre and do
not constitute a health threat. Drinking-water from municipal
water supplies does not contribute more than a few micrograms of
chromium to the daily human intake, but untreated water in certain
areas may be contaminated by runoff or effluents from industrial
sources and may contribute significant amounts of chromium. The
oceans contain less than 1 µg/litre. The daily human intake
through food varies considerably between regions. Typical values
range from 50 to 200 µg/day. They do not represent a toxicity
problem.
However, significant exposures exist in the occupational field.
In the past, chromium-ore mining generated chromium-containing
dusts, levels of which ranged up to 20 mg/m3 in different work-
places. Other exposures ranged up to 150 mg/m3 and consisted of
dust containing as much as 48% chromium (Cr2O3). In production
plants, hexavalent chromium can occur in the airborne state.
However, implementation of protective measures at ferrochromium
production sites reduced the airborne hexavalent chromium to levels
of 30 - 60 µg/m3.
The health effects of the two common oxidation states of
chromium are so fundamentally different that they must be
considered separately. In the form of trivalent compounds,
chromium is an essential nutrient and is relatively non-toxic for
man and other mammalian species. The hexavalent form is man-made
by oxidation of naturally-occurring trivalent chromium minerals and
is widely used. Compounds of hexavalent chromium penetrate
biological membranes easily and can thus interact with essential
constituents of the cells, including genetic material which they
can damage through oxidation and complexation with resulting
trivalent species. On the other hand, oxidation of trivalent
chromium has not been demonstrated in the living organism, and for
practical purposes, the reduction of hexavalent chromium to
trivalent chromium in lungs and other animal tissues is
irreversible.
9.1. Occupational Exposure
A number of effects can result from occupational exposure to
airborne chromium including irritative lesions of the skin and
upper respiratory tract, allergic reactions, and cancers of the
respiratory tract. The data on other effects, e.g., in the
gastrointestinal, cardiovascular, and urogenital systems are
insufficient for evaluation.
Epidemiological studies have shown that workers engaged in the
production of chromate salts and chromate pigments experience an
increased risk of developing bronchial carcinoma. No detailed data
on dose-response relationships are available from epidemiological
studies. Although a suspicion of increased lung cancer risks in
chromium plating workers has been raised, the available data are
inconclusive as are data for other industrial processes where
exposure to chromium occurs. There is insufficient evidence on the
role of chromium as a cause of cancer in any organ other than the
lung. Evidence from studies on laboratory animals shows that
hexavalent chromium compounds, especially those of low solubility,
can induce lung cancer.
In the lymphocytes of workers in chromium-plating factories,
the frequency of sister chromatid exchanges was higher in exposed
than in control groups.
Mutagenicity and related studies have convincingly shown that
hexavalent chromium is genetically active. Hexavalent chromium can
cross cellular membranes and is then reduced to trivalent chromium,
which can cause DNA cross-links and increase the infertility of DNA
implication. Trivalent chromium compounds have been shown to be
genetically inactive in most test systems, except in systems where
they can directly interact with DNA.
9.1.1. Effects other than cancer
9.1.1.1. Respiratory tract
It has been reported that the threshold for acute irritative
effects in the upper respiratory tract is 25 µg/m3 for the most
sensitive individuals. Long-term exposure to doses over 1 µg
chromic acid/m3 can cause nasal irritation, atrophy of the nasal
mucosa, and ulceration of perforation of the nasal septum.
Bronchial asthma was previously attributed to exposure to
chromium compounds, but scientific data are too scarce to draw
conclusions.
9.1.1.2. Skin
Skin rashes, ulcers, sores, and eczema have been reported among
occupationally exposed workers. Both trivalent and hexavalent
chromium compounds can give rise to sensitization of skin,
especially under certain environmental conditions, such as those
encountered in the cement industry, where the high incidence of
chromium-induced skin lesions can be attributed to the alkaline
exposure conditions.
Eczematous dermatitis, which manifests first as a diffuse
erythematous type, progresses in severe cases to an exudative
stage and is associated in 8 - 15% of patients with sensitivity to
chromium, as revealed by skin-patch tests. Although opinion is
divided on the oxidation state of chromium responsible for inducing
sensitization, there is evidence of hexavalent chromium penetrating
the skin as a hapten to be reduced to the trivalent state and
conjugated with proteins and transformed into the full antigen
that initiates the sensitization reaction, involving presumably
only a cell-mediated immune response. It should be noted that
patients suffering from chromium-induced skin allergy tend to
become hypersensitive to cobalt and nickel. Furthermore, 48% of
cases of skin allergy induced by chromium, with or without
clinically observed light sensitivity, showed a significantly more
intense reaction by a standardized photopatch test procedure, when
they were exposed to a 4/5 minimal erythema dose of irradiation.
9.1.1.3. Kidney
After high-dose, short-term oral ingestion of chromium, acute
nephritis and tubular necrosis were observed. A few
epidemiological studies on workers in chrome-plating industries
include data on diseases of the kidney, most of them without giving
exact exposure levels. A recent study related increased urinary-beta2
microglobulin levels to exposure ranges between 2 and 20 µg/m3.
The dose-response relationship observed in this study needs
confirmation on a larger number of exposed workers.
9.1.1.4 Other organs and systems
There are no conclusive data to evaluate the effects of
chromium compounds on the liver or gastrointestinal and circulatory
systems.
9.1.2. Teratogenicity
Both oxidation states, when injected at high levels
parenterally into animals, are teratogenic, with the hexavalent
form accumulating in the embryos to much higher concentrations than
the trivalent. The Task Group was not aware of any report
indicating teratogenicity in human populations.
9.2. General Population
Persons living in the vicinity of ferro-alloy plants, exposed
to an ambient air concentration of up to 1 µg/m3, did not show
increased lung cancer mortality.
The results of many studies suggest that exposure to chromium
through inhalation and skin contact can pose health problems for
the general population. Very little information is available on
the health effects of chromium ingested through untreated drinking-
water, though, in a single study, a correlation was observed
between frequency of malformation in the central nervous system and
the chromium content of water samples (Morton & Elwood, 1974). In
order to assess the nature of the magnitude of these problems,
there appears to be a need for more general population studies on
the effects of inhaled, absorbed, or ingested chromium on
respiratory, cardiovascular, and renal functions, and on the skin.
Studies on animals and man have established trivalent chromium
as an essential micronutrient that interacts with insulin and
enhances the physiological effects of the hormone. Supplementation
trials in a number of countries including Finland, Jordan, Nigeria,
Sweden, Turkey, and the USA have shown that segments of the
population could improve their glucose metabolism and, in some
instances, fat metabolism, by ingesting trivalent chromium
compounds. These observations suggest that some populations are at
risk of chromium deficiency. Children suffering from protein-
energy malnutrition may be at special risk.
As chromium concentrations in the body fluids of unexposed
persons are generally less than 1 µg/litre, analysis for chromium
requires the strictest quality control, including measures to
exclude sample contamination. Even with adequate analytical
methods, it is not possible, as yet, to diagnose chromium
deficiency in individuals by chemical or biochemical methods alone.
Therefore, a quantitative assessment of the prevalence of chromium
deficiency in human populations is not yet possible.
REFERENCES
ABRAHAM, A.S., SONNENBLICK, M., EINI, M., SHEMESH, O., & BATT,
A.P. (1980) The effect of chromium on established athero-
sclerotic plaques in rabbits. Am. J. clin. Nutr., 33:
2294-2298.
AITIO, A., JARVISALO, J., KULUNEN, M., TOSSAVAINEN, A., &
VAITTINEN, P. (1984) Urinary excretion of chromium as an
indicator of exposure to trivalent chromium sulfate in leather
tanning. Int. Arch. occup. environ. Health, 54: 241-249.
ALDERSON, M.R., RATTAN, N.S., & BIDSTRUP, L. (1981) Health
of workmen in the chromate-producing industry in Britain. Br.
J. ind. Med., 38: 117-124.
ALEKSANDROV, A.I. & KAINOV, V.I. (1975) [Crocoite from the
Pervomaisk-Zverevo deposit at the Central Urals.] Tr. Sverdl.
Gorn. Inst., 106: 152-155 (in Russian).
AMA (1979) Guidelines for essential trace element
preparations for parenteral use. J. Am. Med. Assoc., 241:
2051-2054.
AMERICAN PUBLIC HEALTH ASSOCIATION, AMERICAN WATER WORKS
ASSOCIATION, AND WATER POLLUTION CONTROL FEDERATION (1971)
117A. Hexavalent chromium. In: Standard methods for the
examination of water and wastewater, 13th ed., Washington DC,
American Public Health Association, pp. 156-157.
AMES, B.N., DURSTON, E., YAMASAKI, E., & LEE, F.D. (1973)
Carcinogens as mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci. (USA), 70: 2281-2285.
ANDERSON, F.E. (1960) Cement and oil dermatitis. The part
played by chrome sensitivity. Br. J. Derm., 72: 108-117.
ANDERSON, N. (1982) The environmental impact of chromium.
Final draft March 1982, Maine, Department of Environmental
Protection, Bureau of Air Quality Control, 126 pp.
ANDERSON, R.A. & POLANSKY, M.M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Tr. El. Res., 3: 1-5.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., & REAMER, D.C. (1982a) Effect of exercise
(running) on serum glucose, insulin, glucagon, and chromium
excretion. Diabetes, 31: 212-216.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., VEILLON, C., & GLINSMANN, W. (1982b)
Urinary chromium excretion of human subjects: effects of
chromium supplementation and glucose loading. Am. J. clin.
Nutr., 36: 1184-1193.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., PATTERSON, K.Y.,
VEILLON, C., & GLINSMANN, W. (1983) Effects of chromium
supplementation on urinary Cr excretion of human subjects and
correlation of Cr excretion with selected clinical parameters.
J. Nutr., 113: 276-281.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
MERTZ, W., & GLINSMANN, W. (1983b) Chromium supplementation
of human subjects: effects on glucose, insulin, and lipid
variables. Metabolism, 32: 894-899.
ANDERSSON, A. & NILSSON, K.O. (1972) Enrichment of trace
elements from sewage sludge fertilizer in soils and plants.
Ambio, 1: 176-179.
ANGERER, J., SCHALLER, K.-H., & HEINRICH, R. (1981)
[Commercially-available blood and urine samples for quality
control in toxicology.] Arbeitsmed. Sozialmed. Praeventivmed.,
16: 125-127 (in German).
ANIMAL RESEARCH DIVISION OF THE NEW ZEALAND DEPARTMENT OF
AGRICULTURE (1954) Annual report 1953-54, Wellington, R.E.
Owen Government Printer, 40 pp.
ANKE, M., DIETTRICH, M., WICKE, G., PFLUG, D., & SCHUELER, D.
(1971) Absorption, excretion, and distribution of 51chromium
given by mouth to lactating ruminants. Arch. Tierern., 21:
599-607.
AXELSSON, G. & RYLANDER, R. (1980) Environmental chromium
dust and lung cancer mortality. Environ. Res., 23: 469-476.
AXELSSON, G., RYLANDER, R., & SCHMIDT, A. (1980) Mortality
and incidence of tumours among ferrochromium workers. Br. J.
ind. Med., 37: 121-127.
BAER, H. (1920) [Dermatitis resulting from the use of a
beard trainer made partly of artificial leather.] Münch. med.
Wschr., 67: 874 (in German).
BAETJER, A.M. (1950a) Pulmonary carcinoma in chromate
workers. I. A review of the literature and report of cases.
Arch. ind. Hyg. occup. Med., 2: 487-504.
BAETJER, A.M. (1950b) Pulmonary carcinoma in chromate
workers. II. Incidence on basis of hospital records. Arch.
ind. Hyg. occup. Med., 2: 505-516.
BAETJER, A.M., LOWNEY, J.F., STEFFEE, H., & BUDACZ, V.
(1959a) Effect of chromium on incidence of lung tumours in
mice and rats. Am. Med. Assoc. Arch. Ind. Health, 20: 124-135.
BAETJER, A.M., DAMRON, C., & BUDACZ, V. (1959b) The
distribution and retention of chromium in man and animals. Am.
Med. Assoc. Arch. Ind. Health, 20: 136-150.
BAKER, R.S.V. (1984) Evaluation of metals in in vitro
assays: interpretation of data and possible mechanisms of
action. Toxicol. environ. Chem., 7: 191-212.
BEHNE, D. & BRATTER, P. (1979) [Problems of trace element
analysis in medicine.] In: Gladtke, E., Heimann, G., & Eckert,
I., ed. [Trace elements], Stuttgart, Thieme, pp. 42-52 (in
German).
BEHNE, D. & DIEHL, F. (1972) Relations between carbohydrate
and trace element metabolism investigated by neutron
activation analysis. In: Nuclear Activation Techniques in the
Life Sciences. Proceedings of a Symposium, Bled, Yugoslavia,
Vienna, International Atomic Energy Agency, pp. 407-414.
BEHNE, D., BRATTER, P., GESSNER, H., HUBE, G., MERTZ, W., &
ROSICK, U. (1976) Problems in determination of chromium in
biological materials: comparison of flameless atomic
absorption spectrometry and neutron activation analysis. Z.
anal. Chem., 278: 269-272.
BEHRBOHM, P. (1957) [Allergic contact dermatitis caused by
anticorrosives containing chromium.] Berufsdermatosen, 5:
271-283 (in German).
BENJANUVATRA, N.K. & BENNION, M. (1975) Hair chromium
concentration of Thai subjects with and without diabetes
mellitus. Nutr. Rep. Int., 12: 325-330.
BERGMANN, H. & HARDT, K. (1979) Analysis of dissolved Cr(VI)
in water by APDC-MIBK extraction and atomic absorption
spectrometry. Z. anal. Chem., 297: 381-383.
BERING, F. & ZITZKE, E. (1935) [Occupational skin diseases,]
Leipzig, Voss (in German).
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric., 23: 93-100.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BERTRAND, D. & DEWOLF, A. (1968) Requirement for the trace
element chromium in the growth of potatoes. C R Ser. D., 266:
1494-1495.
BEYERMANN, K. (1962) The analytical behaviour of very small
amounts of chromium. Part I. Z. anal. Chem., 190: 4-33. Part
II. Z. anal. Chem., 191: 345-369.
BEYERSMANN, D., KOSTER, A., BUTTNER, B., & FLESSEL, P.
(1984) Model reactions of chromium compounds with mammalian
and bacterial cells. Toxicol. environ. Chem., 8: 279-286.
BIANCHI, V., DAL TOSO, R., DEBETTO, P., LEVIS, A.G., LUCIANI,
S., MAJONE, F., & TAMINO, G. (1980) Mechanisms of chromium
toxicity in mamalian cell cultures. Toxicology, 17: 219-224.
BIANCHI, V., LEVIS, A.G., & SAGGIORO, D. (1979) Differential
cytotoxic activity of potassium dichromate on nucleoside
uptake in BHK fibroblasts. Chem.-biol.Interact., 24: 137-151.
BIANCHI, V., CELOTTI, L., LANFRANCHI, G., MAJONE, F., MARIN,
G., MONTALDI, G., SPONZA, G., TAMINO, G., VENIER, P.,
ZANTEDESCHI, A., & LEVIS, A.G. (1983) Genetic effects of
chromium compounds. Mutat. Res., 117: 279-300.
BIANCHI, V., ZANTEDESCHI, A., MONTALDI, A., & MAJONE, F.
(1984) Trivalent chromium is neither cytotoxic nor mutagenic
in permeabilized hamster fibroblasts. Toxicol. Lett., 23:
51-59.
BIDSTRUP, P.L. & CASE, R.A.M. (1956) Carcinoma of the lung
in workmen in the bichromates-producing industry in Great
Britain. Br. J. ind. Med., 13: 260-264.
BIGALIEV, A.B., ELEMESOVA, M.S., TUREBAEV, M.N., & BIGALIEVA,
R.K. (1978) [Cytogenetic study of the mutagenic activity of
industrial substances.] Zdravookhr. Kaz., 8: 48-50 (in
Russian).
BITTERSOHL, G. (1972) [Health and the working environment-
Epidemiology of carcinomatosis in the chemical industry.] In:
Proceedings of the 5th Merseburger Symposium, 17-18 November
(in German).
BLACK, C.A. (1968) Soil-plant relationships, New York, John
Wiley and Sons, 792 pp.
BLOKIN, V.S. & TROP, F.S. (1977) [Dynamics of the morpho-
logical changes in animals subjected to inhalational dust
treatment using chromium oxide and trisubstituted chromium
phosphate.] In: Dubinin, N.P., ed. [Genetic effects of the
pollution of the environment,] Moscow, Nauka, pp. 173-176 (in
Russian).
BLOOMFIELD, J.J. & BLUM, W. (1928) Health hazards in
chromium plating. Public Health Rep., 43: 2330-2347.
BONATTI, S., MEINI, M., & ABBONDANDOLO, A. (1976) Genetic
effects of potassium dichromate. Mutat. Res., 38: 147-150.
BOND, R.G., STRAUB, C.P., & PROBER, R., ed. (1973) Handbook
of environmental control. III. Water supply and treatment,
Cleveland, Ohio, CRC Press, pp. 763-764.
BONNEVIE, P. (1939) [Etiology and pathogenesis of eczemas,]
Copenhagen, Busck, Leipzig, Barth (in German).
BORGHETTI, A., MUTTI, A., CAVATORTA, A., FALZOI, M., CIGALA,
F., & FRANCHINI, I. (1977) Indices rénaux d'exposition aiguë
et d'imprégnation chronique par le chrome. Med. Lav., 68(5):
355-363.
BORY, R. & LECOCQ, I. (1953) Eczéma par cuivre chromé. Arch.
Mal. prof., 14: 144.
BOSTROM, G. (1936) [Hypersensitivity test for chromium
allergy.] Am. Derm. Vener., 17: 631-632 (in German).
BOVET, P., LOB, M., & GRANDJEAN, M. (1977) Spirometric
alterations in workers in the chromium electroplating
industry. Int. Arch. occup. environ. Health, 40: 25-32.
BOWEN, H.J.M. (1966) Trace elements in biochemistry, New
York, London, Academic Press, 241 pp.
BRARD, D. (1935) Toxicologie du chrome, Paris, Herrmann &
Co., 80 pp.
BRIEGER, H. (1920) [Clinical aspects of acute chromate
poisoning.] Z. exp. Path. Ther., 21: 393-408 (in German).
BROCH, C. (1949) [Bronchial asthma caused by chromic oxide.]
Nord. Med., 41: 996-997 (in Norwegian).
BROWN, E., SKOUGSTAD, M.W., & FISHMAN, M.J. (1970) Methods
for collection and analysis of water samples for dissolved
minerals and gases. In: Techniques of water resources
investigation of the United States geological survey,
Washington DC, US Department Interior, 160 pp.
BROWN, H.G., HENSLEY, C.P., MCKINNEY, G.L., & ROBINSON, J.L.
(1973) Efficiency of heavy metals removal in municipal sewage
treatment plants. Environ. Lett., 5: 103-114.
BRUCKDORFER, K.R., KHAN, I.H., & YUDKIN, J. (1971) Chromium
and sucrose-induced hyperglyceridemia. Proc. Nutr. Soc., 30:
74A.
BRUNE, D., NORDBERG, G., & WESTER, P.O. (1980) Distribution
of 23 elements in the kidney, liver, and lungs of workers from
a smeltery and refinery in north Sweden exposed to a number of
elements and of a control group. Sci. total Environ., 16:
13-35.
BURCKHARD, W. (1962) [Occupational skin diseases.] In:
Jadassohn, W., ed. [Handbook of skin and venereal diseases,]
Berlin, Springer, Vol. II(1), pp. 368-474.
BURROWS, D., ed. (1983) Chromium: metabolism and toxicity,
Boca Raton, Florida, CRC Press, 172 pp.
BYERRUM, R.U. (1961) Some studies on the chronic toxicity of
cadmium and hexavalent chromium in drinking-water. Eng. Bull.
Purdue Univ. Serv., 106: 1-8.
CALNAN, C.D. & HARMAN, R.R.M. (1961) Studies in contact
dermatitis. XIII. Diesel coolant chromate dermatitis. Trans.
St. John's Hosp. Derm. Soc., 46: 13.
CALOW, P. & FLETCHER, C.R. (1972) A new radiotracer
technique involving 14C and 51Cr for estimating the
assimilation efficiencies of aquatic, primary consumers.
Oecologia (Berlin), 9: 155-170.
CAMPBELL, W.J. & MERTZ, W. (1963) Interaction of insulin and
chromium (III) on mitochondrial swelling. Am. J. Physiol.,
204: 1028-1030.
CANADA, NATIONAL HEALTH AND WELFARE (1980) Chromium
drinking-water criteria review. In: Guidelines for Canadian
drinking-water quality, 1978. Supporting documentation,
Ottawa, Canada, National Health and Welfare, pp. 255-268.
CANFIELD, W.K. & DOISY, R.J. (1976) Chromium and diabetes in
the aged. In: Hsu, J.M., Davis, R.L., & Neithammer, R.W., ed.
The biomedical role of trace elements in aging, St. Peters-
burg, Florida, Eckert College, pp. 119-128.
CARD, W.I. (1935) A case of asthma sensitivity to chromates.
Lancet, 2: 1348-1349.
CARTER, J.P., KATTAB, A., ABD-EL-HADI, K., DAVIS, J., EL
GHOLMY, A., & PATWARDHAN, V.N. (1968) Chromium (III) in
hypoglycemia and in impaired glucose utilization in
kwashiorkor. Am. J. clin. Nutr., 21: 195-202.
CARY, F.E., ALLWAY, W.H., & OLSON, O.E. (1977) Control of
chromium concentrations in food plants. I. Absorption and
translocations of chromium by plants. J. agric. food Chem.,
25: 300-304. II. Chemistry of chromium in soils and its
availability to plants. J. agric. food Chem., 25: 305-309.
CASEY, C.E. & HAMBIDGE, K.M. (1984) Chromium in human milk
from American mothers. Br. J. Nutr., 52: 73-77.
CASTO, B.C., PIECZYNSKI, W.J., NELSON, R.L., & DI PAOLO, J.
(1976) In vitro transformation and enhancement of viral
transformation with metals. Proc. Am. Assoc. Cancer Res., 17:
12-20.
CASTO, B.C., MEYERS, J., & DIPAOLO, J.A. (1979) Enhancement
of viral transformation for evaluation of the carcinogenic or
mutagenic potential of inorganic metal salts. Cancer Res., 39:
193-198.
CAWSE, P.A. (1974) A survey of atmospheric trace elements in
the UK, 1972-1973, Harwell, England, Atomic Energy Research
Establishment, 95 pp.
CHANEY, R.L. (1973) Crop and food chain effects of toxic
elements in sludges and effluents. In: Recycling Municipal
Sludges and Effluents on Land. Proceedings of a Joint
Committee, National Association of State Universities and Land
Grant Colleges, Washington DC, July 9-13, 1973, pp. 129-141.
CHEN, N.S.G., TSAI, A., & DYER, I.A. (1973) Effect of
chelating agents on chromium absorption in rats. J. Nutr.,
103: 1182-1186.
CHEN, K.Y., YOUNG, C.S., JAN, T.K., & ROHATGI, N. (1974)
Trace metals in waste water effluents. J. Water Pollut.
Control Fed., 46: 2663-2675.
CHEREMISINOFF, P.N. & HABIB, Y.H. (1972) Cadmium, chromium,
lead, mercury: a plenary account for water pollution. Part I.
Occurrence, toxicity, and detection. Water Sewage Works, 119:
73-86.
CHEREVATY, V.S., EREMEEVA, E.A., & SMAGULOV, S.K. (1965) [On
the nature of nasal microflora in the workers of a chromium
compound plant.] In: Proceedings of 4th Scientific Session of
Aktubinsk State Medical Institute, Alma-Ata, Kazakhstan,
pp. 337-342 (in Russian).
CHIAZZE, L., FERENCE, L.D., & WOLF, P.H. (1980) Mortality
among automobile assembly workers. I. Spray painters. J.
occup. Med., 22: 520-526.
CHRISTIAN, G.D., KNOBLOCK, E.G., PURDY, W.C., & MERTZ, W.
(1963) A polarographic study of chromium-insulin-
mitochondrial interaction. Biochem. Biophys. Acta, 66: 420-423.
CLARK, R. & KUNITSCH, G. (1972) [Statistical studies of
cross allergy in metal salts.] Berufsdermatosen, 20(5):
222-238 (in German).
COLLINS, R.J., FROMM, P.O., & COLLINGS, W.D. (1961) Chromium
excretion in the dog. Am. J. Physiol., 201(5): 795-798.
CONDE-SALAZAR, L., MARTO-CASTANO, A., & ARROYO VICENTE, M.
(1980) Determination of chrome in rubber gloves. Contact
Dermatit., 6: 237-238.
COTTON, F.A. & WILKINSON, G. (1966) Advanced inorganic
chemistry, New York, Interscience, 1136 pp.
CRESSER, M.S. & HARGITT, R. (1976) The determination of
chromium (III) and chromium (VI) by total anion exchange and
atomic absorption spectrometry. Anal. Chim. Acta, 81: 196-198.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
DALAGER, N.A., MASON, T.J., FRAUMENI, J.F., HOOVER, R., &
PAYNE, W.W. (1980) Cancer mortality among workers exposed to
zinc chromate paints. J. occup. Med., 22: 25-29.
DANA, P. (1971) Crocoite-PbCrO4 In: Hurlbut, C.S., ed.
Dana's manual of mineralogy, 18th ed., New York, J. Wiley and
Sons Inc., pp. 346-347.
DANIELSSON, B.R.G., HASSOUN, E., & DENCKER, L. (1982)
Embryotoxicity of chromium: distribution in pregnant mice and
effects on embryonic cells in vitro. Arch. Toxicol., 51:
233-245.
DAVIDSON, I.W.F. & BLACKWELL, W.L. (1968) Changes in
carbohydrate metabolism of squirrel monkeys with chromium
dietary supplementation. Proc. Soc. Exp. Biol. Med., 127:
66-70.
DAVIDSON, I.W.F. & BURT, R.L. (1973) Physiological changes
in plasma chromium of normal and pregnant women: effect of
glucose load. Am. J. Obstet. Gynecol., 116: 601-608.
DAVIES, J.C.A. (1974) Inhalation hazards in the manufacture
of silicon alloys. Cent. Afr. J. Med., 20: 140-143.
DAVIES, J.M.G. (1972) The fibrogenic effects of mineral
dusts injected into the pleural cavity of mice. Br. J. exp.
Pathol., 53: 190-201.
DAVIES, J.M.G. (1979) Lung cancer mortality of workers in
chromate pigment manufacture: an epidemiological survey. J.
Oil. Col. Chem. Assoc., 62: 157-163.
DAVIES. J.M.G. (1984) Lung cancer mortality among workers
making lead chromate and zinc chromate pigments at three
English factories. Br. J. Ind. Med., 41: 158-169.
DAVIS, M.H. & GROSSMANN, V.B. (1971) A sensitive method for
determination of chromium in biological materials. Anal.
Biochem., 44: 339-344.
DEFLORA, S. (1978) Metabolic deactivation of mutagens in
the Salmonella microsome test. Nature (Lond.), 271: 455-456.
DEFLORA, S. (1981) Study of 106 organic and inorganic
compounds in the Salmonella microsome test. Carcinogenesis, 2:
283-298.
DEFLORA, S. & BOIDO, V. (1980) Effect of human gastric
juice on the mutagenicity of chemicals. Mutat. Res., 77:
307-315.
DEFLORA, S., COPPOLA, R., CAMOIRANO, A., BATTAGLIA, M.A., &
BENNICELLI, C. (1980) Mutagenicity of chromyl chloride and
its vapours. Carcinogenesis, 1(7): 583-587.
DEFLORA, S., BIANCHI, V., & LEVIS, A.G. (1984) Distinctive
mechanisms for interaction of hexavalent and trivalent
chromium with DNA? Toxicol. environ. Chem., 8: 287-294.
DE GROOT, A.J. & ALLERSMA, E. (1973) Field observations on
the transport of heavy metals in sediments. In: Proceedings of
the Conference on Heavy Metals in the Aquatic Environment,
Nashville, Tennessee, December, Oxford, Pergamon Press,
pp. 1-16.
DEICHMANN, W.B. & GERARDE, H.W. (1969) Toxicology of drugs
and chemicals, 4th ed., New York, London, Academic Press,
167 pp.
DI PAOLO, J.A. & CASTO, B.C. (1979) Quantitative studies of
in vitro morphological transformation of Syrian hamster cells
by inorganic metal salts. Cancer Res., 32: 1008-1013.
DJAHANSHIRI, H. (1976) [Nutritional physiology problems
concerning the trace element chromiun in the rat. V. Effects
on overall metabolic performande.] Z. Tierphysiol. Tierernähr.
Futtermittelkd., 37: 298-315 (in German).
DOBROLYUBSKII, O.K. (1959) [Changes in the biochemical
process in grapes under the influence of a microfertilizer
which contains chromium.] Biochemistry (USSR), 24: 577-582 (in
Russian).
DOISY, R.J., STREETEN, D.H.P., LEVINE, R.A., & CHODOS, R.B.
(1968) Effects and metabolism of chromium in normals, elderly
subjects, and diabetics. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
Univerisity of Missouri, Vol. 2, 75 pp.
DOISY, R.J., STREETEN D.H.P., SOUMA, M.L., KALAFER, M.E.,
REKANT, S.L., & DALAKOS, T.G. (1971) Metabolism of
51chromium in human subjects. In: Mertz, W. & Cornatzer, W.E.,
ed. Newer trace elements in nutrition, New York, Marcel
Dekker, pp. 155-169.
DOISY, R.J., STREETEN, D.H.P., FREIBERG, J.M., & SCHNEIDER,
A.J. (1976) Chromium metabolism in man and biochemical
effects. In: Prasad, A.S., ed. Trace elements in human health
and disease, New York, London, Academic Press, Vol. 2, pp.
79-104.
DOMASEVA, T.V. (1971) [On the effect produced by chromium
compounds on the skin.] In: [Occupational skin diseases,]
Leningrad, Leningrad Institute of Sanitation and Hygiene,
Vol. 93, pp. 168-173 (in Russian).
DONALDSON, R.M., Jr & BARRERAS, R.F. (1966) Intestinal
absorption of trace quantities of chromium. J. lab. clin.
Med., 68: 484-493.
DOUGLAS, G.R., BELL, R.D.K., GRANT, C.E., WYTSMA, J.M., &
BORA, K.C. (1980) Effect of lead chromate on chromosome
aberration, sister-chromatid exchange, and DNA damage in
mammalian cells in vitro. Mutat. Res., 77: 157-163.
DURFOR, C.N. & BECKER, E. (1964) Public water supplies of
the 100 largest cities in the United States, 1962, Washington
DC, US Geological Survey, 364 pp (Water Supply Paper No. 1812).
DURUM, W.H. & HAFFTY, J. (1963) Implications of the
minor-element content of some major streams in the world.
Geochim. Cosmochim Acta, 27: 1-11.
EATON, D.L., STACEY, N.H., WONG, K.L., & KLAASSEN, C.D.
(1980) Dose-response effects of various metal ions on rat
liver metallothionein, glutathione, heme oxygenase, and
cytochrome P-450. Toxicol. appl. Pharmacol., 55: 393-402.
EDMUNDSON, W.F. (1951) Chrome ulcers of the skin and nasal
septum and their relation to patch testing. J. Invest. Derm.,
17: 17-19.
EIFAC (1983) Water quality criteria for european freshwater
fish. Report on chromium and freshwater fish, European Inland
Fisheries Advisory Commission, Working Party on Water Quality
Criteria for European Freshwater Fish, 31 pp (EIFAC Technical
Paper No. 43).
ELIAS, Z., SCHNEIDER, O., AUBRY, F., DANIERE, M.C., & POIROT,
O. (1983) Sister chromatid exchanges in chinese hamster V79
cells treated with the trivalent chromium compounds chromic
chloride and chromic oxide. Carcinogenesis, 3: 605-611.
ENTERLINE, P.E. (1974) Respiratory cancer among chromate
workers. J. occup. Med., 16: 523-526.
ENVIRONMENTAL INSTRUMENTATION GROUP (1973) Instrumentation
for environmental monitoring: biomedical. IV, Berkeley,
California, Lawrence Berkeley Laboratory.
EQUITABLE ENVIRONMENTAL HEALTH, INC. (1976) An epidemiologic
study of workers in lead chromate plants: Final report
(prepared for the Dry Color Manufacturer's Association,
Berkeley California).
ESSING, H.G., SZADKOWSKI, D., & VALENTIN, H. (1971)
[Significance of the valence gradations of chromium compounds
in occupational medicine examinations.] Med. Sachverständige,
67: 35-39 (in German).
ETMANOVA, Z.J. (1965) [On the functional state of the liver
in workers of Aktubinsk chromium compound plant.] Gig. Tr.
prof. Zabol., 10: 55-56 (in Russian).
EVAN, A.P. & DAIL, W.G. (1974) The effect of sodium chromate
on the proximal tubules of the rat kidney. Lab. Invest.,
30(6): 704-715.
FANG, Y. (1983) [The normal value of chromium in urine for
299 unexposed persons in Yanjian city.] J. Yanbian Med.
College, 6 (1): 35-37 (in Chinese).
FARKAS, T.G. & ROBERSON, S.L. (1965) The effect of Cr(III)
on the glucose utilization of isolated lenses. Exp. Eye Res.,
4: 124-126.
FELDMAN, F.J. (1968) The state of chromium in biological
materials. Fed. Proc., 27: 482.
FELDMAN, F.J., KNOBLOCK, E.C., & PURDY, W.C. (1967) The
determination of chromium in biological materials by atomic
absorption spectroscopy. Anal. Chim. Acta, 38: 489-497.
FRADKIN, A., JANOFF, A., LANE, B.P., & KUSCHNER, M. (1975)
In vitro transformation of BHK21 cells grown in the presence
of calcium chromate. Cancer Res., 35: 1058-1061.
FRANCHINI, I., MUTTI, A., GARDINI, F., & BORGHETTI, A.
(1975) [Excretion and renal elimination of chromium compared
to degree and length of occupational exposure.] In: Roche, L.
& Trager, J., ed. Rein et Toxique. Réunion de la Société
Francaise de Toxicologie (Compte rendu), Paris, Groupement
Francais Des Centre Anti-Poisons, pp. 271-281.
FRANCHINI, I., MUTTI, A., CAVATORTA, A., CORRADI, A., COSI,
A., OLIVETTI, A., & BORGHETTI, A. (1978) Nephrotoxicity of
chromium. Control Nephrol., 10: 98-110.
FRANCHINI, J., MAGNANI, F., & MUTTI, A. (1983) Mortality
experience among chrome plating workers. Scand. J. Work
environ. Health, 9: 247-252.
FREGERT, S. (1975) Occupational dermatitis in a 10-year
material. Contact Dermatit., 1: 96-107.
FREGERT, S. (1981) Manual of contact dermatitis, 2nd ed.,
Copenhagen, Munksgaard, 139 pp.
FREGERT, S. & GRUVBERGER, B. (1972) Chemical properties of
cement. Berufsdermatosen, 20(5): 238-245.
FREGERT, S. & GRUVBERGER, B. (1979) Chromium in industrial
leather gloves. Contact Dermatit., 5: 189.
FREGERT, S. & RORSMAN, H. (1964) Allergy to trivalent
chromium. Arch. Dermatol., 90: 4-6.
FREGERT, S., HJORTH, N., MAGNUSSON, B., BANDMANN, H.-J.,
CALNAN, C.D., CRONIN, E., MALTEN, K., MENEGHINI, C.L., PIRILA,
V., & WILKINSON, D.S. (1969) Epidemiology of contact
dermatitis. Trans. St. John's Hosp. Dermatol. Soc., 55: 17-35.
FRENTZEL-BEYME, R. (1983) Lung cancer mortality of workers
employed in chromate pigment factories. J. Cancer Res. clin.
Oncol., 105: 183-188.
FREUND, H., ATAMIAN, S., & FISCHER, J.E. (1979) Chromium
deficiency during total parenteral nutrition. J. Am. Med.
Assoc., 241: 496-498.
FSA (1953) Health workers in chromate producing industry. A
study, Washington DC, Federal Security Agency, 131 pp (US PHS
Publication No. 192).
FUKAI, R. (1967) Valency state of chromium in seawater.
Nature (Lond.), 213: 901.
FUKUNAGA, M., KURACHI, Y., & MIZUGUCHI, Y. (1982) Action of
some metal ions on yeast chromosomes. Chem. pharm. Bull., 30:
3017-3019.
FURST, A., SCHLAUDER, M., & SASMORE, D.P. (1976) Tumour-
igenic activity of lead chromate. Cancer Res., 36: 1779-1783.
GALE, T.F. (1974) Effects of chromium on the hamster embryo.
Teratology, 9: A-17.
GALE, T.F. (1978) Embryotoxic effects of chromium trioxide
in hamsters. Environ. Res., 16: 101-109.
GALE, T.F. (1982) The embryotoxic response to maternal
chromium trioxide exposure in different strains of hamsters.
Environ. Res., 29: 196-203.
GALE, T.F. & BUNCH, J.D. (1979) The effect of the time of
administration of chromium trioxide on the embryotoxic
response in hamsters. Teratology, 19: 81-86.
GARCIA, J.D. & WETTERHAHN JENNETTE, K. (1981) Electron-
transport cytochrome P-450 system is involved in the
microsomal metabolism of the carcinogen chromate. J. inorg.
Biochem., 14: 281-295.
GEISER, J.D., JEANNERET, J.P., & DELACRETAZ, J. (1960) Eczema
due to cement and sensitization to cobalt. Dermatologica
(Basel), 121: 1-7.
GEMMELL, R.P. (1973) Revegetation of derelict land polluted
by a chromate smelter. Part 1. Chemical factors causing
substrate toxicity in chromate smelter waste. Environ.
Pollut., 5: 181-197.
GEMMELL, R.P. (1974) Revegetation of derelict land polluted
by a chromate smelter. Part 2. Techniques of revegetation of
chromate smelter waste. Environ. Pollut., 6: 31-37.
GENTILE, J.M., HYDE, K., & SCHUBERT, J. (1981) Chromium
genotoxicity as influenced by complexation and rate effects.
Toxicol. Lett., 7: 439-448.
GERAUER, A. (1954) [On the growing importance of
epicutaneous allergy to chromium.] Z. Haut-Geschlechtskr., 17:
97-102 (in German).
GILANI, S.H. & MARANO, M. (1979) Chromium poisoning and
chick embryogenesis. Environ. Res., 29: 427-431.
GERICKE, S. (1943) Studies on the effect of the trace
element chromium on the growth of plants. Bodenkd.
Pflanzenernaehr., 33: 114-128.
GLASER, U., HOCHRAINER, D., KLOPPEL, H., KORDEL, W., & KUHNEN,
H. (1984) [Inhalation studies with Wistar rats and patho-
physiological effects of chromium,] Berlin, 156 pp (Report to
Umweltbundesamt, D-1 UFOPLAN F+E 10606007/2) (in German).
GLASER, U., HOCHRAINER, D., KLOPPEL, H., & KUHNEN, H. (1985)
Low-level chromium VI inhalation effects on alveolar macro-
phages and immune functions in Wistar rats. Arch. Toxicol.,
57: 250-256.
GLINSMANN, W.H., FELDMAN, F.J., & MERTZ, W. (1966) Plasma
chromium after glucose administration. Science, 152: 1243.
GLINSMANN, W.H. & MERTZ, W. (1966) Effect of trivalent
chromium on glucose tolerance. Metabolism, 15: 510-520.
GMELIN, C.G. (1826) Experiments on the effects of baryta,
strontia, chrome, molybdenum, tungsten, tellurium, titanium,
osmium, platinum, iridium, rhodium, palladium, nickel, cobalt,
uranium, cerium, iron, and manganese, on the animal system.
Edinburgh med. Surg. J., 26: 131-139.
GOMES, E.R. (1972) Incidence of chromium-induced lesions
among electroplating workers in Brazil. Ind. Med., 41(12):
21-25.
GOMEZ-ARROYO, S., ALTAMIRANO, M., & VILLALOBOS-PIETRINI, R.
(1981) Sister-chromatid exchanges induced by some chromium
compounds in human lymphocytes in vitro. Mutat. Res., 90:
425-431.
GONCHAROV, A.T. (1968) [Role of chromium in the nutrition of
animals and man.] Vopr. Pitaniya, 27: 59-66 (in Russian).
GONCHAROV, A.T. (1978) [Maximum physiological dose of
chromium in food for human consumption.] Kazanskij med. Z.,
59(2): 90-93 (in Russian).
GRAY, S.J. & STERLING, K. (1950) The tagging of red cells
and plasma proteins with radioactive chromium. J. clin.
Invest., 29: 1604-1613.
GREBE, W.-H., KASTNER, H., KIPPENBERGER, C., KRAUSS, U.,
KRUSZONA, M., SCHMIDT, H., LIEBRUCKS, M., RUMBERGER, M., &
WETTIG, E. (1975) [Investigation on supply and demand for
mineral raw materials. VII. Chromium,] Berlin, Hannover,
Bundesanstalt für Gewissenschaften und Rohstoffe/Deutsches
Institut für Wirtschaftsforschung, 147 pp.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38: 33-42.
GROSS, W.G. & HELLER, V.G. (1946) Chromates in animal
nutrition. J. ind. Hyg. Toxicol., 28: 52-56.
GROSS, E. & KOLSCH, F. (1943) [Lung cancer in the chromate
dye industry.] Gewerbepathol. Gewerbehyg., 12: 164-170 (in
German).
GURSON, C.T. (1977) The metabolic significance of dietary
chromium. In: Draper, H.H., ed. Advances in nutritional
research, New York, Plenum Publishing Company, Vol. 1,
pp. 23-53.
GURSON, C.T. & SANER, G. (1971) Effect of chromium on
glucose utilization in marasmic protein-calorie malnutrition.
Am. J. clin. Nutr., 24: 1313-1319.
GURSON, C.T. & SANER, G. (1973) Effect of chromium
supplementation on growth in marasmic protein-calorie
malnutrition. Am. J. clin. Nutr., 26: 988-991.
GURSON, C.T. & SANER, G. (1978) The effect of glucose
loading on urinary excretion of chromium in normal adults,
individuals from diabetic families, and in diabetics. Am. J.
clin. Nutr., 31: 1158-1161.
GUTHRIE, B.E. (1973) Daily dietary intakes of zinc, copper,
manganese, chromium, and cadmium by some New Zealand women.
Proc. Univ. Otago Med. School., 51: 47-49.
GUTHRIE, B.E., WOLF, W.R., & VEILLON, C. (1978) Background
correction and related problems in the determination of
chromium in urine by graphite furnace atomic absorption
spectrometry. Anal. Chem., 50: 1900-1902.
GUTHRIE, B.E., WOLF, W.R., VEILLON, C., & MERTZ, W. (1979)
Chromium in urine. In: Hemphill, D.D., ed. Trace substances in
environmental health, Columbia, Missouri, University of
Missouri, Vol. XII, pp. 490-492.
GUY, W.B. (1954) Dermatologic problems in the railroad
industry resulting from conversion to Diesel power. Am. Med.
Assoc. Arch. Derm. Syph., 70: 289-292.
GYLSETH, B., GUNDERSEN, N., & LANGORD, S. (1977) Evaluation
of chromium exposure based on a simplified method for urinary
chromium determination. Scand. J. Work environ. Health, 3:
28-31.
HAMBIDGE, K.M. (1971) Chromium nutrition in the mother and
in the growing child. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 169-194.
HAMBIDGE, K.M. (1974) Chromium nutrition in man. Am. J.
clin. Nutr., 27: 505-514.
HAMBIDGE, K.M. & BAUM, J.D. (1972) Hair chromium concen-
trations of human newborn and changes during infancy. Am. J.
clin. Nutr., 25: 376-379.
HAMBIDGE, K.M. & RODGERSON, D.O. (1969) A comparison of the
hair chromium levels of nulliparous and parous women. Am. J.
Obstet. Gynecol., 103: 320-321.
HAMBIDGE, K.M., RODGERSON, D.O., & O'BRIEN, D. (1968)
Concentration of chromium in the hair of normal children and
children with juvenile mellitus. Diabetes, 17: 517-519.
HARA, N. (1982) [The change of valence of chromium by acid
treatment.] Sangyo Igaku, 24: 658-662 (in Japanese).
HARRISON, D.L. & STAPLES, E.L.J. (1955) Zinc chromate
poisoning in calves. N.Z. vet. J., 3: 63-73.
HASEGAWA, M. (1938) Studies on the detexicating hormone of
the liver (Yakriton). Toholu J. exp. Med., 32: 163-176.
HAYES, R.B. (1982) Carcinogenic effects of chromium, In:
Langard, S., ed. Biological and environmental aspects of
chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 221-247.
HAYES, R.B., LILIENFELD, A.M., & SNELL, L.M. (1979)
Mortality in chromium chemical production workers: a
prospective study. Int. J. Epidemiol., 8: 365-374.
HEATH, J.C., FREEMAN, M.A.R., & SWANSON, S.A.V. (1971)
Carcinogenic properties of wear particles from prostheses made
in cobalt-chromium alloy. Lancet, 1: 564-566.
HENNIG, A., ANKE, M., DIETRICH, M., HARTMANN, G., & HOFFMANN,
G. (1971) Absorption and distribution of 51chromium after
oral administration to laying hens as a function of time.
Arch. Tierern., 21: 609-615.
HEPLER, O.E. & SIMONDS, J.P. (1946) Experimental nephro-
pathies. IV. Glycosuria in dogs poisoned with uranyl nitrate,
mercury bichloride and potassium dichromate. Arch. Path., 41:
42-49.
HERNBERG, S., WESTERHOLM, P., SCHULTZ-LARSEN, K., DEGERTH,
R., KUOSMA, E., ENGLUND, A., ENGZELL, U., SAND HANSEN, H., &
MUTANEN, P. (1983) Nasal and sinonasal cancer: connection
with occupational exposures in Denmark, Finland, and Sweden.
Scand. J. Work environ. Health, 9: 315-326.
HERTEL, R.F. (1982) [Chromium as a problem in physiology,
epidemiology and biological monitoring.] Staub-Reinhalt. Luft,
42: 135-137 (in German).
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand
cultures. J. exp. Bot., 4: 59-64.
HILT, G. (1954) La dermite du chrome hexavalent dans le
cadre des dermites eczémateuses par sensibilisation aux
métaux. Dermatologica (Basel), 109: 143-174.
HINTZE, C. (1930) [Handbook of mineralogy,] Berlin, Leipzig,
De Gruyter, Vol. 1. III/2, pp. 4012-4030.
HOPKINS, L.L., Jr (1965) Distribution in the rat of physio-
logical amounts of injected 51Cr(III) with time. Am. J.
Physiol., 209: 731-735.
HOPKINS, L.L., Jr & SCHWARZ, K. (1964) Chromium (III)
binding to serum proteins, specifically siderophidin. Biochem.
Biophys. Acta, 90: 484-491.
HOPKINS, L.L., Jr, RANSOME-KUTI, O., & MAJAJ, A.S. (1968)
Improvement of impaired carbohydrate metabolism by chromium
(III) in malnourished infants. Am. J. clin. Nutr., 21: 203-211.
HUCKABEE, J.W., CARTAN, F.O., & KENNINGTON, G.S. (1972)
Environmental influence on trace elements in hair of 15
species of mammals, Oak Ridge, Tennessee, Oak Ridge National
Laboratory, 36 pp (ORNL-TM-3747).
HUEPER, W.C. (1958) Experimental studies in metal
cancerogenesis. X. Cancerigenic effects of chromite ore roast
deposited in muscle tissue and pleural cavity of rats. Arch.
ind. Health, 18: 284-291.
HUEPER, W.C. (1961) Environmental carcinogenisis and cancer.
Cancer Res., 21: 842-857.
HUNTER, W.C. & ROBERTS, J.M. (1933) Experimental study of
the effects of potassium bichromate in the monkey's kidney.
Am. J. Pathol., 9: 133-147.
HYODO, K., SUZUKI, S., FURUYA, N., & MESHIZUKA, K. (1980) An
analysis of chromium, copper, and zinc in organs of a chromate
worker. Int. Arch. occup. environ. Health, 46: 141-150.
IARC (1980) Some metals and metallic compounds, Lyons,
International Agency for Research on Cancer, pp. 205-323 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 23).
IIJIMA, S., MATSUMOTO, N., LU, C.C., & KATSUNUMA, H. (1975)
Placental transfer of CrCl3 and its effects on fetal growth
and development and development in mice. Teratology, 12: 198.
IMBUS, H.R., CHOLAK, J., MILLER, L.H., & STERLING, T. (1963)
Boron, cadmium, chromium, and nickel in blood and urine. Arch.
environ. Health, 6: 112-121.
IMREH, ST. & RADULESCU, D. (1982) Cytogenic effects of
chromium in vivo and in vitro. Mutat. Res., 97: 192-193.
IVANKOVIC, S. & PREUSSMANN, R. (1975) Absence of toxic and
carcinogenic effects after administration of high doses of
chromium oxide pigment in subacute and long-term feeding
experiments in rats. Food Cosmet. Toxicol., 13: 347-351.
JAEGER, H. & PELLONI, E. (1950) Tests épicutanés aux
bichromates, positifs dans l'eczéma au ciment. Dermatologica
(Basel), 100: 207-216.
JEEJEEBHOY, K.N., CHU, R.G., MARLISS, E.B., GREENBERG, G.R., &
BRUCE-ROBERTSON, A. (1977) Chromium deficiency, glucose
intolerance, and neuropathy reversed by chromium supplement-
ation, in a patient receiving long-term total parenteral
nutrition. Am. J. clin. Nutr., 30: 531-538.
JENNETTE, K.W. (1979) Chromate metabolism in liver micro-
some. Biol. Trace Elem. Res., 1: 55-62.
JENSEN, L.S., MAURICE, D.V., & MURRAY, M.W. (1978) Evidence
for a new biological function of chromium. Fed. Proc., 37: 404.
JINDRICHOVA, J. (1978) [Chromium damage in arc welders.] Z.
Ges. Hyg., 24: 86-89 (in German).
JOHNSON, J.L. (1974) Characterization of air-borne partic-
ulates. Microchem. J., 19:168-178.
JONES, G.B., BUCKLEY, R.A., & CHANDLER, C.S. (1975) The
volability of chromium from brewer's yeast during assay. Anal.
Chim. Acta, 80: 389-392.
JOSCHI, A.P. & NEEB, R. (1980) Gas chromatographic elemental
analysis via di(trifluorethyl)dithiocarbamatochelates. Z.
anal. Chem., 303: 389-393.
KAPLAN, I. & ZELIGMAN, I. (1962) Occupational dermatitis in
railroad workers. Am. Med. Assoc. Arch. Derm., 85: 135-142.
KAUFMANN, D.B., DINICOLA, W., & MCINTOSH, R. (1970) Acute
potassium dichromate poisoning. Am. J. dis. Child, 119:
374-376.
KANEKO, T. (1976) Chromosome damage in cultured human
leucocytes induced by chromium chloride and chromium trioxide.
Jpn. J. ind. Health, 18: 136-137.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec assay and
mutagenicity studies on metal compounds. Mutat. Res., 77:
109-116.
KAZANTZIS, G. & LILLY, L. (1979) Mutagenic and carcinogenic
effects of metals. In: Friedberg, L., Nordberg, G.F., & Vouk,
V.B., ed. Handbook on the toxicology of metals, Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 237-273.
KELLER, C. (1980) [Activation analysis.] In: Bartholomé, E.,
Biekert, E., Hellmann, H., Ley, H., Weigert, M., & Weise, E.,
ed. [Ullmann's encyclopaedia of technical chemistry,] 4th ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
Vol. 5, pp. 685-707 (in German).
KEMP, K., JENSEN, F.P., MOELLER, J.T., & HANSEN, G. (1974)
Trace multi-element analysis of biological tissue by proton-
induced X-ray fluorescence spectroscope, Roskilde, Denmark,
Danish Atomic Energy Commission, 22 pp (Report Riso-M-1732).
KEMPF, T. & SONNEBORN, M. (1976) [Determination of Pb, Cd,
Cr, and Zn according to the drinking-water regulation Federal
Republic of Germany.] In: Aurand, K., Hässelbarth, U., Müller,
G., Schumacher, W., & Steuer, W., ed. [The drinking-water
regulation,] Berlin, Erich Schmidt Verlag, pp. 119-125 (in
German).
KEMPF, T. & SONNEBORN, M. (1981) [Chemical composition of
drinking-waters in various regions of the Federal Republic of
Germany.] Vom Wasser, 57: 83-94 (in German).
KESKINEN, H., KALLIOMAKI, P.-L., & ALANKO, K. (1980) Occupa-
tional asthma due to stainless steel welding fumes. Clin.
Allerg., 10: 151-159.
KIRCHGESSNER, M., MERZ, G., & OELSCHLAEGER, W. (1960)
[Effect of vegetation state on content of macro and micro
elements of three species of grass.] Arch. Tierern., 10:
414-427 (in German).
KITTELBERGER, F. (1973) [Contribution to residue analysis in
fish.] Tierärztl. Prax., 1: 465-479.
KLEIN, L.A., LANG, M., NASH, N., & KIRSCHNER, S.L. (1974)
Sources of metals in New York City wastewater. J. Water
Pollut. Control Fed., 46: 2653-2662.
KLEINER, A.M., STOLBUN, B.M., LIKHACHEVA, E.L., & BELJAEVA,
L.N. (1970) [Some indices of the functional state of the
myocardium and hemodynamics in chronic intoxication with
chromium compounds.] Gig. Tr. prof. Zabol., 12: 7-10 (in
Russian).
KNUDSEN, I. (1980) The mammalian spot test and its use for
the testing of potential carcinogenicity of welding fume
particles and hexavalent chromium. Acta pharmacol. toxicol.,
47: 66-70.
KOGAN, V.Y. (1968) [The basophil degranulation as an index
of skin sensitization to cobalt compounds.] In: Belenky, G.B.,
ed. [Modern aspects of clinical and experimental dermato-
venerology. Works of the clinic of skin and venerological
diseases,] Moscow, Medizina, pp. 100-104 (in Russian).
KOGAN, V.Y. (1971) [Chromium as a specific cause for
occupation dermatoses.] In: Babayants, R.S., ed. [Topical
questions of dermato-venerology. First Medical Institute,
All-Union Conference, Moscow, I.A. Sechenov, pp. 172-176
(in Russian).
KOGAN, V.Y, ARUTYUNYAN, K.E., & KOCHINYAN, Y.E. (1972) [A
comparative characteristic of chromium, nickel, and cobalt
salts as a causative factor in allergic contact dermatosis.]
In: [Clinical Pathology of Chemical Aetiology. Materials of
the 1st All-Union Conference, Kiev, USSR, 24-25 October,
1972,] Kiev, pp. 144 and 146 (in Russian).
KOHLER, H. (1951) [Constitutional examinations of subjects
with skin diseases in the offset department of a printing
works (Doctoral thesis, Leipzig.)] Berufsdermatosen, 4: 93 (in
German).
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace
metals during the ashing of biological materials. Analyst,
101: 870-875.
KOIVISTOINEN, P., ed. (1980) Mineral element composition of
Finnish foods. Acta agric. Scand., Suppl. 22: 171
pp.
KOPP, J.E. & KRONER, R.C. (1968) Trace metals in waters of
the United States: a five-year summary of trace metals in
rivers and lakes of the United States (October 1 1962 -
September 30, 1967), Cincinnati, Ohio, US Department of the
Interior, 32 pp.
KORALLUS, U. & LOENHOFF, N. (1981) [Industrial medical and
epidemiological experience with the manufacture and processing
of chromates.] Arbeitsmed. Sozialmed. Praeventivmed., 16:
285-289 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974a)
[Trivalent chromium compounds. Results of a study in
occupational medicine. I. General; Technology; Preliminary
study.] Arbeitsmed. Sozialmed. Praeventivmed., 9: 51-54 (in
German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974b)
[Trivalent chromium compounds: results of a study in
occupational medicine. II. Disease status analysis.]
Arbeitsmed. Sozialmed. Praeventivmed., 9: 76-79 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974c)
[Trivalent chromium compounds: Results of a study in
occupational medicine. III. Clinical studies.] Arbeitsmed.
Sozialmed. Praeventivmed., 9: 248-252 (in German).
KORALLUS, U., LANGE, H.-J., NEISS, A., WUESTEFELD, E., &
ZWINGERS, T. (1982) [Correlations between reorganization
measures and bronchial carcinoma mortality in the chromate-
manufacturing industry.] Arbeitsmed. Sozialmed. Praevent-
ivmed., 7: 159-167 (in German).
KOSHI, K. (1979) Effects of fume particles from stainless
steel welding on sister chromatid exchanges and chromosome
aberrations in cultured Chinese hamster cells. Ind. Health.,
17: 39-49.
KOTZ, L., KAISER, G., TSCHOPEL, P., & TOLG, G. (1972)
[Clarification of biological matrices for determining very low
contents of trace elements when limited amounts are weighed
with nitric acid under pressure in a teflon vessel.] Z. anal.
Chem., 260: 207-209 (in German).
KOUTRAS, G.A., HATTORY, M., SCHNEIDER, A.S., EBAUGH, F.G., &
VALENTINE, W.N. (1964) Studies on chromated erythrocytes:
effect of sodium chromate on erythrocyte glutathione reduc-
tase. J. clin. Invest., 43: 323-331.
KRISHNAJA, A.P. & REGE, M.S. (1982) Induction of chromosomal
aberrations in fish Boleophthalmus dussumieri after exposure
in vivo to mitomycin C and heavy metals mercury, selenium, and
chromium. Mutat. Res., 102: 71-82.
KROEPFLI, P. & SCHUPPLI, R. (1955) [Detergent eczema.]
Dermatologica (Basel), 110: 1-7 (in German).
KRUGER, H. & DORN, H. (1956) [Clinical observations on
detergent eczema in association with chromium and nickel
allergy.] Z. Haut-Geschlechtskr., 20: 307-314 (in German).
KUCHER, I.M. (1966) In: [Proceedings of the 5th Scientific
Session of the Aktiubinsk Medical Institute, Alma-Ata,
Kazakhstan,] pp. 24-26 (in Russian).
KUHN, J.K. (1973) Trace elements in whole coal determined by
X-ray fluorescence. Norelco Rep., 20(3): 7-10.
KUMPULAINEN, J.T., WOLF, W.R., VEILLON, C., & MERTZ, W.
(1979) Determination of chromium in selected United States
diets. J. agric. food Chem., 27: 490-494.
KUMPULAINEN, J., LEHTO, J., KOIVISTOINEN, P., UUSITUPA, M., &
VUORI, E. (1983) Determination of chromium in human milk
serum and urine by electrothermal atomic absorption spectro-
metry without preliminary ashing. Sci. total Environ., 31:
71-80.
KURYLOWICZ, B. & GASIOROWSKI, S. (1958) [Agrochemical study
on calcinated phosphate-containing admixtures of chromium
compounds.] Przemysl Chem., 37: 797-800 (in Polish).
LANGARD, S. (1977) The fate of chromium after intravenous
administration of Na251 CrO4 and CrCl3 x 6H2 O to the rat,
Guildford, England, University of Surrey, 66 pp (M.Sc.
Toxicology Thesis).
LANGARD, S. (1980a) Chromium. In: Waldron, H.A., ed. Metals
in the environment, London, New York, Academic Press,
pp. 111-132.
LANGARD, S. (1980b) A survey of respiratory symptoms and
lung function in ferrochromium and ferrosilicon workers. Int.
Arch. occup. environ. Health, 46: 1-9.
LANGARD, S., ed. (1982) Biological and environmental aspects
of chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, 285 pp.
LANGARD, S. & HENSTEN-PETTERSEN, A. (1981) Chromium
toxicology. In: Williams, D.F., ed. Systemic aspects of
biocompatibility, Boca Raton, Florida, CRC Press, Vol. 1, pp.
144-161.
LANGARD, S. & NORSETH, T. (1975) A short study of bronchial
carcinomas in workers producing chromate pigments. Br. J. ind.
Med., 32: 62-65.
LANGARD, S. & NORSETH, T. (1979) Chromium. In: Friberg, L.,
Nordberg, G.F., & Vouk, V.B., ed. Handbook on the toxicology
of metals, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 383-397.
LANGARD, S. & STERN, R.M. (1985) Nickel in welding fumes: a
cancer hazard to welders? In: Sunderman, F.W.J., Aitio, A.,
Berlin, A., Bishop, C., Buringh, E., Davis, W., Gounar, M.,
Jacquignon, P.C., Mastromatteo, E., Rigaut, J.P., Rosenfeld,
C., Saracci, R., & Sors, A., ed. Nickel in the human
environment, Lyons, International Agency for Research on
Cancer, pp. 95-103 (IARC Scientific Publications No. 53).
LANGARD, S. & VIGANDER, T. (1983) Occurrence of lung cancer
in workers producing chromium pigments. Br. J. ind. Med., 40:
71-74.
LANGARD, S., GUNDERSEN, N., TSALEV, D.L., & GYLSETH, B.
(1978) Whole blood chromium level and chromium excretion in
the rat after zinc chromate inhalation. Acta pharmacol.
toxicol., 42: 142-149.
LANGARD, S., ANDERSEN, A., & GYLSETH, B. (1980) Incidence of
cancer among ferrosilicon workers. Br. J. ind. Med., 37:
114-120.
LASKIN, S. (1972) Research in environmental sciences,
Washington DC, Institute of Environmental Medicine, pp. 92-97.
LASKIN, S., KUSCHNER, M., & DREW, R.T. (1970) Studies in
pulmonary carcinogenesis. In: Hanna, M.G., Jr, Nettesheim, P.,
& Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 321-351 (US Atomic Energy
Commission Symposium Series No. 18).
LAUTNER, G.M., CARVER, J.C., & KONZEN, R.B. (1978)
Measurement of chromium (VI) and chromium (III) in stainless
steel welding fumes with electron spectroscopy for chemical
analysis and neutron activation analysis. Am. Ind. Hyg. Assoc.
J., 39: 651-660.
LEHMANN, K.B. (1914) [Historical and critical account of our
present knowledge of the effect of small doses of chromate.]
In: Gesamtgebiet gewerbehygiene, Berlin, Springer Verlag, pp.
1-119 (in German).
LEHMANN, K.B. (1932) [Does the occurrence of lung cancer in
chromium workers give grounds for any particular anxiety?]
Zentralbl. Gewerbehyg., 9: 168-170 (in German).
LERICHE, H.H. (1968) Metal contamination of soil in the
Woburn market-garden experiment resulting from the application
of sewage sludge. J. agric. Sci. Camb., 71: 205-208.
LETTERER, E., NEIDHARDT, K., & KLETT, H. (1944) [Chromate
lung cancer and chromate pneumoconiosis. A clinical, morbid
anatomical and industrial-hygiene-related study.] Arch.
Gewerbepath. Gewerbehyg., 12: 323-361 (in German).
LEVIN, H.M., BRUNNER, M.J., & RATTNER, H. (1959)
Lithographer's dermatitis. J. Am. Med. Assoc., 169: 566-569.
LEVINE, R.A., STREETEN, D.H.P., & DOISY, R.J. (1968) Effects
of oral chromium supplementation on the glucose tolerance of
elderly human subjects. Metabolism, 17: 114-125.
LEVIS, A.G. & BIANCHI, V. (1982) Mutagenic and cytogenetic
effects of chromium compounds. In: Langard, S., ed. Biological
and environmental aspects of chromium, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 171-208.
LEVIS, A.G. & BUTTIGNOL, M. (1977) Effects of potassium
dichromate on DNA Synthesis in hamster fibroblast. Br. J.
Cancer, 35: 496-499.
LEVIS, A.G. & MAJONE, F. (1979) Cytotoxic and caryogenic
effects of soluble chromium compounds on mammalian cell
cultures. Br. J. Cancer, 40: 523-533.
LEVIS, A.G. & MAJONE, F. (1981) Cytologic and clastogenic
effects of soluble and insoluble compounds containing
hexavalent and trivalent chromium. Br. J. Cancer, 44: 219-235.
LEVIS, A.G., BUTTIGNOL, M., BIANCHI, V., & SPONZA, G. (1978)
Effects of potassium dichromate on nucleic acid and protein
synthesis and on precursor uptake in BHK fibroblasts. Cancer
Res., 38: 110-116.
LEVY, L.S. & MARTIN, P.A. (1983) The effects of a range of
chromium-containing materials on rat lung, Birmingham,
England, University of Aston, Department of Environmental and
Occupational Health, p. 224 and suppl (Prepared for Dry Color
Manufacturers Association, Alexandria, Virginia, USA).
LEVY, L.S. & VENITT, S. (1975) Carcinogenic and mutagenic
activity of chromium-containing materials. Br. J. Cancer, 32:
254-255.
LIEBER, M., PERLMUTTER, N.M., & FRAUENTHAL, H.L. (1964)
Cadmium and hexavalent chromium in Nassau County ground
water. J. Am. Water Works Assoc., 56: 739-747.
LIFSCHITZ, M.L., WALLACH, S., PEABODY, R.A., VERCH, R.L., &
AGRAWAL, R. (1980) Radiochromium distribution in thyroid
and parathyroid deficiency. Am. J. clin. Nutr., 33: 57-62.
LINDBERG, E. & HEDENSTIERNA, G. (1983) Chrome plating.
Symptoms, findings in the upper airways, and effects on lung
function. Arch. environ. Health, 38: 367-374.
LINDBERG, E. & VESTERBERG, O. (1983a) Monitoring exposure to
chromic acid in chromeplating by measuring chromium in urine.
Scand. J. Work environ. Health, 9: 333-340.
LINDBERG, E. & VESTERBERG, O. (1983b) Urinary excretion of
proteins in chromplaters, previously exposed chromplaters, and
referents. Scand. J. Work. environ. Health, 9: 505-510.
LINDBERG, E., EKHOLM, U. & ULFVARSON, U. (1985) Extent and
conditions of exposure in the Swedish chrome plating industry.
Int. Arch. Occup. Environ. Health, 56: 197-205.
LIU, V.J.K. & MORRIS, J.S. (1978) Relative chromium response
as an indicator of chromium status. Am. J. clin. Nutr., 31:
972-976.
LOFROTH, G. (1978) The mutagenicity of hexavalent chromium
is decreased by microsomal metabolism. Naturwissenschaften,
65: 207-208.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LUKANIN, W.P. (1930) [The pathology of chromate pneumo-
coniosis.] Arch. Hyg. Bakt., 104: 166-174 (in German).
LUZHNIKOV, E.A., SHIMANKO, I.I., KOSTOMAROVA, L.G., MALYGINA,
S.I., & TRAKHTENGARTS, M.I. (1976) [Acute poisoning with
chromium compounds.] Terapeut. Arkh., 48(7): 121-125 (in
Russian).
LYON, G.L., BROOKS, R.R., PETERSON, P.J., & BUTLER, G.W.
(1970) Some trace elements in plants from serpentine soils.
N.Z. J. Sci., 13: 133-139.
MACHLE, W. & GREGORIUS, F. (1948) Cancer of the respiratory
system in the United States chromate-producing industry.
Public Health Rep., 63: 1114-1127.
MCCLENDON, L.T. (1978) Determination of chromium in
biological matrices by neutron activation: application to
standard reference materials. J. radioanal. Chem., 42: 85-91.
MACKENZIE, R.D., BYERRUM, R.U., DECKER, C.F., HOPPERT, C.A., &
LANGHAM, R.F. (1958) Chronic toxicity studies. II. Hexa-
valent and trivalent chromium administered in drinking-water
to rats. Am. Med. Assoc. Arch. ind. Health, 18: 232-234.
MCRAE, W.D., WHITING, R.F., & STICH, H.F. (1979) Sister-
chromatid exchanges induced in cultured mammalian cells by
chromate. Chem.-biol. Interact., 26: 281-286.
MAHALKO, J.R. & BENNION, M. (1976) The effect of parity and
time between pregnancies on maternal hair chromium concentra-
tion. Am. J. clin. Nutr., 29: 1069-1072.
MAJONE, F. & LEVIS, A.G. (1979) Chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster cells treated in
vitro with hexavalent chromium compounds. Mutat. Res., 67:
231-238.
MAJONE, F. & RENSI, D. (1979) Mitotic alterations, chromo-
some aberrations, and sister-chromatid exchanges induced by
hexavalent and trivalent chromium on mammalian cells in vitro.
Caryologia, 32: 379-392.
MALTONI, C. (1974) Occupational carcinogenesis. Excerpta
Med. Int. Congr. Ser., 322: 19-26.
MALTONI, C. (1976) Precursor lesions in exposed populations
as indicators of occupational cancer risk. Ann. NY Acad. Sci.,
271: 444-447.
MANCUSO, T.F. (1951) Occupational cancer and other health
hazards in a chromate plant: a medical appraisal. II. Clinical
and toxicologic aspects. Ind. Med. Surg., 20: 393-407.
MANCUSO, T.F. (1975) Consideration of chromium as an
industrial carcinogen. In: Hutchinson, T.C., ed. Proceedings
of the International Conference on Heavy Metals in the
Environment, Toronto, Canada, October 27-31, 1975, Toronto,
Institute for Environmental Studies, pp. 343-356.
MANCUSO, T.F. & HUEPER, W.C. (1951) Occupational cancer and
other health hazards in a chromate plant: a medical appraisal.
I. Lung cancer in chromate workers. Ind. Med. Surg., 20:
358-363.
MARTIN, J.H. & KNAUER, G.A. (1973) The elemental composition
of plankton. Geochim. Cosmochim. Acta, 3: 1639-1653.
MATHIS, B.J. & CUMMINGS, T.F. (1973) Selected metals in
sediments, water, and biota in the Illinois river. J. Water
Pollut. Control Fed., 45: 1573-1583.
MATHUR, R.K. & DOISY, R.J. (1972) Effect of diabetes and
diet on the distribution of tracer doses of chromium in rats.
Proc. Soc. Exp. Biol. Med., 139: 836-838.
MATSUMOTO, N., IIJIMA, S., & KATSUNUMA, H. (1976) Placental
transfer of chromic chloride and its teratogenic potential in
embryonic mice. J. toxicol. Sci., 2: 1-13.
MAYER, R. (1983) Interaction of forest canopies with atmo-
spheric constituents: aluminium and heavy metals. In: Ulrich,
B. & Pankrath, J., ed. Effects of accumulation of air
pollutants in forest ecosystems, Dordrecht, Boston, London,
D. Reidel, pp. 47-55.
MAZGON, R. (1932) [Therapeutic effect of common salt on
experimental chromium nephritis in the rabbit.] Z. Gesamte
exp. Med., 81: 195-207 (in German).
MERIAN, E. (1984) Introduction on environmental chemistry
and global cycles of arsenic, beryllium, cadmium, chromium,
cobald, nickel, selenium, and their derivatives. Toxicol.
environ. Chem., 8: 9-38.
MERKURIEVA, R.V., KRASOVSKY, G.N., BURMANTOVA, N.P.,
SHATERNIKOVA, I.L., VARSHAVSKAYA, S.P., VASIUKOVICH, L.YA.,
KOGANOVA, Z.I., BUSHINSKAYA, L.I., KONSTANTINOVA, I.N.,
DOLINSKAYA, S.I., ASTAKHOVA, L.F., & VOTYAKOV, A.V. (1980a)
[Changes in various enzyme systems resulting from exposure to
some chemical substances.] Gig. i. Sanit., N1: 25-28 (in
Russian).
MERKURIEVA, R.V., KRASOVSKY, G.N., VOTYAROV, A.V., BURMANTOVA,
N.P., MUCHAMBETOVA, L.H., & KONSTANTINOVA, I.N. (1980b)
[Enzyme disarrangement of various subcellular structures under
the influence of the general toxic and gonadotoxic effects of
environmental chemical factors.] Zdravookhranenie belorus.,
N2: 28-29 (in Russian).
MERKURIEVA, R.V., KOGANOVA, Z.I., GABDULLINA, M.H., TSAPKOVA,
N.N., DUKHATEROV, O.N., & DOLINSKAYA, S.I. (1982) [A
comparative study of metabolic reactions at various routes of
chromium6+ administration in the organism of laboratory
animals.] Gig. i Sanit., N8: 75-78 (in Russian).
MERRITT, W.F. (1971) Identification and measurement of trace
elements in fresh water by neutron activation. In: Proceedings
of the International Symposium on Identification and Measure-
ment of Environmental Pollution, 358-362.
MERTZ, W. (1969) Chromium occurrence and function in
biological systems. Physiol. Rev., 49: 163-239.
MERTZ, W. (1981) The interaction between chromium and
insulin. Adv. Physiol. Sci., 12: 101-105.
MERTZ, W. & ROGINSKI, E.E. (1963) The effect of trivalent
chromium in galactose entry in rat epidymal fat tissue. J.
biol. Chem., 238: 868-872.
MERTZ, W. & ROGINSKI, E.E. (1969) Effects of chromium (III)
supplementation on growth and survival under stress in rats
fed low protein diets. J. Nutr., 97: 531-536.
MERTZ, W. & ROGINSKI, E.E. (1971) Chromium metabolsm: the
glucose tolerance factor. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 123-153.
MERTZ, W. & SCHWARZ, K. (1959) Relation of glucose tolerance
factor to impaired glucose tolerance in rats on stock diets.
Am. J. Physiol., 196: 614-618.
MERTZ, W., ROGINSKI, E.E., & SCHWARZ, K. (1961) Effect of
trivalent chromium complexes on glucose uptake by epididymal
fat tissue of rats. J. biol. Chem., 236: 318-322.
MERTZ, W., ROGINSKI, E.E., & REBA, R.C. (1965a) Biological
activity and fate of intravenous chromium (III) in the rat.
Am. J. Physiol., 209: 489-494.
MERTZ, W., ROGINSKI, E.E., & SCHROEDER, H.A. (1965b) Some
aspects of glucose metabolism of chromium-deficient rats
raised in a strictly controlled environment. J. Nutr., 86:
107-112.
MERTZ, W., ROGINSKI, E.E., FELDMAN, R.J., & THURMAN, D.E.
(1969) Dependence of chromium transfer into the rat embryo on
the chemical form. J. Nutr., 99: 363-367.
MERTZ, W., TOEPFER, E.W., ROGINSKI, E.E., & POLANSKY, M.M.
(1974) Present knowledge of the role of chromium. Fed. Proc.,
33: 2275-2280.
MEYERS, J.B. (1950) Acute pulmonary complications following
inhalations of chromic acid mist. Arch. ind. Hyg. occup. Med.,
2: 742-747.
MICKAIL, T.H., HABIB, Y.A., GABRIAL, G.N., & MORCOS, S.R.
(1976) The effect of chromium III on the intravenous glucose
tolerance of rats given diets of defficient protein values. Z.
Ernährungswiss., 15: 182-187.
MIESCHER, G., AMREIN, H.P., & LEDER, M. (1955) [Chromate
hypersensitivity and cement eczema.] Dermatologica (Basel),
110: 266-283 (in German).
MIGAI, K.V. (1975) [Hygiene and safety of labor in electro
welding works in shipbuilding,] Leningrad, pp. 27-35 (in
Russian).
MITMAN, F.W., WOLF, W.R., KELSAY, J.L., & PRATHER, E.S.
(1975) Urinary chromium levels of nine young women eating
freely chosen diets. J. Nutr., 105: 64-68.
MORGAN, J.M. (1972) Hepatic chromium content in diabetic
subjects. Metabolism, 21: 313-316.
MORRIS, G.E. (1955) Chromate dermatitis from chrome glue and
other aspects of the chrome problem. Am. Med. Assoc. Arch.
Ind. Health, 11: 368-371.
MORRIS, G.E. (1956) The manufacture of leather. Its chemical
and dermatological aspects. Ann. Meet. Am. Acad. Derm. Syph.
(Chicago).
MORRIS, G.E. (1958) Chrome dermatitis. A study of the
chemistry of shoe leather with particular reference to basic
chromic sulfate. Am. Med. Assoc. Arch. Derm., 78: 612-618.
MORRIS, G.E. (1959) [The basic form of chromic sulfate.
Results of epicutaneous testing of a chromium-III salt.]
Berufsdermatosen, 7: 126-131 (in German).
MORROW, R.W. & MCELHANEY, R.J. (1974) Determination of
chromium in industrial effluent water by flameless atomic
absorption spectroscopy. At. Absorpt. Newsl., 13(2): 45-46.
MORTON, M.S. & ELWOOD, P.C. (1974) CNS malformations and
trace elements in water. Teratology, 10: 318.
MOSINGER, M. & FIORENTINI, H. (1954) Sur la pathologie due
aux chromates: Premières recherches expérimentales. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 15: 187-199.
MULLER-WIEGAND, B., STEUER, D., & HEESSELS, P. (1983) Blood
and urine samples for quality control of heavy metals. Chem.
Toxicol. Clin. Chem. Met. Proc. Int. Conf., 2nd: 73-76.
MURAKAMI, Y., SUZUKI, Y., YAMAGATA, T., & YAMAGATA, N. (1965)
Cr and Mn in Japanese diet. J. Radiat. Res., 6: 105-110.
MURRMAN, R.P., WINTERS, R.W., & MARTIN, T.G. (1971)
Determination of trace elements in soils and clay minerals by
resonnance neutron activation analysis. Soil. Sci. Soc. Am.
Proc., 35: 647-652.
MUTTI, A., CAVATORTA, A., PEDRONI, C., BORGHI, A., GIAROLI,
C., & FRANCHINI, I. (1979) The role of chromium accumulation
in the relationship between airborne and urinary chromium in
welders. Int. Arch. occup. environ. Health, 43: 123-133.
MYSLYAEVA, A.V. (1965) [Changes in the peripheral blood in
workers of a chromium compound plant.] In: [Proceedings of
the 4th Scientific Conference of Aktubinsk State Medical
Institute, Alma-Ata,] Kazakhstan, Alma-Ata, Aktubinsk State
Medical Institute, pp. 331-332 (in Russian).
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., & KURATA, H. (1978)
Comparative studies of chromosomal aberrations and muta-
genicity of trivalent and hexavalent chromium. Mutat. Res.,
58: 175-181.
NASH, D.T., ELWOOD, I.G., & STREETEN, D.H.P. (1979) The
effect of brewer's yeast containing chromium-rich glucose
tolerance factor on serum lipids. In: Proceedings of the 5th
International Symposium on Atherosclerosis, Houston, Texas.
NATER, J.P. (1963) Possible causes of chromate eczema.
Dermatologica (Basel), 126: 160-166.
NATH, R. (1976) Assessment of chromium metabolism in
maturity onset and juvenile diabetics using chromium: 51Cr and
therapeutic response of chromium administration. Excerpta Med.
Int. Congr. Ser., 400: 194.
NEIKO, E.M. & DELVA, IU.V. (1978) [Chromium balance in
chronic ischemic heart disease.] Klinicheskaia Meditsina
(Mosk.), 56(4): 97-99 (in Russian).
NESTMANN, E.R., MATULA, T.I., DOUGLAS, G.R., BORA, K.C., &
KOWBEL, D.J. (1979) Detection of the mutagenic activity of
lead chromate using a battery of microbial tests. Mutat. Res.,
66: 357-365.
NETTESHEIM, P., HANNA, M.G., Jr., DOHERTY, D.G., NEWELL, R.F.,
& HELLMANN, A. (1970) Effects of chronic exposure to
artificial smog and chromium oxide dust on the incidence of
lung tumours in mice. In: Hanna, M.G., Jr, Nettesheim, P., &
Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 305-320 (US Atomic Energy
Commission Symposium Series No. 18).
NETTESHEIM, P., HANNA, M.G., DOHERTY, D.G., NEWELL, R.F., &
HELLMAN, A. (1971) Effect of calcium chromate dust,
influenza virus, and 100R whole-body X-radiation on lung
tumour incidence in mice. J. Natl Cancer Inst., 47: 1129-1144.
NEWBOLD, R.F., AMOS, J., & CONNELL, J.R. (1979) The
cytotoxic, mutagenic, and clastogenic effects of chromium-
containing compounds on mammalian cells in culture. Mutat.
Res., 67: 55-63.
NEWMAN, H.A.I., LEIGHTON, R.F., LANESE, R.R., & FREELAND N.A.
(1978) Serum chromium and angiographically-determined
coronary artery disease. Clin. Chem., 24: 541-544.
NISHIOKA, H. (1975) Mutagenic activities of metal compounds
in bacteria. Mutat. Res., 31: 185-189.
NOLLENDORF, A., PAKALNE, E., & UPITIS, V. (1972) [Little
known trace elements in Chlorella culture.] Latv. PSR Zinat.
Akad. Vestis (Latvian SSR), 7: 33-43 (in Russian).
NOMIYAMA, H., YOTORIYAMA, M., & NOMIYAMA, K. (1980b) Normal
chromium levels in urine and blood of Japanese subjects
determined by direct flameless atomic absorption spectrophoto-
metry, and valency of chromium in urine after exposure to
hexavalent chromium. Am. Ind. Hyg. Assoc. J., 41: 98-102.
NOMIYAMA, K., MATSUI, K., & NOMIYAMA, H. (1980a) Environ-
mental temperature: a factor modifying the acute toxicity of
organic solvents, heavy metals, and agricultural chemicals.
Toxicol. Lett., 6: 67-70.
NORSETH, T. (1978) Health effects of nickel and chromium.
In: Elvira di Ferrante, E., ed. Trace metals: exposure and
health effects, Oxford, New York, Toronto, Sydney, Paris,
Frankfurt, Pergamon Press, pp. 135-146.
NORSETH, T. (1980) Cancer hazards caused by nickel and
chromium exposure. J. Toxicol. environ. Health, 6: 1218-1227.
NOVAKOVA, S., MAUTNER, G., & DINOEVA, A. (1974) [Content of
hexavalent chromium in water supplies and its effect on the
development of experimental atherosclerosis in warm-blooded
animals.] Gig. i Sanit., 5: 78-80 (in Russian).
NOVEY, H.S., HABIB, M., WELLS, J.A. (1983) Asthma and JgE
antibodies induced by chromium and nickel salts. J. Allergy
clin. Immunol., 72: 407-412.
OFFENBACHER, E.G. & PI-SUNYER, F.X. (1980) Benefical effect
of chromium-rich yeast on glucose tolerance and blood lipids
in elderly subjects. Diabetes, 29: 919-925.
OHNO, H., HANAOKA, F., & YAMADA, M. (1982) Inducibility of
sister-chromatid exchanges by heavy-metal ions. Mutat. Res.,
104: 141-145.
OHTA, F. (1940) Studies on the detoxicating hormone of the
liver (yakriton). Contribution to the usage of yakriton
against experimental chromate nephritis. Tohoku J. exp. Med.,
39: 37-46.
OKADA, S., OHBA, H., & TANIYAMA, M. (1981) Alterations in
ribonucleic acid synthesis by chromium (III). J. inorg.
Biochem., 15: 223-231.
OKADA, S., TRUKADA, H., & OHBA, H. (1983) Enhancement of
ribonucleic acid synthesis by chromium (III) in mouse liver.
J. inorg. Biochem., 19: 95-103
OKADA, S., TSUKADA, H., & OHBA, H. (1984) Enhancement of
nucleolar RNA synthesis by chromium (III) in regenerating rat
liver. J. inorg. Biochem., 21, 113-124.
OPHULS, W. (1911a) Experimental nephritis in guinea-pigs by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9: 11-13.
OPHULS, W. (1911b) Experimental nephritis in rabbits by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9:13.
OTTINGER, R.S., BLUMENTHAL, J.L., DALPORTO, D.F., GURBER,
G.I., SANTY, M.J., & SHIH, C.C. (1973) Recommended methods
of reduction, neutralization, recovery or disposal of
hazardous waste, Cincinnati, Ohio, US Environmental Protection
Agency, Vol. 6, pp. 143-162 (EPA 670/2-73-053-F).
PAGE, A.L. (1974) Fate and effects of trace elements in
sewage sludge when applied to agricultural lands, Cincinnati,
Ohio, US Environmental Protection Agency (EPA 670/2-74-005).
PAIXAO, L.M. & YOE, J.H. (1959) Spectochemical determination
of magnesium, chromium, nickel, copper, and zinc in human
plasma and red cells. Clin. Chim. Acta, 4: 507-514.
PARR, R.M. (1977) Problems of chromium analysis in
biological materials: an international perspective with
special reference to results for analytical quality control
samples. J. radioanal. Chem., 39: 421-433.
PASCALE, L.R., WALDSTEIN, S.S., ENGBRING, G., DUBIN, A., &
SZANTO, P.B. (1952) Chromium intoxication with special
reference to hepatic injury. J. Am. Med. Assoc., 149:
1385-1389.
PASHIN, Y.V. & KOZACHENKO, V.J. (1981) [Mutagenic activity
of chromium compounds.] Gig. i. Sanit., 5: 46-49 (in Russian).
PASHIN, Y.V., ZACEPILOVA, T.A., & KOZACHENKO, V.I. (1982)
Induction of dominant lethal mutations in male mice by
potassium dichromate. Mutat. Res., 103: 345-347.
PASTERNAK, K. (1973) The spreading of heavy metals in
flowing waters in the region of occurrence of natural deposits
of the zinc and lead industry. Acta hydrobiol., 15: 145-166.
PEKAREK, R.S., HAUER, E.C., RAYFIELD, E.J., WANNEMACHER, R.W.,
& BIESEL, W.R. (1975) Relationship between serum chromium
concentrations and glucose utilization in normal and infected
subjects. Diabetes, 24: 350-353.
PENG, H.Z. (1982) [The investigation of nasal disease
morbidity of chromium exposed workers.] J. Shan-Xie Med.
College, 3: 19-21 (in Chinese).
PERLMUTTER, N.M. & LIEBER, M. (1970) Dispersal of plating
wastes and sewage contaminants in ground water and surface
water, Washington DC, US Government Printing Office, 67 pp
(Geological Survey Water Supply Paper 1879-G).
PERONE, V.B., MOFFITT, A.A., POSSICK, P.A., KEY, M.M.,
DANZINGER, S.J., & GELLIN, G.A. (1974) The chromium, cobalt,
and nickel contents of American cement and their relationship
to cement dermatitis. Am. Ind. Hyg. Assoc. J., 35: 301-306.
PETRILLI, F.L. & DE FLORA, S. (1977) Toxicity and mutagenicity of
hexavalent chromium on Salmonella typhimurium. Appl. environ.
Microbiol., 33: 805-809.
PETRILLI, F.L. & DE FLORA, S. (1978a) Oxidation of inactive
trivalent chromium to the mutagenic hexavalent form. Mutat.
Res., 58: 167-173.
PETRILLI, F.L. & DE FLORA, S. (1978b) Metabolic deactivation
of hexavalent chromium mutagenicity. Mutat. Res., 54: 139-147.
PETRILLI, F.L. & DE FLORA, S. (1980) Mutagenicity of
chromium compounds. In: Chromates Symposium 80, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 76-99.
PETRILLI, F.L. & DE FLORA, S. (1982) Interpretations on
chromium mutagenicity and carcinogenicity. In: Sorsa, M. &
Vainmio, H., ed. Mutagens in our environment, New York, Alan
R. Liss, pp. 453-464.
PIERCE, J.V. & SCHEEL, L.D. (1965) Toxicity of alloys of
chromium. Arch. environ. Health, 10: 870-876.
PIRILA, V. & KILPIO, O. (1949) On dermatoses caused by
bichromates. Acta derm. venerol., 29: 550-563.
POHLAND, F.G. (1975) Sanitary landfill stabilization with
leachate recycle and residual treatment, Cincinnati, Ohio, US
Environmental Protection Agency, 106 pp (EPA 600/2-75-043).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1973) [On carcinogenous
hazards in the product of chromium ferroalloys.] Gig. Tr.
prof. Zabol., 10: 23-26 (in Russian).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1974) [Epidemiological
data on malignant neoplasms in workers who are in contact with
different chromium compounds.] In: Shabad, L.M., Velich-
kovskii, B.T. & Kogan, F.M., ed. [Occupational cancer (a
collection of scientific works),] Moscow, Academic Academy of
Medical Science, pp. 64-71 (in Russian).
POKROVSKAYA, L.V., TJUSHUYAKOVA, A.V., GORODNOVA, N.V.,
ZALOMOVA, E.N., & ANDREEVA, T.D. (1976) [Dust factor and
occupational pathology in workers engaged in the open
excavation of chromium ores.] In: [Occupational diseases of
dust etiology (collection of works),] Vol. 3, pp. 38-43 (in
Russian).
POLAK, L., TURK, J.L., & FREY, J.R. (1973) Studies on
contact hypersensitivity to chromium compounds. In: Kallos,
P., Waksman, B.H., & DeWeck, A., ed. Progress in allergy,
Basel, Karger, Vol. 17, pp. 145-226.
PRESTON, A.M., DOWDY, R.P., PRESTON, M.A., & FREEMAN, J.N.
(1976) Effects of dietary chromium on glucose and serum
cholesterol in guinea-pigs. J. Nutr., 106: 1391-1397.
PRIESTLEY, J. (1877) Observations on the physiological
action of chromium. J. Anat. Physiol., 11: 285-301.
PRINCI, F., MILLER, L.H., DAVIS, A., & CHOLAK, J. (1962)
Pulmonary disease of ferrochromium workers. J. occup. Med., 4:
301-310.
PUNSAR, S., ERAMETSA, O., KARVONEN, M.I., RYHANEN, A., HILSKA,
P., & WORNAMO, H. (1975) Coronary heart disease and
drinking-water. A search in two Finnish male cohorts for
epidemiologic evidence of a water factor. J. chron. Dis., 28:
259-287.
RABEAU, H. & UKRAINCZYK, V. (1939) Dermites des
blanchisseuses. Rôle du chrome et du chlore. Ann. Derm. Syph.,
10: 656-680.
RADIAN CORPORATION (1983) Estimates of population exposure
to ambient chromium emissions, Research Triangle Park, North
Carolina, US Environmental Protection Agency (Final Report to
K.L. Blanchard) (EPA Contract No. 68-02-3818).
RAFFETTO, G. (1977) Direct interaction with cellular targets
as the mechanism for chromium carcinogenesis. Tumori, 63:
503-512.
RAFFETTO, G., PARODI, S., DE FERRARI, M., PARODI, C., TROIANO,
R., & BRAMBILLA, G. (1978) Direct interaction with cellular
targets as the mechanism for chromium carcinogensis. Tumori,
73: 503-512.
REDISKE, J.H. (1956) Chromium toxicity in plants. In:
Biology research: annual report, 1955, Richland, Washington,
Hamford Atomic Products Operations, pp. 48-52 (Report
HW-41500).
REDISKE, J.H., CLINE, J.F., & SELDERS, A.A. (1955) Chromium
toxicity in plants. In: Biology research: annual report, 1954,
Richland, Washington, Hamford Atomic Products Operations,
pp. 32-39 (Report HW-35917).
REGGIANI, A., LOTTI, M., DE ROSA, E., & SAIA, B. (1973)
[Impairments of respiratory functions in subjects exposed to
chromium. Note 1. Spirographic changes.] Lav. Umano, 25: 23-27
(in Italian).
REICHENBACH-KLINKE, H.H. (1977) [To the ecology of the
accumulation of heavy metals in fishes.] In: Reichenbach-
Klinke, H.H., ed. [Contributions to fish ecology: fish and the
environment,] Stuttgart, New York, Gustav Fischer, pp. 7-11
(in German).
REICHENBACH-KLINKE, H.H. (1980) [Fish diseases and harm
suffered by fish,] 2nd ed., Stuttgart, New York, Gustav
Fischer, 474 pp (in German).
RETNEV, V.M. (1960) [On the effect produced by chromium
compounds contained in cement dust on the development of
bronchial asthma.] Gig. Tr. prof. Zabol., 7: 29-32 (in
Russian).
RIALES, R. (1979) Influence of brewer's yeast on lipoprotein
cholesterol concentrations. A preliminary report. In:
Shapcott, D. & Hubert, I., ed. Chromium in nutrition and
metabolism. Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 199-212.
ROBERTS, K.R. & MARZLUF, G.A. (1971) The specific inter-
action of chromate with the dual sulfate permease systems of
Neurospora crassa. Arch. Biochem. Biophys., 142: 651-659.
ROBINSON, W.D. & EDINGTON, G. (1935) Chemical studies of
infertile soils derived from rocks high in magnesium and
generally high in chromium and nickel, Washington DC, US
Department of Agriculture, pp. 1-29 (US Department of
Agriculture Technical Bulletin No. 471).
ROGINSKI, E.E. & MERTZ, W. (1967) An eye lesion in rats fed
low-chromium rations. J. Nutr., 93: 249-251.
ROGINSKI, E.E. & MERTZ, W. (1969) Effects of chromium (III)
supplementation on glucose and amino acid metabolism in rats
fed a low protein diet. J. Nutr., 97: 525-530.
ROHDE, G., TISCHENDORF, G., LEONHARDT, J., & DAMASCHUN, F.
(1978) [Study of crocoite occurrence in Callenberg (Saxony)
(Germany).] Z. angew. Geol., 24: 168-173 (in German).
ROMOSER, G.L., DUDLEY, W.A., MACHLIN, L.J., & LOVELESS, L.
(1961) Toxicity of vanadium and chromium for the growing
chick. Poultry Sci., 40: 1171-1173.
ROOK, H.L. & WOLF, W.R. (1977) The quantitative
determination of volatile trace elements in NBS biological
standard reference materials. In: Hemphill, D.D., ed.
Proceedings of the Eleventh Annual Conference on Trace
Substances in Environmental Health, Columbia, Missouri, the
Curators of the University of Missouri, pp. 324-333.
ROSCHINA, T.A. (1959) [Experimental study on the effects of
magnezite chromium and chromite magnezite dust exposure in the
production of refrectories.] Gig. Tr. prof. Zabol., 4: 28-32
(in Russian).
ROSCHINA, T.A. (1964) [A comparative evaluation of the
effects of carbide dust and chromium boride on the organism.]
Gig. i Sanit., 8: 25-28 (in Russian).
ROSCHINA, T.A. (1976) [The toxicity of chrominum (sic)
hexacarbonyl.] Gig. Tr. Prof. Zabol., 2: 38-42 (in Russian).
ROSEBROUGH, R.W. & STEELE, N.C. (1981) Effect of
supplemental dietary chromium or nicotinic acid on
carbohydrate metabolism during basal, starvation, and
refeeding periods in poults. Poultry Sci., 60: 407-417.
ROSS, D.S., SJOGREN, R.E., & BARTLETT, R.J. (1981) Behaviour
of chromium in soils. IV. Toxicity to microorganisms. J.
environ. Qual., 10: 145-148.
ROSTENBERG, A., Jr & PERKINS, A.J. (1951) Nickel and cobalt
dermatitis. J. Allerg., 22: 466-474.
RTECS (1978) Registry of toxic effects of chemical substances,
Washington DC, National Institute for Occupational Safety and
Health, pp. 450.
SAMITZ, M.H. (1955) Some dermatologic aspects of the
chromate problem. Arch. ind. Health, 11: 361-367.
SAMITZ, M.H. (1956) Studies of chromate dermatitis. Arch.
ind. Health, 14: 269-271.
SAMITZ, M.H. & EPSTEIN, E. (1962) Experimental cutaneous
chrome ulcers in guinea-pigs. Arch. environ. Health, 5:
463-468.
SAMITZ, M.H. & GROSS, S. (1960) Extraction by sweat of
chromium from chrome tanned leathers. J. occup. Med., 2: 12-14.
SAMITZ, M.H. & GROSS, S. (1961) Effects of hexavalent and
trivalent chromium compounds on the skin. Am. Med. Assoc.
Arch. Dermatol., 84: 404-409.
SAMITZ, M.H., KATZ, S., & GROSS, S. (1960) Nature of the
chromium extracted from leather by sweat. J. occup. Med., 2:
435-436.
SANDERS, C.L., JACKSON, T.A., ADEE, R.R., POWERS, G.J., &
WEHNER, A.P. (1971) Distribution of inhaled metal oxide
particles in pulmonary alveoli. Arch. intern. Med., 127:
1085-1089.
SARGENT, T., III, LIM, T.H., & JENSON, R.L. (1979) Reduced
chromium retention in patients with hemochromatosis, a
possible basis of hemochromatotic diabetes. Metabolism, 28:
70-79.
SARTO, F., LEVIS, A.G., & PAULON, C. (1980) Clastogenic
activity of hexavalent and trivalent chromium in cultured
human lymphocytes. Caryologia, 33: 239-250.
SARTO, F., COMINATO, J., BIANCHI, V., & LEVIS, A.G. (1982)
Increased incidence of chromosomal aberrations and sister
chromatid exchanges in workers exposed to chromic acid (CrO3)
in electroplating factories. Carcinogenesis, 3: 1011-1016.
SASSI, C. (1956) [Occupational disease in a chromate
factory.] Med. Lavoro, 47: 314-326 (in Italian).
SATOH, K., FUKUDA, Y., TORII, K., & KATSUNO, N. (1981)
Epidemiological study of workers engaged in the manufacture of
chromium compounds. J. occup. Med., 23: 835-838.
SAVORY, J., MUSHAK, P., & SUNDERMAN, F.W. Jr (1969) Gas
Chromatographic determination of chromium in serum. J.
chromatogr. Sci., 7: 674-679.
SCHALLER, K.H. & ZOBER, A. (1982) [Renal excretion of
toxicologically relevant metals in occupationally non-exposed
individuals.] Arztl. Lab., 28: 209-214 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1972) [Quantitative chromium determination in urine by
flameless atomic absorption spectrometry.] Z. klin. Chem.
klin. Biochem., 10: 434-437 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1973) The quantitative determination of chromium in urine by
flameless atomic absorption spectroscopy. At. Absorpt.
Newsl., 12(6): 147-150.
SCHELENZ, R. (1977) Dietary intake of 25 elements by man
estimated by neutron activation analysis. J. radioanal. Chem.,
37: 539-548.
SCHLETTWEIN-GSELL, D. & MOMMSEN-STRAUB, S. (1971) [Review
trace elements in foods. III. Chromium.] Int. J. Vitam. Nutr.
Res., 41: 116-125 and (1973) Suppl. 13, 188 pp (in German).
SCHOENTAL, R. (1975) Chromium carcinogenesis formation of
epoxyaldehydes and tanning. Br. J. Cancer, 32: 403-404.
SCHROEDER, H.A. (1966) Chromium deficiency in rats: a
syndrome simulating diabetes mellities with retarded growth.
J. Nutr., 88: 439-445.
SCHROEDER, H.A. (1970) Chromium, Washington DC, American
Petroleum Institute, 28 pp (Air Quality Monograph No. 70-15).
SCHROEDER, H.A. & BALASSA, J.J. (1965) Influence of
chromium, cadmium, and lead on rat aortic lipids and
circulating cholesterol. Am. J. Physiol., 209: 433-437.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1962)
Abnormal trace metals in man: chromium. J. chron. Dis., 15:
941-964.
SCHROEDER, H.A., VINTON, W.H., Jr, & BALASSA, J.J. (1963)
Effects of chromium, cadmium, and other trace metals on the
growth and survival of mice. J. Nutr., 80: 39-47.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1964)
Chromium, lead, cadmium, nickel, and titanium in mice: effect
on mortality, tumours, and tissue levels. J. Nutr., 83:
239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1965)
Chromium, cadmium, and lead in rats: effects on life span,
tumours, and tissue levels. J. Nutr., 86: 51-66.
SCHROEDER, H.A., NASON, A.P., & TIPTON, I.H. (1970) Chromium
deficiency as a factor in atherosclerosis. J. chron. Dis., 23:
123-142.
SCHROEDER, K.H. (1955) [Nickel eczema in a blueline (ozalid)
machine operator.] Berufsdermatosen, 3: 170-171 (in German).
SCHUBERT, G.E., GEBHARD, K., & HOENLEIN, F. (1970) [Kidney
damage following injection of serotonin and potassium
dichromate for chronic sodium overloading or restriction.]
Virchows Arch. Abt. A Path. Anat., 351: 68-82 (in German).
SCHULTHEISS, E. (1959) [Rubber and eczema,], Aulendorf i.
Württemberg, Cantor, 213 pp (in German).
SCHWARZ, K. & MERTZ, W. (1959) Chromium (III) and the
glucose tolerance factor. Arch. Biochem. Biophys., 85: 292-295.
SCHWARTZ, L. & DUNN, J.E. (1942) Dermatitis occurring among
operators of air conditioning equipment. Ind. Med. Surg., 11:
375.
SCHWARTZ, L., TULIPAN, L., & PECK, S. (1947) Occupational
diseases of the skin, 2nd ed., Philadelphia, Pennsylvania, Lea
& Febiger.
SEEBER, H., FIKENTSCHER, R., & ROSEBURG, B. (1976)
[Disorders of smell and taste in chromium dye workers.] Z.
Gesamte Hyg. Grenzgeb., 22: 820-822 (in German).
SEELEY, J.L. & SKOGERBOE, R.K. (1974) Combined sampling-
analysis method for the determination of trace elements in
atmospheric particulates. Anal. Chem., 46: 415-421.
SEELING, W., GRUENERT, A., KIENLE, K.H., OPFERKUCH, R., &
SWOBODNIK, M. (1979) [Determination of chromium in human
serum and plasma by flameless atomic absorption spectro-
photometry.] Z. anal. Chem., 299: 368-374 (in German).
SEITZ, W.R., SUYDAM, W.W., & HERCULES, D.M. (1972)
Determination of trace amounts of chromium (III) using
chemiluminescence analysis. Anal. Chem., 44(6): 957-963.
SHACKLETTE, H.T., SAUER, H.I., & MIESCH, A.T. (1970)
Geochemical environments and cardiovascular mortality rates in
Georgia, Washington DC, US Governmental Printing Office, 39 pp
(US Geological Survey Professional Paper No. 574-C).
SHAPCOTT, D., KHOURI, K., DEMERS, P.P., VOBECKY, J., &
VOBECKY, J. (1977) The measurement of volatile chromium in
biological materials. Clin. Biochem, 10: 178-180.
SHEFFET, A., THIND, J., MILLER, A.M., & LOURIA, D.B. (1982)
Cancer mortality in a pigment plant utilizing lead and zinc
chromates. Arch. environ. Health, 37: 44-52.
SHENDRIKAR, A.D. & WEST, P.A. (1974) A study of adsorption
characteristics of traces of chromium (III) and (VI) on
selected surfaces. Anal. Chim. Acta, 72: 91-96.
SHERMAN, L., GLENNON, J.A., BRECK, W.J., KLOMBERG, G.H., &
GORDON, E.S. (1968) Failure of trivalent chromium to improve
hyperglycemia in diabetes mellitus. Metabolism, 17: 439-442.
SHIMKIN, M.M. & LEITER, J. (1940) Induced pulmonary tumours
in mice. III. Role of chromic irritation in the production of
pulmonary tumours in strain A mice. J. Natl Cancer Inst., 1:
241-254.
SHMITOVA, L.A. (1978) In: [The impact of job-related factors
on specific function of the female organism,] Svedlovsk, pp.
105-108 (in Russian).
SHMITOVA, L.A. (1980) Gig. Tr. prof. zabol., 2:
33-35 (in Russian).
SIDI, E. & LONGUEVILLE, R. (1953) Dermites au ciment. Rôle
des sels de chrome. Arch. Mal. prof., 14: 41.
SIEGENTHALER, U., LAINE, A., POLAK, L. (1983) Studies on
contact sensitivity to chromium in the guinea-pig. The role
of valence in the formation of the antigenic determinant. J.
Invest. Dermatol., 80: 44-47.
SIMONOFF, M., LLABADOR, Y. HAMON, C., PEERS; A.M., & SIMONOFF,
G.N. (1984) Low plasma chromium in patients with coronary
artery and heart diseases. Biol. Trace Element Res., 6:
431-439.
SIMONDS, J.P. & HEPLER, O.E. (1945) Experimental nephro-
pathies. I. A method of producing controlled selective injury
of renal units by means of chemical agents. Arch. Path., 39:
103-108.
SIROVER, M.A. & LOEB, L.A. (1976) Infidelity of DNA
synthesis in vitro: screening for potential metal mutagens or
carcinogens. Science, 194: 1434-1436.
SJOGREN, B. (1980) A retrospective cohort study of mortality
among stainless steel welders. Scand. J. Work. environ.
Health, 6: 197-200.
SKOGERBOE, R.K. (1974) Monitoring trace metal particulates:
an evaluation of the sampling and analysis problems. In:
Instrumentation for monitoring air quality, Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp.
125-136.
SLUIS-CREMER, G.K. & DU TOIT, R.S. (1968) Pneumoconiosis in
chromite miners in South Africa. Br. J. ind. Med., 25: 63-67.
SPIER, H.W. (1956) [Wood preservatives and chromate eczema.]
Desinfekt. Gesundheitswes., 4: 58 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil ( Myriophyllum spicatum L.). Arch.
environ. Contam. Toxicol., 2: 331-341.
STAUB, H.W., REUSSNER, G., & THIESSEN, R., Jr (1969) Serum
cholesterol reduction by chromium in hypercholesteremic rats.
Science, 166: 746-747.
STEELE, N.C. & ROSEBROUGH, R.W. (1979) Trivalent chromium
and nicotinic acid supplementation for the turkey poult.
Poultry Sci., 58: 983-984.
STEELE, N.G., ALTHEN, T.G., & FROBISH, L.T. (1977a) Bio-
logical activity of glucose tolerance factor in swine. J.
anim. Sci., 45: 1341-1345.
STEELE, N.C., PELURA, I., & FROBISH, L.T. (1977b) Effect of
dietary protein source on glucose utilization and hepatic
insulin-receptor characteristics of the rat. Nutr. Rep. Int.,
15: 429-435.
STELLA, M., MENTALDI, A., ROSSI, R., ROSSI, G., & LEVIS, A.G.
(1982) Clastogenic effects of chromium on human lymphocytes
in vitro and in vivo. Mutat. Res., 101: 151-164.
STEFFEE, C.H. & BAETJER, A.M. (1965) Histopathologic effects
of chromate chemicals. Report of studies in rabbits, guinea-
pigs, rats, and mice. Arch. environ. Health, 11: 66-75.
STEINHOFF, D., GAD, S.C., HATFIELD, G.K., & MOHR, U. (1983)
Testing sodium dichromate and soluble calcium chromate for
carcinogenicity in rats. Report to the Industrial Health
Foundation, Wuppertal, Bayer AG Institute of Toxicology,
126 pp.
STEREKHOVA, N.P., ZELENEVA, N.I., SOLOMINA, S.N.,
TYUSHNYAKOVA, N.V., MYASNIKOVA, A.G., FOKINA, G.P., & YARINA,
A.L. (1978) [Gastric pathology in workers engaged in the
production of chromium salts.] Gig. Tr. prof. Zabol., 3: 19-23
(in Russian).
STERN, R.M. (1981) Process-dependent risk of delayed health
effects for welders. Environ. Health Perspect., 41: 235-253.
STERN, R.M. (1982) Chromium compounds: production and
occupational exposure. In: Langard, S., ed. Biological and
environmental aspects of chromium, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 5-47.
STRIK, J.J.T.W.A., DE IONGH, H.H., VAN RIJN VAN ALKEMADE,
J.W.A., & WIUTE, T.P. (1975) Toxicity of chromium (VI) in
fish, with special reference to organoweights, liver and
plasma enzyme activities, blood parameters, and histological
alterations. In: Koeman, S.H. & Strik, J.J.T.W.A., ed.
Sublethal effects of toxic chemicals on aquatic animals,
Amsterdam, New York, Oxford, Elsevier Science Publishers,
pp. 31-41.
STROUT, R.W. (1977) The relationship of abnormal circulating
insulin levels to atherosclerosis. Atherosclerosis, 27: 1-13.
SULLIVAN, R.J. (1969) Air pollution aspects of chromium and
its compounds, Raleigh, North Carolina, US Department of
Health, Education and Welfare, 75 pp (National Air Pollution
Control Administration Publication No. APTD 69-34).
SUSA, N., FURUKAWA, YO., & TSUBAKI, S. (1977) Comparative
cytotoxic effects of hexavalent and trivalent chromium
compounds on cultured cells. Jap. J. vet. Sci., 39: 325-336.
SUZUKI, K., ESASHI, T., SUZUE, R., NISHIMURA, H., & MATSUMOTO,
H. (1979) Tissue concentrations of cadmium, lead, and
chromium in Japanese ranging from the fetus to elder subjects.
Toxicol. Lett., 4: 481-484.
SWAINE, D.J. & MITCHELL, R.L. (1960) Trace element
distribution in soil profiles. J. soil Sci., 11 (2): 347-368.
SWENSSON, A. (1977) [Experimental research on the
fibrogenetic effect of chromite.] Arbete och Haelsa, 2: 1-14
(in Swedish).
TABOR, E.C. & WARREN, W.V. (1958) Distribution of certain
metals in the atmosphere of some American cities. Arch. ind.
Hyg., 17: 145-151.
TAMARO, M., BANFI, E., VENTURINI, S., & MONTI-BRAGADIN, C.
(1975) [Hexavalent chromium compounds are mutagens for
bacteria.] In: Proceedings of the XVII Congress Naz. Soc. It.
Microbiol., Padova, pp. 411-415 (in Italian).
TANDON, S.K., SAXENA, D.K., GAUR, J.S., & CHANDRA, S.W.
(1978) Comparative toxicity of trivalent and hexavalent
chromium. Environ. Res., 15: 90-99.
TANDON, S.K., BEHARI, J.R., & KACHRU, D.N. (1979) Distri-
bution of chromium in poisoned rats. Toxicology, 13: 29-34.
TANDON, S.K., MATHUR, A.K., & GAUR, J.S. (1977) Urinary
excretion of chromium and nickel among electroplaters and
pigment industry workers. Int. Arch. occup. environ. Health,
40: 71-76.
TATARU, C., MARINSECU, A., CAPUSAN, I., RIPAN, R., & LITEANU,
C. (1957) [Skin damage during the industrial recovery of
potassium dichromate.] Berufsdermatosen, 5: 283-295 (in
German).
TAYLOR, A.E., GUYTON, M.O., & BISHOP, V.S. (1965)
Permeability of the alveolar membrane to solutes. Circ. Res.,
16: 353-362.
TAYLOR, F.G., Jr, MANN, L.K., DAHLMANN, R.C., & MILLER, F.L.
(1975) Environmental effects of chromium and zinc in
cooling-water drift. In: Cooling tower environment, 1974,
Washington DC, US Energy Research and Development
Administration, pp. 408-426 (Report Conf. No. 740302).
TAYLOR, F.H. (1966) The relationship of mortality and
duration of employment as reflected by a cohort of chromate
workers. Am. J. public Health, 56: 218-229.
TAZELAAR, D.J. (1970) Hypersensitivity to chromium in a
light-blue Tattoo. Dermatologica (Basel), 141: 282-287.
THOMSON, A.G. (1982) Chromite, Mining Annual Review,
pp. 70-71.
THORMANN, J., JESPERSEN, N.B., & JOENSEN, H.D. (1979)
Persistence of contact allergy to chromium. Contact Dermatit.,
5: 261-264.
TOEPFER, E.W., MERTZ, W., ROGINSKI, E.E., & POLANSKY, M.M.
(1973) Chromium in foods in relation to biological activity.
J. agric. food Chem., 21: 69-73.
TOEPFER, E.W., MERTZ, W., POLANSKY, M.M., ROGINSKI, E.E., &
WOLF, W.R. (1977) Preparation of chromium-containing
material of glucose tolerance factor activity from brewer's
yeast extracts and by synthesis. J. agric. food Chem., 25:
162-166.
TOLA, S., KILPIO, J., VIRTAMO, M., & HAAPA, K. (1977)
Urinary chromium as an indicator of the exposure of welders to
chromium. Scand. J. Work environ. Health, 3: 192-202.
TOLG, G. (1974) Recent problems and limitations in the
analytical characterization of high-purity material. Talanta,
21: 327-345.
TSUDA, H. & KATO, K. (1976) Potassium dichromate: induced
chromosome aberrations and its control with sodium sulfite in
hamster embryonic cells in vitro. Gann, 67: 469-470.
TSUDA, H. & KATO, K. (1977) Chromosomal aberrations and
morphological transformation in hamster embryonic cells
treated with potassium dichromate in vitro. Mutat. Res., 46:
87-94.
TUMAN, R.W. & DOISY, R.J. (1977) Metabolic effects of the
glucose tolerance factor (GTF) in normal and genetically
diabetic mice. Diabetes, 26: 820-826.
TUMAN, R.W., BILBO, J.T., & DOISY, R.J. (1978) Comparison
and effects of natural and synthetic glucose tolerance factor
in normal and genetically diabetic mice. Diabetes, 27: 49-56.
TURNER, M.A. & RUST, R.H. (1971) Effects of chromium on
growth and mineral nutrition of soybeans. Soil Sci. Soc. Am.
Proc., 35: 755-758.
TYUSHNYAKOVA, N.V., ZISLIN, D.M., SHABYNINA, N.K., & BELJAEVA,
L.N. (1974) [Criteria of occupational cancer in the
production of chromium salts and some ideas concerning its
pathogenesis.] In: Occupational cancer (collection of
scientific works), Sverdlovsk, pp. 56-63 (in Russian).
UMEDA, M. & NISHIMURA, M. (1979) Inducibility of chromosomal
aberrations by metal compounds in cultured mammalian cells.
Mutat. Res., 67: 221-229.
URCHS, O. (1953) [Occupational skin diseases,] Cantor,
Aulendorf i. Württemberg, 108 pp.
US DHEW (1975) Criteria for a recommended standard.
Occupational exposure to chromium (VI), Washington DC, US
Department of Health, Education and Welfare, 200 pp (US DHEW
Publication No. NIOSH 76-129).
US DHEW (1977) Occupational diseases: a guide to their
recognition, revised ed., Washington DC, US Department of
Health, Education and Welfare, pp. 352-355 (Stock No.
017-033-00266-5).
US EPA (1978) Reviews of the environmental effects of
pollutants. III. Chromium, Washington DC, US Environmental
Protection Agency, 285 pp (ORNL/EIS-80; EPA 600/1-78-023).
US EPA (1980) Ambient water quality criteria for chromium,
Washington DC, US Environmental Protection Agency, pp. A1-C48.
US NAS (1974a) Chromium, Washington DC, US National Academy
of Sciences, 155 pp.
US NAS (1974b) Geochemistry and the environment. I. The
relation of selected trace elements to health and disease,
Washington DC, US National Academy of Sciences, 113 pp.
US NAS/NAE (1972) Water quality criteria 1972, Washington
DC, US Environmental Protection Agency, US National Academy of
Sciences, US National Academy of Engineering, 533 pp.
US PHS (1953) Health of workers in chromate producing
industry, Washington DC, US Department of Health, Education
and Welfare, US Public Health Service, 131 pp (Publication No.
192).
VAKHRUSHEVA, V.A. (1960) [Effect of Mn and Cr as trace
elements on the level of blood sugar in white mice.] Tr.
Izhevskogo Otd. Fiziol. Obschchestva, 1: 184-190 (in Russian).
VALER, M. & RACZ, J. (1971) Investigations concerning the
sensitizing effect of trivalent chromium salts. I. Correlation
between concentration and skin penetration of trivalent
chromium and between the number of positive results obtained
in patch tests. J. ind. Med., 41(12): 302-317.
VEILLON, C., WOLF, W.R., GUTHRIE, B.E. (1979) Determination
of chromium in biological materials by stable isotope
dilution. Anal. Chem., 51: 1022-1024.
VELICHKOVSKY, B.T. & POKROVSKAJA, L.V. (1973) [Labour
hygiene in a modern production of multy-tonnage ferrolloys.]
In: [Proceedings of the National Symposium on Labor Hygiene
and Prophylaxis of Occupational Diseases in the Production of
Ferroalloys,] Sverdlovsk, pp. 3-8 (in Russian).
VENITT, S. & LEVY, L.S. (1974) Mutagenicity of chromates in
bacteria and its relevance to chromate carcinogenesis. Nature
(Lond.), 250: 493-494.
VENCGUJIV, A.J. (1974) Zones of display of biological and
pharmacological action of trace elements. In: Hoekstra, W.G.,
Gantherl, H.E., & Mertz, W., ed. Trace elements metabolism in
animals. II, Baltimore, London, Tokyo, University Park Press,
pp. 295-310.
VERFAILLIE, G.R.M. (1974) Kinetics of chromium absorption by
intact rice plants. In: Comparative studies of food and
environmental contamination, Vienna, International Atomic
Energy Agency, pp. 315-331.
VERSIECK, J.M.J. & SPEECKE, A.B.H. (1972) Contaminations
induced by collection of liver biopsies and human blood. In:
Nuclear Activation Techniques in the Life Sciences.
Proceedings of a Symposium, Bled, Yugoslavia, Vienna
International Atomic Energy Agency, pp. 39-49.
VERSIECK, I., HOSTE, J., BARBIER, F., STEYAERT, H., DERUDDER,
I., & MICHELS, H. (1978) Determination of chromium and
cobalt in human serum by neutron activation analysis. Clin.
Chem., 24: 303-308.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experience of the
occupational health department with some industrial threshold
limit values for industrial poisons.] Arch. Gewerbepath.
Gewerbehyg., 13: 528-534 (in German).
VISEK, W.J., WHITNEY, I.B., KUHN, U.S.G., & COMAR, C.L.
(1953) Metabolism of 51Cr by animals as influenced by
chemical state. Proc. Soc. Exp. Biol. Med., 84: 610-615.
VOELKL, A. (1971) [The daily excretion of chromium in normal
persons.] Zentralbl. Arbeitsmed., 21: 122 (in German).
VOTAVA, H.J., HAHN, C.J., & EVAN, G.W. (1973) Isolation and
partial purification of a 51Cr complex from brewer's yeast.
Biochem. Biophys. Res. Comm., 55: 312-319.
WACKER, W.E.C. & VALLEE, B.L. (1959) Nucleic acids and
metals. I. Chromium mangenese, nickel, iron, and other metals
in ribonucleic acid from diverse biological sources. J. biol.
Chem., 234: 3257-3262.
WAHBA, A. & COHEN, T. (1979) Chrome sensitivity in Israel.
Contact Dermatit., 5: 101-107.
WAHLBERG, J.E. & WENNERSTEN, G. (1977) Light sensitivity and
chromium dermatitis. Br. J. Dermatol., 97: 411-416.
WALSH, E.N. (1953) Chromate hazards in industry. J. Am. Med.
Assoc., 153: 1305-1308.
WARREN, G., SCHULTZ, P., BANCROFT, D., BENNETT, K., ABBOTT,
E.H., & ROGERS, S. (1981) Mutagenicity of a series of
hexacoordinate chromium (III) compounds. Mutat. Res., 90:
111-118.
WATANABE, S. & FUKUCHI, Y. (1975) An epidemiological survey
on lung cancer in workers of a chromate-producing industry in
Hokkaido, Japan (Presented at: The XVIII International Congress
on Occupational Health, Brighton, England, 14-19 September.
WEILER, R.R. & CHAWLA, V.K. (1969) Dissolved mineral and
quality of Great Lakes waters. In: Proceedings of the 12th
Conference on Great Lakes Res., 801.
WELZ, B. (1980) [Use of AAS and ICP. Relative advantage of
each method.] Chem. Techn., 9: 161-170. (in German).
WELZ, B. (1983) [Atomic absorption spectrometry,] 3rd ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
527 pp (in German).
WENDELBERGER, J. & WEITGASSER, H. (1956) [The role of alkali
and degreasing agents in the development of contact eczemas in
electroplasters.] Berufsdermatosen, 4: 16-21 (in German).
WHANGER, P.D. & WESWIG, P.H. (1975) Effects of selenium,
chromium, antioxidants on growth, eye cataracts, plasma
cholesterol, and blood glucose in selenium-deficient, vitamin
E-supplemented rats. Nutr. Rep. Int., 12: 345-348.
WHITE, L.R., JAKOBSEN, K., & 0STGAAD, K. (1979) Comparative
toxicity studies of chromium rich welding fumes and chromium
on an established human cell line. Environ. Res., 20: 366-374.
WHITING, R.F., STICH, H.F., & KOROPATNICK, D.J. (1979) DNA
damage and DNA repair in cultured human cells exposed to
chromate. Chem.-biol. Interact., 26: 267-280.
WHO (1983) EHC 27: Guidelines on studies in environmental
e pidemiology, Geneva, World Health Organization, 351 pp.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984a)
Disposition of intratracheally administered chromium (III) and
chromium (VI) in rabbits. Toxicol. Lett., 22: 273-276.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984b) The
reduction of chromium (VI) to chromium (III) by glutathione:
an intracellular redox pathway in the metabolism of the
carcinogen chromate. Toxicology, 33: 341-348.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1985)
[Determination of chromium in human red blood cells. Basis for
a concept of biological monitoring.] Arbeitsmed. Sozialmed.
Praeventivmed., 20: 1-4 (in German).
WILD, D. (1978) Cytogenetic effects in the mouse of 17
chemical mutagens and carcinogens evaluated by the micronuclei
test. Mutat. Res., 56: 319-327.
WILLIAMS, C.D. (1969) Asthma related to chromium compounds.
Report of two cases and review of the literature on chromate
diseases. NC med. J., 30: 482-491.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1960) The Merck index. An encyclopedia of chemicals and
drugs, 7th ed., Rahway, New Jersey, Merck and Co., 139 pp.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) The Merck index. An encyclopedia of chemicals and
drugs, 9th ed., Rahway, New Jersey, Merck and Co., 1313 pp.
WINSTON, J.R. & WALSH, E.N. (1951) Chromate dermatitis in
railroad employees working with diesel locomotives. J. Am.
Med. Assoc., 147: 1133-1134.
WITHEY, J. (1983) Memorandum addressed to Si Duk Lee, dated
March 4, 1983, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office.
WOLF, W.R. (1976) Coupled gas chromatography/atomic
absorption spectrometry for the nanogram determination of
chromium. Anal. Chem., 48: 1717-1720.
WOLF, W.R. & GREENE, F.E. (1976) Preparation of biological
materials for chromium analysis. In: Accuracy in trace
analysis: sampling, sample handling, and analysis,
Gaithersburg, Maryland, National Bureau of Standards, pp.
605-610 (Special Publication No. 422).
WOLF, W.R., MERTZ, W., & MASIRONI, R. (1974) Determination
of chromium in refined and unrefined sugars by oxygen plasma
ashing flameless atomic absorption. J. agric. food Chem., 22:
1037-1042.
WOLF, W.R., TAYLOR, M.L., HUGHES, B.M., TIERNAN, T.O., &
SIEVERS, R.E. (1972) Determination of chromium and beryllium
at the picogram level by gas chromatography/mass spectrometry.
Anal. Chem., 44: 616-618.
WU, G.-Y. (1981) [The research on the purification and the
chemical structure of the specific chromium binding
materials.] Jpn. J. Ind. Health, 33: 505 (in Chinese).
YAGI, T. & NISHIOKA, H. (1977) DNA damage and its degrada-
tion by metal compounds. Sci. Eng. Rev. Doshisha Univ., 18:
63-70.
YANG, G.L. (1956) [An investigation of occupational disease
among chromium plating workers.] Acta Acad. Med. primae
Shanghai, 2: 36-46 (in Chinese).
YOPP, J.H., SCHMID, W.E., & HOLST, R.W. (1974) Determination
of maximum permissible levels of selected chemicals that exert
toxic effects on plants of economic importance in Illinois,
Chicago, Illinois, Institute for Environmental Quality,
pp. 89-96.
YUNISOVA, H.K. & PAVLOVSKAJA, G.S. (1975) The effect of
regimen of chrome plating on the properties of chromine.
Protect. Metal., 2, 248-250.
ZANDER, A.T., O'HAVER, T.C., & KELIHER, P.N. (1977)
Correction for interferences of spectral origin with continuum
source, echelle wavelength modulated atomic absorption
spectrometry. Anal. Chem., 49: 838-842.
ZHURKOV, U.S. (1981) [Approaches to the regulation of
environmental chemical pollutants with mutagenic activity.]
In: Sidorenko, G.I.M., ed. [Medical problems of environmental
protection,] Moscow, USSR Academy of Medical Sciences,
pp. 88-95 (in Russian).
ZOBER, A. (1979) [On the problems of evaluating bronchial
carcinoma after exposition to chromium compounds.] Int. Arch.
occup. environ. Health, 43(2): 107-121 (in German).
ZOBER, A. (1982) [Possible dangers to the respiratory tract
from welding fumer: methods of approach in an industrial
health care context and results.] Schweissen & Schneiden,
34(2): 77-81 (in German).
ZOBER, A., KICK, K., SCHALLER, K.H., SCHELLMANN, B., &
VALENTIN, H. (1984) ["Normal values" of chromium and nickel
in human lung-, kidney-, blood-, and urine samples.] Zbl.
Bakt. Hyg. I. Abt. Orig. B, 179: 80-95 (in German).
Tuman & Doisy (1977) studied the effects of yeast concentrates
of high chromium (glucose tolerance factor) content and of
synthetic chromium complexes with GTF activity (Tuman et al., 1978)
in mice, raised on a presumably complete commercial stock diet.
Six animals were used for each test, either genetically diabetic
mice or their control litter mates of the C57Bl-KSI strain.
Injections of either 5 mg of the GTF-containing yeast extracts or
0.1 mg of the synthetic chromium complex, acutely reduced the
elevated plasma-glucose levels in the diabetic and the non-fasting
normal mice by 10 - 38% of the initial values ( P < 0.01) and the
plasma-triglycerides by 26 - 56% ( P < 0.01), compared with control
mice injected with saline. Injection of insulin into diabetic mice
produced only an 11 - 18% decrease in plasma-glucose levels,
whereas injection of the GTF-containing extract together with
insulin reduced plasma-glucose levels by 39 - 51% and plasma-
triglyceride levels by 76% (Table 11).
The results suggest either a much higher increase in the
chromium requirement of the genetically diabetic mice or their
inability to use chromium in the diet.
Table 11. Acute effects of GTF and exogenous insulin on non-
fasting plasma-glucose and plasma-triglyceride (TG) concentrations
in 19-week-old genetically diabetic micea
------------------------------------------------------------------
Treatment Plasma- deltaGlucose Plasma- deltaTG
glucoseb triglyceridesb
(mg/litre) (mg/litre)
------------------------------------------------------------------
saline 11 120 ± 3960 ± 160(5)c
490(5)c
GTF 9320 ± -180 (16%) 2790 ± 170(5)d -117 (30%)
280(5)d
insulin 9840 ± -128 (12%) 3020 ± 400(5) - 94 (24%)
410(5)
insulin 7060 ± -406 (37%) 940 ± 220(5)e -302 (76%)
and GTF 840(5)e
------------------------------------------------------------------
a Modified from: Tuman & Doisy (1977).
b Glucose and triglyceride values represent mean ± SEM for 6 mice in
each treatment group. Dose of GTF was 5 mg (WL-10-AT) administered
intraperitoneally, 12 h prior to collection of blood. Lente insulin
(0.1 U per mouse) was administered subcutaneously, 12 h prior to
collection of blood. Data were treated by analysis of variance to
detect differences between the various treatment groups; independent
orthogonal comparisons were performed in the following groups ( P value
indicates level of significance for each comparison).
c Saline versus all other treatments, P < 0.005.
d GTF versus insulin, P < 0.05.
e GTF and insulin alone versus combined GTF and insulin, P < 0.005.
Thus, GTF and insulin > GTF = insulin > saline.
Steele & Rosebrough (1979) reported a significant stimulation
of the growth rate of one-week-old turkey poults (both sexes) by
supplementation of a practical ration with 20 mg chromium (as
chloride)/kg. The weight gains within the 2-week study were 235 g
and 267 g for the 60 controls and 60 supplemented turkeys,
respectively ( P < 0.001). As the practical ration contained
ground yellow corn and soybean meal, limestone, and dicalcium
phosphate, chromium deficiency would appear unlikely. The amount
of the chromium supplement (20 mg/kg diet) is quite high, and
further studies are needed to decide whether the observed effects
were of a pharmaco-dynamic nature or truly nutritional, i.e.,
correcting a deficiency. A similar interpretation should be
applied to a report of improved egg quality in the laying hen
(Jensen et al., 1978).
The quantitative aspects of the effects of chromium on animals
can be summarized as follows: normal rats fed semi-purified, semi-
synthetic rations, with Torula yeast or individual proteins and
sucrose or starch as the source of carbohydrates, develop mild
signs of deficiency at a dietary level of 100 - 150 µg chromium/kg.
To prevent deficiency, most authors used very high supplements of
several mg/kg diet and did not determine the biological
availability of the chromium complexes used for the
supplementation. Diets containing raw ingredients and supplying
chromium levels between 0.5 and 1 mg/kg do not induce signs of
deficiency and probably meet the requirement of the rat. A very
tentative estimate of the dietary chromium need of the rat and
probably the mouse would be approximately 0.5 mg/kg diet. This
estimate should be interpreted with caution, because of the lack of
knowledge concerning the biological availability of chromium and of
its interaction with dietary constituents.
7.2. Toxicity Studies
The toxicology of chromium compounds has been reviewed by the
US National Academy of Science (US NAS, 1974a), Langard & Norseth
(1979), the International Agency for Research on Cancer (IARC,
1980), Langard (1980a, 1982), and Burrows (1983).
In discussing toxicological problems, it is important to
differentiate between the various oxidation states of chromium and
its compounds. Trivalent chromium, when administered to animals in
food or water, does not appear to induce any harmful effects, even
when given in large doses (US NAS, 1974a) (section 7.2.1). Acute
and chronic toxic effects of chromium are mainly caused by
hexavalent compounds. Since it has been shown that both industrial
trivalent chromium compounds as well as reagent-grade trivalent
chromium compounds can be contaminated by hexavalent chromium
(Petrilli & DeFlora, 1978a; Levis & Majone, 1979), the evaluation
of experimental studies becomes difficult, especially when the
purity of the chemical compounds used is not known.
Discrimination between the biological effects, caused by
hexavalent chromium and trivalent chromium is difficult, because,
after penetration of membranes in tissues, hexavalent chromium is
immediately reduced to trivalent chromium (Gray & Sterling, 1950;
US NAS, 1974a), and it is not evident whether the observed
phenomena are caused by this reduction or even by the trapping of
trivalent chromium by ligands after uptake in the cells. Another
problem in evaluating the data is associated with the route of
administration. Hexavalent chromium, introduced by the oral route,
is partly reduced to trivalent chromium by acidic gastric juice
(Donaldson & Barreras, 1966; DeFlora & Boido, 1980); thus, the
effects or lack of effects observed may be caused mainly by
trivalent chromium and not by the hexavalent chromium, actually
administered.
7.2.1. Effects on experimental animals
Many local effects on human beings have been reported (section
8.3), but only a few studies have verified these effects in
experimental animals. A comprehensive survey of hexavalent
chromium-induced effects is given in Table 12 (US NAS, 1974a). For
most studies, details were not given of the length of exposure,
number of treated animals and controls, etc. Diagnoses were stated
without presenting all the original data. Thus, in this section,
some papers will be discussed that refer to the most prominent
local and systemic effects to support and clarify the effects shown
in human beings.
It is evident that the toxicity of hexavalent chromium in
animals varies with the route of entry into the body. Low
concentrations of hexavalent chromium may be tolerated, when
administered in the feed or drinking-water, the extent of
absorption being a factor of importance. For example, rats
tolerated hexavalent chromium in drinking-water at 25 mg/litre, for
1 year, and dogs did not show any effects from chromium
administered as potassium chromate at 0.45 - 11.2 mg/litre over a
4-year period (US NAS, 1974a). However, oral exposure of both male
and female rabbits to sodium dichromate (0.1% solution, 0.2 - 5
mg/kg body weight, for up to 545 days) resulted in significant
morphological changes in the gonads, including atrophy of the
epithelium and dystrophic alterations of the Sertoli and Leydig
cells in the testes, and sclerotic and atrophic changes in ovaries
(Kucher, 1966).
Larger doses of hexavalent chromium are highly toxic and may
cause death, especially when injected iv, subcutaneously (sc), or
intragastrically. The LD50 of chromium compounds was determined
for several experimental animal species. The LD50 of potassium
dichromate (hexavalent chromium), administered orally (stomach
tube) to rats, was 177 mg/kg body weight in males and 149 mg/kg
body weight in females (Hertel, 1982). When injected iv in mice
(sex not given), the LD50 of chromium carbonyl was 30 mg/kg body
weight (IARC, 1980).
Performing a life-time inhalation study on the rat, Glaser et
al. (1984) found an LC50 for Na2Cr2O7 of 28.1 mg/m3 (range, 16.7 -
47.3 mg/m3). Assuming a deposition rate in the lung of 30% of the
dose administered, the LC50 dose was 1 mg/kg body weight in male
and 1.2 mg/kg in female rats.
Table 12. Effects of hexavalent chromium in animalsa
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit, inhalation chromates 1 - 50 mg/m3 14 h/day for pathological Lukanin (1930)
cat 1 - 8 months changes
in the lungs
Rabbit inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day none Lehmann (1914)
as dichromate for 5 days
Cat inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day bronchitis, Lehmann (1914)
as dichromate for 5 days pneumonia
perforation of
nasal septum
Mouse inhalation mixed dust 1.5 mg/m3 4 h/day, no tumours Baetjer et al.
containing as CrO3 5 days/week, (1959a);
chromates for 1 year Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 16 - 27 mg/m3 1/2 h/day tumours in Baetjer et al.
containing as CrO3 intermit- some strains (1959a);
chromates tently Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 7 mg/m3 37 h over increased Baetjer et al.
containing as CrO3 10 days tumour rate (1959a);
chromates Steffee &
Baetjer (1965)
Rat inhalation mixed dust 7 mg/m3 37 h over barely Baetjer et al.
containing as CrO3 10 days toleratedb (1959a);
chromates Steffee &
Baetjer (1965)
Rabbit, inhalation mixed dust 5 mg/m3 4 h/day, none marked Baetjer et al.
guinea- containing as CrO3 5 days/week, (1959a);
pig chromates for 1 year Steffee &
Baetjer (1965)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rat, inhalation hexacarbonyl 1.6 mg/m3 4 months, anaemia; lipid Roschina (1976)
rabbit 4 h, 5 days and/or protein
a week dystrophia
in liver
and kidneys
Rat, inhalation hexacarbonyl 0.16 mg/m3 4 months, anaemia; no Roschina (1976)
rabbit 4 h, 5 days biochemical
a week or morphological
effects
Rat inhalation hexacarbonyl 35 mg/m3 30 min 100% death Roschina (1976)
Rat inhalation dichromates 0.006 - 0.2 28 days/ increase in Glaser et al.
mg/m3 90 days lung-macrophages (1985)
23 h/day
7 days/week lymphocytes
immunoglobulin,
reduced Fe2O3
lung clearance
Rat intratra- dichromates 5 per week up to 30 toleratedc Steinhoff
cheal in- 0.01 - 0.25 months et al. (1983)
stillation mg/kg
Rat intratra- dichromates 1 per week up to 30 tolerated; Steinhoff
cheal in- 0.05 - 1.25 months 1.25 mg/kg et al. (1983)
stillation mg/kg harmful
Rat oral potassium 500 mg/litre daily maximal Gross & Heller
chromate in non-toxic (1946)
drinking-water concentration
Rat, oral zinc chromate 10 g/kg daily maximal Gross & Heller
mouse in feed non-toxic (1946)
(mature) concentration
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit oral sodium di- 0.2 - 5.0 mg/kg 545 days morphological Kucher (1966)
chromate changes in
gonads (testes:
atrophy of
epithelium,
dystrophic
alterations of
Sertoli &
Leydiz alls.
Ovaries:
sclerotic and
atrophic
changes)
Rat oral zinc chromate 1.2 g/kg daily maximal Gross & Heller
(young) in feed non-toxic (1946)
concentration
Rat oral potassium 1.2 g/kg daily maximal Gross & Heller
(young) chromate non-toxic (1946)
in feed concentration
Dog, oral monochromate 1.9 - 5.5 mg 29 - 685 none harmful Lehmann (1914)
cat, or dichromates chromium/kg days
rabbit body weight per
day 1 mg chrom-
ium equivalent
to 2.83 mg
K2Cr2O7
or 3.8 mg
K2CrO4
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog oral potassium 1 - 2 g as daily fatal in 3 Brard (1935)
dichromate chromium months anaemia
Dog stomach potassium 1 - 10 g as - rapidly fatald Brard (1935)
tube dichromate chromium
Monkey subcutan- potassium 0.02 - 0.7 g in - fatald Hunter & Roberts
eous dichromate 2% solution (1933)
Dog subcutan- potassium 210 mg as - rapidly fatal Brard (1935)
eous dichromate chromium
Guinea- subcutan- potassium 10 mg - lethald Ophüls (1911a)
pig eous dichromate Ophüls (1911b)
Rabbit subcutan- potassium 1.5 cc of 1% - 80% fatald Hasegawa (1938)
eous dichromate solution/kg body
weight
Rabbit subcutan- potassium 20 mg - lethald Ohta (1940)
eous dichromate
Rabbit subcutan- potassium 0.5 - 1 cc of - nephritisd Ohta (1940)
eous dichromate 0.5% solution/kg
body weight
Rabbit, subcutan- sodium 0.1 - 0.3 g as - rapid deathd Priestley
guinea- eous or chromate CrO3 fall in blood (1877)
pig intravenous pressure
Rabbit intra- potassium 0.7 cc of 2% - lethald Mazgon (1932)
venous solution/kg 8-10 days after
body weight injection
Dog intra- potassium 10 grains - instant death Gmelin (1826)
venous chromate
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog intra- potassium 1 grain - none marked Gmelin (1826)
venous chromate
Dog intra- potassium 210 mg as - rapidly fatal Brard (1935)
venous dichromate chromium
Dog intra- potassium 3 mg/100 cc 2 doses marked renal Hepler & Simonds
venous dichromate blood per dose damage (1946); Simonds
& Hepler (1945)
---------------------------------------------------------------------------------------------------------
a Modifed from: US NAS (1974a).
b Pathological changes in experimental and control rats, 101 weeks after exposure.
c The same weekly dose distributed over 5 days was clearly better tolerated than a single weekly
administration.
d Renal damage.
A local corrosive action of hexavalent chromium on the skin,
similar to that seen in man, was described by Samitz & Epstein
(1962), who induced chrome ulcers in guinea-pigs at 4 trauma sites,
with daily exposure to 0.34 MK2Cr2O7 solution for 3 days. Mosinger
& Fiorentini (1954) showed the same effects using potassium
chromates.
Following parenteral administration, the most common systemic
effects of chromium were parenchymatous changes in the liver and
kidney (Mosinger & Fiorentini, 1954). Later studies showed
selective damage in the renal proximal convoluted tubules, without
evidence of glomerular damage, as demonstrated after one single sc
injection of potassium dichromate of 10 mg/kg body weight (Schubert
et al., 1970) or after one single intraperitoneal (ip) injection of
sodium chromate of 10 or 20 mg/kg body weight (Evan & Dail, 1974).
Effects have also been found in fish (Strik et al., 1975). After 32
days of continuous exposure to 0.1, 1, or 10 mg hexavalent chromium
(as potassium dichromate)/litre, the fish Rutilus rutilus developed
lysis of the intestinal epithelium with haemorrhages as well as
hypertrophy and hyperplasia of the gill epithelium.
Franchini et al. (1978) found an increase in urinary protein,
lysozyme, glucose, and beta-glucuronidase in rats after a single sc
injection of potassium dichromate at 15 mg/kg body weight. After
sc injection (3 mg/kg body weight), every other day for 2 - 8
weeks, the authors observed a correlation between the chromium
contents of the renal cortex and chromium clearance.
Five-week-old male Wistar rats of the strain TNO-W-74 were
continuously exposed in inhalation chambers to submicron aerosols
of sodium dichromate at concentrations ranging from 25 (low level)
to 200 µg chromium/m3 (high level) (Glaser et al., 1985). Exposure
for 28 days to 25 or 50 µg chromium/m3 resulted in "activated"
alveolar macrophages with stimulated phagocytic activity, and
significantly elevated antibody responses to injected sheep red
blood cells. After 90 days of low-level exposure, there was a more
pronounced effect on the activation of the alveolar macrophages,
with increased phagocytic activity. However, inhibited phagocytic
function of the alveolar macrophages was seen at the high
hexavalent chromium exposure level (200 µg/m3). In rats exposed to
this chromium aerosol concentration for 42 days, the lung clearance
of inert iron oxide was significantly reduced. The humoral immune
system was still stimulated at a low chromium aerosol concentration
of 100 µg/m3, but significantly depressed at 200 µg chromium/m3.
Exposure of rats, through inhalation, to chromium carbide or
chromium boride dust at very high levels (300 - 350 mg/m3 for each
substance) for 3 months (2 h/day) resulted in effects on the
vascular system of the lungs, e.g., endothelial hyperplasia.
Bronchitis and a decrease in the blood-haemoglobin concentration
were also observed (Roschina, 1964). The effects of chromium boride
were more pronounced than those of chromium carbide.
Steinhoff et al. (1983) performed an intracheal injection study
on rats (930 rats, 30 months, 0.05 - 1.25 mg Na2Cr2O7/kg body
weight per week; 1.25 mg CaCrO4/kg body weight per week). Half of
the rats were intratracheally injected once a week and the other
half received the same weekly dose distributed over 5 injections
per week. In rats receiving doses 5 times a week, there were some
significant changes in levels of total plasma-protein and
-cholesterol, in some haematological variables, in organ weights,
and in survival times in females. Only male rats receiving 1.25 mg
sodium dichromate/kg body weight, once a week, showed a sharp
reduction in body weight, female rats being less affected. Rats
receiving calcium chromate in the same dose showed reduced body
weight but to a lesser extent. The weight of lung and trachea was
increased by both substances in all doses.
Marked histopathological changes (congestion, fairly large
areas of focal necrosis, bile duct proliferation) described after
long-term exposure of rabbits to hexavalent chromium (ip injection
of 2 mg chromium/kg body weight per day for 6 weeks) (Tandon et
al., 1978), as well as the increase in hepatic metallothionein and
decrease in cytochrome P-450 levels after ip injection of 400 µmol
chromium/kg per day (type of chromium compound not mentioned)
(Eaton et al., 1980) need further confirmation. The finding of an
accumulation of hexavalent chromium in the reticuloendothelial
system including bone marrow (Baetjer et al., 1959b; Langard, 1977)
may be of importance for a disturbed blood picture.
Merkurieva et al. (1980a,b) studied the effects of potassium
dichromate in the drinking-water on the activities of different
enzymes in rats. Exposure included daily doses of 0.0005, 0.005,
0.05, or 0.5 mg/kg body weight for up to 6 months. After 20 days
of exposure at the highest dose level, enzyme activities increased
by 15 - 28% in liver microsomes (inosine-5-diphosphatase),
lysosomes (beta-D-galactosidase), and cytosol (lactate dehydrogenase
(EC 1.1.1.27)). For some of these enzymes, as well as for
acetylesterase (EC 3.1.1.6), increases in activity of up to 54%
were found in the gonads, kidneys, seminal fluid, and serum. At a
dose of 0.05 mg/kg, the only statistically significant finding was
a 54% increase in the activity of acetylesterase in the gonads.
After a 6-month exposure at this dose level, there was a 70%
increase in lactate dehydrogenase activity in the seminal fluid as
well as a 50% increase in free beta-galactosidase in the liver. There
were no changes in enzyme activities at the two lowest dose levels.
Painting of rat skin with an aqueous solution of potassium
dichromate (0.5%), daily, for 20 days, resulted in a local
inflammatory reaction, an increased level of hexose glyco-proteins
in the skin and serum, and an elevated concentration of serotonin
in the skin and liver (Merkurieva et al., 1982). Most of these
effects were also seen earlier in the exposure period, though they
were not as pronounced. Ten days after the start of exposure, a
nearly 3-fold increase in the serum-acetylcholine concentration
occurred together with decreased acetylcholinesterase activity.
Thus, the data of Merkurieva et al. show systemic effects following
both oral and dermal exposure to hexavalent chromium.
Cats fed chromic phosphate or oxydicarbonate at 50 - 1000 mg/day
for 80 days did not exhibit signs of illness or tissue damage.
Similarly, toxic reactions were not observed in rats administered
drinking-water containing 25 mg trivalent chromium/litre, for 1
year, or 5 mg trivalent chromium/litre throughout their lifetime
(US NAS, 1974a). The toxicity of trivalent chromium is so low that
even by parenteral administration, a chromic acetate level of 2.29
g/kg body weight or a chromic chloride level of 0.8 g/kg body
weight is required to kill mice. Even very large doses given
intragastrically were not fatal for dogs. Brard (1935) reported
that 10 or 15 g of chromium as chromic chloride proved fatal in one
dog (US NAS, 1974a). Some fatal doses of trivalent chromium
compounds reported in the literature are listed in Table 13.
Rats exposed through inhalation to chromic oxide (trivalent
chromium) at 42 mg/m3 or to chromic phosphate at 43 mg/m3 (5 h/day
for 5 days/week) for 4 months developed chronic irritation of the
bronchus and lung parenchyma, and dystrophic changes in the liver
and kidney (Blokin & Trop, 1977).
Inhalation exposure of rats (number not given) to dusts
containing 36 or 50% chromite for 4 months (2 h/day), at
concentrations of 375 - 400 mg/m3, resulted in thickening of the
walls of pulmonary vessels and bronchi (Roschina, 1959). The high
exposure levels in these studies make it difficult to evaluate the
effects.
Inhalation studies have also been performed with chromium
carbonyl, where chromium is in the 0 oxidation state (Roschina,
1976). Twelve rabbits and 48 rats were exposed for 4 months (4
h/day, 6 days/week) at a concentration of 1.6 or 0.16 mg/m3. At
both exposure levels, there was loss of body weight (25 and 12%,
respectively) as well as anaemia and leukocytosis. In the higher
exposure group, the animals showed an elevated gamma-globulin level
in serum and an increased transaminase activity. The contents of
cholesterol and SH-groups were reduced, and there was a decrease in
cholinesterase (EC 3.1.1.7) activity. Lipid and/or protein
dystrophy were noted in several organs, e.g., in the liver and
kidneys. No such effects were detected in the animals in the low-
exposure group.
Table 13. Fatal doses of trivalent chromium in animalsa
---------------------------------------------------------------------------------------------
Animal Number of Routeb Compound Chromium Effect Reference
animals dose (g/kg)
---------------------------------------------------------------------------------------------
Dog 2 sc chromic chloride 0.8 fatal Brard (1935)
Rabbit 1 sc chromic chloride 0.52 fatal Brard (1935)
Rat 38 iv chrome alum 0.01 - 0.018 LD50 Mertz et al.
chromium-hexaurea (1965a)
chloride
Mouse -c iv chromic chloride 0.8 MLDd Windholz et al. (1960)
Mouse -c iv chromic acetate 2.29 MLD Windholz et al. (1960)
Mouse -c iv chromic chloride 0.4 MLD Schroeder (1970)
Mouse -c iv trivalent chromium ? 0.25 - 2.3 MLD Windholz et al. (1960)
Mouse -c iv chromic sulfate 0.247 MLD Windholz et al. (1976)
Mouse -c iv chromic sulfate 0.085 MLD Schroeder (1970)
Mouse -c iv chromium carbonyl 0.03 LD50 Schroeder (1970)
---------------------------------------------------------------------------------------------
a Modified from: US NAS (1974a).
b sc = subcutaneous; iv = intravenous.
c No figures given.
d Minimum lethal dose.
7.2.1.1. Carcinogenicity
Various types of chromium chemicals, methods of administration,
and species of animals have been studied (IARC, 1980a).
Ideally, carcinogenicity should be tested with the methods
recommended by IARC (1980b), but, in many of the early studies,
this was not done. Carcinomas of the lung have been reported in
animals as a result of the administration of chromium chemicals.
Hueper (1958) found 2 squamous cell carcinomas and one
carcinosarcoma in 25 rats following intra-pleural injection of
chromite ore roast. After intrapleural implantation of strontium
chromate lasting 27 months, Hueper (1961) found tumours (type
unspecified) in 17/28 rats. Laskin et al. (1970) and Levy & Venitt
(1975) produced a number of bronchogenic carcinomas by implanting
pellets of cholesterol mixed with various chromium compounds
encased in a wire mesh cage in the bronchi of rats. Calcium and
zinc potassium chromate produced a number of bronchogenic
carcinomas, but soluble chromates and trivalent chromium chemicals
failed to produce cancer.
Using the same technique, Levy & Martin (1983) tested 21
different chromium-containing materials (pigments, intermediates,
and residues from the bichromate-producing industry, relatively
pure crystalline compounds) in 2250 random-bred rats and found that
chromates, described as sparingly soluble, were carcinogenic in the
rat lung. These materials included strontium and calcium chromate
and, to a far lesser extent, certain forms of zinc chromate.
Barium and lead chromate evoked only a very weak carcinogenic
response compared with strontium and calcium chromate. In the
study of Laskin et al. (1970), it was shown that chromium trioxide
produced hepatocellular carcinomas in 2/100 rats (controls, 0/24).
After inhalation of 13 mg calcium chromate/m3 (5 h/day, 5
days/week, for lifetime), Nettesheim et al. (1971) found 14 lung
adenomas in 136 treated mice and 5 in 136 untreated controls, but
no carcinomas. Steffee & Baetjer (1965), performing inhalation
studies on rats, mice, guinea-pigs, and rabbits (inhalation of
mixed chromate dust, corresponding to 3 - 4 mg CrO3/m3, 4 - 5
h/day, 4 days/week, for lifetime, or 50 months, respectively) could
only find 3 alveologenic adenomas in 50 treated guinea-pigs.
Laskin (1972) and Laskin et al. (1970) found 1 squamous cell
carcinoma of the lung, 1 of the larynx and 1 "peritruncal tumor" in
rats (inhalation of calcium chromate, 2 mg/m3, 589 exposures of 5 h
over 891 days) and 1 squamous cell carcinoma and 1 papilloma of the
larynx in hamsters. The number of treated animals was not
specified in either paper. Steinhoff et al. (1983) performed
intratracheal instillations of chromates in rats for 30 months with
one treatment/week and the same weekly dose distributed over 5
treatments/week (Table 14). In 880 exposed rats, 28 adenomas of
alveolar-bronchiolar origin (benign) and 12 malignant tumours (3
adenocarcinomas and 9 squamous cell carcinomas) were found. All
lung tumours developed very late and were only detected at the end
of the lifetime study, often in lungs with callosities. The
tumours were tiny and none of them caused the animal to die.
Sodium dichromate was not carcinogenic after exposure on 5
days/week. With calcium chromate, the carcinogenic effect was more
pronounced after treatment once per week, than after treatment 5
times per week.
Table 14. Incidence of benign and malignant lung tumours among
880 rats intracheally injected with Na dichromate and Ca chromatea
----------------------------------------------------------------
Dose Number of Incidence of lung tumours
(mg/kg) injections benign malignant
per week
----------------------------------------------------------------
Na2Cr2O7 x 2H20 0.25 5 none none
Na2Cr2O7 x 2H20 1.25 1 12 8
CaCrO4 0.25 5 5 1
CaCrO4 1.25 1 11 3
----------------------------------------------------------------
a From: Steinhoff et al. (1983).
Hueper (1961) reported unspecified tumours at implantation
sites in 12/34 rats, following intramuscular (im) implantation of
sintered calcium chromate (25 mg). No tumours were observed in 32
control animals. The same author found implantation-site tumours in
15/33 animals treated with strontium chromate compared with no
tumours in 32 control animals. Single sc injections of 30 mg lead
chromate (Maltoni 1974, 1976) resulted in injection-site sarcomas
in 26/40 treated rats. After administration of the same amount of
lead chromate oxide, injection-site sarcomas were found in 27/40
rats; no sarcomas were found in 60 vehicle controls. Following 9
im injections of 8 mg lead chromate, Furst et al. (1976) found
injection-site sarcomas in 31/47 treated rats, and renal carcinomas
in 3/24 treated rats; no sarcomas were found in 0/22 vehicle
controls. Heath et al. (1971) found injection-site sarcomas and
other tumours in 7/74 treated rats after im injection of 28 mg
cobalt-chromium alloy.
Administration of low levels of trivalent chromium acetate (5
mg/litre in the drinking-water) to mice and rats for the lifetime
did not result in increased tumour incidences compared with
controls (Schroeder et al., 1964, 1965).
Trivalent chromium oxide incorporated in bread at
concentrations of 10, 20, or 50 g/kg and fed to BD rats did not
increase tumour incidence compared with controls (Ivankovic &
Preussmann, 1975). Hexavalent chromium compounds have not been
tested by oral administration.
IARC (1980a) summarized the data of all available references
and concluded as follows: "There is sufficient evidence for the
carcinogenicity of calcium chromate and some relatively insoluble
chromium (VI) compounds (sintered calcium chromate, lead chromate,
strontium chromate, sintered chromium trioxide and zinc chromate)
in rats. There is limited evidence for the carcinogenicity of lead
chromate (VI) oxide and cobalt-chromium alloy in rats. The data
were inadequate for the evaluation of the carcinogenicity of other
chromium (VI) compounds and of chromium (III) compounds".
(a) Interaction with other factors
The possibility that chromium may act synergistically with
other agents in the production of cancer has been tested in several
studies. Nettesheim et al. (1970) pretreated one group of mice
with 100 R whole-body radiation and infected another group with PR8
influenza virus. These mice and control mice were exposed through
inhalation to chromium oxide dust for 6 - 18 months. No measurable
effects of either of the pretreatments were found on the incidence
of tumours. In a similar study with inhalation of calcium chromate
(CaCrO4, 5 h/day, 5 days per week for the lifetime), pre-exposure
to X-rays did not affect the tumour rate, but PR8 influenza
infection reduced tumour incidence (Nettesheim et al., 1971).
Steffee & Baetjer (1965) also found that the infection of rats with
PR8 influenza virus associated with intratracheal injection of
chromates did not lead to the production of tumours. Chromite ore
injected iv did not affect the development of tumours induced by iv
injection of 20-methyl-cholanthrene (Shimkin & Leiter, 1940).
Thus, at present, there are not sufficient data to suggest that
chromium acts synergistically with virus infections, ionizing
radiation, or other chemical carcinogens to produce cancers.
7.2.1.2. Genotoxicity
The mutagenicity of chromium compounds was reviewed by IARC
(1980a), Petrilli & DeFlora (1980), Levis & Bianchi (1982), and
Baker (1984). Present knowledge, in the light of recent findings,
is summarized in Table 15 (hexavalent chromium) and Table 16
(trivalent chromium).
When evaluating the results of chromium mutagenicity tests, it
is necessary to take into consideration several properties of the
tested compound (oxidation state, solubility, ability to penetrate
cell membranes, intracellular stability, and reactivity with
cellular components). Most of the in vitro mutagenicity
experiments have been performed with chromium chemicals, following
the introduction of the reverse mutation test by Ames (1973).
Bacterial strains of Salmonella typhimurium (Petrilli & DeFlora,
1977) containing mutants that require histidine for growth, and
Escherichia coli (Venitt & Levy, 1974) with mutants requiring
tryptophan for growth have been used. In each case, the bacterial
cultures have been incubated with the test chromium compounds and
the number of revertant colonies scored. The chromium compounds
studied included both hexavalent and trivalent compounds; a survey
of the results is given in Table 17 (DeFlora, 1981). Zhurkov
(1981) performed an Ames test to study the mutagenic activity of
potassium dichromate (K2Cr2O7) with S. typhimurium TA 1535 and TA
1538. A medium degree of mutagenic activity without metabolic
activation was shown by the strain TA 1535.
Only hexavalent chromium compounds have shown mutagenic
effects. Using the Salmonella /mammalian microsome tests of Ames
et al. (1973), DeFlora (1978) and Löfroth (1978) both showed a
decrease in the mutagenicity of hexavalent chromium in the presence
of an NADPH-generating system, suggesting reduction of mutagenic
hexavalent chromium to trivalent chromium. In this chromate
metabolism, cytochrome P-450 functions as the reductase (Garcia &
Wetterhahn Jennette, 1981). In recent in vitro studies, Wiegand et
al. (1984b) showed that glutathione could reduce hexavalent
chromium to trivalent chromium, without any further cofactors or
metabolizing enzymes. In rats, the most efficient tissue in
decreasing hexavalent chromium mutagenicity was the liver, followed
by the suprarenal glands, kidney, stomach, and lung (Petrilli &
DeFlora, 1980). These studies suggest a possible way of
intracellular detoxification of hexavalent chromium in low doses.
Schoental (1975) suggested that hexavalent chromium caused the
formation of epoxyaldehydes having mutagenic potential. Venitt &
Levy (1974) assumed that chromates belong to the transition-
inducing class of mutagens. They cause both frameshift and base-
pair substitutions (Petrilli & DeFlora, 1980). The hypothesis of
Kazantzis & Lilly (1979), that chromates cause guanine-cytosine to
adenine-thymine transitions in the subsequent round of DNA-
replication, needs further confirmation.
Table 15. Genotoxic activity of chromium (VI) compoundsa
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
DNA degradation CaCrO4 - Casto et al. (1976)
K2Cr2O7 - Bianchi et al. (1983)
PbCrO4 - Douglas et al. (1980)
K2CrO4 + Whiting et al. (1979)
Decreased fidelity PbCrO4, + (1) Levis & Majone (1981)
of DNA synthesis K2Cr2O7
CrO3 + Sirover & Loeb (1976)
K2Cr2O7 + Bianchi et al. (1983)
Microbial DNA K2CrO4, + Yagi & NishioKa (1977)
repair K2Cr2O7
CrO3, K2CrO4, + Kanematsu et al. (1980)
K2Cr2O7
K2CrO4, + Nishioka (1975)
K2Cr2O7
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
CrO3, + Gentile et al. (1981)
K2Cr2O7,
Na2Cr2O7, + Gentile et al. (1981)
(NH4)2Cr2O7
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Microbial gene K2Cr2O7 + (2) Bonatti et al. (1976)
mutation CrO3 + (2) Fukunaga et al. (1982)
Na2Cr2O7, +/-(3) Petrilli & De Flora
CrO3, +/-(3) (1978a,b)
ZnCrO4xZn(OH)2 +/-(3)
K2Cr2O7 + Zhurkov (1981)
Na2Cr2O7 +/-(3) DeFlora (1978)
chromate (4) +/-(3) Löfroth (1978)
dichromate +/-(3)
K2Cr2O7 - Kanematsu et al. (1980)
K2CrO4, + Löfroth & Ames (1978)
K2Cr2O7 +
K2Cr2O7 + Nishioka (1975)
K2CrO4, CaCrO4 + DeFlora (1981)
(NH4)2CrO4,
CrO3,
Na2CrO4, + Venitt & Levy (1974)
K2CrO4
CaCrO4
K2Cr2O7 + Bianchi et al. (1983)
Na2Cr2O7,
ZnCrO4
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
K2CrO4, CaCrO4 + Petrilli & DeFlora
CrO3 (1977)
Na2Cr2O7
K2CrO4 + Green et al. (1976)
PbCrO4, CrO3 + Nestmann et al. (1979)
Mammalian cell K2Cr2O7 + Bianchi et al. (1983)
gene mutation K2Cr2O7, + Newbold et al. (1979)
ZnCrO4 +
PbCrO4 -
K2Cr2O7 + (5) Bonatti et al. (1976)
K2Cr2O7 + Pashin et al. (1982)
NaeCr2O + Pashin & Kozachenko
(NHy)2Cr2O7 + (1981)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell K2CrO4, + Umeda & Nishimura (1979)
chromosomal K2Cr2O7 +
mutation K2Cr2O7 + Bigaliev et al. (1978)
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7 +
Na2CrO4, + Majone & Levis (1979)
K2CrO4 +
Na2Cr2O7, Levis & Majone (1979)
K2Cr2O7
CrO3, CaCrO4
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CrO3 + Kaneko (1976)
Na2Cr2O7 + Sarto et al. (1980)
K2Cr2O7 +
CrCl3 +
CrO3 + Tsuda & Kato (1977)
K2Cr2O7 + Raffetto (1977)
K2Cr2O7 + Stella et al. (1982)
Na2Cr2O7, + Majone & Levis (1979)
K2Cr2O7 +
K2CrO4 + Wild (1978)
Na2Cr2O7 + (7) Krishnaja & Rige (1982)
Mammalian cell CrO3, K2CrO4, + Ohno et al. (1982)
SCE K2Cr2O7 +
K2Cr2O7 + Bianchi et al. (1983)
K2CrO4,
Na2CrO4 + Levis & Majone (1979)
CrO3, +
K2Cr2O7, + Majone & Levis (1979)
Na2Cr2O7 +
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CaCrO4, CrO3, + Gomez-Arroyo et al.
K2Cr2O7 + (1981)
K2Cr2O7 + Majone & Rensi (1979)
CrO3 + (6) Stella et al. (1982)
K2Cr2O4, + Elias et al. (1983)
Na2CrO4, +
Na2Cr2O7 +
K2Cr2O7,
K2CrO4 + McRae et al. (1979)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell CaCrO4, K2CrO4 + (8) Casto et al. (1979)
transformation ZnCrO4 + (9)
Na2CrO4,
CaCrO4 + Casto et al. (1976)
+ Di Paolo & Casto (1979)
K2Cr2O7 + Bianchi et al. (1983)
K2Cr2O7 + Tsuda & Kato (1977)
CaCrO4 + Fradkin et al. (1975)
K2Cr2O7 + Raffetto (1977)
---------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) As industrial pigments (Cr-yellow-orange; Zn-yellow; Mo-orange).
(2) Gene conversion.
(3) No mutagenicity in the presence of mammalian microsomal activation
system (S-9 mix).
(4) No compounds given.
(5) Forward mutation was observed in 7/480 054 colonies (controls 0/84
546). A significant increase can be stated if comparison is made
with the historical spontaneous mutation rate, i.e., no mutants in
106 colonies.
(6) Occupatinal exposure.
(7) Induction of chromosomal aberrations in gill cells of the fish
Boleophthalmus dussumieri (Goby).
(8) 2 times control levels.
(9) 4 times control levels.
The results summarized in Table 17 show that the chromate ion
(hexavalent chromium) induces different kinds of genetic damage,
even at low doses, whereas the chromosome-damaging capacity of
trivalent chromium was only observed when it was tested in very
high doses.
In assays with whole-cell systems, trivalent chromium is
inactive, unless there is a direct interaction with DNA, e.g., in
studies in which purified DNA was exposed to trivalent chromium,
where modifications of the physical and chemical properties, as
well as decreased fidelity of DNA synthesis, were produced (Levis &
Bianchi, 1982). According to Warren et al. (1981), it appeared
that trivalent chromium in a proper ligand environment could have
considerable genetic toxicity. However, in recent studies, Bianchi
et al. (1984) found that trivalent chromium (CrCl3, 10-3 to 10-5 M)
was neither cytotoxic nor mutagenic in permeabilized hamster
fibroblasts (BHK were incubated for 30 min in Ea or BSS made
hypertonic with 4.2% NaCl).
Table 16. Genotoxic activity of chromium (III) compoundsa
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
DNA degradation CrCl3 + Bianchi et al.
(1983)
Decreased fidelity Cr2O3 + Levis & Majone
of DNA synthesis (1981)
Microbial DNA cis-[Cr(bipy)2ox] + Warren et al. (1981)
repair I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 + Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O + Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O + Warren et al. (1981)
trans-[Cr(en)2 + Warren et al. (1981)
(SCN)2]SCN
CrCl3, Cr2O3, - Yagi & Nishioka
Cr(OH)3 (1977)
K2Cr2(SO4)4 - Yagi & Nishioka
(1977)
K2Cr2(SO4)4 - Kanematsu et al.
(1980)
Cr2(SO4)3 - Kanematsu et al.
(1980)
CrCl3 - Nishioka (1975)
Cr(NO3)3, + De Flora (1981)
Cr(CH3COO)3
CrCl3 - Nakamuro et al.
(1978)
CrCl3 + Gentile et al.
(1981)
CrCl3, KCr(SO4)2, - Gentile et al.
(1981)
Cr2(SO4)3 - Gentile et al.
(1981)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Microbial gene CrCl3, Cr(NO3)3 - Löfroth & Ames
mutation (1978)
Cr(CIO4)3 - Löfroth & Ames
(1978)
CrCl3, Cr(NO3)3 - Venitt & Levy (1974)
Cr2SO4, - Venitt & Levy (1974)
K2SO4 x 2H2O
Cr(CH3COO)3 + Nakamuro et al.
(1978)
Cr(NO3)3 + Nakamuro et al.
(1978)
CrCl3 - Nakamuro et al.
(1978)
CrK(SO4)2 x 12H2O - Petrilli & De Flora
(1977)
CrCl3 x 6H2O - Petrilli & De Flora
(1978a)
Cr(NO3)3 x 9H2O - Petrilli & De Flora
(1978b)
Microbial gene cis-[Cr(bipy)2ox] + Warren et al. (1981)
mutation I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 - Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O - Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O - Warren et al. (1981)
trans-[Cr(en)2 - Warren et al. (1981)
(SCN)2]SCN
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
gene mutation (1983)
Cr(CH3COO)3 - Newbold et al.
(1979)
Mammalian cell Cr2(SO4)3 - Umeda & Nishimura
chromosomal (1979)
mutation
Cr(CH3COO)3 + Nakamuro et al.
(1978)
CrCl3, Cr(NO3)3 - Nakamuro et al.
(1978)
Cr(NO3)3, + Levis & Majone
(1979)
KCr(SO4)2, + Levis & Majone
(1979)
Cr(CH3COO)3 + Levis & Majone
(1979)
CrCl3 + Kaneko (1976)
CrCl3 - Sarto et al. (1980)
Cr2(SO4)3, CrCl3 - Tsuda & Kato (1977)
CrCl3 + Raffetto (1977)
CrCl3 - Stella et al. (1982)
CrCl3 - (1) Bianchi et al.
(1984)
Mammalian cell CrCl3, Cr2O3 + Elias et al. (1983)
SCE
Cr2O3 + Levis & Majone
(1981)
Cr(NO3)3, - Levis & Majone
KCr(SO4)2 (1979)
CrCl3, Cr(CH3COO)3 - Levis & Majone
(1979)
CrCl3 + (2) Ohno et al. (1982)
Cr2(SO4)3 - Ohno et al. (1982)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
SCE (contd). (1983)
CrCl3 - Majone & Rensi
(1979)
Mammalian cell CrCl3 - Bianchi et al.
transformation (1983)
---------------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) Thymidine uptake, DNA replication, damage and repair and SCE were
tested in permeabilized hamster fibroblasts.
(2) Weakly positive.
Levis & Buttignol (1977) and Levis et al. (1978) raised the
question of whether trivalent chromium could be an active mutagenic
agent within a cell. Others, such as Bianchi et al. (1980),
supported this theory, suggesting that trivalent chromium is bound
to genetic material after reduction of the hexavalent form by an
NADPH-dependent microsomal enzyme system (Norseth, 1978; Jennette,
1979). The studies of Sirover & Loeb (1976), showing a 30% error
in DNA synthesis after application of 0.64 mM trivalent chromium
compared with 16 mM of hexavalent chromium, support this theory.
Pashin et al. (1982) demonstrated the induction of dominant
lethal mutations in mice. They treated male mice with a single
injection of potassium dichromate (0.5 - 20 mg/kg body weight, ip)
or with daily injections (1 and 2 mg/kg, ip, for 21 days). The
single injections (0.5 - 2 mg/kg) did not induce any effects;
repeated or higher dosages of potassium dichromate induced a
statistically significant decrease in the survival of embryos from
cells treated at the early spermatid and late spermatocyte stages.
Karyological alterations in mammalian cells were induced after
exposure to concentrations of hexavalent chromium that were more
than 100 times lower than the concentration of trivalent chromium
causing the same changes (Majone & Rensi, 1979).
Analysing the effects of fume particles from stainless steel
welding on sister chromatid exchanges and chromosome aberrations in
cultured Chinese hamster cells, Koshi (1979) found an increase in
cytogenic effects with increasing fume doses, due to the dissolved
hexavalent chromium. Hexavalent chromium and stainless steel
welding fume particles containing chromium compounds produced
positive results in the "mammalian spot test", a somatic tissue
assay (Knudsen, 1980).
Table 17. Mutagenicity of chromium compoundsa
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
L. Hexavalent chromium compounds
1) Sodium dichromate (Merck) - +w +w +w + 50 - 250 4.4 decrease toxic and mutagenic
Na2Cr2O7 x 2H2O Cr6+ was converted
into inactive Cr3+
2) Potassium chromate (BDH) - +w +w +w + 80 - 410 2.9 decrease by reducing
K2CrO4 chemicals (ascorbic
acid, sodium
3) Calcium chromate (BDH) - +w +w +w + 60 - 290 3.2 decrease sulfite) or
CaCrO4 metabolites (NADH,
NADPH, GSH), by
4) Ammonium chromate - +w +w +w + 50 - 320 3.7 decrease human gastric juice
(Carlo Erba) and erythrocyte
(NH4)2CrO4 lysates, by S9 mix
containing human
liver S9 fractions
5) Chromium trioxide or - +w +w +w + 40 - 220 5.1 decrease or rat tissue S9
chromic acid (Merck) fractions (in order
CrO3 of efficiency:
liver>suprarenal
glands>kidney>
stomach>lung);
pretreatment of
rats (Aroclor(R)
1254), ether,
ethanol) influenced
the efficiency of
metabolic systems;
conversely, Cr6+
mutagenicity was not
affected by human
serum or plasma nor
by S9 mix containing
other rat (spleen,
colon, bladder,
striated muscle) S9
fractions
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
6) Zinc yellow (Montedison) - +w +w +w + 90 - 590 1.4 decrease the mutagenic effects
ZnCrO4 x Zn(OH)2 + of Cr6+ compounds and
10% CrO3 benzo( a)pyrene were
less than additive
7) Chromyl chloride (BDH) - +w +w +w + 100 - 430 1.8 decrease liquid volatile
Cl2CrO2 compound; vapours
were also toxic and
mutagenic
8) Chromium orange or basic - - - - +b 2 mg the three pigments
lead (spot test) containing PbCrO4
PbCrO4 x PbO were scarcely (L8),
chromate (Montedison) very scarcely (L9),
or totally (L10)
insoluble in water;
they were assayed
by directly spotting
these compounds at
the centre of plates
9) Molybdenum orange or - - - - +b 2 mg
lead solfomolybdo- (spot test)
chromat (Montedison)
PbCrO4 72-77%
PbSO4 4-6%
Al2O3 2%
PbMoO4 12-14%
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
10) Chromium yellow or - - - - -b
lead solfochromate
(Montedison)
PbCrO4 41-85%
PbSO4 4-45%
SiO2 0.1-3%
Al2O3 2-6%
11) Chromium - - - - - -2.3x103 - - hexacoordinated
hexacarbonyl (BDH) compound; formally,
Cr(CO)6 its oxidation state
is 0; insoluble in
water, dissolved in
ether
M. Trivalent chromium compounds
1) Chromic chloride - - - - - -3x104 - - inactive Cr3+
(BDH) CrCl3 x 6H2O compounds could be
converted into toxic
and mutagenic Cr6+
only in the presence
of oxidizing chemicals
(potassium
permanganate), while a
variety of metabolic
systems were
ineffective
2) Chromic nitrate (Riedel- - - - - - -2x104 - -
de Haen)
Cr(NO3)3 x 9H2O
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
3) Chromic potassium - - - - - -1.6x104 -
sulfate (BDH)
CrK(SO4)2 x 12H2O
4) Chromic acetate (BDH) - - - - - -7x104 - -
Cr(CH3COO)3
5) Neochromium (Montedison) - - - - - -5x104 - -
Cr(OH)SO4 56-58%
Na2SO4 23-24%
H2O 18-21%
6) Chromium alum (Montedison) - - - - - -4.3x104 - -
Cr2(SO4)3 37-39%
K2SO4 16-18%
H2O 43-37%
7) Chromite (Montedison) - - - - + 2 mg - Contaminated with Cr6+
Cr2O3 44-46% (spot test) traces ( sim-diphenyl-
Fe2O3 29-30% carbazide reagent);
Al2O3 15-16% scarcely soluble in
SiO3 0.5-3% water; assayed by
CoO 0.5-2% directly spotting the
powder at the centre
of plates
--------------------------------------------------------------------------------------------------------
Table 17 (contd.)
Note:
Sensitivity of Salmonella strains: Range of activity:
In the assays carried out in this study, This column indicates the lower and
the numbers of spontaneous revertants of the upper limits (in nmol per plate)
Salmonella tester strains fell within the of the mutagenic response, as in-
following ranges: ferred from dose-response curves
TA 1535: 10-25, both with and without S9 mix; with the most sensitive bacterial
TA 1537: 3-18, both with and without S9 mix; strain and under the most favourable
TA 1538: 10-25 without S9 mix; 15-35 with S9 mix; metabolic situation (i.e., with or
TA 98: 20-35 without S9 mix; 25-40 with S9 mix; without S9 mix) for each positive
TA 100: 150-200 without S9 mix; 140-180 with S9 mix compound; for negative compounds,
the maximum dose tested is indicated
The key for the interpretation of symbols in the
column "Reverted strains" is the following: Mutagenic potency:
+ clearly positive result indicated by a dose-
related and reproducible increase of his+ the values presented have
revertants over controls (at least a 3-fold been calculated by dividing the number
increase); of revertants in excess of controls
+w weak positivity indicated by an increase of by the corresponding amount of compounds
revertants 2 - 3 times in controls; (in nmoles); the number of net revertants
± reproducible but less than 2-fold increase was determinated at the top level of the
of revertants; linear part of dose-response curves, which
No symbol: no conclusive experiment carried out were drawn as indicated under the previous
with the corresponding strain. sub-heading.
Effects of S9 mix
The effect of S9 mix containing
liver S9 fractions from
Arochlor(R) pretreated rats on
the mutagenicity of test
compounds is reported
--------------------------------------------------------------------------------------------------------
a From: DeFlora (1981).
b L8, L9, and L10, as well as PbCrO4, were positive in the plate test when
dissolved in 0.5 N NAOH.
Embryonic fibroblast cell cultures showed significant
chromosome aberrations, when potassium dichromate was added to the
medium (Tsuda & Kato, 1976). When the potassium dichromate was
reduced with sodium disulfite (Na2SO3), no significant aberrations
occurred, even with 100 times the chromium concentrations.
At present, it is widely accepted that hexavalent chromium is
genetically active, because of its ability to cross the membranes
and enter the cells. If reduction of hexavalent chromium takes
place outside the cell (or even outside the cell nucleus, e.g., in
mitochondria or microsomes) its genetic activity is suppressed; if
the reduction takes place inside the nucleus (near, or at, the
target DNA molecules) alterations in DNA can occur, depending on
the oxidation power of hexavalent chromium or the formation of
trivalent chromium complexes with nucleophilic sites of the DNA;
thus, trivalent chromium could be the ultimate mutagenic form of
chromium (Levis & Bianchi, 1982). More recent investigations
(Wiegand et al., 1984) have shown that hexavalent chromium may
enter the body unreduced via the lung and be partly deposited in
erythrocytes over a prolonged period of time. From a DNA-repair
test in bacteria, DeFlora et al. (1984) concluded that hexavalent
chromium causes irritation in nucleotide pools and, due to its
oxidizing power, induces single-strand breaks in DNA, while
trivalent chromium induces DNA-protein and DNA-DNA cross-links and
decreases the fidelity of DNA replication.
7.2.1.3. Developmental toxicity and other reproductive effects
The teratogenicity of hexavalent chromium as chromium trioxide
was tested in golden hamsters (Gale, 1974, 1978, 1982; Gale &
Bunch, 1979) and that of trivalent chromium as chromic-chloride in
ICR mice (Iijima et al., 1975; Matsumoto et al., 1976). Injections
of hexavalent chromium into the fertilized chick egg increased
embryolethality and produced malformations in the survivors (Gilani
& Marano, 1979).
The golden hamsters were treated with a single iv injection of
chromium trioxide (5, 7.5, 10, or 15 mg/kg body weight) on the 8th
day of gestation (Gale, 1978).
Dose-response relationships were demonstrated between the rate
of absorption and the rate of malformations including delayed
ossification of the skeletal system. At 5 mg/kg body weight, 4% of
the fetuses showed external abnormalities (oedema, exencephaly),
and 34% showed cleft palate (controls: 2%), which increased to 85%
with the higher doses of 7.5 or 10 mg/kg body weight. Thirty-one
percent of fetuses were retarded following treatment with 7.5 mg/kg
body weight and 49% following 10 mg/kg body weight. The dose of 15
mg/kg body weight was lethal for 75% of the dams. Gale & Bunch
(1979) injected a single dose of 8 mg CrO3/kg body weight on day 7,
8, 9, 10, or 11 of gestation in female golden hamsters (Table 18).
The treated females were adversely affected (body weight loss,
tubular necrosis of the kidneys). In a later study, Gale (1982)
injected 8 mg CrO3/kg iv in hamsters on day 8 of gestation. An
increased incidence of cleft palate was seen in strains LVG, LSH,
and MHA, but no effects were reported in strains CB, LHC, and PD4.
ICR mice were injected sc with chromic chloride (Iijima et al.,
1975; Matsumoto et al., 1976). In the first series (animals
administered 9.8 or 19.5 mg/kg body weight, every other day, from
the 0th to 16th day of gestation), according to the authors, a
slight increase in the rate of malformations could not be excluded.
In the second series, with ip injections of 9.8 - 24.4 mg/kg body
weight, on the 8th day of gestation, the authors reported a dose-
dependent increase in the frequency of exencephaly, anencephaly,
and the occurrence of rib fusion.
Danielsson et al. (1982) studied the rate of embryonic or fetal
uptake of trivalent chromium and hexavalent chromium in early and
late gestational mice (Table 19) and found placental passage of
chromium. Hexavalent chromium appeared in the fetus in high enough
concentrations for a direct effect on embryonic structures to be
likely, trivalent chromium may act on placental structures.
Gilani & Marano (1979) injected chromium trioxide into 840
embryonating chicken eggs (air sack) at doses ranging from 0.002 to
0.05 mg per egg, on days 0, 1, 2, 3, and 4 of incubation (0.1 ml
physiol. saline injections per control egg). All embryos were
examined on day 8. The following malformations were observed in
the 720 eggs affected: short and twisted limbs, microphthalamia,
exencephaly, short and twisted neck, everted viscera, oedema, and
reduced body size. However, the incidence of abnormalities in the
120 controls was reported to be "low".
Inhalation studies were conducted by Glaser et al. (1984) on
Wistar rats (TNO-W-74 SPF) to study the effects of sodium
dichromate on reproduction and teratogenicity in 3 generations.
Whole-body exposures to sodium dichromate aerosols (0.2 mg
chromium/m3) were performed with an exposure time of 130 days per
generation. No effects on reproduction were found. All
teratogenicity tests were negative, and there was no increase in
fetal chromium content. However, from generation to generation,
there was an increase in immunosuppression and hyperplasia of
organs (especially in the lungs) and changes in haematological
variables.
Table 18. Embryotoxicity following maternal exposure to CrO3 on different days
of gestation in hamstersa
-------------------------------------------------------------------------------------
Findings in live fetuses
Day of No. of No. of No. of Frequency External Internal Cleft
gestation females implantation living resorptions defects defects palate
treated sites fetuses No. %b No. %b No. %b No. %b
-------------------------------------------------------------------------------------
Treated CrO3 8 mg/kg
7 6 64 10 54 84 1 10 1c 10 5 50
8 6 88 54 14 20 8d 15 4e 7 17 31
9 6 77 67 10 13 18f 27 0 0 25 37
10 6 77 77 0 0 0 0 0 0 0 0
11 6 68 67 1 1 0 0 0 0 0 0
Controls H2O 5 ml/kg
7 3 35 33 2 6 0 0 0 0 0 0
8 3 44 41 3 7 0 0 0 0 0 0
9 3 37 36 1 3 0 0 0 0 0 0
10 2 20 19 1 5 0 0 0 0 0 0
11 3 34 33 1 3 0 0 0 0 0 0
-------------------------------------------------------------------------------------
a From: Gale & Bunch (1979).
b Underlined %, significantly different statistically from controls.
b Right hindlimb with two digits.
c Right kidney absent.
d Oedema (7), tail short (1).
e Small kidneys (3), right kidney absent (1).
f Oedema (17), omphalocele (1).
Table 19. Comparison of concentrations of trivalent and hexavalent
chromium in fetuses, placentas, and maternal organsa,b
------------------------------------------------------------------
Day 13 Day 16
Hexavalent Trivalent Hexavalent Trivalent
chromium chromium chromium chromium
------------------------------------------------------------------
Fetus 0.3 ± 0.01 0.03 ± 0.002 0.5 ± 0.04 0.1 ± 0.01
Placenta - - 3.0 ± 0.2 2.4 ± 0.2
Liver 29.4 ± 2.2 30.8 ± 6.5 33.9 ± 3.9 27.7 ± 3.5
Kidney 38.1 ± 1.9 5.3 ± 0.7 36.0 ± 4.3 8.7 ± 2.3
Serum 2.6 ± 0.2 9.3 ± 0.9 2.8 ± 0.4 11.0 ± 1.6
------------------------------------------------------------------
a From: Danielsson et al. (1982).
b Trivalent chromium (51CrCl3) and hexavalent chromium
(Na251Cr2O7) were injected iv on day 13 and day 16 of gestation
at doses of 10 mg chromium/kg body weight. The pregnant animals
were autopsied after 1 h. Concentrations are expressed in
mg/litre or g ± SEM (N = 4).
7.2.1.4. Cytotoxicity and micromolecular syntheses
Wacker & Vallee (1959) reported that trivalent chromium was
present in RNA from all sources examined; they hypothesized that
chromium might contribute to the stabilization of the structure.
Administration of trivalent chromium to mice (ip injection of
CrCl3 at 5 or 0.5 mg chromium/kg body weight; P < 0.01) caused
accumulation of chromium in the cell nucleus, which amounted to
about 20% of the accumulated chromium content of the liver cell,
and also enhanced RNA synthesis. Hexavalent chromium inhibited RNA
synthesis (K2CrO4 at 5 or 0.5 mg chromium/kg body weight;
P < 0.01) (Okada et al., 1983). In further studies, Okada et al.
(1984) pretreated rats with CrCl3 (ip injection of 5 mg chromium/kg
body weight) and then partially hepatectomized the rats. Chromium
accumulated in the regenerating liver, and hepatic RNA synthesis
was accelerated compared with that of partially hepatectomiced
controls. These findings suggest a direct participation of
chromium in RNA synthesis.
Potassium dichromate enhanced the passive uptake of ribo-and
desoxyribonucleosides (Levis et al., 1978; Bianchi et al., 1979),
caused an inhibition of DNA replication to BHK fibroblasts, and
reduced the colony-forming ability of BHK cells (doses: 10-4 - 20-7
mol). Hexavalent chromium (potassium dichromate with a
concentration of 5 x 10-7 mol/litre) reduced by half the
proliferation of NHIK 3025 cells originating from a human cervix
carcinoma (White et al., 1979).
The addition of calcium chromate to BHK21 cell cultures altered
their growth characteristics. Whereas these cells normally grow as
elongated fibroblast cells in parallel orientation, the chromium-
exposed cells grew as shortened fibroblasts with enlarged nuclei
and granular cytoplasm, randomly orientated. When grown in
carboxymethyl cellulose, the chromate-exposed cells underwent many
divisions in contrast to the 1 or 2 divisions of normal cells.
These alterations in growth properties persisted, even after
transfer to a normal medium (Fradkin et al., 1975).
Cultures of HeLa and rat embryonic cells were incubated with
various hexavalent and trivalent chromium compounds for 3 days and
the 50% inhibitory dose (ID50) for cell growth was determined. The
ID50 for sodium and potassium chromate and dichromate and calcium
chromate varied from 0.34 to 0.78 µg/ml of the medium. The ID50
for the trivalent compounds varied over a wider range: chromic
oxalate, 4; acetate, 52; nitrate, 720; chloride, 1030; and bromide,
1500 mg/litre. Similar results were found in rat embryonic cell
cultures (Susa et al., 1977).
7.2.1.5. Fibrogenicity
Because of the well-known fibrogenic effects of silica and
asbestos minerals, studies were initiated to determine whether
chromite and various other dusts exhibited similar properties. A
single intratracheal injection in rats of 40 mg chromite particles
(33% chromium, 0.7% SiO2, 99.9% of the particles being smaller than
5 µm) suspended in 1 ml Ringer's solution caused a moderate
cellular reaction that reached a peak after 2 months and then
decreased. After 8 months (end of observation), the lung weight
was within normal limits. Only an increase in fibrils that was not
significant was observed. The tissue reaction in regional lymph
nodes was minimal (Swensson, 1977).
Chromite FeO(CrAl)2O3 (10 mg), ground to an average particle
diameter of 1 µm, was injected into the pleural cavities of 25
mice. The animals were killed at intervals ranging from 2 weeks to
18 months after injection. Chromite produced the least reaction of
all the 15 dusts tested. This material attracted very few cells
and the eventual production of collagen was minimal (Davies, 1972).
7.2.2. Observations in farm animals
Systemic poisoning of farm animals with chromates is rare and
is mainly suspected to be a consequence of accidental industrial
contamination of drinking-water. The acute lethal dose for mature
cattle is approximately 600 - 800 mg/kg body weight; chronic
poisoning in young calves was produced by a daily dose of 30 - 40
mg/kg body weight for one month (NZ Department of Agriculture,
1954) with a clinical picture of profuse scouring, severe
dehydration, low blood pressure, changes in the alimentary tract,
including congestion and inflammation, and the rumen and abomasum
showing ulceration near perforation.
High chromium levels were found in the blood and liver of
calves that had died from poisoning. The minimum tissue levels
causing poisoning were 30 mg/kg in the liver and 4 mg/litre in
whole blood (Harrison & Staples, 1955). According to Romoser et al.
(1961), a level of 100 mg hexavalent chromium (as sodium
chromate)/kg did not affect growing chicks.
8. EFFECTS ON MAN
8.1. Nutritional Role of Chromium
The physiological role of chromium and the need for small
amounts of dietary chromium are indicated by:
(a) chromium-responsive cases of impaired glucose
metabolism in malnourished children and middle-aged
persons;
(b) 2 cases of proved chromium deficiency in patients fed
exclusively by parenteral alimentation; and
(c) epidemiological studies suggesting a link between
chromium deficiency and risk factors for cardiovascular
diseases.
8.1.1. Biological measurements and their interpretation
Biochemical methods for the diagnosis of the human nutritional
chromium status are still at the developmental stage. Blood- or
urine-chromium concentrations are not indicative of chromium
status. Several studies suggest the validity of the "relative
chromium response" in plasma or serum to the ingestion of 50 - 100
g of glucose within 30 - 120 min; the response also appears in the
urine. Lack of this response suggests chromium deficiency,
especially when combined with impaired glucose tolerance in the
presence of normal or elevated plasma-insulin levels.
8.1.2. Chromium deficiency
8.1.2.1. Adults
Chromium deficiency is diagnosed mainly through therapeutic
trials in which an impaired function is restored by supplementation
of the diet with small amounts of chromium close to the estimated
human requirement. Glinsmann & Mertz (1966) studied 4 male
diabetic patients for several months to 1 year, under the strictly
controlled conditions of a metabolic ward. The subjects, who were
fed identical portions of a diet prepared before the start of the
study, were allowed a measured amount of physical activity and were
stabilized under these conditions before the beginning of the
control periods. Three of the 4 patients responded to a chromium
level of 180 - 1000 µg/day as CrCl3 x 6H2O with a significant
improvement in oral glucose tolerance; the fourth showed a very
slight lowering of the glycaemic curve, which was not statistically
significant. A representative example is given in Table 20.
Table 20. Effect of chromium on mean glucose tolerancea,b
------------------------------------------------------------------------
Supplementation Days Number Mean blood glucose concentration
of ---------------------------------
tests Fast- Min after glucose load
ing 30 60 90 120
------------------------------------------------------------------------
None 0 - 32 10 840 2170 2290 1950 1560
60 µg, 3 x per day 33 - 74 12 850 2240 2380 2090 1620
60 µg, 3 x per day 75 - 119 13 840 2100 2130 1860 1410
60 µg, 3 x per day 120 - 140 5 840 2030 2010c 1750 1120c
None 180 - 194 1 960 2070 2900 2930 2370
60 µg, 3 x per day 195 - 211 5 830 1960 1930c 1750 1180c
None 256 - 313 10 870 2200 2450 2240 1660
1 mg, 3 x per day 337 - 340 1 680 1480 1620 1510 960
------------------------------------------------------------------------
a Modified from: Glinsmann & Mertz (1966).
b A 46-year-old man with maturity-onset diabetes, controlled on diet;
oral glucose tolerance test consisted of constant noon meal and 100 g
glucose.
c Mean values significantly different from control ( P < 0.025).
The condition of one of 2 diabetic out-patients, observed over
several months, was improved by chromium supplementation, the
condition of the other was not. Short-term treatment of 7 diabetic
patients for 1 - 7 days with 1 mg chromium did not affect any
variables of glucose metabolism, nor did chromium supplementation
change the normal glucose tolerance tests of 10 healthy, young
volunteers.
Glinsmann's positive results were not confirmed in a double-
blind crossover study on 4 normal volunteers and 10 diabetic
persons, with a crossover after 16 weeks. No effects of chromium
were detected on glucose tolerance or on fasting or postprandial
blood-glucose levels (Sherman et al., 1968). Schroeder et al.
(1970), while reporting that 2 - 10 mg chromium/day "almost always"
restored the impaired glucose tolerance in older, non-diabetic
subjects to normal, found only erratic and mild improvement in 4
out of 12 diabetic out-patients. On the basis of these
observations, Mertz (1969) concluded that chromium should not be
considered a therapeutic agent for diabetic patients.
On the other hand, it was reported in a later study on chromium
metabolism that chromium administration (500 µg/day, for 2 weeks)
produced a marked improvement in glucose utilization in both
juvenile and maturity-onset diabetic patients. Five of the
patients were followed for more than 1 year after exposure and were
found to maintain their improved glucose metabolism (Nath, 1976).
Of 10 non-diabetic elderly subjects with impaired glucose
tolerance, 4 responded to 150 µg chromium/day with complete
normalization of oral glucose tolerance tests. The remaining 6
subjects were not affected by the supplements (Levine et al.,
1968). Significant effects of a GTF-chromium-containing yeast
preparation were reported by Doisy et al. (1976) in 6 out of 12
elderly subjects with impaired glucose tolerance
Table 21. Effect of GTF supplementation on glucose tolerance tests in
elderly subjects with impaired tolerancea,b
-------------------------------------------------------------------------
Mean plasma-glucose levels Cholesterol Triglyceride
(mg/litre) (mg/litre) (mg/litre)
0 h 1 h 2 h
-------------------------------------------------------------------------
Before GTF 1060 ± 40c 2010 ± 70 1780 ± 80 2450 ± 90 1210 ± 80
11 tests
After GTF 990 ± 40 1620 ± 110 1320 ± 50 2050 ± 100 1120 ± 120
9 tests
Signifi- NS < 0.01 < 0.001 < 0.01 NS
canced
-------------------------------------------------------------------------
Mean serum-insulin levels
(microunits/ml)
0 h 1 h 2 h
Before GTF 24 ± 6 78 ± 17 118 ± 17
after GTF 26 ± 12 70 ± 8 83 ± 8
-------------------------------------------------------------------------
a From: Doisy et al. (1976).
b Supplement: 4 g yeast per min/day (Yeastamin Powder 95, Staley Co.,
Chicago, Illinois, USA). GTT: 100 g oral load.
c Mean ± SEM.
d Using paired t test, difference is significant.
In addition to the improvement in glucose tolerance in the
presence of a reduced insulin response, plasma-cholesterol
concentrations were significantly reduced. Several other
individual cases are reported in the publication of Doisy et al.
(1976), all of whom showed a lower serum-insulin response to a
glucose tolerance test during chromium supplementation.
Liu & Morris (1978) performed a similar study on the effects of
a yeast-chromium supplementation in 27 women, 40 - 75 years old, of
whom 15 had a normal and 12 an abnormal glucose tolerance test. In
11 of the normal subjects (73%), the integrated insulin response
was significantly reduced from 436 to 335 µU/ml ( P < 0.025) during
supplementation, without a statistically significant change in
glucose tolerance test. Significant reductions in the total glucose
response (10 650 - 9820 mg/litre; P < 0.001) and in the total
insulin response to glucose were observed in the subjects with
impaired glucose tolerance (1288 - 831 µU/ml; P < 0.001). The
glucose tolerance improved in 7 out of 12 women, but all 12 showed
a reduction in plasma-insulin.
The effects of supplementation of the diet with 200 µg
chromium/day (as chloride) on the glucose tolerance of 76 normal
adult volunteers was investigated in a double-blind cross over
study, with each period lasting 3 months. All 18 subjects entering
the study with impaired glucose tolerance (serum-glucose equal to
or greater than 1 g/litre, 90 min after a glucose load), showed
significant improvement during chromium supplements, but not with a
placebo, with an average decline in the 90-min serum-glucose levels
of 180 mg/litre ( P < 0.01) (Anderson et al., 1983b).
In another placebo-controlled study, 24 elderly volunteers were
given a daily supplement of either chromium-rich brewer's yeast or
chromium-deficient Torula yeast (9 g/day, for 8 weeks). The
glucose tolerance of the subjects given brewer's yeast
significantly improved with a concomitant decrease in plasma-
insulin levels, and a significant decline in plasma-cholesterol as
well as total plasma-lipids. No significant changes were observed
in the control group given the chromium-poor Torula yeast
(Offenbacher & Pi-Sunyer, 1980).
Additional, preliminary studies suggest a specific effect of
brewer's yeast on individual lipoprotein cholesterol fractions.
Twenty normal human subjects responded to daily supplementation
with 18 g of brewer's yeast over a 8-week period with a significant
reduction in low-density lipoprotein-cholesterol ( P < 0.01) and
with a simultaneous increase of 14% in the high-density lipoprotein
( P < 0.01) (Nash et al., 1979).
Similar results were observed in a 6-week supplementation trial
involving 8 physician volunteers, each of whom received 7 g/day of
brewer's yeast. Low-density lipoprotein-cholesterol declined by
nearly 18% ( P < 0.01), whereas the high-density cholesterol
fraction increased by 17.6%, resulting in a significant
( P < 0.001) decrease in the LDL/HDL ratio of 28% (Riales, 1979).
These studies suggest that the primary effect of the
supplementation must have been an increase in the effectiveness of
the insulin, because glucose tolerance was maintained or improved
in spite of lower insulin responses. It is important to note that
while the reduction in insulin levels occurred in all cases, an
improvement in glucose tolerance occurred in only a fraction. This
suggests two possible interpretations of all the results discussed
here, including those showing improvement in glucose tolerance in
only some of the subjects or no improvement at all. Lack of a
response to chromium could suggest that the subjects were not
chromium deficient and that their impaired glucose metabolism was
the result of other causes. Second, they may have responded with a
reduction in circulating insulin levels, as did all the subjects
with impaired glucose tolerance in whom the insulin response was
measured. Unfortunately, these measurements were not performed in
the earlier studies.
8.1.2.2. Malnourished children
Chromium deficiency has been implicated as a factor
contributing to the impairment of glucose tolerance in children
with protein calorie malnutrition in 3 countries, Jordan, Nigeria,
and Turkey. Hopkins et al. (1968) measured intravenous glucose
tolerance in 10 malnourished infants from the mountainous area of
Jordan and 9 equally malnourished infants from the valley area.
They found a severely depressed glucose removal rate of 0.7%/min in
the first group and a normal rate in the second, 3.8%/min (Table
22). The protein intake of both groups prior to hospitalization
had been identical; it was supplied in the form of a milk powder
with a chromium content of 18 µg/litre, when reconstituted.
However, analysis of 10 water samples revealed 3 times as much
chromium in the samples from the valley as in those from the
mountainous area (0.5 versus 1.6 µg/litre). This suggested the
possibility of chromium being a major determinant in the glucose
metabolism of these children, and the effects of supplementation
were measured in 6 children from the hills, with a severely
impaired tolerance test. The morning after oral administration of
250 µg chromium, as CrCl3 x 6H2O, the glucose removal rate had
markedly improved to 2.9%/min from a pretreatment level of
0.6%/min. Similar results were obtained in Nigeria (Table 22);
these tests also included control children who did not receive
chromium.
The immediate effects of chromium on these children were
remarkable and different from the lag phase always present in the
studies on adults. Equally important, was the short duration of
the effect of one dose demonstrated in a Jordanian child observed
for a longer period of time. These observations suggest that a
marked depletion in chromium stores must have existed in the
children.
Table 22. Significance of the effect of chromium on the impaired glucose
tolerances of malnourished infantsa
---------------------------------------------------------------------------
Subjects Number Glucose Significance
of removal of
infants rate difference
(%/min)
---------------------------------------------------------------------------
Jordanian infants from hill area with
0.5 µg chromium/litre in drinking-water 10 0.7
Jordanian infants from valley with
1.6 µg chromium/litre in drinking-water 9 3.8 P < 0.001
Jordanian infants;
initial glucose tolerance test 6 0.6 P < 0.001
after chromium treatment 6 2.9
Nigerian infants;
initial glucose tolerance test 6 1.2
after chromium treatment 6 2.9 P < 0.05
Non-treated infants:
Initial glucose tolerance test 5 1.9 not signi-
Repeated glucose tolerance test 5 2.1 ficant
---------------------------------------------------------------------------
a From: Hopkins et al. (1968).
Similar results were reported from a study in the Istanbul area
of Turkey (Gürson & Saner, 1971). Fourteen malnourished children
with an impaired glucose removal rate of 1.71%/min were given 250
µg of chromium (CrCl3 x 6H2O), and a second test was performed
after 15 h. The removal rate increased to 3.91%/min ( P < 0.001).
This average increase was due to a pronounced increase in 9 of the
children; no change was observed in the remaining 5. No
improvement in glucose tolerance occurred in 5 control children
receiving identical hospital treatment, except for the
administration of the chromium. The children receiving the
chromium supplementation grew significantly more than the control
children during the following 30 days, with the greatest growth
rates found in the 9 children who had responded to administration
of chromium with an improvement in glucose tolerance.
The fact that chromium deficiency interacts with protein-
calorie malnutrition only in certain geographical areas was
confirmed by Carter et al. (1968), who gave chromium
supplementation (250 µg/day as CrCl3 x 6H2O) without effects on
glucose tolerance in 9 Egyptian children tested. Plasma-chromium
levels were in the normal range, and chromium levels in
representative foods were high, ranging from 200 to 390 µg/kg.
These findings suggest that the chromium status of these subjects
was normal, which would explain the lack of an effect.
8.1.2.3. Patients on total parenteral alimentation
A female patient, maintained on total parenteral alimentation
since the age of 35 years, developed an unexpected weight loss of
15% after 5 years combined with peripheral neuropathy, impaired
intravenous glucose tolerance, in spite of normal insulin response,
and a decreased respiratory quotient of 0.66. Insulin treatment
(45 U/day) was ineffective. A strongly negative chromium balance
and low plasma- and hair-chromium levels suggested that the daily
estimated chromium intake from the infusion fluids of 5.3 µg had
not met the patient's requirement and resulted in chromium
deficiency. The insulin infusions were stopped and replaced by the
daily infusion of 250 µg chromium (as CrCl3 x 6H2O) for 2 weeks.
The result was normalization of glucose tolerance and respiratory
quotient, disappearance of signs and symptoms of neuropathy, return
to the previous normal body weight, in spite of reduced caloric
intake, disappearance of the requirement for exogenous insulin, and
a positive chromium balance. The nitrogen balance, which had been
erratic and often negative, became consistently positive following
chromium supplementation. Reduction of the daily supplement to 20
µg chromium proved to be sufficient to maintain the patient in a
state of well-being (Jeejeebhoy et al., 1977).
This case is the best demonstrated example of chromium
deficiency in a human subject. In addition to confirming the well-
known signs of chromium deficiency reported earlier, such as
insulin resistance and its consequences, it was the first case to
demonstrate the occurrence of peripheral neuropathy and disturbance
of nitrogen balance as a result of deficiency. These disturbances
require further study. A second case of chromium deficiency as a
consequence of long-term total parenteral alimentation was reported
by Freund et al. (1979). Signs and symptoms were similar to those
of the first case, except that central encephalopathy was present
instead of peripheral neural disorder. Supplementation with 150 µg
of chromium/day resulted in normalization of the deficiency signs.
Chromium at that time was not routinely added to intravenous
solutions, therefore it is possible that more cases of deficiency
may exist in patients on long-term infusion treatment. As an
outcome of this study, an Expert Panel of the American Medical
Association (AMA, 1979) suggested that the stable adult patient on
total parenteral alimentation should receive 10 - 15 µg chromium
daily.
8.1.2.4. Epidemiological studies
Two epidemiological studies suggest a link between chromium
status and cardiovascular diseases (Schroeder et al., 1970; Punsar
et al., 1975). Lack of chromium is associated with impaired
glucose tolerance, elevation of circulating cholesterol, and aortic
plaques. All of these variables are recognized risk factors for
cardiovascular disease. It has also been suggested that the
elevated insulin levels, often present in chromium-deficient human
subjects (Liu & Morris, 1978) represent a substantial risk for the
development of cardiovascular disease (Strout, 1977).
Schroeder et al. (1970) have summarized their studies of
chromium concentrations in normal and diseased tissues,
particularly in aortas (Table 23). The total body burden of
chromium, excluding the lungs, was lower in the USA, where there is
a higher incidence of cardiovascular disease, compared with other
regions, 1.72 - 1.86 mg/kg ash weight in the USA compared with 7.7,
9.6, and 2.7 mg in Africa, the Far East, and the Near East,
respectively. The only significant difference in tissue-chromium
concentrations in deaths from heart disease compared with deaths
from accidental causes was in the aorta (Table 23). Concentrations
in aortas from Africa, the Far and Near East were generally higher
and did not show such a difference.
Table 23. Chromium in aortasa
----------------------------------------------------------
Location Chromium (mg/kg dry weight)
Accident P Heart disease
----------------------------------------------------------
Africa 0.072 (5)b NS 0.116 (2)
Far East 0.97 (8) NS 0.246 (5)
Middle East 0.36 (8) NS 0.216 (3)
USA (Nine cities) 0.26 (103) 0.005 0.052 (13)
USA (San Francisco) 0.228 (10) 0.005 0.048 (15)
----------------------------------------------------------
a From: Schroeder et al. (1970).
b The number in parenthesis denotes the number of cases
observed.
The second epidemiological study was part of a cohort study
conducted in Finland beginning in 1959 (Punsar et al., 1975). A
total of 327 drinking-water samples were analysed for 22
characteristics including pH, anions, bulk minerals, and several
trace elements including chromium. The study included 504 and 622
men in the Western and Eastern areas of Finland, respectively,
whose domicile had not changed since 1959.
The coronary heart disease mortality rate is much higher in the
east of Finland than in the west. As seen in Table 24, the
chromium levels in drinking-water were much lower in the east than
in the west, whereas the opposite was true for copper. The metal
concentrations in the drinking-water of those with heart disease
and others only differed slightly (Table 24), but, according to the
authors, the data suggest that low chromium levels may play a role
in the high heart disease death rate of eastern Finland.
Table 24. Concentrations of copper and chromium in the
drinking-water of men who died between 1969 and 1973, and
of those who were alive in 1973a
-------------------------------------------------------------
Alive
Deaths (Clinical status in 1969)
CHDb Other CHDb Other No
heart heart
disease disease
-------------------------------------------------------------
West N = 19 N = 23 N = 63 N = 31 N = 288
Copper X 22.15c 20.17 20.05 20.94 20.15
(µg/litre) SD 3.92 4.69 4.69 5.12 4.74
Chromium X 7.52 9.38 9.45 9.74 8.45
(µg/litre) SD 2.06 3.42 4.01 4.44 3.31
East N = 23 N = 26 N = 129 N = 44 N = 279
Copper X 41.59 41.24 42.67 39.50 36.32
(µg/litre) SD 29.91 30.43 33.65 28.74 28.66
Chromium X 2.20 2.09 2.04d 2.49 3.01
(µg/litre) SD 2.17 2.41 2.08 2.17 2.37
-------------------------------------------------------------
a From: Punsar et al. (1975).
b CHD = coronary heart disease.
c P < 0.05.
d P < 0.001.
Note: Levels of significance refer to differences observed
when the concentrations were compared with those in the
"no heart disease" category.
Newman et al. (1978) measured serum-chromium concentrations in
32 patients, 18 male and 14 female, aged 25 - 65 years, in whom
coronary artery cine-arteriography was performed for medical
reasons. Serum-chromium was significantly lower in the 15 subjects
with coronary artery disease than in the 17 subjects without
involvement (2.51 versus 6.09 µg/litre; P < 0.01), whereas serum-
cholesterol, weight index, and systolic or diastolic blood pressure
did not differ among the groups. Serum triglycerides were higher
in the group with coronary artery disease (1.84 versus 1.12
g/litre; P < 0.05). No subject with a serum-chromium
concentration of more than 6 µg/litre exhibited coronary artery
involve- ment, and by regression analysis, serum-chromium was
judged to explain 17% of the total variance of the disease.
These results were independently confirmed in a second study in
which plasma chromium concentrations of 23 healthy persons were
more than 8 times greater than those of 67 patients with coronary
artery disease, as confirmed by cine-angiography (Simonoff et al.,
1984).
8.1.3. Mode of Action
As in experimental animals, the best known action of chromium
is the potentiation of insulin. This is shown by the
supplementation studies of Doisy et al. (1976) and Liu & Morris
(1978) in which a reduction in insulin levels in response to
glucose was observed, together with an improvement in glucose
tolerance. Direct proof, for example from in vitro studies with
human biopsy tissue, has not yet been obtained. Furthermore, it is
not known whether the potentiation of insulin is responsible for
all the effects observed during chromium supplementation, including
the reduction of circulating cholesterol and the growth stimulation
observed in malnourished children and during parenteral nutrition.
In the healthy, chromium-sufficient human subject, chromium
levels in the bloodstream increases acutely following a glucose
load or an injection of insulin. Glinsmann et al. (1966) observed
acute increases in 5 young, healthy volunteers during a glucose
tolerance test, but not when the same subjects were given an
equivalent volume of water. Maturity-onset diabetic patients did
not exhibit this chromium response initially, but did show a rise
in plasma-chromium, after several weeks of chromium
supplementation. These observations were confirmed by Levine et
al. (1968), Hambidge (1971), Behne & Diel (1972), and Liu & Morris
(1978), who saw the acute chromium rise in some, but not all,
subjects. In the study of Liu & Morris (1978), which was the most
detailed, the relative chromium response (CrR) was measured by
neutron activation analysis in 15 women with normal glucose balance
and in 12 with impaired tolerance.
(1-h serum-chromium)
CrR = ------------------------ x 100
(fasting serum-chromium)
Initially, the normal group had a mild positive response (CrR =
107), compared with a negative response (CrR = 81) in the impaired
subjects. Following three months of supplementation with a
chromium-rich yeast extract, the CrR of the normal subjects
increased to 140, and that of the impaired group became positive
with CrR = 149. As discussed in section 8.1.2, these changes were
accompanied by a significant decrease in the insulin response to
glucose and an improvement in glucose tolerance. The results of
these studies are in apparent contrast with those of Davidson &
Burt (1973), who found a significant decline in plasma-chromium
levels following a glucose load in normal, non-pregnant women (mean
age 26 years), but a mild to moderate increase in all but 1 of 10
pregnant women (mean age 20 years). As the time of the acute rise
varied between women, statistical analysis at any one time did not
produce any significant results. Hambidge (1971) and Behne & Diel
(1972) demonstrated an acute plasma-chromium response following
injection of insulin; it can therefore be assumed that the effect
of glucose on the chromium response is associated with
hyperinsulinaemia. The acute "relative chromium response" is
believed to reflect the chromium status of human subjects. It does
not appear when stores from which the chromium increment must come
are depleted. Depleted stores can be replenished by
supplementation, with a resulting reappearance of the response
shown in the two previously discussed studies. As it has been
shown that chromium levels in urine increase following a glucose
load (Wolf et al. 1974; Gürson & Saner, 1978), it is likely that a
fraction or all of the chromium increment is subsequently excreted.
8.2. Acute Toxic Effects
In adults, the lethal oral dose of soluble chromates is
considered to be between 50 mg/kg body weight (RTECS, 1978) and 70
mg/kg body weight (Deichmann & Gerarde, 1969). The clinical
features of toxicity are vomiting, diarrhoea, haemorrhagic
diathesis, and blood loss into the gastrointestinal tract causing
cardiovascular shock. If the patient survives about 8 days, the
outstanding effects are liver necrosis (Brieger, 1920), tubular
necrosis of kidneys, and poisoning of blood-forming organs
(Langard, 1980). A 14-year-old boy, who had ingested about 1.5 g
of potassium dichromate, died 8 days later. Kaufmann et al. (1970)
also reported enlargement and oedema in the boy's brain.
8.3. Chronic Toxic Effects
The chronic effects of trivalent and hexavalent chromium have
been reviewed by Langard & Norseth (1979). The most important
features are changes in the skin and mucous membranes, and allergic
dermal and broncho-pulmonary effects. Important systemic effects
occur in the kidneys, liver, gastrointestinal tract, and
circulatory system.
Mancuso (1951) studied several biological variables in persons
occupationally exposed to chromate and related them to clinical
signs and symptoms. He found occasional leukocytosis or
leukopenia, monocytosis, eosinophilia, reduced haemoglobin
concentrations and prolonged bleeding times, but none of them were
consistent enough to serve as reliable diagnostic tests.
Mild normochronic anaemia was reported by Myslyaeva (1965) in
94 subjects exposed to chromium compounds with 68 of them showing
signs of chronic toxicity. Though the report of Baetjer et al.
(1959b) was based only on 3 cases, it indicated that chromate
attached preferentially to red blood cells. Koutras et al. (1964)
reported the inhibition of the red blood cell enzyme gluthathione
reductase by chromate concentrations of 5 - 25 mg/kg body weight.
However, the lowest effective dose is far in excess of levels
found, even in heavily exposed persons. Thus, all attempts made so
far have failed to identify a specific sensitive biochemical test
for the assessment of chronic chromium toxicity.
Long-term surveillance of pregnant women working at a plant
producing dichromates and those living in its immediate vicinity
(Shmitova, 1980) revealed increased chromium levels in the blood
and urine, compared with those in the control group. At 32 weeks
of pregnancy, the blood concentrations of chromium were,
respectively, 2.5 mg/litre in the women workers, 1.2 mg/litre in
the women living near the plant, and 0.19 mg/litre in the controls;
the levels in the urine were 1, 0.47, and 0.045 mg/litre,
respectively. Umbilical venous blood contained 1.8, 0.66, and 0.37
mg chromium/litre, respectively. The placental chromium contents
in the surveyed women were 162, 179, and 40 µg/kg, respectively,
and the breast-milk levels were 119, 20, and 31 µg/litre, which
means that chromium is capable of transplacental entry into breast
milk and into the fetal and infant organism. Women working at the
bichromate plant exhibited a high incidence of obstetric pathology
and geotoses. Chromium levels were estimated in fetal and
placental tissues (abortive material) derived on the 12th week of
pregnancy from women workers of the dichromate facility and those
not exposed to chromium. In the test group, chromium levels were
1140 µg/kg in the fetal tissues and 135 µg/kg in the placental
tissue, whereas, in the controls, they were 928 and 30 µg/kg,
respectively. No teratogenic effects have been revealed (Shmitova,
1980).
8.3.1. Effects on skin and mucous membranes
Several different types of effects on skin and mucous membranes
may result from exposure to chromium chemicals, and the main
features are:
(a) primary irritation: ulcers (corrosive reactions),
scarred and non-ulcerative contact dermatitis;
(b) allergic contact dermatitis: eczematous and
noneczematous.
A general survey of the nature and cause of contact dermatitis
is given by Fregert (1981).
8.3.1.1. Primary irritation of the skin and mucous membranes
(a) Skin
Skin irritation, manifested by a sharp hyperaemia as well as by
vesicular, papular, and rash pattern, may result from contact with
chromates. However, the major problems in the primary irritation
dermatoses are ulcers, often called chrome holes or chrome sores.
Ulceration is likely to occur among workers who have contact with
high concentrations of chromic acid, sodium or potassium dichromate
or chromate, or ammonium dichromate. It does not result from
contact with trivalent chromium compounds. An ulcer may develop if
the chromium compound, either a dust or a liquid, comes into
contact with any break in the skin such as an abrasion, scratch,
puncture, or a laceration. Favoured sites for ulcer development
are the nailroot areas, the creases over the knuckles, finger webs,
the backs of the hands, and the forearms (Bloomfield & Blum, 1928).
Primary irritation by chromium can be attributed to hexavalent
chromium, as the salts of trivalent chromium are not considered
capable of producing such effects (Fregert, 1981). However,
prolonged exposure to trivalent chromium salts has been reported to
cause skin lesions, less marked than those following exposure to
hexavalent chromium salts (Domaseva, 1971). Findings in 164
patients with eczema showed that skin tests on people who had
contact with trivalent chromium did not give positive results with
dichromate (Valer & Racz, 1971).
Ordinarily, a chrome sore, if not deep, persists for about 3
weeks after exposure is discontinued. There is no evidence that
the ulcers undergo malignant transformation. Furthermore, the
presence of chromium ulcers did not influence the development of
sensitization to chromates (Edmundson, 1951), evidenced by patch
testing with a 0.5% solution of K2Cr2O7.
(b) Nasal septal mucosa
Perforation of the nasal septum was one of the main chronic
effects in workers who had contact with chromates and chromic acid
(Bloomfield & Blum, 1928; Langard & Norseth, 1979). In a group of
100 persons constantly in contact with chromium compounds, 44 had
superficial ulceration of the anterior nasal septal mucosa.
Twenty-one people had profound ulcers and 5 had perforations of
various sizes in the cartilaginous part of the nasal septum.
Perforation of the nasal septum and cicatricial alterations were
observed in people with 3 or more years of service, whereas
ulcerous lesions arose in 2 years of work with chromium compounds.
An additional consequence of the effect on the nasal mucous
membranes is a loss of the senses of smell and taste (Seeber et
al., 1976). In workers with profound ulcerous lesions of the nasal
septal mucosa, strains of white staphylococcus with elevated
resistance to chromium are sometimes isolated (Cherevaty et al.,
1965).
Pokrovskaya et al. (1976) studied 561 workers engaged in the
open excavation of chromium ores (chromium content was 62% in the
form of a complex compound of magnesium chromite and other chromium
ores). Almost half of the workers had worked there for more than 5
years, and about 40% had been working there for 6 - 15 years.
Changes in the nasal septal mucosa, typical of the effect of
chromium compounds, were found in 8.2% of the workers. The longer
the service record, the greater the prevalence of these lesions.
After 5, 11, and 15 years of work, the prevalence was 2.3%, 8.3%,
and 11.2%, respectively.
The prevalence of nasal septum perforation in chromium plating
workers was very high (24%) in the early 1950s in China (Yang,
1956). With the introduction of control measures, the prevalence
dropped so that, in 1982, only 2 cases of nasal septum perforations
were found among 393 chromium-exposed workers (Peng, 1982).
One hundred and four subjects (85 male, 19 female) exposed to
chrome plating and 19 unexposed controls were studied by Lindberg &
Hedenstierna (1983) with regard to changes in the nasal septum.
Exposed subjects were employed at 13 different companies and
included all who were working on the day of study at the particular
company. The median exposure time was 4.5 years (range, 0.1 - 36
years). Forty-three subjects were exposed almost exclusively to
chromic acid and constituted both a "low exposure" group (8-h mean
below 2 µg/m3, N = 22) and a "high exposure" group (2 µg/m3 or
more, N = 21). Their median exposure time was 2.5 years (range,
0.2 - 23.6 years). The other 61 subjects were exposed to a mixture
of chromic acid (0.2 - 1.7 µg/m3) and other pollutants, such as
nitric, hydrochloric, and boric acids, as well as caustic soda,
nickel, and copper salts. The last group was included to disclose
any additive or synergistic effects of chromic acid and other
pollutants and was studied with regard to lung function only. The
breakdown of subjects into various groups is shown in Table 25.
Nasal septal ulceration and perforation were seen in 10 out of 14
subjects exposed to peak levels of 20 - 46 µg chromic acid/m3 or
more; no one in the control group showed signs of atrophy,
ulcerations, or perforations.
8.3.1.2. Allergic contact dermatoses
Chromium is a very important skin sensitizer. Sensitization
is reported to require about 6 - 9 months, but can occur in less
than 3 months (Pirilä & Kilpio, 1949). Genetic factors appear to
contribute to the appearance of chrome sensitivity within the
Jewish population (Wahba & Cohen, 1979).
Apart from the primary irritation and ulceration effects,
direct contact with small amounts of the chromates may cause
allergic dermatosis (dermatitis and eczema). As this allergic
reaction is cell-mediated, i.e., antibody formation is intra-
cellular and not humoral, delayed allergic reactions are rarely
observed.
Fregert et al. (1969) reported that the skin-patch test for
chromium was positive in 8 - 15% of all patients suffering from
eczema. The dermatitis caused by chromium is described as a
diffuse erythematous type with severe cases progressing to an
exudative stage. Persons sensitive to chromium react to patch and
intracutaneous tests with nonirritant concentrations of potassium
dichromate (K2Cr2O7). Opinion is divided about the role of the
oxidation state of chromium as a causal agent in allergic contact
dermatoses (Morris, 1958; Fregert & Rorsman, 1964; Kogan, 1971).
In a review, Polak et al. (1973) showed that hexavalent chromium,
which penetrates the skin by a direct effect of a hapten, is
reduced to the trivalent state, chiefly by sulfhydryl groups of
aminoacids. It then conjugates with proteins thus forming the full
antigen that initiates sensitization.
Guinea-pigs sensitized with either trivalent chromium chloride
or hexavalent potassium dichromate are capable of reacting in vivo
and in vitro to challenges with both chromium salts. This double
reactivity is also found after repeated stimulation with only one
of these chromium compounds. Since it is not possible to select
lymphocytes directed specifically against a chromium determinant of
a particular valence, it is concluded that, by sensitization with
chromium salts of different valences, a common determinant or
closely related determinants are formed. It is suggested that this
determinant is formed by chromium in the trivalent form
(Siegenthaler et al., 1983).
Two to three years after medical treatment, a follow-up study
on 555 patients with contact dermatitis was completed by means of
questionnaires (Fregert, 1975). The eczema had healed in one-
quarter of the patients, one-half had periodic symptoms, and one-
quarter had permanent symptoms. The prognosis for persons who
changed their work or stopped working was the same as that for
those who continued eczema-inducing work.
From 48 positively tested patients (0.5% potassium dichromate),
38 persons, i.e., 79%, still had a positive patch test after 4 - 7
years. In 72% of the cases, a history of occupational exposure to
chromates could be proved (Thormann et al., 1979).
A fairly rapid transformation of dermatitis into eczema is a
characteristic feature of chromium-induced skin lesions. Kogan
(1971) recognized the following important properties: (a) absence
of adaptation, which makes it necessary to discontinue chromium
handling until the slightest clinical manifestations have
disappeared; (b) a persistent chronic development of an eczematous
process; (c) a long-term latent period; and (d) absence of a direct
correlation between the incidence of sensibilization in the exposed
workers and the chromium concentration to which they have been
exposed.
Table 25. Series of exposed subjects and referencesa
--------------------------------------------------------------------------------------------------------
Exposed subjects
Mixed exposure to
Low exposure to High exposure to chromic acid
chromic acid chromic acid (< 2 µg/m3) and other
(< 2 µg Cr6+/m3) (> 2 µg Cr6+/m3) acids and metallic salts References
----------------- ------------------ ----------------------- -----------------
Nb Age (years)c N Age (years) N Age (years) N Age (years)
--------------------------------------------------------------------------------------------------
Men
Non-smokers 10 35.8 ± 16.7 6 33.4 ± 14.1 12 33.6 ± 12.2 52 34.2 ± 13.2
Smokers 6 30.8 ± 9.7 16 30.2 ± 11.8 36 42.8 ± 13.1 67 30.5 ± 8.9
Women
Non-smokers 3 30.7(17-46) 0 9 48.3 ± 13.0
Smokers 2 44.5(29-60) 1 51.2 4 31.0(19-43)
--------------------------------------------------------------------------------------------------
a From: Lindberg & Hedenstierna (1983).
b When N < 5, the range is given instead of SD.
c X ± SD.
Patients suffering from chromium-induced allergic skin
dermatoses had a tendency to develop cross-correlated hyper-
sensitivity to other metals, in particular, to cobalt and nickel
(Rostenberg & Perkins, 1951; Geiser et al., 1960; Clark & Kunitsch,
1972; Kogan et al., 1972).
Increased sensitivity to chromium is proved by skin reaction
tests. In a number of cases, additional immuno-haematological
bioassays are made to confirm the specificity of these tests. A
method for studying the basophil (mast-cells) degranulation in vivo,
at the site of positive compress skin tests, was suggested by Kogan
(1968). The method was used as a basis for proving the specificity
of cutaneous tests for chromium and other sensitising metals of the
chromium group (cobalt and nickel) (Kogan, 1968; Kogan et al.,
1972).
Photosensitivity is sometimes observed in patients with chronic
dermatitis. Wahlberg & Wennerstein (1977) investigated patients
allergic to chromium, with or without clinically observed light
sensitivity, by a standardized photopatch test procedure. A
significantly more intense reaction was observed in 48% of the
cases in the UV-irradiated sites (4/5 minimal erythema dose)
compared with the non-irradiated control sites.
No cases of cancer of the skin have been reported to result
from exposure to any form of chromium (Fregert, 1981).
8.3.2. Effects on the lung
Airborne chromium trioxide is rapidly absorbed in the broncho-
pulmonary tract causing corrosive reactions (Borghetti et al.,
1977). The absorption of chromium aerosols may depend on the
physical and chemical properties of the particles (Taylor et al.,
1965), as well as on the activity of alveolar macrophages, and the
lymphatic drainage (Sanders et al. 1971).
Both Jindrichova (1978) and Keskinen et al. (1980) reported
that welders using chromium-containing electrodes suffered from
bronchitis. Zober (1982) studied arc welders who had used filler
metals containing chromium and nickel for some years. Compared
with controls, the welders reported more previous ailments of the
respiratory system, mainly in the form of acute bronchitis. In
X-rays of welders, more roundish shadows were found, probably
indicative of benign accumulations of ferrous dust. The greater
incidence of significant findings was clearly apparent in welders
who smoked (probably a combined effect of tobacco smoke and welding
fumes). Reggiani et al. (1973) studied 101 electroplaters by means
of spirometric tests and found an obstructive disease pattern in
13% of them. After inhalation of acetylcholine, a bronchospastic
reaction occurred in 23% of the workers. In another spirometry
study (Bovet et al., 1977) on 44 chromium electroplaters, an
obstructive respiratory syndrome was found among the workers with a
high urinary-chromium content. The authors concluded that the
influence of tobacco smoking on the spirometric tests was much less
than the influence of chromium.
Studying lung function and generalized obstructive lung
diseases in electrofurnace workers in a ferrochromium and
ferrosilicon-producing plant, Langard (1980b) found a reduced
forced vital capacity (FVC) and an increased prevalence of
obstructive lung diseases. Unable to document a connection between
the presence of chromates and increased lung diseases, the author
suggested that the effects were due to high levels of total dust,
especially amorphous silica dust.
Lindberg & Hedenstierna (1983) studied lung function in more
than 100 subjects exposed to chrome plating and in 119 car
mechanics as controls (no car painters or welders). In the exposed
subjects, FVC and the forced expired volume in one second (FEV1)
were both reduced by 0.2 litre, and the forced mid-expiratory flow
was reduced by 0.4 litre/second. The authors concluded that an 8-h
mean exposure level of more than 2 µg/m3 might cause a transient
decrease in lung function.
Pneumoconiosis was diagnosed in Germany (Letterer et al., 1944)
and again in chromite miners in South Africa (Sluis-Cremer & du
Toit, 1968). However, American medical officers could not confirm
this feature when examining 897 workers in chromate-producing
plants in the USA (US PHS, 1953). It should be noted that chromium
dusts had a poor fibrogenic potency in experimental animal studies
(section 7.2.1.5). Davies (1974) X-rayed workers employed in the
manufacture of ferro-chrome and found results similar to miliary
lung tuberculosis. The workers were in the immediate area of the
furnace and were exposed to dust-particles, 90% of which were less
than 2 µm in diameter. The dust deposited on girders consisted of
95% SiO2 and 2.4% Cr2O3. The author did not find any symptoms or
signs of tuberculosis, but there was sufficient evidence to
indicate an inhalation hazard in the ferro-chrome industry, thus
underlining earlier observations of Princi et al. (1962).
8.3.2.1. Bronchial irritation and sensitization
Chromium irritates mucous membranes, as has been seen with
exposure to airborne chromium trioxide fume (Meyers, 1950) or mixed
dust (Card, 1935; Broch, 1949; Williams, 1969). The first symptom
was an irritating cough, 4 - 8 h after exposure. Chromium causes
sensitization that can result in asthmatic attacks. These can last
for 24 - 36 h, without treatment (Langard & Norseth, 1979). An
attack may recur on later exposure, even when this exposure is to a
much lower concentration (US NAS, 1974a).
A double-blind experimental exposure study on an asthmatic
patient, who had been exposed to chromium and nickel, showed that
chromium was the main cause of the asthma (Novey et al., 1983).
Keskinen et al. (1980) pretreated their patients, stainless steel
welders, with both disodium cromoglycate and betamethasone and
found an inhibition of this reaction. Placebo medication failed to
produce this inhibition.
8.3.3. Effects on the kidney
In the early part of this century, chromates and chromic acid
were occasionally used as therapeutic chemicals for some skin
lesions. Fatal cases of acute nephritis occurred with albumin,
hyaline, and granular casts and red cells appearing in the urine,
accompanied by oliguria. Autopsies revealed tubular necrosis, but
not glomerular damage (Kaufman et al., 1970). Acute toxic
gastroenteritis developed, followed by arterial hypotonia, in
suicide cases a few hours after swallowing 1.5 - 10 g potassium
dichromate. Within 2 - 4 days, all patients developed signs of
acute renal insufficiency with oliguria, anuria, and hyperhydration
and also evidence of acute toxic hepatitis (Luzhnikov et al.,
1976). Kidney damage has also occurred in industry, as a result of
accidental exposure of the skin to large amounts of chromate,
especially when associated with extensive skin damage (Luzhnikov et
al., 1976).
The mortality from kidney diseases among workers in the
chromium industry does not appear to have increased. In one study,
no deaths from nephritis and uraemia were found in 4 chromate-
producing plants; in only 1 plant was the mortality rate above that
for the controls (Machle & Gregorius, 1948). Hayes et al. (1979)
reported that 4 deaths from nephritis and nephrosis had occurred
between 1945 and 1977 in workers in a chromate-producing plant,
compared with 4.95 cases in the control population. Satoh et al.
(1981) found one case of nephritis and nephrosis in a group of
chromate workers compared with an expected number of 2.18.
Some authors (Franchini et al., 1975; Borghetti et al., 1977;
Gylseth et al., 1977; Tola et al., 1977) have concluded that
urinary excretion and renal clearance of diffusible chromium can be
used as biological indicators for evaluating the extent of exposure
to airborne chromium and the body burden. However, a dose-effect
relationship could not be established between duration of exposure
and either the level of chromium excretion (Tandon et al., 1977) or
early nephrotoxic indicators (Mutti et al., 1979). A dose-
response relationship for hexavalent chromium effects on the kidney
was described by Mutti et al. (1979), measuring beta-glucuronidase,
protein, and lysozyme in the urine. Abnormal patterns were not
observed in 39 stainless-steel welders during 4.5 ± 3.2 years of
exposure. In another group of workers employed in welding armoured
steel with special electrodes for 1 ± 0.4 years, 22% showed an
increase in beta-glucoronidase and 10% in protein in the urine. In a
study on 24 workers in the chromium-plating industry who were
exposed for 8.9 ± 7.3 years, the authors found increased urinary
levels of beta-glucoronidase in 37% of the workers, an increase in
protein in 17%, and an elevated level of lysozyme in 4%. Urinary
beta2-microglobulin was also studied by Lindberg & Vesterberg (1983b)
in chromeplaters (24 males, mean age 36 years, working in 4
different factories). The exposure was measured using personal air
samplers. The 8-h mean value ranged between 2 and 20 µg hexavalent
chromium/m3 and averaged 6 µg/m3. Most of the workers showed
symptoms of airway irritation, 2 of them had ulcerated nasal septum
and another 2, complete perforation. Significantly higher urine
beta2-microglobulin levels (0.23 mg/litre; range, 0.04 - 1.24 mg/litre;
SD, 0.27) were found in this group of workers in comparison with a
reference group of workers of a comparable age (mean age, 38 years;
mean urinary beta2-microglobulin level, 0.15 mg/litre; range, 0.06 -
0.19 mg/litre; SD, 0.08). A relationship was observed (Table 26)
between the range of exposure and prevalence of urinary beta2-micro-
globulin levels exceeding 0.3 mg/litre. The 2 workers with
perforated nasal septum were in the highest exposure group. A
difference in urinary beta2-microglobulin levels was not found between
a group of workers employed several years earlier in an old
chromeplating plant (mean, 0.25 mg/litre) and a group of workers of
comparable age (mean, 0.29 mg/litre), but it should be noted that
the mean age of both these groups exceeded 52 years.
Table 26. beta2-microglobulin U-beta2 levels in urine of workers
related to present exposure levelsa
-------------------------------------------------------------------------
Plant Exposure Number Age Persons with an "elevated" U-beta2
No. range of (mean) (U-beta2 > 0.30 mg/litre) range
(µg/m3) workers concentration (mg/litre)
No. Ages
-------------------------------------------------------------------------
1 11 - 20 5 39 3 21, 37, 58 0.23 - 1.30
2 - 3 14 - 8 13 37 2 20, 21 0.04 - 0.44
4 2 - 3 6 6 - - 0.06 - 0.18
-------------------------------------------------------------------------
a From: Lindberg & Vesterberg (1983b).
8.3.4. Effects on the liver
Statistics on diseases of the liver are seldom published
separately; they are usually included in the group of diseases of
the digestive system. The chromate industry was reported to have
the same rate in this group of diseases as many other industries
(US PHS, 1953). The Federal Security Agency in the USA
investigated 14 chromate workers reported to have "enlarged livers"
but found that these cases were not related to the time spent in
the industry. Acute hepatitis with jaundice was described in one
worker employed in a chromium-plating plant.
In an epidemiological study, Satoh et al. (1981) reported a low
rate of cirrhosis of the liver in chromium workers and liver
function test results that did not differ significantly from those
of the controls.
However, following ingestion of high doses of potassium
dichromate, liver necrosis (Brieger, 1920) and congested liver
(Kaufman et al., 1970) with loss of its architecture have been
described. There are also case studies of liver function and
histology in electroplaters (Pascale et al., 1952) and chromate
chemical plant workers (Etmanova, 1965), but the results are
difficult to interpret.
8.3.5. Effects on the gastrointestinal tract
The following symptoms and signs were reported in workers
engaged in the production of chromium salts (Sterekhova et al.,
1978): hyperchlorhydria, elevated pepsin and pepsinogen level,
oedema, hyperaemia and erosion of the mucosa, polyposis,
dyskinesia, and gastritis. However, Satoh et al. (1981) reported
that the incidence of peptic ulcer in chromate workers was below
the expected rate, and another study showed that the mortality rate
for diseases of the digestive system was lower in chromate workers
than in the control population (Hayes et al., 1979).
The gastric juice has been shown to play a role in the
detoxification of ingested hexavalent chromium by reducing it to
trivalent chromium, which is poorly absorbed and eliminated with
the faeces (Donaldson & Barreras, 1966; DeFlora & Boido, 1980).
8.3.6. Effects on the circulatory system
A clinical study was performed by Kleiner et al. (1970) on
myocardial function in more than 200 workers in the potassium
chromate industry, suffering from pulmonary or gastrointestinal
diseases, attributed by the authors to chromium poisoning. A
control group of 70 healthy individuals was included. Pathological
changes in the ECG and in other tests of heart function indicated
disturbance of right heart function, which the authors considered
secondary to the pulmonary pathology. The full paper was not
available and, consequently, the data could not be evaluated in
detail. It should be noted that no unexpected findings in
cardiovascular mortality were cited in the epidemiological studies
described in section 7.3.9.
8.3.7. Teratogenicity
The only human data available concerning this topic were
reported in an abstract (Morton & Elwood, 1974). The authors did
not find any correlation between frequency of malformations in the
central nervous system and the chromium content in water samples
collected in South Wales. Furthermore, Suzuki et al. (1979) did
not find any indication of accumulation of chromium in fetal
tissues.
8.3.8. Mutagenicity and other short-term tests
Few reports exist dealing with chromium-induced mutagenicity in
human subjects, and only hexavalent chromium causes any effects.
Bigaliev et al. (1978) found a 3 - 8% increase in chromosomal
aberrations of peripheral blood leukocytes in workers handling
chromium-compounds, compared with 2% in unexposed controls.
When classified according to the Papanicolaou system,
cytological samples of the sputum of 116 workers in the chromate-
production industry did not show any stages of tumour development
(Maltoni, 1976). However, intermediate stage lesions found in 30
workers were described as atypical adenomatous proliferation,
squamous-cell, and basal-cell dysplasia.
Studying cultured lymphocytes obtained from workers
occupationally exposed to CrO3, Sarto et al. (1982) found an
increased frequency of sister chromatid exchanges compared with
controls, which was correlated with urinary-chromium levels and
enhanced by smoking habits.
8.3.9. Carcinogenicity
8.3.9.1. Lung cancer
(a) Epidemiological studies in chromate-producing plants
The first recognition that cancer of the respiratory tract
might be related to chromium exposures resulted from the reporting
in 1932 of 2 cases of bronchogenic carcinoma that had occurred in
an old German chromate chemical plant (Lehmann, 1932). Gross &
Kölsch (1943) reported 10 deaths from lung cancer in small plants
producing the chromates of lead and zinc. By 1947, 52 such cases
had been reported from this industry and 10 cases from a chrome
pigment plant, also in the Federal Republic of Germany (Baetjer,
1950a).
The first epidemiological study was reported in the USA in
1948. The mortality data were based on the records of the group
life insurance policies of 6 chromate-producing plants in the USA
compared with similar data from a non-chromium industry or from a
life insurance company. The study reported that 21.8% of all
deaths between 1938 and 1947 in the chromate plants were due to
cancer of the respiratory tract compared with 1.4% in the control
group. The crude death rate for cancer of the bronchi and lungs
was 28 times that for the control groups (Machle & Gregorius,
1948). In one of these plants (Mancuso & Hueper, 1951), the cancer
death rate was 15 times that of the general population in the
county where the plant was located. The US PHS (1953) also studied
the respiratory cancer risk in these 6 plants and estimated a
relative risk of 28.9 using the average cancer mortality rates for
US males between 1940 and 1948 as a comparison.
In addition to these studies, where the cause of death was
taken from insurance or death certificate records, a case-control
study, using only cases diagnosed by autopsy or biopsy, was
conducted by Baetjer (1950b). Two hospitals near the largest
chromate plant in the USA were asked to supply the histories of all
cases diagnosed in 1924-46 as cancer of the lung bronchi, and to
confirm histopathologically. A group of non-cancer cases matched
individually by age, sex, and date of admission was selected as
controls. Of the 290 confirmed cancer cases, 3.26% had been
employed in the chromate plant, whereas none of the 900 control
cases was found to have had chromate exposure. The percentage of
chromate workers in the lung cancer series was 28.6 times the
percentage of chromate workers among the employed male population
of the city. The plant involved in this study was rebuilt in 1950,
following the discovery of the cancer problems. A new study of
this plant was made by Hayes et al. (1979) to determine whether the
risk of cancer had been eliminated. The investigation covered 438
deaths among 2101 males, who were initially employed between 1945
and 1974 for more than 90 days, and who died between 1945 and 1977.
The SMRa for malignant neoplasms of the trachea bronchi and lungs
was 202. Cancer deaths in the city in which the plant was located
were used to calculate the expected number of cases. No cases of
respiratory cancer occurred in the plant between 1960 and 1977
among workers employed only in the new plant. Because of the long
latent period, further follow-up is necessary.
Increased lung cancer rates among chromate workers in the USA
have been confirmed by Taylor (1966), Enterline (1974), and Mancuso
(1975), who also showed that the risks were especially high during
the early years of the study of the cohorts, e.g., before 1950.
Studies conducted in other countries have shown similar results.
In a 1956 study on workers in chromium-producing plants in the
United Kingdom, the rate of lung cancer mortality was 3.6 times the
rate for England and Wales (Bidstrup & Case, 1956). In a follow-up
study on 2715 men who had worked at the 3 chromate-producing
factories in the United Kingdom from 1948 to 1977, Alderson et al.
(1981) found 116 deaths from lung cancer (expected, 48). Since
modification of the one plant that is still in operation, the
relative risk of lung cancer has decreased from over 3 to about
1.8.
Two epidemiological studies have been conducted in the Japanese
chromate industry. In one plant, 5 lung cancer deaths occurred
between 1960 and 1973 in 136 workers who had been exposed for more
than 9 years; the SMR was 1510 (Watanabe & Fukuchi, 1975). In
another plant, the mortality rate for respiratory cancer was
determined for 896 males who worked in the plant from 1918 to 1975
and were followed to 1978; the SMR was 920 (expected deaths based
on 1975 age-cause-specific mortality rates for Japanese males)
(Satoh et al., 1981).
In the Federal Republic of Germany, a study was conducted in 2
chromate-producing plants (Korallus et al., 1982). Comparing the
SMR for lung cancer during the period 1948-79 for these 2 groups, a
clearly decreasing tendency was observed. In the first plant, for
1948-52, the SMR was 512 and, for 1978-79, it was 98; for the other
plant, the corresponding figures were 1905 and 150, respectively.
The newest data indicate a decreasing risk approaching the
frequency in unexposed people (Korallus & Loenhoff, 1981). This is
a result of occupational hygienic and technological measures,
especially of a change in manufacture namely the "on line"
--------------------------------------------------------------------
a The SMR (standardized mortality ratio) is the ratio of
observed to expected deaths times 100.
processing of the chrome ore, which has minimized the contents of
the carcinogenic hexavalent chromium compounds within the process
(Korallus et al., 1982).
Bittersohl (1972) described the results of a study on 30 000
employees of a large chemical unit for the period 1921-70 in the
German Democratic Republic. In particular, 588 malignancies in men
and 170 in women were evaluated for the period 1957-70. In 1971,
108 new malignancies in men and 29 in women came to light. In a
chromate factory, the rate of all cancers was far above average.
The factory manufactured catalysts through the reaction of chromic
acid and iron (III) oxide and nitric acid. The airborne
concentrations of chromium were not reported in detail, but it was
stated that short-term exposures above 400 µg/m3 occurred. The
incidence of malignant neoplasms in employees in the chromate
factory was 852 per 10 000 employees. In "unexposed" personnel,
the incidence of malignant neoplasms was 84 per 10 000 employees.
Approximately 86% of all those with malignant neoplasms were
smokers, and 78% of those without malignant neoplasms were also
smokers, indicating that smoking is not likely to be a confounding
factor.
In the USSR, a high incidence of cancer of the lungs was
reported for men engaged in the production of chrome salts. The
ratio of lung cancer in the plant to that in the control population
was 6.4 for ages 50 - 59 (748 observed, 116 expected) and 15.7 for
ages 60 - 69 (2657 observed, 170 expected) (Tyushnyakova et al.,
1974).
A survey was conducted in China in 1982 to investigate the
problem of lung cancer among chromate-producing workers. It
covered 7 cities and included 2184 male and 798 female workers who
had worked for at least one year in the industry. The preliminary
report revealed that 11 of the 101 deaths among male workers were
due to lung cancer, but none of the 13 deaths among female workers
was due to this cause.a
(b) Epidemiological studies - chrome pigment industry
The first epidemiological study, conducted in 1973, covered 3
small chrome pigment-manufacturing plants in the USA. The
percentage of lung cancer deaths was tentatively reported to be
about 3 times that in unexposed workers (Equitable Environmental
Health Inc., 1976).
Lung cancer mortality was studied among 1152 men working at 3
chromate pigment factories in the United Kingom from the 1930s or
1940s until 1981 (Davies, 1979, 1984). Among workers at factory A,
which produced both zinc and lead chromates, entrants to the
factory between 1932-45 with high and medium exposures had excess
---------------------------------------------------------------------------
a Report of an Investigation Team for Cancer in Chromate
Workers (1983).
risks of lung cancer (SMR = 223). A similar excess was seen in
1946-54 entrants, but the excess risk fell just short of
statistical significance. Highly significant excess risks of lung
cancer were seen among 1947-64 entrants that had high or medium
exposures at factory B, which also produced lead and zinc
chromates. Excesses were more severe in workers employed for more
than 10 years. In factory C, which produced only lead chromate, no
excess of lung cancer was found. However, the number of workers
and the power of the study were small.
Twenty-four male workers, who had been employed in a small
Norwegian company for more than 3 years between 1948 and 1972, were
identified and followed up by Langard & Norseth (1975), because
their principal exposure was to zinc chromate dust (measured
routinely with readings occasionally up to 0.5 mg hexavalent
chromium/m3). By December 1980, 6 cases of lung cancer had been
diagnosed, giving a relative risk of 44 compared with that of the
general population in the country. The authors stated that 5 out of
the 6 patients were smokers and only one had been exposed to
chromates other than zinc chromates (Langard & Vigander, 1983).
Sheffet et al. (1982) undertook a detailed mortality study on
workers employed in a pigment plant in Newark, where lead and zinc
chromates were used. Observed deaths from each cause among 1296
white and 650 non-white males employed between 1940 and 1969, were
compared with expected deaths, as computed from cause-, age-, and
time-specific standard death rates for the USA. A statistically-
significant relative risk of 1.6 for lung cancer among white
males, employed for 10 years or more, was found. A relative risk
of 1.9 was noted for individuals employed for at least 2 years, who
were at least moderately exposed to chromates.
An epidemiological study covering several centres in Europe was
conducted by Frentzel-Beyme (1983). This study was designed to
quantify the mortality from cancer and other diseases among workers
in European factories producing chromate pigments (3 German, 2
Dutch factories). Observed deaths in factories were compared with
expected deaths calculated on the basis of mortality figures for
the region in which a given factory was located. The overall
mortality did not deviate from the expected rates. Lung cancer
rates were always in excess of expected numbers, but only in one
cohort was the excess statistically significant. The pattern of
duration of exposure indicates that the lung-cancer risk was not
clearly correlated with length of employment.
A slight excess of lung cancer risk in chrome pigment (zinc
chromate) spray painters from 2 maintenance bases in the USA was
reported by Dalager et al. (1980). Among the 202 deaths
identified, 21 were due to respiratory cancer (11.4 expected;
comparison population: proportionate mortality, US males). No
statistically significant excess of lung cancer was found in a
group of automobile painters in which 226 deaths, including 22
cancer cases, were analysed using proportionate mortality for areas
in which the plants were located as a comparison (Chiazze et al.,
1980).
(c) Epidemiological studies - ferro-chrome industry
Langard et al. (1980) reported that 7 cases of bronchogenic
carcinoma occurred among 976 workers, who started work before
January 1, 1960. The expected number was 3.1 using national rates,
1.8 using local rates, and < 1 when using an international
reference rate for comparison. The difference was not significant
for the national and local rates, but was significant for the
international rate.
A study in Sweden did not reveal any significant excess of
respiratory cancer in ferro-chrome workers (Axelsson et al., 1980).
The incidence of cancer among 1932 workers employed for at least
one year between 1930 and 1975 was compared with the expected
number based on the National Cancer Registry data for the county in
which the plant was located. Five cases of respiratory cancer were
found against an expected 7.2 cases.
An excessive rate of lung cancer was reported for a ferro-
chrome plant in Russia (Pokrovskaya & Shabynina, 1973). The number
of deaths from cancer between 1955 and 1969 was higher than that in
the town in which the plant was located. The authors considered
that both hexavalent and trivalent chromium exposures were
responsible for the excess, since similar excessive rates were
found in a nearby ore-crushing plant, where only trivalent chromium
was present. The number of cases and the rates involved in these
studies were not given.
Hexavalent chromium is produced in some types of welding. In
section 7.2.1.2, it was mentioned that welding fume particles were
positive in the mammalian spot test, indicating a mutagenic and
possible carcinogenic potential. The incidence of lung cancer was
determined by Sjögren (1980), in a cohort of 234 welders, who had
welded stainless steel for more than 5 years between 1950 and 1965,
and were followed to 1977. Three welders died from lung cancer in
comparison with an expected number of 0.68 in the general
population ( P = 0.03).
Comparing lung cancer mortality in 2 parishes with ferro-alloy
industries to mortality in other parishes of similar size without
such industries from the same county, Axelson & Rylander (1980)
were unable to detect an increased incidence of death due to lung
cancer mortality. The concentration of chromium in the ambient air
of the most polluted areas ranged from 0.1 to 0.4 µg/m3. It should
be noted that several possible confounding factors were not under
control, e.g., migration, occupational exposure, and smoking
habits.
(d) Epidemiological studies - chromium-plating workers
Epidemiological studies on workers in the chromium-plating and
related industries were reviewed by Hayes (1982). The relevant
data from these studies are presented in Table 27. According to
Hayes, the epidemiological evidence is not sufficient to determine
the risk of cancer associated with industrial exposure to chromic
acid and, if an excess risk exists, it is probably lower than that
typically described for employment in the chromium-chemical
producing industry.
In a more recent paper, Franchini et al. (1983) conducted a
retrospective cohort study in 9 chromium-plating plants to examine
the mortality of workers employed for at least one year during the
period January 1951 to December 1981. The study group totalled 178
individuals; vital status ascertainment was 97% complete. The
total number of deaths was close to the expected figure (15
observed, 15.2 expected), whereas death from lung cancer exceeded
the expected number (3 observed, 0.7 expected; P = 0.03). The
increased mortality from lung cancer among chromium-platers seemed
to be related to exposure intensity.
8.3.9.2. Cancer in organs other than lungs
A few cancers of the upper respiratory tract and oral region,
but no excessive rates have been reported to occur in the chromate-
producing industry. The cancers have involved the buccal cavity,
pharynx, and oesophagus. Cancers of the nasal septum have not been
documented, with the exception of one case in Italy (Vigliani &
Zurlo, 1955).
In a joint Danish-Finnish-Swedish case-reference investigation,
initiated in 1977, the connection between nasal and sinonasal
cancer and various occupational exposures was studied. All new
cases of nasal and sinonasal cancer were collected from the
national cancer registers (Finland and Sweden) or from the hospitals
(Denmark). The results showed associations between nasal and
sinonasal cancer and exposure to chromium, welding, flame-cutting,
and soldering, hardwood or mixed wood dust, and softwood dust alone
(13.4) (Hernberg et al., 1983).
In 5 plants in the USA, during the early years of extremely
high exposures, the rate of cancer of the digestive tract varied
from 0 to 3.04/1000 compared with a rate of 0.59/1000 for the
controls (Machle & Gregorius, 1948). The mortality rates from
stomach cancer have been reported for chromate-producing plants in
Japan (SMR = 90) (Satoh et al., 1981) the USA (SMR = 40) (Hayes et
al., 1979). No significant differences were reported between the
incidence of stomach cancer in a Russian plant (Pokrovskaya &
Shabynina, 1973) and the incidence in the surrounding area.
Studying cancer mortality in a pigment plant using lead and
zinc chromates, Sheffet et al. (1982) found an increased (but not
statistically risk of stomach (SMR = 170) and pancreas (SMR = 200)
cancer among the total cohort (1946 employees).
Table 27. Summary of epidemiological studies on respiratory cancer in
workers employed in the chromium plating and related industriesa
------------------------------------------------------------------------------------------------
Study population Follow-up Respiratory cancer Comparison population
Number Estimated
relative risk
------------------------------------------------------------------------------------------------
Chromium plating
1056 workers employed Deaths in former and 24 1.8b Unexposed workers in
> 3 months in 54 plants current (followed 2 plants and in 2 non-
in the United Kingdom years) workers, plating industries
about 80% traced
About 5000 workers 49 1.4c Mortality analysis,
employed since 1945 in method not specified
1 plating factory in
the United Kingdom
889 workers exposed > 6 Reports from management 0 < 1b Tokyo mortality
months in Tokyo chromium of plating firms and rates
plating plants, 1970-76 follow-up of retired
workers; 19 total deaths
reported
Related industries
1292 deaths among US Union death benefit 62 1.1b Proportionate
metal polishers and claims mortality, US males
platers, union members,
1951-69
238 deaths among employees Pension, insurance and 39 2.1c Proportionate
for 10 years in one US benefit records mortality, US
plant for metal die
casting, finishing, and
electroplating, 1974-78.
------------------------------------------------------------------------------------------------
a Adapted from: Hayes (1982).
b Not statistically significant.
c P < 0.05, calculated using an assumption that the observed number is distributed as a
Poisson random variable.
8.3.9.3. Relationship between cancer risk and type of chromium
compound
As the exposures are often poorly defined, it is sometimes not
certain whether workers have been exposed to hexavalent chromium,
trivalent chromium, or a combination of the two. Some authors have
concluded that there is no increased risk of cancer mortality due
to trivalent chromium compounds (Korallus et al., 1974 a,b,c;
Axelsson et al., 1980; Langard et al., 1980; Norseth, 1980),
whereas others do not exclude that trivalent chromium compounds
have a carcinogenic capacity (Essing et al., 1971; Mancuso, 1975;
Zober 1979).
Summing up the data from case reports as well as
epidemiological studies in chromate-producing, chromate-pigment,
chromium-plating, and ferrochromium industries, the IARC Working
Group on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans (IARC, 1980) came to the conclusion that "there is
sufficient evidence of respiratory carcinogenicity in men
occupationally exposed during chromate production. Data on lung
cancer risk in other chromium-associated occupations and for cancer
at other sites are insufficient. The epidemiological data do not
allow an evaluation of the relative contributions to carcinogenic
risk of metallic chromium, trivalent chromium and hexavalent
chromium or of soluble versus insoluble chromium compounds".
9. EVALUATION OF HEALTH RISKS FOR MAN
Low levels of chromium are omnipresent in the environment.
Under normal conditions, human exposure to chromium does not
represent a toxicological risk, but it should be pointed out that
too low an intake of chromium may lead to deficiency. Airborne
concentrations of chromium, predominantly as trivalent chromium,
are usually below 0.1 µg/m3 and there are many places where the
concentration is below the detection limit. Concentrations in
river water are typically in the range of 1 - 10 µg/litre and do
not constitute a health threat. Drinking-water from municipal
water supplies does not contribute more than a few micrograms of
chromium to the daily human intake, but untreated water in certain
areas may be contaminated by runoff or effluents from industrial
sources and may contribute significant amounts of chromium. The
oceans contain less than 1 µg/litre. The daily human intake
through food varies considerably between regions. Typical values
range from 50 to 200 µg/day. They do not represent a toxicity
problem.
However, significant exposures exist in the occupational field.
In the past, chromium-ore mining generated chromium-containing
dusts, levels of which ranged up to 20 mg/m3 in different work-
places. Other exposures ranged up to 150 mg/m3 and consisted of
dust containing as much as 48% chromium (Cr2O3). In production
plants, hexavalent chromium can occur in the airborne state.
However, implementation of protective measures at ferrochromium
production sites reduced the airborne hexavalent chromium to levels
of 30 - 60 µg/m3.
The health effects of the two common oxidation states of
chromium are so fundamentally different that they must be
considered separately. In the form of trivalent compounds,
chromium is an essential nutrient and is relatively non-toxic for
man and other mammalian species. The hexavalent form is man-made
by oxidation of naturally-occurring trivalent chromium minerals and
is widely used. Compounds of hexavalent chromium penetrate
biological membranes easily and can thus interact with essential
constituents of the cells, including genetic material which they
can damage through oxidation and complexation with resulting
trivalent species. On the other hand, oxidation of trivalent
chromium has not been demonstrated in the living organism, and for
practical purposes, the reduction of hexavalent chromium to
trivalent chromium in lungs and other animal tissues is
irreversible.
9.1. Occupational Exposure
A number of effects can result from occupational exposure to
airborne chromium including irritative lesions of the skin and
upper respiratory tract, allergic reactions, and cancers of the
respiratory tract. The data on other effects, e.g., in the
gastrointestinal, cardiovascular, and urogenital systems are
insufficient for evaluation.
Epidemiological studies have shown that workers engaged in the
production of chromate salts and chromate pigments experience an
increased risk of developing bronchial carcinoma. No detailed data
on dose-response relationships are available from epidemiological
studies. Although a suspicion of increased lung cancer risks in
chromium plating workers has been raised, the available data are
inconclusive as are data for other industrial processes where
exposure to chromium occurs. There is insufficient evidence on the
role of chromium as a cause of cancer in any organ other than the
lung. Evidence from studies on laboratory animals shows that
hexavalent chromium compounds, especially those of low solubility,
can induce lung cancer.
In the lymphocytes of workers in chromium-plating factories,
the frequency of sister chromatid exchanges was higher in exposed
than in control groups.
Mutagenicity and related studies have convincingly shown that
hexavalent chromium is genetically active. Hexavalent chromium can
cross cellular membranes and is then reduced to trivalent chromium,
which can cause DNA cross-links and increase the infertility of DNA
implication. Trivalent chromium compounds have been shown to be
genetically inactive in most test systems, except in systems where
they can directly interact with DNA.
9.1.1. Effects other than cancer
9.1.1.1. Respiratory tract
It has been reported that the threshold for acute irritative
effects in the upper respiratory tract is 25 µg/m3 for the most
sensitive individuals. Long-term exposure to doses over 1 µg
chromic acid/m3 can cause nasal irritation, atrophy of the nasal
mucosa, and ulceration of perforation of the nasal septum.
Bronchial asthma was previously attributed to exposure to
chromium compounds, but scientific data are too scarce to draw
conclusions.
9.1.1.2. Skin
Skin rashes, ulcers, sores, and eczema have been reported among
occupationally exposed workers. Both trivalent and hexavalent
chromium compounds can give rise to sensitization of skin,
especially under certain environmental conditions, such as those
encountered in the cement industry, where the high incidence of
chromium-induced skin lesions can be attributed to the alkaline
exposure conditions.
Eczematous dermatitis, which manifests first as a diffuse
erythematous type, progresses in severe cases to an exudative
stage and is associated in 8 - 15% of patients with sensitivity to
chromium, as revealed by skin-patch tests. Although opinion is
divided on the oxidation state of chromium responsible for inducing
sensitization, there is evidence of hexavalent chromium penetrating
the skin as a hapten to be reduced to the trivalent state and
conjugated with proteins and transformed into the full antigen
that initiates the sensitization reaction, involving presumably
only a cell-mediated immune response. It should be noted that
patients suffering from chromium-induced skin allergy tend to
become hypersensitive to cobalt and nickel. Furthermore, 48% of
cases of skin allergy induced by chromium, with or without
clinically observed light sensitivity, showed a significantly more
intense reaction by a standardized photopatch test procedure, when
they were exposed to a 4/5 minimal erythema dose of irradiation.
9.1.1.3. Kidney
After high-dose, short-term oral ingestion of chromium, acute
nephritis and tubular necrosis were observed. A few
epidemiological studies on workers in chrome-plating industries
include data on diseases of the kidney, most of them without giving
exact exposure levels. A recent study related increased urinary-beta2
microglobulin levels to exposure ranges between 2 and 20 µg/m3.
The dose-response relationship observed in this study needs
confirmation on a larger number of exposed workers.
9.1.1.4 Other organs and systems
There are no conclusive data to evaluate the effects of
chromium compounds on the liver or gastrointestinal and circulatory
systems.
9.1.2. Teratogenicity
Both oxidation states, when injected at high levels
parenterally into animals, are teratogenic, with the hexavalent
form accumulating in the embryos to much higher concentrations than
the trivalent. The Task Group was not aware of any report
indicating teratogenicity in human populations.
9.2. General Population
Persons living in the vicinity of ferro-alloy plants, exposed
to an ambient air concentration of up to 1 µg/m3, did not show
increased lung cancer mortality.
The results of many studies suggest that exposure to chromium
through inhalation and skin contact can pose health problems for
the general population. Very little information is available on
the health effects of chromium ingested through untreated drinking-
water, though, in a single study, a correlation was observed
between frequency of malformation in the central nervous system and
the chromium content of water samples (Morton & Elwood, 1974). In
order to assess the nature of the magnitude of these problems,
there appears to be a need for more general population studies on
the effects of inhaled, absorbed, or ingested chromium on
respiratory, cardiovascular, and renal functions, and on the skin.
Studies on animals and man have established trivalent chromium
as an essential micronutrient that interacts with insulin and
enhances the physiological effects of the hormone. Supplementation
trials in a number of countries including Finland, Jordan, Nigeria,
Sweden, Turkey, and the USA have shown that segments of the
population could improve their glucose metabolism and, in some
instances, fat metabolism, by ingesting trivalent chromium
compounds. These observations suggest that some populations are at
risk of chromium deficiency. Children suffering from protein-
energy malnutrition may be at special risk.
As chromium concentrations in the body fluids of unexposed
persons are generally less than 1 µg/litre, analysis for chromium
requires the strictest quality control, including measures to
exclude sample contamination. Even with adequate analytical
methods, it is not possible, as yet, to diagnose chromium
deficiency in individuals by chemical or biochemical methods alone.
Therefore, a quantitative assessment of the prevalence of chromium
deficiency in human populations is not yet possible.
REFERENCES
ABRAHAM, A.S., SONNENBLICK, M., EINI, M., SHEMESH, O., & BATT,
A.P. (1980) The effect of chromium on established athero-
sclerotic plaques in rabbits. Am. J. clin. Nutr., 33:
2294-2298.
AITIO, A., JARVISALO, J., KULUNEN, M., TOSSAVAINEN, A., &
VAITTINEN, P. (1984) Urinary excretion of chromium as an
indicator of exposure to trivalent chromium sulfate in leather
tanning. Int. Arch. occup. environ. Health, 54: 241-249.
ALDERSON, M.R., RATTAN, N.S., & BIDSTRUP, L. (1981) Health
of workmen in the chromate-producing industry in Britain. Br.
J. ind. Med., 38: 117-124.
ALEKSANDROV, A.I. & KAINOV, V.I. (1975) [Crocoite from the
Pervomaisk-Zverevo deposit at the Central Urals.] Tr. Sverdl.
Gorn. Inst., 106: 152-155 (in Russian).
AMA (1979) Guidelines for essential trace element
preparations for parenteral use. J. Am. Med. Assoc., 241:
2051-2054.
AMERICAN PUBLIC HEALTH ASSOCIATION, AMERICAN WATER WORKS
ASSOCIATION, AND WATER POLLUTION CONTROL FEDERATION (1971)
117A. Hexavalent chromium. In: Standard methods for the
examination of water and wastewater, 13th ed., Washington DC,
American Public Health Association, pp. 156-157.
AMES, B.N., DURSTON, E., YAMASAKI, E., & LEE, F.D. (1973)
Carcinogens as mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci. (USA), 70: 2281-2285.
ANDERSON, F.E. (1960) Cement and oil dermatitis. The part
played by chrome sensitivity. Br. J. Derm., 72: 108-117.
ANDERSON, N. (1982) The environmental impact of chromium.
Final draft March 1982, Maine, Department of Environmental
Protection, Bureau of Air Quality Control, 126 pp.
ANDERSON, R.A. & POLANSKY, M.M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Tr. El. Res., 3: 1-5.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., & REAMER, D.C. (1982a) Effect of exercise
(running) on serum glucose, insulin, glucagon, and chromium
excretion. Diabetes, 31: 212-216.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., VEILLON, C., & GLINSMANN, W. (1982b)
Urinary chromium excretion of human subjects: effects of
chromium supplementation and glucose loading. Am. J. clin.
Nutr., 36: 1184-1193.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., PATTERSON, K.Y.,
VEILLON, C., & GLINSMANN, W. (1983) Effects of chromium
supplementation on urinary Cr excretion of human subjects and
correlation of Cr excretion with selected clinical parameters.
J. Nutr., 113: 276-281.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
MERTZ, W., & GLINSMANN, W. (1983b) Chromium supplementation
of human subjects: effects on glucose, insulin, and lipid
variables. Metabolism, 32: 894-899.
ANDERSSON, A. & NILSSON, K.O. (1972) Enrichment of trace
elements from sewage sludge fertilizer in soils and plants.
Ambio, 1: 176-179.
ANGERER, J., SCHALLER, K.-H., & HEINRICH, R. (1981)
[Commercially-available blood and urine samples for quality
control in toxicology.] Arbeitsmed. Sozialmed. Praeventivmed.,
16: 125-127 (in German).
ANIMAL RESEARCH DIVISION OF THE NEW ZEALAND DEPARTMENT OF
AGRICULTURE (1954) Annual report 1953-54, Wellington, R.E.
Owen Government Printer, 40 pp.
ANKE, M., DIETTRICH, M., WICKE, G., PFLUG, D., & SCHUELER, D.
(1971) Absorption, excretion, and distribution of 51chromium
given by mouth to lactating ruminants. Arch. Tierern., 21:
599-607.
AXELSSON, G. & RYLANDER, R. (1980) Environmental chromium
dust and lung cancer mortality. Environ. Res., 23: 469-476.
AXELSSON, G., RYLANDER, R., & SCHMIDT, A. (1980) Mortality
and incidence of tumours among ferrochromium workers. Br. J.
ind. Med., 37: 121-127.
BAER, H. (1920) [Dermatitis resulting from the use of a
beard trainer made partly of artificial leather.] Münch. med.
Wschr., 67: 874 (in German).
BAETJER, A.M. (1950a) Pulmonary carcinoma in chromate
workers. I. A review of the literature and report of cases.
Arch. ind. Hyg. occup. Med., 2: 487-504.
BAETJER, A.M. (1950b) Pulmonary carcinoma in chromate
workers. II. Incidence on basis of hospital records. Arch.
ind. Hyg. occup. Med., 2: 505-516.
BAETJER, A.M., LOWNEY, J.F., STEFFEE, H., & BUDACZ, V.
(1959a) Effect of chromium on incidence of lung tumours in
mice and rats. Am. Med. Assoc. Arch. Ind. Health, 20: 124-135.
BAETJER, A.M., DAMRON, C., & BUDACZ, V. (1959b) The
distribution and retention of chromium in man and animals. Am.
Med. Assoc. Arch. Ind. Health, 20: 136-150.
BAKER, R.S.V. (1984) Evaluation of metals in in vitro
assays: interpretation of data and possible mechanisms of
action. Toxicol. environ. Chem., 7: 191-212.
BEHNE, D. & BRATTER, P. (1979) [Problems of trace element
analysis in medicine.] In: Gladtke, E., Heimann, G., & Eckert,
I., ed. [Trace elements], Stuttgart, Thieme, pp. 42-52 (in
German).
BEHNE, D. & DIEHL, F. (1972) Relations between carbohydrate
and trace element metabolism investigated by neutron
activation analysis. In: Nuclear Activation Techniques in the
Life Sciences. Proceedings of a Symposium, Bled, Yugoslavia,
Vienna, International Atomic Energy Agency, pp. 407-414.
BEHNE, D., BRATTER, P., GESSNER, H., HUBE, G., MERTZ, W., &
ROSICK, U. (1976) Problems in determination of chromium in
biological materials: comparison of flameless atomic
absorption spectrometry and neutron activation analysis. Z.
anal. Chem., 278: 269-272.
BEHRBOHM, P. (1957) [Allergic contact dermatitis caused by
anticorrosives containing chromium.] Berufsdermatosen, 5:
271-283 (in German).
BENJANUVATRA, N.K. & BENNION, M. (1975) Hair chromium
concentration of Thai subjects with and without diabetes
mellitus. Nutr. Rep. Int., 12: 325-330.
BERGMANN, H. & HARDT, K. (1979) Analysis of dissolved Cr(VI)
in water by APDC-MIBK extraction and atomic absorption
spectrometry. Z. anal. Chem., 297: 381-383.
BERING, F. & ZITZKE, E. (1935) [Occupational skin diseases,]
Leipzig, Voss (in German).
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric., 23: 93-100.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BERTRAND, D. & DEWOLF, A. (1968) Requirement for the trace
element chromium in the growth of potatoes. C R Ser. D., 266:
1494-1495.
BEYERMANN, K. (1962) The analytical behaviour of very small
amounts of chromium. Part I. Z. anal. Chem., 190: 4-33. Part
II. Z. anal. Chem., 191: 345-369.
BEYERSMANN, D., KOSTER, A., BUTTNER, B., & FLESSEL, P.
(1984) Model reactions of chromium compounds with mammalian
and bacterial cells. Toxicol. environ. Chem., 8: 279-286.
BIANCHI, V., DAL TOSO, R., DEBETTO, P., LEVIS, A.G., LUCIANI,
S., MAJONE, F., & TAMINO, G. (1980) Mechanisms of chromium
toxicity in mamalian cell cultures. Toxicology, 17: 219-224.
BIANCHI, V., LEVIS, A.G., & SAGGIORO, D. (1979) Differential
cytotoxic activity of potassium dichromate on nucleoside
uptake in BHK fibroblasts. Chem.-biol.Interact., 24: 137-151.
BIANCHI, V., CELOTTI, L., LANFRANCHI, G., MAJONE, F., MARIN,
G., MONTALDI, G., SPONZA, G., TAMINO, G., VENIER, P.,
ZANTEDESCHI, A., & LEVIS, A.G. (1983) Genetic effects of
chromium compounds. Mutat. Res., 117: 279-300.
BIANCHI, V., ZANTEDESCHI, A., MONTALDI, A., & MAJONE, F.
(1984) Trivalent chromium is neither cytotoxic nor mutagenic
in permeabilized hamster fibroblasts. Toxicol. Lett., 23:
51-59.
BIDSTRUP, P.L. & CASE, R.A.M. (1956) Carcinoma of the lung
in workmen in the bichromates-producing industry in Great
Britain. Br. J. ind. Med., 13: 260-264.
BIGALIEV, A.B., ELEMESOVA, M.S., TUREBAEV, M.N., & BIGALIEVA,
R.K. (1978) [Cytogenetic study of the mutagenic activity of
industrial substances.] Zdravookhr. Kaz., 8: 48-50 (in
Russian).
BITTERSOHL, G. (1972) [Health and the working environment-
Epidemiology of carcinomatosis in the chemical industry.] In:
Proceedings of the 5th Merseburger Symposium, 17-18 November
(in German).
BLACK, C.A. (1968) Soil-plant relationships, New York, John
Wiley and Sons, 792 pp.
BLOKIN, V.S. & TROP, F.S. (1977) [Dynamics of the morpho-
logical changes in animals subjected to inhalational dust
treatment using chromium oxide and trisubstituted chromium
phosphate.] In: Dubinin, N.P., ed. [Genetic effects of the
pollution of the environment,] Moscow, Nauka, pp. 173-176 (in
Russian).
BLOOMFIELD, J.J. & BLUM, W. (1928) Health hazards in
chromium plating. Public Health Rep., 43: 2330-2347.
BONATTI, S., MEINI, M., & ABBONDANDOLO, A. (1976) Genetic
effects of potassium dichromate. Mutat. Res., 38: 147-150.
BOND, R.G., STRAUB, C.P., & PROBER, R., ed. (1973) Handbook
of environmental control. III. Water supply and treatment,
Cleveland, Ohio, CRC Press, pp. 763-764.
BONNEVIE, P. (1939) [Etiology and pathogenesis of eczemas,]
Copenhagen, Busck, Leipzig, Barth (in German).
BORGHETTI, A., MUTTI, A., CAVATORTA, A., FALZOI, M., CIGALA,
F., & FRANCHINI, I. (1977) Indices rénaux d'exposition aiguë
et d'imprégnation chronique par le chrome. Med. Lav., 68(5):
355-363.
BORY, R. & LECOCQ, I. (1953) Eczéma par cuivre chromé. Arch.
Mal. prof., 14: 144.
BOSTROM, G. (1936) [Hypersensitivity test for chromium
allergy.] Am. Derm. Vener., 17: 631-632 (in German).
BOVET, P., LOB, M., & GRANDJEAN, M. (1977) Spirometric
alterations in workers in the chromium electroplating
industry. Int. Arch. occup. environ. Health, 40: 25-32.
BOWEN, H.J.M. (1966) Trace elements in biochemistry, New
York, London, Academic Press, 241 pp.
BRARD, D. (1935) Toxicologie du chrome, Paris, Herrmann &
Co., 80 pp.
BRIEGER, H. (1920) [Clinical aspects of acute chromate
poisoning.] Z. exp. Path. Ther., 21: 393-408 (in German).
BROCH, C. (1949) [Bronchial asthma caused by chromic oxide.]
Nord. Med., 41: 996-997 (in Norwegian).
BROWN, E., SKOUGSTAD, M.W., & FISHMAN, M.J. (1970) Methods
for collection and analysis of water samples for dissolved
minerals and gases. In: Techniques of water resources
investigation of the United States geological survey,
Washington DC, US Department Interior, 160 pp.
BROWN, H.G., HENSLEY, C.P., MCKINNEY, G.L., & ROBINSON, J.L.
(1973) Efficiency of heavy metals removal in municipal sewage
treatment plants. Environ. Lett., 5: 103-114.
BRUCKDORFER, K.R., KHAN, I.H., & YUDKIN, J. (1971) Chromium
and sucrose-induced hyperglyceridemia. Proc. Nutr. Soc., 30:
74A.
BRUNE, D., NORDBERG, G., & WESTER, P.O. (1980) Distribution
of 23 elements in the kidney, liver, and lungs of workers from
a smeltery and refinery in north Sweden exposed to a number of
elements and of a control group. Sci. total Environ., 16:
13-35.
BURCKHARD, W. (1962) [Occupational skin diseases.] In:
Jadassohn, W., ed. [Handbook of skin and venereal diseases,]
Berlin, Springer, Vol. II(1), pp. 368-474.
BURROWS, D., ed. (1983) Chromium: metabolism and toxicity,
Boca Raton, Florida, CRC Press, 172 pp.
BYERRUM, R.U. (1961) Some studies on the chronic toxicity of
cadmium and hexavalent chromium in drinking-water. Eng. Bull.
Purdue Univ. Serv., 106: 1-8.
CALNAN, C.D. & HARMAN, R.R.M. (1961) Studies in contact
dermatitis. XIII. Diesel coolant chromate dermatitis. Trans.
St. John's Hosp. Derm. Soc., 46: 13.
CALOW, P. & FLETCHER, C.R. (1972) A new radiotracer
technique involving 14C and 51Cr for estimating the
assimilation efficiencies of aquatic, primary consumers.
Oecologia (Berlin), 9: 155-170.
CAMPBELL, W.J. & MERTZ, W. (1963) Interaction of insulin and
chromium (III) on mitochondrial swelling. Am. J. Physiol.,
204: 1028-1030.
CANADA, NATIONAL HEALTH AND WELFARE (1980) Chromium
drinking-water criteria review. In: Guidelines for Canadian
drinking-water quality, 1978. Supporting documentation,
Ottawa, Canada, National Health and Welfare, pp. 255-268.
CANFIELD, W.K. & DOISY, R.J. (1976) Chromium and diabetes in
the aged. In: Hsu, J.M., Davis, R.L., & Neithammer, R.W., ed.
The biomedical role of trace elements in aging, St. Peters-
burg, Florida, Eckert College, pp. 119-128.
CARD, W.I. (1935) A case of asthma sensitivity to chromates.
Lancet, 2: 1348-1349.
CARTER, J.P., KATTAB, A., ABD-EL-HADI, K., DAVIS, J., EL
GHOLMY, A., & PATWARDHAN, V.N. (1968) Chromium (III) in
hypoglycemia and in impaired glucose utilization in
kwashiorkor. Am. J. clin. Nutr., 21: 195-202.
CARY, F.E., ALLWAY, W.H., & OLSON, O.E. (1977) Control of
chromium concentrations in food plants. I. Absorption and
translocations of chromium by plants. J. agric. food Chem.,
25: 300-304. II. Chemistry of chromium in soils and its
availability to plants. J. agric. food Chem., 25: 305-309.
CASEY, C.E. & HAMBIDGE, K.M. (1984) Chromium in human milk
from American mothers. Br. J. Nutr., 52: 73-77.
CASTO, B.C., PIECZYNSKI, W.J., NELSON, R.L., & DI PAOLO, J.
(1976) In vitro transformation and enhancement of viral
transformation with metals. Proc. Am. Assoc. Cancer Res., 17:
12-20.
CASTO, B.C., MEYERS, J., & DIPAOLO, J.A. (1979) Enhancement
of viral transformation for evaluation of the carcinogenic or
mutagenic potential of inorganic metal salts. Cancer Res., 39:
193-198.
CAWSE, P.A. (1974) A survey of atmospheric trace elements in
the UK, 1972-1973, Harwell, England, Atomic Energy Research
Establishment, 95 pp.
CHANEY, R.L. (1973) Crop and food chain effects of toxic
elements in sludges and effluents. In: Recycling Municipal
Sludges and Effluents on Land. Proceedings of a Joint
Committee, National Association of State Universities and Land
Grant Colleges, Washington DC, July 9-13, 1973, pp. 129-141.
CHEN, N.S.G., TSAI, A., & DYER, I.A. (1973) Effect of
chelating agents on chromium absorption in rats. J. Nutr.,
103: 1182-1186.
CHEN, K.Y., YOUNG, C.S., JAN, T.K., & ROHATGI, N. (1974)
Trace metals in waste water effluents. J. Water Pollut.
Control Fed., 46: 2663-2675.
CHEREMISINOFF, P.N. & HABIB, Y.H. (1972) Cadmium, chromium,
lead, mercury: a plenary account for water pollution. Part I.
Occurrence, toxicity, and detection. Water Sewage Works, 119:
73-86.
CHEREVATY, V.S., EREMEEVA, E.A., & SMAGULOV, S.K. (1965) [On
the nature of nasal microflora in the workers of a chromium
compound plant.] In: Proceedings of 4th Scientific Session of
Aktubinsk State Medical Institute, Alma-Ata, Kazakhstan,
pp. 337-342 (in Russian).
CHIAZZE, L., FERENCE, L.D., & WOLF, P.H. (1980) Mortality
among automobile assembly workers. I. Spray painters. J.
occup. Med., 22: 520-526.
CHRISTIAN, G.D., KNOBLOCK, E.G., PURDY, W.C., & MERTZ, W.
(1963) A polarographic study of chromium-insulin-
mitochondrial interaction. Biochem. Biophys. Acta, 66: 420-423.
CLARK, R. & KUNITSCH, G. (1972) [Statistical studies of
cross allergy in metal salts.] Berufsdermatosen, 20(5):
222-238 (in German).
COLLINS, R.J., FROMM, P.O., & COLLINGS, W.D. (1961) Chromium
excretion in the dog. Am. J. Physiol., 201(5): 795-798.
CONDE-SALAZAR, L., MARTO-CASTANO, A., & ARROYO VICENTE, M.
(1980) Determination of chrome in rubber gloves. Contact
Dermatit., 6: 237-238.
COTTON, F.A. & WILKINSON, G. (1966) Advanced inorganic
chemistry, New York, Interscience, 1136 pp.
CRESSER, M.S. & HARGITT, R. (1976) The determination of
chromium (III) and chromium (VI) by total anion exchange and
atomic absorption spectrometry. Anal. Chim. Acta, 81: 196-198.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
DALAGER, N.A., MASON, T.J., FRAUMENI, J.F., HOOVER, R., &
PAYNE, W.W. (1980) Cancer mortality among workers exposed to
zinc chromate paints. J. occup. Med., 22: 25-29.
DANA, P. (1971) Crocoite-PbCrO4 In: Hurlbut, C.S., ed.
Dana's manual of mineralogy, 18th ed., New York, J. Wiley and
Sons Inc., pp. 346-347.
DANIELSSON, B.R.G., HASSOUN, E., & DENCKER, L. (1982)
Embryotoxicity of chromium: distribution in pregnant mice and
effects on embryonic cells in vitro. Arch. Toxicol., 51:
233-245.
DAVIDSON, I.W.F. & BLACKWELL, W.L. (1968) Changes in
carbohydrate metabolism of squirrel monkeys with chromium
dietary supplementation. Proc. Soc. Exp. Biol. Med., 127:
66-70.
DAVIDSON, I.W.F. & BURT, R.L. (1973) Physiological changes
in plasma chromium of normal and pregnant women: effect of
glucose load. Am. J. Obstet. Gynecol., 116: 601-608.
DAVIES, J.C.A. (1974) Inhalation hazards in the manufacture
of silicon alloys. Cent. Afr. J. Med., 20: 140-143.
DAVIES, J.M.G. (1972) The fibrogenic effects of mineral
dusts injected into the pleural cavity of mice. Br. J. exp.
Pathol., 53: 190-201.
DAVIES, J.M.G. (1979) Lung cancer mortality of workers in
chromate pigment manufacture: an epidemiological survey. J.
Oil. Col. Chem. Assoc., 62: 157-163.
DAVIES. J.M.G. (1984) Lung cancer mortality among workers
making lead chromate and zinc chromate pigments at three
English factories. Br. J. Ind. Med., 41: 158-169.
DAVIS, M.H. & GROSSMANN, V.B. (1971) A sensitive method for
determination of chromium in biological materials. Anal.
Biochem., 44: 339-344.
DEFLORA, S. (1978) Metabolic deactivation of mutagens in
the Salmonella microsome test. Nature (Lond.), 271: 455-456.
DEFLORA, S. (1981) Study of 106 organic and inorganic
compounds in the Salmonella microsome test. Carcinogenesis, 2:
283-298.
DEFLORA, S. & BOIDO, V. (1980) Effect of human gastric
juice on the mutagenicity of chemicals. Mutat. Res., 77:
307-315.
DEFLORA, S., COPPOLA, R., CAMOIRANO, A., BATTAGLIA, M.A., &
BENNICELLI, C. (1980) Mutagenicity of chromyl chloride and
its vapours. Carcinogenesis, 1(7): 583-587.
DEFLORA, S., BIANCHI, V., & LEVIS, A.G. (1984) Distinctive
mechanisms for interaction of hexavalent and trivalent
chromium with DNA? Toxicol. environ. Chem., 8: 287-294.
DE GROOT, A.J. & ALLERSMA, E. (1973) Field observations on
the transport of heavy metals in sediments. In: Proceedings of
the Conference on Heavy Metals in the Aquatic Environment,
Nashville, Tennessee, December, Oxford, Pergamon Press,
pp. 1-16.
DEICHMANN, W.B. & GERARDE, H.W. (1969) Toxicology of drugs
and chemicals, 4th ed., New York, London, Academic Press,
167 pp.
DI PAOLO, J.A. & CASTO, B.C. (1979) Quantitative studies of
in vitro morphological transformation of Syrian hamster cells
by inorganic metal salts. Cancer Res., 32: 1008-1013.
DJAHANSHIRI, H. (1976) [Nutritional physiology problems
concerning the trace element chromiun in the rat. V. Effects
on overall metabolic performande.] Z. Tierphysiol. Tierernähr.
Futtermittelkd., 37: 298-315 (in German).
DOBROLYUBSKII, O.K. (1959) [Changes in the biochemical
process in grapes under the influence of a microfertilizer
which contains chromium.] Biochemistry (USSR), 24: 577-582 (in
Russian).
DOISY, R.J., STREETEN, D.H.P., LEVINE, R.A., & CHODOS, R.B.
(1968) Effects and metabolism of chromium in normals, elderly
subjects, and diabetics. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
Univerisity of Missouri, Vol. 2, 75 pp.
DOISY, R.J., STREETEN D.H.P., SOUMA, M.L., KALAFER, M.E.,
REKANT, S.L., & DALAKOS, T.G. (1971) Metabolism of
51chromium in human subjects. In: Mertz, W. & Cornatzer, W.E.,
ed. Newer trace elements in nutrition, New York, Marcel
Dekker, pp. 155-169.
DOISY, R.J., STREETEN, D.H.P., FREIBERG, J.M., & SCHNEIDER,
A.J. (1976) Chromium metabolism in man and biochemical
effects. In: Prasad, A.S., ed. Trace elements in human health
and disease, New York, London, Academic Press, Vol. 2, pp.
79-104.
DOMASEVA, T.V. (1971) [On the effect produced by chromium
compounds on the skin.] In: [Occupational skin diseases,]
Leningrad, Leningrad Institute of Sanitation and Hygiene,
Vol. 93, pp. 168-173 (in Russian).
DONALDSON, R.M., Jr & BARRERAS, R.F. (1966) Intestinal
absorption of trace quantities of chromium. J. lab. clin.
Med., 68: 484-493.
DOUGLAS, G.R., BELL, R.D.K., GRANT, C.E., WYTSMA, J.M., &
BORA, K.C. (1980) Effect of lead chromate on chromosome
aberration, sister-chromatid exchange, and DNA damage in
mammalian cells in vitro. Mutat. Res., 77: 157-163.
DURFOR, C.N. & BECKER, E. (1964) Public water supplies of
the 100 largest cities in the United States, 1962, Washington
DC, US Geological Survey, 364 pp (Water Supply Paper No. 1812).
DURUM, W.H. & HAFFTY, J. (1963) Implications of the
minor-element content of some major streams in the world.
Geochim. Cosmochim Acta, 27: 1-11.
EATON, D.L., STACEY, N.H., WONG, K.L., & KLAASSEN, C.D.
(1980) Dose-response effects of various metal ions on rat
liver metallothionein, glutathione, heme oxygenase, and
cytochrome P-450. Toxicol. appl. Pharmacol., 55: 393-402.
EDMUNDSON, W.F. (1951) Chrome ulcers of the skin and nasal
septum and their relation to patch testing. J. Invest. Derm.,
17: 17-19.
EIFAC (1983) Water quality criteria for european freshwater
fish. Report on chromium and freshwater fish, European Inland
Fisheries Advisory Commission, Working Party on Water Quality
Criteria for European Freshwater Fish, 31 pp (EIFAC Technical
Paper No. 43).
ELIAS, Z., SCHNEIDER, O., AUBRY, F., DANIERE, M.C., & POIROT,
O. (1983) Sister chromatid exchanges in chinese hamster V79
cells treated with the trivalent chromium compounds chromic
chloride and chromic oxide. Carcinogenesis, 3: 605-611.
ENTERLINE, P.E. (1974) Respiratory cancer among chromate
workers. J. occup. Med., 16: 523-526.
ENVIRONMENTAL INSTRUMENTATION GROUP (1973) Instrumentation
for environmental monitoring: biomedical. IV, Berkeley,
California, Lawrence Berkeley Laboratory.
EQUITABLE ENVIRONMENTAL HEALTH, INC. (1976) An epidemiologic
study of workers in lead chromate plants: Final report
(prepared for the Dry Color Manufacturer's Association,
Berkeley California).
ESSING, H.G., SZADKOWSKI, D., & VALENTIN, H. (1971)
[Significance of the valence gradations of chromium compounds
in occupational medicine examinations.] Med. Sachverständige,
67: 35-39 (in German).
ETMANOVA, Z.J. (1965) [On the functional state of the liver
in workers of Aktubinsk chromium compound plant.] Gig. Tr.
prof. Zabol., 10: 55-56 (in Russian).
EVAN, A.P. & DAIL, W.G. (1974) The effect of sodium chromate
on the proximal tubules of the rat kidney. Lab. Invest.,
30(6): 704-715.
FANG, Y. (1983) [The normal value of chromium in urine for
299 unexposed persons in Yanjian city.] J. Yanbian Med.
College, 6 (1): 35-37 (in Chinese).
FARKAS, T.G. & ROBERSON, S.L. (1965) The effect of Cr(III)
on the glucose utilization of isolated lenses. Exp. Eye Res.,
4: 124-126.
FELDMAN, F.J. (1968) The state of chromium in biological
materials. Fed. Proc., 27: 482.
FELDMAN, F.J., KNOBLOCK, E.C., & PURDY, W.C. (1967) The
determination of chromium in biological materials by atomic
absorption spectroscopy. Anal. Chim. Acta, 38: 489-497.
FRADKIN, A., JANOFF, A., LANE, B.P., & KUSCHNER, M. (1975)
In vitro transformation of BHK21 cells grown in the presence
of calcium chromate. Cancer Res., 35: 1058-1061.
FRANCHINI, I., MUTTI, A., GARDINI, F., & BORGHETTI, A.
(1975) [Excretion and renal elimination of chromium compared
to degree and length of occupational exposure.] In: Roche, L.
& Trager, J., ed. Rein et Toxique. Réunion de la Société
Francaise de Toxicologie (Compte rendu), Paris, Groupement
Francais Des Centre Anti-Poisons, pp. 271-281.
FRANCHINI, I., MUTTI, A., CAVATORTA, A., CORRADI, A., COSI,
A., OLIVETTI, A., & BORGHETTI, A. (1978) Nephrotoxicity of
chromium. Control Nephrol., 10: 98-110.
FRANCHINI, J., MAGNANI, F., & MUTTI, A. (1983) Mortality
experience among chrome plating workers. Scand. J. Work
environ. Health, 9: 247-252.
FREGERT, S. (1975) Occupational dermatitis in a 10-year
material. Contact Dermatit., 1: 96-107.
FREGERT, S. (1981) Manual of contact dermatitis, 2nd ed.,
Copenhagen, Munksgaard, 139 pp.
FREGERT, S. & GRUVBERGER, B. (1972) Chemical properties of
cement. Berufsdermatosen, 20(5): 238-245.
FREGERT, S. & GRUVBERGER, B. (1979) Chromium in industrial
leather gloves. Contact Dermatit., 5: 189.
FREGERT, S. & RORSMAN, H. (1964) Allergy to trivalent
chromium. Arch. Dermatol., 90: 4-6.
FREGERT, S., HJORTH, N., MAGNUSSON, B., BANDMANN, H.-J.,
CALNAN, C.D., CRONIN, E., MALTEN, K., MENEGHINI, C.L., PIRILA,
V., & WILKINSON, D.S. (1969) Epidemiology of contact
dermatitis. Trans. St. John's Hosp. Dermatol. Soc., 55: 17-35.
FRENTZEL-BEYME, R. (1983) Lung cancer mortality of workers
employed in chromate pigment factories. J. Cancer Res. clin.
Oncol., 105: 183-188.
FREUND, H., ATAMIAN, S., & FISCHER, J.E. (1979) Chromium
deficiency during total parenteral nutrition. J. Am. Med.
Assoc., 241: 496-498.
FSA (1953) Health workers in chromate producing industry. A
study, Washington DC, Federal Security Agency, 131 pp (US PHS
Publication No. 192).
FUKAI, R. (1967) Valency state of chromium in seawater.
Nature (Lond.), 213: 901.
FUKUNAGA, M., KURACHI, Y., & MIZUGUCHI, Y. (1982) Action of
some metal ions on yeast chromosomes. Chem. pharm. Bull., 30:
3017-3019.
FURST, A., SCHLAUDER, M., & SASMORE, D.P. (1976) Tumour-
igenic activity of lead chromate. Cancer Res., 36: 1779-1783.
GALE, T.F. (1974) Effects of chromium on the hamster embryo.
Teratology, 9: A-17.
GALE, T.F. (1978) Embryotoxic effects of chromium trioxide
in hamsters. Environ. Res., 16: 101-109.
GALE, T.F. (1982) The embryotoxic response to maternal
chromium trioxide exposure in different strains of hamsters.
Environ. Res., 29: 196-203.
GALE, T.F. & BUNCH, J.D. (1979) The effect of the time of
administration of chromium trioxide on the embryotoxic
response in hamsters. Teratology, 19: 81-86.
GARCIA, J.D. & WETTERHAHN JENNETTE, K. (1981) Electron-
transport cytochrome P-450 system is involved in the
microsomal metabolism of the carcinogen chromate. J. inorg.
Biochem., 14: 281-295.
GEISER, J.D., JEANNERET, J.P., & DELACRETAZ, J. (1960) Eczema
due to cement and sensitization to cobalt. Dermatologica
(Basel), 121: 1-7.
GEMMELL, R.P. (1973) Revegetation of derelict land polluted
by a chromate smelter. Part 1. Chemical factors causing
substrate toxicity in chromate smelter waste. Environ.
Pollut., 5: 181-197.
GEMMELL, R.P. (1974) Revegetation of derelict land polluted
by a chromate smelter. Part 2. Techniques of revegetation of
chromate smelter waste. Environ. Pollut., 6: 31-37.
GENTILE, J.M., HYDE, K., & SCHUBERT, J. (1981) Chromium
genotoxicity as influenced by complexation and rate effects.
Toxicol. Lett., 7: 439-448.
GERAUER, A. (1954) [On the growing importance of
epicutaneous allergy to chromium.] Z. Haut-Geschlechtskr., 17:
97-102 (in German).
GILANI, S.H. & MARANO, M. (1979) Chromium poisoning and
chick embryogenesis. Environ. Res., 29: 427-431.
GERICKE, S. (1943) Studies on the effect of the trace
element chromium on the growth of plants. Bodenkd.
Pflanzenernaehr., 33: 114-128.
GLASER, U., HOCHRAINER, D., KLOPPEL, H., KORDEL, W., & KUHNEN,
H. (1984) [Inhalation studies with Wistar rats and patho-
physiological effects of chromium,] Berlin, 156 pp (Report to
Umweltbundesamt, D-1 UFOPLAN F+E 10606007/2) (in German).
GLASER, U., HOCHRAINER, D., KLOPPEL, H., & KUHNEN, H. (1985)
Low-level chromium VI inhalation effects on alveolar macro-
phages and immune functions in Wistar rats. Arch. Toxicol.,
57: 250-256.
GLINSMANN, W.H., FELDMAN, F.J., & MERTZ, W. (1966) Plasma
chromium after glucose administration. Science, 152: 1243.
GLINSMANN, W.H. & MERTZ, W. (1966) Effect of trivalent
chromium on glucose tolerance. Metabolism, 15: 510-520.
GMELIN, C.G. (1826) Experiments on the effects of baryta,
strontia, chrome, molybdenum, tungsten, tellurium, titanium,
osmium, platinum, iridium, rhodium, palladium, nickel, cobalt,
uranium, cerium, iron, and manganese, on the animal system.
Edinburgh med. Surg. J., 26: 131-139.
GOMES, E.R. (1972) Incidence of chromium-induced lesions
among electroplating workers in Brazil. Ind. Med., 41(12):
21-25.
GOMEZ-ARROYO, S., ALTAMIRANO, M., & VILLALOBOS-PIETRINI, R.
(1981) Sister-chromatid exchanges induced by some chromium
compounds in human lymphocytes in vitro. Mutat. Res., 90:
425-431.
GONCHAROV, A.T. (1968) [Role of chromium in the nutrition of
animals and man.] Vopr. Pitaniya, 27: 59-66 (in Russian).
GONCHAROV, A.T. (1978) [Maximum physiological dose of
chromium in food for human consumption.] Kazanskij med. Z.,
59(2): 90-93 (in Russian).
GRAY, S.J. & STERLING, K. (1950) The tagging of red cells
and plasma proteins with radioactive chromium. J. clin.
Invest., 29: 1604-1613.
GREBE, W.-H., KASTNER, H., KIPPENBERGER, C., KRAUSS, U.,
KRUSZONA, M., SCHMIDT, H., LIEBRUCKS, M., RUMBERGER, M., &
WETTIG, E. (1975) [Investigation on supply and demand for
mineral raw materials. VII. Chromium,] Berlin, Hannover,
Bundesanstalt für Gewissenschaften und Rohstoffe/Deutsches
Institut für Wirtschaftsforschung, 147 pp.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38: 33-42.
GROSS, W.G. & HELLER, V.G. (1946) Chromates in animal
nutrition. J. ind. Hyg. Toxicol., 28: 52-56.
GROSS, E. & KOLSCH, F. (1943) [Lung cancer in the chromate
dye industry.] Gewerbepathol. Gewerbehyg., 12: 164-170 (in
German).
GURSON, C.T. (1977) The metabolic significance of dietary
chromium. In: Draper, H.H., ed. Advances in nutritional
research, New York, Plenum Publishing Company, Vol. 1,
pp. 23-53.
GURSON, C.T. & SANER, G. (1971) Effect of chromium on
glucose utilization in marasmic protein-calorie malnutrition.
Am. J. clin. Nutr., 24: 1313-1319.
GURSON, C.T. & SANER, G. (1973) Effect of chromium
supplementation on growth in marasmic protein-calorie
malnutrition. Am. J. clin. Nutr., 26: 988-991.
GURSON, C.T. & SANER, G. (1978) The effect of glucose
loading on urinary excretion of chromium in normal adults,
individuals from diabetic families, and in diabetics. Am. J.
clin. Nutr., 31: 1158-1161.
GUTHRIE, B.E. (1973) Daily dietary intakes of zinc, copper,
manganese, chromium, and cadmium by some New Zealand women.
Proc. Univ. Otago Med. School., 51: 47-49.
GUTHRIE, B.E., WOLF, W.R., & VEILLON, C. (1978) Background
correction and related problems in the determination of
chromium in urine by graphite furnace atomic absorption
spectrometry. Anal. Chem., 50: 1900-1902.
GUTHRIE, B.E., WOLF, W.R., VEILLON, C., & MERTZ, W. (1979)
Chromium in urine. In: Hemphill, D.D., ed. Trace substances in
environmental health, Columbia, Missouri, University of
Missouri, Vol. XII, pp. 490-492.
GUY, W.B. (1954) Dermatologic problems in the railroad
industry resulting from conversion to Diesel power. Am. Med.
Assoc. Arch. Derm. Syph., 70: 289-292.
GYLSETH, B., GUNDERSEN, N., & LANGORD, S. (1977) Evaluation
of chromium exposure based on a simplified method for urinary
chromium determination. Scand. J. Work environ. Health, 3:
28-31.
HAMBIDGE, K.M. (1971) Chromium nutrition in the mother and
in the growing child. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 169-194.
HAMBIDGE, K.M. (1974) Chromium nutrition in man. Am. J.
clin. Nutr., 27: 505-514.
HAMBIDGE, K.M. & BAUM, J.D. (1972) Hair chromium concen-
trations of human newborn and changes during infancy. Am. J.
clin. Nutr., 25: 376-379.
HAMBIDGE, K.M. & RODGERSON, D.O. (1969) A comparison of the
hair chromium levels of nulliparous and parous women. Am. J.
Obstet. Gynecol., 103: 320-321.
HAMBIDGE, K.M., RODGERSON, D.O., & O'BRIEN, D. (1968)
Concentration of chromium in the hair of normal children and
children with juvenile mellitus. Diabetes, 17: 517-519.
HARA, N. (1982) [The change of valence of chromium by acid
treatment.] Sangyo Igaku, 24: 658-662 (in Japanese).
HARRISON, D.L. & STAPLES, E.L.J. (1955) Zinc chromate
poisoning in calves. N.Z. vet. J., 3: 63-73.
HASEGAWA, M. (1938) Studies on the detexicating hormone of
the liver (Yakriton). Toholu J. exp. Med., 32: 163-176.
HAYES, R.B. (1982) Carcinogenic effects of chromium, In:
Langard, S., ed. Biological and environmental aspects of
chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 221-247.
HAYES, R.B., LILIENFELD, A.M., & SNELL, L.M. (1979)
Mortality in chromium chemical production workers: a
prospective study. Int. J. Epidemiol., 8: 365-374.
HEATH, J.C., FREEMAN, M.A.R., & SWANSON, S.A.V. (1971)
Carcinogenic properties of wear particles from prostheses made
in cobalt-chromium alloy. Lancet, 1: 564-566.
HENNIG, A., ANKE, M., DIETRICH, M., HARTMANN, G., & HOFFMANN,
G. (1971) Absorption and distribution of 51chromium after
oral administration to laying hens as a function of time.
Arch. Tierern., 21: 609-615.
HEPLER, O.E. & SIMONDS, J.P. (1946) Experimental nephro-
pathies. IV. Glycosuria in dogs poisoned with uranyl nitrate,
mercury bichloride and potassium dichromate. Arch. Path., 41:
42-49.
HERNBERG, S., WESTERHOLM, P., SCHULTZ-LARSEN, K., DEGERTH,
R., KUOSMA, E., ENGLUND, A., ENGZELL, U., SAND HANSEN, H., &
MUTANEN, P. (1983) Nasal and sinonasal cancer: connection
with occupational exposures in Denmark, Finland, and Sweden.
Scand. J. Work environ. Health, 9: 315-326.
HERTEL, R.F. (1982) [Chromium as a problem in physiology,
epidemiology and biological monitoring.] Staub-Reinhalt. Luft,
42: 135-137 (in German).
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand
cultures. J. exp. Bot., 4: 59-64.
HILT, G. (1954) La dermite du chrome hexavalent dans le
cadre des dermites eczémateuses par sensibilisation aux
métaux. Dermatologica (Basel), 109: 143-174.
HINTZE, C. (1930) [Handbook of mineralogy,] Berlin, Leipzig,
De Gruyter, Vol. 1. III/2, pp. 4012-4030.
HOPKINS, L.L., Jr (1965) Distribution in the rat of physio-
logical amounts of injected 51Cr(III) with time. Am. J.
Physiol., 209: 731-735.
HOPKINS, L.L., Jr & SCHWARZ, K. (1964) Chromium (III)
binding to serum proteins, specifically siderophidin. Biochem.
Biophys. Acta, 90: 484-491.
HOPKINS, L.L., Jr, RANSOME-KUTI, O., & MAJAJ, A.S. (1968)
Improvement of impaired carbohydrate metabolism by chromium
(III) in malnourished infants. Am. J. clin. Nutr., 21: 203-211.
HUCKABEE, J.W., CARTAN, F.O., & KENNINGTON, G.S. (1972)
Environmental influence on trace elements in hair of 15
species of mammals, Oak Ridge, Tennessee, Oak Ridge National
Laboratory, 36 pp (ORNL-TM-3747).
HUEPER, W.C. (1958) Experimental studies in metal
cancerogenesis. X. Cancerigenic effects of chromite ore roast
deposited in muscle tissue and pleural cavity of rats. Arch.
ind. Health, 18: 284-291.
HUEPER, W.C. (1961) Environmental carcinogenisis and cancer.
Cancer Res., 21: 842-857.
HUNTER, W.C. & ROBERTS, J.M. (1933) Experimental study of
the effects of potassium bichromate in the monkey's kidney.
Am. J. Pathol., 9: 133-147.
HYODO, K., SUZUKI, S., FURUYA, N., & MESHIZUKA, K. (1980) An
analysis of chromium, copper, and zinc in organs of a chromate
worker. Int. Arch. occup. environ. Health, 46: 141-150.
IARC (1980) Some metals and metallic compounds, Lyons,
International Agency for Research on Cancer, pp. 205-323 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 23).
IIJIMA, S., MATSUMOTO, N., LU, C.C., & KATSUNUMA, H. (1975)
Placental transfer of CrCl3 and its effects on fetal growth
and development and development in mice. Teratology, 12: 198.
IMBUS, H.R., CHOLAK, J., MILLER, L.H., & STERLING, T. (1963)
Boron, cadmium, chromium, and nickel in blood and urine. Arch.
environ. Health, 6: 112-121.
IMREH, ST. & RADULESCU, D. (1982) Cytogenic effects of
chromium in vivo and in vitro. Mutat. Res., 97: 192-193.
IVANKOVIC, S. & PREUSSMANN, R. (1975) Absence of toxic and
carcinogenic effects after administration of high doses of
chromium oxide pigment in subacute and long-term feeding
experiments in rats. Food Cosmet. Toxicol., 13: 347-351.
JAEGER, H. & PELLONI, E. (1950) Tests épicutanés aux
bichromates, positifs dans l'eczéma au ciment. Dermatologica
(Basel), 100: 207-216.
JEEJEEBHOY, K.N., CHU, R.G., MARLISS, E.B., GREENBERG, G.R., &
BRUCE-ROBERTSON, A. (1977) Chromium deficiency, glucose
intolerance, and neuropathy reversed by chromium supplement-
ation, in a patient receiving long-term total parenteral
nutrition. Am. J. clin. Nutr., 30: 531-538.
JENNETTE, K.W. (1979) Chromate metabolism in liver micro-
some. Biol. Trace Elem. Res., 1: 55-62.
JENSEN, L.S., MAURICE, D.V., & MURRAY, M.W. (1978) Evidence
for a new biological function of chromium. Fed. Proc., 37: 404.
JINDRICHOVA, J. (1978) [Chromium damage in arc welders.] Z.
Ges. Hyg., 24: 86-89 (in German).
JOHNSON, J.L. (1974) Characterization of air-borne partic-
ulates. Microchem. J., 19:168-178.
JONES, G.B., BUCKLEY, R.A., & CHANDLER, C.S. (1975) The
volability of chromium from brewer's yeast during assay. Anal.
Chim. Acta, 80: 389-392.
JOSCHI, A.P. & NEEB, R. (1980) Gas chromatographic elemental
analysis via di(trifluorethyl)dithiocarbamatochelates. Z.
anal. Chem., 303: 389-393.
KAPLAN, I. & ZELIGMAN, I. (1962) Occupational dermatitis in
railroad workers. Am. Med. Assoc. Arch. Derm., 85: 135-142.
KAUFMANN, D.B., DINICOLA, W., & MCINTOSH, R. (1970) Acute
potassium dichromate poisoning. Am. J. dis. Child, 119:
374-376.
KANEKO, T. (1976) Chromosome damage in cultured human
leucocytes induced by chromium chloride and chromium trioxide.
Jpn. J. ind. Health, 18: 136-137.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec assay and
mutagenicity studies on metal compounds. Mutat. Res., 77:
109-116.
KAZANTZIS, G. & LILLY, L. (1979) Mutagenic and carcinogenic
effects of metals. In: Friedberg, L., Nordberg, G.F., & Vouk,
V.B., ed. Handbook on the toxicology of metals, Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 237-273.
KELLER, C. (1980) [Activation analysis.] In: Bartholomé, E.,
Biekert, E., Hellmann, H., Ley, H., Weigert, M., & Weise, E.,
ed. [Ullmann's encyclopaedia of technical chemistry,] 4th ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
Vol. 5, pp. 685-707 (in German).
KEMP, K., JENSEN, F.P., MOELLER, J.T., & HANSEN, G. (1974)
Trace multi-element analysis of biological tissue by proton-
induced X-ray fluorescence spectroscope, Roskilde, Denmark,
Danish Atomic Energy Commission, 22 pp (Report Riso-M-1732).
KEMPF, T. & SONNEBORN, M. (1976) [Determination of Pb, Cd,
Cr, and Zn according to the drinking-water regulation Federal
Republic of Germany.] In: Aurand, K., Hässelbarth, U., Müller,
G., Schumacher, W., & Steuer, W., ed. [The drinking-water
regulation,] Berlin, Erich Schmidt Verlag, pp. 119-125 (in
German).
KEMPF, T. & SONNEBORN, M. (1981) [Chemical composition of
drinking-waters in various regions of the Federal Republic of
Germany.] Vom Wasser, 57: 83-94 (in German).
KESKINEN, H., KALLIOMAKI, P.-L., & ALANKO, K. (1980) Occupa-
tional asthma due to stainless steel welding fumes. Clin.
Allerg., 10: 151-159.
KIRCHGESSNER, M., MERZ, G., & OELSCHLAEGER, W. (1960)
[Effect of vegetation state on content of macro and micro
elements of three species of grass.] Arch. Tierern., 10:
414-427 (in German).
KITTELBERGER, F. (1973) [Contribution to residue analysis in
fish.] Tierärztl. Prax., 1: 465-479.
KLEIN, L.A., LANG, M., NASH, N., & KIRSCHNER, S.L. (1974)
Sources of metals in New York City wastewater. J. Water
Pollut. Control Fed., 46: 2653-2662.
KLEINER, A.M., STOLBUN, B.M., LIKHACHEVA, E.L., & BELJAEVA,
L.N. (1970) [Some indices of the functional state of the
myocardium and hemodynamics in chronic intoxication with
chromium compounds.] Gig. Tr. prof. Zabol., 12: 7-10 (in
Russian).
KNUDSEN, I. (1980) The mammalian spot test and its use for
the testing of potential carcinogenicity of welding fume
particles and hexavalent chromium. Acta pharmacol. toxicol.,
47: 66-70.
KOGAN, V.Y. (1968) [The basophil degranulation as an index
of skin sensitization to cobalt compounds.] In: Belenky, G.B.,
ed. [Modern aspects of clinical and experimental dermato-
venerology. Works of the clinic of skin and venerological
diseases,] Moscow, Medizina, pp. 100-104 (in Russian).
KOGAN, V.Y. (1971) [Chromium as a specific cause for
occupation dermatoses.] In: Babayants, R.S., ed. [Topical
questions of dermato-venerology. First Medical Institute,
All-Union Conference, Moscow, I.A. Sechenov, pp. 172-176
(in Russian).
KOGAN, V.Y, ARUTYUNYAN, K.E., & KOCHINYAN, Y.E. (1972) [A
comparative characteristic of chromium, nickel, and cobalt
salts as a causative factor in allergic contact dermatosis.]
In: [Clinical Pathology of Chemical Aetiology. Materials of
the 1st All-Union Conference, Kiev, USSR, 24-25 October,
1972,] Kiev, pp. 144 and 146 (in Russian).
KOHLER, H. (1951) [Constitutional examinations of subjects
with skin diseases in the offset department of a printing
works (Doctoral thesis, Leipzig.)] Berufsdermatosen, 4: 93 (in
German).
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace
metals during the ashing of biological materials. Analyst,
101: 870-875.
KOIVISTOINEN, P., ed. (1980) Mineral element composition of
Finnish foods. Acta agric. Scand., Suppl. 22: 171
pp.
KOPP, J.E. & KRONER, R.C. (1968) Trace metals in waters of
the United States: a five-year summary of trace metals in
rivers and lakes of the United States (October 1 1962 -
September 30, 1967), Cincinnati, Ohio, US Department of the
Interior, 32 pp.
KORALLUS, U. & LOENHOFF, N. (1981) [Industrial medical and
epidemiological experience with the manufacture and processing
of chromates.] Arbeitsmed. Sozialmed. Praeventivmed., 16:
285-289 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974a)
[Trivalent chromium compounds. Results of a study in
occupational medicine. I. General; Technology; Preliminary
study.] Arbeitsmed. Sozialmed. Praeventivmed., 9: 51-54 (in
German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974b)
[Trivalent chromium compounds: results of a study in
occupational medicine. II. Disease status analysis.]
Arbeitsmed. Sozialmed. Praeventivmed., 9: 76-79 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974c)
[Trivalent chromium compounds: Results of a study in
occupational medicine. III. Clinical studies.] Arbeitsmed.
Sozialmed. Praeventivmed., 9: 248-252 (in German).
KORALLUS, U., LANGE, H.-J., NEISS, A., WUESTEFELD, E., &
ZWINGERS, T. (1982) [Correlations between reorganization
measures and bronchial carcinoma mortality in the chromate-
manufacturing industry.] Arbeitsmed. Sozialmed. Praevent-
ivmed., 7: 159-167 (in German).
KOSHI, K. (1979) Effects of fume particles from stainless
steel welding on sister chromatid exchanges and chromosome
aberrations in cultured Chinese hamster cells. Ind. Health.,
17: 39-49.
KOTZ, L., KAISER, G., TSCHOPEL, P., & TOLG, G. (1972)
[Clarification of biological matrices for determining very low
contents of trace elements when limited amounts are weighed
with nitric acid under pressure in a teflon vessel.] Z. anal.
Chem., 260: 207-209 (in German).
KOUTRAS, G.A., HATTORY, M., SCHNEIDER, A.S., EBAUGH, F.G., &
VALENTINE, W.N. (1964) Studies on chromated erythrocytes:
effect of sodium chromate on erythrocyte glutathione reduc-
tase. J. clin. Invest., 43: 323-331.
KRISHNAJA, A.P. & REGE, M.S. (1982) Induction of chromosomal
aberrations in fish Boleophthalmus dussumieri after exposure
in vivo to mitomycin C and heavy metals mercury, selenium, and
chromium. Mutat. Res., 102: 71-82.
KROEPFLI, P. & SCHUPPLI, R. (1955) [Detergent eczema.]
Dermatologica (Basel), 110: 1-7 (in German).
KRUGER, H. & DORN, H. (1956) [Clinical observations on
detergent eczema in association with chromium and nickel
allergy.] Z. Haut-Geschlechtskr., 20: 307-314 (in German).
KUCHER, I.M. (1966) In: [Proceedings of the 5th Scientific
Session of the Aktiubinsk Medical Institute, Alma-Ata,
Kazakhstan,] pp. 24-26 (in Russian).
KUHN, J.K. (1973) Trace elements in whole coal determined by
X-ray fluorescence. Norelco Rep., 20(3): 7-10.
KUMPULAINEN, J.T., WOLF, W.R., VEILLON, C., & MERTZ, W.
(1979) Determination of chromium in selected United States
diets. J. agric. food Chem., 27: 490-494.
KUMPULAINEN, J., LEHTO, J., KOIVISTOINEN, P., UUSITUPA, M., &
VUORI, E. (1983) Determination of chromium in human milk
serum and urine by electrothermal atomic absorption spectro-
metry without preliminary ashing. Sci. total Environ., 31:
71-80.
KURYLOWICZ, B. & GASIOROWSKI, S. (1958) [Agrochemical study
on calcinated phosphate-containing admixtures of chromium
compounds.] Przemysl Chem., 37: 797-800 (in Polish).
LANGARD, S. (1977) The fate of chromium after intravenous
administration of Na251 CrO4 and CrCl3 x 6H2 O to the rat,
Guildford, England, University of Surrey, 66 pp (M.Sc.
Toxicology Thesis).
LANGARD, S. (1980a) Chromium. In: Waldron, H.A., ed. Metals
in the environment, London, New York, Academic Press,
pp. 111-132.
LANGARD, S. (1980b) A survey of respiratory symptoms and
lung function in ferrochromium and ferrosilicon workers. Int.
Arch. occup. environ. Health, 46: 1-9.
LANGARD, S., ed. (1982) Biological and environmental aspects
of chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, 285 pp.
LANGARD, S. & HENSTEN-PETTERSEN, A. (1981) Chromium
toxicology. In: Williams, D.F., ed. Systemic aspects of
biocompatibility, Boca Raton, Florida, CRC Press, Vol. 1, pp.
144-161.
LANGARD, S. & NORSETH, T. (1975) A short study of bronchial
carcinomas in workers producing chromate pigments. Br. J. ind.
Med., 32: 62-65.
LANGARD, S. & NORSETH, T. (1979) Chromium. In: Friberg, L.,
Nordberg, G.F., & Vouk, V.B., ed. Handbook on the toxicology
of metals, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 383-397.
LANGARD, S. & STERN, R.M. (1985) Nickel in welding fumes: a
cancer hazard to welders? In: Sunderman, F.W.J., Aitio, A.,
Berlin, A., Bishop, C., Buringh, E., Davis, W., Gounar, M.,
Jacquignon, P.C., Mastromatteo, E., Rigaut, J.P., Rosenfeld,
C., Saracci, R., & Sors, A., ed. Nickel in the human
environment, Lyons, International Agency for Research on
Cancer, pp. 95-103 (IARC Scientific Publications No. 53).
LANGARD, S. & VIGANDER, T. (1983) Occurrence of lung cancer
in workers producing chromium pigments. Br. J. ind. Med., 40:
71-74.
LANGARD, S., GUNDERSEN, N., TSALEV, D.L., & GYLSETH, B.
(1978) Whole blood chromium level and chromium excretion in
the rat after zinc chromate inhalation. Acta pharmacol.
toxicol., 42: 142-149.
LANGARD, S., ANDERSEN, A., & GYLSETH, B. (1980) Incidence of
cancer among ferrosilicon workers. Br. J. ind. Med., 37:
114-120.
LASKIN, S. (1972) Research in environmental sciences,
Washington DC, Institute of Environmental Medicine, pp. 92-97.
LASKIN, S., KUSCHNER, M., & DREW, R.T. (1970) Studies in
pulmonary carcinogenesis. In: Hanna, M.G., Jr, Nettesheim, P.,
& Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 321-351 (US Atomic Energy
Commission Symposium Series No. 18).
LAUTNER, G.M., CARVER, J.C., & KONZEN, R.B. (1978)
Measurement of chromium (VI) and chromium (III) in stainless
steel welding fumes with electron spectroscopy for chemical
analysis and neutron activation analysis. Am. Ind. Hyg. Assoc.
J., 39: 651-660.
LEHMANN, K.B. (1914) [Historical and critical account of our
present knowledge of the effect of small doses of chromate.]
In: Gesamtgebiet gewerbehygiene, Berlin, Springer Verlag, pp.
1-119 (in German).
LEHMANN, K.B. (1932) [Does the occurrence of lung cancer in
chromium workers give grounds for any particular anxiety?]
Zentralbl. Gewerbehyg., 9: 168-170 (in German).
LERICHE, H.H. (1968) Metal contamination of soil in the
Woburn market-garden experiment resulting from the application
of sewage sludge. J. agric. Sci. Camb., 71: 205-208.
LETTERER, E., NEIDHARDT, K., & KLETT, H. (1944) [Chromate
lung cancer and chromate pneumoconiosis. A clinical, morbid
anatomical and industrial-hygiene-related study.] Arch.
Gewerbepath. Gewerbehyg., 12: 323-361 (in German).
LEVIN, H.M., BRUNNER, M.J., & RATTNER, H. (1959)
Lithographer's dermatitis. J. Am. Med. Assoc., 169: 566-569.
LEVINE, R.A., STREETEN, D.H.P., & DOISY, R.J. (1968) Effects
of oral chromium supplementation on the glucose tolerance of
elderly human subjects. Metabolism, 17: 114-125.
LEVIS, A.G. & BIANCHI, V. (1982) Mutagenic and cytogenetic
effects of chromium compounds. In: Langard, S., ed. Biological
and environmental aspects of chromium, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 171-208.
LEVIS, A.G. & BUTTIGNOL, M. (1977) Effects of potassium
dichromate on DNA Synthesis in hamster fibroblast. Br. J.
Cancer, 35: 496-499.
LEVIS, A.G. & MAJONE, F. (1979) Cytotoxic and caryogenic
effects of soluble chromium compounds on mammalian cell
cultures. Br. J. Cancer, 40: 523-533.
LEVIS, A.G. & MAJONE, F. (1981) Cytologic and clastogenic
effects of soluble and insoluble compounds containing
hexavalent and trivalent chromium. Br. J. Cancer, 44: 219-235.
LEVIS, A.G., BUTTIGNOL, M., BIANCHI, V., & SPONZA, G. (1978)
Effects of potassium dichromate on nucleic acid and protein
synthesis and on precursor uptake in BHK fibroblasts. Cancer
Res., 38: 110-116.
LEVY, L.S. & MARTIN, P.A. (1983) The effects of a range of
chromium-containing materials on rat lung, Birmingham,
England, University of Aston, Department of Environmental and
Occupational Health, p. 224 and suppl (Prepared for Dry Color
Manufacturers Association, Alexandria, Virginia, USA).
LEVY, L.S. & VENITT, S. (1975) Carcinogenic and mutagenic
activity of chromium-containing materials. Br. J. Cancer, 32:
254-255.
LIEBER, M., PERLMUTTER, N.M., & FRAUENTHAL, H.L. (1964)
Cadmium and hexavalent chromium in Nassau County ground
water. J. Am. Water Works Assoc., 56: 739-747.
LIFSCHITZ, M.L., WALLACH, S., PEABODY, R.A., VERCH, R.L., &
AGRAWAL, R. (1980) Radiochromium distribution in thyroid
and parathyroid deficiency. Am. J. clin. Nutr., 33: 57-62.
LINDBERG, E. & HEDENSTIERNA, G. (1983) Chrome plating.
Symptoms, findings in the upper airways, and effects on lung
function. Arch. environ. Health, 38: 367-374.
LINDBERG, E. & VESTERBERG, O. (1983a) Monitoring exposure to
chromic acid in chromeplating by measuring chromium in urine.
Scand. J. Work environ. Health, 9: 333-340.
LINDBERG, E. & VESTERBERG, O. (1983b) Urinary excretion of
proteins in chromplaters, previously exposed chromplaters, and
referents. Scand. J. Work. environ. Health, 9: 505-510.
LINDBERG, E., EKHOLM, U. & ULFVARSON, U. (1985) Extent and
conditions of exposure in the Swedish chrome plating industry.
Int. Arch. Occup. Environ. Health, 56: 197-205.
LIU, V.J.K. & MORRIS, J.S. (1978) Relative chromium response
as an indicator of chromium status. Am. J. clin. Nutr., 31:
972-976.
LOFROTH, G. (1978) The mutagenicity of hexavalent chromium
is decreased by microsomal metabolism. Naturwissenschaften,
65: 207-208.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LUKANIN, W.P. (1930) [The pathology of chromate pneumo-
coniosis.] Arch. Hyg. Bakt., 104: 166-174 (in German).
LUZHNIKOV, E.A., SHIMANKO, I.I., KOSTOMAROVA, L.G., MALYGINA,
S.I., & TRAKHTENGARTS, M.I. (1976) [Acute poisoning with
chromium compounds.] Terapeut. Arkh., 48(7): 121-125 (in
Russian).
LYON, G.L., BROOKS, R.R., PETERSON, P.J., & BUTLER, G.W.
(1970) Some trace elements in plants from serpentine soils.
N.Z. J. Sci., 13: 133-139.
MACHLE, W. & GREGORIUS, F. (1948) Cancer of the respiratory
system in the United States chromate-producing industry.
Public Health Rep., 63: 1114-1127.
MCCLENDON, L.T. (1978) Determination of chromium in
biological matrices by neutron activation: application to
standard reference materials. J. radioanal. Chem., 42: 85-91.
MACKENZIE, R.D., BYERRUM, R.U., DECKER, C.F., HOPPERT, C.A., &
LANGHAM, R.F. (1958) Chronic toxicity studies. II. Hexa-
valent and trivalent chromium administered in drinking-water
to rats. Am. Med. Assoc. Arch. ind. Health, 18: 232-234.
MCRAE, W.D., WHITING, R.F., & STICH, H.F. (1979) Sister-
chromatid exchanges induced in cultured mammalian cells by
chromate. Chem.-biol. Interact., 26: 281-286.
MAHALKO, J.R. & BENNION, M. (1976) The effect of parity and
time between pregnancies on maternal hair chromium concentra-
tion. Am. J. clin. Nutr., 29: 1069-1072.
MAJONE, F. & LEVIS, A.G. (1979) Chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster cells treated in
vitro with hexavalent chromium compounds. Mutat. Res., 67:
231-238.
MAJONE, F. & RENSI, D. (1979) Mitotic alterations, chromo-
some aberrations, and sister-chromatid exchanges induced by
hexavalent and trivalent chromium on mammalian cells in vitro.
Caryologia, 32: 379-392.
MALTONI, C. (1974) Occupational carcinogenesis. Excerpta
Med. Int. Congr. Ser., 322: 19-26.
MALTONI, C. (1976) Precursor lesions in exposed populations
as indicators of occupational cancer risk. Ann. NY Acad. Sci.,
271: 444-447.
MANCUSO, T.F. (1951) Occupational cancer and other health
hazards in a chromate plant: a medical appraisal. II. Clinical
and toxicologic aspects. Ind. Med. Surg., 20: 393-407.
MANCUSO, T.F. (1975) Consideration of chromium as an
industrial carcinogen. In: Hutchinson, T.C., ed. Proceedings
of the International Conference on Heavy Metals in the
Environment, Toronto, Canada, October 27-31, 1975, Toronto,
Institute for Environmental Studies, pp. 343-356.
MANCUSO, T.F. & HUEPER, W.C. (1951) Occupational cancer and
other health hazards in a chromate plant: a medical appraisal.
I. Lung cancer in chromate workers. Ind. Med. Surg., 20:
358-363.
MARTIN, J.H. & KNAUER, G.A. (1973) The elemental composition
of plankton. Geochim. Cosmochim. Acta, 3: 1639-1653.
MATHIS, B.J. & CUMMINGS, T.F. (1973) Selected metals in
sediments, water, and biota in the Illinois river. J. Water
Pollut. Control Fed., 45: 1573-1583.
MATHUR, R.K. & DOISY, R.J. (1972) Effect of diabetes and
diet on the distribution of tracer doses of chromium in rats.
Proc. Soc. Exp. Biol. Med., 139: 836-838.
MATSUMOTO, N., IIJIMA, S., & KATSUNUMA, H. (1976) Placental
transfer of chromic chloride and its teratogenic potential in
embryonic mice. J. toxicol. Sci., 2: 1-13.
MAYER, R. (1983) Interaction of forest canopies with atmo-
spheric constituents: aluminium and heavy metals. In: Ulrich,
B. & Pankrath, J., ed. Effects of accumulation of air
pollutants in forest ecosystems, Dordrecht, Boston, London,
D. Reidel, pp. 47-55.
MAZGON, R. (1932) [Therapeutic effect of common salt on
experimental chromium nephritis in the rabbit.] Z. Gesamte
exp. Med., 81: 195-207 (in German).
MERIAN, E. (1984) Introduction on environmental chemistry
and global cycles of arsenic, beryllium, cadmium, chromium,
cobald, nickel, selenium, and their derivatives. Toxicol.
environ. Chem., 8: 9-38.
MERKURIEVA, R.V., KRASOVSKY, G.N., BURMANTOVA, N.P.,
SHATERNIKOVA, I.L., VARSHAVSKAYA, S.P., VASIUKOVICH, L.YA.,
KOGANOVA, Z.I., BUSHINSKAYA, L.I., KONSTANTINOVA, I.N.,
DOLINSKAYA, S.I., ASTAKHOVA, L.F., & VOTYAKOV, A.V. (1980a)
[Changes in various enzyme systems resulting from exposure to
some chemical substances.] Gig. i. Sanit., N1: 25-28 (in
Russian).
MERKURIEVA, R.V., KRASOVSKY, G.N., VOTYAROV, A.V., BURMANTOVA,
N.P., MUCHAMBETOVA, L.H., & KONSTANTINOVA, I.N. (1980b)
[Enzyme disarrangement of various subcellular structures under
the influence of the general toxic and gonadotoxic effects of
environmental chemical factors.] Zdravookhranenie belorus.,
N2: 28-29 (in Russian).
MERKURIEVA, R.V., KOGANOVA, Z.I., GABDULLINA, M.H., TSAPKOVA,
N.N., DUKHATEROV, O.N., & DOLINSKAYA, S.I. (1982) [A
comparative study of metabolic reactions at various routes of
chromium6+ administration in the organism of laboratory
animals.] Gig. i Sanit., N8: 75-78 (in Russian).
MERRITT, W.F. (1971) Identification and measurement of trace
elements in fresh water by neutron activation. In: Proceedings
of the International Symposium on Identification and Measure-
ment of Environmental Pollution, 358-362.
MERTZ, W. (1969) Chromium occurrence and function in
biological systems. Physiol. Rev., 49: 163-239.
MERTZ, W. (1981) The interaction between chromium and
insulin. Adv. Physiol. Sci., 12: 101-105.
MERTZ, W. & ROGINSKI, E.E. (1963) The effect of trivalent
chromium in galactose entry in rat epidymal fat tissue. J.
biol. Chem., 238: 868-872.
MERTZ, W. & ROGINSKI, E.E. (1969) Effects of chromium (III)
supplementation on growth and survival under stress in rats
fed low protein diets. J. Nutr., 97: 531-536.
MERTZ, W. & ROGINSKI, E.E. (1971) Chromium metabolsm: the
glucose tolerance factor. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 123-153.
MERTZ, W. & SCHWARZ, K. (1959) Relation of glucose tolerance
factor to impaired glucose tolerance in rats on stock diets.
Am. J. Physiol., 196: 614-618.
MERTZ, W., ROGINSKI, E.E., & SCHWARZ, K. (1961) Effect of
trivalent chromium complexes on glucose uptake by epididymal
fat tissue of rats. J. biol. Chem., 236: 318-322.
MERTZ, W., ROGINSKI, E.E., & REBA, R.C. (1965a) Biological
activity and fate of intravenous chromium (III) in the rat.
Am. J. Physiol., 209: 489-494.
MERTZ, W., ROGINSKI, E.E., & SCHROEDER, H.A. (1965b) Some
aspects of glucose metabolism of chromium-deficient rats
raised in a strictly controlled environment. J. Nutr., 86:
107-112.
MERTZ, W., ROGINSKI, E.E., FELDMAN, R.J., & THURMAN, D.E.
(1969) Dependence of chromium transfer into the rat embryo on
the chemical form. J. Nutr., 99: 363-367.
MERTZ, W., TOEPFER, E.W., ROGINSKI, E.E., & POLANSKY, M.M.
(1974) Present knowledge of the role of chromium. Fed. Proc.,
33: 2275-2280.
MEYERS, J.B. (1950) Acute pulmonary complications following
inhalations of chromic acid mist. Arch. ind. Hyg. occup. Med.,
2: 742-747.
MICKAIL, T.H., HABIB, Y.A., GABRIAL, G.N., & MORCOS, S.R.
(1976) The effect of chromium III on the intravenous glucose
tolerance of rats given diets of defficient protein values. Z.
Ernährungswiss., 15: 182-187.
MIESCHER, G., AMREIN, H.P., & LEDER, M. (1955) [Chromate
hypersensitivity and cement eczema.] Dermatologica (Basel),
110: 266-283 (in German).
MIGAI, K.V. (1975) [Hygiene and safety of labor in electro
welding works in shipbuilding,] Leningrad, pp. 27-35 (in
Russian).
MITMAN, F.W., WOLF, W.R., KELSAY, J.L., & PRATHER, E.S.
(1975) Urinary chromium levels of nine young women eating
freely chosen diets. J. Nutr., 105: 64-68.
MORGAN, J.M. (1972) Hepatic chromium content in diabetic
subjects. Metabolism, 21: 313-316.
MORRIS, G.E. (1955) Chromate dermatitis from chrome glue and
other aspects of the chrome problem. Am. Med. Assoc. Arch.
Ind. Health, 11: 368-371.
MORRIS, G.E. (1956) The manufacture of leather. Its chemical
and dermatological aspects. Ann. Meet. Am. Acad. Derm. Syph.
(Chicago).
MORRIS, G.E. (1958) Chrome dermatitis. A study of the
chemistry of shoe leather with particular reference to basic
chromic sulfate. Am. Med. Assoc. Arch. Derm., 78: 612-618.
MORRIS, G.E. (1959) [The basic form of chromic sulfate.
Results of epicutaneous testing of a chromium-III salt.]
Berufsdermatosen, 7: 126-131 (in German).
MORROW, R.W. & MCELHANEY, R.J. (1974) Determination of
chromium in industrial effluent water by flameless atomic
absorption spectroscopy. At. Absorpt. Newsl., 13(2): 45-46.
MORTON, M.S. & ELWOOD, P.C. (1974) CNS malformations and
trace elements in water. Teratology, 10: 318.
MOSINGER, M. & FIORENTINI, H. (1954) Sur la pathologie due
aux chromates: Premières recherches expérimentales. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 15: 187-199.
MULLER-WIEGAND, B., STEUER, D., & HEESSELS, P. (1983) Blood
and urine samples for quality control of heavy metals. Chem.
Toxicol. Clin. Chem. Met. Proc. Int. Conf., 2nd: 73-76.
MURAKAMI, Y., SUZUKI, Y., YAMAGATA, T., & YAMAGATA, N. (1965)
Cr and Mn in Japanese diet. J. Radiat. Res., 6: 105-110.
MURRMAN, R.P., WINTERS, R.W., & MARTIN, T.G. (1971)
Determination of trace elements in soils and clay minerals by
resonnance neutron activation analysis. Soil. Sci. Soc. Am.
Proc., 35: 647-652.
MUTTI, A., CAVATORTA, A., PEDRONI, C., BORGHI, A., GIAROLI,
C., & FRANCHINI, I. (1979) The role of chromium accumulation
in the relationship between airborne and urinary chromium in
welders. Int. Arch. occup. environ. Health, 43: 123-133.
MYSLYAEVA, A.V. (1965) [Changes in the peripheral blood in
workers of a chromium compound plant.] In: [Proceedings of
the 4th Scientific Conference of Aktubinsk State Medical
Institute, Alma-Ata,] Kazakhstan, Alma-Ata, Aktubinsk State
Medical Institute, pp. 331-332 (in Russian).
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., & KURATA, H. (1978)
Comparative studies of chromosomal aberrations and muta-
genicity of trivalent and hexavalent chromium. Mutat. Res.,
58: 175-181.
NASH, D.T., ELWOOD, I.G., & STREETEN, D.H.P. (1979) The
effect of brewer's yeast containing chromium-rich glucose
tolerance factor on serum lipids. In: Proceedings of the 5th
International Symposium on Atherosclerosis, Houston, Texas.
NATER, J.P. (1963) Possible causes of chromate eczema.
Dermatologica (Basel), 126: 160-166.
NATH, R. (1976) Assessment of chromium metabolism in
maturity onset and juvenile diabetics using chromium: 51Cr and
therapeutic response of chromium administration. Excerpta Med.
Int. Congr. Ser., 400: 194.
NEIKO, E.M. & DELVA, IU.V. (1978) [Chromium balance in
chronic ischemic heart disease.] Klinicheskaia Meditsina
(Mosk.), 56(4): 97-99 (in Russian).
NESTMANN, E.R., MATULA, T.I., DOUGLAS, G.R., BORA, K.C., &
KOWBEL, D.J. (1979) Detection of the mutagenic activity of
lead chromate using a battery of microbial tests. Mutat. Res.,
66: 357-365.
NETTESHEIM, P., HANNA, M.G., Jr., DOHERTY, D.G., NEWELL, R.F.,
& HELLMANN, A. (1970) Effects of chronic exposure to
artificial smog and chromium oxide dust on the incidence of
lung tumours in mice. In: Hanna, M.G., Jr, Nettesheim, P., &
Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 305-320 (US Atomic Energy
Commission Symposium Series No. 18).
NETTESHEIM, P., HANNA, M.G., DOHERTY, D.G., NEWELL, R.F., &
HELLMAN, A. (1971) Effect of calcium chromate dust,
influenza virus, and 100R whole-body X-radiation on lung
tumour incidence in mice. J. Natl Cancer Inst., 47: 1129-1144.
NEWBOLD, R.F., AMOS, J., & CONNELL, J.R. (1979) The
cytotoxic, mutagenic, and clastogenic effects of chromium-
containing compounds on mammalian cells in culture. Mutat.
Res., 67: 55-63.
NEWMAN, H.A.I., LEIGHTON, R.F., LANESE, R.R., & FREELAND N.A.
(1978) Serum chromium and angiographically-determined
coronary artery disease. Clin. Chem., 24: 541-544.
NISHIOKA, H. (1975) Mutagenic activities of metal compounds
in bacteria. Mutat. Res., 31: 185-189.
NOLLENDORF, A., PAKALNE, E., & UPITIS, V. (1972) [Little
known trace elements in Chlorella culture.] Latv. PSR Zinat.
Akad. Vestis (Latvian SSR), 7: 33-43 (in Russian).
NOMIYAMA, H., YOTORIYAMA, M., & NOMIYAMA, K. (1980b) Normal
chromium levels in urine and blood of Japanese subjects
determined by direct flameless atomic absorption spectrophoto-
metry, and valency of chromium in urine after exposure to
hexavalent chromium. Am. Ind. Hyg. Assoc. J., 41: 98-102.
NOMIYAMA, K., MATSUI, K., & NOMIYAMA, H. (1980a) Environ-
mental temperature: a factor modifying the acute toxicity of
organic solvents, heavy metals, and agricultural chemicals.
Toxicol. Lett., 6: 67-70.
NORSETH, T. (1978) Health effects of nickel and chromium.
In: Elvira di Ferrante, E., ed. Trace metals: exposure and
health effects, Oxford, New York, Toronto, Sydney, Paris,
Frankfurt, Pergamon Press, pp. 135-146.
NORSETH, T. (1980) Cancer hazards caused by nickel and
chromium exposure. J. Toxicol. environ. Health, 6: 1218-1227.
NOVAKOVA, S., MAUTNER, G., & DINOEVA, A. (1974) [Content of
hexavalent chromium in water supplies and its effect on the
development of experimental atherosclerosis in warm-blooded
animals.] Gig. i Sanit., 5: 78-80 (in Russian).
NOVEY, H.S., HABIB, M., WELLS, J.A. (1983) Asthma and JgE
antibodies induced by chromium and nickel salts. J. Allergy
clin. Immunol., 72: 407-412.
OFFENBACHER, E.G. & PI-SUNYER, F.X. (1980) Benefical effect
of chromium-rich yeast on glucose tolerance and blood lipids
in elderly subjects. Diabetes, 29: 919-925.
OHNO, H., HANAOKA, F., & YAMADA, M. (1982) Inducibility of
sister-chromatid exchanges by heavy-metal ions. Mutat. Res.,
104: 141-145.
OHTA, F. (1940) Studies on the detoxicating hormone of the
liver (yakriton). Contribution to the usage of yakriton
against experimental chromate nephritis. Tohoku J. exp. Med.,
39: 37-46.
OKADA, S., OHBA, H., & TANIYAMA, M. (1981) Alterations in
ribonucleic acid synthesis by chromium (III). J. inorg.
Biochem., 15: 223-231.
OKADA, S., TRUKADA, H., & OHBA, H. (1983) Enhancement of
ribonucleic acid synthesis by chromium (III) in mouse liver.
J. inorg. Biochem., 19: 95-103
OKADA, S., TSUKADA, H., & OHBA, H. (1984) Enhancement of
nucleolar RNA synthesis by chromium (III) in regenerating rat
liver. J. inorg. Biochem., 21, 113-124.
OPHULS, W. (1911a) Experimental nephritis in guinea-pigs by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9: 11-13.
OPHULS, W. (1911b) Experimental nephritis in rabbits by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9:13.
OTTINGER, R.S., BLUMENTHAL, J.L., DALPORTO, D.F., GURBER,
G.I., SANTY, M.J., & SHIH, C.C. (1973) Recommended methods
of reduction, neutralization, recovery or disposal of
hazardous waste, Cincinnati, Ohio, US Environmental Protection
Agency, Vol. 6, pp. 143-162 (EPA 670/2-73-053-F).
PAGE, A.L. (1974) Fate and effects of trace elements in
sewage sludge when applied to agricultural lands, Cincinnati,
Ohio, US Environmental Protection Agency (EPA 670/2-74-005).
PAIXAO, L.M. & YOE, J.H. (1959) Spectochemical determination
of magnesium, chromium, nickel, copper, and zinc in human
plasma and red cells. Clin. Chim. Acta, 4: 507-514.
PARR, R.M. (1977) Problems of chromium analysis in
biological materials: an international perspective with
special reference to results for analytical quality control
samples. J. radioanal. Chem., 39: 421-433.
PASCALE, L.R., WALDSTEIN, S.S., ENGBRING, G., DUBIN, A., &
SZANTO, P.B. (1952) Chromium intoxication with special
reference to hepatic injury. J. Am. Med. Assoc., 149:
1385-1389.
PASHIN, Y.V. & KOZACHENKO, V.J. (1981) [Mutagenic activity
of chromium compounds.] Gig. i. Sanit., 5: 46-49 (in Russian).
PASHIN, Y.V., ZACEPILOVA, T.A., & KOZACHENKO, V.I. (1982)
Induction of dominant lethal mutations in male mice by
potassium dichromate. Mutat. Res., 103: 345-347.
PASTERNAK, K. (1973) The spreading of heavy metals in
flowing waters in the region of occurrence of natural deposits
of the zinc and lead industry. Acta hydrobiol., 15: 145-166.
PEKAREK, R.S., HAUER, E.C., RAYFIELD, E.J., WANNEMACHER, R.W.,
& BIESEL, W.R. (1975) Relationship between serum chromium
concentrations and glucose utilization in normal and infected
subjects. Diabetes, 24: 350-353.
PENG, H.Z. (1982) [The investigation of nasal disease
morbidity of chromium exposed workers.] J. Shan-Xie Med.
College, 3: 19-21 (in Chinese).
PERLMUTTER, N.M. & LIEBER, M. (1970) Dispersal of plating
wastes and sewage contaminants in ground water and surface
water, Washington DC, US Government Printing Office, 67 pp
(Geological Survey Water Supply Paper 1879-G).
PERONE, V.B., MOFFITT, A.A., POSSICK, P.A., KEY, M.M.,
DANZINGER, S.J., & GELLIN, G.A. (1974) The chromium, cobalt,
and nickel contents of American cement and their relationship
to cement dermatitis. Am. Ind. Hyg. Assoc. J., 35: 301-306.
PETRILLI, F.L. & DE FLORA, S. (1977) Toxicity and mutagenicity of
hexavalent chromium on Salmonella typhimurium. Appl. environ.
Microbiol., 33: 805-809.
PETRILLI, F.L. & DE FLORA, S. (1978a) Oxidation of inactive
trivalent chromium to the mutagenic hexavalent form. Mutat.
Res., 58: 167-173.
PETRILLI, F.L. & DE FLORA, S. (1978b) Metabolic deactivation
of hexavalent chromium mutagenicity. Mutat. Res., 54: 139-147.
PETRILLI, F.L. & DE FLORA, S. (1980) Mutagenicity of
chromium compounds. In: Chromates Symposium 80, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 76-99.
PETRILLI, F.L. & DE FLORA, S. (1982) Interpretations on
chromium mutagenicity and carcinogenicity. In: Sorsa, M. &
Vainmio, H., ed. Mutagens in our environment, New York, Alan
R. Liss, pp. 453-464.
PIERCE, J.V. & SCHEEL, L.D. (1965) Toxicity of alloys of
chromium. Arch. environ. Health, 10: 870-876.
PIRILA, V. & KILPIO, O. (1949) On dermatoses caused by
bichromates. Acta derm. venerol., 29: 550-563.
POHLAND, F.G. (1975) Sanitary landfill stabilization with
leachate recycle and residual treatment, Cincinnati, Ohio, US
Environmental Protection Agency, 106 pp (EPA 600/2-75-043).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1973) [On carcinogenous
hazards in the product of chromium ferroalloys.] Gig. Tr.
prof. Zabol., 10: 23-26 (in Russian).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1974) [Epidemiological
data on malignant neoplasms in workers who are in contact with
different chromium compounds.] In: Shabad, L.M., Velich-
kovskii, B.T. & Kogan, F.M., ed. [Occupational cancer (a
collection of scientific works),] Moscow, Academic Academy of
Medical Science, pp. 64-71 (in Russian).
POKROVSKAYA, L.V., TJUSHUYAKOVA, A.V., GORODNOVA, N.V.,
ZALOMOVA, E.N., & ANDREEVA, T.D. (1976) [Dust factor and
occupational pathology in workers engaged in the open
excavation of chromium ores.] In: [Occupational diseases of
dust etiology (collection of works),] Vol. 3, pp. 38-43 (in
Russian).
POLAK, L., TURK, J.L., & FREY, J.R. (1973) Studies on
contact hypersensitivity to chromium compounds. In: Kallos,
P., Waksman, B.H., & DeWeck, A., ed. Progress in allergy,
Basel, Karger, Vol. 17, pp. 145-226.
PRESTON, A.M., DOWDY, R.P., PRESTON, M.A., & FREEMAN, J.N.
(1976) Effects of dietary chromium on glucose and serum
cholesterol in guinea-pigs. J. Nutr., 106: 1391-1397.
PRIESTLEY, J. (1877) Observations on the physiological
action of chromium. J. Anat. Physiol., 11: 285-301.
PRINCI, F., MILLER, L.H., DAVIS, A., & CHOLAK, J. (1962)
Pulmonary disease of ferrochromium workers. J. occup. Med., 4:
301-310.
PUNSAR, S., ERAMETSA, O., KARVONEN, M.I., RYHANEN, A., HILSKA,
P., & WORNAMO, H. (1975) Coronary heart disease and
drinking-water. A search in two Finnish male cohorts for
epidemiologic evidence of a water factor. J. chron. Dis., 28:
259-287.
RABEAU, H. & UKRAINCZYK, V. (1939) Dermites des
blanchisseuses. Rôle du chrome et du chlore. Ann. Derm. Syph.,
10: 656-680.
RADIAN CORPORATION (1983) Estimates of population exposure
to ambient chromium emissions, Research Triangle Park, North
Carolina, US Environmental Protection Agency (Final Report to
K.L. Blanchard) (EPA Contract No. 68-02-3818).
RAFFETTO, G. (1977) Direct interaction with cellular targets
as the mechanism for chromium carcinogenesis. Tumori, 63:
503-512.
RAFFETTO, G., PARODI, S., DE FERRARI, M., PARODI, C., TROIANO,
R., & BRAMBILLA, G. (1978) Direct interaction with cellular
targets as the mechanism for chromium carcinogensis. Tumori,
73: 503-512.
REDISKE, J.H. (1956) Chromium toxicity in plants. In:
Biology research: annual report, 1955, Richland, Washington,
Hamford Atomic Products Operations, pp. 48-52 (Report
HW-41500).
REDISKE, J.H., CLINE, J.F., & SELDERS, A.A. (1955) Chromium
toxicity in plants. In: Biology research: annual report, 1954,
Richland, Washington, Hamford Atomic Products Operations,
pp. 32-39 (Report HW-35917).
REGGIANI, A., LOTTI, M., DE ROSA, E., & SAIA, B. (1973)
[Impairments of respiratory functions in subjects exposed to
chromium. Note 1. Spirographic changes.] Lav. Umano, 25: 23-27
(in Italian).
REICHENBACH-KLINKE, H.H. (1977) [To the ecology of the
accumulation of heavy metals in fishes.] In: Reichenbach-
Klinke, H.H., ed. [Contributions to fish ecology: fish and the
environment,] Stuttgart, New York, Gustav Fischer, pp. 7-11
(in German).
REICHENBACH-KLINKE, H.H. (1980) [Fish diseases and harm
suffered by fish,] 2nd ed., Stuttgart, New York, Gustav
Fischer, 474 pp (in German).
RETNEV, V.M. (1960) [On the effect produced by chromium
compounds contained in cement dust on the development of
bronchial asthma.] Gig. Tr. prof. Zabol., 7: 29-32 (in
Russian).
RIALES, R. (1979) Influence of brewer's yeast on lipoprotein
cholesterol concentrations. A preliminary report. In:
Shapcott, D. & Hubert, I., ed. Chromium in nutrition and
metabolism. Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 199-212.
ROBERTS, K.R. & MARZLUF, G.A. (1971) The specific inter-
action of chromate with the dual sulfate permease systems of
Neurospora crassa. Arch. Biochem. Biophys., 142: 651-659.
ROBINSON, W.D. & EDINGTON, G. (1935) Chemical studies of
infertile soils derived from rocks high in magnesium and
generally high in chromium and nickel, Washington DC, US
Department of Agriculture, pp. 1-29 (US Department of
Agriculture Technical Bulletin No. 471).
ROGINSKI, E.E. & MERTZ, W. (1967) An eye lesion in rats fed
low-chromium rations. J. Nutr., 93: 249-251.
ROGINSKI, E.E. & MERTZ, W. (1969) Effects of chromium (III)
supplementation on glucose and amino acid metabolism in rats
fed a low protein diet. J. Nutr., 97: 525-530.
ROHDE, G., TISCHENDORF, G., LEONHARDT, J., & DAMASCHUN, F.
(1978) [Study of crocoite occurrence in Callenberg (Saxony)
(Germany).] Z. angew. Geol., 24: 168-173 (in German).
ROMOSER, G.L., DUDLEY, W.A., MACHLIN, L.J., & LOVELESS, L.
(1961) Toxicity of vanadium and chromium for the growing
chick. Poultry Sci., 40: 1171-1173.
ROOK, H.L. & WOLF, W.R. (1977) The quantitative
determination of volatile trace elements in NBS biological
standard reference materials. In: Hemphill, D.D., ed.
Proceedings of the Eleventh Annual Conference on Trace
Substances in Environmental Health, Columbia, Missouri, the
Curators of the University of Missouri, pp. 324-333.
ROSCHINA, T.A. (1959) [Experimental study on the effects of
magnezite chromium and chromite magnezite dust exposure in the
production of refrectories.] Gig. Tr. prof. Zabol., 4: 28-32
(in Russian).
ROSCHINA, T.A. (1964) [A comparative evaluation of the
effects of carbide dust and chromium boride on the organism.]
Gig. i Sanit., 8: 25-28 (in Russian).
ROSCHINA, T.A. (1976) [The toxicity of chrominum (sic)
hexacarbonyl.] Gig. Tr. Prof. Zabol., 2: 38-42 (in Russian).
ROSEBROUGH, R.W. & STEELE, N.C. (1981) Effect of
supplemental dietary chromium or nicotinic acid on
carbohydrate metabolism during basal, starvation, and
refeeding periods in poults. Poultry Sci., 60: 407-417.
ROSS, D.S., SJOGREN, R.E., & BARTLETT, R.J. (1981) Behaviour
of chromium in soils. IV. Toxicity to microorganisms. J.
environ. Qual., 10: 145-148.
ROSTENBERG, A., Jr & PERKINS, A.J. (1951) Nickel and cobalt
dermatitis. J. Allerg., 22: 466-474.
RTECS (1978) Registry of toxic effects of chemical substances,
Washington DC, National Institute for Occupational Safety and
Health, pp. 450.
SAMITZ, M.H. (1955) Some dermatologic aspects of the
chromate problem. Arch. ind. Health, 11: 361-367.
SAMITZ, M.H. (1956) Studies of chromate dermatitis. Arch.
ind. Health, 14: 269-271.
SAMITZ, M.H. & EPSTEIN, E. (1962) Experimental cutaneous
chrome ulcers in guinea-pigs. Arch. environ. Health, 5:
463-468.
SAMITZ, M.H. & GROSS, S. (1960) Extraction by sweat of
chromium from chrome tanned leathers. J. occup. Med., 2: 12-14.
SAMITZ, M.H. & GROSS, S. (1961) Effects of hexavalent and
trivalent chromium compounds on the skin. Am. Med. Assoc.
Arch. Dermatol., 84: 404-409.
SAMITZ, M.H., KATZ, S., & GROSS, S. (1960) Nature of the
chromium extracted from leather by sweat. J. occup. Med., 2:
435-436.
SANDERS, C.L., JACKSON, T.A., ADEE, R.R., POWERS, G.J., &
WEHNER, A.P. (1971) Distribution of inhaled metal oxide
particles in pulmonary alveoli. Arch. intern. Med., 127:
1085-1089.
SARGENT, T., III, LIM, T.H., & JENSON, R.L. (1979) Reduced
chromium retention in patients with hemochromatosis, a
possible basis of hemochromatotic diabetes. Metabolism, 28:
70-79.
SARTO, F., LEVIS, A.G., & PAULON, C. (1980) Clastogenic
activity of hexavalent and trivalent chromium in cultured
human lymphocytes. Caryologia, 33: 239-250.
SARTO, F., COMINATO, J., BIANCHI, V., & LEVIS, A.G. (1982)
Increased incidence of chromosomal aberrations and sister
chromatid exchanges in workers exposed to chromic acid (CrO3)
in electroplating factories. Carcinogenesis, 3: 1011-1016.
SASSI, C. (1956) [Occupational disease in a chromate
factory.] Med. Lavoro, 47: 314-326 (in Italian).
SATOH, K., FUKUDA, Y., TORII, K., & KATSUNO, N. (1981)
Epidemiological study of workers engaged in the manufacture of
chromium compounds. J. occup. Med., 23: 835-838.
SAVORY, J., MUSHAK, P., & SUNDERMAN, F.W. Jr (1969) Gas
Chromatographic determination of chromium in serum. J.
chromatogr. Sci., 7: 674-679.
SCHALLER, K.H. & ZOBER, A. (1982) [Renal excretion of
toxicologically relevant metals in occupationally non-exposed
individuals.] Arztl. Lab., 28: 209-214 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1972) [Quantitative chromium determination in urine by
flameless atomic absorption spectrometry.] Z. klin. Chem.
klin. Biochem., 10: 434-437 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1973) The quantitative determination of chromium in urine by
flameless atomic absorption spectroscopy. At. Absorpt.
Newsl., 12(6): 147-150.
SCHELENZ, R. (1977) Dietary intake of 25 elements by man
estimated by neutron activation analysis. J. radioanal. Chem.,
37: 539-548.
SCHLETTWEIN-GSELL, D. & MOMMSEN-STRAUB, S. (1971) [Review
trace elements in foods. III. Chromium.] Int. J. Vitam. Nutr.
Res., 41: 116-125 and (1973) Suppl. 13, 188 pp (in German).
SCHOENTAL, R. (1975) Chromium carcinogenesis formation of
epoxyaldehydes and tanning. Br. J. Cancer, 32: 403-404.
SCHROEDER, H.A. (1966) Chromium deficiency in rats: a
syndrome simulating diabetes mellities with retarded growth.
J. Nutr., 88: 439-445.
SCHROEDER, H.A. (1970) Chromium, Washington DC, American
Petroleum Institute, 28 pp (Air Quality Monograph No. 70-15).
SCHROEDER, H.A. & BALASSA, J.J. (1965) Influence of
chromium, cadmium, and lead on rat aortic lipids and
circulating cholesterol. Am. J. Physiol., 209: 433-437.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1962)
Abnormal trace metals in man: chromium. J. chron. Dis., 15:
941-964.
SCHROEDER, H.A., VINTON, W.H., Jr, & BALASSA, J.J. (1963)
Effects of chromium, cadmium, and other trace metals on the
growth and survival of mice. J. Nutr., 80: 39-47.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1964)
Chromium, lead, cadmium, nickel, and titanium in mice: effect
on mortality, tumours, and tissue levels. J. Nutr., 83:
239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1965)
Chromium, cadmium, and lead in rats: effects on life span,
tumours, and tissue levels. J. Nutr., 86: 51-66.
SCHROEDER, H.A., NASON, A.P., & TIPTON, I.H. (1970) Chromium
deficiency as a factor in atherosclerosis. J. chron. Dis., 23:
123-142.
SCHROEDER, K.H. (1955) [Nickel eczema in a blueline (ozalid)
machine operator.] Berufsdermatosen, 3: 170-171 (in German).
SCHUBERT, G.E., GEBHARD, K., & HOENLEIN, F. (1970) [Kidney
damage following injection of serotonin and potassium
dichromate for chronic sodium overloading or restriction.]
Virchows Arch. Abt. A Path. Anat., 351: 68-82 (in German).
SCHULTHEISS, E. (1959) [Rubber and eczema,], Aulendorf i.
Württemberg, Cantor, 213 pp (in German).
SCHWARZ, K. & MERTZ, W. (1959) Chromium (III) and the
glucose tolerance factor. Arch. Biochem. Biophys., 85: 292-295.
SCHWARTZ, L. & DUNN, J.E. (1942) Dermatitis occurring among
operators of air conditioning equipment. Ind. Med. Surg., 11:
375.
SCHWARTZ, L., TULIPAN, L., & PECK, S. (1947) Occupational
diseases of the skin, 2nd ed., Philadelphia, Pennsylvania, Lea
& Febiger.
SEEBER, H., FIKENTSCHER, R., & ROSEBURG, B. (1976)
[Disorders of smell and taste in chromium dye workers.] Z.
Gesamte Hyg. Grenzgeb., 22: 820-822 (in German).
SEELEY, J.L. & SKOGERBOE, R.K. (1974) Combined sampling-
analysis method for the determination of trace elements in
atmospheric particulates. Anal. Chem., 46: 415-421.
SEELING, W., GRUENERT, A., KIENLE, K.H., OPFERKUCH, R., &
SWOBODNIK, M. (1979) [Determination of chromium in human
serum and plasma by flameless atomic absorption spectro-
photometry.] Z. anal. Chem., 299: 368-374 (in German).
SEITZ, W.R., SUYDAM, W.W., & HERCULES, D.M. (1972)
Determination of trace amounts of chromium (III) using
chemiluminescence analysis. Anal. Chem., 44(6): 957-963.
SHACKLETTE, H.T., SAUER, H.I., & MIESCH, A.T. (1970)
Geochemical environments and cardiovascular mortality rates in
Georgia, Washington DC, US Governmental Printing Office, 39 pp
(US Geological Survey Professional Paper No. 574-C).
SHAPCOTT, D., KHOURI, K., DEMERS, P.P., VOBECKY, J., &
VOBECKY, J. (1977) The measurement of volatile chromium in
biological materials. Clin. Biochem, 10: 178-180.
SHEFFET, A., THIND, J., MILLER, A.M., & LOURIA, D.B. (1982)
Cancer mortality in a pigment plant utilizing lead and zinc
chromates. Arch. environ. Health, 37: 44-52.
SHENDRIKAR, A.D. & WEST, P.A. (1974) A study of adsorption
characteristics of traces of chromium (III) and (VI) on
selected surfaces. Anal. Chim. Acta, 72: 91-96.
SHERMAN, L., GLENNON, J.A., BRECK, W.J., KLOMBERG, G.H., &
GORDON, E.S. (1968) Failure of trivalent chromium to improve
hyperglycemia in diabetes mellitus. Metabolism, 17: 439-442.
SHIMKIN, M.M. & LEITER, J. (1940) Induced pulmonary tumours
in mice. III. Role of chromic irritation in the production of
pulmonary tumours in strain A mice. J. Natl Cancer Inst., 1:
241-254.
SHMITOVA, L.A. (1978) In: [The impact of job-related factors
on specific function of the female organism,] Svedlovsk, pp.
105-108 (in Russian).
SHMITOVA, L.A. (1980) Gig. Tr. prof. zabol., 2:
33-35 (in Russian).
SIDI, E. & LONGUEVILLE, R. (1953) Dermites au ciment. Rôle
des sels de chrome. Arch. Mal. prof., 14: 41.
SIEGENTHALER, U., LAINE, A., POLAK, L. (1983) Studies on
contact sensitivity to chromium in the guinea-pig. The role
of valence in the formation of the antigenic determinant. J.
Invest. Dermatol., 80: 44-47.
SIMONOFF, M., LLABADOR, Y. HAMON, C., PEERS; A.M., & SIMONOFF,
G.N. (1984) Low plasma chromium in patients with coronary
artery and heart diseases. Biol. Trace Element Res., 6:
431-439.
SIMONDS, J.P. & HEPLER, O.E. (1945) Experimental nephro-
pathies. I. A method of producing controlled selective injury
of renal units by means of chemical agents. Arch. Path., 39:
103-108.
SIROVER, M.A. & LOEB, L.A. (1976) Infidelity of DNA
synthesis in vitro: screening for potential metal mutagens or
carcinogens. Science, 194: 1434-1436.
SJOGREN, B. (1980) A retrospective cohort study of mortality
among stainless steel welders. Scand. J. Work. environ.
Health, 6: 197-200.
SKOGERBOE, R.K. (1974) Monitoring trace metal particulates:
an evaluation of the sampling and analysis problems. In:
Instrumentation for monitoring air quality, Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp.
125-136.
SLUIS-CREMER, G.K. & DU TOIT, R.S. (1968) Pneumoconiosis in
chromite miners in South Africa. Br. J. ind. Med., 25: 63-67.
SPIER, H.W. (1956) [Wood preservatives and chromate eczema.]
Desinfekt. Gesundheitswes., 4: 58 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil ( Myriophyllum spicatum L.). Arch.
environ. Contam. Toxicol., 2: 331-341.
STAUB, H.W., REUSSNER, G., & THIESSEN, R., Jr (1969) Serum
cholesterol reduction by chromium in hypercholesteremic rats.
Science, 166: 746-747.
STEELE, N.C. & ROSEBROUGH, R.W. (1979) Trivalent chromium
and nicotinic acid supplementation for the turkey poult.
Poultry Sci., 58: 983-984.
STEELE, N.G., ALTHEN, T.G., & FROBISH, L.T. (1977a) Bio-
logical activity of glucose tolerance factor in swine. J.
anim. Sci., 45: 1341-1345.
STEELE, N.C., PELURA, I., & FROBISH, L.T. (1977b) Effect of
dietary protein source on glucose utilization and hepatic
insulin-receptor characteristics of the rat. Nutr. Rep. Int.,
15: 429-435.
STELLA, M., MENTALDI, A., ROSSI, R., ROSSI, G., & LEVIS, A.G.
(1982) Clastogenic effects of chromium on human lymphocytes
in vitro and in vivo. Mutat. Res., 101: 151-164.
STEFFEE, C.H. & BAETJER, A.M. (1965) Histopathologic effects
of chromate chemicals. Report of studies in rabbits, guinea-
pigs, rats, and mice. Arch. environ. Health, 11: 66-75.
STEINHOFF, D., GAD, S.C., HATFIELD, G.K., & MOHR, U. (1983)
Testing sodium dichromate and soluble calcium chromate for
carcinogenicity in rats. Report to the Industrial Health
Foundation, Wuppertal, Bayer AG Institute of Toxicology,
126 pp.
STEREKHOVA, N.P., ZELENEVA, N.I., SOLOMINA, S.N.,
TYUSHNYAKOVA, N.V., MYASNIKOVA, A.G., FOKINA, G.P., & YARINA,
A.L. (1978) [Gastric pathology in workers engaged in the
production of chromium salts.] Gig. Tr. prof. Zabol., 3: 19-23
(in Russian).
STERN, R.M. (1981) Process-dependent risk of delayed health
effects for welders. Environ. Health Perspect., 41: 235-253.
STERN, R.M. (1982) Chromium compounds: production and
occupational exposure. In: Langard, S., ed. Biological and
environmental aspects of chromium, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 5-47.
STRIK, J.J.T.W.A., DE IONGH, H.H., VAN RIJN VAN ALKEMADE,
J.W.A., & WIUTE, T.P. (1975) Toxicity of chromium (VI) in
fish, with special reference to organoweights, liver and
plasma enzyme activities, blood parameters, and histological
alterations. In: Koeman, S.H. & Strik, J.J.T.W.A., ed.
Sublethal effects of toxic chemicals on aquatic animals,
Amsterdam, New York, Oxford, Elsevier Science Publishers,
pp. 31-41.
STROUT, R.W. (1977) The relationship of abnormal circulating
insulin levels to atherosclerosis. Atherosclerosis, 27: 1-13.
SULLIVAN, R.J. (1969) Air pollution aspects of chromium and
its compounds, Raleigh, North Carolina, US Department of
Health, Education and Welfare, 75 pp (National Air Pollution
Control Administration Publication No. APTD 69-34).
SUSA, N., FURUKAWA, YO., & TSUBAKI, S. (1977) Comparative
cytotoxic effects of hexavalent and trivalent chromium
compounds on cultured cells. Jap. J. vet. Sci., 39: 325-336.
SUZUKI, K., ESASHI, T., SUZUE, R., NISHIMURA, H., & MATSUMOTO,
H. (1979) Tissue concentrations of cadmium, lead, and
chromium in Japanese ranging from the fetus to elder subjects.
Toxicol. Lett., 4: 481-484.
SWAINE, D.J. & MITCHELL, R.L. (1960) Trace element
distribution in soil profiles. J. soil Sci., 11 (2): 347-368.
SWENSSON, A. (1977) [Experimental research on the
fibrogenetic effect of chromite.] Arbete och Haelsa, 2: 1-14
(in Swedish).
TABOR, E.C. & WARREN, W.V. (1958) Distribution of certain
metals in the atmosphere of some American cities. Arch. ind.
Hyg., 17: 145-151.
TAMARO, M., BANFI, E., VENTURINI, S., & MONTI-BRAGADIN, C.
(1975) [Hexavalent chromium compounds are mutagens for
bacteria.] In: Proceedings of the XVII Congress Naz. Soc. It.
Microbiol., Padova, pp. 411-415 (in Italian).
TANDON, S.K., SAXENA, D.K., GAUR, J.S., & CHANDRA, S.W.
(1978) Comparative toxicity of trivalent and hexavalent
chromium. Environ. Res., 15: 90-99.
TANDON, S.K., BEHARI, J.R., & KACHRU, D.N. (1979) Distri-
bution of chromium in poisoned rats. Toxicology, 13: 29-34.
TANDON, S.K., MATHUR, A.K., & GAUR, J.S. (1977) Urinary
excretion of chromium and nickel among electroplaters and
pigment industry workers. Int. Arch. occup. environ. Health,
40: 71-76.
TATARU, C., MARINSECU, A., CAPUSAN, I., RIPAN, R., & LITEANU,
C. (1957) [Skin damage during the industrial recovery of
potassium dichromate.] Berufsdermatosen, 5: 283-295 (in
German).
TAYLOR, A.E., GUYTON, M.O., & BISHOP, V.S. (1965)
Permeability of the alveolar membrane to solutes. Circ. Res.,
16: 353-362.
TAYLOR, F.G., Jr, MANN, L.K., DAHLMANN, R.C., & MILLER, F.L.
(1975) Environmental effects of chromium and zinc in
cooling-water drift. In: Cooling tower environment, 1974,
Washington DC, US Energy Research and Development
Administration, pp. 408-426 (Report Conf. No. 740302).
TAYLOR, F.H. (1966) The relationship of mortality and
duration of employment as reflected by a cohort of chromate
workers. Am. J. public Health, 56: 218-229.
TAZELAAR, D.J. (1970) Hypersensitivity to chromium in a
light-blue Tattoo. Dermatologica (Basel), 141: 282-287.
THOMSON, A.G. (1982) Chromite, Mining Annual Review,
pp. 70-71.
THORMANN, J., JESPERSEN, N.B., & JOENSEN, H.D. (1979)
Persistence of contact allergy to chromium. Contact Dermatit.,
5: 261-264.
TOEPFER, E.W., MERTZ, W., ROGINSKI, E.E., & POLANSKY, M.M.
(1973) Chromium in foods in relation to biological activity.
J. agric. food Chem., 21: 69-73.
TOEPFER, E.W., MERTZ, W., POLANSKY, M.M., ROGINSKI, E.E., &
WOLF, W.R. (1977) Preparation of chromium-containing
material of glucose tolerance factor activity from brewer's
yeast extracts and by synthesis. J. agric. food Chem., 25:
162-166.
TOLA, S., KILPIO, J., VIRTAMO, M., & HAAPA, K. (1977)
Urinary chromium as an indicator of the exposure of welders to
chromium. Scand. J. Work environ. Health, 3: 192-202.
TOLG, G. (1974) Recent problems and limitations in the
analytical characterization of high-purity material. Talanta,
21: 327-345.
TSUDA, H. & KATO, K. (1976) Potassium dichromate: induced
chromosome aberrations and its control with sodium sulfite in
hamster embryonic cells in vitro. Gann, 67: 469-470.
TSUDA, H. & KATO, K. (1977) Chromosomal aberrations and
morphological transformation in hamster embryonic cells
treated with potassium dichromate in vitro. Mutat. Res., 46:
87-94.
TUMAN, R.W. & DOISY, R.J. (1977) Metabolic effects of the
glucose tolerance factor (GTF) in normal and genetically
diabetic mice. Diabetes, 26: 820-826.
TUMAN, R.W., BILBO, J.T., & DOISY, R.J. (1978) Comparison
and effects of natural and synthetic glucose tolerance factor
in normal and genetically diabetic mice. Diabetes, 27: 49-56.
TURNER, M.A. & RUST, R.H. (1971) Effects of chromium on
growth and mineral nutrition of soybeans. Soil Sci. Soc. Am.
Proc., 35: 755-758.
TYUSHNYAKOVA, N.V., ZISLIN, D.M., SHABYNINA, N.K., & BELJAEVA,
L.N. (1974) [Criteria of occupational cancer in the
production of chromium salts and some ideas concerning its
pathogenesis.] In: Occupational cancer (collection of
scientific works), Sverdlovsk, pp. 56-63 (in Russian).
UMEDA, M. & NISHIMURA, M. (1979) Inducibility of chromosomal
aberrations by metal compounds in cultured mammalian cells.
Mutat. Res., 67: 221-229.
URCHS, O. (1953) [Occupational skin diseases,] Cantor,
Aulendorf i. Württemberg, 108 pp.
US DHEW (1975) Criteria for a recommended standard.
Occupational exposure to chromium (VI), Washington DC, US
Department of Health, Education and Welfare, 200 pp (US DHEW
Publication No. NIOSH 76-129).
US DHEW (1977) Occupational diseases: a guide to their
recognition, revised ed., Washington DC, US Department of
Health, Education and Welfare, pp. 352-355 (Stock No.
017-033-00266-5).
US EPA (1978) Reviews of the environmental effects of
pollutants. III. Chromium, Washington DC, US Environmental
Protection Agency, 285 pp (ORNL/EIS-80; EPA 600/1-78-023).
US EPA (1980) Ambient water quality criteria for chromium,
Washington DC, US Environmental Protection Agency, pp. A1-C48.
US NAS (1974a) Chromium, Washington DC, US National Academy
of Sciences, 155 pp.
US NAS (1974b) Geochemistry and the environment. I. The
relation of selected trace elements to health and disease,
Washington DC, US National Academy of Sciences, 113 pp.
US NAS/NAE (1972) Water quality criteria 1972, Washington
DC, US Environmental Protection Agency, US National Academy of
Sciences, US National Academy of Engineering, 533 pp.
US PHS (1953) Health of workers in chromate producing
industry, Washington DC, US Department of Health, Education
and Welfare, US Public Health Service, 131 pp (Publication No.
192).
VAKHRUSHEVA, V.A. (1960) [Effect of Mn and Cr as trace
elements on the level of blood sugar in white mice.] Tr.
Izhevskogo Otd. Fiziol. Obschchestva, 1: 184-190 (in Russian).
VALER, M. & RACZ, J. (1971) Investigations concerning the
sensitizing effect of trivalent chromium salts. I. Correlation
between concentration and skin penetration of trivalent
chromium and between the number of positive results obtained
in patch tests. J. ind. Med., 41(12): 302-317.
VEILLON, C., WOLF, W.R., GUTHRIE, B.E. (1979) Determination
of chromium in biological materials by stable isotope
dilution. Anal. Chem., 51: 1022-1024.
VELICHKOVSKY, B.T. & POKROVSKAJA, L.V. (1973) [Labour
hygiene in a modern production of multy-tonnage ferrolloys.]
In: [Proceedings of the National Symposium on Labor Hygiene
and Prophylaxis of Occupational Diseases in the Production of
Ferroalloys,] Sverdlovsk, pp. 3-8 (in Russian).
VENITT, S. & LEVY, L.S. (1974) Mutagenicity of chromates in
bacteria and its relevance to chromate carcinogenesis. Nature
(Lond.), 250: 493-494.
VENCGUJIV, A.J. (1974) Zones of display of biological and
pharmacological action of trace elements. In: Hoekstra, W.G.,
Gantherl, H.E., & Mertz, W., ed. Trace elements metabolism in
animals. II, Baltimore, London, Tokyo, University Park Press,
pp. 295-310.
VERFAILLIE, G.R.M. (1974) Kinetics of chromium absorption by
intact rice plants. In: Comparative studies of food and
environmental contamination, Vienna, International Atomic
Energy Agency, pp. 315-331.
VERSIECK, J.M.J. & SPEECKE, A.B.H. (1972) Contaminations
induced by collection of liver biopsies and human blood. In:
Nuclear Activation Techniques in the Life Sciences.
Proceedings of a Symposium, Bled, Yugoslavia, Vienna
International Atomic Energy Agency, pp. 39-49.
VERSIECK, I., HOSTE, J., BARBIER, F., STEYAERT, H., DERUDDER,
I., & MICHELS, H. (1978) Determination of chromium and
cobalt in human serum by neutron activation analysis. Clin.
Chem., 24: 303-308.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experience of the
occupational health department with some industrial threshold
limit values for industrial poisons.] Arch. Gewerbepath.
Gewerbehyg., 13: 528-534 (in German).
VISEK, W.J., WHITNEY, I.B., KUHN, U.S.G., & COMAR, C.L.
(1953) Metabolism of 51Cr by animals as influenced by
chemical state. Proc. Soc. Exp. Biol. Med., 84: 610-615.
VOELKL, A. (1971) [The daily excretion of chromium in normal
persons.] Zentralbl. Arbeitsmed., 21: 122 (in German).
VOTAVA, H.J., HAHN, C.J., & EVAN, G.W. (1973) Isolation and
partial purification of a 51Cr complex from brewer's yeast.
Biochem. Biophys. Res. Comm., 55: 312-319.
WACKER, W.E.C. & VALLEE, B.L. (1959) Nucleic acids and
metals. I. Chromium mangenese, nickel, iron, and other metals
in ribonucleic acid from diverse biological sources. J. biol.
Chem., 234: 3257-3262.
WAHBA, A. & COHEN, T. (1979) Chrome sensitivity in Israel.
Contact Dermatit., 5: 101-107.
WAHLBERG, J.E. & WENNERSTEN, G. (1977) Light sensitivity and
chromium dermatitis. Br. J. Dermatol., 97: 411-416.
WALSH, E.N. (1953) Chromate hazards in industry. J. Am. Med.
Assoc., 153: 1305-1308.
WARREN, G., SCHULTZ, P., BANCROFT, D., BENNETT, K., ABBOTT,
E.H., & ROGERS, S. (1981) Mutagenicity of a series of
hexacoordinate chromium (III) compounds. Mutat. Res., 90:
111-118.
WATANABE, S. & FUKUCHI, Y. (1975) An epidemiological survey
on lung cancer in workers of a chromate-producing industry in
Hokkaido, Japan (Presented at: The XVIII International Congress
on Occupational Health, Brighton, England, 14-19 September.
WEILER, R.R. & CHAWLA, V.K. (1969) Dissolved mineral and
quality of Great Lakes waters. In: Proceedings of the 12th
Conference on Great Lakes Res., 801.
WELZ, B. (1980) [Use of AAS and ICP. Relative advantage of
each method.] Chem. Techn., 9: 161-170. (in German).
WELZ, B. (1983) [Atomic absorption spectrometry,] 3rd ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
527 pp (in German).
WENDELBERGER, J. & WEITGASSER, H. (1956) [The role of alkali
and degreasing agents in the development of contact eczemas in
electroplasters.] Berufsdermatosen, 4: 16-21 (in German).
WHANGER, P.D. & WESWIG, P.H. (1975) Effects of selenium,
chromium, antioxidants on growth, eye cataracts, plasma
cholesterol, and blood glucose in selenium-deficient, vitamin
E-supplemented rats. Nutr. Rep. Int., 12: 345-348.
WHITE, L.R., JAKOBSEN, K., & 0STGAAD, K. (1979) Comparative
toxicity studies of chromium rich welding fumes and chromium
on an established human cell line. Environ. Res., 20: 366-374.
WHITING, R.F., STICH, H.F., & KOROPATNICK, D.J. (1979) DNA
damage and DNA repair in cultured human cells exposed to
chromate. Chem.-biol. Interact., 26: 267-280.
WHO (1983) EHC 27: Guidelines on studies in environmental
e pidemiology, Geneva, World Health Organization, 351 pp.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984a)
Disposition of intratracheally administered chromium (III) and
chromium (VI) in rabbits. Toxicol. Lett., 22: 273-276.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984b) The
reduction of chromium (VI) to chromium (III) by glutathione:
an intracellular redox pathway in the metabolism of the
carcinogen chromate. Toxicology, 33: 341-348.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1985)
[Determination of chromium in human red blood cells. Basis for
a concept of biological monitoring.] Arbeitsmed. Sozialmed.
Praeventivmed., 20: 1-4 (in German).
WILD, D. (1978) Cytogenetic effects in the mouse of 17
chemical mutagens and carcinogens evaluated by the micronuclei
test. Mutat. Res., 56: 319-327.
WILLIAMS, C.D. (1969) Asthma related to chromium compounds.
Report of two cases and review of the literature on chromate
diseases. NC med. J., 30: 482-491.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1960) The Merck index. An encyclopedia of chemicals and
drugs, 7th ed., Rahway, New Jersey, Merck and Co., 139 pp.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) The Merck index. An encyclopedia of chemicals and
drugs, 9th ed., Rahway, New Jersey, Merck and Co., 1313 pp.
WINSTON, J.R. & WALSH, E.N. (1951) Chromate dermatitis in
railroad employees working with diesel locomotives. J. Am.
Med. Assoc., 147: 1133-1134.
WITHEY, J. (1983) Memorandum addressed to Si Duk Lee, dated
March 4, 1983, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office.
WOLF, W.R. (1976) Coupled gas chromatography/atomic
absorption spectrometry for the nanogram determination of
chromium. Anal. Chem., 48: 1717-1720.
WOLF, W.R. & GREENE, F.E. (1976) Preparation of biological
materials for chromium analysis. In: Accuracy in trace
analysis: sampling, sample handling, and analysis,
Gaithersburg, Maryland, National Bureau of Standards, pp.
605-610 (Special Publication No. 422).
WOLF, W.R., MERTZ, W., & MASIRONI, R. (1974) Determination
of chromium in refined and unrefined sugars by oxygen plasma
ashing flameless atomic absorption. J. agric. food Chem., 22:
1037-1042.
WOLF, W.R., TAYLOR, M.L., HUGHES, B.M., TIERNAN, T.O., &
SIEVERS, R.E. (1972) Determination of chromium and beryllium
at the picogram level by gas chromatography/mass spectrometry.
Anal. Chem., 44: 616-618.
WU, G.-Y. (1981) [The research on the purification and the
chemical structure of the specific chromium binding
materials.] Jpn. J. Ind. Health, 33: 505 (in Chinese).
YAGI, T. & NISHIOKA, H. (1977) DNA damage and its degrada-
tion by metal compounds. Sci. Eng. Rev. Doshisha Univ., 18:
63-70.
YANG, G.L. (1956) [An investigation of occupational disease
among chromium plating workers.] Acta Acad. Med. primae
Shanghai, 2: 36-46 (in Chinese).
YOPP, J.H., SCHMID, W.E., & HOLST, R.W. (1974) Determination
of maximum permissible levels of selected chemicals that exert
toxic effects on plants of economic importance in Illinois,
Chicago, Illinois, Institute for Environmental Quality,
pp. 89-96.
YUNISOVA, H.K. & PAVLOVSKAJA, G.S. (1975) The effect of
regimen of chrome plating on the properties of chromine.
Protect. Metal., 2, 248-250.
ZANDER, A.T., O'HAVER, T.C., & KELIHER, P.N. (1977)
Correction for interferences of spectral origin with continuum
source, echelle wavelength modulated atomic absorption
spectrometry. Anal. Chem., 49: 838-842.
ZHURKOV, U.S. (1981) [Approaches to the regulation of
environmental chemical pollutants with mutagenic activity.]
In: Sidorenko, G.I.M., ed. [Medical problems of environmental
protection,] Moscow, USSR Academy of Medical Sciences,
pp. 88-95 (in Russian).
ZOBER, A. (1979) [On the problems of evaluating bronchial
carcinoma after exposition to chromium compounds.] Int. Arch.
occup. environ. Health, 43(2): 107-121 (in German).
ZOBER, A. (1982) [Possible dangers to the respiratory tract
from welding fumer: methods of approach in an industrial
health care context and results.] Schweissen & Schneiden,
34(2): 77-81 (in German).
ZOBER, A., KICK, K., SCHALLER, K.H., SCHELLMANN, B., &
VALENTIN, H. (1984) ["Normal values" of chromium and nickel
in human lung-, kidney-, blood-, and urine samples.] Zbl.
Bakt. Hyg. I. Abt. Orig. B, 179: 80-95 (in German).
1969). For these reasons, chromium must be considered an essential
micro-nutrient. It is physiologically active in the trivalent
oxidation state at concentrations of approximately 100 µg/kg diet.
7.1.1. Effects of deficiency on glucose metabolism
Semipurified rations, containing Torula yeast as the source of
protein, were fed to groups of 10 male Sprague Dawley rats at
weaning, and intravenous glucose tolerance tests were performed by
injecting 1250 mg glucose/kg body weight and measuring the
subsequent decline in blood-glucose levels. The rate of glucose
disappearance can be calculated from the straight line plot of log
increment glucose (excess of glucose at any time (t) over fasting
glucose), versus time. The disappearance constant k is expressed
as % decline of the increment glucose per min; it is a measure of
the efficiency of glucose utilization. In weaning rats, the rate
constant was found to decline, within 3 weeks or less, from an
average of 4%/min to 2.6%/min in animals fed the Torula yeast diet,
but not in animals administered a diet in which 4 or 8% of Torula
yeast was replaced by an equal amount of brewer's yeast. This
observation suggested that brewer's yeast, but not Torula yeast,
contained an unknown substance necessary for the maintenance of
normal glucose tolerance. Because the only known effect of the
substance was that on glucose tolerance, it was named "Glucose
Tolerance Factor" (GTF) (Mertz & Schwarz, 1959). GTF was extracted
from brewer's yeast and pork kidney powder, concentrated and
purified, and its active ingredient was identified as trivalent
chromium (Schwarz & Mertz, 1959). Chromium in the form of most
common complexes (except for very stable ones) cured the impairment
of glucose tolerance in deficient rats, either as one oral dose of
200 µg/kg body weight, or as an intravenous (iv) injection of 2.5
µg/kg body weight. Chromium in the diet also prevented the
impairment of glucose tolerance.
In order to produce more pronounced deficiencies of chromium
and other trace elements, Schroeder et al. (1963) constructed a
special animal house on a mountain top in Vermont, USA, far removed
from traffic and industry. The interior was specially coated with
organic resins to reduce any metallic exposure. Air was introduced
through special filters, and strict practices were enforced to
avoid the introduction of dust and dirt. This will be referred to
as the "controlled environment". Under these conditions, a more
severe chromium deficiency resulted in very low glucose removal
rates of 1.12%/min in 6 rats and of 0.18%/min in 4 female breeder
rats (482 days old), after 11 days on a chromium-deficient diet.
The removal rate in 4 control rats receiving the same diet with a
chromium supplement improved from 0.51 to 1.39% during the same
period (Mertz et al., 1965b). This strong impairment of glucose
tolerance in rats kept in the "controlled environment" was
reflected in their fasting blood-glucose levels, compared with
those of chromium-supplemented controls: 1370 ± 68 versus 1170 ± 17
mg/litre in males and 1390 ± 68 versus 960 ± 65 mg/litre in
females. More than half of 185 deficient rats excreted more than
0.25% glucose in the urine, whereas glycosuria was found in only 9
of the chromium-supplemented controls (Schroeder, 1966). A
significant reduction in the intravenous glucose removal rate was
also observed in 8 rats in plastic cages, fed an EDTA-washed low-
protein diet (7% casein), compared with 8 controls receiving 50 µg
chromium as the chloride, by stomach tube, daily for 2 weeks (2.1
versus 3.6%/min; P < 0.01). However, chromium supplements did not
improve the near-normal removal rates in rats receiving a 20%
casein diet (Mickail et al., 1976). Significantly lower plasma-
glucose levels, due to supplementation with chromium, were reported
in rats fed the chromium-deficient Torula yeast diet (Whanger &
Weswig, 1975). In another series of studies, only a slight
reduction in blood-glucose (from 1240 to 1180 µg/litre) was found
in 10 rats receiving a diet supplemented with chromium (10 µg/kg)
(Djahanshiri, 1976).
Impaired glucose tolerance in squirrel monkeys, fed a
commercial laboratory chow, was shown to respond to chromium
supplementation. Of 9 monkeys with an impaired glucose removal
rate (1.38%/min), 8 responded after 22 weeks of supplementation of
their drinking-water (chromium acetate, 10 mg/litre) with a
normalization of their glucose tolerance (average removal rate, N =
9, 2.33 ± 0.3%/min) (Davidson & Blackwell, 1968). There was no
effect on food consumption, growth rate, or serum-insulin
concentrations. The chromium content of the commercial chow was
stated to be 3.3 mg/kg, a very high concentration. In view of the
uncertainties of methods of analysis for chromium (section 2.2), it
is not possible to interpret the results as indicative of a very
high chromium requirement of the squirrel monkey or of an unusually
poor bioavailability of the chromium in that particular ration. A
marginal chromium deficiency may have existed in mice fed a bread
and milk diet, as daily administration of 10 µg chromium for 16
days to 8 months produced a 10 - 30% decline in blood-glucose
levels (Vakhrusheva, 1960). However, this effect was not specific
for chromium, as it was also observed when manganese was
administered.
The glucose tolerance of guinea-pigs did not differ
significantly between groups fed diets containing chromium at
0.125, 0.625, or 50 mg/kg, even though the animals fed the 2 higher
levels exhibited a lower mortality rate during pregnancy (Preston
et al., 1976).
Although the administration of synthetic glucose tolerance
factor (a chromium-dinicotinic acid-glutathione complex) to 6 pigs
did not affect glucose tolerance tests, it resulted in a
significant increase in the hypoglycaemic effect of insulin
injected at 0.1 U/kg body weight (Steele et al., 1977a).
Turkey poults, fed a practical ration containing chromium
levels of 5 mg/kg, responded to chromium supplementation (20 mg/kg
diet) with a significant increase in liver glycogen and in glycogen
formation, following a fast, and with a significant increase in
glycogen synthetase (EC 2.4.1.11) activity in the liver (Rosebrough
& Steele, 1981).
It can be concluded that the impairment of glucose tolerance in
rats fed a low-chromium Torula yeast diet is due to chromium
deficiency. The effects of chromium in squirrel monkeys, pigs, and
turkeys, though statistically significant, are somewhat difficult
to interpret, because of the reported high chromium content of the
basal diet. No evidence for chromium deficiency has yet been
obtained through glucose tolerance tests on other animal species.
7.1.2. Effects of deficiency on lipid metabolism
Though trivalent chromium in high doses (2.5 mg/kg body weight)
has been shown to increase the synthesis of fatty acids and
cholesterol in the liver (Curran, 1954), lower, physiological doses
appear to decrease serum-cholesterol concentrations in rats.
Schroeder & Balassa (1965) found an average level of 927 mg
cholesterol/litre serum in 24- to 26-month-old male rats, kept in a
controlled environment and administered chromium in the drinking-
water at a concentration of 5 mg/litre, compared with an average of
1229 mg/litre in controls not receiving chromium ( P < 0.01). The
effects in female rats were ambigous, one study producing the
expected reduction in cholesterol due to chromium, another showing
an elevation in the supplemented female rats. Schroeder's
observations of a cholesterol-reducing effect of chromium in male
rats were confirmed by Staub et al. (1969) and by Whanger & Weswig
(1975) but were contradicted by results of a third study in which
there were not any significant effects of chromium on sucrose-
induced triglyceridaemia and cholesterolaemia (Bruckdorfer et al.,
1971). Perhaps more significant than the effect on circulating
cholesterol is the direct effect of chromium on the occurrence of
aortic plaques. Schroeder & Balassa (1965) observed 6 plaques in 54
male and female chromium-deficient rats, but only one plaque in 48
animals receiving 5 mg chromium/litre in drinking-water. These
results are in agreement with those from a subsequent report of the
protective effect of the natural chromium content of water (60 -
215 µg/litre) against atherosclerosis in cholesterol-fed rabbits
(Novakova et al., 1974). Abraham et al. (1980) extended these
observations by demonstrating that daily chromium injections (20 µg
K2CrO4) reversed the established atherosclerosis in the aorta of 11
cholesterol-fed rabbits, compared with 12 controls. The mean
plaque area was reduced from 95% to 63%, the total aortic
cholesterol from 729 mg to 458 mg, and the atheromatous lesions, as
measured by technetium incorporation from 285 000 cpm to 114 000
cpm, all differences being statistically significant. These
results are reinforced by observations in man discussed in section
8.1.
7.1.3. Effects of deficiency on life span, growth, and
reproduction
The mortality of male, but not of female mice, raised in a
"controlled environment" (section 7.1.1) was reduced by trivalent
chromium administered as acetate in the drinking-water at a
concentration of 5 mg/litre (Schroeder et al., 1964). The survival
rates at 12 months were 92.6% and 68.8% ( P < 0.0001) in
supplemented and deficient animals, respectively. Similary, male,
but not female, rats receiving a chromium concentration of 5
mg/litre in the drinking-water had longer life spans than deficient
controls. The mean age of the last surviving 10% of animals was
1249 days, compared with 1141 days in the deficient animals ( P <
0.01). Survival of male rats fed a low-chromium (< 100 µg/kg),
low-protein ration and subjected to a controlled acute haemorrhage
was significantly less than that of chromium-supplemented rats, in
2 studies (67 versus 92%; P < 0.05 and 27 versus 60%; P < 0.01,
respectively) (Mertz & Roginski, 1969).
In Schroeder's study, growth rates in treated mice and rats of
both sexes raised in a "controlled environment", were higher after
6 and 12 months, with highly significant differences in body weight
ranging from 9 to 17% ( P < 0.005) compared with the controls.
Again, the effects of chromium supplementation were greater in
males than in females (Schroeder et al., 1964, 1965).
Similar results were reported by Djahanschiri (1976), who
studied a total of 2750 rats of a special inbred strain (Hk51) fed
a basal diet (0.15 mg chromium/kg diet) and chromium supplements
ranging from 10 to 500 mg/kg diet. At 12 weeks, the average
weights of the chromium-supplemented animals, regardless of dose
level, were significantly higher (by 6% in the males and 3% in the
females) than those of the animals on the basal diet. The same
author reported a progressive diminution in both the milk
production of lactating rats and weight gain in 3 consecutive
generations fed the low-chromium diet, compared with rats receiving
chromium supplementation. Increased mortality was reported in
pregnant guinea-pigs fed a low-chromium diet (125 µg/kg diet)
compared with animals receiving a chromium supplement of either 625
µg/kg or 50 mg/kg (Preston et al., 1976).
When rats raised on a low-chromium Torula yeast diet (< 100
g/kg) mated with those on a normal diet, they were able to
impregnate the females at a 100% conception rate only up to the age
of 4 months. After this age, the conception rate declined to 25%,
25%, and 0%, at the age of 7, 8, and 9 months, respectively. This
decline was accompanied by a significant ( P < 0.01) decrease in
the sperm count in the chromium-deficient males to approximately
half of the count in supplemented controls at the age of 8 months
(Anderson & Polansky, 1981).
7.1.4. Other effects of deficiency
Male weanling rats, fed a 10% soya protein ration with a
chromium content of less than 100 µg/kg, developed a visible
opacity of the cornea in one or both eyes. In several studies, the
incidence of this effect ranged from 10 to 15% in deficient rats.
No opacities developed in control animals receiving 2 mg
chromium/kg diet (Roginski & Mertz, 1967).
Chromium deficiency has been shown to reduce the physical
performance of rats under stress. Ten male rats raised on a
chromium-deficient diet (150 µg/kg diet) swam for an average of 250
min, until exhaustion, in contrast with 10 rats receiving a
supplement of 10 mg chromium/kg diet, which were exhausted only
after 320 min (Djahanschiri, 1976).
7.1.5. Mechanism of action of chromium as an essential nutrient
7.1.5.1. Enzymes, nucleic acids, and thyroid
Chromium is present in nucleic acids in very high
concentrations, but the function of these is not clear at present
(Mertz, 1969). However, recent work suggests a biological function
of chromium in nucleic acid metabolism (Okada et al., 1984).
Ribonucleic acid synthesis in mouse liver was significantly
increased by as little as 1 µmol trivalent chromium, in the
presence of DNA or chromatin (Okada et al., 1981). These effects
were also present when the DNA or chromatin were first complexed
with chromium prior to incubation. However, prior complexation of
RNA polymerase with chromium depressed activity. These effects
were obtained in vitro with a concentration (52 µg/litre) that is
similar to physiological levels. Goncharov (1968) presented data
suggesting that chromium is involved in the function of the thyroid
gland. These findings have been supported by Lifschitz et al.
(1980).
An oligopeptide with a relative molecular mass of 1480, which
was crystallized from liver tissue, had a specific affinity for
chromium (Wu, 1981).
7.1.5.2. Interaction of chromium with insulin
The interaction of chromium with insulin has been extensively
studied and can therefore be presented in some detail, but this
does not imply that this is the only, or the most important,
function of chromium.
The effects of chromium in vitro, and probably in vivo, depend
on the presence of endogenous or exogenous insulin, no effects
having been demonstrated in in vitro systems that did not either
depend on, or contain, insulin. Chromium deficiency causes an
impaired response to added insulin in rat epididymal fat tissue,
and, when glucose uptake or glucose oxidation or utilization for
lipid synthesis is measured, the dose-effect curve is flat.
Addition of suitable chromium compounds significantly increases the
slope of the curve (Fig. 3). This demonstrates the true
potentiation of the insulin action and indicates that chromium
alone does not act as an insulin-like substance (Mertz et al.,
1961; Mertz & Roginski, 1971; Mertz, 1981). Chromium was also
shown to stimulate the transport of D-galactose into epididymal fat
cells. This suggests cell transport, the first step of sugar
metabolism, as a major site of action for chromium (Mertz &
Roginski, 1963). Insulin-potentiating effects have also been
observed on the swelling of liver mitochondria (Campbell & Mertz,
1963) and on glucose utilization in isolated rat lens (Farkas &
Roberson, 1965).
Fig. 3
Stimulation of the effects of insulin has been observed in a
glucose-independent, but insulin-responsive, system. Significantly
more alpha-amino isobutyric acid (a non-metabolizable amino acid
analogue) was incorporated into the heart and liver tissue of
chromium-supplemented male rats than in the tissues of chromium-
deficient controls, in response to the in vivo injection of the
labelled analogue and insulin (Roginski & Mertz, 1969).
These observations suggest a peripheral action of chromium to
facilitate the action of insulin; no evidence has been produced
indicating that chromium plays any role in the production, storage,
or release of insulin by the pancreas. Thus, the primary result of
chromium deficiency is a diminution in the effectiveness of
insulin. The resulting metabolic impairment may be compensated for
by increased insulin production in some cases, resulting in
elevated concentrations of the hormone, but not enough data exist
from experimental animal studies to assess the action of the
element on insulin metabolism. More information is available for
human subjects and this is discussed in section 8.1. The
interaction between chromium, insulin, and receptor sites of liver
mitochondrial membranes was studied using polarographic techniques.
The results formed the basis for the hypothesis that chromium may
facilitate bond formation between the intra-chain disulfide of
insulin and sulfur-containing groups of the receptors, by
participating in a ternary complex (Christian et al., 1963).
This hypothesis is consistent with results of studies on rats
fed, either a low-chromium Torula yeast diet or a brewer's yeast
diet known to be adequate in chromium. While the insulin-binding
capacity of hepatocytes was not significantly different, the
insulin affinity of the cells was significantly greater ( P < 0.01)
for the chromium-adequate rats than for the deficient Torula yeast
rats (Steele et al., 1977b).
7.1.6. Chromium nutritional requirements of animals
In the preceding sections, studies were evaluated in which the
effects of chromium supplementation were determined in animals that
were at least marginally chromium deficient. In other studies, the
effects of chromium were investigated in animal systems in which
the existence of chromium deficiency was either not ascertained or
not investigated. Before these studies are described and
interpreted for the determination of chromium nutritional
requirements of animals, it is helpful to consider them against the
background of Venchikov's (1974) model. This model is generally
applicable to trace element effects defining 3 zones of action, the
zone of biological action, that of pharmacodynamic action, and that
of toxicity (Fig. 4). The biological zone, in response to
supplements with low amounts of an element, represents the
correction of a deficiency and the resulting level of biological
activity is that of optimal function. Increasing the amount of
supplement further may lead to a certain depression, followed by a
zone of new, increased activity, in which the element no longer
acts as an essential nutrient, but as a drug. Still greater
supplements, beyond the homeostatic control capability of the
organism produce toxic effects and death. Because all the studies
described subsequently involved amounts of chromium supplements
that were higher than the levels normally needed to correct a
deficiency, it is possible, according to Venchikov's definition,
that the observed effects might be pharmacological.
Fig. 4
Tuman & Doisy (1977) studied the effects of yeast concentrates
of high chromium (glucose tolerance factor) content and of
synthetic chromium complexes with GTF activity (Tuman et al., 1978)
in mice, raised on a presumably complete commercial stock diet.
Six animals were used for each test, either genetically diabetic
mice or their control litter mates of the C57Bl-KSI strain.
Injections of either 5 mg of the GTF-containing yeast extracts or
0.1 mg of the synthetic chromium complex, acutely reduced the
elevated plasma-glucose levels in the diabetic and the non-fasting
normal mice by 10 - 38% of the initial values ( P < 0.01) and the
plasma-triglycerides by 26 - 56% ( P < 0.01), compared with control
mice injected with saline. Injection of insulin into diabetic mice
produced only an 11 - 18% decrease in plasma-glucose levels,
whereas injection of the GTF-containing extract together with
insulin reduced plasma-glucose levels by 39 - 51% and plasma-
triglyceride levels by 76% (Table 11).
The results suggest either a much higher increase in the
chromium requirement of the genetically diabetic mice or their
inability to use chromium in the diet.
Table 11. Acute effects of GTF and exogenous insulin on non-
fasting plasma-glucose and plasma-triglyceride (TG) concentrations
in 19-week-old genetically diabetic micea
------------------------------------------------------------------
Treatment Plasma- deltaGlucose Plasma- deltaTG
glucoseb triglyceridesb
(mg/litre) (mg/litre)
------------------------------------------------------------------
saline 11 120 ± 3960 ± 160(5)c
490(5)c
GTF 9320 ± -180 (16%) 2790 ± 170(5)d -117 (30%)
280(5)d
insulin 9840 ± -128 (12%) 3020 ± 400(5) - 94 (24%)
410(5)
insulin 7060 ± -406 (37%) 940 ± 220(5)e -302 (76%)
and GTF 840(5)e
------------------------------------------------------------------
a Modified from: Tuman & Doisy (1977).
b Glucose and triglyceride values represent mean ± SEM for 6 mice in
each treatment group. Dose of GTF was 5 mg (WL-10-AT) administered
intraperitoneally, 12 h prior to collection of blood. Lente insulin
(0.1 U per mouse) was administered subcutaneously, 12 h prior to
collection of blood. Data were treated by analysis of variance to
detect differences between the various treatment groups; independent
orthogonal comparisons were performed in the following groups ( P value
indicates level of significance for each comparison).
c Saline versus all other treatments, P < 0.005.
d GTF versus insulin, P < 0.05.
e GTF and insulin alone versus combined GTF and insulin, P < 0.005.
Thus, GTF and insulin > GTF = insulin > saline.
Steele & Rosebrough (1979) reported a significant stimulation
of the growth rate of one-week-old turkey poults (both sexes) by
supplementation of a practical ration with 20 mg chromium (as
chloride)/kg. The weight gains within the 2-week study were 235 g
and 267 g for the 60 controls and 60 supplemented turkeys,
respectively ( P < 0.001). As the practical ration contained
ground yellow corn and soybean meal, limestone, and dicalcium
phosphate, chromium deficiency would appear unlikely. The amount
of the chromium supplement (20 mg/kg diet) is quite high, and
further studies are needed to decide whether the observed effects
were of a pharmaco-dynamic nature or truly nutritional, i.e.,
correcting a deficiency. A similar interpretation should be
applied to a report of improved egg quality in the laying hen
(Jensen et al., 1978).
The quantitative aspects of the effects of chromium on animals
can be summarized as follows: normal rats fed semi-purified, semi-
synthetic rations, with Torula yeast or individual proteins and
sucrose or starch as the source of carbohydrates, develop mild
signs of deficiency at a dietary level of 100 - 150 µg chromium/kg.
To prevent deficiency, most authors used very high supplements of
several mg/kg diet and did not determine the biological
availability of the chromium complexes used for the
supplementation. Diets containing raw ingredients and supplying
chromium levels between 0.5 and 1 mg/kg do not induce signs of
deficiency and probably meet the requirement of the rat. A very
tentative estimate of the dietary chromium need of the rat and
probably the mouse would be approximately 0.5 mg/kg diet. This
estimate should be interpreted with caution, because of the lack of
knowledge concerning the biological availability of chromium and of
its interaction with dietary constituents.
7.2. Toxicity Studies
The toxicology of chromium compounds has been reviewed by the
US National Academy of Science (US NAS, 1974a), Langard & Norseth
(1979), the International Agency for Research on Cancer (IARC,
1980), Langard (1980a, 1982), and Burrows (1983).
In discussing toxicological problems, it is important to
differentiate between the various oxidation states of chromium and
its compounds. Trivalent chromium, when administered to animals in
food or water, does not appear to induce any harmful effects, even
when given in large doses (US NAS, 1974a) (section 7.2.1). Acute
and chronic toxic effects of chromium are mainly caused by
hexavalent compounds. Since it has been shown that both industrial
trivalent chromium compounds as well as reagent-grade trivalent
chromium compounds can be contaminated by hexavalent chromium
(Petrilli & DeFlora, 1978a; Levis & Majone, 1979), the evaluation
of experimental studies becomes difficult, especially when the
purity of the chemical compounds used is not known.
Discrimination between the biological effects, caused by
hexavalent chromium and trivalent chromium is difficult, because,
after penetration of membranes in tissues, hexavalent chromium is
immediately reduced to trivalent chromium (Gray & Sterling, 1950;
US NAS, 1974a), and it is not evident whether the observed
phenomena are caused by this reduction or even by the trapping of
trivalent chromium by ligands after uptake in the cells. Another
problem in evaluating the data is associated with the route of
administration. Hexavalent chromium, introduced by the oral route,
is partly reduced to trivalent chromium by acidic gastric juice
(Donaldson & Barreras, 1966; DeFlora & Boido, 1980); thus, the
effects or lack of effects observed may be caused mainly by
trivalent chromium and not by the hexavalent chromium, actually
administered.
7.2.1. Effects on experimental animals
Many local effects on human beings have been reported (section
8.3), but only a few studies have verified these effects in
experimental animals. A comprehensive survey of hexavalent
chromium-induced effects is given in Table 12 (US NAS, 1974a). For
most studies, details were not given of the length of exposure,
number of treated animals and controls, etc. Diagnoses were stated
without presenting all the original data. Thus, in this section,
some papers will be discussed that refer to the most prominent
local and systemic effects to support and clarify the effects shown
in human beings.
It is evident that the toxicity of hexavalent chromium in
animals varies with the route of entry into the body. Low
concentrations of hexavalent chromium may be tolerated, when
administered in the feed or drinking-water, the extent of
absorption being a factor of importance. For example, rats
tolerated hexavalent chromium in drinking-water at 25 mg/litre, for
1 year, and dogs did not show any effects from chromium
administered as potassium chromate at 0.45 - 11.2 mg/litre over a
4-year period (US NAS, 1974a). However, oral exposure of both male
and female rabbits to sodium dichromate (0.1% solution, 0.2 - 5
mg/kg body weight, for up to 545 days) resulted in significant
morphological changes in the gonads, including atrophy of the
epithelium and dystrophic alterations of the Sertoli and Leydig
cells in the testes, and sclerotic and atrophic changes in ovaries
(Kucher, 1966).
Larger doses of hexavalent chromium are highly toxic and may
cause death, especially when injected iv, subcutaneously (sc), or
intragastrically. The LD50 of chromium compounds was determined
for several experimental animal species. The LD50 of potassium
dichromate (hexavalent chromium), administered orally (stomach
tube) to rats, was 177 mg/kg body weight in males and 149 mg/kg
body weight in females (Hertel, 1982). When injected iv in mice
(sex not given), the LD50 of chromium carbonyl was 30 mg/kg body
weight (IARC, 1980).
Performing a life-time inhalation study on the rat, Glaser et
al. (1984) found an LC50 for Na2Cr2O7 of 28.1 mg/m3 (range, 16.7 -
47.3 mg/m3). Assuming a deposition rate in the lung of 30% of the
dose administered, the LC50 dose was 1 mg/kg body weight in male
and 1.2 mg/kg in female rats.
A local corrosive action of hexavalent chromium on the skin,
similar to that seen in man, was described by Samitz & Epstein
(1962), who induced chrome ulcers in guinea-pigs at 4 trauma sites,
with daily exposure to 0.34 MK2Cr2O7 solution for 3 days. Mosinger
& Fiorentini (1954) showed the same effects using potassium
chromates.
Table 12. Effects of hexavalent chromium in animalsa
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit, inhalation chromates 1 - 50 mg/m3 14 h/day for pathological Lukanin (1930)
cat 1 - 8 months changes
in the lungs
Rabbit inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day none Lehmann (1914)
as dichromate for 5 days
Cat inhalation dichromates 11 - 23 mg/m3 2 - 3 h/day bronchitis, Lehmann (1914)
as dichromate for 5 days pneumonia
perforation of
nasal septum
Mouse inhalation mixed dust 1.5 mg/m3 4 h/day, no tumours Baetjer et al.
containing as CrO3 5 days/week, (1959a);
chromates for 1 year Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 16 - 27 mg/m3 1/2 h/day tumours in Baetjer et al.
containing as CrO3 intermit- some strains (1959a);
chromates tently Steffee &
Baetjer (1965)
Mouse inhalation mixed dust 7 mg/m3 37 h over increased Baetjer et al.
containing as CrO3 10 days tumour rate (1959a);
chromates Steffee &
Baetjer (1965)
Rat inhalation mixed dust 7 mg/m3 37 h over barely Baetjer et al.
containing as CrO3 10 days toleratedb (1959a);
chromates Steffee &
Baetjer (1965)
Rabbit, inhalation mixed dust 5 mg/m3 4 h/day, none marked Baetjer et al.
guinea- containing as CrO3 5 days/week, (1959a);
pig chromates for 1 year Steffee &
Baetjer (1965)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rat, inhalation hexacarbonyl 1.6 mg/m3 4 months, anaemia; lipid Roschina (1976)
rabbit 4 h, 5 days and/or protein
a week dystrophia
in liver
and kidneys
Rat, inhalation hexacarbonyl 0.16 mg/m3 4 months, anaemia; no Roschina (1976)
rabbit 4 h, 5 days biochemical
a week or morphological
effects
Rat inhalation hexacarbonyl 35 mg/m3 30 min 100% death Roschina (1976)
Rat inhalation dichromates 0.006 - 0.2 28 days/ increase in Glaser et al.
mg/m3 90 days lung-macrophages (1985)
23 h/day
7 days/week lymphocytes
immunoglobulin,
reduced Fe2O3
lung clearance
Rat intratra- dichromates 5 per week up to 30 toleratedc Steinhoff
cheal in- 0.01 - 0.25 months et al. (1983)
stillation mg/kg
Rat intratra- dichromates 1 per week up to 30 tolerated; Steinhoff
cheal in- 0.05 - 1.25 months 1.25 mg/kg et al. (1983)
stillation mg/kg harmful
Rat oral potassium 500 mg/litre daily maximal Gross & Heller
chromate in non-toxic (1946)
drinking-water concentration
Rat, oral zinc chromate 10 g/kg daily maximal Gross & Heller
mouse in feed non-toxic (1946)
(mature) concentration
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Rabbit oral sodium di- 0.2 - 5.0 mg/kg 545 days morphological Kucher (1966)
chromate changes in
gonads (testes:
atrophy of
epithelium,
dystrophic
alterations of
Sertoli &
Leydiz alls.
Ovaries:
sclerotic and
atrophic
changes)
Rat oral zinc chromate 1.2 g/kg daily maximal Gross & Heller
(young) in feed non-toxic (1946)
concentration
Rat oral potassium 1.2 g/kg daily maximal Gross & Heller
(young) chromate non-toxic (1946)
in feed concentration
Dog, oral monochromate 1.9 - 5.5 mg 29 - 685 none harmful Lehmann (1914)
cat, or dichromates chromium/kg days
rabbit body weight per
day 1 mg chrom-
ium equivalent
to 2.83 mg
K2Cr2O7
or 3.8 mg
K2CrO4
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog oral potassium 1 - 2 g as daily fatal in 3 Brard (1935)
dichromate chromium months anaemia
Dog stomach potassium 1 - 10 g as - rapidly fatald Brard (1935)
tube dichromate chromium
Monkey subcutan- potassium 0.02 - 0.7 g in - fatald Hunter & Roberts
eous dichromate 2% solution (1933)
Dog subcutan- potassium 210 mg as - rapidly fatal Brard (1935)
eous dichromate chromium
Guinea- subcutan- potassium 10 mg - lethald Ophüls (1911a)
pig eous dichromate Ophüls (1911b)
Rabbit subcutan- potassium 1.5 cc of 1% - 80% fatald Hasegawa (1938)
eous dichromate solution/kg body
weight
Rabbit subcutan- potassium 20 mg - lethald Ohta (1940)
eous dichromate
Rabbit subcutan- potassium 0.5 - 1 cc of - nephritisd Ohta (1940)
eous dichromate 0.5% solution/kg
body weight
Rabbit, subcutan- sodium 0.1 - 0.3 g as - rapid deathd Priestley
guinea- eous or chromate CrO3 fall in blood (1877)
pig intravenous pressure
Rabbit intra- potassium 0.7 cc of 2% - lethald Mazgon (1932)
venous solution/kg 8-10 days after
body weight injection
Dog intra- potassium 10 grains - instant death Gmelin (1826)
venous chromate
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Animal Route Compound(s) Average dose Duration Effect Reference
or concentration
---------------------------------------------------------------------------------------------------------
Dog intra- potassium 1 grain - none marked Gmelin (1826)
venous chromate
Dog intra- potassium 210 mg as - rapidly fatal Brard (1935)
venous dichromate chromium
Dog intra- potassium 3 mg/100 cc 2 doses marked renal Hepler & Simonds
venous dichromate blood per dose damage (1946); Simonds
& Hepler (1945)
---------------------------------------------------------------------------------------------------------
a Modifed from: US NAS (1974a).
b Pathological changes in experimental and control rats, 101 weeks after exposure.
c The same weekly dose distributed over 5 days was clearly better tolerated than a single weekly
administration.
d Renal damage.
Following parenteral administration, the most common systemic
effects of chromium were parenchymatous changes in the liver and
kidney (Mosinger & Fiorentini, 1954). Later studies showed
selective damage in the renal proximal convoluted tubules, without
evidence of glomerular damage, as demonstrated after one single sc
injection of potassium dichromate of 10 mg/kg body weight (Schubert
et al., 1970) or after one single intraperitoneal (ip) injection of
sodium chromate of 10 or 20 mg/kg body weight (Evan & Dail, 1974).
Effects have also been found in fish (Strik et al., 1975). After 32
days of continuous exposure to 0.1, 1, or 10 mg hexavalent chromium
(as potassium dichromate)/litre, the fish Rutilus rutilus developed
lysis of the intestinal epithelium with haemorrhages as well as
hypertrophy and hyperplasia of the gill epithelium.
Franchini et al. (1978) found an increase in urinary protein,
lysozyme, glucose, and beta-glucuronidase in rats after a single sc
injection of potassium dichromate at 15 mg/kg body weight. After
sc injection (3 mg/kg body weight), every other day for 2 - 8
weeks, the authors observed a correlation between the chromium
contents of the renal cortex and chromium clearance.
Five-week-old male Wistar rats of the strain TNO-W-74 were
continuously exposed in inhalation chambers to submicron aerosols
of sodium dichromate at concentrations ranging from 25 (low level)
to 200 µg chromium/m3 (high level) (Glaser et al., 1985). Exposure
for 28 days to 25 or 50 µg chromium/m3 resulted in "activated"
alveolar macrophages with stimulated phagocytic activity, and
significantly elevated antibody responses to injected sheep red
blood cells. After 90 days of low-level exposure, there was a more
pronounced effect on the activation of the alveolar macrophages,
with increased phagocytic activity. However, inhibited phagocytic
function of the alveolar macrophages was seen at the high
hexavalent chromium exposure level (200 µg/m3). In rats exposed to
this chromium aerosol concentration for 42 days, the lung clearance
of inert iron oxide was significantly reduced. The humoral immune
system was still stimulated at a low chromium aerosol concentration
of 100 µg/m3, but significantly depressed at 200 µg chromium/m3.
Exposure of rats, through inhalation, to chromium carbide or
chromium boride dust at very high levels (300 - 350 mg/m3 for each
substance) for 3 months (2 h/day) resulted in effects on the
vascular system of the lungs, e.g., endothelial hyperplasia.
Bronchitis and a decrease in the blood-haemoglobin concentration
were also observed (Roschina, 1964). The effects of chromium boride
were more pronounced than those of chromium carbide.
Steinhoff et al. (1983) performed an intracheal injection study
on rats (930 rats, 30 months, 0.05 - 1.25 mg Na2Cr2O7/kg body
weight per week; 1.25 mg CaCrO4/kg body weight per week). Half of
the rats were intratracheally injected once a week and the other
half received the same weekly dose distributed over 5 injections
per week. In rats receiving doses 5 times a week, there were some
significant changes in levels of total plasma-protein and
-cholesterol, in some haematological variables, in organ weights,
and in survival times in females. Only male rats receiving 1.25 mg
sodium dichromate/kg body weight, once a week, showed a sharp
reduction in body weight, female rats being less affected. Rats
receiving calcium chromate in the same dose showed reduced body
weight but to a lesser extent. The weight of lung and trachea was
increased by both substances in all doses.
Marked histopathological changes (congestion, fairly large
areas of focal necrosis, bile duct proliferation) described after
long-term exposure of rabbits to hexavalent chromium (ip injection
of 2 mg chromium/kg body weight per day for 6 weeks) (Tandon et
al., 1978), as well as the increase in hepatic metallothionein and
decrease in cytochrome P-450 levels after ip injection of 400 µmol
chromium/kg per day (type of chromium compound not mentioned)
(Eaton et al., 1980) need further confirmation. The finding of an
accumulation of hexavalent chromium in the reticuloendothelial
system including bone marrow (Baetjer et al., 1959b; Langard, 1977)
may be of importance for a disturbed blood picture.
Merkurieva et al. (1980a,b) studied the effects of potassium
dichromate in the drinking-water on the activities of different
enzymes in rats. Exposure included daily doses of 0.0005, 0.005,
0.05, or 0.5 mg/kg body weight for up to 6 months. After 20 days
of exposure at the highest dose level, enzyme activities increased
by 15 - 28% in liver microsomes (inosine-5-diphosphatase),
lysosomes (beta-D-galactosidase), and cytosol (lactate dehydrogenase
(EC 1.1.1.27)). For some of these enzymes, as well as for
acetylesterase (EC 3.1.1.6), increases in activity of up to 54%
were found in the gonads, kidneys, seminal fluid, and serum. At a
dose of 0.05 mg/kg, the only statistically significant finding was
a 54% increase in the activity of acetylesterase in the gonads.
After a 6-month exposure at this dose level, there was a 70%
increase in lactate dehydrogenase activity in the seminal fluid as
well as a 50% increase in free beta-galactosidase in the liver. There
were no changes in enzyme activities at the two lowest dose levels.
Painting of rat skin with an aqueous solution of potassium
dichromate (0.5%), daily, for 20 days, resulted in a local
inflammatory reaction, an increased level of hexose glyco-proteins
in the skin and serum, and an elevated concentration of serotonin
in the skin and liver (Merkurieva et al., 1982). Most of these
effects were also seen earlier in the exposure period, though they
were not as pronounced. Ten days after the start of exposure, a
nearly 3-fold increase in the serum-acetylcholine concentration
occurred together with decreased acetylcholinesterase activity.
Thus, the data of Merkurieva et al. show systemic effects following
both oral and dermal exposure to hexavalent chromium.
Cats fed chromic phosphate or oxydicarbonate at 50 - 1000 mg/day
for 80 days did not exhibit signs of illness or tissue damage.
Similarly, toxic reactions were not observed in rats administered
drinking-water containing 25 mg trivalent chromium/litre, for 1
year, or 5 mg trivalent chromium/litre throughout their lifetime
(US NAS, 1974a). The toxicity of trivalent chromium is so low that
even by parenteral administration, a chromic acetate level of 2.29
g/kg body weight or a chromic chloride level of 0.8 g/kg body
weight is required to kill mice. Even very large doses given
intragastrically were not fatal for dogs. Brard (1935) reported
that 10 or 15 g of chromium as chromic chloride proved fatal in one
dog (US NAS, 1974a). Some fatal doses of trivalent chromium
compounds reported in the literature are listed in Table 13.
Rats exposed through inhalation to chromic oxide (trivalent
chromium) at 42 mg/m3 or to chromic phosphate at 43 mg/m3 (5 h/day
for 5 days/week) for 4 months developed chronic irritation of the
bronchus and lung parenchyma, and dystrophic changes in the liver
and kidney (Blokin & Trop, 1977).
Inhalation exposure of rats (number not given) to dusts
containing 36 or 50% chromite for 4 months (2 h/day), at
concentrations of 375 - 400 mg/m3, resulted in thickening of the
walls of pulmonary vessels and bronchi (Roschina, 1959). The high
exposure levels in these studies make it difficult to evaluate the
effects.
Inhalation studies have also been performed with chromium
carbonyl, where chromium is in the 0 oxidation state (Roschina,
1976). Twelve rabbits and 48 rats were exposed for 4 months (4
h/day, 6 days/week) at a concentration of 1.6 or 0.16 mg/m3. At
both exposure levels, there was loss of body weight (25 and 12%,
respectively) as well as anaemia and leukocytosis. In the higher
exposure group, the animals showed an elevated gamma-globulin level
in serum and an increased transaminase activity. The contents of
cholesterol and SH-groups were reduced, and there was a decrease in
cholinesterase (EC 3.1.1.7) activity. Lipid and/or protein
dystrophy were noted in several organs, e.g., in the liver and
kidneys. No such effects were detected in the animals in the low-
exposure group.
Table 13. Fatal doses of trivalent chromium in animalsa
---------------------------------------------------------------------------------------------
Animal Number of Routeb Compound Chromium Effect Reference
animals dose (g/kg)
---------------------------------------------------------------------------------------------
Dog 2 sc chromic chloride 0.8 fatal Brard (1935)
Rabbit 1 sc chromic chloride 0.52 fatal Brard (1935)
Rat 38 iv chrome alum 0.01 - 0.018 LD50 Mertz et al.
chromium-hexaurea (1965a)
chloride
Mouse -c iv chromic chloride 0.8 MLDd Windholz et al. (1960)
Mouse -c iv chromic acetate 2.29 MLD Windholz et al. (1960)
Mouse -c iv chromic chloride 0.4 MLD Schroeder (1970)
Mouse -c iv trivalent chromium ? 0.25 - 2.3 MLD Windholz et al. (1960)
Mouse -c iv chromic sulfate 0.247 MLD Windholz et al. (1976)
Mouse -c iv chromic sulfate 0.085 MLD Schroeder (1970)
Mouse -c iv chromium carbonyl 0.03 LD50 Schroeder (1970)
---------------------------------------------------------------------------------------------
a Modified from: US NAS (1974a).
b sc = subcutaneous; iv = intravenous.
c No figures given.
d Minimum lethal dose.
7.2.1.1. Carcinogenicity
Various types of chromium chemicals, methods of administration,
and species of animals have been studied (IARC, 1980a).
Ideally, carcinogenicity should be tested with the methods
recommended by IARC (1980b), but, in many of the early studies,
this was not done. Carcinomas of the lung have been reported in
animals as a result of the administration of chromium chemicals.
Hueper (1958) found 2 squamous cell carcinomas and one
carcinosarcoma in 25 rats following intra-pleural injection of
chromite ore roast. After intrapleural implantation of strontium
chromate lasting 27 months, Hueper (1961) found tumours (type
unspecified) in 17/28 rats. Laskin et al. (1970) and Levy & Venitt
(1975) produced a number of bronchogenic carcinomas by implanting
pellets of cholesterol mixed with various chromium compounds
encased in a wire mesh cage in the bronchi of rats. Calcium and
zinc potassium chromate produced a number of bronchogenic
carcinomas, but soluble chromates and trivalent chromium chemicals
failed to produce cancer.
Using the same technique, Levy & Martin (1983) tested 21
different chromium-containing materials (pigments, intermediates,
and residues from the bichromate-producing industry, relatively
pure crystalline compounds) in 2250 random-bred rats and found that
chromates, described as sparingly soluble, were carcinogenic in the
rat lung. These materials included strontium and calcium chromate
and, to a far lesser extent, certain forms of zinc chromate.
Barium and lead chromate evoked only a very weak carcinogenic
response compared with strontium and calcium chromate. In the
study of Laskin et al. (1970), it was shown that chromium trioxide
produced hepatocellular carcinomas in 2/100 rats (controls, 0/24).
After inhalation of 13 mg calcium chromate/m3 (5 h/day, 5
days/week, for lifetime), Nettesheim et al. (1971) found 14 lung
adenomas in 136 treated mice and 5 in 136 untreated controls, but
no carcinomas. Steffee & Baetjer (1965), performing inhalation
studies on rats, mice, guinea-pigs, and rabbits (inhalation of
mixed chromate dust, corresponding to 3 - 4 mg CrO3/m3, 4 - 5
h/day, 4 days/week, for lifetime, or 50 months, respectively) could
only find 3 alveologenic adenomas in 50 treated guinea-pigs.
Laskin (1972) and Laskin et al. (1970) found 1 squamous cell
carcinoma of the lung, 1 of the larynx and 1 "peritruncal tumor" in
rats (inhalation of calcium chromate, 2 mg/m3, 589 exposures of 5 h
over 891 days) and 1 squamous cell carcinoma and 1 papilloma of the
larynx in hamsters. The number of treated animals was not
specified in either paper. Steinhoff et al. (1983) performed
intratracheal instillations of chromates in rats for 30 months with
one treatment/week and the same weekly dose distributed over 5
treatments/week (Table 14). In 880 exposed rats, 28 adenomas of
alveolar-bronchiolar origin (benign) and 12 malignant tumours (3
adenocarcinomas and 9 squamous cell carcinomas) were found. All
lung tumours developed very late and were only detected at the end
of the lifetime study, often in lungs with callosities. The
tumours were tiny and none of them caused the animal to die.
Sodium dichromate was not carcinogenic after exposure on 5
days/week. With calcium chromate, the carcinogenic effect was more
pronounced after treatment once per week, than after treatment 5
times per week.
Table 14. Incidence of benign and malignant lung tumours among
880 rats intracheally injected with Na dichromate and Ca chromatea
----------------------------------------------------------------
Dose Number of Incidence of lung tumours
(mg/kg) injections benign malignant
per week
----------------------------------------------------------------
Na2Cr2O7 x 2H20 0.25 5 none none
Na2Cr2O7 x 2H20 1.25 1 12 8
CaCrO4 0.25 5 5 1
CaCrO4 1.25 1 11 3
----------------------------------------------------------------
a From: Steinhoff et al. (1983).
Hueper (1961) reported unspecified tumours at implantation
sites in 12/34 rats, following intramuscular (im) implantation of
sintered calcium chromate (25 mg). No tumours were observed in 32
control animals. The same author found implantation-site tumours in
15/33 animals treated with strontium chromate compared with no
tumours in 32 control animals. Single sc injections of 30 mg lead
chromate (Maltoni 1974, 1976) resulted in injection-site sarcomas
in 26/40 treated rats. After administration of the same amount of
lead chromate oxide, injection-site sarcomas were found in 27/40
rats; no sarcomas were found in 60 vehicle controls. Following 9
im injections of 8 mg lead chromate, Furst et al. (1976) found
injection-site sarcomas in 31/47 treated rats, and renal carcinomas
in 3/24 treated rats; no sarcomas were found in 0/22 vehicle
controls. Heath et al. (1971) found injection-site sarcomas and
other tumours in 7/74 treated rats after im injection of 28 mg
cobalt-chromium alloy.
Administration of low levels of trivalent chromium acetate (5
mg/litre in the drinking-water) to mice and rats for the lifetime
did not result in increased tumour incidences compared with
controls (Schroeder et al., 1964, 1965).
Trivalent chromium oxide incorporated in bread at
concentrations of 10, 20, or 50 g/kg and fed to BD rats did not
increase tumour incidence compared with controls (Ivankovic &
Preussmann, 1975). Hexavalent chromium compounds have not been
tested by oral administration.
IARC (1980a) summarized the data of all available references
and concluded as follows: "There is sufficient evidence for the
carcinogenicity of calcium chromate and some relatively insoluble
chromium (VI) compounds (sintered calcium chromate, lead chromate,
strontium chromate, sintered chromium trioxide and zinc chromate)
in rats. There is limited evidence for the carcinogenicity of lead
chromate (VI) oxide and cobalt-chromium alloy in rats. The data
were inadequate for the evaluation of the carcinogenicity of other
chromium (VI) compounds and of chromium (III) compounds".
(a) Interaction with other factors
The possibility that chromium may act synergistically with
other agents in the production of cancer has been tested in several
studies. Nettesheim et al. (1970) pretreated one group of mice
with 100 R whole-body radiation and infected another group with PR8
influenza virus. These mice and control mice were exposed through
inhalation to chromium oxide dust for 6 - 18 months. No measurable
effects of either of the pretreatments were found on the incidence
of tumours. In a similar study with inhalation of calcium chromate
(CaCrO4, 5 h/day, 5 days per week for the lifetime), pre-exposure
to X-rays did not affect the tumour rate, but PR8 influenza
infection reduced tumour incidence (Nettesheim et al., 1971).
Steffee & Baetjer (1965) also found that the infection of rats with
PR8 influenza virus associated with intratracheal injection of
chromates did not lead to the production of tumours. Chromite ore
injected iv did not affect the development of tumours induced by iv
injection of 20-methyl-cholanthrene (Shimkin & Leiter, 1940).
Thus, at present, there are not sufficient data to suggest that
chromium acts synergistically with virus infections, ionizing
radiation, or other chemical carcinogens to produce cancers.
7.2.1.2. Genotoxicity
The mutagenicity of chromium compounds was reviewed by IARC
(1980a), Petrilli & DeFlora (1980), Levis & Bianchi (1982), and
Baker (1984). Present knowledge, in the light of recent findings,
is summarized in Table 15 (hexavalent chromium) and Table 16
(trivalent chromium).
When evaluating the results of chromium mutagenicity tests, it
is necessary to take into consideration several properties of the
tested compound (oxidation state, solubility, ability to penetrate
cell membranes, intracellular stability, and reactivity with
cellular components). Most of the in vitro mutagenicity
experiments have been performed with chromium chemicals, following
the introduction of the reverse mutation test by Ames (1973).
Bacterial strains of Salmonella typhimurium (Petrilli & DeFlora,
1977) containing mutants that require histidine for growth, and
Escherichia coli (Venitt & Levy, 1974) with mutants requiring
tryptophan for growth have been used. In each case, the bacterial
cultures have been incubated with the test chromium compounds and
the number of revertant colonies scored. The chromium compounds
studied included both hexavalent and trivalent compounds; a survey
of the results is given in Table 17 (DeFlora, 1981). Zhurkov
(1981) performed an Ames test to study the mutagenic activity of
potassium dichromate (K2Cr2O7) with S. typhimurium TA 1535 and TA
1538. A medium degree of mutagenic activity without metabolic
activation was shown by the strain TA 1535.
Only hexavalent chromium compounds have shown mutagenic
effects. Using the Salmonella /mammalian microsome tests of Ames
et al. (1973), DeFlora (1978) and Löfroth (1978) both showed a
decrease in the mutagenicity of hexavalent chromium in the presence
of an NADPH-generating system, suggesting reduction of mutagenic
hexavalent chromium to trivalent chromium. In this chromate
metabolism, cytochrome P-450 functions as the reductase (Garcia &
Wetterhahn Jennette, 1981). In recent in vitro studies, Wiegand et
al. (1984b) showed that glutathione could reduce hexavalent
chromium to trivalent chromium, without any further cofactors or
metabolizing enzymes. In rats, the most efficient tissue in
decreasing hexavalent chromium mutagenicity was the liver, followed
by the suprarenal glands, kidney, stomach, and lung (Petrilli &
DeFlora, 1980). These studies suggest a possible way of
intracellular detoxification of hexavalent chromium in low doses.
Schoental (1975) suggested that hexavalent chromium caused the
formation of epoxyaldehydes having mutagenic potential. Venitt &
Levy (1974) assumed that chromates belong to the transition-
inducing class of mutagens. They cause both frameshift and base-
pair substitutions (Petrilli & DeFlora, 1980). The hypothesis of
Kazantzis & Lilly (1979), that chromates cause guanine-cytosine to
adenine-thymine transitions in the subsequent round of DNA-
replication, needs further confirmation.
Table 15. Genotoxic activity of chromium (VI) compoundsa
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
DNA degradation CaCrO4 - Casto et al. (1976)
K2Cr2O7 - Bianchi et al. (1983)
PbCrO4 - Douglas et al. (1980)
K2CrO4 + Whiting et al. (1979)
Decreased fidelity PbCrO4, + (1) Levis & Majone (1981)
of DNA synthesis K2Cr2O7
CrO3 + Sirover & Loeb (1976)
K2Cr2O7 + Bianchi et al. (1983)
Microbial DNA K2CrO4, + Yagi & NishioKa (1977)
repair K2Cr2O7
CrO3, K2CrO4, + Kanematsu et al. (1980)
K2Cr2O7
K2CrO4, + Nishioka (1975)
K2Cr2O7
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
CrO3, + Gentile et al. (1981)
K2Cr2O7,
Na2Cr2O7, + Gentile et al. (1981)
(NH4)2Cr2O7
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Microbial gene K2Cr2O7 + (2) Bonatti et al. (1976)
mutation CrO3 + (2) Fukunaga et al. (1982)
Na2Cr2O7, +/-(3) Petrilli & De Flora
CrO3, +/-(3) (1978a,b)
ZnCrO4xZn(OH)2 +/-(3)
K2Cr2O7 + Zhurkov (1981)
Na2Cr2O7 +/-(3) DeFlora (1978)
chromate (4) +/-(3) Löfroth (1978)
dichromate +/-(3)
K2Cr2O7 - Kanematsu et al. (1980)
K2CrO4, + Löfroth & Ames (1978)
K2Cr2O7 +
K2Cr2O7 + Nishioka (1975)
K2CrO4, CaCrO4 + DeFlora (1981)
(NH4)2CrO4,
CrO3,
Na2CrO4, + Venitt & Levy (1974)
K2CrO4
CaCrO4
K2Cr2O7 + Bianchi et al. (1983)
Na2Cr2O7,
ZnCrO4
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7
K2CrO4, CaCrO4 + Petrilli & DeFlora
CrO3 (1977)
Na2Cr2O7
K2CrO4 + Green et al. (1976)
PbCrO4, CrO3 + Nestmann et al. (1979)
Mammalian cell K2Cr2O7 + Bianchi et al. (1983)
gene mutation K2Cr2O7, + Newbold et al. (1979)
ZnCrO4 +
PbCrO4 -
K2Cr2O7 + (5) Bonatti et al. (1976)
K2Cr2O7 + Pashin et al. (1982)
NaeCr2O + Pashin & Kozachenko
(NHy)2Cr2O7 + (1981)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell K2CrO4, + Umeda & Nishimura (1979)
chromosomal K2Cr2O7 +
mutation K2Cr2O7 + Bigaliev et al. (1978)
K2CrO4, + Nakamuro et al. (1978)
K2Cr2O7 +
Na2CrO4, + Majone & Levis (1979)
K2CrO4 +
Na2Cr2O7, Levis & Majone (1979)
K2Cr2O7
CrO3, CaCrO4
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CrO3 + Kaneko (1976)
Na2Cr2O7 + Sarto et al. (1980)
K2Cr2O7 +
CrCl3 +
CrO3 + Tsuda & Kato (1977)
K2Cr2O7 + Raffetto (1977)
K2Cr2O7 + Stella et al. (1982)
Na2Cr2O7, + Majone & Levis (1979)
K2Cr2O7 +
K2CrO4 + Wild (1978)
Na2Cr2O7 + (7) Krishnaja & Rige (1982)
Mammalian cell CrO3, K2CrO4, + Ohno et al. (1982)
SCE K2Cr2O7 +
K2Cr2O7 + Bianchi et al. (1983)
K2CrO4,
Na2CrO4 + Levis & Majone (1979)
CrO3, +
K2Cr2O7, + Majone & Levis (1979)
Na2Cr2O7 +
PbCrO4 + Douglas et al. (1980)
K2Cr2O7 + Imreh & Radulescu (1982)
CaCrO4, CrO3, + Gomez-Arroyo et al.
K2Cr2O7 + (1981)
K2Cr2O7 + Majone & Rensi (1979)
CrO3 + (6) Stella et al. (1982)
K2Cr2O4, + Elias et al. (1983)
Na2CrO4, +
Na2Cr2O7 +
K2Cr2O7,
K2CrO4 + McRae et al. (1979)
---------------------------------------------------------------------
Table 15. (contd.)
---------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------
Mammalian cell CaCrO4, K2CrO4 + (8) Casto et al. (1979)
transformation ZnCrO4 + (9)
Na2CrO4,
CaCrO4 + Casto et al. (1976)
+ Di Paolo & Casto (1979)
K2Cr2O7 + Bianchi et al. (1983)
K2Cr2O7 + Tsuda & Kato (1977)
CaCrO4 + Fradkin et al. (1975)
K2Cr2O7 + Raffetto (1977)
---------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) As industrial pigments (Cr-yellow-orange; Zn-yellow; Mo-orange).
(2) Gene conversion.
(3) No mutagenicity in the presence of mammalian microsomal activation
system (S-9 mix).
(4) No compounds given.
(5) Forward mutation was observed in 7/480 054 colonies (controls 0/84
546). A significant increase can be stated if comparison is made
with the historical spontaneous mutation rate, i.e., no mutants in
106 colonies.
(6) Occupatinal exposure.
(7) Induction of chromosomal aberrations in gill cells of the fish
Boleophthalmus dussumieri (Goby).
(8) 2 times control levels.
(9) 4 times control levels.
The results summarized in Table 17 show that the chromate ion
(hexavalent chromium) induces different kinds of genetic damage,
even at low doses, whereas the chromosome-damaging capacity of
trivalent chromium was only observed when it was tested in very
high doses.
In assays with whole-cell systems, trivalent chromium is
inactive, unless there is a direct interaction with DNA, e.g., in
studies in which purified DNA was exposed to trivalent chromium,
where modifications of the physical and chemical properties, as
well as decreased fidelity of DNA synthesis, were produced (Levis &
Bianchi, 1982). According to Warren et al. (1981), it appeared
that trivalent chromium in a proper ligand environment could have
considerable genetic toxicity. However, in recent studies, Bianchi
et al. (1984) found that trivalent chromium (CrCl3, 10-3 to 10-5 M)
was neither cytotoxic nor mutagenic in permeabilized hamster
fibroblasts (BHK were incubated for 30 min in Ea or BSS made
hypertonic with 4.2% NaCl).
Table 16. Genotoxic activity of chromium (III) compoundsa
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
DNA degradation CrCl3 + Bianchi et al.
(1983)
Decreased fidelity Cr2O3 + Levis & Majone
of DNA synthesis (1981)
Microbial DNA cis-[Cr(bipy)2ox] + Warren et al. (1981)
repair I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 + Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O + Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O + Warren et al. (1981)
trans-[Cr(en)2 + Warren et al. (1981)
(SCN)2]SCN
CrCl3, Cr2O3, - Yagi & Nishioka
Cr(OH)3 (1977)
K2Cr2(SO4)4 - Yagi & Nishioka
(1977)
K2Cr2(SO4)4 - Kanematsu et al.
(1980)
Cr2(SO4)3 - Kanematsu et al.
(1980)
CrCl3 - Nishioka (1975)
Cr(NO3)3, + De Flora (1981)
Cr(CH3COO)3
CrCl3 - Nakamuro et al.
(1978)
CrCl3 + Gentile et al.
(1981)
CrCl3, KCr(SO4)2, - Gentile et al.
(1981)
Cr2(SO4)3 - Gentile et al.
(1981)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Microbial gene CrCl3, Cr(NO3)3 - Löfroth & Ames
mutation (1978)
Cr(CIO4)3 - Löfroth & Ames
(1978)
CrCl3, Cr(NO3)3 - Venitt & Levy (1974)
Cr2SO4, - Venitt & Levy (1974)
K2SO4 x 2H2O
Cr(CH3COO)3 + Nakamuro et al.
(1978)
Cr(NO3)3 + Nakamuro et al.
(1978)
CrCl3 - Nakamuro et al.
(1978)
CrK(SO4)2 x 12H2O - Petrilli & De Flora
(1977)
CrCl3 x 6H2O - Petrilli & De Flora
(1978a)
Cr(NO3)3 x 9H2O - Petrilli & De Flora
(1978b)
Microbial gene cis-[Cr(bipy)2ox] + Warren et al. (1981)
mutation I x 4H2O
cis-[Cr(bipy)2Cl2] + Warren et al. (1981)
Cl x 2H2O
cis-[Cr(phen)2Cl2] + Warren et al. (1981)
Cl x 2,5H2O
[Cr(urea)6] + Warren et al. (1981)
Cl3 x 3H2O
[Cr(H2O)6]Cl3 - Warren et al. (1981)
[Cr(en)3](SCN)3 - Warren et al. (1981)
[Cr(en)3]Cl3 x 3H2O - Warren et al. (1981)
[Cr(pn)3]Cl3 x 3H2O - Warren et al. (1981)
trans-[Cr(en)2 - Warren et al. (1981)
(SCN)2]SCN
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
gene mutation (1983)
Cr(CH3COO)3 - Newbold et al.
(1979)
Mammalian cell Cr2(SO4)3 - Umeda & Nishimura
chromosomal (1979)
mutation
Cr(CH3COO)3 + Nakamuro et al.
(1978)
CrCl3, Cr(NO3)3 - Nakamuro et al.
(1978)
Cr(NO3)3, + Levis & Majone
(1979)
KCr(SO4)2, + Levis & Majone
(1979)
Cr(CH3COO)3 + Levis & Majone
(1979)
CrCl3 + Kaneko (1976)
CrCl3 - Sarto et al. (1980)
Cr2(SO4)3, CrCl3 - Tsuda & Kato (1977)
CrCl3 + Raffetto (1977)
CrCl3 - Stella et al. (1982)
CrCl3 - (1) Bianchi et al.
(1984)
Mammalian cell CrCl3, Cr2O3 + Elias et al. (1983)
SCE
Cr2O3 + Levis & Majone
(1981)
Cr(NO3)3, - Levis & Majone
KCr(SO4)2 (1979)
CrCl3, Cr(CH3COO)3 - Levis & Majone
(1979)
CrCl3 + (2) Ohno et al. (1982)
Cr2(SO4)3 - Ohno et al. (1982)
---------------------------------------------------------------------------
Table 16. (contd.)
---------------------------------------------------------------------------
Assay Compounds Activity Reference
---------------------------------------------------------------------------
Mammalian cell CrCl3 - Bianchi et al.
SCE (contd). (1983)
CrCl3 - Majone & Rensi
(1979)
Mammalian cell CrCl3 - Bianchi et al.
transformation (1983)
---------------------------------------------------------------------------
a Modified from: Baker (1984).
Note:
(1) Thymidine uptake, DNA replication, damage and repair and SCE were
tested in permeabilized hamster fibroblasts.
(2) Weakly positive.
Levis & Buttignol (1977) and Levis et al. (1978) raised the
question of whether trivalent chromium could be an active mutagenic
agent within a cell. Others, such as Bianchi et al. (1980),
supported this theory, suggesting that trivalent chromium is bound
to genetic material after reduction of the hexavalent form by an
NADPH-dependent microsomal enzyme system (Norseth, 1978; Jennette,
1979). The studies of Sirover & Loeb (1976), showing a 30% error
in DNA synthesis after application of 0.64 mM trivalent chromium
compared with 16 mM of hexavalent chromium, support this theory.
Pashin et al. (1982) demonstrated the induction of dominant
lethal mutations in mice. They treated male mice with a single
injection of potassium dichromate (0.5 - 20 mg/kg body weight, ip)
or with daily injections (1 and 2 mg/kg, ip, for 21 days). The
single injections (0.5 - 2 mg/kg) did not induce any effects;
repeated or higher dosages of potassium dichromate induced a
statistically significant decrease in the survival of embryos from
cells treated at the early spermatid and late spermatocyte stages.
Karyological alterations in mammalian cells were induced after
exposure to concentrations of hexavalent chromium that were more
than 100 times lower than the concentration of trivalent chromium
causing the same changes (Majone & Rensi, 1979).
Analysing the effects of fume particles from stainless steel
welding on sister chromatid exchanges and chromosome aberrations in
cultured Chinese hamster cells, Koshi (1979) found an increase in
cytogenic effects with increasing fume doses, due to the dissolved
hexavalent chromium. Hexavalent chromium and stainless steel
welding fume particles containing chromium compounds produced
positive results in the "mammalian spot test", a somatic tissue
assay (Knudsen, 1980).
Table 17. Mutagenicity of chromium compoundsa
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
L. Hexavalent chromium compounds
1) Sodium dichromate (Merck) - +w +w +w + 50 - 250 4.4 decrease toxic and mutagenic
Na2Cr2O7 x 2H2O Cr6+ was converted
into inactive Cr3+
2) Potassium chromate (BDH) - +w +w +w + 80 - 410 2.9 decrease by reducing
K2CrO4 chemicals (ascorbic
acid, sodium
3) Calcium chromate (BDH) - +w +w +w + 60 - 290 3.2 decrease sulfite) or
CaCrO4 metabolites (NADH,
NADPH, GSH), by
4) Ammonium chromate - +w +w +w + 50 - 320 3.7 decrease human gastric juice
(Carlo Erba) and erythrocyte
(NH4)2CrO4 lysates, by S9 mix
containing human
liver S9 fractions
5) Chromium trioxide or - +w +w +w + 40 - 220 5.1 decrease or rat tissue S9
chromic acid (Merck) fractions (in order
CrO3 of efficiency:
liver>suprarenal
glands>kidney>
stomach>lung);
pretreatment of
rats (Aroclor(R)
1254), ether,
ethanol) influenced
the efficiency of
metabolic systems;
conversely, Cr6+
mutagenicity was not
affected by human
serum or plasma nor
by S9 mix containing
other rat (spleen,
colon, bladder,
striated muscle) S9
fractions
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
6) Zinc yellow (Montedison) - +w +w +w + 90 - 590 1.4 decrease the mutagenic effects
ZnCrO4 x Zn(OH)2 + of Cr6+ compounds and
10% CrO3 benzo( a)pyrene were
less than additive
7) Chromyl chloride (BDH) - +w +w +w + 100 - 430 1.8 decrease liquid volatile
Cl2CrO2 compound; vapours
were also toxic and
mutagenic
8) Chromium orange or basic - - - - +b 2 mg the three pigments
lead (spot test) containing PbCrO4
PbCrO4 x PbO were scarcely (L8),
chromate (Montedison) very scarcely (L9),
or totally (L10)
insoluble in water;
they were assayed
by directly spotting
these compounds at
the centre of plates
9) Molybdenum orange or - - - - +b 2 mg
lead solfomolybdo- (spot test)
chromat (Montedison)
PbCrO4 72-77%
PbSO4 4-6%
Al2O3 2%
PbMoO4 12-14%
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
10) Chromium yellow or - - - - -b
lead solfochromate
(Montedison)
PbCrO4 41-85%
PbSO4 4-45%
SiO2 0.1-3%
Al2O3 2-6%
11) Chromium - - - - - -2.3x103 - - hexacoordinated
hexacarbonyl (BDH) compound; formally,
Cr(CO)6 its oxidation state
is 0; insoluble in
water, dissolved in
ether
M. Trivalent chromium compounds
1) Chromic chloride - - - - - -3x104 - - inactive Cr3+
(BDH) CrCl3 x 6H2O compounds could be
converted into toxic
and mutagenic Cr6+
only in the presence
of oxidizing chemicals
(potassium
permanganate), while a
variety of metabolic
systems were
ineffective
2) Chromic nitrate (Riedel- - - - - - -2x104 - -
de Haen)
Cr(NO3)3 x 9H2O
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Compound (source) Reverted strains Range of Mutagenic Effect of Remarks
activity potency S9 mix
(nmoles/ (revs/ (rat
plate) nmol) liver
Aroclor(R))
---------------------------------------------------------------------------------------------------------
3) Chromic potassium - - - - - -1.6x104 -
sulfate (BDH)
CrK(SO4)2 x 12H2O
4) Chromic acetate (BDH) - - - - - -7x104 - -
Cr(CH3COO)3
5) Neochromium (Montedison) - - - - - -5x104 - -
Cr(OH)SO4 56-58%
Na2SO4 23-24%
H2O 18-21%
6) Chromium alum (Montedison) - - - - - -4.3x104 - -
Cr2(SO4)3 37-39%
K2SO4 16-18%
H2O 43-37%
7) Chromite (Montedison) - - - - + 2 mg - Contaminated with Cr6+
Cr2O3 44-46% (spot test) traces ( sim-diphenyl-
Fe2O3 29-30% carbazide reagent);
Al2O3 15-16% scarcely soluble in
SiO3 0.5-3% water; assayed by
CoO 0.5-2% directly spotting the
powder at the centre
of plates
--------------------------------------------------------------------------------------------------------
Table 17 (contd.)
Note:
Sensitivity of Salmonella strains: Range of activity:
In the assays carried out in this study, This column indicates the lower and
the numbers of spontaneous revertants of the upper limits (in nmol per plate)
Salmonella tester strains fell within the of the mutagenic response, as in-
following ranges: ferred from dose-response curves
TA 1535: 10-25, both with and without S9 mix; with the most sensitive bacterial
TA 1537: 3-18, both with and without S9 mix; strain and under the most favourable
TA 1538: 10-25 without S9 mix; 15-35 with S9 mix; metabolic situation (i.e., with or
TA 98: 20-35 without S9 mix; 25-40 with S9 mix; without S9 mix) for each positive
TA 100: 150-200 without S9 mix; 140-180 with S9 mix compound; for negative compounds,
the maximum dose tested is indicated
The key for the interpretation of symbols in the
column "Reverted strains" is the following: Mutagenic potency:
+ clearly positive result indicated by a dose-
related and reproducible increase of his+ the values presented have
revertants over controls (at least a 3-fold been calculated by dividing the number
increase); of revertants in excess of controls
+w weak positivity indicated by an increase of by the corresponding amount of compounds
revertants 2 - 3 times in controls; (in nmoles); the number of net revertants
± reproducible but less than 2-fold increase was determinated at the top level of the
of revertants; linear part of dose-response curves, which
No symbol: no conclusive experiment carried out were drawn as indicated under the previous
with the corresponding strain. sub-heading.
Effects of S9 mix
The effect of S9 mix containing
liver S9 fractions from
Arochlor(R) pretreated rats on
the mutagenicity of test
compounds is reported
--------------------------------------------------------------------------------------------------------
a From: DeFlora (1981).
b L8, L9, and L10, as well as PbCrO4, were positive in the plate test when
dissolved in 0.5 N NAOH.
Embryonic fibroblast cell cultures showed significant
chromosome aberrations, when potassium dichromate was added to the
medium (Tsuda & Kato, 1976). When the potassium dichromate was
reduced with sodium disulfite (Na2SO3), no significant aberrations
occurred, even with 100 times the chromium concentrations.
At present, it is widely accepted that hexavalent chromium is
genetically active, because of its ability to cross the membranes
and enter the cells. If reduction of hexavalent chromium takes
place outside the cell (or even outside the cell nucleus, e.g., in
mitochondria or microsomes) its genetic activity is suppressed; if
the reduction takes place inside the nucleus (near, or at, the
target DNA molecules) alterations in DNA can occur, depending on
the oxidation power of hexavalent chromium or the formation of
trivalent chromium complexes with nucleophilic sites of the DNA;
thus, trivalent chromium could be the ultimate mutagenic form of
chromium (Levis & Bianchi, 1982). More recent investigations
(Wiegand et al., 1984) have shown that hexavalent chromium may
enter the body unreduced via the lung and be partly deposited in
erythrocytes over a prolonged period of time. From a DNA-repair
test in bacteria, DeFlora et al. (1984) concluded that hexavalent
chromium causes irritation in nucleotide pools and, due to its
oxidizing power, induces single-strand breaks in DNA, while
trivalent chromium induces DNA-protein and DNA-DNA cross-links and
decreases the fidelity of DNA replication.
7.2.1.3. Developmental toxicity and other reproductive effects
The teratogenicity of hexavalent chromium as chromium trioxide
was tested in golden hamsters (Gale, 1974, 1978, 1982; Gale &
Bunch, 1979) and that of trivalent chromium as chromic-chloride in
ICR mice (Iijima et al., 1975; Matsumoto et al., 1976). Injections
of hexavalent chromium into the fertilized chick egg increased
embryolethality and produced malformations in the survivors (Gilani
& Marano, 1979).
The golden hamsters were treated with a single iv injection of
chromium trioxide (5, 7.5, 10, or 15 mg/kg body weight) on the 8th
day of gestation (Gale, 1978).
Dose-response relationships were demonstrated between the rate
of absorption and the rate of malformations including delayed
ossification of the skeletal system. At 5 mg/kg body weight, 4% of
the fetuses showed external abnormalities (oedema, exencephaly),
and 34% showed cleft palate (controls: 2%), which increased to 85%
with the higher doses of 7.5 or 10 mg/kg body weight. Thirty-one
percent of fetuses were retarded following treatment with 7.5 mg/kg
body weight and 49% following 10 mg/kg body weight. The dose of 15
mg/kg body weight was lethal for 75% of the dams. Gale & Bunch
(1979) injected a single dose of 8 mg CrO3/kg body weight on day 7,
8, 9, 10, or 11 of gestation in female golden hamsters (Table 18).
The treated females were adversely affected (body weight loss,
tubular necrosis of the kidneys). In a later study, Gale (1982)
injected 8 mg CrO3/kg iv in hamsters on day 8 of gestation. An
increased incidence of cleft palate was seen in strains LVG, LSH,
and MHA, but no effects were reported in strains CB, LHC, and PD4.
ICR mice were injected sc with chromic chloride (Iijima et al.,
1975; Matsumoto et al., 1976). In the first series (animals
administered 9.8 or 19.5 mg/kg body weight, every other day, from
the 0th to 16th day of gestation), according to the authors, a
slight increase in the rate of malformations could not be excluded.
In the second series, with ip injections of 9.8 - 24.4 mg/kg body
weight, on the 8th day of gestation, the authors reported a dose-
dependent increase in the frequency of exencephaly, anencephaly,
and the occurrence of rib fusion.
Danielsson et al. (1982) studied the rate of embryonic or fetal
uptake of trivalent chromium and hexavalent chromium in early and
late gestational mice (Table 19) and found placental passage of
chromium. Hexavalent chromium appeared in the fetus in high enough
concentrations for a direct effect on embryonic structures to be
likely, trivalent chromium may act on placental structures.
Gilani & Marano (1979) injected chromium trioxide into 840
embryonating chicken eggs (air sack) at doses ranging from 0.002 to
0.05 mg per egg, on days 0, 1, 2, 3, and 4 of incubation (0.1 ml
physiol. saline injections per control egg). All embryos were
examined on day 8. The following malformations were observed in
the 720 eggs affected: short and twisted limbs, microphthalamia,
exencephaly, short and twisted neck, everted viscera, oedema, and
reduced body size. However, the incidence of abnormalities in the
120 controls was reported to be "low".
Inhalation studies were conducted by Glaser et al. (1984) on
Wistar rats (TNO-W-74 SPF) to study the effects of sodium
dichromate on reproduction and teratogenicity in 3 generations.
Whole-body exposures to sodium dichromate aerosols (0.2 mg
chromium/m3) were performed with an exposure time of 130 days per
generation. No effects on reproduction were found. All
teratogenicity tests were negative, and there was no increase in
fetal chromium content. However, from generation to generation,
there was an increase in immunosuppression and hyperplasia of
organs (especially in the lungs) and changes in haematological
variables.
Table 18. Embryotoxicity following maternal exposure to CrO3 on different days
of gestation in hamstersa
-------------------------------------------------------------------------------------
Findings in live fetuses
Day of No. of No. of No. of Frequency External Internal Cleft
gestation females implantation living resorptions defects defects palate
treated sites fetuses No. %b No. %b No. %b No. %b
-------------------------------------------------------------------------------------
Treated CrO3 8 mg/kg
7 6 64 10 54 84 1 10 1c 10 5 50
8 6 88 54 14 20 8d 15 4e 7 17 31
9 6 77 67 10 13 18f 27 0 0 25 37
10 6 77 77 0 0 0 0 0 0 0 0
11 6 68 67 1 1 0 0 0 0 0 0
Controls H2O 5 ml/kg
7 3 35 33 2 6 0 0 0 0 0 0
8 3 44 41 3 7 0 0 0 0 0 0
9 3 37 36 1 3 0 0 0 0 0 0
10 2 20 19 1 5 0 0 0 0 0 0
11 3 34 33 1 3 0 0 0 0 0 0
-------------------------------------------------------------------------------------
a From: Gale & Bunch (1979).
b Underlined %, significantly different statistically from controls.
b Right hindlimb with two digits.
c Right kidney absent.
d Oedema (7), tail short (1).
e Small kidneys (3), right kidney absent (1).
f Oedema (17), omphalocele (1).
Table 19. Comparison of concentrations of trivalent and hexavalent
chromium in fetuses, placentas, and maternal organsa,b
------------------------------------------------------------------
Day 13 Day 16
Hexavalent Trivalent Hexavalent Trivalent
chromium chromium chromium chromium
------------------------------------------------------------------
Fetus 0.3 ± 0.01 0.03 ± 0.002 0.5 ± 0.04 0.1 ± 0.01
Placenta - - 3.0 ± 0.2 2.4 ± 0.2
Liver 29.4 ± 2.2 30.8 ± 6.5 33.9 ± 3.9 27.7 ± 3.5
Kidney 38.1 ± 1.9 5.3 ± 0.7 36.0 ± 4.3 8.7 ± 2.3
Serum 2.6 ± 0.2 9.3 ± 0.9 2.8 ± 0.4 11.0 ± 1.6
------------------------------------------------------------------
a From: Danielsson et al. (1982).
b Trivalent chromium (51CrCl3) and hexavalent chromium
(Na251Cr2O7) were injected iv on day 13 and day 16 of gestation
at doses of 10 mg chromium/kg body weight. The pregnant animals
were autopsied after 1 h. Concentrations are expressed in
mg/litre or g ± SEM (N = 4).
7.2.1.4. Cytotoxicity and micromolecular syntheses
Wacker & Vallee (1959) reported that trivalent chromium was
present in RNA from all sources examined; they hypothesized that
chromium might contribute to the stabilization of the structure.
Administration of trivalent chromium to mice (ip injection of
CrCl3 at 5 or 0.5 mg chromium/kg body weight; P < 0.01) caused
accumulation of chromium in the cell nucleus, which amounted to
about 20% of the accumulated chromium content of the liver cell,
and also enhanced RNA synthesis. Hexavalent chromium inhibited RNA
synthesis (K2CrO4 at 5 or 0.5 mg chromium/kg body weight;
P < 0.01) (Okada et al., 1983). In further studies, Okada et al.
(1984) pretreated rats with CrCl3 (ip injection of 5 mg chromium/kg
body weight) and then partially hepatectomized the rats. Chromium
accumulated in the regenerating liver, and hepatic RNA synthesis
was accelerated compared with that of partially hepatectomiced
controls. These findings suggest a direct participation of
chromium in RNA synthesis.
Potassium dichromate enhanced the passive uptake of ribo-and
desoxyribonucleosides (Levis et al., 1978; Bianchi et al., 1979),
caused an inhibition of DNA replication to BHK fibroblasts, and
reduced the colony-forming ability of BHK cells (doses: 10-4 - 20-7
mol). Hexavalent chromium (potassium dichromate with a
concentration of 5 x 10-7 mol/litre) reduced by half the
proliferation of NHIK 3025 cells originating from a human cervix
carcinoma (White et al., 1979).
The addition of calcium chromate to BHK21 cell cultures altered
their growth characteristics. Whereas these cells normally grow as
elongated fibroblast cells in parallel orientation, the chromium-
exposed cells grew as shortened fibroblasts with enlarged nuclei
and granular cytoplasm, randomly orientated. When grown in
carboxymethyl cellulose, the chromate-exposed cells underwent many
divisions in contrast to the 1 or 2 divisions of normal cells.
These alterations in growth properties persisted, even after
transfer to a normal medium (Fradkin et al., 1975).
Cultures of HeLa and rat embryonic cells were incubated with
various hexavalent and trivalent chromium compounds for 3 days and
the 50% inhibitory dose (ID50) for cell growth was determined. The
ID50 for sodium and potassium chromate and dichromate and calcium
chromate varied from 0.34 to 0.78 µg/ml of the medium. The ID50
for the trivalent compounds varied over a wider range: chromic
oxalate, 4; acetate, 52; nitrate, 720; chloride, 1030; and bromide,
1500 mg/litre. Similar results were found in rat embryonic cell
cultures (Susa et al., 1977).
7.2.1.5. Fibrogenicity
Because of the well-known fibrogenic effects of silica and
asbestos minerals, studies were initiated to determine whether
chromite and various other dusts exhibited similar properties. A
single intratracheal injection in rats of 40 mg chromite particles
(33% chromium, 0.7% SiO2, 99.9% of the particles being smaller than
5 µm) suspended in 1 ml Ringer's solution caused a moderate
cellular reaction that reached a peak after 2 months and then
decreased. After 8 months (end of observation), the lung weight
was within normal limits. Only an increase in fibrils that was not
significant was observed. The tissue reaction in regional lymph
nodes was minimal (Swensson, 1977).
Chromite FeO(CrAl)2O3 (10 mg), ground to an average particle
diameter of 1 µm, was injected into the pleural cavities of 25
mice. The animals were killed at intervals ranging from 2 weeks to
18 months after injection. Chromite produced the least reaction of
all the 15 dusts tested. This material attracted very few cells
and the eventual production of collagen was minimal (Davies, 1972).
7.2.2. Observations in farm animals
Systemic poisoning of farm animals with chromates is rare and
is mainly suspected to be a consequence of accidental industrial
contamination of drinking-water. The acute lethal dose for mature
cattle is approximately 600 - 800 mg/kg body weight; chronic
poisoning in young calves was produced by a daily dose of 30 - 40
mg/kg body weight for one month (NZ Department of Agriculture,
1954) with a clinical picture of profuse scouring, severe
dehydration, low blood pressure, changes in the alimentary tract,
including congestion and inflammation, and the rumen and abomasum
showing ulceration near perforation.
High chromium levels were found in the blood and liver of
calves that had died from poisoning. The minimum tissue levels
causing poisoning were 30 mg/kg in the liver and 4 mg/litre in
whole blood (Harrison & Staples, 1955). According to Romoser et al.
(1961), a level of 100 mg hexavalent chromium (as sodium
chromate)/kg did not affect growing chicks.
8. EFFECTS ON MAN
8.1. Nutritional Role of Chromium
The physiological role of chromium and the need for small
amounts of dietary chromium are indicated by:
(a) chromium-responsive cases of impaired glucose
metabolism in malnourished children and middle-aged
persons;
(b) 2 cases of proved chromium deficiency in patients fed
exclusively by parenteral alimentation; and
(c) epidemiological studies suggesting a link between
chromium deficiency and risk factors for cardiovascular
diseases.
8.1.1. Biological measurements and their interpretation
Biochemical methods for the diagnosis of the human nutritional
chromium status are still at the developmental stage. Blood- or
urine-chromium concentrations are not indicative of chromium
status. Several studies suggest the validity of the "relative
chromium response" in plasma or serum to the ingestion of 50 - 100
g of glucose within 30 - 120 min; the response also appears in the
urine. Lack of this response suggests chromium deficiency,
especially when combined with impaired glucose tolerance in the
presence of normal or elevated plasma-insulin levels.
8.1.2. Chromium deficiency
8.1.2.1. Adults
Chromium deficiency is diagnosed mainly through therapeutic
trials in which an impaired function is restored by supplementation
of the diet with small amounts of chromium close to the estimated
human requirement. Glinsmann & Mertz (1966) studied 4 male
diabetic patients for several months to 1 year, under the strictly
controlled conditions of a metabolic ward. The subjects, who were
fed identical portions of a diet prepared before the start of the
study, were allowed a measured amount of physical activity and were
stabilized under these conditions before the beginning of the
control periods. Three of the 4 patients responded to a chromium
level of 180 - 1000 µg/day as CrCl3 x 6H2O with a significant
improvement in oral glucose tolerance; the fourth showed a very
slight lowering of the glycaemic curve, which was not statistically
significant. A representative example is given in Table 20.
Table 20. Effect of chromium on mean glucose tolerancea,b
------------------------------------------------------------------------
Supplementation Days Number Mean blood glucose concentration
of ---------------------------------
tests Fast- Min after glucose load
ing 30 60 90 120
------------------------------------------------------------------------
None 0 - 32 10 840 2170 2290 1950 1560
60 µg, 3 x per day 33 - 74 12 850 2240 2380 2090 1620
60 µg, 3 x per day 75 - 119 13 840 2100 2130 1860 1410
60 µg, 3 x per day 120 - 140 5 840 2030 2010c 1750 1120c
None 180 - 194 1 960 2070 2900 2930 2370
60 µg, 3 x per day 195 - 211 5 830 1960 1930c 1750 1180c
None 256 - 313 10 870 2200 2450 2240 1660
1 mg, 3 x per day 337 - 340 1 680 1480 1620 1510 960
------------------------------------------------------------------------
a Modified from: Glinsmann & Mertz (1966).
b A 46-year-old man with maturity-onset diabetes, controlled on diet;
oral glucose tolerance test consisted of constant noon meal and 100 g
glucose.
c Mean values significantly different from control ( P < 0.025).
The condition of one of 2 diabetic out-patients, observed over
several months, was improved by chromium supplementation, the
condition of the other was not. Short-term treatment of 7 diabetic
patients for 1 - 7 days with 1 mg chromium did not affect any
variables of glucose metabolism, nor did chromium supplementation
change the normal glucose tolerance tests of 10 healthy, young
volunteers.
Glinsmann's positive results were not confirmed in a double-
blind crossover study on 4 normal volunteers and 10 diabetic
persons, with a crossover after 16 weeks. No effects of chromium
were detected on glucose tolerance or on fasting or postprandial
blood-glucose levels (Sherman et al., 1968). Schroeder et al.
(1970), while reporting that 2 - 10 mg chromium/day "almost always"
restored the impaired glucose tolerance in older, non-diabetic
subjects to normal, found only erratic and mild improvement in 4
out of 12 diabetic out-patients. On the basis of these
observations, Mertz (1969) concluded that chromium should not be
considered a therapeutic agent for diabetic patients.
On the other hand, it was reported in a later study on chromium
metabolism that chromium administration (500 µg/day, for 2 weeks)
produced a marked improvement in glucose utilization in both
juvenile and maturity-onset diabetic patients. Five of the
patients were followed for more than 1 year after exposure and were
found to maintain their improved glucose metabolism (Nath, 1976).
Of 10 non-diabetic elderly subjects with impaired glucose
tolerance, 4 responded to 150 µg chromium/day with complete
normalization of oral glucose tolerance tests. The remaining 6
subjects were not affected by the supplements (Levine et al.,
1968). Significant effects of a GTF-chromium-containing yeast
preparation were reported by Doisy et al. (1976) in 6 out of 12
elderly subjects with impaired glucose tolerance
Table 21. Effect of GTF supplementation on glucose tolerance tests in
elderly subjects with impaired tolerancea,b
-------------------------------------------------------------------------
Mean plasma-glucose levels Cholesterol Triglyceride
(mg/litre) (mg/litre) (mg/litre)
0 h 1 h 2 h
-------------------------------------------------------------------------
Before GTF 1060 ± 40c 2010 ± 70 1780 ± 80 2450 ± 90 1210 ± 80
11 tests
After GTF 990 ± 40 1620 ± 110 1320 ± 50 2050 ± 100 1120 ± 120
9 tests
Signifi- NS < 0.01 < 0.001 < 0.01 NS
canced
-------------------------------------------------------------------------
Mean serum-insulin levels
(microunits/ml)
0 h 1 h 2 h
Before GTF 24 ± 6 78 ± 17 118 ± 17
after GTF 26 ± 12 70 ± 8 83 ± 8
-------------------------------------------------------------------------
a From: Doisy et al. (1976).
b Supplement: 4 g yeast per min/day (Yeastamin Powder 95, Staley Co.,
Chicago, Illinois, USA). GTT: 100 g oral load.
c Mean ± SEM.
d Using paired t test, difference is significant.
In addition to the improvement in glucose tolerance in the
presence of a reduced insulin response, plasma-cholesterol
concentrations were significantly reduced. Several other
individual cases are reported in the publication of Doisy et al.
(1976), all of whom showed a lower serum-insulin response to a
glucose tolerance test during chromium supplementation.
Liu & Morris (1978) performed a similar study on the effects of
a yeast-chromium supplementation in 27 women, 40 - 75 years old, of
whom 15 had a normal and 12 an abnormal glucose tolerance test. In
11 of the normal subjects (73%), the integrated insulin response
was significantly reduced from 436 to 335 µU/ml ( P < 0.025) during
supplementation, without a statistically significant change in
glucose tolerance test. Significant reductions in the total glucose
response (10 650 - 9820 mg/litre; P < 0.001) and in the total
insulin response to glucose were observed in the subjects with
impaired glucose tolerance (1288 - 831 µU/ml; P < 0.001). The
glucose tolerance improved in 7 out of 12 women, but all 12 showed
a reduction in plasma-insulin.
The effects of supplementation of the diet with 200 µg
chromium/day (as chloride) on the glucose tolerance of 76 normal
adult volunteers was investigated in a double-blind cross over
study, with each period lasting 3 months. All 18 subjects entering
the study with impaired glucose tolerance (serum-glucose equal to
or greater than 1 g/litre, 90 min after a glucose load), showed
significant improvement during chromium supplements, but not with a
placebo, with an average decline in the 90-min serum-glucose levels
of 180 mg/litre ( P < 0.01) (Anderson et al., 1983b).
In another placebo-controlled study, 24 elderly volunteers were
given a daily supplement of either chromium-rich brewer's yeast or
chromium-deficient Torula yeast (9 g/day, for 8 weeks). The
glucose tolerance of the subjects given brewer's yeast
significantly improved with a concomitant decrease in plasma-
insulin levels, and a significant decline in plasma-cholesterol as
well as total plasma-lipids. No significant changes were observed
in the control group given the chromium-poor Torula yeast
(Offenbacher & Pi-Sunyer, 1980).
Additional, preliminary studies suggest a specific effect of
brewer's yeast on individual lipoprotein cholesterol fractions.
Twenty normal human subjects responded to daily supplementation
with 18 g of brewer's yeast over a 8-week period with a significant
reduction in low-density lipoprotein-cholesterol ( P < 0.01) and
with a simultaneous increase of 14% in the high-density lipoprotein
( P < 0.01) (Nash et al., 1979).
Similar results were observed in a 6-week supplementation trial
involving 8 physician volunteers, each of whom received 7 g/day of
brewer's yeast. Low-density lipoprotein-cholesterol declined by
nearly 18% ( P < 0.01), whereas the high-density cholesterol
fraction increased by 17.6%, resulting in a significant
( P < 0.001) decrease in the LDL/HDL ratio of 28% (Riales, 1979).
These studies suggest that the primary effect of the
supplementation must have been an increase in the effectiveness of
the insulin, because glucose tolerance was maintained or improved
in spite of lower insulin responses. It is important to note that
while the reduction in insulin levels occurred in all cases, an
improvement in glucose tolerance occurred in only a fraction. This
suggests two possible interpretations of all the results discussed
here, including those showing improvement in glucose tolerance in
only some of the subjects or no improvement at all. Lack of a
response to chromium could suggest that the subjects were not
chromium deficient and that their impaired glucose metabolism was
the result of other causes. Second, they may have responded with a
reduction in circulating insulin levels, as did all the subjects
with impaired glucose tolerance in whom the insulin response was
measured. Unfortunately, these measurements were not performed in
the earlier studies.
8.1.2.2. Malnourished children
Chromium deficiency has been implicated as a factor
contributing to the impairment of glucose tolerance in children
with protein calorie malnutrition in 3 countries, Jordan, Nigeria,
and Turkey. Hopkins et al. (1968) measured intravenous glucose
tolerance in 10 malnourished infants from the mountainous area of
Jordan and 9 equally malnourished infants from the valley area.
They found a severely depressed glucose removal rate of 0.7%/min in
the first group and a normal rate in the second, 3.8%/min (Table
22). The protein intake of both groups prior to hospitalization
had been identical; it was supplied in the form of a milk powder
with a chromium content of 18 µg/litre, when reconstituted.
However, analysis of 10 water samples revealed 3 times as much
chromium in the samples from the valley as in those from the
mountainous area (0.5 versus 1.6 µg/litre). This suggested the
possibility of chromium being a major determinant in the glucose
metabolism of these children, and the effects of supplementation
were measured in 6 children from the hills, with a severely
impaired tolerance test. The morning after oral administration of
250 µg chromium, as CrCl3 x 6H2O, the glucose removal rate had
markedly improved to 2.9%/min from a pretreatment level of
0.6%/min. Similar results were obtained in Nigeria (Table 22);
these tests also included control children who did not receive
chromium.
The immediate effects of chromium on these children were
remarkable and different from the lag phase always present in the
studies on adults. Equally important, was the short duration of
the effect of one dose demonstrated in a Jordanian child observed
for a longer period of time. These observations suggest that a
marked depletion in chromium stores must have existed in the
children.
Table 22. Significance of the effect of chromium on the impaired glucose
tolerances of malnourished infantsa
---------------------------------------------------------------------------
Subjects Number Glucose Significance
of removal of
infants rate difference
(%/min)
---------------------------------------------------------------------------
Jordanian infants from hill area with
0.5 µg chromium/litre in drinking-water 10 0.7
Jordanian infants from valley with
1.6 µg chromium/litre in drinking-water 9 3.8 P < 0.001
Jordanian infants;
initial glucose tolerance test 6 0.6 P < 0.001
after chromium treatment 6 2.9
Nigerian infants;
initial glucose tolerance test 6 1.2
after chromium treatment 6 2.9 P < 0.05
Non-treated infants:
Initial glucose tolerance test 5 1.9 not signi-
Repeated glucose tolerance test 5 2.1 ficant
---------------------------------------------------------------------------
a From: Hopkins et al. (1968).
Similar results were reported from a study in the Istanbul area
of Turkey (Gürson & Saner, 1971). Fourteen malnourished children
with an impaired glucose removal rate of 1.71%/min were given 250
µg of chromium (CrCl3 x 6H2O), and a second test was performed
after 15 h. The removal rate increased to 3.91%/min ( P < 0.001).
This average increase was due to a pronounced increase in 9 of the
children; no change was observed in the remaining 5. No
improvement in glucose tolerance occurred in 5 control children
receiving identical hospital treatment, except for the
administration of the chromium. The children receiving the
chromium supplementation grew significantly more than the control
children during the following 30 days, with the greatest growth
rates found in the 9 children who had responded to administration
of chromium with an improvement in glucose tolerance.
The fact that chromium deficiency interacts with protein-
calorie malnutrition only in certain geographical areas was
confirmed by Carter et al. (1968), who gave chromium
supplementation (250 µg/day as CrCl3 x 6H2O) without effects on
glucose tolerance in 9 Egyptian children tested. Plasma-chromium
levels were in the normal range, and chromium levels in
representative foods were high, ranging from 200 to 390 µg/kg.
These findings suggest that the chromium status of these subjects
was normal, which would explain the lack of an effect.
8.1.2.3. Patients on total parenteral alimentation
A female patient, maintained on total parenteral alimentation
since the age of 35 years, developed an unexpected weight loss of
15% after 5 years combined with peripheral neuropathy, impaired
intravenous glucose tolerance, in spite of normal insulin response,
and a decreased respiratory quotient of 0.66. Insulin treatment
(45 U/day) was ineffective. A strongly negative chromium balance
and low plasma- and hair-chromium levels suggested that the daily
estimated chromium intake from the infusion fluids of 5.3 µg had
not met the patient's requirement and resulted in chromium
deficiency. The insulin infusions were stopped and replaced by the
daily infusion of 250 µg chromium (as CrCl3 x 6H2O) for 2 weeks.
The result was normalization of glucose tolerance and respiratory
quotient, disappearance of signs and symptoms of neuropathy, return
to the previous normal body weight, in spite of reduced caloric
intake, disappearance of the requirement for exogenous insulin, and
a positive chromium balance. The nitrogen balance, which had been
erratic and often negative, became consistently positive following
chromium supplementation. Reduction of the daily supplement to 20
µg chromium proved to be sufficient to maintain the patient in a
state of well-being (Jeejeebhoy et al., 1977).
This case is the best demonstrated example of chromium
deficiency in a human subject. In addition to confirming the well-
known signs of chromium deficiency reported earlier, such as
insulin resistance and its consequences, it was the first case to
demonstrate the occurrence of peripheral neuropathy and disturbance
of nitrogen balance as a result of deficiency. These disturbances
require further study. A second case of chromium deficiency as a
consequence of long-term total parenteral alimentation was reported
by Freund et al. (1979). Signs and symptoms were similar to those
of the first case, except that central encephalopathy was present
instead of peripheral neural disorder. Supplementation with 150 µg
of chromium/day resulted in normalization of the deficiency signs.
Chromium at that time was not routinely added to intravenous
solutions, therefore it is possible that more cases of deficiency
may exist in patients on long-term infusion treatment. As an
outcome of this study, an Expert Panel of the American Medical
Association (AMA, 1979) suggested that the stable adult patient on
total parenteral alimentation should receive 10 - 15 µg chromium
daily.
8.1.2.4. Epidemiological studies
Two epidemiological studies suggest a link between chromium
status and cardiovascular diseases (Schroeder et al., 1970; Punsar
et al., 1975). Lack of chromium is associated with impaired
glucose tolerance, elevation of circulating cholesterol, and aortic
plaques. All of these variables are recognized risk factors for
cardiovascular disease. It has also been suggested that the
elevated insulin levels, often present in chromium-deficient human
subjects (Liu & Morris, 1978) represent a substantial risk for the
development of cardiovascular disease (Strout, 1977).
Schroeder et al. (1970) have summarized their studies of
chromium concentrations in normal and diseased tissues,
particularly in aortas (Table 23). The total body burden of
chromium, excluding the lungs, was lower in the USA, where there is
a higher incidence of cardiovascular disease, compared with other
regions, 1.72 - 1.86 mg/kg ash weight in the USA compared with 7.7,
9.6, and 2.7 mg in Africa, the Far East, and the Near East,
respectively. The only significant difference in tissue-chromium
concentrations in deaths from heart disease compared with deaths
from accidental causes was in the aorta (Table 23). Concentrations
in aortas from Africa, the Far and Near East were generally higher
and did not show such a difference.
Table 23. Chromium in aortasa
----------------------------------------------------------
Location Chromium (mg/kg dry weight)
Accident P Heart disease
----------------------------------------------------------
Africa 0.072 (5)b NS 0.116 (2)
Far East 0.97 (8) NS 0.246 (5)
Middle East 0.36 (8) NS 0.216 (3)
USA (Nine cities) 0.26 (103) 0.005 0.052 (13)
USA (San Francisco) 0.228 (10) 0.005 0.048 (15)
----------------------------------------------------------
a From: Schroeder et al. (1970).
b The number in parenthesis denotes the number of cases
observed.
The second epidemiological study was part of a cohort study
conducted in Finland beginning in 1959 (Punsar et al., 1975). A
total of 327 drinking-water samples were analysed for 22
characteristics including pH, anions, bulk minerals, and several
trace elements including chromium. The study included 504 and 622
men in the Western and Eastern areas of Finland, respectively,
whose domicile had not changed since 1959.
The coronary heart disease mortality rate is much higher in the
east of Finland than in the west. As seen in Table 24, the
chromium levels in drinking-water were much lower in the east than
in the west, whereas the opposite was true for copper. The metal
concentrations in the drinking-water of those with heart disease
and others only differed slightly (Table 24), but, according to the
authors, the data suggest that low chromium levels may play a role
in the high heart disease death rate of eastern Finland.
Table 24. Concentrations of copper and chromium in the
drinking-water of men who died between 1969 and 1973, and
of those who were alive in 1973a
-------------------------------------------------------------
Alive
Deaths (Clinical status in 1969)
CHDb Other CHDb Other No
heart heart
disease disease
-------------------------------------------------------------
West N = 19 N = 23 N = 63 N = 31 N = 288
Copper X 22.15c 20.17 20.05 20.94 20.15
(µg/litre) SD 3.92 4.69 4.69 5.12 4.74
Chromium X 7.52 9.38 9.45 9.74 8.45
(µg/litre) SD 2.06 3.42 4.01 4.44 3.31
East N = 23 N = 26 N = 129 N = 44 N = 279
Copper X 41.59 41.24 42.67 39.50 36.32
(µg/litre) SD 29.91 30.43 33.65 28.74 28.66
Chromium X 2.20 2.09 2.04d 2.49 3.01
(µg/litre) SD 2.17 2.41 2.08 2.17 2.37
-------------------------------------------------------------
a From: Punsar et al. (1975).
b CHD = coronary heart disease.
c P < 0.05.
d P < 0.001.
Note: Levels of significance refer to differences observed
when the concentrations were compared with those in the
"no heart disease" category.
Newman et al. (1978) measured serum-chromium concentrations in
32 patients, 18 male and 14 female, aged 25 - 65 years, in whom
coronary artery cine-arteriography was performed for medical
reasons. Serum-chromium was significantly lower in the 15 subjects
with coronary artery disease than in the 17 subjects without
involvement (2.51 versus 6.09 µg/litre; P < 0.01), whereas serum-
cholesterol, weight index, and systolic or diastolic blood pressure
did not differ among the groups. Serum triglycerides were higher
in the group with coronary artery disease (1.84 versus 1.12
g/litre; P < 0.05). No subject with a serum-chromium
concentration of more than 6 µg/litre exhibited coronary artery
involve- ment, and by regression analysis, serum-chromium was
judged to explain 17% of the total variance of the disease.
These results were independently confirmed in a second study in
which plasma chromium concentrations of 23 healthy persons were
more than 8 times greater than those of 67 patients with coronary
artery disease, as confirmed by cine-angiography (Simonoff et al.,
1984).
8.1.3. Mode of Action
As in experimental animals, the best known action of chromium
is the potentiation of insulin. This is shown by the
supplementation studies of Doisy et al. (1976) and Liu & Morris
(1978) in which a reduction in insulin levels in response to
glucose was observed, together with an improvement in glucose
tolerance. Direct proof, for example from in vitro studies with
human biopsy tissue, has not yet been obtained. Furthermore, it is
not known whether the potentiation of insulin is responsible for
all the effects observed during chromium supplementation, including
the reduction of circulating cholesterol and the growth stimulation
observed in malnourished children and during parenteral nutrition.
In the healthy, chromium-sufficient human subject, chromium
levels in the bloodstream increases acutely following a glucose
load or an injection of insulin. Glinsmann et al. (1966) observed
acute increases in 5 young, healthy volunteers during a glucose
tolerance test, but not when the same subjects were given an
equivalent volume of water. Maturity-onset diabetic patients did
not exhibit this chromium response initially, but did show a rise
in plasma-chromium, after several weeks of chromium
supplementation. These observations were confirmed by Levine et
al. (1968), Hambidge (1971), Behne & Diel (1972), and Liu & Morris
(1978), who saw the acute chromium rise in some, but not all,
subjects. In the study of Liu & Morris (1978), which was the most
detailed, the relative chromium response (CrR) was measured by
neutron activation analysis in 15 women with normal glucose balance
and in 12 with impaired tolerance.
(1-h serum-chromium)
CrR = ------------------------ x 100
(fasting serum-chromium)
Initially, the normal group had a mild positive response (CrR =
107), compared with a negative response (CrR = 81) in the impaired
subjects. Following three months of supplementation with a
chromium-rich yeast extract, the CrR of the normal subjects
increased to 140, and that of the impaired group became positive
with CrR = 149. As discussed in section 8.1.2, these changes were
accompanied by a significant decrease in the insulin response to
glucose and an improvement in glucose tolerance. The results of
these studies are in apparent contrast with those of Davidson &
Burt (1973), who found a significant decline in plasma-chromium
levels following a glucose load in normal, non-pregnant women (mean
age 26 years), but a mild to moderate increase in all but 1 of 10
pregnant women (mean age 20 years). As the time of the acute rise
varied between women, statistical analysis at any one time did not
produce any significant results. Hambidge (1971) and Behne & Diel
(1972) demonstrated an acute plasma-chromium response following
injection of insulin; it can therefore be assumed that the effect
of glucose on the chromium response is associated with
hyperinsulinaemia. The acute "relative chromium response" is
believed to reflect the chromium status of human subjects. It does
not appear when stores from which the chromium increment must come
are depleted. Depleted stores can be replenished by
supplementation, with a resulting reappearance of the response
shown in the two previously discussed studies. As it has been
shown that chromium levels in urine increase following a glucose
load (Wolf et al. 1974; Gürson & Saner, 1978), it is likely that a
fraction or all of the chromium increment is subsequently excreted.
8.2. Acute Toxic Effects
In adults, the lethal oral dose of soluble chromates is
considered to be between 50 mg/kg body weight (RTECS, 1978) and 70
mg/kg body weight (Deichmann & Gerarde, 1969). The clinical
features of toxicity are vomiting, diarrhoea, haemorrhagic
diathesis, and blood loss into the gastrointestinal tract causing
cardiovascular shock. If the patient survives about 8 days, the
outstanding effects are liver necrosis (Brieger, 1920), tubular
necrosis of kidneys, and poisoning of blood-forming organs
(Langard, 1980). A 14-year-old boy, who had ingested about 1.5 g
of potassium dichromate, died 8 days later. Kaufmann et al. (1970)
also reported enlargement and oedema in the boy's brain.
8.3. Chronic Toxic Effects
The chronic effects of trivalent and hexavalent chromium have
been reviewed by Langard & Norseth (1979). The most important
features are changes in the skin and mucous membranes, and allergic
dermal and broncho-pulmonary effects. Important systemic effects
occur in the kidneys, liver, gastrointestinal tract, and
circulatory system.
Mancuso (1951) studied several biological variables in persons
occupationally exposed to chromate and related them to clinical
signs and symptoms. He found occasional leukocytosis or
leukopenia, monocytosis, eosinophilia, reduced haemoglobin
concentrations and prolonged bleeding times, but none of them were
consistent enough to serve as reliable diagnostic tests.
Mild normochronic anaemia was reported by Myslyaeva (1965) in
94 subjects exposed to chromium compounds with 68 of them showing
signs of chronic toxicity. Though the report of Baetjer et al.
(1959b) was based only on 3 cases, it indicated that chromate
attached preferentially to red blood cells. Koutras et al. (1964)
reported the inhibition of the red blood cell enzyme gluthathione
reductase by chromate concentrations of 5 - 25 mg/kg body weight.
However, the lowest effective dose is far in excess of levels
found, even in heavily exposed persons. Thus, all attempts made so
far have failed to identify a specific sensitive biochemical test
for the assessment of chronic chromium toxicity.
Long-term surveillance of pregnant women working at a plant
producing dichromates and those living in its immediate vicinity
(Shmitova, 1980) revealed increased chromium levels in the blood
and urine, compared with those in the control group. At 32 weeks
of pregnancy, the blood concentrations of chromium were,
respectively, 2.5 mg/litre in the women workers, 1.2 mg/litre in
the women living near the plant, and 0.19 mg/litre in the controls;
the levels in the urine were 1, 0.47, and 0.045 mg/litre,
respectively. Umbilical venous blood contained 1.8, 0.66, and 0.37
mg chromium/litre, respectively. The placental chromium contents
in the surveyed women were 162, 179, and 40 µg/kg, respectively,
and the breast-milk levels were 119, 20, and 31 µg/litre, which
means that chromium is capable of transplacental entry into breast
milk and into the fetal and infant organism. Women working at the
bichromate plant exhibited a high incidence of obstetric pathology
and geotoses. Chromium levels were estimated in fetal and
placental tissues (abortive material) derived on the 12th week of
pregnancy from women workers of the dichromate facility and those
not exposed to chromium. In the test group, chromium levels were
1140 µg/kg in the fetal tissues and 135 µg/kg in the placental
tissue, whereas, in the controls, they were 928 and 30 µg/kg,
respectively. No teratogenic effects have been revealed (Shmitova,
1980).
8.3.1. Effects on skin and mucous membranes
Several different types of effects on skin and mucous membranes
may result from exposure to chromium chemicals, and the main
features are:
(a) primary irritation: ulcers (corrosive reactions),
scarred and non-ulcerative contact dermatitis;
(b) allergic contact dermatitis: eczematous and
noneczematous.
A general survey of the nature and cause of contact dermatitis
is given by Fregert (1981).
8.3.1.1. Primary irritation of the skin and mucous membranes
(a) Skin
Skin irritation, manifested by a sharp hyperaemia as well as by
vesicular, papular, and rash pattern, may result from contact with
chromates. However, the major problems in the primary irritation
dermatoses are ulcers, often called chrome holes or chrome sores.
Ulceration is likely to occur among workers who have contact with
high concentrations of chromic acid, sodium or potassium dichromate
or chromate, or ammonium dichromate. It does not result from
contact with trivalent chromium compounds. An ulcer may develop if
the chromium compound, either a dust or a liquid, comes into
contact with any break in the skin such as an abrasion, scratch,
puncture, or a laceration. Favoured sites for ulcer development
are the nailroot areas, the creases over the knuckles, finger webs,
the backs of the hands, and the forearms (Bloomfield & Blum, 1928).
Primary irritation by chromium can be attributed to hexavalent
chromium, as the salts of trivalent chromium are not considered
capable of producing such effects (Fregert, 1981). However,
prolonged exposure to trivalent chromium salts has been reported to
cause skin lesions, less marked than those following exposure to
hexavalent chromium salts (Domaseva, 1971). Findings in 164
patients with eczema showed that skin tests on people who had
contact with trivalent chromium did not give positive results with
dichromate (Valer & Racz, 1971).
Ordinarily, a chrome sore, if not deep, persists for about 3
weeks after exposure is discontinued. There is no evidence that
the ulcers undergo malignant transformation. Furthermore, the
presence of chromium ulcers did not influence the development of
sensitization to chromates (Edmundson, 1951), evidenced by patch
testing with a 0.5% solution of K2Cr2O7.
(b) Nasal septal mucosa
Perforation of the nasal septum was one of the main chronic
effects in workers who had contact with chromates and chromic acid
(Bloomfield & Blum, 1928; Langard & Norseth, 1979). In a group of
100 persons constantly in contact with chromium compounds, 44 had
superficial ulceration of the anterior nasal septal mucosa.
Twenty-one people had profound ulcers and 5 had perforations of
various sizes in the cartilaginous part of the nasal septum.
Perforation of the nasal septum and cicatricial alterations were
observed in people with 3 or more years of service, whereas
ulcerous lesions arose in 2 years of work with chromium compounds.
An additional consequence of the effect on the nasal mucous
membranes is a loss of the senses of smell and taste (Seeber et
al., 1976). In workers with profound ulcerous lesions of the nasal
septal mucosa, strains of white staphylococcus with elevated
resistance to chromium are sometimes isolated (Cherevaty et al.,
1965).
Pokrovskaya et al. (1976) studied 561 workers engaged in the
open excavation of chromium ores (chromium content was 62% in the
form of a complex compound of magnesium chromite and other chromium
ores). Almost half of the workers had worked there for more than 5
years, and about 40% had been working there for 6 - 15 years.
Changes in the nasal septal mucosa, typical of the effect of
chromium compounds, were found in 8.2% of the workers. The longer
the service record, the greater the prevalence of these lesions.
After 5, 11, and 15 years of work, the prevalence was 2.3%, 8.3%,
and 11.2%, respectively.
The prevalence of nasal septum perforation in chromium plating
workers was very high (24%) in the early 1950s in China (Yang,
1956). With the introduction of control measures, the prevalence
dropped so that, in 1982, only 2 cases of nasal septum perforations
were found among 393 chromium-exposed workers (Peng, 1982).
One hundred and four subjects (85 male, 19 female) exposed to
chrome plating and 19 unexposed controls were studied by Lindberg &
Hedenstierna (1983) with regard to changes in the nasal septum.
Exposed subjects were employed at 13 different companies and
included all who were working on the day of study at the particular
company. The median exposure time was 4.5 years (range, 0.1 - 36
years). Forty-three subjects were exposed almost exclusively to
chromic acid and constituted both a "low exposure" group (8-h mean
below 2 µg/m3, N = 22) and a "high exposure" group (2 µg/m3 or
more, N = 21). Their median exposure time was 2.5 years (range,
0.2 - 23.6 years). The other 61 subjects were exposed to a mixture
of chromic acid (0.2 - 1.7 µg/m3) and other pollutants, such as
nitric, hydrochloric, and boric acids, as well as caustic soda,
nickel, and copper salts. The last group was included to disclose
any additive or synergistic effects of chromic acid and other
pollutants and was studied with regard to lung function only. The
breakdown of subjects into various groups is shown in Table 25.
Nasal septal ulceration and perforation were seen in 10 out of 14
subjects exposed to peak levels of 20 - 46 µg chromic acid/m3 or
more; no one in the control group showed signs of atrophy,
ulcerations, or perforations.
8.3.1.2. Allergic contact dermatoses
Chromium is a very important skin sensitizer. Sensitization
is reported to require about 6 - 9 months, but can occur in less
than 3 months (Pirilä & Kilpio, 1949). Genetic factors appear to
contribute to the appearance of chrome sensitivity within the
Jewish population (Wahba & Cohen, 1979).
Apart from the primary irritation and ulceration effects,
direct contact with small amounts of the chromates may cause
allergic dermatosis (dermatitis and eczema). As this allergic
reaction is cell-mediated, i.e., antibody formation is intra-
cellular and not humoral, delayed allergic reactions are rarely
observed.
Fregert et al. (1969) reported that the skin-patch test for
chromium was positive in 8 - 15% of all patients suffering from
eczema. The dermatitis caused by chromium is described as a
diffuse erythematous type with severe cases progressing to an
exudative stage. Persons sensitive to chromium react to patch and
intracutaneous tests with nonirritant concentrations of potassium
dichromate (K2Cr2O7). Opinion is divided about the role of the
oxidation state of chromium as a causal agent in allergic contact
dermatoses (Morris, 1958; Fregert & Rorsman, 1964; Kogan, 1971).
In a review, Polak et al. (1973) showed that hexavalent chromium,
which penetrates the skin by a direct effect of a hapten, is
reduced to the trivalent state, chiefly by sulfhydryl groups of
aminoacids. It then conjugates with proteins thus forming the full
antigen that initiates sensitization.
Guinea-pigs sensitized with either trivalent chromium chloride
or hexavalent potassium dichromate are capable of reacting in vivo
and in vitro to challenges with both chromium salts. This double
reactivity is also found after repeated stimulation with only one
of these chromium compounds. Since it is not possible to select
lymphocytes directed specifically against a chromium determinant of
a particular valence, it is concluded that, by sensitization with
chromium salts of different valences, a common determinant or
closely related determinants are formed. It is suggested that this
determinant is formed by chromium in the trivalent form
(Siegenthaler et al., 1983).
Two to three years after medical treatment, a follow-up study
on 555 patients with contact dermatitis was completed by means of
questionnaires (Fregert, 1975). The eczema had healed in one-
quarter of the patients, one-half had periodic symptoms, and one-
quarter had permanent symptoms. The prognosis for persons who
changed their work or stopped working was the same as that for
those who continued eczema-inducing work.
From 48 positively tested patients (0.5% potassium dichromate),
38 persons, i.e., 79%, still had a positive patch test after 4 - 7
years. In 72% of the cases, a history of occupational exposure to
chromates could be proved (Thormann et al., 1979).
A fairly rapid transformation of dermatitis into eczema is a
characteristic feature of chromium-induced skin lesions. Kogan
(1971) recognized the following important properties: (a) absence
of adaptation, which makes it necessary to discontinue chromium
handling until the slightest clinical manifestations have
disappeared; (b) a persistent chronic development of an eczematous
process; (c) a long-term latent period; and (d) absence of a direct
correlation between the incidence of sensibilization in the exposed
workers and the chromium concentration to which they have been
exposed.
Table 25. Series of exposed subjects and referencesa
--------------------------------------------------------------------------------------------------------
Exposed subjects
Mixed exposure to
Low exposure to High exposure to chromic acid
chromic acid chromic acid (< 2 µg/m3) and other
(< 2 µg Cr6+/m3) (> 2 µg Cr6+/m3) acids and metallic salts References
----------------- ------------------ ----------------------- -----------------
Nb Age (years)c N Age (years) N Age (years) N Age (years)
--------------------------------------------------------------------------------------------------
Men
Non-smokers 10 35.8 ± 16.7 6 33.4 ± 14.1 12 33.6 ± 12.2 52 34.2 ± 13.2
Smokers 6 30.8 ± 9.7 16 30.2 ± 11.8 36 42.8 ± 13.1 67 30.5 ± 8.9
Women
Non-smokers 3 30.7(17-46) 0 9 48.3 ± 13.0
Smokers 2 44.5(29-60) 1 51.2 4 31.0(19-43)
--------------------------------------------------------------------------------------------------
a From: Lindberg & Hedenstierna (1983).
b When N < 5, the range is given instead of SD.
c X ± SD.
Patients suffering from chromium-induced allergic skin
dermatoses had a tendency to develop cross-correlated hyper-
sensitivity to other metals, in particular, to cobalt and nickel
(Rostenberg & Perkins, 1951; Geiser et al., 1960; Clark & Kunitsch,
1972; Kogan et al., 1972).
Increased sensitivity to chromium is proved by skin reaction
tests. In a number of cases, additional immuno-haematological
bioassays are made to confirm the specificity of these tests. A
method for studying the basophil (mast-cells) degranulation in vivo,
at the site of positive compress skin tests, was suggested by Kogan
(1968). The method was used as a basis for proving the specificity
of cutaneous tests for chromium and other sensitising metals of the
chromium group (cobalt and nickel) (Kogan, 1968; Kogan et al.,
1972).
Photosensitivity is sometimes observed in patients with chronic
dermatitis. Wahlberg & Wennerstein (1977) investigated patients
allergic to chromium, with or without clinically observed light
sensitivity, by a standardized photopatch test procedure. A
significantly more intense reaction was observed in 48% of the
cases in the UV-irradiated sites (4/5 minimal erythema dose)
compared with the non-irradiated control sites.
No cases of cancer of the skin have been reported to result
from exposure to any form of chromium (Fregert, 1981).
8.3.2. Effects on the lung
Airborne chromium trioxide is rapidly absorbed in the broncho-
pulmonary tract causing corrosive reactions (Borghetti et al.,
1977). The absorption of chromium aerosols may depend on the
physical and chemical properties of the particles (Taylor et al.,
1965), as well as on the activity of alveolar macrophages, and the
lymphatic drainage (Sanders et al. 1971).
Both Jindrichova (1978) and Keskinen et al. (1980) reported
that welders using chromium-containing electrodes suffered from
bronchitis. Zober (1982) studied arc welders who had used filler
metals containing chromium and nickel for some years. Compared
with controls, the welders reported more previous ailments of the
respiratory system, mainly in the form of acute bronchitis. In
X-rays of welders, more roundish shadows were found, probably
indicative of benign accumulations of ferrous dust. The greater
incidence of significant findings was clearly apparent in welders
who smoked (probably a combined effect of tobacco smoke and welding
fumes). Reggiani et al. (1973) studied 101 electroplaters by means
of spirometric tests and found an obstructive disease pattern in
13% of them. After inhalation of acetylcholine, a bronchospastic
reaction occurred in 23% of the workers. In another spirometry
study (Bovet et al., 1977) on 44 chromium electroplaters, an
obstructive respiratory syndrome was found among the workers with a
high urinary-chromium content. The authors concluded that the
influence of tobacco smoking on the spirometric tests was much less
than the influence of chromium.
Studying lung function and generalized obstructive lung
diseases in electrofurnace workers in a ferrochromium and
ferrosilicon-producing plant, Langard (1980b) found a reduced
forced vital capacity (FVC) and an increased prevalence of
obstructive lung diseases. Unable to document a connection between
the presence of chromates and increased lung diseases, the author
suggested that the effects were due to high levels of total dust,
especially amorphous silica dust.
Lindberg & Hedenstierna (1983) studied lung function in more
than 100 subjects exposed to chrome plating and in 119 car
mechanics as controls (no car painters or welders). In the exposed
subjects, FVC and the forced expired volume in one second (FEV1)
were both reduced by 0.2 litre, and the forced mid-expiratory flow
was reduced by 0.4 litre/second. The authors concluded that an 8-h
mean exposure level of more than 2 µg/m3 might cause a transient
decrease in lung function.
Pneumoconiosis was diagnosed in Germany (Letterer et al., 1944)
and again in chromite miners in South Africa (Sluis-Cremer & du
Toit, 1968). However, American medical officers could not confirm
this feature when examining 897 workers in chromate-producing
plants in the USA (US PHS, 1953). It should be noted that chromium
dusts had a poor fibrogenic potency in experimental animal studies
(section 7.2.1.5). Davies (1974) X-rayed workers employed in the
manufacture of ferro-chrome and found results similar to miliary
lung tuberculosis. The workers were in the immediate area of the
furnace and were exposed to dust-particles, 90% of which were less
than 2 µm in diameter. The dust deposited on girders consisted of
95% SiO2 and 2.4% Cr2O3. The author did not find any symptoms or
signs of tuberculosis, but there was sufficient evidence to
indicate an inhalation hazard in the ferro-chrome industry, thus
underlining earlier observations of Princi et al. (1962).
8.3.2.1. Bronchial irritation and sensitization
Chromium irritates mucous membranes, as has been seen with
exposure to airborne chromium trioxide fume (Meyers, 1950) or mixed
dust (Card, 1935; Broch, 1949; Williams, 1969). The first symptom
was an irritating cough, 4 - 8 h after exposure. Chromium causes
sensitization that can result in asthmatic attacks. These can last
for 24 - 36 h, without treatment (Langard & Norseth, 1979). An
attack may recur on later exposure, even when this exposure is to a
much lower concentration (US NAS, 1974a).
A double-blind experimental exposure study on an asthmatic
patient, who had been exposed to chromium and nickel, showed that
chromium was the main cause of the asthma (Novey et al., 1983).
Keskinen et al. (1980) pretreated their patients, stainless steel
welders, with both disodium cromoglycate and betamethasone and
found an inhibition of this reaction. Placebo medication failed to
produce this inhibition.
8.3.3. Effects on the kidney
In the early part of this century, chromates and chromic acid
were occasionally used as therapeutic chemicals for some skin
lesions. Fatal cases of acute nephritis occurred with albumin,
hyaline, and granular casts and red cells appearing in the urine,
accompanied by oliguria. Autopsies revealed tubular necrosis, but
not glomerular damage (Kaufman et al., 1970). Acute toxic
gastroenteritis developed, followed by arterial hypotonia, in
suicide cases a few hours after swallowing 1.5 - 10 g potassium
dichromate. Within 2 - 4 days, all patients developed signs of
acute renal insufficiency with oliguria, anuria, and hyperhydration
and also evidence of acute toxic hepatitis (Luzhnikov et al.,
1976). Kidney damage has also occurred in industry, as a result of
accidental exposure of the skin to large amounts of chromate,
especially when associated with extensive skin damage (Luzhnikov et
al., 1976).
The mortality from kidney diseases among workers in the
chromium industry does not appear to have increased. In one study,
no deaths from nephritis and uraemia were found in 4 chromate-
producing plants; in only 1 plant was the mortality rate above that
for the controls (Machle & Gregorius, 1948). Hayes et al. (1979)
reported that 4 deaths from nephritis and nephrosis had occurred
between 1945 and 1977 in workers in a chromate-producing plant,
compared with 4.95 cases in the control population. Satoh et al.
(1981) found one case of nephritis and nephrosis in a group of
chromate workers compared with an expected number of 2.18.
Some authors (Franchini et al., 1975; Borghetti et al., 1977;
Gylseth et al., 1977; Tola et al., 1977) have concluded that
urinary excretion and renal clearance of diffusible chromium can be
used as biological indicators for evaluating the extent of exposure
to airborne chromium and the body burden. However, a dose-effect
relationship could not be established between duration of exposure
and either the level of chromium excretion (Tandon et al., 1977) or
early nephrotoxic indicators (Mutti et al., 1979). A dose-
response relationship for hexavalent chromium effects on the kidney
was described by Mutti et al. (1979), measuring beta-glucuronidase,
protein, and lysozyme in the urine. Abnormal patterns were not
observed in 39 stainless-steel welders during 4.5 ± 3.2 years of
exposure. In another group of workers employed in welding armoured
steel with special electrodes for 1 ± 0.4 years, 22% showed an
increase in beta-glucoronidase and 10% in protein in the urine. In a
study on 24 workers in the chromium-plating industry who were
exposed for 8.9 ± 7.3 years, the authors found increased urinary
levels of beta-glucoronidase in 37% of the workers, an increase in
protein in 17%, and an elevated level of lysozyme in 4%. Urinary
beta2-microglobulin was also studied by Lindberg & Vesterberg (1983b)
in chromeplaters (24 males, mean age 36 years, working in 4
different factories). The exposure was measured using personal air
samplers. The 8-h mean value ranged between 2 and 20 µg hexavalent
chromium/m3 and averaged 6 µg/m3. Most of the workers showed
symptoms of airway irritation, 2 of them had ulcerated nasal septum
and another 2, complete perforation. Significantly higher urine
beta2-microglobulin levels (0.23 mg/litre; range, 0.04 - 1.24 mg/litre;
SD, 0.27) were found in this group of workers in comparison with a
reference group of workers of a comparable age (mean age, 38 years;
mean urinary beta2-microglobulin level, 0.15 mg/litre; range, 0.06 -
0.19 mg/litre; SD, 0.08). A relationship was observed (Table 26)
between the range of exposure and prevalence of urinary beta2-micro-
globulin levels exceeding 0.3 mg/litre. The 2 workers with
perforated nasal septum were in the highest exposure group. A
difference in urinary beta2-microglobulin levels was not found between
a group of workers employed several years earlier in an old
chromeplating plant (mean, 0.25 mg/litre) and a group of workers of
comparable age (mean, 0.29 mg/litre), but it should be noted that
the mean age of both these groups exceeded 52 years.
Table 26. beta2-microglobulin U-beta2 levels in urine of workers
related to present exposure levelsa
-------------------------------------------------------------------------
Plant Exposure Number Age Persons with an "elevated" U-beta2
No. range of (mean) (U-beta2 > 0.30 mg/litre) range
(µg/m3) workers concentration (mg/litre)
No. Ages
-------------------------------------------------------------------------
1 11 - 20 5 39 3 21, 37, 58 0.23 - 1.30
2 - 3 14 - 8 13 37 2 20, 21 0.04 - 0.44
4 2 - 3 6 6 - - 0.06 - 0.18
-------------------------------------------------------------------------
a From: Lindberg & Vesterberg (1983b).
8.3.4. Effects on the liver
Statistics on diseases of the liver are seldom published
separately; they are usually included in the group of diseases of
the digestive system. The chromate industry was reported to have
the same rate in this group of diseases as many other industries
(US PHS, 1953). The Federal Security Agency in the USA
investigated 14 chromate workers reported to have "enlarged livers"
but found that these cases were not related to the time spent in
the industry. Acute hepatitis with jaundice was described in one
worker employed in a chromium-plating plant.
In an epidemiological study, Satoh et al. (1981) reported a low
rate of cirrhosis of the liver in chromium workers and liver
function test results that did not differ significantly from those
of the controls.
However, following ingestion of high doses of potassium
dichromate, liver necrosis (Brieger, 1920) and congested liver
(Kaufman et al., 1970) with loss of its architecture have been
described. There are also case studies of liver function and
histology in electroplaters (Pascale et al., 1952) and chromate
chemical plant workers (Etmanova, 1965), but the results are
difficult to interpret.
8.3.5. Effects on the gastrointestinal tract
The following symptoms and signs were reported in workers
engaged in the production of chromium salts (Sterekhova et al.,
1978): hyperchlorhydria, elevated pepsin and pepsinogen level,
oedema, hyperaemia and erosion of the mucosa, polyposis,
dyskinesia, and gastritis. However, Satoh et al. (1981) reported
that the incidence of peptic ulcer in chromate workers was below
the expected rate, and another study showed that the mortality rate
for diseases of the digestive system was lower in chromate workers
than in the control population (Hayes et al., 1979).
The gastric juice has been shown to play a role in the
detoxification of ingested hexavalent chromium by reducing it to
trivalent chromium, which is poorly absorbed and eliminated with
the faeces (Donaldson & Barreras, 1966; DeFlora & Boido, 1980).
8.3.6. Effects on the circulatory system
A clinical study was performed by Kleiner et al. (1970) on
myocardial function in more than 200 workers in the potassium
chromate industry, suffering from pulmonary or gastrointestinal
diseases, attributed by the authors to chromium poisoning. A
control group of 70 healthy individuals was included. Pathological
changes in the ECG and in other tests of heart function indicated
disturbance of right heart function, which the authors considered
secondary to the pulmonary pathology. The full paper was not
available and, consequently, the data could not be evaluated in
detail. It should be noted that no unexpected findings in
cardiovascular mortality were cited in the epidemiological studies
described in section 7.3.9.
8.3.7. Teratogenicity
The only human data available concerning this topic were
reported in an abstract (Morton & Elwood, 1974). The authors did
not find any correlation between frequency of malformations in the
central nervous system and the chromium content in water samples
collected in South Wales. Furthermore, Suzuki et al. (1979) did
not find any indication of accumulation of chromium in fetal
tissues.
8.3.8. Mutagenicity and other short-term tests
Few reports exist dealing with chromium-induced mutagenicity in
human subjects, and only hexavalent chromium causes any effects.
Bigaliev et al. (1978) found a 3 - 8% increase in chromosomal
aberrations of peripheral blood leukocytes in workers handling
chromium-compounds, compared with 2% in unexposed controls.
When classified according to the Papanicolaou system,
cytological samples of the sputum of 116 workers in the chromate-
production industry did not show any stages of tumour development
(Maltoni, 1976). However, intermediate stage lesions found in 30
workers were described as atypical adenomatous proliferation,
squamous-cell, and basal-cell dysplasia.
Studying cultured lymphocytes obtained from workers
occupationally exposed to CrO3, Sarto et al. (1982) found an
increased frequency of sister chromatid exchanges compared with
controls, which was correlated with urinary-chromium levels and
enhanced by smoking habits.
8.3.9. Carcinogenicity
8.3.9.1. Lung cancer
(a) Epidemiological studies in chromate-producing plants
The first recognition that cancer of the respiratory tract
might be related to chromium exposures resulted from the reporting
in 1932 of 2 cases of bronchogenic carcinoma that had occurred in
an old German chromate chemical plant (Lehmann, 1932). Gross &
Kölsch (1943) reported 10 deaths from lung cancer in small plants
producing the chromates of lead and zinc. By 1947, 52 such cases
had been reported from this industry and 10 cases from a chrome
pigment plant, also in the Federal Republic of Germany (Baetjer,
1950a).
The first epidemiological study was reported in the USA in
1948. The mortality data were based on the records of the group
life insurance policies of 6 chromate-producing plants in the USA
compared with similar data from a non-chromium industry or from a
life insurance company. The study reported that 21.8% of all
deaths between 1938 and 1947 in the chromate plants were due to
cancer of the respiratory tract compared with 1.4% in the control
group. The crude death rate for cancer of the bronchi and lungs
was 28 times that for the control groups (Machle & Gregorius,
1948). In one of these plants (Mancuso & Hueper, 1951), the cancer
death rate was 15 times that of the general population in the
county where the plant was located. The US PHS (1953) also studied
the respiratory cancer risk in these 6 plants and estimated a
relative risk of 28.9 using the average cancer mortality rates for
US males between 1940 and 1948 as a comparison.
In addition to these studies, where the cause of death was
taken from insurance or death certificate records, a case-control
study, using only cases diagnosed by autopsy or biopsy, was
conducted by Baetjer (1950b). Two hospitals near the largest
chromate plant in the USA were asked to supply the histories of all
cases diagnosed in 1924-46 as cancer of the lung bronchi, and to
confirm histopathologically. A group of non-cancer cases matched
individually by age, sex, and date of admission was selected as
controls. Of the 290 confirmed cancer cases, 3.26% had been
employed in the chromate plant, whereas none of the 900 control
cases was found to have had chromate exposure. The percentage of
chromate workers in the lung cancer series was 28.6 times the
percentage of chromate workers among the employed male population
of the city. The plant involved in this study was rebuilt in 1950,
following the discovery of the cancer problems. A new study of
this plant was made by Hayes et al. (1979) to determine whether the
risk of cancer had been eliminated. The investigation covered 438
deaths among 2101 males, who were initially employed between 1945
and 1974 for more than 90 days, and who died between 1945 and 1977.
The SMRa for malignant neoplasms of the trachea bronchi and lungs
was 202. Cancer deaths in the city in which the plant was located
were used to calculate the expected number of cases. No cases of
respiratory cancer occurred in the plant between 1960 and 1977
among workers employed only in the new plant. Because of the long
latent period, further follow-up is necessary.
Increased lung cancer rates among chromate workers in the USA
have been confirmed by Taylor (1966), Enterline (1974), and Mancuso
(1975), who also showed that the risks were especially high during
the early years of the study of the cohorts, e.g., before 1950.
Studies conducted in other countries have shown similar results.
In a 1956 study on workers in chromium-producing plants in the
United Kingdom, the rate of lung cancer mortality was 3.6 times the
rate for England and Wales (Bidstrup & Case, 1956). In a follow-up
study on 2715 men who had worked at the 3 chromate-producing
factories in the United Kingdom from 1948 to 1977, Alderson et al.
(1981) found 116 deaths from lung cancer (expected, 48). Since
modification of the one plant that is still in operation, the
relative risk of lung cancer has decreased from over 3 to about
1.8.
Two epidemiological studies have been conducted in the Japanese
chromate industry. In one plant, 5 lung cancer deaths occurred
between 1960 and 1973 in 136 workers who had been exposed for more
than 9 years; the SMR was 1510 (Watanabe & Fukuchi, 1975). In
another plant, the mortality rate for respiratory cancer was
determined for 896 males who worked in the plant from 1918 to 1975
and were followed to 1978; the SMR was 920 (expected deaths based
on 1975 age-cause-specific mortality rates for Japanese males)
(Satoh et al., 1981).
In the Federal Republic of Germany, a study was conducted in 2
chromate-producing plants (Korallus et al., 1982). Comparing the
SMR for lung cancer during the period 1948-79 for these 2 groups, a
clearly decreasing tendency was observed. In the first plant, for
1948-52, the SMR was 512 and, for 1978-79, it was 98; for the other
plant, the corresponding figures were 1905 and 150, respectively.
The newest data indicate a decreasing risk approaching the
frequency in unexposed people (Korallus & Loenhoff, 1981). This is
a result of occupational hygienic and technological measures,
especially of a change in manufacture namely the "on line"
--------------------------------------------------------------------
a The SMR (standardized mortality ratio) is the ratio of
observed to expected deaths times 100.
processing of the chrome ore, which has minimized the contents of
the carcinogenic hexavalent chromium compounds within the process
(Korallus et al., 1982).
Bittersohl (1972) described the results of a study on 30 000
employees of a large chemical unit for the period 1921-70 in the
German Democratic Republic. In particular, 588 malignancies in men
and 170 in women were evaluated for the period 1957-70. In 1971,
108 new malignancies in men and 29 in women came to light. In a
chromate factory, the rate of all cancers was far above average.
The factory manufactured catalysts through the reaction of chromic
acid and iron (III) oxide and nitric acid. The airborne
concentrations of chromium were not reported in detail, but it was
stated that short-term exposures above 400 µg/m3 occurred. The
incidence of malignant neoplasms in employees in the chromate
factory was 852 per 10 000 employees. In "unexposed" personnel,
the incidence of malignant neoplasms was 84 per 10 000 employees.
Approximately 86% of all those with malignant neoplasms were
smokers, and 78% of those without malignant neoplasms were also
smokers, indicating that smoking is not likely to be a confounding
factor.
In the USSR, a high incidence of cancer of the lungs was
reported for men engaged in the production of chrome salts. The
ratio of lung cancer in the plant to that in the control population
was 6.4 for ages 50 - 59 (748 observed, 116 expected) and 15.7 for
ages 60 - 69 (2657 observed, 170 expected) (Tyushnyakova et al.,
1974).
A survey was conducted in China in 1982 to investigate the
problem of lung cancer among chromate-producing workers. It
covered 7 cities and included 2184 male and 798 female workers who
had worked for at least one year in the industry. The preliminary
report revealed that 11 of the 101 deaths among male workers were
due to lung cancer, but none of the 13 deaths among female workers
was due to this cause.a
(b) Epidemiological studies - chrome pigment industry
The first epidemiological study, conducted in 1973, covered 3
small chrome pigment-manufacturing plants in the USA. The
percentage of lung cancer deaths was tentatively reported to be
about 3 times that in unexposed workers (Equitable Environmental
Health Inc., 1976).
Lung cancer mortality was studied among 1152 men working at 3
chromate pigment factories in the United Kingom from the 1930s or
1940s until 1981 (Davies, 1979, 1984). Among workers at factory A,
which produced both zinc and lead chromates, entrants to the
factory between 1932-45 with high and medium exposures had excess
---------------------------------------------------------------------------
a Report of an Investigation Team for Cancer in Chromate
Workers (1983).
risks of lung cancer (SMR = 223). A similar excess was seen in
1946-54 entrants, but the excess risk fell just short of
statistical significance. Highly significant excess risks of lung
cancer were seen among 1947-64 entrants that had high or medium
exposures at factory B, which also produced lead and zinc
chromates. Excesses were more severe in workers employed for more
than 10 years. In factory C, which produced only lead chromate, no
excess of lung cancer was found. However, the number of workers
and the power of the study were small.
Twenty-four male workers, who had been employed in a small
Norwegian company for more than 3 years between 1948 and 1972, were
identified and followed up by Langard & Norseth (1975), because
their principal exposure was to zinc chromate dust (measured
routinely with readings occasionally up to 0.5 mg hexavalent
chromium/m3). By December 1980, 6 cases of lung cancer had been
diagnosed, giving a relative risk of 44 compared with that of the
general population in the country. The authors stated that 5 out of
the 6 patients were smokers and only one had been exposed to
chromates other than zinc chromates (Langard & Vigander, 1983).
Sheffet et al. (1982) undertook a detailed mortality study on
workers employed in a pigment plant in Newark, where lead and zinc
chromates were used. Observed deaths from each cause among 1296
white and 650 non-white males employed between 1940 and 1969, were
compared with expected deaths, as computed from cause-, age-, and
time-specific standard death rates for the USA. A statistically-
significant relative risk of 1.6 for lung cancer among white
males, employed for 10 years or more, was found. A relative risk
of 1.9 was noted for individuals employed for at least 2 years, who
were at least moderately exposed to chromates.
An epidemiological study covering several centres in Europe was
conducted by Frentzel-Beyme (1983). This study was designed to
quantify the mortality from cancer and other diseases among workers
in European factories producing chromate pigments (3 German, 2
Dutch factories). Observed deaths in factories were compared with
expected deaths calculated on the basis of mortality figures for
the region in which a given factory was located. The overall
mortality did not deviate from the expected rates. Lung cancer
rates were always in excess of expected numbers, but only in one
cohort was the excess statistically significant. The pattern of
duration of exposure indicates that the lung-cancer risk was not
clearly correlated with length of employment.
A slight excess of lung cancer risk in chrome pigment (zinc
chromate) spray painters from 2 maintenance bases in the USA was
reported by Dalager et al. (1980). Among the 202 deaths
identified, 21 were due to respiratory cancer (11.4 expected;
comparison population: proportionate mortality, US males). No
statistically significant excess of lung cancer was found in a
group of automobile painters in which 226 deaths, including 22
cancer cases, were analysed using proportionate mortality for areas
in which the plants were located as a comparison (Chiazze et al.,
1980).
(c) Epidemiological studies - ferro-chrome industry
Langard et al. (1980) reported that 7 cases of bronchogenic
carcinoma occurred among 976 workers, who started work before
January 1, 1960. The expected number was 3.1 using national rates,
1.8 using local rates, and < 1 when using an international
reference rate for comparison. The difference was not significant
for the national and local rates, but was significant for the
international rate.
A study in Sweden did not reveal any significant excess of
respiratory cancer in ferro-chrome workers (Axelsson et al., 1980).
The incidence of cancer among 1932 workers employed for at least
one year between 1930 and 1975 was compared with the expected
number based on the National Cancer Registry data for the county in
which the plant was located. Five cases of respiratory cancer were
found against an expected 7.2 cases.
An excessive rate of lung cancer was reported for a ferro-
chrome plant in Russia (Pokrovskaya & Shabynina, 1973). The number
of deaths from cancer between 1955 and 1969 was higher than that in
the town in which the plant was located. The authors considered
that both hexavalent and trivalent chromium exposures were
responsible for the excess, since similar excessive rates were
found in a nearby ore-crushing plant, where only trivalent chromium
was present. The number of cases and the rates involved in these
studies were not given.
Hexavalent chromium is produced in some types of welding. In
section 7.2.1.2, it was mentioned that welding fume particles were
positive in the mammalian spot test, indicating a mutagenic and
possible carcinogenic potential. The incidence of lung cancer was
determined by Sjögren (1980), in a cohort of 234 welders, who had
welded stainless steel for more than 5 years between 1950 and 1965,
and were followed to 1977. Three welders died from lung cancer in
comparison with an expected number of 0.68 in the general
population ( P = 0.03).
Comparing lung cancer mortality in 2 parishes with ferro-alloy
industries to mortality in other parishes of similar size without
such industries from the same county, Axelson & Rylander (1980)
were unable to detect an increased incidence of death due to lung
cancer mortality. The concentration of chromium in the ambient air
of the most polluted areas ranged from 0.1 to 0.4 µg/m3. It should
be noted that several possible confounding factors were not under
control, e.g., migration, occupational exposure, and smoking
habits.
(d) Epidemiological studies - chromium-plating workers
Epidemiological studies on workers in the chromium-plating and
related industries were reviewed by Hayes (1982). The relevant
data from these studies are presented in Table 27. According to
Hayes, the epidemiological evidence is not sufficient to determine
the risk of cancer associated with industrial exposure to chromic
acid and, if an excess risk exists, it is probably lower than that
typically described for employment in the chromium-chemical
producing industry.
In a more recent paper, Franchini et al. (1983) conducted a
retrospective cohort study in 9 chromium-plating plants to examine
the mortality of workers employed for at least one year during the
period January 1951 to December 1981. The study group totalled 178
individuals; vital status ascertainment was 97% complete. The
total number of deaths was close to the expected figure (15
observed, 15.2 expected), whereas death from lung cancer exceeded
the expected number (3 observed, 0.7 expected; P = 0.03). The
increased mortality from lung cancer among chromium-platers seemed
to be related to exposure intensity.
8.3.9.2. Cancer in organs other than lungs
A few cancers of the upper respiratory tract and oral region,
but no excessive rates have been reported to occur in the chromate-
producing industry. The cancers have involved the buccal cavity,
pharynx, and oesophagus. Cancers of the nasal septum have not been
documented, with the exception of one case in Italy (Vigliani &
Zurlo, 1955).
In a joint Danish-Finnish-Swedish case-reference investigation,
initiated in 1977, the connection between nasal and sinonasal
cancer and various occupational exposures was studied. All new
cases of nasal and sinonasal cancer were collected from the
national cancer registers (Finland and Sweden) or from the hospitals
(Denmark). The results showed associations between nasal and
sinonasal cancer and exposure to chromium, welding, flame-cutting,
and soldering, hardwood or mixed wood dust, and softwood dust alone
(13.4) (Hernberg et al., 1983).
In 5 plants in the USA, during the early years of extremely
high exposures, the rate of cancer of the digestive tract varied
from 0 to 3.04/1000 compared with a rate of 0.59/1000 for the
controls (Machle & Gregorius, 1948). The mortality rates from
stomach cancer have been reported for chromate-producing plants in
Japan (SMR = 90) (Satoh et al., 1981) the USA (SMR = 40) (Hayes et
al., 1979). No significant differences were reported between the
incidence of stomach cancer in a Russian plant (Pokrovskaya &
Shabynina, 1973) and the incidence in the surrounding area.
Studying cancer mortality in a pigment plant using lead and
zinc chromates, Sheffet et al. (1982) found an increased (but not
statistically risk of stomach (SMR = 170) and pancreas (SMR = 200)
cancer among the total cohort (1946 employees).
Table 27. Summary of epidemiological studies on respiratory cancer in
workers employed in the chromium plating and related industriesa
------------------------------------------------------------------------------------------------
Study population Follow-up Respiratory cancer Comparison population
Number Estimated
relative risk
------------------------------------------------------------------------------------------------
Chromium plating
1056 workers employed Deaths in former and 24 1.8b Unexposed workers in
> 3 months in 54 plants current (followed 2 plants and in 2 non-
in the United Kingdom years) workers, plating industries
about 80% traced
About 5000 workers 49 1.4c Mortality analysis,
employed since 1945 in method not specified
1 plating factory in
the United Kingdom
889 workers exposed > 6 Reports from management 0 < 1b Tokyo mortality
months in Tokyo chromium of plating firms and rates
plating plants, 1970-76 follow-up of retired
workers; 19 total deaths
reported
Related industries
1292 deaths among US Union death benefit 62 1.1b Proportionate
metal polishers and claims mortality, US males
platers, union members,
1951-69
238 deaths among employees Pension, insurance and 39 2.1c Proportionate
for 10 years in one US benefit records mortality, US
plant for metal die
casting, finishing, and
electroplating, 1974-78.
------------------------------------------------------------------------------------------------
a Adapted from: Hayes (1982).
b Not statistically significant.
c P < 0.05, calculated using an assumption that the observed number is distributed as a
Poisson random variable.
8.3.9.3. Relationship between cancer risk and type of chromium
compound
As the exposures are often poorly defined, it is sometimes not
certain whether workers have been exposed to hexavalent chromium,
trivalent chromium, or a combination of the two. Some authors have
concluded that there is no increased risk of cancer mortality due
to trivalent chromium compounds (Korallus et al., 1974 a,b,c;
Axelsson et al., 1980; Langard et al., 1980; Norseth, 1980),
whereas others do not exclude that trivalent chromium compounds
have a carcinogenic capacity (Essing et al., 1971; Mancuso, 1975;
Zober 1979).
Summing up the data from case reports as well as
epidemiological studies in chromate-producing, chromate-pigment,
chromium-plating, and ferrochromium industries, the IARC Working
Group on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans (IARC, 1980) came to the conclusion that "there is
sufficient evidence of respiratory carcinogenicity in men
occupationally exposed during chromate production. Data on lung
cancer risk in other chromium-associated occupations and for cancer
at other sites are insufficient. The epidemiological data do not
allow an evaluation of the relative contributions to carcinogenic
risk of metallic chromium, trivalent chromium and hexavalent
chromium or of soluble versus insoluble chromium compounds".
9. EVALUATION OF HEALTH RISKS FOR MAN
Low levels of chromium are omnipresent in the environment.
Under normal conditions, human exposure to chromium does not
represent a toxicological risk, but it should be pointed out that
too low an intake of chromium may lead to deficiency. Airborne
concentrations of chromium, predominantly as trivalent chromium,
are usually below 0.1 µg/m3 and there are many places where the
concentration is below the detection limit. Concentrations in
river water are typically in the range of 1 - 10 µg/litre and do
not constitute a health threat. Drinking-water from municipal
water supplies does not contribute more than a few micrograms of
chromium to the daily human intake, but untreated water in certain
areas may be contaminated by runoff or effluents from industrial
sources and may contribute significant amounts of chromium. The
oceans contain less than 1 µg/litre. The daily human intake
through food varies considerably between regions. Typical values
range from 50 to 200 µg/day. They do not represent a toxicity
problem.
However, significant exposures exist in the occupational field.
In the past, chromium-ore mining generated chromium-containing
dusts, levels of which ranged up to 20 mg/m3 in different work-
places. Other exposures ranged up to 150 mg/m3 and consisted of
dust containing as much as 48% chromium (Cr2O3). In production
plants, hexavalent chromium can occur in the airborne state.
However, implementation of protective measures at ferrochromium
production sites reduced the airborne hexavalent chromium to levels
of 30 - 60 µg/m3.
The health effects of the two common oxidation states of
chromium are so fundamentally different that they must be
considered separately. In the form of trivalent compounds,
chromium is an essential nutrient and is relatively non-toxic for
man and other mammalian species. The hexavalent form is man-made
by oxidation of naturally-occurring trivalent chromium minerals and
is widely used. Compounds of hexavalent chromium penetrate
biological membranes easily and can thus interact with essential
constituents of the cells, including genetic material which they
can damage through oxidation and complexation with resulting
trivalent species. On the other hand, oxidation of trivalent
chromium has not been demonstrated in the living organism, and for
practical purposes, the reduction of hexavalent chromium to
trivalent chromium in lungs and other animal tissues is
irreversible.
9.1. Occupational Exposure
A number of effects can result from occupational exposure to
airborne chromium including irritative lesions of the skin and
upper respiratory tract, allergic reactions, and cancers of the
respiratory tract. The data on other effects, e.g., in the
gastrointestinal, cardiovascular, and urogenital systems are
insufficient for evaluation.
Epidemiological studies have shown that workers engaged in the
production of chromate salts and chromate pigments experience an
increased risk of developing bronchial carcinoma. No detailed data
on dose-response relationships are available from epidemiological
studies. Although a suspicion of increased lung cancer risks in
chromium plating workers has been raised, the available data are
inconclusive as are data for other industrial processes where
exposure to chromium occurs. There is insufficient evidence on the
role of chromium as a cause of cancer in any organ other than the
lung. Evidence from studies on laboratory animals shows that
hexavalent chromium compounds, especially those of low solubility,
can induce lung cancer.
In the lymphocytes of workers in chromium-plating factories,
the frequency of sister chromatid exchanges was higher in exposed
than in control groups.
Mutagenicity and related studies have convincingly shown that
hexavalent chromium is genetically active. Hexavalent chromium can
cross cellular membranes and is then reduced to trivalent chromium,
which can cause DNA cross-links and increase the infertility of DNA
implication. Trivalent chromium compounds have been shown to be
genetically inactive in most test systems, except in systems where
they can directly interact with DNA.
9.1.1. Effects other than cancer
9.1.1.1. Respiratory tract
It has been reported that the threshold for acute irritative
effects in the upper respiratory tract is 25 µg/m3 for the most
sensitive individuals. Long-term exposure to doses over 1 µg
chromic acid/m3 can cause nasal irritation, atrophy of the nasal
mucosa, and ulceration of perforation of the nasal septum.
Bronchial asthma was previously attributed to exposure to
chromium compounds, but scientific data are too scarce to draw
conclusions.
9.1.1.2. Skin
Skin rashes, ulcers, sores, and eczema have been reported among
occupationally exposed workers. Both trivalent and hexavalent
chromium compounds can give rise to sensitization of skin,
especially under certain environmental conditions, such as those
encountered in the cement industry, where the high incidence of
chromium-induced skin lesions can be attributed to the alkaline
exposure conditions.
Eczematous dermatitis, which manifests first as a diffuse
erythematous type, progresses in severe cases to an exudative
stage and is associated in 8 - 15% of patients with sensitivity to
chromium, as revealed by skin-patch tests. Although opinion is
divided on the oxidation state of chromium responsible for inducing
sensitization, there is evidence of hexavalent chromium penetrating
the skin as a hapten to be reduced to the trivalent state and
conjugated with proteins and transformed into the full antigen
that initiates the sensitization reaction, involving presumably
only a cell-mediated immune response. It should be noted that
patients suffering from chromium-induced skin allergy tend to
become hypersensitive to cobalt and nickel. Furthermore, 48% of
cases of skin allergy induced by chromium, with or without
clinically observed light sensitivity, showed a significantly more
intense reaction by a standardized photopatch test procedure, when
they were exposed to a 4/5 minimal erythema dose of irradiation.
9.1.1.3. Kidney
After high-dose, short-term oral ingestion of chromium, acute
nephritis and tubular necrosis were observed. A few
epidemiological studies on workers in chrome-plating industries
include data on diseases of the kidney, most of them without giving
exact exposure levels. A recent study related increased urinary-beta2
microglobulin levels to exposure ranges between 2 and 20 µg/m3.
The dose-response relationship observed in this study needs
confirmation on a larger number of exposed workers.
9.1.1.4 Other organs and systems
There are no conclusive data to evaluate the effects of
chromium compounds on the liver or gastrointestinal and circulatory
systems.
9.1.2. Teratogenicity
Both oxidation states, when injected at high levels
parenterally into animals, are teratogenic, with the hexavalent
form accumulating in the embryos to much higher concentrations than
the trivalent. The Task Group was not aware of any report
indicating teratogenicity in human populations.
9.2. General Population
Persons living in the vicinity of ferro-alloy plants, exposed
to an ambient air concentration of up to 1 µg/m3, did not show
increased lung cancer mortality.
The results of many studies suggest that exposure to chromium
through inhalation and skin contact can pose health problems for
the general population. Very little information is available on
the health effects of chromium ingested through untreated drinking-
water, though, in a single study, a correlation was observed
between frequency of malformation in the central nervous system and
the chromium content of water samples (Morton & Elwood, 1974). In
order to assess the nature of the magnitude of these problems,
there appears to be a need for more general population studies on
the effects of inhaled, absorbed, or ingested chromium on
respiratory, cardiovascular, and renal functions, and on the skin.
Studies on animals and man have established trivalent chromium
as an essential micronutrient that interacts with insulin and
enhances the physiological effects of the hormone. Supplementation
trials in a number of countries including Finland, Jordan, Nigeria,
Sweden, Turkey, and the USA have shown that segments of the
population could improve their glucose metabolism and, in some
instances, fat metabolism, by ingesting trivalent chromium
compounds. These observations suggest that some populations are at
risk of chromium deficiency. Children suffering from protein-
energy malnutrition may be at special risk.
As chromium concentrations in the body fluids of unexposed
persons are generally less than 1 µg/litre, analysis for chromium
requires the strictest quality control, including measures to
exclude sample contamination. Even with adequate analytical
methods, it is not possible, as yet, to diagnose chromium
deficiency in individuals by chemical or biochemical methods alone.
Therefore, a quantitative assessment of the prevalence of chromium
deficiency in human populations is not yet possible.
REFERENCES
ABRAHAM, A.S., SONNENBLICK, M., EINI, M., SHEMESH, O., & BATT,
A.P. (1980) The effect of chromium on established athero-
sclerotic plaques in rabbits. Am. J. clin. Nutr., 33:
2294-2298.
AITIO, A., JARVISALO, J., KULUNEN, M., TOSSAVAINEN, A., &
VAITTINEN, P. (1984) Urinary excretion of chromium as an
indicator of exposure to trivalent chromium sulfate in leather
tanning. Int. Arch. occup. environ. Health, 54: 241-249.
ALDERSON, M.R., RATTAN, N.S., & BIDSTRUP, L. (1981) Health
of workmen in the chromate-producing industry in Britain. Br.
J. ind. Med., 38: 117-124.
ALEKSANDROV, A.I. & KAINOV, V.I. (1975) [Crocoite from the
Pervomaisk-Zverevo deposit at the Central Urals.] Tr. Sverdl.
Gorn. Inst., 106: 152-155 (in Russian).
AMA (1979) Guidelines for essential trace element
preparations for parenteral use. J. Am. Med. Assoc., 241:
2051-2054.
AMERICAN PUBLIC HEALTH ASSOCIATION, AMERICAN WATER WORKS
ASSOCIATION, AND WATER POLLUTION CONTROL FEDERATION (1971)
117A. Hexavalent chromium. In: Standard methods for the
examination of water and wastewater, 13th ed., Washington DC,
American Public Health Association, pp. 156-157.
AMES, B.N., DURSTON, E., YAMASAKI, E., & LEE, F.D. (1973)
Carcinogens as mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci. (USA), 70: 2281-2285.
ANDERSON, F.E. (1960) Cement and oil dermatitis. The part
played by chrome sensitivity. Br. J. Derm., 72: 108-117.
ANDERSON, N. (1982) The environmental impact of chromium.
Final draft March 1982, Maine, Department of Environmental
Protection, Bureau of Air Quality Control, 126 pp.
ANDERSON, R.A. & POLANSKY, M.M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Tr. El. Res., 3: 1-5.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., & REAMER, D.C. (1982a) Effect of exercise
(running) on serum glucose, insulin, glucagon, and chromium
excretion. Diabetes, 31: 212-216.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
PATTERSON, K.Y., VEILLON, C., & GLINSMANN, W. (1982b)
Urinary chromium excretion of human subjects: effects of
chromium supplementation and glucose loading. Am. J. clin.
Nutr., 36: 1184-1193.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., PATTERSON, K.Y.,
VEILLON, C., & GLINSMANN, W. (1983) Effects of chromium
supplementation on urinary Cr excretion of human subjects and
correlation of Cr excretion with selected clinical parameters.
J. Nutr., 113: 276-281.
ANDERSON, R.A., POLANSKY, M.M., BRYDEN, N.A., ROGINSKI, E.E.,
MERTZ, W., & GLINSMANN, W. (1983b) Chromium supplementation
of human subjects: effects on glucose, insulin, and lipid
variables. Metabolism, 32: 894-899.
ANDERSSON, A. & NILSSON, K.O. (1972) Enrichment of trace
elements from sewage sludge fertilizer in soils and plants.
Ambio, 1: 176-179.
ANGERER, J., SCHALLER, K.-H., & HEINRICH, R. (1981)
[Commercially-available blood and urine samples for quality
control in toxicology.] Arbeitsmed. Sozialmed. Praeventivmed.,
16: 125-127 (in German).
ANIMAL RESEARCH DIVISION OF THE NEW ZEALAND DEPARTMENT OF
AGRICULTURE (1954) Annual report 1953-54, Wellington, R.E.
Owen Government Printer, 40 pp.
ANKE, M., DIETTRICH, M., WICKE, G., PFLUG, D., & SCHUELER, D.
(1971) Absorption, excretion, and distribution of 51chromium
given by mouth to lactating ruminants. Arch. Tierern., 21:
599-607.
AXELSSON, G. & RYLANDER, R. (1980) Environmental chromium
dust and lung cancer mortality. Environ. Res., 23: 469-476.
AXELSSON, G., RYLANDER, R., & SCHMIDT, A. (1980) Mortality
and incidence of tumours among ferrochromium workers. Br. J.
ind. Med., 37: 121-127.
BAER, H. (1920) [Dermatitis resulting from the use of a
beard trainer made partly of artificial leather.] Münch. med.
Wschr., 67: 874 (in German).
BAETJER, A.M. (1950a) Pulmonary carcinoma in chromate
workers. I. A review of the literature and report of cases.
Arch. ind. Hyg. occup. Med., 2: 487-504.
BAETJER, A.M. (1950b) Pulmonary carcinoma in chromate
workers. II. Incidence on basis of hospital records. Arch.
ind. Hyg. occup. Med., 2: 505-516.
BAETJER, A.M., LOWNEY, J.F., STEFFEE, H., & BUDACZ, V.
(1959a) Effect of chromium on incidence of lung tumours in
mice and rats. Am. Med. Assoc. Arch. Ind. Health, 20: 124-135.
BAETJER, A.M., DAMRON, C., & BUDACZ, V. (1959b) The
distribution and retention of chromium in man and animals. Am.
Med. Assoc. Arch. Ind. Health, 20: 136-150.
BAKER, R.S.V. (1984) Evaluation of metals in in vitro
assays: interpretation of data and possible mechanisms of
action. Toxicol. environ. Chem., 7: 191-212.
BEHNE, D. & BRATTER, P. (1979) [Problems of trace element
analysis in medicine.] In: Gladtke, E., Heimann, G., & Eckert,
I., ed. [Trace elements], Stuttgart, Thieme, pp. 42-52 (in
German).
BEHNE, D. & DIEHL, F. (1972) Relations between carbohydrate
and trace element metabolism investigated by neutron
activation analysis. In: Nuclear Activation Techniques in the
Life Sciences. Proceedings of a Symposium, Bled, Yugoslavia,
Vienna, International Atomic Energy Agency, pp. 407-414.
BEHNE, D., BRATTER, P., GESSNER, H., HUBE, G., MERTZ, W., &
ROSICK, U. (1976) Problems in determination of chromium in
biological materials: comparison of flameless atomic
absorption spectrometry and neutron activation analysis. Z.
anal. Chem., 278: 269-272.
BEHRBOHM, P. (1957) [Allergic contact dermatitis caused by
anticorrosives containing chromium.] Berufsdermatosen, 5:
271-283 (in German).
BENJANUVATRA, N.K. & BENNION, M. (1975) Hair chromium
concentration of Thai subjects with and without diabetes
mellitus. Nutr. Rep. Int., 12: 325-330.
BERGMANN, H. & HARDT, K. (1979) Analysis of dissolved Cr(VI)
in water by APDC-MIBK extraction and atomic absorption
spectrometry. Z. anal. Chem., 297: 381-383.
BERING, F. & ZITZKE, E. (1935) [Occupational skin diseases,]
Leipzig, Voss (in German).
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric., 23: 93-100.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BERTRAND, D. & DEWOLF, A. (1968) Requirement for the trace
element chromium in the growth of potatoes. C R Ser. D., 266:
1494-1495.
BEYERMANN, K. (1962) The analytical behaviour of very small
amounts of chromium. Part I. Z. anal. Chem., 190: 4-33. Part
II. Z. anal. Chem., 191: 345-369.
BEYERSMANN, D., KOSTER, A., BUTTNER, B., & FLESSEL, P.
(1984) Model reactions of chromium compounds with mammalian
and bacterial cells. Toxicol. environ. Chem., 8: 279-286.
BIANCHI, V., DAL TOSO, R., DEBETTO, P., LEVIS, A.G., LUCIANI,
S., MAJONE, F., & TAMINO, G. (1980) Mechanisms of chromium
toxicity in mamalian cell cultures. Toxicology, 17: 219-224.
BIANCHI, V., LEVIS, A.G., & SAGGIORO, D. (1979) Differential
cytotoxic activity of potassium dichromate on nucleoside
uptake in BHK fibroblasts. Chem.-biol.Interact., 24: 137-151.
BIANCHI, V., CELOTTI, L., LANFRANCHI, G., MAJONE, F., MARIN,
G., MONTALDI, G., SPONZA, G., TAMINO, G., VENIER, P.,
ZANTEDESCHI, A., & LEVIS, A.G. (1983) Genetic effects of
chromium compounds. Mutat. Res., 117: 279-300.
BIANCHI, V., ZANTEDESCHI, A., MONTALDI, A., & MAJONE, F.
(1984) Trivalent chromium is neither cytotoxic nor mutagenic
in permeabilized hamster fibroblasts. Toxicol. Lett., 23:
51-59.
BIDSTRUP, P.L. & CASE, R.A.M. (1956) Carcinoma of the lung
in workmen in the bichromates-producing industry in Great
Britain. Br. J. ind. Med., 13: 260-264.
BIGALIEV, A.B., ELEMESOVA, M.S., TUREBAEV, M.N., & BIGALIEVA,
R.K. (1978) [Cytogenetic study of the mutagenic activity of
industrial substances.] Zdravookhr. Kaz., 8: 48-50 (in
Russian).
BITTERSOHL, G. (1972) [Health and the working environment-
Epidemiology of carcinomatosis in the chemical industry.] In:
Proceedings of the 5th Merseburger Symposium, 17-18 November
(in German).
BLACK, C.A. (1968) Soil-plant relationships, New York, John
Wiley and Sons, 792 pp.
BLOKIN, V.S. & TROP, F.S. (1977) [Dynamics of the morpho-
logical changes in animals subjected to inhalational dust
treatment using chromium oxide and trisubstituted chromium
phosphate.] In: Dubinin, N.P., ed. [Genetic effects of the
pollution of the environment,] Moscow, Nauka, pp. 173-176 (in
Russian).
BLOOMFIELD, J.J. & BLUM, W. (1928) Health hazards in
chromium plating. Public Health Rep., 43: 2330-2347.
BONATTI, S., MEINI, M., & ABBONDANDOLO, A. (1976) Genetic
effects of potassium dichromate. Mutat. Res., 38: 147-150.
BOND, R.G., STRAUB, C.P., & PROBER, R., ed. (1973) Handbook
of environmental control. III. Water supply and treatment,
Cleveland, Ohio, CRC Press, pp. 763-764.
BONNEVIE, P. (1939) [Etiology and pathogenesis of eczemas,]
Copenhagen, Busck, Leipzig, Barth (in German).
BORGHETTI, A., MUTTI, A., CAVATORTA, A., FALZOI, M., CIGALA,
F., & FRANCHINI, I. (1977) Indices rénaux d'exposition aiguë
et d'imprégnation chronique par le chrome. Med. Lav., 68(5):
355-363.
BORY, R. & LECOCQ, I. (1953) Eczéma par cuivre chromé. Arch.
Mal. prof., 14: 144.
BOSTROM, G. (1936) [Hypersensitivity test for chromium
allergy.] Am. Derm. Vener., 17: 631-632 (in German).
BOVET, P., LOB, M., & GRANDJEAN, M. (1977) Spirometric
alterations in workers in the chromium electroplating
industry. Int. Arch. occup. environ. Health, 40: 25-32.
BOWEN, H.J.M. (1966) Trace elements in biochemistry, New
York, London, Academic Press, 241 pp.
BRARD, D. (1935) Toxicologie du chrome, Paris, Herrmann &
Co., 80 pp.
BRIEGER, H. (1920) [Clinical aspects of acute chromate
poisoning.] Z. exp. Path. Ther., 21: 393-408 (in German).
BROCH, C. (1949) [Bronchial asthma caused by chromic oxide.]
Nord. Med., 41: 996-997 (in Norwegian).
BROWN, E., SKOUGSTAD, M.W., & FISHMAN, M.J. (1970) Methods
for collection and analysis of water samples for dissolved
minerals and gases. In: Techniques of water resources
investigation of the United States geological survey,
Washington DC, US Department Interior, 160 pp.
BROWN, H.G., HENSLEY, C.P., MCKINNEY, G.L., & ROBINSON, J.L.
(1973) Efficiency of heavy metals removal in municipal sewage
treatment plants. Environ. Lett., 5: 103-114.
BRUCKDORFER, K.R., KHAN, I.H., & YUDKIN, J. (1971) Chromium
and sucrose-induced hyperglyceridemia. Proc. Nutr. Soc., 30:
74A.
BRUNE, D., NORDBERG, G., & WESTER, P.O. (1980) Distribution
of 23 elements in the kidney, liver, and lungs of workers from
a smeltery and refinery in north Sweden exposed to a number of
elements and of a control group. Sci. total Environ., 16:
13-35.
BURCKHARD, W. (1962) [Occupational skin diseases.] In:
Jadassohn, W., ed. [Handbook of skin and venereal diseases,]
Berlin, Springer, Vol. II(1), pp. 368-474.
BURROWS, D., ed. (1983) Chromium: metabolism and toxicity,
Boca Raton, Florida, CRC Press, 172 pp.
BYERRUM, R.U. (1961) Some studies on the chronic toxicity of
cadmium and hexavalent chromium in drinking-water. Eng. Bull.
Purdue Univ. Serv., 106: 1-8.
CALNAN, C.D. & HARMAN, R.R.M. (1961) Studies in contact
dermatitis. XIII. Diesel coolant chromate dermatitis. Trans.
St. John's Hosp. Derm. Soc., 46: 13.
CALOW, P. & FLETCHER, C.R. (1972) A new radiotracer
technique involving 14C and 51Cr for estimating the
assimilation efficiencies of aquatic, primary consumers.
Oecologia (Berlin), 9: 155-170.
CAMPBELL, W.J. & MERTZ, W. (1963) Interaction of insulin and
chromium (III) on mitochondrial swelling. Am. J. Physiol.,
204: 1028-1030.
CANADA, NATIONAL HEALTH AND WELFARE (1980) Chromium
drinking-water criteria review. In: Guidelines for Canadian
drinking-water quality, 1978. Supporting documentation,
Ottawa, Canada, National Health and Welfare, pp. 255-268.
CANFIELD, W.K. & DOISY, R.J. (1976) Chromium and diabetes in
the aged. In: Hsu, J.M., Davis, R.L., & Neithammer, R.W., ed.
The biomedical role of trace elements in aging, St. Peters-
burg, Florida, Eckert College, pp. 119-128.
CARD, W.I. (1935) A case of asthma sensitivity to chromates.
Lancet, 2: 1348-1349.
CARTER, J.P., KATTAB, A., ABD-EL-HADI, K., DAVIS, J., EL
GHOLMY, A., & PATWARDHAN, V.N. (1968) Chromium (III) in
hypoglycemia and in impaired glucose utilization in
kwashiorkor. Am. J. clin. Nutr., 21: 195-202.
CARY, F.E., ALLWAY, W.H., & OLSON, O.E. (1977) Control of
chromium concentrations in food plants. I. Absorption and
translocations of chromium by plants. J. agric. food Chem.,
25: 300-304. II. Chemistry of chromium in soils and its
availability to plants. J. agric. food Chem., 25: 305-309.
CASEY, C.E. & HAMBIDGE, K.M. (1984) Chromium in human milk
from American mothers. Br. J. Nutr., 52: 73-77.
CASTO, B.C., PIECZYNSKI, W.J., NELSON, R.L., & DI PAOLO, J.
(1976) In vitro transformation and enhancement of viral
transformation with metals. Proc. Am. Assoc. Cancer Res., 17:
12-20.
CASTO, B.C., MEYERS, J., & DIPAOLO, J.A. (1979) Enhancement
of viral transformation for evaluation of the carcinogenic or
mutagenic potential of inorganic metal salts. Cancer Res., 39:
193-198.
CAWSE, P.A. (1974) A survey of atmospheric trace elements in
the UK, 1972-1973, Harwell, England, Atomic Energy Research
Establishment, 95 pp.
CHANEY, R.L. (1973) Crop and food chain effects of toxic
elements in sludges and effluents. In: Recycling Municipal
Sludges and Effluents on Land. Proceedings of a Joint
Committee, National Association of State Universities and Land
Grant Colleges, Washington DC, July 9-13, 1973, pp. 129-141.
CHEN, N.S.G., TSAI, A., & DYER, I.A. (1973) Effect of
chelating agents on chromium absorption in rats. J. Nutr.,
103: 1182-1186.
CHEN, K.Y., YOUNG, C.S., JAN, T.K., & ROHATGI, N. (1974)
Trace metals in waste water effluents. J. Water Pollut.
Control Fed., 46: 2663-2675.
CHEREMISINOFF, P.N. & HABIB, Y.H. (1972) Cadmium, chromium,
lead, mercury: a plenary account for water pollution. Part I.
Occurrence, toxicity, and detection. Water Sewage Works, 119:
73-86.
CHEREVATY, V.S., EREMEEVA, E.A., & SMAGULOV, S.K. (1965) [On
the nature of nasal microflora in the workers of a chromium
compound plant.] In: Proceedings of 4th Scientific Session of
Aktubinsk State Medical Institute, Alma-Ata, Kazakhstan,
pp. 337-342 (in Russian).
CHIAZZE, L., FERENCE, L.D., & WOLF, P.H. (1980) Mortality
among automobile assembly workers. I. Spray painters. J.
occup. Med., 22: 520-526.
CHRISTIAN, G.D., KNOBLOCK, E.G., PURDY, W.C., & MERTZ, W.
(1963) A polarographic study of chromium-insulin-
mitochondrial interaction. Biochem. Biophys. Acta, 66: 420-423.
CLARK, R. & KUNITSCH, G. (1972) [Statistical studies of
cross allergy in metal salts.] Berufsdermatosen, 20(5):
222-238 (in German).
COLLINS, R.J., FROMM, P.O., & COLLINGS, W.D. (1961) Chromium
excretion in the dog. Am. J. Physiol., 201(5): 795-798.
CONDE-SALAZAR, L., MARTO-CASTANO, A., & ARROYO VICENTE, M.
(1980) Determination of chrome in rubber gloves. Contact
Dermatit., 6: 237-238.
COTTON, F.A. & WILKINSON, G. (1966) Advanced inorganic
chemistry, New York, Interscience, 1136 pp.
CRESSER, M.S. & HARGITT, R. (1976) The determination of
chromium (III) and chromium (VI) by total anion exchange and
atomic absorption spectrometry. Anal. Chim. Acta, 81: 196-198.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
DALAGER, N.A., MASON, T.J., FRAUMENI, J.F., HOOVER, R., &
PAYNE, W.W. (1980) Cancer mortality among workers exposed to
zinc chromate paints. J. occup. Med., 22: 25-29.
DANA, P. (1971) Crocoite-PbCrO4 In: Hurlbut, C.S., ed.
Dana's manual of mineralogy, 18th ed., New York, J. Wiley and
Sons Inc., pp. 346-347.
DANIELSSON, B.R.G., HASSOUN, E., & DENCKER, L. (1982)
Embryotoxicity of chromium: distribution in pregnant mice and
effects on embryonic cells in vitro. Arch. Toxicol., 51:
233-245.
DAVIDSON, I.W.F. & BLACKWELL, W.L. (1968) Changes in
carbohydrate metabolism of squirrel monkeys with chromium
dietary supplementation. Proc. Soc. Exp. Biol. Med., 127:
66-70.
DAVIDSON, I.W.F. & BURT, R.L. (1973) Physiological changes
in plasma chromium of normal and pregnant women: effect of
glucose load. Am. J. Obstet. Gynecol., 116: 601-608.
DAVIES, J.C.A. (1974) Inhalation hazards in the manufacture
of silicon alloys. Cent. Afr. J. Med., 20: 140-143.
DAVIES, J.M.G. (1972) The fibrogenic effects of mineral
dusts injected into the pleural cavity of mice. Br. J. exp.
Pathol., 53: 190-201.
DAVIES, J.M.G. (1979) Lung cancer mortality of workers in
chromate pigment manufacture: an epidemiological survey. J.
Oil. Col. Chem. Assoc., 62: 157-163.
DAVIES. J.M.G. (1984) Lung cancer mortality among workers
making lead chromate and zinc chromate pigments at three
English factories. Br. J. Ind. Med., 41: 158-169.
DAVIS, M.H. & GROSSMANN, V.B. (1971) A sensitive method for
determination of chromium in biological materials. Anal.
Biochem., 44: 339-344.
DEFLORA, S. (1978) Metabolic deactivation of mutagens in
the Salmonella microsome test. Nature (Lond.), 271: 455-456.
DEFLORA, S. (1981) Study of 106 organic and inorganic
compounds in the Salmonella microsome test. Carcinogenesis, 2:
283-298.
DEFLORA, S. & BOIDO, V. (1980) Effect of human gastric
juice on the mutagenicity of chemicals. Mutat. Res., 77:
307-315.
DEFLORA, S., COPPOLA, R., CAMOIRANO, A., BATTAGLIA, M.A., &
BENNICELLI, C. (1980) Mutagenicity of chromyl chloride and
its vapours. Carcinogenesis, 1(7): 583-587.
DEFLORA, S., BIANCHI, V., & LEVIS, A.G. (1984) Distinctive
mechanisms for interaction of hexavalent and trivalent
chromium with DNA? Toxicol. environ. Chem., 8: 287-294.
DE GROOT, A.J. & ALLERSMA, E. (1973) Field observations on
the transport of heavy metals in sediments. In: Proceedings of
the Conference on Heavy Metals in the Aquatic Environment,
Nashville, Tennessee, December, Oxford, Pergamon Press,
pp. 1-16.
DEICHMANN, W.B. & GERARDE, H.W. (1969) Toxicology of drugs
and chemicals, 4th ed., New York, London, Academic Press,
167 pp.
DI PAOLO, J.A. & CASTO, B.C. (1979) Quantitative studies of
in vitro morphological transformation of Syrian hamster cells
by inorganic metal salts. Cancer Res., 32: 1008-1013.
DJAHANSHIRI, H. (1976) [Nutritional physiology problems
concerning the trace element chromiun in the rat. V. Effects
on overall metabolic performande.] Z. Tierphysiol. Tierernähr.
Futtermittelkd., 37: 298-315 (in German).
DOBROLYUBSKII, O.K. (1959) [Changes in the biochemical
process in grapes under the influence of a microfertilizer
which contains chromium.] Biochemistry (USSR), 24: 577-582 (in
Russian).
DOISY, R.J., STREETEN, D.H.P., LEVINE, R.A., & CHODOS, R.B.
(1968) Effects and metabolism of chromium in normals, elderly
subjects, and diabetics. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
Univerisity of Missouri, Vol. 2, 75 pp.
DOISY, R.J., STREETEN D.H.P., SOUMA, M.L., KALAFER, M.E.,
REKANT, S.L., & DALAKOS, T.G. (1971) Metabolism of
51chromium in human subjects. In: Mertz, W. & Cornatzer, W.E.,
ed. Newer trace elements in nutrition, New York, Marcel
Dekker, pp. 155-169.
DOISY, R.J., STREETEN, D.H.P., FREIBERG, J.M., & SCHNEIDER,
A.J. (1976) Chromium metabolism in man and biochemical
effects. In: Prasad, A.S., ed. Trace elements in human health
and disease, New York, London, Academic Press, Vol. 2, pp.
79-104.
DOMASEVA, T.V. (1971) [On the effect produced by chromium
compounds on the skin.] In: [Occupational skin diseases,]
Leningrad, Leningrad Institute of Sanitation and Hygiene,
Vol. 93, pp. 168-173 (in Russian).
DONALDSON, R.M., Jr & BARRERAS, R.F. (1966) Intestinal
absorption of trace quantities of chromium. J. lab. clin.
Med., 68: 484-493.
DOUGLAS, G.R., BELL, R.D.K., GRANT, C.E., WYTSMA, J.M., &
BORA, K.C. (1980) Effect of lead chromate on chromosome
aberration, sister-chromatid exchange, and DNA damage in
mammalian cells in vitro. Mutat. Res., 77: 157-163.
DURFOR, C.N. & BECKER, E. (1964) Public water supplies of
the 100 largest cities in the United States, 1962, Washington
DC, US Geological Survey, 364 pp (Water Supply Paper No. 1812).
DURUM, W.H. & HAFFTY, J. (1963) Implications of the
minor-element content of some major streams in the world.
Geochim. Cosmochim Acta, 27: 1-11.
EATON, D.L., STACEY, N.H., WONG, K.L., & KLAASSEN, C.D.
(1980) Dose-response effects of various metal ions on rat
liver metallothionein, glutathione, heme oxygenase, and
cytochrome P-450. Toxicol. appl. Pharmacol., 55: 393-402.
EDMUNDSON, W.F. (1951) Chrome ulcers of the skin and nasal
septum and their relation to patch testing. J. Invest. Derm.,
17: 17-19.
EIFAC (1983) Water quality criteria for european freshwater
fish. Report on chromium and freshwater fish, European Inland
Fisheries Advisory Commission, Working Party on Water Quality
Criteria for European Freshwater Fish, 31 pp (EIFAC Technical
Paper No. 43).
ELIAS, Z., SCHNEIDER, O., AUBRY, F., DANIERE, M.C., & POIROT,
O. (1983) Sister chromatid exchanges in chinese hamster V79
cells treated with the trivalent chromium compounds chromic
chloride and chromic oxide. Carcinogenesis, 3: 605-611.
ENTERLINE, P.E. (1974) Respiratory cancer among chromate
workers. J. occup. Med., 16: 523-526.
ENVIRONMENTAL INSTRUMENTATION GROUP (1973) Instrumentation
for environmental monitoring: biomedical. IV, Berkeley,
California, Lawrence Berkeley Laboratory.
EQUITABLE ENVIRONMENTAL HEALTH, INC. (1976) An epidemiologic
study of workers in lead chromate plants: Final report
(prepared for the Dry Color Manufacturer's Association,
Berkeley California).
ESSING, H.G., SZADKOWSKI, D., & VALENTIN, H. (1971)
[Significance of the valence gradations of chromium compounds
in occupational medicine examinations.] Med. Sachverständige,
67: 35-39 (in German).
ETMANOVA, Z.J. (1965) [On the functional state of the liver
in workers of Aktubinsk chromium compound plant.] Gig. Tr.
prof. Zabol., 10: 55-56 (in Russian).
EVAN, A.P. & DAIL, W.G. (1974) The effect of sodium chromate
on the proximal tubules of the rat kidney. Lab. Invest.,
30(6): 704-715.
FANG, Y. (1983) [The normal value of chromium in urine for
299 unexposed persons in Yanjian city.] J. Yanbian Med.
College, 6 (1): 35-37 (in Chinese).
FARKAS, T.G. & ROBERSON, S.L. (1965) The effect of Cr(III)
on the glucose utilization of isolated lenses. Exp. Eye Res.,
4: 124-126.
FELDMAN, F.J. (1968) The state of chromium in biological
materials. Fed. Proc., 27: 482.
FELDMAN, F.J., KNOBLOCK, E.C., & PURDY, W.C. (1967) The
determination of chromium in biological materials by atomic
absorption spectroscopy. Anal. Chim. Acta, 38: 489-497.
FRADKIN, A., JANOFF, A., LANE, B.P., & KUSCHNER, M. (1975)
In vitro transformation of BHK21 cells grown in the presence
of calcium chromate. Cancer Res., 35: 1058-1061.
FRANCHINI, I., MUTTI, A., GARDINI, F., & BORGHETTI, A.
(1975) [Excretion and renal elimination of chromium compared
to degree and length of occupational exposure.] In: Roche, L.
& Trager, J., ed. Rein et Toxique. Réunion de la Société
Francaise de Toxicologie (Compte rendu), Paris, Groupement
Francais Des Centre Anti-Poisons, pp. 271-281.
FRANCHINI, I., MUTTI, A., CAVATORTA, A., CORRADI, A., COSI,
A., OLIVETTI, A., & BORGHETTI, A. (1978) Nephrotoxicity of
chromium. Control Nephrol., 10: 98-110.
FRANCHINI, J., MAGNANI, F., & MUTTI, A. (1983) Mortality
experience among chrome plating workers. Scand. J. Work
environ. Health, 9: 247-252.
FREGERT, S. (1975) Occupational dermatitis in a 10-year
material. Contact Dermatit., 1: 96-107.
FREGERT, S. (1981) Manual of contact dermatitis, 2nd ed.,
Copenhagen, Munksgaard, 139 pp.
FREGERT, S. & GRUVBERGER, B. (1972) Chemical properties of
cement. Berufsdermatosen, 20(5): 238-245.
FREGERT, S. & GRUVBERGER, B. (1979) Chromium in industrial
leather gloves. Contact Dermatit., 5: 189.
FREGERT, S. & RORSMAN, H. (1964) Allergy to trivalent
chromium. Arch. Dermatol., 90: 4-6.
FREGERT, S., HJORTH, N., MAGNUSSON, B., BANDMANN, H.-J.,
CALNAN, C.D., CRONIN, E., MALTEN, K., MENEGHINI, C.L., PIRILA,
V., & WILKINSON, D.S. (1969) Epidemiology of contact
dermatitis. Trans. St. John's Hosp. Dermatol. Soc., 55: 17-35.
FRENTZEL-BEYME, R. (1983) Lung cancer mortality of workers
employed in chromate pigment factories. J. Cancer Res. clin.
Oncol., 105: 183-188.
FREUND, H., ATAMIAN, S., & FISCHER, J.E. (1979) Chromium
deficiency during total parenteral nutrition. J. Am. Med.
Assoc., 241: 496-498.
FSA (1953) Health workers in chromate producing industry. A
study, Washington DC, Federal Security Agency, 131 pp (US PHS
Publication No. 192).
FUKAI, R. (1967) Valency state of chromium in seawater.
Nature (Lond.), 213: 901.
FUKUNAGA, M., KURACHI, Y., & MIZUGUCHI, Y. (1982) Action of
some metal ions on yeast chromosomes. Chem. pharm. Bull., 30:
3017-3019.
FURST, A., SCHLAUDER, M., & SASMORE, D.P. (1976) Tumour-
igenic activity of lead chromate. Cancer Res., 36: 1779-1783.
GALE, T.F. (1974) Effects of chromium on the hamster embryo.
Teratology, 9: A-17.
GALE, T.F. (1978) Embryotoxic effects of chromium trioxide
in hamsters. Environ. Res., 16: 101-109.
GALE, T.F. (1982) The embryotoxic response to maternal
chromium trioxide exposure in different strains of hamsters.
Environ. Res., 29: 196-203.
GALE, T.F. & BUNCH, J.D. (1979) The effect of the time of
administration of chromium trioxide on the embryotoxic
response in hamsters. Teratology, 19: 81-86.
GARCIA, J.D. & WETTERHAHN JENNETTE, K. (1981) Electron-
transport cytochrome P-450 system is involved in the
microsomal metabolism of the carcinogen chromate. J. inorg.
Biochem., 14: 281-295.
GEISER, J.D., JEANNERET, J.P., & DELACRETAZ, J. (1960) Eczema
due to cement and sensitization to cobalt. Dermatologica
(Basel), 121: 1-7.
GEMMELL, R.P. (1973) Revegetation of derelict land polluted
by a chromate smelter. Part 1. Chemical factors causing
substrate toxicity in chromate smelter waste. Environ.
Pollut., 5: 181-197.
GEMMELL, R.P. (1974) Revegetation of derelict land polluted
by a chromate smelter. Part 2. Techniques of revegetation of
chromate smelter waste. Environ. Pollut., 6: 31-37.
GENTILE, J.M., HYDE, K., & SCHUBERT, J. (1981) Chromium
genotoxicity as influenced by complexation and rate effects.
Toxicol. Lett., 7: 439-448.
GERAUER, A. (1954) [On the growing importance of
epicutaneous allergy to chromium.] Z. Haut-Geschlechtskr., 17:
97-102 (in German).
GILANI, S.H. & MARANO, M. (1979) Chromium poisoning and
chick embryogenesis. Environ. Res., 29: 427-431.
GERICKE, S. (1943) Studies on the effect of the trace
element chromium on the growth of plants. Bodenkd.
Pflanzenernaehr., 33: 114-128.
GLASER, U., HOCHRAINER, D., KLOPPEL, H., KORDEL, W., & KUHNEN,
H. (1984) [Inhalation studies with Wistar rats and patho-
physiological effects of chromium,] Berlin, 156 pp (Report to
Umweltbundesamt, D-1 UFOPLAN F+E 10606007/2) (in German).
GLASER, U., HOCHRAINER, D., KLOPPEL, H., & KUHNEN, H. (1985)
Low-level chromium VI inhalation effects on alveolar macro-
phages and immune functions in Wistar rats. Arch. Toxicol.,
57: 250-256.
GLINSMANN, W.H., FELDMAN, F.J., & MERTZ, W. (1966) Plasma
chromium after glucose administration. Science, 152: 1243.
GLINSMANN, W.H. & MERTZ, W. (1966) Effect of trivalent
chromium on glucose tolerance. Metabolism, 15: 510-520.
GMELIN, C.G. (1826) Experiments on the effects of baryta,
strontia, chrome, molybdenum, tungsten, tellurium, titanium,
osmium, platinum, iridium, rhodium, palladium, nickel, cobalt,
uranium, cerium, iron, and manganese, on the animal system.
Edinburgh med. Surg. J., 26: 131-139.
GOMES, E.R. (1972) Incidence of chromium-induced lesions
among electroplating workers in Brazil. Ind. Med., 41(12):
21-25.
GOMEZ-ARROYO, S., ALTAMIRANO, M., & VILLALOBOS-PIETRINI, R.
(1981) Sister-chromatid exchanges induced by some chromium
compounds in human lymphocytes in vitro. Mutat. Res., 90:
425-431.
GONCHAROV, A.T. (1968) [Role of chromium in the nutrition of
animals and man.] Vopr. Pitaniya, 27: 59-66 (in Russian).
GONCHAROV, A.T. (1978) [Maximum physiological dose of
chromium in food for human consumption.] Kazanskij med. Z.,
59(2): 90-93 (in Russian).
GRAY, S.J. & STERLING, K. (1950) The tagging of red cells
and plasma proteins with radioactive chromium. J. clin.
Invest., 29: 1604-1613.
GREBE, W.-H., KASTNER, H., KIPPENBERGER, C., KRAUSS, U.,
KRUSZONA, M., SCHMIDT, H., LIEBRUCKS, M., RUMBERGER, M., &
WETTIG, E. (1975) [Investigation on supply and demand for
mineral raw materials. VII. Chromium,] Berlin, Hannover,
Bundesanstalt für Gewissenschaften und Rohstoffe/Deutsches
Institut für Wirtschaftsforschung, 147 pp.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38: 33-42.
GROSS, W.G. & HELLER, V.G. (1946) Chromates in animal
nutrition. J. ind. Hyg. Toxicol., 28: 52-56.
GROSS, E. & KOLSCH, F. (1943) [Lung cancer in the chromate
dye industry.] Gewerbepathol. Gewerbehyg., 12: 164-170 (in
German).
GURSON, C.T. (1977) The metabolic significance of dietary
chromium. In: Draper, H.H., ed. Advances in nutritional
research, New York, Plenum Publishing Company, Vol. 1,
pp. 23-53.
GURSON, C.T. & SANER, G. (1971) Effect of chromium on
glucose utilization in marasmic protein-calorie malnutrition.
Am. J. clin. Nutr., 24: 1313-1319.
GURSON, C.T. & SANER, G. (1973) Effect of chromium
supplementation on growth in marasmic protein-calorie
malnutrition. Am. J. clin. Nutr., 26: 988-991.
GURSON, C.T. & SANER, G. (1978) The effect of glucose
loading on urinary excretion of chromium in normal adults,
individuals from diabetic families, and in diabetics. Am. J.
clin. Nutr., 31: 1158-1161.
GUTHRIE, B.E. (1973) Daily dietary intakes of zinc, copper,
manganese, chromium, and cadmium by some New Zealand women.
Proc. Univ. Otago Med. School., 51: 47-49.
GUTHRIE, B.E., WOLF, W.R., & VEILLON, C. (1978) Background
correction and related problems in the determination of
chromium in urine by graphite furnace atomic absorption
spectrometry. Anal. Chem., 50: 1900-1902.
GUTHRIE, B.E., WOLF, W.R., VEILLON, C., & MERTZ, W. (1979)
Chromium in urine. In: Hemphill, D.D., ed. Trace substances in
environmental health, Columbia, Missouri, University of
Missouri, Vol. XII, pp. 490-492.
GUY, W.B. (1954) Dermatologic problems in the railroad
industry resulting from conversion to Diesel power. Am. Med.
Assoc. Arch. Derm. Syph., 70: 289-292.
GYLSETH, B., GUNDERSEN, N., & LANGORD, S. (1977) Evaluation
of chromium exposure based on a simplified method for urinary
chromium determination. Scand. J. Work environ. Health, 3:
28-31.
HAMBIDGE, K.M. (1971) Chromium nutrition in the mother and
in the growing child. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 169-194.
HAMBIDGE, K.M. (1974) Chromium nutrition in man. Am. J.
clin. Nutr., 27: 505-514.
HAMBIDGE, K.M. & BAUM, J.D. (1972) Hair chromium concen-
trations of human newborn and changes during infancy. Am. J.
clin. Nutr., 25: 376-379.
HAMBIDGE, K.M. & RODGERSON, D.O. (1969) A comparison of the
hair chromium levels of nulliparous and parous women. Am. J.
Obstet. Gynecol., 103: 320-321.
HAMBIDGE, K.M., RODGERSON, D.O., & O'BRIEN, D. (1968)
Concentration of chromium in the hair of normal children and
children with juvenile mellitus. Diabetes, 17: 517-519.
HARA, N. (1982) [The change of valence of chromium by acid
treatment.] Sangyo Igaku, 24: 658-662 (in Japanese).
HARRISON, D.L. & STAPLES, E.L.J. (1955) Zinc chromate
poisoning in calves. N.Z. vet. J., 3: 63-73.
HASEGAWA, M. (1938) Studies on the detexicating hormone of
the liver (Yakriton). Toholu J. exp. Med., 32: 163-176.
HAYES, R.B. (1982) Carcinogenic effects of chromium, In:
Langard, S., ed. Biological and environmental aspects of
chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 221-247.
HAYES, R.B., LILIENFELD, A.M., & SNELL, L.M. (1979)
Mortality in chromium chemical production workers: a
prospective study. Int. J. Epidemiol., 8: 365-374.
HEATH, J.C., FREEMAN, M.A.R., & SWANSON, S.A.V. (1971)
Carcinogenic properties of wear particles from prostheses made
in cobalt-chromium alloy. Lancet, 1: 564-566.
HENNIG, A., ANKE, M., DIETRICH, M., HARTMANN, G., & HOFFMANN,
G. (1971) Absorption and distribution of 51chromium after
oral administration to laying hens as a function of time.
Arch. Tierern., 21: 609-615.
HEPLER, O.E. & SIMONDS, J.P. (1946) Experimental nephro-
pathies. IV. Glycosuria in dogs poisoned with uranyl nitrate,
mercury bichloride and potassium dichromate. Arch. Path., 41:
42-49.
HERNBERG, S., WESTERHOLM, P., SCHULTZ-LARSEN, K., DEGERTH,
R., KUOSMA, E., ENGLUND, A., ENGZELL, U., SAND HANSEN, H., &
MUTANEN, P. (1983) Nasal and sinonasal cancer: connection
with occupational exposures in Denmark, Finland, and Sweden.
Scand. J. Work environ. Health, 9: 315-326.
HERTEL, R.F. (1982) [Chromium as a problem in physiology,
epidemiology and biological monitoring.] Staub-Reinhalt. Luft,
42: 135-137 (in German).
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand
cultures. J. exp. Bot., 4: 59-64.
HILT, G. (1954) La dermite du chrome hexavalent dans le
cadre des dermites eczémateuses par sensibilisation aux
métaux. Dermatologica (Basel), 109: 143-174.
HINTZE, C. (1930) [Handbook of mineralogy,] Berlin, Leipzig,
De Gruyter, Vol. 1. III/2, pp. 4012-4030.
HOPKINS, L.L., Jr (1965) Distribution in the rat of physio-
logical amounts of injected 51Cr(III) with time. Am. J.
Physiol., 209: 731-735.
HOPKINS, L.L., Jr & SCHWARZ, K. (1964) Chromium (III)
binding to serum proteins, specifically siderophidin. Biochem.
Biophys. Acta, 90: 484-491.
HOPKINS, L.L., Jr, RANSOME-KUTI, O., & MAJAJ, A.S. (1968)
Improvement of impaired carbohydrate metabolism by chromium
(III) in malnourished infants. Am. J. clin. Nutr., 21: 203-211.
HUCKABEE, J.W., CARTAN, F.O., & KENNINGTON, G.S. (1972)
Environmental influence on trace elements in hair of 15
species of mammals, Oak Ridge, Tennessee, Oak Ridge National
Laboratory, 36 pp (ORNL-TM-3747).
HUEPER, W.C. (1958) Experimental studies in metal
cancerogenesis. X. Cancerigenic effects of chromite ore roast
deposited in muscle tissue and pleural cavity of rats. Arch.
ind. Health, 18: 284-291.
HUEPER, W.C. (1961) Environmental carcinogenisis and cancer.
Cancer Res., 21: 842-857.
HUNTER, W.C. & ROBERTS, J.M. (1933) Experimental study of
the effects of potassium bichromate in the monkey's kidney.
Am. J. Pathol., 9: 133-147.
HYODO, K., SUZUKI, S., FURUYA, N., & MESHIZUKA, K. (1980) An
analysis of chromium, copper, and zinc in organs of a chromate
worker. Int. Arch. occup. environ. Health, 46: 141-150.
IARC (1980) Some metals and metallic compounds, Lyons,
International Agency for Research on Cancer, pp. 205-323 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 23).
IIJIMA, S., MATSUMOTO, N., LU, C.C., & KATSUNUMA, H. (1975)
Placental transfer of CrCl3 and its effects on fetal growth
and development and development in mice. Teratology, 12: 198.
IMBUS, H.R., CHOLAK, J., MILLER, L.H., & STERLING, T. (1963)
Boron, cadmium, chromium, and nickel in blood and urine. Arch.
environ. Health, 6: 112-121.
IMREH, ST. & RADULESCU, D. (1982) Cytogenic effects of
chromium in vivo and in vitro. Mutat. Res., 97: 192-193.
IVANKOVIC, S. & PREUSSMANN, R. (1975) Absence of toxic and
carcinogenic effects after administration of high doses of
chromium oxide pigment in subacute and long-term feeding
experiments in rats. Food Cosmet. Toxicol., 13: 347-351.
JAEGER, H. & PELLONI, E. (1950) Tests épicutanés aux
bichromates, positifs dans l'eczéma au ciment. Dermatologica
(Basel), 100: 207-216.
JEEJEEBHOY, K.N., CHU, R.G., MARLISS, E.B., GREENBERG, G.R., &
BRUCE-ROBERTSON, A. (1977) Chromium deficiency, glucose
intolerance, and neuropathy reversed by chromium supplement-
ation, in a patient receiving long-term total parenteral
nutrition. Am. J. clin. Nutr., 30: 531-538.
JENNETTE, K.W. (1979) Chromate metabolism in liver micro-
some. Biol. Trace Elem. Res., 1: 55-62.
JENSEN, L.S., MAURICE, D.V., & MURRAY, M.W. (1978) Evidence
for a new biological function of chromium. Fed. Proc., 37: 404.
JINDRICHOVA, J. (1978) [Chromium damage in arc welders.] Z.
Ges. Hyg., 24: 86-89 (in German).
JOHNSON, J.L. (1974) Characterization of air-borne partic-
ulates. Microchem. J., 19:168-178.
JONES, G.B., BUCKLEY, R.A., & CHANDLER, C.S. (1975) The
volability of chromium from brewer's yeast during assay. Anal.
Chim. Acta, 80: 389-392.
JOSCHI, A.P. & NEEB, R. (1980) Gas chromatographic elemental
analysis via di(trifluorethyl)dithiocarbamatochelates. Z.
anal. Chem., 303: 389-393.
KAPLAN, I. & ZELIGMAN, I. (1962) Occupational dermatitis in
railroad workers. Am. Med. Assoc. Arch. Derm., 85: 135-142.
KAUFMANN, D.B., DINICOLA, W., & MCINTOSH, R. (1970) Acute
potassium dichromate poisoning. Am. J. dis. Child, 119:
374-376.
KANEKO, T. (1976) Chromosome damage in cultured human
leucocytes induced by chromium chloride and chromium trioxide.
Jpn. J. ind. Health, 18: 136-137.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec assay and
mutagenicity studies on metal compounds. Mutat. Res., 77:
109-116.
KAZANTZIS, G. & LILLY, L. (1979) Mutagenic and carcinogenic
effects of metals. In: Friedberg, L., Nordberg, G.F., & Vouk,
V.B., ed. Handbook on the toxicology of metals, Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 237-273.
KELLER, C. (1980) [Activation analysis.] In: Bartholomé, E.,
Biekert, E., Hellmann, H., Ley, H., Weigert, M., & Weise, E.,
ed. [Ullmann's encyclopaedia of technical chemistry,] 4th ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
Vol. 5, pp. 685-707 (in German).
KEMP, K., JENSEN, F.P., MOELLER, J.T., & HANSEN, G. (1974)
Trace multi-element analysis of biological tissue by proton-
induced X-ray fluorescence spectroscope, Roskilde, Denmark,
Danish Atomic Energy Commission, 22 pp (Report Riso-M-1732).
KEMPF, T. & SONNEBORN, M. (1976) [Determination of Pb, Cd,
Cr, and Zn according to the drinking-water regulation Federal
Republic of Germany.] In: Aurand, K., Hässelbarth, U., Müller,
G., Schumacher, W., & Steuer, W., ed. [The drinking-water
regulation,] Berlin, Erich Schmidt Verlag, pp. 119-125 (in
German).
KEMPF, T. & SONNEBORN, M. (1981) [Chemical composition of
drinking-waters in various regions of the Federal Republic of
Germany.] Vom Wasser, 57: 83-94 (in German).
KESKINEN, H., KALLIOMAKI, P.-L., & ALANKO, K. (1980) Occupa-
tional asthma due to stainless steel welding fumes. Clin.
Allerg., 10: 151-159.
KIRCHGESSNER, M., MERZ, G., & OELSCHLAEGER, W. (1960)
[Effect of vegetation state on content of macro and micro
elements of three species of grass.] Arch. Tierern., 10:
414-427 (in German).
KITTELBERGER, F. (1973) [Contribution to residue analysis in
fish.] Tierärztl. Prax., 1: 465-479.
KLEIN, L.A., LANG, M., NASH, N., & KIRSCHNER, S.L. (1974)
Sources of metals in New York City wastewater. J. Water
Pollut. Control Fed., 46: 2653-2662.
KLEINER, A.M., STOLBUN, B.M., LIKHACHEVA, E.L., & BELJAEVA,
L.N. (1970) [Some indices of the functional state of the
myocardium and hemodynamics in chronic intoxication with
chromium compounds.] Gig. Tr. prof. Zabol., 12: 7-10 (in
Russian).
KNUDSEN, I. (1980) The mammalian spot test and its use for
the testing of potential carcinogenicity of welding fume
particles and hexavalent chromium. Acta pharmacol. toxicol.,
47: 66-70.
KOGAN, V.Y. (1968) [The basophil degranulation as an index
of skin sensitization to cobalt compounds.] In: Belenky, G.B.,
ed. [Modern aspects of clinical and experimental dermato-
venerology. Works of the clinic of skin and venerological
diseases,] Moscow, Medizina, pp. 100-104 (in Russian).
KOGAN, V.Y. (1971) [Chromium as a specific cause for
occupation dermatoses.] In: Babayants, R.S., ed. [Topical
questions of dermato-venerology. First Medical Institute,
All-Union Conference, Moscow, I.A. Sechenov, pp. 172-176
(in Russian).
KOGAN, V.Y, ARUTYUNYAN, K.E., & KOCHINYAN, Y.E. (1972) [A
comparative characteristic of chromium, nickel, and cobalt
salts as a causative factor in allergic contact dermatosis.]
In: [Clinical Pathology of Chemical Aetiology. Materials of
the 1st All-Union Conference, Kiev, USSR, 24-25 October,
1972,] Kiev, pp. 144 and 146 (in Russian).
KOHLER, H. (1951) [Constitutional examinations of subjects
with skin diseases in the offset department of a printing
works (Doctoral thesis, Leipzig.)] Berufsdermatosen, 4: 93 (in
German).
KOIRTYOHANN, S.R. & HOPKINS, C.A. (1976) Losses of trace
metals during the ashing of biological materials. Analyst,
101: 870-875.
KOIVISTOINEN, P., ed. (1980) Mineral element composition of
Finnish foods. Acta agric. Scand., Suppl. 22: 171
pp.
KOPP, J.E. & KRONER, R.C. (1968) Trace metals in waters of
the United States: a five-year summary of trace metals in
rivers and lakes of the United States (October 1 1962 -
September 30, 1967), Cincinnati, Ohio, US Department of the
Interior, 32 pp.
KORALLUS, U. & LOENHOFF, N. (1981) [Industrial medical and
epidemiological experience with the manufacture and processing
of chromates.] Arbeitsmed. Sozialmed. Praeventivmed., 16:
285-289 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974a)
[Trivalent chromium compounds. Results of a study in
occupational medicine. I. General; Technology; Preliminary
study.] Arbeitsmed. Sozialmed. Praeventivmed., 9: 51-54 (in
German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974b)
[Trivalent chromium compounds: results of a study in
occupational medicine. II. Disease status analysis.]
Arbeitsmed. Sozialmed. Praeventivmed., 9: 76-79 (in German).
KORALLUS, U., EHRLICHER, H., & WUESTEFELD, E. (1974c)
[Trivalent chromium compounds: Results of a study in
occupational medicine. III. Clinical studies.] Arbeitsmed.
Sozialmed. Praeventivmed., 9: 248-252 (in German).
KORALLUS, U., LANGE, H.-J., NEISS, A., WUESTEFELD, E., &
ZWINGERS, T. (1982) [Correlations between reorganization
measures and bronchial carcinoma mortality in the chromate-
manufacturing industry.] Arbeitsmed. Sozialmed. Praevent-
ivmed., 7: 159-167 (in German).
KOSHI, K. (1979) Effects of fume particles from stainless
steel welding on sister chromatid exchanges and chromosome
aberrations in cultured Chinese hamster cells. Ind. Health.,
17: 39-49.
KOTZ, L., KAISER, G., TSCHOPEL, P., & TOLG, G. (1972)
[Clarification of biological matrices for determining very low
contents of trace elements when limited amounts are weighed
with nitric acid under pressure in a teflon vessel.] Z. anal.
Chem., 260: 207-209 (in German).
KOUTRAS, G.A., HATTORY, M., SCHNEIDER, A.S., EBAUGH, F.G., &
VALENTINE, W.N. (1964) Studies on chromated erythrocytes:
effect of sodium chromate on erythrocyte glutathione reduc-
tase. J. clin. Invest., 43: 323-331.
KRISHNAJA, A.P. & REGE, M.S. (1982) Induction of chromosomal
aberrations in fish Boleophthalmus dussumieri after exposure
in vivo to mitomycin C and heavy metals mercury, selenium, and
chromium. Mutat. Res., 102: 71-82.
KROEPFLI, P. & SCHUPPLI, R. (1955) [Detergent eczema.]
Dermatologica (Basel), 110: 1-7 (in German).
KRUGER, H. & DORN, H. (1956) [Clinical observations on
detergent eczema in association with chromium and nickel
allergy.] Z. Haut-Geschlechtskr., 20: 307-314 (in German).
KUCHER, I.M. (1966) In: [Proceedings of the 5th Scientific
Session of the Aktiubinsk Medical Institute, Alma-Ata,
Kazakhstan,] pp. 24-26 (in Russian).
KUHN, J.K. (1973) Trace elements in whole coal determined by
X-ray fluorescence. Norelco Rep., 20(3): 7-10.
KUMPULAINEN, J.T., WOLF, W.R., VEILLON, C., & MERTZ, W.
(1979) Determination of chromium in selected United States
diets. J. agric. food Chem., 27: 490-494.
KUMPULAINEN, J., LEHTO, J., KOIVISTOINEN, P., UUSITUPA, M., &
VUORI, E. (1983) Determination of chromium in human milk
serum and urine by electrothermal atomic absorption spectro-
metry without preliminary ashing. Sci. total Environ., 31:
71-80.
KURYLOWICZ, B. & GASIOROWSKI, S. (1958) [Agrochemical study
on calcinated phosphate-containing admixtures of chromium
compounds.] Przemysl Chem., 37: 797-800 (in Polish).
LANGARD, S. (1977) The fate of chromium after intravenous
administration of Na251 CrO4 and CrCl3 x 6H2 O to the rat,
Guildford, England, University of Surrey, 66 pp (M.Sc.
Toxicology Thesis).
LANGARD, S. (1980a) Chromium. In: Waldron, H.A., ed. Metals
in the environment, London, New York, Academic Press,
pp. 111-132.
LANGARD, S. (1980b) A survey of respiratory symptoms and
lung function in ferrochromium and ferrosilicon workers. Int.
Arch. occup. environ. Health, 46: 1-9.
LANGARD, S., ed. (1982) Biological and environmental aspects
of chromium, Amsterdam, New York, Oxford, Elsevier Science
Publishers, 285 pp.
LANGARD, S. & HENSTEN-PETTERSEN, A. (1981) Chromium
toxicology. In: Williams, D.F., ed. Systemic aspects of
biocompatibility, Boca Raton, Florida, CRC Press, Vol. 1, pp.
144-161.
LANGARD, S. & NORSETH, T. (1975) A short study of bronchial
carcinomas in workers producing chromate pigments. Br. J. ind.
Med., 32: 62-65.
LANGARD, S. & NORSETH, T. (1979) Chromium. In: Friberg, L.,
Nordberg, G.F., & Vouk, V.B., ed. Handbook on the toxicology
of metals, Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 383-397.
LANGARD, S. & STERN, R.M. (1985) Nickel in welding fumes: a
cancer hazard to welders? In: Sunderman, F.W.J., Aitio, A.,
Berlin, A., Bishop, C., Buringh, E., Davis, W., Gounar, M.,
Jacquignon, P.C., Mastromatteo, E., Rigaut, J.P., Rosenfeld,
C., Saracci, R., & Sors, A., ed. Nickel in the human
environment, Lyons, International Agency for Research on
Cancer, pp. 95-103 (IARC Scientific Publications No. 53).
LANGARD, S. & VIGANDER, T. (1983) Occurrence of lung cancer
in workers producing chromium pigments. Br. J. ind. Med., 40:
71-74.
LANGARD, S., GUNDERSEN, N., TSALEV, D.L., & GYLSETH, B.
(1978) Whole blood chromium level and chromium excretion in
the rat after zinc chromate inhalation. Acta pharmacol.
toxicol., 42: 142-149.
LANGARD, S., ANDERSEN, A., & GYLSETH, B. (1980) Incidence of
cancer among ferrosilicon workers. Br. J. ind. Med., 37:
114-120.
LASKIN, S. (1972) Research in environmental sciences,
Washington DC, Institute of Environmental Medicine, pp. 92-97.
LASKIN, S., KUSCHNER, M., & DREW, R.T. (1970) Studies in
pulmonary carcinogenesis. In: Hanna, M.G., Jr, Nettesheim, P.,
& Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 321-351 (US Atomic Energy
Commission Symposium Series No. 18).
LAUTNER, G.M., CARVER, J.C., & KONZEN, R.B. (1978)
Measurement of chromium (VI) and chromium (III) in stainless
steel welding fumes with electron spectroscopy for chemical
analysis and neutron activation analysis. Am. Ind. Hyg. Assoc.
J., 39: 651-660.
LEHMANN, K.B. (1914) [Historical and critical account of our
present knowledge of the effect of small doses of chromate.]
In: Gesamtgebiet gewerbehygiene, Berlin, Springer Verlag, pp.
1-119 (in German).
LEHMANN, K.B. (1932) [Does the occurrence of lung cancer in
chromium workers give grounds for any particular anxiety?]
Zentralbl. Gewerbehyg., 9: 168-170 (in German).
LERICHE, H.H. (1968) Metal contamination of soil in the
Woburn market-garden experiment resulting from the application
of sewage sludge. J. agric. Sci. Camb., 71: 205-208.
LETTERER, E., NEIDHARDT, K., & KLETT, H. (1944) [Chromate
lung cancer and chromate pneumoconiosis. A clinical, morbid
anatomical and industrial-hygiene-related study.] Arch.
Gewerbepath. Gewerbehyg., 12: 323-361 (in German).
LEVIN, H.M., BRUNNER, M.J., & RATTNER, H. (1959)
Lithographer's dermatitis. J. Am. Med. Assoc., 169: 566-569.
LEVINE, R.A., STREETEN, D.H.P., & DOISY, R.J. (1968) Effects
of oral chromium supplementation on the glucose tolerance of
elderly human subjects. Metabolism, 17: 114-125.
LEVIS, A.G. & BIANCHI, V. (1982) Mutagenic and cytogenetic
effects of chromium compounds. In: Langard, S., ed. Biological
and environmental aspects of chromium, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 171-208.
LEVIS, A.G. & BUTTIGNOL, M. (1977) Effects of potassium
dichromate on DNA Synthesis in hamster fibroblast. Br. J.
Cancer, 35: 496-499.
LEVIS, A.G. & MAJONE, F. (1979) Cytotoxic and caryogenic
effects of soluble chromium compounds on mammalian cell
cultures. Br. J. Cancer, 40: 523-533.
LEVIS, A.G. & MAJONE, F. (1981) Cytologic and clastogenic
effects of soluble and insoluble compounds containing
hexavalent and trivalent chromium. Br. J. Cancer, 44: 219-235.
LEVIS, A.G., BUTTIGNOL, M., BIANCHI, V., & SPONZA, G. (1978)
Effects of potassium dichromate on nucleic acid and protein
synthesis and on precursor uptake in BHK fibroblasts. Cancer
Res., 38: 110-116.
LEVY, L.S. & MARTIN, P.A. (1983) The effects of a range of
chromium-containing materials on rat lung, Birmingham,
England, University of Aston, Department of Environmental and
Occupational Health, p. 224 and suppl (Prepared for Dry Color
Manufacturers Association, Alexandria, Virginia, USA).
LEVY, L.S. & VENITT, S. (1975) Carcinogenic and mutagenic
activity of chromium-containing materials. Br. J. Cancer, 32:
254-255.
LIEBER, M., PERLMUTTER, N.M., & FRAUENTHAL, H.L. (1964)
Cadmium and hexavalent chromium in Nassau County ground
water. J. Am. Water Works Assoc., 56: 739-747.
LIFSCHITZ, M.L., WALLACH, S., PEABODY, R.A., VERCH, R.L., &
AGRAWAL, R. (1980) Radiochromium distribution in thyroid
and parathyroid deficiency. Am. J. clin. Nutr., 33: 57-62.
LINDBERG, E. & HEDENSTIERNA, G. (1983) Chrome plating.
Symptoms, findings in the upper airways, and effects on lung
function. Arch. environ. Health, 38: 367-374.
LINDBERG, E. & VESTERBERG, O. (1983a) Monitoring exposure to
chromic acid in chromeplating by measuring chromium in urine.
Scand. J. Work environ. Health, 9: 333-340.
LINDBERG, E. & VESTERBERG, O. (1983b) Urinary excretion of
proteins in chromplaters, previously exposed chromplaters, and
referents. Scand. J. Work. environ. Health, 9: 505-510.
LINDBERG, E., EKHOLM, U. & ULFVARSON, U. (1985) Extent and
conditions of exposure in the Swedish chrome plating industry.
Int. Arch. Occup. Environ. Health, 56: 197-205.
LIU, V.J.K. & MORRIS, J.S. (1978) Relative chromium response
as an indicator of chromium status. Am. J. clin. Nutr., 31:
972-976.
LOFROTH, G. (1978) The mutagenicity of hexavalent chromium
is decreased by microsomal metabolism. Naturwissenschaften,
65: 207-208.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LUKANIN, W.P. (1930) [The pathology of chromate pneumo-
coniosis.] Arch. Hyg. Bakt., 104: 166-174 (in German).
LUZHNIKOV, E.A., SHIMANKO, I.I., KOSTOMAROVA, L.G., MALYGINA,
S.I., & TRAKHTENGARTS, M.I. (1976) [Acute poisoning with
chromium compounds.] Terapeut. Arkh., 48(7): 121-125 (in
Russian).
LYON, G.L., BROOKS, R.R., PETERSON, P.J., & BUTLER, G.W.
(1970) Some trace elements in plants from serpentine soils.
N.Z. J. Sci., 13: 133-139.
MACHLE, W. & GREGORIUS, F. (1948) Cancer of the respiratory
system in the United States chromate-producing industry.
Public Health Rep., 63: 1114-1127.
MCCLENDON, L.T. (1978) Determination of chromium in
biological matrices by neutron activation: application to
standard reference materials. J. radioanal. Chem., 42: 85-91.
MACKENZIE, R.D., BYERRUM, R.U., DECKER, C.F., HOPPERT, C.A., &
LANGHAM, R.F. (1958) Chronic toxicity studies. II. Hexa-
valent and trivalent chromium administered in drinking-water
to rats. Am. Med. Assoc. Arch. ind. Health, 18: 232-234.
MCRAE, W.D., WHITING, R.F., & STICH, H.F. (1979) Sister-
chromatid exchanges induced in cultured mammalian cells by
chromate. Chem.-biol. Interact., 26: 281-286.
MAHALKO, J.R. & BENNION, M. (1976) The effect of parity and
time between pregnancies on maternal hair chromium concentra-
tion. Am. J. clin. Nutr., 29: 1069-1072.
MAJONE, F. & LEVIS, A.G. (1979) Chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster cells treated in
vitro with hexavalent chromium compounds. Mutat. Res., 67:
231-238.
MAJONE, F. & RENSI, D. (1979) Mitotic alterations, chromo-
some aberrations, and sister-chromatid exchanges induced by
hexavalent and trivalent chromium on mammalian cells in vitro.
Caryologia, 32: 379-392.
MALTONI, C. (1974) Occupational carcinogenesis. Excerpta
Med. Int. Congr. Ser., 322: 19-26.
MALTONI, C. (1976) Precursor lesions in exposed populations
as indicators of occupational cancer risk. Ann. NY Acad. Sci.,
271: 444-447.
MANCUSO, T.F. (1951) Occupational cancer and other health
hazards in a chromate plant: a medical appraisal. II. Clinical
and toxicologic aspects. Ind. Med. Surg., 20: 393-407.
MANCUSO, T.F. (1975) Consideration of chromium as an
industrial carcinogen. In: Hutchinson, T.C., ed. Proceedings
of the International Conference on Heavy Metals in the
Environment, Toronto, Canada, October 27-31, 1975, Toronto,
Institute for Environmental Studies, pp. 343-356.
MANCUSO, T.F. & HUEPER, W.C. (1951) Occupational cancer and
other health hazards in a chromate plant: a medical appraisal.
I. Lung cancer in chromate workers. Ind. Med. Surg., 20:
358-363.
MARTIN, J.H. & KNAUER, G.A. (1973) The elemental composition
of plankton. Geochim. Cosmochim. Acta, 3: 1639-1653.
MATHIS, B.J. & CUMMINGS, T.F. (1973) Selected metals in
sediments, water, and biota in the Illinois river. J. Water
Pollut. Control Fed., 45: 1573-1583.
MATHUR, R.K. & DOISY, R.J. (1972) Effect of diabetes and
diet on the distribution of tracer doses of chromium in rats.
Proc. Soc. Exp. Biol. Med., 139: 836-838.
MATSUMOTO, N., IIJIMA, S., & KATSUNUMA, H. (1976) Placental
transfer of chromic chloride and its teratogenic potential in
embryonic mice. J. toxicol. Sci., 2: 1-13.
MAYER, R. (1983) Interaction of forest canopies with atmo-
spheric constituents: aluminium and heavy metals. In: Ulrich,
B. & Pankrath, J., ed. Effects of accumulation of air
pollutants in forest ecosystems, Dordrecht, Boston, London,
D. Reidel, pp. 47-55.
MAZGON, R. (1932) [Therapeutic effect of common salt on
experimental chromium nephritis in the rabbit.] Z. Gesamte
exp. Med., 81: 195-207 (in German).
MERIAN, E. (1984) Introduction on environmental chemistry
and global cycles of arsenic, beryllium, cadmium, chromium,
cobald, nickel, selenium, and their derivatives. Toxicol.
environ. Chem., 8: 9-38.
MERKURIEVA, R.V., KRASOVSKY, G.N., BURMANTOVA, N.P.,
SHATERNIKOVA, I.L., VARSHAVSKAYA, S.P., VASIUKOVICH, L.YA.,
KOGANOVA, Z.I., BUSHINSKAYA, L.I., KONSTANTINOVA, I.N.,
DOLINSKAYA, S.I., ASTAKHOVA, L.F., & VOTYAKOV, A.V. (1980a)
[Changes in various enzyme systems resulting from exposure to
some chemical substances.] Gig. i. Sanit., N1: 25-28 (in
Russian).
MERKURIEVA, R.V., KRASOVSKY, G.N., VOTYAROV, A.V., BURMANTOVA,
N.P., MUCHAMBETOVA, L.H., & KONSTANTINOVA, I.N. (1980b)
[Enzyme disarrangement of various subcellular structures under
the influence of the general toxic and gonadotoxic effects of
environmental chemical factors.] Zdravookhranenie belorus.,
N2: 28-29 (in Russian).
MERKURIEVA, R.V., KOGANOVA, Z.I., GABDULLINA, M.H., TSAPKOVA,
N.N., DUKHATEROV, O.N., & DOLINSKAYA, S.I. (1982) [A
comparative study of metabolic reactions at various routes of
chromium6+ administration in the organism of laboratory
animals.] Gig. i Sanit., N8: 75-78 (in Russian).
MERRITT, W.F. (1971) Identification and measurement of trace
elements in fresh water by neutron activation. In: Proceedings
of the International Symposium on Identification and Measure-
ment of Environmental Pollution, 358-362.
MERTZ, W. (1969) Chromium occurrence and function in
biological systems. Physiol. Rev., 49: 163-239.
MERTZ, W. (1981) The interaction between chromium and
insulin. Adv. Physiol. Sci., 12: 101-105.
MERTZ, W. & ROGINSKI, E.E. (1963) The effect of trivalent
chromium in galactose entry in rat epidymal fat tissue. J.
biol. Chem., 238: 868-872.
MERTZ, W. & ROGINSKI, E.E. (1969) Effects of chromium (III)
supplementation on growth and survival under stress in rats
fed low protein diets. J. Nutr., 97: 531-536.
MERTZ, W. & ROGINSKI, E.E. (1971) Chromium metabolsm: the
glucose tolerance factor. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition, New York, Marcel Dekker,
pp. 123-153.
MERTZ, W. & SCHWARZ, K. (1959) Relation of glucose tolerance
factor to impaired glucose tolerance in rats on stock diets.
Am. J. Physiol., 196: 614-618.
MERTZ, W., ROGINSKI, E.E., & SCHWARZ, K. (1961) Effect of
trivalent chromium complexes on glucose uptake by epididymal
fat tissue of rats. J. biol. Chem., 236: 318-322.
MERTZ, W., ROGINSKI, E.E., & REBA, R.C. (1965a) Biological
activity and fate of intravenous chromium (III) in the rat.
Am. J. Physiol., 209: 489-494.
MERTZ, W., ROGINSKI, E.E., & SCHROEDER, H.A. (1965b) Some
aspects of glucose metabolism of chromium-deficient rats
raised in a strictly controlled environment. J. Nutr., 86:
107-112.
MERTZ, W., ROGINSKI, E.E., FELDMAN, R.J., & THURMAN, D.E.
(1969) Dependence of chromium transfer into the rat embryo on
the chemical form. J. Nutr., 99: 363-367.
MERTZ, W., TOEPFER, E.W., ROGINSKI, E.E., & POLANSKY, M.M.
(1974) Present knowledge of the role of chromium. Fed. Proc.,
33: 2275-2280.
MEYERS, J.B. (1950) Acute pulmonary complications following
inhalations of chromic acid mist. Arch. ind. Hyg. occup. Med.,
2: 742-747.
MICKAIL, T.H., HABIB, Y.A., GABRIAL, G.N., & MORCOS, S.R.
(1976) The effect of chromium III on the intravenous glucose
tolerance of rats given diets of defficient protein values. Z.
Ernährungswiss., 15: 182-187.
MIESCHER, G., AMREIN, H.P., & LEDER, M. (1955) [Chromate
hypersensitivity and cement eczema.] Dermatologica (Basel),
110: 266-283 (in German).
MIGAI, K.V. (1975) [Hygiene and safety of labor in electro
welding works in shipbuilding,] Leningrad, pp. 27-35 (in
Russian).
MITMAN, F.W., WOLF, W.R., KELSAY, J.L., & PRATHER, E.S.
(1975) Urinary chromium levels of nine young women eating
freely chosen diets. J. Nutr., 105: 64-68.
MORGAN, J.M. (1972) Hepatic chromium content in diabetic
subjects. Metabolism, 21: 313-316.
MORRIS, G.E. (1955) Chromate dermatitis from chrome glue and
other aspects of the chrome problem. Am. Med. Assoc. Arch.
Ind. Health, 11: 368-371.
MORRIS, G.E. (1956) The manufacture of leather. Its chemical
and dermatological aspects. Ann. Meet. Am. Acad. Derm. Syph.
(Chicago).
MORRIS, G.E. (1958) Chrome dermatitis. A study of the
chemistry of shoe leather with particular reference to basic
chromic sulfate. Am. Med. Assoc. Arch. Derm., 78: 612-618.
MORRIS, G.E. (1959) [The basic form of chromic sulfate.
Results of epicutaneous testing of a chromium-III salt.]
Berufsdermatosen, 7: 126-131 (in German).
MORROW, R.W. & MCELHANEY, R.J. (1974) Determination of
chromium in industrial effluent water by flameless atomic
absorption spectroscopy. At. Absorpt. Newsl., 13(2): 45-46.
MORTON, M.S. & ELWOOD, P.C. (1974) CNS malformations and
trace elements in water. Teratology, 10: 318.
MOSINGER, M. & FIORENTINI, H. (1954) Sur la pathologie due
aux chromates: Premières recherches expérimentales. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 15: 187-199.
MULLER-WIEGAND, B., STEUER, D., & HEESSELS, P. (1983) Blood
and urine samples for quality control of heavy metals. Chem.
Toxicol. Clin. Chem. Met. Proc. Int. Conf., 2nd: 73-76.
MURAKAMI, Y., SUZUKI, Y., YAMAGATA, T., & YAMAGATA, N. (1965)
Cr and Mn in Japanese diet. J. Radiat. Res., 6: 105-110.
MURRMAN, R.P., WINTERS, R.W., & MARTIN, T.G. (1971)
Determination of trace elements in soils and clay minerals by
resonnance neutron activation analysis. Soil. Sci. Soc. Am.
Proc., 35: 647-652.
MUTTI, A., CAVATORTA, A., PEDRONI, C., BORGHI, A., GIAROLI,
C., & FRANCHINI, I. (1979) The role of chromium accumulation
in the relationship between airborne and urinary chromium in
welders. Int. Arch. occup. environ. Health, 43: 123-133.
MYSLYAEVA, A.V. (1965) [Changes in the peripheral blood in
workers of a chromium compound plant.] In: [Proceedings of
the 4th Scientific Conference of Aktubinsk State Medical
Institute, Alma-Ata,] Kazakhstan, Alma-Ata, Aktubinsk State
Medical Institute, pp. 331-332 (in Russian).
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., & KURATA, H. (1978)
Comparative studies of chromosomal aberrations and muta-
genicity of trivalent and hexavalent chromium. Mutat. Res.,
58: 175-181.
NASH, D.T., ELWOOD, I.G., & STREETEN, D.H.P. (1979) The
effect of brewer's yeast containing chromium-rich glucose
tolerance factor on serum lipids. In: Proceedings of the 5th
International Symposium on Atherosclerosis, Houston, Texas.
NATER, J.P. (1963) Possible causes of chromate eczema.
Dermatologica (Basel), 126: 160-166.
NATH, R. (1976) Assessment of chromium metabolism in
maturity onset and juvenile diabetics using chromium: 51Cr and
therapeutic response of chromium administration. Excerpta Med.
Int. Congr. Ser., 400: 194.
NEIKO, E.M. & DELVA, IU.V. (1978) [Chromium balance in
chronic ischemic heart disease.] Klinicheskaia Meditsina
(Mosk.), 56(4): 97-99 (in Russian).
NESTMANN, E.R., MATULA, T.I., DOUGLAS, G.R., BORA, K.C., &
KOWBEL, D.J. (1979) Detection of the mutagenic activity of
lead chromate using a battery of microbial tests. Mutat. Res.,
66: 357-365.
NETTESHEIM, P., HANNA, M.G., Jr., DOHERTY, D.G., NEWELL, R.F.,
& HELLMANN, A. (1970) Effects of chronic exposure to
artificial smog and chromium oxide dust on the incidence of
lung tumours in mice. In: Hanna, M.G., Jr, Nettesheim, P., &
Gilbert, J.R., ed. Inhalation carcinogenesis, Oak Ridge,
Tennessee, US Atomic Energy Commission, Division of Technical
Information Extension, pp. 305-320 (US Atomic Energy
Commission Symposium Series No. 18).
NETTESHEIM, P., HANNA, M.G., DOHERTY, D.G., NEWELL, R.F., &
HELLMAN, A. (1971) Effect of calcium chromate dust,
influenza virus, and 100R whole-body X-radiation on lung
tumour incidence in mice. J. Natl Cancer Inst., 47: 1129-1144.
NEWBOLD, R.F., AMOS, J., & CONNELL, J.R. (1979) The
cytotoxic, mutagenic, and clastogenic effects of chromium-
containing compounds on mammalian cells in culture. Mutat.
Res., 67: 55-63.
NEWMAN, H.A.I., LEIGHTON, R.F., LANESE, R.R., & FREELAND N.A.
(1978) Serum chromium and angiographically-determined
coronary artery disease. Clin. Chem., 24: 541-544.
NISHIOKA, H. (1975) Mutagenic activities of metal compounds
in bacteria. Mutat. Res., 31: 185-189.
NOLLENDORF, A., PAKALNE, E., & UPITIS, V. (1972) [Little
known trace elements in Chlorella culture.] Latv. PSR Zinat.
Akad. Vestis (Latvian SSR), 7: 33-43 (in Russian).
NOMIYAMA, H., YOTORIYAMA, M., & NOMIYAMA, K. (1980b) Normal
chromium levels in urine and blood of Japanese subjects
determined by direct flameless atomic absorption spectrophoto-
metry, and valency of chromium in urine after exposure to
hexavalent chromium. Am. Ind. Hyg. Assoc. J., 41: 98-102.
NOMIYAMA, K., MATSUI, K., & NOMIYAMA, H. (1980a) Environ-
mental temperature: a factor modifying the acute toxicity of
organic solvents, heavy metals, and agricultural chemicals.
Toxicol. Lett., 6: 67-70.
NORSETH, T. (1978) Health effects of nickel and chromium.
In: Elvira di Ferrante, E., ed. Trace metals: exposure and
health effects, Oxford, New York, Toronto, Sydney, Paris,
Frankfurt, Pergamon Press, pp. 135-146.
NORSETH, T. (1980) Cancer hazards caused by nickel and
chromium exposure. J. Toxicol. environ. Health, 6: 1218-1227.
NOVAKOVA, S., MAUTNER, G., & DINOEVA, A. (1974) [Content of
hexavalent chromium in water supplies and its effect on the
development of experimental atherosclerosis in warm-blooded
animals.] Gig. i Sanit., 5: 78-80 (in Russian).
NOVEY, H.S., HABIB, M., WELLS, J.A. (1983) Asthma and JgE
antibodies induced by chromium and nickel salts. J. Allergy
clin. Immunol., 72: 407-412.
OFFENBACHER, E.G. & PI-SUNYER, F.X. (1980) Benefical effect
of chromium-rich yeast on glucose tolerance and blood lipids
in elderly subjects. Diabetes, 29: 919-925.
OHNO, H., HANAOKA, F., & YAMADA, M. (1982) Inducibility of
sister-chromatid exchanges by heavy-metal ions. Mutat. Res.,
104: 141-145.
OHTA, F. (1940) Studies on the detoxicating hormone of the
liver (yakriton). Contribution to the usage of yakriton
against experimental chromate nephritis. Tohoku J. exp. Med.,
39: 37-46.
OKADA, S., OHBA, H., & TANIYAMA, M. (1981) Alterations in
ribonucleic acid synthesis by chromium (III). J. inorg.
Biochem., 15: 223-231.
OKADA, S., TRUKADA, H., & OHBA, H. (1983) Enhancement of
ribonucleic acid synthesis by chromium (III) in mouse liver.
J. inorg. Biochem., 19: 95-103
OKADA, S., TSUKADA, H., & OHBA, H. (1984) Enhancement of
nucleolar RNA synthesis by chromium (III) in regenerating rat
liver. J. inorg. Biochem., 21, 113-124.
OPHULS, W. (1911a) Experimental nephritis in guinea-pigs by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9: 11-13.
OPHULS, W. (1911b) Experimental nephritis in rabbits by
subcutaneous injections of chromates. Proc. Soc. Exp. Biol.
Med., 9:13.
OTTINGER, R.S., BLUMENTHAL, J.L., DALPORTO, D.F., GURBER,
G.I., SANTY, M.J., & SHIH, C.C. (1973) Recommended methods
of reduction, neutralization, recovery or disposal of
hazardous waste, Cincinnati, Ohio, US Environmental Protection
Agency, Vol. 6, pp. 143-162 (EPA 670/2-73-053-F).
PAGE, A.L. (1974) Fate and effects of trace elements in
sewage sludge when applied to agricultural lands, Cincinnati,
Ohio, US Environmental Protection Agency (EPA 670/2-74-005).
PAIXAO, L.M. & YOE, J.H. (1959) Spectochemical determination
of magnesium, chromium, nickel, copper, and zinc in human
plasma and red cells. Clin. Chim. Acta, 4: 507-514.
PARR, R.M. (1977) Problems of chromium analysis in
biological materials: an international perspective with
special reference to results for analytical quality control
samples. J. radioanal. Chem., 39: 421-433.
PASCALE, L.R., WALDSTEIN, S.S., ENGBRING, G., DUBIN, A., &
SZANTO, P.B. (1952) Chromium intoxication with special
reference to hepatic injury. J. Am. Med. Assoc., 149:
1385-1389.
PASHIN, Y.V. & KOZACHENKO, V.J. (1981) [Mutagenic activity
of chromium compounds.] Gig. i. Sanit., 5: 46-49 (in Russian).
PASHIN, Y.V., ZACEPILOVA, T.A., & KOZACHENKO, V.I. (1982)
Induction of dominant lethal mutations in male mice by
potassium dichromate. Mutat. Res., 103: 345-347.
PASTERNAK, K. (1973) The spreading of heavy metals in
flowing waters in the region of occurrence of natural deposits
of the zinc and lead industry. Acta hydrobiol., 15: 145-166.
PEKAREK, R.S., HAUER, E.C., RAYFIELD, E.J., WANNEMACHER, R.W.,
& BIESEL, W.R. (1975) Relationship between serum chromium
concentrations and glucose utilization in normal and infected
subjects. Diabetes, 24: 350-353.
PENG, H.Z. (1982) [The investigation of nasal disease
morbidity of chromium exposed workers.] J. Shan-Xie Med.
College, 3: 19-21 (in Chinese).
PERLMUTTER, N.M. & LIEBER, M. (1970) Dispersal of plating
wastes and sewage contaminants in ground water and surface
water, Washington DC, US Government Printing Office, 67 pp
(Geological Survey Water Supply Paper 1879-G).
PERONE, V.B., MOFFITT, A.A., POSSICK, P.A., KEY, M.M.,
DANZINGER, S.J., & GELLIN, G.A. (1974) The chromium, cobalt,
and nickel contents of American cement and their relationship
to cement dermatitis. Am. Ind. Hyg. Assoc. J., 35: 301-306.
PETRILLI, F.L. & DE FLORA, S. (1977) Toxicity and mutagenicity of
hexavalent chromium on Salmonella typhimurium. Appl. environ.
Microbiol., 33: 805-809.
PETRILLI, F.L. & DE FLORA, S. (1978a) Oxidation of inactive
trivalent chromium to the mutagenic hexavalent form. Mutat.
Res., 58: 167-173.
PETRILLI, F.L. & DE FLORA, S. (1978b) Metabolic deactivation
of hexavalent chromium mutagenicity. Mutat. Res., 54: 139-147.
PETRILLI, F.L. & DE FLORA, S. (1980) Mutagenicity of
chromium compounds. In: Chromates Symposium 80, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 76-99.
PETRILLI, F.L. & DE FLORA, S. (1982) Interpretations on
chromium mutagenicity and carcinogenicity. In: Sorsa, M. &
Vainmio, H., ed. Mutagens in our environment, New York, Alan
R. Liss, pp. 453-464.
PIERCE, J.V. & SCHEEL, L.D. (1965) Toxicity of alloys of
chromium. Arch. environ. Health, 10: 870-876.
PIRILA, V. & KILPIO, O. (1949) On dermatoses caused by
bichromates. Acta derm. venerol., 29: 550-563.
POHLAND, F.G. (1975) Sanitary landfill stabilization with
leachate recycle and residual treatment, Cincinnati, Ohio, US
Environmental Protection Agency, 106 pp (EPA 600/2-75-043).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1973) [On carcinogenous
hazards in the product of chromium ferroalloys.] Gig. Tr.
prof. Zabol., 10: 23-26 (in Russian).
POKROVSKAYA, L.V. & SHABYNINA, N.K. (1974) [Epidemiological
data on malignant neoplasms in workers who are in contact with
different chromium compounds.] In: Shabad, L.M., Velich-
kovskii, B.T. & Kogan, F.M., ed. [Occupational cancer (a
collection of scientific works),] Moscow, Academic Academy of
Medical Science, pp. 64-71 (in Russian).
POKROVSKAYA, L.V., TJUSHUYAKOVA, A.V., GORODNOVA, N.V.,
ZALOMOVA, E.N., & ANDREEVA, T.D. (1976) [Dust factor and
occupational pathology in workers engaged in the open
excavation of chromium ores.] In: [Occupational diseases of
dust etiology (collection of works),] Vol. 3, pp. 38-43 (in
Russian).
POLAK, L., TURK, J.L., & FREY, J.R. (1973) Studies on
contact hypersensitivity to chromium compounds. In: Kallos,
P., Waksman, B.H., & DeWeck, A., ed. Progress in allergy,
Basel, Karger, Vol. 17, pp. 145-226.
PRESTON, A.M., DOWDY, R.P., PRESTON, M.A., & FREEMAN, J.N.
(1976) Effects of dietary chromium on glucose and serum
cholesterol in guinea-pigs. J. Nutr., 106: 1391-1397.
PRIESTLEY, J. (1877) Observations on the physiological
action of chromium. J. Anat. Physiol., 11: 285-301.
PRINCI, F., MILLER, L.H., DAVIS, A., & CHOLAK, J. (1962)
Pulmonary disease of ferrochromium workers. J. occup. Med., 4:
301-310.
PUNSAR, S., ERAMETSA, O., KARVONEN, M.I., RYHANEN, A., HILSKA,
P., & WORNAMO, H. (1975) Coronary heart disease and
drinking-water. A search in two Finnish male cohorts for
epidemiologic evidence of a water factor. J. chron. Dis., 28:
259-287.
RABEAU, H. & UKRAINCZYK, V. (1939) Dermites des
blanchisseuses. Rôle du chrome et du chlore. Ann. Derm. Syph.,
10: 656-680.
RADIAN CORPORATION (1983) Estimates of population exposure
to ambient chromium emissions, Research Triangle Park, North
Carolina, US Environmental Protection Agency (Final Report to
K.L. Blanchard) (EPA Contract No. 68-02-3818).
RAFFETTO, G. (1977) Direct interaction with cellular targets
as the mechanism for chromium carcinogenesis. Tumori, 63:
503-512.
RAFFETTO, G., PARODI, S., DE FERRARI, M., PARODI, C., TROIANO,
R., & BRAMBILLA, G. (1978) Direct interaction with cellular
targets as the mechanism for chromium carcinogensis. Tumori,
73: 503-512.
REDISKE, J.H. (1956) Chromium toxicity in plants. In:
Biology research: annual report, 1955, Richland, Washington,
Hamford Atomic Products Operations, pp. 48-52 (Report
HW-41500).
REDISKE, J.H., CLINE, J.F., & SELDERS, A.A. (1955) Chromium
toxicity in plants. In: Biology research: annual report, 1954,
Richland, Washington, Hamford Atomic Products Operations,
pp. 32-39 (Report HW-35917).
REGGIANI, A., LOTTI, M., DE ROSA, E., & SAIA, B. (1973)
[Impairments of respiratory functions in subjects exposed to
chromium. Note 1. Spirographic changes.] Lav. Umano, 25: 23-27
(in Italian).
REICHENBACH-KLINKE, H.H. (1977) [To the ecology of the
accumulation of heavy metals in fishes.] In: Reichenbach-
Klinke, H.H., ed. [Contributions to fish ecology: fish and the
environment,] Stuttgart, New York, Gustav Fischer, pp. 7-11
(in German).
REICHENBACH-KLINKE, H.H. (1980) [Fish diseases and harm
suffered by fish,] 2nd ed., Stuttgart, New York, Gustav
Fischer, 474 pp (in German).
RETNEV, V.M. (1960) [On the effect produced by chromium
compounds contained in cement dust on the development of
bronchial asthma.] Gig. Tr. prof. Zabol., 7: 29-32 (in
Russian).
RIALES, R. (1979) Influence of brewer's yeast on lipoprotein
cholesterol concentrations. A preliminary report. In:
Shapcott, D. & Hubert, I., ed. Chromium in nutrition and
metabolism. Amsterdam, New York, Oxford, Elsevier Science
Publishers, pp. 199-212.
ROBERTS, K.R. & MARZLUF, G.A. (1971) The specific inter-
action of chromate with the dual sulfate permease systems of
Neurospora crassa. Arch. Biochem. Biophys., 142: 651-659.
ROBINSON, W.D. & EDINGTON, G. (1935) Chemical studies of
infertile soils derived from rocks high in magnesium and
generally high in chromium and nickel, Washington DC, US
Department of Agriculture, pp. 1-29 (US Department of
Agriculture Technical Bulletin No. 471).
ROGINSKI, E.E. & MERTZ, W. (1967) An eye lesion in rats fed
low-chromium rations. J. Nutr., 93: 249-251.
ROGINSKI, E.E. & MERTZ, W. (1969) Effects of chromium (III)
supplementation on glucose and amino acid metabolism in rats
fed a low protein diet. J. Nutr., 97: 525-530.
ROHDE, G., TISCHENDORF, G., LEONHARDT, J., & DAMASCHUN, F.
(1978) [Study of crocoite occurrence in Callenberg (Saxony)
(Germany).] Z. angew. Geol., 24: 168-173 (in German).
ROMOSER, G.L., DUDLEY, W.A., MACHLIN, L.J., & LOVELESS, L.
(1961) Toxicity of vanadium and chromium for the growing
chick. Poultry Sci., 40: 1171-1173.
ROOK, H.L. & WOLF, W.R. (1977) The quantitative
determination of volatile trace elements in NBS biological
standard reference materials. In: Hemphill, D.D., ed.
Proceedings of the Eleventh Annual Conference on Trace
Substances in Environmental Health, Columbia, Missouri, the
Curators of the University of Missouri, pp. 324-333.
ROSCHINA, T.A. (1959) [Experimental study on the effects of
magnezite chromium and chromite magnezite dust exposure in the
production of refrectories.] Gig. Tr. prof. Zabol., 4: 28-32
(in Russian).
ROSCHINA, T.A. (1964) [A comparative evaluation of the
effects of carbide dust and chromium boride on the organism.]
Gig. i Sanit., 8: 25-28 (in Russian).
ROSCHINA, T.A. (1976) [The toxicity of chrominum (sic)
hexacarbonyl.] Gig. Tr. Prof. Zabol., 2: 38-42 (in Russian).
ROSEBROUGH, R.W. & STEELE, N.C. (1981) Effect of
supplemental dietary chromium or nicotinic acid on
carbohydrate metabolism during basal, starvation, and
refeeding periods in poults. Poultry Sci., 60: 407-417.
ROSS, D.S., SJOGREN, R.E., & BARTLETT, R.J. (1981) Behaviour
of chromium in soils. IV. Toxicity to microorganisms. J.
environ. Qual., 10: 145-148.
ROSTENBERG, A., Jr & PERKINS, A.J. (1951) Nickel and cobalt
dermatitis. J. Allerg., 22: 466-474.
RTECS (1978) Registry of toxic effects of chemical substances,
Washington DC, National Institute for Occupational Safety and
Health, pp. 450.
SAMITZ, M.H. (1955) Some dermatologic aspects of the
chromate problem. Arch. ind. Health, 11: 361-367.
SAMITZ, M.H. (1956) Studies of chromate dermatitis. Arch.
ind. Health, 14: 269-271.
SAMITZ, M.H. & EPSTEIN, E. (1962) Experimental cutaneous
chrome ulcers in guinea-pigs. Arch. environ. Health, 5:
463-468.
SAMITZ, M.H. & GROSS, S. (1960) Extraction by sweat of
chromium from chrome tanned leathers. J. occup. Med., 2: 12-14.
SAMITZ, M.H. & GROSS, S. (1961) Effects of hexavalent and
trivalent chromium compounds on the skin. Am. Med. Assoc.
Arch. Dermatol., 84: 404-409.
SAMITZ, M.H., KATZ, S., & GROSS, S. (1960) Nature of the
chromium extracted from leather by sweat. J. occup. Med., 2:
435-436.
SANDERS, C.L., JACKSON, T.A., ADEE, R.R., POWERS, G.J., &
WEHNER, A.P. (1971) Distribution of inhaled metal oxide
particles in pulmonary alveoli. Arch. intern. Med., 127:
1085-1089.
SARGENT, T., III, LIM, T.H., & JENSON, R.L. (1979) Reduced
chromium retention in patients with hemochromatosis, a
possible basis of hemochromatotic diabetes. Metabolism, 28:
70-79.
SARTO, F., LEVIS, A.G., & PAULON, C. (1980) Clastogenic
activity of hexavalent and trivalent chromium in cultured
human lymphocytes. Caryologia, 33: 239-250.
SARTO, F., COMINATO, J., BIANCHI, V., & LEVIS, A.G. (1982)
Increased incidence of chromosomal aberrations and sister
chromatid exchanges in workers exposed to chromic acid (CrO3)
in electroplating factories. Carcinogenesis, 3: 1011-1016.
SASSI, C. (1956) [Occupational disease in a chromate
factory.] Med. Lavoro, 47: 314-326 (in Italian).
SATOH, K., FUKUDA, Y., TORII, K., & KATSUNO, N. (1981)
Epidemiological study of workers engaged in the manufacture of
chromium compounds. J. occup. Med., 23: 835-838.
SAVORY, J., MUSHAK, P., & SUNDERMAN, F.W. Jr (1969) Gas
Chromatographic determination of chromium in serum. J.
chromatogr. Sci., 7: 674-679.
SCHALLER, K.H. & ZOBER, A. (1982) [Renal excretion of
toxicologically relevant metals in occupationally non-exposed
individuals.] Arztl. Lab., 28: 209-214 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1972) [Quantitative chromium determination in urine by
flameless atomic absorption spectrometry.] Z. klin. Chem.
klin. Biochem., 10: 434-437 (in German).
SCHALLER, K.H., ESSING, H.G., VALENTIN, H., & SCHACKE, G.
(1973) The quantitative determination of chromium in urine by
flameless atomic absorption spectroscopy. At. Absorpt.
Newsl., 12(6): 147-150.
SCHELENZ, R. (1977) Dietary intake of 25 elements by man
estimated by neutron activation analysis. J. radioanal. Chem.,
37: 539-548.
SCHLETTWEIN-GSELL, D. & MOMMSEN-STRAUB, S. (1971) [Review
trace elements in foods. III. Chromium.] Int. J. Vitam. Nutr.
Res., 41: 116-125 and (1973) Suppl. 13, 188 pp (in German).
SCHOENTAL, R. (1975) Chromium carcinogenesis formation of
epoxyaldehydes and tanning. Br. J. Cancer, 32: 403-404.
SCHROEDER, H.A. (1966) Chromium deficiency in rats: a
syndrome simulating diabetes mellities with retarded growth.
J. Nutr., 88: 439-445.
SCHROEDER, H.A. (1970) Chromium, Washington DC, American
Petroleum Institute, 28 pp (Air Quality Monograph No. 70-15).
SCHROEDER, H.A. & BALASSA, J.J. (1965) Influence of
chromium, cadmium, and lead on rat aortic lipids and
circulating cholesterol. Am. J. Physiol., 209: 433-437.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1962)
Abnormal trace metals in man: chromium. J. chron. Dis., 15:
941-964.
SCHROEDER, H.A., VINTON, W.H., Jr, & BALASSA, J.J. (1963)
Effects of chromium, cadmium, and other trace metals on the
growth and survival of mice. J. Nutr., 80: 39-47.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1964)
Chromium, lead, cadmium, nickel, and titanium in mice: effect
on mortality, tumours, and tissue levels. J. Nutr., 83:
239-250.
SCHROEDER, H.A., BALASSA, J.J., & VINTON, W.H. (1965)
Chromium, cadmium, and lead in rats: effects on life span,
tumours, and tissue levels. J. Nutr., 86: 51-66.
SCHROEDER, H.A., NASON, A.P., & TIPTON, I.H. (1970) Chromium
deficiency as a factor in atherosclerosis. J. chron. Dis., 23:
123-142.
SCHROEDER, K.H. (1955) [Nickel eczema in a blueline (ozalid)
machine operator.] Berufsdermatosen, 3: 170-171 (in German).
SCHUBERT, G.E., GEBHARD, K., & HOENLEIN, F. (1970) [Kidney
damage following injection of serotonin and potassium
dichromate for chronic sodium overloading or restriction.]
Virchows Arch. Abt. A Path. Anat., 351: 68-82 (in German).
SCHULTHEISS, E. (1959) [Rubber and eczema,], Aulendorf i.
Württemberg, Cantor, 213 pp (in German).
SCHWARZ, K. & MERTZ, W. (1959) Chromium (III) and the
glucose tolerance factor. Arch. Biochem. Biophys., 85: 292-295.
SCHWARTZ, L. & DUNN, J.E. (1942) Dermatitis occurring among
operators of air conditioning equipment. Ind. Med. Surg., 11:
375.
SCHWARTZ, L., TULIPAN, L., & PECK, S. (1947) Occupational
diseases of the skin, 2nd ed., Philadelphia, Pennsylvania, Lea
& Febiger.
SEEBER, H., FIKENTSCHER, R., & ROSEBURG, B. (1976)
[Disorders of smell and taste in chromium dye workers.] Z.
Gesamte Hyg. Grenzgeb., 22: 820-822 (in German).
SEELEY, J.L. & SKOGERBOE, R.K. (1974) Combined sampling-
analysis method for the determination of trace elements in
atmospheric particulates. Anal. Chem., 46: 415-421.
SEELING, W., GRUENERT, A., KIENLE, K.H., OPFERKUCH, R., &
SWOBODNIK, M. (1979) [Determination of chromium in human
serum and plasma by flameless atomic absorption spectro-
photometry.] Z. anal. Chem., 299: 368-374 (in German).
SEITZ, W.R., SUYDAM, W.W., & HERCULES, D.M. (1972)
Determination of trace amounts of chromium (III) using
chemiluminescence analysis. Anal. Chem., 44(6): 957-963.
SHACKLETTE, H.T., SAUER, H.I., & MIESCH, A.T. (1970)
Geochemical environments and cardiovascular mortality rates in
Georgia, Washington DC, US Governmental Printing Office, 39 pp
(US Geological Survey Professional Paper No. 574-C).
SHAPCOTT, D., KHOURI, K., DEMERS, P.P., VOBECKY, J., &
VOBECKY, J. (1977) The measurement of volatile chromium in
biological materials. Clin. Biochem, 10: 178-180.
SHEFFET, A., THIND, J., MILLER, A.M., & LOURIA, D.B. (1982)
Cancer mortality in a pigment plant utilizing lead and zinc
chromates. Arch. environ. Health, 37: 44-52.
SHENDRIKAR, A.D. & WEST, P.A. (1974) A study of adsorption
characteristics of traces of chromium (III) and (VI) on
selected surfaces. Anal. Chim. Acta, 72: 91-96.
SHERMAN, L., GLENNON, J.A., BRECK, W.J., KLOMBERG, G.H., &
GORDON, E.S. (1968) Failure of trivalent chromium to improve
hyperglycemia in diabetes mellitus. Metabolism, 17: 439-442.
SHIMKIN, M.M. & LEITER, J. (1940) Induced pulmonary tumours
in mice. III. Role of chromic irritation in the production of
pulmonary tumours in strain A mice. J. Natl Cancer Inst., 1:
241-254.
SHMITOVA, L.A. (1978) In: [The impact of job-related factors
on specific function of the female organism,] Svedlovsk, pp.
105-108 (in Russian).
SHMITOVA, L.A. (1980) Gig. Tr. prof. zabol., 2:
33-35 (in Russian).
SIDI, E. & LONGUEVILLE, R. (1953) Dermites au ciment. Rôle
des sels de chrome. Arch. Mal. prof., 14: 41.
SIEGENTHALER, U., LAINE, A., POLAK, L. (1983) Studies on
contact sensitivity to chromium in the guinea-pig. The role
of valence in the formation of the antigenic determinant. J.
Invest. Dermatol., 80: 44-47.
SIMONOFF, M., LLABADOR, Y. HAMON, C., PEERS; A.M., & SIMONOFF,
G.N. (1984) Low plasma chromium in patients with coronary
artery and heart diseases. Biol. Trace Element Res., 6:
431-439.
SIMONDS, J.P. & HEPLER, O.E. (1945) Experimental nephro-
pathies. I. A method of producing controlled selective injury
of renal units by means of chemical agents. Arch. Path., 39:
103-108.
SIROVER, M.A. & LOEB, L.A. (1976) Infidelity of DNA
synthesis in vitro: screening for potential metal mutagens or
carcinogens. Science, 194: 1434-1436.
SJOGREN, B. (1980) A retrospective cohort study of mortality
among stainless steel welders. Scand. J. Work. environ.
Health, 6: 197-200.
SKOGERBOE, R.K. (1974) Monitoring trace metal particulates:
an evaluation of the sampling and analysis problems. In:
Instrumentation for monitoring air quality, Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp.
125-136.
SLUIS-CREMER, G.K. & DU TOIT, R.S. (1968) Pneumoconiosis in
chromite miners in South Africa. Br. J. ind. Med., 25: 63-67.
SPIER, H.W. (1956) [Wood preservatives and chromate eczema.]
Desinfekt. Gesundheitswes., 4: 58 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil ( Myriophyllum spicatum L.). Arch.
environ. Contam. Toxicol., 2: 331-341.
STAUB, H.W., REUSSNER, G., & THIESSEN, R., Jr (1969) Serum
cholesterol reduction by chromium in hypercholesteremic rats.
Science, 166: 746-747.
STEELE, N.C. & ROSEBROUGH, R.W. (1979) Trivalent chromium
and nicotinic acid supplementation for the turkey poult.
Poultry Sci., 58: 983-984.
STEELE, N.G., ALTHEN, T.G., & FROBISH, L.T. (1977a) Bio-
logical activity of glucose tolerance factor in swine. J.
anim. Sci., 45: 1341-1345.
STEELE, N.C., PELURA, I., & FROBISH, L.T. (1977b) Effect of
dietary protein source on glucose utilization and hepatic
insulin-receptor characteristics of the rat. Nutr. Rep. Int.,
15: 429-435.
STELLA, M., MENTALDI, A., ROSSI, R., ROSSI, G., & LEVIS, A.G.
(1982) Clastogenic effects of chromium on human lymphocytes
in vitro and in vivo. Mutat. Res., 101: 151-164.
STEFFEE, C.H. & BAETJER, A.M. (1965) Histopathologic effects
of chromate chemicals. Report of studies in rabbits, guinea-
pigs, rats, and mice. Arch. environ. Health, 11: 66-75.
STEINHOFF, D., GAD, S.C., HATFIELD, G.K., & MOHR, U. (1983)
Testing sodium dichromate and soluble calcium chromate for
carcinogenicity in rats. Report to the Industrial Health
Foundation, Wuppertal, Bayer AG Institute of Toxicology,
126 pp.
STEREKHOVA, N.P., ZELENEVA, N.I., SOLOMINA, S.N.,
TYUSHNYAKOVA, N.V., MYASNIKOVA, A.G., FOKINA, G.P., & YARINA,
A.L. (1978) [Gastric pathology in workers engaged in the
production of chromium salts.] Gig. Tr. prof. Zabol., 3: 19-23
(in Russian).
STERN, R.M. (1981) Process-dependent risk of delayed health
effects for welders. Environ. Health Perspect., 41: 235-253.
STERN, R.M. (1982) Chromium compounds: production and
occupational exposure. In: Langard, S., ed. Biological and
environmental aspects of chromium, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 5-47.
STRIK, J.J.T.W.A., DE IONGH, H.H., VAN RIJN VAN ALKEMADE,
J.W.A., & WIUTE, T.P. (1975) Toxicity of chromium (VI) in
fish, with special reference to organoweights, liver and
plasma enzyme activities, blood parameters, and histological
alterations. In: Koeman, S.H. & Strik, J.J.T.W.A., ed.
Sublethal effects of toxic chemicals on aquatic animals,
Amsterdam, New York, Oxford, Elsevier Science Publishers,
pp. 31-41.
STROUT, R.W. (1977) The relationship of abnormal circulating
insulin levels to atherosclerosis. Atherosclerosis, 27: 1-13.
SULLIVAN, R.J. (1969) Air pollution aspects of chromium and
its compounds, Raleigh, North Carolina, US Department of
Health, Education and Welfare, 75 pp (National Air Pollution
Control Administration Publication No. APTD 69-34).
SUSA, N., FURUKAWA, YO., & TSUBAKI, S. (1977) Comparative
cytotoxic effects of hexavalent and trivalent chromium
compounds on cultured cells. Jap. J. vet. Sci., 39: 325-336.
SUZUKI, K., ESASHI, T., SUZUE, R., NISHIMURA, H., & MATSUMOTO,
H. (1979) Tissue concentrations of cadmium, lead, and
chromium in Japanese ranging from the fetus to elder subjects.
Toxicol. Lett., 4: 481-484.
SWAINE, D.J. & MITCHELL, R.L. (1960) Trace element
distribution in soil profiles. J. soil Sci., 11 (2): 347-368.
SWENSSON, A. (1977) [Experimental research on the
fibrogenetic effect of chromite.] Arbete och Haelsa, 2: 1-14
(in Swedish).
TABOR, E.C. & WARREN, W.V. (1958) Distribution of certain
metals in the atmosphere of some American cities. Arch. ind.
Hyg., 17: 145-151.
TAMARO, M., BANFI, E., VENTURINI, S., & MONTI-BRAGADIN, C.
(1975) [Hexavalent chromium compounds are mutagens for
bacteria.] In: Proceedings of the XVII Congress Naz. Soc. It.
Microbiol., Padova, pp. 411-415 (in Italian).
TANDON, S.K., SAXENA, D.K., GAUR, J.S., & CHANDRA, S.W.
(1978) Comparative toxicity of trivalent and hexavalent
chromium. Environ. Res., 15: 90-99.
TANDON, S.K., BEHARI, J.R., & KACHRU, D.N. (1979) Distri-
bution of chromium in poisoned rats. Toxicology, 13: 29-34.
TANDON, S.K., MATHUR, A.K., & GAUR, J.S. (1977) Urinary
excretion of chromium and nickel among electroplaters and
pigment industry workers. Int. Arch. occup. environ. Health,
40: 71-76.
TATARU, C., MARINSECU, A., CAPUSAN, I., RIPAN, R., & LITEANU,
C. (1957) [Skin damage during the industrial recovery of
potassium dichromate.] Berufsdermatosen, 5: 283-295 (in
German).
TAYLOR, A.E., GUYTON, M.O., & BISHOP, V.S. (1965)
Permeability of the alveolar membrane to solutes. Circ. Res.,
16: 353-362.
TAYLOR, F.G., Jr, MANN, L.K., DAHLMANN, R.C., & MILLER, F.L.
(1975) Environmental effects of chromium and zinc in
cooling-water drift. In: Cooling tower environment, 1974,
Washington DC, US Energy Research and Development
Administration, pp. 408-426 (Report Conf. No. 740302).
TAYLOR, F.H. (1966) The relationship of mortality and
duration of employment as reflected by a cohort of chromate
workers. Am. J. public Health, 56: 218-229.
TAZELAAR, D.J. (1970) Hypersensitivity to chromium in a
light-blue Tattoo. Dermatologica (Basel), 141: 282-287.
THOMSON, A.G. (1982) Chromite, Mining Annual Review,
pp. 70-71.
THORMANN, J., JESPERSEN, N.B., & JOENSEN, H.D. (1979)
Persistence of contact allergy to chromium. Contact Dermatit.,
5: 261-264.
TOEPFER, E.W., MERTZ, W., ROGINSKI, E.E., & POLANSKY, M.M.
(1973) Chromium in foods in relation to biological activity.
J. agric. food Chem., 21: 69-73.
TOEPFER, E.W., MERTZ, W., POLANSKY, M.M., ROGINSKI, E.E., &
WOLF, W.R. (1977) Preparation of chromium-containing
material of glucose tolerance factor activity from brewer's
yeast extracts and by synthesis. J. agric. food Chem., 25:
162-166.
TOLA, S., KILPIO, J., VIRTAMO, M., & HAAPA, K. (1977)
Urinary chromium as an indicator of the exposure of welders to
chromium. Scand. J. Work environ. Health, 3: 192-202.
TOLG, G. (1974) Recent problems and limitations in the
analytical characterization of high-purity material. Talanta,
21: 327-345.
TSUDA, H. & KATO, K. (1976) Potassium dichromate: induced
chromosome aberrations and its control with sodium sulfite in
hamster embryonic cells in vitro. Gann, 67: 469-470.
TSUDA, H. & KATO, K. (1977) Chromosomal aberrations and
morphological transformation in hamster embryonic cells
treated with potassium dichromate in vitro. Mutat. Res., 46:
87-94.
TUMAN, R.W. & DOISY, R.J. (1977) Metabolic effects of the
glucose tolerance factor (GTF) in normal and genetically
diabetic mice. Diabetes, 26: 820-826.
TUMAN, R.W., BILBO, J.T., & DOISY, R.J. (1978) Comparison
and effects of natural and synthetic glucose tolerance factor
in normal and genetically diabetic mice. Diabetes, 27: 49-56.
TURNER, M.A. & RUST, R.H. (1971) Effects of chromium on
growth and mineral nutrition of soybeans. Soil Sci. Soc. Am.
Proc., 35: 755-758.
TYUSHNYAKOVA, N.V., ZISLIN, D.M., SHABYNINA, N.K., & BELJAEVA,
L.N. (1974) [Criteria of occupational cancer in the
production of chromium salts and some ideas concerning its
pathogenesis.] In: Occupational cancer (collection of
scientific works), Sverdlovsk, pp. 56-63 (in Russian).
UMEDA, M. & NISHIMURA, M. (1979) Inducibility of chromosomal
aberrations by metal compounds in cultured mammalian cells.
Mutat. Res., 67: 221-229.
URCHS, O. (1953) [Occupational skin diseases,] Cantor,
Aulendorf i. Württemberg, 108 pp.
US DHEW (1975) Criteria for a recommended standard.
Occupational exposure to chromium (VI), Washington DC, US
Department of Health, Education and Welfare, 200 pp (US DHEW
Publication No. NIOSH 76-129).
US DHEW (1977) Occupational diseases: a guide to their
recognition, revised ed., Washington DC, US Department of
Health, Education and Welfare, pp. 352-355 (Stock No.
017-033-00266-5).
US EPA (1978) Reviews of the environmental effects of
pollutants. III. Chromium, Washington DC, US Environmental
Protection Agency, 285 pp (ORNL/EIS-80; EPA 600/1-78-023).
US EPA (1980) Ambient water quality criteria for chromium,
Washington DC, US Environmental Protection Agency, pp. A1-C48.
US NAS (1974a) Chromium, Washington DC, US National Academy
of Sciences, 155 pp.
US NAS (1974b) Geochemistry and the environment. I. The
relation of selected trace elements to health and disease,
Washington DC, US National Academy of Sciences, 113 pp.
US NAS/NAE (1972) Water quality criteria 1972, Washington
DC, US Environmental Protection Agency, US National Academy of
Sciences, US National Academy of Engineering, 533 pp.
US PHS (1953) Health of workers in chromate producing
industry, Washington DC, US Department of Health, Education
and Welfare, US Public Health Service, 131 pp (Publication No.
192).
VAKHRUSHEVA, V.A. (1960) [Effect of Mn and Cr as trace
elements on the level of blood sugar in white mice.] Tr.
Izhevskogo Otd. Fiziol. Obschchestva, 1: 184-190 (in Russian).
VALER, M. & RACZ, J. (1971) Investigations concerning the
sensitizing effect of trivalent chromium salts. I. Correlation
between concentration and skin penetration of trivalent
chromium and between the number of positive results obtained
in patch tests. J. ind. Med., 41(12): 302-317.
VEILLON, C., WOLF, W.R., GUTHRIE, B.E. (1979) Determination
of chromium in biological materials by stable isotope
dilution. Anal. Chem., 51: 1022-1024.
VELICHKOVSKY, B.T. & POKROVSKAJA, L.V. (1973) [Labour
hygiene in a modern production of multy-tonnage ferrolloys.]
In: [Proceedings of the National Symposium on Labor Hygiene
and Prophylaxis of Occupational Diseases in the Production of
Ferroalloys,] Sverdlovsk, pp. 3-8 (in Russian).
VENITT, S. & LEVY, L.S. (1974) Mutagenicity of chromates in
bacteria and its relevance to chromate carcinogenesis. Nature
(Lond.), 250: 493-494.
VENCGUJIV, A.J. (1974) Zones of display of biological and
pharmacological action of trace elements. In: Hoekstra, W.G.,
Gantherl, H.E., & Mertz, W., ed. Trace elements metabolism in
animals. II, Baltimore, London, Tokyo, University Park Press,
pp. 295-310.
VERFAILLIE, G.R.M. (1974) Kinetics of chromium absorption by
intact rice plants. In: Comparative studies of food and
environmental contamination, Vienna, International Atomic
Energy Agency, pp. 315-331.
VERSIECK, J.M.J. & SPEECKE, A.B.H. (1972) Contaminations
induced by collection of liver biopsies and human blood. In:
Nuclear Activation Techniques in the Life Sciences.
Proceedings of a Symposium, Bled, Yugoslavia, Vienna
International Atomic Energy Agency, pp. 39-49.
VERSIECK, I., HOSTE, J., BARBIER, F., STEYAERT, H., DERUDDER,
I., & MICHELS, H. (1978) Determination of chromium and
cobalt in human serum by neutron activation analysis. Clin.
Chem., 24: 303-308.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experience of the
occupational health department with some industrial threshold
limit values for industrial poisons.] Arch. Gewerbepath.
Gewerbehyg., 13: 528-534 (in German).
VISEK, W.J., WHITNEY, I.B., KUHN, U.S.G., & COMAR, C.L.
(1953) Metabolism of 51Cr by animals as influenced by
chemical state. Proc. Soc. Exp. Biol. Med., 84: 610-615.
VOELKL, A. (1971) [The daily excretion of chromium in normal
persons.] Zentralbl. Arbeitsmed., 21: 122 (in German).
VOTAVA, H.J., HAHN, C.J., & EVAN, G.W. (1973) Isolation and
partial purification of a 51Cr complex from brewer's yeast.
Biochem. Biophys. Res. Comm., 55: 312-319.
WACKER, W.E.C. & VALLEE, B.L. (1959) Nucleic acids and
metals. I. Chromium mangenese, nickel, iron, and other metals
in ribonucleic acid from diverse biological sources. J. biol.
Chem., 234: 3257-3262.
WAHBA, A. & COHEN, T. (1979) Chrome sensitivity in Israel.
Contact Dermatit., 5: 101-107.
WAHLBERG, J.E. & WENNERSTEN, G. (1977) Light sensitivity and
chromium dermatitis. Br. J. Dermatol., 97: 411-416.
WALSH, E.N. (1953) Chromate hazards in industry. J. Am. Med.
Assoc., 153: 1305-1308.
WARREN, G., SCHULTZ, P., BANCROFT, D., BENNETT, K., ABBOTT,
E.H., & ROGERS, S. (1981) Mutagenicity of a series of
hexacoordinate chromium (III) compounds. Mutat. Res., 90:
111-118.
WATANABE, S. & FUKUCHI, Y. (1975) An epidemiological survey
on lung cancer in workers of a chromate-producing industry in
Hokkaido, Japan (Presented at: The XVIII International Congress
on Occupational Health, Brighton, England, 14-19 September.
WEILER, R.R. & CHAWLA, V.K. (1969) Dissolved mineral and
quality of Great Lakes waters. In: Proceedings of the 12th
Conference on Great Lakes Res., 801.
WELZ, B. (1980) [Use of AAS and ICP. Relative advantage of
each method.] Chem. Techn., 9: 161-170. (in German).
WELZ, B. (1983) [Atomic absorption spectrometry,] 3rd ed.,
Weinheim, Deerfield Beach, Florida, Basel, Verlag Chemie,
527 pp (in German).
WENDELBERGER, J. & WEITGASSER, H. (1956) [The role of alkali
and degreasing agents in the development of contact eczemas in
electroplasters.] Berufsdermatosen, 4: 16-21 (in German).
WHANGER, P.D. & WESWIG, P.H. (1975) Effects of selenium,
chromium, antioxidants on growth, eye cataracts, plasma
cholesterol, and blood glucose in selenium-deficient, vitamin
E-supplemented rats. Nutr. Rep. Int., 12: 345-348.
WHITE, L.R., JAKOBSEN, K., & 0STGAAD, K. (1979) Comparative
toxicity studies of chromium rich welding fumes and chromium
on an established human cell line. Environ. Res., 20: 366-374.
WHITING, R.F., STICH, H.F., & KOROPATNICK, D.J. (1979) DNA
damage and DNA repair in cultured human cells exposed to
chromate. Chem.-biol. Interact., 26: 267-280.
WHO (1983) EHC 27: Guidelines on studies in environmental
e pidemiology, Geneva, World Health Organization, 351 pp.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984a)
Disposition of intratracheally administered chromium (III) and
chromium (VI) in rabbits. Toxicol. Lett., 22: 273-276.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1984b) The
reduction of chromium (VI) to chromium (III) by glutathione:
an intracellular redox pathway in the metabolism of the
carcinogen chromate. Toxicology, 33: 341-348.
WIEGAND, H.J., OTTENWALDER, H., & BOLT, H.M. (1985)
[Determination of chromium in human red blood cells. Basis for
a concept of biological monitoring.] Arbeitsmed. Sozialmed.
Praeventivmed., 20: 1-4 (in German).
WILD, D. (1978) Cytogenetic effects in the mouse of 17
chemical mutagens and carcinogens evaluated by the micronuclei
test. Mutat. Res., 56: 319-327.
WILLIAMS, C.D. (1969) Asthma related to chromium compounds.
Report of two cases and review of the literature on chromate
diseases. NC med. J., 30: 482-491.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1960) The Merck index. An encyclopedia of chemicals and
drugs, 7th ed., Rahway, New Jersey, Merck and Co., 139 pp.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) The Merck index. An encyclopedia of chemicals and
drugs, 9th ed., Rahway, New Jersey, Merck and Co., 1313 pp.
WINSTON, J.R. & WALSH, E.N. (1951) Chromate dermatitis in
railroad employees working with diesel locomotives. J. Am.
Med. Assoc., 147: 1133-1134.
WITHEY, J. (1983) Memorandum addressed to Si Duk Lee, dated
March 4, 1983, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office.
WOLF, W.R. (1976) Coupled gas chromatography/atomic
absorption spectrometry for the nanogram determination of
chromium. Anal. Chem., 48: 1717-1720.
WOLF, W.R. & GREENE, F.E. (1976) Preparation of biological
materials for chromium analysis. In: Accuracy in trace
analysis: sampling, sample handling, and analysis,
Gaithersburg, Maryland, National Bureau of Standards, pp.
605-610 (Special Publication No. 422).
WOLF, W.R., MERTZ, W., & MASIRONI, R. (1974) Determination
of chromium in refined and unrefined sugars by oxygen plasma
ashing flameless atomic absorption. J. agric. food Chem., 22:
1037-1042.
WOLF, W.R., TAYLOR, M.L., HUGHES, B.M., TIERNAN, T.O., &
SIEVERS, R.E. (1972) Determination of chromium and beryllium
at the picogram level by gas chromatography/mass spectrometry.
Anal. Chem., 44: 616-618.
WU, G.-Y. (1981) [The research on the purification and the
chemical structure of the specific chromium binding
materials.] Jpn. J. Ind. Health, 33: 505 (in Chinese).
YAGI, T. & NISHIOKA, H. (1977) DNA damage and its degrada-
tion by metal compounds. Sci. Eng. Rev. Doshisha Univ., 18:
63-70.
YANG, G.L. (1956) [An investigation of occupational disease
among chromium plating workers.] Acta Acad. Med. primae
Shanghai, 2: 36-46 (in Chinese).
YOPP, J.H., SCHMID, W.E., & HOLST, R.W. (1974) Determination
of maximum permissible levels of selected chemicals that exert
toxic effects on plants of economic importance in Illinois,
Chicago, Illinois, Institute for Environmental Quality,
pp. 89-96.
YUNISOVA, H.K. & PAVLOVSKAJA, G.S. (1975) The effect of
regimen of chrome plating on the properties of chromine.
Protect. Metal., 2, 248-250.
ZANDER, A.T., O'HAVER, T.C., & KELIHER, P.N. (1977)
Correction for interferences of spectral origin with continuum
source, echelle wavelength modulated atomic absorption
spectrometry. Anal. Chem., 49: 838-842.
ZHURKOV, U.S. (1981) [Approaches to the regulation of
environmental chemical pollutants with mutagenic activity.]
In: Sidorenko, G.I.M., ed. [Medical problems of environmental
protection,] Moscow, USSR Academy of Medical Sciences,
pp. 88-95 (in Russian).
ZOBER, A. (1979) [On the problems of evaluating bronchial
carcinoma after exposition to chromium compounds.] Int. Arch.
occup. environ. Health, 43(2): 107-121 (in German).
ZOBER, A. (1982) [Possible dangers to the respiratory tract
from welding fumer: methods of approach in an industrial
health care context and results.] Schweissen & Schneiden,
34(2): 77-81 (in German).
ZOBER, A., KICK, K., SCHALLER, K.H., SCHELLMANN, B., &
VALENTIN, H. (1984) ["Normal values" of chromium and nickel
in human lung-, kidney-, blood-, and urine samples.] Zbl.
Bakt. Hyg. I. Abt. Orig. B, 179: 80-95 (in German).
