
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 58
SELENIUM
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1987
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154258 6
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1987
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEATH CRITERIA FOR SELENIUM
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1. Summary
1.1.1. Properties and analytical methods
1.1.2. Sources, environmental transport, and distribution
1.1.3. Environmental levels and exposures
1.1.4. Selenium metabolism
1.1.4.1 Absorption
1.1.4.2 Total human body selenium content
1.1.4.3 Distribution
1.1.4.4 Metabolic pools of selenium in the body
1.1.4.5 Metabolic conversion
1.1.4.6 Effect of chemical form of selenium on its
metabolism
1.1.4.7 Selenium excretion
1.1.5. Effects on animals
1.1.5.1 Selenium toxicity
1.1.5.2 Selenium deficiency
1.1.6. Effects on man
1.1.6.1 General population exposure
1.1.6.2 Occupational exposure
1.2. Recommendations for further activities
2. CHEMICAL AND PHYSICAL PROPERTIES; ANALYTICAL METHODS
2.1. Properties
2.2. Analytical methods
2.2.1. Sample collection, processing, and storage
2.2.2. Sample decomposition or other preliminary treatment
2.2.2.1 Wet digestion
2.2.2.2 Predigestion
2.2.2.3 Combustion
2.2.2.4 Fusion
2.2.2.5 Concentration
2.2.3. Removal from interfering substances
2.2.4. Detection and identification of selenium
2.2.5. Measurement of selenium
2.2.5.1 Fluorometric analysis
2.2.5.2 Neutron activation analysis
2.2.5.3 Atomic absorption spectrometry
2.2.5.4 Other methods
3. SOURCES, TRANSPORT, AND CYCLING OF SELENIUM IN THE ENVIRONMENT
3.1. Natural sources
3.1.1. Rocks and soils
3.1.2. Natural selenium in the food chain
3.l.3 Water and air
3.2. Man-made sources
3.2.1. Agriculture
3.2.2. Industry
3.3. Environmental transport
3.4. Biological selenium cycle
4. LEVELS IN ENVIRONMENTAL MEDIA
4.1. Levels and chemical forms of selenium in food
4.1.1. Levels in food
4.1.1.1 Natural differences among food commodities
4.1.1.2 Effects of natural differences in the
availability of selenium in the
environment on levels in food
4.1.1.3 Man-induced changes in selenium levels in
food
4.1.2. Chemical forms of selenium in food
4.2. Drinking-water
4.3. Air
5. HUMAN EXPOSURE
5.1. Estimate of general population exposure
5.1.1. Food
5.1.1.1 Geographical variation
5.1.1.2 Food habits (consumption patterns)
5.1.1.3 Elderly people
5.1.1.4 Infants and children
5.1.1.5 Special medical diets
5.2. Occupational exposure
5.2.1. Levels in the workplace air
5.2.2. Biological monitoring
6. METABOLISM OF SELENIUM
6.1. Absorption
6.1.1. Gastrointestinal absorption
6.1.1.1 Animal studies
6.1.1.2 Human studies
6.1.2. Absorption by inhalation
6.1.3. Absorption through the skin
6.2. Distribution in the organism
6.2.1. Transport
6.2.2. Organs
6.2.2.1 Animal studies
6.2.2.2 Human studies
6.2.3. Blood
6.2.4. Total-body selenium content
6.3. Excretion in urine, faeces, and expired air
6.3.1. Animal studies
6.3.2. Human studies
6.3.2.1 Excretion of selenium
6.3.2.2 Balance studies
6.4. Retention and turnover
6.4.1. Animal studies
6.4.2. Controlled human studies
6.5. Metabolic transformation
6.5.1. Animal studies
6.5.1.1 Reduction and methylation
6.5.1.2 Form in proteins
6.5.1.3 Conversion of selenium compounds to
nutritionally-active forms of selenium
6.5.2. Human studies
7. EFFECTS OF SELENIUM ON ANIMALS
7.1. Selenium toxicity
7.1.1. Farm animal diseases associated with a high
selenium intake
7.1.2. Toxicity in experimental animals
7.1.2.1 Acute and subacute toxicity - single or
repeated exposure studies with oral,
intraperitoneal, or cutaneous
administration
7.1.2.2 Effects of long-term oral exposure
7.1.2.3 Inhalation toxicity
7.1.3. Blood levels in toxicity
7.1.4. Effects on reproduction
7.1.5. Effects on dental caries
7.1.6. Factors influencing toxicity
7.1.6.1 Form of selenium
7.1.6.2 Nutritional factors
7.1.6.3 Arsenic
7.1.6.4 Sulfate
7.1.6.5 Adaptation
7.1.7. Mechanism of toxicity
7.2. Selenium deficiency
7.2.1. Animal diseases
7.2.2. Intakes needed to prevent deficiency
7.2.2.1 Quantitative dietary levels
7.2.2.2 Bioavailability
7.2.3. Blood and tissue levels in deficiency
7.2.4. Physiological role: glutathione peroxidase
7.2.4.1 Function of selenium and relationship to
vitamin E
7.2.4.2 Effect of selenium intake on tissue-
glutathione peroxidase, activity
7.2.4.3 Relationship between blood-selenium levels
and erythrocyte-glutathione peroxidase
activity
7.2.4.4 Gluthathione peroxidase as an indicator of
selenium status
7.2.5. Other possible physiological roles
7.2.5.1 Homeostasis of hepatic haem
7.2.5.2 Microsomal and mitochondrial electron
transport
7.2.5.3 The immune response
7.2.5.4 Selenium and vision
7.2.6. Effects on reproduction
7.2.7. Factors influencing deficiency
7.2.7.1 Form of selenium
7.2.7.2 Vitamin E
7.2.7.3 Heavy metals and other minerals
7.2.7.4 Xenobiotics
7.2.7.5 Exercise stress
7.3. Ratio between toxic and sufficient exposures
7.4. Protection against heavy metal toxicity
7.4.1. Mercury
7.4.2. Cadmium
7.4.3. Other heavy metals
7.5. Cytotoxicity, mutagenicity, and anti-mutagenicity
7.5.1. Cytotoxicity and mutagenicity
7.5.2. Anti-mutagenicity
7.6. Teratogenicity
7.7. Carcinogenicity and anti-carcinogenicity
7.7.1. Selenium as a possible carcinogen
7.7.2. Selenium as a possible anti-carcinogen
8. EFFECTS OF SELENIUM ON MAN
8.1. High selenium intake
8.1.1. General population
8.1.1.1 Signs and symptoms
8.1.1.2 Attempts to associate high selenium intake
with human diseases
8.1.2. Reports on health effects associated with
occupational exposure
8.1.2.1 Fumes and dust of selenium and its
compounds
8.1.2.1.1 Selenium dioxide
8.1.2.1.2 Hydrogen selenide
8.1.2.1.3 Selenium oxychloride
8.2. Low selenium intake
8.2.1. Evidence supporting the possible essentiality of
selenium in man
8.2.2. Signs and symptoms of low intake
8.2.3. Dietary levels consistent with good nutrition
8.2.3.1 Quantitative estimates
8.2.3.2 Nutritional bioavailability
8.2.4. Blood and urine levels typical of low intake
8.2.5. Relationship between blood-selenium levels and
erythrocyte-glutathione peroxidase activity
8.2.6. Attempts to associate low selenium intake with
human diseases
8.2.6.1 Keshan disease
8.2.6.2 Kashin-Beck disease
8.2.6.3 Cancer
8.2.6.4 Heart disease
9. EVALUATION OF THE HEALTH RISKS ASSOCIATED WITH EXCESSIVE OR
DEFICIENT SELENIUM EXPOSURE
9.1. The need to consider the essentiality of selenium in
health risk evaluation
9.2. Pathway of selenium exposure for the general population
9.3. Quantitative assessment of human selenium exposure
9.3.1. Analytical methods for selenium
9.3.2. Food intake data
9.3.3. Blood-selenium
9.3.4. Hair-selenium
9.3.5. Urine-selenium
9.3.6. Blood-glutathione peroxidase
9.4. Levels of dietary selenium exposure in the general
population
9.5. Evaluation of health risks - general population
9.5.1. Predictive value of animal studies
9.5.2. Studies on high-exposure effects in the general
population
9.5.3. Studies on low-exposure effects in the general
population
9.5.4. Evaluation of the involvement of selenium in human
diseases of multiple etiopathogenesis
9.5.4.1 Keshan disease
9.5.4.2 Kashin-Beck disease
9.5.4.3 Ischaemic heart disease
9.5.4.4 Studies on the involvement of selenium in
cancer
9.5.4.5 Caries
9.5.4.6 Health effects related to reproduction
9.6. Occupational exposure
REFERENCES
WHO TASK GROUP ON SELENIUM
Members
Dr R.F. Burk, Jr, Department of Medicine, University of Texas
Health Science Center, San Antonio, Texas, USA
Professor A.I. Diplock, Department of Biochemistry, Guy's
Hospital Medical School, London, United Kingdom (Chairman)
Dr H.N.B. Gopalan, University of Nairobi, Department of
Botany, Nairobi, Kenya
Dr J. Chen, Department of Nutrition and Food Hygiene,
Institute of Health, China National Centre for Preventive
Medicine, Beijing, People's Republic of China
Dr G.N. Krasovskij, Sysir Institute of General and Community
Hygiene, Academy of Medical Sciences of the USSR, Moscow,
USSRa
Professor C.R. Krishna Murti, Integrated Environmental
Programme on Heavy Metals, Centre for Environmental
Studies, Anna University, College of Engineering, Guindy,
Madras, Indiaa
Dr O.A. Levander, Vitamin and Mineral Nutrition Institute, US
Department of Agriculture, Beltsville, Maryland, USA
(Rapporteur)
Professor A. Massoud, Department of Community, Environmental
and Occupational Medicine, Faculty of Medicine, Ain Shams
University, Cairo, Egypta
Professor M.F. Robinson, Nutrition Department, University of
Otago, Dunedin, New Zealand
Secretariat
Dr J. Parizek, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)
Dr E.S. Johnson, International Agency for Research on Cancer,
Lyons, France
-------------------------------------------------------------------
a Invited but unable to attend.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR SELENIUM
A WHO Task Group on Environmental Health Criteria for Selenium
was held in Geneva on 2 - 6 December 1985. Dr J. Parizek opened
the meeting on behalf of the Director-General. The Task Group
reviewed and revised the draft criteria document and made an
evaluation of the health risks of exposure to selenium.
DR O.A. LEVANDER of the US Department of Agriculture was
responsible for the preparation of the drafts of the document.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1. Summary
1.1.1. Properties and analytical methods
Selenium exists naturally in several oxidation states and some
of its chemical forms are volatile. Many selenium analogues of
organic sulfur compounds exist in nature.
A number of procedures exist for the determination of selenium.
However, as selenium exists in volatile and unstable forms and
because of the inhomogeniety of many types of materials, the
methods of sampling, preparation, and storage are equally as
important as the analytical methods. Great care is necessary to
avoid contamination or loss of the element.
The most commonly used analytical methods depend on wet
digestion for destroying organic matter and freeing the element.
Some procedures depend on the formation of the piazoselenol; this
is extracted in an organic solvent and the fluorescence determined.
The fluorometric method with a wide variety of modifications is
very sensitive and reliable and can be adapted for most materials.
It is also inexpensive. Atomic absorption spectrometry, especially
with the atomization of the selenium as the hydride, has been
useful, and atomic absorption methods based on the Zeeman effect
background correction show promise for the determination of
selenium in biological matrices, without prior sample digestion.
Neutron activation analysis, particularly when combined with the
chemical separation of selenium, is an excellent method, limited
only by the cost and availability of equipment.
1.1.2. Sources, environmental transport, and distribution
Selenium appears to be ubiquitous. However, its uneven
distribution over the face of the earth results in regions with
very low or very high natural levels of selenium in the
environment. Geophysical, biological, and industrial processes are
involved in the distribution and transport of the element and its
cycling, but the relative importance of these processes has not
been established. However, the natural geophysical and biological
processes are probably almost entirely responsible for the present
status of selenium in the general environment. This must be given
primary consideration in any evaluation of the superimposed effects
of man's activity on selenium in the environment and food chains.
Some human activities are responsible for the redistribution of
selenium in the environment. Industrial sources of selenium stem
initially from copper refining. During this refining and the
purification of the selenium, there can be some loss of the element
into the environment. In addition, industries concerned with the
production of glass, electronic equipment, or certain metals may
emit selenium into the environment in the immediate vicinity of the
factories involved. The inclusion of the element in manufactured
products provides another avenue for its distribution. There is
concern in several countries with regard to the possible health
effects of low and/or decreasing levels of selenium in the soil
(sections 1.1.3, 1.1.5.2 and 1.1.6.1); the use of fertilizers
containing selenium compounds in some Nordic countries is a
remarkable example of intentional human intervention in the
environmental distribution of selenium.
1.1.3. Environmental levels and exposures
The range of selenium levels in different foods can vary
widely, depending on the natural availability of selenium in the
environment and on certain activities of man, such as the direct
addition of selenium to the food supply.
No data are available on the chemical forms of selenium in
foods produced under normal conditions.
The limited analytical data available show that the levels of
selenium typically found in foods (on a wet-weight basis) range
from: 0.4 - 1.5 mg/kg in liver, kidney, and seafood; 0.1 - 0.4
mg/kg in muscle meat; < 0.1 - 0.8 mg/kg or more in cereals or
cereal products; < 0.1 - 0.3 mg/kg in dairy products; and < 0.1
mg/kg in most fruits and vegetables. However, in countries with
low selenium levels in soil, lower selenium levels than the above
were reported, in particular in meats, cereals, and dairy products.
The levels of selenium in baby foods tend to follow the same trends
as in adult foods, i.e., meat and cereal products contain the
highest levels, and fruit and vegetable products the lowest. Meat-
based infant formulae contain more selenium than formulae based on
milk or soy protein. Average selenium concentrations in human milk
range from 0.013 to 0.018 mg/litre. However, in countries with low
selenium levels in soil and food, lower selenium levels in breast
milk were reported.
Special medical diets based on egg albumen contain more
selenium than diets based on casein hydrolysate. Chemically-
defined diets or total parenteral nutrition solutions based on
amino acid mixtures contain very low levels of selenium.
Levels of selenium in air and water are usually very low, i.e.,
less than 10 ng/m3 in air and only a few µg/litre water.
Food constitutes the main route of exposure to selenium for the
general population. Because of geochemical differences, the
estimates of adult human exposure to selenium via the diet range
from 11 to 5000 µg/day, in different parts of the world; however,
dietary intake more usually falls within the range of 20 - 300
µg/day. Food consumption patterns also affect dietary-selenium
intake, and the extreme values observed have occurred in
populations consuming a monotonous diet comprising a limited range
of locally-grown staple foods. The estimated selenium intake of
infants in different parts of the world during the first month of
life ranges from 5 to 55 µg/day, because of the variation in levels
of selenium in milk. Children consuming synthetic diets, as part
of long-term diet therapy for certain metabolic diseases, such as
phenylketonuria and maple syrup urine disease, ingest only 5 - 11
µg/day. Adults, maintained on chemically-defined diets or total
parenteral nutrition solutions based on amino acid mixtures,
receive only 1 or 2 µg/day. There is growing evidence of the
importance of the bioavailability of selenium in different foods
for human beings.
Human occupational exposure to selenium is primarily via the
air. Exposure via direct contact is rarely of importance, unless
local irritation or skin damage caused by vesicant selenium
compounds facilitates cutaneous absorption. Few quantitative data
are available on the actual levels of occupational exposure to
selenium. However, it is likely that analyses carried out several
decades ago yielded higher values (several mg/m3) than those
carried out more recently.
1.1.4. Selenium metabolism
1.1.4.1. Absorption
Potential sites of selenium absorption from the environment
are the gastrointestinal tract, the respiratory tract, and the
skin. Limited animal and no quantitative human data are available
on the pulmonary absorption of selenium. Nevertheless, high
urinary-selenium levels in workers exposed to selenium in air have
been reported and could indicate pulmonary absorption. Selenite
and selenium oxychloride have been shown to be absorbed through the
skin of experimental animals. The assessment of the possible
pulmonary or dermal absorption rates for various selenium compounds
must take into account differences in their physical and chemical
properties.
The results of several experimental animal studies as well as
direct measurements of selenium absorption in man indicate that
selenium compounds can be readily absorbed in the intestinal tract
and there appears to be no physiological control over this
absorption. This statement is based largely on studies of 75Se-
labelled selenite absorption in rats fed widely varying amounts of
selenium in the diet. All groups absorbed more than 95% of the
75Se administered by stomach tube. High absorption of selenium was
reported in women given 1-mg doses of selenium as selenomethionine
or selenite dissolved in water.
1.1.4.2. Total human body selenium contenta
The reported estimates of the amount of selenium in the adult
human body range from 3 to 14.6 mg. The lower estimates, 3.0 or
6.1 mg, were obtained in New Zealand by indirect techniques
following the administration of radiolabelled selenite or
selenomethionine, respectively, to healthy human volunteers. The
higher estimate, 14.6 mg, was obtained in the USA and was based on
-------------------------------------------------------------------
a The Task Group felt that the term "body burden" might be
misleading when applied to an essential trace element.
direct analysis of autopsy material accounting for 91.7% of the
body mass. The difference between these 2 estimates may be due
either to differences in the techniques employed or to differences
in dietary-selenium intake in New Zealand and the USA.
1.1.4.3. Distribution
Under normal conditions, levels of selenium are higher in the
kidney and liver than in the other major body tissues. Although
muscle-selenium levels are lower, muscle is the tissue present in
the greatest amount in the body, and thus accounts for the highest
proportion of the total body selenium. It is generally assumed
that the levels of selenium in the above tissues and in red blood
cells and plasma are related to the total body content of selenium,
but additional studies are needed to establish this point under
various conditions of selenium exposure, and also in man.
1.1.4.4. Metabolic pools of selenium in the body
There is no evidence of a regulated or specific storage form of
selenium, analogous to ferritin-bound iron. However, various data
suggest that several body pools of selenium could be considered as
serving a similar purpose. Evidence for a carry-over effect of
selenium exists. Sheep fed plants containing adequate levels of
selenium for 5 months and then fed low-selenium plants for 10
months produced lambs with higher tissue-selenium contents and
fewer signs of selenium deficiency than sheep fed only low-selenium
plants. North Americans moving to the low-selenium area of New
Zealand experienced a slow drop in blood-selenium contents for
approximately 1 year, before reaching New Zealand levels.
These observations could be explained by sequestration of
selenium in the form of selenomethionine and/or other selenoamino
acids incorporated into the primary structure of proteins
throughout the body. Selenium sequestered in this form would be
released and made available at a rate corresponding to the turnover
of proteins and the catabolism of selenomethionine. This mechanism
could provide selenium for animals temporarily unable to obtain it
in sufficient amounts through their diets.
The Task Group concluded that present knowledge about selenium
biochemistry was consistent with the concept of different selenium
pools in the body, even if direct measurements of the quantitative
aspects regarding the compartmentalization of selenium in the human
body and the related turnover rates were not available.
1.1.4.5. Metabolic conversion
Biological utilization of selenium from different chemical
forms has usually been assessed by determining the ability of such
compounds to prevent selenium-deficiency diseases or to increase
glutathione peroxidase activity in selenium-deficient animals.
Selenite and selenomethionine readily fulfil both these criteria
and can thus be considered convertible to metabolically-active
forms. In addition, it should be recognized that, in several of
the early metabolic studies on selenium, many other selenium-
containing compounds were shown to prevent dietary liver necrosis
and thus to undergo metabolic conversion to nutritionally-active
forms.
Some of the excretory metabolites of selenium have been
identified. Dimethylselenide is excreted in the breath at high
levels of selenium exposure. Trimethylselenonium ion and several
unidentified selenium metabolites are excreted in the urine.
1.1.4.6. Effect of chemical form of selenium on its metabolism
Animal studies indicate that selenite is not converted to
selenomethionine in the body. Nevertheless, both selenite and
selenomethionine are able to satisfy the nutritional requirement
for selenium.
The results of several studies indicate differences in the
metabolism of these two forms of selenium. On the basis of limited
human studies, in which 100 µg selenium/day was given, selenium in
the form of selenomethionine was retained in larger amounts than
selenium given as selenite; it also resulted in higher blood-
selenium levels. Comparison of selenite and selenomethionine in
chick studies demonstrated that retention of selenium was greater
when given as selenomethionine than when given as selenite. When
mice were given toxic levels of selenium compounds, selenomethionine
administration resulted in higher tissue-selenium levels than
administration of the same level of selenium as selenite.
Furthermore, manifestations of toxicity disappeared rapidly when
selenite was withdrawn from the diet but subsided only slowly
following withdrawal of selenomethionine. The results of limited
studies suggest that the metabolism of both selenium-
methylselenocysteine and selenocystine resembles that of selenite
rather than that of selenomethionine. These findings can be
explained by the incorporation of a portion of the selenium
supplied as selenomethionine into the tissue proteins as the amino
acid.
1.1.4.7. Selenium excretion
In human volunteers given tracer doses of inorganic or organic
selenium compounds orally, excretion was mainly by the urinary
route. However, when people consumed naturally-occurring selenium
in foods, approximately equal proportions were excreted in the
urine and faeces. Very little was excreted in the sweat, and
animal studies have shown that significant respiratory excretion of
volatile selenium compounds only occurs in cases of very high
selenium exposure. Selenium loss from the body, as judged by
whole-body counting studies following administration of single
doses of 75Se-labelled compounds, consisted of an initial phase of
rapid decrease of whole body radioactivity followed, after several
days, by a phase of more gradual 75Se excretion. The results of
animal studies indicate that the rate of selenium excretion in the
initial phase is affected by the dose of selenium administered as
well as by selenium status, whereas the second phase of excretion
is mainly affected by selenium status.
1.1.5. Effects on animals
1.1.5.1. Selenium toxicity
A characteristic sign of acute selenium poisoning in animals is
the odour due to the pulmonary excretion of dimethyl selenide.
Other signs of acute selenium poisoning in dogs and rats include:
vomiting, dyspnoea, tetanic spasms, and death from respiratory
failure. Pathological changes include congestion of the liver with
areas of focal necrosis, congestion of the kidney, endocarditis,
myocarditis, and peticheal haemorrhages of the epicardium.
The oral LD50 reported for sodium selenite varies from 2.3 to
13 mg selenium/kg body weight because of species differences and
other variables. Inhalation exposure to various selenium
compounds, including selenium dioxide, hydrogen selenide, and
selenium dust, proved to be toxic, causing damage to the
respiratory tract, liver, and other organs, lethality being
dependent on the level and duration of exposure. Because of the
limited number of studies and differences in species and other
experimental conditions used, evaluation of the dose-response
relationships for the different compounds is difficult.
It has been shown that the amount of dietary selenium needed to
cause chronic toxicity in animals is influenced by many variables
including the form of selenium and the type of diet. When fed in
the diet, elemental selenium has a low order of toxicity because of
its insolubility. Sodium selenite or seleniferous wheat fed at a
level of 6.4 mg selenium/kg diet caused growth inhibition, liver
cirrhosis, and splenomegaly in young growing rats fed the diet for
6 weeks. Growth inhibition at 4.8 mg/kg diet was not statistically
significant. Levels of 8 mg/kg diet or more caused additional
effects such as pancreatic enlargement, anaemia, elevated serum-
bilirubin levels and, in 4 weeks, death. In another study when
rats were fed 16 mg selenium/kg diet as sodium selenate in a
commercial "laboratory chow" type ration, their median survival age
was reported to be 96 days, and the predominant histopathological
lesion was acute toxic hepatitis. The predominant lesion at 8
mg/kg in the chow diet was chronic toxic hepatitis and the median
survival age was 429 days. Only 4 mg/kg were needed to cause acute
toxic hepatitis in rats consuming a semi-purified diet containing
12% casein. Increased activities of serum alkaline phosphatase (EC
3.1.3.1) and glutamic-pyruvic transaminase (EC 2.6.1.2) were
observed in young growing rats fed a semi-purified diet containing
4.5 mg selenium/kg as seleniferous sesame meal.
By using other criteria of toxicity, some research workers have
claimed deleterious effects of selenium at lower levels of intake,
however, there is a need to develop and validate more sensitive and
specific indicators of selenium poisoning. Farm animals raised in
regions where there are high levels of available selenium in the
soil develop toxicity diseases as a result of consuming plants
containing excess selenium. The levels of dietary selenium needed
to cause chronic toxicity are 5 mg/kg or more in cattle and 2 mg/kg
or more in sheep. Blood-selenium levels higher than 2 mg/litre are
generally associated with frank chronic selenium poisoning in
cattle, but borderline toxicity problems may be observed at levels
between 1 and 2 mg/litre. Blood-selenium levels of 0.6 - 0.7
mg/litre are associated with chronic selenosis in sheep.
On the basis of anecdotal reports, it has been suggested that
excess selenium may cause various practical reproduction problems
in farm animals, such as decreased reproductive performance in
livestock or decreased hatchability in chickens because of
deformities, at levels that do not cause obvious manifestations of
toxicity. A marked deterioration in reproductive performance was
noted in a multigeneration study in which mice were given sodium
selenate in the drinking-water at 3 mg selenium/litre, a level that
had no effect on growth or survival in a typical single-generation
toxicity study. There was a considerable decrease in the number of
litters produced by the third-generation mice and a considerable
increase in the number of runts among the mice that were born.
When monkeys were fed a cariogenic diet and given 2 mg
selenium/litre as sodium selenite for 15 months followed by 1
mg/litre for 45 months, the incidence of caries increased when the
selenium was given during tooth development but not when the teeth
were exposed post-eruptively. On the other hand, the effect of a
cariogenic diet given for two months after weaning was decreased in
rats whose mothers had been given, during the second half of
pregnancy and during lactation, drinking water containing selenium
at the level of 0.8 mg/litre in the form of sodium selenite or
selenomethionine.
In two independent studies, it was shown that, under certain
circumstances, selenium compounds were shown to be less toxic to
animals kept on a high dietary intake of selenium, thereby
suggesting possible adaptation to high selenium exposures.
1.1.5.2. Selenium deficiency
Animals raised in regions where there are low levels of
available selenium in the soil develop deficiency diseases through
consuming plants lacking adequate selenium. These diseases can be
prevented by feeding inorganic selenium compounds to the animals.
Deficiency diseases in which both selenium and vitamin E may
play a role include nutritional muscular dystrophy in sheep and
cattle, exudative diathesis in chickens, and liver necrosis in
swine and rats. Signs specific for selenium deficiency in the
absence of vitamin E deficiency include pancreatic degeneration in
chicks, and poor growth, reproductive failure, vascular changes,
and cataracts in rats.
The level of dietary selenium needed to prevent deficiency
diseases in animals depends on the vitamin E content of the diet.
For example, chicks receiving a diet deficient in vitamin E need
0.05 mg selenium/kg diet to prevent exudative diathesis whereas
0.01 mg/kg will be sufficient to prevent the disease if the diet
contains 100 mg vitamin E/kg.
Under normal vitamin E intake, the level of dietary selenium
needed to prevent deficiency is about 0.02 mg/kg for ruminants and
0.03 - 0.05 mg/kg for poultry.
Blood-selenium levels of less than 0.05 mg/litre are usually
associated with signs of selenium deficiency in sheep. Hepatic
selenium levels of less than 0.21 mg/kg (dry basis) are associated
with a high incidence of white muscle disease in lambs. However,
levels indicative of marginal deficiency can be influenced by the
vitamin E status of the animal.
The physiological function of selenium and its nutritional
relationship to the biological antioxidant vitamin E can be largely
explained on the basis of its role as a component of the enzyme
glutathione peroxidase (EC 1.11.1.9), which is responsible for the
destruction of hydrogen peroxide and lipid peroxides.
There is a close association between the level of dietary
intake of selenium and the glutathione peroxidase activity in
several organs. Also, the glutathione peroxidase activity of
erythrocytes is closely associated with the selenium content of the
blood. Because of these close relationships, measurement of
glutathione peroxidase offers a convenient method for assessing
selenium intake. However, the activity of the enzyme is influenced
by several nutritional and environmental factors that must be
considered when using it as an index of selenium status.
The ratio of the toxic level of selenium in the diet to the
nutritional level of selenium in the diet is approximately 100, but
this ratio can be decreased by nutritional or environmental
factors. For example, deficiency of vitamin E increases the
susceptibility of animals to selenium toxicity but also increases
the nutritional need for the element. Furthermore, inorganic
mercury potentiates the toxicity of methylated selenium
metabolites, whereas methyl-mercury potentiates selenium
deficiency.
Dietary selenium can protect against the toxicity of several
heavy metals, such as mercury or cadmium, and certain xenobiotics,
such as paraquat, but the mechanism of these protective effects is
not known.
Selenium has been suspected of being a carcinogen in the past,
but more recent research suggests that it may be able to protect
against certain types of cancers in experimental animals.
1.1.6. Effects on man
1.1.6.1. General population exposure
As stated above, the main environmental pathway of selenium
exposure in the general population is through food. Nutritional
surveys have shown that extreme dietary intakes range from 11 -
5000 µg/day, but on most diets intakes between 20 and 300 µg/day
can be considered as more typical. The extremes in intake are
reflected in extreme levels of selenium in blood, mean reported
values ranging from 0.021 to 3.2 mg/litre. The highest blood-
selenium levels ever observed in the general population were found
in an area of the People's Republic of China in which an episode of
intoxication reported as selenosis had occurred some years earlier.
In this respect, as well as in at least two other studies in over-
exposed populations, hair loss and nail pathology were the most
marked and readily documented toxic signs. The Task Group, being
aware of the hepatotoxicity of selenium compounds observed in
animal studies, noted that no clinical signs of hepatotoxicity were
observed in the studies of people exposed to high levels of dietary
selenium, but concluded that there is a need for more thorough
evaluation of hepatic function in persons with high selenium
exposure. Tooth decay was also observed in several studies on
over-exposed populations, but in evaluating its significance the
Task Group was unable to exclude interference by other
environmental factors. The Task Group recognized the difficulty in
establishing an exact dose-response with respect to selenium in the
above studies. The range of blood values noted was 0.44 - 3.2
mg/litre; in these studies no adverse effects were reported at the
lower level, whereas clear effects on the hair and nails were
observed at and above a level of 0.813 mg/litre.
The lowest blood-selenium levels ever reported in a general
population were seen in regions of the People's Republic of China
where Keshan disease and Kashin-Beck disease are known to be
endemic. The Task Group recognized the intensive research being
carried out concerning the involvement of selenium in the
multifactorial etiology of these diseases and the use of selenium
compounds in their prevention.
The Task Group considered a large number of studies on the
possible relationship between low levels of selenium intake and a
high incidence of cancer. In evaluating the available data, the
Task Group noted both consistencies and inconsistencies, which
made it difficult to draw a firm conclusion. However, some recent
case-control studies within prospective studies suggested the
importance of this approach for a firmer evaluation of the
involvement of the level of selenium intake in cancer prevention.
The Task Group was aware of an intervention trial being carried out
in China which could provide additional information on the
association between selenium exposure and human cancer risk.
1.1.6.2. Occupational exposure
As discussed above, the main environmental pathway of
occupational exposure to selenium is through the air, or in some
cases, by direct dermal contact. The selenium compounds likely to
be encountered include selenium dust, selenium dioxide, and
hydrogen selenide. The toxicological potential of selenium for
human beings employed in industry, can be inferred from respiratory
exposure studies carried out on laboratory animals and from ad hoc
case studies of industrial accidents.
Caution must be exercised when extrapolating the results of
animal studies or industrial accidents to the industrial health
aspects of long-term selenium exposure, because data available from
animal studies dealing with respiratory exposure are limited, the
exposure periods are brief, and industrial accidents involve
situations in which the exposure was brief and at an undetermined
level.
Extrapolation from general-population exposure is also quite
difficult, since selenium ingested in the diet may have
characteristics quite distinct from selenium encountered under
occupational exposure conditions: the chemical and physical forms
are likely to be different and these forms may change after contact
with the moist mucous membranes or with sweat. Knowledge about the
health effects of industrial selenium exposure is rudimentary,
since acute exposures are accidental and must be described on an ad
hoc case study basis. The Task Group did not know of any
epidemiological investigations of the effects of long-term
industrial exposure to selenium that included unexposed control
groups, nor were long-term follow-up studies with appropriate
control groups available. Exposure levels in short- and long-term
exposure studies are often ill-defined and the form of selenium is
not characterized. Confounding factors such as simultaneous
exposure to other toxic materials may exist.
1.2. Recommendations for Further Activities
1. The Task Group recommended that further studies were needed on
the well-identified population segments over-exposed to
selenium to clarify the signs and symptoms of selenium
overexposure in man and establish the relevant dose-response
relationships. The exclusion of hepatotoxic effects seems to
be of particular importance.
2. Further research on Keshan disease and Kashin-Beck disease
should be undertaken, not only to improve prevention of these
diseases, but also to provide basic information on the effects
of low selenium intake in man.
3. Further research is needed on the relationship between the
level of selenium intake and the incidence of cancer.
2. CHEMICAL AND PHYSICAL PROPERTIES; ANALYTICAL METHODS
2.1. Properties
Selenium belongs to group VI of the periodic table, between
sulfur and tellurium. It has similar chemical and physical
properties because the structure of its outer electron shells is
similar. Some chemical and physical properties of selenium are
listed in Table 1.
Table 1. Some chemical and physical properties of seleniuma
------------------------------------------------------------------
Properties Values
------------------------------------------------------------------
Relative atomic mass 78.96
Atomic number 34
Atomic radius 0.14 nm
Covalent radius 0.116 nm
Electronegativity (Pauling's) 2.55
Electron structure [Ar]3d104s24p4
Oxidation states -2, 0, +2, +4, +6
Stable isotopes
Mass 74 76 77 78 80 82
Natural abundance (%) 0.87 9.02 7.85 23.52 49.82 9.19
------------------------------------------------------------------
a From: Rosenfeld & Beath (1964) and Cooper et al. (1974).
All of the oxidation states of the element listed in Table 1
are commonly found in nature except the +2 state. However,
selenium compounds containing the divalent positive ion are known.
The natural isotopic pattern has been useful in identifying
selenium-containing molecular fragments produced in mass
spectrometry. While there are no naturally occurring radioisotopes
of selenium, several can be produced by neutron activation. Of
these, 75Se has the longest half-life (120 days) and is used as a
tracer in experiments as well as in the determination of selenium
by neutron activation analysis. Two relatively short-lived
radioisotopes, 77mSe (17.5-second half-life) and 81Se (18.6 min
half-life) have also been used in neutron activation analysis
(Heath, 1969-70; Alcino & Kowald, 1973).
Selenium in the +6 or selenate state is stable under both
alkaline and oxidizing conditions. Thus, it occurs in alkaline
soils, where it is soluble and easily available to plants. It is
also the most common form of the element found in alkaline waters.
Because of its stability and solubility, it may be potentially the
most environmentally dangerous form of the element.
Selenium in the +4 state occurs naturally as selenite. In
alkaline solution, it tends to oxidize slowly to the +6 state, if
oxygen is present, but not in an acid medium. It is readily
reduced to elemental selenium by a number of reducing reagents,
ascorbic acid or sulfur dioxide being commonly used for this
purpose. It readily reacts with certain o-diamines, and this is
used as the basis for some analytical methods. Selenium dioxide,
the anhydride of selenious acid, sublimes at 317°C. This is
important with regard to air pollution through the combustion of
materials containing the element (Heath, 1969-70; US NAS/NRC,
1976), and also to air sampling procedures.
Selenite binds tightly to iron and aluminium oxides. Thus, it
is quite insoluble in soils and generally not present in waters in
any appreciable amount. The nature of this binding has been
suggested to be (Howard, 1971):
(a) hydroxylation of fracture surfaces of oxides in an
aqueous environment;
(b) development of a pH-dependent charge on the surface
by amphoteric dissociation of the hydroxyl groups;
(c) electrostatic attraction of ions of negative charge
(biselenite, for instance) to the surface when it is
positively charged; and
(d) specific adsorption of the ion through exchange with
surface hydroxyl groups.
Elemental selenium (Seo), like elemental sulfur, exists in
several allotropic forms. At the molecular level, while several Se
aggregates can form, only rings containing 8 selenium atoms (Se8)
and Sen polymeric chains exist at room temperature. Se8 is soluble
in carbon disulfide, but Sen is not. Both forms have a very low
order of solubility in water and dilute acids or bases. Seo exists
in both amorphous and crystalline forms. Colloidal Seo, prepared
by reducing aqueous solutions of selenite, is an amorphous form
with a reddish colour, used in some early methods for determining
selenium at low concentrations. On heating at 60 °C, for a short
time, colloidal selenium crystallizes and its colour changes to
black. Elemental selenium boils at 684 °C, and since it, as well
as hydrogen selenide, may form during the pyrolysis of organic
materials, the use of dry ashing in preparing samples for analysis
has serious limitations. This property also contributes to the
problem of atmospheric contamination with selenium in certain
industrial processes (Crystal, 1973). Elemental selenium is very
stable and highly insoluble. It is formed on the reduction of
selenate as well as of selenite. The stability and insolubility of
elemental selenium render it unavailable to plants. Its formation
by natural processes might, thus, be considered one means by which
the element is removed from active cycling in the environment.
In its -2 state, selenium exists as hydrogen selenide and in a
number of metallic selenides. Hydrogen selenide is a strong
reducing agent and a relatively strong acid with a pKa of 3.73. It
is a gas at room temperature and very toxic. Exposure to hydrogen
selenide results in olfactory fatigue, and individuals exposed to
it may soon be unaware of its presence. In air, it decomposes
rapidly to form Seo and water. Selenides of heavy metals occur
naturally in many minerals, and iron selenide may be one of the
insoluble forms of the element in soils (Sindeeva, 1959; Nazarenko
& Ermakov, 1971; Johnson, 1976; US NAS/NRC, 1976).
A large number of selenium analogues of organic sulfur
compounds are known. Many have been identified in plants, animals,
and microorganisms. However, although some aspects of the
metabolism of selenium resemble those of sulfur, in many cases,
their metabolic pathways diverge considerably (Levander, 1976a).
As in the case of sulfur, many of the selenium compounds are
odoriferous, volatile, and relatively unstable. Because of this
volatility, precautions are necessary in handling some samples for
analysis (Klayman & Gunther, 1973; Irgolic & Kudchadker, 1974).
2.2. Analytical Methods
2.2.1. Sample collection, processing, and storage
Initially, attention must be given to the adequacy of the
method used to analyse for selenium. It is strongly recommended
that the analytical method under consideration be verified against
samples of known certified selenium content such as the standard
reference materials available commercially from the US National
Bureau of Standards. Moreover, adequate analytical quality control
measures should be standard operating practice in any laboratory
conducting analyses for selenium. Equally important is the proper
collection and treatment of samples before analysis. The sample
collected must be representative of the material being studied, and
must be protected from either contamination or loss of selenium
during analysis. These considerations are especially important in
the processing of organic matter containing selenium and in the
determination of trace amounts of selenium in materials of
environmental interest such as soils, air, and water.
In the case of soils, it should be recognized that sampling to
a depth of 1 m is more meaningful than shallower sampling,
especially where soil-selenium levels are excessively high (Olson
et al., 1942). Also, considerable variations will occur in soils
lying only a few metres from each other. In air samples, both
volatile and particulate selenium may be present and a dry filter
with an added liquid filter has been suggested for measuring these
two forms and the total selenium in air (Diplock et al., 1973;
Olson, 1976). Water samples may contain suspended solids and, in
sampling for analysis, it must be decided whether these solids,
which may contribute significant amounts of selenium, should be
included in, or excluded from, the sample. Animals are known to
synthesize volatile selenium compounds, and so it is preferable to
analyse animal specimens without drying.
2.2.2. Sample decomposition or other preliminary treatment
Some methods of analysis can be performed without destroying
the sample. In others, the sample must be treated in some way to
remove organic matter, release the selenium, and bring it to the
proper oxidation state. Several procedures for accomplishing this
have been developed and are discussed below.
2.2.2.1. Wet digestion
Wet digestion is most commonly used for freeing the selenium
and destroying the organic matter. It can be used for a wide
variety of materials, wet or dry, and with many procedures for
measuring the selenium. Different mixtures of nitric, sulfuric,
and perchloric acids, with or without such additives as hydrogen
peroxide, mercury, molybdenum, vanadium, or persulfate, have all
been used with equal success (Olson et al., 1973; Olson, 1976).
Some analysts find it convenient to add nitric acid alone to their
samples and allow them to stand overnight at room temperature.
This procedure facilitates the later steps in the wet digestion and
reduces the likelihood of foaming and/or clarring of the sample.
However, reproducible recoveries of selenium are obtained only if
all traces of nitric acid are removed from the sample digests by
heating until the appearance of perchloric acid fumes for 15-20 min
(Haddad & Smythe, 1974). The trimethylselenonium ion of urine is
somewhat resistant to decomposition by wet digestion, so an
extended period of digestion is recommended for both urine and
certain plant materials that may contain the trimethylselenonium
moiety (Olson et al., 1975). While its presence in significant
amounts has not been demonstrated in most tissues, the
trimethylselenonium moiety may occur in kidney in such an amount
that extended digestion of this tissue may also be wise.
Wet digestion readily adapts to the predigestion procedure
mentioned below and it can be adapted for the handling of large
numbers of samples (Chan, 1976; Whetter & Ullrey, 1978). Special
care is needed to avoid explosions when using perchloric acid,
including the use of a digestion rack with a fume manifold and a
special fume hood. However, a wet digestion technique with
phosphoric acid, nitric acid, and hydrogen peroxide has been
described that avoids the use of perchloric acid (Reamer & Veillon,
1983a). This procedure gives results for plasma and urine samples
similar to those obtained with the traditional fluorometric method
using perchloric acid digestion. However, the method needs to be
validated for other sample matrices.
2.2.2.2. Predigestion
A predigestion process can often reduce sampling error, when
solubilized samples or wet digestion are used in determining
selenium. A sample much larger than that required for the actual
determination is heated with about 10 volumes of concentrated
nitric acid at a slow boil for about 20 min. After cooling and
making to volume with water, an appropriate aliquot can be removed
for analysis. With samples of high fat content, the fat will rise
to the surface on cooling and it can be avoided in sampling for
analysis. The fat contains essentially no selenium at this point.
Feed samples fortified by the addition of sodium selenite or
selenate, as well as premixes containing these compounds, can often
be handled much more successfully with this procedure than by
relying on fine grinding to assure a representative sample (Olson,
1976).
2.2.2.3. Combustion
Open combustion, with or without added fixatives, and low
temperature ashing with excited oxygen have not been successfully
used in selenium determination. Using the Schoeniger flask for
destroying organic matter and oxidizing the selenium gives
excellent results, but the method is somewhat inconvenient, and
this has limited its use (Olson et al., 1973).
2.2.2.4. Fusion
Sodium carbonate or sodium peroxide fusion of soils or rocks
and the Parr bomb fusion of some organic materials have given
satisfactory results in selenium determination. However, these
methods are inconvenient in many respects and are little used
(Olson et al., 1973).
2.2.2.5. Concentration
Materials containing very small amounts of selenium may require
a step for concentrating the element. With water, this can usually
be accomplished by evaporation under alkaline conditions. Other
methods of concentrating selenium include coprecipitation with iron
hydroxide (selenite) and some of the techniques used for separating
the element from interfering substances, which will be discussed in
section 2.2.3.
2.2.3. Removal from interfering substances
In many methods, it is necessary to isolate the selenium or to
remove certain interfering substances before the selenium is
measured. Procedures used include: coprecipitation with arsenic,
tellurium, or ferric hydroxide; ion exchange column chromatography;
solvent extraction of selenium halides, of organic selenium
complexes, or of certain interfering metals; distillation of the
tetrabromide; volatilization as hydrogen selenide; precipitation
as elemental selenium; paper, thin-layer, or gas chromatography;
and the ring oven technique (Alcino & Kowald, 1973; Cooper, 1974;
Olson, 1976).
Interference by certain metal ions has been overcome, to a
large extent, by the addition of complexing reagents such as
oxalate or ethylenediaminetetraacetic acid.
2.2.4. Detection and identification of selenium
Selenium in the form of selenite or selenate can be detected
and identified in a number of ways (Alcino & Kowald, 1973). Such
qualitative tests may be useful in some industrial situations, but
normally quantitative analysis is required for confidence in any
interpretation, and in the end may only be slightly more time-
consuming.
2.2.5. Measurement of selenium
Several reviews of methods for measuring selenium in a wide
variety of materials (Nazarenko & Ermakov, 1971; Alcino & Kowald,
1973; Olson et al., 1973; Cooper, 1974; Olson 1976) have been used
as a basis for the following discussion.
The method used will depend on the sample to be analysed, the
sensitivity required, the accuracy required, other analyses to be
made, the number of samples, and the equipment and expertise
available.
For samples containing high concentrations of the element,
gravimetric or titrimetric methods may be the best. The
gravimetric methods have been based on precipitation of selenium as
the element, the sulfide, the salts of certain heavy metals, an
organic complex, or electrogravimetrically as Cu2Se. Titrations
have been based on iodometry, argentometry, potassium permanganate
reductions by selenite, or back-titrations using thiourea or sodium
thiosulfate.
Other methods, many of them very sensitive, can be classified
as: colorimetric, spectrophotometric, fluorometric, atomic
absorption spectrometric, X-ray fluorescent, gas chromatographic,
neutron activation, proton activation, polarographic, coulometric,
potentiometric, spark source mass spectrometric, gas
chromatograpic, mass spectrometric, anodic stripping voltammetric,
and catalytic.
At one time, the colorimetric determination was the most used
for the analysis of samples containing small amounts of selenium.
The method most commonly used (Robinson, 1933) was specific for
selenium and, for its time, quite sensitive. The titrimetric
method of Klein (1943) superceded it, being somewhat more
reproducible and sensitive, until selenium was found to have a role
as an animal nutrient, when considerably greater sensitivity was
required. Very sensitive methods, most commonly used, include
fluorometry, neutron activation, and atomic absorption
spectrophotometry.
2.2.5.1. Fluorometric analysis
The methods most widely used for the determination of selenium
in natural materials are based on fluorometry. Selenious acid
reacts with 1-diamines to give a piazselenol, which is fluorescent.
The 1-diamine of choice is 2,3-diaminonaphthalene. The piazselenol
is extracted from an acid solution (pH 1 - 2) with either
decahydronaphthalene or cyclohexane, in either of which the
fluorescence yield is good. When the organic matter is destroyed,
the reaction can be specific for selenium. Interference from
copper, iron, and vanadium, and some oxidizing agents can usually
be avoided.
Fluorometric methods have been applied to a number of
materials; those for foods and plants have been subjected to
collaborative studies (Olson, 1969; Ihnat, 1974) and have been
accepted as official first, and final action, by the Association
of Official Analytical Chemists (AOAC, 1975). Critical reviews of
fluorometric methods (Haddad & Smythe, 1974; Michie et al., 1978)
have shown them to be reliable providing that the precautions
described for various adaptations are followed. The methods are
sensitive to about 0.01 µg of selenium in a sample, are adaptable
to large numbers of samples, can be automated (Brown & Watkinson,
1977; Szydlowski & Dunmire, 1979; Watkinson, 1979; Watkinson &
Brown, 1979) and can be simplified (Spallholz et al., 1978; Whetter
& Ullrey, 1978). While isotope dilution procedures can be used
with fluorometric methods (Cukor et al., 1964), they are not
essential for reliable results. Fluorometric analyses rely on
perchloric acid digestion of the samples, and, thus, require a
perchloric acid fume hood. Furthermore, 2,3-diaminonaphthalene of
adequate purity is sometimes difficult to obtain. Improved
sensitivity of the fluorometric analysis of water and biological
samples was achieved by redistilling cyclohexane and extracting
2,3-diaminonaphthalene solution in 0.1 M hydrochloric acid with two
portions of redistilled cyclohexane (Nazarenko et al., 1975;
Nazarenko & Kislova, 1977). Wilkie & Young (1970) have also
published detailed experimental procedures for purifying various
reagents used in fluorometric analysis. The analysis can be
carried out with a simple fluorometer, is adaptable to a wide
variety of materials, is reasonably rapid and reliable, and is
relatively inexpensive to perform.
2.2.5.2. Neutron activation analysis
Thermal neutron activation is the most commonly used procedure
for irradiating samples containing selenium. Of the radionuclides
of selenium that it produces, 75Se (half-life 120 days), 81Se
(half-life 18.6 min), and 77mSe (half-life 17.5 seconds) have been
useful in analysis. 81mSe (half-life 57 min) has been used to
measure carrier selenium after separation and reirradiation
(Kronborg & Steinnes, 1975). Proton-induced X-ray fluorescence
methods have also been reported (Bearse et al., 1974; Barrette et
al., 1976).
Two methods of converting radioactivity measurements to
selenium content are:
(a) the direct method using calculations based on certain
known values and constants; and
(b) the comparator method, based on the irradiation and
counting of a known amount of selenium, as a
standard, together with the samples. The comparator
method is the most accurate and most often used, but
the direct method is often used when short-lived
isotopes are measured. Ruthenium has been
recommended as a multi-isotopic comparator when
multi-element analysis is being performed (Van der
Linden et al., 1974).
Neutron activation analysis can be used without and with sample
destruction. When 75Se is measured by non-destructive analysis, a
period of several weeks or months may be allowed for decay of some
interfering radioisotopes. The 77mSe isotope has been used without
this type of delay with some success. Non-destructive methods have
been used for multi-element analysis, but they have been subject to
more errors, because of interference, than destructive methods
followed by chemical separation. The use of high resolution gamma-
ray spectrometry substantially increases the specificity of the
non-destructive methods and the complete processing of 300 - 400
samples per month is possible (Pelekis et al., 1975). A method has
been developed using irradiation with epithermal neutrons and
multiparameter analysis which eliminates the effect of spurious
interferences in the determination of low selenium concentrations
by instrumental neutron activation analysis. High specificity has
been achieved by the selective response of the system to gamma-
gamma coincidences of 75Se (Vobecky et al., 1977, 1979; Pavlik et
al., 1979).
Destructive neutron activation analysis is used with the
chemical separation of the selenium. In most methods, 75Se is
measured, but 77mSe measurement is not uncommon, and 81Se
measurement has also been used. Carrier selenium is usually added
following the irradiation. Destruction of the sample has been
accomplished by wet digestion, sodium peroxide fusion, or
combustion. Combustion has been accomplished in a closed system,
which provides for the dry distillation of selenium and certain
other elements into a trapping system. Wet digestion is normally
followed by separation of the element by distillation as the
tetrabromide, and/or its precipitation, adsorption on an ion
exchange material, solvent extraction of an organic selenium
complex, or reversed phase extraction chromatography. A semi-
automated method based on wet digestion, separation by
distillation, and the use of ruthenium as a comparator has been
reported (D'Hondt et al., 1977).
Neutron activation analysis can be very accurate, sensitive,
and specific, especially when used with sample destruction and
chemical separation of the selenium. It adapts well to multi-
element analysis. However, it requires sophisticated equipment
that most laboratories do not have. Furthermore, it is time-
consuming, especially when used with chemical separation. Its most
important use may be as a reference method against which other
methods may be evaluated or where good reagents are not available.
2.2.5.3. Atomic absorption spectrometry
Methods for selenium analysis now being most actively studied
are those based on atomic absorption spectrometry (AAS). A wide
variety of techniques has been described. Most require some type
of wet digestion, when organic matter is present. Flame
atomization methods are useful for materials with a high selenium
content. However, other more sensitive methods have been
developed, the most used being based on hydrogen selenide
generation. This has the advantages of separating the selenium
from many other elements and measuring it using techniques that
provide excellent sensitivity. Ihnat (1976) compared the
performance of hydride generation with that of a carbon furnace
atomization-AAS method, finding hydrogen selenide generation to be
superior. In further studies, Ihnat & Miller (1977a,b) found that
the sensitivity of this method was excellent and its accuracy,
fairly good, but that its precision in a collaborative study was
not. McDaniel et al. (1976) found that procedures for hydrogen
selenide generation might liberate as little as 10% of the element
from solution. They suggested means for optimizing hydride
generation to give a simple and sensitive selenium determination,
free from interferences, and using a heated graphite atomizer for
the measurement of the element. The hydride generation technique
has been automated (Goulden & Brooksbank, 1974; Pierce & Brown,
1977; Pyen & Fishman, 1978), and improved equipment for hydride
generation has recently been described (Brodie, 1979). Encouraging
results with graphite furnace atomization in the presence of nickel
(Shum et al., 1977) indicate that this method may also find
considerable use.
Atomic absorption methods based on Zeeman effect background
correction to remove spectral interferences (Pleban et al., 1982;
Carnrick et al., 1983) are currently receiving attention. Such
procedures offer the promise of carrying out selenium analyses in
certain biological matrices directly, i.e., without the need for
sample digestion. Development of such techniques would greatly
increase the speed and convenience of selenium analysis.
2.2.5.4. Other methods
Examples of other methods that have received recent attention
and may find some more use in the future include: energy
dispersive X-ray fluorescence (Holynska & Markowicz, 1977);
differential pulse polarography (Bound & Forbes, 1978); anodic
(Andrews & Johnson, 1976) or cathodic (Blades et al., 1976)
stripping voltammetry; atomic fluorescence spectrometry (Thompson,
1975); gas-liquid chromatography (Ermakov, 1975; Poole et al.,
1977); and spark source mass spectrometric isotope dilution
(Paulsen, 1977). A gas chromatographic, mass spectrometric double
isotope dilution technique based on a volatile chelate of selenium
has recently been described, which not only determines total
selenium in biological materials but also allows stable selenium
isotopes to be followed as tracers in metabolic studies (Reamer &
Veillon, 1983b).
A variety of methods has been proposed and used for identifying
and measuring a number of chemical forms of selenium in different
materials including: paper (Hamilton, 1975) or ion exchange (Martin
& Gerlach, 1969) chromatography for selenium compounds in plants;
gas chromatography for volatile selenium compounds (Doran, 1976);
and ion exchange (Shrift & Virupaksha, 1965) or solvent extraction
separation (Kamada et al., 1978) for measuring selenite and
selenate in solutions.
3. SOURCES, TRANSPORT, AND CYCLING OF SELENIUM IN THE ENVIRONMENT
3.1. Natural Sources
Because selenium is present in natural materials, its
occurrence in any substance cannot be assumed to be the result of
human activity and some knowledge of its natural distribution is
required before evaluating its role as a pollutant.
3.1.1. Rocks and soils
Several reviews of selenium geochemistry have been published
(Sindeeva, 1959; Rosenfeld & Beath, 1964; Lakin & Davidson, 1967;
Cooper et al., 1974). The concentration of selenium in igneous
rocks is low, usually much less than 1 mg/kg, and similar levels
probably occur in metamorphic rocks. Sedimentary rocks, such as
sandstone, limestone, phosphorite, and shales may contain from < 1
to > 100 mg/kg.
The selenium content of a soil reflects, to some extent, that
of the parent material from which the soil has been formed. Thus,
in arid and semi-arid areas, soils of high selenium content have
been derived from sedimentary rocks, usually shales and chalks
(Moxon et al., 1950). These soils are alkaline in reaction,
favouring the formation of selenate (Geering et al., 1968), which
is readily available to plants (Moxon et al., 1950). The selenate
is easily leached from the surface soil, but, with limited
rainfall, it is redeposited in the subsurface soil, where it is
still available to plants (Olson et al., 1942). Thus, surface soil
analysis has not been found to be a reliable measure of the
potential of a soil to produce vegetation containing toxic levels
of selenium.
Coal with an unusually high selenium content (> 80 000 mg/kg;
average 300 mg/kg) was recently identified as the ultimate
environmental source of selenium contaminating soils in a
seleniferous region of Enshi county, Hubei province, the People's
Republic of China (Yang et al., 1983). It was thought that
selenium passed from the coal to the soil through weathering,
leaching, and possibly biological action, thus making it available
to the crops. Lime fertilizers, traditionally used in the area,
would also render the selenium accumulated in the soil more readily
available to plants.
Some soils produce crops nutritionally deficient in selenium
(US NAS/NRC, 1971). The most obvious factor in the formation of
these soils is probably the parent material (Hodder & Watkinson,
1976), but other factors such as rainfall, climate, pH, and soil
composition contribute significantly (Gissel-Nielsen, 1976, 1986).
As with producing plants of high selenium content, soils producing
plants low in the element cannot be identified by analysis of the
surface soil alone (Shacklette et al., 1974). Because of the many
factors affecting availability, plant analysis is also necessary
(Kubota et al., 1967).
Sun et al. (1985) reported that the average total selenium
content (112 µg/kg, range 59 - 190 µg/kg) in the soil of 6 low-
selenium Keshan disease areas in China was significantly lower than
that of the 5 corresponding non-endemic areas (234 µg/kg, range 142
- 318 µg/kg). The average water-soluble selenium content and the
percentage of water-soluble selenium in the total soil-selenium of
the endemic areas (4.0 µg/kg, range 2.2 - 8.7 µg/kg and 39 g/kg,
respectively) were also significantly lower than those of the non-
endemic areas (19.9 µg/kg, range 11.4 - 38.8 µg/kg and 9.2 g/kg,
respectively). The total soil-selenium content of a high-selenium
area in China was 7865 µg/kg (range 6390 - 10 660 µg/kg), but the
percentage of water-soluble selenium was not very high (30 - 40
g/kg).
3.1.2. Natural selenium in the food chain
All plants absorb selenium from the soil, the amount depending
mainly on the species, the stage of growth, and the availability of
the selenium in the soil. Biogeochemical factors influencing the
availability of selenium in the soil, such as pH, iron content,
etc., have been reviewed by Ermakov & Kovalskij (1968), Allaway
(1973), and Kovalskij (1974, 1978). Various plant tissues contain
different selenium levels, generally following protein content.
Certain plants, known as selenium accumulator plants, take up
quantities of selenium great enough to be toxic for animals. The
selenium content of animal tissues reflects that of the feeds
consumed (Allaway, 1973; Kovalskij & Ermakov, 1975). Thus,
naturally-occurring selenium readily passes up through the food
chain via animals to human beings, and the amount of selenium in
the human diet is largely determined by the amount of selenium in
the soil available for absorption by plants. However, as discussed
in section 7, the increased retention of selenium by animals, under
conditions of low selenium intake, and the decreased retention
during high selenium intake ensures that, in different geographical
zones, the range of selenium levels in animal products is less than
that in plant tissues. This "buffering effect" of animals in the
food chain tends to moderate the extremes of selenium intake to
which human beings are exposed, whereas herbivores are subject to
much greater fluctuations in selenium intake.
3.1.3. Water and air
Under natural conditions, the concentration of selenium in
water usually ranges from a few tenths to 2 or 3 µg/litre (Ermakov
& Kovalskij, 1974; US NAS/NRC, 1976). The highest natural
concentration reported to date is 9000 µg/litre, almost all other
values falling below 500 µg/litre (US NAS/NRC, 1976). A garlicky
odour has been noted in waters containing 10 - 25 µg selenium/litre
and an astringent taste can be detected in water samples containing
100 - 200 µg/litre (Pletnikova, 1970). In a study carried out in
Nebraska, USA, which was biased towards finding waters with
elevated selenium contents, it was reported that about one-third of
161 well samples contained over 10 µg selenium/litre and about 4%,
over 100 µg/litre (Engberg, 1973). Surface waters seem much less
likely to contain excessive levels of selenium than ground waters.
Soils, plants, microorganisms, animals, and volcanoes all
contribute selenium to the atmosphere. All of these sources,
and possibly also certain sediments, produce volatile forms,
and soils and volcanoes probably contribute particulate matter
containing the element. Some man-made activities also yield
atmospheric selenium (section 3.2.2), and it is difficult to
establish the proportion of selenium that comes from these
activities and the proportion that comes from natural sources
(US NAS/NRC, 1976). However, data concerning atmospheric
selenium over the poles and over the Atlantic Ocean, as well
as over areas of minimal human activity (Zoller et al., 1974;
Duce et al., 1975; Dams & De Jonge, 1976) suggest that the
average concentration from natural sources should be less than
0.04 ng/m3, except close to centres of volcanic activity.
3.2. Man-Made Sources
3.2.1. Agriculture
Early agricultural uses of selenium compounds as pesticides
were very limited and short-lived. The recent use of selenium
compounds as feed additives or injectables for the prevention of
selenium deficiency diseases in farm animals represents a source
for environmental contamination, but, compared with the levels
already present in most feeds and the amounts found in soils, even
in the deficient areas, this source seems insignificant (US
NAS/NRC, 1976). In New Zealand, little increase in the selenium
content of human foods was observed after the introduction of
selenium supplementation for farm animals (Thomson & Robinson,
1980). The use of fertilizers (Koivistoinen & Huttunen, 1985) and
foliar sprays (Gissel-Nielsen, 1973, 1986) to rectify selenium
deficiency in feeds is being introduced, but they are unlikely to
become a hazard in the environment. Other fertilizers do not
contain enough selenium to contribute significantly to the
environment (Gissel-Nielsen, 1971). Irrigation of seleniferous
lands may result in an increased concentration of selenium in
drainage waters (US NAS/NRC, 1976), but until recently there was no
evidence to suggest that this constitutes a hazard. However, in
the San Joaquin Valley of California, reproductive problems have
now been observed in aquatic birds that used irrigation drain-water
ponds as waterfowl habitat (Ohlendorf et al., 1986). Although the
toxic signs reported in these birds resembled avian selenosis, the
possible role of other water-borne toxins such as high levels of
arsenic or boron has been pointed out.
3.2.2. Industry
The main industrial sources emitting selenium into the
environment include the mining, milling, smelting, and refining of
copper, lead, zinc, phosphate, and uranium, the recovery and
purification of selenium itself, the use of selenium in the
manufacture of various products, and the burning of fossil fuels.
Some problems from selenium emissions might arise in regions near
selenium-producing or coal-burning industries. For instance, in
areas where copper sulfide ores were mined and processed,
atmospheric selenium levels ranging from 0.15 to 6.5 µg/m3 were
reported within 0.5 - 10 km of the ore-processing plant (Seljankina
et al., 1974). Selenium pollution of the air may be important with
regard to possible contamination of open-air water reservoirs;
waterways can be contaminated directly by selenium from mining or
industrial effluents. For example, waste-waters of ore mines and
effluents from a number of non-ferrous industries were reported to
contain selenium in the concentration range of 14 - 56 µg/litre.
Residual selenium impurities in conditionally cleaned effluents
released into open water bodies can contribute some contamination
(Shumaev et al., 1976). Effluents from sewage plants can add
selenium to waters; raw sewage has been reported to contain up to
280 µg selenium/litre, and levels of 45 - 50 µg/litre have been
reported in primary and secondary sewage effluents (Baird et al.,
1972). Emission factors for atmospheric selenium contamination and
solid waste generation for various industrial processes in the USA
are shown in Table 2. The pattern of such emissions will vary
depending on the industrial and mining characteristics of
individual countries. In Canada, for example, over half of the air
pollution due to selenium resulted from primary copper and nickel
production, the combustion of coal contributing less than 25%.
When coal from deposits throughout the USA was analysed (138
analyses), the highest selenium content was 10.65 mg/kg and the
average, was 2.8 mg/kg, a hundred times less than the high-selenium
coals reported in China (Yang et al., 1983) (section 3.1.1). The
chemical form of the element in coal is not known (US NAS/NRC,
1976). The selenium from the coal was released into the
surrounding soils by natural processes, thereby contaminating the
food chain with toxic levels of selenium.
Few data on the selenium content of fuel oils are available.
Most reported values have been below 1 mg/kg (US NAS/NRC, 1976).
Oil shale has been reported to contain selenium (Shendrikar &
Faudel, 1978), but apparently there are no reports of its
occurrence in natural gas.
3.3. Environmental Transport
Because of the lack of quantitative data, it is impossible to
evaluate the relative importance of various processes in the
environmental transport of selenium. However, Bertine & Goldberg
(1971) have estimated that rivers move over 7 x 106 kg of selenium
every year, even though surface waters generally contain little of
the element. The quantity of selenium passing via grain into the
world feed and food supplies would be at least 105 kg, if it is
assumed that the annual global production of cereal grains and corn
is 109 tonnes and that these grains contain an average of 0.1 mg
selenium/kg. Although this simple calculation neglects all other
plants and the large ocean flora, the quantity of selenium moved in
grains alone is equivalent to about 7% of the average world
production of the element for industrial purposes. Thus, natural
geophysical and biological processes probably play dominant roles
in shaping the present status of selenium in the human environment
and must be taken into account in any evaluation of the effects of
man's activities on selenium in the environment.
Table 2. Estimated selenium materials balance for the
USA for 1970a,b
---------------------------------------------------------
Source Selenium Atmospheric Solid
input emissions waste
---------------------------------------------------------
(---------tonne----------)
Industrial production
Mining and milling 2900 5 1633
Smelting and refining 1270 227 181
Selenium refining 877 59 362
Industrial consumption
Glass and ceramics 59 59
Electronics and duplicating 227 c c
Pigments 59 c c
Iron and steel alloys 23 11 c
Other 38 c c
Other
Coal consumption 1315 680 635
Fuel oil consumption 59 59 0
Incineration 36 c 36
Other disposal 322 c 318
Total emissions: 1100 3165
---------------------------------------------------------
a From: US NAS/NRC (1976).
b The input not accounted for as atmospheric emissions or
solid waste would appear in intermediate or commercial
products.
c Less than 1 tonne.
3.4. Biological Selenium Cycle
Several diagrammatic schemes have been presented for the
cycling of selenium in nature (Moxon et al., 1950; Lakin &
Davidson, 1967; Olson, 1967; Frost & Ingvoldstad, 1975; US NAS/NRC,
1976). None of the schemes is complete and none provides good
quantification for the various steps in the cycle. Indeed, a
complete scheme is perhaps too complex to be presented
diagrammatically with clarity, and reasonably accurate
quantification of the various steps would demand much more
background information than is available at present. It appears
that selenium has many pathways for distribution among geological
media, biological media, the atmosphere, and waters by geophysical,
biological, and man-made activities. It also appears that there
are "sinks" into which selenium may fall (elemental selenium,
ferric hydroxy complexes, and metal selenides) from which it may be
only very slowly recycled. These may represent a natural
detoxification process. However, the fact that there are areas of
deficiency and areas of excess of the element over the earth's
surface is evidence that its depletion or concentration can occur
locally. How quickly changes in the selenium status of a region
occur is not known, and there is a distinct need for data from
which an evaluation could be made of the rate and impact of these
changes as well as the influence of man's activities on them. For
example, Allaway (1973) concluded that any soil-plant-animal chain
of food production on neutral or acid soils would eventually become
depleted in biologically effective selenium, though it is not known
how rapidly this might occur. Shrift (1973) suggested the
existence of a biological selenium cycle from the standpoint of
transformations between several oxidation-reduction states, as
shown in Fig. 1. Reduction of the element is common and well
established, but its biological oxidation is less well documented.
However, a missing link on the oxidative side of the biological
selenium cycle was found by Sarathchandra & Watkinson (1981), when
they discovered a strain of Bacillus megaterium, isolated from
soil, that oxidized elemental selenium to selenite and traces of
selenate. The role of non-biological oxidation and reduction of
the element has not yet been elucidated. While a balance may be
maintained in the redox state of selenium throughout the world,
there are regions where oxidation prevails and others where
reduction prevails. Knowledge of the conditions and mechanisms
leading to each situation is essential to understand the
development of regions of selenium excess or deficiency.
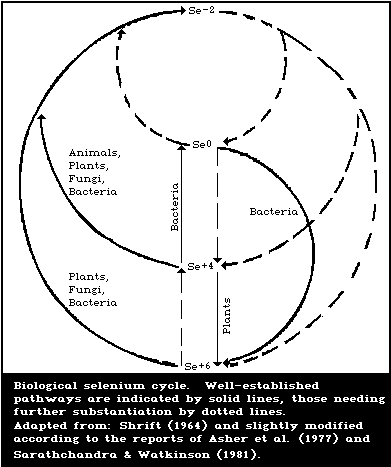 4. LEVELS IN ENVIRONMENTAL MEDIA
4.1. Levels and Chemical Forms of Selenium in Food
4.1.1. Levels in food
In spite of the relatively few data available, some
generalizations concerning the selenium content of foodstuffs can
be made. For example, the level of selenium in food depends on
natural differences among food commodities and the natural
availability of selenium in the environment. Moreover, certain of
man's activities can influence the selenium content of human foods.
4.1.1.1. Natural differences among food commodities
A wide range of values has been reported in the selenium
content of foods (mg/kg wet weight): liver, kidney, and seafood,
0.4 - 1.5; muscle meats, 0.1 - 0.4; cereal and cereal products, <
0.1 - > 0.8; diary products, < 0.1 - 0.3; and fruits and
vegetables, < 0.1) (Oelschlager & Menke, 1969; Morris & Levander,
1970; Schroeder et al., 1970; Suchkov, 1971; Arthur, 1972; Millar &
Sheppard, 1972; Ferretti & Levander, 1974; Sakurai & Tsuchiya,
1975; Abutalybov et al., 1976; Bieri & Ahmad, 1976; Kasimov et al.,
1976; Olson et al., 1978). It should be pointed out that these
values are given for raw foods (food as purchased) rather than
cooked foods (food as eaten). The effects of cooking on the
selenium content of foods are described in section 4.1.1.3.
Moreover, the values presented in these food composition studies
should not be compared from one country to another, because of the
variations in the analytical methods and sampling procedures used
(section 2).
Organ meats, such as kidneys or liver, contain the highest
levels of selenium, but some seafood products contain almost as
much. Muscle meats are significant sources of selenium, though
they do not contain as much as organ meats or seafoods. Certain
semolina, grain, and cereal products can contribute appreciably to
the dietary-selenium intake, but wide variations in selenium
content have been found in different samples of the same foodstuff.
Such variation is typical of many plant foods and the reasons for
this are discussed below. Milk, cheese, and egg samples from
several countries showed low to moderate values for selenium, but
again the results were quite variable. Fruits and vegetables
generally contained very low levels of selenium, though garlic and
mushrooms contained moderate levels of the element.
The selenium contents of baby foods tended to show the same
general pattern as those in adult foods (Morris & Levander, 1970;
Arthur, 1972), i.e., meat and cereal products contained the highest
levels and fruit and vegetable products, the lowest. Shearer &
Hadjimarkos (1975) analysed samples of mature human milk from 241
subjects living in the USA and found that the overall mean selenium
content was 0.018 mg/litre. Over 98% of the samples contained
between 0.007 and 0.033 mg/litre. Grimanis et al. (1978) reported
an average of 0.015 mg/litre in 5 samples of mature human milk
collected in Greece. These authors found slightly higher values in
15 samples of human transitional milk (0.016 mg/litre) and
colostrum (0.048 mg/litre). A slightly lower average selenium
concentration of 0.013 mg/litre, was reported for samples of human
transitional milk in New Zealand (Millar & Sheppard, 1972). The
most extreme values for the selenium content of human milk were
reported from China and ranged from 0.0026 mg/litre in areas where
Keshan disease was prevalent to 0.283 mg/litre in high-selenium
areas (Yang et al., 1986).
It can be seen from Table 3 that meat-based infant formulae
have a higher selenium content than formulae based on milk or soy
protein, and that casein-based powdered formulae for special
medical purposes (diet therapy for errors in amino acid metabolism
or malabsorptive states) contain low levels of selenium. Blended
food tube-feeding formulae containing meat tend to have higher
selenium contents than comparable products containing only milk or
casein and soy protein (Table 3). Chemically-defined diets having
egg albumen as the protein source contained more selenium than
diets based on casein hydrolysate, which, in turn, contained more
selenium than diets based on purified amino acids. Total
parenteral nutrition solutions based on casein hydrolysate also
contain more selenium than solutions based on amino acid mixtures,
which contain very low levels of selenium.
Table 3. Selenium content of commercial formula dietsa
-----------------------------------------------------------------
Product Selenium content
(mg/kg wet (mg/kg dry
weight) weight)
-----------------------------------------------------------------
Infant formulae:
milk-based 0.004 - 0.027
soy-based 0.004 - 0.030
meat-based 0.046 - 0.070
casein-based formulae for special 0.048 - 0.120
medical purposes (powders)
Food supplements and tube-feeding
formulae:
milk-based 0.005 - 0.045
soy-casein-based 0.013 - 0.020
blended foods 0.037 - 0.056
Chemically defined diets:
(powders)
egg albumen low residue 0.224 - 0.351
egg albumen moderate nitrogen 0.388 - 0.886
egg albumen high nitrogen 0.503 - 0.570
casein hydrolysate 0.052 - 0.071
amino acid mixture 0.001 - 0.011
-----------------------------------------------------------------
Table 3. (contd.)
-----------------------------------------------------------------
Product Selenium content
(mg/kg wet (mg/kg dry
weight) weight)
-----------------------------------------------------------------
Total parenteral nutrition
solutions:
casein hydrolysate 0.037 0.324
(0.032 - 0.041)
diluted 1:1 with 50% dextrose 0.019 0.093
solution (0.017 - 0.020)
amino acid mixture 0.001 0.010
-----------------------------------------------------------------
a Adapted from: Zabel et al. (1978).
One of the factors that can influence the amount of selenium in
plant foodstuffs is the nature of the plant itself. Plants have
been divided into 3 groups depending on their tendency to take up
selenium from seleniferous soils (Rosenfeld & Beath, 1964):
Group 1 Primary selenium accumulators - can contain very
high amounts of the element (often over 1000
mg/kg dry weight).
Group 2 Secondary selenium accumulators - rarely contain
more than a few hundred mg/kg.
Group 3 Many weeds and most crop plants, grains, and
grasses - rarely contain more than 30 mg/kg, even
when grown on seleniferous soils; when grown on
normal soils generally contain less than 1 mg/kg.
Plants from Groups 1 and 2 do not usually contribute to the
selenium intake of human beings, since they are not consumed
directly by people and are consumed by animals only when other
feeds are not available. However, plants from Group 3 can
contribute large amounts of selenium, if they are grown in
seleniferous areas (see below).
The concentrations of many nutritionally essential trace
elements are known to be decreased by the milling of grains into
cereals (Czerniejewski et al., 1964) and preliminary analysis of
random supermarket samples of cereal foods suggested that refined
products such as white flour or white bread contained less selenium
than whole grain foods such as whole wheat flour or whole wheat
bread (Morris & Levander, 1970). However, a subsequent more
controlled analytical study in which various grain fractions were
taken from the same production batch showed that milling a variety
of grains decreased the concentration of selenium in the consumer
product by only 10 - 30% (Ferretti & Levander, 1974). Thus, the
selenium content of cereal products is somewhat less than that of
the parent grains but the decreases in concentration are not nearly
as great as those observed with other essential trace minerals.
Selenium tends to be localized mainly in the protein fraction
of plant and animal tissues; thus, the protein content of a food
also influences its selenium content. For example, the
concentration of selenium in a series of soybean products prepared
from a given lot of soybeans increased as the protein content of
the product increased (Ferretti & Levander, 1976). However,
protein content is only an expression of the potential of a food to
contain selenium, and a food high in protein will not necessarily
also be high in selenium. Moreover, non-protein seleno amino acids
occur in some foods and thus, the protein content may not always
reflect the selenium content.
4.1.1.2. Effects of natural differences in the availability of
selenium in the environment on levels in food
The most important factor in determining the selenium content
of plant foods and feeds is the amount of selenium in the soil that
is available for uptake by the plant. Sun et al. (1985) reported
that the selenium content of local crops was significantly
correlated with the selenium content of the local soil, the
correlation coefficients of soybean, corn, and rice being 0.9456,
0.9953, and 0.9954, respectively. Since the water-soluble selenium
in the soil was directly correlated with the pH and inversely
correlated with humin and total iron in the soil, the selenium
content of local crops was also influenced by the amount of water-
soluble selenium in the soil. An analytical survey carried out in
the USA demonstrated that the level of selenium in alfalfa plants
varied from less than 0.01 to more than 5.0 mg/kg, and it was
assumed that these levels reflected the amount of available
selenium in the soil (Kubota et al., 1967). A variation in the
selenium content of wheat from 0.04 to 21.4 mg/kg, depending on
where the plant was grown, was reported by Schroeder et al. (1970).
Samples of several foods bought in local markets in Caracas,
Venezuela contained much more selenium than similar foods purchased
in supermarkets in the eastern USA (Table 4). The likely source of
selenium in the milk, eggs, and meat from Caracas is sesame cake,
since sesame is produced mostly in the seleniferous area of
Venezuela and the pressed cake is widely used as an ingredient in
animal feed. Selenium levels as high as 14 mg/kg in corn and 18
mg/kg in rice have also been observed in certain food samples taken
from high-selenium regions in Venezuela (Jaffe, 1976). These
concentrations of selenium are as high as those reported in foods
from seleniferous zones of the USA (Smith & Westfall, 1937).
Great extremes in the selenium content of staple foods have
also been reported recently from China (Yang et al., 1983). For
example, samples of corn, rice, and soybeans, taken from a high-
selenium area with a history of human intoxication reported as
chronic selenosis (section 8.1.1.1) contained average selenium
levels of 8.1, 4.0, and 11.9 mg/kg, respectively, whereas samples
of the same staples collected in a low-selenium area where Keshan
disease was prevalent (a human selenium-deficiency disease)
(section 8.2.2) contained average selenium levels of only 0.005,
0.007, and 0.010 mg/kg, respectively (Table 5). A low selenium
content in food has also been reported in other countries with low
selenium soils, such as New Zealand and Finland. Typical ranges
for selenium contents in food in such countries include (mg/kg wet
weight): liver, kidney, and seafood, 0.09 - 0.92; muscle meats,
0.01 - 0.06; cereal and cereal products, 0.01 - 0.07; milk, <
0.01; and fruits and vegetables, < 0.01 - 0.02 (Koivistoinen,
1980; Thomson & Robinson, 1980). Samples of staple foods collected
in areas where soils contained intermediate levels of selenium also
contained intermediate levels of selenium.
Table 4. Comparison of selenium contents
of selected foods available in Caracas,
Venezuela, and Beltsville, Maryland, USA
(mg selenium/kg wet weight)a
-----------------------------------------
Food Caracas Beltsville
-----------------------------------------
Powdered milk 0.417 0.169
Whole milk 0.115 0.012
American-type cheese 0.425 0.090
Swiss-type cheese 0.382 0.104
Pork 0.833 0.209
Chicken 0.702 0.106
Egg 1.520 0.116
-----------------------------------------
a From: Mondragon & Jaffe (1976).
As might be expected, the selenium contents of food products of
animal origin depend heavily on the amount of naturally-occurring
selenium in the feed given to the animal. In the USA, it was shown
that the selenium content of swine muscle was highest in areas
known to have a high level of available selenium in the soil and
lowest in areas in which the available soil-selenium was low (Ku et
al., 1972). This indicates that selenium is readily passed up the
soil-plant-animal food chain to human beings.
4.1.1.3. Man-induced changes in selenium levels in food
(a) Human activities that increase selenium levels
Perhaps the most direct way that man's activities can increase
the selenium content of the food supply is the deliberate addition
of selenium to the feeds of poultry and certain livestock. In some
countries, this is now accepted practice to prevent the occurrence
of selenium-deficiency diseases, many of which cause significant
economic losses to farmers throughout the world. In the USA, for
example, farmers are permitted to add 0.1 mg selenium/kg (as sodium
selenite or selenate) complete feed for beef and dairy cattle,
sheep, chickens, ducks, swine (0.3 mg/kg in starter and prestarter
rations), and 0.2 mg/kg for turkeys (US Department of Health and
Human Services, Food and Drug Administration, 1984; Subcommittee on
Selenium - Committee on animal Nutrition, 1983). It has been shown
that the edible tissues of poultry, swine, and sheep, fed diets
fortified with inorganic selenium salts at the regulated levels,
did not contain any more selenium than the tissues of animals fed
diets, naturally adequate in selenium (Allaway, 1973; Ullrey et
al., 1977). The homeostatic mechanisms that appear to limit the
concentrations of selenium in the edible tissues of animals fed
certain levels of sodium selenite are discussed further in section
6.
Table 5. Selenium contents of staple foods grown on soils in areas of China with excess,
moderate, and deficient levels of seleniuma
-----------------------------------------------------------------------------------------
Place Corn Rice Soybean
Number Se content Number Se content Number Se content
of (mg/kg) of (mg/kg) of (mg/kg)
samples samples samples
-----------------------------------------------------------------------------------------
High-selenium area 44 8.1 22 4.0 17 11.9
with a history of (0.5-28.5)b (0.3-20.2)b (5.0-22.2)b
intoxication reported
as chronic selenosis
High-selenium area 2 0.57 2 0.97 2 0.34
reported to be without
selenosis
Moderate-selenium- 82 0.036 76 0.035 31 0.069
adequate area (± 0.056) (± 0.027) (± 0.076)
(Beijing)
Low-selenium area 10 0.009 32 0.022 - -
(± 0.009) (± 0.009)
Low-selenium area 195 0.005 49 0.007 150 0.010
with Keshan disease (± 0.003) (± 0.003) (± 0.008)
-----------------------------------------------------------------------------------------
a Adapted from: Yang et al. (1983).
b Mean ± SD or range shown in parenthesis.
Finland is the first country to decide to increase the selenium
content of Finnish feed and food by the addition of sodium selenate
to fertilizers, to be used in the whole country at a concentration
of 16 or 6 mg/kg for cereal and grassland crops, respectively
(Koivistoinen & Huttunen, 1985). The manufacture of these
selenized fertilizers began in the summer of 1984 and they will be
used at application rates of 10 g selenium/ha per growing season.
It is anticipated that the added selenium in the fertilizers will
be transported to the human food chain, and the selenium level of
the cereal and grassland crops will be raised to 0.1 and 0.15 -
0.20 mg/kg (dry basis), respectively.
Another potential way in which the level of selenium in the
food chain might be increased by man's activities is the proposed
use of selenium-bearing fly ash as a soil supplement. Furr et al.
(1977) analysed cabbages grown on potted soil supplemented with
several different fly ashes and found that the selenium levels in
the cabbages were closely correlated with those in the respective
fly ashes in which the plants were cultured. The ready
bioavailability to animals of the selenium in plants grown on fly
ash was demonstrated in a study (Stoewsand et al., 1978) in which
Japanese quail were fed a complete diet containing 60% winter wheat
that had been grown to maturity on either soil or a deep bed of fly
ash. The soil contained 2.1 mg selenium/kg and the wheat grown
thereon contained 0.02 mg/kg, a level typical of wheat from
selenium-deficient areas. The fly ash contained 21.3 mg
selenium/kg, and the wheat grown on it contained 5.7 mg/kg, a level
sometimes found in wheat from naturally seleniferous areas. The
levels of selenium in the tissues of the quail fed the wheat grown
on fly ash were much higher than those of quail fed the wheat grown
on soil (Table 6). Moreover, the selenium contents of eggs from
quail fed the wheat grown on fly ash were 3.5 mg/kg in the yolk and
almost 10 mg/kg in the white compared with 0.5 and 0.2 mg
selenium/kg, respectively, in eggs from quail fed the control
wheat. Obviously, the use of selenium-bearing fly ash as a soil
supplement to provide nutritionally desirable levels of selenium in
plants should be carried out with care, since inappropriate use
could lead to an unwanted build-up of selenium residues in the food
chain. Also, the application of fly ash to soil at rates
sufficient to correct selenium deficiency in animals may damage the
soil.
Table 6. Selenium in tissues of male Japanese quail fed winter
wheat grown on soil or fly asha
-------------------------------------------------------------------
Wheat grown Tissue selenium
on: brain heart kidney liver muscle
-------------------------------------------------------------------
(mg/kg dry weight)
Soil 0.8 ± 0.1 0.7 ± 0.2 3.6 ± 0.4 1.6 ± 0.0 0.3 ± 0.2
Fly ash 3.4 ± 1.6 4.4 ± 0.7 9.5 ± 1.1 12.7 ± 2.4 4.1 ± 0.6
-------------------------------------------------------------------
a From: Stoewsand et al. (1978).
The possibility has been raised that some selenium may be added
to the food supply as a result of atmospheric contaminants from
the burning of fossil fuel or industrial emissions settling out on
the leaves of plants or on the surface of the soil. The
geographical pattern of selenium levels in rain-water from Denmark
or the USA implicates airborne selenium from the industrial and
domestic uses of fossil fuel as sources (Kubota et al., 1975).
Total industrial emissions of selenium in the USA for the year 1970
were estimated to be about 1.1 million kg, 62% of which was derived
from the burning of coal (US NAS/NRC, 1976). This level of
industrial emission of selenium may be compared with a projected
annual use of selenium as a feed additive for chickens and swine in
the "low-selenium" areas of the USA of 6000 kg (US FDA, 1974). But
there is no evidence that the selenium deposited in rainwater has
any influence on the concentration of selenium in forage plants
grown in Denmark or in the heavily industrialized northeastern USA
(Kubota et al., 1975). Furthermore, selenium deficiency has been
observed in farm livestock grazing near coal-burning electric power
stations (Anonymous, 1975). This suggests that airborne selenium
exists in the form of inert elemental selenium, insoluble selenide
salts, or as selenium dioxide, which would be tightly bound by
acidic, iron-bearing soils. In any case, the selenium would not be
available for uptake by plants; thus, there would appear to be
little prospect of adding appreciable selenium to the food supply
via atmospheric contamination.
(b) Human activities that decrease selenium levels in the food
chain
The metabolic antagonism between sulfur and selenium (Levander,
1976a) led early workers to suggest that borderline selenium
deficiency might be exacerbated by sulfate fertilization (Schubert
et al., 1961). More recent research has demonstrated that sulfate
fertilization can result in decreased selenium concentrations in
forage plants (Pratley & McFarlane, 1974; Westermann & Robbins,
1974). In many cases, this decrease can be largely explained by a
dilution effect caused by a growth response of the plant to the
sulfate fertilization, but decreases in selenium concentration
independent of crop yield have also been reported. Such decreases
could be the result of competitive interference by sulfate with the
uptake of selenate by plants. The use of phosphate fertilizers in
parts of Australia and New Zealand has resulted in an apparent
increase in the incidence of selenium deficiency in livestock
(Judson & Obst, 1975), but others have reported increases in the
selenium content of forages after fertilization with phosphorus
(Robbins & Carter, 1970; Carter et al., 1972). These conflicting
results may be due to differences in the selenium content of the
phosphatic rock from which the fertilizers were prepared.
Another possible way in which man's activities might decrease
selenium in the food chain or render it less available, is through
heavy metal pollution. For example, silver has been demonstrated
to antagonize selenium in a wide variety of situations (Diplock,
1976). In an area where there has been a large amount of silver
mining activity, muscular dystrophy of the type usually associated
with selenium deficiency has been reported in calves fed a ration
based on milk powder, even though the selenium content of the milk
seemed to be adequate. Apparently, the milk powder was derived
from cows that grazed land containing high levels of silver
residues from the old mining industry. Further research is needed
to establish fully the role of silver in potentiating these field
cases of apparent selenium deficiency. Because of the many
interactions of selenium with other environmental pollutants such
as mercury, cadmium, or arsenic (section 7), it is possible that
other cases of heavy metal-induced selenium deficiency will be
discovered.
The influence of cooking on the selenium content of foods was
determined because of the well-known instability and volatility of
many selenium compounds. Certain vegetables that normally contain
high levels of selenium such as asparagus or mushrooms, lost up to
40% of their selenium content as a result of boiling (Higgs et al.,
1972). Majstruk & Suchkov (1978) reported that up to 50% of the
original selenium was lost from vegetables and dairy products
during cooking; the addition of salt and acid pH or extended
cooking, particularly promoted such losses. But, other typical
cooking procedures such as boiling cereals, baking poultry or fish,
or broiling meats had little effect on selenium levels in the food
(Higgs et al., 1972). Similar results were observed by others for
baking fish or broiling meats, though some decrease in selenium
concentration was caused by frying organ meats or fish (Ganapathy
et al., 1975). Thompson et al. (1975) determined the selenium
contents of cooked food composites and found a rough agreement with
the calculated values based on unprepared foods from which they
were derived. On the basis of all these studies, it can be
concluded that usual cooking procedures cause little decrease in
the selenium content of most foods.
4.1.2. Chemical forms of selenium in food
There is little information on the chemical forms of selenium
that occur in human food (Levander, 1986). Cappon & Smith (1982)
reported that canned tuna fish contained variable percentages of
hexavalent selenium, ranging from 7.6 to 44.8%, which was
independent of the total selenium content. The other forms of
selenium in the tuna were di- and quadrivalent selenium. On an
average percentage basis, hexavalent selenium was more easily
extractable by water than di- or quadrivalent selenium. In
recently canned samples, an average of 55.6% of the total selenium
content was water-extractable. However, for the older samples, the
corresponding average extractable level was 48.2%. The results
suggest that sample storage may influence the chemical form of
selenium in canned tuna. Olson et al. (1970) found that, in a
certain portion of high selenium wheat (the pronase hydrolysate of
gluten), about half of the selenium was in the form of
selenomethionine. About 15% of the selenium in water extracts of
seleniferous cabbage leaves was in the form of selenomethionine,
but appreciable amounts of other selenium compounds were also
present (Hamilton, 1975). The chemical forms of selenium in food
are likely to affect the bioavailability of selenium (section 7.2.2
and 8.2.3).
4.2. Drinking-Water
Analysis of samples taken from various public water-supply
systems in the USA showed that less than 0.5% contained selenium
levels that exceeded the US PHS limit of 10 µg/litre (Taylor, 1963;
Lakin & Davidson, 1967; McCabe et al., 1970). Samples from 1280
central water sources providing water for 6.5 million Bulgarians
contained less than 2 µg selenium/litre (Gitsova, 1973). Tap water
from Stockholm, Sweden contained only 0.06 µg/litre (Lindberg,
1968) and tap and mineral waters from Stuttgart, Federal Republic
of Germany contained 1.6 and 5.3 µg/litre, respectively
(Oelschlager & Menke, 1969). Surface water sources in different
subregions of the Cernovici region of the Ukrainian SSR contained
selenium levels ranging from 0.09 ± 0.01 to 3.00 ± 0.40 µg/litre;
ground-water levels ranged from 0.07 ± 0.0 to 4.00 ± 0.85 µg/litre
(Suchkov & Kacap, 1971). A few tenths to several µg per litre were
reported in non-seleniferous regions of the USSR, the maximum
reported value being 5.1 µg/litre. In Argentina, the selenium
content of 22 surface waters varied from < 2 to 19 µg/litre with a
median value of 3 µg/litre (WHO, 1984).
Elevated levels of selenium (> 100 µg/litre) can be found in
seeps, springs, and shallow wells, though waters from deep wells
contain only a few µg/litre (US NAS/NRC, 1976). Highly variable
amounts of selenium were reported in wells from seleniferous areas
in the USA ranging from non-detectable levels to 330 µg/litre
(Smith & Westfall, 1937). Some well waters contained enough
selenium to be considered as poisonous for man or livestock and
loss of hair and nails in children was attributed to selenosis, but
the evidence for this was not considered convincing (US NAS/NRC,
1976).
The average selenium content of 11 samples of drinking-water
taken from a high-selenium area in China with a history of disease
reported as chronic selenosis was 54 µg/litre (Yang et al., 1983).
Four of these samples were surface water from a village with
previous heavy intoxication and these averaged 139 (117 - 159)
µg/litre. Such water would contribute substantial amounts of
selenium, but would still comprise a small fraction of the total
selenium intake in this area, since the contribution from food was
estimated to be about 5000 µg/day (section 5.1.1.1). The other 7
samples of drinking-water originated from several sources and
averaged only 5 µg/litre.
Rosenfeld & Beath (1964) concluded that selenium does not occur
in water in sufficient amounts to produce selenium toxicity in man
or animals, except in isolated cases. This was confirmed and
expanded in the statement by US NAS/NRC (1976, 1980) that waters
are rarely a significant source of selenium from either a
nutritional or a toxicity point of view. The low concentrations of
selenium in drinking-water are probably the result of several
mechanisms that act to decrease the selenium content of waters
(section 2.1).
4.3. Air
Selenium levels in the air breathed by the general population
are probably well below 10 ng/m3, on average (US NAS/NRC, 1976).
Hashimoto & Winchester (1967) found 0.3 - 1.6 ng airborne
selenium/m3 in an urbanized area. Levels of 3.6 - 9.7 ng
selenium/m3 were detected by Pillay et al. (1971), who showed that
over half of the selenium was not retained on filters able to trap
particulates greater than 0.1 µg in diameter. Zoller & Reamer
(1976) concluded that atmospheric levels of selenium in most urban
regions vary from 0.1 to 10 ng/m3. Selenium levels in air around
industries that use selenium can be higher (section 3.2.2), perhaps
of the order of a few µg/m3. Work-place air in selenium industries
apparently contained mg levels of selenium/m3 in the past, but more
recent measurements have indicated lower levels (section 5.2).
5. HUMAN EXPOSURE
5.1. Estimate of General Population Exposure
As discussed in section 4, selenium levels in air are low in
areas without selenium-emitting industries and are not likely to
exceed 10 ng/m3. Assuming that a person respires 20 m3 air daily,
the contribution to daily selenium intake via this route would be
0.2 µg or less. Since this intake is much lower than that through
food (see below), airborne selenium makes a negligible contribution
to the average daily selenium intake of the general population.
However, levels of selenium may be somewhat higher in the
atmosphere around some industrial plants using selenium (section
3.2.2). If it is assumed that 3 µg selenium/m3 is found in the air
near these industries, then exposure to selenium via air would be
60 µg/day.
Other possible exposures of the general population to selenium
via the air include contributions from house dust and tobacco
smoke. Lakin & Davidson (1967) reported that house or office dust
could contain up to 10 mg selenium/kg. Although no data are
available to provide an estimate of human exposure to selenium from
this source, the possibility of such exposure should be considered
because of the increased interest in the quality of indoor air.
Olsen & Frost (1970) found an average of 0.08 mg selenium/kg (range
0.03 - 0.13 mg/kg) in a variety of cigarette tobaccos. If it is
assumed that a cigarette contains 1 g tobacco and that all the
selenium in tobacco is volatilized and inhaled during smoking, it
can be calculated that a person smoking one pack of 20 cigarettes
per day would inhale an average of 1.6 µg from this source.
Most drinking-water supplies contain only small quantities of
selenium, except possibly for those in certain seleniferous areas.
As discussed in section 4.2, most public water supplies contain
much less than 10 µg selenium/litre. Assuming that a person drinks
2 litres of water daily, the intake via this route would be only a
few µg. While this is considerably higher than the intake via air,
in most areas of the world, this is still a small fraction of the
daily intake from food.
5.1.1. Food
5.1.1.1. Geographical variation
Both the amount and the bioavailability of dietary selenium are
determinants of its biological effects. Bioavailability is be
considered in sections 7.2.2 and 8.2.3. The estimated daily intake
of selenium from dietary sources by adult human beings varies
considerably in different parts of the world. The greatest
extremes in intake have been found in China (Table 7) where both
selenium toxicity and deficiency have been reported (section
8.1.1.1, 8.2.2). An average selenium intake of 4990 µg/day was
estimated in a high-selenium area of China with a history of
intoxication reported as chronic selenosis, whereas an average
intake of only 11 µg/day was estimated in an area of China where
Keshan disease, a cardiomyopathy related to low selenium status,
was reported. Intermediate intakes of selenium were found in areas
of China that were not affected with either selenosis or Keshan
disease. It should be pointed out that the dietary-selenium intake
in the high-selenium area with a history of intoxication reported
as selenosis did not overlap with that in the high-selenium area
reported to be without selenosis. Likewise, the dietary-selenium
intake in the low-selenium area with Keshan disease did not overlap
with that in the moderate-selenium area (Beijing).
Table 7. Daily selenium intake of residents living in high-, medium-, and low-selenium
areas of Chinaa
---------------------------------------------------------------------------------------
Number Daily selenium intake Se intake from staple
Place of minimum maximum average cereals as % of total
subjects (µg) daily intake
---------------------------------------------------------------------------------------
High-selenium area with 6 3200 6690 4990 28-70%
a history of intoxication
reported as chronic
selenosis
High-selenium area 3 240 1510 750 25-45%
reported as without
selenosis
Moderate-selenium area 8 42 232 116 various sources
(Beijing)
Low-selenium area with 13 3 22 11 mainly from cereals
Keshan disease
---------------------------------------------------------------------------------------
a Adapted from: Yang et al. (1983).
Less extreme, but still widely diverse intakes of selenium have
been observed in other parts of the world (Table 8). The selenium
intake calculated in New Zealand, a country with low levels of
selenium in the soils in certain areas, averaged 30 µg/day (Thomson
& Robinson, 1980; Watkinson, 1981). Similar low intakes have been
reported by other research workers in New Zealand. For example,
Stewart et al. (1978) showed that the mean dietary-selenium intake
of 4 New Zealand women consuming normal diets ad libitum was
24.2 µg/day, and Griffiths (1973) reported that the daily intake of
selenium by 13 young New Zealand women varied from 6 to 70 µg.
Daily intakes of 30 µg or more in the latter group were associated
with the inclusion of liver, kidney, or fish in the diet. A low
average dietary-selenium intake (30 µg/day) has also been reported
in Finland, another country known to have soils low in selenium
(Varo & Koivistoinen, 1980). Low levels of dietary selenium may
also occur in Italy (Rossi et al., 1976) and Egypt (Waslien, 1976).
The average daily intake of selenium in the United Kingdom was
estimated to be 60 mg (Thorn et al., 1978). In one study, the
dietary-selenium intake in Japan was estimated to be 88 µg/day
(Sakurai & Tsuchiya, 1975). A higher estimate in another study was
arrived at largely because higher selenium contents of food staples
were used in the calculations (Yasumoto et al., 1976). The
estimated dietary intake of selenium in North America ranged from
98 to 224 µg/day. A "Market basket" survey carried out in the USA
from 1974 to 1982 indicated an average overall selenium intake of
108 µg/day (Pennington et al., 1984). An earlier survey had
revealed regional differences in intake, with people living in
western parts of the country consuming 1.3 times as much selenium
as people in the northeastern part of the country (US FDA, 1975).
Duplicate plate analyses of self-selected diets in Maryland, USA
gave a mean intake of 81 ± 41 µg/day (Welsh et al., 1981). The
daily intake in Venezuela was estimated to be 326 µg, but this
figure requires cautious interpretation because North American food
consumption patterns were used in its derivation (Mondragon &
Jaffe, 1976).
5.1.1.2. Food habits (consumption patterns)
Because of the wide variations in the selenium content of
various food groups in different countries, it is possible that
certain food habits or preferences could play an important role in
determining whether a person is at risk with regard to a deficient
or excessive intake of selenium. Persons with a preference for
foods such as fish may consume high levels of selenium in their
diet. Sakurai & Tsuchiya (1975) estimated that Japanese eating
large amounts of seafish may ingest as much as 500 µg of selenium
daily. People residing in seleniferous agricultural zones may
consume large amounts of selenium if locally-produced foods
constitute the bulk of their diet. In Venezuela, for example, corn
and rice samples from high selenium areas contained as much as 14
and 18 mg selenium/kg, respectively (Jaffe, 1976), and values as
high as 180 mg/kg have been reported in wheat from Colombia
(Ancizar-Sordo, 1947). If it is assumed that some segments of the
population living in such seleniferous regions consume, under
certain conditions, about half of their diet in the form of rice or
corn with a similar selenium content to that described above, the
daily intake of selenium from dietary sources could exceed 7000 µg.
However, it should be pointed out that only a few samples from the
high-selenium areas showed these extreme high levels. Obviously,
vegetarian diets could vary tremendously in selenium content.
Ganapathy & Dhanda (1976) showed that a vegetarian diet in the USA
supplied 84 µg selenium/day.
If a particular staple food constitutes a large fraction of the
diet of a population, then the selenium content of that food will
have a great influence on the overall dietary-selenium intake. For
example, one estimated daily dietary intake of selenium in Japan
(Sakurai & Tsuchiya, 1975) of 88 µg, was based on selenium levels
in rice and soybeans of 0.05 and 0.02 mg/kg, respectively, whereas
another estimated intake, 208 µg, was based on rice and soybean
selenium levels of 0.220 and 0.234 mg/kg, respectively (Yasumoto et
al., 1976). Low levels of selenium in rice have been reported in
Bangladesh, and it would be particularly interesting to study the
population there, as they depend on rice as a staple food, but they
have a recognized poor dietary vitamin E status (Bieri & Ahmad,
1976).
Table 8. Estimated human dietary intake of selenium (µg/day)a
----------------------------------------------------------------------------------------------------
Food New Zealand Finland United Japanf Canadag USAh USA Venezuelaj
Dunedinb Hamiltonc 1975d 1979d Kingdome South
Dakotai
----------------------------------------------------------------------------------------------------
Plant
vegetables,
fruits,
and sugars 1 2 1 1 3 6 1-9 5 10 15
cereals 4 3 3 25 30 24 62-113 45 57 88
Animal
dairy
products, 11 11 7 13 5 2 5-28 13 48 70
eggs
meat, fish 12 16 19 19 22 56 25-90 69 101 153
Total 28 32 30 50-60 60 88 98-224 132 216 326
----------------------------------------------------------------------------------------------------
a From: Robinson & Thomson (1983).
b From: Thomson & Robinson (1980).
c From: Watkinson (1981).
d From: Varo & Koivistoinen (1981).
e From: Thorn et al. (1978).
f From: Sakurai & Tsuchiya (1975).
g From: Thompson et al. (1975).
h From: Watkinson (1974) & Levander (1976b).
i From: US NAS/NRC (1980).
j From: Levander (1976b).
The importance of the selenium content of a particular staple
in the food supply of persons dependent on a monotonous diet
consisting of locally-produced foods was recently pointed out in
connection with the occurrence of Keshan disease (section 8.2.2) in
China (Yu, 1982). In the years in which Keshan disease was endemic
in the Fuyu county of Heilongjiang province, about 90% of the diet
consisted of maize, whereas, in the years in which Keshan disease
was not endemic, only about 30% of the diet was maize with much of
the rest coming from millet and wheat. Thus, it seems possible
that over-reliance on this single staple food during certain years
helped to precipitate Keshan disease in this area.
Recently, there has been considerable interest in some
countries in the use of textured soy protein products as
substitutes for meat products. Since meat products are such good
dietary sources of selenium, the possible impact of this trend on
the selenium intake of human beings was examined. Some soy protein-
based meat analogues contained as much selenium as meat products,
but others did not (Ferretti & Levander, 1976). These results show
that meat analogues made from soy or other plant proteins are not
consistent sources of selenium, since these plant-based products
will vary in selenium content depending on where the plant is
grown. In contrast, meat products are more reliable dietary
sources of selenium, because they contain a minimum quantity
compatible with animal life.
5.1.1.3. Elderly people
Thomson et al. (1977a) studied a group of 48 elderly people
(mean age of 78 years) in New Zealand and found that their blood-
selenium levels and red cell glutathione peroxidase (EC 1.11.1.9)
activity were lower than those in 12 young adult controls. It was
not possible for the authors to conclude from the data whether the
depressed blood-selenium levels and enzyme activity were due to
decreased selenium intake or were a general part of the aging
process. Abdulla et al. (1979) analysed 7-day pooled dietary
composites of 20 pensioners in Sweden and found that the average
daily selenium intake was 30.8 µg with a range of 8.7 - 96.3
µg/day.
5.1.1.4. Infants and children
The selenium intake of infants is of interest because of their
rapid growth rate and their heavy reliance on milk, a food that has
a highly variable selenium content, depending on its geographical
origin (Table 9). Millar & Sheppard (1972) assumed that human
and cow's milk in New Zealand contained 0.010 and 0.005 mg
selenium/litre, respectively, and calculated that the selenium
intake of infants for the first month of life would be 5.0 or
2.1 µg/day, depending on whether human or cow's milk was fed.
Williams (1983) measured the selenium content of mature mother's
milk from 10 New Zealand women and calculated an average daily
selenium intake of 5 - 6 µg for an infant consuming 700 ml milk.
If the value for the selenium content of Venezuelan cow's milk
shown in Table 9 is typical, it can be calculated that the selenium
intake of infants in that country would be about 58 µg/day during
the first month of life. The most extreme values for the selenium
content of human milk are from low- and high-selenium areas of
China (Yang et al., 1986) (section 4.1.1.1). For infants consuming
750 ml milk daily, these milks would provide 2 µg and 212 µg
selenium, respectively.
Table 9. Estimated infant daily intake of selenium
from dietary sources in North America (6-month-old,
6.5-kg child)a
-----------------------------------------------------
Daily Selenium Selenium
Food consumption content intake
(g) (mg/kg) (µg/day)
-----------------------------------------------------
Milk 824 0.013 11
Orange juice 122 0.014 2
Dry mixed cereal 10 0.540 5
Egg yolk 17 0.437 7
Strained meat 28 0.097 3
Strained fruit 57 0.002 -
Strained vegetable 57 0.003 -
Total selenium intake 28
-----------------------------------------------------
a Adapted from: Levander (1976b).
It was shown that milk continues to furnish a sizeable portion
of an infant's estimated daily selenium intake of 28 µg, in the
USA, after the child starts to eat solid foods (Table 9). The
estimated daily intake of selenium by 3-month-old infants in the
Federal Republic of Germany was 11 µg (Lombeck et al., 1975) and a
"market basket" survey indicated that the intake of 6-month-old
infants in the USA was about 12 µg/day (Harland et al., 1978). On
the basis of a caloric intake of 700 Kcal/day, infants in the USA
consuming formula diets based on milk, soy protein, casein, or
meat, ingested 8.5, 9.5, 12.6, or 31.5 µg/day, respectively (Zabel
et al., 1978).
As infants grow up in New Zealand, they continue to ingest
relatively low levels of selenium, since the estimated daily intake
over the age span of 6 months to 5 years was 5 - 8 µg (McKenzie et
al., 1978).
5.1.1.5. Special medical diets
Infants and children suffering from metabolic diseases such as
phenylketonuria (PKU) or maple syrup urine disease (MSUD) are fed
synthetic diets that contain little selenium for the first 10 or 12
years of their lives. In New Zealand, the mean daily selenium
intake of 12 subjects consuming such synthetic diets was 5 ± 2 µg
with little difference between the older children and the infants
(McKenzie et al., 1978). The selenium content of diets of 3-month-
old infants with PKU and MSUD in the Federal Republic of Germany
amounted to 4.7 and 5.6 µg/day, respectively (Lombeck et al.,
1975).
A wide variation in the selenium contents of special-purpose
medical diets for adults is related to the source of protein in the
diet (Zabel et al., 1978). On the basis of an intake of 2000
Kcal/day, adults consuming food supplements or tube-feeding
formulae would ingest 29, 47, or 98 µg selenium/day, depending on
whether the diet contained soy protein and casein, milk protein and
casein, or blended foods. At a similar caloric intake, chemically-
defined diets based on egg albumen provided 225 µg/day, whereas
diets based on casein hydrolysate or amino acid mixtures provided
only 28 and 1.5 µg/day, respectively. Total parenteral nutrition
solutions based on casein hydrolysate and amino acid mixtures also
furnished low amounts of selenium of only 32 and < 1 µg/day,
respectively. When levels of selenium supplied by special
therapeutic diets were studied in the Chernovitsi region of the
Ukrainian SSR, selenium intakes were found to vary between 99 ± 6
and 121 ± 8 µg/day (Majstruk & Suchkov, 1978).
5.2. Occupational Exposure
5.2.1. Levels in the workplace air
The main pathway of human occupational exposure to selenium is
through the air. Rarely, and under special circumstances, direct
contact may be of importance, i.e., when cutaneous absorption might
be facilitated by the local irritation and skin damage caused by
vesicant selenium compounds.
Various mechanical processes connected with the mining of
seleniferous ores or the grinding of selenium compounds can
contribute selenium-containing dusts to the atmosphere. In other
industrial activities, the amount of selenium released into the air
depends on the temperature to which it is heated and on the area
available for sublimation and/or vaporization. It is well
established that heating amorphous selenium below its melting point
results in its sublimation. At temperatures of 170 - 180 °C,
traces of selenium can be detected in the air and, at temperatures
of 230 - 240 °C, selenium dioxide is released. Heating of
amorphous selenium and selenium dioxide resulted in the release of
substantial quantities of selenium into the air, e.g., from a
surface of 10 cm2, 2.6 - 3.75 mg was released after 20 min at 230 -
240°C and 23 - 31.6 mg was released after 20 min at 350 - 360 °C
(Izraelson et al., 1973).
Little quantitative information is available in the literature
on actual levels of human exposure to selenium in industry. The
levels of selenium in the workplace depend not only on the nature
of the process involved but also on the control technology in any
given industry. Thus, it can be expected that analyses carried out
several decades ago yielded higher values than analyses carried out
more recently. In 1948, Filatova reported air levels of selenium
and selenium dioxide of 20.6 - 24.8 mg/m3 and 0.46 - 0.78 mg/m3,
respectively, in working zones of selenium-heating processes in a
selenium rectifier plant, whereas levels of 0.55 - 1.1 mg/m3 and up
to 0.11 mg/m3, respectively, were associated with the process of
disc smearing (Filatova, cited in Izraelson et al., 1973). In the
production of selenium-containing photoelements by the vacuum
application of a photosensitive layer, selenium and selenium
dioxide levels did not exceed 2.0 mg/m3 and 0.1 mg/m3, respectively
(Sverdlina & Maslennikova, 1961). Selenium grinding, sulfuric acid
treatment, and cathode alloy coating resulted in air-selenium
levels ranging from 0.133 to 2.0 mg/m3 (Izraelson et al., 1973). A
highly-dispersed aerosol selenium condensate with a particle size
of 0.5 - 2 µm was produced at a level of 0.88 - 6.13 mg/m3, as a
result of the manufacture of selenium-containing steels (Ershov,
1969). In the production of rare metals, the air in industrial
premises can contain as much as 0.4 - 750 mg metallic dust/m3,
which, in addition to other elements, contains from 0.8 - 15.7%
selenium (Burchanov, 1972). During the 1950s, Glover (1967)
carried out an extensive survey in which air-selenium levels were
determined in areas of a selenium rectifier plant in which the
workers had been found to have high urinary-selenium values. In
general, the highest air levels of 1.5 - 5.2 mg selenium/m3 were
found in relation to the grinding process. With the exception of
certain "special processes", air levels in the plant due to all the
other manufacturing activities were less than 0.5 mg/m3. In one
case, a level of 21.3 mg/m3 was observed, but no additional details
were given. In this rectifier plant, a process, in which the
elemental selenium was heated, was used for preparing the selenium
mixture that was later applied to the metal plate. For this
reason, the selenium levels in the air are likely to have been
higher than in other factories where the selenium was deposited
from aqueous solution. In another selenium rectifier plant, air
levels of between 7 and 50 µg selenium/m3 were reported
(Kinnigkeit, 1962). However, in this case, no correlation was
observed between the air levels in the workplace and the selenium
levels in the blood of the workers. Kinnigkeit felt that the air-
selenium levels were not high enough to account for the high
urinary-selenium levels in the workers. He suggested that the
discrepancies might be due to incomplete collection of selenium
during air sampling, transient peaks of selenium exposure that were
not detected by short-term sampling, or contamination of hands,
body, and clothing with selenium, which was then transferred to the
mouth as a result of eating or smoking. Another possible
explanation is that measurements of the levels in the workroom air
did not accurately reflect levels of selenium in the actual
breathing zone of the workers.
Low air levels of selenium have been observed in factories
involved with the use of selenium in certain photocopying devices.
In this type of manufacturing, "clean room" techniques are used,
because strict dust control is needed to produce the quality of
photoreceptor surfaces required. Cannella (1976) reported air
levels of selenium ranging from 6 to 91 µg/m3 in various locations
in a selenium alloy plant that made such photoreceptor products.
5.2.2. Biological monitoring
Although selenium levels in the work-place air have been
determined in several instances, these measurements cannot be used
to draw any conclusions about the exposure history of individual
workers. Glover (1967), who estimated occupational exposure to
selenium indirectly by determining selenium levels in the urine of
workers, acknowledged the limitations associated with monitoring
based on grab samples of urine. The Working Group also recognized
the inadequacy of urinary-selenium concentrations as a monitoring
tool. However, it appears that the use of a more rigorous
programme (e.g., total 24-h urine collections) would have resulted
in losing the cooperation of the workers involved. A tentative
maximum allowable concentration of selenium in urine of 0.1
mg/litre was proposed by Glover (1967). The urine of the workers
was analysed at 3-month intervals, and any employee whose urinary-
selenium value exceeded the limit was transferred to a process not
involving selenium. The urinary-selenium levels of these
individuals were tested weekly.
Two primary factors were responsible for setting the maximum
allowable concentration for selenium in urine at 0.1 mg/litre
(Glover, 1970). First, a limit lower than this would have resulted
in a certain number of "false positives", since selenium levels in
urine are often this high in people not industrially exposed to the
element. Second, it was found that values below 0.1 mg/litre were
invariably accompanied by air levels in the work-place of less than
0.1 mg/m3, which was below the threshold limit value of 0.2 mg/m3
for elemental selenium and its common inorganic compounds (ACGIH,
1971). It must be emphasized that urinary selenium cannot be used
to monitor exposure to such dangerous selenium compounds as
hydrogen selenide, selenium oxychloride, or certain organic
selenium compounds because severe damage to the lungs or skin would
have occurred by the time that the urinary-selenium values were
raised (Glover, 1976).
6. METABOLISM OF SELENIUM
The metabolism of selenium has been most completely studied in
animals and data from human investigations are limited. Thus, in
the following sections, pertinent information from selected animal
studies is presented followed by available information from
controlled human studies.
6.1. Absorption
Since food is the primary environmental medium through which
man and animals are exposed to selenium, most data concerning
selenium absorption deal with the gastrointestinal pathway. Much
less is known about the absorption of selenium through the lungs or
skin.
6.1.1. Gastrointestinal absorption
6.1.1.1. Animal studies
The intestinal absorption of soluble selenium compounds by rats
is highly efficient. It has been shown that these animals absorbed
92, 91, and 81% of doses of selenite, selenomethionine, and
selenocystine, respectively (Thomson & Stewart, 1973; Thomson et
al., 1975a). In a study on rats by Whanger et al. (1976a), the
greatest absorption of selenite or selenomethionine occurred from
the duodenum, with slightly less absorption from the jejunum or
ileum. Virtually no absorption occurred from the stomach. The
absorption of selenite by rats does not appear to be under
homeostatic control, since 95% or more of the dose was absorbed,
regardless of whether the animals were fed a selenium-deficient
diet or a diet containing mildly toxic levels of selenium (Brown et
al., 1972). Wright & Bell (1966) found that selenite was absorbed
from the gastrointestinal tract to a greater extent by monogastric
animals (swine) than by ruminant animals (sheep). The decreased
absorption by sheep may have been due to reduction of the
administered selenite to insoluble or unavailable forms by rumen
microorganisms.
Only limited information is available regarding the absorption
by animals of selenium occurring naturally in foods. Kidney tissue
and fish muscle were chosen for model studies, because these foods
are relatively rich sources of dietary selenium for human beings.
The levels of intestinal absorption by rats of radio-selenium
administered in homogenates of rabbit kidney or fish muscle
injected with selenite, were 87 and 64%, respectively (Thomson et
al., 1975b; Richold et al., 1977). The low absorbability of
"fish selenium" agrees with other reports showing the poor
bioavailability to animals of selenium in certain fish products
(section 5).
Little is known concerning the physiological processes
governing the absorption of even simple selenium compounds, though
McConnell & Cho (1965) showed that selenomethionine was transported
against a concentration gradient, whereas selenite and
selenocystine were not.
6.1.1.2. Human studies
Studies carried out in New Zealand on 3 young female volunteers
indicated that the intestinal absorption of oral doses of
radioactive selenite, containing not more than 10 µg selenium, was
70, 64, and 44% of the dose, respectively (Thomson & Stewart,
1974). In a study conducted 2 years later, in which 2 of the 4
female volunteers were women who had participated in the earlier
study with selenite, the intestinal absorption of oral doses of
75Se-selenomethionine, containing less than 2 µg selenium, averaged
about 96% of the dose (Griffiths et al., 1976). When a larger dose
of 1 mg selenium was given orally in solution, a similar difference
in absorption was obtained, i.e., 97% for selenomethionine (one
subject) (Thomson et al., 1978) and about 60% for sodium selenite
(13 subjects) (Robinson et al., 1985; Thomson & Robinson, 1986).
This value for absorption of selenite-selenium is much lower than
the 92% absorption reported for "selenite" in the earlier paper of
Thomson (1974), which is now known to have been selenate-selenium
and not selenite-selenium. Later studies on 10 volunteers also
showed absorption of 94% for 1 mg selenate-selenium in solution
(Thomson & Robinson, 1986). Thus, selenate-selenium is similar to
selenomethionine-selenium in that it is better absorbed than
selenite-selenium.
Correction of selenate-selenium for selenite selenium in the
paper of Thomson (1974) also removes the "surprising difference"
reported between absorption of selenite given in solid form (60%
for 4 female volunteers) and in solution (60% and not 92%). A
female volunteer given a 1 mg dose of selenium as selenite in
solution daily for 5 consecutive days absorbed 59% of the total 5
mg dose (Thomson et al., 1978). It would appear that human beings
have no homeostatic control to limit their absorption of large
single doses of these selenium compounds (Barbezat et al., 1984).
Robinson et al. (1978b) measured the intestinal absorption of
selenium by a New Zealand female volunteer receiving a daily
supplement of 100 µg selenium as selenomethionine, a male volunteer
supplemented daily with 100 µg selenium as sodium selenite, and a
second female supplemented daily with 65 µg selenium, as it occurs
naturally in mackerel (Scomber japonieus). The selenomethionine
and selenite were given in solution, whereas the mackerel was given
as canned fish. During a 4-week supplementation period, 75% of the
selenium given as selenomethionine was absorbed compared with only
48% of the selenium administered as selenite. The absorption of
the "fish selenium" was 66%. Details of the diets consumed by the
3 subjects were not provided, but the subjects supplemented with
selenomethionine or fish selenium did not consume liver or kidney
throughout the study. However, it was specified only that the
subject supplemented with selenite did not eat liver, kidney, or
fish on the days that weekly urine collections were made or on
the day prior to the collection. Also, the subject receiving
selenomethionine ate fish only occasionally throughout the study.
The subject supplemented with the fish selenium ate fish other than
mackerel on only one occasion. Stewart et al. (1978) found that
the average intestinal absorption of naturally-occurring selenium
in foods was 55% in 4 young New Zealand women freely consuming
normal diets. However, allowing for the endogenous faecal
excretion (half of total faecal output) the average true absorption
of naturally occurring selenium became 79%.
6.1.2. Absorption by inhalation
Certain volatile selenium compounds, such as hydrogen selenide,
are known to be toxic when inhaled (Dudley & Miller, 1941).
Lipinskij (1962) observed increased selenium levels in the liver
and kidneys after respiratory exposure of rabbits to elemental
selenium and selenium dioxide. More recently, Weissman et al.
(1979, 1983) studied the distribution and retention of inhaled
75Se-labelled selenious acid and elementary selenium aerosols.
They used 63 Beagle dogs, which were between 3 and 4 years old at
the time of exposure. All inhalation exposures were through the
nose only. Aerosol particles of both forms were small with median
aerodynamic diameters of 0.5 and 0.7 µm and geometric standard
deviations of 2.4 and 1.5 for selenious acid and elemental
selenium, respectively. The urine and faeces of 4 dogs were
collected daily, for 32 days, and then at regular time intervals
until sacrifice. Selenium from exhaled breath was collected from 3
dogs for several 4-h periods, between 2 and 10 days after exposure.
Whole-body retention of 75Se-selenium was measured immediately
after exposure and at regular intervals up to 320 days after
exposure. Two dogs exposed to each aerosol were sacrificed at
various time intervals for over half a year. The initial 75Se-
selenium body burden (IBB) in dogs that inhaled 75Se-selenium as
selenious acid was 40 ± 17 µg selenium/kg body weight and 22 ± 9 µg
selenium/kg body weight in dogs inhaling elementary selenium. For
both forms of selenium, the retention data of 75Se-selenium could
be expressed by a 3-component negative exponential equation:
Selenious acid:
%IBB = 66e-1.5t + 17e-0.089t + 17e-0.023t
Elemental selenium:
%IBB = 66e-0.090t + 21e-0.059t + 13e-0.018t
The corresponding half time values were 0.46, 7.8, and 30 days and
0.77, 12, and 38 days for selenious acid or elemental selenium
exposure, respectively. Studies on organ distribution showed that,
2 h after exposure to selenious acid, only about 5.3% of the IBB
was retained in the lung compared with 26% of the IBB of elemental
selenium. For the first 32 days following exposure, the urine
accounted for 79 and 66% of the 75Se-selenium excreted by dogs
exposed to selenious acid and elemental selenium aerosols,
respectively. Respiratory excretion of 75Se-selenium was
negligible. The organ distribution of 75Se-selenium as a
percentage of the sacrifice body burden is given in Table 10.
Table 10. 75Se-selenium distribution in organs of dogs
128 days after respiratory exposure to 75Se-selenious acid
or elemental 75Se-selenium aerosolsa
----------------------------------------------------------
Organ 75Se-selenium organ distribution
as % of 75Se-selenium body burden
at sacrifice
----------------------------------
selenious acid elemental selenium
treated treated
----------------------------------------------------------
Lung 1.3 6.2
Liver 11.4 7.4
Kidney 1.6 1.6
Blood 19.7 21.5
Gastrointestinal tract 3.4 3.6
Pelt 9.6 9.1
----------------------------------------------------------
a Adapted from: Weissman et al. (1979).
Comparison of the absorption of these two forms of selenium
through inhalation was also studied in the rat (Medinsky et al.,
1981). The rate of transfer of selenium into the blood was slower
when the elemental form was administered. However, once absorbed,
both chemical forms behaved identically.
6.1.3. Absorption through the skin
Apart from the report of Dutkiewicz et al. (1972), who found
that 10% of a 0.1 mol solution of sodium selenite applied to rat
skin was absorbed in 1 h, there are no quantitative data on the
dermal absorption of water-soluble selenium compounds. However,
Dudley (1938) showed that selenium oxychloride was absorbed through
the skin of rabbits. Selenium sulfide, an insoluble selenium
compound used in certain anti-dandruff shampoos, is not ordinarily
absorbed through the skin, although a garlicky breath odour and
elevated urinary excretion of selenium were noted in a patient with
open scalp lesions who used a selenium sulfide shampoo (Ransone et
al., 1961).
6.2. Distribution in the Organism
6.2.1. Transport
Little is known about the transport of selenium in the body.
However, a plasma selenoprotein has been identified which one group
postulates is involved in selenium transport (Motsenbocker &
Tappel, 1982).
6.2.2. Organs
6.2.2.1. Animal studies
Absorbed selenium is rapidly distributed among the tissues.
Heinrich & Kelsey (1955) found that the liver of mice injected
subcutaneously with sodium selenite contained 17 and 30% of the
dose, 15 and 60 min after injection, respectively. However,
because of the great variation in the metabolic half-time of
selenium in various tissues (Thomson & Stewart, 1973), the
distribution pattern observed will change markedly with the time of
sampling after injection with radio-selenium.
As long as the diet provides nutritionally adequate levels of
selenium, the relative concentrations of selenium in the various
internal organs are quite consistant over a wide range of selenium
intakes. For example, Jones & Godwin (1962) studied the
distribution of selenium in the tissues of mice fed alfalfa
containing nutritional levels of selenium. The concentrations of
selenium in the internal organs decreased in the following order:
kidneys > liver > pancreas >> lungs > heart > spleen >
skin > brain > carcass. Smith et al. (1937) fed toxic levels of
sodium selenite to cats and observed the following order for the
concentration of selenium in the internal organs: liver >
kidneys > spleen > pancreas > heart > lungs.
The distribution of selenium appears to be relatively
independent of the form and route of administration, since the
kidneys and adrenals contained the highest concentrations of radio-
selenium, regardless of whether the rats were given labelled
selenite or selenomethionine orally or by intravenous injection
(Thomson & Stewart, 1973). The greatest amounts of radioactivity
were in the kidneys, liver, and total muscle mass. Similar
distribution patterns were noted in rats dosed orally with
homogenates of tissues from rabbits or from fish that had been
given labelled selenium compounds (Thomson et al., 1975b; Richold
et al., 1977).
The distribution of selenium in tissues was markedly different
when rats were fed a diet deficient in selenium: the testes,
brain, thymus gland, and spleen took up the greatest concentrations
of selenium from a tracer dose of selenite (Burk et al., 1972). A
recent report confirms the ability of the testis to retain selenium
better than other tissues in selenium deficiency (Behne & Hofer-
Bosse, 1984).
The distribution of selenium in the eye was determined
fluorometrically with 2,3-diaminonaphthalene by Gusejnov et al.
(1974), who found the following levels in various parts of the eye
of a bull (mg/kg fresh weight): iris, 1.3; retinal pigment
epithelium, 0.85; retina, 0.50; cornea, 0.3; lens, 0.03; and
vitreous body, 0.01. In some birds (pigeons and crows), the level
of selenium in the retina was about 10 times higher (about 5.0
mg/kg). In studies in which 75Se-labelled sodium selenite in
saline was administered intraperitoneally to frogs, rats, and
rabbits, the distribution of radio-selenium was similar to that of
the naturally-occurring selenium measured in the bull's eye. The
maximum 75Se activity in the eye (5 h after administration) was
found in the pigment with the vascular layer of the retina, whereas
minimum activity was found in the lens and vitreous body.
Histoautoradiography of the eyes also indicated the highest
radioactivity in the vascular and pigment layers (Abdullaev et al.,
1972).
6.2.2.2. Human studies
The distribution of selenium in human organs was examined by
Schroeder et al. (1970), who found the following descending order
for selenium concentrations in tissues: kidney > liver >
spleen > pancreas > testes > heart muscle > intestine >
lung > brain. Blotcky et al. (1976) showed that, in autopsy
samples from 106 persons, the concentrations of selenium were 2-3
times higher in the kidneys than in the liver.
Uptake of an orally-administered dose of radioselenite in the
internal organs occurred in the following relative order: liver >
kidneys > lungs > muscle = articular tissue (Leeb et al., 1977).
Other studies have shown significant accumulation of radio-selenium
by the liver and kidneys after intravenous injections of selenite
or selenomethionine (Lathrop et al., 1972; Falk & Lindhe, 1974;
Nordman, 1974).
6.2.3. Blood
Studies on experimental animals have shown a close correlation
between the level of selenium in the blood and the level of
selenium in the diet (Lindberg & Jacobsson, 1970; Cary et al.,
1973; Ermakov & Kovalskij, 1974; Oh et al., 1976a). Analytical
surveys on human beings have shown significant variations in blood-
selenium levels in different geographical areas, presumably because
of differences in dietary-selenium intake. For example, Allaway et
al. (1968) found that selenium levels in the blood of persons
living in areas of the USA where soils and vegetation were high in
selenium tended to contain more selenium than that of persons
living in areas where soils and plants were generally low in
selenium. Thus, geographical differences in the selenium levels in
human blood are possible, even in a country where large-scale
interregional food shipments would be expected to level out
differences in the amount of selenium in the food supply. These
geographical differences in human blood-selenium levels in the USA
have been confirmed by Howe (1974), who found a mean selenium
content of 0.265 mg/litre (SD = 0.056) for 626 samples collected in
and around the state of South Dakota (a high soil-selenium region),
whereas Schultz & Leklem (1983) reported blood-selenium values in
Oregon (a low soil-selenium region) that were well below those
reported for other areas of the USA.
Analyses of blood samples from persons living in different
regions of the world indicate similar correlations between the
amount of selenium in the blood and the amount of selenium in the
food throughout the world (Table 11). For example, blood-selenium
concentrations tend to be lowest in areas of the world that are
known to have low levels of selenium in the soil (Keshan-disease
areas of China, New Zealand, Scandinavia) and highest in areas that
are known to have high levels of selenium in the soil (Venezuela,
reported selenosis areas of China). Superimposed on these
geographical variations in effects are the effects of general
nutritional status or effects of certain disease states. For
example, children suffering from Kwashiorkor have lower blood-
selenium levels than their well-nourished counterparts (Burk et
al., 1967) and reductions in the selenium content of sera have been
observed in patients with cancer (McConnell et al., 1975). Lower
blood-selenium values were associated with lower serum-albumin
values in surgical patients with and without cancer in New Zealand
(Robinson et al., 1979). Akesson (1985) suggested that alterations
in plasma-selenium may be secondary to changes in plasma-protein
concentration. In a study of Finnish men with one or more risk
factors for coronary heart disease, Miettinen et al. (1983)
observed a strong association between serum-selenium and the
eicosapentanoic acid contents of cholesterol ester and
phospholipids. The authors pointed out that since eicosapentanoate
is a fatty acid peculiar to fish such an association could reflect
the intake of fish at the time of blood sampling.
Blood-selenium levels change if persons travel to countries
with soils of different selenium content. For example, Griffiths &
Thomson (1974) noted that the blood-selenium levels of adults from
the USA declined rapidly on arrival in New Zealand, but, after 1
year, their levels were still higher than the mean value for
permanent New Zealand residents (Fig. 2). Rea et al. (1979) found
that plasma-selenium levels changed with changes in selenium intake
more rapidly than erythrocyte-selenium levels. The latter
reflected longer-term changes in selenium exposure, presumably
because of the relatively long life span of erythrocytes. In a
controlled depletion/repletion metabolic study (section 6.3.2.2),
Levander et al. (1980) stated that the plasma-selenium levels of
healthy young male volunteers in the USA dropped about 19% after
2 weeks on a low-selenium diet and then returned to their previous
levels 11 days after consuming a high-selenium diet (Fig. 3).
4. LEVELS IN ENVIRONMENTAL MEDIA
4.1. Levels and Chemical Forms of Selenium in Food
4.1.1. Levels in food
In spite of the relatively few data available, some
generalizations concerning the selenium content of foodstuffs can
be made. For example, the level of selenium in food depends on
natural differences among food commodities and the natural
availability of selenium in the environment. Moreover, certain of
man's activities can influence the selenium content of human foods.
4.1.1.1. Natural differences among food commodities
A wide range of values has been reported in the selenium
content of foods (mg/kg wet weight): liver, kidney, and seafood,
0.4 - 1.5; muscle meats, 0.1 - 0.4; cereal and cereal products, <
0.1 - > 0.8; diary products, < 0.1 - 0.3; and fruits and
vegetables, < 0.1) (Oelschlager & Menke, 1969; Morris & Levander,
1970; Schroeder et al., 1970; Suchkov, 1971; Arthur, 1972; Millar &
Sheppard, 1972; Ferretti & Levander, 1974; Sakurai & Tsuchiya,
1975; Abutalybov et al., 1976; Bieri & Ahmad, 1976; Kasimov et al.,
1976; Olson et al., 1978). It should be pointed out that these
values are given for raw foods (food as purchased) rather than
cooked foods (food as eaten). The effects of cooking on the
selenium content of foods are described in section 4.1.1.3.
Moreover, the values presented in these food composition studies
should not be compared from one country to another, because of the
variations in the analytical methods and sampling procedures used
(section 2).
Organ meats, such as kidneys or liver, contain the highest
levels of selenium, but some seafood products contain almost as
much. Muscle meats are significant sources of selenium, though
they do not contain as much as organ meats or seafoods. Certain
semolina, grain, and cereal products can contribute appreciably to
the dietary-selenium intake, but wide variations in selenium
content have been found in different samples of the same foodstuff.
Such variation is typical of many plant foods and the reasons for
this are discussed below. Milk, cheese, and egg samples from
several countries showed low to moderate values for selenium, but
again the results were quite variable. Fruits and vegetables
generally contained very low levels of selenium, though garlic and
mushrooms contained moderate levels of the element.
The selenium contents of baby foods tended to show the same
general pattern as those in adult foods (Morris & Levander, 1970;
Arthur, 1972), i.e., meat and cereal products contained the highest
levels and fruit and vegetable products, the lowest. Shearer &
Hadjimarkos (1975) analysed samples of mature human milk from 241
subjects living in the USA and found that the overall mean selenium
content was 0.018 mg/litre. Over 98% of the samples contained
between 0.007 and 0.033 mg/litre. Grimanis et al. (1978) reported
an average of 0.015 mg/litre in 5 samples of mature human milk
collected in Greece. These authors found slightly higher values in
15 samples of human transitional milk (0.016 mg/litre) and
colostrum (0.048 mg/litre). A slightly lower average selenium
concentration of 0.013 mg/litre, was reported for samples of human
transitional milk in New Zealand (Millar & Sheppard, 1972). The
most extreme values for the selenium content of human milk were
reported from China and ranged from 0.0026 mg/litre in areas where
Keshan disease was prevalent to 0.283 mg/litre in high-selenium
areas (Yang et al., 1986).
It can be seen from Table 3 that meat-based infant formulae
have a higher selenium content than formulae based on milk or soy
protein, and that casein-based powdered formulae for special
medical purposes (diet therapy for errors in amino acid metabolism
or malabsorptive states) contain low levels of selenium. Blended
food tube-feeding formulae containing meat tend to have higher
selenium contents than comparable products containing only milk or
casein and soy protein (Table 3). Chemically-defined diets having
egg albumen as the protein source contained more selenium than
diets based on casein hydrolysate, which, in turn, contained more
selenium than diets based on purified amino acids. Total
parenteral nutrition solutions based on casein hydrolysate also
contain more selenium than solutions based on amino acid mixtures,
which contain very low levels of selenium.
Table 3. Selenium content of commercial formula dietsa
-----------------------------------------------------------------
Product Selenium content
(mg/kg wet (mg/kg dry
weight) weight)
-----------------------------------------------------------------
Infant formulae:
milk-based 0.004 - 0.027
soy-based 0.004 - 0.030
meat-based 0.046 - 0.070
casein-based formulae for special 0.048 - 0.120
medical purposes (powders)
Food supplements and tube-feeding
formulae:
milk-based 0.005 - 0.045
soy-casein-based 0.013 - 0.020
blended foods 0.037 - 0.056
Chemically defined diets:
(powders)
egg albumen low residue 0.224 - 0.351
egg albumen moderate nitrogen 0.388 - 0.886
egg albumen high nitrogen 0.503 - 0.570
casein hydrolysate 0.052 - 0.071
amino acid mixture 0.001 - 0.011
-----------------------------------------------------------------
Table 3. (contd.)
-----------------------------------------------------------------
Product Selenium content
(mg/kg wet (mg/kg dry
weight) weight)
-----------------------------------------------------------------
Total parenteral nutrition
solutions:
casein hydrolysate 0.037 0.324
(0.032 - 0.041)
diluted 1:1 with 50% dextrose 0.019 0.093
solution (0.017 - 0.020)
amino acid mixture 0.001 0.010
-----------------------------------------------------------------
a Adapted from: Zabel et al. (1978).
One of the factors that can influence the amount of selenium in
plant foodstuffs is the nature of the plant itself. Plants have
been divided into 3 groups depending on their tendency to take up
selenium from seleniferous soils (Rosenfeld & Beath, 1964):
Group 1 Primary selenium accumulators - can contain very
high amounts of the element (often over 1000
mg/kg dry weight).
Group 2 Secondary selenium accumulators - rarely contain
more than a few hundred mg/kg.
Group 3 Many weeds and most crop plants, grains, and
grasses - rarely contain more than 30 mg/kg, even
when grown on seleniferous soils; when grown on
normal soils generally contain less than 1 mg/kg.
Plants from Groups 1 and 2 do not usually contribute to the
selenium intake of human beings, since they are not consumed
directly by people and are consumed by animals only when other
feeds are not available. However, plants from Group 3 can
contribute large amounts of selenium, if they are grown in
seleniferous areas (see below).
The concentrations of many nutritionally essential trace
elements are known to be decreased by the milling of grains into
cereals (Czerniejewski et al., 1964) and preliminary analysis of
random supermarket samples of cereal foods suggested that refined
products such as white flour or white bread contained less selenium
than whole grain foods such as whole wheat flour or whole wheat
bread (Morris & Levander, 1970). However, a subsequent more
controlled analytical study in which various grain fractions were
taken from the same production batch showed that milling a variety
of grains decreased the concentration of selenium in the consumer
product by only 10 - 30% (Ferretti & Levander, 1974). Thus, the
selenium content of cereal products is somewhat less than that of
the parent grains but the decreases in concentration are not nearly
as great as those observed with other essential trace minerals.
Selenium tends to be localized mainly in the protein fraction
of plant and animal tissues; thus, the protein content of a food
also influences its selenium content. For example, the
concentration of selenium in a series of soybean products prepared
from a given lot of soybeans increased as the protein content of
the product increased (Ferretti & Levander, 1976). However,
protein content is only an expression of the potential of a food to
contain selenium, and a food high in protein will not necessarily
also be high in selenium. Moreover, non-protein seleno amino acids
occur in some foods and thus, the protein content may not always
reflect the selenium content.
4.1.1.2. Effects of natural differences in the availability of
selenium in the environment on levels in food
The most important factor in determining the selenium content
of plant foods and feeds is the amount of selenium in the soil that
is available for uptake by the plant. Sun et al. (1985) reported
that the selenium content of local crops was significantly
correlated with the selenium content of the local soil, the
correlation coefficients of soybean, corn, and rice being 0.9456,
0.9953, and 0.9954, respectively. Since the water-soluble selenium
in the soil was directly correlated with the pH and inversely
correlated with humin and total iron in the soil, the selenium
content of local crops was also influenced by the amount of water-
soluble selenium in the soil. An analytical survey carried out in
the USA demonstrated that the level of selenium in alfalfa plants
varied from less than 0.01 to more than 5.0 mg/kg, and it was
assumed that these levels reflected the amount of available
selenium in the soil (Kubota et al., 1967). A variation in the
selenium content of wheat from 0.04 to 21.4 mg/kg, depending on
where the plant was grown, was reported by Schroeder et al. (1970).
Samples of several foods bought in local markets in Caracas,
Venezuela contained much more selenium than similar foods purchased
in supermarkets in the eastern USA (Table 4). The likely source of
selenium in the milk, eggs, and meat from Caracas is sesame cake,
since sesame is produced mostly in the seleniferous area of
Venezuela and the pressed cake is widely used as an ingredient in
animal feed. Selenium levels as high as 14 mg/kg in corn and 18
mg/kg in rice have also been observed in certain food samples taken
from high-selenium regions in Venezuela (Jaffe, 1976). These
concentrations of selenium are as high as those reported in foods
from seleniferous zones of the USA (Smith & Westfall, 1937).
Great extremes in the selenium content of staple foods have
also been reported recently from China (Yang et al., 1983). For
example, samples of corn, rice, and soybeans, taken from a high-
selenium area with a history of human intoxication reported as
chronic selenosis (section 8.1.1.1) contained average selenium
levels of 8.1, 4.0, and 11.9 mg/kg, respectively, whereas samples
of the same staples collected in a low-selenium area where Keshan
disease was prevalent (a human selenium-deficiency disease)
(section 8.2.2) contained average selenium levels of only 0.005,
0.007, and 0.010 mg/kg, respectively (Table 5). A low selenium
content in food has also been reported in other countries with low
selenium soils, such as New Zealand and Finland. Typical ranges
for selenium contents in food in such countries include (mg/kg wet
weight): liver, kidney, and seafood, 0.09 - 0.92; muscle meats,
0.01 - 0.06; cereal and cereal products, 0.01 - 0.07; milk, <
0.01; and fruits and vegetables, < 0.01 - 0.02 (Koivistoinen,
1980; Thomson & Robinson, 1980). Samples of staple foods collected
in areas where soils contained intermediate levels of selenium also
contained intermediate levels of selenium.
Table 4. Comparison of selenium contents
of selected foods available in Caracas,
Venezuela, and Beltsville, Maryland, USA
(mg selenium/kg wet weight)a
-----------------------------------------
Food Caracas Beltsville
-----------------------------------------
Powdered milk 0.417 0.169
Whole milk 0.115 0.012
American-type cheese 0.425 0.090
Swiss-type cheese 0.382 0.104
Pork 0.833 0.209
Chicken 0.702 0.106
Egg 1.520 0.116
-----------------------------------------
a From: Mondragon & Jaffe (1976).
As might be expected, the selenium contents of food products of
animal origin depend heavily on the amount of naturally-occurring
selenium in the feed given to the animal. In the USA, it was shown
that the selenium content of swine muscle was highest in areas
known to have a high level of available selenium in the soil and
lowest in areas in which the available soil-selenium was low (Ku et
al., 1972). This indicates that selenium is readily passed up the
soil-plant-animal food chain to human beings.
4.1.1.3. Man-induced changes in selenium levels in food
(a) Human activities that increase selenium levels
Perhaps the most direct way that man's activities can increase
the selenium content of the food supply is the deliberate addition
of selenium to the feeds of poultry and certain livestock. In some
countries, this is now accepted practice to prevent the occurrence
of selenium-deficiency diseases, many of which cause significant
economic losses to farmers throughout the world. In the USA, for
example, farmers are permitted to add 0.1 mg selenium/kg (as sodium
selenite or selenate) complete feed for beef and dairy cattle,
sheep, chickens, ducks, swine (0.3 mg/kg in starter and prestarter
rations), and 0.2 mg/kg for turkeys (US Department of Health and
Human Services, Food and Drug Administration, 1984; Subcommittee on
Selenium - Committee on animal Nutrition, 1983). It has been shown
that the edible tissues of poultry, swine, and sheep, fed diets
fortified with inorganic selenium salts at the regulated levels,
did not contain any more selenium than the tissues of animals fed
diets, naturally adequate in selenium (Allaway, 1973; Ullrey et
al., 1977). The homeostatic mechanisms that appear to limit the
concentrations of selenium in the edible tissues of animals fed
certain levels of sodium selenite are discussed further in section
6.
Table 5. Selenium contents of staple foods grown on soils in areas of China with excess,
moderate, and deficient levels of seleniuma
-----------------------------------------------------------------------------------------
Place Corn Rice Soybean
Number Se content Number Se content Number Se content
of (mg/kg) of (mg/kg) of (mg/kg)
samples samples samples
-----------------------------------------------------------------------------------------
High-selenium area 44 8.1 22 4.0 17 11.9
with a history of (0.5-28.5)b (0.3-20.2)b (5.0-22.2)b
intoxication reported
as chronic selenosis
High-selenium area 2 0.57 2 0.97 2 0.34
reported to be without
selenosis
Moderate-selenium- 82 0.036 76 0.035 31 0.069
adequate area (± 0.056) (± 0.027) (± 0.076)
(Beijing)
Low-selenium area 10 0.009 32 0.022 - -
(± 0.009) (± 0.009)
Low-selenium area 195 0.005 49 0.007 150 0.010
with Keshan disease (± 0.003) (± 0.003) (± 0.008)
-----------------------------------------------------------------------------------------
a Adapted from: Yang et al. (1983).
b Mean ± SD or range shown in parenthesis.
Finland is the first country to decide to increase the selenium
content of Finnish feed and food by the addition of sodium selenate
to fertilizers, to be used in the whole country at a concentration
of 16 or 6 mg/kg for cereal and grassland crops, respectively
(Koivistoinen & Huttunen, 1985). The manufacture of these
selenized fertilizers began in the summer of 1984 and they will be
used at application rates of 10 g selenium/ha per growing season.
It is anticipated that the added selenium in the fertilizers will
be transported to the human food chain, and the selenium level of
the cereal and grassland crops will be raised to 0.1 and 0.15 -
0.20 mg/kg (dry basis), respectively.
Another potential way in which the level of selenium in the
food chain might be increased by man's activities is the proposed
use of selenium-bearing fly ash as a soil supplement. Furr et al.
(1977) analysed cabbages grown on potted soil supplemented with
several different fly ashes and found that the selenium levels in
the cabbages were closely correlated with those in the respective
fly ashes in which the plants were cultured. The ready
bioavailability to animals of the selenium in plants grown on fly
ash was demonstrated in a study (Stoewsand et al., 1978) in which
Japanese quail were fed a complete diet containing 60% winter wheat
that had been grown to maturity on either soil or a deep bed of fly
ash. The soil contained 2.1 mg selenium/kg and the wheat grown
thereon contained 0.02 mg/kg, a level typical of wheat from
selenium-deficient areas. The fly ash contained 21.3 mg
selenium/kg, and the wheat grown on it contained 5.7 mg/kg, a level
sometimes found in wheat from naturally seleniferous areas. The
levels of selenium in the tissues of the quail fed the wheat grown
on fly ash were much higher than those of quail fed the wheat grown
on soil (Table 6). Moreover, the selenium contents of eggs from
quail fed the wheat grown on fly ash were 3.5 mg/kg in the yolk and
almost 10 mg/kg in the white compared with 0.5 and 0.2 mg
selenium/kg, respectively, in eggs from quail fed the control
wheat. Obviously, the use of selenium-bearing fly ash as a soil
supplement to provide nutritionally desirable levels of selenium in
plants should be carried out with care, since inappropriate use
could lead to an unwanted build-up of selenium residues in the food
chain. Also, the application of fly ash to soil at rates
sufficient to correct selenium deficiency in animals may damage the
soil.
Table 6. Selenium in tissues of male Japanese quail fed winter
wheat grown on soil or fly asha
-------------------------------------------------------------------
Wheat grown Tissue selenium
on: brain heart kidney liver muscle
-------------------------------------------------------------------
(mg/kg dry weight)
Soil 0.8 ± 0.1 0.7 ± 0.2 3.6 ± 0.4 1.6 ± 0.0 0.3 ± 0.2
Fly ash 3.4 ± 1.6 4.4 ± 0.7 9.5 ± 1.1 12.7 ± 2.4 4.1 ± 0.6
-------------------------------------------------------------------
a From: Stoewsand et al. (1978).
The possibility has been raised that some selenium may be added
to the food supply as a result of atmospheric contaminants from
the burning of fossil fuel or industrial emissions settling out on
the leaves of plants or on the surface of the soil. The
geographical pattern of selenium levels in rain-water from Denmark
or the USA implicates airborne selenium from the industrial and
domestic uses of fossil fuel as sources (Kubota et al., 1975).
Total industrial emissions of selenium in the USA for the year 1970
were estimated to be about 1.1 million kg, 62% of which was derived
from the burning of coal (US NAS/NRC, 1976). This level of
industrial emission of selenium may be compared with a projected
annual use of selenium as a feed additive for chickens and swine in
the "low-selenium" areas of the USA of 6000 kg (US FDA, 1974). But
there is no evidence that the selenium deposited in rainwater has
any influence on the concentration of selenium in forage plants
grown in Denmark or in the heavily industrialized northeastern USA
(Kubota et al., 1975). Furthermore, selenium deficiency has been
observed in farm livestock grazing near coal-burning electric power
stations (Anonymous, 1975). This suggests that airborne selenium
exists in the form of inert elemental selenium, insoluble selenide
salts, or as selenium dioxide, which would be tightly bound by
acidic, iron-bearing soils. In any case, the selenium would not be
available for uptake by plants; thus, there would appear to be
little prospect of adding appreciable selenium to the food supply
via atmospheric contamination.
(b) Human activities that decrease selenium levels in the food
chain
The metabolic antagonism between sulfur and selenium (Levander,
1976a) led early workers to suggest that borderline selenium
deficiency might be exacerbated by sulfate fertilization (Schubert
et al., 1961). More recent research has demonstrated that sulfate
fertilization can result in decreased selenium concentrations in
forage plants (Pratley & McFarlane, 1974; Westermann & Robbins,
1974). In many cases, this decrease can be largely explained by a
dilution effect caused by a growth response of the plant to the
sulfate fertilization, but decreases in selenium concentration
independent of crop yield have also been reported. Such decreases
could be the result of competitive interference by sulfate with the
uptake of selenate by plants. The use of phosphate fertilizers in
parts of Australia and New Zealand has resulted in an apparent
increase in the incidence of selenium deficiency in livestock
(Judson & Obst, 1975), but others have reported increases in the
selenium content of forages after fertilization with phosphorus
(Robbins & Carter, 1970; Carter et al., 1972). These conflicting
results may be due to differences in the selenium content of the
phosphatic rock from which the fertilizers were prepared.
Another possible way in which man's activities might decrease
selenium in the food chain or render it less available, is through
heavy metal pollution. For example, silver has been demonstrated
to antagonize selenium in a wide variety of situations (Diplock,
1976). In an area where there has been a large amount of silver
mining activity, muscular dystrophy of the type usually associated
with selenium deficiency has been reported in calves fed a ration
based on milk powder, even though the selenium content of the milk
seemed to be adequate. Apparently, the milk powder was derived
from cows that grazed land containing high levels of silver
residues from the old mining industry. Further research is needed
to establish fully the role of silver in potentiating these field
cases of apparent selenium deficiency. Because of the many
interactions of selenium with other environmental pollutants such
as mercury, cadmium, or arsenic (section 7), it is possible that
other cases of heavy metal-induced selenium deficiency will be
discovered.
The influence of cooking on the selenium content of foods was
determined because of the well-known instability and volatility of
many selenium compounds. Certain vegetables that normally contain
high levels of selenium such as asparagus or mushrooms, lost up to
40% of their selenium content as a result of boiling (Higgs et al.,
1972). Majstruk & Suchkov (1978) reported that up to 50% of the
original selenium was lost from vegetables and dairy products
during cooking; the addition of salt and acid pH or extended
cooking, particularly promoted such losses. But, other typical
cooking procedures such as boiling cereals, baking poultry or fish,
or broiling meats had little effect on selenium levels in the food
(Higgs et al., 1972). Similar results were observed by others for
baking fish or broiling meats, though some decrease in selenium
concentration was caused by frying organ meats or fish (Ganapathy
et al., 1975). Thompson et al. (1975) determined the selenium
contents of cooked food composites and found a rough agreement with
the calculated values based on unprepared foods from which they
were derived. On the basis of all these studies, it can be
concluded that usual cooking procedures cause little decrease in
the selenium content of most foods.
4.1.2. Chemical forms of selenium in food
There is little information on the chemical forms of selenium
that occur in human food (Levander, 1986). Cappon & Smith (1982)
reported that canned tuna fish contained variable percentages of
hexavalent selenium, ranging from 7.6 to 44.8%, which was
independent of the total selenium content. The other forms of
selenium in the tuna were di- and quadrivalent selenium. On an
average percentage basis, hexavalent selenium was more easily
extractable by water than di- or quadrivalent selenium. In
recently canned samples, an average of 55.6% of the total selenium
content was water-extractable. However, for the older samples, the
corresponding average extractable level was 48.2%. The results
suggest that sample storage may influence the chemical form of
selenium in canned tuna. Olson et al. (1970) found that, in a
certain portion of high selenium wheat (the pronase hydrolysate of
gluten), about half of the selenium was in the form of
selenomethionine. About 15% of the selenium in water extracts of
seleniferous cabbage leaves was in the form of selenomethionine,
but appreciable amounts of other selenium compounds were also
present (Hamilton, 1975). The chemical forms of selenium in food
are likely to affect the bioavailability of selenium (section 7.2.2
and 8.2.3).
4.2. Drinking-Water
Analysis of samples taken from various public water-supply
systems in the USA showed that less than 0.5% contained selenium
levels that exceeded the US PHS limit of 10 µg/litre (Taylor, 1963;
Lakin & Davidson, 1967; McCabe et al., 1970). Samples from 1280
central water sources providing water for 6.5 million Bulgarians
contained less than 2 µg selenium/litre (Gitsova, 1973). Tap water
from Stockholm, Sweden contained only 0.06 µg/litre (Lindberg,
1968) and tap and mineral waters from Stuttgart, Federal Republic
of Germany contained 1.6 and 5.3 µg/litre, respectively
(Oelschlager & Menke, 1969). Surface water sources in different
subregions of the Cernovici region of the Ukrainian SSR contained
selenium levels ranging from 0.09 ± 0.01 to 3.00 ± 0.40 µg/litre;
ground-water levels ranged from 0.07 ± 0.0 to 4.00 ± 0.85 µg/litre
(Suchkov & Kacap, 1971). A few tenths to several µg per litre were
reported in non-seleniferous regions of the USSR, the maximum
reported value being 5.1 µg/litre. In Argentina, the selenium
content of 22 surface waters varied from < 2 to 19 µg/litre with a
median value of 3 µg/litre (WHO, 1984).
Elevated levels of selenium (> 100 µg/litre) can be found in
seeps, springs, and shallow wells, though waters from deep wells
contain only a few µg/litre (US NAS/NRC, 1976). Highly variable
amounts of selenium were reported in wells from seleniferous areas
in the USA ranging from non-detectable levels to 330 µg/litre
(Smith & Westfall, 1937). Some well waters contained enough
selenium to be considered as poisonous for man or livestock and
loss of hair and nails in children was attributed to selenosis, but
the evidence for this was not considered convincing (US NAS/NRC,
1976).
The average selenium content of 11 samples of drinking-water
taken from a high-selenium area in China with a history of disease
reported as chronic selenosis was 54 µg/litre (Yang et al., 1983).
Four of these samples were surface water from a village with
previous heavy intoxication and these averaged 139 (117 - 159)
µg/litre. Such water would contribute substantial amounts of
selenium, but would still comprise a small fraction of the total
selenium intake in this area, since the contribution from food was
estimated to be about 5000 µg/day (section 5.1.1.1). The other 7
samples of drinking-water originated from several sources and
averaged only 5 µg/litre.
Rosenfeld & Beath (1964) concluded that selenium does not occur
in water in sufficient amounts to produce selenium toxicity in man
or animals, except in isolated cases. This was confirmed and
expanded in the statement by US NAS/NRC (1976, 1980) that waters
are rarely a significant source of selenium from either a
nutritional or a toxicity point of view. The low concentrations of
selenium in drinking-water are probably the result of several
mechanisms that act to decrease the selenium content of waters
(section 2.1).
4.3. Air
Selenium levels in the air breathed by the general population
are probably well below 10 ng/m3, on average (US NAS/NRC, 1976).
Hashimoto & Winchester (1967) found 0.3 - 1.6 ng airborne
selenium/m3 in an urbanized area. Levels of 3.6 - 9.7 ng
selenium/m3 were detected by Pillay et al. (1971), who showed that
over half of the selenium was not retained on filters able to trap
particulates greater than 0.1 µg in diameter. Zoller & Reamer
(1976) concluded that atmospheric levels of selenium in most urban
regions vary from 0.1 to 10 ng/m3. Selenium levels in air around
industries that use selenium can be higher (section 3.2.2), perhaps
of the order of a few µg/m3. Work-place air in selenium industries
apparently contained mg levels of selenium/m3 in the past, but more
recent measurements have indicated lower levels (section 5.2).
5. HUMAN EXPOSURE
5.1. Estimate of General Population Exposure
As discussed in section 4, selenium levels in air are low in
areas without selenium-emitting industries and are not likely to
exceed 10 ng/m3. Assuming that a person respires 20 m3 air daily,
the contribution to daily selenium intake via this route would be
0.2 µg or less. Since this intake is much lower than that through
food (see below), airborne selenium makes a negligible contribution
to the average daily selenium intake of the general population.
However, levels of selenium may be somewhat higher in the
atmosphere around some industrial plants using selenium (section
3.2.2). If it is assumed that 3 µg selenium/m3 is found in the air
near these industries, then exposure to selenium via air would be
60 µg/day.
Other possible exposures of the general population to selenium
via the air include contributions from house dust and tobacco
smoke. Lakin & Davidson (1967) reported that house or office dust
could contain up to 10 mg selenium/kg. Although no data are
available to provide an estimate of human exposure to selenium from
this source, the possibility of such exposure should be considered
because of the increased interest in the quality of indoor air.
Olsen & Frost (1970) found an average of 0.08 mg selenium/kg (range
0.03 - 0.13 mg/kg) in a variety of cigarette tobaccos. If it is
assumed that a cigarette contains 1 g tobacco and that all the
selenium in tobacco is volatilized and inhaled during smoking, it
can be calculated that a person smoking one pack of 20 cigarettes
per day would inhale an average of 1.6 µg from this source.
Most drinking-water supplies contain only small quantities of
selenium, except possibly for those in certain seleniferous areas.
As discussed in section 4.2, most public water supplies contain
much less than 10 µg selenium/litre. Assuming that a person drinks
2 litres of water daily, the intake via this route would be only a
few µg. While this is considerably higher than the intake via air,
in most areas of the world, this is still a small fraction of the
daily intake from food.
5.1.1. Food
5.1.1.1. Geographical variation
Both the amount and the bioavailability of dietary selenium are
determinants of its biological effects. Bioavailability is be
considered in sections 7.2.2 and 8.2.3. The estimated daily intake
of selenium from dietary sources by adult human beings varies
considerably in different parts of the world. The greatest
extremes in intake have been found in China (Table 7) where both
selenium toxicity and deficiency have been reported (section
8.1.1.1, 8.2.2). An average selenium intake of 4990 µg/day was
estimated in a high-selenium area of China with a history of
intoxication reported as chronic selenosis, whereas an average
intake of only 11 µg/day was estimated in an area of China where
Keshan disease, a cardiomyopathy related to low selenium status,
was reported. Intermediate intakes of selenium were found in areas
of China that were not affected with either selenosis or Keshan
disease. It should be pointed out that the dietary-selenium intake
in the high-selenium area with a history of intoxication reported
as selenosis did not overlap with that in the high-selenium area
reported to be without selenosis. Likewise, the dietary-selenium
intake in the low-selenium area with Keshan disease did not overlap
with that in the moderate-selenium area (Beijing).
Table 7. Daily selenium intake of residents living in high-, medium-, and low-selenium
areas of Chinaa
---------------------------------------------------------------------------------------
Number Daily selenium intake Se intake from staple
Place of minimum maximum average cereals as % of total
subjects (µg) daily intake
---------------------------------------------------------------------------------------
High-selenium area with 6 3200 6690 4990 28-70%
a history of intoxication
reported as chronic
selenosis
High-selenium area 3 240 1510 750 25-45%
reported as without
selenosis
Moderate-selenium area 8 42 232 116 various sources
(Beijing)
Low-selenium area with 13 3 22 11 mainly from cereals
Keshan disease
---------------------------------------------------------------------------------------
a Adapted from: Yang et al. (1983).
Less extreme, but still widely diverse intakes of selenium have
been observed in other parts of the world (Table 8). The selenium
intake calculated in New Zealand, a country with low levels of
selenium in the soils in certain areas, averaged 30 µg/day (Thomson
& Robinson, 1980; Watkinson, 1981). Similar low intakes have been
reported by other research workers in New Zealand. For example,
Stewart et al. (1978) showed that the mean dietary-selenium intake
of 4 New Zealand women consuming normal diets ad libitum was
24.2 µg/day, and Griffiths (1973) reported that the daily intake of
selenium by 13 young New Zealand women varied from 6 to 70 µg.
Daily intakes of 30 µg or more in the latter group were associated
with the inclusion of liver, kidney, or fish in the diet. A low
average dietary-selenium intake (30 µg/day) has also been reported
in Finland, another country known to have soils low in selenium
(Varo & Koivistoinen, 1980). Low levels of dietary selenium may
also occur in Italy (Rossi et al., 1976) and Egypt (Waslien, 1976).
The average daily intake of selenium in the United Kingdom was
estimated to be 60 mg (Thorn et al., 1978). In one study, the
dietary-selenium intake in Japan was estimated to be 88 µg/day
(Sakurai & Tsuchiya, 1975). A higher estimate in another study was
arrived at largely because higher selenium contents of food staples
were used in the calculations (Yasumoto et al., 1976). The
estimated dietary intake of selenium in North America ranged from
98 to 224 µg/day. A "Market basket" survey carried out in the USA
from 1974 to 1982 indicated an average overall selenium intake of
108 µg/day (Pennington et al., 1984). An earlier survey had
revealed regional differences in intake, with people living in
western parts of the country consuming 1.3 times as much selenium
as people in the northeastern part of the country (US FDA, 1975).
Duplicate plate analyses of self-selected diets in Maryland, USA
gave a mean intake of 81 ± 41 µg/day (Welsh et al., 1981). The
daily intake in Venezuela was estimated to be 326 µg, but this
figure requires cautious interpretation because North American food
consumption patterns were used in its derivation (Mondragon &
Jaffe, 1976).
5.1.1.2. Food habits (consumption patterns)
Because of the wide variations in the selenium content of
various food groups in different countries, it is possible that
certain food habits or preferences could play an important role in
determining whether a person is at risk with regard to a deficient
or excessive intake of selenium. Persons with a preference for
foods such as fish may consume high levels of selenium in their
diet. Sakurai & Tsuchiya (1975) estimated that Japanese eating
large amounts of seafish may ingest as much as 500 µg of selenium
daily. People residing in seleniferous agricultural zones may
consume large amounts of selenium if locally-produced foods
constitute the bulk of their diet. In Venezuela, for example, corn
and rice samples from high selenium areas contained as much as 14
and 18 mg selenium/kg, respectively (Jaffe, 1976), and values as
high as 180 mg/kg have been reported in wheat from Colombia
(Ancizar-Sordo, 1947). If it is assumed that some segments of the
population living in such seleniferous regions consume, under
certain conditions, about half of their diet in the form of rice or
corn with a similar selenium content to that described above, the
daily intake of selenium from dietary sources could exceed 7000 µg.
However, it should be pointed out that only a few samples from the
high-selenium areas showed these extreme high levels. Obviously,
vegetarian diets could vary tremendously in selenium content.
Ganapathy & Dhanda (1976) showed that a vegetarian diet in the USA
supplied 84 µg selenium/day.
If a particular staple food constitutes a large fraction of the
diet of a population, then the selenium content of that food will
have a great influence on the overall dietary-selenium intake. For
example, one estimated daily dietary intake of selenium in Japan
(Sakurai & Tsuchiya, 1975) of 88 µg, was based on selenium levels
in rice and soybeans of 0.05 and 0.02 mg/kg, respectively, whereas
another estimated intake, 208 µg, was based on rice and soybean
selenium levels of 0.220 and 0.234 mg/kg, respectively (Yasumoto et
al., 1976). Low levels of selenium in rice have been reported in
Bangladesh, and it would be particularly interesting to study the
population there, as they depend on rice as a staple food, but they
have a recognized poor dietary vitamin E status (Bieri & Ahmad,
1976).
Table 8. Estimated human dietary intake of selenium (µg/day)a
----------------------------------------------------------------------------------------------------
Food New Zealand Finland United Japanf Canadag USAh USA Venezuelaj
Dunedinb Hamiltonc 1975d 1979d Kingdome South
Dakotai
----------------------------------------------------------------------------------------------------
Plant
vegetables,
fruits,
and sugars 1 2 1 1 3 6 1-9 5 10 15
cereals 4 3 3 25 30 24 62-113 45 57 88
Animal
dairy
products, 11 11 7 13 5 2 5-28 13 48 70
eggs
meat, fish 12 16 19 19 22 56 25-90 69 101 153
Total 28 32 30 50-60 60 88 98-224 132 216 326
----------------------------------------------------------------------------------------------------
a From: Robinson & Thomson (1983).
b From: Thomson & Robinson (1980).
c From: Watkinson (1981).
d From: Varo & Koivistoinen (1981).
e From: Thorn et al. (1978).
f From: Sakurai & Tsuchiya (1975).
g From: Thompson et al. (1975).
h From: Watkinson (1974) & Levander (1976b).
i From: US NAS/NRC (1980).
j From: Levander (1976b).
The importance of the selenium content of a particular staple
in the food supply of persons dependent on a monotonous diet
consisting of locally-produced foods was recently pointed out in
connection with the occurrence of Keshan disease (section 8.2.2) in
China (Yu, 1982). In the years in which Keshan disease was endemic
in the Fuyu county of Heilongjiang province, about 90% of the diet
consisted of maize, whereas, in the years in which Keshan disease
was not endemic, only about 30% of the diet was maize with much of
the rest coming from millet and wheat. Thus, it seems possible
that over-reliance on this single staple food during certain years
helped to precipitate Keshan disease in this area.
Recently, there has been considerable interest in some
countries in the use of textured soy protein products as
substitutes for meat products. Since meat products are such good
dietary sources of selenium, the possible impact of this trend on
the selenium intake of human beings was examined. Some soy protein-
based meat analogues contained as much selenium as meat products,
but others did not (Ferretti & Levander, 1976). These results show
that meat analogues made from soy or other plant proteins are not
consistent sources of selenium, since these plant-based products
will vary in selenium content depending on where the plant is
grown. In contrast, meat products are more reliable dietary
sources of selenium, because they contain a minimum quantity
compatible with animal life.
5.1.1.3. Elderly people
Thomson et al. (1977a) studied a group of 48 elderly people
(mean age of 78 years) in New Zealand and found that their blood-
selenium levels and red cell glutathione peroxidase (EC 1.11.1.9)
activity were lower than those in 12 young adult controls. It was
not possible for the authors to conclude from the data whether the
depressed blood-selenium levels and enzyme activity were due to
decreased selenium intake or were a general part of the aging
process. Abdulla et al. (1979) analysed 7-day pooled dietary
composites of 20 pensioners in Sweden and found that the average
daily selenium intake was 30.8 µg with a range of 8.7 - 96.3
µg/day.
5.1.1.4. Infants and children
The selenium intake of infants is of interest because of their
rapid growth rate and their heavy reliance on milk, a food that has
a highly variable selenium content, depending on its geographical
origin (Table 9). Millar & Sheppard (1972) assumed that human
and cow's milk in New Zealand contained 0.010 and 0.005 mg
selenium/litre, respectively, and calculated that the selenium
intake of infants for the first month of life would be 5.0 or
2.1 µg/day, depending on whether human or cow's milk was fed.
Williams (1983) measured the selenium content of mature mother's
milk from 10 New Zealand women and calculated an average daily
selenium intake of 5 - 6 µg for an infant consuming 700 ml milk.
If the value for the selenium content of Venezuelan cow's milk
shown in Table 9 is typical, it can be calculated that the selenium
intake of infants in that country would be about 58 µg/day during
the first month of life. The most extreme values for the selenium
content of human milk are from low- and high-selenium areas of
China (Yang et al., 1986) (section 4.1.1.1). For infants consuming
750 ml milk daily, these milks would provide 2 µg and 212 µg
selenium, respectively.
Table 9. Estimated infant daily intake of selenium
from dietary sources in North America (6-month-old,
6.5-kg child)a
-----------------------------------------------------
Daily Selenium Selenium
Food consumption content intake
(g) (mg/kg) (µg/day)
-----------------------------------------------------
Milk 824 0.013 11
Orange juice 122 0.014 2
Dry mixed cereal 10 0.540 5
Egg yolk 17 0.437 7
Strained meat 28 0.097 3
Strained fruit 57 0.002 -
Strained vegetable 57 0.003 -
Total selenium intake 28
-----------------------------------------------------
a Adapted from: Levander (1976b).
It was shown that milk continues to furnish a sizeable portion
of an infant's estimated daily selenium intake of 28 µg, in the
USA, after the child starts to eat solid foods (Table 9). The
estimated daily intake of selenium by 3-month-old infants in the
Federal Republic of Germany was 11 µg (Lombeck et al., 1975) and a
"market basket" survey indicated that the intake of 6-month-old
infants in the USA was about 12 µg/day (Harland et al., 1978). On
the basis of a caloric intake of 700 Kcal/day, infants in the USA
consuming formula diets based on milk, soy protein, casein, or
meat, ingested 8.5, 9.5, 12.6, or 31.5 µg/day, respectively (Zabel
et al., 1978).
As infants grow up in New Zealand, they continue to ingest
relatively low levels of selenium, since the estimated daily intake
over the age span of 6 months to 5 years was 5 - 8 µg (McKenzie et
al., 1978).
5.1.1.5. Special medical diets
Infants and children suffering from metabolic diseases such as
phenylketonuria (PKU) or maple syrup urine disease (MSUD) are fed
synthetic diets that contain little selenium for the first 10 or 12
years of their lives. In New Zealand, the mean daily selenium
intake of 12 subjects consuming such synthetic diets was 5 ± 2 µg
with little difference between the older children and the infants
(McKenzie et al., 1978). The selenium content of diets of 3-month-
old infants with PKU and MSUD in the Federal Republic of Germany
amounted to 4.7 and 5.6 µg/day, respectively (Lombeck et al.,
1975).
A wide variation in the selenium contents of special-purpose
medical diets for adults is related to the source of protein in the
diet (Zabel et al., 1978). On the basis of an intake of 2000
Kcal/day, adults consuming food supplements or tube-feeding
formulae would ingest 29, 47, or 98 µg selenium/day, depending on
whether the diet contained soy protein and casein, milk protein and
casein, or blended foods. At a similar caloric intake, chemically-
defined diets based on egg albumen provided 225 µg/day, whereas
diets based on casein hydrolysate or amino acid mixtures provided
only 28 and 1.5 µg/day, respectively. Total parenteral nutrition
solutions based on casein hydrolysate and amino acid mixtures also
furnished low amounts of selenium of only 32 and < 1 µg/day,
respectively. When levels of selenium supplied by special
therapeutic diets were studied in the Chernovitsi region of the
Ukrainian SSR, selenium intakes were found to vary between 99 ± 6
and 121 ± 8 µg/day (Majstruk & Suchkov, 1978).
5.2. Occupational Exposure
5.2.1. Levels in the workplace air
The main pathway of human occupational exposure to selenium is
through the air. Rarely, and under special circumstances, direct
contact may be of importance, i.e., when cutaneous absorption might
be facilitated by the local irritation and skin damage caused by
vesicant selenium compounds.
Various mechanical processes connected with the mining of
seleniferous ores or the grinding of selenium compounds can
contribute selenium-containing dusts to the atmosphere. In other
industrial activities, the amount of selenium released into the air
depends on the temperature to which it is heated and on the area
available for sublimation and/or vaporization. It is well
established that heating amorphous selenium below its melting point
results in its sublimation. At temperatures of 170 - 180 °C,
traces of selenium can be detected in the air and, at temperatures
of 230 - 240 °C, selenium dioxide is released. Heating of
amorphous selenium and selenium dioxide resulted in the release of
substantial quantities of selenium into the air, e.g., from a
surface of 10 cm2, 2.6 - 3.75 mg was released after 20 min at 230 -
240°C and 23 - 31.6 mg was released after 20 min at 350 - 360 °C
(Izraelson et al., 1973).
Little quantitative information is available in the literature
on actual levels of human exposure to selenium in industry. The
levels of selenium in the workplace depend not only on the nature
of the process involved but also on the control technology in any
given industry. Thus, it can be expected that analyses carried out
several decades ago yielded higher values than analyses carried out
more recently. In 1948, Filatova reported air levels of selenium
and selenium dioxide of 20.6 - 24.8 mg/m3 and 0.46 - 0.78 mg/m3,
respectively, in working zones of selenium-heating processes in a
selenium rectifier plant, whereas levels of 0.55 - 1.1 mg/m3 and up
to 0.11 mg/m3, respectively, were associated with the process of
disc smearing (Filatova, cited in Izraelson et al., 1973). In the
production of selenium-containing photoelements by the vacuum
application of a photosensitive layer, selenium and selenium
dioxide levels did not exceed 2.0 mg/m3 and 0.1 mg/m3, respectively
(Sverdlina & Maslennikova, 1961). Selenium grinding, sulfuric acid
treatment, and cathode alloy coating resulted in air-selenium
levels ranging from 0.133 to 2.0 mg/m3 (Izraelson et al., 1973). A
highly-dispersed aerosol selenium condensate with a particle size
of 0.5 - 2 µm was produced at a level of 0.88 - 6.13 mg/m3, as a
result of the manufacture of selenium-containing steels (Ershov,
1969). In the production of rare metals, the air in industrial
premises can contain as much as 0.4 - 750 mg metallic dust/m3,
which, in addition to other elements, contains from 0.8 - 15.7%
selenium (Burchanov, 1972). During the 1950s, Glover (1967)
carried out an extensive survey in which air-selenium levels were
determined in areas of a selenium rectifier plant in which the
workers had been found to have high urinary-selenium values. In
general, the highest air levels of 1.5 - 5.2 mg selenium/m3 were
found in relation to the grinding process. With the exception of
certain "special processes", air levels in the plant due to all the
other manufacturing activities were less than 0.5 mg/m3. In one
case, a level of 21.3 mg/m3 was observed, but no additional details
were given. In this rectifier plant, a process, in which the
elemental selenium was heated, was used for preparing the selenium
mixture that was later applied to the metal plate. For this
reason, the selenium levels in the air are likely to have been
higher than in other factories where the selenium was deposited
from aqueous solution. In another selenium rectifier plant, air
levels of between 7 and 50 µg selenium/m3 were reported
(Kinnigkeit, 1962). However, in this case, no correlation was
observed between the air levels in the workplace and the selenium
levels in the blood of the workers. Kinnigkeit felt that the air-
selenium levels were not high enough to account for the high
urinary-selenium levels in the workers. He suggested that the
discrepancies might be due to incomplete collection of selenium
during air sampling, transient peaks of selenium exposure that were
not detected by short-term sampling, or contamination of hands,
body, and clothing with selenium, which was then transferred to the
mouth as a result of eating or smoking. Another possible
explanation is that measurements of the levels in the workroom air
did not accurately reflect levels of selenium in the actual
breathing zone of the workers.
Low air levels of selenium have been observed in factories
involved with the use of selenium in certain photocopying devices.
In this type of manufacturing, "clean room" techniques are used,
because strict dust control is needed to produce the quality of
photoreceptor surfaces required. Cannella (1976) reported air
levels of selenium ranging from 6 to 91 µg/m3 in various locations
in a selenium alloy plant that made such photoreceptor products.
5.2.2. Biological monitoring
Although selenium levels in the work-place air have been
determined in several instances, these measurements cannot be used
to draw any conclusions about the exposure history of individual
workers. Glover (1967), who estimated occupational exposure to
selenium indirectly by determining selenium levels in the urine of
workers, acknowledged the limitations associated with monitoring
based on grab samples of urine. The Working Group also recognized
the inadequacy of urinary-selenium concentrations as a monitoring
tool. However, it appears that the use of a more rigorous
programme (e.g., total 24-h urine collections) would have resulted
in losing the cooperation of the workers involved. A tentative
maximum allowable concentration of selenium in urine of 0.1
mg/litre was proposed by Glover (1967). The urine of the workers
was analysed at 3-month intervals, and any employee whose urinary-
selenium value exceeded the limit was transferred to a process not
involving selenium. The urinary-selenium levels of these
individuals were tested weekly.
Two primary factors were responsible for setting the maximum
allowable concentration for selenium in urine at 0.1 mg/litre
(Glover, 1970). First, a limit lower than this would have resulted
in a certain number of "false positives", since selenium levels in
urine are often this high in people not industrially exposed to the
element. Second, it was found that values below 0.1 mg/litre were
invariably accompanied by air levels in the work-place of less than
0.1 mg/m3, which was below the threshold limit value of 0.2 mg/m3
for elemental selenium and its common inorganic compounds (ACGIH,
1971). It must be emphasized that urinary selenium cannot be used
to monitor exposure to such dangerous selenium compounds as
hydrogen selenide, selenium oxychloride, or certain organic
selenium compounds because severe damage to the lungs or skin would
have occurred by the time that the urinary-selenium values were
raised (Glover, 1976).
6. METABOLISM OF SELENIUM
The metabolism of selenium has been most completely studied in
animals and data from human investigations are limited. Thus, in
the following sections, pertinent information from selected animal
studies is presented followed by available information from
controlled human studies.
6.1. Absorption
Since food is the primary environmental medium through which
man and animals are exposed to selenium, most data concerning
selenium absorption deal with the gastrointestinal pathway. Much
less is known about the absorption of selenium through the lungs or
skin.
6.1.1. Gastrointestinal absorption
6.1.1.1. Animal studies
The intestinal absorption of soluble selenium compounds by rats
is highly efficient. It has been shown that these animals absorbed
92, 91, and 81% of doses of selenite, selenomethionine, and
selenocystine, respectively (Thomson & Stewart, 1973; Thomson et
al., 1975a). In a study on rats by Whanger et al. (1976a), the
greatest absorption of selenite or selenomethionine occurred from
the duodenum, with slightly less absorption from the jejunum or
ileum. Virtually no absorption occurred from the stomach. The
absorption of selenite by rats does not appear to be under
homeostatic control, since 95% or more of the dose was absorbed,
regardless of whether the animals were fed a selenium-deficient
diet or a diet containing mildly toxic levels of selenium (Brown et
al., 1972). Wright & Bell (1966) found that selenite was absorbed
from the gastrointestinal tract to a greater extent by monogastric
animals (swine) than by ruminant animals (sheep). The decreased
absorption by sheep may have been due to reduction of the
administered selenite to insoluble or unavailable forms by rumen
microorganisms.
Only limited information is available regarding the absorption
by animals of selenium occurring naturally in foods. Kidney tissue
and fish muscle were chosen for model studies, because these foods
are relatively rich sources of dietary selenium for human beings.
The levels of intestinal absorption by rats of radio-selenium
administered in homogenates of rabbit kidney or fish muscle
injected with selenite, were 87 and 64%, respectively (Thomson et
al., 1975b; Richold et al., 1977). The low absorbability of
"fish selenium" agrees with other reports showing the poor
bioavailability to animals of selenium in certain fish products
(section 5).
Little is known concerning the physiological processes
governing the absorption of even simple selenium compounds, though
McConnell & Cho (1965) showed that selenomethionine was transported
against a concentration gradient, whereas selenite and
selenocystine were not.
6.1.1.2. Human studies
Studies carried out in New Zealand on 3 young female volunteers
indicated that the intestinal absorption of oral doses of
radioactive selenite, containing not more than 10 µg selenium, was
70, 64, and 44% of the dose, respectively (Thomson & Stewart,
1974). In a study conducted 2 years later, in which 2 of the 4
female volunteers were women who had participated in the earlier
study with selenite, the intestinal absorption of oral doses of
75Se-selenomethionine, containing less than 2 µg selenium, averaged
about 96% of the dose (Griffiths et al., 1976). When a larger dose
of 1 mg selenium was given orally in solution, a similar difference
in absorption was obtained, i.e., 97% for selenomethionine (one
subject) (Thomson et al., 1978) and about 60% for sodium selenite
(13 subjects) (Robinson et al., 1985; Thomson & Robinson, 1986).
This value for absorption of selenite-selenium is much lower than
the 92% absorption reported for "selenite" in the earlier paper of
Thomson (1974), which is now known to have been selenate-selenium
and not selenite-selenium. Later studies on 10 volunteers also
showed absorption of 94% for 1 mg selenate-selenium in solution
(Thomson & Robinson, 1986). Thus, selenate-selenium is similar to
selenomethionine-selenium in that it is better absorbed than
selenite-selenium.
Correction of selenate-selenium for selenite selenium in the
paper of Thomson (1974) also removes the "surprising difference"
reported between absorption of selenite given in solid form (60%
for 4 female volunteers) and in solution (60% and not 92%). A
female volunteer given a 1 mg dose of selenium as selenite in
solution daily for 5 consecutive days absorbed 59% of the total 5
mg dose (Thomson et al., 1978). It would appear that human beings
have no homeostatic control to limit their absorption of large
single doses of these selenium compounds (Barbezat et al., 1984).
Robinson et al. (1978b) measured the intestinal absorption of
selenium by a New Zealand female volunteer receiving a daily
supplement of 100 µg selenium as selenomethionine, a male volunteer
supplemented daily with 100 µg selenium as sodium selenite, and a
second female supplemented daily with 65 µg selenium, as it occurs
naturally in mackerel (Scomber japonieus). The selenomethionine
and selenite were given in solution, whereas the mackerel was given
as canned fish. During a 4-week supplementation period, 75% of the
selenium given as selenomethionine was absorbed compared with only
48% of the selenium administered as selenite. The absorption of
the "fish selenium" was 66%. Details of the diets consumed by the
3 subjects were not provided, but the subjects supplemented with
selenomethionine or fish selenium did not consume liver or kidney
throughout the study. However, it was specified only that the
subject supplemented with selenite did not eat liver, kidney, or
fish on the days that weekly urine collections were made or on
the day prior to the collection. Also, the subject receiving
selenomethionine ate fish only occasionally throughout the study.
The subject supplemented with the fish selenium ate fish other than
mackerel on only one occasion. Stewart et al. (1978) found that
the average intestinal absorption of naturally-occurring selenium
in foods was 55% in 4 young New Zealand women freely consuming
normal diets. However, allowing for the endogenous faecal
excretion (half of total faecal output) the average true absorption
of naturally occurring selenium became 79%.
6.1.2. Absorption by inhalation
Certain volatile selenium compounds, such as hydrogen selenide,
are known to be toxic when inhaled (Dudley & Miller, 1941).
Lipinskij (1962) observed increased selenium levels in the liver
and kidneys after respiratory exposure of rabbits to elemental
selenium and selenium dioxide. More recently, Weissman et al.
(1979, 1983) studied the distribution and retention of inhaled
75Se-labelled selenious acid and elementary selenium aerosols.
They used 63 Beagle dogs, which were between 3 and 4 years old at
the time of exposure. All inhalation exposures were through the
nose only. Aerosol particles of both forms were small with median
aerodynamic diameters of 0.5 and 0.7 µm and geometric standard
deviations of 2.4 and 1.5 for selenious acid and elemental
selenium, respectively. The urine and faeces of 4 dogs were
collected daily, for 32 days, and then at regular time intervals
until sacrifice. Selenium from exhaled breath was collected from 3
dogs for several 4-h periods, between 2 and 10 days after exposure.
Whole-body retention of 75Se-selenium was measured immediately
after exposure and at regular intervals up to 320 days after
exposure. Two dogs exposed to each aerosol were sacrificed at
various time intervals for over half a year. The initial 75Se-
selenium body burden (IBB) in dogs that inhaled 75Se-selenium as
selenious acid was 40 ± 17 µg selenium/kg body weight and 22 ± 9 µg
selenium/kg body weight in dogs inhaling elementary selenium. For
both forms of selenium, the retention data of 75Se-selenium could
be expressed by a 3-component negative exponential equation:
Selenious acid:
%IBB = 66e-1.5t + 17e-0.089t + 17e-0.023t
Elemental selenium:
%IBB = 66e-0.090t + 21e-0.059t + 13e-0.018t
The corresponding half time values were 0.46, 7.8, and 30 days and
0.77, 12, and 38 days for selenious acid or elemental selenium
exposure, respectively. Studies on organ distribution showed that,
2 h after exposure to selenious acid, only about 5.3% of the IBB
was retained in the lung compared with 26% of the IBB of elemental
selenium. For the first 32 days following exposure, the urine
accounted for 79 and 66% of the 75Se-selenium excreted by dogs
exposed to selenious acid and elemental selenium aerosols,
respectively. Respiratory excretion of 75Se-selenium was
negligible. The organ distribution of 75Se-selenium as a
percentage of the sacrifice body burden is given in Table 10.
Table 10. 75Se-selenium distribution in organs of dogs
128 days after respiratory exposure to 75Se-selenious acid
or elemental 75Se-selenium aerosolsa
----------------------------------------------------------
Organ 75Se-selenium organ distribution
as % of 75Se-selenium body burden
at sacrifice
----------------------------------
selenious acid elemental selenium
treated treated
----------------------------------------------------------
Lung 1.3 6.2
Liver 11.4 7.4
Kidney 1.6 1.6
Blood 19.7 21.5
Gastrointestinal tract 3.4 3.6
Pelt 9.6 9.1
----------------------------------------------------------
a Adapted from: Weissman et al. (1979).
Comparison of the absorption of these two forms of selenium
through inhalation was also studied in the rat (Medinsky et al.,
1981). The rate of transfer of selenium into the blood was slower
when the elemental form was administered. However, once absorbed,
both chemical forms behaved identically.
6.1.3. Absorption through the skin
Apart from the report of Dutkiewicz et al. (1972), who found
that 10% of a 0.1 mol solution of sodium selenite applied to rat
skin was absorbed in 1 h, there are no quantitative data on the
dermal absorption of water-soluble selenium compounds. However,
Dudley (1938) showed that selenium oxychloride was absorbed through
the skin of rabbits. Selenium sulfide, an insoluble selenium
compound used in certain anti-dandruff shampoos, is not ordinarily
absorbed through the skin, although a garlicky breath odour and
elevated urinary excretion of selenium were noted in a patient with
open scalp lesions who used a selenium sulfide shampoo (Ransone et
al., 1961).
6.2. Distribution in the Organism
6.2.1. Transport
Little is known about the transport of selenium in the body.
However, a plasma selenoprotein has been identified which one group
postulates is involved in selenium transport (Motsenbocker &
Tappel, 1982).
6.2.2. Organs
6.2.2.1. Animal studies
Absorbed selenium is rapidly distributed among the tissues.
Heinrich & Kelsey (1955) found that the liver of mice injected
subcutaneously with sodium selenite contained 17 and 30% of the
dose, 15 and 60 min after injection, respectively. However,
because of the great variation in the metabolic half-time of
selenium in various tissues (Thomson & Stewart, 1973), the
distribution pattern observed will change markedly with the time of
sampling after injection with radio-selenium.
As long as the diet provides nutritionally adequate levels of
selenium, the relative concentrations of selenium in the various
internal organs are quite consistant over a wide range of selenium
intakes. For example, Jones & Godwin (1962) studied the
distribution of selenium in the tissues of mice fed alfalfa
containing nutritional levels of selenium. The concentrations of
selenium in the internal organs decreased in the following order:
kidneys > liver > pancreas >> lungs > heart > spleen >
skin > brain > carcass. Smith et al. (1937) fed toxic levels of
sodium selenite to cats and observed the following order for the
concentration of selenium in the internal organs: liver >
kidneys > spleen > pancreas > heart > lungs.
The distribution of selenium appears to be relatively
independent of the form and route of administration, since the
kidneys and adrenals contained the highest concentrations of radio-
selenium, regardless of whether the rats were given labelled
selenite or selenomethionine orally or by intravenous injection
(Thomson & Stewart, 1973). The greatest amounts of radioactivity
were in the kidneys, liver, and total muscle mass. Similar
distribution patterns were noted in rats dosed orally with
homogenates of tissues from rabbits or from fish that had been
given labelled selenium compounds (Thomson et al., 1975b; Richold
et al., 1977).
The distribution of selenium in tissues was markedly different
when rats were fed a diet deficient in selenium: the testes,
brain, thymus gland, and spleen took up the greatest concentrations
of selenium from a tracer dose of selenite (Burk et al., 1972). A
recent report confirms the ability of the testis to retain selenium
better than other tissues in selenium deficiency (Behne & Hofer-
Bosse, 1984).
The distribution of selenium in the eye was determined
fluorometrically with 2,3-diaminonaphthalene by Gusejnov et al.
(1974), who found the following levels in various parts of the eye
of a bull (mg/kg fresh weight): iris, 1.3; retinal pigment
epithelium, 0.85; retina, 0.50; cornea, 0.3; lens, 0.03; and
vitreous body, 0.01. In some birds (pigeons and crows), the level
of selenium in the retina was about 10 times higher (about 5.0
mg/kg). In studies in which 75Se-labelled sodium selenite in
saline was administered intraperitoneally to frogs, rats, and
rabbits, the distribution of radio-selenium was similar to that of
the naturally-occurring selenium measured in the bull's eye. The
maximum 75Se activity in the eye (5 h after administration) was
found in the pigment with the vascular layer of the retina, whereas
minimum activity was found in the lens and vitreous body.
Histoautoradiography of the eyes also indicated the highest
radioactivity in the vascular and pigment layers (Abdullaev et al.,
1972).
6.2.2.2. Human studies
The distribution of selenium in human organs was examined by
Schroeder et al. (1970), who found the following descending order
for selenium concentrations in tissues: kidney > liver >
spleen > pancreas > testes > heart muscle > intestine >
lung > brain. Blotcky et al. (1976) showed that, in autopsy
samples from 106 persons, the concentrations of selenium were 2-3
times higher in the kidneys than in the liver.
Uptake of an orally-administered dose of radioselenite in the
internal organs occurred in the following relative order: liver >
kidneys > lungs > muscle = articular tissue (Leeb et al., 1977).
Other studies have shown significant accumulation of radio-selenium
by the liver and kidneys after intravenous injections of selenite
or selenomethionine (Lathrop et al., 1972; Falk & Lindhe, 1974;
Nordman, 1974).
6.2.3. Blood
Studies on experimental animals have shown a close correlation
between the level of selenium in the blood and the level of
selenium in the diet (Lindberg & Jacobsson, 1970; Cary et al.,
1973; Ermakov & Kovalskij, 1974; Oh et al., 1976a). Analytical
surveys on human beings have shown significant variations in blood-
selenium levels in different geographical areas, presumably because
of differences in dietary-selenium intake. For example, Allaway et
al. (1968) found that selenium levels in the blood of persons
living in areas of the USA where soils and vegetation were high in
selenium tended to contain more selenium than that of persons
living in areas where soils and plants were generally low in
selenium. Thus, geographical differences in the selenium levels in
human blood are possible, even in a country where large-scale
interregional food shipments would be expected to level out
differences in the amount of selenium in the food supply. These
geographical differences in human blood-selenium levels in the USA
have been confirmed by Howe (1974), who found a mean selenium
content of 0.265 mg/litre (SD = 0.056) for 626 samples collected in
and around the state of South Dakota (a high soil-selenium region),
whereas Schultz & Leklem (1983) reported blood-selenium values in
Oregon (a low soil-selenium region) that were well below those
reported for other areas of the USA.
Analyses of blood samples from persons living in different
regions of the world indicate similar correlations between the
amount of selenium in the blood and the amount of selenium in the
food throughout the world (Table 11). For example, blood-selenium
concentrations tend to be lowest in areas of the world that are
known to have low levels of selenium in the soil (Keshan-disease
areas of China, New Zealand, Scandinavia) and highest in areas that
are known to have high levels of selenium in the soil (Venezuela,
reported selenosis areas of China). Superimposed on these
geographical variations in effects are the effects of general
nutritional status or effects of certain disease states. For
example, children suffering from Kwashiorkor have lower blood-
selenium levels than their well-nourished counterparts (Burk et
al., 1967) and reductions in the selenium content of sera have been
observed in patients with cancer (McConnell et al., 1975). Lower
blood-selenium values were associated with lower serum-albumin
values in surgical patients with and without cancer in New Zealand
(Robinson et al., 1979). Akesson (1985) suggested that alterations
in plasma-selenium may be secondary to changes in plasma-protein
concentration. In a study of Finnish men with one or more risk
factors for coronary heart disease, Miettinen et al. (1983)
observed a strong association between serum-selenium and the
eicosapentanoic acid contents of cholesterol ester and
phospholipids. The authors pointed out that since eicosapentanoate
is a fatty acid peculiar to fish such an association could reflect
the intake of fish at the time of blood sampling.
Blood-selenium levels change if persons travel to countries
with soils of different selenium content. For example, Griffiths &
Thomson (1974) noted that the blood-selenium levels of adults from
the USA declined rapidly on arrival in New Zealand, but, after 1
year, their levels were still higher than the mean value for
permanent New Zealand residents (Fig. 2). Rea et al. (1979) found
that plasma-selenium levels changed with changes in selenium intake
more rapidly than erythrocyte-selenium levels. The latter
reflected longer-term changes in selenium exposure, presumably
because of the relatively long life span of erythrocytes. In a
controlled depletion/repletion metabolic study (section 6.3.2.2),
Levander et al. (1980) stated that the plasma-selenium levels of
healthy young male volunteers in the USA dropped about 19% after
2 weeks on a low-selenium diet and then returned to their previous
levels 11 days after consuming a high-selenium diet (Fig. 3).
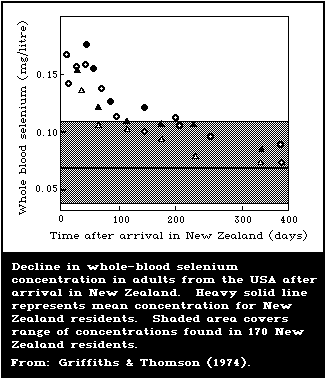
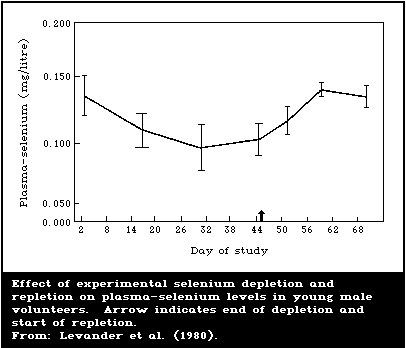 Table 11. Comparison of selenium levels in whole-blood samples
obtained from human beings living in different parts of the world
(values for each country are listed in decreasing numerical order)
-------------------------------------------------------------------
Country or region Reported values Reference
(mg selenium/litre
whole blood)
-------------------------------------------------------------------
Canada
Ontario 0.182 Dickson & Tomlinson
SD ± 0.036 (1967)
The People's Republic of
China
high-selenium area with 3.2 Yang et al. (1983)
a history of intoxication (1.3-7.5)
reported to be chronic
selenosisa
high-selenium area 0.44 Yang et al. (1983)
reported to be without (0.35-0.58)
selenosisa
moderate-selenium 0.095 Yang et al. (1983)
area (Beijing)a SD ± 0.091
low-selenium area 0.027 Yang et al. (1983)
without Keshan disease SD ± 0.009
low-selenium area with 0.021 Yang et al. (1983)
Keshan diseasea SD ± 0.010
Egypt 0.068 Maxia et al. (1972)
(0.054-0.079)
Finland
Helsinki 0.081 Westermarck et al.
SD ± 0.015 (1977)
Lappeenranta 0.056 Westermarck et al.
SD ± 0.017 (1977)
Guatemala 0.23 Burk et al. (1967)
SD ± 0.05
New Zealand
Auckland, North Island 0.083 Robinson & Thomson
SD ± 0.013 (1981)
Dunedin, South Island 0.059 Rea et al. (1979)
SD ± 0.012
-------------------------------------------------------------------
Table 11. (contd.)
-------------------------------------------------------------------
Country or region Reported values Reference
(mg selenium/litre
whole blood)
-------------------------------------------------------------------
Sweden 0.12 Brune et al. (1966)
SD ± 0.02
United Kingdom 0.32 Bowen & Cawse
(0.26-0.37) (1963)
USA
Rapid City, South Dakota 0.256 Allaway et al.
SE ± 0.036 (1968)
Lima, Ohio 0.157
SE ± 0.032
USSR
Ukrainian SSR 0.442 Suchkov (1971)
SE ± 0.034
Azerbeijan SSR 0.11 Abdullaev (1976)
SE ± 0.007
Venezuela
Seleniferous zone 0.813 Jaffe et al.
Villa Bruzual (1972a)
Caracas 0.355
-------------------------------------------------------------------
a These blood samples were collected in areas corresponding to
those shown in Table 8.
The Task Group was aware of one report (Kinnigkeit, 1962) that
presented blood-selenium levels in workers occupationally exposed
to selenium (Table 12). The analytical method used in this study
was a wet chemical technique based on complexing selenium with
diaminobenzidine, and the blood-selenium values obtained by this
procedure from a population not occupationally exposed were below
0.5 mg/litre. These values imply that blood-selenium levels in
workers, at least in the past, were much higher than those found in
the general population (Table 11). Unfortunately no control values
were available (Table 12) so it was not possible to compare the
blood-selenium levels with those obtained using the more recent
analytical technique using diaminonaphthalene instead of
diaminobenzedine (section 2.2.5.1).
6.2.4. Total-body selenium content
Schroeder et al. (1970) estimated the total-body selenium
content of persons in New England, USA, by multiplying the mean
values of the selenium content of human tissues obtained at autopsy
by standard organ weights. In this way, a total-body selenium
content of 14.6 mg (range, 13.0 - 20.3 mg) was calculated for 91.7%
of the body. Stewart et al. (1978) calculated the total-body
selenium content of 4 New Zealand women by 3 different techniques:
using the specific activity of urinary selenium and retained whole-
body radio-selenium; using plasma-selenium and the occupancy of
radio-selenium in whole body and plasma; and using absorbed food-
selenium and the occupancy of absorbed radio-selenium in the whole
body. Depending on whether labelled selenomethionine or selenite
was used in the estimation, the total-body selenium content was
found to be either 6.1 mg (range, 4.1 - 10.0 mg) or 3.0 mg (range,
2.3 - 5.0 mg), respectively, which was less than half that of North
Americans. This was consistent with the selenium contents of
individual tissues (liver, muscle, heart) (Money, 1978; Casey et
al., 1982) of New Zealand subjects, which also contained less than
half that reported in North Americans.
Table 12. Blood-selenium levels reported in
workers from a selenium rectifier planta
---------------------------------------------
Department Number Selenium level
of in blood
workers (mg/litre)
---------------------------------------------
Vaporization 13 4.8 ± 14.8
Measurement 13 15.8 ± 11.8
field
Stamping 12 8.8 ± 13.8
Electric fabrication 18 13.4 ± 12.9
---------------------------------------------
a Geometric mean ± standard error. From: Kinnigkeit (1962).
6.3. Excretion in Urine, Faeces, and Expired Air
6.3.1. Animal studies
Studies on rats have shown that the urinary pathway is the
dominant route for selenium excretion, as long as the dietary
selenium exceeds a certain critical threshold level. For selenium
as selenite, this threshold lies between 0.054 and 0.084 mg/kg
(Burk et al., 1973). As the dietary level of selenium increased
from 0.004 to 1.000 mg/kg, the cumulative 10-day urinary excretion
of a tracer dose of radio-selenite increased from 6 to 67% (Burk et
al., 1972). In contrast, the faecal excretion of selenium remained
constant at about 10% of the dose over this range of dietary-
selenium intake. Significant differences in the urinary excretion
of selenium were demonstrated after dosing rats orally with various
forms of selenium (Richold et al., 1977). For example, the
cumulative levels of selenium excreted in the urine, one week after
dosing with selenite, selenocystine, selenomethionine, "rabbit
kidney" selenium, and "fish muscle" selenium, were 14, 14, 5, 7,
and 6% of the absorbed dose, respectively. In many of these
studies, the endogenous faecal excretion of selenium approached or
exceeded urinary excretion. These decreased urinary/faecal
excretion ratios may have been characteristic of the different
forms of selenium used or may have been due to the low selenium
content of the stock diet fed to the animals (0.050 mg/kg or less).
The urinary-selenium excretion in animals suffering from
chronic selenosis obviously exceeds any dietary threshold
requirement, and cats poisoned with sodium selenite eliminated
50 - 80% of the intake via this pathway, but only 20% or less in
the faeces (Smith et al., 1938). Cats poisoned with organic
selenium in the form of seleniferous wheat protein excreted only
40% of the ingested selenium in the urine, but this decrease in
urinary output was more the result of increased selenium retention
rather than increased faecal excretion.
The main urinary selenium metabolite of rats is trimethyl-
selenonium ion (Byard, 1969; Palmer et al., 1969). This form
accounts for 20 - 50% of the urinary selenium, regardless of the
form of selenium given (Palmer et al., 1970). A recent report
indicates that trimethylselenonium ion accounts for a greater
fraction of urinary selenium under conditions of high selenium
exposure than under conditions of low exposure (Nahapetian et al.,
1983). When selenate is injected into rats, over 35% of the
urinary selenium is in an inorganic form, but less than 3% of the
urinary selenium is inorganic when selenomethionine is injected
into rats. A major urinary metabolite that accounted for 11 - 28%
of the total selenium in rat urine was not identified.
The faecal excretion of selenium is more important in ruminants
than in non-ruminants. For example, sheep given radio-selenite,
orally, excreted 66% of the dose in the faeces, whereas swine
excreted only 15% of the dose via this route (Wright & Bell, 1966).
This increased faecal excretion of selenium by ruminants is the
result of poor absorption rather than elevated endogenous
excretion. The selenite is thought to be reduced to insoluble or
unavailable forms by rumen microorganisms.
The results of studies on rats have demonstrated that excretion
of selenium by the exhalation of volatile compounds is
quantitatively significant only when animals are injected with
doses approaching almost lethal levels of soluble selenium salts
(Olson et al., 1963). A close dose-effect relationship between
selenium exhalation and selenium exposure was observed, since the
amount of selenium exhaled decreased as the amount of selenium
administered decreased. Rats fed toxic levels of selenium in the
diet on a long-term basis, either as seleniferous wheat or sodium
selenite, exhaled less than 2% of the selenium ingested.
6.3.2. Human studies
6.3.2.1. Excretion of selenium
Human volunteers dosed orally with microgram quantities of
selenite or selenomethionine excreted 3 - 4 times more selenium in
the urine than in the faeces over a 2-week collection period (Table
13). Subjects given selenite excreted roughly twice as much total
selenium as subjects given selenomethionine, but the urinary
pathway was dominant in both cases. As might be anticipated from
the low doses administered, losses of selenium via the dermal or
pulmonary routes were negligible.
Table 13. Urinary and endogenous faecal
excretion of radio-selenium by human beings
over a 2-week perioda
----------------------------------------------
Labelled selenium Urinary Faecal excretion
compound excretion (endogenous)
----------------------------------------------
% of absorbed dose
selenite 18 5
selenomethionine 9 2
----------------------------------------------
a Adapted from: Griffiths et al. (1976).
Urinary excretion assumes greater importance when human beings
ingest solutions of milligram quantities of selenium compounds that
are soluble and also well absorbed (Table 14) (section 6.1.1.2).
Volunteers given one milligram of selenium as selenate excreted 81%
in the urine, over 3 times that excreted after similar sized doses
of selenite-selenium, and was still twice as high when expressed as
% absorbed dose. Total excretions in urine and faeces for both
selenite-selenium (63%) and selenate-selenium (90%) suggested that
long-term retention of selenium was not high. Much less of one
milligram selenium as selenomethionine was excreted in urine and
faeces (24% dose), which indicated that selenomethionine was
mainly retained in comparison with selenate- and selenite-selenium.
No pulmonary excretion was observed in one subject given one
milligram of selenium as selenite (Thomson, 1974).
Table 14. Urinary and faecal excretion of one milligram
doses of selenium by human volunteers during a 5-day
perioda
--------------------------------------------------------
Selenium compound Faecal excretion Urinary excretion
% dose % dose % absorbed
dose
--------------------------------------------------------
Selenite 40 23 40
Selenate 8 81 87
Selenomethionine 6 18 20
--------------------------------------------------------
a Adapted from: Thomson & Robinson (1986) (section
6.1.1.2).
Urinary-selenium levels have long been used to monitor
occupational selenium exposure (Glover, 1970), but little is known
about the urinary excretion of selenium derived from foods. The
results of balance studies conducted in New Zealand suggested that
roughly half of the dietary intake of selenium was excreted in the
urine (Robinson et al., 1973; Stewart et al., 1978). However,
these subjects were ingesting small amounts of selenium (18 -
34 µg/day), and this relationship may not hold for persons with
higher selenium intakes.
The importance of the kidneys in the homeostasis of selenium
was emphasized by work from New Zealand that demonstrated that
persons of low selenium status had low renal plasma clearances of
selenium and excreted selenium more sparingly than others (Robinson
et al., 1985).
6.3.2.2. Balance studies
Metabolic trials, conducted on 4 New Zealand female volunteers
in selenium balance, and consuming daily 18 - 34 µg of selenium
occurring naturally in the diet, showed that roughly equivalent
amounts of selenium were voided in the urine and faeces. In the
USA, a controlled depletion/repletion study carried out on 6
healthy young male volunteers in a metabolic unit (Levander et al.,
1980) indicated the rapidity with which both the urinary and faecal
routes adjust to differences in selenium intake (Fig. 4, 5). When
the subjects were brought into the metabolic ward and given a low-
selenium formula diet providing 19 - 24 µg selenium/day (depletion
period), urinary-selenium excretion started to fall immediately and
decreased from an initial level of 54 ± 11 µg/day to 29 ± 5 µg/day
within 14 days. Faecal selenium output also responded rapidly and
declined from 33 ± 13 µg/day to 17 ± 7 µg/day, after only 3 days.
During the time that the subjects were on the low-selenium diet and
were in negative selenium balance, urinary excretion accounted for
about 63% of the total selenium output (Table 15). When the
subjects were fed the low-selenium formula diet plus additional
selenium in the form of seleniferous wheat and/or tuna fish (203 -
224 µg selenium/day), both urinary and faecal selenium excretion
increased rapidly. During this repletion period, the subjects were
in positive selenium balance and urinary excretion comprised about
45% of the total selenium output. Thus, despite a 6-fold
difference in selenium intake and a transition from negative to
positive selenium balance, the proportion of selenium excreted in
the urine remained relatively constant in terms of the total
selenium output, when the selenium was supplied as it occurs
naturally in foods.
The amount of selenium excreted in the sweat was found to be
very low, and was not affected by changes in dietary-selenium
intake (Table 16). In agreement with these results, Griffiths et
al. (1976) found very little radio-selenium in the sweat of
subjects who had been given labelled selenite or selenomethionine.
In the study by Levander et al. (1980), salivary-selenium levels
were also very low, but appeared to reflect differences in dietary-
selenium intakes, since salivary-selenium was somewhat higher at
the end of the repletion period than at the end of the depletion
period (Table 16). Hadjimarkos & Shearer (197l) found 1.1 - 5.2 µg
selenium/litre of saliva in normal children.
Table 11. Comparison of selenium levels in whole-blood samples
obtained from human beings living in different parts of the world
(values for each country are listed in decreasing numerical order)
-------------------------------------------------------------------
Country or region Reported values Reference
(mg selenium/litre
whole blood)
-------------------------------------------------------------------
Canada
Ontario 0.182 Dickson & Tomlinson
SD ± 0.036 (1967)
The People's Republic of
China
high-selenium area with 3.2 Yang et al. (1983)
a history of intoxication (1.3-7.5)
reported to be chronic
selenosisa
high-selenium area 0.44 Yang et al. (1983)
reported to be without (0.35-0.58)
selenosisa
moderate-selenium 0.095 Yang et al. (1983)
area (Beijing)a SD ± 0.091
low-selenium area 0.027 Yang et al. (1983)
without Keshan disease SD ± 0.009
low-selenium area with 0.021 Yang et al. (1983)
Keshan diseasea SD ± 0.010
Egypt 0.068 Maxia et al. (1972)
(0.054-0.079)
Finland
Helsinki 0.081 Westermarck et al.
SD ± 0.015 (1977)
Lappeenranta 0.056 Westermarck et al.
SD ± 0.017 (1977)
Guatemala 0.23 Burk et al. (1967)
SD ± 0.05
New Zealand
Auckland, North Island 0.083 Robinson & Thomson
SD ± 0.013 (1981)
Dunedin, South Island 0.059 Rea et al. (1979)
SD ± 0.012
-------------------------------------------------------------------
Table 11. (contd.)
-------------------------------------------------------------------
Country or region Reported values Reference
(mg selenium/litre
whole blood)
-------------------------------------------------------------------
Sweden 0.12 Brune et al. (1966)
SD ± 0.02
United Kingdom 0.32 Bowen & Cawse
(0.26-0.37) (1963)
USA
Rapid City, South Dakota 0.256 Allaway et al.
SE ± 0.036 (1968)
Lima, Ohio 0.157
SE ± 0.032
USSR
Ukrainian SSR 0.442 Suchkov (1971)
SE ± 0.034
Azerbeijan SSR 0.11 Abdullaev (1976)
SE ± 0.007
Venezuela
Seleniferous zone 0.813 Jaffe et al.
Villa Bruzual (1972a)
Caracas 0.355
-------------------------------------------------------------------
a These blood samples were collected in areas corresponding to
those shown in Table 8.
The Task Group was aware of one report (Kinnigkeit, 1962) that
presented blood-selenium levels in workers occupationally exposed
to selenium (Table 12). The analytical method used in this study
was a wet chemical technique based on complexing selenium with
diaminobenzidine, and the blood-selenium values obtained by this
procedure from a population not occupationally exposed were below
0.5 mg/litre. These values imply that blood-selenium levels in
workers, at least in the past, were much higher than those found in
the general population (Table 11). Unfortunately no control values
were available (Table 12) so it was not possible to compare the
blood-selenium levels with those obtained using the more recent
analytical technique using diaminonaphthalene instead of
diaminobenzedine (section 2.2.5.1).
6.2.4. Total-body selenium content
Schroeder et al. (1970) estimated the total-body selenium
content of persons in New England, USA, by multiplying the mean
values of the selenium content of human tissues obtained at autopsy
by standard organ weights. In this way, a total-body selenium
content of 14.6 mg (range, 13.0 - 20.3 mg) was calculated for 91.7%
of the body. Stewart et al. (1978) calculated the total-body
selenium content of 4 New Zealand women by 3 different techniques:
using the specific activity of urinary selenium and retained whole-
body radio-selenium; using plasma-selenium and the occupancy of
radio-selenium in whole body and plasma; and using absorbed food-
selenium and the occupancy of absorbed radio-selenium in the whole
body. Depending on whether labelled selenomethionine or selenite
was used in the estimation, the total-body selenium content was
found to be either 6.1 mg (range, 4.1 - 10.0 mg) or 3.0 mg (range,
2.3 - 5.0 mg), respectively, which was less than half that of North
Americans. This was consistent with the selenium contents of
individual tissues (liver, muscle, heart) (Money, 1978; Casey et
al., 1982) of New Zealand subjects, which also contained less than
half that reported in North Americans.
Table 12. Blood-selenium levels reported in
workers from a selenium rectifier planta
---------------------------------------------
Department Number Selenium level
of in blood
workers (mg/litre)
---------------------------------------------
Vaporization 13 4.8 ± 14.8
Measurement 13 15.8 ± 11.8
field
Stamping 12 8.8 ± 13.8
Electric fabrication 18 13.4 ± 12.9
---------------------------------------------
a Geometric mean ± standard error. From: Kinnigkeit (1962).
6.3. Excretion in Urine, Faeces, and Expired Air
6.3.1. Animal studies
Studies on rats have shown that the urinary pathway is the
dominant route for selenium excretion, as long as the dietary
selenium exceeds a certain critical threshold level. For selenium
as selenite, this threshold lies between 0.054 and 0.084 mg/kg
(Burk et al., 1973). As the dietary level of selenium increased
from 0.004 to 1.000 mg/kg, the cumulative 10-day urinary excretion
of a tracer dose of radio-selenite increased from 6 to 67% (Burk et
al., 1972). In contrast, the faecal excretion of selenium remained
constant at about 10% of the dose over this range of dietary-
selenium intake. Significant differences in the urinary excretion
of selenium were demonstrated after dosing rats orally with various
forms of selenium (Richold et al., 1977). For example, the
cumulative levels of selenium excreted in the urine, one week after
dosing with selenite, selenocystine, selenomethionine, "rabbit
kidney" selenium, and "fish muscle" selenium, were 14, 14, 5, 7,
and 6% of the absorbed dose, respectively. In many of these
studies, the endogenous faecal excretion of selenium approached or
exceeded urinary excretion. These decreased urinary/faecal
excretion ratios may have been characteristic of the different
forms of selenium used or may have been due to the low selenium
content of the stock diet fed to the animals (0.050 mg/kg or less).
The urinary-selenium excretion in animals suffering from
chronic selenosis obviously exceeds any dietary threshold
requirement, and cats poisoned with sodium selenite eliminated
50 - 80% of the intake via this pathway, but only 20% or less in
the faeces (Smith et al., 1938). Cats poisoned with organic
selenium in the form of seleniferous wheat protein excreted only
40% of the ingested selenium in the urine, but this decrease in
urinary output was more the result of increased selenium retention
rather than increased faecal excretion.
The main urinary selenium metabolite of rats is trimethyl-
selenonium ion (Byard, 1969; Palmer et al., 1969). This form
accounts for 20 - 50% of the urinary selenium, regardless of the
form of selenium given (Palmer et al., 1970). A recent report
indicates that trimethylselenonium ion accounts for a greater
fraction of urinary selenium under conditions of high selenium
exposure than under conditions of low exposure (Nahapetian et al.,
1983). When selenate is injected into rats, over 35% of the
urinary selenium is in an inorganic form, but less than 3% of the
urinary selenium is inorganic when selenomethionine is injected
into rats. A major urinary metabolite that accounted for 11 - 28%
of the total selenium in rat urine was not identified.
The faecal excretion of selenium is more important in ruminants
than in non-ruminants. For example, sheep given radio-selenite,
orally, excreted 66% of the dose in the faeces, whereas swine
excreted only 15% of the dose via this route (Wright & Bell, 1966).
This increased faecal excretion of selenium by ruminants is the
result of poor absorption rather than elevated endogenous
excretion. The selenite is thought to be reduced to insoluble or
unavailable forms by rumen microorganisms.
The results of studies on rats have demonstrated that excretion
of selenium by the exhalation of volatile compounds is
quantitatively significant only when animals are injected with
doses approaching almost lethal levels of soluble selenium salts
(Olson et al., 1963). A close dose-effect relationship between
selenium exhalation and selenium exposure was observed, since the
amount of selenium exhaled decreased as the amount of selenium
administered decreased. Rats fed toxic levels of selenium in the
diet on a long-term basis, either as seleniferous wheat or sodium
selenite, exhaled less than 2% of the selenium ingested.
6.3.2. Human studies
6.3.2.1. Excretion of selenium
Human volunteers dosed orally with microgram quantities of
selenite or selenomethionine excreted 3 - 4 times more selenium in
the urine than in the faeces over a 2-week collection period (Table
13). Subjects given selenite excreted roughly twice as much total
selenium as subjects given selenomethionine, but the urinary
pathway was dominant in both cases. As might be anticipated from
the low doses administered, losses of selenium via the dermal or
pulmonary routes were negligible.
Table 13. Urinary and endogenous faecal
excretion of radio-selenium by human beings
over a 2-week perioda
----------------------------------------------
Labelled selenium Urinary Faecal excretion
compound excretion (endogenous)
----------------------------------------------
% of absorbed dose
selenite 18 5
selenomethionine 9 2
----------------------------------------------
a Adapted from: Griffiths et al. (1976).
Urinary excretion assumes greater importance when human beings
ingest solutions of milligram quantities of selenium compounds that
are soluble and also well absorbed (Table 14) (section 6.1.1.2).
Volunteers given one milligram of selenium as selenate excreted 81%
in the urine, over 3 times that excreted after similar sized doses
of selenite-selenium, and was still twice as high when expressed as
% absorbed dose. Total excretions in urine and faeces for both
selenite-selenium (63%) and selenate-selenium (90%) suggested that
long-term retention of selenium was not high. Much less of one
milligram selenium as selenomethionine was excreted in urine and
faeces (24% dose), which indicated that selenomethionine was
mainly retained in comparison with selenate- and selenite-selenium.
No pulmonary excretion was observed in one subject given one
milligram of selenium as selenite (Thomson, 1974).
Table 14. Urinary and faecal excretion of one milligram
doses of selenium by human volunteers during a 5-day
perioda
--------------------------------------------------------
Selenium compound Faecal excretion Urinary excretion
% dose % dose % absorbed
dose
--------------------------------------------------------
Selenite 40 23 40
Selenate 8 81 87
Selenomethionine 6 18 20
--------------------------------------------------------
a Adapted from: Thomson & Robinson (1986) (section
6.1.1.2).
Urinary-selenium levels have long been used to monitor
occupational selenium exposure (Glover, 1970), but little is known
about the urinary excretion of selenium derived from foods. The
results of balance studies conducted in New Zealand suggested that
roughly half of the dietary intake of selenium was excreted in the
urine (Robinson et al., 1973; Stewart et al., 1978). However,
these subjects were ingesting small amounts of selenium (18 -
34 µg/day), and this relationship may not hold for persons with
higher selenium intakes.
The importance of the kidneys in the homeostasis of selenium
was emphasized by work from New Zealand that demonstrated that
persons of low selenium status had low renal plasma clearances of
selenium and excreted selenium more sparingly than others (Robinson
et al., 1985).
6.3.2.2. Balance studies
Metabolic trials, conducted on 4 New Zealand female volunteers
in selenium balance, and consuming daily 18 - 34 µg of selenium
occurring naturally in the diet, showed that roughly equivalent
amounts of selenium were voided in the urine and faeces. In the
USA, a controlled depletion/repletion study carried out on 6
healthy young male volunteers in a metabolic unit (Levander et al.,
1980) indicated the rapidity with which both the urinary and faecal
routes adjust to differences in selenium intake (Fig. 4, 5). When
the subjects were brought into the metabolic ward and given a low-
selenium formula diet providing 19 - 24 µg selenium/day (depletion
period), urinary-selenium excretion started to fall immediately and
decreased from an initial level of 54 ± 11 µg/day to 29 ± 5 µg/day
within 14 days. Faecal selenium output also responded rapidly and
declined from 33 ± 13 µg/day to 17 ± 7 µg/day, after only 3 days.
During the time that the subjects were on the low-selenium diet and
were in negative selenium balance, urinary excretion accounted for
about 63% of the total selenium output (Table 15). When the
subjects were fed the low-selenium formula diet plus additional
selenium in the form of seleniferous wheat and/or tuna fish (203 -
224 µg selenium/day), both urinary and faecal selenium excretion
increased rapidly. During this repletion period, the subjects were
in positive selenium balance and urinary excretion comprised about
45% of the total selenium output. Thus, despite a 6-fold
difference in selenium intake and a transition from negative to
positive selenium balance, the proportion of selenium excreted in
the urine remained relatively constant in terms of the total
selenium output, when the selenium was supplied as it occurs
naturally in foods.
The amount of selenium excreted in the sweat was found to be
very low, and was not affected by changes in dietary-selenium
intake (Table 16). In agreement with these results, Griffiths et
al. (1976) found very little radio-selenium in the sweat of
subjects who had been given labelled selenite or selenomethionine.
In the study by Levander et al. (1980), salivary-selenium levels
were also very low, but appeared to reflect differences in dietary-
selenium intakes, since salivary-selenium was somewhat higher at
the end of the repletion period than at the end of the depletion
period (Table 16). Hadjimarkos & Shearer (197l) found 1.1 - 5.2 µg
selenium/litre of saliva in normal children.
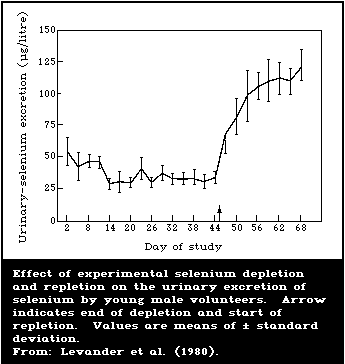
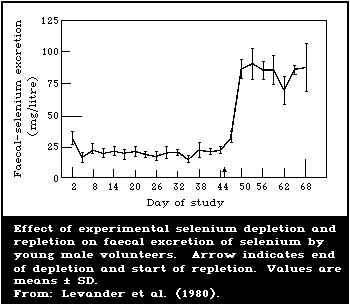 Table 15. Selenium balance during depletion and
repletion periodsa
-----------------------------------------------------
Selenium balance
Depletion period Repletion period
-----------------------------------------------------
(µg) (µg)
Urinary excretion -1665 ± 211 -2435 ± 272
Faecal excretion -985 ± 103 -1919 ± 228
Total excretion -2650 ± 257 -4354 ± 295
Intake +1524 ± 173 +5424 ± 248
Overall balance -1126 ± 268 +1070 ± 482
-----------------------------------------------------
a Data expressed as mean ± standard deviation.
Adapted from: Levander et al. (1981b).
Table 16. Effect of selenium depletion and
repletion on the selenium contents of saliva
and sweat in young mena
---------------------------------------------
Body Selenium content
fluid Start of End of End of
study depletion repletion
---------------------------------------------
(µg selenium/litre)
Saliva 2.8 ± 0.7b,c 1.4 ± 0.4b 4.4 ± 0.4c
Sweat - 1.4 ± 0.2 1.2 ± 0.7
---------------------------------------------
a Adapted from: Levander et al. (1981b).
b,c Means in the same row with different
superscript letters differ significantly
( P < 0.05, Duncan's multiple range test).
Data expressed as mean ± SD.
6.4. Retention and Turnover
6.4.1. Animal studies
Results of studies on rats have shown that the whole-body
retention of a single injected dose of radioactive selenite is
described by a curve that consists of an initial phase, a
transition phase, and an extended phase (Ewan et al., 1967; Burk et
al., 1972). During the initial equilibration phase, there is a
distribution of radioactive selenium throughout the tissues and a
rapid excretion of radio-selenium in the urine, faeces, and, if the
dose is sufficiently high, the expired air. The initial phase is
followed by a transition phase, during which the rate of loss of
radio-selenium is less than that in the initial phase but greater
than that in the final extended phase. The extended phase consists
of a slow constant rate of radio-selenium loss which represents the
long-term whole-body turnover of selenium. Increasing the dose of
radioactive selenite administered decreased the percentage of the
dose retained during the initial phase, but did not have any effect
on radio-selenium retention during the extended phase. Increasing
the level of dietary selenium increased the turnover of radio-
selenium during both the initial and extended phases. For example,
supplementing the diet with 0.95 mg selenium/kg decreased the
biological half-life of radio-selenium, during the extended phase,
from 78 to 27 days. The extended phase of radio-selenium retention
in rats was shown by Richold et al. (1977) to be largely
independent of the route of administration and the chemical form of
selenium given. Thus, after the initial period, it appears that
selenium from a variety of sources is ultimately incorporated into
the same metabolic pool in rats. The apparent "whole-body"
retention of radio-selenium during the extended phase is an average
of several discrete processes, since each internal organ has its
own characteristic rate of selenium turnover. For example, the
biological half-times of radio-selenium in the kidneys, whole body,
and skeletal muscle of rats were 38, 55, and 74 days, respectively
(Thomson & Stewart, 1973).
Information on the retention of selenium by animals became of
practical significance when approval was sought for the use of
selenium as a feed additive to prevent nutritional deficiency
diseases (section 4). Because of the toxicity of high levels of
selenium, there was some concern about the possible build up of
residues in the edible flesh of animals supplemented with selenium.
Scott & Thompson (1971) noted very little increase in the
concentrations of selenium in the tissues of chicks or poults fed
low-selenium diets supplemented with 0.2 - 0.8 mg selenium/kg as
sodium selenite. However, feeding a diet naturally high in
selenium, but not toxic (0.67 mg/kg) resulted in substantially
increased tissue-selenium levels.
Scott & Thompson (1971) also showed that addition of sodium
selenite to a diet that was naturally high in selenium did not
produce any further increase in tissue-selenium levels. In further
studies on turkeys, swine, sheep, and cattle, animal feeds,
naturally low or marginal in selenium, were supplemented with
nutritional levels of selenium (0.1 - 0.5 mg/kg) as sodium selenite.
Resulting tissue-selenium concentrations were not any higher than
those that would be expected if the animals were fed diets
naturally adequate in selenium (Groce et al., 1971; Cantor & Scott,
1975; Ullrey et al., 1977).
These observations are in agreement with those from earlier
work with toxic levels of selenium, which indicated that naturally-
occurring organically-bound selenium was retained in the tissues to
a much greater extent than inorganic selenium (Smith et al., 1938).
Martin & Hurlbut (1976) fed mice high levels of selenium as
selenite, selenium-methylselenocysteine, or selenomethionine.
After 7 weeks, the mice fed selenomethionine had much higher levels
of selenium in their tissues than those fed either selenite or
selenium-methylselenocysteine. Moreover, when the various selenium
compounds were removed from the diet, selenium was retained more
strongly in the tissues of the mice fed the selenomethionine than
in those of the mice fed selenite or selenium-methylselenocysteine.
The results of this study showed that a distinction more precise
than inorganic versus organic must be made when discussing the
metabolism of selenium compounds, since the metabolism of selenium-
methylselenocysteine resembled that of selenite rather than that of
selenomethionine.
Differences in the retention of selenite compared with
selenomethionine were also observed when these 2 forms of selenium
were fed in the diet at nutritional levels. For example, Miller et
al. (1972) showed that the total body selenium content of chicks
was increased by an average of 29.1%, when 0.025 - 0.500 mg of
selenium as selenomethionine was fed per kg of diet, but was
increased only by 17.9% when selenium as selenite was fed at
similar levels.
Selenium retained by female animals can apparently be used
later to protect their offspring against the effects of selenium
deficiency. Allaway et al. (1966) fed ewes alfalfa containing
2.6 mg selenium/kg for 5 months followed by a diet low in selenium.
The ewes were able to transmit levels of selenium to their lambs
that protected them against White Muscle Disease, even though the
lambs were born 10 months after the low-selenium diet was first
administered.
6.4.2. Controlled human studies
As in the case of rats, the total body retention curves of
radioactive selenium in human beings can be resolved into 3
components (Griffiths et al., 1976). However, oral doses of
75Se-selenomethionine are retained more strongly and turned over
more slowly than oral doses of 75Se-selenite (Table 17). This
metabolic difference between selenomethionine and selenite holds
true, even if the compounds are administered by intravenous
injection (Lathrop et al., 1972; Falk & Lindhe, 1974). These
results differ with those obtained in rats in which several forms
of selenium apparently were incorporated into the same long-term
metabolic pool and were thus turned over at similar rates (Richold
et al., 1977). On this basis, it was suggested that
selenomethionine, or food-selenium in a form that produced
selenomethionine after digestion, might prove more effective than
selenite in improving a low-selenium status or in correcting
selenium deficiency in man (Griffiths et al., 1976).
Table 17. Retention of oral doses of selenite and selenomethionine by
human beingsa
----------------------------------------------------------------------
Whole-body selenium Biological half-times for
Form of retention 14 days whole-body selenium retention
selenium after dose phase 1 phase 2 phase 3
given (% of absorbed dose) (days)
----------------------------------------------------------------------
selenite 78 1.0 8 103
selenomethionine 89 1.7 18 234
----------------------------------------------------------------------
a Adapted from: Griffiths et al. (1976).
Thomson et al. (1982) gave 100 µg doses of selenium as
selenomethionine or sodium selenite to 12 New Zealand volunteers
over a period of several weeks. Blood glutathione peroxidase (EC
1.11.19) activity increased in all subjects and, at 17 weeks,
the response was similar in both selenomethionine and selenite
groups. Increases in blood-selenium levels were greater after
supplementation with selenomethionine than with selenite. Selenium
levels tended to plateau in the selenite-treated subjects but kept
increasing in the selenomethionine-treated subjects. Similar
differences in the retention and turnover of selenium as selenate
compared with selenium in selenium-rich yeast or wheat were
observed in a bioavailability trial in Finland (Levander et al.,
1983) (section 5.1.1.6). Thus, it appears that feeding of selenium
bound in organic form (selenomethionine, selenium-rich yeast or
wheat) results in higher blood-selenium levels than the feeding of
selenium as selenate or selenite, but there is no difference in the
glutathione peroxidase activities ultimately achieved during
supplementation. However, when the selenium supplements were
discontinued, the glutathione peroxidase activities remained
somewhat elevated in the groups receiving the wheat or yeast
compared with those receiving selenate. Apparently, the selenium
in the yeast or wheat retained in the tissues could be used for
glutathione peroxidase production after the selenium supplement was
discontinued.
6.5. Metabolic Transformation
Some metabolic transformations of selenium compounds are
outlined in Fig. 6. This scheme tends to emphasize the central
role of selenite, but other forms of selenium may have important
characteristic pathways of their own, under certain conditions
(e.g., the direct incorporation of selenomethionine as such into
tissue proteins).
Table 15. Selenium balance during depletion and
repletion periodsa
-----------------------------------------------------
Selenium balance
Depletion period Repletion period
-----------------------------------------------------
(µg) (µg)
Urinary excretion -1665 ± 211 -2435 ± 272
Faecal excretion -985 ± 103 -1919 ± 228
Total excretion -2650 ± 257 -4354 ± 295
Intake +1524 ± 173 +5424 ± 248
Overall balance -1126 ± 268 +1070 ± 482
-----------------------------------------------------
a Data expressed as mean ± standard deviation.
Adapted from: Levander et al. (1981b).
Table 16. Effect of selenium depletion and
repletion on the selenium contents of saliva
and sweat in young mena
---------------------------------------------
Body Selenium content
fluid Start of End of End of
study depletion repletion
---------------------------------------------
(µg selenium/litre)
Saliva 2.8 ± 0.7b,c 1.4 ± 0.4b 4.4 ± 0.4c
Sweat - 1.4 ± 0.2 1.2 ± 0.7
---------------------------------------------
a Adapted from: Levander et al. (1981b).
b,c Means in the same row with different
superscript letters differ significantly
( P < 0.05, Duncan's multiple range test).
Data expressed as mean ± SD.
6.4. Retention and Turnover
6.4.1. Animal studies
Results of studies on rats have shown that the whole-body
retention of a single injected dose of radioactive selenite is
described by a curve that consists of an initial phase, a
transition phase, and an extended phase (Ewan et al., 1967; Burk et
al., 1972). During the initial equilibration phase, there is a
distribution of radioactive selenium throughout the tissues and a
rapid excretion of radio-selenium in the urine, faeces, and, if the
dose is sufficiently high, the expired air. The initial phase is
followed by a transition phase, during which the rate of loss of
radio-selenium is less than that in the initial phase but greater
than that in the final extended phase. The extended phase consists
of a slow constant rate of radio-selenium loss which represents the
long-term whole-body turnover of selenium. Increasing the dose of
radioactive selenite administered decreased the percentage of the
dose retained during the initial phase, but did not have any effect
on radio-selenium retention during the extended phase. Increasing
the level of dietary selenium increased the turnover of radio-
selenium during both the initial and extended phases. For example,
supplementing the diet with 0.95 mg selenium/kg decreased the
biological half-life of radio-selenium, during the extended phase,
from 78 to 27 days. The extended phase of radio-selenium retention
in rats was shown by Richold et al. (1977) to be largely
independent of the route of administration and the chemical form of
selenium given. Thus, after the initial period, it appears that
selenium from a variety of sources is ultimately incorporated into
the same metabolic pool in rats. The apparent "whole-body"
retention of radio-selenium during the extended phase is an average
of several discrete processes, since each internal organ has its
own characteristic rate of selenium turnover. For example, the
biological half-times of radio-selenium in the kidneys, whole body,
and skeletal muscle of rats were 38, 55, and 74 days, respectively
(Thomson & Stewart, 1973).
Information on the retention of selenium by animals became of
practical significance when approval was sought for the use of
selenium as a feed additive to prevent nutritional deficiency
diseases (section 4). Because of the toxicity of high levels of
selenium, there was some concern about the possible build up of
residues in the edible flesh of animals supplemented with selenium.
Scott & Thompson (1971) noted very little increase in the
concentrations of selenium in the tissues of chicks or poults fed
low-selenium diets supplemented with 0.2 - 0.8 mg selenium/kg as
sodium selenite. However, feeding a diet naturally high in
selenium, but not toxic (0.67 mg/kg) resulted in substantially
increased tissue-selenium levels.
Scott & Thompson (1971) also showed that addition of sodium
selenite to a diet that was naturally high in selenium did not
produce any further increase in tissue-selenium levels. In further
studies on turkeys, swine, sheep, and cattle, animal feeds,
naturally low or marginal in selenium, were supplemented with
nutritional levels of selenium (0.1 - 0.5 mg/kg) as sodium selenite.
Resulting tissue-selenium concentrations were not any higher than
those that would be expected if the animals were fed diets
naturally adequate in selenium (Groce et al., 1971; Cantor & Scott,
1975; Ullrey et al., 1977).
These observations are in agreement with those from earlier
work with toxic levels of selenium, which indicated that naturally-
occurring organically-bound selenium was retained in the tissues to
a much greater extent than inorganic selenium (Smith et al., 1938).
Martin & Hurlbut (1976) fed mice high levels of selenium as
selenite, selenium-methylselenocysteine, or selenomethionine.
After 7 weeks, the mice fed selenomethionine had much higher levels
of selenium in their tissues than those fed either selenite or
selenium-methylselenocysteine. Moreover, when the various selenium
compounds were removed from the diet, selenium was retained more
strongly in the tissues of the mice fed the selenomethionine than
in those of the mice fed selenite or selenium-methylselenocysteine.
The results of this study showed that a distinction more precise
than inorganic versus organic must be made when discussing the
metabolism of selenium compounds, since the metabolism of selenium-
methylselenocysteine resembled that of selenite rather than that of
selenomethionine.
Differences in the retention of selenite compared with
selenomethionine were also observed when these 2 forms of selenium
were fed in the diet at nutritional levels. For example, Miller et
al. (1972) showed that the total body selenium content of chicks
was increased by an average of 29.1%, when 0.025 - 0.500 mg of
selenium as selenomethionine was fed per kg of diet, but was
increased only by 17.9% when selenium as selenite was fed at
similar levels.
Selenium retained by female animals can apparently be used
later to protect their offspring against the effects of selenium
deficiency. Allaway et al. (1966) fed ewes alfalfa containing
2.6 mg selenium/kg for 5 months followed by a diet low in selenium.
The ewes were able to transmit levels of selenium to their lambs
that protected them against White Muscle Disease, even though the
lambs were born 10 months after the low-selenium diet was first
administered.
6.4.2. Controlled human studies
As in the case of rats, the total body retention curves of
radioactive selenium in human beings can be resolved into 3
components (Griffiths et al., 1976). However, oral doses of
75Se-selenomethionine are retained more strongly and turned over
more slowly than oral doses of 75Se-selenite (Table 17). This
metabolic difference between selenomethionine and selenite holds
true, even if the compounds are administered by intravenous
injection (Lathrop et al., 1972; Falk & Lindhe, 1974). These
results differ with those obtained in rats in which several forms
of selenium apparently were incorporated into the same long-term
metabolic pool and were thus turned over at similar rates (Richold
et al., 1977). On this basis, it was suggested that
selenomethionine, or food-selenium in a form that produced
selenomethionine after digestion, might prove more effective than
selenite in improving a low-selenium status or in correcting
selenium deficiency in man (Griffiths et al., 1976).
Table 17. Retention of oral doses of selenite and selenomethionine by
human beingsa
----------------------------------------------------------------------
Whole-body selenium Biological half-times for
Form of retention 14 days whole-body selenium retention
selenium after dose phase 1 phase 2 phase 3
given (% of absorbed dose) (days)
----------------------------------------------------------------------
selenite 78 1.0 8 103
selenomethionine 89 1.7 18 234
----------------------------------------------------------------------
a Adapted from: Griffiths et al. (1976).
Thomson et al. (1982) gave 100 µg doses of selenium as
selenomethionine or sodium selenite to 12 New Zealand volunteers
over a period of several weeks. Blood glutathione peroxidase (EC
1.11.19) activity increased in all subjects and, at 17 weeks,
the response was similar in both selenomethionine and selenite
groups. Increases in blood-selenium levels were greater after
supplementation with selenomethionine than with selenite. Selenium
levels tended to plateau in the selenite-treated subjects but kept
increasing in the selenomethionine-treated subjects. Similar
differences in the retention and turnover of selenium as selenate
compared with selenium in selenium-rich yeast or wheat were
observed in a bioavailability trial in Finland (Levander et al.,
1983) (section 5.1.1.6). Thus, it appears that feeding of selenium
bound in organic form (selenomethionine, selenium-rich yeast or
wheat) results in higher blood-selenium levels than the feeding of
selenium as selenate or selenite, but there is no difference in the
glutathione peroxidase activities ultimately achieved during
supplementation. However, when the selenium supplements were
discontinued, the glutathione peroxidase activities remained
somewhat elevated in the groups receiving the wheat or yeast
compared with those receiving selenate. Apparently, the selenium
in the yeast or wheat retained in the tissues could be used for
glutathione peroxidase production after the selenium supplement was
discontinued.
6.5. Metabolic Transformation
Some metabolic transformations of selenium compounds are
outlined in Fig. 6. This scheme tends to emphasize the central
role of selenite, but other forms of selenium may have important
characteristic pathways of their own, under certain conditions
(e.g., the direct incorporation of selenomethionine as such into
tissue proteins).
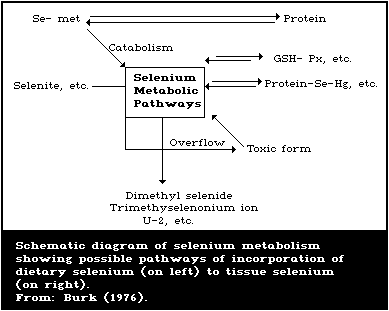 6.5.1. Animal studies
6.5.1.1. Reduction and methylation
The main flow of selenium metabolism in animals is via
reductive pathways, which contrasts with the primarily oxidative
metabolism of sulfur (Levander, 1976a). Selenite can react with
glutathione or protein sulfhydryls to form selenotrisulfides
(Ganther, 1968; Jenkins & Hidiroglou, 1971) which, at least in the
case of the glutathione derivatives, can be reduced further by an
enzymatic mechanism to selenide (Ganther & Hsieh, 1974; Diplock,
1976).
Under normal conditions, selenide is methylated to form
trimethylselenonium ion, the main urinary metabolite of selenium
(section 6.3.1). In cases of selenium toxicity, this pathway is
overloaded and dimethyl selenide is produced. This is the main
volatile selenium metabolite expired via the lungs and is
responsible for the typical "garlicky odour" of animals poisoned
with selenium (McConnell & Portman, 1952a). The last 2 reactions
could be considered detoxication steps, since the methylated end-
products are much less toxic for the organism than selenite
(McConnell & Portman, 1952b; Obermeyer et al., 1971). However,
both of these methylated selenium derivatives have strong
synergistic toxicity with other minerals (section 7) and dimethyl
selenide toxicity in male rats was reported to be inversely related
to the level of previous selenium intake (Parizek et al., 1980).
6.5.1.2. Form in proteins
Early work with animals poisoned with selenium revealed that
much of the selenium in the tissues was associated with protein
(Smith et al., 1938). Since that time there has been considerable
controversy about the exact chemical nature of the selenium in
tissue proteins (Levander, 1976a). Selenomethionine may be
incorporated initially as such into animal proteins (Ochoa-Solano
& Gitler, 1968), but, in rats, it appears to be eventually
catabolized to selenite or selenate (Millar et al., 1973; Thomson
& Stewart, 1973). Non-ruminant animals cannot synthesize
selenomethionine from inorganic selenium compounds (Cummins &
Martin, 1967; Olson & Palmer, 1976), but rabbits and rats can
convert selenite into selenocysteine tissue proteins (Godwin &
Fuss, 1972; Olson & Palmer, 1976). Forstrom et al. (1978) have
proposed that seleno-cysteine is the form of selenium located at
the catalytic site of glutathione peroxidase and others have shown
that selenocysteine is essential for the activity of clostridial
glycine reductase (Cone et al., 1976). The mechanism by which
selenocysteine is formed from selenite is not known but
incorporation of preformed selenocysteine and post-translational
modification of the protein have been considered (Sunde, 1984).
Other possible forms of selenium in proteins include
selenotrisulfides (Ganther & Corcoran, 1969) and "acid-labile"
selenium (Diplock et al., 1973).
6.5.1.3. Conversion of selenium compounds to nutritionally-active
forms of selenium
The pioneering studies of Schwarz & Foltz (1958) demonstrated
that a variety of selenium compounds could protect against dietary
liver necrosis in vitamin E- and selenium-deficient rats. Sodium
selenite, sodium selenate, selenium dioxide, selenic acid, and
potassium selenocyanate were all more or less equally active, but
elemental gray selenium was essentially inactive. Selenocystine
and selenomethionine were about as effective as the active
inorganic selenium compounds, but an organic selenium fraction
isolated from pig kidney powder (Factor 3) was shown to be even
more active. A number of organic derivatives of selenium have
shown some protective effect against liver necrosis, but none was
superior to Factor 3 (Schwarz et al., 1972). However, certain
simple amino-acid derivatives of monoseleno diacetic acid showed
some promise in that they combined high nutritional potency with a
low order of toxicity (Schwarz, 1976). The effects of selenite and
selenomethionine were shown to be roughly similar in inducing
glutathione peroxidase activity in the tissues of rats fed a diet
deficient in selenium (Pierce & Tappel, 1977).
Cantor et al. (1975a) found that while selenite and
selenocystine were equally effective in preventing exudative
diathesis in vitamin E- and selenium-deficient chicks, seleno-
methionine was less effective. This suggests a difference between
rats and chicks in the metabolism of selenomethionine. The degree
of protection against exudative diathesis and the level of plasma
glutathione peroxidase activity were highly correlated, suggesting
that nutritional potency depended on the ability of the chick to
convert various selenium compounds to the enzymatically active
form.
6.5.2. Human studies
Few studies have been carried out to investigate the metabolic
transformation of selenium compounds in human beings, but the
limited data available suggest certain similarities in the
metabolism of selenium by man and animals. For example, human
beings overexposed to selenium develop breath with a smell of
garlic, which is presumably due to the exhalation of dimethyl
selenide (Glover, 1976). Also, the chromatographic pattern of
urinary-selenium metabolites is the same in man and the rat (Burk,
1976). However, species differences in selenium metabolism do
exist, since human beings retain selenomethionine to a much greater
extent than selenite, whereas the retention of the 2 compounds in
rats is about the same (section 6.4.2).
7. EFFECTS OF SELENIUM ON ANIMALS
The considerable biological importance of selenium was first
recognized in the 1930s when it was discovered that certain well-
defined and economically important farm animal diseases were
actually the result of chronic selenium poisoning (section 7.1).
These animal diseases were restricted to agricultural areas in
which large amounts of selenium in the soil were available for
uptake by the plants, which were then consumed by the animals.
Research, over the last 20 years, showed that selenium was an
essential trace element (section 7.2), and selenium deficiency
diseases were rapidly recognized in several species of farm
animals. These deficiency diseases are significant economic
problems in areas of the world where the soil levels of the
element available for uptake by plants are low.
7.1. Selenium Toxicity
7.1.1. Farm animal diseases associated with a high selenium intake
On the basis of field experience, Rosenfeld & Beath (1964)
delineated 3 different types of selenium poisoning in livestock:
(a) acute;
(b) chronic, of the blind staggers type; and
(c) chronic, of the alkali disease type.
Acute poisoning is due to the ingestion of toxic quantities of
selenium in the form of highly seleniferous accumulator plants.
The animal has severe signs of distress such as laboured breathing,
abnormal movement and posture, prostration, and diarrhoea. Death
often follows within a few hours. This type of selenium poisoning
is rather rare under field conditions, since grazing animals
generally avoid the selenium accumulator plants, except in times of
pasture shortage. Acute selenium poisoning has also been produced
by the experimental or accidental administration of selenium
compounds to farm animals (US NAS/NRC, 1976).
Blind staggers has been reported in animals that eat a limited
number of selenium accumulator plants over a period of weeks or
months (Rosenfeld & Beath, 1964). The affected animals wander,
stumble, have impaired vision, and eventually succumb to
respiratory failure. Although this type of poisoning can be
produced experimentally by the administration of water extracts of
accumulator plants, it has not been possible to duplicate this
syndrome by the administration of pure selenium compounds.
Possibly alkaloids or other toxic substances found in many
seleniferous plants may contribute to blind staggers (Maag & Glenn,
1967; Van Kampen & James, 1978).
Alkali disease is associated with the consumption of grains
containing 5 - 40 mg selenium/kg over weeks or months. Animals
exhibit liver cirrhosis, lameness, hoof malformations, loss of
hair, and emaciation. Maag & Glenn (1967) were unable to produce
alkali disease in cattle by feeding inorganic selenium, but several
other studies have shown that the syndrome is causally associated
with seleniferous grains or grasses and can be produced by feeding
inorganic selenium salts (Olson, 1978).
The Task Group noted that most of the work on alkali disease
was concerned with cattle, but was aware of the report by Ermakov &
Kovalskij (1968), which described chronic selenium toxicity of this
type in sheep, under natural conditions. In sheep fed feeds
containing levels of 2 mg selenium/kg feed (fresh weight), the
following characteristic signs were observed: hoof deformation,
loss of hair, hypochromic anaemia, and increases in the activity of
both alkaline and acid phosphatases in various tissues.
7.1.2. Toxicity in experimental animals
The practical significance of selenium poisoning in farm
animals stimulated a great deal of research on both the acute and
chronic effects of selenium in laboratory animals. Interest in the
toxic effects of repeated exposure to selenium via inhalation was
stimulated by concern about the possible effects on human health of
occupational exposure to selenium. Also, studies were carried out
with the aim of establishing the no-observed-adverse-effect dose
level of selenium when administered in the drinking-water. In some
of the studies in which selenium was given via either the air or
water, biochemical and/or behavioural criteria were used to assess
the biological effects of selenium exposure. However, as discussed
in section 7.1.6, the toxicity of selenium compounds can be
influenced by different variables and the results from various
laboratories are often not comparable because of quite different
experimental conditions. Also, the criteria for toxicity are less
developed than the signs of deficiency (section 7.2).
Reviews that deal with various aspects of selenium toxicology
include those by Rosenfeld & Beath (1964), Muth (1966), Izraelson
et al. (1973), Ermakov & Kovalskij (1974), US NAS/NRC (1976), and
Lazarev (1977).
7.1.2.1. Acute and subacute toxicity - single or repeated exposure
studies with oral, intraperitoneal, or cutaneous administration
Perhaps the most characteristic sign of acute selenium
poisoning in animals is the development of the so-called "garlicky
breath odour", which is due to the pulmonary excretion of volatile
selenium compounds, particularly dimethyl selenide, by animals
overexposed to selenium (section 6.3.1). Other signs of acute
selenium poisoning described by Franke & Moxon (1936) in dogs and
rats included: vomiting, dyspnoea, tetanic spasms, and death from
respiratory failure. Pathological changes included congestion of
the liver with areas of focal necrosis, congestion of the kidney,
endocarditis, myocarditis, peticheal haemorrhages of the
epicardium, atony of the smooth muscles of the gastrointestinal
tract, gall-bladder, and bladder, and erosion of the long bones,
especially the tibia. The LD50 values for sodium selenite,
administered orally to various animal species, are given in Table
18 (Pletnikova, 1970).
Table 18. Acute oral toxicity of sodium selenite for various
species of laboratory animalsa
-------------------------------------------------------------
Species LD50 Statistical method
(mg selenium/kg
body weight)
-------------------------------------------------------------
White mouse (male) 7.75 Behrens & Schlosser
7.08 Litchfield & Wilcoxon
Albino rat (female) 10.50 Behrens & Schlosser
13.19 Diechmann & LeBlanc
Guinea-pig (female) 5.06 Deichmann & LeBlanc
Rabbit (female) 2.25 Diechmann & LeBlanc
-------------------------------------------------------------
a From: Pletnikova (1970).
Table 19. Acute toxicity of some selenium compounds administered to rats by
intraperitoneal injection
----------------------------------------------------------------------------
Compound Criterion Toxic dose Reference
of (mg selenium/kg
toxicity body weight)
----------------------------------------------------------------------------
Sodium selenite MLD 3.25 - 3.5 Franke & Moxon (1936)
Sodium selenate MLD 5.25 - 5.75 Franke & Moxon (1936)
DL-selenocystine MLD 4 Moxon (1940)
DL-selenomethionine MLD 4.25 Klug et al. (1950)
Diselenodipropionic LD50 25 - 30 Moxon et al. (1938)
acid
Trimethylselenonium LD50 49.4 Obermeyer et al. (1971)
chloride
Dimethyl selenide LD50 1600 McConnell & Portman (1952b)
----------------------------------------------------------------------------
For a given species, the lethal doses of sodium selenite,
sodium selenate, DL-selenocystine, and DL-selenomethionine are
quite similar (Table 19). Although certain methylated metabolites
of selenium such as dimethylselenide and trimethylselenonium
chloride were considered relatively innocuous (see LD50 values by
McConnell & Portman and Obermeyer et al. in Table 19), more recent
work by Parizek et al. (1976, 1980) has shown that the toxicity of
methylated selenium compounds depends not only on the sex of the
animal (Parizek et al., 1974) but also on the level of previous
selenium intake. For example, 90% mortality was observed in male
rats maintained on a diet containing 0.05 mg selenium/kg, when they
were injected intraperitoneally with 20 µmoles dimethylselenide/kg
body weight, i.e., by a dose of dimethylselenide that is more than
1000 times lower than the LD50 reported by McConnell & Portman
(1952b). Pre-treatment with a small amount of selenite (1 µmol/kg
body weight), intraperitoneally, 6 h, but not 1 h, before
dimethylselenide injection or increased oral intake of selenite
(Table 20), protected male rats against the toxicity of a
subsequent dose of dimethylselenide (Parizek et al., 1976, 1980).
Moreover, the methylated forms of selenium have strong synergistic
toxicities with other minerals (section 7.1.6.3, 7.4.1) and
dimethylselenide can be much more toxic for male rats than for
female rats (section 7.1.6).
Table 20. Dependence of the toxicity of dimethylselenide on
the level of previous oral intake of seleniuma
------------------------------------------------------------
Selenite Single intraperitoneal 24-h mortality (%)
supplement in dose of dimethylselenide Diet A Diet B
drinking-water
(mg selenium/ (mg mole/kg body weight) (n = 20) (n = 10)
litre
------------------------------------------------------------
0 20 90 90
0.1 20 45 30
0.5 20 5 0
1.0 20 0 0
------------------------------------------------------------
a Adapted from: Parizek et al. (1976, 1980).
Male rats (2 months old) given drinking-water with stated
supplement of selenite for 3 days before dimethylselenide
administration. Diet A contained 0.052 ± 0.005 mg selenium/kg and
diet B (semi-synthetic diet) 0.044 ± 0.001 mg selenium/kg.
Smith et al. (1937) reported that the minimum lethal dose
of selenium as sodium selenite or selenate in rabbits, rats,
and cats was 1.5 - 3.0 mg/kg body weight, regardless of whether
the compounds were administered orally, subcutaneously,
intraperitoneally, or intravenously. This lack of effect of the
mode of administration probably reflects the rapid and complete
absorption of soluble selenium compounds, either from the site of
injection or from the gastrointestinal tract.
Cummins & Kimura (1971) described comparative studies on
Sprague Dawley rats and dogs concerning the oral toxicity of the
following selenium compounds: sodium selenate, selenourea,
biphenylselenium, selenium sulfide (1 - 30 µ particle size), and
elemental selenium (1 - 30 µ particle size). The oral LD50 values
in rats for a number of selenium compounds are shown in Table 21
and demonstrate the large variations in LD50 values that occur,
depending on both the oxidation state of the compound and its
aqueous solubility.
Table 21. Comparative solubility and toxicity of various
selenium compounds in ratsa
--------------------------------------------------------------
Compound Solubility in 0.01 N HCl Rat oral LD50 (95% CL)
(mg/kg body weight)
--------------------------------------------------------------
Na2SeO3 700 g/litre 7 (4.4 - 11.2)
H2N-C-NH2 30 g/litre 50 (35.7 - 70.0)
||
Se
SeS2 insoluble (< 1 g/litre) 138 (110 - 172)
Se 5 g/litre 360 (308 - 421)
Elemental Se insoluble (< 1 g/litre) 6700 (6000 - 7300)
--------------------------------------------------------------
a From: Cummins & Kimura (1971).
Male Sprague Dawley rats, each weighing between 50 and 100 g,
were given the above chemicals by gavage as 0.1 - 20% suspensions
in 0.5% methylcellulose. A total of 30-36 animals was used per
compound, in groups of 6 animals per dose. The LD50 values and
the associated confidence limits (CL) were calculated according to
the method of Litchfield & Wilcoxon.
The aqueous solubilities were carried out in 0.01 N HCl to more
closely simulate acidic conditions in the stomach. The least toxic
selenium compound was insoluble elemental selenium with an LD50 of
6.7 g/kg body weight. Toxic signs included pilomotor activity,
decreased body activity, dyspnoea, diarrhoea, anorexia, and
cachexia. Fatalities occurred within 18 - 72 h; survivors appeared
outwardly normal, at the end of the 7-day observation period. The
most toxic of the selenium compounds tested was the highly soluble
sodium selenite with an oral LD50 of 7 mg/kg body weight and the
toxic signs were similar to those seen with high doses of elemental
selenium. Selenium sulfide (a component in shampoos) was about 20
times less toxic than sodium selenite (i.e., an LD50 of 138 mg/kg
compared with 7 mg/kg). It was also found in this study that, with
the exception of biphenyl selenium, toxicity could be correlated
with blood-selenium levels. For example, sodium selenite being the
most toxic, gave the highest blood level followed in descending
order by selenourea, selenium sulfide, and elemental selenium. It
was suggested that the blood-selenium level produced by the
relatively non-toxic biphenyl selenium was high because this
covalently-bound selenium compound is the most lipophilic compound,
which is better absorbed, resists catabolism, and apparently
circulates as the parent compound.
The application of 83 mg of selenium oxychloride to the skin of
rabbits caused the death of the animals in 5 h, and application of
4 mg caused death in 24 h (Dudley, 1938). Lazarev (1977) reported
a minimum lethal dose of selenium oxychloride of 7 mg/kg body
weight, after cutaneous administration to rabbits.
Several papers have shown that injection of selenite in rats in
the early postnatal period in single doses of 1.5 mg selenium/kg
body weight, or in repeated doses of 0.5 mg/kg, induced cataracts
(Ostadalova et al., 1979; Bhuyan et al., 1981; Shearer et al.,
1980; Bunce et al., 1985). Similar effects have not been observed
in hamsters (Shearer et al., 1980). This response is dose
dependant (Table 22) and, thus far, has been observed only when the
selenite was given by parenteral injection.
Table 22. Cataractagenesis by selenite in the rata
--------------------------------------------------
Group Daily dosageb Frequency of cataractsc
(mg selenium/kg (%)
body weight)
--------------------------------------------------
Control 0 0
Na2SeO3 0.25 13
Na2SeO3 0.50 96
Na2SeO3 0.75 96
--------------------------------------------------
a Adapted from: Shearer et al. (1980).
b Dose given to rat pups daily on days 2-18
postpartum.
c Observed at 21 days of age.
7.1.2.2. Effects of long-term oral exposure
Moxon & Rhian (1943) summarized several older studies that
indicated that diets containing 5 mg selenium/kg or more cause
chronic selenosis in several species of animals, such as chickens,
rats, and dogs. In seleniferous areas, 5 mg selenium/kg diet is
generally accepted as the dividing line between toxic and non-toxic
feeds (US NAS/NRC, 1976).
Halverson et al. (1966) fed diets containing 0, 1.6, 3.2, 4.8,
6.4, 8.0, 9.6, or 11.2 mg selenium/kg in the form of sodium
selenite or seleniferous wheat to male Sprague Dawley rats,
initially weighing 60 - 70 g. The rats were divided into groups of
8 and were fed the diets for 6 weeks. The diet consisted of (g/kg)
ground wheat, 809; purified casein 120; USP salt mixture XIV, 20;
USP brewer's yeast, 20; corn oil, 30; and vitamin B12 mix, 1.
Vitamins A, D, and E were provided separately. The criteria used
to assess toxicity were growth depression, liver cirrhosis,
splenomegaly, pancreatic enlargement, anaemia, elevated
serumbilirubin levels, and death. The addition to the diet of 1.6,
3.2, or 4.8 mg selenium/kg did not have any significant effect on
the rats, as judged by any of these criteria. There was a
depression in growth in the group that received 4.8 mg dietary
selenium/kg as sodium selenite, but this was not significant.
Liver cirrhosis, splenomegaly, and significant growth depression
were observed when the rats were fed levels of 6.4 mg/kg or more
from either source of selenium. Diets containing 8.0 mg/kg or more
caused additional effects such as pancreatic enlargement, anaemia,
elevated serum-bilirubin levels, and, after 4 weeks, death.
Diets made either with non-seleniferous or seleniferous sesame
meal were fed to groups of 12 male Sprague Dawley rats, initially
weighing 50 g for a period of 6 weeks (Jaffe et al., 1972b). The
diet made with non-seleniferous sesame meal contained 0.5 mg
selenium/kg and consisted of (g/kg): non-seleniferous sesame meal,
463.3; almidon, 422.7; corn oil, 50; cod liver oil, 10; USP XVI
salts, 40; L-lysine x HCl, 4; and vitamin mix, 10. The diet made
with the seleniferous sesame meal contained 10 mg selenium/kg and
had the same composition as the previous diet except that
seleniferous sesame, which replaced the non-seleniferous sesame
meal, was added at a level of 490.2 g/kg, and the almidon was added
at a level of 395.8 g/kg. The rats fed the diet containing the
seleniferous sesame meal showed decreased survival, impaired weight
gain, higher incidence of liver lesions, elevated hepatic selenium
levels, enlarged spleens, depressed haemoglobin, haematocrit, and
fibrinogen levels, and decreased prothrombin activity (Table 23).
In a separate study, the same workers investigated the effects of
dietary selenium, given as seleniferous sesame meal, on the
activities of various serum enzymes (Table 24). The diet
containing 4.5 mg selenium/kg had the same composition as the
previous diets, except that the seleniferous sesame meal, non-
seleniferous sesame meal, and almidon were added at levels of
230.8, 270.4, and 384.8 g/kg, respectively. Feeding the diet
containing 4.5 mg of selenium/kg for 6 weeks increased the
activities of serum alkaline phosphatase (EC 3.1.3.1) and glutamic
pyruvic transaminase (SGPT) (EC 2.6.1.2), whereas 10.0 mg/kg also
increased the activity of glutamic-oxaloacetic transaminase (SGOT)
(EC 2.6.1.1).
Table 23. Pathological signs in rats fed diets containing seleniferous sesame meala
--------------------------------------------------------------------------------------------------------------------
Dietary Survival Weight Rats Hepatic Spleen Haemo- Haema- Fibrinogen Prothrombin
selenium after gain with selenium weight globin tocrit activity
6 weeks after hepatic level level value
6 weeks lesions
--------------------------------------------------------------------------------------------------------------------
(mg/kg) (g) (mg/kg) (% of body (g/litre (volume %) (mg/litre)
weight) blood)
0.5 12/12 156.8 ± 7.2 0/12 0.72 ± 0.14 0.207 ± 0.01 147 ± 2.1 43.9 ± 0.61 1664 ± 78 963 ± 9.5
10 8/12 61.2 ± 7.43 10/12 7.34 ± 0.97 0.544 ± 0.09 123 ± 3.5 40.8 ± 0.82 655 ± 78 713 ± 76.6
--------------------------------------------------------------------------------------------------------------------
a Adapted from: Jaffe et al. (1972b).
Table 24. Effect of chronic selenium toxicity on the
activity of serum enzymesa
-------------------------------------------------------
Dietary Number Alkaline SGOT SGPT
selenium of rats phosphatase
-------------------------------------------------------
(mg/kg) (units/ml) (units/ml) (units/ml)
0.5 6 6.3 ± 0.2 66 ± 3 20 ± 1
4.5 10 8.8 ± 1.3 59 ± 2 26 ± 2
10 8 18.9 ± 2.3 77 ± 5 43 ± 6
-------------------------------------------------------
a Adapted from: Jaffe et al. (1972b).
Tinsley et al. (1967) and Harr et al. (1967) carried out an
extensive study on the chronic toxicity of selenium in rats.
Although the primary purpose of their research was to investigate
the alleged carcinogenicity of selenium (section 7.7.1), the design
of their study provided an opportunity to examine other aspects of
the toxicity of selenium, such as the influence of selenium
poisoning on growth rate and histopathology. A total of 1437
Wistar rats from a closed random-bred colony was used with the size
of the experimental groups ranging from 10 - 110 animals. The rats
were fed one of 3 different diets: a semipurified diet containing
either 12 or 22% casein or a commercial "laboratory chow" type
ration. The casein-based diets also contained corn oil, 50 g;
H.M.W. salts, 40 g; vitamin mix, 10 g; and glucose monohydrate
(Cerelose) up to a weight of 1 kg. Selenium as sodium selenite or
sodium selenate was added at levels of 0, 0.5, 2.0, 4.0, 8.0, or
16.0 mg/kg. Only a small proportion of the animals fed diets
supplemented with more than 4.0 mg/kg of selenium survived for 12
months. The rats fed the commercial ration were 2 - 3 times more
resistant to the effects of selenium toxicity than the rats fed the
semipurified diet. A calculated maximum body weight was reported
to be depressed by as little as 0.5 mg selenium/kg, but no
statistical evaluation of the results was presented. Usually
moribund animals were killed and necropsied for histopathological
determinations, but 136 rats were killed at specific ages.
Acute toxic hepatitis was the predominant histopathological
lesion in rats fed the commercial ration supplemented with sodium
selenate at 16 mg selenium/kg. These rats had a median survival
age of only 96 days. Acute toxic hepatitis was also observed in
rats fed the 12% casein diet supplemented with sodium selenate at 4
or more mg selenium/kg. The rats were emaciated, pale, and had
poor quality hair coat. Hydrothorax, ascites, pericardial oedema,
and icterus were common. Myocardial hyperaemia, fluid imbalance,
and parenchymal degeneration were often present. The adrenals were
enlarged and the pancreas was oedematous. The failure of normal
chondrocyte proliferation observed in the metaphyses appeared to be
different from that seen in malnutrition, since proliferation was
irregular and not merely reduced.
When rats were fed the commercial ration supplemented with
sodium selenate at 8 mg selenium/kg, the predominant lesion was
chronic toxic hepatitis. The median survival age in this group was
429 days. Other histopathological changes reported included
pancreatic duct hyperplasia, intestinal nephritis, and myocardial
damage, particularly in rats of more than 450 days of age and
receiving selenite.
Harr et al. (1967) reported an increased proliferation of the
hepatic parenchyma when the rats were fed the semi-purified diet
supplemented with 0.5 - 2.0 mg selenium/kg as selenite or selenate.
But a more detailed report from this laboratory (Weswig et al.,
1966) showed that this lesion of "chronic liver and bile duct
hyperplasia" was observed to a greater extent in rats fed the
commercial ration not supplemented with selenium than in rats fed
the semi-purified diet supplemented with 0.5 mg selenium/kg. Thus,
this lesion may not be specifically related to selenium.
Harr & Muth (1972) stated that 0.25 mg/kg was the minimum toxic
level for liver lesions, when selenium was added to a semi-purified
diet. The minimum toxic level was 0.75 mg/kg when the criteria
were longevity or lesions of the heart, kidneys, or spleen.
However, growth was normal in rats fed 0.5 mg selenium/kg diet.
The authors estimated that the dietary threshold for physiological
and pathological effects was 0.4 mg/kg and for pathological and
clinical effects, 3 mg/kg.
Weanling rats of the Long-Evans strain were given either sodium
selenite or selenate at 0 or 2 mg selenium/litre in the drinking-
water for 1 year (Schroeder & Mitchener, 1971b). After 1 year, the
selenium dosage was increased to 3 mg/litre in the selenate group.
The weanlings were born from random-bred females that had been fed
a low-selenium diet (0.05 mg selenium/kg) consisting of: whole rye
flour, 600 g; dry skim milk, 300 g; corn oil, 90 g; and iodized
sodium chloride to which were added vitamins and iron, 1 g/kg. The
same diet was fed to the weanlings during the toxicity study. The
drinking-water was doubly deionized forest spring water to which
had been added: zinc, 50 mg; manganese, 10 mg; chromium(III), 5 mg;
copper, 5 mg; cobalt, 1 mg; and molybdenum, 1 mg/litre. The rats
given the selenium compounds (plus another group not discussed here
given sodium tellurite) were divided into groups totalling 313
animals. There were 105 control rats. By 58 days, half of the
male rats in the selenite group were dead. Fifty percent mortality
in the group of female rats given selenite was not achieved until
348 days and was not achieved in the male and female groups given
selenate until 962 and 1014 days, respectively. On the other hand,
Palmer & Olson (1974) gave 2 or 3 mg selenium/litre drinking-water,
in the form of either sodium selenite or selenate to male weanling
Sprague Dawley rats for 6 weeks and noted small decreases in weight
gain compared with control rats receiving selenium, but no deaths.
Two diets were used in this study, a rye diet similar to that
described by Schroeder & Mitchener above and a corn diet that
consisted of ground corn, 808 g; casein, 120 g; corn oil, 30 g;
U.S.P. XIV salts, 20 g; and vitamin mix, 22 g/kg. The trace
elements zinc, copper, manganese, chromium, cobalt, and molybdenum
were also added to the drinking-water, as suggested by Schroeder &
Mitchener (1971a).
In a study by Jacobs & Forst (1981a), sodium selenite at 0 or 4
mg selenium/litre drinking-water was administered to groups of 17
and 30 male 5-week-old Sprague Dawley rats fed a commercial pellet
ration. After 64 weeks, survival was 94 and 63% in the control and
selenium-treated groups, respectively. In a second study of similar
design, except that the rats were 8 weeks old at the start,
survival was 90 and 95% in the control and selenium-treated groups,
respectively, after 61 weeks.
Pletnikova (1970) investigated the effects of long-term, low-
level administration of sodium selenite in water to rabbits and
rats. For these studies, 32 rabbits and 16 rats were divided into
4 groups and administered, orally, doses of 0, 0.005, 0.0005, or
0.00005 mg selenium/kg body weight for periods of 7 1/2 and 6
months, respectively. Prolonged administration of the maximum dose
investigated (0.005 mg/kg) produced significant alterations in the
rabbits. After 2 months, there was a significant increase in the
concentration of oxidized glutathione in the blood and, after 7
months, there was slower elimination of bromsulphalein by the
liver, and hepatic succinic dehydrogenase activity was decreased.
A dose of 0.0005 mg/kg caused fewer, less pronounced changes,
whereas 0.00005 mg/kg did not produce any statistically significant
effects in any of these tests. A dose of 0.005 mg/kg given to rats
caused a considerable weakening of the capacity for forming new
conditioned reflexes. It can be assumed that a daily dose of 0.005
mg/kg body weight in rats is equivalent to a dietary-selenium level
of about 0.063 mg/kg. Since this level of selenium is within the
physiological range needed to prevent selenium deficiency in
animals (section 7.2.2), the toxicological significance of these
observations is not clear, unless it is assumed that a certain dose
of selenite given in water solution is more toxic than the same
dose given in the diet.
Recently, Csallany et al. (1984) reported that giving sodium
selenite to female mice in the drinking-water at a level of 0.1 mg
selenium/litre increased the amount of hepatic lipid-soluble
lipofuscin pigments, when the animals were sacrificed at 9 months
of age. There were 8 mice in each group and the animals were fed a
diet adequate in vitamin E, which contained 0.05 mg selenium/kg.
Sodium selenite added to the drinking-water at a level of 9 mg
selenium/litre killed all 12 rats fed a diet based on either ground
corn or ground rye in 6 weeks (Palmer & Olson, 1974), whereas
Halverson et al. (1966) found that sodium selenite added to a diet
based on ground wheat, at a level of 9.6 mg/kg, killed only 1 out
of 10 rats in the same period of time. However, the rats used in
the latter study were post-weanling animals weighing 60 - 70 g when
the study started, whereas the rats used in the former study were
21-day-old weanlings and weighed only 35 - 45 g. Other research
workers have shown that the resistance of rats to the toxic effects
of selenium increases markedly after the twenty-first day of life
(Franke & Potter, 1936).
Feng et al. (1985) studied the hepatoxicity of high selenium
corn (7.12 mg/kg) produced in the Enshi county of China, where
human selenium intoxication was reported (section 8.1.1.1). After
feeding a diet that contained 61% of this corn (total selenium
concentration of 4.343 mg/kg) for 16 weeks, liver cirrhosis was
seen in 3 out of 6 male and 5 out of 6 female rats in one group.
No liver damage was found histologically in another group of rats
in the same study, which had consumed a diet containing 30.5% of
the high selenium corn (total selenium concentration of 2.35
mg/kg).
At present, the best indicator of chronic selenium toxicity
appears to be growth inhibition (US NAS/NRC, 1976), and a selenium
level of 4 - 5 mg/kg is necessary to achieve this response in
animals fed a normal diet. In laboratory rats, this exposure to
selenium represents an intake of about 200 - 250 µg/kg body weight
per day. More sensitive and specific criteria of selenium
poisoning to demonstrate effects at lower dose levels, such as
biochemical or histological techniques, would obviously be highly
desirable, but such tests are not available at present.
7.1.2.3. Inhalation toxicity
The effects of respiratory exposure to selenium compounds,
administered under conditions mimicking occupational exposure, have
been described in several papers. Filatova (1951) studied the
toxic effects of respiratory exposure to selenium dioxide (SeO2)
under conditions similar to those that occur in industry, i.e.,
heating of selenium (section 5.2.1). In acute studies, white rats
were exposed to air concentrations of selenium dioxide of 0.15 -
0.6 mg/litre, and all rats died within one-half - 4 h.
Morphological examination of the organs revealed that intraalveolar
and perivascular oedema occurred in the lungs, and haemorrhages and
degenerative changes in the liver, kidney, and heart. In 4
additional studies, all rats survived 4 h when exposed to doses of
0.09, 0.06 - 0.07, or 0.03 - 0.04 mg selenium dioxide/litre, but
all rats exposed to the highest dose (equal to 5 - 5.2 mg/kg body
weight) died within 24 h. In a series of long-term studies, rats
were exposed to repeated doses of selenium dioxide at 0.01 - 0.03,
0.006 - 0.009, or 0.003 - 0.005 mg/litre for 6 h, every other day,
for one month. The lowest dose did not produce any effects on body
weight or on the blood picture, and all the rats survived.
Histological examination revealed degenerative changes in the
liver, renal tubules, dystrophy of heart muscle, and hyperaemia and
hypertrophy of the splenic pulp. At the dose of 0.006 - 0.009
mg/litre, all the rats but one died within 27-33 days. For the
first 2 weeks, there was no difference in body weight between the
exposed and unexposed control rats but, during the last 2 weeks,
the exposed rats lost body weight and all but one of the exposed
rats died. The histopathological changes consisted of multiple
necrosis and degeneration in the liver and myocardial fibres, and
involvement of renal tubules. In the third group of rats, which
was exposed to 0.01 - 0.03 mg/litre, the animals showed respiratory
distress, weight loss, and, in 3 rats, anaemia. All the rats died
between days 8 and 18 of exposure. In the liver, kidneys,
myocardium, and spleen, the changes observed were similar to those
seen at lower doses, but more pronounced. Moreover, lung oedema
similar to that noted in the acute exposure studies was seen.
Lipinskij (1962) exposed 2 groups of 5 rabbits to airborne
amorphous selenium and selenium dioxide in chambers, under
conditions analogous to industrial exposure, except that the doses
were higher. In the first group, the rabbits were exposed to 20 mg
selenium dioxide/litre and 40 mg selenium/m3, for 2 h per day, for
one week, at which time a decrease in blood catalase (EC 1.11.1.6)
activity was noted. The rabbits in the second group were exposed
to 10 µg selenium dioxide/litre and 20 mg selenium/m3, for 2 h
daily, for 12 weeks. After 12 weeks, decreases in total and
reduced glutathione were observed, but there was no change in
levels of oxidized glutathione.
On the basis of studies on 60 white rats weighing 120 - 150 g,
Burchanov et al. (1969) concluded that intratracheal injection of
0.06 ml of a sterile suspension containing 50 mg of highly
dispersed elemental selenium dust in physiological saline, for
1 - 12 months, resulted in decreases in body weight and muscular
strength, and morphological and biochemical alterations in the
respiratory tract. Burchanov (1972) obtained similar results in
inhalation studies on rats exposed to 2 types of polymetallic dusts
found in industry.
The acute toxicity of selenium dust (average mass median
particle diameter, 1.2 µ) for rats, guinea-pigs, and rabbits was
described by Hall et al. (1951). Exposure of these animals, for
16 h, to an atmosphere containing approximately 30 mg selenium
dust/m3 produced mild interstitial pneumonitis in the animals.
Rats exposed to selenium fumes developed acute toxic effects, and
it was suggested that the fumes might have contained some selenium
dioxide.
The acute toxic effects of hydrogen selenide were investigated
in guinea-pigs exposed for 10, 30, or 60 min to concentrations
ranging from 0.002 to 0.57 mg/litre (Dudley & Miller, 1941). All
animals exposed to 0.02 mg/litre, for 60 min, died within 25 days;
93% of those exposed to 0.043 mg/litre, for 30 min, died within 30
days, and all exposed to 0.57 mg/litre, for 10 min, died within 5
days. Decreasing the concentrations of hydrogen selenide, and
increasing the time of exposure to 2, 4, or 8 h produced death in
50% of the guinea-pigs, within 8 h.
Acute studies on the toxicity of selenium hexafluoride (SeF6),
were carried out on the rabbit, guinea-pig, rat, and mouse at 100,
50, 25, 10, 5, and 1 ppm for 4 h. Exposures down to and including
10 ppm (Ct = 40 ppm/h) were uniformly fatal (Kimmerle, 1960).
Exposure to 5 ppm (Ct = 20 ppm/h) resulted in pulmonary oedema from
which the animals recovered, and 1 ppm did not induce any grossly
observable effects. However, with repeated exposure for 1 h daily
at 5 ppm, for 5 days, there were definite signs of pulmonary
injury.
7.1.3. Blood levels in toxicity
Analyses of blood from cattle have shown that, if the average
value of blood-selenium for a herd is over 2 mg/litre, damage from
chronic selenium poisoning is very likely to occur (Dinkel et al.,
1957). Average values of 1 - 2 mg/litre suggest a borderline
problem, especially in reproduction, whereas values below 1
mg/litre indicate that no damage from toxicity should be expected.
Rosenfeld & Beath (1964) commented that typical concentrations of
selenium in the blood in alkali disease, blind staggers, and acute
selenium poisoning were 1 - 2, 1.5 - 4, and up to 25 mg/litre,
respectively. In studies with sodium selenite, Maag et al. (1960)
found that severe selenium toxicity occurred in cattle when the
selenium content of the blood exceeded 3 mg/litre. The selenium
levels in blood associated with chronic toxicity in sheep
corresponded to 0.6 - 0.7 mg/litre (Ermakov & Kovalskij, 1974).
7.1.4. Effects on reproduction
Franke & Potter (1936) fed 7 groups of 6 weanling rats (2 males
and 4 females), 21 days of age, a basal diet consisting of ground
whole wheat, 82%; commercial casein, 10%; pure leaf lard, 3%;
dehydrated yeast, 2%; cod liver oil, 2%; and McCollum's salt
mixture No. 185, 1%. Group 1 was shifted immediately to a toxic
diet in which sufficient control wheat was substituted by
seleniferous wheat to give a final selenium concentration of 24.6
mg/kg diet. Groups 2, 3, 4, 5, and 6 were shifted to the toxic
diet when the rats attained 42, 63, 84, 105, and 186 days of age,
respectively. Group 7 was fed the basal diet throughout the entire
study. No matings were successful in which both males and females
had been fed the toxic diet for 40 days, regardless of the age at
which the rats had been taken off the basal ration. This was true,
even in group 6, in which successful matings had been achieved at
82 and 131 days, while the rats were still being fed the basal
diet. All male rats not placed on the toxic diet until 63 days of
age or more were able to fertilize the normal females. In a later
study in which females fed the toxic diet were mated with normal
males, there were some successful matings, but the pups that were
born generally died or were eaten by the mother soon after birth.
In a study by Munsell et al. (1936), 7 groups of 4-week-old
rats, each containing 2 males and 6 females, were fed a basal diet
consisting of wheat, 58%; skim milk powder, 30%; yeast, 5%; butter,
5%; and cod liver oil, 2%. In each of the groups, a variable
proportion of the control wheat was replaced by toxic wheat so that
the latter contributed 8.7, 6.0, 3.0, 1.5, 0.75, 0.38, or 0 mg
selenium/kg diet. Compared with the controls receiving no toxic
wheat, the number of females having young was decreased by feeding
the diets containing 3.0 mg or more selenium/kg, and the percentage
of young reared was decreased by the diets containing 6.0 mg or
more/kg (Table 25). Feeding the diet containing 0.75 mg/kg
appeared to improve reproductive performance in terms of total
young produced or percentage of young reared. In the second
generation of rats continued on their respective levels of toxic
grain, the diet containing 6.0 mg/kg had a definite deleterious
effect on reproduction, whereas diets containing 0.75 - 1.5 mg/kg
had some beneficial effects. In a second study, the percentage of
young reared was improved in the group fed the diet containing 1.5
mg/kg compared with the control diet group, but the average number
of young per litter and the average number of young per bearing
female were decreased in the group fed the diet containing 0.75
mg/kg.
Halverson (1974) fed 4 groups of 70-day-old ARS/Sprague Dawley
albino rats a basal diet containing glucose, 61.8%; purified
casein, 25%; corn oil, 5%; minerals, 6%; and vitamins, 2.2%. The
groups comprised 2 - 4 males and 6 - 8 females and were given the
basal diet supplemented with sodium selenite at 0, 1.25, 2.50, or
3.75 mg selenium/kg. After 90 days, the rats were mated; no
consistent effects of selenium on reproduction were observed in
these first generation rats. Four groups of second-generation
young were maintained on their respective diets and used as
breeding stock in a second life cycle. In 2 separate studies, the
first and second reproductions of these second generation rats were
successful, regardless of the selenium content of the diet (Table
26). In the third reproduction, however, the unsupplemented rats
showed signs of reproductive failure. Selenium supplementation at
all levels prevented poor litter production in the first study but
only levels of 2.50 and 3.75 mg/kg prevented it in the second.
Neonatal survival was improved in both studies by selenium
administered at 3.75 mg/kg.
Schroeder & Mitchener (1971a) gave selenate at 3 mg
selenium/litre, in the drinking-water, continuously, during a
multigeneration study in mice. Five pairs of Fo mice were given
selenium at weaning and allowed to breed ad libitum for 6 months.
Controls were treated identically but did not receive any selenium
in the water. At weaning, F1 pairs were randomly selected from the
first, second, and third litters and left to breed, to produce the
F2 generation. F2 pairs were similarly selected from the first or
second litters to produce the F3 generation. An increased
male:female ratio was observed in all generations (1.30 - 1.50
versus 0.94 - 1.03 for the controls), but the mechanism of this
effect was not explained. No breeding failures were seen in the
control group, but 2 or 3 such failures occurred in each of the F1,
F2, and F3 generations given selenate. Only 3 litters were
produced in the F3 generation treated with selenate and 16 of 23
mice born were runts, whereas in the control F3 generation, 22
litters were produced with a total of 230 mice and no runts.
Two groups of 5 male and 2 groups of 5 female Wistar rats were
fed a laboratory chow diet and one group of each sex was given
sodium selenate at 0 or 7.5 mg selenium/litre in the drinking-
water, respectively (Rosenfeld & Beath, 1954). The rats receiving
selenium ("selenized rats") were treated from birth to 8 months of
age. Mating of normal males with selenized females was totally
unsuccessful, whereas mating of selenized males with normal females
resulted in normal reproduction and survival. Doses of 1.5 or 2.5
mg/litre did not have any effect on the reproduction of breeding
rats, the number of young reared by the mothers, or the
reproduction of 2 successive generations of males and females.
However, a level of 2.5 mg/litre decreased the number of young
reared by the mothers by about 50%.
Table 25. Effect of seleniferous wheat on reproduction in first- and second-generation ratsa
-------------------------------------------------------------------------------------------------
Level of Generation and Females having Total Total Average Average Young reared
dietary number of females young young litters number number
selenium 1st 2nd young/ young/
(from generation generation litter bearing
wheat) female
--------------------------------------------------------------------------------------------------
(mg/kg) Number % Number Number Number Number Number %
0 6 - 6 100 70 13 5.4 11.7 30 42.9
0.38 6 - 5 83.3 72 13 5.5 14.4 23 31.9
0.75 6 - 6 100 90 18 5.0 15.0 75 83.3
1.5 6 - 5 83.3 42 10 4.2 8.4 15 35.7
3.0 6 - 3 50.0 38 7 5.4 12.7 19 50.0
6.0 6 - 4 66.7 30 8 3.8 7.5 5 16.7
8.7 6 - 2 33.3 7 2 3.5 3.5 0 0
0 - 11 10 90.9 134 19 7.1 13.4 46 34.3
0.38 - 9 6 66.7 63 10 6.3 10.5 31 49.2
0.75 - 41 29 70.7 434 52 8.3 15.0 328 75.6
1.5 - 7 7 100 100 11 9.1 14.3 72 72.0
3.0 - 7 5 71.4 61 9 6.8 12.2 25 41.0
6.0 - 2 2 100 17 3 5.7 8.5 0 0
8.7 - 0 - - - - - - - -
--------------------------------------------------------------------------------------------------
a Adapted from: Munsell et al. (1936).
Table 26. Effect of selenium as selenite on successive reproductions of second-
generation rats maintained on a casein dieta
--------------------------------------------------------------------------------
Study Added Number Measurements in first, second and third reproductions
selenium of brood Number of Number young per 2-day survival of
in diet females litters born newborn litter litter members
1 2 3 1 2 3 1 2 3
--------------------------------------------------------------------------------
(mg/kg) (%)
1 0 3 3 3 0 12 12 - 61 46 -
1.250 6 6 6 5 12 7 8 29 21 26
2.500 5 5 5 5 12 11 8 71 84 30
3.750 6 6 6 6 11 9 8 79 78 78
2 0 7 7 6 2 8 10 6 74 82 33
1.250 7 7 5 2 10 11 4 90 84 00
2.500 9 8 8 6 9 9 5 81 85 25
3.750 8 7 7 4 7 6 6 92 100 92
--------------------------------------------------------------------------------
a Adapted from: Halverson (1974).
Poley & Moxon (1938) fed 4 groups of 15 Rhode Island pullets a
basal laying ration consisting of ground corn, 25%; ground barley,
25%; ground wheat, 15%; wheat bran, 8%; wheat middlings, 8%; meat
and bone scraps, 8%; alfalfa leaf meal, 5%; dried buttermilk, 5%;
salt, 0.5%; and cod liver oil concentrate, 0.5%. Each of the 4
groups was mated with 2 male brothers for the entire study. After
2 weeks, grains containing selenium (corn, barley, and wheat) were
substituted for normal grains in order to contribute 0, 2.5, 5, or
10 mg selenium/kg diet fed to each of the 4 groups, respectively.
Oyster shells and water were provided ad libitum. There were no
significant differences in feed consumption, body weight, egg
production, or egg fertility in any of the groups. After 4 weeks
on the toxic diets, 10 mg of selenium/kg reduced hatchability to
zero. Hatchability was only slightly reduced by 5 mg/kg, and a
level of 2.5 mg/kg had no effect.
In a study by Wahlstrom & Olson (1959), 2 groups of 10 purebred
Duroc gilts, 8 weeks of age, were fed a basal diet supplemented
with sodium selenite at 10 mg selenium/kg diet. Of the 9 gilts in
the unsupplemented group (one was killed accidentally), 8 conceived
with the first service and one failed to conceive after 3 services.
Of the 10 gilts in the selenium-exposed group, 5 conceived with the
first service, 2 with the second service and 3 failed to conceive
after 3 services. The selenium-exposed group farrowed fewer
litters and fewer live pigs, and weaned fewer pigs than the control
group (Table 27). Moreover, the average birth weights and weaning
weights were lower in the selenium-exposed group. The average
litter weight at 56 days for both litters was 131 and 227 pounds
for the selenium-exposed and unexposed groups, respectively.
Table 27. The effect of selenium on reproduction and lactation in swine
-------------------------------------------------------------------------
Items Number Average Average Average Average Average
litters number number birth number weaning
farrowed pigs pigs weight pigs weight
farrowed farrowed (kg) weaned (kg)
alive
-------------------------------------------------------------------------
Basal
First litters 7 8.0 8.0 2.95 7.3 30.5
Second litters 6 11.2 10.5 2.89 8.0 29.0
Average 6.5 9.6 9.25 2.92 7.65 29.75
Basal + selenium
First litters 6 9.8 7.7 2.60b 5.7 23.1c
Second litters 5 8.6 7.2 2.76 5.6 23.5c
Average 5.5 9.2 7.45 2.68 5.65 23.3
-------------------------------------------------------------------------
a Adapted from: Wahlstrom & Olson (1959).
b Significantly less than basal lot ( P < .05)
c Significantly less than basal lot ( P < .01)
Dinkel et al. (1963) reported on the effect of early (May 1
to mid-July) versus late (mid-July to October 1) breeding on the
reproductive performance of beef cattle grazing on a seleniferous
range in South Dakota. With such a programme, it was felt that
early conception would avoid any deleterious effects on
reproduction of the highly seleniferous young range grasses that
grow luxuriantly during the early summer. Data from 152 matings,
75 in the early and 77 in the late breeding group, collected over
5 years, showed that the average conception rate of the early-bred
cows was 60%, whereas that of the late-bred cows was 37.7%.
Moreover, the average calf crop weaned was 52% in the early
breeding group and 32.4% in the late group. The data do not prove,
but suggest, that selenium was the cause of the difference. In
addition to the early breeding effect, the very low overall
reproductive rate should be noted. This is considerably below the
rate that can be attained with similar operations when chronic
selenosis is not a problem, and it has been repeatedly observed
that, on ranches with a selenium problem, the rate of reproduction
is consistently low. This led Olson (1969) to comment that the
effect on reproduction might be the most significant effect of
excessive amounts of the element from an economic standpoint.
7.1.5. Effects on dental caries
Most studies on experimental animals have shown that high doses
of selenium do not have any effect on caries formation when the
selenium is given after tooth formation has already occurred. For
example, Muhler & Shafer (1957) fed 22 male weanling Sprague Dawley
rats a stock corn cariogenic diet that contained 10 mg sodium
selenite/kg. After 6, 8, and 12 weeks, the level of sodium
selenite was raised to 15, 20, and 30 mg/kg diet, respectively. A
control group of 20 rats received the same diet without added
selenium. The animals were given their respective diets and
drinking-water low in fluorine (F = 0.2 mg/litre) ad libitum for
140 days. The selenium-exposed group grew only about 53% as much
as the controls, but the mean number of carious lesions was
essentially the same in both groups (6.4 and 6.9, respectively).
Claycomb et al. (1965) fed 2 groups of 24 weanling rats a high-
carbohydrate diet, with or without 10 mg sodium selenite/kg diet,
for 100 days. In this study, selenium depressed growth by only
11%, but again the average number of carious lesions per rat was
similar in the selenium-treated and control groups (0.92 and 1.08,
respectively). The rather low incidence of dental caries was
thought to be due to genetic influences. Wheatcroft et al. (1951)
maintained 80 white rats, 40 males and 40 females, on a coarse corn
cariogenic diet for 100 days. The rats were divided into 4 groups
of 20 and were given daily intraperitoneal doses of sodium selenite
at 0, 0.2, 0.5, or 1.0 mg selenium/kg body weight. The authors
felt that there was a trend towards an increased incidence of
caries in the group receiving the most selenium, but the 2 highest
doses of selenium were clearly toxic, since these rats had
diarrhoea, affected eyes, and dull, matted fur. There was also
histological evidence of liver damage in over half, and kidney
damage in a third, of the rats. Thirty new-born Wistar rats were
divided into 3 groups receiving, intraperitoneally, 0, 0.5 - 1.0
µg, or 5 - 10 µg sodium selenate, respectively, every day for 53
days (Kaqueler et al., 1977). The group receiving the lower dose
of selenium had a lower total number of caries than the controls,
while the group receiving the higher dose had more caries than the
controls. The authors concluded that post-eruptive administration
of sodium selenate may have either a cariostatic or cariogenic
influence in rats, depending on the dosage administered.
Attempts to induce dental caries by exposure to high levels of
selenium were more successful when the selenium was given during
the time of tooth development. For example, Navia et al. (1968)
fed sodium selenite at 4 mg selenium/kg, to groups of 21 CR-COBS
rats in the drinking-water, in a purified caries-producing diet, or
in the same diet gelled with equal parts of 2% aqueous agar. The
experimental treatments of the mothers and litters began at birth
and continued until the pups were 50 days old. Selenium had no
effect on buccal, sulcal, or proximal caries, when given in the
diet, but caused a 12% increase in sulcal lesions when administered
in the drinking-water. Buttner (1963) fed 3 groups of 8 female,
caries-susceptible rats of the Wistar strain a cariogenic coarse
stock corn diet plus 0, 5, or 10 mg sodium selenite/litre drinking-
water during mating, pregnancy, and lactation. The offspring
received the same concentrations of sodium selenite for 120 days.
Thirty-one pups were born in the control group but, since the
dosages of selenium used partially inhibited reproduction, only 20
and 7 pups were born in the groups receiving 5 or 10 mg sodium
selenite/litre drinking-water, respectively. Growth of the pups
was strongly inhibited and the number and extent of carious lesions
were increased in both selenium-treated groups (Table 28). In a
study by Bowen (1972), 7 female monkeys of the species Macaca irus
were fed a cariogenic diet, with drinking-water sweetened with 3%
sucrose, at night, and phosphate-free icing sugar, 4 times daily.
A second group of 3 females of the same species was treated
identically except that they received selenium, as sodium selenate,
at 2 mg/litre drinking-water. After 15 months, some of the
selenium-treated monkeys experienced slight gastrointestinal
trouble in the form of greenish malodorous stools and the dose of
selenium was reduced to 1 mg/litre for another 45 months. The
second permanent molars and the premolars were formed during the
period of higher selenium exposure and these teeth had a yellow
chalky appearance in the selenium-treated monkeys. On the other
hand, selenium had no effect on the first permanent molars, which
had already formed before the start of the study. Although both
groups of monkeys had severe dental caries, the mean caries score
of the selenium-treated group was about twice that of the controls
(16 and 8.4, respectively). There was no difference between the
times required for caries to develop in the first permanent molars
in the controls and experimental groups, but carious lesions in the
second permanent molars developed more rapidly in the selenium-
exposed group (6.7 versus 21 months for mean caries development
time in selenium-treated compared with control groups). The author
concluded that selenium had a cariogenic effect, when administered
during tooth development and a moderate anti-cariogenic effect,
when given posteruptively.
Table 28. Effect of developmental and postdevelopmental
administration of sodium selenite on dental caries in the rata
-------------------------------------------------------------------
Dose of sodium Sex Number Weight gain Mean number Extent
selenite in of in 120 days of carious of carious
drinking-water rats (g) lesions lesions
(mg/litre)
-------------------------------------------------------------------
0 M 17 330 ± 9 5.3 ± 0.5 11.6 ± 1.4
F 14 207 ± 7
5 M 13 240 ± 5 7.2 ± 0.7 18.7 ± 2.2
F 7 176 ± 7
10 M 5 220 ± 18 8.6 ± 1.1 25.2 ± 3.2
F 2 148 ± 3
-------------------------------------------------------------------
a Adapted from: Buttner (1963).
Britton et al. (1980), however, presented evidence that showed
that, under some conditions, selenium can have a cariostatic
effect, even when given during tooth development. These workers
gave 0, 0.8, or 2.4 mg selenium/litre drinking-water in the form of
selenomethionine or sodium selenite, to rats from the 10th day of
pregnancy until the pups were weaned. At 17 - 19 days of age, the
pups were given oral innoculations of Streptococcus mutans - 6715
in thioglycollate broth. When 19 days old, the pups were weaned,
divided into groups of 13 - 19, and given a 67% sucrose diet and
distilled water ad libitum. At 65 - 68 days of age, the young rats
were killed and the first and second molar teeth were stained with
murexide and scored for caries. The high dose of selenium given to
the mothers, as either compound, did not have any effect on
dental caries, but the middle dose (0.8 mg/litre) had a definite
cariostatic effect, since selenomethionine and sodium selenite
decreased the incidence of total buccal caries by 46.1 and 40.9%,
respectively. The authors suggested that this cariostatic effect
might occur because some selenium is necessary for proper enamal
formation or because of undetermined effects on the oral
environment.
The fact that high doses of selenium have an apparent
cariogenic effect, only when given during tooth development,
is consistent with the results of Shearer (1975) who found that
the incorporation into teeth of radioactive selenite or
selenomethionine given in the drinking-water at a level of 0.2 mg
selenium/litre was much greater in rat pups undergoing dental
development than in their mothers whose teeth had already matured.
The mechanism of the cariogenic effect of high levels of selenium
is not known, but an antagonism to fluoride does not seem to be
involved (Shearer & Ridlington, 1976).
7.1.6. Factors influencing toxicity
7.1.6.1. Form of selenium
Studies comparing the toxicity of different forms of selenium
are described in section 7.1.2.1. This point is particularly
relevant because the forms of selenium in foods have yet to be
characterized.
7.1.6.2. Nutritional factors
High levels of dietary protein protect against selenium
toxicity (Gortner, 1940), but some proteins give better protection
than others (Smith & Stohlman, 1940). Jaffe (1976) found that the
toxicity of seleniferous sesame meal for rats was markedly
decreased when the diets were supplemented with L-lysine, the
limiting essential amino acid in sesame proteins. Lysine was
thought to protect against selenium poisoning indirectly by
improving the biological value of the sesame proteins.
Linseed meal contains a unique non-proteinaceous factor that
is highly effective in protecting against selenium poisoning
(Halverson et al., 1955; Levander et al., 1970). The results of
recent work suggest that the protective activity of linseed meal
may reside in 2 newly-isolated cyanogenic glycosides (Palmer et
al., 1980; Smith et al., 1980). Cyanide has a partially protective
effect against selenium poisoning in rats (Palmer & Olson, 1979)
and increases the occurrence of nutritional myopathy in lambs
(Rudert & Lewis, 1978).
There is conflicting evidence as to the ability of methyl-
donating compounds to protect against selenium toxicity (Rosenfeld
& Beath, 1964). Methionine was shown to protect against chronic
selenite poisoning in rats, but only when vitamin E or other fat-
soluble antioxidants were in the diet (Levander & Morris, 1970).
Vitamin E deficiency increased the susceptibility of rats to
chronic poisoning by selenium as selenite (Witting & Horwitt, 1964)
and pigs deficient in vitamin E and selenium were more susceptible
to acute selenium toxicosis than non-deficient pigs (Van Vleet et
al., 1974).
7.1.6.3. Arsenic
Selenium poisoning caused by feeding rats seleniferous wheat
was decreased by giving sodium arsenite in the drinking-water
(Moxon, 1938). The protective effect of arsenic against selenium
toxicity might be explained by the increased biliary excretion of
selenium caused by arsenic (Levander & Baumann, 1966). However,
the use of arsenic compounds did not prove to be a practical way of
controlling selenium poisoning in livestock (Olson, 1969). Also,
arsenic does not protect against all forms of selenium, since it
potentiates the toxicity of the trimethylselenonium ion (Obermeyer
et al., 1971).
7.1.6.4. Sulfate
Dietary sulfate partially counteracts the toxicity of selenate
in rats, but has little or no effect against selenite or organic
forms of selenium (Halverson et al., 1962). Sulfate apparently
increases the urinary excretion of selenium fed as selenate,
thereby reducing the retention of selenium in the internal organs
(Ganther & Baumann, 1962).
7.1.6.5. Adaptation
Ermakov & Kovalskij (1968) fed 18 adult female sheep from
seleniferous and normal localities their own respective diets or
these diets plus an additional 2 mg selenium, as selenite, daily
for 28 days. The 18 sheep were divided into 6 equal groups,
according to their history of selenium intake and, in the case of
the seleniferous sheep, according to the presence or absence of
signs of selenium toxicity. After 28 days, all the sheep were
killed, and selenium levels and acid and alkaline phosphatase
activities were determined in several tissues. Selenium levels and
alkaline phosphatase activities are presented in Table 29. The
increase in selenium retention in the blood, kidneys, and most of
the other internal organs, produced by supplementing the feed with
selenite, was less in sheep from high-selenium localities than in
animals from control localities. Retention in the liver seemed to
be an exception. The increase in alkaline phosphatase activity due
to the selenite load was much clearer in sheep from the control
localities than in the sheep from the seleniferous localities. The
alkaline phosphatase activity of the pancreas in sheep from the
high-selenium locality, manifesting selenium toxicity signs, was an
exception being much higher after the selenite load (370 ± 57
compared with 84 ± 34). These differences in the response of the
sheep to selenite loading were interpreted by the authors as
evidence of the adaptation of the animals to high levels of
selenium exposure.
Table 29. Effect of high selenium (Se) load on selenium metabolism and alkaline phosphatase activity in sheep
previously exposed to normal and high selenium intakes in foragea
----------------------------------------------------------------------------------------------------------------
History Daily Se intake Skeletal Alkaline phosphatase activity
of sheep Plant Selenite Total Blood muscle Liver Kidney kidney liver skeletal
Se Se Se Se Se Se Se muscle
(µg) (µg) (µg) (µg/litre) (µg/kg) (µg/kg) (µg/kg) (units/100 g tissue)
----------------------------------------------------------------------------------------------------------------
High 1821 2000 3821 411 ± 18 230 ± 24 600 ± 44 870 ± 69 286 ± 148 448 ± 43 13.0 ± 2.7
Se-curved
hoofs
High 1944 0 1944 290 ± 18 180 ± 7 330 ± 20 760 ± 26 362 ± 89 374 ± 117 9.5 ± 3.0
Se-curved
hoofs
High 1774 2000 3774 362 ± 34 210 ± 16 590 ± 90 1000 ± 174 309 ± 103 335 ± 18 9.4 ± 2.9
Se-curved
hoofs
High 2038 0 2038 239 ± 34 170 ± 6 340 ± 20 890 ± 21 349 ± 63 344 ± 56 9.5 ± 1.2
Se-curved
hoofs
Normal Se 376 2000 2376 408 ± 23 170 ± 8 450 ± 38 950 ± 77 496 ± 53 457 ± 57 14.8 ± 3.0
Normal Se 396 0 396 100 ± 12 89 ± 5 190 ± 20 780 ± 69 143 ± 29 254 ± 7 5.5 ± 0.8
----------------------------------------------------------------------------------------------------------------
a Adapted from: Ermakov & Kovalskij (1968).
Jaffe & Mondragon (1969) presented evidence that animals could
adapt to long-term selenium intake. Hepatic-selenium levels
decreased steadily in young rats fed a diet containing 4.5 mg
selenium/kg as seleniferous sesame meal, if the rats came from
mothers that had been fed the same seleniferous diet, but the
levels increased if the rats came from mothers fed a non-
seleniferous stock diet. It was concluded that an adaptation
mechanism existed that allowed rats exposed to long-term selenium
ingestion to store less of this element than previously unexposed
controls. A later report (Jaffe & Mondragon, 1975) confirmed these
results and also indicated that the hepatic lesions, splenomegaly,
and other toxicity signs were significantly decreased in adapted
rats.
One mechanism for a possible adaptive response to high levels
of selenium could involve effects of selenium intake on various
selenium metabolic pathways, since previous selenium exposure has
been shown to have an influence on the whole body retention of
selenium and the urinary and pulmonary excretion of selenium
(Ganther et al., 1966; Ewan et al., 1967; Burk et al., 1973)
(section 6.3, 6.4). However, as discussed in section 7.1.2.1, more
recent work has shown that previous selenium exposure directly
influences the toxicity of final selenium metabolites such as
dimethyl selenide and the trimethylselenonium ion (Parizek et al.,
1976, 1980).
7.1.7. Mechanism of toxicity
The mechanism of selenium toxicity remains unclear and the
modes of action of various selenium compounds, such as hydrogen
selenide, selenomethionine, and selenium dioxide are likely to be
quite different. Thus, no unifying hypothesis regarding the toxic
effects of selenium compounds is possible. Various hypotheses
concerning the mechanisms of selenium toxicity have been presented
(Rosenfeld & Beath, 1964; Izraelson et al., 1973; Ermakov &
Kovalskij, 1974; US NAS/NRC, 1976; Lazarev, 1977; Levander, 1982).
Although it appears unlikely that selenite interferes directly with
sulfhydryl enzymes (Tsen & Tappel, 1958; Tsen & Collier, 1959), it
is possible that selenite may interfere with glutathione metabolism
(Vernie et al., 1978; Chung & Maines, 1981; Vernie, 1984; Anundi et
al., 1984) and that this may affect enzyme activities. The
apparent pro-oxidant nature of high levels of selenite has been
reported by several workers (Witting & Horwitt, 1964; Csallany et
al., 1984), and this may be related to some aspects of selenite
toxicity.
7.2. Selenium Deficiency
7.2.1. Animal diseases
The nutritionally beneficial effects of selenium were first
reported in 1957 by Schwarz, who discovered that sodium selenite
prevented dietary liver necrosis in vitamin E-deficient rats
(Schwarz & Foltz, 1957). This discovery led to the rapid
recognition of vitamin E-related selenium-responsive deficiency
diseases in several species of farm animals. Diseases such as
white muscle disease in sheep and cattle, hepatosis dietetica in
swine, and exudative diathesis in poultry are economically
significant problems in areas of the world where the soil levels of
selenium available for uptake by plants are low. Schwarz & Foltz
(1958) also reported that feeding a diet deficient in both selenium
and vitamin E to mice resulted in multiple necrotic degeneration of
several organs; more recently, Suchkov et al. (1977, 1978) found
that feeding such diets to mice for 13 days caused a decreased
staining intensity for zinc in the pancreas, kidneys, and testes.
The fact that vitamin E as well as selenium tended to protect
against many of these diseases led some research workers to
question whether there was any requirement for selenium in animals
receiving adequate amounts of vitamin E. But, the results of more
recent work have clearly demonstrated the need for selenium, even
in animals given nutritionally adequate levels of vitamin E.
Deficiency signs specific for selenium included alopecia, vascular
changes, cataract, poor growth, and reproductive failure in second
generation selenium-deficient rats (McCoy & Weswig, 1969; Wu et
al., 1979). Pancreatic degeneration occurred in selenium-deficient
chicks with a normal intake of vitamin E (Thompson & Scott, 1970),
but the condition could be prevented by feeding very high
levels (> 300 µg/kg) of vitamin E or other antioxidants (Whiteacre
et al., 1983).
Changes in the myocardial parenchyma and increases in heart
weight have been observed in albino rats fed corn and vegetables
grown in the areas in which Keshan disease is endemic (Su et al.,
1982), and swine fed grain from endemic areas for 6 months
exhibited multiple myocardial necrosis as well as other lesions
(Zhu et al., 1981). Vitamin E deficiency exacerbated the
pathological and histochemical changes in the heart and liver of
piglets fed a low selenium diet composed mainly of cereals grown in
the Keshan disease area (Zhu et al., 1981).
7.2.2. Intakes needed to prevent deficiency
7.2.2.1. Quantitative dietary levels
Schwarz (1961) showed that 0.02 - 0.03 mg selenium/kg diet, in
the form of sodium selenate or selenite, afforded 50% protection
against liver necrosis in vitamin E-deficient rats over an
experimental period of 30 days. Selenite-selenium at 0.10 mg/kg
protected the rats over their entire life span in the absence of
vitamin E. Under these conditions, the rats developed severe
tocopherol deficiency, which mainly afflicted the central nervous
system. Sprinker et al. (1971) noted that 0.10 mg selenium/kg, as
selenite, prevented specific selenium deficiency lesions in rats
that had been fed a vitamin E-supplemented diet for 2 generations.
Thompson & Scott (1969) found that administration of 0.02 - 0.05 mg
selenium/kg, as selenite, prevented death and exudative diathesis
in chicks fed diets containing typical levels of vitamin E
(sections 7.2.4.1 and 7.2.7.2 include discussions on the
nutritional interrelationship of selenium and vitamin E).
Considerable field experience in New Zealand has indicated that
feeds from pastures associated with selenium-responsive
unthriftiness in sheep contain 0.008 - 0.030 mg selenium/kg
(Hartley, 1967). In areas where selenium-responsive diseases do
not occur, the feed levels of selenium range from 0.020 - 0.098
mg/kg. Since there is some overlap in these selenium levels, other
factors may be involved in the etiology of this selenium-responsive
disease (various factors that can influence selenium deficiency
are discussed in section 7.2.7).
It has been concluded that "the critical level for dietary
selenium, below which deficiency symptoms are observed, is
apparently about 0.02 mg/kg for ruminants and 0.03 - 0.05 mg/kg for
poultry" (US NAS/NRC, 1971). However, this US NAS/NRC committee
pointed out that "when supplementary selenium is fed, higher levels
than the minimal requirements have been proposed, both to permit
satisfactory distribution of the element through the large feed
mass and to overcome variations in feed intake by individual
animals". The final recommendation of the committee was that 0.1
and 0.2 mg selenium should be added per kg feed to eliminate
selenium deficiency in livestock and turkeys, respectively. In
laboratory rats, a dietary-selenium level of 0.1 - 0.2 mg/kg would
be equivalent to an intake of about 5 - 10 µg/kg body weight per
day.
7.2.2.2. Bioavailability
7.2.3. Blood and tissue levels in deficiency
Hartley (1967) presented data on the blood-selenium levels of
normal New Zealand lambs and lambs suffering from various selenium-
responsive diseases (Table 30). The mean blood-selenium levels
were lower in the diseased lambs than in the healthy ones, but
there was considerable overlap between the two groups. It was
suggested that other unknown factors might be involved in the
etiology of these diseases. Jacobsson et al. (1970) found blood-
selenium levels of 0.003 mg/litre in cows afflicted with muscular
degeneration, whereas healthy cows had an average of 0.046
mg/litre. Hartley (1967) estimated that blood-selenium levels of
0.05 mg/litre in young sheep could be considered satisfactory.
The same authors compared liver-selenium levels found in normal
lambs with those found in lambs with selenium-responsive
unthriftiness (Table 31). Again, there was some overlap between
the two groups, but the average level of selenium in the livers
from the deficient lambs was much lower than that from the healthy
lambs. Allaway et al. (1966) suggested that 0.21 mg/kg (dry
weight) is the critical minimal hepatic-selenium level below which
a high incidence of white muscle disease can be expected in lambs.
Table 30. Blood-selenium levels in normal
and selenium-deficient lambsa
---------------------------------------------
Group Selenium contentb
(mg/litre)
---------------------------------------------
Normalc 0.026 (0.014 - 0.048)
White muscle diseased 0.016 (0.006 - 0.033)
Normale 0.06 (0.020 - 0.195)
Selenium-responsive 0.01 (0.007 - 0.030)
unthriftinessf
---------------------------------------------
a Adapted from: Hartley (1967).
b Mean with range in parentheses.
c Normal lambs from areas with white muscle
disease.
d Lambs with white muscle disease.
e Lambs from areas where selenium-responsive
diseases are not recognized.
f Lambs with selenium-responsive
unthriftiness.
Table 31. Liver-selenium levels in normal
lambs or lambs with selenium-responsive
unthriftinessa
------------------------------------------
Group Selenium contentb
(mg/kg)
------------------------------------------
Normalc 0.16 (0.03 - 0.40)
Selenium-responsive 0.018 (0.005 - 0.05)
unthriftinessd
------------------------------------------
a Adapted from: Hartley (1967).
b Mean with range in parentheses.
c Lambs from areas where selenium-
responsive diseases are not
recognized.
d Lambs with selenium-responsive
unthriftiness.
7.2.4. Physiological role: glutathione peroxidase
7.2.4.1. Function of selenium and relationship to vitamin E
The discovery that selenium is a component of the enzyme
glutathione peroxidase (Rotruck et al., 1973) provides a logical
explanation for the nutritional interaction of vitamin E and
selenium, a puzzle that perplexed scientists for many years.
Analysis of purified ovine erythrocyte glutathione peroxidase by
fluorometry revealed that the protein contained 4 moles of selenium
per mole of enzyme (Hoekstra et al., 1973) and this stoichiometry
was also found for crystalline bovine erythrocyte-glutathione
peroxidase analysed for selenium by neutron activation analysis
(Flohe et al., 1973). In the scheme postulated by Hoekstra
(1975a), tocopherols act as intracellular antioxidants to prevent
oxidative damage to polyunsaturated fatty acids in biological
membranes by terminating chain reactions of lipid peroxides (Fig.
7). Selenium, as a part of glutathione peroxidase, protects
against oxidative stress either by catalysing the destruction of
hydrogen peroxide or by catalysing the decomposition of lipid
hydroperoxides, thereby interrupting the free radical peroxidative
chain reaction; in this latter role the free fatty acyl
hydroperoxide must be liberated by phospholipan A2 action from the
hydrophobic region of biological membranes, before its reduction
can be catalysed by glutathione peroxidase (Grossman & Wendel,
1983). Thus, both of these nutrients play separate but
interrelated roles in the cellular defence mechanisms against
oxidative damage.
6.5.1. Animal studies
6.5.1.1. Reduction and methylation
The main flow of selenium metabolism in animals is via
reductive pathways, which contrasts with the primarily oxidative
metabolism of sulfur (Levander, 1976a). Selenite can react with
glutathione or protein sulfhydryls to form selenotrisulfides
(Ganther, 1968; Jenkins & Hidiroglou, 1971) which, at least in the
case of the glutathione derivatives, can be reduced further by an
enzymatic mechanism to selenide (Ganther & Hsieh, 1974; Diplock,
1976).
Under normal conditions, selenide is methylated to form
trimethylselenonium ion, the main urinary metabolite of selenium
(section 6.3.1). In cases of selenium toxicity, this pathway is
overloaded and dimethyl selenide is produced. This is the main
volatile selenium metabolite expired via the lungs and is
responsible for the typical "garlicky odour" of animals poisoned
with selenium (McConnell & Portman, 1952a). The last 2 reactions
could be considered detoxication steps, since the methylated end-
products are much less toxic for the organism than selenite
(McConnell & Portman, 1952b; Obermeyer et al., 1971). However,
both of these methylated selenium derivatives have strong
synergistic toxicity with other minerals (section 7) and dimethyl
selenide toxicity in male rats was reported to be inversely related
to the level of previous selenium intake (Parizek et al., 1980).
6.5.1.2. Form in proteins
Early work with animals poisoned with selenium revealed that
much of the selenium in the tissues was associated with protein
(Smith et al., 1938). Since that time there has been considerable
controversy about the exact chemical nature of the selenium in
tissue proteins (Levander, 1976a). Selenomethionine may be
incorporated initially as such into animal proteins (Ochoa-Solano
& Gitler, 1968), but, in rats, it appears to be eventually
catabolized to selenite or selenate (Millar et al., 1973; Thomson
& Stewart, 1973). Non-ruminant animals cannot synthesize
selenomethionine from inorganic selenium compounds (Cummins &
Martin, 1967; Olson & Palmer, 1976), but rabbits and rats can
convert selenite into selenocysteine tissue proteins (Godwin &
Fuss, 1972; Olson & Palmer, 1976). Forstrom et al. (1978) have
proposed that seleno-cysteine is the form of selenium located at
the catalytic site of glutathione peroxidase and others have shown
that selenocysteine is essential for the activity of clostridial
glycine reductase (Cone et al., 1976). The mechanism by which
selenocysteine is formed from selenite is not known but
incorporation of preformed selenocysteine and post-translational
modification of the protein have been considered (Sunde, 1984).
Other possible forms of selenium in proteins include
selenotrisulfides (Ganther & Corcoran, 1969) and "acid-labile"
selenium (Diplock et al., 1973).
6.5.1.3. Conversion of selenium compounds to nutritionally-active
forms of selenium
The pioneering studies of Schwarz & Foltz (1958) demonstrated
that a variety of selenium compounds could protect against dietary
liver necrosis in vitamin E- and selenium-deficient rats. Sodium
selenite, sodium selenate, selenium dioxide, selenic acid, and
potassium selenocyanate were all more or less equally active, but
elemental gray selenium was essentially inactive. Selenocystine
and selenomethionine were about as effective as the active
inorganic selenium compounds, but an organic selenium fraction
isolated from pig kidney powder (Factor 3) was shown to be even
more active. A number of organic derivatives of selenium have
shown some protective effect against liver necrosis, but none was
superior to Factor 3 (Schwarz et al., 1972). However, certain
simple amino-acid derivatives of monoseleno diacetic acid showed
some promise in that they combined high nutritional potency with a
low order of toxicity (Schwarz, 1976). The effects of selenite and
selenomethionine were shown to be roughly similar in inducing
glutathione peroxidase activity in the tissues of rats fed a diet
deficient in selenium (Pierce & Tappel, 1977).
Cantor et al. (1975a) found that while selenite and
selenocystine were equally effective in preventing exudative
diathesis in vitamin E- and selenium-deficient chicks, seleno-
methionine was less effective. This suggests a difference between
rats and chicks in the metabolism of selenomethionine. The degree
of protection against exudative diathesis and the level of plasma
glutathione peroxidase activity were highly correlated, suggesting
that nutritional potency depended on the ability of the chick to
convert various selenium compounds to the enzymatically active
form.
6.5.2. Human studies
Few studies have been carried out to investigate the metabolic
transformation of selenium compounds in human beings, but the
limited data available suggest certain similarities in the
metabolism of selenium by man and animals. For example, human
beings overexposed to selenium develop breath with a smell of
garlic, which is presumably due to the exhalation of dimethyl
selenide (Glover, 1976). Also, the chromatographic pattern of
urinary-selenium metabolites is the same in man and the rat (Burk,
1976). However, species differences in selenium metabolism do
exist, since human beings retain selenomethionine to a much greater
extent than selenite, whereas the retention of the 2 compounds in
rats is about the same (section 6.4.2).
7. EFFECTS OF SELENIUM ON ANIMALS
The considerable biological importance of selenium was first
recognized in the 1930s when it was discovered that certain well-
defined and economically important farm animal diseases were
actually the result of chronic selenium poisoning (section 7.1).
These animal diseases were restricted to agricultural areas in
which large amounts of selenium in the soil were available for
uptake by the plants, which were then consumed by the animals.
Research, over the last 20 years, showed that selenium was an
essential trace element (section 7.2), and selenium deficiency
diseases were rapidly recognized in several species of farm
animals. These deficiency diseases are significant economic
problems in areas of the world where the soil levels of the
element available for uptake by plants are low.
7.1. Selenium Toxicity
7.1.1. Farm animal diseases associated with a high selenium intake
On the basis of field experience, Rosenfeld & Beath (1964)
delineated 3 different types of selenium poisoning in livestock:
(a) acute;
(b) chronic, of the blind staggers type; and
(c) chronic, of the alkali disease type.
Acute poisoning is due to the ingestion of toxic quantities of
selenium in the form of highly seleniferous accumulator plants.
The animal has severe signs of distress such as laboured breathing,
abnormal movement and posture, prostration, and diarrhoea. Death
often follows within a few hours. This type of selenium poisoning
is rather rare under field conditions, since grazing animals
generally avoid the selenium accumulator plants, except in times of
pasture shortage. Acute selenium poisoning has also been produced
by the experimental or accidental administration of selenium
compounds to farm animals (US NAS/NRC, 1976).
Blind staggers has been reported in animals that eat a limited
number of selenium accumulator plants over a period of weeks or
months (Rosenfeld & Beath, 1964). The affected animals wander,
stumble, have impaired vision, and eventually succumb to
respiratory failure. Although this type of poisoning can be
produced experimentally by the administration of water extracts of
accumulator plants, it has not been possible to duplicate this
syndrome by the administration of pure selenium compounds.
Possibly alkaloids or other toxic substances found in many
seleniferous plants may contribute to blind staggers (Maag & Glenn,
1967; Van Kampen & James, 1978).
Alkali disease is associated with the consumption of grains
containing 5 - 40 mg selenium/kg over weeks or months. Animals
exhibit liver cirrhosis, lameness, hoof malformations, loss of
hair, and emaciation. Maag & Glenn (1967) were unable to produce
alkali disease in cattle by feeding inorganic selenium, but several
other studies have shown that the syndrome is causally associated
with seleniferous grains or grasses and can be produced by feeding
inorganic selenium salts (Olson, 1978).
The Task Group noted that most of the work on alkali disease
was concerned with cattle, but was aware of the report by Ermakov &
Kovalskij (1968), which described chronic selenium toxicity of this
type in sheep, under natural conditions. In sheep fed feeds
containing levels of 2 mg selenium/kg feed (fresh weight), the
following characteristic signs were observed: hoof deformation,
loss of hair, hypochromic anaemia, and increases in the activity of
both alkaline and acid phosphatases in various tissues.
7.1.2. Toxicity in experimental animals
The practical significance of selenium poisoning in farm
animals stimulated a great deal of research on both the acute and
chronic effects of selenium in laboratory animals. Interest in the
toxic effects of repeated exposure to selenium via inhalation was
stimulated by concern about the possible effects on human health of
occupational exposure to selenium. Also, studies were carried out
with the aim of establishing the no-observed-adverse-effect dose
level of selenium when administered in the drinking-water. In some
of the studies in which selenium was given via either the air or
water, biochemical and/or behavioural criteria were used to assess
the biological effects of selenium exposure. However, as discussed
in section 7.1.6, the toxicity of selenium compounds can be
influenced by different variables and the results from various
laboratories are often not comparable because of quite different
experimental conditions. Also, the criteria for toxicity are less
developed than the signs of deficiency (section 7.2).
Reviews that deal with various aspects of selenium toxicology
include those by Rosenfeld & Beath (1964), Muth (1966), Izraelson
et al. (1973), Ermakov & Kovalskij (1974), US NAS/NRC (1976), and
Lazarev (1977).
7.1.2.1. Acute and subacute toxicity - single or repeated exposure
studies with oral, intraperitoneal, or cutaneous administration
Perhaps the most characteristic sign of acute selenium
poisoning in animals is the development of the so-called "garlicky
breath odour", which is due to the pulmonary excretion of volatile
selenium compounds, particularly dimethyl selenide, by animals
overexposed to selenium (section 6.3.1). Other signs of acute
selenium poisoning described by Franke & Moxon (1936) in dogs and
rats included: vomiting, dyspnoea, tetanic spasms, and death from
respiratory failure. Pathological changes included congestion of
the liver with areas of focal necrosis, congestion of the kidney,
endocarditis, myocarditis, peticheal haemorrhages of the
epicardium, atony of the smooth muscles of the gastrointestinal
tract, gall-bladder, and bladder, and erosion of the long bones,
especially the tibia. The LD50 values for sodium selenite,
administered orally to various animal species, are given in Table
18 (Pletnikova, 1970).
Table 18. Acute oral toxicity of sodium selenite for various
species of laboratory animalsa
-------------------------------------------------------------
Species LD50 Statistical method
(mg selenium/kg
body weight)
-------------------------------------------------------------
White mouse (male) 7.75 Behrens & Schlosser
7.08 Litchfield & Wilcoxon
Albino rat (female) 10.50 Behrens & Schlosser
13.19 Diechmann & LeBlanc
Guinea-pig (female) 5.06 Deichmann & LeBlanc
Rabbit (female) 2.25 Diechmann & LeBlanc
-------------------------------------------------------------
a From: Pletnikova (1970).
Table 19. Acute toxicity of some selenium compounds administered to rats by
intraperitoneal injection
----------------------------------------------------------------------------
Compound Criterion Toxic dose Reference
of (mg selenium/kg
toxicity body weight)
----------------------------------------------------------------------------
Sodium selenite MLD 3.25 - 3.5 Franke & Moxon (1936)
Sodium selenate MLD 5.25 - 5.75 Franke & Moxon (1936)
DL-selenocystine MLD 4 Moxon (1940)
DL-selenomethionine MLD 4.25 Klug et al. (1950)
Diselenodipropionic LD50 25 - 30 Moxon et al. (1938)
acid
Trimethylselenonium LD50 49.4 Obermeyer et al. (1971)
chloride
Dimethyl selenide LD50 1600 McConnell & Portman (1952b)
----------------------------------------------------------------------------
For a given species, the lethal doses of sodium selenite,
sodium selenate, DL-selenocystine, and DL-selenomethionine are
quite similar (Table 19). Although certain methylated metabolites
of selenium such as dimethylselenide and trimethylselenonium
chloride were considered relatively innocuous (see LD50 values by
McConnell & Portman and Obermeyer et al. in Table 19), more recent
work by Parizek et al. (1976, 1980) has shown that the toxicity of
methylated selenium compounds depends not only on the sex of the
animal (Parizek et al., 1974) but also on the level of previous
selenium intake. For example, 90% mortality was observed in male
rats maintained on a diet containing 0.05 mg selenium/kg, when they
were injected intraperitoneally with 20 µmoles dimethylselenide/kg
body weight, i.e., by a dose of dimethylselenide that is more than
1000 times lower than the LD50 reported by McConnell & Portman
(1952b). Pre-treatment with a small amount of selenite (1 µmol/kg
body weight), intraperitoneally, 6 h, but not 1 h, before
dimethylselenide injection or increased oral intake of selenite
(Table 20), protected male rats against the toxicity of a
subsequent dose of dimethylselenide (Parizek et al., 1976, 1980).
Moreover, the methylated forms of selenium have strong synergistic
toxicities with other minerals (section 7.1.6.3, 7.4.1) and
dimethylselenide can be much more toxic for male rats than for
female rats (section 7.1.6).
Table 20. Dependence of the toxicity of dimethylselenide on
the level of previous oral intake of seleniuma
------------------------------------------------------------
Selenite Single intraperitoneal 24-h mortality (%)
supplement in dose of dimethylselenide Diet A Diet B
drinking-water
(mg selenium/ (mg mole/kg body weight) (n = 20) (n = 10)
litre
------------------------------------------------------------
0 20 90 90
0.1 20 45 30
0.5 20 5 0
1.0 20 0 0
------------------------------------------------------------
a Adapted from: Parizek et al. (1976, 1980).
Male rats (2 months old) given drinking-water with stated
supplement of selenite for 3 days before dimethylselenide
administration. Diet A contained 0.052 ± 0.005 mg selenium/kg and
diet B (semi-synthetic diet) 0.044 ± 0.001 mg selenium/kg.
Smith et al. (1937) reported that the minimum lethal dose
of selenium as sodium selenite or selenate in rabbits, rats,
and cats was 1.5 - 3.0 mg/kg body weight, regardless of whether
the compounds were administered orally, subcutaneously,
intraperitoneally, or intravenously. This lack of effect of the
mode of administration probably reflects the rapid and complete
absorption of soluble selenium compounds, either from the site of
injection or from the gastrointestinal tract.
Cummins & Kimura (1971) described comparative studies on
Sprague Dawley rats and dogs concerning the oral toxicity of the
following selenium compounds: sodium selenate, selenourea,
biphenylselenium, selenium sulfide (1 - 30 µ particle size), and
elemental selenium (1 - 30 µ particle size). The oral LD50 values
in rats for a number of selenium compounds are shown in Table 21
and demonstrate the large variations in LD50 values that occur,
depending on both the oxidation state of the compound and its
aqueous solubility.
Table 21. Comparative solubility and toxicity of various
selenium compounds in ratsa
--------------------------------------------------------------
Compound Solubility in 0.01 N HCl Rat oral LD50 (95% CL)
(mg/kg body weight)
--------------------------------------------------------------
Na2SeO3 700 g/litre 7 (4.4 - 11.2)
H2N-C-NH2 30 g/litre 50 (35.7 - 70.0)
||
Se
SeS2 insoluble (< 1 g/litre) 138 (110 - 172)
Se 5 g/litre 360 (308 - 421)
Elemental Se insoluble (< 1 g/litre) 6700 (6000 - 7300)
--------------------------------------------------------------
a From: Cummins & Kimura (1971).
Male Sprague Dawley rats, each weighing between 50 and 100 g,
were given the above chemicals by gavage as 0.1 - 20% suspensions
in 0.5% methylcellulose. A total of 30-36 animals was used per
compound, in groups of 6 animals per dose. The LD50 values and
the associated confidence limits (CL) were calculated according to
the method of Litchfield & Wilcoxon.
The aqueous solubilities were carried out in 0.01 N HCl to more
closely simulate acidic conditions in the stomach. The least toxic
selenium compound was insoluble elemental selenium with an LD50 of
6.7 g/kg body weight. Toxic signs included pilomotor activity,
decreased body activity, dyspnoea, diarrhoea, anorexia, and
cachexia. Fatalities occurred within 18 - 72 h; survivors appeared
outwardly normal, at the end of the 7-day observation period. The
most toxic of the selenium compounds tested was the highly soluble
sodium selenite with an oral LD50 of 7 mg/kg body weight and the
toxic signs were similar to those seen with high doses of elemental
selenium. Selenium sulfide (a component in shampoos) was about 20
times less toxic than sodium selenite (i.e., an LD50 of 138 mg/kg
compared with 7 mg/kg). It was also found in this study that, with
the exception of biphenyl selenium, toxicity could be correlated
with blood-selenium levels. For example, sodium selenite being the
most toxic, gave the highest blood level followed in descending
order by selenourea, selenium sulfide, and elemental selenium. It
was suggested that the blood-selenium level produced by the
relatively non-toxic biphenyl selenium was high because this
covalently-bound selenium compound is the most lipophilic compound,
which is better absorbed, resists catabolism, and apparently
circulates as the parent compound.
The application of 83 mg of selenium oxychloride to the skin of
rabbits caused the death of the animals in 5 h, and application of
4 mg caused death in 24 h (Dudley, 1938). Lazarev (1977) reported
a minimum lethal dose of selenium oxychloride of 7 mg/kg body
weight, after cutaneous administration to rabbits.
Several papers have shown that injection of selenite in rats in
the early postnatal period in single doses of 1.5 mg selenium/kg
body weight, or in repeated doses of 0.5 mg/kg, induced cataracts
(Ostadalova et al., 1979; Bhuyan et al., 1981; Shearer et al.,
1980; Bunce et al., 1985). Similar effects have not been observed
in hamsters (Shearer et al., 1980). This response is dose
dependant (Table 22) and, thus far, has been observed only when the
selenite was given by parenteral injection.
Table 22. Cataractagenesis by selenite in the rata
--------------------------------------------------
Group Daily dosageb Frequency of cataractsc
(mg selenium/kg (%)
body weight)
--------------------------------------------------
Control 0 0
Na2SeO3 0.25 13
Na2SeO3 0.50 96
Na2SeO3 0.75 96
--------------------------------------------------
a Adapted from: Shearer et al. (1980).
b Dose given to rat pups daily on days 2-18
postpartum.
c Observed at 21 days of age.
7.1.2.2. Effects of long-term oral exposure
Moxon & Rhian (1943) summarized several older studies that
indicated that diets containing 5 mg selenium/kg or more cause
chronic selenosis in several species of animals, such as chickens,
rats, and dogs. In seleniferous areas, 5 mg selenium/kg diet is
generally accepted as the dividing line between toxic and non-toxic
feeds (US NAS/NRC, 1976).
Halverson et al. (1966) fed diets containing 0, 1.6, 3.2, 4.8,
6.4, 8.0, 9.6, or 11.2 mg selenium/kg in the form of sodium
selenite or seleniferous wheat to male Sprague Dawley rats,
initially weighing 60 - 70 g. The rats were divided into groups of
8 and were fed the diets for 6 weeks. The diet consisted of (g/kg)
ground wheat, 809; purified casein 120; USP salt mixture XIV, 20;
USP brewer's yeast, 20; corn oil, 30; and vitamin B12 mix, 1.
Vitamins A, D, and E were provided separately. The criteria used
to assess toxicity were growth depression, liver cirrhosis,
splenomegaly, pancreatic enlargement, anaemia, elevated
serumbilirubin levels, and death. The addition to the diet of 1.6,
3.2, or 4.8 mg selenium/kg did not have any significant effect on
the rats, as judged by any of these criteria. There was a
depression in growth in the group that received 4.8 mg dietary
selenium/kg as sodium selenite, but this was not significant.
Liver cirrhosis, splenomegaly, and significant growth depression
were observed when the rats were fed levels of 6.4 mg/kg or more
from either source of selenium. Diets containing 8.0 mg/kg or more
caused additional effects such as pancreatic enlargement, anaemia,
elevated serum-bilirubin levels, and, after 4 weeks, death.
Diets made either with non-seleniferous or seleniferous sesame
meal were fed to groups of 12 male Sprague Dawley rats, initially
weighing 50 g for a period of 6 weeks (Jaffe et al., 1972b). The
diet made with non-seleniferous sesame meal contained 0.5 mg
selenium/kg and consisted of (g/kg): non-seleniferous sesame meal,
463.3; almidon, 422.7; corn oil, 50; cod liver oil, 10; USP XVI
salts, 40; L-lysine x HCl, 4; and vitamin mix, 10. The diet made
with the seleniferous sesame meal contained 10 mg selenium/kg and
had the same composition as the previous diet except that
seleniferous sesame, which replaced the non-seleniferous sesame
meal, was added at a level of 490.2 g/kg, and the almidon was added
at a level of 395.8 g/kg. The rats fed the diet containing the
seleniferous sesame meal showed decreased survival, impaired weight
gain, higher incidence of liver lesions, elevated hepatic selenium
levels, enlarged spleens, depressed haemoglobin, haematocrit, and
fibrinogen levels, and decreased prothrombin activity (Table 23).
In a separate study, the same workers investigated the effects of
dietary selenium, given as seleniferous sesame meal, on the
activities of various serum enzymes (Table 24). The diet
containing 4.5 mg selenium/kg had the same composition as the
previous diets, except that the seleniferous sesame meal, non-
seleniferous sesame meal, and almidon were added at levels of
230.8, 270.4, and 384.8 g/kg, respectively. Feeding the diet
containing 4.5 mg of selenium/kg for 6 weeks increased the
activities of serum alkaline phosphatase (EC 3.1.3.1) and glutamic
pyruvic transaminase (SGPT) (EC 2.6.1.2), whereas 10.0 mg/kg also
increased the activity of glutamic-oxaloacetic transaminase (SGOT)
(EC 2.6.1.1).
Table 23. Pathological signs in rats fed diets containing seleniferous sesame meala
--------------------------------------------------------------------------------------------------------------------
Dietary Survival Weight Rats Hepatic Spleen Haemo- Haema- Fibrinogen Prothrombin
selenium after gain with selenium weight globin tocrit activity
6 weeks after hepatic level level value
6 weeks lesions
--------------------------------------------------------------------------------------------------------------------
(mg/kg) (g) (mg/kg) (% of body (g/litre (volume %) (mg/litre)
weight) blood)
0.5 12/12 156.8 ± 7.2 0/12 0.72 ± 0.14 0.207 ± 0.01 147 ± 2.1 43.9 ± 0.61 1664 ± 78 963 ± 9.5
10 8/12 61.2 ± 7.43 10/12 7.34 ± 0.97 0.544 ± 0.09 123 ± 3.5 40.8 ± 0.82 655 ± 78 713 ± 76.6
--------------------------------------------------------------------------------------------------------------------
a Adapted from: Jaffe et al. (1972b).
Table 24. Effect of chronic selenium toxicity on the
activity of serum enzymesa
-------------------------------------------------------
Dietary Number Alkaline SGOT SGPT
selenium of rats phosphatase
-------------------------------------------------------
(mg/kg) (units/ml) (units/ml) (units/ml)
0.5 6 6.3 ± 0.2 66 ± 3 20 ± 1
4.5 10 8.8 ± 1.3 59 ± 2 26 ± 2
10 8 18.9 ± 2.3 77 ± 5 43 ± 6
-------------------------------------------------------
a Adapted from: Jaffe et al. (1972b).
Tinsley et al. (1967) and Harr et al. (1967) carried out an
extensive study on the chronic toxicity of selenium in rats.
Although the primary purpose of their research was to investigate
the alleged carcinogenicity of selenium (section 7.7.1), the design
of their study provided an opportunity to examine other aspects of
the toxicity of selenium, such as the influence of selenium
poisoning on growth rate and histopathology. A total of 1437
Wistar rats from a closed random-bred colony was used with the size
of the experimental groups ranging from 10 - 110 animals. The rats
were fed one of 3 different diets: a semipurified diet containing
either 12 or 22% casein or a commercial "laboratory chow" type
ration. The casein-based diets also contained corn oil, 50 g;
H.M.W. salts, 40 g; vitamin mix, 10 g; and glucose monohydrate
(Cerelose) up to a weight of 1 kg. Selenium as sodium selenite or
sodium selenate was added at levels of 0, 0.5, 2.0, 4.0, 8.0, or
16.0 mg/kg. Only a small proportion of the animals fed diets
supplemented with more than 4.0 mg/kg of selenium survived for 12
months. The rats fed the commercial ration were 2 - 3 times more
resistant to the effects of selenium toxicity than the rats fed the
semipurified diet. A calculated maximum body weight was reported
to be depressed by as little as 0.5 mg selenium/kg, but no
statistical evaluation of the results was presented. Usually
moribund animals were killed and necropsied for histopathological
determinations, but 136 rats were killed at specific ages.
Acute toxic hepatitis was the predominant histopathological
lesion in rats fed the commercial ration supplemented with sodium
selenate at 16 mg selenium/kg. These rats had a median survival
age of only 96 days. Acute toxic hepatitis was also observed in
rats fed the 12% casein diet supplemented with sodium selenate at 4
or more mg selenium/kg. The rats were emaciated, pale, and had
poor quality hair coat. Hydrothorax, ascites, pericardial oedema,
and icterus were common. Myocardial hyperaemia, fluid imbalance,
and parenchymal degeneration were often present. The adrenals were
enlarged and the pancreas was oedematous. The failure of normal
chondrocyte proliferation observed in the metaphyses appeared to be
different from that seen in malnutrition, since proliferation was
irregular and not merely reduced.
When rats were fed the commercial ration supplemented with
sodium selenate at 8 mg selenium/kg, the predominant lesion was
chronic toxic hepatitis. The median survival age in this group was
429 days. Other histopathological changes reported included
pancreatic duct hyperplasia, intestinal nephritis, and myocardial
damage, particularly in rats of more than 450 days of age and
receiving selenite.
Harr et al. (1967) reported an increased proliferation of the
hepatic parenchyma when the rats were fed the semi-purified diet
supplemented with 0.5 - 2.0 mg selenium/kg as selenite or selenate.
But a more detailed report from this laboratory (Weswig et al.,
1966) showed that this lesion of "chronic liver and bile duct
hyperplasia" was observed to a greater extent in rats fed the
commercial ration not supplemented with selenium than in rats fed
the semi-purified diet supplemented with 0.5 mg selenium/kg. Thus,
this lesion may not be specifically related to selenium.
Harr & Muth (1972) stated that 0.25 mg/kg was the minimum toxic
level for liver lesions, when selenium was added to a semi-purified
diet. The minimum toxic level was 0.75 mg/kg when the criteria
were longevity or lesions of the heart, kidneys, or spleen.
However, growth was normal in rats fed 0.5 mg selenium/kg diet.
The authors estimated that the dietary threshold for physiological
and pathological effects was 0.4 mg/kg and for pathological and
clinical effects, 3 mg/kg.
Weanling rats of the Long-Evans strain were given either sodium
selenite or selenate at 0 or 2 mg selenium/litre in the drinking-
water for 1 year (Schroeder & Mitchener, 1971b). After 1 year, the
selenium dosage was increased to 3 mg/litre in the selenate group.
The weanlings were born from random-bred females that had been fed
a low-selenium diet (0.05 mg selenium/kg) consisting of: whole rye
flour, 600 g; dry skim milk, 300 g; corn oil, 90 g; and iodized
sodium chloride to which were added vitamins and iron, 1 g/kg. The
same diet was fed to the weanlings during the toxicity study. The
drinking-water was doubly deionized forest spring water to which
had been added: zinc, 50 mg; manganese, 10 mg; chromium(III), 5 mg;
copper, 5 mg; cobalt, 1 mg; and molybdenum, 1 mg/litre. The rats
given the selenium compounds (plus another group not discussed here
given sodium tellurite) were divided into groups totalling 313
animals. There were 105 control rats. By 58 days, half of the
male rats in the selenite group were dead. Fifty percent mortality
in the group of female rats given selenite was not achieved until
348 days and was not achieved in the male and female groups given
selenate until 962 and 1014 days, respectively. On the other hand,
Palmer & Olson (1974) gave 2 or 3 mg selenium/litre drinking-water,
in the form of either sodium selenite or selenate to male weanling
Sprague Dawley rats for 6 weeks and noted small decreases in weight
gain compared with control rats receiving selenium, but no deaths.
Two diets were used in this study, a rye diet similar to that
described by Schroeder & Mitchener above and a corn diet that
consisted of ground corn, 808 g; casein, 120 g; corn oil, 30 g;
U.S.P. XIV salts, 20 g; and vitamin mix, 22 g/kg. The trace
elements zinc, copper, manganese, chromium, cobalt, and molybdenum
were also added to the drinking-water, as suggested by Schroeder &
Mitchener (1971a).
In a study by Jacobs & Forst (1981a), sodium selenite at 0 or 4
mg selenium/litre drinking-water was administered to groups of 17
and 30 male 5-week-old Sprague Dawley rats fed a commercial pellet
ration. After 64 weeks, survival was 94 and 63% in the control and
selenium-treated groups, respectively. In a second study of similar
design, except that the rats were 8 weeks old at the start,
survival was 90 and 95% in the control and selenium-treated groups,
respectively, after 61 weeks.
Pletnikova (1970) investigated the effects of long-term, low-
level administration of sodium selenite in water to rabbits and
rats. For these studies, 32 rabbits and 16 rats were divided into
4 groups and administered, orally, doses of 0, 0.005, 0.0005, or
0.00005 mg selenium/kg body weight for periods of 7 1/2 and 6
months, respectively. Prolonged administration of the maximum dose
investigated (0.005 mg/kg) produced significant alterations in the
rabbits. After 2 months, there was a significant increase in the
concentration of oxidized glutathione in the blood and, after 7
months, there was slower elimination of bromsulphalein by the
liver, and hepatic succinic dehydrogenase activity was decreased.
A dose of 0.0005 mg/kg caused fewer, less pronounced changes,
whereas 0.00005 mg/kg did not produce any statistically significant
effects in any of these tests. A dose of 0.005 mg/kg given to rats
caused a considerable weakening of the capacity for forming new
conditioned reflexes. It can be assumed that a daily dose of 0.005
mg/kg body weight in rats is equivalent to a dietary-selenium level
of about 0.063 mg/kg. Since this level of selenium is within the
physiological range needed to prevent selenium deficiency in
animals (section 7.2.2), the toxicological significance of these
observations is not clear, unless it is assumed that a certain dose
of selenite given in water solution is more toxic than the same
dose given in the diet.
Recently, Csallany et al. (1984) reported that giving sodium
selenite to female mice in the drinking-water at a level of 0.1 mg
selenium/litre increased the amount of hepatic lipid-soluble
lipofuscin pigments, when the animals were sacrificed at 9 months
of age. There were 8 mice in each group and the animals were fed a
diet adequate in vitamin E, which contained 0.05 mg selenium/kg.
Sodium selenite added to the drinking-water at a level of 9 mg
selenium/litre killed all 12 rats fed a diet based on either ground
corn or ground rye in 6 weeks (Palmer & Olson, 1974), whereas
Halverson et al. (1966) found that sodium selenite added to a diet
based on ground wheat, at a level of 9.6 mg/kg, killed only 1 out
of 10 rats in the same period of time. However, the rats used in
the latter study were post-weanling animals weighing 60 - 70 g when
the study started, whereas the rats used in the former study were
21-day-old weanlings and weighed only 35 - 45 g. Other research
workers have shown that the resistance of rats to the toxic effects
of selenium increases markedly after the twenty-first day of life
(Franke & Potter, 1936).
Feng et al. (1985) studied the hepatoxicity of high selenium
corn (7.12 mg/kg) produced in the Enshi county of China, where
human selenium intoxication was reported (section 8.1.1.1). After
feeding a diet that contained 61% of this corn (total selenium
concentration of 4.343 mg/kg) for 16 weeks, liver cirrhosis was
seen in 3 out of 6 male and 5 out of 6 female rats in one group.
No liver damage was found histologically in another group of rats
in the same study, which had consumed a diet containing 30.5% of
the high selenium corn (total selenium concentration of 2.35
mg/kg).
At present, the best indicator of chronic selenium toxicity
appears to be growth inhibition (US NAS/NRC, 1976), and a selenium
level of 4 - 5 mg/kg is necessary to achieve this response in
animals fed a normal diet. In laboratory rats, this exposure to
selenium represents an intake of about 200 - 250 µg/kg body weight
per day. More sensitive and specific criteria of selenium
poisoning to demonstrate effects at lower dose levels, such as
biochemical or histological techniques, would obviously be highly
desirable, but such tests are not available at present.
7.1.2.3. Inhalation toxicity
The effects of respiratory exposure to selenium compounds,
administered under conditions mimicking occupational exposure, have
been described in several papers. Filatova (1951) studied the
toxic effects of respiratory exposure to selenium dioxide (SeO2)
under conditions similar to those that occur in industry, i.e.,
heating of selenium (section 5.2.1). In acute studies, white rats
were exposed to air concentrations of selenium dioxide of 0.15 -
0.6 mg/litre, and all rats died within one-half - 4 h.
Morphological examination of the organs revealed that intraalveolar
and perivascular oedema occurred in the lungs, and haemorrhages and
degenerative changes in the liver, kidney, and heart. In 4
additional studies, all rats survived 4 h when exposed to doses of
0.09, 0.06 - 0.07, or 0.03 - 0.04 mg selenium dioxide/litre, but
all rats exposed to the highest dose (equal to 5 - 5.2 mg/kg body
weight) died within 24 h. In a series of long-term studies, rats
were exposed to repeated doses of selenium dioxide at 0.01 - 0.03,
0.006 - 0.009, or 0.003 - 0.005 mg/litre for 6 h, every other day,
for one month. The lowest dose did not produce any effects on body
weight or on the blood picture, and all the rats survived.
Histological examination revealed degenerative changes in the
liver, renal tubules, dystrophy of heart muscle, and hyperaemia and
hypertrophy of the splenic pulp. At the dose of 0.006 - 0.009
mg/litre, all the rats but one died within 27-33 days. For the
first 2 weeks, there was no difference in body weight between the
exposed and unexposed control rats but, during the last 2 weeks,
the exposed rats lost body weight and all but one of the exposed
rats died. The histopathological changes consisted of multiple
necrosis and degeneration in the liver and myocardial fibres, and
involvement of renal tubules. In the third group of rats, which
was exposed to 0.01 - 0.03 mg/litre, the animals showed respiratory
distress, weight loss, and, in 3 rats, anaemia. All the rats died
between days 8 and 18 of exposure. In the liver, kidneys,
myocardium, and spleen, the changes observed were similar to those
seen at lower doses, but more pronounced. Moreover, lung oedema
similar to that noted in the acute exposure studies was seen.
Lipinskij (1962) exposed 2 groups of 5 rabbits to airborne
amorphous selenium and selenium dioxide in chambers, under
conditions analogous to industrial exposure, except that the doses
were higher. In the first group, the rabbits were exposed to 20 mg
selenium dioxide/litre and 40 mg selenium/m3, for 2 h per day, for
one week, at which time a decrease in blood catalase (EC 1.11.1.6)
activity was noted. The rabbits in the second group were exposed
to 10 µg selenium dioxide/litre and 20 mg selenium/m3, for 2 h
daily, for 12 weeks. After 12 weeks, decreases in total and
reduced glutathione were observed, but there was no change in
levels of oxidized glutathione.
On the basis of studies on 60 white rats weighing 120 - 150 g,
Burchanov et al. (1969) concluded that intratracheal injection of
0.06 ml of a sterile suspension containing 50 mg of highly
dispersed elemental selenium dust in physiological saline, for
1 - 12 months, resulted in decreases in body weight and muscular
strength, and morphological and biochemical alterations in the
respiratory tract. Burchanov (1972) obtained similar results in
inhalation studies on rats exposed to 2 types of polymetallic dusts
found in industry.
The acute toxicity of selenium dust (average mass median
particle diameter, 1.2 µ) for rats, guinea-pigs, and rabbits was
described by Hall et al. (1951). Exposure of these animals, for
16 h, to an atmosphere containing approximately 30 mg selenium
dust/m3 produced mild interstitial pneumonitis in the animals.
Rats exposed to selenium fumes developed acute toxic effects, and
it was suggested that the fumes might have contained some selenium
dioxide.
The acute toxic effects of hydrogen selenide were investigated
in guinea-pigs exposed for 10, 30, or 60 min to concentrations
ranging from 0.002 to 0.57 mg/litre (Dudley & Miller, 1941). All
animals exposed to 0.02 mg/litre, for 60 min, died within 25 days;
93% of those exposed to 0.043 mg/litre, for 30 min, died within 30
days, and all exposed to 0.57 mg/litre, for 10 min, died within 5
days. Decreasing the concentrations of hydrogen selenide, and
increasing the time of exposure to 2, 4, or 8 h produced death in
50% of the guinea-pigs, within 8 h.
Acute studies on the toxicity of selenium hexafluoride (SeF6),
were carried out on the rabbit, guinea-pig, rat, and mouse at 100,
50, 25, 10, 5, and 1 ppm for 4 h. Exposures down to and including
10 ppm (Ct = 40 ppm/h) were uniformly fatal (Kimmerle, 1960).
Exposure to 5 ppm (Ct = 20 ppm/h) resulted in pulmonary oedema from
which the animals recovered, and 1 ppm did not induce any grossly
observable effects. However, with repeated exposure for 1 h daily
at 5 ppm, for 5 days, there were definite signs of pulmonary
injury.
7.1.3. Blood levels in toxicity
Analyses of blood from cattle have shown that, if the average
value of blood-selenium for a herd is over 2 mg/litre, damage from
chronic selenium poisoning is very likely to occur (Dinkel et al.,
1957). Average values of 1 - 2 mg/litre suggest a borderline
problem, especially in reproduction, whereas values below 1
mg/litre indicate that no damage from toxicity should be expected.
Rosenfeld & Beath (1964) commented that typical concentrations of
selenium in the blood in alkali disease, blind staggers, and acute
selenium poisoning were 1 - 2, 1.5 - 4, and up to 25 mg/litre,
respectively. In studies with sodium selenite, Maag et al. (1960)
found that severe selenium toxicity occurred in cattle when the
selenium content of the blood exceeded 3 mg/litre. The selenium
levels in blood associated with chronic toxicity in sheep
corresponded to 0.6 - 0.7 mg/litre (Ermakov & Kovalskij, 1974).
7.1.4. Effects on reproduction
Franke & Potter (1936) fed 7 groups of 6 weanling rats (2 males
and 4 females), 21 days of age, a basal diet consisting of ground
whole wheat, 82%; commercial casein, 10%; pure leaf lard, 3%;
dehydrated yeast, 2%; cod liver oil, 2%; and McCollum's salt
mixture No. 185, 1%. Group 1 was shifted immediately to a toxic
diet in which sufficient control wheat was substituted by
seleniferous wheat to give a final selenium concentration of 24.6
mg/kg diet. Groups 2, 3, 4, 5, and 6 were shifted to the toxic
diet when the rats attained 42, 63, 84, 105, and 186 days of age,
respectively. Group 7 was fed the basal diet throughout the entire
study. No matings were successful in which both males and females
had been fed the toxic diet for 40 days, regardless of the age at
which the rats had been taken off the basal ration. This was true,
even in group 6, in which successful matings had been achieved at
82 and 131 days, while the rats were still being fed the basal
diet. All male rats not placed on the toxic diet until 63 days of
age or more were able to fertilize the normal females. In a later
study in which females fed the toxic diet were mated with normal
males, there were some successful matings, but the pups that were
born generally died or were eaten by the mother soon after birth.
In a study by Munsell et al. (1936), 7 groups of 4-week-old
rats, each containing 2 males and 6 females, were fed a basal diet
consisting of wheat, 58%; skim milk powder, 30%; yeast, 5%; butter,
5%; and cod liver oil, 2%. In each of the groups, a variable
proportion of the control wheat was replaced by toxic wheat so that
the latter contributed 8.7, 6.0, 3.0, 1.5, 0.75, 0.38, or 0 mg
selenium/kg diet. Compared with the controls receiving no toxic
wheat, the number of females having young was decreased by feeding
the diets containing 3.0 mg or more selenium/kg, and the percentage
of young reared was decreased by the diets containing 6.0 mg or
more/kg (Table 25). Feeding the diet containing 0.75 mg/kg
appeared to improve reproductive performance in terms of total
young produced or percentage of young reared. In the second
generation of rats continued on their respective levels of toxic
grain, the diet containing 6.0 mg/kg had a definite deleterious
effect on reproduction, whereas diets containing 0.75 - 1.5 mg/kg
had some beneficial effects. In a second study, the percentage of
young reared was improved in the group fed the diet containing 1.5
mg/kg compared with the control diet group, but the average number
of young per litter and the average number of young per bearing
female were decreased in the group fed the diet containing 0.75
mg/kg.
Halverson (1974) fed 4 groups of 70-day-old ARS/Sprague Dawley
albino rats a basal diet containing glucose, 61.8%; purified
casein, 25%; corn oil, 5%; minerals, 6%; and vitamins, 2.2%. The
groups comprised 2 - 4 males and 6 - 8 females and were given the
basal diet supplemented with sodium selenite at 0, 1.25, 2.50, or
3.75 mg selenium/kg. After 90 days, the rats were mated; no
consistent effects of selenium on reproduction were observed in
these first generation rats. Four groups of second-generation
young were maintained on their respective diets and used as
breeding stock in a second life cycle. In 2 separate studies, the
first and second reproductions of these second generation rats were
successful, regardless of the selenium content of the diet (Table
26). In the third reproduction, however, the unsupplemented rats
showed signs of reproductive failure. Selenium supplementation at
all levels prevented poor litter production in the first study but
only levels of 2.50 and 3.75 mg/kg prevented it in the second.
Neonatal survival was improved in both studies by selenium
administered at 3.75 mg/kg.
Schroeder & Mitchener (1971a) gave selenate at 3 mg
selenium/litre, in the drinking-water, continuously, during a
multigeneration study in mice. Five pairs of Fo mice were given
selenium at weaning and allowed to breed ad libitum for 6 months.
Controls were treated identically but did not receive any selenium
in the water. At weaning, F1 pairs were randomly selected from the
first, second, and third litters and left to breed, to produce the
F2 generation. F2 pairs were similarly selected from the first or
second litters to produce the F3 generation. An increased
male:female ratio was observed in all generations (1.30 - 1.50
versus 0.94 - 1.03 for the controls), but the mechanism of this
effect was not explained. No breeding failures were seen in the
control group, but 2 or 3 such failures occurred in each of the F1,
F2, and F3 generations given selenate. Only 3 litters were
produced in the F3 generation treated with selenate and 16 of 23
mice born were runts, whereas in the control F3 generation, 22
litters were produced with a total of 230 mice and no runts.
Two groups of 5 male and 2 groups of 5 female Wistar rats were
fed a laboratory chow diet and one group of each sex was given
sodium selenate at 0 or 7.5 mg selenium/litre in the drinking-
water, respectively (Rosenfeld & Beath, 1954). The rats receiving
selenium ("selenized rats") were treated from birth to 8 months of
age. Mating of normal males with selenized females was totally
unsuccessful, whereas mating of selenized males with normal females
resulted in normal reproduction and survival. Doses of 1.5 or 2.5
mg/litre did not have any effect on the reproduction of breeding
rats, the number of young reared by the mothers, or the
reproduction of 2 successive generations of males and females.
However, a level of 2.5 mg/litre decreased the number of young
reared by the mothers by about 50%.
Table 25. Effect of seleniferous wheat on reproduction in first- and second-generation ratsa
-------------------------------------------------------------------------------------------------
Level of Generation and Females having Total Total Average Average Young reared
dietary number of females young young litters number number
selenium 1st 2nd young/ young/
(from generation generation litter bearing
wheat) female
--------------------------------------------------------------------------------------------------
(mg/kg) Number % Number Number Number Number Number %
0 6 - 6 100 70 13 5.4 11.7 30 42.9
0.38 6 - 5 83.3 72 13 5.5 14.4 23 31.9
0.75 6 - 6 100 90 18 5.0 15.0 75 83.3
1.5 6 - 5 83.3 42 10 4.2 8.4 15 35.7
3.0 6 - 3 50.0 38 7 5.4 12.7 19 50.0
6.0 6 - 4 66.7 30 8 3.8 7.5 5 16.7
8.7 6 - 2 33.3 7 2 3.5 3.5 0 0
0 - 11 10 90.9 134 19 7.1 13.4 46 34.3
0.38 - 9 6 66.7 63 10 6.3 10.5 31 49.2
0.75 - 41 29 70.7 434 52 8.3 15.0 328 75.6
1.5 - 7 7 100 100 11 9.1 14.3 72 72.0
3.0 - 7 5 71.4 61 9 6.8 12.2 25 41.0
6.0 - 2 2 100 17 3 5.7 8.5 0 0
8.7 - 0 - - - - - - - -
--------------------------------------------------------------------------------------------------
a Adapted from: Munsell et al. (1936).
Table 26. Effect of selenium as selenite on successive reproductions of second-
generation rats maintained on a casein dieta
--------------------------------------------------------------------------------
Study Added Number Measurements in first, second and third reproductions
selenium of brood Number of Number young per 2-day survival of
in diet females litters born newborn litter litter members
1 2 3 1 2 3 1 2 3
--------------------------------------------------------------------------------
(mg/kg) (%)
1 0 3 3 3 0 12 12 - 61 46 -
1.250 6 6 6 5 12 7 8 29 21 26
2.500 5 5 5 5 12 11 8 71 84 30
3.750 6 6 6 6 11 9 8 79 78 78
2 0 7 7 6 2 8 10 6 74 82 33
1.250 7 7 5 2 10 11 4 90 84 00
2.500 9 8 8 6 9 9 5 81 85 25
3.750 8 7 7 4 7 6 6 92 100 92
--------------------------------------------------------------------------------
a Adapted from: Halverson (1974).
Poley & Moxon (1938) fed 4 groups of 15 Rhode Island pullets a
basal laying ration consisting of ground corn, 25%; ground barley,
25%; ground wheat, 15%; wheat bran, 8%; wheat middlings, 8%; meat
and bone scraps, 8%; alfalfa leaf meal, 5%; dried buttermilk, 5%;
salt, 0.5%; and cod liver oil concentrate, 0.5%. Each of the 4
groups was mated with 2 male brothers for the entire study. After
2 weeks, grains containing selenium (corn, barley, and wheat) were
substituted for normal grains in order to contribute 0, 2.5, 5, or
10 mg selenium/kg diet fed to each of the 4 groups, respectively.
Oyster shells and water were provided ad libitum. There were no
significant differences in feed consumption, body weight, egg
production, or egg fertility in any of the groups. After 4 weeks
on the toxic diets, 10 mg of selenium/kg reduced hatchability to
zero. Hatchability was only slightly reduced by 5 mg/kg, and a
level of 2.5 mg/kg had no effect.
In a study by Wahlstrom & Olson (1959), 2 groups of 10 purebred
Duroc gilts, 8 weeks of age, were fed a basal diet supplemented
with sodium selenite at 10 mg selenium/kg diet. Of the 9 gilts in
the unsupplemented group (one was killed accidentally), 8 conceived
with the first service and one failed to conceive after 3 services.
Of the 10 gilts in the selenium-exposed group, 5 conceived with the
first service, 2 with the second service and 3 failed to conceive
after 3 services. The selenium-exposed group farrowed fewer
litters and fewer live pigs, and weaned fewer pigs than the control
group (Table 27). Moreover, the average birth weights and weaning
weights were lower in the selenium-exposed group. The average
litter weight at 56 days for both litters was 131 and 227 pounds
for the selenium-exposed and unexposed groups, respectively.
Table 27. The effect of selenium on reproduction and lactation in swine
-------------------------------------------------------------------------
Items Number Average Average Average Average Average
litters number number birth number weaning
farrowed pigs pigs weight pigs weight
farrowed farrowed (kg) weaned (kg)
alive
-------------------------------------------------------------------------
Basal
First litters 7 8.0 8.0 2.95 7.3 30.5
Second litters 6 11.2 10.5 2.89 8.0 29.0
Average 6.5 9.6 9.25 2.92 7.65 29.75
Basal + selenium
First litters 6 9.8 7.7 2.60b 5.7 23.1c
Second litters 5 8.6 7.2 2.76 5.6 23.5c
Average 5.5 9.2 7.45 2.68 5.65 23.3
-------------------------------------------------------------------------
a Adapted from: Wahlstrom & Olson (1959).
b Significantly less than basal lot ( P < .05)
c Significantly less than basal lot ( P < .01)
Dinkel et al. (1963) reported on the effect of early (May 1
to mid-July) versus late (mid-July to October 1) breeding on the
reproductive performance of beef cattle grazing on a seleniferous
range in South Dakota. With such a programme, it was felt that
early conception would avoid any deleterious effects on
reproduction of the highly seleniferous young range grasses that
grow luxuriantly during the early summer. Data from 152 matings,
75 in the early and 77 in the late breeding group, collected over
5 years, showed that the average conception rate of the early-bred
cows was 60%, whereas that of the late-bred cows was 37.7%.
Moreover, the average calf crop weaned was 52% in the early
breeding group and 32.4% in the late group. The data do not prove,
but suggest, that selenium was the cause of the difference. In
addition to the early breeding effect, the very low overall
reproductive rate should be noted. This is considerably below the
rate that can be attained with similar operations when chronic
selenosis is not a problem, and it has been repeatedly observed
that, on ranches with a selenium problem, the rate of reproduction
is consistently low. This led Olson (1969) to comment that the
effect on reproduction might be the most significant effect of
excessive amounts of the element from an economic standpoint.
7.1.5. Effects on dental caries
Most studies on experimental animals have shown that high doses
of selenium do not have any effect on caries formation when the
selenium is given after tooth formation has already occurred. For
example, Muhler & Shafer (1957) fed 22 male weanling Sprague Dawley
rats a stock corn cariogenic diet that contained 10 mg sodium
selenite/kg. After 6, 8, and 12 weeks, the level of sodium
selenite was raised to 15, 20, and 30 mg/kg diet, respectively. A
control group of 20 rats received the same diet without added
selenium. The animals were given their respective diets and
drinking-water low in fluorine (F = 0.2 mg/litre) ad libitum for
140 days. The selenium-exposed group grew only about 53% as much
as the controls, but the mean number of carious lesions was
essentially the same in both groups (6.4 and 6.9, respectively).
Claycomb et al. (1965) fed 2 groups of 24 weanling rats a high-
carbohydrate diet, with or without 10 mg sodium selenite/kg diet,
for 100 days. In this study, selenium depressed growth by only
11%, but again the average number of carious lesions per rat was
similar in the selenium-treated and control groups (0.92 and 1.08,
respectively). The rather low incidence of dental caries was
thought to be due to genetic influences. Wheatcroft et al. (1951)
maintained 80 white rats, 40 males and 40 females, on a coarse corn
cariogenic diet for 100 days. The rats were divided into 4 groups
of 20 and were given daily intraperitoneal doses of sodium selenite
at 0, 0.2, 0.5, or 1.0 mg selenium/kg body weight. The authors
felt that there was a trend towards an increased incidence of
caries in the group receiving the most selenium, but the 2 highest
doses of selenium were clearly toxic, since these rats had
diarrhoea, affected eyes, and dull, matted fur. There was also
histological evidence of liver damage in over half, and kidney
damage in a third, of the rats. Thirty new-born Wistar rats were
divided into 3 groups receiving, intraperitoneally, 0, 0.5 - 1.0
µg, or 5 - 10 µg sodium selenate, respectively, every day for 53
days (Kaqueler et al., 1977). The group receiving the lower dose
of selenium had a lower total number of caries than the controls,
while the group receiving the higher dose had more caries than the
controls. The authors concluded that post-eruptive administration
of sodium selenate may have either a cariostatic or cariogenic
influence in rats, depending on the dosage administered.
Attempts to induce dental caries by exposure to high levels of
selenium were more successful when the selenium was given during
the time of tooth development. For example, Navia et al. (1968)
fed sodium selenite at 4 mg selenium/kg, to groups of 21 CR-COBS
rats in the drinking-water, in a purified caries-producing diet, or
in the same diet gelled with equal parts of 2% aqueous agar. The
experimental treatments of the mothers and litters began at birth
and continued until the pups were 50 days old. Selenium had no
effect on buccal, sulcal, or proximal caries, when given in the
diet, but caused a 12% increase in sulcal lesions when administered
in the drinking-water. Buttner (1963) fed 3 groups of 8 female,
caries-susceptible rats of the Wistar strain a cariogenic coarse
stock corn diet plus 0, 5, or 10 mg sodium selenite/litre drinking-
water during mating, pregnancy, and lactation. The offspring
received the same concentrations of sodium selenite for 120 days.
Thirty-one pups were born in the control group but, since the
dosages of selenium used partially inhibited reproduction, only 20
and 7 pups were born in the groups receiving 5 or 10 mg sodium
selenite/litre drinking-water, respectively. Growth of the pups
was strongly inhibited and the number and extent of carious lesions
were increased in both selenium-treated groups (Table 28). In a
study by Bowen (1972), 7 female monkeys of the species Macaca irus
were fed a cariogenic diet, with drinking-water sweetened with 3%
sucrose, at night, and phosphate-free icing sugar, 4 times daily.
A second group of 3 females of the same species was treated
identically except that they received selenium, as sodium selenate,
at 2 mg/litre drinking-water. After 15 months, some of the
selenium-treated monkeys experienced slight gastrointestinal
trouble in the form of greenish malodorous stools and the dose of
selenium was reduced to 1 mg/litre for another 45 months. The
second permanent molars and the premolars were formed during the
period of higher selenium exposure and these teeth had a yellow
chalky appearance in the selenium-treated monkeys. On the other
hand, selenium had no effect on the first permanent molars, which
had already formed before the start of the study. Although both
groups of monkeys had severe dental caries, the mean caries score
of the selenium-treated group was about twice that of the controls
(16 and 8.4, respectively). There was no difference between the
times required for caries to develop in the first permanent molars
in the controls and experimental groups, but carious lesions in the
second permanent molars developed more rapidly in the selenium-
exposed group (6.7 versus 21 months for mean caries development
time in selenium-treated compared with control groups). The author
concluded that selenium had a cariogenic effect, when administered
during tooth development and a moderate anti-cariogenic effect,
when given posteruptively.
Table 28. Effect of developmental and postdevelopmental
administration of sodium selenite on dental caries in the rata
-------------------------------------------------------------------
Dose of sodium Sex Number Weight gain Mean number Extent
selenite in of in 120 days of carious of carious
drinking-water rats (g) lesions lesions
(mg/litre)
-------------------------------------------------------------------
0 M 17 330 ± 9 5.3 ± 0.5 11.6 ± 1.4
F 14 207 ± 7
5 M 13 240 ± 5 7.2 ± 0.7 18.7 ± 2.2
F 7 176 ± 7
10 M 5 220 ± 18 8.6 ± 1.1 25.2 ± 3.2
F 2 148 ± 3
-------------------------------------------------------------------
a Adapted from: Buttner (1963).
Britton et al. (1980), however, presented evidence that showed
that, under some conditions, selenium can have a cariostatic
effect, even when given during tooth development. These workers
gave 0, 0.8, or 2.4 mg selenium/litre drinking-water in the form of
selenomethionine or sodium selenite, to rats from the 10th day of
pregnancy until the pups were weaned. At 17 - 19 days of age, the
pups were given oral innoculations of Streptococcus mutans - 6715
in thioglycollate broth. When 19 days old, the pups were weaned,
divided into groups of 13 - 19, and given a 67% sucrose diet and
distilled water ad libitum. At 65 - 68 days of age, the young rats
were killed and the first and second molar teeth were stained with
murexide and scored for caries. The high dose of selenium given to
the mothers, as either compound, did not have any effect on
dental caries, but the middle dose (0.8 mg/litre) had a definite
cariostatic effect, since selenomethionine and sodium selenite
decreased the incidence of total buccal caries by 46.1 and 40.9%,
respectively. The authors suggested that this cariostatic effect
might occur because some selenium is necessary for proper enamal
formation or because of undetermined effects on the oral
environment.
The fact that high doses of selenium have an apparent
cariogenic effect, only when given during tooth development,
is consistent with the results of Shearer (1975) who found that
the incorporation into teeth of radioactive selenite or
selenomethionine given in the drinking-water at a level of 0.2 mg
selenium/litre was much greater in rat pups undergoing dental
development than in their mothers whose teeth had already matured.
The mechanism of the cariogenic effect of high levels of selenium
is not known, but an antagonism to fluoride does not seem to be
involved (Shearer & Ridlington, 1976).
7.1.6. Factors influencing toxicity
7.1.6.1. Form of selenium
Studies comparing the toxicity of different forms of selenium
are described in section 7.1.2.1. This point is particularly
relevant because the forms of selenium in foods have yet to be
characterized.
7.1.6.2. Nutritional factors
High levels of dietary protein protect against selenium
toxicity (Gortner, 1940), but some proteins give better protection
than others (Smith & Stohlman, 1940). Jaffe (1976) found that the
toxicity of seleniferous sesame meal for rats was markedly
decreased when the diets were supplemented with L-lysine, the
limiting essential amino acid in sesame proteins. Lysine was
thought to protect against selenium poisoning indirectly by
improving the biological value of the sesame proteins.
Linseed meal contains a unique non-proteinaceous factor that
is highly effective in protecting against selenium poisoning
(Halverson et al., 1955; Levander et al., 1970). The results of
recent work suggest that the protective activity of linseed meal
may reside in 2 newly-isolated cyanogenic glycosides (Palmer et
al., 1980; Smith et al., 1980). Cyanide has a partially protective
effect against selenium poisoning in rats (Palmer & Olson, 1979)
and increases the occurrence of nutritional myopathy in lambs
(Rudert & Lewis, 1978).
There is conflicting evidence as to the ability of methyl-
donating compounds to protect against selenium toxicity (Rosenfeld
& Beath, 1964). Methionine was shown to protect against chronic
selenite poisoning in rats, but only when vitamin E or other fat-
soluble antioxidants were in the diet (Levander & Morris, 1970).
Vitamin E deficiency increased the susceptibility of rats to
chronic poisoning by selenium as selenite (Witting & Horwitt, 1964)
and pigs deficient in vitamin E and selenium were more susceptible
to acute selenium toxicosis than non-deficient pigs (Van Vleet et
al., 1974).
7.1.6.3. Arsenic
Selenium poisoning caused by feeding rats seleniferous wheat
was decreased by giving sodium arsenite in the drinking-water
(Moxon, 1938). The protective effect of arsenic against selenium
toxicity might be explained by the increased biliary excretion of
selenium caused by arsenic (Levander & Baumann, 1966). However,
the use of arsenic compounds did not prove to be a practical way of
controlling selenium poisoning in livestock (Olson, 1969). Also,
arsenic does not protect against all forms of selenium, since it
potentiates the toxicity of the trimethylselenonium ion (Obermeyer
et al., 1971).
7.1.6.4. Sulfate
Dietary sulfate partially counteracts the toxicity of selenate
in rats, but has little or no effect against selenite or organic
forms of selenium (Halverson et al., 1962). Sulfate apparently
increases the urinary excretion of selenium fed as selenate,
thereby reducing the retention of selenium in the internal organs
(Ganther & Baumann, 1962).
7.1.6.5. Adaptation
Ermakov & Kovalskij (1968) fed 18 adult female sheep from
seleniferous and normal localities their own respective diets or
these diets plus an additional 2 mg selenium, as selenite, daily
for 28 days. The 18 sheep were divided into 6 equal groups,
according to their history of selenium intake and, in the case of
the seleniferous sheep, according to the presence or absence of
signs of selenium toxicity. After 28 days, all the sheep were
killed, and selenium levels and acid and alkaline phosphatase
activities were determined in several tissues. Selenium levels and
alkaline phosphatase activities are presented in Table 29. The
increase in selenium retention in the blood, kidneys, and most of
the other internal organs, produced by supplementing the feed with
selenite, was less in sheep from high-selenium localities than in
animals from control localities. Retention in the liver seemed to
be an exception. The increase in alkaline phosphatase activity due
to the selenite load was much clearer in sheep from the control
localities than in the sheep from the seleniferous localities. The
alkaline phosphatase activity of the pancreas in sheep from the
high-selenium locality, manifesting selenium toxicity signs, was an
exception being much higher after the selenite load (370 ± 57
compared with 84 ± 34). These differences in the response of the
sheep to selenite loading were interpreted by the authors as
evidence of the adaptation of the animals to high levels of
selenium exposure.
Table 29. Effect of high selenium (Se) load on selenium metabolism and alkaline phosphatase activity in sheep
previously exposed to normal and high selenium intakes in foragea
----------------------------------------------------------------------------------------------------------------
History Daily Se intake Skeletal Alkaline phosphatase activity
of sheep Plant Selenite Total Blood muscle Liver Kidney kidney liver skeletal
Se Se Se Se Se Se Se muscle
(µg) (µg) (µg) (µg/litre) (µg/kg) (µg/kg) (µg/kg) (units/100 g tissue)
----------------------------------------------------------------------------------------------------------------
High 1821 2000 3821 411 ± 18 230 ± 24 600 ± 44 870 ± 69 286 ± 148 448 ± 43 13.0 ± 2.7
Se-curved
hoofs
High 1944 0 1944 290 ± 18 180 ± 7 330 ± 20 760 ± 26 362 ± 89 374 ± 117 9.5 ± 3.0
Se-curved
hoofs
High 1774 2000 3774 362 ± 34 210 ± 16 590 ± 90 1000 ± 174 309 ± 103 335 ± 18 9.4 ± 2.9
Se-curved
hoofs
High 2038 0 2038 239 ± 34 170 ± 6 340 ± 20 890 ± 21 349 ± 63 344 ± 56 9.5 ± 1.2
Se-curved
hoofs
Normal Se 376 2000 2376 408 ± 23 170 ± 8 450 ± 38 950 ± 77 496 ± 53 457 ± 57 14.8 ± 3.0
Normal Se 396 0 396 100 ± 12 89 ± 5 190 ± 20 780 ± 69 143 ± 29 254 ± 7 5.5 ± 0.8
----------------------------------------------------------------------------------------------------------------
a Adapted from: Ermakov & Kovalskij (1968).
Jaffe & Mondragon (1969) presented evidence that animals could
adapt to long-term selenium intake. Hepatic-selenium levels
decreased steadily in young rats fed a diet containing 4.5 mg
selenium/kg as seleniferous sesame meal, if the rats came from
mothers that had been fed the same seleniferous diet, but the
levels increased if the rats came from mothers fed a non-
seleniferous stock diet. It was concluded that an adaptation
mechanism existed that allowed rats exposed to long-term selenium
ingestion to store less of this element than previously unexposed
controls. A later report (Jaffe & Mondragon, 1975) confirmed these
results and also indicated that the hepatic lesions, splenomegaly,
and other toxicity signs were significantly decreased in adapted
rats.
One mechanism for a possible adaptive response to high levels
of selenium could involve effects of selenium intake on various
selenium metabolic pathways, since previous selenium exposure has
been shown to have an influence on the whole body retention of
selenium and the urinary and pulmonary excretion of selenium
(Ganther et al., 1966; Ewan et al., 1967; Burk et al., 1973)
(section 6.3, 6.4). However, as discussed in section 7.1.2.1, more
recent work has shown that previous selenium exposure directly
influences the toxicity of final selenium metabolites such as
dimethyl selenide and the trimethylselenonium ion (Parizek et al.,
1976, 1980).
7.1.7. Mechanism of toxicity
The mechanism of selenium toxicity remains unclear and the
modes of action of various selenium compounds, such as hydrogen
selenide, selenomethionine, and selenium dioxide are likely to be
quite different. Thus, no unifying hypothesis regarding the toxic
effects of selenium compounds is possible. Various hypotheses
concerning the mechanisms of selenium toxicity have been presented
(Rosenfeld & Beath, 1964; Izraelson et al., 1973; Ermakov &
Kovalskij, 1974; US NAS/NRC, 1976; Lazarev, 1977; Levander, 1982).
Although it appears unlikely that selenite interferes directly with
sulfhydryl enzymes (Tsen & Tappel, 1958; Tsen & Collier, 1959), it
is possible that selenite may interfere with glutathione metabolism
(Vernie et al., 1978; Chung & Maines, 1981; Vernie, 1984; Anundi et
al., 1984) and that this may affect enzyme activities. The
apparent pro-oxidant nature of high levels of selenite has been
reported by several workers (Witting & Horwitt, 1964; Csallany et
al., 1984), and this may be related to some aspects of selenite
toxicity.
7.2. Selenium Deficiency
7.2.1. Animal diseases
The nutritionally beneficial effects of selenium were first
reported in 1957 by Schwarz, who discovered that sodium selenite
prevented dietary liver necrosis in vitamin E-deficient rats
(Schwarz & Foltz, 1957). This discovery led to the rapid
recognition of vitamin E-related selenium-responsive deficiency
diseases in several species of farm animals. Diseases such as
white muscle disease in sheep and cattle, hepatosis dietetica in
swine, and exudative diathesis in poultry are economically
significant problems in areas of the world where the soil levels of
selenium available for uptake by plants are low. Schwarz & Foltz
(1958) also reported that feeding a diet deficient in both selenium
and vitamin E to mice resulted in multiple necrotic degeneration of
several organs; more recently, Suchkov et al. (1977, 1978) found
that feeding such diets to mice for 13 days caused a decreased
staining intensity for zinc in the pancreas, kidneys, and testes.
The fact that vitamin E as well as selenium tended to protect
against many of these diseases led some research workers to
question whether there was any requirement for selenium in animals
receiving adequate amounts of vitamin E. But, the results of more
recent work have clearly demonstrated the need for selenium, even
in animals given nutritionally adequate levels of vitamin E.
Deficiency signs specific for selenium included alopecia, vascular
changes, cataract, poor growth, and reproductive failure in second
generation selenium-deficient rats (McCoy & Weswig, 1969; Wu et
al., 1979). Pancreatic degeneration occurred in selenium-deficient
chicks with a normal intake of vitamin E (Thompson & Scott, 1970),
but the condition could be prevented by feeding very high
levels (> 300 µg/kg) of vitamin E or other antioxidants (Whiteacre
et al., 1983).
Changes in the myocardial parenchyma and increases in heart
weight have been observed in albino rats fed corn and vegetables
grown in the areas in which Keshan disease is endemic (Su et al.,
1982), and swine fed grain from endemic areas for 6 months
exhibited multiple myocardial necrosis as well as other lesions
(Zhu et al., 1981). Vitamin E deficiency exacerbated the
pathological and histochemical changes in the heart and liver of
piglets fed a low selenium diet composed mainly of cereals grown in
the Keshan disease area (Zhu et al., 1981).
7.2.2. Intakes needed to prevent deficiency
7.2.2.1. Quantitative dietary levels
Schwarz (1961) showed that 0.02 - 0.03 mg selenium/kg diet, in
the form of sodium selenate or selenite, afforded 50% protection
against liver necrosis in vitamin E-deficient rats over an
experimental period of 30 days. Selenite-selenium at 0.10 mg/kg
protected the rats over their entire life span in the absence of
vitamin E. Under these conditions, the rats developed severe
tocopherol deficiency, which mainly afflicted the central nervous
system. Sprinker et al. (1971) noted that 0.10 mg selenium/kg, as
selenite, prevented specific selenium deficiency lesions in rats
that had been fed a vitamin E-supplemented diet for 2 generations.
Thompson & Scott (1969) found that administration of 0.02 - 0.05 mg
selenium/kg, as selenite, prevented death and exudative diathesis
in chicks fed diets containing typical levels of vitamin E
(sections 7.2.4.1 and 7.2.7.2 include discussions on the
nutritional interrelationship of selenium and vitamin E).
Considerable field experience in New Zealand has indicated that
feeds from pastures associated with selenium-responsive
unthriftiness in sheep contain 0.008 - 0.030 mg selenium/kg
(Hartley, 1967). In areas where selenium-responsive diseases do
not occur, the feed levels of selenium range from 0.020 - 0.098
mg/kg. Since there is some overlap in these selenium levels, other
factors may be involved in the etiology of this selenium-responsive
disease (various factors that can influence selenium deficiency
are discussed in section 7.2.7).
It has been concluded that "the critical level for dietary
selenium, below which deficiency symptoms are observed, is
apparently about 0.02 mg/kg for ruminants and 0.03 - 0.05 mg/kg for
poultry" (US NAS/NRC, 1971). However, this US NAS/NRC committee
pointed out that "when supplementary selenium is fed, higher levels
than the minimal requirements have been proposed, both to permit
satisfactory distribution of the element through the large feed
mass and to overcome variations in feed intake by individual
animals". The final recommendation of the committee was that 0.1
and 0.2 mg selenium should be added per kg feed to eliminate
selenium deficiency in livestock and turkeys, respectively. In
laboratory rats, a dietary-selenium level of 0.1 - 0.2 mg/kg would
be equivalent to an intake of about 5 - 10 µg/kg body weight per
day.
7.2.2.2. Bioavailability
7.2.3. Blood and tissue levels in deficiency
Hartley (1967) presented data on the blood-selenium levels of
normal New Zealand lambs and lambs suffering from various selenium-
responsive diseases (Table 30). The mean blood-selenium levels
were lower in the diseased lambs than in the healthy ones, but
there was considerable overlap between the two groups. It was
suggested that other unknown factors might be involved in the
etiology of these diseases. Jacobsson et al. (1970) found blood-
selenium levels of 0.003 mg/litre in cows afflicted with muscular
degeneration, whereas healthy cows had an average of 0.046
mg/litre. Hartley (1967) estimated that blood-selenium levels of
0.05 mg/litre in young sheep could be considered satisfactory.
The same authors compared liver-selenium levels found in normal
lambs with those found in lambs with selenium-responsive
unthriftiness (Table 31). Again, there was some overlap between
the two groups, but the average level of selenium in the livers
from the deficient lambs was much lower than that from the healthy
lambs. Allaway et al. (1966) suggested that 0.21 mg/kg (dry
weight) is the critical minimal hepatic-selenium level below which
a high incidence of white muscle disease can be expected in lambs.
Table 30. Blood-selenium levels in normal
and selenium-deficient lambsa
---------------------------------------------
Group Selenium contentb
(mg/litre)
---------------------------------------------
Normalc 0.026 (0.014 - 0.048)
White muscle diseased 0.016 (0.006 - 0.033)
Normale 0.06 (0.020 - 0.195)
Selenium-responsive 0.01 (0.007 - 0.030)
unthriftinessf
---------------------------------------------
a Adapted from: Hartley (1967).
b Mean with range in parentheses.
c Normal lambs from areas with white muscle
disease.
d Lambs with white muscle disease.
e Lambs from areas where selenium-responsive
diseases are not recognized.
f Lambs with selenium-responsive
unthriftiness.
Table 31. Liver-selenium levels in normal
lambs or lambs with selenium-responsive
unthriftinessa
------------------------------------------
Group Selenium contentb
(mg/kg)
------------------------------------------
Normalc 0.16 (0.03 - 0.40)
Selenium-responsive 0.018 (0.005 - 0.05)
unthriftinessd
------------------------------------------
a Adapted from: Hartley (1967).
b Mean with range in parentheses.
c Lambs from areas where selenium-
responsive diseases are not
recognized.
d Lambs with selenium-responsive
unthriftiness.
7.2.4. Physiological role: glutathione peroxidase
7.2.4.1. Function of selenium and relationship to vitamin E
The discovery that selenium is a component of the enzyme
glutathione peroxidase (Rotruck et al., 1973) provides a logical
explanation for the nutritional interaction of vitamin E and
selenium, a puzzle that perplexed scientists for many years.
Analysis of purified ovine erythrocyte glutathione peroxidase by
fluorometry revealed that the protein contained 4 moles of selenium
per mole of enzyme (Hoekstra et al., 1973) and this stoichiometry
was also found for crystalline bovine erythrocyte-glutathione
peroxidase analysed for selenium by neutron activation analysis
(Flohe et al., 1973). In the scheme postulated by Hoekstra
(1975a), tocopherols act as intracellular antioxidants to prevent
oxidative damage to polyunsaturated fatty acids in biological
membranes by terminating chain reactions of lipid peroxides (Fig.
7). Selenium, as a part of glutathione peroxidase, protects
against oxidative stress either by catalysing the destruction of
hydrogen peroxide or by catalysing the decomposition of lipid
hydroperoxides, thereby interrupting the free radical peroxidative
chain reaction; in this latter role the free fatty acyl
hydroperoxide must be liberated by phospholipan A2 action from the
hydrophobic region of biological membranes, before its reduction
can be catalysed by glutathione peroxidase (Grossman & Wendel,
1983). Thus, both of these nutrients play separate but
interrelated roles in the cellular defence mechanisms against
oxidative damage.
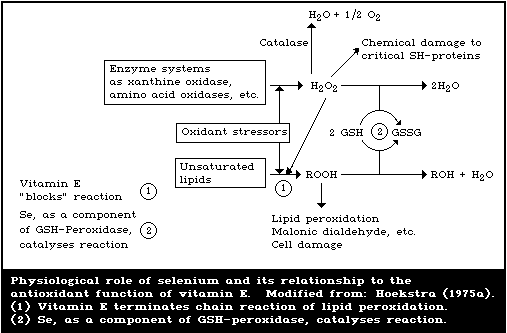 Although glutathione peroxidase accounts for many of the
biological effects of selenium, other functions of this trace
element in the body may yet be discovered. Only 1/5 of the total
selenium in rat brain is in the form of glutathione peroxidase
(Prohaska & Ganther, 1976). Selenium has also been shown to be a
constituent of several enzymes in microorganisms (Stadtman, 1977).
7.2.4.2. Effect of selenium intake on tissue-glutathione peroxidase
activity
The results of several studies have shown a close dose-effect
relationship between the dietary intake of selenium and the
activity of glutathione peroxidase in various tissues. For example,
Hafeman et al. (1974) found that increased dietary selenium caused
corresponding increases in erythrocyte-glutathione peroxidase
activity in rats, even when toxic levels of selenium were fed (Fig.
8). Hepatic-glutathione peroxidase activity increased with
increasing dietary-selenium levels up to 1 mg/kg, but then declined
due to liver damage when toxic levels were fed (Fig. 9). However,
studies on hamsters have shown that consumption of excess selenium
does not always result in increased erythrocyte-glutathione
peroxidase activity, since feeding selenite at more than 10 mg
selenium/kg diet did not cause increases in erythrocyte-enzyme
activity that corresponded to increases in blood-selenium
concentrations (Julius et al., 1980).
Although glutathione peroxidase accounts for many of the
biological effects of selenium, other functions of this trace
element in the body may yet be discovered. Only 1/5 of the total
selenium in rat brain is in the form of glutathione peroxidase
(Prohaska & Ganther, 1976). Selenium has also been shown to be a
constituent of several enzymes in microorganisms (Stadtman, 1977).
7.2.4.2. Effect of selenium intake on tissue-glutathione peroxidase
activity
The results of several studies have shown a close dose-effect
relationship between the dietary intake of selenium and the
activity of glutathione peroxidase in various tissues. For example,
Hafeman et al. (1974) found that increased dietary selenium caused
corresponding increases in erythrocyte-glutathione peroxidase
activity in rats, even when toxic levels of selenium were fed (Fig.
8). Hepatic-glutathione peroxidase activity increased with
increasing dietary-selenium levels up to 1 mg/kg, but then declined
due to liver damage when toxic levels were fed (Fig. 9). However,
studies on hamsters have shown that consumption of excess selenium
does not always result in increased erythrocyte-glutathione
peroxidase activity, since feeding selenite at more than 10 mg
selenium/kg diet did not cause increases in erythrocyte-enzyme
activity that corresponded to increases in blood-selenium
concentrations (Julius et al., 1980).
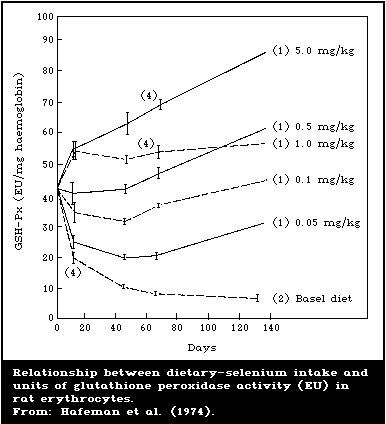
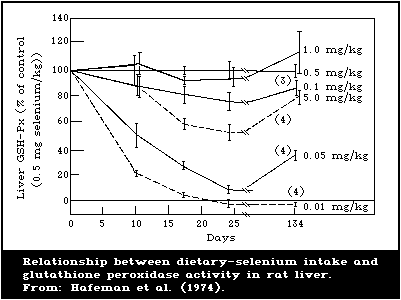 Oh et al. (1976a) investigated the effects of feeding several
different levels of dietary selenium on the activity of glutathione
peroxidase in various tissues in lambs. The activity of the enzyme
tended to plateau in all tissues examined, except red cells and
pancreas, when 0.1 mg was fed per kg diet. This level of dietary
selenium approximates the presumed requirement in this species.
7.2.4.3. Relationship between blood-selenium levels and erythrocyte-
glutathione peroxidase activity
A direct linear relationship between the selenium concentration
of whole blood and the activity of erythrocyte-glutathione
peroxidase has been demonstrated in lambs, sheep, cattle, and swine
(Oh et al., 1976b; Wilson & Judson, 1976; Sivertsen et al., 1977).
This linear relationship in lambs (Fig. 10) was thought to be due
to the fact that glutathione peroxidase accounts for most of the
total selenium in the ovine red cell and that ovine red cells
contain a relatively constant proportion of the total blood-
selenium.
Oh et al. (1976a) investigated the effects of feeding several
different levels of dietary selenium on the activity of glutathione
peroxidase in various tissues in lambs. The activity of the enzyme
tended to plateau in all tissues examined, except red cells and
pancreas, when 0.1 mg was fed per kg diet. This level of dietary
selenium approximates the presumed requirement in this species.
7.2.4.3. Relationship between blood-selenium levels and erythrocyte-
glutathione peroxidase activity
A direct linear relationship between the selenium concentration
of whole blood and the activity of erythrocyte-glutathione
peroxidase has been demonstrated in lambs, sheep, cattle, and swine
(Oh et al., 1976b; Wilson & Judson, 1976; Sivertsen et al., 1977).
This linear relationship in lambs (Fig. 10) was thought to be due
to the fact that glutathione peroxidase accounts for most of the
total selenium in the ovine red cell and that ovine red cells
contain a relatively constant proportion of the total blood-
selenium.
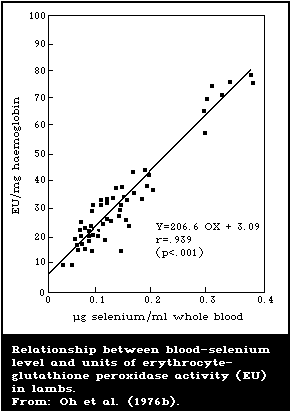 Under certain conditions, some investigators have not found a
significant correlation between blood-selenium levels and blood
glutathione peroxidase activity. For example, Thompson et al.
(1976) described swine that had high blood-selenium levels but low
blood-glutathione peroxidase activities. The reasons for this
discrepancy are not known but could perhaps be related to an
increased level of "non-functional" selenium in the blood, such as
selenomethionine that has been nonspecifically incorporated into
protein as a substitute for methionine.
7.2.4.4. Glutathione peroxidase as an indicator of selenium status
As discussed by Hoekstra (1975b), the measurement of
glutathione peroxidase activity offers several advantages as an
indicator of selenium status. First of all, the enzyme assay is
easier to perform and is less time consuming than the selenium
analysis. Also, the enzyme assay is not subject to contamination
problems. Moreover, glutathione peroxidase represents only
"functional" selenium in the tissues, not selenium that has been
non-specifically incorporated into proteins or that has formed
biologically inactive complexes with heavy metals. Finally, at
least in some tissues, the activity of the enzyme appears to taper
off as the dietary level of selenium approaches the nutritional
requirement of the animal. This "plateau effect" of glutathione
peroxidase activity does not occur in red blood cells, a biological
material that is highly convenient for sampling purposes. However,
as pointed out by Hoekstra (1975b), the absence of plateauing in
red cells may limit the usefulness of erythrocyte-glutathione
peroxidase in accurately establishing selenium requirements, but
does not diminish the usefulness of this source of the enzyme as an
overall index of selenium status.
One complicating factor in the use of glutathione peroxidase to
assess selenium status is the fact that the activity of the enzyme
is influenced by many physiological variables other than selenium
intake (Ganther et al., 1976). Among these are the age and sex of
the animal, starvation, exposure to certain oxidant stressors,
toxicants, or heavy metals, and deficiency in iron and vitamin B12.
Obviously, these variables have to be controlled, or compensated
for, if glutathione peroxidase is to be a valid index of selenium
status.
Another serious complicating factor in the use of glutathione
peroxidase to assess selenium status is the existence of a "non
selenium-dependent" glutathione peroxidase activity that persists,
even in severe selenium deficiency (Lawrence & Burk, 1976). This
enzymic activity has been found in variable amounts in different
rat tissues ranging from 23% of the total glutathione peroxidase
activity in fat to 91% in testis (Lawrence & Burk, 1978). The "non
selenium-dependent" activity accounts for 26 - 38% of the total
glutathione peroxidase activity in rat brain, kidneys, liver, and
adrenals. No such activity is found in rat erythrocytes, spleen,
heart, thymus, intestinal mucosum, skin, or skeletal muscle. The
"non selenium-dependent" glutathione peroxidase activity has been
found in the liver of several different species including the rat,
hamster, guinea-pig, chicken, pig, sheep, and man. In human and
guinea-pig liver, it accounts for the major part of the total
glutathione peroxidase activity. The "non selenium-dependent"
glutathione peroxidase activity differs from the selenium-
containing enzyme in substrate specificity in that it catalyses the
reduction of a range of organic hydroperoxides but not of hydrogen
peroxide. The activity of the "non selenium-dependent" enzyme is
inversely related in rat liver to the dietary level of selenium
(Lawrence & Burk, 1978; Lawrence et al., 1978) and the glutathione-
S-transferase enzymes are responsible for the "non selenium-
dependent" enzyme activity (Prohaska & Ganther, 1977; Lawrence et
al., 1978). The glutathione- S-transferases are not selenium-
containing enzymes; they are all dimeric, and their monomeric
constituents fall into three categories of different relative
molecular mass. The glutathione peroxidase activity of the enzymes
is only shown by the dimeric enzymes that contain one of the three
subunits and, in selenium deficiency, the amount of this subunit
alone is increased (Ketterer et al., 1982; Mehlert & Diplock,
1985).
The physiological significance of the "non selenium-dependent"
activity has been questioned because the seleno-enzyme has a much
higher Vmax and lower KM (Prohaska & Ganther, 1977). However, Burk
et al. (1980a) showed that the "non selenium-dependent enzyme"
removed organic hydroperoxides in intact rat livers perfused with a
haemoglobin-free medium containing high concentrations of
peroxides.
Obviously, any use of glutathione peroxidase activity to
monitor selenium status has to take into account this "non
selenium-dependent" activity. This complication can be avoided by
selecting a tissue for test that has little or none of the
selenium-independent activity, such as erythrocytes, or by using
hydrogen peroxide as the substrate, since this has little activity
with the selenium-independent enzyme.
7.2.5. Other possible physiological roles
7.2.5.1. Homeostasis of hepatic haem
Burk et al. (1974) showed that the induction of hepatic
cytochrome P-450 by daily intraperitoneal injections of 75 mg
phenobarbital/kg body weight, for 4 days, was markedly impaired in
rats that had been fed a selenium-deficient diet for at least 3
months. The induction of ethylmorphine demethylase activity by
phenobarbital was also impaired in the livers of selenium-deficient
rats. However, no such impairment was observed in the induction of
cytochrome b5 or of NADPH-cytochrome c reductase activity or
biphenyl hydroxylase activity, or in pentobarbital sleeping time
(Burk & Masters, 1975). Treatment with phenobarbital stimulated a
6- to 8-fold increase in hepatic microsomal haem oxygenase activity
in selenium-deficient rats but had little or no effect in selenium-
supplemented rats (Correia & Burk, 1976). This suggested that the
abnormalities of cytochrome P-450 induction in selenium-deficient
rats were related to increased degradation rather than decreased
synthesis of hepatic haem. Mackinnon & Simon (1976) reported an
impaired haem synthesis in selenium-deficient phenobarbital-treated
rats, but Burk & Correia (1977) were unable to confirm these
results. Apparently, the pronounced stimulation of microsomal
haem oxygenase (EC 1.14.99.3) in the livers of selenium-deficient
rats by phenobarbital is unrelated to the role of selenium in
glutathione peroxidase, since pretreatment of deficient rats with
a single dose of selenite at 50 mg selenium/rat, 4 - 6 h before
phenobarbital administration, abolished the response in haem
oxygenase but had no effect on cytosolic or mitochondrial
glutathione peroxidase activity (Correia & Burk, 1978). Moreover,
phenobarbital failed to stimulate liver microsomal haem oxygenase
activity or to affect cytochrome P-450 in vitamin E-deficient,
selenium-adequate rats, so this response does not seem to be
related to the direct effects of lipid peroxidation on haem or
cytochrome P-450. There is a marked sex difference in the
phenobarbital stimulation of hepatic microsomal haem oxygenase
activity in selenium-deficient rats. The stimulation was greatest
in males and least in females with intermediate values in castrated
males and testosterone-treated females (Burk et al., 1980a). This
sex difference existed even though hepatic glutathione peroxidase
activity was reduced to the same extent by selenium deficiency in
all groups. However, the exact mechanism by which selenium
influences homeostasis of hepatic haem still needs to be clarified.
Pascoe et al. (1983) showed that both intraluminal iron and
selenium were required by the intestinal mucosa for maintenance of
the intestinal cytochrome P-450 content, and its dependent mixed
function oxidase activity, and that withdrawal of dietary selenium
for one day markedly decreased the level of cytochrome P-450 in the
small intestine, suggesting that intestinal mucosal cells derive
their selenium from the diet rather than from the blood.
A report by Maines & Kappas (1976) indicated that injection of
selenium increased hepatic haem oxygenase activity, but the doses
of selenium used (3.94 mg/kg body weight) exceeded the MLD for
selenium as selenite (Table 19), so that the phenomenon observed by
these authors may have been merely an artifact of acute selenium
poisoning.
7.2.5.2. Microsomal and mitochondrial electron transport
Diplock and co-workers carried out an extensive series of
studies, the results of which suggest a possible role for selenium
and vitamin E in the electron-transfer system of rat-liver
microsomes (Diplock, 1974a,b). First, they showed that appreciable
amounts of volatile selenium evolved from rat liver subcellular
organelles, after acid treatment, only when the rats were adequate
in vitamin E and the tissue homogenization medium contained large
concentrations of anti-oxidants (Diplock et al., 1971). The acid-
volatile selenium was later identified as selenide (Diplock et al.,
1973). It was assumed that this compound was formed in vivo,
presumably by the glutathione reductase pathway (Fig. 9), but
artifactual in vitro formation of such selenide, because of the
presence of mercaptoethanol in the homogenization medium, cannot be
ruled out (Levander, 1976a). An oxidant-labile form of non-haem
iron, dependent on dietary vitamin E and selenium, was observed in
rat liver microsomes (Caygill & Diplock, 1973). It was considered
that this supported the hypothesis that the selenide might form
part of the active centre of a non-haem iron protein functional in
microsomal electron transfer. Alterations in the kinetic
parameters of aminopyrine demethylation by the liver microsomes in
vitamin E-deficient rats also supported this idea (Giasuddin et
al., 1975). However, attempts to isolate an oxidant-labile
selenium- and vitamin E-dependent non-haem, iron-containing protein
fraction were unsuccessful, because of the lability of the
selenide, and it was concluded that clarification of the role of
selenide in microsomes needed additional research (Diplock, 1976,
1979). The effects of prolonged selenium deficiency on a large
number of parameters in mouse liver xenobiotic metabolism were
studied by Reiter & Wendel (1983). They demonstrated a
heterogeneous pattern of effects that did not appear to involve
glutathione peroxidase.
One of the earliest biochemical defects discovered in vitamin
E- and selenium-deficient rats was the inability of liver slices or
homogenates to maintain normal rates of respiration after prolonged
periods of incubation in vitro (Schwarz, 1962). Although this so-
called respiratory decline might be explained on the basis of
generalized mitochondrial membrane damage due to lipid peroxidation
(Yeh & Johnson, 1973), it was also plausible that selenium might
have a more specific role in mitochondrial metabolism. Indeed,
selenium added to the diet, or added in vitro, accelerated the
swelling of rat liver mitochondria induced by certain thiols, and
this swelling could be blocked by the addition of cyanide (Levander
et al., 1973a). Selenium was in fact shown to be an excellent
catalyst for the reduction of cytochrome c by thiols in chemically-
defined systems (Levander et al., 1973b) and a selenoprotein
containing a haem group similar to that of cytochrome c has been
reported in lamb muscle (Whanger et al., 1973). However, more work
is required to establish the possible role of selenium in
mitochondrial electron transfer.
7.2.5.3. The immune response
Dietary vitamin E in excess of nutritionally required levels
has been shown to improve the humoral immune response of several
different species to bacterial and viral antigens (Nockels, 1979).
Because of the close nutritional and biochemical relationship
between selenium and vitamin E (section 7.2.4.1), the effect of
dietary selenium supplementation was tested on the immunological
responses of mice (Spallholz et al., 1973). Mice fed a laboratory
chow diet (thought to contain 0.5 mg selenium/kg) supplemented with
sodium selenite at 0.7 or 2.8 mg selenium/kg had approximately 7-
and 30-fold higher antibody titres, respectively, after challenge
with sheep red blood cells than mice fed the chow diet alone.
Sodium selenite injected into mice intraperitoneally at doses of
3 - 5 µg per animal also increased the primary immune response to
the sheep red-blood-cell antigen and this increase was greatest
when the selenium was given prior to, or simultaneously with, the
antigen (Spallholz et al., 1975). The mechanism by which selenium
stimulates antibody synthesis is not known (Martin & Spallholz,
1976), but the dose of selenium needed to elicit this response is
clearly above the nutritionally required level. Levander (1986)
has reviewed the very limited data on the possible effect of
selenium on the immune response in man.
7.2.5.4. Selenium and vision
The effects of selenium on light perception were studied in 50
male "Grey chinchilla" rabbits weighing 3 - 3.5 kg and maintained
on a standard laboratory diet (Abdullaev et al., 1972, 1974a,b).
Electroretinography was carried out in dark-adapted animals under
conditions of maximally dilated pupil induced by topical
application of 0.5% dikaine - 1% atropine. Light stimulation was
provided with an impulse lamp giving either single or dual flashes
of 150 microseconds. Eight different energy levels of light
intensity were used ranging from 0.016 to 1.4 J. Sodium selenite
was administered subcutaneously at a dose of 0.45 mg selenium/kg
body weight. Control rabbits were injected with sodium sulfite at
a dose of 1 mg/kg. Sodium selenite stimulated light sensitivity,
as judged by increases in the a and b waves of the electro-
retinogram (ERG). The increase in the amplitude of the b wave was
considerably greater than that of the a wave. This stimulating
effect of sodium selenite on light sensitivity persisted for 3 days
in all studies. The same effects were observed when sodium
selenite was given via retrobulbar administration. Sodium sulfite
did not have any effect on the ERG. Similar results were obtained
by Bacharev et al. (1975).
The Task Group recognized that the data on the effects of
selenium on vision were obtained in studies involving high dose
levels (in the case of rabbits, the doses were approximately 20 -
30% of the LD50). The effects on vision of lower doses of selenium
have not been elucidated so far. The possible effect of deficiency
of selenium on vision is also of interest. Model studies with
isolated retinas exposed to a 0.01% concentration of sodium
selenite resulted in an increase in the amplitude of the ERG
(Kulieva et al., 1978). The effect of increased sensitivity of the
eye to light, after administration of sodium selenite, and the rate
of manifestation and duration of the effect are directly
correlated with localization of the substance in the structures of
the eye (section 6.2.2.1). Subcutaneous administration of sodium
selenite to rabbits, at a dose of 0.4 - 2 mg/kg body weight,
inhibited free radical formation as indicated by electron
paramagnetic resonance (EPR) measurements, not only in the lipid
membrane phase but also within the melanoprotein granules of the
retinal epithelium. The EPR signal was decreased in intensity by
selenite treatment in comparison with controls, but the form and
parameters of the EPR lines were not changed (Abdullaev et al.,
1974a,b).
7.2.6. Effects on reproduction
Vitamin E was discovered as a result of the isolation of an
unknown fat-soluble dietary factor essential for reproduction in
rats (Evans & Bishop, 1922), but selenium compounds are not active
in preventing reproductive failure in vitamin E-deficient rats
(Harris et al., 1958). Moreover, female rats weighing 80 - 120 g
and fed a selenium-deficient, but vitamin E-adequate, diet
successfully delivered 3 litters over a period of 6 months,
even though their blood-selenium levels dropped from 0.52 to
0.06 mg/litre during this time (Hurt et al., 1971). Similarly,
McCoy & Weswig (1969) found that rats fed a low-selenium but
adequate-vitamin E diet, for 4 months, reproduced normally.
However, their offspring failed to reproduce if kept on the low
selenium diet. Immobile spermatozoa, with separation of heads from
tails, were observed in 5 out of the 8 male progeny not given
selenium supplements, and no spermatozoa were seen in the other 3
males. The 2 male and 2 female offspring, supplemented with sodium
selenite at 0.1 mg selenium/kg diet, were fertile but delivered
litters of only one or 2 rats, all of which died in a few days.
The authors concluded that selenium supplementation resulted in
some fertilization, but only a few full-term young were delivered
and these were abnormal as they survived for only a brief period.
Wu et al. (1973) found that the motility of spermatozoa from
male rats, born to females fed a selenium-deficient diet, was
always very poor and that most of the sperm cells showed breakage
near the midpiece of the tail. Supplementation of the diet with
high levels of vitamin E or various other antioxidants did not
prevent these selenium deficiency effects. However, in this study,
the body and testicular weights of the rats were so markedly
depressed by selenium deficiency that it was not possible to
establish whether the abnormal sperm were due to a direct effect of
selenium deficiency or to an indirect effect on overall growth and
well-being. In a second series of studies (Wu et al., 1979), using
male rats born to dams fed a diet adequate in selenium, the
depression of growth, and testes weight in the progeny caused by
selenium deficiency was much less, even though impaired sperm
motility and abnormal sperm morphology were still observed (Table
32). The authors also pointed out that there did not appear to be
any correlation between reduction in body or testicular weights and
the characteristic sperm midpiece abnormality among the individual
rats in each treatment group. However, all of the rats used by Wu
and associates were fed diets that were probably low in zinc and
chromium and both of these trace elements are known to play
important roles in sperm formation (Wu et al., 1971; US NAS/NRC,
1979; Anderson & Polansky, 1981).
The mechanism of the effects of selenium on the integrity of
sperm morphology in the rat is not known, but Brown & Burk (1973)
found over 40% of an injected tracer dose of radio-selenite in the
testis-epididymis complex of selenium-deficient rats, after 3
weeks. Autoradiography of epididymal sperm revealed that the
labelled selenium was heavily localized in the midpiece. Calvin
(1978) has isolated a selenopolypeptide, with a relative molecular
mass of 17 000 daltons, from rat sperm, which may have a critical
function in the normal assembly of the sperm tail.
Selenium cannot prevent resorption-gestation in vitamin E-
deficient rats, nor can it improve reproductive performance in
vitamin E-deficient chickens or turkeys (Creger et al., 1960;
Jensen & McGinnis, 1960). However, studies in which severe
depletion of selenium was produced by the long-term feeding of low-
selenium, high-vitamin E diets have shown the beneficial effects of
the element on egg production and hatchability in poultry. For
example, Cantor & Scott (1974) fed 1-day-old Single Comb White
Leghorn chicks semi-purified low-selenium starter, grower, and
developer diets, all of which contained only 0.02 mg naturally-
occurring selenium/kg from 0 to 6, 7 to 12, and 13 to 25 weeks of
age, respectively. Because of the low selenium level, high levels
of vitamin E (50 - 84 IU/kg) were added to the diets to prevent
exudative diathesis during the growth period. From 25 weeks of age
to 10 months, the birds were fed semi-purified low-selenium layer
diets that contained 11 IU of vitamin E/kg. After 10 months, the
hens were fed a low-selenium corn-soybean meal practical laying
ration containing 0.027 mg natural selenium/kg, by analysis. The
calculated vitamin E content was 16 IU/kg. At 12 1/2 months of
age, 4 replicate groups of 5 hens were continued on the low-
selenium diet, whereas a similar number of hens was fed the same
diet supplemented with sodium selenite at 0.1 mg selenium/kg.
Selenium supplementation had beneficial effects on both egg
production and hatchability of fertile eggs (Table 33), but there
was no significant effect of selenium on egg fertility, determined
after 2 - 5 weeks (data not shown).
Table 32. Effects of selenium deficiency on body and testicular weights and
spermatozoan morphology and motility in ratsa
------------------------------------------------------------------------------------
Dietary selenite Number Duration Body Testes Sperm Number of rats
supplement of (month) weight weight motilityb with sperm
(mg selenium/kg) rats (g) (g) midpiece
abnormality
------------------------------------------------------------------------------------
Study 1
0 6 4 452 ± 6 3.29 ± 0.06 6.2 0/6
0.5 6 4 470 ± 18 3.55 ± 0.11 6.2 0/6
0 4c 12 476 ± 17 3.03 ± 0.30 2.5 2/4
0.5 4c 12 494 ± 12 3.57 ± 0.07 5.8 0/4
Study 2
0 10c 11 456 ± 16 3.07 ± 0.09 3.5 5/10
0.4 12 11 543 ± 18 3.50 ± 0.08 8.0 0/12
------------------------------------------------------------------------------------
a Adapted from: Wu et al. (1979).
b Relative activity of sperm observed with a light microscope at 400x at 37 °C and
graded from 0 - 10 with 10 representing the highest motility.
c Two rats died in each of these groups.
Cantor et al. (1978) carried out a similar study on turkeys in
which large white breeder hens and toms were raised from one day of
age until sexual maturity on a series of Torula yeast-containing
diets, low in selenium content (0.025 - 0.047 mg/kg). At 32 weeks
of age, 8 replicate groups, each comprising 6 individually-caged
hens, were fed a low-selenium basal diet (0.033 mg/kg) or the same
diet supplemented with sodium selenite at 0.2 mg selenium/kg.
Eight individually-caged toms were also assigned to each dietary
treatment at 32 weeks of age. Neither the tom nor the hen dietary
treatments had any effect on fertility and the tom dietary
treatment did not have any effects on hatchability. But the
hatchability of fertile eggs from hens fed the unsupplemented and
supplemented diets was about 50 and 59%, respectively. The
mechanism by which dietary selenium improves hatchability in
chickens or turkeys is not known, but the improvement may be due to
increased embryonic selenium levels or to a reduced embryonic need
of vitamin E (Cantor & Scott, 1974).
Table 33. Effect of dietary selenium
supplements on reproductive performance of
12 1/2-month-old selenium-depleted hens fed a
low-selenium, corn-soybean-meal practical
rationa
----------------------------------------------
Selenium supplement None 0.1 mg selenium as
Na2Se03/kg diet
----------------------------------------------
Criterion
Egg production (%)
Weeks 1-2 67.2 76.6
3-4 66.4 78.5
5-6 64.2 71.8
Day 46-76 54.2 79.7
77-107 55.1 76.7
108-137 59.1 75.1
Hatchability of fertile eggs (%)
Week 2 57.3 93.0
3 54.7 97.1
4 54.5 94.9
5 42.4 86.6
17 0.0 90.3
18 8.0 93.6
----------------------------------------------
a Adapted from: Cantor & Scott (1974).
In New Zealand, a close geographical relationship was observed
between the presence of white muscle disease and the incidence of
barren ewes (Table 34). A selenium intervention trial was carried
out on 3 farms where there was a history of white muscle disease
and low lambing percentages (Table 35). The selenium-treated ewes
received 5 mg of selenium, as sodium selenite, at monthly intervals
from one month before tupping until just before lambing; half of
the rams were also given selenium at monthly intervals before and
during tupping (Hartley et al., 1960; Hartley & Grant, 1961).
Selenium-dosed groups on all farms had higher lambing percentages
and fewer barren ewes than the controls. Moreover, white muscle
disease was eliminated in the lambs of the treated ewes. Estrus,
ovulation, fertilization, and early embryonic development all
proceeded normally in selenium-responsive ovine infertility until
between 3 and 4 weeks after conception (at about the time of
implantation) when the embryos perished for unknown reasons
(Hartley, 1963). The actual cause of selenium-responsive
infertility is also unknown and the geographical distribution of
the disease is not always the same as that of other selenium
deficiency diseases, such as white muscle disease or selenium-
responsive unthriftiness (Gardiner et al., 1962). This suggests
either that selenium-responsive infertility occurs only where
intake of selenium is particularly low or that some other factors
must be present that exacerbate the effects of low selenium intake.
For example, mercury has a strong antagonism with selenium (section
7.4.1), and Parizek et al. (1971) showed that inorganic mercury
could block the transport of selenium from mother rats to the
fetuses.
Table 34. Geographical relationship between
congenital white muscle disease and incidence
of barren ewesa
-----------------------------------------------
Congenital Number Incidence of barren ewes
white muscle of up to 11 - 30% over
disease farms 10% 30%
-----------------------------------------------
Present 21 3 8 10
Absent 16 12 4 0
-----------------------------------------------
a Adapted from: Hartley et al. (1960).
Table 35. Effects of selenium (Se) on the reproductive
performance of sheep and white muscle disease in lambsa
-------------------------------------------------------
White muscle
Farm Lambing Barren ewes disease incidence
control Se control Se control Se
-------------------------------------------------------
(%) (%) (%)
C 60.6 94.9 31.1 7.5 37.5 0
D 55.0 86.0 24.3 11.5 22.2 0
E 70.5 93.6 26.1 6.4 12.0 0
-------------------------------------------------------
a Adapted from: Hartley et al. (1960).
7.2.7. Factors influencing deficiency
7.2.7.1. Form of selenium
Schwarz (1961) pointed out that only 0.007 mg naturally-
occurring selenium/kg diet in extracts from pork kidney ("Factor 3
Selenium") was needed to afford 50% protection against dietary
necrotic liver degeneration in rats, whereas 0.020 - 0.030 mg/kg
was required when the selenium was in the form of selenite,
selenate, selenocystine, or selenomethionine. Elemental selenium
was essentially inactive. Many organic derivatives were
synthesized in an attempt to develop selenium compounds that were
not highly toxic but still retained considerable biological potency
against deficiency diseases (Schwarz et al., 1972). Simple amino
acid derivatives of monoseleno diacetic acid appeared promising in
this regard and the hope was expressed that some of these compounds
might make it possible to use selenium in the prevention and cure
of disease (Schwarz, 1976).
Cantor et al. (1975a) studied the ability of selenium in
various poultry feed ingredients to prevent exudative diathesis, a
selenium deficiency disease, in chickens. In general, the selenium
in plant products was more readily available than that in animal
products, but the latter consisted of highly-processed fish or
poultry meals.
Selenium in the form of selenomethionine was less effective
than selenium as sodium selenite for preventing exudative diathesis
in chicks (Noguchi et al., 1973), but the reverse was true for the
prevention of pancreatic fibrosis (Cantor et al., 1975b). In fact,
selenomethionine was four times as effective as either selenite or
selenocystine for this purpose; this phenomenon may have been
related to the peculiar affinity of this compound for the pancreas.
7.2.7.2. Vitamin E
Vitamin E decreases the level of dietary selenium needed to
prevent deficiency diseases in several species of animals. For
example, Scott & Thompson (1971) demonstrated that chicks receiving
100 mg vitamin E/kg diet needed only 0.01 mg selenium/kg diet to
prevent exudative diathesis, whereas chicks not receiving any added
vitamin E needed 0.05 mg/kg. Similarly, Scott et al. (1967) showed
that selenium at 0.18 mg/kg protected against gizzard myopathy in
turkey poults fed diets containing vitamin E, but that 0.28 mg/kg
was needed to protect poults fed diets not supplemented with
vitamin E. Hakkarainen et al. (1978) found that 0.135 mg
selenium/kg was insufficient to prevent deficiency signs in swine
fed a diet containing 1.5 mg vitamin E/kg but was adequate in swine
fed 5.0 mg vitamin E/kg. Increasing the level of dietary vitamin E
decreased the severity of nutritional muscular dystrophy in lambs
fed diets containing selenium at 0.1 mg/kg (Ewan et al., 1968). In
lambs not receiving any supplementary dietary selenium, lambs
receiving low levels of vitamin E died of dystrophy before their
hepatic-selenium levels were depleted to 0.21 mg/kg, the level
considered by Allaway et al. (1966) to be the critical
concentration of selenium in liver for the development of
nutritional muscular dystrophy (section 7.2.3). With the highest
level of vitamin E supplementation, the liver-selenium values were
lower than the critical level suggested by Allaway et al. (1966)
and yet the lambs still survived. These results suggest that the
tissue-selenium level is not the only factor involved, but that the
levels of both tocopherol and selenium determine the appearance of
nutritional muscular dystrophy. The effects of vitamin E in
reducing selenium requirements are readily explainable on the basis
of the close biochemical relationship between these two nutrients
discussed in section 7.2.4.1.
7.2.7.3. Heavy metals and other minerals
Since selenium protects against the toxicity of several heavy
metals (section 7.4), many scientists have examined the effect of
heavy metals on the induction of selenium deficiency. Silver
accelerated the development of liver necrosis in rats (Whanger,
1976) and increased the severity of selenium-vitamin E deficiency
in swine (Van Vleet, 1976; McDowell et al., 1978), presumably by
decreasing the activity of glutathione peroxidase. Inorganic
mercury also decreased glutathione peroxidase activity in the rat
but did not have any effect on the rate of development of liver
necrosis (Whanger, 1976). However, methylmercury accentuated the
development of selenium deficiency in pigs fed a low-selenium diet
(Froseth et al., 1974). In spite of the ability of arsenic to
enhance the biliary excretion of toxic levels of selenium (section
7.1.6.3), several attempts to induce selenium deficiency by feeding
arsenic have been unsuccessful (Levander, 1977; McDowell et al.,
1978). On the other hand, drenching pregnant and lactating ewes
with potassium cyanide, which partially counteracts selenium
toxicity in rats (section 7.1.6.2), increased the incidence of
nutritional myopathy in their lambs (Rudert & Lewis, 1978). It has
been suggested that the use of sulfate fertilizers may decrease
selenium concentrations in forage plants (Pratley & McFarlane,
1974; Westermann & Robbins, 1974), and that this could lead to an
increased incidence of selenium deficiency in farm animals
(Schubert et al., 1961). Dietary sulfate had no effect on the
incidence of white muscle disease but increased the number of lambs
with degenerative lesions of the heart (Whanger et al., 1969).
Neither cadmium nor tellurium had any effect on white muscle
disease in lambs (Whanger et al., 1976b) or vitamin E-selenium
deficiency in swine (McDowell et al., 1978).
7.2.7.4. Xenobiotics
The results of early work by Hove (1948) suggested that vitamin
E could protect rats fed a low-protein diet from death due to
carbon tetrachloride (CCl4) poisoning. The establishment of a
nutritional relationship between selenium and vitamin E stimulated
Seward et al. (1966) to study deficiencies of these nutrients in
relation to poisoning (Table 36). Mortality was higher in the
group deficient in both nutrients than in controls, but selenium
deficiency alone did not increase mortality. Other workers
(Hafeman & Hoekstra, 1977a; Burk & Lane, 1979) have confirmed this
result. The increased mortality due to CCl4 in doubly-deficient
animals could have been due simply to inhibition of eating.
Fasting these animals overnight can precipitate dietary liver
necrosis leading to death (Hafeman & Hoekstra, 1977b).
Table 36. Protective effect of selenium and vitamin E
against carbon tetrachloride toxicity in rats fed a
10% soybean-protein dieta
-----------------------------------------------------
Dietary supplement Weight gain Survival 48 h after
(g/8 weeks) CCl4 injectionb
-----------------------------------------------------
None 62 ± 5 3/10
Vitamin Ec 77 ± 3 9/10
Seleniumd 79 ± 6 8/10
Vitamin E and 80 ± 5 10/10
selenium
-----------------------------------------------------
a Adapted from: Seward et al. (1966).
b All rats injected intraperitoneally with a single
dose at 2 ml/kg body weight.
c Added as a mixture of equal quantities of dl-alpha-
tocopherol and dl-alpha-tocopheryl acetate at 200
mg/kg diet.
d Added at 0.1 mg selenium/kg diet, as sodium selenite.
Paraquat is another xenobiotic that is thought to exert its
toxic effect via increased lipid peroxidation (Bus et al., 1974).
Selenium deficiency worsens lung injury in rats caused by paraquat
(Omaye et al., 1978) and leads to liver injury that would not
otherwise occur in mice exposed to this compound (Cagen & Gibson,
1977). Burk et al. (1980b) showed that selenium-deficient rats
were much more susceptible to paraquat or diquat poisoning than
controls (Table 37). The deficient rats also produced more ethane
and had a higher serum-glutamic-pyruvic transaminase (SGPT)
activity, when treated with paraquat or diquat, than the controls.
The same workers found that injected selenium protected deficient
rats against diquat toxicity before appreciable amounts of
glutathione peroxidase activity appeared in the tissues. They
suggested the existence of a selenium-dependent factor, apart from
glutathione peroxidase which protects against lipid peroxidation.
Combs et al. (1975) noted a similarity between the oedema
produced in chicks fed a practical diet containing polychlorinated
biphenyls (PCBs) and that observed in chicks fed a diet deficient
in selenium and vitamin E (exudative diathesis). When selenium-
depleted hens were fed a diet not containing PCBs, selenite at
0.15 mg selenium/kg diet was sufficient to prevent exudative
diathesis in the chicks, but was not sufficient to prevent the
disease when the hens were fed a diet containing 10 mg PCBs/kg
(Table 38). In another study, addition of 50 mg PCBs/kg diet
increased the incidence of exudative diathesis in vitamin E-
deficient chicks receiving selenite at 0.04 mg selenium/kg diet
from less than 60% in the controls to almost 90% in the PCBs group,
14 days after hatching (Combs & Scott, 1975). Dietary PCBs
depressed plasma-glutathione peroxidase activity and increased the
amount of dietary selenium required to protect liver microsomal
fractions from peroxidation in vitro. On this basis, the authors
concluded that dietary PCBs potentiate vitamin E-selenium
deficiency in chicks by interfering with the biological utilization
of dietary selenium, though some interference with the absorption
and retention of vitamin E was also seen.
Table 37. Effect of selenium deficiency in rats treated with paraquat or
diquata,b
-------------------------------------------------------------------------
Diet Agent Dose Ethane SGPT Survival
group given produced activity time
-------------------------------------------------------------------------
(µmol/kg) (pmol/100 g/h) (mU/ml) (min)
Selenium- paraquat 78 470 ± 93 212 ± 87 297 ± 74
deficient
Control paraquat 78 18 ± 13 32 ± 26 survived 24 h
Control paraquat 390 92 ± 5 45 ± 34 106 ± 30
Selenium- diquat 19.5 1940 ± 420 3490 ± 1940 150 ± 37
deficient
Control diquat 230 56 ± 50 41 ± 5 80 ± 12
-------------------------------------------------------------------------
a Adapted from: Burk et al. (1980b).
b Values are mean ± SD of 4 or 5 values.
Burk & Lane (1979) pointed out the complexity of nutritional
toxicology experimentation involving selenium, vitamin E, and
various drugs and other chemicals. After studying the effects of
numerous xenobiotics on rats that were deficient in either vitamin
E or selenium, they concluded that in vivo lipid peroxidation, as
assessed by ethane production, was not necessarily correlated with
liver necrosis. Moreover, it was concluded that selenium
deficiency was not just a lack of glutathione peroxidase activity
in the tissues, since this condition also elevates hepatic
glutathione S-transferase activity. Thus, selenium deficiency may
actually protect against the hepatotoxicity of certain compounds,
(e.g., acetaminophen and iodipamide) by increasing their
conjugation with glutathione.
Table 38. Effect of PCBs fed to hens on the
incidence of exudative diathesis in progeny fed a
sub-optimal level of seleniuma
------------------------------------------------------
Additions to diets of hensb Exudative Weight gain
-------------------------- diathesis of chicks
PCBsd Seleniume in chicksc (g/2 weeks)
(mg/kg) (mg/kg) (%)
------------------------------------------------------
0 0 35 89
0 0.15 0 102
10 0 30 82
10 0.15 45 78
------------------------------------------------------
a Adapted from: Combs et al. (1975).
b Single Comb White Leghorn hens, 27 weeks old, that
had been reared on low-selenium, semi-purified diets
supplemented with high levels of vitamin E; hens
were on dietary treatments shown in Table for an
additional 5 weeks.
c Determined at 2 weeks of age. Chicks were fed a
selenium-deficient diet (less than 0.02 mg
selenium/kg) supplemented with 0.06 mg selenium, as
Na2Se03/kg. Diet was essentially free of tocopherols.
d Aroclor(R) 1254.
e Added as Na2Se03.
7.2.7.5. Exercise stress
Ancedotal observations from New Zealand suggested that selenium
might be of value against "tying-up" in thoroughbred racehorses
(Hartley & Grant, 1961), a condition that occurs during training
and is associated with exercise. Characteristics include muscle
stiffness and tenderness and disinclination to move. Young &
Keeler (1962) applied mechanical restraint to one foreleg of lambs
born to ewes fed a diet known to produce nutritional muscular
dystrophy and found that lesions either did not occur or occurred
to a much lesser extent in muscles of the restrained limb than in
muscles of the unrestrained limb. Brady et al. (1978) observed
increased levels of malondialdehyde, a product of lipid
peroxidation, in the erythrocytes of 6 horses, immediately after
exercise, but were unable to reproduce this phenomenon in a second
exercise trial with 8 horses. Sodium selenite, given for 4 weeks
in the trace mineral salt at a level calculated to provide 0.15 mg
supplementary selenium/kg diet, did not have any effect on the
elevated plasma-enzyme activities observed in exercised horses.
Furthermore, it did not have any effect on plasma-selenium levels
or erythrocyte-glutathione peroxidase activity. Harthoorn & Young
(1974) commented that the pathological picture in wild animals that
have died following mechanical capture ("capture myopathy" or
"overstraining syndrome") is indistinguishable from white muscle
disease seen in cattle suffering from vitamin E deficiency, but
pointed out that prophylactic and subsequent symptomatic treatment
with vitamin E and selenium-containing preparations did not have
any beneficial effects on captured animals. Brady et al. (1979)
expected to induce death in rats that had been fed a selenium and
vitamin E-deficient diet for 4 weeks, after exercising them to
exhaustion by swimming, but had no success, in spite of markedly
depressed tissue-glutathione peroxidase activity, decreased hepatic
stores of fat-soluble antioxidants, and increased levels of
thiobarbituric acid-reacting substances. The authors concluded
that the rat might be an unsuitable model for an exercise stress-
susceptible species.
7.3. Ratio Between Toxic and Sufficient Exposures
The minimum level of dietary selenium that causes overt signs
of chronic selenium toxicity in most species of animals is of the
order of 4 - 5 mg/kg (US NAS/NRC, 1976). The minimum level of
dietary selenium needed to prevent selenium deficiency diseases in
most species is in the range of 0.02 - 0.05 mg/kg (US NAS/NRC,
1971). Therefore, the ratio of toxic to deficient selenium
exposures is about 100. This difference between the harmful and
beneficial levels of selenium is similar to that between the
harmful and beneficial levels of several nutritionally essential
minerals. However, in the case of selenium, this ratio can be
decreased by certain nutritional or environmental factors. For
example, a deficiency in vitamin E decreases the tolerance of
animals to selenium toxicity (section 7.1.6.2) but increases the
nutritional need for the element (section 7.2.7.2). Also,
inorganic mercury increases the toxicity of methylated selenium
metabolites (section 7.4.1) but methylmercury potentiates selenium
deficiency (section 7.2.7.3). Thus, caution may have to be
exercised in some situations to avoid a diminution in the ratio
between harmful and beneficial intakes of selenium.
7.4. Protection Against Heavy Metal Toxicity
7.4.1. Mercury
Parizek & Ostadalova (1967) first showed that selenium could
protect against the toxicity of mercury in short-term acute
toxicity studies. This led to the suggestion that one of the
nutritional roles of selenium might be protection of the organism
against traces of toxic metals that enter the body, even under
normal conditions (Parizek et al., 1971). Nutritional levels of
dietary selenium have been demonstrated to decrease the chronic
toxicity of the highly hazardous methylmercury (Ganther et al.,
1972) and the selenium in tuna may provide a built-in protective
mechanism against the methylmercury in such fish (Ganther & Sunde,
1974). The possible practical significance of the metabolic
interaction of selenium and mercury has been indicated by the
strong correlations observed between the concentrations of mercury
and selenium in the livers of marine mammals under normal
environmental conditions (Koeman et al., 1975) and in human tissues
following exposure to inorganic mercury (Kosta et al., 1975). The
mechanism by which selenium protects against the toxicity of
methylmercury is not known, but the fact that vitamin E and certain
antioxidants also decrease methylmercury toxicity (Welsh & Soares,
1976; Welsh, 1979) has prompted the hypothesis that these compounds
may diminish methylmercury toxicity by counteracting the damaging
effects of the free radicals generated by its breakdown (Ganther,
1978).
Although inorganic selenium salts protect against the toxicity
of inorganic or methylmercury under a wide variety of conditions,
dimethylselenide has a strong synergistic toxic action with
mercuric chloride (Parizek et al., 1971). The mechanism of this
synergism is unknown.
7.4.2. Cadmium
Parenterally administered selenium protects against the
toxicity of injected doses of cadmium in rats, apparently by
diverting the cadmium from low relative molecular mass target
proteins to other proteins of higher relative molecular mass
(Parizek et al., 1971; Chen et al., 1975). However, selenium does
not cause this diversion when given orally, so any mechanism by
which selenium counteracts cadmium toxicity, under environmental
conditions, is yet to be determined (Whanger, 1976). The
cadmium/selenium antagonism may be important for human health in
that selenium prevents the hypertension caused by long-term cadmium
exposure in rats (Perry et al., 1976).
7.4.3. Other heavy metals
Selenium may interact with lead but the interaction appears to
be much weaker than that with mercury or cadmium, and vitamin E
seems to be more important than selenium in determining the effects
of lead poisoning (Levander, 1979). The interaction of selenium
and silver is of theoretical interest since glutathione peroxidase
activity is depressed by silver (Wagner et al., 1975), but this
interaction does not appear to have any practical significance.
Large doses of selenate protect against thallium toxicity in rats
(Rusiecki & Brzezinski, 1966), but this interaction has not been
investigated in any detail.
7.5. Cytotoxicity, Mutagenicity, and Anti-mutagenicity
7.5.1. Cytotoxicity and mutagenicity
Walker & Ting (1967) treated the soil around barley seedlings
with solutions of sodium selenate to determine the effect on
crossing over. Hoagland's solution containing 0, 0.5, 1.6, or
5.0 mg selenium/litre was applied 10 times, at 2-day intervals, to
each of 4 groups of plants, each treatment consisting of the
application of a 250 ml aliquot of the appropriate solution per
pot. On the day after treatment, the pots were irrigated with
distilled water to prevent lethal accumulation of selenate.
Genetic data indicated a significant effect of the selenate on the
rate of crossing over, consisting of a progressive decline with
increasing concentration.
Walker & Bradley (1969) reared larvae of Drosophila
melanogaster on a semi-defined culture medium containing
selenocystine at concentrations of 0, 2, 10, and 50 µM. The
selenocystine had different effects on crossing over on various
chromosomal segments and the authors concluded that these effects
were mediated through a structural chromosomal protein with a role
in the exchange process.
In studies by Nakamuro et al. (1976), various inorganic
compounds of selenium exhibited clastogenic effects when added in
vitro to cultures of human leukocytes (Table 39). Quadrivalent
selenium compounds were generally more efficient than hexavalent
compounds in inducing chromosome aberrations. Similarly, selenite
was more potent than selenate in causing DNA damage to Bacillus
subtilis that was subject to recombination repair. The same
authors also found that 1.6 x 10-3 mol H2Se03 or Se02 caused about
a 30% inactivation of the tryptophan marker of B. subtilis
transforming DNA, whereas similar concentrations of Na2Se04,
H2Se04, or Na2Se03 were without any significant effect.
Table 39. Chromosome aberrations in leukocytes exposed to selenium compoundsa
-------------------------------------------------------------------------------------
Compound Dose Occurrence of particular chromosome aberrationsb Total
chromatid chromatid iso-chromatid chromatid aberrations
gaps breaks breaks exchanges
-------------------------------------------------------------------------------------
(x 10-5 mol) aberrations per 100 cells
Na2Se04 53 8.4 0.9 0.9 0.9 11.2
26 9.3 0.8 0.8 0.8 11.9
13 11.2 0 0 0 11.2
H2Se04 53 21.0 14.5 3.2 1.6 40.3
26 11.9 0 0 0 11.9
13 9.2 0.8 0 0 10.0
Na2Se03 26 54.0 14.5 1.6 1.6 71.8
13 41.7 8.3 0.9 0.9 51.9
Table 39 (contd.)
-------------------------------------------------------------------------------------
Compound Dose Occurrence of particular chromosome aberrationsb Total
chromatid chromatid iso-chromatid chromatid aberrations
gaps breaks breaks exchanges
-------------------------------------------------------------------------------------
(x 10-5 mol) aberrations per 100 cells
H2Se03 26 113.2 41.2 22.1 5.8 182.4
13 92.3 42.3 12.8 2.6 150.0
6.5 15.2 6.3 5.4 0 26.8
Se02 26 52.1 22.5 7.0 1.4 83.1
13 24.2 2.5 3.3 0 30.0
6.5 15.5 3.6 4.8 0 20.9
None - 7.6 0 0 0 7.6
-------------------------------------------------------------------------------------
a Adapted from: Nakamuro et al. (1976).
b Number of cells examined varied from 62 - 250.
Lo et al. (1978) showed that selenite caused chromosome
aberrations and mitotic inhibition in cultured human fibroblasts
and that incubation with a mouse liver S9 microsomal fraction
increased this capacity of selenite about 5-fold at equimolar
concentrations (Table 40). Selenite also induced DNA fragmentation
and repair synthesis and decreased the clone-forming capacity of
the fibroblasts. Incubation with the S9 fraction increased the
last 2 effects of selenite but slightly decreased the extent of DNA
fragmentation. The response to selenite was similar in cultured
fibroblasts from normal persons and from DNA repair-deficient
xeroderma pigmentosum patients. The ability of selenate to trigger
DNA repair, inhibit the mitotic rate, or cause a lethal effect was
about the same as that of selenite over concentrations varying from
7 x 10-6 to 2 x 10-3 mol. However, when both compounds were
incubated with the S9 fraction, only the activity of the selenite
was potentiated (Table 41).
Table 40. Effect of 1.5 h selenite treatement on chromosome
aberrations and mitotic rate of human fibroblastsa
-------------------------------------------------------------
Sodium Metaphase plates with Mitotic rate
selenite chromosome aberrations without S-9 with S-9
for 1.5 h without S-9 with S-9 (%) (%)
(mol) (%) (%)
-------------------------------------------------------------
Control 1.2 0.8 9.0 8.9
2 x 10-5 1.3 6.9 8.6 9.8
4 x 10-5 1.6 7.4 8.9 9.6
8 x 10-5 3.0 14.3 9.3 9.4
1 x 10-4 3.2 - 8.2 0.1
2 x 10-4 4.7 - 9.5 0.0
4 x 10-4 6.1 - 6.4 0.0
8 x 10-4 14.5 - 6.9 0.0
1 x 10-3 14.2 - 7.6 0.0
3 x 10-3 18.1 - 0.7 0.0
-------------------------------------------------------------
a Adapted from: Lo et al. (1978).
Another example of a tissue fraction influencing the
cytotoxicity of selenite is provided by the work of Ray &
associates (Ray & Altenburg, 1978; Ray et al., 1978). These
workers first showed that sodium selenite tripled the frequency of
sister-chromatid exchanges (SCEs) in lymphocytes cultured with
whole human blood (Table 42). At the concentrations of selenite
used, the selenite could only be present during the final 19 h of
the 96-h culture incubation, otherwise cell death occurred, as
measured by mitotic indices. Later, they found that 7.90 x 10-6
mol Na2Se03 increased SCE frequency, only when the lymphocytes were
cultured in the presence of whole blood or separated red cells.
Lysis of the erythrocytes indicated that the lysate rather than the
red cell ghosts contained the activity necessary for selenite to
raise the lymphocyte SCE frequency. The authors suggested that
exposure of selenite to the lysate possibly resulted in a chemical
modification of the selenite that enabled it to induce SCEs. This
chemical modification of the selenite probably involves reduction
to glutathione selenopersulfide derivatives, since Ray (1984) found
that reduced glutathione (10-3 - 10-4 mol) could substitute for
erythrocytes in activating sodium selenite to its SCE-inducing
form. Whiting et al. (1980) showed that glutathione markedly
stimulated unscheduled DNA synthesis in cultured human skin
fibroblasts and chromosome aberrations in Chinese hamster ovary
cells caused by a variety of inorganic selenium compounds. These
research workers suggested that reduced selenium compounds are the
ultimate mutagens and that their formation depends on reduction by
glutathione.
Table 41. Effect of selenite versus selenate on human
fibroblasts incubated with, or without, mouse liver S9
microsomal fractiona
--------------------------------------------------------------
Treatment DNA repair Mitotic rate Clone forming
(grains/nucleus) (%) capacity (%)
--------------------------------------------------------------
Seleniteb 10.7 5.2 36
Selenite + S9 115.6 0.0 0
Selenate 7.0 4.8 42
Selenate + S9 9.1 3.9 32
--------------------------------------------------------------
a Adapted from: Lo et al. (1978).
b The concentrations used for the estimation of DNA repair,
mitotic rate, and surviving colonies were 8 x 10-4, 2 x 10-7,
and 2 x 10-4 mol, respectively. Selenite and selenate were
applied at equimolar concentrations for 1.5 h.
Table 42. Effect of sodium selenite on
sister-chromatid exchange frequency and
mitotic index in lymphocytes cultured
with whole human blooda
----------------------------------------
Na2Se03 Sister chromatid Average
added exchanges per mitotic
(mol) cellb index
----------------------------------------
Control 6.74 0.03
1.58 x 10-6 7.17 0.03
3.95 x 10-6 7.49 0.03
7.90 x 10-6 20.71 0.02
1.19 x 10-5 21.51 0.03
----------------------------------------
a Adapted from: Ray et al. (1978).
b Average of 4 studies; a total of 100
cells was scored for each Na2Se03
concentration except 75 at the highest
concentration.
In a preliminary note, Lofroth & Ames (1978) stated that
selenite did not give any indication of mutagenicity (<< 0.01
revertants/n mol) whereas selenate gave rise to base-pair
substitutions with about 0.03 revertants/n mol in a Salmonella
plate incorporation test using different histidine-requiring
strains that are reverted to prototrophy by different mechanisms.
On the other hand, Noda et al. (1979) found that both selenite and
selenate were weak mutagens in a similar test, since they gave
rise to base-pair substitution (0.2 and 0.05 revertants/n mol,
respectively). Ray & Altenburg (1980) showed that sodium selenide
and sodium selenite were more active than sodium selenate in their
ability to induce SCEs in human whole-blood cultures. Sirianni &
Huang (1983) found that sodium selenite was the most potent inducer
of SCEs in Chinese hamster V79 cells when S9 mixture was present,
whereas sodium selenide was the most effective inducer in the
absence of S9. For sodium selenate, there was no increase in SCE
rate compared with controls, regardless of whether S9 was present
or absent. At present, it is difficult to provide a biochemical
explanation for the contrasting cytogenic effects exhibited by the
various selenium compounds, when studied in different in vitro
test systems.
Norppa et al. (1980) investigated the chromosomal effects
of sodium selenite when given in vivo. They found that
supplementation of human neuronal ceroid lipofuscinosis patients
with tablets furnishing sodium selenite at a dose of 0.025 mg
selenium/kg body weight for 1 - 13.5 months did not have any
detectable effects on chromosomal aberrations or SCEs in peripheral
blood lymphocytes. Similarly, mice treated with a single dose of
sodium selenite at 0.8 mg selenium/kg body weight did not show any
rise in chromosomal abnormalities in bone-marrow cells or primary
spermatocytes after 24 h. However, there was an increased number
of SCEs and chromosomal aberrations in the bone-marrow cells of
Chinese hamsters, 17 - 19 h after injection with sodium selenite
at a dose of 0.3 - 6.0 mg selenium/kg body weight. It was thought
that the manifestations of chromosomal damage observed in the
second study may have been related to the general toxicity of
selenium at the high doses used.
7.5.2. Anti-mutagenicity
Shamberger et al. (1973a) tested the ability of sodium selenite
and various antioxidants to decrease the chromosomal breakage
induced by 7,12-dimethylbenz[alpha]anthracene (DMBA) in human blood
leukocyte cultures. They found that 0.20 µmol sodium selenite
reduced the breaks caused by 1.6 µmol DMBA by 42% (Table 43). This
concentration of selenite was used because 1 µmol almost completely
inhibited the growth of the cultures. In later studies, Shamberger
et al. (1979) found that sodium selenite at concentrations of
0.67 µmol or less was effective in reducing the mutagenic effects
of malonaldehyde and beta-propiolactone in certain tester strains
of Salmonella typhimurium. Rosin & Stich (1979) showed that sodium
selenite at concentrations of 3 x 10-4 and 1 x 10-3 mol caused
a 50% inhibition of mutagenesis in S. typhimurium induced by N-
methyl- N'-nitro- N-nitrosoguanidine or N-acetoxy-2-acetylamino-
fluorene, respectively. However, 10-2 mol sodium selenite was
toxic to the bacteria in the presence of either carcinogen. Sodium
selenite has also been shown to suppress spontaneous mutagenesis in
yeast cultures (Rosin, 1981).
Table 43. Effects of sodium selenite and various antioxidants on
chromosomal breakage induced by 7,12-dimethylbenz-(alpha)anthracene
(DMBA) in human blood leukocyte culturesa
-----------------------------------------------------------------------
DMBA Antioxidant Number Cells with Breaks Reduction
added added of breaks minus in
(µmol) cells number % control breaks
(%) (%)
-----------------------------------------------------------------------
0 none 211 23 10.9 - -
1.6 none 290 82 28.3 17.4 -
1.6 0.20 µmol Na2Se03 171 37 21.6 10.1 42.0
1.6 10 µmol dl-alpha 156 28 17.9 6.4 63.2
tocopherol
1.6 10 µmol ascorbic acid 127 30 23.6 11.9 31.7
1.6 0.21 µmol butylated 157 29 18.4 6.3 63.8
hydroxytoluene
-----------------------------------------------------------------------
a Adapted from: Shamberger et al. (1973a).
Jacobs et al. (1977b) examined the effects of sodium selenite
on the mutagenicity of 2-acetylaminofluorene (AAF), N-hydroxy-2-
acetyl-aminofluorene (N-OH-AAF), and N-hydroxy-aminofluorene
(N-OH-AF) in the S. typhimurium TA 1538 bacterial tester strain.
Metabolism of AAF and N-OH-AAF to the active mutagen, N-OH-AF was
accomplished by rat liver extracts. Sodium selenite at
concentrations ranging from 0.1 to 40 mmol decreased the
mutagenicity of these compounds (Table 44). However, in the case
of AAF, the authors noted that further decreases in mutagenicity
induced by concentrations of selenium higher than 40 mmol were not
tested, partly because of the formation of a red selenium compound
of low solubility, which can be presumed to have been elemental
selenium.
Ray et al. (1978) determined the frequencies of sister
chromatid exchange (SCE) in lymphocytes resulting from the
simultaneous exposure of whole-blood cultures to different
concentrations of sodium selenite plus 10-4 mol methyl
methanesulfonate (MMS) or 2.7 x 10-5 mol N-hydroxy-2-acetyl-
aminofluorene (N-OH-AAF). The SCE frequency observed as a result
of coexposure to 2 compounds was less than that expected because of
an additive SCE frequency response to each individual compound
(Table 45). In their interpretive analysis of the data, the
authors concluded that the most consistent explanation for the
results was that sodium selenite decreased the SCE-inducing
abilities of MMS and N-OH-AAF.
Table 44. Effect of sodium selenite on the mutagenicity of
2-acetylaminofluorene and its derviatives to Salmonella
typhimuriuma
-------------------------------------------------------------------
Compound Selenium His+ revertants Mutagenic activity
added per plate (± SD) (% of appropriate
(mmol) control)
-------------------------------------------------------------------
4.5 mmol AAF - 1768 ± 149 100
4 1411 ± 41 80
10 1336 ± 69 76
40 1148 ± 71 65
0.45 mmol N-OH-AAF - 2353 ± 12 100
0.4 1891 ± 210 80
4 1598 ± 71 68
10 1247 ± 53 53
40 655 ± 43 28
0.065 mmol N-OH-AF - 1628 ± 41 100
0.1 1280 ± 88 79
10 1233 ± 31 76
20 999 ± 26 61
-------------------------------------------------------------------
a Adapted from: Jacobs et al. (1977b).
Table 45. Effect of methyl methanesulfonate (MMS) or N-hydroxy-2-
acetylaminofluorene (N-OH-AAF), with or without different concentrations of
sodium selenite, on sister chromatid exchange (SCE) frequencies in whole-
blood cultures of lymphocytesa
----------------------------------------------------------------------------
Compound Na2Se03 Total Observed Expected Observed/
(mol) cells SCE/cell SCE/cellb expected
scored (%)
----------------------------------------------------------------------------
None none 100 6.74 ± 0.30 - -
10-4 mol MMS none 100 30.17 ± 0.75 - -
1.58 x 10-6 75 30.60 ± 0.74 30.60 (0.43) 100
3.95 x 10-6 75 30.13 ± 0.96 30.92 (0.75) 97
7.90 x 10-6 75 33.15 ± 1.15 44.14 (13.97) 75
1.19 x 10-5 75 31.60 ± 1.17 44.94 (14.77) 70
None none 125 7.65 ± 0.27 - -
2.7 x 10-5 mol none 125 13.61 ± 0.43 - -
N-OH-AAF 1.58 x 10-6 100 13.79 ± 0.42 13.61 (0.00) 100
7.90 x 10-6 65 22.66 ± 1.10 27.15 (13.54) 83
1.19 x 10-5 25 26.16 ± 1.86 29.24 (15.63) 89
----------------------------------------------------------------------------
a Adapted from: Ray et a1. (1978).
b The expected SCE/cell was determined by adding the observed separate
contributions to SCE frequency due to either MMS or N-OH-AAF to that due
to different concentrations of Na2Se03, as determined in a previous study
(shown in parentheses (see also Table 42)).
Martin et al. (1981) found that sodium selenite could
protect against the mutagenic effects of acridine orange and
7,12-dimethylbenz[alpha]anthracene (DMBA) in the Ames Salmonella/
microsome mutagenicity test. However, Chatterjee & Banerjee (1982)
showed that the influence of sodium selenite on the transformation
of mouse mammary cells, induced by DMBA added in organ culture, was
markedly affected by the level of selenium used. For example,
sodium selenite at concentrations of 10-7 - 10-8 mol increased the
transformation frequency of the cells within the glands. At 10-5
mol, sodium selenite caused an 18 and 84% inhibition of the
frequency of transformed cells at the initiation and promotional
stages, respectively. At 10-4 mol, sodium selenite was toxic to
the cells in vitro. Sodium selenite inhibited the metabolism and
mutagenicity of benzo (a)pyrene to S. typhimurium strain TA 100
in rat (Teel & Kain, 1984) and hamster (Teel, 1984) liver S9
activation systems, but had no inhibitory effect on the
mutagenicity of 1,2-dimethylhydrazine or azoxymethane for S.
typhimurium G46 in the host-mediated assay (Moriya et al., 1979).
On the other hand, sodium selenite at 3.95 x 10-9 - 1.98 x 10-8 mol
protected against chromosomal damage in cultured human lymphocytes
caused by 2.3 x 10-6 mol sodium arsenite (Sweins, 1983). However,
this author reported that the cytotoxic concentration of sodium
selenite in his system was very low (somewhere between 1.98 and
3.95 x 10-8 mol). No explanation was offered for the considerable
variations between laboratories with respect to selenium
cytotoxicity.
Several investigators have now examined the anti-mutagenic
effects of dietary selenium in a variety of experimental systems.
For example, Gairola & Chow (1982) fed rats either a low-selenium
diet based on Torula yeast or the same diet supplemented with
sodium selenite at 2 mg selenium/kg for 20 weeks. Dietary selenium
did not have any effect on the metabolic activation potential of
S9 liver enzymes towards benzo( a)pyrene in the Ames Salmonella/
microsome mutagenicity assay. S9 mixtures from selenium-deficient
rats were more active towards 2-aminoanthracene and less active
towards 2-aminofluorene than mixtures from the selenium-
supplemented rats. The authors concluded that further studies were
needed to elucidate the role and nature of in vitro metabolites
causing mutations in the bacteria. In contrast, Schillaci et al.
(1982) did not find any differences in the mutagenic activation of
7-12-dimethylbenz[alpha]anthracene (DMBA) by liver S9 mixtures in
the Ames test with S. typhimurium strain TA 98, prepared from rats
fed Torula yeast-based diets supplemented with sodium selenite at
0.1, 2.5, or 5.0 mg selenium/kg, from weaning for 3 weeks.
However, if the rats were injected with 20, 50, or 100 mg
Aroclor(R) 1254/kg body weight, 5 days before sacrifice, dietary
selenium at 2.5 or 5.0 mg/kg in the form of sodium selenite
markedly decreased the mutagenic activation of DMBA by liver
microsomes.
Lawson & Birt (1983) measured single-strand breaks (SSB)
produced in pancreatic DNA by injecting 20 mg N-nitrosobis(2-
oxopropyl) amine (BOP)/kg body weight subcutaneously into hamsters
that had been fed a Torula yeast-based diet supplemented with
sodium selenite at 0, 0.1, or 5 mg selenium/kg, for 4 weeks
previously. One hour after injection with BOP, there were 2.26 ±
0.47, 2.83 ± 0.43, and 1.74 ± 0.43 SSB per 108 daltons of DNA (mean
± SEM), respectively, in the 3 dietary groups, and the approximate
half-lives of the SSB were 33, 30, and 8 days, respectively. On
this basis, the authors suggested that high levels of dietary
selenium may stimulate the repair of carcinogen-induced DNA damage.
Olsson et al. (1984) used a novel isolated rat liver/cell
culture system to study the effects of selenium deficiency and
selenium supplementation, within the physiological range, on the
anti-mutagenic effects of the element. Rats were first fed a
Torula yeast-based selenium-deficient diet for 5 - 6 weeks with or
without sodium selenite supplementation at 0.2 mg selenium/litre
drinking-water. The rat livers were then connected to an isolated
liver perfusion system. A glass plate with cultured Chinese
hamster V79 cells was introduced into the perfusion system
immediately before the addition of 5 mmol dimethylnitrosamine (DMN)
and then exposed directly to the circulating perfusate. After 2 h,
this glass plate was exchanged for another with fresh V79 cells,
which were exposed during the subsequent 2 h. This procedure was
adopted to avoid a possible toxic effect that might influence
mutation frequency. As in vitro controls, V79 cells were treated
for 2 h in Krebs-Ringer albumin solution with or without DMN. The
induced mutation frequencies in the 2 successively treated cell
populations were summarized to give the value for each liver. It
was found that the mutagenicity of DMN in Chinese hamster V79
cells, after metabolic activation by the isolated perfused rat
liver, was approximately doubled when livers from selenium-
deficient rats were used compared with livers from rats given the
supplementary selenium. The authors were unable to provide a
precise biochemical explanation for the mechanism by which selenium
deficiency increased the mutagenicity of DMN, but they noted that
microsomal N-oxygenation of N,N-dimethylaniline (DMA) was
decreased in livers from selenium-deficient rats and suggested that
further investigation of the different enzymes involved in DMA- N-
oxygenation appeared warranted. Another application of this liver
perfusion/cell culture system was demonstrated by the work of Beije
et al. (1984) who showed that bile collected from selenium-
deficient livers perfused with a medium containing 5 mmol 1,1-
dimethylhydrazine was much more mutagenic toward Chinese hamster
V79 cells than bile from livers of rats given supplementary
selenium and perfused in a similar manner. The authors suggested
that the increased biliary excretion of reactive mutagenic
metabolites observed in their selenium-deficient rat liver
perfusion system might furnish a potential explanation for some of
the protective effects of selenium reported against chemically-
induced colon cancer in experimental animals.
7.6. Teratogenicity
Franke & Tully (1935) obtained chicken eggs from 2 farms in
South Dakota that had histories of low hatchability. Hatchability
in the 2 test groups was extremely low, being 4% in one and 12% in
another. Examination of the eggs revealed that about 75% of those
that had failed to hatch on the 21st day contained deformed
embryos. Franke et al. (1936) then showed that injection of
selenium salts into the air cell of eggs before incubation resulted
in monsters similar to those occurring naturally on affected farms.
Selenium injected as selenite to give a final concentration in the
egg of 0.6 mg selenium/kg was the most effective in this respect,
higher doses causing embryonic death and lower doses producing
fewer monsters (Table 46). When the toxicity of various selenium
compounds for chick embryos was compared, it was found that
selenate was about twice as toxic as selenite (Palmer et al., 1973)
(Table 47). The toxicity of selenomethionine was about the same as
that of selenate but methylseleninic acid was more than twice as
toxic. The toxicity of trimethylselenonium chloride was relatively
low. The most common embryonic deformities observed in this study
were the underdevelopment of the beak and abnormal development of
the feet and legs, especially a fusing or webbing of the 2 outside
toes. Because of the metabolic relationships between selenium and
arsenic or cadmium, Holmberg & Ferm (1969) investigated the
teratogenic potential of these elements, administered separately or
together to golden hamsters (Table 48). Embryonic malformations
were not observed when pregnant hamsters were injected
intravenously with a barely sub-lethal dose of sodium selenite
alone, and the 6% resorption rate observed was stated to be similar
to the normal rate of resorption in this species. Furthermore,
under these conditions, sodium selenite actually gave partial
protection against the teratogenesis induced by sodium arsenate or
cadmium sulfate.
Table 46. Teratogenicity of sodium selenite
injected into hens' eggsa
--------------------------------------------------
Sodium selenite Total Dead Abnormal Normal
(mg selenium/kg) embryos (%) (%) (%)
--------------------------------------------------
0.9 5 60.0 20.0 20.0
0.8 16 12.5 31.0 56.5
0.7 4 50.0 25.0 25.0
0.6 4 50.0 50.0 0
0.5 12 50.0 8.3 41.7
0.1 131 24.4 2.3 73.3
0.02 78 53.8 3.8 42.3
0.01 64 34.3 9.4 56.3
--------------------------------------------------
a Adapted from: Franke et al. (1936).
Table 47. Comparative toxicity of various selenium compounds for chick
embryosa
----------------------------------------------------------------------------
Compound Dose Livability ratio LD50 with 95%
(mg selenium/kg) (live chicks/ confidence limits
fertile eggs) (mg selenium/kg)
----------------------------------------------------------------------------
sodium selenite 0.0 16/18
0.05 18/18
0.1 16/17 0.3b
0.2 17/18
0.4 2/18
0.8 0/18
sodium selenate 0.0 18/18
0.1 12/18
0.2 4/18 0.13
0.4 1/18 (0.086 - 0.17)
0.8 0/18
1.6 0/18
selenomethionine 0.0 17/18
0.05 18/18
0.1 13/18 0.13
0.15 5/18 (0.095-0.15)
0.2 4/18
0.4 0/18
Table 47 (contd.)
----------------------------------------------------------------------------
Compound Dose Livability ratio LD50 with 95%
(mg selenium/kg) (live chicks/ confidence limits
fertile eggs) (mg selenium/kg)
----------------------------------------------------------------------------
methylseleninic 0.0 23/24
acid 0.025 17/18
0.05 9/18 0.052
0.1 2/24 (0.039-0.065)
0.2 0/24
0.4 0/24
trimethylselenonium 0.0 18/18
chloride 6.0 17/18
12.0 13/18 15.7
18.0 5/17 (12.7 - 19.0)
24.0 5/18
30.0 3/18
----------------------------------------------------------------------------
a Adapted from: Palmer et al. (1973).
b An estimate since the data did not allow calculation by the computer
programme.
7.7. Carcinogenicity and Anti-Carcinogenicity
7.7.1. Selenium as a possible carcinogen
Five investigations have been reported in the literature on the
alleged carcinogenicity of selenium for laboratory animals. Nelson
et al. (1943) fed groups of 18 female rats of an inbred Osborne-
Mendel strain a 12% protein diet consisting of 49% corn, 44% wheat,
3% yeast, and 1% each of cod liver oil, calcium carbonate, sodium
chloride and dried whole liver. The diet was supplemented with 0,
5, 7, or 10 mg selenium/kg, as seleniferous corn or wheat, or 10 mg
selenium/kg, as a mixed inorganic selenide containing ammonium
potassium selenide and ammonium potassium sulfide. Although
cirrhosis was frequently observed after 3 months of selenium
exposure, no tumours or advanced adenomatoid hyperplasia were seen
in any of the 73 selenium-exposed rats that died or were sacrificed
before 18 months. Of the 53 selenium-exposed rats that survived
18 - 24 months, 43 had cirrhosis and 11 developed liver cell
adenoma or low-grade carcinoma without metastasis in cirrhotic
livers. There was no relationship between the extent of cirrhosis
and tumour incidence except that there were no tumours in any of
the 18- to 24-month-old rats that did not have cirrhosis. The
incidence of spontaneous adenoma and low-grade carcinoma of the
liver in the unexposed rats was low and the incidence of
spontaneous hepatic tumours in the rat colony was less than 1% in
18- to 24-month-old rats.
Table 48. Effect of selenium, arsenic, and cadmium on embryonic death and
malformations in the golden hamstera
------------------------------------------------------------------------------------
Treatment Dose Total Number of Number of Malformed Malformed
(mg/kg) number of embryos embryos (%) or resorbed
embryos resorbed malformed (%)
------------------------------------------------------------------------------------
sodium selenite 2 86 5 0 0 6
sodium arsenate 20 177 62 86 49 84
sodium arsenate 20 144 28 28 19 39
+ sodium selenite 2
cadmium sulfate 2 115 24 59 51 72
cadmium sulfate 2 82 2 3 4 6
+ sodium selenite 2
------------------------------------------------------------------------------------
a Adapted from: Holmberg & Ferm (1969).
The results of some of the initial studies of Volgarev &
Tscherkes (1967) seemed to indicate an increased incidence of
tumours in rats fed a low-protein diet supplemented with sodium
selenate at 4.3 mg selenium/kg, but subsequent tests carried out by
these authors failed to confirm these results. Moreover, their
work suffered from the fact that no control groups, consuming diets
without additional selenium, were included in any of their trials.
Schroeder & Mitchener (1971b) reported an increased incidence of
tumours in rats given sodium selenate at 2 mg selenium/litre in the
drinking-water for the first year of life followed by 3 mg/litre
until death. In a previous evaluation of this work (US NAS/NRC,
1976), it was observed that the selenate-treated rats survived
longer than the untreated rats and this could have contributed to
the increased tumour incidence observed in the rats receiving
selenate. Also, as the organs and tissues did not appear to have
been systematically searched, the type and incidence of
histological lesions could not be known with certainty.
Schroeder & Mitchener (1972) carried out 2 studies in which
mice were given selenium in the drinking-water. In both studies,
the mice were fed a diet composed of 60% whole rye flour, 30% dried
skim milk, 9% corn oil, and 1% iodized sodium chloride, to which
were added vitamins and iron. The diet was calculated to contain
24% protein, 65% carbohydrate, and 11% fat (dry weight). The mice
were given doubly deionized water for drinking, which originally
came from a forest spring. Certain essential trace metals were
added to the drinking-water, as soluble salts, in the following
concentrations (mg/litre): zinc, 50; manganese, 10; copper, 5;
cobalt, 1; and molybdenum 1. All mice received these trace
elements in their drinking-water. In addition, chromium was added
to the drinking-water at a level of 1 or 5 mg/litre in the first
and second studies, respectively. In the first study, groups of
100 or more Swiss mice of the Charles River CD strain, containing
equal numbers of each sex, were given, at weaning, sodium selenite
at either 0 or 3 mg selenium/litre of the trace element-fortified
drinking-water. This regimen continued over the entire life span
of the mice. The second study was identical to the first, apart
from the level of chromium given in the drinking-water, and the
fact that the selenium was administered in the form of sodium
selenate. Of the 180 control mice autopsied from both studies, 119
were sectioned. Tumours were found in 23 (19%) of those sectioned
and 10 of the tumours (43%) were malignant. The different forms of
selenium given did not influence the incidence of tumours and, of
the 176 selenium-exposed mice autopsied in both studies, 88 were
sectioned. Tumours were found in 13 (15%) of those sectioned but
all tumours were malignant. It was concluded that selenium had
little tumourigenic or carcinogenic activity in mice, though, when
tumours did appear in the selenium-exposed mice, they were all
malignant.
The results of studies of Harr et al. (1967) (section 7.1.2.2)
failed to demonstrate any tumours attributable to selenium, but
most of the rats fed semi-purified diets containing levels of
selenium greater than 2 mg/kg died within 100 days and almost all
were dead before 2 years. Exceptions included 1 rat fed selenate
at 4 mg selenium/kg diet containing 12% casein and 0.3% DL-
methionine, and 27 rats alternating at weekly intervals between a
control ration and a diet containing sodium selenate at either 4 or
8 mg selenium/kg. However, no hepatic tumours were observed, even
in the 71 rats that survived 2 years or longer at continuous
dietary-selenium levels of 0.5 - 2.0 mg/kg.
The above studies indicate that test animals develop neoplastic
lesions, only when they have liver cirrhosis produced by frank
selenium toxicity (i.e., no hepatomas were observed in the absence
of severe hepatotoxic phenomena). For this reason, it was
concluded that selenium was not, by reason of its capacity to
induce liver damage when consumed at high levels, properly
classified as carcinogenic because of its potential association
with a higher rate of liver cancer (Gardner, 1973).
Jacobs & Forst (1981b) did not observe any signs of neoplasia
in groups of 35 female Swiss mice fed a commercial pelleted diet
and given 0, 1, 4, or 8 mg selenium/litre drinking-water, as sodium
selenite, for 50 weeks.
Three studies have shown carcinogenic effects that appear to be
more a specific effect of a particular selenium compound rather
than an effect of selenium itself. Seifter et al. (1946) found
that 8 white rats that had received 0.05% bis-4-acetamino-phenyl-
selenium dihydroxide in their diet for 105 days had multiple
adenomas of the the thyroid glands and adenomatous hyperplasia of
the liver. Innes et al. (1969) fed the maximal tolerated dose of
selenium diethyldithiocarbamate (Ethyl selenac) to a group of 72
mice containing both sexes of two hybrid strains (C57BL/6 x
C3H/Anf)F and (C57BL/g x AKR)F1. The mice were given this
substance by stomach tube at a dose of 10 mg/kg body weight,
starting at 7 days of age. At 4 weeks of age, the chemical was
mixed directly into the diet at a concentration of 26 mg/kg. After
82 weeks, all the surviving mice were sacrificed; of 69 necropsied,
26, 13, and 5.8% had hepatomas, lymphomas, and pulmonary tumours,
respectively. Among 338 negative control mice, most of which were
sacrificed between 78 and 89 weeks, the incidences of the
corresponding tumours were 4.1, 4.1, and 6.2%, respectively.
A report from the US National Cancer Institute (National Cancer
Institute, 1980) suggested that commercial selenium sulfide, an
ingredient in certain anti-dandruff shampoos, was carcinogenic for
rats and mice. Elemental analysis of the test chemical used in
this study indicated that the material was a mixture of selenium
monosulfide and selenium disulfide. The melting point of the test
sample was closer to that reported for the monosulfide than that
reported for the disulfide and the X-ray diffraction pattern was
consistent with patterns reported for the monosulfide. It was
concluded that the selenium in the test substance used in this
bioassay was present primarily as the monosulfide.
In the rat study, groups of 4-week-old male and female Fischer
F344 rats were fed presterilized lab meal and were given untreated
well water ad libitum for 104 - 105 weeks. During the first 103
weeks, 50 rats of each sex were given one of 4 treatments:
untreated control; vehicle control (received volumes of 0.5%
aqueous carboxymethylcellulose equal to those of the test solutions
administered); low-dose group (3 mg selenium sulfide suspended in
0.5% aqueous carboxymethylcellulose/kg body weight, 5 days per
week, given by gavage); or high-dose group (15 mg selenium
sulfide/kg body weight administered by the same route and
schedule). The results showed an increased incidence of primary
liver tumours in both male and female high-dose groups (Table 49).
Neoplastic nodules were usually single, rather well-defined
areas characterized by altered hepatocytes. In most instances, the
hepatocytes were larger than normal, eosinophilic, and occasionally
vacuolated. The normal architecture was altered, mainly resulting
in a solid mass of hepatocytes or a trabecular pattern rather than
the normal hepatic cords. The mass compressed the adjacent
parenchyma around the periphery. Anaplasia and mitoses were
minimal. Hepatocellular carcinomas were usually large multinodular
masses, often encompassing entire liver lobes or even multiple
lobes. The histological appearance of these neoplasms varied from
areas appearing normal to much more anaplastic areas. The
neoplastic hepatocytes varied from small basophilic cells to very
large eosinophilic and occasionally vacuolated cells. Mitoses were
variable. No distant metastases were observed in any of the rats
bearing hepatocellular carcinomas. No other neoplasms were found
that could be related to the administration of the selenium
sulfide.
Table 49. Effect of selenium sulfide, given orally for 103 weeks, on
the incidence of liver tumours in Fischer F344 ratsa
------------------------------------------------------------------------
Initial Selenium Tumour incidence
Test group Sex number sulfide Hepatocellular Neoplastic
of rats dose carcinoma nodules
------------------------------------------------------------------------
(mg/kg)
Untreated control M 50 0 1/48 (2%) 3/48 (6%)
Vehicle controlb M 50 0 0/50 (0%) 1/50 (2%)
Low-dosec M 50 3 0/50 (0%) 0/50 (0%)
High-dosec M 50 15 14/49 (29%) 15/49 (31%)
Untreated control F 50 0 0/50 (0%) 0/50 (0%)
Vehicle controlb F 50 0 0/50 (0%) 1/50 (2%)
Low-dosec F 50 3 0/50 (0%) 0/50 (0%)
High-dosec F 50 15 21/50 (42%) 25/50 (50%)
------------------------------------------------------------------------
a Adapted from: National Cancer Institute (1980).
b Received only vehicle for dosing (0.5% aqueous carboxymethylcellulose)
5 days per week, by gavage.
c Received stated dose of selenium sulfide suspended in 0.5% aqueous
carboxymethylcellulose, 5 days per week, by gavage.
An increased incidence of focal cellular change in the liver
was noted in high-dose male rats but was essentially comparable in
frequency in the remaining treated and control groups of each sex.
A compound-related increase in pigmentation in the lungs was
observed. This was characterized by the accumulation of dark,
slightly granular-appearing pigment in the interstitial areas and
in some peribronchial areas. In most cases, the pigment appeared
to be located within cells, principally macrophages. No evidence
of inflammation, relative to the pigment deposits, was noted. Lung
pigmentation was found in 47/49 (96%) high-dose males, 1/50 (2%)
low-dose males, 45/50 (90%) high-dose females, and 36/50 (72%) low-
dose females, but not in control males or females.
The protocol for the testing of selenium sulfide in B6C3F1 mice
was similar to that used for rats, except that the doses of the
substance under test were increased (Table 50). There was an
increased incidence of primary liver and lung tumours in the high-
dose female mice and a marginal increase in the incidence of these
tumours in high-dose males.
Hepatocellular carcinomas varied from single nodules to
multinodular masses, often encompassing several liver lobes.
Individual hepatocytes varied considerably in morphology from large
eosinophilic cells to small, darkly-staining hepatocytes. In many
cases, there was marked variation in cell type in different parts
of the neoplasm. The number of mitoses varied. The number of
hepatocellular carcinomas metastasizing to the lungs was comparable
in vehicle-control and high-dose males. No metastases were
observed in the lung in female mice. Alveolar/bronchiolar adenomas
were usually small solitary lesions located in the subpleural area
or immediately adjacent to a bronchiole. The cells involved were
cuboidal to tall columnar and tended to be situated perpendicular
to the basement membrane in a single layer. These cells were
arranged in complex papillary projections forming discrete nodules
and compressing adjacent alveolar walls. Mitoses were rare.
Alveolar/bronchiolar carcinomas, however, were less discrete
lesions and tended to be larger and occasionally multiple,
consisting of a confluence of two or more nodules. The individual
cells tended to be less rigidly arranged along basement membranes
and were often piled up in layers or arranged in solid sheets
without a papillary pattern. The cells often showed increased
basophilia and a moderate mitotic index. Evidence of invasion into
adjacent vessels, or extension into bronchioles and adjacent lung
parenchyma was frequently present.
Other neoplasms that occurred in the mice were similar in
number and kind to those that usually occur in aged B6C3F1 control
mice and could not be related to the long-term administration of
selenium sulfide.
In a comparison study, the effect of the dermal application of
selenium sulfide was examined. No increased incidence of neoplasms
was observed that could be attributed to selenium sulfide.
7.7.2. Selenium as a possible anti-carcinogen
High levels of dietary selenium have been shown to protect
laboratory animals against chemical carcinogenesis, under a wide
variety of conditions. Clayton & Baumann (1949) fed 2 groups of 15
young adult rats, weighing approximately 200 g, a basal diet
consisting of extracted casein, 12 parts; salts, 4 parts; corn oil,
5 parts; and glucose monohydrate (Cerelose) to 100 parts. The diet
was supplemented with thiamin, riboflavin, pyridoxine, calcium
pantothenate, and choline. Fat-soluble vitamins were provided by
giving 2 drops of halibut liver oil per rat every 4 weeks. During
the initial 4-week feeding period, both groups received the basal
diet supplemented with 0.064% 3'-methyl-4-dimethylaminoazobenzene.
This was followed by a 4-week "interruption period" during which
neither group received the azo dye, but one group received the
basal diet supplemented with sodium selenite at 5 mg selenium/kg,
and the other group received the basal diet without any added
selenium. During the third, 4-week feeding period, both groups
received the basal diet supplemented with 0.048% of the azo dye,
but no supplementary selenium. During the final 8-week feeding
period, both groups received the basal diet without either azo dye
or selenium. Two of the 9 surviving rats in the group that
received supplementary selenium during the "interruption period"
had liver tumours (22%), compared with 4 out of 10 survivors (40%)
in the group not supplemented with selenium. The results of a
second similar study showed a liver tumour incidence of 4/13 (31%)
in the selenium-supplemented group compared with 8 out of 13 rats
(62%) in the unsupplemented group.
A preliminary study by Shamberger & Rudolph (1966) indicated
that concomitant dermal application of sodium selenide reduced the
tumour-promoting effect of croton oil in mice initiated with 7,12-
dimethylbenz[alpha]anthracene (DMBA). Riley (1968) found that a
similar application of sodium selenide prevented the mast cell
reaction caused by an active fraction of croton oil in the skin of
DMBA-initiated mice. In a later set of studies (Shamberger, 1970),
2 groups of 30 female ICR Swiss mice, 50 - 55 days old, were
treated once on day one with 0.125 mg DMBA dissolved in 0.25 ml
acetone. On days 2 - 21, the shaved backs of the first group of
mice were treated with 0.25 ml of a 20:80 water-acetone mixture
containing 0.0005% sodium selenide, whereas the second group was
not treated with any anti-oxidant. Subsequently, in 3 separate,
but essentially identical, studies, both groups of mice received
0.25 ml of 0.04% croton oil in acetone daily for 16, 18, and 18
weeks respectively. After the croton oil treatment, the incidence
of papillomas in the selenide-treated groups was 17, 63, and 45%,
respectively, in 3 separate studies, compared with an incidence in
the non-treated groups of 43, 63, and 89%, respectively. The
corresponding number of papillomas per mouse in the 3 studies was
1.5, 3.0, and 1.5, respectively, in the selenide-treated groups and
3.2, 6.0, and 2.3 in the non-treated groups. No papillomas were
observed in 3 DMBA-initiated control groups, which did not receive
either antioxidant or croton oil. A similar protective effect of
sodium selenide was observed in 3 additional studies in which the
promoting agents were croton oil, croton resin, and phenol and in
which the selenide was applied concomitantly with the promoter.
In another study, 0.25 ml of 0.01% 3-methylcholanthrene (MCA)
was applied daily to the shaved backs of one group of mice for 19
weeks; a second group received 0.0005% sodium selenide together
with the MCA. After 19 weeks, the incidence of papillomas was 68%
in the selenide-treated group and 87% in the untreated group, and
the number of papillomas per mouse was 3.2 and 2.2, respectively.
After 30 weeks, the number of mice with cancers was 17/28 and 25/30
in the selenide-treated and untreated groups, respectively, the
total number of cancers per group being 29 and 71, respectively.
Table 50. Effect of selenium sulfide given orally for 103 weeks on the incidence of liver and lung
tumours in B6C3F1 micea
----------------------------------------------------------------------------------------------------
Lung tumour incidence
Initial Selenium Liver tumour incidence Alveolar/ Alveolar/
Test group Sex number sulfide hepatocellular hepatocellular bronchiolar bronchiolar
of mice dose carcinoma adenoma carcinoma adenoma
(mg/kg)
----------------------------------------------------------------------------------------------------
Untreated control M 50 0 17/49 (35%) 3/49 (6%) 1/49 (2%) 8/49 (16%)
Vehicle controlb M 50 0 15/50 (30%) 0/50 (0%) 1/50 (2%) 3/50 (6%)
Low-dosec M 50 20 11/50 (22%) 3/50 (6%) 2/50 (4%) 8/50 (16%)
High-dosec M 50 100 23/50 (46%) 0/50 (0%) 2/50 (4%) 12/50 (24%)
Untreated control F 50 0 2/50 (4%) 1/50 (2%) 0/50 (0%) 2/50 (4%)
Vehicle controlb F 50 0 0/49 (0%) 0/49 (0%) 0/49 (0%) 0/49 (0%)
Low-dosec F 50 20 1/50 (2%) 1/50 (2%) 1/50 (2%) 2/50 (4%)
High-dosec F 50 100 22/49 (45%) 6/49 (12%) 4/49 (8%) 8/49 (16%)
----------------------------------------------------------------------------------------------------
a Adapted from: National Cancer Institute (1980).
b Received only vehicle for dosing (0.5% aqueous carboxymethylcellulose), 5 days per week, by gavage.
c Received stated dose of selenium sulfide suspended in 0.5% aqueous carboxymethylcellulose, 5 days
per week, by gavage.
Shamberger (1970) also carried out 2 dietary studies in which 4
groups of 36 female, albino ICR Swiss mice, 50 - 55 days old, were
fed Torula yeast diets supplemented with sodium selenite at 0, 0.1,
or 1.0 mg selenium/kg or sodium selenide at 0.1 mg selenium/kg.
After 2 weeks on the test diets, 0.125 mg DMBA dissolved in acetone
was applied once to the skin. After 3 weeks, 0.25 ml 0.05% croton
oil in acetone was applied daily for 17 weeks. At this time, 26/36
mice fed the unsupplemented diet had papillomas, compared with
14/35 mice fed the diet supplemented with 1.0 mg selenium/kg, as
selenite. The incidence of papillomas was slightly elevated in the
mice fed the diet supplemented with 0.1 mg selenium/kg, as sodium
selenite, compared with the unsupplemented mice. The incidence of
papillomas in mice fed sodium selenide at 0.1 mg selenium/kg was
intermediate between that of the unsupplemented group and the group
fed sodium selenite at 1.0 mg selenium/kg.
In a second study of similar design, 4 groups of 36 mice were
fed the test diets described above for 2 weeks. Then 0.25 ml of
0.03% benzo( a)pyrene in acetone was applied daily to the shaved
backs of the mice for 27 weeks. At this time, the incidence of
papillomas in the groups fed the torula diet supplemented with 0,
0.1, or 1.0 mg selenium/kg, as selenite, or 0.1 mg selenium/kg, as
selenide, was 14/35, 22/36, 8/33, and 12/35, respectively. The
number of papillomas per mouse in the corresponding groups was 8.1,
9.8, 5.0, and 6.8.
Harr et al. (1972) weaned 80 female OSU-Brown rats at 35 days
of age and divided them into 4 groups of 20. Each group was fed a
low-selenium basal diet (0.018 µg selenium/kg) that included Torula
yeast as the protein source and contained 60 mg vitamin E/kg.
Groups, 1, 2, 3, and 4 were fed the basal diet supplemented with
sodium selenite at 2.5, 0.5, 0.1, and 0 mg selenium/kg,
respectively. All diets contained 150 mg 2-acetylaminofluorene/kg.
The 40 rats in groups 1 and 2 were born from parents reared on a
selenium-depleted regimen, but were clinically normal. The 40 rats
in groups 3 and 4 were from the second generation maintained on the
depeletion regimen and had clinical signs of selenium deficiency.
After 200 days of treatment, the number of mammary adenocarcinomas
in groups 1, 2, 3, and 4 was 0, 1, 8, and 9, respectively. After
320 days, the number of mammary adenocarcinomas in the same groups
was 11, 11, 13, and 12, respectively. Mammary adenocarcinomas in
groups 1, 2, and 3 occurred mainly (90%) in the thoracic region and
were well circumscribed, firm, and easily removed, whereas those in
group 4 occurred mainly (80%) in the pelvic area and were soft and
fluid, contained little connective tissue, and were invasive.
After 240 days of treatment, the number of hepatic carcinomas in
groups 1, 2, 3, and 4 was 0, 1, 9, and 4, respectively. After 320
days, the number of hepatic carcinomas in the same groups was 8,
12, 12, and 6, respectively. Toxic hepatitis was observed in 18 of
the 20 livers from group 1 (selenium added at 2.5 mg/kg), but was
not seen in the other groups.
In studies of Marshall et al. (1978), 2 groups of male albino
Sprague Dawley rats were fed diets containing 2-acetylaminofluorene
at 0.3 g/kg, for 14 weeks. One group received 4 mg selenium, as
sodium selenite/litre drinking-water. The carcinogen was withdrawn
from the diet for an additional 4 weeks and then the rats were
sacrificed. The rats given selenium had about 50% fewer liver
tumours than unsupplemented rats. Control rats given a similar
level of selenium in the water showed normal growth response, liver
weight, and appearance.
Three groups of 15 male Sprague Dawley rats, weighing about
250 g, were fed a basal diet of finely ground Purina Laboratory
Chow and water ad libitum (Griffin & Jacobs, 1977). One group did
not receive any supplementary selenium, a second group received 6
mg selenium, as sodium selenite/litre drinking-water, and a third
group received 6 mg selenium, in the form of a high selenium
yeast/kg diet. After one week on this regimen, all 3 groups were
given 0.05% 3'-methyl-4-dimethyl-aminoazobenzene in the diet for 8
weeks. Following this, all groups were maintained on the
carcinogen-free diets for an additional 4 weeks. The selenium
supplements were maintained for the second and third group
throughout the entire study. At sacrifice, the incidence of liver
tumours in the surviving rats was 92% (11/12) in the unsupplemented
group, 46% (7/15) in the group supplemented with selenite in the
drinking-water, and 64% (9/14) in the group supplemented with
selenium yeast in the diet. In this study, the selenium
supplements had little effect on the growth of the rats.
Histopathological examination of several of the tumours revealed
that they were bile duct adenocarcinomas and liver cell
adenocarcinomas.
Jacobs et al. (1977a) injected 2 groups of 15 male, 8-week-old
Sprague Dawley rats, weekly, with 20 mg sym,-dimethylhydrazine
dihydrochloride (DMH)/kg body weight, for 18 weeks. One group of
rats received sodium selenite at 4 mg selenium/ litre drinking-
water, which was available ad libitum one week prior to, and
throughout, administration of the carcinogen. The other group did
not receive selenium added to the drinking-water. The group
receiving DMH and no added selenium had an 87% incidence (13/15) of
colon tumours, whereas the group receiving both DMH and added
selenium had a 40% incidence (6/15) of colon tumours. The total
number of colon tumours was 39 in rats receiving only DMH and 11 in
rats receiving both DMH and added selenium. In a similar study in
which 2 groups of 15 rats were injected weekly with 20 mg of
(methylazoxyl)-methanol acetate (MAM)/kg body weight for 18 weeks,
no significant differences in the incidence of colon tumours were
apparent between groups with (14/15) and without (14/14) added
selenium in the drinking-water. However, the number of MAM induced
tumours was 73 in the group given MAM alone and 42 in the group
receiving both MAM and added selenium.
Schrauzer and colleagues carried out a series of studies in
which exogenous carcinogens were not used, to test the ability of
supplemental selenium in influencing the development of spontaneous
mouse mammary tumours, presumably of viral origin. In study 1
(Schrauzer & Ishmael, 1974), 2 groups of 30 virgin female C3H/St
mice, 4 - 6 weeks old, were fed a basal diet ("Concord Maid")
consisting of meat scraps, dried skimmed milk, oat groats, ground
wheat, wheat germ meal, vegetable oil, cane molasses, salt,
brewer's yeast, cereal binder, sodium propionate, and calcium
pantothenate. The diet contained about 150 g protein, 5 g fat, and
0.15 mg selenium/kg. One group of mice received plain distilled
water for drinking, while the other group received distilled water
fortified with 2 mg selenium, as selenium dioxide/litre. The
strain of mice used in this study ordinarily has a high incidence
of spontaneous mammary adenocarcinomas and, after 16 months of
treatment, the observed incidence in the group not given
supplemental selenium in the water was 82%, whereas the incidence
in the group given selenium was 10%.
In study 2 (Schrauzer et al., 1976), 3 groups of 30, 30, and 50
female C3H/St mice fed the basal diet described above were also
given selenite at 0, 5, or 15 mg selenium/litre drinking-water,
respectively. Toxicity due to the doses of selenium was indicated
by the average body weights in the 3 groups at 12 months (33, 29,
and 25 g, respectively) and by a higher tumour-unrelated mortality
rate in the selenium-exposed mice. After 26 months, the
corresponding incidence of spontaneous mammary tumours in the 3
groups was 82, 36, and 33% in the mice that survived to the age at
which the first tumours appeared in each group. A fourth group of
20 mice, fed a selenium-deficient diet for 14 months and then the
basal diet until death, had a mammary tumour incidence of 69% which
was not judged to be different from the 82% incidence in the
control group (basal diet throughout life span and no added
selenium in the water).
In study 3 (Schrauzer et al., 1978a), 4 groups of 30 female
C3H/St mice were fed a basal diet ("Wayne F-6 Lab Blox") that was
different from that used in the first 2 studies and consisted of
fish meal, animal liver meal, soybean meal, corn and wheat flakes,
ground corn, wheat red dog, wheat middlings, soybean oil, cane
molasses, salt, brewer's yeast, and various vitamins and minerals.
This diet contained 244.8 g protein, 64.8 g fat, and 0.45 mg
selenium/kg. The 4 groups received selenium dioxide at 0, 0.1,
0.5, or 1.0 mg selenium/litre drinking-water and, after 22 months,
the incidence of mammary tumours was 42, 25, 19, and 10%,
respectively. Selenium supplementation at these levels did not
have any noticeable adverse effects on weight-gain or survival of
the mice. The incidence of spontaneous mammary tumours in the
control (unsupplemented) group was lower in this study than that
observed in the 2 previous studies (42 compared with 82%), and this
was attributed to the higher selenium content of the new basal diet
used in the third study (0.45 versus 0.15 mg/kg).
Medina & Shepherd (1980) fed BALB/cfC3H mice Wayne Lab Blox and
gave them selenium dioxide at 0, 2, or 6 mg selenium/litre
drinking-water, ad libitum, starting at 10 weeks of age and
continuing to the end of the study. In 12-month-old mice, 2 and 6
mg selenium/litre decreased mammary tumour incidence from 82% in
the untreated controls to 48 and 12%, respectively. There were no
effects of the selenium treatment on normal reproductive function
or weight gain in these mice. In another study, samples of 4
different preneoplastic outgrowth lines (D2, C3, C4, and CD-7)
were transplanted into the mammary gland-free fat pads of syngenic
mice. When the implants had filled the mammary fat pads with their
respective outgrowths (8 weeks after implantation), the mice were
given selenium dioxide at 4 mg selenium/litre drinking-water, ad
libitum, for the rest of the study. Such treatment with selenium
delayed the rate of tumour formation only in line C4, increasing
the time for half of the outgrowths to produce tumours from 34 to
44 weeks. In a third study, 2 - 6 mg selenium/litre drinking-water
had no effect on the growth rate of primary tumours transplanted
subcutaneously in BALB/c mice. Since selenium did not have any
effect on tumour formation rate in 3 of 4 preneoplastic mammary
outgrowth lines or on the growth rate of established mammary
tumours, the authors suggested that selenium might act by
inhibiting chemical or viral transformation of normal cells or by
inhibiting expression of initially transformed cells.
Because of the metabolic antagonism between arsenic and
selenium (section 7.1.6.3), Schrauzer and co-workers also
investigated the effect of arsenic on the genesis of the
spontaneous mammary tumours in C3H/St female mice described above.
In their first study on arsenic and selenium (which was part of the
same study described above in Schrauzer & Ishmael (1974)), giving
sodium arsenite at 10 mg arsenic/litre drinking-water to a group of
30 C3H/St mice for 16 months, reduced the incidence of spontaneous
mammary tumours to 27% compared with an incidence of 82% in the
untreated controls (Schrauzer & Ishmael, 1974). However, arsenic
treatment markedly stimulated the growth rate of spontaneous or
transplanted mammary tumours. In a second study concerning
arsenic, sodium arsenite at 80 mg arsenic/litre drinking-water,
administered to a group of 20 C3H/St mice, reduced the incidence of
spontaneous mammary tumours to 40% compared with an incidence of
82% in the untreated controls (Schrauzer et al., 1976), but some of
the tumour-inhibiting effect of arsenic appeared to be masked at
this dose level by its toxicity.
In a third study relating arsenic and selenium (Schrauzer et
al., 1978b), 4 groups of 30 female C3H/St mice were fed the Wayne
F-6 Lab Blox diet described above, which contained 0.29 mg
arsenic/kg. The 4 groups received the following supplements in
their deionized drinking-water: none, arsenic trioxide at 2 mg
arsenic/litre, selenium dioxide at 2 mg selenium/litre, or 2 mg of
both arsenic and selenium. The incidence of mammary tumours in the
4 groups was 41, 36, 17, and 62%, respectively, and the percentage
of multiple mammary tumours was 17, 40, 0, and 28. The age of
tumour onset in the corresponding groups was 4.5, 9, 16, and 8
months. Thus, in this case, treatment with arsenic appeared to
diminish the cancer-protecting effect of selenium. Arsenic also
accelerated tumour growth and increased the incidence of multiple
tumours.
Newberne & Conner (1974) fed 4 groups of male rats of the
Charles River CD strain, weighing about 100 g each, a basal diet
consisting of casein, 200 g/kg; sucrose, 209 g/kg; dextrose, 209
g/kg; dextrin, 209 g/kg; stripped lard, 80 g/kg; Wesson Oil, 20
g/kg; selenium-free salts, 50 g/kg; vitamin mix, 20 g/kg; choline,
3 g/kg; and vitamin B12, 0.05 g/kg. The basal diet contained about
0.03 mg selenium/kg. One group received the unsupplemented basal
diet, whereas the other 3 groups received the basal diet
supplemented with sufficient sodium selenite to attain approximate
dietary levels of 0.1, 1.0, or 5.0 mg selenium/kg, respectively.
Each group was fed the diet for 2 - 3 weeks, before oral
administration of 7 mg aflatoxin B1 in dimethyl sulfoxide/kg body
weight. The rats continued on their respective diets for an
additional 2 weeks, and the mortality rate after this time, in the
groups fed the diets containing 0.03, 0.1, 1.0, and 5.0 mg
selenium/kg was 28/29, 20/30, 7/28, and 27/29, respectively.
Histological examination revealed that rats receiving 1.0 mg
selenium/kg diet were partially protected against the aflatoxin B1
and also had less severe liver lesions. However, the groups fed
diets containing 1.0 or 5.0 mg selenium/kg exhibited a novel renal
lesion associated with acute aflatoxin B1 toxicity, characterized
by marked tubular necrosis at the cortical medullary junction.
Some tubules exhibited hyperplastic changes of the epithelium as
well as necrosis.
In a second study, Grant et al. (1977) gave 25 µg aflatoxin B1
orally, 5 days/week for 4 weeks, to 140 male Sprague Dawley rats.
The rats were fed normal or marginally lipotrope-deficient
semisynthetic basal diets containing sodium selenite at 0.03, 0.10,
0.50, 1.0, 2.5, or 5.0 mg selenium/kg. After 17 months, the
surviving rats were sacrificed and histopathological examination
was carried out. A very low incidence (20%) of hepatocarcinomas
was seen in rats receiving 1.0 mg selenium/kg in the normal basal
diet and 0.10 or 0.50 mg/kg in the lipotrope-deficient diet. No
other tumours were observed, and grading of the hepatic lesions
indicated that there was no significant differences among the
dietary selenium groups. Dietary sodium selenite and repeated
doses of aflatoxin B1 interacted to produce large bizarre cells in
the renal tubules, occurring in the same region of the kidney as
the severe necrosis seen previously in rats fed high levels of
dietary selenium and given an acute dose of aflatoxin B1.
Recently, a study of the effects of selenium on aflatoxin B1-
induced enzyme altered foci in rat liver has been reported (Milks
et al., 1985). Male Sprague Dawley rats were fed a selenium-
deficient diet and given sodium selenite at 5, 2, 0.2, or 1 mg
selenium/litre drinking-water for 3 weeks. Each rat then received
2 µg mol aflatoxin B1/kg body weight by stomach tube. For the next
week the selenium status was "normalized". Rats previously
receiving 5, 2, 0.2, or 0 mg selenium/litre received, respectively,
0. 0.2, 2, or 5 mg/litre. Then rat chow was fed and a promoting
regimen consisting of phenobarbital in the drinking-water, and a
partial hepatectomy was instituted. Eight weeks later, necropsies
were carried out and livers were stained histochemically for
gamma-glutamyl transpeptidase activity. Foci of activity were
counted. The number of foci seen in livers from rats given 5, 2,
0.2, or 0 mg selenium/litre during initiation were 0.62 ± 0.22,
1.97 ± 0.46, 3.35 ± 0.66, and 2.46 ± 0.23 per cm2, respectively.
The data suggested that 5 mg selenium/litre can protect against the
hepatocarcinogenic effects of aflatoxin B1 in the rat.
On the basis of anecdotal observations, Wedderburn (1972)
suggested that a decrease in the number of cases of intestinal
carcinoma in autopsied sheep might be associated with the
widespread veterinary use of selenium to prevent deficiency
diseases in sheep. Simpson (1972a) conducted an investigation into
the epidemiology of carcinomas of the small intestine of sheep in
which the viscera of 32 733 ewes were examined. Carcinomas of the
small intestine were diagnosed in 483 animals, but the prevalence
of these neoplasms in ewes regularly dosed with selenium throughout
their lives was not significantly different from the prevalence in
ewes never treated with selenium (Simpson, 1972b). Furthermore, no
significant differences were found in association with differences
in soil types on the farms from which the sheep originated. It was
concluded that soil-selenium levels and administration of selenium
in the form and at the dose rates used did not have any effects on
the development of intestinal carcinomas in sheep. Underwood
(1977) commented that neoplasias were not observed among the
various lesions attributed to selenium deficiency in animals.
Ip has made and summarized a series of observations on selenium
and carcinogenesis (Ip, 1985a).
Evidence of an interaction between dietary fat and selenium
status in the induction of mammary tumours by dimethylbenz-
[alpha]anthracene in rats has been presented by Ip & Sinha (1981).
They fed 8 groups of 23 - 25 female weanling Sprague Dawley rats
one of the following diets either deficient in selenium or
supplemented with sodium selenite at 0.1 mg selenium/kg: 1% corn
oil, 5% corn oil, 25% corn oil, or 1% corn oil plus 24%
hydrogenated coconut oil. In addition to the fat, the diets
consisted of Torula yeast, dextrose, HMW salt mix, vitamin mix,
alphacel, and DL-methionine. The diets were adjusted so that the
intake of all nutrients would be the same, except for dextrose and
fat, assuming that the rats would consume an equal number of
calories. The corn oil used was stripped of tocopherol and the
unsupplemented diet was deficient in selenium (less than 0.02 mg/kg
by fluorometry). Mammary tumours were induced by the intragastric
administration of 5 mg dimethylbenz[alpha]anthracene at 50 days of
age. Increasing the dietary polyunsaturated-fat level (corn oil)
increased the tumour incidence in rats fed the selenium-
supplemented diets (Table 51). However, the high-saturated-fat
diet was much less active in stimulating tumourigenesis. Selenium
deficiency increased the tumour yield only in rats fed the high
polyunsaturated-fat diet (25% corn oil). Increased tumour
incidence due to selenium deficiency was not seen in the rats fed
the low-fat diets containing polyunsaturated fat (1 or 5% corn
oil) or the high-fat diet containing primarily saturated fat (1%
corn oil with 24% coconut oil). Selenium deficiency, however, did
increase the incidence of mammary tumours in rats fed a low-fat
diet containing polyunsaturated fat (1% corn oil), when larger
doses of dimethylbenz[alpha]anthracene were used (10 or 15 mg -
data not shown).
Table 51. Incidence of palpable mammary tumours in
dimethylbenz(alpha)anthracene-treated rats fed different
levels and types of fats in the diet, with or without
selenium supplementationa
-----------------------------------------------------------
Fats in diet Selenium Incidence of palpable tumours
Corn Coconut in diet 19 weeks after dimethylbenz
oil oil (mg/kg) (alpha)-anthracene administration
(%) (%) Number of rats (%)
-----------------------------------------------------------
1 0 0 4/23 17.4
1 0 0.1 3/24 12.5
5 0 0 11/25 44.0
5 0 0.1 8/24 33.3
25 0 0 24/25 96.0
25 0 0.1 15/25 60.0
1 24 0 7/24 29.2
1 24 0.1 6/25 24.0
----------------------------------------------------------
a Adapted from: Ip & Sinha (1981).
In a recent study, the effects of vitamin E status on the
anticarcinogenic effect of selenium were examined (Ip, 1985b).
Female Sprague Dawley rats were fed a 20% stripped corn oil,
casein-based diet. Four dietary groups were formed: adequate
vitamin E/adequate selenium, adequate vitamin E/high selenium,
deficient vitamin E/adequate selenium, and deficient vitamin E/high
selenium. Adequate and deficient vitamin E diets contained 50 and
10 mg vitamin E/kg diet, respectively. Adequate and high selenium
diets contained Na2SeO3 at 0.1 and 2.5 mg selenium/kg,
respectively. At 50 days of age, rats received 5 mg of
dimethylbenz[alpha]anthracene (DMBA) each by stomach tube. Rats
were killed 20 weeks after DMBA administration. The tumour
incidences in the groups were: adequate vitamin E/adequate
selenium, 76%; adequate vitamin E/high selenium, 40%; deficient
vitamin E/adequate selenium, 84%; deficient vitamin E/high
selenium, 68%. This suggests that selenium protection against
carcinogenesis is decreased in vitamin E deficiency.
Pence & Buddingh (1985) studied the effects of selenium
deficiency on 1,2-dimethylhydrazine (DMH)-induced colon cancer in
the rat. They used male Sprague Dawley rats fed Torula yeast-based
diets containing 2% corn oil. Sodium selenite at 0.1 mg
selenium/kg was added to the diet of the controls. Weanlings were
fed the diets 3 weeks prior the institution of DMH treatment and
were killed after 20 weeks of treatment. No effects of selenium
status were noted on the incidence of colon adenocarcinomas.
The effects of selenium on UVR-induced skin carcinogenesis were
studied by Overvad et al. (1985). Female hairless mice were given
sodium selenite at 0, 2, 4, or 8 mg selenium/litre drinking-water.
Three weeks after selenium exposure began they were exposed to UVR
daily for 22 weeks. Then they were examined weekly for 26 weeks
for skin tumours, and relative tumour onset ratios were calculated.
The 2 mg selenium/litre treatment did not affect tumour onset but
the higher doses did. This suggests that selenium can protect
against UVR-induced skin cancer.
Birt et al. (1984) reported that high levels of selenium in the
diet increased pancreatic carcinogenesis induced by bis-(2-
oxopropyl)-nitrosamine (BOP) in male Syrian hamsters. Torula yeast-
based diets with either 0.1 or 2.5 mg selenium/kg were fed to
hamsters beginning at 4 weeks of age. BOP was given in 4 weekly
injections of 5 mg/kg body weight, beginning at 8 weeks of age.
Hamsters were killed at 78 weeks of age. When dietary fat was low
(11% calories as corn oil), the low-selenium group had 25
pancreatic ductular carcinomas (PCDA) in 18 hamsters, and the high-
selenium group had 63 PCDA in 23 hamsters. When dietary fat was
high (45% of calories as corn oil), the low-selenium group had 27
PCDA in 19 hamsters and the high-selenium group had 44 PCDA in 18
hamsters.
Other studies have been reported. Milner, who showed that
pharmacological doses of selenium inhibited the growth of
transplantable tumours, has recently summarized his work (Milner,
1985), and Thompson has summarized his work on mammary
carcinogenesis (Thompson, 1984). The metabolism of the carcinogen
2-acetylaminofluorene in selenium-deficient rats has been studied
by Besbris et al. (1982). They found that selenium-deficient rats
excreted more N-OH-acetylaminofluorene than controls, suggesting
that selenium might prevent the production of this carcinogenic
metabolite or promote its detoxification.
8. EFFECTS OF SELENIUM ON MAN
8.1. High Selenium Intake
8.1.1. General population
8.1.1.1. Signs and symptoms
When it became apparent that selenium was the toxic factor in
plants that caused alkali disease in livestock raised in
seleniferous areas, public health personnel became interested in
the possible hazards for human health in such regions, since
seleniferous grains or vegetables grown on high-selenium soil could
enter the human food chain. Smith et al. (1936) reasoned that, if
human selenosis were a problem anywhere, it would most likely occur
in farmers living in seleniferous regions, who consumed largely
locally-produced foodstuffs. Therefore, they surveyed a rural
population living on farms or ranches known to have a history of
alkali disease. Their survey inquired into the health status of
111 families and also determined the actual consumption of locally-
produced foods. Wherever possible, general physical examinations
were made and urine samples were collected.
These workers were unable to find any symptom or group of
symptoms or serious illness that could be considered characteristic
of, or could definitely be attributed to, selenium poisoning in
man. However, the incidence of vague symptoms of ill health and
symptoms suggesting damage to the liver, kidneys, skin, and joints
was rather high. But since the causes for such disorders are many,
it was not possible to determine whether selenium played any role
in their causation. Apart from the more vague symptoms of
anorexia, indigestion, general pallor, and malnutrition, the
following more pronounced disease states were observed: bad teeth,
yellowish discoloration of the skin, skin eruptions, chronic
arthritis, diseased nails, and subcutaneous oedema.
The same authors (Smith et al., 1936) were unable to
demonstrate a very definite correlation between the clinical
evidence of selenium intoxication in 127 subjects and the
concentration of selenium in the urine. In fact, they expressed
surprise that there was not any more definite evidence of serious
injury, particularly in subjects with high concentrations of
selenium in the urine. Relatively high urinary-selenium levels
were most often associated with pathological nails,
gastrointestinal disorders, icteroid skin, and bad teeth. The
incidence of high urinary-selenium in individuals with dermatitis
and arthritis was no greater than that in individuals without
symptoms.
Because of the ambiguous findings in their first study, Smith &
Westfall (1937) carried out a second, more detailed and intensive
survey to establish the symptomatology of human selenosis and its
relation to the amount of selenium excreted in the urine. For this
purpose, they examined 100 subjects from 50 families that had high
levels of urinary-selenium in their previous testing. The
percentage frequency of the various signs and symptoms observed is
given as follows: none, 24; gastrointestinal disturbances, 31;
icteroid discolouration of the skin, 28; bad teeth, 27; sallow and
pallid colour, especially in younger individuals, 17; history of
recurrent jaundice, 5; dermatitis, 5; pigmentation of the skin
(chloasma?), 3; pathological nails, 3; rheumatoid arthritis, 3;
cardiorenal disease, 2; vitiligo, 2. It was concluded that none of
these signs or symptoms could be regarded as specific for selenium
poisoning, and it was not certain that any one was the direct
result of the continual ingestion of selenium. Nonetheless, these
workers felt that the high incidence of gastrointestinal
disturbances was of importance. Moreover, the high incidence of
icteroid discolouration of the skin was thought to be related in
some way to the ingestion of selenium, possibly as a result of
liver dysfunction. The possible cariogenic effects of high levels
of selenium is discussed further in section 8.1.1.2. The other
symptoms occurred so infrequently that they did not seem to be
associated with selenium. However, it should be noted that the
value of all these observations is in doubt since there is no
comparative information available concerning the frequency of
occurrence of these signs and symptoms in an appropriate control
group.
Smith & Westfall (1937) estimated that most of their subjects
living in highly seleniferous areas were probably absorbing about
10 - 100 µg/kg body weight per day and that some of their subjects
might have absorbed as much as 200 µg/kg per day. For a 70-kg man,
these rates of absorption would be equivalent to a dietary-selenium
intake of 700 - 14 000 µg/day, assuming that all of the selenium in
the food had been absorbed.
As discussed in section 4.1.2, drinking-water rarely
contributes much selenium to a person's total daily intake, but a
brief note indicated that a family of North American Indians living
in Colorado, USA, suffered hair loss, weakened nails, and
listlessness after consuming well water reported to contain 9000 µg
selenium/litre for about 3 months (Anonymous, 1962). This incident
was thought to be the first authentic case of selenium poisoning in
human beings induced exclusively from a naturally-occurring
underground source of water. Tsongas & Ferguson (1977) studied the
effects of selenium in the drinking-water on the health of 2 groups
of persons living in a rural Colorado community. The first group
("exposed") received a water supply that contained 50 - 125
µg/litre, whereas the second group ("unexposed") received a water
supply that contained levels ranging from non-detectable (1
µg/litre) to 16 µg/litre. Examination of a total of 86 individuals
revealed that there were no significant differences between the
exposed and unexposed groups in the incidence or prevalence of any
of 85 health variables studied.
Lemley (1940) presented a 58-year-old rancher from South
Dakota, thought to be the first described case of chronic selenium
dermatitis in a human being, caused by the ingestion of selenium
from natural sources. However, 2 samples of urine collected from
this patient a week apart, contained only 0.043 and 0.040 mg
selenium/litre, levels that are considered to be within the normal
range. Although some improvement in the patient's condition was
noted when certain seleniferous foods consumed on his ranch were
avoided, the improvement was not marked. Administration of
bromobenzene, now known to be a potent hepatotoxin, cleared up the
dermatitis, but caused only a small increase in the urinary-
selenium output, which reached a peak value of about 0.100
mg/litre, after 4 days. Earlier work by Moxon et al. (1940) had
shown that the rate of excretion of selenium from selenized animals
could be increased by the administration of bromobenzene. The
likelihood that selenium was the cause of the dermatitis in this
patient is doubtful, since, in a later paper, Lemley & Merryman
(194l) claimed that a relapse in this patient's condition was cured
when some canned meat containing 0.40 µg selenium/kg (considered by
them to be a highly toxic amount of selenium) was withdrawn from
the patient's diet. This figure for the level of selenium in the
meat is almost certainly erroneous, since not only is it below that
which would be found in animals suffering from selenium deficiency
but it is also below the sensitivity of the analytical techniques
available at that time.
Other cases of presumed human overexposure to selenium were
reported by Lemley & Merryman (1941). For example, urine samples
from a ranching family living in South Dakota contained 0.200,
0.250, 0.300, 0.550, and 0.600 mg selenium/litre for the father,
mother, daughter, son, and uncle, respectively. Administration of
bromobenzene to the father resulted in a urinary output of selenium
of 1.800 mg/litre within 24 h. No mention of dermatitis was made
in connection with this family. Rather, all family members
suffered from slight, continual dizziness and clouding of the
sensorium, extreme lassitude accompanied by depression, and
moderate emotional instability. The patients tired on exertion and
complained that their powers of concentration were markedly
impaired. After a course of bromobenzene, these people improved
markedly and their general condition remained improved, since they
were instructed not to use the various food products of the ranch
that contained selenium. Lemley & Merryman concluded that the
following facts provided the basis for the diagnosis of selenium
poisoning in these subjects:
(a) knowledge that the subjects lived in a seleniferous
area;
(b) presence of concentrations of selenium in the urine
exceeding 0.100 mg/litre;
(c) increased elimination of selenium in the urine after
bromobenzene administration; and
(d) improvement in the subject's symptoms after
elimination of selenium from the diet.
The same authors also described a 65-year-old rancher from
South Dakota, who suffered alternate bouts of diarrhoea and
constipation. This patient's urinary-selenium concentrations were
as high as 0.250 mg/litre. An exploratory abdominal operation
suggested that the patient had very early cirrhosis of the liver,
which was confirmed by pathological examination. The patient
recovered from the operation and then gradually improved after
being placed on a strict selenium-free diet and given a course of
bromobenzene.
In a later paper, Lemley (1943) expressed the opinion that
human selenium poisoning is common, widespread, and, in certain
localities, of importance for the general public health. More
recently, Kilness (1973) complained that no follow-up public heath
survey with an appropriate control group had been made in South
Dakota since the initial surveys by the United States Public Health
Service in highly seleniferous areas more than 30 years previously.
Jaffe (1976) carried out a field study in Venezuela and
compared 111 children living in a seleniferous area (Villa Bruzual)
with 50 living in Caracas. The overall haemoglobin and haematocrit
values were somewhat lower in Villa Bruzual (128 g/litre and 39
volume %, respectively) than in Caracas (148 g/litre and 42 volume
%, respectively), but no correlations between blood- and urine-
selenium levels and haemoglobin or haematocrit values were found.
The children in Villa Bruzual consumed less meat and milk and had a
higher incidence of intestinal parasite infestation (Jaffe et al.,
1972a) than those in Caracas. Therefore, it was concluded that the
differences in haemoglobin were probably due to differences in
nutritional or parasitological status and not to differences in
selenium intake. Activities of prothrombin and serum alkaline
phosphatase and transaminases, which altered in selenium-poisoned
rats (Jaffe et al., 1972b), were normal in all the children and no
correlation with blood-selenium levels was apparent. Symptoms of
dermatitis, loose hair, and pathological nails were reported as
more frequent among the children in the seleniferous area than in
those living in Caracas, but no quantitative information was given.
The clinical signs of nausea and pathological nails appeared to be
correlated with serum- and urine-selenium levels. However, the
cause of the different incidence of clinical signs was considered
doubtful, especially in the absence of differences in the various
biochemical tests performed (Jaffe et al., 1972a).
The mean blood-selenium level of 111 school children from the
seleniferous area in Venezuela was 0.813 mg/litre (Jaffe et al.,
1972a). A subgroup of 28 children from this area who had blood-
selenium levels of over 1 mg/litre had a mean blood-selenium level
of more than 1.321 mg/litre. There was one child with a blood-
selenium level of 1.8 mg/litre. Another subgroup of 11 children
who had blood-selenium levels of less than 0.4 mg/litre had a mean
blood-selenium level of 0.330 mg/litre. Urinary-selenium levels
tended to reflect blood-selenium levels, since children in the
high-blood-selenium subgroup (> 1 mg/litre) excreted a mean level
of 0.657 mg/litre urine, whereas children in the low-blood-selenium
subgroup (< 0.4 mg/litre) excreted a mean level of 0.266 mg/litre
urine.
Kerdel-Vegas (1966) summarized 9 cases of acute intoxication
due to the ingestion of nuts of the "Coco de Mono" tree (Lecythis
ollaria) from the seleniferous areas of Venezuela. Although the
course of the poisoning differed from patient to patient (probably
because of differences in the amount of nuts consumed and the
length of time the nuts were present in the digestive tract), most
cases experienced nausea, vomiting, and diarrhoea, a few hours
after eating the nuts, followed by hair loss and nail changes, some
weeks after the initial episode. Two patients had foul breath that
was described as being reminiscent of decomposed seaweed or
phosphorus. After a period of time, re-growth of hair and nails
took place and most patients appeared to make a satisfactory
recovery. One fatality was reported, a 2-year-old boy who died, in
spite of proper medical attention and symptomatic treatment for
severe dehydration.
Dickson (1969) described his own personal experience after
eating sapucaia nuts ( Lecythis elliptica H.B.K.) that had grown in
Honduras. He reported hair loss and splitting of finger and
toenails several weeks after consuming the nuts. Although no
mention of selenium was made in his report, he noted that a
peculiar odour was associated with consumption of these nuts, not
only of his breath but of his body as well. Kerdel-Vegas (1966)
pointed out that the sapucaia, or chestnut of Para (which he called
Lecythis paraensis Ducke), is consumed as food in Brazil and other
countries to which it is exported (i.e., Europe and the USA). He
also commented that there is a popular saying in northern Brazil
that eating sapucaia leads to hair loss, but he knew of no
scientific or medical reports from Brazil. Signs of selenium
toxicity have been observed in rats fed diets containing defatted
Brazil nut flour that assayed 51 mg selenium/kg (Chavez, 1966), and
Thorn et al. (1978) found that samples of Brazil nuts marketed in
the United Kingdom contained an average of 22 mg selenium/kg
(range, 2.3 - 53 mg/kg).
As reported recently, an unexplained intoxication characterized
by nail deformation and the loss of hair and nails as the most
common signs was observed in Enshi county of Hubei province of the
People's Republic of China, more than 20 years ago, with a peak
prevalence during the years 1961 - 64 (Yang et al., 1983). In 5
villages with 248 inhabitants, about half of the population was
affected and in one particular village over 80% of the people were
affected. No other quantitative information about the cases was
given but a general description of the signs and symptoms observed
is presented below. The hair became dry and brittle and was easily
broken off at the scalp with retention of intact radicles so that
depigmented and dull hair continued to grow. A scalp rash
accompanied by intolerable itching resulted in hard scratching
which easily removed the hair. The nails became brittle with white
spots and longitudinal streaks on the surface, followed by a break
on the wall of the nail. With new nail growth, the broken nail
advanced and ultimately fell off. Effusian of fluid from around
the nail was common in many cases. The new nail had a rough and
ridged surface and was fragile and thickened.
Other signs of intoxication included skin lesions, tooth decay,
and abnormalities of the nervous system. The skin became red and
swollen and then blistered and eruptive followed, in some cases, by
ulcerations that took a long but unstated time to heal. The
lesions occurred primarily on the limbs and also on the back of the
neck. Mottled teeth were observed in the intoxicated individuals
but this observation may have been confounded by high exposure to
fluoride. In one heavily affected village, nervous system
abnormalities were seen, including peripheral anaesthesia,
acroparaesthesia, and pain in the extremities. Hyperreflexia of
the tendon commonly developed later followed by numbness,
paralysis, and motor disturbances. One case of hemiplegia was also
reported. There was an indication that this intoxication was
related to the locally grown monotonous diet consumed, which later
was shown to contain high levels of selenium. No quantitative data
regarding selenium exposure were obtained at the time of the
outbreak. However, because of circumstantial evidence, the
intoxication was considered by the authors to be selenosis, and, as
discussed in sections 5.1.1.1 and 6.2.3, current levels of exposure
to dietary selenium in an area with a history of intoxication, as
assessed by blood- and hair-selenium levels as well as dietary
intake data, exceeded those ever reported in any non-occupationally
exposed population (Table 8). The authors estimated that the daily
dietary intake of selenium, after the peak prevalence of the
poisoning had subsided, averaged 5 mg, and blood- and hair-selenium
levels averaged 3.2 mg/litre and 32.2 mg/kg, respectively. The
ultimate environmental source of the selenium in this episode of
intoxication was a highly seleniferous coal (average selenium
content of 300 µg/g) which lost its selenium to the soil as a
result of weathering processes. Once in the soil, the selenium was
taken up by plant crops and entered into the food chain.
Because of the nutritionally-beneficial effects of selenium in
animals, some investigators have deliberately given inorganic or
organic forms of the element to people with the aim of producing
some desirable health benefit. For example, Westermarck (1977)
administered selenium, as selenite, in oral doses of 0.05 mg/kg
body weight per day, for more than one year, to patients with
neuronal ceroid lipofuscinosis (NCL) and did not observe any toxic
manifestations. On the contrary, it was felt that some of the NCL
patients showed at least a transitory improvement in their
condition. In some patients, a slight increase in serum aspartate
aminotransferase activity was observed, but apparently this is seen
in a number of patients with NCL.
Schrauzer & White (1978) described 2 individuals who had been
taking commercially-available nutritional supplements consisting of
selenium-containing yeast at doses of 200 and 450 µg selenium,
daily, for 18 months. Together with their dietary intakes, these
individuals received a total of 350 and 600 µg/day. Although some
marginal haematological changes were seen and the serum-glutamic
oxaloacetic transaminase activities were on the borderline of high,
it was concluded that daily intakes of up to 600 µg selenium for 18
months do not induce toxic effects in well-fed individuals.
In a study by Perona et al. (1978), 4 subjects were given,
orally, a 2-mg dose of selenium, as sodium selenite, daily, for
20 - 40 days, to determine the effects of in vivo selenium
administration on the activity of human erythrocyte-glutathione
peroxidase. It was stated that none of the subjects exhibited any
symptoms of selenium poisoning, but the criteria used to judge any
deleterious effects of selenium were not described.
Yang et al. (1983) reported the case of a 62-year-old man who
had taken one tablet containing 2 mg sodium selenite per day for
more than 2 years. The subject did not have any symptoms of
indisposition but presented with thickened, fragile and somewhat
honeycomb-like fingernails. After the oral intake of sodium
selenite was stopped, the surface of the new nail growth became
smooth and gradually recovered. Blood-and hair-selenium levels
were 0.179 mg/litre and 0.828 mg/kg, respectively, on the day the
selenite tablets were discontinued. These tissue-selenium levels
are much lower than those reported by the same authors in Enshi
county of the Hubei province (Yang et al., 1983) suggesting that
the form of selenium ingested must be considered when interpreting
tissue-selenium levels in the diagnosis of selenium poisoning.
Ingestion of superpotent selenium tablets, meant to be consumed
as a "health food" supplement, resulted in 12 cases of human
selenium toxicity in the USA in 1984 (Anonymous, 1984; Jensen et
al., 1984; Helzlsouer et al., 1985). Each tablet contained 27 - 31
mg selenium by analysis (about 182 times more than the level stated
on the label). Approximately 25 mg of the selenium was present as
sodium selenite whereas the rest was elemental and/or organic
selenium. The total doses of selenium estimated to be consumed by
the victims ranged from 27 to 2387 mg. Based on the limited
information available, the symptoms reported in these cases as most
common were nausea and vomiting, nail changes, hair loss, fatigue,
and irritability. Other symptoms included abdominal cramps, watery
diarrhea, paraesthesias, dryness of hair, and garlicky breath.
Eight of the 12 victims did not have any abnormalities in the blood
chemistry, and the results of liver and kidney function tests were
normal.
A level of 500 µg has been proposed as the tentative maximum
acceptable daily intake of selenium for the protection of human
health (Sakurai & Tsuchiya, 1975). This figure was derived from an
initial estimate of the mean normal daily intake of selenium by
human beings of between 50 and 150 µg. It was concluded that
values of 10 - 200 times the normal intake appeared acceptable as
an estimated range for the margin of safety within which the
average human being could tolerate selenium. By taking the lower
values of both these estimates, the lowest level of potentially
dangerous daily intake of selenium was estimated to be 500 µg.
8.1.1.2. Attempts to associate high selenium intake with human
diseases
(a) Dental caries
As already mentioned in section 8.1.1.1, bad teeth was one of
the signs that was thought to be possibly associated with a high
intake of selenium by a rural population living in seleniferous
areas of South Dakota, Wyoming, and Nebraska (Smith et al., 1936)
and by children living in a seleniferous zone in Venezuela (Jaffe,
1976). Several studies have been concerned with the possibility of
a specific association between the incidence or prevalence of
dental caries and residence in a seleniferous area, urinary
excretion of selenium, or the selenium content of teeth.
Hadjimarkos & Storvick (1950) and Hadjimarkos (1956) noted a
geographical difference in the prevalence of dental caries in 2029
children, between the ages 14 and 16 years, residing in 4 different
counties in Oregon. Two different counties, one with the highest
(Clatsop), and one with the lowest (Klamath), rates of caries
experience were selected for further study. Urine samples from 24
and 29 male children who were attending county seat high schools
and who were born and residing in the respective counties were
analysed for selenium content. The mean levels of urinary-selenium
were 0.049 and 0.037 mg/litre in the groups of children from the
counties with the high (14.4 DMF (decayed, missing, or filled)
teeth per child) and low (9.0 DMF teeth per child) prevalence of
dental caries, respectively (Hadjimarkos et al., 1952).
In a second study, 2 additional counties with similar but high
rates of caries experience (Jackson and Josephine, 13.4 and 14.4
DMF teeth per child, respectively) were selected (Hadjimarkos &
Bonhorst, 1958). Analysis of 33 urine specimens from continuously-
resident, male high school children attending a county seat high
school (Jackson) and 46 specimens from similar children attending a
high school serving a rural area (Josephine) gave values (mean ±
SE) of 0.074 ± 0.007 and 0.076 ± 0.005 mg/litre, respectively. It
was concluded that there was an association between the high
prevalence of dental caries and the values of urinary-selenium
excretion, which were double those seen in the previous study in
the county with a lower rate of caries experience.
In order to explain the variations in urinary-selenium levels
observed in the above studies, samples of locally-produced and -
consumed milk and eggs, as well as local drinking-water samples
were collected from 74 farms located in Klamath, Jackson, and
Josephine counties and analysed for their selenium content
(Hadjimarkos & Bonhorst, 1961). The selenium levels were almost 10
times higher in food samples collected from the counties with high
caries prevalence (Jackson and Josephine) than in those from the
county with low caries prevalance (Klamath). The selenium levels
in the milk samples obtained in Jackson and Josephine counties were
higher than those listed in Table 3.
In another study, Tank & Storvick (1960) determined the extent
of dental caries in a population of children from 15 areas of
Wyoming that had been identified previously as seleniferous or non-
seleniferous on the basis of the geological distribution of
selenium, occurrence of the element in vegetation, and the
occurrence of selenosis in livestock. These research workers
claimed that the dental caries rate in the permanent teeth was
higher in children residing in seleniferous areas of Wyoming than
in children living in non-seleniferous areas of Wyoming, but they
were unable to demonstrate any consistent relationship between
calculated selenium intake and caries or urinary-selenium excretion
and caries.
Suchkov et al. (1973) found that the incidence of caries varied
in 3 different geographical zones (mountainous, pre-mountainous,
and forest-steppe) in the Chernovitsi region of the Ukraine.
Analysis of teeth from people residing in rural areas in these 3
zones and mainly consuming locally-produced foodstuffs revealed a
direct correlation between the selenium content of the teeth and
the incidence of dental caries. The people in the mountainous zone
had the highest level of selenium in the teeth and the greatest
incidence of dental caries, whereas the people in the forest-steppe
zone had the lowest level of selenium in the teeth and smallest
incidence of dental caries. This correlation was true for
deciduous teeth as well as for permanent teeth. However, as
pointed out by the authors, the mountainous zone also had the
softest water and the lowest content of fluorine in the drinking-
water, so that factors other than selenium might have been
involved.
It was not possible to demonstrate any association between the
urinary excretion of selenium and the incidence of dental caries in
subjects living on the South Island of New Zealand, an area known
to be low in selenium (Cadell & Cousins, 1960). However,
Hadjimarkos (1960) claimed that the levels of urinary excretion of
selenium in this study (all less than 0.050 mg/litre) were too low
to be associated with an increased incidence of caries.
As emphasized by Schwarz (1967), the levels of urinary-selenium
excretion reported in the Hadjimarkos studies were within the
limits generally found in the normal population without excessive
selenium intake. It may be recalled from section 7 that, in
experimental animal studies, very high toxic levels were used to
demonstrate the cariogenic effect of selenium.
(b) Reproduction
Possible effects of selenium on reproduction were suspected
following old anecdotal reports from Colombia, South America that
women living in areas, later shown to be seleniferous, gave birth
to malformed babies (Rosenfeld & Beath, 1964), but there is a lack
of reliable studies. Robertson (1970) pointed out that, among 6
women preparing microbiological media containing sodium selenite,
one probable and 4 certain pregnancies all ended in abortions, save
one that went to term. The infant was born with bilateral club
foot. However, no differences in urinary-selenium levels were
observed between this group of women and a control group living in
the same area, and inquiries by the author at other laboratories
carrying out comparable work did not show any evidence of similar
trouble. Jaffe & Velez (1973) could not demonstrate any
correlation between the selenium level in the urine of school
children in different Venezuelan states and the incidence of infant
mortality due to congenital malformations, on the basis of
published public health statistics. Jaffe (1973) concluded that no
recent observations of a teratogenic action of dietary selenium in
human beings had been reported at that time.
(c) Amyotrophic lateral sclerosis
Kilness & Hochberg (1977) reported an unusual cluster of 4
cases of amyotrophic lateral sclerosis (ALS) in male farmers living
in a seleniferous area and indicated that selenium might be an
environmental factor predisposing to the disease. But Schwarz
(1977) pointed out that the frequency of ALS was at least as high
if not higher in areas that were selenium-deficient as in those
with normal or elevated levels and Kurland (1977) suggested that
the cluster was more indicative of a chance occurrence than of a
new etiological lead in ALS. Moreover, Norris & Sang (1978) found
that 19 out of 20 well-established cases of ALS had urinary-
selenium levels lower than the mean for unexposed persons and
therefore concluded that selenium exposure was of no concern in the
average case.
8.1.2. Reports on health effects associated with occupational
exposure
Although the toxicological potential of selenium for human
beings can be inferred from studies carried out with laboratory
animals, certain precautions must be taken in applying the results
of animal studies to the industrial health aspects of selenium.
First, the number of studies dealing with the respiratory exposure
of animals to selenium compounds is limited, and the dose levels
employed have usually been higher than those encountered in
industry. On the other hand, the period of exposure used in the
studies reviewed in section 7.1.2.3 did not exceed one month. The
specific chemical form and physical state of the selenium must be
considered as well as the fact that the chemical form of selenium
may change when in contact with moist mucous membranes or with
sweat. These factors, plus several others, must be taken into
account when considering the health effects of various selenium
compounds under occupational exposure conditions.
The Task Group recognized that, for various reasons, knowledge
on the health effects of industrial exposure to selenium compounds
was not complete. Acute exposures to selenium are the result of
accidents and are, of necessity, described on an ad hoc case study
basis. Regarding the effects of long-term selenium exposure in
industry, there is a lack of epidemiological studies that include
unexposed control groups. Also, no follow-up studies are available
comparing the health status of a sufficiently large number of
workers, previously exposed to selenium, with that of the unexposed
population. Furthermore, the exposure level was not known with
certainty in either acute or long-term studies, and, in some cases,
the form of selenium was not established. In many cases,
simultaneous exposure to other noxious agents occurred.
Nevertheless, the Task Group felt that some preliminary assessment
of the toxicological potential of selenium in industry could be
made on the basis of the occupational experience available with
selenium compounds.
Since Hamilton's first observation on the effects of selenium
exposure in industry in 1917 (Hamilton, 1927 - 34), several hundred
persons have been described in the literature who have been
directly affected by the vapour, fumes, or dust of selenium and its
compounds. In addition, there is a systematic study of a group of
over 100 selenium workers for a period of 2 years (Glover, 1967).
Systematic attention has also been given to the health of workers
exposed to selenium in the USSR (Filatova, 1948; Monaenkova &
Glotova, 1963; Gracianskaja & Kovshilo, 1977). Several reviews
evaluating the health effects of selenium from the point of view of
occupational health have been presented (Glover, 1954 - 70, 1976;
Cooper, 1967; Izraelson et al., 1973; Cooper & Glover, 1974).
8.1.2.1. Fumes and dust of selenium and its compounds
Workers in several industries, such as the production or
recovery of selenium itself, or the manufacture of glass or
rectifiers can be exposed to the fumes and dust of selenium and its
compounds. In addition, direct contact with powders or solutions
containing selenium compounds is possible and the biological
effects of such contact will be discussed in this section.
The chemical form of selenium in the fumes and dusts found in
the above-mentioned industries, consists of elemental selenium plus
various amounts of selenium dioxide, the only oxide of selenium
found in the industrial environment. However, in some cases, the
possible occurrence of other selenium compounds, such as hydrogen
selenide, cannot be excluded. Instances where hydrogen selenide
was the main selenium compound of concern will be discussed
separately in section 8.1.2.1.2.
8.1.2.1.1. Selenium dioxide
Selenium dioxide mainly occurs in industry:
(a) during the production of the compound, usually by the
oxidation of elemental selenium; and
(b) whenever selenium is heated in air, purposely or
accidently, above its melting point. "Selenium fume"
rising under these conditions is a mixture containing
red elemental selenium and about 20 - 80% of selenium
dioxide.
Glover (1954, 1970, 1976) has summarized the acute local,
systemic, and possible long-term effects of occupational exposure
to selenium dioxide. Acute local effects are seen in the lungs,
gastric mucosa, skin, nails, and eyes. Sudden inhalation of large
amounts of selenium dioxide produces pulmonary oedema, due to a
local irritant effect on lung alveoli. Working in an atmosphere
containing selenium dioxide can increase indigestion. Contact with
the skin results in burns or dermatitis. Occasionally, a true
urticarial type of generalized allergic body rash may occur, in
which case the individual must be removed from any work involving
selenium. The selenium dioxide may penetrate under the nails and
cause excruciating pain in the nail beds. If selenium dioxide
enters the eyes, prompt flushing with water will prevent
conjunctivitis. "Rose eye", a pink discoloration of the skin of
the eyelids, which often become puffy, is sometimes seen in persons
who work in an atmosphere of selenium dioxide dust. Systemic
effects of selenium dioxide exposure include garlicky-smelling
breath, metallic taste on the tongue, and indefinite
sociopsychological effects. The garlicky breath is the first and
most characteristic sign and has been used by occupational
hygienists as a way of monitoring exposure, even though the odour
is not always a reliable index in this respect. The metallic taste
is an earlier symptom but, being more subtle, is often overlooked
by workers. Sociopsychological effects such as lassitude and
irritability have also been associated with selenium exposure.
Since the effects of selenium overexposure lead to hepatic damage
in animals, Glover (1954) concluded that it would be prudent to
watch for such damage in human beings.
(a) Reports on health effects connected with short-term
accidental exposures
An industrial incident in a plant engaged in smelting scrap
aluminium and other non-ferrous metals was reported by Clinton
(1947). The aluminium scrap included more than 100 kg of aluminium
rectifier plates coated on one side with metallic selenium overlaid
with a coating of an alloy of bismuth, cadmium, and tin. When an
attempt was made to skim the dross prior to pouring, a cloud of
reddish fume arose. The fumes were intensely irritating to the
eyes, nose, and throat and the plant was evacuated. No workers
were exposed for more than 2 min.
All exposed workers noticed immediate and intense irritation
of the eyes, nose, and throat, and an unpleasant sour, garlic-like
odour to the fumes. The more heavily exposed workers complained of
a severe burning sensation in the nostrils, and dryness of the
throat; followed after 2 - 4 h by severe headache, mainly frontal
in location, lasting until the following day. Several men observed
immediate sneezing, coughing, and headache, followed for 4 - 8 h by
nasal congestion, dizziness, and redness of the eyes. Most of the
men noticed a bad taste in their mouths and an unpleasant odour to
their skin and clothing. Other people, however, did not note any
unpleasant odour in the breath of the exposed workers.
Two labourers who had attempted to skim the dross from the
furnace and therefore underwent intense exposure were hospitalized
for observation. On arrival at the hospital, they complained of
soreness of the eyes and lachrymation, pain in the nose, slight
difficulty in breathing, and frontal headache. Physical
examination on admission revealed conjunctival injection,
congestion of the mucous membranes of the nose and throat, and
oedema of the uvula. One man had a few fine rales audible in the
right base; roentgenological examination of the chest did not
reveal any abnormalities. The temperature, pulse, and respiration
were normal. Both workers were discharged 2 days after the
accident, at which time they were asymptomatic and had no positive
physical findings. Another worker, a foreman, underwent a more
severe exposure than most of the workers. He was not hospitalized,
but, about 8 - 12 h after the accident, he developed severe
dyspnoea accompanied by a slight elevation in temperature, in
addition to a headache and sore throat. Physical examination
revealed fine rales in both bases, as well as scattered asthmatic-
like wheezes throughout the chest. These signs and symptoms
cleared in about 24 h, without specific therapy. All persons
exposed to the fumes had recovered entirely in 3 days, and no
sequelae have been encountered.
In the same year, Lauer (1947) described an episode that
occurred when 15 men were exposed to fumes arising from an
explosion and fire, which occurred in a pot when selenium became
overheated in contact with aluminium. The 2 elements produced a
high-temperature reaction, and fumes and vapours were dispersed
throughout the work area. The lengths of exposure varied, some
victims were using masks part of the time, and others were almost
without protection.
The exposed men reported to the medical dispensary about 15 -
45 min after the accident. All complained of soreness and burning
of the nose and throat, and 8 out of 15 cases had some dyspnoea.
Two of these cases were moderately severe, requiring almost
continuous oxygen therapy. Headache and dizziness and a burning
sensation in the eyes occurred in 6 cases. Three others complained
of substernal burning and tightness of the chest. In 4 there was
nausea and vomiting. All were hospitalized. Physical examination
showed reddening of nasal and pharyngeal mucosa, wheezes, and
musical rales in the lungs (12 cases). These symptoms abated
quickly. In about 10 days, there were few subjective complaints.
All apparently recovered satisfactorily with no known
complications. To assess possible liver affection, Hanger's
cephalin cholesterol tests and Icterus Index determinations were
performed on admission to the hospital and again at the end of the
first, second, third, and seventh week. On the day of admission to
the hospital, Hanger's tests were made on 13 workers, 4 of which
were positive and 9, negative. At the end of the first week
following exposure, there were 10 positives and 3 negatives. At
the end of the second week, there were 11 positives and 3 negatives
out of a group of 14 tested, and the same result was obtained at
the end of the third week. By the end of the seventh week, only 4
of 11 tested were positive. The average value of the Icterus Index
on admission to the hospital was 7.3 units. One week after
exposure, it was 13.5. In 2 weeks, it averaged 20.7. After the
third week, it had fallen to 9.9 and, by the end of the seventh
week, the average Icterus Index was 7.2.
An accident connected with a fire in a selenium rectifier plant
was described by Wilson (1962). Twenty-eight of the employees were
exposed directly to the smoke and fumes containing selenium
dioxide. The length of exposure to the fumes varied with the
individual, but no one was exposed for more than 20 min. Oxygen
was administered by mask to the men lying on the ground, who
experienced bronchial spasm with coughing, gagging, and, in some
instances, transient loss of consciousness.
The initial signs and symptoms were a feeling of constriction
in the chest, accompanied by burning and irritation of the upper
respiratory passages, violent coughing and gagging with nausea and
vomiting, and a bitter acid taste in the mouth. During the acute
episode, there were mild signs of shock, with a drop in blood
pressure and an elevated pulse and respiratory rate. Other
symptoms, experienced during this stage, were burning of the skin,
conjunctivae, and mucous membranes of the upper respiratory
passages. Within 4 h, all patients had apparently recovered from
the acute episode and a recheck of their blood pressure, pulse,
respiratory rate, and general condition was found to be within
normal limits.
Within 6 h of the exposure, the victims began complaining of
secondary symptoms with the onset of generalized chills accompanied
by nausea and vomiting, diarrhoea, malaise, dyspnoea, and headache.
The onset of secondary symptoms was delayed in some of the victims
for several hours after the initial exposure. Within 12 h, all
exposed personnel began experiencing the symptoms described. The
following morning, the first patient, a 30-year-old male, was
admitted to the hospital. He was cyanotic and in moderate
respiratory distress. He complained of chest pain bilaterally,
and was experiencing bronchial spasms. A chest roentgenogram
revealed extensive bilateral consolidation of the lung fields,
indicating pneumonia. The white blood cell count was elevated to
153 000 with a marked prominence of neutrophils. He experienced a
stormy course, requiring constant oxygen for the first 9 days of
hospitalization. By the fifth hospital day, X-ray films revealed a
further extension of the previously noted bilateral pneumonic
consolidation. Two weeks following admission, comparison of a
chest film with previous films revealed marked improvement
bilaterally. He was free of respiratory distress, his white blood
cell count had returned to within normal limits, and he was
discharged home for convalescence. It is interesting to note that
this was the only employee of the 5 hospitalized cases who did not
receive oxygen on the afternoon of the fire. On the same day, the
second victim was admitted to the hospital in a similar dyspnoeic,
cyanotic state with an elevated white blood cell count of 24 600.
X-ray again revealed bilateral atelectasis and consolidation. This
patient required continuous oxygen for 6 days, and was discharged
after 9 days of hospitalization: his white blood cell count had
returned to normal and a repeat roentgenogram revealed improvement.
This was the second most involved and prolonged illness, and he
received only about 6 breaths of oxygen following his exposure.
Three days after the accident, a third employee was admitted to the
hospital in less respiratory distress, with a normal white blood
cell count and a chest film that revealed bilateral elevation of
the diaphram with extensive peribronchial infiltration and some
consolidation at the lung bases. On the same day, another worker
was admitted to the hospital with a normal white blood cell count
and a chest film revealing bilateral increase in lung markings
consistent with bronchitis; however, no consolidation was evident.
The final patient to be hospitalized was admitted 4 days after the
accident with a white cell count of 12 800 and a prominence of
neutrophils. The X-ray film indicated bilateral pneumonia.
Four days after exposure, all other employees with any upper
respiratory complaints were examined. Thirty-two of the 53
employees examined were found to have a residual bronchitis, of
minimal to moderate degree, that required medication. These were
treated on an outpatient basis. Within 1 week, all were
asymptomatic and free of any respiratory signs.
Both Monaenkova & Glotova (1963) and Skornjakova et al. (1969)
have described occupational incidents of short-term selenium
dioxide exposure. In the former case, 6 workers, 20 - 52 years
old, were exposed from 5 to 20 min in a room with a leaky selenium
still. The workers suffered acute pain in the eyes, hoarseness in
the throat, painful cough, and a heavy feeling in the chest. Acute
conjuctivitis, laryngotracheitis, and bronchitis were also noted
and gastroenterocolitis was common to all. Transient fever was
seen in 2 workers. The various signs and symptoms disappeared
within 5 - 7 days, but, on return to work involving selenium, 2
workers developed chronic bronchitis. In the report of acute
selenium dioxide exposure by Skornjakova et al. (1969), there was
irritation of the eyes and mucous membranes, coughing without
expectoration, nasal secretion, headache, vertigo, nausea,
vomiting, garlicky-smelling breath and skin, general weakness, loss
of consciousness, and collapse. Two weeks after exposure, an
allergic whole body rash occurred that disappeared after removal of
the worker from contact with selenium.
(b) Effects of short-term and/or repeated dermal exposure
Selenium dioxide is more than a primary irritant, it causes
extremely painful burns on the skin which, however, always heal
without a scar. Theoretically, the selenium dioxide powder itself
does not burn the skin (and if dropped on to the skin should be
immediately brushed off dry). However, in practice, in the
industrial environment, there is sufficient moisture on the skin
from sweating for this very deliquescent white solid to form a
sticky solution of selenious acid within seconds, or at the most,
minutes, of coming into contact with the skin. Selenious acid is
so soluble that, if the skin is immediately washed with a lot of
water, there will be no burn or even rash from accidental contact.
When the selenious acid does burn the skin, there is little to see
or feel for several hours. After 4 h with a 50% solution, an
unremitting intense pain begins, small petechial areas occur, and a
faintly orange coloration occurs, which indicates reduction of some
of the selenious acid to elemental red selenium. The pain and
necrosis can be prevented by the application of a reducing solution
or ointment, such as 10% sodium thiosulfate (Glover, 1954). If the
burn remains untreated it may go on to ulceration, but this is
rare. Högger & Böhm (1944) described a case of a woman who
suffered pain and reddening of the middle, ring, and little fingers
of one hand when selenium dioxide crystals penetrated into the
protective rubber glove that she was wearing.
The first accurate description of a case of selenium fumes
causing allergic dermatitis was published by Duvoir et al. (1937).
A chemical engineer, who had handled selenium for 3 years in the
past, developed swelling of the face with a hard urticarial oedema
of the nose and cheeks extending on the neck down to his shirt
collar following a 3-day exposure to selenium vapours. There was a
semi-circle of vesicles on the lower lids, but the forehead and
ears escaped completely. His hands showed discrete lesions.
Within 24 h, the genital oedema had disappeared, after 3 days, the
rash started to go, and, by 9 days, there was only desquamation
left. The 5-cm plaque, occurring 1 h after a patch test with 10%
potassium selenite, showed a greater than average contact
sensitivity.
Another case was described (Halter, 1938) involving a glass
worker whose job was to add a mixture of red selenium and sodium
selenite to the glass. This man was in daily contact with selenite
powder for 36 years. He complained of an oedematous erythema of
the face and neck, and several hard infiltrated raised plaques on
the dorsi of the hand and fingers. On examination, his nasal and
laryngeal mucosae were found to be reddened, and there was a very
slight conjunctivitis. There were no changes in the skin of areas
protected by clothing. His only other complaint was of headache.
There were no symptoms or signs referrable to the nervous or
gastrointestinal systems. Selenium was detected in the urine (no
actual values were given). His liver was found to be enlarged and
there was an increase of porphyrins in the urine.
Pringle (1942) has described several cases of acute dermatitis
resulting from contact with dry selenium dioxide or selenium
dioxide dissolved in water, and 2 cases of acutely painful
paronychia after accidental contact with dry selenium dioxide. In
addition, several cases of mild rash on the anterior surface of
both forearms, but principally affecting the bend of the elbows,
were seen among employees working with heated selenium dioxide in
the fume cupboards. Only their fingers, protected at that time
with gloves, entered the fume cupboard, which apparently was
adequately protected. The dermatitis resembled a seborrhoeic
condition and there was frequently a thin watery discharge. In the
course of a year, 2 cases developed a high degree of sensitivity to
selenium dioxide, so that working in the same department, though
not actually on selenium, caused repeated recurrences and they had
to be removed permanently to another part of the factory.
(c) Studies on the health effects of long-term exposure
Filatova (1948) reported that workers exposed over a long
period of time to selenium aerosols containing elemental selenium
at levels of 0.35 - 24.8 mg/m3 and selenium dioxide at levels of
0.11 - 0.78 mg/m3 developed rhinitis, nasal bleeding, headaches,
loss of weight, irritability, and pain in the extremities
(Izraelson et al., 1973).
Conditions of exposure and the related health effects in a
rectifier factory were investigated by Kinnigkeit (1962). Air-
selenium levels were determined in various workplaces and blood-
selenium levels were measured in 62 workers exposed to selenium.
Urinary-selenium levels were also determined in 22 workers. The
reported air-selenium levels did not exceed 0.05 mg/m3 and were
less than the threshold limit value. However, as discussed in
section 5.2.1 there was a clearcut discrepancy between the air
values and the selenium levels observed in blood and urine,
indicating that the actual workers' exposure must have been
considerably higher. Of 62 workers, over half (35) complained of
irritability, sleeplessness, loss of appetite, and nausea. Twenty
six had headaches and 3 had cramplike pains in their limbs.
Clinical examination revealed irritation of the mucosa in 9
workers, with conjunctivitis and slight tracheal bronchitis. Of
the 2 workers that had unavoidable skin contact with selenium, one
had excematous lesions of the forearms and the other had bluish-red
urticarial exanthema.
The Takata reaction and the thymol test were performed on 61 of
these workers, and the results were within the normal range in all
employees, with the remarkable exception of workers engaged in the
electrical testing of the rectifier plates. In this group of 13
people, abnormal results suggesting impaired liver function, were
obtained in 8 workers, the only employees from the plant showing
this pathological reaction. As underlined by Kinnigkeit, workers
carrying out this process handled the plates by hand and were
frequently directly exposed to fumes containing selenium dioxide
arising from the burning out of plates during electrical testing.
As discussed previously, this group was characterized by a very
high mean blood-selenium level (geometrical mean ± SE: 15.8 ± 11.8
mg/litre). A great interindividual variability in selenium-blood
levels existed with this and other groups, but no attempt was made
to relate these individual values to the results of liver function
tests. Another group of workers had blood-selenium levels that
were almost as high (Table 13) (section 6.2.3), but no evidence of
liver dysfunction was observed in this group. However, it cannot
be assumed that the form and pathway of the exposure to selenium
was the same in both groups.
Monaenkova & Glotova (1963) summed up results of clinical
observations on 12 people, aged 30 - 50 years, who had been
occupationally exposed to selenium for 3 - 16 years and who
manifested the symptoms of chronic selenium and selenium dioxide
poisoning. Ten of the 12 persons had been previously engaged in
enterprises producing selenium rectifiers and were exposed to both
elementary selenium and selenium dioxide. The patients complained
of pain in the right hypochondrium, dyspeptic phenomena, undue
fatigability, dyspnoea, weakness, and sleeplessness. Several
patients complained of cough and, in 3 of them, chronic bronchitis
or moderate emphysaema were established. One patient had asthmatic
bronchitis. Almost all patients had various impairments of the
liver and gastrointestinal tract (toxic hepatitis in 6 patients,
dyskinesis of gallbladder in 4 patients, cholecystis in 4 patients,
and spastic colitis in 4 patients). The patients showed an
astheno-vegetative syndrome, pigmentation of exposed areas of the
skin, and, in 8 persons signs of a hyperfunction of the thyroid
gland (radioiodine uptake measurements) were found. Elevation of
the basal metabolic rate or hyperfunction of the thyroid gland were
mentioned also briefly in a previous case report (Halter, 1938) and
in a review (Holstein, 1951), respectively.
A group of selenium workers in a rectifier factory was followed
by Glover (1967, 1970) in 1953 - 56. Every 3 months, urinary-
selenium levels were determined, and the workers were asked about
their health and examined for the presence of garlicky-smelling
breath and skin rashes. Other routine medical checks were not
performed. Workers complained of indigestion and epigastric pain;
severe haematemesis (with hospitalization) occurred in one case.
Several men noticed symptoms of lassitude and irritability, when on
selenium work, which cleared whenever they were taken away from
selenium. A strong odour of garlic on the breath was detected in
most workers with selenium-urinary levels of 0.5 - 1.0 mg litre,
but not below these levels. The breath odour disappeared in 7 - 10
days following the removal of workers from contact with selenium.
Glover (1967, 1970) was able to trace 17 deaths, occurring within
10 years, among selenium-exposed workers in the same factory (Table
52). The author recognized that the list of deaths was not
complete and that the group was too small to permit far-reaching
conclusions. The length of exposure to selenium in individual
cases was not given. Carcinomas arose in various organs: 2
bronchus, 1 stomach, 1 colon, 1 ovary, and 1 testis.
Table 52. Comparison of the certified causes of death of
seventeen selenium workers with the expected distribution
by cause based on the experience throughout England and
Wales
----------------------------------------------------------
International Cause of death Observed Expectedb
list number
----------------------------------------------------------
001 - 019 tuberculosis 0 0.4
140 - 205 malignant neoplasms 6 5.1
330 - 334 vascular lesions 0 1.4
410 - 416 chronic rheumatic 2 0.6
heart disease
420 - 422 arteriosclerotic 3 3.8
heart disease
----------------------------------------------------------
Table 52. (contd.)
----------------------------------------------------------
International Cause of death Observed Expectedb
list number
----------------------------------------------------------
430 - 434 "other" heart disease 0 0.2
440 - 443 hypertensive heart 0 0.2
disease
444 - 447 "other" hypertensive 1 0.2
disease
450 - 456 disease of arteries 0 0.2
480 - 483 influenza 0 0.1
490 - 493 pneumonia 0 0.5
500 - 502 bronchitis 2 0.9
- all other causes 3 3.5
- all causes 17 17.1
----------------------------------------------------------
a From: Glover (1967).
b Taking into consideration the age and sex of those who
died, and the year of death. The Table shows no
evidence of excess mortality in the selenium workers
from any of these main groups of causes.
8.1.2.1.2. Hydrogen selenide
(a) Short-term exposure
There are few cases of occupational poisoning described in
which hydrogen selenide was identified as the sole causative agent.
Reported cases of occupational intoxication by hydrogen selenide
resulted usually from short-term accidental exposures in the
chemical laboratory or industry. Apparently, the first account of
an individual requiring hospital treatment after sudden exposure
to this gas was by Senf (1941), describing the effects of the
accidental laboratory exposure of a chemist to hydrogen selenide.
The patient noticed the characteristic smell and the gas caused her
eyes to weep and her nose to run. Within a few hours, her voice
became hoarse and increasing dyspnoea caused her admission to
hospital. On examination she was found to have a bluish-red
erythema of the skin, conjunctivitis, and injection of the nasal
mucous membrane. The lung oedema, ECG myocardial changes, the dark
red erythema, and porphyrinuria disappeared within 10 days. In
1941, Painter described a case of a single inhalation of hydrogen
selenide. After a brief "metallic" sensation, no ill effects were
felt for about 4 h. Then a copious discharge from the nasal
passages began. This persisted with violent sneezing for 3 or 4
days. No ill effects were noted later.
A case of pure hydrogen selenide poisoning in a works chemist,
producing hydrogen selenide in order to form selenides, was
described by Symanski (1950). Apparently only one inhalation of
the gas was followed by a sudden feeling of constriction in the
chest with coughing, tears, and burning of the nose, all of which
disappeared in a few moments. The patient thought that he had lost
his sense of smell. Four or 5 h later, a severe cough and dyspnoea
developed. At medical examination (8 h after the accident) he was
dyspnoeic and febrile. He continued to cough up blood-stained
frothy sputum all night, and by morning the acute symptoms had
disappeared leaving him apparently with bronchitis and an
irritating cough. A week later he had a recurrence of the fever
with chest pain and the signs of bronchopneumonia. After 4 more
days, the fever disappeared and 9 weeks later he was fit and well
again. A man, 5 h after cleaning out apparatus used for making
hydrogen selenide, developed severe dyspnoea, difficulty in
expiration, painful cough, and yellowish sputum (Bonard & Koralink,
1958). His condition became worse and the next day he was admitted
to the hospital. He was cyanosed, had severe expiratory dyspnoea,
discrete rhinitis and conjunctivitis, and was in considerable
distress. He had no headache or visual troubles. His pharynx was
hyperaemic, his buccal mucosa was dry, and he had a furred tongue.
Four days later, the chest roentgenogram was normal, but lung
function tests were depressed. Rohmer et al. (1950) reported the
case of a chemist exposed to a high level of hydrogen selenide,
calling attention to the development of severe hyperglycaemia that
could only be controlled by increasingly large doses of insulin. A
24-year-old white man accidentally inhaled hydrogen selenide while
transferring the gas from one cylinder to another (Schecter et al.,
1980). He immediately experienced burning in his eyes and throat
followed by coughing and wheezing. He was given oxygen and
improved over a period of 2 h. However, 18 h later, because of
recurrent cough and progressive dyspnoea, he was hospitalized.
Pneumomediastinum developed in this patient and pulmonary function
tests revealed restrictive and obstructive airways disease, which
slowly improved.
Glover (1970), who reviewed 8 cases of acute hydrogen selenide
poisoning (5 laboratory workers and 3 from industrial accidents;
some of them probably identical with those mentioned above), and
summarized the following sequence of events. The first effects
observed after exposure are signs of irritation of the mucous
membranes, i.e., running nose, running eyes, cough, sneeze,
followed by a slight tightness of the chest. This clears, and
there may be a latent period of 6 - 8 h. The patient, who has
often returned home by this time, usually wakes up in bed
breathless with signs and symptoms of pulmonary oedema. Glover, at
the same time, underlined the importance of oxygen therapy in these
intoxications, particularly with regard to the prevention of the
development of pulmonary oedema.
Lazarev & Gadaskina (1977) referred to 5 cases of hydrogen
selenide intoxication at an exposure level of approximately
7 mg/m3. These cases manifested nausea, vomiting, vertigo, and
extreme tiredness, in addition to effects on the respiratory tract
and conjunctiva. The same author mentioned one case of laboratory
exposure to hydrogen selenide in which a chemist developed lung
oedema and long-lasting cyanosis with respiratory difficulties. On
the 22nd day, thrombophlebitis was observed and within 52 days
signs of myocardial damage were noted.
(b) Prolonged and/or repeated exposure
An important point when considering prolonged low-level
exposure to hydrogen selenide was recognized by Dudley & Miller
(1941). In their experience, acute exposure levels of 5 µg/litre
resulted in such eye and nose irritation in workers that the men
could not continue their duties without the protection of a gas
mask. At 1 µg/litre, this irritation did not occur for several
minutes, though the presence of the gas was detected by its odour.
However, continued exposure at these levels results in olfactory
fatigue so that workers lose their ability to smell the gas and are
thus disarmed against subsequent increases in levels of the gas in
the work-place.
The effects of prolonged exposure to hydrogen selenide were
described by Buchan (1947) in workers who were engaged in a process
involving the etching of a pattern on steel stripping. In this
operation, steel stripping is passed between rubber imprinting
wheels with a raised pattern on the periphery. The pattern is in
constant contact with a wick immersed in the etching ink. After
imprinting, the steel stripping passes between felt wipers and dips
into a rust-preventive oil bath passing then to a reel. The odour
of hydrogen selenide was detectable at the etching machine and was
particularly offensive in the sludge of the oil bath. Selenium was
identified, qualitatively, in the sludge. Apparently, the hydrogen
selenide was generated as a result of the reaction of the excess
selenium deposited on the steel stripping in an acid medium, i.e.,
the oil bath, which was further acidified by contamination with the
etching ink carried by the steel stripping. Five out of 25 workers
complained of nausea, vomiting, metallic taste in the mouth,
dizziness, extreme lassitude, and fatigability. The symptoms
lasted 2 weeks and were said to be increasing in severity. Until
one month before the onset of symptoms, an etching ink containing
nitric acid and silver was used, but then a new ink containing
selenious acid at 52 mg selenium/litre was substituted.
Air samples were taken at 6 representative sampling points.
During the analysis, qualitative detection of selenium was made,
but, because of the lack of sensitivity of the titrimetric method
used, it was not possible to measure the selenium quantitatively,
and the results were recorded as less than 0.2 parts per million.
Spot and 24-h specimens of workers' urine contained 0 - 131 µg
selenium/litre or an average of 61.6 µg/litre. So-called control
specimens contained similar amounts, but the controls were the
professional staff collecting the air samples and they had been
exposed to the same environment as the workers for almost 5 h.
Also, it should be realized that hydrogen selenide produces
symptoms at exposures too low to increase the urinary excretion
above normal values. When the selenium ink was replaced by silver
ink, there was a gradual regression of symptoms and, within 6
months, all complaints had ceased and there was no recurrence.
The Task Group was not aware of any other reports dealing with
the prolonged exposure of human beings to hydrogen selenide.
8.1.2.1.3. Selenium oxychloride
Less than 5 µg of pure selenium oxychloride on the skin of the
hand resulted in a painful reaction within a few minutes, exythema
surrounding the central necrotic area (Dudley, 1938). Within 1
month, the skin defect healed with a scar.
8.2. Low Selenium Intake
8.2.1. Evidence supporting the possible essentiality of selenium in
man
Beneficial nutritional effects of selenium have been observed
in several different species (Schwarz, 1976), and specific signs of
selenium deficiency in the presence of adequate intake of vitamin E
have been demonstrated in rats and chicks (section 7.2.1). Thus,
the question of whether selenium is essential for man arises. The
identification of any human disease due to low selenium intake is
difficult, because selenium deficiency in animals is characterized
by a wide variety of signs involving several different organ
systems.
Although a clear-cut pathological condition attributable to
selenium deficiency alone has not yet been demonstrated in human
beings, certain evidence suggests that selenium may be essential
for man.
For example, purified glutathione peroxidase, from human red
blood cells, contains quantities of selenium similar to those found
in the enzyme isolated from animals (Awasthi et al., 1975).
Moreover, selenium is necessary for the optimal growth of human
fibroblasts in purified cell culture media (McKeehan et al., 1976).
Blood-selenium levels are depressed in children suffering from
kwashiorkor (Burk et al., 1967; Levine & Olson, 1970) and Hopkins &
Majaj (1967) obtained a reticulocyte response in malnourished
infants treated with a physiological dose of selenium (25 µg
selenium, as sodium selenite). Finally, on the basis of the
distribution of selenium in various human tissues, Liebscher &
Smith (1968) concluded that selenium must be an essential element
for man.
8.2.2. Signs and symptoms of low intake
The greatest possibility of a hazard due to inadequate selenium
intake would be expected in low-selenium areas. Nutritionists and
public health officials in several countries are aware of the low-
selenium status of their populations and are attempting to identify
any human health problems associated with it. For instance, in
the south island of New Zealand, one of the first regions where a
low selenium status in animals was recognized, some farmers, noting
supposed similarities between their own symptoms and those of the
white muscle disease affecting their livestock, claimed improvement
in their muscular complaints after self-medication with selenium
(Hickey, 1968). However, such anecdotal reports are not supported
by the results of 3 separate trials involving a total of 120
patients suffering from "muscular complaints", carried out in
several low-selenium areas of the south island of New Zealand
(Robinson et al., 198l). In these studies, the patients were given
either sodium selenite or selenomethionine at a number of dose
levels and schedules for various periods of time. Some subjects
also received vitamin E with the selenium supplement. In all
subjects who received selenium, blood-selenium levels and
glutathione peroxidase activity increased, whereas little change
was seen in the control group. Clinical assessment of muscular
symptoms showed that approximately equal numbers of patients in the
test and control groups exhibited an improvement in their muscular
condition. On the basis of these results, the authors concluded
that there was no evidence of any response, under the conditions of
the trials, to selenium supplements for the relief of muscular
complaints.
Total parenteral nutrition (TPN) fluids for intravenous feeding
contain very low levels of selenium (section 4.1.1.1), and patients
sustained by such techniques would seem to be at risk of developing
selenium deficiency. One such patient on TPN in New Zealand
developed muscular discomfort that disappeared after selenium
supplementation (van Rij et al., 1979). This patient was a 37-
year-old female who lived in a rural area of the south island of
New Zealand where the soils were low in selenium and there was a
history of endemic white muscle disease in sheep. She presented
with a perforated small intestine with peritonitis, after
radiotherapy 2 years previously for carcinoma of the cervix. Five
days after abdominal exploration and the start of intensive
antibiotic therapy she developed enterocutaneous and vaginal
fistulae. Gastric stress ulcer followed and necessitated a 1.5-
litre blood transfusion. Elevated temperatures persisted with
intraabdominal sepsis. Ten days after admission to hospital, TPN
was begun and resulted in a general improvement and a 6 kg weight
gain over the next 20 days. Regular plasma and albumin infusions
treated hypoproteinemia. After 20 days of TPN, early clinical
signs of fatty acid deficiency (dry flaky skin on hands and feet)
were noted, which responded rapidly to intralipid infusion. After
30 days of TPN, the patient complained of increasing bilateral
muscular discomfort in her quadriceps and hamstring muscles.
Muscle pain was present at rest as a persistent ache and with
tenderness on palpation. The muscle pain was aggravated by walking
until she found it distressing, even to move beyond her room. On
examination, there was tenderness of the quadriceps, hamstrings,
and less markedly of the calf muscles of both legs. Both active
and passive movements of these muscle groups were painful,
particularly of the hamstrings. The upper limb girdle was
unaffected. A generalized muscle wasting of all the limbs was
observed after the prolonged catabolic stress, despite TPN. No
muscle fasciculation or neurological deficits were observed.
Supplementation with selenium was begun with no other
modification to the patient's management. Each day 100 µg of
selenium, as selenomethionine, was infused intravenously with the
TPN solution. During the next week, muscle pain at rest,
tenderness to palpation, and pain on active and passive movement
disappeared. A return to full mobility followed. This symptomatic
response associated with selenium supplementation plus the
extremely low blood-selenium levels initially observed in this
patient (discussed further in section 8.2.4) led the authors to
conclude that this case could be the first clinical report
supporting the essential role of selenium in human nutrition.
Because of their increased metabolic requirements and faster
growth rates, infants and children might be particularly vulnerable
to selenium deficiency. McKenzie et al. (1978) analysed blood from
230 healthy adults and 83 healthy children from various areas of
New Zealand and found that children in Auckland and Tapanui had
lower blood-selenium concentrations (0.064 and 0.048 mg/litre) than
adults from the same areas (0.083 and 0.060 mg/litre). Red-blood-
cell glutathione peroxidase activity was also lower in Auckland
children than in adults (10.6 versus 12.9 units/g haemoglobin). A
specific population of infants and children that might be
especially at risk regarding low selenium intake includes those who
suffer from phenylketonuria (PKU) and maple syrup urine disease
(MSUD) and consume only special synthetic diets that are very low
in selenium (section 4). McKenzie et al. (1978) reported that the
mean blood-selenium level in 12 such children was 0.038 ± 0.013
mg/litre. One 13-year-old patient had 0.016 mg selenium/litre
whole blood and 0.009 mg selenium/litre plasma, but clinical
examination indicated that he was in good health. Lombeck et al.
(1978) found that the serum-selenium content in 36 children
receiving diet therapy for PKU and MSUD in the Federal Republic of
Germany ranged from 0.007 - 0.028 mg/litre. The selenium content
of the hair was lower in the patients (0.062 mg/kg) than in healthy
children (0.429 mg/kg) and the erythrocyte glutathione peroxidase
activity was reduced in comparison with normal values (4.6 versus
8.8 units/g haemoglobin). And yet all the patients thrived during
the time of observation and did not exhibit any increased rate of
haemolysis or oxidation of haemoglobin to methaemoglobin after
incubation of their erythrocytes with sodium azide.
Gross (1976) studied 4 groups of premature infants who were fed
4 different formulae based on cow's milk containing a high
concentration of polyunsaturated fatty acid (PUFA), with and
without iron, or a low PUFA concentration, with and without iron.
The tocopherol content was the same in all 4 formulae and was
judged adequate in terms of maintaining serum-vitamin E levels.
Both glutathione peroxidase activity and plasma-selenium levels
were similar in all 4 groups. The former declined from 4.2 units/g
haemoglobin at one week of age to 2.7 units/g haemoglobin at 7
weeks of age, whereas the latter declined from 0.08 to 0.035
mg/litre. Although all infants exhibited the anaemia typical of
the prematurely newborn, the decreases in haemoglobin and increases
in reticulocyte levels were greatest in the group of infants given
the formula high in PUFA and iron. These haemolytic events in
vitamin E-sufficient premature infants fed a diet rich in PUFA and
iron were thought to be due to the oxidative stress of the formula
coupled with the poor nutritional status of the infants with regard
to selenium (Gross, 1976).
8.2.3. Dietary levels consistent with good nutrition
8.2.3.1. Quantitative estimates
Since no clear-cut pathological condition attributable to
selenium deficiency alone has yet been observed in man, it is not
possible to define a precise dietary requirement level for human
beings. However, 0.1 - 0.2 mg selenium/kg diet is a nutritionally
generous level for most species of animals (US NAS/NRC, 1971). If
these animal data are extrapolated, a 70-kg man consuming 500 g of
diet per day (dry basis), would need a daily intake of 50 - 100 µg.
The US National Research Council has estimated that the safe and
adequate range of the daily intake of selenium for adults is 50 -
200 µg, with correspondingly lower intakes for infants and children
(Table 53). On this basis, the recommended intake for a 70-kg man
would be equivalent to 0.7 - 2.8 µg/kg body weight per day. Any
daily intake within the recommended range is considered adequate
and safe, but the recommendations do not imply that intakes at the
upper limit of the range are more desirable or beneficial than
those at the lower limit. The lower recommended intake for infants
and children is consistent with the observation that
supplementation of malnourished children with sodium selenite at
30 µg selenium daily, produced weight gain and reticulocyte
responses without any untoward signs (Hopkins & Majaj, 1967).
Table 53. Estimated safe and
adequate range of selenium intakea
-----------------------------------
Group Age Daily selenium
(years) intake (µg)
-----------------------------------
Infants 0 - 0.5 10 - 40
0.5 - 1 20 - 60
Children 1 - 3 20 - 80
4 - 6 30 - 120
7+ 50 - 200
Adults 50 - 200
-----------------------------------
a Adapted from: US NAS/NRC (1980).
Three approaches have now been used to estimate human
nutritional requirements for selenium. Nutritionists have long
used metabolic balance studies to determine the human requirements
for a variety of minerals. In the case of selenium, healthy North
American men needed about 80 µg dietary selenium/day to maintain
balance, but women needed only 57 µg/day (Levander & Morris, 1984).
The difference between men and women in the selenium intake needed
to achieve balance was considered to be due to differences in body
weight. Expressing the balance data on a body weight basis
revealed that both men and women needed about 1 µg selenium per kg
body weight per day to stay in balance. However, other research
groups showed that selenium balance in New Zealand women or Chinese
men could be reached on intakes as low as 27 and 9 µg/day,
respectively (Stewart et al., 1978; Luo et al., 1985). This
demonstrates the great effect of prior dietary-selenium intake on
the amount of selenium needed to achieve balance in people and
shows that balance studies may not be valid techniques for
estimating human selenium requirements.
Yang et al. (1985) estimated human selenium requirements on the
basis of a comparison of dietary intakes in areas with and without
Keshan disease. Dietary-selenium intakes of 7.7 and 6.6 µg/day in
endemic and 19.1 and 13.3 µg/day in non-endemic Keshan disease
areas were reported for adult men and women, respectively. These
estimates should be considered minimum daily adult requirements for
selenium.
The depletion/repletion study is another approach used by
nutritionists to estimate human selenium requirements. The
relatively large body pool of selenium in North Americans prevented
their plasma-selenium levels from dropping to values commonly found
in persons from low-selenium areas (Finland, New Zealand), even
when depleted for almost 7 weeks (Levander et al., 1981a,b).
Chinese men of naturally-low-selenium status (dietary intake about
10 µg/day) were given graded supplements of selenomethionine and
their plasma-glutathione peroxidase activity was followed (Yang et
al., 1985). The activity of the enzyme plateaued at the same level
in all men receiving 30 µg or more of supplementary selenium daily.
From this study, a physiological selenium requirement of about 40
µg/day (diet plus supplement) was suggested for Chinese adult
males, which may require adjustment to account for body weight
differences in other populations including women.
8.2.3.2. Nutritional bioavailability
None of the studies discussed in section 8.2.3.1 have
specifically addressed the question concerning the nutritional
bioavailability of the selenium in foods for human beings. Using
an animal model, Douglass et al. (1981) found that the selenium in
freeze-dried, water-packed canned tuna for human consumption was
only 57% as effective as selenite in restoring liver glutathione
peroxidase activity in rats previously deficient in selenium,
whereas selenium as cooked freeze-dried beef kidney or seleniferous
wheat had 97 and 83% of the activity of selenite, respectively.
The selenium in the tuna was also less effective than selenite in
raising hepatic-selenium levels in the deficient rats. Similar
results concerning the relative bioavailability of the selenium in
tuna compared with wheat were obtained by Alexander et al. (1983)
in rats, using the slope-ratio technique. On the other hand,
Chansler et al. (1983) found that the selenium in mushrooms was
only about 4% as available as that in selenite for restoring
hepatic glutathione peroxidase activity in selenium-depleted rats.
It has been pointed out that, in addition to absorption and
retention, factors such as utilizability within the body are
apparently important in determining selenium bioavailability
(Levander, 1983).
Although data are accumulating on the absorption by human
beings of the selenium in various compounds or in foods (section
6.1.1.2), there have been few studies examining the bioavailability
(i.e., utilization, consisting of transport, conversion to a
metabolically-active form, retention, etc., in addition to
absorption) of the selenium in foods for human beings. One such
study was carried out in a low-selenium area of central Finland
(Levander et al., 1983). Three groups of 10 men of low-selenium
status (mean plasma-selenium level of 70 µg/litre) were
supplemented with 200 µg of selenium, daily, as selenium-rich
wheat, selenium-rich yeast, or sodium selenate, for 11 weeks.
Twenty unsupplemented subjects served as controls. Plasma-selenium
levels increased steadily in the wheat and yeast groups for 11
weeks to around 160 µg/litre with no sign of plateauing, whereas,
in the selenate group, plasma-selenium plateaued at about 110
µg/litre, after 4 weeks. Red blood cell-selenium levels also
increased steadily in the wheat and yeast groups for 11 weeks from
90 to 190 µg/litre, again with no sign of plateauing. Red blood
cell-selenium levels were unaffected in the selenate group.
Platelet glutathione peroxidase activity (glutathione peroxidase is
an index of selenium status) (section 7.2.4.4) roughly doubled
after 4 weeks of supplementation with wheat or selenate and then
plateaued. Platelet glutathione peroxidase increased more slowly
in the yeast group. Plasma-glutathione peroxidase activity did not
respond to selenium supplementation. Ten weeks after the
supplements were stopped, platelet glutathione peroxidase remained
higher in the wheat and yeast groups than in the selenate group.
This suggested that the selenium in yeast or wheat was, to some
extent, deposited in the tissues in a form that could be used later
for glutathione peroxidase biosynthesis, once the dietary
supplement was discontinued. The results of this bioavailability
trial indicated that there are several different aspects to the
nutritional availability of selenium. Complete assessment may
require several measurements including: short-term platelet
glutathione peroxidase activity, to estimate immediate
availability; medium-term plasma-selenium levels, to determine
retention; and long-term platelet glutathione peroxidase activity,
after discontinuation of supplements, to estimate the
convertibility of tissue-selenium stores to metabolically active
selenium.
Griffiths & Thomson (1974) also noted that the blood-selenium
levels of adults from the USA declined rapidly on arrival in New
Zealand, but, after a year, their levels were still higher than the
mean value for permanent New Zealand residents (Fig. 11). When
selenium levels were measured in the whole blood, erythrocytes, and
plasma of postoperative surgical patients receiving TPN in New
Zealand, selenium concentrations decreased as TPN was continued
(Table 54) (van Rij et al., 1979). The plasma-selenium
concentration of the New Zealand TPN patient, discussed in section
8.2.2, was 0.025 mg/litre and fell to 0.009 mg/litre just before
selenium supplementation was begun. The lowest value for blood-
selenium concentration in areas of China not affected by Keshan
disease was around 0.040 mg/litre, while, in affected areas, the
blood-selenium concentration often dropped below 0.010 mg/litre
(Keshan Disease Research Group, 1979b).
Under certain conditions, some investigators have not found a
significant correlation between blood-selenium levels and blood
glutathione peroxidase activity. For example, Thompson et al.
(1976) described swine that had high blood-selenium levels but low
blood-glutathione peroxidase activities. The reasons for this
discrepancy are not known but could perhaps be related to an
increased level of "non-functional" selenium in the blood, such as
selenomethionine that has been nonspecifically incorporated into
protein as a substitute for methionine.
7.2.4.4. Glutathione peroxidase as an indicator of selenium status
As discussed by Hoekstra (1975b), the measurement of
glutathione peroxidase activity offers several advantages as an
indicator of selenium status. First of all, the enzyme assay is
easier to perform and is less time consuming than the selenium
analysis. Also, the enzyme assay is not subject to contamination
problems. Moreover, glutathione peroxidase represents only
"functional" selenium in the tissues, not selenium that has been
non-specifically incorporated into proteins or that has formed
biologically inactive complexes with heavy metals. Finally, at
least in some tissues, the activity of the enzyme appears to taper
off as the dietary level of selenium approaches the nutritional
requirement of the animal. This "plateau effect" of glutathione
peroxidase activity does not occur in red blood cells, a biological
material that is highly convenient for sampling purposes. However,
as pointed out by Hoekstra (1975b), the absence of plateauing in
red cells may limit the usefulness of erythrocyte-glutathione
peroxidase in accurately establishing selenium requirements, but
does not diminish the usefulness of this source of the enzyme as an
overall index of selenium status.
One complicating factor in the use of glutathione peroxidase to
assess selenium status is the fact that the activity of the enzyme
is influenced by many physiological variables other than selenium
intake (Ganther et al., 1976). Among these are the age and sex of
the animal, starvation, exposure to certain oxidant stressors,
toxicants, or heavy metals, and deficiency in iron and vitamin B12.
Obviously, these variables have to be controlled, or compensated
for, if glutathione peroxidase is to be a valid index of selenium
status.
Another serious complicating factor in the use of glutathione
peroxidase to assess selenium status is the existence of a "non
selenium-dependent" glutathione peroxidase activity that persists,
even in severe selenium deficiency (Lawrence & Burk, 1976). This
enzymic activity has been found in variable amounts in different
rat tissues ranging from 23% of the total glutathione peroxidase
activity in fat to 91% in testis (Lawrence & Burk, 1978). The "non
selenium-dependent" activity accounts for 26 - 38% of the total
glutathione peroxidase activity in rat brain, kidneys, liver, and
adrenals. No such activity is found in rat erythrocytes, spleen,
heart, thymus, intestinal mucosum, skin, or skeletal muscle. The
"non selenium-dependent" glutathione peroxidase activity has been
found in the liver of several different species including the rat,
hamster, guinea-pig, chicken, pig, sheep, and man. In human and
guinea-pig liver, it accounts for the major part of the total
glutathione peroxidase activity. The "non selenium-dependent"
glutathione peroxidase activity differs from the selenium-
containing enzyme in substrate specificity in that it catalyses the
reduction of a range of organic hydroperoxides but not of hydrogen
peroxide. The activity of the "non selenium-dependent" enzyme is
inversely related in rat liver to the dietary level of selenium
(Lawrence & Burk, 1978; Lawrence et al., 1978) and the glutathione-
S-transferase enzymes are responsible for the "non selenium-
dependent" enzyme activity (Prohaska & Ganther, 1977; Lawrence et
al., 1978). The glutathione- S-transferases are not selenium-
containing enzymes; they are all dimeric, and their monomeric
constituents fall into three categories of different relative
molecular mass. The glutathione peroxidase activity of the enzymes
is only shown by the dimeric enzymes that contain one of the three
subunits and, in selenium deficiency, the amount of this subunit
alone is increased (Ketterer et al., 1982; Mehlert & Diplock,
1985).
The physiological significance of the "non selenium-dependent"
activity has been questioned because the seleno-enzyme has a much
higher Vmax and lower KM (Prohaska & Ganther, 1977). However, Burk
et al. (1980a) showed that the "non selenium-dependent enzyme"
removed organic hydroperoxides in intact rat livers perfused with a
haemoglobin-free medium containing high concentrations of
peroxides.
Obviously, any use of glutathione peroxidase activity to
monitor selenium status has to take into account this "non
selenium-dependent" activity. This complication can be avoided by
selecting a tissue for test that has little or none of the
selenium-independent activity, such as erythrocytes, or by using
hydrogen peroxide as the substrate, since this has little activity
with the selenium-independent enzyme.
7.2.5. Other possible physiological roles
7.2.5.1. Homeostasis of hepatic haem
Burk et al. (1974) showed that the induction of hepatic
cytochrome P-450 by daily intraperitoneal injections of 75 mg
phenobarbital/kg body weight, for 4 days, was markedly impaired in
rats that had been fed a selenium-deficient diet for at least 3
months. The induction of ethylmorphine demethylase activity by
phenobarbital was also impaired in the livers of selenium-deficient
rats. However, no such impairment was observed in the induction of
cytochrome b5 or of NADPH-cytochrome c reductase activity or
biphenyl hydroxylase activity, or in pentobarbital sleeping time
(Burk & Masters, 1975). Treatment with phenobarbital stimulated a
6- to 8-fold increase in hepatic microsomal haem oxygenase activity
in selenium-deficient rats but had little or no effect in selenium-
supplemented rats (Correia & Burk, 1976). This suggested that the
abnormalities of cytochrome P-450 induction in selenium-deficient
rats were related to increased degradation rather than decreased
synthesis of hepatic haem. Mackinnon & Simon (1976) reported an
impaired haem synthesis in selenium-deficient phenobarbital-treated
rats, but Burk & Correia (1977) were unable to confirm these
results. Apparently, the pronounced stimulation of microsomal
haem oxygenase (EC 1.14.99.3) in the livers of selenium-deficient
rats by phenobarbital is unrelated to the role of selenium in
glutathione peroxidase, since pretreatment of deficient rats with
a single dose of selenite at 50 mg selenium/rat, 4 - 6 h before
phenobarbital administration, abolished the response in haem
oxygenase but had no effect on cytosolic or mitochondrial
glutathione peroxidase activity (Correia & Burk, 1978). Moreover,
phenobarbital failed to stimulate liver microsomal haem oxygenase
activity or to affect cytochrome P-450 in vitamin E-deficient,
selenium-adequate rats, so this response does not seem to be
related to the direct effects of lipid peroxidation on haem or
cytochrome P-450. There is a marked sex difference in the
phenobarbital stimulation of hepatic microsomal haem oxygenase
activity in selenium-deficient rats. The stimulation was greatest
in males and least in females with intermediate values in castrated
males and testosterone-treated females (Burk et al., 1980a). This
sex difference existed even though hepatic glutathione peroxidase
activity was reduced to the same extent by selenium deficiency in
all groups. However, the exact mechanism by which selenium
influences homeostasis of hepatic haem still needs to be clarified.
Pascoe et al. (1983) showed that both intraluminal iron and
selenium were required by the intestinal mucosa for maintenance of
the intestinal cytochrome P-450 content, and its dependent mixed
function oxidase activity, and that withdrawal of dietary selenium
for one day markedly decreased the level of cytochrome P-450 in the
small intestine, suggesting that intestinal mucosal cells derive
their selenium from the diet rather than from the blood.
A report by Maines & Kappas (1976) indicated that injection of
selenium increased hepatic haem oxygenase activity, but the doses
of selenium used (3.94 mg/kg body weight) exceeded the MLD for
selenium as selenite (Table 19), so that the phenomenon observed by
these authors may have been merely an artifact of acute selenium
poisoning.
7.2.5.2. Microsomal and mitochondrial electron transport
Diplock and co-workers carried out an extensive series of
studies, the results of which suggest a possible role for selenium
and vitamin E in the electron-transfer system of rat-liver
microsomes (Diplock, 1974a,b). First, they showed that appreciable
amounts of volatile selenium evolved from rat liver subcellular
organelles, after acid treatment, only when the rats were adequate
in vitamin E and the tissue homogenization medium contained large
concentrations of anti-oxidants (Diplock et al., 1971). The acid-
volatile selenium was later identified as selenide (Diplock et al.,
1973). It was assumed that this compound was formed in vivo,
presumably by the glutathione reductase pathway (Fig. 9), but
artifactual in vitro formation of such selenide, because of the
presence of mercaptoethanol in the homogenization medium, cannot be
ruled out (Levander, 1976a). An oxidant-labile form of non-haem
iron, dependent on dietary vitamin E and selenium, was observed in
rat liver microsomes (Caygill & Diplock, 1973). It was considered
that this supported the hypothesis that the selenide might form
part of the active centre of a non-haem iron protein functional in
microsomal electron transfer. Alterations in the kinetic
parameters of aminopyrine demethylation by the liver microsomes in
vitamin E-deficient rats also supported this idea (Giasuddin et
al., 1975). However, attempts to isolate an oxidant-labile
selenium- and vitamin E-dependent non-haem, iron-containing protein
fraction were unsuccessful, because of the lability of the
selenide, and it was concluded that clarification of the role of
selenide in microsomes needed additional research (Diplock, 1976,
1979). The effects of prolonged selenium deficiency on a large
number of parameters in mouse liver xenobiotic metabolism were
studied by Reiter & Wendel (1983). They demonstrated a
heterogeneous pattern of effects that did not appear to involve
glutathione peroxidase.
One of the earliest biochemical defects discovered in vitamin
E- and selenium-deficient rats was the inability of liver slices or
homogenates to maintain normal rates of respiration after prolonged
periods of incubation in vitro (Schwarz, 1962). Although this so-
called respiratory decline might be explained on the basis of
generalized mitochondrial membrane damage due to lipid peroxidation
(Yeh & Johnson, 1973), it was also plausible that selenium might
have a more specific role in mitochondrial metabolism. Indeed,
selenium added to the diet, or added in vitro, accelerated the
swelling of rat liver mitochondria induced by certain thiols, and
this swelling could be blocked by the addition of cyanide (Levander
et al., 1973a). Selenium was in fact shown to be an excellent
catalyst for the reduction of cytochrome c by thiols in chemically-
defined systems (Levander et al., 1973b) and a selenoprotein
containing a haem group similar to that of cytochrome c has been
reported in lamb muscle (Whanger et al., 1973). However, more work
is required to establish the possible role of selenium in
mitochondrial electron transfer.
7.2.5.3. The immune response
Dietary vitamin E in excess of nutritionally required levels
has been shown to improve the humoral immune response of several
different species to bacterial and viral antigens (Nockels, 1979).
Because of the close nutritional and biochemical relationship
between selenium and vitamin E (section 7.2.4.1), the effect of
dietary selenium supplementation was tested on the immunological
responses of mice (Spallholz et al., 1973). Mice fed a laboratory
chow diet (thought to contain 0.5 mg selenium/kg) supplemented with
sodium selenite at 0.7 or 2.8 mg selenium/kg had approximately 7-
and 30-fold higher antibody titres, respectively, after challenge
with sheep red blood cells than mice fed the chow diet alone.
Sodium selenite injected into mice intraperitoneally at doses of
3 - 5 µg per animal also increased the primary immune response to
the sheep red-blood-cell antigen and this increase was greatest
when the selenium was given prior to, or simultaneously with, the
antigen (Spallholz et al., 1975). The mechanism by which selenium
stimulates antibody synthesis is not known (Martin & Spallholz,
1976), but the dose of selenium needed to elicit this response is
clearly above the nutritionally required level. Levander (1986)
has reviewed the very limited data on the possible effect of
selenium on the immune response in man.
7.2.5.4. Selenium and vision
The effects of selenium on light perception were studied in 50
male "Grey chinchilla" rabbits weighing 3 - 3.5 kg and maintained
on a standard laboratory diet (Abdullaev et al., 1972, 1974a,b).
Electroretinography was carried out in dark-adapted animals under
conditions of maximally dilated pupil induced by topical
application of 0.5% dikaine - 1% atropine. Light stimulation was
provided with an impulse lamp giving either single or dual flashes
of 150 microseconds. Eight different energy levels of light
intensity were used ranging from 0.016 to 1.4 J. Sodium selenite
was administered subcutaneously at a dose of 0.45 mg selenium/kg
body weight. Control rabbits were injected with sodium sulfite at
a dose of 1 mg/kg. Sodium selenite stimulated light sensitivity,
as judged by increases in the a and b waves of the electro-
retinogram (ERG). The increase in the amplitude of the b wave was
considerably greater than that of the a wave. This stimulating
effect of sodium selenite on light sensitivity persisted for 3 days
in all studies. The same effects were observed when sodium
selenite was given via retrobulbar administration. Sodium sulfite
did not have any effect on the ERG. Similar results were obtained
by Bacharev et al. (1975).
The Task Group recognized that the data on the effects of
selenium on vision were obtained in studies involving high dose
levels (in the case of rabbits, the doses were approximately 20 -
30% of the LD50). The effects on vision of lower doses of selenium
have not been elucidated so far. The possible effect of deficiency
of selenium on vision is also of interest. Model studies with
isolated retinas exposed to a 0.01% concentration of sodium
selenite resulted in an increase in the amplitude of the ERG
(Kulieva et al., 1978). The effect of increased sensitivity of the
eye to light, after administration of sodium selenite, and the rate
of manifestation and duration of the effect are directly
correlated with localization of the substance in the structures of
the eye (section 6.2.2.1). Subcutaneous administration of sodium
selenite to rabbits, at a dose of 0.4 - 2 mg/kg body weight,
inhibited free radical formation as indicated by electron
paramagnetic resonance (EPR) measurements, not only in the lipid
membrane phase but also within the melanoprotein granules of the
retinal epithelium. The EPR signal was decreased in intensity by
selenite treatment in comparison with controls, but the form and
parameters of the EPR lines were not changed (Abdullaev et al.,
1974a,b).
7.2.6. Effects on reproduction
Vitamin E was discovered as a result of the isolation of an
unknown fat-soluble dietary factor essential for reproduction in
rats (Evans & Bishop, 1922), but selenium compounds are not active
in preventing reproductive failure in vitamin E-deficient rats
(Harris et al., 1958). Moreover, female rats weighing 80 - 120 g
and fed a selenium-deficient, but vitamin E-adequate, diet
successfully delivered 3 litters over a period of 6 months,
even though their blood-selenium levels dropped from 0.52 to
0.06 mg/litre during this time (Hurt et al., 1971). Similarly,
McCoy & Weswig (1969) found that rats fed a low-selenium but
adequate-vitamin E diet, for 4 months, reproduced normally.
However, their offspring failed to reproduce if kept on the low
selenium diet. Immobile spermatozoa, with separation of heads from
tails, were observed in 5 out of the 8 male progeny not given
selenium supplements, and no spermatozoa were seen in the other 3
males. The 2 male and 2 female offspring, supplemented with sodium
selenite at 0.1 mg selenium/kg diet, were fertile but delivered
litters of only one or 2 rats, all of which died in a few days.
The authors concluded that selenium supplementation resulted in
some fertilization, but only a few full-term young were delivered
and these were abnormal as they survived for only a brief period.
Wu et al. (1973) found that the motility of spermatozoa from
male rats, born to females fed a selenium-deficient diet, was
always very poor and that most of the sperm cells showed breakage
near the midpiece of the tail. Supplementation of the diet with
high levels of vitamin E or various other antioxidants did not
prevent these selenium deficiency effects. However, in this study,
the body and testicular weights of the rats were so markedly
depressed by selenium deficiency that it was not possible to
establish whether the abnormal sperm were due to a direct effect of
selenium deficiency or to an indirect effect on overall growth and
well-being. In a second series of studies (Wu et al., 1979), using
male rats born to dams fed a diet adequate in selenium, the
depression of growth, and testes weight in the progeny caused by
selenium deficiency was much less, even though impaired sperm
motility and abnormal sperm morphology were still observed (Table
32). The authors also pointed out that there did not appear to be
any correlation between reduction in body or testicular weights and
the characteristic sperm midpiece abnormality among the individual
rats in each treatment group. However, all of the rats used by Wu
and associates were fed diets that were probably low in zinc and
chromium and both of these trace elements are known to play
important roles in sperm formation (Wu et al., 1971; US NAS/NRC,
1979; Anderson & Polansky, 1981).
The mechanism of the effects of selenium on the integrity of
sperm morphology in the rat is not known, but Brown & Burk (1973)
found over 40% of an injected tracer dose of radio-selenite in the
testis-epididymis complex of selenium-deficient rats, after 3
weeks. Autoradiography of epididymal sperm revealed that the
labelled selenium was heavily localized in the midpiece. Calvin
(1978) has isolated a selenopolypeptide, with a relative molecular
mass of 17 000 daltons, from rat sperm, which may have a critical
function in the normal assembly of the sperm tail.
Selenium cannot prevent resorption-gestation in vitamin E-
deficient rats, nor can it improve reproductive performance in
vitamin E-deficient chickens or turkeys (Creger et al., 1960;
Jensen & McGinnis, 1960). However, studies in which severe
depletion of selenium was produced by the long-term feeding of low-
selenium, high-vitamin E diets have shown the beneficial effects of
the element on egg production and hatchability in poultry. For
example, Cantor & Scott (1974) fed 1-day-old Single Comb White
Leghorn chicks semi-purified low-selenium starter, grower, and
developer diets, all of which contained only 0.02 mg naturally-
occurring selenium/kg from 0 to 6, 7 to 12, and 13 to 25 weeks of
age, respectively. Because of the low selenium level, high levels
of vitamin E (50 - 84 IU/kg) were added to the diets to prevent
exudative diathesis during the growth period. From 25 weeks of age
to 10 months, the birds were fed semi-purified low-selenium layer
diets that contained 11 IU of vitamin E/kg. After 10 months, the
hens were fed a low-selenium corn-soybean meal practical laying
ration containing 0.027 mg natural selenium/kg, by analysis. The
calculated vitamin E content was 16 IU/kg. At 12 1/2 months of
age, 4 replicate groups of 5 hens were continued on the low-
selenium diet, whereas a similar number of hens was fed the same
diet supplemented with sodium selenite at 0.1 mg selenium/kg.
Selenium supplementation had beneficial effects on both egg
production and hatchability of fertile eggs (Table 33), but there
was no significant effect of selenium on egg fertility, determined
after 2 - 5 weeks (data not shown).
Table 32. Effects of selenium deficiency on body and testicular weights and
spermatozoan morphology and motility in ratsa
------------------------------------------------------------------------------------
Dietary selenite Number Duration Body Testes Sperm Number of rats
supplement of (month) weight weight motilityb with sperm
(mg selenium/kg) rats (g) (g) midpiece
abnormality
------------------------------------------------------------------------------------
Study 1
0 6 4 452 ± 6 3.29 ± 0.06 6.2 0/6
0.5 6 4 470 ± 18 3.55 ± 0.11 6.2 0/6
0 4c 12 476 ± 17 3.03 ± 0.30 2.5 2/4
0.5 4c 12 494 ± 12 3.57 ± 0.07 5.8 0/4
Study 2
0 10c 11 456 ± 16 3.07 ± 0.09 3.5 5/10
0.4 12 11 543 ± 18 3.50 ± 0.08 8.0 0/12
------------------------------------------------------------------------------------
a Adapted from: Wu et al. (1979).
b Relative activity of sperm observed with a light microscope at 400x at 37 °C and
graded from 0 - 10 with 10 representing the highest motility.
c Two rats died in each of these groups.
Cantor et al. (1978) carried out a similar study on turkeys in
which large white breeder hens and toms were raised from one day of
age until sexual maturity on a series of Torula yeast-containing
diets, low in selenium content (0.025 - 0.047 mg/kg). At 32 weeks
of age, 8 replicate groups, each comprising 6 individually-caged
hens, were fed a low-selenium basal diet (0.033 mg/kg) or the same
diet supplemented with sodium selenite at 0.2 mg selenium/kg.
Eight individually-caged toms were also assigned to each dietary
treatment at 32 weeks of age. Neither the tom nor the hen dietary
treatments had any effect on fertility and the tom dietary
treatment did not have any effects on hatchability. But the
hatchability of fertile eggs from hens fed the unsupplemented and
supplemented diets was about 50 and 59%, respectively. The
mechanism by which dietary selenium improves hatchability in
chickens or turkeys is not known, but the improvement may be due to
increased embryonic selenium levels or to a reduced embryonic need
of vitamin E (Cantor & Scott, 1974).
Table 33. Effect of dietary selenium
supplements on reproductive performance of
12 1/2-month-old selenium-depleted hens fed a
low-selenium, corn-soybean-meal practical
rationa
----------------------------------------------
Selenium supplement None 0.1 mg selenium as
Na2Se03/kg diet
----------------------------------------------
Criterion
Egg production (%)
Weeks 1-2 67.2 76.6
3-4 66.4 78.5
5-6 64.2 71.8
Day 46-76 54.2 79.7
77-107 55.1 76.7
108-137 59.1 75.1
Hatchability of fertile eggs (%)
Week 2 57.3 93.0
3 54.7 97.1
4 54.5 94.9
5 42.4 86.6
17 0.0 90.3
18 8.0 93.6
----------------------------------------------
a Adapted from: Cantor & Scott (1974).
In New Zealand, a close geographical relationship was observed
between the presence of white muscle disease and the incidence of
barren ewes (Table 34). A selenium intervention trial was carried
out on 3 farms where there was a history of white muscle disease
and low lambing percentages (Table 35). The selenium-treated ewes
received 5 mg of selenium, as sodium selenite, at monthly intervals
from one month before tupping until just before lambing; half of
the rams were also given selenium at monthly intervals before and
during tupping (Hartley et al., 1960; Hartley & Grant, 1961).
Selenium-dosed groups on all farms had higher lambing percentages
and fewer barren ewes than the controls. Moreover, white muscle
disease was eliminated in the lambs of the treated ewes. Estrus,
ovulation, fertilization, and early embryonic development all
proceeded normally in selenium-responsive ovine infertility until
between 3 and 4 weeks after conception (at about the time of
implantation) when the embryos perished for unknown reasons
(Hartley, 1963). The actual cause of selenium-responsive
infertility is also unknown and the geographical distribution of
the disease is not always the same as that of other selenium
deficiency diseases, such as white muscle disease or selenium-
responsive unthriftiness (Gardiner et al., 1962). This suggests
either that selenium-responsive infertility occurs only where
intake of selenium is particularly low or that some other factors
must be present that exacerbate the effects of low selenium intake.
For example, mercury has a strong antagonism with selenium (section
7.4.1), and Parizek et al. (1971) showed that inorganic mercury
could block the transport of selenium from mother rats to the
fetuses.
Table 34. Geographical relationship between
congenital white muscle disease and incidence
of barren ewesa
-----------------------------------------------
Congenital Number Incidence of barren ewes
white muscle of up to 11 - 30% over
disease farms 10% 30%
-----------------------------------------------
Present 21 3 8 10
Absent 16 12 4 0
-----------------------------------------------
a Adapted from: Hartley et al. (1960).
Table 35. Effects of selenium (Se) on the reproductive
performance of sheep and white muscle disease in lambsa
-------------------------------------------------------
White muscle
Farm Lambing Barren ewes disease incidence
control Se control Se control Se
-------------------------------------------------------
(%) (%) (%)
C 60.6 94.9 31.1 7.5 37.5 0
D 55.0 86.0 24.3 11.5 22.2 0
E 70.5 93.6 26.1 6.4 12.0 0
-------------------------------------------------------
a Adapted from: Hartley et al. (1960).
7.2.7. Factors influencing deficiency
7.2.7.1. Form of selenium
Schwarz (1961) pointed out that only 0.007 mg naturally-
occurring selenium/kg diet in extracts from pork kidney ("Factor 3
Selenium") was needed to afford 50% protection against dietary
necrotic liver degeneration in rats, whereas 0.020 - 0.030 mg/kg
was required when the selenium was in the form of selenite,
selenate, selenocystine, or selenomethionine. Elemental selenium
was essentially inactive. Many organic derivatives were
synthesized in an attempt to develop selenium compounds that were
not highly toxic but still retained considerable biological potency
against deficiency diseases (Schwarz et al., 1972). Simple amino
acid derivatives of monoseleno diacetic acid appeared promising in
this regard and the hope was expressed that some of these compounds
might make it possible to use selenium in the prevention and cure
of disease (Schwarz, 1976).
Cantor et al. (1975a) studied the ability of selenium in
various poultry feed ingredients to prevent exudative diathesis, a
selenium deficiency disease, in chickens. In general, the selenium
in plant products was more readily available than that in animal
products, but the latter consisted of highly-processed fish or
poultry meals.
Selenium in the form of selenomethionine was less effective
than selenium as sodium selenite for preventing exudative diathesis
in chicks (Noguchi et al., 1973), but the reverse was true for the
prevention of pancreatic fibrosis (Cantor et al., 1975b). In fact,
selenomethionine was four times as effective as either selenite or
selenocystine for this purpose; this phenomenon may have been
related to the peculiar affinity of this compound for the pancreas.
7.2.7.2. Vitamin E
Vitamin E decreases the level of dietary selenium needed to
prevent deficiency diseases in several species of animals. For
example, Scott & Thompson (1971) demonstrated that chicks receiving
100 mg vitamin E/kg diet needed only 0.01 mg selenium/kg diet to
prevent exudative diathesis, whereas chicks not receiving any added
vitamin E needed 0.05 mg/kg. Similarly, Scott et al. (1967) showed
that selenium at 0.18 mg/kg protected against gizzard myopathy in
turkey poults fed diets containing vitamin E, but that 0.28 mg/kg
was needed to protect poults fed diets not supplemented with
vitamin E. Hakkarainen et al. (1978) found that 0.135 mg
selenium/kg was insufficient to prevent deficiency signs in swine
fed a diet containing 1.5 mg vitamin E/kg but was adequate in swine
fed 5.0 mg vitamin E/kg. Increasing the level of dietary vitamin E
decreased the severity of nutritional muscular dystrophy in lambs
fed diets containing selenium at 0.1 mg/kg (Ewan et al., 1968). In
lambs not receiving any supplementary dietary selenium, lambs
receiving low levels of vitamin E died of dystrophy before their
hepatic-selenium levels were depleted to 0.21 mg/kg, the level
considered by Allaway et al. (1966) to be the critical
concentration of selenium in liver for the development of
nutritional muscular dystrophy (section 7.2.3). With the highest
level of vitamin E supplementation, the liver-selenium values were
lower than the critical level suggested by Allaway et al. (1966)
and yet the lambs still survived. These results suggest that the
tissue-selenium level is not the only factor involved, but that the
levels of both tocopherol and selenium determine the appearance of
nutritional muscular dystrophy. The effects of vitamin E in
reducing selenium requirements are readily explainable on the basis
of the close biochemical relationship between these two nutrients
discussed in section 7.2.4.1.
7.2.7.3. Heavy metals and other minerals
Since selenium protects against the toxicity of several heavy
metals (section 7.4), many scientists have examined the effect of
heavy metals on the induction of selenium deficiency. Silver
accelerated the development of liver necrosis in rats (Whanger,
1976) and increased the severity of selenium-vitamin E deficiency
in swine (Van Vleet, 1976; McDowell et al., 1978), presumably by
decreasing the activity of glutathione peroxidase. Inorganic
mercury also decreased glutathione peroxidase activity in the rat
but did not have any effect on the rate of development of liver
necrosis (Whanger, 1976). However, methylmercury accentuated the
development of selenium deficiency in pigs fed a low-selenium diet
(Froseth et al., 1974). In spite of the ability of arsenic to
enhance the biliary excretion of toxic levels of selenium (section
7.1.6.3), several attempts to induce selenium deficiency by feeding
arsenic have been unsuccessful (Levander, 1977; McDowell et al.,
1978). On the other hand, drenching pregnant and lactating ewes
with potassium cyanide, which partially counteracts selenium
toxicity in rats (section 7.1.6.2), increased the incidence of
nutritional myopathy in their lambs (Rudert & Lewis, 1978). It has
been suggested that the use of sulfate fertilizers may decrease
selenium concentrations in forage plants (Pratley & McFarlane,
1974; Westermann & Robbins, 1974), and that this could lead to an
increased incidence of selenium deficiency in farm animals
(Schubert et al., 1961). Dietary sulfate had no effect on the
incidence of white muscle disease but increased the number of lambs
with degenerative lesions of the heart (Whanger et al., 1969).
Neither cadmium nor tellurium had any effect on white muscle
disease in lambs (Whanger et al., 1976b) or vitamin E-selenium
deficiency in swine (McDowell et al., 1978).
7.2.7.4. Xenobiotics
The results of early work by Hove (1948) suggested that vitamin
E could protect rats fed a low-protein diet from death due to
carbon tetrachloride (CCl4) poisoning. The establishment of a
nutritional relationship between selenium and vitamin E stimulated
Seward et al. (1966) to study deficiencies of these nutrients in
relation to poisoning (Table 36). Mortality was higher in the
group deficient in both nutrients than in controls, but selenium
deficiency alone did not increase mortality. Other workers
(Hafeman & Hoekstra, 1977a; Burk & Lane, 1979) have confirmed this
result. The increased mortality due to CCl4 in doubly-deficient
animals could have been due simply to inhibition of eating.
Fasting these animals overnight can precipitate dietary liver
necrosis leading to death (Hafeman & Hoekstra, 1977b).
Table 36. Protective effect of selenium and vitamin E
against carbon tetrachloride toxicity in rats fed a
10% soybean-protein dieta
-----------------------------------------------------
Dietary supplement Weight gain Survival 48 h after
(g/8 weeks) CCl4 injectionb
-----------------------------------------------------
None 62 ± 5 3/10
Vitamin Ec 77 ± 3 9/10
Seleniumd 79 ± 6 8/10
Vitamin E and 80 ± 5 10/10
selenium
-----------------------------------------------------
a Adapted from: Seward et al. (1966).
b All rats injected intraperitoneally with a single
dose at 2 ml/kg body weight.
c Added as a mixture of equal quantities of dl-alpha-
tocopherol and dl-alpha-tocopheryl acetate at 200
mg/kg diet.
d Added at 0.1 mg selenium/kg diet, as sodium selenite.
Paraquat is another xenobiotic that is thought to exert its
toxic effect via increased lipid peroxidation (Bus et al., 1974).
Selenium deficiency worsens lung injury in rats caused by paraquat
(Omaye et al., 1978) and leads to liver injury that would not
otherwise occur in mice exposed to this compound (Cagen & Gibson,
1977). Burk et al. (1980b) showed that selenium-deficient rats
were much more susceptible to paraquat or diquat poisoning than
controls (Table 37). The deficient rats also produced more ethane
and had a higher serum-glutamic-pyruvic transaminase (SGPT)
activity, when treated with paraquat or diquat, than the controls.
The same workers found that injected selenium protected deficient
rats against diquat toxicity before appreciable amounts of
glutathione peroxidase activity appeared in the tissues. They
suggested the existence of a selenium-dependent factor, apart from
glutathione peroxidase which protects against lipid peroxidation.
Combs et al. (1975) noted a similarity between the oedema
produced in chicks fed a practical diet containing polychlorinated
biphenyls (PCBs) and that observed in chicks fed a diet deficient
in selenium and vitamin E (exudative diathesis). When selenium-
depleted hens were fed a diet not containing PCBs, selenite at
0.15 mg selenium/kg diet was sufficient to prevent exudative
diathesis in the chicks, but was not sufficient to prevent the
disease when the hens were fed a diet containing 10 mg PCBs/kg
(Table 38). In another study, addition of 50 mg PCBs/kg diet
increased the incidence of exudative diathesis in vitamin E-
deficient chicks receiving selenite at 0.04 mg selenium/kg diet
from less than 60% in the controls to almost 90% in the PCBs group,
14 days after hatching (Combs & Scott, 1975). Dietary PCBs
depressed plasma-glutathione peroxidase activity and increased the
amount of dietary selenium required to protect liver microsomal
fractions from peroxidation in vitro. On this basis, the authors
concluded that dietary PCBs potentiate vitamin E-selenium
deficiency in chicks by interfering with the biological utilization
of dietary selenium, though some interference with the absorption
and retention of vitamin E was also seen.
Table 37. Effect of selenium deficiency in rats treated with paraquat or
diquata,b
-------------------------------------------------------------------------
Diet Agent Dose Ethane SGPT Survival
group given produced activity time
-------------------------------------------------------------------------
(µmol/kg) (pmol/100 g/h) (mU/ml) (min)
Selenium- paraquat 78 470 ± 93 212 ± 87 297 ± 74
deficient
Control paraquat 78 18 ± 13 32 ± 26 survived 24 h
Control paraquat 390 92 ± 5 45 ± 34 106 ± 30
Selenium- diquat 19.5 1940 ± 420 3490 ± 1940 150 ± 37
deficient
Control diquat 230 56 ± 50 41 ± 5 80 ± 12
-------------------------------------------------------------------------
a Adapted from: Burk et al. (1980b).
b Values are mean ± SD of 4 or 5 values.
Burk & Lane (1979) pointed out the complexity of nutritional
toxicology experimentation involving selenium, vitamin E, and
various drugs and other chemicals. After studying the effects of
numerous xenobiotics on rats that were deficient in either vitamin
E or selenium, they concluded that in vivo lipid peroxidation, as
assessed by ethane production, was not necessarily correlated with
liver necrosis. Moreover, it was concluded that selenium
deficiency was not just a lack of glutathione peroxidase activity
in the tissues, since this condition also elevates hepatic
glutathione S-transferase activity. Thus, selenium deficiency may
actually protect against the hepatotoxicity of certain compounds,
(e.g., acetaminophen and iodipamide) by increasing their
conjugation with glutathione.
Table 38. Effect of PCBs fed to hens on the
incidence of exudative diathesis in progeny fed a
sub-optimal level of seleniuma
------------------------------------------------------
Additions to diets of hensb Exudative Weight gain
-------------------------- diathesis of chicks
PCBsd Seleniume in chicksc (g/2 weeks)
(mg/kg) (mg/kg) (%)
------------------------------------------------------
0 0 35 89
0 0.15 0 102
10 0 30 82
10 0.15 45 78
------------------------------------------------------
a Adapted from: Combs et al. (1975).
b Single Comb White Leghorn hens, 27 weeks old, that
had been reared on low-selenium, semi-purified diets
supplemented with high levels of vitamin E; hens
were on dietary treatments shown in Table for an
additional 5 weeks.
c Determined at 2 weeks of age. Chicks were fed a
selenium-deficient diet (less than 0.02 mg
selenium/kg) supplemented with 0.06 mg selenium, as
Na2Se03/kg. Diet was essentially free of tocopherols.
d Aroclor(R) 1254.
e Added as Na2Se03.
7.2.7.5. Exercise stress
Ancedotal observations from New Zealand suggested that selenium
might be of value against "tying-up" in thoroughbred racehorses
(Hartley & Grant, 1961), a condition that occurs during training
and is associated with exercise. Characteristics include muscle
stiffness and tenderness and disinclination to move. Young &
Keeler (1962) applied mechanical restraint to one foreleg of lambs
born to ewes fed a diet known to produce nutritional muscular
dystrophy and found that lesions either did not occur or occurred
to a much lesser extent in muscles of the restrained limb than in
muscles of the unrestrained limb. Brady et al. (1978) observed
increased levels of malondialdehyde, a product of lipid
peroxidation, in the erythrocytes of 6 horses, immediately after
exercise, but were unable to reproduce this phenomenon in a second
exercise trial with 8 horses. Sodium selenite, given for 4 weeks
in the trace mineral salt at a level calculated to provide 0.15 mg
supplementary selenium/kg diet, did not have any effect on the
elevated plasma-enzyme activities observed in exercised horses.
Furthermore, it did not have any effect on plasma-selenium levels
or erythrocyte-glutathione peroxidase activity. Harthoorn & Young
(1974) commented that the pathological picture in wild animals that
have died following mechanical capture ("capture myopathy" or
"overstraining syndrome") is indistinguishable from white muscle
disease seen in cattle suffering from vitamin E deficiency, but
pointed out that prophylactic and subsequent symptomatic treatment
with vitamin E and selenium-containing preparations did not have
any beneficial effects on captured animals. Brady et al. (1979)
expected to induce death in rats that had been fed a selenium and
vitamin E-deficient diet for 4 weeks, after exercising them to
exhaustion by swimming, but had no success, in spite of markedly
depressed tissue-glutathione peroxidase activity, decreased hepatic
stores of fat-soluble antioxidants, and increased levels of
thiobarbituric acid-reacting substances. The authors concluded
that the rat might be an unsuitable model for an exercise stress-
susceptible species.
7.3. Ratio Between Toxic and Sufficient Exposures
The minimum level of dietary selenium that causes overt signs
of chronic selenium toxicity in most species of animals is of the
order of 4 - 5 mg/kg (US NAS/NRC, 1976). The minimum level of
dietary selenium needed to prevent selenium deficiency diseases in
most species is in the range of 0.02 - 0.05 mg/kg (US NAS/NRC,
1971). Therefore, the ratio of toxic to deficient selenium
exposures is about 100. This difference between the harmful and
beneficial levels of selenium is similar to that between the
harmful and beneficial levels of several nutritionally essential
minerals. However, in the case of selenium, this ratio can be
decreased by certain nutritional or environmental factors. For
example, a deficiency in vitamin E decreases the tolerance of
animals to selenium toxicity (section 7.1.6.2) but increases the
nutritional need for the element (section 7.2.7.2). Also,
inorganic mercury increases the toxicity of methylated selenium
metabolites (section 7.4.1) but methylmercury potentiates selenium
deficiency (section 7.2.7.3). Thus, caution may have to be
exercised in some situations to avoid a diminution in the ratio
between harmful and beneficial intakes of selenium.
7.4. Protection Against Heavy Metal Toxicity
7.4.1. Mercury
Parizek & Ostadalova (1967) first showed that selenium could
protect against the toxicity of mercury in short-term acute
toxicity studies. This led to the suggestion that one of the
nutritional roles of selenium might be protection of the organism
against traces of toxic metals that enter the body, even under
normal conditions (Parizek et al., 1971). Nutritional levels of
dietary selenium have been demonstrated to decrease the chronic
toxicity of the highly hazardous methylmercury (Ganther et al.,
1972) and the selenium in tuna may provide a built-in protective
mechanism against the methylmercury in such fish (Ganther & Sunde,
1974). The possible practical significance of the metabolic
interaction of selenium and mercury has been indicated by the
strong correlations observed between the concentrations of mercury
and selenium in the livers of marine mammals under normal
environmental conditions (Koeman et al., 1975) and in human tissues
following exposure to inorganic mercury (Kosta et al., 1975). The
mechanism by which selenium protects against the toxicity of
methylmercury is not known, but the fact that vitamin E and certain
antioxidants also decrease methylmercury toxicity (Welsh & Soares,
1976; Welsh, 1979) has prompted the hypothesis that these compounds
may diminish methylmercury toxicity by counteracting the damaging
effects of the free radicals generated by its breakdown (Ganther,
1978).
Although inorganic selenium salts protect against the toxicity
of inorganic or methylmercury under a wide variety of conditions,
dimethylselenide has a strong synergistic toxic action with
mercuric chloride (Parizek et al., 1971). The mechanism of this
synergism is unknown.
7.4.2. Cadmium
Parenterally administered selenium protects against the
toxicity of injected doses of cadmium in rats, apparently by
diverting the cadmium from low relative molecular mass target
proteins to other proteins of higher relative molecular mass
(Parizek et al., 1971; Chen et al., 1975). However, selenium does
not cause this diversion when given orally, so any mechanism by
which selenium counteracts cadmium toxicity, under environmental
conditions, is yet to be determined (Whanger, 1976). The
cadmium/selenium antagonism may be important for human health in
that selenium prevents the hypertension caused by long-term cadmium
exposure in rats (Perry et al., 1976).
7.4.3. Other heavy metals
Selenium may interact with lead but the interaction appears to
be much weaker than that with mercury or cadmium, and vitamin E
seems to be more important than selenium in determining the effects
of lead poisoning (Levander, 1979). The interaction of selenium
and silver is of theoretical interest since glutathione peroxidase
activity is depressed by silver (Wagner et al., 1975), but this
interaction does not appear to have any practical significance.
Large doses of selenate protect against thallium toxicity in rats
(Rusiecki & Brzezinski, 1966), but this interaction has not been
investigated in any detail.
7.5. Cytotoxicity, Mutagenicity, and Anti-mutagenicity
7.5.1. Cytotoxicity and mutagenicity
Walker & Ting (1967) treated the soil around barley seedlings
with solutions of sodium selenate to determine the effect on
crossing over. Hoagland's solution containing 0, 0.5, 1.6, or
5.0 mg selenium/litre was applied 10 times, at 2-day intervals, to
each of 4 groups of plants, each treatment consisting of the
application of a 250 ml aliquot of the appropriate solution per
pot. On the day after treatment, the pots were irrigated with
distilled water to prevent lethal accumulation of selenate.
Genetic data indicated a significant effect of the selenate on the
rate of crossing over, consisting of a progressive decline with
increasing concentration.
Walker & Bradley (1969) reared larvae of Drosophila
melanogaster on a semi-defined culture medium containing
selenocystine at concentrations of 0, 2, 10, and 50 µM. The
selenocystine had different effects on crossing over on various
chromosomal segments and the authors concluded that these effects
were mediated through a structural chromosomal protein with a role
in the exchange process.
In studies by Nakamuro et al. (1976), various inorganic
compounds of selenium exhibited clastogenic effects when added in
vitro to cultures of human leukocytes (Table 39). Quadrivalent
selenium compounds were generally more efficient than hexavalent
compounds in inducing chromosome aberrations. Similarly, selenite
was more potent than selenate in causing DNA damage to Bacillus
subtilis that was subject to recombination repair. The same
authors also found that 1.6 x 10-3 mol H2Se03 or Se02 caused about
a 30% inactivation of the tryptophan marker of B. subtilis
transforming DNA, whereas similar concentrations of Na2Se04,
H2Se04, or Na2Se03 were without any significant effect.
Table 39. Chromosome aberrations in leukocytes exposed to selenium compoundsa
-------------------------------------------------------------------------------------
Compound Dose Occurrence of particular chromosome aberrationsb Total
chromatid chromatid iso-chromatid chromatid aberrations
gaps breaks breaks exchanges
-------------------------------------------------------------------------------------
(x 10-5 mol) aberrations per 100 cells
Na2Se04 53 8.4 0.9 0.9 0.9 11.2
26 9.3 0.8 0.8 0.8 11.9
13 11.2 0 0 0 11.2
H2Se04 53 21.0 14.5 3.2 1.6 40.3
26 11.9 0 0 0 11.9
13 9.2 0.8 0 0 10.0
Na2Se03 26 54.0 14.5 1.6 1.6 71.8
13 41.7 8.3 0.9 0.9 51.9
Table 39 (contd.)
-------------------------------------------------------------------------------------
Compound Dose Occurrence of particular chromosome aberrationsb Total
chromatid chromatid iso-chromatid chromatid aberrations
gaps breaks breaks exchanges
-------------------------------------------------------------------------------------
(x 10-5 mol) aberrations per 100 cells
H2Se03 26 113.2 41.2 22.1 5.8 182.4
13 92.3 42.3 12.8 2.6 150.0
6.5 15.2 6.3 5.4 0 26.8
Se02 26 52.1 22.5 7.0 1.4 83.1
13 24.2 2.5 3.3 0 30.0
6.5 15.5 3.6 4.8 0 20.9
None - 7.6 0 0 0 7.6
-------------------------------------------------------------------------------------
a Adapted from: Nakamuro et al. (1976).
b Number of cells examined varied from 62 - 250.
Lo et al. (1978) showed that selenite caused chromosome
aberrations and mitotic inhibition in cultured human fibroblasts
and that incubation with a mouse liver S9 microsomal fraction
increased this capacity of selenite about 5-fold at equimolar
concentrations (Table 40). Selenite also induced DNA fragmentation
and repair synthesis and decreased the clone-forming capacity of
the fibroblasts. Incubation with the S9 fraction increased the
last 2 effects of selenite but slightly decreased the extent of DNA
fragmentation. The response to selenite was similar in cultured
fibroblasts from normal persons and from DNA repair-deficient
xeroderma pigmentosum patients. The ability of selenate to trigger
DNA repair, inhibit the mitotic rate, or cause a lethal effect was
about the same as that of selenite over concentrations varying from
7 x 10-6 to 2 x 10-3 mol. However, when both compounds were
incubated with the S9 fraction, only the activity of the selenite
was potentiated (Table 41).
Table 40. Effect of 1.5 h selenite treatement on chromosome
aberrations and mitotic rate of human fibroblastsa
-------------------------------------------------------------
Sodium Metaphase plates with Mitotic rate
selenite chromosome aberrations without S-9 with S-9
for 1.5 h without S-9 with S-9 (%) (%)
(mol) (%) (%)
-------------------------------------------------------------
Control 1.2 0.8 9.0 8.9
2 x 10-5 1.3 6.9 8.6 9.8
4 x 10-5 1.6 7.4 8.9 9.6
8 x 10-5 3.0 14.3 9.3 9.4
1 x 10-4 3.2 - 8.2 0.1
2 x 10-4 4.7 - 9.5 0.0
4 x 10-4 6.1 - 6.4 0.0
8 x 10-4 14.5 - 6.9 0.0
1 x 10-3 14.2 - 7.6 0.0
3 x 10-3 18.1 - 0.7 0.0
-------------------------------------------------------------
a Adapted from: Lo et al. (1978).
Another example of a tissue fraction influencing the
cytotoxicity of selenite is provided by the work of Ray &
associates (Ray & Altenburg, 1978; Ray et al., 1978). These
workers first showed that sodium selenite tripled the frequency of
sister-chromatid exchanges (SCEs) in lymphocytes cultured with
whole human blood (Table 42). At the concentrations of selenite
used, the selenite could only be present during the final 19 h of
the 96-h culture incubation, otherwise cell death occurred, as
measured by mitotic indices. Later, they found that 7.90 x 10-6
mol Na2Se03 increased SCE frequency, only when the lymphocytes were
cultured in the presence of whole blood or separated red cells.
Lysis of the erythrocytes indicated that the lysate rather than the
red cell ghosts contained the activity necessary for selenite to
raise the lymphocyte SCE frequency. The authors suggested that
exposure of selenite to the lysate possibly resulted in a chemical
modification of the selenite that enabled it to induce SCEs. This
chemical modification of the selenite probably involves reduction
to glutathione selenopersulfide derivatives, since Ray (1984) found
that reduced glutathione (10-3 - 10-4 mol) could substitute for
erythrocytes in activating sodium selenite to its SCE-inducing
form. Whiting et al. (1980) showed that glutathione markedly
stimulated unscheduled DNA synthesis in cultured human skin
fibroblasts and chromosome aberrations in Chinese hamster ovary
cells caused by a variety of inorganic selenium compounds. These
research workers suggested that reduced selenium compounds are the
ultimate mutagens and that their formation depends on reduction by
glutathione.
Table 41. Effect of selenite versus selenate on human
fibroblasts incubated with, or without, mouse liver S9
microsomal fractiona
--------------------------------------------------------------
Treatment DNA repair Mitotic rate Clone forming
(grains/nucleus) (%) capacity (%)
--------------------------------------------------------------
Seleniteb 10.7 5.2 36
Selenite + S9 115.6 0.0 0
Selenate 7.0 4.8 42
Selenate + S9 9.1 3.9 32
--------------------------------------------------------------
a Adapted from: Lo et al. (1978).
b The concentrations used for the estimation of DNA repair,
mitotic rate, and surviving colonies were 8 x 10-4, 2 x 10-7,
and 2 x 10-4 mol, respectively. Selenite and selenate were
applied at equimolar concentrations for 1.5 h.
Table 42. Effect of sodium selenite on
sister-chromatid exchange frequency and
mitotic index in lymphocytes cultured
with whole human blooda
----------------------------------------
Na2Se03 Sister chromatid Average
added exchanges per mitotic
(mol) cellb index
----------------------------------------
Control 6.74 0.03
1.58 x 10-6 7.17 0.03
3.95 x 10-6 7.49 0.03
7.90 x 10-6 20.71 0.02
1.19 x 10-5 21.51 0.03
----------------------------------------
a Adapted from: Ray et al. (1978).
b Average of 4 studies; a total of 100
cells was scored for each Na2Se03
concentration except 75 at the highest
concentration.
In a preliminary note, Lofroth & Ames (1978) stated that
selenite did not give any indication of mutagenicity (<< 0.01
revertants/n mol) whereas selenate gave rise to base-pair
substitutions with about 0.03 revertants/n mol in a Salmonella
plate incorporation test using different histidine-requiring
strains that are reverted to prototrophy by different mechanisms.
On the other hand, Noda et al. (1979) found that both selenite and
selenate were weak mutagens in a similar test, since they gave
rise to base-pair substitution (0.2 and 0.05 revertants/n mol,
respectively). Ray & Altenburg (1980) showed that sodium selenide
and sodium selenite were more active than sodium selenate in their
ability to induce SCEs in human whole-blood cultures. Sirianni &
Huang (1983) found that sodium selenite was the most potent inducer
of SCEs in Chinese hamster V79 cells when S9 mixture was present,
whereas sodium selenide was the most effective inducer in the
absence of S9. For sodium selenate, there was no increase in SCE
rate compared with controls, regardless of whether S9 was present
or absent. At present, it is difficult to provide a biochemical
explanation for the contrasting cytogenic effects exhibited by the
various selenium compounds, when studied in different in vitro
test systems.
Norppa et al. (1980) investigated the chromosomal effects
of sodium selenite when given in vivo. They found that
supplementation of human neuronal ceroid lipofuscinosis patients
with tablets furnishing sodium selenite at a dose of 0.025 mg
selenium/kg body weight for 1 - 13.5 months did not have any
detectable effects on chromosomal aberrations or SCEs in peripheral
blood lymphocytes. Similarly, mice treated with a single dose of
sodium selenite at 0.8 mg selenium/kg body weight did not show any
rise in chromosomal abnormalities in bone-marrow cells or primary
spermatocytes after 24 h. However, there was an increased number
of SCEs and chromosomal aberrations in the bone-marrow cells of
Chinese hamsters, 17 - 19 h after injection with sodium selenite
at a dose of 0.3 - 6.0 mg selenium/kg body weight. It was thought
that the manifestations of chromosomal damage observed in the
second study may have been related to the general toxicity of
selenium at the high doses used.
7.5.2. Anti-mutagenicity
Shamberger et al. (1973a) tested the ability of sodium selenite
and various antioxidants to decrease the chromosomal breakage
induced by 7,12-dimethylbenz[alpha]anthracene (DMBA) in human blood
leukocyte cultures. They found that 0.20 µmol sodium selenite
reduced the breaks caused by 1.6 µmol DMBA by 42% (Table 43). This
concentration of selenite was used because 1 µmol almost completely
inhibited the growth of the cultures. In later studies, Shamberger
et al. (1979) found that sodium selenite at concentrations of
0.67 µmol or less was effective in reducing the mutagenic effects
of malonaldehyde and beta-propiolactone in certain tester strains
of Salmonella typhimurium. Rosin & Stich (1979) showed that sodium
selenite at concentrations of 3 x 10-4 and 1 x 10-3 mol caused
a 50% inhibition of mutagenesis in S. typhimurium induced by N-
methyl- N'-nitro- N-nitrosoguanidine or N-acetoxy-2-acetylamino-
fluorene, respectively. However, 10-2 mol sodium selenite was
toxic to the bacteria in the presence of either carcinogen. Sodium
selenite has also been shown to suppress spontaneous mutagenesis in
yeast cultures (Rosin, 1981).
Table 43. Effects of sodium selenite and various antioxidants on
chromosomal breakage induced by 7,12-dimethylbenz-(alpha)anthracene
(DMBA) in human blood leukocyte culturesa
-----------------------------------------------------------------------
DMBA Antioxidant Number Cells with Breaks Reduction
added added of breaks minus in
(µmol) cells number % control breaks
(%) (%)
-----------------------------------------------------------------------
0 none 211 23 10.9 - -
1.6 none 290 82 28.3 17.4 -
1.6 0.20 µmol Na2Se03 171 37 21.6 10.1 42.0
1.6 10 µmol dl-alpha 156 28 17.9 6.4 63.2
tocopherol
1.6 10 µmol ascorbic acid 127 30 23.6 11.9 31.7
1.6 0.21 µmol butylated 157 29 18.4 6.3 63.8
hydroxytoluene
-----------------------------------------------------------------------
a Adapted from: Shamberger et al. (1973a).
Jacobs et al. (1977b) examined the effects of sodium selenite
on the mutagenicity of 2-acetylaminofluorene (AAF), N-hydroxy-2-
acetyl-aminofluorene (N-OH-AAF), and N-hydroxy-aminofluorene
(N-OH-AF) in the S. typhimurium TA 1538 bacterial tester strain.
Metabolism of AAF and N-OH-AAF to the active mutagen, N-OH-AF was
accomplished by rat liver extracts. Sodium selenite at
concentrations ranging from 0.1 to 40 mmol decreased the
mutagenicity of these compounds (Table 44). However, in the case
of AAF, the authors noted that further decreases in mutagenicity
induced by concentrations of selenium higher than 40 mmol were not
tested, partly because of the formation of a red selenium compound
of low solubility, which can be presumed to have been elemental
selenium.
Ray et al. (1978) determined the frequencies of sister
chromatid exchange (SCE) in lymphocytes resulting from the
simultaneous exposure of whole-blood cultures to different
concentrations of sodium selenite plus 10-4 mol methyl
methanesulfonate (MMS) or 2.7 x 10-5 mol N-hydroxy-2-acetyl-
aminofluorene (N-OH-AAF). The SCE frequency observed as a result
of coexposure to 2 compounds was less than that expected because of
an additive SCE frequency response to each individual compound
(Table 45). In their interpretive analysis of the data, the
authors concluded that the most consistent explanation for the
results was that sodium selenite decreased the SCE-inducing
abilities of MMS and N-OH-AAF.
Table 44. Effect of sodium selenite on the mutagenicity of
2-acetylaminofluorene and its derviatives to Salmonella
typhimuriuma
-------------------------------------------------------------------
Compound Selenium His+ revertants Mutagenic activity
added per plate (± SD) (% of appropriate
(mmol) control)
-------------------------------------------------------------------
4.5 mmol AAF - 1768 ± 149 100
4 1411 ± 41 80
10 1336 ± 69 76
40 1148 ± 71 65
0.45 mmol N-OH-AAF - 2353 ± 12 100
0.4 1891 ± 210 80
4 1598 ± 71 68
10 1247 ± 53 53
40 655 ± 43 28
0.065 mmol N-OH-AF - 1628 ± 41 100
0.1 1280 ± 88 79
10 1233 ± 31 76
20 999 ± 26 61
-------------------------------------------------------------------
a Adapted from: Jacobs et al. (1977b).
Table 45. Effect of methyl methanesulfonate (MMS) or N-hydroxy-2-
acetylaminofluorene (N-OH-AAF), with or without different concentrations of
sodium selenite, on sister chromatid exchange (SCE) frequencies in whole-
blood cultures of lymphocytesa
----------------------------------------------------------------------------
Compound Na2Se03 Total Observed Expected Observed/
(mol) cells SCE/cell SCE/cellb expected
scored (%)
----------------------------------------------------------------------------
None none 100 6.74 ± 0.30 - -
10-4 mol MMS none 100 30.17 ± 0.75 - -
1.58 x 10-6 75 30.60 ± 0.74 30.60 (0.43) 100
3.95 x 10-6 75 30.13 ± 0.96 30.92 (0.75) 97
7.90 x 10-6 75 33.15 ± 1.15 44.14 (13.97) 75
1.19 x 10-5 75 31.60 ± 1.17 44.94 (14.77) 70
None none 125 7.65 ± 0.27 - -
2.7 x 10-5 mol none 125 13.61 ± 0.43 - -
N-OH-AAF 1.58 x 10-6 100 13.79 ± 0.42 13.61 (0.00) 100
7.90 x 10-6 65 22.66 ± 1.10 27.15 (13.54) 83
1.19 x 10-5 25 26.16 ± 1.86 29.24 (15.63) 89
----------------------------------------------------------------------------
a Adapted from: Ray et a1. (1978).
b The expected SCE/cell was determined by adding the observed separate
contributions to SCE frequency due to either MMS or N-OH-AAF to that due
to different concentrations of Na2Se03, as determined in a previous study
(shown in parentheses (see also Table 42)).
Martin et al. (1981) found that sodium selenite could
protect against the mutagenic effects of acridine orange and
7,12-dimethylbenz[alpha]anthracene (DMBA) in the Ames Salmonella/
microsome mutagenicity test. However, Chatterjee & Banerjee (1982)
showed that the influence of sodium selenite on the transformation
of mouse mammary cells, induced by DMBA added in organ culture, was
markedly affected by the level of selenium used. For example,
sodium selenite at concentrations of 10-7 - 10-8 mol increased the
transformation frequency of the cells within the glands. At 10-5
mol, sodium selenite caused an 18 and 84% inhibition of the
frequency of transformed cells at the initiation and promotional
stages, respectively. At 10-4 mol, sodium selenite was toxic to
the cells in vitro. Sodium selenite inhibited the metabolism and
mutagenicity of benzo (a)pyrene to S. typhimurium strain TA 100
in rat (Teel & Kain, 1984) and hamster (Teel, 1984) liver S9
activation systems, but had no inhibitory effect on the
mutagenicity of 1,2-dimethylhydrazine or azoxymethane for S.
typhimurium G46 in the host-mediated assay (Moriya et al., 1979).
On the other hand, sodium selenite at 3.95 x 10-9 - 1.98 x 10-8 mol
protected against chromosomal damage in cultured human lymphocytes
caused by 2.3 x 10-6 mol sodium arsenite (Sweins, 1983). However,
this author reported that the cytotoxic concentration of sodium
selenite in his system was very low (somewhere between 1.98 and
3.95 x 10-8 mol). No explanation was offered for the considerable
variations between laboratories with respect to selenium
cytotoxicity.
Several investigators have now examined the anti-mutagenic
effects of dietary selenium in a variety of experimental systems.
For example, Gairola & Chow (1982) fed rats either a low-selenium
diet based on Torula yeast or the same diet supplemented with
sodium selenite at 2 mg selenium/kg for 20 weeks. Dietary selenium
did not have any effect on the metabolic activation potential of
S9 liver enzymes towards benzo( a)pyrene in the Ames Salmonella/
microsome mutagenicity assay. S9 mixtures from selenium-deficient
rats were more active towards 2-aminoanthracene and less active
towards 2-aminofluorene than mixtures from the selenium-
supplemented rats. The authors concluded that further studies were
needed to elucidate the role and nature of in vitro metabolites
causing mutations in the bacteria. In contrast, Schillaci et al.
(1982) did not find any differences in the mutagenic activation of
7-12-dimethylbenz[alpha]anthracene (DMBA) by liver S9 mixtures in
the Ames test with S. typhimurium strain TA 98, prepared from rats
fed Torula yeast-based diets supplemented with sodium selenite at
0.1, 2.5, or 5.0 mg selenium/kg, from weaning for 3 weeks.
However, if the rats were injected with 20, 50, or 100 mg
Aroclor(R) 1254/kg body weight, 5 days before sacrifice, dietary
selenium at 2.5 or 5.0 mg/kg in the form of sodium selenite
markedly decreased the mutagenic activation of DMBA by liver
microsomes.
Lawson & Birt (1983) measured single-strand breaks (SSB)
produced in pancreatic DNA by injecting 20 mg N-nitrosobis(2-
oxopropyl) amine (BOP)/kg body weight subcutaneously into hamsters
that had been fed a Torula yeast-based diet supplemented with
sodium selenite at 0, 0.1, or 5 mg selenium/kg, for 4 weeks
previously. One hour after injection with BOP, there were 2.26 ±
0.47, 2.83 ± 0.43, and 1.74 ± 0.43 SSB per 108 daltons of DNA (mean
± SEM), respectively, in the 3 dietary groups, and the approximate
half-lives of the SSB were 33, 30, and 8 days, respectively. On
this basis, the authors suggested that high levels of dietary
selenium may stimulate the repair of carcinogen-induced DNA damage.
Olsson et al. (1984) used a novel isolated rat liver/cell
culture system to study the effects of selenium deficiency and
selenium supplementation, within the physiological range, on the
anti-mutagenic effects of the element. Rats were first fed a
Torula yeast-based selenium-deficient diet for 5 - 6 weeks with or
without sodium selenite supplementation at 0.2 mg selenium/litre
drinking-water. The rat livers were then connected to an isolated
liver perfusion system. A glass plate with cultured Chinese
hamster V79 cells was introduced into the perfusion system
immediately before the addition of 5 mmol dimethylnitrosamine (DMN)
and then exposed directly to the circulating perfusate. After 2 h,
this glass plate was exchanged for another with fresh V79 cells,
which were exposed during the subsequent 2 h. This procedure was
adopted to avoid a possible toxic effect that might influence
mutation frequency. As in vitro controls, V79 cells were treated
for 2 h in Krebs-Ringer albumin solution with or without DMN. The
induced mutation frequencies in the 2 successively treated cell
populations were summarized to give the value for each liver. It
was found that the mutagenicity of DMN in Chinese hamster V79
cells, after metabolic activation by the isolated perfused rat
liver, was approximately doubled when livers from selenium-
deficient rats were used compared with livers from rats given the
supplementary selenium. The authors were unable to provide a
precise biochemical explanation for the mechanism by which selenium
deficiency increased the mutagenicity of DMN, but they noted that
microsomal N-oxygenation of N,N-dimethylaniline (DMA) was
decreased in livers from selenium-deficient rats and suggested that
further investigation of the different enzymes involved in DMA- N-
oxygenation appeared warranted. Another application of this liver
perfusion/cell culture system was demonstrated by the work of Beije
et al. (1984) who showed that bile collected from selenium-
deficient livers perfused with a medium containing 5 mmol 1,1-
dimethylhydrazine was much more mutagenic toward Chinese hamster
V79 cells than bile from livers of rats given supplementary
selenium and perfused in a similar manner. The authors suggested
that the increased biliary excretion of reactive mutagenic
metabolites observed in their selenium-deficient rat liver
perfusion system might furnish a potential explanation for some of
the protective effects of selenium reported against chemically-
induced colon cancer in experimental animals.
7.6. Teratogenicity
Franke & Tully (1935) obtained chicken eggs from 2 farms in
South Dakota that had histories of low hatchability. Hatchability
in the 2 test groups was extremely low, being 4% in one and 12% in
another. Examination of the eggs revealed that about 75% of those
that had failed to hatch on the 21st day contained deformed
embryos. Franke et al. (1936) then showed that injection of
selenium salts into the air cell of eggs before incubation resulted
in monsters similar to those occurring naturally on affected farms.
Selenium injected as selenite to give a final concentration in the
egg of 0.6 mg selenium/kg was the most effective in this respect,
higher doses causing embryonic death and lower doses producing
fewer monsters (Table 46). When the toxicity of various selenium
compounds for chick embryos was compared, it was found that
selenate was about twice as toxic as selenite (Palmer et al., 1973)
(Table 47). The toxicity of selenomethionine was about the same as
that of selenate but methylseleninic acid was more than twice as
toxic. The toxicity of trimethylselenonium chloride was relatively
low. The most common embryonic deformities observed in this study
were the underdevelopment of the beak and abnormal development of
the feet and legs, especially a fusing or webbing of the 2 outside
toes. Because of the metabolic relationships between selenium and
arsenic or cadmium, Holmberg & Ferm (1969) investigated the
teratogenic potential of these elements, administered separately or
together to golden hamsters (Table 48). Embryonic malformations
were not observed when pregnant hamsters were injected
intravenously with a barely sub-lethal dose of sodium selenite
alone, and the 6% resorption rate observed was stated to be similar
to the normal rate of resorption in this species. Furthermore,
under these conditions, sodium selenite actually gave partial
protection against the teratogenesis induced by sodium arsenate or
cadmium sulfate.
Table 46. Teratogenicity of sodium selenite
injected into hens' eggsa
--------------------------------------------------
Sodium selenite Total Dead Abnormal Normal
(mg selenium/kg) embryos (%) (%) (%)
--------------------------------------------------
0.9 5 60.0 20.0 20.0
0.8 16 12.5 31.0 56.5
0.7 4 50.0 25.0 25.0
0.6 4 50.0 50.0 0
0.5 12 50.0 8.3 41.7
0.1 131 24.4 2.3 73.3
0.02 78 53.8 3.8 42.3
0.01 64 34.3 9.4 56.3
--------------------------------------------------
a Adapted from: Franke et al. (1936).
Table 47. Comparative toxicity of various selenium compounds for chick
embryosa
----------------------------------------------------------------------------
Compound Dose Livability ratio LD50 with 95%
(mg selenium/kg) (live chicks/ confidence limits
fertile eggs) (mg selenium/kg)
----------------------------------------------------------------------------
sodium selenite 0.0 16/18
0.05 18/18
0.1 16/17 0.3b
0.2 17/18
0.4 2/18
0.8 0/18
sodium selenate 0.0 18/18
0.1 12/18
0.2 4/18 0.13
0.4 1/18 (0.086 - 0.17)
0.8 0/18
1.6 0/18
selenomethionine 0.0 17/18
0.05 18/18
0.1 13/18 0.13
0.15 5/18 (0.095-0.15)
0.2 4/18
0.4 0/18
Table 47 (contd.)
----------------------------------------------------------------------------
Compound Dose Livability ratio LD50 with 95%
(mg selenium/kg) (live chicks/ confidence limits
fertile eggs) (mg selenium/kg)
----------------------------------------------------------------------------
methylseleninic 0.0 23/24
acid 0.025 17/18
0.05 9/18 0.052
0.1 2/24 (0.039-0.065)
0.2 0/24
0.4 0/24
trimethylselenonium 0.0 18/18
chloride 6.0 17/18
12.0 13/18 15.7
18.0 5/17 (12.7 - 19.0)
24.0 5/18
30.0 3/18
----------------------------------------------------------------------------
a Adapted from: Palmer et al. (1973).
b An estimate since the data did not allow calculation by the computer
programme.
7.7. Carcinogenicity and Anti-Carcinogenicity
7.7.1. Selenium as a possible carcinogen
Five investigations have been reported in the literature on the
alleged carcinogenicity of selenium for laboratory animals. Nelson
et al. (1943) fed groups of 18 female rats of an inbred Osborne-
Mendel strain a 12% protein diet consisting of 49% corn, 44% wheat,
3% yeast, and 1% each of cod liver oil, calcium carbonate, sodium
chloride and dried whole liver. The diet was supplemented with 0,
5, 7, or 10 mg selenium/kg, as seleniferous corn or wheat, or 10 mg
selenium/kg, as a mixed inorganic selenide containing ammonium
potassium selenide and ammonium potassium sulfide. Although
cirrhosis was frequently observed after 3 months of selenium
exposure, no tumours or advanced adenomatoid hyperplasia were seen
in any of the 73 selenium-exposed rats that died or were sacrificed
before 18 months. Of the 53 selenium-exposed rats that survived
18 - 24 months, 43 had cirrhosis and 11 developed liver cell
adenoma or low-grade carcinoma without metastasis in cirrhotic
livers. There was no relationship between the extent of cirrhosis
and tumour incidence except that there were no tumours in any of
the 18- to 24-month-old rats that did not have cirrhosis. The
incidence of spontaneous adenoma and low-grade carcinoma of the
liver in the unexposed rats was low and the incidence of
spontaneous hepatic tumours in the rat colony was less than 1% in
18- to 24-month-old rats.
Table 48. Effect of selenium, arsenic, and cadmium on embryonic death and
malformations in the golden hamstera
------------------------------------------------------------------------------------
Treatment Dose Total Number of Number of Malformed Malformed
(mg/kg) number of embryos embryos (%) or resorbed
embryos resorbed malformed (%)
------------------------------------------------------------------------------------
sodium selenite 2 86 5 0 0 6
sodium arsenate 20 177 62 86 49 84
sodium arsenate 20 144 28 28 19 39
+ sodium selenite 2
cadmium sulfate 2 115 24 59 51 72
cadmium sulfate 2 82 2 3 4 6
+ sodium selenite 2
------------------------------------------------------------------------------------
a Adapted from: Holmberg & Ferm (1969).
The results of some of the initial studies of Volgarev &
Tscherkes (1967) seemed to indicate an increased incidence of
tumours in rats fed a low-protein diet supplemented with sodium
selenate at 4.3 mg selenium/kg, but subsequent tests carried out by
these authors failed to confirm these results. Moreover, their
work suffered from the fact that no control groups, consuming diets
without additional selenium, were included in any of their trials.
Schroeder & Mitchener (1971b) reported an increased incidence of
tumours in rats given sodium selenate at 2 mg selenium/litre in the
drinking-water for the first year of life followed by 3 mg/litre
until death. In a previous evaluation of this work (US NAS/NRC,
1976), it was observed that the selenate-treated rats survived
longer than the untreated rats and this could have contributed to
the increased tumour incidence observed in the rats receiving
selenate. Also, as the organs and tissues did not appear to have
been systematically searched, the type and incidence of
histological lesions could not be known with certainty.
Schroeder & Mitchener (1972) carried out 2 studies in which
mice were given selenium in the drinking-water. In both studies,
the mice were fed a diet composed of 60% whole rye flour, 30% dried
skim milk, 9% corn oil, and 1% iodized sodium chloride, to which
were added vitamins and iron. The diet was calculated to contain
24% protein, 65% carbohydrate, and 11% fat (dry weight). The mice
were given doubly deionized water for drinking, which originally
came from a forest spring. Certain essential trace metals were
added to the drinking-water, as soluble salts, in the following
concentrations (mg/litre): zinc, 50; manganese, 10; copper, 5;
cobalt, 1; and molybdenum 1. All mice received these trace
elements in their drinking-water. In addition, chromium was added
to the drinking-water at a level of 1 or 5 mg/litre in the first
and second studies, respectively. In the first study, groups of
100 or more Swiss mice of the Charles River CD strain, containing
equal numbers of each sex, were given, at weaning, sodium selenite
at either 0 or 3 mg selenium/litre of the trace element-fortified
drinking-water. This regimen continued over the entire life span
of the mice. The second study was identical to the first, apart
from the level of chromium given in the drinking-water, and the
fact that the selenium was administered in the form of sodium
selenate. Of the 180 control mice autopsied from both studies, 119
were sectioned. Tumours were found in 23 (19%) of those sectioned
and 10 of the tumours (43%) were malignant. The different forms of
selenium given did not influence the incidence of tumours and, of
the 176 selenium-exposed mice autopsied in both studies, 88 were
sectioned. Tumours were found in 13 (15%) of those sectioned but
all tumours were malignant. It was concluded that selenium had
little tumourigenic or carcinogenic activity in mice, though, when
tumours did appear in the selenium-exposed mice, they were all
malignant.
The results of studies of Harr et al. (1967) (section 7.1.2.2)
failed to demonstrate any tumours attributable to selenium, but
most of the rats fed semi-purified diets containing levels of
selenium greater than 2 mg/kg died within 100 days and almost all
were dead before 2 years. Exceptions included 1 rat fed selenate
at 4 mg selenium/kg diet containing 12% casein and 0.3% DL-
methionine, and 27 rats alternating at weekly intervals between a
control ration and a diet containing sodium selenate at either 4 or
8 mg selenium/kg. However, no hepatic tumours were observed, even
in the 71 rats that survived 2 years or longer at continuous
dietary-selenium levels of 0.5 - 2.0 mg/kg.
The above studies indicate that test animals develop neoplastic
lesions, only when they have liver cirrhosis produced by frank
selenium toxicity (i.e., no hepatomas were observed in the absence
of severe hepatotoxic phenomena). For this reason, it was
concluded that selenium was not, by reason of its capacity to
induce liver damage when consumed at high levels, properly
classified as carcinogenic because of its potential association
with a higher rate of liver cancer (Gardner, 1973).
Jacobs & Forst (1981b) did not observe any signs of neoplasia
in groups of 35 female Swiss mice fed a commercial pelleted diet
and given 0, 1, 4, or 8 mg selenium/litre drinking-water, as sodium
selenite, for 50 weeks.
Three studies have shown carcinogenic effects that appear to be
more a specific effect of a particular selenium compound rather
than an effect of selenium itself. Seifter et al. (1946) found
that 8 white rats that had received 0.05% bis-4-acetamino-phenyl-
selenium dihydroxide in their diet for 105 days had multiple
adenomas of the the thyroid glands and adenomatous hyperplasia of
the liver. Innes et al. (1969) fed the maximal tolerated dose of
selenium diethyldithiocarbamate (Ethyl selenac) to a group of 72
mice containing both sexes of two hybrid strains (C57BL/6 x
C3H/Anf)F and (C57BL/g x AKR)F1. The mice were given this
substance by stomach tube at a dose of 10 mg/kg body weight,
starting at 7 days of age. At 4 weeks of age, the chemical was
mixed directly into the diet at a concentration of 26 mg/kg. After
82 weeks, all the surviving mice were sacrificed; of 69 necropsied,
26, 13, and 5.8% had hepatomas, lymphomas, and pulmonary tumours,
respectively. Among 338 negative control mice, most of which were
sacrificed between 78 and 89 weeks, the incidences of the
corresponding tumours were 4.1, 4.1, and 6.2%, respectively.
A report from the US National Cancer Institute (National Cancer
Institute, 1980) suggested that commercial selenium sulfide, an
ingredient in certain anti-dandruff shampoos, was carcinogenic for
rats and mice. Elemental analysis of the test chemical used in
this study indicated that the material was a mixture of selenium
monosulfide and selenium disulfide. The melting point of the test
sample was closer to that reported for the monosulfide than that
reported for the disulfide and the X-ray diffraction pattern was
consistent with patterns reported for the monosulfide. It was
concluded that the selenium in the test substance used in this
bioassay was present primarily as the monosulfide.
In the rat study, groups of 4-week-old male and female Fischer
F344 rats were fed presterilized lab meal and were given untreated
well water ad libitum for 104 - 105 weeks. During the first 103
weeks, 50 rats of each sex were given one of 4 treatments:
untreated control; vehicle control (received volumes of 0.5%
aqueous carboxymethylcellulose equal to those of the test solutions
administered); low-dose group (3 mg selenium sulfide suspended in
0.5% aqueous carboxymethylcellulose/kg body weight, 5 days per
week, given by gavage); or high-dose group (15 mg selenium
sulfide/kg body weight administered by the same route and
schedule). The results showed an increased incidence of primary
liver tumours in both male and female high-dose groups (Table 49).
Neoplastic nodules were usually single, rather well-defined
areas characterized by altered hepatocytes. In most instances, the
hepatocytes were larger than normal, eosinophilic, and occasionally
vacuolated. The normal architecture was altered, mainly resulting
in a solid mass of hepatocytes or a trabecular pattern rather than
the normal hepatic cords. The mass compressed the adjacent
parenchyma around the periphery. Anaplasia and mitoses were
minimal. Hepatocellular carcinomas were usually large multinodular
masses, often encompassing entire liver lobes or even multiple
lobes. The histological appearance of these neoplasms varied from
areas appearing normal to much more anaplastic areas. The
neoplastic hepatocytes varied from small basophilic cells to very
large eosinophilic and occasionally vacuolated cells. Mitoses were
variable. No distant metastases were observed in any of the rats
bearing hepatocellular carcinomas. No other neoplasms were found
that could be related to the administration of the selenium
sulfide.
Table 49. Effect of selenium sulfide, given orally for 103 weeks, on
the incidence of liver tumours in Fischer F344 ratsa
------------------------------------------------------------------------
Initial Selenium Tumour incidence
Test group Sex number sulfide Hepatocellular Neoplastic
of rats dose carcinoma nodules
------------------------------------------------------------------------
(mg/kg)
Untreated control M 50 0 1/48 (2%) 3/48 (6%)
Vehicle controlb M 50 0 0/50 (0%) 1/50 (2%)
Low-dosec M 50 3 0/50 (0%) 0/50 (0%)
High-dosec M 50 15 14/49 (29%) 15/49 (31%)
Untreated control F 50 0 0/50 (0%) 0/50 (0%)
Vehicle controlb F 50 0 0/50 (0%) 1/50 (2%)
Low-dosec F 50 3 0/50 (0%) 0/50 (0%)
High-dosec F 50 15 21/50 (42%) 25/50 (50%)
------------------------------------------------------------------------
a Adapted from: National Cancer Institute (1980).
b Received only vehicle for dosing (0.5% aqueous carboxymethylcellulose)
5 days per week, by gavage.
c Received stated dose of selenium sulfide suspended in 0.5% aqueous
carboxymethylcellulose, 5 days per week, by gavage.
An increased incidence of focal cellular change in the liver
was noted in high-dose male rats but was essentially comparable in
frequency in the remaining treated and control groups of each sex.
A compound-related increase in pigmentation in the lungs was
observed. This was characterized by the accumulation of dark,
slightly granular-appearing pigment in the interstitial areas and
in some peribronchial areas. In most cases, the pigment appeared
to be located within cells, principally macrophages. No evidence
of inflammation, relative to the pigment deposits, was noted. Lung
pigmentation was found in 47/49 (96%) high-dose males, 1/50 (2%)
low-dose males, 45/50 (90%) high-dose females, and 36/50 (72%) low-
dose females, but not in control males or females.
The protocol for the testing of selenium sulfide in B6C3F1 mice
was similar to that used for rats, except that the doses of the
substance under test were increased (Table 50). There was an
increased incidence of primary liver and lung tumours in the high-
dose female mice and a marginal increase in the incidence of these
tumours in high-dose males.
Hepatocellular carcinomas varied from single nodules to
multinodular masses, often encompassing several liver lobes.
Individual hepatocytes varied considerably in morphology from large
eosinophilic cells to small, darkly-staining hepatocytes. In many
cases, there was marked variation in cell type in different parts
of the neoplasm. The number of mitoses varied. The number of
hepatocellular carcinomas metastasizing to the lungs was comparable
in vehicle-control and high-dose males. No metastases were
observed in the lung in female mice. Alveolar/bronchiolar adenomas
were usually small solitary lesions located in the subpleural area
or immediately adjacent to a bronchiole. The cells involved were
cuboidal to tall columnar and tended to be situated perpendicular
to the basement membrane in a single layer. These cells were
arranged in complex papillary projections forming discrete nodules
and compressing adjacent alveolar walls. Mitoses were rare.
Alveolar/bronchiolar carcinomas, however, were less discrete
lesions and tended to be larger and occasionally multiple,
consisting of a confluence of two or more nodules. The individual
cells tended to be less rigidly arranged along basement membranes
and were often piled up in layers or arranged in solid sheets
without a papillary pattern. The cells often showed increased
basophilia and a moderate mitotic index. Evidence of invasion into
adjacent vessels, or extension into bronchioles and adjacent lung
parenchyma was frequently present.
Other neoplasms that occurred in the mice were similar in
number and kind to those that usually occur in aged B6C3F1 control
mice and could not be related to the long-term administration of
selenium sulfide.
In a comparison study, the effect of the dermal application of
selenium sulfide was examined. No increased incidence of neoplasms
was observed that could be attributed to selenium sulfide.
7.7.2. Selenium as a possible anti-carcinogen
High levels of dietary selenium have been shown to protect
laboratory animals against chemical carcinogenesis, under a wide
variety of conditions. Clayton & Baumann (1949) fed 2 groups of 15
young adult rats, weighing approximately 200 g, a basal diet
consisting of extracted casein, 12 parts; salts, 4 parts; corn oil,
5 parts; and glucose monohydrate (Cerelose) to 100 parts. The diet
was supplemented with thiamin, riboflavin, pyridoxine, calcium
pantothenate, and choline. Fat-soluble vitamins were provided by
giving 2 drops of halibut liver oil per rat every 4 weeks. During
the initial 4-week feeding period, both groups received the basal
diet supplemented with 0.064% 3'-methyl-4-dimethylaminoazobenzene.
This was followed by a 4-week "interruption period" during which
neither group received the azo dye, but one group received the
basal diet supplemented with sodium selenite at 5 mg selenium/kg,
and the other group received the basal diet without any added
selenium. During the third, 4-week feeding period, both groups
received the basal diet supplemented with 0.048% of the azo dye,
but no supplementary selenium. During the final 8-week feeding
period, both groups received the basal diet without either azo dye
or selenium. Two of the 9 surviving rats in the group that
received supplementary selenium during the "interruption period"
had liver tumours (22%), compared with 4 out of 10 survivors (40%)
in the group not supplemented with selenium. The results of a
second similar study showed a liver tumour incidence of 4/13 (31%)
in the selenium-supplemented group compared with 8 out of 13 rats
(62%) in the unsupplemented group.
A preliminary study by Shamberger & Rudolph (1966) indicated
that concomitant dermal application of sodium selenide reduced the
tumour-promoting effect of croton oil in mice initiated with 7,12-
dimethylbenz[alpha]anthracene (DMBA). Riley (1968) found that a
similar application of sodium selenide prevented the mast cell
reaction caused by an active fraction of croton oil in the skin of
DMBA-initiated mice. In a later set of studies (Shamberger, 1970),
2 groups of 30 female ICR Swiss mice, 50 - 55 days old, were
treated once on day one with 0.125 mg DMBA dissolved in 0.25 ml
acetone. On days 2 - 21, the shaved backs of the first group of
mice were treated with 0.25 ml of a 20:80 water-acetone mixture
containing 0.0005% sodium selenide, whereas the second group was
not treated with any anti-oxidant. Subsequently, in 3 separate,
but essentially identical, studies, both groups of mice received
0.25 ml of 0.04% croton oil in acetone daily for 16, 18, and 18
weeks respectively. After the croton oil treatment, the incidence
of papillomas in the selenide-treated groups was 17, 63, and 45%,
respectively, in 3 separate studies, compared with an incidence in
the non-treated groups of 43, 63, and 89%, respectively. The
corresponding number of papillomas per mouse in the 3 studies was
1.5, 3.0, and 1.5, respectively, in the selenide-treated groups and
3.2, 6.0, and 2.3 in the non-treated groups. No papillomas were
observed in 3 DMBA-initiated control groups, which did not receive
either antioxidant or croton oil. A similar protective effect of
sodium selenide was observed in 3 additional studies in which the
promoting agents were croton oil, croton resin, and phenol and in
which the selenide was applied concomitantly with the promoter.
In another study, 0.25 ml of 0.01% 3-methylcholanthrene (MCA)
was applied daily to the shaved backs of one group of mice for 19
weeks; a second group received 0.0005% sodium selenide together
with the MCA. After 19 weeks, the incidence of papillomas was 68%
in the selenide-treated group and 87% in the untreated group, and
the number of papillomas per mouse was 3.2 and 2.2, respectively.
After 30 weeks, the number of mice with cancers was 17/28 and 25/30
in the selenide-treated and untreated groups, respectively, the
total number of cancers per group being 29 and 71, respectively.
Table 50. Effect of selenium sulfide given orally for 103 weeks on the incidence of liver and lung
tumours in B6C3F1 micea
----------------------------------------------------------------------------------------------------
Lung tumour incidence
Initial Selenium Liver tumour incidence Alveolar/ Alveolar/
Test group Sex number sulfide hepatocellular hepatocellular bronchiolar bronchiolar
of mice dose carcinoma adenoma carcinoma adenoma
(mg/kg)
----------------------------------------------------------------------------------------------------
Untreated control M 50 0 17/49 (35%) 3/49 (6%) 1/49 (2%) 8/49 (16%)
Vehicle controlb M 50 0 15/50 (30%) 0/50 (0%) 1/50 (2%) 3/50 (6%)
Low-dosec M 50 20 11/50 (22%) 3/50 (6%) 2/50 (4%) 8/50 (16%)
High-dosec M 50 100 23/50 (46%) 0/50 (0%) 2/50 (4%) 12/50 (24%)
Untreated control F 50 0 2/50 (4%) 1/50 (2%) 0/50 (0%) 2/50 (4%)
Vehicle controlb F 50 0 0/49 (0%) 0/49 (0%) 0/49 (0%) 0/49 (0%)
Low-dosec F 50 20 1/50 (2%) 1/50 (2%) 1/50 (2%) 2/50 (4%)
High-dosec F 50 100 22/49 (45%) 6/49 (12%) 4/49 (8%) 8/49 (16%)
----------------------------------------------------------------------------------------------------
a Adapted from: National Cancer Institute (1980).
b Received only vehicle for dosing (0.5% aqueous carboxymethylcellulose), 5 days per week, by gavage.
c Received stated dose of selenium sulfide suspended in 0.5% aqueous carboxymethylcellulose, 5 days
per week, by gavage.
Shamberger (1970) also carried out 2 dietary studies in which 4
groups of 36 female, albino ICR Swiss mice, 50 - 55 days old, were
fed Torula yeast diets supplemented with sodium selenite at 0, 0.1,
or 1.0 mg selenium/kg or sodium selenide at 0.1 mg selenium/kg.
After 2 weeks on the test diets, 0.125 mg DMBA dissolved in acetone
was applied once to the skin. After 3 weeks, 0.25 ml 0.05% croton
oil in acetone was applied daily for 17 weeks. At this time, 26/36
mice fed the unsupplemented diet had papillomas, compared with
14/35 mice fed the diet supplemented with 1.0 mg selenium/kg, as
selenite. The incidence of papillomas was slightly elevated in the
mice fed the diet supplemented with 0.1 mg selenium/kg, as sodium
selenite, compared with the unsupplemented mice. The incidence of
papillomas in mice fed sodium selenide at 0.1 mg selenium/kg was
intermediate between that of the unsupplemented group and the group
fed sodium selenite at 1.0 mg selenium/kg.
In a second study of similar design, 4 groups of 36 mice were
fed the test diets described above for 2 weeks. Then 0.25 ml of
0.03% benzo( a)pyrene in acetone was applied daily to the shaved
backs of the mice for 27 weeks. At this time, the incidence of
papillomas in the groups fed the torula diet supplemented with 0,
0.1, or 1.0 mg selenium/kg, as selenite, or 0.1 mg selenium/kg, as
selenide, was 14/35, 22/36, 8/33, and 12/35, respectively. The
number of papillomas per mouse in the corresponding groups was 8.1,
9.8, 5.0, and 6.8.
Harr et al. (1972) weaned 80 female OSU-Brown rats at 35 days
of age and divided them into 4 groups of 20. Each group was fed a
low-selenium basal diet (0.018 µg selenium/kg) that included Torula
yeast as the protein source and contained 60 mg vitamin E/kg.
Groups, 1, 2, 3, and 4 were fed the basal diet supplemented with
sodium selenite at 2.5, 0.5, 0.1, and 0 mg selenium/kg,
respectively. All diets contained 150 mg 2-acetylaminofluorene/kg.
The 40 rats in groups 1 and 2 were born from parents reared on a
selenium-depleted regimen, but were clinically normal. The 40 rats
in groups 3 and 4 were from the second generation maintained on the
depeletion regimen and had clinical signs of selenium deficiency.
After 200 days of treatment, the number of mammary adenocarcinomas
in groups 1, 2, 3, and 4 was 0, 1, 8, and 9, respectively. After
320 days, the number of mammary adenocarcinomas in the same groups
was 11, 11, 13, and 12, respectively. Mammary adenocarcinomas in
groups 1, 2, and 3 occurred mainly (90%) in the thoracic region and
were well circumscribed, firm, and easily removed, whereas those in
group 4 occurred mainly (80%) in the pelvic area and were soft and
fluid, contained little connective tissue, and were invasive.
After 240 days of treatment, the number of hepatic carcinomas in
groups 1, 2, 3, and 4 was 0, 1, 9, and 4, respectively. After 320
days, the number of hepatic carcinomas in the same groups was 8,
12, 12, and 6, respectively. Toxic hepatitis was observed in 18 of
the 20 livers from group 1 (selenium added at 2.5 mg/kg), but was
not seen in the other groups.
In studies of Marshall et al. (1978), 2 groups of male albino
Sprague Dawley rats were fed diets containing 2-acetylaminofluorene
at 0.3 g/kg, for 14 weeks. One group received 4 mg selenium, as
sodium selenite/litre drinking-water. The carcinogen was withdrawn
from the diet for an additional 4 weeks and then the rats were
sacrificed. The rats given selenium had about 50% fewer liver
tumours than unsupplemented rats. Control rats given a similar
level of selenium in the water showed normal growth response, liver
weight, and appearance.
Three groups of 15 male Sprague Dawley rats, weighing about
250 g, were fed a basal diet of finely ground Purina Laboratory
Chow and water ad libitum (Griffin & Jacobs, 1977). One group did
not receive any supplementary selenium, a second group received 6
mg selenium, as sodium selenite/litre drinking-water, and a third
group received 6 mg selenium, in the form of a high selenium
yeast/kg diet. After one week on this regimen, all 3 groups were
given 0.05% 3'-methyl-4-dimethyl-aminoazobenzene in the diet for 8
weeks. Following this, all groups were maintained on the
carcinogen-free diets for an additional 4 weeks. The selenium
supplements were maintained for the second and third group
throughout the entire study. At sacrifice, the incidence of liver
tumours in the surviving rats was 92% (11/12) in the unsupplemented
group, 46% (7/15) in the group supplemented with selenite in the
drinking-water, and 64% (9/14) in the group supplemented with
selenium yeast in the diet. In this study, the selenium
supplements had little effect on the growth of the rats.
Histopathological examination of several of the tumours revealed
that they were bile duct adenocarcinomas and liver cell
adenocarcinomas.
Jacobs et al. (1977a) injected 2 groups of 15 male, 8-week-old
Sprague Dawley rats, weekly, with 20 mg sym,-dimethylhydrazine
dihydrochloride (DMH)/kg body weight, for 18 weeks. One group of
rats received sodium selenite at 4 mg selenium/ litre drinking-
water, which was available ad libitum one week prior to, and
throughout, administration of the carcinogen. The other group did
not receive selenium added to the drinking-water. The group
receiving DMH and no added selenium had an 87% incidence (13/15) of
colon tumours, whereas the group receiving both DMH and added
selenium had a 40% incidence (6/15) of colon tumours. The total
number of colon tumours was 39 in rats receiving only DMH and 11 in
rats receiving both DMH and added selenium. In a similar study in
which 2 groups of 15 rats were injected weekly with 20 mg of
(methylazoxyl)-methanol acetate (MAM)/kg body weight for 18 weeks,
no significant differences in the incidence of colon tumours were
apparent between groups with (14/15) and without (14/14) added
selenium in the drinking-water. However, the number of MAM induced
tumours was 73 in the group given MAM alone and 42 in the group
receiving both MAM and added selenium.
Schrauzer and colleagues carried out a series of studies in
which exogenous carcinogens were not used, to test the ability of
supplemental selenium in influencing the development of spontaneous
mouse mammary tumours, presumably of viral origin. In study 1
(Schrauzer & Ishmael, 1974), 2 groups of 30 virgin female C3H/St
mice, 4 - 6 weeks old, were fed a basal diet ("Concord Maid")
consisting of meat scraps, dried skimmed milk, oat groats, ground
wheat, wheat germ meal, vegetable oil, cane molasses, salt,
brewer's yeast, cereal binder, sodium propionate, and calcium
pantothenate. The diet contained about 150 g protein, 5 g fat, and
0.15 mg selenium/kg. One group of mice received plain distilled
water for drinking, while the other group received distilled water
fortified with 2 mg selenium, as selenium dioxide/litre. The
strain of mice used in this study ordinarily has a high incidence
of spontaneous mammary adenocarcinomas and, after 16 months of
treatment, the observed incidence in the group not given
supplemental selenium in the water was 82%, whereas the incidence
in the group given selenium was 10%.
In study 2 (Schrauzer et al., 1976), 3 groups of 30, 30, and 50
female C3H/St mice fed the basal diet described above were also
given selenite at 0, 5, or 15 mg selenium/litre drinking-water,
respectively. Toxicity due to the doses of selenium was indicated
by the average body weights in the 3 groups at 12 months (33, 29,
and 25 g, respectively) and by a higher tumour-unrelated mortality
rate in the selenium-exposed mice. After 26 months, the
corresponding incidence of spontaneous mammary tumours in the 3
groups was 82, 36, and 33% in the mice that survived to the age at
which the first tumours appeared in each group. A fourth group of
20 mice, fed a selenium-deficient diet for 14 months and then the
basal diet until death, had a mammary tumour incidence of 69% which
was not judged to be different from the 82% incidence in the
control group (basal diet throughout life span and no added
selenium in the water).
In study 3 (Schrauzer et al., 1978a), 4 groups of 30 female
C3H/St mice were fed a basal diet ("Wayne F-6 Lab Blox") that was
different from that used in the first 2 studies and consisted of
fish meal, animal liver meal, soybean meal, corn and wheat flakes,
ground corn, wheat red dog, wheat middlings, soybean oil, cane
molasses, salt, brewer's yeast, and various vitamins and minerals.
This diet contained 244.8 g protein, 64.8 g fat, and 0.45 mg
selenium/kg. The 4 groups received selenium dioxide at 0, 0.1,
0.5, or 1.0 mg selenium/litre drinking-water and, after 22 months,
the incidence of mammary tumours was 42, 25, 19, and 10%,
respectively. Selenium supplementation at these levels did not
have any noticeable adverse effects on weight-gain or survival of
the mice. The incidence of spontaneous mammary tumours in the
control (unsupplemented) group was lower in this study than that
observed in the 2 previous studies (42 compared with 82%), and this
was attributed to the higher selenium content of the new basal diet
used in the third study (0.45 versus 0.15 mg/kg).
Medina & Shepherd (1980) fed BALB/cfC3H mice Wayne Lab Blox and
gave them selenium dioxide at 0, 2, or 6 mg selenium/litre
drinking-water, ad libitum, starting at 10 weeks of age and
continuing to the end of the study. In 12-month-old mice, 2 and 6
mg selenium/litre decreased mammary tumour incidence from 82% in
the untreated controls to 48 and 12%, respectively. There were no
effects of the selenium treatment on normal reproductive function
or weight gain in these mice. In another study, samples of 4
different preneoplastic outgrowth lines (D2, C3, C4, and CD-7)
were transplanted into the mammary gland-free fat pads of syngenic
mice. When the implants had filled the mammary fat pads with their
respective outgrowths (8 weeks after implantation), the mice were
given selenium dioxide at 4 mg selenium/litre drinking-water, ad
libitum, for the rest of the study. Such treatment with selenium
delayed the rate of tumour formation only in line C4, increasing
the time for half of the outgrowths to produce tumours from 34 to
44 weeks. In a third study, 2 - 6 mg selenium/litre drinking-water
had no effect on the growth rate of primary tumours transplanted
subcutaneously in BALB/c mice. Since selenium did not have any
effect on tumour formation rate in 3 of 4 preneoplastic mammary
outgrowth lines or on the growth rate of established mammary
tumours, the authors suggested that selenium might act by
inhibiting chemical or viral transformation of normal cells or by
inhibiting expression of initially transformed cells.
Because of the metabolic antagonism between arsenic and
selenium (section 7.1.6.3), Schrauzer and co-workers also
investigated the effect of arsenic on the genesis of the
spontaneous mammary tumours in C3H/St female mice described above.
In their first study on arsenic and selenium (which was part of the
same study described above in Schrauzer & Ishmael (1974)), giving
sodium arsenite at 10 mg arsenic/litre drinking-water to a group of
30 C3H/St mice for 16 months, reduced the incidence of spontaneous
mammary tumours to 27% compared with an incidence of 82% in the
untreated controls (Schrauzer & Ishmael, 1974). However, arsenic
treatment markedly stimulated the growth rate of spontaneous or
transplanted mammary tumours. In a second study concerning
arsenic, sodium arsenite at 80 mg arsenic/litre drinking-water,
administered to a group of 20 C3H/St mice, reduced the incidence of
spontaneous mammary tumours to 40% compared with an incidence of
82% in the untreated controls (Schrauzer et al., 1976), but some of
the tumour-inhibiting effect of arsenic appeared to be masked at
this dose level by its toxicity.
In a third study relating arsenic and selenium (Schrauzer et
al., 1978b), 4 groups of 30 female C3H/St mice were fed the Wayne
F-6 Lab Blox diet described above, which contained 0.29 mg
arsenic/kg. The 4 groups received the following supplements in
their deionized drinking-water: none, arsenic trioxide at 2 mg
arsenic/litre, selenium dioxide at 2 mg selenium/litre, or 2 mg of
both arsenic and selenium. The incidence of mammary tumours in the
4 groups was 41, 36, 17, and 62%, respectively, and the percentage
of multiple mammary tumours was 17, 40, 0, and 28. The age of
tumour onset in the corresponding groups was 4.5, 9, 16, and 8
months. Thus, in this case, treatment with arsenic appeared to
diminish the cancer-protecting effect of selenium. Arsenic also
accelerated tumour growth and increased the incidence of multiple
tumours.
Newberne & Conner (1974) fed 4 groups of male rats of the
Charles River CD strain, weighing about 100 g each, a basal diet
consisting of casein, 200 g/kg; sucrose, 209 g/kg; dextrose, 209
g/kg; dextrin, 209 g/kg; stripped lard, 80 g/kg; Wesson Oil, 20
g/kg; selenium-free salts, 50 g/kg; vitamin mix, 20 g/kg; choline,
3 g/kg; and vitamin B12, 0.05 g/kg. The basal diet contained about
0.03 mg selenium/kg. One group received the unsupplemented basal
diet, whereas the other 3 groups received the basal diet
supplemented with sufficient sodium selenite to attain approximate
dietary levels of 0.1, 1.0, or 5.0 mg selenium/kg, respectively.
Each group was fed the diet for 2 - 3 weeks, before oral
administration of 7 mg aflatoxin B1 in dimethyl sulfoxide/kg body
weight. The rats continued on their respective diets for an
additional 2 weeks, and the mortality rate after this time, in the
groups fed the diets containing 0.03, 0.1, 1.0, and 5.0 mg
selenium/kg was 28/29, 20/30, 7/28, and 27/29, respectively.
Histological examination revealed that rats receiving 1.0 mg
selenium/kg diet were partially protected against the aflatoxin B1
and also had less severe liver lesions. However, the groups fed
diets containing 1.0 or 5.0 mg selenium/kg exhibited a novel renal
lesion associated with acute aflatoxin B1 toxicity, characterized
by marked tubular necrosis at the cortical medullary junction.
Some tubules exhibited hyperplastic changes of the epithelium as
well as necrosis.
In a second study, Grant et al. (1977) gave 25 µg aflatoxin B1
orally, 5 days/week for 4 weeks, to 140 male Sprague Dawley rats.
The rats were fed normal or marginally lipotrope-deficient
semisynthetic basal diets containing sodium selenite at 0.03, 0.10,
0.50, 1.0, 2.5, or 5.0 mg selenium/kg. After 17 months, the
surviving rats were sacrificed and histopathological examination
was carried out. A very low incidence (20%) of hepatocarcinomas
was seen in rats receiving 1.0 mg selenium/kg in the normal basal
diet and 0.10 or 0.50 mg/kg in the lipotrope-deficient diet. No
other tumours were observed, and grading of the hepatic lesions
indicated that there was no significant differences among the
dietary selenium groups. Dietary sodium selenite and repeated
doses of aflatoxin B1 interacted to produce large bizarre cells in
the renal tubules, occurring in the same region of the kidney as
the severe necrosis seen previously in rats fed high levels of
dietary selenium and given an acute dose of aflatoxin B1.
Recently, a study of the effects of selenium on aflatoxin B1-
induced enzyme altered foci in rat liver has been reported (Milks
et al., 1985). Male Sprague Dawley rats were fed a selenium-
deficient diet and given sodium selenite at 5, 2, 0.2, or 1 mg
selenium/litre drinking-water for 3 weeks. Each rat then received
2 µg mol aflatoxin B1/kg body weight by stomach tube. For the next
week the selenium status was "normalized". Rats previously
receiving 5, 2, 0.2, or 0 mg selenium/litre received, respectively,
0. 0.2, 2, or 5 mg/litre. Then rat chow was fed and a promoting
regimen consisting of phenobarbital in the drinking-water, and a
partial hepatectomy was instituted. Eight weeks later, necropsies
were carried out and livers were stained histochemically for
gamma-glutamyl transpeptidase activity. Foci of activity were
counted. The number of foci seen in livers from rats given 5, 2,
0.2, or 0 mg selenium/litre during initiation were 0.62 ± 0.22,
1.97 ± 0.46, 3.35 ± 0.66, and 2.46 ± 0.23 per cm2, respectively.
The data suggested that 5 mg selenium/litre can protect against the
hepatocarcinogenic effects of aflatoxin B1 in the rat.
On the basis of anecdotal observations, Wedderburn (1972)
suggested that a decrease in the number of cases of intestinal
carcinoma in autopsied sheep might be associated with the
widespread veterinary use of selenium to prevent deficiency
diseases in sheep. Simpson (1972a) conducted an investigation into
the epidemiology of carcinomas of the small intestine of sheep in
which the viscera of 32 733 ewes were examined. Carcinomas of the
small intestine were diagnosed in 483 animals, but the prevalence
of these neoplasms in ewes regularly dosed with selenium throughout
their lives was not significantly different from the prevalence in
ewes never treated with selenium (Simpson, 1972b). Furthermore, no
significant differences were found in association with differences
in soil types on the farms from which the sheep originated. It was
concluded that soil-selenium levels and administration of selenium
in the form and at the dose rates used did not have any effects on
the development of intestinal carcinomas in sheep. Underwood
(1977) commented that neoplasias were not observed among the
various lesions attributed to selenium deficiency in animals.
Ip has made and summarized a series of observations on selenium
and carcinogenesis (Ip, 1985a).
Evidence of an interaction between dietary fat and selenium
status in the induction of mammary tumours by dimethylbenz-
[alpha]anthracene in rats has been presented by Ip & Sinha (1981).
They fed 8 groups of 23 - 25 female weanling Sprague Dawley rats
one of the following diets either deficient in selenium or
supplemented with sodium selenite at 0.1 mg selenium/kg: 1% corn
oil, 5% corn oil, 25% corn oil, or 1% corn oil plus 24%
hydrogenated coconut oil. In addition to the fat, the diets
consisted of Torula yeast, dextrose, HMW salt mix, vitamin mix,
alphacel, and DL-methionine. The diets were adjusted so that the
intake of all nutrients would be the same, except for dextrose and
fat, assuming that the rats would consume an equal number of
calories. The corn oil used was stripped of tocopherol and the
unsupplemented diet was deficient in selenium (less than 0.02 mg/kg
by fluorometry). Mammary tumours were induced by the intragastric
administration of 5 mg dimethylbenz[alpha]anthracene at 50 days of
age. Increasing the dietary polyunsaturated-fat level (corn oil)
increased the tumour incidence in rats fed the selenium-
supplemented diets (Table 51). However, the high-saturated-fat
diet was much less active in stimulating tumourigenesis. Selenium
deficiency increased the tumour yield only in rats fed the high
polyunsaturated-fat diet (25% corn oil). Increased tumour
incidence due to selenium deficiency was not seen in the rats fed
the low-fat diets containing polyunsaturated fat (1 or 5% corn
oil) or the high-fat diet containing primarily saturated fat (1%
corn oil with 24% coconut oil). Selenium deficiency, however, did
increase the incidence of mammary tumours in rats fed a low-fat
diet containing polyunsaturated fat (1% corn oil), when larger
doses of dimethylbenz[alpha]anthracene were used (10 or 15 mg -
data not shown).
Table 51. Incidence of palpable mammary tumours in
dimethylbenz(alpha)anthracene-treated rats fed different
levels and types of fats in the diet, with or without
selenium supplementationa
-----------------------------------------------------------
Fats in diet Selenium Incidence of palpable tumours
Corn Coconut in diet 19 weeks after dimethylbenz
oil oil (mg/kg) (alpha)-anthracene administration
(%) (%) Number of rats (%)
-----------------------------------------------------------
1 0 0 4/23 17.4
1 0 0.1 3/24 12.5
5 0 0 11/25 44.0
5 0 0.1 8/24 33.3
25 0 0 24/25 96.0
25 0 0.1 15/25 60.0
1 24 0 7/24 29.2
1 24 0.1 6/25 24.0
----------------------------------------------------------
a Adapted from: Ip & Sinha (1981).
In a recent study, the effects of vitamin E status on the
anticarcinogenic effect of selenium were examined (Ip, 1985b).
Female Sprague Dawley rats were fed a 20% stripped corn oil,
casein-based diet. Four dietary groups were formed: adequate
vitamin E/adequate selenium, adequate vitamin E/high selenium,
deficient vitamin E/adequate selenium, and deficient vitamin E/high
selenium. Adequate and deficient vitamin E diets contained 50 and
10 mg vitamin E/kg diet, respectively. Adequate and high selenium
diets contained Na2SeO3 at 0.1 and 2.5 mg selenium/kg,
respectively. At 50 days of age, rats received 5 mg of
dimethylbenz[alpha]anthracene (DMBA) each by stomach tube. Rats
were killed 20 weeks after DMBA administration. The tumour
incidences in the groups were: adequate vitamin E/adequate
selenium, 76%; adequate vitamin E/high selenium, 40%; deficient
vitamin E/adequate selenium, 84%; deficient vitamin E/high
selenium, 68%. This suggests that selenium protection against
carcinogenesis is decreased in vitamin E deficiency.
Pence & Buddingh (1985) studied the effects of selenium
deficiency on 1,2-dimethylhydrazine (DMH)-induced colon cancer in
the rat. They used male Sprague Dawley rats fed Torula yeast-based
diets containing 2% corn oil. Sodium selenite at 0.1 mg
selenium/kg was added to the diet of the controls. Weanlings were
fed the diets 3 weeks prior the institution of DMH treatment and
were killed after 20 weeks of treatment. No effects of selenium
status were noted on the incidence of colon adenocarcinomas.
The effects of selenium on UVR-induced skin carcinogenesis were
studied by Overvad et al. (1985). Female hairless mice were given
sodium selenite at 0, 2, 4, or 8 mg selenium/litre drinking-water.
Three weeks after selenium exposure began they were exposed to UVR
daily for 22 weeks. Then they were examined weekly for 26 weeks
for skin tumours, and relative tumour onset ratios were calculated.
The 2 mg selenium/litre treatment did not affect tumour onset but
the higher doses did. This suggests that selenium can protect
against UVR-induced skin cancer.
Birt et al. (1984) reported that high levels of selenium in the
diet increased pancreatic carcinogenesis induced by bis-(2-
oxopropyl)-nitrosamine (BOP) in male Syrian hamsters. Torula yeast-
based diets with either 0.1 or 2.5 mg selenium/kg were fed to
hamsters beginning at 4 weeks of age. BOP was given in 4 weekly
injections of 5 mg/kg body weight, beginning at 8 weeks of age.
Hamsters were killed at 78 weeks of age. When dietary fat was low
(11% calories as corn oil), the low-selenium group had 25
pancreatic ductular carcinomas (PCDA) in 18 hamsters, and the high-
selenium group had 63 PCDA in 23 hamsters. When dietary fat was
high (45% of calories as corn oil), the low-selenium group had 27
PCDA in 19 hamsters and the high-selenium group had 44 PCDA in 18
hamsters.
Other studies have been reported. Milner, who showed that
pharmacological doses of selenium inhibited the growth of
transplantable tumours, has recently summarized his work (Milner,
1985), and Thompson has summarized his work on mammary
carcinogenesis (Thompson, 1984). The metabolism of the carcinogen
2-acetylaminofluorene in selenium-deficient rats has been studied
by Besbris et al. (1982). They found that selenium-deficient rats
excreted more N-OH-acetylaminofluorene than controls, suggesting
that selenium might prevent the production of this carcinogenic
metabolite or promote its detoxification.
8. EFFECTS OF SELENIUM ON MAN
8.1. High Selenium Intake
8.1.1. General population
8.1.1.1. Signs and symptoms
When it became apparent that selenium was the toxic factor in
plants that caused alkali disease in livestock raised in
seleniferous areas, public health personnel became interested in
the possible hazards for human health in such regions, since
seleniferous grains or vegetables grown on high-selenium soil could
enter the human food chain. Smith et al. (1936) reasoned that, if
human selenosis were a problem anywhere, it would most likely occur
in farmers living in seleniferous regions, who consumed largely
locally-produced foodstuffs. Therefore, they surveyed a rural
population living on farms or ranches known to have a history of
alkali disease. Their survey inquired into the health status of
111 families and also determined the actual consumption of locally-
produced foods. Wherever possible, general physical examinations
were made and urine samples were collected.
These workers were unable to find any symptom or group of
symptoms or serious illness that could be considered characteristic
of, or could definitely be attributed to, selenium poisoning in
man. However, the incidence of vague symptoms of ill health and
symptoms suggesting damage to the liver, kidneys, skin, and joints
was rather high. But since the causes for such disorders are many,
it was not possible to determine whether selenium played any role
in their causation. Apart from the more vague symptoms of
anorexia, indigestion, general pallor, and malnutrition, the
following more pronounced disease states were observed: bad teeth,
yellowish discoloration of the skin, skin eruptions, chronic
arthritis, diseased nails, and subcutaneous oedema.
The same authors (Smith et al., 1936) were unable to
demonstrate a very definite correlation between the clinical
evidence of selenium intoxication in 127 subjects and the
concentration of selenium in the urine. In fact, they expressed
surprise that there was not any more definite evidence of serious
injury, particularly in subjects with high concentrations of
selenium in the urine. Relatively high urinary-selenium levels
were most often associated with pathological nails,
gastrointestinal disorders, icteroid skin, and bad teeth. The
incidence of high urinary-selenium in individuals with dermatitis
and arthritis was no greater than that in individuals without
symptoms.
Because of the ambiguous findings in their first study, Smith &
Westfall (1937) carried out a second, more detailed and intensive
survey to establish the symptomatology of human selenosis and its
relation to the amount of selenium excreted in the urine. For this
purpose, they examined 100 subjects from 50 families that had high
levels of urinary-selenium in their previous testing. The
percentage frequency of the various signs and symptoms observed is
given as follows: none, 24; gastrointestinal disturbances, 31;
icteroid discolouration of the skin, 28; bad teeth, 27; sallow and
pallid colour, especially in younger individuals, 17; history of
recurrent jaundice, 5; dermatitis, 5; pigmentation of the skin
(chloasma?), 3; pathological nails, 3; rheumatoid arthritis, 3;
cardiorenal disease, 2; vitiligo, 2. It was concluded that none of
these signs or symptoms could be regarded as specific for selenium
poisoning, and it was not certain that any one was the direct
result of the continual ingestion of selenium. Nonetheless, these
workers felt that the high incidence of gastrointestinal
disturbances was of importance. Moreover, the high incidence of
icteroid discolouration of the skin was thought to be related in
some way to the ingestion of selenium, possibly as a result of
liver dysfunction. The possible cariogenic effects of high levels
of selenium is discussed further in section 8.1.1.2. The other
symptoms occurred so infrequently that they did not seem to be
associated with selenium. However, it should be noted that the
value of all these observations is in doubt since there is no
comparative information available concerning the frequency of
occurrence of these signs and symptoms in an appropriate control
group.
Smith & Westfall (1937) estimated that most of their subjects
living in highly seleniferous areas were probably absorbing about
10 - 100 µg/kg body weight per day and that some of their subjects
might have absorbed as much as 200 µg/kg per day. For a 70-kg man,
these rates of absorption would be equivalent to a dietary-selenium
intake of 700 - 14 000 µg/day, assuming that all of the selenium in
the food had been absorbed.
As discussed in section 4.1.2, drinking-water rarely
contributes much selenium to a person's total daily intake, but a
brief note indicated that a family of North American Indians living
in Colorado, USA, suffered hair loss, weakened nails, and
listlessness after consuming well water reported to contain 9000 µg
selenium/litre for about 3 months (Anonymous, 1962). This incident
was thought to be the first authentic case of selenium poisoning in
human beings induced exclusively from a naturally-occurring
underground source of water. Tsongas & Ferguson (1977) studied the
effects of selenium in the drinking-water on the health of 2 groups
of persons living in a rural Colorado community. The first group
("exposed") received a water supply that contained 50 - 125
µg/litre, whereas the second group ("unexposed") received a water
supply that contained levels ranging from non-detectable (1
µg/litre) to 16 µg/litre. Examination of a total of 86 individuals
revealed that there were no significant differences between the
exposed and unexposed groups in the incidence or prevalence of any
of 85 health variables studied.
Lemley (1940) presented a 58-year-old rancher from South
Dakota, thought to be the first described case of chronic selenium
dermatitis in a human being, caused by the ingestion of selenium
from natural sources. However, 2 samples of urine collected from
this patient a week apart, contained only 0.043 and 0.040 mg
selenium/litre, levels that are considered to be within the normal
range. Although some improvement in the patient's condition was
noted when certain seleniferous foods consumed on his ranch were
avoided, the improvement was not marked. Administration of
bromobenzene, now known to be a potent hepatotoxin, cleared up the
dermatitis, but caused only a small increase in the urinary-
selenium output, which reached a peak value of about 0.100
mg/litre, after 4 days. Earlier work by Moxon et al. (1940) had
shown that the rate of excretion of selenium from selenized animals
could be increased by the administration of bromobenzene. The
likelihood that selenium was the cause of the dermatitis in this
patient is doubtful, since, in a later paper, Lemley & Merryman
(194l) claimed that a relapse in this patient's condition was cured
when some canned meat containing 0.40 µg selenium/kg (considered by
them to be a highly toxic amount of selenium) was withdrawn from
the patient's diet. This figure for the level of selenium in the
meat is almost certainly erroneous, since not only is it below that
which would be found in animals suffering from selenium deficiency
but it is also below the sensitivity of the analytical techniques
available at that time.
Other cases of presumed human overexposure to selenium were
reported by Lemley & Merryman (1941). For example, urine samples
from a ranching family living in South Dakota contained 0.200,
0.250, 0.300, 0.550, and 0.600 mg selenium/litre for the father,
mother, daughter, son, and uncle, respectively. Administration of
bromobenzene to the father resulted in a urinary output of selenium
of 1.800 mg/litre within 24 h. No mention of dermatitis was made
in connection with this family. Rather, all family members
suffered from slight, continual dizziness and clouding of the
sensorium, extreme lassitude accompanied by depression, and
moderate emotional instability. The patients tired on exertion and
complained that their powers of concentration were markedly
impaired. After a course of bromobenzene, these people improved
markedly and their general condition remained improved, since they
were instructed not to use the various food products of the ranch
that contained selenium. Lemley & Merryman concluded that the
following facts provided the basis for the diagnosis of selenium
poisoning in these subjects:
(a) knowledge that the subjects lived in a seleniferous
area;
(b) presence of concentrations of selenium in the urine
exceeding 0.100 mg/litre;
(c) increased elimination of selenium in the urine after
bromobenzene administration; and
(d) improvement in the subject's symptoms after
elimination of selenium from the diet.
The same authors also described a 65-year-old rancher from
South Dakota, who suffered alternate bouts of diarrhoea and
constipation. This patient's urinary-selenium concentrations were
as high as 0.250 mg/litre. An exploratory abdominal operation
suggested that the patient had very early cirrhosis of the liver,
which was confirmed by pathological examination. The patient
recovered from the operation and then gradually improved after
being placed on a strict selenium-free diet and given a course of
bromobenzene.
In a later paper, Lemley (1943) expressed the opinion that
human selenium poisoning is common, widespread, and, in certain
localities, of importance for the general public health. More
recently, Kilness (1973) complained that no follow-up public heath
survey with an appropriate control group had been made in South
Dakota since the initial surveys by the United States Public Health
Service in highly seleniferous areas more than 30 years previously.
Jaffe (1976) carried out a field study in Venezuela and
compared 111 children living in a seleniferous area (Villa Bruzual)
with 50 living in Caracas. The overall haemoglobin and haematocrit
values were somewhat lower in Villa Bruzual (128 g/litre and 39
volume %, respectively) than in Caracas (148 g/litre and 42 volume
%, respectively), but no correlations between blood- and urine-
selenium levels and haemoglobin or haematocrit values were found.
The children in Villa Bruzual consumed less meat and milk and had a
higher incidence of intestinal parasite infestation (Jaffe et al.,
1972a) than those in Caracas. Therefore, it was concluded that the
differences in haemoglobin were probably due to differences in
nutritional or parasitological status and not to differences in
selenium intake. Activities of prothrombin and serum alkaline
phosphatase and transaminases, which altered in selenium-poisoned
rats (Jaffe et al., 1972b), were normal in all the children and no
correlation with blood-selenium levels was apparent. Symptoms of
dermatitis, loose hair, and pathological nails were reported as
more frequent among the children in the seleniferous area than in
those living in Caracas, but no quantitative information was given.
The clinical signs of nausea and pathological nails appeared to be
correlated with serum- and urine-selenium levels. However, the
cause of the different incidence of clinical signs was considered
doubtful, especially in the absence of differences in the various
biochemical tests performed (Jaffe et al., 1972a).
The mean blood-selenium level of 111 school children from the
seleniferous area in Venezuela was 0.813 mg/litre (Jaffe et al.,
1972a). A subgroup of 28 children from this area who had blood-
selenium levels of over 1 mg/litre had a mean blood-selenium level
of more than 1.321 mg/litre. There was one child with a blood-
selenium level of 1.8 mg/litre. Another subgroup of 11 children
who had blood-selenium levels of less than 0.4 mg/litre had a mean
blood-selenium level of 0.330 mg/litre. Urinary-selenium levels
tended to reflect blood-selenium levels, since children in the
high-blood-selenium subgroup (> 1 mg/litre) excreted a mean level
of 0.657 mg/litre urine, whereas children in the low-blood-selenium
subgroup (< 0.4 mg/litre) excreted a mean level of 0.266 mg/litre
urine.
Kerdel-Vegas (1966) summarized 9 cases of acute intoxication
due to the ingestion of nuts of the "Coco de Mono" tree (Lecythis
ollaria) from the seleniferous areas of Venezuela. Although the
course of the poisoning differed from patient to patient (probably
because of differences in the amount of nuts consumed and the
length of time the nuts were present in the digestive tract), most
cases experienced nausea, vomiting, and diarrhoea, a few hours
after eating the nuts, followed by hair loss and nail changes, some
weeks after the initial episode. Two patients had foul breath that
was described as being reminiscent of decomposed seaweed or
phosphorus. After a period of time, re-growth of hair and nails
took place and most patients appeared to make a satisfactory
recovery. One fatality was reported, a 2-year-old boy who died, in
spite of proper medical attention and symptomatic treatment for
severe dehydration.
Dickson (1969) described his own personal experience after
eating sapucaia nuts ( Lecythis elliptica H.B.K.) that had grown in
Honduras. He reported hair loss and splitting of finger and
toenails several weeks after consuming the nuts. Although no
mention of selenium was made in his report, he noted that a
peculiar odour was associated with consumption of these nuts, not
only of his breath but of his body as well. Kerdel-Vegas (1966)
pointed out that the sapucaia, or chestnut of Para (which he called
Lecythis paraensis Ducke), is consumed as food in Brazil and other
countries to which it is exported (i.e., Europe and the USA). He
also commented that there is a popular saying in northern Brazil
that eating sapucaia leads to hair loss, but he knew of no
scientific or medical reports from Brazil. Signs of selenium
toxicity have been observed in rats fed diets containing defatted
Brazil nut flour that assayed 51 mg selenium/kg (Chavez, 1966), and
Thorn et al. (1978) found that samples of Brazil nuts marketed in
the United Kingdom contained an average of 22 mg selenium/kg
(range, 2.3 - 53 mg/kg).
As reported recently, an unexplained intoxication characterized
by nail deformation and the loss of hair and nails as the most
common signs was observed in Enshi county of Hubei province of the
People's Republic of China, more than 20 years ago, with a peak
prevalence during the years 1961 - 64 (Yang et al., 1983). In 5
villages with 248 inhabitants, about half of the population was
affected and in one particular village over 80% of the people were
affected. No other quantitative information about the cases was
given but a general description of the signs and symptoms observed
is presented below. The hair became dry and brittle and was easily
broken off at the scalp with retention of intact radicles so that
depigmented and dull hair continued to grow. A scalp rash
accompanied by intolerable itching resulted in hard scratching
which easily removed the hair. The nails became brittle with white
spots and longitudinal streaks on the surface, followed by a break
on the wall of the nail. With new nail growth, the broken nail
advanced and ultimately fell off. Effusian of fluid from around
the nail was common in many cases. The new nail had a rough and
ridged surface and was fragile and thickened.
Other signs of intoxication included skin lesions, tooth decay,
and abnormalities of the nervous system. The skin became red and
swollen and then blistered and eruptive followed, in some cases, by
ulcerations that took a long but unstated time to heal. The
lesions occurred primarily on the limbs and also on the back of the
neck. Mottled teeth were observed in the intoxicated individuals
but this observation may have been confounded by high exposure to
fluoride. In one heavily affected village, nervous system
abnormalities were seen, including peripheral anaesthesia,
acroparaesthesia, and pain in the extremities. Hyperreflexia of
the tendon commonly developed later followed by numbness,
paralysis, and motor disturbances. One case of hemiplegia was also
reported. There was an indication that this intoxication was
related to the locally grown monotonous diet consumed, which later
was shown to contain high levels of selenium. No quantitative data
regarding selenium exposure were obtained at the time of the
outbreak. However, because of circumstantial evidence, the
intoxication was considered by the authors to be selenosis, and, as
discussed in sections 5.1.1.1 and 6.2.3, current levels of exposure
to dietary selenium in an area with a history of intoxication, as
assessed by blood- and hair-selenium levels as well as dietary
intake data, exceeded those ever reported in any non-occupationally
exposed population (Table 8). The authors estimated that the daily
dietary intake of selenium, after the peak prevalence of the
poisoning had subsided, averaged 5 mg, and blood- and hair-selenium
levels averaged 3.2 mg/litre and 32.2 mg/kg, respectively. The
ultimate environmental source of the selenium in this episode of
intoxication was a highly seleniferous coal (average selenium
content of 300 µg/g) which lost its selenium to the soil as a
result of weathering processes. Once in the soil, the selenium was
taken up by plant crops and entered into the food chain.
Because of the nutritionally-beneficial effects of selenium in
animals, some investigators have deliberately given inorganic or
organic forms of the element to people with the aim of producing
some desirable health benefit. For example, Westermarck (1977)
administered selenium, as selenite, in oral doses of 0.05 mg/kg
body weight per day, for more than one year, to patients with
neuronal ceroid lipofuscinosis (NCL) and did not observe any toxic
manifestations. On the contrary, it was felt that some of the NCL
patients showed at least a transitory improvement in their
condition. In some patients, a slight increase in serum aspartate
aminotransferase activity was observed, but apparently this is seen
in a number of patients with NCL.
Schrauzer & White (1978) described 2 individuals who had been
taking commercially-available nutritional supplements consisting of
selenium-containing yeast at doses of 200 and 450 µg selenium,
daily, for 18 months. Together with their dietary intakes, these
individuals received a total of 350 and 600 µg/day. Although some
marginal haematological changes were seen and the serum-glutamic
oxaloacetic transaminase activities were on the borderline of high,
it was concluded that daily intakes of up to 600 µg selenium for 18
months do not induce toxic effects in well-fed individuals.
In a study by Perona et al. (1978), 4 subjects were given,
orally, a 2-mg dose of selenium, as sodium selenite, daily, for
20 - 40 days, to determine the effects of in vivo selenium
administration on the activity of human erythrocyte-glutathione
peroxidase. It was stated that none of the subjects exhibited any
symptoms of selenium poisoning, but the criteria used to judge any
deleterious effects of selenium were not described.
Yang et al. (1983) reported the case of a 62-year-old man who
had taken one tablet containing 2 mg sodium selenite per day for
more than 2 years. The subject did not have any symptoms of
indisposition but presented with thickened, fragile and somewhat
honeycomb-like fingernails. After the oral intake of sodium
selenite was stopped, the surface of the new nail growth became
smooth and gradually recovered. Blood-and hair-selenium levels
were 0.179 mg/litre and 0.828 mg/kg, respectively, on the day the
selenite tablets were discontinued. These tissue-selenium levels
are much lower than those reported by the same authors in Enshi
county of the Hubei province (Yang et al., 1983) suggesting that
the form of selenium ingested must be considered when interpreting
tissue-selenium levels in the diagnosis of selenium poisoning.
Ingestion of superpotent selenium tablets, meant to be consumed
as a "health food" supplement, resulted in 12 cases of human
selenium toxicity in the USA in 1984 (Anonymous, 1984; Jensen et
al., 1984; Helzlsouer et al., 1985). Each tablet contained 27 - 31
mg selenium by analysis (about 182 times more than the level stated
on the label). Approximately 25 mg of the selenium was present as
sodium selenite whereas the rest was elemental and/or organic
selenium. The total doses of selenium estimated to be consumed by
the victims ranged from 27 to 2387 mg. Based on the limited
information available, the symptoms reported in these cases as most
common were nausea and vomiting, nail changes, hair loss, fatigue,
and irritability. Other symptoms included abdominal cramps, watery
diarrhea, paraesthesias, dryness of hair, and garlicky breath.
Eight of the 12 victims did not have any abnormalities in the blood
chemistry, and the results of liver and kidney function tests were
normal.
A level of 500 µg has been proposed as the tentative maximum
acceptable daily intake of selenium for the protection of human
health (Sakurai & Tsuchiya, 1975). This figure was derived from an
initial estimate of the mean normal daily intake of selenium by
human beings of between 50 and 150 µg. It was concluded that
values of 10 - 200 times the normal intake appeared acceptable as
an estimated range for the margin of safety within which the
average human being could tolerate selenium. By taking the lower
values of both these estimates, the lowest level of potentially
dangerous daily intake of selenium was estimated to be 500 µg.
8.1.1.2. Attempts to associate high selenium intake with human
diseases
(a) Dental caries
As already mentioned in section 8.1.1.1, bad teeth was one of
the signs that was thought to be possibly associated with a high
intake of selenium by a rural population living in seleniferous
areas of South Dakota, Wyoming, and Nebraska (Smith et al., 1936)
and by children living in a seleniferous zone in Venezuela (Jaffe,
1976). Several studies have been concerned with the possibility of
a specific association between the incidence or prevalence of
dental caries and residence in a seleniferous area, urinary
excretion of selenium, or the selenium content of teeth.
Hadjimarkos & Storvick (1950) and Hadjimarkos (1956) noted a
geographical difference in the prevalence of dental caries in 2029
children, between the ages 14 and 16 years, residing in 4 different
counties in Oregon. Two different counties, one with the highest
(Clatsop), and one with the lowest (Klamath), rates of caries
experience were selected for further study. Urine samples from 24
and 29 male children who were attending county seat high schools
and who were born and residing in the respective counties were
analysed for selenium content. The mean levels of urinary-selenium
were 0.049 and 0.037 mg/litre in the groups of children from the
counties with the high (14.4 DMF (decayed, missing, or filled)
teeth per child) and low (9.0 DMF teeth per child) prevalence of
dental caries, respectively (Hadjimarkos et al., 1952).
In a second study, 2 additional counties with similar but high
rates of caries experience (Jackson and Josephine, 13.4 and 14.4
DMF teeth per child, respectively) were selected (Hadjimarkos &
Bonhorst, 1958). Analysis of 33 urine specimens from continuously-
resident, male high school children attending a county seat high
school (Jackson) and 46 specimens from similar children attending a
high school serving a rural area (Josephine) gave values (mean ±
SE) of 0.074 ± 0.007 and 0.076 ± 0.005 mg/litre, respectively. It
was concluded that there was an association between the high
prevalence of dental caries and the values of urinary-selenium
excretion, which were double those seen in the previous study in
the county with a lower rate of caries experience.
In order to explain the variations in urinary-selenium levels
observed in the above studies, samples of locally-produced and -
consumed milk and eggs, as well as local drinking-water samples
were collected from 74 farms located in Klamath, Jackson, and
Josephine counties and analysed for their selenium content
(Hadjimarkos & Bonhorst, 1961). The selenium levels were almost 10
times higher in food samples collected from the counties with high
caries prevalence (Jackson and Josephine) than in those from the
county with low caries prevalance (Klamath). The selenium levels
in the milk samples obtained in Jackson and Josephine counties were
higher than those listed in Table 3.
In another study, Tank & Storvick (1960) determined the extent
of dental caries in a population of children from 15 areas of
Wyoming that had been identified previously as seleniferous or non-
seleniferous on the basis of the geological distribution of
selenium, occurrence of the element in vegetation, and the
occurrence of selenosis in livestock. These research workers
claimed that the dental caries rate in the permanent teeth was
higher in children residing in seleniferous areas of Wyoming than
in children living in non-seleniferous areas of Wyoming, but they
were unable to demonstrate any consistent relationship between
calculated selenium intake and caries or urinary-selenium excretion
and caries.
Suchkov et al. (1973) found that the incidence of caries varied
in 3 different geographical zones (mountainous, pre-mountainous,
and forest-steppe) in the Chernovitsi region of the Ukraine.
Analysis of teeth from people residing in rural areas in these 3
zones and mainly consuming locally-produced foodstuffs revealed a
direct correlation between the selenium content of the teeth and
the incidence of dental caries. The people in the mountainous zone
had the highest level of selenium in the teeth and the greatest
incidence of dental caries, whereas the people in the forest-steppe
zone had the lowest level of selenium in the teeth and smallest
incidence of dental caries. This correlation was true for
deciduous teeth as well as for permanent teeth. However, as
pointed out by the authors, the mountainous zone also had the
softest water and the lowest content of fluorine in the drinking-
water, so that factors other than selenium might have been
involved.
It was not possible to demonstrate any association between the
urinary excretion of selenium and the incidence of dental caries in
subjects living on the South Island of New Zealand, an area known
to be low in selenium (Cadell & Cousins, 1960). However,
Hadjimarkos (1960) claimed that the levels of urinary excretion of
selenium in this study (all less than 0.050 mg/litre) were too low
to be associated with an increased incidence of caries.
As emphasized by Schwarz (1967), the levels of urinary-selenium
excretion reported in the Hadjimarkos studies were within the
limits generally found in the normal population without excessive
selenium intake. It may be recalled from section 7 that, in
experimental animal studies, very high toxic levels were used to
demonstrate the cariogenic effect of selenium.
(b) Reproduction
Possible effects of selenium on reproduction were suspected
following old anecdotal reports from Colombia, South America that
women living in areas, later shown to be seleniferous, gave birth
to malformed babies (Rosenfeld & Beath, 1964), but there is a lack
of reliable studies. Robertson (1970) pointed out that, among 6
women preparing microbiological media containing sodium selenite,
one probable and 4 certain pregnancies all ended in abortions, save
one that went to term. The infant was born with bilateral club
foot. However, no differences in urinary-selenium levels were
observed between this group of women and a control group living in
the same area, and inquiries by the author at other laboratories
carrying out comparable work did not show any evidence of similar
trouble. Jaffe & Velez (1973) could not demonstrate any
correlation between the selenium level in the urine of school
children in different Venezuelan states and the incidence of infant
mortality due to congenital malformations, on the basis of
published public health statistics. Jaffe (1973) concluded that no
recent observations of a teratogenic action of dietary selenium in
human beings had been reported at that time.
(c) Amyotrophic lateral sclerosis
Kilness & Hochberg (1977) reported an unusual cluster of 4
cases of amyotrophic lateral sclerosis (ALS) in male farmers living
in a seleniferous area and indicated that selenium might be an
environmental factor predisposing to the disease. But Schwarz
(1977) pointed out that the frequency of ALS was at least as high
if not higher in areas that were selenium-deficient as in those
with normal or elevated levels and Kurland (1977) suggested that
the cluster was more indicative of a chance occurrence than of a
new etiological lead in ALS. Moreover, Norris & Sang (1978) found
that 19 out of 20 well-established cases of ALS had urinary-
selenium levels lower than the mean for unexposed persons and
therefore concluded that selenium exposure was of no concern in the
average case.
8.1.2. Reports on health effects associated with occupational
exposure
Although the toxicological potential of selenium for human
beings can be inferred from studies carried out with laboratory
animals, certain precautions must be taken in applying the results
of animal studies to the industrial health aspects of selenium.
First, the number of studies dealing with the respiratory exposure
of animals to selenium compounds is limited, and the dose levels
employed have usually been higher than those encountered in
industry. On the other hand, the period of exposure used in the
studies reviewed in section 7.1.2.3 did not exceed one month. The
specific chemical form and physical state of the selenium must be
considered as well as the fact that the chemical form of selenium
may change when in contact with moist mucous membranes or with
sweat. These factors, plus several others, must be taken into
account when considering the health effects of various selenium
compounds under occupational exposure conditions.
The Task Group recognized that, for various reasons, knowledge
on the health effects of industrial exposure to selenium compounds
was not complete. Acute exposures to selenium are the result of
accidents and are, of necessity, described on an ad hoc case study
basis. Regarding the effects of long-term selenium exposure in
industry, there is a lack of epidemiological studies that include
unexposed control groups. Also, no follow-up studies are available
comparing the health status of a sufficiently large number of
workers, previously exposed to selenium, with that of the unexposed
population. Furthermore, the exposure level was not known with
certainty in either acute or long-term studies, and, in some cases,
the form of selenium was not established. In many cases,
simultaneous exposure to other noxious agents occurred.
Nevertheless, the Task Group felt that some preliminary assessment
of the toxicological potential of selenium in industry could be
made on the basis of the occupational experience available with
selenium compounds.
Since Hamilton's first observation on the effects of selenium
exposure in industry in 1917 (Hamilton, 1927 - 34), several hundred
persons have been described in the literature who have been
directly affected by the vapour, fumes, or dust of selenium and its
compounds. In addition, there is a systematic study of a group of
over 100 selenium workers for a period of 2 years (Glover, 1967).
Systematic attention has also been given to the health of workers
exposed to selenium in the USSR (Filatova, 1948; Monaenkova &
Glotova, 1963; Gracianskaja & Kovshilo, 1977). Several reviews
evaluating the health effects of selenium from the point of view of
occupational health have been presented (Glover, 1954 - 70, 1976;
Cooper, 1967; Izraelson et al., 1973; Cooper & Glover, 1974).
8.1.2.1. Fumes and dust of selenium and its compounds
Workers in several industries, such as the production or
recovery of selenium itself, or the manufacture of glass or
rectifiers can be exposed to the fumes and dust of selenium and its
compounds. In addition, direct contact with powders or solutions
containing selenium compounds is possible and the biological
effects of such contact will be discussed in this section.
The chemical form of selenium in the fumes and dusts found in
the above-mentioned industries, consists of elemental selenium plus
various amounts of selenium dioxide, the only oxide of selenium
found in the industrial environment. However, in some cases, the
possible occurrence of other selenium compounds, such as hydrogen
selenide, cannot be excluded. Instances where hydrogen selenide
was the main selenium compound of concern will be discussed
separately in section 8.1.2.1.2.
8.1.2.1.1. Selenium dioxide
Selenium dioxide mainly occurs in industry:
(a) during the production of the compound, usually by the
oxidation of elemental selenium; and
(b) whenever selenium is heated in air, purposely or
accidently, above its melting point. "Selenium fume"
rising under these conditions is a mixture containing
red elemental selenium and about 20 - 80% of selenium
dioxide.
Glover (1954, 1970, 1976) has summarized the acute local,
systemic, and possible long-term effects of occupational exposure
to selenium dioxide. Acute local effects are seen in the lungs,
gastric mucosa, skin, nails, and eyes. Sudden inhalation of large
amounts of selenium dioxide produces pulmonary oedema, due to a
local irritant effect on lung alveoli. Working in an atmosphere
containing selenium dioxide can increase indigestion. Contact with
the skin results in burns or dermatitis. Occasionally, a true
urticarial type of generalized allergic body rash may occur, in
which case the individual must be removed from any work involving
selenium. The selenium dioxide may penetrate under the nails and
cause excruciating pain in the nail beds. If selenium dioxide
enters the eyes, prompt flushing with water will prevent
conjunctivitis. "Rose eye", a pink discoloration of the skin of
the eyelids, which often become puffy, is sometimes seen in persons
who work in an atmosphere of selenium dioxide dust. Systemic
effects of selenium dioxide exposure include garlicky-smelling
breath, metallic taste on the tongue, and indefinite
sociopsychological effects. The garlicky breath is the first and
most characteristic sign and has been used by occupational
hygienists as a way of monitoring exposure, even though the odour
is not always a reliable index in this respect. The metallic taste
is an earlier symptom but, being more subtle, is often overlooked
by workers. Sociopsychological effects such as lassitude and
irritability have also been associated with selenium exposure.
Since the effects of selenium overexposure lead to hepatic damage
in animals, Glover (1954) concluded that it would be prudent to
watch for such damage in human beings.
(a) Reports on health effects connected with short-term
accidental exposures
An industrial incident in a plant engaged in smelting scrap
aluminium and other non-ferrous metals was reported by Clinton
(1947). The aluminium scrap included more than 100 kg of aluminium
rectifier plates coated on one side with metallic selenium overlaid
with a coating of an alloy of bismuth, cadmium, and tin. When an
attempt was made to skim the dross prior to pouring, a cloud of
reddish fume arose. The fumes were intensely irritating to the
eyes, nose, and throat and the plant was evacuated. No workers
were exposed for more than 2 min.
All exposed workers noticed immediate and intense irritation
of the eyes, nose, and throat, and an unpleasant sour, garlic-like
odour to the fumes. The more heavily exposed workers complained of
a severe burning sensation in the nostrils, and dryness of the
throat; followed after 2 - 4 h by severe headache, mainly frontal
in location, lasting until the following day. Several men observed
immediate sneezing, coughing, and headache, followed for 4 - 8 h by
nasal congestion, dizziness, and redness of the eyes. Most of the
men noticed a bad taste in their mouths and an unpleasant odour to
their skin and clothing. Other people, however, did not note any
unpleasant odour in the breath of the exposed workers.
Two labourers who had attempted to skim the dross from the
furnace and therefore underwent intense exposure were hospitalized
for observation. On arrival at the hospital, they complained of
soreness of the eyes and lachrymation, pain in the nose, slight
difficulty in breathing, and frontal headache. Physical
examination on admission revealed conjunctival injection,
congestion of the mucous membranes of the nose and throat, and
oedema of the uvula. One man had a few fine rales audible in the
right base; roentgenological examination of the chest did not
reveal any abnormalities. The temperature, pulse, and respiration
were normal. Both workers were discharged 2 days after the
accident, at which time they were asymptomatic and had no positive
physical findings. Another worker, a foreman, underwent a more
severe exposure than most of the workers. He was not hospitalized,
but, about 8 - 12 h after the accident, he developed severe
dyspnoea accompanied by a slight elevation in temperature, in
addition to a headache and sore throat. Physical examination
revealed fine rales in both bases, as well as scattered asthmatic-
like wheezes throughout the chest. These signs and symptoms
cleared in about 24 h, without specific therapy. All persons
exposed to the fumes had recovered entirely in 3 days, and no
sequelae have been encountered.
In the same year, Lauer (1947) described an episode that
occurred when 15 men were exposed to fumes arising from an
explosion and fire, which occurred in a pot when selenium became
overheated in contact with aluminium. The 2 elements produced a
high-temperature reaction, and fumes and vapours were dispersed
throughout the work area. The lengths of exposure varied, some
victims were using masks part of the time, and others were almost
without protection.
The exposed men reported to the medical dispensary about 15 -
45 min after the accident. All complained of soreness and burning
of the nose and throat, and 8 out of 15 cases had some dyspnoea.
Two of these cases were moderately severe, requiring almost
continuous oxygen therapy. Headache and dizziness and a burning
sensation in the eyes occurred in 6 cases. Three others complained
of substernal burning and tightness of the chest. In 4 there was
nausea and vomiting. All were hospitalized. Physical examination
showed reddening of nasal and pharyngeal mucosa, wheezes, and
musical rales in the lungs (12 cases). These symptoms abated
quickly. In about 10 days, there were few subjective complaints.
All apparently recovered satisfactorily with no known
complications. To assess possible liver affection, Hanger's
cephalin cholesterol tests and Icterus Index determinations were
performed on admission to the hospital and again at the end of the
first, second, third, and seventh week. On the day of admission to
the hospital, Hanger's tests were made on 13 workers, 4 of which
were positive and 9, negative. At the end of the first week
following exposure, there were 10 positives and 3 negatives. At
the end of the second week, there were 11 positives and 3 negatives
out of a group of 14 tested, and the same result was obtained at
the end of the third week. By the end of the seventh week, only 4
of 11 tested were positive. The average value of the Icterus Index
on admission to the hospital was 7.3 units. One week after
exposure, it was 13.5. In 2 weeks, it averaged 20.7. After the
third week, it had fallen to 9.9 and, by the end of the seventh
week, the average Icterus Index was 7.2.
An accident connected with a fire in a selenium rectifier plant
was described by Wilson (1962). Twenty-eight of the employees were
exposed directly to the smoke and fumes containing selenium
dioxide. The length of exposure to the fumes varied with the
individual, but no one was exposed for more than 20 min. Oxygen
was administered by mask to the men lying on the ground, who
experienced bronchial spasm with coughing, gagging, and, in some
instances, transient loss of consciousness.
The initial signs and symptoms were a feeling of constriction
in the chest, accompanied by burning and irritation of the upper
respiratory passages, violent coughing and gagging with nausea and
vomiting, and a bitter acid taste in the mouth. During the acute
episode, there were mild signs of shock, with a drop in blood
pressure and an elevated pulse and respiratory rate. Other
symptoms, experienced during this stage, were burning of the skin,
conjunctivae, and mucous membranes of the upper respiratory
passages. Within 4 h, all patients had apparently recovered from
the acute episode and a recheck of their blood pressure, pulse,
respiratory rate, and general condition was found to be within
normal limits.
Within 6 h of the exposure, the victims began complaining of
secondary symptoms with the onset of generalized chills accompanied
by nausea and vomiting, diarrhoea, malaise, dyspnoea, and headache.
The onset of secondary symptoms was delayed in some of the victims
for several hours after the initial exposure. Within 12 h, all
exposed personnel began experiencing the symptoms described. The
following morning, the first patient, a 30-year-old male, was
admitted to the hospital. He was cyanotic and in moderate
respiratory distress. He complained of chest pain bilaterally,
and was experiencing bronchial spasms. A chest roentgenogram
revealed extensive bilateral consolidation of the lung fields,
indicating pneumonia. The white blood cell count was elevated to
153 000 with a marked prominence of neutrophils. He experienced a
stormy course, requiring constant oxygen for the first 9 days of
hospitalization. By the fifth hospital day, X-ray films revealed a
further extension of the previously noted bilateral pneumonic
consolidation. Two weeks following admission, comparison of a
chest film with previous films revealed marked improvement
bilaterally. He was free of respiratory distress, his white blood
cell count had returned to within normal limits, and he was
discharged home for convalescence. It is interesting to note that
this was the only employee of the 5 hospitalized cases who did not
receive oxygen on the afternoon of the fire. On the same day, the
second victim was admitted to the hospital in a similar dyspnoeic,
cyanotic state with an elevated white blood cell count of 24 600.
X-ray again revealed bilateral atelectasis and consolidation. This
patient required continuous oxygen for 6 days, and was discharged
after 9 days of hospitalization: his white blood cell count had
returned to normal and a repeat roentgenogram revealed improvement.
This was the second most involved and prolonged illness, and he
received only about 6 breaths of oxygen following his exposure.
Three days after the accident, a third employee was admitted to the
hospital in less respiratory distress, with a normal white blood
cell count and a chest film that revealed bilateral elevation of
the diaphram with extensive peribronchial infiltration and some
consolidation at the lung bases. On the same day, another worker
was admitted to the hospital with a normal white blood cell count
and a chest film revealing bilateral increase in lung markings
consistent with bronchitis; however, no consolidation was evident.
The final patient to be hospitalized was admitted 4 days after the
accident with a white cell count of 12 800 and a prominence of
neutrophils. The X-ray film indicated bilateral pneumonia.
Four days after exposure, all other employees with any upper
respiratory complaints were examined. Thirty-two of the 53
employees examined were found to have a residual bronchitis, of
minimal to moderate degree, that required medication. These were
treated on an outpatient basis. Within 1 week, all were
asymptomatic and free of any respiratory signs.
Both Monaenkova & Glotova (1963) and Skornjakova et al. (1969)
have described occupational incidents of short-term selenium
dioxide exposure. In the former case, 6 workers, 20 - 52 years
old, were exposed from 5 to 20 min in a room with a leaky selenium
still. The workers suffered acute pain in the eyes, hoarseness in
the throat, painful cough, and a heavy feeling in the chest. Acute
conjuctivitis, laryngotracheitis, and bronchitis were also noted
and gastroenterocolitis was common to all. Transient fever was
seen in 2 workers. The various signs and symptoms disappeared
within 5 - 7 days, but, on return to work involving selenium, 2
workers developed chronic bronchitis. In the report of acute
selenium dioxide exposure by Skornjakova et al. (1969), there was
irritation of the eyes and mucous membranes, coughing without
expectoration, nasal secretion, headache, vertigo, nausea,
vomiting, garlicky-smelling breath and skin, general weakness, loss
of consciousness, and collapse. Two weeks after exposure, an
allergic whole body rash occurred that disappeared after removal of
the worker from contact with selenium.
(b) Effects of short-term and/or repeated dermal exposure
Selenium dioxide is more than a primary irritant, it causes
extremely painful burns on the skin which, however, always heal
without a scar. Theoretically, the selenium dioxide powder itself
does not burn the skin (and if dropped on to the skin should be
immediately brushed off dry). However, in practice, in the
industrial environment, there is sufficient moisture on the skin
from sweating for this very deliquescent white solid to form a
sticky solution of selenious acid within seconds, or at the most,
minutes, of coming into contact with the skin. Selenious acid is
so soluble that, if the skin is immediately washed with a lot of
water, there will be no burn or even rash from accidental contact.
When the selenious acid does burn the skin, there is little to see
or feel for several hours. After 4 h with a 50% solution, an
unremitting intense pain begins, small petechial areas occur, and a
faintly orange coloration occurs, which indicates reduction of some
of the selenious acid to elemental red selenium. The pain and
necrosis can be prevented by the application of a reducing solution
or ointment, such as 10% sodium thiosulfate (Glover, 1954). If the
burn remains untreated it may go on to ulceration, but this is
rare. Högger & Böhm (1944) described a case of a woman who
suffered pain and reddening of the middle, ring, and little fingers
of one hand when selenium dioxide crystals penetrated into the
protective rubber glove that she was wearing.
The first accurate description of a case of selenium fumes
causing allergic dermatitis was published by Duvoir et al. (1937).
A chemical engineer, who had handled selenium for 3 years in the
past, developed swelling of the face with a hard urticarial oedema
of the nose and cheeks extending on the neck down to his shirt
collar following a 3-day exposure to selenium vapours. There was a
semi-circle of vesicles on the lower lids, but the forehead and
ears escaped completely. His hands showed discrete lesions.
Within 24 h, the genital oedema had disappeared, after 3 days, the
rash started to go, and, by 9 days, there was only desquamation
left. The 5-cm plaque, occurring 1 h after a patch test with 10%
potassium selenite, showed a greater than average contact
sensitivity.
Another case was described (Halter, 1938) involving a glass
worker whose job was to add a mixture of red selenium and sodium
selenite to the glass. This man was in daily contact with selenite
powder for 36 years. He complained of an oedematous erythema of
the face and neck, and several hard infiltrated raised plaques on
the dorsi of the hand and fingers. On examination, his nasal and
laryngeal mucosae were found to be reddened, and there was a very
slight conjunctivitis. There were no changes in the skin of areas
protected by clothing. His only other complaint was of headache.
There were no symptoms or signs referrable to the nervous or
gastrointestinal systems. Selenium was detected in the urine (no
actual values were given). His liver was found to be enlarged and
there was an increase of porphyrins in the urine.
Pringle (1942) has described several cases of acute dermatitis
resulting from contact with dry selenium dioxide or selenium
dioxide dissolved in water, and 2 cases of acutely painful
paronychia after accidental contact with dry selenium dioxide. In
addition, several cases of mild rash on the anterior surface of
both forearms, but principally affecting the bend of the elbows,
were seen among employees working with heated selenium dioxide in
the fume cupboards. Only their fingers, protected at that time
with gloves, entered the fume cupboard, which apparently was
adequately protected. The dermatitis resembled a seborrhoeic
condition and there was frequently a thin watery discharge. In the
course of a year, 2 cases developed a high degree of sensitivity to
selenium dioxide, so that working in the same department, though
not actually on selenium, caused repeated recurrences and they had
to be removed permanently to another part of the factory.
(c) Studies on the health effects of long-term exposure
Filatova (1948) reported that workers exposed over a long
period of time to selenium aerosols containing elemental selenium
at levels of 0.35 - 24.8 mg/m3 and selenium dioxide at levels of
0.11 - 0.78 mg/m3 developed rhinitis, nasal bleeding, headaches,
loss of weight, irritability, and pain in the extremities
(Izraelson et al., 1973).
Conditions of exposure and the related health effects in a
rectifier factory were investigated by Kinnigkeit (1962). Air-
selenium levels were determined in various workplaces and blood-
selenium levels were measured in 62 workers exposed to selenium.
Urinary-selenium levels were also determined in 22 workers. The
reported air-selenium levels did not exceed 0.05 mg/m3 and were
less than the threshold limit value. However, as discussed in
section 5.2.1 there was a clearcut discrepancy between the air
values and the selenium levels observed in blood and urine,
indicating that the actual workers' exposure must have been
considerably higher. Of 62 workers, over half (35) complained of
irritability, sleeplessness, loss of appetite, and nausea. Twenty
six had headaches and 3 had cramplike pains in their limbs.
Clinical examination revealed irritation of the mucosa in 9
workers, with conjunctivitis and slight tracheal bronchitis. Of
the 2 workers that had unavoidable skin contact with selenium, one
had excematous lesions of the forearms and the other had bluish-red
urticarial exanthema.
The Takata reaction and the thymol test were performed on 61 of
these workers, and the results were within the normal range in all
employees, with the remarkable exception of workers engaged in the
electrical testing of the rectifier plates. In this group of 13
people, abnormal results suggesting impaired liver function, were
obtained in 8 workers, the only employees from the plant showing
this pathological reaction. As underlined by Kinnigkeit, workers
carrying out this process handled the plates by hand and were
frequently directly exposed to fumes containing selenium dioxide
arising from the burning out of plates during electrical testing.
As discussed previously, this group was characterized by a very
high mean blood-selenium level (geometrical mean ± SE: 15.8 ± 11.8
mg/litre). A great interindividual variability in selenium-blood
levels existed with this and other groups, but no attempt was made
to relate these individual values to the results of liver function
tests. Another group of workers had blood-selenium levels that
were almost as high (Table 13) (section 6.2.3), but no evidence of
liver dysfunction was observed in this group. However, it cannot
be assumed that the form and pathway of the exposure to selenium
was the same in both groups.
Monaenkova & Glotova (1963) summed up results of clinical
observations on 12 people, aged 30 - 50 years, who had been
occupationally exposed to selenium for 3 - 16 years and who
manifested the symptoms of chronic selenium and selenium dioxide
poisoning. Ten of the 12 persons had been previously engaged in
enterprises producing selenium rectifiers and were exposed to both
elementary selenium and selenium dioxide. The patients complained
of pain in the right hypochondrium, dyspeptic phenomena, undue
fatigability, dyspnoea, weakness, and sleeplessness. Several
patients complained of cough and, in 3 of them, chronic bronchitis
or moderate emphysaema were established. One patient had asthmatic
bronchitis. Almost all patients had various impairments of the
liver and gastrointestinal tract (toxic hepatitis in 6 patients,
dyskinesis of gallbladder in 4 patients, cholecystis in 4 patients,
and spastic colitis in 4 patients). The patients showed an
astheno-vegetative syndrome, pigmentation of exposed areas of the
skin, and, in 8 persons signs of a hyperfunction of the thyroid
gland (radioiodine uptake measurements) were found. Elevation of
the basal metabolic rate or hyperfunction of the thyroid gland were
mentioned also briefly in a previous case report (Halter, 1938) and
in a review (Holstein, 1951), respectively.
A group of selenium workers in a rectifier factory was followed
by Glover (1967, 1970) in 1953 - 56. Every 3 months, urinary-
selenium levels were determined, and the workers were asked about
their health and examined for the presence of garlicky-smelling
breath and skin rashes. Other routine medical checks were not
performed. Workers complained of indigestion and epigastric pain;
severe haematemesis (with hospitalization) occurred in one case.
Several men noticed symptoms of lassitude and irritability, when on
selenium work, which cleared whenever they were taken away from
selenium. A strong odour of garlic on the breath was detected in
most workers with selenium-urinary levels of 0.5 - 1.0 mg litre,
but not below these levels. The breath odour disappeared in 7 - 10
days following the removal of workers from contact with selenium.
Glover (1967, 1970) was able to trace 17 deaths, occurring within
10 years, among selenium-exposed workers in the same factory (Table
52). The author recognized that the list of deaths was not
complete and that the group was too small to permit far-reaching
conclusions. The length of exposure to selenium in individual
cases was not given. Carcinomas arose in various organs: 2
bronchus, 1 stomach, 1 colon, 1 ovary, and 1 testis.
Table 52. Comparison of the certified causes of death of
seventeen selenium workers with the expected distribution
by cause based on the experience throughout England and
Wales
----------------------------------------------------------
International Cause of death Observed Expectedb
list number
----------------------------------------------------------
001 - 019 tuberculosis 0 0.4
140 - 205 malignant neoplasms 6 5.1
330 - 334 vascular lesions 0 1.4
410 - 416 chronic rheumatic 2 0.6
heart disease
420 - 422 arteriosclerotic 3 3.8
heart disease
----------------------------------------------------------
Table 52. (contd.)
----------------------------------------------------------
International Cause of death Observed Expectedb
list number
----------------------------------------------------------
430 - 434 "other" heart disease 0 0.2
440 - 443 hypertensive heart 0 0.2
disease
444 - 447 "other" hypertensive 1 0.2
disease
450 - 456 disease of arteries 0 0.2
480 - 483 influenza 0 0.1
490 - 493 pneumonia 0 0.5
500 - 502 bronchitis 2 0.9
- all other causes 3 3.5
- all causes 17 17.1
----------------------------------------------------------
a From: Glover (1967).
b Taking into consideration the age and sex of those who
died, and the year of death. The Table shows no
evidence of excess mortality in the selenium workers
from any of these main groups of causes.
8.1.2.1.2. Hydrogen selenide
(a) Short-term exposure
There are few cases of occupational poisoning described in
which hydrogen selenide was identified as the sole causative agent.
Reported cases of occupational intoxication by hydrogen selenide
resulted usually from short-term accidental exposures in the
chemical laboratory or industry. Apparently, the first account of
an individual requiring hospital treatment after sudden exposure
to this gas was by Senf (1941), describing the effects of the
accidental laboratory exposure of a chemist to hydrogen selenide.
The patient noticed the characteristic smell and the gas caused her
eyes to weep and her nose to run. Within a few hours, her voice
became hoarse and increasing dyspnoea caused her admission to
hospital. On examination she was found to have a bluish-red
erythema of the skin, conjunctivitis, and injection of the nasal
mucous membrane. The lung oedema, ECG myocardial changes, the dark
red erythema, and porphyrinuria disappeared within 10 days. In
1941, Painter described a case of a single inhalation of hydrogen
selenide. After a brief "metallic" sensation, no ill effects were
felt for about 4 h. Then a copious discharge from the nasal
passages began. This persisted with violent sneezing for 3 or 4
days. No ill effects were noted later.
A case of pure hydrogen selenide poisoning in a works chemist,
producing hydrogen selenide in order to form selenides, was
described by Symanski (1950). Apparently only one inhalation of
the gas was followed by a sudden feeling of constriction in the
chest with coughing, tears, and burning of the nose, all of which
disappeared in a few moments. The patient thought that he had lost
his sense of smell. Four or 5 h later, a severe cough and dyspnoea
developed. At medical examination (8 h after the accident) he was
dyspnoeic and febrile. He continued to cough up blood-stained
frothy sputum all night, and by morning the acute symptoms had
disappeared leaving him apparently with bronchitis and an
irritating cough. A week later he had a recurrence of the fever
with chest pain and the signs of bronchopneumonia. After 4 more
days, the fever disappeared and 9 weeks later he was fit and well
again. A man, 5 h after cleaning out apparatus used for making
hydrogen selenide, developed severe dyspnoea, difficulty in
expiration, painful cough, and yellowish sputum (Bonard & Koralink,
1958). His condition became worse and the next day he was admitted
to the hospital. He was cyanosed, had severe expiratory dyspnoea,
discrete rhinitis and conjunctivitis, and was in considerable
distress. He had no headache or visual troubles. His pharynx was
hyperaemic, his buccal mucosa was dry, and he had a furred tongue.
Four days later, the chest roentgenogram was normal, but lung
function tests were depressed. Rohmer et al. (1950) reported the
case of a chemist exposed to a high level of hydrogen selenide,
calling attention to the development of severe hyperglycaemia that
could only be controlled by increasingly large doses of insulin. A
24-year-old white man accidentally inhaled hydrogen selenide while
transferring the gas from one cylinder to another (Schecter et al.,
1980). He immediately experienced burning in his eyes and throat
followed by coughing and wheezing. He was given oxygen and
improved over a period of 2 h. However, 18 h later, because of
recurrent cough and progressive dyspnoea, he was hospitalized.
Pneumomediastinum developed in this patient and pulmonary function
tests revealed restrictive and obstructive airways disease, which
slowly improved.
Glover (1970), who reviewed 8 cases of acute hydrogen selenide
poisoning (5 laboratory workers and 3 from industrial accidents;
some of them probably identical with those mentioned above), and
summarized the following sequence of events. The first effects
observed after exposure are signs of irritation of the mucous
membranes, i.e., running nose, running eyes, cough, sneeze,
followed by a slight tightness of the chest. This clears, and
there may be a latent period of 6 - 8 h. The patient, who has
often returned home by this time, usually wakes up in bed
breathless with signs and symptoms of pulmonary oedema. Glover, at
the same time, underlined the importance of oxygen therapy in these
intoxications, particularly with regard to the prevention of the
development of pulmonary oedema.
Lazarev & Gadaskina (1977) referred to 5 cases of hydrogen
selenide intoxication at an exposure level of approximately
7 mg/m3. These cases manifested nausea, vomiting, vertigo, and
extreme tiredness, in addition to effects on the respiratory tract
and conjunctiva. The same author mentioned one case of laboratory
exposure to hydrogen selenide in which a chemist developed lung
oedema and long-lasting cyanosis with respiratory difficulties. On
the 22nd day, thrombophlebitis was observed and within 52 days
signs of myocardial damage were noted.
(b) Prolonged and/or repeated exposure
An important point when considering prolonged low-level
exposure to hydrogen selenide was recognized by Dudley & Miller
(1941). In their experience, acute exposure levels of 5 µg/litre
resulted in such eye and nose irritation in workers that the men
could not continue their duties without the protection of a gas
mask. At 1 µg/litre, this irritation did not occur for several
minutes, though the presence of the gas was detected by its odour.
However, continued exposure at these levels results in olfactory
fatigue so that workers lose their ability to smell the gas and are
thus disarmed against subsequent increases in levels of the gas in
the work-place.
The effects of prolonged exposure to hydrogen selenide were
described by Buchan (1947) in workers who were engaged in a process
involving the etching of a pattern on steel stripping. In this
operation, steel stripping is passed between rubber imprinting
wheels with a raised pattern on the periphery. The pattern is in
constant contact with a wick immersed in the etching ink. After
imprinting, the steel stripping passes between felt wipers and dips
into a rust-preventive oil bath passing then to a reel. The odour
of hydrogen selenide was detectable at the etching machine and was
particularly offensive in the sludge of the oil bath. Selenium was
identified, qualitatively, in the sludge. Apparently, the hydrogen
selenide was generated as a result of the reaction of the excess
selenium deposited on the steel stripping in an acid medium, i.e.,
the oil bath, which was further acidified by contamination with the
etching ink carried by the steel stripping. Five out of 25 workers
complained of nausea, vomiting, metallic taste in the mouth,
dizziness, extreme lassitude, and fatigability. The symptoms
lasted 2 weeks and were said to be increasing in severity. Until
one month before the onset of symptoms, an etching ink containing
nitric acid and silver was used, but then a new ink containing
selenious acid at 52 mg selenium/litre was substituted.
Air samples were taken at 6 representative sampling points.
During the analysis, qualitative detection of selenium was made,
but, because of the lack of sensitivity of the titrimetric method
used, it was not possible to measure the selenium quantitatively,
and the results were recorded as less than 0.2 parts per million.
Spot and 24-h specimens of workers' urine contained 0 - 131 µg
selenium/litre or an average of 61.6 µg/litre. So-called control
specimens contained similar amounts, but the controls were the
professional staff collecting the air samples and they had been
exposed to the same environment as the workers for almost 5 h.
Also, it should be realized that hydrogen selenide produces
symptoms at exposures too low to increase the urinary excretion
above normal values. When the selenium ink was replaced by silver
ink, there was a gradual regression of symptoms and, within 6
months, all complaints had ceased and there was no recurrence.
The Task Group was not aware of any other reports dealing with
the prolonged exposure of human beings to hydrogen selenide.
8.1.2.1.3. Selenium oxychloride
Less than 5 µg of pure selenium oxychloride on the skin of the
hand resulted in a painful reaction within a few minutes, exythema
surrounding the central necrotic area (Dudley, 1938). Within 1
month, the skin defect healed with a scar.
8.2. Low Selenium Intake
8.2.1. Evidence supporting the possible essentiality of selenium in
man
Beneficial nutritional effects of selenium have been observed
in several different species (Schwarz, 1976), and specific signs of
selenium deficiency in the presence of adequate intake of vitamin E
have been demonstrated in rats and chicks (section 7.2.1). Thus,
the question of whether selenium is essential for man arises. The
identification of any human disease due to low selenium intake is
difficult, because selenium deficiency in animals is characterized
by a wide variety of signs involving several different organ
systems.
Although a clear-cut pathological condition attributable to
selenium deficiency alone has not yet been demonstrated in human
beings, certain evidence suggests that selenium may be essential
for man.
For example, purified glutathione peroxidase, from human red
blood cells, contains quantities of selenium similar to those found
in the enzyme isolated from animals (Awasthi et al., 1975).
Moreover, selenium is necessary for the optimal growth of human
fibroblasts in purified cell culture media (McKeehan et al., 1976).
Blood-selenium levels are depressed in children suffering from
kwashiorkor (Burk et al., 1967; Levine & Olson, 1970) and Hopkins &
Majaj (1967) obtained a reticulocyte response in malnourished
infants treated with a physiological dose of selenium (25 µg
selenium, as sodium selenite). Finally, on the basis of the
distribution of selenium in various human tissues, Liebscher &
Smith (1968) concluded that selenium must be an essential element
for man.
8.2.2. Signs and symptoms of low intake
The greatest possibility of a hazard due to inadequate selenium
intake would be expected in low-selenium areas. Nutritionists and
public health officials in several countries are aware of the low-
selenium status of their populations and are attempting to identify
any human health problems associated with it. For instance, in
the south island of New Zealand, one of the first regions where a
low selenium status in animals was recognized, some farmers, noting
supposed similarities between their own symptoms and those of the
white muscle disease affecting their livestock, claimed improvement
in their muscular complaints after self-medication with selenium
(Hickey, 1968). However, such anecdotal reports are not supported
by the results of 3 separate trials involving a total of 120
patients suffering from "muscular complaints", carried out in
several low-selenium areas of the south island of New Zealand
(Robinson et al., 198l). In these studies, the patients were given
either sodium selenite or selenomethionine at a number of dose
levels and schedules for various periods of time. Some subjects
also received vitamin E with the selenium supplement. In all
subjects who received selenium, blood-selenium levels and
glutathione peroxidase activity increased, whereas little change
was seen in the control group. Clinical assessment of muscular
symptoms showed that approximately equal numbers of patients in the
test and control groups exhibited an improvement in their muscular
condition. On the basis of these results, the authors concluded
that there was no evidence of any response, under the conditions of
the trials, to selenium supplements for the relief of muscular
complaints.
Total parenteral nutrition (TPN) fluids for intravenous feeding
contain very low levels of selenium (section 4.1.1.1), and patients
sustained by such techniques would seem to be at risk of developing
selenium deficiency. One such patient on TPN in New Zealand
developed muscular discomfort that disappeared after selenium
supplementation (van Rij et al., 1979). This patient was a 37-
year-old female who lived in a rural area of the south island of
New Zealand where the soils were low in selenium and there was a
history of endemic white muscle disease in sheep. She presented
with a perforated small intestine with peritonitis, after
radiotherapy 2 years previously for carcinoma of the cervix. Five
days after abdominal exploration and the start of intensive
antibiotic therapy she developed enterocutaneous and vaginal
fistulae. Gastric stress ulcer followed and necessitated a 1.5-
litre blood transfusion. Elevated temperatures persisted with
intraabdominal sepsis. Ten days after admission to hospital, TPN
was begun and resulted in a general improvement and a 6 kg weight
gain over the next 20 days. Regular plasma and albumin infusions
treated hypoproteinemia. After 20 days of TPN, early clinical
signs of fatty acid deficiency (dry flaky skin on hands and feet)
were noted, which responded rapidly to intralipid infusion. After
30 days of TPN, the patient complained of increasing bilateral
muscular discomfort in her quadriceps and hamstring muscles.
Muscle pain was present at rest as a persistent ache and with
tenderness on palpation. The muscle pain was aggravated by walking
until she found it distressing, even to move beyond her room. On
examination, there was tenderness of the quadriceps, hamstrings,
and less markedly of the calf muscles of both legs. Both active
and passive movements of these muscle groups were painful,
particularly of the hamstrings. The upper limb girdle was
unaffected. A generalized muscle wasting of all the limbs was
observed after the prolonged catabolic stress, despite TPN. No
muscle fasciculation or neurological deficits were observed.
Supplementation with selenium was begun with no other
modification to the patient's management. Each day 100 µg of
selenium, as selenomethionine, was infused intravenously with the
TPN solution. During the next week, muscle pain at rest,
tenderness to palpation, and pain on active and passive movement
disappeared. A return to full mobility followed. This symptomatic
response associated with selenium supplementation plus the
extremely low blood-selenium levels initially observed in this
patient (discussed further in section 8.2.4) led the authors to
conclude that this case could be the first clinical report
supporting the essential role of selenium in human nutrition.
Because of their increased metabolic requirements and faster
growth rates, infants and children might be particularly vulnerable
to selenium deficiency. McKenzie et al. (1978) analysed blood from
230 healthy adults and 83 healthy children from various areas of
New Zealand and found that children in Auckland and Tapanui had
lower blood-selenium concentrations (0.064 and 0.048 mg/litre) than
adults from the same areas (0.083 and 0.060 mg/litre). Red-blood-
cell glutathione peroxidase activity was also lower in Auckland
children than in adults (10.6 versus 12.9 units/g haemoglobin). A
specific population of infants and children that might be
especially at risk regarding low selenium intake includes those who
suffer from phenylketonuria (PKU) and maple syrup urine disease
(MSUD) and consume only special synthetic diets that are very low
in selenium (section 4). McKenzie et al. (1978) reported that the
mean blood-selenium level in 12 such children was 0.038 ± 0.013
mg/litre. One 13-year-old patient had 0.016 mg selenium/litre
whole blood and 0.009 mg selenium/litre plasma, but clinical
examination indicated that he was in good health. Lombeck et al.
(1978) found that the serum-selenium content in 36 children
receiving diet therapy for PKU and MSUD in the Federal Republic of
Germany ranged from 0.007 - 0.028 mg/litre. The selenium content
of the hair was lower in the patients (0.062 mg/kg) than in healthy
children (0.429 mg/kg) and the erythrocyte glutathione peroxidase
activity was reduced in comparison with normal values (4.6 versus
8.8 units/g haemoglobin). And yet all the patients thrived during
the time of observation and did not exhibit any increased rate of
haemolysis or oxidation of haemoglobin to methaemoglobin after
incubation of their erythrocytes with sodium azide.
Gross (1976) studied 4 groups of premature infants who were fed
4 different formulae based on cow's milk containing a high
concentration of polyunsaturated fatty acid (PUFA), with and
without iron, or a low PUFA concentration, with and without iron.
The tocopherol content was the same in all 4 formulae and was
judged adequate in terms of maintaining serum-vitamin E levels.
Both glutathione peroxidase activity and plasma-selenium levels
were similar in all 4 groups. The former declined from 4.2 units/g
haemoglobin at one week of age to 2.7 units/g haemoglobin at 7
weeks of age, whereas the latter declined from 0.08 to 0.035
mg/litre. Although all infants exhibited the anaemia typical of
the prematurely newborn, the decreases in haemoglobin and increases
in reticulocyte levels were greatest in the group of infants given
the formula high in PUFA and iron. These haemolytic events in
vitamin E-sufficient premature infants fed a diet rich in PUFA and
iron were thought to be due to the oxidative stress of the formula
coupled with the poor nutritional status of the infants with regard
to selenium (Gross, 1976).
8.2.3. Dietary levels consistent with good nutrition
8.2.3.1. Quantitative estimates
Since no clear-cut pathological condition attributable to
selenium deficiency alone has yet been observed in man, it is not
possible to define a precise dietary requirement level for human
beings. However, 0.1 - 0.2 mg selenium/kg diet is a nutritionally
generous level for most species of animals (US NAS/NRC, 1971). If
these animal data are extrapolated, a 70-kg man consuming 500 g of
diet per day (dry basis), would need a daily intake of 50 - 100 µg.
The US National Research Council has estimated that the safe and
adequate range of the daily intake of selenium for adults is 50 -
200 µg, with correspondingly lower intakes for infants and children
(Table 53). On this basis, the recommended intake for a 70-kg man
would be equivalent to 0.7 - 2.8 µg/kg body weight per day. Any
daily intake within the recommended range is considered adequate
and safe, but the recommendations do not imply that intakes at the
upper limit of the range are more desirable or beneficial than
those at the lower limit. The lower recommended intake for infants
and children is consistent with the observation that
supplementation of malnourished children with sodium selenite at
30 µg selenium daily, produced weight gain and reticulocyte
responses without any untoward signs (Hopkins & Majaj, 1967).
Table 53. Estimated safe and
adequate range of selenium intakea
-----------------------------------
Group Age Daily selenium
(years) intake (µg)
-----------------------------------
Infants 0 - 0.5 10 - 40
0.5 - 1 20 - 60
Children 1 - 3 20 - 80
4 - 6 30 - 120
7+ 50 - 200
Adults 50 - 200
-----------------------------------
a Adapted from: US NAS/NRC (1980).
Three approaches have now been used to estimate human
nutritional requirements for selenium. Nutritionists have long
used metabolic balance studies to determine the human requirements
for a variety of minerals. In the case of selenium, healthy North
American men needed about 80 µg dietary selenium/day to maintain
balance, but women needed only 57 µg/day (Levander & Morris, 1984).
The difference between men and women in the selenium intake needed
to achieve balance was considered to be due to differences in body
weight. Expressing the balance data on a body weight basis
revealed that both men and women needed about 1 µg selenium per kg
body weight per day to stay in balance. However, other research
groups showed that selenium balance in New Zealand women or Chinese
men could be reached on intakes as low as 27 and 9 µg/day,
respectively (Stewart et al., 1978; Luo et al., 1985). This
demonstrates the great effect of prior dietary-selenium intake on
the amount of selenium needed to achieve balance in people and
shows that balance studies may not be valid techniques for
estimating human selenium requirements.
Yang et al. (1985) estimated human selenium requirements on the
basis of a comparison of dietary intakes in areas with and without
Keshan disease. Dietary-selenium intakes of 7.7 and 6.6 µg/day in
endemic and 19.1 and 13.3 µg/day in non-endemic Keshan disease
areas were reported for adult men and women, respectively. These
estimates should be considered minimum daily adult requirements for
selenium.
The depletion/repletion study is another approach used by
nutritionists to estimate human selenium requirements. The
relatively large body pool of selenium in North Americans prevented
their plasma-selenium levels from dropping to values commonly found
in persons from low-selenium areas (Finland, New Zealand), even
when depleted for almost 7 weeks (Levander et al., 1981a,b).
Chinese men of naturally-low-selenium status (dietary intake about
10 µg/day) were given graded supplements of selenomethionine and
their plasma-glutathione peroxidase activity was followed (Yang et
al., 1985). The activity of the enzyme plateaued at the same level
in all men receiving 30 µg or more of supplementary selenium daily.
From this study, a physiological selenium requirement of about 40
µg/day (diet plus supplement) was suggested for Chinese adult
males, which may require adjustment to account for body weight
differences in other populations including women.
8.2.3.2. Nutritional bioavailability
None of the studies discussed in section 8.2.3.1 have
specifically addressed the question concerning the nutritional
bioavailability of the selenium in foods for human beings. Using
an animal model, Douglass et al. (1981) found that the selenium in
freeze-dried, water-packed canned tuna for human consumption was
only 57% as effective as selenite in restoring liver glutathione
peroxidase activity in rats previously deficient in selenium,
whereas selenium as cooked freeze-dried beef kidney or seleniferous
wheat had 97 and 83% of the activity of selenite, respectively.
The selenium in the tuna was also less effective than selenite in
raising hepatic-selenium levels in the deficient rats. Similar
results concerning the relative bioavailability of the selenium in
tuna compared with wheat were obtained by Alexander et al. (1983)
in rats, using the slope-ratio technique. On the other hand,
Chansler et al. (1983) found that the selenium in mushrooms was
only about 4% as available as that in selenite for restoring
hepatic glutathione peroxidase activity in selenium-depleted rats.
It has been pointed out that, in addition to absorption and
retention, factors such as utilizability within the body are
apparently important in determining selenium bioavailability
(Levander, 1983).
Although data are accumulating on the absorption by human
beings of the selenium in various compounds or in foods (section
6.1.1.2), there have been few studies examining the bioavailability
(i.e., utilization, consisting of transport, conversion to a
metabolically-active form, retention, etc., in addition to
absorption) of the selenium in foods for human beings. One such
study was carried out in a low-selenium area of central Finland
(Levander et al., 1983). Three groups of 10 men of low-selenium
status (mean plasma-selenium level of 70 µg/litre) were
supplemented with 200 µg of selenium, daily, as selenium-rich
wheat, selenium-rich yeast, or sodium selenate, for 11 weeks.
Twenty unsupplemented subjects served as controls. Plasma-selenium
levels increased steadily in the wheat and yeast groups for 11
weeks to around 160 µg/litre with no sign of plateauing, whereas,
in the selenate group, plasma-selenium plateaued at about 110
µg/litre, after 4 weeks. Red blood cell-selenium levels also
increased steadily in the wheat and yeast groups for 11 weeks from
90 to 190 µg/litre, again with no sign of plateauing. Red blood
cell-selenium levels were unaffected in the selenate group.
Platelet glutathione peroxidase activity (glutathione peroxidase is
an index of selenium status) (section 7.2.4.4) roughly doubled
after 4 weeks of supplementation with wheat or selenate and then
plateaued. Platelet glutathione peroxidase increased more slowly
in the yeast group. Plasma-glutathione peroxidase activity did not
respond to selenium supplementation. Ten weeks after the
supplements were stopped, platelet glutathione peroxidase remained
higher in the wheat and yeast groups than in the selenate group.
This suggested that the selenium in yeast or wheat was, to some
extent, deposited in the tissues in a form that could be used later
for glutathione peroxidase biosynthesis, once the dietary
supplement was discontinued. The results of this bioavailability
trial indicated that there are several different aspects to the
nutritional availability of selenium. Complete assessment may
require several measurements including: short-term platelet
glutathione peroxidase activity, to estimate immediate
availability; medium-term plasma-selenium levels, to determine
retention; and long-term platelet glutathione peroxidase activity,
after discontinuation of supplements, to estimate the
convertibility of tissue-selenium stores to metabolically active
selenium.
Griffiths & Thomson (1974) also noted that the blood-selenium
levels of adults from the USA declined rapidly on arrival in New
Zealand, but, after a year, their levels were still higher than the
mean value for permanent New Zealand residents (Fig. 11). When
selenium levels were measured in the whole blood, erythrocytes, and
plasma of postoperative surgical patients receiving TPN in New
Zealand, selenium concentrations decreased as TPN was continued
(Table 54) (van Rij et al., 1979). The plasma-selenium
concentration of the New Zealand TPN patient, discussed in section
8.2.2, was 0.025 mg/litre and fell to 0.009 mg/litre just before
selenium supplementation was begun. The lowest value for blood-
selenium concentration in areas of China not affected by Keshan
disease was around 0.040 mg/litre, while, in affected areas, the
blood-selenium concentration often dropped below 0.010 mg/litre
(Keshan Disease Research Group, 1979b).
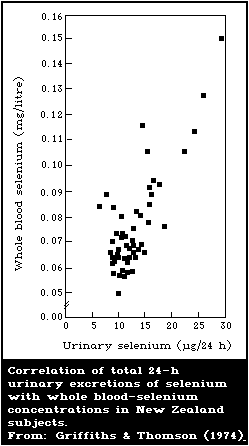 Table 54. Effects of total parenteral nutrition on
the concentration of selenium in whole blood,
erythrocytes, and plasma of postoperative surgical
patients in New Zealanda
------------------------------------------------------
Duration Selenium concentration
of TPN Whole blood Erythrocyte Plasma
(days) (mg/litre) (mg/litre) (mg/litre)
------------------------------------------------------
0 0.050 ± 0.006 0.077 ± 0.008 0.031 ± 0.004
10-20 0.040 ± 0.003 0.060 ± 0.004 0.022 ± 0.002
> 20 0.025 ± 0.003 0.043 ± 0.003 0.015 ± 0.004
------------------------------------------------------
a Adapted from: van Rij et al. (1979).
The urinary excretion of selenium by New Zealand residents is
quite low, reflects their low intake, and is related to whole
blood-selenium levels (Fig. 11). Similar relationships between
24-h urinary-selenium excretion and plasma-selenium concentrations
have been observed in New Zealand residents, New Zealand patients
with TPN, and Swedish patients with TPN (van Rij et al., 1979).
8.2.4. Blood and urine levels typical of low intake
In separate but simultaneous publications, Griffiths & Thomson
(1974) and Watkinson (1974) reported that the mean selenium content
of whole blood from New Zealand subjects was 0.068 and 0.069
mg/litre, respectively. However, even in New Zealand, where the
residents generally have a low selenium status, it is apparently
possible to observe regional differences in blood-selenium levels
(Table 55).
Table 55. Selenium
concentration in whole blood
of human beings residing in
different areas of New Zealanda
-------------------------------
Area of Blood-selenium
New Zealand concentration
(mg/litre)
-------------------------------
Heriot 0.057 ± 0.012
Dunedin 0.062 ± 0.013
Kurow 0.070 ± 0.009
Oamaru 0.074 ± 0.012
-------------------------------
a Adapted from: Griffiths &
Thomson (1974).
8.2.5. Relationship between blood-selenium levels and erythrocyte-
glutathione peroxidase activity
There is an excellent correlation between human whole blood-
selenium concentrations and glutathione peroxidase activity, at
concentrations below about 0.10 mg/litre (Thomson et al., 1977b).
However, above this concentration, activity of the enzyme is not
noticeably increased (Fig. 12), which suggests either that this
concentration of selenium is optimal and that an intake that
maintains this concentration is adequate for function as measured
by glutathione peroxidase activity, or, that above this
concentration, other factors might play a greater role in
influencing glutathione peroxidase activity. Rea et al. (1979)
showed that there was also an excellent correlation between human
erythrocyte-selenium concentrations and whole blood-glutathione
peroxidase activity, as long as the former was less than 0.14
mg/litre (Fig. 13). Schrauzer & White (1978) did not observe any
correlation between glutathione peroxidase activity and selenium
concentrations in the blood of subjects whose blood-selenium levels
were all over 0.10 mg/litre. Moreover, the activity of the enzyme
did not increase after these subjects were supplemented with a
selenized yeast preparation, even though blood-selenium levels
responded to such treatment. Only about 10% of the total selenium
in human red cells is associated with glutathione peroxidase
(Behne & Wolters, 1979) whereas, in sheep red cells, most of the
selenium appears to be associated with the enzyme (Oh et al.,
1976b). Thus, the role of selenium in glutathione peroxidase may
not be the only function of the element. Whether the non-
glutathione peroxidase selenium in human red blood cells truly
represents other functional forms of the element or is merely non-
functional selenium non-specifically incorporated into tissue
proteins cannot be answered at this time. But the usefulness of
the glutathione peroxidase assay as a means of assessing selenium
intake in human beings whose whole blood-selenium concentration
exceeds 0.10 mg/litre currently appears to be an open question.
Table 54. Effects of total parenteral nutrition on
the concentration of selenium in whole blood,
erythrocytes, and plasma of postoperative surgical
patients in New Zealanda
------------------------------------------------------
Duration Selenium concentration
of TPN Whole blood Erythrocyte Plasma
(days) (mg/litre) (mg/litre) (mg/litre)
------------------------------------------------------
0 0.050 ± 0.006 0.077 ± 0.008 0.031 ± 0.004
10-20 0.040 ± 0.003 0.060 ± 0.004 0.022 ± 0.002
> 20 0.025 ± 0.003 0.043 ± 0.003 0.015 ± 0.004
------------------------------------------------------
a Adapted from: van Rij et al. (1979).
The urinary excretion of selenium by New Zealand residents is
quite low, reflects their low intake, and is related to whole
blood-selenium levels (Fig. 11). Similar relationships between
24-h urinary-selenium excretion and plasma-selenium concentrations
have been observed in New Zealand residents, New Zealand patients
with TPN, and Swedish patients with TPN (van Rij et al., 1979).
8.2.4. Blood and urine levels typical of low intake
In separate but simultaneous publications, Griffiths & Thomson
(1974) and Watkinson (1974) reported that the mean selenium content
of whole blood from New Zealand subjects was 0.068 and 0.069
mg/litre, respectively. However, even in New Zealand, where the
residents generally have a low selenium status, it is apparently
possible to observe regional differences in blood-selenium levels
(Table 55).
Table 55. Selenium
concentration in whole blood
of human beings residing in
different areas of New Zealanda
-------------------------------
Area of Blood-selenium
New Zealand concentration
(mg/litre)
-------------------------------
Heriot 0.057 ± 0.012
Dunedin 0.062 ± 0.013
Kurow 0.070 ± 0.009
Oamaru 0.074 ± 0.012
-------------------------------
a Adapted from: Griffiths &
Thomson (1974).
8.2.5. Relationship between blood-selenium levels and erythrocyte-
glutathione peroxidase activity
There is an excellent correlation between human whole blood-
selenium concentrations and glutathione peroxidase activity, at
concentrations below about 0.10 mg/litre (Thomson et al., 1977b).
However, above this concentration, activity of the enzyme is not
noticeably increased (Fig. 12), which suggests either that this
concentration of selenium is optimal and that an intake that
maintains this concentration is adequate for function as measured
by glutathione peroxidase activity, or, that above this
concentration, other factors might play a greater role in
influencing glutathione peroxidase activity. Rea et al. (1979)
showed that there was also an excellent correlation between human
erythrocyte-selenium concentrations and whole blood-glutathione
peroxidase activity, as long as the former was less than 0.14
mg/litre (Fig. 13). Schrauzer & White (1978) did not observe any
correlation between glutathione peroxidase activity and selenium
concentrations in the blood of subjects whose blood-selenium levels
were all over 0.10 mg/litre. Moreover, the activity of the enzyme
did not increase after these subjects were supplemented with a
selenized yeast preparation, even though blood-selenium levels
responded to such treatment. Only about 10% of the total selenium
in human red cells is associated with glutathione peroxidase
(Behne & Wolters, 1979) whereas, in sheep red cells, most of the
selenium appears to be associated with the enzyme (Oh et al.,
1976b). Thus, the role of selenium in glutathione peroxidase may
not be the only function of the element. Whether the non-
glutathione peroxidase selenium in human red blood cells truly
represents other functional forms of the element or is merely non-
functional selenium non-specifically incorporated into tissue
proteins cannot be answered at this time. But the usefulness of
the glutathione peroxidase assay as a means of assessing selenium
intake in human beings whose whole blood-selenium concentration
exceeds 0.10 mg/litre currently appears to be an open question.
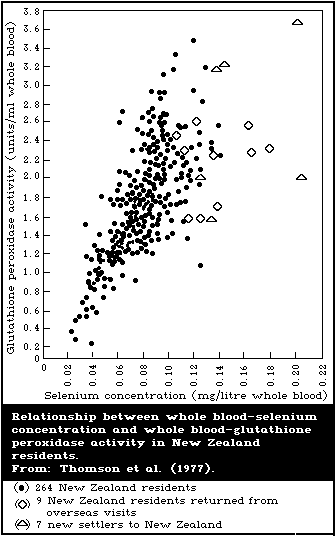
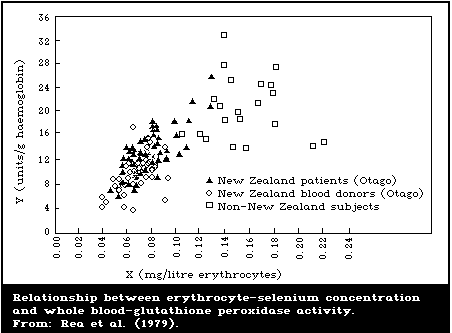 8.2.6. Attempts to associate low selenium intake with human diseases
8.2.6.1. Keshan Disease
Results of research in China have suggested a relationship
between low selenium status and the prevalence of Keshan disease,
an endemic cardiomyopathy that primarily affects children (Keshan
Disease Research Group, 1979a,b). Cases of Keshan disease were
recorded as early as 1907 in Heilongjiang Province of northeastern
China (Gu, 1983). Since the etiology of the disease was not known,
it was named after the locality in which it was originally
observed, Keshan County.
Epidemiologically, the disease exhibits a regional distribution
and occurs in a belt-like zone reaching from northeastern China to
the southwestern part of the country. There is a marked seasonal
fluctuation in the disease with more cases appearing during winter
in the north and during summer in the south. There is also a great
annual variation in the incidence of Keshan disease. Recently, the
incidence has decreased sharply such that from 1978 to 1980 less
than one death was reported per 100 000 members of the population
(Gu, 1983). The overall recent decline in the incidence of Keshan
disease has been attributed, at least in part, to the general
increase in the living standards of the people, such as better
sanitation, more medical attention, and improved quality of the
diet (He, 1979). There is also a shifting of epidemic foci from
year to year. Rural peasants constitute the population at risk
with children below 10 years of age and women of child-bearing age
most susceptible. According to Gu (1983), migrants from non-
affected areas will not contract the disease unless they have lived
in the endemic area for at least 3 months.
The criteria for diagnosing Keshan disease include acute or
chronic cardiac insufficiency, heart enlargement, gallop rhythm,
arrhythmia, and ECG changes (Chen et al., 1980). The disease is
classified into four types: acute (cardiogenic shock), subacute,
chronic (low output pump failure), and latent (normal heart
function but mild enlargement). There is no symptom or sign
specific for identifying the disease (Gu, 1983).
Histopathologically, Keshan disease is characterized by multifocal
necrosis and fibrous replacement of the myocardium.
In the past, many different hypotheses were advanced in an
attempt to explain the etiology of Keshan disease. For example,
there were theories of poisoning due to rhodamine, silica,
digitalis, carbon monoxide, barium, nitrite, and Fusarium
mycotoxins. However, clear-cut proof of these toxicants as a cause
of the disease has not been obtained despite much analytical effort
(Yang et al., 1984). Several nutritional problems have also been
proposed to play a role in Keshan disease such as deficiency of
protein, lipids, thiamin, magnesium, or molybdenum. A hypothesis
concerning Keshan disease etiology was proposed linking the disease
to selenium deficiency, after it was noted that severely endemic
areas coincided with areas where the incidence of enzootic selenium
deficiency diseases in farm animals was also high (Zhu et al.,
1981). On this basis, selenium supplements were used as a
preventive measure for Keshan disease. This hypothesis is very
appealing since cardiomyopathy is a prime feature of selenium-
vitamin E deficiency in many species of animals, including cattle,
sheep, and swine.
Since the initial proposal of the selenium-Keshan disease
connection, much evidence has been gathered in support of this
concept. For example, the average blood-selenium content was 0.021
± 0.001 mg/litre for the affected areas and 0.095 ± 0.088 mg/litre
for non-affected areas (Yang et al., 1984). The average hair-
selenium content was below 0.12 mg/kg in affected areas, whereas
hair-selenium levels in neighbouring but unaffected areas ranged
between 0.12 and 0.2 mg/kg. Average hair-selenium contents in
areas removed from the affected belt were between 0.25 and 0.6
mg/kg. The selenium level in several staple foods (rice, maize,
wheat, soybeans, black beans, and sweet potatoes) was lower in
areas affected with Keshan disease than in unaffected areas. It
was stated that an area could be considered to be unaffected
wherever the selenium content of grains was 0.04 mg/kg or more.
The Chinese workers stated that the amount of selenium needed to
prevent the disease was about 20 µg/day (Yang et al., 1984).
In some affected areas, there were highly localized pockets
(so-called "safety islands") that were free from the disease.
Apparently, these islands were protected because of the higher
selenium content of their crops in the immediate vicinity. For
example, the average selenium contents of rice and soybeans in one
such island were 0.020 and 0.025 mg/kg, respectively, whereas the
values for the corresponding crops in a nearby affected area were
0.0078 and 0.0057 mg/kg. It was also noted that the children in
the unaffected spot liked to catch shrimp from local streams and
that the selenium content of these dried shrimp was as high as 1
mg/kg (Keshan Disease Research Group, 1979b).
These relationships between selenium and Keshan disease led the
Chinese workers to conduct a randomized intervention trial to test
the possible prophylactic effect of selenium against this condition
in the population at risk (i.e., children 1 - 9 years old). In a
trial in 1974, 4510 children took sodium selenite and 3985 children
took the placebo (Table 56). The treated children took 0.5 mg
sodium selenite per week if 1 - 5 years old, or 1.0 mg per week if
6 - 9 years old. The morbidity rate due to Keshan disease was
1.35% in the placebo group (54 cases out of 3985 children) but only
0.22% in the treated group (10/4510). Since a significant
difference was also shown in the 1975 trial (0.95% morbidity rate
in the placebo group compared with 0.1% in those treated) the
placebo groups were abolished in 1976 and 1977. As a result, the
case rate dropped to 0.034% and 0% in these 2 years, respectively.
However, in 1976, there was one case out of 212 children who failed
to take the treatment.
No untoward side effects due to the sodium selenite were
observed, except for some individual cases of nausea, which could
be overcome by taking the medicine after meals. Physical
examinations and liver function tests indicated that the liver was
undamaged after continuous ingestion of the selenium tablets for
3 - 4 years.
Table 56. Effect of selenium on Keshan disease in childrena
-------------------------------------------------------------------
Treatment Year Number Number Outcome of alive cases Death
of of Turned Improved Turned
subjects cases latent chronic
-------------------------------------------------------------------
Placebo 1974 3985 54 16 9 2 27
1975 5445 52 13 10 3 26
Sodium 1974 4510 10 9 0 1 0
selenite 1975 6767 7 6 0 0 1
-------------------------------------------------------------------
a Adapted from: Keshan Disease Research Group (1979a).
Since 1976, more extensive intervention trials with sodium
selenite have been carried out in 5 counties in the same area of
Sichuan Province (Yang et al., 1984). All children, 1 to 12 years
of age, in some of the most severely affected communes, were
treated with selenium as described above, while untreated children
in nearby communes served as controls. The incidence rate of
Keshan disease in the selenium-treated children was lower in each
year of the five-year period than among the untreated children
(Table 57).
Table 57. Keshan disease incidence rates in selenium-treated and
untreated children in five counties of Sichuan provincea
-------------------------------------------------------------------------
Treated children Untreated children
Year Number of Number of Incidence Number of Number of Incidence
subjects cases (per 1000) subjects cases (per 1000)
-------------------------------------------------------------------------
1976 45 515 8 0.17 243 649 448 2.00
1977 67 754 15 0.22 222 944 350 1.57
1978 65 953 10 0.15 220 599 373 1.69
1979 69 910 33 0.47 223 280 300 1.34
1980 74 740 22 0.29 197 096 202 1.07
Total 323 872 88 0.27 1 107 568 1713 1.55
-------------------------------------------------------------------------
a Taken from: Yang et al. (1984).
Selenium intervention has proved to be very effective in the
prophylaxis of Keshan disease and it is very likely that selenium
insufficiency plays an important role in its etiology (Yang et al.,
1984). Nevertheless, the Chinese workers recognized that there
were certain epidemiological characteristics of the disease, which
suggested that additional etiological factors were involved. The
selenium hypothesis, for example, does not adequately explain the
seasonal variation in the disease, the occurrence of epidemic
years, or the annual shifting of epidemic foci. Such
characteristics are more compatible with an infectious theory and,
in fact, a hypothesis that the disease is a form of viral
myocarditis has been put forward (He, 1979). Perhaps the best way
to account for all the characteristics of the disease is to assume
that the disease has a multifactorial etiology and that a
combination of several factors may be involved (Yang et al., 1984).
There are some animal studies that favour such a possibility, since
selenium-deficient mice were less resistant to the cardiotoxic
effects of a Coxsackie B4 virus isolated from a patient with Keshan
disease (Bai et al., 1980). If selenium-deficient human beings are
also less resistant to viral infection, this phenomenon could
provide a reasonable explanation for many of the apparently
conflicting features of Keshan disease. In this view, selenium
deficiency would be the fundamental underlying condition that would
predispose persons to viral attack, possibly by impairing normal
immune function (Yang et al., 1984).
8.2.6.2. Kashin-Beck disease
Kashin-Beck disease is an endemic osteoarthropathy that occurs
in eastern Siberia and in certain parts of China, which is
characterized as a chronic, disabling, and degenerative
osteoarthrosis that mainly involves children (Sokoloff, 1985).
Although the etiology of this disease has not been fully
established, present works show that selenium deficiency might be
one of the main causes. This concept is based on the following
evidence. First, in China most of the endemic areas are located in
the same low-selenium zone, from northeast to southwest, as the
Keshan disease (section 8.2.6.1) (Tan et al., in press). For the
same reason, residents in these areas have low-selenium status
characterized by low blood- and hair-selenium levels, low blood
glutathione peroxidase activity, and low urinary-selenium
excretion. A survey carried out in Heilongjiang province of China
(one of the most heavily affected province) showed that the average
hair-selenium levels in 151 children in endemic areas (0.096 ± SD
0.026 mg/kg) was significantly lower than that of the 235 children
in the non-endemic areas (0.223 ± 0.083 mg/kg) (Wang et al., 1985).
The authors concluded that the low selenium status of children was
due to the low selenium content of the locally produced staple
food. The average selenium content of corn, wheat, and millet in
the endemic and non-endemic areas shown in the paper were: 0.0056
± SD 0.0038 (n = 262) versus 0.015 ± 0.015 (176), 0.0091 ± 0.0096
(225) versus 0.0235 ± 0.022 (120), and 0.0064 ± 0.0053 (14) versus
0.0197 ± 0.0144 (11) mg/kg, respectively. Second, sodium selenite
is reported to have both therapeutic and prophylactic effects on
this disease. Liang (1985) reported that 325 cases of Kashin-Beck
disease in Shaanxi province of China were randomly divided into a
treated and control group. The treated group was given sodium
selenite (1 mg/week for children 3 - 10 years of age and 2 mg/week
for children of 11 - 13 years of age) and the control group was
given a placebo. After one year, X-ray examination of the
metaphyseal changes of fingers showed that 81.9% of the cases in
the treated group had improved, none of the cases were getting
worse, and that 18.1% showed no change, while, in the control
group, only 39.6% of the cases had improved, 30% were getting
worse, and 41.5% showed no change. Li et al. (in press) reported
that children of 1 - 5 and 6 - 10 years of age in an endemic area
of Gansu province in China were supplemented with 0.5 and 1.0 mg
sodium selenite, respectively, per week over a period of 6 years.
X-ray examination showed that the incidence of Kashin-Beck disease
declined from 42% to 4% after the selenium intervention.
However, other information suggests that other factors in the
environment may also play some role in the disease. For example,
the possible role of mycotoxin contamination of cereals by certain
Fusarium strains in China and high phosphate and manganese
contents in the soil, food, and drinking-water in endemic areas of
the USSR have been suggested. More studies are required to clarify
the relationship between these hypotheses and Kashin-Beck disease.
8.2.6.3. Cancer
(a) Ecological studies
Shamberger & Frost (1969) first pointed out the inverse
relationship between selenium levels in forage crops and human
blood, and cancer death rates in various regions of the USA.
Subsequent papers expanded this concept (Shamberger & Willis, 1971;
Shamberger et al., 1976). From a number of comparisons, the
cancers frequently found at one time or another to have high
mortality associated with low-selenium areas or low blood-selenium
levels, included cancer of the tongue, oesophagus, stomach, colon,
rectum, liver, pancreas, larynx, lungs, kidneys, bladder, and
Hodgkin's disease and lymphoma. However, the high mortality of
some of these same cancers and others, was also found to be
associated with high-selenium areas or high blood-selenium levels;
these include cancer of the lung, prostate, pancreas, breast, lip,
skin, eye and dermal melanoma, and leukaemia/aleukaemia. Some of
these studies have been criticized as lacking strength and
consistency, particularly because the states for which cancer
mortality was calculated did not coincide directly with the natural
geographical units on which the estimates of selenium levels in
forage crops were made, thus leading frequently to
misclassification of the different selenium areas (Allaway, 1972,
1978). However, in a study attempting to minimize this problem by
using county data, similar inverse correlations were observed
between counties classified as intermediate or high for selenium
levels in forage, and cancers of the lung, colon, rectum, bladder,
oesophagus, pancreas, and all sites combined, for both males and
females, and cancers of the breast, ovary, and cervix (Clark,
1985).
Schrauzer (1976) found that the mortality rates due to several
cancers, including those of the large intestine, rectum, and breast
(so-called Type A cancers) were directly correlated with the
consumption of meat, eggs, milk, fat, and/or sugar and were
inversely correlated with the consumption of cereals and fish.
Just the opposite correlations were found for certain other cancers
such as hepatic and stomach cancer. Since cereals and seafoods are
good sources of dietary selenium, it was suggested that selenium
might be the factor in these foods that protected against Type A
cancers. Schrauzer et al. (1977) extended these studies to data
from 27 countries including the USA; New Zealand was intentionally
excluded. Dietary intake of selenium was found to be inversely
correlated with total age-adjusted cancer mortality, r = -0.46 ( P <
0.01) for males and r = -0.60 ( P < 0.001) for females. Significant
inverse correlations were observed between dietary-selenium intake
and mortality from cancers of the colon, rectum, prostate, female
breast, ovary, lung (males), and from leukaemia. Weak inverse
relationships were found with mortality from cancers of the
pancreas ( P = 0.06), bladder ( P = 0.1), and skin ( P = 0.1, males).
Other cancers including those of the stomach, oesophagus, and liver
did not show any significant direct or inverse correlations with
dietary-selenium intake. In this study, dietary-selenium intake
was calculated, assuming that the same average concentration of
selenium was present in the foods consumed in all countries (the
Task Group questioned the validity of such an assumption).
Mortality from cancers of the colon, rectum, prostate, lung, skin,
bladder (all Type A), and leukaemia showed significant inverse
correlations with blood-selenium levels in males (USA excluded).
However in the data from the USA, this relationship was not
observed for cancer of the prostate, lung, skin, or bladder, and
leukaemia, and similar inconsistencies were observed for other
cancers and in data for females.
Jansson et al. (1978) postulated an inverse relationship
between dietary-selenium and the rate of colorectal and breast
cancer, but found a direct correlation between the concentration
of selenium in the drinking-water and the rate of colorectal
cancer. Moreover, these workers commented that the same
statistical associations that indicated a protective effect of
dietary selenium against colon, rectum, and breast cancers also
indicated an increased risk of liver and stomach cancer due to
selenium.
In a recent study in China, Yu et al. (1985) obtained a mean
serum-selenium level in the whole blood of 1458 donors from 24
regions, of 107 µg/litre (range 22 - 314 µg/litre). Blood-selenium
levels were inversely correlated with age-adjusted total cancer
mortality for both males and females, r = -0.64 ( P < 0.01) and
r = -0.60 ( P < 0.01), respectively. Analysis by cancer sites
revealed significant negative correlations between blood-selenium
levels and stomach and oesophageal cancers in both sexes. On
reclassifying regions according to low-, moderate-, and high-
selenium areas on the basis of blood levels, significantly lower
total cancer death rates were observed in regions with high
selenium levels and the mortality from cancer of the stomach,
oesophagus, and liver was particularly increased in the low-
selenium areas. In an area where primary liver cancer was very
common, a statistically significant negative correlation between
primary liver cancer incidence and selenium levels in grain was
observed (r = -0.623 for maize, and -0.631 for barley corn). An
inverse correlation between age-adjusted primary liver cancer and
blood-selenium levels of residents in the area was also observed.
The authors concluded that the results indicated that selenium
might play an important role in the etiology of liver cancer, and
that though selenium deficiency was not a cause of primary liver
cancer, low selenium intake apparently reduced the ability of the
body to withstand cancer-causing stress.
It has been pointed out (Levander, 1986) that the age-adjusted
mortality rates for breast cancer and colon cancer reported in
Finland are considerably lower than those reported in the USA,
despite the well-documented lower dietary-selenium intakes in
Finland.
(b) Case-control studies
Shamberger et al. (1973b) reported that blood-selenium levels
in patients with cancer of the colon, pancreas, stomach, and in
Hodgkin's disease and liver metastases were statistically
significantly lower than those in normal controls. However, of 29
patients with rectal cancers, 6 had lower selenium levels than
controls, and 23 had normal levels. Similarly, normal levels were
observed in patients with breast cancer and in patients with other
types of carcinoma.
McConnell et al. (1975), compared blood-selenium levels in 110
patients with carcinomas, 36 patients with primary neoplasm of the
reticuloendothelial system, 28 hospitalized patients with no
malignancy, and 18 non-hospitalized healthy individuals. The mean
selenium concentration for the hospitalized non-malignant patients
was 1.49 ± 0.06 mg/kg and for healthy controls 1.48 ± 0.07 mg/kg.
The mean level of 1.27 ± 0.03 mg/kg for patients with carcinomas
was significantly different from that of the healthy control group
( P = 0.01). The mean serum-selenium level of 1.14 ± 0.08 mg/kg
obtained for gastrointestinal cancer was significantly different
from that of healthy controls ( P < 0.005). In about one-third of
the 110 cancer patients having the lowest selenium levels,
disseminated tumour, recurrences of the primary lesions, the
incidence of multiple primaries, and shortened patient survival
time, were more frequently observed than in the third of the
patients having the highest serum levels ( P > 0.001). The mean
serum-selenium level for the primary malignancies of the
reticuloendothelial system was 1.76 ± 0.24 mg/kg, which though
higher, was not significantly different from that for the healthy
controls.
Broghamer et al. (1976) reported no difference in serum-
selenium levels measured as µg/ml between 110 cancer patients and
controls. However, patients with the lowest serum-selenium levels
had shorter survival, higher incidence of multiple primary
malignancies, higher rate of recurrence of the primary lesion, and
were more likely to have dissemination of cancer than those with
the highest serum-selenium levels. In a study of 59 patients with
primary malignant reticuloendothelial tumours and controls,
Broghamer et al. (1978) found no difference in serum-selenium
levels measured as µg/ml between the two groups. McConnell et al.
(1980) in their study found statistically significant lower levels
of serum-selenium in 35 breast cancer patients compared with a
control group of women free of the disease. On the other hand, van
Rij et al. (1979) and Robinson et al. (1978a) did not find any
differences in the blood-selenium levels of surgical patients with
and without cancer. Although nutritional status, age, and severity
and duration of disease influenced the selenium levels in the
patients studied, low selenium levels were not characteristic for
the cancer patients and it was suggested that the low-selenium
status of cancer patients was more likely a consequence of their
illness rather than the cause of the cancer.
Sundstrom et al. (1984a) found that 44 patients with
gynaecological cancer had lower serum-selenium concentrations (1.15
± 0.04 µmol/litre, P < 0.05) and serum-glutathione peroxidase
activity (404 ± 13 units/litre, P < 0.01) than 56 control subjects
(1.25 ± 0.03 µmol/litre and 444 ± 8 units/litre, respectively). It
was observed that in association with cytotoxic chemotherapy,
selenium alone ( P < 0.05), vitamin E alone ( P < 0.05), and the 2
combined ( P < 0.001) decreased the plasma concentration of lipid
peroxides; the combination of selenium and vitamin E also increased
the activity of serum GSH-Px ( P < 0.01). The authors stated that
during placebo treatment, cytotoxic chemotherapy did not affect
plasma-lipid peroxides but decreased ( P < 0.001) the activity of
GSH-Px. Selenium inhibited this effect. The authors concluded
that this suggested that the anti-oxidative mechanisms of patients
with these types of cancer might be defective and that treatment
with selenium and vitamin E resulted in changes in biochemical
factors related to lipid peroxidation.
In another study, Sundstrom et al. (1984b) reported that
patients with ovarian cancer had significantly lower serum-selenium
concentrations (mean 0.93 ± 0.04 µmol/litre, P < 0.001) than
matched controls (mean 1.22 ± 0.03 µmol/litre). Clinical stage IV
patients had lower levels of selenium (0.82 ± 0.07 µmol/litre) than
clinical stage I and II combined (1.00 ± 0.04 µmol/litre).
Moreover, levels tended to decrease with progressive disease and
increase with remission, probably related to nutrition.
Goodwin et al. (1983) studied blood-selenium levels, and blood
and tissue GSH-Px activity in 50 patients with untreated cancer of
the oral cavity and oropharynx. Mean erythrocyte-selenium and
-glutathione peroxidase were significantly depressed compared with
those in age-matched controls. Mean plasma-selenium, on the other
hand, was significantly elevated in the cancer group. Also,
although not significant, mean erythrocyte-selenium levels tended
to be lower in patients who had never smoked or who had recently
given up smoking. No correlation between dietary selenium as
determined by recall history and plasma- or erythrocyte-selenium
levels in the cancer patients was observed.
Stead et al. (1985) reported significantly lower serum-selenium
concentrations in 20 patients with cystic fibrosis, two of whom had
cancer, than in controls. The two cancer cases had mean serum-
selenium levels of 1.01 µmol/litre and 0.62 µmol/litre,
respectively, compared with 1.41 ± 0.20 µmol/litre in the controls.
Serum-vitamin E levels were also found to be low in patients, but
did not show any correlation with serum-selenium levels.
(c) Case-control studies within prospective studies
As part of the Hypertention Detection Follow-up Programme,
10 940 men and women with diastolic blood pressures of at least 90
mm Hg were identified and enrolled between 1973-74, and followed-up
for 5 years (Willett et al., 1983). Venous blood samples were
collected from all participants at the beginning of the study. A
total of 111 new cases of cancer occurred in the group during the
period of observation. For each case, 2 controls without cancer
were selected who most closely matched the case in age, sex, race,
smoking history, month of blood collection, initial blood pressure,
hypertensive medication, randomisation assignment, and (in women)
parity and menopausal status. Cases and controls were comparable
for most of the confounding factors, although serum-cholesterol and
albumin were slightly lower among cases than controls ( P = 0.05 and
0.06, respectively). Serum-selenium levels did not vary with age
or sex in controls, but black subjects had lower selenium levels
than white. The mean serum-selenium level in cancer cases (0.129 ±
0.002 mg/litre) was significantly lower than that in controls
(0.136 ± 0.002 mg/litre). The P value was 0.02. The increased
risk of cancer in the lowest quintile of baseline selenium was
twice that in the highest quintile (confidence limits 1.1 to 3.3).
Cases were too few to examine by cancer site, but a consistent
trend of lower selenium levels among cases was observed for the
following groups of cancers: lung, breast, prostate,
lymphoma/leukaemia, gastrointestinal cancer, and others. However,
statistically significant differences were observed only for
gastrointestinal cancer. Selenium level remained a significant
predictor of risk, even when the effects of serum-retinol, vitamin
E, and lipid levels, as well as age, sex, and race were taken into
account. On examination of the data according to race, sex, and
smoking status, separately, significant differences in the mean
serum-selenium between cases and controls were observed in blacks
but not whites, in males but not females, and in current smokers,
but not in past-smokers or in persons who had never smoked. The
risk associated with low selenium was greater among those in the
lowest tertile of serum-vitamin E, and a similar inverse
relationship was observed for serum-retinol. A very strong effect
of low selenium (relative risk = 6.2 for lowest versus highest
tertiles) was observed for subjects who had both low serum-vitamin
E and -retinol levels. The authors concluded that, although their
findings supported the overall hypothesis that low selenium intake
increases the risk of cancer, they believed that the observed
differences between cancer sites should be treated as hypotheses to
be tested in other data sets, and that the differences in the
effects of selenium according to age, race, sex, and smoking
status, needed to be examined further (Willett et al., 1983).
Salonen et al. (1984b) identified a cohort of 8113 men and
women in 1972, randomly selected from 2 counties in Finland, who
had no history of cancer in the 12 months preceding this date. The
blood samples were collected from each subject at the beginning of
the study in 1972, and stored at -20 °C. The cohort was followed-
up for cancer occurrence and death up to the end of 1978, during
which time 43 deaths from cancer occurred and an additional 85
persons developed cancer (total cancers = 128). Each cancer case
or cancer death was matched for sex, age, number of cigarettes
smoked and total serum-cholesterol, to a control selected from the
rest of the same population of 8113 persons. In 1983, selenium
concentration was estimated in blood collected from each case and
control at the time of enrolment in the study in 1972 before the
development of cancer. The mean concentration of selenium was
50.5 µg/litre (SD = 12.5) for all 128 cases, and 54.3 µg/litre
(SD = 11.8) for all controls ( P < 0.012). This difference between
cases and control persisted for the following cancer sites:
gastrointestinal, respiratory, and haematological cancers,
miscellaneous cancers, and secondary cancers. No such difference
was observed for skin cancer, skeletal cancers, and urogenital
cancer. Using the lowest (35 µg/litre) and third deciles as cut-
off points, relative risks were computed for the 2 lowest levels of
serum-selenium, with the highest selenium stratum (45 µg/litre) as
the reference. The relative risk of cancer associated with a
serum-selenium level of less than 35 µg/litre was 3.0 and that
associated with a level of 35 - 44 µg/litre was 2.4 (both
statistically significant). The authors concluded that their data
provided additional support for the hypothesis that selenium
deficiency increases the risk of most non-hormone-dependent
cancers in middle-aged persons, though they cautioned that the
number of cancer cases in their study was insufficient to draw
definite conclusions about the effect of selenium for cancers at
specific sites.
Salonen et al. (1985) in a further study of a subgroup of 51
patients, who died from cancer, among the same study population as
above, each matched to a control for age, sex, and smoking,
obtained a mean pre-follow-up serum-selenium concentration in
subjects who died from cancer during the study period of 53.7 ± 1.8
µg/litre and that in controls of 60.9 ± 1.8 µg/litre; the
difference was statistically significant. A statistically
significant difference in the mean serum-selenium concentration
between cases and controls was observed in men ( P = 0.002), but not
in women (half of the male pairs were smokers, but none of the 21
female pairs were smokers). Similarly, the difference in the mean
selenium concentration was significant in smokers ( P = 0.013) but
not significant in non-smokers. Years of smoking showed an inverse
correlation with serum-selenium in the cases (r = -0.30) but not in
controls. Analysis according to cancer site revealed that a
statistically significant difference in serum-selenium levels
between cases and controls was recorded only for respiratory
cancers, though a non-significant difference also existed for
gastrointestinal sites and other cancers. An association between
low serum retinol concentration and increased risk of cancer was
observed only among smoking men. This sex difference was
attributed to smoking as there were no smoking women in the study.
Vitamin E and serum-retinol did not show any association with a
specific cancer site, and although vitamin E had only a weak
independent effect on the risk of cancer, it showed a strong
synergistic relationship with selenium on the risk of fatal cancer.
A serum-selenium concentration of 47 µg/litre or less (the lowest
tertile) was associated with a relative risk of death from cancer
of 5.8 (95% confidence interval 1.2 - 29.0). The authors concluded
that, although their findings indicated that dietary-selenium
deficiency increased the risk of cancer, owing to their study
design, they could not rule out the possibility that the substances
measured in the serum were not truly the protective factors but
were merely indicators of some other compounds or nutrients that
were directly involved in the causal relationship. Furthermore,
the authors recognized that there was need to investigate further
the modification and the confounding of effects by sex, smoking,
and other factors.
8.2.6.4. Heart disease
Using the same ecological approach discussed above for cancer,
Shamberger et al. (1975) concluded that the age-specific death
rates for a number of heart diseases were significantly lower in
the high-selenium regions of the USA than in the low-selenium
regions. However, results of a WHO/IAEA research programme showed
no difference in tissue-selenium concentrations between patients
who died with, or without, myocardial infarction (Masironi & Parr,
1976). Furthermore, Shamberger (1978) found that the kidney-
selenium levels of patients with atherosclerosis and hypertension
did not differ from those of patients with a variety of other
diseases. The blood-selenium levels of patients with acute
myocardial infarction were lower than those of healthy adults, but
there was no difference in heart- or liver-selenium levels of
patients who died from myocardial infarction and those who died
from other diseases (Westermarck, 1977).
A recent case-control study from Finland suggested a possible
association between the serum-selenium level and the risk of death
from acute coronary heart disease as well as the risk of fatal and
non-fatal myocardial infarction (Salonen et al., 1982). The case-
control pairs were derived from 11 000 persons residing in eastern
Finland, an area with a very high incidence of death from
cardiovascular disease. The cases were middle-aged persons who had
died of coronary heart disease or other cardiovascular disease or
suffered a non-fatal myocardial infarction over a 7-year follow-up
period. Attempts were made to control for potential confounding
factors by using controls matched for 6 major coronary heart
disease risk factors: age, sex, serum-cholesterol, diastolic blood
pressure, smoking, and history of angina pectoris, but the cases
had slightly higher blood pressure than the controls. The mean
serum-selenium levels were 51.8 and 55.3 µg/litre for cases and
controls, respectively. A serum-selenium level of less than 45
µg/litre was associated with an increased risk of coronary and
cardiovascular death and myocardial infarction. Although few
necropsies were done, the authors felt that it was unlikely that
the excess cardiovascular mortality observed in their subjects was
due to Keshan disease. The authors cautioned that the apparent
association between low serum-selenium levels and cardiovascular
risk might be spurious since serum-selenium might only be, for
example, a marker for other dietary factors more directly related
to increased coronary heart disease. The authors also emphasized
that, even if their results truly reflected a causal relationship
between low selenium intake and increased ischaemic heart disease,
most such disease is still due to the other well-known risk factors
of elevated cholesterol, high blood pressure, and smoking.
Moreover, it was pointed out that any association between low
serum-selenium levels and ischaemic heart disease is likely to be
of significance only for populations in areas where the dietary
intake of selenium is very low.
In 3 other studies from Finland little or no association was
found between the risk of death from ischemic heart disease and low
selenium status (Miettinen et al., 1983; Salonen et al., 1985;
Virtamo et al., 1985). However, a critique of these studies
(Salonen, 1985) indicated that the first and third were
characterized by low statistical power and in the first the mean
serum-selenium level was relatively high compared with typical
Finnish values (73 µg/litre), probably due to importation of grain
high in selenium during the late 1970s (Mutanen & Koivistoinen,
1983). Salonen (1985) stated that all 4 Finnish studies discussed
above supported the concept of an increased risk of ischemic heart
disease due to low selenium intake as indicated by serum-selenium
levels of less than 60 µg/litre.
In the USA, an inverse correlation was reported between the
plasma-selenium level and the severity of coronaryatherosclerosis
as documented arteriographically (Moore et al., 1984). The mean
plasma-selenium levels of patients with "zero-vessel" disease (no
visible narrowing as much as 50% of any coronary arteriol lumen) or
"three-vessel" disease (as much as a more than 50% narrowing in the
three major coronary arteries or their branches) were 136 ± 7 or
105 ± 4 µg/litre, respectively. On the other hand, neither Ellis
et al. (1984) in the United Kingdom nor Robinson et al. (1983) in
New Zealand were able to demonstrate any correlation between the
traditional risk factors for cardiovascular disease and blood-
selenium levels or glutathione peroxidase activity.
9. EVALUATION OF THE HEALTH RISKS ASSOCIATED WITH EXCESSIVE OR
DEFICIENT SELENIUM EXPOSURE
9.1. The Need to Consider the Essentiality of Selenium in the Health
Risk Evaluation
The data presented in the preceding sections show evidence that
selenium is a functional component of an enzyme (glutathione
peroxidase) in animals and man, and can prevent certain diseases in
animals and responsive conditions in man. The Task Group concluded
that selenium meets the criteria of essentiality for man. As is
true for all essential elements, not only deficient but also
excessive exposure results in adverse health effects.
The effects of selenium deficiency as well as toxicity are well
known in several animal species. In contrast to the effects seen
in animals, health effects in man resulting from deficiency or
excess of selenium are less well defined, but available evidence
has been described. They can occur at low and excessive exposure
levels that may be expected to correspond broadly with those having
deleterious effects on the health of animals. Between these
extremes is a range of safe and adequate exposures (intakes) that
can be defined as free from toxicity and adequate to meet
nutritional requirements. Exposures outside this range increase
the risk of adverse health effects. Therefore, the health risk
evaluation of both selenium deficiency and excess is important.
However, it should be noted that the safe and adequate range may be
modified by certain dietary and other environmental conditions.
The aim of the Task Group was not to evaluate the need for, and
safety of, medication by selenium compounds as a preventive or
therapeutic measure. However, the Task Group felt that some of the
observations presented might be of relevance whenever such a
question might be addressed by any other body responsible for the
appropriate risk-benefit evaluation of administration of selenium
compounds to human beings.
9.2. Pathway of Selenium Exposure for the General Population
Reproducible and accurate methods are available for the
collection of environmental and biological samples and the
measurement of their selenium content. These methods, though they
require special skills and equipment and are time-consuming, have
been employed to assess human selenium exposure. However, the data
are incomplete and there are no data available on selenium exposure
levels for the general population in many countries and regions of
the world.
In spite of these limitations, it can be concluded that, for
the general population, the main source of selenium exposure is
food. In nutritional surveys, extreme mean values for the
calculated selenium intake from food by adult human beings varied
from 11 to 5000 µg/day. However, on the basis of the data
available from most areas, the Task Group concluded that dietary-
selenium intakes usually fall within the range of 20 - 300 µg/day.
Exposure via drinking-water is much less and rarely exceeds a few
µg/day. Limited data on selenium levels in air indicate that about
0.2 µg can be inspired daily by individual members of the general
population.
9.3. Quantitative Assessment of Human Selenium Exposure
9.3.1. Analytical methods for selenium
Several methods of analysis for selenium are available. Those
based on fluorometry, neutron activation analysis, or atomic
absorption spectrometry have been the most thoroughly studied and
used. While simplified and automated procedures have been
developed, all of these methods require skilled analysts to obtain
consistently accurate results. The analysis of NBS Standard
Reference Materials and the exchange of a variety of samples with
another laboratory known to be accomplished in analysis for this
element, should be used to verify the adequacy of results, prior to
any study of human exposure. The method of choice will depend on
the availability of equipment and of the pure chemicals that may be
required, as well as on certain other factors. Where a high degree
of accuracy is required, comparison of results obtained by two or
more different techniques is helpful.
The reliability of the estimate of selenium exposure will
depend, not only on the adequacy of the method, but also on the
adequacy of sampling, storing, subsampling, and preparing the
subsamples for selenium determination. Failure to plan for, and
observe, proper practices in these steps can negate the reliability
of results by even the most highly accurate method of measurement.
9.3.2. Food intake data
Because of the wide variation in the selenium content of foods
in different regions, special techniques must be used to assess
human selenium exposure on the basis of food intake data. The most
accurate method is to determine selenium levels in duplicate diets
(food-on-the-plate-method) made from the same food that is consumed
by the subjects. This method may be suitable for studies in small
subpopulations and where a high degree of accuracy is necessary; it
has the disadvantage of being expensive and time-consuming.
The use of a nutrition survey approach with calculation of
selenium intake from food tables can be used if an important rule
is observed, i.e., the food tables used must be formulated from
data on the selenium contents of the food sources of the population
being studied and of the food as eaten by the subjects under study.
This is important to avoid the errors introduced by the wide
variation in selenium content of a given foodstuff, depending on
its origin.
9.3.3. Blood-selenium
The selenium content of whole blood, serum, or plasma are the
most commonly used measurements of human selenium exposure. Blood
is relatively easy to sample and contamination can be controlled.
Studies on animals have shown that blood-selenium values are a good
indication of selenium deficiency and excess. However, variations
in selenium deficiency signs have been observed in animals with
similar whole blood-selenium levels. This may be due to variations
in the vitamin E content of the diet or exposure to other dietary
or environmental variables. Several factors besides selenium
intake have been identified that may affect blood-selenium content.
Exposure to inorganic mercury or cadmium can lead to deposition of
selenium in the blood attached to protein in combination with the
metal. Human beings exposed to selenium in the form of
selenomethionine or selenium-rich wheat or yeast, for 10 - 11
weeks, had higher blood-selenium levels than human beings exposed
to the same amount of selenium in the form of selenite or selenate.
Blood can be fractionated and plasma-selenium and red blood
cell-selenium can be measured separately. A recent study on the
effects of a low-selenium diet on human beings indicated that a
decrease in plasma-selenium content might occur before a decrease
in red blood cell-selenium can be detected. These data suggest
that it may be possible to use plasma-selenium levels to assess
short-term selenium exposure but that red blood cell- and whole
blood-selenium reflect long-term exposure.
9.3.4. Hair-selenium
Measurement of the selenium content in hair has been used in
animals for the assessment of selenium status, with regard to both
deficiency and excess. It has not been generally adopted for use
in human beings, but, in special circumstances where external
contamination can be excluded, hair-selenium content is useful for
assessing selenium status.
9.3.5. Urine-selenium
The results of animal studies and a few human studies indicate
that the urinary excretion of selenium can be useful for assessing
very recent selenium exposure (i.e., within the past 24 h).
However, determination of selenium in incomplete urine collections
or expression of urinary-selenium per unit volume of urine cannot
provide valid information about selenium exposure in the general
population. An understanding of the reservations associated with
this technique is necessary in its application and then it may only
be useful in conjunction with other measurements discussed in this
section.
9.3.6. Blood-glutathione peroxidase
There is no suitable, simple field method for assessing the
selenium exposure of the general population. The Task Group
concluded that approaches based on the glutathione peroxidase
activity of blood components might provide the basis for a suitable
screening method to detect low human exposure to selenium.
Blood-glutathione peroxidase activity is useful for detecting
selenium deficiency, because it represents a functional form of
selenium and its assay is more rapid than the measurement of
selenium in blood.
However, the Task Group recognized that there are several
difficulties and limitations associated with the determination of
glutathione peroxidase activity. For example, the enzyme is not
stable and hence its activity cannot be determined in samples
stored for periods of time, whereas measurements of selenium can be
made on stored samples. Moreover, large differences in blood-
glutathione peroxidase activity have been reported from different
laboratories using similar methods. The discrepancies can be so
great that interlaboratory comparisons are impossible without
suitable controls. Furthermore, results from New Zealand suggest
that human blood-glutathione peroxidase activity may be useful in
assessing selenium intake at low, but not necessarily at
intermediate or high levels, because the activity of the enzyme is
correlated with whole blood-selenium levels, only when the latter
are less than 0.10 mg/litre. It is not known whether excessive
selenium exposure can increase glutathione peroxidase activity in
human blood, above normal values. Animal studies indicate that
iron deficiency decreases blood glutathione peroxidase activity,
thus possibly further confounding selenium status assessment by
this method.
Human plasma contains glutathione peroxidase but its activity
is very low and difficult to detect and haemolysis during sample
collection should be excluded. Nevertheless, if these reservations
are borne in mind, the technique is promising. Measurement of
platelet-glutathione peroxidase is also a promising technique for
measuring short-term changes in selenium status because of the
short half-life of this blood component.
9.4. Levels of Dietary Selenium Exposure in the General Population
The selenium content of food is highly variable in relation to
several factors. Levels of selenium in soil available for uptake
by plants vary markedly in different locations and this is
reflected in differences in the selenium contents of feeds and
foodstuffs. Countries in which food-stuffs are shipped between
regions tend to avoid extremes in dietary-selenium intake by
averaging foodstuffs with high- and low-selenium contents but some
differences in regional intakes are still observed.
Animal tissues usually do not have as high a selenium content
as the plants in high-selenium areas or as low a selenium content
as plants in low-selenium areas. This is probably because of the
ability of animals to conserve selenium when it is in short supply,
and to excrete it when an excess is present. This may protect
consumers of animal products from extremes of selenium intake.
Available analytical data show that the levels of selenium
typically found in foods, are in the range of 0.4 - 1.5 mg/kg in
liver, kidney, and seafood; 0.1 - 0.4 mg/kg in muscle meats; from
less than 0.1 to over 0.8 mg/kg in cereals or cereal products; less
than 0.1 - 0.3 mg/kg in dairy products; and less than 0.1 mg/kg in
most fruits and vegetables. The very large variation in the
selenium contents of foodstuffs of the same type, depending on
their origin, makes a food table approach to estimating dietary-
selenium intake potentially misleading as discussed in section
9.3.2. More accurate intake estimates could be derived from assay
data on samples of the food items actually being consumed (food-on-
the-plate analysis).
The practice of supplementing the diets of livestock and
poultry in low-selenium areas with selenite or selenate causes
little increase in muscle-selenium content over levels encountered
in animals raised in areas of adequate, but not excessive, selenium
supply. With some exceptions, food processing and preparation
generally do not cause major losses of selenium.
There are few data that indicate the chemical form that
selenium takes in normal human foods. One available study of
seleniferous wheat has shown that a significant fraction of the
selenium is in the form of selenomethionine.
Various forms of selenium differ in their nutritional
availability. The absorption of selenium from foods appears quite
efficient (about 80%).
Daily selenium intake can be markedly influenced by food
consumption patterns. For example, in many countries, consumption
of large amounts of fish, kidney, or liver could raise an
individual's selenium intake substantially above the highest daily
intake shown in Table 8.
The greatest extremes in dietary-selenium intake have been
reported in areas in which the diet was monotonous and consisted
largely of locally-produced staple food.
The wide range of geographically-related selenium intake due to
variations in the selenium contents of the diet is reflected in the
wide range of selenium levels observed in human whole blood.
The extreme mean blood-selenium values reported for groups
dependent on locally-grown foods of different selenium content
ranged from 0.02 - 3.2 mg/litre (Table 11). Limited studies from
New Zealand have shown that children and older individuals had
lower whole blood-selenium levels, but it is not known whether
these lower levels are due to low dietary intakes or to changes
connected with growth and/or aging.
9.5. Evaluation of Health Risks - General Population
9.5.1. Predictive value of animal studies
The Task Group concluded that, in view of the still fragmentary
data concerning the possible health effects of either deficient or
excess selenium exposure on human beings, any evaluation of the
human health effects of selenium exposure must take into account
results arising from animal studies. For this purpose, these can
be summarized as follows:
(a) Selenium deficiency combined with concurrent low
vitamin E status has resulted in deleterious effects
in all animal species tested so far (mice, rats,
chicks, ducks, swine, sheep, cattle, and monkeys).
In rats, specific signs of selenium deficiency have
been produced in animals fed diets adequate in
vitamin E.
(b) Acute and chronic selenium toxicity have been
demonstrated in a wide variety of species, under a
wide variety of conditions (section 7.1.2). In
evaluating the toxic effects of different selenium
compounds, the Task Group felt that it was useful to
distinguish between effects that are strictly
dependent on the selenium in the molecule and cannot
be duplicated by, e.g., homologous compounds of
sulfur, and compounds where the toxicity is, in
principle, similar for homologues containing either
selenium or sulfur. In addition, the Task Group
recognized that the similarity of some of the effects
of different selenium compounds could be related to
the formation of certain common intermediates.
(c) Dose-response relationships have been demonstrated in
acute and chronic toxicity, as well as in selenium
deficiency. Comparative studies have shown that acute
toxicity is similar in parenteral and oral exposure
(section 7.1.2.1), which is in good agreement with the
recognized high absorbability of selenium compounds
from the gastrointestinal tract (section 6.1.1).
(d) As indicated in section 7.1.2.2, the borderline
level of dietary selenium needed to cause growth
depression, due to overt chronic selenium toxicity in
rats, is in the range of 4 - 5 mg selenium/kg diet.
However, the dietary level of selenium needed to
cause chronic toxicity can be influenced by several
environmental factors such as previous selenium
intake (section 7.1.6).
(e) The effects of various environmental factors on
selenium dose-response relationships is even more
dramatically illustrated in situations of low-
selenium intake leading to deficiency. One of the
primary factors influencing the nutritional
requirements of animals for selenium is the vitamin E
status. The minimum dietary level of selenium needed
to prevent deficiency diseases in various animal
species is in the range of 0.02 - 0.05 mg/kg diet.
(f) Animal diseases associated with both high- and
low-selenium intake have been reported in certain
areas of the world in which the animals were
consuming feeds primarily of local origin.
The possibility of a human disease due to selenium deficiency
or excess should be looked for under similar conditions of
restricted dietary consumption (monotonous diets based on locally
grown foods). In addition, the Task Group concluded that, in view
of the dependence of selenium deficiency or toxicity effects on
various environmental factors, the possible involvement of selenium
in human diseases of multifactorial etiopathogenesis deserves
special attention.
9.5.2. Studies on high-exposure effects in the general population
Studies on this type of exposure are few and, as they are
confined to subpopulations in high-selenium areas, some do not
include comparison groups. Symptoms and signs of illness elicited
are frequently mild and not clearly related to selenium. In
practically all studies (section 8.1.1.1), nail pathology was
reported and, in several studies, hair loss and increased dental
decay. Some of the studies included reports of gastrointestinal
disturbances and icteroid skin. Other possible causes of illness
in these studies cannot be excluded and the dependence of severity
of effects on gradation of selenium exposure cannot be evaluated.
Examination of exposed individuals showed increased levels of
selenium in the blood and urine.
Three studies on populations living in seleniferous areas and
dependent on locally-produced food deserve particular attention.
Examination of individuals exposed in these areas revealed highly
increased levels of selenium in the blood and/or urine. A study
from South Dakota reported various signs and symptoms in people
with long-term overexposure to selenium, as revealed by elevated
urinary-selenium excretion. In these areas, farm animals were
affected by chronic selenium poisoning.
In a seleniferous zone of Venezuela (Villa Bruzual) 111
children (average blood-selenium level = 0.813 mg/litre) were
studied and compared with 50 children (blood-selenium level = 0.355
mg/litre) not over-exposed to selenium (Caracas). The children
from the seleniferous area had some loss of hair and some
abnormalities of skin and nails; the authors noted some differences
in socio-economic factors between the 2 groups.
In a recent report from China, past incidents of intoxication
were described that were thought to be due to chronic selenium
poisoning, with hair loss and nail pathology as the most common
signs. No quantitative data are available from the period 1961 -
64, which was the time of peak prevalence of the intoxication.
Recent diet samples collected from high-selenium areas with, and
without, a history of this intoxication showed mean daily selenium
intakes of about 5 and 0.75 mg, respectively (Table 7). Blood
samples taken from the same areas had mean selenium levels of 3.2
and 0.44 mg/litre, respectively (Table 11).
The above studies of populations ingesting high selenium levels
in Venezuela and China have not revealed abnormal serum
transaminase activity in the subjects concerned. In individuals
exposed to high levels of selenium in the recent outbreak in the
USA; there was no evidence of impaired liver function, but loss of
hair and pathological changes in the nails were observed, as well
as some other signs and symptoms described in more detail in
section 8.1.1.1. However, the Task Group recognized that this is
insufficient evidence to exclude the occurrence of liver damage and
recognized the need for more thorough evaluation of the liver in
persons with high selenium exposure.
The Task group noted that the signs and symptoms of selenium
overexposure in human beings were not well defined. However the
Task Group was aware of 3 situations concerning elevated selenium
exposure, which could form the basis for estimating a dose-response
to high levels of selenium. In one area of Enshi county in China,
blood-selenium levels in adults of 0.44 mg/litre were associated
with no reported effects. In Venezuelan children with blood-
selenium levels of 0.813 mg/litre, hair and nail changes of an
unspecified nature and incidence were reported. In another area of
Enshi county in China, definite hair and nail changes and symptoms
and signs consistent with neuropathy were reported in adults with
blood levels of 3.2 mg/litre. The Task Group, however, could not
be certain of how signs and symptoms were searched for, how their
absence was ascertained, and how the control populations were
selected in these studies.
9.5.3. Studies on low-exposure effects in the general population
Very low blood-selenium levels observed in human subjects
(section 8.2.4), approach those observed in animals with selenium
responsive diseases. One woman from a general population known to
have a low exposure to selenium and maintained on total parenteral
nutrition, which provided less than 1 µg selenium/day, had muscular
pain and dysfunction that responded to selenium supplementation.
However, similar symptoms have not been observed in other patients
with similar low blood-selenium levels. Also, in another study, no
muscular symptomatology was reported in children fed exclusively
special medical diets containing very low levels of selenium, for
long periods of time. On the other hand, the Task Group assigned a
high significance to recent reports describing an association
between Keshan Disease (section 9.5.4.1) and poor selenium status
as indicated by low selenium content of grain, low blood and hair
levels of selenium, low blood-glutathione peroxidase activity, as
well as a positive response to an intervention study with sodium
selenite. As discussed in section 9.5.1, the results of several
experimental animal studies indicate that many different factors
may contribute to the development of selenium deficiency diseases.
These studies point to the conclusion that a lack of selenium may
be only one of several causative factors responsible for the
occurrence of certain diseases of complex multiple
etiopathogenesis. Such a conclusion was also fully recognized in
the reports from China on the Keshan Disease.
9.5.4. Evaluation of the involvement of selenium in human diseases
of multiple etiopathogenesis
9.5.4.1. Keshan disease
A suitable animal model of the Keshan disease is not available,
even though heart damage is a feature of combined vitamin E and
selenium deficiency in several species of animals.
The ecological evidence strongly favours a relationship between
low selenium status and the incidence of Keshan disease. Such
evidence includes low blood-, hair-, and urine-selenium levels in
the affected areas as well as a low selenium content in the staple
foods raised and consumed in the affected areas. A randomized
intervention trial carried out in China showed that children who
received sodium selenite had a lower incidence of Keshan disease
than those who received a placebo. However, in this randomized
trial there may have been an underlying trend in the placebo group
towards a decline in the incidence of the disease. Also,
information on the effectiveness of the randomization was not
available and the incidence rates were not adjusted for age. The
results of the much larger but non-randomized intervention trial
involving at least a quarter of a million children on a yearly
basis indicated a clear-cut beneficial effect of sodium selenite in
the prevention of the Keshan disease. Thus, the Task Group
concluded that Keshan disease is a condition in human beings that
is related to low selenium status but that additional research is
needed to clarify the role of other factors in the etiopathogenesis
of the disease.
The Task Group also recognized that in section 8.2.3.1 this
disease was used as the basis for estimating a minimal human
nutritional selenium requirement, suggested by the authors to be 19
and 14 µg/day for men and women, respectively.
9.5.4.2. Kashin-Beck disease
An additional disease found in certain low-selenium areas of
China is an endemic osteoarthropathy known as Kashin-Beck disease
that occurs mainly in children. There is some evidence linking low
selenium status with the incidence of Kashin-Beck disease, but the
Task Group concluded that additional research, particularly in
relation to intervention studies, is required before a definite
statement can be made concerning the role of selenium in the
etiology of the disease.
9.5.4.3. Ischaemic heart disease
The Task Group was aware of studies that examined the
possibility of an association between low selenium status and
myocardial ischaemia and atherosclerosis. However, because of the
limited data available, the Task Group was not prepared to come to
any conclusion regarding the role of selenium in ischaemic heart
disease.
9.5.4.4. Studies on the involvement of selenium in cancer
(a) Carcinogenicity studies
The Task Group concluded that the studies described in section
7.7 suggesting a carcinogenic effect of selenite or selenate were
invalid, because, in one study, the results were statistically
insignificant and, in the other studies, there were no controls or
systematic pathological examinations. In another systematic study,
no differences were observed in the incidence of tumours in rats
surviving 2 years or longer and exposed to 0.5 - 2.0 mg selenium/kg
diet.
The Task Group was aware of one study that demonstrated the
carcinogenicity of ethyl selenac (selenium diethyldithiocarbamate)
and another that showed the carcinogenicity of selenium sulfide,
given by gavage. The latter result is of possible interest both as
regards human dermal exposure to selenium sulfide and/or its
possible formation within the body, but the Task Group was not
aware of any studies demonstrating carcinogenic effects of other
selenium compounds.
The Task Group felt that the evaluation published by IARC in
1975 was still valid in concluding that the available animal data
were insufficient to allow an evaluation of the carcinogenicity of
selenium compounds (IARC, 1975).
(b) Experimental evidence of anticarcinogenic effects of
selenium compounds
The results of studies on laboratory animals provide evidence
of a preventive effect of selenium dioxide, sodium selenite, or
"selenized" yeast, given in the food or drinking-water, against
chemically-induced cancers or certain spontaneous, presumably
viral-induced cancers (section 7.7). Generally, the level required
to demonstrate such effects ranges from 1 - 6 mg/kg food or per
litre drinking-water and thus is considerably in excess of the
animal's nutritional needs. In one report, selenium dioxide at
0.1 mg selenium/litre water had some beneficial effect against
spontaneous mammary tumours in mice, but, in this case, the diet
itself contained 0.45 mg selenium/kg. Other studies have shown the
beneficial effects of sodium selenide when applied concomitantly to
the skin with certain carcinogens. In rats fed diets high in
polyunsaturated fats, selenium deficiency increased the incidence
of mammary tumours, after treatment with dimethylbenz[alpha]
anthracene. Therefore, the Task Group concluded that
pharmacological levels of selenium compounds exhibit in many cases
a favourable influence against the development of cancer in various
animal model systems. However, the Task Group was aware of one
study in which administration of high levels of selenium was
associated with an increased incidence of chemically-induced
cancer.
(c) Epidemiological evidence regarding the possible anti-
carcinogenic effects of selenium in human beings
The Task Group acknowledged that apparent negative correlations
have been drawn between cancer death rates and certain general
population characteristics, such as blood-selenium levels or the
average level of selenium in diets in specific geographical zones
(section 8.2.6.1). However, these apparent correlations have not
been consistent with specific cancer sites and are subject to
ecological fallacy. Moreover, such epidemiological data are
subject to question regarding the adequacy of sampling, the
interpretation of blood-selenium data, and the precision of the
estimates of dietary-selenium intake in various countries and
different geographical areas within the USA.
Of the 7 case-control studies examined by the Task Group, no
consistent association was apparent between low selenium status and
risk of cancer. For a given cancer site, there were few replicate
studies. On the majority of occasions, no association was found
between low selenium status and cancer risk. In fact, in at least
one situation a positive correlation was found. In several
countries, low blood- or plasma-selenium levels were found in
patients with several different types of cancer. However, the
effect of the malignant disease itself in lowering blood-selenium
levels (e.g., by decreasing absorption and/or worsening the
generally debilitated nutritional state of the patient) cannot be
excluded as a partial or total explanation for these results.
The Task Group was aware of 3 studies in which blood samples
had been taken for other purposes prior to the diagnosis of any
cancer. Some time later, the samples were retrieved from storage
banks and analysed for selenium. All 3 studies showed a consistent
inverse association between the prediagnostic serum-selenium level
and the risk of cancer. This association between low serum-
selenium level and cancer risk was observed only in smokers, only
in males, and in one study only in blacks but not in whites. In
one study, the association between low selenium level and cancer
risk showed some site specificity, but the second and third studies
were unable to confirm or rebut this because of inadequate sample
size.
The Task Group was aware that in many studies showing a
protective effect of selenium against cancer in animals
pharmacological doses of selenium compounds were used. Therefore,
there were difficulties in relating the animal and human studies,
but the Task Group was aware of an intervention trial being carried
out in China which it is hoped will enable a more precise
evaluation of the association between selenium status and human
cancer risk in the future.
Thus, the Task Group concluded that existing data are
insufficient to determine whether the level of selenium intake is
indeed correlated with the incidence of cancer in man.
9.5.4.5. Caries
Although an association between high selenium intake and dental
caries has been reported in at least one animal study, there is no
clear-cut evidence of such an association in man. The Task Group
concluded that difficulties associated with the interpretation of
urinary-selenium excretion, expressed as mass-concentration per
volume and not expressed as total daily urinary excretion of
selenium (section 9.3.5), as well as the inability to exclude
interference by other environmental factors such as fluoride,
precluded any significant conclusions. A better understanding of
the impact of selenium on the incidence of caries will require a
more comprehensive estimation of selenium exposure and of the other
confounding factors in the respective subpopulations under study.
9.5.4.6. Health effects related to reproduction
The Task Group concluded that at present there is no evidence
that selenium has significant effects on reproductive function in
man.
9.6. Occupational Exposure
In contrast to general population exposure, occupational
exposure usually occurs through direct contact and/or through
inhalation, i.e., dermal and respiratory exposure predominate.
Exposure to selenium and its compounds occurs in primary
industries, i.e., those that extract, mine, treat, or process
selenium-bearing minerals, e.g., copper, zinc, or lead ores and in
secondary industries, i.e., those that use selenium in
manufacturing processes. The physical and chemical form of
selenium under these circumstances varies and is determined by the
industrial processes. The Task Group recognized that industrial
exposure has not been adequately studied with respect to levels of
exposure and there is a need for such studies to be carried out.
For acute occupational selenium exposures, the effects vary
according to to the chemical form of selenium. In contrast to
elemental selenium, which does not appear to be toxic unless
oxidized or reduced, hydrogen selenide and selenium dioxide are
highly toxic, causing irritation of the respiratory tract, which
may be followed by pulmonary oedema.
The Task Group recognized that evaluation of long-term
occupational exposure to selenium must take into account other
dietary and environmental substances because of their recognized or
potential interactions with selenium. An additional factor that
needs to be considered is the fact that preventive measures usually
result in the removal of exposed workers with the appearance of the
first sign of selenium over-exposure (garlic-like odour).
Monitoring of selenium in the urine is also used to identify those
who should be removed from further overexposure.
The Task Group concluded that no studies were available on the
dose-response relationship with regard to occupational exposure to
selenium.
REFERENCES
ABDULLA, M., KOLAR, K., & SVENSSON, S. (1979) Selenium.
Scand. J. Gastroenterol., 14(suppl. 52): 181-184.
ABDULLAEV, G.M. (1976) [Selenium levels in healthy subjects
and those with certain haematological diseases.] In: Selen v
biologii, Vol. 1, Baku, Elm Publishing House, pp. 136-139 (in
Russian).
ABDULLAEV, G.B., GADZHIEVA, N.A., GASANOV, G.G., DZHAFAROV,
A.I., & PERELYGIN, V.V. (1972) [Selenium and vision,] Baku,
Elm Publishing House, 50 pp (in Russian).
ABDULLAEV, G.B., GADZHIEVA, N.A., GASANOV, G.G., MAMEDOVA,
S.A., DMITRENKO, A.I., GULIEVA, L.I., & RODIONOV, V.P.
(1974a) [The effect of selenium compounds on electroretino-
gram under different experimental conditions.] In: [Selen v
biologii,] Baku, Elm Publishing House, pp. 11-22 (in Russian).
ABDULLAEV, G.B., MAMEDOV, SH.V., DZHAFAROV, A.I., PERELYGIN,
V.V., & MAGOMEDOV, N.M. (1974b) [Possible regulation of free
radicals in the retina by selenium compounds.] In: [Selen v
biologii,] Baku, Elm Publishing House, pp. 54-57 (in Russian).
ABU-ERREISH, G.M. (1967) On the nature of some selenium
losses from soils and waters, Brookings, South Dakota, South
Dakota State University (MS Thesis).
ABU-ERREISH, G.M., WHITEHEAD, E.I., & OLSON, O.E. (1968)
Evolution of volatile selenium from soils. Soil Sci., 106:
415-420.
ABUTALYBOV, M.G., VEZIROVA, N.B., FATALIEVA, C.M., KHALILOV,
E.KH., & MUSAEV, S.G. (1976) [Selenium content in some bean
plants of Azerbajdzhan.] In: [Selen v biologii,] Vol. 2, Baku,
Elm Publishing House, pp. 140-142 (in Russian).
ACGIH (1971) Documentation of the threshold limit values for
substances in workroom air, 3rd ed., Cincinnati, Ohio,
American Conference of Governmental Industrial Hygienists,
pp. 224-225.
AKESSON, B. (1985) Plasma selenium in patients with abnormal
plasma protein patterns. In: Proceedings of the XIII Inter-
national Congress of Nutrition, p. 152 (Abstract).
ALEXANDER, A.R., WHANGER, P.D., & MILLER, L.T. (1983)
Bioavailability to rats of selenium in various tuna and wheat
products. J. Nutr., 113(1): 196-204.
ALCINO, J.F. & KOWALD, J.A. (1973) Analytical methods. In:
Klayman, D.L. & Gunther, W.H.H., ed. Organic selenium
compounds, their chemistry and biology, New York, John Wiley
and Sons, pp. 1049-1081.
ALLAWAY, W.H. (1972) An overview of distribution patterns of
trace elements in soils and plants. Ann. NY Acad. Sci., 199:
17-25.
ALLAWAY, W.H. (1973) Selenium in the food chain. The Cornell
Veterinarian, 63: 151-170.
ALLAWAY, W.H. (1978) Perspectives on trace elements in soil
and human health. In: Hemphill, D.D., ed. Trace substances in
environmental health. XII, Columbia, Missouri, University of
Missouri Press, pp. 3-10.
ALLAWAY, W.H. & CARY, E.E. (1964) Determination of
submicrogram amounts of selenium in biological materials.
Anal. Chem., 36: 1359-1362.
ALLAWAY, W.H., MOORE, D.P., OLDFIELD, J.E., & MUTH, O.H.
(1966) Movement of physiological levels of selenium from
soils through plants to animals. J. Nutr., 88: 411-4l8.
ALLAWAY, W.H., CARY, E.E., & EHLIG, C.F. (1967) The cycling
of low levels of selenium in soils, plants, and animals. In:
Muth, O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 273-296.
ALLAWAY, W.H., KUBOTA, J., LOSEE, F., & ROTH, M. (1968)
Selenium, molybdenum, and vanadium in human blood. Arch.
environ. Health, 16: 342-348.
AMOR, A.J. & PRINGLE, P. (1945) A review of selenium as an
industrial hazard. Bull. Hyg., 20: 239-241.
ANCIZAR-SORDO, J. (1947) Occurrence of selenium in soils and
plants of Columbia, South America. Soil Sci., 63: 437.
ANDERSON, R.A. & POLANSKY, M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Trace Elem. Res., 3: 1-5.
ANDREWS, R.W. & JOHNSON, D.C. (1976) Determination of
selenium (IV) by anodic stripping voltammetry in flow system
with ion exchange separation. Anal. Chem., 48: 1056-1060.
ANONYMOUS (1962) Selenium poisons Indians. Sci. News Lett.,
81: 254.
ANONYMOUS (1975) New links in the selenium cycle. Agric.
Res., 24: 6-7.
ANONYMOUS (1984) Washington DC, US Food and Drug
Administration, pp. 19 (FDA Bulletin No. 14).
ANUNDI, I., STAHL, A., & HOGBERG, J. (1984) Chem.-biol.
Interact., 50: 277.
AOAC (1975) Official methods of analysis, 12th ed.,
Washington DC, Association of Official Analytical Chemists.
ARTHUR, D. (1972) Selenium content of Canadian foods. Can.
Inst. Food Sci. Technol. J., 5: 165-169.
ASHER, C.J., BUTLER, G.W., & PETERSON, P.J. (1977) Selenium
transport in root systems of tomato. J. exp. Bot., 28: 279-291.
AWASTHI, Y.C., BEUTLER, E., & SRIVASTAVA, S.K. (1975)
Purification and properties of human erythrocyte glutathione
peroxidase. J. biol. Chem., 250: 5144-5149.
BACHAREV, V.D., BOCHAROVA, M.A., & SHOSTAK, V.I. (1975) [On
the effect of selenium on the light perception of the eye.]
Fiziol. Zhurn., 61(1): 150-153 (in Russian).
BAI, J., WU, S.Q., GE, K.Y., DENG, X.J., & SU, C.Q. (1980)
The combined effect of selenium deficiency and viral infection
on the myocardium of mice (preliminary study). Acta Acad. Med.
Sinicae, 2: 29-31.
BAIRD, R.B., POURIAN, S., & GABRIELIAN, S.M. (1972) Determination
of trace amounts of selenium in waste waters by carbon rod
atomization. Anal. Chem., 44: 1887-1889.
BARBEZAT, G.O., CASEY, C.E., REASBECK, P.G., ROBINSON, M.F., &
THOMSON, C.D. (1984) Selenium. In: Rosenberg, I. &
Solomons, N.W., ed. Absorption and malabsorption of mineral
nutrients, New York, Alan R. Liss., pp. 231-258.
BARRETTE, M., LAMOUREUX, G., LEBEL, E., LECOMTE, R., PARADIS,
P., & MONARO, S. (1976) Trace element analysis of freeze-
dried blood serum by proton and alpha-induced X-rays. Nucl.
Instrum. Methods, 134: 189-196.
BEARSE, R.C., CLOSE, D.A., MALANIFY, J.J., & UMBARGER, C.J.
(1974) Elemental analysis of whole blood using proton-induced
X-ray emission. Anal. Chem., 46: 499-503.
BEATH, O.A., EPPSON, H.F., & GILBERT, C.S. (1935). Selenium
and other toxic minerals in soils and vegetation, Wyoming
Experimental Station, 55 pp (Bulletin No. 206).
BEHNE, D. & HOFER-BOSSE, T. (1984) Effects of a low selenium
status on the distribution and retention of selenium in the
rat. J. Nutr., 114: 1289-1296.
BEHNE, D. & WOLTERS, W. (1979) Selenium content and gluta-
thione peroxidase activity in the plasma and erythrocytes of
non-pregnant and pregnant women. J. clin. Chem. clin.
Biochem., 17: 133-135.
BEIJE, B., ONFELT, A., & OLSSON, U. (1984) Influence of
dietary selenium on the mutagenic activity of perfusate and
bile from rat liver, perfused with 1,1-dimethylhydrazine.
Mutat. Res., 130: 121-126.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BESBRIS, H.J., WORTZMAN, M.S., & COHEN, A.M. (1982) Effect
of dietary selenium on the metabolism and excretion of
2-acetylaminofluorene in the rat. J. Toxicol. environ.
Health, 9: 63-76.
BHUYAN, K.C., BHUYAN, D.K., & PODOS, S.M. (1981) Selenium-
induced cataract: biochemical mechanism. In: Spallholz, J.E.,
Martin, J.L., & Ganther, H.E., ed. Selenium in biology and
medicine, Westport, Connecticut, Avi Publishing Company,
pp. 403-412.
BIERI, J.G. & AHMAD, K. (1976) Selenium content of
Bangladeshi rice by chemical and biological assay. J. agric.
food Chem., 24: 1073-1074.
BIRT, D.F., JULINS, A.D., & POUR, P.M. (1984) Increased
pancreatic carcinogenesis in Syrian hamsters fed high selenium
diets. Proc. Am. Assoc. Cancer Res., 25: 133.
BLADES, M.W., DALZIEL, J.A., & ELSON, C.M. (1976) Cathodic
stripping voltammetry of nanogram amounts of selenium in
biological material. J. Assoc. Off. Anal. Chem., 59: 1234-1239.
BLOTCKY, A.J., SULLIVAN, J.F., SHUMAN, M.S., WOODWARD, G.P.,
VOORS, A.W., & JOHNSON, W.D. (1976) Selenium levels in liver
and kidney. In: Hemphill, D.D., ed. Trace substances in
environmental health. X, Columbia, Missouri, University of
Missouri Press, pp. 97-103.
BONARD, E.C. & KORALINK, K.D. (1958) Intoxication aiguë par
vapeurs d'hydrogene selenide. Praxis, 47(i): 533-534.
BOUND, G.P. & FORBES, S. (1978) Differential-pulse
polarography of selenium (IV) in the presence of metal ions.
Analyst, 103: 176-179.
BOWEN, H.J.M. & CAWSE, P.A. (1963) The determination of
selenium in biological material by radioactivation. Analyst,
88: 721-726.
BOWEN, W.H. (1972) The effects of selenium and vanadium on
caries activity in monkeys (M. irus). J. Irish Dent. Assoc.,
18: 83-89.
BRADY, P.S., KU, P.K., & ULLREY, D.E. (1978) Lack of effect
of selenium supplementation on the response of the equine
erythrocyte glutathione system and plasma enzymes to exercise.
J. anal. Sci., 47: 492-496.
BRADY, P.S., BRADY, L.J., & ULLREY, D.E. (1979) Selenium,
vitamin E, and the response to swimming stress in the rat. J.
Nutr., 109: 1103-1109.
BRITTON, J.L., SHEARER, T.R., & DE SART, D.J. (1980) Cario-
stasis by moderate doses of selenium in the rat model. Arch.
environ. Health, 35: 74-76.
BRODIE, K.G. (1979) Analysis of arsenic and other trace
elements by vapor generation. Am. Lab. (June issue): 58-66.
BROGHAMER, W.L., MCCONNELL, K.P., & BLOTCKY, A.L. (1976)
Relationship between serum selenium levels and patients with
carcinoma. Cancer, 37: 1384-1388.
BROGHAMER, W.L., MCCONNELL, K.P., GRIMALDI, M., & BLOTCKY,
A.J. (1978) Serum selenium and reticuloendothelial tumours.
Cancer, 41: 1462-1466.
BROWN, D.G. & BURK, R.F. (1973) Selenium retention in
tissues and sperm of rats fed a torula yeast diet. J. Nutr.,
103: 102-108.
BROWN, D.G., BURK, R.F., SEELY, R.J., & KIKER, K.W. (1972)
Effect of dietary selenium on the gastrointestinal absorption
of 75Se03 in the rat. Int. J. Vit. nutr. Res., 42: 588-591.
BROWN, M.W. & WATKINSON, J.H. (1977) An automated
fluorimetric method for the determination of nanogram
quantities of selenium. Anal. Chim. Acta, 89: 29-35.
BRUNE, D., SAMSAHL, K., & WESTER, P.O. (1966) A comparison
between the amounts of As, Au, Br, Cu, Fe, Mo, Se, and Zn in
normal and uraemic human whole blood by means of neutron
activation analysis. Clin. Chim. Acta, 13: 285-291.
BUCHAN, R.F. (1947) Industrial selenosis. Occup. Med., 3:
439-456.
BUNCE, G.E., HESS, J.L., GURLEY, R., BATRA, R., & TARNAWSKA,
E. (1985) Lens energy metabolism and Ca content in selenite
induced cataract. In: Mills, C.F., Bremner, I., & Chesters,
J.K., ed. Trace elements in man and animals - TEMA 5, Slough,
Commonwealth Agricultural Bureaux, pp. 250-254.
BURCHANOV, A.I. (1972) [On regulation of complex chemical
dusts in rare metals production.] Vopr. Gig. Trud. Profzabol.
Kazajenade, Kazah NII Gig. Trud. Profazabol., pp. 72-74 (in
Russian).
BURCHANOV, A.I. & ZHAKENOVA, R.K. (1973) [Pulmonary changes
following intratracheal administration of elementary selenium
to white rats.] Zavavoohr. Kazah., 3: 52-53 (in Russian).
BURCHANOV, A.I., SALEHOV, M.I., DOROFEEVA, O.N., & ZHAKENOVA,
R.K. (1969) [On the effects of metal selenium dust on the
organism.] In: [Proceedings of a Concluding Scientific
Conference on Labour Hygiene and Occupational Disease, 21-22
October, 1969,] Karaganda, Kazah., NII Gig. Trud. Profzabol,
pp. 77-80 (in Russian).
BURK, R.F. (1976) Selenium in man. In: Prasad, A.S., ed.
Trace elements in human health and disease. II. Essential and
toxic elements, New York, Academic Press, pp. 105-133.
BURK, R.F. & CORREIA, M.A. (1977) Accelerated hepatic haem
catabolism in the selenium-deficient rat. Biochem. J., 168:
105-111.
BURK, R.F. & LANE, J.M. (1979) Ethane production and liver
necrosis in rats after administration of drugs and other
chemicals. Toxicol. appl. Pharmacol., 50: 467-478.
BURK, R.F & MASTERS, B.S.S. (1975) Some effects of selenium
deficiency on the hepatic microsomal cytochrome P-450 system
in the rat. Arch. Biochem. Biophys., 170: 124-131.
BURK, R.F., Jr, PEARSON, W.N., WOOD, R.P., II, & VITERI, F.
(1967) Blood selenium levels and in vitro red blood cell
uptake of 75Se in kwashiorkor. Am. J. clin. Nutr., 20: 723-733.
BURK, R.F., BROWN, D.G., SEELY, R.J., & SCAIEF, C.C., III
(1972) Influence of dietary and injected selenium on whole-
body retention, route of excretion, and tissue retention of
75Se03 2- in the rat. J. Nutr., 102: 1049-1055.
BURK, R.F., SEELEY, R.J., & KIKER, K.W. (1973) Selenium:
dietary threshold for urinary excretion in the rat. In:
Proceedings of the Society of Experimental and Biological
Medicine, Vol. 142, pp. 214-216.
BURK, R.F., MACKINNON, A.M., & SIMON, F.R. (1974) Selenium
and hepatic microsomal hemoproteins. Biochem. Biophys. Res.
Commun., 56: 431-436.
BURK, R.F., NISHIKI, K., LAWRENCE, R.A., & CHANCE, B. (1978)
Peroxide removal by selenium-dependent and selenium independent
glutathione peroxidases in hemoglobin-free perfused rat liver.
J. biol. Chem., 253: 43-46.
BURK, R.F., LAWRENCE, R.A., & CORREIA, M.A. (1980a) Sex
differences in biochemical manifestations of selenium
deficiency in rat liver with special reference to heme
metabolism. Biochem. Pharmacol., 29: 39-42.
BURK, R.F., LAWRENCE, R.A., & LANE, J.M. (1980b) Liver
necrosis and lipid peroxidation in the rat as the result of
paraquat and diquat administration. J. clin. Invest., 65:
1024-1031.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1974) Superoxide- and
singlet oxygen-catalyzed lipid peroxidation as a possible
mechanism for paraquat (methyl viologen) toxicity. Biochem.
Biophys. Res. Commun., 58: 749-755.
BUTLER, G.W. & PETERSON, P.J. (1961) Aspects of the faecal
excretion of selenium by sheep. N.Z. J. agric. Res., 4:
484-491.
BUTTNER, W. (1963) Action of trace elements on the
metabolism of fluoride. J. dent. Res., 42: 453-460.
BYARD, J.L. (1969) Trimethyl selenide: a urinary metabolite
of selenite. Arch. Biochem. Biophys., 130: 556-560.
CADELL, P.B. & COUSINS, F.B. (1960) Urinary selenium and
dental caries. Nature (Lond.), 185: 863-864.
CAGEN, S.Z. & GIBSON, J.E. (1977) Liver damage following
paraquat in selenium-deficient and diethyl maleate-pretreated
mice. Toxicol. appl. Pharmacol., 40: 193-200.
CALVIN, H.I. (1978) Selective incorporation of selenium-75
into a polypeptide of the rat sperm tail. J. exp. Zool., 204:
445-452.
CANNELLA, J.M. (1976) Surveillance of employees in selenium
alloying operations. In: Proceedings of the Symposium on
Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 343-348.
CANTOR, A.H. & SCOTT, M.L. (1974) The effect of selenium in
the hen's diet on egg production, hatchability, performance of
progeny, and selenium concentration in eggs. Poultry Sci., 53:
1870-1880.
CANTOR, A.H. & SCOTT, M.L. (1975) Influence of dietary
selenium on tissue selenium levels in turkeys. Poultry Sci.,
54: 262-265.
CANTOR, A.H., SCOTT, M.L., & NOGUCHI, T. (1975a) Biological
availability of selenium in feedstuffs and selenium compounds
for prevention of exudative diathesis in chicks. J. Nutr.,
105: 96-105.
CANTOR, A.H., LANGEVIN, M.L., NOGUCHI, T., & SCOTT, M.L.
(1975b) Efficacy of selenium in selenium compounds and
feedstuffs for prevention of pancreatic fibrosis in chicks. J.
Nutr., 105(1): 106-111.
CANTOR, A.H., MOORHEAD, P.D., & BROWN, K.I. (1978) Influence
of dietary selenium upon reproductive performance of male and
female breeder turkeys. Poultry Sci., 57: 1337-1345.
CAPPON, C.J. & SMITH, J.C. (1982) Chemical form and
distribution of mercury and selenium in canned tuna. J. appl.
Toxicol., 2: 181-189.
CARNRICK, G.R., MANNING, D.C., & SLAVIN, W. (1983)
Determination of selenium in biological materials with
platform furnace atomic absorption spectroscopy and Zeeman
background correction. Analyst, 108: 1297-1312.
CARTER, D.L., ROBBINS, C.W., & BROWN, M.J. (1972) Effect of
phosphorus fertilization on the selenium concentration in
alfalfa (Medicago sativa). Soil Sci. Soc. Am. Proc., 36:
624-628.
CARY, E.E., ALLAWAY, W.H., & MILLER, M. (1973) Utilization
of different forms of dietary selenium. J. anal. Sci., 36:
285-292.
CASEY, C.E., GUTHINE, B.E., FREUD, G.M., & ROBINSON, M.F.
(1982) Selenium in human tissues from New Zealand. Arch.
environ. Health., 37: 133-135.
CAYGILL, C.P.J. & DIPLOCK, A.T. (1973) The dependence on
dietary selenium and vitamin E of oxidant-labile liver
microsomal non-haem iron. FEBS Lett., 33: 172-176.
CHAN, C.C.Y. (1976) Improvement in the fluorimetric
determination of selenium in plant materials with
2,3-diaminonaphthalene. Anal. Chim. Acta, 82: 213-215.
CHANSLER, M.W., MORRIS, V.C., & LEVANDER, O.A. (1983)
Bioavailability to rats of selenium in Brazil nuts and
mushrooms. Fed. Proc., 42(4): 927.
CHATTERJEE, M. & BANERJEE, M.R. (1982) Selenium mediated
dose-inhibition of 7-12-dimethylbenz(alpha)anthracene-induced
transformation of mammary cells in organ culture. Cancer
Lett., 17: 187-195.
CHAU, Y.K., WONG, P.T.S., SILVERBERG, B.A., LUXON, P.L., &
BENGERT, G.A. (1976) Methylation of selenium in the aquatic
environment. Science, 192: 1130-1131.
CHAVEZ, J.F. (1966) Studies on the toxicity of a sample of
Brazil nuts with a high content of selenium. Bol. Soc. Quim.
Peru, 32: 195-203.
CHEN, R.W., WHANGER, P.D., & WESWIG, P.H. (1975) Selenium-
induced redistribution of cadmium binding to tissue proteins:
a possible mechanism of protection against cadmium toxicity.
Bioinorgan. Chem., 4: 125-133.
CHEN, X., YANG, G., CHEN, J., CHEN, X., WEN, Z., & GE, K.
(1980) Studies on the relations of selenium and Keshan
disease. Biol. Trace Elem. Res., 2: 91-107.
CHERRY, D.S. & GUTHRIE, R.K. (1977) Toxic metals in surface
waters from coal ash. Water Resour. Bull., 13: 1227-1236.
CHUNG, A. & MAINES, M.D. (1981) Effect of selenium on
glutathione metabolism. Induction of -glutamylcysteine
synthetase and glutathione reductase in the rat liver.
Biochem. Pharmacol., 30(23): 3217-3223.
CLARK, L.C. (1985) The epidemiology of selenium and cancer.
Fed. Proc., 44(9): 2584-2589.
CLAYCOMB, C.K., SUMMERS, G.W., & JUMP, E.B. (1965) Effect of
dietary selenium on dental caries in Sprague Dawley rats. J.
dent. Res., 44: 826.
CLAYTON, C.C. & BAUMANN, C.A. (1949) Diet and azo dye
tumors: effect of diet during a period when the dye is not
fed. Cancer Res., 9: 575-582.
CLINTON, M., Jr (1947) Selenium fume exposure. J. ind. Hyg.
Toxicol., 29: 225-226.
COMBS, G.F., Jr & SCOTT, M.L. (1975) Polychlorinated
biphenyl-stimulated selenium deficiency in the chick.
Poultry. Sci., 54: 1152-1158.
COMBS, G.F., Jr, CANTOR, A.H., & SCOTT, M.L. (1975) Effects
of dietary polychlorinated biphenyls on vitamin E and selenium
nutrition in the chick. Poultry Sci., 54: 1143-1152.
CONE, J.E., MARTIN DEL RIO, R., DAVIS, J.N., & STADTMAN, T.C.
(1976) Chemical characterization of the selenoprotein
component of clostridial glycine reductase: identification of
selenocysteine as the organoselenium moiety. Proc. Natl Acad.
Sci. (USA), 73: 2659-2663.
COOPER, W.C. (1967) Selenium toxicity in man. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 185-199.
COOPER, W.C. (1974) Analytical chemistry of selenium. In:
Zingaro, R.A. & Cooper, W.C., ed. Selenium, New York, Van
Nostrand Reinhold Co., pp. 615-653.
COOPER, W.C. & GLOVER, J.R. (1974) The toxicology of
selenium and its compounds. III. In: Zingaro, R.A. & Cooper,
W.C., ed. Selenium, New York, Van Nostrand Reinhold Co., pp.
654-674.
COOPER, W.C., BENNETT, K.G., & CROXTON, F.C. (1974) The
history, occurrence, and properties of selenium. In: Zingaro,
R.A. & Cooper, W.C., ed. Selenium, New York, Van Nostrand
Reinhold Co., pp. 1-31.
CORREIA, M.A. & BURK, R.F. (1976) Hepatic heme metabolism in
selenium-deficient rats: effect of phenobarbital. Arch.
Biochem. Biophys., 177: 642-644.
CORREIA, M.A. & BURK, R.F. (1978) Rapid stimulation of
hepatic microsomal heme oxygenase in selenium-deficient rats:
an effect of phenobarbital. J. biol. Chem., 253: 6203-6210.
COWGILL, U.M. (1974) Trace elements and the birth rate in
the continental United States. In: Hemphill, D.D., ed. Trace
substances in environmental health. VIII, Columbia, Missouri,
University of Missouri Press, pp. 15-21.
COWGILL, U.M. (1976) Selenium and human fertility. In:
Proceedings of the Symposium on Selenium and Tellurium in the
Environment, Pittsburg, Pennsylvania, Industrial Health
Foundation, pp. 300-315.
CREGER, C.R., MITCHELL, R.H., ATKINSON, R.L., FERGUSON, T.M.,
REID, B.L., & COUCH, J.R. (1960) Vitamin E activity of
selenium in turkey hatchability. Poultry Sci., 39: 59-63.
CRYSTAL, R.G. (1973) Elemental selenium: structure and
properties. In: Klayman, D.L. & Gunther, W.H.H., ed. Organic
selenium compounds: their chemistry and biology, New York,
John Wiley and Sons, Inc, pp. 13-27.
CSALLANY, A.S., SU, L.-C., & MENKEN, B.Z. (1984) Effect of
selenite, vitamin E, and n,n'-diphenyl-p-phenylenediamine on
liver organic solvent-soluble lipofuscin pigments in mice.
J. Nutr., 114: 1582-1587.
CUKOR, P., WALZCYK, J., & LOTT, P.F. (1964) The application
of isotopic dilution analysis to the fluorimetric
determination of selenium in plant material. Anal. Chim. Acta,
30: 473-482.
CUMMINS, L.M. & KIMURA, E.T. (1971) Safety evaluation of
selenium sulfide antidandruff shampoos. Toxicol. appl.
Pharmacol., 20: 89-96.
CUMMINS, L.M. & MARTIN, J.L. (1967) Are selenocystine and
selenomethionine synthesized in vivo from sodium selenite in
mammals? Biochemistry, 6: 3162-3168.
CZERNIEJEWSKI, C.P., SHANK, C.W., BECHTEL, W.G., & BRADLEY,
W.B. (1964) The minerals of wheat, flour, and bread. Cereal
Chem., 41: 65-72.
D'HONDT, P., LIEVENS, P., VERSIECK, J., & HOSTE, J. (1977)
Determination of trace elements in animal and human muscle by
semi-automated radiochemical neutron activation analysis.
Radiochem. Radioanal. Lett., 31: 231-240.
DAMS, R. & DE JONGE, J. (1976) Chemical composition of Swiss
aerosols from the Jungfraujoch. Atmos. Environ., 10: 1079-1084.
DE JONG, D., MORSE, R.A., GUTENMANN, W.H., & LISK, D.J.
(1977) Selenium in pollen gathered by bees foraging on fly
ash-grown plants. Bull. environ. Contamin. Toxicol., 18:
442-444.
DE WITT, W.B. & SCHWARZ, K. (1958) Multiple dietary necrotic
degeneration in the mouse. Experientia, 14: 28-34.
DICKSON, J.D. (1969) Notes on hair and nail loss after
ingesting Sapucaia nuts (Lecythis elliptica). Econ. Bot., 23:
133-134.
DICKSON, R.C. & TOMLINSON, R.H. (1967) Selenium in blood and
human tissues. Clin. Chim. Acta, 16: 311-321.
DINKEL, C.A., MINYARD, J.A., WHITEHEAD, E.I., & OLSON, O.E.
(1957) Agricultural research at the Reed Ranch substation,
Brookings, South Dakota, South Dakota State College of
Agriculture and Mechanic Arts, 35 pp (Agricultural Experiment
Station Circular No. 135).
DINKEL, C.A., MINYARD, J.A., & RAY, D.E. (1963) Effects of
season of breeding on reproductive and weaning performance of
beef cattle grazing seleniferous range. J. anim. Sci., 22:
1043-1045.
DIPLOCK, A.T. (1974a) A possible role for trace amounts of
selenium and vitamin E in the electron-transfer system of
rat-liver microsomes. In: Hoekstra, W.G., Suttie, J.W.,
Ganther, H.E., & Mertz, W., ed. Trace element metabolism in
animals. II, Baltimore, Maryland, University Park Press, pp.
147-160.
DIPLOCK, A.T. (1974b) Possible stabilizing effect of vitamin
E on microsomal, membrane-bound, selenide-containing proteins
and drug-metabolizing enzyme systems. Am. J. clin. Nutr., 27:
995-1004.
DIPLOCK, A.T. (1976) Metabolic aspects of selenium action
and toxicity. CRC Crit. Rev. Toxicol., 4: 271-329.
DIPLOCK, A.T. (1979) The influence of selenium and vitamin E
on oxidative demethylation reactions. In: Olive, G., ed. Adv.
Pharmacol. Therap., Proceedings of the 7th International
Congress on Pharmacology, Vol. 8, pp. 25-33.
DIPLOCK, A.T., BAUM, H., & LUCY, J.A. (1971) The effect of
vitamin E on the oxidation state of selenium in rat liver.
Biochem. J., 123: 72l-729.
DIPLOCK, A.T., CAYGILL, C.P.J., JEFFERY, E.H., & THOMAS, C.
(1973) The nature of the acid-volatile selenium in the liver
of the male rat. Biochem. J., 134: 283-293.
DONALDSON, W.E. (1977) Selenium inhibition of avian fatty
acid synthetase complex. Chem.-biol. Interac., 17: 313-320.
DONGHERTY, J.J. & HOEKSTRA, W.G. (1982) Stimulation of lipid
peroxidation in vivo by injected selenite and lack of
stimulation by selenate. Proc. Soc. Exp. Biol. Med., 169(2):
209-215.
DORAN, J.W. (1976) Microbial transformations of selenium in
soil and culture, Ithaca, New York, Cornell University
(Ph.D. Thesis).
DOUGLASS, J.S., MORRIS, V.C., SOARES, J.H., Jr, & LEVANDER,
O.A. (1981) Nutritional availability to rats of selenium in
tuna, beef kidney, and wheat. J. Nutr., 111: 2180-2187.
DUCE, R.A., HOFFMAN, G.L., & ZOLLER, W.H. (1975) Atmospheric
trace metals at remote northern and southern hemisphere sites:
pollution or natural? Science, 187: 59-61.
DUDLEY, H.C. (1938) Toxicology of selenium. V. Toxic and
vesicant properties of selenium oxychloride. Public Health
Rep., 53: 94-98.
DUDLEY, H.C. & MILLER, J.W. (194l) Toxicology of selenium.
VI. Effects of subacute exposure to hydrogen selenide. J. ind.
Hyg. Toxicol., 23: 470-477.
DUTKIEWICZ, T., DUTKIEWICZ, B., & BALCERSKA, I. (1972)
Dynamics of organ and tissue distribution of selenium after
intragastric and dermal administration of sodium selenite.
Bromatol. Chem. Toksykol., 4: 475-481.
DUVOIR, M., POLLETT, L., & HERRENSCHMIDT, J.L. (1937) Eczéma
professionnel dû au sélénium. Bull. Soc. Franc. Dermat.
Syphil., 44: 88-95.
DYER, D.G., SCHUETT, V.E., & GANTHER, H.E. (1977) Blood
selenium and glutathione peroxidase in children with PKU on
diet therapy. In: Abstracts of the Western Hemisphere
Nutrition Congress. V, Quebec, pp. 417.
EGAN, A., KERR, S., & MINSKI, M.J. (1977) Instrumental
neutron activation analysis of selenium using 77µSe (T 1/2 =
17s) in biological materials. In: Brown, S.S., ed. Clinical
chemistry and chemical toxicology of metals, Amsterdam,
Elsevier North-Holland Biomedical Press, pp. 353-356.
ELLIS, N., LLOYD, B., LLOYD, R.S., & CLAYTON, B.E. (1984)
Selenium and vitamin E in relation to risk factors for
coronary heart disease. J. Clin. Pathol, 37(2): 200-206.
ENGBERG, R.A. (1973) Selenium in Nebraska's groundwater and
streams, Lincoln, Nebraska, University of Nebraska (Nebraska
Water Survey Paper No. 35).
ERMAKOV, V.V. (1975) [Selenium determination in biological
materials by fluorometry and gas-chromatography.] In:
[Vitamins. VIII. Biochemistry of vitamin E and selenium,]
Kiev, Naukova Dumka Publishing House, pp. 141-146 (in Russian).
ERMAKOV, V.V. & KOVALSKIJ, V.V. (1968) [The geochemical
ecology of organisms at high selenium levels in the
environment.] In: [Transactions of the biogeochemical
laboratory,] Moscow, Nauka Publishing House, Vol. 12, pp.
204-237 (in Russian).
ERMAKOV, V.V. & KOVALSKIJ, V.V. (1974) [The biological
importance of selenium,] Moscow, Nauka Publishing House, 298
pp (in Russian).
ERSHOV, V.P. (1969) [Hygienic aspects of selenium containing
steel production and the prevention of selenium intoxication.]
Gig. Tr. Prof. Zabol., 12: 29-33 (in Russian).
EVANS, C.S., ASHER, C.J., & JOHNSON, C.M. (1968) Isolation
of dimethyldiselenide and other volatile selenium compounds
from Astragalus racemosus (Pursh.). Austral. J. biol. Sci.,
21: 13-20.
EVANS, H.M. & BISHOP, K.S. (1922) On the existence of a
hitherto unrecognized dietary factor essential for
reproduction. Science, 56: 650-651.
EWAN, R.C., POPE, A.L., & BAUMANN, C.A. (1967) Elimination
of fixed selenium by the rat. J. Nutr., 91: 547-554.
EWAN, R.C., BAUMANN, C.A., & POPE, A.L. (1968) Retention of
selenium by growing lambs. J. agric. food Chem., 16: 216-219.
EXON, J.H., KOLLER, L.D., & ELLIOTT, S.C. (1976) Effect of
dietary selenium on tumor induction by an oncogenic virus.
Clin. Toxicol., 9: 273-279.
FALK, R. & LINDHE, J.C. (1974) Radiation dose received by
humans from intravenously-administered sodium selenite marked
with selenium-75, Los Alamos, New Mexico, Los Alamos
Scientific Laboratory (Los Alamos Translation LA-TR-76-5).
FENG, Z., WANG, X., & HAN, C. (1985) [Studies on the
toxicity of diets with different selenium levels in rats.]
Food Hyg. Res., 3(2): 14-20 (in Chinese).
FERRETTI, R.J. & LEVANDER, O.A. (1974) Effect of milling and
processing on the selenium content of grains and cereal
products. J. agric. food Chem., 22: 1049-1051.
FERRETTI, R.J. & LEVANDER, O.A. (1976) Selenium content of
soybean foods. J. agric. food Chem., 24: 54-56.
FILATOVA, V.S. (1948) [Characteristics of selenium as an
industrial intoxicant.] Can. Med. Thesis (in Russian).
FILATOVA, V.S. (1951) [On the toxicity of selenium
chloride.] Gig. i Sanit., 5: 18-23 (in Russian).
FLEMING, G.A. (1962) Selenium in Irish soils and plants.
Soil Sci., 94: 28-35.
FLEMING, G.A. & WALSH, T. (1957) Selenium occurrence in
certain Irish soils and its toxic effects on animals. Proc.
Royal Irish Acad., 58: 151-166.
FLOHE, L., GUNZLER, W.A., & SCHOEKS, H.H. (1973) Glutathione
peroxidase: a selenoenzyme. FEBS Lett., 32: 132-134.
FORBES, S. & BOUND, G.P. (1977) Some investigations into the
electroanalytical chemistry of selenium. Proc. Anal. Div.
Chem. Soc., 14: 253-256.
FORSTROM, J.W., ZAKOWSKI, J.J., & TAPPEL, A.L. (1978)
Identification of the catalytic site of rat liver glutathione
peroxidase as selenocysteine. Biochemistry, 17: 2639-2644.
FRANKE, K.W. & MOXON, A.L. (1936) A comparison of the
minimum fatal doses of selenium, tellurium, arsenic, and
vanadium. J. Pharmacol. exp. Ther., 58: 454-459.
FRANKE, K.W. & POTTER, V.R. (1936) The effect of selenium
containing foodstuffs on growth and reproduction of rats at
various ages. J. Nutr., 12: 205-214.
FRANKE, K.W. & TULLY, W.C. (1935) A new toxicant occurring
naturally in certain samples of plant foodstuffs. V. Low
hatchability due to deformities in chicks. Poultry Sci., 14:
273-279.
FRANKE, K.W., MOXON, A.L., POLEY, W.E., & TULLY, W.C. (1936)
Monstrosities produced by the injection of selenium salts into
hen's eggs. Anat. Rec., 65: 15-22.
FROSETH, J.A., PIPER, R.C., & CARLSON, J.R. (1974)
Relationship of dietary selenium and oral methyl mercury to
blood and tissue selenium and mercury concentrations and
deficiency: toxicity signs in swine. Fed. Proc., 33: 660.
FROST, D.V. & INGVOLDSTAD, D. (1975) Ecological aspects of
selenium and tellurium in human and animal health. Chemica
Scripta, 8A: 96-107.
FURR, A.K., PARKINSON, T.F., HINRICHS, R.A., VAN CAMPEN, D.R.,
BACHE, C.A., GUTENMANN, W.H., ST. JOHN, L.E., Jr, PAKKALA,
I.S., & LISK, D.J. (1977) National survey of elements and
radioactivity in fly ashes. Absorption of elements by cabbage
grown in fly ash-soil mixtures. Environ. Sci. Technol., 11:
1194-1201.
FURR, A.K., PARKINSON, T.F., GUTENMANN, W.H., PAKKALA, I.S., &
LISK, D.J. (1978a) Elemental content of vegetables, grains,
and forages field-grown on fly ash amended soil. J. agric.
food Chem., 26: 357-359.
FURR, A.K., PARKINSON, T.F., HEFFRON, C.L., REID, J.T.,
HASCHEK, W.M., GUTENMANN, W.H., PAKKALA, I.S., & LISK, D.J.
(1978b) Elemental content of tissues of sheep fed rations
containing coal fly ash. J. agric. food Chem., 26: 1271-1274.
GAIROLA, C. & CHOW, C.K. (1982) Dietary selenium, hepatic
arylhydrocarbon hydroxylase, and mutagenic activation of
benzo( a)pyrene, 2-aminoanthracene, and 2-aminofluorene.
Toxicol. Lett., 11: 281-287.
GANAPATHY, S. & DHANDA, R. (1976) Selenium content of
omniverous and vegetarian diets. Fed. Proc., 35: 360.
GANAPATHY, S.N., JOYNER, B.T., & HAFNER, K.M. (1975) Effect
of baking, broiling, and frying on the selenium content of
selected beef, pork, chicken and fish foods. In: Proceedings
of the 10th International Congress of Nutrition - Abstracts,
Kyoto, Japan, 3-9 August, 1975, p. 277.
GANTHER, H.E. (1968) Selenotrisulfides. Formation by the
reaction of thiols with selenious acid. Biochemistry, 7:
2898-2905.
GANTHER, H.E. (1971) Reduction of the selenotrisulfide
derivative of glutathione to a persulfide analog by
glutathione reductase. Biochemistry, 10: 4089-4098.
GANTHER, H.E. (1978) Modification of methylmercury toxicity
and metabolism by selenium and vitamin E: possible mechanisms.
Environ. Health Perspect., 25: 71-76.
GANTHER, H.E. (1979) Metabolism of hydrogen selenide and
methylated selenides. Adv. Nutr. Res., 2: 107-128.
GANTHER, H.E. & BAUMANN, C.A. (1962) Selenium metabolism.
II. Modifying effects of sulfate. J. Nutr., 77: 408-414.
GANTHER, H.E. & CORCORAN, C. (1969) Selenotrisulfides. II.
Cross-linking of reduced pancreatic ribonuclease with
selenium. Biochemistry, 8: 2557-2563.
GANTHER, H.E. & HSIEH, H.S. (1974) Mechanisms for the
conversion of selenite to selenides in mammalian tissues. In:
Hoekstra, W.G., Suttie, J.W., Ganther, H.E., & Mertz, W., ed.
Trace element metabolism in animals. 2, Baltimore, Maryland,
University Park Press, pp. 339-353.
GANTHER, H.E. & SUNDE, M.L. (1974) Effect of tuna fish and
selenium on the toxicity of methylmercury: a progress report.
J. food Sci., 39: 1-5.
GANTHER, H.E., LEVANDER, O.A., & BAUMANN, C.A. (1966)
Dietary control of selenium volatilization in the rat. J.
Nutr., 88: 55-60.
GANTHER, H.E., GOUDIE, C., SUNDE, M.L., KOPECKY, M.J., WAGNER,
P., OH, S., & HOEKSTRA, W.G. (1972) Selenium: relation to
decreased toxicity of methylmercury added to diets containing
tuna. Science, 175: 1122-1124.
GANTHER, H.E., HAFEMAN, D.G., LAWRENCE, R.A., SERFASS, R.E., &
HOEKSTRA, W.G. (1976) Selenium and glutathione peroxidase in
health and disease: a review. In: Prasad, H.H., ed. Trace
elements in human health and disease. II. Essential and toxic
elements, New York, Academic Press pp. 165-234.
GARDINER, M.R., ARMSTRONG, J., FELS, H., & GLENCROSS, R.N.
(1962) A preliminary report on selenium and animal health in
Western Australia. Aust. J. exp. Agric. Animal Husb., 2:
261-269.
GARDNER, S. (1973) Selenium in animal feed. Proposed food
additive regulation. Fed. Reg., 38: 10458-10460.
GE, K., XUE, A., BAI, J., & WANG, S. (1983) Keshan disease -
an endemic cardiomyopathy in China. Virchows Arch. (Pathol.
Anat.), 401: 1-15.
GEERING, H.R., CARY, E.E., JONES, L.H.P., & ALLAWAY, W.H.
(1968) Solubility and redox criteria for the possible forms
of selenium in soils. Soil Sci. Soc. Am. Proc., 32: 35-40.
GIASUDDIN, A.S.M., CAYGILL, C.P.J., DIPLOCK, A.T., & JEFFERY,
E.H. (1975) The dependence on vitamin E and selenium of drug
demethylation in rat liver microsomal fractions. Biochem. J.,
146: 339-350.
GISSEL-NIELSEN, G. (1971) Selenium content of some
fertilizers and their influence on uptake of selenium in
plants. J. agric. food Chem., 19: 564-566.
GISSEL-NIELSEN, G. (1973) Ecological effects of selenium
application to field crops. Ambio, 2: 114-117.
GISSEL-NIELSEN, G. (1976) Selenium in soils and plants. In:
Proceedings of the Symposium on Se-Te in the Environment,
Pittsburgh, Pennsylvania, Industrial Health Foundation,
pp. 10-25.
GISSEL-NIELSEN, G. (1986) Selenium fertilizers and foliar
application, Danish experiments. Ann. clin. Res., 18: 61-64.
GISSEL-NIELSEN, M. & GISSEL-NIELSEN, G. (1975) Selenium in
soil-animal relationships. Pedobiologia, 15: 65-67.
GITSOVA, S. (1973) Presence of selenium in drinking waters
in Bulgaria. Khig. Zdraveopazvane, 16: 557-561.
GLOVER, J.R. (1954) Some medical problems concerning
selenium in industry. Trans. Assoc. Ind. Med. Off., 4: 94-96.
GLOVER, J.R. (1967) Selenium in human urine: a tentative
maximum allowable concentration for industrial and rural
populations. Ann. occup. Hyg., 10: 3-14.
GLOVER, J.R. (1970) Selenium and its industrial toxicology.
Ind. Med. Surg., 39: 50-54.
GLOVER, J.R. (1976) Environmental health aspects of selenium
and tellurium. In: Proceedings of the Symposium on
Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 279-292.
GODWIN, K.O. & FUSS, C.N. (1972) The entry of selenium into
rabbit protein following the administration of Na2 75Se03.
Aust. J. biol. Sci., 25: 865-871.
GOODWIN, W.J., LANE, H.W., BRADFORD, K., MARSHALL, M.V.,
GRIFFIN, A.C., GEOPFERT, H., & JESSE, R.H. (1983) Selenium
and glutathione peroxidase levels in patients with epidermoid
carcinoma of the oral cavity and orophasynx. Cancer, 51:
110-115.
GORTNER, R.A., Jr (1940) Chronic selenium poisoning of rats
as influenced by dietary protein. J. Nutr., 19: 105-112.
GOULDEN, P.D. & BROOKSBANK, P. (1974) Automated atomic
absorption determination of arsenic, antimony, and selenium in
natural waters. Anal. Chem., 46: 1431-1436.
GRACIANSKAJA, L.N. & KOVSHILO, V.E., ed. (1977) [Selenium
and its compounds]. In: [Handbook of occupational pathology,]
Moscow, Medicina Publishing House pp. 330-332 (in Russian).
GRANT, K.E., CONNER, M.W., & NEWBERNE, P.M. (1977) Effect of
dietary sodium selenite upon lesions induced by repeated small
doses of aflatoxin B1. Toxicol. appl. Pharmacol., 41: 166.
GREEN, J., BUNYAN, J., CAWTHORNE, M.A., & DIPLOCK, A.T.
(1969) Vitamin E and hepatotoxic agents. I. Carbon
tetrachloride and lipid peroxidation in the rat. Br. J. Nutr.,
23: 297-307.
GRIFFIN, A.C. & JACOBS, M.M. (1977) Effects of selenium on
azo dye hepatocarcinogenesis. Cancer Lett., 3: 177-181.
GRIFFITHS, N.M. (1973) Dietary intake and urinary excretion
of selenium in some New Zealand women. Proc. Univ. Otago Med.
School, 51: 8-9.
GRIFFITHS, N.M. & THOMSON, C.D. (1974) Selenium in the whole
blood of New Zealand residents. N.Z. med. J., 80: 199-202.
GRIFFITHS, N.M., STEWART, R.D.H., & ROBINSON, M.F. (1976)
The metabolism of 75Se-selenomethionine in four women. Br. J.
Nutr., 35: 373-382.
GRIMANIS, A.P., VASSILAKI-GRIMANI, M., ALEXIOU, D., &
PAPADATOS, C. (1978) Determination of seven trace elements in
human milk, powdered cow's milk, and infant foods by neutron
activation analysis. In: Nuclear activation techniques in the
life sciences, Vienna, International Atomic Energy Agency,
pp. 241-253.
GROCE, A.W., MILLER, E.R., KEAHEY, K.K., ULLREY, D.E., &
ELLIS, D. J. (1971) Selenium supplementation of practical
diets for growing-finishing swine. J. Ann. Sci., 32: 905-911.
GROSS, S. (1976) Hemolytic anemia in premature infants:
Relationship to vitamin E, selenium, glutathione peroxidase,
and erythrocyte lipids. Semin. Hematol., 13: 187-199.
GROSSMAN, A. & WENDEL, A. (1983) Non-reactivity of the
selenoenzyme glutathione peroxidase with enzymatically hydro-
peroxidized phospholipids. Eur. J. Biochem., 135(3): 549-552.
GU, B. (1983) Pathology of Keshan disease: a comprehensive
review. Chin. med. J., 96: 251-261.
GUSEJNOV, T.M., ZHAFAROV, A.I., PERELYGIN, V.V., & KARAEV,
M.A. (1974) [On the localization of endogenous selenium in
structural slements of bull's eye.] In: [Selenium in biology,]
Baku, Elm Publishing House, pp. 47-49.
GUTENMANN, W.H., BACHE, C.A., YOUNGS, W.D., & LISK, D.J.
(1976) Selenium in fly ash. Science, 191: 966-967.
HADDAD, P.R. & SMYTHE, L.E. (1974) A critical evaluation of
fluorometric methods for determination of selenium in plant
materials with 2,3-diaminoaphthalene. Talanta, 21: 859-865.
HADJIMARKOS, D.M. (1956) Geographic variations of dental
caries in Oregon. VII. Caries prevalence among children in the
blue mountains region. J. Pediatr., 48: 195-201.
HADJIMARKOS, D.M. (1960) Urinary selenium and dental caries.
Nature (Lond.), 188: 677.
HADJIMARKOS, D.M. & BONHORST, C.W. (1958) The trace element
selenium and its influence on dental caries susceptibility. J.
Pediatr., 52: 274-278.
HADJIMARKOS, D.M. & BONHORST, C.W. (1961) The selenium
content of eggs, milk, and water in relation to dental caries
in children. J. Pediatr., 59: 256-259.
HADJIMARKOS, D.M. & SHEARER, T.R. (1971) Selenium
concentration in human saliva. Am. J. clin. Nutr., 24: 1210.
HADJIMARKOS, D.M. & STORVICK, C.A. (1950) Geographic
variations of dental caries in Oregon. IV. Am. J. public
Health, 40: 1552-1555.
HADJIMARKOS, D.M., STORVICK, C.A., & REMMERT, L.F. (1952)
Selenium and dental caries. An investigation among school
children of Oregon. J. Pediatr., 40: 451-455.
HAFEMAN, D.G. & HOEKSTRA, W.G. (1977a) Protection against
carbon tetrachloride-induced lipid peroxidation in the rat by
dietary vitamin E, selenium, and methionine as measured by
ethane evolution. J. Nutr., 107: 656-665.
HAFEMAN, D.G. & HOEKSTRA, W.G. (1977b) Lipid peroxidation in
vivo during vitamin E and selenium deficiency in the rat as
monitored by ethane evolution. J. Nutr., 107: 666-672.
HAFEMAN, D.G., SUNDE, R.A., & HOEKSTRA, W.G. (1974) Effect
of dietary selenium on erythrocyte and liver glutathione
peroxidase in the rat. J. Nutr., 104: 580-587.
HAKKARAINEN, J., LINDBERG, P., BENGTSSON, G., JONSSON, L., &
LANNEK, N. (1978) Requirement for selenium (as selenite) and
vitamin E (as alpha-tocopherol) in weaned pigs. III. The effect on
the development of the VESD syndrome of varying selenium
levels in a low-tocopherol diet. J. anal. Sci., 46: 1001-1008.
HALL, R.H., LASKIN, S., FRANK, P., MAYNARD, E.A., & HODGE,
C.H. (1951) Arch. ind. Hyg. occup. Med., 4: 458-464.
HALTER, K. (1938) [Selenium poisoning, especially skin
changes accompanied by secondary porphysia.] Arch. Dermatol.,
178: 340 (in German).
HALVERSON, A.W. (1974) Growth and reproduction with rats fed
selenite-Se. Proc. S. Dak. Acad. Sci., 53: 167-177.
HALVERSON, A.W., HENDRICK, C.M., & OLSON, O.E. (1955) Observations
on the protective effect of linseed oil meal and some extracts
against chronic selenium poisoning in rats. J. Nutr., 56: 51-60.
HALVERSON, A.W., GUSS, P.L., & OLSON, O.E. (1962) Effect of
sulfur salts on selenium poisoning in the rat. J. Nutr., 77:
459-464.
HALVERSON, A.W., PALMER, I.S., & GUSS, P.L. (1966) Toxicity
of selenium to post-weanling rats. Toxicol. appl. Pharmacol.,
9: 477-484.
HAMDY, A.A. & GISSEL-NIELSEN, G. (1976) Volatilization of
selenium from soils. Z. Pflanzenernaehr. Bodenkd., 6: 671-678.
HAMILTON, A. (1927) Industrial poisons in the United States,
Macmillan Company, p. 111.
HAMILTON, A. (1934) Industrial toxicology, New York, Harpers
Publishing Company, p. 111.
HAMILTON, J.W. (1975) Chemical examination of seleniferous
cabbage Brassica oleracea capitata. J. agric. food Chem., 23:
1150-1152.
HARLAND, B.F., PROSKY, L., & VANDERVEEN, J.E. (1978)
Nutritional adequacy of current levels of Ca, Cu, Fe, I, Mg,
Mn, P, Se, and Zn in the American food supply for adults,
infants, and toddlers. In: Kirchgessner, M., ed. Trace element
metabolism in man and animals. 3, Freising- Weihenstephan,
West Germany, Arbeitskreis fur Tierernährungsforschung
Weihenstephan, pp. 311-315.
HARR, J.R. & MUTH, O.H. (1972) Selenium poisoning in
domestic animals and its relationship to man. Clin. Toxicol.,
5: 175-186.
HARR, J.R., BONE, J.F., TINSLEY, I.J., WESWIG, P.H., &
YAMAMOTO, R.S. (1967) Selenium toxicity in rats. II.
Histopathology. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI Publishing Co., Inc, pp. 153-178.
HARR, J.R., EXON, J.H., WHANGER, P.D., & WESWIG, P.H. (1972)
Effect of dietary selenium on N-2-fluorenyl-acetamide
(FAA)-induced cancer in vitamin E-supplemented, selenium
depleted rats. Clin. Toxicol., 5: 187-194.
HARRIS, P.L., LUDWIG, M.I., & SCHWARZ, K. (1958)
Ineffectiveness of Factor 3-active selenium compounds in
resorption-gestation bioassay for vitamin E. Proc. Soc. Exp.
Biol. Med., 97: 686-688.
HARTHOORN, A.M. & YOUNG, E. (1974) A relationship between
acid-base balance and capture myopathy in zebra (Equus
burchelli) and an apparent therapy. Vet. Res., 95: 337-342.
HARTLEY, W.J. (1963) Selenium and ewe fertility. Proc. N.Z.
Soc. Anim. Prod., 23: 20-27.
HARTLEY, W.J. (1967) Levels of selenium in animal tissues
and methods of selenium administration . In: Muth, O.H.,
Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 79-96.
HARTLEY, W.J. & GRANT, A.B. (1961) A review of selenium
responsive diseases of New Zealand livestock. Fed. Proc., 20:
679-688.
HARTLEY, W.J., GRANT, A.B., & DRAKE, C. (1960) Control of
white muscle disease and ill thrift with selenium. N.Z. J.
Agric., 101: 343-345.
HASHIMOTO, Y. & WINCHESTER, J.W. (1967) Selenium in the
atmosphere. Environ. Sci. Technol., 1: 338-340.
HEATH, R.L. (1969-70) Handbook of chemistry and physics,
50th ed., Cleveland, Ohio, The Chemical Rubber Publishing Co.
HE, G. (1979) On the etiology of Keshan disease: two
hypotheses. Chin. med. J., 92: 416-422.
HEINRICH, M., Jr & KELSEY, F.E. (1955) Studies on selenium
metabolism: the distribution of selenium in the tissues of the
mouse. J. Pharmacol. exp. Therap., 114: 28-32.
HELZLSOUER, K., JACOBS, R., & MORRIS, S. (1985) Acute
selenium intoxication in the United States. Fed. Proc., 44(5):
1670.
HICKEY, F. (1968) Selenium in human and animal nutrition.
N.Z. Agric., 18: 1-2.
HICKEY, F. (1977) Human medication with selenium. Including
brief comments on the comparative pathology of White Muscle
Disease in livestock and human muscular dystrophies. In:
Hamilton, N.Z., ed. Trace elements in human and animal health
in New Zealand, Hamilton, New Zealand, Waikato University
Press, pp. 92-99.
HIGGS, D.J., MORRIS, V.C., & LEVANDER, O.A. (1972) Effect of
cooking on selenium content of foods. J. agric. food Chem.,
20: 678-680.
HODDER, A.P.W. & WATKINSON, J.H. (1976) Low selenium levels
in tephra-derived soil - inherent or pedogenetic? N.Z. J.
Sci., 19: 397-400.
HOEKSTRA, W.G. (1975a) Biochemical function of selenium and
its relation to vitamin E. Fed. Proc., 34: 2083-2089.
HOEKSTRA, W.G. (1975b) Glutathione peroxidase activity of
animal tissues as an index of selenium status. In: Hemphill,
D.D., ed. Trace substances in environmental health. IX,
Columbia, Missouri, University of Missouri Press, pp. 331-337.
HOEKSTRA, W.G., HAFEMAN, O., OH, S.H., SUNDER, R.A., & GANTHER,
H.E. (1973) Effect of dietary selenium on liver and erythrocyte
glutathione peroxidase in the rat. Fed. Proc., 32: 885.
HOGGER, D. & BOHM, C. (1944) [On dermal injury produced by
selenite]. Dermatologica, 90: 217-223 (in German).
HOLMBERG, R.E., & FERM, V.H. (1969) Interrelationships of
selenium, cadmium, and arsenic in mammalian teratogenesis.
Arch. environ. Health, 18: 873-877.
HOLSTEIN, E. (1951) [The effects of occupational exposure to
selenium.] Zentrblt. Arbeitsmed. Arbeitschutz, 1: 102-104 (in
German).
HOLYNSKA, B. & MARKOWICZ, A. (1977) Application of energy
dispersive X-ray fluorescence for the determination of
selenium in blood and tissue. Radiochem. radioanal. Lett., 31:
165-170.
HOPKINS, L.L. & MAJAJ, A.S. (1967) Selenium in human
nutrition. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H.,
ed. Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 203-214.
HOVE, E.L. (1948) Interrelation between alpha-tocopherol and
protein metabolism. III. The protective effect of vitamin E
and certain nitrogenous compounds against CCl4 poisoning in
rats. Arch. Biochem., 17: 467-474.
HOWARD, J.H., III (1971) Control of geochemical behavior of
selenium in natural waters by adsorption on hydrous ferric
oxides. In: Hemphill, D.D., ed. Trace substances in
environmental health. V, Columbia, Missouri, University of
Missouri Press, pp. 485-495.
HOWE, M. (1974) Selenium in the blood of south Dakotans.
Arch. environ. Health, 34: 444-448.
HURT, H.D., CARY E.E., & VISEK, W.J. (1971) Growth,
reproduction, and tissue concentrations of selenium in the
selenium-depleted rat. J. Nutr., 101: 761-766.
IARC (1975) Some aziridines, N,-S-, and O-mustards and
selenium, Lyons, International Agency for Research on Cancer,
pp. 245-260 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, Vol. 9).
IHNAT, M. (1974) Collaborative study of a fluorometric
method for determining selenium in foods. J. Assoc. Off. Anal.
Chem., 57: 373-378.
IHNAT, M. (1976) Selenium in foods: evaluation of atomic
absorption spectrometric techniques involving hydrogen
selenide generation and carbon furnace atomization. J. Assoc.
Off. Anal. Chem., 59: 911-922.
IHNAT, M. & MILLER, H.J. (1977a) Analysis of foods for
arsenic and selenium by acid digestion, hydride evolution
atomic absorption spectrophotometry. J. Assoc. Off. Anal.
Chem., 60: 813-825.
IHNAT, M. & MILLER, H.J. (1977b) Acid digestion, hydride
evolution atomic absorption spectrophotometric method for
determining arsenic and selenium in foods: collaborative
study. I. J. Assoc. Off. Anal. Chem., 60: 1414-1433.
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J.
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IP, C. (1985a) Selenium inhibition of chemical
carcinogenesis. Fed. Proc., 44: 2573-2578.
IP, C. (1985b) Attenuation of the anticarcinogenic action of
selenium by vitamin E deficiency. Cancer Lett., 25: 325-331.
IP, C. & SINHA, D.K. (1981) Enhancement of mammary
tumorigenesis by dietary selenium deficiency in rats with a
high polyunsaturated fat intake. Cancer Res., 41: 31-34.
IRGOLIC, K.J. & KUDCHADKER, M.H. (1974) Organic chemistry of
selenium. In: Zingaro, R.A. & Cooper, W.C., ed. Selenium, New
York, Van Nostrand Reinhold Co., pp. 408-545.
IZRAELSON, Z.I., MOGILEVSKAJA, O.Ja., & SUVOROV, S.W. (1973)
[Selenium.] In: [Problems of occupational hygiene and
occupational pathology connected with the work with toxic
metals,] Moscow, Medicina Publishing House pp. 245-257 (in
Russian).
JACOBS, M.M. (1976) Selenium: a possible inhibitor of colon
and rectum cancer. II. Biochemical aspects. In: Proceedings of
the Symposium on Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 329-340.
JACOBS, M.M. (1977) Inhibitory effects of selenium on 1,2-
dimethylhydrazine and methylazoxymethanol colon carcinogenesis.
Cancer, 40: 2557-2564.
JACOBS, M.M. & FORST, C. (1981a) Toxicological effects of
sodium selenite in Sprague-Dawley rats. J. Toxicol. environ.
Health, 8: 575-585.
JACOBS, M.M. & FORST, C. (1981b) Toxicological effects of
sodium selenite in Swiss mice. J. Toxicol. environ. Health, 8:
587-598.
JACOBS, M.M., JANSSON, B., & GRIFFIN, A.C. (1977a) Inhibitory
effects of selenium on 1,2-dimethylhydrazine and methylazoxy-
methanol acetate induction of colon tumours. Cancer Lett., 2:
133-138.
JACOBS, M.M., MATNEY, T.S., & GRIFFIN, A.C. (1977b) Inhibitory
effects of selenium on the mutagenicity of 2-acetylamino-
fluorene (AAF) and AAF derivatives. Cancer Lett., 2: 319-322.
JACOBSSON, S.O., LIDMAN, S., & LINDBERG, P. (1970) Blood
selenium in a beef herd affected with muscular degeneration.
Acta vet. Scand., 11: 324-326.
JAFFE, W.G. (1973) Selenium in food plants and feeds.
Toxicology and nutrition. Qualitas Plantarum. Plant foods hum.
Nutr., 23: 191-204.
JAFFE, W.G. (1976) Effect of selenium intake in humans and
in rats. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 188-193.
JAFFE, W.G. & MONDRAGON, M.C. (1969) Adaptation of rats to
selenium intake. J. Nutr., 97: 431-436.
JAFFE, W.G. & MONDRAGON, C. (1975) Effects of ingestion of
organic selenium in adapted and non-adapted rats. Br. J.
Nutr., 33: 387-397.
JAFFE, W.G. & VELEZ, B.F. (1973) Selenium intake and
congenital malformations in humans. Arch. Latinoam. Nutr., 23:
514-516.
JAFFE, W.G., RUPHAEL, M.D., MONDRAGON, M.C., & CUEVAS, M.A.
(1972a) Clinical and biochemical studies on school children
from a seleniferous zone. Arch. Latinoam. Nutr., 22: 595-611.
JAFFE, W.G., MONDRAGON, M.C., LAYRISSE, M., & OJEDA, A.
(1972b) Toxicity symptoms in rats fed organic selenium. Arch.
Latinoam. Nutr., 22: 467-481.
JANSSON, B., JACOBS, M.M., & GRIFFIN, A.C. (1978)
Gastrointestinal cancer: epidemiology and experimental
studies. In: Schrauzer, G.N., ed. Inorganic and nutritional
aspects of cancer, New York, Plenum Press, pp. 305-322.
JENKINS, K.J. & HIDIROGLOU, M. (1971) Comparative uptake of
selenium by low cystine and high cystine proteins. Can. J.
Biochem., 49: 468-472.
JENSEN, L.S. & MCGINNIS, J. (1960) Influence of selenium,
antioxidants, and type of yeast on vitamin E deficiency in the
adult chicken. J. Nutr., 72: 23-28.
JENSEN, R., CLOSSON, W., & ROTHENBERG, R. (1984) Selenium
intoxication - New York. Mobid. Mortal. Weekly Rep., 33:
157-158.
JOHNSON, C.M. (1976) Selenium in the environment. Res. Rev.,
62: 101-130.
JONES, G.B. & GODWIN, K.O. (1962) Distribution of
radioactive selenium in mice. Nature (Lond.), 196: 1294-1296.
JUDSON, G.J. & OBST, J.M. (1975) Diagnosis and treatment of
selenium inadequacies in the grazing ruminant. In: Nicholas,
D.J.D. & Egan, A.R., ed. Trace elements in soil-plant-animal
systems, New York, Academic Press, Inc, pp. 385-405.
JULIUS, A.D., DAVIES, M.H., & BIRT, D.F. (1980) Relationship
between blood selenium and erythrocyte glutathione peroxidase
activity with excess dietary selenium in Syrian golden
hamsters. Fed. Proc., 39: 555.
KAMADA, T., SHIRAISHI, T., & YAMAMOTO, Y. (1978)
Differential determination of selenium (IV) and selenium (VI)
with sodium diethyldithiocarbamate, ammonium pyrrolidinedi-
thiocarbamate and dithizone by atomic-absorption spectrophoto-
metry with a carbon-tube atomizer. Talanta, 25: 15-19.
KAQUELER, J.C., MALOIGNE, E., & BONIFAY, P. (1977) Effects
of sodium selenate on caries incidence on the rat. J. dent.
Res., D 56 (Special Issue): D151.
KASIMOV, R.Ju., RUSTAMOVA, Sh.A., GUSEJNOV, T.M., & KIAZIMOV,
S.K. (1976) [Dynamics of selenium distribution in some organs
of young fish from selected valuable edible species.] In:
[Selenium in biology,] Baku, Elm Publishing House, Vol. 2, pp.
143-144 (in Russian).
KERDEL-VEGAS, F. (1966) The depilatory and cytotoxic action
of "Coco de Mono" (Lecythis ollaria) and its relationship to
chronic seleniosis. Econ. Bot., 20: 187-195.
KESHAN DISEASE RESEARCH GROUP (1979a) Observations on effect
of sodium selenite in prevention of Keshan disease. Chinese
med. J., 92: 471-476.
KESHAN DISEASE RESEARCH GROUP (1979b) Epidemiologic studies
on the etiologic relationship of selenium and Keshan disease.
Chinese med. J., 92: 477-482.
KETTERER, B., BEALE, D., & MEYER, D. (1982) The structure
and multiple functions of glutathione transferases. Biochem.
Soc. Trans., 10(2): 82-84.
KILNESS, A.W. (1973) Selenium and public health. S.D. J.
Med., 26: 17-19.
KILNESS, A.W. & HOCHBERG, F.H. (1977) Amyotrophic lateral
sclerosis in a high selenium environment. J. Am. Med. Assoc.,
237: 2843-2844.
KIMMERLE, G. (1960) [Comparative studies on the inhalation
toxicity of sulfur-selenium and tellurium hexafluoride.] Arch.
Toxikol., 18: 140-144 (in German).
KINNIGKEIT, G. (1962) [Studies on workers exposed to selenium
in a rectifier plant.] Z. Gesamte Hyg. Grenzgeb., 8: 350-362
(in German).
KLAYMAN, D.L. & GUNTHER, W.H.H. (1973) Organic selenium
compounds: their chemistry and biology, New York, John Wiley
and Sons, Inc, 1188 pp.
KLEIN, A.K. (1943) Report on selenium. J. Assoc. Offic.
Anal. Chem., 26: 346-352.
KLUG, H.L., PETERSEN, D.F., & MOXON, A.L. (1949) The
toxicity of selenium analogues of cystine and methionine.
Proc. S.D. Acad. Sci., 28: 117-120.
KLUG, H.L., MOXON, A.L., PETERSEN, D.F, & POTTER, V.R. (1950)
The in vivo inhibition of succinic dehydrogenase by selenium
and its release by arsenic. Arch. Biochem. Biophys., 28:
253-259.
KOEMAN, J.H., VAN DE VEN, W.S.M., DE GOEIJ, J.J.M., TJIOE,
P.S., & VAN HAAFTEN, J.L. (1975) Mercury and selenium in
marine mammals and birds. Sci. total Environ., 3: 279-287.
KOIVISTOINEN, P., ed. (1980) Mineral elements composition of
Finnish foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni,
Cr F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb, and ash. Acta
agric. Scand., Suppl. 2: 17.
KOIVISTOINEN, P. & HUTTUNEN, J.K. (1985) Selenium deficiency
in Finnish food and nutrition: research strategy and measures.
In: Mills, C.F., Bremner, I., & Chesters, J.K., ed., Trace
elements in man and animals-TEMA 5, Slough, Commonwealth
Agricultural Bureaux, pp. 925-928.
KOIVISTOINEN, P. & HUTTUNEN, J.K. (1986) Selenium in food
and nutrition in Finland. An overview on research and action.
Ann. clin. Res., 18: 13-17.
KOSTA, L., BYRNE, A.R., & ZELENKO, V. (1975) Correlation
between selenium and mercury in man following exposure to
inorganic mercury. Nature (Lond.), 254: 238-239.
KOVALSKIJ, V.V. (1974) [Geochemical ecology,] Moscow, Nauka
Publishing House, 299 pp (in Russian).
KOVALSKIJ, V.V. (1978) [Geochemical ecology: a basis for a
system of biogeochemical regional characterization.] In:
[Biochemical regional characterization: a method for studying
the ecological structure of the biosphere,] Moscow, Nauka
Publishing House, Vol. 15, pp. 3-21 (in Russian).
KOVALSKIJ, V.V. & ERMAKOV, V.V. (1975) [Problems of
experimental geochemical ecology of animals under the
conditions of selenium regions and some approaches to the
study of the biological role of selenium.] In: [Vitamins. VII.
Biochemistry of vitamin E and selenium,] Kiev, Nakove Dunka
Publishing House, pp. 80-87 (in Russian).
KRAUSKOPF, K.B. (1955) Sedimentary deposits of rare
elements. Econ. Geol. Fiftieth Anniversary, (Part I): 411-463.
KRONBORG, O.J. & STEINNES, E. (1975) Simultaneous determination
of arsenic and selenium in soil by neutron-activation analysis.
Analyst, 100: 835-837.
KU, P.K., ELY, W.T., GROCE, A.W., & ULLREY, D.E. (1972)
Natural dietary selenium alpha-tocopherol and effect on tissue
selenium. J. Ann. Sci., 34: 208-211.
KUBOTA, J., ALLAWAY, W.H., CARTER, D.L., CARY, E.E., & LAZAR,
V.A. (1967) Selenium in crops in the United States in
relation to selenium-responsive diseases of animals. J. agric.
food Chem., 15: 448-453.
KUBOTA, J., CARY, E.E., & GISSEL-NIELSEN, G. (1975) Selenium
in rainwater of the United States and Denmark. In: Hemphill,
D.D., ed. Trace substances in environmental health. IX,
Columbia, Missouri, University of Missouri Press, pp. 123-130.
KULIEVA, E.M., PERELIGIN, V.V., & DZHAFAROV, A.I. (1978)
[Isolated retina electroretinogram (ERG) under the conditions
of induced lipoperoxidation.] Dokl. Akad. Nauk. Azarbayidzh
SSR, 34(2): 85-89 (in Russian).
KURLAND, L.T. (1977) Amyotrophic lateral sclerosis and
selenium. J. Am. Med. Assoc., 238: 2365-2366.
LAKIN, H.W. & BYERS, H.G. (194l) Selenium occurrence in
certain soils in the United States, with a discussion of
related topics, sixth report, Washington DC, US Department of
Agriculture, 26 pp (USDA Technical Bulletin No. 783).
LAKIN, H.W. & DAVIDSON, D.F. (1967) The relation of the geo-
chemistry of selenium to its occurrence in soils. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 27-56.
LATHROP, K.A., JOHNSTON, R.E., BLAU, M., & ROTHSCHILD, E.O.
(1972) Radiation dose to humans from 75Se-L-seleno-
methionine. J. Nucl. Med., 13 (suppl. No. 6): 7-30.
LAUER D.J. (1947) Selenium poisoning. A review, with
emphasis on its industrial aspects, Pittsburgh, Pennsylvania,
University of Pittsburgh School of Medicine, pp. 1-47 (Thesis).
LAWRENCE, R.A. & BURK, R.F. (1976) Glutathione peroxidase
activity in selenium-deficient rat liver. Biochem. Biophys.
Res. Commun., 71: 952-958.
LAWRENCE, R.A. & BURK, R.F. (1978) Species, tissue, and
subcellular distribution of non Se-dependent glutathione
peroxidase activity. J. Nutr., 108: 211-215.
LAWRENCE, R.A., PARKHILL, L.K., & BURK, R.F. (1978) Hepatic
cytosolic non selenium-dependent glutathione peroxidase
activity: its nature and the effect of selenium deficiency. J.
Nutr., 108: 981-987.
LAWSON, T. & BIRT, D.F. (1983) Enhancement of the repair of
carcinogen-induced DNA damage in the hamster pancreas by
dietary selenium. Chem. Biol. Interact., 45: 95-104.
LAZAREV, N.V. (1977) [ Harmful compounds in the industry,]
7th ed., Leningrad, Chimija, Vol. 3, pp. 74-82 (in Russian).
LAZAREV, N.V. & GADASKINA, I.D., ed. (1977) [Selenium and
its compounds.] In: [Noxious substances in industry,] Lenin-
grad, Chimija Publishing House, pp. 75-82 (in Russian).
LEEB, J., BAUMGARTNER, W.A., LYONS, K., & LORBER, A. (1977)
Uptake, distribution, and excretion of sodium selenite in
rheumatoid subjects. In: Biological implications of metals in
the environment, Technical Information Center, Energy Research
and Development Administration, pp. 536-546.
LEMLEY, R.E. (1940) Selenium poisoning in the human. Lancet,
60: 528-531.
LEMLEY, R.E. (1943) Observations on selenium poisoning in
South and North America. Lancet, 63: 257-258.
LEMLEY, R.E. & MERRYMAN, M.P. (194l) Selenium poisoning in
the human. Lancet, 61: 435-438.
LEVANDER, O.A. (1976a) Selected aspects of the comparative
metabolism and biochemistry of selenium and sulfur. In:
Prasad, A.S., ed. Trace elements in human health and disease,
Vol. 2, Essential and toxic elements, New York, Academic
Press, pp. 135-163.
LEVANDER, O.A. (1976b) Selenium in foods. In: Proceedings of
the Symposium on Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 26-53.
LEVANDER, O.A. (1977) Metabolic interrelationships between
arsenic and selenium. Environ. Health Perspect., 19: 159-164.
LEVANDER, O.A. (1979) Lead toxicity and nutritional
deficiencies. Environ. Health Perspect., 29: 115-125.
LEVANDER, O.A. (1982) Selenium: biochemical actions, inter-
actions, and some human health implications. In: Clinical,
biochemical, and nutritional aspects of trace elements, pp.
345-368.
LEVANDER, O.A. (1983) Consideration in the design of
selenium bioavailability studies. Fed. Proc., 42: 1721-1725.
LEVANDER, O.A. (1986) Human and animal nutrition. In: Mertz,
M., ed. Trace elements, Vol. 2, New York, Academic Press, pp.
209-279.
LEVANDER, O.A. & BAUMANN, C.A. (1966) Selenium metabolism.
VI. Effect of arsenic on the excretion of selenium in the
bile. Toxicol. appl. Pharmacol., 9: 106-115.
LEVANDER, O.A. & MORRIS, V.C. (1970) Interactions of
methionine, vitamin E, and antioxidants in selenium toxicity
in the rat. J. Nutr., 100: 1111-1118.
LEVANDER, O.A. & MORRIS (1984) Dietary selenium levels
needed to maintain balance in North American adults consuming
self-selected diets. Am. J. clin. Nutr., 39: 809-815.
LEVANDER, O.A., YOUNG, M.L., & MEEKS, S.A. (1970) Studies on
the binding of selenium by liver homogenates from rats fed
diets containing either casein or casein plus linseed oil
meal. Toxicol. appl. Pharmacol., 16: 79-87.
LEVANDER, O.A., MORRIS, V.C., & HIGGS, D.J. (1973a)
Acceleration of thiol-induced swelling of rat liver
mitochondria by selenium. Biochemistry, 12: 4586-4590.
LEVANDER, O.A., MORRIS, V.C., & HIGGS, D.J. (1973b) Selenium
as a catalyst for the reduction of cytochrome c by glutathione.
Biochemistry, 12: 4591-4595.
LEVANDER, O.A., WELSH, S.O., & MORRIS, V.C. (1980) Erythrocyte
deformability as affected by vitamin E deficiency and lead
toxicity. Ann. N.Y. Acad. Sci., 355: 227.
LEVANDER, O.A., SUTHERLAND, B., MORRIS, V.C., & KING, J.C.
(1981a) Selenium metabolism in human nutrition. In: Spallhoz,
J.E., Martin, J.L., & Ganther, H.E., ed. Selenium in biology
and medicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 256-268.
LEVANDER, O.A., SUTHERLAND, B., MORRIS, V.C., & KING, J.C.
(1981b) Selenium balance young men during selenium depletion
and repletion. Am. J. clin. Nutr., 34: 2662-2669.
LEVANDER, O.A., ALFTHAN, G., ARVILOMMI, H., GREF, C.G.,
HUTTUNEN, J.K., KATAJA, M., KOIVISTOINEN, P., & PIKKARAINEN,
J. (1983) Bioavailability of selenium to Finnish men as
assessed by platelet glutathione peroxidase activity and other
blood parameters. Am. J. clin. Nutr., 37: 887-897.
LEVINE, R.J. & OLSON, R.E. (1970) Blood selenium in Thai
children with protein-calorie malnutrition. Proc. Soc. Exp.
Biol. Med., 134: 1030-1034.
LEWIS, B.G., JOHNSON, C.M., & BROYER, T.C. (1974) Volatile
selenium in higher plants: the production of dimethyl selenide
in cabbage leaves by enzymatic cleavage of Se-methyl seleno-
methionine selenonium salt. Plant Soil, 40: 107-118.
LI, C., HUANG, J., & LI, C. (in press) Observational report
on the effects of taking sodium selenite for six years
continuously as a preventive for Kashin-Beck disease as shown
in X-ray studies. In: Proceedings of the Third International
Symposium on Selenium in Biology and Medicine.
LIANG, S. (1985) The prophylactic and curing effect of
selenium (Se) in combatting of the Kaschin-Beck's disease.
LIEBSCHER, K. & SMITH, H. (1968) Essential and non-essential
trace elements. A method of determining whether an element is
essential or nonessential in human tissue. Arch. environ.
Health, 17: 881-890.
LINDBERG, P. (1968) Selenium determination in plant and
animal material, and in water. A methodological study. Acta
vet. Scand. (suppl. 23): 48 pp.
LINDBERG, P. & JACOBSSON, S.O. (1970) Relationship between
selenium content of forage, blood, and organs of sheep, and
lamb mortality rate. Acta vet. Scand., 11: 49-58.
LIPINSKIJ, S. (1962) [Background for evaluation of selenium
as an industrial toxicant.] Gig. i Sanit., 1: 91-93 (in
Russian).
LO, L.W., KOROPATNICK, J., & STICH, H.F. (1978) The
mutagenicity and cytotoxicity of selenite, "activated"
selenite, and selenate for normal and DNA repair-deficient
human fibroblasts. Mutat. Res., 49: 305-312.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LOMBECK, I., KASPEREK, K., FEINENDEGEN, L.E., & BREMER, H.J.
(1975) Serum-selenium concentrations in patients with
maple-syrup-urine disease and phenylketonuria under
dieto-therapy. Clin. Chim. Acta, 64: 57-61.
LOMBECK, I., KASPEREK, K., HARBISCH, H.D., BECKER, K.,
SCHUMANN, E., SCHROTER, W., FEINENDEGEN, L.E., & BREMER, H.J.
(1978) The selenium state of children. II. Selenium content
of serum, whole blood, hair and the activity of erythrocyte
glutathione peroxidase in dietetically treated patients with
phenylketonuria and maple-syrup-urine disease. Eur. J.
Pediatr., 128: 213-223.
LUO, X., WEI, H., YANG, C., XING, J., LIU, X., QIAO, C., FENG,
Y., LIU, J., LIU, Y., WU, Q., LIU, X., GUO, J., STOECKER,
B.J., SPALLHOLTZ, J.E., & YANG, S.P. (1985) Bioavailability
of selenium to residents in a low-selenium area of China. Am.
J. clin. Nutr., 42: 439-448.
MAAG, D.D. & GLENN, M.W. (1967) Toxicity of selenium: farm
animals. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H., ed.
Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 127-140.
MAAG, D.D., ORSBORN, J.S., & CLOPTON, J.R. (1960) The effect
of sodium selenite on cattle. Am. J. vet. Res., 21: 1049-l053.
MCCABE, L.J., SYMONS, J.M., LEE, R.D., & ROBECK, G.G. (1970)
Survey of community water supply systems. J. Am. Water Works
Assoc., 62: 670-687.
MCCONNELL, K.P. & CHO, G.J. (1965) Transmucosal movement of
selenium. Am. J. Physiol., 208: 1191-1195.
MCCONNELL, K.P. & PORTMAN, O.W. (1952a). Excretion of
dimethyl selenide by the rat. J. Biol. Chem., 195: 277-282.
MCCONNELL, K.P. & PORTMAN, O.W. (1952b) Toxicity of dimethyl
selenide in the rat and mouse. Proc. Soc. Exp. Biol. Med., 79:
230-231.
MCCONNELL, K.P., BROGHAMER, W.L., Jr, BLOTCKY, A.J., & HURT,
O.J. (1975) Selenium levels in human blood and tissues in
health and in disease. J. Nutr., 105: 1026-1031.
MCCONNELL, K.P., JAGER, R.M., BLAND, K.I., & BLOCTCKY, A.J.
(1980) The relationship of dietary selenium and breast
cancer. J. surg. Oncol., 15: 67-70.
MCCOY, K.E.M. & WESWIG, P.H. (1969) Some selenium responses
in the rat not related to vitamin E. J. Nutr., 98: 383-389.
MCDANIEL, M., SHENDRIKAR, A.D., REISZNER, K.D., & WEST, P.W.
(1976) Concentration and determination of selenium from
environmental samples. Anal. Chem., 48: 2240-2243.
MCDOWELL, L.R., FROSETH, J.A., & PIPER, R.C. (1978)
Influence of arsenic, sulfur, cadmium, tellurium, silver, and
selenium on the selenium-vitamin E deficiency in the pig.
Nutr. Rep. Int., 17: 19-33.
MCKEEHAN, W.L., HAMILTON, W.G., & HAM, R.G. (1976) Selenium
is an essential trace nutrient for growth of WI-38 diploid
human fibroblasts. Proc. Natl Acad. Sci. (USA), 73: 2023-2027.
MCKENZIE, J.M. (1977) Trace elements in total parenteral
nutrition in New Zealand. In: Trace elements in human and
animal health in New Zealand, Hamilton, New Zealand, Waikato
University Press, pp. 59-69.
MCKENZIE, R.L., REA, H.M., THOMSON, C.D., & ROBINSON, M.F.
(1978) Selenium concentration and glutathione peroxidase
activity in blood of New Zealand infants and children. Am. J.
clin. Nutr., 3l: l4l3-l4l8.
MACKINNON, A.M. & SIMON, F.R. (1976) Impaired hepatic heme
synthesis in the phenobarbital-stimulated selenium-deficient
rat. Proc. Soc. Exp. Biol. Med., 152: 568-572.
MCLEAN, A.E.M. (1967) The effect of diet and vitamin E on
liver injury due to carbon tetrachloride. Br. J. exp. Path.,
48: 632-636.
MAINES, M.D. & KAPPAS, A. (1976) Selenium regulation of
hepatic heme metabolism: induction of sigma-aminolevulinate
synthase and heme oxygenase. Proc. Natl Acad. Sci.(USA), 73:
4428-443l.
MAJSTRUK, P.N. & SUCHKOV, B.P. (1978) [Sanitary hygienic
control of selenium levels in the main food commodities and
the daily intake of selenium,] Kiev, Kievskij, NII gig. Pit.,
4 pp (in Russian).
MARSHALL, M.V., JACOBS, M.M., & GRIFFIN, A.C. (1978)
Reduction in acetylaminofluorene (AAF) hepatocarcinogenesis by
selenium. Proc. Am. Assoc. Cancer Res., l9: 75.
MARTIN, J.L. & GERLACH, M.L. (1969) Separate elution by
ion-exchange chromatography of some biologically important
selenoamino acids. Anal. Biochem., 29: 257-264.
MARTIN, J.L. & HURLBUT, J.A. (1976) Tissue selenium levels
and growth responses of mice fed selenomethionine,
Se-methylselenocysteine, or sodium selenite. Phosphorus
Sulfur, 1: 295-300.
MARTIN, J.L. & SPALLHOLZ, J.S. (1976) Selenium in the immune
response. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburg, Pennsylvania,
Industrial Health Foundation, pp. 204-225.
MARTIN, S.E., ADAMS, G.H., SCHILLACI, M., & MILNER, J.A.
(1981) Antimutagenic effect of selenium on acridine orange
and 7,12-dimethylbenz(alpha)anthracene in the Ames Salmonella
microsomal system. Mutat. Res., 82: 41-46.
MASIRONI, R. & PARR, R. (1976) Selenium and cardiovascular
diseases: preliminary results of the WHO/IAEA joint research
programme. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 3l6-325.
MAXIA, V., MELONI, S., ROLLIER, M.A., BRANDONE, A.,
PATWARDHAN, V.N., WASLIEN, C.I., & SHAMI, S.E. (1972)
Selenium and chromium assay in Egyptian foods and in blood of
Egyptian children by activation analysis. In: Nuclear
activation techniques in the life sciences, Vienna,
International Atomic Energy Agency, pp. 527-550.
MEDINA, D. & SHEPHERD, F. (1980) Selenium-mediated
inhibition of mouse mammary tumorigenesis. Cancer Lett., 8:
241-245.
MEDINSKY, M.A., CUDDIHY, R.G., GRIFFITH, W.C., & MCCLELLAN,
R.O. (1981) A simulation model describing the metabolism of
inhaled and ingested selenium compounds. Toxicol. appl.
Pharmacol., 59: 54-63.
MEHLERT, A. & DIPLOCK, A.T. (1985) The glutathione-S-
transferases in selenium and vitamin E deficiency. Biochem.
J., 227: 823-831.
MICHIE, N.D., DIXON, E.J., & BUNTON, N.G. (1978) Critical
review of AOAC fluorometric method for determining selenium in
foods. J. Assoc. Off. Anal. Chem., 61: 48-51.
MIETTINEN, T.A., ALFTHAN, G., HUTTUNEN, J.K., PIKKARAINEN, J.,
NAUKKARINEN, V., MATTILA, S., & KUMLIN, T. (1983) Serum
selenium concentration related to myocardial infarction and
fatty acid content of serum lipids. Brit. med. J., 287:
517-519.
MILKS, M.M., WILT, S.R., ALI, I.I., & COURI, D. (1985) The
effects of selenium on the emergence of aflatoxin B1-induced
enzyme-altered foci in rat liver. Fundam. appl. Toxicol., 5:
320-326.
MILLAR, K.R. & SHEPPARD, A.D. (1972) alpha-Tocopherol and
selenium levels in human and cow's milk. N.Z. J. Sci., 15:
3-15.
MILLAR, K.R., GARDINER, M.A., & SHEPPARD, A.D. (1973) A
comparison of the metabolism of intravenously injected sodium
selenite, sodium selenate, and selenomethionine in rats. N.Z.
J. agric. Res., 16: 115-127.
MILLER, D., SOARES, J.H., Jr, BAUERSFELD, P., Jr, & CUPPETT,
S.L. (1972) Comparative selenium retention by chicks fed
sodium selenite, selenomethionine, fish meal, and fish
solubles. Poultry Sci., 51: 1669-1673.
MILNER, J.A. (1985) Effect of selenium on virally induced
and transplantable tumour models. Fed. Proc., 44: 2568-2572.
MONAENKOVA, A.M. & GLOTOVA, K.V. (1963) [Selenium
intoxication.] Gig. i Sanit., 6: 41-44 (in Russian).
MONDRAGON, M.C. & JAFFE, W.G. (1976) Ingestion of selenium
in Caracas, compared with some other cities. Arch. Latinoam.
Nutr., 26: 34l-352.
MONEY, D.F.L. (1970) Vitamin E and selenium deficiencies and
their possible aetiological role in the sudden death in
infants syndrome. N.Z. med. J., 7l: 32-34.
MONEY, D.F.L. (1978) Vitamin E, selenium, iron and vitamin A
content of livers from sudden infant death syndrome and
control children: interrelationships and possible
significance. N.Z. J. Sci Teel., 21: 41-55.
MOORE, J.A., NOIVA, R., & WELLS, I.C. (1984) Selenium
concentrations in plasma of patients with arteriographically
defined coronary atherosclerosis. Clin. Chem., 30(7):
1171-1173.
MORIYA, M., OHTA, T., WATANABE, K., WATANABE, Y., SUGIYAMA,
F., MIYAZAWA, T., & SHIRASU, Y. (1979) Inhibitors for the
mutagenicities of colon carcinogens, 1,2-dimethylhydrazine and
azoxymethane, in the host-mediated assay. Cancer Lett., 7:
325-330.
MORRIS, V.C. & LEVANDER, O.A. (1970) Selenium content of
foods. J. Nutr., 100: 1383-1388.
MOTSENBOCKER, M.A. & TAPPEL, A.L. (1982) A
selenocysteine-containing selenium-transport protein in rat
plasma. Biochim. Biophys. Acta, 719: 147-153.
MOXON, A.L. (1938) The effect of arsenic on the toxicity of
seleniferous grains. Science, 88: 81.
MOXON, A.L. (1940) Toxicity of selenium-cystine and some
other organic selenium compounds. J. Am. Pharm. Assoc. Sci.
Ed., 29: 249-250.
MOXON, A.L. (1976) Natural occurrence of selenium. In:
Proceedings of the Symposium on Selenium-Tellurium in the
Environment, Pittsburgh, Pennsylvania, Industrial Health
Foundation, pp. 1-9.
MOXON, A.L. & RHIAN, M. (1943) Selenium poisoning. Physiol.
Rev., 23: 305-337.
MOXON, A.L., ANDERSON, H.D., & PAINTER, E.P. (1938) The
toxicity of some organic selenium compounds. J. Pharmacol.
exp. Ther., 63: 357-368.
MOXON, A.L., OLSON, O.E., & SEARIGHT, W.V. (1939) Selenium
in rocks, soils, and plants. S. Dak. Agric. Exp. Sta. Tech.
Bull., 2: 94 pp.
MOXON, A.L., SCHAEFER, A.E., LARDY, H.A., DUBOIS, K.P., &
OLSON, O.E. (1940) Increasing the rate of excretion of
selenium from selenized animals by the administration of
p-bromobenzene. J. biol. Chem., l32: 785-786.
MOXON, A.L., OLSON, O.E., & SEARIGHT, W.V. (1950) Selenium
in rocks, soils, and plants. S. Dak. Agric. Exp. Sta. Tech.
Bull., 2.
MUHLER, J.C. & SHAFER, W.G. (1957) The effect of selenium on
the incidence of dental caries in rats. J. dent. Res., 36:
895-896.
MUNSELL, H.E., DEVANEY, G.M., & KENNEDY, M.H. (1936)
Toxicity of food containing selenium as shown by its effect on
the rat, Washington DC, US Department of Agriculture, 25 pp
(US Department of Agriculture Technical Bulletin No. 534).
MUTANEN, M. & KOIVISTOINEN, P. (1983) The role of imported
grain on the selenium intake of Finnish population 1941-1981.
Int. J. Vit. Nutr. Res., 53: 34-38.
MUTH, O.H. (1960) Carbon tetrachloride poisoning of ewes on
a low selenium ration. Am. J. vet. Res., 21: 86-87.
MUTH, O.H., ed. (1966) Selenium in biomedicine. In: Proceedings
of the First International Symposium at Oregon State University,
Westport, Connecticut, AVI Publishing Co., 445 pp.
MUTH, O.H., WESWIG, P.H., WHANGER, P.D., & OLDFIELD, J.E.
(197l) Effect of feeding selenium-deficient ration to the
subhuman primate (Saimiri sciureus). Am. J. vet. Res., 32:
l603-l605.
NAHAPETIAN, A.T., JANGHORBANI, M., & YOUNG, V.R. (1983)
Urinary trimethylselenonium excretion by the rat: effect of
level and source of selenium-75. J. Nutr., 113: 401-411.
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., KURATA, H., TONOMURA,
M., & TONOMURA, A. (1976) Studies on selenium-related
compounds. V. Cytogenetic effect and reactivity with DNA.
Mutat. Res., 40: 177-184.
NATIONAL CANCER INSTITUTE (1980) Bioassay of selenium
sulfide for possible carcinogenicity (gavage study), Bethesda,
Maryland, US Department of Health and Human Services, Public
Health Service, National Institutes of Health, 132 pp (DHHS
Publication No. (NIH) 80-1750).
NAVIA, J.M., MENAKER, L., SELTZER, J., & HARRIS, R.S. (1968)
Effect of Na2Se03 supplemented in the diet or the water on
dental caries of rats. Fed. Proc., 27: 676.
NAZARENKO, I.I. & ERMAKOV, A.N. (1971) Analytical chemistry
of selenium and tellurium, New York, Halsted Press.
NAZARENKO, I.I. & KISLOVA, I.V. (1977) [A highly sensitive
method for the analysis of migratory forms of selenium in
natural waters.] El. Viems. Labor. Technol. issled. obogashch.
min. syrja, 6: 1-8 (in Russian).
NAZARENKO, I.I., KISLOVA, I.V., GUSEJNOV, T.M., MKRTCHJAN,
M.A., & KISLOV, A.M. (1975) [Fluorometric determination of
selenium in biological material by 2,3-diaminonaphthalene].
Zh. Anal. Chim., 30(4): 733-737 (in Russian).
NELSON, A.A., FITZHUGH, O.G., & CALVERY, H.O. (1943) Liver
tumors following cirrhosis caused by selenium in rats. Cancer
Res., 3: 230-236.
NEWBERNE, P.M. & CONNER, M.W. (1974) Effect of selenium on
acute response to aflatoxin B1. In: Hemphill, D.D., ed. Trace
substances in environmental health. VIII, Columbia, Missouri,
University of Missouri Press, pp. 323-328.
NOCKELS, C.F. (1979) Protective effects of supplemental
vitamin E against infection. Fed. Proc., 38: 2134-2138.
NODA, M., TAKANO, T., & SAKURAI, H. (1979) Mutagenic
activity of selenium compounds. Mutat. Res., 66: 175-179.
NOGUCHI, T., CANTOR, A.H., & SCOTT, M.L. (1973) Mode of
action of selenium and vitamin E in prevention of exudative
drathesis in chicks. J. Nutr., 103(10): 1502-1511.
NORDMAN, E. (1974) Sodium selenite (selenium-75)
scintigraphy in diagnosis of tumours. Acta radiol.,
340(suppl.): 78 pp.
NORPPA, H., ET AL. (1980) Chromosomal effects of sodium
selenite in vivo. Hereditas, 93: 93-105.
NORRIS, F.H. & SANG, K. (1978) Amyotrophic lateral sclerosis
and low urinary selenium levels. J. Am. Med. Assoc., 239: 404.
OBERMEYER, B.D., PALMER, I.S., OLSON, O.E., & HALVERSON, A.W.
(1971) Toxicity of trimethylselenonium chloride in the rat
with and without arsenite. Toxicol. appl. Pharmacol., 20:
135-146.
OCHOA-SOLANO, A. & GITLER, C. (1968) Incorporation of
75Se-selenomethionine and 35S-methionine into chicken egg
white proteins. J. Nutr., 94: 243-248.
OELSCHLAGER, W. & MENKE, K.H. (1969) Concerning the selenium
content of plant, animal, and other materials. II. The
selenium and sulfur content of foods. Z. Ernahrungswiss., 9:
216-222.
OH, S.H., SUNDE, R.A., POPE, A.L., & HOEKSTRA, W.G. (1976a)
Glutathione peroxidase response to selenium intake in lambs
fed a Torula yeast-based, artificial milk. J. anal. Sci., 42:
977-983.
OH, S.H., POPE, A.L., & HOEKSTRA, W.G. (1976b) Dietary
selenium requirement of sheep fed a practical-type diet as
assessed by tissue glutathione peroxidase and other criteria.
J. anal. Sci., 42: 984-992.
OHLENDORF, H.M., HOFFMAN, D.J., SAIKI, M.K., & ALDRICH, T.W.
(1986) Embryonic mortality and abnormalities of aquatic
birds: apparent impact of selenium from irrigation drain
water. Sci. total Environ., 52: 49-63.
OLSON, O.E. (1967) Soil, plant, animal cycling of excessive
levels of selenium. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI Publishing Co., Inc, pp. 297-3l2.
OLSON, O.E. (1969) Selenium as a toxic factor in animal
nutrition. In: Proceedings of the Georgia Nutrition
Conference, Atlanta, Georgia, pp. 68-78.
OLSON, O.E. (1970) Selenium in feedstuffs: deficiencies and
excesses. Proceedings of the 31st Minnesota Nutrition
Conference, Minneapolis, University of Minnesota, pp. 7-l3.
OLSON, O.E. (1976) Methods of analysis for selenium. A
review. In: Proceedings of the Symposium on Selenium-Tellurium
in the Environment, Pittsburgh, Pennsylvania, Industrial
Health Foundation, pp. 67-84.
OLSON, O.E. (1978) Selenium in plants as a cause of
livestock poisoning. In: Keeler, R.F., Van Kampen, K.R., &
James, L.F., ed. Effects of poisonous plants on livestock, New
York, Academic Press, pp. 121-133.
OLSON, O.E. & FROST, D.V. (1970) Selenium in papers and
tabaccos. Environ. Sci. Technol., 4: 686-687.
OLSON, O.E. & PALMER, I.S. (1976) Selenoamino acids in
tissues of rats administered inorganic selenium. Metabolism,
25: 299-306.
OLSON, O.E., WHITEHEAD, E.I., & MOXON, A.L. (1942)
Occurrence of soluble selenium in soils and its availability
to plants. Soil Sci., 54: 47-53.
OLSON, O.E., SCHULTE, B.M., WHITEHEAD, E.I., & HALVERSON,
A.W. (1963) Effect of arsenic on selenium metabolism in
rats. J. agric. food Chem., 11: 531-534.
OLSON, O.E., NOVACEK, E.J., WHITEHEAD, E.I., & PALMER, I.S.
(1970) Investigations on selenium in wheat. Phytochemistry,
9: 1181-1188.
OLSON, O.E., PALMER, I.S., & WHITEHEAD, E.I. (1973)
Determination of selenium in biological materials. In: Glick,
D., ed. Methods of biochemical analysis, New York, John Wiley
and Sons, Inc, Vol. 21, pp. 39-78.
OLSON, O.E., PALMER, I.S., & CARY, E.E. (1975) Modification
of the official fluorometric method for selenium in plants. J.
Assoc. Off. Anal. Chem., 58: 117-121.
OLSON, O.E., CARY, E.E., & ALLAWAY, W.H. (1976) Fixation and
volatilization by soils of selenium from trimethylselenonium.
Agron. J., 68: 839-843.
OLSON, O.E., PALMER, I.S., & HOWE, M. (1978) Selenium in
foods consumed by South Dakotans. Proc. S.D. Acad. Sci., 57:
113-121.
OLSSON, U., ONFELT, A., & BEIJE, B. (1984) Dietary selenium
deficiency causes decreased N-oxygenation of N'N-dimethyl-
aniline and increased mutagenicity of dimethylnitrosamine in
the isolated rat liver/cell cultrue system. Mutat. Res., 126:
73-80.
OMAYE, S.T., REDDY, K.A., & CROSS, C.E. (1978) Enhanced lung
toxicity of paraquat in selenium-deficient rats. Toxicol.
appl. Pharmacol., 43: 237-247.
OSTADALOVA, I., BABICKY, A., & OBENBERGER, J. (1979)
Cataractogenic and lethal effect of selenite in rats during
postnatal ontogenesis. Physiol. Bohemoslov., 28: 393-397.
OVERVAD, K., THORLING, E.B., BJERRING, P., & EBBESEN, P.
(1985) Selenium inhibits UV-light-induced skin carcinogenesis
in hairless mice. Cancer Lett., 27: 163-170.
PAINTER, E.P. (1941) The chemistry and toxicity of selenium
compounds with special reference to the selenium problem.
Chem. Rev., 28: 179-213.
PALMER, I.S. & OLSON, O.E. (1974) Relative toxicities of
selenite and selenate in the drinking-water of rats. J. Nutr.,
104(3): 306-314.
PALMER, I.S. & OLSON, O.E. (1979) Partial prevention by
cyanide of selenium poisoning in rats. Biochem. Biophys. Res.
Commun., 90: 1379-1386.
PALMER, I.S., FISCHER, D.D., HALVERSON, A.W., & OLSON, O.E.
(1969) Identification of a major selenium excretory product
in rat urine. Biochim. Biophys. Acta, 177: 336-342.
PALMER, I.S., GUNSALUS, R.P., HALVERSON, A.W., & OLSON, O.E.
(1970) Trimethylselenonium ion as a general excretory product
from selenium metabolism in the rat. Biochim. Biophys. Acta,
208: 260-266.
PALMER, I.S., ARNOLD, R.L., & CARLSON, C.W. (1973) Toxicity
of various selenium derivatives to chick embryos. Poultry
Sci., 52: 1841-l846.
PALMER, I.S., OLSON, O.E., HALVERSON, A.W., MILLER, R., &
SMITH, C. (1980) Isolation of factors in linseed oil meal
protective against chronic selenosis in rats. J. Nutr., 110:
145-150.
PARIZEK, J. & OSTADALOVA, I. (1967) The protective effect of
small amounts of selenite in sublimate intoxication. Experientia
(Basel), 23: 142-143.
PARIZEK, J., OSTADALOVA, I., KALOUSKOVA, J., BABICKY, A., &
BENES, J. (1971) The detoxifying effects of selenium.
Interrelations between compounds of selenium and certain
metals. In: Mertz, W. & Cornatzer, W.E., ed. Newer trace
elements in nutrition, New York, Marcel Dekker, Inc,
pp. 85-122.
PARIZEK, J., ET AL. (1974) Some hormonal and environmental
factors influencing selenium metabolism and action. In:
Proceedings of the Ninth International Congress in Nutrition,
Mexico, 1972 (Coop. Nutr. Cong., 16: 94-97).
PARIZEK, J., KALOUSKOVA, J., KORUNOVA, V., BENES, J., &
PAVLIK, L. (1976) The protective effect of pretreatment with
selenite on the toxicity of dimethylselenide. Physiol.
Bohemoslov., 25: 573-576.
PARIZEK, J., KALOUSKOVA, J., BENES, J., & PAVLIK, L. (1980)
Interactions of selenium-mercury and selenium-selenium
compounds. Ann. N.Y. Acad. Sci., 355: 347-360.
PASCOE, G.A. & CORREIA, M.A. (1985) Structural and
functional assembly of rat intestinal cytochrome P-450
isozymes: effects of dietary iron and selenium. Biochem.
Pharmacol., 34: 599-608.
PASCOE, G.A., SAKAI-WONG, J., SOLIVEN, E., & CORREIA, M.A.
(1983) Regulation of intestinal cytochrome P-450 and Heme by
dietary nutrients. Critical role of selenium. Biochem.
Pharmacol., 32(20): 3027-3035.
PAULSEN, P.J. (1977) Determination of cadmium, copper, iron,
lead, mercury, molybdenum, nickel, selenium, silver,
tellurium, thallium, and zinc. Natl Bur. Stand. Special Publ.
(USA), 492: 33-48.
PAVLIK, L., KALOUSKOVA, J., VOBECKY, M., DEDINA, BENES, J., &
PARIZEK, J. (1979) Selenium levels in the kidneys of male
and female rats. In: Nuclear activation techniques in the life
sciences, 1978, Vienna, International Atomic Energy Agency,
pp. 213-223.
PELEKIS, E.E., PELEKIS, L.L., & TAURE, I. JA. (1975)
[Instrumental neutron-activation method of selenium determination
in biological materials.] Vitaminy, 8: 146-150 (in Russian).
PENCE, B.C. & BUDDINGH, F. (1985) Effect of selenium
deficiency on incidence and size of 1,2-dimethylhydrazine-
induced colon cancer in rats. J. Nutr., 115: 1196-1202.
PENNINGTON, J.A.T., WILSON, D.B., NEWELL, R.F., HARLAND, B.F.,
JOHNSON, R.D., & VANDERVEEN, J.E. (1984) Selected minerals
in foods surveys, 1974 to 1981/82. J. Am. Diet. Assoc., 84:
771-780.
PERONA, G., GUIDI, G.C., PIGA, A., CELLERINO, R., MENNA, R., &
ZATTI, M. (1978) In vivo and in vitro variations of human
erythrocyte glutathione peroxidase activity as result of cells
ageing, selenium availability and peroxide activation. Br. J.
Haematol., 39: 399-408.
PERRY, H.M., Jr, ERLANGER, M.W., & PERRY, E.F. (1976)
Limiting conditions for the induction of hypertension in rats
by cadmium. In: Hemphill, D.D., ed. Trace substances in
environmental health. X, Columbia, Missouri, University of
Missouri Press, pp. 459-467.
PIERCE, F.D. & BROWN, H.R. (1977) Comparison of inorganic
interferences in atomic absorption spectrometric determination
of arsenic and selenium. Anal. Chem., 49: 1417-1422.
PIERCE, S. & TAPPEL, A.L. (1977) Effects of selenite and
selenomethionine on glutathione peroxidase in the rat. J.
Nutr., 107: 475-479.
PILLAY, K.K.S., THOMAS, C.C., Jr, & SONDEL, J.A. (1971)
Activation analysis of airborne selenium as a possible
indicator of atmospheric sulfur pollutants. Environ. Sci.
Technol., 5: 74-77.
PLAA, G.L. & WITSCHI, H. (1976) Chemicals, drugs, and lipid
peroxidation. Ann. Rev. Pharmacol. Toxicol., 16: 125-141.
PLEBAN, P.A., MUNYANI, A., & BEACHUM, J. (1982)
Determination of selenium concentration and glutathine
peroxidase activity in plasma and erythrocytes. Clin. Chem.,
28: 311-316.
PLETNIKOVA, I.P. (1970) Biological effect and safe
concentration of selenium in drinking-water. Hyg. Sanit., 35:
176-181.
POLEY, W.E. & MOXON, A.L. (1938) Tolerance levels of
seleniferous grains in laying rations. Poultry Sci., 17: 72-76.
POOLE, C.F., EVANS, N.J., & WIBBERLEY, D.G. (1977)
Determination of selenium in biological samples by gas-liquid
chromatography with electron-capture detection. J.
Chromatogr., 136: 73-83.
PRATLEY, J.E. & MCFARLANE, J.D. (1974) The effect of
sulphate on the selenium content of pasture plants. Aust. J.
exp. Agric. Anim. Husb., 14: 533-538.
PRINGLE, P. (1942) Occupational dermatitis following
exposure to inorganic selenium compounds. Br. J. Dermatol.
Syphil., 54: 54-58.
PROHASKA, J.R. & GANTHER, H.E. (1976) Selenium and
glutathione peroxidase in developing rat brain. J. Neurochem.,
27: 1379-1387.
PROHASKA, J.R. & GANTHER, H.E. (1977) Glutathione peroxidase
activity of glutathione-S-transferases purified from rat
liver. Biochem. Biophys. Res. Commun., 76: 437-445.
PYEN, G. & FISHMAN, M. (1978) Automated determination of
selenium in water. At. Absorpt. Newslett., 17: 47-48.
RANDOLPH, W.F. (1978) Food additives permitted in feed and
drinking water of animals. Selenium. Fed. Reg., 43:
11700-11701.
RANSONE, J.W., SCOTT, N.M., Jr, & KNOBLOCK, E.C. (1961)
Selenium sulfide intoxication. New Engl. J. Med., 264: 384-385.
RAVIKOVITCH, S. & MARGOLIN, M. (1957) Selenium in soils and
plants. Ktavim. Rec. agric. Res. Sta., 7: 41-52.
RAY, J.H. (1984) Sister-chromatid exchange induction by
sodium selenite: reduced glutathione converts Na2SeO3 to its
SCE-inducing form. Mutat. Res., 141: 49-53.
RAY, J.H. & ALTENBURG, L.C. (1978) Sister-chromatid exchange
induction by sodium selenite: dependence on the presence of
red blood cells or red blood cell lysate. Mutat. Res., 54:
343-354.
RAY, J.H. & ALTENBURG, L.C. (1980) Dependence of the
sister-chromatid exchange-inducing abilities of inorganic
selenium compounds on the valence state of selenium. Mutat.
Res., 78: 261-266.
RAY, J.H., ALTENBURG, L.C., & JACOBS, M.M. (1978) Effect of
sodium selenite and methyl methanesulfonate or N-hydroxy-2-
acetylamino-fluorene co-exposure on sister-chromatid exchange
production in human whole blood cultures. Mutat. Res., 57:
359-368.
REA, H.M., THOMSON, C.D., CAMPBELL, D.R., & ROBINSON, M.F.
(1979) Relation between erythrocyte selenium concentrations
and glutathione peroxidase (EC 1.11.1.9) activities of New
Zealand residents and visitors to New Zealand. Br. J. Nutr.,
42: 201-208.
REAMER, D.C. & VEILLON, C. (1983a) Letter to the editor.
Anal. Chem., in press.
REAMER, D.C. & VEILLON, C. (1983b) A double isotape dilution
method for using stable selenium isotapes in metabolic tracer
studies: analysis by gas chromatography/mass spectrometry
(GC/MS). J. Nutr., 113: 786-792.
REITER, R. & WENDEL, A. (1983) Selenium and drug metabolism.
Multiple modulations of mouse liver enzymes. Biochem.
Pharmacol., 32(20): 3063-3067.
RHEAD, W.J., CARY, E.E., ALLAWAY, W.H., SALTZSTEIN, S.L., &
SCHRAUZER, G.N. (1972) The vitamin E and selenium status of
infants and the sudden infant death syndrome. Bioinorg. Chem.,
1: 289-294.
RICHOLD, M., ROBINSON, M.F., & STEWART, R.D.H. (1977)
Metabolic studies in rats of 75Se incorporated in vivo into
fish muscle. Br. J. Nutr., 38: 19-29.
RIELY, C.A., COHEN, G., & LIEBERMAN, M. (1974) Ethane
evolution: a new index of lipid peroxidation. Science, 183:
208-210.
RILEY, J.F. (1968) Mast cells, co-carcinogenesis and
anti-carcinogenesis in the skin of mice. Experientia (Basel),
24: 1237-1238.
ROBBINS, C.W. & CARTER, D.L. (1970) Selenium concentrations
in phosphorus fertilizer materials and associated uptake by
plants. Soil Sci. Soc. Am. Proc., 34: 506-509.
ROBERTSON, D.S.F. (1970) Selenium, a possible teratogen?
Lancet, 1: 518-519.
ROBINSON, J.R., ROBINSON, M.F., LEVANDER, O.A., & THOMSON,
C.D. (1985) Urinary excretion of selenium by New Zealand and
North American human subjects on differing intakes. Am. J.
clin. Nutr., 41: 1023-1031.
ROBINSON, M.F. & THOMSON, C.D. (1981) Selenium levels in
humans vs environmental sources. In: Spallholz, J.E., Martin,
J.L., & Ganther, H.E., ed. Selenium in biology and medicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 283-302
ROBINSON, M.F. & THOMSON, C.D. (1983) The role of selenium
in the diet. Nutr. Abstr. Rev., 53: 3-26.
ROBINSON, M.F. & THOMSON, C.D. (1986) Selenium status of the
food supply and residents of New Zealand (Beijing Conference).
ROBINSON, M.F., MCKENZIE, J.M., THOMSON, C.D., & VAN RIJ, A.L.
(1973) Metabolic balance of zinc, copper, cadmium, iron,
molybdenum and selenium in young New Zealand women. Br. J.
Nutr., 30: 195-205.
ROBINSON, M.F., GODFREY, P.J., THOMSON, C.D., REA, H.M., & VAN
RIJ, A.M. (1978a) Blood selenium and glutathione peroxidase
activity in normal subjects and in surgical patients with and
without cancer in New Zealand. Am. J. clin. Nutr., 32:
1477-1485.
ROBINSON, M.F., REA, H.M., FRIEND, G.M., STEWART, R.D.H.,
SNOW, P.C., & THOMSON, C.D. (1978b) On supplementing the
selenium intake of New Zealanders. II. Prolonged metabolic
experiments with daily supplements of selenomethionine,
selenite, and fish. Br. J. Nutr., 39: 589-600.
ROBINSON, M.F., GODFREY, J.P., THOMSON, C.D., REA, H.M., & VAN
RIJ, A.M. (1979) Blood selenium and glutathione peroxidase
activity in normal subjects and in surgical patients with and
without cancer in New Zealand. Am. J. clin. Nutr., 32:
1477-1485.
ROBINSON, M.F., CAMPBELL, D.R., STEWART, R.D.H., REA, H.M.,
THOMSON, C.D., SNOW, P.G., & SQUIRES, I.H.W. (1981) Effect
of daily supplements of selenium on patients with muscular
complaints in Otago and Canterbury. N.Z. med. J., 93: 289-292.
ROBINSON, M.F., CAMPBELL, D.R., SUTHERLAND, W.H.F., HERBISON,
P.G., PAULIN, J.M., & SIMPSON, F.O. (1983) Selenium and risk
factors for cardiovascular disease in New Zealand. N.Z. med.
J., 96: 755-757.
ROBINSON, M.F., THOMSON, C.D., & HUEMMER, P.K. (1985) Effect
of a megadose of ascorbic acid, a meal and orange juice on the
absorption of selenium as sodium selenite. N.Z. med. J., 98:
627-629.
ROBINSON, W.O. (1933) Determination of selenium in wheat and
soils. J. Assoc. Off. Agric. Chem., 16: 423-424.
ROHMER, R., CARROT, E., & GOUFFAULT, J. (1950) Nouvel aspect
de l'intoxication par les composés du sélénium. Bull. Soc.
Chim. France, 5(17): 275-278.
ROSIN, M.P. & STICH, H.F. (1979) Assessment of the use of
the Salmonella mutagenesis assay to determine the influence of
antioxidants on carcinogen-induced mutagenesis. Int. J.
Cancer, 23: 722-727.
ROSENFELD, I. & BEATH, O.A. (1954) Effect of selenium on
reproduction in rats. Proc. Soc. Exp. Biol. Med., 87: 295-297.
ROSENFELD, I. & BEATH, O.A. (1964) Selenium geobotany,
biochemistry, toxicity and nutrition, New York, Academic
Press, 411 pp.
ROSIN, M.P. (1981) Inhibition of spontaneous mutagenesis in
yeast cultures by selenite, selenate, and selenide. Cancer
Lett., 13: 7-14.
ROSSI, L.C., CLEMENTE, G.F., & SANTARONI, G. (1976) Mercury
and selenium distribution in a defined area and in its
population. Arch. environ. Health, 31: 160-165.
ROTRUCK, J.T., POPE, A.L., GANTHER, H.E., SWANSON, A.B.,
HAFEMAN, D.G., & HOEKSTRA, W.G. (1973) Selenium: biochemical
role as a component of glutathione peroxidase. Science, 179:
588-590.
RUDERT, C.P. & LEWIS, A.R. (1978) The effect of potassium
cyanide on the occurrence of nutritional myopathy in lambs.
Rhod. J. agric. Res., 16: 109-116.
RUSIECKI, W. & BRZEZINSKI, J. (1966) Influence of sodium
selenate on acute thallium poisonings. Acta Pol. pharm., 23:
74-80.
SAKURAI, H. & TSUCHIYA, K. (1975) A tentative recommendation
for the maximum daily intake of selenium. Environ. Physiol.
Biochem., 5: 107-118.
SALONEN, J.T. (1985) Selenium in cardiovascular diseases and
cancer. Epidemiologic findings from Finland. In: Boström, H.
& Ljungstedt, N., ed. Trace elements in health disease,
Stockholm, Sweden, Almqvist & Wiksell International, pp.
172-186.
SALONEN, J.T., ALFTHAN, G., HUTTUNEN, J.K., PIKKARAINEN, J., &
PUSKA, P. (1982) Association between cardiovascular death
and myocardial infarction and serum selenium in a watched-pair
longitudinal study. Lancet, 2: 175-179.
SALONEN, J.T., ALFTHAN, G., HUTTUNEN, J.K., & PUSKA, P.
(1984a) Association between serum selenium and the risk of
cancer. Am. J. Epidemiol., 120(3): 342-349.
SALONEN, J.T., SALONEN, R., PENTTILA, I., HERRANEN, J., JAUHIAINEN,
J., KANTOLA, M., KAURANEN, P., LAPPETELAINEN, R., MAENPAA, P., &
PUSKA, P. (1984b) Serum fatty acids, apolipoproteins, selenium
and vitamin antioxidants and the risk of death from ischaemic heart
disease. Am. J. Cardiol., 56(2): 226.
SALONEN, J.T., SALONEN, R., LAPPETELAINEN, R., MAENPAA, P.H.,
ALFTHAN, G., & PUSKA, P. (1985) Risk of cancer in relation
to serum concentrations of selenium and vitamins A and E:
matched case-control analysis of prospective data. Brit. med.
J., 290: 417-420.
SARATHCHANDRA, S.U. & WATKINSON, J.H. (1981) Oxidation of
elemental selenium to selenite by Bacillus megaterium.
Science, 211: 600-601.
SARATHCHANDRASCHMIDT, K. & HELLER, W. (1976) Selenium
concentration and activity of glutathione peroxidase in
lysates of human erythrocytes. Blut, 33: 247-251.
SCHECTER, A., SHANSKE, W., STENZLER, A., QUINTILIAN, H., &
STEINBERG, H. (1980) Acute hydrogen selenide intoxication.
Chest, 77: 554-555.
SCHILLACI, M., MARTIN, S.E., & MILNER, J.A. (1982) The
effects of dietary selenium on the biotransformation of
7,12-dimethylbenz(alpha)anthracene. Mutat. Res., 101: 31-37.
SCHRAUZER, G.N. (1976) Cancer mortality correlation studies.
II. Regional associations of mortalities with the consumptions
of foods and other commodities. Medic. Hypoth., 2: 39-49.
SCHRAUZER, G.N. & ISHMAEL, D. (1974) Effects of selenium and
of arsenic on the genesis of spontaneous mammary tumors in
inbred C3H mice. Ann. clin. Lab. Sci., 4: 441-447.
SCHRAUZER, G.N. & WHITE, D.A. (1978) Selenium in human
nutrition: dietary intakes and effects of supplementation.
Bioinorg. Chem., 8: 303-318.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1976)
Inhibition of the genesis of spontaneous mammary tumors in
C3H mice: effects of selenium and of selenium-antagonistic
elements and their possible role in human breast cancer.
Bioinorg. Chem., 6: 265-270.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1977)
Cancer mortality correlation studies. III. Statistical
associations with dietary selenium intakes. Bioinorg. Chem.,
7: 23-34.
SCHRAUZER, G.N, WHITE, D.A., & SCHNEIDER, C.J. (1978a)
Effects of selenium, arsenic, and zinc on the genesis of
spontaneous mammary tumors in inbred female C3H mice. In:
Kirchgessner, M., ed. Trace element metabolism in man and
animals. III, Freising-Weihenstephan, West Germany,
Arbeitskreis für Tierernährungsforschung Weihenstephan, pp.
387-390.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1978b)
Selenium and cancer: Effects of selenium and of the diet on
the genesis of spontaneous mammary tumors in virgin inbred
female C3H/St mice. Bioinorg. Chem., 8: 387-396.
SCHRAUZER, G.N., WHITE, D.A., MCGINNESS, J.E., SCHNEIDER,
C.J., & BELL, L.J. (1978c) Arsenic and cancer: effects of
joint administration of arsenite and selenite on the genesis
of mammary adenocarcinoma in inbred female C3H/St mice.
Bioinorg. Chem., 9: 245-253.
SCHROEDER, H.A. & MITCHENER, M. (1971a) Toxic effects of
trace elements on the reproduction of mice and rats. Arch.
environ. Health, 23: 102-106.
SCHROEDER, H.A. & MITCHENER, M. (1971b) Selenium and
tellurium in rats: effect on growth, survival and tumors. J.
Nutr., 101: 1531-1540.
SCHROEDER, H.A. & MITCHENER, M. (1972) Selenium and
tellurium in mice. Effects on growth, survival, and tumors.
Arch. environ. Health, 24: 66-71.
SCHROEDER, H.A., FROST, D.V., & BALASSA, J.J. (1970)
Essential trace metals in man: selenium. J. chron. Dis., 23:
227-243.
SCHUBERT, J.R., MUTH, O.H., OLDFIELD, J.E., & REMMERT, L.F.
(196l) Experimental results with selenium in white muscle
disease of lambs and calves. Fed. Proc., 20: 689-694.
SCHULTZ, T.D. & LEKLEM, J.E. (1983) Selenium status of
vegetarians, nonvegetarians, and hormone-dependent cancer
subjects. Am. J. clin. Nutr., 37: 114-118.
SCHWARZ, K. (1961) Development and status of experimental
work on Factor 3-selenium. Fed. Proc., 20: 666-673.
SCHWARZ, K. (1962) Vitamin E, trace elements, and sulfhydryl
groups in respiratory decline. Vitam. Horm., 20: 463-484.
SCHWARZ, K. (1967) Discussion comments. In: Muth, O.H.,
Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc,
pp. 225-226.
SCHWARZ, K. (1976) The discovery of the essentiality of
selenium, and related topics (a personal account). In:
Proceedings of the Symposium on Selenium-Tellurium in the
Environment, Pittsburgh, Pennsylvania, Industrial Health
Foundation, pp. 349-376.
SCHWARZ, K. (1977) Amyotrophic lateral sclerosis and
selenium. J. Am. Med. Assoc., 238: 2365.
SCHWARZ, K. & FOLTZ, C.M. (1957) Selenium as an integral
part of Factor 3 against dietary necrotic liver degeneration.
J. Am. Chem. Soc., 79: 3292-3293.
SCHWARZ, K. & FOLTZ, C.M. (1958) Factor 3 activity of
selenium compounds. J. biol. Chem., 233: 245-251.
SCHWARZ, K., PORTER, L.A., & FREDGA, A. (1972) Some
regularities in the structure-function relationship of
organoselenium compounds effective against dietary liver
necrosis. Ann. NY Acad. Sci., 192: 200-214.
SCOTT, M.L. & THOMPSON, J.N. (1971) Selenium content of
feedstuffs and effects of dietary selenium levels upon tissue
selenium in chicks and poults. Poultry Sci., 50: 1742-1748.
SCOTT, M.L., OLSON, G., KROOK, L., & BROWN, W.R. (1967)
Selenium-responsive myopathies of myocardium and of smooth
muscle in the young poult. J. Nutr., 91: 573-583.
SEIFTER, J., EHRICH, W.E., HUDYMA, G., & MUELLER, G. (1946)
Thyroid adenomas in rats receiving selenium. Science, 103: 762.
SELJANKINA, K.P., JAHIMOVICH, N.P., ALEKSEEVA, L.S., & PETINA,
A.A. (1974) [Selenium and tellurium levels in environmental
media.] In: [Hygiene and occupational diseases,] Moscow, Nauka
Publishing House, Vol 21, pp. 69-71 (in Russian).
SENF, H.W. (1941) [A case of poisoning by hydrogen
selenide]. Dtsch. Med. Wochenschr., 67: 1094-1096 (in German).
SEWARD, C.R., VAUGHAN, G., & HOVE, E.L. (1966) Effect of
selenium on incisor depigmentation and carbon tetrachloride
poisoning in vitamin E-deficient rats. Proc. Soc. Exp. Biol.
Med., 121: 850-852.
SHACKLETTE, H.T., BOERNGEN, J.G., & KEITH, J.R. (1974)
Selenium, fluorine, and arsenic in surficial materials of the
conterminous United States, 14 pp (US Geological Survey
Circular, 692).
SHAMBERGER, R.J. (1970) Relationship of selenium to cancer.
I. Inhibitory effect of selenium on carcinogenesis. J. Natl
Cancer Inst., 44: 931-936.
SHAMBERGER, R.J. (1971) Is selenium a teratogen? Lancet, 2:
1316.
SHAMBERGER, R.J. (1978) Antioxidants and cancer. VIII.
Cadmium-selenium levels in kidneys. In: Kirchgessner, M., ed.
Trace element metabolism in man and animals. III,
Freising-Weihenstephan, West Germany, Arbeitskreis für
Tierernährungsforschung Weihenstephan, pp. 391-392.
SHAMBERGER, R.J. & FROST, D.V. (1969) Possible protective
effect of selenium against human cancer. Can. Med. Assoc. J.,
100: 682.
SHAMBERGER, R.J. & RUDOLPH, G. (1966) Protection against
cocarcinogenesis by antioxidants. Experientia (Basel), 22: 116.
SHAMBERGER, R.J. & WILLIS, C.E. (1971) Selenium distribution
and human cancer mortality. Crit. Rev. clin. Lab. Sci., 2:
211-221.
SHAMBERGER, R.J., BAUGHMAN, F.F., KALCHERT, S.L., WILLIS,
C.E., & HOFFMAN, G.C. (1973a) Carcinogen-induced chromosomal
breakage decreased by antioxidants. Proc. Natl Acad. Sci.
(USA), 70: 1461-1463.
SHAMBERGER, R.J., RUKOVENA, E., LONGFIELD, A.K., TYTKO, S.A.,
DEODHAR, S., & WILLIS, C.E. (1973b) Antioxidants and cancer.
I. Selenium in the blood of normals and cancer patients. J.
Natl Cancer Inst., 50(4): 863-870.
SHAMBERGER, R.J., TYTKO, S.A., & WILLIS, C.E. (1975)
Selenium and heart disease. In: Hemphill, D.D., ed. Trace
substances in environmental health. IX, Columbia, Missouri,
University of Missouri Press, pp. 15-22.
SHAMBERGER, R.J., TYTKO, S.A., & WILLIS, C.E. (1976)
Antioxidants and cancer. VI. Selenium and age-adjusted human
cancer mortality. Arch. environ. Health, 31: 231-235.
SHAMBERGER, R.J., CORLETT, C.L., BEAMAN, K.D., & KASTEN, B.L.
(1979) Antioxidants reduce the mutagenic effect of malonaldehyde
and beta-propiolactone. Partix, antioxidants, and cancer.
Mutat. Res., 66: 349-355.
SHAMBERGER, R.J., WILLIS, C.E., & MCCORMACK, L.J. (1979)
Selenium and heart disease. III. Blood selenium and heart
mortality in 19 states. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
University of Missouri, pp. 59-63.
SHAPIRO, J.R. (1972) Selenium and carcinogenesis: a review.
Ann. NY Acad. Sci., 192: 215-219.
SHEARER, T.R. (1973) Lack of effect of selenium on
glycolytic and citric acid cycle intermediates in rat kidney
and liver. Proc. Soc. Exp. Biol. Med., 144: 688-691.
SHEARER, T.R. (1975) Developmental and postdevelopmental
uptake of dietary organic and inorganic selenium into the
molar teeth of rats. J. Nutr., 105: 338-347.
SHEARER, T.R. & HADJIMARKOS, D.M. (1975) Geographic distribution
of selenium in human milk. Arch. environ. Health, 30: 230-233.
SHEARER, T.R. & RIDLINGTON, J.W. (1976) Fluoride-selenium
interaction in the hard and soft tissues of the rat. J. Nutr.,
106: 451-456.
SHEARER, T.R., MCCORMACK, D.W., DESART, D.J., BRITTON, J.L., &
LOPEZ, M.T. (1980) Histological evaluation of selenium
induced cataracts. Exp. Eye Res., 31: 327-333.
SHENDRIKAR, A.D. (1974) Critical evaluation of analytical
methods for the determination of selenium in air, water, and
biological materials. Sci. total Environ., 3: 155-168.
SHENDRIKAR, A.D. & FAUDEL, G.B. (1978) Distribution of trace
metals during oil shale retorting. Environ. Sci. Technol., 12:
332-334.
SHRIFT, A. (1964) A selenium cycle in nature? Nature
(Lond.), 201: 1304-1305.
SHRIFT, A. (1973) Selenium compounds in nature and medicine.
Metabolism of selenium by plants and microorganisms. In:
Klayman, D.L. & Gunther, W.H.H., ed. Organic selenium
compounds: their chemistry and biology, New York, John Wiley
and Sons, Inc, pp. 763-814.
SHRIFT, A. & VIRUPAKSHA, T.K. (1965) Seleno-amino acids in
selenium-accumulating plants. Biochim. Biophys. Acta, 100:
67-75.
SHUM, G.T.C., FREEMAN, H.C., & UTHE, J.F. (1977) Flameless
atomic absorption spectrophotometry of selenium in fish and
food products. J. Assoc. Off. Anal. Chem., 60: 1010-1014.
SHUMAEV, V.D., MAKUSHINSKAJA, N.D., & CHIGRINA, T.A. (1976)
[Hygiene aspects of industrial waste water pollution in some
non-ferrous metal enterprises in Kazahstan by chemicals
regulated for the point of sanitary toxicological risk
characteristics.] Zolvavoohram Kazah, 4: 36-38 (in Russian).
SIMPSON, B.H. (1972a) An epidemiological study of carcinoma
of the small intestine in New Zealand sheep. N.Z. vet. J., 20:
91-97.
SIMPSON, B.H. (1972b) The possible relationship of selenium
and superphosphate to the frequencey of occurrence of
intestinal carcinomas in sheep. N.Z. vet. J., 20: 224.
SINDEEVA, N.D. (1959) [Minerology, occurrence and main
characteristics of the geochemistry of selenium and
tellurium,] Moscow, Publishing House of the Academy of
Sciences of the USSR, 257 pp (in Russian).
SIRIANNI, S.R. & HUANG, C.C. (1983) Induction of sister
chromatid exchange by various selenium compounds in Chinese
hamster cells in the presence and absence of S9 mixture.
Cancer Lett., 18: 109-116.
SIVERTSEN, T., KARLSEN, J.T., & FROSLIE, A. (1977) The
relationship of erythrocyte glutathione peroxidase to blood
selenium in swine. Acta vet. Scand., 18: 494-500.
SKORNJAKOVA, L.V., BURCHANOV, A.I., & SALECHOV, M.I. (1969)
[On the acute manifestations of selenium poisoning.] Gig. Tr.
Prof. Zabol., 11: 45-46 (in Russian).
SMITH, C.R., Jr, WEISLEDER, D., MILLER, R.W., PALMER, I.S., &
OLSON, O.E. (1980) Linustatin and neolinustatin: cyanogenic
glycosides of linseed meal that protect animals against
selenium toxicity. J. org. Chem., 45: 507-510.
SMITH, M.I. & STOHLMAN, E.F. (1940) Further observations on
the influence of dietary protein on the toxicity of selenium.
J. Pharmacol. exp. Ther., 70: 270-278.
SMITH, M.I. & WESTFALL, B.B. (1937) Further field studies on
the selenium problem in relation to public health. US Public
Health Rep., 52: 1375-1384.
SMITH, M.I., FRANKE, K.W., & WESTFALL, B.B. (1936) The
selenium problem in relation to public health. A preliminary
survey to determine the possibility of selenium intoxication
in the rural population living on seleniferous soil. US Public
Health Rep., 51: 1496-1505.
SMITH, M.I., WESTFALL, B.B., & STOHLMAN, E.F., Jr (1937) The
elmination of selenium and its distribution in the tissues. US
Public Health Rep., 52: 1171-1177.
SMITH, M.I., WESTFALL, B.B., & STOHLMAN, E.F., Jr (1938)
Studies on the fate of selenium in the organism. US Public
Health Rep., 53: 1199-1216.
SOKOLOFF, L. (1985) Endemic forms of osteoarthritis. Clin.
rheum. Dis., 11: 187-202.
SPALLHOLZ, J.E., MARTIN, J.L., GERLACH, M.L., & HEINZERLING,
R.H. (1973) Immunologic responses of mice fed diets
supplemented with selenite selenium. Proc. Soc. Exp. Biol.
Med., 143: 685-689.
SPALLHOLZ, J.E., MARTIN, J.L., GERLACH, M.L., & HEINZERLING,
R.H. (1975) Injectable selenium: effect on the primary
immune response of mice. Proc. Soc. Exp. Biol. Med., 148:
37-40.
SPALLHOLZ, J.E., COLLINS, G.E., & SCHWARZ, K. (1978) A
single test-tube method for the fluorometric micro-
determination of selenium. Bioinorg. Chem., 9: 453-459.
SPRINKER, L.H., HARR, J.R., NEWBERNE, P.M., WHANGER, P.D., &
WESWIG, P.H. (197l) Selenium deficiency lesions in rats fed
vitamin E supplemented rations. Nutr. Rep. Int., 4: 335-340.
STADTMAN, T.C. (1977) Biological function of selenium. Nutr.
Rev., 35: 161-166.
STEAD, R.J., HINKS, L.J., HODSON, M.E., REDINGTON, A.N.,
CLAYTON, B.E., & BATTEN, J.C. (1985) Selenium deficiency and
possible increased risk of carcinoma in adults with cystic
fibrosis. Lancet (October): 862-863.
STEWART, R.D.H., GRIFFITHS, N.M., THOMSON, C.D., & ROBINSON,
M.F. (1978) Quantitative selenium metabolism in normal New
Zealand women. Br. J. Nutr., 40: 45-54.
STOEWSAND, G.S., GUTENMANN, W.H., & LISK, D.J. (1978) Wheat
grown on fly ash: high selenium uptake and response when fed
to Japanese quail. J. agric. food Chem., 26: 757-759.
SU, Y. & YU, W. (1983) Nutritional bio-geochemical etiology
of Keshan disease. Chin. med. J., 96: 594-596.
SU, Y., CUI, S., GU, B., ZENG, X., & YU, W. (1982)
Experimental study of the effects of corn and vegetables from
Keshan disease endemic districts on the growth and myocardium
in rats. Acta nutr. Sinica, 4: 261-269.
SUBCOMMITTEE ON SELENIUM - COMMITTEE ON ANIMAL NUTRITION
(1983) Selenium in nutrition, revised ed., Washington DC,
Board on Agriculture, National Research Council, National
Academy of Sciences, 174 pp.
SUCHKOV, B.P. (1971) [Selenium content in major nutrients
consumed by the population of the Ukrainian SSR.] Vopr.
Pitan., 30(b): 75-77 (in Russian).
SUCHKOV, B.P. & KACAP, I.M. (1971) [Dental caries in the
population of the Chernovitsk region in relation with the
impact of microelements.] Gig. i Sanit., 3: 91-93 (in Russian).
SUCHKOV, B.P. & ZHIVECKIJ, A.V. (1973) [Selenium levels in
the blood of healthy people and people with neoplasms in a
Chernovici region republican interdepartmental collective.]
In: [Microelements in medicine,] Kiev, Zdarovie, Zdarovie
Publishing House, Vol. 4, pp. 69-73 (in Russian).
SUCHKOV, B.P., KACAP, I.M., & GULGASENKO, A.I. (1973)
[Affection of the population of the Chernovitsi region with
caries in association with selenium content in the teeth].
Stomatologia, 52: 21-23 (in Russian).
SUCHKOV, B.P., SHEVCHUK, I.A., & MARBAR, A.I. (1977)
[Histopathomorphological changes in the organs and tissues of
laboratory animals on a synthetic diet with a low content of
selenium and vitamin E.] Vopr. Pitan., 2: 33-41 (in Russian).
SUCHKOV, B.P., SHTUTMAN, C.M., & HALMURADOV, A.G. (1978)
[The biochemical role of selenium in animal organisms.]
Ukrain. Biochem., 50: 659-671 (in Russian).
SUN, S., ZHAI, F., ZHOU, L., & YANG, G. (1985) The
bioavailability of soil selenium in Keshan disease and high
selenium areas. Chinese J. end. Dis., 4: 21-28.
SUNDE, R.A. (1984) Selenoproteins. J. Am. Oil Chem. Soc.,
61: 1891-1898.
SUNDSTROM, H., KORPELA, H., VIINIKKA, L., & KAUPPILA, A.
(1984a) Serum selenium and glutathione peroxidase, and plasma
lipid peroxides in uterine, ovarian or vulvar cancer, and
their responses to antioxidants in patients with ovarian
cancer. Cancer Lett., 24: 1-10.
SUNDSTROM, H., YRJANHEIKKI, E., & KAUPPILA, A. (1984b) Serum
selenium in patients with ovarian cancer during and after
therapy. Carcinogenesis, 5(6): 731-734.
SVERDLINA, N.T & MASLENNIKOVA, V.S. (1961) [Hygienic
evaluation of working conditions in production of selenium
photocells.] Leningrad, Mater. manch sessin Leningrad NII gig.
truda prof. Zab, pp. 73-75 (in Russian).
SWEINS, A. (1983) Protective effect of selenium against
arsenic-induced chromosomal damage in cultured human lympho-
cytes. Hereditas, 98: 249-252.
SYMANSKI, H. (1950) [A case of hygrogen selenide poisoning.]
Dtsch. Med. Wochenschr., 75: 1730 (in German).
SZYDLOWSKI, F.J. & DUNMIRE, D.L. (1979) Semi-automatic
digestion and automatic analysis for selenium in animal feeds.
Anal. Chim Acta, 105: 445-449.
TAN, J., ZHENG, D., HOU, S., ZHU, W., LI, R., ZHU, Z., & WANG,
W. (in press) Selenium ecological chemico-geography and
endemic Keshan disease and Kashin-Beck disease in China. In:
Proceedings of the Third International Symposium on Selenium
in Biology and Medicine.
TANK, G. & STORVICK, C.A. (1960) Effect of naturally
occurring selenium and vanadium on dental caries. J. dent.
Res., 39: 473-488.
TAYLOR, F.B. (1963) Significance of trace elements in public
finished water supplies. J. Am. Water Works Assoc., 55:
619-623.
TEEL, R.W. (1984) A comparison of the effect of selenium on
the mutagenicity and metabolism of benzo(alpha)pyrene in rat and
hamster liver S9 activation systems. Cancer Lett., 24: 281-289.
TEEL, R.W. & KAIN, S.R. (1984) Selenium modified mutagenicity
and metabolism of benzo(alpha)pyrene in an S9-dependent system.
Mutat. Res., 127: 9-14.
THOMPSON, H.J. (1984) Selenium as an anticarcinogen. J.
agric. food Chem., 32: 422-425.
THOMPSON, J.N. & SCOTT, M.L. (1969) Role of selenium in the
nutrition of the chick. J. Nutr., 97: 335-342.
THOMPSON, J.N. & SCOTT, M.L. (1970) Impaired lipid and
vitamin E absorption related to atrophy of the pancreas in
selenium-deficient chicks. J. Nutr., 100: 797-809.
THOMPSON, J.N., ERDODY, P., & SMITH, D.C. (1975) Selenium
content of food consumed by Canadians. J. Nutr., 105: 274-277.
THOMPSON, K.C. (1975) The atomic-fluorescence determination
of antimony, arsenic, selenium and tellurium by using the
hydride generation technique. Analyst, 100: 307-310.
THOMPSON, R.H., MCMURRAY, C.H., & BLANCHFLOWER, W.J. (1976)
The levels of selenium and glutathione peroxidase activity in
blood of sheep, cows and pigs. Res. vet. Sci., 20: 229-231.
THOMSON, C.D. (1974) Recovery of large doses of selenium
given as sodium selenite with or without vitamin E. N.Z. med.
J., 80: 163-168.
THOMSON, C.D. (1985) Selenium dependent and non-selenium
dependent glutathione peroxidase in human tissues of New
Zealand residents. Biochem. Int., 10: 673-679.
THOMSON, C.D. & ROBINSON, M.F. (1980) Selenium in human
health with emphasis on those aspects peculiar to New
Zealand. Am. J. clin. Nutr., 33: 303-323.
THOMSON, C.D. & ROBINSON, M.F. (1986) Urinary and faecal
excretions and absorption of a large supplement of selenium as
selenite or as selenate. Am. J. clin. Nutr. (submitted for
publication).
THOMSON, C.D. & STEWART, R.D.H. (1973) Metabolic studies of
75Se selenomethionine and 75Se selenite in the rat. Br. J.
Nutr., 30: 139-147.
THOMSON, C.D. & STEWART, R.D.H. (1974) The metabolism of
75Se selenite in young women. Br. J. Nutr., 32: 47-57.
THOMSON, C.D., ROBINSON, B.A., STEWART, R.D.H., & ROBINSON,
M.F. (1975a) Metabolic studies of 75Se selenocystine and
75Se selenomethionine in the rat. Br. J. Nutr., 34: 501-509.
THOMSON, C.D., STEWART, R.D.H., & ROBINSON, M.F. (1975b)
Metabolic studies in rats of 75Se selenomethionine and of 75Se
incorporated in vivo into rabbit kidney. Br. J. Nutr., 33:
45-54.
THOMSON, C.D., REA, H.M., ROBINSON, M.F., & CHAPMAN, O.W.
(1977a) Low blood selenium concentrations and glutathione
peroxidase activities in elderly people. Proc. Univ. Otago
Med. Sch., 55: 18-19.
THOMSON, C.D., REA, H.M., DOESBURG, V.M., & ROBINSON, M.F.
(1977b) Selenium concentrations and glutathione peroxidase
activities in whole blood of New Zealand residents. Br. J.
Nutr., 37: 457-460.
THOMSON, C.D., BURTON, C.E., & ROBINSON, M.F. (1978) On
supplementing the selenium intake of New Zealanders. I. Short
experiments with large doses of selenite or selenomethionine.
Br. J. Nutr., 39: 579-587.
THOMSON, C.D., ROBINSON, M.F., CAMPBELL, D.R., & REA, H.M.
(1982) Effect of prolonged supplementation with daily
supplements of selenomethionine and sodium selenite on
glutathione peroxidase activity in blood of New Zealand
residents. Am. J. clin. Nutr., 36: 24-31.
THOMSON, C.D., ONG, L.K., & ROBINSON, M.F. (1985) Effect of
supplementation with high-selenium wheat bread on selenium,
glutathione peroxidase and related enzymes in blood components
of New Zealand residents. Am. J. clin. Nutr., 41: 1015-1022.
THORN, J., ROBERTSON, J., & BUSS, D.H. (1978) Trace
nutrients. Selenium in British food. Br. J. Nutr., 39: 391-396.
TINSLEY, I.J., HARR, J.R., BONE, J.F., WESWIG, P.H., &
YAMAMOTO, R.S. (1967) Selenium toxicity in rats. I. Growth
and longevity. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H.,
ed. Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 141-152.
TSEN, C.C. & COLLIER, H.B. (1959) Selenite as a relatively
weak inhibitor of some sulphydryl enzymes. Nature (Lond.),
183: 1327-1328.
TSEN, C.C. & TAPPEL, A.L. (1958) Catalytic oxidation of
glutathione and other sulfhydryl compounds by selenite. J.
biol. Chem., 233(5): 1230-1232.
TSONGAS, T.A. & FERGUSON, S.W. (1977) Human health effects
of selenium in a rural Colorado drinking-water supply. In:
Hemphill, D.D., ed. Trace substances in environmental health.
XI, Columbia, Missouri, University of Missouri Press, pp.
30-35.
ULLREY, D.E., BRADY, P.S., WHETTER, P.A., KU, P.K., & MAGEE,
W.T. (1977) Selenium supplementation of diets for sheep and
beef cattle. J. anal. Sci., 45: 559-565.
UNDERWOOD, E.J. (1977) Trace elements in human and animal
nutrition, 4th ed., New York, Academic Press, 545 pp.
US DEPARTMENT OF HEALTH AND HUMAN SERVICES, FOOD AND DRUG
ADMINISTRATION (1984) 21 CFR Pt.573.920 Selenium. Fed. Reg.,
49: 627-628.
US FDA (1974) Final environmental impact statement rule
making on selenium in animal feeds, Washington DC, US Food and
Drug Administration, 131 pp.
US FDA (1975) Bureau of Foods Compliance Program Evaluation
Report. Total diet studies, Fiscal Year 1974, Washington DC,
US Food and Drug Administration, 32 pp.
US NAS/NRC (197l) Selenium in nutrition, Washington DC,
National Academy of Science, National Research Council,
Agricultural Board, Committee on Animal Nutrition,
Subcommittee on Selenium, 79 pp.
US NAS/NRC (1976) Selenium, Washington DC, National Academy
of Science, National Research Council, Assembly of Life
Sciences, Medical and Biological Effects of Environmental
Pollutants, 203 pp.
US NAS/NRC (1979) Zinc, Baltimore, Maryland, National
Academy of Science, National Research Council, Assembly of
Life Sciences, Committee on Medical and Biologic Effects of
Environmental Pollutants, 471 pp.
US NAS/NRC (1980) Recommended dietary allowances, Washington
DC, National Academy of Science, National Research Council,
Food and Nutrition Board, Committee on Dietary Allowances, 185
pp.
VAN DER LINDEN, R., DE CORTE, F., & HOSTE, J. (1974)
Activation analysis of biological material with ruthenium as a
multi-element comparator. Anal. Chim. Acta, 71: 263-275.
VAN KAMPEN, K.R. & JAMES, L.F. (1978) Manifestations of
intoxication by selenium-accumulating plants. In: Keeler,
R.F., Van Kampen, K.R., & James, L.F., ed. Effects of
poisonous plants on livestock, New York, Academic Press,
pp. 135-138.
VAN RIJ, A.M., ROBINSON, M.F., GODFREY, P.J., THOMSON, C.D., &
RHEA, H.M. (1978) Selenium blood levels in cancer and other
diseases in surgery. In: Hemphill, D.D., ed. Trace substances
in environmental health. XII, Columbia, Missouri, University
of Missouri Press, pp. 157-163.
VAN RIJ, A.M., THOMSON, C.D., MCKENZIE, J.M., & ROBINSON,
M.F. (1979) Selenium deficiency in total parenteral
nutrition. Am. J. clin. Nutr., 32: 2076-2085.
VAN VLEET, J.F. (1976) Induction of lesions of selenium-
vitamin E deficiency in pigs fed silver. Am. J. vet. Res., 37:
1415-1420.
VAN VLEET, J.F., MEYER, K.B., & OLANDER, H.J. (1974) Acute
selenium toxicosis induced in baby pigs by parenteral
administration of selenium-vitamin E preparations. J. Am. Vet.
Med. Assoc., 165: 543-547.
VARO, P. & KOIVISTOINEN, P. (1980) Mineral element
composition of Finnish foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn,
Zn, Mo, Co, Ni, Cr, F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb,
and ash. XII. General discussion and nutritional evaluation.
Acta agric. Scand. Suppl., 22: 165.
VARO, P. & KOIVISTOINEN, P. (1981) Annual variations in the
average selenium intake in Finland: cereal products and milk
as sources of selenium in 1979/80. Int. J. Vit. Nutr. Res.,
51: 79-84.
VERNIE, L.N. (1984) Selenium in carcinogenesis. Biochim.
Biophys. Acta, 738(4): 203-217.
VERNIE, L.N., BONT, W.S., GINJAAR, H.B., & EMMELOT, P.
(1975) Elongation factor 2 as the target of the reaction
product between sodium selenite and glutathione (GSSeSG) in
the inhibiting of amino acid incorporation in vitro. Biochim.
Biophys. Acta, 414: 283-292.
VERNIE, L.N., GINJARR, H.B., WILDERS, I.T., & BONT, W.S.
(1978) Amino acid incorporation in a cell-free system derived
from rat liver studied with the aid of selenodiglutathione.
Biochim. Biophys. Acta, 518(3): 507-517.
VIRTAMO, J., VALKEILA, E., ALFTHAN, G., PUNSAR, S., HUTTUNEN,
J.K., & KARVONEN, M. (1985) Serum selenium and the risk of
coronary heart disease and stroke. Am. J. Epidemiol., 122(2):
276-282.
VOBECKY, M., PAVLIK, L., & BENES, J. (1977) Non-destructive
neutron activation assay of submicrogram quantities of
selenium. Radiochem. Radioanal. Lett., 29(4): 159-164.
VOBECKY, M., DEDINA, J., PAVLIK, L., & VALASEK, J. (1979)
Gamma-ray interferences in the determination of selenium by
the Inaa method. Radiochem. Radioanal. Lett., 38(3): 197-204.
VOLGAREV, M.N. & TSCHERKES, L.A. (1967) Further studies in
tissue changes associated with sodium selenate. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 179-184.
WAGNER, P.A., HOEKSTRA, W.G., & GANTHER, H.E. (1975)
Alleviation of silver toxicity by selenite in the rat in
relation to tissue glutathione peroxidase. Proc. Soc. Exp.
Biol. Med., 148: 1106-1110.
WAHLSTROM, R.C. & OLSON, O.E. (1959) The effect of selenium
on reproduction in swine. J. anim. Sci., 18: 141-145.
WALKER, G.W.R. & BRADLEY, A.M. (1969) Interacting effects of
sodium monohydrogenarsenate and selenocystine on crossing over
in Drosophila melanogaster. Can. J. Genet. Cytol., 11: 677-688.
WALKER, G.W.R. & TING, K.P. (1967) Effect of selenium on
recombination in barley. Can. J. Genet. Cytol., 9: 314-320.
WANG, Z., LI, C., & LI, L. (1985) An epidemiological
investigation on the selenium content of water, cereals and
hair of children in Heilongjiang province. Chinese J. end.
Dis., 4: 330-333.
WARRINGTON, P.B. (1979) Selenium. Eng. Min. J., 180: 149-150.
WASLIEN, C.I. (1976) Human intake of trace elements. In:
Prasad, A.S., ed. Trace elements in human health and disease.
II. Essential and toxic elements, New York, Academic Press,
pp. 347-370.
WATKINSON, J.H. (1967) Analytical methods for selenium in
biological material. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI publishing Co., Inc, pp. 97-117.
WATKINSON, J.H. (1974) The selenium status of New
Zealanders. N.Z. med. J., 80: 202-205.
WATKINSON, J.H. (1979) Semi-automated fluorimetric
determination of nanogram quantities of selenium in biological
materials. Anal. Chim. Acta, 105: 319-325.
WATKINSON, J.H. (1981) Changes of blood selenium in New
Zealand adults with time and importation of Australian wheat.
Am. J. clin. Nutr., 34: 936-942.
WATKINSON, J.H. & BROWN, M.W. (1979) New phase-separating
device and other improvements in the semi-automated
fluorimetric determination of selenium. Anal. Chim. Acta, 105:
451-454.
WEDDERBURN, J.F. (1972) Selenium and cancer. N.Z. vet. J.,
20: 56-57.
WEISSMAN, S.H., CUDDIHY, R.G., & BURKSTALLER, M.A. (1979)
Distribution and retention of inhaled selenious acid and
selenium metal aerosols in Beagle dogs. In: Hemphill, D.D.,
ed. Trace substances in environmental health. XIII, Columbia,
Missouri, University of Missouri Press, pp. 477-482.
WEISSMAN, S.H., CUDDIHY, R.G., & MEDINSKY, M.A. (1983)
Absorption, distribution, and retention of inhaled selenious
acid and selenium metal aerosols in Beagle dogs. Toxicol.
appl. Pharmacol., 67: 331-337.
WELSH, S.O. (1979) The protective effect of vitamin E and
N,N'-diphenyl- p-phenylenediamine (DPPD) against methyl mercury
toxicity in the rat. J. Nutr., 109: 1673-1681.
WELSH, S.O. & SOARES, J.H., Jr (1976) The protective effect
of vitamin E and selenium against methyl mercury toxicity in
the Japanese quail. Nutr. Rep. Int., 13: 43-51.
WELSH, S.O., HOLDEN, J.W., WOLF, W.R., & LEVANDER, O.A.
(1981) Selenium intake of Maryland residents consuming
self-selected diets. J. Am. Diet. Assoc., 79: 277-285.
WESTERMANN, D.T. & ROBBINS, C.W. (1974) Effect of S04-S
fertilization on Se concentration of alfalfa (Medicago sativa
L). Agron. J., 66: 207-208.
WESTERMARCK, T. (1977) Selenium content of tissues in
Finnish infants and adults with various diseases, and studies
on the effects of selenium supplementation in neuronal ceroid
lipofuscinosis patients. Acta pharmacol. toxicol., 41: 121-128.
WESTERMARCK, T., RAUNU, P., KIRJARINTA, M., & LAPPALAINEN, L.
(1977) Selenium content of whole blood and serum in adults
and children of different ages from different parts of
Finland. Acta pharmacol. toxicol., 40: 465-475.
WESWIG, P.H., TINSLEY, I.J., HARR, J.R., BONE, J.F., YAMAMOTO,
R.S., & FALK, H. (1966) Bioassay of selenium compounds for
carcinogenesis in rats. Final report, Corvallis, Oregon State
University, Departments of Agricultral Chemistry and
Veterinary Medicine, 131 pp.
WHANGER, P.D. (1976) Selenium versus metal toxicity in
animals. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 234-252.
WHANGER, P.D., MUTH, O.H., OLDFIELD, J.E., & WESWIG, P.H.
(1969) Influence of sulfur on incidence of white muscle
disease in lambs. J. Nutr., 97: 553-562.
WHANGER, P.D., PEDERSEN, N.D., & WESWIG, P.H. (1973)
Selenium proteins in ovine tissues. II. Spectral properties of
a 10,000 molecular weight selenium protein. Biochem. Biophys.
Res. Commun., 53: 1031-1035.
WHANGER, P.D., PEDERSON, N.D., HATFIELD, J., & WESWIG, P.H.
(1976a) Absorption of selenite and selenomethionine from
ligated digestive tract segments in rats. Proc. Soc. Exp.
Biol. Med., 153: 295-297.
WHANGER, P.D., WESWIG, P.H., SCHMITZ, J.A., & OLDFIELD, J.E.
(1976b) Effects of selenium, cadmium, mercury, tellurium,
arsenic, silver, and cobalt on White Muscle Disease in lambs
and effect of dietary forms of arsenic on its accumulation in
tissues. Nutr. Rep. Int., 14: 63-72.
WHEATCROFT, M.G., ENGLISH, J.A., & SCHLACK, C.A. (1951)
Effects of selenium on the incidence of dental caries in white
rats. J. dent. Res., 30: 523-524.
WHETTER, P.A. & ULLREY, D.E. (1978) Improved fluorometric
method for determining selenium. J. Assoc. Off. Anal. Chem.,
61: 927-930.
WHITEACRE, M.E., COMBS, G.F., & PARKER, R.S. (1983)
Peroxidative damage in nutritional pancreatic atrophy due to
selenium-deficiency in the chick. Fed. Proc. Fed. Am. Soc.
Exp. Biol., 42: 928.
WHITING, R.F., WEI, L., & STICH, H.F. (1980) Unscheduled DNA
synthesis and chromosome aberrations induced by inorganic and
organic selenium compounds in the presence of glutathione.
Mutat. Res., 78: 159-169.
WHO (1984) Guidelines for drinking water quality. Volume 2:
Health criteria and other supporting information, Geneva,
World Health Organization, 335 pp.
WILKIE, J.B. & YOUNG, M. (1970) Improvement in the 2,3-
diaminoaphthalene reagent for microfluorescent. Determination
of selenium in biological materials. J. agric. food Chem., 18:
944-945.
WILLETT, W.C., MORRIS, J.S., PRESSEL, S., TAYLOR, J.O., POLK,
B.F., STAMPFER, M.J., ROSNER, B., SCHNEIDER, K., & HAMES,
C.G. (1983) Prediagnostic serum selenium and risk of cancer.
Lancet (July): 130-134.
WILLIAMS, K.T. & BYERS, H.G. (1935) Occurrence of selenium
in the Colorado River and some of its tributaries. Ind. Eng.
Chem. Anal. Ed., 7: 431-432.
WILLIAMS, M.M.I. (1983) Selenium and glutathione peroxidase
in mature human milk. Proc. Univ. Otago Med. Sch., 61: 20-21.
WILSON, H.M. (1962) Selenium oxide poisoning. N.C. med. J.,
23: 73-75.
WILSON, P.S. & JUDSON, G.J. (1976) Glutathione peroxidase
activity in bovine and ovine erythrocytes in relation to blood
selenium concentration. Br. vet. J., 132: 428-434.
WITTING, L.A. & HORWITT, M.K. (1964) Effects of dietary
selenium, methionine, fat level, and tocopherol on rat growth.
J. Nutr., 84: 351-360.
WRIGHT, P.L. & BELL, M.C. (1966) Comparative metabolism of
selenium and tellurium in sheep and swine. Am. J. Physiol.,
211: 6-10.
WU, A.S.H., OLDFIELD, J.E., & WHANGER, P.D. (197l) Effect of
selenium, chromium and vitamin E on spermatogensis. J. anal.
Sci., 33: 273.
WU, A.S.H., OLDFIELD, J.E., WHANGER, P.D., & WESWIG, P.H.
(1973) Effect of selenium, vitamin E, and antioxidants on
testicular function in rats. Biol. Reprod., 8: 625-629.
WU, A.S.H., OLDFIELD, J.E., SHULL, L.R., & CHEEKE, P.R.
(1979) Specific effect of selenium deficiency on rat sperm.
Biol. Reprod., 20: 793-798.
XIA, X. & YU, S. (in press) Studies on the anti-carcinogenic
mechanisms of selenium - The effects of glucose oxidation and
related enzymes. Chinese J. Cancer.
YANG, G.Q., WANG, S., ZHOU, R., & SUN, S. (1983) Endemic
selenium intoxication of humans in China. Am. J. clin. Nutr.,
37: 872-881.
YANG, G., CHEN, J., WEN, Z., GE, K., ZHU, L., CHEN, X., &
CHEN, X. (1984) The role of selenium in Keshan disease. In:
Draper, H.H., ed. Advances in nutritional research, New York,
Plenum Press, pp. 203-231.
YANG, G., ZHOU, R., YIN, S., PIAO, J., ZHU, L., LIN, S., & GU,
L., MAI, R., XU, J., QIAG, F., LU, M., DENG, X., HUANG, J.,
LIN, & ZHOU, W. (1985) [Studies on the selenium requirement
of Chinese people. I. Physiological requirement, minimum
requirement, and minimum allowance.] J. Inst. Health, 14:
24-28 (in Chinese).
YANG, G.Q., ZHU, L.Z., LIU, S.J., GU, L.Z., QIAN, P.C., HUANG,
J.H., & LU, M.D. (1986) Studies of human selenium requirements
in China. In: Combs, G.F., Spallholz, J.E., Levander, O.A., &
Oldfield, J.E., ed. Selenium in biology and medicine, 3rd
Symposium, Westport, Connecticut, AVI Publishing Company.
YASUMOTO, K., IWAMI, K., YOSHIDA, M., & MITSUDA, H. (1976)
Selenium content of foods and its average daily intake in
Japan. Eiyo To Shokuryo, 29: 511-515.
YEH, Y. & JOHNSON, R.M. (1973) Vitamin E deficiency in the
rat. IV. Alteration in mitochondrial membrane and its relation
to respiratory decline. Arch. Biochem. Biophys., 159: 821-831.
YOUNG, S. & KEELER, R.F. (1962) Nutritional muscular dystrophy
in lambs. The effect of muscular activity on the symmetrical
distribution of lesions. Am. J. vet. Res., 23: 966-971.
YU, W.H. (1982) A study of nutritional and bio-geochemical
factors in the occurrence and development of Keshan disease.
Jpn Circ. J., 46: 1201-1207.
YU, S., CHU, Y., GONG, X., & HOU, C. (1985) Region variation
of cancer mortality incidence and its relation to selenium
levels in China. Ecol. trace Element Res., 7: 21-23.
YU, S., ZHU, Y., HON, C., & HUANG, C. (in press) Selective
effects of selenium on the function and structure of
mitochondria isolated from hepatoma and normal liver. In:
Proceedings of the Third International Symposium of Selenium
in Biology and Medicine.
ZABEL, N.L., HARLAND, J., GORMICAN, A.T., & GANTHER, H.E.
(1978) Selenium content of commercial formula diets. Am. J.
clin. Nutr., 31: 850-858.
ZHU, L. & LU, Z. et al. (1981) Effects in pigs fed the crops
grown in Keshan disease affected province of China. In:
Howell, J.M., Gawthone, J.M., & White, C.L., ed. Trace
Element Metabolism in Man and Animals (TEMA 4).
ZOLLER, W.H. & REAMER, D.C. (1976) Selenium in the
atmosphere. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 54-66.
ZOLLER, W.H., GLADNEY, E.S., & DUCE, R.A. (1974) Atmospheric
concentrations and sources of trace metals at the South Pole.
Science, 183: 198-200.
8.2.6. Attempts to associate low selenium intake with human diseases
8.2.6.1. Keshan Disease
Results of research in China have suggested a relationship
between low selenium status and the prevalence of Keshan disease,
an endemic cardiomyopathy that primarily affects children (Keshan
Disease Research Group, 1979a,b). Cases of Keshan disease were
recorded as early as 1907 in Heilongjiang Province of northeastern
China (Gu, 1983). Since the etiology of the disease was not known,
it was named after the locality in which it was originally
observed, Keshan County.
Epidemiologically, the disease exhibits a regional distribution
and occurs in a belt-like zone reaching from northeastern China to
the southwestern part of the country. There is a marked seasonal
fluctuation in the disease with more cases appearing during winter
in the north and during summer in the south. There is also a great
annual variation in the incidence of Keshan disease. Recently, the
incidence has decreased sharply such that from 1978 to 1980 less
than one death was reported per 100 000 members of the population
(Gu, 1983). The overall recent decline in the incidence of Keshan
disease has been attributed, at least in part, to the general
increase in the living standards of the people, such as better
sanitation, more medical attention, and improved quality of the
diet (He, 1979). There is also a shifting of epidemic foci from
year to year. Rural peasants constitute the population at risk
with children below 10 years of age and women of child-bearing age
most susceptible. According to Gu (1983), migrants from non-
affected areas will not contract the disease unless they have lived
in the endemic area for at least 3 months.
The criteria for diagnosing Keshan disease include acute or
chronic cardiac insufficiency, heart enlargement, gallop rhythm,
arrhythmia, and ECG changes (Chen et al., 1980). The disease is
classified into four types: acute (cardiogenic shock), subacute,
chronic (low output pump failure), and latent (normal heart
function but mild enlargement). There is no symptom or sign
specific for identifying the disease (Gu, 1983).
Histopathologically, Keshan disease is characterized by multifocal
necrosis and fibrous replacement of the myocardium.
In the past, many different hypotheses were advanced in an
attempt to explain the etiology of Keshan disease. For example,
there were theories of poisoning due to rhodamine, silica,
digitalis, carbon monoxide, barium, nitrite, and Fusarium
mycotoxins. However, clear-cut proof of these toxicants as a cause
of the disease has not been obtained despite much analytical effort
(Yang et al., 1984). Several nutritional problems have also been
proposed to play a role in Keshan disease such as deficiency of
protein, lipids, thiamin, magnesium, or molybdenum. A hypothesis
concerning Keshan disease etiology was proposed linking the disease
to selenium deficiency, after it was noted that severely endemic
areas coincided with areas where the incidence of enzootic selenium
deficiency diseases in farm animals was also high (Zhu et al.,
1981). On this basis, selenium supplements were used as a
preventive measure for Keshan disease. This hypothesis is very
appealing since cardiomyopathy is a prime feature of selenium-
vitamin E deficiency in many species of animals, including cattle,
sheep, and swine.
Since the initial proposal of the selenium-Keshan disease
connection, much evidence has been gathered in support of this
concept. For example, the average blood-selenium content was 0.021
± 0.001 mg/litre for the affected areas and 0.095 ± 0.088 mg/litre
for non-affected areas (Yang et al., 1984). The average hair-
selenium content was below 0.12 mg/kg in affected areas, whereas
hair-selenium levels in neighbouring but unaffected areas ranged
between 0.12 and 0.2 mg/kg. Average hair-selenium contents in
areas removed from the affected belt were between 0.25 and 0.6
mg/kg. The selenium level in several staple foods (rice, maize,
wheat, soybeans, black beans, and sweet potatoes) was lower in
areas affected with Keshan disease than in unaffected areas. It
was stated that an area could be considered to be unaffected
wherever the selenium content of grains was 0.04 mg/kg or more.
The Chinese workers stated that the amount of selenium needed to
prevent the disease was about 20 µg/day (Yang et al., 1984).
In some affected areas, there were highly localized pockets
(so-called "safety islands") that were free from the disease.
Apparently, these islands were protected because of the higher
selenium content of their crops in the immediate vicinity. For
example, the average selenium contents of rice and soybeans in one
such island were 0.020 and 0.025 mg/kg, respectively, whereas the
values for the corresponding crops in a nearby affected area were
0.0078 and 0.0057 mg/kg. It was also noted that the children in
the unaffected spot liked to catch shrimp from local streams and
that the selenium content of these dried shrimp was as high as 1
mg/kg (Keshan Disease Research Group, 1979b).
These relationships between selenium and Keshan disease led the
Chinese workers to conduct a randomized intervention trial to test
the possible prophylactic effect of selenium against this condition
in the population at risk (i.e., children 1 - 9 years old). In a
trial in 1974, 4510 children took sodium selenite and 3985 children
took the placebo (Table 56). The treated children took 0.5 mg
sodium selenite per week if 1 - 5 years old, or 1.0 mg per week if
6 - 9 years old. The morbidity rate due to Keshan disease was
1.35% in the placebo group (54 cases out of 3985 children) but only
0.22% in the treated group (10/4510). Since a significant
difference was also shown in the 1975 trial (0.95% morbidity rate
in the placebo group compared with 0.1% in those treated) the
placebo groups were abolished in 1976 and 1977. As a result, the
case rate dropped to 0.034% and 0% in these 2 years, respectively.
However, in 1976, there was one case out of 212 children who failed
to take the treatment.
No untoward side effects due to the sodium selenite were
observed, except for some individual cases of nausea, which could
be overcome by taking the medicine after meals. Physical
examinations and liver function tests indicated that the liver was
undamaged after continuous ingestion of the selenium tablets for
3 - 4 years.
Table 56. Effect of selenium on Keshan disease in childrena
-------------------------------------------------------------------
Treatment Year Number Number Outcome of alive cases Death
of of Turned Improved Turned
subjects cases latent chronic
-------------------------------------------------------------------
Placebo 1974 3985 54 16 9 2 27
1975 5445 52 13 10 3 26
Sodium 1974 4510 10 9 0 1 0
selenite 1975 6767 7 6 0 0 1
-------------------------------------------------------------------
a Adapted from: Keshan Disease Research Group (1979a).
Since 1976, more extensive intervention trials with sodium
selenite have been carried out in 5 counties in the same area of
Sichuan Province (Yang et al., 1984). All children, 1 to 12 years
of age, in some of the most severely affected communes, were
treated with selenium as described above, while untreated children
in nearby communes served as controls. The incidence rate of
Keshan disease in the selenium-treated children was lower in each
year of the five-year period than among the untreated children
(Table 57).
Table 57. Keshan disease incidence rates in selenium-treated and
untreated children in five counties of Sichuan provincea
-------------------------------------------------------------------------
Treated children Untreated children
Year Number of Number of Incidence Number of Number of Incidence
subjects cases (per 1000) subjects cases (per 1000)
-------------------------------------------------------------------------
1976 45 515 8 0.17 243 649 448 2.00
1977 67 754 15 0.22 222 944 350 1.57
1978 65 953 10 0.15 220 599 373 1.69
1979 69 910 33 0.47 223 280 300 1.34
1980 74 740 22 0.29 197 096 202 1.07
Total 323 872 88 0.27 1 107 568 1713 1.55
-------------------------------------------------------------------------
a Taken from: Yang et al. (1984).
Selenium intervention has proved to be very effective in the
prophylaxis of Keshan disease and it is very likely that selenium
insufficiency plays an important role in its etiology (Yang et al.,
1984). Nevertheless, the Chinese workers recognized that there
were certain epidemiological characteristics of the disease, which
suggested that additional etiological factors were involved. The
selenium hypothesis, for example, does not adequately explain the
seasonal variation in the disease, the occurrence of epidemic
years, or the annual shifting of epidemic foci. Such
characteristics are more compatible with an infectious theory and,
in fact, a hypothesis that the disease is a form of viral
myocarditis has been put forward (He, 1979). Perhaps the best way
to account for all the characteristics of the disease is to assume
that the disease has a multifactorial etiology and that a
combination of several factors may be involved (Yang et al., 1984).
There are some animal studies that favour such a possibility, since
selenium-deficient mice were less resistant to the cardiotoxic
effects of a Coxsackie B4 virus isolated from a patient with Keshan
disease (Bai et al., 1980). If selenium-deficient human beings are
also less resistant to viral infection, this phenomenon could
provide a reasonable explanation for many of the apparently
conflicting features of Keshan disease. In this view, selenium
deficiency would be the fundamental underlying condition that would
predispose persons to viral attack, possibly by impairing normal
immune function (Yang et al., 1984).
8.2.6.2. Kashin-Beck disease
Kashin-Beck disease is an endemic osteoarthropathy that occurs
in eastern Siberia and in certain parts of China, which is
characterized as a chronic, disabling, and degenerative
osteoarthrosis that mainly involves children (Sokoloff, 1985).
Although the etiology of this disease has not been fully
established, present works show that selenium deficiency might be
one of the main causes. This concept is based on the following
evidence. First, in China most of the endemic areas are located in
the same low-selenium zone, from northeast to southwest, as the
Keshan disease (section 8.2.6.1) (Tan et al., in press). For the
same reason, residents in these areas have low-selenium status
characterized by low blood- and hair-selenium levels, low blood
glutathione peroxidase activity, and low urinary-selenium
excretion. A survey carried out in Heilongjiang province of China
(one of the most heavily affected province) showed that the average
hair-selenium levels in 151 children in endemic areas (0.096 ± SD
0.026 mg/kg) was significantly lower than that of the 235 children
in the non-endemic areas (0.223 ± 0.083 mg/kg) (Wang et al., 1985).
The authors concluded that the low selenium status of children was
due to the low selenium content of the locally produced staple
food. The average selenium content of corn, wheat, and millet in
the endemic and non-endemic areas shown in the paper were: 0.0056
± SD 0.0038 (n = 262) versus 0.015 ± 0.015 (176), 0.0091 ± 0.0096
(225) versus 0.0235 ± 0.022 (120), and 0.0064 ± 0.0053 (14) versus
0.0197 ± 0.0144 (11) mg/kg, respectively. Second, sodium selenite
is reported to have both therapeutic and prophylactic effects on
this disease. Liang (1985) reported that 325 cases of Kashin-Beck
disease in Shaanxi province of China were randomly divided into a
treated and control group. The treated group was given sodium
selenite (1 mg/week for children 3 - 10 years of age and 2 mg/week
for children of 11 - 13 years of age) and the control group was
given a placebo. After one year, X-ray examination of the
metaphyseal changes of fingers showed that 81.9% of the cases in
the treated group had improved, none of the cases were getting
worse, and that 18.1% showed no change, while, in the control
group, only 39.6% of the cases had improved, 30% were getting
worse, and 41.5% showed no change. Li et al. (in press) reported
that children of 1 - 5 and 6 - 10 years of age in an endemic area
of Gansu province in China were supplemented with 0.5 and 1.0 mg
sodium selenite, respectively, per week over a period of 6 years.
X-ray examination showed that the incidence of Kashin-Beck disease
declined from 42% to 4% after the selenium intervention.
However, other information suggests that other factors in the
environment may also play some role in the disease. For example,
the possible role of mycotoxin contamination of cereals by certain
Fusarium strains in China and high phosphate and manganese
contents in the soil, food, and drinking-water in endemic areas of
the USSR have been suggested. More studies are required to clarify
the relationship between these hypotheses and Kashin-Beck disease.
8.2.6.3. Cancer
(a) Ecological studies
Shamberger & Frost (1969) first pointed out the inverse
relationship between selenium levels in forage crops and human
blood, and cancer death rates in various regions of the USA.
Subsequent papers expanded this concept (Shamberger & Willis, 1971;
Shamberger et al., 1976). From a number of comparisons, the
cancers frequently found at one time or another to have high
mortality associated with low-selenium areas or low blood-selenium
levels, included cancer of the tongue, oesophagus, stomach, colon,
rectum, liver, pancreas, larynx, lungs, kidneys, bladder, and
Hodgkin's disease and lymphoma. However, the high mortality of
some of these same cancers and others, was also found to be
associated with high-selenium areas or high blood-selenium levels;
these include cancer of the lung, prostate, pancreas, breast, lip,
skin, eye and dermal melanoma, and leukaemia/aleukaemia. Some of
these studies have been criticized as lacking strength and
consistency, particularly because the states for which cancer
mortality was calculated did not coincide directly with the natural
geographical units on which the estimates of selenium levels in
forage crops were made, thus leading frequently to
misclassification of the different selenium areas (Allaway, 1972,
1978). However, in a study attempting to minimize this problem by
using county data, similar inverse correlations were observed
between counties classified as intermediate or high for selenium
levels in forage, and cancers of the lung, colon, rectum, bladder,
oesophagus, pancreas, and all sites combined, for both males and
females, and cancers of the breast, ovary, and cervix (Clark,
1985).
Schrauzer (1976) found that the mortality rates due to several
cancers, including those of the large intestine, rectum, and breast
(so-called Type A cancers) were directly correlated with the
consumption of meat, eggs, milk, fat, and/or sugar and were
inversely correlated with the consumption of cereals and fish.
Just the opposite correlations were found for certain other cancers
such as hepatic and stomach cancer. Since cereals and seafoods are
good sources of dietary selenium, it was suggested that selenium
might be the factor in these foods that protected against Type A
cancers. Schrauzer et al. (1977) extended these studies to data
from 27 countries including the USA; New Zealand was intentionally
excluded. Dietary intake of selenium was found to be inversely
correlated with total age-adjusted cancer mortality, r = -0.46 ( P <
0.01) for males and r = -0.60 ( P < 0.001) for females. Significant
inverse correlations were observed between dietary-selenium intake
and mortality from cancers of the colon, rectum, prostate, female
breast, ovary, lung (males), and from leukaemia. Weak inverse
relationships were found with mortality from cancers of the
pancreas ( P = 0.06), bladder ( P = 0.1), and skin ( P = 0.1, males).
Other cancers including those of the stomach, oesophagus, and liver
did not show any significant direct or inverse correlations with
dietary-selenium intake. In this study, dietary-selenium intake
was calculated, assuming that the same average concentration of
selenium was present in the foods consumed in all countries (the
Task Group questioned the validity of such an assumption).
Mortality from cancers of the colon, rectum, prostate, lung, skin,
bladder (all Type A), and leukaemia showed significant inverse
correlations with blood-selenium levels in males (USA excluded).
However in the data from the USA, this relationship was not
observed for cancer of the prostate, lung, skin, or bladder, and
leukaemia, and similar inconsistencies were observed for other
cancers and in data for females.
Jansson et al. (1978) postulated an inverse relationship
between dietary-selenium and the rate of colorectal and breast
cancer, but found a direct correlation between the concentration
of selenium in the drinking-water and the rate of colorectal
cancer. Moreover, these workers commented that the same
statistical associations that indicated a protective effect of
dietary selenium against colon, rectum, and breast cancers also
indicated an increased risk of liver and stomach cancer due to
selenium.
In a recent study in China, Yu et al. (1985) obtained a mean
serum-selenium level in the whole blood of 1458 donors from 24
regions, of 107 µg/litre (range 22 - 314 µg/litre). Blood-selenium
levels were inversely correlated with age-adjusted total cancer
mortality for both males and females, r = -0.64 ( P < 0.01) and
r = -0.60 ( P < 0.01), respectively. Analysis by cancer sites
revealed significant negative correlations between blood-selenium
levels and stomach and oesophageal cancers in both sexes. On
reclassifying regions according to low-, moderate-, and high-
selenium areas on the basis of blood levels, significantly lower
total cancer death rates were observed in regions with high
selenium levels and the mortality from cancer of the stomach,
oesophagus, and liver was particularly increased in the low-
selenium areas. In an area where primary liver cancer was very
common, a statistically significant negative correlation between
primary liver cancer incidence and selenium levels in grain was
observed (r = -0.623 for maize, and -0.631 for barley corn). An
inverse correlation between age-adjusted primary liver cancer and
blood-selenium levels of residents in the area was also observed.
The authors concluded that the results indicated that selenium
might play an important role in the etiology of liver cancer, and
that though selenium deficiency was not a cause of primary liver
cancer, low selenium intake apparently reduced the ability of the
body to withstand cancer-causing stress.
It has been pointed out (Levander, 1986) that the age-adjusted
mortality rates for breast cancer and colon cancer reported in
Finland are considerably lower than those reported in the USA,
despite the well-documented lower dietary-selenium intakes in
Finland.
(b) Case-control studies
Shamberger et al. (1973b) reported that blood-selenium levels
in patients with cancer of the colon, pancreas, stomach, and in
Hodgkin's disease and liver metastases were statistically
significantly lower than those in normal controls. However, of 29
patients with rectal cancers, 6 had lower selenium levels than
controls, and 23 had normal levels. Similarly, normal levels were
observed in patients with breast cancer and in patients with other
types of carcinoma.
McConnell et al. (1975), compared blood-selenium levels in 110
patients with carcinomas, 36 patients with primary neoplasm of the
reticuloendothelial system, 28 hospitalized patients with no
malignancy, and 18 non-hospitalized healthy individuals. The mean
selenium concentration for the hospitalized non-malignant patients
was 1.49 ± 0.06 mg/kg and for healthy controls 1.48 ± 0.07 mg/kg.
The mean level of 1.27 ± 0.03 mg/kg for patients with carcinomas
was significantly different from that of the healthy control group
( P = 0.01). The mean serum-selenium level of 1.14 ± 0.08 mg/kg
obtained for gastrointestinal cancer was significantly different
from that of healthy controls ( P < 0.005). In about one-third of
the 110 cancer patients having the lowest selenium levels,
disseminated tumour, recurrences of the primary lesions, the
incidence of multiple primaries, and shortened patient survival
time, were more frequently observed than in the third of the
patients having the highest serum levels ( P > 0.001). The mean
serum-selenium level for the primary malignancies of the
reticuloendothelial system was 1.76 ± 0.24 mg/kg, which though
higher, was not significantly different from that for the healthy
controls.
Broghamer et al. (1976) reported no difference in serum-
selenium levels measured as µg/ml between 110 cancer patients and
controls. However, patients with the lowest serum-selenium levels
had shorter survival, higher incidence of multiple primary
malignancies, higher rate of recurrence of the primary lesion, and
were more likely to have dissemination of cancer than those with
the highest serum-selenium levels. In a study of 59 patients with
primary malignant reticuloendothelial tumours and controls,
Broghamer et al. (1978) found no difference in serum-selenium
levels measured as µg/ml between the two groups. McConnell et al.
(1980) in their study found statistically significant lower levels
of serum-selenium in 35 breast cancer patients compared with a
control group of women free of the disease. On the other hand, van
Rij et al. (1979) and Robinson et al. (1978a) did not find any
differences in the blood-selenium levels of surgical patients with
and without cancer. Although nutritional status, age, and severity
and duration of disease influenced the selenium levels in the
patients studied, low selenium levels were not characteristic for
the cancer patients and it was suggested that the low-selenium
status of cancer patients was more likely a consequence of their
illness rather than the cause of the cancer.
Sundstrom et al. (1984a) found that 44 patients with
gynaecological cancer had lower serum-selenium concentrations (1.15
± 0.04 µmol/litre, P < 0.05) and serum-glutathione peroxidase
activity (404 ± 13 units/litre, P < 0.01) than 56 control subjects
(1.25 ± 0.03 µmol/litre and 444 ± 8 units/litre, respectively). It
was observed that in association with cytotoxic chemotherapy,
selenium alone ( P < 0.05), vitamin E alone ( P < 0.05), and the 2
combined ( P < 0.001) decreased the plasma concentration of lipid
peroxides; the combination of selenium and vitamin E also increased
the activity of serum GSH-Px ( P < 0.01). The authors stated that
during placebo treatment, cytotoxic chemotherapy did not affect
plasma-lipid peroxides but decreased ( P < 0.001) the activity of
GSH-Px. Selenium inhibited this effect. The authors concluded
that this suggested that the anti-oxidative mechanisms of patients
with these types of cancer might be defective and that treatment
with selenium and vitamin E resulted in changes in biochemical
factors related to lipid peroxidation.
In another study, Sundstrom et al. (1984b) reported that
patients with ovarian cancer had significantly lower serum-selenium
concentrations (mean 0.93 ± 0.04 µmol/litre, P < 0.001) than
matched controls (mean 1.22 ± 0.03 µmol/litre). Clinical stage IV
patients had lower levels of selenium (0.82 ± 0.07 µmol/litre) than
clinical stage I and II combined (1.00 ± 0.04 µmol/litre).
Moreover, levels tended to decrease with progressive disease and
increase with remission, probably related to nutrition.
Goodwin et al. (1983) studied blood-selenium levels, and blood
and tissue GSH-Px activity in 50 patients with untreated cancer of
the oral cavity and oropharynx. Mean erythrocyte-selenium and
-glutathione peroxidase were significantly depressed compared with
those in age-matched controls. Mean plasma-selenium, on the other
hand, was significantly elevated in the cancer group. Also,
although not significant, mean erythrocyte-selenium levels tended
to be lower in patients who had never smoked or who had recently
given up smoking. No correlation between dietary selenium as
determined by recall history and plasma- or erythrocyte-selenium
levels in the cancer patients was observed.
Stead et al. (1985) reported significantly lower serum-selenium
concentrations in 20 patients with cystic fibrosis, two of whom had
cancer, than in controls. The two cancer cases had mean serum-
selenium levels of 1.01 µmol/litre and 0.62 µmol/litre,
respectively, compared with 1.41 ± 0.20 µmol/litre in the controls.
Serum-vitamin E levels were also found to be low in patients, but
did not show any correlation with serum-selenium levels.
(c) Case-control studies within prospective studies
As part of the Hypertention Detection Follow-up Programme,
10 940 men and women with diastolic blood pressures of at least 90
mm Hg were identified and enrolled between 1973-74, and followed-up
for 5 years (Willett et al., 1983). Venous blood samples were
collected from all participants at the beginning of the study. A
total of 111 new cases of cancer occurred in the group during the
period of observation. For each case, 2 controls without cancer
were selected who most closely matched the case in age, sex, race,
smoking history, month of blood collection, initial blood pressure,
hypertensive medication, randomisation assignment, and (in women)
parity and menopausal status. Cases and controls were comparable
for most of the confounding factors, although serum-cholesterol and
albumin were slightly lower among cases than controls ( P = 0.05 and
0.06, respectively). Serum-selenium levels did not vary with age
or sex in controls, but black subjects had lower selenium levels
than white. The mean serum-selenium level in cancer cases (0.129 ±
0.002 mg/litre) was significantly lower than that in controls
(0.136 ± 0.002 mg/litre). The P value was 0.02. The increased
risk of cancer in the lowest quintile of baseline selenium was
twice that in the highest quintile (confidence limits 1.1 to 3.3).
Cases were too few to examine by cancer site, but a consistent
trend of lower selenium levels among cases was observed for the
following groups of cancers: lung, breast, prostate,
lymphoma/leukaemia, gastrointestinal cancer, and others. However,
statistically significant differences were observed only for
gastrointestinal cancer. Selenium level remained a significant
predictor of risk, even when the effects of serum-retinol, vitamin
E, and lipid levels, as well as age, sex, and race were taken into
account. On examination of the data according to race, sex, and
smoking status, separately, significant differences in the mean
serum-selenium between cases and controls were observed in blacks
but not whites, in males but not females, and in current smokers,
but not in past-smokers or in persons who had never smoked. The
risk associated with low selenium was greater among those in the
lowest tertile of serum-vitamin E, and a similar inverse
relationship was observed for serum-retinol. A very strong effect
of low selenium (relative risk = 6.2 for lowest versus highest
tertiles) was observed for subjects who had both low serum-vitamin
E and -retinol levels. The authors concluded that, although their
findings supported the overall hypothesis that low selenium intake
increases the risk of cancer, they believed that the observed
differences between cancer sites should be treated as hypotheses to
be tested in other data sets, and that the differences in the
effects of selenium according to age, race, sex, and smoking
status, needed to be examined further (Willett et al., 1983).
Salonen et al. (1984b) identified a cohort of 8113 men and
women in 1972, randomly selected from 2 counties in Finland, who
had no history of cancer in the 12 months preceding this date. The
blood samples were collected from each subject at the beginning of
the study in 1972, and stored at -20 °C. The cohort was followed-
up for cancer occurrence and death up to the end of 1978, during
which time 43 deaths from cancer occurred and an additional 85
persons developed cancer (total cancers = 128). Each cancer case
or cancer death was matched for sex, age, number of cigarettes
smoked and total serum-cholesterol, to a control selected from the
rest of the same population of 8113 persons. In 1983, selenium
concentration was estimated in blood collected from each case and
control at the time of enrolment in the study in 1972 before the
development of cancer. The mean concentration of selenium was
50.5 µg/litre (SD = 12.5) for all 128 cases, and 54.3 µg/litre
(SD = 11.8) for all controls ( P < 0.012). This difference between
cases and control persisted for the following cancer sites:
gastrointestinal, respiratory, and haematological cancers,
miscellaneous cancers, and secondary cancers. No such difference
was observed for skin cancer, skeletal cancers, and urogenital
cancer. Using the lowest (35 µg/litre) and third deciles as cut-
off points, relative risks were computed for the 2 lowest levels of
serum-selenium, with the highest selenium stratum (45 µg/litre) as
the reference. The relative risk of cancer associated with a
serum-selenium level of less than 35 µg/litre was 3.0 and that
associated with a level of 35 - 44 µg/litre was 2.4 (both
statistically significant). The authors concluded that their data
provided additional support for the hypothesis that selenium
deficiency increases the risk of most non-hormone-dependent
cancers in middle-aged persons, though they cautioned that the
number of cancer cases in their study was insufficient to draw
definite conclusions about the effect of selenium for cancers at
specific sites.
Salonen et al. (1985) in a further study of a subgroup of 51
patients, who died from cancer, among the same study population as
above, each matched to a control for age, sex, and smoking,
obtained a mean pre-follow-up serum-selenium concentration in
subjects who died from cancer during the study period of 53.7 ± 1.8
µg/litre and that in controls of 60.9 ± 1.8 µg/litre; the
difference was statistically significant. A statistically
significant difference in the mean serum-selenium concentration
between cases and controls was observed in men ( P = 0.002), but not
in women (half of the male pairs were smokers, but none of the 21
female pairs were smokers). Similarly, the difference in the mean
selenium concentration was significant in smokers ( P = 0.013) but
not significant in non-smokers. Years of smoking showed an inverse
correlation with serum-selenium in the cases (r = -0.30) but not in
controls. Analysis according to cancer site revealed that a
statistically significant difference in serum-selenium levels
between cases and controls was recorded only for respiratory
cancers, though a non-significant difference also existed for
gastrointestinal sites and other cancers. An association between
low serum retinol concentration and increased risk of cancer was
observed only among smoking men. This sex difference was
attributed to smoking as there were no smoking women in the study.
Vitamin E and serum-retinol did not show any association with a
specific cancer site, and although vitamin E had only a weak
independent effect on the risk of cancer, it showed a strong
synergistic relationship with selenium on the risk of fatal cancer.
A serum-selenium concentration of 47 µg/litre or less (the lowest
tertile) was associated with a relative risk of death from cancer
of 5.8 (95% confidence interval 1.2 - 29.0). The authors concluded
that, although their findings indicated that dietary-selenium
deficiency increased the risk of cancer, owing to their study
design, they could not rule out the possibility that the substances
measured in the serum were not truly the protective factors but
were merely indicators of some other compounds or nutrients that
were directly involved in the causal relationship. Furthermore,
the authors recognized that there was need to investigate further
the modification and the confounding of effects by sex, smoking,
and other factors.
8.2.6.4. Heart disease
Using the same ecological approach discussed above for cancer,
Shamberger et al. (1975) concluded that the age-specific death
rates for a number of heart diseases were significantly lower in
the high-selenium regions of the USA than in the low-selenium
regions. However, results of a WHO/IAEA research programme showed
no difference in tissue-selenium concentrations between patients
who died with, or without, myocardial infarction (Masironi & Parr,
1976). Furthermore, Shamberger (1978) found that the kidney-
selenium levels of patients with atherosclerosis and hypertension
did not differ from those of patients with a variety of other
diseases. The blood-selenium levels of patients with acute
myocardial infarction were lower than those of healthy adults, but
there was no difference in heart- or liver-selenium levels of
patients who died from myocardial infarction and those who died
from other diseases (Westermarck, 1977).
A recent case-control study from Finland suggested a possible
association between the serum-selenium level and the risk of death
from acute coronary heart disease as well as the risk of fatal and
non-fatal myocardial infarction (Salonen et al., 1982). The case-
control pairs were derived from 11 000 persons residing in eastern
Finland, an area with a very high incidence of death from
cardiovascular disease. The cases were middle-aged persons who had
died of coronary heart disease or other cardiovascular disease or
suffered a non-fatal myocardial infarction over a 7-year follow-up
period. Attempts were made to control for potential confounding
factors by using controls matched for 6 major coronary heart
disease risk factors: age, sex, serum-cholesterol, diastolic blood
pressure, smoking, and history of angina pectoris, but the cases
had slightly higher blood pressure than the controls. The mean
serum-selenium levels were 51.8 and 55.3 µg/litre for cases and
controls, respectively. A serum-selenium level of less than 45
µg/litre was associated with an increased risk of coronary and
cardiovascular death and myocardial infarction. Although few
necropsies were done, the authors felt that it was unlikely that
the excess cardiovascular mortality observed in their subjects was
due to Keshan disease. The authors cautioned that the apparent
association between low serum-selenium levels and cardiovascular
risk might be spurious since serum-selenium might only be, for
example, a marker for other dietary factors more directly related
to increased coronary heart disease. The authors also emphasized
that, even if their results truly reflected a causal relationship
between low selenium intake and increased ischaemic heart disease,
most such disease is still due to the other well-known risk factors
of elevated cholesterol, high blood pressure, and smoking.
Moreover, it was pointed out that any association between low
serum-selenium levels and ischaemic heart disease is likely to be
of significance only for populations in areas where the dietary
intake of selenium is very low.
In 3 other studies from Finland little or no association was
found between the risk of death from ischemic heart disease and low
selenium status (Miettinen et al., 1983; Salonen et al., 1985;
Virtamo et al., 1985). However, a critique of these studies
(Salonen, 1985) indicated that the first and third were
characterized by low statistical power and in the first the mean
serum-selenium level was relatively high compared with typical
Finnish values (73 µg/litre), probably due to importation of grain
high in selenium during the late 1970s (Mutanen & Koivistoinen,
1983). Salonen (1985) stated that all 4 Finnish studies discussed
above supported the concept of an increased risk of ischemic heart
disease due to low selenium intake as indicated by serum-selenium
levels of less than 60 µg/litre.
In the USA, an inverse correlation was reported between the
plasma-selenium level and the severity of coronaryatherosclerosis
as documented arteriographically (Moore et al., 1984). The mean
plasma-selenium levels of patients with "zero-vessel" disease (no
visible narrowing as much as 50% of any coronary arteriol lumen) or
"three-vessel" disease (as much as a more than 50% narrowing in the
three major coronary arteries or their branches) were 136 ± 7 or
105 ± 4 µg/litre, respectively. On the other hand, neither Ellis
et al. (1984) in the United Kingdom nor Robinson et al. (1983) in
New Zealand were able to demonstrate any correlation between the
traditional risk factors for cardiovascular disease and blood-
selenium levels or glutathione peroxidase activity.
9. EVALUATION OF THE HEALTH RISKS ASSOCIATED WITH EXCESSIVE OR
DEFICIENT SELENIUM EXPOSURE
9.1. The Need to Consider the Essentiality of Selenium in the Health
Risk Evaluation
The data presented in the preceding sections show evidence that
selenium is a functional component of an enzyme (glutathione
peroxidase) in animals and man, and can prevent certain diseases in
animals and responsive conditions in man. The Task Group concluded
that selenium meets the criteria of essentiality for man. As is
true for all essential elements, not only deficient but also
excessive exposure results in adverse health effects.
The effects of selenium deficiency as well as toxicity are well
known in several animal species. In contrast to the effects seen
in animals, health effects in man resulting from deficiency or
excess of selenium are less well defined, but available evidence
has been described. They can occur at low and excessive exposure
levels that may be expected to correspond broadly with those having
deleterious effects on the health of animals. Between these
extremes is a range of safe and adequate exposures (intakes) that
can be defined as free from toxicity and adequate to meet
nutritional requirements. Exposures outside this range increase
the risk of adverse health effects. Therefore, the health risk
evaluation of both selenium deficiency and excess is important.
However, it should be noted that the safe and adequate range may be
modified by certain dietary and other environmental conditions.
The aim of the Task Group was not to evaluate the need for, and
safety of, medication by selenium compounds as a preventive or
therapeutic measure. However, the Task Group felt that some of the
observations presented might be of relevance whenever such a
question might be addressed by any other body responsible for the
appropriate risk-benefit evaluation of administration of selenium
compounds to human beings.
9.2. Pathway of Selenium Exposure for the General Population
Reproducible and accurate methods are available for the
collection of environmental and biological samples and the
measurement of their selenium content. These methods, though they
require special skills and equipment and are time-consuming, have
been employed to assess human selenium exposure. However, the data
are incomplete and there are no data available on selenium exposure
levels for the general population in many countries and regions of
the world.
In spite of these limitations, it can be concluded that, for
the general population, the main source of selenium exposure is
food. In nutritional surveys, extreme mean values for the
calculated selenium intake from food by adult human beings varied
from 11 to 5000 µg/day. However, on the basis of the data
available from most areas, the Task Group concluded that dietary-
selenium intakes usually fall within the range of 20 - 300 µg/day.
Exposure via drinking-water is much less and rarely exceeds a few
µg/day. Limited data on selenium levels in air indicate that about
0.2 µg can be inspired daily by individual members of the general
population.
9.3. Quantitative Assessment of Human Selenium Exposure
9.3.1. Analytical methods for selenium
Several methods of analysis for selenium are available. Those
based on fluorometry, neutron activation analysis, or atomic
absorption spectrometry have been the most thoroughly studied and
used. While simplified and automated procedures have been
developed, all of these methods require skilled analysts to obtain
consistently accurate results. The analysis of NBS Standard
Reference Materials and the exchange of a variety of samples with
another laboratory known to be accomplished in analysis for this
element, should be used to verify the adequacy of results, prior to
any study of human exposure. The method of choice will depend on
the availability of equipment and of the pure chemicals that may be
required, as well as on certain other factors. Where a high degree
of accuracy is required, comparison of results obtained by two or
more different techniques is helpful.
The reliability of the estimate of selenium exposure will
depend, not only on the adequacy of the method, but also on the
adequacy of sampling, storing, subsampling, and preparing the
subsamples for selenium determination. Failure to plan for, and
observe, proper practices in these steps can negate the reliability
of results by even the most highly accurate method of measurement.
9.3.2. Food intake data
Because of the wide variation in the selenium content of foods
in different regions, special techniques must be used to assess
human selenium exposure on the basis of food intake data. The most
accurate method is to determine selenium levels in duplicate diets
(food-on-the-plate-method) made from the same food that is consumed
by the subjects. This method may be suitable for studies in small
subpopulations and where a high degree of accuracy is necessary; it
has the disadvantage of being expensive and time-consuming.
The use of a nutrition survey approach with calculation of
selenium intake from food tables can be used if an important rule
is observed, i.e., the food tables used must be formulated from
data on the selenium contents of the food sources of the population
being studied and of the food as eaten by the subjects under study.
This is important to avoid the errors introduced by the wide
variation in selenium content of a given foodstuff, depending on
its origin.
9.3.3. Blood-selenium
The selenium content of whole blood, serum, or plasma are the
most commonly used measurements of human selenium exposure. Blood
is relatively easy to sample and contamination can be controlled.
Studies on animals have shown that blood-selenium values are a good
indication of selenium deficiency and excess. However, variations
in selenium deficiency signs have been observed in animals with
similar whole blood-selenium levels. This may be due to variations
in the vitamin E content of the diet or exposure to other dietary
or environmental variables. Several factors besides selenium
intake have been identified that may affect blood-selenium content.
Exposure to inorganic mercury or cadmium can lead to deposition of
selenium in the blood attached to protein in combination with the
metal. Human beings exposed to selenium in the form of
selenomethionine or selenium-rich wheat or yeast, for 10 - 11
weeks, had higher blood-selenium levels than human beings exposed
to the same amount of selenium in the form of selenite or selenate.
Blood can be fractionated and plasma-selenium and red blood
cell-selenium can be measured separately. A recent study on the
effects of a low-selenium diet on human beings indicated that a
decrease in plasma-selenium content might occur before a decrease
in red blood cell-selenium can be detected. These data suggest
that it may be possible to use plasma-selenium levels to assess
short-term selenium exposure but that red blood cell- and whole
blood-selenium reflect long-term exposure.
9.3.4. Hair-selenium
Measurement of the selenium content in hair has been used in
animals for the assessment of selenium status, with regard to both
deficiency and excess. It has not been generally adopted for use
in human beings, but, in special circumstances where external
contamination can be excluded, hair-selenium content is useful for
assessing selenium status.
9.3.5. Urine-selenium
The results of animal studies and a few human studies indicate
that the urinary excretion of selenium can be useful for assessing
very recent selenium exposure (i.e., within the past 24 h).
However, determination of selenium in incomplete urine collections
or expression of urinary-selenium per unit volume of urine cannot
provide valid information about selenium exposure in the general
population. An understanding of the reservations associated with
this technique is necessary in its application and then it may only
be useful in conjunction with other measurements discussed in this
section.
9.3.6. Blood-glutathione peroxidase
There is no suitable, simple field method for assessing the
selenium exposure of the general population. The Task Group
concluded that approaches based on the glutathione peroxidase
activity of blood components might provide the basis for a suitable
screening method to detect low human exposure to selenium.
Blood-glutathione peroxidase activity is useful for detecting
selenium deficiency, because it represents a functional form of
selenium and its assay is more rapid than the measurement of
selenium in blood.
However, the Task Group recognized that there are several
difficulties and limitations associated with the determination of
glutathione peroxidase activity. For example, the enzyme is not
stable and hence its activity cannot be determined in samples
stored for periods of time, whereas measurements of selenium can be
made on stored samples. Moreover, large differences in blood-
glutathione peroxidase activity have been reported from different
laboratories using similar methods. The discrepancies can be so
great that interlaboratory comparisons are impossible without
suitable controls. Furthermore, results from New Zealand suggest
that human blood-glutathione peroxidase activity may be useful in
assessing selenium intake at low, but not necessarily at
intermediate or high levels, because the activity of the enzyme is
correlated with whole blood-selenium levels, only when the latter
are less than 0.10 mg/litre. It is not known whether excessive
selenium exposure can increase glutathione peroxidase activity in
human blood, above normal values. Animal studies indicate that
iron deficiency decreases blood glutathione peroxidase activity,
thus possibly further confounding selenium status assessment by
this method.
Human plasma contains glutathione peroxidase but its activity
is very low and difficult to detect and haemolysis during sample
collection should be excluded. Nevertheless, if these reservations
are borne in mind, the technique is promising. Measurement of
platelet-glutathione peroxidase is also a promising technique for
measuring short-term changes in selenium status because of the
short half-life of this blood component.
9.4. Levels of Dietary Selenium Exposure in the General Population
The selenium content of food is highly variable in relation to
several factors. Levels of selenium in soil available for uptake
by plants vary markedly in different locations and this is
reflected in differences in the selenium contents of feeds and
foodstuffs. Countries in which food-stuffs are shipped between
regions tend to avoid extremes in dietary-selenium intake by
averaging foodstuffs with high- and low-selenium contents but some
differences in regional intakes are still observed.
Animal tissues usually do not have as high a selenium content
as the plants in high-selenium areas or as low a selenium content
as plants in low-selenium areas. This is probably because of the
ability of animals to conserve selenium when it is in short supply,
and to excrete it when an excess is present. This may protect
consumers of animal products from extremes of selenium intake.
Available analytical data show that the levels of selenium
typically found in foods, are in the range of 0.4 - 1.5 mg/kg in
liver, kidney, and seafood; 0.1 - 0.4 mg/kg in muscle meats; from
less than 0.1 to over 0.8 mg/kg in cereals or cereal products; less
than 0.1 - 0.3 mg/kg in dairy products; and less than 0.1 mg/kg in
most fruits and vegetables. The very large variation in the
selenium contents of foodstuffs of the same type, depending on
their origin, makes a food table approach to estimating dietary-
selenium intake potentially misleading as discussed in section
9.3.2. More accurate intake estimates could be derived from assay
data on samples of the food items actually being consumed (food-on-
the-plate analysis).
The practice of supplementing the diets of livestock and
poultry in low-selenium areas with selenite or selenate causes
little increase in muscle-selenium content over levels encountered
in animals raised in areas of adequate, but not excessive, selenium
supply. With some exceptions, food processing and preparation
generally do not cause major losses of selenium.
There are few data that indicate the chemical form that
selenium takes in normal human foods. One available study of
seleniferous wheat has shown that a significant fraction of the
selenium is in the form of selenomethionine.
Various forms of selenium differ in their nutritional
availability. The absorption of selenium from foods appears quite
efficient (about 80%).
Daily selenium intake can be markedly influenced by food
consumption patterns. For example, in many countries, consumption
of large amounts of fish, kidney, or liver could raise an
individual's selenium intake substantially above the highest daily
intake shown in Table 8.
The greatest extremes in dietary-selenium intake have been
reported in areas in which the diet was monotonous and consisted
largely of locally-produced staple food.
The wide range of geographically-related selenium intake due to
variations in the selenium contents of the diet is reflected in the
wide range of selenium levels observed in human whole blood.
The extreme mean blood-selenium values reported for groups
dependent on locally-grown foods of different selenium content
ranged from 0.02 - 3.2 mg/litre (Table 11). Limited studies from
New Zealand have shown that children and older individuals had
lower whole blood-selenium levels, but it is not known whether
these lower levels are due to low dietary intakes or to changes
connected with growth and/or aging.
9.5. Evaluation of Health Risks - General Population
9.5.1. Predictive value of animal studies
The Task Group concluded that, in view of the still fragmentary
data concerning the possible health effects of either deficient or
excess selenium exposure on human beings, any evaluation of the
human health effects of selenium exposure must take into account
results arising from animal studies. For this purpose, these can
be summarized as follows:
(a) Selenium deficiency combined with concurrent low
vitamin E status has resulted in deleterious effects
in all animal species tested so far (mice, rats,
chicks, ducks, swine, sheep, cattle, and monkeys).
In rats, specific signs of selenium deficiency have
been produced in animals fed diets adequate in
vitamin E.
(b) Acute and chronic selenium toxicity have been
demonstrated in a wide variety of species, under a
wide variety of conditions (section 7.1.2). In
evaluating the toxic effects of different selenium
compounds, the Task Group felt that it was useful to
distinguish between effects that are strictly
dependent on the selenium in the molecule and cannot
be duplicated by, e.g., homologous compounds of
sulfur, and compounds where the toxicity is, in
principle, similar for homologues containing either
selenium or sulfur. In addition, the Task Group
recognized that the similarity of some of the effects
of different selenium compounds could be related to
the formation of certain common intermediates.
(c) Dose-response relationships have been demonstrated in
acute and chronic toxicity, as well as in selenium
deficiency. Comparative studies have shown that acute
toxicity is similar in parenteral and oral exposure
(section 7.1.2.1), which is in good agreement with the
recognized high absorbability of selenium compounds
from the gastrointestinal tract (section 6.1.1).
(d) As indicated in section 7.1.2.2, the borderline
level of dietary selenium needed to cause growth
depression, due to overt chronic selenium toxicity in
rats, is in the range of 4 - 5 mg selenium/kg diet.
However, the dietary level of selenium needed to
cause chronic toxicity can be influenced by several
environmental factors such as previous selenium
intake (section 7.1.6).
(e) The effects of various environmental factors on
selenium dose-response relationships is even more
dramatically illustrated in situations of low-
selenium intake leading to deficiency. One of the
primary factors influencing the nutritional
requirements of animals for selenium is the vitamin E
status. The minimum dietary level of selenium needed
to prevent deficiency diseases in various animal
species is in the range of 0.02 - 0.05 mg/kg diet.
(f) Animal diseases associated with both high- and
low-selenium intake have been reported in certain
areas of the world in which the animals were
consuming feeds primarily of local origin.
The possibility of a human disease due to selenium deficiency
or excess should be looked for under similar conditions of
restricted dietary consumption (monotonous diets based on locally
grown foods). In addition, the Task Group concluded that, in view
of the dependence of selenium deficiency or toxicity effects on
various environmental factors, the possible involvement of selenium
in human diseases of multifactorial etiopathogenesis deserves
special attention.
9.5.2. Studies on high-exposure effects in the general population
Studies on this type of exposure are few and, as they are
confined to subpopulations in high-selenium areas, some do not
include comparison groups. Symptoms and signs of illness elicited
are frequently mild and not clearly related to selenium. In
practically all studies (section 8.1.1.1), nail pathology was
reported and, in several studies, hair loss and increased dental
decay. Some of the studies included reports of gastrointestinal
disturbances and icteroid skin. Other possible causes of illness
in these studies cannot be excluded and the dependence of severity
of effects on gradation of selenium exposure cannot be evaluated.
Examination of exposed individuals showed increased levels of
selenium in the blood and urine.
Three studies on populations living in seleniferous areas and
dependent on locally-produced food deserve particular attention.
Examination of individuals exposed in these areas revealed highly
increased levels of selenium in the blood and/or urine. A study
from South Dakota reported various signs and symptoms in people
with long-term overexposure to selenium, as revealed by elevated
urinary-selenium excretion. In these areas, farm animals were
affected by chronic selenium poisoning.
In a seleniferous zone of Venezuela (Villa Bruzual) 111
children (average blood-selenium level = 0.813 mg/litre) were
studied and compared with 50 children (blood-selenium level = 0.355
mg/litre) not over-exposed to selenium (Caracas). The children
from the seleniferous area had some loss of hair and some
abnormalities of skin and nails; the authors noted some differences
in socio-economic factors between the 2 groups.
In a recent report from China, past incidents of intoxication
were described that were thought to be due to chronic selenium
poisoning, with hair loss and nail pathology as the most common
signs. No quantitative data are available from the period 1961 -
64, which was the time of peak prevalence of the intoxication.
Recent diet samples collected from high-selenium areas with, and
without, a history of this intoxication showed mean daily selenium
intakes of about 5 and 0.75 mg, respectively (Table 7). Blood
samples taken from the same areas had mean selenium levels of 3.2
and 0.44 mg/litre, respectively (Table 11).
The above studies of populations ingesting high selenium levels
in Venezuela and China have not revealed abnormal serum
transaminase activity in the subjects concerned. In individuals
exposed to high levels of selenium in the recent outbreak in the
USA; there was no evidence of impaired liver function, but loss of
hair and pathological changes in the nails were observed, as well
as some other signs and symptoms described in more detail in
section 8.1.1.1. However, the Task Group recognized that this is
insufficient evidence to exclude the occurrence of liver damage and
recognized the need for more thorough evaluation of the liver in
persons with high selenium exposure.
The Task group noted that the signs and symptoms of selenium
overexposure in human beings were not well defined. However the
Task Group was aware of 3 situations concerning elevated selenium
exposure, which could form the basis for estimating a dose-response
to high levels of selenium. In one area of Enshi county in China,
blood-selenium levels in adults of 0.44 mg/litre were associated
with no reported effects. In Venezuelan children with blood-
selenium levels of 0.813 mg/litre, hair and nail changes of an
unspecified nature and incidence were reported. In another area of
Enshi county in China, definite hair and nail changes and symptoms
and signs consistent with neuropathy were reported in adults with
blood levels of 3.2 mg/litre. The Task Group, however, could not
be certain of how signs and symptoms were searched for, how their
absence was ascertained, and how the control populations were
selected in these studies.
9.5.3. Studies on low-exposure effects in the general population
Very low blood-selenium levels observed in human subjects
(section 8.2.4), approach those observed in animals with selenium
responsive diseases. One woman from a general population known to
have a low exposure to selenium and maintained on total parenteral
nutrition, which provided less than 1 µg selenium/day, had muscular
pain and dysfunction that responded to selenium supplementation.
However, similar symptoms have not been observed in other patients
with similar low blood-selenium levels. Also, in another study, no
muscular symptomatology was reported in children fed exclusively
special medical diets containing very low levels of selenium, for
long periods of time. On the other hand, the Task Group assigned a
high significance to recent reports describing an association
between Keshan Disease (section 9.5.4.1) and poor selenium status
as indicated by low selenium content of grain, low blood and hair
levels of selenium, low blood-glutathione peroxidase activity, as
well as a positive response to an intervention study with sodium
selenite. As discussed in section 9.5.1, the results of several
experimental animal studies indicate that many different factors
may contribute to the development of selenium deficiency diseases.
These studies point to the conclusion that a lack of selenium may
be only one of several causative factors responsible for the
occurrence of certain diseases of complex multiple
etiopathogenesis. Such a conclusion was also fully recognized in
the reports from China on the Keshan Disease.
9.5.4. Evaluation of the involvement of selenium in human diseases
of multiple etiopathogenesis
9.5.4.1. Keshan disease
A suitable animal model of the Keshan disease is not available,
even though heart damage is a feature of combined vitamin E and
selenium deficiency in several species of animals.
The ecological evidence strongly favours a relationship between
low selenium status and the incidence of Keshan disease. Such
evidence includes low blood-, hair-, and urine-selenium levels in
the affected areas as well as a low selenium content in the staple
foods raised and consumed in the affected areas. A randomized
intervention trial carried out in China showed that children who
received sodium selenite had a lower incidence of Keshan disease
than those who received a placebo. However, in this randomized
trial there may have been an underlying trend in the placebo group
towards a decline in the incidence of the disease. Also,
information on the effectiveness of the randomization was not
available and the incidence rates were not adjusted for age. The
results of the much larger but non-randomized intervention trial
involving at least a quarter of a million children on a yearly
basis indicated a clear-cut beneficial effect of sodium selenite in
the prevention of the Keshan disease. Thus, the Task Group
concluded that Keshan disease is a condition in human beings that
is related to low selenium status but that additional research is
needed to clarify the role of other factors in the etiopathogenesis
of the disease.
The Task Group also recognized that in section 8.2.3.1 this
disease was used as the basis for estimating a minimal human
nutritional selenium requirement, suggested by the authors to be 19
and 14 µg/day for men and women, respectively.
9.5.4.2. Kashin-Beck disease
An additional disease found in certain low-selenium areas of
China is an endemic osteoarthropathy known as Kashin-Beck disease
that occurs mainly in children. There is some evidence linking low
selenium status with the incidence of Kashin-Beck disease, but the
Task Group concluded that additional research, particularly in
relation to intervention studies, is required before a definite
statement can be made concerning the role of selenium in the
etiology of the disease.
9.5.4.3. Ischaemic heart disease
The Task Group was aware of studies that examined the
possibility of an association between low selenium status and
myocardial ischaemia and atherosclerosis. However, because of the
limited data available, the Task Group was not prepared to come to
any conclusion regarding the role of selenium in ischaemic heart
disease.
9.5.4.4. Studies on the involvement of selenium in cancer
(a) Carcinogenicity studies
The Task Group concluded that the studies described in section
7.7 suggesting a carcinogenic effect of selenite or selenate were
invalid, because, in one study, the results were statistically
insignificant and, in the other studies, there were no controls or
systematic pathological examinations. In another systematic study,
no differences were observed in the incidence of tumours in rats
surviving 2 years or longer and exposed to 0.5 - 2.0 mg selenium/kg
diet.
The Task Group was aware of one study that demonstrated the
carcinogenicity of ethyl selenac (selenium diethyldithiocarbamate)
and another that showed the carcinogenicity of selenium sulfide,
given by gavage. The latter result is of possible interest both as
regards human dermal exposure to selenium sulfide and/or its
possible formation within the body, but the Task Group was not
aware of any studies demonstrating carcinogenic effects of other
selenium compounds.
The Task Group felt that the evaluation published by IARC in
1975 was still valid in concluding that the available animal data
were insufficient to allow an evaluation of the carcinogenicity of
selenium compounds (IARC, 1975).
(b) Experimental evidence of anticarcinogenic effects of
selenium compounds
The results of studies on laboratory animals provide evidence
of a preventive effect of selenium dioxide, sodium selenite, or
"selenized" yeast, given in the food or drinking-water, against
chemically-induced cancers or certain spontaneous, presumably
viral-induced cancers (section 7.7). Generally, the level required
to demonstrate such effects ranges from 1 - 6 mg/kg food or per
litre drinking-water and thus is considerably in excess of the
animal's nutritional needs. In one report, selenium dioxide at
0.1 mg selenium/litre water had some beneficial effect against
spontaneous mammary tumours in mice, but, in this case, the diet
itself contained 0.45 mg selenium/kg. Other studies have shown the
beneficial effects of sodium selenide when applied concomitantly to
the skin with certain carcinogens. In rats fed diets high in
polyunsaturated fats, selenium deficiency increased the incidence
of mammary tumours, after treatment with dimethylbenz[alpha]
anthracene. Therefore, the Task Group concluded that
pharmacological levels of selenium compounds exhibit in many cases
a favourable influence against the development of cancer in various
animal model systems. However, the Task Group was aware of one
study in which administration of high levels of selenium was
associated with an increased incidence of chemically-induced
cancer.
(c) Epidemiological evidence regarding the possible anti-
carcinogenic effects of selenium in human beings
The Task Group acknowledged that apparent negative correlations
have been drawn between cancer death rates and certain general
population characteristics, such as blood-selenium levels or the
average level of selenium in diets in specific geographical zones
(section 8.2.6.1). However, these apparent correlations have not
been consistent with specific cancer sites and are subject to
ecological fallacy. Moreover, such epidemiological data are
subject to question regarding the adequacy of sampling, the
interpretation of blood-selenium data, and the precision of the
estimates of dietary-selenium intake in various countries and
different geographical areas within the USA.
Of the 7 case-control studies examined by the Task Group, no
consistent association was apparent between low selenium status and
risk of cancer. For a given cancer site, there were few replicate
studies. On the majority of occasions, no association was found
between low selenium status and cancer risk. In fact, in at least
one situation a positive correlation was found. In several
countries, low blood- or plasma-selenium levels were found in
patients with several different types of cancer. However, the
effect of the malignant disease itself in lowering blood-selenium
levels (e.g., by decreasing absorption and/or worsening the
generally debilitated nutritional state of the patient) cannot be
excluded as a partial or total explanation for these results.
The Task Group was aware of 3 studies in which blood samples
had been taken for other purposes prior to the diagnosis of any
cancer. Some time later, the samples were retrieved from storage
banks and analysed for selenium. All 3 studies showed a consistent
inverse association between the prediagnostic serum-selenium level
and the risk of cancer. This association between low serum-
selenium level and cancer risk was observed only in smokers, only
in males, and in one study only in blacks but not in whites. In
one study, the association between low selenium level and cancer
risk showed some site specificity, but the second and third studies
were unable to confirm or rebut this because of inadequate sample
size.
The Task Group was aware that in many studies showing a
protective effect of selenium against cancer in animals
pharmacological doses of selenium compounds were used. Therefore,
there were difficulties in relating the animal and human studies,
but the Task Group was aware of an intervention trial being carried
out in China which it is hoped will enable a more precise
evaluation of the association between selenium status and human
cancer risk in the future.
Thus, the Task Group concluded that existing data are
insufficient to determine whether the level of selenium intake is
indeed correlated with the incidence of cancer in man.
9.5.4.5. Caries
Although an association between high selenium intake and dental
caries has been reported in at least one animal study, there is no
clear-cut evidence of such an association in man. The Task Group
concluded that difficulties associated with the interpretation of
urinary-selenium excretion, expressed as mass-concentration per
volume and not expressed as total daily urinary excretion of
selenium (section 9.3.5), as well as the inability to exclude
interference by other environmental factors such as fluoride,
precluded any significant conclusions. A better understanding of
the impact of selenium on the incidence of caries will require a
more comprehensive estimation of selenium exposure and of the other
confounding factors in the respective subpopulations under study.
9.5.4.6. Health effects related to reproduction
The Task Group concluded that at present there is no evidence
that selenium has significant effects on reproductive function in
man.
9.6. Occupational Exposure
In contrast to general population exposure, occupational
exposure usually occurs through direct contact and/or through
inhalation, i.e., dermal and respiratory exposure predominate.
Exposure to selenium and its compounds occurs in primary
industries, i.e., those that extract, mine, treat, or process
selenium-bearing minerals, e.g., copper, zinc, or lead ores and in
secondary industries, i.e., those that use selenium in
manufacturing processes. The physical and chemical form of
selenium under these circumstances varies and is determined by the
industrial processes. The Task Group recognized that industrial
exposure has not been adequately studied with respect to levels of
exposure and there is a need for such studies to be carried out.
For acute occupational selenium exposures, the effects vary
according to to the chemical form of selenium. In contrast to
elemental selenium, which does not appear to be toxic unless
oxidized or reduced, hydrogen selenide and selenium dioxide are
highly toxic, causing irritation of the respiratory tract, which
may be followed by pulmonary oedema.
The Task Group recognized that evaluation of long-term
occupational exposure to selenium must take into account other
dietary and environmental substances because of their recognized or
potential interactions with selenium. An additional factor that
needs to be considered is the fact that preventive measures usually
result in the removal of exposed workers with the appearance of the
first sign of selenium over-exposure (garlic-like odour).
Monitoring of selenium in the urine is also used to identify those
who should be removed from further overexposure.
The Task Group concluded that no studies were available on the
dose-response relationship with regard to occupational exposure to
selenium.
REFERENCES
ABDULLA, M., KOLAR, K., & SVENSSON, S. (1979) Selenium.
Scand. J. Gastroenterol., 14(suppl. 52): 181-184.
ABDULLAEV, G.M. (1976) [Selenium levels in healthy subjects
and those with certain haematological diseases.] In: Selen v
biologii, Vol. 1, Baku, Elm Publishing House, pp. 136-139 (in
Russian).
ABDULLAEV, G.B., GADZHIEVA, N.A., GASANOV, G.G., DZHAFAROV,
A.I., & PERELYGIN, V.V. (1972) [Selenium and vision,] Baku,
Elm Publishing House, 50 pp (in Russian).
ABDULLAEV, G.B., GADZHIEVA, N.A., GASANOV, G.G., MAMEDOVA,
S.A., DMITRENKO, A.I., GULIEVA, L.I., & RODIONOV, V.P.
(1974a) [The effect of selenium compounds on electroretino-
gram under different experimental conditions.] In: [Selen v
biologii,] Baku, Elm Publishing House, pp. 11-22 (in Russian).
ABDULLAEV, G.B., MAMEDOV, SH.V., DZHAFAROV, A.I., PERELYGIN,
V.V., & MAGOMEDOV, N.M. (1974b) [Possible regulation of free
radicals in the retina by selenium compounds.] In: [Selen v
biologii,] Baku, Elm Publishing House, pp. 54-57 (in Russian).
ABU-ERREISH, G.M. (1967) On the nature of some selenium
losses from soils and waters, Brookings, South Dakota, South
Dakota State University (MS Thesis).
ABU-ERREISH, G.M., WHITEHEAD, E.I., & OLSON, O.E. (1968)
Evolution of volatile selenium from soils. Soil Sci., 106:
415-420.
ABUTALYBOV, M.G., VEZIROVA, N.B., FATALIEVA, C.M., KHALILOV,
E.KH., & MUSAEV, S.G. (1976) [Selenium content in some bean
plants of Azerbajdzhan.] In: [Selen v biologii,] Vol. 2, Baku,
Elm Publishing House, pp. 140-142 (in Russian).
ACGIH (1971) Documentation of the threshold limit values for
substances in workroom air, 3rd ed., Cincinnati, Ohio,
American Conference of Governmental Industrial Hygienists,
pp. 224-225.
AKESSON, B. (1985) Plasma selenium in patients with abnormal
plasma protein patterns. In: Proceedings of the XIII Inter-
national Congress of Nutrition, p. 152 (Abstract).
ALEXANDER, A.R., WHANGER, P.D., & MILLER, L.T. (1983)
Bioavailability to rats of selenium in various tuna and wheat
products. J. Nutr., 113(1): 196-204.
ALCINO, J.F. & KOWALD, J.A. (1973) Analytical methods. In:
Klayman, D.L. & Gunther, W.H.H., ed. Organic selenium
compounds, their chemistry and biology, New York, John Wiley
and Sons, pp. 1049-1081.
ALLAWAY, W.H. (1972) An overview of distribution patterns of
trace elements in soils and plants. Ann. NY Acad. Sci., 199:
17-25.
ALLAWAY, W.H. (1973) Selenium in the food chain. The Cornell
Veterinarian, 63: 151-170.
ALLAWAY, W.H. (1978) Perspectives on trace elements in soil
and human health. In: Hemphill, D.D., ed. Trace substances in
environmental health. XII, Columbia, Missouri, University of
Missouri Press, pp. 3-10.
ALLAWAY, W.H. & CARY, E.E. (1964) Determination of
submicrogram amounts of selenium in biological materials.
Anal. Chem., 36: 1359-1362.
ALLAWAY, W.H., MOORE, D.P., OLDFIELD, J.E., & MUTH, O.H.
(1966) Movement of physiological levels of selenium from
soils through plants to animals. J. Nutr., 88: 411-4l8.
ALLAWAY, W.H., CARY, E.E., & EHLIG, C.F. (1967) The cycling
of low levels of selenium in soils, plants, and animals. In:
Muth, O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 273-296.
ALLAWAY, W.H., KUBOTA, J., LOSEE, F., & ROTH, M. (1968)
Selenium, molybdenum, and vanadium in human blood. Arch.
environ. Health, 16: 342-348.
AMOR, A.J. & PRINGLE, P. (1945) A review of selenium as an
industrial hazard. Bull. Hyg., 20: 239-241.
ANCIZAR-SORDO, J. (1947) Occurrence of selenium in soils and
plants of Columbia, South America. Soil Sci., 63: 437.
ANDERSON, R.A. & POLANSKY, M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Trace Elem. Res., 3: 1-5.
ANDREWS, R.W. & JOHNSON, D.C. (1976) Determination of
selenium (IV) by anodic stripping voltammetry in flow system
with ion exchange separation. Anal. Chem., 48: 1056-1060.
ANONYMOUS (1962) Selenium poisons Indians. Sci. News Lett.,
81: 254.
ANONYMOUS (1975) New links in the selenium cycle. Agric.
Res., 24: 6-7.
ANONYMOUS (1984) Washington DC, US Food and Drug
Administration, pp. 19 (FDA Bulletin No. 14).
ANUNDI, I., STAHL, A., & HOGBERG, J. (1984) Chem.-biol.
Interact., 50: 277.
AOAC (1975) Official methods of analysis, 12th ed.,
Washington DC, Association of Official Analytical Chemists.
ARTHUR, D. (1972) Selenium content of Canadian foods. Can.
Inst. Food Sci. Technol. J., 5: 165-169.
ASHER, C.J., BUTLER, G.W., & PETERSON, P.J. (1977) Selenium
transport in root systems of tomato. J. exp. Bot., 28: 279-291.
AWASTHI, Y.C., BEUTLER, E., & SRIVASTAVA, S.K. (1975)
Purification and properties of human erythrocyte glutathione
peroxidase. J. biol. Chem., 250: 5144-5149.
BACHAREV, V.D., BOCHAROVA, M.A., & SHOSTAK, V.I. (1975) [On
the effect of selenium on the light perception of the eye.]
Fiziol. Zhurn., 61(1): 150-153 (in Russian).
BAI, J., WU, S.Q., GE, K.Y., DENG, X.J., & SU, C.Q. (1980)
The combined effect of selenium deficiency and viral infection
on the myocardium of mice (preliminary study). Acta Acad. Med.
Sinicae, 2: 29-31.
BAIRD, R.B., POURIAN, S., & GABRIELIAN, S.M. (1972) Determination
of trace amounts of selenium in waste waters by carbon rod
atomization. Anal. Chem., 44: 1887-1889.
BARBEZAT, G.O., CASEY, C.E., REASBECK, P.G., ROBINSON, M.F., &
THOMSON, C.D. (1984) Selenium. In: Rosenberg, I. &
Solomons, N.W., ed. Absorption and malabsorption of mineral
nutrients, New York, Alan R. Liss., pp. 231-258.
BARRETTE, M., LAMOUREUX, G., LEBEL, E., LECOMTE, R., PARADIS,
P., & MONARO, S. (1976) Trace element analysis of freeze-
dried blood serum by proton and alpha-induced X-rays. Nucl.
Instrum. Methods, 134: 189-196.
BEARSE, R.C., CLOSE, D.A., MALANIFY, J.J., & UMBARGER, C.J.
(1974) Elemental analysis of whole blood using proton-induced
X-ray emission. Anal. Chem., 46: 499-503.
BEATH, O.A., EPPSON, H.F., & GILBERT, C.S. (1935). Selenium
and other toxic minerals in soils and vegetation, Wyoming
Experimental Station, 55 pp (Bulletin No. 206).
BEHNE, D. & HOFER-BOSSE, T. (1984) Effects of a low selenium
status on the distribution and retention of selenium in the
rat. J. Nutr., 114: 1289-1296.
BEHNE, D. & WOLTERS, W. (1979) Selenium content and gluta-
thione peroxidase activity in the plasma and erythrocytes of
non-pregnant and pregnant women. J. clin. Chem. clin.
Biochem., 17: 133-135.
BEIJE, B., ONFELT, A., & OLSSON, U. (1984) Influence of
dietary selenium on the mutagenic activity of perfusate and
bile from rat liver, perfused with 1,1-dimethylhydrazine.
Mutat. Res., 130: 121-126.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BESBRIS, H.J., WORTZMAN, M.S., & COHEN, A.M. (1982) Effect
of dietary selenium on the metabolism and excretion of
2-acetylaminofluorene in the rat. J. Toxicol. environ.
Health, 9: 63-76.
BHUYAN, K.C., BHUYAN, D.K., & PODOS, S.M. (1981) Selenium-
induced cataract: biochemical mechanism. In: Spallholz, J.E.,
Martin, J.L., & Ganther, H.E., ed. Selenium in biology and
medicine, Westport, Connecticut, Avi Publishing Company,
pp. 403-412.
BIERI, J.G. & AHMAD, K. (1976) Selenium content of
Bangladeshi rice by chemical and biological assay. J. agric.
food Chem., 24: 1073-1074.
BIRT, D.F., JULINS, A.D., & POUR, P.M. (1984) Increased
pancreatic carcinogenesis in Syrian hamsters fed high selenium
diets. Proc. Am. Assoc. Cancer Res., 25: 133.
BLADES, M.W., DALZIEL, J.A., & ELSON, C.M. (1976) Cathodic
stripping voltammetry of nanogram amounts of selenium in
biological material. J. Assoc. Off. Anal. Chem., 59: 1234-1239.
BLOTCKY, A.J., SULLIVAN, J.F., SHUMAN, M.S., WOODWARD, G.P.,
VOORS, A.W., & JOHNSON, W.D. (1976) Selenium levels in liver
and kidney. In: Hemphill, D.D., ed. Trace substances in
environmental health. X, Columbia, Missouri, University of
Missouri Press, pp. 97-103.
BONARD, E.C. & KORALINK, K.D. (1958) Intoxication aiguë par
vapeurs d'hydrogene selenide. Praxis, 47(i): 533-534.
BOUND, G.P. & FORBES, S. (1978) Differential-pulse
polarography of selenium (IV) in the presence of metal ions.
Analyst, 103: 176-179.
BOWEN, H.J.M. & CAWSE, P.A. (1963) The determination of
selenium in biological material by radioactivation. Analyst,
88: 721-726.
BOWEN, W.H. (1972) The effects of selenium and vanadium on
caries activity in monkeys (M. irus). J. Irish Dent. Assoc.,
18: 83-89.
BRADY, P.S., KU, P.K., & ULLREY, D.E. (1978) Lack of effect
of selenium supplementation on the response of the equine
erythrocyte glutathione system and plasma enzymes to exercise.
J. anal. Sci., 47: 492-496.
BRADY, P.S., BRADY, L.J., & ULLREY, D.E. (1979) Selenium,
vitamin E, and the response to swimming stress in the rat. J.
Nutr., 109: 1103-1109.
BRITTON, J.L., SHEARER, T.R., & DE SART, D.J. (1980) Cario-
stasis by moderate doses of selenium in the rat model. Arch.
environ. Health, 35: 74-76.
BRODIE, K.G. (1979) Analysis of arsenic and other trace
elements by vapor generation. Am. Lab. (June issue): 58-66.
BROGHAMER, W.L., MCCONNELL, K.P., & BLOTCKY, A.L. (1976)
Relationship between serum selenium levels and patients with
carcinoma. Cancer, 37: 1384-1388.
BROGHAMER, W.L., MCCONNELL, K.P., GRIMALDI, M., & BLOTCKY,
A.J. (1978) Serum selenium and reticuloendothelial tumours.
Cancer, 41: 1462-1466.
BROWN, D.G. & BURK, R.F. (1973) Selenium retention in
tissues and sperm of rats fed a torula yeast diet. J. Nutr.,
103: 102-108.
BROWN, D.G., BURK, R.F., SEELY, R.J., & KIKER, K.W. (1972)
Effect of dietary selenium on the gastrointestinal absorption
of 75Se03 in the rat. Int. J. Vit. nutr. Res., 42: 588-591.
BROWN, M.W. & WATKINSON, J.H. (1977) An automated
fluorimetric method for the determination of nanogram
quantities of selenium. Anal. Chim. Acta, 89: 29-35.
BRUNE, D., SAMSAHL, K., & WESTER, P.O. (1966) A comparison
between the amounts of As, Au, Br, Cu, Fe, Mo, Se, and Zn in
normal and uraemic human whole blood by means of neutron
activation analysis. Clin. Chim. Acta, 13: 285-291.
BUCHAN, R.F. (1947) Industrial selenosis. Occup. Med., 3:
439-456.
BUNCE, G.E., HESS, J.L., GURLEY, R., BATRA, R., & TARNAWSKA,
E. (1985) Lens energy metabolism and Ca content in selenite
induced cataract. In: Mills, C.F., Bremner, I., & Chesters,
J.K., ed. Trace elements in man and animals - TEMA 5, Slough,
Commonwealth Agricultural Bureaux, pp. 250-254.
BURCHANOV, A.I. (1972) [On regulation of complex chemical
dusts in rare metals production.] Vopr. Gig. Trud. Profzabol.
Kazajenade, Kazah NII Gig. Trud. Profazabol., pp. 72-74 (in
Russian).
BURCHANOV, A.I. & ZHAKENOVA, R.K. (1973) [Pulmonary changes
following intratracheal administration of elementary selenium
to white rats.] Zavavoohr. Kazah., 3: 52-53 (in Russian).
BURCHANOV, A.I., SALEHOV, M.I., DOROFEEVA, O.N., & ZHAKENOVA,
R.K. (1969) [On the effects of metal selenium dust on the
organism.] In: [Proceedings of a Concluding Scientific
Conference on Labour Hygiene and Occupational Disease, 21-22
October, 1969,] Karaganda, Kazah., NII Gig. Trud. Profzabol,
pp. 77-80 (in Russian).
BURK, R.F. (1976) Selenium in man. In: Prasad, A.S., ed.
Trace elements in human health and disease. II. Essential and
toxic elements, New York, Academic Press, pp. 105-133.
BURK, R.F. & CORREIA, M.A. (1977) Accelerated hepatic haem
catabolism in the selenium-deficient rat. Biochem. J., 168:
105-111.
BURK, R.F. & LANE, J.M. (1979) Ethane production and liver
necrosis in rats after administration of drugs and other
chemicals. Toxicol. appl. Pharmacol., 50: 467-478.
BURK, R.F & MASTERS, B.S.S. (1975) Some effects of selenium
deficiency on the hepatic microsomal cytochrome P-450 system
in the rat. Arch. Biochem. Biophys., 170: 124-131.
BURK, R.F., Jr, PEARSON, W.N., WOOD, R.P., II, & VITERI, F.
(1967) Blood selenium levels and in vitro red blood cell
uptake of 75Se in kwashiorkor. Am. J. clin. Nutr., 20: 723-733.
BURK, R.F., BROWN, D.G., SEELY, R.J., & SCAIEF, C.C., III
(1972) Influence of dietary and injected selenium on whole-
body retention, route of excretion, and tissue retention of
75Se03 2- in the rat. J. Nutr., 102: 1049-1055.
BURK, R.F., SEELEY, R.J., & KIKER, K.W. (1973) Selenium:
dietary threshold for urinary excretion in the rat. In:
Proceedings of the Society of Experimental and Biological
Medicine, Vol. 142, pp. 214-216.
BURK, R.F., MACKINNON, A.M., & SIMON, F.R. (1974) Selenium
and hepatic microsomal hemoproteins. Biochem. Biophys. Res.
Commun., 56: 431-436.
BURK, R.F., NISHIKI, K., LAWRENCE, R.A., & CHANCE, B. (1978)
Peroxide removal by selenium-dependent and selenium independent
glutathione peroxidases in hemoglobin-free perfused rat liver.
J. biol. Chem., 253: 43-46.
BURK, R.F., LAWRENCE, R.A., & CORREIA, M.A. (1980a) Sex
differences in biochemical manifestations of selenium
deficiency in rat liver with special reference to heme
metabolism. Biochem. Pharmacol., 29: 39-42.
BURK, R.F., LAWRENCE, R.A., & LANE, J.M. (1980b) Liver
necrosis and lipid peroxidation in the rat as the result of
paraquat and diquat administration. J. clin. Invest., 65:
1024-1031.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1974) Superoxide- and
singlet oxygen-catalyzed lipid peroxidation as a possible
mechanism for paraquat (methyl viologen) toxicity. Biochem.
Biophys. Res. Commun., 58: 749-755.
BUTLER, G.W. & PETERSON, P.J. (1961) Aspects of the faecal
excretion of selenium by sheep. N.Z. J. agric. Res., 4:
484-491.
BUTTNER, W. (1963) Action of trace elements on the
metabolism of fluoride. J. dent. Res., 42: 453-460.
BYARD, J.L. (1969) Trimethyl selenide: a urinary metabolite
of selenite. Arch. Biochem. Biophys., 130: 556-560.
CADELL, P.B. & COUSINS, F.B. (1960) Urinary selenium and
dental caries. Nature (Lond.), 185: 863-864.
CAGEN, S.Z. & GIBSON, J.E. (1977) Liver damage following
paraquat in selenium-deficient and diethyl maleate-pretreated
mice. Toxicol. appl. Pharmacol., 40: 193-200.
CALVIN, H.I. (1978) Selective incorporation of selenium-75
into a polypeptide of the rat sperm tail. J. exp. Zool., 204:
445-452.
CANNELLA, J.M. (1976) Surveillance of employees in selenium
alloying operations. In: Proceedings of the Symposium on
Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 343-348.
CANTOR, A.H. & SCOTT, M.L. (1974) The effect of selenium in
the hen's diet on egg production, hatchability, performance of
progeny, and selenium concentration in eggs. Poultry Sci., 53:
1870-1880.
CANTOR, A.H. & SCOTT, M.L. (1975) Influence of dietary
selenium on tissue selenium levels in turkeys. Poultry Sci.,
54: 262-265.
CANTOR, A.H., SCOTT, M.L., & NOGUCHI, T. (1975a) Biological
availability of selenium in feedstuffs and selenium compounds
for prevention of exudative diathesis in chicks. J. Nutr.,
105: 96-105.
CANTOR, A.H., LANGEVIN, M.L., NOGUCHI, T., & SCOTT, M.L.
(1975b) Efficacy of selenium in selenium compounds and
feedstuffs for prevention of pancreatic fibrosis in chicks. J.
Nutr., 105(1): 106-111.
CANTOR, A.H., MOORHEAD, P.D., & BROWN, K.I. (1978) Influence
of dietary selenium upon reproductive performance of male and
female breeder turkeys. Poultry Sci., 57: 1337-1345.
CAPPON, C.J. & SMITH, J.C. (1982) Chemical form and
distribution of mercury and selenium in canned tuna. J. appl.
Toxicol., 2: 181-189.
CARNRICK, G.R., MANNING, D.C., & SLAVIN, W. (1983)
Determination of selenium in biological materials with
platform furnace atomic absorption spectroscopy and Zeeman
background correction. Analyst, 108: 1297-1312.
CARTER, D.L., ROBBINS, C.W., & BROWN, M.J. (1972) Effect of
phosphorus fertilization on the selenium concentration in
alfalfa (Medicago sativa). Soil Sci. Soc. Am. Proc., 36:
624-628.
CARY, E.E., ALLAWAY, W.H., & MILLER, M. (1973) Utilization
of different forms of dietary selenium. J. anal. Sci., 36:
285-292.
CASEY, C.E., GUTHINE, B.E., FREUD, G.M., & ROBINSON, M.F.
(1982) Selenium in human tissues from New Zealand. Arch.
environ. Health., 37: 133-135.
CAYGILL, C.P.J. & DIPLOCK, A.T. (1973) The dependence on
dietary selenium and vitamin E of oxidant-labile liver
microsomal non-haem iron. FEBS Lett., 33: 172-176.
CHAN, C.C.Y. (1976) Improvement in the fluorimetric
determination of selenium in plant materials with
2,3-diaminonaphthalene. Anal. Chim. Acta, 82: 213-215.
CHANSLER, M.W., MORRIS, V.C., & LEVANDER, O.A. (1983)
Bioavailability to rats of selenium in Brazil nuts and
mushrooms. Fed. Proc., 42(4): 927.
CHATTERJEE, M. & BANERJEE, M.R. (1982) Selenium mediated
dose-inhibition of 7-12-dimethylbenz(alpha)anthracene-induced
transformation of mammary cells in organ culture. Cancer
Lett., 17: 187-195.
CHAU, Y.K., WONG, P.T.S., SILVERBERG, B.A., LUXON, P.L., &
BENGERT, G.A. (1976) Methylation of selenium in the aquatic
environment. Science, 192: 1130-1131.
CHAVEZ, J.F. (1966) Studies on the toxicity of a sample of
Brazil nuts with a high content of selenium. Bol. Soc. Quim.
Peru, 32: 195-203.
CHEN, R.W., WHANGER, P.D., & WESWIG, P.H. (1975) Selenium-
induced redistribution of cadmium binding to tissue proteins:
a possible mechanism of protection against cadmium toxicity.
Bioinorgan. Chem., 4: 125-133.
CHEN, X., YANG, G., CHEN, J., CHEN, X., WEN, Z., & GE, K.
(1980) Studies on the relations of selenium and Keshan
disease. Biol. Trace Elem. Res., 2: 91-107.
CHERRY, D.S. & GUTHRIE, R.K. (1977) Toxic metals in surface
waters from coal ash. Water Resour. Bull., 13: 1227-1236.
CHUNG, A. & MAINES, M.D. (1981) Effect of selenium on
glutathione metabolism. Induction of -glutamylcysteine
synthetase and glutathione reductase in the rat liver.
Biochem. Pharmacol., 30(23): 3217-3223.
CLARK, L.C. (1985) The epidemiology of selenium and cancer.
Fed. Proc., 44(9): 2584-2589.
CLAYCOMB, C.K., SUMMERS, G.W., & JUMP, E.B. (1965) Effect of
dietary selenium on dental caries in Sprague Dawley rats. J.
dent. Res., 44: 826.
CLAYTON, C.C. & BAUMANN, C.A. (1949) Diet and azo dye
tumors: effect of diet during a period when the dye is not
fed. Cancer Res., 9: 575-582.
CLINTON, M., Jr (1947) Selenium fume exposure. J. ind. Hyg.
Toxicol., 29: 225-226.
COMBS, G.F., Jr & SCOTT, M.L. (1975) Polychlorinated
biphenyl-stimulated selenium deficiency in the chick.
Poultry. Sci., 54: 1152-1158.
COMBS, G.F., Jr, CANTOR, A.H., & SCOTT, M.L. (1975) Effects
of dietary polychlorinated biphenyls on vitamin E and selenium
nutrition in the chick. Poultry Sci., 54: 1143-1152.
CONE, J.E., MARTIN DEL RIO, R., DAVIS, J.N., & STADTMAN, T.C.
(1976) Chemical characterization of the selenoprotein
component of clostridial glycine reductase: identification of
selenocysteine as the organoselenium moiety. Proc. Natl Acad.
Sci. (USA), 73: 2659-2663.
COOPER, W.C. (1967) Selenium toxicity in man. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 185-199.
COOPER, W.C. (1974) Analytical chemistry of selenium. In:
Zingaro, R.A. & Cooper, W.C., ed. Selenium, New York, Van
Nostrand Reinhold Co., pp. 615-653.
COOPER, W.C. & GLOVER, J.R. (1974) The toxicology of
selenium and its compounds. III. In: Zingaro, R.A. & Cooper,
W.C., ed. Selenium, New York, Van Nostrand Reinhold Co., pp.
654-674.
COOPER, W.C., BENNETT, K.G., & CROXTON, F.C. (1974) The
history, occurrence, and properties of selenium. In: Zingaro,
R.A. & Cooper, W.C., ed. Selenium, New York, Van Nostrand
Reinhold Co., pp. 1-31.
CORREIA, M.A. & BURK, R.F. (1976) Hepatic heme metabolism in
selenium-deficient rats: effect of phenobarbital. Arch.
Biochem. Biophys., 177: 642-644.
CORREIA, M.A. & BURK, R.F. (1978) Rapid stimulation of
hepatic microsomal heme oxygenase in selenium-deficient rats:
an effect of phenobarbital. J. biol. Chem., 253: 6203-6210.
COWGILL, U.M. (1974) Trace elements and the birth rate in
the continental United States. In: Hemphill, D.D., ed. Trace
substances in environmental health. VIII, Columbia, Missouri,
University of Missouri Press, pp. 15-21.
COWGILL, U.M. (1976) Selenium and human fertility. In:
Proceedings of the Symposium on Selenium and Tellurium in the
Environment, Pittsburg, Pennsylvania, Industrial Health
Foundation, pp. 300-315.
CREGER, C.R., MITCHELL, R.H., ATKINSON, R.L., FERGUSON, T.M.,
REID, B.L., & COUCH, J.R. (1960) Vitamin E activity of
selenium in turkey hatchability. Poultry Sci., 39: 59-63.
CRYSTAL, R.G. (1973) Elemental selenium: structure and
properties. In: Klayman, D.L. & Gunther, W.H.H., ed. Organic
selenium compounds: their chemistry and biology, New York,
John Wiley and Sons, Inc, pp. 13-27.
CSALLANY, A.S., SU, L.-C., & MENKEN, B.Z. (1984) Effect of
selenite, vitamin E, and n,n'-diphenyl-p-phenylenediamine on
liver organic solvent-soluble lipofuscin pigments in mice.
J. Nutr., 114: 1582-1587.
CUKOR, P., WALZCYK, J., & LOTT, P.F. (1964) The application
of isotopic dilution analysis to the fluorimetric
determination of selenium in plant material. Anal. Chim. Acta,
30: 473-482.
CUMMINS, L.M. & KIMURA, E.T. (1971) Safety evaluation of
selenium sulfide antidandruff shampoos. Toxicol. appl.
Pharmacol., 20: 89-96.
CUMMINS, L.M. & MARTIN, J.L. (1967) Are selenocystine and
selenomethionine synthesized in vivo from sodium selenite in
mammals? Biochemistry, 6: 3162-3168.
CZERNIEJEWSKI, C.P., SHANK, C.W., BECHTEL, W.G., & BRADLEY,
W.B. (1964) The minerals of wheat, flour, and bread. Cereal
Chem., 41: 65-72.
D'HONDT, P., LIEVENS, P., VERSIECK, J., & HOSTE, J. (1977)
Determination of trace elements in animal and human muscle by
semi-automated radiochemical neutron activation analysis.
Radiochem. Radioanal. Lett., 31: 231-240.
DAMS, R. & DE JONGE, J. (1976) Chemical composition of Swiss
aerosols from the Jungfraujoch. Atmos. Environ., 10: 1079-1084.
DE JONG, D., MORSE, R.A., GUTENMANN, W.H., & LISK, D.J.
(1977) Selenium in pollen gathered by bees foraging on fly
ash-grown plants. Bull. environ. Contamin. Toxicol., 18:
442-444.
DE WITT, W.B. & SCHWARZ, K. (1958) Multiple dietary necrotic
degeneration in the mouse. Experientia, 14: 28-34.
DICKSON, J.D. (1969) Notes on hair and nail loss after
ingesting Sapucaia nuts (Lecythis elliptica). Econ. Bot., 23:
133-134.
DICKSON, R.C. & TOMLINSON, R.H. (1967) Selenium in blood and
human tissues. Clin. Chim. Acta, 16: 311-321.
DINKEL, C.A., MINYARD, J.A., WHITEHEAD, E.I., & OLSON, O.E.
(1957) Agricultural research at the Reed Ranch substation,
Brookings, South Dakota, South Dakota State College of
Agriculture and Mechanic Arts, 35 pp (Agricultural Experiment
Station Circular No. 135).
DINKEL, C.A., MINYARD, J.A., & RAY, D.E. (1963) Effects of
season of breeding on reproductive and weaning performance of
beef cattle grazing seleniferous range. J. anim. Sci., 22:
1043-1045.
DIPLOCK, A.T. (1974a) A possible role for trace amounts of
selenium and vitamin E in the electron-transfer system of
rat-liver microsomes. In: Hoekstra, W.G., Suttie, J.W.,
Ganther, H.E., & Mertz, W., ed. Trace element metabolism in
animals. II, Baltimore, Maryland, University Park Press, pp.
147-160.
DIPLOCK, A.T. (1974b) Possible stabilizing effect of vitamin
E on microsomal, membrane-bound, selenide-containing proteins
and drug-metabolizing enzyme systems. Am. J. clin. Nutr., 27:
995-1004.
DIPLOCK, A.T. (1976) Metabolic aspects of selenium action
and toxicity. CRC Crit. Rev. Toxicol., 4: 271-329.
DIPLOCK, A.T. (1979) The influence of selenium and vitamin E
on oxidative demethylation reactions. In: Olive, G., ed. Adv.
Pharmacol. Therap., Proceedings of the 7th International
Congress on Pharmacology, Vol. 8, pp. 25-33.
DIPLOCK, A.T., BAUM, H., & LUCY, J.A. (1971) The effect of
vitamin E on the oxidation state of selenium in rat liver.
Biochem. J., 123: 72l-729.
DIPLOCK, A.T., CAYGILL, C.P.J., JEFFERY, E.H., & THOMAS, C.
(1973) The nature of the acid-volatile selenium in the liver
of the male rat. Biochem. J., 134: 283-293.
DONALDSON, W.E. (1977) Selenium inhibition of avian fatty
acid synthetase complex. Chem.-biol. Interac., 17: 313-320.
DONGHERTY, J.J. & HOEKSTRA, W.G. (1982) Stimulation of lipid
peroxidation in vivo by injected selenite and lack of
stimulation by selenate. Proc. Soc. Exp. Biol. Med., 169(2):
209-215.
DORAN, J.W. (1976) Microbial transformations of selenium in
soil and culture, Ithaca, New York, Cornell University
(Ph.D. Thesis).
DOUGLASS, J.S., MORRIS, V.C., SOARES, J.H., Jr, & LEVANDER,
O.A. (1981) Nutritional availability to rats of selenium in
tuna, beef kidney, and wheat. J. Nutr., 111: 2180-2187.
DUCE, R.A., HOFFMAN, G.L., & ZOLLER, W.H. (1975) Atmospheric
trace metals at remote northern and southern hemisphere sites:
pollution or natural? Science, 187: 59-61.
DUDLEY, H.C. (1938) Toxicology of selenium. V. Toxic and
vesicant properties of selenium oxychloride. Public Health
Rep., 53: 94-98.
DUDLEY, H.C. & MILLER, J.W. (194l) Toxicology of selenium.
VI. Effects of subacute exposure to hydrogen selenide. J. ind.
Hyg. Toxicol., 23: 470-477.
DUTKIEWICZ, T., DUTKIEWICZ, B., & BALCERSKA, I. (1972)
Dynamics of organ and tissue distribution of selenium after
intragastric and dermal administration of sodium selenite.
Bromatol. Chem. Toksykol., 4: 475-481.
DUVOIR, M., POLLETT, L., & HERRENSCHMIDT, J.L. (1937) Eczéma
professionnel dû au sélénium. Bull. Soc. Franc. Dermat.
Syphil., 44: 88-95.
DYER, D.G., SCHUETT, V.E., & GANTHER, H.E. (1977) Blood
selenium and glutathione peroxidase in children with PKU on
diet therapy. In: Abstracts of the Western Hemisphere
Nutrition Congress. V, Quebec, pp. 417.
EGAN, A., KERR, S., & MINSKI, M.J. (1977) Instrumental
neutron activation analysis of selenium using 77µSe (T 1/2 =
17s) in biological materials. In: Brown, S.S., ed. Clinical
chemistry and chemical toxicology of metals, Amsterdam,
Elsevier North-Holland Biomedical Press, pp. 353-356.
ELLIS, N., LLOYD, B., LLOYD, R.S., & CLAYTON, B.E. (1984)
Selenium and vitamin E in relation to risk factors for
coronary heart disease. J. Clin. Pathol, 37(2): 200-206.
ENGBERG, R.A. (1973) Selenium in Nebraska's groundwater and
streams, Lincoln, Nebraska, University of Nebraska (Nebraska
Water Survey Paper No. 35).
ERMAKOV, V.V. (1975) [Selenium determination in biological
materials by fluorometry and gas-chromatography.] In:
[Vitamins. VIII. Biochemistry of vitamin E and selenium,]
Kiev, Naukova Dumka Publishing House, pp. 141-146 (in Russian).
ERMAKOV, V.V. & KOVALSKIJ, V.V. (1968) [The geochemical
ecology of organisms at high selenium levels in the
environment.] In: [Transactions of the biogeochemical
laboratory,] Moscow, Nauka Publishing House, Vol. 12, pp.
204-237 (in Russian).
ERMAKOV, V.V. & KOVALSKIJ, V.V. (1974) [The biological
importance of selenium,] Moscow, Nauka Publishing House, 298
pp (in Russian).
ERSHOV, V.P. (1969) [Hygienic aspects of selenium containing
steel production and the prevention of selenium intoxication.]
Gig. Tr. Prof. Zabol., 12: 29-33 (in Russian).
EVANS, C.S., ASHER, C.J., & JOHNSON, C.M. (1968) Isolation
of dimethyldiselenide and other volatile selenium compounds
from Astragalus racemosus (Pursh.). Austral. J. biol. Sci.,
21: 13-20.
EVANS, H.M. & BISHOP, K.S. (1922) On the existence of a
hitherto unrecognized dietary factor essential for
reproduction. Science, 56: 650-651.
EWAN, R.C., POPE, A.L., & BAUMANN, C.A. (1967) Elimination
of fixed selenium by the rat. J. Nutr., 91: 547-554.
EWAN, R.C., BAUMANN, C.A., & POPE, A.L. (1968) Retention of
selenium by growing lambs. J. agric. food Chem., 16: 216-219.
EXON, J.H., KOLLER, L.D., & ELLIOTT, S.C. (1976) Effect of
dietary selenium on tumor induction by an oncogenic virus.
Clin. Toxicol., 9: 273-279.
FALK, R. & LINDHE, J.C. (1974) Radiation dose received by
humans from intravenously-administered sodium selenite marked
with selenium-75, Los Alamos, New Mexico, Los Alamos
Scientific Laboratory (Los Alamos Translation LA-TR-76-5).
FENG, Z., WANG, X., & HAN, C. (1985) [Studies on the
toxicity of diets with different selenium levels in rats.]
Food Hyg. Res., 3(2): 14-20 (in Chinese).
FERRETTI, R.J. & LEVANDER, O.A. (1974) Effect of milling and
processing on the selenium content of grains and cereal
products. J. agric. food Chem., 22: 1049-1051.
FERRETTI, R.J. & LEVANDER, O.A. (1976) Selenium content of
soybean foods. J. agric. food Chem., 24: 54-56.
FILATOVA, V.S. (1948) [Characteristics of selenium as an
industrial intoxicant.] Can. Med. Thesis (in Russian).
FILATOVA, V.S. (1951) [On the toxicity of selenium
chloride.] Gig. i Sanit., 5: 18-23 (in Russian).
FLEMING, G.A. (1962) Selenium in Irish soils and plants.
Soil Sci., 94: 28-35.
FLEMING, G.A. & WALSH, T. (1957) Selenium occurrence in
certain Irish soils and its toxic effects on animals. Proc.
Royal Irish Acad., 58: 151-166.
FLOHE, L., GUNZLER, W.A., & SCHOEKS, H.H. (1973) Glutathione
peroxidase: a selenoenzyme. FEBS Lett., 32: 132-134.
FORBES, S. & BOUND, G.P. (1977) Some investigations into the
electroanalytical chemistry of selenium. Proc. Anal. Div.
Chem. Soc., 14: 253-256.
FORSTROM, J.W., ZAKOWSKI, J.J., & TAPPEL, A.L. (1978)
Identification of the catalytic site of rat liver glutathione
peroxidase as selenocysteine. Biochemistry, 17: 2639-2644.
FRANKE, K.W. & MOXON, A.L. (1936) A comparison of the
minimum fatal doses of selenium, tellurium, arsenic, and
vanadium. J. Pharmacol. exp. Ther., 58: 454-459.
FRANKE, K.W. & POTTER, V.R. (1936) The effect of selenium
containing foodstuffs on growth and reproduction of rats at
various ages. J. Nutr., 12: 205-214.
FRANKE, K.W. & TULLY, W.C. (1935) A new toxicant occurring
naturally in certain samples of plant foodstuffs. V. Low
hatchability due to deformities in chicks. Poultry Sci., 14:
273-279.
FRANKE, K.W., MOXON, A.L., POLEY, W.E., & TULLY, W.C. (1936)
Monstrosities produced by the injection of selenium salts into
hen's eggs. Anat. Rec., 65: 15-22.
FROSETH, J.A., PIPER, R.C., & CARLSON, J.R. (1974)
Relationship of dietary selenium and oral methyl mercury to
blood and tissue selenium and mercury concentrations and
deficiency: toxicity signs in swine. Fed. Proc., 33: 660.
FROST, D.V. & INGVOLDSTAD, D. (1975) Ecological aspects of
selenium and tellurium in human and animal health. Chemica
Scripta, 8A: 96-107.
FURR, A.K., PARKINSON, T.F., HINRICHS, R.A., VAN CAMPEN, D.R.,
BACHE, C.A., GUTENMANN, W.H., ST. JOHN, L.E., Jr, PAKKALA,
I.S., & LISK, D.J. (1977) National survey of elements and
radioactivity in fly ashes. Absorption of elements by cabbage
grown in fly ash-soil mixtures. Environ. Sci. Technol., 11:
1194-1201.
FURR, A.K., PARKINSON, T.F., GUTENMANN, W.H., PAKKALA, I.S., &
LISK, D.J. (1978a) Elemental content of vegetables, grains,
and forages field-grown on fly ash amended soil. J. agric.
food Chem., 26: 357-359.
FURR, A.K., PARKINSON, T.F., HEFFRON, C.L., REID, J.T.,
HASCHEK, W.M., GUTENMANN, W.H., PAKKALA, I.S., & LISK, D.J.
(1978b) Elemental content of tissues of sheep fed rations
containing coal fly ash. J. agric. food Chem., 26: 1271-1274.
GAIROLA, C. & CHOW, C.K. (1982) Dietary selenium, hepatic
arylhydrocarbon hydroxylase, and mutagenic activation of
benzo( a)pyrene, 2-aminoanthracene, and 2-aminofluorene.
Toxicol. Lett., 11: 281-287.
GANAPATHY, S. & DHANDA, R. (1976) Selenium content of
omniverous and vegetarian diets. Fed. Proc., 35: 360.
GANAPATHY, S.N., JOYNER, B.T., & HAFNER, K.M. (1975) Effect
of baking, broiling, and frying on the selenium content of
selected beef, pork, chicken and fish foods. In: Proceedings
of the 10th International Congress of Nutrition - Abstracts,
Kyoto, Japan, 3-9 August, 1975, p. 277.
GANTHER, H.E. (1968) Selenotrisulfides. Formation by the
reaction of thiols with selenious acid. Biochemistry, 7:
2898-2905.
GANTHER, H.E. (1971) Reduction of the selenotrisulfide
derivative of glutathione to a persulfide analog by
glutathione reductase. Biochemistry, 10: 4089-4098.
GANTHER, H.E. (1978) Modification of methylmercury toxicity
and metabolism by selenium and vitamin E: possible mechanisms.
Environ. Health Perspect., 25: 71-76.
GANTHER, H.E. (1979) Metabolism of hydrogen selenide and
methylated selenides. Adv. Nutr. Res., 2: 107-128.
GANTHER, H.E. & BAUMANN, C.A. (1962) Selenium metabolism.
II. Modifying effects of sulfate. J. Nutr., 77: 408-414.
GANTHER, H.E. & CORCORAN, C. (1969) Selenotrisulfides. II.
Cross-linking of reduced pancreatic ribonuclease with
selenium. Biochemistry, 8: 2557-2563.
GANTHER, H.E. & HSIEH, H.S. (1974) Mechanisms for the
conversion of selenite to selenides in mammalian tissues. In:
Hoekstra, W.G., Suttie, J.W., Ganther, H.E., & Mertz, W., ed.
Trace element metabolism in animals. 2, Baltimore, Maryland,
University Park Press, pp. 339-353.
GANTHER, H.E. & SUNDE, M.L. (1974) Effect of tuna fish and
selenium on the toxicity of methylmercury: a progress report.
J. food Sci., 39: 1-5.
GANTHER, H.E., LEVANDER, O.A., & BAUMANN, C.A. (1966)
Dietary control of selenium volatilization in the rat. J.
Nutr., 88: 55-60.
GANTHER, H.E., GOUDIE, C., SUNDE, M.L., KOPECKY, M.J., WAGNER,
P., OH, S., & HOEKSTRA, W.G. (1972) Selenium: relation to
decreased toxicity of methylmercury added to diets containing
tuna. Science, 175: 1122-1124.
GANTHER, H.E., HAFEMAN, D.G., LAWRENCE, R.A., SERFASS, R.E., &
HOEKSTRA, W.G. (1976) Selenium and glutathione peroxidase in
health and disease: a review. In: Prasad, H.H., ed. Trace
elements in human health and disease. II. Essential and toxic
elements, New York, Academic Press pp. 165-234.
GARDINER, M.R., ARMSTRONG, J., FELS, H., & GLENCROSS, R.N.
(1962) A preliminary report on selenium and animal health in
Western Australia. Aust. J. exp. Agric. Animal Husb., 2:
261-269.
GARDNER, S. (1973) Selenium in animal feed. Proposed food
additive regulation. Fed. Reg., 38: 10458-10460.
GE, K., XUE, A., BAI, J., & WANG, S. (1983) Keshan disease -
an endemic cardiomyopathy in China. Virchows Arch. (Pathol.
Anat.), 401: 1-15.
GEERING, H.R., CARY, E.E., JONES, L.H.P., & ALLAWAY, W.H.
(1968) Solubility and redox criteria for the possible forms
of selenium in soils. Soil Sci. Soc. Am. Proc., 32: 35-40.
GIASUDDIN, A.S.M., CAYGILL, C.P.J., DIPLOCK, A.T., & JEFFERY,
E.H. (1975) The dependence on vitamin E and selenium of drug
demethylation in rat liver microsomal fractions. Biochem. J.,
146: 339-350.
GISSEL-NIELSEN, G. (1971) Selenium content of some
fertilizers and their influence on uptake of selenium in
plants. J. agric. food Chem., 19: 564-566.
GISSEL-NIELSEN, G. (1973) Ecological effects of selenium
application to field crops. Ambio, 2: 114-117.
GISSEL-NIELSEN, G. (1976) Selenium in soils and plants. In:
Proceedings of the Symposium on Se-Te in the Environment,
Pittsburgh, Pennsylvania, Industrial Health Foundation,
pp. 10-25.
GISSEL-NIELSEN, G. (1986) Selenium fertilizers and foliar
application, Danish experiments. Ann. clin. Res., 18: 61-64.
GISSEL-NIELSEN, M. & GISSEL-NIELSEN, G. (1975) Selenium in
soil-animal relationships. Pedobiologia, 15: 65-67.
GITSOVA, S. (1973) Presence of selenium in drinking waters
in Bulgaria. Khig. Zdraveopazvane, 16: 557-561.
GLOVER, J.R. (1954) Some medical problems concerning
selenium in industry. Trans. Assoc. Ind. Med. Off., 4: 94-96.
GLOVER, J.R. (1967) Selenium in human urine: a tentative
maximum allowable concentration for industrial and rural
populations. Ann. occup. Hyg., 10: 3-14.
GLOVER, J.R. (1970) Selenium and its industrial toxicology.
Ind. Med. Surg., 39: 50-54.
GLOVER, J.R. (1976) Environmental health aspects of selenium
and tellurium. In: Proceedings of the Symposium on
Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 279-292.
GODWIN, K.O. & FUSS, C.N. (1972) The entry of selenium into
rabbit protein following the administration of Na2 75Se03.
Aust. J. biol. Sci., 25: 865-871.
GOODWIN, W.J., LANE, H.W., BRADFORD, K., MARSHALL, M.V.,
GRIFFIN, A.C., GEOPFERT, H., & JESSE, R.H. (1983) Selenium
and glutathione peroxidase levels in patients with epidermoid
carcinoma of the oral cavity and orophasynx. Cancer, 51:
110-115.
GORTNER, R.A., Jr (1940) Chronic selenium poisoning of rats
as influenced by dietary protein. J. Nutr., 19: 105-112.
GOULDEN, P.D. & BROOKSBANK, P. (1974) Automated atomic
absorption determination of arsenic, antimony, and selenium in
natural waters. Anal. Chem., 46: 1431-1436.
GRACIANSKAJA, L.N. & KOVSHILO, V.E., ed. (1977) [Selenium
and its compounds]. In: [Handbook of occupational pathology,]
Moscow, Medicina Publishing House pp. 330-332 (in Russian).
GRANT, K.E., CONNER, M.W., & NEWBERNE, P.M. (1977) Effect of
dietary sodium selenite upon lesions induced by repeated small
doses of aflatoxin B1. Toxicol. appl. Pharmacol., 41: 166.
GREEN, J., BUNYAN, J., CAWTHORNE, M.A., & DIPLOCK, A.T.
(1969) Vitamin E and hepatotoxic agents. I. Carbon
tetrachloride and lipid peroxidation in the rat. Br. J. Nutr.,
23: 297-307.
GRIFFIN, A.C. & JACOBS, M.M. (1977) Effects of selenium on
azo dye hepatocarcinogenesis. Cancer Lett., 3: 177-181.
GRIFFITHS, N.M. (1973) Dietary intake and urinary excretion
of selenium in some New Zealand women. Proc. Univ. Otago Med.
School, 51: 8-9.
GRIFFITHS, N.M. & THOMSON, C.D. (1974) Selenium in the whole
blood of New Zealand residents. N.Z. med. J., 80: 199-202.
GRIFFITHS, N.M., STEWART, R.D.H., & ROBINSON, M.F. (1976)
The metabolism of 75Se-selenomethionine in four women. Br. J.
Nutr., 35: 373-382.
GRIMANIS, A.P., VASSILAKI-GRIMANI, M., ALEXIOU, D., &
PAPADATOS, C. (1978) Determination of seven trace elements in
human milk, powdered cow's milk, and infant foods by neutron
activation analysis. In: Nuclear activation techniques in the
life sciences, Vienna, International Atomic Energy Agency,
pp. 241-253.
GROCE, A.W., MILLER, E.R., KEAHEY, K.K., ULLREY, D.E., &
ELLIS, D. J. (1971) Selenium supplementation of practical
diets for growing-finishing swine. J. Ann. Sci., 32: 905-911.
GROSS, S. (1976) Hemolytic anemia in premature infants:
Relationship to vitamin E, selenium, glutathione peroxidase,
and erythrocyte lipids. Semin. Hematol., 13: 187-199.
GROSSMAN, A. & WENDEL, A. (1983) Non-reactivity of the
selenoenzyme glutathione peroxidase with enzymatically hydro-
peroxidized phospholipids. Eur. J. Biochem., 135(3): 549-552.
GU, B. (1983) Pathology of Keshan disease: a comprehensive
review. Chin. med. J., 96: 251-261.
GUSEJNOV, T.M., ZHAFAROV, A.I., PERELYGIN, V.V., & KARAEV,
M.A. (1974) [On the localization of endogenous selenium in
structural slements of bull's eye.] In: [Selenium in biology,]
Baku, Elm Publishing House, pp. 47-49.
GUTENMANN, W.H., BACHE, C.A., YOUNGS, W.D., & LISK, D.J.
(1976) Selenium in fly ash. Science, 191: 966-967.
HADDAD, P.R. & SMYTHE, L.E. (1974) A critical evaluation of
fluorometric methods for determination of selenium in plant
materials with 2,3-diaminoaphthalene. Talanta, 21: 859-865.
HADJIMARKOS, D.M. (1956) Geographic variations of dental
caries in Oregon. VII. Caries prevalence among children in the
blue mountains region. J. Pediatr., 48: 195-201.
HADJIMARKOS, D.M. (1960) Urinary selenium and dental caries.
Nature (Lond.), 188: 677.
HADJIMARKOS, D.M. & BONHORST, C.W. (1958) The trace element
selenium and its influence on dental caries susceptibility. J.
Pediatr., 52: 274-278.
HADJIMARKOS, D.M. & BONHORST, C.W. (1961) The selenium
content of eggs, milk, and water in relation to dental caries
in children. J. Pediatr., 59: 256-259.
HADJIMARKOS, D.M. & SHEARER, T.R. (1971) Selenium
concentration in human saliva. Am. J. clin. Nutr., 24: 1210.
HADJIMARKOS, D.M. & STORVICK, C.A. (1950) Geographic
variations of dental caries in Oregon. IV. Am. J. public
Health, 40: 1552-1555.
HADJIMARKOS, D.M., STORVICK, C.A., & REMMERT, L.F. (1952)
Selenium and dental caries. An investigation among school
children of Oregon. J. Pediatr., 40: 451-455.
HAFEMAN, D.G. & HOEKSTRA, W.G. (1977a) Protection against
carbon tetrachloride-induced lipid peroxidation in the rat by
dietary vitamin E, selenium, and methionine as measured by
ethane evolution. J. Nutr., 107: 656-665.
HAFEMAN, D.G. & HOEKSTRA, W.G. (1977b) Lipid peroxidation in
vivo during vitamin E and selenium deficiency in the rat as
monitored by ethane evolution. J. Nutr., 107: 666-672.
HAFEMAN, D.G., SUNDE, R.A., & HOEKSTRA, W.G. (1974) Effect
of dietary selenium on erythrocyte and liver glutathione
peroxidase in the rat. J. Nutr., 104: 580-587.
HAKKARAINEN, J., LINDBERG, P., BENGTSSON, G., JONSSON, L., &
LANNEK, N. (1978) Requirement for selenium (as selenite) and
vitamin E (as alpha-tocopherol) in weaned pigs. III. The effect on
the development of the VESD syndrome of varying selenium
levels in a low-tocopherol diet. J. anal. Sci., 46: 1001-1008.
HALL, R.H., LASKIN, S., FRANK, P., MAYNARD, E.A., & HODGE,
C.H. (1951) Arch. ind. Hyg. occup. Med., 4: 458-464.
HALTER, K. (1938) [Selenium poisoning, especially skin
changes accompanied by secondary porphysia.] Arch. Dermatol.,
178: 340 (in German).
HALVERSON, A.W. (1974) Growth and reproduction with rats fed
selenite-Se. Proc. S. Dak. Acad. Sci., 53: 167-177.
HALVERSON, A.W., HENDRICK, C.M., & OLSON, O.E. (1955) Observations
on the protective effect of linseed oil meal and some extracts
against chronic selenium poisoning in rats. J. Nutr., 56: 51-60.
HALVERSON, A.W., GUSS, P.L., & OLSON, O.E. (1962) Effect of
sulfur salts on selenium poisoning in the rat. J. Nutr., 77:
459-464.
HALVERSON, A.W., PALMER, I.S., & GUSS, P.L. (1966) Toxicity
of selenium to post-weanling rats. Toxicol. appl. Pharmacol.,
9: 477-484.
HAMDY, A.A. & GISSEL-NIELSEN, G. (1976) Volatilization of
selenium from soils. Z. Pflanzenernaehr. Bodenkd., 6: 671-678.
HAMILTON, A. (1927) Industrial poisons in the United States,
Macmillan Company, p. 111.
HAMILTON, A. (1934) Industrial toxicology, New York, Harpers
Publishing Company, p. 111.
HAMILTON, J.W. (1975) Chemical examination of seleniferous
cabbage Brassica oleracea capitata. J. agric. food Chem., 23:
1150-1152.
HARLAND, B.F., PROSKY, L., & VANDERVEEN, J.E. (1978)
Nutritional adequacy of current levels of Ca, Cu, Fe, I, Mg,
Mn, P, Se, and Zn in the American food supply for adults,
infants, and toddlers. In: Kirchgessner, M., ed. Trace element
metabolism in man and animals. 3, Freising- Weihenstephan,
West Germany, Arbeitskreis fur Tierernährungsforschung
Weihenstephan, pp. 311-315.
HARR, J.R. & MUTH, O.H. (1972) Selenium poisoning in
domestic animals and its relationship to man. Clin. Toxicol.,
5: 175-186.
HARR, J.R., BONE, J.F., TINSLEY, I.J., WESWIG, P.H., &
YAMAMOTO, R.S. (1967) Selenium toxicity in rats. II.
Histopathology. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI Publishing Co., Inc, pp. 153-178.
HARR, J.R., EXON, J.H., WHANGER, P.D., & WESWIG, P.H. (1972)
Effect of dietary selenium on N-2-fluorenyl-acetamide
(FAA)-induced cancer in vitamin E-supplemented, selenium
depleted rats. Clin. Toxicol., 5: 187-194.
HARRIS, P.L., LUDWIG, M.I., & SCHWARZ, K. (1958)
Ineffectiveness of Factor 3-active selenium compounds in
resorption-gestation bioassay for vitamin E. Proc. Soc. Exp.
Biol. Med., 97: 686-688.
HARTHOORN, A.M. & YOUNG, E. (1974) A relationship between
acid-base balance and capture myopathy in zebra (Equus
burchelli) and an apparent therapy. Vet. Res., 95: 337-342.
HARTLEY, W.J. (1963) Selenium and ewe fertility. Proc. N.Z.
Soc. Anim. Prod., 23: 20-27.
HARTLEY, W.J. (1967) Levels of selenium in animal tissues
and methods of selenium administration . In: Muth, O.H.,
Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 79-96.
HARTLEY, W.J. & GRANT, A.B. (1961) A review of selenium
responsive diseases of New Zealand livestock. Fed. Proc., 20:
679-688.
HARTLEY, W.J., GRANT, A.B., & DRAKE, C. (1960) Control of
white muscle disease and ill thrift with selenium. N.Z. J.
Agric., 101: 343-345.
HASHIMOTO, Y. & WINCHESTER, J.W. (1967) Selenium in the
atmosphere. Environ. Sci. Technol., 1: 338-340.
HEATH, R.L. (1969-70) Handbook of chemistry and physics,
50th ed., Cleveland, Ohio, The Chemical Rubber Publishing Co.
HE, G. (1979) On the etiology of Keshan disease: two
hypotheses. Chin. med. J., 92: 416-422.
HEINRICH, M., Jr & KELSEY, F.E. (1955) Studies on selenium
metabolism: the distribution of selenium in the tissues of the
mouse. J. Pharmacol. exp. Therap., 114: 28-32.
HELZLSOUER, K., JACOBS, R., & MORRIS, S. (1985) Acute
selenium intoxication in the United States. Fed. Proc., 44(5):
1670.
HICKEY, F. (1968) Selenium in human and animal nutrition.
N.Z. Agric., 18: 1-2.
HICKEY, F. (1977) Human medication with selenium. Including
brief comments on the comparative pathology of White Muscle
Disease in livestock and human muscular dystrophies. In:
Hamilton, N.Z., ed. Trace elements in human and animal health
in New Zealand, Hamilton, New Zealand, Waikato University
Press, pp. 92-99.
HIGGS, D.J., MORRIS, V.C., & LEVANDER, O.A. (1972) Effect of
cooking on selenium content of foods. J. agric. food Chem.,
20: 678-680.
HODDER, A.P.W. & WATKINSON, J.H. (1976) Low selenium levels
in tephra-derived soil - inherent or pedogenetic? N.Z. J.
Sci., 19: 397-400.
HOEKSTRA, W.G. (1975a) Biochemical function of selenium and
its relation to vitamin E. Fed. Proc., 34: 2083-2089.
HOEKSTRA, W.G. (1975b) Glutathione peroxidase activity of
animal tissues as an index of selenium status. In: Hemphill,
D.D., ed. Trace substances in environmental health. IX,
Columbia, Missouri, University of Missouri Press, pp. 331-337.
HOEKSTRA, W.G., HAFEMAN, O., OH, S.H., SUNDER, R.A., & GANTHER,
H.E. (1973) Effect of dietary selenium on liver and erythrocyte
glutathione peroxidase in the rat. Fed. Proc., 32: 885.
HOGGER, D. & BOHM, C. (1944) [On dermal injury produced by
selenite]. Dermatologica, 90: 217-223 (in German).
HOLMBERG, R.E., & FERM, V.H. (1969) Interrelationships of
selenium, cadmium, and arsenic in mammalian teratogenesis.
Arch. environ. Health, 18: 873-877.
HOLSTEIN, E. (1951) [The effects of occupational exposure to
selenium.] Zentrblt. Arbeitsmed. Arbeitschutz, 1: 102-104 (in
German).
HOLYNSKA, B. & MARKOWICZ, A. (1977) Application of energy
dispersive X-ray fluorescence for the determination of
selenium in blood and tissue. Radiochem. radioanal. Lett., 31:
165-170.
HOPKINS, L.L. & MAJAJ, A.S. (1967) Selenium in human
nutrition. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H.,
ed. Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 203-214.
HOVE, E.L. (1948) Interrelation between alpha-tocopherol and
protein metabolism. III. The protective effect of vitamin E
and certain nitrogenous compounds against CCl4 poisoning in
rats. Arch. Biochem., 17: 467-474.
HOWARD, J.H., III (1971) Control of geochemical behavior of
selenium in natural waters by adsorption on hydrous ferric
oxides. In: Hemphill, D.D., ed. Trace substances in
environmental health. V, Columbia, Missouri, University of
Missouri Press, pp. 485-495.
HOWE, M. (1974) Selenium in the blood of south Dakotans.
Arch. environ. Health, 34: 444-448.
HURT, H.D., CARY E.E., & VISEK, W.J. (1971) Growth,
reproduction, and tissue concentrations of selenium in the
selenium-depleted rat. J. Nutr., 101: 761-766.
IARC (1975) Some aziridines, N,-S-, and O-mustards and
selenium, Lyons, International Agency for Research on Cancer,
pp. 245-260 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, Vol. 9).
IHNAT, M. (1974) Collaborative study of a fluorometric
method for determining selenium in foods. J. Assoc. Off. Anal.
Chem., 57: 373-378.
IHNAT, M. (1976) Selenium in foods: evaluation of atomic
absorption spectrometric techniques involving hydrogen
selenide generation and carbon furnace atomization. J. Assoc.
Off. Anal. Chem., 59: 911-922.
IHNAT, M. & MILLER, H.J. (1977a) Analysis of foods for
arsenic and selenium by acid digestion, hydride evolution
atomic absorption spectrophotometry. J. Assoc. Off. Anal.
Chem., 60: 813-825.
IHNAT, M. & MILLER, H.J. (1977b) Acid digestion, hydride
evolution atomic absorption spectrophotometric method for
determining arsenic and selenium in foods: collaborative
study. I. J. Assoc. Off. Anal. Chem., 60: 1414-1433.
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J.
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IP, C. (1985a) Selenium inhibition of chemical
carcinogenesis. Fed. Proc., 44: 2573-2578.
IP, C. (1985b) Attenuation of the anticarcinogenic action of
selenium by vitamin E deficiency. Cancer Lett., 25: 325-331.
IP, C. & SINHA, D.K. (1981) Enhancement of mammary
tumorigenesis by dietary selenium deficiency in rats with a
high polyunsaturated fat intake. Cancer Res., 41: 31-34.
IRGOLIC, K.J. & KUDCHADKER, M.H. (1974) Organic chemistry of
selenium. In: Zingaro, R.A. & Cooper, W.C., ed. Selenium, New
York, Van Nostrand Reinhold Co., pp. 408-545.
IZRAELSON, Z.I., MOGILEVSKAJA, O.Ja., & SUVOROV, S.W. (1973)
[Selenium.] In: [Problems of occupational hygiene and
occupational pathology connected with the work with toxic
metals,] Moscow, Medicina Publishing House pp. 245-257 (in
Russian).
JACOBS, M.M. (1976) Selenium: a possible inhibitor of colon
and rectum cancer. II. Biochemical aspects. In: Proceedings of
the Symposium on Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 329-340.
JACOBS, M.M. (1977) Inhibitory effects of selenium on 1,2-
dimethylhydrazine and methylazoxymethanol colon carcinogenesis.
Cancer, 40: 2557-2564.
JACOBS, M.M. & FORST, C. (1981a) Toxicological effects of
sodium selenite in Sprague-Dawley rats. J. Toxicol. environ.
Health, 8: 575-585.
JACOBS, M.M. & FORST, C. (1981b) Toxicological effects of
sodium selenite in Swiss mice. J. Toxicol. environ. Health, 8:
587-598.
JACOBS, M.M., JANSSON, B., & GRIFFIN, A.C. (1977a) Inhibitory
effects of selenium on 1,2-dimethylhydrazine and methylazoxy-
methanol acetate induction of colon tumours. Cancer Lett., 2:
133-138.
JACOBS, M.M., MATNEY, T.S., & GRIFFIN, A.C. (1977b) Inhibitory
effects of selenium on the mutagenicity of 2-acetylamino-
fluorene (AAF) and AAF derivatives. Cancer Lett., 2: 319-322.
JACOBSSON, S.O., LIDMAN, S., & LINDBERG, P. (1970) Blood
selenium in a beef herd affected with muscular degeneration.
Acta vet. Scand., 11: 324-326.
JAFFE, W.G. (1973) Selenium in food plants and feeds.
Toxicology and nutrition. Qualitas Plantarum. Plant foods hum.
Nutr., 23: 191-204.
JAFFE, W.G. (1976) Effect of selenium intake in humans and
in rats. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 188-193.
JAFFE, W.G. & MONDRAGON, M.C. (1969) Adaptation of rats to
selenium intake. J. Nutr., 97: 431-436.
JAFFE, W.G. & MONDRAGON, C. (1975) Effects of ingestion of
organic selenium in adapted and non-adapted rats. Br. J.
Nutr., 33: 387-397.
JAFFE, W.G. & VELEZ, B.F. (1973) Selenium intake and
congenital malformations in humans. Arch. Latinoam. Nutr., 23:
514-516.
JAFFE, W.G., RUPHAEL, M.D., MONDRAGON, M.C., & CUEVAS, M.A.
(1972a) Clinical and biochemical studies on school children
from a seleniferous zone. Arch. Latinoam. Nutr., 22: 595-611.
JAFFE, W.G., MONDRAGON, M.C., LAYRISSE, M., & OJEDA, A.
(1972b) Toxicity symptoms in rats fed organic selenium. Arch.
Latinoam. Nutr., 22: 467-481.
JANSSON, B., JACOBS, M.M., & GRIFFIN, A.C. (1978)
Gastrointestinal cancer: epidemiology and experimental
studies. In: Schrauzer, G.N., ed. Inorganic and nutritional
aspects of cancer, New York, Plenum Press, pp. 305-322.
JENKINS, K.J. & HIDIROGLOU, M. (1971) Comparative uptake of
selenium by low cystine and high cystine proteins. Can. J.
Biochem., 49: 468-472.
JENSEN, L.S. & MCGINNIS, J. (1960) Influence of selenium,
antioxidants, and type of yeast on vitamin E deficiency in the
adult chicken. J. Nutr., 72: 23-28.
JENSEN, R., CLOSSON, W., & ROTHENBERG, R. (1984) Selenium
intoxication - New York. Mobid. Mortal. Weekly Rep., 33:
157-158.
JOHNSON, C.M. (1976) Selenium in the environment. Res. Rev.,
62: 101-130.
JONES, G.B. & GODWIN, K.O. (1962) Distribution of
radioactive selenium in mice. Nature (Lond.), 196: 1294-1296.
JUDSON, G.J. & OBST, J.M. (1975) Diagnosis and treatment of
selenium inadequacies in the grazing ruminant. In: Nicholas,
D.J.D. & Egan, A.R., ed. Trace elements in soil-plant-animal
systems, New York, Academic Press, Inc, pp. 385-405.
JULIUS, A.D., DAVIES, M.H., & BIRT, D.F. (1980) Relationship
between blood selenium and erythrocyte glutathione peroxidase
activity with excess dietary selenium in Syrian golden
hamsters. Fed. Proc., 39: 555.
KAMADA, T., SHIRAISHI, T., & YAMAMOTO, Y. (1978)
Differential determination of selenium (IV) and selenium (VI)
with sodium diethyldithiocarbamate, ammonium pyrrolidinedi-
thiocarbamate and dithizone by atomic-absorption spectrophoto-
metry with a carbon-tube atomizer. Talanta, 25: 15-19.
KAQUELER, J.C., MALOIGNE, E., & BONIFAY, P. (1977) Effects
of sodium selenate on caries incidence on the rat. J. dent.
Res., D 56 (Special Issue): D151.
KASIMOV, R.Ju., RUSTAMOVA, Sh.A., GUSEJNOV, T.M., & KIAZIMOV,
S.K. (1976) [Dynamics of selenium distribution in some organs
of young fish from selected valuable edible species.] In:
[Selenium in biology,] Baku, Elm Publishing House, Vol. 2, pp.
143-144 (in Russian).
KERDEL-VEGAS, F. (1966) The depilatory and cytotoxic action
of "Coco de Mono" (Lecythis ollaria) and its relationship to
chronic seleniosis. Econ. Bot., 20: 187-195.
KESHAN DISEASE RESEARCH GROUP (1979a) Observations on effect
of sodium selenite in prevention of Keshan disease. Chinese
med. J., 92: 471-476.
KESHAN DISEASE RESEARCH GROUP (1979b) Epidemiologic studies
on the etiologic relationship of selenium and Keshan disease.
Chinese med. J., 92: 477-482.
KETTERER, B., BEALE, D., & MEYER, D. (1982) The structure
and multiple functions of glutathione transferases. Biochem.
Soc. Trans., 10(2): 82-84.
KILNESS, A.W. (1973) Selenium and public health. S.D. J.
Med., 26: 17-19.
KILNESS, A.W. & HOCHBERG, F.H. (1977) Amyotrophic lateral
sclerosis in a high selenium environment. J. Am. Med. Assoc.,
237: 2843-2844.
KIMMERLE, G. (1960) [Comparative studies on the inhalation
toxicity of sulfur-selenium and tellurium hexafluoride.] Arch.
Toxikol., 18: 140-144 (in German).
KINNIGKEIT, G. (1962) [Studies on workers exposed to selenium
in a rectifier plant.] Z. Gesamte Hyg. Grenzgeb., 8: 350-362
(in German).
KLAYMAN, D.L. & GUNTHER, W.H.H. (1973) Organic selenium
compounds: their chemistry and biology, New York, John Wiley
and Sons, Inc, 1188 pp.
KLEIN, A.K. (1943) Report on selenium. J. Assoc. Offic.
Anal. Chem., 26: 346-352.
KLUG, H.L., PETERSEN, D.F., & MOXON, A.L. (1949) The
toxicity of selenium analogues of cystine and methionine.
Proc. S.D. Acad. Sci., 28: 117-120.
KLUG, H.L., MOXON, A.L., PETERSEN, D.F, & POTTER, V.R. (1950)
The in vivo inhibition of succinic dehydrogenase by selenium
and its release by arsenic. Arch. Biochem. Biophys., 28:
253-259.
KOEMAN, J.H., VAN DE VEN, W.S.M., DE GOEIJ, J.J.M., TJIOE,
P.S., & VAN HAAFTEN, J.L. (1975) Mercury and selenium in
marine mammals and birds. Sci. total Environ., 3: 279-287.
KOIVISTOINEN, P., ed. (1980) Mineral elements composition of
Finnish foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni,
Cr F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb, and ash. Acta
agric. Scand., Suppl. 2: 17.
KOIVISTOINEN, P. & HUTTUNEN, J.K. (1985) Selenium deficiency
in Finnish food and nutrition: research strategy and measures.
In: Mills, C.F., Bremner, I., & Chesters, J.K., ed., Trace
elements in man and animals-TEMA 5, Slough, Commonwealth
Agricultural Bureaux, pp. 925-928.
KOIVISTOINEN, P. & HUTTUNEN, J.K. (1986) Selenium in food
and nutrition in Finland. An overview on research and action.
Ann. clin. Res., 18: 13-17.
KOSTA, L., BYRNE, A.R., & ZELENKO, V. (1975) Correlation
between selenium and mercury in man following exposure to
inorganic mercury. Nature (Lond.), 254: 238-239.
KOVALSKIJ, V.V. (1974) [Geochemical ecology,] Moscow, Nauka
Publishing House, 299 pp (in Russian).
KOVALSKIJ, V.V. (1978) [Geochemical ecology: a basis for a
system of biogeochemical regional characterization.] In:
[Biochemical regional characterization: a method for studying
the ecological structure of the biosphere,] Moscow, Nauka
Publishing House, Vol. 15, pp. 3-21 (in Russian).
KOVALSKIJ, V.V. & ERMAKOV, V.V. (1975) [Problems of
experimental geochemical ecology of animals under the
conditions of selenium regions and some approaches to the
study of the biological role of selenium.] In: [Vitamins. VII.
Biochemistry of vitamin E and selenium,] Kiev, Nakove Dunka
Publishing House, pp. 80-87 (in Russian).
KRAUSKOPF, K.B. (1955) Sedimentary deposits of rare
elements. Econ. Geol. Fiftieth Anniversary, (Part I): 411-463.
KRONBORG, O.J. & STEINNES, E. (1975) Simultaneous determination
of arsenic and selenium in soil by neutron-activation analysis.
Analyst, 100: 835-837.
KU, P.K., ELY, W.T., GROCE, A.W., & ULLREY, D.E. (1972)
Natural dietary selenium alpha-tocopherol and effect on tissue
selenium. J. Ann. Sci., 34: 208-211.
KUBOTA, J., ALLAWAY, W.H., CARTER, D.L., CARY, E.E., & LAZAR,
V.A. (1967) Selenium in crops in the United States in
relation to selenium-responsive diseases of animals. J. agric.
food Chem., 15: 448-453.
KUBOTA, J., CARY, E.E., & GISSEL-NIELSEN, G. (1975) Selenium
in rainwater of the United States and Denmark. In: Hemphill,
D.D., ed. Trace substances in environmental health. IX,
Columbia, Missouri, University of Missouri Press, pp. 123-130.
KULIEVA, E.M., PERELIGIN, V.V., & DZHAFAROV, A.I. (1978)
[Isolated retina electroretinogram (ERG) under the conditions
of induced lipoperoxidation.] Dokl. Akad. Nauk. Azarbayidzh
SSR, 34(2): 85-89 (in Russian).
KURLAND, L.T. (1977) Amyotrophic lateral sclerosis and
selenium. J. Am. Med. Assoc., 238: 2365-2366.
LAKIN, H.W. & BYERS, H.G. (194l) Selenium occurrence in
certain soils in the United States, with a discussion of
related topics, sixth report, Washington DC, US Department of
Agriculture, 26 pp (USDA Technical Bulletin No. 783).
LAKIN, H.W. & DAVIDSON, D.F. (1967) The relation of the geo-
chemistry of selenium to its occurrence in soils. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 27-56.
LATHROP, K.A., JOHNSTON, R.E., BLAU, M., & ROTHSCHILD, E.O.
(1972) Radiation dose to humans from 75Se-L-seleno-
methionine. J. Nucl. Med., 13 (suppl. No. 6): 7-30.
LAUER D.J. (1947) Selenium poisoning. A review, with
emphasis on its industrial aspects, Pittsburgh, Pennsylvania,
University of Pittsburgh School of Medicine, pp. 1-47 (Thesis).
LAWRENCE, R.A. & BURK, R.F. (1976) Glutathione peroxidase
activity in selenium-deficient rat liver. Biochem. Biophys.
Res. Commun., 71: 952-958.
LAWRENCE, R.A. & BURK, R.F. (1978) Species, tissue, and
subcellular distribution of non Se-dependent glutathione
peroxidase activity. J. Nutr., 108: 211-215.
LAWRENCE, R.A., PARKHILL, L.K., & BURK, R.F. (1978) Hepatic
cytosolic non selenium-dependent glutathione peroxidase
activity: its nature and the effect of selenium deficiency. J.
Nutr., 108: 981-987.
LAWSON, T. & BIRT, D.F. (1983) Enhancement of the repair of
carcinogen-induced DNA damage in the hamster pancreas by
dietary selenium. Chem. Biol. Interact., 45: 95-104.
LAZAREV, N.V. (1977) [ Harmful compounds in the industry,]
7th ed., Leningrad, Chimija, Vol. 3, pp. 74-82 (in Russian).
LAZAREV, N.V. & GADASKINA, I.D., ed. (1977) [Selenium and
its compounds.] In: [Noxious substances in industry,] Lenin-
grad, Chimija Publishing House, pp. 75-82 (in Russian).
LEEB, J., BAUMGARTNER, W.A., LYONS, K., & LORBER, A. (1977)
Uptake, distribution, and excretion of sodium selenite in
rheumatoid subjects. In: Biological implications of metals in
the environment, Technical Information Center, Energy Research
and Development Administration, pp. 536-546.
LEMLEY, R.E. (1940) Selenium poisoning in the human. Lancet,
60: 528-531.
LEMLEY, R.E. (1943) Observations on selenium poisoning in
South and North America. Lancet, 63: 257-258.
LEMLEY, R.E. & MERRYMAN, M.P. (194l) Selenium poisoning in
the human. Lancet, 61: 435-438.
LEVANDER, O.A. (1976a) Selected aspects of the comparative
metabolism and biochemistry of selenium and sulfur. In:
Prasad, A.S., ed. Trace elements in human health and disease,
Vol. 2, Essential and toxic elements, New York, Academic
Press, pp. 135-163.
LEVANDER, O.A. (1976b) Selenium in foods. In: Proceedings of
the Symposium on Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 26-53.
LEVANDER, O.A. (1977) Metabolic interrelationships between
arsenic and selenium. Environ. Health Perspect., 19: 159-164.
LEVANDER, O.A. (1979) Lead toxicity and nutritional
deficiencies. Environ. Health Perspect., 29: 115-125.
LEVANDER, O.A. (1982) Selenium: biochemical actions, inter-
actions, and some human health implications. In: Clinical,
biochemical, and nutritional aspects of trace elements, pp.
345-368.
LEVANDER, O.A. (1983) Consideration in the design of
selenium bioavailability studies. Fed. Proc., 42: 1721-1725.
LEVANDER, O.A. (1986) Human and animal nutrition. In: Mertz,
M., ed. Trace elements, Vol. 2, New York, Academic Press, pp.
209-279.
LEVANDER, O.A. & BAUMANN, C.A. (1966) Selenium metabolism.
VI. Effect of arsenic on the excretion of selenium in the
bile. Toxicol. appl. Pharmacol., 9: 106-115.
LEVANDER, O.A. & MORRIS, V.C. (1970) Interactions of
methionine, vitamin E, and antioxidants in selenium toxicity
in the rat. J. Nutr., 100: 1111-1118.
LEVANDER, O.A. & MORRIS (1984) Dietary selenium levels
needed to maintain balance in North American adults consuming
self-selected diets. Am. J. clin. Nutr., 39: 809-815.
LEVANDER, O.A., YOUNG, M.L., & MEEKS, S.A. (1970) Studies on
the binding of selenium by liver homogenates from rats fed
diets containing either casein or casein plus linseed oil
meal. Toxicol. appl. Pharmacol., 16: 79-87.
LEVANDER, O.A., MORRIS, V.C., & HIGGS, D.J. (1973a)
Acceleration of thiol-induced swelling of rat liver
mitochondria by selenium. Biochemistry, 12: 4586-4590.
LEVANDER, O.A., MORRIS, V.C., & HIGGS, D.J. (1973b) Selenium
as a catalyst for the reduction of cytochrome c by glutathione.
Biochemistry, 12: 4591-4595.
LEVANDER, O.A., WELSH, S.O., & MORRIS, V.C. (1980) Erythrocyte
deformability as affected by vitamin E deficiency and lead
toxicity. Ann. N.Y. Acad. Sci., 355: 227.
LEVANDER, O.A., SUTHERLAND, B., MORRIS, V.C., & KING, J.C.
(1981a) Selenium metabolism in human nutrition. In: Spallhoz,
J.E., Martin, J.L., & Ganther, H.E., ed. Selenium in biology
and medicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 256-268.
LEVANDER, O.A., SUTHERLAND, B., MORRIS, V.C., & KING, J.C.
(1981b) Selenium balance young men during selenium depletion
and repletion. Am. J. clin. Nutr., 34: 2662-2669.
LEVANDER, O.A., ALFTHAN, G., ARVILOMMI, H., GREF, C.G.,
HUTTUNEN, J.K., KATAJA, M., KOIVISTOINEN, P., & PIKKARAINEN,
J. (1983) Bioavailability of selenium to Finnish men as
assessed by platelet glutathione peroxidase activity and other
blood parameters. Am. J. clin. Nutr., 37: 887-897.
LEVINE, R.J. & OLSON, R.E. (1970) Blood selenium in Thai
children with protein-calorie malnutrition. Proc. Soc. Exp.
Biol. Med., 134: 1030-1034.
LEWIS, B.G., JOHNSON, C.M., & BROYER, T.C. (1974) Volatile
selenium in higher plants: the production of dimethyl selenide
in cabbage leaves by enzymatic cleavage of Se-methyl seleno-
methionine selenonium salt. Plant Soil, 40: 107-118.
LI, C., HUANG, J., & LI, C. (in press) Observational report
on the effects of taking sodium selenite for six years
continuously as a preventive for Kashin-Beck disease as shown
in X-ray studies. In: Proceedings of the Third International
Symposium on Selenium in Biology and Medicine.
LIANG, S. (1985) The prophylactic and curing effect of
selenium (Se) in combatting of the Kaschin-Beck's disease.
LIEBSCHER, K. & SMITH, H. (1968) Essential and non-essential
trace elements. A method of determining whether an element is
essential or nonessential in human tissue. Arch. environ.
Health, 17: 881-890.
LINDBERG, P. (1968) Selenium determination in plant and
animal material, and in water. A methodological study. Acta
vet. Scand. (suppl. 23): 48 pp.
LINDBERG, P. & JACOBSSON, S.O. (1970) Relationship between
selenium content of forage, blood, and organs of sheep, and
lamb mortality rate. Acta vet. Scand., 11: 49-58.
LIPINSKIJ, S. (1962) [Background for evaluation of selenium
as an industrial toxicant.] Gig. i Sanit., 1: 91-93 (in
Russian).
LO, L.W., KOROPATNICK, J., & STICH, H.F. (1978) The
mutagenicity and cytotoxicity of selenite, "activated"
selenite, and selenate for normal and DNA repair-deficient
human fibroblasts. Mutat. Res., 49: 305-312.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LOMBECK, I., KASPEREK, K., FEINENDEGEN, L.E., & BREMER, H.J.
(1975) Serum-selenium concentrations in patients with
maple-syrup-urine disease and phenylketonuria under
dieto-therapy. Clin. Chim. Acta, 64: 57-61.
LOMBECK, I., KASPEREK, K., HARBISCH, H.D., BECKER, K.,
SCHUMANN, E., SCHROTER, W., FEINENDEGEN, L.E., & BREMER, H.J.
(1978) The selenium state of children. II. Selenium content
of serum, whole blood, hair and the activity of erythrocyte
glutathione peroxidase in dietetically treated patients with
phenylketonuria and maple-syrup-urine disease. Eur. J.
Pediatr., 128: 213-223.
LUO, X., WEI, H., YANG, C., XING, J., LIU, X., QIAO, C., FENG,
Y., LIU, J., LIU, Y., WU, Q., LIU, X., GUO, J., STOECKER,
B.J., SPALLHOLTZ, J.E., & YANG, S.P. (1985) Bioavailability
of selenium to residents in a low-selenium area of China. Am.
J. clin. Nutr., 42: 439-448.
MAAG, D.D. & GLENN, M.W. (1967) Toxicity of selenium: farm
animals. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H., ed.
Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 127-140.
MAAG, D.D., ORSBORN, J.S., & CLOPTON, J.R. (1960) The effect
of sodium selenite on cattle. Am. J. vet. Res., 21: 1049-l053.
MCCABE, L.J., SYMONS, J.M., LEE, R.D., & ROBECK, G.G. (1970)
Survey of community water supply systems. J. Am. Water Works
Assoc., 62: 670-687.
MCCONNELL, K.P. & CHO, G.J. (1965) Transmucosal movement of
selenium. Am. J. Physiol., 208: 1191-1195.
MCCONNELL, K.P. & PORTMAN, O.W. (1952a). Excretion of
dimethyl selenide by the rat. J. Biol. Chem., 195: 277-282.
MCCONNELL, K.P. & PORTMAN, O.W. (1952b) Toxicity of dimethyl
selenide in the rat and mouse. Proc. Soc. Exp. Biol. Med., 79:
230-231.
MCCONNELL, K.P., BROGHAMER, W.L., Jr, BLOTCKY, A.J., & HURT,
O.J. (1975) Selenium levels in human blood and tissues in
health and in disease. J. Nutr., 105: 1026-1031.
MCCONNELL, K.P., JAGER, R.M., BLAND, K.I., & BLOCTCKY, A.J.
(1980) The relationship of dietary selenium and breast
cancer. J. surg. Oncol., 15: 67-70.
MCCOY, K.E.M. & WESWIG, P.H. (1969) Some selenium responses
in the rat not related to vitamin E. J. Nutr., 98: 383-389.
MCDANIEL, M., SHENDRIKAR, A.D., REISZNER, K.D., & WEST, P.W.
(1976) Concentration and determination of selenium from
environmental samples. Anal. Chem., 48: 2240-2243.
MCDOWELL, L.R., FROSETH, J.A., & PIPER, R.C. (1978)
Influence of arsenic, sulfur, cadmium, tellurium, silver, and
selenium on the selenium-vitamin E deficiency in the pig.
Nutr. Rep. Int., 17: 19-33.
MCKEEHAN, W.L., HAMILTON, W.G., & HAM, R.G. (1976) Selenium
is an essential trace nutrient for growth of WI-38 diploid
human fibroblasts. Proc. Natl Acad. Sci. (USA), 73: 2023-2027.
MCKENZIE, J.M. (1977) Trace elements in total parenteral
nutrition in New Zealand. In: Trace elements in human and
animal health in New Zealand, Hamilton, New Zealand, Waikato
University Press, pp. 59-69.
MCKENZIE, R.L., REA, H.M., THOMSON, C.D., & ROBINSON, M.F.
(1978) Selenium concentration and glutathione peroxidase
activity in blood of New Zealand infants and children. Am. J.
clin. Nutr., 3l: l4l3-l4l8.
MACKINNON, A.M. & SIMON, F.R. (1976) Impaired hepatic heme
synthesis in the phenobarbital-stimulated selenium-deficient
rat. Proc. Soc. Exp. Biol. Med., 152: 568-572.
MCLEAN, A.E.M. (1967) The effect of diet and vitamin E on
liver injury due to carbon tetrachloride. Br. J. exp. Path.,
48: 632-636.
MAINES, M.D. & KAPPAS, A. (1976) Selenium regulation of
hepatic heme metabolism: induction of sigma-aminolevulinate
synthase and heme oxygenase. Proc. Natl Acad. Sci.(USA), 73:
4428-443l.
MAJSTRUK, P.N. & SUCHKOV, B.P. (1978) [Sanitary hygienic
control of selenium levels in the main food commodities and
the daily intake of selenium,] Kiev, Kievskij, NII gig. Pit.,
4 pp (in Russian).
MARSHALL, M.V., JACOBS, M.M., & GRIFFIN, A.C. (1978)
Reduction in acetylaminofluorene (AAF) hepatocarcinogenesis by
selenium. Proc. Am. Assoc. Cancer Res., l9: 75.
MARTIN, J.L. & GERLACH, M.L. (1969) Separate elution by
ion-exchange chromatography of some biologically important
selenoamino acids. Anal. Biochem., 29: 257-264.
MARTIN, J.L. & HURLBUT, J.A. (1976) Tissue selenium levels
and growth responses of mice fed selenomethionine,
Se-methylselenocysteine, or sodium selenite. Phosphorus
Sulfur, 1: 295-300.
MARTIN, J.L. & SPALLHOLZ, J.S. (1976) Selenium in the immune
response. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburg, Pennsylvania,
Industrial Health Foundation, pp. 204-225.
MARTIN, S.E., ADAMS, G.H., SCHILLACI, M., & MILNER, J.A.
(1981) Antimutagenic effect of selenium on acridine orange
and 7,12-dimethylbenz(alpha)anthracene in the Ames Salmonella
microsomal system. Mutat. Res., 82: 41-46.
MASIRONI, R. & PARR, R. (1976) Selenium and cardiovascular
diseases: preliminary results of the WHO/IAEA joint research
programme. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 3l6-325.
MAXIA, V., MELONI, S., ROLLIER, M.A., BRANDONE, A.,
PATWARDHAN, V.N., WASLIEN, C.I., & SHAMI, S.E. (1972)
Selenium and chromium assay in Egyptian foods and in blood of
Egyptian children by activation analysis. In: Nuclear
activation techniques in the life sciences, Vienna,
International Atomic Energy Agency, pp. 527-550.
MEDINA, D. & SHEPHERD, F. (1980) Selenium-mediated
inhibition of mouse mammary tumorigenesis. Cancer Lett., 8:
241-245.
MEDINSKY, M.A., CUDDIHY, R.G., GRIFFITH, W.C., & MCCLELLAN,
R.O. (1981) A simulation model describing the metabolism of
inhaled and ingested selenium compounds. Toxicol. appl.
Pharmacol., 59: 54-63.
MEHLERT, A. & DIPLOCK, A.T. (1985) The glutathione-S-
transferases in selenium and vitamin E deficiency. Biochem.
J., 227: 823-831.
MICHIE, N.D., DIXON, E.J., & BUNTON, N.G. (1978) Critical
review of AOAC fluorometric method for determining selenium in
foods. J. Assoc. Off. Anal. Chem., 61: 48-51.
MIETTINEN, T.A., ALFTHAN, G., HUTTUNEN, J.K., PIKKARAINEN, J.,
NAUKKARINEN, V., MATTILA, S., & KUMLIN, T. (1983) Serum
selenium concentration related to myocardial infarction and
fatty acid content of serum lipids. Brit. med. J., 287:
517-519.
MILKS, M.M., WILT, S.R., ALI, I.I., & COURI, D. (1985) The
effects of selenium on the emergence of aflatoxin B1-induced
enzyme-altered foci in rat liver. Fundam. appl. Toxicol., 5:
320-326.
MILLAR, K.R. & SHEPPARD, A.D. (1972) alpha-Tocopherol and
selenium levels in human and cow's milk. N.Z. J. Sci., 15:
3-15.
MILLAR, K.R., GARDINER, M.A., & SHEPPARD, A.D. (1973) A
comparison of the metabolism of intravenously injected sodium
selenite, sodium selenate, and selenomethionine in rats. N.Z.
J. agric. Res., 16: 115-127.
MILLER, D., SOARES, J.H., Jr, BAUERSFELD, P., Jr, & CUPPETT,
S.L. (1972) Comparative selenium retention by chicks fed
sodium selenite, selenomethionine, fish meal, and fish
solubles. Poultry Sci., 51: 1669-1673.
MILNER, J.A. (1985) Effect of selenium on virally induced
and transplantable tumour models. Fed. Proc., 44: 2568-2572.
MONAENKOVA, A.M. & GLOTOVA, K.V. (1963) [Selenium
intoxication.] Gig. i Sanit., 6: 41-44 (in Russian).
MONDRAGON, M.C. & JAFFE, W.G. (1976) Ingestion of selenium
in Caracas, compared with some other cities. Arch. Latinoam.
Nutr., 26: 34l-352.
MONEY, D.F.L. (1970) Vitamin E and selenium deficiencies and
their possible aetiological role in the sudden death in
infants syndrome. N.Z. med. J., 7l: 32-34.
MONEY, D.F.L. (1978) Vitamin E, selenium, iron and vitamin A
content of livers from sudden infant death syndrome and
control children: interrelationships and possible
significance. N.Z. J. Sci Teel., 21: 41-55.
MOORE, J.A., NOIVA, R., & WELLS, I.C. (1984) Selenium
concentrations in plasma of patients with arteriographically
defined coronary atherosclerosis. Clin. Chem., 30(7):
1171-1173.
MORIYA, M., OHTA, T., WATANABE, K., WATANABE, Y., SUGIYAMA,
F., MIYAZAWA, T., & SHIRASU, Y. (1979) Inhibitors for the
mutagenicities of colon carcinogens, 1,2-dimethylhydrazine and
azoxymethane, in the host-mediated assay. Cancer Lett., 7:
325-330.
MORRIS, V.C. & LEVANDER, O.A. (1970) Selenium content of
foods. J. Nutr., 100: 1383-1388.
MOTSENBOCKER, M.A. & TAPPEL, A.L. (1982) A
selenocysteine-containing selenium-transport protein in rat
plasma. Biochim. Biophys. Acta, 719: 147-153.
MOXON, A.L. (1938) The effect of arsenic on the toxicity of
seleniferous grains. Science, 88: 81.
MOXON, A.L. (1940) Toxicity of selenium-cystine and some
other organic selenium compounds. J. Am. Pharm. Assoc. Sci.
Ed., 29: 249-250.
MOXON, A.L. (1976) Natural occurrence of selenium. In:
Proceedings of the Symposium on Selenium-Tellurium in the
Environment, Pittsburgh, Pennsylvania, Industrial Health
Foundation, pp. 1-9.
MOXON, A.L. & RHIAN, M. (1943) Selenium poisoning. Physiol.
Rev., 23: 305-337.
MOXON, A.L., ANDERSON, H.D., & PAINTER, E.P. (1938) The
toxicity of some organic selenium compounds. J. Pharmacol.
exp. Ther., 63: 357-368.
MOXON, A.L., OLSON, O.E., & SEARIGHT, W.V. (1939) Selenium
in rocks, soils, and plants. S. Dak. Agric. Exp. Sta. Tech.
Bull., 2: 94 pp.
MOXON, A.L., SCHAEFER, A.E., LARDY, H.A., DUBOIS, K.P., &
OLSON, O.E. (1940) Increasing the rate of excretion of
selenium from selenized animals by the administration of
p-bromobenzene. J. biol. Chem., l32: 785-786.
MOXON, A.L., OLSON, O.E., & SEARIGHT, W.V. (1950) Selenium
in rocks, soils, and plants. S. Dak. Agric. Exp. Sta. Tech.
Bull., 2.
MUHLER, J.C. & SHAFER, W.G. (1957) The effect of selenium on
the incidence of dental caries in rats. J. dent. Res., 36:
895-896.
MUNSELL, H.E., DEVANEY, G.M., & KENNEDY, M.H. (1936)
Toxicity of food containing selenium as shown by its effect on
the rat, Washington DC, US Department of Agriculture, 25 pp
(US Department of Agriculture Technical Bulletin No. 534).
MUTANEN, M. & KOIVISTOINEN, P. (1983) The role of imported
grain on the selenium intake of Finnish population 1941-1981.
Int. J. Vit. Nutr. Res., 53: 34-38.
MUTH, O.H. (1960) Carbon tetrachloride poisoning of ewes on
a low selenium ration. Am. J. vet. Res., 21: 86-87.
MUTH, O.H., ed. (1966) Selenium in biomedicine. In: Proceedings
of the First International Symposium at Oregon State University,
Westport, Connecticut, AVI Publishing Co., 445 pp.
MUTH, O.H., WESWIG, P.H., WHANGER, P.D., & OLDFIELD, J.E.
(197l) Effect of feeding selenium-deficient ration to the
subhuman primate (Saimiri sciureus). Am. J. vet. Res., 32:
l603-l605.
NAHAPETIAN, A.T., JANGHORBANI, M., & YOUNG, V.R. (1983)
Urinary trimethylselenonium excretion by the rat: effect of
level and source of selenium-75. J. Nutr., 113: 401-411.
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., KURATA, H., TONOMURA,
M., & TONOMURA, A. (1976) Studies on selenium-related
compounds. V. Cytogenetic effect and reactivity with DNA.
Mutat. Res., 40: 177-184.
NATIONAL CANCER INSTITUTE (1980) Bioassay of selenium
sulfide for possible carcinogenicity (gavage study), Bethesda,
Maryland, US Department of Health and Human Services, Public
Health Service, National Institutes of Health, 132 pp (DHHS
Publication No. (NIH) 80-1750).
NAVIA, J.M., MENAKER, L., SELTZER, J., & HARRIS, R.S. (1968)
Effect of Na2Se03 supplemented in the diet or the water on
dental caries of rats. Fed. Proc., 27: 676.
NAZARENKO, I.I. & ERMAKOV, A.N. (1971) Analytical chemistry
of selenium and tellurium, New York, Halsted Press.
NAZARENKO, I.I. & KISLOVA, I.V. (1977) [A highly sensitive
method for the analysis of migratory forms of selenium in
natural waters.] El. Viems. Labor. Technol. issled. obogashch.
min. syrja, 6: 1-8 (in Russian).
NAZARENKO, I.I., KISLOVA, I.V., GUSEJNOV, T.M., MKRTCHJAN,
M.A., & KISLOV, A.M. (1975) [Fluorometric determination of
selenium in biological material by 2,3-diaminonaphthalene].
Zh. Anal. Chim., 30(4): 733-737 (in Russian).
NELSON, A.A., FITZHUGH, O.G., & CALVERY, H.O. (1943) Liver
tumors following cirrhosis caused by selenium in rats. Cancer
Res., 3: 230-236.
NEWBERNE, P.M. & CONNER, M.W. (1974) Effect of selenium on
acute response to aflatoxin B1. In: Hemphill, D.D., ed. Trace
substances in environmental health. VIII, Columbia, Missouri,
University of Missouri Press, pp. 323-328.
NOCKELS, C.F. (1979) Protective effects of supplemental
vitamin E against infection. Fed. Proc., 38: 2134-2138.
NODA, M., TAKANO, T., & SAKURAI, H. (1979) Mutagenic
activity of selenium compounds. Mutat. Res., 66: 175-179.
NOGUCHI, T., CANTOR, A.H., & SCOTT, M.L. (1973) Mode of
action of selenium and vitamin E in prevention of exudative
drathesis in chicks. J. Nutr., 103(10): 1502-1511.
NORDMAN, E. (1974) Sodium selenite (selenium-75)
scintigraphy in diagnosis of tumours. Acta radiol.,
340(suppl.): 78 pp.
NORPPA, H., ET AL. (1980) Chromosomal effects of sodium
selenite in vivo. Hereditas, 93: 93-105.
NORRIS, F.H. & SANG, K. (1978) Amyotrophic lateral sclerosis
and low urinary selenium levels. J. Am. Med. Assoc., 239: 404.
OBERMEYER, B.D., PALMER, I.S., OLSON, O.E., & HALVERSON, A.W.
(1971) Toxicity of trimethylselenonium chloride in the rat
with and without arsenite. Toxicol. appl. Pharmacol., 20:
135-146.
OCHOA-SOLANO, A. & GITLER, C. (1968) Incorporation of
75Se-selenomethionine and 35S-methionine into chicken egg
white proteins. J. Nutr., 94: 243-248.
OELSCHLAGER, W. & MENKE, K.H. (1969) Concerning the selenium
content of plant, animal, and other materials. II. The
selenium and sulfur content of foods. Z. Ernahrungswiss., 9:
216-222.
OH, S.H., SUNDE, R.A., POPE, A.L., & HOEKSTRA, W.G. (1976a)
Glutathione peroxidase response to selenium intake in lambs
fed a Torula yeast-based, artificial milk. J. anal. Sci., 42:
977-983.
OH, S.H., POPE, A.L., & HOEKSTRA, W.G. (1976b) Dietary
selenium requirement of sheep fed a practical-type diet as
assessed by tissue glutathione peroxidase and other criteria.
J. anal. Sci., 42: 984-992.
OHLENDORF, H.M., HOFFMAN, D.J., SAIKI, M.K., & ALDRICH, T.W.
(1986) Embryonic mortality and abnormalities of aquatic
birds: apparent impact of selenium from irrigation drain
water. Sci. total Environ., 52: 49-63.
OLSON, O.E. (1967) Soil, plant, animal cycling of excessive
levels of selenium. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI Publishing Co., Inc, pp. 297-3l2.
OLSON, O.E. (1969) Selenium as a toxic factor in animal
nutrition. In: Proceedings of the Georgia Nutrition
Conference, Atlanta, Georgia, pp. 68-78.
OLSON, O.E. (1970) Selenium in feedstuffs: deficiencies and
excesses. Proceedings of the 31st Minnesota Nutrition
Conference, Minneapolis, University of Minnesota, pp. 7-l3.
OLSON, O.E. (1976) Methods of analysis for selenium. A
review. In: Proceedings of the Symposium on Selenium-Tellurium
in the Environment, Pittsburgh, Pennsylvania, Industrial
Health Foundation, pp. 67-84.
OLSON, O.E. (1978) Selenium in plants as a cause of
livestock poisoning. In: Keeler, R.F., Van Kampen, K.R., &
James, L.F., ed. Effects of poisonous plants on livestock, New
York, Academic Press, pp. 121-133.
OLSON, O.E. & FROST, D.V. (1970) Selenium in papers and
tabaccos. Environ. Sci. Technol., 4: 686-687.
OLSON, O.E. & PALMER, I.S. (1976) Selenoamino acids in
tissues of rats administered inorganic selenium. Metabolism,
25: 299-306.
OLSON, O.E., WHITEHEAD, E.I., & MOXON, A.L. (1942)
Occurrence of soluble selenium in soils and its availability
to plants. Soil Sci., 54: 47-53.
OLSON, O.E., SCHULTE, B.M., WHITEHEAD, E.I., & HALVERSON,
A.W. (1963) Effect of arsenic on selenium metabolism in
rats. J. agric. food Chem., 11: 531-534.
OLSON, O.E., NOVACEK, E.J., WHITEHEAD, E.I., & PALMER, I.S.
(1970) Investigations on selenium in wheat. Phytochemistry,
9: 1181-1188.
OLSON, O.E., PALMER, I.S., & WHITEHEAD, E.I. (1973)
Determination of selenium in biological materials. In: Glick,
D., ed. Methods of biochemical analysis, New York, John Wiley
and Sons, Inc, Vol. 21, pp. 39-78.
OLSON, O.E., PALMER, I.S., & CARY, E.E. (1975) Modification
of the official fluorometric method for selenium in plants. J.
Assoc. Off. Anal. Chem., 58: 117-121.
OLSON, O.E., CARY, E.E., & ALLAWAY, W.H. (1976) Fixation and
volatilization by soils of selenium from trimethylselenonium.
Agron. J., 68: 839-843.
OLSON, O.E., PALMER, I.S., & HOWE, M. (1978) Selenium in
foods consumed by South Dakotans. Proc. S.D. Acad. Sci., 57:
113-121.
OLSSON, U., ONFELT, A., & BEIJE, B. (1984) Dietary selenium
deficiency causes decreased N-oxygenation of N'N-dimethyl-
aniline and increased mutagenicity of dimethylnitrosamine in
the isolated rat liver/cell cultrue system. Mutat. Res., 126:
73-80.
OMAYE, S.T., REDDY, K.A., & CROSS, C.E. (1978) Enhanced lung
toxicity of paraquat in selenium-deficient rats. Toxicol.
appl. Pharmacol., 43: 237-247.
OSTADALOVA, I., BABICKY, A., & OBENBERGER, J. (1979)
Cataractogenic and lethal effect of selenite in rats during
postnatal ontogenesis. Physiol. Bohemoslov., 28: 393-397.
OVERVAD, K., THORLING, E.B., BJERRING, P., & EBBESEN, P.
(1985) Selenium inhibits UV-light-induced skin carcinogenesis
in hairless mice. Cancer Lett., 27: 163-170.
PAINTER, E.P. (1941) The chemistry and toxicity of selenium
compounds with special reference to the selenium problem.
Chem. Rev., 28: 179-213.
PALMER, I.S. & OLSON, O.E. (1974) Relative toxicities of
selenite and selenate in the drinking-water of rats. J. Nutr.,
104(3): 306-314.
PALMER, I.S. & OLSON, O.E. (1979) Partial prevention by
cyanide of selenium poisoning in rats. Biochem. Biophys. Res.
Commun., 90: 1379-1386.
PALMER, I.S., FISCHER, D.D., HALVERSON, A.W., & OLSON, O.E.
(1969) Identification of a major selenium excretory product
in rat urine. Biochim. Biophys. Acta, 177: 336-342.
PALMER, I.S., GUNSALUS, R.P., HALVERSON, A.W., & OLSON, O.E.
(1970) Trimethylselenonium ion as a general excretory product
from selenium metabolism in the rat. Biochim. Biophys. Acta,
208: 260-266.
PALMER, I.S., ARNOLD, R.L., & CARLSON, C.W. (1973) Toxicity
of various selenium derivatives to chick embryos. Poultry
Sci., 52: 1841-l846.
PALMER, I.S., OLSON, O.E., HALVERSON, A.W., MILLER, R., &
SMITH, C. (1980) Isolation of factors in linseed oil meal
protective against chronic selenosis in rats. J. Nutr., 110:
145-150.
PARIZEK, J. & OSTADALOVA, I. (1967) The protective effect of
small amounts of selenite in sublimate intoxication. Experientia
(Basel), 23: 142-143.
PARIZEK, J., OSTADALOVA, I., KALOUSKOVA, J., BABICKY, A., &
BENES, J. (1971) The detoxifying effects of selenium.
Interrelations between compounds of selenium and certain
metals. In: Mertz, W. & Cornatzer, W.E., ed. Newer trace
elements in nutrition, New York, Marcel Dekker, Inc,
pp. 85-122.
PARIZEK, J., ET AL. (1974) Some hormonal and environmental
factors influencing selenium metabolism and action. In:
Proceedings of the Ninth International Congress in Nutrition,
Mexico, 1972 (Coop. Nutr. Cong., 16: 94-97).
PARIZEK, J., KALOUSKOVA, J., KORUNOVA, V., BENES, J., &
PAVLIK, L. (1976) The protective effect of pretreatment with
selenite on the toxicity of dimethylselenide. Physiol.
Bohemoslov., 25: 573-576.
PARIZEK, J., KALOUSKOVA, J., BENES, J., & PAVLIK, L. (1980)
Interactions of selenium-mercury and selenium-selenium
compounds. Ann. N.Y. Acad. Sci., 355: 347-360.
PASCOE, G.A. & CORREIA, M.A. (1985) Structural and
functional assembly of rat intestinal cytochrome P-450
isozymes: effects of dietary iron and selenium. Biochem.
Pharmacol., 34: 599-608.
PASCOE, G.A., SAKAI-WONG, J., SOLIVEN, E., & CORREIA, M.A.
(1983) Regulation of intestinal cytochrome P-450 and Heme by
dietary nutrients. Critical role of selenium. Biochem.
Pharmacol., 32(20): 3027-3035.
PAULSEN, P.J. (1977) Determination of cadmium, copper, iron,
lead, mercury, molybdenum, nickel, selenium, silver,
tellurium, thallium, and zinc. Natl Bur. Stand. Special Publ.
(USA), 492: 33-48.
PAVLIK, L., KALOUSKOVA, J., VOBECKY, M., DEDINA, BENES, J., &
PARIZEK, J. (1979) Selenium levels in the kidneys of male
and female rats. In: Nuclear activation techniques in the life
sciences, 1978, Vienna, International Atomic Energy Agency,
pp. 213-223.
PELEKIS, E.E., PELEKIS, L.L., & TAURE, I. JA. (1975)
[Instrumental neutron-activation method of selenium determination
in biological materials.] Vitaminy, 8: 146-150 (in Russian).
PENCE, B.C. & BUDDINGH, F. (1985) Effect of selenium
deficiency on incidence and size of 1,2-dimethylhydrazine-
induced colon cancer in rats. J. Nutr., 115: 1196-1202.
PENNINGTON, J.A.T., WILSON, D.B., NEWELL, R.F., HARLAND, B.F.,
JOHNSON, R.D., & VANDERVEEN, J.E. (1984) Selected minerals
in foods surveys, 1974 to 1981/82. J. Am. Diet. Assoc., 84:
771-780.
PERONA, G., GUIDI, G.C., PIGA, A., CELLERINO, R., MENNA, R., &
ZATTI, M. (1978) In vivo and in vitro variations of human
erythrocyte glutathione peroxidase activity as result of cells
ageing, selenium availability and peroxide activation. Br. J.
Haematol., 39: 399-408.
PERRY, H.M., Jr, ERLANGER, M.W., & PERRY, E.F. (1976)
Limiting conditions for the induction of hypertension in rats
by cadmium. In: Hemphill, D.D., ed. Trace substances in
environmental health. X, Columbia, Missouri, University of
Missouri Press, pp. 459-467.
PIERCE, F.D. & BROWN, H.R. (1977) Comparison of inorganic
interferences in atomic absorption spectrometric determination
of arsenic and selenium. Anal. Chem., 49: 1417-1422.
PIERCE, S. & TAPPEL, A.L. (1977) Effects of selenite and
selenomethionine on glutathione peroxidase in the rat. J.
Nutr., 107: 475-479.
PILLAY, K.K.S., THOMAS, C.C., Jr, & SONDEL, J.A. (1971)
Activation analysis of airborne selenium as a possible
indicator of atmospheric sulfur pollutants. Environ. Sci.
Technol., 5: 74-77.
PLAA, G.L. & WITSCHI, H. (1976) Chemicals, drugs, and lipid
peroxidation. Ann. Rev. Pharmacol. Toxicol., 16: 125-141.
PLEBAN, P.A., MUNYANI, A., & BEACHUM, J. (1982)
Determination of selenium concentration and glutathine
peroxidase activity in plasma and erythrocytes. Clin. Chem.,
28: 311-316.
PLETNIKOVA, I.P. (1970) Biological effect and safe
concentration of selenium in drinking-water. Hyg. Sanit., 35:
176-181.
POLEY, W.E. & MOXON, A.L. (1938) Tolerance levels of
seleniferous grains in laying rations. Poultry Sci., 17: 72-76.
POOLE, C.F., EVANS, N.J., & WIBBERLEY, D.G. (1977)
Determination of selenium in biological samples by gas-liquid
chromatography with electron-capture detection. J.
Chromatogr., 136: 73-83.
PRATLEY, J.E. & MCFARLANE, J.D. (1974) The effect of
sulphate on the selenium content of pasture plants. Aust. J.
exp. Agric. Anim. Husb., 14: 533-538.
PRINGLE, P. (1942) Occupational dermatitis following
exposure to inorganic selenium compounds. Br. J. Dermatol.
Syphil., 54: 54-58.
PROHASKA, J.R. & GANTHER, H.E. (1976) Selenium and
glutathione peroxidase in developing rat brain. J. Neurochem.,
27: 1379-1387.
PROHASKA, J.R. & GANTHER, H.E. (1977) Glutathione peroxidase
activity of glutathione-S-transferases purified from rat
liver. Biochem. Biophys. Res. Commun., 76: 437-445.
PYEN, G. & FISHMAN, M. (1978) Automated determination of
selenium in water. At. Absorpt. Newslett., 17: 47-48.
RANDOLPH, W.F. (1978) Food additives permitted in feed and
drinking water of animals. Selenium. Fed. Reg., 43:
11700-11701.
RANSONE, J.W., SCOTT, N.M., Jr, & KNOBLOCK, E.C. (1961)
Selenium sulfide intoxication. New Engl. J. Med., 264: 384-385.
RAVIKOVITCH, S. & MARGOLIN, M. (1957) Selenium in soils and
plants. Ktavim. Rec. agric. Res. Sta., 7: 41-52.
RAY, J.H. (1984) Sister-chromatid exchange induction by
sodium selenite: reduced glutathione converts Na2SeO3 to its
SCE-inducing form. Mutat. Res., 141: 49-53.
RAY, J.H. & ALTENBURG, L.C. (1978) Sister-chromatid exchange
induction by sodium selenite: dependence on the presence of
red blood cells or red blood cell lysate. Mutat. Res., 54:
343-354.
RAY, J.H. & ALTENBURG, L.C. (1980) Dependence of the
sister-chromatid exchange-inducing abilities of inorganic
selenium compounds on the valence state of selenium. Mutat.
Res., 78: 261-266.
RAY, J.H., ALTENBURG, L.C., & JACOBS, M.M. (1978) Effect of
sodium selenite and methyl methanesulfonate or N-hydroxy-2-
acetylamino-fluorene co-exposure on sister-chromatid exchange
production in human whole blood cultures. Mutat. Res., 57:
359-368.
REA, H.M., THOMSON, C.D., CAMPBELL, D.R., & ROBINSON, M.F.
(1979) Relation between erythrocyte selenium concentrations
and glutathione peroxidase (EC 1.11.1.9) activities of New
Zealand residents and visitors to New Zealand. Br. J. Nutr.,
42: 201-208.
REAMER, D.C. & VEILLON, C. (1983a) Letter to the editor.
Anal. Chem., in press.
REAMER, D.C. & VEILLON, C. (1983b) A double isotape dilution
method for using stable selenium isotapes in metabolic tracer
studies: analysis by gas chromatography/mass spectrometry
(GC/MS). J. Nutr., 113: 786-792.
REITER, R. & WENDEL, A. (1983) Selenium and drug metabolism.
Multiple modulations of mouse liver enzymes. Biochem.
Pharmacol., 32(20): 3063-3067.
RHEAD, W.J., CARY, E.E., ALLAWAY, W.H., SALTZSTEIN, S.L., &
SCHRAUZER, G.N. (1972) The vitamin E and selenium status of
infants and the sudden infant death syndrome. Bioinorg. Chem.,
1: 289-294.
RICHOLD, M., ROBINSON, M.F., & STEWART, R.D.H. (1977)
Metabolic studies in rats of 75Se incorporated in vivo into
fish muscle. Br. J. Nutr., 38: 19-29.
RIELY, C.A., COHEN, G., & LIEBERMAN, M. (1974) Ethane
evolution: a new index of lipid peroxidation. Science, 183:
208-210.
RILEY, J.F. (1968) Mast cells, co-carcinogenesis and
anti-carcinogenesis in the skin of mice. Experientia (Basel),
24: 1237-1238.
ROBBINS, C.W. & CARTER, D.L. (1970) Selenium concentrations
in phosphorus fertilizer materials and associated uptake by
plants. Soil Sci. Soc. Am. Proc., 34: 506-509.
ROBERTSON, D.S.F. (1970) Selenium, a possible teratogen?
Lancet, 1: 518-519.
ROBINSON, J.R., ROBINSON, M.F., LEVANDER, O.A., & THOMSON,
C.D. (1985) Urinary excretion of selenium by New Zealand and
North American human subjects on differing intakes. Am. J.
clin. Nutr., 41: 1023-1031.
ROBINSON, M.F. & THOMSON, C.D. (1981) Selenium levels in
humans vs environmental sources. In: Spallholz, J.E., Martin,
J.L., & Ganther, H.E., ed. Selenium in biology and medicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 283-302
ROBINSON, M.F. & THOMSON, C.D. (1983) The role of selenium
in the diet. Nutr. Abstr. Rev., 53: 3-26.
ROBINSON, M.F. & THOMSON, C.D. (1986) Selenium status of the
food supply and residents of New Zealand (Beijing Conference).
ROBINSON, M.F., MCKENZIE, J.M., THOMSON, C.D., & VAN RIJ, A.L.
(1973) Metabolic balance of zinc, copper, cadmium, iron,
molybdenum and selenium in young New Zealand women. Br. J.
Nutr., 30: 195-205.
ROBINSON, M.F., GODFREY, P.J., THOMSON, C.D., REA, H.M., & VAN
RIJ, A.M. (1978a) Blood selenium and glutathione peroxidase
activity in normal subjects and in surgical patients with and
without cancer in New Zealand. Am. J. clin. Nutr., 32:
1477-1485.
ROBINSON, M.F., REA, H.M., FRIEND, G.M., STEWART, R.D.H.,
SNOW, P.C., & THOMSON, C.D. (1978b) On supplementing the
selenium intake of New Zealanders. II. Prolonged metabolic
experiments with daily supplements of selenomethionine,
selenite, and fish. Br. J. Nutr., 39: 589-600.
ROBINSON, M.F., GODFREY, J.P., THOMSON, C.D., REA, H.M., & VAN
RIJ, A.M. (1979) Blood selenium and glutathione peroxidase
activity in normal subjects and in surgical patients with and
without cancer in New Zealand. Am. J. clin. Nutr., 32:
1477-1485.
ROBINSON, M.F., CAMPBELL, D.R., STEWART, R.D.H., REA, H.M.,
THOMSON, C.D., SNOW, P.G., & SQUIRES, I.H.W. (1981) Effect
of daily supplements of selenium on patients with muscular
complaints in Otago and Canterbury. N.Z. med. J., 93: 289-292.
ROBINSON, M.F., CAMPBELL, D.R., SUTHERLAND, W.H.F., HERBISON,
P.G., PAULIN, J.M., & SIMPSON, F.O. (1983) Selenium and risk
factors for cardiovascular disease in New Zealand. N.Z. med.
J., 96: 755-757.
ROBINSON, M.F., THOMSON, C.D., & HUEMMER, P.K. (1985) Effect
of a megadose of ascorbic acid, a meal and orange juice on the
absorption of selenium as sodium selenite. N.Z. med. J., 98:
627-629.
ROBINSON, W.O. (1933) Determination of selenium in wheat and
soils. J. Assoc. Off. Agric. Chem., 16: 423-424.
ROHMER, R., CARROT, E., & GOUFFAULT, J. (1950) Nouvel aspect
de l'intoxication par les composés du sélénium. Bull. Soc.
Chim. France, 5(17): 275-278.
ROSIN, M.P. & STICH, H.F. (1979) Assessment of the use of
the Salmonella mutagenesis assay to determine the influence of
antioxidants on carcinogen-induced mutagenesis. Int. J.
Cancer, 23: 722-727.
ROSENFELD, I. & BEATH, O.A. (1954) Effect of selenium on
reproduction in rats. Proc. Soc. Exp. Biol. Med., 87: 295-297.
ROSENFELD, I. & BEATH, O.A. (1964) Selenium geobotany,
biochemistry, toxicity and nutrition, New York, Academic
Press, 411 pp.
ROSIN, M.P. (1981) Inhibition of spontaneous mutagenesis in
yeast cultures by selenite, selenate, and selenide. Cancer
Lett., 13: 7-14.
ROSSI, L.C., CLEMENTE, G.F., & SANTARONI, G. (1976) Mercury
and selenium distribution in a defined area and in its
population. Arch. environ. Health, 31: 160-165.
ROTRUCK, J.T., POPE, A.L., GANTHER, H.E., SWANSON, A.B.,
HAFEMAN, D.G., & HOEKSTRA, W.G. (1973) Selenium: biochemical
role as a component of glutathione peroxidase. Science, 179:
588-590.
RUDERT, C.P. & LEWIS, A.R. (1978) The effect of potassium
cyanide on the occurrence of nutritional myopathy in lambs.
Rhod. J. agric. Res., 16: 109-116.
RUSIECKI, W. & BRZEZINSKI, J. (1966) Influence of sodium
selenate on acute thallium poisonings. Acta Pol. pharm., 23:
74-80.
SAKURAI, H. & TSUCHIYA, K. (1975) A tentative recommendation
for the maximum daily intake of selenium. Environ. Physiol.
Biochem., 5: 107-118.
SALONEN, J.T. (1985) Selenium in cardiovascular diseases and
cancer. Epidemiologic findings from Finland. In: Boström, H.
& Ljungstedt, N., ed. Trace elements in health disease,
Stockholm, Sweden, Almqvist & Wiksell International, pp.
172-186.
SALONEN, J.T., ALFTHAN, G., HUTTUNEN, J.K., PIKKARAINEN, J., &
PUSKA, P. (1982) Association between cardiovascular death
and myocardial infarction and serum selenium in a watched-pair
longitudinal study. Lancet, 2: 175-179.
SALONEN, J.T., ALFTHAN, G., HUTTUNEN, J.K., & PUSKA, P.
(1984a) Association between serum selenium and the risk of
cancer. Am. J. Epidemiol., 120(3): 342-349.
SALONEN, J.T., SALONEN, R., PENTTILA, I., HERRANEN, J., JAUHIAINEN,
J., KANTOLA, M., KAURANEN, P., LAPPETELAINEN, R., MAENPAA, P., &
PUSKA, P. (1984b) Serum fatty acids, apolipoproteins, selenium
and vitamin antioxidants and the risk of death from ischaemic heart
disease. Am. J. Cardiol., 56(2): 226.
SALONEN, J.T., SALONEN, R., LAPPETELAINEN, R., MAENPAA, P.H.,
ALFTHAN, G., & PUSKA, P. (1985) Risk of cancer in relation
to serum concentrations of selenium and vitamins A and E:
matched case-control analysis of prospective data. Brit. med.
J., 290: 417-420.
SARATHCHANDRA, S.U. & WATKINSON, J.H. (1981) Oxidation of
elemental selenium to selenite by Bacillus megaterium.
Science, 211: 600-601.
SARATHCHANDRASCHMIDT, K. & HELLER, W. (1976) Selenium
concentration and activity of glutathione peroxidase in
lysates of human erythrocytes. Blut, 33: 247-251.
SCHECTER, A., SHANSKE, W., STENZLER, A., QUINTILIAN, H., &
STEINBERG, H. (1980) Acute hydrogen selenide intoxication.
Chest, 77: 554-555.
SCHILLACI, M., MARTIN, S.E., & MILNER, J.A. (1982) The
effects of dietary selenium on the biotransformation of
7,12-dimethylbenz(alpha)anthracene. Mutat. Res., 101: 31-37.
SCHRAUZER, G.N. (1976) Cancer mortality correlation studies.
II. Regional associations of mortalities with the consumptions
of foods and other commodities. Medic. Hypoth., 2: 39-49.
SCHRAUZER, G.N. & ISHMAEL, D. (1974) Effects of selenium and
of arsenic on the genesis of spontaneous mammary tumors in
inbred C3H mice. Ann. clin. Lab. Sci., 4: 441-447.
SCHRAUZER, G.N. & WHITE, D.A. (1978) Selenium in human
nutrition: dietary intakes and effects of supplementation.
Bioinorg. Chem., 8: 303-318.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1976)
Inhibition of the genesis of spontaneous mammary tumors in
C3H mice: effects of selenium and of selenium-antagonistic
elements and their possible role in human breast cancer.
Bioinorg. Chem., 6: 265-270.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1977)
Cancer mortality correlation studies. III. Statistical
associations with dietary selenium intakes. Bioinorg. Chem.,
7: 23-34.
SCHRAUZER, G.N, WHITE, D.A., & SCHNEIDER, C.J. (1978a)
Effects of selenium, arsenic, and zinc on the genesis of
spontaneous mammary tumors in inbred female C3H mice. In:
Kirchgessner, M., ed. Trace element metabolism in man and
animals. III, Freising-Weihenstephan, West Germany,
Arbeitskreis für Tierernährungsforschung Weihenstephan, pp.
387-390.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1978b)
Selenium and cancer: Effects of selenium and of the diet on
the genesis of spontaneous mammary tumors in virgin inbred
female C3H/St mice. Bioinorg. Chem., 8: 387-396.
SCHRAUZER, G.N., WHITE, D.A., MCGINNESS, J.E., SCHNEIDER,
C.J., & BELL, L.J. (1978c) Arsenic and cancer: effects of
joint administration of arsenite and selenite on the genesis
of mammary adenocarcinoma in inbred female C3H/St mice.
Bioinorg. Chem., 9: 245-253.
SCHROEDER, H.A. & MITCHENER, M. (1971a) Toxic effects of
trace elements on the reproduction of mice and rats. Arch.
environ. Health, 23: 102-106.
SCHROEDER, H.A. & MITCHENER, M. (1971b) Selenium and
tellurium in rats: effect on growth, survival and tumors. J.
Nutr., 101: 1531-1540.
SCHROEDER, H.A. & MITCHENER, M. (1972) Selenium and
tellurium in mice. Effects on growth, survival, and tumors.
Arch. environ. Health, 24: 66-71.
SCHROEDER, H.A., FROST, D.V., & BALASSA, J.J. (1970)
Essential trace metals in man: selenium. J. chron. Dis., 23:
227-243.
SCHUBERT, J.R., MUTH, O.H., OLDFIELD, J.E., & REMMERT, L.F.
(196l) Experimental results with selenium in white muscle
disease of lambs and calves. Fed. Proc., 20: 689-694.
SCHULTZ, T.D. & LEKLEM, J.E. (1983) Selenium status of
vegetarians, nonvegetarians, and hormone-dependent cancer
subjects. Am. J. clin. Nutr., 37: 114-118.
SCHWARZ, K. (1961) Development and status of experimental
work on Factor 3-selenium. Fed. Proc., 20: 666-673.
SCHWARZ, K. (1962) Vitamin E, trace elements, and sulfhydryl
groups in respiratory decline. Vitam. Horm., 20: 463-484.
SCHWARZ, K. (1967) Discussion comments. In: Muth, O.H.,
Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc,
pp. 225-226.
SCHWARZ, K. (1976) The discovery of the essentiality of
selenium, and related topics (a personal account). In:
Proceedings of the Symposium on Selenium-Tellurium in the
Environment, Pittsburgh, Pennsylvania, Industrial Health
Foundation, pp. 349-376.
SCHWARZ, K. (1977) Amyotrophic lateral sclerosis and
selenium. J. Am. Med. Assoc., 238: 2365.
SCHWARZ, K. & FOLTZ, C.M. (1957) Selenium as an integral
part of Factor 3 against dietary necrotic liver degeneration.
J. Am. Chem. Soc., 79: 3292-3293.
SCHWARZ, K. & FOLTZ, C.M. (1958) Factor 3 activity of
selenium compounds. J. biol. Chem., 233: 245-251.
SCHWARZ, K., PORTER, L.A., & FREDGA, A. (1972) Some
regularities in the structure-function relationship of
organoselenium compounds effective against dietary liver
necrosis. Ann. NY Acad. Sci., 192: 200-214.
SCOTT, M.L. & THOMPSON, J.N. (1971) Selenium content of
feedstuffs and effects of dietary selenium levels upon tissue
selenium in chicks and poults. Poultry Sci., 50: 1742-1748.
SCOTT, M.L., OLSON, G., KROOK, L., & BROWN, W.R. (1967)
Selenium-responsive myopathies of myocardium and of smooth
muscle in the young poult. J. Nutr., 91: 573-583.
SEIFTER, J., EHRICH, W.E., HUDYMA, G., & MUELLER, G. (1946)
Thyroid adenomas in rats receiving selenium. Science, 103: 762.
SELJANKINA, K.P., JAHIMOVICH, N.P., ALEKSEEVA, L.S., & PETINA,
A.A. (1974) [Selenium and tellurium levels in environmental
media.] In: [Hygiene and occupational diseases,] Moscow, Nauka
Publishing House, Vol 21, pp. 69-71 (in Russian).
SENF, H.W. (1941) [A case of poisoning by hydrogen
selenide]. Dtsch. Med. Wochenschr., 67: 1094-1096 (in German).
SEWARD, C.R., VAUGHAN, G., & HOVE, E.L. (1966) Effect of
selenium on incisor depigmentation and carbon tetrachloride
poisoning in vitamin E-deficient rats. Proc. Soc. Exp. Biol.
Med., 121: 850-852.
SHACKLETTE, H.T., BOERNGEN, J.G., & KEITH, J.R. (1974)
Selenium, fluorine, and arsenic in surficial materials of the
conterminous United States, 14 pp (US Geological Survey
Circular, 692).
SHAMBERGER, R.J. (1970) Relationship of selenium to cancer.
I. Inhibitory effect of selenium on carcinogenesis. J. Natl
Cancer Inst., 44: 931-936.
SHAMBERGER, R.J. (1971) Is selenium a teratogen? Lancet, 2:
1316.
SHAMBERGER, R.J. (1978) Antioxidants and cancer. VIII.
Cadmium-selenium levels in kidneys. In: Kirchgessner, M., ed.
Trace element metabolism in man and animals. III,
Freising-Weihenstephan, West Germany, Arbeitskreis für
Tierernährungsforschung Weihenstephan, pp. 391-392.
SHAMBERGER, R.J. & FROST, D.V. (1969) Possible protective
effect of selenium against human cancer. Can. Med. Assoc. J.,
100: 682.
SHAMBERGER, R.J. & RUDOLPH, G. (1966) Protection against
cocarcinogenesis by antioxidants. Experientia (Basel), 22: 116.
SHAMBERGER, R.J. & WILLIS, C.E. (1971) Selenium distribution
and human cancer mortality. Crit. Rev. clin. Lab. Sci., 2:
211-221.
SHAMBERGER, R.J., BAUGHMAN, F.F., KALCHERT, S.L., WILLIS,
C.E., & HOFFMAN, G.C. (1973a) Carcinogen-induced chromosomal
breakage decreased by antioxidants. Proc. Natl Acad. Sci.
(USA), 70: 1461-1463.
SHAMBERGER, R.J., RUKOVENA, E., LONGFIELD, A.K., TYTKO, S.A.,
DEODHAR, S., & WILLIS, C.E. (1973b) Antioxidants and cancer.
I. Selenium in the blood of normals and cancer patients. J.
Natl Cancer Inst., 50(4): 863-870.
SHAMBERGER, R.J., TYTKO, S.A., & WILLIS, C.E. (1975)
Selenium and heart disease. In: Hemphill, D.D., ed. Trace
substances in environmental health. IX, Columbia, Missouri,
University of Missouri Press, pp. 15-22.
SHAMBERGER, R.J., TYTKO, S.A., & WILLIS, C.E. (1976)
Antioxidants and cancer. VI. Selenium and age-adjusted human
cancer mortality. Arch. environ. Health, 31: 231-235.
SHAMBERGER, R.J., CORLETT, C.L., BEAMAN, K.D., & KASTEN, B.L.
(1979) Antioxidants reduce the mutagenic effect of malonaldehyde
and beta-propiolactone. Partix, antioxidants, and cancer.
Mutat. Res., 66: 349-355.
SHAMBERGER, R.J., WILLIS, C.E., & MCCORMACK, L.J. (1979)
Selenium and heart disease. III. Blood selenium and heart
mortality in 19 states. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
University of Missouri, pp. 59-63.
SHAPIRO, J.R. (1972) Selenium and carcinogenesis: a review.
Ann. NY Acad. Sci., 192: 215-219.
SHEARER, T.R. (1973) Lack of effect of selenium on
glycolytic and citric acid cycle intermediates in rat kidney
and liver. Proc. Soc. Exp. Biol. Med., 144: 688-691.
SHEARER, T.R. (1975) Developmental and postdevelopmental
uptake of dietary organic and inorganic selenium into the
molar teeth of rats. J. Nutr., 105: 338-347.
SHEARER, T.R. & HADJIMARKOS, D.M. (1975) Geographic distribution
of selenium in human milk. Arch. environ. Health, 30: 230-233.
SHEARER, T.R. & RIDLINGTON, J.W. (1976) Fluoride-selenium
interaction in the hard and soft tissues of the rat. J. Nutr.,
106: 451-456.
SHEARER, T.R., MCCORMACK, D.W., DESART, D.J., BRITTON, J.L., &
LOPEZ, M.T. (1980) Histological evaluation of selenium
induced cataracts. Exp. Eye Res., 31: 327-333.
SHENDRIKAR, A.D. (1974) Critical evaluation of analytical
methods for the determination of selenium in air, water, and
biological materials. Sci. total Environ., 3: 155-168.
SHENDRIKAR, A.D. & FAUDEL, G.B. (1978) Distribution of trace
metals during oil shale retorting. Environ. Sci. Technol., 12:
332-334.
SHRIFT, A. (1964) A selenium cycle in nature? Nature
(Lond.), 201: 1304-1305.
SHRIFT, A. (1973) Selenium compounds in nature and medicine.
Metabolism of selenium by plants and microorganisms. In:
Klayman, D.L. & Gunther, W.H.H., ed. Organic selenium
compounds: their chemistry and biology, New York, John Wiley
and Sons, Inc, pp. 763-814.
SHRIFT, A. & VIRUPAKSHA, T.K. (1965) Seleno-amino acids in
selenium-accumulating plants. Biochim. Biophys. Acta, 100:
67-75.
SHUM, G.T.C., FREEMAN, H.C., & UTHE, J.F. (1977) Flameless
atomic absorption spectrophotometry of selenium in fish and
food products. J. Assoc. Off. Anal. Chem., 60: 1010-1014.
SHUMAEV, V.D., MAKUSHINSKAJA, N.D., & CHIGRINA, T.A. (1976)
[Hygiene aspects of industrial waste water pollution in some
non-ferrous metal enterprises in Kazahstan by chemicals
regulated for the point of sanitary toxicological risk
characteristics.] Zolvavoohram Kazah, 4: 36-38 (in Russian).
SIMPSON, B.H. (1972a) An epidemiological study of carcinoma
of the small intestine in New Zealand sheep. N.Z. vet. J., 20:
91-97.
SIMPSON, B.H. (1972b) The possible relationship of selenium
and superphosphate to the frequencey of occurrence of
intestinal carcinomas in sheep. N.Z. vet. J., 20: 224.
SINDEEVA, N.D. (1959) [Minerology, occurrence and main
characteristics of the geochemistry of selenium and
tellurium,] Moscow, Publishing House of the Academy of
Sciences of the USSR, 257 pp (in Russian).
SIRIANNI, S.R. & HUANG, C.C. (1983) Induction of sister
chromatid exchange by various selenium compounds in Chinese
hamster cells in the presence and absence of S9 mixture.
Cancer Lett., 18: 109-116.
SIVERTSEN, T., KARLSEN, J.T., & FROSLIE, A. (1977) The
relationship of erythrocyte glutathione peroxidase to blood
selenium in swine. Acta vet. Scand., 18: 494-500.
SKORNJAKOVA, L.V., BURCHANOV, A.I., & SALECHOV, M.I. (1969)
[On the acute manifestations of selenium poisoning.] Gig. Tr.
Prof. Zabol., 11: 45-46 (in Russian).
SMITH, C.R., Jr, WEISLEDER, D., MILLER, R.W., PALMER, I.S., &
OLSON, O.E. (1980) Linustatin and neolinustatin: cyanogenic
glycosides of linseed meal that protect animals against
selenium toxicity. J. org. Chem., 45: 507-510.
SMITH, M.I. & STOHLMAN, E.F. (1940) Further observations on
the influence of dietary protein on the toxicity of selenium.
J. Pharmacol. exp. Ther., 70: 270-278.
SMITH, M.I. & WESTFALL, B.B. (1937) Further field studies on
the selenium problem in relation to public health. US Public
Health Rep., 52: 1375-1384.
SMITH, M.I., FRANKE, K.W., & WESTFALL, B.B. (1936) The
selenium problem in relation to public health. A preliminary
survey to determine the possibility of selenium intoxication
in the rural population living on seleniferous soil. US Public
Health Rep., 51: 1496-1505.
SMITH, M.I., WESTFALL, B.B., & STOHLMAN, E.F., Jr (1937) The
elmination of selenium and its distribution in the tissues. US
Public Health Rep., 52: 1171-1177.
SMITH, M.I., WESTFALL, B.B., & STOHLMAN, E.F., Jr (1938)
Studies on the fate of selenium in the organism. US Public
Health Rep., 53: 1199-1216.
SOKOLOFF, L. (1985) Endemic forms of osteoarthritis. Clin.
rheum. Dis., 11: 187-202.
SPALLHOLZ, J.E., MARTIN, J.L., GERLACH, M.L., & HEINZERLING,
R.H. (1973) Immunologic responses of mice fed diets
supplemented with selenite selenium. Proc. Soc. Exp. Biol.
Med., 143: 685-689.
SPALLHOLZ, J.E., MARTIN, J.L., GERLACH, M.L., & HEINZERLING,
R.H. (1975) Injectable selenium: effect on the primary
immune response of mice. Proc. Soc. Exp. Biol. Med., 148:
37-40.
SPALLHOLZ, J.E., COLLINS, G.E., & SCHWARZ, K. (1978) A
single test-tube method for the fluorometric micro-
determination of selenium. Bioinorg. Chem., 9: 453-459.
SPRINKER, L.H., HARR, J.R., NEWBERNE, P.M., WHANGER, P.D., &
WESWIG, P.H. (197l) Selenium deficiency lesions in rats fed
vitamin E supplemented rations. Nutr. Rep. Int., 4: 335-340.
STADTMAN, T.C. (1977) Biological function of selenium. Nutr.
Rev., 35: 161-166.
STEAD, R.J., HINKS, L.J., HODSON, M.E., REDINGTON, A.N.,
CLAYTON, B.E., & BATTEN, J.C. (1985) Selenium deficiency and
possible increased risk of carcinoma in adults with cystic
fibrosis. Lancet (October): 862-863.
STEWART, R.D.H., GRIFFITHS, N.M., THOMSON, C.D., & ROBINSON,
M.F. (1978) Quantitative selenium metabolism in normal New
Zealand women. Br. J. Nutr., 40: 45-54.
STOEWSAND, G.S., GUTENMANN, W.H., & LISK, D.J. (1978) Wheat
grown on fly ash: high selenium uptake and response when fed
to Japanese quail. J. agric. food Chem., 26: 757-759.
SU, Y. & YU, W. (1983) Nutritional bio-geochemical etiology
of Keshan disease. Chin. med. J., 96: 594-596.
SU, Y., CUI, S., GU, B., ZENG, X., & YU, W. (1982)
Experimental study of the effects of corn and vegetables from
Keshan disease endemic districts on the growth and myocardium
in rats. Acta nutr. Sinica, 4: 261-269.
SUBCOMMITTEE ON SELENIUM - COMMITTEE ON ANIMAL NUTRITION
(1983) Selenium in nutrition, revised ed., Washington DC,
Board on Agriculture, National Research Council, National
Academy of Sciences, 174 pp.
SUCHKOV, B.P. (1971) [Selenium content in major nutrients
consumed by the population of the Ukrainian SSR.] Vopr.
Pitan., 30(b): 75-77 (in Russian).
SUCHKOV, B.P. & KACAP, I.M. (1971) [Dental caries in the
population of the Chernovitsk region in relation with the
impact of microelements.] Gig. i Sanit., 3: 91-93 (in Russian).
SUCHKOV, B.P. & ZHIVECKIJ, A.V. (1973) [Selenium levels in
the blood of healthy people and people with neoplasms in a
Chernovici region republican interdepartmental collective.]
In: [Microelements in medicine,] Kiev, Zdarovie, Zdarovie
Publishing House, Vol. 4, pp. 69-73 (in Russian).
SUCHKOV, B.P., KACAP, I.M., & GULGASENKO, A.I. (1973)
[Affection of the population of the Chernovitsi region with
caries in association with selenium content in the teeth].
Stomatologia, 52: 21-23 (in Russian).
SUCHKOV, B.P., SHEVCHUK, I.A., & MARBAR, A.I. (1977)
[Histopathomorphological changes in the organs and tissues of
laboratory animals on a synthetic diet with a low content of
selenium and vitamin E.] Vopr. Pitan., 2: 33-41 (in Russian).
SUCHKOV, B.P., SHTUTMAN, C.M., & HALMURADOV, A.G. (1978)
[The biochemical role of selenium in animal organisms.]
Ukrain. Biochem., 50: 659-671 (in Russian).
SUN, S., ZHAI, F., ZHOU, L., & YANG, G. (1985) The
bioavailability of soil selenium in Keshan disease and high
selenium areas. Chinese J. end. Dis., 4: 21-28.
SUNDE, R.A. (1984) Selenoproteins. J. Am. Oil Chem. Soc.,
61: 1891-1898.
SUNDSTROM, H., KORPELA, H., VIINIKKA, L., & KAUPPILA, A.
(1984a) Serum selenium and glutathione peroxidase, and plasma
lipid peroxides in uterine, ovarian or vulvar cancer, and
their responses to antioxidants in patients with ovarian
cancer. Cancer Lett., 24: 1-10.
SUNDSTROM, H., YRJANHEIKKI, E., & KAUPPILA, A. (1984b) Serum
selenium in patients with ovarian cancer during and after
therapy. Carcinogenesis, 5(6): 731-734.
SVERDLINA, N.T & MASLENNIKOVA, V.S. (1961) [Hygienic
evaluation of working conditions in production of selenium
photocells.] Leningrad, Mater. manch sessin Leningrad NII gig.
truda prof. Zab, pp. 73-75 (in Russian).
SWEINS, A. (1983) Protective effect of selenium against
arsenic-induced chromosomal damage in cultured human lympho-
cytes. Hereditas, 98: 249-252.
SYMANSKI, H. (1950) [A case of hygrogen selenide poisoning.]
Dtsch. Med. Wochenschr., 75: 1730 (in German).
SZYDLOWSKI, F.J. & DUNMIRE, D.L. (1979) Semi-automatic
digestion and automatic analysis for selenium in animal feeds.
Anal. Chim Acta, 105: 445-449.
TAN, J., ZHENG, D., HOU, S., ZHU, W., LI, R., ZHU, Z., & WANG,
W. (in press) Selenium ecological chemico-geography and
endemic Keshan disease and Kashin-Beck disease in China. In:
Proceedings of the Third International Symposium on Selenium
in Biology and Medicine.
TANK, G. & STORVICK, C.A. (1960) Effect of naturally
occurring selenium and vanadium on dental caries. J. dent.
Res., 39: 473-488.
TAYLOR, F.B. (1963) Significance of trace elements in public
finished water supplies. J. Am. Water Works Assoc., 55:
619-623.
TEEL, R.W. (1984) A comparison of the effect of selenium on
the mutagenicity and metabolism of benzo(alpha)pyrene in rat and
hamster liver S9 activation systems. Cancer Lett., 24: 281-289.
TEEL, R.W. & KAIN, S.R. (1984) Selenium modified mutagenicity
and metabolism of benzo(alpha)pyrene in an S9-dependent system.
Mutat. Res., 127: 9-14.
THOMPSON, H.J. (1984) Selenium as an anticarcinogen. J.
agric. food Chem., 32: 422-425.
THOMPSON, J.N. & SCOTT, M.L. (1969) Role of selenium in the
nutrition of the chick. J. Nutr., 97: 335-342.
THOMPSON, J.N. & SCOTT, M.L. (1970) Impaired lipid and
vitamin E absorption related to atrophy of the pancreas in
selenium-deficient chicks. J. Nutr., 100: 797-809.
THOMPSON, J.N., ERDODY, P., & SMITH, D.C. (1975) Selenium
content of food consumed by Canadians. J. Nutr., 105: 274-277.
THOMPSON, K.C. (1975) The atomic-fluorescence determination
of antimony, arsenic, selenium and tellurium by using the
hydride generation technique. Analyst, 100: 307-310.
THOMPSON, R.H., MCMURRAY, C.H., & BLANCHFLOWER, W.J. (1976)
The levels of selenium and glutathione peroxidase activity in
blood of sheep, cows and pigs. Res. vet. Sci., 20: 229-231.
THOMSON, C.D. (1974) Recovery of large doses of selenium
given as sodium selenite with or without vitamin E. N.Z. med.
J., 80: 163-168.
THOMSON, C.D. (1985) Selenium dependent and non-selenium
dependent glutathione peroxidase in human tissues of New
Zealand residents. Biochem. Int., 10: 673-679.
THOMSON, C.D. & ROBINSON, M.F. (1980) Selenium in human
health with emphasis on those aspects peculiar to New
Zealand. Am. J. clin. Nutr., 33: 303-323.
THOMSON, C.D. & ROBINSON, M.F. (1986) Urinary and faecal
excretions and absorption of a large supplement of selenium as
selenite or as selenate. Am. J. clin. Nutr. (submitted for
publication).
THOMSON, C.D. & STEWART, R.D.H. (1973) Metabolic studies of
75Se selenomethionine and 75Se selenite in the rat. Br. J.
Nutr., 30: 139-147.
THOMSON, C.D. & STEWART, R.D.H. (1974) The metabolism of
75Se selenite in young women. Br. J. Nutr., 32: 47-57.
THOMSON, C.D., ROBINSON, B.A., STEWART, R.D.H., & ROBINSON,
M.F. (1975a) Metabolic studies of 75Se selenocystine and
75Se selenomethionine in the rat. Br. J. Nutr., 34: 501-509.
THOMSON, C.D., STEWART, R.D.H., & ROBINSON, M.F. (1975b)
Metabolic studies in rats of 75Se selenomethionine and of 75Se
incorporated in vivo into rabbit kidney. Br. J. Nutr., 33:
45-54.
THOMSON, C.D., REA, H.M., ROBINSON, M.F., & CHAPMAN, O.W.
(1977a) Low blood selenium concentrations and glutathione
peroxidase activities in elderly people. Proc. Univ. Otago
Med. Sch., 55: 18-19.
THOMSON, C.D., REA, H.M., DOESBURG, V.M., & ROBINSON, M.F.
(1977b) Selenium concentrations and glutathione peroxidase
activities in whole blood of New Zealand residents. Br. J.
Nutr., 37: 457-460.
THOMSON, C.D., BURTON, C.E., & ROBINSON, M.F. (1978) On
supplementing the selenium intake of New Zealanders. I. Short
experiments with large doses of selenite or selenomethionine.
Br. J. Nutr., 39: 579-587.
THOMSON, C.D., ROBINSON, M.F., CAMPBELL, D.R., & REA, H.M.
(1982) Effect of prolonged supplementation with daily
supplements of selenomethionine and sodium selenite on
glutathione peroxidase activity in blood of New Zealand
residents. Am. J. clin. Nutr., 36: 24-31.
THOMSON, C.D., ONG, L.K., & ROBINSON, M.F. (1985) Effect of
supplementation with high-selenium wheat bread on selenium,
glutathione peroxidase and related enzymes in blood components
of New Zealand residents. Am. J. clin. Nutr., 41: 1015-1022.
THORN, J., ROBERTSON, J., & BUSS, D.H. (1978) Trace
nutrients. Selenium in British food. Br. J. Nutr., 39: 391-396.
TINSLEY, I.J., HARR, J.R., BONE, J.F., WESWIG, P.H., &
YAMAMOTO, R.S. (1967) Selenium toxicity in rats. I. Growth
and longevity. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H.,
ed. Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 141-152.
TSEN, C.C. & COLLIER, H.B. (1959) Selenite as a relatively
weak inhibitor of some sulphydryl enzymes. Nature (Lond.),
183: 1327-1328.
TSEN, C.C. & TAPPEL, A.L. (1958) Catalytic oxidation of
glutathione and other sulfhydryl compounds by selenite. J.
biol. Chem., 233(5): 1230-1232.
TSONGAS, T.A. & FERGUSON, S.W. (1977) Human health effects
of selenium in a rural Colorado drinking-water supply. In:
Hemphill, D.D., ed. Trace substances in environmental health.
XI, Columbia, Missouri, University of Missouri Press, pp.
30-35.
ULLREY, D.E., BRADY, P.S., WHETTER, P.A., KU, P.K., & MAGEE,
W.T. (1977) Selenium supplementation of diets for sheep and
beef cattle. J. anal. Sci., 45: 559-565.
UNDERWOOD, E.J. (1977) Trace elements in human and animal
nutrition, 4th ed., New York, Academic Press, 545 pp.
US DEPARTMENT OF HEALTH AND HUMAN SERVICES, FOOD AND DRUG
ADMINISTRATION (1984) 21 CFR Pt.573.920 Selenium. Fed. Reg.,
49: 627-628.
US FDA (1974) Final environmental impact statement rule
making on selenium in animal feeds, Washington DC, US Food and
Drug Administration, 131 pp.
US FDA (1975) Bureau of Foods Compliance Program Evaluation
Report. Total diet studies, Fiscal Year 1974, Washington DC,
US Food and Drug Administration, 32 pp.
US NAS/NRC (197l) Selenium in nutrition, Washington DC,
National Academy of Science, National Research Council,
Agricultural Board, Committee on Animal Nutrition,
Subcommittee on Selenium, 79 pp.
US NAS/NRC (1976) Selenium, Washington DC, National Academy
of Science, National Research Council, Assembly of Life
Sciences, Medical and Biological Effects of Environmental
Pollutants, 203 pp.
US NAS/NRC (1979) Zinc, Baltimore, Maryland, National
Academy of Science, National Research Council, Assembly of
Life Sciences, Committee on Medical and Biologic Effects of
Environmental Pollutants, 471 pp.
US NAS/NRC (1980) Recommended dietary allowances, Washington
DC, National Academy of Science, National Research Council,
Food and Nutrition Board, Committee on Dietary Allowances, 185
pp.
VAN DER LINDEN, R., DE CORTE, F., & HOSTE, J. (1974)
Activation analysis of biological material with ruthenium as a
multi-element comparator. Anal. Chim. Acta, 71: 263-275.
VAN KAMPEN, K.R. & JAMES, L.F. (1978) Manifestations of
intoxication by selenium-accumulating plants. In: Keeler,
R.F., Van Kampen, K.R., & James, L.F., ed. Effects of
poisonous plants on livestock, New York, Academic Press,
pp. 135-138.
VAN RIJ, A.M., ROBINSON, M.F., GODFREY, P.J., THOMSON, C.D., &
RHEA, H.M. (1978) Selenium blood levels in cancer and other
diseases in surgery. In: Hemphill, D.D., ed. Trace substances
in environmental health. XII, Columbia, Missouri, University
of Missouri Press, pp. 157-163.
VAN RIJ, A.M., THOMSON, C.D., MCKENZIE, J.M., & ROBINSON,
M.F. (1979) Selenium deficiency in total parenteral
nutrition. Am. J. clin. Nutr., 32: 2076-2085.
VAN VLEET, J.F. (1976) Induction of lesions of selenium-
vitamin E deficiency in pigs fed silver. Am. J. vet. Res., 37:
1415-1420.
VAN VLEET, J.F., MEYER, K.B., & OLANDER, H.J. (1974) Acute
selenium toxicosis induced in baby pigs by parenteral
administration of selenium-vitamin E preparations. J. Am. Vet.
Med. Assoc., 165: 543-547.
VARO, P. & KOIVISTOINEN, P. (1980) Mineral element
composition of Finnish foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn,
Zn, Mo, Co, Ni, Cr, F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb,
and ash. XII. General discussion and nutritional evaluation.
Acta agric. Scand. Suppl., 22: 165.
VARO, P. & KOIVISTOINEN, P. (1981) Annual variations in the
average selenium intake in Finland: cereal products and milk
as sources of selenium in 1979/80. Int. J. Vit. Nutr. Res.,
51: 79-84.
VERNIE, L.N. (1984) Selenium in carcinogenesis. Biochim.
Biophys. Acta, 738(4): 203-217.
VERNIE, L.N., BONT, W.S., GINJAAR, H.B., & EMMELOT, P.
(1975) Elongation factor 2 as the target of the reaction
product between sodium selenite and glutathione (GSSeSG) in
the inhibiting of amino acid incorporation in vitro. Biochim.
Biophys. Acta, 414: 283-292.
VERNIE, L.N., GINJARR, H.B., WILDERS, I.T., & BONT, W.S.
(1978) Amino acid incorporation in a cell-free system derived
from rat liver studied with the aid of selenodiglutathione.
Biochim. Biophys. Acta, 518(3): 507-517.
VIRTAMO, J., VALKEILA, E., ALFTHAN, G., PUNSAR, S., HUTTUNEN,
J.K., & KARVONEN, M. (1985) Serum selenium and the risk of
coronary heart disease and stroke. Am. J. Epidemiol., 122(2):
276-282.
VOBECKY, M., PAVLIK, L., & BENES, J. (1977) Non-destructive
neutron activation assay of submicrogram quantities of
selenium. Radiochem. Radioanal. Lett., 29(4): 159-164.
VOBECKY, M., DEDINA, J., PAVLIK, L., & VALASEK, J. (1979)
Gamma-ray interferences in the determination of selenium by
the Inaa method. Radiochem. Radioanal. Lett., 38(3): 197-204.
VOLGAREV, M.N. & TSCHERKES, L.A. (1967) Further studies in
tissue changes associated with sodium selenate. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 179-184.
WAGNER, P.A., HOEKSTRA, W.G., & GANTHER, H.E. (1975)
Alleviation of silver toxicity by selenite in the rat in
relation to tissue glutathione peroxidase. Proc. Soc. Exp.
Biol. Med., 148: 1106-1110.
WAHLSTROM, R.C. & OLSON, O.E. (1959) The effect of selenium
on reproduction in swine. J. anim. Sci., 18: 141-145.
WALKER, G.W.R. & BRADLEY, A.M. (1969) Interacting effects of
sodium monohydrogenarsenate and selenocystine on crossing over
in Drosophila melanogaster. Can. J. Genet. Cytol., 11: 677-688.
WALKER, G.W.R. & TING, K.P. (1967) Effect of selenium on
recombination in barley. Can. J. Genet. Cytol., 9: 314-320.
WANG, Z., LI, C., & LI, L. (1985) An epidemiological
investigation on the selenium content of water, cereals and
hair of children in Heilongjiang province. Chinese J. end.
Dis., 4: 330-333.
WARRINGTON, P.B. (1979) Selenium. Eng. Min. J., 180: 149-150.
WASLIEN, C.I. (1976) Human intake of trace elements. In:
Prasad, A.S., ed. Trace elements in human health and disease.
II. Essential and toxic elements, New York, Academic Press,
pp. 347-370.
WATKINSON, J.H. (1967) Analytical methods for selenium in
biological material. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI publishing Co., Inc, pp. 97-117.
WATKINSON, J.H. (1974) The selenium status of New
Zealanders. N.Z. med. J., 80: 202-205.
WATKINSON, J.H. (1979) Semi-automated fluorimetric
determination of nanogram quantities of selenium in biological
materials. Anal. Chim. Acta, 105: 319-325.
WATKINSON, J.H. (1981) Changes of blood selenium in New
Zealand adults with time and importation of Australian wheat.
Am. J. clin. Nutr., 34: 936-942.
WATKINSON, J.H. & BROWN, M.W. (1979) New phase-separating
device and other improvements in the semi-automated
fluorimetric determination of selenium. Anal. Chim. Acta, 105:
451-454.
WEDDERBURN, J.F. (1972) Selenium and cancer. N.Z. vet. J.,
20: 56-57.
WEISSMAN, S.H., CUDDIHY, R.G., & BURKSTALLER, M.A. (1979)
Distribution and retention of inhaled selenious acid and
selenium metal aerosols in Beagle dogs. In: Hemphill, D.D.,
ed. Trace substances in environmental health. XIII, Columbia,
Missouri, University of Missouri Press, pp. 477-482.
WEISSMAN, S.H., CUDDIHY, R.G., & MEDINSKY, M.A. (1983)
Absorption, distribution, and retention of inhaled selenious
acid and selenium metal aerosols in Beagle dogs. Toxicol.
appl. Pharmacol., 67: 331-337.
WELSH, S.O. (1979) The protective effect of vitamin E and
N,N'-diphenyl- p-phenylenediamine (DPPD) against methyl mercury
toxicity in the rat. J. Nutr., 109: 1673-1681.
WELSH, S.O. & SOARES, J.H., Jr (1976) The protective effect
of vitamin E and selenium against methyl mercury toxicity in
the Japanese quail. Nutr. Rep. Int., 13: 43-51.
WELSH, S.O., HOLDEN, J.W., WOLF, W.R., & LEVANDER, O.A.
(1981) Selenium intake of Maryland residents consuming
self-selected diets. J. Am. Diet. Assoc., 79: 277-285.
WESTERMANN, D.T. & ROBBINS, C.W. (1974) Effect of S04-S
fertilization on Se concentration of alfalfa (Medicago sativa
L). Agron. J., 66: 207-208.
WESTERMARCK, T. (1977) Selenium content of tissues in
Finnish infants and adults with various diseases, and studies
on the effects of selenium supplementation in neuronal ceroid
lipofuscinosis patients. Acta pharmacol. toxicol., 41: 121-128.
WESTERMARCK, T., RAUNU, P., KIRJARINTA, M., & LAPPALAINEN, L.
(1977) Selenium content of whole blood and serum in adults
and children of different ages from different parts of
Finland. Acta pharmacol. toxicol., 40: 465-475.
WESWIG, P.H., TINSLEY, I.J., HARR, J.R., BONE, J.F., YAMAMOTO,
R.S., & FALK, H. (1966) Bioassay of selenium compounds for
carcinogenesis in rats. Final report, Corvallis, Oregon State
University, Departments of Agricultral Chemistry and
Veterinary Medicine, 131 pp.
WHANGER, P.D. (1976) Selenium versus metal toxicity in
animals. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 234-252.
WHANGER, P.D., MUTH, O.H., OLDFIELD, J.E., & WESWIG, P.H.
(1969) Influence of sulfur on incidence of white muscle
disease in lambs. J. Nutr., 97: 553-562.
WHANGER, P.D., PEDERSEN, N.D., & WESWIG, P.H. (1973)
Selenium proteins in ovine tissues. II. Spectral properties of
a 10,000 molecular weight selenium protein. Biochem. Biophys.
Res. Commun., 53: 1031-1035.
WHANGER, P.D., PEDERSON, N.D., HATFIELD, J., & WESWIG, P.H.
(1976a) Absorption of selenite and selenomethionine from
ligated digestive tract segments in rats. Proc. Soc. Exp.
Biol. Med., 153: 295-297.
WHANGER, P.D., WESWIG, P.H., SCHMITZ, J.A., & OLDFIELD, J.E.
(1976b) Effects of selenium, cadmium, mercury, tellurium,
arsenic, silver, and cobalt on White Muscle Disease in lambs
and effect of dietary forms of arsenic on its accumulation in
tissues. Nutr. Rep. Int., 14: 63-72.
WHEATCROFT, M.G., ENGLISH, J.A., & SCHLACK, C.A. (1951)
Effects of selenium on the incidence of dental caries in white
rats. J. dent. Res., 30: 523-524.
WHETTER, P.A. & ULLREY, D.E. (1978) Improved fluorometric
method for determining selenium. J. Assoc. Off. Anal. Chem.,
61: 927-930.
WHITEACRE, M.E., COMBS, G.F., & PARKER, R.S. (1983)
Peroxidative damage in nutritional pancreatic atrophy due to
selenium-deficiency in the chick. Fed. Proc. Fed. Am. Soc.
Exp. Biol., 42: 928.
WHITING, R.F., WEI, L., & STICH, H.F. (1980) Unscheduled DNA
synthesis and chromosome aberrations induced by inorganic and
organic selenium compounds in the presence of glutathione.
Mutat. Res., 78: 159-169.
WHO (1984) Guidelines for drinking water quality. Volume 2:
Health criteria and other supporting information, Geneva,
World Health Organization, 335 pp.
WILKIE, J.B. & YOUNG, M. (1970) Improvement in the 2,3-
diaminoaphthalene reagent for microfluorescent. Determination
of selenium in biological materials. J. agric. food Chem., 18:
944-945.
WILLETT, W.C., MORRIS, J.S., PRESSEL, S., TAYLOR, J.O., POLK,
B.F., STAMPFER, M.J., ROSNER, B., SCHNEIDER, K., & HAMES,
C.G. (1983) Prediagnostic serum selenium and risk of cancer.
Lancet (July): 130-134.
WILLIAMS, K.T. & BYERS, H.G. (1935) Occurrence of selenium
in the Colorado River and some of its tributaries. Ind. Eng.
Chem. Anal. Ed., 7: 431-432.
WILLIAMS, M.M.I. (1983) Selenium and glutathione peroxidase
in mature human milk. Proc. Univ. Otago Med. Sch., 61: 20-21.
WILSON, H.M. (1962) Selenium oxide poisoning. N.C. med. J.,
23: 73-75.
WILSON, P.S. & JUDSON, G.J. (1976) Glutathione peroxidase
activity in bovine and ovine erythrocytes in relation to blood
selenium concentration. Br. vet. J., 132: 428-434.
WITTING, L.A. & HORWITT, M.K. (1964) Effects of dietary
selenium, methionine, fat level, and tocopherol on rat growth.
J. Nutr., 84: 351-360.
WRIGHT, P.L. & BELL, M.C. (1966) Comparative metabolism of
selenium and tellurium in sheep and swine. Am. J. Physiol.,
211: 6-10.
WU, A.S.H., OLDFIELD, J.E., & WHANGER, P.D. (197l) Effect of
selenium, chromium and vitamin E on spermatogensis. J. anal.
Sci., 33: 273.
WU, A.S.H., OLDFIELD, J.E., WHANGER, P.D., & WESWIG, P.H.
(1973) Effect of selenium, vitamin E, and antioxidants on
testicular function in rats. Biol. Reprod., 8: 625-629.
WU, A.S.H., OLDFIELD, J.E., SHULL, L.R., & CHEEKE, P.R.
(1979) Specific effect of selenium deficiency on rat sperm.
Biol. Reprod., 20: 793-798.
XIA, X. & YU, S. (in press) Studies on the anti-carcinogenic
mechanisms of selenium - The effects of glucose oxidation and
related enzymes. Chinese J. Cancer.
YANG, G.Q., WANG, S., ZHOU, R., & SUN, S. (1983) Endemic
selenium intoxication of humans in China. Am. J. clin. Nutr.,
37: 872-881.
YANG, G., CHEN, J., WEN, Z., GE, K., ZHU, L., CHEN, X., &
CHEN, X. (1984) The role of selenium in Keshan disease. In:
Draper, H.H., ed. Advances in nutritional research, New York,
Plenum Press, pp. 203-231.
YANG, G., ZHOU, R., YIN, S., PIAO, J., ZHU, L., LIN, S., & GU,
L., MAI, R., XU, J., QIAG, F., LU, M., DENG, X., HUANG, J.,
LIN, & ZHOU, W. (1985) [Studies on the selenium requirement
of Chinese people. I. Physiological requirement, minimum
requirement, and minimum allowance.] J. Inst. Health, 14:
24-28 (in Chinese).
YANG, G.Q., ZHU, L.Z., LIU, S.J., GU, L.Z., QIAN, P.C., HUANG,
J.H., & LU, M.D. (1986) Studies of human selenium requirements
in China. In: Combs, G.F., Spallholz, J.E., Levander, O.A., &
Oldfield, J.E., ed. Selenium in biology and medicine, 3rd
Symposium, Westport, Connecticut, AVI Publishing Company.
YASUMOTO, K., IWAMI, K., YOSHIDA, M., & MITSUDA, H. (1976)
Selenium content of foods and its average daily intake in
Japan. Eiyo To Shokuryo, 29: 511-515.
YEH, Y. & JOHNSON, R.M. (1973) Vitamin E deficiency in the
rat. IV. Alteration in mitochondrial membrane and its relation
to respiratory decline. Arch. Biochem. Biophys., 159: 821-831.
YOUNG, S. & KEELER, R.F. (1962) Nutritional muscular dystrophy
in lambs. The effect of muscular activity on the symmetrical
distribution of lesions. Am. J. vet. Res., 23: 966-971.
YU, W.H. (1982) A study of nutritional and bio-geochemical
factors in the occurrence and development of Keshan disease.
Jpn Circ. J., 46: 1201-1207.
YU, S., CHU, Y., GONG, X., & HOU, C. (1985) Region variation
of cancer mortality incidence and its relation to selenium
levels in China. Ecol. trace Element Res., 7: 21-23.
YU, S., ZHU, Y., HON, C., & HUANG, C. (in press) Selective
effects of selenium on the function and structure of
mitochondria isolated from hepatoma and normal liver. In:
Proceedings of the Third International Symposium of Selenium
in Biology and Medicine.
ZABEL, N.L., HARLAND, J., GORMICAN, A.T., & GANTHER, H.E.
(1978) Selenium content of commercial formula diets. Am. J.
clin. Nutr., 31: 850-858.
ZHU, L. & LU, Z. et al. (1981) Effects in pigs fed the crops
grown in Keshan disease affected province of China. In:
Howell, J.M., Gawthone, J.M., & White, C.L., ed. Trace
Element Metabolism in Man and Animals (TEMA 4).
ZOLLER, W.H. & REAMER, D.C. (1976) Selenium in the
atmosphere. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 54-66.
ZOLLER, W.H., GLADNEY, E.S., & DUCE, R.A. (1974) Atmospheric
concentrations and sources of trace metals at the South Pole.
Science, 183: 198-200.
See Also:
Toxicological Abbreviations
Selenium (ICSC)
Selenium (PIM 483)
 4. LEVELS IN ENVIRONMENTAL MEDIA
4.1. Levels and Chemical Forms of Selenium in Food
4.1.1. Levels in food
In spite of the relatively few data available, some
generalizations concerning the selenium content of foodstuffs can
be made. For example, the level of selenium in food depends on
natural differences among food commodities and the natural
availability of selenium in the environment. Moreover, certain of
man's activities can influence the selenium content of human foods.
4.1.1.1. Natural differences among food commodities
A wide range of values has been reported in the selenium
content of foods (mg/kg wet weight): liver, kidney, and seafood,
0.4 - 1.5; muscle meats, 0.1 - 0.4; cereal and cereal products, <
0.1 - > 0.8; diary products, < 0.1 - 0.3; and fruits and
vegetables, < 0.1) (Oelschlager & Menke, 1969; Morris & Levander,
1970; Schroeder et al., 1970; Suchkov, 1971; Arthur, 1972; Millar &
Sheppard, 1972; Ferretti & Levander, 1974; Sakurai & Tsuchiya,
1975; Abutalybov et al., 1976; Bieri & Ahmad, 1976; Kasimov et al.,
1976; Olson et al., 1978). It should be pointed out that these
values are given for raw foods (food as purchased) rather than
cooked foods (food as eaten). The effects of cooking on the
selenium content of foods are described in section 4.1.1.3.
Moreover, the values presented in these food composition studies
should not be compared from one country to another, because of the
variations in the analytical methods and sampling procedures used
(section 2).
Organ meats, such as kidneys or liver, contain the highest
levels of selenium, but some seafood products contain almost as
much. Muscle meats are significant sources of selenium, though
they do not contain as much as organ meats or seafoods. Certain
semolina, grain, and cereal products can contribute appreciably to
the dietary-selenium intake, but wide variations in selenium
content have been found in different samples of the same foodstuff.
Such variation is typical of many plant foods and the reasons for
this are discussed below. Milk, cheese, and egg samples from
several countries showed low to moderate values for selenium, but
again the results were quite variable. Fruits and vegetables
generally contained very low levels of selenium, though garlic and
mushrooms contained moderate levels of the element.
The selenium contents of baby foods tended to show the same
general pattern as those in adult foods (Morris & Levander, 1970;
Arthur, 1972), i.e., meat and cereal products contained the highest
levels and fruit and vegetable products, the lowest. Shearer &
Hadjimarkos (1975) analysed samples of mature human milk from 241
subjects living in the USA and found that the overall mean selenium
content was 0.018 mg/litre. Over 98% of the samples contained
between 0.007 and 0.033 mg/litre. Grimanis et al. (1978) reported
an average of 0.015 mg/litre in 5 samples of mature human milk
collected in Greece. These authors found slightly higher values in
15 samples of human transitional milk (0.016 mg/litre) and
colostrum (0.048 mg/litre). A slightly lower average selenium
concentration of 0.013 mg/litre, was reported for samples of human
transitional milk in New Zealand (Millar & Sheppard, 1972). The
most extreme values for the selenium content of human milk were
reported from China and ranged from 0.0026 mg/litre in areas where
Keshan disease was prevalent to 0.283 mg/litre in high-selenium
areas (Yang et al., 1986).
It can be seen from Table 3 that meat-based infant formulae
have a higher selenium content than formulae based on milk or soy
protein, and that casein-based powdered formulae for special
medical purposes (diet therapy for errors in amino acid metabolism
or malabsorptive states) contain low levels of selenium. Blended
food tube-feeding formulae containing meat tend to have higher
selenium contents than comparable products containing only milk or
casein and soy protein (Table 3). Chemically-defined diets having
egg albumen as the protein source contained more selenium than
diets based on casein hydrolysate, which, in turn, contained more
selenium than diets based on purified amino acids. Total
parenteral nutrition solutions based on casein hydrolysate also
contain more selenium than solutions based on amino acid mixtures,
which contain very low levels of selenium.
Table 3. Selenium content of commercial formula dietsa
-----------------------------------------------------------------
Product Selenium content
(mg/kg wet (mg/kg dry
weight) weight)
-----------------------------------------------------------------
Infant formulae:
milk-based 0.004 - 0.027
soy-based 0.004 - 0.030
meat-based 0.046 - 0.070
casein-based formulae for special 0.048 - 0.120
medical purposes (powders)
Food supplements and tube-feeding
formulae:
milk-based 0.005 - 0.045
soy-casein-based 0.013 - 0.020
blended foods 0.037 - 0.056
Chemically defined diets:
(powders)
egg albumen low residue 0.224 - 0.351
egg albumen moderate nitrogen 0.388 - 0.886
egg albumen high nitrogen 0.503 - 0.570
casein hydrolysate 0.052 - 0.071
amino acid mixture 0.001 - 0.011
-----------------------------------------------------------------
Table 3. (contd.)
-----------------------------------------------------------------
Product Selenium content
(mg/kg wet (mg/kg dry
weight) weight)
-----------------------------------------------------------------
Total parenteral nutrition
solutions:
casein hydrolysate 0.037 0.324
(0.032 - 0.041)
diluted 1:1 with 50% dextrose 0.019 0.093
solution (0.017 - 0.020)
amino acid mixture 0.001 0.010
-----------------------------------------------------------------
a Adapted from: Zabel et al. (1978).
One of the factors that can influence the amount of selenium in
plant foodstuffs is the nature of the plant itself. Plants have
been divided into 3 groups depending on their tendency to take up
selenium from seleniferous soils (Rosenfeld & Beath, 1964):
Group 1 Primary selenium accumulators - can contain very
high amounts of the element (often over 1000
mg/kg dry weight).
Group 2 Secondary selenium accumulators - rarely contain
more than a few hundred mg/kg.
Group 3 Many weeds and most crop plants, grains, and
grasses - rarely contain more than 30 mg/kg, even
when grown on seleniferous soils; when grown on
normal soils generally contain less than 1 mg/kg.
Plants from Groups 1 and 2 do not usually contribute to the
selenium intake of human beings, since they are not consumed
directly by people and are consumed by animals only when other
feeds are not available. However, plants from Group 3 can
contribute large amounts of selenium, if they are grown in
seleniferous areas (see below).
The concentrations of many nutritionally essential trace
elements are known to be decreased by the milling of grains into
cereals (Czerniejewski et al., 1964) and preliminary analysis of
random supermarket samples of cereal foods suggested that refined
products such as white flour or white bread contained less selenium
than whole grain foods such as whole wheat flour or whole wheat
bread (Morris & Levander, 1970). However, a subsequent more
controlled analytical study in which various grain fractions were
taken from the same production batch showed that milling a variety
of grains decreased the concentration of selenium in the consumer
product by only 10 - 30% (Ferretti & Levander, 1974). Thus, the
selenium content of cereal products is somewhat less than that of
the parent grains but the decreases in concentration are not nearly
as great as those observed with other essential trace minerals.
Selenium tends to be localized mainly in the protein fraction
of plant and animal tissues; thus, the protein content of a food
also influences its selenium content. For example, the
concentration of selenium in a series of soybean products prepared
from a given lot of soybeans increased as the protein content of
the product increased (Ferretti & Levander, 1976). However,
protein content is only an expression of the potential of a food to
contain selenium, and a food high in protein will not necessarily
also be high in selenium. Moreover, non-protein seleno amino acids
occur in some foods and thus, the protein content may not always
reflect the selenium content.
4.1.1.2. Effects of natural differences in the availability of
selenium in the environment on levels in food
The most important factor in determining the selenium content
of plant foods and feeds is the amount of selenium in the soil that
is available for uptake by the plant. Sun et al. (1985) reported
that the selenium content of local crops was significantly
correlated with the selenium content of the local soil, the
correlation coefficients of soybean, corn, and rice being 0.9456,
0.9953, and 0.9954, respectively. Since the water-soluble selenium
in the soil was directly correlated with the pH and inversely
correlated with humin and total iron in the soil, the selenium
content of local crops was also influenced by the amount of water-
soluble selenium in the soil. An analytical survey carried out in
the USA demonstrated that the level of selenium in alfalfa plants
varied from less than 0.01 to more than 5.0 mg/kg, and it was
assumed that these levels reflected the amount of available
selenium in the soil (Kubota et al., 1967). A variation in the
selenium content of wheat from 0.04 to 21.4 mg/kg, depending on
where the plant was grown, was reported by Schroeder et al. (1970).
Samples of several foods bought in local markets in Caracas,
Venezuela contained much more selenium than similar foods purchased
in supermarkets in the eastern USA (Table 4). The likely source of
selenium in the milk, eggs, and meat from Caracas is sesame cake,
since sesame is produced mostly in the seleniferous area of
Venezuela and the pressed cake is widely used as an ingredient in
animal feed. Selenium levels as high as 14 mg/kg in corn and 18
mg/kg in rice have also been observed in certain food samples taken
from high-selenium regions in Venezuela (Jaffe, 1976). These
concentrations of selenium are as high as those reported in foods
from seleniferous zones of the USA (Smith & Westfall, 1937).
Great extremes in the selenium content of staple foods have
also been reported recently from China (Yang et al., 1983). For
example, samples of corn, rice, and soybeans, taken from a high-
selenium area with a history of human intoxication reported as
chronic selenosis (section 8.1.1.1) contained average selenium
levels of 8.1, 4.0, and 11.9 mg/kg, respectively, whereas samples
of the same staples collected in a low-selenium area where Keshan
disease was prevalent (a human selenium-deficiency disease)
(section 8.2.2) contained average selenium levels of only 0.005,
0.007, and 0.010 mg/kg, respectively (Table 5). A low selenium
content in food has also been reported in other countries with low
selenium soils, such as New Zealand and Finland. Typical ranges
for selenium contents in food in such countries include (mg/kg wet
weight): liver, kidney, and seafood, 0.09 - 0.92; muscle meats,
0.01 - 0.06; cereal and cereal products, 0.01 - 0.07; milk, <
0.01; and fruits and vegetables, < 0.01 - 0.02 (Koivistoinen,
1980; Thomson & Robinson, 1980). Samples of staple foods collected
in areas where soils contained intermediate levels of selenium also
contained intermediate levels of selenium.
Table 4. Comparison of selenium contents
of selected foods available in Caracas,
Venezuela, and Beltsville, Maryland, USA
(mg selenium/kg wet weight)a
-----------------------------------------
Food Caracas Beltsville
-----------------------------------------
Powdered milk 0.417 0.169
Whole milk 0.115 0.012
American-type cheese 0.425 0.090
Swiss-type cheese 0.382 0.104
Pork 0.833 0.209
Chicken 0.702 0.106
Egg 1.520 0.116
-----------------------------------------
a From: Mondragon & Jaffe (1976).
As might be expected, the selenium contents of food products of
animal origin depend heavily on the amount of naturally-occurring
selenium in the feed given to the animal. In the USA, it was shown
that the selenium content of swine muscle was highest in areas
known to have a high level of available selenium in the soil and
lowest in areas in which the available soil-selenium was low (Ku et
al., 1972). This indicates that selenium is readily passed up the
soil-plant-animal food chain to human beings.
4.1.1.3. Man-induced changes in selenium levels in food
(a) Human activities that increase selenium levels
Perhaps the most direct way that man's activities can increase
the selenium content of the food supply is the deliberate addition
of selenium to the feeds of poultry and certain livestock. In some
countries, this is now accepted practice to prevent the occurrence
of selenium-deficiency diseases, many of which cause significant
economic losses to farmers throughout the world. In the USA, for
example, farmers are permitted to add 0.1 mg selenium/kg (as sodium
selenite or selenate) complete feed for beef and dairy cattle,
sheep, chickens, ducks, swine (0.3 mg/kg in starter and prestarter
rations), and 0.2 mg/kg for turkeys (US Department of Health and
Human Services, Food and Drug Administration, 1984; Subcommittee on
Selenium - Committee on animal Nutrition, 1983). It has been shown
that the edible tissues of poultry, swine, and sheep, fed diets
fortified with inorganic selenium salts at the regulated levels,
did not contain any more selenium than the tissues of animals fed
diets, naturally adequate in selenium (Allaway, 1973; Ullrey et
al., 1977). The homeostatic mechanisms that appear to limit the
concentrations of selenium in the edible tissues of animals fed
certain levels of sodium selenite are discussed further in section
6.
Table 5. Selenium contents of staple foods grown on soils in areas of China with excess,
moderate, and deficient levels of seleniuma
-----------------------------------------------------------------------------------------
Place Corn Rice Soybean
Number Se content Number Se content Number Se content
of (mg/kg) of (mg/kg) of (mg/kg)
samples samples samples
-----------------------------------------------------------------------------------------
High-selenium area 44 8.1 22 4.0 17 11.9
with a history of (0.5-28.5)b (0.3-20.2)b (5.0-22.2)b
intoxication reported
as chronic selenosis
High-selenium area 2 0.57 2 0.97 2 0.34
reported to be without
selenosis
Moderate-selenium- 82 0.036 76 0.035 31 0.069
adequate area (± 0.056) (± 0.027) (± 0.076)
(Beijing)
Low-selenium area 10 0.009 32 0.022 - -
(± 0.009) (± 0.009)
Low-selenium area 195 0.005 49 0.007 150 0.010
with Keshan disease (± 0.003) (± 0.003) (± 0.008)
-----------------------------------------------------------------------------------------
a Adapted from: Yang et al. (1983).
b Mean ± SD or range shown in parenthesis.
Finland is the first country to decide to increase the selenium
content of Finnish feed and food by the addition of sodium selenate
to fertilizers, to be used in the whole country at a concentration
of 16 or 6 mg/kg for cereal and grassland crops, respectively
(Koivistoinen & Huttunen, 1985). The manufacture of these
selenized fertilizers began in the summer of 1984 and they will be
used at application rates of 10 g selenium/ha per growing season.
It is anticipated that the added selenium in the fertilizers will
be transported to the human food chain, and the selenium level of
the cereal and grassland crops will be raised to 0.1 and 0.15 -
0.20 mg/kg (dry basis), respectively.
Another potential way in which the level of selenium in the
food chain might be increased by man's activities is the proposed
use of selenium-bearing fly ash as a soil supplement. Furr et al.
(1977) analysed cabbages grown on potted soil supplemented with
several different fly ashes and found that the selenium levels in
the cabbages were closely correlated with those in the respective
fly ashes in which the plants were cultured. The ready
bioavailability to animals of the selenium in plants grown on fly
ash was demonstrated in a study (Stoewsand et al., 1978) in which
Japanese quail were fed a complete diet containing 60% winter wheat
that had been grown to maturity on either soil or a deep bed of fly
ash. The soil contained 2.1 mg selenium/kg and the wheat grown
thereon contained 0.02 mg/kg, a level typical of wheat from
selenium-deficient areas. The fly ash contained 21.3 mg
selenium/kg, and the wheat grown on it contained 5.7 mg/kg, a level
sometimes found in wheat from naturally seleniferous areas. The
levels of selenium in the tissues of the quail fed the wheat grown
on fly ash were much higher than those of quail fed the wheat grown
on soil (Table 6). Moreover, the selenium contents of eggs from
quail fed the wheat grown on fly ash were 3.5 mg/kg in the yolk and
almost 10 mg/kg in the white compared with 0.5 and 0.2 mg
selenium/kg, respectively, in eggs from quail fed the control
wheat. Obviously, the use of selenium-bearing fly ash as a soil
supplement to provide nutritionally desirable levels of selenium in
plants should be carried out with care, since inappropriate use
could lead to an unwanted build-up of selenium residues in the food
chain. Also, the application of fly ash to soil at rates
sufficient to correct selenium deficiency in animals may damage the
soil.
Table 6. Selenium in tissues of male Japanese quail fed winter
wheat grown on soil or fly asha
-------------------------------------------------------------------
Wheat grown Tissue selenium
on: brain heart kidney liver muscle
-------------------------------------------------------------------
(mg/kg dry weight)
Soil 0.8 ± 0.1 0.7 ± 0.2 3.6 ± 0.4 1.6 ± 0.0 0.3 ± 0.2
Fly ash 3.4 ± 1.6 4.4 ± 0.7 9.5 ± 1.1 12.7 ± 2.4 4.1 ± 0.6
-------------------------------------------------------------------
a From: Stoewsand et al. (1978).
The possibility has been raised that some selenium may be added
to the food supply as a result of atmospheric contaminants from
the burning of fossil fuel or industrial emissions settling out on
the leaves of plants or on the surface of the soil. The
geographical pattern of selenium levels in rain-water from Denmark
or the USA implicates airborne selenium from the industrial and
domestic uses of fossil fuel as sources (Kubota et al., 1975).
Total industrial emissions of selenium in the USA for the year 1970
were estimated to be about 1.1 million kg, 62% of which was derived
from the burning of coal (US NAS/NRC, 1976). This level of
industrial emission of selenium may be compared with a projected
annual use of selenium as a feed additive for chickens and swine in
the "low-selenium" areas of the USA of 6000 kg (US FDA, 1974). But
there is no evidence that the selenium deposited in rainwater has
any influence on the concentration of selenium in forage plants
grown in Denmark or in the heavily industrialized northeastern USA
(Kubota et al., 1975). Furthermore, selenium deficiency has been
observed in farm livestock grazing near coal-burning electric power
stations (Anonymous, 1975). This suggests that airborne selenium
exists in the form of inert elemental selenium, insoluble selenide
salts, or as selenium dioxide, which would be tightly bound by
acidic, iron-bearing soils. In any case, the selenium would not be
available for uptake by plants; thus, there would appear to be
little prospect of adding appreciable selenium to the food supply
via atmospheric contamination.
(b) Human activities that decrease selenium levels in the food
chain
The metabolic antagonism between sulfur and selenium (Levander,
1976a) led early workers to suggest that borderline selenium
deficiency might be exacerbated by sulfate fertilization (Schubert
et al., 1961). More recent research has demonstrated that sulfate
fertilization can result in decreased selenium concentrations in
forage plants (Pratley & McFarlane, 1974; Westermann & Robbins,
1974). In many cases, this decrease can be largely explained by a
dilution effect caused by a growth response of the plant to the
sulfate fertilization, but decreases in selenium concentration
independent of crop yield have also been reported. Such decreases
could be the result of competitive interference by sulfate with the
uptake of selenate by plants. The use of phosphate fertilizers in
parts of Australia and New Zealand has resulted in an apparent
increase in the incidence of selenium deficiency in livestock
(Judson & Obst, 1975), but others have reported increases in the
selenium content of forages after fertilization with phosphorus
(Robbins & Carter, 1970; Carter et al., 1972). These conflicting
results may be due to differences in the selenium content of the
phosphatic rock from which the fertilizers were prepared.
Another possible way in which man's activities might decrease
selenium in the food chain or render it less available, is through
heavy metal pollution. For example, silver has been demonstrated
to antagonize selenium in a wide variety of situations (Diplock,
1976). In an area where there has been a large amount of silver
mining activity, muscular dystrophy of the type usually associated
with selenium deficiency has been reported in calves fed a ration
based on milk powder, even though the selenium content of the milk
seemed to be adequate. Apparently, the milk powder was derived
from cows that grazed land containing high levels of silver
residues from the old mining industry. Further research is needed
to establish fully the role of silver in potentiating these field
cases of apparent selenium deficiency. Because of the many
interactions of selenium with other environmental pollutants such
as mercury, cadmium, or arsenic (section 7), it is possible that
other cases of heavy metal-induced selenium deficiency will be
discovered.
The influence of cooking on the selenium content of foods was
determined because of the well-known instability and volatility of
many selenium compounds. Certain vegetables that normally contain
high levels of selenium such as asparagus or mushrooms, lost up to
40% of their selenium content as a result of boiling (Higgs et al.,
1972). Majstruk & Suchkov (1978) reported that up to 50% of the
original selenium was lost from vegetables and dairy products
during cooking; the addition of salt and acid pH or extended
cooking, particularly promoted such losses. But, other typical
cooking procedures such as boiling cereals, baking poultry or fish,
or broiling meats had little effect on selenium levels in the food
(Higgs et al., 1972). Similar results were observed by others for
baking fish or broiling meats, though some decrease in selenium
concentration was caused by frying organ meats or fish (Ganapathy
et al., 1975). Thompson et al. (1975) determined the selenium
contents of cooked food composites and found a rough agreement with
the calculated values based on unprepared foods from which they
were derived. On the basis of all these studies, it can be
concluded that usual cooking procedures cause little decrease in
the selenium content of most foods.
4.1.2. Chemical forms of selenium in food
There is little information on the chemical forms of selenium
that occur in human food (Levander, 1986). Cappon & Smith (1982)
reported that canned tuna fish contained variable percentages of
hexavalent selenium, ranging from 7.6 to 44.8%, which was
independent of the total selenium content. The other forms of
selenium in the tuna were di- and quadrivalent selenium. On an
average percentage basis, hexavalent selenium was more easily
extractable by water than di- or quadrivalent selenium. In
recently canned samples, an average of 55.6% of the total selenium
content was water-extractable. However, for the older samples, the
corresponding average extractable level was 48.2%. The results
suggest that sample storage may influence the chemical form of
selenium in canned tuna. Olson et al. (1970) found that, in a
certain portion of high selenium wheat (the pronase hydrolysate of
gluten), about half of the selenium was in the form of
selenomethionine. About 15% of the selenium in water extracts of
seleniferous cabbage leaves was in the form of selenomethionine,
but appreciable amounts of other selenium compounds were also
present (Hamilton, 1975). The chemical forms of selenium in food
are likely to affect the bioavailability of selenium (section 7.2.2
and 8.2.3).
4.2. Drinking-Water
Analysis of samples taken from various public water-supply
systems in the USA showed that less than 0.5% contained selenium
levels that exceeded the US PHS limit of 10 µg/litre (Taylor, 1963;
Lakin & Davidson, 1967; McCabe et al., 1970). Samples from 1280
central water sources providing water for 6.5 million Bulgarians
contained less than 2 µg selenium/litre (Gitsova, 1973). Tap water
from Stockholm, Sweden contained only 0.06 µg/litre (Lindberg,
1968) and tap and mineral waters from Stuttgart, Federal Republic
of Germany contained 1.6 and 5.3 µg/litre, respectively
(Oelschlager & Menke, 1969). Surface water sources in different
subregions of the Cernovici region of the Ukrainian SSR contained
selenium levels ranging from 0.09 ± 0.01 to 3.00 ± 0.40 µg/litre;
ground-water levels ranged from 0.07 ± 0.0 to 4.00 ± 0.85 µg/litre
(Suchkov & Kacap, 1971). A few tenths to several µg per litre were
reported in non-seleniferous regions of the USSR, the maximum
reported value being 5.1 µg/litre. In Argentina, the selenium
content of 22 surface waters varied from < 2 to 19 µg/litre with a
median value of 3 µg/litre (WHO, 1984).
Elevated levels of selenium (> 100 µg/litre) can be found in
seeps, springs, and shallow wells, though waters from deep wells
contain only a few µg/litre (US NAS/NRC, 1976). Highly variable
amounts of selenium were reported in wells from seleniferous areas
in the USA ranging from non-detectable levels to 330 µg/litre
(Smith & Westfall, 1937). Some well waters contained enough
selenium to be considered as poisonous for man or livestock and
loss of hair and nails in children was attributed to selenosis, but
the evidence for this was not considered convincing (US NAS/NRC,
1976).
The average selenium content of 11 samples of drinking-water
taken from a high-selenium area in China with a history of disease
reported as chronic selenosis was 54 µg/litre (Yang et al., 1983).
Four of these samples were surface water from a village with
previous heavy intoxication and these averaged 139 (117 - 159)
µg/litre. Such water would contribute substantial amounts of
selenium, but would still comprise a small fraction of the total
selenium intake in this area, since the contribution from food was
estimated to be about 5000 µg/day (section 5.1.1.1). The other 7
samples of drinking-water originated from several sources and
averaged only 5 µg/litre.
Rosenfeld & Beath (1964) concluded that selenium does not occur
in water in sufficient amounts to produce selenium toxicity in man
or animals, except in isolated cases. This was confirmed and
expanded in the statement by US NAS/NRC (1976, 1980) that waters
are rarely a significant source of selenium from either a
nutritional or a toxicity point of view. The low concentrations of
selenium in drinking-water are probably the result of several
mechanisms that act to decrease the selenium content of waters
(section 2.1).
4.3. Air
Selenium levels in the air breathed by the general population
are probably well below 10 ng/m3, on average (US NAS/NRC, 1976).
Hashimoto & Winchester (1967) found 0.3 - 1.6 ng airborne
selenium/m3 in an urbanized area. Levels of 3.6 - 9.7 ng
selenium/m3 were detected by Pillay et al. (1971), who showed that
over half of the selenium was not retained on filters able to trap
particulates greater than 0.1 µg in diameter. Zoller & Reamer
(1976) concluded that atmospheric levels of selenium in most urban
regions vary from 0.1 to 10 ng/m3. Selenium levels in air around
industries that use selenium can be higher (section 3.2.2), perhaps
of the order of a few µg/m3. Work-place air in selenium industries
apparently contained mg levels of selenium/m3 in the past, but more
recent measurements have indicated lower levels (section 5.2).
5. HUMAN EXPOSURE
5.1. Estimate of General Population Exposure
As discussed in section 4, selenium levels in air are low in
areas without selenium-emitting industries and are not likely to
exceed 10 ng/m3. Assuming that a person respires 20 m3 air daily,
the contribution to daily selenium intake via this route would be
0.2 µg or less. Since this intake is much lower than that through
food (see below), airborne selenium makes a negligible contribution
to the average daily selenium intake of the general population.
However, levels of selenium may be somewhat higher in the
atmosphere around some industrial plants using selenium (section
3.2.2). If it is assumed that 3 µg selenium/m3 is found in the air
near these industries, then exposure to selenium via air would be
60 µg/day.
Other possible exposures of the general population to selenium
via the air include contributions from house dust and tobacco
smoke. Lakin & Davidson (1967) reported that house or office dust
could contain up to 10 mg selenium/kg. Although no data are
available to provide an estimate of human exposure to selenium from
this source, the possibility of such exposure should be considered
because of the increased interest in the quality of indoor air.
Olsen & Frost (1970) found an average of 0.08 mg selenium/kg (range
0.03 - 0.13 mg/kg) in a variety of cigarette tobaccos. If it is
assumed that a cigarette contains 1 g tobacco and that all the
selenium in tobacco is volatilized and inhaled during smoking, it
can be calculated that a person smoking one pack of 20 cigarettes
per day would inhale an average of 1.6 µg from this source.
Most drinking-water supplies contain only small quantities of
selenium, except possibly for those in certain seleniferous areas.
As discussed in section 4.2, most public water supplies contain
much less than 10 µg selenium/litre. Assuming that a person drinks
2 litres of water daily, the intake via this route would be only a
few µg. While this is considerably higher than the intake via air,
in most areas of the world, this is still a small fraction of the
daily intake from food.
5.1.1. Food
5.1.1.1. Geographical variation
Both the amount and the bioavailability of dietary selenium are
determinants of its biological effects. Bioavailability is be
considered in sections 7.2.2 and 8.2.3. The estimated daily intake
of selenium from dietary sources by adult human beings varies
considerably in different parts of the world. The greatest
extremes in intake have been found in China (Table 7) where both
selenium toxicity and deficiency have been reported (section
8.1.1.1, 8.2.2). An average selenium intake of 4990 µg/day was
estimated in a high-selenium area of China with a history of
intoxication reported as chronic selenosis, whereas an average
intake of only 11 µg/day was estimated in an area of China where
Keshan disease, a cardiomyopathy related to low selenium status,
was reported. Intermediate intakes of selenium were found in areas
of China that were not affected with either selenosis or Keshan
disease. It should be pointed out that the dietary-selenium intake
in the high-selenium area with a history of intoxication reported
as selenosis did not overlap with that in the high-selenium area
reported to be without selenosis. Likewise, the dietary-selenium
intake in the low-selenium area with Keshan disease did not overlap
with that in the moderate-selenium area (Beijing).
Table 7. Daily selenium intake of residents living in high-, medium-, and low-selenium
areas of Chinaa
---------------------------------------------------------------------------------------
Number Daily selenium intake Se intake from staple
Place of minimum maximum average cereals as % of total
subjects (µg) daily intake
---------------------------------------------------------------------------------------
High-selenium area with 6 3200 6690 4990 28-70%
a history of intoxication
reported as chronic
selenosis
High-selenium area 3 240 1510 750 25-45%
reported as without
selenosis
Moderate-selenium area 8 42 232 116 various sources
(Beijing)
Low-selenium area with 13 3 22 11 mainly from cereals
Keshan disease
---------------------------------------------------------------------------------------
a Adapted from: Yang et al. (1983).
Less extreme, but still widely diverse intakes of selenium have
been observed in other parts of the world (Table 8). The selenium
intake calculated in New Zealand, a country with low levels of
selenium in the soils in certain areas, averaged 30 µg/day (Thomson
& Robinson, 1980; Watkinson, 1981). Similar low intakes have been
reported by other research workers in New Zealand. For example,
Stewart et al. (1978) showed that the mean dietary-selenium intake
of 4 New Zealand women consuming normal diets ad libitum was
24.2 µg/day, and Griffiths (1973) reported that the daily intake of
selenium by 13 young New Zealand women varied from 6 to 70 µg.
Daily intakes of 30 µg or more in the latter group were associated
with the inclusion of liver, kidney, or fish in the diet. A low
average dietary-selenium intake (30 µg/day) has also been reported
in Finland, another country known to have soils low in selenium
(Varo & Koivistoinen, 1980). Low levels of dietary selenium may
also occur in Italy (Rossi et al., 1976) and Egypt (Waslien, 1976).
The average daily intake of selenium in the United Kingdom was
estimated to be 60 mg (Thorn et al., 1978). In one study, the
dietary-selenium intake in Japan was estimated to be 88 µg/day
(Sakurai & Tsuchiya, 1975). A higher estimate in another study was
arrived at largely because higher selenium contents of food staples
were used in the calculations (Yasumoto et al., 1976). The
estimated dietary intake of selenium in North America ranged from
98 to 224 µg/day. A "Market basket" survey carried out in the USA
from 1974 to 1982 indicated an average overall selenium intake of
108 µg/day (Pennington et al., 1984). An earlier survey had
revealed regional differences in intake, with people living in
western parts of the country consuming 1.3 times as much selenium
as people in the northeastern part of the country (US FDA, 1975).
Duplicate plate analyses of self-selected diets in Maryland, USA
gave a mean intake of 81 ± 41 µg/day (Welsh et al., 1981). The
daily intake in Venezuela was estimated to be 326 µg, but this
figure requires cautious interpretation because North American food
consumption patterns were used in its derivation (Mondragon &
Jaffe, 1976).
5.1.1.2. Food habits (consumption patterns)
Because of the wide variations in the selenium content of
various food groups in different countries, it is possible that
certain food habits or preferences could play an important role in
determining whether a person is at risk with regard to a deficient
or excessive intake of selenium. Persons with a preference for
foods such as fish may consume high levels of selenium in their
diet. Sakurai & Tsuchiya (1975) estimated that Japanese eating
large amounts of seafish may ingest as much as 500 µg of selenium
daily. People residing in seleniferous agricultural zones may
consume large amounts of selenium if locally-produced foods
constitute the bulk of their diet. In Venezuela, for example, corn
and rice samples from high selenium areas contained as much as 14
and 18 mg selenium/kg, respectively (Jaffe, 1976), and values as
high as 180 mg/kg have been reported in wheat from Colombia
(Ancizar-Sordo, 1947). If it is assumed that some segments of the
population living in such seleniferous regions consume, under
certain conditions, about half of their diet in the form of rice or
corn with a similar selenium content to that described above, the
daily intake of selenium from dietary sources could exceed 7000 µg.
However, it should be pointed out that only a few samples from the
high-selenium areas showed these extreme high levels. Obviously,
vegetarian diets could vary tremendously in selenium content.
Ganapathy & Dhanda (1976) showed that a vegetarian diet in the USA
supplied 84 µg selenium/day.
If a particular staple food constitutes a large fraction of the
diet of a population, then the selenium content of that food will
have a great influence on the overall dietary-selenium intake. For
example, one estimated daily dietary intake of selenium in Japan
(Sakurai & Tsuchiya, 1975) of 88 µg, was based on selenium levels
in rice and soybeans of 0.05 and 0.02 mg/kg, respectively, whereas
another estimated intake, 208 µg, was based on rice and soybean
selenium levels of 0.220 and 0.234 mg/kg, respectively (Yasumoto et
al., 1976). Low levels of selenium in rice have been reported in
Bangladesh, and it would be particularly interesting to study the
population there, as they depend on rice as a staple food, but they
have a recognized poor dietary vitamin E status (Bieri & Ahmad,
1976).
Table 8. Estimated human dietary intake of selenium (µg/day)a
----------------------------------------------------------------------------------------------------
Food New Zealand Finland United Japanf Canadag USAh USA Venezuelaj
Dunedinb Hamiltonc 1975d 1979d Kingdome South
Dakotai
----------------------------------------------------------------------------------------------------
Plant
vegetables,
fruits,
and sugars 1 2 1 1 3 6 1-9 5 10 15
cereals 4 3 3 25 30 24 62-113 45 57 88
Animal
dairy
products, 11 11 7 13 5 2 5-28 13 48 70
eggs
meat, fish 12 16 19 19 22 56 25-90 69 101 153
Total 28 32 30 50-60 60 88 98-224 132 216 326
----------------------------------------------------------------------------------------------------
a From: Robinson & Thomson (1983).
b From: Thomson & Robinson (1980).
c From: Watkinson (1981).
d From: Varo & Koivistoinen (1981).
e From: Thorn et al. (1978).
f From: Sakurai & Tsuchiya (1975).
g From: Thompson et al. (1975).
h From: Watkinson (1974) & Levander (1976b).
i From: US NAS/NRC (1980).
j From: Levander (1976b).
The importance of the selenium content of a particular staple
in the food supply of persons dependent on a monotonous diet
consisting of locally-produced foods was recently pointed out in
connection with the occurrence of Keshan disease (section 8.2.2) in
China (Yu, 1982). In the years in which Keshan disease was endemic
in the Fuyu county of Heilongjiang province, about 90% of the diet
consisted of maize, whereas, in the years in which Keshan disease
was not endemic, only about 30% of the diet was maize with much of
the rest coming from millet and wheat. Thus, it seems possible
that over-reliance on this single staple food during certain years
helped to precipitate Keshan disease in this area.
Recently, there has been considerable interest in some
countries in the use of textured soy protein products as
substitutes for meat products. Since meat products are such good
dietary sources of selenium, the possible impact of this trend on
the selenium intake of human beings was examined. Some soy protein-
based meat analogues contained as much selenium as meat products,
but others did not (Ferretti & Levander, 1976). These results show
that meat analogues made from soy or other plant proteins are not
consistent sources of selenium, since these plant-based products
will vary in selenium content depending on where the plant is
grown. In contrast, meat products are more reliable dietary
sources of selenium, because they contain a minimum quantity
compatible with animal life.
5.1.1.3. Elderly people
Thomson et al. (1977a) studied a group of 48 elderly people
(mean age of 78 years) in New Zealand and found that their blood-
selenium levels and red cell glutathione peroxidase (EC 1.11.1.9)
activity were lower than those in 12 young adult controls. It was
not possible for the authors to conclude from the data whether the
depressed blood-selenium levels and enzyme activity were due to
decreased selenium intake or were a general part of the aging
process. Abdulla et al. (1979) analysed 7-day pooled dietary
composites of 20 pensioners in Sweden and found that the average
daily selenium intake was 30.8 µg with a range of 8.7 - 96.3
µg/day.
5.1.1.4. Infants and children
The selenium intake of infants is of interest because of their
rapid growth rate and their heavy reliance on milk, a food that has
a highly variable selenium content, depending on its geographical
origin (Table 9). Millar & Sheppard (1972) assumed that human
and cow's milk in New Zealand contained 0.010 and 0.005 mg
selenium/litre, respectively, and calculated that the selenium
intake of infants for the first month of life would be 5.0 or
2.1 µg/day, depending on whether human or cow's milk was fed.
Williams (1983) measured the selenium content of mature mother's
milk from 10 New Zealand women and calculated an average daily
selenium intake of 5 - 6 µg for an infant consuming 700 ml milk.
If the value for the selenium content of Venezuelan cow's milk
shown in Table 9 is typical, it can be calculated that the selenium
intake of infants in that country would be about 58 µg/day during
the first month of life. The most extreme values for the selenium
content of human milk are from low- and high-selenium areas of
China (Yang et al., 1986) (section 4.1.1.1). For infants consuming
750 ml milk daily, these milks would provide 2 µg and 212 µg
selenium, respectively.
Table 9. Estimated infant daily intake of selenium
from dietary sources in North America (6-month-old,
6.5-kg child)a
-----------------------------------------------------
Daily Selenium Selenium
Food consumption content intake
(g) (mg/kg) (µg/day)
-----------------------------------------------------
Milk 824 0.013 11
Orange juice 122 0.014 2
Dry mixed cereal 10 0.540 5
Egg yolk 17 0.437 7
Strained meat 28 0.097 3
Strained fruit 57 0.002 -
Strained vegetable 57 0.003 -
Total selenium intake 28
-----------------------------------------------------
a Adapted from: Levander (1976b).
It was shown that milk continues to furnish a sizeable portion
of an infant's estimated daily selenium intake of 28 µg, in the
USA, after the child starts to eat solid foods (Table 9). The
estimated daily intake of selenium by 3-month-old infants in the
Federal Republic of Germany was 11 µg (Lombeck et al., 1975) and a
"market basket" survey indicated that the intake of 6-month-old
infants in the USA was about 12 µg/day (Harland et al., 1978). On
the basis of a caloric intake of 700 Kcal/day, infants in the USA
consuming formula diets based on milk, soy protein, casein, or
meat, ingested 8.5, 9.5, 12.6, or 31.5 µg/day, respectively (Zabel
et al., 1978).
As infants grow up in New Zealand, they continue to ingest
relatively low levels of selenium, since the estimated daily intake
over the age span of 6 months to 5 years was 5 - 8 µg (McKenzie et
al., 1978).
5.1.1.5. Special medical diets
Infants and children suffering from metabolic diseases such as
phenylketonuria (PKU) or maple syrup urine disease (MSUD) are fed
synthetic diets that contain little selenium for the first 10 or 12
years of their lives. In New Zealand, the mean daily selenium
intake of 12 subjects consuming such synthetic diets was 5 ± 2 µg
with little difference between the older children and the infants
(McKenzie et al., 1978). The selenium content of diets of 3-month-
old infants with PKU and MSUD in the Federal Republic of Germany
amounted to 4.7 and 5.6 µg/day, respectively (Lombeck et al.,
1975).
A wide variation in the selenium contents of special-purpose
medical diets for adults is related to the source of protein in the
diet (Zabel et al., 1978). On the basis of an intake of 2000
Kcal/day, adults consuming food supplements or tube-feeding
formulae would ingest 29, 47, or 98 µg selenium/day, depending on
whether the diet contained soy protein and casein, milk protein and
casein, or blended foods. At a similar caloric intake, chemically-
defined diets based on egg albumen provided 225 µg/day, whereas
diets based on casein hydrolysate or amino acid mixtures provided
only 28 and 1.5 µg/day, respectively. Total parenteral nutrition
solutions based on casein hydrolysate and amino acid mixtures also
furnished low amounts of selenium of only 32 and < 1 µg/day,
respectively. When levels of selenium supplied by special
therapeutic diets were studied in the Chernovitsi region of the
Ukrainian SSR, selenium intakes were found to vary between 99 ± 6
and 121 ± 8 µg/day (Majstruk & Suchkov, 1978).
5.2. Occupational Exposure
5.2.1. Levels in the workplace air
The main pathway of human occupational exposure to selenium is
through the air. Rarely, and under special circumstances, direct
contact may be of importance, i.e., when cutaneous absorption might
be facilitated by the local irritation and skin damage caused by
vesicant selenium compounds.
Various mechanical processes connected with the mining of
seleniferous ores or the grinding of selenium compounds can
contribute selenium-containing dusts to the atmosphere. In other
industrial activities, the amount of selenium released into the air
depends on the temperature to which it is heated and on the area
available for sublimation and/or vaporization. It is well
established that heating amorphous selenium below its melting point
results in its sublimation. At temperatures of 170 - 180 °C,
traces of selenium can be detected in the air and, at temperatures
of 230 - 240 °C, selenium dioxide is released. Heating of
amorphous selenium and selenium dioxide resulted in the release of
substantial quantities of selenium into the air, e.g., from a
surface of 10 cm2, 2.6 - 3.75 mg was released after 20 min at 230 -
240°C and 23 - 31.6 mg was released after 20 min at 350 - 360 °C
(Izraelson et al., 1973).
Little quantitative information is available in the literature
on actual levels of human exposure to selenium in industry. The
levels of selenium in the workplace depend not only on the nature
of the process involved but also on the control technology in any
given industry. Thus, it can be expected that analyses carried out
several decades ago yielded higher values than analyses carried out
more recently. In 1948, Filatova reported air levels of selenium
and selenium dioxide of 20.6 - 24.8 mg/m3 and 0.46 - 0.78 mg/m3,
respectively, in working zones of selenium-heating processes in a
selenium rectifier plant, whereas levels of 0.55 - 1.1 mg/m3 and up
to 0.11 mg/m3, respectively, were associated with the process of
disc smearing (Filatova, cited in Izraelson et al., 1973). In the
production of selenium-containing photoelements by the vacuum
application of a photosensitive layer, selenium and selenium
dioxide levels did not exceed 2.0 mg/m3 and 0.1 mg/m3, respectively
(Sverdlina & Maslennikova, 1961). Selenium grinding, sulfuric acid
treatment, and cathode alloy coating resulted in air-selenium
levels ranging from 0.133 to 2.0 mg/m3 (Izraelson et al., 1973). A
highly-dispersed aerosol selenium condensate with a particle size
of 0.5 - 2 µm was produced at a level of 0.88 - 6.13 mg/m3, as a
result of the manufacture of selenium-containing steels (Ershov,
1969). In the production of rare metals, the air in industrial
premises can contain as much as 0.4 - 750 mg metallic dust/m3,
which, in addition to other elements, contains from 0.8 - 15.7%
selenium (Burchanov, 1972). During the 1950s, Glover (1967)
carried out an extensive survey in which air-selenium levels were
determined in areas of a selenium rectifier plant in which the
workers had been found to have high urinary-selenium values. In
general, the highest air levels of 1.5 - 5.2 mg selenium/m3 were
found in relation to the grinding process. With the exception of
certain "special processes", air levels in the plant due to all the
other manufacturing activities were less than 0.5 mg/m3. In one
case, a level of 21.3 mg/m3 was observed, but no additional details
were given. In this rectifier plant, a process, in which the
elemental selenium was heated, was used for preparing the selenium
mixture that was later applied to the metal plate. For this
reason, the selenium levels in the air are likely to have been
higher than in other factories where the selenium was deposited
from aqueous solution. In another selenium rectifier plant, air
levels of between 7 and 50 µg selenium/m3 were reported
(Kinnigkeit, 1962). However, in this case, no correlation was
observed between the air levels in the workplace and the selenium
levels in the blood of the workers. Kinnigkeit felt that the air-
selenium levels were not high enough to account for the high
urinary-selenium levels in the workers. He suggested that the
discrepancies might be due to incomplete collection of selenium
during air sampling, transient peaks of selenium exposure that were
not detected by short-term sampling, or contamination of hands,
body, and clothing with selenium, which was then transferred to the
mouth as a result of eating or smoking. Another possible
explanation is that measurements of the levels in the workroom air
did not accurately reflect levels of selenium in the actual
breathing zone of the workers.
Low air levels of selenium have been observed in factories
involved with the use of selenium in certain photocopying devices.
In this type of manufacturing, "clean room" techniques are used,
because strict dust control is needed to produce the quality of
photoreceptor surfaces required. Cannella (1976) reported air
levels of selenium ranging from 6 to 91 µg/m3 in various locations
in a selenium alloy plant that made such photoreceptor products.
5.2.2. Biological monitoring
Although selenium levels in the work-place air have been
determined in several instances, these measurements cannot be used
to draw any conclusions about the exposure history of individual
workers. Glover (1967), who estimated occupational exposure to
selenium indirectly by determining selenium levels in the urine of
workers, acknowledged the limitations associated with monitoring
based on grab samples of urine. The Working Group also recognized
the inadequacy of urinary-selenium concentrations as a monitoring
tool. However, it appears that the use of a more rigorous
programme (e.g., total 24-h urine collections) would have resulted
in losing the cooperation of the workers involved. A tentative
maximum allowable concentration of selenium in urine of 0.1
mg/litre was proposed by Glover (1967). The urine of the workers
was analysed at 3-month intervals, and any employee whose urinary-
selenium value exceeded the limit was transferred to a process not
involving selenium. The urinary-selenium levels of these
individuals were tested weekly.
Two primary factors were responsible for setting the maximum
allowable concentration for selenium in urine at 0.1 mg/litre
(Glover, 1970). First, a limit lower than this would have resulted
in a certain number of "false positives", since selenium levels in
urine are often this high in people not industrially exposed to the
element. Second, it was found that values below 0.1 mg/litre were
invariably accompanied by air levels in the work-place of less than
0.1 mg/m3, which was below the threshold limit value of 0.2 mg/m3
for elemental selenium and its common inorganic compounds (ACGIH,
1971). It must be emphasized that urinary selenium cannot be used
to monitor exposure to such dangerous selenium compounds as
hydrogen selenide, selenium oxychloride, or certain organic
selenium compounds because severe damage to the lungs or skin would
have occurred by the time that the urinary-selenium values were
raised (Glover, 1976).
6. METABOLISM OF SELENIUM
The metabolism of selenium has been most completely studied in
animals and data from human investigations are limited. Thus, in
the following sections, pertinent information from selected animal
studies is presented followed by available information from
controlled human studies.
6.1. Absorption
Since food is the primary environmental medium through which
man and animals are exposed to selenium, most data concerning
selenium absorption deal with the gastrointestinal pathway. Much
less is known about the absorption of selenium through the lungs or
skin.
6.1.1. Gastrointestinal absorption
6.1.1.1. Animal studies
The intestinal absorption of soluble selenium compounds by rats
is highly efficient. It has been shown that these animals absorbed
92, 91, and 81% of doses of selenite, selenomethionine, and
selenocystine, respectively (Thomson & Stewart, 1973; Thomson et
al., 1975a). In a study on rats by Whanger et al. (1976a), the
greatest absorption of selenite or selenomethionine occurred from
the duodenum, with slightly less absorption from the jejunum or
ileum. Virtually no absorption occurred from the stomach. The
absorption of selenite by rats does not appear to be under
homeostatic control, since 95% or more of the dose was absorbed,
regardless of whether the animals were fed a selenium-deficient
diet or a diet containing mildly toxic levels of selenium (Brown et
al., 1972). Wright & Bell (1966) found that selenite was absorbed
from the gastrointestinal tract to a greater extent by monogastric
animals (swine) than by ruminant animals (sheep). The decreased
absorption by sheep may have been due to reduction of the
administered selenite to insoluble or unavailable forms by rumen
microorganisms.
Only limited information is available regarding the absorption
by animals of selenium occurring naturally in foods. Kidney tissue
and fish muscle were chosen for model studies, because these foods
are relatively rich sources of dietary selenium for human beings.
The levels of intestinal absorption by rats of radio-selenium
administered in homogenates of rabbit kidney or fish muscle
injected with selenite, were 87 and 64%, respectively (Thomson et
al., 1975b; Richold et al., 1977). The low absorbability of
"fish selenium" agrees with other reports showing the poor
bioavailability to animals of selenium in certain fish products
(section 5).
Little is known concerning the physiological processes
governing the absorption of even simple selenium compounds, though
McConnell & Cho (1965) showed that selenomethionine was transported
against a concentration gradient, whereas selenite and
selenocystine were not.
6.1.1.2. Human studies
Studies carried out in New Zealand on 3 young female volunteers
indicated that the intestinal absorption of oral doses of
radioactive selenite, containing not more than 10 µg selenium, was
70, 64, and 44% of the dose, respectively (Thomson & Stewart,
1974). In a study conducted 2 years later, in which 2 of the 4
female volunteers were women who had participated in the earlier
study with selenite, the intestinal absorption of oral doses of
75Se-selenomethionine, containing less than 2 µg selenium, averaged
about 96% of the dose (Griffiths et al., 1976). When a larger dose
of 1 mg selenium was given orally in solution, a similar difference
in absorption was obtained, i.e., 97% for selenomethionine (one
subject) (Thomson et al., 1978) and about 60% for sodium selenite
(13 subjects) (Robinson et al., 1985; Thomson & Robinson, 1986).
This value for absorption of selenite-selenium is much lower than
the 92% absorption reported for "selenite" in the earlier paper of
Thomson (1974), which is now known to have been selenate-selenium
and not selenite-selenium. Later studies on 10 volunteers also
showed absorption of 94% for 1 mg selenate-selenium in solution
(Thomson & Robinson, 1986). Thus, selenate-selenium is similar to
selenomethionine-selenium in that it is better absorbed than
selenite-selenium.
Correction of selenate-selenium for selenite selenium in the
paper of Thomson (1974) also removes the "surprising difference"
reported between absorption of selenite given in solid form (60%
for 4 female volunteers) and in solution (60% and not 92%). A
female volunteer given a 1 mg dose of selenium as selenite in
solution daily for 5 consecutive days absorbed 59% of the total 5
mg dose (Thomson et al., 1978). It would appear that human beings
have no homeostatic control to limit their absorption of large
single doses of these selenium compounds (Barbezat et al., 1984).
Robinson et al. (1978b) measured the intestinal absorption of
selenium by a New Zealand female volunteer receiving a daily
supplement of 100 µg selenium as selenomethionine, a male volunteer
supplemented daily with 100 µg selenium as sodium selenite, and a
second female supplemented daily with 65 µg selenium, as it occurs
naturally in mackerel (Scomber japonieus). The selenomethionine
and selenite were given in solution, whereas the mackerel was given
as canned fish. During a 4-week supplementation period, 75% of the
selenium given as selenomethionine was absorbed compared with only
48% of the selenium administered as selenite. The absorption of
the "fish selenium" was 66%. Details of the diets consumed by the
3 subjects were not provided, but the subjects supplemented with
selenomethionine or fish selenium did not consume liver or kidney
throughout the study. However, it was specified only that the
subject supplemented with selenite did not eat liver, kidney, or
fish on the days that weekly urine collections were made or on
the day prior to the collection. Also, the subject receiving
selenomethionine ate fish only occasionally throughout the study.
The subject supplemented with the fish selenium ate fish other than
mackerel on only one occasion. Stewart et al. (1978) found that
the average intestinal absorption of naturally-occurring selenium
in foods was 55% in 4 young New Zealand women freely consuming
normal diets. However, allowing for the endogenous faecal
excretion (half of total faecal output) the average true absorption
of naturally occurring selenium became 79%.
6.1.2. Absorption by inhalation
Certain volatile selenium compounds, such as hydrogen selenide,
are known to be toxic when inhaled (Dudley & Miller, 1941).
Lipinskij (1962) observed increased selenium levels in the liver
and kidneys after respiratory exposure of rabbits to elemental
selenium and selenium dioxide. More recently, Weissman et al.
(1979, 1983) studied the distribution and retention of inhaled
75Se-labelled selenious acid and elementary selenium aerosols.
They used 63 Beagle dogs, which were between 3 and 4 years old at
the time of exposure. All inhalation exposures were through the
nose only. Aerosol particles of both forms were small with median
aerodynamic diameters of 0.5 and 0.7 µm and geometric standard
deviations of 2.4 and 1.5 for selenious acid and elemental
selenium, respectively. The urine and faeces of 4 dogs were
collected daily, for 32 days, and then at regular time intervals
until sacrifice. Selenium from exhaled breath was collected from 3
dogs for several 4-h periods, between 2 and 10 days after exposure.
Whole-body retention of 75Se-selenium was measured immediately
after exposure and at regular intervals up to 320 days after
exposure. Two dogs exposed to each aerosol were sacrificed at
various time intervals for over half a year. The initial 75Se-
selenium body burden (IBB) in dogs that inhaled 75Se-selenium as
selenious acid was 40 ± 17 µg selenium/kg body weight and 22 ± 9 µg
selenium/kg body weight in dogs inhaling elementary selenium. For
both forms of selenium, the retention data of 75Se-selenium could
be expressed by a 3-component negative exponential equation:
Selenious acid:
%IBB = 66e-1.5t + 17e-0.089t + 17e-0.023t
Elemental selenium:
%IBB = 66e-0.090t + 21e-0.059t + 13e-0.018t
The corresponding half time values were 0.46, 7.8, and 30 days and
0.77, 12, and 38 days for selenious acid or elemental selenium
exposure, respectively. Studies on organ distribution showed that,
2 h after exposure to selenious acid, only about 5.3% of the IBB
was retained in the lung compared with 26% of the IBB of elemental
selenium. For the first 32 days following exposure, the urine
accounted for 79 and 66% of the 75Se-selenium excreted by dogs
exposed to selenious acid and elemental selenium aerosols,
respectively. Respiratory excretion of 75Se-selenium was
negligible. The organ distribution of 75Se-selenium as a
percentage of the sacrifice body burden is given in Table 10.
Table 10. 75Se-selenium distribution in organs of dogs
128 days after respiratory exposure to 75Se-selenious acid
or elemental 75Se-selenium aerosolsa
----------------------------------------------------------
Organ 75Se-selenium organ distribution
as % of 75Se-selenium body burden
at sacrifice
----------------------------------
selenious acid elemental selenium
treated treated
----------------------------------------------------------
Lung 1.3 6.2
Liver 11.4 7.4
Kidney 1.6 1.6
Blood 19.7 21.5
Gastrointestinal tract 3.4 3.6
Pelt 9.6 9.1
----------------------------------------------------------
a Adapted from: Weissman et al. (1979).
Comparison of the absorption of these two forms of selenium
through inhalation was also studied in the rat (Medinsky et al.,
1981). The rate of transfer of selenium into the blood was slower
when the elemental form was administered. However, once absorbed,
both chemical forms behaved identically.
6.1.3. Absorption through the skin
Apart from the report of Dutkiewicz et al. (1972), who found
that 10% of a 0.1 mol solution of sodium selenite applied to rat
skin was absorbed in 1 h, there are no quantitative data on the
dermal absorption of water-soluble selenium compounds. However,
Dudley (1938) showed that selenium oxychloride was absorbed through
the skin of rabbits. Selenium sulfide, an insoluble selenium
compound used in certain anti-dandruff shampoos, is not ordinarily
absorbed through the skin, although a garlicky breath odour and
elevated urinary excretion of selenium were noted in a patient with
open scalp lesions who used a selenium sulfide shampoo (Ransone et
al., 1961).
6.2. Distribution in the Organism
6.2.1. Transport
Little is known about the transport of selenium in the body.
However, a plasma selenoprotein has been identified which one group
postulates is involved in selenium transport (Motsenbocker &
Tappel, 1982).
6.2.2. Organs
6.2.2.1. Animal studies
Absorbed selenium is rapidly distributed among the tissues.
Heinrich & Kelsey (1955) found that the liver of mice injected
subcutaneously with sodium selenite contained 17 and 30% of the
dose, 15 and 60 min after injection, respectively. However,
because of the great variation in the metabolic half-time of
selenium in various tissues (Thomson & Stewart, 1973), the
distribution pattern observed will change markedly with the time of
sampling after injection with radio-selenium.
As long as the diet provides nutritionally adequate levels of
selenium, the relative concentrations of selenium in the various
internal organs are quite consistant over a wide range of selenium
intakes. For example, Jones & Godwin (1962) studied the
distribution of selenium in the tissues of mice fed alfalfa
containing nutritional levels of selenium. The concentrations of
selenium in the internal organs decreased in the following order:
kidneys > liver > pancreas >> lungs > heart > spleen >
skin > brain > carcass. Smith et al. (1937) fed toxic levels of
sodium selenite to cats and observed the following order for the
concentration of selenium in the internal organs: liver >
kidneys > spleen > pancreas > heart > lungs.
The distribution of selenium appears to be relatively
independent of the form and route of administration, since the
kidneys and adrenals contained the highest concentrations of radio-
selenium, regardless of whether the rats were given labelled
selenite or selenomethionine orally or by intravenous injection
(Thomson & Stewart, 1973). The greatest amounts of radioactivity
were in the kidneys, liver, and total muscle mass. Similar
distribution patterns were noted in rats dosed orally with
homogenates of tissues from rabbits or from fish that had been
given labelled selenium compounds (Thomson et al., 1975b; Richold
et al., 1977).
The distribution of selenium in tissues was markedly different
when rats were fed a diet deficient in selenium: the testes,
brain, thymus gland, and spleen took up the greatest concentrations
of selenium from a tracer dose of selenite (Burk et al., 1972). A
recent report confirms the ability of the testis to retain selenium
better than other tissues in selenium deficiency (Behne & Hofer-
Bosse, 1984).
The distribution of selenium in the eye was determined
fluorometrically with 2,3-diaminonaphthalene by Gusejnov et al.
(1974), who found the following levels in various parts of the eye
of a bull (mg/kg fresh weight): iris, 1.3; retinal pigment
epithelium, 0.85; retina, 0.50; cornea, 0.3; lens, 0.03; and
vitreous body, 0.01. In some birds (pigeons and crows), the level
of selenium in the retina was about 10 times higher (about 5.0
mg/kg). In studies in which 75Se-labelled sodium selenite in
saline was administered intraperitoneally to frogs, rats, and
rabbits, the distribution of radio-selenium was similar to that of
the naturally-occurring selenium measured in the bull's eye. The
maximum 75Se activity in the eye (5 h after administration) was
found in the pigment with the vascular layer of the retina, whereas
minimum activity was found in the lens and vitreous body.
Histoautoradiography of the eyes also indicated the highest
radioactivity in the vascular and pigment layers (Abdullaev et al.,
1972).
6.2.2.2. Human studies
The distribution of selenium in human organs was examined by
Schroeder et al. (1970), who found the following descending order
for selenium concentrations in tissues: kidney > liver >
spleen > pancreas > testes > heart muscle > intestine >
lung > brain. Blotcky et al. (1976) showed that, in autopsy
samples from 106 persons, the concentrations of selenium were 2-3
times higher in the kidneys than in the liver.
Uptake of an orally-administered dose of radioselenite in the
internal organs occurred in the following relative order: liver >
kidneys > lungs > muscle = articular tissue (Leeb et al., 1977).
Other studies have shown significant accumulation of radio-selenium
by the liver and kidneys after intravenous injections of selenite
or selenomethionine (Lathrop et al., 1972; Falk & Lindhe, 1974;
Nordman, 1974).
6.2.3. Blood
Studies on experimental animals have shown a close correlation
between the level of selenium in the blood and the level of
selenium in the diet (Lindberg & Jacobsson, 1970; Cary et al.,
1973; Ermakov & Kovalskij, 1974; Oh et al., 1976a). Analytical
surveys on human beings have shown significant variations in blood-
selenium levels in different geographical areas, presumably because
of differences in dietary-selenium intake. For example, Allaway et
al. (1968) found that selenium levels in the blood of persons
living in areas of the USA where soils and vegetation were high in
selenium tended to contain more selenium than that of persons
living in areas where soils and plants were generally low in
selenium. Thus, geographical differences in the selenium levels in
human blood are possible, even in a country where large-scale
interregional food shipments would be expected to level out
differences in the amount of selenium in the food supply. These
geographical differences in human blood-selenium levels in the USA
have been confirmed by Howe (1974), who found a mean selenium
content of 0.265 mg/litre (SD = 0.056) for 626 samples collected in
and around the state of South Dakota (a high soil-selenium region),
whereas Schultz & Leklem (1983) reported blood-selenium values in
Oregon (a low soil-selenium region) that were well below those
reported for other areas of the USA.
Analyses of blood samples from persons living in different
regions of the world indicate similar correlations between the
amount of selenium in the blood and the amount of selenium in the
food throughout the world (Table 11). For example, blood-selenium
concentrations tend to be lowest in areas of the world that are
known to have low levels of selenium in the soil (Keshan-disease
areas of China, New Zealand, Scandinavia) and highest in areas that
are known to have high levels of selenium in the soil (Venezuela,
reported selenosis areas of China). Superimposed on these
geographical variations in effects are the effects of general
nutritional status or effects of certain disease states. For
example, children suffering from Kwashiorkor have lower blood-
selenium levels than their well-nourished counterparts (Burk et
al., 1967) and reductions in the selenium content of sera have been
observed in patients with cancer (McConnell et al., 1975). Lower
blood-selenium values were associated with lower serum-albumin
values in surgical patients with and without cancer in New Zealand
(Robinson et al., 1979). Akesson (1985) suggested that alterations
in plasma-selenium may be secondary to changes in plasma-protein
concentration. In a study of Finnish men with one or more risk
factors for coronary heart disease, Miettinen et al. (1983)
observed a strong association between serum-selenium and the
eicosapentanoic acid contents of cholesterol ester and
phospholipids. The authors pointed out that since eicosapentanoate
is a fatty acid peculiar to fish such an association could reflect
the intake of fish at the time of blood sampling.
Blood-selenium levels change if persons travel to countries
with soils of different selenium content. For example, Griffiths &
Thomson (1974) noted that the blood-selenium levels of adults from
the USA declined rapidly on arrival in New Zealand, but, after 1
year, their levels were still higher than the mean value for
permanent New Zealand residents (Fig. 2). Rea et al. (1979) found
that plasma-selenium levels changed with changes in selenium intake
more rapidly than erythrocyte-selenium levels. The latter
reflected longer-term changes in selenium exposure, presumably
because of the relatively long life span of erythrocytes. In a
controlled depletion/repletion metabolic study (section 6.3.2.2),
Levander et al. (1980) stated that the plasma-selenium levels of
healthy young male volunteers in the USA dropped about 19% after
2 weeks on a low-selenium diet and then returned to their previous
levels 11 days after consuming a high-selenium diet (Fig. 3).
4. LEVELS IN ENVIRONMENTAL MEDIA
4.1. Levels and Chemical Forms of Selenium in Food
4.1.1. Levels in food
In spite of the relatively few data available, some
generalizations concerning the selenium content of foodstuffs can
be made. For example, the level of selenium in food depends on
natural differences among food commodities and the natural
availability of selenium in the environment. Moreover, certain of
man's activities can influence the selenium content of human foods.
4.1.1.1. Natural differences among food commodities
A wide range of values has been reported in the selenium
content of foods (mg/kg wet weight): liver, kidney, and seafood,
0.4 - 1.5; muscle meats, 0.1 - 0.4; cereal and cereal products, <
0.1 - > 0.8; diary products, < 0.1 - 0.3; and fruits and
vegetables, < 0.1) (Oelschlager & Menke, 1969; Morris & Levander,
1970; Schroeder et al., 1970; Suchkov, 1971; Arthur, 1972; Millar &
Sheppard, 1972; Ferretti & Levander, 1974; Sakurai & Tsuchiya,
1975; Abutalybov et al., 1976; Bieri & Ahmad, 1976; Kasimov et al.,
1976; Olson et al., 1978). It should be pointed out that these
values are given for raw foods (food as purchased) rather than
cooked foods (food as eaten). The effects of cooking on the
selenium content of foods are described in section 4.1.1.3.
Moreover, the values presented in these food composition studies
should not be compared from one country to another, because of the
variations in the analytical methods and sampling procedures used
(section 2).
Organ meats, such as kidneys or liver, contain the highest
levels of selenium, but some seafood products contain almost as
much. Muscle meats are significant sources of selenium, though
they do not contain as much as organ meats or seafoods. Certain
semolina, grain, and cereal products can contribute appreciably to
the dietary-selenium intake, but wide variations in selenium
content have been found in different samples of the same foodstuff.
Such variation is typical of many plant foods and the reasons for
this are discussed below. Milk, cheese, and egg samples from
several countries showed low to moderate values for selenium, but
again the results were quite variable. Fruits and vegetables
generally contained very low levels of selenium, though garlic and
mushrooms contained moderate levels of the element.
The selenium contents of baby foods tended to show the same
general pattern as those in adult foods (Morris & Levander, 1970;
Arthur, 1972), i.e., meat and cereal products contained the highest
levels and fruit and vegetable products, the lowest. Shearer &
Hadjimarkos (1975) analysed samples of mature human milk from 241
subjects living in the USA and found that the overall mean selenium
content was 0.018 mg/litre. Over 98% of the samples contained
between 0.007 and 0.033 mg/litre. Grimanis et al. (1978) reported
an average of 0.015 mg/litre in 5 samples of mature human milk
collected in Greece. These authors found slightly higher values in
15 samples of human transitional milk (0.016 mg/litre) and
colostrum (0.048 mg/litre). A slightly lower average selenium
concentration of 0.013 mg/litre, was reported for samples of human
transitional milk in New Zealand (Millar & Sheppard, 1972). The
most extreme values for the selenium content of human milk were
reported from China and ranged from 0.0026 mg/litre in areas where
Keshan disease was prevalent to 0.283 mg/litre in high-selenium
areas (Yang et al., 1986).
It can be seen from Table 3 that meat-based infant formulae
have a higher selenium content than formulae based on milk or soy
protein, and that casein-based powdered formulae for special
medical purposes (diet therapy for errors in amino acid metabolism
or malabsorptive states) contain low levels of selenium. Blended
food tube-feeding formulae containing meat tend to have higher
selenium contents than comparable products containing only milk or
casein and soy protein (Table 3). Chemically-defined diets having
egg albumen as the protein source contained more selenium than
diets based on casein hydrolysate, which, in turn, contained more
selenium than diets based on purified amino acids. Total
parenteral nutrition solutions based on casein hydrolysate also
contain more selenium than solutions based on amino acid mixtures,
which contain very low levels of selenium.
Table 3. Selenium content of commercial formula dietsa
-----------------------------------------------------------------
Product Selenium content
(mg/kg wet (mg/kg dry
weight) weight)
-----------------------------------------------------------------
Infant formulae:
milk-based 0.004 - 0.027
soy-based 0.004 - 0.030
meat-based 0.046 - 0.070
casein-based formulae for special 0.048 - 0.120
medical purposes (powders)
Food supplements and tube-feeding
formulae:
milk-based 0.005 - 0.045
soy-casein-based 0.013 - 0.020
blended foods 0.037 - 0.056
Chemically defined diets:
(powders)
egg albumen low residue 0.224 - 0.351
egg albumen moderate nitrogen 0.388 - 0.886
egg albumen high nitrogen 0.503 - 0.570
casein hydrolysate 0.052 - 0.071
amino acid mixture 0.001 - 0.011
-----------------------------------------------------------------
Table 3. (contd.)
-----------------------------------------------------------------
Product Selenium content
(mg/kg wet (mg/kg dry
weight) weight)
-----------------------------------------------------------------
Total parenteral nutrition
solutions:
casein hydrolysate 0.037 0.324
(0.032 - 0.041)
diluted 1:1 with 50% dextrose 0.019 0.093
solution (0.017 - 0.020)
amino acid mixture 0.001 0.010
-----------------------------------------------------------------
a Adapted from: Zabel et al. (1978).
One of the factors that can influence the amount of selenium in
plant foodstuffs is the nature of the plant itself. Plants have
been divided into 3 groups depending on their tendency to take up
selenium from seleniferous soils (Rosenfeld & Beath, 1964):
Group 1 Primary selenium accumulators - can contain very
high amounts of the element (often over 1000
mg/kg dry weight).
Group 2 Secondary selenium accumulators - rarely contain
more than a few hundred mg/kg.
Group 3 Many weeds and most crop plants, grains, and
grasses - rarely contain more than 30 mg/kg, even
when grown on seleniferous soils; when grown on
normal soils generally contain less than 1 mg/kg.
Plants from Groups 1 and 2 do not usually contribute to the
selenium intake of human beings, since they are not consumed
directly by people and are consumed by animals only when other
feeds are not available. However, plants from Group 3 can
contribute large amounts of selenium, if they are grown in
seleniferous areas (see below).
The concentrations of many nutritionally essential trace
elements are known to be decreased by the milling of grains into
cereals (Czerniejewski et al., 1964) and preliminary analysis of
random supermarket samples of cereal foods suggested that refined
products such as white flour or white bread contained less selenium
than whole grain foods such as whole wheat flour or whole wheat
bread (Morris & Levander, 1970). However, a subsequent more
controlled analytical study in which various grain fractions were
taken from the same production batch showed that milling a variety
of grains decreased the concentration of selenium in the consumer
product by only 10 - 30% (Ferretti & Levander, 1974). Thus, the
selenium content of cereal products is somewhat less than that of
the parent grains but the decreases in concentration are not nearly
as great as those observed with other essential trace minerals.
Selenium tends to be localized mainly in the protein fraction
of plant and animal tissues; thus, the protein content of a food
also influences its selenium content. For example, the
concentration of selenium in a series of soybean products prepared
from a given lot of soybeans increased as the protein content of
the product increased (Ferretti & Levander, 1976). However,
protein content is only an expression of the potential of a food to
contain selenium, and a food high in protein will not necessarily
also be high in selenium. Moreover, non-protein seleno amino acids
occur in some foods and thus, the protein content may not always
reflect the selenium content.
4.1.1.2. Effects of natural differences in the availability of
selenium in the environment on levels in food
The most important factor in determining the selenium content
of plant foods and feeds is the amount of selenium in the soil that
is available for uptake by the plant. Sun et al. (1985) reported
that the selenium content of local crops was significantly
correlated with the selenium content of the local soil, the
correlation coefficients of soybean, corn, and rice being 0.9456,
0.9953, and 0.9954, respectively. Since the water-soluble selenium
in the soil was directly correlated with the pH and inversely
correlated with humin and total iron in the soil, the selenium
content of local crops was also influenced by the amount of water-
soluble selenium in the soil. An analytical survey carried out in
the USA demonstrated that the level of selenium in alfalfa plants
varied from less than 0.01 to more than 5.0 mg/kg, and it was
assumed that these levels reflected the amount of available
selenium in the soil (Kubota et al., 1967). A variation in the
selenium content of wheat from 0.04 to 21.4 mg/kg, depending on
where the plant was grown, was reported by Schroeder et al. (1970).
Samples of several foods bought in local markets in Caracas,
Venezuela contained much more selenium than similar foods purchased
in supermarkets in the eastern USA (Table 4). The likely source of
selenium in the milk, eggs, and meat from Caracas is sesame cake,
since sesame is produced mostly in the seleniferous area of
Venezuela and the pressed cake is widely used as an ingredient in
animal feed. Selenium levels as high as 14 mg/kg in corn and 18
mg/kg in rice have also been observed in certain food samples taken
from high-selenium regions in Venezuela (Jaffe, 1976). These
concentrations of selenium are as high as those reported in foods
from seleniferous zones of the USA (Smith & Westfall, 1937).
Great extremes in the selenium content of staple foods have
also been reported recently from China (Yang et al., 1983). For
example, samples of corn, rice, and soybeans, taken from a high-
selenium area with a history of human intoxication reported as
chronic selenosis (section 8.1.1.1) contained average selenium
levels of 8.1, 4.0, and 11.9 mg/kg, respectively, whereas samples
of the same staples collected in a low-selenium area where Keshan
disease was prevalent (a human selenium-deficiency disease)
(section 8.2.2) contained average selenium levels of only 0.005,
0.007, and 0.010 mg/kg, respectively (Table 5). A low selenium
content in food has also been reported in other countries with low
selenium soils, such as New Zealand and Finland. Typical ranges
for selenium contents in food in such countries include (mg/kg wet
weight): liver, kidney, and seafood, 0.09 - 0.92; muscle meats,
0.01 - 0.06; cereal and cereal products, 0.01 - 0.07; milk, <
0.01; and fruits and vegetables, < 0.01 - 0.02 (Koivistoinen,
1980; Thomson & Robinson, 1980). Samples of staple foods collected
in areas where soils contained intermediate levels of selenium also
contained intermediate levels of selenium.
Table 4. Comparison of selenium contents
of selected foods available in Caracas,
Venezuela, and Beltsville, Maryland, USA
(mg selenium/kg wet weight)a
-----------------------------------------
Food Caracas Beltsville
-----------------------------------------
Powdered milk 0.417 0.169
Whole milk 0.115 0.012
American-type cheese 0.425 0.090
Swiss-type cheese 0.382 0.104
Pork 0.833 0.209
Chicken 0.702 0.106
Egg 1.520 0.116
-----------------------------------------
a From: Mondragon & Jaffe (1976).
As might be expected, the selenium contents of food products of
animal origin depend heavily on the amount of naturally-occurring
selenium in the feed given to the animal. In the USA, it was shown
that the selenium content of swine muscle was highest in areas
known to have a high level of available selenium in the soil and
lowest in areas in which the available soil-selenium was low (Ku et
al., 1972). This indicates that selenium is readily passed up the
soil-plant-animal food chain to human beings.
4.1.1.3. Man-induced changes in selenium levels in food
(a) Human activities that increase selenium levels
Perhaps the most direct way that man's activities can increase
the selenium content of the food supply is the deliberate addition
of selenium to the feeds of poultry and certain livestock. In some
countries, this is now accepted practice to prevent the occurrence
of selenium-deficiency diseases, many of which cause significant
economic losses to farmers throughout the world. In the USA, for
example, farmers are permitted to add 0.1 mg selenium/kg (as sodium
selenite or selenate) complete feed for beef and dairy cattle,
sheep, chickens, ducks, swine (0.3 mg/kg in starter and prestarter
rations), and 0.2 mg/kg for turkeys (US Department of Health and
Human Services, Food and Drug Administration, 1984; Subcommittee on
Selenium - Committee on animal Nutrition, 1983). It has been shown
that the edible tissues of poultry, swine, and sheep, fed diets
fortified with inorganic selenium salts at the regulated levels,
did not contain any more selenium than the tissues of animals fed
diets, naturally adequate in selenium (Allaway, 1973; Ullrey et
al., 1977). The homeostatic mechanisms that appear to limit the
concentrations of selenium in the edible tissues of animals fed
certain levels of sodium selenite are discussed further in section
6.
Table 5. Selenium contents of staple foods grown on soils in areas of China with excess,
moderate, and deficient levels of seleniuma
-----------------------------------------------------------------------------------------
Place Corn Rice Soybean
Number Se content Number Se content Number Se content
of (mg/kg) of (mg/kg) of (mg/kg)
samples samples samples
-----------------------------------------------------------------------------------------
High-selenium area 44 8.1 22 4.0 17 11.9
with a history of (0.5-28.5)b (0.3-20.2)b (5.0-22.2)b
intoxication reported
as chronic selenosis
High-selenium area 2 0.57 2 0.97 2 0.34
reported to be without
selenosis
Moderate-selenium- 82 0.036 76 0.035 31 0.069
adequate area (± 0.056) (± 0.027) (± 0.076)
(Beijing)
Low-selenium area 10 0.009 32 0.022 - -
(± 0.009) (± 0.009)
Low-selenium area 195 0.005 49 0.007 150 0.010
with Keshan disease (± 0.003) (± 0.003) (± 0.008)
-----------------------------------------------------------------------------------------
a Adapted from: Yang et al. (1983).
b Mean ± SD or range shown in parenthesis.
Finland is the first country to decide to increase the selenium
content of Finnish feed and food by the addition of sodium selenate
to fertilizers, to be used in the whole country at a concentration
of 16 or 6 mg/kg for cereal and grassland crops, respectively
(Koivistoinen & Huttunen, 1985). The manufacture of these
selenized fertilizers began in the summer of 1984 and they will be
used at application rates of 10 g selenium/ha per growing season.
It is anticipated that the added selenium in the fertilizers will
be transported to the human food chain, and the selenium level of
the cereal and grassland crops will be raised to 0.1 and 0.15 -
0.20 mg/kg (dry basis), respectively.
Another potential way in which the level of selenium in the
food chain might be increased by man's activities is the proposed
use of selenium-bearing fly ash as a soil supplement. Furr et al.
(1977) analysed cabbages grown on potted soil supplemented with
several different fly ashes and found that the selenium levels in
the cabbages were closely correlated with those in the respective
fly ashes in which the plants were cultured. The ready
bioavailability to animals of the selenium in plants grown on fly
ash was demonstrated in a study (Stoewsand et al., 1978) in which
Japanese quail were fed a complete diet containing 60% winter wheat
that had been grown to maturity on either soil or a deep bed of fly
ash. The soil contained 2.1 mg selenium/kg and the wheat grown
thereon contained 0.02 mg/kg, a level typical of wheat from
selenium-deficient areas. The fly ash contained 21.3 mg
selenium/kg, and the wheat grown on it contained 5.7 mg/kg, a level
sometimes found in wheat from naturally seleniferous areas. The
levels of selenium in the tissues of the quail fed the wheat grown
on fly ash were much higher than those of quail fed the wheat grown
on soil (Table 6). Moreover, the selenium contents of eggs from
quail fed the wheat grown on fly ash were 3.5 mg/kg in the yolk and
almost 10 mg/kg in the white compared with 0.5 and 0.2 mg
selenium/kg, respectively, in eggs from quail fed the control
wheat. Obviously, the use of selenium-bearing fly ash as a soil
supplement to provide nutritionally desirable levels of selenium in
plants should be carried out with care, since inappropriate use
could lead to an unwanted build-up of selenium residues in the food
chain. Also, the application of fly ash to soil at rates
sufficient to correct selenium deficiency in animals may damage the
soil.
Table 6. Selenium in tissues of male Japanese quail fed winter
wheat grown on soil or fly asha
-------------------------------------------------------------------
Wheat grown Tissue selenium
on: brain heart kidney liver muscle
-------------------------------------------------------------------
(mg/kg dry weight)
Soil 0.8 ± 0.1 0.7 ± 0.2 3.6 ± 0.4 1.6 ± 0.0 0.3 ± 0.2
Fly ash 3.4 ± 1.6 4.4 ± 0.7 9.5 ± 1.1 12.7 ± 2.4 4.1 ± 0.6
-------------------------------------------------------------------
a From: Stoewsand et al. (1978).
The possibility has been raised that some selenium may be added
to the food supply as a result of atmospheric contaminants from
the burning of fossil fuel or industrial emissions settling out on
the leaves of plants or on the surface of the soil. The
geographical pattern of selenium levels in rain-water from Denmark
or the USA implicates airborne selenium from the industrial and
domestic uses of fossil fuel as sources (Kubota et al., 1975).
Total industrial emissions of selenium in the USA for the year 1970
were estimated to be about 1.1 million kg, 62% of which was derived
from the burning of coal (US NAS/NRC, 1976). This level of
industrial emission of selenium may be compared with a projected
annual use of selenium as a feed additive for chickens and swine in
the "low-selenium" areas of the USA of 6000 kg (US FDA, 1974). But
there is no evidence that the selenium deposited in rainwater has
any influence on the concentration of selenium in forage plants
grown in Denmark or in the heavily industrialized northeastern USA
(Kubota et al., 1975). Furthermore, selenium deficiency has been
observed in farm livestock grazing near coal-burning electric power
stations (Anonymous, 1975). This suggests that airborne selenium
exists in the form of inert elemental selenium, insoluble selenide
salts, or as selenium dioxide, which would be tightly bound by
acidic, iron-bearing soils. In any case, the selenium would not be
available for uptake by plants; thus, there would appear to be
little prospect of adding appreciable selenium to the food supply
via atmospheric contamination.
(b) Human activities that decrease selenium levels in the food
chain
The metabolic antagonism between sulfur and selenium (Levander,
1976a) led early workers to suggest that borderline selenium
deficiency might be exacerbated by sulfate fertilization (Schubert
et al., 1961). More recent research has demonstrated that sulfate
fertilization can result in decreased selenium concentrations in
forage plants (Pratley & McFarlane, 1974; Westermann & Robbins,
1974). In many cases, this decrease can be largely explained by a
dilution effect caused by a growth response of the plant to the
sulfate fertilization, but decreases in selenium concentration
independent of crop yield have also been reported. Such decreases
could be the result of competitive interference by sulfate with the
uptake of selenate by plants. The use of phosphate fertilizers in
parts of Australia and New Zealand has resulted in an apparent
increase in the incidence of selenium deficiency in livestock
(Judson & Obst, 1975), but others have reported increases in the
selenium content of forages after fertilization with phosphorus
(Robbins & Carter, 1970; Carter et al., 1972). These conflicting
results may be due to differences in the selenium content of the
phosphatic rock from which the fertilizers were prepared.
Another possible way in which man's activities might decrease
selenium in the food chain or render it less available, is through
heavy metal pollution. For example, silver has been demonstrated
to antagonize selenium in a wide variety of situations (Diplock,
1976). In an area where there has been a large amount of silver
mining activity, muscular dystrophy of the type usually associated
with selenium deficiency has been reported in calves fed a ration
based on milk powder, even though the selenium content of the milk
seemed to be adequate. Apparently, the milk powder was derived
from cows that grazed land containing high levels of silver
residues from the old mining industry. Further research is needed
to establish fully the role of silver in potentiating these field
cases of apparent selenium deficiency. Because of the many
interactions of selenium with other environmental pollutants such
as mercury, cadmium, or arsenic (section 7), it is possible that
other cases of heavy metal-induced selenium deficiency will be
discovered.
The influence of cooking on the selenium content of foods was
determined because of the well-known instability and volatility of
many selenium compounds. Certain vegetables that normally contain
high levels of selenium such as asparagus or mushrooms, lost up to
40% of their selenium content as a result of boiling (Higgs et al.,
1972). Majstruk & Suchkov (1978) reported that up to 50% of the
original selenium was lost from vegetables and dairy products
during cooking; the addition of salt and acid pH or extended
cooking, particularly promoted such losses. But, other typical
cooking procedures such as boiling cereals, baking poultry or fish,
or broiling meats had little effect on selenium levels in the food
(Higgs et al., 1972). Similar results were observed by others for
baking fish or broiling meats, though some decrease in selenium
concentration was caused by frying organ meats or fish (Ganapathy
et al., 1975). Thompson et al. (1975) determined the selenium
contents of cooked food composites and found a rough agreement with
the calculated values based on unprepared foods from which they
were derived. On the basis of all these studies, it can be
concluded that usual cooking procedures cause little decrease in
the selenium content of most foods.
4.1.2. Chemical forms of selenium in food
There is little information on the chemical forms of selenium
that occur in human food (Levander, 1986). Cappon & Smith (1982)
reported that canned tuna fish contained variable percentages of
hexavalent selenium, ranging from 7.6 to 44.8%, which was
independent of the total selenium content. The other forms of
selenium in the tuna were di- and quadrivalent selenium. On an
average percentage basis, hexavalent selenium was more easily
extractable by water than di- or quadrivalent selenium. In
recently canned samples, an average of 55.6% of the total selenium
content was water-extractable. However, for the older samples, the
corresponding average extractable level was 48.2%. The results
suggest that sample storage may influence the chemical form of
selenium in canned tuna. Olson et al. (1970) found that, in a
certain portion of high selenium wheat (the pronase hydrolysate of
gluten), about half of the selenium was in the form of
selenomethionine. About 15% of the selenium in water extracts of
seleniferous cabbage leaves was in the form of selenomethionine,
but appreciable amounts of other selenium compounds were also
present (Hamilton, 1975). The chemical forms of selenium in food
are likely to affect the bioavailability of selenium (section 7.2.2
and 8.2.3).
4.2. Drinking-Water
Analysis of samples taken from various public water-supply
systems in the USA showed that less than 0.5% contained selenium
levels that exceeded the US PHS limit of 10 µg/litre (Taylor, 1963;
Lakin & Davidson, 1967; McCabe et al., 1970). Samples from 1280
central water sources providing water for 6.5 million Bulgarians
contained less than 2 µg selenium/litre (Gitsova, 1973). Tap water
from Stockholm, Sweden contained only 0.06 µg/litre (Lindberg,
1968) and tap and mineral waters from Stuttgart, Federal Republic
of Germany contained 1.6 and 5.3 µg/litre, respectively
(Oelschlager & Menke, 1969). Surface water sources in different
subregions of the Cernovici region of the Ukrainian SSR contained
selenium levels ranging from 0.09 ± 0.01 to 3.00 ± 0.40 µg/litre;
ground-water levels ranged from 0.07 ± 0.0 to 4.00 ± 0.85 µg/litre
(Suchkov & Kacap, 1971). A few tenths to several µg per litre were
reported in non-seleniferous regions of the USSR, the maximum
reported value being 5.1 µg/litre. In Argentina, the selenium
content of 22 surface waters varied from < 2 to 19 µg/litre with a
median value of 3 µg/litre (WHO, 1984).
Elevated levels of selenium (> 100 µg/litre) can be found in
seeps, springs, and shallow wells, though waters from deep wells
contain only a few µg/litre (US NAS/NRC, 1976). Highly variable
amounts of selenium were reported in wells from seleniferous areas
in the USA ranging from non-detectable levels to 330 µg/litre
(Smith & Westfall, 1937). Some well waters contained enough
selenium to be considered as poisonous for man or livestock and
loss of hair and nails in children was attributed to selenosis, but
the evidence for this was not considered convincing (US NAS/NRC,
1976).
The average selenium content of 11 samples of drinking-water
taken from a high-selenium area in China with a history of disease
reported as chronic selenosis was 54 µg/litre (Yang et al., 1983).
Four of these samples were surface water from a village with
previous heavy intoxication and these averaged 139 (117 - 159)
µg/litre. Such water would contribute substantial amounts of
selenium, but would still comprise a small fraction of the total
selenium intake in this area, since the contribution from food was
estimated to be about 5000 µg/day (section 5.1.1.1). The other 7
samples of drinking-water originated from several sources and
averaged only 5 µg/litre.
Rosenfeld & Beath (1964) concluded that selenium does not occur
in water in sufficient amounts to produce selenium toxicity in man
or animals, except in isolated cases. This was confirmed and
expanded in the statement by US NAS/NRC (1976, 1980) that waters
are rarely a significant source of selenium from either a
nutritional or a toxicity point of view. The low concentrations of
selenium in drinking-water are probably the result of several
mechanisms that act to decrease the selenium content of waters
(section 2.1).
4.3. Air
Selenium levels in the air breathed by the general population
are probably well below 10 ng/m3, on average (US NAS/NRC, 1976).
Hashimoto & Winchester (1967) found 0.3 - 1.6 ng airborne
selenium/m3 in an urbanized area. Levels of 3.6 - 9.7 ng
selenium/m3 were detected by Pillay et al. (1971), who showed that
over half of the selenium was not retained on filters able to trap
particulates greater than 0.1 µg in diameter. Zoller & Reamer
(1976) concluded that atmospheric levels of selenium in most urban
regions vary from 0.1 to 10 ng/m3. Selenium levels in air around
industries that use selenium can be higher (section 3.2.2), perhaps
of the order of a few µg/m3. Work-place air in selenium industries
apparently contained mg levels of selenium/m3 in the past, but more
recent measurements have indicated lower levels (section 5.2).
5. HUMAN EXPOSURE
5.1. Estimate of General Population Exposure
As discussed in section 4, selenium levels in air are low in
areas without selenium-emitting industries and are not likely to
exceed 10 ng/m3. Assuming that a person respires 20 m3 air daily,
the contribution to daily selenium intake via this route would be
0.2 µg or less. Since this intake is much lower than that through
food (see below), airborne selenium makes a negligible contribution
to the average daily selenium intake of the general population.
However, levels of selenium may be somewhat higher in the
atmosphere around some industrial plants using selenium (section
3.2.2). If it is assumed that 3 µg selenium/m3 is found in the air
near these industries, then exposure to selenium via air would be
60 µg/day.
Other possible exposures of the general population to selenium
via the air include contributions from house dust and tobacco
smoke. Lakin & Davidson (1967) reported that house or office dust
could contain up to 10 mg selenium/kg. Although no data are
available to provide an estimate of human exposure to selenium from
this source, the possibility of such exposure should be considered
because of the increased interest in the quality of indoor air.
Olsen & Frost (1970) found an average of 0.08 mg selenium/kg (range
0.03 - 0.13 mg/kg) in a variety of cigarette tobaccos. If it is
assumed that a cigarette contains 1 g tobacco and that all the
selenium in tobacco is volatilized and inhaled during smoking, it
can be calculated that a person smoking one pack of 20 cigarettes
per day would inhale an average of 1.6 µg from this source.
Most drinking-water supplies contain only small quantities of
selenium, except possibly for those in certain seleniferous areas.
As discussed in section 4.2, most public water supplies contain
much less than 10 µg selenium/litre. Assuming that a person drinks
2 litres of water daily, the intake via this route would be only a
few µg. While this is considerably higher than the intake via air,
in most areas of the world, this is still a small fraction of the
daily intake from food.
5.1.1. Food
5.1.1.1. Geographical variation
Both the amount and the bioavailability of dietary selenium are
determinants of its biological effects. Bioavailability is be
considered in sections 7.2.2 and 8.2.3. The estimated daily intake
of selenium from dietary sources by adult human beings varies
considerably in different parts of the world. The greatest
extremes in intake have been found in China (Table 7) where both
selenium toxicity and deficiency have been reported (section
8.1.1.1, 8.2.2). An average selenium intake of 4990 µg/day was
estimated in a high-selenium area of China with a history of
intoxication reported as chronic selenosis, whereas an average
intake of only 11 µg/day was estimated in an area of China where
Keshan disease, a cardiomyopathy related to low selenium status,
was reported. Intermediate intakes of selenium were found in areas
of China that were not affected with either selenosis or Keshan
disease. It should be pointed out that the dietary-selenium intake
in the high-selenium area with a history of intoxication reported
as selenosis did not overlap with that in the high-selenium area
reported to be without selenosis. Likewise, the dietary-selenium
intake in the low-selenium area with Keshan disease did not overlap
with that in the moderate-selenium area (Beijing).
Table 7. Daily selenium intake of residents living in high-, medium-, and low-selenium
areas of Chinaa
---------------------------------------------------------------------------------------
Number Daily selenium intake Se intake from staple
Place of minimum maximum average cereals as % of total
subjects (µg) daily intake
---------------------------------------------------------------------------------------
High-selenium area with 6 3200 6690 4990 28-70%
a history of intoxication
reported as chronic
selenosis
High-selenium area 3 240 1510 750 25-45%
reported as without
selenosis
Moderate-selenium area 8 42 232 116 various sources
(Beijing)
Low-selenium area with 13 3 22 11 mainly from cereals
Keshan disease
---------------------------------------------------------------------------------------
a Adapted from: Yang et al. (1983).
Less extreme, but still widely diverse intakes of selenium have
been observed in other parts of the world (Table 8). The selenium
intake calculated in New Zealand, a country with low levels of
selenium in the soils in certain areas, averaged 30 µg/day (Thomson
& Robinson, 1980; Watkinson, 1981). Similar low intakes have been
reported by other research workers in New Zealand. For example,
Stewart et al. (1978) showed that the mean dietary-selenium intake
of 4 New Zealand women consuming normal diets ad libitum was
24.2 µg/day, and Griffiths (1973) reported that the daily intake of
selenium by 13 young New Zealand women varied from 6 to 70 µg.
Daily intakes of 30 µg or more in the latter group were associated
with the inclusion of liver, kidney, or fish in the diet. A low
average dietary-selenium intake (30 µg/day) has also been reported
in Finland, another country known to have soils low in selenium
(Varo & Koivistoinen, 1980). Low levels of dietary selenium may
also occur in Italy (Rossi et al., 1976) and Egypt (Waslien, 1976).
The average daily intake of selenium in the United Kingdom was
estimated to be 60 mg (Thorn et al., 1978). In one study, the
dietary-selenium intake in Japan was estimated to be 88 µg/day
(Sakurai & Tsuchiya, 1975). A higher estimate in another study was
arrived at largely because higher selenium contents of food staples
were used in the calculations (Yasumoto et al., 1976). The
estimated dietary intake of selenium in North America ranged from
98 to 224 µg/day. A "Market basket" survey carried out in the USA
from 1974 to 1982 indicated an average overall selenium intake of
108 µg/day (Pennington et al., 1984). An earlier survey had
revealed regional differences in intake, with people living in
western parts of the country consuming 1.3 times as much selenium
as people in the northeastern part of the country (US FDA, 1975).
Duplicate plate analyses of self-selected diets in Maryland, USA
gave a mean intake of 81 ± 41 µg/day (Welsh et al., 1981). The
daily intake in Venezuela was estimated to be 326 µg, but this
figure requires cautious interpretation because North American food
consumption patterns were used in its derivation (Mondragon &
Jaffe, 1976).
5.1.1.2. Food habits (consumption patterns)
Because of the wide variations in the selenium content of
various food groups in different countries, it is possible that
certain food habits or preferences could play an important role in
determining whether a person is at risk with regard to a deficient
or excessive intake of selenium. Persons with a preference for
foods such as fish may consume high levels of selenium in their
diet. Sakurai & Tsuchiya (1975) estimated that Japanese eating
large amounts of seafish may ingest as much as 500 µg of selenium
daily. People residing in seleniferous agricultural zones may
consume large amounts of selenium if locally-produced foods
constitute the bulk of their diet. In Venezuela, for example, corn
and rice samples from high selenium areas contained as much as 14
and 18 mg selenium/kg, respectively (Jaffe, 1976), and values as
high as 180 mg/kg have been reported in wheat from Colombia
(Ancizar-Sordo, 1947). If it is assumed that some segments of the
population living in such seleniferous regions consume, under
certain conditions, about half of their diet in the form of rice or
corn with a similar selenium content to that described above, the
daily intake of selenium from dietary sources could exceed 7000 µg.
However, it should be pointed out that only a few samples from the
high-selenium areas showed these extreme high levels. Obviously,
vegetarian diets could vary tremendously in selenium content.
Ganapathy & Dhanda (1976) showed that a vegetarian diet in the USA
supplied 84 µg selenium/day.
If a particular staple food constitutes a large fraction of the
diet of a population, then the selenium content of that food will
have a great influence on the overall dietary-selenium intake. For
example, one estimated daily dietary intake of selenium in Japan
(Sakurai & Tsuchiya, 1975) of 88 µg, was based on selenium levels
in rice and soybeans of 0.05 and 0.02 mg/kg, respectively, whereas
another estimated intake, 208 µg, was based on rice and soybean
selenium levels of 0.220 and 0.234 mg/kg, respectively (Yasumoto et
al., 1976). Low levels of selenium in rice have been reported in
Bangladesh, and it would be particularly interesting to study the
population there, as they depend on rice as a staple food, but they
have a recognized poor dietary vitamin E status (Bieri & Ahmad,
1976).
Table 8. Estimated human dietary intake of selenium (µg/day)a
----------------------------------------------------------------------------------------------------
Food New Zealand Finland United Japanf Canadag USAh USA Venezuelaj
Dunedinb Hamiltonc 1975d 1979d Kingdome South
Dakotai
----------------------------------------------------------------------------------------------------
Plant
vegetables,
fruits,
and sugars 1 2 1 1 3 6 1-9 5 10 15
cereals 4 3 3 25 30 24 62-113 45 57 88
Animal
dairy
products, 11 11 7 13 5 2 5-28 13 48 70
eggs
meat, fish 12 16 19 19 22 56 25-90 69 101 153
Total 28 32 30 50-60 60 88 98-224 132 216 326
----------------------------------------------------------------------------------------------------
a From: Robinson & Thomson (1983).
b From: Thomson & Robinson (1980).
c From: Watkinson (1981).
d From: Varo & Koivistoinen (1981).
e From: Thorn et al. (1978).
f From: Sakurai & Tsuchiya (1975).
g From: Thompson et al. (1975).
h From: Watkinson (1974) & Levander (1976b).
i From: US NAS/NRC (1980).
j From: Levander (1976b).
The importance of the selenium content of a particular staple
in the food supply of persons dependent on a monotonous diet
consisting of locally-produced foods was recently pointed out in
connection with the occurrence of Keshan disease (section 8.2.2) in
China (Yu, 1982). In the years in which Keshan disease was endemic
in the Fuyu county of Heilongjiang province, about 90% of the diet
consisted of maize, whereas, in the years in which Keshan disease
was not endemic, only about 30% of the diet was maize with much of
the rest coming from millet and wheat. Thus, it seems possible
that over-reliance on this single staple food during certain years
helped to precipitate Keshan disease in this area.
Recently, there has been considerable interest in some
countries in the use of textured soy protein products as
substitutes for meat products. Since meat products are such good
dietary sources of selenium, the possible impact of this trend on
the selenium intake of human beings was examined. Some soy protein-
based meat analogues contained as much selenium as meat products,
but others did not (Ferretti & Levander, 1976). These results show
that meat analogues made from soy or other plant proteins are not
consistent sources of selenium, since these plant-based products
will vary in selenium content depending on where the plant is
grown. In contrast, meat products are more reliable dietary
sources of selenium, because they contain a minimum quantity
compatible with animal life.
5.1.1.3. Elderly people
Thomson et al. (1977a) studied a group of 48 elderly people
(mean age of 78 years) in New Zealand and found that their blood-
selenium levels and red cell glutathione peroxidase (EC 1.11.1.9)
activity were lower than those in 12 young adult controls. It was
not possible for the authors to conclude from the data whether the
depressed blood-selenium levels and enzyme activity were due to
decreased selenium intake or were a general part of the aging
process. Abdulla et al. (1979) analysed 7-day pooled dietary
composites of 20 pensioners in Sweden and found that the average
daily selenium intake was 30.8 µg with a range of 8.7 - 96.3
µg/day.
5.1.1.4. Infants and children
The selenium intake of infants is of interest because of their
rapid growth rate and their heavy reliance on milk, a food that has
a highly variable selenium content, depending on its geographical
origin (Table 9). Millar & Sheppard (1972) assumed that human
and cow's milk in New Zealand contained 0.010 and 0.005 mg
selenium/litre, respectively, and calculated that the selenium
intake of infants for the first month of life would be 5.0 or
2.1 µg/day, depending on whether human or cow's milk was fed.
Williams (1983) measured the selenium content of mature mother's
milk from 10 New Zealand women and calculated an average daily
selenium intake of 5 - 6 µg for an infant consuming 700 ml milk.
If the value for the selenium content of Venezuelan cow's milk
shown in Table 9 is typical, it can be calculated that the selenium
intake of infants in that country would be about 58 µg/day during
the first month of life. The most extreme values for the selenium
content of human milk are from low- and high-selenium areas of
China (Yang et al., 1986) (section 4.1.1.1). For infants consuming
750 ml milk daily, these milks would provide 2 µg and 212 µg
selenium, respectively.
Table 9. Estimated infant daily intake of selenium
from dietary sources in North America (6-month-old,
6.5-kg child)a
-----------------------------------------------------
Daily Selenium Selenium
Food consumption content intake
(g) (mg/kg) (µg/day)
-----------------------------------------------------
Milk 824 0.013 11
Orange juice 122 0.014 2
Dry mixed cereal 10 0.540 5
Egg yolk 17 0.437 7
Strained meat 28 0.097 3
Strained fruit 57 0.002 -
Strained vegetable 57 0.003 -
Total selenium intake 28
-----------------------------------------------------
a Adapted from: Levander (1976b).
It was shown that milk continues to furnish a sizeable portion
of an infant's estimated daily selenium intake of 28 µg, in the
USA, after the child starts to eat solid foods (Table 9). The
estimated daily intake of selenium by 3-month-old infants in the
Federal Republic of Germany was 11 µg (Lombeck et al., 1975) and a
"market basket" survey indicated that the intake of 6-month-old
infants in the USA was about 12 µg/day (Harland et al., 1978). On
the basis of a caloric intake of 700 Kcal/day, infants in the USA
consuming formula diets based on milk, soy protein, casein, or
meat, ingested 8.5, 9.5, 12.6, or 31.5 µg/day, respectively (Zabel
et al., 1978).
As infants grow up in New Zealand, they continue to ingest
relatively low levels of selenium, since the estimated daily intake
over the age span of 6 months to 5 years was 5 - 8 µg (McKenzie et
al., 1978).
5.1.1.5. Special medical diets
Infants and children suffering from metabolic diseases such as
phenylketonuria (PKU) or maple syrup urine disease (MSUD) are fed
synthetic diets that contain little selenium for the first 10 or 12
years of their lives. In New Zealand, the mean daily selenium
intake of 12 subjects consuming such synthetic diets was 5 ± 2 µg
with little difference between the older children and the infants
(McKenzie et al., 1978). The selenium content of diets of 3-month-
old infants with PKU and MSUD in the Federal Republic of Germany
amounted to 4.7 and 5.6 µg/day, respectively (Lombeck et al.,
1975).
A wide variation in the selenium contents of special-purpose
medical diets for adults is related to the source of protein in the
diet (Zabel et al., 1978). On the basis of an intake of 2000
Kcal/day, adults consuming food supplements or tube-feeding
formulae would ingest 29, 47, or 98 µg selenium/day, depending on
whether the diet contained soy protein and casein, milk protein and
casein, or blended foods. At a similar caloric intake, chemically-
defined diets based on egg albumen provided 225 µg/day, whereas
diets based on casein hydrolysate or amino acid mixtures provided
only 28 and 1.5 µg/day, respectively. Total parenteral nutrition
solutions based on casein hydrolysate and amino acid mixtures also
furnished low amounts of selenium of only 32 and < 1 µg/day,
respectively. When levels of selenium supplied by special
therapeutic diets were studied in the Chernovitsi region of the
Ukrainian SSR, selenium intakes were found to vary between 99 ± 6
and 121 ± 8 µg/day (Majstruk & Suchkov, 1978).
5.2. Occupational Exposure
5.2.1. Levels in the workplace air
The main pathway of human occupational exposure to selenium is
through the air. Rarely, and under special circumstances, direct
contact may be of importance, i.e., when cutaneous absorption might
be facilitated by the local irritation and skin damage caused by
vesicant selenium compounds.
Various mechanical processes connected with the mining of
seleniferous ores or the grinding of selenium compounds can
contribute selenium-containing dusts to the atmosphere. In other
industrial activities, the amount of selenium released into the air
depends on the temperature to which it is heated and on the area
available for sublimation and/or vaporization. It is well
established that heating amorphous selenium below its melting point
results in its sublimation. At temperatures of 170 - 180 °C,
traces of selenium can be detected in the air and, at temperatures
of 230 - 240 °C, selenium dioxide is released. Heating of
amorphous selenium and selenium dioxide resulted in the release of
substantial quantities of selenium into the air, e.g., from a
surface of 10 cm2, 2.6 - 3.75 mg was released after 20 min at 230 -
240°C and 23 - 31.6 mg was released after 20 min at 350 - 360 °C
(Izraelson et al., 1973).
Little quantitative information is available in the literature
on actual levels of human exposure to selenium in industry. The
levels of selenium in the workplace depend not only on the nature
of the process involved but also on the control technology in any
given industry. Thus, it can be expected that analyses carried out
several decades ago yielded higher values than analyses carried out
more recently. In 1948, Filatova reported air levels of selenium
and selenium dioxide of 20.6 - 24.8 mg/m3 and 0.46 - 0.78 mg/m3,
respectively, in working zones of selenium-heating processes in a
selenium rectifier plant, whereas levels of 0.55 - 1.1 mg/m3 and up
to 0.11 mg/m3, respectively, were associated with the process of
disc smearing (Filatova, cited in Izraelson et al., 1973). In the
production of selenium-containing photoelements by the vacuum
application of a photosensitive layer, selenium and selenium
dioxide levels did not exceed 2.0 mg/m3 and 0.1 mg/m3, respectively
(Sverdlina & Maslennikova, 1961). Selenium grinding, sulfuric acid
treatment, and cathode alloy coating resulted in air-selenium
levels ranging from 0.133 to 2.0 mg/m3 (Izraelson et al., 1973). A
highly-dispersed aerosol selenium condensate with a particle size
of 0.5 - 2 µm was produced at a level of 0.88 - 6.13 mg/m3, as a
result of the manufacture of selenium-containing steels (Ershov,
1969). In the production of rare metals, the air in industrial
premises can contain as much as 0.4 - 750 mg metallic dust/m3,
which, in addition to other elements, contains from 0.8 - 15.7%
selenium (Burchanov, 1972). During the 1950s, Glover (1967)
carried out an extensive survey in which air-selenium levels were
determined in areas of a selenium rectifier plant in which the
workers had been found to have high urinary-selenium values. In
general, the highest air levels of 1.5 - 5.2 mg selenium/m3 were
found in relation to the grinding process. With the exception of
certain "special processes", air levels in the plant due to all the
other manufacturing activities were less than 0.5 mg/m3. In one
case, a level of 21.3 mg/m3 was observed, but no additional details
were given. In this rectifier plant, a process, in which the
elemental selenium was heated, was used for preparing the selenium
mixture that was later applied to the metal plate. For this
reason, the selenium levels in the air are likely to have been
higher than in other factories where the selenium was deposited
from aqueous solution. In another selenium rectifier plant, air
levels of between 7 and 50 µg selenium/m3 were reported
(Kinnigkeit, 1962). However, in this case, no correlation was
observed between the air levels in the workplace and the selenium
levels in the blood of the workers. Kinnigkeit felt that the air-
selenium levels were not high enough to account for the high
urinary-selenium levels in the workers. He suggested that the
discrepancies might be due to incomplete collection of selenium
during air sampling, transient peaks of selenium exposure that were
not detected by short-term sampling, or contamination of hands,
body, and clothing with selenium, which was then transferred to the
mouth as a result of eating or smoking. Another possible
explanation is that measurements of the levels in the workroom air
did not accurately reflect levels of selenium in the actual
breathing zone of the workers.
Low air levels of selenium have been observed in factories
involved with the use of selenium in certain photocopying devices.
In this type of manufacturing, "clean room" techniques are used,
because strict dust control is needed to produce the quality of
photoreceptor surfaces required. Cannella (1976) reported air
levels of selenium ranging from 6 to 91 µg/m3 in various locations
in a selenium alloy plant that made such photoreceptor products.
5.2.2. Biological monitoring
Although selenium levels in the work-place air have been
determined in several instances, these measurements cannot be used
to draw any conclusions about the exposure history of individual
workers. Glover (1967), who estimated occupational exposure to
selenium indirectly by determining selenium levels in the urine of
workers, acknowledged the limitations associated with monitoring
based on grab samples of urine. The Working Group also recognized
the inadequacy of urinary-selenium concentrations as a monitoring
tool. However, it appears that the use of a more rigorous
programme (e.g., total 24-h urine collections) would have resulted
in losing the cooperation of the workers involved. A tentative
maximum allowable concentration of selenium in urine of 0.1
mg/litre was proposed by Glover (1967). The urine of the workers
was analysed at 3-month intervals, and any employee whose urinary-
selenium value exceeded the limit was transferred to a process not
involving selenium. The urinary-selenium levels of these
individuals were tested weekly.
Two primary factors were responsible for setting the maximum
allowable concentration for selenium in urine at 0.1 mg/litre
(Glover, 1970). First, a limit lower than this would have resulted
in a certain number of "false positives", since selenium levels in
urine are often this high in people not industrially exposed to the
element. Second, it was found that values below 0.1 mg/litre were
invariably accompanied by air levels in the work-place of less than
0.1 mg/m3, which was below the threshold limit value of 0.2 mg/m3
for elemental selenium and its common inorganic compounds (ACGIH,
1971). It must be emphasized that urinary selenium cannot be used
to monitor exposure to such dangerous selenium compounds as
hydrogen selenide, selenium oxychloride, or certain organic
selenium compounds because severe damage to the lungs or skin would
have occurred by the time that the urinary-selenium values were
raised (Glover, 1976).
6. METABOLISM OF SELENIUM
The metabolism of selenium has been most completely studied in
animals and data from human investigations are limited. Thus, in
the following sections, pertinent information from selected animal
studies is presented followed by available information from
controlled human studies.
6.1. Absorption
Since food is the primary environmental medium through which
man and animals are exposed to selenium, most data concerning
selenium absorption deal with the gastrointestinal pathway. Much
less is known about the absorption of selenium through the lungs or
skin.
6.1.1. Gastrointestinal absorption
6.1.1.1. Animal studies
The intestinal absorption of soluble selenium compounds by rats
is highly efficient. It has been shown that these animals absorbed
92, 91, and 81% of doses of selenite, selenomethionine, and
selenocystine, respectively (Thomson & Stewart, 1973; Thomson et
al., 1975a). In a study on rats by Whanger et al. (1976a), the
greatest absorption of selenite or selenomethionine occurred from
the duodenum, with slightly less absorption from the jejunum or
ileum. Virtually no absorption occurred from the stomach. The
absorption of selenite by rats does not appear to be under
homeostatic control, since 95% or more of the dose was absorbed,
regardless of whether the animals were fed a selenium-deficient
diet or a diet containing mildly toxic levels of selenium (Brown et
al., 1972). Wright & Bell (1966) found that selenite was absorbed
from the gastrointestinal tract to a greater extent by monogastric
animals (swine) than by ruminant animals (sheep). The decreased
absorption by sheep may have been due to reduction of the
administered selenite to insoluble or unavailable forms by rumen
microorganisms.
Only limited information is available regarding the absorption
by animals of selenium occurring naturally in foods. Kidney tissue
and fish muscle were chosen for model studies, because these foods
are relatively rich sources of dietary selenium for human beings.
The levels of intestinal absorption by rats of radio-selenium
administered in homogenates of rabbit kidney or fish muscle
injected with selenite, were 87 and 64%, respectively (Thomson et
al., 1975b; Richold et al., 1977). The low absorbability of
"fish selenium" agrees with other reports showing the poor
bioavailability to animals of selenium in certain fish products
(section 5).
Little is known concerning the physiological processes
governing the absorption of even simple selenium compounds, though
McConnell & Cho (1965) showed that selenomethionine was transported
against a concentration gradient, whereas selenite and
selenocystine were not.
6.1.1.2. Human studies
Studies carried out in New Zealand on 3 young female volunteers
indicated that the intestinal absorption of oral doses of
radioactive selenite, containing not more than 10 µg selenium, was
70, 64, and 44% of the dose, respectively (Thomson & Stewart,
1974). In a study conducted 2 years later, in which 2 of the 4
female volunteers were women who had participated in the earlier
study with selenite, the intestinal absorption of oral doses of
75Se-selenomethionine, containing less than 2 µg selenium, averaged
about 96% of the dose (Griffiths et al., 1976). When a larger dose
of 1 mg selenium was given orally in solution, a similar difference
in absorption was obtained, i.e., 97% for selenomethionine (one
subject) (Thomson et al., 1978) and about 60% for sodium selenite
(13 subjects) (Robinson et al., 1985; Thomson & Robinson, 1986).
This value for absorption of selenite-selenium is much lower than
the 92% absorption reported for "selenite" in the earlier paper of
Thomson (1974), which is now known to have been selenate-selenium
and not selenite-selenium. Later studies on 10 volunteers also
showed absorption of 94% for 1 mg selenate-selenium in solution
(Thomson & Robinson, 1986). Thus, selenate-selenium is similar to
selenomethionine-selenium in that it is better absorbed than
selenite-selenium.
Correction of selenate-selenium for selenite selenium in the
paper of Thomson (1974) also removes the "surprising difference"
reported between absorption of selenite given in solid form (60%
for 4 female volunteers) and in solution (60% and not 92%). A
female volunteer given a 1 mg dose of selenium as selenite in
solution daily for 5 consecutive days absorbed 59% of the total 5
mg dose (Thomson et al., 1978). It would appear that human beings
have no homeostatic control to limit their absorption of large
single doses of these selenium compounds (Barbezat et al., 1984).
Robinson et al. (1978b) measured the intestinal absorption of
selenium by a New Zealand female volunteer receiving a daily
supplement of 100 µg selenium as selenomethionine, a male volunteer
supplemented daily with 100 µg selenium as sodium selenite, and a
second female supplemented daily with 65 µg selenium, as it occurs
naturally in mackerel (Scomber japonieus). The selenomethionine
and selenite were given in solution, whereas the mackerel was given
as canned fish. During a 4-week supplementation period, 75% of the
selenium given as selenomethionine was absorbed compared with only
48% of the selenium administered as selenite. The absorption of
the "fish selenium" was 66%. Details of the diets consumed by the
3 subjects were not provided, but the subjects supplemented with
selenomethionine or fish selenium did not consume liver or kidney
throughout the study. However, it was specified only that the
subject supplemented with selenite did not eat liver, kidney, or
fish on the days that weekly urine collections were made or on
the day prior to the collection. Also, the subject receiving
selenomethionine ate fish only occasionally throughout the study.
The subject supplemented with the fish selenium ate fish other than
mackerel on only one occasion. Stewart et al. (1978) found that
the average intestinal absorption of naturally-occurring selenium
in foods was 55% in 4 young New Zealand women freely consuming
normal diets. However, allowing for the endogenous faecal
excretion (half of total faecal output) the average true absorption
of naturally occurring selenium became 79%.
6.1.2. Absorption by inhalation
Certain volatile selenium compounds, such as hydrogen selenide,
are known to be toxic when inhaled (Dudley & Miller, 1941).
Lipinskij (1962) observed increased selenium levels in the liver
and kidneys after respiratory exposure of rabbits to elemental
selenium and selenium dioxide. More recently, Weissman et al.
(1979, 1983) studied the distribution and retention of inhaled
75Se-labelled selenious acid and elementary selenium aerosols.
They used 63 Beagle dogs, which were between 3 and 4 years old at
the time of exposure. All inhalation exposures were through the
nose only. Aerosol particles of both forms were small with median
aerodynamic diameters of 0.5 and 0.7 µm and geometric standard
deviations of 2.4 and 1.5 for selenious acid and elemental
selenium, respectively. The urine and faeces of 4 dogs were
collected daily, for 32 days, and then at regular time intervals
until sacrifice. Selenium from exhaled breath was collected from 3
dogs for several 4-h periods, between 2 and 10 days after exposure.
Whole-body retention of 75Se-selenium was measured immediately
after exposure and at regular intervals up to 320 days after
exposure. Two dogs exposed to each aerosol were sacrificed at
various time intervals for over half a year. The initial 75Se-
selenium body burden (IBB) in dogs that inhaled 75Se-selenium as
selenious acid was 40 ± 17 µg selenium/kg body weight and 22 ± 9 µg
selenium/kg body weight in dogs inhaling elementary selenium. For
both forms of selenium, the retention data of 75Se-selenium could
be expressed by a 3-component negative exponential equation:
Selenious acid:
%IBB = 66e-1.5t + 17e-0.089t + 17e-0.023t
Elemental selenium:
%IBB = 66e-0.090t + 21e-0.059t + 13e-0.018t
The corresponding half time values were 0.46, 7.8, and 30 days and
0.77, 12, and 38 days for selenious acid or elemental selenium
exposure, respectively. Studies on organ distribution showed that,
2 h after exposure to selenious acid, only about 5.3% of the IBB
was retained in the lung compared with 26% of the IBB of elemental
selenium. For the first 32 days following exposure, the urine
accounted for 79 and 66% of the 75Se-selenium excreted by dogs
exposed to selenious acid and elemental selenium aerosols,
respectively. Respiratory excretion of 75Se-selenium was
negligible. The organ distribution of 75Se-selenium as a
percentage of the sacrifice body burden is given in Table 10.
Table 10. 75Se-selenium distribution in organs of dogs
128 days after respiratory exposure to 75Se-selenious acid
or elemental 75Se-selenium aerosolsa
----------------------------------------------------------
Organ 75Se-selenium organ distribution
as % of 75Se-selenium body burden
at sacrifice
----------------------------------
selenious acid elemental selenium
treated treated
----------------------------------------------------------
Lung 1.3 6.2
Liver 11.4 7.4
Kidney 1.6 1.6
Blood 19.7 21.5
Gastrointestinal tract 3.4 3.6
Pelt 9.6 9.1
----------------------------------------------------------
a Adapted from: Weissman et al. (1979).
Comparison of the absorption of these two forms of selenium
through inhalation was also studied in the rat (Medinsky et al.,
1981). The rate of transfer of selenium into the blood was slower
when the elemental form was administered. However, once absorbed,
both chemical forms behaved identically.
6.1.3. Absorption through the skin
Apart from the report of Dutkiewicz et al. (1972), who found
that 10% of a 0.1 mol solution of sodium selenite applied to rat
skin was absorbed in 1 h, there are no quantitative data on the
dermal absorption of water-soluble selenium compounds. However,
Dudley (1938) showed that selenium oxychloride was absorbed through
the skin of rabbits. Selenium sulfide, an insoluble selenium
compound used in certain anti-dandruff shampoos, is not ordinarily
absorbed through the skin, although a garlicky breath odour and
elevated urinary excretion of selenium were noted in a patient with
open scalp lesions who used a selenium sulfide shampoo (Ransone et
al., 1961).
6.2. Distribution in the Organism
6.2.1. Transport
Little is known about the transport of selenium in the body.
However, a plasma selenoprotein has been identified which one group
postulates is involved in selenium transport (Motsenbocker &
Tappel, 1982).
6.2.2. Organs
6.2.2.1. Animal studies
Absorbed selenium is rapidly distributed among the tissues.
Heinrich & Kelsey (1955) found that the liver of mice injected
subcutaneously with sodium selenite contained 17 and 30% of the
dose, 15 and 60 min after injection, respectively. However,
because of the great variation in the metabolic half-time of
selenium in various tissues (Thomson & Stewart, 1973), the
distribution pattern observed will change markedly with the time of
sampling after injection with radio-selenium.
As long as the diet provides nutritionally adequate levels of
selenium, the relative concentrations of selenium in the various
internal organs are quite consistant over a wide range of selenium
intakes. For example, Jones & Godwin (1962) studied the
distribution of selenium in the tissues of mice fed alfalfa
containing nutritional levels of selenium. The concentrations of
selenium in the internal organs decreased in the following order:
kidneys > liver > pancreas >> lungs > heart > spleen >
skin > brain > carcass. Smith et al. (1937) fed toxic levels of
sodium selenite to cats and observed the following order for the
concentration of selenium in the internal organs: liver >
kidneys > spleen > pancreas > heart > lungs.
The distribution of selenium appears to be relatively
independent of the form and route of administration, since the
kidneys and adrenals contained the highest concentrations of radio-
selenium, regardless of whether the rats were given labelled
selenite or selenomethionine orally or by intravenous injection
(Thomson & Stewart, 1973). The greatest amounts of radioactivity
were in the kidneys, liver, and total muscle mass. Similar
distribution patterns were noted in rats dosed orally with
homogenates of tissues from rabbits or from fish that had been
given labelled selenium compounds (Thomson et al., 1975b; Richold
et al., 1977).
The distribution of selenium in tissues was markedly different
when rats were fed a diet deficient in selenium: the testes,
brain, thymus gland, and spleen took up the greatest concentrations
of selenium from a tracer dose of selenite (Burk et al., 1972). A
recent report confirms the ability of the testis to retain selenium
better than other tissues in selenium deficiency (Behne & Hofer-
Bosse, 1984).
The distribution of selenium in the eye was determined
fluorometrically with 2,3-diaminonaphthalene by Gusejnov et al.
(1974), who found the following levels in various parts of the eye
of a bull (mg/kg fresh weight): iris, 1.3; retinal pigment
epithelium, 0.85; retina, 0.50; cornea, 0.3; lens, 0.03; and
vitreous body, 0.01. In some birds (pigeons and crows), the level
of selenium in the retina was about 10 times higher (about 5.0
mg/kg). In studies in which 75Se-labelled sodium selenite in
saline was administered intraperitoneally to frogs, rats, and
rabbits, the distribution of radio-selenium was similar to that of
the naturally-occurring selenium measured in the bull's eye. The
maximum 75Se activity in the eye (5 h after administration) was
found in the pigment with the vascular layer of the retina, whereas
minimum activity was found in the lens and vitreous body.
Histoautoradiography of the eyes also indicated the highest
radioactivity in the vascular and pigment layers (Abdullaev et al.,
1972).
6.2.2.2. Human studies
The distribution of selenium in human organs was examined by
Schroeder et al. (1970), who found the following descending order
for selenium concentrations in tissues: kidney > liver >
spleen > pancreas > testes > heart muscle > intestine >
lung > brain. Blotcky et al. (1976) showed that, in autopsy
samples from 106 persons, the concentrations of selenium were 2-3
times higher in the kidneys than in the liver.
Uptake of an orally-administered dose of radioselenite in the
internal organs occurred in the following relative order: liver >
kidneys > lungs > muscle = articular tissue (Leeb et al., 1977).
Other studies have shown significant accumulation of radio-selenium
by the liver and kidneys after intravenous injections of selenite
or selenomethionine (Lathrop et al., 1972; Falk & Lindhe, 1974;
Nordman, 1974).
6.2.3. Blood
Studies on experimental animals have shown a close correlation
between the level of selenium in the blood and the level of
selenium in the diet (Lindberg & Jacobsson, 1970; Cary et al.,
1973; Ermakov & Kovalskij, 1974; Oh et al., 1976a). Analytical
surveys on human beings have shown significant variations in blood-
selenium levels in different geographical areas, presumably because
of differences in dietary-selenium intake. For example, Allaway et
al. (1968) found that selenium levels in the blood of persons
living in areas of the USA where soils and vegetation were high in
selenium tended to contain more selenium than that of persons
living in areas where soils and plants were generally low in
selenium. Thus, geographical differences in the selenium levels in
human blood are possible, even in a country where large-scale
interregional food shipments would be expected to level out
differences in the amount of selenium in the food supply. These
geographical differences in human blood-selenium levels in the USA
have been confirmed by Howe (1974), who found a mean selenium
content of 0.265 mg/litre (SD = 0.056) for 626 samples collected in
and around the state of South Dakota (a high soil-selenium region),
whereas Schultz & Leklem (1983) reported blood-selenium values in
Oregon (a low soil-selenium region) that were well below those
reported for other areas of the USA.
Analyses of blood samples from persons living in different
regions of the world indicate similar correlations between the
amount of selenium in the blood and the amount of selenium in the
food throughout the world (Table 11). For example, blood-selenium
concentrations tend to be lowest in areas of the world that are
known to have low levels of selenium in the soil (Keshan-disease
areas of China, New Zealand, Scandinavia) and highest in areas that
are known to have high levels of selenium in the soil (Venezuela,
reported selenosis areas of China). Superimposed on these
geographical variations in effects are the effects of general
nutritional status or effects of certain disease states. For
example, children suffering from Kwashiorkor have lower blood-
selenium levels than their well-nourished counterparts (Burk et
al., 1967) and reductions in the selenium content of sera have been
observed in patients with cancer (McConnell et al., 1975). Lower
blood-selenium values were associated with lower serum-albumin
values in surgical patients with and without cancer in New Zealand
(Robinson et al., 1979). Akesson (1985) suggested that alterations
in plasma-selenium may be secondary to changes in plasma-protein
concentration. In a study of Finnish men with one or more risk
factors for coronary heart disease, Miettinen et al. (1983)
observed a strong association between serum-selenium and the
eicosapentanoic acid contents of cholesterol ester and
phospholipids. The authors pointed out that since eicosapentanoate
is a fatty acid peculiar to fish such an association could reflect
the intake of fish at the time of blood sampling.
Blood-selenium levels change if persons travel to countries
with soils of different selenium content. For example, Griffiths &
Thomson (1974) noted that the blood-selenium levels of adults from
the USA declined rapidly on arrival in New Zealand, but, after 1
year, their levels were still higher than the mean value for
permanent New Zealand residents (Fig. 2). Rea et al. (1979) found
that plasma-selenium levels changed with changes in selenium intake
more rapidly than erythrocyte-selenium levels. The latter
reflected longer-term changes in selenium exposure, presumably
because of the relatively long life span of erythrocytes. In a
controlled depletion/repletion metabolic study (section 6.3.2.2),
Levander et al. (1980) stated that the plasma-selenium levels of
healthy young male volunteers in the USA dropped about 19% after
2 weeks on a low-selenium diet and then returned to their previous
levels 11 days after consuming a high-selenium diet (Fig. 3).

 Table 11. Comparison of selenium levels in whole-blood samples
obtained from human beings living in different parts of the world
(values for each country are listed in decreasing numerical order)
-------------------------------------------------------------------
Country or region Reported values Reference
(mg selenium/litre
whole blood)
-------------------------------------------------------------------
Canada
Ontario 0.182 Dickson & Tomlinson
SD ± 0.036 (1967)
The People's Republic of
China
high-selenium area with 3.2 Yang et al. (1983)
a history of intoxication (1.3-7.5)
reported to be chronic
selenosisa
high-selenium area 0.44 Yang et al. (1983)
reported to be without (0.35-0.58)
selenosisa
moderate-selenium 0.095 Yang et al. (1983)
area (Beijing)a SD ± 0.091
low-selenium area 0.027 Yang et al. (1983)
without Keshan disease SD ± 0.009
low-selenium area with 0.021 Yang et al. (1983)
Keshan diseasea SD ± 0.010
Egypt 0.068 Maxia et al. (1972)
(0.054-0.079)
Finland
Helsinki 0.081 Westermarck et al.
SD ± 0.015 (1977)
Lappeenranta 0.056 Westermarck et al.
SD ± 0.017 (1977)
Guatemala 0.23 Burk et al. (1967)
SD ± 0.05
New Zealand
Auckland, North Island 0.083 Robinson & Thomson
SD ± 0.013 (1981)
Dunedin, South Island 0.059 Rea et al. (1979)
SD ± 0.012
-------------------------------------------------------------------
Table 11. (contd.)
-------------------------------------------------------------------
Country or region Reported values Reference
(mg selenium/litre
whole blood)
-------------------------------------------------------------------
Sweden 0.12 Brune et al. (1966)
SD ± 0.02
United Kingdom 0.32 Bowen & Cawse
(0.26-0.37) (1963)
USA
Rapid City, South Dakota 0.256 Allaway et al.
SE ± 0.036 (1968)
Lima, Ohio 0.157
SE ± 0.032
USSR
Ukrainian SSR 0.442 Suchkov (1971)
SE ± 0.034
Azerbeijan SSR 0.11 Abdullaev (1976)
SE ± 0.007
Venezuela
Seleniferous zone 0.813 Jaffe et al.
Villa Bruzual (1972a)
Caracas 0.355
-------------------------------------------------------------------
a These blood samples were collected in areas corresponding to
those shown in Table 8.
The Task Group was aware of one report (Kinnigkeit, 1962) that
presented blood-selenium levels in workers occupationally exposed
to selenium (Table 12). The analytical method used in this study
was a wet chemical technique based on complexing selenium with
diaminobenzidine, and the blood-selenium values obtained by this
procedure from a population not occupationally exposed were below
0.5 mg/litre. These values imply that blood-selenium levels in
workers, at least in the past, were much higher than those found in
the general population (Table 11). Unfortunately no control values
were available (Table 12) so it was not possible to compare the
blood-selenium levels with those obtained using the more recent
analytical technique using diaminonaphthalene instead of
diaminobenzedine (section 2.2.5.1).
6.2.4. Total-body selenium content
Schroeder et al. (1970) estimated the total-body selenium
content of persons in New England, USA, by multiplying the mean
values of the selenium content of human tissues obtained at autopsy
by standard organ weights. In this way, a total-body selenium
content of 14.6 mg (range, 13.0 - 20.3 mg) was calculated for 91.7%
of the body. Stewart et al. (1978) calculated the total-body
selenium content of 4 New Zealand women by 3 different techniques:
using the specific activity of urinary selenium and retained whole-
body radio-selenium; using plasma-selenium and the occupancy of
radio-selenium in whole body and plasma; and using absorbed food-
selenium and the occupancy of absorbed radio-selenium in the whole
body. Depending on whether labelled selenomethionine or selenite
was used in the estimation, the total-body selenium content was
found to be either 6.1 mg (range, 4.1 - 10.0 mg) or 3.0 mg (range,
2.3 - 5.0 mg), respectively, which was less than half that of North
Americans. This was consistent with the selenium contents of
individual tissues (liver, muscle, heart) (Money, 1978; Casey et
al., 1982) of New Zealand subjects, which also contained less than
half that reported in North Americans.
Table 12. Blood-selenium levels reported in
workers from a selenium rectifier planta
---------------------------------------------
Department Number Selenium level
of in blood
workers (mg/litre)
---------------------------------------------
Vaporization 13 4.8 ± 14.8
Measurement 13 15.8 ± 11.8
field
Stamping 12 8.8 ± 13.8
Electric fabrication 18 13.4 ± 12.9
---------------------------------------------
a Geometric mean ± standard error. From: Kinnigkeit (1962).
6.3. Excretion in Urine, Faeces, and Expired Air
6.3.1. Animal studies
Studies on rats have shown that the urinary pathway is the
dominant route for selenium excretion, as long as the dietary
selenium exceeds a certain critical threshold level. For selenium
as selenite, this threshold lies between 0.054 and 0.084 mg/kg
(Burk et al., 1973). As the dietary level of selenium increased
from 0.004 to 1.000 mg/kg, the cumulative 10-day urinary excretion
of a tracer dose of radio-selenite increased from 6 to 67% (Burk et
al., 1972). In contrast, the faecal excretion of selenium remained
constant at about 10% of the dose over this range of dietary-
selenium intake. Significant differences in the urinary excretion
of selenium were demonstrated after dosing rats orally with various
forms of selenium (Richold et al., 1977). For example, the
cumulative levels of selenium excreted in the urine, one week after
dosing with selenite, selenocystine, selenomethionine, "rabbit
kidney" selenium, and "fish muscle" selenium, were 14, 14, 5, 7,
and 6% of the absorbed dose, respectively. In many of these
studies, the endogenous faecal excretion of selenium approached or
exceeded urinary excretion. These decreased urinary/faecal
excretion ratios may have been characteristic of the different
forms of selenium used or may have been due to the low selenium
content of the stock diet fed to the animals (0.050 mg/kg or less).
The urinary-selenium excretion in animals suffering from
chronic selenosis obviously exceeds any dietary threshold
requirement, and cats poisoned with sodium selenite eliminated
50 - 80% of the intake via this pathway, but only 20% or less in
the faeces (Smith et al., 1938). Cats poisoned with organic
selenium in the form of seleniferous wheat protein excreted only
40% of the ingested selenium in the urine, but this decrease in
urinary output was more the result of increased selenium retention
rather than increased faecal excretion.
The main urinary selenium metabolite of rats is trimethyl-
selenonium ion (Byard, 1969; Palmer et al., 1969). This form
accounts for 20 - 50% of the urinary selenium, regardless of the
form of selenium given (Palmer et al., 1970). A recent report
indicates that trimethylselenonium ion accounts for a greater
fraction of urinary selenium under conditions of high selenium
exposure than under conditions of low exposure (Nahapetian et al.,
1983). When selenate is injected into rats, over 35% of the
urinary selenium is in an inorganic form, but less than 3% of the
urinary selenium is inorganic when selenomethionine is injected
into rats. A major urinary metabolite that accounted for 11 - 28%
of the total selenium in rat urine was not identified.
The faecal excretion of selenium is more important in ruminants
than in non-ruminants. For example, sheep given radio-selenite,
orally, excreted 66% of the dose in the faeces, whereas swine
excreted only 15% of the dose via this route (Wright & Bell, 1966).
This increased faecal excretion of selenium by ruminants is the
result of poor absorption rather than elevated endogenous
excretion. The selenite is thought to be reduced to insoluble or
unavailable forms by rumen microorganisms.
The results of studies on rats have demonstrated that excretion
of selenium by the exhalation of volatile compounds is
quantitatively significant only when animals are injected with
doses approaching almost lethal levels of soluble selenium salts
(Olson et al., 1963). A close dose-effect relationship between
selenium exhalation and selenium exposure was observed, since the
amount of selenium exhaled decreased as the amount of selenium
administered decreased. Rats fed toxic levels of selenium in the
diet on a long-term basis, either as seleniferous wheat or sodium
selenite, exhaled less than 2% of the selenium ingested.
6.3.2. Human studies
6.3.2.1. Excretion of selenium
Human volunteers dosed orally with microgram quantities of
selenite or selenomethionine excreted 3 - 4 times more selenium in
the urine than in the faeces over a 2-week collection period (Table
13). Subjects given selenite excreted roughly twice as much total
selenium as subjects given selenomethionine, but the urinary
pathway was dominant in both cases. As might be anticipated from
the low doses administered, losses of selenium via the dermal or
pulmonary routes were negligible.
Table 13. Urinary and endogenous faecal
excretion of radio-selenium by human beings
over a 2-week perioda
----------------------------------------------
Labelled selenium Urinary Faecal excretion
compound excretion (endogenous)
----------------------------------------------
% of absorbed dose
selenite 18 5
selenomethionine 9 2
----------------------------------------------
a Adapted from: Griffiths et al. (1976).
Urinary excretion assumes greater importance when human beings
ingest solutions of milligram quantities of selenium compounds that
are soluble and also well absorbed (Table 14) (section 6.1.1.2).
Volunteers given one milligram of selenium as selenate excreted 81%
in the urine, over 3 times that excreted after similar sized doses
of selenite-selenium, and was still twice as high when expressed as
% absorbed dose. Total excretions in urine and faeces for both
selenite-selenium (63%) and selenate-selenium (90%) suggested that
long-term retention of selenium was not high. Much less of one
milligram selenium as selenomethionine was excreted in urine and
faeces (24% dose), which indicated that selenomethionine was
mainly retained in comparison with selenate- and selenite-selenium.
No pulmonary excretion was observed in one subject given one
milligram of selenium as selenite (Thomson, 1974).
Table 14. Urinary and faecal excretion of one milligram
doses of selenium by human volunteers during a 5-day
perioda
--------------------------------------------------------
Selenium compound Faecal excretion Urinary excretion
% dose % dose % absorbed
dose
--------------------------------------------------------
Selenite 40 23 40
Selenate 8 81 87
Selenomethionine 6 18 20
--------------------------------------------------------
a Adapted from: Thomson & Robinson (1986) (section
6.1.1.2).
Urinary-selenium levels have long been used to monitor
occupational selenium exposure (Glover, 1970), but little is known
about the urinary excretion of selenium derived from foods. The
results of balance studies conducted in New Zealand suggested that
roughly half of the dietary intake of selenium was excreted in the
urine (Robinson et al., 1973; Stewart et al., 1978). However,
these subjects were ingesting small amounts of selenium (18 -
34 µg/day), and this relationship may not hold for persons with
higher selenium intakes.
The importance of the kidneys in the homeostasis of selenium
was emphasized by work from New Zealand that demonstrated that
persons of low selenium status had low renal plasma clearances of
selenium and excreted selenium more sparingly than others (Robinson
et al., 1985).
6.3.2.2. Balance studies
Metabolic trials, conducted on 4 New Zealand female volunteers
in selenium balance, and consuming daily 18 - 34 µg of selenium
occurring naturally in the diet, showed that roughly equivalent
amounts of selenium were voided in the urine and faeces. In the
USA, a controlled depletion/repletion study carried out on 6
healthy young male volunteers in a metabolic unit (Levander et al.,
1980) indicated the rapidity with which both the urinary and faecal
routes adjust to differences in selenium intake (Fig. 4, 5). When
the subjects were brought into the metabolic ward and given a low-
selenium formula diet providing 19 - 24 µg selenium/day (depletion
period), urinary-selenium excretion started to fall immediately and
decreased from an initial level of 54 ± 11 µg/day to 29 ± 5 µg/day
within 14 days. Faecal selenium output also responded rapidly and
declined from 33 ± 13 µg/day to 17 ± 7 µg/day, after only 3 days.
During the time that the subjects were on the low-selenium diet and
were in negative selenium balance, urinary excretion accounted for
about 63% of the total selenium output (Table 15). When the
subjects were fed the low-selenium formula diet plus additional
selenium in the form of seleniferous wheat and/or tuna fish (203 -
224 µg selenium/day), both urinary and faecal selenium excretion
increased rapidly. During this repletion period, the subjects were
in positive selenium balance and urinary excretion comprised about
45% of the total selenium output. Thus, despite a 6-fold
difference in selenium intake and a transition from negative to
positive selenium balance, the proportion of selenium excreted in
the urine remained relatively constant in terms of the total
selenium output, when the selenium was supplied as it occurs
naturally in foods.
The amount of selenium excreted in the sweat was found to be
very low, and was not affected by changes in dietary-selenium
intake (Table 16). In agreement with these results, Griffiths et
al. (1976) found very little radio-selenium in the sweat of
subjects who had been given labelled selenite or selenomethionine.
In the study by Levander et al. (1980), salivary-selenium levels
were also very low, but appeared to reflect differences in dietary-
selenium intakes, since salivary-selenium was somewhat higher at
the end of the repletion period than at the end of the depletion
period (Table 16). Hadjimarkos & Shearer (197l) found 1.1 - 5.2 µg
selenium/litre of saliva in normal children.
Table 11. Comparison of selenium levels in whole-blood samples
obtained from human beings living in different parts of the world
(values for each country are listed in decreasing numerical order)
-------------------------------------------------------------------
Country or region Reported values Reference
(mg selenium/litre
whole blood)
-------------------------------------------------------------------
Canada
Ontario 0.182 Dickson & Tomlinson
SD ± 0.036 (1967)
The People's Republic of
China
high-selenium area with 3.2 Yang et al. (1983)
a history of intoxication (1.3-7.5)
reported to be chronic
selenosisa
high-selenium area 0.44 Yang et al. (1983)
reported to be without (0.35-0.58)
selenosisa
moderate-selenium 0.095 Yang et al. (1983)
area (Beijing)a SD ± 0.091
low-selenium area 0.027 Yang et al. (1983)
without Keshan disease SD ± 0.009
low-selenium area with 0.021 Yang et al. (1983)
Keshan diseasea SD ± 0.010
Egypt 0.068 Maxia et al. (1972)
(0.054-0.079)
Finland
Helsinki 0.081 Westermarck et al.
SD ± 0.015 (1977)
Lappeenranta 0.056 Westermarck et al.
SD ± 0.017 (1977)
Guatemala 0.23 Burk et al. (1967)
SD ± 0.05
New Zealand
Auckland, North Island 0.083 Robinson & Thomson
SD ± 0.013 (1981)
Dunedin, South Island 0.059 Rea et al. (1979)
SD ± 0.012
-------------------------------------------------------------------
Table 11. (contd.)
-------------------------------------------------------------------
Country or region Reported values Reference
(mg selenium/litre
whole blood)
-------------------------------------------------------------------
Sweden 0.12 Brune et al. (1966)
SD ± 0.02
United Kingdom 0.32 Bowen & Cawse
(0.26-0.37) (1963)
USA
Rapid City, South Dakota 0.256 Allaway et al.
SE ± 0.036 (1968)
Lima, Ohio 0.157
SE ± 0.032
USSR
Ukrainian SSR 0.442 Suchkov (1971)
SE ± 0.034
Azerbeijan SSR 0.11 Abdullaev (1976)
SE ± 0.007
Venezuela
Seleniferous zone 0.813 Jaffe et al.
Villa Bruzual (1972a)
Caracas 0.355
-------------------------------------------------------------------
a These blood samples were collected in areas corresponding to
those shown in Table 8.
The Task Group was aware of one report (Kinnigkeit, 1962) that
presented blood-selenium levels in workers occupationally exposed
to selenium (Table 12). The analytical method used in this study
was a wet chemical technique based on complexing selenium with
diaminobenzidine, and the blood-selenium values obtained by this
procedure from a population not occupationally exposed were below
0.5 mg/litre. These values imply that blood-selenium levels in
workers, at least in the past, were much higher than those found in
the general population (Table 11). Unfortunately no control values
were available (Table 12) so it was not possible to compare the
blood-selenium levels with those obtained using the more recent
analytical technique using diaminonaphthalene instead of
diaminobenzedine (section 2.2.5.1).
6.2.4. Total-body selenium content
Schroeder et al. (1970) estimated the total-body selenium
content of persons in New England, USA, by multiplying the mean
values of the selenium content of human tissues obtained at autopsy
by standard organ weights. In this way, a total-body selenium
content of 14.6 mg (range, 13.0 - 20.3 mg) was calculated for 91.7%
of the body. Stewart et al. (1978) calculated the total-body
selenium content of 4 New Zealand women by 3 different techniques:
using the specific activity of urinary selenium and retained whole-
body radio-selenium; using plasma-selenium and the occupancy of
radio-selenium in whole body and plasma; and using absorbed food-
selenium and the occupancy of absorbed radio-selenium in the whole
body. Depending on whether labelled selenomethionine or selenite
was used in the estimation, the total-body selenium content was
found to be either 6.1 mg (range, 4.1 - 10.0 mg) or 3.0 mg (range,
2.3 - 5.0 mg), respectively, which was less than half that of North
Americans. This was consistent with the selenium contents of
individual tissues (liver, muscle, heart) (Money, 1978; Casey et
al., 1982) of New Zealand subjects, which also contained less than
half that reported in North Americans.
Table 12. Blood-selenium levels reported in
workers from a selenium rectifier planta
---------------------------------------------
Department Number Selenium level
of in blood
workers (mg/litre)
---------------------------------------------
Vaporization 13 4.8 ± 14.8
Measurement 13 15.8 ± 11.8
field
Stamping 12 8.8 ± 13.8
Electric fabrication 18 13.4 ± 12.9
---------------------------------------------
a Geometric mean ± standard error. From: Kinnigkeit (1962).
6.3. Excretion in Urine, Faeces, and Expired Air
6.3.1. Animal studies
Studies on rats have shown that the urinary pathway is the
dominant route for selenium excretion, as long as the dietary
selenium exceeds a certain critical threshold level. For selenium
as selenite, this threshold lies between 0.054 and 0.084 mg/kg
(Burk et al., 1973). As the dietary level of selenium increased
from 0.004 to 1.000 mg/kg, the cumulative 10-day urinary excretion
of a tracer dose of radio-selenite increased from 6 to 67% (Burk et
al., 1972). In contrast, the faecal excretion of selenium remained
constant at about 10% of the dose over this range of dietary-
selenium intake. Significant differences in the urinary excretion
of selenium were demonstrated after dosing rats orally with various
forms of selenium (Richold et al., 1977). For example, the
cumulative levels of selenium excreted in the urine, one week after
dosing with selenite, selenocystine, selenomethionine, "rabbit
kidney" selenium, and "fish muscle" selenium, were 14, 14, 5, 7,
and 6% of the absorbed dose, respectively. In many of these
studies, the endogenous faecal excretion of selenium approached or
exceeded urinary excretion. These decreased urinary/faecal
excretion ratios may have been characteristic of the different
forms of selenium used or may have been due to the low selenium
content of the stock diet fed to the animals (0.050 mg/kg or less).
The urinary-selenium excretion in animals suffering from
chronic selenosis obviously exceeds any dietary threshold
requirement, and cats poisoned with sodium selenite eliminated
50 - 80% of the intake via this pathway, but only 20% or less in
the faeces (Smith et al., 1938). Cats poisoned with organic
selenium in the form of seleniferous wheat protein excreted only
40% of the ingested selenium in the urine, but this decrease in
urinary output was more the result of increased selenium retention
rather than increased faecal excretion.
The main urinary selenium metabolite of rats is trimethyl-
selenonium ion (Byard, 1969; Palmer et al., 1969). This form
accounts for 20 - 50% of the urinary selenium, regardless of the
form of selenium given (Palmer et al., 1970). A recent report
indicates that trimethylselenonium ion accounts for a greater
fraction of urinary selenium under conditions of high selenium
exposure than under conditions of low exposure (Nahapetian et al.,
1983). When selenate is injected into rats, over 35% of the
urinary selenium is in an inorganic form, but less than 3% of the
urinary selenium is inorganic when selenomethionine is injected
into rats. A major urinary metabolite that accounted for 11 - 28%
of the total selenium in rat urine was not identified.
The faecal excretion of selenium is more important in ruminants
than in non-ruminants. For example, sheep given radio-selenite,
orally, excreted 66% of the dose in the faeces, whereas swine
excreted only 15% of the dose via this route (Wright & Bell, 1966).
This increased faecal excretion of selenium by ruminants is the
result of poor absorption rather than elevated endogenous
excretion. The selenite is thought to be reduced to insoluble or
unavailable forms by rumen microorganisms.
The results of studies on rats have demonstrated that excretion
of selenium by the exhalation of volatile compounds is
quantitatively significant only when animals are injected with
doses approaching almost lethal levels of soluble selenium salts
(Olson et al., 1963). A close dose-effect relationship between
selenium exhalation and selenium exposure was observed, since the
amount of selenium exhaled decreased as the amount of selenium
administered decreased. Rats fed toxic levels of selenium in the
diet on a long-term basis, either as seleniferous wheat or sodium
selenite, exhaled less than 2% of the selenium ingested.
6.3.2. Human studies
6.3.2.1. Excretion of selenium
Human volunteers dosed orally with microgram quantities of
selenite or selenomethionine excreted 3 - 4 times more selenium in
the urine than in the faeces over a 2-week collection period (Table
13). Subjects given selenite excreted roughly twice as much total
selenium as subjects given selenomethionine, but the urinary
pathway was dominant in both cases. As might be anticipated from
the low doses administered, losses of selenium via the dermal or
pulmonary routes were negligible.
Table 13. Urinary and endogenous faecal
excretion of radio-selenium by human beings
over a 2-week perioda
----------------------------------------------
Labelled selenium Urinary Faecal excretion
compound excretion (endogenous)
----------------------------------------------
% of absorbed dose
selenite 18 5
selenomethionine 9 2
----------------------------------------------
a Adapted from: Griffiths et al. (1976).
Urinary excretion assumes greater importance when human beings
ingest solutions of milligram quantities of selenium compounds that
are soluble and also well absorbed (Table 14) (section 6.1.1.2).
Volunteers given one milligram of selenium as selenate excreted 81%
in the urine, over 3 times that excreted after similar sized doses
of selenite-selenium, and was still twice as high when expressed as
% absorbed dose. Total excretions in urine and faeces for both
selenite-selenium (63%) and selenate-selenium (90%) suggested that
long-term retention of selenium was not high. Much less of one
milligram selenium as selenomethionine was excreted in urine and
faeces (24% dose), which indicated that selenomethionine was
mainly retained in comparison with selenate- and selenite-selenium.
No pulmonary excretion was observed in one subject given one
milligram of selenium as selenite (Thomson, 1974).
Table 14. Urinary and faecal excretion of one milligram
doses of selenium by human volunteers during a 5-day
perioda
--------------------------------------------------------
Selenium compound Faecal excretion Urinary excretion
% dose % dose % absorbed
dose
--------------------------------------------------------
Selenite 40 23 40
Selenate 8 81 87
Selenomethionine 6 18 20
--------------------------------------------------------
a Adapted from: Thomson & Robinson (1986) (section
6.1.1.2).
Urinary-selenium levels have long been used to monitor
occupational selenium exposure (Glover, 1970), but little is known
about the urinary excretion of selenium derived from foods. The
results of balance studies conducted in New Zealand suggested that
roughly half of the dietary intake of selenium was excreted in the
urine (Robinson et al., 1973; Stewart et al., 1978). However,
these subjects were ingesting small amounts of selenium (18 -
34 µg/day), and this relationship may not hold for persons with
higher selenium intakes.
The importance of the kidneys in the homeostasis of selenium
was emphasized by work from New Zealand that demonstrated that
persons of low selenium status had low renal plasma clearances of
selenium and excreted selenium more sparingly than others (Robinson
et al., 1985).
6.3.2.2. Balance studies
Metabolic trials, conducted on 4 New Zealand female volunteers
in selenium balance, and consuming daily 18 - 34 µg of selenium
occurring naturally in the diet, showed that roughly equivalent
amounts of selenium were voided in the urine and faeces. In the
USA, a controlled depletion/repletion study carried out on 6
healthy young male volunteers in a metabolic unit (Levander et al.,
1980) indicated the rapidity with which both the urinary and faecal
routes adjust to differences in selenium intake (Fig. 4, 5). When
the subjects were brought into the metabolic ward and given a low-
selenium formula diet providing 19 - 24 µg selenium/day (depletion
period), urinary-selenium excretion started to fall immediately and
decreased from an initial level of 54 ± 11 µg/day to 29 ± 5 µg/day
within 14 days. Faecal selenium output also responded rapidly and
declined from 33 ± 13 µg/day to 17 ± 7 µg/day, after only 3 days.
During the time that the subjects were on the low-selenium diet and
were in negative selenium balance, urinary excretion accounted for
about 63% of the total selenium output (Table 15). When the
subjects were fed the low-selenium formula diet plus additional
selenium in the form of seleniferous wheat and/or tuna fish (203 -
224 µg selenium/day), both urinary and faecal selenium excretion
increased rapidly. During this repletion period, the subjects were
in positive selenium balance and urinary excretion comprised about
45% of the total selenium output. Thus, despite a 6-fold
difference in selenium intake and a transition from negative to
positive selenium balance, the proportion of selenium excreted in
the urine remained relatively constant in terms of the total
selenium output, when the selenium was supplied as it occurs
naturally in foods.
The amount of selenium excreted in the sweat was found to be
very low, and was not affected by changes in dietary-selenium
intake (Table 16). In agreement with these results, Griffiths et
al. (1976) found very little radio-selenium in the sweat of
subjects who had been given labelled selenite or selenomethionine.
In the study by Levander et al. (1980), salivary-selenium levels
were also very low, but appeared to reflect differences in dietary-
selenium intakes, since salivary-selenium was somewhat higher at
the end of the repletion period than at the end of the depletion
period (Table 16). Hadjimarkos & Shearer (197l) found 1.1 - 5.2 µg
selenium/litre of saliva in normal children.

 Table 15. Selenium balance during depletion and
repletion periodsa
-----------------------------------------------------
Selenium balance
Depletion period Repletion period
-----------------------------------------------------
(µg) (µg)
Urinary excretion -1665 ± 211 -2435 ± 272
Faecal excretion -985 ± 103 -1919 ± 228
Total excretion -2650 ± 257 -4354 ± 295
Intake +1524 ± 173 +5424 ± 248
Overall balance -1126 ± 268 +1070 ± 482
-----------------------------------------------------
a Data expressed as mean ± standard deviation.
Adapted from: Levander et al. (1981b).
Table 16. Effect of selenium depletion and
repletion on the selenium contents of saliva
and sweat in young mena
---------------------------------------------
Body Selenium content
fluid Start of End of End of
study depletion repletion
---------------------------------------------
(µg selenium/litre)
Saliva 2.8 ± 0.7b,c 1.4 ± 0.4b 4.4 ± 0.4c
Sweat - 1.4 ± 0.2 1.2 ± 0.7
---------------------------------------------
a Adapted from: Levander et al. (1981b).
b,c Means in the same row with different
superscript letters differ significantly
( P < 0.05, Duncan's multiple range test).
Data expressed as mean ± SD.
6.4. Retention and Turnover
6.4.1. Animal studies
Results of studies on rats have shown that the whole-body
retention of a single injected dose of radioactive selenite is
described by a curve that consists of an initial phase, a
transition phase, and an extended phase (Ewan et al., 1967; Burk et
al., 1972). During the initial equilibration phase, there is a
distribution of radioactive selenium throughout the tissues and a
rapid excretion of radio-selenium in the urine, faeces, and, if the
dose is sufficiently high, the expired air. The initial phase is
followed by a transition phase, during which the rate of loss of
radio-selenium is less than that in the initial phase but greater
than that in the final extended phase. The extended phase consists
of a slow constant rate of radio-selenium loss which represents the
long-term whole-body turnover of selenium. Increasing the dose of
radioactive selenite administered decreased the percentage of the
dose retained during the initial phase, but did not have any effect
on radio-selenium retention during the extended phase. Increasing
the level of dietary selenium increased the turnover of radio-
selenium during both the initial and extended phases. For example,
supplementing the diet with 0.95 mg selenium/kg decreased the
biological half-life of radio-selenium, during the extended phase,
from 78 to 27 days. The extended phase of radio-selenium retention
in rats was shown by Richold et al. (1977) to be largely
independent of the route of administration and the chemical form of
selenium given. Thus, after the initial period, it appears that
selenium from a variety of sources is ultimately incorporated into
the same metabolic pool in rats. The apparent "whole-body"
retention of radio-selenium during the extended phase is an average
of several discrete processes, since each internal organ has its
own characteristic rate of selenium turnover. For example, the
biological half-times of radio-selenium in the kidneys, whole body,
and skeletal muscle of rats were 38, 55, and 74 days, respectively
(Thomson & Stewart, 1973).
Information on the retention of selenium by animals became of
practical significance when approval was sought for the use of
selenium as a feed additive to prevent nutritional deficiency
diseases (section 4). Because of the toxicity of high levels of
selenium, there was some concern about the possible build up of
residues in the edible flesh of animals supplemented with selenium.
Scott & Thompson (1971) noted very little increase in the
concentrations of selenium in the tissues of chicks or poults fed
low-selenium diets supplemented with 0.2 - 0.8 mg selenium/kg as
sodium selenite. However, feeding a diet naturally high in
selenium, but not toxic (0.67 mg/kg) resulted in substantially
increased tissue-selenium levels.
Scott & Thompson (1971) also showed that addition of sodium
selenite to a diet that was naturally high in selenium did not
produce any further increase in tissue-selenium levels. In further
studies on turkeys, swine, sheep, and cattle, animal feeds,
naturally low or marginal in selenium, were supplemented with
nutritional levels of selenium (0.1 - 0.5 mg/kg) as sodium selenite.
Resulting tissue-selenium concentrations were not any higher than
those that would be expected if the animals were fed diets
naturally adequate in selenium (Groce et al., 1971; Cantor & Scott,
1975; Ullrey et al., 1977).
These observations are in agreement with those from earlier
work with toxic levels of selenium, which indicated that naturally-
occurring organically-bound selenium was retained in the tissues to
a much greater extent than inorganic selenium (Smith et al., 1938).
Martin & Hurlbut (1976) fed mice high levels of selenium as
selenite, selenium-methylselenocysteine, or selenomethionine.
After 7 weeks, the mice fed selenomethionine had much higher levels
of selenium in their tissues than those fed either selenite or
selenium-methylselenocysteine. Moreover, when the various selenium
compounds were removed from the diet, selenium was retained more
strongly in the tissues of the mice fed the selenomethionine than
in those of the mice fed selenite or selenium-methylselenocysteine.
The results of this study showed that a distinction more precise
than inorganic versus organic must be made when discussing the
metabolism of selenium compounds, since the metabolism of selenium-
methylselenocysteine resembled that of selenite rather than that of
selenomethionine.
Differences in the retention of selenite compared with
selenomethionine were also observed when these 2 forms of selenium
were fed in the diet at nutritional levels. For example, Miller et
al. (1972) showed that the total body selenium content of chicks
was increased by an average of 29.1%, when 0.025 - 0.500 mg of
selenium as selenomethionine was fed per kg of diet, but was
increased only by 17.9% when selenium as selenite was fed at
similar levels.
Selenium retained by female animals can apparently be used
later to protect their offspring against the effects of selenium
deficiency. Allaway et al. (1966) fed ewes alfalfa containing
2.6 mg selenium/kg for 5 months followed by a diet low in selenium.
The ewes were able to transmit levels of selenium to their lambs
that protected them against White Muscle Disease, even though the
lambs were born 10 months after the low-selenium diet was first
administered.
6.4.2. Controlled human studies
As in the case of rats, the total body retention curves of
radioactive selenium in human beings can be resolved into 3
components (Griffiths et al., 1976). However, oral doses of
75Se-selenomethionine are retained more strongly and turned over
more slowly than oral doses of 75Se-selenite (Table 17). This
metabolic difference between selenomethionine and selenite holds
true, even if the compounds are administered by intravenous
injection (Lathrop et al., 1972; Falk & Lindhe, 1974). These
results differ with those obtained in rats in which several forms
of selenium apparently were incorporated into the same long-term
metabolic pool and were thus turned over at similar rates (Richold
et al., 1977). On this basis, it was suggested that
selenomethionine, or food-selenium in a form that produced
selenomethionine after digestion, might prove more effective than
selenite in improving a low-selenium status or in correcting
selenium deficiency in man (Griffiths et al., 1976).
Table 17. Retention of oral doses of selenite and selenomethionine by
human beingsa
----------------------------------------------------------------------
Whole-body selenium Biological half-times for
Form of retention 14 days whole-body selenium retention
selenium after dose phase 1 phase 2 phase 3
given (% of absorbed dose) (days)
----------------------------------------------------------------------
selenite 78 1.0 8 103
selenomethionine 89 1.7 18 234
----------------------------------------------------------------------
a Adapted from: Griffiths et al. (1976).
Thomson et al. (1982) gave 100 µg doses of selenium as
selenomethionine or sodium selenite to 12 New Zealand volunteers
over a period of several weeks. Blood glutathione peroxidase (EC
1.11.19) activity increased in all subjects and, at 17 weeks,
the response was similar in both selenomethionine and selenite
groups. Increases in blood-selenium levels were greater after
supplementation with selenomethionine than with selenite. Selenium
levels tended to plateau in the selenite-treated subjects but kept
increasing in the selenomethionine-treated subjects. Similar
differences in the retention and turnover of selenium as selenate
compared with selenium in selenium-rich yeast or wheat were
observed in a bioavailability trial in Finland (Levander et al.,
1983) (section 5.1.1.6). Thus, it appears that feeding of selenium
bound in organic form (selenomethionine, selenium-rich yeast or
wheat) results in higher blood-selenium levels than the feeding of
selenium as selenate or selenite, but there is no difference in the
glutathione peroxidase activities ultimately achieved during
supplementation. However, when the selenium supplements were
discontinued, the glutathione peroxidase activities remained
somewhat elevated in the groups receiving the wheat or yeast
compared with those receiving selenate. Apparently, the selenium
in the yeast or wheat retained in the tissues could be used for
glutathione peroxidase production after the selenium supplement was
discontinued.
6.5. Metabolic Transformation
Some metabolic transformations of selenium compounds are
outlined in Fig. 6. This scheme tends to emphasize the central
role of selenite, but other forms of selenium may have important
characteristic pathways of their own, under certain conditions
(e.g., the direct incorporation of selenomethionine as such into
tissue proteins).
Table 15. Selenium balance during depletion and
repletion periodsa
-----------------------------------------------------
Selenium balance
Depletion period Repletion period
-----------------------------------------------------
(µg) (µg)
Urinary excretion -1665 ± 211 -2435 ± 272
Faecal excretion -985 ± 103 -1919 ± 228
Total excretion -2650 ± 257 -4354 ± 295
Intake +1524 ± 173 +5424 ± 248
Overall balance -1126 ± 268 +1070 ± 482
-----------------------------------------------------
a Data expressed as mean ± standard deviation.
Adapted from: Levander et al. (1981b).
Table 16. Effect of selenium depletion and
repletion on the selenium contents of saliva
and sweat in young mena
---------------------------------------------
Body Selenium content
fluid Start of End of End of
study depletion repletion
---------------------------------------------
(µg selenium/litre)
Saliva 2.8 ± 0.7b,c 1.4 ± 0.4b 4.4 ± 0.4c
Sweat - 1.4 ± 0.2 1.2 ± 0.7
---------------------------------------------
a Adapted from: Levander et al. (1981b).
b,c Means in the same row with different
superscript letters differ significantly
( P < 0.05, Duncan's multiple range test).
Data expressed as mean ± SD.
6.4. Retention and Turnover
6.4.1. Animal studies
Results of studies on rats have shown that the whole-body
retention of a single injected dose of radioactive selenite is
described by a curve that consists of an initial phase, a
transition phase, and an extended phase (Ewan et al., 1967; Burk et
al., 1972). During the initial equilibration phase, there is a
distribution of radioactive selenium throughout the tissues and a
rapid excretion of radio-selenium in the urine, faeces, and, if the
dose is sufficiently high, the expired air. The initial phase is
followed by a transition phase, during which the rate of loss of
radio-selenium is less than that in the initial phase but greater
than that in the final extended phase. The extended phase consists
of a slow constant rate of radio-selenium loss which represents the
long-term whole-body turnover of selenium. Increasing the dose of
radioactive selenite administered decreased the percentage of the
dose retained during the initial phase, but did not have any effect
on radio-selenium retention during the extended phase. Increasing
the level of dietary selenium increased the turnover of radio-
selenium during both the initial and extended phases. For example,
supplementing the diet with 0.95 mg selenium/kg decreased the
biological half-life of radio-selenium, during the extended phase,
from 78 to 27 days. The extended phase of radio-selenium retention
in rats was shown by Richold et al. (1977) to be largely
independent of the route of administration and the chemical form of
selenium given. Thus, after the initial period, it appears that
selenium from a variety of sources is ultimately incorporated into
the same metabolic pool in rats. The apparent "whole-body"
retention of radio-selenium during the extended phase is an average
of several discrete processes, since each internal organ has its
own characteristic rate of selenium turnover. For example, the
biological half-times of radio-selenium in the kidneys, whole body,
and skeletal muscle of rats were 38, 55, and 74 days, respectively
(Thomson & Stewart, 1973).
Information on the retention of selenium by animals became of
practical significance when approval was sought for the use of
selenium as a feed additive to prevent nutritional deficiency
diseases (section 4). Because of the toxicity of high levels of
selenium, there was some concern about the possible build up of
residues in the edible flesh of animals supplemented with selenium.
Scott & Thompson (1971) noted very little increase in the
concentrations of selenium in the tissues of chicks or poults fed
low-selenium diets supplemented with 0.2 - 0.8 mg selenium/kg as
sodium selenite. However, feeding a diet naturally high in
selenium, but not toxic (0.67 mg/kg) resulted in substantially
increased tissue-selenium levels.
Scott & Thompson (1971) also showed that addition of sodium
selenite to a diet that was naturally high in selenium did not
produce any further increase in tissue-selenium levels. In further
studies on turkeys, swine, sheep, and cattle, animal feeds,
naturally low or marginal in selenium, were supplemented with
nutritional levels of selenium (0.1 - 0.5 mg/kg) as sodium selenite.
Resulting tissue-selenium concentrations were not any higher than
those that would be expected if the animals were fed diets
naturally adequate in selenium (Groce et al., 1971; Cantor & Scott,
1975; Ullrey et al., 1977).
These observations are in agreement with those from earlier
work with toxic levels of selenium, which indicated that naturally-
occurring organically-bound selenium was retained in the tissues to
a much greater extent than inorganic selenium (Smith et al., 1938).
Martin & Hurlbut (1976) fed mice high levels of selenium as
selenite, selenium-methylselenocysteine, or selenomethionine.
After 7 weeks, the mice fed selenomethionine had much higher levels
of selenium in their tissues than those fed either selenite or
selenium-methylselenocysteine. Moreover, when the various selenium
compounds were removed from the diet, selenium was retained more
strongly in the tissues of the mice fed the selenomethionine than
in those of the mice fed selenite or selenium-methylselenocysteine.
The results of this study showed that a distinction more precise
than inorganic versus organic must be made when discussing the
metabolism of selenium compounds, since the metabolism of selenium-
methylselenocysteine resembled that of selenite rather than that of
selenomethionine.
Differences in the retention of selenite compared with
selenomethionine were also observed when these 2 forms of selenium
were fed in the diet at nutritional levels. For example, Miller et
al. (1972) showed that the total body selenium content of chicks
was increased by an average of 29.1%, when 0.025 - 0.500 mg of
selenium as selenomethionine was fed per kg of diet, but was
increased only by 17.9% when selenium as selenite was fed at
similar levels.
Selenium retained by female animals can apparently be used
later to protect their offspring against the effects of selenium
deficiency. Allaway et al. (1966) fed ewes alfalfa containing
2.6 mg selenium/kg for 5 months followed by a diet low in selenium.
The ewes were able to transmit levels of selenium to their lambs
that protected them against White Muscle Disease, even though the
lambs were born 10 months after the low-selenium diet was first
administered.
6.4.2. Controlled human studies
As in the case of rats, the total body retention curves of
radioactive selenium in human beings can be resolved into 3
components (Griffiths et al., 1976). However, oral doses of
75Se-selenomethionine are retained more strongly and turned over
more slowly than oral doses of 75Se-selenite (Table 17). This
metabolic difference between selenomethionine and selenite holds
true, even if the compounds are administered by intravenous
injection (Lathrop et al., 1972; Falk & Lindhe, 1974). These
results differ with those obtained in rats in which several forms
of selenium apparently were incorporated into the same long-term
metabolic pool and were thus turned over at similar rates (Richold
et al., 1977). On this basis, it was suggested that
selenomethionine, or food-selenium in a form that produced
selenomethionine after digestion, might prove more effective than
selenite in improving a low-selenium status or in correcting
selenium deficiency in man (Griffiths et al., 1976).
Table 17. Retention of oral doses of selenite and selenomethionine by
human beingsa
----------------------------------------------------------------------
Whole-body selenium Biological half-times for
Form of retention 14 days whole-body selenium retention
selenium after dose phase 1 phase 2 phase 3
given (% of absorbed dose) (days)
----------------------------------------------------------------------
selenite 78 1.0 8 103
selenomethionine 89 1.7 18 234
----------------------------------------------------------------------
a Adapted from: Griffiths et al. (1976).
Thomson et al. (1982) gave 100 µg doses of selenium as
selenomethionine or sodium selenite to 12 New Zealand volunteers
over a period of several weeks. Blood glutathione peroxidase (EC
1.11.19) activity increased in all subjects and, at 17 weeks,
the response was similar in both selenomethionine and selenite
groups. Increases in blood-selenium levels were greater after
supplementation with selenomethionine than with selenite. Selenium
levels tended to plateau in the selenite-treated subjects but kept
increasing in the selenomethionine-treated subjects. Similar
differences in the retention and turnover of selenium as selenate
compared with selenium in selenium-rich yeast or wheat were
observed in a bioavailability trial in Finland (Levander et al.,
1983) (section 5.1.1.6). Thus, it appears that feeding of selenium
bound in organic form (selenomethionine, selenium-rich yeast or
wheat) results in higher blood-selenium levels than the feeding of
selenium as selenate or selenite, but there is no difference in the
glutathione peroxidase activities ultimately achieved during
supplementation. However, when the selenium supplements were
discontinued, the glutathione peroxidase activities remained
somewhat elevated in the groups receiving the wheat or yeast
compared with those receiving selenate. Apparently, the selenium
in the yeast or wheat retained in the tissues could be used for
glutathione peroxidase production after the selenium supplement was
discontinued.
6.5. Metabolic Transformation
Some metabolic transformations of selenium compounds are
outlined in Fig. 6. This scheme tends to emphasize the central
role of selenite, but other forms of selenium may have important
characteristic pathways of their own, under certain conditions
(e.g., the direct incorporation of selenomethionine as such into
tissue proteins).
 6.5.1. Animal studies
6.5.1.1. Reduction and methylation
The main flow of selenium metabolism in animals is via
reductive pathways, which contrasts with the primarily oxidative
metabolism of sulfur (Levander, 1976a). Selenite can react with
glutathione or protein sulfhydryls to form selenotrisulfides
(Ganther, 1968; Jenkins & Hidiroglou, 1971) which, at least in the
case of the glutathione derivatives, can be reduced further by an
enzymatic mechanism to selenide (Ganther & Hsieh, 1974; Diplock,
1976).
Under normal conditions, selenide is methylated to form
trimethylselenonium ion, the main urinary metabolite of selenium
(section 6.3.1). In cases of selenium toxicity, this pathway is
overloaded and dimethyl selenide is produced. This is the main
volatile selenium metabolite expired via the lungs and is
responsible for the typical "garlicky odour" of animals poisoned
with selenium (McConnell & Portman, 1952a). The last 2 reactions
could be considered detoxication steps, since the methylated end-
products are much less toxic for the organism than selenite
(McConnell & Portman, 1952b; Obermeyer et al., 1971). However,
both of these methylated selenium derivatives have strong
synergistic toxicity with other minerals (section 7) and dimethyl
selenide toxicity in male rats was reported to be inversely related
to the level of previous selenium intake (Parizek et al., 1980).
6.5.1.2. Form in proteins
Early work with animals poisoned with selenium revealed that
much of the selenium in the tissues was associated with protein
(Smith et al., 1938). Since that time there has been considerable
controversy about the exact chemical nature of the selenium in
tissue proteins (Levander, 1976a). Selenomethionine may be
incorporated initially as such into animal proteins (Ochoa-Solano
& Gitler, 1968), but, in rats, it appears to be eventually
catabolized to selenite or selenate (Millar et al., 1973; Thomson
& Stewart, 1973). Non-ruminant animals cannot synthesize
selenomethionine from inorganic selenium compounds (Cummins &
Martin, 1967; Olson & Palmer, 1976), but rabbits and rats can
convert selenite into selenocysteine tissue proteins (Godwin &
Fuss, 1972; Olson & Palmer, 1976). Forstrom et al. (1978) have
proposed that seleno-cysteine is the form of selenium located at
the catalytic site of glutathione peroxidase and others have shown
that selenocysteine is essential for the activity of clostridial
glycine reductase (Cone et al., 1976). The mechanism by which
selenocysteine is formed from selenite is not known but
incorporation of preformed selenocysteine and post-translational
modification of the protein have been considered (Sunde, 1984).
Other possible forms of selenium in proteins include
selenotrisulfides (Ganther & Corcoran, 1969) and "acid-labile"
selenium (Diplock et al., 1973).
6.5.1.3. Conversion of selenium compounds to nutritionally-active
forms of selenium
The pioneering studies of Schwarz & Foltz (1958) demonstrated
that a variety of selenium compounds could protect against dietary
liver necrosis in vitamin E- and selenium-deficient rats. Sodium
selenite, sodium selenate, selenium dioxide, selenic acid, and
potassium selenocyanate were all more or less equally active, but
elemental gray selenium was essentially inactive. Selenocystine
and selenomethionine were about as effective as the active
inorganic selenium compounds, but an organic selenium fraction
isolated from pig kidney powder (Factor 3) was shown to be even
more active. A number of organic derivatives of selenium have
shown some protective effect against liver necrosis, but none was
superior to Factor 3 (Schwarz et al., 1972). However, certain
simple amino-acid derivatives of monoseleno diacetic acid showed
some promise in that they combined high nutritional potency with a
low order of toxicity (Schwarz, 1976). The effects of selenite and
selenomethionine were shown to be roughly similar in inducing
glutathione peroxidase activity in the tissues of rats fed a diet
deficient in selenium (Pierce & Tappel, 1977).
Cantor et al. (1975a) found that while selenite and
selenocystine were equally effective in preventing exudative
diathesis in vitamin E- and selenium-deficient chicks, seleno-
methionine was less effective. This suggests a difference between
rats and chicks in the metabolism of selenomethionine. The degree
of protection against exudative diathesis and the level of plasma
glutathione peroxidase activity were highly correlated, suggesting
that nutritional potency depended on the ability of the chick to
convert various selenium compounds to the enzymatically active
form.
6.5.2. Human studies
Few studies have been carried out to investigate the metabolic
transformation of selenium compounds in human beings, but the
limited data available suggest certain similarities in the
metabolism of selenium by man and animals. For example, human
beings overexposed to selenium develop breath with a smell of
garlic, which is presumably due to the exhalation of dimethyl
selenide (Glover, 1976). Also, the chromatographic pattern of
urinary-selenium metabolites is the same in man and the rat (Burk,
1976). However, species differences in selenium metabolism do
exist, since human beings retain selenomethionine to a much greater
extent than selenite, whereas the retention of the 2 compounds in
rats is about the same (section 6.4.2).
7. EFFECTS OF SELENIUM ON ANIMALS
The considerable biological importance of selenium was first
recognized in the 1930s when it was discovered that certain well-
defined and economically important farm animal diseases were
actually the result of chronic selenium poisoning (section 7.1).
These animal diseases were restricted to agricultural areas in
which large amounts of selenium in the soil were available for
uptake by the plants, which were then consumed by the animals.
Research, over the last 20 years, showed that selenium was an
essential trace element (section 7.2), and selenium deficiency
diseases were rapidly recognized in several species of farm
animals. These deficiency diseases are significant economic
problems in areas of the world where the soil levels of the
element available for uptake by plants are low.
7.1. Selenium Toxicity
7.1.1. Farm animal diseases associated with a high selenium intake
On the basis of field experience, Rosenfeld & Beath (1964)
delineated 3 different types of selenium poisoning in livestock:
(a) acute;
(b) chronic, of the blind staggers type; and
(c) chronic, of the alkali disease type.
Acute poisoning is due to the ingestion of toxic quantities of
selenium in the form of highly seleniferous accumulator plants.
The animal has severe signs of distress such as laboured breathing,
abnormal movement and posture, prostration, and diarrhoea. Death
often follows within a few hours. This type of selenium poisoning
is rather rare under field conditions, since grazing animals
generally avoid the selenium accumulator plants, except in times of
pasture shortage. Acute selenium poisoning has also been produced
by the experimental or accidental administration of selenium
compounds to farm animals (US NAS/NRC, 1976).
Blind staggers has been reported in animals that eat a limited
number of selenium accumulator plants over a period of weeks or
months (Rosenfeld & Beath, 1964). The affected animals wander,
stumble, have impaired vision, and eventually succumb to
respiratory failure. Although this type of poisoning can be
produced experimentally by the administration of water extracts of
accumulator plants, it has not been possible to duplicate this
syndrome by the administration of pure selenium compounds.
Possibly alkaloids or other toxic substances found in many
seleniferous plants may contribute to blind staggers (Maag & Glenn,
1967; Van Kampen & James, 1978).
Alkali disease is associated with the consumption of grains
containing 5 - 40 mg selenium/kg over weeks or months. Animals
exhibit liver cirrhosis, lameness, hoof malformations, loss of
hair, and emaciation. Maag & Glenn (1967) were unable to produce
alkali disease in cattle by feeding inorganic selenium, but several
other studies have shown that the syndrome is causally associated
with seleniferous grains or grasses and can be produced by feeding
inorganic selenium salts (Olson, 1978).
The Task Group noted that most of the work on alkali disease
was concerned with cattle, but was aware of the report by Ermakov &
Kovalskij (1968), which described chronic selenium toxicity of this
type in sheep, under natural conditions. In sheep fed feeds
containing levels of 2 mg selenium/kg feed (fresh weight), the
following characteristic signs were observed: hoof deformation,
loss of hair, hypochromic anaemia, and increases in the activity of
both alkaline and acid phosphatases in various tissues.
7.1.2. Toxicity in experimental animals
The practical significance of selenium poisoning in farm
animals stimulated a great deal of research on both the acute and
chronic effects of selenium in laboratory animals. Interest in the
toxic effects of repeated exposure to selenium via inhalation was
stimulated by concern about the possible effects on human health of
occupational exposure to selenium. Also, studies were carried out
with the aim of establishing the no-observed-adverse-effect dose
level of selenium when administered in the drinking-water. In some
of the studies in which selenium was given via either the air or
water, biochemical and/or behavioural criteria were used to assess
the biological effects of selenium exposure. However, as discussed
in section 7.1.6, the toxicity of selenium compounds can be
influenced by different variables and the results from various
laboratories are often not comparable because of quite different
experimental conditions. Also, the criteria for toxicity are less
developed than the signs of deficiency (section 7.2).
Reviews that deal with various aspects of selenium toxicology
include those by Rosenfeld & Beath (1964), Muth (1966), Izraelson
et al. (1973), Ermakov & Kovalskij (1974), US NAS/NRC (1976), and
Lazarev (1977).
7.1.2.1. Acute and subacute toxicity - single or repeated exposure
studies with oral, intraperitoneal, or cutaneous administration
Perhaps the most characteristic sign of acute selenium
poisoning in animals is the development of the so-called "garlicky
breath odour", which is due to the pulmonary excretion of volatile
selenium compounds, particularly dimethyl selenide, by animals
overexposed to selenium (section 6.3.1). Other signs of acute
selenium poisoning described by Franke & Moxon (1936) in dogs and
rats included: vomiting, dyspnoea, tetanic spasms, and death from
respiratory failure. Pathological changes included congestion of
the liver with areas of focal necrosis, congestion of the kidney,
endocarditis, myocarditis, peticheal haemorrhages of the
epicardium, atony of the smooth muscles of the gastrointestinal
tract, gall-bladder, and bladder, and erosion of the long bones,
especially the tibia. The LD50 values for sodium selenite,
administered orally to various animal species, are given in Table
18 (Pletnikova, 1970).
Table 18. Acute oral toxicity of sodium selenite for various
species of laboratory animalsa
-------------------------------------------------------------
Species LD50 Statistical method
(mg selenium/kg
body weight)
-------------------------------------------------------------
White mouse (male) 7.75 Behrens & Schlosser
7.08 Litchfield & Wilcoxon
Albino rat (female) 10.50 Behrens & Schlosser
13.19 Diechmann & LeBlanc
Guinea-pig (female) 5.06 Deichmann & LeBlanc
Rabbit (female) 2.25 Diechmann & LeBlanc
-------------------------------------------------------------
a From: Pletnikova (1970).
Table 19. Acute toxicity of some selenium compounds administered to rats by
intraperitoneal injection
----------------------------------------------------------------------------
Compound Criterion Toxic dose Reference
of (mg selenium/kg
toxicity body weight)
----------------------------------------------------------------------------
Sodium selenite MLD 3.25 - 3.5 Franke & Moxon (1936)
Sodium selenate MLD 5.25 - 5.75 Franke & Moxon (1936)
DL-selenocystine MLD 4 Moxon (1940)
DL-selenomethionine MLD 4.25 Klug et al. (1950)
Diselenodipropionic LD50 25 - 30 Moxon et al. (1938)
acid
Trimethylselenonium LD50 49.4 Obermeyer et al. (1971)
chloride
Dimethyl selenide LD50 1600 McConnell & Portman (1952b)
----------------------------------------------------------------------------
For a given species, the lethal doses of sodium selenite,
sodium selenate, DL-selenocystine, and DL-selenomethionine are
quite similar (Table 19). Although certain methylated metabolites
of selenium such as dimethylselenide and trimethylselenonium
chloride were considered relatively innocuous (see LD50 values by
McConnell & Portman and Obermeyer et al. in Table 19), more recent
work by Parizek et al. (1976, 1980) has shown that the toxicity of
methylated selenium compounds depends not only on the sex of the
animal (Parizek et al., 1974) but also on the level of previous
selenium intake. For example, 90% mortality was observed in male
rats maintained on a diet containing 0.05 mg selenium/kg, when they
were injected intraperitoneally with 20 µmoles dimethylselenide/kg
body weight, i.e., by a dose of dimethylselenide that is more than
1000 times lower than the LD50 reported by McConnell & Portman
(1952b). Pre-treatment with a small amount of selenite (1 µmol/kg
body weight), intraperitoneally, 6 h, but not 1 h, before
dimethylselenide injection or increased oral intake of selenite
(Table 20), protected male rats against the toxicity of a
subsequent dose of dimethylselenide (Parizek et al., 1976, 1980).
Moreover, the methylated forms of selenium have strong synergistic
toxicities with other minerals (section 7.1.6.3, 7.4.1) and
dimethylselenide can be much more toxic for male rats than for
female rats (section 7.1.6).
Table 20. Dependence of the toxicity of dimethylselenide on
the level of previous oral intake of seleniuma
------------------------------------------------------------
Selenite Single intraperitoneal 24-h mortality (%)
supplement in dose of dimethylselenide Diet A Diet B
drinking-water
(mg selenium/ (mg mole/kg body weight) (n = 20) (n = 10)
litre
------------------------------------------------------------
0 20 90 90
0.1 20 45 30
0.5 20 5 0
1.0 20 0 0
------------------------------------------------------------
a Adapted from: Parizek et al. (1976, 1980).
Male rats (2 months old) given drinking-water with stated
supplement of selenite for 3 days before dimethylselenide
administration. Diet A contained 0.052 ± 0.005 mg selenium/kg and
diet B (semi-synthetic diet) 0.044 ± 0.001 mg selenium/kg.
Smith et al. (1937) reported that the minimum lethal dose
of selenium as sodium selenite or selenate in rabbits, rats,
and cats was 1.5 - 3.0 mg/kg body weight, regardless of whether
the compounds were administered orally, subcutaneously,
intraperitoneally, or intravenously. This lack of effect of the
mode of administration probably reflects the rapid and complete
absorption of soluble selenium compounds, either from the site of
injection or from the gastrointestinal tract.
Cummins & Kimura (1971) described comparative studies on
Sprague Dawley rats and dogs concerning the oral toxicity of the
following selenium compounds: sodium selenate, selenourea,
biphenylselenium, selenium sulfide (1 - 30 µ particle size), and
elemental selenium (1 - 30 µ particle size). The oral LD50 values
in rats for a number of selenium compounds are shown in Table 21
and demonstrate the large variations in LD50 values that occur,
depending on both the oxidation state of the compound and its
aqueous solubility.
Table 21. Comparative solubility and toxicity of various
selenium compounds in ratsa
--------------------------------------------------------------
Compound Solubility in 0.01 N HCl Rat oral LD50 (95% CL)
(mg/kg body weight)
--------------------------------------------------------------
Na2SeO3 700 g/litre 7 (4.4 - 11.2)
H2N-C-NH2 30 g/litre 50 (35.7 - 70.0)
||
Se
SeS2 insoluble (< 1 g/litre) 138 (110 - 172)
Se 5 g/litre 360 (308 - 421)
Elemental Se insoluble (< 1 g/litre) 6700 (6000 - 7300)
--------------------------------------------------------------
a From: Cummins & Kimura (1971).
Male Sprague Dawley rats, each weighing between 50 and 100 g,
were given the above chemicals by gavage as 0.1 - 20% suspensions
in 0.5% methylcellulose. A total of 30-36 animals was used per
compound, in groups of 6 animals per dose. The LD50 values and
the associated confidence limits (CL) were calculated according to
the method of Litchfield & Wilcoxon.
The aqueous solubilities were carried out in 0.01 N HCl to more
closely simulate acidic conditions in the stomach. The least toxic
selenium compound was insoluble elemental selenium with an LD50 of
6.7 g/kg body weight. Toxic signs included pilomotor activity,
decreased body activity, dyspnoea, diarrhoea, anorexia, and
cachexia. Fatalities occurred within 18 - 72 h; survivors appeared
outwardly normal, at the end of the 7-day observation period. The
most toxic of the selenium compounds tested was the highly soluble
sodium selenite with an oral LD50 of 7 mg/kg body weight and the
toxic signs were similar to those seen with high doses of elemental
selenium. Selenium sulfide (a component in shampoos) was about 20
times less toxic than sodium selenite (i.e., an LD50 of 138 mg/kg
compared with 7 mg/kg). It was also found in this study that, with
the exception of biphenyl selenium, toxicity could be correlated
with blood-selenium levels. For example, sodium selenite being the
most toxic, gave the highest blood level followed in descending
order by selenourea, selenium sulfide, and elemental selenium. It
was suggested that the blood-selenium level produced by the
relatively non-toxic biphenyl selenium was high because this
covalently-bound selenium compound is the most lipophilic compound,
which is better absorbed, resists catabolism, and apparently
circulates as the parent compound.
The application of 83 mg of selenium oxychloride to the skin of
rabbits caused the death of the animals in 5 h, and application of
4 mg caused death in 24 h (Dudley, 1938). Lazarev (1977) reported
a minimum lethal dose of selenium oxychloride of 7 mg/kg body
weight, after cutaneous administration to rabbits.
Several papers have shown that injection of selenite in rats in
the early postnatal period in single doses of 1.5 mg selenium/kg
body weight, or in repeated doses of 0.5 mg/kg, induced cataracts
(Ostadalova et al., 1979; Bhuyan et al., 1981; Shearer et al.,
1980; Bunce et al., 1985). Similar effects have not been observed
in hamsters (Shearer et al., 1980). This response is dose
dependant (Table 22) and, thus far, has been observed only when the
selenite was given by parenteral injection.
Table 22. Cataractagenesis by selenite in the rata
--------------------------------------------------
Group Daily dosageb Frequency of cataractsc
(mg selenium/kg (%)
body weight)
--------------------------------------------------
Control 0 0
Na2SeO3 0.25 13
Na2SeO3 0.50 96
Na2SeO3 0.75 96
--------------------------------------------------
a Adapted from: Shearer et al. (1980).
b Dose given to rat pups daily on days 2-18
postpartum.
c Observed at 21 days of age.
7.1.2.2. Effects of long-term oral exposure
Moxon & Rhian (1943) summarized several older studies that
indicated that diets containing 5 mg selenium/kg or more cause
chronic selenosis in several species of animals, such as chickens,
rats, and dogs. In seleniferous areas, 5 mg selenium/kg diet is
generally accepted as the dividing line between toxic and non-toxic
feeds (US NAS/NRC, 1976).
Halverson et al. (1966) fed diets containing 0, 1.6, 3.2, 4.8,
6.4, 8.0, 9.6, or 11.2 mg selenium/kg in the form of sodium
selenite or seleniferous wheat to male Sprague Dawley rats,
initially weighing 60 - 70 g. The rats were divided into groups of
8 and were fed the diets for 6 weeks. The diet consisted of (g/kg)
ground wheat, 809; purified casein 120; USP salt mixture XIV, 20;
USP brewer's yeast, 20; corn oil, 30; and vitamin B12 mix, 1.
Vitamins A, D, and E were provided separately. The criteria used
to assess toxicity were growth depression, liver cirrhosis,
splenomegaly, pancreatic enlargement, anaemia, elevated
serumbilirubin levels, and death. The addition to the diet of 1.6,
3.2, or 4.8 mg selenium/kg did not have any significant effect on
the rats, as judged by any of these criteria. There was a
depression in growth in the group that received 4.8 mg dietary
selenium/kg as sodium selenite, but this was not significant.
Liver cirrhosis, splenomegaly, and significant growth depression
were observed when the rats were fed levels of 6.4 mg/kg or more
from either source of selenium. Diets containing 8.0 mg/kg or more
caused additional effects such as pancreatic enlargement, anaemia,
elevated serum-bilirubin levels, and, after 4 weeks, death.
Diets made either with non-seleniferous or seleniferous sesame
meal were fed to groups of 12 male Sprague Dawley rats, initially
weighing 50 g for a period of 6 weeks (Jaffe et al., 1972b). The
diet made with non-seleniferous sesame meal contained 0.5 mg
selenium/kg and consisted of (g/kg): non-seleniferous sesame meal,
463.3; almidon, 422.7; corn oil, 50; cod liver oil, 10; USP XVI
salts, 40; L-lysine x HCl, 4; and vitamin mix, 10. The diet made
with the seleniferous sesame meal contained 10 mg selenium/kg and
had the same composition as the previous diet except that
seleniferous sesame, which replaced the non-seleniferous sesame
meal, was added at a level of 490.2 g/kg, and the almidon was added
at a level of 395.8 g/kg. The rats fed the diet containing the
seleniferous sesame meal showed decreased survival, impaired weight
gain, higher incidence of liver lesions, elevated hepatic selenium
levels, enlarged spleens, depressed haemoglobin, haematocrit, and
fibrinogen levels, and decreased prothrombin activity (Table 23).
In a separate study, the same workers investigated the effects of
dietary selenium, given as seleniferous sesame meal, on the
activities of various serum enzymes (Table 24). The diet
containing 4.5 mg selenium/kg had the same composition as the
previous diets, except that the seleniferous sesame meal, non-
seleniferous sesame meal, and almidon were added at levels of
230.8, 270.4, and 384.8 g/kg, respectively. Feeding the diet
containing 4.5 mg of selenium/kg for 6 weeks increased the
activities of serum alkaline phosphatase (EC 3.1.3.1) and glutamic
pyruvic transaminase (SGPT) (EC 2.6.1.2), whereas 10.0 mg/kg also
increased the activity of glutamic-oxaloacetic transaminase (SGOT)
(EC 2.6.1.1).
Table 23. Pathological signs in rats fed diets containing seleniferous sesame meala
--------------------------------------------------------------------------------------------------------------------
Dietary Survival Weight Rats Hepatic Spleen Haemo- Haema- Fibrinogen Prothrombin
selenium after gain with selenium weight globin tocrit activity
6 weeks after hepatic level level value
6 weeks lesions
--------------------------------------------------------------------------------------------------------------------
(mg/kg) (g) (mg/kg) (% of body (g/litre (volume %) (mg/litre)
weight) blood)
0.5 12/12 156.8 ± 7.2 0/12 0.72 ± 0.14 0.207 ± 0.01 147 ± 2.1 43.9 ± 0.61 1664 ± 78 963 ± 9.5
10 8/12 61.2 ± 7.43 10/12 7.34 ± 0.97 0.544 ± 0.09 123 ± 3.5 40.8 ± 0.82 655 ± 78 713 ± 76.6
--------------------------------------------------------------------------------------------------------------------
a Adapted from: Jaffe et al. (1972b).
Table 24. Effect of chronic selenium toxicity on the
activity of serum enzymesa
-------------------------------------------------------
Dietary Number Alkaline SGOT SGPT
selenium of rats phosphatase
-------------------------------------------------------
(mg/kg) (units/ml) (units/ml) (units/ml)
0.5 6 6.3 ± 0.2 66 ± 3 20 ± 1
4.5 10 8.8 ± 1.3 59 ± 2 26 ± 2
10 8 18.9 ± 2.3 77 ± 5 43 ± 6
-------------------------------------------------------
a Adapted from: Jaffe et al. (1972b).
Tinsley et al. (1967) and Harr et al. (1967) carried out an
extensive study on the chronic toxicity of selenium in rats.
Although the primary purpose of their research was to investigate
the alleged carcinogenicity of selenium (section 7.7.1), the design
of their study provided an opportunity to examine other aspects of
the toxicity of selenium, such as the influence of selenium
poisoning on growth rate and histopathology. A total of 1437
Wistar rats from a closed random-bred colony was used with the size
of the experimental groups ranging from 10 - 110 animals. The rats
were fed one of 3 different diets: a semipurified diet containing
either 12 or 22% casein or a commercial "laboratory chow" type
ration. The casein-based diets also contained corn oil, 50 g;
H.M.W. salts, 40 g; vitamin mix, 10 g; and glucose monohydrate
(Cerelose) up to a weight of 1 kg. Selenium as sodium selenite or
sodium selenate was added at levels of 0, 0.5, 2.0, 4.0, 8.0, or
16.0 mg/kg. Only a small proportion of the animals fed diets
supplemented with more than 4.0 mg/kg of selenium survived for 12
months. The rats fed the commercial ration were 2 - 3 times more
resistant to the effects of selenium toxicity than the rats fed the
semipurified diet. A calculated maximum body weight was reported
to be depressed by as little as 0.5 mg selenium/kg, but no
statistical evaluation of the results was presented. Usually
moribund animals were killed and necropsied for histopathological
determinations, but 136 rats were killed at specific ages.
Acute toxic hepatitis was the predominant histopathological
lesion in rats fed the commercial ration supplemented with sodium
selenate at 16 mg selenium/kg. These rats had a median survival
age of only 96 days. Acute toxic hepatitis was also observed in
rats fed the 12% casein diet supplemented with sodium selenate at 4
or more mg selenium/kg. The rats were emaciated, pale, and had
poor quality hair coat. Hydrothorax, ascites, pericardial oedema,
and icterus were common. Myocardial hyperaemia, fluid imbalance,
and parenchymal degeneration were often present. The adrenals were
enlarged and the pancreas was oedematous. The failure of normal
chondrocyte proliferation observed in the metaphyses appeared to be
different from that seen in malnutrition, since proliferation was
irregular and not merely reduced.
When rats were fed the commercial ration supplemented with
sodium selenate at 8 mg selenium/kg, the predominant lesion was
chronic toxic hepatitis. The median survival age in this group was
429 days. Other histopathological changes reported included
pancreatic duct hyperplasia, intestinal nephritis, and myocardial
damage, particularly in rats of more than 450 days of age and
receiving selenite.
Harr et al. (1967) reported an increased proliferation of the
hepatic parenchyma when the rats were fed the semi-purified diet
supplemented with 0.5 - 2.0 mg selenium/kg as selenite or selenate.
But a more detailed report from this laboratory (Weswig et al.,
1966) showed that this lesion of "chronic liver and bile duct
hyperplasia" was observed to a greater extent in rats fed the
commercial ration not supplemented with selenium than in rats fed
the semi-purified diet supplemented with 0.5 mg selenium/kg. Thus,
this lesion may not be specifically related to selenium.
Harr & Muth (1972) stated that 0.25 mg/kg was the minimum toxic
level for liver lesions, when selenium was added to a semi-purified
diet. The minimum toxic level was 0.75 mg/kg when the criteria
were longevity or lesions of the heart, kidneys, or spleen.
However, growth was normal in rats fed 0.5 mg selenium/kg diet.
The authors estimated that the dietary threshold for physiological
and pathological effects was 0.4 mg/kg and for pathological and
clinical effects, 3 mg/kg.
Weanling rats of the Long-Evans strain were given either sodium
selenite or selenate at 0 or 2 mg selenium/litre in the drinking-
water for 1 year (Schroeder & Mitchener, 1971b). After 1 year, the
selenium dosage was increased to 3 mg/litre in the selenate group.
The weanlings were born from random-bred females that had been fed
a low-selenium diet (0.05 mg selenium/kg) consisting of: whole rye
flour, 600 g; dry skim milk, 300 g; corn oil, 90 g; and iodized
sodium chloride to which were added vitamins and iron, 1 g/kg. The
same diet was fed to the weanlings during the toxicity study. The
drinking-water was doubly deionized forest spring water to which
had been added: zinc, 50 mg; manganese, 10 mg; chromium(III), 5 mg;
copper, 5 mg; cobalt, 1 mg; and molybdenum, 1 mg/litre. The rats
given the selenium compounds (plus another group not discussed here
given sodium tellurite) were divided into groups totalling 313
animals. There were 105 control rats. By 58 days, half of the
male rats in the selenite group were dead. Fifty percent mortality
in the group of female rats given selenite was not achieved until
348 days and was not achieved in the male and female groups given
selenate until 962 and 1014 days, respectively. On the other hand,
Palmer & Olson (1974) gave 2 or 3 mg selenium/litre drinking-water,
in the form of either sodium selenite or selenate to male weanling
Sprague Dawley rats for 6 weeks and noted small decreases in weight
gain compared with control rats receiving selenium, but no deaths.
Two diets were used in this study, a rye diet similar to that
described by Schroeder & Mitchener above and a corn diet that
consisted of ground corn, 808 g; casein, 120 g; corn oil, 30 g;
U.S.P. XIV salts, 20 g; and vitamin mix, 22 g/kg. The trace
elements zinc, copper, manganese, chromium, cobalt, and molybdenum
were also added to the drinking-water, as suggested by Schroeder &
Mitchener (1971a).
In a study by Jacobs & Forst (1981a), sodium selenite at 0 or 4
mg selenium/litre drinking-water was administered to groups of 17
and 30 male 5-week-old Sprague Dawley rats fed a commercial pellet
ration. After 64 weeks, survival was 94 and 63% in the control and
selenium-treated groups, respectively. In a second study of similar
design, except that the rats were 8 weeks old at the start,
survival was 90 and 95% in the control and selenium-treated groups,
respectively, after 61 weeks.
Pletnikova (1970) investigated the effects of long-term, low-
level administration of sodium selenite in water to rabbits and
rats. For these studies, 32 rabbits and 16 rats were divided into
4 groups and administered, orally, doses of 0, 0.005, 0.0005, or
0.00005 mg selenium/kg body weight for periods of 7 1/2 and 6
months, respectively. Prolonged administration of the maximum dose
investigated (0.005 mg/kg) produced significant alterations in the
rabbits. After 2 months, there was a significant increase in the
concentration of oxidized glutathione in the blood and, after 7
months, there was slower elimination of bromsulphalein by the
liver, and hepatic succinic dehydrogenase activity was decreased.
A dose of 0.0005 mg/kg caused fewer, less pronounced changes,
whereas 0.00005 mg/kg did not produce any statistically significant
effects in any of these tests. A dose of 0.005 mg/kg given to rats
caused a considerable weakening of the capacity for forming new
conditioned reflexes. It can be assumed that a daily dose of 0.005
mg/kg body weight in rats is equivalent to a dietary-selenium level
of about 0.063 mg/kg. Since this level of selenium is within the
physiological range needed to prevent selenium deficiency in
animals (section 7.2.2), the toxicological significance of these
observations is not clear, unless it is assumed that a certain dose
of selenite given in water solution is more toxic than the same
dose given in the diet.
Recently, Csallany et al. (1984) reported that giving sodium
selenite to female mice in the drinking-water at a level of 0.1 mg
selenium/litre increased the amount of hepatic lipid-soluble
lipofuscin pigments, when the animals were sacrificed at 9 months
of age. There were 8 mice in each group and the animals were fed a
diet adequate in vitamin E, which contained 0.05 mg selenium/kg.
Sodium selenite added to the drinking-water at a level of 9 mg
selenium/litre killed all 12 rats fed a diet based on either ground
corn or ground rye in 6 weeks (Palmer & Olson, 1974), whereas
Halverson et al. (1966) found that sodium selenite added to a diet
based on ground wheat, at a level of 9.6 mg/kg, killed only 1 out
of 10 rats in the same period of time. However, the rats used in
the latter study were post-weanling animals weighing 60 - 70 g when
the study started, whereas the rats used in the former study were
21-day-old weanlings and weighed only 35 - 45 g. Other research
workers have shown that the resistance of rats to the toxic effects
of selenium increases markedly after the twenty-first day of life
(Franke & Potter, 1936).
Feng et al. (1985) studied the hepatoxicity of high selenium
corn (7.12 mg/kg) produced in the Enshi county of China, where
human selenium intoxication was reported (section 8.1.1.1). After
feeding a diet that contained 61% of this corn (total selenium
concentration of 4.343 mg/kg) for 16 weeks, liver cirrhosis was
seen in 3 out of 6 male and 5 out of 6 female rats in one group.
No liver damage was found histologically in another group of rats
in the same study, which had consumed a diet containing 30.5% of
the high selenium corn (total selenium concentration of 2.35
mg/kg).
At present, the best indicator of chronic selenium toxicity
appears to be growth inhibition (US NAS/NRC, 1976), and a selenium
level of 4 - 5 mg/kg is necessary to achieve this response in
animals fed a normal diet. In laboratory rats, this exposure to
selenium represents an intake of about 200 - 250 µg/kg body weight
per day. More sensitive and specific criteria of selenium
poisoning to demonstrate effects at lower dose levels, such as
biochemical or histological techniques, would obviously be highly
desirable, but such tests are not available at present.
7.1.2.3. Inhalation toxicity
The effects of respiratory exposure to selenium compounds,
administered under conditions mimicking occupational exposure, have
been described in several papers. Filatova (1951) studied the
toxic effects of respiratory exposure to selenium dioxide (SeO2)
under conditions similar to those that occur in industry, i.e.,
heating of selenium (section 5.2.1). In acute studies, white rats
were exposed to air concentrations of selenium dioxide of 0.15 -
0.6 mg/litre, and all rats died within one-half - 4 h.
Morphological examination of the organs revealed that intraalveolar
and perivascular oedema occurred in the lungs, and haemorrhages and
degenerative changes in the liver, kidney, and heart. In 4
additional studies, all rats survived 4 h when exposed to doses of
0.09, 0.06 - 0.07, or 0.03 - 0.04 mg selenium dioxide/litre, but
all rats exposed to the highest dose (equal to 5 - 5.2 mg/kg body
weight) died within 24 h. In a series of long-term studies, rats
were exposed to repeated doses of selenium dioxide at 0.01 - 0.03,
0.006 - 0.009, or 0.003 - 0.005 mg/litre for 6 h, every other day,
for one month. The lowest dose did not produce any effects on body
weight or on the blood picture, and all the rats survived.
Histological examination revealed degenerative changes in the
liver, renal tubules, dystrophy of heart muscle, and hyperaemia and
hypertrophy of the splenic pulp. At the dose of 0.006 - 0.009
mg/litre, all the rats but one died within 27-33 days. For the
first 2 weeks, there was no difference in body weight between the
exposed and unexposed control rats but, during the last 2 weeks,
the exposed rats lost body weight and all but one of the exposed
rats died. The histopathological changes consisted of multiple
necrosis and degeneration in the liver and myocardial fibres, and
involvement of renal tubules. In the third group of rats, which
was exposed to 0.01 - 0.03 mg/litre, the animals showed respiratory
distress, weight loss, and, in 3 rats, anaemia. All the rats died
between days 8 and 18 of exposure. In the liver, kidneys,
myocardium, and spleen, the changes observed were similar to those
seen at lower doses, but more pronounced. Moreover, lung oedema
similar to that noted in the acute exposure studies was seen.
Lipinskij (1962) exposed 2 groups of 5 rabbits to airborne
amorphous selenium and selenium dioxide in chambers, under
conditions analogous to industrial exposure, except that the doses
were higher. In the first group, the rabbits were exposed to 20 mg
selenium dioxide/litre and 40 mg selenium/m3, for 2 h per day, for
one week, at which time a decrease in blood catalase (EC 1.11.1.6)
activity was noted. The rabbits in the second group were exposed
to 10 µg selenium dioxide/litre and 20 mg selenium/m3, for 2 h
daily, for 12 weeks. After 12 weeks, decreases in total and
reduced glutathione were observed, but there was no change in
levels of oxidized glutathione.
On the basis of studies on 60 white rats weighing 120 - 150 g,
Burchanov et al. (1969) concluded that intratracheal injection of
0.06 ml of a sterile suspension containing 50 mg of highly
dispersed elemental selenium dust in physiological saline, for
1 - 12 months, resulted in decreases in body weight and muscular
strength, and morphological and biochemical alterations in the
respiratory tract. Burchanov (1972) obtained similar results in
inhalation studies on rats exposed to 2 types of polymetallic dusts
found in industry.
The acute toxicity of selenium dust (average mass median
particle diameter, 1.2 µ) for rats, guinea-pigs, and rabbits was
described by Hall et al. (1951). Exposure of these animals, for
16 h, to an atmosphere containing approximately 30 mg selenium
dust/m3 produced mild interstitial pneumonitis in the animals.
Rats exposed to selenium fumes developed acute toxic effects, and
it was suggested that the fumes might have contained some selenium
dioxide.
The acute toxic effects of hydrogen selenide were investigated
in guinea-pigs exposed for 10, 30, or 60 min to concentrations
ranging from 0.002 to 0.57 mg/litre (Dudley & Miller, 1941). All
animals exposed to 0.02 mg/litre, for 60 min, died within 25 days;
93% of those exposed to 0.043 mg/litre, for 30 min, died within 30
days, and all exposed to 0.57 mg/litre, for 10 min, died within 5
days. Decreasing the concentrations of hydrogen selenide, and
increasing the time of exposure to 2, 4, or 8 h produced death in
50% of the guinea-pigs, within 8 h.
Acute studies on the toxicity of selenium hexafluoride (SeF6),
were carried out on the rabbit, guinea-pig, rat, and mouse at 100,
50, 25, 10, 5, and 1 ppm for 4 h. Exposures down to and including
10 ppm (Ct = 40 ppm/h) were uniformly fatal (Kimmerle, 1960).
Exposure to 5 ppm (Ct = 20 ppm/h) resulted in pulmonary oedema from
which the animals recovered, and 1 ppm did not induce any grossly
observable effects. However, with repeated exposure for 1 h daily
at 5 ppm, for 5 days, there were definite signs of pulmonary
injury.
7.1.3. Blood levels in toxicity
Analyses of blood from cattle have shown that, if the average
value of blood-selenium for a herd is over 2 mg/litre, damage from
chronic selenium poisoning is very likely to occur (Dinkel et al.,
1957). Average values of 1 - 2 mg/litre suggest a borderline
problem, especially in reproduction, whereas values below 1
mg/litre indicate that no damage from toxicity should be expected.
Rosenfeld & Beath (1964) commented that typical concentrations of
selenium in the blood in alkali disease, blind staggers, and acute
selenium poisoning were 1 - 2, 1.5 - 4, and up to 25 mg/litre,
respectively. In studies with sodium selenite, Maag et al. (1960)
found that severe selenium toxicity occurred in cattle when the
selenium content of the blood exceeded 3 mg/litre. The selenium
levels in blood associated with chronic toxicity in sheep
corresponded to 0.6 - 0.7 mg/litre (Ermakov & Kovalskij, 1974).
7.1.4. Effects on reproduction
Franke & Potter (1936) fed 7 groups of 6 weanling rats (2 males
and 4 females), 21 days of age, a basal diet consisting of ground
whole wheat, 82%; commercial casein, 10%; pure leaf lard, 3%;
dehydrated yeast, 2%; cod liver oil, 2%; and McCollum's salt
mixture No. 185, 1%. Group 1 was shifted immediately to a toxic
diet in which sufficient control wheat was substituted by
seleniferous wheat to give a final selenium concentration of 24.6
mg/kg diet. Groups 2, 3, 4, 5, and 6 were shifted to the toxic
diet when the rats attained 42, 63, 84, 105, and 186 days of age,
respectively. Group 7 was fed the basal diet throughout the entire
study. No matings were successful in which both males and females
had been fed the toxic diet for 40 days, regardless of the age at
which the rats had been taken off the basal ration. This was true,
even in group 6, in which successful matings had been achieved at
82 and 131 days, while the rats were still being fed the basal
diet. All male rats not placed on the toxic diet until 63 days of
age or more were able to fertilize the normal females. In a later
study in which females fed the toxic diet were mated with normal
males, there were some successful matings, but the pups that were
born generally died or were eaten by the mother soon after birth.
In a study by Munsell et al. (1936), 7 groups of 4-week-old
rats, each containing 2 males and 6 females, were fed a basal diet
consisting of wheat, 58%; skim milk powder, 30%; yeast, 5%; butter,
5%; and cod liver oil, 2%. In each of the groups, a variable
proportion of the control wheat was replaced by toxic wheat so that
the latter contributed 8.7, 6.0, 3.0, 1.5, 0.75, 0.38, or 0 mg
selenium/kg diet. Compared with the controls receiving no toxic
wheat, the number of females having young was decreased by feeding
the diets containing 3.0 mg or more selenium/kg, and the percentage
of young reared was decreased by the diets containing 6.0 mg or
more/kg (Table 25). Feeding the diet containing 0.75 mg/kg
appeared to improve reproductive performance in terms of total
young produced or percentage of young reared. In the second
generation of rats continued on their respective levels of toxic
grain, the diet containing 6.0 mg/kg had a definite deleterious
effect on reproduction, whereas diets containing 0.75 - 1.5 mg/kg
had some beneficial effects. In a second study, the percentage of
young reared was improved in the group fed the diet containing 1.5
mg/kg compared with the control diet group, but the average number
of young per litter and the average number of young per bearing
female were decreased in the group fed the diet containing 0.75
mg/kg.
Halverson (1974) fed 4 groups of 70-day-old ARS/Sprague Dawley
albino rats a basal diet containing glucose, 61.8%; purified
casein, 25%; corn oil, 5%; minerals, 6%; and vitamins, 2.2%. The
groups comprised 2 - 4 males and 6 - 8 females and were given the
basal diet supplemented with sodium selenite at 0, 1.25, 2.50, or
3.75 mg selenium/kg. After 90 days, the rats were mated; no
consistent effects of selenium on reproduction were observed in
these first generation rats. Four groups of second-generation
young were maintained on their respective diets and used as
breeding stock in a second life cycle. In 2 separate studies, the
first and second reproductions of these second generation rats were
successful, regardless of the selenium content of the diet (Table
26). In the third reproduction, however, the unsupplemented rats
showed signs of reproductive failure. Selenium supplementation at
all levels prevented poor litter production in the first study but
only levels of 2.50 and 3.75 mg/kg prevented it in the second.
Neonatal survival was improved in both studies by selenium
administered at 3.75 mg/kg.
Schroeder & Mitchener (1971a) gave selenate at 3 mg
selenium/litre, in the drinking-water, continuously, during a
multigeneration study in mice. Five pairs of Fo mice were given
selenium at weaning and allowed to breed ad libitum for 6 months.
Controls were treated identically but did not receive any selenium
in the water. At weaning, F1 pairs were randomly selected from the
first, second, and third litters and left to breed, to produce the
F2 generation. F2 pairs were similarly selected from the first or
second litters to produce the F3 generation. An increased
male:female ratio was observed in all generations (1.30 - 1.50
versus 0.94 - 1.03 for the controls), but the mechanism of this
effect was not explained. No breeding failures were seen in the
control group, but 2 or 3 such failures occurred in each of the F1,
F2, and F3 generations given selenate. Only 3 litters were
produced in the F3 generation treated with selenate and 16 of 23
mice born were runts, whereas in the control F3 generation, 22
litters were produced with a total of 230 mice and no runts.
Two groups of 5 male and 2 groups of 5 female Wistar rats were
fed a laboratory chow diet and one group of each sex was given
sodium selenate at 0 or 7.5 mg selenium/litre in the drinking-
water, respectively (Rosenfeld & Beath, 1954). The rats receiving
selenium ("selenized rats") were treated from birth to 8 months of
age. Mating of normal males with selenized females was totally
unsuccessful, whereas mating of selenized males with normal females
resulted in normal reproduction and survival. Doses of 1.5 or 2.5
mg/litre did not have any effect on the reproduction of breeding
rats, the number of young reared by the mothers, or the
reproduction of 2 successive generations of males and females.
However, a level of 2.5 mg/litre decreased the number of young
reared by the mothers by about 50%.
Table 25. Effect of seleniferous wheat on reproduction in first- and second-generation ratsa
-------------------------------------------------------------------------------------------------
Level of Generation and Females having Total Total Average Average Young reared
dietary number of females young young litters number number
selenium 1st 2nd young/ young/
(from generation generation litter bearing
wheat) female
--------------------------------------------------------------------------------------------------
(mg/kg) Number % Number Number Number Number Number %
0 6 - 6 100 70 13 5.4 11.7 30 42.9
0.38 6 - 5 83.3 72 13 5.5 14.4 23 31.9
0.75 6 - 6 100 90 18 5.0 15.0 75 83.3
1.5 6 - 5 83.3 42 10 4.2 8.4 15 35.7
3.0 6 - 3 50.0 38 7 5.4 12.7 19 50.0
6.0 6 - 4 66.7 30 8 3.8 7.5 5 16.7
8.7 6 - 2 33.3 7 2 3.5 3.5 0 0
0 - 11 10 90.9 134 19 7.1 13.4 46 34.3
0.38 - 9 6 66.7 63 10 6.3 10.5 31 49.2
0.75 - 41 29 70.7 434 52 8.3 15.0 328 75.6
1.5 - 7 7 100 100 11 9.1 14.3 72 72.0
3.0 - 7 5 71.4 61 9 6.8 12.2 25 41.0
6.0 - 2 2 100 17 3 5.7 8.5 0 0
8.7 - 0 - - - - - - - -
--------------------------------------------------------------------------------------------------
a Adapted from: Munsell et al. (1936).
Table 26. Effect of selenium as selenite on successive reproductions of second-
generation rats maintained on a casein dieta
--------------------------------------------------------------------------------
Study Added Number Measurements in first, second and third reproductions
selenium of brood Number of Number young per 2-day survival of
in diet females litters born newborn litter litter members
1 2 3 1 2 3 1 2 3
--------------------------------------------------------------------------------
(mg/kg) (%)
1 0 3 3 3 0 12 12 - 61 46 -
1.250 6 6 6 5 12 7 8 29 21 26
2.500 5 5 5 5 12 11 8 71 84 30
3.750 6 6 6 6 11 9 8 79 78 78
2 0 7 7 6 2 8 10 6 74 82 33
1.250 7 7 5 2 10 11 4 90 84 00
2.500 9 8 8 6 9 9 5 81 85 25
3.750 8 7 7 4 7 6 6 92 100 92
--------------------------------------------------------------------------------
a Adapted from: Halverson (1974).
Poley & Moxon (1938) fed 4 groups of 15 Rhode Island pullets a
basal laying ration consisting of ground corn, 25%; ground barley,
25%; ground wheat, 15%; wheat bran, 8%; wheat middlings, 8%; meat
and bone scraps, 8%; alfalfa leaf meal, 5%; dried buttermilk, 5%;
salt, 0.5%; and cod liver oil concentrate, 0.5%. Each of the 4
groups was mated with 2 male brothers for the entire study. After
2 weeks, grains containing selenium (corn, barley, and wheat) were
substituted for normal grains in order to contribute 0, 2.5, 5, or
10 mg selenium/kg diet fed to each of the 4 groups, respectively.
Oyster shells and water were provided ad libitum. There were no
significant differences in feed consumption, body weight, egg
production, or egg fertility in any of the groups. After 4 weeks
on the toxic diets, 10 mg of selenium/kg reduced hatchability to
zero. Hatchability was only slightly reduced by 5 mg/kg, and a
level of 2.5 mg/kg had no effect.
In a study by Wahlstrom & Olson (1959), 2 groups of 10 purebred
Duroc gilts, 8 weeks of age, were fed a basal diet supplemented
with sodium selenite at 10 mg selenium/kg diet. Of the 9 gilts in
the unsupplemented group (one was killed accidentally), 8 conceived
with the first service and one failed to conceive after 3 services.
Of the 10 gilts in the selenium-exposed group, 5 conceived with the
first service, 2 with the second service and 3 failed to conceive
after 3 services. The selenium-exposed group farrowed fewer
litters and fewer live pigs, and weaned fewer pigs than the control
group (Table 27). Moreover, the average birth weights and weaning
weights were lower in the selenium-exposed group. The average
litter weight at 56 days for both litters was 131 and 227 pounds
for the selenium-exposed and unexposed groups, respectively.
Table 27. The effect of selenium on reproduction and lactation in swine
-------------------------------------------------------------------------
Items Number Average Average Average Average Average
litters number number birth number weaning
farrowed pigs pigs weight pigs weight
farrowed farrowed (kg) weaned (kg)
alive
-------------------------------------------------------------------------
Basal
First litters 7 8.0 8.0 2.95 7.3 30.5
Second litters 6 11.2 10.5 2.89 8.0 29.0
Average 6.5 9.6 9.25 2.92 7.65 29.75
Basal + selenium
First litters 6 9.8 7.7 2.60b 5.7 23.1c
Second litters 5 8.6 7.2 2.76 5.6 23.5c
Average 5.5 9.2 7.45 2.68 5.65 23.3
-------------------------------------------------------------------------
a Adapted from: Wahlstrom & Olson (1959).
b Significantly less than basal lot ( P < .05)
c Significantly less than basal lot ( P < .01)
Dinkel et al. (1963) reported on the effect of early (May 1
to mid-July) versus late (mid-July to October 1) breeding on the
reproductive performance of beef cattle grazing on a seleniferous
range in South Dakota. With such a programme, it was felt that
early conception would avoid any deleterious effects on
reproduction of the highly seleniferous young range grasses that
grow luxuriantly during the early summer. Data from 152 matings,
75 in the early and 77 in the late breeding group, collected over
5 years, showed that the average conception rate of the early-bred
cows was 60%, whereas that of the late-bred cows was 37.7%.
Moreover, the average calf crop weaned was 52% in the early
breeding group and 32.4% in the late group. The data do not prove,
but suggest, that selenium was the cause of the difference. In
addition to the early breeding effect, the very low overall
reproductive rate should be noted. This is considerably below the
rate that can be attained with similar operations when chronic
selenosis is not a problem, and it has been repeatedly observed
that, on ranches with a selenium problem, the rate of reproduction
is consistently low. This led Olson (1969) to comment that the
effect on reproduction might be the most significant effect of
excessive amounts of the element from an economic standpoint.
7.1.5. Effects on dental caries
Most studies on experimental animals have shown that high doses
of selenium do not have any effect on caries formation when the
selenium is given after tooth formation has already occurred. For
example, Muhler & Shafer (1957) fed 22 male weanling Sprague Dawley
rats a stock corn cariogenic diet that contained 10 mg sodium
selenite/kg. After 6, 8, and 12 weeks, the level of sodium
selenite was raised to 15, 20, and 30 mg/kg diet, respectively. A
control group of 20 rats received the same diet without added
selenium. The animals were given their respective diets and
drinking-water low in fluorine (F = 0.2 mg/litre) ad libitum for
140 days. The selenium-exposed group grew only about 53% as much
as the controls, but the mean number of carious lesions was
essentially the same in both groups (6.4 and 6.9, respectively).
Claycomb et al. (1965) fed 2 groups of 24 weanling rats a high-
carbohydrate diet, with or without 10 mg sodium selenite/kg diet,
for 100 days. In this study, selenium depressed growth by only
11%, but again the average number of carious lesions per rat was
similar in the selenium-treated and control groups (0.92 and 1.08,
respectively). The rather low incidence of dental caries was
thought to be due to genetic influences. Wheatcroft et al. (1951)
maintained 80 white rats, 40 males and 40 females, on a coarse corn
cariogenic diet for 100 days. The rats were divided into 4 groups
of 20 and were given daily intraperitoneal doses of sodium selenite
at 0, 0.2, 0.5, or 1.0 mg selenium/kg body weight. The authors
felt that there was a trend towards an increased incidence of
caries in the group receiving the most selenium, but the 2 highest
doses of selenium were clearly toxic, since these rats had
diarrhoea, affected eyes, and dull, matted fur. There was also
histological evidence of liver damage in over half, and kidney
damage in a third, of the rats. Thirty new-born Wistar rats were
divided into 3 groups receiving, intraperitoneally, 0, 0.5 - 1.0
µg, or 5 - 10 µg sodium selenate, respectively, every day for 53
days (Kaqueler et al., 1977). The group receiving the lower dose
of selenium had a lower total number of caries than the controls,
while the group receiving the higher dose had more caries than the
controls. The authors concluded that post-eruptive administration
of sodium selenate may have either a cariostatic or cariogenic
influence in rats, depending on the dosage administered.
Attempts to induce dental caries by exposure to high levels of
selenium were more successful when the selenium was given during
the time of tooth development. For example, Navia et al. (1968)
fed sodium selenite at 4 mg selenium/kg, to groups of 21 CR-COBS
rats in the drinking-water, in a purified caries-producing diet, or
in the same diet gelled with equal parts of 2% aqueous agar. The
experimental treatments of the mothers and litters began at birth
and continued until the pups were 50 days old. Selenium had no
effect on buccal, sulcal, or proximal caries, when given in the
diet, but caused a 12% increase in sulcal lesions when administered
in the drinking-water. Buttner (1963) fed 3 groups of 8 female,
caries-susceptible rats of the Wistar strain a cariogenic coarse
stock corn diet plus 0, 5, or 10 mg sodium selenite/litre drinking-
water during mating, pregnancy, and lactation. The offspring
received the same concentrations of sodium selenite for 120 days.
Thirty-one pups were born in the control group but, since the
dosages of selenium used partially inhibited reproduction, only 20
and 7 pups were born in the groups receiving 5 or 10 mg sodium
selenite/litre drinking-water, respectively. Growth of the pups
was strongly inhibited and the number and extent of carious lesions
were increased in both selenium-treated groups (Table 28). In a
study by Bowen (1972), 7 female monkeys of the species Macaca irus
were fed a cariogenic diet, with drinking-water sweetened with 3%
sucrose, at night, and phosphate-free icing sugar, 4 times daily.
A second group of 3 females of the same species was treated
identically except that they received selenium, as sodium selenate,
at 2 mg/litre drinking-water. After 15 months, some of the
selenium-treated monkeys experienced slight gastrointestinal
trouble in the form of greenish malodorous stools and the dose of
selenium was reduced to 1 mg/litre for another 45 months. The
second permanent molars and the premolars were formed during the
period of higher selenium exposure and these teeth had a yellow
chalky appearance in the selenium-treated monkeys. On the other
hand, selenium had no effect on the first permanent molars, which
had already formed before the start of the study. Although both
groups of monkeys had severe dental caries, the mean caries score
of the selenium-treated group was about twice that of the controls
(16 and 8.4, respectively). There was no difference between the
times required for caries to develop in the first permanent molars
in the controls and experimental groups, but carious lesions in the
second permanent molars developed more rapidly in the selenium-
exposed group (6.7 versus 21 months for mean caries development
time in selenium-treated compared with control groups). The author
concluded that selenium had a cariogenic effect, when administered
during tooth development and a moderate anti-cariogenic effect,
when given posteruptively.
Table 28. Effect of developmental and postdevelopmental
administration of sodium selenite on dental caries in the rata
-------------------------------------------------------------------
Dose of sodium Sex Number Weight gain Mean number Extent
selenite in of in 120 days of carious of carious
drinking-water rats (g) lesions lesions
(mg/litre)
-------------------------------------------------------------------
0 M 17 330 ± 9 5.3 ± 0.5 11.6 ± 1.4
F 14 207 ± 7
5 M 13 240 ± 5 7.2 ± 0.7 18.7 ± 2.2
F 7 176 ± 7
10 M 5 220 ± 18 8.6 ± 1.1 25.2 ± 3.2
F 2 148 ± 3
-------------------------------------------------------------------
a Adapted from: Buttner (1963).
Britton et al. (1980), however, presented evidence that showed
that, under some conditions, selenium can have a cariostatic
effect, even when given during tooth development. These workers
gave 0, 0.8, or 2.4 mg selenium/litre drinking-water in the form of
selenomethionine or sodium selenite, to rats from the 10th day of
pregnancy until the pups were weaned. At 17 - 19 days of age, the
pups were given oral innoculations of Streptococcus mutans - 6715
in thioglycollate broth. When 19 days old, the pups were weaned,
divided into groups of 13 - 19, and given a 67% sucrose diet and
distilled water ad libitum. At 65 - 68 days of age, the young rats
were killed and the first and second molar teeth were stained with
murexide and scored for caries. The high dose of selenium given to
the mothers, as either compound, did not have any effect on
dental caries, but the middle dose (0.8 mg/litre) had a definite
cariostatic effect, since selenomethionine and sodium selenite
decreased the incidence of total buccal caries by 46.1 and 40.9%,
respectively. The authors suggested that this cariostatic effect
might occur because some selenium is necessary for proper enamal
formation or because of undetermined effects on the oral
environment.
The fact that high doses of selenium have an apparent
cariogenic effect, only when given during tooth development,
is consistent with the results of Shearer (1975) who found that
the incorporation into teeth of radioactive selenite or
selenomethionine given in the drinking-water at a level of 0.2 mg
selenium/litre was much greater in rat pups undergoing dental
development than in their mothers whose teeth had already matured.
The mechanism of the cariogenic effect of high levels of selenium
is not known, but an antagonism to fluoride does not seem to be
involved (Shearer & Ridlington, 1976).
7.1.6. Factors influencing toxicity
7.1.6.1. Form of selenium
Studies comparing the toxicity of different forms of selenium
are described in section 7.1.2.1. This point is particularly
relevant because the forms of selenium in foods have yet to be
characterized.
7.1.6.2. Nutritional factors
High levels of dietary protein protect against selenium
toxicity (Gortner, 1940), but some proteins give better protection
than others (Smith & Stohlman, 1940). Jaffe (1976) found that the
toxicity of seleniferous sesame meal for rats was markedly
decreased when the diets were supplemented with L-lysine, the
limiting essential amino acid in sesame proteins. Lysine was
thought to protect against selenium poisoning indirectly by
improving the biological value of the sesame proteins.
Linseed meal contains a unique non-proteinaceous factor that
is highly effective in protecting against selenium poisoning
(Halverson et al., 1955; Levander et al., 1970). The results of
recent work suggest that the protective activity of linseed meal
may reside in 2 newly-isolated cyanogenic glycosides (Palmer et
al., 1980; Smith et al., 1980). Cyanide has a partially protective
effect against selenium poisoning in rats (Palmer & Olson, 1979)
and increases the occurrence of nutritional myopathy in lambs
(Rudert & Lewis, 1978).
There is conflicting evidence as to the ability of methyl-
donating compounds to protect against selenium toxicity (Rosenfeld
& Beath, 1964). Methionine was shown to protect against chronic
selenite poisoning in rats, but only when vitamin E or other fat-
soluble antioxidants were in the diet (Levander & Morris, 1970).
Vitamin E deficiency increased the susceptibility of rats to
chronic poisoning by selenium as selenite (Witting & Horwitt, 1964)
and pigs deficient in vitamin E and selenium were more susceptible
to acute selenium toxicosis than non-deficient pigs (Van Vleet et
al., 1974).
7.1.6.3. Arsenic
Selenium poisoning caused by feeding rats seleniferous wheat
was decreased by giving sodium arsenite in the drinking-water
(Moxon, 1938). The protective effect of arsenic against selenium
toxicity might be explained by the increased biliary excretion of
selenium caused by arsenic (Levander & Baumann, 1966). However,
the use of arsenic compounds did not prove to be a practical way of
controlling selenium poisoning in livestock (Olson, 1969). Also,
arsenic does not protect against all forms of selenium, since it
potentiates the toxicity of the trimethylselenonium ion (Obermeyer
et al., 1971).
7.1.6.4. Sulfate
Dietary sulfate partially counteracts the toxicity of selenate
in rats, but has little or no effect against selenite or organic
forms of selenium (Halverson et al., 1962). Sulfate apparently
increases the urinary excretion of selenium fed as selenate,
thereby reducing the retention of selenium in the internal organs
(Ganther & Baumann, 1962).
7.1.6.5. Adaptation
Ermakov & Kovalskij (1968) fed 18 adult female sheep from
seleniferous and normal localities their own respective diets or
these diets plus an additional 2 mg selenium, as selenite, daily
for 28 days. The 18 sheep were divided into 6 equal groups,
according to their history of selenium intake and, in the case of
the seleniferous sheep, according to the presence or absence of
signs of selenium toxicity. After 28 days, all the sheep were
killed, and selenium levels and acid and alkaline phosphatase
activities were determined in several tissues. Selenium levels and
alkaline phosphatase activities are presented in Table 29. The
increase in selenium retention in the blood, kidneys, and most of
the other internal organs, produced by supplementing the feed with
selenite, was less in sheep from high-selenium localities than in
animals from control localities. Retention in the liver seemed to
be an exception. The increase in alkaline phosphatase activity due
to the selenite load was much clearer in sheep from the control
localities than in the sheep from the seleniferous localities. The
alkaline phosphatase activity of the pancreas in sheep from the
high-selenium locality, manifesting selenium toxicity signs, was an
exception being much higher after the selenite load (370 ± 57
compared with 84 ± 34). These differences in the response of the
sheep to selenite loading were interpreted by the authors as
evidence of the adaptation of the animals to high levels of
selenium exposure.
Table 29. Effect of high selenium (Se) load on selenium metabolism and alkaline phosphatase activity in sheep
previously exposed to normal and high selenium intakes in foragea
----------------------------------------------------------------------------------------------------------------
History Daily Se intake Skeletal Alkaline phosphatase activity
of sheep Plant Selenite Total Blood muscle Liver Kidney kidney liver skeletal
Se Se Se Se Se Se Se muscle
(µg) (µg) (µg) (µg/litre) (µg/kg) (µg/kg) (µg/kg) (units/100 g tissue)
----------------------------------------------------------------------------------------------------------------
High 1821 2000 3821 411 ± 18 230 ± 24 600 ± 44 870 ± 69 286 ± 148 448 ± 43 13.0 ± 2.7
Se-curved
hoofs
High 1944 0 1944 290 ± 18 180 ± 7 330 ± 20 760 ± 26 362 ± 89 374 ± 117 9.5 ± 3.0
Se-curved
hoofs
High 1774 2000 3774 362 ± 34 210 ± 16 590 ± 90 1000 ± 174 309 ± 103 335 ± 18 9.4 ± 2.9
Se-curved
hoofs
High 2038 0 2038 239 ± 34 170 ± 6 340 ± 20 890 ± 21 349 ± 63 344 ± 56 9.5 ± 1.2
Se-curved
hoofs
Normal Se 376 2000 2376 408 ± 23 170 ± 8 450 ± 38 950 ± 77 496 ± 53 457 ± 57 14.8 ± 3.0
Normal Se 396 0 396 100 ± 12 89 ± 5 190 ± 20 780 ± 69 143 ± 29 254 ± 7 5.5 ± 0.8
----------------------------------------------------------------------------------------------------------------
a Adapted from: Ermakov & Kovalskij (1968).
Jaffe & Mondragon (1969) presented evidence that animals could
adapt to long-term selenium intake. Hepatic-selenium levels
decreased steadily in young rats fed a diet containing 4.5 mg
selenium/kg as seleniferous sesame meal, if the rats came from
mothers that had been fed the same seleniferous diet, but the
levels increased if the rats came from mothers fed a non-
seleniferous stock diet. It was concluded that an adaptation
mechanism existed that allowed rats exposed to long-term selenium
ingestion to store less of this element than previously unexposed
controls. A later report (Jaffe & Mondragon, 1975) confirmed these
results and also indicated that the hepatic lesions, splenomegaly,
and other toxicity signs were significantly decreased in adapted
rats.
One mechanism for a possible adaptive response to high levels
of selenium could involve effects of selenium intake on various
selenium metabolic pathways, since previous selenium exposure has
been shown to have an influence on the whole body retention of
selenium and the urinary and pulmonary excretion of selenium
(Ganther et al., 1966; Ewan et al., 1967; Burk et al., 1973)
(section 6.3, 6.4). However, as discussed in section 7.1.2.1, more
recent work has shown that previous selenium exposure directly
influences the toxicity of final selenium metabolites such as
dimethyl selenide and the trimethylselenonium ion (Parizek et al.,
1976, 1980).
7.1.7. Mechanism of toxicity
The mechanism of selenium toxicity remains unclear and the
modes of action of various selenium compounds, such as hydrogen
selenide, selenomethionine, and selenium dioxide are likely to be
quite different. Thus, no unifying hypothesis regarding the toxic
effects of selenium compounds is possible. Various hypotheses
concerning the mechanisms of selenium toxicity have been presented
(Rosenfeld & Beath, 1964; Izraelson et al., 1973; Ermakov &
Kovalskij, 1974; US NAS/NRC, 1976; Lazarev, 1977; Levander, 1982).
Although it appears unlikely that selenite interferes directly with
sulfhydryl enzymes (Tsen & Tappel, 1958; Tsen & Collier, 1959), it
is possible that selenite may interfere with glutathione metabolism
(Vernie et al., 1978; Chung & Maines, 1981; Vernie, 1984; Anundi et
al., 1984) and that this may affect enzyme activities. The
apparent pro-oxidant nature of high levels of selenite has been
reported by several workers (Witting & Horwitt, 1964; Csallany et
al., 1984), and this may be related to some aspects of selenite
toxicity.
7.2. Selenium Deficiency
7.2.1. Animal diseases
The nutritionally beneficial effects of selenium were first
reported in 1957 by Schwarz, who discovered that sodium selenite
prevented dietary liver necrosis in vitamin E-deficient rats
(Schwarz & Foltz, 1957). This discovery led to the rapid
recognition of vitamin E-related selenium-responsive deficiency
diseases in several species of farm animals. Diseases such as
white muscle disease in sheep and cattle, hepatosis dietetica in
swine, and exudative diathesis in poultry are economically
significant problems in areas of the world where the soil levels of
selenium available for uptake by plants are low. Schwarz & Foltz
(1958) also reported that feeding a diet deficient in both selenium
and vitamin E to mice resulted in multiple necrotic degeneration of
several organs; more recently, Suchkov et al. (1977, 1978) found
that feeding such diets to mice for 13 days caused a decreased
staining intensity for zinc in the pancreas, kidneys, and testes.
The fact that vitamin E as well as selenium tended to protect
against many of these diseases led some research workers to
question whether there was any requirement for selenium in animals
receiving adequate amounts of vitamin E. But, the results of more
recent work have clearly demonstrated the need for selenium, even
in animals given nutritionally adequate levels of vitamin E.
Deficiency signs specific for selenium included alopecia, vascular
changes, cataract, poor growth, and reproductive failure in second
generation selenium-deficient rats (McCoy & Weswig, 1969; Wu et
al., 1979). Pancreatic degeneration occurred in selenium-deficient
chicks with a normal intake of vitamin E (Thompson & Scott, 1970),
but the condition could be prevented by feeding very high
levels (> 300 µg/kg) of vitamin E or other antioxidants (Whiteacre
et al., 1983).
Changes in the myocardial parenchyma and increases in heart
weight have been observed in albino rats fed corn and vegetables
grown in the areas in which Keshan disease is endemic (Su et al.,
1982), and swine fed grain from endemic areas for 6 months
exhibited multiple myocardial necrosis as well as other lesions
(Zhu et al., 1981). Vitamin E deficiency exacerbated the
pathological and histochemical changes in the heart and liver of
piglets fed a low selenium diet composed mainly of cereals grown in
the Keshan disease area (Zhu et al., 1981).
7.2.2. Intakes needed to prevent deficiency
7.2.2.1. Quantitative dietary levels
Schwarz (1961) showed that 0.02 - 0.03 mg selenium/kg diet, in
the form of sodium selenate or selenite, afforded 50% protection
against liver necrosis in vitamin E-deficient rats over an
experimental period of 30 days. Selenite-selenium at 0.10 mg/kg
protected the rats over their entire life span in the absence of
vitamin E. Under these conditions, the rats developed severe
tocopherol deficiency, which mainly afflicted the central nervous
system. Sprinker et al. (1971) noted that 0.10 mg selenium/kg, as
selenite, prevented specific selenium deficiency lesions in rats
that had been fed a vitamin E-supplemented diet for 2 generations.
Thompson & Scott (1969) found that administration of 0.02 - 0.05 mg
selenium/kg, as selenite, prevented death and exudative diathesis
in chicks fed diets containing typical levels of vitamin E
(sections 7.2.4.1 and 7.2.7.2 include discussions on the
nutritional interrelationship of selenium and vitamin E).
Considerable field experience in New Zealand has indicated that
feeds from pastures associated with selenium-responsive
unthriftiness in sheep contain 0.008 - 0.030 mg selenium/kg
(Hartley, 1967). In areas where selenium-responsive diseases do
not occur, the feed levels of selenium range from 0.020 - 0.098
mg/kg. Since there is some overlap in these selenium levels, other
factors may be involved in the etiology of this selenium-responsive
disease (various factors that can influence selenium deficiency
are discussed in section 7.2.7).
It has been concluded that "the critical level for dietary
selenium, below which deficiency symptoms are observed, is
apparently about 0.02 mg/kg for ruminants and 0.03 - 0.05 mg/kg for
poultry" (US NAS/NRC, 1971). However, this US NAS/NRC committee
pointed out that "when supplementary selenium is fed, higher levels
than the minimal requirements have been proposed, both to permit
satisfactory distribution of the element through the large feed
mass and to overcome variations in feed intake by individual
animals". The final recommendation of the committee was that 0.1
and 0.2 mg selenium should be added per kg feed to eliminate
selenium deficiency in livestock and turkeys, respectively. In
laboratory rats, a dietary-selenium level of 0.1 - 0.2 mg/kg would
be equivalent to an intake of about 5 - 10 µg/kg body weight per
day.
7.2.2.2. Bioavailability
7.2.3. Blood and tissue levels in deficiency
Hartley (1967) presented data on the blood-selenium levels of
normal New Zealand lambs and lambs suffering from various selenium-
responsive diseases (Table 30). The mean blood-selenium levels
were lower in the diseased lambs than in the healthy ones, but
there was considerable overlap between the two groups. It was
suggested that other unknown factors might be involved in the
etiology of these diseases. Jacobsson et al. (1970) found blood-
selenium levels of 0.003 mg/litre in cows afflicted with muscular
degeneration, whereas healthy cows had an average of 0.046
mg/litre. Hartley (1967) estimated that blood-selenium levels of
0.05 mg/litre in young sheep could be considered satisfactory.
The same authors compared liver-selenium levels found in normal
lambs with those found in lambs with selenium-responsive
unthriftiness (Table 31). Again, there was some overlap between
the two groups, but the average level of selenium in the livers
from the deficient lambs was much lower than that from the healthy
lambs. Allaway et al. (1966) suggested that 0.21 mg/kg (dry
weight) is the critical minimal hepatic-selenium level below which
a high incidence of white muscle disease can be expected in lambs.
Table 30. Blood-selenium levels in normal
and selenium-deficient lambsa
---------------------------------------------
Group Selenium contentb
(mg/litre)
---------------------------------------------
Normalc 0.026 (0.014 - 0.048)
White muscle diseased 0.016 (0.006 - 0.033)
Normale 0.06 (0.020 - 0.195)
Selenium-responsive 0.01 (0.007 - 0.030)
unthriftinessf
---------------------------------------------
a Adapted from: Hartley (1967).
b Mean with range in parentheses.
c Normal lambs from areas with white muscle
disease.
d Lambs with white muscle disease.
e Lambs from areas where selenium-responsive
diseases are not recognized.
f Lambs with selenium-responsive
unthriftiness.
Table 31. Liver-selenium levels in normal
lambs or lambs with selenium-responsive
unthriftinessa
------------------------------------------
Group Selenium contentb
(mg/kg)
------------------------------------------
Normalc 0.16 (0.03 - 0.40)
Selenium-responsive 0.018 (0.005 - 0.05)
unthriftinessd
------------------------------------------
a Adapted from: Hartley (1967).
b Mean with range in parentheses.
c Lambs from areas where selenium-
responsive diseases are not
recognized.
d Lambs with selenium-responsive
unthriftiness.
7.2.4. Physiological role: glutathione peroxidase
7.2.4.1. Function of selenium and relationship to vitamin E
The discovery that selenium is a component of the enzyme
glutathione peroxidase (Rotruck et al., 1973) provides a logical
explanation for the nutritional interaction of vitamin E and
selenium, a puzzle that perplexed scientists for many years.
Analysis of purified ovine erythrocyte glutathione peroxidase by
fluorometry revealed that the protein contained 4 moles of selenium
per mole of enzyme (Hoekstra et al., 1973) and this stoichiometry
was also found for crystalline bovine erythrocyte-glutathione
peroxidase analysed for selenium by neutron activation analysis
(Flohe et al., 1973). In the scheme postulated by Hoekstra
(1975a), tocopherols act as intracellular antioxidants to prevent
oxidative damage to polyunsaturated fatty acids in biological
membranes by terminating chain reactions of lipid peroxides (Fig.
7). Selenium, as a part of glutathione peroxidase, protects
against oxidative stress either by catalysing the destruction of
hydrogen peroxide or by catalysing the decomposition of lipid
hydroperoxides, thereby interrupting the free radical peroxidative
chain reaction; in this latter role the free fatty acyl
hydroperoxide must be liberated by phospholipan A2 action from the
hydrophobic region of biological membranes, before its reduction
can be catalysed by glutathione peroxidase (Grossman & Wendel,
1983). Thus, both of these nutrients play separate but
interrelated roles in the cellular defence mechanisms against
oxidative damage.
6.5.1. Animal studies
6.5.1.1. Reduction and methylation
The main flow of selenium metabolism in animals is via
reductive pathways, which contrasts with the primarily oxidative
metabolism of sulfur (Levander, 1976a). Selenite can react with
glutathione or protein sulfhydryls to form selenotrisulfides
(Ganther, 1968; Jenkins & Hidiroglou, 1971) which, at least in the
case of the glutathione derivatives, can be reduced further by an
enzymatic mechanism to selenide (Ganther & Hsieh, 1974; Diplock,
1976).
Under normal conditions, selenide is methylated to form
trimethylselenonium ion, the main urinary metabolite of selenium
(section 6.3.1). In cases of selenium toxicity, this pathway is
overloaded and dimethyl selenide is produced. This is the main
volatile selenium metabolite expired via the lungs and is
responsible for the typical "garlicky odour" of animals poisoned
with selenium (McConnell & Portman, 1952a). The last 2 reactions
could be considered detoxication steps, since the methylated end-
products are much less toxic for the organism than selenite
(McConnell & Portman, 1952b; Obermeyer et al., 1971). However,
both of these methylated selenium derivatives have strong
synergistic toxicity with other minerals (section 7) and dimethyl
selenide toxicity in male rats was reported to be inversely related
to the level of previous selenium intake (Parizek et al., 1980).
6.5.1.2. Form in proteins
Early work with animals poisoned with selenium revealed that
much of the selenium in the tissues was associated with protein
(Smith et al., 1938). Since that time there has been considerable
controversy about the exact chemical nature of the selenium in
tissue proteins (Levander, 1976a). Selenomethionine may be
incorporated initially as such into animal proteins (Ochoa-Solano
& Gitler, 1968), but, in rats, it appears to be eventually
catabolized to selenite or selenate (Millar et al., 1973; Thomson
& Stewart, 1973). Non-ruminant animals cannot synthesize
selenomethionine from inorganic selenium compounds (Cummins &
Martin, 1967; Olson & Palmer, 1976), but rabbits and rats can
convert selenite into selenocysteine tissue proteins (Godwin &
Fuss, 1972; Olson & Palmer, 1976). Forstrom et al. (1978) have
proposed that seleno-cysteine is the form of selenium located at
the catalytic site of glutathione peroxidase and others have shown
that selenocysteine is essential for the activity of clostridial
glycine reductase (Cone et al., 1976). The mechanism by which
selenocysteine is formed from selenite is not known but
incorporation of preformed selenocysteine and post-translational
modification of the protein have been considered (Sunde, 1984).
Other possible forms of selenium in proteins include
selenotrisulfides (Ganther & Corcoran, 1969) and "acid-labile"
selenium (Diplock et al., 1973).
6.5.1.3. Conversion of selenium compounds to nutritionally-active
forms of selenium
The pioneering studies of Schwarz & Foltz (1958) demonstrated
that a variety of selenium compounds could protect against dietary
liver necrosis in vitamin E- and selenium-deficient rats. Sodium
selenite, sodium selenate, selenium dioxide, selenic acid, and
potassium selenocyanate were all more or less equally active, but
elemental gray selenium was essentially inactive. Selenocystine
and selenomethionine were about as effective as the active
inorganic selenium compounds, but an organic selenium fraction
isolated from pig kidney powder (Factor 3) was shown to be even
more active. A number of organic derivatives of selenium have
shown some protective effect against liver necrosis, but none was
superior to Factor 3 (Schwarz et al., 1972). However, certain
simple amino-acid derivatives of monoseleno diacetic acid showed
some promise in that they combined high nutritional potency with a
low order of toxicity (Schwarz, 1976). The effects of selenite and
selenomethionine were shown to be roughly similar in inducing
glutathione peroxidase activity in the tissues of rats fed a diet
deficient in selenium (Pierce & Tappel, 1977).
Cantor et al. (1975a) found that while selenite and
selenocystine were equally effective in preventing exudative
diathesis in vitamin E- and selenium-deficient chicks, seleno-
methionine was less effective. This suggests a difference between
rats and chicks in the metabolism of selenomethionine. The degree
of protection against exudative diathesis and the level of plasma
glutathione peroxidase activity were highly correlated, suggesting
that nutritional potency depended on the ability of the chick to
convert various selenium compounds to the enzymatically active
form.
6.5.2. Human studies
Few studies have been carried out to investigate the metabolic
transformation of selenium compounds in human beings, but the
limited data available suggest certain similarities in the
metabolism of selenium by man and animals. For example, human
beings overexposed to selenium develop breath with a smell of
garlic, which is presumably due to the exhalation of dimethyl
selenide (Glover, 1976). Also, the chromatographic pattern of
urinary-selenium metabolites is the same in man and the rat (Burk,
1976). However, species differences in selenium metabolism do
exist, since human beings retain selenomethionine to a much greater
extent than selenite, whereas the retention of the 2 compounds in
rats is about the same (section 6.4.2).
7. EFFECTS OF SELENIUM ON ANIMALS
The considerable biological importance of selenium was first
recognized in the 1930s when it was discovered that certain well-
defined and economically important farm animal diseases were
actually the result of chronic selenium poisoning (section 7.1).
These animal diseases were restricted to agricultural areas in
which large amounts of selenium in the soil were available for
uptake by the plants, which were then consumed by the animals.
Research, over the last 20 years, showed that selenium was an
essential trace element (section 7.2), and selenium deficiency
diseases were rapidly recognized in several species of farm
animals. These deficiency diseases are significant economic
problems in areas of the world where the soil levels of the
element available for uptake by plants are low.
7.1. Selenium Toxicity
7.1.1. Farm animal diseases associated with a high selenium intake
On the basis of field experience, Rosenfeld & Beath (1964)
delineated 3 different types of selenium poisoning in livestock:
(a) acute;
(b) chronic, of the blind staggers type; and
(c) chronic, of the alkali disease type.
Acute poisoning is due to the ingestion of toxic quantities of
selenium in the form of highly seleniferous accumulator plants.
The animal has severe signs of distress such as laboured breathing,
abnormal movement and posture, prostration, and diarrhoea. Death
often follows within a few hours. This type of selenium poisoning
is rather rare under field conditions, since grazing animals
generally avoid the selenium accumulator plants, except in times of
pasture shortage. Acute selenium poisoning has also been produced
by the experimental or accidental administration of selenium
compounds to farm animals (US NAS/NRC, 1976).
Blind staggers has been reported in animals that eat a limited
number of selenium accumulator plants over a period of weeks or
months (Rosenfeld & Beath, 1964). The affected animals wander,
stumble, have impaired vision, and eventually succumb to
respiratory failure. Although this type of poisoning can be
produced experimentally by the administration of water extracts of
accumulator plants, it has not been possible to duplicate this
syndrome by the administration of pure selenium compounds.
Possibly alkaloids or other toxic substances found in many
seleniferous plants may contribute to blind staggers (Maag & Glenn,
1967; Van Kampen & James, 1978).
Alkali disease is associated with the consumption of grains
containing 5 - 40 mg selenium/kg over weeks or months. Animals
exhibit liver cirrhosis, lameness, hoof malformations, loss of
hair, and emaciation. Maag & Glenn (1967) were unable to produce
alkali disease in cattle by feeding inorganic selenium, but several
other studies have shown that the syndrome is causally associated
with seleniferous grains or grasses and can be produced by feeding
inorganic selenium salts (Olson, 1978).
The Task Group noted that most of the work on alkali disease
was concerned with cattle, but was aware of the report by Ermakov &
Kovalskij (1968), which described chronic selenium toxicity of this
type in sheep, under natural conditions. In sheep fed feeds
containing levels of 2 mg selenium/kg feed (fresh weight), the
following characteristic signs were observed: hoof deformation,
loss of hair, hypochromic anaemia, and increases in the activity of
both alkaline and acid phosphatases in various tissues.
7.1.2. Toxicity in experimental animals
The practical significance of selenium poisoning in farm
animals stimulated a great deal of research on both the acute and
chronic effects of selenium in laboratory animals. Interest in the
toxic effects of repeated exposure to selenium via inhalation was
stimulated by concern about the possible effects on human health of
occupational exposure to selenium. Also, studies were carried out
with the aim of establishing the no-observed-adverse-effect dose
level of selenium when administered in the drinking-water. In some
of the studies in which selenium was given via either the air or
water, biochemical and/or behavioural criteria were used to assess
the biological effects of selenium exposure. However, as discussed
in section 7.1.6, the toxicity of selenium compounds can be
influenced by different variables and the results from various
laboratories are often not comparable because of quite different
experimental conditions. Also, the criteria for toxicity are less
developed than the signs of deficiency (section 7.2).
Reviews that deal with various aspects of selenium toxicology
include those by Rosenfeld & Beath (1964), Muth (1966), Izraelson
et al. (1973), Ermakov & Kovalskij (1974), US NAS/NRC (1976), and
Lazarev (1977).
7.1.2.1. Acute and subacute toxicity - single or repeated exposure
studies with oral, intraperitoneal, or cutaneous administration
Perhaps the most characteristic sign of acute selenium
poisoning in animals is the development of the so-called "garlicky
breath odour", which is due to the pulmonary excretion of volatile
selenium compounds, particularly dimethyl selenide, by animals
overexposed to selenium (section 6.3.1). Other signs of acute
selenium poisoning described by Franke & Moxon (1936) in dogs and
rats included: vomiting, dyspnoea, tetanic spasms, and death from
respiratory failure. Pathological changes included congestion of
the liver with areas of focal necrosis, congestion of the kidney,
endocarditis, myocarditis, peticheal haemorrhages of the
epicardium, atony of the smooth muscles of the gastrointestinal
tract, gall-bladder, and bladder, and erosion of the long bones,
especially the tibia. The LD50 values for sodium selenite,
administered orally to various animal species, are given in Table
18 (Pletnikova, 1970).
Table 18. Acute oral toxicity of sodium selenite for various
species of laboratory animalsa
-------------------------------------------------------------
Species LD50 Statistical method
(mg selenium/kg
body weight)
-------------------------------------------------------------
White mouse (male) 7.75 Behrens & Schlosser
7.08 Litchfield & Wilcoxon
Albino rat (female) 10.50 Behrens & Schlosser
13.19 Diechmann & LeBlanc
Guinea-pig (female) 5.06 Deichmann & LeBlanc
Rabbit (female) 2.25 Diechmann & LeBlanc
-------------------------------------------------------------
a From: Pletnikova (1970).
Table 19. Acute toxicity of some selenium compounds administered to rats by
intraperitoneal injection
----------------------------------------------------------------------------
Compound Criterion Toxic dose Reference
of (mg selenium/kg
toxicity body weight)
----------------------------------------------------------------------------
Sodium selenite MLD 3.25 - 3.5 Franke & Moxon (1936)
Sodium selenate MLD 5.25 - 5.75 Franke & Moxon (1936)
DL-selenocystine MLD 4 Moxon (1940)
DL-selenomethionine MLD 4.25 Klug et al. (1950)
Diselenodipropionic LD50 25 - 30 Moxon et al. (1938)
acid
Trimethylselenonium LD50 49.4 Obermeyer et al. (1971)
chloride
Dimethyl selenide LD50 1600 McConnell & Portman (1952b)
----------------------------------------------------------------------------
For a given species, the lethal doses of sodium selenite,
sodium selenate, DL-selenocystine, and DL-selenomethionine are
quite similar (Table 19). Although certain methylated metabolites
of selenium such as dimethylselenide and trimethylselenonium
chloride were considered relatively innocuous (see LD50 values by
McConnell & Portman and Obermeyer et al. in Table 19), more recent
work by Parizek et al. (1976, 1980) has shown that the toxicity of
methylated selenium compounds depends not only on the sex of the
animal (Parizek et al., 1974) but also on the level of previous
selenium intake. For example, 90% mortality was observed in male
rats maintained on a diet containing 0.05 mg selenium/kg, when they
were injected intraperitoneally with 20 µmoles dimethylselenide/kg
body weight, i.e., by a dose of dimethylselenide that is more than
1000 times lower than the LD50 reported by McConnell & Portman
(1952b). Pre-treatment with a small amount of selenite (1 µmol/kg
body weight), intraperitoneally, 6 h, but not 1 h, before
dimethylselenide injection or increased oral intake of selenite
(Table 20), protected male rats against the toxicity of a
subsequent dose of dimethylselenide (Parizek et al., 1976, 1980).
Moreover, the methylated forms of selenium have strong synergistic
toxicities with other minerals (section 7.1.6.3, 7.4.1) and
dimethylselenide can be much more toxic for male rats than for
female rats (section 7.1.6).
Table 20. Dependence of the toxicity of dimethylselenide on
the level of previous oral intake of seleniuma
------------------------------------------------------------
Selenite Single intraperitoneal 24-h mortality (%)
supplement in dose of dimethylselenide Diet A Diet B
drinking-water
(mg selenium/ (mg mole/kg body weight) (n = 20) (n = 10)
litre
------------------------------------------------------------
0 20 90 90
0.1 20 45 30
0.5 20 5 0
1.0 20 0 0
------------------------------------------------------------
a Adapted from: Parizek et al. (1976, 1980).
Male rats (2 months old) given drinking-water with stated
supplement of selenite for 3 days before dimethylselenide
administration. Diet A contained 0.052 ± 0.005 mg selenium/kg and
diet B (semi-synthetic diet) 0.044 ± 0.001 mg selenium/kg.
Smith et al. (1937) reported that the minimum lethal dose
of selenium as sodium selenite or selenate in rabbits, rats,
and cats was 1.5 - 3.0 mg/kg body weight, regardless of whether
the compounds were administered orally, subcutaneously,
intraperitoneally, or intravenously. This lack of effect of the
mode of administration probably reflects the rapid and complete
absorption of soluble selenium compounds, either from the site of
injection or from the gastrointestinal tract.
Cummins & Kimura (1971) described comparative studies on
Sprague Dawley rats and dogs concerning the oral toxicity of the
following selenium compounds: sodium selenate, selenourea,
biphenylselenium, selenium sulfide (1 - 30 µ particle size), and
elemental selenium (1 - 30 µ particle size). The oral LD50 values
in rats for a number of selenium compounds are shown in Table 21
and demonstrate the large variations in LD50 values that occur,
depending on both the oxidation state of the compound and its
aqueous solubility.
Table 21. Comparative solubility and toxicity of various
selenium compounds in ratsa
--------------------------------------------------------------
Compound Solubility in 0.01 N HCl Rat oral LD50 (95% CL)
(mg/kg body weight)
--------------------------------------------------------------
Na2SeO3 700 g/litre 7 (4.4 - 11.2)
H2N-C-NH2 30 g/litre 50 (35.7 - 70.0)
||
Se
SeS2 insoluble (< 1 g/litre) 138 (110 - 172)
Se 5 g/litre 360 (308 - 421)
Elemental Se insoluble (< 1 g/litre) 6700 (6000 - 7300)
--------------------------------------------------------------
a From: Cummins & Kimura (1971).
Male Sprague Dawley rats, each weighing between 50 and 100 g,
were given the above chemicals by gavage as 0.1 - 20% suspensions
in 0.5% methylcellulose. A total of 30-36 animals was used per
compound, in groups of 6 animals per dose. The LD50 values and
the associated confidence limits (CL) were calculated according to
the method of Litchfield & Wilcoxon.
The aqueous solubilities were carried out in 0.01 N HCl to more
closely simulate acidic conditions in the stomach. The least toxic
selenium compound was insoluble elemental selenium with an LD50 of
6.7 g/kg body weight. Toxic signs included pilomotor activity,
decreased body activity, dyspnoea, diarrhoea, anorexia, and
cachexia. Fatalities occurred within 18 - 72 h; survivors appeared
outwardly normal, at the end of the 7-day observation period. The
most toxic of the selenium compounds tested was the highly soluble
sodium selenite with an oral LD50 of 7 mg/kg body weight and the
toxic signs were similar to those seen with high doses of elemental
selenium. Selenium sulfide (a component in shampoos) was about 20
times less toxic than sodium selenite (i.e., an LD50 of 138 mg/kg
compared with 7 mg/kg). It was also found in this study that, with
the exception of biphenyl selenium, toxicity could be correlated
with blood-selenium levels. For example, sodium selenite being the
most toxic, gave the highest blood level followed in descending
order by selenourea, selenium sulfide, and elemental selenium. It
was suggested that the blood-selenium level produced by the
relatively non-toxic biphenyl selenium was high because this
covalently-bound selenium compound is the most lipophilic compound,
which is better absorbed, resists catabolism, and apparently
circulates as the parent compound.
The application of 83 mg of selenium oxychloride to the skin of
rabbits caused the death of the animals in 5 h, and application of
4 mg caused death in 24 h (Dudley, 1938). Lazarev (1977) reported
a minimum lethal dose of selenium oxychloride of 7 mg/kg body
weight, after cutaneous administration to rabbits.
Several papers have shown that injection of selenite in rats in
the early postnatal period in single doses of 1.5 mg selenium/kg
body weight, or in repeated doses of 0.5 mg/kg, induced cataracts
(Ostadalova et al., 1979; Bhuyan et al., 1981; Shearer et al.,
1980; Bunce et al., 1985). Similar effects have not been observed
in hamsters (Shearer et al., 1980). This response is dose
dependant (Table 22) and, thus far, has been observed only when the
selenite was given by parenteral injection.
Table 22. Cataractagenesis by selenite in the rata
--------------------------------------------------
Group Daily dosageb Frequency of cataractsc
(mg selenium/kg (%)
body weight)
--------------------------------------------------
Control 0 0
Na2SeO3 0.25 13
Na2SeO3 0.50 96
Na2SeO3 0.75 96
--------------------------------------------------
a Adapted from: Shearer et al. (1980).
b Dose given to rat pups daily on days 2-18
postpartum.
c Observed at 21 days of age.
7.1.2.2. Effects of long-term oral exposure
Moxon & Rhian (1943) summarized several older studies that
indicated that diets containing 5 mg selenium/kg or more cause
chronic selenosis in several species of animals, such as chickens,
rats, and dogs. In seleniferous areas, 5 mg selenium/kg diet is
generally accepted as the dividing line between toxic and non-toxic
feeds (US NAS/NRC, 1976).
Halverson et al. (1966) fed diets containing 0, 1.6, 3.2, 4.8,
6.4, 8.0, 9.6, or 11.2 mg selenium/kg in the form of sodium
selenite or seleniferous wheat to male Sprague Dawley rats,
initially weighing 60 - 70 g. The rats were divided into groups of
8 and were fed the diets for 6 weeks. The diet consisted of (g/kg)
ground wheat, 809; purified casein 120; USP salt mixture XIV, 20;
USP brewer's yeast, 20; corn oil, 30; and vitamin B12 mix, 1.
Vitamins A, D, and E were provided separately. The criteria used
to assess toxicity were growth depression, liver cirrhosis,
splenomegaly, pancreatic enlargement, anaemia, elevated
serumbilirubin levels, and death. The addition to the diet of 1.6,
3.2, or 4.8 mg selenium/kg did not have any significant effect on
the rats, as judged by any of these criteria. There was a
depression in growth in the group that received 4.8 mg dietary
selenium/kg as sodium selenite, but this was not significant.
Liver cirrhosis, splenomegaly, and significant growth depression
were observed when the rats were fed levels of 6.4 mg/kg or more
from either source of selenium. Diets containing 8.0 mg/kg or more
caused additional effects such as pancreatic enlargement, anaemia,
elevated serum-bilirubin levels, and, after 4 weeks, death.
Diets made either with non-seleniferous or seleniferous sesame
meal were fed to groups of 12 male Sprague Dawley rats, initially
weighing 50 g for a period of 6 weeks (Jaffe et al., 1972b). The
diet made with non-seleniferous sesame meal contained 0.5 mg
selenium/kg and consisted of (g/kg): non-seleniferous sesame meal,
463.3; almidon, 422.7; corn oil, 50; cod liver oil, 10; USP XVI
salts, 40; L-lysine x HCl, 4; and vitamin mix, 10. The diet made
with the seleniferous sesame meal contained 10 mg selenium/kg and
had the same composition as the previous diet except that
seleniferous sesame, which replaced the non-seleniferous sesame
meal, was added at a level of 490.2 g/kg, and the almidon was added
at a level of 395.8 g/kg. The rats fed the diet containing the
seleniferous sesame meal showed decreased survival, impaired weight
gain, higher incidence of liver lesions, elevated hepatic selenium
levels, enlarged spleens, depressed haemoglobin, haematocrit, and
fibrinogen levels, and decreased prothrombin activity (Table 23).
In a separate study, the same workers investigated the effects of
dietary selenium, given as seleniferous sesame meal, on the
activities of various serum enzymes (Table 24). The diet
containing 4.5 mg selenium/kg had the same composition as the
previous diets, except that the seleniferous sesame meal, non-
seleniferous sesame meal, and almidon were added at levels of
230.8, 270.4, and 384.8 g/kg, respectively. Feeding the diet
containing 4.5 mg of selenium/kg for 6 weeks increased the
activities of serum alkaline phosphatase (EC 3.1.3.1) and glutamic
pyruvic transaminase (SGPT) (EC 2.6.1.2), whereas 10.0 mg/kg also
increased the activity of glutamic-oxaloacetic transaminase (SGOT)
(EC 2.6.1.1).
Table 23. Pathological signs in rats fed diets containing seleniferous sesame meala
--------------------------------------------------------------------------------------------------------------------
Dietary Survival Weight Rats Hepatic Spleen Haemo- Haema- Fibrinogen Prothrombin
selenium after gain with selenium weight globin tocrit activity
6 weeks after hepatic level level value
6 weeks lesions
--------------------------------------------------------------------------------------------------------------------
(mg/kg) (g) (mg/kg) (% of body (g/litre (volume %) (mg/litre)
weight) blood)
0.5 12/12 156.8 ± 7.2 0/12 0.72 ± 0.14 0.207 ± 0.01 147 ± 2.1 43.9 ± 0.61 1664 ± 78 963 ± 9.5
10 8/12 61.2 ± 7.43 10/12 7.34 ± 0.97 0.544 ± 0.09 123 ± 3.5 40.8 ± 0.82 655 ± 78 713 ± 76.6
--------------------------------------------------------------------------------------------------------------------
a Adapted from: Jaffe et al. (1972b).
Table 24. Effect of chronic selenium toxicity on the
activity of serum enzymesa
-------------------------------------------------------
Dietary Number Alkaline SGOT SGPT
selenium of rats phosphatase
-------------------------------------------------------
(mg/kg) (units/ml) (units/ml) (units/ml)
0.5 6 6.3 ± 0.2 66 ± 3 20 ± 1
4.5 10 8.8 ± 1.3 59 ± 2 26 ± 2
10 8 18.9 ± 2.3 77 ± 5 43 ± 6
-------------------------------------------------------
a Adapted from: Jaffe et al. (1972b).
Tinsley et al. (1967) and Harr et al. (1967) carried out an
extensive study on the chronic toxicity of selenium in rats.
Although the primary purpose of their research was to investigate
the alleged carcinogenicity of selenium (section 7.7.1), the design
of their study provided an opportunity to examine other aspects of
the toxicity of selenium, such as the influence of selenium
poisoning on growth rate and histopathology. A total of 1437
Wistar rats from a closed random-bred colony was used with the size
of the experimental groups ranging from 10 - 110 animals. The rats
were fed one of 3 different diets: a semipurified diet containing
either 12 or 22% casein or a commercial "laboratory chow" type
ration. The casein-based diets also contained corn oil, 50 g;
H.M.W. salts, 40 g; vitamin mix, 10 g; and glucose monohydrate
(Cerelose) up to a weight of 1 kg. Selenium as sodium selenite or
sodium selenate was added at levels of 0, 0.5, 2.0, 4.0, 8.0, or
16.0 mg/kg. Only a small proportion of the animals fed diets
supplemented with more than 4.0 mg/kg of selenium survived for 12
months. The rats fed the commercial ration were 2 - 3 times more
resistant to the effects of selenium toxicity than the rats fed the
semipurified diet. A calculated maximum body weight was reported
to be depressed by as little as 0.5 mg selenium/kg, but no
statistical evaluation of the results was presented. Usually
moribund animals were killed and necropsied for histopathological
determinations, but 136 rats were killed at specific ages.
Acute toxic hepatitis was the predominant histopathological
lesion in rats fed the commercial ration supplemented with sodium
selenate at 16 mg selenium/kg. These rats had a median survival
age of only 96 days. Acute toxic hepatitis was also observed in
rats fed the 12% casein diet supplemented with sodium selenate at 4
or more mg selenium/kg. The rats were emaciated, pale, and had
poor quality hair coat. Hydrothorax, ascites, pericardial oedema,
and icterus were common. Myocardial hyperaemia, fluid imbalance,
and parenchymal degeneration were often present. The adrenals were
enlarged and the pancreas was oedematous. The failure of normal
chondrocyte proliferation observed in the metaphyses appeared to be
different from that seen in malnutrition, since proliferation was
irregular and not merely reduced.
When rats were fed the commercial ration supplemented with
sodium selenate at 8 mg selenium/kg, the predominant lesion was
chronic toxic hepatitis. The median survival age in this group was
429 days. Other histopathological changes reported included
pancreatic duct hyperplasia, intestinal nephritis, and myocardial
damage, particularly in rats of more than 450 days of age and
receiving selenite.
Harr et al. (1967) reported an increased proliferation of the
hepatic parenchyma when the rats were fed the semi-purified diet
supplemented with 0.5 - 2.0 mg selenium/kg as selenite or selenate.
But a more detailed report from this laboratory (Weswig et al.,
1966) showed that this lesion of "chronic liver and bile duct
hyperplasia" was observed to a greater extent in rats fed the
commercial ration not supplemented with selenium than in rats fed
the semi-purified diet supplemented with 0.5 mg selenium/kg. Thus,
this lesion may not be specifically related to selenium.
Harr & Muth (1972) stated that 0.25 mg/kg was the minimum toxic
level for liver lesions, when selenium was added to a semi-purified
diet. The minimum toxic level was 0.75 mg/kg when the criteria
were longevity or lesions of the heart, kidneys, or spleen.
However, growth was normal in rats fed 0.5 mg selenium/kg diet.
The authors estimated that the dietary threshold for physiological
and pathological effects was 0.4 mg/kg and for pathological and
clinical effects, 3 mg/kg.
Weanling rats of the Long-Evans strain were given either sodium
selenite or selenate at 0 or 2 mg selenium/litre in the drinking-
water for 1 year (Schroeder & Mitchener, 1971b). After 1 year, the
selenium dosage was increased to 3 mg/litre in the selenate group.
The weanlings were born from random-bred females that had been fed
a low-selenium diet (0.05 mg selenium/kg) consisting of: whole rye
flour, 600 g; dry skim milk, 300 g; corn oil, 90 g; and iodized
sodium chloride to which were added vitamins and iron, 1 g/kg. The
same diet was fed to the weanlings during the toxicity study. The
drinking-water was doubly deionized forest spring water to which
had been added: zinc, 50 mg; manganese, 10 mg; chromium(III), 5 mg;
copper, 5 mg; cobalt, 1 mg; and molybdenum, 1 mg/litre. The rats
given the selenium compounds (plus another group not discussed here
given sodium tellurite) were divided into groups totalling 313
animals. There were 105 control rats. By 58 days, half of the
male rats in the selenite group were dead. Fifty percent mortality
in the group of female rats given selenite was not achieved until
348 days and was not achieved in the male and female groups given
selenate until 962 and 1014 days, respectively. On the other hand,
Palmer & Olson (1974) gave 2 or 3 mg selenium/litre drinking-water,
in the form of either sodium selenite or selenate to male weanling
Sprague Dawley rats for 6 weeks and noted small decreases in weight
gain compared with control rats receiving selenium, but no deaths.
Two diets were used in this study, a rye diet similar to that
described by Schroeder & Mitchener above and a corn diet that
consisted of ground corn, 808 g; casein, 120 g; corn oil, 30 g;
U.S.P. XIV salts, 20 g; and vitamin mix, 22 g/kg. The trace
elements zinc, copper, manganese, chromium, cobalt, and molybdenum
were also added to the drinking-water, as suggested by Schroeder &
Mitchener (1971a).
In a study by Jacobs & Forst (1981a), sodium selenite at 0 or 4
mg selenium/litre drinking-water was administered to groups of 17
and 30 male 5-week-old Sprague Dawley rats fed a commercial pellet
ration. After 64 weeks, survival was 94 and 63% in the control and
selenium-treated groups, respectively. In a second study of similar
design, except that the rats were 8 weeks old at the start,
survival was 90 and 95% in the control and selenium-treated groups,
respectively, after 61 weeks.
Pletnikova (1970) investigated the effects of long-term, low-
level administration of sodium selenite in water to rabbits and
rats. For these studies, 32 rabbits and 16 rats were divided into
4 groups and administered, orally, doses of 0, 0.005, 0.0005, or
0.00005 mg selenium/kg body weight for periods of 7 1/2 and 6
months, respectively. Prolonged administration of the maximum dose
investigated (0.005 mg/kg) produced significant alterations in the
rabbits. After 2 months, there was a significant increase in the
concentration of oxidized glutathione in the blood and, after 7
months, there was slower elimination of bromsulphalein by the
liver, and hepatic succinic dehydrogenase activity was decreased.
A dose of 0.0005 mg/kg caused fewer, less pronounced changes,
whereas 0.00005 mg/kg did not produce any statistically significant
effects in any of these tests. A dose of 0.005 mg/kg given to rats
caused a considerable weakening of the capacity for forming new
conditioned reflexes. It can be assumed that a daily dose of 0.005
mg/kg body weight in rats is equivalent to a dietary-selenium level
of about 0.063 mg/kg. Since this level of selenium is within the
physiological range needed to prevent selenium deficiency in
animals (section 7.2.2), the toxicological significance of these
observations is not clear, unless it is assumed that a certain dose
of selenite given in water solution is more toxic than the same
dose given in the diet.
Recently, Csallany et al. (1984) reported that giving sodium
selenite to female mice in the drinking-water at a level of 0.1 mg
selenium/litre increased the amount of hepatic lipid-soluble
lipofuscin pigments, when the animals were sacrificed at 9 months
of age. There were 8 mice in each group and the animals were fed a
diet adequate in vitamin E, which contained 0.05 mg selenium/kg.
Sodium selenite added to the drinking-water at a level of 9 mg
selenium/litre killed all 12 rats fed a diet based on either ground
corn or ground rye in 6 weeks (Palmer & Olson, 1974), whereas
Halverson et al. (1966) found that sodium selenite added to a diet
based on ground wheat, at a level of 9.6 mg/kg, killed only 1 out
of 10 rats in the same period of time. However, the rats used in
the latter study were post-weanling animals weighing 60 - 70 g when
the study started, whereas the rats used in the former study were
21-day-old weanlings and weighed only 35 - 45 g. Other research
workers have shown that the resistance of rats to the toxic effects
of selenium increases markedly after the twenty-first day of life
(Franke & Potter, 1936).
Feng et al. (1985) studied the hepatoxicity of high selenium
corn (7.12 mg/kg) produced in the Enshi county of China, where
human selenium intoxication was reported (section 8.1.1.1). After
feeding a diet that contained 61% of this corn (total selenium
concentration of 4.343 mg/kg) for 16 weeks, liver cirrhosis was
seen in 3 out of 6 male and 5 out of 6 female rats in one group.
No liver damage was found histologically in another group of rats
in the same study, which had consumed a diet containing 30.5% of
the high selenium corn (total selenium concentration of 2.35
mg/kg).
At present, the best indicator of chronic selenium toxicity
appears to be growth inhibition (US NAS/NRC, 1976), and a selenium
level of 4 - 5 mg/kg is necessary to achieve this response in
animals fed a normal diet. In laboratory rats, this exposure to
selenium represents an intake of about 200 - 250 µg/kg body weight
per day. More sensitive and specific criteria of selenium
poisoning to demonstrate effects at lower dose levels, such as
biochemical or histological techniques, would obviously be highly
desirable, but such tests are not available at present.
7.1.2.3. Inhalation toxicity
The effects of respiratory exposure to selenium compounds,
administered under conditions mimicking occupational exposure, have
been described in several papers. Filatova (1951) studied the
toxic effects of respiratory exposure to selenium dioxide (SeO2)
under conditions similar to those that occur in industry, i.e.,
heating of selenium (section 5.2.1). In acute studies, white rats
were exposed to air concentrations of selenium dioxide of 0.15 -
0.6 mg/litre, and all rats died within one-half - 4 h.
Morphological examination of the organs revealed that intraalveolar
and perivascular oedema occurred in the lungs, and haemorrhages and
degenerative changes in the liver, kidney, and heart. In 4
additional studies, all rats survived 4 h when exposed to doses of
0.09, 0.06 - 0.07, or 0.03 - 0.04 mg selenium dioxide/litre, but
all rats exposed to the highest dose (equal to 5 - 5.2 mg/kg body
weight) died within 24 h. In a series of long-term studies, rats
were exposed to repeated doses of selenium dioxide at 0.01 - 0.03,
0.006 - 0.009, or 0.003 - 0.005 mg/litre for 6 h, every other day,
for one month. The lowest dose did not produce any effects on body
weight or on the blood picture, and all the rats survived.
Histological examination revealed degenerative changes in the
liver, renal tubules, dystrophy of heart muscle, and hyperaemia and
hypertrophy of the splenic pulp. At the dose of 0.006 - 0.009
mg/litre, all the rats but one died within 27-33 days. For the
first 2 weeks, there was no difference in body weight between the
exposed and unexposed control rats but, during the last 2 weeks,
the exposed rats lost body weight and all but one of the exposed
rats died. The histopathological changes consisted of multiple
necrosis and degeneration in the liver and myocardial fibres, and
involvement of renal tubules. In the third group of rats, which
was exposed to 0.01 - 0.03 mg/litre, the animals showed respiratory
distress, weight loss, and, in 3 rats, anaemia. All the rats died
between days 8 and 18 of exposure. In the liver, kidneys,
myocardium, and spleen, the changes observed were similar to those
seen at lower doses, but more pronounced. Moreover, lung oedema
similar to that noted in the acute exposure studies was seen.
Lipinskij (1962) exposed 2 groups of 5 rabbits to airborne
amorphous selenium and selenium dioxide in chambers, under
conditions analogous to industrial exposure, except that the doses
were higher. In the first group, the rabbits were exposed to 20 mg
selenium dioxide/litre and 40 mg selenium/m3, for 2 h per day, for
one week, at which time a decrease in blood catalase (EC 1.11.1.6)
activity was noted. The rabbits in the second group were exposed
to 10 µg selenium dioxide/litre and 20 mg selenium/m3, for 2 h
daily, for 12 weeks. After 12 weeks, decreases in total and
reduced glutathione were observed, but there was no change in
levels of oxidized glutathione.
On the basis of studies on 60 white rats weighing 120 - 150 g,
Burchanov et al. (1969) concluded that intratracheal injection of
0.06 ml of a sterile suspension containing 50 mg of highly
dispersed elemental selenium dust in physiological saline, for
1 - 12 months, resulted in decreases in body weight and muscular
strength, and morphological and biochemical alterations in the
respiratory tract. Burchanov (1972) obtained similar results in
inhalation studies on rats exposed to 2 types of polymetallic dusts
found in industry.
The acute toxicity of selenium dust (average mass median
particle diameter, 1.2 µ) for rats, guinea-pigs, and rabbits was
described by Hall et al. (1951). Exposure of these animals, for
16 h, to an atmosphere containing approximately 30 mg selenium
dust/m3 produced mild interstitial pneumonitis in the animals.
Rats exposed to selenium fumes developed acute toxic effects, and
it was suggested that the fumes might have contained some selenium
dioxide.
The acute toxic effects of hydrogen selenide were investigated
in guinea-pigs exposed for 10, 30, or 60 min to concentrations
ranging from 0.002 to 0.57 mg/litre (Dudley & Miller, 1941). All
animals exposed to 0.02 mg/litre, for 60 min, died within 25 days;
93% of those exposed to 0.043 mg/litre, for 30 min, died within 30
days, and all exposed to 0.57 mg/litre, for 10 min, died within 5
days. Decreasing the concentrations of hydrogen selenide, and
increasing the time of exposure to 2, 4, or 8 h produced death in
50% of the guinea-pigs, within 8 h.
Acute studies on the toxicity of selenium hexafluoride (SeF6),
were carried out on the rabbit, guinea-pig, rat, and mouse at 100,
50, 25, 10, 5, and 1 ppm for 4 h. Exposures down to and including
10 ppm (Ct = 40 ppm/h) were uniformly fatal (Kimmerle, 1960).
Exposure to 5 ppm (Ct = 20 ppm/h) resulted in pulmonary oedema from
which the animals recovered, and 1 ppm did not induce any grossly
observable effects. However, with repeated exposure for 1 h daily
at 5 ppm, for 5 days, there were definite signs of pulmonary
injury.
7.1.3. Blood levels in toxicity
Analyses of blood from cattle have shown that, if the average
value of blood-selenium for a herd is over 2 mg/litre, damage from
chronic selenium poisoning is very likely to occur (Dinkel et al.,
1957). Average values of 1 - 2 mg/litre suggest a borderline
problem, especially in reproduction, whereas values below 1
mg/litre indicate that no damage from toxicity should be expected.
Rosenfeld & Beath (1964) commented that typical concentrations of
selenium in the blood in alkali disease, blind staggers, and acute
selenium poisoning were 1 - 2, 1.5 - 4, and up to 25 mg/litre,
respectively. In studies with sodium selenite, Maag et al. (1960)
found that severe selenium toxicity occurred in cattle when the
selenium content of the blood exceeded 3 mg/litre. The selenium
levels in blood associated with chronic toxicity in sheep
corresponded to 0.6 - 0.7 mg/litre (Ermakov & Kovalskij, 1974).
7.1.4. Effects on reproduction
Franke & Potter (1936) fed 7 groups of 6 weanling rats (2 males
and 4 females), 21 days of age, a basal diet consisting of ground
whole wheat, 82%; commercial casein, 10%; pure leaf lard, 3%;
dehydrated yeast, 2%; cod liver oil, 2%; and McCollum's salt
mixture No. 185, 1%. Group 1 was shifted immediately to a toxic
diet in which sufficient control wheat was substituted by
seleniferous wheat to give a final selenium concentration of 24.6
mg/kg diet. Groups 2, 3, 4, 5, and 6 were shifted to the toxic
diet when the rats attained 42, 63, 84, 105, and 186 days of age,
respectively. Group 7 was fed the basal diet throughout the entire
study. No matings were successful in which both males and females
had been fed the toxic diet for 40 days, regardless of the age at
which the rats had been taken off the basal ration. This was true,
even in group 6, in which successful matings had been achieved at
82 and 131 days, while the rats were still being fed the basal
diet. All male rats not placed on the toxic diet until 63 days of
age or more were able to fertilize the normal females. In a later
study in which females fed the toxic diet were mated with normal
males, there were some successful matings, but the pups that were
born generally died or were eaten by the mother soon after birth.
In a study by Munsell et al. (1936), 7 groups of 4-week-old
rats, each containing 2 males and 6 females, were fed a basal diet
consisting of wheat, 58%; skim milk powder, 30%; yeast, 5%; butter,
5%; and cod liver oil, 2%. In each of the groups, a variable
proportion of the control wheat was replaced by toxic wheat so that
the latter contributed 8.7, 6.0, 3.0, 1.5, 0.75, 0.38, or 0 mg
selenium/kg diet. Compared with the controls receiving no toxic
wheat, the number of females having young was decreased by feeding
the diets containing 3.0 mg or more selenium/kg, and the percentage
of young reared was decreased by the diets containing 6.0 mg or
more/kg (Table 25). Feeding the diet containing 0.75 mg/kg
appeared to improve reproductive performance in terms of total
young produced or percentage of young reared. In the second
generation of rats continued on their respective levels of toxic
grain, the diet containing 6.0 mg/kg had a definite deleterious
effect on reproduction, whereas diets containing 0.75 - 1.5 mg/kg
had some beneficial effects. In a second study, the percentage of
young reared was improved in the group fed the diet containing 1.5
mg/kg compared with the control diet group, but the average number
of young per litter and the average number of young per bearing
female were decreased in the group fed the diet containing 0.75
mg/kg.
Halverson (1974) fed 4 groups of 70-day-old ARS/Sprague Dawley
albino rats a basal diet containing glucose, 61.8%; purified
casein, 25%; corn oil, 5%; minerals, 6%; and vitamins, 2.2%. The
groups comprised 2 - 4 males and 6 - 8 females and were given the
basal diet supplemented with sodium selenite at 0, 1.25, 2.50, or
3.75 mg selenium/kg. After 90 days, the rats were mated; no
consistent effects of selenium on reproduction were observed in
these first generation rats. Four groups of second-generation
young were maintained on their respective diets and used as
breeding stock in a second life cycle. In 2 separate studies, the
first and second reproductions of these second generation rats were
successful, regardless of the selenium content of the diet (Table
26). In the third reproduction, however, the unsupplemented rats
showed signs of reproductive failure. Selenium supplementation at
all levels prevented poor litter production in the first study but
only levels of 2.50 and 3.75 mg/kg prevented it in the second.
Neonatal survival was improved in both studies by selenium
administered at 3.75 mg/kg.
Schroeder & Mitchener (1971a) gave selenate at 3 mg
selenium/litre, in the drinking-water, continuously, during a
multigeneration study in mice. Five pairs of Fo mice were given
selenium at weaning and allowed to breed ad libitum for 6 months.
Controls were treated identically but did not receive any selenium
in the water. At weaning, F1 pairs were randomly selected from the
first, second, and third litters and left to breed, to produce the
F2 generation. F2 pairs were similarly selected from the first or
second litters to produce the F3 generation. An increased
male:female ratio was observed in all generations (1.30 - 1.50
versus 0.94 - 1.03 for the controls), but the mechanism of this
effect was not explained. No breeding failures were seen in the
control group, but 2 or 3 such failures occurred in each of the F1,
F2, and F3 generations given selenate. Only 3 litters were
produced in the F3 generation treated with selenate and 16 of 23
mice born were runts, whereas in the control F3 generation, 22
litters were produced with a total of 230 mice and no runts.
Two groups of 5 male and 2 groups of 5 female Wistar rats were
fed a laboratory chow diet and one group of each sex was given
sodium selenate at 0 or 7.5 mg selenium/litre in the drinking-
water, respectively (Rosenfeld & Beath, 1954). The rats receiving
selenium ("selenized rats") were treated from birth to 8 months of
age. Mating of normal males with selenized females was totally
unsuccessful, whereas mating of selenized males with normal females
resulted in normal reproduction and survival. Doses of 1.5 or 2.5
mg/litre did not have any effect on the reproduction of breeding
rats, the number of young reared by the mothers, or the
reproduction of 2 successive generations of males and females.
However, a level of 2.5 mg/litre decreased the number of young
reared by the mothers by about 50%.
Table 25. Effect of seleniferous wheat on reproduction in first- and second-generation ratsa
-------------------------------------------------------------------------------------------------
Level of Generation and Females having Total Total Average Average Young reared
dietary number of females young young litters number number
selenium 1st 2nd young/ young/
(from generation generation litter bearing
wheat) female
--------------------------------------------------------------------------------------------------
(mg/kg) Number % Number Number Number Number Number %
0 6 - 6 100 70 13 5.4 11.7 30 42.9
0.38 6 - 5 83.3 72 13 5.5 14.4 23 31.9
0.75 6 - 6 100 90 18 5.0 15.0 75 83.3
1.5 6 - 5 83.3 42 10 4.2 8.4 15 35.7
3.0 6 - 3 50.0 38 7 5.4 12.7 19 50.0
6.0 6 - 4 66.7 30 8 3.8 7.5 5 16.7
8.7 6 - 2 33.3 7 2 3.5 3.5 0 0
0 - 11 10 90.9 134 19 7.1 13.4 46 34.3
0.38 - 9 6 66.7 63 10 6.3 10.5 31 49.2
0.75 - 41 29 70.7 434 52 8.3 15.0 328 75.6
1.5 - 7 7 100 100 11 9.1 14.3 72 72.0
3.0 - 7 5 71.4 61 9 6.8 12.2 25 41.0
6.0 - 2 2 100 17 3 5.7 8.5 0 0
8.7 - 0 - - - - - - - -
--------------------------------------------------------------------------------------------------
a Adapted from: Munsell et al. (1936).
Table 26. Effect of selenium as selenite on successive reproductions of second-
generation rats maintained on a casein dieta
--------------------------------------------------------------------------------
Study Added Number Measurements in first, second and third reproductions
selenium of brood Number of Number young per 2-day survival of
in diet females litters born newborn litter litter members
1 2 3 1 2 3 1 2 3
--------------------------------------------------------------------------------
(mg/kg) (%)
1 0 3 3 3 0 12 12 - 61 46 -
1.250 6 6 6 5 12 7 8 29 21 26
2.500 5 5 5 5 12 11 8 71 84 30
3.750 6 6 6 6 11 9 8 79 78 78
2 0 7 7 6 2 8 10 6 74 82 33
1.250 7 7 5 2 10 11 4 90 84 00
2.500 9 8 8 6 9 9 5 81 85 25
3.750 8 7 7 4 7 6 6 92 100 92
--------------------------------------------------------------------------------
a Adapted from: Halverson (1974).
Poley & Moxon (1938) fed 4 groups of 15 Rhode Island pullets a
basal laying ration consisting of ground corn, 25%; ground barley,
25%; ground wheat, 15%; wheat bran, 8%; wheat middlings, 8%; meat
and bone scraps, 8%; alfalfa leaf meal, 5%; dried buttermilk, 5%;
salt, 0.5%; and cod liver oil concentrate, 0.5%. Each of the 4
groups was mated with 2 male brothers for the entire study. After
2 weeks, grains containing selenium (corn, barley, and wheat) were
substituted for normal grains in order to contribute 0, 2.5, 5, or
10 mg selenium/kg diet fed to each of the 4 groups, respectively.
Oyster shells and water were provided ad libitum. There were no
significant differences in feed consumption, body weight, egg
production, or egg fertility in any of the groups. After 4 weeks
on the toxic diets, 10 mg of selenium/kg reduced hatchability to
zero. Hatchability was only slightly reduced by 5 mg/kg, and a
level of 2.5 mg/kg had no effect.
In a study by Wahlstrom & Olson (1959), 2 groups of 10 purebred
Duroc gilts, 8 weeks of age, were fed a basal diet supplemented
with sodium selenite at 10 mg selenium/kg diet. Of the 9 gilts in
the unsupplemented group (one was killed accidentally), 8 conceived
with the first service and one failed to conceive after 3 services.
Of the 10 gilts in the selenium-exposed group, 5 conceived with the
first service, 2 with the second service and 3 failed to conceive
after 3 services. The selenium-exposed group farrowed fewer
litters and fewer live pigs, and weaned fewer pigs than the control
group (Table 27). Moreover, the average birth weights and weaning
weights were lower in the selenium-exposed group. The average
litter weight at 56 days for both litters was 131 and 227 pounds
for the selenium-exposed and unexposed groups, respectively.
Table 27. The effect of selenium on reproduction and lactation in swine
-------------------------------------------------------------------------
Items Number Average Average Average Average Average
litters number number birth number weaning
farrowed pigs pigs weight pigs weight
farrowed farrowed (kg) weaned (kg)
alive
-------------------------------------------------------------------------
Basal
First litters 7 8.0 8.0 2.95 7.3 30.5
Second litters 6 11.2 10.5 2.89 8.0 29.0
Average 6.5 9.6 9.25 2.92 7.65 29.75
Basal + selenium
First litters 6 9.8 7.7 2.60b 5.7 23.1c
Second litters 5 8.6 7.2 2.76 5.6 23.5c
Average 5.5 9.2 7.45 2.68 5.65 23.3
-------------------------------------------------------------------------
a Adapted from: Wahlstrom & Olson (1959).
b Significantly less than basal lot ( P < .05)
c Significantly less than basal lot ( P < .01)
Dinkel et al. (1963) reported on the effect of early (May 1
to mid-July) versus late (mid-July to October 1) breeding on the
reproductive performance of beef cattle grazing on a seleniferous
range in South Dakota. With such a programme, it was felt that
early conception would avoid any deleterious effects on
reproduction of the highly seleniferous young range grasses that
grow luxuriantly during the early summer. Data from 152 matings,
75 in the early and 77 in the late breeding group, collected over
5 years, showed that the average conception rate of the early-bred
cows was 60%, whereas that of the late-bred cows was 37.7%.
Moreover, the average calf crop weaned was 52% in the early
breeding group and 32.4% in the late group. The data do not prove,
but suggest, that selenium was the cause of the difference. In
addition to the early breeding effect, the very low overall
reproductive rate should be noted. This is considerably below the
rate that can be attained with similar operations when chronic
selenosis is not a problem, and it has been repeatedly observed
that, on ranches with a selenium problem, the rate of reproduction
is consistently low. This led Olson (1969) to comment that the
effect on reproduction might be the most significant effect of
excessive amounts of the element from an economic standpoint.
7.1.5. Effects on dental caries
Most studies on experimental animals have shown that high doses
of selenium do not have any effect on caries formation when the
selenium is given after tooth formation has already occurred. For
example, Muhler & Shafer (1957) fed 22 male weanling Sprague Dawley
rats a stock corn cariogenic diet that contained 10 mg sodium
selenite/kg. After 6, 8, and 12 weeks, the level of sodium
selenite was raised to 15, 20, and 30 mg/kg diet, respectively. A
control group of 20 rats received the same diet without added
selenium. The animals were given their respective diets and
drinking-water low in fluorine (F = 0.2 mg/litre) ad libitum for
140 days. The selenium-exposed group grew only about 53% as much
as the controls, but the mean number of carious lesions was
essentially the same in both groups (6.4 and 6.9, respectively).
Claycomb et al. (1965) fed 2 groups of 24 weanling rats a high-
carbohydrate diet, with or without 10 mg sodium selenite/kg diet,
for 100 days. In this study, selenium depressed growth by only
11%, but again the average number of carious lesions per rat was
similar in the selenium-treated and control groups (0.92 and 1.08,
respectively). The rather low incidence of dental caries was
thought to be due to genetic influences. Wheatcroft et al. (1951)
maintained 80 white rats, 40 males and 40 females, on a coarse corn
cariogenic diet for 100 days. The rats were divided into 4 groups
of 20 and were given daily intraperitoneal doses of sodium selenite
at 0, 0.2, 0.5, or 1.0 mg selenium/kg body weight. The authors
felt that there was a trend towards an increased incidence of
caries in the group receiving the most selenium, but the 2 highest
doses of selenium were clearly toxic, since these rats had
diarrhoea, affected eyes, and dull, matted fur. There was also
histological evidence of liver damage in over half, and kidney
damage in a third, of the rats. Thirty new-born Wistar rats were
divided into 3 groups receiving, intraperitoneally, 0, 0.5 - 1.0
µg, or 5 - 10 µg sodium selenate, respectively, every day for 53
days (Kaqueler et al., 1977). The group receiving the lower dose
of selenium had a lower total number of caries than the controls,
while the group receiving the higher dose had more caries than the
controls. The authors concluded that post-eruptive administration
of sodium selenate may have either a cariostatic or cariogenic
influence in rats, depending on the dosage administered.
Attempts to induce dental caries by exposure to high levels of
selenium were more successful when the selenium was given during
the time of tooth development. For example, Navia et al. (1968)
fed sodium selenite at 4 mg selenium/kg, to groups of 21 CR-COBS
rats in the drinking-water, in a purified caries-producing diet, or
in the same diet gelled with equal parts of 2% aqueous agar. The
experimental treatments of the mothers and litters began at birth
and continued until the pups were 50 days old. Selenium had no
effect on buccal, sulcal, or proximal caries, when given in the
diet, but caused a 12% increase in sulcal lesions when administered
in the drinking-water. Buttner (1963) fed 3 groups of 8 female,
caries-susceptible rats of the Wistar strain a cariogenic coarse
stock corn diet plus 0, 5, or 10 mg sodium selenite/litre drinking-
water during mating, pregnancy, and lactation. The offspring
received the same concentrations of sodium selenite for 120 days.
Thirty-one pups were born in the control group but, since the
dosages of selenium used partially inhibited reproduction, only 20
and 7 pups were born in the groups receiving 5 or 10 mg sodium
selenite/litre drinking-water, respectively. Growth of the pups
was strongly inhibited and the number and extent of carious lesions
were increased in both selenium-treated groups (Table 28). In a
study by Bowen (1972), 7 female monkeys of the species Macaca irus
were fed a cariogenic diet, with drinking-water sweetened with 3%
sucrose, at night, and phosphate-free icing sugar, 4 times daily.
A second group of 3 females of the same species was treated
identically except that they received selenium, as sodium selenate,
at 2 mg/litre drinking-water. After 15 months, some of the
selenium-treated monkeys experienced slight gastrointestinal
trouble in the form of greenish malodorous stools and the dose of
selenium was reduced to 1 mg/litre for another 45 months. The
second permanent molars and the premolars were formed during the
period of higher selenium exposure and these teeth had a yellow
chalky appearance in the selenium-treated monkeys. On the other
hand, selenium had no effect on the first permanent molars, which
had already formed before the start of the study. Although both
groups of monkeys had severe dental caries, the mean caries score
of the selenium-treated group was about twice that of the controls
(16 and 8.4, respectively). There was no difference between the
times required for caries to develop in the first permanent molars
in the controls and experimental groups, but carious lesions in the
second permanent molars developed more rapidly in the selenium-
exposed group (6.7 versus 21 months for mean caries development
time in selenium-treated compared with control groups). The author
concluded that selenium had a cariogenic effect, when administered
during tooth development and a moderate anti-cariogenic effect,
when given posteruptively.
Table 28. Effect of developmental and postdevelopmental
administration of sodium selenite on dental caries in the rata
-------------------------------------------------------------------
Dose of sodium Sex Number Weight gain Mean number Extent
selenite in of in 120 days of carious of carious
drinking-water rats (g) lesions lesions
(mg/litre)
-------------------------------------------------------------------
0 M 17 330 ± 9 5.3 ± 0.5 11.6 ± 1.4
F 14 207 ± 7
5 M 13 240 ± 5 7.2 ± 0.7 18.7 ± 2.2
F 7 176 ± 7
10 M 5 220 ± 18 8.6 ± 1.1 25.2 ± 3.2
F 2 148 ± 3
-------------------------------------------------------------------
a Adapted from: Buttner (1963).
Britton et al. (1980), however, presented evidence that showed
that, under some conditions, selenium can have a cariostatic
effect, even when given during tooth development. These workers
gave 0, 0.8, or 2.4 mg selenium/litre drinking-water in the form of
selenomethionine or sodium selenite, to rats from the 10th day of
pregnancy until the pups were weaned. At 17 - 19 days of age, the
pups were given oral innoculations of Streptococcus mutans - 6715
in thioglycollate broth. When 19 days old, the pups were weaned,
divided into groups of 13 - 19, and given a 67% sucrose diet and
distilled water ad libitum. At 65 - 68 days of age, the young rats
were killed and the first and second molar teeth were stained with
murexide and scored for caries. The high dose of selenium given to
the mothers, as either compound, did not have any effect on
dental caries, but the middle dose (0.8 mg/litre) had a definite
cariostatic effect, since selenomethionine and sodium selenite
decreased the incidence of total buccal caries by 46.1 and 40.9%,
respectively. The authors suggested that this cariostatic effect
might occur because some selenium is necessary for proper enamal
formation or because of undetermined effects on the oral
environment.
The fact that high doses of selenium have an apparent
cariogenic effect, only when given during tooth development,
is consistent with the results of Shearer (1975) who found that
the incorporation into teeth of radioactive selenite or
selenomethionine given in the drinking-water at a level of 0.2 mg
selenium/litre was much greater in rat pups undergoing dental
development than in their mothers whose teeth had already matured.
The mechanism of the cariogenic effect of high levels of selenium
is not known, but an antagonism to fluoride does not seem to be
involved (Shearer & Ridlington, 1976).
7.1.6. Factors influencing toxicity
7.1.6.1. Form of selenium
Studies comparing the toxicity of different forms of selenium
are described in section 7.1.2.1. This point is particularly
relevant because the forms of selenium in foods have yet to be
characterized.
7.1.6.2. Nutritional factors
High levels of dietary protein protect against selenium
toxicity (Gortner, 1940), but some proteins give better protection
than others (Smith & Stohlman, 1940). Jaffe (1976) found that the
toxicity of seleniferous sesame meal for rats was markedly
decreased when the diets were supplemented with L-lysine, the
limiting essential amino acid in sesame proteins. Lysine was
thought to protect against selenium poisoning indirectly by
improving the biological value of the sesame proteins.
Linseed meal contains a unique non-proteinaceous factor that
is highly effective in protecting against selenium poisoning
(Halverson et al., 1955; Levander et al., 1970). The results of
recent work suggest that the protective activity of linseed meal
may reside in 2 newly-isolated cyanogenic glycosides (Palmer et
al., 1980; Smith et al., 1980). Cyanide has a partially protective
effect against selenium poisoning in rats (Palmer & Olson, 1979)
and increases the occurrence of nutritional myopathy in lambs
(Rudert & Lewis, 1978).
There is conflicting evidence as to the ability of methyl-
donating compounds to protect against selenium toxicity (Rosenfeld
& Beath, 1964). Methionine was shown to protect against chronic
selenite poisoning in rats, but only when vitamin E or other fat-
soluble antioxidants were in the diet (Levander & Morris, 1970).
Vitamin E deficiency increased the susceptibility of rats to
chronic poisoning by selenium as selenite (Witting & Horwitt, 1964)
and pigs deficient in vitamin E and selenium were more susceptible
to acute selenium toxicosis than non-deficient pigs (Van Vleet et
al., 1974).
7.1.6.3. Arsenic
Selenium poisoning caused by feeding rats seleniferous wheat
was decreased by giving sodium arsenite in the drinking-water
(Moxon, 1938). The protective effect of arsenic against selenium
toxicity might be explained by the increased biliary excretion of
selenium caused by arsenic (Levander & Baumann, 1966). However,
the use of arsenic compounds did not prove to be a practical way of
controlling selenium poisoning in livestock (Olson, 1969). Also,
arsenic does not protect against all forms of selenium, since it
potentiates the toxicity of the trimethylselenonium ion (Obermeyer
et al., 1971).
7.1.6.4. Sulfate
Dietary sulfate partially counteracts the toxicity of selenate
in rats, but has little or no effect against selenite or organic
forms of selenium (Halverson et al., 1962). Sulfate apparently
increases the urinary excretion of selenium fed as selenate,
thereby reducing the retention of selenium in the internal organs
(Ganther & Baumann, 1962).
7.1.6.5. Adaptation
Ermakov & Kovalskij (1968) fed 18 adult female sheep from
seleniferous and normal localities their own respective diets or
these diets plus an additional 2 mg selenium, as selenite, daily
for 28 days. The 18 sheep were divided into 6 equal groups,
according to their history of selenium intake and, in the case of
the seleniferous sheep, according to the presence or absence of
signs of selenium toxicity. After 28 days, all the sheep were
killed, and selenium levels and acid and alkaline phosphatase
activities were determined in several tissues. Selenium levels and
alkaline phosphatase activities are presented in Table 29. The
increase in selenium retention in the blood, kidneys, and most of
the other internal organs, produced by supplementing the feed with
selenite, was less in sheep from high-selenium localities than in
animals from control localities. Retention in the liver seemed to
be an exception. The increase in alkaline phosphatase activity due
to the selenite load was much clearer in sheep from the control
localities than in the sheep from the seleniferous localities. The
alkaline phosphatase activity of the pancreas in sheep from the
high-selenium locality, manifesting selenium toxicity signs, was an
exception being much higher after the selenite load (370 ± 57
compared with 84 ± 34). These differences in the response of the
sheep to selenite loading were interpreted by the authors as
evidence of the adaptation of the animals to high levels of
selenium exposure.
Table 29. Effect of high selenium (Se) load on selenium metabolism and alkaline phosphatase activity in sheep
previously exposed to normal and high selenium intakes in foragea
----------------------------------------------------------------------------------------------------------------
History Daily Se intake Skeletal Alkaline phosphatase activity
of sheep Plant Selenite Total Blood muscle Liver Kidney kidney liver skeletal
Se Se Se Se Se Se Se muscle
(µg) (µg) (µg) (µg/litre) (µg/kg) (µg/kg) (µg/kg) (units/100 g tissue)
----------------------------------------------------------------------------------------------------------------
High 1821 2000 3821 411 ± 18 230 ± 24 600 ± 44 870 ± 69 286 ± 148 448 ± 43 13.0 ± 2.7
Se-curved
hoofs
High 1944 0 1944 290 ± 18 180 ± 7 330 ± 20 760 ± 26 362 ± 89 374 ± 117 9.5 ± 3.0
Se-curved
hoofs
High 1774 2000 3774 362 ± 34 210 ± 16 590 ± 90 1000 ± 174 309 ± 103 335 ± 18 9.4 ± 2.9
Se-curved
hoofs
High 2038 0 2038 239 ± 34 170 ± 6 340 ± 20 890 ± 21 349 ± 63 344 ± 56 9.5 ± 1.2
Se-curved
hoofs
Normal Se 376 2000 2376 408 ± 23 170 ± 8 450 ± 38 950 ± 77 496 ± 53 457 ± 57 14.8 ± 3.0
Normal Se 396 0 396 100 ± 12 89 ± 5 190 ± 20 780 ± 69 143 ± 29 254 ± 7 5.5 ± 0.8
----------------------------------------------------------------------------------------------------------------
a Adapted from: Ermakov & Kovalskij (1968).
Jaffe & Mondragon (1969) presented evidence that animals could
adapt to long-term selenium intake. Hepatic-selenium levels
decreased steadily in young rats fed a diet containing 4.5 mg
selenium/kg as seleniferous sesame meal, if the rats came from
mothers that had been fed the same seleniferous diet, but the
levels increased if the rats came from mothers fed a non-
seleniferous stock diet. It was concluded that an adaptation
mechanism existed that allowed rats exposed to long-term selenium
ingestion to store less of this element than previously unexposed
controls. A later report (Jaffe & Mondragon, 1975) confirmed these
results and also indicated that the hepatic lesions, splenomegaly,
and other toxicity signs were significantly decreased in adapted
rats.
One mechanism for a possible adaptive response to high levels
of selenium could involve effects of selenium intake on various
selenium metabolic pathways, since previous selenium exposure has
been shown to have an influence on the whole body retention of
selenium and the urinary and pulmonary excretion of selenium
(Ganther et al., 1966; Ewan et al., 1967; Burk et al., 1973)
(section 6.3, 6.4). However, as discussed in section 7.1.2.1, more
recent work has shown that previous selenium exposure directly
influences the toxicity of final selenium metabolites such as
dimethyl selenide and the trimethylselenonium ion (Parizek et al.,
1976, 1980).
7.1.7. Mechanism of toxicity
The mechanism of selenium toxicity remains unclear and the
modes of action of various selenium compounds, such as hydrogen
selenide, selenomethionine, and selenium dioxide are likely to be
quite different. Thus, no unifying hypothesis regarding the toxic
effects of selenium compounds is possible. Various hypotheses
concerning the mechanisms of selenium toxicity have been presented
(Rosenfeld & Beath, 1964; Izraelson et al., 1973; Ermakov &
Kovalskij, 1974; US NAS/NRC, 1976; Lazarev, 1977; Levander, 1982).
Although it appears unlikely that selenite interferes directly with
sulfhydryl enzymes (Tsen & Tappel, 1958; Tsen & Collier, 1959), it
is possible that selenite may interfere with glutathione metabolism
(Vernie et al., 1978; Chung & Maines, 1981; Vernie, 1984; Anundi et
al., 1984) and that this may affect enzyme activities. The
apparent pro-oxidant nature of high levels of selenite has been
reported by several workers (Witting & Horwitt, 1964; Csallany et
al., 1984), and this may be related to some aspects of selenite
toxicity.
7.2. Selenium Deficiency
7.2.1. Animal diseases
The nutritionally beneficial effects of selenium were first
reported in 1957 by Schwarz, who discovered that sodium selenite
prevented dietary liver necrosis in vitamin E-deficient rats
(Schwarz & Foltz, 1957). This discovery led to the rapid
recognition of vitamin E-related selenium-responsive deficiency
diseases in several species of farm animals. Diseases such as
white muscle disease in sheep and cattle, hepatosis dietetica in
swine, and exudative diathesis in poultry are economically
significant problems in areas of the world where the soil levels of
selenium available for uptake by plants are low. Schwarz & Foltz
(1958) also reported that feeding a diet deficient in both selenium
and vitamin E to mice resulted in multiple necrotic degeneration of
several organs; more recently, Suchkov et al. (1977, 1978) found
that feeding such diets to mice for 13 days caused a decreased
staining intensity for zinc in the pancreas, kidneys, and testes.
The fact that vitamin E as well as selenium tended to protect
against many of these diseases led some research workers to
question whether there was any requirement for selenium in animals
receiving adequate amounts of vitamin E. But, the results of more
recent work have clearly demonstrated the need for selenium, even
in animals given nutritionally adequate levels of vitamin E.
Deficiency signs specific for selenium included alopecia, vascular
changes, cataract, poor growth, and reproductive failure in second
generation selenium-deficient rats (McCoy & Weswig, 1969; Wu et
al., 1979). Pancreatic degeneration occurred in selenium-deficient
chicks with a normal intake of vitamin E (Thompson & Scott, 1970),
but the condition could be prevented by feeding very high
levels (> 300 µg/kg) of vitamin E or other antioxidants (Whiteacre
et al., 1983).
Changes in the myocardial parenchyma and increases in heart
weight have been observed in albino rats fed corn and vegetables
grown in the areas in which Keshan disease is endemic (Su et al.,
1982), and swine fed grain from endemic areas for 6 months
exhibited multiple myocardial necrosis as well as other lesions
(Zhu et al., 1981). Vitamin E deficiency exacerbated the
pathological and histochemical changes in the heart and liver of
piglets fed a low selenium diet composed mainly of cereals grown in
the Keshan disease area (Zhu et al., 1981).
7.2.2. Intakes needed to prevent deficiency
7.2.2.1. Quantitative dietary levels
Schwarz (1961) showed that 0.02 - 0.03 mg selenium/kg diet, in
the form of sodium selenate or selenite, afforded 50% protection
against liver necrosis in vitamin E-deficient rats over an
experimental period of 30 days. Selenite-selenium at 0.10 mg/kg
protected the rats over their entire life span in the absence of
vitamin E. Under these conditions, the rats developed severe
tocopherol deficiency, which mainly afflicted the central nervous
system. Sprinker et al. (1971) noted that 0.10 mg selenium/kg, as
selenite, prevented specific selenium deficiency lesions in rats
that had been fed a vitamin E-supplemented diet for 2 generations.
Thompson & Scott (1969) found that administration of 0.02 - 0.05 mg
selenium/kg, as selenite, prevented death and exudative diathesis
in chicks fed diets containing typical levels of vitamin E
(sections 7.2.4.1 and 7.2.7.2 include discussions on the
nutritional interrelationship of selenium and vitamin E).
Considerable field experience in New Zealand has indicated that
feeds from pastures associated with selenium-responsive
unthriftiness in sheep contain 0.008 - 0.030 mg selenium/kg
(Hartley, 1967). In areas where selenium-responsive diseases do
not occur, the feed levels of selenium range from 0.020 - 0.098
mg/kg. Since there is some overlap in these selenium levels, other
factors may be involved in the etiology of this selenium-responsive
disease (various factors that can influence selenium deficiency
are discussed in section 7.2.7).
It has been concluded that "the critical level for dietary
selenium, below which deficiency symptoms are observed, is
apparently about 0.02 mg/kg for ruminants and 0.03 - 0.05 mg/kg for
poultry" (US NAS/NRC, 1971). However, this US NAS/NRC committee
pointed out that "when supplementary selenium is fed, higher levels
than the minimal requirements have been proposed, both to permit
satisfactory distribution of the element through the large feed
mass and to overcome variations in feed intake by individual
animals". The final recommendation of the committee was that 0.1
and 0.2 mg selenium should be added per kg feed to eliminate
selenium deficiency in livestock and turkeys, respectively. In
laboratory rats, a dietary-selenium level of 0.1 - 0.2 mg/kg would
be equivalent to an intake of about 5 - 10 µg/kg body weight per
day.
7.2.2.2. Bioavailability
7.2.3. Blood and tissue levels in deficiency
Hartley (1967) presented data on the blood-selenium levels of
normal New Zealand lambs and lambs suffering from various selenium-
responsive diseases (Table 30). The mean blood-selenium levels
were lower in the diseased lambs than in the healthy ones, but
there was considerable overlap between the two groups. It was
suggested that other unknown factors might be involved in the
etiology of these diseases. Jacobsson et al. (1970) found blood-
selenium levels of 0.003 mg/litre in cows afflicted with muscular
degeneration, whereas healthy cows had an average of 0.046
mg/litre. Hartley (1967) estimated that blood-selenium levels of
0.05 mg/litre in young sheep could be considered satisfactory.
The same authors compared liver-selenium levels found in normal
lambs with those found in lambs with selenium-responsive
unthriftiness (Table 31). Again, there was some overlap between
the two groups, but the average level of selenium in the livers
from the deficient lambs was much lower than that from the healthy
lambs. Allaway et al. (1966) suggested that 0.21 mg/kg (dry
weight) is the critical minimal hepatic-selenium level below which
a high incidence of white muscle disease can be expected in lambs.
Table 30. Blood-selenium levels in normal
and selenium-deficient lambsa
---------------------------------------------
Group Selenium contentb
(mg/litre)
---------------------------------------------
Normalc 0.026 (0.014 - 0.048)
White muscle diseased 0.016 (0.006 - 0.033)
Normale 0.06 (0.020 - 0.195)
Selenium-responsive 0.01 (0.007 - 0.030)
unthriftinessf
---------------------------------------------
a Adapted from: Hartley (1967).
b Mean with range in parentheses.
c Normal lambs from areas with white muscle
disease.
d Lambs with white muscle disease.
e Lambs from areas where selenium-responsive
diseases are not recognized.
f Lambs with selenium-responsive
unthriftiness.
Table 31. Liver-selenium levels in normal
lambs or lambs with selenium-responsive
unthriftinessa
------------------------------------------
Group Selenium contentb
(mg/kg)
------------------------------------------
Normalc 0.16 (0.03 - 0.40)
Selenium-responsive 0.018 (0.005 - 0.05)
unthriftinessd
------------------------------------------
a Adapted from: Hartley (1967).
b Mean with range in parentheses.
c Lambs from areas where selenium-
responsive diseases are not
recognized.
d Lambs with selenium-responsive
unthriftiness.
7.2.4. Physiological role: glutathione peroxidase
7.2.4.1. Function of selenium and relationship to vitamin E
The discovery that selenium is a component of the enzyme
glutathione peroxidase (Rotruck et al., 1973) provides a logical
explanation for the nutritional interaction of vitamin E and
selenium, a puzzle that perplexed scientists for many years.
Analysis of purified ovine erythrocyte glutathione peroxidase by
fluorometry revealed that the protein contained 4 moles of selenium
per mole of enzyme (Hoekstra et al., 1973) and this stoichiometry
was also found for crystalline bovine erythrocyte-glutathione
peroxidase analysed for selenium by neutron activation analysis
(Flohe et al., 1973). In the scheme postulated by Hoekstra
(1975a), tocopherols act as intracellular antioxidants to prevent
oxidative damage to polyunsaturated fatty acids in biological
membranes by terminating chain reactions of lipid peroxides (Fig.
7). Selenium, as a part of glutathione peroxidase, protects
against oxidative stress either by catalysing the destruction of
hydrogen peroxide or by catalysing the decomposition of lipid
hydroperoxides, thereby interrupting the free radical peroxidative
chain reaction; in this latter role the free fatty acyl
hydroperoxide must be liberated by phospholipan A2 action from the
hydrophobic region of biological membranes, before its reduction
can be catalysed by glutathione peroxidase (Grossman & Wendel,
1983). Thus, both of these nutrients play separate but
interrelated roles in the cellular defence mechanisms against
oxidative damage.
 Although glutathione peroxidase accounts for many of the
biological effects of selenium, other functions of this trace
element in the body may yet be discovered. Only 1/5 of the total
selenium in rat brain is in the form of glutathione peroxidase
(Prohaska & Ganther, 1976). Selenium has also been shown to be a
constituent of several enzymes in microorganisms (Stadtman, 1977).
7.2.4.2. Effect of selenium intake on tissue-glutathione peroxidase
activity
The results of several studies have shown a close dose-effect
relationship between the dietary intake of selenium and the
activity of glutathione peroxidase in various tissues. For example,
Hafeman et al. (1974) found that increased dietary selenium caused
corresponding increases in erythrocyte-glutathione peroxidase
activity in rats, even when toxic levels of selenium were fed (Fig.
8). Hepatic-glutathione peroxidase activity increased with
increasing dietary-selenium levels up to 1 mg/kg, but then declined
due to liver damage when toxic levels were fed (Fig. 9). However,
studies on hamsters have shown that consumption of excess selenium
does not always result in increased erythrocyte-glutathione
peroxidase activity, since feeding selenite at more than 10 mg
selenium/kg diet did not cause increases in erythrocyte-enzyme
activity that corresponded to increases in blood-selenium
concentrations (Julius et al., 1980).
Although glutathione peroxidase accounts for many of the
biological effects of selenium, other functions of this trace
element in the body may yet be discovered. Only 1/5 of the total
selenium in rat brain is in the form of glutathione peroxidase
(Prohaska & Ganther, 1976). Selenium has also been shown to be a
constituent of several enzymes in microorganisms (Stadtman, 1977).
7.2.4.2. Effect of selenium intake on tissue-glutathione peroxidase
activity
The results of several studies have shown a close dose-effect
relationship between the dietary intake of selenium and the
activity of glutathione peroxidase in various tissues. For example,
Hafeman et al. (1974) found that increased dietary selenium caused
corresponding increases in erythrocyte-glutathione peroxidase
activity in rats, even when toxic levels of selenium were fed (Fig.
8). Hepatic-glutathione peroxidase activity increased with
increasing dietary-selenium levels up to 1 mg/kg, but then declined
due to liver damage when toxic levels were fed (Fig. 9). However,
studies on hamsters have shown that consumption of excess selenium
does not always result in increased erythrocyte-glutathione
peroxidase activity, since feeding selenite at more than 10 mg
selenium/kg diet did not cause increases in erythrocyte-enzyme
activity that corresponded to increases in blood-selenium
concentrations (Julius et al., 1980).

 Oh et al. (1976a) investigated the effects of feeding several
different levels of dietary selenium on the activity of glutathione
peroxidase in various tissues in lambs. The activity of the enzyme
tended to plateau in all tissues examined, except red cells and
pancreas, when 0.1 mg was fed per kg diet. This level of dietary
selenium approximates the presumed requirement in this species.
7.2.4.3. Relationship between blood-selenium levels and erythrocyte-
glutathione peroxidase activity
A direct linear relationship between the selenium concentration
of whole blood and the activity of erythrocyte-glutathione
peroxidase has been demonstrated in lambs, sheep, cattle, and swine
(Oh et al., 1976b; Wilson & Judson, 1976; Sivertsen et al., 1977).
This linear relationship in lambs (Fig. 10) was thought to be due
to the fact that glutathione peroxidase accounts for most of the
total selenium in the ovine red cell and that ovine red cells
contain a relatively constant proportion of the total blood-
selenium.
Oh et al. (1976a) investigated the effects of feeding several
different levels of dietary selenium on the activity of glutathione
peroxidase in various tissues in lambs. The activity of the enzyme
tended to plateau in all tissues examined, except red cells and
pancreas, when 0.1 mg was fed per kg diet. This level of dietary
selenium approximates the presumed requirement in this species.
7.2.4.3. Relationship between blood-selenium levels and erythrocyte-
glutathione peroxidase activity
A direct linear relationship between the selenium concentration
of whole blood and the activity of erythrocyte-glutathione
peroxidase has been demonstrated in lambs, sheep, cattle, and swine
(Oh et al., 1976b; Wilson & Judson, 1976; Sivertsen et al., 1977).
This linear relationship in lambs (Fig. 10) was thought to be due
to the fact that glutathione peroxidase accounts for most of the
total selenium in the ovine red cell and that ovine red cells
contain a relatively constant proportion of the total blood-
selenium.
 Under certain conditions, some investigators have not found a
significant correlation between blood-selenium levels and blood
glutathione peroxidase activity. For example, Thompson et al.
(1976) described swine that had high blood-selenium levels but low
blood-glutathione peroxidase activities. The reasons for this
discrepancy are not known but could perhaps be related to an
increased level of "non-functional" selenium in the blood, such as
selenomethionine that has been nonspecifically incorporated into
protein as a substitute for methionine.
7.2.4.4. Glutathione peroxidase as an indicator of selenium status
As discussed by Hoekstra (1975b), the measurement of
glutathione peroxidase activity offers several advantages as an
indicator of selenium status. First of all, the enzyme assay is
easier to perform and is less time consuming than the selenium
analysis. Also, the enzyme assay is not subject to contamination
problems. Moreover, glutathione peroxidase represents only
"functional" selenium in the tissues, not selenium that has been
non-specifically incorporated into proteins or that has formed
biologically inactive complexes with heavy metals. Finally, at
least in some tissues, the activity of the enzyme appears to taper
off as the dietary level of selenium approaches the nutritional
requirement of the animal. This "plateau effect" of glutathione
peroxidase activity does not occur in red blood cells, a biological
material that is highly convenient for sampling purposes. However,
as pointed out by Hoekstra (1975b), the absence of plateauing in
red cells may limit the usefulness of erythrocyte-glutathione
peroxidase in accurately establishing selenium requirements, but
does not diminish the usefulness of this source of the enzyme as an
overall index of selenium status.
One complicating factor in the use of glutathione peroxidase to
assess selenium status is the fact that the activity of the enzyme
is influenced by many physiological variables other than selenium
intake (Ganther et al., 1976). Among these are the age and sex of
the animal, starvation, exposure to certain oxidant stressors,
toxicants, or heavy metals, and deficiency in iron and vitamin B12.
Obviously, these variables have to be controlled, or compensated
for, if glutathione peroxidase is to be a valid index of selenium
status.
Another serious complicating factor in the use of glutathione
peroxidase to assess selenium status is the existence of a "non
selenium-dependent" glutathione peroxidase activity that persists,
even in severe selenium deficiency (Lawrence & Burk, 1976). This
enzymic activity has been found in variable amounts in different
rat tissues ranging from 23% of the total glutathione peroxidase
activity in fat to 91% in testis (Lawrence & Burk, 1978). The "non
selenium-dependent" activity accounts for 26 - 38% of the total
glutathione peroxidase activity in rat brain, kidneys, liver, and
adrenals. No such activity is found in rat erythrocytes, spleen,
heart, thymus, intestinal mucosum, skin, or skeletal muscle. The
"non selenium-dependent" glutathione peroxidase activity has been
found in the liver of several different species including the rat,
hamster, guinea-pig, chicken, pig, sheep, and man. In human and
guinea-pig liver, it accounts for the major part of the total
glutathione peroxidase activity. The "non selenium-dependent"
glutathione peroxidase activity differs from the selenium-
containing enzyme in substrate specificity in that it catalyses the
reduction of a range of organic hydroperoxides but not of hydrogen
peroxide. The activity of the "non selenium-dependent" enzyme is
inversely related in rat liver to the dietary level of selenium
(Lawrence & Burk, 1978; Lawrence et al., 1978) and the glutathione-
S-transferase enzymes are responsible for the "non selenium-
dependent" enzyme activity (Prohaska & Ganther, 1977; Lawrence et
al., 1978). The glutathione- S-transferases are not selenium-
containing enzymes; they are all dimeric, and their monomeric
constituents fall into three categories of different relative
molecular mass. The glutathione peroxidase activity of the enzymes
is only shown by the dimeric enzymes that contain one of the three
subunits and, in selenium deficiency, the amount of this subunit
alone is increased (Ketterer et al., 1982; Mehlert & Diplock,
1985).
The physiological significance of the "non selenium-dependent"
activity has been questioned because the seleno-enzyme has a much
higher Vmax and lower KM (Prohaska & Ganther, 1977). However, Burk
et al. (1980a) showed that the "non selenium-dependent enzyme"
removed organic hydroperoxides in intact rat livers perfused with a
haemoglobin-free medium containing high concentrations of
peroxides.
Obviously, any use of glutathione peroxidase activity to
monitor selenium status has to take into account this "non
selenium-dependent" activity. This complication can be avoided by
selecting a tissue for test that has little or none of the
selenium-independent activity, such as erythrocytes, or by using
hydrogen peroxide as the substrate, since this has little activity
with the selenium-independent enzyme.
7.2.5. Other possible physiological roles
7.2.5.1. Homeostasis of hepatic haem
Burk et al. (1974) showed that the induction of hepatic
cytochrome P-450 by daily intraperitoneal injections of 75 mg
phenobarbital/kg body weight, for 4 days, was markedly impaired in
rats that had been fed a selenium-deficient diet for at least 3
months. The induction of ethylmorphine demethylase activity by
phenobarbital was also impaired in the livers of selenium-deficient
rats. However, no such impairment was observed in the induction of
cytochrome b5 or of NADPH-cytochrome c reductase activity or
biphenyl hydroxylase activity, or in pentobarbital sleeping time
(Burk & Masters, 1975). Treatment with phenobarbital stimulated a
6- to 8-fold increase in hepatic microsomal haem oxygenase activity
in selenium-deficient rats but had little or no effect in selenium-
supplemented rats (Correia & Burk, 1976). This suggested that the
abnormalities of cytochrome P-450 induction in selenium-deficient
rats were related to increased degradation rather than decreased
synthesis of hepatic haem. Mackinnon & Simon (1976) reported an
impaired haem synthesis in selenium-deficient phenobarbital-treated
rats, but Burk & Correia (1977) were unable to confirm these
results. Apparently, the pronounced stimulation of microsomal
haem oxygenase (EC 1.14.99.3) in the livers of selenium-deficient
rats by phenobarbital is unrelated to the role of selenium in
glutathione peroxidase, since pretreatment of deficient rats with
a single dose of selenite at 50 mg selenium/rat, 4 - 6 h before
phenobarbital administration, abolished the response in haem
oxygenase but had no effect on cytosolic or mitochondrial
glutathione peroxidase activity (Correia & Burk, 1978). Moreover,
phenobarbital failed to stimulate liver microsomal haem oxygenase
activity or to affect cytochrome P-450 in vitamin E-deficient,
selenium-adequate rats, so this response does not seem to be
related to the direct effects of lipid peroxidation on haem or
cytochrome P-450. There is a marked sex difference in the
phenobarbital stimulation of hepatic microsomal haem oxygenase
activity in selenium-deficient rats. The stimulation was greatest
in males and least in females with intermediate values in castrated
males and testosterone-treated females (Burk et al., 1980a). This
sex difference existed even though hepatic glutathione peroxidase
activity was reduced to the same extent by selenium deficiency in
all groups. However, the exact mechanism by which selenium
influences homeostasis of hepatic haem still needs to be clarified.
Pascoe et al. (1983) showed that both intraluminal iron and
selenium were required by the intestinal mucosa for maintenance of
the intestinal cytochrome P-450 content, and its dependent mixed
function oxidase activity, and that withdrawal of dietary selenium
for one day markedly decreased the level of cytochrome P-450 in the
small intestine, suggesting that intestinal mucosal cells derive
their selenium from the diet rather than from the blood.
A report by Maines & Kappas (1976) indicated that injection of
selenium increased hepatic haem oxygenase activity, but the doses
of selenium used (3.94 mg/kg body weight) exceeded the MLD for
selenium as selenite (Table 19), so that the phenomenon observed by
these authors may have been merely an artifact of acute selenium
poisoning.
7.2.5.2. Microsomal and mitochondrial electron transport
Diplock and co-workers carried out an extensive series of
studies, the results of which suggest a possible role for selenium
and vitamin E in the electron-transfer system of rat-liver
microsomes (Diplock, 1974a,b). First, they showed that appreciable
amounts of volatile selenium evolved from rat liver subcellular
organelles, after acid treatment, only when the rats were adequate
in vitamin E and the tissue homogenization medium contained large
concentrations of anti-oxidants (Diplock et al., 1971). The acid-
volatile selenium was later identified as selenide (Diplock et al.,
1973). It was assumed that this compound was formed in vivo,
presumably by the glutathione reductase pathway (Fig. 9), but
artifactual in vitro formation of such selenide, because of the
presence of mercaptoethanol in the homogenization medium, cannot be
ruled out (Levander, 1976a). An oxidant-labile form of non-haem
iron, dependent on dietary vitamin E and selenium, was observed in
rat liver microsomes (Caygill & Diplock, 1973). It was considered
that this supported the hypothesis that the selenide might form
part of the active centre of a non-haem iron protein functional in
microsomal electron transfer. Alterations in the kinetic
parameters of aminopyrine demethylation by the liver microsomes in
vitamin E-deficient rats also supported this idea (Giasuddin et
al., 1975). However, attempts to isolate an oxidant-labile
selenium- and vitamin E-dependent non-haem, iron-containing protein
fraction were unsuccessful, because of the lability of the
selenide, and it was concluded that clarification of the role of
selenide in microsomes needed additional research (Diplock, 1976,
1979). The effects of prolonged selenium deficiency on a large
number of parameters in mouse liver xenobiotic metabolism were
studied by Reiter & Wendel (1983). They demonstrated a
heterogeneous pattern of effects that did not appear to involve
glutathione peroxidase.
One of the earliest biochemical defects discovered in vitamin
E- and selenium-deficient rats was the inability of liver slices or
homogenates to maintain normal rates of respiration after prolonged
periods of incubation in vitro (Schwarz, 1962). Although this so-
called respiratory decline might be explained on the basis of
generalized mitochondrial membrane damage due to lipid peroxidation
(Yeh & Johnson, 1973), it was also plausible that selenium might
have a more specific role in mitochondrial metabolism. Indeed,
selenium added to the diet, or added in vitro, accelerated the
swelling of rat liver mitochondria induced by certain thiols, and
this swelling could be blocked by the addition of cyanide (Levander
et al., 1973a). Selenium was in fact shown to be an excellent
catalyst for the reduction of cytochrome c by thiols in chemically-
defined systems (Levander et al., 1973b) and a selenoprotein
containing a haem group similar to that of cytochrome c has been
reported in lamb muscle (Whanger et al., 1973). However, more work
is required to establish the possible role of selenium in
mitochondrial electron transfer.
7.2.5.3. The immune response
Dietary vitamin E in excess of nutritionally required levels
has been shown to improve the humoral immune response of several
different species to bacterial and viral antigens (Nockels, 1979).
Because of the close nutritional and biochemical relationship
between selenium and vitamin E (section 7.2.4.1), the effect of
dietary selenium supplementation was tested on the immunological
responses of mice (Spallholz et al., 1973). Mice fed a laboratory
chow diet (thought to contain 0.5 mg selenium/kg) supplemented with
sodium selenite at 0.7 or 2.8 mg selenium/kg had approximately 7-
and 30-fold higher antibody titres, respectively, after challenge
with sheep red blood cells than mice fed the chow diet alone.
Sodium selenite injected into mice intraperitoneally at doses of
3 - 5 µg per animal also increased the primary immune response to
the sheep red-blood-cell antigen and this increase was greatest
when the selenium was given prior to, or simultaneously with, the
antigen (Spallholz et al., 1975). The mechanism by which selenium
stimulates antibody synthesis is not known (Martin & Spallholz,
1976), but the dose of selenium needed to elicit this response is
clearly above the nutritionally required level. Levander (1986)
has reviewed the very limited data on the possible effect of
selenium on the immune response in man.
7.2.5.4. Selenium and vision
The effects of selenium on light perception were studied in 50
male "Grey chinchilla" rabbits weighing 3 - 3.5 kg and maintained
on a standard laboratory diet (Abdullaev et al., 1972, 1974a,b).
Electroretinography was carried out in dark-adapted animals under
conditions of maximally dilated pupil induced by topical
application of 0.5% dikaine - 1% atropine. Light stimulation was
provided with an impulse lamp giving either single or dual flashes
of 150 microseconds. Eight different energy levels of light
intensity were used ranging from 0.016 to 1.4 J. Sodium selenite
was administered subcutaneously at a dose of 0.45 mg selenium/kg
body weight. Control rabbits were injected with sodium sulfite at
a dose of 1 mg/kg. Sodium selenite stimulated light sensitivity,
as judged by increases in the a and b waves of the electro-
retinogram (ERG). The increase in the amplitude of the b wave was
considerably greater than that of the a wave. This stimulating
effect of sodium selenite on light sensitivity persisted for 3 days
in all studies. The same effects were observed when sodium
selenite was given via retrobulbar administration. Sodium sulfite
did not have any effect on the ERG. Similar results were obtained
by Bacharev et al. (1975).
The Task Group recognized that the data on the effects of
selenium on vision were obtained in studies involving high dose
levels (in the case of rabbits, the doses were approximately 20 -
30% of the LD50). The effects on vision of lower doses of selenium
have not been elucidated so far. The possible effect of deficiency
of selenium on vision is also of interest. Model studies with
isolated retinas exposed to a 0.01% concentration of sodium
selenite resulted in an increase in the amplitude of the ERG
(Kulieva et al., 1978). The effect of increased sensitivity of the
eye to light, after administration of sodium selenite, and the rate
of manifestation and duration of the effect are directly
correlated with localization of the substance in the structures of
the eye (section 6.2.2.1). Subcutaneous administration of sodium
selenite to rabbits, at a dose of 0.4 - 2 mg/kg body weight,
inhibited free radical formation as indicated by electron
paramagnetic resonance (EPR) measurements, not only in the lipid
membrane phase but also within the melanoprotein granules of the
retinal epithelium. The EPR signal was decreased in intensity by
selenite treatment in comparison with controls, but the form and
parameters of the EPR lines were not changed (Abdullaev et al.,
1974a,b).
7.2.6. Effects on reproduction
Vitamin E was discovered as a result of the isolation of an
unknown fat-soluble dietary factor essential for reproduction in
rats (Evans & Bishop, 1922), but selenium compounds are not active
in preventing reproductive failure in vitamin E-deficient rats
(Harris et al., 1958). Moreover, female rats weighing 80 - 120 g
and fed a selenium-deficient, but vitamin E-adequate, diet
successfully delivered 3 litters over a period of 6 months,
even though their blood-selenium levels dropped from 0.52 to
0.06 mg/litre during this time (Hurt et al., 1971). Similarly,
McCoy & Weswig (1969) found that rats fed a low-selenium but
adequate-vitamin E diet, for 4 months, reproduced normally.
However, their offspring failed to reproduce if kept on the low
selenium diet. Immobile spermatozoa, with separation of heads from
tails, were observed in 5 out of the 8 male progeny not given
selenium supplements, and no spermatozoa were seen in the other 3
males. The 2 male and 2 female offspring, supplemented with sodium
selenite at 0.1 mg selenium/kg diet, were fertile but delivered
litters of only one or 2 rats, all of which died in a few days.
The authors concluded that selenium supplementation resulted in
some fertilization, but only a few full-term young were delivered
and these were abnormal as they survived for only a brief period.
Wu et al. (1973) found that the motility of spermatozoa from
male rats, born to females fed a selenium-deficient diet, was
always very poor and that most of the sperm cells showed breakage
near the midpiece of the tail. Supplementation of the diet with
high levels of vitamin E or various other antioxidants did not
prevent these selenium deficiency effects. However, in this study,
the body and testicular weights of the rats were so markedly
depressed by selenium deficiency that it was not possible to
establish whether the abnormal sperm were due to a direct effect of
selenium deficiency or to an indirect effect on overall growth and
well-being. In a second series of studies (Wu et al., 1979), using
male rats born to dams fed a diet adequate in selenium, the
depression of growth, and testes weight in the progeny caused by
selenium deficiency was much less, even though impaired sperm
motility and abnormal sperm morphology were still observed (Table
32). The authors also pointed out that there did not appear to be
any correlation between reduction in body or testicular weights and
the characteristic sperm midpiece abnormality among the individual
rats in each treatment group. However, all of the rats used by Wu
and associates were fed diets that were probably low in zinc and
chromium and both of these trace elements are known to play
important roles in sperm formation (Wu et al., 1971; US NAS/NRC,
1979; Anderson & Polansky, 1981).
The mechanism of the effects of selenium on the integrity of
sperm morphology in the rat is not known, but Brown & Burk (1973)
found over 40% of an injected tracer dose of radio-selenite in the
testis-epididymis complex of selenium-deficient rats, after 3
weeks. Autoradiography of epididymal sperm revealed that the
labelled selenium was heavily localized in the midpiece. Calvin
(1978) has isolated a selenopolypeptide, with a relative molecular
mass of 17 000 daltons, from rat sperm, which may have a critical
function in the normal assembly of the sperm tail.
Selenium cannot prevent resorption-gestation in vitamin E-
deficient rats, nor can it improve reproductive performance in
vitamin E-deficient chickens or turkeys (Creger et al., 1960;
Jensen & McGinnis, 1960). However, studies in which severe
depletion of selenium was produced by the long-term feeding of low-
selenium, high-vitamin E diets have shown the beneficial effects of
the element on egg production and hatchability in poultry. For
example, Cantor & Scott (1974) fed 1-day-old Single Comb White
Leghorn chicks semi-purified low-selenium starter, grower, and
developer diets, all of which contained only 0.02 mg naturally-
occurring selenium/kg from 0 to 6, 7 to 12, and 13 to 25 weeks of
age, respectively. Because of the low selenium level, high levels
of vitamin E (50 - 84 IU/kg) were added to the diets to prevent
exudative diathesis during the growth period. From 25 weeks of age
to 10 months, the birds were fed semi-purified low-selenium layer
diets that contained 11 IU of vitamin E/kg. After 10 months, the
hens were fed a low-selenium corn-soybean meal practical laying
ration containing 0.027 mg natural selenium/kg, by analysis. The
calculated vitamin E content was 16 IU/kg. At 12 1/2 months of
age, 4 replicate groups of 5 hens were continued on the low-
selenium diet, whereas a similar number of hens was fed the same
diet supplemented with sodium selenite at 0.1 mg selenium/kg.
Selenium supplementation had beneficial effects on both egg
production and hatchability of fertile eggs (Table 33), but there
was no significant effect of selenium on egg fertility, determined
after 2 - 5 weeks (data not shown).
Table 32. Effects of selenium deficiency on body and testicular weights and
spermatozoan morphology and motility in ratsa
------------------------------------------------------------------------------------
Dietary selenite Number Duration Body Testes Sperm Number of rats
supplement of (month) weight weight motilityb with sperm
(mg selenium/kg) rats (g) (g) midpiece
abnormality
------------------------------------------------------------------------------------
Study 1
0 6 4 452 ± 6 3.29 ± 0.06 6.2 0/6
0.5 6 4 470 ± 18 3.55 ± 0.11 6.2 0/6
0 4c 12 476 ± 17 3.03 ± 0.30 2.5 2/4
0.5 4c 12 494 ± 12 3.57 ± 0.07 5.8 0/4
Study 2
0 10c 11 456 ± 16 3.07 ± 0.09 3.5 5/10
0.4 12 11 543 ± 18 3.50 ± 0.08 8.0 0/12
------------------------------------------------------------------------------------
a Adapted from: Wu et al. (1979).
b Relative activity of sperm observed with a light microscope at 400x at 37 °C and
graded from 0 - 10 with 10 representing the highest motility.
c Two rats died in each of these groups.
Cantor et al. (1978) carried out a similar study on turkeys in
which large white breeder hens and toms were raised from one day of
age until sexual maturity on a series of Torula yeast-containing
diets, low in selenium content (0.025 - 0.047 mg/kg). At 32 weeks
of age, 8 replicate groups, each comprising 6 individually-caged
hens, were fed a low-selenium basal diet (0.033 mg/kg) or the same
diet supplemented with sodium selenite at 0.2 mg selenium/kg.
Eight individually-caged toms were also assigned to each dietary
treatment at 32 weeks of age. Neither the tom nor the hen dietary
treatments had any effect on fertility and the tom dietary
treatment did not have any effects on hatchability. But the
hatchability of fertile eggs from hens fed the unsupplemented and
supplemented diets was about 50 and 59%, respectively. The
mechanism by which dietary selenium improves hatchability in
chickens or turkeys is not known, but the improvement may be due to
increased embryonic selenium levels or to a reduced embryonic need
of vitamin E (Cantor & Scott, 1974).
Table 33. Effect of dietary selenium
supplements on reproductive performance of
12 1/2-month-old selenium-depleted hens fed a
low-selenium, corn-soybean-meal practical
rationa
----------------------------------------------
Selenium supplement None 0.1 mg selenium as
Na2Se03/kg diet
----------------------------------------------
Criterion
Egg production (%)
Weeks 1-2 67.2 76.6
3-4 66.4 78.5
5-6 64.2 71.8
Day 46-76 54.2 79.7
77-107 55.1 76.7
108-137 59.1 75.1
Hatchability of fertile eggs (%)
Week 2 57.3 93.0
3 54.7 97.1
4 54.5 94.9
5 42.4 86.6
17 0.0 90.3
18 8.0 93.6
----------------------------------------------
a Adapted from: Cantor & Scott (1974).
In New Zealand, a close geographical relationship was observed
between the presence of white muscle disease and the incidence of
barren ewes (Table 34). A selenium intervention trial was carried
out on 3 farms where there was a history of white muscle disease
and low lambing percentages (Table 35). The selenium-treated ewes
received 5 mg of selenium, as sodium selenite, at monthly intervals
from one month before tupping until just before lambing; half of
the rams were also given selenium at monthly intervals before and
during tupping (Hartley et al., 1960; Hartley & Grant, 1961).
Selenium-dosed groups on all farms had higher lambing percentages
and fewer barren ewes than the controls. Moreover, white muscle
disease was eliminated in the lambs of the treated ewes. Estrus,
ovulation, fertilization, and early embryonic development all
proceeded normally in selenium-responsive ovine infertility until
between 3 and 4 weeks after conception (at about the time of
implantation) when the embryos perished for unknown reasons
(Hartley, 1963). The actual cause of selenium-responsive
infertility is also unknown and the geographical distribution of
the disease is not always the same as that of other selenium
deficiency diseases, such as white muscle disease or selenium-
responsive unthriftiness (Gardiner et al., 1962). This suggests
either that selenium-responsive infertility occurs only where
intake of selenium is particularly low or that some other factors
must be present that exacerbate the effects of low selenium intake.
For example, mercury has a strong antagonism with selenium (section
7.4.1), and Parizek et al. (1971) showed that inorganic mercury
could block the transport of selenium from mother rats to the
fetuses.
Table 34. Geographical relationship between
congenital white muscle disease and incidence
of barren ewesa
-----------------------------------------------
Congenital Number Incidence of barren ewes
white muscle of up to 11 - 30% over
disease farms 10% 30%
-----------------------------------------------
Present 21 3 8 10
Absent 16 12 4 0
-----------------------------------------------
a Adapted from: Hartley et al. (1960).
Table 35. Effects of selenium (Se) on the reproductive
performance of sheep and white muscle disease in lambsa
-------------------------------------------------------
White muscle
Farm Lambing Barren ewes disease incidence
control Se control Se control Se
-------------------------------------------------------
(%) (%) (%)
C 60.6 94.9 31.1 7.5 37.5 0
D 55.0 86.0 24.3 11.5 22.2 0
E 70.5 93.6 26.1 6.4 12.0 0
-------------------------------------------------------
a Adapted from: Hartley et al. (1960).
7.2.7. Factors influencing deficiency
7.2.7.1. Form of selenium
Schwarz (1961) pointed out that only 0.007 mg naturally-
occurring selenium/kg diet in extracts from pork kidney ("Factor 3
Selenium") was needed to afford 50% protection against dietary
necrotic liver degeneration in rats, whereas 0.020 - 0.030 mg/kg
was required when the selenium was in the form of selenite,
selenate, selenocystine, or selenomethionine. Elemental selenium
was essentially inactive. Many organic derivatives were
synthesized in an attempt to develop selenium compounds that were
not highly toxic but still retained considerable biological potency
against deficiency diseases (Schwarz et al., 1972). Simple amino
acid derivatives of monoseleno diacetic acid appeared promising in
this regard and the hope was expressed that some of these compounds
might make it possible to use selenium in the prevention and cure
of disease (Schwarz, 1976).
Cantor et al. (1975a) studied the ability of selenium in
various poultry feed ingredients to prevent exudative diathesis, a
selenium deficiency disease, in chickens. In general, the selenium
in plant products was more readily available than that in animal
products, but the latter consisted of highly-processed fish or
poultry meals.
Selenium in the form of selenomethionine was less effective
than selenium as sodium selenite for preventing exudative diathesis
in chicks (Noguchi et al., 1973), but the reverse was true for the
prevention of pancreatic fibrosis (Cantor et al., 1975b). In fact,
selenomethionine was four times as effective as either selenite or
selenocystine for this purpose; this phenomenon may have been
related to the peculiar affinity of this compound for the pancreas.
7.2.7.2. Vitamin E
Vitamin E decreases the level of dietary selenium needed to
prevent deficiency diseases in several species of animals. For
example, Scott & Thompson (1971) demonstrated that chicks receiving
100 mg vitamin E/kg diet needed only 0.01 mg selenium/kg diet to
prevent exudative diathesis, whereas chicks not receiving any added
vitamin E needed 0.05 mg/kg. Similarly, Scott et al. (1967) showed
that selenium at 0.18 mg/kg protected against gizzard myopathy in
turkey poults fed diets containing vitamin E, but that 0.28 mg/kg
was needed to protect poults fed diets not supplemented with
vitamin E. Hakkarainen et al. (1978) found that 0.135 mg
selenium/kg was insufficient to prevent deficiency signs in swine
fed a diet containing 1.5 mg vitamin E/kg but was adequate in swine
fed 5.0 mg vitamin E/kg. Increasing the level of dietary vitamin E
decreased the severity of nutritional muscular dystrophy in lambs
fed diets containing selenium at 0.1 mg/kg (Ewan et al., 1968). In
lambs not receiving any supplementary dietary selenium, lambs
receiving low levels of vitamin E died of dystrophy before their
hepatic-selenium levels were depleted to 0.21 mg/kg, the level
considered by Allaway et al. (1966) to be the critical
concentration of selenium in liver for the development of
nutritional muscular dystrophy (section 7.2.3). With the highest
level of vitamin E supplementation, the liver-selenium values were
lower than the critical level suggested by Allaway et al. (1966)
and yet the lambs still survived. These results suggest that the
tissue-selenium level is not the only factor involved, but that the
levels of both tocopherol and selenium determine the appearance of
nutritional muscular dystrophy. The effects of vitamin E in
reducing selenium requirements are readily explainable on the basis
of the close biochemical relationship between these two nutrients
discussed in section 7.2.4.1.
7.2.7.3. Heavy metals and other minerals
Since selenium protects against the toxicity of several heavy
metals (section 7.4), many scientists have examined the effect of
heavy metals on the induction of selenium deficiency. Silver
accelerated the development of liver necrosis in rats (Whanger,
1976) and increased the severity of selenium-vitamin E deficiency
in swine (Van Vleet, 1976; McDowell et al., 1978), presumably by
decreasing the activity of glutathione peroxidase. Inorganic
mercury also decreased glutathione peroxidase activity in the rat
but did not have any effect on the rate of development of liver
necrosis (Whanger, 1976). However, methylmercury accentuated the
development of selenium deficiency in pigs fed a low-selenium diet
(Froseth et al., 1974). In spite of the ability of arsenic to
enhance the biliary excretion of toxic levels of selenium (section
7.1.6.3), several attempts to induce selenium deficiency by feeding
arsenic have been unsuccessful (Levander, 1977; McDowell et al.,
1978). On the other hand, drenching pregnant and lactating ewes
with potassium cyanide, which partially counteracts selenium
toxicity in rats (section 7.1.6.2), increased the incidence of
nutritional myopathy in their lambs (Rudert & Lewis, 1978). It has
been suggested that the use of sulfate fertilizers may decrease
selenium concentrations in forage plants (Pratley & McFarlane,
1974; Westermann & Robbins, 1974), and that this could lead to an
increased incidence of selenium deficiency in farm animals
(Schubert et al., 1961). Dietary sulfate had no effect on the
incidence of white muscle disease but increased the number of lambs
with degenerative lesions of the heart (Whanger et al., 1969).
Neither cadmium nor tellurium had any effect on white muscle
disease in lambs (Whanger et al., 1976b) or vitamin E-selenium
deficiency in swine (McDowell et al., 1978).
7.2.7.4. Xenobiotics
The results of early work by Hove (1948) suggested that vitamin
E could protect rats fed a low-protein diet from death due to
carbon tetrachloride (CCl4) poisoning. The establishment of a
nutritional relationship between selenium and vitamin E stimulated
Seward et al. (1966) to study deficiencies of these nutrients in
relation to poisoning (Table 36). Mortality was higher in the
group deficient in both nutrients than in controls, but selenium
deficiency alone did not increase mortality. Other workers
(Hafeman & Hoekstra, 1977a; Burk & Lane, 1979) have confirmed this
result. The increased mortality due to CCl4 in doubly-deficient
animals could have been due simply to inhibition of eating.
Fasting these animals overnight can precipitate dietary liver
necrosis leading to death (Hafeman & Hoekstra, 1977b).
Table 36. Protective effect of selenium and vitamin E
against carbon tetrachloride toxicity in rats fed a
10% soybean-protein dieta
-----------------------------------------------------
Dietary supplement Weight gain Survival 48 h after
(g/8 weeks) CCl4 injectionb
-----------------------------------------------------
None 62 ± 5 3/10
Vitamin Ec 77 ± 3 9/10
Seleniumd 79 ± 6 8/10
Vitamin E and 80 ± 5 10/10
selenium
-----------------------------------------------------
a Adapted from: Seward et al. (1966).
b All rats injected intraperitoneally with a single
dose at 2 ml/kg body weight.
c Added as a mixture of equal quantities of dl-alpha-
tocopherol and dl-alpha-tocopheryl acetate at 200
mg/kg diet.
d Added at 0.1 mg selenium/kg diet, as sodium selenite.
Paraquat is another xenobiotic that is thought to exert its
toxic effect via increased lipid peroxidation (Bus et al., 1974).
Selenium deficiency worsens lung injury in rats caused by paraquat
(Omaye et al., 1978) and leads to liver injury that would not
otherwise occur in mice exposed to this compound (Cagen & Gibson,
1977). Burk et al. (1980b) showed that selenium-deficient rats
were much more susceptible to paraquat or diquat poisoning than
controls (Table 37). The deficient rats also produced more ethane
and had a higher serum-glutamic-pyruvic transaminase (SGPT)
activity, when treated with paraquat or diquat, than the controls.
The same workers found that injected selenium protected deficient
rats against diquat toxicity before appreciable amounts of
glutathione peroxidase activity appeared in the tissues. They
suggested the existence of a selenium-dependent factor, apart from
glutathione peroxidase which protects against lipid peroxidation.
Combs et al. (1975) noted a similarity between the oedema
produced in chicks fed a practical diet containing polychlorinated
biphenyls (PCBs) and that observed in chicks fed a diet deficient
in selenium and vitamin E (exudative diathesis). When selenium-
depleted hens were fed a diet not containing PCBs, selenite at
0.15 mg selenium/kg diet was sufficient to prevent exudative
diathesis in the chicks, but was not sufficient to prevent the
disease when the hens were fed a diet containing 10 mg PCBs/kg
(Table 38). In another study, addition of 50 mg PCBs/kg diet
increased the incidence of exudative diathesis in vitamin E-
deficient chicks receiving selenite at 0.04 mg selenium/kg diet
from less than 60% in the controls to almost 90% in the PCBs group,
14 days after hatching (Combs & Scott, 1975). Dietary PCBs
depressed plasma-glutathione peroxidase activity and increased the
amount of dietary selenium required to protect liver microsomal
fractions from peroxidation in vitro. On this basis, the authors
concluded that dietary PCBs potentiate vitamin E-selenium
deficiency in chicks by interfering with the biological utilization
of dietary selenium, though some interference with the absorption
and retention of vitamin E was also seen.
Table 37. Effect of selenium deficiency in rats treated with paraquat or
diquata,b
-------------------------------------------------------------------------
Diet Agent Dose Ethane SGPT Survival
group given produced activity time
-------------------------------------------------------------------------
(µmol/kg) (pmol/100 g/h) (mU/ml) (min)
Selenium- paraquat 78 470 ± 93 212 ± 87 297 ± 74
deficient
Control paraquat 78 18 ± 13 32 ± 26 survived 24 h
Control paraquat 390 92 ± 5 45 ± 34 106 ± 30
Selenium- diquat 19.5 1940 ± 420 3490 ± 1940 150 ± 37
deficient
Control diquat 230 56 ± 50 41 ± 5 80 ± 12
-------------------------------------------------------------------------
a Adapted from: Burk et al. (1980b).
b Values are mean ± SD of 4 or 5 values.
Burk & Lane (1979) pointed out the complexity of nutritional
toxicology experimentation involving selenium, vitamin E, and
various drugs and other chemicals. After studying the effects of
numerous xenobiotics on rats that were deficient in either vitamin
E or selenium, they concluded that in vivo lipid peroxidation, as
assessed by ethane production, was not necessarily correlated with
liver necrosis. Moreover, it was concluded that selenium
deficiency was not just a lack of glutathione peroxidase activity
in the tissues, since this condition also elevates hepatic
glutathione S-transferase activity. Thus, selenium deficiency may
actually protect against the hepatotoxicity of certain compounds,
(e.g., acetaminophen and iodipamide) by increasing their
conjugation with glutathione.
Table 38. Effect of PCBs fed to hens on the
incidence of exudative diathesis in progeny fed a
sub-optimal level of seleniuma
------------------------------------------------------
Additions to diets of hensb Exudative Weight gain
-------------------------- diathesis of chicks
PCBsd Seleniume in chicksc (g/2 weeks)
(mg/kg) (mg/kg) (%)
------------------------------------------------------
0 0 35 89
0 0.15 0 102
10 0 30 82
10 0.15 45 78
------------------------------------------------------
a Adapted from: Combs et al. (1975).
b Single Comb White Leghorn hens, 27 weeks old, that
had been reared on low-selenium, semi-purified diets
supplemented with high levels of vitamin E; hens
were on dietary treatments shown in Table for an
additional 5 weeks.
c Determined at 2 weeks of age. Chicks were fed a
selenium-deficient diet (less than 0.02 mg
selenium/kg) supplemented with 0.06 mg selenium, as
Na2Se03/kg. Diet was essentially free of tocopherols.
d Aroclor(R) 1254.
e Added as Na2Se03.
7.2.7.5. Exercise stress
Ancedotal observations from New Zealand suggested that selenium
might be of value against "tying-up" in thoroughbred racehorses
(Hartley & Grant, 1961), a condition that occurs during training
and is associated with exercise. Characteristics include muscle
stiffness and tenderness and disinclination to move. Young &
Keeler (1962) applied mechanical restraint to one foreleg of lambs
born to ewes fed a diet known to produce nutritional muscular
dystrophy and found that lesions either did not occur or occurred
to a much lesser extent in muscles of the restrained limb than in
muscles of the unrestrained limb. Brady et al. (1978) observed
increased levels of malondialdehyde, a product of lipid
peroxidation, in the erythrocytes of 6 horses, immediately after
exercise, but were unable to reproduce this phenomenon in a second
exercise trial with 8 horses. Sodium selenite, given for 4 weeks
in the trace mineral salt at a level calculated to provide 0.15 mg
supplementary selenium/kg diet, did not have any effect on the
elevated plasma-enzyme activities observed in exercised horses.
Furthermore, it did not have any effect on plasma-selenium levels
or erythrocyte-glutathione peroxidase activity. Harthoorn & Young
(1974) commented that the pathological picture in wild animals that
have died following mechanical capture ("capture myopathy" or
"overstraining syndrome") is indistinguishable from white muscle
disease seen in cattle suffering from vitamin E deficiency, but
pointed out that prophylactic and subsequent symptomatic treatment
with vitamin E and selenium-containing preparations did not have
any beneficial effects on captured animals. Brady et al. (1979)
expected to induce death in rats that had been fed a selenium and
vitamin E-deficient diet for 4 weeks, after exercising them to
exhaustion by swimming, but had no success, in spite of markedly
depressed tissue-glutathione peroxidase activity, decreased hepatic
stores of fat-soluble antioxidants, and increased levels of
thiobarbituric acid-reacting substances. The authors concluded
that the rat might be an unsuitable model for an exercise stress-
susceptible species.
7.3. Ratio Between Toxic and Sufficient Exposures
The minimum level of dietary selenium that causes overt signs
of chronic selenium toxicity in most species of animals is of the
order of 4 - 5 mg/kg (US NAS/NRC, 1976). The minimum level of
dietary selenium needed to prevent selenium deficiency diseases in
most species is in the range of 0.02 - 0.05 mg/kg (US NAS/NRC,
1971). Therefore, the ratio of toxic to deficient selenium
exposures is about 100. This difference between the harmful and
beneficial levels of selenium is similar to that between the
harmful and beneficial levels of several nutritionally essential
minerals. However, in the case of selenium, this ratio can be
decreased by certain nutritional or environmental factors. For
example, a deficiency in vitamin E decreases the tolerance of
animals to selenium toxicity (section 7.1.6.2) but increases the
nutritional need for the element (section 7.2.7.2). Also,
inorganic mercury increases the toxicity of methylated selenium
metabolites (section 7.4.1) but methylmercury potentiates selenium
deficiency (section 7.2.7.3). Thus, caution may have to be
exercised in some situations to avoid a diminution in the ratio
between harmful and beneficial intakes of selenium.
7.4. Protection Against Heavy Metal Toxicity
7.4.1. Mercury
Parizek & Ostadalova (1967) first showed that selenium could
protect against the toxicity of mercury in short-term acute
toxicity studies. This led to the suggestion that one of the
nutritional roles of selenium might be protection of the organism
against traces of toxic metals that enter the body, even under
normal conditions (Parizek et al., 1971). Nutritional levels of
dietary selenium have been demonstrated to decrease the chronic
toxicity of the highly hazardous methylmercury (Ganther et al.,
1972) and the selenium in tuna may provide a built-in protective
mechanism against the methylmercury in such fish (Ganther & Sunde,
1974). The possible practical significance of the metabolic
interaction of selenium and mercury has been indicated by the
strong correlations observed between the concentrations of mercury
and selenium in the livers of marine mammals under normal
environmental conditions (Koeman et al., 1975) and in human tissues
following exposure to inorganic mercury (Kosta et al., 1975). The
mechanism by which selenium protects against the toxicity of
methylmercury is not known, but the fact that vitamin E and certain
antioxidants also decrease methylmercury toxicity (Welsh & Soares,
1976; Welsh, 1979) has prompted the hypothesis that these compounds
may diminish methylmercury toxicity by counteracting the damaging
effects of the free radicals generated by its breakdown (Ganther,
1978).
Although inorganic selenium salts protect against the toxicity
of inorganic or methylmercury under a wide variety of conditions,
dimethylselenide has a strong synergistic toxic action with
mercuric chloride (Parizek et al., 1971). The mechanism of this
synergism is unknown.
7.4.2. Cadmium
Parenterally administered selenium protects against the
toxicity of injected doses of cadmium in rats, apparently by
diverting the cadmium from low relative molecular mass target
proteins to other proteins of higher relative molecular mass
(Parizek et al., 1971; Chen et al., 1975). However, selenium does
not cause this diversion when given orally, so any mechanism by
which selenium counteracts cadmium toxicity, under environmental
conditions, is yet to be determined (Whanger, 1976). The
cadmium/selenium antagonism may be important for human health in
that selenium prevents the hypertension caused by long-term cadmium
exposure in rats (Perry et al., 1976).
7.4.3. Other heavy metals
Selenium may interact with lead but the interaction appears to
be much weaker than that with mercury or cadmium, and vitamin E
seems to be more important than selenium in determining the effects
of lead poisoning (Levander, 1979). The interaction of selenium
and silver is of theoretical interest since glutathione peroxidase
activity is depressed by silver (Wagner et al., 1975), but this
interaction does not appear to have any practical significance.
Large doses of selenate protect against thallium toxicity in rats
(Rusiecki & Brzezinski, 1966), but this interaction has not been
investigated in any detail.
7.5. Cytotoxicity, Mutagenicity, and Anti-mutagenicity
7.5.1. Cytotoxicity and mutagenicity
Walker & Ting (1967) treated the soil around barley seedlings
with solutions of sodium selenate to determine the effect on
crossing over. Hoagland's solution containing 0, 0.5, 1.6, or
5.0 mg selenium/litre was applied 10 times, at 2-day intervals, to
each of 4 groups of plants, each treatment consisting of the
application of a 250 ml aliquot of the appropriate solution per
pot. On the day after treatment, the pots were irrigated with
distilled water to prevent lethal accumulation of selenate.
Genetic data indicated a significant effect of the selenate on the
rate of crossing over, consisting of a progressive decline with
increasing concentration.
Walker & Bradley (1969) reared larvae of Drosophila
melanogaster on a semi-defined culture medium containing
selenocystine at concentrations of 0, 2, 10, and 50 µM. The
selenocystine had different effects on crossing over on various
chromosomal segments and the authors concluded that these effects
were mediated through a structural chromosomal protein with a role
in the exchange process.
In studies by Nakamuro et al. (1976), various inorganic
compounds of selenium exhibited clastogenic effects when added in
vitro to cultures of human leukocytes (Table 39). Quadrivalent
selenium compounds were generally more efficient than hexavalent
compounds in inducing chromosome aberrations. Similarly, selenite
was more potent than selenate in causing DNA damage to Bacillus
subtilis that was subject to recombination repair. The same
authors also found that 1.6 x 10-3 mol H2Se03 or Se02 caused about
a 30% inactivation of the tryptophan marker of B. subtilis
transforming DNA, whereas similar concentrations of Na2Se04,
H2Se04, or Na2Se03 were without any significant effect.
Table 39. Chromosome aberrations in leukocytes exposed to selenium compoundsa
-------------------------------------------------------------------------------------
Compound Dose Occurrence of particular chromosome aberrationsb Total
chromatid chromatid iso-chromatid chromatid aberrations
gaps breaks breaks exchanges
-------------------------------------------------------------------------------------
(x 10-5 mol) aberrations per 100 cells
Na2Se04 53 8.4 0.9 0.9 0.9 11.2
26 9.3 0.8 0.8 0.8 11.9
13 11.2 0 0 0 11.2
H2Se04 53 21.0 14.5 3.2 1.6 40.3
26 11.9 0 0 0 11.9
13 9.2 0.8 0 0 10.0
Na2Se03 26 54.0 14.5 1.6 1.6 71.8
13 41.7 8.3 0.9 0.9 51.9
Table 39 (contd.)
-------------------------------------------------------------------------------------
Compound Dose Occurrence of particular chromosome aberrationsb Total
chromatid chromatid iso-chromatid chromatid aberrations
gaps breaks breaks exchanges
-------------------------------------------------------------------------------------
(x 10-5 mol) aberrations per 100 cells
H2Se03 26 113.2 41.2 22.1 5.8 182.4
13 92.3 42.3 12.8 2.6 150.0
6.5 15.2 6.3 5.4 0 26.8
Se02 26 52.1 22.5 7.0 1.4 83.1
13 24.2 2.5 3.3 0 30.0
6.5 15.5 3.6 4.8 0 20.9
None - 7.6 0 0 0 7.6
-------------------------------------------------------------------------------------
a Adapted from: Nakamuro et al. (1976).
b Number of cells examined varied from 62 - 250.
Lo et al. (1978) showed that selenite caused chromosome
aberrations and mitotic inhibition in cultured human fibroblasts
and that incubation with a mouse liver S9 microsomal fraction
increased this capacity of selenite about 5-fold at equimolar
concentrations (Table 40). Selenite also induced DNA fragmentation
and repair synthesis and decreased the clone-forming capacity of
the fibroblasts. Incubation with the S9 fraction increased the
last 2 effects of selenite but slightly decreased the extent of DNA
fragmentation. The response to selenite was similar in cultured
fibroblasts from normal persons and from DNA repair-deficient
xeroderma pigmentosum patients. The ability of selenate to trigger
DNA repair, inhibit the mitotic rate, or cause a lethal effect was
about the same as that of selenite over concentrations varying from
7 x 10-6 to 2 x 10-3 mol. However, when both compounds were
incubated with the S9 fraction, only the activity of the selenite
was potentiated (Table 41).
Table 40. Effect of 1.5 h selenite treatement on chromosome
aberrations and mitotic rate of human fibroblastsa
-------------------------------------------------------------
Sodium Metaphase plates with Mitotic rate
selenite chromosome aberrations without S-9 with S-9
for 1.5 h without S-9 with S-9 (%) (%)
(mol) (%) (%)
-------------------------------------------------------------
Control 1.2 0.8 9.0 8.9
2 x 10-5 1.3 6.9 8.6 9.8
4 x 10-5 1.6 7.4 8.9 9.6
8 x 10-5 3.0 14.3 9.3 9.4
1 x 10-4 3.2 - 8.2 0.1
2 x 10-4 4.7 - 9.5 0.0
4 x 10-4 6.1 - 6.4 0.0
8 x 10-4 14.5 - 6.9 0.0
1 x 10-3 14.2 - 7.6 0.0
3 x 10-3 18.1 - 0.7 0.0
-------------------------------------------------------------
a Adapted from: Lo et al. (1978).
Another example of a tissue fraction influencing the
cytotoxicity of selenite is provided by the work of Ray &
associates (Ray & Altenburg, 1978; Ray et al., 1978). These
workers first showed that sodium selenite tripled the frequency of
sister-chromatid exchanges (SCEs) in lymphocytes cultured with
whole human blood (Table 42). At the concentrations of selenite
used, the selenite could only be present during the final 19 h of
the 96-h culture incubation, otherwise cell death occurred, as
measured by mitotic indices. Later, they found that 7.90 x 10-6
mol Na2Se03 increased SCE frequency, only when the lymphocytes were
cultured in the presence of whole blood or separated red cells.
Lysis of the erythrocytes indicated that the lysate rather than the
red cell ghosts contained the activity necessary for selenite to
raise the lymphocyte SCE frequency. The authors suggested that
exposure of selenite to the lysate possibly resulted in a chemical
modification of the selenite that enabled it to induce SCEs. This
chemical modification of the selenite probably involves reduction
to glutathione selenopersulfide derivatives, since Ray (1984) found
that reduced glutathione (10-3 - 10-4 mol) could substitute for
erythrocytes in activating sodium selenite to its SCE-inducing
form. Whiting et al. (1980) showed that glutathione markedly
stimulated unscheduled DNA synthesis in cultured human skin
fibroblasts and chromosome aberrations in Chinese hamster ovary
cells caused by a variety of inorganic selenium compounds. These
research workers suggested that reduced selenium compounds are the
ultimate mutagens and that their formation depends on reduction by
glutathione.
Table 41. Effect of selenite versus selenate on human
fibroblasts incubated with, or without, mouse liver S9
microsomal fractiona
--------------------------------------------------------------
Treatment DNA repair Mitotic rate Clone forming
(grains/nucleus) (%) capacity (%)
--------------------------------------------------------------
Seleniteb 10.7 5.2 36
Selenite + S9 115.6 0.0 0
Selenate 7.0 4.8 42
Selenate + S9 9.1 3.9 32
--------------------------------------------------------------
a Adapted from: Lo et al. (1978).
b The concentrations used for the estimation of DNA repair,
mitotic rate, and surviving colonies were 8 x 10-4, 2 x 10-7,
and 2 x 10-4 mol, respectively. Selenite and selenate were
applied at equimolar concentrations for 1.5 h.
Table 42. Effect of sodium selenite on
sister-chromatid exchange frequency and
mitotic index in lymphocytes cultured
with whole human blooda
----------------------------------------
Na2Se03 Sister chromatid Average
added exchanges per mitotic
(mol) cellb index
----------------------------------------
Control 6.74 0.03
1.58 x 10-6 7.17 0.03
3.95 x 10-6 7.49 0.03
7.90 x 10-6 20.71 0.02
1.19 x 10-5 21.51 0.03
----------------------------------------
a Adapted from: Ray et al. (1978).
b Average of 4 studies; a total of 100
cells was scored for each Na2Se03
concentration except 75 at the highest
concentration.
In a preliminary note, Lofroth & Ames (1978) stated that
selenite did not give any indication of mutagenicity (<< 0.01
revertants/n mol) whereas selenate gave rise to base-pair
substitutions with about 0.03 revertants/n mol in a Salmonella
plate incorporation test using different histidine-requiring
strains that are reverted to prototrophy by different mechanisms.
On the other hand, Noda et al. (1979) found that both selenite and
selenate were weak mutagens in a similar test, since they gave
rise to base-pair substitution (0.2 and 0.05 revertants/n mol,
respectively). Ray & Altenburg (1980) showed that sodium selenide
and sodium selenite were more active than sodium selenate in their
ability to induce SCEs in human whole-blood cultures. Sirianni &
Huang (1983) found that sodium selenite was the most potent inducer
of SCEs in Chinese hamster V79 cells when S9 mixture was present,
whereas sodium selenide was the most effective inducer in the
absence of S9. For sodium selenate, there was no increase in SCE
rate compared with controls, regardless of whether S9 was present
or absent. At present, it is difficult to provide a biochemical
explanation for the contrasting cytogenic effects exhibited by the
various selenium compounds, when studied in different in vitro
test systems.
Norppa et al. (1980) investigated the chromosomal effects
of sodium selenite when given in vivo. They found that
supplementation of human neuronal ceroid lipofuscinosis patients
with tablets furnishing sodium selenite at a dose of 0.025 mg
selenium/kg body weight for 1 - 13.5 months did not have any
detectable effects on chromosomal aberrations or SCEs in peripheral
blood lymphocytes. Similarly, mice treated with a single dose of
sodium selenite at 0.8 mg selenium/kg body weight did not show any
rise in chromosomal abnormalities in bone-marrow cells or primary
spermatocytes after 24 h. However, there was an increased number
of SCEs and chromosomal aberrations in the bone-marrow cells of
Chinese hamsters, 17 - 19 h after injection with sodium selenite
at a dose of 0.3 - 6.0 mg selenium/kg body weight. It was thought
that the manifestations of chromosomal damage observed in the
second study may have been related to the general toxicity of
selenium at the high doses used.
7.5.2. Anti-mutagenicity
Shamberger et al. (1973a) tested the ability of sodium selenite
and various antioxidants to decrease the chromosomal breakage
induced by 7,12-dimethylbenz[alpha]anthracene (DMBA) in human blood
leukocyte cultures. They found that 0.20 µmol sodium selenite
reduced the breaks caused by 1.6 µmol DMBA by 42% (Table 43). This
concentration of selenite was used because 1 µmol almost completely
inhibited the growth of the cultures. In later studies, Shamberger
et al. (1979) found that sodium selenite at concentrations of
0.67 µmol or less was effective in reducing the mutagenic effects
of malonaldehyde and beta-propiolactone in certain tester strains
of Salmonella typhimurium. Rosin & Stich (1979) showed that sodium
selenite at concentrations of 3 x 10-4 and 1 x 10-3 mol caused
a 50% inhibition of mutagenesis in S. typhimurium induced by N-
methyl- N'-nitro- N-nitrosoguanidine or N-acetoxy-2-acetylamino-
fluorene, respectively. However, 10-2 mol sodium selenite was
toxic to the bacteria in the presence of either carcinogen. Sodium
selenite has also been shown to suppress spontaneous mutagenesis in
yeast cultures (Rosin, 1981).
Table 43. Effects of sodium selenite and various antioxidants on
chromosomal breakage induced by 7,12-dimethylbenz-(alpha)anthracene
(DMBA) in human blood leukocyte culturesa
-----------------------------------------------------------------------
DMBA Antioxidant Number Cells with Breaks Reduction
added added of breaks minus in
(µmol) cells number % control breaks
(%) (%)
-----------------------------------------------------------------------
0 none 211 23 10.9 - -
1.6 none 290 82 28.3 17.4 -
1.6 0.20 µmol Na2Se03 171 37 21.6 10.1 42.0
1.6 10 µmol dl-alpha 156 28 17.9 6.4 63.2
tocopherol
1.6 10 µmol ascorbic acid 127 30 23.6 11.9 31.7
1.6 0.21 µmol butylated 157 29 18.4 6.3 63.8
hydroxytoluene
-----------------------------------------------------------------------
a Adapted from: Shamberger et al. (1973a).
Jacobs et al. (1977b) examined the effects of sodium selenite
on the mutagenicity of 2-acetylaminofluorene (AAF), N-hydroxy-2-
acetyl-aminofluorene (N-OH-AAF), and N-hydroxy-aminofluorene
(N-OH-AF) in the S. typhimurium TA 1538 bacterial tester strain.
Metabolism of AAF and N-OH-AAF to the active mutagen, N-OH-AF was
accomplished by rat liver extracts. Sodium selenite at
concentrations ranging from 0.1 to 40 mmol decreased the
mutagenicity of these compounds (Table 44). However, in the case
of AAF, the authors noted that further decreases in mutagenicity
induced by concentrations of selenium higher than 40 mmol were not
tested, partly because of the formation of a red selenium compound
of low solubility, which can be presumed to have been elemental
selenium.
Ray et al. (1978) determined the frequencies of sister
chromatid exchange (SCE) in lymphocytes resulting from the
simultaneous exposure of whole-blood cultures to different
concentrations of sodium selenite plus 10-4 mol methyl
methanesulfonate (MMS) or 2.7 x 10-5 mol N-hydroxy-2-acetyl-
aminofluorene (N-OH-AAF). The SCE frequency observed as a result
of coexposure to 2 compounds was less than that expected because of
an additive SCE frequency response to each individual compound
(Table 45). In their interpretive analysis of the data, the
authors concluded that the most consistent explanation for the
results was that sodium selenite decreased the SCE-inducing
abilities of MMS and N-OH-AAF.
Table 44. Effect of sodium selenite on the mutagenicity of
2-acetylaminofluorene and its derviatives to Salmonella
typhimuriuma
-------------------------------------------------------------------
Compound Selenium His+ revertants Mutagenic activity
added per plate (± SD) (% of appropriate
(mmol) control)
-------------------------------------------------------------------
4.5 mmol AAF - 1768 ± 149 100
4 1411 ± 41 80
10 1336 ± 69 76
40 1148 ± 71 65
0.45 mmol N-OH-AAF - 2353 ± 12 100
0.4 1891 ± 210 80
4 1598 ± 71 68
10 1247 ± 53 53
40 655 ± 43 28
0.065 mmol N-OH-AF - 1628 ± 41 100
0.1 1280 ± 88 79
10 1233 ± 31 76
20 999 ± 26 61
-------------------------------------------------------------------
a Adapted from: Jacobs et al. (1977b).
Table 45. Effect of methyl methanesulfonate (MMS) or N-hydroxy-2-
acetylaminofluorene (N-OH-AAF), with or without different concentrations of
sodium selenite, on sister chromatid exchange (SCE) frequencies in whole-
blood cultures of lymphocytesa
----------------------------------------------------------------------------
Compound Na2Se03 Total Observed Expected Observed/
(mol) cells SCE/cell SCE/cellb expected
scored (%)
----------------------------------------------------------------------------
None none 100 6.74 ± 0.30 - -
10-4 mol MMS none 100 30.17 ± 0.75 - -
1.58 x 10-6 75 30.60 ± 0.74 30.60 (0.43) 100
3.95 x 10-6 75 30.13 ± 0.96 30.92 (0.75) 97
7.90 x 10-6 75 33.15 ± 1.15 44.14 (13.97) 75
1.19 x 10-5 75 31.60 ± 1.17 44.94 (14.77) 70
None none 125 7.65 ± 0.27 - -
2.7 x 10-5 mol none 125 13.61 ± 0.43 - -
N-OH-AAF 1.58 x 10-6 100 13.79 ± 0.42 13.61 (0.00) 100
7.90 x 10-6 65 22.66 ± 1.10 27.15 (13.54) 83
1.19 x 10-5 25 26.16 ± 1.86 29.24 (15.63) 89
----------------------------------------------------------------------------
a Adapted from: Ray et a1. (1978).
b The expected SCE/cell was determined by adding the observed separate
contributions to SCE frequency due to either MMS or N-OH-AAF to that due
to different concentrations of Na2Se03, as determined in a previous study
(shown in parentheses (see also Table 42)).
Martin et al. (1981) found that sodium selenite could
protect against the mutagenic effects of acridine orange and
7,12-dimethylbenz[alpha]anthracene (DMBA) in the Ames Salmonella/
microsome mutagenicity test. However, Chatterjee & Banerjee (1982)
showed that the influence of sodium selenite on the transformation
of mouse mammary cells, induced by DMBA added in organ culture, was
markedly affected by the level of selenium used. For example,
sodium selenite at concentrations of 10-7 - 10-8 mol increased the
transformation frequency of the cells within the glands. At 10-5
mol, sodium selenite caused an 18 and 84% inhibition of the
frequency of transformed cells at the initiation and promotional
stages, respectively. At 10-4 mol, sodium selenite was toxic to
the cells in vitro. Sodium selenite inhibited the metabolism and
mutagenicity of benzo (a)pyrene to S. typhimurium strain TA 100
in rat (Teel & Kain, 1984) and hamster (Teel, 1984) liver S9
activation systems, but had no inhibitory effect on the
mutagenicity of 1,2-dimethylhydrazine or azoxymethane for S.
typhimurium G46 in the host-mediated assay (Moriya et al., 1979).
On the other hand, sodium selenite at 3.95 x 10-9 - 1.98 x 10-8 mol
protected against chromosomal damage in cultured human lymphocytes
caused by 2.3 x 10-6 mol sodium arsenite (Sweins, 1983). However,
this author reported that the cytotoxic concentration of sodium
selenite in his system was very low (somewhere between 1.98 and
3.95 x 10-8 mol). No explanation was offered for the considerable
variations between laboratories with respect to selenium
cytotoxicity.
Several investigators have now examined the anti-mutagenic
effects of dietary selenium in a variety of experimental systems.
For example, Gairola & Chow (1982) fed rats either a low-selenium
diet based on Torula yeast or the same diet supplemented with
sodium selenite at 2 mg selenium/kg for 20 weeks. Dietary selenium
did not have any effect on the metabolic activation potential of
S9 liver enzymes towards benzo( a)pyrene in the Ames Salmonella/
microsome mutagenicity assay. S9 mixtures from selenium-deficient
rats were more active towards 2-aminoanthracene and less active
towards 2-aminofluorene than mixtures from the selenium-
supplemented rats. The authors concluded that further studies were
needed to elucidate the role and nature of in vitro metabolites
causing mutations in the bacteria. In contrast, Schillaci et al.
(1982) did not find any differences in the mutagenic activation of
7-12-dimethylbenz[alpha]anthracene (DMBA) by liver S9 mixtures in
the Ames test with S. typhimurium strain TA 98, prepared from rats
fed Torula yeast-based diets supplemented with sodium selenite at
0.1, 2.5, or 5.0 mg selenium/kg, from weaning for 3 weeks.
However, if the rats were injected with 20, 50, or 100 mg
Aroclor(R) 1254/kg body weight, 5 days before sacrifice, dietary
selenium at 2.5 or 5.0 mg/kg in the form of sodium selenite
markedly decreased the mutagenic activation of DMBA by liver
microsomes.
Lawson & Birt (1983) measured single-strand breaks (SSB)
produced in pancreatic DNA by injecting 20 mg N-nitrosobis(2-
oxopropyl) amine (BOP)/kg body weight subcutaneously into hamsters
that had been fed a Torula yeast-based diet supplemented with
sodium selenite at 0, 0.1, or 5 mg selenium/kg, for 4 weeks
previously. One hour after injection with BOP, there were 2.26 ±
0.47, 2.83 ± 0.43, and 1.74 ± 0.43 SSB per 108 daltons of DNA (mean
± SEM), respectively, in the 3 dietary groups, and the approximate
half-lives of the SSB were 33, 30, and 8 days, respectively. On
this basis, the authors suggested that high levels of dietary
selenium may stimulate the repair of carcinogen-induced DNA damage.
Olsson et al. (1984) used a novel isolated rat liver/cell
culture system to study the effects of selenium deficiency and
selenium supplementation, within the physiological range, on the
anti-mutagenic effects of the element. Rats were first fed a
Torula yeast-based selenium-deficient diet for 5 - 6 weeks with or
without sodium selenite supplementation at 0.2 mg selenium/litre
drinking-water. The rat livers were then connected to an isolated
liver perfusion system. A glass plate with cultured Chinese
hamster V79 cells was introduced into the perfusion system
immediately before the addition of 5 mmol dimethylnitrosamine (DMN)
and then exposed directly to the circulating perfusate. After 2 h,
this glass plate was exchanged for another with fresh V79 cells,
which were exposed during the subsequent 2 h. This procedure was
adopted to avoid a possible toxic effect that might influence
mutation frequency. As in vitro controls, V79 cells were treated
for 2 h in Krebs-Ringer albumin solution with or without DMN. The
induced mutation frequencies in the 2 successively treated cell
populations were summarized to give the value for each liver. It
was found that the mutagenicity of DMN in Chinese hamster V79
cells, after metabolic activation by the isolated perfused rat
liver, was approximately doubled when livers from selenium-
deficient rats were used compared with livers from rats given the
supplementary selenium. The authors were unable to provide a
precise biochemical explanation for the mechanism by which selenium
deficiency increased the mutagenicity of DMN, but they noted that
microsomal N-oxygenation of N,N-dimethylaniline (DMA) was
decreased in livers from selenium-deficient rats and suggested that
further investigation of the different enzymes involved in DMA- N-
oxygenation appeared warranted. Another application of this liver
perfusion/cell culture system was demonstrated by the work of Beije
et al. (1984) who showed that bile collected from selenium-
deficient livers perfused with a medium containing 5 mmol 1,1-
dimethylhydrazine was much more mutagenic toward Chinese hamster
V79 cells than bile from livers of rats given supplementary
selenium and perfused in a similar manner. The authors suggested
that the increased biliary excretion of reactive mutagenic
metabolites observed in their selenium-deficient rat liver
perfusion system might furnish a potential explanation for some of
the protective effects of selenium reported against chemically-
induced colon cancer in experimental animals.
7.6. Teratogenicity
Franke & Tully (1935) obtained chicken eggs from 2 farms in
South Dakota that had histories of low hatchability. Hatchability
in the 2 test groups was extremely low, being 4% in one and 12% in
another. Examination of the eggs revealed that about 75% of those
that had failed to hatch on the 21st day contained deformed
embryos. Franke et al. (1936) then showed that injection of
selenium salts into the air cell of eggs before incubation resulted
in monsters similar to those occurring naturally on affected farms.
Selenium injected as selenite to give a final concentration in the
egg of 0.6 mg selenium/kg was the most effective in this respect,
higher doses causing embryonic death and lower doses producing
fewer monsters (Table 46). When the toxicity of various selenium
compounds for chick embryos was compared, it was found that
selenate was about twice as toxic as selenite (Palmer et al., 1973)
(Table 47). The toxicity of selenomethionine was about the same as
that of selenate but methylseleninic acid was more than twice as
toxic. The toxicity of trimethylselenonium chloride was relatively
low. The most common embryonic deformities observed in this study
were the underdevelopment of the beak and abnormal development of
the feet and legs, especially a fusing or webbing of the 2 outside
toes. Because of the metabolic relationships between selenium and
arsenic or cadmium, Holmberg & Ferm (1969) investigated the
teratogenic potential of these elements, administered separately or
together to golden hamsters (Table 48). Embryonic malformations
were not observed when pregnant hamsters were injected
intravenously with a barely sub-lethal dose of sodium selenite
alone, and the 6% resorption rate observed was stated to be similar
to the normal rate of resorption in this species. Furthermore,
under these conditions, sodium selenite actually gave partial
protection against the teratogenesis induced by sodium arsenate or
cadmium sulfate.
Table 46. Teratogenicity of sodium selenite
injected into hens' eggsa
--------------------------------------------------
Sodium selenite Total Dead Abnormal Normal
(mg selenium/kg) embryos (%) (%) (%)
--------------------------------------------------
0.9 5 60.0 20.0 20.0
0.8 16 12.5 31.0 56.5
0.7 4 50.0 25.0 25.0
0.6 4 50.0 50.0 0
0.5 12 50.0 8.3 41.7
0.1 131 24.4 2.3 73.3
0.02 78 53.8 3.8 42.3
0.01 64 34.3 9.4 56.3
--------------------------------------------------
a Adapted from: Franke et al. (1936).
Table 47. Comparative toxicity of various selenium compounds for chick
embryosa
----------------------------------------------------------------------------
Compound Dose Livability ratio LD50 with 95%
(mg selenium/kg) (live chicks/ confidence limits
fertile eggs) (mg selenium/kg)
----------------------------------------------------------------------------
sodium selenite 0.0 16/18
0.05 18/18
0.1 16/17 0.3b
0.2 17/18
0.4 2/18
0.8 0/18
sodium selenate 0.0 18/18
0.1 12/18
0.2 4/18 0.13
0.4 1/18 (0.086 - 0.17)
0.8 0/18
1.6 0/18
selenomethionine 0.0 17/18
0.05 18/18
0.1 13/18 0.13
0.15 5/18 (0.095-0.15)
0.2 4/18
0.4 0/18
Table 47 (contd.)
----------------------------------------------------------------------------
Compound Dose Livability ratio LD50 with 95%
(mg selenium/kg) (live chicks/ confidence limits
fertile eggs) (mg selenium/kg)
----------------------------------------------------------------------------
methylseleninic 0.0 23/24
acid 0.025 17/18
0.05 9/18 0.052
0.1 2/24 (0.039-0.065)
0.2 0/24
0.4 0/24
trimethylselenonium 0.0 18/18
chloride 6.0 17/18
12.0 13/18 15.7
18.0 5/17 (12.7 - 19.0)
24.0 5/18
30.0 3/18
----------------------------------------------------------------------------
a Adapted from: Palmer et al. (1973).
b An estimate since the data did not allow calculation by the computer
programme.
7.7. Carcinogenicity and Anti-Carcinogenicity
7.7.1. Selenium as a possible carcinogen
Five investigations have been reported in the literature on the
alleged carcinogenicity of selenium for laboratory animals. Nelson
et al. (1943) fed groups of 18 female rats of an inbred Osborne-
Mendel strain a 12% protein diet consisting of 49% corn, 44% wheat,
3% yeast, and 1% each of cod liver oil, calcium carbonate, sodium
chloride and dried whole liver. The diet was supplemented with 0,
5, 7, or 10 mg selenium/kg, as seleniferous corn or wheat, or 10 mg
selenium/kg, as a mixed inorganic selenide containing ammonium
potassium selenide and ammonium potassium sulfide. Although
cirrhosis was frequently observed after 3 months of selenium
exposure, no tumours or advanced adenomatoid hyperplasia were seen
in any of the 73 selenium-exposed rats that died or were sacrificed
before 18 months. Of the 53 selenium-exposed rats that survived
18 - 24 months, 43 had cirrhosis and 11 developed liver cell
adenoma or low-grade carcinoma without metastasis in cirrhotic
livers. There was no relationship between the extent of cirrhosis
and tumour incidence except that there were no tumours in any of
the 18- to 24-month-old rats that did not have cirrhosis. The
incidence of spontaneous adenoma and low-grade carcinoma of the
liver in the unexposed rats was low and the incidence of
spontaneous hepatic tumours in the rat colony was less than 1% in
18- to 24-month-old rats.
Table 48. Effect of selenium, arsenic, and cadmium on embryonic death and
malformations in the golden hamstera
------------------------------------------------------------------------------------
Treatment Dose Total Number of Number of Malformed Malformed
(mg/kg) number of embryos embryos (%) or resorbed
embryos resorbed malformed (%)
------------------------------------------------------------------------------------
sodium selenite 2 86 5 0 0 6
sodium arsenate 20 177 62 86 49 84
sodium arsenate 20 144 28 28 19 39
+ sodium selenite 2
cadmium sulfate 2 115 24 59 51 72
cadmium sulfate 2 82 2 3 4 6
+ sodium selenite 2
------------------------------------------------------------------------------------
a Adapted from: Holmberg & Ferm (1969).
The results of some of the initial studies of Volgarev &
Tscherkes (1967) seemed to indicate an increased incidence of
tumours in rats fed a low-protein diet supplemented with sodium
selenate at 4.3 mg selenium/kg, but subsequent tests carried out by
these authors failed to confirm these results. Moreover, their
work suffered from the fact that no control groups, consuming diets
without additional selenium, were included in any of their trials.
Schroeder & Mitchener (1971b) reported an increased incidence of
tumours in rats given sodium selenate at 2 mg selenium/litre in the
drinking-water for the first year of life followed by 3 mg/litre
until death. In a previous evaluation of this work (US NAS/NRC,
1976), it was observed that the selenate-treated rats survived
longer than the untreated rats and this could have contributed to
the increased tumour incidence observed in the rats receiving
selenate. Also, as the organs and tissues did not appear to have
been systematically searched, the type and incidence of
histological lesions could not be known with certainty.
Schroeder & Mitchener (1972) carried out 2 studies in which
mice were given selenium in the drinking-water. In both studies,
the mice were fed a diet composed of 60% whole rye flour, 30% dried
skim milk, 9% corn oil, and 1% iodized sodium chloride, to which
were added vitamins and iron. The diet was calculated to contain
24% protein, 65% carbohydrate, and 11% fat (dry weight). The mice
were given doubly deionized water for drinking, which originally
came from a forest spring. Certain essential trace metals were
added to the drinking-water, as soluble salts, in the following
concentrations (mg/litre): zinc, 50; manganese, 10; copper, 5;
cobalt, 1; and molybdenum 1. All mice received these trace
elements in their drinking-water. In addition, chromium was added
to the drinking-water at a level of 1 or 5 mg/litre in the first
and second studies, respectively. In the first study, groups of
100 or more Swiss mice of the Charles River CD strain, containing
equal numbers of each sex, were given, at weaning, sodium selenite
at either 0 or 3 mg selenium/litre of the trace element-fortified
drinking-water. This regimen continued over the entire life span
of the mice. The second study was identical to the first, apart
from the level of chromium given in the drinking-water, and the
fact that the selenium was administered in the form of sodium
selenate. Of the 180 control mice autopsied from both studies, 119
were sectioned. Tumours were found in 23 (19%) of those sectioned
and 10 of the tumours (43%) were malignant. The different forms of
selenium given did not influence the incidence of tumours and, of
the 176 selenium-exposed mice autopsied in both studies, 88 were
sectioned. Tumours were found in 13 (15%) of those sectioned but
all tumours were malignant. It was concluded that selenium had
little tumourigenic or carcinogenic activity in mice, though, when
tumours did appear in the selenium-exposed mice, they were all
malignant.
The results of studies of Harr et al. (1967) (section 7.1.2.2)
failed to demonstrate any tumours attributable to selenium, but
most of the rats fed semi-purified diets containing levels of
selenium greater than 2 mg/kg died within 100 days and almost all
were dead before 2 years. Exceptions included 1 rat fed selenate
at 4 mg selenium/kg diet containing 12% casein and 0.3% DL-
methionine, and 27 rats alternating at weekly intervals between a
control ration and a diet containing sodium selenate at either 4 or
8 mg selenium/kg. However, no hepatic tumours were observed, even
in the 71 rats that survived 2 years or longer at continuous
dietary-selenium levels of 0.5 - 2.0 mg/kg.
The above studies indicate that test animals develop neoplastic
lesions, only when they have liver cirrhosis produced by frank
selenium toxicity (i.e., no hepatomas were observed in the absence
of severe hepatotoxic phenomena). For this reason, it was
concluded that selenium was not, by reason of its capacity to
induce liver damage when consumed at high levels, properly
classified as carcinogenic because of its potential association
with a higher rate of liver cancer (Gardner, 1973).
Jacobs & Forst (1981b) did not observe any signs of neoplasia
in groups of 35 female Swiss mice fed a commercial pelleted diet
and given 0, 1, 4, or 8 mg selenium/litre drinking-water, as sodium
selenite, for 50 weeks.
Three studies have shown carcinogenic effects that appear to be
more a specific effect of a particular selenium compound rather
than an effect of selenium itself. Seifter et al. (1946) found
that 8 white rats that had received 0.05% bis-4-acetamino-phenyl-
selenium dihydroxide in their diet for 105 days had multiple
adenomas of the the thyroid glands and adenomatous hyperplasia of
the liver. Innes et al. (1969) fed the maximal tolerated dose of
selenium diethyldithiocarbamate (Ethyl selenac) to a group of 72
mice containing both sexes of two hybrid strains (C57BL/6 x
C3H/Anf)F and (C57BL/g x AKR)F1. The mice were given this
substance by stomach tube at a dose of 10 mg/kg body weight,
starting at 7 days of age. At 4 weeks of age, the chemical was
mixed directly into the diet at a concentration of 26 mg/kg. After
82 weeks, all the surviving mice were sacrificed; of 69 necropsied,
26, 13, and 5.8% had hepatomas, lymphomas, and pulmonary tumours,
respectively. Among 338 negative control mice, most of which were
sacrificed between 78 and 89 weeks, the incidences of the
corresponding tumours were 4.1, 4.1, and 6.2%, respectively.
A report from the US National Cancer Institute (National Cancer
Institute, 1980) suggested that commercial selenium sulfide, an
ingredient in certain anti-dandruff shampoos, was carcinogenic for
rats and mice. Elemental analysis of the test chemical used in
this study indicated that the material was a mixture of selenium
monosulfide and selenium disulfide. The melting point of the test
sample was closer to that reported for the monosulfide than that
reported for the disulfide and the X-ray diffraction pattern was
consistent with patterns reported for the monosulfide. It was
concluded that the selenium in the test substance used in this
bioassay was present primarily as the monosulfide.
In the rat study, groups of 4-week-old male and female Fischer
F344 rats were fed presterilized lab meal and were given untreated
well water ad libitum for 104 - 105 weeks. During the first 103
weeks, 50 rats of each sex were given one of 4 treatments:
untreated control; vehicle control (received volumes of 0.5%
aqueous carboxymethylcellulose equal to those of the test solutions
administered); low-dose group (3 mg selenium sulfide suspended in
0.5% aqueous carboxymethylcellulose/kg body weight, 5 days per
week, given by gavage); or high-dose group (15 mg selenium
sulfide/kg body weight administered by the same route and
schedule). The results showed an increased incidence of primary
liver tumours in both male and female high-dose groups (Table 49).
Neoplastic nodules were usually single, rather well-defined
areas characterized by altered hepatocytes. In most instances, the
hepatocytes were larger than normal, eosinophilic, and occasionally
vacuolated. The normal architecture was altered, mainly resulting
in a solid mass of hepatocytes or a trabecular pattern rather than
the normal hepatic cords. The mass compressed the adjacent
parenchyma around the periphery. Anaplasia and mitoses were
minimal. Hepatocellular carcinomas were usually large multinodular
masses, often encompassing entire liver lobes or even multiple
lobes. The histological appearance of these neoplasms varied from
areas appearing normal to much more anaplastic areas. The
neoplastic hepatocytes varied from small basophilic cells to very
large eosinophilic and occasionally vacuolated cells. Mitoses were
variable. No distant metastases were observed in any of the rats
bearing hepatocellular carcinomas. No other neoplasms were found
that could be related to the administration of the selenium
sulfide.
Table 49. Effect of selenium sulfide, given orally for 103 weeks, on
the incidence of liver tumours in Fischer F344 ratsa
------------------------------------------------------------------------
Initial Selenium Tumour incidence
Test group Sex number sulfide Hepatocellular Neoplastic
of rats dose carcinoma nodules
------------------------------------------------------------------------
(mg/kg)
Untreated control M 50 0 1/48 (2%) 3/48 (6%)
Vehicle controlb M 50 0 0/50 (0%) 1/50 (2%)
Low-dosec M 50 3 0/50 (0%) 0/50 (0%)
High-dosec M 50 15 14/49 (29%) 15/49 (31%)
Untreated control F 50 0 0/50 (0%) 0/50 (0%)
Vehicle controlb F 50 0 0/50 (0%) 1/50 (2%)
Low-dosec F 50 3 0/50 (0%) 0/50 (0%)
High-dosec F 50 15 21/50 (42%) 25/50 (50%)
------------------------------------------------------------------------
a Adapted from: National Cancer Institute (1980).
b Received only vehicle for dosing (0.5% aqueous carboxymethylcellulose)
5 days per week, by gavage.
c Received stated dose of selenium sulfide suspended in 0.5% aqueous
carboxymethylcellulose, 5 days per week, by gavage.
An increased incidence of focal cellular change in the liver
was noted in high-dose male rats but was essentially comparable in
frequency in the remaining treated and control groups of each sex.
A compound-related increase in pigmentation in the lungs was
observed. This was characterized by the accumulation of dark,
slightly granular-appearing pigment in the interstitial areas and
in some peribronchial areas. In most cases, the pigment appeared
to be located within cells, principally macrophages. No evidence
of inflammation, relative to the pigment deposits, was noted. Lung
pigmentation was found in 47/49 (96%) high-dose males, 1/50 (2%)
low-dose males, 45/50 (90%) high-dose females, and 36/50 (72%) low-
dose females, but not in control males or females.
The protocol for the testing of selenium sulfide in B6C3F1 mice
was similar to that used for rats, except that the doses of the
substance under test were increased (Table 50). There was an
increased incidence of primary liver and lung tumours in the high-
dose female mice and a marginal increase in the incidence of these
tumours in high-dose males.
Hepatocellular carcinomas varied from single nodules to
multinodular masses, often encompassing several liver lobes.
Individual hepatocytes varied considerably in morphology from large
eosinophilic cells to small, darkly-staining hepatocytes. In many
cases, there was marked variation in cell type in different parts
of the neoplasm. The number of mitoses varied. The number of
hepatocellular carcinomas metastasizing to the lungs was comparable
in vehicle-control and high-dose males. No metastases were
observed in the lung in female mice. Alveolar/bronchiolar adenomas
were usually small solitary lesions located in the subpleural area
or immediately adjacent to a bronchiole. The cells involved were
cuboidal to tall columnar and tended to be situated perpendicular
to the basement membrane in a single layer. These cells were
arranged in complex papillary projections forming discrete nodules
and compressing adjacent alveolar walls. Mitoses were rare.
Alveolar/bronchiolar carcinomas, however, were less discrete
lesions and tended to be larger and occasionally multiple,
consisting of a confluence of two or more nodules. The individual
cells tended to be less rigidly arranged along basement membranes
and were often piled up in layers or arranged in solid sheets
without a papillary pattern. The cells often showed increased
basophilia and a moderate mitotic index. Evidence of invasion into
adjacent vessels, or extension into bronchioles and adjacent lung
parenchyma was frequently present.
Other neoplasms that occurred in the mice were similar in
number and kind to those that usually occur in aged B6C3F1 control
mice and could not be related to the long-term administration of
selenium sulfide.
In a comparison study, the effect of the dermal application of
selenium sulfide was examined. No increased incidence of neoplasms
was observed that could be attributed to selenium sulfide.
7.7.2. Selenium as a possible anti-carcinogen
High levels of dietary selenium have been shown to protect
laboratory animals against chemical carcinogenesis, under a wide
variety of conditions. Clayton & Baumann (1949) fed 2 groups of 15
young adult rats, weighing approximately 200 g, a basal diet
consisting of extracted casein, 12 parts; salts, 4 parts; corn oil,
5 parts; and glucose monohydrate (Cerelose) to 100 parts. The diet
was supplemented with thiamin, riboflavin, pyridoxine, calcium
pantothenate, and choline. Fat-soluble vitamins were provided by
giving 2 drops of halibut liver oil per rat every 4 weeks. During
the initial 4-week feeding period, both groups received the basal
diet supplemented with 0.064% 3'-methyl-4-dimethylaminoazobenzene.
This was followed by a 4-week "interruption period" during which
neither group received the azo dye, but one group received the
basal diet supplemented with sodium selenite at 5 mg selenium/kg,
and the other group received the basal diet without any added
selenium. During the third, 4-week feeding period, both groups
received the basal diet supplemented with 0.048% of the azo dye,
but no supplementary selenium. During the final 8-week feeding
period, both groups received the basal diet without either azo dye
or selenium. Two of the 9 surviving rats in the group that
received supplementary selenium during the "interruption period"
had liver tumours (22%), compared with 4 out of 10 survivors (40%)
in the group not supplemented with selenium. The results of a
second similar study showed a liver tumour incidence of 4/13 (31%)
in the selenium-supplemented group compared with 8 out of 13 rats
(62%) in the unsupplemented group.
A preliminary study by Shamberger & Rudolph (1966) indicated
that concomitant dermal application of sodium selenide reduced the
tumour-promoting effect of croton oil in mice initiated with 7,12-
dimethylbenz[alpha]anthracene (DMBA). Riley (1968) found that a
similar application of sodium selenide prevented the mast cell
reaction caused by an active fraction of croton oil in the skin of
DMBA-initiated mice. In a later set of studies (Shamberger, 1970),
2 groups of 30 female ICR Swiss mice, 50 - 55 days old, were
treated once on day one with 0.125 mg DMBA dissolved in 0.25 ml
acetone. On days 2 - 21, the shaved backs of the first group of
mice were treated with 0.25 ml of a 20:80 water-acetone mixture
containing 0.0005% sodium selenide, whereas the second group was
not treated with any anti-oxidant. Subsequently, in 3 separate,
but essentially identical, studies, both groups of mice received
0.25 ml of 0.04% croton oil in acetone daily for 16, 18, and 18
weeks respectively. After the croton oil treatment, the incidence
of papillomas in the selenide-treated groups was 17, 63, and 45%,
respectively, in 3 separate studies, compared with an incidence in
the non-treated groups of 43, 63, and 89%, respectively. The
corresponding number of papillomas per mouse in the 3 studies was
1.5, 3.0, and 1.5, respectively, in the selenide-treated groups and
3.2, 6.0, and 2.3 in the non-treated groups. No papillomas were
observed in 3 DMBA-initiated control groups, which did not receive
either antioxidant or croton oil. A similar protective effect of
sodium selenide was observed in 3 additional studies in which the
promoting agents were croton oil, croton resin, and phenol and in
which the selenide was applied concomitantly with the promoter.
In another study, 0.25 ml of 0.01% 3-methylcholanthrene (MCA)
was applied daily to the shaved backs of one group of mice for 19
weeks; a second group received 0.0005% sodium selenide together
with the MCA. After 19 weeks, the incidence of papillomas was 68%
in the selenide-treated group and 87% in the untreated group, and
the number of papillomas per mouse was 3.2 and 2.2, respectively.
After 30 weeks, the number of mice with cancers was 17/28 and 25/30
in the selenide-treated and untreated groups, respectively, the
total number of cancers per group being 29 and 71, respectively.
Table 50. Effect of selenium sulfide given orally for 103 weeks on the incidence of liver and lung
tumours in B6C3F1 micea
----------------------------------------------------------------------------------------------------
Lung tumour incidence
Initial Selenium Liver tumour incidence Alveolar/ Alveolar/
Test group Sex number sulfide hepatocellular hepatocellular bronchiolar bronchiolar
of mice dose carcinoma adenoma carcinoma adenoma
(mg/kg)
----------------------------------------------------------------------------------------------------
Untreated control M 50 0 17/49 (35%) 3/49 (6%) 1/49 (2%) 8/49 (16%)
Vehicle controlb M 50 0 15/50 (30%) 0/50 (0%) 1/50 (2%) 3/50 (6%)
Low-dosec M 50 20 11/50 (22%) 3/50 (6%) 2/50 (4%) 8/50 (16%)
High-dosec M 50 100 23/50 (46%) 0/50 (0%) 2/50 (4%) 12/50 (24%)
Untreated control F 50 0 2/50 (4%) 1/50 (2%) 0/50 (0%) 2/50 (4%)
Vehicle controlb F 50 0 0/49 (0%) 0/49 (0%) 0/49 (0%) 0/49 (0%)
Low-dosec F 50 20 1/50 (2%) 1/50 (2%) 1/50 (2%) 2/50 (4%)
High-dosec F 50 100 22/49 (45%) 6/49 (12%) 4/49 (8%) 8/49 (16%)
----------------------------------------------------------------------------------------------------
a Adapted from: National Cancer Institute (1980).
b Received only vehicle for dosing (0.5% aqueous carboxymethylcellulose), 5 days per week, by gavage.
c Received stated dose of selenium sulfide suspended in 0.5% aqueous carboxymethylcellulose, 5 days
per week, by gavage.
Shamberger (1970) also carried out 2 dietary studies in which 4
groups of 36 female, albino ICR Swiss mice, 50 - 55 days old, were
fed Torula yeast diets supplemented with sodium selenite at 0, 0.1,
or 1.0 mg selenium/kg or sodium selenide at 0.1 mg selenium/kg.
After 2 weeks on the test diets, 0.125 mg DMBA dissolved in acetone
was applied once to the skin. After 3 weeks, 0.25 ml 0.05% croton
oil in acetone was applied daily for 17 weeks. At this time, 26/36
mice fed the unsupplemented diet had papillomas, compared with
14/35 mice fed the diet supplemented with 1.0 mg selenium/kg, as
selenite. The incidence of papillomas was slightly elevated in the
mice fed the diet supplemented with 0.1 mg selenium/kg, as sodium
selenite, compared with the unsupplemented mice. The incidence of
papillomas in mice fed sodium selenide at 0.1 mg selenium/kg was
intermediate between that of the unsupplemented group and the group
fed sodium selenite at 1.0 mg selenium/kg.
In a second study of similar design, 4 groups of 36 mice were
fed the test diets described above for 2 weeks. Then 0.25 ml of
0.03% benzo( a)pyrene in acetone was applied daily to the shaved
backs of the mice for 27 weeks. At this time, the incidence of
papillomas in the groups fed the torula diet supplemented with 0,
0.1, or 1.0 mg selenium/kg, as selenite, or 0.1 mg selenium/kg, as
selenide, was 14/35, 22/36, 8/33, and 12/35, respectively. The
number of papillomas per mouse in the corresponding groups was 8.1,
9.8, 5.0, and 6.8.
Harr et al. (1972) weaned 80 female OSU-Brown rats at 35 days
of age and divided them into 4 groups of 20. Each group was fed a
low-selenium basal diet (0.018 µg selenium/kg) that included Torula
yeast as the protein source and contained 60 mg vitamin E/kg.
Groups, 1, 2, 3, and 4 were fed the basal diet supplemented with
sodium selenite at 2.5, 0.5, 0.1, and 0 mg selenium/kg,
respectively. All diets contained 150 mg 2-acetylaminofluorene/kg.
The 40 rats in groups 1 and 2 were born from parents reared on a
selenium-depleted regimen, but were clinically normal. The 40 rats
in groups 3 and 4 were from the second generation maintained on the
depeletion regimen and had clinical signs of selenium deficiency.
After 200 days of treatment, the number of mammary adenocarcinomas
in groups 1, 2, 3, and 4 was 0, 1, 8, and 9, respectively. After
320 days, the number of mammary adenocarcinomas in the same groups
was 11, 11, 13, and 12, respectively. Mammary adenocarcinomas in
groups 1, 2, and 3 occurred mainly (90%) in the thoracic region and
were well circumscribed, firm, and easily removed, whereas those in
group 4 occurred mainly (80%) in the pelvic area and were soft and
fluid, contained little connective tissue, and were invasive.
After 240 days of treatment, the number of hepatic carcinomas in
groups 1, 2, 3, and 4 was 0, 1, 9, and 4, respectively. After 320
days, the number of hepatic carcinomas in the same groups was 8,
12, 12, and 6, respectively. Toxic hepatitis was observed in 18 of
the 20 livers from group 1 (selenium added at 2.5 mg/kg), but was
not seen in the other groups.
In studies of Marshall et al. (1978), 2 groups of male albino
Sprague Dawley rats were fed diets containing 2-acetylaminofluorene
at 0.3 g/kg, for 14 weeks. One group received 4 mg selenium, as
sodium selenite/litre drinking-water. The carcinogen was withdrawn
from the diet for an additional 4 weeks and then the rats were
sacrificed. The rats given selenium had about 50% fewer liver
tumours than unsupplemented rats. Control rats given a similar
level of selenium in the water showed normal growth response, liver
weight, and appearance.
Three groups of 15 male Sprague Dawley rats, weighing about
250 g, were fed a basal diet of finely ground Purina Laboratory
Chow and water ad libitum (Griffin & Jacobs, 1977). One group did
not receive any supplementary selenium, a second group received 6
mg selenium, as sodium selenite/litre drinking-water, and a third
group received 6 mg selenium, in the form of a high selenium
yeast/kg diet. After one week on this regimen, all 3 groups were
given 0.05% 3'-methyl-4-dimethyl-aminoazobenzene in the diet for 8
weeks. Following this, all groups were maintained on the
carcinogen-free diets for an additional 4 weeks. The selenium
supplements were maintained for the second and third group
throughout the entire study. At sacrifice, the incidence of liver
tumours in the surviving rats was 92% (11/12) in the unsupplemented
group, 46% (7/15) in the group supplemented with selenite in the
drinking-water, and 64% (9/14) in the group supplemented with
selenium yeast in the diet. In this study, the selenium
supplements had little effect on the growth of the rats.
Histopathological examination of several of the tumours revealed
that they were bile duct adenocarcinomas and liver cell
adenocarcinomas.
Jacobs et al. (1977a) injected 2 groups of 15 male, 8-week-old
Sprague Dawley rats, weekly, with 20 mg sym,-dimethylhydrazine
dihydrochloride (DMH)/kg body weight, for 18 weeks. One group of
rats received sodium selenite at 4 mg selenium/ litre drinking-
water, which was available ad libitum one week prior to, and
throughout, administration of the carcinogen. The other group did
not receive selenium added to the drinking-water. The group
receiving DMH and no added selenium had an 87% incidence (13/15) of
colon tumours, whereas the group receiving both DMH and added
selenium had a 40% incidence (6/15) of colon tumours. The total
number of colon tumours was 39 in rats receiving only DMH and 11 in
rats receiving both DMH and added selenium. In a similar study in
which 2 groups of 15 rats were injected weekly with 20 mg of
(methylazoxyl)-methanol acetate (MAM)/kg body weight for 18 weeks,
no significant differences in the incidence of colon tumours were
apparent between groups with (14/15) and without (14/14) added
selenium in the drinking-water. However, the number of MAM induced
tumours was 73 in the group given MAM alone and 42 in the group
receiving both MAM and added selenium.
Schrauzer and colleagues carried out a series of studies in
which exogenous carcinogens were not used, to test the ability of
supplemental selenium in influencing the development of spontaneous
mouse mammary tumours, presumably of viral origin. In study 1
(Schrauzer & Ishmael, 1974), 2 groups of 30 virgin female C3H/St
mice, 4 - 6 weeks old, were fed a basal diet ("Concord Maid")
consisting of meat scraps, dried skimmed milk, oat groats, ground
wheat, wheat germ meal, vegetable oil, cane molasses, salt,
brewer's yeast, cereal binder, sodium propionate, and calcium
pantothenate. The diet contained about 150 g protein, 5 g fat, and
0.15 mg selenium/kg. One group of mice received plain distilled
water for drinking, while the other group received distilled water
fortified with 2 mg selenium, as selenium dioxide/litre. The
strain of mice used in this study ordinarily has a high incidence
of spontaneous mammary adenocarcinomas and, after 16 months of
treatment, the observed incidence in the group not given
supplemental selenium in the water was 82%, whereas the incidence
in the group given selenium was 10%.
In study 2 (Schrauzer et al., 1976), 3 groups of 30, 30, and 50
female C3H/St mice fed the basal diet described above were also
given selenite at 0, 5, or 15 mg selenium/litre drinking-water,
respectively. Toxicity due to the doses of selenium was indicated
by the average body weights in the 3 groups at 12 months (33, 29,
and 25 g, respectively) and by a higher tumour-unrelated mortality
rate in the selenium-exposed mice. After 26 months, the
corresponding incidence of spontaneous mammary tumours in the 3
groups was 82, 36, and 33% in the mice that survived to the age at
which the first tumours appeared in each group. A fourth group of
20 mice, fed a selenium-deficient diet for 14 months and then the
basal diet until death, had a mammary tumour incidence of 69% which
was not judged to be different from the 82% incidence in the
control group (basal diet throughout life span and no added
selenium in the water).
In study 3 (Schrauzer et al., 1978a), 4 groups of 30 female
C3H/St mice were fed a basal diet ("Wayne F-6 Lab Blox") that was
different from that used in the first 2 studies and consisted of
fish meal, animal liver meal, soybean meal, corn and wheat flakes,
ground corn, wheat red dog, wheat middlings, soybean oil, cane
molasses, salt, brewer's yeast, and various vitamins and minerals.
This diet contained 244.8 g protein, 64.8 g fat, and 0.45 mg
selenium/kg. The 4 groups received selenium dioxide at 0, 0.1,
0.5, or 1.0 mg selenium/litre drinking-water and, after 22 months,
the incidence of mammary tumours was 42, 25, 19, and 10%,
respectively. Selenium supplementation at these levels did not
have any noticeable adverse effects on weight-gain or survival of
the mice. The incidence of spontaneous mammary tumours in the
control (unsupplemented) group was lower in this study than that
observed in the 2 previous studies (42 compared with 82%), and this
was attributed to the higher selenium content of the new basal diet
used in the third study (0.45 versus 0.15 mg/kg).
Medina & Shepherd (1980) fed BALB/cfC3H mice Wayne Lab Blox and
gave them selenium dioxide at 0, 2, or 6 mg selenium/litre
drinking-water, ad libitum, starting at 10 weeks of age and
continuing to the end of the study. In 12-month-old mice, 2 and 6
mg selenium/litre decreased mammary tumour incidence from 82% in
the untreated controls to 48 and 12%, respectively. There were no
effects of the selenium treatment on normal reproductive function
or weight gain in these mice. In another study, samples of 4
different preneoplastic outgrowth lines (D2, C3, C4, and CD-7)
were transplanted into the mammary gland-free fat pads of syngenic
mice. When the implants had filled the mammary fat pads with their
respective outgrowths (8 weeks after implantation), the mice were
given selenium dioxide at 4 mg selenium/litre drinking-water, ad
libitum, for the rest of the study. Such treatment with selenium
delayed the rate of tumour formation only in line C4, increasing
the time for half of the outgrowths to produce tumours from 34 to
44 weeks. In a third study, 2 - 6 mg selenium/litre drinking-water
had no effect on the growth rate of primary tumours transplanted
subcutaneously in BALB/c mice. Since selenium did not have any
effect on tumour formation rate in 3 of 4 preneoplastic mammary
outgrowth lines or on the growth rate of established mammary
tumours, the authors suggested that selenium might act by
inhibiting chemical or viral transformation of normal cells or by
inhibiting expression of initially transformed cells.
Because of the metabolic antagonism between arsenic and
selenium (section 7.1.6.3), Schrauzer and co-workers also
investigated the effect of arsenic on the genesis of the
spontaneous mammary tumours in C3H/St female mice described above.
In their first study on arsenic and selenium (which was part of the
same study described above in Schrauzer & Ishmael (1974)), giving
sodium arsenite at 10 mg arsenic/litre drinking-water to a group of
30 C3H/St mice for 16 months, reduced the incidence of spontaneous
mammary tumours to 27% compared with an incidence of 82% in the
untreated controls (Schrauzer & Ishmael, 1974). However, arsenic
treatment markedly stimulated the growth rate of spontaneous or
transplanted mammary tumours. In a second study concerning
arsenic, sodium arsenite at 80 mg arsenic/litre drinking-water,
administered to a group of 20 C3H/St mice, reduced the incidence of
spontaneous mammary tumours to 40% compared with an incidence of
82% in the untreated controls (Schrauzer et al., 1976), but some of
the tumour-inhibiting effect of arsenic appeared to be masked at
this dose level by its toxicity.
In a third study relating arsenic and selenium (Schrauzer et
al., 1978b), 4 groups of 30 female C3H/St mice were fed the Wayne
F-6 Lab Blox diet described above, which contained 0.29 mg
arsenic/kg. The 4 groups received the following supplements in
their deionized drinking-water: none, arsenic trioxide at 2 mg
arsenic/litre, selenium dioxide at 2 mg selenium/litre, or 2 mg of
both arsenic and selenium. The incidence of mammary tumours in the
4 groups was 41, 36, 17, and 62%, respectively, and the percentage
of multiple mammary tumours was 17, 40, 0, and 28. The age of
tumour onset in the corresponding groups was 4.5, 9, 16, and 8
months. Thus, in this case, treatment with arsenic appeared to
diminish the cancer-protecting effect of selenium. Arsenic also
accelerated tumour growth and increased the incidence of multiple
tumours.
Newberne & Conner (1974) fed 4 groups of male rats of the
Charles River CD strain, weighing about 100 g each, a basal diet
consisting of casein, 200 g/kg; sucrose, 209 g/kg; dextrose, 209
g/kg; dextrin, 209 g/kg; stripped lard, 80 g/kg; Wesson Oil, 20
g/kg; selenium-free salts, 50 g/kg; vitamin mix, 20 g/kg; choline,
3 g/kg; and vitamin B12, 0.05 g/kg. The basal diet contained about
0.03 mg selenium/kg. One group received the unsupplemented basal
diet, whereas the other 3 groups received the basal diet
supplemented with sufficient sodium selenite to attain approximate
dietary levels of 0.1, 1.0, or 5.0 mg selenium/kg, respectively.
Each group was fed the diet for 2 - 3 weeks, before oral
administration of 7 mg aflatoxin B1 in dimethyl sulfoxide/kg body
weight. The rats continued on their respective diets for an
additional 2 weeks, and the mortality rate after this time, in the
groups fed the diets containing 0.03, 0.1, 1.0, and 5.0 mg
selenium/kg was 28/29, 20/30, 7/28, and 27/29, respectively.
Histological examination revealed that rats receiving 1.0 mg
selenium/kg diet were partially protected against the aflatoxin B1
and also had less severe liver lesions. However, the groups fed
diets containing 1.0 or 5.0 mg selenium/kg exhibited a novel renal
lesion associated with acute aflatoxin B1 toxicity, characterized
by marked tubular necrosis at the cortical medullary junction.
Some tubules exhibited hyperplastic changes of the epithelium as
well as necrosis.
In a second study, Grant et al. (1977) gave 25 µg aflatoxin B1
orally, 5 days/week for 4 weeks, to 140 male Sprague Dawley rats.
The rats were fed normal or marginally lipotrope-deficient
semisynthetic basal diets containing sodium selenite at 0.03, 0.10,
0.50, 1.0, 2.5, or 5.0 mg selenium/kg. After 17 months, the
surviving rats were sacrificed and histopathological examination
was carried out. A very low incidence (20%) of hepatocarcinomas
was seen in rats receiving 1.0 mg selenium/kg in the normal basal
diet and 0.10 or 0.50 mg/kg in the lipotrope-deficient diet. No
other tumours were observed, and grading of the hepatic lesions
indicated that there was no significant differences among the
dietary selenium groups. Dietary sodium selenite and repeated
doses of aflatoxin B1 interacted to produce large bizarre cells in
the renal tubules, occurring in the same region of the kidney as
the severe necrosis seen previously in rats fed high levels of
dietary selenium and given an acute dose of aflatoxin B1.
Recently, a study of the effects of selenium on aflatoxin B1-
induced enzyme altered foci in rat liver has been reported (Milks
et al., 1985). Male Sprague Dawley rats were fed a selenium-
deficient diet and given sodium selenite at 5, 2, 0.2, or 1 mg
selenium/litre drinking-water for 3 weeks. Each rat then received
2 µg mol aflatoxin B1/kg body weight by stomach tube. For the next
week the selenium status was "normalized". Rats previously
receiving 5, 2, 0.2, or 0 mg selenium/litre received, respectively,
0. 0.2, 2, or 5 mg/litre. Then rat chow was fed and a promoting
regimen consisting of phenobarbital in the drinking-water, and a
partial hepatectomy was instituted. Eight weeks later, necropsies
were carried out and livers were stained histochemically for
gamma-glutamyl transpeptidase activity. Foci of activity were
counted. The number of foci seen in livers from rats given 5, 2,
0.2, or 0 mg selenium/litre during initiation were 0.62 ± 0.22,
1.97 ± 0.46, 3.35 ± 0.66, and 2.46 ± 0.23 per cm2, respectively.
The data suggested that 5 mg selenium/litre can protect against the
hepatocarcinogenic effects of aflatoxin B1 in the rat.
On the basis of anecdotal observations, Wedderburn (1972)
suggested that a decrease in the number of cases of intestinal
carcinoma in autopsied sheep might be associated with the
widespread veterinary use of selenium to prevent deficiency
diseases in sheep. Simpson (1972a) conducted an investigation into
the epidemiology of carcinomas of the small intestine of sheep in
which the viscera of 32 733 ewes were examined. Carcinomas of the
small intestine were diagnosed in 483 animals, but the prevalence
of these neoplasms in ewes regularly dosed with selenium throughout
their lives was not significantly different from the prevalence in
ewes never treated with selenium (Simpson, 1972b). Furthermore, no
significant differences were found in association with differences
in soil types on the farms from which the sheep originated. It was
concluded that soil-selenium levels and administration of selenium
in the form and at the dose rates used did not have any effects on
the development of intestinal carcinomas in sheep. Underwood
(1977) commented that neoplasias were not observed among the
various lesions attributed to selenium deficiency in animals.
Ip has made and summarized a series of observations on selenium
and carcinogenesis (Ip, 1985a).
Evidence of an interaction between dietary fat and selenium
status in the induction of mammary tumours by dimethylbenz-
[alpha]anthracene in rats has been presented by Ip & Sinha (1981).
They fed 8 groups of 23 - 25 female weanling Sprague Dawley rats
one of the following diets either deficient in selenium or
supplemented with sodium selenite at 0.1 mg selenium/kg: 1% corn
oil, 5% corn oil, 25% corn oil, or 1% corn oil plus 24%
hydrogenated coconut oil. In addition to the fat, the diets
consisted of Torula yeast, dextrose, HMW salt mix, vitamin mix,
alphacel, and DL-methionine. The diets were adjusted so that the
intake of all nutrients would be the same, except for dextrose and
fat, assuming that the rats would consume an equal number of
calories. The corn oil used was stripped of tocopherol and the
unsupplemented diet was deficient in selenium (less than 0.02 mg/kg
by fluorometry). Mammary tumours were induced by the intragastric
administration of 5 mg dimethylbenz[alpha]anthracene at 50 days of
age. Increasing the dietary polyunsaturated-fat level (corn oil)
increased the tumour incidence in rats fed the selenium-
supplemented diets (Table 51). However, the high-saturated-fat
diet was much less active in stimulating tumourigenesis. Selenium
deficiency increased the tumour yield only in rats fed the high
polyunsaturated-fat diet (25% corn oil). Increased tumour
incidence due to selenium deficiency was not seen in the rats fed
the low-fat diets containing polyunsaturated fat (1 or 5% corn
oil) or the high-fat diet containing primarily saturated fat (1%
corn oil with 24% coconut oil). Selenium deficiency, however, did
increase the incidence of mammary tumours in rats fed a low-fat
diet containing polyunsaturated fat (1% corn oil), when larger
doses of dimethylbenz[alpha]anthracene were used (10 or 15 mg -
data not shown).
Table 51. Incidence of palpable mammary tumours in
dimethylbenz(alpha)anthracene-treated rats fed different
levels and types of fats in the diet, with or without
selenium supplementationa
-----------------------------------------------------------
Fats in diet Selenium Incidence of palpable tumours
Corn Coconut in diet 19 weeks after dimethylbenz
oil oil (mg/kg) (alpha)-anthracene administration
(%) (%) Number of rats (%)
-----------------------------------------------------------
1 0 0 4/23 17.4
1 0 0.1 3/24 12.5
5 0 0 11/25 44.0
5 0 0.1 8/24 33.3
25 0 0 24/25 96.0
25 0 0.1 15/25 60.0
1 24 0 7/24 29.2
1 24 0.1 6/25 24.0
----------------------------------------------------------
a Adapted from: Ip & Sinha (1981).
In a recent study, the effects of vitamin E status on the
anticarcinogenic effect of selenium were examined (Ip, 1985b).
Female Sprague Dawley rats were fed a 20% stripped corn oil,
casein-based diet. Four dietary groups were formed: adequate
vitamin E/adequate selenium, adequate vitamin E/high selenium,
deficient vitamin E/adequate selenium, and deficient vitamin E/high
selenium. Adequate and deficient vitamin E diets contained 50 and
10 mg vitamin E/kg diet, respectively. Adequate and high selenium
diets contained Na2SeO3 at 0.1 and 2.5 mg selenium/kg,
respectively. At 50 days of age, rats received 5 mg of
dimethylbenz[alpha]anthracene (DMBA) each by stomach tube. Rats
were killed 20 weeks after DMBA administration. The tumour
incidences in the groups were: adequate vitamin E/adequate
selenium, 76%; adequate vitamin E/high selenium, 40%; deficient
vitamin E/adequate selenium, 84%; deficient vitamin E/high
selenium, 68%. This suggests that selenium protection against
carcinogenesis is decreased in vitamin E deficiency.
Pence & Buddingh (1985) studied the effects of selenium
deficiency on 1,2-dimethylhydrazine (DMH)-induced colon cancer in
the rat. They used male Sprague Dawley rats fed Torula yeast-based
diets containing 2% corn oil. Sodium selenite at 0.1 mg
selenium/kg was added to the diet of the controls. Weanlings were
fed the diets 3 weeks prior the institution of DMH treatment and
were killed after 20 weeks of treatment. No effects of selenium
status were noted on the incidence of colon adenocarcinomas.
The effects of selenium on UVR-induced skin carcinogenesis were
studied by Overvad et al. (1985). Female hairless mice were given
sodium selenite at 0, 2, 4, or 8 mg selenium/litre drinking-water.
Three weeks after selenium exposure began they were exposed to UVR
daily for 22 weeks. Then they were examined weekly for 26 weeks
for skin tumours, and relative tumour onset ratios were calculated.
The 2 mg selenium/litre treatment did not affect tumour onset but
the higher doses did. This suggests that selenium can protect
against UVR-induced skin cancer.
Birt et al. (1984) reported that high levels of selenium in the
diet increased pancreatic carcinogenesis induced by bis-(2-
oxopropyl)-nitrosamine (BOP) in male Syrian hamsters. Torula yeast-
based diets with either 0.1 or 2.5 mg selenium/kg were fed to
hamsters beginning at 4 weeks of age. BOP was given in 4 weekly
injections of 5 mg/kg body weight, beginning at 8 weeks of age.
Hamsters were killed at 78 weeks of age. When dietary fat was low
(11% calories as corn oil), the low-selenium group had 25
pancreatic ductular carcinomas (PCDA) in 18 hamsters, and the high-
selenium group had 63 PCDA in 23 hamsters. When dietary fat was
high (45% of calories as corn oil), the low-selenium group had 27
PCDA in 19 hamsters and the high-selenium group had 44 PCDA in 18
hamsters.
Other studies have been reported. Milner, who showed that
pharmacological doses of selenium inhibited the growth of
transplantable tumours, has recently summarized his work (Milner,
1985), and Thompson has summarized his work on mammary
carcinogenesis (Thompson, 1984). The metabolism of the carcinogen
2-acetylaminofluorene in selenium-deficient rats has been studied
by Besbris et al. (1982). They found that selenium-deficient rats
excreted more N-OH-acetylaminofluorene than controls, suggesting
that selenium might prevent the production of this carcinogenic
metabolite or promote its detoxification.
8. EFFECTS OF SELENIUM ON MAN
8.1. High Selenium Intake
8.1.1. General population
8.1.1.1. Signs and symptoms
When it became apparent that selenium was the toxic factor in
plants that caused alkali disease in livestock raised in
seleniferous areas, public health personnel became interested in
the possible hazards for human health in such regions, since
seleniferous grains or vegetables grown on high-selenium soil could
enter the human food chain. Smith et al. (1936) reasoned that, if
human selenosis were a problem anywhere, it would most likely occur
in farmers living in seleniferous regions, who consumed largely
locally-produced foodstuffs. Therefore, they surveyed a rural
population living on farms or ranches known to have a history of
alkali disease. Their survey inquired into the health status of
111 families and also determined the actual consumption of locally-
produced foods. Wherever possible, general physical examinations
were made and urine samples were collected.
These workers were unable to find any symptom or group of
symptoms or serious illness that could be considered characteristic
of, or could definitely be attributed to, selenium poisoning in
man. However, the incidence of vague symptoms of ill health and
symptoms suggesting damage to the liver, kidneys, skin, and joints
was rather high. But since the causes for such disorders are many,
it was not possible to determine whether selenium played any role
in their causation. Apart from the more vague symptoms of
anorexia, indigestion, general pallor, and malnutrition, the
following more pronounced disease states were observed: bad teeth,
yellowish discoloration of the skin, skin eruptions, chronic
arthritis, diseased nails, and subcutaneous oedema.
The same authors (Smith et al., 1936) were unable to
demonstrate a very definite correlation between the clinical
evidence of selenium intoxication in 127 subjects and the
concentration of selenium in the urine. In fact, they expressed
surprise that there was not any more definite evidence of serious
injury, particularly in subjects with high concentrations of
selenium in the urine. Relatively high urinary-selenium levels
were most often associated with pathological nails,
gastrointestinal disorders, icteroid skin, and bad teeth. The
incidence of high urinary-selenium in individuals with dermatitis
and arthritis was no greater than that in individuals without
symptoms.
Because of the ambiguous findings in their first study, Smith &
Westfall (1937) carried out a second, more detailed and intensive
survey to establish the symptomatology of human selenosis and its
relation to the amount of selenium excreted in the urine. For this
purpose, they examined 100 subjects from 50 families that had high
levels of urinary-selenium in their previous testing. The
percentage frequency of the various signs and symptoms observed is
given as follows: none, 24; gastrointestinal disturbances, 31;
icteroid discolouration of the skin, 28; bad teeth, 27; sallow and
pallid colour, especially in younger individuals, 17; history of
recurrent jaundice, 5; dermatitis, 5; pigmentation of the skin
(chloasma?), 3; pathological nails, 3; rheumatoid arthritis, 3;
cardiorenal disease, 2; vitiligo, 2. It was concluded that none of
these signs or symptoms could be regarded as specific for selenium
poisoning, and it was not certain that any one was the direct
result of the continual ingestion of selenium. Nonetheless, these
workers felt that the high incidence of gastrointestinal
disturbances was of importance. Moreover, the high incidence of
icteroid discolouration of the skin was thought to be related in
some way to the ingestion of selenium, possibly as a result of
liver dysfunction. The possible cariogenic effects of high levels
of selenium is discussed further in section 8.1.1.2. The other
symptoms occurred so infrequently that they did not seem to be
associated with selenium. However, it should be noted that the
value of all these observations is in doubt since there is no
comparative information available concerning the frequency of
occurrence of these signs and symptoms in an appropriate control
group.
Smith & Westfall (1937) estimated that most of their subjects
living in highly seleniferous areas were probably absorbing about
10 - 100 µg/kg body weight per day and that some of their subjects
might have absorbed as much as 200 µg/kg per day. For a 70-kg man,
these rates of absorption would be equivalent to a dietary-selenium
intake of 700 - 14 000 µg/day, assuming that all of the selenium in
the food had been absorbed.
As discussed in section 4.1.2, drinking-water rarely
contributes much selenium to a person's total daily intake, but a
brief note indicated that a family of North American Indians living
in Colorado, USA, suffered hair loss, weakened nails, and
listlessness after consuming well water reported to contain 9000 µg
selenium/litre for about 3 months (Anonymous, 1962). This incident
was thought to be the first authentic case of selenium poisoning in
human beings induced exclusively from a naturally-occurring
underground source of water. Tsongas & Ferguson (1977) studied the
effects of selenium in the drinking-water on the health of 2 groups
of persons living in a rural Colorado community. The first group
("exposed") received a water supply that contained 50 - 125
µg/litre, whereas the second group ("unexposed") received a water
supply that contained levels ranging from non-detectable (1
µg/litre) to 16 µg/litre. Examination of a total of 86 individuals
revealed that there were no significant differences between the
exposed and unexposed groups in the incidence or prevalence of any
of 85 health variables studied.
Lemley (1940) presented a 58-year-old rancher from South
Dakota, thought to be the first described case of chronic selenium
dermatitis in a human being, caused by the ingestion of selenium
from natural sources. However, 2 samples of urine collected from
this patient a week apart, contained only 0.043 and 0.040 mg
selenium/litre, levels that are considered to be within the normal
range. Although some improvement in the patient's condition was
noted when certain seleniferous foods consumed on his ranch were
avoided, the improvement was not marked. Administration of
bromobenzene, now known to be a potent hepatotoxin, cleared up the
dermatitis, but caused only a small increase in the urinary-
selenium output, which reached a peak value of about 0.100
mg/litre, after 4 days. Earlier work by Moxon et al. (1940) had
shown that the rate of excretion of selenium from selenized animals
could be increased by the administration of bromobenzene. The
likelihood that selenium was the cause of the dermatitis in this
patient is doubtful, since, in a later paper, Lemley & Merryman
(194l) claimed that a relapse in this patient's condition was cured
when some canned meat containing 0.40 µg selenium/kg (considered by
them to be a highly toxic amount of selenium) was withdrawn from
the patient's diet. This figure for the level of selenium in the
meat is almost certainly erroneous, since not only is it below that
which would be found in animals suffering from selenium deficiency
but it is also below the sensitivity of the analytical techniques
available at that time.
Other cases of presumed human overexposure to selenium were
reported by Lemley & Merryman (1941). For example, urine samples
from a ranching family living in South Dakota contained 0.200,
0.250, 0.300, 0.550, and 0.600 mg selenium/litre for the father,
mother, daughter, son, and uncle, respectively. Administration of
bromobenzene to the father resulted in a urinary output of selenium
of 1.800 mg/litre within 24 h. No mention of dermatitis was made
in connection with this family. Rather, all family members
suffered from slight, continual dizziness and clouding of the
sensorium, extreme lassitude accompanied by depression, and
moderate emotional instability. The patients tired on exertion and
complained that their powers of concentration were markedly
impaired. After a course of bromobenzene, these people improved
markedly and their general condition remained improved, since they
were instructed not to use the various food products of the ranch
that contained selenium. Lemley & Merryman concluded that the
following facts provided the basis for the diagnosis of selenium
poisoning in these subjects:
(a) knowledge that the subjects lived in a seleniferous
area;
(b) presence of concentrations of selenium in the urine
exceeding 0.100 mg/litre;
(c) increased elimination of selenium in the urine after
bromobenzene administration; and
(d) improvement in the subject's symptoms after
elimination of selenium from the diet.
The same authors also described a 65-year-old rancher from
South Dakota, who suffered alternate bouts of diarrhoea and
constipation. This patient's urinary-selenium concentrations were
as high as 0.250 mg/litre. An exploratory abdominal operation
suggested that the patient had very early cirrhosis of the liver,
which was confirmed by pathological examination. The patient
recovered from the operation and then gradually improved after
being placed on a strict selenium-free diet and given a course of
bromobenzene.
In a later paper, Lemley (1943) expressed the opinion that
human selenium poisoning is common, widespread, and, in certain
localities, of importance for the general public health. More
recently, Kilness (1973) complained that no follow-up public heath
survey with an appropriate control group had been made in South
Dakota since the initial surveys by the United States Public Health
Service in highly seleniferous areas more than 30 years previously.
Jaffe (1976) carried out a field study in Venezuela and
compared 111 children living in a seleniferous area (Villa Bruzual)
with 50 living in Caracas. The overall haemoglobin and haematocrit
values were somewhat lower in Villa Bruzual (128 g/litre and 39
volume %, respectively) than in Caracas (148 g/litre and 42 volume
%, respectively), but no correlations between blood- and urine-
selenium levels and haemoglobin or haematocrit values were found.
The children in Villa Bruzual consumed less meat and milk and had a
higher incidence of intestinal parasite infestation (Jaffe et al.,
1972a) than those in Caracas. Therefore, it was concluded that the
differences in haemoglobin were probably due to differences in
nutritional or parasitological status and not to differences in
selenium intake. Activities of prothrombin and serum alkaline
phosphatase and transaminases, which altered in selenium-poisoned
rats (Jaffe et al., 1972b), were normal in all the children and no
correlation with blood-selenium levels was apparent. Symptoms of
dermatitis, loose hair, and pathological nails were reported as
more frequent among the children in the seleniferous area than in
those living in Caracas, but no quantitative information was given.
The clinical signs of nausea and pathological nails appeared to be
correlated with serum- and urine-selenium levels. However, the
cause of the different incidence of clinical signs was considered
doubtful, especially in the absence of differences in the various
biochemical tests performed (Jaffe et al., 1972a).
The mean blood-selenium level of 111 school children from the
seleniferous area in Venezuela was 0.813 mg/litre (Jaffe et al.,
1972a). A subgroup of 28 children from this area who had blood-
selenium levels of over 1 mg/litre had a mean blood-selenium level
of more than 1.321 mg/litre. There was one child with a blood-
selenium level of 1.8 mg/litre. Another subgroup of 11 children
who had blood-selenium levels of less than 0.4 mg/litre had a mean
blood-selenium level of 0.330 mg/litre. Urinary-selenium levels
tended to reflect blood-selenium levels, since children in the
high-blood-selenium subgroup (> 1 mg/litre) excreted a mean level
of 0.657 mg/litre urine, whereas children in the low-blood-selenium
subgroup (< 0.4 mg/litre) excreted a mean level of 0.266 mg/litre
urine.
Kerdel-Vegas (1966) summarized 9 cases of acute intoxication
due to the ingestion of nuts of the "Coco de Mono" tree (Lecythis
ollaria) from the seleniferous areas of Venezuela. Although the
course of the poisoning differed from patient to patient (probably
because of differences in the amount of nuts consumed and the
length of time the nuts were present in the digestive tract), most
cases experienced nausea, vomiting, and diarrhoea, a few hours
after eating the nuts, followed by hair loss and nail changes, some
weeks after the initial episode. Two patients had foul breath that
was described as being reminiscent of decomposed seaweed or
phosphorus. After a period of time, re-growth of hair and nails
took place and most patients appeared to make a satisfactory
recovery. One fatality was reported, a 2-year-old boy who died, in
spite of proper medical attention and symptomatic treatment for
severe dehydration.
Dickson (1969) described his own personal experience after
eating sapucaia nuts ( Lecythis elliptica H.B.K.) that had grown in
Honduras. He reported hair loss and splitting of finger and
toenails several weeks after consuming the nuts. Although no
mention of selenium was made in his report, he noted that a
peculiar odour was associated with consumption of these nuts, not
only of his breath but of his body as well. Kerdel-Vegas (1966)
pointed out that the sapucaia, or chestnut of Para (which he called
Lecythis paraensis Ducke), is consumed as food in Brazil and other
countries to which it is exported (i.e., Europe and the USA). He
also commented that there is a popular saying in northern Brazil
that eating sapucaia leads to hair loss, but he knew of no
scientific or medical reports from Brazil. Signs of selenium
toxicity have been observed in rats fed diets containing defatted
Brazil nut flour that assayed 51 mg selenium/kg (Chavez, 1966), and
Thorn et al. (1978) found that samples of Brazil nuts marketed in
the United Kingdom contained an average of 22 mg selenium/kg
(range, 2.3 - 53 mg/kg).
As reported recently, an unexplained intoxication characterized
by nail deformation and the loss of hair and nails as the most
common signs was observed in Enshi county of Hubei province of the
People's Republic of China, more than 20 years ago, with a peak
prevalence during the years 1961 - 64 (Yang et al., 1983). In 5
villages with 248 inhabitants, about half of the population was
affected and in one particular village over 80% of the people were
affected. No other quantitative information about the cases was
given but a general description of the signs and symptoms observed
is presented below. The hair became dry and brittle and was easily
broken off at the scalp with retention of intact radicles so that
depigmented and dull hair continued to grow. A scalp rash
accompanied by intolerable itching resulted in hard scratching
which easily removed the hair. The nails became brittle with white
spots and longitudinal streaks on the surface, followed by a break
on the wall of the nail. With new nail growth, the broken nail
advanced and ultimately fell off. Effusian of fluid from around
the nail was common in many cases. The new nail had a rough and
ridged surface and was fragile and thickened.
Other signs of intoxication included skin lesions, tooth decay,
and abnormalities of the nervous system. The skin became red and
swollen and then blistered and eruptive followed, in some cases, by
ulcerations that took a long but unstated time to heal. The
lesions occurred primarily on the limbs and also on the back of the
neck. Mottled teeth were observed in the intoxicated individuals
but this observation may have been confounded by high exposure to
fluoride. In one heavily affected village, nervous system
abnormalities were seen, including peripheral anaesthesia,
acroparaesthesia, and pain in the extremities. Hyperreflexia of
the tendon commonly developed later followed by numbness,
paralysis, and motor disturbances. One case of hemiplegia was also
reported. There was an indication that this intoxication was
related to the locally grown monotonous diet consumed, which later
was shown to contain high levels of selenium. No quantitative data
regarding selenium exposure were obtained at the time of the
outbreak. However, because of circumstantial evidence, the
intoxication was considered by the authors to be selenosis, and, as
discussed in sections 5.1.1.1 and 6.2.3, current levels of exposure
to dietary selenium in an area with a history of intoxication, as
assessed by blood- and hair-selenium levels as well as dietary
intake data, exceeded those ever reported in any non-occupationally
exposed population (Table 8). The authors estimated that the daily
dietary intake of selenium, after the peak prevalence of the
poisoning had subsided, averaged 5 mg, and blood- and hair-selenium
levels averaged 3.2 mg/litre and 32.2 mg/kg, respectively. The
ultimate environmental source of the selenium in this episode of
intoxication was a highly seleniferous coal (average selenium
content of 300 µg/g) which lost its selenium to the soil as a
result of weathering processes. Once in the soil, the selenium was
taken up by plant crops and entered into the food chain.
Because of the nutritionally-beneficial effects of selenium in
animals, some investigators have deliberately given inorganic or
organic forms of the element to people with the aim of producing
some desirable health benefit. For example, Westermarck (1977)
administered selenium, as selenite, in oral doses of 0.05 mg/kg
body weight per day, for more than one year, to patients with
neuronal ceroid lipofuscinosis (NCL) and did not observe any toxic
manifestations. On the contrary, it was felt that some of the NCL
patients showed at least a transitory improvement in their
condition. In some patients, a slight increase in serum aspartate
aminotransferase activity was observed, but apparently this is seen
in a number of patients with NCL.
Schrauzer & White (1978) described 2 individuals who had been
taking commercially-available nutritional supplements consisting of
selenium-containing yeast at doses of 200 and 450 µg selenium,
daily, for 18 months. Together with their dietary intakes, these
individuals received a total of 350 and 600 µg/day. Although some
marginal haematological changes were seen and the serum-glutamic
oxaloacetic transaminase activities were on the borderline of high,
it was concluded that daily intakes of up to 600 µg selenium for 18
months do not induce toxic effects in well-fed individuals.
In a study by Perona et al. (1978), 4 subjects were given,
orally, a 2-mg dose of selenium, as sodium selenite, daily, for
20 - 40 days, to determine the effects of in vivo selenium
administration on the activity of human erythrocyte-glutathione
peroxidase. It was stated that none of the subjects exhibited any
symptoms of selenium poisoning, but the criteria used to judge any
deleterious effects of selenium were not described.
Yang et al. (1983) reported the case of a 62-year-old man who
had taken one tablet containing 2 mg sodium selenite per day for
more than 2 years. The subject did not have any symptoms of
indisposition but presented with thickened, fragile and somewhat
honeycomb-like fingernails. After the oral intake of sodium
selenite was stopped, the surface of the new nail growth became
smooth and gradually recovered. Blood-and hair-selenium levels
were 0.179 mg/litre and 0.828 mg/kg, respectively, on the day the
selenite tablets were discontinued. These tissue-selenium levels
are much lower than those reported by the same authors in Enshi
county of the Hubei province (Yang et al., 1983) suggesting that
the form of selenium ingested must be considered when interpreting
tissue-selenium levels in the diagnosis of selenium poisoning.
Ingestion of superpotent selenium tablets, meant to be consumed
as a "health food" supplement, resulted in 12 cases of human
selenium toxicity in the USA in 1984 (Anonymous, 1984; Jensen et
al., 1984; Helzlsouer et al., 1985). Each tablet contained 27 - 31
mg selenium by analysis (about 182 times more than the level stated
on the label). Approximately 25 mg of the selenium was present as
sodium selenite whereas the rest was elemental and/or organic
selenium. The total doses of selenium estimated to be consumed by
the victims ranged from 27 to 2387 mg. Based on the limited
information available, the symptoms reported in these cases as most
common were nausea and vomiting, nail changes, hair loss, fatigue,
and irritability. Other symptoms included abdominal cramps, watery
diarrhea, paraesthesias, dryness of hair, and garlicky breath.
Eight of the 12 victims did not have any abnormalities in the blood
chemistry, and the results of liver and kidney function tests were
normal.
A level of 500 µg has been proposed as the tentative maximum
acceptable daily intake of selenium for the protection of human
health (Sakurai & Tsuchiya, 1975). This figure was derived from an
initial estimate of the mean normal daily intake of selenium by
human beings of between 50 and 150 µg. It was concluded that
values of 10 - 200 times the normal intake appeared acceptable as
an estimated range for the margin of safety within which the
average human being could tolerate selenium. By taking the lower
values of both these estimates, the lowest level of potentially
dangerous daily intake of selenium was estimated to be 500 µg.
8.1.1.2. Attempts to associate high selenium intake with human
diseases
(a) Dental caries
As already mentioned in section 8.1.1.1, bad teeth was one of
the signs that was thought to be possibly associated with a high
intake of selenium by a rural population living in seleniferous
areas of South Dakota, Wyoming, and Nebraska (Smith et al., 1936)
and by children living in a seleniferous zone in Venezuela (Jaffe,
1976). Several studies have been concerned with the possibility of
a specific association between the incidence or prevalence of
dental caries and residence in a seleniferous area, urinary
excretion of selenium, or the selenium content of teeth.
Hadjimarkos & Storvick (1950) and Hadjimarkos (1956) noted a
geographical difference in the prevalence of dental caries in 2029
children, between the ages 14 and 16 years, residing in 4 different
counties in Oregon. Two different counties, one with the highest
(Clatsop), and one with the lowest (Klamath), rates of caries
experience were selected for further study. Urine samples from 24
and 29 male children who were attending county seat high schools
and who were born and residing in the respective counties were
analysed for selenium content. The mean levels of urinary-selenium
were 0.049 and 0.037 mg/litre in the groups of children from the
counties with the high (14.4 DMF (decayed, missing, or filled)
teeth per child) and low (9.0 DMF teeth per child) prevalence of
dental caries, respectively (Hadjimarkos et al., 1952).
In a second study, 2 additional counties with similar but high
rates of caries experience (Jackson and Josephine, 13.4 and 14.4
DMF teeth per child, respectively) were selected (Hadjimarkos &
Bonhorst, 1958). Analysis of 33 urine specimens from continuously-
resident, male high school children attending a county seat high
school (Jackson) and 46 specimens from similar children attending a
high school serving a rural area (Josephine) gave values (mean ±
SE) of 0.074 ± 0.007 and 0.076 ± 0.005 mg/litre, respectively. It
was concluded that there was an association between the high
prevalence of dental caries and the values of urinary-selenium
excretion, which were double those seen in the previous study in
the county with a lower rate of caries experience.
In order to explain the variations in urinary-selenium levels
observed in the above studies, samples of locally-produced and -
consumed milk and eggs, as well as local drinking-water samples
were collected from 74 farms located in Klamath, Jackson, and
Josephine counties and analysed for their selenium content
(Hadjimarkos & Bonhorst, 1961). The selenium levels were almost 10
times higher in food samples collected from the counties with high
caries prevalence (Jackson and Josephine) than in those from the
county with low caries prevalance (Klamath). The selenium levels
in the milk samples obtained in Jackson and Josephine counties were
higher than those listed in Table 3.
In another study, Tank & Storvick (1960) determined the extent
of dental caries in a population of children from 15 areas of
Wyoming that had been identified previously as seleniferous or non-
seleniferous on the basis of the geological distribution of
selenium, occurrence of the element in vegetation, and the
occurrence of selenosis in livestock. These research workers
claimed that the dental caries rate in the permanent teeth was
higher in children residing in seleniferous areas of Wyoming than
in children living in non-seleniferous areas of Wyoming, but they
were unable to demonstrate any consistent relationship between
calculated selenium intake and caries or urinary-selenium excretion
and caries.
Suchkov et al. (1973) found that the incidence of caries varied
in 3 different geographical zones (mountainous, pre-mountainous,
and forest-steppe) in the Chernovitsi region of the Ukraine.
Analysis of teeth from people residing in rural areas in these 3
zones and mainly consuming locally-produced foodstuffs revealed a
direct correlation between the selenium content of the teeth and
the incidence of dental caries. The people in the mountainous zone
had the highest level of selenium in the teeth and the greatest
incidence of dental caries, whereas the people in the forest-steppe
zone had the lowest level of selenium in the teeth and smallest
incidence of dental caries. This correlation was true for
deciduous teeth as well as for permanent teeth. However, as
pointed out by the authors, the mountainous zone also had the
softest water and the lowest content of fluorine in the drinking-
water, so that factors other than selenium might have been
involved.
It was not possible to demonstrate any association between the
urinary excretion of selenium and the incidence of dental caries in
subjects living on the South Island of New Zealand, an area known
to be low in selenium (Cadell & Cousins, 1960). However,
Hadjimarkos (1960) claimed that the levels of urinary excretion of
selenium in this study (all less than 0.050 mg/litre) were too low
to be associated with an increased incidence of caries.
As emphasized by Schwarz (1967), the levels of urinary-selenium
excretion reported in the Hadjimarkos studies were within the
limits generally found in the normal population without excessive
selenium intake. It may be recalled from section 7 that, in
experimental animal studies, very high toxic levels were used to
demonstrate the cariogenic effect of selenium.
(b) Reproduction
Possible effects of selenium on reproduction were suspected
following old anecdotal reports from Colombia, South America that
women living in areas, later shown to be seleniferous, gave birth
to malformed babies (Rosenfeld & Beath, 1964), but there is a lack
of reliable studies. Robertson (1970) pointed out that, among 6
women preparing microbiological media containing sodium selenite,
one probable and 4 certain pregnancies all ended in abortions, save
one that went to term. The infant was born with bilateral club
foot. However, no differences in urinary-selenium levels were
observed between this group of women and a control group living in
the same area, and inquiries by the author at other laboratories
carrying out comparable work did not show any evidence of similar
trouble. Jaffe & Velez (1973) could not demonstrate any
correlation between the selenium level in the urine of school
children in different Venezuelan states and the incidence of infant
mortality due to congenital malformations, on the basis of
published public health statistics. Jaffe (1973) concluded that no
recent observations of a teratogenic action of dietary selenium in
human beings had been reported at that time.
(c) Amyotrophic lateral sclerosis
Kilness & Hochberg (1977) reported an unusual cluster of 4
cases of amyotrophic lateral sclerosis (ALS) in male farmers living
in a seleniferous area and indicated that selenium might be an
environmental factor predisposing to the disease. But Schwarz
(1977) pointed out that the frequency of ALS was at least as high
if not higher in areas that were selenium-deficient as in those
with normal or elevated levels and Kurland (1977) suggested that
the cluster was more indicative of a chance occurrence than of a
new etiological lead in ALS. Moreover, Norris & Sang (1978) found
that 19 out of 20 well-established cases of ALS had urinary-
selenium levels lower than the mean for unexposed persons and
therefore concluded that selenium exposure was of no concern in the
average case.
8.1.2. Reports on health effects associated with occupational
exposure
Although the toxicological potential of selenium for human
beings can be inferred from studies carried out with laboratory
animals, certain precautions must be taken in applying the results
of animal studies to the industrial health aspects of selenium.
First, the number of studies dealing with the respiratory exposure
of animals to selenium compounds is limited, and the dose levels
employed have usually been higher than those encountered in
industry. On the other hand, the period of exposure used in the
studies reviewed in section 7.1.2.3 did not exceed one month. The
specific chemical form and physical state of the selenium must be
considered as well as the fact that the chemical form of selenium
may change when in contact with moist mucous membranes or with
sweat. These factors, plus several others, must be taken into
account when considering the health effects of various selenium
compounds under occupational exposure conditions.
The Task Group recognized that, for various reasons, knowledge
on the health effects of industrial exposure to selenium compounds
was not complete. Acute exposures to selenium are the result of
accidents and are, of necessity, described on an ad hoc case study
basis. Regarding the effects of long-term selenium exposure in
industry, there is a lack of epidemiological studies that include
unexposed control groups. Also, no follow-up studies are available
comparing the health status of a sufficiently large number of
workers, previously exposed to selenium, with that of the unexposed
population. Furthermore, the exposure level was not known with
certainty in either acute or long-term studies, and, in some cases,
the form of selenium was not established. In many cases,
simultaneous exposure to other noxious agents occurred.
Nevertheless, the Task Group felt that some preliminary assessment
of the toxicological potential of selenium in industry could be
made on the basis of the occupational experience available with
selenium compounds.
Since Hamilton's first observation on the effects of selenium
exposure in industry in 1917 (Hamilton, 1927 - 34), several hundred
persons have been described in the literature who have been
directly affected by the vapour, fumes, or dust of selenium and its
compounds. In addition, there is a systematic study of a group of
over 100 selenium workers for a period of 2 years (Glover, 1967).
Systematic attention has also been given to the health of workers
exposed to selenium in the USSR (Filatova, 1948; Monaenkova &
Glotova, 1963; Gracianskaja & Kovshilo, 1977). Several reviews
evaluating the health effects of selenium from the point of view of
occupational health have been presented (Glover, 1954 - 70, 1976;
Cooper, 1967; Izraelson et al., 1973; Cooper & Glover, 1974).
8.1.2.1. Fumes and dust of selenium and its compounds
Workers in several industries, such as the production or
recovery of selenium itself, or the manufacture of glass or
rectifiers can be exposed to the fumes and dust of selenium and its
compounds. In addition, direct contact with powders or solutions
containing selenium compounds is possible and the biological
effects of such contact will be discussed in this section.
The chemical form of selenium in the fumes and dusts found in
the above-mentioned industries, consists of elemental selenium plus
various amounts of selenium dioxide, the only oxide of selenium
found in the industrial environment. However, in some cases, the
possible occurrence of other selenium compounds, such as hydrogen
selenide, cannot be excluded. Instances where hydrogen selenide
was the main selenium compound of concern will be discussed
separately in section 8.1.2.1.2.
8.1.2.1.1. Selenium dioxide
Selenium dioxide mainly occurs in industry:
(a) during the production of the compound, usually by the
oxidation of elemental selenium; and
(b) whenever selenium is heated in air, purposely or
accidently, above its melting point. "Selenium fume"
rising under these conditions is a mixture containing
red elemental selenium and about 20 - 80% of selenium
dioxide.
Glover (1954, 1970, 1976) has summarized the acute local,
systemic, and possible long-term effects of occupational exposure
to selenium dioxide. Acute local effects are seen in the lungs,
gastric mucosa, skin, nails, and eyes. Sudden inhalation of large
amounts of selenium dioxide produces pulmonary oedema, due to a
local irritant effect on lung alveoli. Working in an atmosphere
containing selenium dioxide can increase indigestion. Contact with
the skin results in burns or dermatitis. Occasionally, a true
urticarial type of generalized allergic body rash may occur, in
which case the individual must be removed from any work involving
selenium. The selenium dioxide may penetrate under the nails and
cause excruciating pain in the nail beds. If selenium dioxide
enters the eyes, prompt flushing with water will prevent
conjunctivitis. "Rose eye", a pink discoloration of the skin of
the eyelids, which often become puffy, is sometimes seen in persons
who work in an atmosphere of selenium dioxide dust. Systemic
effects of selenium dioxide exposure include garlicky-smelling
breath, metallic taste on the tongue, and indefinite
sociopsychological effects. The garlicky breath is the first and
most characteristic sign and has been used by occupational
hygienists as a way of monitoring exposure, even though the odour
is not always a reliable index in this respect. The metallic taste
is an earlier symptom but, being more subtle, is often overlooked
by workers. Sociopsychological effects such as lassitude and
irritability have also been associated with selenium exposure.
Since the effects of selenium overexposure lead to hepatic damage
in animals, Glover (1954) concluded that it would be prudent to
watch for such damage in human beings.
(a) Reports on health effects connected with short-term
accidental exposures
An industrial incident in a plant engaged in smelting scrap
aluminium and other non-ferrous metals was reported by Clinton
(1947). The aluminium scrap included more than 100 kg of aluminium
rectifier plates coated on one side with metallic selenium overlaid
with a coating of an alloy of bismuth, cadmium, and tin. When an
attempt was made to skim the dross prior to pouring, a cloud of
reddish fume arose. The fumes were intensely irritating to the
eyes, nose, and throat and the plant was evacuated. No workers
were exposed for more than 2 min.
All exposed workers noticed immediate and intense irritation
of the eyes, nose, and throat, and an unpleasant sour, garlic-like
odour to the fumes. The more heavily exposed workers complained of
a severe burning sensation in the nostrils, and dryness of the
throat; followed after 2 - 4 h by severe headache, mainly frontal
in location, lasting until the following day. Several men observed
immediate sneezing, coughing, and headache, followed for 4 - 8 h by
nasal congestion, dizziness, and redness of the eyes. Most of the
men noticed a bad taste in their mouths and an unpleasant odour to
their skin and clothing. Other people, however, did not note any
unpleasant odour in the breath of the exposed workers.
Two labourers who had attempted to skim the dross from the
furnace and therefore underwent intense exposure were hospitalized
for observation. On arrival at the hospital, they complained of
soreness of the eyes and lachrymation, pain in the nose, slight
difficulty in breathing, and frontal headache. Physical
examination on admission revealed conjunctival injection,
congestion of the mucous membranes of the nose and throat, and
oedema of the uvula. One man had a few fine rales audible in the
right base; roentgenological examination of the chest did not
reveal any abnormalities. The temperature, pulse, and respiration
were normal. Both workers were discharged 2 days after the
accident, at which time they were asymptomatic and had no positive
physical findings. Another worker, a foreman, underwent a more
severe exposure than most of the workers. He was not hospitalized,
but, about 8 - 12 h after the accident, he developed severe
dyspnoea accompanied by a slight elevation in temperature, in
addition to a headache and sore throat. Physical examination
revealed fine rales in both bases, as well as scattered asthmatic-
like wheezes throughout the chest. These signs and symptoms
cleared in about 24 h, without specific therapy. All persons
exposed to the fumes had recovered entirely in 3 days, and no
sequelae have been encountered.
In the same year, Lauer (1947) described an episode that
occurred when 15 men were exposed to fumes arising from an
explosion and fire, which occurred in a pot when selenium became
overheated in contact with aluminium. The 2 elements produced a
high-temperature reaction, and fumes and vapours were dispersed
throughout the work area. The lengths of exposure varied, some
victims were using masks part of the time, and others were almost
without protection.
The exposed men reported to the medical dispensary about 15 -
45 min after the accident. All complained of soreness and burning
of the nose and throat, and 8 out of 15 cases had some dyspnoea.
Two of these cases were moderately severe, requiring almost
continuous oxygen therapy. Headache and dizziness and a burning
sensation in the eyes occurred in 6 cases. Three others complained
of substernal burning and tightness of the chest. In 4 there was
nausea and vomiting. All were hospitalized. Physical examination
showed reddening of nasal and pharyngeal mucosa, wheezes, and
musical rales in the lungs (12 cases). These symptoms abated
quickly. In about 10 days, there were few subjective complaints.
All apparently recovered satisfactorily with no known
complications. To assess possible liver affection, Hanger's
cephalin cholesterol tests and Icterus Index determinations were
performed on admission to the hospital and again at the end of the
first, second, third, and seventh week. On the day of admission to
the hospital, Hanger's tests were made on 13 workers, 4 of which
were positive and 9, negative. At the end of the first week
following exposure, there were 10 positives and 3 negatives. At
the end of the second week, there were 11 positives and 3 negatives
out of a group of 14 tested, and the same result was obtained at
the end of the third week. By the end of the seventh week, only 4
of 11 tested were positive. The average value of the Icterus Index
on admission to the hospital was 7.3 units. One week after
exposure, it was 13.5. In 2 weeks, it averaged 20.7. After the
third week, it had fallen to 9.9 and, by the end of the seventh
week, the average Icterus Index was 7.2.
An accident connected with a fire in a selenium rectifier plant
was described by Wilson (1962). Twenty-eight of the employees were
exposed directly to the smoke and fumes containing selenium
dioxide. The length of exposure to the fumes varied with the
individual, but no one was exposed for more than 20 min. Oxygen
was administered by mask to the men lying on the ground, who
experienced bronchial spasm with coughing, gagging, and, in some
instances, transient loss of consciousness.
The initial signs and symptoms were a feeling of constriction
in the chest, accompanied by burning and irritation of the upper
respiratory passages, violent coughing and gagging with nausea and
vomiting, and a bitter acid taste in the mouth. During the acute
episode, there were mild signs of shock, with a drop in blood
pressure and an elevated pulse and respiratory rate. Other
symptoms, experienced during this stage, were burning of the skin,
conjunctivae, and mucous membranes of the upper respiratory
passages. Within 4 h, all patients had apparently recovered from
the acute episode and a recheck of their blood pressure, pulse,
respiratory rate, and general condition was found to be within
normal limits.
Within 6 h of the exposure, the victims began complaining of
secondary symptoms with the onset of generalized chills accompanied
by nausea and vomiting, diarrhoea, malaise, dyspnoea, and headache.
The onset of secondary symptoms was delayed in some of the victims
for several hours after the initial exposure. Within 12 h, all
exposed personnel began experiencing the symptoms described. The
following morning, the first patient, a 30-year-old male, was
admitted to the hospital. He was cyanotic and in moderate
respiratory distress. He complained of chest pain bilaterally,
and was experiencing bronchial spasms. A chest roentgenogram
revealed extensive bilateral consolidation of the lung fields,
indicating pneumonia. The white blood cell count was elevated to
153 000 with a marked prominence of neutrophils. He experienced a
stormy course, requiring constant oxygen for the first 9 days of
hospitalization. By the fifth hospital day, X-ray films revealed a
further extension of the previously noted bilateral pneumonic
consolidation. Two weeks following admission, comparison of a
chest film with previous films revealed marked improvement
bilaterally. He was free of respiratory distress, his white blood
cell count had returned to within normal limits, and he was
discharged home for convalescence. It is interesting to note that
this was the only employee of the 5 hospitalized cases who did not
receive oxygen on the afternoon of the fire. On the same day, the
second victim was admitted to the hospital in a similar dyspnoeic,
cyanotic state with an elevated white blood cell count of 24 600.
X-ray again revealed bilateral atelectasis and consolidation. This
patient required continuous oxygen for 6 days, and was discharged
after 9 days of hospitalization: his white blood cell count had
returned to normal and a repeat roentgenogram revealed improvement.
This was the second most involved and prolonged illness, and he
received only about 6 breaths of oxygen following his exposure.
Three days after the accident, a third employee was admitted to the
hospital in less respiratory distress, with a normal white blood
cell count and a chest film that revealed bilateral elevation of
the diaphram with extensive peribronchial infiltration and some
consolidation at the lung bases. On the same day, another worker
was admitted to the hospital with a normal white blood cell count
and a chest film revealing bilateral increase in lung markings
consistent with bronchitis; however, no consolidation was evident.
The final patient to be hospitalized was admitted 4 days after the
accident with a white cell count of 12 800 and a prominence of
neutrophils. The X-ray film indicated bilateral pneumonia.
Four days after exposure, all other employees with any upper
respiratory complaints were examined. Thirty-two of the 53
employees examined were found to have a residual bronchitis, of
minimal to moderate degree, that required medication. These were
treated on an outpatient basis. Within 1 week, all were
asymptomatic and free of any respiratory signs.
Both Monaenkova & Glotova (1963) and Skornjakova et al. (1969)
have described occupational incidents of short-term selenium
dioxide exposure. In the former case, 6 workers, 20 - 52 years
old, were exposed from 5 to 20 min in a room with a leaky selenium
still. The workers suffered acute pain in the eyes, hoarseness in
the throat, painful cough, and a heavy feeling in the chest. Acute
conjuctivitis, laryngotracheitis, and bronchitis were also noted
and gastroenterocolitis was common to all. Transient fever was
seen in 2 workers. The various signs and symptoms disappeared
within 5 - 7 days, but, on return to work involving selenium, 2
workers developed chronic bronchitis. In the report of acute
selenium dioxide exposure by Skornjakova et al. (1969), there was
irritation of the eyes and mucous membranes, coughing without
expectoration, nasal secretion, headache, vertigo, nausea,
vomiting, garlicky-smelling breath and skin, general weakness, loss
of consciousness, and collapse. Two weeks after exposure, an
allergic whole body rash occurred that disappeared after removal of
the worker from contact with selenium.
(b) Effects of short-term and/or repeated dermal exposure
Selenium dioxide is more than a primary irritant, it causes
extremely painful burns on the skin which, however, always heal
without a scar. Theoretically, the selenium dioxide powder itself
does not burn the skin (and if dropped on to the skin should be
immediately brushed off dry). However, in practice, in the
industrial environment, there is sufficient moisture on the skin
from sweating for this very deliquescent white solid to form a
sticky solution of selenious acid within seconds, or at the most,
minutes, of coming into contact with the skin. Selenious acid is
so soluble that, if the skin is immediately washed with a lot of
water, there will be no burn or even rash from accidental contact.
When the selenious acid does burn the skin, there is little to see
or feel for several hours. After 4 h with a 50% solution, an
unremitting intense pain begins, small petechial areas occur, and a
faintly orange coloration occurs, which indicates reduction of some
of the selenious acid to elemental red selenium. The pain and
necrosis can be prevented by the application of a reducing solution
or ointment, such as 10% sodium thiosulfate (Glover, 1954). If the
burn remains untreated it may go on to ulceration, but this is
rare. Högger & Böhm (1944) described a case of a woman who
suffered pain and reddening of the middle, ring, and little fingers
of one hand when selenium dioxide crystals penetrated into the
protective rubber glove that she was wearing.
The first accurate description of a case of selenium fumes
causing allergic dermatitis was published by Duvoir et al. (1937).
A chemical engineer, who had handled selenium for 3 years in the
past, developed swelling of the face with a hard urticarial oedema
of the nose and cheeks extending on the neck down to his shirt
collar following a 3-day exposure to selenium vapours. There was a
semi-circle of vesicles on the lower lids, but the forehead and
ears escaped completely. His hands showed discrete lesions.
Within 24 h, the genital oedema had disappeared, after 3 days, the
rash started to go, and, by 9 days, there was only desquamation
left. The 5-cm plaque, occurring 1 h after a patch test with 10%
potassium selenite, showed a greater than average contact
sensitivity.
Another case was described (Halter, 1938) involving a glass
worker whose job was to add a mixture of red selenium and sodium
selenite to the glass. This man was in daily contact with selenite
powder for 36 years. He complained of an oedematous erythema of
the face and neck, and several hard infiltrated raised plaques on
the dorsi of the hand and fingers. On examination, his nasal and
laryngeal mucosae were found to be reddened, and there was a very
slight conjunctivitis. There were no changes in the skin of areas
protected by clothing. His only other complaint was of headache.
There were no symptoms or signs referrable to the nervous or
gastrointestinal systems. Selenium was detected in the urine (no
actual values were given). His liver was found to be enlarged and
there was an increase of porphyrins in the urine.
Pringle (1942) has described several cases of acute dermatitis
resulting from contact with dry selenium dioxide or selenium
dioxide dissolved in water, and 2 cases of acutely painful
paronychia after accidental contact with dry selenium dioxide. In
addition, several cases of mild rash on the anterior surface of
both forearms, but principally affecting the bend of the elbows,
were seen among employees working with heated selenium dioxide in
the fume cupboards. Only their fingers, protected at that time
with gloves, entered the fume cupboard, which apparently was
adequately protected. The dermatitis resembled a seborrhoeic
condition and there was frequently a thin watery discharge. In the
course of a year, 2 cases developed a high degree of sensitivity to
selenium dioxide, so that working in the same department, though
not actually on selenium, caused repeated recurrences and they had
to be removed permanently to another part of the factory.
(c) Studies on the health effects of long-term exposure
Filatova (1948) reported that workers exposed over a long
period of time to selenium aerosols containing elemental selenium
at levels of 0.35 - 24.8 mg/m3 and selenium dioxide at levels of
0.11 - 0.78 mg/m3 developed rhinitis, nasal bleeding, headaches,
loss of weight, irritability, and pain in the extremities
(Izraelson et al., 1973).
Conditions of exposure and the related health effects in a
rectifier factory were investigated by Kinnigkeit (1962). Air-
selenium levels were determined in various workplaces and blood-
selenium levels were measured in 62 workers exposed to selenium.
Urinary-selenium levels were also determined in 22 workers. The
reported air-selenium levels did not exceed 0.05 mg/m3 and were
less than the threshold limit value. However, as discussed in
section 5.2.1 there was a clearcut discrepancy between the air
values and the selenium levels observed in blood and urine,
indicating that the actual workers' exposure must have been
considerably higher. Of 62 workers, over half (35) complained of
irritability, sleeplessness, loss of appetite, and nausea. Twenty
six had headaches and 3 had cramplike pains in their limbs.
Clinical examination revealed irritation of the mucosa in 9
workers, with conjunctivitis and slight tracheal bronchitis. Of
the 2 workers that had unavoidable skin contact with selenium, one
had excematous lesions of the forearms and the other had bluish-red
urticarial exanthema.
The Takata reaction and the thymol test were performed on 61 of
these workers, and the results were within the normal range in all
employees, with the remarkable exception of workers engaged in the
electrical testing of the rectifier plates. In this group of 13
people, abnormal results suggesting impaired liver function, were
obtained in 8 workers, the only employees from the plant showing
this pathological reaction. As underlined by Kinnigkeit, workers
carrying out this process handled the plates by hand and were
frequently directly exposed to fumes containing selenium dioxide
arising from the burning out of plates during electrical testing.
As discussed previously, this group was characterized by a very
high mean blood-selenium level (geometrical mean ± SE: 15.8 ± 11.8
mg/litre). A great interindividual variability in selenium-blood
levels existed with this and other groups, but no attempt was made
to relate these individual values to the results of liver function
tests. Another group of workers had blood-selenium levels that
were almost as high (Table 13) (section 6.2.3), but no evidence of
liver dysfunction was observed in this group. However, it cannot
be assumed that the form and pathway of the exposure to selenium
was the same in both groups.
Monaenkova & Glotova (1963) summed up results of clinical
observations on 12 people, aged 30 - 50 years, who had been
occupationally exposed to selenium for 3 - 16 years and who
manifested the symptoms of chronic selenium and selenium dioxide
poisoning. Ten of the 12 persons had been previously engaged in
enterprises producing selenium rectifiers and were exposed to both
elementary selenium and selenium dioxide. The patients complained
of pain in the right hypochondrium, dyspeptic phenomena, undue
fatigability, dyspnoea, weakness, and sleeplessness. Several
patients complained of cough and, in 3 of them, chronic bronchitis
or moderate emphysaema were established. One patient had asthmatic
bronchitis. Almost all patients had various impairments of the
liver and gastrointestinal tract (toxic hepatitis in 6 patients,
dyskinesis of gallbladder in 4 patients, cholecystis in 4 patients,
and spastic colitis in 4 patients). The patients showed an
astheno-vegetative syndrome, pigmentation of exposed areas of the
skin, and, in 8 persons signs of a hyperfunction of the thyroid
gland (radioiodine uptake measurements) were found. Elevation of
the basal metabolic rate or hyperfunction of the thyroid gland were
mentioned also briefly in a previous case report (Halter, 1938) and
in a review (Holstein, 1951), respectively.
A group of selenium workers in a rectifier factory was followed
by Glover (1967, 1970) in 1953 - 56. Every 3 months, urinary-
selenium levels were determined, and the workers were asked about
their health and examined for the presence of garlicky-smelling
breath and skin rashes. Other routine medical checks were not
performed. Workers complained of indigestion and epigastric pain;
severe haematemesis (with hospitalization) occurred in one case.
Several men noticed symptoms of lassitude and irritability, when on
selenium work, which cleared whenever they were taken away from
selenium. A strong odour of garlic on the breath was detected in
most workers with selenium-urinary levels of 0.5 - 1.0 mg litre,
but not below these levels. The breath odour disappeared in 7 - 10
days following the removal of workers from contact with selenium.
Glover (1967, 1970) was able to trace 17 deaths, occurring within
10 years, among selenium-exposed workers in the same factory (Table
52). The author recognized that the list of deaths was not
complete and that the group was too small to permit far-reaching
conclusions. The length of exposure to selenium in individual
cases was not given. Carcinomas arose in various organs: 2
bronchus, 1 stomach, 1 colon, 1 ovary, and 1 testis.
Table 52. Comparison of the certified causes of death of
seventeen selenium workers with the expected distribution
by cause based on the experience throughout England and
Wales
----------------------------------------------------------
International Cause of death Observed Expectedb
list number
----------------------------------------------------------
001 - 019 tuberculosis 0 0.4
140 - 205 malignant neoplasms 6 5.1
330 - 334 vascular lesions 0 1.4
410 - 416 chronic rheumatic 2 0.6
heart disease
420 - 422 arteriosclerotic 3 3.8
heart disease
----------------------------------------------------------
Table 52. (contd.)
----------------------------------------------------------
International Cause of death Observed Expectedb
list number
----------------------------------------------------------
430 - 434 "other" heart disease 0 0.2
440 - 443 hypertensive heart 0 0.2
disease
444 - 447 "other" hypertensive 1 0.2
disease
450 - 456 disease of arteries 0 0.2
480 - 483 influenza 0 0.1
490 - 493 pneumonia 0 0.5
500 - 502 bronchitis 2 0.9
- all other causes 3 3.5
- all causes 17 17.1
----------------------------------------------------------
a From: Glover (1967).
b Taking into consideration the age and sex of those who
died, and the year of death. The Table shows no
evidence of excess mortality in the selenium workers
from any of these main groups of causes.
8.1.2.1.2. Hydrogen selenide
(a) Short-term exposure
There are few cases of occupational poisoning described in
which hydrogen selenide was identified as the sole causative agent.
Reported cases of occupational intoxication by hydrogen selenide
resulted usually from short-term accidental exposures in the
chemical laboratory or industry. Apparently, the first account of
an individual requiring hospital treatment after sudden exposure
to this gas was by Senf (1941), describing the effects of the
accidental laboratory exposure of a chemist to hydrogen selenide.
The patient noticed the characteristic smell and the gas caused her
eyes to weep and her nose to run. Within a few hours, her voice
became hoarse and increasing dyspnoea caused her admission to
hospital. On examination she was found to have a bluish-red
erythema of the skin, conjunctivitis, and injection of the nasal
mucous membrane. The lung oedema, ECG myocardial changes, the dark
red erythema, and porphyrinuria disappeared within 10 days. In
1941, Painter described a case of a single inhalation of hydrogen
selenide. After a brief "metallic" sensation, no ill effects were
felt for about 4 h. Then a copious discharge from the nasal
passages began. This persisted with violent sneezing for 3 or 4
days. No ill effects were noted later.
A case of pure hydrogen selenide poisoning in a works chemist,
producing hydrogen selenide in order to form selenides, was
described by Symanski (1950). Apparently only one inhalation of
the gas was followed by a sudden feeling of constriction in the
chest with coughing, tears, and burning of the nose, all of which
disappeared in a few moments. The patient thought that he had lost
his sense of smell. Four or 5 h later, a severe cough and dyspnoea
developed. At medical examination (8 h after the accident) he was
dyspnoeic and febrile. He continued to cough up blood-stained
frothy sputum all night, and by morning the acute symptoms had
disappeared leaving him apparently with bronchitis and an
irritating cough. A week later he had a recurrence of the fever
with chest pain and the signs of bronchopneumonia. After 4 more
days, the fever disappeared and 9 weeks later he was fit and well
again. A man, 5 h after cleaning out apparatus used for making
hydrogen selenide, developed severe dyspnoea, difficulty in
expiration, painful cough, and yellowish sputum (Bonard & Koralink,
1958). His condition became worse and the next day he was admitted
to the hospital. He was cyanosed, had severe expiratory dyspnoea,
discrete rhinitis and conjunctivitis, and was in considerable
distress. He had no headache or visual troubles. His pharynx was
hyperaemic, his buccal mucosa was dry, and he had a furred tongue.
Four days later, the chest roentgenogram was normal, but lung
function tests were depressed. Rohmer et al. (1950) reported the
case of a chemist exposed to a high level of hydrogen selenide,
calling attention to the development of severe hyperglycaemia that
could only be controlled by increasingly large doses of insulin. A
24-year-old white man accidentally inhaled hydrogen selenide while
transferring the gas from one cylinder to another (Schecter et al.,
1980). He immediately experienced burning in his eyes and throat
followed by coughing and wheezing. He was given oxygen and
improved over a period of 2 h. However, 18 h later, because of
recurrent cough and progressive dyspnoea, he was hospitalized.
Pneumomediastinum developed in this patient and pulmonary function
tests revealed restrictive and obstructive airways disease, which
slowly improved.
Glover (1970), who reviewed 8 cases of acute hydrogen selenide
poisoning (5 laboratory workers and 3 from industrial accidents;
some of them probably identical with those mentioned above), and
summarized the following sequence of events. The first effects
observed after exposure are signs of irritation of the mucous
membranes, i.e., running nose, running eyes, cough, sneeze,
followed by a slight tightness of the chest. This clears, and
there may be a latent period of 6 - 8 h. The patient, who has
often returned home by this time, usually wakes up in bed
breathless with signs and symptoms of pulmonary oedema. Glover, at
the same time, underlined the importance of oxygen therapy in these
intoxications, particularly with regard to the prevention of the
development of pulmonary oedema.
Lazarev & Gadaskina (1977) referred to 5 cases of hydrogen
selenide intoxication at an exposure level of approximately
7 mg/m3. These cases manifested nausea, vomiting, vertigo, and
extreme tiredness, in addition to effects on the respiratory tract
and conjunctiva. The same author mentioned one case of laboratory
exposure to hydrogen selenide in which a chemist developed lung
oedema and long-lasting cyanosis with respiratory difficulties. On
the 22nd day, thrombophlebitis was observed and within 52 days
signs of myocardial damage were noted.
(b) Prolonged and/or repeated exposure
An important point when considering prolonged low-level
exposure to hydrogen selenide was recognized by Dudley & Miller
(1941). In their experience, acute exposure levels of 5 µg/litre
resulted in such eye and nose irritation in workers that the men
could not continue their duties without the protection of a gas
mask. At 1 µg/litre, this irritation did not occur for several
minutes, though the presence of the gas was detected by its odour.
However, continued exposure at these levels results in olfactory
fatigue so that workers lose their ability to smell the gas and are
thus disarmed against subsequent increases in levels of the gas in
the work-place.
The effects of prolonged exposure to hydrogen selenide were
described by Buchan (1947) in workers who were engaged in a process
involving the etching of a pattern on steel stripping. In this
operation, steel stripping is passed between rubber imprinting
wheels with a raised pattern on the periphery. The pattern is in
constant contact with a wick immersed in the etching ink. After
imprinting, the steel stripping passes between felt wipers and dips
into a rust-preventive oil bath passing then to a reel. The odour
of hydrogen selenide was detectable at the etching machine and was
particularly offensive in the sludge of the oil bath. Selenium was
identified, qualitatively, in the sludge. Apparently, the hydrogen
selenide was generated as a result of the reaction of the excess
selenium deposited on the steel stripping in an acid medium, i.e.,
the oil bath, which was further acidified by contamination with the
etching ink carried by the steel stripping. Five out of 25 workers
complained of nausea, vomiting, metallic taste in the mouth,
dizziness, extreme lassitude, and fatigability. The symptoms
lasted 2 weeks and were said to be increasing in severity. Until
one month before the onset of symptoms, an etching ink containing
nitric acid and silver was used, but then a new ink containing
selenious acid at 52 mg selenium/litre was substituted.
Air samples were taken at 6 representative sampling points.
During the analysis, qualitative detection of selenium was made,
but, because of the lack of sensitivity of the titrimetric method
used, it was not possible to measure the selenium quantitatively,
and the results were recorded as less than 0.2 parts per million.
Spot and 24-h specimens of workers' urine contained 0 - 131 µg
selenium/litre or an average of 61.6 µg/litre. So-called control
specimens contained similar amounts, but the controls were the
professional staff collecting the air samples and they had been
exposed to the same environment as the workers for almost 5 h.
Also, it should be realized that hydrogen selenide produces
symptoms at exposures too low to increase the urinary excretion
above normal values. When the selenium ink was replaced by silver
ink, there was a gradual regression of symptoms and, within 6
months, all complaints had ceased and there was no recurrence.
The Task Group was not aware of any other reports dealing with
the prolonged exposure of human beings to hydrogen selenide.
8.1.2.1.3. Selenium oxychloride
Less than 5 µg of pure selenium oxychloride on the skin of the
hand resulted in a painful reaction within a few minutes, exythema
surrounding the central necrotic area (Dudley, 1938). Within 1
month, the skin defect healed with a scar.
8.2. Low Selenium Intake
8.2.1. Evidence supporting the possible essentiality of selenium in
man
Beneficial nutritional effects of selenium have been observed
in several different species (Schwarz, 1976), and specific signs of
selenium deficiency in the presence of adequate intake of vitamin E
have been demonstrated in rats and chicks (section 7.2.1). Thus,
the question of whether selenium is essential for man arises. The
identification of any human disease due to low selenium intake is
difficult, because selenium deficiency in animals is characterized
by a wide variety of signs involving several different organ
systems.
Although a clear-cut pathological condition attributable to
selenium deficiency alone has not yet been demonstrated in human
beings, certain evidence suggests that selenium may be essential
for man.
For example, purified glutathione peroxidase, from human red
blood cells, contains quantities of selenium similar to those found
in the enzyme isolated from animals (Awasthi et al., 1975).
Moreover, selenium is necessary for the optimal growth of human
fibroblasts in purified cell culture media (McKeehan et al., 1976).
Blood-selenium levels are depressed in children suffering from
kwashiorkor (Burk et al., 1967; Levine & Olson, 1970) and Hopkins &
Majaj (1967) obtained a reticulocyte response in malnourished
infants treated with a physiological dose of selenium (25 µg
selenium, as sodium selenite). Finally, on the basis of the
distribution of selenium in various human tissues, Liebscher &
Smith (1968) concluded that selenium must be an essential element
for man.
8.2.2. Signs and symptoms of low intake
The greatest possibility of a hazard due to inadequate selenium
intake would be expected in low-selenium areas. Nutritionists and
public health officials in several countries are aware of the low-
selenium status of their populations and are attempting to identify
any human health problems associated with it. For instance, in
the south island of New Zealand, one of the first regions where a
low selenium status in animals was recognized, some farmers, noting
supposed similarities between their own symptoms and those of the
white muscle disease affecting their livestock, claimed improvement
in their muscular complaints after self-medication with selenium
(Hickey, 1968). However, such anecdotal reports are not supported
by the results of 3 separate trials involving a total of 120
patients suffering from "muscular complaints", carried out in
several low-selenium areas of the south island of New Zealand
(Robinson et al., 198l). In these studies, the patients were given
either sodium selenite or selenomethionine at a number of dose
levels and schedules for various periods of time. Some subjects
also received vitamin E with the selenium supplement. In all
subjects who received selenium, blood-selenium levels and
glutathione peroxidase activity increased, whereas little change
was seen in the control group. Clinical assessment of muscular
symptoms showed that approximately equal numbers of patients in the
test and control groups exhibited an improvement in their muscular
condition. On the basis of these results, the authors concluded
that there was no evidence of any response, under the conditions of
the trials, to selenium supplements for the relief of muscular
complaints.
Total parenteral nutrition (TPN) fluids for intravenous feeding
contain very low levels of selenium (section 4.1.1.1), and patients
sustained by such techniques would seem to be at risk of developing
selenium deficiency. One such patient on TPN in New Zealand
developed muscular discomfort that disappeared after selenium
supplementation (van Rij et al., 1979). This patient was a 37-
year-old female who lived in a rural area of the south island of
New Zealand where the soils were low in selenium and there was a
history of endemic white muscle disease in sheep. She presented
with a perforated small intestine with peritonitis, after
radiotherapy 2 years previously for carcinoma of the cervix. Five
days after abdominal exploration and the start of intensive
antibiotic therapy she developed enterocutaneous and vaginal
fistulae. Gastric stress ulcer followed and necessitated a 1.5-
litre blood transfusion. Elevated temperatures persisted with
intraabdominal sepsis. Ten days after admission to hospital, TPN
was begun and resulted in a general improvement and a 6 kg weight
gain over the next 20 days. Regular plasma and albumin infusions
treated hypoproteinemia. After 20 days of TPN, early clinical
signs of fatty acid deficiency (dry flaky skin on hands and feet)
were noted, which responded rapidly to intralipid infusion. After
30 days of TPN, the patient complained of increasing bilateral
muscular discomfort in her quadriceps and hamstring muscles.
Muscle pain was present at rest as a persistent ache and with
tenderness on palpation. The muscle pain was aggravated by walking
until she found it distressing, even to move beyond her room. On
examination, there was tenderness of the quadriceps, hamstrings,
and less markedly of the calf muscles of both legs. Both active
and passive movements of these muscle groups were painful,
particularly of the hamstrings. The upper limb girdle was
unaffected. A generalized muscle wasting of all the limbs was
observed after the prolonged catabolic stress, despite TPN. No
muscle fasciculation or neurological deficits were observed.
Supplementation with selenium was begun with no other
modification to the patient's management. Each day 100 µg of
selenium, as selenomethionine, was infused intravenously with the
TPN solution. During the next week, muscle pain at rest,
tenderness to palpation, and pain on active and passive movement
disappeared. A return to full mobility followed. This symptomatic
response associated with selenium supplementation plus the
extremely low blood-selenium levels initially observed in this
patient (discussed further in section 8.2.4) led the authors to
conclude that this case could be the first clinical report
supporting the essential role of selenium in human nutrition.
Because of their increased metabolic requirements and faster
growth rates, infants and children might be particularly vulnerable
to selenium deficiency. McKenzie et al. (1978) analysed blood from
230 healthy adults and 83 healthy children from various areas of
New Zealand and found that children in Auckland and Tapanui had
lower blood-selenium concentrations (0.064 and 0.048 mg/litre) than
adults from the same areas (0.083 and 0.060 mg/litre). Red-blood-
cell glutathione peroxidase activity was also lower in Auckland
children than in adults (10.6 versus 12.9 units/g haemoglobin). A
specific population of infants and children that might be
especially at risk regarding low selenium intake includes those who
suffer from phenylketonuria (PKU) and maple syrup urine disease
(MSUD) and consume only special synthetic diets that are very low
in selenium (section 4). McKenzie et al. (1978) reported that the
mean blood-selenium level in 12 such children was 0.038 ± 0.013
mg/litre. One 13-year-old patient had 0.016 mg selenium/litre
whole blood and 0.009 mg selenium/litre plasma, but clinical
examination indicated that he was in good health. Lombeck et al.
(1978) found that the serum-selenium content in 36 children
receiving diet therapy for PKU and MSUD in the Federal Republic of
Germany ranged from 0.007 - 0.028 mg/litre. The selenium content
of the hair was lower in the patients (0.062 mg/kg) than in healthy
children (0.429 mg/kg) and the erythrocyte glutathione peroxidase
activity was reduced in comparison with normal values (4.6 versus
8.8 units/g haemoglobin). And yet all the patients thrived during
the time of observation and did not exhibit any increased rate of
haemolysis or oxidation of haemoglobin to methaemoglobin after
incubation of their erythrocytes with sodium azide.
Gross (1976) studied 4 groups of premature infants who were fed
4 different formulae based on cow's milk containing a high
concentration of polyunsaturated fatty acid (PUFA), with and
without iron, or a low PUFA concentration, with and without iron.
The tocopherol content was the same in all 4 formulae and was
judged adequate in terms of maintaining serum-vitamin E levels.
Both glutathione peroxidase activity and plasma-selenium levels
were similar in all 4 groups. The former declined from 4.2 units/g
haemoglobin at one week of age to 2.7 units/g haemoglobin at 7
weeks of age, whereas the latter declined from 0.08 to 0.035
mg/litre. Although all infants exhibited the anaemia typical of
the prematurely newborn, the decreases in haemoglobin and increases
in reticulocyte levels were greatest in the group of infants given
the formula high in PUFA and iron. These haemolytic events in
vitamin E-sufficient premature infants fed a diet rich in PUFA and
iron were thought to be due to the oxidative stress of the formula
coupled with the poor nutritional status of the infants with regard
to selenium (Gross, 1976).
8.2.3. Dietary levels consistent with good nutrition
8.2.3.1. Quantitative estimates
Since no clear-cut pathological condition attributable to
selenium deficiency alone has yet been observed in man, it is not
possible to define a precise dietary requirement level for human
beings. However, 0.1 - 0.2 mg selenium/kg diet is a nutritionally
generous level for most species of animals (US NAS/NRC, 1971). If
these animal data are extrapolated, a 70-kg man consuming 500 g of
diet per day (dry basis), would need a daily intake of 50 - 100 µg.
The US National Research Council has estimated that the safe and
adequate range of the daily intake of selenium for adults is 50 -
200 µg, with correspondingly lower intakes for infants and children
(Table 53). On this basis, the recommended intake for a 70-kg man
would be equivalent to 0.7 - 2.8 µg/kg body weight per day. Any
daily intake within the recommended range is considered adequate
and safe, but the recommendations do not imply that intakes at the
upper limit of the range are more desirable or beneficial than
those at the lower limit. The lower recommended intake for infants
and children is consistent with the observation that
supplementation of malnourished children with sodium selenite at
30 µg selenium daily, produced weight gain and reticulocyte
responses without any untoward signs (Hopkins & Majaj, 1967).
Table 53. Estimated safe and
adequate range of selenium intakea
-----------------------------------
Group Age Daily selenium
(years) intake (µg)
-----------------------------------
Infants 0 - 0.5 10 - 40
0.5 - 1 20 - 60
Children 1 - 3 20 - 80
4 - 6 30 - 120
7+ 50 - 200
Adults 50 - 200
-----------------------------------
a Adapted from: US NAS/NRC (1980).
Three approaches have now been used to estimate human
nutritional requirements for selenium. Nutritionists have long
used metabolic balance studies to determine the human requirements
for a variety of minerals. In the case of selenium, healthy North
American men needed about 80 µg dietary selenium/day to maintain
balance, but women needed only 57 µg/day (Levander & Morris, 1984).
The difference between men and women in the selenium intake needed
to achieve balance was considered to be due to differences in body
weight. Expressing the balance data on a body weight basis
revealed that both men and women needed about 1 µg selenium per kg
body weight per day to stay in balance. However, other research
groups showed that selenium balance in New Zealand women or Chinese
men could be reached on intakes as low as 27 and 9 µg/day,
respectively (Stewart et al., 1978; Luo et al., 1985). This
demonstrates the great effect of prior dietary-selenium intake on
the amount of selenium needed to achieve balance in people and
shows that balance studies may not be valid techniques for
estimating human selenium requirements.
Yang et al. (1985) estimated human selenium requirements on the
basis of a comparison of dietary intakes in areas with and without
Keshan disease. Dietary-selenium intakes of 7.7 and 6.6 µg/day in
endemic and 19.1 and 13.3 µg/day in non-endemic Keshan disease
areas were reported for adult men and women, respectively. These
estimates should be considered minimum daily adult requirements for
selenium.
The depletion/repletion study is another approach used by
nutritionists to estimate human selenium requirements. The
relatively large body pool of selenium in North Americans prevented
their plasma-selenium levels from dropping to values commonly found
in persons from low-selenium areas (Finland, New Zealand), even
when depleted for almost 7 weeks (Levander et al., 1981a,b).
Chinese men of naturally-low-selenium status (dietary intake about
10 µg/day) were given graded supplements of selenomethionine and
their plasma-glutathione peroxidase activity was followed (Yang et
al., 1985). The activity of the enzyme plateaued at the same level
in all men receiving 30 µg or more of supplementary selenium daily.
From this study, a physiological selenium requirement of about 40
µg/day (diet plus supplement) was suggested for Chinese adult
males, which may require adjustment to account for body weight
differences in other populations including women.
8.2.3.2. Nutritional bioavailability
None of the studies discussed in section 8.2.3.1 have
specifically addressed the question concerning the nutritional
bioavailability of the selenium in foods for human beings. Using
an animal model, Douglass et al. (1981) found that the selenium in
freeze-dried, water-packed canned tuna for human consumption was
only 57% as effective as selenite in restoring liver glutathione
peroxidase activity in rats previously deficient in selenium,
whereas selenium as cooked freeze-dried beef kidney or seleniferous
wheat had 97 and 83% of the activity of selenite, respectively.
The selenium in the tuna was also less effective than selenite in
raising hepatic-selenium levels in the deficient rats. Similar
results concerning the relative bioavailability of the selenium in
tuna compared with wheat were obtained by Alexander et al. (1983)
in rats, using the slope-ratio technique. On the other hand,
Chansler et al. (1983) found that the selenium in mushrooms was
only about 4% as available as that in selenite for restoring
hepatic glutathione peroxidase activity in selenium-depleted rats.
It has been pointed out that, in addition to absorption and
retention, factors such as utilizability within the body are
apparently important in determining selenium bioavailability
(Levander, 1983).
Although data are accumulating on the absorption by human
beings of the selenium in various compounds or in foods (section
6.1.1.2), there have been few studies examining the bioavailability
(i.e., utilization, consisting of transport, conversion to a
metabolically-active form, retention, etc., in addition to
absorption) of the selenium in foods for human beings. One such
study was carried out in a low-selenium area of central Finland
(Levander et al., 1983). Three groups of 10 men of low-selenium
status (mean plasma-selenium level of 70 µg/litre) were
supplemented with 200 µg of selenium, daily, as selenium-rich
wheat, selenium-rich yeast, or sodium selenate, for 11 weeks.
Twenty unsupplemented subjects served as controls. Plasma-selenium
levels increased steadily in the wheat and yeast groups for 11
weeks to around 160 µg/litre with no sign of plateauing, whereas,
in the selenate group, plasma-selenium plateaued at about 110
µg/litre, after 4 weeks. Red blood cell-selenium levels also
increased steadily in the wheat and yeast groups for 11 weeks from
90 to 190 µg/litre, again with no sign of plateauing. Red blood
cell-selenium levels were unaffected in the selenate group.
Platelet glutathione peroxidase activity (glutathione peroxidase is
an index of selenium status) (section 7.2.4.4) roughly doubled
after 4 weeks of supplementation with wheat or selenate and then
plateaued. Platelet glutathione peroxidase increased more slowly
in the yeast group. Plasma-glutathione peroxidase activity did not
respond to selenium supplementation. Ten weeks after the
supplements were stopped, platelet glutathione peroxidase remained
higher in the wheat and yeast groups than in the selenate group.
This suggested that the selenium in yeast or wheat was, to some
extent, deposited in the tissues in a form that could be used later
for glutathione peroxidase biosynthesis, once the dietary
supplement was discontinued. The results of this bioavailability
trial indicated that there are several different aspects to the
nutritional availability of selenium. Complete assessment may
require several measurements including: short-term platelet
glutathione peroxidase activity, to estimate immediate
availability; medium-term plasma-selenium levels, to determine
retention; and long-term platelet glutathione peroxidase activity,
after discontinuation of supplements, to estimate the
convertibility of tissue-selenium stores to metabolically active
selenium.
Griffiths & Thomson (1974) also noted that the blood-selenium
levels of adults from the USA declined rapidly on arrival in New
Zealand, but, after a year, their levels were still higher than the
mean value for permanent New Zealand residents (Fig. 11). When
selenium levels were measured in the whole blood, erythrocytes, and
plasma of postoperative surgical patients receiving TPN in New
Zealand, selenium concentrations decreased as TPN was continued
(Table 54) (van Rij et al., 1979). The plasma-selenium
concentration of the New Zealand TPN patient, discussed in section
8.2.2, was 0.025 mg/litre and fell to 0.009 mg/litre just before
selenium supplementation was begun. The lowest value for blood-
selenium concentration in areas of China not affected by Keshan
disease was around 0.040 mg/litre, while, in affected areas, the
blood-selenium concentration often dropped below 0.010 mg/litre
(Keshan Disease Research Group, 1979b).
Under certain conditions, some investigators have not found a
significant correlation between blood-selenium levels and blood
glutathione peroxidase activity. For example, Thompson et al.
(1976) described swine that had high blood-selenium levels but low
blood-glutathione peroxidase activities. The reasons for this
discrepancy are not known but could perhaps be related to an
increased level of "non-functional" selenium in the blood, such as
selenomethionine that has been nonspecifically incorporated into
protein as a substitute for methionine.
7.2.4.4. Glutathione peroxidase as an indicator of selenium status
As discussed by Hoekstra (1975b), the measurement of
glutathione peroxidase activity offers several advantages as an
indicator of selenium status. First of all, the enzyme assay is
easier to perform and is less time consuming than the selenium
analysis. Also, the enzyme assay is not subject to contamination
problems. Moreover, glutathione peroxidase represents only
"functional" selenium in the tissues, not selenium that has been
non-specifically incorporated into proteins or that has formed
biologically inactive complexes with heavy metals. Finally, at
least in some tissues, the activity of the enzyme appears to taper
off as the dietary level of selenium approaches the nutritional
requirement of the animal. This "plateau effect" of glutathione
peroxidase activity does not occur in red blood cells, a biological
material that is highly convenient for sampling purposes. However,
as pointed out by Hoekstra (1975b), the absence of plateauing in
red cells may limit the usefulness of erythrocyte-glutathione
peroxidase in accurately establishing selenium requirements, but
does not diminish the usefulness of this source of the enzyme as an
overall index of selenium status.
One complicating factor in the use of glutathione peroxidase to
assess selenium status is the fact that the activity of the enzyme
is influenced by many physiological variables other than selenium
intake (Ganther et al., 1976). Among these are the age and sex of
the animal, starvation, exposure to certain oxidant stressors,
toxicants, or heavy metals, and deficiency in iron and vitamin B12.
Obviously, these variables have to be controlled, or compensated
for, if glutathione peroxidase is to be a valid index of selenium
status.
Another serious complicating factor in the use of glutathione
peroxidase to assess selenium status is the existence of a "non
selenium-dependent" glutathione peroxidase activity that persists,
even in severe selenium deficiency (Lawrence & Burk, 1976). This
enzymic activity has been found in variable amounts in different
rat tissues ranging from 23% of the total glutathione peroxidase
activity in fat to 91% in testis (Lawrence & Burk, 1978). The "non
selenium-dependent" activity accounts for 26 - 38% of the total
glutathione peroxidase activity in rat brain, kidneys, liver, and
adrenals. No such activity is found in rat erythrocytes, spleen,
heart, thymus, intestinal mucosum, skin, or skeletal muscle. The
"non selenium-dependent" glutathione peroxidase activity has been
found in the liver of several different species including the rat,
hamster, guinea-pig, chicken, pig, sheep, and man. In human and
guinea-pig liver, it accounts for the major part of the total
glutathione peroxidase activity. The "non selenium-dependent"
glutathione peroxidase activity differs from the selenium-
containing enzyme in substrate specificity in that it catalyses the
reduction of a range of organic hydroperoxides but not of hydrogen
peroxide. The activity of the "non selenium-dependent" enzyme is
inversely related in rat liver to the dietary level of selenium
(Lawrence & Burk, 1978; Lawrence et al., 1978) and the glutathione-
S-transferase enzymes are responsible for the "non selenium-
dependent" enzyme activity (Prohaska & Ganther, 1977; Lawrence et
al., 1978). The glutathione- S-transferases are not selenium-
containing enzymes; they are all dimeric, and their monomeric
constituents fall into three categories of different relative
molecular mass. The glutathione peroxidase activity of the enzymes
is only shown by the dimeric enzymes that contain one of the three
subunits and, in selenium deficiency, the amount of this subunit
alone is increased (Ketterer et al., 1982; Mehlert & Diplock,
1985).
The physiological significance of the "non selenium-dependent"
activity has been questioned because the seleno-enzyme has a much
higher Vmax and lower KM (Prohaska & Ganther, 1977). However, Burk
et al. (1980a) showed that the "non selenium-dependent enzyme"
removed organic hydroperoxides in intact rat livers perfused with a
haemoglobin-free medium containing high concentrations of
peroxides.
Obviously, any use of glutathione peroxidase activity to
monitor selenium status has to take into account this "non
selenium-dependent" activity. This complication can be avoided by
selecting a tissue for test that has little or none of the
selenium-independent activity, such as erythrocytes, or by using
hydrogen peroxide as the substrate, since this has little activity
with the selenium-independent enzyme.
7.2.5. Other possible physiological roles
7.2.5.1. Homeostasis of hepatic haem
Burk et al. (1974) showed that the induction of hepatic
cytochrome P-450 by daily intraperitoneal injections of 75 mg
phenobarbital/kg body weight, for 4 days, was markedly impaired in
rats that had been fed a selenium-deficient diet for at least 3
months. The induction of ethylmorphine demethylase activity by
phenobarbital was also impaired in the livers of selenium-deficient
rats. However, no such impairment was observed in the induction of
cytochrome b5 or of NADPH-cytochrome c reductase activity or
biphenyl hydroxylase activity, or in pentobarbital sleeping time
(Burk & Masters, 1975). Treatment with phenobarbital stimulated a
6- to 8-fold increase in hepatic microsomal haem oxygenase activity
in selenium-deficient rats but had little or no effect in selenium-
supplemented rats (Correia & Burk, 1976). This suggested that the
abnormalities of cytochrome P-450 induction in selenium-deficient
rats were related to increased degradation rather than decreased
synthesis of hepatic haem. Mackinnon & Simon (1976) reported an
impaired haem synthesis in selenium-deficient phenobarbital-treated
rats, but Burk & Correia (1977) were unable to confirm these
results. Apparently, the pronounced stimulation of microsomal
haem oxygenase (EC 1.14.99.3) in the livers of selenium-deficient
rats by phenobarbital is unrelated to the role of selenium in
glutathione peroxidase, since pretreatment of deficient rats with
a single dose of selenite at 50 mg selenium/rat, 4 - 6 h before
phenobarbital administration, abolished the response in haem
oxygenase but had no effect on cytosolic or mitochondrial
glutathione peroxidase activity (Correia & Burk, 1978). Moreover,
phenobarbital failed to stimulate liver microsomal haem oxygenase
activity or to affect cytochrome P-450 in vitamin E-deficient,
selenium-adequate rats, so this response does not seem to be
related to the direct effects of lipid peroxidation on haem or
cytochrome P-450. There is a marked sex difference in the
phenobarbital stimulation of hepatic microsomal haem oxygenase
activity in selenium-deficient rats. The stimulation was greatest
in males and least in females with intermediate values in castrated
males and testosterone-treated females (Burk et al., 1980a). This
sex difference existed even though hepatic glutathione peroxidase
activity was reduced to the same extent by selenium deficiency in
all groups. However, the exact mechanism by which selenium
influences homeostasis of hepatic haem still needs to be clarified.
Pascoe et al. (1983) showed that both intraluminal iron and
selenium were required by the intestinal mucosa for maintenance of
the intestinal cytochrome P-450 content, and its dependent mixed
function oxidase activity, and that withdrawal of dietary selenium
for one day markedly decreased the level of cytochrome P-450 in the
small intestine, suggesting that intestinal mucosal cells derive
their selenium from the diet rather than from the blood.
A report by Maines & Kappas (1976) indicated that injection of
selenium increased hepatic haem oxygenase activity, but the doses
of selenium used (3.94 mg/kg body weight) exceeded the MLD for
selenium as selenite (Table 19), so that the phenomenon observed by
these authors may have been merely an artifact of acute selenium
poisoning.
7.2.5.2. Microsomal and mitochondrial electron transport
Diplock and co-workers carried out an extensive series of
studies, the results of which suggest a possible role for selenium
and vitamin E in the electron-transfer system of rat-liver
microsomes (Diplock, 1974a,b). First, they showed that appreciable
amounts of volatile selenium evolved from rat liver subcellular
organelles, after acid treatment, only when the rats were adequate
in vitamin E and the tissue homogenization medium contained large
concentrations of anti-oxidants (Diplock et al., 1971). The acid-
volatile selenium was later identified as selenide (Diplock et al.,
1973). It was assumed that this compound was formed in vivo,
presumably by the glutathione reductase pathway (Fig. 9), but
artifactual in vitro formation of such selenide, because of the
presence of mercaptoethanol in the homogenization medium, cannot be
ruled out (Levander, 1976a). An oxidant-labile form of non-haem
iron, dependent on dietary vitamin E and selenium, was observed in
rat liver microsomes (Caygill & Diplock, 1973). It was considered
that this supported the hypothesis that the selenide might form
part of the active centre of a non-haem iron protein functional in
microsomal electron transfer. Alterations in the kinetic
parameters of aminopyrine demethylation by the liver microsomes in
vitamin E-deficient rats also supported this idea (Giasuddin et
al., 1975). However, attempts to isolate an oxidant-labile
selenium- and vitamin E-dependent non-haem, iron-containing protein
fraction were unsuccessful, because of the lability of the
selenide, and it was concluded that clarification of the role of
selenide in microsomes needed additional research (Diplock, 1976,
1979). The effects of prolonged selenium deficiency on a large
number of parameters in mouse liver xenobiotic metabolism were
studied by Reiter & Wendel (1983). They demonstrated a
heterogeneous pattern of effects that did not appear to involve
glutathione peroxidase.
One of the earliest biochemical defects discovered in vitamin
E- and selenium-deficient rats was the inability of liver slices or
homogenates to maintain normal rates of respiration after prolonged
periods of incubation in vitro (Schwarz, 1962). Although this so-
called respiratory decline might be explained on the basis of
generalized mitochondrial membrane damage due to lipid peroxidation
(Yeh & Johnson, 1973), it was also plausible that selenium might
have a more specific role in mitochondrial metabolism. Indeed,
selenium added to the diet, or added in vitro, accelerated the
swelling of rat liver mitochondria induced by certain thiols, and
this swelling could be blocked by the addition of cyanide (Levander
et al., 1973a). Selenium was in fact shown to be an excellent
catalyst for the reduction of cytochrome c by thiols in chemically-
defined systems (Levander et al., 1973b) and a selenoprotein
containing a haem group similar to that of cytochrome c has been
reported in lamb muscle (Whanger et al., 1973). However, more work
is required to establish the possible role of selenium in
mitochondrial electron transfer.
7.2.5.3. The immune response
Dietary vitamin E in excess of nutritionally required levels
has been shown to improve the humoral immune response of several
different species to bacterial and viral antigens (Nockels, 1979).
Because of the close nutritional and biochemical relationship
between selenium and vitamin E (section 7.2.4.1), the effect of
dietary selenium supplementation was tested on the immunological
responses of mice (Spallholz et al., 1973). Mice fed a laboratory
chow diet (thought to contain 0.5 mg selenium/kg) supplemented with
sodium selenite at 0.7 or 2.8 mg selenium/kg had approximately 7-
and 30-fold higher antibody titres, respectively, after challenge
with sheep red blood cells than mice fed the chow diet alone.
Sodium selenite injected into mice intraperitoneally at doses of
3 - 5 µg per animal also increased the primary immune response to
the sheep red-blood-cell antigen and this increase was greatest
when the selenium was given prior to, or simultaneously with, the
antigen (Spallholz et al., 1975). The mechanism by which selenium
stimulates antibody synthesis is not known (Martin & Spallholz,
1976), but the dose of selenium needed to elicit this response is
clearly above the nutritionally required level. Levander (1986)
has reviewed the very limited data on the possible effect of
selenium on the immune response in man.
7.2.5.4. Selenium and vision
The effects of selenium on light perception were studied in 50
male "Grey chinchilla" rabbits weighing 3 - 3.5 kg and maintained
on a standard laboratory diet (Abdullaev et al., 1972, 1974a,b).
Electroretinography was carried out in dark-adapted animals under
conditions of maximally dilated pupil induced by topical
application of 0.5% dikaine - 1% atropine. Light stimulation was
provided with an impulse lamp giving either single or dual flashes
of 150 microseconds. Eight different energy levels of light
intensity were used ranging from 0.016 to 1.4 J. Sodium selenite
was administered subcutaneously at a dose of 0.45 mg selenium/kg
body weight. Control rabbits were injected with sodium sulfite at
a dose of 1 mg/kg. Sodium selenite stimulated light sensitivity,
as judged by increases in the a and b waves of the electro-
retinogram (ERG). The increase in the amplitude of the b wave was
considerably greater than that of the a wave. This stimulating
effect of sodium selenite on light sensitivity persisted for 3 days
in all studies. The same effects were observed when sodium
selenite was given via retrobulbar administration. Sodium sulfite
did not have any effect on the ERG. Similar results were obtained
by Bacharev et al. (1975).
The Task Group recognized that the data on the effects of
selenium on vision were obtained in studies involving high dose
levels (in the case of rabbits, the doses were approximately 20 -
30% of the LD50). The effects on vision of lower doses of selenium
have not been elucidated so far. The possible effect of deficiency
of selenium on vision is also of interest. Model studies with
isolated retinas exposed to a 0.01% concentration of sodium
selenite resulted in an increase in the amplitude of the ERG
(Kulieva et al., 1978). The effect of increased sensitivity of the
eye to light, after administration of sodium selenite, and the rate
of manifestation and duration of the effect are directly
correlated with localization of the substance in the structures of
the eye (section 6.2.2.1). Subcutaneous administration of sodium
selenite to rabbits, at a dose of 0.4 - 2 mg/kg body weight,
inhibited free radical formation as indicated by electron
paramagnetic resonance (EPR) measurements, not only in the lipid
membrane phase but also within the melanoprotein granules of the
retinal epithelium. The EPR signal was decreased in intensity by
selenite treatment in comparison with controls, but the form and
parameters of the EPR lines were not changed (Abdullaev et al.,
1974a,b).
7.2.6. Effects on reproduction
Vitamin E was discovered as a result of the isolation of an
unknown fat-soluble dietary factor essential for reproduction in
rats (Evans & Bishop, 1922), but selenium compounds are not active
in preventing reproductive failure in vitamin E-deficient rats
(Harris et al., 1958). Moreover, female rats weighing 80 - 120 g
and fed a selenium-deficient, but vitamin E-adequate, diet
successfully delivered 3 litters over a period of 6 months,
even though their blood-selenium levels dropped from 0.52 to
0.06 mg/litre during this time (Hurt et al., 1971). Similarly,
McCoy & Weswig (1969) found that rats fed a low-selenium but
adequate-vitamin E diet, for 4 months, reproduced normally.
However, their offspring failed to reproduce if kept on the low
selenium diet. Immobile spermatozoa, with separation of heads from
tails, were observed in 5 out of the 8 male progeny not given
selenium supplements, and no spermatozoa were seen in the other 3
males. The 2 male and 2 female offspring, supplemented with sodium
selenite at 0.1 mg selenium/kg diet, were fertile but delivered
litters of only one or 2 rats, all of which died in a few days.
The authors concluded that selenium supplementation resulted in
some fertilization, but only a few full-term young were delivered
and these were abnormal as they survived for only a brief period.
Wu et al. (1973) found that the motility of spermatozoa from
male rats, born to females fed a selenium-deficient diet, was
always very poor and that most of the sperm cells showed breakage
near the midpiece of the tail. Supplementation of the diet with
high levels of vitamin E or various other antioxidants did not
prevent these selenium deficiency effects. However, in this study,
the body and testicular weights of the rats were so markedly
depressed by selenium deficiency that it was not possible to
establish whether the abnormal sperm were due to a direct effect of
selenium deficiency or to an indirect effect on overall growth and
well-being. In a second series of studies (Wu et al., 1979), using
male rats born to dams fed a diet adequate in selenium, the
depression of growth, and testes weight in the progeny caused by
selenium deficiency was much less, even though impaired sperm
motility and abnormal sperm morphology were still observed (Table
32). The authors also pointed out that there did not appear to be
any correlation between reduction in body or testicular weights and
the characteristic sperm midpiece abnormality among the individual
rats in each treatment group. However, all of the rats used by Wu
and associates were fed diets that were probably low in zinc and
chromium and both of these trace elements are known to play
important roles in sperm formation (Wu et al., 1971; US NAS/NRC,
1979; Anderson & Polansky, 1981).
The mechanism of the effects of selenium on the integrity of
sperm morphology in the rat is not known, but Brown & Burk (1973)
found over 40% of an injected tracer dose of radio-selenite in the
testis-epididymis complex of selenium-deficient rats, after 3
weeks. Autoradiography of epididymal sperm revealed that the
labelled selenium was heavily localized in the midpiece. Calvin
(1978) has isolated a selenopolypeptide, with a relative molecular
mass of 17 000 daltons, from rat sperm, which may have a critical
function in the normal assembly of the sperm tail.
Selenium cannot prevent resorption-gestation in vitamin E-
deficient rats, nor can it improve reproductive performance in
vitamin E-deficient chickens or turkeys (Creger et al., 1960;
Jensen & McGinnis, 1960). However, studies in which severe
depletion of selenium was produced by the long-term feeding of low-
selenium, high-vitamin E diets have shown the beneficial effects of
the element on egg production and hatchability in poultry. For
example, Cantor & Scott (1974) fed 1-day-old Single Comb White
Leghorn chicks semi-purified low-selenium starter, grower, and
developer diets, all of which contained only 0.02 mg naturally-
occurring selenium/kg from 0 to 6, 7 to 12, and 13 to 25 weeks of
age, respectively. Because of the low selenium level, high levels
of vitamin E (50 - 84 IU/kg) were added to the diets to prevent
exudative diathesis during the growth period. From 25 weeks of age
to 10 months, the birds were fed semi-purified low-selenium layer
diets that contained 11 IU of vitamin E/kg. After 10 months, the
hens were fed a low-selenium corn-soybean meal practical laying
ration containing 0.027 mg natural selenium/kg, by analysis. The
calculated vitamin E content was 16 IU/kg. At 12 1/2 months of
age, 4 replicate groups of 5 hens were continued on the low-
selenium diet, whereas a similar number of hens was fed the same
diet supplemented with sodium selenite at 0.1 mg selenium/kg.
Selenium supplementation had beneficial effects on both egg
production and hatchability of fertile eggs (Table 33), but there
was no significant effect of selenium on egg fertility, determined
after 2 - 5 weeks (data not shown).
Table 32. Effects of selenium deficiency on body and testicular weights and
spermatozoan morphology and motility in ratsa
------------------------------------------------------------------------------------
Dietary selenite Number Duration Body Testes Sperm Number of rats
supplement of (month) weight weight motilityb with sperm
(mg selenium/kg) rats (g) (g) midpiece
abnormality
------------------------------------------------------------------------------------
Study 1
0 6 4 452 ± 6 3.29 ± 0.06 6.2 0/6
0.5 6 4 470 ± 18 3.55 ± 0.11 6.2 0/6
0 4c 12 476 ± 17 3.03 ± 0.30 2.5 2/4
0.5 4c 12 494 ± 12 3.57 ± 0.07 5.8 0/4
Study 2
0 10c 11 456 ± 16 3.07 ± 0.09 3.5 5/10
0.4 12 11 543 ± 18 3.50 ± 0.08 8.0 0/12
------------------------------------------------------------------------------------
a Adapted from: Wu et al. (1979).
b Relative activity of sperm observed with a light microscope at 400x at 37 °C and
graded from 0 - 10 with 10 representing the highest motility.
c Two rats died in each of these groups.
Cantor et al. (1978) carried out a similar study on turkeys in
which large white breeder hens and toms were raised from one day of
age until sexual maturity on a series of Torula yeast-containing
diets, low in selenium content (0.025 - 0.047 mg/kg). At 32 weeks
of age, 8 replicate groups, each comprising 6 individually-caged
hens, were fed a low-selenium basal diet (0.033 mg/kg) or the same
diet supplemented with sodium selenite at 0.2 mg selenium/kg.
Eight individually-caged toms were also assigned to each dietary
treatment at 32 weeks of age. Neither the tom nor the hen dietary
treatments had any effect on fertility and the tom dietary
treatment did not have any effects on hatchability. But the
hatchability of fertile eggs from hens fed the unsupplemented and
supplemented diets was about 50 and 59%, respectively. The
mechanism by which dietary selenium improves hatchability in
chickens or turkeys is not known, but the improvement may be due to
increased embryonic selenium levels or to a reduced embryonic need
of vitamin E (Cantor & Scott, 1974).
Table 33. Effect of dietary selenium
supplements on reproductive performance of
12 1/2-month-old selenium-depleted hens fed a
low-selenium, corn-soybean-meal practical
rationa
----------------------------------------------
Selenium supplement None 0.1 mg selenium as
Na2Se03/kg diet
----------------------------------------------
Criterion
Egg production (%)
Weeks 1-2 67.2 76.6
3-4 66.4 78.5
5-6 64.2 71.8
Day 46-76 54.2 79.7
77-107 55.1 76.7
108-137 59.1 75.1
Hatchability of fertile eggs (%)
Week 2 57.3 93.0
3 54.7 97.1
4 54.5 94.9
5 42.4 86.6
17 0.0 90.3
18 8.0 93.6
----------------------------------------------
a Adapted from: Cantor & Scott (1974).
In New Zealand, a close geographical relationship was observed
between the presence of white muscle disease and the incidence of
barren ewes (Table 34). A selenium intervention trial was carried
out on 3 farms where there was a history of white muscle disease
and low lambing percentages (Table 35). The selenium-treated ewes
received 5 mg of selenium, as sodium selenite, at monthly intervals
from one month before tupping until just before lambing; half of
the rams were also given selenium at monthly intervals before and
during tupping (Hartley et al., 1960; Hartley & Grant, 1961).
Selenium-dosed groups on all farms had higher lambing percentages
and fewer barren ewes than the controls. Moreover, white muscle
disease was eliminated in the lambs of the treated ewes. Estrus,
ovulation, fertilization, and early embryonic development all
proceeded normally in selenium-responsive ovine infertility until
between 3 and 4 weeks after conception (at about the time of
implantation) when the embryos perished for unknown reasons
(Hartley, 1963). The actual cause of selenium-responsive
infertility is also unknown and the geographical distribution of
the disease is not always the same as that of other selenium
deficiency diseases, such as white muscle disease or selenium-
responsive unthriftiness (Gardiner et al., 1962). This suggests
either that selenium-responsive infertility occurs only where
intake of selenium is particularly low or that some other factors
must be present that exacerbate the effects of low selenium intake.
For example, mercury has a strong antagonism with selenium (section
7.4.1), and Parizek et al. (1971) showed that inorganic mercury
could block the transport of selenium from mother rats to the
fetuses.
Table 34. Geographical relationship between
congenital white muscle disease and incidence
of barren ewesa
-----------------------------------------------
Congenital Number Incidence of barren ewes
white muscle of up to 11 - 30% over
disease farms 10% 30%
-----------------------------------------------
Present 21 3 8 10
Absent 16 12 4 0
-----------------------------------------------
a Adapted from: Hartley et al. (1960).
Table 35. Effects of selenium (Se) on the reproductive
performance of sheep and white muscle disease in lambsa
-------------------------------------------------------
White muscle
Farm Lambing Barren ewes disease incidence
control Se control Se control Se
-------------------------------------------------------
(%) (%) (%)
C 60.6 94.9 31.1 7.5 37.5 0
D 55.0 86.0 24.3 11.5 22.2 0
E 70.5 93.6 26.1 6.4 12.0 0
-------------------------------------------------------
a Adapted from: Hartley et al. (1960).
7.2.7. Factors influencing deficiency
7.2.7.1. Form of selenium
Schwarz (1961) pointed out that only 0.007 mg naturally-
occurring selenium/kg diet in extracts from pork kidney ("Factor 3
Selenium") was needed to afford 50% protection against dietary
necrotic liver degeneration in rats, whereas 0.020 - 0.030 mg/kg
was required when the selenium was in the form of selenite,
selenate, selenocystine, or selenomethionine. Elemental selenium
was essentially inactive. Many organic derivatives were
synthesized in an attempt to develop selenium compounds that were
not highly toxic but still retained considerable biological potency
against deficiency diseases (Schwarz et al., 1972). Simple amino
acid derivatives of monoseleno diacetic acid appeared promising in
this regard and the hope was expressed that some of these compounds
might make it possible to use selenium in the prevention and cure
of disease (Schwarz, 1976).
Cantor et al. (1975a) studied the ability of selenium in
various poultry feed ingredients to prevent exudative diathesis, a
selenium deficiency disease, in chickens. In general, the selenium
in plant products was more readily available than that in animal
products, but the latter consisted of highly-processed fish or
poultry meals.
Selenium in the form of selenomethionine was less effective
than selenium as sodium selenite for preventing exudative diathesis
in chicks (Noguchi et al., 1973), but the reverse was true for the
prevention of pancreatic fibrosis (Cantor et al., 1975b). In fact,
selenomethionine was four times as effective as either selenite or
selenocystine for this purpose; this phenomenon may have been
related to the peculiar affinity of this compound for the pancreas.
7.2.7.2. Vitamin E
Vitamin E decreases the level of dietary selenium needed to
prevent deficiency diseases in several species of animals. For
example, Scott & Thompson (1971) demonstrated that chicks receiving
100 mg vitamin E/kg diet needed only 0.01 mg selenium/kg diet to
prevent exudative diathesis, whereas chicks not receiving any added
vitamin E needed 0.05 mg/kg. Similarly, Scott et al. (1967) showed
that selenium at 0.18 mg/kg protected against gizzard myopathy in
turkey poults fed diets containing vitamin E, but that 0.28 mg/kg
was needed to protect poults fed diets not supplemented with
vitamin E. Hakkarainen et al. (1978) found that 0.135 mg
selenium/kg was insufficient to prevent deficiency signs in swine
fed a diet containing 1.5 mg vitamin E/kg but was adequate in swine
fed 5.0 mg vitamin E/kg. Increasing the level of dietary vitamin E
decreased the severity of nutritional muscular dystrophy in lambs
fed diets containing selenium at 0.1 mg/kg (Ewan et al., 1968). In
lambs not receiving any supplementary dietary selenium, lambs
receiving low levels of vitamin E died of dystrophy before their
hepatic-selenium levels were depleted to 0.21 mg/kg, the level
considered by Allaway et al. (1966) to be the critical
concentration of selenium in liver for the development of
nutritional muscular dystrophy (section 7.2.3). With the highest
level of vitamin E supplementation, the liver-selenium values were
lower than the critical level suggested by Allaway et al. (1966)
and yet the lambs still survived. These results suggest that the
tissue-selenium level is not the only factor involved, but that the
levels of both tocopherol and selenium determine the appearance of
nutritional muscular dystrophy. The effects of vitamin E in
reducing selenium requirements are readily explainable on the basis
of the close biochemical relationship between these two nutrients
discussed in section 7.2.4.1.
7.2.7.3. Heavy metals and other minerals
Since selenium protects against the toxicity of several heavy
metals (section 7.4), many scientists have examined the effect of
heavy metals on the induction of selenium deficiency. Silver
accelerated the development of liver necrosis in rats (Whanger,
1976) and increased the severity of selenium-vitamin E deficiency
in swine (Van Vleet, 1976; McDowell et al., 1978), presumably by
decreasing the activity of glutathione peroxidase. Inorganic
mercury also decreased glutathione peroxidase activity in the rat
but did not have any effect on the rate of development of liver
necrosis (Whanger, 1976). However, methylmercury accentuated the
development of selenium deficiency in pigs fed a low-selenium diet
(Froseth et al., 1974). In spite of the ability of arsenic to
enhance the biliary excretion of toxic levels of selenium (section
7.1.6.3), several attempts to induce selenium deficiency by feeding
arsenic have been unsuccessful (Levander, 1977; McDowell et al.,
1978). On the other hand, drenching pregnant and lactating ewes
with potassium cyanide, which partially counteracts selenium
toxicity in rats (section 7.1.6.2), increased the incidence of
nutritional myopathy in their lambs (Rudert & Lewis, 1978). It has
been suggested that the use of sulfate fertilizers may decrease
selenium concentrations in forage plants (Pratley & McFarlane,
1974; Westermann & Robbins, 1974), and that this could lead to an
increased incidence of selenium deficiency in farm animals
(Schubert et al., 1961). Dietary sulfate had no effect on the
incidence of white muscle disease but increased the number of lambs
with degenerative lesions of the heart (Whanger et al., 1969).
Neither cadmium nor tellurium had any effect on white muscle
disease in lambs (Whanger et al., 1976b) or vitamin E-selenium
deficiency in swine (McDowell et al., 1978).
7.2.7.4. Xenobiotics
The results of early work by Hove (1948) suggested that vitamin
E could protect rats fed a low-protein diet from death due to
carbon tetrachloride (CCl4) poisoning. The establishment of a
nutritional relationship between selenium and vitamin E stimulated
Seward et al. (1966) to study deficiencies of these nutrients in
relation to poisoning (Table 36). Mortality was higher in the
group deficient in both nutrients than in controls, but selenium
deficiency alone did not increase mortality. Other workers
(Hafeman & Hoekstra, 1977a; Burk & Lane, 1979) have confirmed this
result. The increased mortality due to CCl4 in doubly-deficient
animals could have been due simply to inhibition of eating.
Fasting these animals overnight can precipitate dietary liver
necrosis leading to death (Hafeman & Hoekstra, 1977b).
Table 36. Protective effect of selenium and vitamin E
against carbon tetrachloride toxicity in rats fed a
10% soybean-protein dieta
-----------------------------------------------------
Dietary supplement Weight gain Survival 48 h after
(g/8 weeks) CCl4 injectionb
-----------------------------------------------------
None 62 ± 5 3/10
Vitamin Ec 77 ± 3 9/10
Seleniumd 79 ± 6 8/10
Vitamin E and 80 ± 5 10/10
selenium
-----------------------------------------------------
a Adapted from: Seward et al. (1966).
b All rats injected intraperitoneally with a single
dose at 2 ml/kg body weight.
c Added as a mixture of equal quantities of dl-alpha-
tocopherol and dl-alpha-tocopheryl acetate at 200
mg/kg diet.
d Added at 0.1 mg selenium/kg diet, as sodium selenite.
Paraquat is another xenobiotic that is thought to exert its
toxic effect via increased lipid peroxidation (Bus et al., 1974).
Selenium deficiency worsens lung injury in rats caused by paraquat
(Omaye et al., 1978) and leads to liver injury that would not
otherwise occur in mice exposed to this compound (Cagen & Gibson,
1977). Burk et al. (1980b) showed that selenium-deficient rats
were much more susceptible to paraquat or diquat poisoning than
controls (Table 37). The deficient rats also produced more ethane
and had a higher serum-glutamic-pyruvic transaminase (SGPT)
activity, when treated with paraquat or diquat, than the controls.
The same workers found that injected selenium protected deficient
rats against diquat toxicity before appreciable amounts of
glutathione peroxidase activity appeared in the tissues. They
suggested the existence of a selenium-dependent factor, apart from
glutathione peroxidase which protects against lipid peroxidation.
Combs et al. (1975) noted a similarity between the oedema
produced in chicks fed a practical diet containing polychlorinated
biphenyls (PCBs) and that observed in chicks fed a diet deficient
in selenium and vitamin E (exudative diathesis). When selenium-
depleted hens were fed a diet not containing PCBs, selenite at
0.15 mg selenium/kg diet was sufficient to prevent exudative
diathesis in the chicks, but was not sufficient to prevent the
disease when the hens were fed a diet containing 10 mg PCBs/kg
(Table 38). In another study, addition of 50 mg PCBs/kg diet
increased the incidence of exudative diathesis in vitamin E-
deficient chicks receiving selenite at 0.04 mg selenium/kg diet
from less than 60% in the controls to almost 90% in the PCBs group,
14 days after hatching (Combs & Scott, 1975). Dietary PCBs
depressed plasma-glutathione peroxidase activity and increased the
amount of dietary selenium required to protect liver microsomal
fractions from peroxidation in vitro. On this basis, the authors
concluded that dietary PCBs potentiate vitamin E-selenium
deficiency in chicks by interfering with the biological utilization
of dietary selenium, though some interference with the absorption
and retention of vitamin E was also seen.
Table 37. Effect of selenium deficiency in rats treated with paraquat or
diquata,b
-------------------------------------------------------------------------
Diet Agent Dose Ethane SGPT Survival
group given produced activity time
-------------------------------------------------------------------------
(µmol/kg) (pmol/100 g/h) (mU/ml) (min)
Selenium- paraquat 78 470 ± 93 212 ± 87 297 ± 74
deficient
Control paraquat 78 18 ± 13 32 ± 26 survived 24 h
Control paraquat 390 92 ± 5 45 ± 34 106 ± 30
Selenium- diquat 19.5 1940 ± 420 3490 ± 1940 150 ± 37
deficient
Control diquat 230 56 ± 50 41 ± 5 80 ± 12
-------------------------------------------------------------------------
a Adapted from: Burk et al. (1980b).
b Values are mean ± SD of 4 or 5 values.
Burk & Lane (1979) pointed out the complexity of nutritional
toxicology experimentation involving selenium, vitamin E, and
various drugs and other chemicals. After studying the effects of
numerous xenobiotics on rats that were deficient in either vitamin
E or selenium, they concluded that in vivo lipid peroxidation, as
assessed by ethane production, was not necessarily correlated with
liver necrosis. Moreover, it was concluded that selenium
deficiency was not just a lack of glutathione peroxidase activity
in the tissues, since this condition also elevates hepatic
glutathione S-transferase activity. Thus, selenium deficiency may
actually protect against the hepatotoxicity of certain compounds,
(e.g., acetaminophen and iodipamide) by increasing their
conjugation with glutathione.
Table 38. Effect of PCBs fed to hens on the
incidence of exudative diathesis in progeny fed a
sub-optimal level of seleniuma
------------------------------------------------------
Additions to diets of hensb Exudative Weight gain
-------------------------- diathesis of chicks
PCBsd Seleniume in chicksc (g/2 weeks)
(mg/kg) (mg/kg) (%)
------------------------------------------------------
0 0 35 89
0 0.15 0 102
10 0 30 82
10 0.15 45 78
------------------------------------------------------
a Adapted from: Combs et al. (1975).
b Single Comb White Leghorn hens, 27 weeks old, that
had been reared on low-selenium, semi-purified diets
supplemented with high levels of vitamin E; hens
were on dietary treatments shown in Table for an
additional 5 weeks.
c Determined at 2 weeks of age. Chicks were fed a
selenium-deficient diet (less than 0.02 mg
selenium/kg) supplemented with 0.06 mg selenium, as
Na2Se03/kg. Diet was essentially free of tocopherols.
d Aroclor(R) 1254.
e Added as Na2Se03.
7.2.7.5. Exercise stress
Ancedotal observations from New Zealand suggested that selenium
might be of value against "tying-up" in thoroughbred racehorses
(Hartley & Grant, 1961), a condition that occurs during training
and is associated with exercise. Characteristics include muscle
stiffness and tenderness and disinclination to move. Young &
Keeler (1962) applied mechanical restraint to one foreleg of lambs
born to ewes fed a diet known to produce nutritional muscular
dystrophy and found that lesions either did not occur or occurred
to a much lesser extent in muscles of the restrained limb than in
muscles of the unrestrained limb. Brady et al. (1978) observed
increased levels of malondialdehyde, a product of lipid
peroxidation, in the erythrocytes of 6 horses, immediately after
exercise, but were unable to reproduce this phenomenon in a second
exercise trial with 8 horses. Sodium selenite, given for 4 weeks
in the trace mineral salt at a level calculated to provide 0.15 mg
supplementary selenium/kg diet, did not have any effect on the
elevated plasma-enzyme activities observed in exercised horses.
Furthermore, it did not have any effect on plasma-selenium levels
or erythrocyte-glutathione peroxidase activity. Harthoorn & Young
(1974) commented that the pathological picture in wild animals that
have died following mechanical capture ("capture myopathy" or
"overstraining syndrome") is indistinguishable from white muscle
disease seen in cattle suffering from vitamin E deficiency, but
pointed out that prophylactic and subsequent symptomatic treatment
with vitamin E and selenium-containing preparations did not have
any beneficial effects on captured animals. Brady et al. (1979)
expected to induce death in rats that had been fed a selenium and
vitamin E-deficient diet for 4 weeks, after exercising them to
exhaustion by swimming, but had no success, in spite of markedly
depressed tissue-glutathione peroxidase activity, decreased hepatic
stores of fat-soluble antioxidants, and increased levels of
thiobarbituric acid-reacting substances. The authors concluded
that the rat might be an unsuitable model for an exercise stress-
susceptible species.
7.3. Ratio Between Toxic and Sufficient Exposures
The minimum level of dietary selenium that causes overt signs
of chronic selenium toxicity in most species of animals is of the
order of 4 - 5 mg/kg (US NAS/NRC, 1976). The minimum level of
dietary selenium needed to prevent selenium deficiency diseases in
most species is in the range of 0.02 - 0.05 mg/kg (US NAS/NRC,
1971). Therefore, the ratio of toxic to deficient selenium
exposures is about 100. This difference between the harmful and
beneficial levels of selenium is similar to that between the
harmful and beneficial levels of several nutritionally essential
minerals. However, in the case of selenium, this ratio can be
decreased by certain nutritional or environmental factors. For
example, a deficiency in vitamin E decreases the tolerance of
animals to selenium toxicity (section 7.1.6.2) but increases the
nutritional need for the element (section 7.2.7.2). Also,
inorganic mercury increases the toxicity of methylated selenium
metabolites (section 7.4.1) but methylmercury potentiates selenium
deficiency (section 7.2.7.3). Thus, caution may have to be
exercised in some situations to avoid a diminution in the ratio
between harmful and beneficial intakes of selenium.
7.4. Protection Against Heavy Metal Toxicity
7.4.1. Mercury
Parizek & Ostadalova (1967) first showed that selenium could
protect against the toxicity of mercury in short-term acute
toxicity studies. This led to the suggestion that one of the
nutritional roles of selenium might be protection of the organism
against traces of toxic metals that enter the body, even under
normal conditions (Parizek et al., 1971). Nutritional levels of
dietary selenium have been demonstrated to decrease the chronic
toxicity of the highly hazardous methylmercury (Ganther et al.,
1972) and the selenium in tuna may provide a built-in protective
mechanism against the methylmercury in such fish (Ganther & Sunde,
1974). The possible practical significance of the metabolic
interaction of selenium and mercury has been indicated by the
strong correlations observed between the concentrations of mercury
and selenium in the livers of marine mammals under normal
environmental conditions (Koeman et al., 1975) and in human tissues
following exposure to inorganic mercury (Kosta et al., 1975). The
mechanism by which selenium protects against the toxicity of
methylmercury is not known, but the fact that vitamin E and certain
antioxidants also decrease methylmercury toxicity (Welsh & Soares,
1976; Welsh, 1979) has prompted the hypothesis that these compounds
may diminish methylmercury toxicity by counteracting the damaging
effects of the free radicals generated by its breakdown (Ganther,
1978).
Although inorganic selenium salts protect against the toxicity
of inorganic or methylmercury under a wide variety of conditions,
dimethylselenide has a strong synergistic toxic action with
mercuric chloride (Parizek et al., 1971). The mechanism of this
synergism is unknown.
7.4.2. Cadmium
Parenterally administered selenium protects against the
toxicity of injected doses of cadmium in rats, apparently by
diverting the cadmium from low relative molecular mass target
proteins to other proteins of higher relative molecular mass
(Parizek et al., 1971; Chen et al., 1975). However, selenium does
not cause this diversion when given orally, so any mechanism by
which selenium counteracts cadmium toxicity, under environmental
conditions, is yet to be determined (Whanger, 1976). The
cadmium/selenium antagonism may be important for human health in
that selenium prevents the hypertension caused by long-term cadmium
exposure in rats (Perry et al., 1976).
7.4.3. Other heavy metals
Selenium may interact with lead but the interaction appears to
be much weaker than that with mercury or cadmium, and vitamin E
seems to be more important than selenium in determining the effects
of lead poisoning (Levander, 1979). The interaction of selenium
and silver is of theoretical interest since glutathione peroxidase
activity is depressed by silver (Wagner et al., 1975), but this
interaction does not appear to have any practical significance.
Large doses of selenate protect against thallium toxicity in rats
(Rusiecki & Brzezinski, 1966), but this interaction has not been
investigated in any detail.
7.5. Cytotoxicity, Mutagenicity, and Anti-mutagenicity
7.5.1. Cytotoxicity and mutagenicity
Walker & Ting (1967) treated the soil around barley seedlings
with solutions of sodium selenate to determine the effect on
crossing over. Hoagland's solution containing 0, 0.5, 1.6, or
5.0 mg selenium/litre was applied 10 times, at 2-day intervals, to
each of 4 groups of plants, each treatment consisting of the
application of a 250 ml aliquot of the appropriate solution per
pot. On the day after treatment, the pots were irrigated with
distilled water to prevent lethal accumulation of selenate.
Genetic data indicated a significant effect of the selenate on the
rate of crossing over, consisting of a progressive decline with
increasing concentration.
Walker & Bradley (1969) reared larvae of Drosophila
melanogaster on a semi-defined culture medium containing
selenocystine at concentrations of 0, 2, 10, and 50 µM. The
selenocystine had different effects on crossing over on various
chromosomal segments and the authors concluded that these effects
were mediated through a structural chromosomal protein with a role
in the exchange process.
In studies by Nakamuro et al. (1976), various inorganic
compounds of selenium exhibited clastogenic effects when added in
vitro to cultures of human leukocytes (Table 39). Quadrivalent
selenium compounds were generally more efficient than hexavalent
compounds in inducing chromosome aberrations. Similarly, selenite
was more potent than selenate in causing DNA damage to Bacillus
subtilis that was subject to recombination repair. The same
authors also found that 1.6 x 10-3 mol H2Se03 or Se02 caused about
a 30% inactivation of the tryptophan marker of B. subtilis
transforming DNA, whereas similar concentrations of Na2Se04,
H2Se04, or Na2Se03 were without any significant effect.
Table 39. Chromosome aberrations in leukocytes exposed to selenium compoundsa
-------------------------------------------------------------------------------------
Compound Dose Occurrence of particular chromosome aberrationsb Total
chromatid chromatid iso-chromatid chromatid aberrations
gaps breaks breaks exchanges
-------------------------------------------------------------------------------------
(x 10-5 mol) aberrations per 100 cells
Na2Se04 53 8.4 0.9 0.9 0.9 11.2
26 9.3 0.8 0.8 0.8 11.9
13 11.2 0 0 0 11.2
H2Se04 53 21.0 14.5 3.2 1.6 40.3
26 11.9 0 0 0 11.9
13 9.2 0.8 0 0 10.0
Na2Se03 26 54.0 14.5 1.6 1.6 71.8
13 41.7 8.3 0.9 0.9 51.9
Table 39 (contd.)
-------------------------------------------------------------------------------------
Compound Dose Occurrence of particular chromosome aberrationsb Total
chromatid chromatid iso-chromatid chromatid aberrations
gaps breaks breaks exchanges
-------------------------------------------------------------------------------------
(x 10-5 mol) aberrations per 100 cells
H2Se03 26 113.2 41.2 22.1 5.8 182.4
13 92.3 42.3 12.8 2.6 150.0
6.5 15.2 6.3 5.4 0 26.8
Se02 26 52.1 22.5 7.0 1.4 83.1
13 24.2 2.5 3.3 0 30.0
6.5 15.5 3.6 4.8 0 20.9
None - 7.6 0 0 0 7.6
-------------------------------------------------------------------------------------
a Adapted from: Nakamuro et al. (1976).
b Number of cells examined varied from 62 - 250.
Lo et al. (1978) showed that selenite caused chromosome
aberrations and mitotic inhibition in cultured human fibroblasts
and that incubation with a mouse liver S9 microsomal fraction
increased this capacity of selenite about 5-fold at equimolar
concentrations (Table 40). Selenite also induced DNA fragmentation
and repair synthesis and decreased the clone-forming capacity of
the fibroblasts. Incubation with the S9 fraction increased the
last 2 effects of selenite but slightly decreased the extent of DNA
fragmentation. The response to selenite was similar in cultured
fibroblasts from normal persons and from DNA repair-deficient
xeroderma pigmentosum patients. The ability of selenate to trigger
DNA repair, inhibit the mitotic rate, or cause a lethal effect was
about the same as that of selenite over concentrations varying from
7 x 10-6 to 2 x 10-3 mol. However, when both compounds were
incubated with the S9 fraction, only the activity of the selenite
was potentiated (Table 41).
Table 40. Effect of 1.5 h selenite treatement on chromosome
aberrations and mitotic rate of human fibroblastsa
-------------------------------------------------------------
Sodium Metaphase plates with Mitotic rate
selenite chromosome aberrations without S-9 with S-9
for 1.5 h without S-9 with S-9 (%) (%)
(mol) (%) (%)
-------------------------------------------------------------
Control 1.2 0.8 9.0 8.9
2 x 10-5 1.3 6.9 8.6 9.8
4 x 10-5 1.6 7.4 8.9 9.6
8 x 10-5 3.0 14.3 9.3 9.4
1 x 10-4 3.2 - 8.2 0.1
2 x 10-4 4.7 - 9.5 0.0
4 x 10-4 6.1 - 6.4 0.0
8 x 10-4 14.5 - 6.9 0.0
1 x 10-3 14.2 - 7.6 0.0
3 x 10-3 18.1 - 0.7 0.0
-------------------------------------------------------------
a Adapted from: Lo et al. (1978).
Another example of a tissue fraction influencing the
cytotoxicity of selenite is provided by the work of Ray &
associates (Ray & Altenburg, 1978; Ray et al., 1978). These
workers first showed that sodium selenite tripled the frequency of
sister-chromatid exchanges (SCEs) in lymphocytes cultured with
whole human blood (Table 42). At the concentrations of selenite
used, the selenite could only be present during the final 19 h of
the 96-h culture incubation, otherwise cell death occurred, as
measured by mitotic indices. Later, they found that 7.90 x 10-6
mol Na2Se03 increased SCE frequency, only when the lymphocytes were
cultured in the presence of whole blood or separated red cells.
Lysis of the erythrocytes indicated that the lysate rather than the
red cell ghosts contained the activity necessary for selenite to
raise the lymphocyte SCE frequency. The authors suggested that
exposure of selenite to the lysate possibly resulted in a chemical
modification of the selenite that enabled it to induce SCEs. This
chemical modification of the selenite probably involves reduction
to glutathione selenopersulfide derivatives, since Ray (1984) found
that reduced glutathione (10-3 - 10-4 mol) could substitute for
erythrocytes in activating sodium selenite to its SCE-inducing
form. Whiting et al. (1980) showed that glutathione markedly
stimulated unscheduled DNA synthesis in cultured human skin
fibroblasts and chromosome aberrations in Chinese hamster ovary
cells caused by a variety of inorganic selenium compounds. These
research workers suggested that reduced selenium compounds are the
ultimate mutagens and that their formation depends on reduction by
glutathione.
Table 41. Effect of selenite versus selenate on human
fibroblasts incubated with, or without, mouse liver S9
microsomal fractiona
--------------------------------------------------------------
Treatment DNA repair Mitotic rate Clone forming
(grains/nucleus) (%) capacity (%)
--------------------------------------------------------------
Seleniteb 10.7 5.2 36
Selenite + S9 115.6 0.0 0
Selenate 7.0 4.8 42
Selenate + S9 9.1 3.9 32
--------------------------------------------------------------
a Adapted from: Lo et al. (1978).
b The concentrations used for the estimation of DNA repair,
mitotic rate, and surviving colonies were 8 x 10-4, 2 x 10-7,
and 2 x 10-4 mol, respectively. Selenite and selenate were
applied at equimolar concentrations for 1.5 h.
Table 42. Effect of sodium selenite on
sister-chromatid exchange frequency and
mitotic index in lymphocytes cultured
with whole human blooda
----------------------------------------
Na2Se03 Sister chromatid Average
added exchanges per mitotic
(mol) cellb index
----------------------------------------
Control 6.74 0.03
1.58 x 10-6 7.17 0.03
3.95 x 10-6 7.49 0.03
7.90 x 10-6 20.71 0.02
1.19 x 10-5 21.51 0.03
----------------------------------------
a Adapted from: Ray et al. (1978).
b Average of 4 studies; a total of 100
cells was scored for each Na2Se03
concentration except 75 at the highest
concentration.
In a preliminary note, Lofroth & Ames (1978) stated that
selenite did not give any indication of mutagenicity (<< 0.01
revertants/n mol) whereas selenate gave rise to base-pair
substitutions with about 0.03 revertants/n mol in a Salmonella
plate incorporation test using different histidine-requiring
strains that are reverted to prototrophy by different mechanisms.
On the other hand, Noda et al. (1979) found that both selenite and
selenate were weak mutagens in a similar test, since they gave
rise to base-pair substitution (0.2 and 0.05 revertants/n mol,
respectively). Ray & Altenburg (1980) showed that sodium selenide
and sodium selenite were more active than sodium selenate in their
ability to induce SCEs in human whole-blood cultures. Sirianni &
Huang (1983) found that sodium selenite was the most potent inducer
of SCEs in Chinese hamster V79 cells when S9 mixture was present,
whereas sodium selenide was the most effective inducer in the
absence of S9. For sodium selenate, there was no increase in SCE
rate compared with controls, regardless of whether S9 was present
or absent. At present, it is difficult to provide a biochemical
explanation for the contrasting cytogenic effects exhibited by the
various selenium compounds, when studied in different in vitro
test systems.
Norppa et al. (1980) investigated the chromosomal effects
of sodium selenite when given in vivo. They found that
supplementation of human neuronal ceroid lipofuscinosis patients
with tablets furnishing sodium selenite at a dose of 0.025 mg
selenium/kg body weight for 1 - 13.5 months did not have any
detectable effects on chromosomal aberrations or SCEs in peripheral
blood lymphocytes. Similarly, mice treated with a single dose of
sodium selenite at 0.8 mg selenium/kg body weight did not show any
rise in chromosomal abnormalities in bone-marrow cells or primary
spermatocytes after 24 h. However, there was an increased number
of SCEs and chromosomal aberrations in the bone-marrow cells of
Chinese hamsters, 17 - 19 h after injection with sodium selenite
at a dose of 0.3 - 6.0 mg selenium/kg body weight. It was thought
that the manifestations of chromosomal damage observed in the
second study may have been related to the general toxicity of
selenium at the high doses used.
7.5.2. Anti-mutagenicity
Shamberger et al. (1973a) tested the ability of sodium selenite
and various antioxidants to decrease the chromosomal breakage
induced by 7,12-dimethylbenz[alpha]anthracene (DMBA) in human blood
leukocyte cultures. They found that 0.20 µmol sodium selenite
reduced the breaks caused by 1.6 µmol DMBA by 42% (Table 43). This
concentration of selenite was used because 1 µmol almost completely
inhibited the growth of the cultures. In later studies, Shamberger
et al. (1979) found that sodium selenite at concentrations of
0.67 µmol or less was effective in reducing the mutagenic effects
of malonaldehyde and beta-propiolactone in certain tester strains
of Salmonella typhimurium. Rosin & Stich (1979) showed that sodium
selenite at concentrations of 3 x 10-4 and 1 x 10-3 mol caused
a 50% inhibition of mutagenesis in S. typhimurium induced by N-
methyl- N'-nitro- N-nitrosoguanidine or N-acetoxy-2-acetylamino-
fluorene, respectively. However, 10-2 mol sodium selenite was
toxic to the bacteria in the presence of either carcinogen. Sodium
selenite has also been shown to suppress spontaneous mutagenesis in
yeast cultures (Rosin, 1981).
Table 43. Effects of sodium selenite and various antioxidants on
chromosomal breakage induced by 7,12-dimethylbenz-(alpha)anthracene
(DMBA) in human blood leukocyte culturesa
-----------------------------------------------------------------------
DMBA Antioxidant Number Cells with Breaks Reduction
added added of breaks minus in
(µmol) cells number % control breaks
(%) (%)
-----------------------------------------------------------------------
0 none 211 23 10.9 - -
1.6 none 290 82 28.3 17.4 -
1.6 0.20 µmol Na2Se03 171 37 21.6 10.1 42.0
1.6 10 µmol dl-alpha 156 28 17.9 6.4 63.2
tocopherol
1.6 10 µmol ascorbic acid 127 30 23.6 11.9 31.7
1.6 0.21 µmol butylated 157 29 18.4 6.3 63.8
hydroxytoluene
-----------------------------------------------------------------------
a Adapted from: Shamberger et al. (1973a).
Jacobs et al. (1977b) examined the effects of sodium selenite
on the mutagenicity of 2-acetylaminofluorene (AAF), N-hydroxy-2-
acetyl-aminofluorene (N-OH-AAF), and N-hydroxy-aminofluorene
(N-OH-AF) in the S. typhimurium TA 1538 bacterial tester strain.
Metabolism of AAF and N-OH-AAF to the active mutagen, N-OH-AF was
accomplished by rat liver extracts. Sodium selenite at
concentrations ranging from 0.1 to 40 mmol decreased the
mutagenicity of these compounds (Table 44). However, in the case
of AAF, the authors noted that further decreases in mutagenicity
induced by concentrations of selenium higher than 40 mmol were not
tested, partly because of the formation of a red selenium compound
of low solubility, which can be presumed to have been elemental
selenium.
Ray et al. (1978) determined the frequencies of sister
chromatid exchange (SCE) in lymphocytes resulting from the
simultaneous exposure of whole-blood cultures to different
concentrations of sodium selenite plus 10-4 mol methyl
methanesulfonate (MMS) or 2.7 x 10-5 mol N-hydroxy-2-acetyl-
aminofluorene (N-OH-AAF). The SCE frequency observed as a result
of coexposure to 2 compounds was less than that expected because of
an additive SCE frequency response to each individual compound
(Table 45). In their interpretive analysis of the data, the
authors concluded that the most consistent explanation for the
results was that sodium selenite decreased the SCE-inducing
abilities of MMS and N-OH-AAF.
Table 44. Effect of sodium selenite on the mutagenicity of
2-acetylaminofluorene and its derviatives to Salmonella
typhimuriuma
-------------------------------------------------------------------
Compound Selenium His+ revertants Mutagenic activity
added per plate (± SD) (% of appropriate
(mmol) control)
-------------------------------------------------------------------
4.5 mmol AAF - 1768 ± 149 100
4 1411 ± 41 80
10 1336 ± 69 76
40 1148 ± 71 65
0.45 mmol N-OH-AAF - 2353 ± 12 100
0.4 1891 ± 210 80
4 1598 ± 71 68
10 1247 ± 53 53
40 655 ± 43 28
0.065 mmol N-OH-AF - 1628 ± 41 100
0.1 1280 ± 88 79
10 1233 ± 31 76
20 999 ± 26 61
-------------------------------------------------------------------
a Adapted from: Jacobs et al. (1977b).
Table 45. Effect of methyl methanesulfonate (MMS) or N-hydroxy-2-
acetylaminofluorene (N-OH-AAF), with or without different concentrations of
sodium selenite, on sister chromatid exchange (SCE) frequencies in whole-
blood cultures of lymphocytesa
----------------------------------------------------------------------------
Compound Na2Se03 Total Observed Expected Observed/
(mol) cells SCE/cell SCE/cellb expected
scored (%)
----------------------------------------------------------------------------
None none 100 6.74 ± 0.30 - -
10-4 mol MMS none 100 30.17 ± 0.75 - -
1.58 x 10-6 75 30.60 ± 0.74 30.60 (0.43) 100
3.95 x 10-6 75 30.13 ± 0.96 30.92 (0.75) 97
7.90 x 10-6 75 33.15 ± 1.15 44.14 (13.97) 75
1.19 x 10-5 75 31.60 ± 1.17 44.94 (14.77) 70
None none 125 7.65 ± 0.27 - -
2.7 x 10-5 mol none 125 13.61 ± 0.43 - -
N-OH-AAF 1.58 x 10-6 100 13.79 ± 0.42 13.61 (0.00) 100
7.90 x 10-6 65 22.66 ± 1.10 27.15 (13.54) 83
1.19 x 10-5 25 26.16 ± 1.86 29.24 (15.63) 89
----------------------------------------------------------------------------
a Adapted from: Ray et a1. (1978).
b The expected SCE/cell was determined by adding the observed separate
contributions to SCE frequency due to either MMS or N-OH-AAF to that due
to different concentrations of Na2Se03, as determined in a previous study
(shown in parentheses (see also Table 42)).
Martin et al. (1981) found that sodium selenite could
protect against the mutagenic effects of acridine orange and
7,12-dimethylbenz[alpha]anthracene (DMBA) in the Ames Salmonella/
microsome mutagenicity test. However, Chatterjee & Banerjee (1982)
showed that the influence of sodium selenite on the transformation
of mouse mammary cells, induced by DMBA added in organ culture, was
markedly affected by the level of selenium used. For example,
sodium selenite at concentrations of 10-7 - 10-8 mol increased the
transformation frequency of the cells within the glands. At 10-5
mol, sodium selenite caused an 18 and 84% inhibition of the
frequency of transformed cells at the initiation and promotional
stages, respectively. At 10-4 mol, sodium selenite was toxic to
the cells in vitro. Sodium selenite inhibited the metabolism and
mutagenicity of benzo (a)pyrene to S. typhimurium strain TA 100
in rat (Teel & Kain, 1984) and hamster (Teel, 1984) liver S9
activation systems, but had no inhibitory effect on the
mutagenicity of 1,2-dimethylhydrazine or azoxymethane for S.
typhimurium G46 in the host-mediated assay (Moriya et al., 1979).
On the other hand, sodium selenite at 3.95 x 10-9 - 1.98 x 10-8 mol
protected against chromosomal damage in cultured human lymphocytes
caused by 2.3 x 10-6 mol sodium arsenite (Sweins, 1983). However,
this author reported that the cytotoxic concentration of sodium
selenite in his system was very low (somewhere between 1.98 and
3.95 x 10-8 mol). No explanation was offered for the considerable
variations between laboratories with respect to selenium
cytotoxicity.
Several investigators have now examined the anti-mutagenic
effects of dietary selenium in a variety of experimental systems.
For example, Gairola & Chow (1982) fed rats either a low-selenium
diet based on Torula yeast or the same diet supplemented with
sodium selenite at 2 mg selenium/kg for 20 weeks. Dietary selenium
did not have any effect on the metabolic activation potential of
S9 liver enzymes towards benzo( a)pyrene in the Ames Salmonella/
microsome mutagenicity assay. S9 mixtures from selenium-deficient
rats were more active towards 2-aminoanthracene and less active
towards 2-aminofluorene than mixtures from the selenium-
supplemented rats. The authors concluded that further studies were
needed to elucidate the role and nature of in vitro metabolites
causing mutations in the bacteria. In contrast, Schillaci et al.
(1982) did not find any differences in the mutagenic activation of
7-12-dimethylbenz[alpha]anthracene (DMBA) by liver S9 mixtures in
the Ames test with S. typhimurium strain TA 98, prepared from rats
fed Torula yeast-based diets supplemented with sodium selenite at
0.1, 2.5, or 5.0 mg selenium/kg, from weaning for 3 weeks.
However, if the rats were injected with 20, 50, or 100 mg
Aroclor(R) 1254/kg body weight, 5 days before sacrifice, dietary
selenium at 2.5 or 5.0 mg/kg in the form of sodium selenite
markedly decreased the mutagenic activation of DMBA by liver
microsomes.
Lawson & Birt (1983) measured single-strand breaks (SSB)
produced in pancreatic DNA by injecting 20 mg N-nitrosobis(2-
oxopropyl) amine (BOP)/kg body weight subcutaneously into hamsters
that had been fed a Torula yeast-based diet supplemented with
sodium selenite at 0, 0.1, or 5 mg selenium/kg, for 4 weeks
previously. One hour after injection with BOP, there were 2.26 ±
0.47, 2.83 ± 0.43, and 1.74 ± 0.43 SSB per 108 daltons of DNA (mean
± SEM), respectively, in the 3 dietary groups, and the approximate
half-lives of the SSB were 33, 30, and 8 days, respectively. On
this basis, the authors suggested that high levels of dietary
selenium may stimulate the repair of carcinogen-induced DNA damage.
Olsson et al. (1984) used a novel isolated rat liver/cell
culture system to study the effects of selenium deficiency and
selenium supplementation, within the physiological range, on the
anti-mutagenic effects of the element. Rats were first fed a
Torula yeast-based selenium-deficient diet for 5 - 6 weeks with or
without sodium selenite supplementation at 0.2 mg selenium/litre
drinking-water. The rat livers were then connected to an isolated
liver perfusion system. A glass plate with cultured Chinese
hamster V79 cells was introduced into the perfusion system
immediately before the addition of 5 mmol dimethylnitrosamine (DMN)
and then exposed directly to the circulating perfusate. After 2 h,
this glass plate was exchanged for another with fresh V79 cells,
which were exposed during the subsequent 2 h. This procedure was
adopted to avoid a possible toxic effect that might influence
mutation frequency. As in vitro controls, V79 cells were treated
for 2 h in Krebs-Ringer albumin solution with or without DMN. The
induced mutation frequencies in the 2 successively treated cell
populations were summarized to give the value for each liver. It
was found that the mutagenicity of DMN in Chinese hamster V79
cells, after metabolic activation by the isolated perfused rat
liver, was approximately doubled when livers from selenium-
deficient rats were used compared with livers from rats given the
supplementary selenium. The authors were unable to provide a
precise biochemical explanation for the mechanism by which selenium
deficiency increased the mutagenicity of DMN, but they noted that
microsomal N-oxygenation of N,N-dimethylaniline (DMA) was
decreased in livers from selenium-deficient rats and suggested that
further investigation of the different enzymes involved in DMA- N-
oxygenation appeared warranted. Another application of this liver
perfusion/cell culture system was demonstrated by the work of Beije
et al. (1984) who showed that bile collected from selenium-
deficient livers perfused with a medium containing 5 mmol 1,1-
dimethylhydrazine was much more mutagenic toward Chinese hamster
V79 cells than bile from livers of rats given supplementary
selenium and perfused in a similar manner. The authors suggested
that the increased biliary excretion of reactive mutagenic
metabolites observed in their selenium-deficient rat liver
perfusion system might furnish a potential explanation for some of
the protective effects of selenium reported against chemically-
induced colon cancer in experimental animals.
7.6. Teratogenicity
Franke & Tully (1935) obtained chicken eggs from 2 farms in
South Dakota that had histories of low hatchability. Hatchability
in the 2 test groups was extremely low, being 4% in one and 12% in
another. Examination of the eggs revealed that about 75% of those
that had failed to hatch on the 21st day contained deformed
embryos. Franke et al. (1936) then showed that injection of
selenium salts into the air cell of eggs before incubation resulted
in monsters similar to those occurring naturally on affected farms.
Selenium injected as selenite to give a final concentration in the
egg of 0.6 mg selenium/kg was the most effective in this respect,
higher doses causing embryonic death and lower doses producing
fewer monsters (Table 46). When the toxicity of various selenium
compounds for chick embryos was compared, it was found that
selenate was about twice as toxic as selenite (Palmer et al., 1973)
(Table 47). The toxicity of selenomethionine was about the same as
that of selenate but methylseleninic acid was more than twice as
toxic. The toxicity of trimethylselenonium chloride was relatively
low. The most common embryonic deformities observed in this study
were the underdevelopment of the beak and abnormal development of
the feet and legs, especially a fusing or webbing of the 2 outside
toes. Because of the metabolic relationships between selenium and
arsenic or cadmium, Holmberg & Ferm (1969) investigated the
teratogenic potential of these elements, administered separately or
together to golden hamsters (Table 48). Embryonic malformations
were not observed when pregnant hamsters were injected
intravenously with a barely sub-lethal dose of sodium selenite
alone, and the 6% resorption rate observed was stated to be similar
to the normal rate of resorption in this species. Furthermore,
under these conditions, sodium selenite actually gave partial
protection against the teratogenesis induced by sodium arsenate or
cadmium sulfate.
Table 46. Teratogenicity of sodium selenite
injected into hens' eggsa
--------------------------------------------------
Sodium selenite Total Dead Abnormal Normal
(mg selenium/kg) embryos (%) (%) (%)
--------------------------------------------------
0.9 5 60.0 20.0 20.0
0.8 16 12.5 31.0 56.5
0.7 4 50.0 25.0 25.0
0.6 4 50.0 50.0 0
0.5 12 50.0 8.3 41.7
0.1 131 24.4 2.3 73.3
0.02 78 53.8 3.8 42.3
0.01 64 34.3 9.4 56.3
--------------------------------------------------
a Adapted from: Franke et al. (1936).
Table 47. Comparative toxicity of various selenium compounds for chick
embryosa
----------------------------------------------------------------------------
Compound Dose Livability ratio LD50 with 95%
(mg selenium/kg) (live chicks/ confidence limits
fertile eggs) (mg selenium/kg)
----------------------------------------------------------------------------
sodium selenite 0.0 16/18
0.05 18/18
0.1 16/17 0.3b
0.2 17/18
0.4 2/18
0.8 0/18
sodium selenate 0.0 18/18
0.1 12/18
0.2 4/18 0.13
0.4 1/18 (0.086 - 0.17)
0.8 0/18
1.6 0/18
selenomethionine 0.0 17/18
0.05 18/18
0.1 13/18 0.13
0.15 5/18 (0.095-0.15)
0.2 4/18
0.4 0/18
Table 47 (contd.)
----------------------------------------------------------------------------
Compound Dose Livability ratio LD50 with 95%
(mg selenium/kg) (live chicks/ confidence limits
fertile eggs) (mg selenium/kg)
----------------------------------------------------------------------------
methylseleninic 0.0 23/24
acid 0.025 17/18
0.05 9/18 0.052
0.1 2/24 (0.039-0.065)
0.2 0/24
0.4 0/24
trimethylselenonium 0.0 18/18
chloride 6.0 17/18
12.0 13/18 15.7
18.0 5/17 (12.7 - 19.0)
24.0 5/18
30.0 3/18
----------------------------------------------------------------------------
a Adapted from: Palmer et al. (1973).
b An estimate since the data did not allow calculation by the computer
programme.
7.7. Carcinogenicity and Anti-Carcinogenicity
7.7.1. Selenium as a possible carcinogen
Five investigations have been reported in the literature on the
alleged carcinogenicity of selenium for laboratory animals. Nelson
et al. (1943) fed groups of 18 female rats of an inbred Osborne-
Mendel strain a 12% protein diet consisting of 49% corn, 44% wheat,
3% yeast, and 1% each of cod liver oil, calcium carbonate, sodium
chloride and dried whole liver. The diet was supplemented with 0,
5, 7, or 10 mg selenium/kg, as seleniferous corn or wheat, or 10 mg
selenium/kg, as a mixed inorganic selenide containing ammonium
potassium selenide and ammonium potassium sulfide. Although
cirrhosis was frequently observed after 3 months of selenium
exposure, no tumours or advanced adenomatoid hyperplasia were seen
in any of the 73 selenium-exposed rats that died or were sacrificed
before 18 months. Of the 53 selenium-exposed rats that survived
18 - 24 months, 43 had cirrhosis and 11 developed liver cell
adenoma or low-grade carcinoma without metastasis in cirrhotic
livers. There was no relationship between the extent of cirrhosis
and tumour incidence except that there were no tumours in any of
the 18- to 24-month-old rats that did not have cirrhosis. The
incidence of spontaneous adenoma and low-grade carcinoma of the
liver in the unexposed rats was low and the incidence of
spontaneous hepatic tumours in the rat colony was less than 1% in
18- to 24-month-old rats.
Table 48. Effect of selenium, arsenic, and cadmium on embryonic death and
malformations in the golden hamstera
------------------------------------------------------------------------------------
Treatment Dose Total Number of Number of Malformed Malformed
(mg/kg) number of embryos embryos (%) or resorbed
embryos resorbed malformed (%)
------------------------------------------------------------------------------------
sodium selenite 2 86 5 0 0 6
sodium arsenate 20 177 62 86 49 84
sodium arsenate 20 144 28 28 19 39
+ sodium selenite 2
cadmium sulfate 2 115 24 59 51 72
cadmium sulfate 2 82 2 3 4 6
+ sodium selenite 2
------------------------------------------------------------------------------------
a Adapted from: Holmberg & Ferm (1969).
The results of some of the initial studies of Volgarev &
Tscherkes (1967) seemed to indicate an increased incidence of
tumours in rats fed a low-protein diet supplemented with sodium
selenate at 4.3 mg selenium/kg, but subsequent tests carried out by
these authors failed to confirm these results. Moreover, their
work suffered from the fact that no control groups, consuming diets
without additional selenium, were included in any of their trials.
Schroeder & Mitchener (1971b) reported an increased incidence of
tumours in rats given sodium selenate at 2 mg selenium/litre in the
drinking-water for the first year of life followed by 3 mg/litre
until death. In a previous evaluation of this work (US NAS/NRC,
1976), it was observed that the selenate-treated rats survived
longer than the untreated rats and this could have contributed to
the increased tumour incidence observed in the rats receiving
selenate. Also, as the organs and tissues did not appear to have
been systematically searched, the type and incidence of
histological lesions could not be known with certainty.
Schroeder & Mitchener (1972) carried out 2 studies in which
mice were given selenium in the drinking-water. In both studies,
the mice were fed a diet composed of 60% whole rye flour, 30% dried
skim milk, 9% corn oil, and 1% iodized sodium chloride, to which
were added vitamins and iron. The diet was calculated to contain
24% protein, 65% carbohydrate, and 11% fat (dry weight). The mice
were given doubly deionized water for drinking, which originally
came from a forest spring. Certain essential trace metals were
added to the drinking-water, as soluble salts, in the following
concentrations (mg/litre): zinc, 50; manganese, 10; copper, 5;
cobalt, 1; and molybdenum 1. All mice received these trace
elements in their drinking-water. In addition, chromium was added
to the drinking-water at a level of 1 or 5 mg/litre in the first
and second studies, respectively. In the first study, groups of
100 or more Swiss mice of the Charles River CD strain, containing
equal numbers of each sex, were given, at weaning, sodium selenite
at either 0 or 3 mg selenium/litre of the trace element-fortified
drinking-water. This regimen continued over the entire life span
of the mice. The second study was identical to the first, apart
from the level of chromium given in the drinking-water, and the
fact that the selenium was administered in the form of sodium
selenate. Of the 180 control mice autopsied from both studies, 119
were sectioned. Tumours were found in 23 (19%) of those sectioned
and 10 of the tumours (43%) were malignant. The different forms of
selenium given did not influence the incidence of tumours and, of
the 176 selenium-exposed mice autopsied in both studies, 88 were
sectioned. Tumours were found in 13 (15%) of those sectioned but
all tumours were malignant. It was concluded that selenium had
little tumourigenic or carcinogenic activity in mice, though, when
tumours did appear in the selenium-exposed mice, they were all
malignant.
The results of studies of Harr et al. (1967) (section 7.1.2.2)
failed to demonstrate any tumours attributable to selenium, but
most of the rats fed semi-purified diets containing levels of
selenium greater than 2 mg/kg died within 100 days and almost all
were dead before 2 years. Exceptions included 1 rat fed selenate
at 4 mg selenium/kg diet containing 12% casein and 0.3% DL-
methionine, and 27 rats alternating at weekly intervals between a
control ration and a diet containing sodium selenate at either 4 or
8 mg selenium/kg. However, no hepatic tumours were observed, even
in the 71 rats that survived 2 years or longer at continuous
dietary-selenium levels of 0.5 - 2.0 mg/kg.
The above studies indicate that test animals develop neoplastic
lesions, only when they have liver cirrhosis produced by frank
selenium toxicity (i.e., no hepatomas were observed in the absence
of severe hepatotoxic phenomena). For this reason, it was
concluded that selenium was not, by reason of its capacity to
induce liver damage when consumed at high levels, properly
classified as carcinogenic because of its potential association
with a higher rate of liver cancer (Gardner, 1973).
Jacobs & Forst (1981b) did not observe any signs of neoplasia
in groups of 35 female Swiss mice fed a commercial pelleted diet
and given 0, 1, 4, or 8 mg selenium/litre drinking-water, as sodium
selenite, for 50 weeks.
Three studies have shown carcinogenic effects that appear to be
more a specific effect of a particular selenium compound rather
than an effect of selenium itself. Seifter et al. (1946) found
that 8 white rats that had received 0.05% bis-4-acetamino-phenyl-
selenium dihydroxide in their diet for 105 days had multiple
adenomas of the the thyroid glands and adenomatous hyperplasia of
the liver. Innes et al. (1969) fed the maximal tolerated dose of
selenium diethyldithiocarbamate (Ethyl selenac) to a group of 72
mice containing both sexes of two hybrid strains (C57BL/6 x
C3H/Anf)F and (C57BL/g x AKR)F1. The mice were given this
substance by stomach tube at a dose of 10 mg/kg body weight,
starting at 7 days of age. At 4 weeks of age, the chemical was
mixed directly into the diet at a concentration of 26 mg/kg. After
82 weeks, all the surviving mice were sacrificed; of 69 necropsied,
26, 13, and 5.8% had hepatomas, lymphomas, and pulmonary tumours,
respectively. Among 338 negative control mice, most of which were
sacrificed between 78 and 89 weeks, the incidences of the
corresponding tumours were 4.1, 4.1, and 6.2%, respectively.
A report from the US National Cancer Institute (National Cancer
Institute, 1980) suggested that commercial selenium sulfide, an
ingredient in certain anti-dandruff shampoos, was carcinogenic for
rats and mice. Elemental analysis of the test chemical used in
this study indicated that the material was a mixture of selenium
monosulfide and selenium disulfide. The melting point of the test
sample was closer to that reported for the monosulfide than that
reported for the disulfide and the X-ray diffraction pattern was
consistent with patterns reported for the monosulfide. It was
concluded that the selenium in the test substance used in this
bioassay was present primarily as the monosulfide.
In the rat study, groups of 4-week-old male and female Fischer
F344 rats were fed presterilized lab meal and were given untreated
well water ad libitum for 104 - 105 weeks. During the first 103
weeks, 50 rats of each sex were given one of 4 treatments:
untreated control; vehicle control (received volumes of 0.5%
aqueous carboxymethylcellulose equal to those of the test solutions
administered); low-dose group (3 mg selenium sulfide suspended in
0.5% aqueous carboxymethylcellulose/kg body weight, 5 days per
week, given by gavage); or high-dose group (15 mg selenium
sulfide/kg body weight administered by the same route and
schedule). The results showed an increased incidence of primary
liver tumours in both male and female high-dose groups (Table 49).
Neoplastic nodules were usually single, rather well-defined
areas characterized by altered hepatocytes. In most instances, the
hepatocytes were larger than normal, eosinophilic, and occasionally
vacuolated. The normal architecture was altered, mainly resulting
in a solid mass of hepatocytes or a trabecular pattern rather than
the normal hepatic cords. The mass compressed the adjacent
parenchyma around the periphery. Anaplasia and mitoses were
minimal. Hepatocellular carcinomas were usually large multinodular
masses, often encompassing entire liver lobes or even multiple
lobes. The histological appearance of these neoplasms varied from
areas appearing normal to much more anaplastic areas. The
neoplastic hepatocytes varied from small basophilic cells to very
large eosinophilic and occasionally vacuolated cells. Mitoses were
variable. No distant metastases were observed in any of the rats
bearing hepatocellular carcinomas. No other neoplasms were found
that could be related to the administration of the selenium
sulfide.
Table 49. Effect of selenium sulfide, given orally for 103 weeks, on
the incidence of liver tumours in Fischer F344 ratsa
------------------------------------------------------------------------
Initial Selenium Tumour incidence
Test group Sex number sulfide Hepatocellular Neoplastic
of rats dose carcinoma nodules
------------------------------------------------------------------------
(mg/kg)
Untreated control M 50 0 1/48 (2%) 3/48 (6%)
Vehicle controlb M 50 0 0/50 (0%) 1/50 (2%)
Low-dosec M 50 3 0/50 (0%) 0/50 (0%)
High-dosec M 50 15 14/49 (29%) 15/49 (31%)
Untreated control F 50 0 0/50 (0%) 0/50 (0%)
Vehicle controlb F 50 0 0/50 (0%) 1/50 (2%)
Low-dosec F 50 3 0/50 (0%) 0/50 (0%)
High-dosec F 50 15 21/50 (42%) 25/50 (50%)
------------------------------------------------------------------------
a Adapted from: National Cancer Institute (1980).
b Received only vehicle for dosing (0.5% aqueous carboxymethylcellulose)
5 days per week, by gavage.
c Received stated dose of selenium sulfide suspended in 0.5% aqueous
carboxymethylcellulose, 5 days per week, by gavage.
An increased incidence of focal cellular change in the liver
was noted in high-dose male rats but was essentially comparable in
frequency in the remaining treated and control groups of each sex.
A compound-related increase in pigmentation in the lungs was
observed. This was characterized by the accumulation of dark,
slightly granular-appearing pigment in the interstitial areas and
in some peribronchial areas. In most cases, the pigment appeared
to be located within cells, principally macrophages. No evidence
of inflammation, relative to the pigment deposits, was noted. Lung
pigmentation was found in 47/49 (96%) high-dose males, 1/50 (2%)
low-dose males, 45/50 (90%) high-dose females, and 36/50 (72%) low-
dose females, but not in control males or females.
The protocol for the testing of selenium sulfide in B6C3F1 mice
was similar to that used for rats, except that the doses of the
substance under test were increased (Table 50). There was an
increased incidence of primary liver and lung tumours in the high-
dose female mice and a marginal increase in the incidence of these
tumours in high-dose males.
Hepatocellular carcinomas varied from single nodules to
multinodular masses, often encompassing several liver lobes.
Individual hepatocytes varied considerably in morphology from large
eosinophilic cells to small, darkly-staining hepatocytes. In many
cases, there was marked variation in cell type in different parts
of the neoplasm. The number of mitoses varied. The number of
hepatocellular carcinomas metastasizing to the lungs was comparable
in vehicle-control and high-dose males. No metastases were
observed in the lung in female mice. Alveolar/bronchiolar adenomas
were usually small solitary lesions located in the subpleural area
or immediately adjacent to a bronchiole. The cells involved were
cuboidal to tall columnar and tended to be situated perpendicular
to the basement membrane in a single layer. These cells were
arranged in complex papillary projections forming discrete nodules
and compressing adjacent alveolar walls. Mitoses were rare.
Alveolar/bronchiolar carcinomas, however, were less discrete
lesions and tended to be larger and occasionally multiple,
consisting of a confluence of two or more nodules. The individual
cells tended to be less rigidly arranged along basement membranes
and were often piled up in layers or arranged in solid sheets
without a papillary pattern. The cells often showed increased
basophilia and a moderate mitotic index. Evidence of invasion into
adjacent vessels, or extension into bronchioles and adjacent lung
parenchyma was frequently present.
Other neoplasms that occurred in the mice were similar in
number and kind to those that usually occur in aged B6C3F1 control
mice and could not be related to the long-term administration of
selenium sulfide.
In a comparison study, the effect of the dermal application of
selenium sulfide was examined. No increased incidence of neoplasms
was observed that could be attributed to selenium sulfide.
7.7.2. Selenium as a possible anti-carcinogen
High levels of dietary selenium have been shown to protect
laboratory animals against chemical carcinogenesis, under a wide
variety of conditions. Clayton & Baumann (1949) fed 2 groups of 15
young adult rats, weighing approximately 200 g, a basal diet
consisting of extracted casein, 12 parts; salts, 4 parts; corn oil,
5 parts; and glucose monohydrate (Cerelose) to 100 parts. The diet
was supplemented with thiamin, riboflavin, pyridoxine, calcium
pantothenate, and choline. Fat-soluble vitamins were provided by
giving 2 drops of halibut liver oil per rat every 4 weeks. During
the initial 4-week feeding period, both groups received the basal
diet supplemented with 0.064% 3'-methyl-4-dimethylaminoazobenzene.
This was followed by a 4-week "interruption period" during which
neither group received the azo dye, but one group received the
basal diet supplemented with sodium selenite at 5 mg selenium/kg,
and the other group received the basal diet without any added
selenium. During the third, 4-week feeding period, both groups
received the basal diet supplemented with 0.048% of the azo dye,
but no supplementary selenium. During the final 8-week feeding
period, both groups received the basal diet without either azo dye
or selenium. Two of the 9 surviving rats in the group that
received supplementary selenium during the "interruption period"
had liver tumours (22%), compared with 4 out of 10 survivors (40%)
in the group not supplemented with selenium. The results of a
second similar study showed a liver tumour incidence of 4/13 (31%)
in the selenium-supplemented group compared with 8 out of 13 rats
(62%) in the unsupplemented group.
A preliminary study by Shamberger & Rudolph (1966) indicated
that concomitant dermal application of sodium selenide reduced the
tumour-promoting effect of croton oil in mice initiated with 7,12-
dimethylbenz[alpha]anthracene (DMBA). Riley (1968) found that a
similar application of sodium selenide prevented the mast cell
reaction caused by an active fraction of croton oil in the skin of
DMBA-initiated mice. In a later set of studies (Shamberger, 1970),
2 groups of 30 female ICR Swiss mice, 50 - 55 days old, were
treated once on day one with 0.125 mg DMBA dissolved in 0.25 ml
acetone. On days 2 - 21, the shaved backs of the first group of
mice were treated with 0.25 ml of a 20:80 water-acetone mixture
containing 0.0005% sodium selenide, whereas the second group was
not treated with any anti-oxidant. Subsequently, in 3 separate,
but essentially identical, studies, both groups of mice received
0.25 ml of 0.04% croton oil in acetone daily for 16, 18, and 18
weeks respectively. After the croton oil treatment, the incidence
of papillomas in the selenide-treated groups was 17, 63, and 45%,
respectively, in 3 separate studies, compared with an incidence in
the non-treated groups of 43, 63, and 89%, respectively. The
corresponding number of papillomas per mouse in the 3 studies was
1.5, 3.0, and 1.5, respectively, in the selenide-treated groups and
3.2, 6.0, and 2.3 in the non-treated groups. No papillomas were
observed in 3 DMBA-initiated control groups, which did not receive
either antioxidant or croton oil. A similar protective effect of
sodium selenide was observed in 3 additional studies in which the
promoting agents were croton oil, croton resin, and phenol and in
which the selenide was applied concomitantly with the promoter.
In another study, 0.25 ml of 0.01% 3-methylcholanthrene (MCA)
was applied daily to the shaved backs of one group of mice for 19
weeks; a second group received 0.0005% sodium selenide together
with the MCA. After 19 weeks, the incidence of papillomas was 68%
in the selenide-treated group and 87% in the untreated group, and
the number of papillomas per mouse was 3.2 and 2.2, respectively.
After 30 weeks, the number of mice with cancers was 17/28 and 25/30
in the selenide-treated and untreated groups, respectively, the
total number of cancers per group being 29 and 71, respectively.
Table 50. Effect of selenium sulfide given orally for 103 weeks on the incidence of liver and lung
tumours in B6C3F1 micea
----------------------------------------------------------------------------------------------------
Lung tumour incidence
Initial Selenium Liver tumour incidence Alveolar/ Alveolar/
Test group Sex number sulfide hepatocellular hepatocellular bronchiolar bronchiolar
of mice dose carcinoma adenoma carcinoma adenoma
(mg/kg)
----------------------------------------------------------------------------------------------------
Untreated control M 50 0 17/49 (35%) 3/49 (6%) 1/49 (2%) 8/49 (16%)
Vehicle controlb M 50 0 15/50 (30%) 0/50 (0%) 1/50 (2%) 3/50 (6%)
Low-dosec M 50 20 11/50 (22%) 3/50 (6%) 2/50 (4%) 8/50 (16%)
High-dosec M 50 100 23/50 (46%) 0/50 (0%) 2/50 (4%) 12/50 (24%)
Untreated control F 50 0 2/50 (4%) 1/50 (2%) 0/50 (0%) 2/50 (4%)
Vehicle controlb F 50 0 0/49 (0%) 0/49 (0%) 0/49 (0%) 0/49 (0%)
Low-dosec F 50 20 1/50 (2%) 1/50 (2%) 1/50 (2%) 2/50 (4%)
High-dosec F 50 100 22/49 (45%) 6/49 (12%) 4/49 (8%) 8/49 (16%)
----------------------------------------------------------------------------------------------------
a Adapted from: National Cancer Institute (1980).
b Received only vehicle for dosing (0.5% aqueous carboxymethylcellulose), 5 days per week, by gavage.
c Received stated dose of selenium sulfide suspended in 0.5% aqueous carboxymethylcellulose, 5 days
per week, by gavage.
Shamberger (1970) also carried out 2 dietary studies in which 4
groups of 36 female, albino ICR Swiss mice, 50 - 55 days old, were
fed Torula yeast diets supplemented with sodium selenite at 0, 0.1,
or 1.0 mg selenium/kg or sodium selenide at 0.1 mg selenium/kg.
After 2 weeks on the test diets, 0.125 mg DMBA dissolved in acetone
was applied once to the skin. After 3 weeks, 0.25 ml 0.05% croton
oil in acetone was applied daily for 17 weeks. At this time, 26/36
mice fed the unsupplemented diet had papillomas, compared with
14/35 mice fed the diet supplemented with 1.0 mg selenium/kg, as
selenite. The incidence of papillomas was slightly elevated in the
mice fed the diet supplemented with 0.1 mg selenium/kg, as sodium
selenite, compared with the unsupplemented mice. The incidence of
papillomas in mice fed sodium selenide at 0.1 mg selenium/kg was
intermediate between that of the unsupplemented group and the group
fed sodium selenite at 1.0 mg selenium/kg.
In a second study of similar design, 4 groups of 36 mice were
fed the test diets described above for 2 weeks. Then 0.25 ml of
0.03% benzo( a)pyrene in acetone was applied daily to the shaved
backs of the mice for 27 weeks. At this time, the incidence of
papillomas in the groups fed the torula diet supplemented with 0,
0.1, or 1.0 mg selenium/kg, as selenite, or 0.1 mg selenium/kg, as
selenide, was 14/35, 22/36, 8/33, and 12/35, respectively. The
number of papillomas per mouse in the corresponding groups was 8.1,
9.8, 5.0, and 6.8.
Harr et al. (1972) weaned 80 female OSU-Brown rats at 35 days
of age and divided them into 4 groups of 20. Each group was fed a
low-selenium basal diet (0.018 µg selenium/kg) that included Torula
yeast as the protein source and contained 60 mg vitamin E/kg.
Groups, 1, 2, 3, and 4 were fed the basal diet supplemented with
sodium selenite at 2.5, 0.5, 0.1, and 0 mg selenium/kg,
respectively. All diets contained 150 mg 2-acetylaminofluorene/kg.
The 40 rats in groups 1 and 2 were born from parents reared on a
selenium-depleted regimen, but were clinically normal. The 40 rats
in groups 3 and 4 were from the second generation maintained on the
depeletion regimen and had clinical signs of selenium deficiency.
After 200 days of treatment, the number of mammary adenocarcinomas
in groups 1, 2, 3, and 4 was 0, 1, 8, and 9, respectively. After
320 days, the number of mammary adenocarcinomas in the same groups
was 11, 11, 13, and 12, respectively. Mammary adenocarcinomas in
groups 1, 2, and 3 occurred mainly (90%) in the thoracic region and
were well circumscribed, firm, and easily removed, whereas those in
group 4 occurred mainly (80%) in the pelvic area and were soft and
fluid, contained little connective tissue, and were invasive.
After 240 days of treatment, the number of hepatic carcinomas in
groups 1, 2, 3, and 4 was 0, 1, 9, and 4, respectively. After 320
days, the number of hepatic carcinomas in the same groups was 8,
12, 12, and 6, respectively. Toxic hepatitis was observed in 18 of
the 20 livers from group 1 (selenium added at 2.5 mg/kg), but was
not seen in the other groups.
In studies of Marshall et al. (1978), 2 groups of male albino
Sprague Dawley rats were fed diets containing 2-acetylaminofluorene
at 0.3 g/kg, for 14 weeks. One group received 4 mg selenium, as
sodium selenite/litre drinking-water. The carcinogen was withdrawn
from the diet for an additional 4 weeks and then the rats were
sacrificed. The rats given selenium had about 50% fewer liver
tumours than unsupplemented rats. Control rats given a similar
level of selenium in the water showed normal growth response, liver
weight, and appearance.
Three groups of 15 male Sprague Dawley rats, weighing about
250 g, were fed a basal diet of finely ground Purina Laboratory
Chow and water ad libitum (Griffin & Jacobs, 1977). One group did
not receive any supplementary selenium, a second group received 6
mg selenium, as sodium selenite/litre drinking-water, and a third
group received 6 mg selenium, in the form of a high selenium
yeast/kg diet. After one week on this regimen, all 3 groups were
given 0.05% 3'-methyl-4-dimethyl-aminoazobenzene in the diet for 8
weeks. Following this, all groups were maintained on the
carcinogen-free diets for an additional 4 weeks. The selenium
supplements were maintained for the second and third group
throughout the entire study. At sacrifice, the incidence of liver
tumours in the surviving rats was 92% (11/12) in the unsupplemented
group, 46% (7/15) in the group supplemented with selenite in the
drinking-water, and 64% (9/14) in the group supplemented with
selenium yeast in the diet. In this study, the selenium
supplements had little effect on the growth of the rats.
Histopathological examination of several of the tumours revealed
that they were bile duct adenocarcinomas and liver cell
adenocarcinomas.
Jacobs et al. (1977a) injected 2 groups of 15 male, 8-week-old
Sprague Dawley rats, weekly, with 20 mg sym,-dimethylhydrazine
dihydrochloride (DMH)/kg body weight, for 18 weeks. One group of
rats received sodium selenite at 4 mg selenium/ litre drinking-
water, which was available ad libitum one week prior to, and
throughout, administration of the carcinogen. The other group did
not receive selenium added to the drinking-water. The group
receiving DMH and no added selenium had an 87% incidence (13/15) of
colon tumours, whereas the group receiving both DMH and added
selenium had a 40% incidence (6/15) of colon tumours. The total
number of colon tumours was 39 in rats receiving only DMH and 11 in
rats receiving both DMH and added selenium. In a similar study in
which 2 groups of 15 rats were injected weekly with 20 mg of
(methylazoxyl)-methanol acetate (MAM)/kg body weight for 18 weeks,
no significant differences in the incidence of colon tumours were
apparent between groups with (14/15) and without (14/14) added
selenium in the drinking-water. However, the number of MAM induced
tumours was 73 in the group given MAM alone and 42 in the group
receiving both MAM and added selenium.
Schrauzer and colleagues carried out a series of studies in
which exogenous carcinogens were not used, to test the ability of
supplemental selenium in influencing the development of spontaneous
mouse mammary tumours, presumably of viral origin. In study 1
(Schrauzer & Ishmael, 1974), 2 groups of 30 virgin female C3H/St
mice, 4 - 6 weeks old, were fed a basal diet ("Concord Maid")
consisting of meat scraps, dried skimmed milk, oat groats, ground
wheat, wheat germ meal, vegetable oil, cane molasses, salt,
brewer's yeast, cereal binder, sodium propionate, and calcium
pantothenate. The diet contained about 150 g protein, 5 g fat, and
0.15 mg selenium/kg. One group of mice received plain distilled
water for drinking, while the other group received distilled water
fortified with 2 mg selenium, as selenium dioxide/litre. The
strain of mice used in this study ordinarily has a high incidence
of spontaneous mammary adenocarcinomas and, after 16 months of
treatment, the observed incidence in the group not given
supplemental selenium in the water was 82%, whereas the incidence
in the group given selenium was 10%.
In study 2 (Schrauzer et al., 1976), 3 groups of 30, 30, and 50
female C3H/St mice fed the basal diet described above were also
given selenite at 0, 5, or 15 mg selenium/litre drinking-water,
respectively. Toxicity due to the doses of selenium was indicated
by the average body weights in the 3 groups at 12 months (33, 29,
and 25 g, respectively) and by a higher tumour-unrelated mortality
rate in the selenium-exposed mice. After 26 months, the
corresponding incidence of spontaneous mammary tumours in the 3
groups was 82, 36, and 33% in the mice that survived to the age at
which the first tumours appeared in each group. A fourth group of
20 mice, fed a selenium-deficient diet for 14 months and then the
basal diet until death, had a mammary tumour incidence of 69% which
was not judged to be different from the 82% incidence in the
control group (basal diet throughout life span and no added
selenium in the water).
In study 3 (Schrauzer et al., 1978a), 4 groups of 30 female
C3H/St mice were fed a basal diet ("Wayne F-6 Lab Blox") that was
different from that used in the first 2 studies and consisted of
fish meal, animal liver meal, soybean meal, corn and wheat flakes,
ground corn, wheat red dog, wheat middlings, soybean oil, cane
molasses, salt, brewer's yeast, and various vitamins and minerals.
This diet contained 244.8 g protein, 64.8 g fat, and 0.45 mg
selenium/kg. The 4 groups received selenium dioxide at 0, 0.1,
0.5, or 1.0 mg selenium/litre drinking-water and, after 22 months,
the incidence of mammary tumours was 42, 25, 19, and 10%,
respectively. Selenium supplementation at these levels did not
have any noticeable adverse effects on weight-gain or survival of
the mice. The incidence of spontaneous mammary tumours in the
control (unsupplemented) group was lower in this study than that
observed in the 2 previous studies (42 compared with 82%), and this
was attributed to the higher selenium content of the new basal diet
used in the third study (0.45 versus 0.15 mg/kg).
Medina & Shepherd (1980) fed BALB/cfC3H mice Wayne Lab Blox and
gave them selenium dioxide at 0, 2, or 6 mg selenium/litre
drinking-water, ad libitum, starting at 10 weeks of age and
continuing to the end of the study. In 12-month-old mice, 2 and 6
mg selenium/litre decreased mammary tumour incidence from 82% in
the untreated controls to 48 and 12%, respectively. There were no
effects of the selenium treatment on normal reproductive function
or weight gain in these mice. In another study, samples of 4
different preneoplastic outgrowth lines (D2, C3, C4, and CD-7)
were transplanted into the mammary gland-free fat pads of syngenic
mice. When the implants had filled the mammary fat pads with their
respective outgrowths (8 weeks after implantation), the mice were
given selenium dioxide at 4 mg selenium/litre drinking-water, ad
libitum, for the rest of the study. Such treatment with selenium
delayed the rate of tumour formation only in line C4, increasing
the time for half of the outgrowths to produce tumours from 34 to
44 weeks. In a third study, 2 - 6 mg selenium/litre drinking-water
had no effect on the growth rate of primary tumours transplanted
subcutaneously in BALB/c mice. Since selenium did not have any
effect on tumour formation rate in 3 of 4 preneoplastic mammary
outgrowth lines or on the growth rate of established mammary
tumours, the authors suggested that selenium might act by
inhibiting chemical or viral transformation of normal cells or by
inhibiting expression of initially transformed cells.
Because of the metabolic antagonism between arsenic and
selenium (section 7.1.6.3), Schrauzer and co-workers also
investigated the effect of arsenic on the genesis of the
spontaneous mammary tumours in C3H/St female mice described above.
In their first study on arsenic and selenium (which was part of the
same study described above in Schrauzer & Ishmael (1974)), giving
sodium arsenite at 10 mg arsenic/litre drinking-water to a group of
30 C3H/St mice for 16 months, reduced the incidence of spontaneous
mammary tumours to 27% compared with an incidence of 82% in the
untreated controls (Schrauzer & Ishmael, 1974). However, arsenic
treatment markedly stimulated the growth rate of spontaneous or
transplanted mammary tumours. In a second study concerning
arsenic, sodium arsenite at 80 mg arsenic/litre drinking-water,
administered to a group of 20 C3H/St mice, reduced the incidence of
spontaneous mammary tumours to 40% compared with an incidence of
82% in the untreated controls (Schrauzer et al., 1976), but some of
the tumour-inhibiting effect of arsenic appeared to be masked at
this dose level by its toxicity.
In a third study relating arsenic and selenium (Schrauzer et
al., 1978b), 4 groups of 30 female C3H/St mice were fed the Wayne
F-6 Lab Blox diet described above, which contained 0.29 mg
arsenic/kg. The 4 groups received the following supplements in
their deionized drinking-water: none, arsenic trioxide at 2 mg
arsenic/litre, selenium dioxide at 2 mg selenium/litre, or 2 mg of
both arsenic and selenium. The incidence of mammary tumours in the
4 groups was 41, 36, 17, and 62%, respectively, and the percentage
of multiple mammary tumours was 17, 40, 0, and 28. The age of
tumour onset in the corresponding groups was 4.5, 9, 16, and 8
months. Thus, in this case, treatment with arsenic appeared to
diminish the cancer-protecting effect of selenium. Arsenic also
accelerated tumour growth and increased the incidence of multiple
tumours.
Newberne & Conner (1974) fed 4 groups of male rats of the
Charles River CD strain, weighing about 100 g each, a basal diet
consisting of casein, 200 g/kg; sucrose, 209 g/kg; dextrose, 209
g/kg; dextrin, 209 g/kg; stripped lard, 80 g/kg; Wesson Oil, 20
g/kg; selenium-free salts, 50 g/kg; vitamin mix, 20 g/kg; choline,
3 g/kg; and vitamin B12, 0.05 g/kg. The basal diet contained about
0.03 mg selenium/kg. One group received the unsupplemented basal
diet, whereas the other 3 groups received the basal diet
supplemented with sufficient sodium selenite to attain approximate
dietary levels of 0.1, 1.0, or 5.0 mg selenium/kg, respectively.
Each group was fed the diet for 2 - 3 weeks, before oral
administration of 7 mg aflatoxin B1 in dimethyl sulfoxide/kg body
weight. The rats continued on their respective diets for an
additional 2 weeks, and the mortality rate after this time, in the
groups fed the diets containing 0.03, 0.1, 1.0, and 5.0 mg
selenium/kg was 28/29, 20/30, 7/28, and 27/29, respectively.
Histological examination revealed that rats receiving 1.0 mg
selenium/kg diet were partially protected against the aflatoxin B1
and also had less severe liver lesions. However, the groups fed
diets containing 1.0 or 5.0 mg selenium/kg exhibited a novel renal
lesion associated with acute aflatoxin B1 toxicity, characterized
by marked tubular necrosis at the cortical medullary junction.
Some tubules exhibited hyperplastic changes of the epithelium as
well as necrosis.
In a second study, Grant et al. (1977) gave 25 µg aflatoxin B1
orally, 5 days/week for 4 weeks, to 140 male Sprague Dawley rats.
The rats were fed normal or marginally lipotrope-deficient
semisynthetic basal diets containing sodium selenite at 0.03, 0.10,
0.50, 1.0, 2.5, or 5.0 mg selenium/kg. After 17 months, the
surviving rats were sacrificed and histopathological examination
was carried out. A very low incidence (20%) of hepatocarcinomas
was seen in rats receiving 1.0 mg selenium/kg in the normal basal
diet and 0.10 or 0.50 mg/kg in the lipotrope-deficient diet. No
other tumours were observed, and grading of the hepatic lesions
indicated that there was no significant differences among the
dietary selenium groups. Dietary sodium selenite and repeated
doses of aflatoxin B1 interacted to produce large bizarre cells in
the renal tubules, occurring in the same region of the kidney as
the severe necrosis seen previously in rats fed high levels of
dietary selenium and given an acute dose of aflatoxin B1.
Recently, a study of the effects of selenium on aflatoxin B1-
induced enzyme altered foci in rat liver has been reported (Milks
et al., 1985). Male Sprague Dawley rats were fed a selenium-
deficient diet and given sodium selenite at 5, 2, 0.2, or 1 mg
selenium/litre drinking-water for 3 weeks. Each rat then received
2 µg mol aflatoxin B1/kg body weight by stomach tube. For the next
week the selenium status was "normalized". Rats previously
receiving 5, 2, 0.2, or 0 mg selenium/litre received, respectively,
0. 0.2, 2, or 5 mg/litre. Then rat chow was fed and a promoting
regimen consisting of phenobarbital in the drinking-water, and a
partial hepatectomy was instituted. Eight weeks later, necropsies
were carried out and livers were stained histochemically for
gamma-glutamyl transpeptidase activity. Foci of activity were
counted. The number of foci seen in livers from rats given 5, 2,
0.2, or 0 mg selenium/litre during initiation were 0.62 ± 0.22,
1.97 ± 0.46, 3.35 ± 0.66, and 2.46 ± 0.23 per cm2, respectively.
The data suggested that 5 mg selenium/litre can protect against the
hepatocarcinogenic effects of aflatoxin B1 in the rat.
On the basis of anecdotal observations, Wedderburn (1972)
suggested that a decrease in the number of cases of intestinal
carcinoma in autopsied sheep might be associated with the
widespread veterinary use of selenium to prevent deficiency
diseases in sheep. Simpson (1972a) conducted an investigation into
the epidemiology of carcinomas of the small intestine of sheep in
which the viscera of 32 733 ewes were examined. Carcinomas of the
small intestine were diagnosed in 483 animals, but the prevalence
of these neoplasms in ewes regularly dosed with selenium throughout
their lives was not significantly different from the prevalence in
ewes never treated with selenium (Simpson, 1972b). Furthermore, no
significant differences were found in association with differences
in soil types on the farms from which the sheep originated. It was
concluded that soil-selenium levels and administration of selenium
in the form and at the dose rates used did not have any effects on
the development of intestinal carcinomas in sheep. Underwood
(1977) commented that neoplasias were not observed among the
various lesions attributed to selenium deficiency in animals.
Ip has made and summarized a series of observations on selenium
and carcinogenesis (Ip, 1985a).
Evidence of an interaction between dietary fat and selenium
status in the induction of mammary tumours by dimethylbenz-
[alpha]anthracene in rats has been presented by Ip & Sinha (1981).
They fed 8 groups of 23 - 25 female weanling Sprague Dawley rats
one of the following diets either deficient in selenium or
supplemented with sodium selenite at 0.1 mg selenium/kg: 1% corn
oil, 5% corn oil, 25% corn oil, or 1% corn oil plus 24%
hydrogenated coconut oil. In addition to the fat, the diets
consisted of Torula yeast, dextrose, HMW salt mix, vitamin mix,
alphacel, and DL-methionine. The diets were adjusted so that the
intake of all nutrients would be the same, except for dextrose and
fat, assuming that the rats would consume an equal number of
calories. The corn oil used was stripped of tocopherol and the
unsupplemented diet was deficient in selenium (less than 0.02 mg/kg
by fluorometry). Mammary tumours were induced by the intragastric
administration of 5 mg dimethylbenz[alpha]anthracene at 50 days of
age. Increasing the dietary polyunsaturated-fat level (corn oil)
increased the tumour incidence in rats fed the selenium-
supplemented diets (Table 51). However, the high-saturated-fat
diet was much less active in stimulating tumourigenesis. Selenium
deficiency increased the tumour yield only in rats fed the high
polyunsaturated-fat diet (25% corn oil). Increased tumour
incidence due to selenium deficiency was not seen in the rats fed
the low-fat diets containing polyunsaturated fat (1 or 5% corn
oil) or the high-fat diet containing primarily saturated fat (1%
corn oil with 24% coconut oil). Selenium deficiency, however, did
increase the incidence of mammary tumours in rats fed a low-fat
diet containing polyunsaturated fat (1% corn oil), when larger
doses of dimethylbenz[alpha]anthracene were used (10 or 15 mg -
data not shown).
Table 51. Incidence of palpable mammary tumours in
dimethylbenz(alpha)anthracene-treated rats fed different
levels and types of fats in the diet, with or without
selenium supplementationa
-----------------------------------------------------------
Fats in diet Selenium Incidence of palpable tumours
Corn Coconut in diet 19 weeks after dimethylbenz
oil oil (mg/kg) (alpha)-anthracene administration
(%) (%) Number of rats (%)
-----------------------------------------------------------
1 0 0 4/23 17.4
1 0 0.1 3/24 12.5
5 0 0 11/25 44.0
5 0 0.1 8/24 33.3
25 0 0 24/25 96.0
25 0 0.1 15/25 60.0
1 24 0 7/24 29.2
1 24 0.1 6/25 24.0
----------------------------------------------------------
a Adapted from: Ip & Sinha (1981).
In a recent study, the effects of vitamin E status on the
anticarcinogenic effect of selenium were examined (Ip, 1985b).
Female Sprague Dawley rats were fed a 20% stripped corn oil,
casein-based diet. Four dietary groups were formed: adequate
vitamin E/adequate selenium, adequate vitamin E/high selenium,
deficient vitamin E/adequate selenium, and deficient vitamin E/high
selenium. Adequate and deficient vitamin E diets contained 50 and
10 mg vitamin E/kg diet, respectively. Adequate and high selenium
diets contained Na2SeO3 at 0.1 and 2.5 mg selenium/kg,
respectively. At 50 days of age, rats received 5 mg of
dimethylbenz[alpha]anthracene (DMBA) each by stomach tube. Rats
were killed 20 weeks after DMBA administration. The tumour
incidences in the groups were: adequate vitamin E/adequate
selenium, 76%; adequate vitamin E/high selenium, 40%; deficient
vitamin E/adequate selenium, 84%; deficient vitamin E/high
selenium, 68%. This suggests that selenium protection against
carcinogenesis is decreased in vitamin E deficiency.
Pence & Buddingh (1985) studied the effects of selenium
deficiency on 1,2-dimethylhydrazine (DMH)-induced colon cancer in
the rat. They used male Sprague Dawley rats fed Torula yeast-based
diets containing 2% corn oil. Sodium selenite at 0.1 mg
selenium/kg was added to the diet of the controls. Weanlings were
fed the diets 3 weeks prior the institution of DMH treatment and
were killed after 20 weeks of treatment. No effects of selenium
status were noted on the incidence of colon adenocarcinomas.
The effects of selenium on UVR-induced skin carcinogenesis were
studied by Overvad et al. (1985). Female hairless mice were given
sodium selenite at 0, 2, 4, or 8 mg selenium/litre drinking-water.
Three weeks after selenium exposure began they were exposed to UVR
daily for 22 weeks. Then they were examined weekly for 26 weeks
for skin tumours, and relative tumour onset ratios were calculated.
The 2 mg selenium/litre treatment did not affect tumour onset but
the higher doses did. This suggests that selenium can protect
against UVR-induced skin cancer.
Birt et al. (1984) reported that high levels of selenium in the
diet increased pancreatic carcinogenesis induced by bis-(2-
oxopropyl)-nitrosamine (BOP) in male Syrian hamsters. Torula yeast-
based diets with either 0.1 or 2.5 mg selenium/kg were fed to
hamsters beginning at 4 weeks of age. BOP was given in 4 weekly
injections of 5 mg/kg body weight, beginning at 8 weeks of age.
Hamsters were killed at 78 weeks of age. When dietary fat was low
(11% calories as corn oil), the low-selenium group had 25
pancreatic ductular carcinomas (PCDA) in 18 hamsters, and the high-
selenium group had 63 PCDA in 23 hamsters. When dietary fat was
high (45% of calories as corn oil), the low-selenium group had 27
PCDA in 19 hamsters and the high-selenium group had 44 PCDA in 18
hamsters.
Other studies have been reported. Milner, who showed that
pharmacological doses of selenium inhibited the growth of
transplantable tumours, has recently summarized his work (Milner,
1985), and Thompson has summarized his work on mammary
carcinogenesis (Thompson, 1984). The metabolism of the carcinogen
2-acetylaminofluorene in selenium-deficient rats has been studied
by Besbris et al. (1982). They found that selenium-deficient rats
excreted more N-OH-acetylaminofluorene than controls, suggesting
that selenium might prevent the production of this carcinogenic
metabolite or promote its detoxification.
8. EFFECTS OF SELENIUM ON MAN
8.1. High Selenium Intake
8.1.1. General population
8.1.1.1. Signs and symptoms
When it became apparent that selenium was the toxic factor in
plants that caused alkali disease in livestock raised in
seleniferous areas, public health personnel became interested in
the possible hazards for human health in such regions, since
seleniferous grains or vegetables grown on high-selenium soil could
enter the human food chain. Smith et al. (1936) reasoned that, if
human selenosis were a problem anywhere, it would most likely occur
in farmers living in seleniferous regions, who consumed largely
locally-produced foodstuffs. Therefore, they surveyed a rural
population living on farms or ranches known to have a history of
alkali disease. Their survey inquired into the health status of
111 families and also determined the actual consumption of locally-
produced foods. Wherever possible, general physical examinations
were made and urine samples were collected.
These workers were unable to find any symptom or group of
symptoms or serious illness that could be considered characteristic
of, or could definitely be attributed to, selenium poisoning in
man. However, the incidence of vague symptoms of ill health and
symptoms suggesting damage to the liver, kidneys, skin, and joints
was rather high. But since the causes for such disorders are many,
it was not possible to determine whether selenium played any role
in their causation. Apart from the more vague symptoms of
anorexia, indigestion, general pallor, and malnutrition, the
following more pronounced disease states were observed: bad teeth,
yellowish discoloration of the skin, skin eruptions, chronic
arthritis, diseased nails, and subcutaneous oedema.
The same authors (Smith et al., 1936) were unable to
demonstrate a very definite correlation between the clinical
evidence of selenium intoxication in 127 subjects and the
concentration of selenium in the urine. In fact, they expressed
surprise that there was not any more definite evidence of serious
injury, particularly in subjects with high concentrations of
selenium in the urine. Relatively high urinary-selenium levels
were most often associated with pathological nails,
gastrointestinal disorders, icteroid skin, and bad teeth. The
incidence of high urinary-selenium in individuals with dermatitis
and arthritis was no greater than that in individuals without
symptoms.
Because of the ambiguous findings in their first study, Smith &
Westfall (1937) carried out a second, more detailed and intensive
survey to establish the symptomatology of human selenosis and its
relation to the amount of selenium excreted in the urine. For this
purpose, they examined 100 subjects from 50 families that had high
levels of urinary-selenium in their previous testing. The
percentage frequency of the various signs and symptoms observed is
given as follows: none, 24; gastrointestinal disturbances, 31;
icteroid discolouration of the skin, 28; bad teeth, 27; sallow and
pallid colour, especially in younger individuals, 17; history of
recurrent jaundice, 5; dermatitis, 5; pigmentation of the skin
(chloasma?), 3; pathological nails, 3; rheumatoid arthritis, 3;
cardiorenal disease, 2; vitiligo, 2. It was concluded that none of
these signs or symptoms could be regarded as specific for selenium
poisoning, and it was not certain that any one was the direct
result of the continual ingestion of selenium. Nonetheless, these
workers felt that the high incidence of gastrointestinal
disturbances was of importance. Moreover, the high incidence of
icteroid discolouration of the skin was thought to be related in
some way to the ingestion of selenium, possibly as a result of
liver dysfunction. The possible cariogenic effects of high levels
of selenium is discussed further in section 8.1.1.2. The other
symptoms occurred so infrequently that they did not seem to be
associated with selenium. However, it should be noted that the
value of all these observations is in doubt since there is no
comparative information available concerning the frequency of
occurrence of these signs and symptoms in an appropriate control
group.
Smith & Westfall (1937) estimated that most of their subjects
living in highly seleniferous areas were probably absorbing about
10 - 100 µg/kg body weight per day and that some of their subjects
might have absorbed as much as 200 µg/kg per day. For a 70-kg man,
these rates of absorption would be equivalent to a dietary-selenium
intake of 700 - 14 000 µg/day, assuming that all of the selenium in
the food had been absorbed.
As discussed in section 4.1.2, drinking-water rarely
contributes much selenium to a person's total daily intake, but a
brief note indicated that a family of North American Indians living
in Colorado, USA, suffered hair loss, weakened nails, and
listlessness after consuming well water reported to contain 9000 µg
selenium/litre for about 3 months (Anonymous, 1962). This incident
was thought to be the first authentic case of selenium poisoning in
human beings induced exclusively from a naturally-occurring
underground source of water. Tsongas & Ferguson (1977) studied the
effects of selenium in the drinking-water on the health of 2 groups
of persons living in a rural Colorado community. The first group
("exposed") received a water supply that contained 50 - 125
µg/litre, whereas the second group ("unexposed") received a water
supply that contained levels ranging from non-detectable (1
µg/litre) to 16 µg/litre. Examination of a total of 86 individuals
revealed that there were no significant differences between the
exposed and unexposed groups in the incidence or prevalence of any
of 85 health variables studied.
Lemley (1940) presented a 58-year-old rancher from South
Dakota, thought to be the first described case of chronic selenium
dermatitis in a human being, caused by the ingestion of selenium
from natural sources. However, 2 samples of urine collected from
this patient a week apart, contained only 0.043 and 0.040 mg
selenium/litre, levels that are considered to be within the normal
range. Although some improvement in the patient's condition was
noted when certain seleniferous foods consumed on his ranch were
avoided, the improvement was not marked. Administration of
bromobenzene, now known to be a potent hepatotoxin, cleared up the
dermatitis, but caused only a small increase in the urinary-
selenium output, which reached a peak value of about 0.100
mg/litre, after 4 days. Earlier work by Moxon et al. (1940) had
shown that the rate of excretion of selenium from selenized animals
could be increased by the administration of bromobenzene. The
likelihood that selenium was the cause of the dermatitis in this
patient is doubtful, since, in a later paper, Lemley & Merryman
(194l) claimed that a relapse in this patient's condition was cured
when some canned meat containing 0.40 µg selenium/kg (considered by
them to be a highly toxic amount of selenium) was withdrawn from
the patient's diet. This figure for the level of selenium in the
meat is almost certainly erroneous, since not only is it below that
which would be found in animals suffering from selenium deficiency
but it is also below the sensitivity of the analytical techniques
available at that time.
Other cases of presumed human overexposure to selenium were
reported by Lemley & Merryman (1941). For example, urine samples
from a ranching family living in South Dakota contained 0.200,
0.250, 0.300, 0.550, and 0.600 mg selenium/litre for the father,
mother, daughter, son, and uncle, respectively. Administration of
bromobenzene to the father resulted in a urinary output of selenium
of 1.800 mg/litre within 24 h. No mention of dermatitis was made
in connection with this family. Rather, all family members
suffered from slight, continual dizziness and clouding of the
sensorium, extreme lassitude accompanied by depression, and
moderate emotional instability. The patients tired on exertion and
complained that their powers of concentration were markedly
impaired. After a course of bromobenzene, these people improved
markedly and their general condition remained improved, since they
were instructed not to use the various food products of the ranch
that contained selenium. Lemley & Merryman concluded that the
following facts provided the basis for the diagnosis of selenium
poisoning in these subjects:
(a) knowledge that the subjects lived in a seleniferous
area;
(b) presence of concentrations of selenium in the urine
exceeding 0.100 mg/litre;
(c) increased elimination of selenium in the urine after
bromobenzene administration; and
(d) improvement in the subject's symptoms after
elimination of selenium from the diet.
The same authors also described a 65-year-old rancher from
South Dakota, who suffered alternate bouts of diarrhoea and
constipation. This patient's urinary-selenium concentrations were
as high as 0.250 mg/litre. An exploratory abdominal operation
suggested that the patient had very early cirrhosis of the liver,
which was confirmed by pathological examination. The patient
recovered from the operation and then gradually improved after
being placed on a strict selenium-free diet and given a course of
bromobenzene.
In a later paper, Lemley (1943) expressed the opinion that
human selenium poisoning is common, widespread, and, in certain
localities, of importance for the general public health. More
recently, Kilness (1973) complained that no follow-up public heath
survey with an appropriate control group had been made in South
Dakota since the initial surveys by the United States Public Health
Service in highly seleniferous areas more than 30 years previously.
Jaffe (1976) carried out a field study in Venezuela and
compared 111 children living in a seleniferous area (Villa Bruzual)
with 50 living in Caracas. The overall haemoglobin and haematocrit
values were somewhat lower in Villa Bruzual (128 g/litre and 39
volume %, respectively) than in Caracas (148 g/litre and 42 volume
%, respectively), but no correlations between blood- and urine-
selenium levels and haemoglobin or haematocrit values were found.
The children in Villa Bruzual consumed less meat and milk and had a
higher incidence of intestinal parasite infestation (Jaffe et al.,
1972a) than those in Caracas. Therefore, it was concluded that the
differences in haemoglobin were probably due to differences in
nutritional or parasitological status and not to differences in
selenium intake. Activities of prothrombin and serum alkaline
phosphatase and transaminases, which altered in selenium-poisoned
rats (Jaffe et al., 1972b), were normal in all the children and no
correlation with blood-selenium levels was apparent. Symptoms of
dermatitis, loose hair, and pathological nails were reported as
more frequent among the children in the seleniferous area than in
those living in Caracas, but no quantitative information was given.
The clinical signs of nausea and pathological nails appeared to be
correlated with serum- and urine-selenium levels. However, the
cause of the different incidence of clinical signs was considered
doubtful, especially in the absence of differences in the various
biochemical tests performed (Jaffe et al., 1972a).
The mean blood-selenium level of 111 school children from the
seleniferous area in Venezuela was 0.813 mg/litre (Jaffe et al.,
1972a). A subgroup of 28 children from this area who had blood-
selenium levels of over 1 mg/litre had a mean blood-selenium level
of more than 1.321 mg/litre. There was one child with a blood-
selenium level of 1.8 mg/litre. Another subgroup of 11 children
who had blood-selenium levels of less than 0.4 mg/litre had a mean
blood-selenium level of 0.330 mg/litre. Urinary-selenium levels
tended to reflect blood-selenium levels, since children in the
high-blood-selenium subgroup (> 1 mg/litre) excreted a mean level
of 0.657 mg/litre urine, whereas children in the low-blood-selenium
subgroup (< 0.4 mg/litre) excreted a mean level of 0.266 mg/litre
urine.
Kerdel-Vegas (1966) summarized 9 cases of acute intoxication
due to the ingestion of nuts of the "Coco de Mono" tree (Lecythis
ollaria) from the seleniferous areas of Venezuela. Although the
course of the poisoning differed from patient to patient (probably
because of differences in the amount of nuts consumed and the
length of time the nuts were present in the digestive tract), most
cases experienced nausea, vomiting, and diarrhoea, a few hours
after eating the nuts, followed by hair loss and nail changes, some
weeks after the initial episode. Two patients had foul breath that
was described as being reminiscent of decomposed seaweed or
phosphorus. After a period of time, re-growth of hair and nails
took place and most patients appeared to make a satisfactory
recovery. One fatality was reported, a 2-year-old boy who died, in
spite of proper medical attention and symptomatic treatment for
severe dehydration.
Dickson (1969) described his own personal experience after
eating sapucaia nuts ( Lecythis elliptica H.B.K.) that had grown in
Honduras. He reported hair loss and splitting of finger and
toenails several weeks after consuming the nuts. Although no
mention of selenium was made in his report, he noted that a
peculiar odour was associated with consumption of these nuts, not
only of his breath but of his body as well. Kerdel-Vegas (1966)
pointed out that the sapucaia, or chestnut of Para (which he called
Lecythis paraensis Ducke), is consumed as food in Brazil and other
countries to which it is exported (i.e., Europe and the USA). He
also commented that there is a popular saying in northern Brazil
that eating sapucaia leads to hair loss, but he knew of no
scientific or medical reports from Brazil. Signs of selenium
toxicity have been observed in rats fed diets containing defatted
Brazil nut flour that assayed 51 mg selenium/kg (Chavez, 1966), and
Thorn et al. (1978) found that samples of Brazil nuts marketed in
the United Kingdom contained an average of 22 mg selenium/kg
(range, 2.3 - 53 mg/kg).
As reported recently, an unexplained intoxication characterized
by nail deformation and the loss of hair and nails as the most
common signs was observed in Enshi county of Hubei province of the
People's Republic of China, more than 20 years ago, with a peak
prevalence during the years 1961 - 64 (Yang et al., 1983). In 5
villages with 248 inhabitants, about half of the population was
affected and in one particular village over 80% of the people were
affected. No other quantitative information about the cases was
given but a general description of the signs and symptoms observed
is presented below. The hair became dry and brittle and was easily
broken off at the scalp with retention of intact radicles so that
depigmented and dull hair continued to grow. A scalp rash
accompanied by intolerable itching resulted in hard scratching
which easily removed the hair. The nails became brittle with white
spots and longitudinal streaks on the surface, followed by a break
on the wall of the nail. With new nail growth, the broken nail
advanced and ultimately fell off. Effusian of fluid from around
the nail was common in many cases. The new nail had a rough and
ridged surface and was fragile and thickened.
Other signs of intoxication included skin lesions, tooth decay,
and abnormalities of the nervous system. The skin became red and
swollen and then blistered and eruptive followed, in some cases, by
ulcerations that took a long but unstated time to heal. The
lesions occurred primarily on the limbs and also on the back of the
neck. Mottled teeth were observed in the intoxicated individuals
but this observation may have been confounded by high exposure to
fluoride. In one heavily affected village, nervous system
abnormalities were seen, including peripheral anaesthesia,
acroparaesthesia, and pain in the extremities. Hyperreflexia of
the tendon commonly developed later followed by numbness,
paralysis, and motor disturbances. One case of hemiplegia was also
reported. There was an indication that this intoxication was
related to the locally grown monotonous diet consumed, which later
was shown to contain high levels of selenium. No quantitative data
regarding selenium exposure were obtained at the time of the
outbreak. However, because of circumstantial evidence, the
intoxication was considered by the authors to be selenosis, and, as
discussed in sections 5.1.1.1 and 6.2.3, current levels of exposure
to dietary selenium in an area with a history of intoxication, as
assessed by blood- and hair-selenium levels as well as dietary
intake data, exceeded those ever reported in any non-occupationally
exposed population (Table 8). The authors estimated that the daily
dietary intake of selenium, after the peak prevalence of the
poisoning had subsided, averaged 5 mg, and blood- and hair-selenium
levels averaged 3.2 mg/litre and 32.2 mg/kg, respectively. The
ultimate environmental source of the selenium in this episode of
intoxication was a highly seleniferous coal (average selenium
content of 300 µg/g) which lost its selenium to the soil as a
result of weathering processes. Once in the soil, the selenium was
taken up by plant crops and entered into the food chain.
Because of the nutritionally-beneficial effects of selenium in
animals, some investigators have deliberately given inorganic or
organic forms of the element to people with the aim of producing
some desirable health benefit. For example, Westermarck (1977)
administered selenium, as selenite, in oral doses of 0.05 mg/kg
body weight per day, for more than one year, to patients with
neuronal ceroid lipofuscinosis (NCL) and did not observe any toxic
manifestations. On the contrary, it was felt that some of the NCL
patients showed at least a transitory improvement in their
condition. In some patients, a slight increase in serum aspartate
aminotransferase activity was observed, but apparently this is seen
in a number of patients with NCL.
Schrauzer & White (1978) described 2 individuals who had been
taking commercially-available nutritional supplements consisting of
selenium-containing yeast at doses of 200 and 450 µg selenium,
daily, for 18 months. Together with their dietary intakes, these
individuals received a total of 350 and 600 µg/day. Although some
marginal haematological changes were seen and the serum-glutamic
oxaloacetic transaminase activities were on the borderline of high,
it was concluded that daily intakes of up to 600 µg selenium for 18
months do not induce toxic effects in well-fed individuals.
In a study by Perona et al. (1978), 4 subjects were given,
orally, a 2-mg dose of selenium, as sodium selenite, daily, for
20 - 40 days, to determine the effects of in vivo selenium
administration on the activity of human erythrocyte-glutathione
peroxidase. It was stated that none of the subjects exhibited any
symptoms of selenium poisoning, but the criteria used to judge any
deleterious effects of selenium were not described.
Yang et al. (1983) reported the case of a 62-year-old man who
had taken one tablet containing 2 mg sodium selenite per day for
more than 2 years. The subject did not have any symptoms of
indisposition but presented with thickened, fragile and somewhat
honeycomb-like fingernails. After the oral intake of sodium
selenite was stopped, the surface of the new nail growth became
smooth and gradually recovered. Blood-and hair-selenium levels
were 0.179 mg/litre and 0.828 mg/kg, respectively, on the day the
selenite tablets were discontinued. These tissue-selenium levels
are much lower than those reported by the same authors in Enshi
county of the Hubei province (Yang et al., 1983) suggesting that
the form of selenium ingested must be considered when interpreting
tissue-selenium levels in the diagnosis of selenium poisoning.
Ingestion of superpotent selenium tablets, meant to be consumed
as a "health food" supplement, resulted in 12 cases of human
selenium toxicity in the USA in 1984 (Anonymous, 1984; Jensen et
al., 1984; Helzlsouer et al., 1985). Each tablet contained 27 - 31
mg selenium by analysis (about 182 times more than the level stated
on the label). Approximately 25 mg of the selenium was present as
sodium selenite whereas the rest was elemental and/or organic
selenium. The total doses of selenium estimated to be consumed by
the victims ranged from 27 to 2387 mg. Based on the limited
information available, the symptoms reported in these cases as most
common were nausea and vomiting, nail changes, hair loss, fatigue,
and irritability. Other symptoms included abdominal cramps, watery
diarrhea, paraesthesias, dryness of hair, and garlicky breath.
Eight of the 12 victims did not have any abnormalities in the blood
chemistry, and the results of liver and kidney function tests were
normal.
A level of 500 µg has been proposed as the tentative maximum
acceptable daily intake of selenium for the protection of human
health (Sakurai & Tsuchiya, 1975). This figure was derived from an
initial estimate of the mean normal daily intake of selenium by
human beings of between 50 and 150 µg. It was concluded that
values of 10 - 200 times the normal intake appeared acceptable as
an estimated range for the margin of safety within which the
average human being could tolerate selenium. By taking the lower
values of both these estimates, the lowest level of potentially
dangerous daily intake of selenium was estimated to be 500 µg.
8.1.1.2. Attempts to associate high selenium intake with human
diseases
(a) Dental caries
As already mentioned in section 8.1.1.1, bad teeth was one of
the signs that was thought to be possibly associated with a high
intake of selenium by a rural population living in seleniferous
areas of South Dakota, Wyoming, and Nebraska (Smith et al., 1936)
and by children living in a seleniferous zone in Venezuela (Jaffe,
1976). Several studies have been concerned with the possibility of
a specific association between the incidence or prevalence of
dental caries and residence in a seleniferous area, urinary
excretion of selenium, or the selenium content of teeth.
Hadjimarkos & Storvick (1950) and Hadjimarkos (1956) noted a
geographical difference in the prevalence of dental caries in 2029
children, between the ages 14 and 16 years, residing in 4 different
counties in Oregon. Two different counties, one with the highest
(Clatsop), and one with the lowest (Klamath), rates of caries
experience were selected for further study. Urine samples from 24
and 29 male children who were attending county seat high schools
and who were born and residing in the respective counties were
analysed for selenium content. The mean levels of urinary-selenium
were 0.049 and 0.037 mg/litre in the groups of children from the
counties with the high (14.4 DMF (decayed, missing, or filled)
teeth per child) and low (9.0 DMF teeth per child) prevalence of
dental caries, respectively (Hadjimarkos et al., 1952).
In a second study, 2 additional counties with similar but high
rates of caries experience (Jackson and Josephine, 13.4 and 14.4
DMF teeth per child, respectively) were selected (Hadjimarkos &
Bonhorst, 1958). Analysis of 33 urine specimens from continuously-
resident, male high school children attending a county seat high
school (Jackson) and 46 specimens from similar children attending a
high school serving a rural area (Josephine) gave values (mean ±
SE) of 0.074 ± 0.007 and 0.076 ± 0.005 mg/litre, respectively. It
was concluded that there was an association between the high
prevalence of dental caries and the values of urinary-selenium
excretion, which were double those seen in the previous study in
the county with a lower rate of caries experience.
In order to explain the variations in urinary-selenium levels
observed in the above studies, samples of locally-produced and -
consumed milk and eggs, as well as local drinking-water samples
were collected from 74 farms located in Klamath, Jackson, and
Josephine counties and analysed for their selenium content
(Hadjimarkos & Bonhorst, 1961). The selenium levels were almost 10
times higher in food samples collected from the counties with high
caries prevalence (Jackson and Josephine) than in those from the
county with low caries prevalance (Klamath). The selenium levels
in the milk samples obtained in Jackson and Josephine counties were
higher than those listed in Table 3.
In another study, Tank & Storvick (1960) determined the extent
of dental caries in a population of children from 15 areas of
Wyoming that had been identified previously as seleniferous or non-
seleniferous on the basis of the geological distribution of
selenium, occurrence of the element in vegetation, and the
occurrence of selenosis in livestock. These research workers
claimed that the dental caries rate in the permanent teeth was
higher in children residing in seleniferous areas of Wyoming than
in children living in non-seleniferous areas of Wyoming, but they
were unable to demonstrate any consistent relationship between
calculated selenium intake and caries or urinary-selenium excretion
and caries.
Suchkov et al. (1973) found that the incidence of caries varied
in 3 different geographical zones (mountainous, pre-mountainous,
and forest-steppe) in the Chernovitsi region of the Ukraine.
Analysis of teeth from people residing in rural areas in these 3
zones and mainly consuming locally-produced foodstuffs revealed a
direct correlation between the selenium content of the teeth and
the incidence of dental caries. The people in the mountainous zone
had the highest level of selenium in the teeth and the greatest
incidence of dental caries, whereas the people in the forest-steppe
zone had the lowest level of selenium in the teeth and smallest
incidence of dental caries. This correlation was true for
deciduous teeth as well as for permanent teeth. However, as
pointed out by the authors, the mountainous zone also had the
softest water and the lowest content of fluorine in the drinking-
water, so that factors other than selenium might have been
involved.
It was not possible to demonstrate any association between the
urinary excretion of selenium and the incidence of dental caries in
subjects living on the South Island of New Zealand, an area known
to be low in selenium (Cadell & Cousins, 1960). However,
Hadjimarkos (1960) claimed that the levels of urinary excretion of
selenium in this study (all less than 0.050 mg/litre) were too low
to be associated with an increased incidence of caries.
As emphasized by Schwarz (1967), the levels of urinary-selenium
excretion reported in the Hadjimarkos studies were within the
limits generally found in the normal population without excessive
selenium intake. It may be recalled from section 7 that, in
experimental animal studies, very high toxic levels were used to
demonstrate the cariogenic effect of selenium.
(b) Reproduction
Possible effects of selenium on reproduction were suspected
following old anecdotal reports from Colombia, South America that
women living in areas, later shown to be seleniferous, gave birth
to malformed babies (Rosenfeld & Beath, 1964), but there is a lack
of reliable studies. Robertson (1970) pointed out that, among 6
women preparing microbiological media containing sodium selenite,
one probable and 4 certain pregnancies all ended in abortions, save
one that went to term. The infant was born with bilateral club
foot. However, no differences in urinary-selenium levels were
observed between this group of women and a control group living in
the same area, and inquiries by the author at other laboratories
carrying out comparable work did not show any evidence of similar
trouble. Jaffe & Velez (1973) could not demonstrate any
correlation between the selenium level in the urine of school
children in different Venezuelan states and the incidence of infant
mortality due to congenital malformations, on the basis of
published public health statistics. Jaffe (1973) concluded that no
recent observations of a teratogenic action of dietary selenium in
human beings had been reported at that time.
(c) Amyotrophic lateral sclerosis
Kilness & Hochberg (1977) reported an unusual cluster of 4
cases of amyotrophic lateral sclerosis (ALS) in male farmers living
in a seleniferous area and indicated that selenium might be an
environmental factor predisposing to the disease. But Schwarz
(1977) pointed out that the frequency of ALS was at least as high
if not higher in areas that were selenium-deficient as in those
with normal or elevated levels and Kurland (1977) suggested that
the cluster was more indicative of a chance occurrence than of a
new etiological lead in ALS. Moreover, Norris & Sang (1978) found
that 19 out of 20 well-established cases of ALS had urinary-
selenium levels lower than the mean for unexposed persons and
therefore concluded that selenium exposure was of no concern in the
average case.
8.1.2. Reports on health effects associated with occupational
exposure
Although the toxicological potential of selenium for human
beings can be inferred from studies carried out with laboratory
animals, certain precautions must be taken in applying the results
of animal studies to the industrial health aspects of selenium.
First, the number of studies dealing with the respiratory exposure
of animals to selenium compounds is limited, and the dose levels
employed have usually been higher than those encountered in
industry. On the other hand, the period of exposure used in the
studies reviewed in section 7.1.2.3 did not exceed one month. The
specific chemical form and physical state of the selenium must be
considered as well as the fact that the chemical form of selenium
may change when in contact with moist mucous membranes or with
sweat. These factors, plus several others, must be taken into
account when considering the health effects of various selenium
compounds under occupational exposure conditions.
The Task Group recognized that, for various reasons, knowledge
on the health effects of industrial exposure to selenium compounds
was not complete. Acute exposures to selenium are the result of
accidents and are, of necessity, described on an ad hoc case study
basis. Regarding the effects of long-term selenium exposure in
industry, there is a lack of epidemiological studies that include
unexposed control groups. Also, no follow-up studies are available
comparing the health status of a sufficiently large number of
workers, previously exposed to selenium, with that of the unexposed
population. Furthermore, the exposure level was not known with
certainty in either acute or long-term studies, and, in some cases,
the form of selenium was not established. In many cases,
simultaneous exposure to other noxious agents occurred.
Nevertheless, the Task Group felt that some preliminary assessment
of the toxicological potential of selenium in industry could be
made on the basis of the occupational experience available with
selenium compounds.
Since Hamilton's first observation on the effects of selenium
exposure in industry in 1917 (Hamilton, 1927 - 34), several hundred
persons have been described in the literature who have been
directly affected by the vapour, fumes, or dust of selenium and its
compounds. In addition, there is a systematic study of a group of
over 100 selenium workers for a period of 2 years (Glover, 1967).
Systematic attention has also been given to the health of workers
exposed to selenium in the USSR (Filatova, 1948; Monaenkova &
Glotova, 1963; Gracianskaja & Kovshilo, 1977). Several reviews
evaluating the health effects of selenium from the point of view of
occupational health have been presented (Glover, 1954 - 70, 1976;
Cooper, 1967; Izraelson et al., 1973; Cooper & Glover, 1974).
8.1.2.1. Fumes and dust of selenium and its compounds
Workers in several industries, such as the production or
recovery of selenium itself, or the manufacture of glass or
rectifiers can be exposed to the fumes and dust of selenium and its
compounds. In addition, direct contact with powders or solutions
containing selenium compounds is possible and the biological
effects of such contact will be discussed in this section.
The chemical form of selenium in the fumes and dusts found in
the above-mentioned industries, consists of elemental selenium plus
various amounts of selenium dioxide, the only oxide of selenium
found in the industrial environment. However, in some cases, the
possible occurrence of other selenium compounds, such as hydrogen
selenide, cannot be excluded. Instances where hydrogen selenide
was the main selenium compound of concern will be discussed
separately in section 8.1.2.1.2.
8.1.2.1.1. Selenium dioxide
Selenium dioxide mainly occurs in industry:
(a) during the production of the compound, usually by the
oxidation of elemental selenium; and
(b) whenever selenium is heated in air, purposely or
accidently, above its melting point. "Selenium fume"
rising under these conditions is a mixture containing
red elemental selenium and about 20 - 80% of selenium
dioxide.
Glover (1954, 1970, 1976) has summarized the acute local,
systemic, and possible long-term effects of occupational exposure
to selenium dioxide. Acute local effects are seen in the lungs,
gastric mucosa, skin, nails, and eyes. Sudden inhalation of large
amounts of selenium dioxide produces pulmonary oedema, due to a
local irritant effect on lung alveoli. Working in an atmosphere
containing selenium dioxide can increase indigestion. Contact with
the skin results in burns or dermatitis. Occasionally, a true
urticarial type of generalized allergic body rash may occur, in
which case the individual must be removed from any work involving
selenium. The selenium dioxide may penetrate under the nails and
cause excruciating pain in the nail beds. If selenium dioxide
enters the eyes, prompt flushing with water will prevent
conjunctivitis. "Rose eye", a pink discoloration of the skin of
the eyelids, which often become puffy, is sometimes seen in persons
who work in an atmosphere of selenium dioxide dust. Systemic
effects of selenium dioxide exposure include garlicky-smelling
breath, metallic taste on the tongue, and indefinite
sociopsychological effects. The garlicky breath is the first and
most characteristic sign and has been used by occupational
hygienists as a way of monitoring exposure, even though the odour
is not always a reliable index in this respect. The metallic taste
is an earlier symptom but, being more subtle, is often overlooked
by workers. Sociopsychological effects such as lassitude and
irritability have also been associated with selenium exposure.
Since the effects of selenium overexposure lead to hepatic damage
in animals, Glover (1954) concluded that it would be prudent to
watch for such damage in human beings.
(a) Reports on health effects connected with short-term
accidental exposures
An industrial incident in a plant engaged in smelting scrap
aluminium and other non-ferrous metals was reported by Clinton
(1947). The aluminium scrap included more than 100 kg of aluminium
rectifier plates coated on one side with metallic selenium overlaid
with a coating of an alloy of bismuth, cadmium, and tin. When an
attempt was made to skim the dross prior to pouring, a cloud of
reddish fume arose. The fumes were intensely irritating to the
eyes, nose, and throat and the plant was evacuated. No workers
were exposed for more than 2 min.
All exposed workers noticed immediate and intense irritation
of the eyes, nose, and throat, and an unpleasant sour, garlic-like
odour to the fumes. The more heavily exposed workers complained of
a severe burning sensation in the nostrils, and dryness of the
throat; followed after 2 - 4 h by severe headache, mainly frontal
in location, lasting until the following day. Several men observed
immediate sneezing, coughing, and headache, followed for 4 - 8 h by
nasal congestion, dizziness, and redness of the eyes. Most of the
men noticed a bad taste in their mouths and an unpleasant odour to
their skin and clothing. Other people, however, did not note any
unpleasant odour in the breath of the exposed workers.
Two labourers who had attempted to skim the dross from the
furnace and therefore underwent intense exposure were hospitalized
for observation. On arrival at the hospital, they complained of
soreness of the eyes and lachrymation, pain in the nose, slight
difficulty in breathing, and frontal headache. Physical
examination on admission revealed conjunctival injection,
congestion of the mucous membranes of the nose and throat, and
oedema of the uvula. One man had a few fine rales audible in the
right base; roentgenological examination of the chest did not
reveal any abnormalities. The temperature, pulse, and respiration
were normal. Both workers were discharged 2 days after the
accident, at which time they were asymptomatic and had no positive
physical findings. Another worker, a foreman, underwent a more
severe exposure than most of the workers. He was not hospitalized,
but, about 8 - 12 h after the accident, he developed severe
dyspnoea accompanied by a slight elevation in temperature, in
addition to a headache and sore throat. Physical examination
revealed fine rales in both bases, as well as scattered asthmatic-
like wheezes throughout the chest. These signs and symptoms
cleared in about 24 h, without specific therapy. All persons
exposed to the fumes had recovered entirely in 3 days, and no
sequelae have been encountered.
In the same year, Lauer (1947) described an episode that
occurred when 15 men were exposed to fumes arising from an
explosion and fire, which occurred in a pot when selenium became
overheated in contact with aluminium. The 2 elements produced a
high-temperature reaction, and fumes and vapours were dispersed
throughout the work area. The lengths of exposure varied, some
victims were using masks part of the time, and others were almost
without protection.
The exposed men reported to the medical dispensary about 15 -
45 min after the accident. All complained of soreness and burning
of the nose and throat, and 8 out of 15 cases had some dyspnoea.
Two of these cases were moderately severe, requiring almost
continuous oxygen therapy. Headache and dizziness and a burning
sensation in the eyes occurred in 6 cases. Three others complained
of substernal burning and tightness of the chest. In 4 there was
nausea and vomiting. All were hospitalized. Physical examination
showed reddening of nasal and pharyngeal mucosa, wheezes, and
musical rales in the lungs (12 cases). These symptoms abated
quickly. In about 10 days, there were few subjective complaints.
All apparently recovered satisfactorily with no known
complications. To assess possible liver affection, Hanger's
cephalin cholesterol tests and Icterus Index determinations were
performed on admission to the hospital and again at the end of the
first, second, third, and seventh week. On the day of admission to
the hospital, Hanger's tests were made on 13 workers, 4 of which
were positive and 9, negative. At the end of the first week
following exposure, there were 10 positives and 3 negatives. At
the end of the second week, there were 11 positives and 3 negatives
out of a group of 14 tested, and the same result was obtained at
the end of the third week. By the end of the seventh week, only 4
of 11 tested were positive. The average value of the Icterus Index
on admission to the hospital was 7.3 units. One week after
exposure, it was 13.5. In 2 weeks, it averaged 20.7. After the
third week, it had fallen to 9.9 and, by the end of the seventh
week, the average Icterus Index was 7.2.
An accident connected with a fire in a selenium rectifier plant
was described by Wilson (1962). Twenty-eight of the employees were
exposed directly to the smoke and fumes containing selenium
dioxide. The length of exposure to the fumes varied with the
individual, but no one was exposed for more than 20 min. Oxygen
was administered by mask to the men lying on the ground, who
experienced bronchial spasm with coughing, gagging, and, in some
instances, transient loss of consciousness.
The initial signs and symptoms were a feeling of constriction
in the chest, accompanied by burning and irritation of the upper
respiratory passages, violent coughing and gagging with nausea and
vomiting, and a bitter acid taste in the mouth. During the acute
episode, there were mild signs of shock, with a drop in blood
pressure and an elevated pulse and respiratory rate. Other
symptoms, experienced during this stage, were burning of the skin,
conjunctivae, and mucous membranes of the upper respiratory
passages. Within 4 h, all patients had apparently recovered from
the acute episode and a recheck of their blood pressure, pulse,
respiratory rate, and general condition was found to be within
normal limits.
Within 6 h of the exposure, the victims began complaining of
secondary symptoms with the onset of generalized chills accompanied
by nausea and vomiting, diarrhoea, malaise, dyspnoea, and headache.
The onset of secondary symptoms was delayed in some of the victims
for several hours after the initial exposure. Within 12 h, all
exposed personnel began experiencing the symptoms described. The
following morning, the first patient, a 30-year-old male, was
admitted to the hospital. He was cyanotic and in moderate
respiratory distress. He complained of chest pain bilaterally,
and was experiencing bronchial spasms. A chest roentgenogram
revealed extensive bilateral consolidation of the lung fields,
indicating pneumonia. The white blood cell count was elevated to
153 000 with a marked prominence of neutrophils. He experienced a
stormy course, requiring constant oxygen for the first 9 days of
hospitalization. By the fifth hospital day, X-ray films revealed a
further extension of the previously noted bilateral pneumonic
consolidation. Two weeks following admission, comparison of a
chest film with previous films revealed marked improvement
bilaterally. He was free of respiratory distress, his white blood
cell count had returned to within normal limits, and he was
discharged home for convalescence. It is interesting to note that
this was the only employee of the 5 hospitalized cases who did not
receive oxygen on the afternoon of the fire. On the same day, the
second victim was admitted to the hospital in a similar dyspnoeic,
cyanotic state with an elevated white blood cell count of 24 600.
X-ray again revealed bilateral atelectasis and consolidation. This
patient required continuous oxygen for 6 days, and was discharged
after 9 days of hospitalization: his white blood cell count had
returned to normal and a repeat roentgenogram revealed improvement.
This was the second most involved and prolonged illness, and he
received only about 6 breaths of oxygen following his exposure.
Three days after the accident, a third employee was admitted to the
hospital in less respiratory distress, with a normal white blood
cell count and a chest film that revealed bilateral elevation of
the diaphram with extensive peribronchial infiltration and some
consolidation at the lung bases. On the same day, another worker
was admitted to the hospital with a normal white blood cell count
and a chest film revealing bilateral increase in lung markings
consistent with bronchitis; however, no consolidation was evident.
The final patient to be hospitalized was admitted 4 days after the
accident with a white cell count of 12 800 and a prominence of
neutrophils. The X-ray film indicated bilateral pneumonia.
Four days after exposure, all other employees with any upper
respiratory complaints were examined. Thirty-two of the 53
employees examined were found to have a residual bronchitis, of
minimal to moderate degree, that required medication. These were
treated on an outpatient basis. Within 1 week, all were
asymptomatic and free of any respiratory signs.
Both Monaenkova & Glotova (1963) and Skornjakova et al. (1969)
have described occupational incidents of short-term selenium
dioxide exposure. In the former case, 6 workers, 20 - 52 years
old, were exposed from 5 to 20 min in a room with a leaky selenium
still. The workers suffered acute pain in the eyes, hoarseness in
the throat, painful cough, and a heavy feeling in the chest. Acute
conjuctivitis, laryngotracheitis, and bronchitis were also noted
and gastroenterocolitis was common to all. Transient fever was
seen in 2 workers. The various signs and symptoms disappeared
within 5 - 7 days, but, on return to work involving selenium, 2
workers developed chronic bronchitis. In the report of acute
selenium dioxide exposure by Skornjakova et al. (1969), there was
irritation of the eyes and mucous membranes, coughing without
expectoration, nasal secretion, headache, vertigo, nausea,
vomiting, garlicky-smelling breath and skin, general weakness, loss
of consciousness, and collapse. Two weeks after exposure, an
allergic whole body rash occurred that disappeared after removal of
the worker from contact with selenium.
(b) Effects of short-term and/or repeated dermal exposure
Selenium dioxide is more than a primary irritant, it causes
extremely painful burns on the skin which, however, always heal
without a scar. Theoretically, the selenium dioxide powder itself
does not burn the skin (and if dropped on to the skin should be
immediately brushed off dry). However, in practice, in the
industrial environment, there is sufficient moisture on the skin
from sweating for this very deliquescent white solid to form a
sticky solution of selenious acid within seconds, or at the most,
minutes, of coming into contact with the skin. Selenious acid is
so soluble that, if the skin is immediately washed with a lot of
water, there will be no burn or even rash from accidental contact.
When the selenious acid does burn the skin, there is little to see
or feel for several hours. After 4 h with a 50% solution, an
unremitting intense pain begins, small petechial areas occur, and a
faintly orange coloration occurs, which indicates reduction of some
of the selenious acid to elemental red selenium. The pain and
necrosis can be prevented by the application of a reducing solution
or ointment, such as 10% sodium thiosulfate (Glover, 1954). If the
burn remains untreated it may go on to ulceration, but this is
rare. Högger & Böhm (1944) described a case of a woman who
suffered pain and reddening of the middle, ring, and little fingers
of one hand when selenium dioxide crystals penetrated into the
protective rubber glove that she was wearing.
The first accurate description of a case of selenium fumes
causing allergic dermatitis was published by Duvoir et al. (1937).
A chemical engineer, who had handled selenium for 3 years in the
past, developed swelling of the face with a hard urticarial oedema
of the nose and cheeks extending on the neck down to his shirt
collar following a 3-day exposure to selenium vapours. There was a
semi-circle of vesicles on the lower lids, but the forehead and
ears escaped completely. His hands showed discrete lesions.
Within 24 h, the genital oedema had disappeared, after 3 days, the
rash started to go, and, by 9 days, there was only desquamation
left. The 5-cm plaque, occurring 1 h after a patch test with 10%
potassium selenite, showed a greater than average contact
sensitivity.
Another case was described (Halter, 1938) involving a glass
worker whose job was to add a mixture of red selenium and sodium
selenite to the glass. This man was in daily contact with selenite
powder for 36 years. He complained of an oedematous erythema of
the face and neck, and several hard infiltrated raised plaques on
the dorsi of the hand and fingers. On examination, his nasal and
laryngeal mucosae were found to be reddened, and there was a very
slight conjunctivitis. There were no changes in the skin of areas
protected by clothing. His only other complaint was of headache.
There were no symptoms or signs referrable to the nervous or
gastrointestinal systems. Selenium was detected in the urine (no
actual values were given). His liver was found to be enlarged and
there was an increase of porphyrins in the urine.
Pringle (1942) has described several cases of acute dermatitis
resulting from contact with dry selenium dioxide or selenium
dioxide dissolved in water, and 2 cases of acutely painful
paronychia after accidental contact with dry selenium dioxide. In
addition, several cases of mild rash on the anterior surface of
both forearms, but principally affecting the bend of the elbows,
were seen among employees working with heated selenium dioxide in
the fume cupboards. Only their fingers, protected at that time
with gloves, entered the fume cupboard, which apparently was
adequately protected. The dermatitis resembled a seborrhoeic
condition and there was frequently a thin watery discharge. In the
course of a year, 2 cases developed a high degree of sensitivity to
selenium dioxide, so that working in the same department, though
not actually on selenium, caused repeated recurrences and they had
to be removed permanently to another part of the factory.
(c) Studies on the health effects of long-term exposure
Filatova (1948) reported that workers exposed over a long
period of time to selenium aerosols containing elemental selenium
at levels of 0.35 - 24.8 mg/m3 and selenium dioxide at levels of
0.11 - 0.78 mg/m3 developed rhinitis, nasal bleeding, headaches,
loss of weight, irritability, and pain in the extremities
(Izraelson et al., 1973).
Conditions of exposure and the related health effects in a
rectifier factory were investigated by Kinnigkeit (1962). Air-
selenium levels were determined in various workplaces and blood-
selenium levels were measured in 62 workers exposed to selenium.
Urinary-selenium levels were also determined in 22 workers. The
reported air-selenium levels did not exceed 0.05 mg/m3 and were
less than the threshold limit value. However, as discussed in
section 5.2.1 there was a clearcut discrepancy between the air
values and the selenium levels observed in blood and urine,
indicating that the actual workers' exposure must have been
considerably higher. Of 62 workers, over half (35) complained of
irritability, sleeplessness, loss of appetite, and nausea. Twenty
six had headaches and 3 had cramplike pains in their limbs.
Clinical examination revealed irritation of the mucosa in 9
workers, with conjunctivitis and slight tracheal bronchitis. Of
the 2 workers that had unavoidable skin contact with selenium, one
had excematous lesions of the forearms and the other had bluish-red
urticarial exanthema.
The Takata reaction and the thymol test were performed on 61 of
these workers, and the results were within the normal range in all
employees, with the remarkable exception of workers engaged in the
electrical testing of the rectifier plates. In this group of 13
people, abnormal results suggesting impaired liver function, were
obtained in 8 workers, the only employees from the plant showing
this pathological reaction. As underlined by Kinnigkeit, workers
carrying out this process handled the plates by hand and were
frequently directly exposed to fumes containing selenium dioxide
arising from the burning out of plates during electrical testing.
As discussed previously, this group was characterized by a very
high mean blood-selenium level (geometrical mean ± SE: 15.8 ± 11.8
mg/litre). A great interindividual variability in selenium-blood
levels existed with this and other groups, but no attempt was made
to relate these individual values to the results of liver function
tests. Another group of workers had blood-selenium levels that
were almost as high (Table 13) (section 6.2.3), but no evidence of
liver dysfunction was observed in this group. However, it cannot
be assumed that the form and pathway of the exposure to selenium
was the same in both groups.
Monaenkova & Glotova (1963) summed up results of clinical
observations on 12 people, aged 30 - 50 years, who had been
occupationally exposed to selenium for 3 - 16 years and who
manifested the symptoms of chronic selenium and selenium dioxide
poisoning. Ten of the 12 persons had been previously engaged in
enterprises producing selenium rectifiers and were exposed to both
elementary selenium and selenium dioxide. The patients complained
of pain in the right hypochondrium, dyspeptic phenomena, undue
fatigability, dyspnoea, weakness, and sleeplessness. Several
patients complained of cough and, in 3 of them, chronic bronchitis
or moderate emphysaema were established. One patient had asthmatic
bronchitis. Almost all patients had various impairments of the
liver and gastrointestinal tract (toxic hepatitis in 6 patients,
dyskinesis of gallbladder in 4 patients, cholecystis in 4 patients,
and spastic colitis in 4 patients). The patients showed an
astheno-vegetative syndrome, pigmentation of exposed areas of the
skin, and, in 8 persons signs of a hyperfunction of the thyroid
gland (radioiodine uptake measurements) were found. Elevation of
the basal metabolic rate or hyperfunction of the thyroid gland were
mentioned also briefly in a previous case report (Halter, 1938) and
in a review (Holstein, 1951), respectively.
A group of selenium workers in a rectifier factory was followed
by Glover (1967, 1970) in 1953 - 56. Every 3 months, urinary-
selenium levels were determined, and the workers were asked about
their health and examined for the presence of garlicky-smelling
breath and skin rashes. Other routine medical checks were not
performed. Workers complained of indigestion and epigastric pain;
severe haematemesis (with hospitalization) occurred in one case.
Several men noticed symptoms of lassitude and irritability, when on
selenium work, which cleared whenever they were taken away from
selenium. A strong odour of garlic on the breath was detected in
most workers with selenium-urinary levels of 0.5 - 1.0 mg litre,
but not below these levels. The breath odour disappeared in 7 - 10
days following the removal of workers from contact with selenium.
Glover (1967, 1970) was able to trace 17 deaths, occurring within
10 years, among selenium-exposed workers in the same factory (Table
52). The author recognized that the list of deaths was not
complete and that the group was too small to permit far-reaching
conclusions. The length of exposure to selenium in individual
cases was not given. Carcinomas arose in various organs: 2
bronchus, 1 stomach, 1 colon, 1 ovary, and 1 testis.
Table 52. Comparison of the certified causes of death of
seventeen selenium workers with the expected distribution
by cause based on the experience throughout England and
Wales
----------------------------------------------------------
International Cause of death Observed Expectedb
list number
----------------------------------------------------------
001 - 019 tuberculosis 0 0.4
140 - 205 malignant neoplasms 6 5.1
330 - 334 vascular lesions 0 1.4
410 - 416 chronic rheumatic 2 0.6
heart disease
420 - 422 arteriosclerotic 3 3.8
heart disease
----------------------------------------------------------
Table 52. (contd.)
----------------------------------------------------------
International Cause of death Observed Expectedb
list number
----------------------------------------------------------
430 - 434 "other" heart disease 0 0.2
440 - 443 hypertensive heart 0 0.2
disease
444 - 447 "other" hypertensive 1 0.2
disease
450 - 456 disease of arteries 0 0.2
480 - 483 influenza 0 0.1
490 - 493 pneumonia 0 0.5
500 - 502 bronchitis 2 0.9
- all other causes 3 3.5
- all causes 17 17.1
----------------------------------------------------------
a From: Glover (1967).
b Taking into consideration the age and sex of those who
died, and the year of death. The Table shows no
evidence of excess mortality in the selenium workers
from any of these main groups of causes.
8.1.2.1.2. Hydrogen selenide
(a) Short-term exposure
There are few cases of occupational poisoning described in
which hydrogen selenide was identified as the sole causative agent.
Reported cases of occupational intoxication by hydrogen selenide
resulted usually from short-term accidental exposures in the
chemical laboratory or industry. Apparently, the first account of
an individual requiring hospital treatment after sudden exposure
to this gas was by Senf (1941), describing the effects of the
accidental laboratory exposure of a chemist to hydrogen selenide.
The patient noticed the characteristic smell and the gas caused her
eyes to weep and her nose to run. Within a few hours, her voice
became hoarse and increasing dyspnoea caused her admission to
hospital. On examination she was found to have a bluish-red
erythema of the skin, conjunctivitis, and injection of the nasal
mucous membrane. The lung oedema, ECG myocardial changes, the dark
red erythema, and porphyrinuria disappeared within 10 days. In
1941, Painter described a case of a single inhalation of hydrogen
selenide. After a brief "metallic" sensation, no ill effects were
felt for about 4 h. Then a copious discharge from the nasal
passages began. This persisted with violent sneezing for 3 or 4
days. No ill effects were noted later.
A case of pure hydrogen selenide poisoning in a works chemist,
producing hydrogen selenide in order to form selenides, was
described by Symanski (1950). Apparently only one inhalation of
the gas was followed by a sudden feeling of constriction in the
chest with coughing, tears, and burning of the nose, all of which
disappeared in a few moments. The patient thought that he had lost
his sense of smell. Four or 5 h later, a severe cough and dyspnoea
developed. At medical examination (8 h after the accident) he was
dyspnoeic and febrile. He continued to cough up blood-stained
frothy sputum all night, and by morning the acute symptoms had
disappeared leaving him apparently with bronchitis and an
irritating cough. A week later he had a recurrence of the fever
with chest pain and the signs of bronchopneumonia. After 4 more
days, the fever disappeared and 9 weeks later he was fit and well
again. A man, 5 h after cleaning out apparatus used for making
hydrogen selenide, developed severe dyspnoea, difficulty in
expiration, painful cough, and yellowish sputum (Bonard & Koralink,
1958). His condition became worse and the next day he was admitted
to the hospital. He was cyanosed, had severe expiratory dyspnoea,
discrete rhinitis and conjunctivitis, and was in considerable
distress. He had no headache or visual troubles. His pharynx was
hyperaemic, his buccal mucosa was dry, and he had a furred tongue.
Four days later, the chest roentgenogram was normal, but lung
function tests were depressed. Rohmer et al. (1950) reported the
case of a chemist exposed to a high level of hydrogen selenide,
calling attention to the development of severe hyperglycaemia that
could only be controlled by increasingly large doses of insulin. A
24-year-old white man accidentally inhaled hydrogen selenide while
transferring the gas from one cylinder to another (Schecter et al.,
1980). He immediately experienced burning in his eyes and throat
followed by coughing and wheezing. He was given oxygen and
improved over a period of 2 h. However, 18 h later, because of
recurrent cough and progressive dyspnoea, he was hospitalized.
Pneumomediastinum developed in this patient and pulmonary function
tests revealed restrictive and obstructive airways disease, which
slowly improved.
Glover (1970), who reviewed 8 cases of acute hydrogen selenide
poisoning (5 laboratory workers and 3 from industrial accidents;
some of them probably identical with those mentioned above), and
summarized the following sequence of events. The first effects
observed after exposure are signs of irritation of the mucous
membranes, i.e., running nose, running eyes, cough, sneeze,
followed by a slight tightness of the chest. This clears, and
there may be a latent period of 6 - 8 h. The patient, who has
often returned home by this time, usually wakes up in bed
breathless with signs and symptoms of pulmonary oedema. Glover, at
the same time, underlined the importance of oxygen therapy in these
intoxications, particularly with regard to the prevention of the
development of pulmonary oedema.
Lazarev & Gadaskina (1977) referred to 5 cases of hydrogen
selenide intoxication at an exposure level of approximately
7 mg/m3. These cases manifested nausea, vomiting, vertigo, and
extreme tiredness, in addition to effects on the respiratory tract
and conjunctiva. The same author mentioned one case of laboratory
exposure to hydrogen selenide in which a chemist developed lung
oedema and long-lasting cyanosis with respiratory difficulties. On
the 22nd day, thrombophlebitis was observed and within 52 days
signs of myocardial damage were noted.
(b) Prolonged and/or repeated exposure
An important point when considering prolonged low-level
exposure to hydrogen selenide was recognized by Dudley & Miller
(1941). In their experience, acute exposure levels of 5 µg/litre
resulted in such eye and nose irritation in workers that the men
could not continue their duties without the protection of a gas
mask. At 1 µg/litre, this irritation did not occur for several
minutes, though the presence of the gas was detected by its odour.
However, continued exposure at these levels results in olfactory
fatigue so that workers lose their ability to smell the gas and are
thus disarmed against subsequent increases in levels of the gas in
the work-place.
The effects of prolonged exposure to hydrogen selenide were
described by Buchan (1947) in workers who were engaged in a process
involving the etching of a pattern on steel stripping. In this
operation, steel stripping is passed between rubber imprinting
wheels with a raised pattern on the periphery. The pattern is in
constant contact with a wick immersed in the etching ink. After
imprinting, the steel stripping passes between felt wipers and dips
into a rust-preventive oil bath passing then to a reel. The odour
of hydrogen selenide was detectable at the etching machine and was
particularly offensive in the sludge of the oil bath. Selenium was
identified, qualitatively, in the sludge. Apparently, the hydrogen
selenide was generated as a result of the reaction of the excess
selenium deposited on the steel stripping in an acid medium, i.e.,
the oil bath, which was further acidified by contamination with the
etching ink carried by the steel stripping. Five out of 25 workers
complained of nausea, vomiting, metallic taste in the mouth,
dizziness, extreme lassitude, and fatigability. The symptoms
lasted 2 weeks and were said to be increasing in severity. Until
one month before the onset of symptoms, an etching ink containing
nitric acid and silver was used, but then a new ink containing
selenious acid at 52 mg selenium/litre was substituted.
Air samples were taken at 6 representative sampling points.
During the analysis, qualitative detection of selenium was made,
but, because of the lack of sensitivity of the titrimetric method
used, it was not possible to measure the selenium quantitatively,
and the results were recorded as less than 0.2 parts per million.
Spot and 24-h specimens of workers' urine contained 0 - 131 µg
selenium/litre or an average of 61.6 µg/litre. So-called control
specimens contained similar amounts, but the controls were the
professional staff collecting the air samples and they had been
exposed to the same environment as the workers for almost 5 h.
Also, it should be realized that hydrogen selenide produces
symptoms at exposures too low to increase the urinary excretion
above normal values. When the selenium ink was replaced by silver
ink, there was a gradual regression of symptoms and, within 6
months, all complaints had ceased and there was no recurrence.
The Task Group was not aware of any other reports dealing with
the prolonged exposure of human beings to hydrogen selenide.
8.1.2.1.3. Selenium oxychloride
Less than 5 µg of pure selenium oxychloride on the skin of the
hand resulted in a painful reaction within a few minutes, exythema
surrounding the central necrotic area (Dudley, 1938). Within 1
month, the skin defect healed with a scar.
8.2. Low Selenium Intake
8.2.1. Evidence supporting the possible essentiality of selenium in
man
Beneficial nutritional effects of selenium have been observed
in several different species (Schwarz, 1976), and specific signs of
selenium deficiency in the presence of adequate intake of vitamin E
have been demonstrated in rats and chicks (section 7.2.1). Thus,
the question of whether selenium is essential for man arises. The
identification of any human disease due to low selenium intake is
difficult, because selenium deficiency in animals is characterized
by a wide variety of signs involving several different organ
systems.
Although a clear-cut pathological condition attributable to
selenium deficiency alone has not yet been demonstrated in human
beings, certain evidence suggests that selenium may be essential
for man.
For example, purified glutathione peroxidase, from human red
blood cells, contains quantities of selenium similar to those found
in the enzyme isolated from animals (Awasthi et al., 1975).
Moreover, selenium is necessary for the optimal growth of human
fibroblasts in purified cell culture media (McKeehan et al., 1976).
Blood-selenium levels are depressed in children suffering from
kwashiorkor (Burk et al., 1967; Levine & Olson, 1970) and Hopkins &
Majaj (1967) obtained a reticulocyte response in malnourished
infants treated with a physiological dose of selenium (25 µg
selenium, as sodium selenite). Finally, on the basis of the
distribution of selenium in various human tissues, Liebscher &
Smith (1968) concluded that selenium must be an essential element
for man.
8.2.2. Signs and symptoms of low intake
The greatest possibility of a hazard due to inadequate selenium
intake would be expected in low-selenium areas. Nutritionists and
public health officials in several countries are aware of the low-
selenium status of their populations and are attempting to identify
any human health problems associated with it. For instance, in
the south island of New Zealand, one of the first regions where a
low selenium status in animals was recognized, some farmers, noting
supposed similarities between their own symptoms and those of the
white muscle disease affecting their livestock, claimed improvement
in their muscular complaints after self-medication with selenium
(Hickey, 1968). However, such anecdotal reports are not supported
by the results of 3 separate trials involving a total of 120
patients suffering from "muscular complaints", carried out in
several low-selenium areas of the south island of New Zealand
(Robinson et al., 198l). In these studies, the patients were given
either sodium selenite or selenomethionine at a number of dose
levels and schedules for various periods of time. Some subjects
also received vitamin E with the selenium supplement. In all
subjects who received selenium, blood-selenium levels and
glutathione peroxidase activity increased, whereas little change
was seen in the control group. Clinical assessment of muscular
symptoms showed that approximately equal numbers of patients in the
test and control groups exhibited an improvement in their muscular
condition. On the basis of these results, the authors concluded
that there was no evidence of any response, under the conditions of
the trials, to selenium supplements for the relief of muscular
complaints.
Total parenteral nutrition (TPN) fluids for intravenous feeding
contain very low levels of selenium (section 4.1.1.1), and patients
sustained by such techniques would seem to be at risk of developing
selenium deficiency. One such patient on TPN in New Zealand
developed muscular discomfort that disappeared after selenium
supplementation (van Rij et al., 1979). This patient was a 37-
year-old female who lived in a rural area of the south island of
New Zealand where the soils were low in selenium and there was a
history of endemic white muscle disease in sheep. She presented
with a perforated small intestine with peritonitis, after
radiotherapy 2 years previously for carcinoma of the cervix. Five
days after abdominal exploration and the start of intensive
antibiotic therapy she developed enterocutaneous and vaginal
fistulae. Gastric stress ulcer followed and necessitated a 1.5-
litre blood transfusion. Elevated temperatures persisted with
intraabdominal sepsis. Ten days after admission to hospital, TPN
was begun and resulted in a general improvement and a 6 kg weight
gain over the next 20 days. Regular plasma and albumin infusions
treated hypoproteinemia. After 20 days of TPN, early clinical
signs of fatty acid deficiency (dry flaky skin on hands and feet)
were noted, which responded rapidly to intralipid infusion. After
30 days of TPN, the patient complained of increasing bilateral
muscular discomfort in her quadriceps and hamstring muscles.
Muscle pain was present at rest as a persistent ache and with
tenderness on palpation. The muscle pain was aggravated by walking
until she found it distressing, even to move beyond her room. On
examination, there was tenderness of the quadriceps, hamstrings,
and less markedly of the calf muscles of both legs. Both active
and passive movements of these muscle groups were painful,
particularly of the hamstrings. The upper limb girdle was
unaffected. A generalized muscle wasting of all the limbs was
observed after the prolonged catabolic stress, despite TPN. No
muscle fasciculation or neurological deficits were observed.
Supplementation with selenium was begun with no other
modification to the patient's management. Each day 100 µg of
selenium, as selenomethionine, was infused intravenously with the
TPN solution. During the next week, muscle pain at rest,
tenderness to palpation, and pain on active and passive movement
disappeared. A return to full mobility followed. This symptomatic
response associated with selenium supplementation plus the
extremely low blood-selenium levels initially observed in this
patient (discussed further in section 8.2.4) led the authors to
conclude that this case could be the first clinical report
supporting the essential role of selenium in human nutrition.
Because of their increased metabolic requirements and faster
growth rates, infants and children might be particularly vulnerable
to selenium deficiency. McKenzie et al. (1978) analysed blood from
230 healthy adults and 83 healthy children from various areas of
New Zealand and found that children in Auckland and Tapanui had
lower blood-selenium concentrations (0.064 and 0.048 mg/litre) than
adults from the same areas (0.083 and 0.060 mg/litre). Red-blood-
cell glutathione peroxidase activity was also lower in Auckland
children than in adults (10.6 versus 12.9 units/g haemoglobin). A
specific population of infants and children that might be
especially at risk regarding low selenium intake includes those who
suffer from phenylketonuria (PKU) and maple syrup urine disease
(MSUD) and consume only special synthetic diets that are very low
in selenium (section 4). McKenzie et al. (1978) reported that the
mean blood-selenium level in 12 such children was 0.038 ± 0.013
mg/litre. One 13-year-old patient had 0.016 mg selenium/litre
whole blood and 0.009 mg selenium/litre plasma, but clinical
examination indicated that he was in good health. Lombeck et al.
(1978) found that the serum-selenium content in 36 children
receiving diet therapy for PKU and MSUD in the Federal Republic of
Germany ranged from 0.007 - 0.028 mg/litre. The selenium content
of the hair was lower in the patients (0.062 mg/kg) than in healthy
children (0.429 mg/kg) and the erythrocyte glutathione peroxidase
activity was reduced in comparison with normal values (4.6 versus
8.8 units/g haemoglobin). And yet all the patients thrived during
the time of observation and did not exhibit any increased rate of
haemolysis or oxidation of haemoglobin to methaemoglobin after
incubation of their erythrocytes with sodium azide.
Gross (1976) studied 4 groups of premature infants who were fed
4 different formulae based on cow's milk containing a high
concentration of polyunsaturated fatty acid (PUFA), with and
without iron, or a low PUFA concentration, with and without iron.
The tocopherol content was the same in all 4 formulae and was
judged adequate in terms of maintaining serum-vitamin E levels.
Both glutathione peroxidase activity and plasma-selenium levels
were similar in all 4 groups. The former declined from 4.2 units/g
haemoglobin at one week of age to 2.7 units/g haemoglobin at 7
weeks of age, whereas the latter declined from 0.08 to 0.035
mg/litre. Although all infants exhibited the anaemia typical of
the prematurely newborn, the decreases in haemoglobin and increases
in reticulocyte levels were greatest in the group of infants given
the formula high in PUFA and iron. These haemolytic events in
vitamin E-sufficient premature infants fed a diet rich in PUFA and
iron were thought to be due to the oxidative stress of the formula
coupled with the poor nutritional status of the infants with regard
to selenium (Gross, 1976).
8.2.3. Dietary levels consistent with good nutrition
8.2.3.1. Quantitative estimates
Since no clear-cut pathological condition attributable to
selenium deficiency alone has yet been observed in man, it is not
possible to define a precise dietary requirement level for human
beings. However, 0.1 - 0.2 mg selenium/kg diet is a nutritionally
generous level for most species of animals (US NAS/NRC, 1971). If
these animal data are extrapolated, a 70-kg man consuming 500 g of
diet per day (dry basis), would need a daily intake of 50 - 100 µg.
The US National Research Council has estimated that the safe and
adequate range of the daily intake of selenium for adults is 50 -
200 µg, with correspondingly lower intakes for infants and children
(Table 53). On this basis, the recommended intake for a 70-kg man
would be equivalent to 0.7 - 2.8 µg/kg body weight per day. Any
daily intake within the recommended range is considered adequate
and safe, but the recommendations do not imply that intakes at the
upper limit of the range are more desirable or beneficial than
those at the lower limit. The lower recommended intake for infants
and children is consistent with the observation that
supplementation of malnourished children with sodium selenite at
30 µg selenium daily, produced weight gain and reticulocyte
responses without any untoward signs (Hopkins & Majaj, 1967).
Table 53. Estimated safe and
adequate range of selenium intakea
-----------------------------------
Group Age Daily selenium
(years) intake (µg)
-----------------------------------
Infants 0 - 0.5 10 - 40
0.5 - 1 20 - 60
Children 1 - 3 20 - 80
4 - 6 30 - 120
7+ 50 - 200
Adults 50 - 200
-----------------------------------
a Adapted from: US NAS/NRC (1980).
Three approaches have now been used to estimate human
nutritional requirements for selenium. Nutritionists have long
used metabolic balance studies to determine the human requirements
for a variety of minerals. In the case of selenium, healthy North
American men needed about 80 µg dietary selenium/day to maintain
balance, but women needed only 57 µg/day (Levander & Morris, 1984).
The difference between men and women in the selenium intake needed
to achieve balance was considered to be due to differences in body
weight. Expressing the balance data on a body weight basis
revealed that both men and women needed about 1 µg selenium per kg
body weight per day to stay in balance. However, other research
groups showed that selenium balance in New Zealand women or Chinese
men could be reached on intakes as low as 27 and 9 µg/day,
respectively (Stewart et al., 1978; Luo et al., 1985). This
demonstrates the great effect of prior dietary-selenium intake on
the amount of selenium needed to achieve balance in people and
shows that balance studies may not be valid techniques for
estimating human selenium requirements.
Yang et al. (1985) estimated human selenium requirements on the
basis of a comparison of dietary intakes in areas with and without
Keshan disease. Dietary-selenium intakes of 7.7 and 6.6 µg/day in
endemic and 19.1 and 13.3 µg/day in non-endemic Keshan disease
areas were reported for adult men and women, respectively. These
estimates should be considered minimum daily adult requirements for
selenium.
The depletion/repletion study is another approach used by
nutritionists to estimate human selenium requirements. The
relatively large body pool of selenium in North Americans prevented
their plasma-selenium levels from dropping to values commonly found
in persons from low-selenium areas (Finland, New Zealand), even
when depleted for almost 7 weeks (Levander et al., 1981a,b).
Chinese men of naturally-low-selenium status (dietary intake about
10 µg/day) were given graded supplements of selenomethionine and
their plasma-glutathione peroxidase activity was followed (Yang et
al., 1985). The activity of the enzyme plateaued at the same level
in all men receiving 30 µg or more of supplementary selenium daily.
From this study, a physiological selenium requirement of about 40
µg/day (diet plus supplement) was suggested for Chinese adult
males, which may require adjustment to account for body weight
differences in other populations including women.
8.2.3.2. Nutritional bioavailability
None of the studies discussed in section 8.2.3.1 have
specifically addressed the question concerning the nutritional
bioavailability of the selenium in foods for human beings. Using
an animal model, Douglass et al. (1981) found that the selenium in
freeze-dried, water-packed canned tuna for human consumption was
only 57% as effective as selenite in restoring liver glutathione
peroxidase activity in rats previously deficient in selenium,
whereas selenium as cooked freeze-dried beef kidney or seleniferous
wheat had 97 and 83% of the activity of selenite, respectively.
The selenium in the tuna was also less effective than selenite in
raising hepatic-selenium levels in the deficient rats. Similar
results concerning the relative bioavailability of the selenium in
tuna compared with wheat were obtained by Alexander et al. (1983)
in rats, using the slope-ratio technique. On the other hand,
Chansler et al. (1983) found that the selenium in mushrooms was
only about 4% as available as that in selenite for restoring
hepatic glutathione peroxidase activity in selenium-depleted rats.
It has been pointed out that, in addition to absorption and
retention, factors such as utilizability within the body are
apparently important in determining selenium bioavailability
(Levander, 1983).
Although data are accumulating on the absorption by human
beings of the selenium in various compounds or in foods (section
6.1.1.2), there have been few studies examining the bioavailability
(i.e., utilization, consisting of transport, conversion to a
metabolically-active form, retention, etc., in addition to
absorption) of the selenium in foods for human beings. One such
study was carried out in a low-selenium area of central Finland
(Levander et al., 1983). Three groups of 10 men of low-selenium
status (mean plasma-selenium level of 70 µg/litre) were
supplemented with 200 µg of selenium, daily, as selenium-rich
wheat, selenium-rich yeast, or sodium selenate, for 11 weeks.
Twenty unsupplemented subjects served as controls. Plasma-selenium
levels increased steadily in the wheat and yeast groups for 11
weeks to around 160 µg/litre with no sign of plateauing, whereas,
in the selenate group, plasma-selenium plateaued at about 110
µg/litre, after 4 weeks. Red blood cell-selenium levels also
increased steadily in the wheat and yeast groups for 11 weeks from
90 to 190 µg/litre, again with no sign of plateauing. Red blood
cell-selenium levels were unaffected in the selenate group.
Platelet glutathione peroxidase activity (glutathione peroxidase is
an index of selenium status) (section 7.2.4.4) roughly doubled
after 4 weeks of supplementation with wheat or selenate and then
plateaued. Platelet glutathione peroxidase increased more slowly
in the yeast group. Plasma-glutathione peroxidase activity did not
respond to selenium supplementation. Ten weeks after the
supplements were stopped, platelet glutathione peroxidase remained
higher in the wheat and yeast groups than in the selenate group.
This suggested that the selenium in yeast or wheat was, to some
extent, deposited in the tissues in a form that could be used later
for glutathione peroxidase biosynthesis, once the dietary
supplement was discontinued. The results of this bioavailability
trial indicated that there are several different aspects to the
nutritional availability of selenium. Complete assessment may
require several measurements including: short-term platelet
glutathione peroxidase activity, to estimate immediate
availability; medium-term plasma-selenium levels, to determine
retention; and long-term platelet glutathione peroxidase activity,
after discontinuation of supplements, to estimate the
convertibility of tissue-selenium stores to metabolically active
selenium.
Griffiths & Thomson (1974) also noted that the blood-selenium
levels of adults from the USA declined rapidly on arrival in New
Zealand, but, after a year, their levels were still higher than the
mean value for permanent New Zealand residents (Fig. 11). When
selenium levels were measured in the whole blood, erythrocytes, and
plasma of postoperative surgical patients receiving TPN in New
Zealand, selenium concentrations decreased as TPN was continued
(Table 54) (van Rij et al., 1979). The plasma-selenium
concentration of the New Zealand TPN patient, discussed in section
8.2.2, was 0.025 mg/litre and fell to 0.009 mg/litre just before
selenium supplementation was begun. The lowest value for blood-
selenium concentration in areas of China not affected by Keshan
disease was around 0.040 mg/litre, while, in affected areas, the
blood-selenium concentration often dropped below 0.010 mg/litre
(Keshan Disease Research Group, 1979b).
 Table 54. Effects of total parenteral nutrition on
the concentration of selenium in whole blood,
erythrocytes, and plasma of postoperative surgical
patients in New Zealanda
------------------------------------------------------
Duration Selenium concentration
of TPN Whole blood Erythrocyte Plasma
(days) (mg/litre) (mg/litre) (mg/litre)
------------------------------------------------------
0 0.050 ± 0.006 0.077 ± 0.008 0.031 ± 0.004
10-20 0.040 ± 0.003 0.060 ± 0.004 0.022 ± 0.002
> 20 0.025 ± 0.003 0.043 ± 0.003 0.015 ± 0.004
------------------------------------------------------
a Adapted from: van Rij et al. (1979).
The urinary excretion of selenium by New Zealand residents is
quite low, reflects their low intake, and is related to whole
blood-selenium levels (Fig. 11). Similar relationships between
24-h urinary-selenium excretion and plasma-selenium concentrations
have been observed in New Zealand residents, New Zealand patients
with TPN, and Swedish patients with TPN (van Rij et al., 1979).
8.2.4. Blood and urine levels typical of low intake
In separate but simultaneous publications, Griffiths & Thomson
(1974) and Watkinson (1974) reported that the mean selenium content
of whole blood from New Zealand subjects was 0.068 and 0.069
mg/litre, respectively. However, even in New Zealand, where the
residents generally have a low selenium status, it is apparently
possible to observe regional differences in blood-selenium levels
(Table 55).
Table 55. Selenium
concentration in whole blood
of human beings residing in
different areas of New Zealanda
-------------------------------
Area of Blood-selenium
New Zealand concentration
(mg/litre)
-------------------------------
Heriot 0.057 ± 0.012
Dunedin 0.062 ± 0.013
Kurow 0.070 ± 0.009
Oamaru 0.074 ± 0.012
-------------------------------
a Adapted from: Griffiths &
Thomson (1974).
8.2.5. Relationship between blood-selenium levels and erythrocyte-
glutathione peroxidase activity
There is an excellent correlation between human whole blood-
selenium concentrations and glutathione peroxidase activity, at
concentrations below about 0.10 mg/litre (Thomson et al., 1977b).
However, above this concentration, activity of the enzyme is not
noticeably increased (Fig. 12), which suggests either that this
concentration of selenium is optimal and that an intake that
maintains this concentration is adequate for function as measured
by glutathione peroxidase activity, or, that above this
concentration, other factors might play a greater role in
influencing glutathione peroxidase activity. Rea et al. (1979)
showed that there was also an excellent correlation between human
erythrocyte-selenium concentrations and whole blood-glutathione
peroxidase activity, as long as the former was less than 0.14
mg/litre (Fig. 13). Schrauzer & White (1978) did not observe any
correlation between glutathione peroxidase activity and selenium
concentrations in the blood of subjects whose blood-selenium levels
were all over 0.10 mg/litre. Moreover, the activity of the enzyme
did not increase after these subjects were supplemented with a
selenized yeast preparation, even though blood-selenium levels
responded to such treatment. Only about 10% of the total selenium
in human red cells is associated with glutathione peroxidase
(Behne & Wolters, 1979) whereas, in sheep red cells, most of the
selenium appears to be associated with the enzyme (Oh et al.,
1976b). Thus, the role of selenium in glutathione peroxidase may
not be the only function of the element. Whether the non-
glutathione peroxidase selenium in human red blood cells truly
represents other functional forms of the element or is merely non-
functional selenium non-specifically incorporated into tissue
proteins cannot be answered at this time. But the usefulness of
the glutathione peroxidase assay as a means of assessing selenium
intake in human beings whose whole blood-selenium concentration
exceeds 0.10 mg/litre currently appears to be an open question.
Table 54. Effects of total parenteral nutrition on
the concentration of selenium in whole blood,
erythrocytes, and plasma of postoperative surgical
patients in New Zealanda
------------------------------------------------------
Duration Selenium concentration
of TPN Whole blood Erythrocyte Plasma
(days) (mg/litre) (mg/litre) (mg/litre)
------------------------------------------------------
0 0.050 ± 0.006 0.077 ± 0.008 0.031 ± 0.004
10-20 0.040 ± 0.003 0.060 ± 0.004 0.022 ± 0.002
> 20 0.025 ± 0.003 0.043 ± 0.003 0.015 ± 0.004
------------------------------------------------------
a Adapted from: van Rij et al. (1979).
The urinary excretion of selenium by New Zealand residents is
quite low, reflects their low intake, and is related to whole
blood-selenium levels (Fig. 11). Similar relationships between
24-h urinary-selenium excretion and plasma-selenium concentrations
have been observed in New Zealand residents, New Zealand patients
with TPN, and Swedish patients with TPN (van Rij et al., 1979).
8.2.4. Blood and urine levels typical of low intake
In separate but simultaneous publications, Griffiths & Thomson
(1974) and Watkinson (1974) reported that the mean selenium content
of whole blood from New Zealand subjects was 0.068 and 0.069
mg/litre, respectively. However, even in New Zealand, where the
residents generally have a low selenium status, it is apparently
possible to observe regional differences in blood-selenium levels
(Table 55).
Table 55. Selenium
concentration in whole blood
of human beings residing in
different areas of New Zealanda
-------------------------------
Area of Blood-selenium
New Zealand concentration
(mg/litre)
-------------------------------
Heriot 0.057 ± 0.012
Dunedin 0.062 ± 0.013
Kurow 0.070 ± 0.009
Oamaru 0.074 ± 0.012
-------------------------------
a Adapted from: Griffiths &
Thomson (1974).
8.2.5. Relationship between blood-selenium levels and erythrocyte-
glutathione peroxidase activity
There is an excellent correlation between human whole blood-
selenium concentrations and glutathione peroxidase activity, at
concentrations below about 0.10 mg/litre (Thomson et al., 1977b).
However, above this concentration, activity of the enzyme is not
noticeably increased (Fig. 12), which suggests either that this
concentration of selenium is optimal and that an intake that
maintains this concentration is adequate for function as measured
by glutathione peroxidase activity, or, that above this
concentration, other factors might play a greater role in
influencing glutathione peroxidase activity. Rea et al. (1979)
showed that there was also an excellent correlation between human
erythrocyte-selenium concentrations and whole blood-glutathione
peroxidase activity, as long as the former was less than 0.14
mg/litre (Fig. 13). Schrauzer & White (1978) did not observe any
correlation between glutathione peroxidase activity and selenium
concentrations in the blood of subjects whose blood-selenium levels
were all over 0.10 mg/litre. Moreover, the activity of the enzyme
did not increase after these subjects were supplemented with a
selenized yeast preparation, even though blood-selenium levels
responded to such treatment. Only about 10% of the total selenium
in human red cells is associated with glutathione peroxidase
(Behne & Wolters, 1979) whereas, in sheep red cells, most of the
selenium appears to be associated with the enzyme (Oh et al.,
1976b). Thus, the role of selenium in glutathione peroxidase may
not be the only function of the element. Whether the non-
glutathione peroxidase selenium in human red blood cells truly
represents other functional forms of the element or is merely non-
functional selenium non-specifically incorporated into tissue
proteins cannot be answered at this time. But the usefulness of
the glutathione peroxidase assay as a means of assessing selenium
intake in human beings whose whole blood-selenium concentration
exceeds 0.10 mg/litre currently appears to be an open question.

 8.2.6. Attempts to associate low selenium intake with human diseases
8.2.6.1. Keshan Disease
Results of research in China have suggested a relationship
between low selenium status and the prevalence of Keshan disease,
an endemic cardiomyopathy that primarily affects children (Keshan
Disease Research Group, 1979a,b). Cases of Keshan disease were
recorded as early as 1907 in Heilongjiang Province of northeastern
China (Gu, 1983). Since the etiology of the disease was not known,
it was named after the locality in which it was originally
observed, Keshan County.
Epidemiologically, the disease exhibits a regional distribution
and occurs in a belt-like zone reaching from northeastern China to
the southwestern part of the country. There is a marked seasonal
fluctuation in the disease with more cases appearing during winter
in the north and during summer in the south. There is also a great
annual variation in the incidence of Keshan disease. Recently, the
incidence has decreased sharply such that from 1978 to 1980 less
than one death was reported per 100 000 members of the population
(Gu, 1983). The overall recent decline in the incidence of Keshan
disease has been attributed, at least in part, to the general
increase in the living standards of the people, such as better
sanitation, more medical attention, and improved quality of the
diet (He, 1979). There is also a shifting of epidemic foci from
year to year. Rural peasants constitute the population at risk
with children below 10 years of age and women of child-bearing age
most susceptible. According to Gu (1983), migrants from non-
affected areas will not contract the disease unless they have lived
in the endemic area for at least 3 months.
The criteria for diagnosing Keshan disease include acute or
chronic cardiac insufficiency, heart enlargement, gallop rhythm,
arrhythmia, and ECG changes (Chen et al., 1980). The disease is
classified into four types: acute (cardiogenic shock), subacute,
chronic (low output pump failure), and latent (normal heart
function but mild enlargement). There is no symptom or sign
specific for identifying the disease (Gu, 1983).
Histopathologically, Keshan disease is characterized by multifocal
necrosis and fibrous replacement of the myocardium.
In the past, many different hypotheses were advanced in an
attempt to explain the etiology of Keshan disease. For example,
there were theories of poisoning due to rhodamine, silica,
digitalis, carbon monoxide, barium, nitrite, and Fusarium
mycotoxins. However, clear-cut proof of these toxicants as a cause
of the disease has not been obtained despite much analytical effort
(Yang et al., 1984). Several nutritional problems have also been
proposed to play a role in Keshan disease such as deficiency of
protein, lipids, thiamin, magnesium, or molybdenum. A hypothesis
concerning Keshan disease etiology was proposed linking the disease
to selenium deficiency, after it was noted that severely endemic
areas coincided with areas where the incidence of enzootic selenium
deficiency diseases in farm animals was also high (Zhu et al.,
1981). On this basis, selenium supplements were used as a
preventive measure for Keshan disease. This hypothesis is very
appealing since cardiomyopathy is a prime feature of selenium-
vitamin E deficiency in many species of animals, including cattle,
sheep, and swine.
Since the initial proposal of the selenium-Keshan disease
connection, much evidence has been gathered in support of this
concept. For example, the average blood-selenium content was 0.021
± 0.001 mg/litre for the affected areas and 0.095 ± 0.088 mg/litre
for non-affected areas (Yang et al., 1984). The average hair-
selenium content was below 0.12 mg/kg in affected areas, whereas
hair-selenium levels in neighbouring but unaffected areas ranged
between 0.12 and 0.2 mg/kg. Average hair-selenium contents in
areas removed from the affected belt were between 0.25 and 0.6
mg/kg. The selenium level in several staple foods (rice, maize,
wheat, soybeans, black beans, and sweet potatoes) was lower in
areas affected with Keshan disease than in unaffected areas. It
was stated that an area could be considered to be unaffected
wherever the selenium content of grains was 0.04 mg/kg or more.
The Chinese workers stated that the amount of selenium needed to
prevent the disease was about 20 µg/day (Yang et al., 1984).
In some affected areas, there were highly localized pockets
(so-called "safety islands") that were free from the disease.
Apparently, these islands were protected because of the higher
selenium content of their crops in the immediate vicinity. For
example, the average selenium contents of rice and soybeans in one
such island were 0.020 and 0.025 mg/kg, respectively, whereas the
values for the corresponding crops in a nearby affected area were
0.0078 and 0.0057 mg/kg. It was also noted that the children in
the unaffected spot liked to catch shrimp from local streams and
that the selenium content of these dried shrimp was as high as 1
mg/kg (Keshan Disease Research Group, 1979b).
These relationships between selenium and Keshan disease led the
Chinese workers to conduct a randomized intervention trial to test
the possible prophylactic effect of selenium against this condition
in the population at risk (i.e., children 1 - 9 years old). In a
trial in 1974, 4510 children took sodium selenite and 3985 children
took the placebo (Table 56). The treated children took 0.5 mg
sodium selenite per week if 1 - 5 years old, or 1.0 mg per week if
6 - 9 years old. The morbidity rate due to Keshan disease was
1.35% in the placebo group (54 cases out of 3985 children) but only
0.22% in the treated group (10/4510). Since a significant
difference was also shown in the 1975 trial (0.95% morbidity rate
in the placebo group compared with 0.1% in those treated) the
placebo groups were abolished in 1976 and 1977. As a result, the
case rate dropped to 0.034% and 0% in these 2 years, respectively.
However, in 1976, there was one case out of 212 children who failed
to take the treatment.
No untoward side effects due to the sodium selenite were
observed, except for some individual cases of nausea, which could
be overcome by taking the medicine after meals. Physical
examinations and liver function tests indicated that the liver was
undamaged after continuous ingestion of the selenium tablets for
3 - 4 years.
Table 56. Effect of selenium on Keshan disease in childrena
-------------------------------------------------------------------
Treatment Year Number Number Outcome of alive cases Death
of of Turned Improved Turned
subjects cases latent chronic
-------------------------------------------------------------------
Placebo 1974 3985 54 16 9 2 27
1975 5445 52 13 10 3 26
Sodium 1974 4510 10 9 0 1 0
selenite 1975 6767 7 6 0 0 1
-------------------------------------------------------------------
a Adapted from: Keshan Disease Research Group (1979a).
Since 1976, more extensive intervention trials with sodium
selenite have been carried out in 5 counties in the same area of
Sichuan Province (Yang et al., 1984). All children, 1 to 12 years
of age, in some of the most severely affected communes, were
treated with selenium as described above, while untreated children
in nearby communes served as controls. The incidence rate of
Keshan disease in the selenium-treated children was lower in each
year of the five-year period than among the untreated children
(Table 57).
Table 57. Keshan disease incidence rates in selenium-treated and
untreated children in five counties of Sichuan provincea
-------------------------------------------------------------------------
Treated children Untreated children
Year Number of Number of Incidence Number of Number of Incidence
subjects cases (per 1000) subjects cases (per 1000)
-------------------------------------------------------------------------
1976 45 515 8 0.17 243 649 448 2.00
1977 67 754 15 0.22 222 944 350 1.57
1978 65 953 10 0.15 220 599 373 1.69
1979 69 910 33 0.47 223 280 300 1.34
1980 74 740 22 0.29 197 096 202 1.07
Total 323 872 88 0.27 1 107 568 1713 1.55
-------------------------------------------------------------------------
a Taken from: Yang et al. (1984).
Selenium intervention has proved to be very effective in the
prophylaxis of Keshan disease and it is very likely that selenium
insufficiency plays an important role in its etiology (Yang et al.,
1984). Nevertheless, the Chinese workers recognized that there
were certain epidemiological characteristics of the disease, which
suggested that additional etiological factors were involved. The
selenium hypothesis, for example, does not adequately explain the
seasonal variation in the disease, the occurrence of epidemic
years, or the annual shifting of epidemic foci. Such
characteristics are more compatible with an infectious theory and,
in fact, a hypothesis that the disease is a form of viral
myocarditis has been put forward (He, 1979). Perhaps the best way
to account for all the characteristics of the disease is to assume
that the disease has a multifactorial etiology and that a
combination of several factors may be involved (Yang et al., 1984).
There are some animal studies that favour such a possibility, since
selenium-deficient mice were less resistant to the cardiotoxic
effects of a Coxsackie B4 virus isolated from a patient with Keshan
disease (Bai et al., 1980). If selenium-deficient human beings are
also less resistant to viral infection, this phenomenon could
provide a reasonable explanation for many of the apparently
conflicting features of Keshan disease. In this view, selenium
deficiency would be the fundamental underlying condition that would
predispose persons to viral attack, possibly by impairing normal
immune function (Yang et al., 1984).
8.2.6.2. Kashin-Beck disease
Kashin-Beck disease is an endemic osteoarthropathy that occurs
in eastern Siberia and in certain parts of China, which is
characterized as a chronic, disabling, and degenerative
osteoarthrosis that mainly involves children (Sokoloff, 1985).
Although the etiology of this disease has not been fully
established, present works show that selenium deficiency might be
one of the main causes. This concept is based on the following
evidence. First, in China most of the endemic areas are located in
the same low-selenium zone, from northeast to southwest, as the
Keshan disease (section 8.2.6.1) (Tan et al., in press). For the
same reason, residents in these areas have low-selenium status
characterized by low blood- and hair-selenium levels, low blood
glutathione peroxidase activity, and low urinary-selenium
excretion. A survey carried out in Heilongjiang province of China
(one of the most heavily affected province) showed that the average
hair-selenium levels in 151 children in endemic areas (0.096 ± SD
0.026 mg/kg) was significantly lower than that of the 235 children
in the non-endemic areas (0.223 ± 0.083 mg/kg) (Wang et al., 1985).
The authors concluded that the low selenium status of children was
due to the low selenium content of the locally produced staple
food. The average selenium content of corn, wheat, and millet in
the endemic and non-endemic areas shown in the paper were: 0.0056
± SD 0.0038 (n = 262) versus 0.015 ± 0.015 (176), 0.0091 ± 0.0096
(225) versus 0.0235 ± 0.022 (120), and 0.0064 ± 0.0053 (14) versus
0.0197 ± 0.0144 (11) mg/kg, respectively. Second, sodium selenite
is reported to have both therapeutic and prophylactic effects on
this disease. Liang (1985) reported that 325 cases of Kashin-Beck
disease in Shaanxi province of China were randomly divided into a
treated and control group. The treated group was given sodium
selenite (1 mg/week for children 3 - 10 years of age and 2 mg/week
for children of 11 - 13 years of age) and the control group was
given a placebo. After one year, X-ray examination of the
metaphyseal changes of fingers showed that 81.9% of the cases in
the treated group had improved, none of the cases were getting
worse, and that 18.1% showed no change, while, in the control
group, only 39.6% of the cases had improved, 30% were getting
worse, and 41.5% showed no change. Li et al. (in press) reported
that children of 1 - 5 and 6 - 10 years of age in an endemic area
of Gansu province in China were supplemented with 0.5 and 1.0 mg
sodium selenite, respectively, per week over a period of 6 years.
X-ray examination showed that the incidence of Kashin-Beck disease
declined from 42% to 4% after the selenium intervention.
However, other information suggests that other factors in the
environment may also play some role in the disease. For example,
the possible role of mycotoxin contamination of cereals by certain
Fusarium strains in China and high phosphate and manganese
contents in the soil, food, and drinking-water in endemic areas of
the USSR have been suggested. More studies are required to clarify
the relationship between these hypotheses and Kashin-Beck disease.
8.2.6.3. Cancer
(a) Ecological studies
Shamberger & Frost (1969) first pointed out the inverse
relationship between selenium levels in forage crops and human
blood, and cancer death rates in various regions of the USA.
Subsequent papers expanded this concept (Shamberger & Willis, 1971;
Shamberger et al., 1976). From a number of comparisons, the
cancers frequently found at one time or another to have high
mortality associated with low-selenium areas or low blood-selenium
levels, included cancer of the tongue, oesophagus, stomach, colon,
rectum, liver, pancreas, larynx, lungs, kidneys, bladder, and
Hodgkin's disease and lymphoma. However, the high mortality of
some of these same cancers and others, was also found to be
associated with high-selenium areas or high blood-selenium levels;
these include cancer of the lung, prostate, pancreas, breast, lip,
skin, eye and dermal melanoma, and leukaemia/aleukaemia. Some of
these studies have been criticized as lacking strength and
consistency, particularly because the states for which cancer
mortality was calculated did not coincide directly with the natural
geographical units on which the estimates of selenium levels in
forage crops were made, thus leading frequently to
misclassification of the different selenium areas (Allaway, 1972,
1978). However, in a study attempting to minimize this problem by
using county data, similar inverse correlations were observed
between counties classified as intermediate or high for selenium
levels in forage, and cancers of the lung, colon, rectum, bladder,
oesophagus, pancreas, and all sites combined, for both males and
females, and cancers of the breast, ovary, and cervix (Clark,
1985).
Schrauzer (1976) found that the mortality rates due to several
cancers, including those of the large intestine, rectum, and breast
(so-called Type A cancers) were directly correlated with the
consumption of meat, eggs, milk, fat, and/or sugar and were
inversely correlated with the consumption of cereals and fish.
Just the opposite correlations were found for certain other cancers
such as hepatic and stomach cancer. Since cereals and seafoods are
good sources of dietary selenium, it was suggested that selenium
might be the factor in these foods that protected against Type A
cancers. Schrauzer et al. (1977) extended these studies to data
from 27 countries including the USA; New Zealand was intentionally
excluded. Dietary intake of selenium was found to be inversely
correlated with total age-adjusted cancer mortality, r = -0.46 ( P <
0.01) for males and r = -0.60 ( P < 0.001) for females. Significant
inverse correlations were observed between dietary-selenium intake
and mortality from cancers of the colon, rectum, prostate, female
breast, ovary, lung (males), and from leukaemia. Weak inverse
relationships were found with mortality from cancers of the
pancreas ( P = 0.06), bladder ( P = 0.1), and skin ( P = 0.1, males).
Other cancers including those of the stomach, oesophagus, and liver
did not show any significant direct or inverse correlations with
dietary-selenium intake. In this study, dietary-selenium intake
was calculated, assuming that the same average concentration of
selenium was present in the foods consumed in all countries (the
Task Group questioned the validity of such an assumption).
Mortality from cancers of the colon, rectum, prostate, lung, skin,
bladder (all Type A), and leukaemia showed significant inverse
correlations with blood-selenium levels in males (USA excluded).
However in the data from the USA, this relationship was not
observed for cancer of the prostate, lung, skin, or bladder, and
leukaemia, and similar inconsistencies were observed for other
cancers and in data for females.
Jansson et al. (1978) postulated an inverse relationship
between dietary-selenium and the rate of colorectal and breast
cancer, but found a direct correlation between the concentration
of selenium in the drinking-water and the rate of colorectal
cancer. Moreover, these workers commented that the same
statistical associations that indicated a protective effect of
dietary selenium against colon, rectum, and breast cancers also
indicated an increased risk of liver and stomach cancer due to
selenium.
In a recent study in China, Yu et al. (1985) obtained a mean
serum-selenium level in the whole blood of 1458 donors from 24
regions, of 107 µg/litre (range 22 - 314 µg/litre). Blood-selenium
levels were inversely correlated with age-adjusted total cancer
mortality for both males and females, r = -0.64 ( P < 0.01) and
r = -0.60 ( P < 0.01), respectively. Analysis by cancer sites
revealed significant negative correlations between blood-selenium
levels and stomach and oesophageal cancers in both sexes. On
reclassifying regions according to low-, moderate-, and high-
selenium areas on the basis of blood levels, significantly lower
total cancer death rates were observed in regions with high
selenium levels and the mortality from cancer of the stomach,
oesophagus, and liver was particularly increased in the low-
selenium areas. In an area where primary liver cancer was very
common, a statistically significant negative correlation between
primary liver cancer incidence and selenium levels in grain was
observed (r = -0.623 for maize, and -0.631 for barley corn). An
inverse correlation between age-adjusted primary liver cancer and
blood-selenium levels of residents in the area was also observed.
The authors concluded that the results indicated that selenium
might play an important role in the etiology of liver cancer, and
that though selenium deficiency was not a cause of primary liver
cancer, low selenium intake apparently reduced the ability of the
body to withstand cancer-causing stress.
It has been pointed out (Levander, 1986) that the age-adjusted
mortality rates for breast cancer and colon cancer reported in
Finland are considerably lower than those reported in the USA,
despite the well-documented lower dietary-selenium intakes in
Finland.
(b) Case-control studies
Shamberger et al. (1973b) reported that blood-selenium levels
in patients with cancer of the colon, pancreas, stomach, and in
Hodgkin's disease and liver metastases were statistically
significantly lower than those in normal controls. However, of 29
patients with rectal cancers, 6 had lower selenium levels than
controls, and 23 had normal levels. Similarly, normal levels were
observed in patients with breast cancer and in patients with other
types of carcinoma.
McConnell et al. (1975), compared blood-selenium levels in 110
patients with carcinomas, 36 patients with primary neoplasm of the
reticuloendothelial system, 28 hospitalized patients with no
malignancy, and 18 non-hospitalized healthy individuals. The mean
selenium concentration for the hospitalized non-malignant patients
was 1.49 ± 0.06 mg/kg and for healthy controls 1.48 ± 0.07 mg/kg.
The mean level of 1.27 ± 0.03 mg/kg for patients with carcinomas
was significantly different from that of the healthy control group
( P = 0.01). The mean serum-selenium level of 1.14 ± 0.08 mg/kg
obtained for gastrointestinal cancer was significantly different
from that of healthy controls ( P < 0.005). In about one-third of
the 110 cancer patients having the lowest selenium levels,
disseminated tumour, recurrences of the primary lesions, the
incidence of multiple primaries, and shortened patient survival
time, were more frequently observed than in the third of the
patients having the highest serum levels ( P > 0.001). The mean
serum-selenium level for the primary malignancies of the
reticuloendothelial system was 1.76 ± 0.24 mg/kg, which though
higher, was not significantly different from that for the healthy
controls.
Broghamer et al. (1976) reported no difference in serum-
selenium levels measured as µg/ml between 110 cancer patients and
controls. However, patients with the lowest serum-selenium levels
had shorter survival, higher incidence of multiple primary
malignancies, higher rate of recurrence of the primary lesion, and
were more likely to have dissemination of cancer than those with
the highest serum-selenium levels. In a study of 59 patients with
primary malignant reticuloendothelial tumours and controls,
Broghamer et al. (1978) found no difference in serum-selenium
levels measured as µg/ml between the two groups. McConnell et al.
(1980) in their study found statistically significant lower levels
of serum-selenium in 35 breast cancer patients compared with a
control group of women free of the disease. On the other hand, van
Rij et al. (1979) and Robinson et al. (1978a) did not find any
differences in the blood-selenium levels of surgical patients with
and without cancer. Although nutritional status, age, and severity
and duration of disease influenced the selenium levels in the
patients studied, low selenium levels were not characteristic for
the cancer patients and it was suggested that the low-selenium
status of cancer patients was more likely a consequence of their
illness rather than the cause of the cancer.
Sundstrom et al. (1984a) found that 44 patients with
gynaecological cancer had lower serum-selenium concentrations (1.15
± 0.04 µmol/litre, P < 0.05) and serum-glutathione peroxidase
activity (404 ± 13 units/litre, P < 0.01) than 56 control subjects
(1.25 ± 0.03 µmol/litre and 444 ± 8 units/litre, respectively). It
was observed that in association with cytotoxic chemotherapy,
selenium alone ( P < 0.05), vitamin E alone ( P < 0.05), and the 2
combined ( P < 0.001) decreased the plasma concentration of lipid
peroxides; the combination of selenium and vitamin E also increased
the activity of serum GSH-Px ( P < 0.01). The authors stated that
during placebo treatment, cytotoxic chemotherapy did not affect
plasma-lipid peroxides but decreased ( P < 0.001) the activity of
GSH-Px. Selenium inhibited this effect. The authors concluded
that this suggested that the anti-oxidative mechanisms of patients
with these types of cancer might be defective and that treatment
with selenium and vitamin E resulted in changes in biochemical
factors related to lipid peroxidation.
In another study, Sundstrom et al. (1984b) reported that
patients with ovarian cancer had significantly lower serum-selenium
concentrations (mean 0.93 ± 0.04 µmol/litre, P < 0.001) than
matched controls (mean 1.22 ± 0.03 µmol/litre). Clinical stage IV
patients had lower levels of selenium (0.82 ± 0.07 µmol/litre) than
clinical stage I and II combined (1.00 ± 0.04 µmol/litre).
Moreover, levels tended to decrease with progressive disease and
increase with remission, probably related to nutrition.
Goodwin et al. (1983) studied blood-selenium levels, and blood
and tissue GSH-Px activity in 50 patients with untreated cancer of
the oral cavity and oropharynx. Mean erythrocyte-selenium and
-glutathione peroxidase were significantly depressed compared with
those in age-matched controls. Mean plasma-selenium, on the other
hand, was significantly elevated in the cancer group. Also,
although not significant, mean erythrocyte-selenium levels tended
to be lower in patients who had never smoked or who had recently
given up smoking. No correlation between dietary selenium as
determined by recall history and plasma- or erythrocyte-selenium
levels in the cancer patients was observed.
Stead et al. (1985) reported significantly lower serum-selenium
concentrations in 20 patients with cystic fibrosis, two of whom had
cancer, than in controls. The two cancer cases had mean serum-
selenium levels of 1.01 µmol/litre and 0.62 µmol/litre,
respectively, compared with 1.41 ± 0.20 µmol/litre in the controls.
Serum-vitamin E levels were also found to be low in patients, but
did not show any correlation with serum-selenium levels.
(c) Case-control studies within prospective studies
As part of the Hypertention Detection Follow-up Programme,
10 940 men and women with diastolic blood pressures of at least 90
mm Hg were identified and enrolled between 1973-74, and followed-up
for 5 years (Willett et al., 1983). Venous blood samples were
collected from all participants at the beginning of the study. A
total of 111 new cases of cancer occurred in the group during the
period of observation. For each case, 2 controls without cancer
were selected who most closely matched the case in age, sex, race,
smoking history, month of blood collection, initial blood pressure,
hypertensive medication, randomisation assignment, and (in women)
parity and menopausal status. Cases and controls were comparable
for most of the confounding factors, although serum-cholesterol and
albumin were slightly lower among cases than controls ( P = 0.05 and
0.06, respectively). Serum-selenium levels did not vary with age
or sex in controls, but black subjects had lower selenium levels
than white. The mean serum-selenium level in cancer cases (0.129 ±
0.002 mg/litre) was significantly lower than that in controls
(0.136 ± 0.002 mg/litre). The P value was 0.02. The increased
risk of cancer in the lowest quintile of baseline selenium was
twice that in the highest quintile (confidence limits 1.1 to 3.3).
Cases were too few to examine by cancer site, but a consistent
trend of lower selenium levels among cases was observed for the
following groups of cancers: lung, breast, prostate,
lymphoma/leukaemia, gastrointestinal cancer, and others. However,
statistically significant differences were observed only for
gastrointestinal cancer. Selenium level remained a significant
predictor of risk, even when the effects of serum-retinol, vitamin
E, and lipid levels, as well as age, sex, and race were taken into
account. On examination of the data according to race, sex, and
smoking status, separately, significant differences in the mean
serum-selenium between cases and controls were observed in blacks
but not whites, in males but not females, and in current smokers,
but not in past-smokers or in persons who had never smoked. The
risk associated with low selenium was greater among those in the
lowest tertile of serum-vitamin E, and a similar inverse
relationship was observed for serum-retinol. A very strong effect
of low selenium (relative risk = 6.2 for lowest versus highest
tertiles) was observed for subjects who had both low serum-vitamin
E and -retinol levels. The authors concluded that, although their
findings supported the overall hypothesis that low selenium intake
increases the risk of cancer, they believed that the observed
differences between cancer sites should be treated as hypotheses to
be tested in other data sets, and that the differences in the
effects of selenium according to age, race, sex, and smoking
status, needed to be examined further (Willett et al., 1983).
Salonen et al. (1984b) identified a cohort of 8113 men and
women in 1972, randomly selected from 2 counties in Finland, who
had no history of cancer in the 12 months preceding this date. The
blood samples were collected from each subject at the beginning of
the study in 1972, and stored at -20 °C. The cohort was followed-
up for cancer occurrence and death up to the end of 1978, during
which time 43 deaths from cancer occurred and an additional 85
persons developed cancer (total cancers = 128). Each cancer case
or cancer death was matched for sex, age, number of cigarettes
smoked and total serum-cholesterol, to a control selected from the
rest of the same population of 8113 persons. In 1983, selenium
concentration was estimated in blood collected from each case and
control at the time of enrolment in the study in 1972 before the
development of cancer. The mean concentration of selenium was
50.5 µg/litre (SD = 12.5) for all 128 cases, and 54.3 µg/litre
(SD = 11.8) for all controls ( P < 0.012). This difference between
cases and control persisted for the following cancer sites:
gastrointestinal, respiratory, and haematological cancers,
miscellaneous cancers, and secondary cancers. No such difference
was observed for skin cancer, skeletal cancers, and urogenital
cancer. Using the lowest (35 µg/litre) and third deciles as cut-
off points, relative risks were computed for the 2 lowest levels of
serum-selenium, with the highest selenium stratum (45 µg/litre) as
the reference. The relative risk of cancer associated with a
serum-selenium level of less than 35 µg/litre was 3.0 and that
associated with a level of 35 - 44 µg/litre was 2.4 (both
statistically significant). The authors concluded that their data
provided additional support for the hypothesis that selenium
deficiency increases the risk of most non-hormone-dependent
cancers in middle-aged persons, though they cautioned that the
number of cancer cases in their study was insufficient to draw
definite conclusions about the effect of selenium for cancers at
specific sites.
Salonen et al. (1985) in a further study of a subgroup of 51
patients, who died from cancer, among the same study population as
above, each matched to a control for age, sex, and smoking,
obtained a mean pre-follow-up serum-selenium concentration in
subjects who died from cancer during the study period of 53.7 ± 1.8
µg/litre and that in controls of 60.9 ± 1.8 µg/litre; the
difference was statistically significant. A statistically
significant difference in the mean serum-selenium concentration
between cases and controls was observed in men ( P = 0.002), but not
in women (half of the male pairs were smokers, but none of the 21
female pairs were smokers). Similarly, the difference in the mean
selenium concentration was significant in smokers ( P = 0.013) but
not significant in non-smokers. Years of smoking showed an inverse
correlation with serum-selenium in the cases (r = -0.30) but not in
controls. Analysis according to cancer site revealed that a
statistically significant difference in serum-selenium levels
between cases and controls was recorded only for respiratory
cancers, though a non-significant difference also existed for
gastrointestinal sites and other cancers. An association between
low serum retinol concentration and increased risk of cancer was
observed only among smoking men. This sex difference was
attributed to smoking as there were no smoking women in the study.
Vitamin E and serum-retinol did not show any association with a
specific cancer site, and although vitamin E had only a weak
independent effect on the risk of cancer, it showed a strong
synergistic relationship with selenium on the risk of fatal cancer.
A serum-selenium concentration of 47 µg/litre or less (the lowest
tertile) was associated with a relative risk of death from cancer
of 5.8 (95% confidence interval 1.2 - 29.0). The authors concluded
that, although their findings indicated that dietary-selenium
deficiency increased the risk of cancer, owing to their study
design, they could not rule out the possibility that the substances
measured in the serum were not truly the protective factors but
were merely indicators of some other compounds or nutrients that
were directly involved in the causal relationship. Furthermore,
the authors recognized that there was need to investigate further
the modification and the confounding of effects by sex, smoking,
and other factors.
8.2.6.4. Heart disease
Using the same ecological approach discussed above for cancer,
Shamberger et al. (1975) concluded that the age-specific death
rates for a number of heart diseases were significantly lower in
the high-selenium regions of the USA than in the low-selenium
regions. However, results of a WHO/IAEA research programme showed
no difference in tissue-selenium concentrations between patients
who died with, or without, myocardial infarction (Masironi & Parr,
1976). Furthermore, Shamberger (1978) found that the kidney-
selenium levels of patients with atherosclerosis and hypertension
did not differ from those of patients with a variety of other
diseases. The blood-selenium levels of patients with acute
myocardial infarction were lower than those of healthy adults, but
there was no difference in heart- or liver-selenium levels of
patients who died from myocardial infarction and those who died
from other diseases (Westermarck, 1977).
A recent case-control study from Finland suggested a possible
association between the serum-selenium level and the risk of death
from acute coronary heart disease as well as the risk of fatal and
non-fatal myocardial infarction (Salonen et al., 1982). The case-
control pairs were derived from 11 000 persons residing in eastern
Finland, an area with a very high incidence of death from
cardiovascular disease. The cases were middle-aged persons who had
died of coronary heart disease or other cardiovascular disease or
suffered a non-fatal myocardial infarction over a 7-year follow-up
period. Attempts were made to control for potential confounding
factors by using controls matched for 6 major coronary heart
disease risk factors: age, sex, serum-cholesterol, diastolic blood
pressure, smoking, and history of angina pectoris, but the cases
had slightly higher blood pressure than the controls. The mean
serum-selenium levels were 51.8 and 55.3 µg/litre for cases and
controls, respectively. A serum-selenium level of less than 45
µg/litre was associated with an increased risk of coronary and
cardiovascular death and myocardial infarction. Although few
necropsies were done, the authors felt that it was unlikely that
the excess cardiovascular mortality observed in their subjects was
due to Keshan disease. The authors cautioned that the apparent
association between low serum-selenium levels and cardiovascular
risk might be spurious since serum-selenium might only be, for
example, a marker for other dietary factors more directly related
to increased coronary heart disease. The authors also emphasized
that, even if their results truly reflected a causal relationship
between low selenium intake and increased ischaemic heart disease,
most such disease is still due to the other well-known risk factors
of elevated cholesterol, high blood pressure, and smoking.
Moreover, it was pointed out that any association between low
serum-selenium levels and ischaemic heart disease is likely to be
of significance only for populations in areas where the dietary
intake of selenium is very low.
In 3 other studies from Finland little or no association was
found between the risk of death from ischemic heart disease and low
selenium status (Miettinen et al., 1983; Salonen et al., 1985;
Virtamo et al., 1985). However, a critique of these studies
(Salonen, 1985) indicated that the first and third were
characterized by low statistical power and in the first the mean
serum-selenium level was relatively high compared with typical
Finnish values (73 µg/litre), probably due to importation of grain
high in selenium during the late 1970s (Mutanen & Koivistoinen,
1983). Salonen (1985) stated that all 4 Finnish studies discussed
above supported the concept of an increased risk of ischemic heart
disease due to low selenium intake as indicated by serum-selenium
levels of less than 60 µg/litre.
In the USA, an inverse correlation was reported between the
plasma-selenium level and the severity of coronaryatherosclerosis
as documented arteriographically (Moore et al., 1984). The mean
plasma-selenium levels of patients with "zero-vessel" disease (no
visible narrowing as much as 50% of any coronary arteriol lumen) or
"three-vessel" disease (as much as a more than 50% narrowing in the
three major coronary arteries or their branches) were 136 ± 7 or
105 ± 4 µg/litre, respectively. On the other hand, neither Ellis
et al. (1984) in the United Kingdom nor Robinson et al. (1983) in
New Zealand were able to demonstrate any correlation between the
traditional risk factors for cardiovascular disease and blood-
selenium levels or glutathione peroxidase activity.
9. EVALUATION OF THE HEALTH RISKS ASSOCIATED WITH EXCESSIVE OR
DEFICIENT SELENIUM EXPOSURE
9.1. The Need to Consider the Essentiality of Selenium in the Health
Risk Evaluation
The data presented in the preceding sections show evidence that
selenium is a functional component of an enzyme (glutathione
peroxidase) in animals and man, and can prevent certain diseases in
animals and responsive conditions in man. The Task Group concluded
that selenium meets the criteria of essentiality for man. As is
true for all essential elements, not only deficient but also
excessive exposure results in adverse health effects.
The effects of selenium deficiency as well as toxicity are well
known in several animal species. In contrast to the effects seen
in animals, health effects in man resulting from deficiency or
excess of selenium are less well defined, but available evidence
has been described. They can occur at low and excessive exposure
levels that may be expected to correspond broadly with those having
deleterious effects on the health of animals. Between these
extremes is a range of safe and adequate exposures (intakes) that
can be defined as free from toxicity and adequate to meet
nutritional requirements. Exposures outside this range increase
the risk of adverse health effects. Therefore, the health risk
evaluation of both selenium deficiency and excess is important.
However, it should be noted that the safe and adequate range may be
modified by certain dietary and other environmental conditions.
The aim of the Task Group was not to evaluate the need for, and
safety of, medication by selenium compounds as a preventive or
therapeutic measure. However, the Task Group felt that some of the
observations presented might be of relevance whenever such a
question might be addressed by any other body responsible for the
appropriate risk-benefit evaluation of administration of selenium
compounds to human beings.
9.2. Pathway of Selenium Exposure for the General Population
Reproducible and accurate methods are available for the
collection of environmental and biological samples and the
measurement of their selenium content. These methods, though they
require special skills and equipment and are time-consuming, have
been employed to assess human selenium exposure. However, the data
are incomplete and there are no data available on selenium exposure
levels for the general population in many countries and regions of
the world.
In spite of these limitations, it can be concluded that, for
the general population, the main source of selenium exposure is
food. In nutritional surveys, extreme mean values for the
calculated selenium intake from food by adult human beings varied
from 11 to 5000 µg/day. However, on the basis of the data
available from most areas, the Task Group concluded that dietary-
selenium intakes usually fall within the range of 20 - 300 µg/day.
Exposure via drinking-water is much less and rarely exceeds a few
µg/day. Limited data on selenium levels in air indicate that about
0.2 µg can be inspired daily by individual members of the general
population.
9.3. Quantitative Assessment of Human Selenium Exposure
9.3.1. Analytical methods for selenium
Several methods of analysis for selenium are available. Those
based on fluorometry, neutron activation analysis, or atomic
absorption spectrometry have been the most thoroughly studied and
used. While simplified and automated procedures have been
developed, all of these methods require skilled analysts to obtain
consistently accurate results. The analysis of NBS Standard
Reference Materials and the exchange of a variety of samples with
another laboratory known to be accomplished in analysis for this
element, should be used to verify the adequacy of results, prior to
any study of human exposure. The method of choice will depend on
the availability of equipment and of the pure chemicals that may be
required, as well as on certain other factors. Where a high degree
of accuracy is required, comparison of results obtained by two or
more different techniques is helpful.
The reliability of the estimate of selenium exposure will
depend, not only on the adequacy of the method, but also on the
adequacy of sampling, storing, subsampling, and preparing the
subsamples for selenium determination. Failure to plan for, and
observe, proper practices in these steps can negate the reliability
of results by even the most highly accurate method of measurement.
9.3.2. Food intake data
Because of the wide variation in the selenium content of foods
in different regions, special techniques must be used to assess
human selenium exposure on the basis of food intake data. The most
accurate method is to determine selenium levels in duplicate diets
(food-on-the-plate-method) made from the same food that is consumed
by the subjects. This method may be suitable for studies in small
subpopulations and where a high degree of accuracy is necessary; it
has the disadvantage of being expensive and time-consuming.
The use of a nutrition survey approach with calculation of
selenium intake from food tables can be used if an important rule
is observed, i.e., the food tables used must be formulated from
data on the selenium contents of the food sources of the population
being studied and of the food as eaten by the subjects under study.
This is important to avoid the errors introduced by the wide
variation in selenium content of a given foodstuff, depending on
its origin.
9.3.3. Blood-selenium
The selenium content of whole blood, serum, or plasma are the
most commonly used measurements of human selenium exposure. Blood
is relatively easy to sample and contamination can be controlled.
Studies on animals have shown that blood-selenium values are a good
indication of selenium deficiency and excess. However, variations
in selenium deficiency signs have been observed in animals with
similar whole blood-selenium levels. This may be due to variations
in the vitamin E content of the diet or exposure to other dietary
or environmental variables. Several factors besides selenium
intake have been identified that may affect blood-selenium content.
Exposure to inorganic mercury or cadmium can lead to deposition of
selenium in the blood attached to protein in combination with the
metal. Human beings exposed to selenium in the form of
selenomethionine or selenium-rich wheat or yeast, for 10 - 11
weeks, had higher blood-selenium levels than human beings exposed
to the same amount of selenium in the form of selenite or selenate.
Blood can be fractionated and plasma-selenium and red blood
cell-selenium can be measured separately. A recent study on the
effects of a low-selenium diet on human beings indicated that a
decrease in plasma-selenium content might occur before a decrease
in red blood cell-selenium can be detected. These data suggest
that it may be possible to use plasma-selenium levels to assess
short-term selenium exposure but that red blood cell- and whole
blood-selenium reflect long-term exposure.
9.3.4. Hair-selenium
Measurement of the selenium content in hair has been used in
animals for the assessment of selenium status, with regard to both
deficiency and excess. It has not been generally adopted for use
in human beings, but, in special circumstances where external
contamination can be excluded, hair-selenium content is useful for
assessing selenium status.
9.3.5. Urine-selenium
The results of animal studies and a few human studies indicate
that the urinary excretion of selenium can be useful for assessing
very recent selenium exposure (i.e., within the past 24 h).
However, determination of selenium in incomplete urine collections
or expression of urinary-selenium per unit volume of urine cannot
provide valid information about selenium exposure in the general
population. An understanding of the reservations associated with
this technique is necessary in its application and then it may only
be useful in conjunction with other measurements discussed in this
section.
9.3.6. Blood-glutathione peroxidase
There is no suitable, simple field method for assessing the
selenium exposure of the general population. The Task Group
concluded that approaches based on the glutathione peroxidase
activity of blood components might provide the basis for a suitable
screening method to detect low human exposure to selenium.
Blood-glutathione peroxidase activity is useful for detecting
selenium deficiency, because it represents a functional form of
selenium and its assay is more rapid than the measurement of
selenium in blood.
However, the Task Group recognized that there are several
difficulties and limitations associated with the determination of
glutathione peroxidase activity. For example, the enzyme is not
stable and hence its activity cannot be determined in samples
stored for periods of time, whereas measurements of selenium can be
made on stored samples. Moreover, large differences in blood-
glutathione peroxidase activity have been reported from different
laboratories using similar methods. The discrepancies can be so
great that interlaboratory comparisons are impossible without
suitable controls. Furthermore, results from New Zealand suggest
that human blood-glutathione peroxidase activity may be useful in
assessing selenium intake at low, but not necessarily at
intermediate or high levels, because the activity of the enzyme is
correlated with whole blood-selenium levels, only when the latter
are less than 0.10 mg/litre. It is not known whether excessive
selenium exposure can increase glutathione peroxidase activity in
human blood, above normal values. Animal studies indicate that
iron deficiency decreases blood glutathione peroxidase activity,
thus possibly further confounding selenium status assessment by
this method.
Human plasma contains glutathione peroxidase but its activity
is very low and difficult to detect and haemolysis during sample
collection should be excluded. Nevertheless, if these reservations
are borne in mind, the technique is promising. Measurement of
platelet-glutathione peroxidase is also a promising technique for
measuring short-term changes in selenium status because of the
short half-life of this blood component.
9.4. Levels of Dietary Selenium Exposure in the General Population
The selenium content of food is highly variable in relation to
several factors. Levels of selenium in soil available for uptake
by plants vary markedly in different locations and this is
reflected in differences in the selenium contents of feeds and
foodstuffs. Countries in which food-stuffs are shipped between
regions tend to avoid extremes in dietary-selenium intake by
averaging foodstuffs with high- and low-selenium contents but some
differences in regional intakes are still observed.
Animal tissues usually do not have as high a selenium content
as the plants in high-selenium areas or as low a selenium content
as plants in low-selenium areas. This is probably because of the
ability of animals to conserve selenium when it is in short supply,
and to excrete it when an excess is present. This may protect
consumers of animal products from extremes of selenium intake.
Available analytical data show that the levels of selenium
typically found in foods, are in the range of 0.4 - 1.5 mg/kg in
liver, kidney, and seafood; 0.1 - 0.4 mg/kg in muscle meats; from
less than 0.1 to over 0.8 mg/kg in cereals or cereal products; less
than 0.1 - 0.3 mg/kg in dairy products; and less than 0.1 mg/kg in
most fruits and vegetables. The very large variation in the
selenium contents of foodstuffs of the same type, depending on
their origin, makes a food table approach to estimating dietary-
selenium intake potentially misleading as discussed in section
9.3.2. More accurate intake estimates could be derived from assay
data on samples of the food items actually being consumed (food-on-
the-plate analysis).
The practice of supplementing the diets of livestock and
poultry in low-selenium areas with selenite or selenate causes
little increase in muscle-selenium content over levels encountered
in animals raised in areas of adequate, but not excessive, selenium
supply. With some exceptions, food processing and preparation
generally do not cause major losses of selenium.
There are few data that indicate the chemical form that
selenium takes in normal human foods. One available study of
seleniferous wheat has shown that a significant fraction of the
selenium is in the form of selenomethionine.
Various forms of selenium differ in their nutritional
availability. The absorption of selenium from foods appears quite
efficient (about 80%).
Daily selenium intake can be markedly influenced by food
consumption patterns. For example, in many countries, consumption
of large amounts of fish, kidney, or liver could raise an
individual's selenium intake substantially above the highest daily
intake shown in Table 8.
The greatest extremes in dietary-selenium intake have been
reported in areas in which the diet was monotonous and consisted
largely of locally-produced staple food.
The wide range of geographically-related selenium intake due to
variations in the selenium contents of the diet is reflected in the
wide range of selenium levels observed in human whole blood.
The extreme mean blood-selenium values reported for groups
dependent on locally-grown foods of different selenium content
ranged from 0.02 - 3.2 mg/litre (Table 11). Limited studies from
New Zealand have shown that children and older individuals had
lower whole blood-selenium levels, but it is not known whether
these lower levels are due to low dietary intakes or to changes
connected with growth and/or aging.
9.5. Evaluation of Health Risks - General Population
9.5.1. Predictive value of animal studies
The Task Group concluded that, in view of the still fragmentary
data concerning the possible health effects of either deficient or
excess selenium exposure on human beings, any evaluation of the
human health effects of selenium exposure must take into account
results arising from animal studies. For this purpose, these can
be summarized as follows:
(a) Selenium deficiency combined with concurrent low
vitamin E status has resulted in deleterious effects
in all animal species tested so far (mice, rats,
chicks, ducks, swine, sheep, cattle, and monkeys).
In rats, specific signs of selenium deficiency have
been produced in animals fed diets adequate in
vitamin E.
(b) Acute and chronic selenium toxicity have been
demonstrated in a wide variety of species, under a
wide variety of conditions (section 7.1.2). In
evaluating the toxic effects of different selenium
compounds, the Task Group felt that it was useful to
distinguish between effects that are strictly
dependent on the selenium in the molecule and cannot
be duplicated by, e.g., homologous compounds of
sulfur, and compounds where the toxicity is, in
principle, similar for homologues containing either
selenium or sulfur. In addition, the Task Group
recognized that the similarity of some of the effects
of different selenium compounds could be related to
the formation of certain common intermediates.
(c) Dose-response relationships have been demonstrated in
acute and chronic toxicity, as well as in selenium
deficiency. Comparative studies have shown that acute
toxicity is similar in parenteral and oral exposure
(section 7.1.2.1), which is in good agreement with the
recognized high absorbability of selenium compounds
from the gastrointestinal tract (section 6.1.1).
(d) As indicated in section 7.1.2.2, the borderline
level of dietary selenium needed to cause growth
depression, due to overt chronic selenium toxicity in
rats, is in the range of 4 - 5 mg selenium/kg diet.
However, the dietary level of selenium needed to
cause chronic toxicity can be influenced by several
environmental factors such as previous selenium
intake (section 7.1.6).
(e) The effects of various environmental factors on
selenium dose-response relationships is even more
dramatically illustrated in situations of low-
selenium intake leading to deficiency. One of the
primary factors influencing the nutritional
requirements of animals for selenium is the vitamin E
status. The minimum dietary level of selenium needed
to prevent deficiency diseases in various animal
species is in the range of 0.02 - 0.05 mg/kg diet.
(f) Animal diseases associated with both high- and
low-selenium intake have been reported in certain
areas of the world in which the animals were
consuming feeds primarily of local origin.
The possibility of a human disease due to selenium deficiency
or excess should be looked for under similar conditions of
restricted dietary consumption (monotonous diets based on locally
grown foods). In addition, the Task Group concluded that, in view
of the dependence of selenium deficiency or toxicity effects on
various environmental factors, the possible involvement of selenium
in human diseases of multifactorial etiopathogenesis deserves
special attention.
9.5.2. Studies on high-exposure effects in the general population
Studies on this type of exposure are few and, as they are
confined to subpopulations in high-selenium areas, some do not
include comparison groups. Symptoms and signs of illness elicited
are frequently mild and not clearly related to selenium. In
practically all studies (section 8.1.1.1), nail pathology was
reported and, in several studies, hair loss and increased dental
decay. Some of the studies included reports of gastrointestinal
disturbances and icteroid skin. Other possible causes of illness
in these studies cannot be excluded and the dependence of severity
of effects on gradation of selenium exposure cannot be evaluated.
Examination of exposed individuals showed increased levels of
selenium in the blood and urine.
Three studies on populations living in seleniferous areas and
dependent on locally-produced food deserve particular attention.
Examination of individuals exposed in these areas revealed highly
increased levels of selenium in the blood and/or urine. A study
from South Dakota reported various signs and symptoms in people
with long-term overexposure to selenium, as revealed by elevated
urinary-selenium excretion. In these areas, farm animals were
affected by chronic selenium poisoning.
In a seleniferous zone of Venezuela (Villa Bruzual) 111
children (average blood-selenium level = 0.813 mg/litre) were
studied and compared with 50 children (blood-selenium level = 0.355
mg/litre) not over-exposed to selenium (Caracas). The children
from the seleniferous area had some loss of hair and some
abnormalities of skin and nails; the authors noted some differences
in socio-economic factors between the 2 groups.
In a recent report from China, past incidents of intoxication
were described that were thought to be due to chronic selenium
poisoning, with hair loss and nail pathology as the most common
signs. No quantitative data are available from the period 1961 -
64, which was the time of peak prevalence of the intoxication.
Recent diet samples collected from high-selenium areas with, and
without, a history of this intoxication showed mean daily selenium
intakes of about 5 and 0.75 mg, respectively (Table 7). Blood
samples taken from the same areas had mean selenium levels of 3.2
and 0.44 mg/litre, respectively (Table 11).
The above studies of populations ingesting high selenium levels
in Venezuela and China have not revealed abnormal serum
transaminase activity in the subjects concerned. In individuals
exposed to high levels of selenium in the recent outbreak in the
USA; there was no evidence of impaired liver function, but loss of
hair and pathological changes in the nails were observed, as well
as some other signs and symptoms described in more detail in
section 8.1.1.1. However, the Task Group recognized that this is
insufficient evidence to exclude the occurrence of liver damage and
recognized the need for more thorough evaluation of the liver in
persons with high selenium exposure.
The Task group noted that the signs and symptoms of selenium
overexposure in human beings were not well defined. However the
Task Group was aware of 3 situations concerning elevated selenium
exposure, which could form the basis for estimating a dose-response
to high levels of selenium. In one area of Enshi county in China,
blood-selenium levels in adults of 0.44 mg/litre were associated
with no reported effects. In Venezuelan children with blood-
selenium levels of 0.813 mg/litre, hair and nail changes of an
unspecified nature and incidence were reported. In another area of
Enshi county in China, definite hair and nail changes and symptoms
and signs consistent with neuropathy were reported in adults with
blood levels of 3.2 mg/litre. The Task Group, however, could not
be certain of how signs and symptoms were searched for, how their
absence was ascertained, and how the control populations were
selected in these studies.
9.5.3. Studies on low-exposure effects in the general population
Very low blood-selenium levels observed in human subjects
(section 8.2.4), approach those observed in animals with selenium
responsive diseases. One woman from a general population known to
have a low exposure to selenium and maintained on total parenteral
nutrition, which provided less than 1 µg selenium/day, had muscular
pain and dysfunction that responded to selenium supplementation.
However, similar symptoms have not been observed in other patients
with similar low blood-selenium levels. Also, in another study, no
muscular symptomatology was reported in children fed exclusively
special medical diets containing very low levels of selenium, for
long periods of time. On the other hand, the Task Group assigned a
high significance to recent reports describing an association
between Keshan Disease (section 9.5.4.1) and poor selenium status
as indicated by low selenium content of grain, low blood and hair
levels of selenium, low blood-glutathione peroxidase activity, as
well as a positive response to an intervention study with sodium
selenite. As discussed in section 9.5.1, the results of several
experimental animal studies indicate that many different factors
may contribute to the development of selenium deficiency diseases.
These studies point to the conclusion that a lack of selenium may
be only one of several causative factors responsible for the
occurrence of certain diseases of complex multiple
etiopathogenesis. Such a conclusion was also fully recognized in
the reports from China on the Keshan Disease.
9.5.4. Evaluation of the involvement of selenium in human diseases
of multiple etiopathogenesis
9.5.4.1. Keshan disease
A suitable animal model of the Keshan disease is not available,
even though heart damage is a feature of combined vitamin E and
selenium deficiency in several species of animals.
The ecological evidence strongly favours a relationship between
low selenium status and the incidence of Keshan disease. Such
evidence includes low blood-, hair-, and urine-selenium levels in
the affected areas as well as a low selenium content in the staple
foods raised and consumed in the affected areas. A randomized
intervention trial carried out in China showed that children who
received sodium selenite had a lower incidence of Keshan disease
than those who received a placebo. However, in this randomized
trial there may have been an underlying trend in the placebo group
towards a decline in the incidence of the disease. Also,
information on the effectiveness of the randomization was not
available and the incidence rates were not adjusted for age. The
results of the much larger but non-randomized intervention trial
involving at least a quarter of a million children on a yearly
basis indicated a clear-cut beneficial effect of sodium selenite in
the prevention of the Keshan disease. Thus, the Task Group
concluded that Keshan disease is a condition in human beings that
is related to low selenium status but that additional research is
needed to clarify the role of other factors in the etiopathogenesis
of the disease.
The Task Group also recognized that in section 8.2.3.1 this
disease was used as the basis for estimating a minimal human
nutritional selenium requirement, suggested by the authors to be 19
and 14 µg/day for men and women, respectively.
9.5.4.2. Kashin-Beck disease
An additional disease found in certain low-selenium areas of
China is an endemic osteoarthropathy known as Kashin-Beck disease
that occurs mainly in children. There is some evidence linking low
selenium status with the incidence of Kashin-Beck disease, but the
Task Group concluded that additional research, particularly in
relation to intervention studies, is required before a definite
statement can be made concerning the role of selenium in the
etiology of the disease.
9.5.4.3. Ischaemic heart disease
The Task Group was aware of studies that examined the
possibility of an association between low selenium status and
myocardial ischaemia and atherosclerosis. However, because of the
limited data available, the Task Group was not prepared to come to
any conclusion regarding the role of selenium in ischaemic heart
disease.
9.5.4.4. Studies on the involvement of selenium in cancer
(a) Carcinogenicity studies
The Task Group concluded that the studies described in section
7.7 suggesting a carcinogenic effect of selenite or selenate were
invalid, because, in one study, the results were statistically
insignificant and, in the other studies, there were no controls or
systematic pathological examinations. In another systematic study,
no differences were observed in the incidence of tumours in rats
surviving 2 years or longer and exposed to 0.5 - 2.0 mg selenium/kg
diet.
The Task Group was aware of one study that demonstrated the
carcinogenicity of ethyl selenac (selenium diethyldithiocarbamate)
and another that showed the carcinogenicity of selenium sulfide,
given by gavage. The latter result is of possible interest both as
regards human dermal exposure to selenium sulfide and/or its
possible formation within the body, but the Task Group was not
aware of any studies demonstrating carcinogenic effects of other
selenium compounds.
The Task Group felt that the evaluation published by IARC in
1975 was still valid in concluding that the available animal data
were insufficient to allow an evaluation of the carcinogenicity of
selenium compounds (IARC, 1975).
(b) Experimental evidence of anticarcinogenic effects of
selenium compounds
The results of studies on laboratory animals provide evidence
of a preventive effect of selenium dioxide, sodium selenite, or
"selenized" yeast, given in the food or drinking-water, against
chemically-induced cancers or certain spontaneous, presumably
viral-induced cancers (section 7.7). Generally, the level required
to demonstrate such effects ranges from 1 - 6 mg/kg food or per
litre drinking-water and thus is considerably in excess of the
animal's nutritional needs. In one report, selenium dioxide at
0.1 mg selenium/litre water had some beneficial effect against
spontaneous mammary tumours in mice, but, in this case, the diet
itself contained 0.45 mg selenium/kg. Other studies have shown the
beneficial effects of sodium selenide when applied concomitantly to
the skin with certain carcinogens. In rats fed diets high in
polyunsaturated fats, selenium deficiency increased the incidence
of mammary tumours, after treatment with dimethylbenz[alpha]
anthracene. Therefore, the Task Group concluded that
pharmacological levels of selenium compounds exhibit in many cases
a favourable influence against the development of cancer in various
animal model systems. However, the Task Group was aware of one
study in which administration of high levels of selenium was
associated with an increased incidence of chemically-induced
cancer.
(c) Epidemiological evidence regarding the possible anti-
carcinogenic effects of selenium in human beings
The Task Group acknowledged that apparent negative correlations
have been drawn between cancer death rates and certain general
population characteristics, such as blood-selenium levels or the
average level of selenium in diets in specific geographical zones
(section 8.2.6.1). However, these apparent correlations have not
been consistent with specific cancer sites and are subject to
ecological fallacy. Moreover, such epidemiological data are
subject to question regarding the adequacy of sampling, the
interpretation of blood-selenium data, and the precision of the
estimates of dietary-selenium intake in various countries and
different geographical areas within the USA.
Of the 7 case-control studies examined by the Task Group, no
consistent association was apparent between low selenium status and
risk of cancer. For a given cancer site, there were few replicate
studies. On the majority of occasions, no association was found
between low selenium status and cancer risk. In fact, in at least
one situation a positive correlation was found. In several
countries, low blood- or plasma-selenium levels were found in
patients with several different types of cancer. However, the
effect of the malignant disease itself in lowering blood-selenium
levels (e.g., by decreasing absorption and/or worsening the
generally debilitated nutritional state of the patient) cannot be
excluded as a partial or total explanation for these results.
The Task Group was aware of 3 studies in which blood samples
had been taken for other purposes prior to the diagnosis of any
cancer. Some time later, the samples were retrieved from storage
banks and analysed for selenium. All 3 studies showed a consistent
inverse association between the prediagnostic serum-selenium level
and the risk of cancer. This association between low serum-
selenium level and cancer risk was observed only in smokers, only
in males, and in one study only in blacks but not in whites. In
one study, the association between low selenium level and cancer
risk showed some site specificity, but the second and third studies
were unable to confirm or rebut this because of inadequate sample
size.
The Task Group was aware that in many studies showing a
protective effect of selenium against cancer in animals
pharmacological doses of selenium compounds were used. Therefore,
there were difficulties in relating the animal and human studies,
but the Task Group was aware of an intervention trial being carried
out in China which it is hoped will enable a more precise
evaluation of the association between selenium status and human
cancer risk in the future.
Thus, the Task Group concluded that existing data are
insufficient to determine whether the level of selenium intake is
indeed correlated with the incidence of cancer in man.
9.5.4.5. Caries
Although an association between high selenium intake and dental
caries has been reported in at least one animal study, there is no
clear-cut evidence of such an association in man. The Task Group
concluded that difficulties associated with the interpretation of
urinary-selenium excretion, expressed as mass-concentration per
volume and not expressed as total daily urinary excretion of
selenium (section 9.3.5), as well as the inability to exclude
interference by other environmental factors such as fluoride,
precluded any significant conclusions. A better understanding of
the impact of selenium on the incidence of caries will require a
more comprehensive estimation of selenium exposure and of the other
confounding factors in the respective subpopulations under study.
9.5.4.6. Health effects related to reproduction
The Task Group concluded that at present there is no evidence
that selenium has significant effects on reproductive function in
man.
9.6. Occupational Exposure
In contrast to general population exposure, occupational
exposure usually occurs through direct contact and/or through
inhalation, i.e., dermal and respiratory exposure predominate.
Exposure to selenium and its compounds occurs in primary
industries, i.e., those that extract, mine, treat, or process
selenium-bearing minerals, e.g., copper, zinc, or lead ores and in
secondary industries, i.e., those that use selenium in
manufacturing processes. The physical and chemical form of
selenium under these circumstances varies and is determined by the
industrial processes. The Task Group recognized that industrial
exposure has not been adequately studied with respect to levels of
exposure and there is a need for such studies to be carried out.
For acute occupational selenium exposures, the effects vary
according to to the chemical form of selenium. In contrast to
elemental selenium, which does not appear to be toxic unless
oxidized or reduced, hydrogen selenide and selenium dioxide are
highly toxic, causing irritation of the respiratory tract, which
may be followed by pulmonary oedema.
The Task Group recognized that evaluation of long-term
occupational exposure to selenium must take into account other
dietary and environmental substances because of their recognized or
potential interactions with selenium. An additional factor that
needs to be considered is the fact that preventive measures usually
result in the removal of exposed workers with the appearance of the
first sign of selenium over-exposure (garlic-like odour).
Monitoring of selenium in the urine is also used to identify those
who should be removed from further overexposure.
The Task Group concluded that no studies were available on the
dose-response relationship with regard to occupational exposure to
selenium.
REFERENCES
ABDULLA, M., KOLAR, K., & SVENSSON, S. (1979) Selenium.
Scand. J. Gastroenterol., 14(suppl. 52): 181-184.
ABDULLAEV, G.M. (1976) [Selenium levels in healthy subjects
and those with certain haematological diseases.] In: Selen v
biologii, Vol. 1, Baku, Elm Publishing House, pp. 136-139 (in
Russian).
ABDULLAEV, G.B., GADZHIEVA, N.A., GASANOV, G.G., DZHAFAROV,
A.I., & PERELYGIN, V.V. (1972) [Selenium and vision,] Baku,
Elm Publishing House, 50 pp (in Russian).
ABDULLAEV, G.B., GADZHIEVA, N.A., GASANOV, G.G., MAMEDOVA,
S.A., DMITRENKO, A.I., GULIEVA, L.I., & RODIONOV, V.P.
(1974a) [The effect of selenium compounds on electroretino-
gram under different experimental conditions.] In: [Selen v
biologii,] Baku, Elm Publishing House, pp. 11-22 (in Russian).
ABDULLAEV, G.B., MAMEDOV, SH.V., DZHAFAROV, A.I., PERELYGIN,
V.V., & MAGOMEDOV, N.M. (1974b) [Possible regulation of free
radicals in the retina by selenium compounds.] In: [Selen v
biologii,] Baku, Elm Publishing House, pp. 54-57 (in Russian).
ABU-ERREISH, G.M. (1967) On the nature of some selenium
losses from soils and waters, Brookings, South Dakota, South
Dakota State University (MS Thesis).
ABU-ERREISH, G.M., WHITEHEAD, E.I., & OLSON, O.E. (1968)
Evolution of volatile selenium from soils. Soil Sci., 106:
415-420.
ABUTALYBOV, M.G., VEZIROVA, N.B., FATALIEVA, C.M., KHALILOV,
E.KH., & MUSAEV, S.G. (1976) [Selenium content in some bean
plants of Azerbajdzhan.] In: [Selen v biologii,] Vol. 2, Baku,
Elm Publishing House, pp. 140-142 (in Russian).
ACGIH (1971) Documentation of the threshold limit values for
substances in workroom air, 3rd ed., Cincinnati, Ohio,
American Conference of Governmental Industrial Hygienists,
pp. 224-225.
AKESSON, B. (1985) Plasma selenium in patients with abnormal
plasma protein patterns. In: Proceedings of the XIII Inter-
national Congress of Nutrition, p. 152 (Abstract).
ALEXANDER, A.R., WHANGER, P.D., & MILLER, L.T. (1983)
Bioavailability to rats of selenium in various tuna and wheat
products. J. Nutr., 113(1): 196-204.
ALCINO, J.F. & KOWALD, J.A. (1973) Analytical methods. In:
Klayman, D.L. & Gunther, W.H.H., ed. Organic selenium
compounds, their chemistry and biology, New York, John Wiley
and Sons, pp. 1049-1081.
ALLAWAY, W.H. (1972) An overview of distribution patterns of
trace elements in soils and plants. Ann. NY Acad. Sci., 199:
17-25.
ALLAWAY, W.H. (1973) Selenium in the food chain. The Cornell
Veterinarian, 63: 151-170.
ALLAWAY, W.H. (1978) Perspectives on trace elements in soil
and human health. In: Hemphill, D.D., ed. Trace substances in
environmental health. XII, Columbia, Missouri, University of
Missouri Press, pp. 3-10.
ALLAWAY, W.H. & CARY, E.E. (1964) Determination of
submicrogram amounts of selenium in biological materials.
Anal. Chem., 36: 1359-1362.
ALLAWAY, W.H., MOORE, D.P., OLDFIELD, J.E., & MUTH, O.H.
(1966) Movement of physiological levels of selenium from
soils through plants to animals. J. Nutr., 88: 411-4l8.
ALLAWAY, W.H., CARY, E.E., & EHLIG, C.F. (1967) The cycling
of low levels of selenium in soils, plants, and animals. In:
Muth, O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 273-296.
ALLAWAY, W.H., KUBOTA, J., LOSEE, F., & ROTH, M. (1968)
Selenium, molybdenum, and vanadium in human blood. Arch.
environ. Health, 16: 342-348.
AMOR, A.J. & PRINGLE, P. (1945) A review of selenium as an
industrial hazard. Bull. Hyg., 20: 239-241.
ANCIZAR-SORDO, J. (1947) Occurrence of selenium in soils and
plants of Columbia, South America. Soil Sci., 63: 437.
ANDERSON, R.A. & POLANSKY, M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Trace Elem. Res., 3: 1-5.
ANDREWS, R.W. & JOHNSON, D.C. (1976) Determination of
selenium (IV) by anodic stripping voltammetry in flow system
with ion exchange separation. Anal. Chem., 48: 1056-1060.
ANONYMOUS (1962) Selenium poisons Indians. Sci. News Lett.,
81: 254.
ANONYMOUS (1975) New links in the selenium cycle. Agric.
Res., 24: 6-7.
ANONYMOUS (1984) Washington DC, US Food and Drug
Administration, pp. 19 (FDA Bulletin No. 14).
ANUNDI, I., STAHL, A., & HOGBERG, J. (1984) Chem.-biol.
Interact., 50: 277.
AOAC (1975) Official methods of analysis, 12th ed.,
Washington DC, Association of Official Analytical Chemists.
ARTHUR, D. (1972) Selenium content of Canadian foods. Can.
Inst. Food Sci. Technol. J., 5: 165-169.
ASHER, C.J., BUTLER, G.W., & PETERSON, P.J. (1977) Selenium
transport in root systems of tomato. J. exp. Bot., 28: 279-291.
AWASTHI, Y.C., BEUTLER, E., & SRIVASTAVA, S.K. (1975)
Purification and properties of human erythrocyte glutathione
peroxidase. J. biol. Chem., 250: 5144-5149.
BACHAREV, V.D., BOCHAROVA, M.A., & SHOSTAK, V.I. (1975) [On
the effect of selenium on the light perception of the eye.]
Fiziol. Zhurn., 61(1): 150-153 (in Russian).
BAI, J., WU, S.Q., GE, K.Y., DENG, X.J., & SU, C.Q. (1980)
The combined effect of selenium deficiency and viral infection
on the myocardium of mice (preliminary study). Acta Acad. Med.
Sinicae, 2: 29-31.
BAIRD, R.B., POURIAN, S., & GABRIELIAN, S.M. (1972) Determination
of trace amounts of selenium in waste waters by carbon rod
atomization. Anal. Chem., 44: 1887-1889.
BARBEZAT, G.O., CASEY, C.E., REASBECK, P.G., ROBINSON, M.F., &
THOMSON, C.D. (1984) Selenium. In: Rosenberg, I. &
Solomons, N.W., ed. Absorption and malabsorption of mineral
nutrients, New York, Alan R. Liss., pp. 231-258.
BARRETTE, M., LAMOUREUX, G., LEBEL, E., LECOMTE, R., PARADIS,
P., & MONARO, S. (1976) Trace element analysis of freeze-
dried blood serum by proton and alpha-induced X-rays. Nucl.
Instrum. Methods, 134: 189-196.
BEARSE, R.C., CLOSE, D.A., MALANIFY, J.J., & UMBARGER, C.J.
(1974) Elemental analysis of whole blood using proton-induced
X-ray emission. Anal. Chem., 46: 499-503.
BEATH, O.A., EPPSON, H.F., & GILBERT, C.S. (1935). Selenium
and other toxic minerals in soils and vegetation, Wyoming
Experimental Station, 55 pp (Bulletin No. 206).
BEHNE, D. & HOFER-BOSSE, T. (1984) Effects of a low selenium
status on the distribution and retention of selenium in the
rat. J. Nutr., 114: 1289-1296.
BEHNE, D. & WOLTERS, W. (1979) Selenium content and gluta-
thione peroxidase activity in the plasma and erythrocytes of
non-pregnant and pregnant women. J. clin. Chem. clin.
Biochem., 17: 133-135.
BEIJE, B., ONFELT, A., & OLSSON, U. (1984) Influence of
dietary selenium on the mutagenic activity of perfusate and
bile from rat liver, perfused with 1,1-dimethylhydrazine.
Mutat. Res., 130: 121-126.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BESBRIS, H.J., WORTZMAN, M.S., & COHEN, A.M. (1982) Effect
of dietary selenium on the metabolism and excretion of
2-acetylaminofluorene in the rat. J. Toxicol. environ.
Health, 9: 63-76.
BHUYAN, K.C., BHUYAN, D.K., & PODOS, S.M. (1981) Selenium-
induced cataract: biochemical mechanism. In: Spallholz, J.E.,
Martin, J.L., & Ganther, H.E., ed. Selenium in biology and
medicine, Westport, Connecticut, Avi Publishing Company,
pp. 403-412.
BIERI, J.G. & AHMAD, K. (1976) Selenium content of
Bangladeshi rice by chemical and biological assay. J. agric.
food Chem., 24: 1073-1074.
BIRT, D.F., JULINS, A.D., & POUR, P.M. (1984) Increased
pancreatic carcinogenesis in Syrian hamsters fed high selenium
diets. Proc. Am. Assoc. Cancer Res., 25: 133.
BLADES, M.W., DALZIEL, J.A., & ELSON, C.M. (1976) Cathodic
stripping voltammetry of nanogram amounts of selenium in
biological material. J. Assoc. Off. Anal. Chem., 59: 1234-1239.
BLOTCKY, A.J., SULLIVAN, J.F., SHUMAN, M.S., WOODWARD, G.P.,
VOORS, A.W., & JOHNSON, W.D. (1976) Selenium levels in liver
and kidney. In: Hemphill, D.D., ed. Trace substances in
environmental health. X, Columbia, Missouri, University of
Missouri Press, pp. 97-103.
BONARD, E.C. & KORALINK, K.D. (1958) Intoxication aiguë par
vapeurs d'hydrogene selenide. Praxis, 47(i): 533-534.
BOUND, G.P. & FORBES, S. (1978) Differential-pulse
polarography of selenium (IV) in the presence of metal ions.
Analyst, 103: 176-179.
BOWEN, H.J.M. & CAWSE, P.A. (1963) The determination of
selenium in biological material by radioactivation. Analyst,
88: 721-726.
BOWEN, W.H. (1972) The effects of selenium and vanadium on
caries activity in monkeys (M. irus). J. Irish Dent. Assoc.,
18: 83-89.
BRADY, P.S., KU, P.K., & ULLREY, D.E. (1978) Lack of effect
of selenium supplementation on the response of the equine
erythrocyte glutathione system and plasma enzymes to exercise.
J. anal. Sci., 47: 492-496.
BRADY, P.S., BRADY, L.J., & ULLREY, D.E. (1979) Selenium,
vitamin E, and the response to swimming stress in the rat. J.
Nutr., 109: 1103-1109.
BRITTON, J.L., SHEARER, T.R., & DE SART, D.J. (1980) Cario-
stasis by moderate doses of selenium in the rat model. Arch.
environ. Health, 35: 74-76.
BRODIE, K.G. (1979) Analysis of arsenic and other trace
elements by vapor generation. Am. Lab. (June issue): 58-66.
BROGHAMER, W.L., MCCONNELL, K.P., & BLOTCKY, A.L. (1976)
Relationship between serum selenium levels and patients with
carcinoma. Cancer, 37: 1384-1388.
BROGHAMER, W.L., MCCONNELL, K.P., GRIMALDI, M., & BLOTCKY,
A.J. (1978) Serum selenium and reticuloendothelial tumours.
Cancer, 41: 1462-1466.
BROWN, D.G. & BURK, R.F. (1973) Selenium retention in
tissues and sperm of rats fed a torula yeast diet. J. Nutr.,
103: 102-108.
BROWN, D.G., BURK, R.F., SEELY, R.J., & KIKER, K.W. (1972)
Effect of dietary selenium on the gastrointestinal absorption
of 75Se03 in the rat. Int. J. Vit. nutr. Res., 42: 588-591.
BROWN, M.W. & WATKINSON, J.H. (1977) An automated
fluorimetric method for the determination of nanogram
quantities of selenium. Anal. Chim. Acta, 89: 29-35.
BRUNE, D., SAMSAHL, K., & WESTER, P.O. (1966) A comparison
between the amounts of As, Au, Br, Cu, Fe, Mo, Se, and Zn in
normal and uraemic human whole blood by means of neutron
activation analysis. Clin. Chim. Acta, 13: 285-291.
BUCHAN, R.F. (1947) Industrial selenosis. Occup. Med., 3:
439-456.
BUNCE, G.E., HESS, J.L., GURLEY, R., BATRA, R., & TARNAWSKA,
E. (1985) Lens energy metabolism and Ca content in selenite
induced cataract. In: Mills, C.F., Bremner, I., & Chesters,
J.K., ed. Trace elements in man and animals - TEMA 5, Slough,
Commonwealth Agricultural Bureaux, pp. 250-254.
BURCHANOV, A.I. (1972) [On regulation of complex chemical
dusts in rare metals production.] Vopr. Gig. Trud. Profzabol.
Kazajenade, Kazah NII Gig. Trud. Profazabol., pp. 72-74 (in
Russian).
BURCHANOV, A.I. & ZHAKENOVA, R.K. (1973) [Pulmonary changes
following intratracheal administration of elementary selenium
to white rats.] Zavavoohr. Kazah., 3: 52-53 (in Russian).
BURCHANOV, A.I., SALEHOV, M.I., DOROFEEVA, O.N., & ZHAKENOVA,
R.K. (1969) [On the effects of metal selenium dust on the
organism.] In: [Proceedings of a Concluding Scientific
Conference on Labour Hygiene and Occupational Disease, 21-22
October, 1969,] Karaganda, Kazah., NII Gig. Trud. Profzabol,
pp. 77-80 (in Russian).
BURK, R.F. (1976) Selenium in man. In: Prasad, A.S., ed.
Trace elements in human health and disease. II. Essential and
toxic elements, New York, Academic Press, pp. 105-133.
BURK, R.F. & CORREIA, M.A. (1977) Accelerated hepatic haem
catabolism in the selenium-deficient rat. Biochem. J., 168:
105-111.
BURK, R.F. & LANE, J.M. (1979) Ethane production and liver
necrosis in rats after administration of drugs and other
chemicals. Toxicol. appl. Pharmacol., 50: 467-478.
BURK, R.F & MASTERS, B.S.S. (1975) Some effects of selenium
deficiency on the hepatic microsomal cytochrome P-450 system
in the rat. Arch. Biochem. Biophys., 170: 124-131.
BURK, R.F., Jr, PEARSON, W.N., WOOD, R.P., II, & VITERI, F.
(1967) Blood selenium levels and in vitro red blood cell
uptake of 75Se in kwashiorkor. Am. J. clin. Nutr., 20: 723-733.
BURK, R.F., BROWN, D.G., SEELY, R.J., & SCAIEF, C.C., III
(1972) Influence of dietary and injected selenium on whole-
body retention, route of excretion, and tissue retention of
75Se03 2- in the rat. J. Nutr., 102: 1049-1055.
BURK, R.F., SEELEY, R.J., & KIKER, K.W. (1973) Selenium:
dietary threshold for urinary excretion in the rat. In:
Proceedings of the Society of Experimental and Biological
Medicine, Vol. 142, pp. 214-216.
BURK, R.F., MACKINNON, A.M., & SIMON, F.R. (1974) Selenium
and hepatic microsomal hemoproteins. Biochem. Biophys. Res.
Commun., 56: 431-436.
BURK, R.F., NISHIKI, K., LAWRENCE, R.A., & CHANCE, B. (1978)
Peroxide removal by selenium-dependent and selenium independent
glutathione peroxidases in hemoglobin-free perfused rat liver.
J. biol. Chem., 253: 43-46.
BURK, R.F., LAWRENCE, R.A., & CORREIA, M.A. (1980a) Sex
differences in biochemical manifestations of selenium
deficiency in rat liver with special reference to heme
metabolism. Biochem. Pharmacol., 29: 39-42.
BURK, R.F., LAWRENCE, R.A., & LANE, J.M. (1980b) Liver
necrosis and lipid peroxidation in the rat as the result of
paraquat and diquat administration. J. clin. Invest., 65:
1024-1031.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1974) Superoxide- and
singlet oxygen-catalyzed lipid peroxidation as a possible
mechanism for paraquat (methyl viologen) toxicity. Biochem.
Biophys. Res. Commun., 58: 749-755.
BUTLER, G.W. & PETERSON, P.J. (1961) Aspects of the faecal
excretion of selenium by sheep. N.Z. J. agric. Res., 4:
484-491.
BUTTNER, W. (1963) Action of trace elements on the
metabolism of fluoride. J. dent. Res., 42: 453-460.
BYARD, J.L. (1969) Trimethyl selenide: a urinary metabolite
of selenite. Arch. Biochem. Biophys., 130: 556-560.
CADELL, P.B. & COUSINS, F.B. (1960) Urinary selenium and
dental caries. Nature (Lond.), 185: 863-864.
CAGEN, S.Z. & GIBSON, J.E. (1977) Liver damage following
paraquat in selenium-deficient and diethyl maleate-pretreated
mice. Toxicol. appl. Pharmacol., 40: 193-200.
CALVIN, H.I. (1978) Selective incorporation of selenium-75
into a polypeptide of the rat sperm tail. J. exp. Zool., 204:
445-452.
CANNELLA, J.M. (1976) Surveillance of employees in selenium
alloying operations. In: Proceedings of the Symposium on
Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 343-348.
CANTOR, A.H. & SCOTT, M.L. (1974) The effect of selenium in
the hen's diet on egg production, hatchability, performance of
progeny, and selenium concentration in eggs. Poultry Sci., 53:
1870-1880.
CANTOR, A.H. & SCOTT, M.L. (1975) Influence of dietary
selenium on tissue selenium levels in turkeys. Poultry Sci.,
54: 262-265.
CANTOR, A.H., SCOTT, M.L., & NOGUCHI, T. (1975a) Biological
availability of selenium in feedstuffs and selenium compounds
for prevention of exudative diathesis in chicks. J. Nutr.,
105: 96-105.
CANTOR, A.H., LANGEVIN, M.L., NOGUCHI, T., & SCOTT, M.L.
(1975b) Efficacy of selenium in selenium compounds and
feedstuffs for prevention of pancreatic fibrosis in chicks. J.
Nutr., 105(1): 106-111.
CANTOR, A.H., MOORHEAD, P.D., & BROWN, K.I. (1978) Influence
of dietary selenium upon reproductive performance of male and
female breeder turkeys. Poultry Sci., 57: 1337-1345.
CAPPON, C.J. & SMITH, J.C. (1982) Chemical form and
distribution of mercury and selenium in canned tuna. J. appl.
Toxicol., 2: 181-189.
CARNRICK, G.R., MANNING, D.C., & SLAVIN, W. (1983)
Determination of selenium in biological materials with
platform furnace atomic absorption spectroscopy and Zeeman
background correction. Analyst, 108: 1297-1312.
CARTER, D.L., ROBBINS, C.W., & BROWN, M.J. (1972) Effect of
phosphorus fertilization on the selenium concentration in
alfalfa (Medicago sativa). Soil Sci. Soc. Am. Proc., 36:
624-628.
CARY, E.E., ALLAWAY, W.H., & MILLER, M. (1973) Utilization
of different forms of dietary selenium. J. anal. Sci., 36:
285-292.
CASEY, C.E., GUTHINE, B.E., FREUD, G.M., & ROBINSON, M.F.
(1982) Selenium in human tissues from New Zealand. Arch.
environ. Health., 37: 133-135.
CAYGILL, C.P.J. & DIPLOCK, A.T. (1973) The dependence on
dietary selenium and vitamin E of oxidant-labile liver
microsomal non-haem iron. FEBS Lett., 33: 172-176.
CHAN, C.C.Y. (1976) Improvement in the fluorimetric
determination of selenium in plant materials with
2,3-diaminonaphthalene. Anal. Chim. Acta, 82: 213-215.
CHANSLER, M.W., MORRIS, V.C., & LEVANDER, O.A. (1983)
Bioavailability to rats of selenium in Brazil nuts and
mushrooms. Fed. Proc., 42(4): 927.
CHATTERJEE, M. & BANERJEE, M.R. (1982) Selenium mediated
dose-inhibition of 7-12-dimethylbenz(alpha)anthracene-induced
transformation of mammary cells in organ culture. Cancer
Lett., 17: 187-195.
CHAU, Y.K., WONG, P.T.S., SILVERBERG, B.A., LUXON, P.L., &
BENGERT, G.A. (1976) Methylation of selenium in the aquatic
environment. Science, 192: 1130-1131.
CHAVEZ, J.F. (1966) Studies on the toxicity of a sample of
Brazil nuts with a high content of selenium. Bol. Soc. Quim.
Peru, 32: 195-203.
CHEN, R.W., WHANGER, P.D., & WESWIG, P.H. (1975) Selenium-
induced redistribution of cadmium binding to tissue proteins:
a possible mechanism of protection against cadmium toxicity.
Bioinorgan. Chem., 4: 125-133.
CHEN, X., YANG, G., CHEN, J., CHEN, X., WEN, Z., & GE, K.
(1980) Studies on the relations of selenium and Keshan
disease. Biol. Trace Elem. Res., 2: 91-107.
CHERRY, D.S. & GUTHRIE, R.K. (1977) Toxic metals in surface
waters from coal ash. Water Resour. Bull., 13: 1227-1236.
CHUNG, A. & MAINES, M.D. (1981) Effect of selenium on
glutathione metabolism. Induction of -glutamylcysteine
synthetase and glutathione reductase in the rat liver.
Biochem. Pharmacol., 30(23): 3217-3223.
CLARK, L.C. (1985) The epidemiology of selenium and cancer.
Fed. Proc., 44(9): 2584-2589.
CLAYCOMB, C.K., SUMMERS, G.W., & JUMP, E.B. (1965) Effect of
dietary selenium on dental caries in Sprague Dawley rats. J.
dent. Res., 44: 826.
CLAYTON, C.C. & BAUMANN, C.A. (1949) Diet and azo dye
tumors: effect of diet during a period when the dye is not
fed. Cancer Res., 9: 575-582.
CLINTON, M., Jr (1947) Selenium fume exposure. J. ind. Hyg.
Toxicol., 29: 225-226.
COMBS, G.F., Jr & SCOTT, M.L. (1975) Polychlorinated
biphenyl-stimulated selenium deficiency in the chick.
Poultry. Sci., 54: 1152-1158.
COMBS, G.F., Jr, CANTOR, A.H., & SCOTT, M.L. (1975) Effects
of dietary polychlorinated biphenyls on vitamin E and selenium
nutrition in the chick. Poultry Sci., 54: 1143-1152.
CONE, J.E., MARTIN DEL RIO, R., DAVIS, J.N., & STADTMAN, T.C.
(1976) Chemical characterization of the selenoprotein
component of clostridial glycine reductase: identification of
selenocysteine as the organoselenium moiety. Proc. Natl Acad.
Sci. (USA), 73: 2659-2663.
COOPER, W.C. (1967) Selenium toxicity in man. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 185-199.
COOPER, W.C. (1974) Analytical chemistry of selenium. In:
Zingaro, R.A. & Cooper, W.C., ed. Selenium, New York, Van
Nostrand Reinhold Co., pp. 615-653.
COOPER, W.C. & GLOVER, J.R. (1974) The toxicology of
selenium and its compounds. III. In: Zingaro, R.A. & Cooper,
W.C., ed. Selenium, New York, Van Nostrand Reinhold Co., pp.
654-674.
COOPER, W.C., BENNETT, K.G., & CROXTON, F.C. (1974) The
history, occurrence, and properties of selenium. In: Zingaro,
R.A. & Cooper, W.C., ed. Selenium, New York, Van Nostrand
Reinhold Co., pp. 1-31.
CORREIA, M.A. & BURK, R.F. (1976) Hepatic heme metabolism in
selenium-deficient rats: effect of phenobarbital. Arch.
Biochem. Biophys., 177: 642-644.
CORREIA, M.A. & BURK, R.F. (1978) Rapid stimulation of
hepatic microsomal heme oxygenase in selenium-deficient rats:
an effect of phenobarbital. J. biol. Chem., 253: 6203-6210.
COWGILL, U.M. (1974) Trace elements and the birth rate in
the continental United States. In: Hemphill, D.D., ed. Trace
substances in environmental health. VIII, Columbia, Missouri,
University of Missouri Press, pp. 15-21.
COWGILL, U.M. (1976) Selenium and human fertility. In:
Proceedings of the Symposium on Selenium and Tellurium in the
Environment, Pittsburg, Pennsylvania, Industrial Health
Foundation, pp. 300-315.
CREGER, C.R., MITCHELL, R.H., ATKINSON, R.L., FERGUSON, T.M.,
REID, B.L., & COUCH, J.R. (1960) Vitamin E activity of
selenium in turkey hatchability. Poultry Sci., 39: 59-63.
CRYSTAL, R.G. (1973) Elemental selenium: structure and
properties. In: Klayman, D.L. & Gunther, W.H.H., ed. Organic
selenium compounds: their chemistry and biology, New York,
John Wiley and Sons, Inc, pp. 13-27.
CSALLANY, A.S., SU, L.-C., & MENKEN, B.Z. (1984) Effect of
selenite, vitamin E, and n,n'-diphenyl-p-phenylenediamine on
liver organic solvent-soluble lipofuscin pigments in mice.
J. Nutr., 114: 1582-1587.
CUKOR, P., WALZCYK, J., & LOTT, P.F. (1964) The application
of isotopic dilution analysis to the fluorimetric
determination of selenium in plant material. Anal. Chim. Acta,
30: 473-482.
CUMMINS, L.M. & KIMURA, E.T. (1971) Safety evaluation of
selenium sulfide antidandruff shampoos. Toxicol. appl.
Pharmacol., 20: 89-96.
CUMMINS, L.M. & MARTIN, J.L. (1967) Are selenocystine and
selenomethionine synthesized in vivo from sodium selenite in
mammals? Biochemistry, 6: 3162-3168.
CZERNIEJEWSKI, C.P., SHANK, C.W., BECHTEL, W.G., & BRADLEY,
W.B. (1964) The minerals of wheat, flour, and bread. Cereal
Chem., 41: 65-72.
D'HONDT, P., LIEVENS, P., VERSIECK, J., & HOSTE, J. (1977)
Determination of trace elements in animal and human muscle by
semi-automated radiochemical neutron activation analysis.
Radiochem. Radioanal. Lett., 31: 231-240.
DAMS, R. & DE JONGE, J. (1976) Chemical composition of Swiss
aerosols from the Jungfraujoch. Atmos. Environ., 10: 1079-1084.
DE JONG, D., MORSE, R.A., GUTENMANN, W.H., & LISK, D.J.
(1977) Selenium in pollen gathered by bees foraging on fly
ash-grown plants. Bull. environ. Contamin. Toxicol., 18:
442-444.
DE WITT, W.B. & SCHWARZ, K. (1958) Multiple dietary necrotic
degeneration in the mouse. Experientia, 14: 28-34.
DICKSON, J.D. (1969) Notes on hair and nail loss after
ingesting Sapucaia nuts (Lecythis elliptica). Econ. Bot., 23:
133-134.
DICKSON, R.C. & TOMLINSON, R.H. (1967) Selenium in blood and
human tissues. Clin. Chim. Acta, 16: 311-321.
DINKEL, C.A., MINYARD, J.A., WHITEHEAD, E.I., & OLSON, O.E.
(1957) Agricultural research at the Reed Ranch substation,
Brookings, South Dakota, South Dakota State College of
Agriculture and Mechanic Arts, 35 pp (Agricultural Experiment
Station Circular No. 135).
DINKEL, C.A., MINYARD, J.A., & RAY, D.E. (1963) Effects of
season of breeding on reproductive and weaning performance of
beef cattle grazing seleniferous range. J. anim. Sci., 22:
1043-1045.
DIPLOCK, A.T. (1974a) A possible role for trace amounts of
selenium and vitamin E in the electron-transfer system of
rat-liver microsomes. In: Hoekstra, W.G., Suttie, J.W.,
Ganther, H.E., & Mertz, W., ed. Trace element metabolism in
animals. II, Baltimore, Maryland, University Park Press, pp.
147-160.
DIPLOCK, A.T. (1974b) Possible stabilizing effect of vitamin
E on microsomal, membrane-bound, selenide-containing proteins
and drug-metabolizing enzyme systems. Am. J. clin. Nutr., 27:
995-1004.
DIPLOCK, A.T. (1976) Metabolic aspects of selenium action
and toxicity. CRC Crit. Rev. Toxicol., 4: 271-329.
DIPLOCK, A.T. (1979) The influence of selenium and vitamin E
on oxidative demethylation reactions. In: Olive, G., ed. Adv.
Pharmacol. Therap., Proceedings of the 7th International
Congress on Pharmacology, Vol. 8, pp. 25-33.
DIPLOCK, A.T., BAUM, H., & LUCY, J.A. (1971) The effect of
vitamin E on the oxidation state of selenium in rat liver.
Biochem. J., 123: 72l-729.
DIPLOCK, A.T., CAYGILL, C.P.J., JEFFERY, E.H., & THOMAS, C.
(1973) The nature of the acid-volatile selenium in the liver
of the male rat. Biochem. J., 134: 283-293.
DONALDSON, W.E. (1977) Selenium inhibition of avian fatty
acid synthetase complex. Chem.-biol. Interac., 17: 313-320.
DONGHERTY, J.J. & HOEKSTRA, W.G. (1982) Stimulation of lipid
peroxidation in vivo by injected selenite and lack of
stimulation by selenate. Proc. Soc. Exp. Biol. Med., 169(2):
209-215.
DORAN, J.W. (1976) Microbial transformations of selenium in
soil and culture, Ithaca, New York, Cornell University
(Ph.D. Thesis).
DOUGLASS, J.S., MORRIS, V.C., SOARES, J.H., Jr, & LEVANDER,
O.A. (1981) Nutritional availability to rats of selenium in
tuna, beef kidney, and wheat. J. Nutr., 111: 2180-2187.
DUCE, R.A., HOFFMAN, G.L., & ZOLLER, W.H. (1975) Atmospheric
trace metals at remote northern and southern hemisphere sites:
pollution or natural? Science, 187: 59-61.
DUDLEY, H.C. (1938) Toxicology of selenium. V. Toxic and
vesicant properties of selenium oxychloride. Public Health
Rep., 53: 94-98.
DUDLEY, H.C. & MILLER, J.W. (194l) Toxicology of selenium.
VI. Effects of subacute exposure to hydrogen selenide. J. ind.
Hyg. Toxicol., 23: 470-477.
DUTKIEWICZ, T., DUTKIEWICZ, B., & BALCERSKA, I. (1972)
Dynamics of organ and tissue distribution of selenium after
intragastric and dermal administration of sodium selenite.
Bromatol. Chem. Toksykol., 4: 475-481.
DUVOIR, M., POLLETT, L., & HERRENSCHMIDT, J.L. (1937) Eczéma
professionnel dû au sélénium. Bull. Soc. Franc. Dermat.
Syphil., 44: 88-95.
DYER, D.G., SCHUETT, V.E., & GANTHER, H.E. (1977) Blood
selenium and glutathione peroxidase in children with PKU on
diet therapy. In: Abstracts of the Western Hemisphere
Nutrition Congress. V, Quebec, pp. 417.
EGAN, A., KERR, S., & MINSKI, M.J. (1977) Instrumental
neutron activation analysis of selenium using 77µSe (T 1/2 =
17s) in biological materials. In: Brown, S.S., ed. Clinical
chemistry and chemical toxicology of metals, Amsterdam,
Elsevier North-Holland Biomedical Press, pp. 353-356.
ELLIS, N., LLOYD, B., LLOYD, R.S., & CLAYTON, B.E. (1984)
Selenium and vitamin E in relation to risk factors for
coronary heart disease. J. Clin. Pathol, 37(2): 200-206.
ENGBERG, R.A. (1973) Selenium in Nebraska's groundwater and
streams, Lincoln, Nebraska, University of Nebraska (Nebraska
Water Survey Paper No. 35).
ERMAKOV, V.V. (1975) [Selenium determination in biological
materials by fluorometry and gas-chromatography.] In:
[Vitamins. VIII. Biochemistry of vitamin E and selenium,]
Kiev, Naukova Dumka Publishing House, pp. 141-146 (in Russian).
ERMAKOV, V.V. & KOVALSKIJ, V.V. (1968) [The geochemical
ecology of organisms at high selenium levels in the
environment.] In: [Transactions of the biogeochemical
laboratory,] Moscow, Nauka Publishing House, Vol. 12, pp.
204-237 (in Russian).
ERMAKOV, V.V. & KOVALSKIJ, V.V. (1974) [The biological
importance of selenium,] Moscow, Nauka Publishing House, 298
pp (in Russian).
ERSHOV, V.P. (1969) [Hygienic aspects of selenium containing
steel production and the prevention of selenium intoxication.]
Gig. Tr. Prof. Zabol., 12: 29-33 (in Russian).
EVANS, C.S., ASHER, C.J., & JOHNSON, C.M. (1968) Isolation
of dimethyldiselenide and other volatile selenium compounds
from Astragalus racemosus (Pursh.). Austral. J. biol. Sci.,
21: 13-20.
EVANS, H.M. & BISHOP, K.S. (1922) On the existence of a
hitherto unrecognized dietary factor essential for
reproduction. Science, 56: 650-651.
EWAN, R.C., POPE, A.L., & BAUMANN, C.A. (1967) Elimination
of fixed selenium by the rat. J. Nutr., 91: 547-554.
EWAN, R.C., BAUMANN, C.A., & POPE, A.L. (1968) Retention of
selenium by growing lambs. J. agric. food Chem., 16: 216-219.
EXON, J.H., KOLLER, L.D., & ELLIOTT, S.C. (1976) Effect of
dietary selenium on tumor induction by an oncogenic virus.
Clin. Toxicol., 9: 273-279.
FALK, R. & LINDHE, J.C. (1974) Radiation dose received by
humans from intravenously-administered sodium selenite marked
with selenium-75, Los Alamos, New Mexico, Los Alamos
Scientific Laboratory (Los Alamos Translation LA-TR-76-5).
FENG, Z., WANG, X., & HAN, C. (1985) [Studies on the
toxicity of diets with different selenium levels in rats.]
Food Hyg. Res., 3(2): 14-20 (in Chinese).
FERRETTI, R.J. & LEVANDER, O.A. (1974) Effect of milling and
processing on the selenium content of grains and cereal
products. J. agric. food Chem., 22: 1049-1051.
FERRETTI, R.J. & LEVANDER, O.A. (1976) Selenium content of
soybean foods. J. agric. food Chem., 24: 54-56.
FILATOVA, V.S. (1948) [Characteristics of selenium as an
industrial intoxicant.] Can. Med. Thesis (in Russian).
FILATOVA, V.S. (1951) [On the toxicity of selenium
chloride.] Gig. i Sanit., 5: 18-23 (in Russian).
FLEMING, G.A. (1962) Selenium in Irish soils and plants.
Soil Sci., 94: 28-35.
FLEMING, G.A. & WALSH, T. (1957) Selenium occurrence in
certain Irish soils and its toxic effects on animals. Proc.
Royal Irish Acad., 58: 151-166.
FLOHE, L., GUNZLER, W.A., & SCHOEKS, H.H. (1973) Glutathione
peroxidase: a selenoenzyme. FEBS Lett., 32: 132-134.
FORBES, S. & BOUND, G.P. (1977) Some investigations into the
electroanalytical chemistry of selenium. Proc. Anal. Div.
Chem. Soc., 14: 253-256.
FORSTROM, J.W., ZAKOWSKI, J.J., & TAPPEL, A.L. (1978)
Identification of the catalytic site of rat liver glutathione
peroxidase as selenocysteine. Biochemistry, 17: 2639-2644.
FRANKE, K.W. & MOXON, A.L. (1936) A comparison of the
minimum fatal doses of selenium, tellurium, arsenic, and
vanadium. J. Pharmacol. exp. Ther., 58: 454-459.
FRANKE, K.W. & POTTER, V.R. (1936) The effect of selenium
containing foodstuffs on growth and reproduction of rats at
various ages. J. Nutr., 12: 205-214.
FRANKE, K.W. & TULLY, W.C. (1935) A new toxicant occurring
naturally in certain samples of plant foodstuffs. V. Low
hatchability due to deformities in chicks. Poultry Sci., 14:
273-279.
FRANKE, K.W., MOXON, A.L., POLEY, W.E., & TULLY, W.C. (1936)
Monstrosities produced by the injection of selenium salts into
hen's eggs. Anat. Rec., 65: 15-22.
FROSETH, J.A., PIPER, R.C., & CARLSON, J.R. (1974)
Relationship of dietary selenium and oral methyl mercury to
blood and tissue selenium and mercury concentrations and
deficiency: toxicity signs in swine. Fed. Proc., 33: 660.
FROST, D.V. & INGVOLDSTAD, D. (1975) Ecological aspects of
selenium and tellurium in human and animal health. Chemica
Scripta, 8A: 96-107.
FURR, A.K., PARKINSON, T.F., HINRICHS, R.A., VAN CAMPEN, D.R.,
BACHE, C.A., GUTENMANN, W.H., ST. JOHN, L.E., Jr, PAKKALA,
I.S., & LISK, D.J. (1977) National survey of elements and
radioactivity in fly ashes. Absorption of elements by cabbage
grown in fly ash-soil mixtures. Environ. Sci. Technol., 11:
1194-1201.
FURR, A.K., PARKINSON, T.F., GUTENMANN, W.H., PAKKALA, I.S., &
LISK, D.J. (1978a) Elemental content of vegetables, grains,
and forages field-grown on fly ash amended soil. J. agric.
food Chem., 26: 357-359.
FURR, A.K., PARKINSON, T.F., HEFFRON, C.L., REID, J.T.,
HASCHEK, W.M., GUTENMANN, W.H., PAKKALA, I.S., & LISK, D.J.
(1978b) Elemental content of tissues of sheep fed rations
containing coal fly ash. J. agric. food Chem., 26: 1271-1274.
GAIROLA, C. & CHOW, C.K. (1982) Dietary selenium, hepatic
arylhydrocarbon hydroxylase, and mutagenic activation of
benzo( a)pyrene, 2-aminoanthracene, and 2-aminofluorene.
Toxicol. Lett., 11: 281-287.
GANAPATHY, S. & DHANDA, R. (1976) Selenium content of
omniverous and vegetarian diets. Fed. Proc., 35: 360.
GANAPATHY, S.N., JOYNER, B.T., & HAFNER, K.M. (1975) Effect
of baking, broiling, and frying on the selenium content of
selected beef, pork, chicken and fish foods. In: Proceedings
of the 10th International Congress of Nutrition - Abstracts,
Kyoto, Japan, 3-9 August, 1975, p. 277.
GANTHER, H.E. (1968) Selenotrisulfides. Formation by the
reaction of thiols with selenious acid. Biochemistry, 7:
2898-2905.
GANTHER, H.E. (1971) Reduction of the selenotrisulfide
derivative of glutathione to a persulfide analog by
glutathione reductase. Biochemistry, 10: 4089-4098.
GANTHER, H.E. (1978) Modification of methylmercury toxicity
and metabolism by selenium and vitamin E: possible mechanisms.
Environ. Health Perspect., 25: 71-76.
GANTHER, H.E. (1979) Metabolism of hydrogen selenide and
methylated selenides. Adv. Nutr. Res., 2: 107-128.
GANTHER, H.E. & BAUMANN, C.A. (1962) Selenium metabolism.
II. Modifying effects of sulfate. J. Nutr., 77: 408-414.
GANTHER, H.E. & CORCORAN, C. (1969) Selenotrisulfides. II.
Cross-linking of reduced pancreatic ribonuclease with
selenium. Biochemistry, 8: 2557-2563.
GANTHER, H.E. & HSIEH, H.S. (1974) Mechanisms for the
conversion of selenite to selenides in mammalian tissues. In:
Hoekstra, W.G., Suttie, J.W., Ganther, H.E., & Mertz, W., ed.
Trace element metabolism in animals. 2, Baltimore, Maryland,
University Park Press, pp. 339-353.
GANTHER, H.E. & SUNDE, M.L. (1974) Effect of tuna fish and
selenium on the toxicity of methylmercury: a progress report.
J. food Sci., 39: 1-5.
GANTHER, H.E., LEVANDER, O.A., & BAUMANN, C.A. (1966)
Dietary control of selenium volatilization in the rat. J.
Nutr., 88: 55-60.
GANTHER, H.E., GOUDIE, C., SUNDE, M.L., KOPECKY, M.J., WAGNER,
P., OH, S., & HOEKSTRA, W.G. (1972) Selenium: relation to
decreased toxicity of methylmercury added to diets containing
tuna. Science, 175: 1122-1124.
GANTHER, H.E., HAFEMAN, D.G., LAWRENCE, R.A., SERFASS, R.E., &
HOEKSTRA, W.G. (1976) Selenium and glutathione peroxidase in
health and disease: a review. In: Prasad, H.H., ed. Trace
elements in human health and disease. II. Essential and toxic
elements, New York, Academic Press pp. 165-234.
GARDINER, M.R., ARMSTRONG, J., FELS, H., & GLENCROSS, R.N.
(1962) A preliminary report on selenium and animal health in
Western Australia. Aust. J. exp. Agric. Animal Husb., 2:
261-269.
GARDNER, S. (1973) Selenium in animal feed. Proposed food
additive regulation. Fed. Reg., 38: 10458-10460.
GE, K., XUE, A., BAI, J., & WANG, S. (1983) Keshan disease -
an endemic cardiomyopathy in China. Virchows Arch. (Pathol.
Anat.), 401: 1-15.
GEERING, H.R., CARY, E.E., JONES, L.H.P., & ALLAWAY, W.H.
(1968) Solubility and redox criteria for the possible forms
of selenium in soils. Soil Sci. Soc. Am. Proc., 32: 35-40.
GIASUDDIN, A.S.M., CAYGILL, C.P.J., DIPLOCK, A.T., & JEFFERY,
E.H. (1975) The dependence on vitamin E and selenium of drug
demethylation in rat liver microsomal fractions. Biochem. J.,
146: 339-350.
GISSEL-NIELSEN, G. (1971) Selenium content of some
fertilizers and their influence on uptake of selenium in
plants. J. agric. food Chem., 19: 564-566.
GISSEL-NIELSEN, G. (1973) Ecological effects of selenium
application to field crops. Ambio, 2: 114-117.
GISSEL-NIELSEN, G. (1976) Selenium in soils and plants. In:
Proceedings of the Symposium on Se-Te in the Environment,
Pittsburgh, Pennsylvania, Industrial Health Foundation,
pp. 10-25.
GISSEL-NIELSEN, G. (1986) Selenium fertilizers and foliar
application, Danish experiments. Ann. clin. Res., 18: 61-64.
GISSEL-NIELSEN, M. & GISSEL-NIELSEN, G. (1975) Selenium in
soil-animal relationships. Pedobiologia, 15: 65-67.
GITSOVA, S. (1973) Presence of selenium in drinking waters
in Bulgaria. Khig. Zdraveopazvane, 16: 557-561.
GLOVER, J.R. (1954) Some medical problems concerning
selenium in industry. Trans. Assoc. Ind. Med. Off., 4: 94-96.
GLOVER, J.R. (1967) Selenium in human urine: a tentative
maximum allowable concentration for industrial and rural
populations. Ann. occup. Hyg., 10: 3-14.
GLOVER, J.R. (1970) Selenium and its industrial toxicology.
Ind. Med. Surg., 39: 50-54.
GLOVER, J.R. (1976) Environmental health aspects of selenium
and tellurium. In: Proceedings of the Symposium on
Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 279-292.
GODWIN, K.O. & FUSS, C.N. (1972) The entry of selenium into
rabbit protein following the administration of Na2 75Se03.
Aust. J. biol. Sci., 25: 865-871.
GOODWIN, W.J., LANE, H.W., BRADFORD, K., MARSHALL, M.V.,
GRIFFIN, A.C., GEOPFERT, H., & JESSE, R.H. (1983) Selenium
and glutathione peroxidase levels in patients with epidermoid
carcinoma of the oral cavity and orophasynx. Cancer, 51:
110-115.
GORTNER, R.A., Jr (1940) Chronic selenium poisoning of rats
as influenced by dietary protein. J. Nutr., 19: 105-112.
GOULDEN, P.D. & BROOKSBANK, P. (1974) Automated atomic
absorption determination of arsenic, antimony, and selenium in
natural waters. Anal. Chem., 46: 1431-1436.
GRACIANSKAJA, L.N. & KOVSHILO, V.E., ed. (1977) [Selenium
and its compounds]. In: [Handbook of occupational pathology,]
Moscow, Medicina Publishing House pp. 330-332 (in Russian).
GRANT, K.E., CONNER, M.W., & NEWBERNE, P.M. (1977) Effect of
dietary sodium selenite upon lesions induced by repeated small
doses of aflatoxin B1. Toxicol. appl. Pharmacol., 41: 166.
GREEN, J., BUNYAN, J., CAWTHORNE, M.A., & DIPLOCK, A.T.
(1969) Vitamin E and hepatotoxic agents. I. Carbon
tetrachloride and lipid peroxidation in the rat. Br. J. Nutr.,
23: 297-307.
GRIFFIN, A.C. & JACOBS, M.M. (1977) Effects of selenium on
azo dye hepatocarcinogenesis. Cancer Lett., 3: 177-181.
GRIFFITHS, N.M. (1973) Dietary intake and urinary excretion
of selenium in some New Zealand women. Proc. Univ. Otago Med.
School, 51: 8-9.
GRIFFITHS, N.M. & THOMSON, C.D. (1974) Selenium in the whole
blood of New Zealand residents. N.Z. med. J., 80: 199-202.
GRIFFITHS, N.M., STEWART, R.D.H., & ROBINSON, M.F. (1976)
The metabolism of 75Se-selenomethionine in four women. Br. J.
Nutr., 35: 373-382.
GRIMANIS, A.P., VASSILAKI-GRIMANI, M., ALEXIOU, D., &
PAPADATOS, C. (1978) Determination of seven trace elements in
human milk, powdered cow's milk, and infant foods by neutron
activation analysis. In: Nuclear activation techniques in the
life sciences, Vienna, International Atomic Energy Agency,
pp. 241-253.
GROCE, A.W., MILLER, E.R., KEAHEY, K.K., ULLREY, D.E., &
ELLIS, D. J. (1971) Selenium supplementation of practical
diets for growing-finishing swine. J. Ann. Sci., 32: 905-911.
GROSS, S. (1976) Hemolytic anemia in premature infants:
Relationship to vitamin E, selenium, glutathione peroxidase,
and erythrocyte lipids. Semin. Hematol., 13: 187-199.
GROSSMAN, A. & WENDEL, A. (1983) Non-reactivity of the
selenoenzyme glutathione peroxidase with enzymatically hydro-
peroxidized phospholipids. Eur. J. Biochem., 135(3): 549-552.
GU, B. (1983) Pathology of Keshan disease: a comprehensive
review. Chin. med. J., 96: 251-261.
GUSEJNOV, T.M., ZHAFAROV, A.I., PERELYGIN, V.V., & KARAEV,
M.A. (1974) [On the localization of endogenous selenium in
structural slements of bull's eye.] In: [Selenium in biology,]
Baku, Elm Publishing House, pp. 47-49.
GUTENMANN, W.H., BACHE, C.A., YOUNGS, W.D., & LISK, D.J.
(1976) Selenium in fly ash. Science, 191: 966-967.
HADDAD, P.R. & SMYTHE, L.E. (1974) A critical evaluation of
fluorometric methods for determination of selenium in plant
materials with 2,3-diaminoaphthalene. Talanta, 21: 859-865.
HADJIMARKOS, D.M. (1956) Geographic variations of dental
caries in Oregon. VII. Caries prevalence among children in the
blue mountains region. J. Pediatr., 48: 195-201.
HADJIMARKOS, D.M. (1960) Urinary selenium and dental caries.
Nature (Lond.), 188: 677.
HADJIMARKOS, D.M. & BONHORST, C.W. (1958) The trace element
selenium and its influence on dental caries susceptibility. J.
Pediatr., 52: 274-278.
HADJIMARKOS, D.M. & BONHORST, C.W. (1961) The selenium
content of eggs, milk, and water in relation to dental caries
in children. J. Pediatr., 59: 256-259.
HADJIMARKOS, D.M. & SHEARER, T.R. (1971) Selenium
concentration in human saliva. Am. J. clin. Nutr., 24: 1210.
HADJIMARKOS, D.M. & STORVICK, C.A. (1950) Geographic
variations of dental caries in Oregon. IV. Am. J. public
Health, 40: 1552-1555.
HADJIMARKOS, D.M., STORVICK, C.A., & REMMERT, L.F. (1952)
Selenium and dental caries. An investigation among school
children of Oregon. J. Pediatr., 40: 451-455.
HAFEMAN, D.G. & HOEKSTRA, W.G. (1977a) Protection against
carbon tetrachloride-induced lipid peroxidation in the rat by
dietary vitamin E, selenium, and methionine as measured by
ethane evolution. J. Nutr., 107: 656-665.
HAFEMAN, D.G. & HOEKSTRA, W.G. (1977b) Lipid peroxidation in
vivo during vitamin E and selenium deficiency in the rat as
monitored by ethane evolution. J. Nutr., 107: 666-672.
HAFEMAN, D.G., SUNDE, R.A., & HOEKSTRA, W.G. (1974) Effect
of dietary selenium on erythrocyte and liver glutathione
peroxidase in the rat. J. Nutr., 104: 580-587.
HAKKARAINEN, J., LINDBERG, P., BENGTSSON, G., JONSSON, L., &
LANNEK, N. (1978) Requirement for selenium (as selenite) and
vitamin E (as alpha-tocopherol) in weaned pigs. III. The effect on
the development of the VESD syndrome of varying selenium
levels in a low-tocopherol diet. J. anal. Sci., 46: 1001-1008.
HALL, R.H., LASKIN, S., FRANK, P., MAYNARD, E.A., & HODGE,
C.H. (1951) Arch. ind. Hyg. occup. Med., 4: 458-464.
HALTER, K. (1938) [Selenium poisoning, especially skin
changes accompanied by secondary porphysia.] Arch. Dermatol.,
178: 340 (in German).
HALVERSON, A.W. (1974) Growth and reproduction with rats fed
selenite-Se. Proc. S. Dak. Acad. Sci., 53: 167-177.
HALVERSON, A.W., HENDRICK, C.M., & OLSON, O.E. (1955) Observations
on the protective effect of linseed oil meal and some extracts
against chronic selenium poisoning in rats. J. Nutr., 56: 51-60.
HALVERSON, A.W., GUSS, P.L., & OLSON, O.E. (1962) Effect of
sulfur salts on selenium poisoning in the rat. J. Nutr., 77:
459-464.
HALVERSON, A.W., PALMER, I.S., & GUSS, P.L. (1966) Toxicity
of selenium to post-weanling rats. Toxicol. appl. Pharmacol.,
9: 477-484.
HAMDY, A.A. & GISSEL-NIELSEN, G. (1976) Volatilization of
selenium from soils. Z. Pflanzenernaehr. Bodenkd., 6: 671-678.
HAMILTON, A. (1927) Industrial poisons in the United States,
Macmillan Company, p. 111.
HAMILTON, A. (1934) Industrial toxicology, New York, Harpers
Publishing Company, p. 111.
HAMILTON, J.W. (1975) Chemical examination of seleniferous
cabbage Brassica oleracea capitata. J. agric. food Chem., 23:
1150-1152.
HARLAND, B.F., PROSKY, L., & VANDERVEEN, J.E. (1978)
Nutritional adequacy of current levels of Ca, Cu, Fe, I, Mg,
Mn, P, Se, and Zn in the American food supply for adults,
infants, and toddlers. In: Kirchgessner, M., ed. Trace element
metabolism in man and animals. 3, Freising- Weihenstephan,
West Germany, Arbeitskreis fur Tierernährungsforschung
Weihenstephan, pp. 311-315.
HARR, J.R. & MUTH, O.H. (1972) Selenium poisoning in
domestic animals and its relationship to man. Clin. Toxicol.,
5: 175-186.
HARR, J.R., BONE, J.F., TINSLEY, I.J., WESWIG, P.H., &
YAMAMOTO, R.S. (1967) Selenium toxicity in rats. II.
Histopathology. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI Publishing Co., Inc, pp. 153-178.
HARR, J.R., EXON, J.H., WHANGER, P.D., & WESWIG, P.H. (1972)
Effect of dietary selenium on N-2-fluorenyl-acetamide
(FAA)-induced cancer in vitamin E-supplemented, selenium
depleted rats. Clin. Toxicol., 5: 187-194.
HARRIS, P.L., LUDWIG, M.I., & SCHWARZ, K. (1958)
Ineffectiveness of Factor 3-active selenium compounds in
resorption-gestation bioassay for vitamin E. Proc. Soc. Exp.
Biol. Med., 97: 686-688.
HARTHOORN, A.M. & YOUNG, E. (1974) A relationship between
acid-base balance and capture myopathy in zebra (Equus
burchelli) and an apparent therapy. Vet. Res., 95: 337-342.
HARTLEY, W.J. (1963) Selenium and ewe fertility. Proc. N.Z.
Soc. Anim. Prod., 23: 20-27.
HARTLEY, W.J. (1967) Levels of selenium in animal tissues
and methods of selenium administration . In: Muth, O.H.,
Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 79-96.
HARTLEY, W.J. & GRANT, A.B. (1961) A review of selenium
responsive diseases of New Zealand livestock. Fed. Proc., 20:
679-688.
HARTLEY, W.J., GRANT, A.B., & DRAKE, C. (1960) Control of
white muscle disease and ill thrift with selenium. N.Z. J.
Agric., 101: 343-345.
HASHIMOTO, Y. & WINCHESTER, J.W. (1967) Selenium in the
atmosphere. Environ. Sci. Technol., 1: 338-340.
HEATH, R.L. (1969-70) Handbook of chemistry and physics,
50th ed., Cleveland, Ohio, The Chemical Rubber Publishing Co.
HE, G. (1979) On the etiology of Keshan disease: two
hypotheses. Chin. med. J., 92: 416-422.
HEINRICH, M., Jr & KELSEY, F.E. (1955) Studies on selenium
metabolism: the distribution of selenium in the tissues of the
mouse. J. Pharmacol. exp. Therap., 114: 28-32.
HELZLSOUER, K., JACOBS, R., & MORRIS, S. (1985) Acute
selenium intoxication in the United States. Fed. Proc., 44(5):
1670.
HICKEY, F. (1968) Selenium in human and animal nutrition.
N.Z. Agric., 18: 1-2.
HICKEY, F. (1977) Human medication with selenium. Including
brief comments on the comparative pathology of White Muscle
Disease in livestock and human muscular dystrophies. In:
Hamilton, N.Z., ed. Trace elements in human and animal health
in New Zealand, Hamilton, New Zealand, Waikato University
Press, pp. 92-99.
HIGGS, D.J., MORRIS, V.C., & LEVANDER, O.A. (1972) Effect of
cooking on selenium content of foods. J. agric. food Chem.,
20: 678-680.
HODDER, A.P.W. & WATKINSON, J.H. (1976) Low selenium levels
in tephra-derived soil - inherent or pedogenetic? N.Z. J.
Sci., 19: 397-400.
HOEKSTRA, W.G. (1975a) Biochemical function of selenium and
its relation to vitamin E. Fed. Proc., 34: 2083-2089.
HOEKSTRA, W.G. (1975b) Glutathione peroxidase activity of
animal tissues as an index of selenium status. In: Hemphill,
D.D., ed. Trace substances in environmental health. IX,
Columbia, Missouri, University of Missouri Press, pp. 331-337.
HOEKSTRA, W.G., HAFEMAN, O., OH, S.H., SUNDER, R.A., & GANTHER,
H.E. (1973) Effect of dietary selenium on liver and erythrocyte
glutathione peroxidase in the rat. Fed. Proc., 32: 885.
HOGGER, D. & BOHM, C. (1944) [On dermal injury produced by
selenite]. Dermatologica, 90: 217-223 (in German).
HOLMBERG, R.E., & FERM, V.H. (1969) Interrelationships of
selenium, cadmium, and arsenic in mammalian teratogenesis.
Arch. environ. Health, 18: 873-877.
HOLSTEIN, E. (1951) [The effects of occupational exposure to
selenium.] Zentrblt. Arbeitsmed. Arbeitschutz, 1: 102-104 (in
German).
HOLYNSKA, B. & MARKOWICZ, A. (1977) Application of energy
dispersive X-ray fluorescence for the determination of
selenium in blood and tissue. Radiochem. radioanal. Lett., 31:
165-170.
HOPKINS, L.L. & MAJAJ, A.S. (1967) Selenium in human
nutrition. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H.,
ed. Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 203-214.
HOVE, E.L. (1948) Interrelation between alpha-tocopherol and
protein metabolism. III. The protective effect of vitamin E
and certain nitrogenous compounds against CCl4 poisoning in
rats. Arch. Biochem., 17: 467-474.
HOWARD, J.H., III (1971) Control of geochemical behavior of
selenium in natural waters by adsorption on hydrous ferric
oxides. In: Hemphill, D.D., ed. Trace substances in
environmental health. V, Columbia, Missouri, University of
Missouri Press, pp. 485-495.
HOWE, M. (1974) Selenium in the blood of south Dakotans.
Arch. environ. Health, 34: 444-448.
HURT, H.D., CARY E.E., & VISEK, W.J. (1971) Growth,
reproduction, and tissue concentrations of selenium in the
selenium-depleted rat. J. Nutr., 101: 761-766.
IARC (1975) Some aziridines, N,-S-, and O-mustards and
selenium, Lyons, International Agency for Research on Cancer,
pp. 245-260 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, Vol. 9).
IHNAT, M. (1974) Collaborative study of a fluorometric
method for determining selenium in foods. J. Assoc. Off. Anal.
Chem., 57: 373-378.
IHNAT, M. (1976) Selenium in foods: evaluation of atomic
absorption spectrometric techniques involving hydrogen
selenide generation and carbon furnace atomization. J. Assoc.
Off. Anal. Chem., 59: 911-922.
IHNAT, M. & MILLER, H.J. (1977a) Analysis of foods for
arsenic and selenium by acid digestion, hydride evolution
atomic absorption spectrophotometry. J. Assoc. Off. Anal.
Chem., 60: 813-825.
IHNAT, M. & MILLER, H.J. (1977b) Acid digestion, hydride
evolution atomic absorption spectrophotometric method for
determining arsenic and selenium in foods: collaborative
study. I. J. Assoc. Off. Anal. Chem., 60: 1414-1433.
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J.
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IP, C. (1985a) Selenium inhibition of chemical
carcinogenesis. Fed. Proc., 44: 2573-2578.
IP, C. (1985b) Attenuation of the anticarcinogenic action of
selenium by vitamin E deficiency. Cancer Lett., 25: 325-331.
IP, C. & SINHA, D.K. (1981) Enhancement of mammary
tumorigenesis by dietary selenium deficiency in rats with a
high polyunsaturated fat intake. Cancer Res., 41: 31-34.
IRGOLIC, K.J. & KUDCHADKER, M.H. (1974) Organic chemistry of
selenium. In: Zingaro, R.A. & Cooper, W.C., ed. Selenium, New
York, Van Nostrand Reinhold Co., pp. 408-545.
IZRAELSON, Z.I., MOGILEVSKAJA, O.Ja., & SUVOROV, S.W. (1973)
[Selenium.] In: [Problems of occupational hygiene and
occupational pathology connected with the work with toxic
metals,] Moscow, Medicina Publishing House pp. 245-257 (in
Russian).
JACOBS, M.M. (1976) Selenium: a possible inhibitor of colon
and rectum cancer. II. Biochemical aspects. In: Proceedings of
the Symposium on Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 329-340.
JACOBS, M.M. (1977) Inhibitory effects of selenium on 1,2-
dimethylhydrazine and methylazoxymethanol colon carcinogenesis.
Cancer, 40: 2557-2564.
JACOBS, M.M. & FORST, C. (1981a) Toxicological effects of
sodium selenite in Sprague-Dawley rats. J. Toxicol. environ.
Health, 8: 575-585.
JACOBS, M.M. & FORST, C. (1981b) Toxicological effects of
sodium selenite in Swiss mice. J. Toxicol. environ. Health, 8:
587-598.
JACOBS, M.M., JANSSON, B., & GRIFFIN, A.C. (1977a) Inhibitory
effects of selenium on 1,2-dimethylhydrazine and methylazoxy-
methanol acetate induction of colon tumours. Cancer Lett., 2:
133-138.
JACOBS, M.M., MATNEY, T.S., & GRIFFIN, A.C. (1977b) Inhibitory
effects of selenium on the mutagenicity of 2-acetylamino-
fluorene (AAF) and AAF derivatives. Cancer Lett., 2: 319-322.
JACOBSSON, S.O., LIDMAN, S., & LINDBERG, P. (1970) Blood
selenium in a beef herd affected with muscular degeneration.
Acta vet. Scand., 11: 324-326.
JAFFE, W.G. (1973) Selenium in food plants and feeds.
Toxicology and nutrition. Qualitas Plantarum. Plant foods hum.
Nutr., 23: 191-204.
JAFFE, W.G. (1976) Effect of selenium intake in humans and
in rats. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 188-193.
JAFFE, W.G. & MONDRAGON, M.C. (1969) Adaptation of rats to
selenium intake. J. Nutr., 97: 431-436.
JAFFE, W.G. & MONDRAGON, C. (1975) Effects of ingestion of
organic selenium in adapted and non-adapted rats. Br. J.
Nutr., 33: 387-397.
JAFFE, W.G. & VELEZ, B.F. (1973) Selenium intake and
congenital malformations in humans. Arch. Latinoam. Nutr., 23:
514-516.
JAFFE, W.G., RUPHAEL, M.D., MONDRAGON, M.C., & CUEVAS, M.A.
(1972a) Clinical and biochemical studies on school children
from a seleniferous zone. Arch. Latinoam. Nutr., 22: 595-611.
JAFFE, W.G., MONDRAGON, M.C., LAYRISSE, M., & OJEDA, A.
(1972b) Toxicity symptoms in rats fed organic selenium. Arch.
Latinoam. Nutr., 22: 467-481.
JANSSON, B., JACOBS, M.M., & GRIFFIN, A.C. (1978)
Gastrointestinal cancer: epidemiology and experimental
studies. In: Schrauzer, G.N., ed. Inorganic and nutritional
aspects of cancer, New York, Plenum Press, pp. 305-322.
JENKINS, K.J. & HIDIROGLOU, M. (1971) Comparative uptake of
selenium by low cystine and high cystine proteins. Can. J.
Biochem., 49: 468-472.
JENSEN, L.S. & MCGINNIS, J. (1960) Influence of selenium,
antioxidants, and type of yeast on vitamin E deficiency in the
adult chicken. J. Nutr., 72: 23-28.
JENSEN, R., CLOSSON, W., & ROTHENBERG, R. (1984) Selenium
intoxication - New York. Mobid. Mortal. Weekly Rep., 33:
157-158.
JOHNSON, C.M. (1976) Selenium in the environment. Res. Rev.,
62: 101-130.
JONES, G.B. & GODWIN, K.O. (1962) Distribution of
radioactive selenium in mice. Nature (Lond.), 196: 1294-1296.
JUDSON, G.J. & OBST, J.M. (1975) Diagnosis and treatment of
selenium inadequacies in the grazing ruminant. In: Nicholas,
D.J.D. & Egan, A.R., ed. Trace elements in soil-plant-animal
systems, New York, Academic Press, Inc, pp. 385-405.
JULIUS, A.D., DAVIES, M.H., & BIRT, D.F. (1980) Relationship
between blood selenium and erythrocyte glutathione peroxidase
activity with excess dietary selenium in Syrian golden
hamsters. Fed. Proc., 39: 555.
KAMADA, T., SHIRAISHI, T., & YAMAMOTO, Y. (1978)
Differential determination of selenium (IV) and selenium (VI)
with sodium diethyldithiocarbamate, ammonium pyrrolidinedi-
thiocarbamate and dithizone by atomic-absorption spectrophoto-
metry with a carbon-tube atomizer. Talanta, 25: 15-19.
KAQUELER, J.C., MALOIGNE, E., & BONIFAY, P. (1977) Effects
of sodium selenate on caries incidence on the rat. J. dent.
Res., D 56 (Special Issue): D151.
KASIMOV, R.Ju., RUSTAMOVA, Sh.A., GUSEJNOV, T.M., & KIAZIMOV,
S.K. (1976) [Dynamics of selenium distribution in some organs
of young fish from selected valuable edible species.] In:
[Selenium in biology,] Baku, Elm Publishing House, Vol. 2, pp.
143-144 (in Russian).
KERDEL-VEGAS, F. (1966) The depilatory and cytotoxic action
of "Coco de Mono" (Lecythis ollaria) and its relationship to
chronic seleniosis. Econ. Bot., 20: 187-195.
KESHAN DISEASE RESEARCH GROUP (1979a) Observations on effect
of sodium selenite in prevention of Keshan disease. Chinese
med. J., 92: 471-476.
KESHAN DISEASE RESEARCH GROUP (1979b) Epidemiologic studies
on the etiologic relationship of selenium and Keshan disease.
Chinese med. J., 92: 477-482.
KETTERER, B., BEALE, D., & MEYER, D. (1982) The structure
and multiple functions of glutathione transferases. Biochem.
Soc. Trans., 10(2): 82-84.
KILNESS, A.W. (1973) Selenium and public health. S.D. J.
Med., 26: 17-19.
KILNESS, A.W. & HOCHBERG, F.H. (1977) Amyotrophic lateral
sclerosis in a high selenium environment. J. Am. Med. Assoc.,
237: 2843-2844.
KIMMERLE, G. (1960) [Comparative studies on the inhalation
toxicity of sulfur-selenium and tellurium hexafluoride.] Arch.
Toxikol., 18: 140-144 (in German).
KINNIGKEIT, G. (1962) [Studies on workers exposed to selenium
in a rectifier plant.] Z. Gesamte Hyg. Grenzgeb., 8: 350-362
(in German).
KLAYMAN, D.L. & GUNTHER, W.H.H. (1973) Organic selenium
compounds: their chemistry and biology, New York, John Wiley
and Sons, Inc, 1188 pp.
KLEIN, A.K. (1943) Report on selenium. J. Assoc. Offic.
Anal. Chem., 26: 346-352.
KLUG, H.L., PETERSEN, D.F., & MOXON, A.L. (1949) The
toxicity of selenium analogues of cystine and methionine.
Proc. S.D. Acad. Sci., 28: 117-120.
KLUG, H.L., MOXON, A.L., PETERSEN, D.F, & POTTER, V.R. (1950)
The in vivo inhibition of succinic dehydrogenase by selenium
and its release by arsenic. Arch. Biochem. Biophys., 28:
253-259.
KOEMAN, J.H., VAN DE VEN, W.S.M., DE GOEIJ, J.J.M., TJIOE,
P.S., & VAN HAAFTEN, J.L. (1975) Mercury and selenium in
marine mammals and birds. Sci. total Environ., 3: 279-287.
KOIVISTOINEN, P., ed. (1980) Mineral elements composition of
Finnish foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni,
Cr F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb, and ash. Acta
agric. Scand., Suppl. 2: 17.
KOIVISTOINEN, P. & HUTTUNEN, J.K. (1985) Selenium deficiency
in Finnish food and nutrition: research strategy and measures.
In: Mills, C.F., Bremner, I., & Chesters, J.K., ed., Trace
elements in man and animals-TEMA 5, Slough, Commonwealth
Agricultural Bureaux, pp. 925-928.
KOIVISTOINEN, P. & HUTTUNEN, J.K. (1986) Selenium in food
and nutrition in Finland. An overview on research and action.
Ann. clin. Res., 18: 13-17.
KOSTA, L., BYRNE, A.R., & ZELENKO, V. (1975) Correlation
between selenium and mercury in man following exposure to
inorganic mercury. Nature (Lond.), 254: 238-239.
KOVALSKIJ, V.V. (1974) [Geochemical ecology,] Moscow, Nauka
Publishing House, 299 pp (in Russian).
KOVALSKIJ, V.V. (1978) [Geochemical ecology: a basis for a
system of biogeochemical regional characterization.] In:
[Biochemical regional characterization: a method for studying
the ecological structure of the biosphere,] Moscow, Nauka
Publishing House, Vol. 15, pp. 3-21 (in Russian).
KOVALSKIJ, V.V. & ERMAKOV, V.V. (1975) [Problems of
experimental geochemical ecology of animals under the
conditions of selenium regions and some approaches to the
study of the biological role of selenium.] In: [Vitamins. VII.
Biochemistry of vitamin E and selenium,] Kiev, Nakove Dunka
Publishing House, pp. 80-87 (in Russian).
KRAUSKOPF, K.B. (1955) Sedimentary deposits of rare
elements. Econ. Geol. Fiftieth Anniversary, (Part I): 411-463.
KRONBORG, O.J. & STEINNES, E. (1975) Simultaneous determination
of arsenic and selenium in soil by neutron-activation analysis.
Analyst, 100: 835-837.
KU, P.K., ELY, W.T., GROCE, A.W., & ULLREY, D.E. (1972)
Natural dietary selenium alpha-tocopherol and effect on tissue
selenium. J. Ann. Sci., 34: 208-211.
KUBOTA, J., ALLAWAY, W.H., CARTER, D.L., CARY, E.E., & LAZAR,
V.A. (1967) Selenium in crops in the United States in
relation to selenium-responsive diseases of animals. J. agric.
food Chem., 15: 448-453.
KUBOTA, J., CARY, E.E., & GISSEL-NIELSEN, G. (1975) Selenium
in rainwater of the United States and Denmark. In: Hemphill,
D.D., ed. Trace substances in environmental health. IX,
Columbia, Missouri, University of Missouri Press, pp. 123-130.
KULIEVA, E.M., PERELIGIN, V.V., & DZHAFAROV, A.I. (1978)
[Isolated retina electroretinogram (ERG) under the conditions
of induced lipoperoxidation.] Dokl. Akad. Nauk. Azarbayidzh
SSR, 34(2): 85-89 (in Russian).
KURLAND, L.T. (1977) Amyotrophic lateral sclerosis and
selenium. J. Am. Med. Assoc., 238: 2365-2366.
LAKIN, H.W. & BYERS, H.G. (194l) Selenium occurrence in
certain soils in the United States, with a discussion of
related topics, sixth report, Washington DC, US Department of
Agriculture, 26 pp (USDA Technical Bulletin No. 783).
LAKIN, H.W. & DAVIDSON, D.F. (1967) The relation of the geo-
chemistry of selenium to its occurrence in soils. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 27-56.
LATHROP, K.A., JOHNSTON, R.E., BLAU, M., & ROTHSCHILD, E.O.
(1972) Radiation dose to humans from 75Se-L-seleno-
methionine. J. Nucl. Med., 13 (suppl. No. 6): 7-30.
LAUER D.J. (1947) Selenium poisoning. A review, with
emphasis on its industrial aspects, Pittsburgh, Pennsylvania,
University of Pittsburgh School of Medicine, pp. 1-47 (Thesis).
LAWRENCE, R.A. & BURK, R.F. (1976) Glutathione peroxidase
activity in selenium-deficient rat liver. Biochem. Biophys.
Res. Commun., 71: 952-958.
LAWRENCE, R.A. & BURK, R.F. (1978) Species, tissue, and
subcellular distribution of non Se-dependent glutathione
peroxidase activity. J. Nutr., 108: 211-215.
LAWRENCE, R.A., PARKHILL, L.K., & BURK, R.F. (1978) Hepatic
cytosolic non selenium-dependent glutathione peroxidase
activity: its nature and the effect of selenium deficiency. J.
Nutr., 108: 981-987.
LAWSON, T. & BIRT, D.F. (1983) Enhancement of the repair of
carcinogen-induced DNA damage in the hamster pancreas by
dietary selenium. Chem. Biol. Interact., 45: 95-104.
LAZAREV, N.V. (1977) [ Harmful compounds in the industry,]
7th ed., Leningrad, Chimija, Vol. 3, pp. 74-82 (in Russian).
LAZAREV, N.V. & GADASKINA, I.D., ed. (1977) [Selenium and
its compounds.] In: [Noxious substances in industry,] Lenin-
grad, Chimija Publishing House, pp. 75-82 (in Russian).
LEEB, J., BAUMGARTNER, W.A., LYONS, K., & LORBER, A. (1977)
Uptake, distribution, and excretion of sodium selenite in
rheumatoid subjects. In: Biological implications of metals in
the environment, Technical Information Center, Energy Research
and Development Administration, pp. 536-546.
LEMLEY, R.E. (1940) Selenium poisoning in the human. Lancet,
60: 528-531.
LEMLEY, R.E. (1943) Observations on selenium poisoning in
South and North America. Lancet, 63: 257-258.
LEMLEY, R.E. & MERRYMAN, M.P. (194l) Selenium poisoning in
the human. Lancet, 61: 435-438.
LEVANDER, O.A. (1976a) Selected aspects of the comparative
metabolism and biochemistry of selenium and sulfur. In:
Prasad, A.S., ed. Trace elements in human health and disease,
Vol. 2, Essential and toxic elements, New York, Academic
Press, pp. 135-163.
LEVANDER, O.A. (1976b) Selenium in foods. In: Proceedings of
the Symposium on Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 26-53.
LEVANDER, O.A. (1977) Metabolic interrelationships between
arsenic and selenium. Environ. Health Perspect., 19: 159-164.
LEVANDER, O.A. (1979) Lead toxicity and nutritional
deficiencies. Environ. Health Perspect., 29: 115-125.
LEVANDER, O.A. (1982) Selenium: biochemical actions, inter-
actions, and some human health implications. In: Clinical,
biochemical, and nutritional aspects of trace elements, pp.
345-368.
LEVANDER, O.A. (1983) Consideration in the design of
selenium bioavailability studies. Fed. Proc., 42: 1721-1725.
LEVANDER, O.A. (1986) Human and animal nutrition. In: Mertz,
M., ed. Trace elements, Vol. 2, New York, Academic Press, pp.
209-279.
LEVANDER, O.A. & BAUMANN, C.A. (1966) Selenium metabolism.
VI. Effect of arsenic on the excretion of selenium in the
bile. Toxicol. appl. Pharmacol., 9: 106-115.
LEVANDER, O.A. & MORRIS, V.C. (1970) Interactions of
methionine, vitamin E, and antioxidants in selenium toxicity
in the rat. J. Nutr., 100: 1111-1118.
LEVANDER, O.A. & MORRIS (1984) Dietary selenium levels
needed to maintain balance in North American adults consuming
self-selected diets. Am. J. clin. Nutr., 39: 809-815.
LEVANDER, O.A., YOUNG, M.L., & MEEKS, S.A. (1970) Studies on
the binding of selenium by liver homogenates from rats fed
diets containing either casein or casein plus linseed oil
meal. Toxicol. appl. Pharmacol., 16: 79-87.
LEVANDER, O.A., MORRIS, V.C., & HIGGS, D.J. (1973a)
Acceleration of thiol-induced swelling of rat liver
mitochondria by selenium. Biochemistry, 12: 4586-4590.
LEVANDER, O.A., MORRIS, V.C., & HIGGS, D.J. (1973b) Selenium
as a catalyst for the reduction of cytochrome c by glutathione.
Biochemistry, 12: 4591-4595.
LEVANDER, O.A., WELSH, S.O., & MORRIS, V.C. (1980) Erythrocyte
deformability as affected by vitamin E deficiency and lead
toxicity. Ann. N.Y. Acad. Sci., 355: 227.
LEVANDER, O.A., SUTHERLAND, B., MORRIS, V.C., & KING, J.C.
(1981a) Selenium metabolism in human nutrition. In: Spallhoz,
J.E., Martin, J.L., & Ganther, H.E., ed. Selenium in biology
and medicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 256-268.
LEVANDER, O.A., SUTHERLAND, B., MORRIS, V.C., & KING, J.C.
(1981b) Selenium balance young men during selenium depletion
and repletion. Am. J. clin. Nutr., 34: 2662-2669.
LEVANDER, O.A., ALFTHAN, G., ARVILOMMI, H., GREF, C.G.,
HUTTUNEN, J.K., KATAJA, M., KOIVISTOINEN, P., & PIKKARAINEN,
J. (1983) Bioavailability of selenium to Finnish men as
assessed by platelet glutathione peroxidase activity and other
blood parameters. Am. J. clin. Nutr., 37: 887-897.
LEVINE, R.J. & OLSON, R.E. (1970) Blood selenium in Thai
children with protein-calorie malnutrition. Proc. Soc. Exp.
Biol. Med., 134: 1030-1034.
LEWIS, B.G., JOHNSON, C.M., & BROYER, T.C. (1974) Volatile
selenium in higher plants: the production of dimethyl selenide
in cabbage leaves by enzymatic cleavage of Se-methyl seleno-
methionine selenonium salt. Plant Soil, 40: 107-118.
LI, C., HUANG, J., & LI, C. (in press) Observational report
on the effects of taking sodium selenite for six years
continuously as a preventive for Kashin-Beck disease as shown
in X-ray studies. In: Proceedings of the Third International
Symposium on Selenium in Biology and Medicine.
LIANG, S. (1985) The prophylactic and curing effect of
selenium (Se) in combatting of the Kaschin-Beck's disease.
LIEBSCHER, K. & SMITH, H. (1968) Essential and non-essential
trace elements. A method of determining whether an element is
essential or nonessential in human tissue. Arch. environ.
Health, 17: 881-890.
LINDBERG, P. (1968) Selenium determination in plant and
animal material, and in water. A methodological study. Acta
vet. Scand. (suppl. 23): 48 pp.
LINDBERG, P. & JACOBSSON, S.O. (1970) Relationship between
selenium content of forage, blood, and organs of sheep, and
lamb mortality rate. Acta vet. Scand., 11: 49-58.
LIPINSKIJ, S. (1962) [Background for evaluation of selenium
as an industrial toxicant.] Gig. i Sanit., 1: 91-93 (in
Russian).
LO, L.W., KOROPATNICK, J., & STICH, H.F. (1978) The
mutagenicity and cytotoxicity of selenite, "activated"
selenite, and selenate for normal and DNA repair-deficient
human fibroblasts. Mutat. Res., 49: 305-312.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LOMBECK, I., KASPEREK, K., FEINENDEGEN, L.E., & BREMER, H.J.
(1975) Serum-selenium concentrations in patients with
maple-syrup-urine disease and phenylketonuria under
dieto-therapy. Clin. Chim. Acta, 64: 57-61.
LOMBECK, I., KASPEREK, K., HARBISCH, H.D., BECKER, K.,
SCHUMANN, E., SCHROTER, W., FEINENDEGEN, L.E., & BREMER, H.J.
(1978) The selenium state of children. II. Selenium content
of serum, whole blood, hair and the activity of erythrocyte
glutathione peroxidase in dietetically treated patients with
phenylketonuria and maple-syrup-urine disease. Eur. J.
Pediatr., 128: 213-223.
LUO, X., WEI, H., YANG, C., XING, J., LIU, X., QIAO, C., FENG,
Y., LIU, J., LIU, Y., WU, Q., LIU, X., GUO, J., STOECKER,
B.J., SPALLHOLTZ, J.E., & YANG, S.P. (1985) Bioavailability
of selenium to residents in a low-selenium area of China. Am.
J. clin. Nutr., 42: 439-448.
MAAG, D.D. & GLENN, M.W. (1967) Toxicity of selenium: farm
animals. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H., ed.
Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 127-140.
MAAG, D.D., ORSBORN, J.S., & CLOPTON, J.R. (1960) The effect
of sodium selenite on cattle. Am. J. vet. Res., 21: 1049-l053.
MCCABE, L.J., SYMONS, J.M., LEE, R.D., & ROBECK, G.G. (1970)
Survey of community water supply systems. J. Am. Water Works
Assoc., 62: 670-687.
MCCONNELL, K.P. & CHO, G.J. (1965) Transmucosal movement of
selenium. Am. J. Physiol., 208: 1191-1195.
MCCONNELL, K.P. & PORTMAN, O.W. (1952a). Excretion of
dimethyl selenide by the rat. J. Biol. Chem., 195: 277-282.
MCCONNELL, K.P. & PORTMAN, O.W. (1952b) Toxicity of dimethyl
selenide in the rat and mouse. Proc. Soc. Exp. Biol. Med., 79:
230-231.
MCCONNELL, K.P., BROGHAMER, W.L., Jr, BLOTCKY, A.J., & HURT,
O.J. (1975) Selenium levels in human blood and tissues in
health and in disease. J. Nutr., 105: 1026-1031.
MCCONNELL, K.P., JAGER, R.M., BLAND, K.I., & BLOCTCKY, A.J.
(1980) The relationship of dietary selenium and breast
cancer. J. surg. Oncol., 15: 67-70.
MCCOY, K.E.M. & WESWIG, P.H. (1969) Some selenium responses
in the rat not related to vitamin E. J. Nutr., 98: 383-389.
MCDANIEL, M., SHENDRIKAR, A.D., REISZNER, K.D., & WEST, P.W.
(1976) Concentration and determination of selenium from
environmental samples. Anal. Chem., 48: 2240-2243.
MCDOWELL, L.R., FROSETH, J.A., & PIPER, R.C. (1978)
Influence of arsenic, sulfur, cadmium, tellurium, silver, and
selenium on the selenium-vitamin E deficiency in the pig.
Nutr. Rep. Int., 17: 19-33.
MCKEEHAN, W.L., HAMILTON, W.G., & HAM, R.G. (1976) Selenium
is an essential trace nutrient for growth of WI-38 diploid
human fibroblasts. Proc. Natl Acad. Sci. (USA), 73: 2023-2027.
MCKENZIE, J.M. (1977) Trace elements in total parenteral
nutrition in New Zealand. In: Trace elements in human and
animal health in New Zealand, Hamilton, New Zealand, Waikato
University Press, pp. 59-69.
MCKENZIE, R.L., REA, H.M., THOMSON, C.D., & ROBINSON, M.F.
(1978) Selenium concentration and glutathione peroxidase
activity in blood of New Zealand infants and children. Am. J.
clin. Nutr., 3l: l4l3-l4l8.
MACKINNON, A.M. & SIMON, F.R. (1976) Impaired hepatic heme
synthesis in the phenobarbital-stimulated selenium-deficient
rat. Proc. Soc. Exp. Biol. Med., 152: 568-572.
MCLEAN, A.E.M. (1967) The effect of diet and vitamin E on
liver injury due to carbon tetrachloride. Br. J. exp. Path.,
48: 632-636.
MAINES, M.D. & KAPPAS, A. (1976) Selenium regulation of
hepatic heme metabolism: induction of sigma-aminolevulinate
synthase and heme oxygenase. Proc. Natl Acad. Sci.(USA), 73:
4428-443l.
MAJSTRUK, P.N. & SUCHKOV, B.P. (1978) [Sanitary hygienic
control of selenium levels in the main food commodities and
the daily intake of selenium,] Kiev, Kievskij, NII gig. Pit.,
4 pp (in Russian).
MARSHALL, M.V., JACOBS, M.M., & GRIFFIN, A.C. (1978)
Reduction in acetylaminofluorene (AAF) hepatocarcinogenesis by
selenium. Proc. Am. Assoc. Cancer Res., l9: 75.
MARTIN, J.L. & GERLACH, M.L. (1969) Separate elution by
ion-exchange chromatography of some biologically important
selenoamino acids. Anal. Biochem., 29: 257-264.
MARTIN, J.L. & HURLBUT, J.A. (1976) Tissue selenium levels
and growth responses of mice fed selenomethionine,
Se-methylselenocysteine, or sodium selenite. Phosphorus
Sulfur, 1: 295-300.
MARTIN, J.L. & SPALLHOLZ, J.S. (1976) Selenium in the immune
response. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburg, Pennsylvania,
Industrial Health Foundation, pp. 204-225.
MARTIN, S.E., ADAMS, G.H., SCHILLACI, M., & MILNER, J.A.
(1981) Antimutagenic effect of selenium on acridine orange
and 7,12-dimethylbenz(alpha)anthracene in the Ames Salmonella
microsomal system. Mutat. Res., 82: 41-46.
MASIRONI, R. & PARR, R. (1976) Selenium and cardiovascular
diseases: preliminary results of the WHO/IAEA joint research
programme. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 3l6-325.
MAXIA, V., MELONI, S., ROLLIER, M.A., BRANDONE, A.,
PATWARDHAN, V.N., WASLIEN, C.I., & SHAMI, S.E. (1972)
Selenium and chromium assay in Egyptian foods and in blood of
Egyptian children by activation analysis. In: Nuclear
activation techniques in the life sciences, Vienna,
International Atomic Energy Agency, pp. 527-550.
MEDINA, D. & SHEPHERD, F. (1980) Selenium-mediated
inhibition of mouse mammary tumorigenesis. Cancer Lett., 8:
241-245.
MEDINSKY, M.A., CUDDIHY, R.G., GRIFFITH, W.C., & MCCLELLAN,
R.O. (1981) A simulation model describing the metabolism of
inhaled and ingested selenium compounds. Toxicol. appl.
Pharmacol., 59: 54-63.
MEHLERT, A. & DIPLOCK, A.T. (1985) The glutathione-S-
transferases in selenium and vitamin E deficiency. Biochem.
J., 227: 823-831.
MICHIE, N.D., DIXON, E.J., & BUNTON, N.G. (1978) Critical
review of AOAC fluorometric method for determining selenium in
foods. J. Assoc. Off. Anal. Chem., 61: 48-51.
MIETTINEN, T.A., ALFTHAN, G., HUTTUNEN, J.K., PIKKARAINEN, J.,
NAUKKARINEN, V., MATTILA, S., & KUMLIN, T. (1983) Serum
selenium concentration related to myocardial infarction and
fatty acid content of serum lipids. Brit. med. J., 287:
517-519.
MILKS, M.M., WILT, S.R., ALI, I.I., & COURI, D. (1985) The
effects of selenium on the emergence of aflatoxin B1-induced
enzyme-altered foci in rat liver. Fundam. appl. Toxicol., 5:
320-326.
MILLAR, K.R. & SHEPPARD, A.D. (1972) alpha-Tocopherol and
selenium levels in human and cow's milk. N.Z. J. Sci., 15:
3-15.
MILLAR, K.R., GARDINER, M.A., & SHEPPARD, A.D. (1973) A
comparison of the metabolism of intravenously injected sodium
selenite, sodium selenate, and selenomethionine in rats. N.Z.
J. agric. Res., 16: 115-127.
MILLER, D., SOARES, J.H., Jr, BAUERSFELD, P., Jr, & CUPPETT,
S.L. (1972) Comparative selenium retention by chicks fed
sodium selenite, selenomethionine, fish meal, and fish
solubles. Poultry Sci., 51: 1669-1673.
MILNER, J.A. (1985) Effect of selenium on virally induced
and transplantable tumour models. Fed. Proc., 44: 2568-2572.
MONAENKOVA, A.M. & GLOTOVA, K.V. (1963) [Selenium
intoxication.] Gig. i Sanit., 6: 41-44 (in Russian).
MONDRAGON, M.C. & JAFFE, W.G. (1976) Ingestion of selenium
in Caracas, compared with some other cities. Arch. Latinoam.
Nutr., 26: 34l-352.
MONEY, D.F.L. (1970) Vitamin E and selenium deficiencies and
their possible aetiological role in the sudden death in
infants syndrome. N.Z. med. J., 7l: 32-34.
MONEY, D.F.L. (1978) Vitamin E, selenium, iron and vitamin A
content of livers from sudden infant death syndrome and
control children: interrelationships and possible
significance. N.Z. J. Sci Teel., 21: 41-55.
MOORE, J.A., NOIVA, R., & WELLS, I.C. (1984) Selenium
concentrations in plasma of patients with arteriographically
defined coronary atherosclerosis. Clin. Chem., 30(7):
1171-1173.
MORIYA, M., OHTA, T., WATANABE, K., WATANABE, Y., SUGIYAMA,
F., MIYAZAWA, T., & SHIRASU, Y. (1979) Inhibitors for the
mutagenicities of colon carcinogens, 1,2-dimethylhydrazine and
azoxymethane, in the host-mediated assay. Cancer Lett., 7:
325-330.
MORRIS, V.C. & LEVANDER, O.A. (1970) Selenium content of
foods. J. Nutr., 100: 1383-1388.
MOTSENBOCKER, M.A. & TAPPEL, A.L. (1982) A
selenocysteine-containing selenium-transport protein in rat
plasma. Biochim. Biophys. Acta, 719: 147-153.
MOXON, A.L. (1938) The effect of arsenic on the toxicity of
seleniferous grains. Science, 88: 81.
MOXON, A.L. (1940) Toxicity of selenium-cystine and some
other organic selenium compounds. J. Am. Pharm. Assoc. Sci.
Ed., 29: 249-250.
MOXON, A.L. (1976) Natural occurrence of selenium. In:
Proceedings of the Symposium on Selenium-Tellurium in the
Environment, Pittsburgh, Pennsylvania, Industrial Health
Foundation, pp. 1-9.
MOXON, A.L. & RHIAN, M. (1943) Selenium poisoning. Physiol.
Rev., 23: 305-337.
MOXON, A.L., ANDERSON, H.D., & PAINTER, E.P. (1938) The
toxicity of some organic selenium compounds. J. Pharmacol.
exp. Ther., 63: 357-368.
MOXON, A.L., OLSON, O.E., & SEARIGHT, W.V. (1939) Selenium
in rocks, soils, and plants. S. Dak. Agric. Exp. Sta. Tech.
Bull., 2: 94 pp.
MOXON, A.L., SCHAEFER, A.E., LARDY, H.A., DUBOIS, K.P., &
OLSON, O.E. (1940) Increasing the rate of excretion of
selenium from selenized animals by the administration of
p-bromobenzene. J. biol. Chem., l32: 785-786.
MOXON, A.L., OLSON, O.E., & SEARIGHT, W.V. (1950) Selenium
in rocks, soils, and plants. S. Dak. Agric. Exp. Sta. Tech.
Bull., 2.
MUHLER, J.C. & SHAFER, W.G. (1957) The effect of selenium on
the incidence of dental caries in rats. J. dent. Res., 36:
895-896.
MUNSELL, H.E., DEVANEY, G.M., & KENNEDY, M.H. (1936)
Toxicity of food containing selenium as shown by its effect on
the rat, Washington DC, US Department of Agriculture, 25 pp
(US Department of Agriculture Technical Bulletin No. 534).
MUTANEN, M. & KOIVISTOINEN, P. (1983) The role of imported
grain on the selenium intake of Finnish population 1941-1981.
Int. J. Vit. Nutr. Res., 53: 34-38.
MUTH, O.H. (1960) Carbon tetrachloride poisoning of ewes on
a low selenium ration. Am. J. vet. Res., 21: 86-87.
MUTH, O.H., ed. (1966) Selenium in biomedicine. In: Proceedings
of the First International Symposium at Oregon State University,
Westport, Connecticut, AVI Publishing Co., 445 pp.
MUTH, O.H., WESWIG, P.H., WHANGER, P.D., & OLDFIELD, J.E.
(197l) Effect of feeding selenium-deficient ration to the
subhuman primate (Saimiri sciureus). Am. J. vet. Res., 32:
l603-l605.
NAHAPETIAN, A.T., JANGHORBANI, M., & YOUNG, V.R. (1983)
Urinary trimethylselenonium excretion by the rat: effect of
level and source of selenium-75. J. Nutr., 113: 401-411.
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., KURATA, H., TONOMURA,
M., & TONOMURA, A. (1976) Studies on selenium-related
compounds. V. Cytogenetic effect and reactivity with DNA.
Mutat. Res., 40: 177-184.
NATIONAL CANCER INSTITUTE (1980) Bioassay of selenium
sulfide for possible carcinogenicity (gavage study), Bethesda,
Maryland, US Department of Health and Human Services, Public
Health Service, National Institutes of Health, 132 pp (DHHS
Publication No. (NIH) 80-1750).
NAVIA, J.M., MENAKER, L., SELTZER, J., & HARRIS, R.S. (1968)
Effect of Na2Se03 supplemented in the diet or the water on
dental caries of rats. Fed. Proc., 27: 676.
NAZARENKO, I.I. & ERMAKOV, A.N. (1971) Analytical chemistry
of selenium and tellurium, New York, Halsted Press.
NAZARENKO, I.I. & KISLOVA, I.V. (1977) [A highly sensitive
method for the analysis of migratory forms of selenium in
natural waters.] El. Viems. Labor. Technol. issled. obogashch.
min. syrja, 6: 1-8 (in Russian).
NAZARENKO, I.I., KISLOVA, I.V., GUSEJNOV, T.M., MKRTCHJAN,
M.A., & KISLOV, A.M. (1975) [Fluorometric determination of
selenium in biological material by 2,3-diaminonaphthalene].
Zh. Anal. Chim., 30(4): 733-737 (in Russian).
NELSON, A.A., FITZHUGH, O.G., & CALVERY, H.O. (1943) Liver
tumors following cirrhosis caused by selenium in rats. Cancer
Res., 3: 230-236.
NEWBERNE, P.M. & CONNER, M.W. (1974) Effect of selenium on
acute response to aflatoxin B1. In: Hemphill, D.D., ed. Trace
substances in environmental health. VIII, Columbia, Missouri,
University of Missouri Press, pp. 323-328.
NOCKELS, C.F. (1979) Protective effects of supplemental
vitamin E against infection. Fed. Proc., 38: 2134-2138.
NODA, M., TAKANO, T., & SAKURAI, H. (1979) Mutagenic
activity of selenium compounds. Mutat. Res., 66: 175-179.
NOGUCHI, T., CANTOR, A.H., & SCOTT, M.L. (1973) Mode of
action of selenium and vitamin E in prevention of exudative
drathesis in chicks. J. Nutr., 103(10): 1502-1511.
NORDMAN, E. (1974) Sodium selenite (selenium-75)
scintigraphy in diagnosis of tumours. Acta radiol.,
340(suppl.): 78 pp.
NORPPA, H., ET AL. (1980) Chromosomal effects of sodium
selenite in vivo. Hereditas, 93: 93-105.
NORRIS, F.H. & SANG, K. (1978) Amyotrophic lateral sclerosis
and low urinary selenium levels. J. Am. Med. Assoc., 239: 404.
OBERMEYER, B.D., PALMER, I.S., OLSON, O.E., & HALVERSON, A.W.
(1971) Toxicity of trimethylselenonium chloride in the rat
with and without arsenite. Toxicol. appl. Pharmacol., 20:
135-146.
OCHOA-SOLANO, A. & GITLER, C. (1968) Incorporation of
75Se-selenomethionine and 35S-methionine into chicken egg
white proteins. J. Nutr., 94: 243-248.
OELSCHLAGER, W. & MENKE, K.H. (1969) Concerning the selenium
content of plant, animal, and other materials. II. The
selenium and sulfur content of foods. Z. Ernahrungswiss., 9:
216-222.
OH, S.H., SUNDE, R.A., POPE, A.L., & HOEKSTRA, W.G. (1976a)
Glutathione peroxidase response to selenium intake in lambs
fed a Torula yeast-based, artificial milk. J. anal. Sci., 42:
977-983.
OH, S.H., POPE, A.L., & HOEKSTRA, W.G. (1976b) Dietary
selenium requirement of sheep fed a practical-type diet as
assessed by tissue glutathione peroxidase and other criteria.
J. anal. Sci., 42: 984-992.
OHLENDORF, H.M., HOFFMAN, D.J., SAIKI, M.K., & ALDRICH, T.W.
(1986) Embryonic mortality and abnormalities of aquatic
birds: apparent impact of selenium from irrigation drain
water. Sci. total Environ., 52: 49-63.
OLSON, O.E. (1967) Soil, plant, animal cycling of excessive
levels of selenium. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI Publishing Co., Inc, pp. 297-3l2.
OLSON, O.E. (1969) Selenium as a toxic factor in animal
nutrition. In: Proceedings of the Georgia Nutrition
Conference, Atlanta, Georgia, pp. 68-78.
OLSON, O.E. (1970) Selenium in feedstuffs: deficiencies and
excesses. Proceedings of the 31st Minnesota Nutrition
Conference, Minneapolis, University of Minnesota, pp. 7-l3.
OLSON, O.E. (1976) Methods of analysis for selenium. A
review. In: Proceedings of the Symposium on Selenium-Tellurium
in the Environment, Pittsburgh, Pennsylvania, Industrial
Health Foundation, pp. 67-84.
OLSON, O.E. (1978) Selenium in plants as a cause of
livestock poisoning. In: Keeler, R.F., Van Kampen, K.R., &
James, L.F., ed. Effects of poisonous plants on livestock, New
York, Academic Press, pp. 121-133.
OLSON, O.E. & FROST, D.V. (1970) Selenium in papers and
tabaccos. Environ. Sci. Technol., 4: 686-687.
OLSON, O.E. & PALMER, I.S. (1976) Selenoamino acids in
tissues of rats administered inorganic selenium. Metabolism,
25: 299-306.
OLSON, O.E., WHITEHEAD, E.I., & MOXON, A.L. (1942)
Occurrence of soluble selenium in soils and its availability
to plants. Soil Sci., 54: 47-53.
OLSON, O.E., SCHULTE, B.M., WHITEHEAD, E.I., & HALVERSON,
A.W. (1963) Effect of arsenic on selenium metabolism in
rats. J. agric. food Chem., 11: 531-534.
OLSON, O.E., NOVACEK, E.J., WHITEHEAD, E.I., & PALMER, I.S.
(1970) Investigations on selenium in wheat. Phytochemistry,
9: 1181-1188.
OLSON, O.E., PALMER, I.S., & WHITEHEAD, E.I. (1973)
Determination of selenium in biological materials. In: Glick,
D., ed. Methods of biochemical analysis, New York, John Wiley
and Sons, Inc, Vol. 21, pp. 39-78.
OLSON, O.E., PALMER, I.S., & CARY, E.E. (1975) Modification
of the official fluorometric method for selenium in plants. J.
Assoc. Off. Anal. Chem., 58: 117-121.
OLSON, O.E., CARY, E.E., & ALLAWAY, W.H. (1976) Fixation and
volatilization by soils of selenium from trimethylselenonium.
Agron. J., 68: 839-843.
OLSON, O.E., PALMER, I.S., & HOWE, M. (1978) Selenium in
foods consumed by South Dakotans. Proc. S.D. Acad. Sci., 57:
113-121.
OLSSON, U., ONFELT, A., & BEIJE, B. (1984) Dietary selenium
deficiency causes decreased N-oxygenation of N'N-dimethyl-
aniline and increased mutagenicity of dimethylnitrosamine in
the isolated rat liver/cell cultrue system. Mutat. Res., 126:
73-80.
OMAYE, S.T., REDDY, K.A., & CROSS, C.E. (1978) Enhanced lung
toxicity of paraquat in selenium-deficient rats. Toxicol.
appl. Pharmacol., 43: 237-247.
OSTADALOVA, I., BABICKY, A., & OBENBERGER, J. (1979)
Cataractogenic and lethal effect of selenite in rats during
postnatal ontogenesis. Physiol. Bohemoslov., 28: 393-397.
OVERVAD, K., THORLING, E.B., BJERRING, P., & EBBESEN, P.
(1985) Selenium inhibits UV-light-induced skin carcinogenesis
in hairless mice. Cancer Lett., 27: 163-170.
PAINTER, E.P. (1941) The chemistry and toxicity of selenium
compounds with special reference to the selenium problem.
Chem. Rev., 28: 179-213.
PALMER, I.S. & OLSON, O.E. (1974) Relative toxicities of
selenite and selenate in the drinking-water of rats. J. Nutr.,
104(3): 306-314.
PALMER, I.S. & OLSON, O.E. (1979) Partial prevention by
cyanide of selenium poisoning in rats. Biochem. Biophys. Res.
Commun., 90: 1379-1386.
PALMER, I.S., FISCHER, D.D., HALVERSON, A.W., & OLSON, O.E.
(1969) Identification of a major selenium excretory product
in rat urine. Biochim. Biophys. Acta, 177: 336-342.
PALMER, I.S., GUNSALUS, R.P., HALVERSON, A.W., & OLSON, O.E.
(1970) Trimethylselenonium ion as a general excretory product
from selenium metabolism in the rat. Biochim. Biophys. Acta,
208: 260-266.
PALMER, I.S., ARNOLD, R.L., & CARLSON, C.W. (1973) Toxicity
of various selenium derivatives to chick embryos. Poultry
Sci., 52: 1841-l846.
PALMER, I.S., OLSON, O.E., HALVERSON, A.W., MILLER, R., &
SMITH, C. (1980) Isolation of factors in linseed oil meal
protective against chronic selenosis in rats. J. Nutr., 110:
145-150.
PARIZEK, J. & OSTADALOVA, I. (1967) The protective effect of
small amounts of selenite in sublimate intoxication. Experientia
(Basel), 23: 142-143.
PARIZEK, J., OSTADALOVA, I., KALOUSKOVA, J., BABICKY, A., &
BENES, J. (1971) The detoxifying effects of selenium.
Interrelations between compounds of selenium and certain
metals. In: Mertz, W. & Cornatzer, W.E., ed. Newer trace
elements in nutrition, New York, Marcel Dekker, Inc,
pp. 85-122.
PARIZEK, J., ET AL. (1974) Some hormonal and environmental
factors influencing selenium metabolism and action. In:
Proceedings of the Ninth International Congress in Nutrition,
Mexico, 1972 (Coop. Nutr. Cong., 16: 94-97).
PARIZEK, J., KALOUSKOVA, J., KORUNOVA, V., BENES, J., &
PAVLIK, L. (1976) The protective effect of pretreatment with
selenite on the toxicity of dimethylselenide. Physiol.
Bohemoslov., 25: 573-576.
PARIZEK, J., KALOUSKOVA, J., BENES, J., & PAVLIK, L. (1980)
Interactions of selenium-mercury and selenium-selenium
compounds. Ann. N.Y. Acad. Sci., 355: 347-360.
PASCOE, G.A. & CORREIA, M.A. (1985) Structural and
functional assembly of rat intestinal cytochrome P-450
isozymes: effects of dietary iron and selenium. Biochem.
Pharmacol., 34: 599-608.
PASCOE, G.A., SAKAI-WONG, J., SOLIVEN, E., & CORREIA, M.A.
(1983) Regulation of intestinal cytochrome P-450 and Heme by
dietary nutrients. Critical role of selenium. Biochem.
Pharmacol., 32(20): 3027-3035.
PAULSEN, P.J. (1977) Determination of cadmium, copper, iron,
lead, mercury, molybdenum, nickel, selenium, silver,
tellurium, thallium, and zinc. Natl Bur. Stand. Special Publ.
(USA), 492: 33-48.
PAVLIK, L., KALOUSKOVA, J., VOBECKY, M., DEDINA, BENES, J., &
PARIZEK, J. (1979) Selenium levels in the kidneys of male
and female rats. In: Nuclear activation techniques in the life
sciences, 1978, Vienna, International Atomic Energy Agency,
pp. 213-223.
PELEKIS, E.E., PELEKIS, L.L., & TAURE, I. JA. (1975)
[Instrumental neutron-activation method of selenium determination
in biological materials.] Vitaminy, 8: 146-150 (in Russian).
PENCE, B.C. & BUDDINGH, F. (1985) Effect of selenium
deficiency on incidence and size of 1,2-dimethylhydrazine-
induced colon cancer in rats. J. Nutr., 115: 1196-1202.
PENNINGTON, J.A.T., WILSON, D.B., NEWELL, R.F., HARLAND, B.F.,
JOHNSON, R.D., & VANDERVEEN, J.E. (1984) Selected minerals
in foods surveys, 1974 to 1981/82. J. Am. Diet. Assoc., 84:
771-780.
PERONA, G., GUIDI, G.C., PIGA, A., CELLERINO, R., MENNA, R., &
ZATTI, M. (1978) In vivo and in vitro variations of human
erythrocyte glutathione peroxidase activity as result of cells
ageing, selenium availability and peroxide activation. Br. J.
Haematol., 39: 399-408.
PERRY, H.M., Jr, ERLANGER, M.W., & PERRY, E.F. (1976)
Limiting conditions for the induction of hypertension in rats
by cadmium. In: Hemphill, D.D., ed. Trace substances in
environmental health. X, Columbia, Missouri, University of
Missouri Press, pp. 459-467.
PIERCE, F.D. & BROWN, H.R. (1977) Comparison of inorganic
interferences in atomic absorption spectrometric determination
of arsenic and selenium. Anal. Chem., 49: 1417-1422.
PIERCE, S. & TAPPEL, A.L. (1977) Effects of selenite and
selenomethionine on glutathione peroxidase in the rat. J.
Nutr., 107: 475-479.
PILLAY, K.K.S., THOMAS, C.C., Jr, & SONDEL, J.A. (1971)
Activation analysis of airborne selenium as a possible
indicator of atmospheric sulfur pollutants. Environ. Sci.
Technol., 5: 74-77.
PLAA, G.L. & WITSCHI, H. (1976) Chemicals, drugs, and lipid
peroxidation. Ann. Rev. Pharmacol. Toxicol., 16: 125-141.
PLEBAN, P.A., MUNYANI, A., & BEACHUM, J. (1982)
Determination of selenium concentration and glutathine
peroxidase activity in plasma and erythrocytes. Clin. Chem.,
28: 311-316.
PLETNIKOVA, I.P. (1970) Biological effect and safe
concentration of selenium in drinking-water. Hyg. Sanit., 35:
176-181.
POLEY, W.E. & MOXON, A.L. (1938) Tolerance levels of
seleniferous grains in laying rations. Poultry Sci., 17: 72-76.
POOLE, C.F., EVANS, N.J., & WIBBERLEY, D.G. (1977)
Determination of selenium in biological samples by gas-liquid
chromatography with electron-capture detection. J.
Chromatogr., 136: 73-83.
PRATLEY, J.E. & MCFARLANE, J.D. (1974) The effect of
sulphate on the selenium content of pasture plants. Aust. J.
exp. Agric. Anim. Husb., 14: 533-538.
PRINGLE, P. (1942) Occupational dermatitis following
exposure to inorganic selenium compounds. Br. J. Dermatol.
Syphil., 54: 54-58.
PROHASKA, J.R. & GANTHER, H.E. (1976) Selenium and
glutathione peroxidase in developing rat brain. J. Neurochem.,
27: 1379-1387.
PROHASKA, J.R. & GANTHER, H.E. (1977) Glutathione peroxidase
activity of glutathione-S-transferases purified from rat
liver. Biochem. Biophys. Res. Commun., 76: 437-445.
PYEN, G. & FISHMAN, M. (1978) Automated determination of
selenium in water. At. Absorpt. Newslett., 17: 47-48.
RANDOLPH, W.F. (1978) Food additives permitted in feed and
drinking water of animals. Selenium. Fed. Reg., 43:
11700-11701.
RANSONE, J.W., SCOTT, N.M., Jr, & KNOBLOCK, E.C. (1961)
Selenium sulfide intoxication. New Engl. J. Med., 264: 384-385.
RAVIKOVITCH, S. & MARGOLIN, M. (1957) Selenium in soils and
plants. Ktavim. Rec. agric. Res. Sta., 7: 41-52.
RAY, J.H. (1984) Sister-chromatid exchange induction by
sodium selenite: reduced glutathione converts Na2SeO3 to its
SCE-inducing form. Mutat. Res., 141: 49-53.
RAY, J.H. & ALTENBURG, L.C. (1978) Sister-chromatid exchange
induction by sodium selenite: dependence on the presence of
red blood cells or red blood cell lysate. Mutat. Res., 54:
343-354.
RAY, J.H. & ALTENBURG, L.C. (1980) Dependence of the
sister-chromatid exchange-inducing abilities of inorganic
selenium compounds on the valence state of selenium. Mutat.
Res., 78: 261-266.
RAY, J.H., ALTENBURG, L.C., & JACOBS, M.M. (1978) Effect of
sodium selenite and methyl methanesulfonate or N-hydroxy-2-
acetylamino-fluorene co-exposure on sister-chromatid exchange
production in human whole blood cultures. Mutat. Res., 57:
359-368.
REA, H.M., THOMSON, C.D., CAMPBELL, D.R., & ROBINSON, M.F.
(1979) Relation between erythrocyte selenium concentrations
and glutathione peroxidase (EC 1.11.1.9) activities of New
Zealand residents and visitors to New Zealand. Br. J. Nutr.,
42: 201-208.
REAMER, D.C. & VEILLON, C. (1983a) Letter to the editor.
Anal. Chem., in press.
REAMER, D.C. & VEILLON, C. (1983b) A double isotape dilution
method for using stable selenium isotapes in metabolic tracer
studies: analysis by gas chromatography/mass spectrometry
(GC/MS). J. Nutr., 113: 786-792.
REITER, R. & WENDEL, A. (1983) Selenium and drug metabolism.
Multiple modulations of mouse liver enzymes. Biochem.
Pharmacol., 32(20): 3063-3067.
RHEAD, W.J., CARY, E.E., ALLAWAY, W.H., SALTZSTEIN, S.L., &
SCHRAUZER, G.N. (1972) The vitamin E and selenium status of
infants and the sudden infant death syndrome. Bioinorg. Chem.,
1: 289-294.
RICHOLD, M., ROBINSON, M.F., & STEWART, R.D.H. (1977)
Metabolic studies in rats of 75Se incorporated in vivo into
fish muscle. Br. J. Nutr., 38: 19-29.
RIELY, C.A., COHEN, G., & LIEBERMAN, M. (1974) Ethane
evolution: a new index of lipid peroxidation. Science, 183:
208-210.
RILEY, J.F. (1968) Mast cells, co-carcinogenesis and
anti-carcinogenesis in the skin of mice. Experientia (Basel),
24: 1237-1238.
ROBBINS, C.W. & CARTER, D.L. (1970) Selenium concentrations
in phosphorus fertilizer materials and associated uptake by
plants. Soil Sci. Soc. Am. Proc., 34: 506-509.
ROBERTSON, D.S.F. (1970) Selenium, a possible teratogen?
Lancet, 1: 518-519.
ROBINSON, J.R., ROBINSON, M.F., LEVANDER, O.A., & THOMSON,
C.D. (1985) Urinary excretion of selenium by New Zealand and
North American human subjects on differing intakes. Am. J.
clin. Nutr., 41: 1023-1031.
ROBINSON, M.F. & THOMSON, C.D. (1981) Selenium levels in
humans vs environmental sources. In: Spallholz, J.E., Martin,
J.L., & Ganther, H.E., ed. Selenium in biology and medicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 283-302
ROBINSON, M.F. & THOMSON, C.D. (1983) The role of selenium
in the diet. Nutr. Abstr. Rev., 53: 3-26.
ROBINSON, M.F. & THOMSON, C.D. (1986) Selenium status of the
food supply and residents of New Zealand (Beijing Conference).
ROBINSON, M.F., MCKENZIE, J.M., THOMSON, C.D., & VAN RIJ, A.L.
(1973) Metabolic balance of zinc, copper, cadmium, iron,
molybdenum and selenium in young New Zealand women. Br. J.
Nutr., 30: 195-205.
ROBINSON, M.F., GODFREY, P.J., THOMSON, C.D., REA, H.M., & VAN
RIJ, A.M. (1978a) Blood selenium and glutathione peroxidase
activity in normal subjects and in surgical patients with and
without cancer in New Zealand. Am. J. clin. Nutr., 32:
1477-1485.
ROBINSON, M.F., REA, H.M., FRIEND, G.M., STEWART, R.D.H.,
SNOW, P.C., & THOMSON, C.D. (1978b) On supplementing the
selenium intake of New Zealanders. II. Prolonged metabolic
experiments with daily supplements of selenomethionine,
selenite, and fish. Br. J. Nutr., 39: 589-600.
ROBINSON, M.F., GODFREY, J.P., THOMSON, C.D., REA, H.M., & VAN
RIJ, A.M. (1979) Blood selenium and glutathione peroxidase
activity in normal subjects and in surgical patients with and
without cancer in New Zealand. Am. J. clin. Nutr., 32:
1477-1485.
ROBINSON, M.F., CAMPBELL, D.R., STEWART, R.D.H., REA, H.M.,
THOMSON, C.D., SNOW, P.G., & SQUIRES, I.H.W. (1981) Effect
of daily supplements of selenium on patients with muscular
complaints in Otago and Canterbury. N.Z. med. J., 93: 289-292.
ROBINSON, M.F., CAMPBELL, D.R., SUTHERLAND, W.H.F., HERBISON,
P.G., PAULIN, J.M., & SIMPSON, F.O. (1983) Selenium and risk
factors for cardiovascular disease in New Zealand. N.Z. med.
J., 96: 755-757.
ROBINSON, M.F., THOMSON, C.D., & HUEMMER, P.K. (1985) Effect
of a megadose of ascorbic acid, a meal and orange juice on the
absorption of selenium as sodium selenite. N.Z. med. J., 98:
627-629.
ROBINSON, W.O. (1933) Determination of selenium in wheat and
soils. J. Assoc. Off. Agric. Chem., 16: 423-424.
ROHMER, R., CARROT, E., & GOUFFAULT, J. (1950) Nouvel aspect
de l'intoxication par les composés du sélénium. Bull. Soc.
Chim. France, 5(17): 275-278.
ROSIN, M.P. & STICH, H.F. (1979) Assessment of the use of
the Salmonella mutagenesis assay to determine the influence of
antioxidants on carcinogen-induced mutagenesis. Int. J.
Cancer, 23: 722-727.
ROSENFELD, I. & BEATH, O.A. (1954) Effect of selenium on
reproduction in rats. Proc. Soc. Exp. Biol. Med., 87: 295-297.
ROSENFELD, I. & BEATH, O.A. (1964) Selenium geobotany,
biochemistry, toxicity and nutrition, New York, Academic
Press, 411 pp.
ROSIN, M.P. (1981) Inhibition of spontaneous mutagenesis in
yeast cultures by selenite, selenate, and selenide. Cancer
Lett., 13: 7-14.
ROSSI, L.C., CLEMENTE, G.F., & SANTARONI, G. (1976) Mercury
and selenium distribution in a defined area and in its
population. Arch. environ. Health, 31: 160-165.
ROTRUCK, J.T., POPE, A.L., GANTHER, H.E., SWANSON, A.B.,
HAFEMAN, D.G., & HOEKSTRA, W.G. (1973) Selenium: biochemical
role as a component of glutathione peroxidase. Science, 179:
588-590.
RUDERT, C.P. & LEWIS, A.R. (1978) The effect of potassium
cyanide on the occurrence of nutritional myopathy in lambs.
Rhod. J. agric. Res., 16: 109-116.
RUSIECKI, W. & BRZEZINSKI, J. (1966) Influence of sodium
selenate on acute thallium poisonings. Acta Pol. pharm., 23:
74-80.
SAKURAI, H. & TSUCHIYA, K. (1975) A tentative recommendation
for the maximum daily intake of selenium. Environ. Physiol.
Biochem., 5: 107-118.
SALONEN, J.T. (1985) Selenium in cardiovascular diseases and
cancer. Epidemiologic findings from Finland. In: Boström, H.
& Ljungstedt, N., ed. Trace elements in health disease,
Stockholm, Sweden, Almqvist & Wiksell International, pp.
172-186.
SALONEN, J.T., ALFTHAN, G., HUTTUNEN, J.K., PIKKARAINEN, J., &
PUSKA, P. (1982) Association between cardiovascular death
and myocardial infarction and serum selenium in a watched-pair
longitudinal study. Lancet, 2: 175-179.
SALONEN, J.T., ALFTHAN, G., HUTTUNEN, J.K., & PUSKA, P.
(1984a) Association between serum selenium and the risk of
cancer. Am. J. Epidemiol., 120(3): 342-349.
SALONEN, J.T., SALONEN, R., PENTTILA, I., HERRANEN, J., JAUHIAINEN,
J., KANTOLA, M., KAURANEN, P., LAPPETELAINEN, R., MAENPAA, P., &
PUSKA, P. (1984b) Serum fatty acids, apolipoproteins, selenium
and vitamin antioxidants and the risk of death from ischaemic heart
disease. Am. J. Cardiol., 56(2): 226.
SALONEN, J.T., SALONEN, R., LAPPETELAINEN, R., MAENPAA, P.H.,
ALFTHAN, G., & PUSKA, P. (1985) Risk of cancer in relation
to serum concentrations of selenium and vitamins A and E:
matched case-control analysis of prospective data. Brit. med.
J., 290: 417-420.
SARATHCHANDRA, S.U. & WATKINSON, J.H. (1981) Oxidation of
elemental selenium to selenite by Bacillus megaterium.
Science, 211: 600-601.
SARATHCHANDRASCHMIDT, K. & HELLER, W. (1976) Selenium
concentration and activity of glutathione peroxidase in
lysates of human erythrocytes. Blut, 33: 247-251.
SCHECTER, A., SHANSKE, W., STENZLER, A., QUINTILIAN, H., &
STEINBERG, H. (1980) Acute hydrogen selenide intoxication.
Chest, 77: 554-555.
SCHILLACI, M., MARTIN, S.E., & MILNER, J.A. (1982) The
effects of dietary selenium on the biotransformation of
7,12-dimethylbenz(alpha)anthracene. Mutat. Res., 101: 31-37.
SCHRAUZER, G.N. (1976) Cancer mortality correlation studies.
II. Regional associations of mortalities with the consumptions
of foods and other commodities. Medic. Hypoth., 2: 39-49.
SCHRAUZER, G.N. & ISHMAEL, D. (1974) Effects of selenium and
of arsenic on the genesis of spontaneous mammary tumors in
inbred C3H mice. Ann. clin. Lab. Sci., 4: 441-447.
SCHRAUZER, G.N. & WHITE, D.A. (1978) Selenium in human
nutrition: dietary intakes and effects of supplementation.
Bioinorg. Chem., 8: 303-318.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1976)
Inhibition of the genesis of spontaneous mammary tumors in
C3H mice: effects of selenium and of selenium-antagonistic
elements and their possible role in human breast cancer.
Bioinorg. Chem., 6: 265-270.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1977)
Cancer mortality correlation studies. III. Statistical
associations with dietary selenium intakes. Bioinorg. Chem.,
7: 23-34.
SCHRAUZER, G.N, WHITE, D.A., & SCHNEIDER, C.J. (1978a)
Effects of selenium, arsenic, and zinc on the genesis of
spontaneous mammary tumors in inbred female C3H mice. In:
Kirchgessner, M., ed. Trace element metabolism in man and
animals. III, Freising-Weihenstephan, West Germany,
Arbeitskreis für Tierernährungsforschung Weihenstephan, pp.
387-390.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1978b)
Selenium and cancer: Effects of selenium and of the diet on
the genesis of spontaneous mammary tumors in virgin inbred
female C3H/St mice. Bioinorg. Chem., 8: 387-396.
SCHRAUZER, G.N., WHITE, D.A., MCGINNESS, J.E., SCHNEIDER,
C.J., & BELL, L.J. (1978c) Arsenic and cancer: effects of
joint administration of arsenite and selenite on the genesis
of mammary adenocarcinoma in inbred female C3H/St mice.
Bioinorg. Chem., 9: 245-253.
SCHROEDER, H.A. & MITCHENER, M. (1971a) Toxic effects of
trace elements on the reproduction of mice and rats. Arch.
environ. Health, 23: 102-106.
SCHROEDER, H.A. & MITCHENER, M. (1971b) Selenium and
tellurium in rats: effect on growth, survival and tumors. J.
Nutr., 101: 1531-1540.
SCHROEDER, H.A. & MITCHENER, M. (1972) Selenium and
tellurium in mice. Effects on growth, survival, and tumors.
Arch. environ. Health, 24: 66-71.
SCHROEDER, H.A., FROST, D.V., & BALASSA, J.J. (1970)
Essential trace metals in man: selenium. J. chron. Dis., 23:
227-243.
SCHUBERT, J.R., MUTH, O.H., OLDFIELD, J.E., & REMMERT, L.F.
(196l) Experimental results with selenium in white muscle
disease of lambs and calves. Fed. Proc., 20: 689-694.
SCHULTZ, T.D. & LEKLEM, J.E. (1983) Selenium status of
vegetarians, nonvegetarians, and hormone-dependent cancer
subjects. Am. J. clin. Nutr., 37: 114-118.
SCHWARZ, K. (1961) Development and status of experimental
work on Factor 3-selenium. Fed. Proc., 20: 666-673.
SCHWARZ, K. (1962) Vitamin E, trace elements, and sulfhydryl
groups in respiratory decline. Vitam. Horm., 20: 463-484.
SCHWARZ, K. (1967) Discussion comments. In: Muth, O.H.,
Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc,
pp. 225-226.
SCHWARZ, K. (1976) The discovery of the essentiality of
selenium, and related topics (a personal account). In:
Proceedings of the Symposium on Selenium-Tellurium in the
Environment, Pittsburgh, Pennsylvania, Industrial Health
Foundation, pp. 349-376.
SCHWARZ, K. (1977) Amyotrophic lateral sclerosis and
selenium. J. Am. Med. Assoc., 238: 2365.
SCHWARZ, K. & FOLTZ, C.M. (1957) Selenium as an integral
part of Factor 3 against dietary necrotic liver degeneration.
J. Am. Chem. Soc., 79: 3292-3293.
SCHWARZ, K. & FOLTZ, C.M. (1958) Factor 3 activity of
selenium compounds. J. biol. Chem., 233: 245-251.
SCHWARZ, K., PORTER, L.A., & FREDGA, A. (1972) Some
regularities in the structure-function relationship of
organoselenium compounds effective against dietary liver
necrosis. Ann. NY Acad. Sci., 192: 200-214.
SCOTT, M.L. & THOMPSON, J.N. (1971) Selenium content of
feedstuffs and effects of dietary selenium levels upon tissue
selenium in chicks and poults. Poultry Sci., 50: 1742-1748.
SCOTT, M.L., OLSON, G., KROOK, L., & BROWN, W.R. (1967)
Selenium-responsive myopathies of myocardium and of smooth
muscle in the young poult. J. Nutr., 91: 573-583.
SEIFTER, J., EHRICH, W.E., HUDYMA, G., & MUELLER, G. (1946)
Thyroid adenomas in rats receiving selenium. Science, 103: 762.
SELJANKINA, K.P., JAHIMOVICH, N.P., ALEKSEEVA, L.S., & PETINA,
A.A. (1974) [Selenium and tellurium levels in environmental
media.] In: [Hygiene and occupational diseases,] Moscow, Nauka
Publishing House, Vol 21, pp. 69-71 (in Russian).
SENF, H.W. (1941) [A case of poisoning by hydrogen
selenide]. Dtsch. Med. Wochenschr., 67: 1094-1096 (in German).
SEWARD, C.R., VAUGHAN, G., & HOVE, E.L. (1966) Effect of
selenium on incisor depigmentation and carbon tetrachloride
poisoning in vitamin E-deficient rats. Proc. Soc. Exp. Biol.
Med., 121: 850-852.
SHACKLETTE, H.T., BOERNGEN, J.G., & KEITH, J.R. (1974)
Selenium, fluorine, and arsenic in surficial materials of the
conterminous United States, 14 pp (US Geological Survey
Circular, 692).
SHAMBERGER, R.J. (1970) Relationship of selenium to cancer.
I. Inhibitory effect of selenium on carcinogenesis. J. Natl
Cancer Inst., 44: 931-936.
SHAMBERGER, R.J. (1971) Is selenium a teratogen? Lancet, 2:
1316.
SHAMBERGER, R.J. (1978) Antioxidants and cancer. VIII.
Cadmium-selenium levels in kidneys. In: Kirchgessner, M., ed.
Trace element metabolism in man and animals. III,
Freising-Weihenstephan, West Germany, Arbeitskreis für
Tierernährungsforschung Weihenstephan, pp. 391-392.
SHAMBERGER, R.J. & FROST, D.V. (1969) Possible protective
effect of selenium against human cancer. Can. Med. Assoc. J.,
100: 682.
SHAMBERGER, R.J. & RUDOLPH, G. (1966) Protection against
cocarcinogenesis by antioxidants. Experientia (Basel), 22: 116.
SHAMBERGER, R.J. & WILLIS, C.E. (1971) Selenium distribution
and human cancer mortality. Crit. Rev. clin. Lab. Sci., 2:
211-221.
SHAMBERGER, R.J., BAUGHMAN, F.F., KALCHERT, S.L., WILLIS,
C.E., & HOFFMAN, G.C. (1973a) Carcinogen-induced chromosomal
breakage decreased by antioxidants. Proc. Natl Acad. Sci.
(USA), 70: 1461-1463.
SHAMBERGER, R.J., RUKOVENA, E., LONGFIELD, A.K., TYTKO, S.A.,
DEODHAR, S., & WILLIS, C.E. (1973b) Antioxidants and cancer.
I. Selenium in the blood of normals and cancer patients. J.
Natl Cancer Inst., 50(4): 863-870.
SHAMBERGER, R.J., TYTKO, S.A., & WILLIS, C.E. (1975)
Selenium and heart disease. In: Hemphill, D.D., ed. Trace
substances in environmental health. IX, Columbia, Missouri,
University of Missouri Press, pp. 15-22.
SHAMBERGER, R.J., TYTKO, S.A., & WILLIS, C.E. (1976)
Antioxidants and cancer. VI. Selenium and age-adjusted human
cancer mortality. Arch. environ. Health, 31: 231-235.
SHAMBERGER, R.J., CORLETT, C.L., BEAMAN, K.D., & KASTEN, B.L.
(1979) Antioxidants reduce the mutagenic effect of malonaldehyde
and beta-propiolactone. Partix, antioxidants, and cancer.
Mutat. Res., 66: 349-355.
SHAMBERGER, R.J., WILLIS, C.E., & MCCORMACK, L.J. (1979)
Selenium and heart disease. III. Blood selenium and heart
mortality in 19 states. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
University of Missouri, pp. 59-63.
SHAPIRO, J.R. (1972) Selenium and carcinogenesis: a review.
Ann. NY Acad. Sci., 192: 215-219.
SHEARER, T.R. (1973) Lack of effect of selenium on
glycolytic and citric acid cycle intermediates in rat kidney
and liver. Proc. Soc. Exp. Biol. Med., 144: 688-691.
SHEARER, T.R. (1975) Developmental and postdevelopmental
uptake of dietary organic and inorganic selenium into the
molar teeth of rats. J. Nutr., 105: 338-347.
SHEARER, T.R. & HADJIMARKOS, D.M. (1975) Geographic distribution
of selenium in human milk. Arch. environ. Health, 30: 230-233.
SHEARER, T.R. & RIDLINGTON, J.W. (1976) Fluoride-selenium
interaction in the hard and soft tissues of the rat. J. Nutr.,
106: 451-456.
SHEARER, T.R., MCCORMACK, D.W., DESART, D.J., BRITTON, J.L., &
LOPEZ, M.T. (1980) Histological evaluation of selenium
induced cataracts. Exp. Eye Res., 31: 327-333.
SHENDRIKAR, A.D. (1974) Critical evaluation of analytical
methods for the determination of selenium in air, water, and
biological materials. Sci. total Environ., 3: 155-168.
SHENDRIKAR, A.D. & FAUDEL, G.B. (1978) Distribution of trace
metals during oil shale retorting. Environ. Sci. Technol., 12:
332-334.
SHRIFT, A. (1964) A selenium cycle in nature? Nature
(Lond.), 201: 1304-1305.
SHRIFT, A. (1973) Selenium compounds in nature and medicine.
Metabolism of selenium by plants and microorganisms. In:
Klayman, D.L. & Gunther, W.H.H., ed. Organic selenium
compounds: their chemistry and biology, New York, John Wiley
and Sons, Inc, pp. 763-814.
SHRIFT, A. & VIRUPAKSHA, T.K. (1965) Seleno-amino acids in
selenium-accumulating plants. Biochim. Biophys. Acta, 100:
67-75.
SHUM, G.T.C., FREEMAN, H.C., & UTHE, J.F. (1977) Flameless
atomic absorption spectrophotometry of selenium in fish and
food products. J. Assoc. Off. Anal. Chem., 60: 1010-1014.
SHUMAEV, V.D., MAKUSHINSKAJA, N.D., & CHIGRINA, T.A. (1976)
[Hygiene aspects of industrial waste water pollution in some
non-ferrous metal enterprises in Kazahstan by chemicals
regulated for the point of sanitary toxicological risk
characteristics.] Zolvavoohram Kazah, 4: 36-38 (in Russian).
SIMPSON, B.H. (1972a) An epidemiological study of carcinoma
of the small intestine in New Zealand sheep. N.Z. vet. J., 20:
91-97.
SIMPSON, B.H. (1972b) The possible relationship of selenium
and superphosphate to the frequencey of occurrence of
intestinal carcinomas in sheep. N.Z. vet. J., 20: 224.
SINDEEVA, N.D. (1959) [Minerology, occurrence and main
characteristics of the geochemistry of selenium and
tellurium,] Moscow, Publishing House of the Academy of
Sciences of the USSR, 257 pp (in Russian).
SIRIANNI, S.R. & HUANG, C.C. (1983) Induction of sister
chromatid exchange by various selenium compounds in Chinese
hamster cells in the presence and absence of S9 mixture.
Cancer Lett., 18: 109-116.
SIVERTSEN, T., KARLSEN, J.T., & FROSLIE, A. (1977) The
relationship of erythrocyte glutathione peroxidase to blood
selenium in swine. Acta vet. Scand., 18: 494-500.
SKORNJAKOVA, L.V., BURCHANOV, A.I., & SALECHOV, M.I. (1969)
[On the acute manifestations of selenium poisoning.] Gig. Tr.
Prof. Zabol., 11: 45-46 (in Russian).
SMITH, C.R., Jr, WEISLEDER, D., MILLER, R.W., PALMER, I.S., &
OLSON, O.E. (1980) Linustatin and neolinustatin: cyanogenic
glycosides of linseed meal that protect animals against
selenium toxicity. J. org. Chem., 45: 507-510.
SMITH, M.I. & STOHLMAN, E.F. (1940) Further observations on
the influence of dietary protein on the toxicity of selenium.
J. Pharmacol. exp. Ther., 70: 270-278.
SMITH, M.I. & WESTFALL, B.B. (1937) Further field studies on
the selenium problem in relation to public health. US Public
Health Rep., 52: 1375-1384.
SMITH, M.I., FRANKE, K.W., & WESTFALL, B.B. (1936) The
selenium problem in relation to public health. A preliminary
survey to determine the possibility of selenium intoxication
in the rural population living on seleniferous soil. US Public
Health Rep., 51: 1496-1505.
SMITH, M.I., WESTFALL, B.B., & STOHLMAN, E.F., Jr (1937) The
elmination of selenium and its distribution in the tissues. US
Public Health Rep., 52: 1171-1177.
SMITH, M.I., WESTFALL, B.B., & STOHLMAN, E.F., Jr (1938)
Studies on the fate of selenium in the organism. US Public
Health Rep., 53: 1199-1216.
SOKOLOFF, L. (1985) Endemic forms of osteoarthritis. Clin.
rheum. Dis., 11: 187-202.
SPALLHOLZ, J.E., MARTIN, J.L., GERLACH, M.L., & HEINZERLING,
R.H. (1973) Immunologic responses of mice fed diets
supplemented with selenite selenium. Proc. Soc. Exp. Biol.
Med., 143: 685-689.
SPALLHOLZ, J.E., MARTIN, J.L., GERLACH, M.L., & HEINZERLING,
R.H. (1975) Injectable selenium: effect on the primary
immune response of mice. Proc. Soc. Exp. Biol. Med., 148:
37-40.
SPALLHOLZ, J.E., COLLINS, G.E., & SCHWARZ, K. (1978) A
single test-tube method for the fluorometric micro-
determination of selenium. Bioinorg. Chem., 9: 453-459.
SPRINKER, L.H., HARR, J.R., NEWBERNE, P.M., WHANGER, P.D., &
WESWIG, P.H. (197l) Selenium deficiency lesions in rats fed
vitamin E supplemented rations. Nutr. Rep. Int., 4: 335-340.
STADTMAN, T.C. (1977) Biological function of selenium. Nutr.
Rev., 35: 161-166.
STEAD, R.J., HINKS, L.J., HODSON, M.E., REDINGTON, A.N.,
CLAYTON, B.E., & BATTEN, J.C. (1985) Selenium deficiency and
possible increased risk of carcinoma in adults with cystic
fibrosis. Lancet (October): 862-863.
STEWART, R.D.H., GRIFFITHS, N.M., THOMSON, C.D., & ROBINSON,
M.F. (1978) Quantitative selenium metabolism in normal New
Zealand women. Br. J. Nutr., 40: 45-54.
STOEWSAND, G.S., GUTENMANN, W.H., & LISK, D.J. (1978) Wheat
grown on fly ash: high selenium uptake and response when fed
to Japanese quail. J. agric. food Chem., 26: 757-759.
SU, Y. & YU, W. (1983) Nutritional bio-geochemical etiology
of Keshan disease. Chin. med. J., 96: 594-596.
SU, Y., CUI, S., GU, B., ZENG, X., & YU, W. (1982)
Experimental study of the effects of corn and vegetables from
Keshan disease endemic districts on the growth and myocardium
in rats. Acta nutr. Sinica, 4: 261-269.
SUBCOMMITTEE ON SELENIUM - COMMITTEE ON ANIMAL NUTRITION
(1983) Selenium in nutrition, revised ed., Washington DC,
Board on Agriculture, National Research Council, National
Academy of Sciences, 174 pp.
SUCHKOV, B.P. (1971) [Selenium content in major nutrients
consumed by the population of the Ukrainian SSR.] Vopr.
Pitan., 30(b): 75-77 (in Russian).
SUCHKOV, B.P. & KACAP, I.M. (1971) [Dental caries in the
population of the Chernovitsk region in relation with the
impact of microelements.] Gig. i Sanit., 3: 91-93 (in Russian).
SUCHKOV, B.P. & ZHIVECKIJ, A.V. (1973) [Selenium levels in
the blood of healthy people and people with neoplasms in a
Chernovici region republican interdepartmental collective.]
In: [Microelements in medicine,] Kiev, Zdarovie, Zdarovie
Publishing House, Vol. 4, pp. 69-73 (in Russian).
SUCHKOV, B.P., KACAP, I.M., & GULGASENKO, A.I. (1973)
[Affection of the population of the Chernovitsi region with
caries in association with selenium content in the teeth].
Stomatologia, 52: 21-23 (in Russian).
SUCHKOV, B.P., SHEVCHUK, I.A., & MARBAR, A.I. (1977)
[Histopathomorphological changes in the organs and tissues of
laboratory animals on a synthetic diet with a low content of
selenium and vitamin E.] Vopr. Pitan., 2: 33-41 (in Russian).
SUCHKOV, B.P., SHTUTMAN, C.M., & HALMURADOV, A.G. (1978)
[The biochemical role of selenium in animal organisms.]
Ukrain. Biochem., 50: 659-671 (in Russian).
SUN, S., ZHAI, F., ZHOU, L., & YANG, G. (1985) The
bioavailability of soil selenium in Keshan disease and high
selenium areas. Chinese J. end. Dis., 4: 21-28.
SUNDE, R.A. (1984) Selenoproteins. J. Am. Oil Chem. Soc.,
61: 1891-1898.
SUNDSTROM, H., KORPELA, H., VIINIKKA, L., & KAUPPILA, A.
(1984a) Serum selenium and glutathione peroxidase, and plasma
lipid peroxides in uterine, ovarian or vulvar cancer, and
their responses to antioxidants in patients with ovarian
cancer. Cancer Lett., 24: 1-10.
SUNDSTROM, H., YRJANHEIKKI, E., & KAUPPILA, A. (1984b) Serum
selenium in patients with ovarian cancer during and after
therapy. Carcinogenesis, 5(6): 731-734.
SVERDLINA, N.T & MASLENNIKOVA, V.S. (1961) [Hygienic
evaluation of working conditions in production of selenium
photocells.] Leningrad, Mater. manch sessin Leningrad NII gig.
truda prof. Zab, pp. 73-75 (in Russian).
SWEINS, A. (1983) Protective effect of selenium against
arsenic-induced chromosomal damage in cultured human lympho-
cytes. Hereditas, 98: 249-252.
SYMANSKI, H. (1950) [A case of hygrogen selenide poisoning.]
Dtsch. Med. Wochenschr., 75: 1730 (in German).
SZYDLOWSKI, F.J. & DUNMIRE, D.L. (1979) Semi-automatic
digestion and automatic analysis for selenium in animal feeds.
Anal. Chim Acta, 105: 445-449.
TAN, J., ZHENG, D., HOU, S., ZHU, W., LI, R., ZHU, Z., & WANG,
W. (in press) Selenium ecological chemico-geography and
endemic Keshan disease and Kashin-Beck disease in China. In:
Proceedings of the Third International Symposium on Selenium
in Biology and Medicine.
TANK, G. & STORVICK, C.A. (1960) Effect of naturally
occurring selenium and vanadium on dental caries. J. dent.
Res., 39: 473-488.
TAYLOR, F.B. (1963) Significance of trace elements in public
finished water supplies. J. Am. Water Works Assoc., 55:
619-623.
TEEL, R.W. (1984) A comparison of the effect of selenium on
the mutagenicity and metabolism of benzo(alpha)pyrene in rat and
hamster liver S9 activation systems. Cancer Lett., 24: 281-289.
TEEL, R.W. & KAIN, S.R. (1984) Selenium modified mutagenicity
and metabolism of benzo(alpha)pyrene in an S9-dependent system.
Mutat. Res., 127: 9-14.
THOMPSON, H.J. (1984) Selenium as an anticarcinogen. J.
agric. food Chem., 32: 422-425.
THOMPSON, J.N. & SCOTT, M.L. (1969) Role of selenium in the
nutrition of the chick. J. Nutr., 97: 335-342.
THOMPSON, J.N. & SCOTT, M.L. (1970) Impaired lipid and
vitamin E absorption related to atrophy of the pancreas in
selenium-deficient chicks. J. Nutr., 100: 797-809.
THOMPSON, J.N., ERDODY, P., & SMITH, D.C. (1975) Selenium
content of food consumed by Canadians. J. Nutr., 105: 274-277.
THOMPSON, K.C. (1975) The atomic-fluorescence determination
of antimony, arsenic, selenium and tellurium by using the
hydride generation technique. Analyst, 100: 307-310.
THOMPSON, R.H., MCMURRAY, C.H., & BLANCHFLOWER, W.J. (1976)
The levels of selenium and glutathione peroxidase activity in
blood of sheep, cows and pigs. Res. vet. Sci., 20: 229-231.
THOMSON, C.D. (1974) Recovery of large doses of selenium
given as sodium selenite with or without vitamin E. N.Z. med.
J., 80: 163-168.
THOMSON, C.D. (1985) Selenium dependent and non-selenium
dependent glutathione peroxidase in human tissues of New
Zealand residents. Biochem. Int., 10: 673-679.
THOMSON, C.D. & ROBINSON, M.F. (1980) Selenium in human
health with emphasis on those aspects peculiar to New
Zealand. Am. J. clin. Nutr., 33: 303-323.
THOMSON, C.D. & ROBINSON, M.F. (1986) Urinary and faecal
excretions and absorption of a large supplement of selenium as
selenite or as selenate. Am. J. clin. Nutr. (submitted for
publication).
THOMSON, C.D. & STEWART, R.D.H. (1973) Metabolic studies of
75Se selenomethionine and 75Se selenite in the rat. Br. J.
Nutr., 30: 139-147.
THOMSON, C.D. & STEWART, R.D.H. (1974) The metabolism of
75Se selenite in young women. Br. J. Nutr., 32: 47-57.
THOMSON, C.D., ROBINSON, B.A., STEWART, R.D.H., & ROBINSON,
M.F. (1975a) Metabolic studies of 75Se selenocystine and
75Se selenomethionine in the rat. Br. J. Nutr., 34: 501-509.
THOMSON, C.D., STEWART, R.D.H., & ROBINSON, M.F. (1975b)
Metabolic studies in rats of 75Se selenomethionine and of 75Se
incorporated in vivo into rabbit kidney. Br. J. Nutr., 33:
45-54.
THOMSON, C.D., REA, H.M., ROBINSON, M.F., & CHAPMAN, O.W.
(1977a) Low blood selenium concentrations and glutathione
peroxidase activities in elderly people. Proc. Univ. Otago
Med. Sch., 55: 18-19.
THOMSON, C.D., REA, H.M., DOESBURG, V.M., & ROBINSON, M.F.
(1977b) Selenium concentrations and glutathione peroxidase
activities in whole blood of New Zealand residents. Br. J.
Nutr., 37: 457-460.
THOMSON, C.D., BURTON, C.E., & ROBINSON, M.F. (1978) On
supplementing the selenium intake of New Zealanders. I. Short
experiments with large doses of selenite or selenomethionine.
Br. J. Nutr., 39: 579-587.
THOMSON, C.D., ROBINSON, M.F., CAMPBELL, D.R., & REA, H.M.
(1982) Effect of prolonged supplementation with daily
supplements of selenomethionine and sodium selenite on
glutathione peroxidase activity in blood of New Zealand
residents. Am. J. clin. Nutr., 36: 24-31.
THOMSON, C.D., ONG, L.K., & ROBINSON, M.F. (1985) Effect of
supplementation with high-selenium wheat bread on selenium,
glutathione peroxidase and related enzymes in blood components
of New Zealand residents. Am. J. clin. Nutr., 41: 1015-1022.
THORN, J., ROBERTSON, J., & BUSS, D.H. (1978) Trace
nutrients. Selenium in British food. Br. J. Nutr., 39: 391-396.
TINSLEY, I.J., HARR, J.R., BONE, J.F., WESWIG, P.H., &
YAMAMOTO, R.S. (1967) Selenium toxicity in rats. I. Growth
and longevity. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H.,
ed. Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 141-152.
TSEN, C.C. & COLLIER, H.B. (1959) Selenite as a relatively
weak inhibitor of some sulphydryl enzymes. Nature (Lond.),
183: 1327-1328.
TSEN, C.C. & TAPPEL, A.L. (1958) Catalytic oxidation of
glutathione and other sulfhydryl compounds by selenite. J.
biol. Chem., 233(5): 1230-1232.
TSONGAS, T.A. & FERGUSON, S.W. (1977) Human health effects
of selenium in a rural Colorado drinking-water supply. In:
Hemphill, D.D., ed. Trace substances in environmental health.
XI, Columbia, Missouri, University of Missouri Press, pp.
30-35.
ULLREY, D.E., BRADY, P.S., WHETTER, P.A., KU, P.K., & MAGEE,
W.T. (1977) Selenium supplementation of diets for sheep and
beef cattle. J. anal. Sci., 45: 559-565.
UNDERWOOD, E.J. (1977) Trace elements in human and animal
nutrition, 4th ed., New York, Academic Press, 545 pp.
US DEPARTMENT OF HEALTH AND HUMAN SERVICES, FOOD AND DRUG
ADMINISTRATION (1984) 21 CFR Pt.573.920 Selenium. Fed. Reg.,
49: 627-628.
US FDA (1974) Final environmental impact statement rule
making on selenium in animal feeds, Washington DC, US Food and
Drug Administration, 131 pp.
US FDA (1975) Bureau of Foods Compliance Program Evaluation
Report. Total diet studies, Fiscal Year 1974, Washington DC,
US Food and Drug Administration, 32 pp.
US NAS/NRC (197l) Selenium in nutrition, Washington DC,
National Academy of Science, National Research Council,
Agricultural Board, Committee on Animal Nutrition,
Subcommittee on Selenium, 79 pp.
US NAS/NRC (1976) Selenium, Washington DC, National Academy
of Science, National Research Council, Assembly of Life
Sciences, Medical and Biological Effects of Environmental
Pollutants, 203 pp.
US NAS/NRC (1979) Zinc, Baltimore, Maryland, National
Academy of Science, National Research Council, Assembly of
Life Sciences, Committee on Medical and Biologic Effects of
Environmental Pollutants, 471 pp.
US NAS/NRC (1980) Recommended dietary allowances, Washington
DC, National Academy of Science, National Research Council,
Food and Nutrition Board, Committee on Dietary Allowances, 185
pp.
VAN DER LINDEN, R., DE CORTE, F., & HOSTE, J. (1974)
Activation analysis of biological material with ruthenium as a
multi-element comparator. Anal. Chim. Acta, 71: 263-275.
VAN KAMPEN, K.R. & JAMES, L.F. (1978) Manifestations of
intoxication by selenium-accumulating plants. In: Keeler,
R.F., Van Kampen, K.R., & James, L.F., ed. Effects of
poisonous plants on livestock, New York, Academic Press,
pp. 135-138.
VAN RIJ, A.M., ROBINSON, M.F., GODFREY, P.J., THOMSON, C.D., &
RHEA, H.M. (1978) Selenium blood levels in cancer and other
diseases in surgery. In: Hemphill, D.D., ed. Trace substances
in environmental health. XII, Columbia, Missouri, University
of Missouri Press, pp. 157-163.
VAN RIJ, A.M., THOMSON, C.D., MCKENZIE, J.M., & ROBINSON,
M.F. (1979) Selenium deficiency in total parenteral
nutrition. Am. J. clin. Nutr., 32: 2076-2085.
VAN VLEET, J.F. (1976) Induction of lesions of selenium-
vitamin E deficiency in pigs fed silver. Am. J. vet. Res., 37:
1415-1420.
VAN VLEET, J.F., MEYER, K.B., & OLANDER, H.J. (1974) Acute
selenium toxicosis induced in baby pigs by parenteral
administration of selenium-vitamin E preparations. J. Am. Vet.
Med. Assoc., 165: 543-547.
VARO, P. & KOIVISTOINEN, P. (1980) Mineral element
composition of Finnish foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn,
Zn, Mo, Co, Ni, Cr, F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb,
and ash. XII. General discussion and nutritional evaluation.
Acta agric. Scand. Suppl., 22: 165.
VARO, P. & KOIVISTOINEN, P. (1981) Annual variations in the
average selenium intake in Finland: cereal products and milk
as sources of selenium in 1979/80. Int. J. Vit. Nutr. Res.,
51: 79-84.
VERNIE, L.N. (1984) Selenium in carcinogenesis. Biochim.
Biophys. Acta, 738(4): 203-217.
VERNIE, L.N., BONT, W.S., GINJAAR, H.B., & EMMELOT, P.
(1975) Elongation factor 2 as the target of the reaction
product between sodium selenite and glutathione (GSSeSG) in
the inhibiting of amino acid incorporation in vitro. Biochim.
Biophys. Acta, 414: 283-292.
VERNIE, L.N., GINJARR, H.B., WILDERS, I.T., & BONT, W.S.
(1978) Amino acid incorporation in a cell-free system derived
from rat liver studied with the aid of selenodiglutathione.
Biochim. Biophys. Acta, 518(3): 507-517.
VIRTAMO, J., VALKEILA, E., ALFTHAN, G., PUNSAR, S., HUTTUNEN,
J.K., & KARVONEN, M. (1985) Serum selenium and the risk of
coronary heart disease and stroke. Am. J. Epidemiol., 122(2):
276-282.
VOBECKY, M., PAVLIK, L., & BENES, J. (1977) Non-destructive
neutron activation assay of submicrogram quantities of
selenium. Radiochem. Radioanal. Lett., 29(4): 159-164.
VOBECKY, M., DEDINA, J., PAVLIK, L., & VALASEK, J. (1979)
Gamma-ray interferences in the determination of selenium by
the Inaa method. Radiochem. Radioanal. Lett., 38(3): 197-204.
VOLGAREV, M.N. & TSCHERKES, L.A. (1967) Further studies in
tissue changes associated with sodium selenate. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 179-184.
WAGNER, P.A., HOEKSTRA, W.G., & GANTHER, H.E. (1975)
Alleviation of silver toxicity by selenite in the rat in
relation to tissue glutathione peroxidase. Proc. Soc. Exp.
Biol. Med., 148: 1106-1110.
WAHLSTROM, R.C. & OLSON, O.E. (1959) The effect of selenium
on reproduction in swine. J. anim. Sci., 18: 141-145.
WALKER, G.W.R. & BRADLEY, A.M. (1969) Interacting effects of
sodium monohydrogenarsenate and selenocystine on crossing over
in Drosophila melanogaster. Can. J. Genet. Cytol., 11: 677-688.
WALKER, G.W.R. & TING, K.P. (1967) Effect of selenium on
recombination in barley. Can. J. Genet. Cytol., 9: 314-320.
WANG, Z., LI, C., & LI, L. (1985) An epidemiological
investigation on the selenium content of water, cereals and
hair of children in Heilongjiang province. Chinese J. end.
Dis., 4: 330-333.
WARRINGTON, P.B. (1979) Selenium. Eng. Min. J., 180: 149-150.
WASLIEN, C.I. (1976) Human intake of trace elements. In:
Prasad, A.S., ed. Trace elements in human health and disease.
II. Essential and toxic elements, New York, Academic Press,
pp. 347-370.
WATKINSON, J.H. (1967) Analytical methods for selenium in
biological material. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI publishing Co., Inc, pp. 97-117.
WATKINSON, J.H. (1974) The selenium status of New
Zealanders. N.Z. med. J., 80: 202-205.
WATKINSON, J.H. (1979) Semi-automated fluorimetric
determination of nanogram quantities of selenium in biological
materials. Anal. Chim. Acta, 105: 319-325.
WATKINSON, J.H. (1981) Changes of blood selenium in New
Zealand adults with time and importation of Australian wheat.
Am. J. clin. Nutr., 34: 936-942.
WATKINSON, J.H. & BROWN, M.W. (1979) New phase-separating
device and other improvements in the semi-automated
fluorimetric determination of selenium. Anal. Chim. Acta, 105:
451-454.
WEDDERBURN, J.F. (1972) Selenium and cancer. N.Z. vet. J.,
20: 56-57.
WEISSMAN, S.H., CUDDIHY, R.G., & BURKSTALLER, M.A. (1979)
Distribution and retention of inhaled selenious acid and
selenium metal aerosols in Beagle dogs. In: Hemphill, D.D.,
ed. Trace substances in environmental health. XIII, Columbia,
Missouri, University of Missouri Press, pp. 477-482.
WEISSMAN, S.H., CUDDIHY, R.G., & MEDINSKY, M.A. (1983)
Absorption, distribution, and retention of inhaled selenious
acid and selenium metal aerosols in Beagle dogs. Toxicol.
appl. Pharmacol., 67: 331-337.
WELSH, S.O. (1979) The protective effect of vitamin E and
N,N'-diphenyl- p-phenylenediamine (DPPD) against methyl mercury
toxicity in the rat. J. Nutr., 109: 1673-1681.
WELSH, S.O. & SOARES, J.H., Jr (1976) The protective effect
of vitamin E and selenium against methyl mercury toxicity in
the Japanese quail. Nutr. Rep. Int., 13: 43-51.
WELSH, S.O., HOLDEN, J.W., WOLF, W.R., & LEVANDER, O.A.
(1981) Selenium intake of Maryland residents consuming
self-selected diets. J. Am. Diet. Assoc., 79: 277-285.
WESTERMANN, D.T. & ROBBINS, C.W. (1974) Effect of S04-S
fertilization on Se concentration of alfalfa (Medicago sativa
L). Agron. J., 66: 207-208.
WESTERMARCK, T. (1977) Selenium content of tissues in
Finnish infants and adults with various diseases, and studies
on the effects of selenium supplementation in neuronal ceroid
lipofuscinosis patients. Acta pharmacol. toxicol., 41: 121-128.
WESTERMARCK, T., RAUNU, P., KIRJARINTA, M., & LAPPALAINEN, L.
(1977) Selenium content of whole blood and serum in adults
and children of different ages from different parts of
Finland. Acta pharmacol. toxicol., 40: 465-475.
WESWIG, P.H., TINSLEY, I.J., HARR, J.R., BONE, J.F., YAMAMOTO,
R.S., & FALK, H. (1966) Bioassay of selenium compounds for
carcinogenesis in rats. Final report, Corvallis, Oregon State
University, Departments of Agricultral Chemistry and
Veterinary Medicine, 131 pp.
WHANGER, P.D. (1976) Selenium versus metal toxicity in
animals. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 234-252.
WHANGER, P.D., MUTH, O.H., OLDFIELD, J.E., & WESWIG, P.H.
(1969) Influence of sulfur on incidence of white muscle
disease in lambs. J. Nutr., 97: 553-562.
WHANGER, P.D., PEDERSEN, N.D., & WESWIG, P.H. (1973)
Selenium proteins in ovine tissues. II. Spectral properties of
a 10,000 molecular weight selenium protein. Biochem. Biophys.
Res. Commun., 53: 1031-1035.
WHANGER, P.D., PEDERSON, N.D., HATFIELD, J., & WESWIG, P.H.
(1976a) Absorption of selenite and selenomethionine from
ligated digestive tract segments in rats. Proc. Soc. Exp.
Biol. Med., 153: 295-297.
WHANGER, P.D., WESWIG, P.H., SCHMITZ, J.A., & OLDFIELD, J.E.
(1976b) Effects of selenium, cadmium, mercury, tellurium,
arsenic, silver, and cobalt on White Muscle Disease in lambs
and effect of dietary forms of arsenic on its accumulation in
tissues. Nutr. Rep. Int., 14: 63-72.
WHEATCROFT, M.G., ENGLISH, J.A., & SCHLACK, C.A. (1951)
Effects of selenium on the incidence of dental caries in white
rats. J. dent. Res., 30: 523-524.
WHETTER, P.A. & ULLREY, D.E. (1978) Improved fluorometric
method for determining selenium. J. Assoc. Off. Anal. Chem.,
61: 927-930.
WHITEACRE, M.E., COMBS, G.F., & PARKER, R.S. (1983)
Peroxidative damage in nutritional pancreatic atrophy due to
selenium-deficiency in the chick. Fed. Proc. Fed. Am. Soc.
Exp. Biol., 42: 928.
WHITING, R.F., WEI, L., & STICH, H.F. (1980) Unscheduled DNA
synthesis and chromosome aberrations induced by inorganic and
organic selenium compounds in the presence of glutathione.
Mutat. Res., 78: 159-169.
WHO (1984) Guidelines for drinking water quality. Volume 2:
Health criteria and other supporting information, Geneva,
World Health Organization, 335 pp.
WILKIE, J.B. & YOUNG, M. (1970) Improvement in the 2,3-
diaminoaphthalene reagent for microfluorescent. Determination
of selenium in biological materials. J. agric. food Chem., 18:
944-945.
WILLETT, W.C., MORRIS, J.S., PRESSEL, S., TAYLOR, J.O., POLK,
B.F., STAMPFER, M.J., ROSNER, B., SCHNEIDER, K., & HAMES,
C.G. (1983) Prediagnostic serum selenium and risk of cancer.
Lancet (July): 130-134.
WILLIAMS, K.T. & BYERS, H.G. (1935) Occurrence of selenium
in the Colorado River and some of its tributaries. Ind. Eng.
Chem. Anal. Ed., 7: 431-432.
WILLIAMS, M.M.I. (1983) Selenium and glutathione peroxidase
in mature human milk. Proc. Univ. Otago Med. Sch., 61: 20-21.
WILSON, H.M. (1962) Selenium oxide poisoning. N.C. med. J.,
23: 73-75.
WILSON, P.S. & JUDSON, G.J. (1976) Glutathione peroxidase
activity in bovine and ovine erythrocytes in relation to blood
selenium concentration. Br. vet. J., 132: 428-434.
WITTING, L.A. & HORWITT, M.K. (1964) Effects of dietary
selenium, methionine, fat level, and tocopherol on rat growth.
J. Nutr., 84: 351-360.
WRIGHT, P.L. & BELL, M.C. (1966) Comparative metabolism of
selenium and tellurium in sheep and swine. Am. J. Physiol.,
211: 6-10.
WU, A.S.H., OLDFIELD, J.E., & WHANGER, P.D. (197l) Effect of
selenium, chromium and vitamin E on spermatogensis. J. anal.
Sci., 33: 273.
WU, A.S.H., OLDFIELD, J.E., WHANGER, P.D., & WESWIG, P.H.
(1973) Effect of selenium, vitamin E, and antioxidants on
testicular function in rats. Biol. Reprod., 8: 625-629.
WU, A.S.H., OLDFIELD, J.E., SHULL, L.R., & CHEEKE, P.R.
(1979) Specific effect of selenium deficiency on rat sperm.
Biol. Reprod., 20: 793-798.
XIA, X. & YU, S. (in press) Studies on the anti-carcinogenic
mechanisms of selenium - The effects of glucose oxidation and
related enzymes. Chinese J. Cancer.
YANG, G.Q., WANG, S., ZHOU, R., & SUN, S. (1983) Endemic
selenium intoxication of humans in China. Am. J. clin. Nutr.,
37: 872-881.
YANG, G., CHEN, J., WEN, Z., GE, K., ZHU, L., CHEN, X., &
CHEN, X. (1984) The role of selenium in Keshan disease. In:
Draper, H.H., ed. Advances in nutritional research, New York,
Plenum Press, pp. 203-231.
YANG, G., ZHOU, R., YIN, S., PIAO, J., ZHU, L., LIN, S., & GU,
L., MAI, R., XU, J., QIAG, F., LU, M., DENG, X., HUANG, J.,
LIN, & ZHOU, W. (1985) [Studies on the selenium requirement
of Chinese people. I. Physiological requirement, minimum
requirement, and minimum allowance.] J. Inst. Health, 14:
24-28 (in Chinese).
YANG, G.Q., ZHU, L.Z., LIU, S.J., GU, L.Z., QIAN, P.C., HUANG,
J.H., & LU, M.D. (1986) Studies of human selenium requirements
in China. In: Combs, G.F., Spallholz, J.E., Levander, O.A., &
Oldfield, J.E., ed. Selenium in biology and medicine, 3rd
Symposium, Westport, Connecticut, AVI Publishing Company.
YASUMOTO, K., IWAMI, K., YOSHIDA, M., & MITSUDA, H. (1976)
Selenium content of foods and its average daily intake in
Japan. Eiyo To Shokuryo, 29: 511-515.
YEH, Y. & JOHNSON, R.M. (1973) Vitamin E deficiency in the
rat. IV. Alteration in mitochondrial membrane and its relation
to respiratory decline. Arch. Biochem. Biophys., 159: 821-831.
YOUNG, S. & KEELER, R.F. (1962) Nutritional muscular dystrophy
in lambs. The effect of muscular activity on the symmetrical
distribution of lesions. Am. J. vet. Res., 23: 966-971.
YU, W.H. (1982) A study of nutritional and bio-geochemical
factors in the occurrence and development of Keshan disease.
Jpn Circ. J., 46: 1201-1207.
YU, S., CHU, Y., GONG, X., & HOU, C. (1985) Region variation
of cancer mortality incidence and its relation to selenium
levels in China. Ecol. trace Element Res., 7: 21-23.
YU, S., ZHU, Y., HON, C., & HUANG, C. (in press) Selective
effects of selenium on the function and structure of
mitochondria isolated from hepatoma and normal liver. In:
Proceedings of the Third International Symposium of Selenium
in Biology and Medicine.
ZABEL, N.L., HARLAND, J., GORMICAN, A.T., & GANTHER, H.E.
(1978) Selenium content of commercial formula diets. Am. J.
clin. Nutr., 31: 850-858.
ZHU, L. & LU, Z. et al. (1981) Effects in pigs fed the crops
grown in Keshan disease affected province of China. In:
Howell, J.M., Gawthone, J.M., & White, C.L., ed. Trace
Element Metabolism in Man and Animals (TEMA 4).
ZOLLER, W.H. & REAMER, D.C. (1976) Selenium in the
atmosphere. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 54-66.
ZOLLER, W.H., GLADNEY, E.S., & DUCE, R.A. (1974) Atmospheric
concentrations and sources of trace metals at the South Pole.
Science, 183: 198-200.
8.2.6. Attempts to associate low selenium intake with human diseases
8.2.6.1. Keshan Disease
Results of research in China have suggested a relationship
between low selenium status and the prevalence of Keshan disease,
an endemic cardiomyopathy that primarily affects children (Keshan
Disease Research Group, 1979a,b). Cases of Keshan disease were
recorded as early as 1907 in Heilongjiang Province of northeastern
China (Gu, 1983). Since the etiology of the disease was not known,
it was named after the locality in which it was originally
observed, Keshan County.
Epidemiologically, the disease exhibits a regional distribution
and occurs in a belt-like zone reaching from northeastern China to
the southwestern part of the country. There is a marked seasonal
fluctuation in the disease with more cases appearing during winter
in the north and during summer in the south. There is also a great
annual variation in the incidence of Keshan disease. Recently, the
incidence has decreased sharply such that from 1978 to 1980 less
than one death was reported per 100 000 members of the population
(Gu, 1983). The overall recent decline in the incidence of Keshan
disease has been attributed, at least in part, to the general
increase in the living standards of the people, such as better
sanitation, more medical attention, and improved quality of the
diet (He, 1979). There is also a shifting of epidemic foci from
year to year. Rural peasants constitute the population at risk
with children below 10 years of age and women of child-bearing age
most susceptible. According to Gu (1983), migrants from non-
affected areas will not contract the disease unless they have lived
in the endemic area for at least 3 months.
The criteria for diagnosing Keshan disease include acute or
chronic cardiac insufficiency, heart enlargement, gallop rhythm,
arrhythmia, and ECG changes (Chen et al., 1980). The disease is
classified into four types: acute (cardiogenic shock), subacute,
chronic (low output pump failure), and latent (normal heart
function but mild enlargement). There is no symptom or sign
specific for identifying the disease (Gu, 1983).
Histopathologically, Keshan disease is characterized by multifocal
necrosis and fibrous replacement of the myocardium.
In the past, many different hypotheses were advanced in an
attempt to explain the etiology of Keshan disease. For example,
there were theories of poisoning due to rhodamine, silica,
digitalis, carbon monoxide, barium, nitrite, and Fusarium
mycotoxins. However, clear-cut proof of these toxicants as a cause
of the disease has not been obtained despite much analytical effort
(Yang et al., 1984). Several nutritional problems have also been
proposed to play a role in Keshan disease such as deficiency of
protein, lipids, thiamin, magnesium, or molybdenum. A hypothesis
concerning Keshan disease etiology was proposed linking the disease
to selenium deficiency, after it was noted that severely endemic
areas coincided with areas where the incidence of enzootic selenium
deficiency diseases in farm animals was also high (Zhu et al.,
1981). On this basis, selenium supplements were used as a
preventive measure for Keshan disease. This hypothesis is very
appealing since cardiomyopathy is a prime feature of selenium-
vitamin E deficiency in many species of animals, including cattle,
sheep, and swine.
Since the initial proposal of the selenium-Keshan disease
connection, much evidence has been gathered in support of this
concept. For example, the average blood-selenium content was 0.021
± 0.001 mg/litre for the affected areas and 0.095 ± 0.088 mg/litre
for non-affected areas (Yang et al., 1984). The average hair-
selenium content was below 0.12 mg/kg in affected areas, whereas
hair-selenium levels in neighbouring but unaffected areas ranged
between 0.12 and 0.2 mg/kg. Average hair-selenium contents in
areas removed from the affected belt were between 0.25 and 0.6
mg/kg. The selenium level in several staple foods (rice, maize,
wheat, soybeans, black beans, and sweet potatoes) was lower in
areas affected with Keshan disease than in unaffected areas. It
was stated that an area could be considered to be unaffected
wherever the selenium content of grains was 0.04 mg/kg or more.
The Chinese workers stated that the amount of selenium needed to
prevent the disease was about 20 µg/day (Yang et al., 1984).
In some affected areas, there were highly localized pockets
(so-called "safety islands") that were free from the disease.
Apparently, these islands were protected because of the higher
selenium content of their crops in the immediate vicinity. For
example, the average selenium contents of rice and soybeans in one
such island were 0.020 and 0.025 mg/kg, respectively, whereas the
values for the corresponding crops in a nearby affected area were
0.0078 and 0.0057 mg/kg. It was also noted that the children in
the unaffected spot liked to catch shrimp from local streams and
that the selenium content of these dried shrimp was as high as 1
mg/kg (Keshan Disease Research Group, 1979b).
These relationships between selenium and Keshan disease led the
Chinese workers to conduct a randomized intervention trial to test
the possible prophylactic effect of selenium against this condition
in the population at risk (i.e., children 1 - 9 years old). In a
trial in 1974, 4510 children took sodium selenite and 3985 children
took the placebo (Table 56). The treated children took 0.5 mg
sodium selenite per week if 1 - 5 years old, or 1.0 mg per week if
6 - 9 years old. The morbidity rate due to Keshan disease was
1.35% in the placebo group (54 cases out of 3985 children) but only
0.22% in the treated group (10/4510). Since a significant
difference was also shown in the 1975 trial (0.95% morbidity rate
in the placebo group compared with 0.1% in those treated) the
placebo groups were abolished in 1976 and 1977. As a result, the
case rate dropped to 0.034% and 0% in these 2 years, respectively.
However, in 1976, there was one case out of 212 children who failed
to take the treatment.
No untoward side effects due to the sodium selenite were
observed, except for some individual cases of nausea, which could
be overcome by taking the medicine after meals. Physical
examinations and liver function tests indicated that the liver was
undamaged after continuous ingestion of the selenium tablets for
3 - 4 years.
Table 56. Effect of selenium on Keshan disease in childrena
-------------------------------------------------------------------
Treatment Year Number Number Outcome of alive cases Death
of of Turned Improved Turned
subjects cases latent chronic
-------------------------------------------------------------------
Placebo 1974 3985 54 16 9 2 27
1975 5445 52 13 10 3 26
Sodium 1974 4510 10 9 0 1 0
selenite 1975 6767 7 6 0 0 1
-------------------------------------------------------------------
a Adapted from: Keshan Disease Research Group (1979a).
Since 1976, more extensive intervention trials with sodium
selenite have been carried out in 5 counties in the same area of
Sichuan Province (Yang et al., 1984). All children, 1 to 12 years
of age, in some of the most severely affected communes, were
treated with selenium as described above, while untreated children
in nearby communes served as controls. The incidence rate of
Keshan disease in the selenium-treated children was lower in each
year of the five-year period than among the untreated children
(Table 57).
Table 57. Keshan disease incidence rates in selenium-treated and
untreated children in five counties of Sichuan provincea
-------------------------------------------------------------------------
Treated children Untreated children
Year Number of Number of Incidence Number of Number of Incidence
subjects cases (per 1000) subjects cases (per 1000)
-------------------------------------------------------------------------
1976 45 515 8 0.17 243 649 448 2.00
1977 67 754 15 0.22 222 944 350 1.57
1978 65 953 10 0.15 220 599 373 1.69
1979 69 910 33 0.47 223 280 300 1.34
1980 74 740 22 0.29 197 096 202 1.07
Total 323 872 88 0.27 1 107 568 1713 1.55
-------------------------------------------------------------------------
a Taken from: Yang et al. (1984).
Selenium intervention has proved to be very effective in the
prophylaxis of Keshan disease and it is very likely that selenium
insufficiency plays an important role in its etiology (Yang et al.,
1984). Nevertheless, the Chinese workers recognized that there
were certain epidemiological characteristics of the disease, which
suggested that additional etiological factors were involved. The
selenium hypothesis, for example, does not adequately explain the
seasonal variation in the disease, the occurrence of epidemic
years, or the annual shifting of epidemic foci. Such
characteristics are more compatible with an infectious theory and,
in fact, a hypothesis that the disease is a form of viral
myocarditis has been put forward (He, 1979). Perhaps the best way
to account for all the characteristics of the disease is to assume
that the disease has a multifactorial etiology and that a
combination of several factors may be involved (Yang et al., 1984).
There are some animal studies that favour such a possibility, since
selenium-deficient mice were less resistant to the cardiotoxic
effects of a Coxsackie B4 virus isolated from a patient with Keshan
disease (Bai et al., 1980). If selenium-deficient human beings are
also less resistant to viral infection, this phenomenon could
provide a reasonable explanation for many of the apparently
conflicting features of Keshan disease. In this view, selenium
deficiency would be the fundamental underlying condition that would
predispose persons to viral attack, possibly by impairing normal
immune function (Yang et al., 1984).
8.2.6.2. Kashin-Beck disease
Kashin-Beck disease is an endemic osteoarthropathy that occurs
in eastern Siberia and in certain parts of China, which is
characterized as a chronic, disabling, and degenerative
osteoarthrosis that mainly involves children (Sokoloff, 1985).
Although the etiology of this disease has not been fully
established, present works show that selenium deficiency might be
one of the main causes. This concept is based on the following
evidence. First, in China most of the endemic areas are located in
the same low-selenium zone, from northeast to southwest, as the
Keshan disease (section 8.2.6.1) (Tan et al., in press). For the
same reason, residents in these areas have low-selenium status
characterized by low blood- and hair-selenium levels, low blood
glutathione peroxidase activity, and low urinary-selenium
excretion. A survey carried out in Heilongjiang province of China
(one of the most heavily affected province) showed that the average
hair-selenium levels in 151 children in endemic areas (0.096 ± SD
0.026 mg/kg) was significantly lower than that of the 235 children
in the non-endemic areas (0.223 ± 0.083 mg/kg) (Wang et al., 1985).
The authors concluded that the low selenium status of children was
due to the low selenium content of the locally produced staple
food. The average selenium content of corn, wheat, and millet in
the endemic and non-endemic areas shown in the paper were: 0.0056
± SD 0.0038 (n = 262) versus 0.015 ± 0.015 (176), 0.0091 ± 0.0096
(225) versus 0.0235 ± 0.022 (120), and 0.0064 ± 0.0053 (14) versus
0.0197 ± 0.0144 (11) mg/kg, respectively. Second, sodium selenite
is reported to have both therapeutic and prophylactic effects on
this disease. Liang (1985) reported that 325 cases of Kashin-Beck
disease in Shaanxi province of China were randomly divided into a
treated and control group. The treated group was given sodium
selenite (1 mg/week for children 3 - 10 years of age and 2 mg/week
for children of 11 - 13 years of age) and the control group was
given a placebo. After one year, X-ray examination of the
metaphyseal changes of fingers showed that 81.9% of the cases in
the treated group had improved, none of the cases were getting
worse, and that 18.1% showed no change, while, in the control
group, only 39.6% of the cases had improved, 30% were getting
worse, and 41.5% showed no change. Li et al. (in press) reported
that children of 1 - 5 and 6 - 10 years of age in an endemic area
of Gansu province in China were supplemented with 0.5 and 1.0 mg
sodium selenite, respectively, per week over a period of 6 years.
X-ray examination showed that the incidence of Kashin-Beck disease
declined from 42% to 4% after the selenium intervention.
However, other information suggests that other factors in the
environment may also play some role in the disease. For example,
the possible role of mycotoxin contamination of cereals by certain
Fusarium strains in China and high phosphate and manganese
contents in the soil, food, and drinking-water in endemic areas of
the USSR have been suggested. More studies are required to clarify
the relationship between these hypotheses and Kashin-Beck disease.
8.2.6.3. Cancer
(a) Ecological studies
Shamberger & Frost (1969) first pointed out the inverse
relationship between selenium levels in forage crops and human
blood, and cancer death rates in various regions of the USA.
Subsequent papers expanded this concept (Shamberger & Willis, 1971;
Shamberger et al., 1976). From a number of comparisons, the
cancers frequently found at one time or another to have high
mortality associated with low-selenium areas or low blood-selenium
levels, included cancer of the tongue, oesophagus, stomach, colon,
rectum, liver, pancreas, larynx, lungs, kidneys, bladder, and
Hodgkin's disease and lymphoma. However, the high mortality of
some of these same cancers and others, was also found to be
associated with high-selenium areas or high blood-selenium levels;
these include cancer of the lung, prostate, pancreas, breast, lip,
skin, eye and dermal melanoma, and leukaemia/aleukaemia. Some of
these studies have been criticized as lacking strength and
consistency, particularly because the states for which cancer
mortality was calculated did not coincide directly with the natural
geographical units on which the estimates of selenium levels in
forage crops were made, thus leading frequently to
misclassification of the different selenium areas (Allaway, 1972,
1978). However, in a study attempting to minimize this problem by
using county data, similar inverse correlations were observed
between counties classified as intermediate or high for selenium
levels in forage, and cancers of the lung, colon, rectum, bladder,
oesophagus, pancreas, and all sites combined, for both males and
females, and cancers of the breast, ovary, and cervix (Clark,
1985).
Schrauzer (1976) found that the mortality rates due to several
cancers, including those of the large intestine, rectum, and breast
(so-called Type A cancers) were directly correlated with the
consumption of meat, eggs, milk, fat, and/or sugar and were
inversely correlated with the consumption of cereals and fish.
Just the opposite correlations were found for certain other cancers
such as hepatic and stomach cancer. Since cereals and seafoods are
good sources of dietary selenium, it was suggested that selenium
might be the factor in these foods that protected against Type A
cancers. Schrauzer et al. (1977) extended these studies to data
from 27 countries including the USA; New Zealand was intentionally
excluded. Dietary intake of selenium was found to be inversely
correlated with total age-adjusted cancer mortality, r = -0.46 ( P <
0.01) for males and r = -0.60 ( P < 0.001) for females. Significant
inverse correlations were observed between dietary-selenium intake
and mortality from cancers of the colon, rectum, prostate, female
breast, ovary, lung (males), and from leukaemia. Weak inverse
relationships were found with mortality from cancers of the
pancreas ( P = 0.06), bladder ( P = 0.1), and skin ( P = 0.1, males).
Other cancers including those of the stomach, oesophagus, and liver
did not show any significant direct or inverse correlations with
dietary-selenium intake. In this study, dietary-selenium intake
was calculated, assuming that the same average concentration of
selenium was present in the foods consumed in all countries (the
Task Group questioned the validity of such an assumption).
Mortality from cancers of the colon, rectum, prostate, lung, skin,
bladder (all Type A), and leukaemia showed significant inverse
correlations with blood-selenium levels in males (USA excluded).
However in the data from the USA, this relationship was not
observed for cancer of the prostate, lung, skin, or bladder, and
leukaemia, and similar inconsistencies were observed for other
cancers and in data for females.
Jansson et al. (1978) postulated an inverse relationship
between dietary-selenium and the rate of colorectal and breast
cancer, but found a direct correlation between the concentration
of selenium in the drinking-water and the rate of colorectal
cancer. Moreover, these workers commented that the same
statistical associations that indicated a protective effect of
dietary selenium against colon, rectum, and breast cancers also
indicated an increased risk of liver and stomach cancer due to
selenium.
In a recent study in China, Yu et al. (1985) obtained a mean
serum-selenium level in the whole blood of 1458 donors from 24
regions, of 107 µg/litre (range 22 - 314 µg/litre). Blood-selenium
levels were inversely correlated with age-adjusted total cancer
mortality for both males and females, r = -0.64 ( P < 0.01) and
r = -0.60 ( P < 0.01), respectively. Analysis by cancer sites
revealed significant negative correlations between blood-selenium
levels and stomach and oesophageal cancers in both sexes. On
reclassifying regions according to low-, moderate-, and high-
selenium areas on the basis of blood levels, significantly lower
total cancer death rates were observed in regions with high
selenium levels and the mortality from cancer of the stomach,
oesophagus, and liver was particularly increased in the low-
selenium areas. In an area where primary liver cancer was very
common, a statistically significant negative correlation between
primary liver cancer incidence and selenium levels in grain was
observed (r = -0.623 for maize, and -0.631 for barley corn). An
inverse correlation between age-adjusted primary liver cancer and
blood-selenium levels of residents in the area was also observed.
The authors concluded that the results indicated that selenium
might play an important role in the etiology of liver cancer, and
that though selenium deficiency was not a cause of primary liver
cancer, low selenium intake apparently reduced the ability of the
body to withstand cancer-causing stress.
It has been pointed out (Levander, 1986) that the age-adjusted
mortality rates for breast cancer and colon cancer reported in
Finland are considerably lower than those reported in the USA,
despite the well-documented lower dietary-selenium intakes in
Finland.
(b) Case-control studies
Shamberger et al. (1973b) reported that blood-selenium levels
in patients with cancer of the colon, pancreas, stomach, and in
Hodgkin's disease and liver metastases were statistically
significantly lower than those in normal controls. However, of 29
patients with rectal cancers, 6 had lower selenium levels than
controls, and 23 had normal levels. Similarly, normal levels were
observed in patients with breast cancer and in patients with other
types of carcinoma.
McConnell et al. (1975), compared blood-selenium levels in 110
patients with carcinomas, 36 patients with primary neoplasm of the
reticuloendothelial system, 28 hospitalized patients with no
malignancy, and 18 non-hospitalized healthy individuals. The mean
selenium concentration for the hospitalized non-malignant patients
was 1.49 ± 0.06 mg/kg and for healthy controls 1.48 ± 0.07 mg/kg.
The mean level of 1.27 ± 0.03 mg/kg for patients with carcinomas
was significantly different from that of the healthy control group
( P = 0.01). The mean serum-selenium level of 1.14 ± 0.08 mg/kg
obtained for gastrointestinal cancer was significantly different
from that of healthy controls ( P < 0.005). In about one-third of
the 110 cancer patients having the lowest selenium levels,
disseminated tumour, recurrences of the primary lesions, the
incidence of multiple primaries, and shortened patient survival
time, were more frequently observed than in the third of the
patients having the highest serum levels ( P > 0.001). The mean
serum-selenium level for the primary malignancies of the
reticuloendothelial system was 1.76 ± 0.24 mg/kg, which though
higher, was not significantly different from that for the healthy
controls.
Broghamer et al. (1976) reported no difference in serum-
selenium levels measured as µg/ml between 110 cancer patients and
controls. However, patients with the lowest serum-selenium levels
had shorter survival, higher incidence of multiple primary
malignancies, higher rate of recurrence of the primary lesion, and
were more likely to have dissemination of cancer than those with
the highest serum-selenium levels. In a study of 59 patients with
primary malignant reticuloendothelial tumours and controls,
Broghamer et al. (1978) found no difference in serum-selenium
levels measured as µg/ml between the two groups. McConnell et al.
(1980) in their study found statistically significant lower levels
of serum-selenium in 35 breast cancer patients compared with a
control group of women free of the disease. On the other hand, van
Rij et al. (1979) and Robinson et al. (1978a) did not find any
differences in the blood-selenium levels of surgical patients with
and without cancer. Although nutritional status, age, and severity
and duration of disease influenced the selenium levels in the
patients studied, low selenium levels were not characteristic for
the cancer patients and it was suggested that the low-selenium
status of cancer patients was more likely a consequence of their
illness rather than the cause of the cancer.
Sundstrom et al. (1984a) found that 44 patients with
gynaecological cancer had lower serum-selenium concentrations (1.15
± 0.04 µmol/litre, P < 0.05) and serum-glutathione peroxidase
activity (404 ± 13 units/litre, P < 0.01) than 56 control subjects
(1.25 ± 0.03 µmol/litre and 444 ± 8 units/litre, respectively). It
was observed that in association with cytotoxic chemotherapy,
selenium alone ( P < 0.05), vitamin E alone ( P < 0.05), and the 2
combined ( P < 0.001) decreased the plasma concentration of lipid
peroxides; the combination of selenium and vitamin E also increased
the activity of serum GSH-Px ( P < 0.01). The authors stated that
during placebo treatment, cytotoxic chemotherapy did not affect
plasma-lipid peroxides but decreased ( P < 0.001) the activity of
GSH-Px. Selenium inhibited this effect. The authors concluded
that this suggested that the anti-oxidative mechanisms of patients
with these types of cancer might be defective and that treatment
with selenium and vitamin E resulted in changes in biochemical
factors related to lipid peroxidation.
In another study, Sundstrom et al. (1984b) reported that
patients with ovarian cancer had significantly lower serum-selenium
concentrations (mean 0.93 ± 0.04 µmol/litre, P < 0.001) than
matched controls (mean 1.22 ± 0.03 µmol/litre). Clinical stage IV
patients had lower levels of selenium (0.82 ± 0.07 µmol/litre) than
clinical stage I and II combined (1.00 ± 0.04 µmol/litre).
Moreover, levels tended to decrease with progressive disease and
increase with remission, probably related to nutrition.
Goodwin et al. (1983) studied blood-selenium levels, and blood
and tissue GSH-Px activity in 50 patients with untreated cancer of
the oral cavity and oropharynx. Mean erythrocyte-selenium and
-glutathione peroxidase were significantly depressed compared with
those in age-matched controls. Mean plasma-selenium, on the other
hand, was significantly elevated in the cancer group. Also,
although not significant, mean erythrocyte-selenium levels tended
to be lower in patients who had never smoked or who had recently
given up smoking. No correlation between dietary selenium as
determined by recall history and plasma- or erythrocyte-selenium
levels in the cancer patients was observed.
Stead et al. (1985) reported significantly lower serum-selenium
concentrations in 20 patients with cystic fibrosis, two of whom had
cancer, than in controls. The two cancer cases had mean serum-
selenium levels of 1.01 µmol/litre and 0.62 µmol/litre,
respectively, compared with 1.41 ± 0.20 µmol/litre in the controls.
Serum-vitamin E levels were also found to be low in patients, but
did not show any correlation with serum-selenium levels.
(c) Case-control studies within prospective studies
As part of the Hypertention Detection Follow-up Programme,
10 940 men and women with diastolic blood pressures of at least 90
mm Hg were identified and enrolled between 1973-74, and followed-up
for 5 years (Willett et al., 1983). Venous blood samples were
collected from all participants at the beginning of the study. A
total of 111 new cases of cancer occurred in the group during the
period of observation. For each case, 2 controls without cancer
were selected who most closely matched the case in age, sex, race,
smoking history, month of blood collection, initial blood pressure,
hypertensive medication, randomisation assignment, and (in women)
parity and menopausal status. Cases and controls were comparable
for most of the confounding factors, although serum-cholesterol and
albumin were slightly lower among cases than controls ( P = 0.05 and
0.06, respectively). Serum-selenium levels did not vary with age
or sex in controls, but black subjects had lower selenium levels
than white. The mean serum-selenium level in cancer cases (0.129 ±
0.002 mg/litre) was significantly lower than that in controls
(0.136 ± 0.002 mg/litre). The P value was 0.02. The increased
risk of cancer in the lowest quintile of baseline selenium was
twice that in the highest quintile (confidence limits 1.1 to 3.3).
Cases were too few to examine by cancer site, but a consistent
trend of lower selenium levels among cases was observed for the
following groups of cancers: lung, breast, prostate,
lymphoma/leukaemia, gastrointestinal cancer, and others. However,
statistically significant differences were observed only for
gastrointestinal cancer. Selenium level remained a significant
predictor of risk, even when the effects of serum-retinol, vitamin
E, and lipid levels, as well as age, sex, and race were taken into
account. On examination of the data according to race, sex, and
smoking status, separately, significant differences in the mean
serum-selenium between cases and controls were observed in blacks
but not whites, in males but not females, and in current smokers,
but not in past-smokers or in persons who had never smoked. The
risk associated with low selenium was greater among those in the
lowest tertile of serum-vitamin E, and a similar inverse
relationship was observed for serum-retinol. A very strong effect
of low selenium (relative risk = 6.2 for lowest versus highest
tertiles) was observed for subjects who had both low serum-vitamin
E and -retinol levels. The authors concluded that, although their
findings supported the overall hypothesis that low selenium intake
increases the risk of cancer, they believed that the observed
differences between cancer sites should be treated as hypotheses to
be tested in other data sets, and that the differences in the
effects of selenium according to age, race, sex, and smoking
status, needed to be examined further (Willett et al., 1983).
Salonen et al. (1984b) identified a cohort of 8113 men and
women in 1972, randomly selected from 2 counties in Finland, who
had no history of cancer in the 12 months preceding this date. The
blood samples were collected from each subject at the beginning of
the study in 1972, and stored at -20 °C. The cohort was followed-
up for cancer occurrence and death up to the end of 1978, during
which time 43 deaths from cancer occurred and an additional 85
persons developed cancer (total cancers = 128). Each cancer case
or cancer death was matched for sex, age, number of cigarettes
smoked and total serum-cholesterol, to a control selected from the
rest of the same population of 8113 persons. In 1983, selenium
concentration was estimated in blood collected from each case and
control at the time of enrolment in the study in 1972 before the
development of cancer. The mean concentration of selenium was
50.5 µg/litre (SD = 12.5) for all 128 cases, and 54.3 µg/litre
(SD = 11.8) for all controls ( P < 0.012). This difference between
cases and control persisted for the following cancer sites:
gastrointestinal, respiratory, and haematological cancers,
miscellaneous cancers, and secondary cancers. No such difference
was observed for skin cancer, skeletal cancers, and urogenital
cancer. Using the lowest (35 µg/litre) and third deciles as cut-
off points, relative risks were computed for the 2 lowest levels of
serum-selenium, with the highest selenium stratum (45 µg/litre) as
the reference. The relative risk of cancer associated with a
serum-selenium level of less than 35 µg/litre was 3.0 and that
associated with a level of 35 - 44 µg/litre was 2.4 (both
statistically significant). The authors concluded that their data
provided additional support for the hypothesis that selenium
deficiency increases the risk of most non-hormone-dependent
cancers in middle-aged persons, though they cautioned that the
number of cancer cases in their study was insufficient to draw
definite conclusions about the effect of selenium for cancers at
specific sites.
Salonen et al. (1985) in a further study of a subgroup of 51
patients, who died from cancer, among the same study population as
above, each matched to a control for age, sex, and smoking,
obtained a mean pre-follow-up serum-selenium concentration in
subjects who died from cancer during the study period of 53.7 ± 1.8
µg/litre and that in controls of 60.9 ± 1.8 µg/litre; the
difference was statistically significant. A statistically
significant difference in the mean serum-selenium concentration
between cases and controls was observed in men ( P = 0.002), but not
in women (half of the male pairs were smokers, but none of the 21
female pairs were smokers). Similarly, the difference in the mean
selenium concentration was significant in smokers ( P = 0.013) but
not significant in non-smokers. Years of smoking showed an inverse
correlation with serum-selenium in the cases (r = -0.30) but not in
controls. Analysis according to cancer site revealed that a
statistically significant difference in serum-selenium levels
between cases and controls was recorded only for respiratory
cancers, though a non-significant difference also existed for
gastrointestinal sites and other cancers. An association between
low serum retinol concentration and increased risk of cancer was
observed only among smoking men. This sex difference was
attributed to smoking as there were no smoking women in the study.
Vitamin E and serum-retinol did not show any association with a
specific cancer site, and although vitamin E had only a weak
independent effect on the risk of cancer, it showed a strong
synergistic relationship with selenium on the risk of fatal cancer.
A serum-selenium concentration of 47 µg/litre or less (the lowest
tertile) was associated with a relative risk of death from cancer
of 5.8 (95% confidence interval 1.2 - 29.0). The authors concluded
that, although their findings indicated that dietary-selenium
deficiency increased the risk of cancer, owing to their study
design, they could not rule out the possibility that the substances
measured in the serum were not truly the protective factors but
were merely indicators of some other compounds or nutrients that
were directly involved in the causal relationship. Furthermore,
the authors recognized that there was need to investigate further
the modification and the confounding of effects by sex, smoking,
and other factors.
8.2.6.4. Heart disease
Using the same ecological approach discussed above for cancer,
Shamberger et al. (1975) concluded that the age-specific death
rates for a number of heart diseases were significantly lower in
the high-selenium regions of the USA than in the low-selenium
regions. However, results of a WHO/IAEA research programme showed
no difference in tissue-selenium concentrations between patients
who died with, or without, myocardial infarction (Masironi & Parr,
1976). Furthermore, Shamberger (1978) found that the kidney-
selenium levels of patients with atherosclerosis and hypertension
did not differ from those of patients with a variety of other
diseases. The blood-selenium levels of patients with acute
myocardial infarction were lower than those of healthy adults, but
there was no difference in heart- or liver-selenium levels of
patients who died from myocardial infarction and those who died
from other diseases (Westermarck, 1977).
A recent case-control study from Finland suggested a possible
association between the serum-selenium level and the risk of death
from acute coronary heart disease as well as the risk of fatal and
non-fatal myocardial infarction (Salonen et al., 1982). The case-
control pairs were derived from 11 000 persons residing in eastern
Finland, an area with a very high incidence of death from
cardiovascular disease. The cases were middle-aged persons who had
died of coronary heart disease or other cardiovascular disease or
suffered a non-fatal myocardial infarction over a 7-year follow-up
period. Attempts were made to control for potential confounding
factors by using controls matched for 6 major coronary heart
disease risk factors: age, sex, serum-cholesterol, diastolic blood
pressure, smoking, and history of angina pectoris, but the cases
had slightly higher blood pressure than the controls. The mean
serum-selenium levels were 51.8 and 55.3 µg/litre for cases and
controls, respectively. A serum-selenium level of less than 45
µg/litre was associated with an increased risk of coronary and
cardiovascular death and myocardial infarction. Although few
necropsies were done, the authors felt that it was unlikely that
the excess cardiovascular mortality observed in their subjects was
due to Keshan disease. The authors cautioned that the apparent
association between low serum-selenium levels and cardiovascular
risk might be spurious since serum-selenium might only be, for
example, a marker for other dietary factors more directly related
to increased coronary heart disease. The authors also emphasized
that, even if their results truly reflected a causal relationship
between low selenium intake and increased ischaemic heart disease,
most such disease is still due to the other well-known risk factors
of elevated cholesterol, high blood pressure, and smoking.
Moreover, it was pointed out that any association between low
serum-selenium levels and ischaemic heart disease is likely to be
of significance only for populations in areas where the dietary
intake of selenium is very low.
In 3 other studies from Finland little or no association was
found between the risk of death from ischemic heart disease and low
selenium status (Miettinen et al., 1983; Salonen et al., 1985;
Virtamo et al., 1985). However, a critique of these studies
(Salonen, 1985) indicated that the first and third were
characterized by low statistical power and in the first the mean
serum-selenium level was relatively high compared with typical
Finnish values (73 µg/litre), probably due to importation of grain
high in selenium during the late 1970s (Mutanen & Koivistoinen,
1983). Salonen (1985) stated that all 4 Finnish studies discussed
above supported the concept of an increased risk of ischemic heart
disease due to low selenium intake as indicated by serum-selenium
levels of less than 60 µg/litre.
In the USA, an inverse correlation was reported between the
plasma-selenium level and the severity of coronaryatherosclerosis
as documented arteriographically (Moore et al., 1984). The mean
plasma-selenium levels of patients with "zero-vessel" disease (no
visible narrowing as much as 50% of any coronary arteriol lumen) or
"three-vessel" disease (as much as a more than 50% narrowing in the
three major coronary arteries or their branches) were 136 ± 7 or
105 ± 4 µg/litre, respectively. On the other hand, neither Ellis
et al. (1984) in the United Kingdom nor Robinson et al. (1983) in
New Zealand were able to demonstrate any correlation between the
traditional risk factors for cardiovascular disease and blood-
selenium levels or glutathione peroxidase activity.
9. EVALUATION OF THE HEALTH RISKS ASSOCIATED WITH EXCESSIVE OR
DEFICIENT SELENIUM EXPOSURE
9.1. The Need to Consider the Essentiality of Selenium in the Health
Risk Evaluation
The data presented in the preceding sections show evidence that
selenium is a functional component of an enzyme (glutathione
peroxidase) in animals and man, and can prevent certain diseases in
animals and responsive conditions in man. The Task Group concluded
that selenium meets the criteria of essentiality for man. As is
true for all essential elements, not only deficient but also
excessive exposure results in adverse health effects.
The effects of selenium deficiency as well as toxicity are well
known in several animal species. In contrast to the effects seen
in animals, health effects in man resulting from deficiency or
excess of selenium are less well defined, but available evidence
has been described. They can occur at low and excessive exposure
levels that may be expected to correspond broadly with those having
deleterious effects on the health of animals. Between these
extremes is a range of safe and adequate exposures (intakes) that
can be defined as free from toxicity and adequate to meet
nutritional requirements. Exposures outside this range increase
the risk of adverse health effects. Therefore, the health risk
evaluation of both selenium deficiency and excess is important.
However, it should be noted that the safe and adequate range may be
modified by certain dietary and other environmental conditions.
The aim of the Task Group was not to evaluate the need for, and
safety of, medication by selenium compounds as a preventive or
therapeutic measure. However, the Task Group felt that some of the
observations presented might be of relevance whenever such a
question might be addressed by any other body responsible for the
appropriate risk-benefit evaluation of administration of selenium
compounds to human beings.
9.2. Pathway of Selenium Exposure for the General Population
Reproducible and accurate methods are available for the
collection of environmental and biological samples and the
measurement of their selenium content. These methods, though they
require special skills and equipment and are time-consuming, have
been employed to assess human selenium exposure. However, the data
are incomplete and there are no data available on selenium exposure
levels for the general population in many countries and regions of
the world.
In spite of these limitations, it can be concluded that, for
the general population, the main source of selenium exposure is
food. In nutritional surveys, extreme mean values for the
calculated selenium intake from food by adult human beings varied
from 11 to 5000 µg/day. However, on the basis of the data
available from most areas, the Task Group concluded that dietary-
selenium intakes usually fall within the range of 20 - 300 µg/day.
Exposure via drinking-water is much less and rarely exceeds a few
µg/day. Limited data on selenium levels in air indicate that about
0.2 µg can be inspired daily by individual members of the general
population.
9.3. Quantitative Assessment of Human Selenium Exposure
9.3.1. Analytical methods for selenium
Several methods of analysis for selenium are available. Those
based on fluorometry, neutron activation analysis, or atomic
absorption spectrometry have been the most thoroughly studied and
used. While simplified and automated procedures have been
developed, all of these methods require skilled analysts to obtain
consistently accurate results. The analysis of NBS Standard
Reference Materials and the exchange of a variety of samples with
another laboratory known to be accomplished in analysis for this
element, should be used to verify the adequacy of results, prior to
any study of human exposure. The method of choice will depend on
the availability of equipment and of the pure chemicals that may be
required, as well as on certain other factors. Where a high degree
of accuracy is required, comparison of results obtained by two or
more different techniques is helpful.
The reliability of the estimate of selenium exposure will
depend, not only on the adequacy of the method, but also on the
adequacy of sampling, storing, subsampling, and preparing the
subsamples for selenium determination. Failure to plan for, and
observe, proper practices in these steps can negate the reliability
of results by even the most highly accurate method of measurement.
9.3.2. Food intake data
Because of the wide variation in the selenium content of foods
in different regions, special techniques must be used to assess
human selenium exposure on the basis of food intake data. The most
accurate method is to determine selenium levels in duplicate diets
(food-on-the-plate-method) made from the same food that is consumed
by the subjects. This method may be suitable for studies in small
subpopulations and where a high degree of accuracy is necessary; it
has the disadvantage of being expensive and time-consuming.
The use of a nutrition survey approach with calculation of
selenium intake from food tables can be used if an important rule
is observed, i.e., the food tables used must be formulated from
data on the selenium contents of the food sources of the population
being studied and of the food as eaten by the subjects under study.
This is important to avoid the errors introduced by the wide
variation in selenium content of a given foodstuff, depending on
its origin.
9.3.3. Blood-selenium
The selenium content of whole blood, serum, or plasma are the
most commonly used measurements of human selenium exposure. Blood
is relatively easy to sample and contamination can be controlled.
Studies on animals have shown that blood-selenium values are a good
indication of selenium deficiency and excess. However, variations
in selenium deficiency signs have been observed in animals with
similar whole blood-selenium levels. This may be due to variations
in the vitamin E content of the diet or exposure to other dietary
or environmental variables. Several factors besides selenium
intake have been identified that may affect blood-selenium content.
Exposure to inorganic mercury or cadmium can lead to deposition of
selenium in the blood attached to protein in combination with the
metal. Human beings exposed to selenium in the form of
selenomethionine or selenium-rich wheat or yeast, for 10 - 11
weeks, had higher blood-selenium levels than human beings exposed
to the same amount of selenium in the form of selenite or selenate.
Blood can be fractionated and plasma-selenium and red blood
cell-selenium can be measured separately. A recent study on the
effects of a low-selenium diet on human beings indicated that a
decrease in plasma-selenium content might occur before a decrease
in red blood cell-selenium can be detected. These data suggest
that it may be possible to use plasma-selenium levels to assess
short-term selenium exposure but that red blood cell- and whole
blood-selenium reflect long-term exposure.
9.3.4. Hair-selenium
Measurement of the selenium content in hair has been used in
animals for the assessment of selenium status, with regard to both
deficiency and excess. It has not been generally adopted for use
in human beings, but, in special circumstances where external
contamination can be excluded, hair-selenium content is useful for
assessing selenium status.
9.3.5. Urine-selenium
The results of animal studies and a few human studies indicate
that the urinary excretion of selenium can be useful for assessing
very recent selenium exposure (i.e., within the past 24 h).
However, determination of selenium in incomplete urine collections
or expression of urinary-selenium per unit volume of urine cannot
provide valid information about selenium exposure in the general
population. An understanding of the reservations associated with
this technique is necessary in its application and then it may only
be useful in conjunction with other measurements discussed in this
section.
9.3.6. Blood-glutathione peroxidase
There is no suitable, simple field method for assessing the
selenium exposure of the general population. The Task Group
concluded that approaches based on the glutathione peroxidase
activity of blood components might provide the basis for a suitable
screening method to detect low human exposure to selenium.
Blood-glutathione peroxidase activity is useful for detecting
selenium deficiency, because it represents a functional form of
selenium and its assay is more rapid than the measurement of
selenium in blood.
However, the Task Group recognized that there are several
difficulties and limitations associated with the determination of
glutathione peroxidase activity. For example, the enzyme is not
stable and hence its activity cannot be determined in samples
stored for periods of time, whereas measurements of selenium can be
made on stored samples. Moreover, large differences in blood-
glutathione peroxidase activity have been reported from different
laboratories using similar methods. The discrepancies can be so
great that interlaboratory comparisons are impossible without
suitable controls. Furthermore, results from New Zealand suggest
that human blood-glutathione peroxidase activity may be useful in
assessing selenium intake at low, but not necessarily at
intermediate or high levels, because the activity of the enzyme is
correlated with whole blood-selenium levels, only when the latter
are less than 0.10 mg/litre. It is not known whether excessive
selenium exposure can increase glutathione peroxidase activity in
human blood, above normal values. Animal studies indicate that
iron deficiency decreases blood glutathione peroxidase activity,
thus possibly further confounding selenium status assessment by
this method.
Human plasma contains glutathione peroxidase but its activity
is very low and difficult to detect and haemolysis during sample
collection should be excluded. Nevertheless, if these reservations
are borne in mind, the technique is promising. Measurement of
platelet-glutathione peroxidase is also a promising technique for
measuring short-term changes in selenium status because of the
short half-life of this blood component.
9.4. Levels of Dietary Selenium Exposure in the General Population
The selenium content of food is highly variable in relation to
several factors. Levels of selenium in soil available for uptake
by plants vary markedly in different locations and this is
reflected in differences in the selenium contents of feeds and
foodstuffs. Countries in which food-stuffs are shipped between
regions tend to avoid extremes in dietary-selenium intake by
averaging foodstuffs with high- and low-selenium contents but some
differences in regional intakes are still observed.
Animal tissues usually do not have as high a selenium content
as the plants in high-selenium areas or as low a selenium content
as plants in low-selenium areas. This is probably because of the
ability of animals to conserve selenium when it is in short supply,
and to excrete it when an excess is present. This may protect
consumers of animal products from extremes of selenium intake.
Available analytical data show that the levels of selenium
typically found in foods, are in the range of 0.4 - 1.5 mg/kg in
liver, kidney, and seafood; 0.1 - 0.4 mg/kg in muscle meats; from
less than 0.1 to over 0.8 mg/kg in cereals or cereal products; less
than 0.1 - 0.3 mg/kg in dairy products; and less than 0.1 mg/kg in
most fruits and vegetables. The very large variation in the
selenium contents of foodstuffs of the same type, depending on
their origin, makes a food table approach to estimating dietary-
selenium intake potentially misleading as discussed in section
9.3.2. More accurate intake estimates could be derived from assay
data on samples of the food items actually being consumed (food-on-
the-plate analysis).
The practice of supplementing the diets of livestock and
poultry in low-selenium areas with selenite or selenate causes
little increase in muscle-selenium content over levels encountered
in animals raised in areas of adequate, but not excessive, selenium
supply. With some exceptions, food processing and preparation
generally do not cause major losses of selenium.
There are few data that indicate the chemical form that
selenium takes in normal human foods. One available study of
seleniferous wheat has shown that a significant fraction of the
selenium is in the form of selenomethionine.
Various forms of selenium differ in their nutritional
availability. The absorption of selenium from foods appears quite
efficient (about 80%).
Daily selenium intake can be markedly influenced by food
consumption patterns. For example, in many countries, consumption
of large amounts of fish, kidney, or liver could raise an
individual's selenium intake substantially above the highest daily
intake shown in Table 8.
The greatest extremes in dietary-selenium intake have been
reported in areas in which the diet was monotonous and consisted
largely of locally-produced staple food.
The wide range of geographically-related selenium intake due to
variations in the selenium contents of the diet is reflected in the
wide range of selenium levels observed in human whole blood.
The extreme mean blood-selenium values reported for groups
dependent on locally-grown foods of different selenium content
ranged from 0.02 - 3.2 mg/litre (Table 11). Limited studies from
New Zealand have shown that children and older individuals had
lower whole blood-selenium levels, but it is not known whether
these lower levels are due to low dietary intakes or to changes
connected with growth and/or aging.
9.5. Evaluation of Health Risks - General Population
9.5.1. Predictive value of animal studies
The Task Group concluded that, in view of the still fragmentary
data concerning the possible health effects of either deficient or
excess selenium exposure on human beings, any evaluation of the
human health effects of selenium exposure must take into account
results arising from animal studies. For this purpose, these can
be summarized as follows:
(a) Selenium deficiency combined with concurrent low
vitamin E status has resulted in deleterious effects
in all animal species tested so far (mice, rats,
chicks, ducks, swine, sheep, cattle, and monkeys).
In rats, specific signs of selenium deficiency have
been produced in animals fed diets adequate in
vitamin E.
(b) Acute and chronic selenium toxicity have been
demonstrated in a wide variety of species, under a
wide variety of conditions (section 7.1.2). In
evaluating the toxic effects of different selenium
compounds, the Task Group felt that it was useful to
distinguish between effects that are strictly
dependent on the selenium in the molecule and cannot
be duplicated by, e.g., homologous compounds of
sulfur, and compounds where the toxicity is, in
principle, similar for homologues containing either
selenium or sulfur. In addition, the Task Group
recognized that the similarity of some of the effects
of different selenium compounds could be related to
the formation of certain common intermediates.
(c) Dose-response relationships have been demonstrated in
acute and chronic toxicity, as well as in selenium
deficiency. Comparative studies have shown that acute
toxicity is similar in parenteral and oral exposure
(section 7.1.2.1), which is in good agreement with the
recognized high absorbability of selenium compounds
from the gastrointestinal tract (section 6.1.1).
(d) As indicated in section 7.1.2.2, the borderline
level of dietary selenium needed to cause growth
depression, due to overt chronic selenium toxicity in
rats, is in the range of 4 - 5 mg selenium/kg diet.
However, the dietary level of selenium needed to
cause chronic toxicity can be influenced by several
environmental factors such as previous selenium
intake (section 7.1.6).
(e) The effects of various environmental factors on
selenium dose-response relationships is even more
dramatically illustrated in situations of low-
selenium intake leading to deficiency. One of the
primary factors influencing the nutritional
requirements of animals for selenium is the vitamin E
status. The minimum dietary level of selenium needed
to prevent deficiency diseases in various animal
species is in the range of 0.02 - 0.05 mg/kg diet.
(f) Animal diseases associated with both high- and
low-selenium intake have been reported in certain
areas of the world in which the animals were
consuming feeds primarily of local origin.
The possibility of a human disease due to selenium deficiency
or excess should be looked for under similar conditions of
restricted dietary consumption (monotonous diets based on locally
grown foods). In addition, the Task Group concluded that, in view
of the dependence of selenium deficiency or toxicity effects on
various environmental factors, the possible involvement of selenium
in human diseases of multifactorial etiopathogenesis deserves
special attention.
9.5.2. Studies on high-exposure effects in the general population
Studies on this type of exposure are few and, as they are
confined to subpopulations in high-selenium areas, some do not
include comparison groups. Symptoms and signs of illness elicited
are frequently mild and not clearly related to selenium. In
practically all studies (section 8.1.1.1), nail pathology was
reported and, in several studies, hair loss and increased dental
decay. Some of the studies included reports of gastrointestinal
disturbances and icteroid skin. Other possible causes of illness
in these studies cannot be excluded and the dependence of severity
of effects on gradation of selenium exposure cannot be evaluated.
Examination of exposed individuals showed increased levels of
selenium in the blood and urine.
Three studies on populations living in seleniferous areas and
dependent on locally-produced food deserve particular attention.
Examination of individuals exposed in these areas revealed highly
increased levels of selenium in the blood and/or urine. A study
from South Dakota reported various signs and symptoms in people
with long-term overexposure to selenium, as revealed by elevated
urinary-selenium excretion. In these areas, farm animals were
affected by chronic selenium poisoning.
In a seleniferous zone of Venezuela (Villa Bruzual) 111
children (average blood-selenium level = 0.813 mg/litre) were
studied and compared with 50 children (blood-selenium level = 0.355
mg/litre) not over-exposed to selenium (Caracas). The children
from the seleniferous area had some loss of hair and some
abnormalities of skin and nails; the authors noted some differences
in socio-economic factors between the 2 groups.
In a recent report from China, past incidents of intoxication
were described that were thought to be due to chronic selenium
poisoning, with hair loss and nail pathology as the most common
signs. No quantitative data are available from the period 1961 -
64, which was the time of peak prevalence of the intoxication.
Recent diet samples collected from high-selenium areas with, and
without, a history of this intoxication showed mean daily selenium
intakes of about 5 and 0.75 mg, respectively (Table 7). Blood
samples taken from the same areas had mean selenium levels of 3.2
and 0.44 mg/litre, respectively (Table 11).
The above studies of populations ingesting high selenium levels
in Venezuela and China have not revealed abnormal serum
transaminase activity in the subjects concerned. In individuals
exposed to high levels of selenium in the recent outbreak in the
USA; there was no evidence of impaired liver function, but loss of
hair and pathological changes in the nails were observed, as well
as some other signs and symptoms described in more detail in
section 8.1.1.1. However, the Task Group recognized that this is
insufficient evidence to exclude the occurrence of liver damage and
recognized the need for more thorough evaluation of the liver in
persons with high selenium exposure.
The Task group noted that the signs and symptoms of selenium
overexposure in human beings were not well defined. However the
Task Group was aware of 3 situations concerning elevated selenium
exposure, which could form the basis for estimating a dose-response
to high levels of selenium. In one area of Enshi county in China,
blood-selenium levels in adults of 0.44 mg/litre were associated
with no reported effects. In Venezuelan children with blood-
selenium levels of 0.813 mg/litre, hair and nail changes of an
unspecified nature and incidence were reported. In another area of
Enshi county in China, definite hair and nail changes and symptoms
and signs consistent with neuropathy were reported in adults with
blood levels of 3.2 mg/litre. The Task Group, however, could not
be certain of how signs and symptoms were searched for, how their
absence was ascertained, and how the control populations were
selected in these studies.
9.5.3. Studies on low-exposure effects in the general population
Very low blood-selenium levels observed in human subjects
(section 8.2.4), approach those observed in animals with selenium
responsive diseases. One woman from a general population known to
have a low exposure to selenium and maintained on total parenteral
nutrition, which provided less than 1 µg selenium/day, had muscular
pain and dysfunction that responded to selenium supplementation.
However, similar symptoms have not been observed in other patients
with similar low blood-selenium levels. Also, in another study, no
muscular symptomatology was reported in children fed exclusively
special medical diets containing very low levels of selenium, for
long periods of time. On the other hand, the Task Group assigned a
high significance to recent reports describing an association
between Keshan Disease (section 9.5.4.1) and poor selenium status
as indicated by low selenium content of grain, low blood and hair
levels of selenium, low blood-glutathione peroxidase activity, as
well as a positive response to an intervention study with sodium
selenite. As discussed in section 9.5.1, the results of several
experimental animal studies indicate that many different factors
may contribute to the development of selenium deficiency diseases.
These studies point to the conclusion that a lack of selenium may
be only one of several causative factors responsible for the
occurrence of certain diseases of complex multiple
etiopathogenesis. Such a conclusion was also fully recognized in
the reports from China on the Keshan Disease.
9.5.4. Evaluation of the involvement of selenium in human diseases
of multiple etiopathogenesis
9.5.4.1. Keshan disease
A suitable animal model of the Keshan disease is not available,
even though heart damage is a feature of combined vitamin E and
selenium deficiency in several species of animals.
The ecological evidence strongly favours a relationship between
low selenium status and the incidence of Keshan disease. Such
evidence includes low blood-, hair-, and urine-selenium levels in
the affected areas as well as a low selenium content in the staple
foods raised and consumed in the affected areas. A randomized
intervention trial carried out in China showed that children who
received sodium selenite had a lower incidence of Keshan disease
than those who received a placebo. However, in this randomized
trial there may have been an underlying trend in the placebo group
towards a decline in the incidence of the disease. Also,
information on the effectiveness of the randomization was not
available and the incidence rates were not adjusted for age. The
results of the much larger but non-randomized intervention trial
involving at least a quarter of a million children on a yearly
basis indicated a clear-cut beneficial effect of sodium selenite in
the prevention of the Keshan disease. Thus, the Task Group
concluded that Keshan disease is a condition in human beings that
is related to low selenium status but that additional research is
needed to clarify the role of other factors in the etiopathogenesis
of the disease.
The Task Group also recognized that in section 8.2.3.1 this
disease was used as the basis for estimating a minimal human
nutritional selenium requirement, suggested by the authors to be 19
and 14 µg/day for men and women, respectively.
9.5.4.2. Kashin-Beck disease
An additional disease found in certain low-selenium areas of
China is an endemic osteoarthropathy known as Kashin-Beck disease
that occurs mainly in children. There is some evidence linking low
selenium status with the incidence of Kashin-Beck disease, but the
Task Group concluded that additional research, particularly in
relation to intervention studies, is required before a definite
statement can be made concerning the role of selenium in the
etiology of the disease.
9.5.4.3. Ischaemic heart disease
The Task Group was aware of studies that examined the
possibility of an association between low selenium status and
myocardial ischaemia and atherosclerosis. However, because of the
limited data available, the Task Group was not prepared to come to
any conclusion regarding the role of selenium in ischaemic heart
disease.
9.5.4.4. Studies on the involvement of selenium in cancer
(a) Carcinogenicity studies
The Task Group concluded that the studies described in section
7.7 suggesting a carcinogenic effect of selenite or selenate were
invalid, because, in one study, the results were statistically
insignificant and, in the other studies, there were no controls or
systematic pathological examinations. In another systematic study,
no differences were observed in the incidence of tumours in rats
surviving 2 years or longer and exposed to 0.5 - 2.0 mg selenium/kg
diet.
The Task Group was aware of one study that demonstrated the
carcinogenicity of ethyl selenac (selenium diethyldithiocarbamate)
and another that showed the carcinogenicity of selenium sulfide,
given by gavage. The latter result is of possible interest both as
regards human dermal exposure to selenium sulfide and/or its
possible formation within the body, but the Task Group was not
aware of any studies demonstrating carcinogenic effects of other
selenium compounds.
The Task Group felt that the evaluation published by IARC in
1975 was still valid in concluding that the available animal data
were insufficient to allow an evaluation of the carcinogenicity of
selenium compounds (IARC, 1975).
(b) Experimental evidence of anticarcinogenic effects of
selenium compounds
The results of studies on laboratory animals provide evidence
of a preventive effect of selenium dioxide, sodium selenite, or
"selenized" yeast, given in the food or drinking-water, against
chemically-induced cancers or certain spontaneous, presumably
viral-induced cancers (section 7.7). Generally, the level required
to demonstrate such effects ranges from 1 - 6 mg/kg food or per
litre drinking-water and thus is considerably in excess of the
animal's nutritional needs. In one report, selenium dioxide at
0.1 mg selenium/litre water had some beneficial effect against
spontaneous mammary tumours in mice, but, in this case, the diet
itself contained 0.45 mg selenium/kg. Other studies have shown the
beneficial effects of sodium selenide when applied concomitantly to
the skin with certain carcinogens. In rats fed diets high in
polyunsaturated fats, selenium deficiency increased the incidence
of mammary tumours, after treatment with dimethylbenz[alpha]
anthracene. Therefore, the Task Group concluded that
pharmacological levels of selenium compounds exhibit in many cases
a favourable influence against the development of cancer in various
animal model systems. However, the Task Group was aware of one
study in which administration of high levels of selenium was
associated with an increased incidence of chemically-induced
cancer.
(c) Epidemiological evidence regarding the possible anti-
carcinogenic effects of selenium in human beings
The Task Group acknowledged that apparent negative correlations
have been drawn between cancer death rates and certain general
population characteristics, such as blood-selenium levels or the
average level of selenium in diets in specific geographical zones
(section 8.2.6.1). However, these apparent correlations have not
been consistent with specific cancer sites and are subject to
ecological fallacy. Moreover, such epidemiological data are
subject to question regarding the adequacy of sampling, the
interpretation of blood-selenium data, and the precision of the
estimates of dietary-selenium intake in various countries and
different geographical areas within the USA.
Of the 7 case-control studies examined by the Task Group, no
consistent association was apparent between low selenium status and
risk of cancer. For a given cancer site, there were few replicate
studies. On the majority of occasions, no association was found
between low selenium status and cancer risk. In fact, in at least
one situation a positive correlation was found. In several
countries, low blood- or plasma-selenium levels were found in
patients with several different types of cancer. However, the
effect of the malignant disease itself in lowering blood-selenium
levels (e.g., by decreasing absorption and/or worsening the
generally debilitated nutritional state of the patient) cannot be
excluded as a partial or total explanation for these results.
The Task Group was aware of 3 studies in which blood samples
had been taken for other purposes prior to the diagnosis of any
cancer. Some time later, the samples were retrieved from storage
banks and analysed for selenium. All 3 studies showed a consistent
inverse association between the prediagnostic serum-selenium level
and the risk of cancer. This association between low serum-
selenium level and cancer risk was observed only in smokers, only
in males, and in one study only in blacks but not in whites. In
one study, the association between low selenium level and cancer
risk showed some site specificity, but the second and third studies
were unable to confirm or rebut this because of inadequate sample
size.
The Task Group was aware that in many studies showing a
protective effect of selenium against cancer in animals
pharmacological doses of selenium compounds were used. Therefore,
there were difficulties in relating the animal and human studies,
but the Task Group was aware of an intervention trial being carried
out in China which it is hoped will enable a more precise
evaluation of the association between selenium status and human
cancer risk in the future.
Thus, the Task Group concluded that existing data are
insufficient to determine whether the level of selenium intake is
indeed correlated with the incidence of cancer in man.
9.5.4.5. Caries
Although an association between high selenium intake and dental
caries has been reported in at least one animal study, there is no
clear-cut evidence of such an association in man. The Task Group
concluded that difficulties associated with the interpretation of
urinary-selenium excretion, expressed as mass-concentration per
volume and not expressed as total daily urinary excretion of
selenium (section 9.3.5), as well as the inability to exclude
interference by other environmental factors such as fluoride,
precluded any significant conclusions. A better understanding of
the impact of selenium on the incidence of caries will require a
more comprehensive estimation of selenium exposure and of the other
confounding factors in the respective subpopulations under study.
9.5.4.6. Health effects related to reproduction
The Task Group concluded that at present there is no evidence
that selenium has significant effects on reproductive function in
man.
9.6. Occupational Exposure
In contrast to general population exposure, occupational
exposure usually occurs through direct contact and/or through
inhalation, i.e., dermal and respiratory exposure predominate.
Exposure to selenium and its compounds occurs in primary
industries, i.e., those that extract, mine, treat, or process
selenium-bearing minerals, e.g., copper, zinc, or lead ores and in
secondary industries, i.e., those that use selenium in
manufacturing processes. The physical and chemical form of
selenium under these circumstances varies and is determined by the
industrial processes. The Task Group recognized that industrial
exposure has not been adequately studied with respect to levels of
exposure and there is a need for such studies to be carried out.
For acute occupational selenium exposures, the effects vary
according to to the chemical form of selenium. In contrast to
elemental selenium, which does not appear to be toxic unless
oxidized or reduced, hydrogen selenide and selenium dioxide are
highly toxic, causing irritation of the respiratory tract, which
may be followed by pulmonary oedema.
The Task Group recognized that evaluation of long-term
occupational exposure to selenium must take into account other
dietary and environmental substances because of their recognized or
potential interactions with selenium. An additional factor that
needs to be considered is the fact that preventive measures usually
result in the removal of exposed workers with the appearance of the
first sign of selenium over-exposure (garlic-like odour).
Monitoring of selenium in the urine is also used to identify those
who should be removed from further overexposure.
The Task Group concluded that no studies were available on the
dose-response relationship with regard to occupational exposure to
selenium.
REFERENCES
ABDULLA, M., KOLAR, K., & SVENSSON, S. (1979) Selenium.
Scand. J. Gastroenterol., 14(suppl. 52): 181-184.
ABDULLAEV, G.M. (1976) [Selenium levels in healthy subjects
and those with certain haematological diseases.] In: Selen v
biologii, Vol. 1, Baku, Elm Publishing House, pp. 136-139 (in
Russian).
ABDULLAEV, G.B., GADZHIEVA, N.A., GASANOV, G.G., DZHAFAROV,
A.I., & PERELYGIN, V.V. (1972) [Selenium and vision,] Baku,
Elm Publishing House, 50 pp (in Russian).
ABDULLAEV, G.B., GADZHIEVA, N.A., GASANOV, G.G., MAMEDOVA,
S.A., DMITRENKO, A.I., GULIEVA, L.I., & RODIONOV, V.P.
(1974a) [The effect of selenium compounds on electroretino-
gram under different experimental conditions.] In: [Selen v
biologii,] Baku, Elm Publishing House, pp. 11-22 (in Russian).
ABDULLAEV, G.B., MAMEDOV, SH.V., DZHAFAROV, A.I., PERELYGIN,
V.V., & MAGOMEDOV, N.M. (1974b) [Possible regulation of free
radicals in the retina by selenium compounds.] In: [Selen v
biologii,] Baku, Elm Publishing House, pp. 54-57 (in Russian).
ABU-ERREISH, G.M. (1967) On the nature of some selenium
losses from soils and waters, Brookings, South Dakota, South
Dakota State University (MS Thesis).
ABU-ERREISH, G.M., WHITEHEAD, E.I., & OLSON, O.E. (1968)
Evolution of volatile selenium from soils. Soil Sci., 106:
415-420.
ABUTALYBOV, M.G., VEZIROVA, N.B., FATALIEVA, C.M., KHALILOV,
E.KH., & MUSAEV, S.G. (1976) [Selenium content in some bean
plants of Azerbajdzhan.] In: [Selen v biologii,] Vol. 2, Baku,
Elm Publishing House, pp. 140-142 (in Russian).
ACGIH (1971) Documentation of the threshold limit values for
substances in workroom air, 3rd ed., Cincinnati, Ohio,
American Conference of Governmental Industrial Hygienists,
pp. 224-225.
AKESSON, B. (1985) Plasma selenium in patients with abnormal
plasma protein patterns. In: Proceedings of the XIII Inter-
national Congress of Nutrition, p. 152 (Abstract).
ALEXANDER, A.R., WHANGER, P.D., & MILLER, L.T. (1983)
Bioavailability to rats of selenium in various tuna and wheat
products. J. Nutr., 113(1): 196-204.
ALCINO, J.F. & KOWALD, J.A. (1973) Analytical methods. In:
Klayman, D.L. & Gunther, W.H.H., ed. Organic selenium
compounds, their chemistry and biology, New York, John Wiley
and Sons, pp. 1049-1081.
ALLAWAY, W.H. (1972) An overview of distribution patterns of
trace elements in soils and plants. Ann. NY Acad. Sci., 199:
17-25.
ALLAWAY, W.H. (1973) Selenium in the food chain. The Cornell
Veterinarian, 63: 151-170.
ALLAWAY, W.H. (1978) Perspectives on trace elements in soil
and human health. In: Hemphill, D.D., ed. Trace substances in
environmental health. XII, Columbia, Missouri, University of
Missouri Press, pp. 3-10.
ALLAWAY, W.H. & CARY, E.E. (1964) Determination of
submicrogram amounts of selenium in biological materials.
Anal. Chem., 36: 1359-1362.
ALLAWAY, W.H., MOORE, D.P., OLDFIELD, J.E., & MUTH, O.H.
(1966) Movement of physiological levels of selenium from
soils through plants to animals. J. Nutr., 88: 411-4l8.
ALLAWAY, W.H., CARY, E.E., & EHLIG, C.F. (1967) The cycling
of low levels of selenium in soils, plants, and animals. In:
Muth, O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 273-296.
ALLAWAY, W.H., KUBOTA, J., LOSEE, F., & ROTH, M. (1968)
Selenium, molybdenum, and vanadium in human blood. Arch.
environ. Health, 16: 342-348.
AMOR, A.J. & PRINGLE, P. (1945) A review of selenium as an
industrial hazard. Bull. Hyg., 20: 239-241.
ANCIZAR-SORDO, J. (1947) Occurrence of selenium in soils and
plants of Columbia, South America. Soil Sci., 63: 437.
ANDERSON, R.A. & POLANSKY, M. (1981) Dietary chromium
deficiency. Effect on sperm count and fertility in rats. Biol.
Trace Elem. Res., 3: 1-5.
ANDREWS, R.W. & JOHNSON, D.C. (1976) Determination of
selenium (IV) by anodic stripping voltammetry in flow system
with ion exchange separation. Anal. Chem., 48: 1056-1060.
ANONYMOUS (1962) Selenium poisons Indians. Sci. News Lett.,
81: 254.
ANONYMOUS (1975) New links in the selenium cycle. Agric.
Res., 24: 6-7.
ANONYMOUS (1984) Washington DC, US Food and Drug
Administration, pp. 19 (FDA Bulletin No. 14).
ANUNDI, I., STAHL, A., & HOGBERG, J. (1984) Chem.-biol.
Interact., 50: 277.
AOAC (1975) Official methods of analysis, 12th ed.,
Washington DC, Association of Official Analytical Chemists.
ARTHUR, D. (1972) Selenium content of Canadian foods. Can.
Inst. Food Sci. Technol. J., 5: 165-169.
ASHER, C.J., BUTLER, G.W., & PETERSON, P.J. (1977) Selenium
transport in root systems of tomato. J. exp. Bot., 28: 279-291.
AWASTHI, Y.C., BEUTLER, E., & SRIVASTAVA, S.K. (1975)
Purification and properties of human erythrocyte glutathione
peroxidase. J. biol. Chem., 250: 5144-5149.
BACHAREV, V.D., BOCHAROVA, M.A., & SHOSTAK, V.I. (1975) [On
the effect of selenium on the light perception of the eye.]
Fiziol. Zhurn., 61(1): 150-153 (in Russian).
BAI, J., WU, S.Q., GE, K.Y., DENG, X.J., & SU, C.Q. (1980)
The combined effect of selenium deficiency and viral infection
on the myocardium of mice (preliminary study). Acta Acad. Med.
Sinicae, 2: 29-31.
BAIRD, R.B., POURIAN, S., & GABRIELIAN, S.M. (1972) Determination
of trace amounts of selenium in waste waters by carbon rod
atomization. Anal. Chem., 44: 1887-1889.
BARBEZAT, G.O., CASEY, C.E., REASBECK, P.G., ROBINSON, M.F., &
THOMSON, C.D. (1984) Selenium. In: Rosenberg, I. &
Solomons, N.W., ed. Absorption and malabsorption of mineral
nutrients, New York, Alan R. Liss., pp. 231-258.
BARRETTE, M., LAMOUREUX, G., LEBEL, E., LECOMTE, R., PARADIS,
P., & MONARO, S. (1976) Trace element analysis of freeze-
dried blood serum by proton and alpha-induced X-rays. Nucl.
Instrum. Methods, 134: 189-196.
BEARSE, R.C., CLOSE, D.A., MALANIFY, J.J., & UMBARGER, C.J.
(1974) Elemental analysis of whole blood using proton-induced
X-ray emission. Anal. Chem., 46: 499-503.
BEATH, O.A., EPPSON, H.F., & GILBERT, C.S. (1935). Selenium
and other toxic minerals in soils and vegetation, Wyoming
Experimental Station, 55 pp (Bulletin No. 206).
BEHNE, D. & HOFER-BOSSE, T. (1984) Effects of a low selenium
status on the distribution and retention of selenium in the
rat. J. Nutr., 114: 1289-1296.
BEHNE, D. & WOLTERS, W. (1979) Selenium content and gluta-
thione peroxidase activity in the plasma and erythrocytes of
non-pregnant and pregnant women. J. clin. Chem. clin.
Biochem., 17: 133-135.
BEIJE, B., ONFELT, A., & OLSSON, U. (1984) Influence of
dietary selenium on the mutagenic activity of perfusate and
bile from rat liver, perfused with 1,1-dimethylhydrazine.
Mutat. Res., 130: 121-126.
BERTINE, K.K. & GOLDBERG, E.D. (1971) Fossil fuel combustion
and the major sedimentary cycle. Science, 173: 233-235.
BESBRIS, H.J., WORTZMAN, M.S., & COHEN, A.M. (1982) Effect
of dietary selenium on the metabolism and excretion of
2-acetylaminofluorene in the rat. J. Toxicol. environ.
Health, 9: 63-76.
BHUYAN, K.C., BHUYAN, D.K., & PODOS, S.M. (1981) Selenium-
induced cataract: biochemical mechanism. In: Spallholz, J.E.,
Martin, J.L., & Ganther, H.E., ed. Selenium in biology and
medicine, Westport, Connecticut, Avi Publishing Company,
pp. 403-412.
BIERI, J.G. & AHMAD, K. (1976) Selenium content of
Bangladeshi rice by chemical and biological assay. J. agric.
food Chem., 24: 1073-1074.
BIRT, D.F., JULINS, A.D., & POUR, P.M. (1984) Increased
pancreatic carcinogenesis in Syrian hamsters fed high selenium
diets. Proc. Am. Assoc. Cancer Res., 25: 133.
BLADES, M.W., DALZIEL, J.A., & ELSON, C.M. (1976) Cathodic
stripping voltammetry of nanogram amounts of selenium in
biological material. J. Assoc. Off. Anal. Chem., 59: 1234-1239.
BLOTCKY, A.J., SULLIVAN, J.F., SHUMAN, M.S., WOODWARD, G.P.,
VOORS, A.W., & JOHNSON, W.D. (1976) Selenium levels in liver
and kidney. In: Hemphill, D.D., ed. Trace substances in
environmental health. X, Columbia, Missouri, University of
Missouri Press, pp. 97-103.
BONARD, E.C. & KORALINK, K.D. (1958) Intoxication aiguë par
vapeurs d'hydrogene selenide. Praxis, 47(i): 533-534.
BOUND, G.P. & FORBES, S. (1978) Differential-pulse
polarography of selenium (IV) in the presence of metal ions.
Analyst, 103: 176-179.
BOWEN, H.J.M. & CAWSE, P.A. (1963) The determination of
selenium in biological material by radioactivation. Analyst,
88: 721-726.
BOWEN, W.H. (1972) The effects of selenium and vanadium on
caries activity in monkeys (M. irus). J. Irish Dent. Assoc.,
18: 83-89.
BRADY, P.S., KU, P.K., & ULLREY, D.E. (1978) Lack of effect
of selenium supplementation on the response of the equine
erythrocyte glutathione system and plasma enzymes to exercise.
J. anal. Sci., 47: 492-496.
BRADY, P.S., BRADY, L.J., & ULLREY, D.E. (1979) Selenium,
vitamin E, and the response to swimming stress in the rat. J.
Nutr., 109: 1103-1109.
BRITTON, J.L., SHEARER, T.R., & DE SART, D.J. (1980) Cario-
stasis by moderate doses of selenium in the rat model. Arch.
environ. Health, 35: 74-76.
BRODIE, K.G. (1979) Analysis of arsenic and other trace
elements by vapor generation. Am. Lab. (June issue): 58-66.
BROGHAMER, W.L., MCCONNELL, K.P., & BLOTCKY, A.L. (1976)
Relationship between serum selenium levels and patients with
carcinoma. Cancer, 37: 1384-1388.
BROGHAMER, W.L., MCCONNELL, K.P., GRIMALDI, M., & BLOTCKY,
A.J. (1978) Serum selenium and reticuloendothelial tumours.
Cancer, 41: 1462-1466.
BROWN, D.G. & BURK, R.F. (1973) Selenium retention in
tissues and sperm of rats fed a torula yeast diet. J. Nutr.,
103: 102-108.
BROWN, D.G., BURK, R.F., SEELY, R.J., & KIKER, K.W. (1972)
Effect of dietary selenium on the gastrointestinal absorption
of 75Se03 in the rat. Int. J. Vit. nutr. Res., 42: 588-591.
BROWN, M.W. & WATKINSON, J.H. (1977) An automated
fluorimetric method for the determination of nanogram
quantities of selenium. Anal. Chim. Acta, 89: 29-35.
BRUNE, D., SAMSAHL, K., & WESTER, P.O. (1966) A comparison
between the amounts of As, Au, Br, Cu, Fe, Mo, Se, and Zn in
normal and uraemic human whole blood by means of neutron
activation analysis. Clin. Chim. Acta, 13: 285-291.
BUCHAN, R.F. (1947) Industrial selenosis. Occup. Med., 3:
439-456.
BUNCE, G.E., HESS, J.L., GURLEY, R., BATRA, R., & TARNAWSKA,
E. (1985) Lens energy metabolism and Ca content in selenite
induced cataract. In: Mills, C.F., Bremner, I., & Chesters,
J.K., ed. Trace elements in man and animals - TEMA 5, Slough,
Commonwealth Agricultural Bureaux, pp. 250-254.
BURCHANOV, A.I. (1972) [On regulation of complex chemical
dusts in rare metals production.] Vopr. Gig. Trud. Profzabol.
Kazajenade, Kazah NII Gig. Trud. Profazabol., pp. 72-74 (in
Russian).
BURCHANOV, A.I. & ZHAKENOVA, R.K. (1973) [Pulmonary changes
following intratracheal administration of elementary selenium
to white rats.] Zavavoohr. Kazah., 3: 52-53 (in Russian).
BURCHANOV, A.I., SALEHOV, M.I., DOROFEEVA, O.N., & ZHAKENOVA,
R.K. (1969) [On the effects of metal selenium dust on the
organism.] In: [Proceedings of a Concluding Scientific
Conference on Labour Hygiene and Occupational Disease, 21-22
October, 1969,] Karaganda, Kazah., NII Gig. Trud. Profzabol,
pp. 77-80 (in Russian).
BURK, R.F. (1976) Selenium in man. In: Prasad, A.S., ed.
Trace elements in human health and disease. II. Essential and
toxic elements, New York, Academic Press, pp. 105-133.
BURK, R.F. & CORREIA, M.A. (1977) Accelerated hepatic haem
catabolism in the selenium-deficient rat. Biochem. J., 168:
105-111.
BURK, R.F. & LANE, J.M. (1979) Ethane production and liver
necrosis in rats after administration of drugs and other
chemicals. Toxicol. appl. Pharmacol., 50: 467-478.
BURK, R.F & MASTERS, B.S.S. (1975) Some effects of selenium
deficiency on the hepatic microsomal cytochrome P-450 system
in the rat. Arch. Biochem. Biophys., 170: 124-131.
BURK, R.F., Jr, PEARSON, W.N., WOOD, R.P., II, & VITERI, F.
(1967) Blood selenium levels and in vitro red blood cell
uptake of 75Se in kwashiorkor. Am. J. clin. Nutr., 20: 723-733.
BURK, R.F., BROWN, D.G., SEELY, R.J., & SCAIEF, C.C., III
(1972) Influence of dietary and injected selenium on whole-
body retention, route of excretion, and tissue retention of
75Se03 2- in the rat. J. Nutr., 102: 1049-1055.
BURK, R.F., SEELEY, R.J., & KIKER, K.W. (1973) Selenium:
dietary threshold for urinary excretion in the rat. In:
Proceedings of the Society of Experimental and Biological
Medicine, Vol. 142, pp. 214-216.
BURK, R.F., MACKINNON, A.M., & SIMON, F.R. (1974) Selenium
and hepatic microsomal hemoproteins. Biochem. Biophys. Res.
Commun., 56: 431-436.
BURK, R.F., NISHIKI, K., LAWRENCE, R.A., & CHANCE, B. (1978)
Peroxide removal by selenium-dependent and selenium independent
glutathione peroxidases in hemoglobin-free perfused rat liver.
J. biol. Chem., 253: 43-46.
BURK, R.F., LAWRENCE, R.A., & CORREIA, M.A. (1980a) Sex
differences in biochemical manifestations of selenium
deficiency in rat liver with special reference to heme
metabolism. Biochem. Pharmacol., 29: 39-42.
BURK, R.F., LAWRENCE, R.A., & LANE, J.M. (1980b) Liver
necrosis and lipid peroxidation in the rat as the result of
paraquat and diquat administration. J. clin. Invest., 65:
1024-1031.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1974) Superoxide- and
singlet oxygen-catalyzed lipid peroxidation as a possible
mechanism for paraquat (methyl viologen) toxicity. Biochem.
Biophys. Res. Commun., 58: 749-755.
BUTLER, G.W. & PETERSON, P.J. (1961) Aspects of the faecal
excretion of selenium by sheep. N.Z. J. agric. Res., 4:
484-491.
BUTTNER, W. (1963) Action of trace elements on the
metabolism of fluoride. J. dent. Res., 42: 453-460.
BYARD, J.L. (1969) Trimethyl selenide: a urinary metabolite
of selenite. Arch. Biochem. Biophys., 130: 556-560.
CADELL, P.B. & COUSINS, F.B. (1960) Urinary selenium and
dental caries. Nature (Lond.), 185: 863-864.
CAGEN, S.Z. & GIBSON, J.E. (1977) Liver damage following
paraquat in selenium-deficient and diethyl maleate-pretreated
mice. Toxicol. appl. Pharmacol., 40: 193-200.
CALVIN, H.I. (1978) Selective incorporation of selenium-75
into a polypeptide of the rat sperm tail. J. exp. Zool., 204:
445-452.
CANNELLA, J.M. (1976) Surveillance of employees in selenium
alloying operations. In: Proceedings of the Symposium on
Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 343-348.
CANTOR, A.H. & SCOTT, M.L. (1974) The effect of selenium in
the hen's diet on egg production, hatchability, performance of
progeny, and selenium concentration in eggs. Poultry Sci., 53:
1870-1880.
CANTOR, A.H. & SCOTT, M.L. (1975) Influence of dietary
selenium on tissue selenium levels in turkeys. Poultry Sci.,
54: 262-265.
CANTOR, A.H., SCOTT, M.L., & NOGUCHI, T. (1975a) Biological
availability of selenium in feedstuffs and selenium compounds
for prevention of exudative diathesis in chicks. J. Nutr.,
105: 96-105.
CANTOR, A.H., LANGEVIN, M.L., NOGUCHI, T., & SCOTT, M.L.
(1975b) Efficacy of selenium in selenium compounds and
feedstuffs for prevention of pancreatic fibrosis in chicks. J.
Nutr., 105(1): 106-111.
CANTOR, A.H., MOORHEAD, P.D., & BROWN, K.I. (1978) Influence
of dietary selenium upon reproductive performance of male and
female breeder turkeys. Poultry Sci., 57: 1337-1345.
CAPPON, C.J. & SMITH, J.C. (1982) Chemical form and
distribution of mercury and selenium in canned tuna. J. appl.
Toxicol., 2: 181-189.
CARNRICK, G.R., MANNING, D.C., & SLAVIN, W. (1983)
Determination of selenium in biological materials with
platform furnace atomic absorption spectroscopy and Zeeman
background correction. Analyst, 108: 1297-1312.
CARTER, D.L., ROBBINS, C.W., & BROWN, M.J. (1972) Effect of
phosphorus fertilization on the selenium concentration in
alfalfa (Medicago sativa). Soil Sci. Soc. Am. Proc., 36:
624-628.
CARY, E.E., ALLAWAY, W.H., & MILLER, M. (1973) Utilization
of different forms of dietary selenium. J. anal. Sci., 36:
285-292.
CASEY, C.E., GUTHINE, B.E., FREUD, G.M., & ROBINSON, M.F.
(1982) Selenium in human tissues from New Zealand. Arch.
environ. Health., 37: 133-135.
CAYGILL, C.P.J. & DIPLOCK, A.T. (1973) The dependence on
dietary selenium and vitamin E of oxidant-labile liver
microsomal non-haem iron. FEBS Lett., 33: 172-176.
CHAN, C.C.Y. (1976) Improvement in the fluorimetric
determination of selenium in plant materials with
2,3-diaminonaphthalene. Anal. Chim. Acta, 82: 213-215.
CHANSLER, M.W., MORRIS, V.C., & LEVANDER, O.A. (1983)
Bioavailability to rats of selenium in Brazil nuts and
mushrooms. Fed. Proc., 42(4): 927.
CHATTERJEE, M. & BANERJEE, M.R. (1982) Selenium mediated
dose-inhibition of 7-12-dimethylbenz(alpha)anthracene-induced
transformation of mammary cells in organ culture. Cancer
Lett., 17: 187-195.
CHAU, Y.K., WONG, P.T.S., SILVERBERG, B.A., LUXON, P.L., &
BENGERT, G.A. (1976) Methylation of selenium in the aquatic
environment. Science, 192: 1130-1131.
CHAVEZ, J.F. (1966) Studies on the toxicity of a sample of
Brazil nuts with a high content of selenium. Bol. Soc. Quim.
Peru, 32: 195-203.
CHEN, R.W., WHANGER, P.D., & WESWIG, P.H. (1975) Selenium-
induced redistribution of cadmium binding to tissue proteins:
a possible mechanism of protection against cadmium toxicity.
Bioinorgan. Chem., 4: 125-133.
CHEN, X., YANG, G., CHEN, J., CHEN, X., WEN, Z., & GE, K.
(1980) Studies on the relations of selenium and Keshan
disease. Biol. Trace Elem. Res., 2: 91-107.
CHERRY, D.S. & GUTHRIE, R.K. (1977) Toxic metals in surface
waters from coal ash. Water Resour. Bull., 13: 1227-1236.
CHUNG, A. & MAINES, M.D. (1981) Effect of selenium on
glutathione metabolism. Induction of -glutamylcysteine
synthetase and glutathione reductase in the rat liver.
Biochem. Pharmacol., 30(23): 3217-3223.
CLARK, L.C. (1985) The epidemiology of selenium and cancer.
Fed. Proc., 44(9): 2584-2589.
CLAYCOMB, C.K., SUMMERS, G.W., & JUMP, E.B. (1965) Effect of
dietary selenium on dental caries in Sprague Dawley rats. J.
dent. Res., 44: 826.
CLAYTON, C.C. & BAUMANN, C.A. (1949) Diet and azo dye
tumors: effect of diet during a period when the dye is not
fed. Cancer Res., 9: 575-582.
CLINTON, M., Jr (1947) Selenium fume exposure. J. ind. Hyg.
Toxicol., 29: 225-226.
COMBS, G.F., Jr & SCOTT, M.L. (1975) Polychlorinated
biphenyl-stimulated selenium deficiency in the chick.
Poultry. Sci., 54: 1152-1158.
COMBS, G.F., Jr, CANTOR, A.H., & SCOTT, M.L. (1975) Effects
of dietary polychlorinated biphenyls on vitamin E and selenium
nutrition in the chick. Poultry Sci., 54: 1143-1152.
CONE, J.E., MARTIN DEL RIO, R., DAVIS, J.N., & STADTMAN, T.C.
(1976) Chemical characterization of the selenoprotein
component of clostridial glycine reductase: identification of
selenocysteine as the organoselenium moiety. Proc. Natl Acad.
Sci. (USA), 73: 2659-2663.
COOPER, W.C. (1967) Selenium toxicity in man. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 185-199.
COOPER, W.C. (1974) Analytical chemistry of selenium. In:
Zingaro, R.A. & Cooper, W.C., ed. Selenium, New York, Van
Nostrand Reinhold Co., pp. 615-653.
COOPER, W.C. & GLOVER, J.R. (1974) The toxicology of
selenium and its compounds. III. In: Zingaro, R.A. & Cooper,
W.C., ed. Selenium, New York, Van Nostrand Reinhold Co., pp.
654-674.
COOPER, W.C., BENNETT, K.G., & CROXTON, F.C. (1974) The
history, occurrence, and properties of selenium. In: Zingaro,
R.A. & Cooper, W.C., ed. Selenium, New York, Van Nostrand
Reinhold Co., pp. 1-31.
CORREIA, M.A. & BURK, R.F. (1976) Hepatic heme metabolism in
selenium-deficient rats: effect of phenobarbital. Arch.
Biochem. Biophys., 177: 642-644.
CORREIA, M.A. & BURK, R.F. (1978) Rapid stimulation of
hepatic microsomal heme oxygenase in selenium-deficient rats:
an effect of phenobarbital. J. biol. Chem., 253: 6203-6210.
COWGILL, U.M. (1974) Trace elements and the birth rate in
the continental United States. In: Hemphill, D.D., ed. Trace
substances in environmental health. VIII, Columbia, Missouri,
University of Missouri Press, pp. 15-21.
COWGILL, U.M. (1976) Selenium and human fertility. In:
Proceedings of the Symposium on Selenium and Tellurium in the
Environment, Pittsburg, Pennsylvania, Industrial Health
Foundation, pp. 300-315.
CREGER, C.R., MITCHELL, R.H., ATKINSON, R.L., FERGUSON, T.M.,
REID, B.L., & COUCH, J.R. (1960) Vitamin E activity of
selenium in turkey hatchability. Poultry Sci., 39: 59-63.
CRYSTAL, R.G. (1973) Elemental selenium: structure and
properties. In: Klayman, D.L. & Gunther, W.H.H., ed. Organic
selenium compounds: their chemistry and biology, New York,
John Wiley and Sons, Inc, pp. 13-27.
CSALLANY, A.S., SU, L.-C., & MENKEN, B.Z. (1984) Effect of
selenite, vitamin E, and n,n'-diphenyl-p-phenylenediamine on
liver organic solvent-soluble lipofuscin pigments in mice.
J. Nutr., 114: 1582-1587.
CUKOR, P., WALZCYK, J., & LOTT, P.F. (1964) The application
of isotopic dilution analysis to the fluorimetric
determination of selenium in plant material. Anal. Chim. Acta,
30: 473-482.
CUMMINS, L.M. & KIMURA, E.T. (1971) Safety evaluation of
selenium sulfide antidandruff shampoos. Toxicol. appl.
Pharmacol., 20: 89-96.
CUMMINS, L.M. & MARTIN, J.L. (1967) Are selenocystine and
selenomethionine synthesized in vivo from sodium selenite in
mammals? Biochemistry, 6: 3162-3168.
CZERNIEJEWSKI, C.P., SHANK, C.W., BECHTEL, W.G., & BRADLEY,
W.B. (1964) The minerals of wheat, flour, and bread. Cereal
Chem., 41: 65-72.
D'HONDT, P., LIEVENS, P., VERSIECK, J., & HOSTE, J. (1977)
Determination of trace elements in animal and human muscle by
semi-automated radiochemical neutron activation analysis.
Radiochem. Radioanal. Lett., 31: 231-240.
DAMS, R. & DE JONGE, J. (1976) Chemical composition of Swiss
aerosols from the Jungfraujoch. Atmos. Environ., 10: 1079-1084.
DE JONG, D., MORSE, R.A., GUTENMANN, W.H., & LISK, D.J.
(1977) Selenium in pollen gathered by bees foraging on fly
ash-grown plants. Bull. environ. Contamin. Toxicol., 18:
442-444.
DE WITT, W.B. & SCHWARZ, K. (1958) Multiple dietary necrotic
degeneration in the mouse. Experientia, 14: 28-34.
DICKSON, J.D. (1969) Notes on hair and nail loss after
ingesting Sapucaia nuts (Lecythis elliptica). Econ. Bot., 23:
133-134.
DICKSON, R.C. & TOMLINSON, R.H. (1967) Selenium in blood and
human tissues. Clin. Chim. Acta, 16: 311-321.
DINKEL, C.A., MINYARD, J.A., WHITEHEAD, E.I., & OLSON, O.E.
(1957) Agricultural research at the Reed Ranch substation,
Brookings, South Dakota, South Dakota State College of
Agriculture and Mechanic Arts, 35 pp (Agricultural Experiment
Station Circular No. 135).
DINKEL, C.A., MINYARD, J.A., & RAY, D.E. (1963) Effects of
season of breeding on reproductive and weaning performance of
beef cattle grazing seleniferous range. J. anim. Sci., 22:
1043-1045.
DIPLOCK, A.T. (1974a) A possible role for trace amounts of
selenium and vitamin E in the electron-transfer system of
rat-liver microsomes. In: Hoekstra, W.G., Suttie, J.W.,
Ganther, H.E., & Mertz, W., ed. Trace element metabolism in
animals. II, Baltimore, Maryland, University Park Press, pp.
147-160.
DIPLOCK, A.T. (1974b) Possible stabilizing effect of vitamin
E on microsomal, membrane-bound, selenide-containing proteins
and drug-metabolizing enzyme systems. Am. J. clin. Nutr., 27:
995-1004.
DIPLOCK, A.T. (1976) Metabolic aspects of selenium action
and toxicity. CRC Crit. Rev. Toxicol., 4: 271-329.
DIPLOCK, A.T. (1979) The influence of selenium and vitamin E
on oxidative demethylation reactions. In: Olive, G., ed. Adv.
Pharmacol. Therap., Proceedings of the 7th International
Congress on Pharmacology, Vol. 8, pp. 25-33.
DIPLOCK, A.T., BAUM, H., & LUCY, J.A. (1971) The effect of
vitamin E on the oxidation state of selenium in rat liver.
Biochem. J., 123: 72l-729.
DIPLOCK, A.T., CAYGILL, C.P.J., JEFFERY, E.H., & THOMAS, C.
(1973) The nature of the acid-volatile selenium in the liver
of the male rat. Biochem. J., 134: 283-293.
DONALDSON, W.E. (1977) Selenium inhibition of avian fatty
acid synthetase complex. Chem.-biol. Interac., 17: 313-320.
DONGHERTY, J.J. & HOEKSTRA, W.G. (1982) Stimulation of lipid
peroxidation in vivo by injected selenite and lack of
stimulation by selenate. Proc. Soc. Exp. Biol. Med., 169(2):
209-215.
DORAN, J.W. (1976) Microbial transformations of selenium in
soil and culture, Ithaca, New York, Cornell University
(Ph.D. Thesis).
DOUGLASS, J.S., MORRIS, V.C., SOARES, J.H., Jr, & LEVANDER,
O.A. (1981) Nutritional availability to rats of selenium in
tuna, beef kidney, and wheat. J. Nutr., 111: 2180-2187.
DUCE, R.A., HOFFMAN, G.L., & ZOLLER, W.H. (1975) Atmospheric
trace metals at remote northern and southern hemisphere sites:
pollution or natural? Science, 187: 59-61.
DUDLEY, H.C. (1938) Toxicology of selenium. V. Toxic and
vesicant properties of selenium oxychloride. Public Health
Rep., 53: 94-98.
DUDLEY, H.C. & MILLER, J.W. (194l) Toxicology of selenium.
VI. Effects of subacute exposure to hydrogen selenide. J. ind.
Hyg. Toxicol., 23: 470-477.
DUTKIEWICZ, T., DUTKIEWICZ, B., & BALCERSKA, I. (1972)
Dynamics of organ and tissue distribution of selenium after
intragastric and dermal administration of sodium selenite.
Bromatol. Chem. Toksykol., 4: 475-481.
DUVOIR, M., POLLETT, L., & HERRENSCHMIDT, J.L. (1937) Eczéma
professionnel dû au sélénium. Bull. Soc. Franc. Dermat.
Syphil., 44: 88-95.
DYER, D.G., SCHUETT, V.E., & GANTHER, H.E. (1977) Blood
selenium and glutathione peroxidase in children with PKU on
diet therapy. In: Abstracts of the Western Hemisphere
Nutrition Congress. V, Quebec, pp. 417.
EGAN, A., KERR, S., & MINSKI, M.J. (1977) Instrumental
neutron activation analysis of selenium using 77µSe (T 1/2 =
17s) in biological materials. In: Brown, S.S., ed. Clinical
chemistry and chemical toxicology of metals, Amsterdam,
Elsevier North-Holland Biomedical Press, pp. 353-356.
ELLIS, N., LLOYD, B., LLOYD, R.S., & CLAYTON, B.E. (1984)
Selenium and vitamin E in relation to risk factors for
coronary heart disease. J. Clin. Pathol, 37(2): 200-206.
ENGBERG, R.A. (1973) Selenium in Nebraska's groundwater and
streams, Lincoln, Nebraska, University of Nebraska (Nebraska
Water Survey Paper No. 35).
ERMAKOV, V.V. (1975) [Selenium determination in biological
materials by fluorometry and gas-chromatography.] In:
[Vitamins. VIII. Biochemistry of vitamin E and selenium,]
Kiev, Naukova Dumka Publishing House, pp. 141-146 (in Russian).
ERMAKOV, V.V. & KOVALSKIJ, V.V. (1968) [The geochemical
ecology of organisms at high selenium levels in the
environment.] In: [Transactions of the biogeochemical
laboratory,] Moscow, Nauka Publishing House, Vol. 12, pp.
204-237 (in Russian).
ERMAKOV, V.V. & KOVALSKIJ, V.V. (1974) [The biological
importance of selenium,] Moscow, Nauka Publishing House, 298
pp (in Russian).
ERSHOV, V.P. (1969) [Hygienic aspects of selenium containing
steel production and the prevention of selenium intoxication.]
Gig. Tr. Prof. Zabol., 12: 29-33 (in Russian).
EVANS, C.S., ASHER, C.J., & JOHNSON, C.M. (1968) Isolation
of dimethyldiselenide and other volatile selenium compounds
from Astragalus racemosus (Pursh.). Austral. J. biol. Sci.,
21: 13-20.
EVANS, H.M. & BISHOP, K.S. (1922) On the existence of a
hitherto unrecognized dietary factor essential for
reproduction. Science, 56: 650-651.
EWAN, R.C., POPE, A.L., & BAUMANN, C.A. (1967) Elimination
of fixed selenium by the rat. J. Nutr., 91: 547-554.
EWAN, R.C., BAUMANN, C.A., & POPE, A.L. (1968) Retention of
selenium by growing lambs. J. agric. food Chem., 16: 216-219.
EXON, J.H., KOLLER, L.D., & ELLIOTT, S.C. (1976) Effect of
dietary selenium on tumor induction by an oncogenic virus.
Clin. Toxicol., 9: 273-279.
FALK, R. & LINDHE, J.C. (1974) Radiation dose received by
humans from intravenously-administered sodium selenite marked
with selenium-75, Los Alamos, New Mexico, Los Alamos
Scientific Laboratory (Los Alamos Translation LA-TR-76-5).
FENG, Z., WANG, X., & HAN, C. (1985) [Studies on the
toxicity of diets with different selenium levels in rats.]
Food Hyg. Res., 3(2): 14-20 (in Chinese).
FERRETTI, R.J. & LEVANDER, O.A. (1974) Effect of milling and
processing on the selenium content of grains and cereal
products. J. agric. food Chem., 22: 1049-1051.
FERRETTI, R.J. & LEVANDER, O.A. (1976) Selenium content of
soybean foods. J. agric. food Chem., 24: 54-56.
FILATOVA, V.S. (1948) [Characteristics of selenium as an
industrial intoxicant.] Can. Med. Thesis (in Russian).
FILATOVA, V.S. (1951) [On the toxicity of selenium
chloride.] Gig. i Sanit., 5: 18-23 (in Russian).
FLEMING, G.A. (1962) Selenium in Irish soils and plants.
Soil Sci., 94: 28-35.
FLEMING, G.A. & WALSH, T. (1957) Selenium occurrence in
certain Irish soils and its toxic effects on animals. Proc.
Royal Irish Acad., 58: 151-166.
FLOHE, L., GUNZLER, W.A., & SCHOEKS, H.H. (1973) Glutathione
peroxidase: a selenoenzyme. FEBS Lett., 32: 132-134.
FORBES, S. & BOUND, G.P. (1977) Some investigations into the
electroanalytical chemistry of selenium. Proc. Anal. Div.
Chem. Soc., 14: 253-256.
FORSTROM, J.W., ZAKOWSKI, J.J., & TAPPEL, A.L. (1978)
Identification of the catalytic site of rat liver glutathione
peroxidase as selenocysteine. Biochemistry, 17: 2639-2644.
FRANKE, K.W. & MOXON, A.L. (1936) A comparison of the
minimum fatal doses of selenium, tellurium, arsenic, and
vanadium. J. Pharmacol. exp. Ther., 58: 454-459.
FRANKE, K.W. & POTTER, V.R. (1936) The effect of selenium
containing foodstuffs on growth and reproduction of rats at
various ages. J. Nutr., 12: 205-214.
FRANKE, K.W. & TULLY, W.C. (1935) A new toxicant occurring
naturally in certain samples of plant foodstuffs. V. Low
hatchability due to deformities in chicks. Poultry Sci., 14:
273-279.
FRANKE, K.W., MOXON, A.L., POLEY, W.E., & TULLY, W.C. (1936)
Monstrosities produced by the injection of selenium salts into
hen's eggs. Anat. Rec., 65: 15-22.
FROSETH, J.A., PIPER, R.C., & CARLSON, J.R. (1974)
Relationship of dietary selenium and oral methyl mercury to
blood and tissue selenium and mercury concentrations and
deficiency: toxicity signs in swine. Fed. Proc., 33: 660.
FROST, D.V. & INGVOLDSTAD, D. (1975) Ecological aspects of
selenium and tellurium in human and animal health. Chemica
Scripta, 8A: 96-107.
FURR, A.K., PARKINSON, T.F., HINRICHS, R.A., VAN CAMPEN, D.R.,
BACHE, C.A., GUTENMANN, W.H., ST. JOHN, L.E., Jr, PAKKALA,
I.S., & LISK, D.J. (1977) National survey of elements and
radioactivity in fly ashes. Absorption of elements by cabbage
grown in fly ash-soil mixtures. Environ. Sci. Technol., 11:
1194-1201.
FURR, A.K., PARKINSON, T.F., GUTENMANN, W.H., PAKKALA, I.S., &
LISK, D.J. (1978a) Elemental content of vegetables, grains,
and forages field-grown on fly ash amended soil. J. agric.
food Chem., 26: 357-359.
FURR, A.K., PARKINSON, T.F., HEFFRON, C.L., REID, J.T.,
HASCHEK, W.M., GUTENMANN, W.H., PAKKALA, I.S., & LISK, D.J.
(1978b) Elemental content of tissues of sheep fed rations
containing coal fly ash. J. agric. food Chem., 26: 1271-1274.
GAIROLA, C. & CHOW, C.K. (1982) Dietary selenium, hepatic
arylhydrocarbon hydroxylase, and mutagenic activation of
benzo( a)pyrene, 2-aminoanthracene, and 2-aminofluorene.
Toxicol. Lett., 11: 281-287.
GANAPATHY, S. & DHANDA, R. (1976) Selenium content of
omniverous and vegetarian diets. Fed. Proc., 35: 360.
GANAPATHY, S.N., JOYNER, B.T., & HAFNER, K.M. (1975) Effect
of baking, broiling, and frying on the selenium content of
selected beef, pork, chicken and fish foods. In: Proceedings
of the 10th International Congress of Nutrition - Abstracts,
Kyoto, Japan, 3-9 August, 1975, p. 277.
GANTHER, H.E. (1968) Selenotrisulfides. Formation by the
reaction of thiols with selenious acid. Biochemistry, 7:
2898-2905.
GANTHER, H.E. (1971) Reduction of the selenotrisulfide
derivative of glutathione to a persulfide analog by
glutathione reductase. Biochemistry, 10: 4089-4098.
GANTHER, H.E. (1978) Modification of methylmercury toxicity
and metabolism by selenium and vitamin E: possible mechanisms.
Environ. Health Perspect., 25: 71-76.
GANTHER, H.E. (1979) Metabolism of hydrogen selenide and
methylated selenides. Adv. Nutr. Res., 2: 107-128.
GANTHER, H.E. & BAUMANN, C.A. (1962) Selenium metabolism.
II. Modifying effects of sulfate. J. Nutr., 77: 408-414.
GANTHER, H.E. & CORCORAN, C. (1969) Selenotrisulfides. II.
Cross-linking of reduced pancreatic ribonuclease with
selenium. Biochemistry, 8: 2557-2563.
GANTHER, H.E. & HSIEH, H.S. (1974) Mechanisms for the
conversion of selenite to selenides in mammalian tissues. In:
Hoekstra, W.G., Suttie, J.W., Ganther, H.E., & Mertz, W., ed.
Trace element metabolism in animals. 2, Baltimore, Maryland,
University Park Press, pp. 339-353.
GANTHER, H.E. & SUNDE, M.L. (1974) Effect of tuna fish and
selenium on the toxicity of methylmercury: a progress report.
J. food Sci., 39: 1-5.
GANTHER, H.E., LEVANDER, O.A., & BAUMANN, C.A. (1966)
Dietary control of selenium volatilization in the rat. J.
Nutr., 88: 55-60.
GANTHER, H.E., GOUDIE, C., SUNDE, M.L., KOPECKY, M.J., WAGNER,
P., OH, S., & HOEKSTRA, W.G. (1972) Selenium: relation to
decreased toxicity of methylmercury added to diets containing
tuna. Science, 175: 1122-1124.
GANTHER, H.E., HAFEMAN, D.G., LAWRENCE, R.A., SERFASS, R.E., &
HOEKSTRA, W.G. (1976) Selenium and glutathione peroxidase in
health and disease: a review. In: Prasad, H.H., ed. Trace
elements in human health and disease. II. Essential and toxic
elements, New York, Academic Press pp. 165-234.
GARDINER, M.R., ARMSTRONG, J., FELS, H., & GLENCROSS, R.N.
(1962) A preliminary report on selenium and animal health in
Western Australia. Aust. J. exp. Agric. Animal Husb., 2:
261-269.
GARDNER, S. (1973) Selenium in animal feed. Proposed food
additive regulation. Fed. Reg., 38: 10458-10460.
GE, K., XUE, A., BAI, J., & WANG, S. (1983) Keshan disease -
an endemic cardiomyopathy in China. Virchows Arch. (Pathol.
Anat.), 401: 1-15.
GEERING, H.R., CARY, E.E., JONES, L.H.P., & ALLAWAY, W.H.
(1968) Solubility and redox criteria for the possible forms
of selenium in soils. Soil Sci. Soc. Am. Proc., 32: 35-40.
GIASUDDIN, A.S.M., CAYGILL, C.P.J., DIPLOCK, A.T., & JEFFERY,
E.H. (1975) The dependence on vitamin E and selenium of drug
demethylation in rat liver microsomal fractions. Biochem. J.,
146: 339-350.
GISSEL-NIELSEN, G. (1971) Selenium content of some
fertilizers and their influence on uptake of selenium in
plants. J. agric. food Chem., 19: 564-566.
GISSEL-NIELSEN, G. (1973) Ecological effects of selenium
application to field crops. Ambio, 2: 114-117.
GISSEL-NIELSEN, G. (1976) Selenium in soils and plants. In:
Proceedings of the Symposium on Se-Te in the Environment,
Pittsburgh, Pennsylvania, Industrial Health Foundation,
pp. 10-25.
GISSEL-NIELSEN, G. (1986) Selenium fertilizers and foliar
application, Danish experiments. Ann. clin. Res., 18: 61-64.
GISSEL-NIELSEN, M. & GISSEL-NIELSEN, G. (1975) Selenium in
soil-animal relationships. Pedobiologia, 15: 65-67.
GITSOVA, S. (1973) Presence of selenium in drinking waters
in Bulgaria. Khig. Zdraveopazvane, 16: 557-561.
GLOVER, J.R. (1954) Some medical problems concerning
selenium in industry. Trans. Assoc. Ind. Med. Off., 4: 94-96.
GLOVER, J.R. (1967) Selenium in human urine: a tentative
maximum allowable concentration for industrial and rural
populations. Ann. occup. Hyg., 10: 3-14.
GLOVER, J.R. (1970) Selenium and its industrial toxicology.
Ind. Med. Surg., 39: 50-54.
GLOVER, J.R. (1976) Environmental health aspects of selenium
and tellurium. In: Proceedings of the Symposium on
Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 279-292.
GODWIN, K.O. & FUSS, C.N. (1972) The entry of selenium into
rabbit protein following the administration of Na2 75Se03.
Aust. J. biol. Sci., 25: 865-871.
GOODWIN, W.J., LANE, H.W., BRADFORD, K., MARSHALL, M.V.,
GRIFFIN, A.C., GEOPFERT, H., & JESSE, R.H. (1983) Selenium
and glutathione peroxidase levels in patients with epidermoid
carcinoma of the oral cavity and orophasynx. Cancer, 51:
110-115.
GORTNER, R.A., Jr (1940) Chronic selenium poisoning of rats
as influenced by dietary protein. J. Nutr., 19: 105-112.
GOULDEN, P.D. & BROOKSBANK, P. (1974) Automated atomic
absorption determination of arsenic, antimony, and selenium in
natural waters. Anal. Chem., 46: 1431-1436.
GRACIANSKAJA, L.N. & KOVSHILO, V.E., ed. (1977) [Selenium
and its compounds]. In: [Handbook of occupational pathology,]
Moscow, Medicina Publishing House pp. 330-332 (in Russian).
GRANT, K.E., CONNER, M.W., & NEWBERNE, P.M. (1977) Effect of
dietary sodium selenite upon lesions induced by repeated small
doses of aflatoxin B1. Toxicol. appl. Pharmacol., 41: 166.
GREEN, J., BUNYAN, J., CAWTHORNE, M.A., & DIPLOCK, A.T.
(1969) Vitamin E and hepatotoxic agents. I. Carbon
tetrachloride and lipid peroxidation in the rat. Br. J. Nutr.,
23: 297-307.
GRIFFIN, A.C. & JACOBS, M.M. (1977) Effects of selenium on
azo dye hepatocarcinogenesis. Cancer Lett., 3: 177-181.
GRIFFITHS, N.M. (1973) Dietary intake and urinary excretion
of selenium in some New Zealand women. Proc. Univ. Otago Med.
School, 51: 8-9.
GRIFFITHS, N.M. & THOMSON, C.D. (1974) Selenium in the whole
blood of New Zealand residents. N.Z. med. J., 80: 199-202.
GRIFFITHS, N.M., STEWART, R.D.H., & ROBINSON, M.F. (1976)
The metabolism of 75Se-selenomethionine in four women. Br. J.
Nutr., 35: 373-382.
GRIMANIS, A.P., VASSILAKI-GRIMANI, M., ALEXIOU, D., &
PAPADATOS, C. (1978) Determination of seven trace elements in
human milk, powdered cow's milk, and infant foods by neutron
activation analysis. In: Nuclear activation techniques in the
life sciences, Vienna, International Atomic Energy Agency,
pp. 241-253.
GROCE, A.W., MILLER, E.R., KEAHEY, K.K., ULLREY, D.E., &
ELLIS, D. J. (1971) Selenium supplementation of practical
diets for growing-finishing swine. J. Ann. Sci., 32: 905-911.
GROSS, S. (1976) Hemolytic anemia in premature infants:
Relationship to vitamin E, selenium, glutathione peroxidase,
and erythrocyte lipids. Semin. Hematol., 13: 187-199.
GROSSMAN, A. & WENDEL, A. (1983) Non-reactivity of the
selenoenzyme glutathione peroxidase with enzymatically hydro-
peroxidized phospholipids. Eur. J. Biochem., 135(3): 549-552.
GU, B. (1983) Pathology of Keshan disease: a comprehensive
review. Chin. med. J., 96: 251-261.
GUSEJNOV, T.M., ZHAFAROV, A.I., PERELYGIN, V.V., & KARAEV,
M.A. (1974) [On the localization of endogenous selenium in
structural slements of bull's eye.] In: [Selenium in biology,]
Baku, Elm Publishing House, pp. 47-49.
GUTENMANN, W.H., BACHE, C.A., YOUNGS, W.D., & LISK, D.J.
(1976) Selenium in fly ash. Science, 191: 966-967.
HADDAD, P.R. & SMYTHE, L.E. (1974) A critical evaluation of
fluorometric methods for determination of selenium in plant
materials with 2,3-diaminoaphthalene. Talanta, 21: 859-865.
HADJIMARKOS, D.M. (1956) Geographic variations of dental
caries in Oregon. VII. Caries prevalence among children in the
blue mountains region. J. Pediatr., 48: 195-201.
HADJIMARKOS, D.M. (1960) Urinary selenium and dental caries.
Nature (Lond.), 188: 677.
HADJIMARKOS, D.M. & BONHORST, C.W. (1958) The trace element
selenium and its influence on dental caries susceptibility. J.
Pediatr., 52: 274-278.
HADJIMARKOS, D.M. & BONHORST, C.W. (1961) The selenium
content of eggs, milk, and water in relation to dental caries
in children. J. Pediatr., 59: 256-259.
HADJIMARKOS, D.M. & SHEARER, T.R. (1971) Selenium
concentration in human saliva. Am. J. clin. Nutr., 24: 1210.
HADJIMARKOS, D.M. & STORVICK, C.A. (1950) Geographic
variations of dental caries in Oregon. IV. Am. J. public
Health, 40: 1552-1555.
HADJIMARKOS, D.M., STORVICK, C.A., & REMMERT, L.F. (1952)
Selenium and dental caries. An investigation among school
children of Oregon. J. Pediatr., 40: 451-455.
HAFEMAN, D.G. & HOEKSTRA, W.G. (1977a) Protection against
carbon tetrachloride-induced lipid peroxidation in the rat by
dietary vitamin E, selenium, and methionine as measured by
ethane evolution. J. Nutr., 107: 656-665.
HAFEMAN, D.G. & HOEKSTRA, W.G. (1977b) Lipid peroxidation in
vivo during vitamin E and selenium deficiency in the rat as
monitored by ethane evolution. J. Nutr., 107: 666-672.
HAFEMAN, D.G., SUNDE, R.A., & HOEKSTRA, W.G. (1974) Effect
of dietary selenium on erythrocyte and liver glutathione
peroxidase in the rat. J. Nutr., 104: 580-587.
HAKKARAINEN, J., LINDBERG, P., BENGTSSON, G., JONSSON, L., &
LANNEK, N. (1978) Requirement for selenium (as selenite) and
vitamin E (as alpha-tocopherol) in weaned pigs. III. The effect on
the development of the VESD syndrome of varying selenium
levels in a low-tocopherol diet. J. anal. Sci., 46: 1001-1008.
HALL, R.H., LASKIN, S., FRANK, P., MAYNARD, E.A., & HODGE,
C.H. (1951) Arch. ind. Hyg. occup. Med., 4: 458-464.
HALTER, K. (1938) [Selenium poisoning, especially skin
changes accompanied by secondary porphysia.] Arch. Dermatol.,
178: 340 (in German).
HALVERSON, A.W. (1974) Growth and reproduction with rats fed
selenite-Se. Proc. S. Dak. Acad. Sci., 53: 167-177.
HALVERSON, A.W., HENDRICK, C.M., & OLSON, O.E. (1955) Observations
on the protective effect of linseed oil meal and some extracts
against chronic selenium poisoning in rats. J. Nutr., 56: 51-60.
HALVERSON, A.W., GUSS, P.L., & OLSON, O.E. (1962) Effect of
sulfur salts on selenium poisoning in the rat. J. Nutr., 77:
459-464.
HALVERSON, A.W., PALMER, I.S., & GUSS, P.L. (1966) Toxicity
of selenium to post-weanling rats. Toxicol. appl. Pharmacol.,
9: 477-484.
HAMDY, A.A. & GISSEL-NIELSEN, G. (1976) Volatilization of
selenium from soils. Z. Pflanzenernaehr. Bodenkd., 6: 671-678.
HAMILTON, A. (1927) Industrial poisons in the United States,
Macmillan Company, p. 111.
HAMILTON, A. (1934) Industrial toxicology, New York, Harpers
Publishing Company, p. 111.
HAMILTON, J.W. (1975) Chemical examination of seleniferous
cabbage Brassica oleracea capitata. J. agric. food Chem., 23:
1150-1152.
HARLAND, B.F., PROSKY, L., & VANDERVEEN, J.E. (1978)
Nutritional adequacy of current levels of Ca, Cu, Fe, I, Mg,
Mn, P, Se, and Zn in the American food supply for adults,
infants, and toddlers. In: Kirchgessner, M., ed. Trace element
metabolism in man and animals. 3, Freising- Weihenstephan,
West Germany, Arbeitskreis fur Tierernährungsforschung
Weihenstephan, pp. 311-315.
HARR, J.R. & MUTH, O.H. (1972) Selenium poisoning in
domestic animals and its relationship to man. Clin. Toxicol.,
5: 175-186.
HARR, J.R., BONE, J.F., TINSLEY, I.J., WESWIG, P.H., &
YAMAMOTO, R.S. (1967) Selenium toxicity in rats. II.
Histopathology. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI Publishing Co., Inc, pp. 153-178.
HARR, J.R., EXON, J.H., WHANGER, P.D., & WESWIG, P.H. (1972)
Effect of dietary selenium on N-2-fluorenyl-acetamide
(FAA)-induced cancer in vitamin E-supplemented, selenium
depleted rats. Clin. Toxicol., 5: 187-194.
HARRIS, P.L., LUDWIG, M.I., & SCHWARZ, K. (1958)
Ineffectiveness of Factor 3-active selenium compounds in
resorption-gestation bioassay for vitamin E. Proc. Soc. Exp.
Biol. Med., 97: 686-688.
HARTHOORN, A.M. & YOUNG, E. (1974) A relationship between
acid-base balance and capture myopathy in zebra (Equus
burchelli) and an apparent therapy. Vet. Res., 95: 337-342.
HARTLEY, W.J. (1963) Selenium and ewe fertility. Proc. N.Z.
Soc. Anim. Prod., 23: 20-27.
HARTLEY, W.J. (1967) Levels of selenium in animal tissues
and methods of selenium administration . In: Muth, O.H.,
Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 79-96.
HARTLEY, W.J. & GRANT, A.B. (1961) A review of selenium
responsive diseases of New Zealand livestock. Fed. Proc., 20:
679-688.
HARTLEY, W.J., GRANT, A.B., & DRAKE, C. (1960) Control of
white muscle disease and ill thrift with selenium. N.Z. J.
Agric., 101: 343-345.
HASHIMOTO, Y. & WINCHESTER, J.W. (1967) Selenium in the
atmosphere. Environ. Sci. Technol., 1: 338-340.
HEATH, R.L. (1969-70) Handbook of chemistry and physics,
50th ed., Cleveland, Ohio, The Chemical Rubber Publishing Co.
HE, G. (1979) On the etiology of Keshan disease: two
hypotheses. Chin. med. J., 92: 416-422.
HEINRICH, M., Jr & KELSEY, F.E. (1955) Studies on selenium
metabolism: the distribution of selenium in the tissues of the
mouse. J. Pharmacol. exp. Therap., 114: 28-32.
HELZLSOUER, K., JACOBS, R., & MORRIS, S. (1985) Acute
selenium intoxication in the United States. Fed. Proc., 44(5):
1670.
HICKEY, F. (1968) Selenium in human and animal nutrition.
N.Z. Agric., 18: 1-2.
HICKEY, F. (1977) Human medication with selenium. Including
brief comments on the comparative pathology of White Muscle
Disease in livestock and human muscular dystrophies. In:
Hamilton, N.Z., ed. Trace elements in human and animal health
in New Zealand, Hamilton, New Zealand, Waikato University
Press, pp. 92-99.
HIGGS, D.J., MORRIS, V.C., & LEVANDER, O.A. (1972) Effect of
cooking on selenium content of foods. J. agric. food Chem.,
20: 678-680.
HODDER, A.P.W. & WATKINSON, J.H. (1976) Low selenium levels
in tephra-derived soil - inherent or pedogenetic? N.Z. J.
Sci., 19: 397-400.
HOEKSTRA, W.G. (1975a) Biochemical function of selenium and
its relation to vitamin E. Fed. Proc., 34: 2083-2089.
HOEKSTRA, W.G. (1975b) Glutathione peroxidase activity of
animal tissues as an index of selenium status. In: Hemphill,
D.D., ed. Trace substances in environmental health. IX,
Columbia, Missouri, University of Missouri Press, pp. 331-337.
HOEKSTRA, W.G., HAFEMAN, O., OH, S.H., SUNDER, R.A., & GANTHER,
H.E. (1973) Effect of dietary selenium on liver and erythrocyte
glutathione peroxidase in the rat. Fed. Proc., 32: 885.
HOGGER, D. & BOHM, C. (1944) [On dermal injury produced by
selenite]. Dermatologica, 90: 217-223 (in German).
HOLMBERG, R.E., & FERM, V.H. (1969) Interrelationships of
selenium, cadmium, and arsenic in mammalian teratogenesis.
Arch. environ. Health, 18: 873-877.
HOLSTEIN, E. (1951) [The effects of occupational exposure to
selenium.] Zentrblt. Arbeitsmed. Arbeitschutz, 1: 102-104 (in
German).
HOLYNSKA, B. & MARKOWICZ, A. (1977) Application of energy
dispersive X-ray fluorescence for the determination of
selenium in blood and tissue. Radiochem. radioanal. Lett., 31:
165-170.
HOPKINS, L.L. & MAJAJ, A.S. (1967) Selenium in human
nutrition. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H.,
ed. Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 203-214.
HOVE, E.L. (1948) Interrelation between alpha-tocopherol and
protein metabolism. III. The protective effect of vitamin E
and certain nitrogenous compounds against CCl4 poisoning in
rats. Arch. Biochem., 17: 467-474.
HOWARD, J.H., III (1971) Control of geochemical behavior of
selenium in natural waters by adsorption on hydrous ferric
oxides. In: Hemphill, D.D., ed. Trace substances in
environmental health. V, Columbia, Missouri, University of
Missouri Press, pp. 485-495.
HOWE, M. (1974) Selenium in the blood of south Dakotans.
Arch. environ. Health, 34: 444-448.
HURT, H.D., CARY E.E., & VISEK, W.J. (1971) Growth,
reproduction, and tissue concentrations of selenium in the
selenium-depleted rat. J. Nutr., 101: 761-766.
IARC (1975) Some aziridines, N,-S-, and O-mustards and
selenium, Lyons, International Agency for Research on Cancer,
pp. 245-260 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, Vol. 9).
IHNAT, M. (1974) Collaborative study of a fluorometric
method for determining selenium in foods. J. Assoc. Off. Anal.
Chem., 57: 373-378.
IHNAT, M. (1976) Selenium in foods: evaluation of atomic
absorption spectrometric techniques involving hydrogen
selenide generation and carbon furnace atomization. J. Assoc.
Off. Anal. Chem., 59: 911-922.
IHNAT, M. & MILLER, H.J. (1977a) Analysis of foods for
arsenic and selenium by acid digestion, hydride evolution
atomic absorption spectrophotometry. J. Assoc. Off. Anal.
Chem., 60: 813-825.
IHNAT, M. & MILLER, H.J. (1977b) Acid digestion, hydride
evolution atomic absorption spectrophotometric method for
determining arsenic and selenium in foods: collaborative
study. I. J. Assoc. Off. Anal. Chem., 60: 1414-1433.
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J.
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IP, C. (1985a) Selenium inhibition of chemical
carcinogenesis. Fed. Proc., 44: 2573-2578.
IP, C. (1985b) Attenuation of the anticarcinogenic action of
selenium by vitamin E deficiency. Cancer Lett., 25: 325-331.
IP, C. & SINHA, D.K. (1981) Enhancement of mammary
tumorigenesis by dietary selenium deficiency in rats with a
high polyunsaturated fat intake. Cancer Res., 41: 31-34.
IRGOLIC, K.J. & KUDCHADKER, M.H. (1974) Organic chemistry of
selenium. In: Zingaro, R.A. & Cooper, W.C., ed. Selenium, New
York, Van Nostrand Reinhold Co., pp. 408-545.
IZRAELSON, Z.I., MOGILEVSKAJA, O.Ja., & SUVOROV, S.W. (1973)
[Selenium.] In: [Problems of occupational hygiene and
occupational pathology connected with the work with toxic
metals,] Moscow, Medicina Publishing House pp. 245-257 (in
Russian).
JACOBS, M.M. (1976) Selenium: a possible inhibitor of colon
and rectum cancer. II. Biochemical aspects. In: Proceedings of
the Symposium on Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 329-340.
JACOBS, M.M. (1977) Inhibitory effects of selenium on 1,2-
dimethylhydrazine and methylazoxymethanol colon carcinogenesis.
Cancer, 40: 2557-2564.
JACOBS, M.M. & FORST, C. (1981a) Toxicological effects of
sodium selenite in Sprague-Dawley rats. J. Toxicol. environ.
Health, 8: 575-585.
JACOBS, M.M. & FORST, C. (1981b) Toxicological effects of
sodium selenite in Swiss mice. J. Toxicol. environ. Health, 8:
587-598.
JACOBS, M.M., JANSSON, B., & GRIFFIN, A.C. (1977a) Inhibitory
effects of selenium on 1,2-dimethylhydrazine and methylazoxy-
methanol acetate induction of colon tumours. Cancer Lett., 2:
133-138.
JACOBS, M.M., MATNEY, T.S., & GRIFFIN, A.C. (1977b) Inhibitory
effects of selenium on the mutagenicity of 2-acetylamino-
fluorene (AAF) and AAF derivatives. Cancer Lett., 2: 319-322.
JACOBSSON, S.O., LIDMAN, S., & LINDBERG, P. (1970) Blood
selenium in a beef herd affected with muscular degeneration.
Acta vet. Scand., 11: 324-326.
JAFFE, W.G. (1973) Selenium in food plants and feeds.
Toxicology and nutrition. Qualitas Plantarum. Plant foods hum.
Nutr., 23: 191-204.
JAFFE, W.G. (1976) Effect of selenium intake in humans and
in rats. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 188-193.
JAFFE, W.G. & MONDRAGON, M.C. (1969) Adaptation of rats to
selenium intake. J. Nutr., 97: 431-436.
JAFFE, W.G. & MONDRAGON, C. (1975) Effects of ingestion of
organic selenium in adapted and non-adapted rats. Br. J.
Nutr., 33: 387-397.
JAFFE, W.G. & VELEZ, B.F. (1973) Selenium intake and
congenital malformations in humans. Arch. Latinoam. Nutr., 23:
514-516.
JAFFE, W.G., RUPHAEL, M.D., MONDRAGON, M.C., & CUEVAS, M.A.
(1972a) Clinical and biochemical studies on school children
from a seleniferous zone. Arch. Latinoam. Nutr., 22: 595-611.
JAFFE, W.G., MONDRAGON, M.C., LAYRISSE, M., & OJEDA, A.
(1972b) Toxicity symptoms in rats fed organic selenium. Arch.
Latinoam. Nutr., 22: 467-481.
JANSSON, B., JACOBS, M.M., & GRIFFIN, A.C. (1978)
Gastrointestinal cancer: epidemiology and experimental
studies. In: Schrauzer, G.N., ed. Inorganic and nutritional
aspects of cancer, New York, Plenum Press, pp. 305-322.
JENKINS, K.J. & HIDIROGLOU, M. (1971) Comparative uptake of
selenium by low cystine and high cystine proteins. Can. J.
Biochem., 49: 468-472.
JENSEN, L.S. & MCGINNIS, J. (1960) Influence of selenium,
antioxidants, and type of yeast on vitamin E deficiency in the
adult chicken. J. Nutr., 72: 23-28.
JENSEN, R., CLOSSON, W., & ROTHENBERG, R. (1984) Selenium
intoxication - New York. Mobid. Mortal. Weekly Rep., 33:
157-158.
JOHNSON, C.M. (1976) Selenium in the environment. Res. Rev.,
62: 101-130.
JONES, G.B. & GODWIN, K.O. (1962) Distribution of
radioactive selenium in mice. Nature (Lond.), 196: 1294-1296.
JUDSON, G.J. & OBST, J.M. (1975) Diagnosis and treatment of
selenium inadequacies in the grazing ruminant. In: Nicholas,
D.J.D. & Egan, A.R., ed. Trace elements in soil-plant-animal
systems, New York, Academic Press, Inc, pp. 385-405.
JULIUS, A.D., DAVIES, M.H., & BIRT, D.F. (1980) Relationship
between blood selenium and erythrocyte glutathione peroxidase
activity with excess dietary selenium in Syrian golden
hamsters. Fed. Proc., 39: 555.
KAMADA, T., SHIRAISHI, T., & YAMAMOTO, Y. (1978)
Differential determination of selenium (IV) and selenium (VI)
with sodium diethyldithiocarbamate, ammonium pyrrolidinedi-
thiocarbamate and dithizone by atomic-absorption spectrophoto-
metry with a carbon-tube atomizer. Talanta, 25: 15-19.
KAQUELER, J.C., MALOIGNE, E., & BONIFAY, P. (1977) Effects
of sodium selenate on caries incidence on the rat. J. dent.
Res., D 56 (Special Issue): D151.
KASIMOV, R.Ju., RUSTAMOVA, Sh.A., GUSEJNOV, T.M., & KIAZIMOV,
S.K. (1976) [Dynamics of selenium distribution in some organs
of young fish from selected valuable edible species.] In:
[Selenium in biology,] Baku, Elm Publishing House, Vol. 2, pp.
143-144 (in Russian).
KERDEL-VEGAS, F. (1966) The depilatory and cytotoxic action
of "Coco de Mono" (Lecythis ollaria) and its relationship to
chronic seleniosis. Econ. Bot., 20: 187-195.
KESHAN DISEASE RESEARCH GROUP (1979a) Observations on effect
of sodium selenite in prevention of Keshan disease. Chinese
med. J., 92: 471-476.
KESHAN DISEASE RESEARCH GROUP (1979b) Epidemiologic studies
on the etiologic relationship of selenium and Keshan disease.
Chinese med. J., 92: 477-482.
KETTERER, B., BEALE, D., & MEYER, D. (1982) The structure
and multiple functions of glutathione transferases. Biochem.
Soc. Trans., 10(2): 82-84.
KILNESS, A.W. (1973) Selenium and public health. S.D. J.
Med., 26: 17-19.
KILNESS, A.W. & HOCHBERG, F.H. (1977) Amyotrophic lateral
sclerosis in a high selenium environment. J. Am. Med. Assoc.,
237: 2843-2844.
KIMMERLE, G. (1960) [Comparative studies on the inhalation
toxicity of sulfur-selenium and tellurium hexafluoride.] Arch.
Toxikol., 18: 140-144 (in German).
KINNIGKEIT, G. (1962) [Studies on workers exposed to selenium
in a rectifier plant.] Z. Gesamte Hyg. Grenzgeb., 8: 350-362
(in German).
KLAYMAN, D.L. & GUNTHER, W.H.H. (1973) Organic selenium
compounds: their chemistry and biology, New York, John Wiley
and Sons, Inc, 1188 pp.
KLEIN, A.K. (1943) Report on selenium. J. Assoc. Offic.
Anal. Chem., 26: 346-352.
KLUG, H.L., PETERSEN, D.F., & MOXON, A.L. (1949) The
toxicity of selenium analogues of cystine and methionine.
Proc. S.D. Acad. Sci., 28: 117-120.
KLUG, H.L., MOXON, A.L., PETERSEN, D.F, & POTTER, V.R. (1950)
The in vivo inhibition of succinic dehydrogenase by selenium
and its release by arsenic. Arch. Biochem. Biophys., 28:
253-259.
KOEMAN, J.H., VAN DE VEN, W.S.M., DE GOEIJ, J.J.M., TJIOE,
P.S., & VAN HAAFTEN, J.L. (1975) Mercury and selenium in
marine mammals and birds. Sci. total Environ., 3: 279-287.
KOIVISTOINEN, P., ed. (1980) Mineral elements composition of
Finnish foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni,
Cr F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb, and ash. Acta
agric. Scand., Suppl. 2: 17.
KOIVISTOINEN, P. & HUTTUNEN, J.K. (1985) Selenium deficiency
in Finnish food and nutrition: research strategy and measures.
In: Mills, C.F., Bremner, I., & Chesters, J.K., ed., Trace
elements in man and animals-TEMA 5, Slough, Commonwealth
Agricultural Bureaux, pp. 925-928.
KOIVISTOINEN, P. & HUTTUNEN, J.K. (1986) Selenium in food
and nutrition in Finland. An overview on research and action.
Ann. clin. Res., 18: 13-17.
KOSTA, L., BYRNE, A.R., & ZELENKO, V. (1975) Correlation
between selenium and mercury in man following exposure to
inorganic mercury. Nature (Lond.), 254: 238-239.
KOVALSKIJ, V.V. (1974) [Geochemical ecology,] Moscow, Nauka
Publishing House, 299 pp (in Russian).
KOVALSKIJ, V.V. (1978) [Geochemical ecology: a basis for a
system of biogeochemical regional characterization.] In:
[Biochemical regional characterization: a method for studying
the ecological structure of the biosphere,] Moscow, Nauka
Publishing House, Vol. 15, pp. 3-21 (in Russian).
KOVALSKIJ, V.V. & ERMAKOV, V.V. (1975) [Problems of
experimental geochemical ecology of animals under the
conditions of selenium regions and some approaches to the
study of the biological role of selenium.] In: [Vitamins. VII.
Biochemistry of vitamin E and selenium,] Kiev, Nakove Dunka
Publishing House, pp. 80-87 (in Russian).
KRAUSKOPF, K.B. (1955) Sedimentary deposits of rare
elements. Econ. Geol. Fiftieth Anniversary, (Part I): 411-463.
KRONBORG, O.J. & STEINNES, E. (1975) Simultaneous determination
of arsenic and selenium in soil by neutron-activation analysis.
Analyst, 100: 835-837.
KU, P.K., ELY, W.T., GROCE, A.W., & ULLREY, D.E. (1972)
Natural dietary selenium alpha-tocopherol and effect on tissue
selenium. J. Ann. Sci., 34: 208-211.
KUBOTA, J., ALLAWAY, W.H., CARTER, D.L., CARY, E.E., & LAZAR,
V.A. (1967) Selenium in crops in the United States in
relation to selenium-responsive diseases of animals. J. agric.
food Chem., 15: 448-453.
KUBOTA, J., CARY, E.E., & GISSEL-NIELSEN, G. (1975) Selenium
in rainwater of the United States and Denmark. In: Hemphill,
D.D., ed. Trace substances in environmental health. IX,
Columbia, Missouri, University of Missouri Press, pp. 123-130.
KULIEVA, E.M., PERELIGIN, V.V., & DZHAFAROV, A.I. (1978)
[Isolated retina electroretinogram (ERG) under the conditions
of induced lipoperoxidation.] Dokl. Akad. Nauk. Azarbayidzh
SSR, 34(2): 85-89 (in Russian).
KURLAND, L.T. (1977) Amyotrophic lateral sclerosis and
selenium. J. Am. Med. Assoc., 238: 2365-2366.
LAKIN, H.W. & BYERS, H.G. (194l) Selenium occurrence in
certain soils in the United States, with a discussion of
related topics, sixth report, Washington DC, US Department of
Agriculture, 26 pp (USDA Technical Bulletin No. 783).
LAKIN, H.W. & DAVIDSON, D.F. (1967) The relation of the geo-
chemistry of selenium to its occurrence in soils. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 27-56.
LATHROP, K.A., JOHNSTON, R.E., BLAU, M., & ROTHSCHILD, E.O.
(1972) Radiation dose to humans from 75Se-L-seleno-
methionine. J. Nucl. Med., 13 (suppl. No. 6): 7-30.
LAUER D.J. (1947) Selenium poisoning. A review, with
emphasis on its industrial aspects, Pittsburgh, Pennsylvania,
University of Pittsburgh School of Medicine, pp. 1-47 (Thesis).
LAWRENCE, R.A. & BURK, R.F. (1976) Glutathione peroxidase
activity in selenium-deficient rat liver. Biochem. Biophys.
Res. Commun., 71: 952-958.
LAWRENCE, R.A. & BURK, R.F. (1978) Species, tissue, and
subcellular distribution of non Se-dependent glutathione
peroxidase activity. J. Nutr., 108: 211-215.
LAWRENCE, R.A., PARKHILL, L.K., & BURK, R.F. (1978) Hepatic
cytosolic non selenium-dependent glutathione peroxidase
activity: its nature and the effect of selenium deficiency. J.
Nutr., 108: 981-987.
LAWSON, T. & BIRT, D.F. (1983) Enhancement of the repair of
carcinogen-induced DNA damage in the hamster pancreas by
dietary selenium. Chem. Biol. Interact., 45: 95-104.
LAZAREV, N.V. (1977) [ Harmful compounds in the industry,]
7th ed., Leningrad, Chimija, Vol. 3, pp. 74-82 (in Russian).
LAZAREV, N.V. & GADASKINA, I.D., ed. (1977) [Selenium and
its compounds.] In: [Noxious substances in industry,] Lenin-
grad, Chimija Publishing House, pp. 75-82 (in Russian).
LEEB, J., BAUMGARTNER, W.A., LYONS, K., & LORBER, A. (1977)
Uptake, distribution, and excretion of sodium selenite in
rheumatoid subjects. In: Biological implications of metals in
the environment, Technical Information Center, Energy Research
and Development Administration, pp. 536-546.
LEMLEY, R.E. (1940) Selenium poisoning in the human. Lancet,
60: 528-531.
LEMLEY, R.E. (1943) Observations on selenium poisoning in
South and North America. Lancet, 63: 257-258.
LEMLEY, R.E. & MERRYMAN, M.P. (194l) Selenium poisoning in
the human. Lancet, 61: 435-438.
LEVANDER, O.A. (1976a) Selected aspects of the comparative
metabolism and biochemistry of selenium and sulfur. In:
Prasad, A.S., ed. Trace elements in human health and disease,
Vol. 2, Essential and toxic elements, New York, Academic
Press, pp. 135-163.
LEVANDER, O.A. (1976b) Selenium in foods. In: Proceedings of
the Symposium on Selenium-Tellurium in the Environment, Pittsburgh,
Pennsylvania, Industrial Health Foundation, pp. 26-53.
LEVANDER, O.A. (1977) Metabolic interrelationships between
arsenic and selenium. Environ. Health Perspect., 19: 159-164.
LEVANDER, O.A. (1979) Lead toxicity and nutritional
deficiencies. Environ. Health Perspect., 29: 115-125.
LEVANDER, O.A. (1982) Selenium: biochemical actions, inter-
actions, and some human health implications. In: Clinical,
biochemical, and nutritional aspects of trace elements, pp.
345-368.
LEVANDER, O.A. (1983) Consideration in the design of
selenium bioavailability studies. Fed. Proc., 42: 1721-1725.
LEVANDER, O.A. (1986) Human and animal nutrition. In: Mertz,
M., ed. Trace elements, Vol. 2, New York, Academic Press, pp.
209-279.
LEVANDER, O.A. & BAUMANN, C.A. (1966) Selenium metabolism.
VI. Effect of arsenic on the excretion of selenium in the
bile. Toxicol. appl. Pharmacol., 9: 106-115.
LEVANDER, O.A. & MORRIS, V.C. (1970) Interactions of
methionine, vitamin E, and antioxidants in selenium toxicity
in the rat. J. Nutr., 100: 1111-1118.
LEVANDER, O.A. & MORRIS (1984) Dietary selenium levels
needed to maintain balance in North American adults consuming
self-selected diets. Am. J. clin. Nutr., 39: 809-815.
LEVANDER, O.A., YOUNG, M.L., & MEEKS, S.A. (1970) Studies on
the binding of selenium by liver homogenates from rats fed
diets containing either casein or casein plus linseed oil
meal. Toxicol. appl. Pharmacol., 16: 79-87.
LEVANDER, O.A., MORRIS, V.C., & HIGGS, D.J. (1973a)
Acceleration of thiol-induced swelling of rat liver
mitochondria by selenium. Biochemistry, 12: 4586-4590.
LEVANDER, O.A., MORRIS, V.C., & HIGGS, D.J. (1973b) Selenium
as a catalyst for the reduction of cytochrome c by glutathione.
Biochemistry, 12: 4591-4595.
LEVANDER, O.A., WELSH, S.O., & MORRIS, V.C. (1980) Erythrocyte
deformability as affected by vitamin E deficiency and lead
toxicity. Ann. N.Y. Acad. Sci., 355: 227.
LEVANDER, O.A., SUTHERLAND, B., MORRIS, V.C., & KING, J.C.
(1981a) Selenium metabolism in human nutrition. In: Spallhoz,
J.E., Martin, J.L., & Ganther, H.E., ed. Selenium in biology
and medicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 256-268.
LEVANDER, O.A., SUTHERLAND, B., MORRIS, V.C., & KING, J.C.
(1981b) Selenium balance young men during selenium depletion
and repletion. Am. J. clin. Nutr., 34: 2662-2669.
LEVANDER, O.A., ALFTHAN, G., ARVILOMMI, H., GREF, C.G.,
HUTTUNEN, J.K., KATAJA, M., KOIVISTOINEN, P., & PIKKARAINEN,
J. (1983) Bioavailability of selenium to Finnish men as
assessed by platelet glutathione peroxidase activity and other
blood parameters. Am. J. clin. Nutr., 37: 887-897.
LEVINE, R.J. & OLSON, R.E. (1970) Blood selenium in Thai
children with protein-calorie malnutrition. Proc. Soc. Exp.
Biol. Med., 134: 1030-1034.
LEWIS, B.G., JOHNSON, C.M., & BROYER, T.C. (1974) Volatile
selenium in higher plants: the production of dimethyl selenide
in cabbage leaves by enzymatic cleavage of Se-methyl seleno-
methionine selenonium salt. Plant Soil, 40: 107-118.
LI, C., HUANG, J., & LI, C. (in press) Observational report
on the effects of taking sodium selenite for six years
continuously as a preventive for Kashin-Beck disease as shown
in X-ray studies. In: Proceedings of the Third International
Symposium on Selenium in Biology and Medicine.
LIANG, S. (1985) The prophylactic and curing effect of
selenium (Se) in combatting of the Kaschin-Beck's disease.
LIEBSCHER, K. & SMITH, H. (1968) Essential and non-essential
trace elements. A method of determining whether an element is
essential or nonessential in human tissue. Arch. environ.
Health, 17: 881-890.
LINDBERG, P. (1968) Selenium determination in plant and
animal material, and in water. A methodological study. Acta
vet. Scand. (suppl. 23): 48 pp.
LINDBERG, P. & JACOBSSON, S.O. (1970) Relationship between
selenium content of forage, blood, and organs of sheep, and
lamb mortality rate. Acta vet. Scand., 11: 49-58.
LIPINSKIJ, S. (1962) [Background for evaluation of selenium
as an industrial toxicant.] Gig. i Sanit., 1: 91-93 (in
Russian).
LO, L.W., KOROPATNICK, J., & STICH, H.F. (1978) The
mutagenicity and cytotoxicity of selenite, "activated"
selenite, and selenate for normal and DNA repair-deficient
human fibroblasts. Mutat. Res., 49: 305-312.
LOFROTH, G. & AMES, B.N. (1978) Mutagenicity of inorganic
compounds in Salmonella typhimurium: arsenic, chromium, and
selenium. Mutat. Res., 53: 65-66.
LOMBECK, I., KASPEREK, K., FEINENDEGEN, L.E., & BREMER, H.J.
(1975) Serum-selenium concentrations in patients with
maple-syrup-urine disease and phenylketonuria under
dieto-therapy. Clin. Chim. Acta, 64: 57-61.
LOMBECK, I., KASPEREK, K., HARBISCH, H.D., BECKER, K.,
SCHUMANN, E., SCHROTER, W., FEINENDEGEN, L.E., & BREMER, H.J.
(1978) The selenium state of children. II. Selenium content
of serum, whole blood, hair and the activity of erythrocyte
glutathione peroxidase in dietetically treated patients with
phenylketonuria and maple-syrup-urine disease. Eur. J.
Pediatr., 128: 213-223.
LUO, X., WEI, H., YANG, C., XING, J., LIU, X., QIAO, C., FENG,
Y., LIU, J., LIU, Y., WU, Q., LIU, X., GUO, J., STOECKER,
B.J., SPALLHOLTZ, J.E., & YANG, S.P. (1985) Bioavailability
of selenium to residents in a low-selenium area of China. Am.
J. clin. Nutr., 42: 439-448.
MAAG, D.D. & GLENN, M.W. (1967) Toxicity of selenium: farm
animals. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H., ed.
Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 127-140.
MAAG, D.D., ORSBORN, J.S., & CLOPTON, J.R. (1960) The effect
of sodium selenite on cattle. Am. J. vet. Res., 21: 1049-l053.
MCCABE, L.J., SYMONS, J.M., LEE, R.D., & ROBECK, G.G. (1970)
Survey of community water supply systems. J. Am. Water Works
Assoc., 62: 670-687.
MCCONNELL, K.P. & CHO, G.J. (1965) Transmucosal movement of
selenium. Am. J. Physiol., 208: 1191-1195.
MCCONNELL, K.P. & PORTMAN, O.W. (1952a). Excretion of
dimethyl selenide by the rat. J. Biol. Chem., 195: 277-282.
MCCONNELL, K.P. & PORTMAN, O.W. (1952b) Toxicity of dimethyl
selenide in the rat and mouse. Proc. Soc. Exp. Biol. Med., 79:
230-231.
MCCONNELL, K.P., BROGHAMER, W.L., Jr, BLOTCKY, A.J., & HURT,
O.J. (1975) Selenium levels in human blood and tissues in
health and in disease. J. Nutr., 105: 1026-1031.
MCCONNELL, K.P., JAGER, R.M., BLAND, K.I., & BLOCTCKY, A.J.
(1980) The relationship of dietary selenium and breast
cancer. J. surg. Oncol., 15: 67-70.
MCCOY, K.E.M. & WESWIG, P.H. (1969) Some selenium responses
in the rat not related to vitamin E. J. Nutr., 98: 383-389.
MCDANIEL, M., SHENDRIKAR, A.D., REISZNER, K.D., & WEST, P.W.
(1976) Concentration and determination of selenium from
environmental samples. Anal. Chem., 48: 2240-2243.
MCDOWELL, L.R., FROSETH, J.A., & PIPER, R.C. (1978)
Influence of arsenic, sulfur, cadmium, tellurium, silver, and
selenium on the selenium-vitamin E deficiency in the pig.
Nutr. Rep. Int., 17: 19-33.
MCKEEHAN, W.L., HAMILTON, W.G., & HAM, R.G. (1976) Selenium
is an essential trace nutrient for growth of WI-38 diploid
human fibroblasts. Proc. Natl Acad. Sci. (USA), 73: 2023-2027.
MCKENZIE, J.M. (1977) Trace elements in total parenteral
nutrition in New Zealand. In: Trace elements in human and
animal health in New Zealand, Hamilton, New Zealand, Waikato
University Press, pp. 59-69.
MCKENZIE, R.L., REA, H.M., THOMSON, C.D., & ROBINSON, M.F.
(1978) Selenium concentration and glutathione peroxidase
activity in blood of New Zealand infants and children. Am. J.
clin. Nutr., 3l: l4l3-l4l8.
MACKINNON, A.M. & SIMON, F.R. (1976) Impaired hepatic heme
synthesis in the phenobarbital-stimulated selenium-deficient
rat. Proc. Soc. Exp. Biol. Med., 152: 568-572.
MCLEAN, A.E.M. (1967) The effect of diet and vitamin E on
liver injury due to carbon tetrachloride. Br. J. exp. Path.,
48: 632-636.
MAINES, M.D. & KAPPAS, A. (1976) Selenium regulation of
hepatic heme metabolism: induction of sigma-aminolevulinate
synthase and heme oxygenase. Proc. Natl Acad. Sci.(USA), 73:
4428-443l.
MAJSTRUK, P.N. & SUCHKOV, B.P. (1978) [Sanitary hygienic
control of selenium levels in the main food commodities and
the daily intake of selenium,] Kiev, Kievskij, NII gig. Pit.,
4 pp (in Russian).
MARSHALL, M.V., JACOBS, M.M., & GRIFFIN, A.C. (1978)
Reduction in acetylaminofluorene (AAF) hepatocarcinogenesis by
selenium. Proc. Am. Assoc. Cancer Res., l9: 75.
MARTIN, J.L. & GERLACH, M.L. (1969) Separate elution by
ion-exchange chromatography of some biologically important
selenoamino acids. Anal. Biochem., 29: 257-264.
MARTIN, J.L. & HURLBUT, J.A. (1976) Tissue selenium levels
and growth responses of mice fed selenomethionine,
Se-methylselenocysteine, or sodium selenite. Phosphorus
Sulfur, 1: 295-300.
MARTIN, J.L. & SPALLHOLZ, J.S. (1976) Selenium in the immune
response. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburg, Pennsylvania,
Industrial Health Foundation, pp. 204-225.
MARTIN, S.E., ADAMS, G.H., SCHILLACI, M., & MILNER, J.A.
(1981) Antimutagenic effect of selenium on acridine orange
and 7,12-dimethylbenz(alpha)anthracene in the Ames Salmonella
microsomal system. Mutat. Res., 82: 41-46.
MASIRONI, R. & PARR, R. (1976) Selenium and cardiovascular
diseases: preliminary results of the WHO/IAEA joint research
programme. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 3l6-325.
MAXIA, V., MELONI, S., ROLLIER, M.A., BRANDONE, A.,
PATWARDHAN, V.N., WASLIEN, C.I., & SHAMI, S.E. (1972)
Selenium and chromium assay in Egyptian foods and in blood of
Egyptian children by activation analysis. In: Nuclear
activation techniques in the life sciences, Vienna,
International Atomic Energy Agency, pp. 527-550.
MEDINA, D. & SHEPHERD, F. (1980) Selenium-mediated
inhibition of mouse mammary tumorigenesis. Cancer Lett., 8:
241-245.
MEDINSKY, M.A., CUDDIHY, R.G., GRIFFITH, W.C., & MCCLELLAN,
R.O. (1981) A simulation model describing the metabolism of
inhaled and ingested selenium compounds. Toxicol. appl.
Pharmacol., 59: 54-63.
MEHLERT, A. & DIPLOCK, A.T. (1985) The glutathione-S-
transferases in selenium and vitamin E deficiency. Biochem.
J., 227: 823-831.
MICHIE, N.D., DIXON, E.J., & BUNTON, N.G. (1978) Critical
review of AOAC fluorometric method for determining selenium in
foods. J. Assoc. Off. Anal. Chem., 61: 48-51.
MIETTINEN, T.A., ALFTHAN, G., HUTTUNEN, J.K., PIKKARAINEN, J.,
NAUKKARINEN, V., MATTILA, S., & KUMLIN, T. (1983) Serum
selenium concentration related to myocardial infarction and
fatty acid content of serum lipids. Brit. med. J., 287:
517-519.
MILKS, M.M., WILT, S.R., ALI, I.I., & COURI, D. (1985) The
effects of selenium on the emergence of aflatoxin B1-induced
enzyme-altered foci in rat liver. Fundam. appl. Toxicol., 5:
320-326.
MILLAR, K.R. & SHEPPARD, A.D. (1972) alpha-Tocopherol and
selenium levels in human and cow's milk. N.Z. J. Sci., 15:
3-15.
MILLAR, K.R., GARDINER, M.A., & SHEPPARD, A.D. (1973) A
comparison of the metabolism of intravenously injected sodium
selenite, sodium selenate, and selenomethionine in rats. N.Z.
J. agric. Res., 16: 115-127.
MILLER, D., SOARES, J.H., Jr, BAUERSFELD, P., Jr, & CUPPETT,
S.L. (1972) Comparative selenium retention by chicks fed
sodium selenite, selenomethionine, fish meal, and fish
solubles. Poultry Sci., 51: 1669-1673.
MILNER, J.A. (1985) Effect of selenium on virally induced
and transplantable tumour models. Fed. Proc., 44: 2568-2572.
MONAENKOVA, A.M. & GLOTOVA, K.V. (1963) [Selenium
intoxication.] Gig. i Sanit., 6: 41-44 (in Russian).
MONDRAGON, M.C. & JAFFE, W.G. (1976) Ingestion of selenium
in Caracas, compared with some other cities. Arch. Latinoam.
Nutr., 26: 34l-352.
MONEY, D.F.L. (1970) Vitamin E and selenium deficiencies and
their possible aetiological role in the sudden death in
infants syndrome. N.Z. med. J., 7l: 32-34.
MONEY, D.F.L. (1978) Vitamin E, selenium, iron and vitamin A
content of livers from sudden infant death syndrome and
control children: interrelationships and possible
significance. N.Z. J. Sci Teel., 21: 41-55.
MOORE, J.A., NOIVA, R., & WELLS, I.C. (1984) Selenium
concentrations in plasma of patients with arteriographically
defined coronary atherosclerosis. Clin. Chem., 30(7):
1171-1173.
MORIYA, M., OHTA, T., WATANABE, K., WATANABE, Y., SUGIYAMA,
F., MIYAZAWA, T., & SHIRASU, Y. (1979) Inhibitors for the
mutagenicities of colon carcinogens, 1,2-dimethylhydrazine and
azoxymethane, in the host-mediated assay. Cancer Lett., 7:
325-330.
MORRIS, V.C. & LEVANDER, O.A. (1970) Selenium content of
foods. J. Nutr., 100: 1383-1388.
MOTSENBOCKER, M.A. & TAPPEL, A.L. (1982) A
selenocysteine-containing selenium-transport protein in rat
plasma. Biochim. Biophys. Acta, 719: 147-153.
MOXON, A.L. (1938) The effect of arsenic on the toxicity of
seleniferous grains. Science, 88: 81.
MOXON, A.L. (1940) Toxicity of selenium-cystine and some
other organic selenium compounds. J. Am. Pharm. Assoc. Sci.
Ed., 29: 249-250.
MOXON, A.L. (1976) Natural occurrence of selenium. In:
Proceedings of the Symposium on Selenium-Tellurium in the
Environment, Pittsburgh, Pennsylvania, Industrial Health
Foundation, pp. 1-9.
MOXON, A.L. & RHIAN, M. (1943) Selenium poisoning. Physiol.
Rev., 23: 305-337.
MOXON, A.L., ANDERSON, H.D., & PAINTER, E.P. (1938) The
toxicity of some organic selenium compounds. J. Pharmacol.
exp. Ther., 63: 357-368.
MOXON, A.L., OLSON, O.E., & SEARIGHT, W.V. (1939) Selenium
in rocks, soils, and plants. S. Dak. Agric. Exp. Sta. Tech.
Bull., 2: 94 pp.
MOXON, A.L., SCHAEFER, A.E., LARDY, H.A., DUBOIS, K.P., &
OLSON, O.E. (1940) Increasing the rate of excretion of
selenium from selenized animals by the administration of
p-bromobenzene. J. biol. Chem., l32: 785-786.
MOXON, A.L., OLSON, O.E., & SEARIGHT, W.V. (1950) Selenium
in rocks, soils, and plants. S. Dak. Agric. Exp. Sta. Tech.
Bull., 2.
MUHLER, J.C. & SHAFER, W.G. (1957) The effect of selenium on
the incidence of dental caries in rats. J. dent. Res., 36:
895-896.
MUNSELL, H.E., DEVANEY, G.M., & KENNEDY, M.H. (1936)
Toxicity of food containing selenium as shown by its effect on
the rat, Washington DC, US Department of Agriculture, 25 pp
(US Department of Agriculture Technical Bulletin No. 534).
MUTANEN, M. & KOIVISTOINEN, P. (1983) The role of imported
grain on the selenium intake of Finnish population 1941-1981.
Int. J. Vit. Nutr. Res., 53: 34-38.
MUTH, O.H. (1960) Carbon tetrachloride poisoning of ewes on
a low selenium ration. Am. J. vet. Res., 21: 86-87.
MUTH, O.H., ed. (1966) Selenium in biomedicine. In: Proceedings
of the First International Symposium at Oregon State University,
Westport, Connecticut, AVI Publishing Co., 445 pp.
MUTH, O.H., WESWIG, P.H., WHANGER, P.D., & OLDFIELD, J.E.
(197l) Effect of feeding selenium-deficient ration to the
subhuman primate (Saimiri sciureus). Am. J. vet. Res., 32:
l603-l605.
NAHAPETIAN, A.T., JANGHORBANI, M., & YOUNG, V.R. (1983)
Urinary trimethylselenonium excretion by the rat: effect of
level and source of selenium-75. J. Nutr., 113: 401-411.
NAKAMURO, K., YOSHIKAWA, K., SAYATO, Y., KURATA, H., TONOMURA,
M., & TONOMURA, A. (1976) Studies on selenium-related
compounds. V. Cytogenetic effect and reactivity with DNA.
Mutat. Res., 40: 177-184.
NATIONAL CANCER INSTITUTE (1980) Bioassay of selenium
sulfide for possible carcinogenicity (gavage study), Bethesda,
Maryland, US Department of Health and Human Services, Public
Health Service, National Institutes of Health, 132 pp (DHHS
Publication No. (NIH) 80-1750).
NAVIA, J.M., MENAKER, L., SELTZER, J., & HARRIS, R.S. (1968)
Effect of Na2Se03 supplemented in the diet or the water on
dental caries of rats. Fed. Proc., 27: 676.
NAZARENKO, I.I. & ERMAKOV, A.N. (1971) Analytical chemistry
of selenium and tellurium, New York, Halsted Press.
NAZARENKO, I.I. & KISLOVA, I.V. (1977) [A highly sensitive
method for the analysis of migratory forms of selenium in
natural waters.] El. Viems. Labor. Technol. issled. obogashch.
min. syrja, 6: 1-8 (in Russian).
NAZARENKO, I.I., KISLOVA, I.V., GUSEJNOV, T.M., MKRTCHJAN,
M.A., & KISLOV, A.M. (1975) [Fluorometric determination of
selenium in biological material by 2,3-diaminonaphthalene].
Zh. Anal. Chim., 30(4): 733-737 (in Russian).
NELSON, A.A., FITZHUGH, O.G., & CALVERY, H.O. (1943) Liver
tumors following cirrhosis caused by selenium in rats. Cancer
Res., 3: 230-236.
NEWBERNE, P.M. & CONNER, M.W. (1974) Effect of selenium on
acute response to aflatoxin B1. In: Hemphill, D.D., ed. Trace
substances in environmental health. VIII, Columbia, Missouri,
University of Missouri Press, pp. 323-328.
NOCKELS, C.F. (1979) Protective effects of supplemental
vitamin E against infection. Fed. Proc., 38: 2134-2138.
NODA, M., TAKANO, T., & SAKURAI, H. (1979) Mutagenic
activity of selenium compounds. Mutat. Res., 66: 175-179.
NOGUCHI, T., CANTOR, A.H., & SCOTT, M.L. (1973) Mode of
action of selenium and vitamin E in prevention of exudative
drathesis in chicks. J. Nutr., 103(10): 1502-1511.
NORDMAN, E. (1974) Sodium selenite (selenium-75)
scintigraphy in diagnosis of tumours. Acta radiol.,
340(suppl.): 78 pp.
NORPPA, H., ET AL. (1980) Chromosomal effects of sodium
selenite in vivo. Hereditas, 93: 93-105.
NORRIS, F.H. & SANG, K. (1978) Amyotrophic lateral sclerosis
and low urinary selenium levels. J. Am. Med. Assoc., 239: 404.
OBERMEYER, B.D., PALMER, I.S., OLSON, O.E., & HALVERSON, A.W.
(1971) Toxicity of trimethylselenonium chloride in the rat
with and without arsenite. Toxicol. appl. Pharmacol., 20:
135-146.
OCHOA-SOLANO, A. & GITLER, C. (1968) Incorporation of
75Se-selenomethionine and 35S-methionine into chicken egg
white proteins. J. Nutr., 94: 243-248.
OELSCHLAGER, W. & MENKE, K.H. (1969) Concerning the selenium
content of plant, animal, and other materials. II. The
selenium and sulfur content of foods. Z. Ernahrungswiss., 9:
216-222.
OH, S.H., SUNDE, R.A., POPE, A.L., & HOEKSTRA, W.G. (1976a)
Glutathione peroxidase response to selenium intake in lambs
fed a Torula yeast-based, artificial milk. J. anal. Sci., 42:
977-983.
OH, S.H., POPE, A.L., & HOEKSTRA, W.G. (1976b) Dietary
selenium requirement of sheep fed a practical-type diet as
assessed by tissue glutathione peroxidase and other criteria.
J. anal. Sci., 42: 984-992.
OHLENDORF, H.M., HOFFMAN, D.J., SAIKI, M.K., & ALDRICH, T.W.
(1986) Embryonic mortality and abnormalities of aquatic
birds: apparent impact of selenium from irrigation drain
water. Sci. total Environ., 52: 49-63.
OLSON, O.E. (1967) Soil, plant, animal cycling of excessive
levels of selenium. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI Publishing Co., Inc, pp. 297-3l2.
OLSON, O.E. (1969) Selenium as a toxic factor in animal
nutrition. In: Proceedings of the Georgia Nutrition
Conference, Atlanta, Georgia, pp. 68-78.
OLSON, O.E. (1970) Selenium in feedstuffs: deficiencies and
excesses. Proceedings of the 31st Minnesota Nutrition
Conference, Minneapolis, University of Minnesota, pp. 7-l3.
OLSON, O.E. (1976) Methods of analysis for selenium. A
review. In: Proceedings of the Symposium on Selenium-Tellurium
in the Environment, Pittsburgh, Pennsylvania, Industrial
Health Foundation, pp. 67-84.
OLSON, O.E. (1978) Selenium in plants as a cause of
livestock poisoning. In: Keeler, R.F., Van Kampen, K.R., &
James, L.F., ed. Effects of poisonous plants on livestock, New
York, Academic Press, pp. 121-133.
OLSON, O.E. & FROST, D.V. (1970) Selenium in papers and
tabaccos. Environ. Sci. Technol., 4: 686-687.
OLSON, O.E. & PALMER, I.S. (1976) Selenoamino acids in
tissues of rats administered inorganic selenium. Metabolism,
25: 299-306.
OLSON, O.E., WHITEHEAD, E.I., & MOXON, A.L. (1942)
Occurrence of soluble selenium in soils and its availability
to plants. Soil Sci., 54: 47-53.
OLSON, O.E., SCHULTE, B.M., WHITEHEAD, E.I., & HALVERSON,
A.W. (1963) Effect of arsenic on selenium metabolism in
rats. J. agric. food Chem., 11: 531-534.
OLSON, O.E., NOVACEK, E.J., WHITEHEAD, E.I., & PALMER, I.S.
(1970) Investigations on selenium in wheat. Phytochemistry,
9: 1181-1188.
OLSON, O.E., PALMER, I.S., & WHITEHEAD, E.I. (1973)
Determination of selenium in biological materials. In: Glick,
D., ed. Methods of biochemical analysis, New York, John Wiley
and Sons, Inc, Vol. 21, pp. 39-78.
OLSON, O.E., PALMER, I.S., & CARY, E.E. (1975) Modification
of the official fluorometric method for selenium in plants. J.
Assoc. Off. Anal. Chem., 58: 117-121.
OLSON, O.E., CARY, E.E., & ALLAWAY, W.H. (1976) Fixation and
volatilization by soils of selenium from trimethylselenonium.
Agron. J., 68: 839-843.
OLSON, O.E., PALMER, I.S., & HOWE, M. (1978) Selenium in
foods consumed by South Dakotans. Proc. S.D. Acad. Sci., 57:
113-121.
OLSSON, U., ONFELT, A., & BEIJE, B. (1984) Dietary selenium
deficiency causes decreased N-oxygenation of N'N-dimethyl-
aniline and increased mutagenicity of dimethylnitrosamine in
the isolated rat liver/cell cultrue system. Mutat. Res., 126:
73-80.
OMAYE, S.T., REDDY, K.A., & CROSS, C.E. (1978) Enhanced lung
toxicity of paraquat in selenium-deficient rats. Toxicol.
appl. Pharmacol., 43: 237-247.
OSTADALOVA, I., BABICKY, A., & OBENBERGER, J. (1979)
Cataractogenic and lethal effect of selenite in rats during
postnatal ontogenesis. Physiol. Bohemoslov., 28: 393-397.
OVERVAD, K., THORLING, E.B., BJERRING, P., & EBBESEN, P.
(1985) Selenium inhibits UV-light-induced skin carcinogenesis
in hairless mice. Cancer Lett., 27: 163-170.
PAINTER, E.P. (1941) The chemistry and toxicity of selenium
compounds with special reference to the selenium problem.
Chem. Rev., 28: 179-213.
PALMER, I.S. & OLSON, O.E. (1974) Relative toxicities of
selenite and selenate in the drinking-water of rats. J. Nutr.,
104(3): 306-314.
PALMER, I.S. & OLSON, O.E. (1979) Partial prevention by
cyanide of selenium poisoning in rats. Biochem. Biophys. Res.
Commun., 90: 1379-1386.
PALMER, I.S., FISCHER, D.D., HALVERSON, A.W., & OLSON, O.E.
(1969) Identification of a major selenium excretory product
in rat urine. Biochim. Biophys. Acta, 177: 336-342.
PALMER, I.S., GUNSALUS, R.P., HALVERSON, A.W., & OLSON, O.E.
(1970) Trimethylselenonium ion as a general excretory product
from selenium metabolism in the rat. Biochim. Biophys. Acta,
208: 260-266.
PALMER, I.S., ARNOLD, R.L., & CARLSON, C.W. (1973) Toxicity
of various selenium derivatives to chick embryos. Poultry
Sci., 52: 1841-l846.
PALMER, I.S., OLSON, O.E., HALVERSON, A.W., MILLER, R., &
SMITH, C. (1980) Isolation of factors in linseed oil meal
protective against chronic selenosis in rats. J. Nutr., 110:
145-150.
PARIZEK, J. & OSTADALOVA, I. (1967) The protective effect of
small amounts of selenite in sublimate intoxication. Experientia
(Basel), 23: 142-143.
PARIZEK, J., OSTADALOVA, I., KALOUSKOVA, J., BABICKY, A., &
BENES, J. (1971) The detoxifying effects of selenium.
Interrelations between compounds of selenium and certain
metals. In: Mertz, W. & Cornatzer, W.E., ed. Newer trace
elements in nutrition, New York, Marcel Dekker, Inc,
pp. 85-122.
PARIZEK, J., ET AL. (1974) Some hormonal and environmental
factors influencing selenium metabolism and action. In:
Proceedings of the Ninth International Congress in Nutrition,
Mexico, 1972 (Coop. Nutr. Cong., 16: 94-97).
PARIZEK, J., KALOUSKOVA, J., KORUNOVA, V., BENES, J., &
PAVLIK, L. (1976) The protective effect of pretreatment with
selenite on the toxicity of dimethylselenide. Physiol.
Bohemoslov., 25: 573-576.
PARIZEK, J., KALOUSKOVA, J., BENES, J., & PAVLIK, L. (1980)
Interactions of selenium-mercury and selenium-selenium
compounds. Ann. N.Y. Acad. Sci., 355: 347-360.
PASCOE, G.A. & CORREIA, M.A. (1985) Structural and
functional assembly of rat intestinal cytochrome P-450
isozymes: effects of dietary iron and selenium. Biochem.
Pharmacol., 34: 599-608.
PASCOE, G.A., SAKAI-WONG, J., SOLIVEN, E., & CORREIA, M.A.
(1983) Regulation of intestinal cytochrome P-450 and Heme by
dietary nutrients. Critical role of selenium. Biochem.
Pharmacol., 32(20): 3027-3035.
PAULSEN, P.J. (1977) Determination of cadmium, copper, iron,
lead, mercury, molybdenum, nickel, selenium, silver,
tellurium, thallium, and zinc. Natl Bur. Stand. Special Publ.
(USA), 492: 33-48.
PAVLIK, L., KALOUSKOVA, J., VOBECKY, M., DEDINA, BENES, J., &
PARIZEK, J. (1979) Selenium levels in the kidneys of male
and female rats. In: Nuclear activation techniques in the life
sciences, 1978, Vienna, International Atomic Energy Agency,
pp. 213-223.
PELEKIS, E.E., PELEKIS, L.L., & TAURE, I. JA. (1975)
[Instrumental neutron-activation method of selenium determination
in biological materials.] Vitaminy, 8: 146-150 (in Russian).
PENCE, B.C. & BUDDINGH, F. (1985) Effect of selenium
deficiency on incidence and size of 1,2-dimethylhydrazine-
induced colon cancer in rats. J. Nutr., 115: 1196-1202.
PENNINGTON, J.A.T., WILSON, D.B., NEWELL, R.F., HARLAND, B.F.,
JOHNSON, R.D., & VANDERVEEN, J.E. (1984) Selected minerals
in foods surveys, 1974 to 1981/82. J. Am. Diet. Assoc., 84:
771-780.
PERONA, G., GUIDI, G.C., PIGA, A., CELLERINO, R., MENNA, R., &
ZATTI, M. (1978) In vivo and in vitro variations of human
erythrocyte glutathione peroxidase activity as result of cells
ageing, selenium availability and peroxide activation. Br. J.
Haematol., 39: 399-408.
PERRY, H.M., Jr, ERLANGER, M.W., & PERRY, E.F. (1976)
Limiting conditions for the induction of hypertension in rats
by cadmium. In: Hemphill, D.D., ed. Trace substances in
environmental health. X, Columbia, Missouri, University of
Missouri Press, pp. 459-467.
PIERCE, F.D. & BROWN, H.R. (1977) Comparison of inorganic
interferences in atomic absorption spectrometric determination
of arsenic and selenium. Anal. Chem., 49: 1417-1422.
PIERCE, S. & TAPPEL, A.L. (1977) Effects of selenite and
selenomethionine on glutathione peroxidase in the rat. J.
Nutr., 107: 475-479.
PILLAY, K.K.S., THOMAS, C.C., Jr, & SONDEL, J.A. (1971)
Activation analysis of airborne selenium as a possible
indicator of atmospheric sulfur pollutants. Environ. Sci.
Technol., 5: 74-77.
PLAA, G.L. & WITSCHI, H. (1976) Chemicals, drugs, and lipid
peroxidation. Ann. Rev. Pharmacol. Toxicol., 16: 125-141.
PLEBAN, P.A., MUNYANI, A., & BEACHUM, J. (1982)
Determination of selenium concentration and glutathine
peroxidase activity in plasma and erythrocytes. Clin. Chem.,
28: 311-316.
PLETNIKOVA, I.P. (1970) Biological effect and safe
concentration of selenium in drinking-water. Hyg. Sanit., 35:
176-181.
POLEY, W.E. & MOXON, A.L. (1938) Tolerance levels of
seleniferous grains in laying rations. Poultry Sci., 17: 72-76.
POOLE, C.F., EVANS, N.J., & WIBBERLEY, D.G. (1977)
Determination of selenium in biological samples by gas-liquid
chromatography with electron-capture detection. J.
Chromatogr., 136: 73-83.
PRATLEY, J.E. & MCFARLANE, J.D. (1974) The effect of
sulphate on the selenium content of pasture plants. Aust. J.
exp. Agric. Anim. Husb., 14: 533-538.
PRINGLE, P. (1942) Occupational dermatitis following
exposure to inorganic selenium compounds. Br. J. Dermatol.
Syphil., 54: 54-58.
PROHASKA, J.R. & GANTHER, H.E. (1976) Selenium and
glutathione peroxidase in developing rat brain. J. Neurochem.,
27: 1379-1387.
PROHASKA, J.R. & GANTHER, H.E. (1977) Glutathione peroxidase
activity of glutathione-S-transferases purified from rat
liver. Biochem. Biophys. Res. Commun., 76: 437-445.
PYEN, G. & FISHMAN, M. (1978) Automated determination of
selenium in water. At. Absorpt. Newslett., 17: 47-48.
RANDOLPH, W.F. (1978) Food additives permitted in feed and
drinking water of animals. Selenium. Fed. Reg., 43:
11700-11701.
RANSONE, J.W., SCOTT, N.M., Jr, & KNOBLOCK, E.C. (1961)
Selenium sulfide intoxication. New Engl. J. Med., 264: 384-385.
RAVIKOVITCH, S. & MARGOLIN, M. (1957) Selenium in soils and
plants. Ktavim. Rec. agric. Res. Sta., 7: 41-52.
RAY, J.H. (1984) Sister-chromatid exchange induction by
sodium selenite: reduced glutathione converts Na2SeO3 to its
SCE-inducing form. Mutat. Res., 141: 49-53.
RAY, J.H. & ALTENBURG, L.C. (1978) Sister-chromatid exchange
induction by sodium selenite: dependence on the presence of
red blood cells or red blood cell lysate. Mutat. Res., 54:
343-354.
RAY, J.H. & ALTENBURG, L.C. (1980) Dependence of the
sister-chromatid exchange-inducing abilities of inorganic
selenium compounds on the valence state of selenium. Mutat.
Res., 78: 261-266.
RAY, J.H., ALTENBURG, L.C., & JACOBS, M.M. (1978) Effect of
sodium selenite and methyl methanesulfonate or N-hydroxy-2-
acetylamino-fluorene co-exposure on sister-chromatid exchange
production in human whole blood cultures. Mutat. Res., 57:
359-368.
REA, H.M., THOMSON, C.D., CAMPBELL, D.R., & ROBINSON, M.F.
(1979) Relation between erythrocyte selenium concentrations
and glutathione peroxidase (EC 1.11.1.9) activities of New
Zealand residents and visitors to New Zealand. Br. J. Nutr.,
42: 201-208.
REAMER, D.C. & VEILLON, C. (1983a) Letter to the editor.
Anal. Chem., in press.
REAMER, D.C. & VEILLON, C. (1983b) A double isotape dilution
method for using stable selenium isotapes in metabolic tracer
studies: analysis by gas chromatography/mass spectrometry
(GC/MS). J. Nutr., 113: 786-792.
REITER, R. & WENDEL, A. (1983) Selenium and drug metabolism.
Multiple modulations of mouse liver enzymes. Biochem.
Pharmacol., 32(20): 3063-3067.
RHEAD, W.J., CARY, E.E., ALLAWAY, W.H., SALTZSTEIN, S.L., &
SCHRAUZER, G.N. (1972) The vitamin E and selenium status of
infants and the sudden infant death syndrome. Bioinorg. Chem.,
1: 289-294.
RICHOLD, M., ROBINSON, M.F., & STEWART, R.D.H. (1977)
Metabolic studies in rats of 75Se incorporated in vivo into
fish muscle. Br. J. Nutr., 38: 19-29.
RIELY, C.A., COHEN, G., & LIEBERMAN, M. (1974) Ethane
evolution: a new index of lipid peroxidation. Science, 183:
208-210.
RILEY, J.F. (1968) Mast cells, co-carcinogenesis and
anti-carcinogenesis in the skin of mice. Experientia (Basel),
24: 1237-1238.
ROBBINS, C.W. & CARTER, D.L. (1970) Selenium concentrations
in phosphorus fertilizer materials and associated uptake by
plants. Soil Sci. Soc. Am. Proc., 34: 506-509.
ROBERTSON, D.S.F. (1970) Selenium, a possible teratogen?
Lancet, 1: 518-519.
ROBINSON, J.R., ROBINSON, M.F., LEVANDER, O.A., & THOMSON,
C.D. (1985) Urinary excretion of selenium by New Zealand and
North American human subjects on differing intakes. Am. J.
clin. Nutr., 41: 1023-1031.
ROBINSON, M.F. & THOMSON, C.D. (1981) Selenium levels in
humans vs environmental sources. In: Spallholz, J.E., Martin,
J.L., & Ganther, H.E., ed. Selenium in biology and medicine,
Westport, Connecticut, The AVI Publishing Co., Inc, pp. 283-302
ROBINSON, M.F. & THOMSON, C.D. (1983) The role of selenium
in the diet. Nutr. Abstr. Rev., 53: 3-26.
ROBINSON, M.F. & THOMSON, C.D. (1986) Selenium status of the
food supply and residents of New Zealand (Beijing Conference).
ROBINSON, M.F., MCKENZIE, J.M., THOMSON, C.D., & VAN RIJ, A.L.
(1973) Metabolic balance of zinc, copper, cadmium, iron,
molybdenum and selenium in young New Zealand women. Br. J.
Nutr., 30: 195-205.
ROBINSON, M.F., GODFREY, P.J., THOMSON, C.D., REA, H.M., & VAN
RIJ, A.M. (1978a) Blood selenium and glutathione peroxidase
activity in normal subjects and in surgical patients with and
without cancer in New Zealand. Am. J. clin. Nutr., 32:
1477-1485.
ROBINSON, M.F., REA, H.M., FRIEND, G.M., STEWART, R.D.H.,
SNOW, P.C., & THOMSON, C.D. (1978b) On supplementing the
selenium intake of New Zealanders. II. Prolonged metabolic
experiments with daily supplements of selenomethionine,
selenite, and fish. Br. J. Nutr., 39: 589-600.
ROBINSON, M.F., GODFREY, J.P., THOMSON, C.D., REA, H.M., & VAN
RIJ, A.M. (1979) Blood selenium and glutathione peroxidase
activity in normal subjects and in surgical patients with and
without cancer in New Zealand. Am. J. clin. Nutr., 32:
1477-1485.
ROBINSON, M.F., CAMPBELL, D.R., STEWART, R.D.H., REA, H.M.,
THOMSON, C.D., SNOW, P.G., & SQUIRES, I.H.W. (1981) Effect
of daily supplements of selenium on patients with muscular
complaints in Otago and Canterbury. N.Z. med. J., 93: 289-292.
ROBINSON, M.F., CAMPBELL, D.R., SUTHERLAND, W.H.F., HERBISON,
P.G., PAULIN, J.M., & SIMPSON, F.O. (1983) Selenium and risk
factors for cardiovascular disease in New Zealand. N.Z. med.
J., 96: 755-757.
ROBINSON, M.F., THOMSON, C.D., & HUEMMER, P.K. (1985) Effect
of a megadose of ascorbic acid, a meal and orange juice on the
absorption of selenium as sodium selenite. N.Z. med. J., 98:
627-629.
ROBINSON, W.O. (1933) Determination of selenium in wheat and
soils. J. Assoc. Off. Agric. Chem., 16: 423-424.
ROHMER, R., CARROT, E., & GOUFFAULT, J. (1950) Nouvel aspect
de l'intoxication par les composés du sélénium. Bull. Soc.
Chim. France, 5(17): 275-278.
ROSIN, M.P. & STICH, H.F. (1979) Assessment of the use of
the Salmonella mutagenesis assay to determine the influence of
antioxidants on carcinogen-induced mutagenesis. Int. J.
Cancer, 23: 722-727.
ROSENFELD, I. & BEATH, O.A. (1954) Effect of selenium on
reproduction in rats. Proc. Soc. Exp. Biol. Med., 87: 295-297.
ROSENFELD, I. & BEATH, O.A. (1964) Selenium geobotany,
biochemistry, toxicity and nutrition, New York, Academic
Press, 411 pp.
ROSIN, M.P. (1981) Inhibition of spontaneous mutagenesis in
yeast cultures by selenite, selenate, and selenide. Cancer
Lett., 13: 7-14.
ROSSI, L.C., CLEMENTE, G.F., & SANTARONI, G. (1976) Mercury
and selenium distribution in a defined area and in its
population. Arch. environ. Health, 31: 160-165.
ROTRUCK, J.T., POPE, A.L., GANTHER, H.E., SWANSON, A.B.,
HAFEMAN, D.G., & HOEKSTRA, W.G. (1973) Selenium: biochemical
role as a component of glutathione peroxidase. Science, 179:
588-590.
RUDERT, C.P. & LEWIS, A.R. (1978) The effect of potassium
cyanide on the occurrence of nutritional myopathy in lambs.
Rhod. J. agric. Res., 16: 109-116.
RUSIECKI, W. & BRZEZINSKI, J. (1966) Influence of sodium
selenate on acute thallium poisonings. Acta Pol. pharm., 23:
74-80.
SAKURAI, H. & TSUCHIYA, K. (1975) A tentative recommendation
for the maximum daily intake of selenium. Environ. Physiol.
Biochem., 5: 107-118.
SALONEN, J.T. (1985) Selenium in cardiovascular diseases and
cancer. Epidemiologic findings from Finland. In: Boström, H.
& Ljungstedt, N., ed. Trace elements in health disease,
Stockholm, Sweden, Almqvist & Wiksell International, pp.
172-186.
SALONEN, J.T., ALFTHAN, G., HUTTUNEN, J.K., PIKKARAINEN, J., &
PUSKA, P. (1982) Association between cardiovascular death
and myocardial infarction and serum selenium in a watched-pair
longitudinal study. Lancet, 2: 175-179.
SALONEN, J.T., ALFTHAN, G., HUTTUNEN, J.K., & PUSKA, P.
(1984a) Association between serum selenium and the risk of
cancer. Am. J. Epidemiol., 120(3): 342-349.
SALONEN, J.T., SALONEN, R., PENTTILA, I., HERRANEN, J., JAUHIAINEN,
J., KANTOLA, M., KAURANEN, P., LAPPETELAINEN, R., MAENPAA, P., &
PUSKA, P. (1984b) Serum fatty acids, apolipoproteins, selenium
and vitamin antioxidants and the risk of death from ischaemic heart
disease. Am. J. Cardiol., 56(2): 226.
SALONEN, J.T., SALONEN, R., LAPPETELAINEN, R., MAENPAA, P.H.,
ALFTHAN, G., & PUSKA, P. (1985) Risk of cancer in relation
to serum concentrations of selenium and vitamins A and E:
matched case-control analysis of prospective data. Brit. med.
J., 290: 417-420.
SARATHCHANDRA, S.U. & WATKINSON, J.H. (1981) Oxidation of
elemental selenium to selenite by Bacillus megaterium.
Science, 211: 600-601.
SARATHCHANDRASCHMIDT, K. & HELLER, W. (1976) Selenium
concentration and activity of glutathione peroxidase in
lysates of human erythrocytes. Blut, 33: 247-251.
SCHECTER, A., SHANSKE, W., STENZLER, A., QUINTILIAN, H., &
STEINBERG, H. (1980) Acute hydrogen selenide intoxication.
Chest, 77: 554-555.
SCHILLACI, M., MARTIN, S.E., & MILNER, J.A. (1982) The
effects of dietary selenium on the biotransformation of
7,12-dimethylbenz(alpha)anthracene. Mutat. Res., 101: 31-37.
SCHRAUZER, G.N. (1976) Cancer mortality correlation studies.
II. Regional associations of mortalities with the consumptions
of foods and other commodities. Medic. Hypoth., 2: 39-49.
SCHRAUZER, G.N. & ISHMAEL, D. (1974) Effects of selenium and
of arsenic on the genesis of spontaneous mammary tumors in
inbred C3H mice. Ann. clin. Lab. Sci., 4: 441-447.
SCHRAUZER, G.N. & WHITE, D.A. (1978) Selenium in human
nutrition: dietary intakes and effects of supplementation.
Bioinorg. Chem., 8: 303-318.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1976)
Inhibition of the genesis of spontaneous mammary tumors in
C3H mice: effects of selenium and of selenium-antagonistic
elements and their possible role in human breast cancer.
Bioinorg. Chem., 6: 265-270.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1977)
Cancer mortality correlation studies. III. Statistical
associations with dietary selenium intakes. Bioinorg. Chem.,
7: 23-34.
SCHRAUZER, G.N, WHITE, D.A., & SCHNEIDER, C.J. (1978a)
Effects of selenium, arsenic, and zinc on the genesis of
spontaneous mammary tumors in inbred female C3H mice. In:
Kirchgessner, M., ed. Trace element metabolism in man and
animals. III, Freising-Weihenstephan, West Germany,
Arbeitskreis für Tierernährungsforschung Weihenstephan, pp.
387-390.
SCHRAUZER, G.N., WHITE, D.A., & SCHNEIDER, C.J. (1978b)
Selenium and cancer: Effects of selenium and of the diet on
the genesis of spontaneous mammary tumors in virgin inbred
female C3H/St mice. Bioinorg. Chem., 8: 387-396.
SCHRAUZER, G.N., WHITE, D.A., MCGINNESS, J.E., SCHNEIDER,
C.J., & BELL, L.J. (1978c) Arsenic and cancer: effects of
joint administration of arsenite and selenite on the genesis
of mammary adenocarcinoma in inbred female C3H/St mice.
Bioinorg. Chem., 9: 245-253.
SCHROEDER, H.A. & MITCHENER, M. (1971a) Toxic effects of
trace elements on the reproduction of mice and rats. Arch.
environ. Health, 23: 102-106.
SCHROEDER, H.A. & MITCHENER, M. (1971b) Selenium and
tellurium in rats: effect on growth, survival and tumors. J.
Nutr., 101: 1531-1540.
SCHROEDER, H.A. & MITCHENER, M. (1972) Selenium and
tellurium in mice. Effects on growth, survival, and tumors.
Arch. environ. Health, 24: 66-71.
SCHROEDER, H.A., FROST, D.V., & BALASSA, J.J. (1970)
Essential trace metals in man: selenium. J. chron. Dis., 23:
227-243.
SCHUBERT, J.R., MUTH, O.H., OLDFIELD, J.E., & REMMERT, L.F.
(196l) Experimental results with selenium in white muscle
disease of lambs and calves. Fed. Proc., 20: 689-694.
SCHULTZ, T.D. & LEKLEM, J.E. (1983) Selenium status of
vegetarians, nonvegetarians, and hormone-dependent cancer
subjects. Am. J. clin. Nutr., 37: 114-118.
SCHWARZ, K. (1961) Development and status of experimental
work on Factor 3-selenium. Fed. Proc., 20: 666-673.
SCHWARZ, K. (1962) Vitamin E, trace elements, and sulfhydryl
groups in respiratory decline. Vitam. Horm., 20: 463-484.
SCHWARZ, K. (1967) Discussion comments. In: Muth, O.H.,
Oldfield, J.E., & Weswig, P.H., ed. Selenium in biomedicine,
Westport, Connecticut, The AVI Publishing Co., Inc,
pp. 225-226.
SCHWARZ, K. (1976) The discovery of the essentiality of
selenium, and related topics (a personal account). In:
Proceedings of the Symposium on Selenium-Tellurium in the
Environment, Pittsburgh, Pennsylvania, Industrial Health
Foundation, pp. 349-376.
SCHWARZ, K. (1977) Amyotrophic lateral sclerosis and
selenium. J. Am. Med. Assoc., 238: 2365.
SCHWARZ, K. & FOLTZ, C.M. (1957) Selenium as an integral
part of Factor 3 against dietary necrotic liver degeneration.
J. Am. Chem. Soc., 79: 3292-3293.
SCHWARZ, K. & FOLTZ, C.M. (1958) Factor 3 activity of
selenium compounds. J. biol. Chem., 233: 245-251.
SCHWARZ, K., PORTER, L.A., & FREDGA, A. (1972) Some
regularities in the structure-function relationship of
organoselenium compounds effective against dietary liver
necrosis. Ann. NY Acad. Sci., 192: 200-214.
SCOTT, M.L. & THOMPSON, J.N. (1971) Selenium content of
feedstuffs and effects of dietary selenium levels upon tissue
selenium in chicks and poults. Poultry Sci., 50: 1742-1748.
SCOTT, M.L., OLSON, G., KROOK, L., & BROWN, W.R. (1967)
Selenium-responsive myopathies of myocardium and of smooth
muscle in the young poult. J. Nutr., 91: 573-583.
SEIFTER, J., EHRICH, W.E., HUDYMA, G., & MUELLER, G. (1946)
Thyroid adenomas in rats receiving selenium. Science, 103: 762.
SELJANKINA, K.P., JAHIMOVICH, N.P., ALEKSEEVA, L.S., & PETINA,
A.A. (1974) [Selenium and tellurium levels in environmental
media.] In: [Hygiene and occupational diseases,] Moscow, Nauka
Publishing House, Vol 21, pp. 69-71 (in Russian).
SENF, H.W. (1941) [A case of poisoning by hydrogen
selenide]. Dtsch. Med. Wochenschr., 67: 1094-1096 (in German).
SEWARD, C.R., VAUGHAN, G., & HOVE, E.L. (1966) Effect of
selenium on incisor depigmentation and carbon tetrachloride
poisoning in vitamin E-deficient rats. Proc. Soc. Exp. Biol.
Med., 121: 850-852.
SHACKLETTE, H.T., BOERNGEN, J.G., & KEITH, J.R. (1974)
Selenium, fluorine, and arsenic in surficial materials of the
conterminous United States, 14 pp (US Geological Survey
Circular, 692).
SHAMBERGER, R.J. (1970) Relationship of selenium to cancer.
I. Inhibitory effect of selenium on carcinogenesis. J. Natl
Cancer Inst., 44: 931-936.
SHAMBERGER, R.J. (1971) Is selenium a teratogen? Lancet, 2:
1316.
SHAMBERGER, R.J. (1978) Antioxidants and cancer. VIII.
Cadmium-selenium levels in kidneys. In: Kirchgessner, M., ed.
Trace element metabolism in man and animals. III,
Freising-Weihenstephan, West Germany, Arbeitskreis für
Tierernährungsforschung Weihenstephan, pp. 391-392.
SHAMBERGER, R.J. & FROST, D.V. (1969) Possible protective
effect of selenium against human cancer. Can. Med. Assoc. J.,
100: 682.
SHAMBERGER, R.J. & RUDOLPH, G. (1966) Protection against
cocarcinogenesis by antioxidants. Experientia (Basel), 22: 116.
SHAMBERGER, R.J. & WILLIS, C.E. (1971) Selenium distribution
and human cancer mortality. Crit. Rev. clin. Lab. Sci., 2:
211-221.
SHAMBERGER, R.J., BAUGHMAN, F.F., KALCHERT, S.L., WILLIS,
C.E., & HOFFMAN, G.C. (1973a) Carcinogen-induced chromosomal
breakage decreased by antioxidants. Proc. Natl Acad. Sci.
(USA), 70: 1461-1463.
SHAMBERGER, R.J., RUKOVENA, E., LONGFIELD, A.K., TYTKO, S.A.,
DEODHAR, S., & WILLIS, C.E. (1973b) Antioxidants and cancer.
I. Selenium in the blood of normals and cancer patients. J.
Natl Cancer Inst., 50(4): 863-870.
SHAMBERGER, R.J., TYTKO, S.A., & WILLIS, C.E. (1975)
Selenium and heart disease. In: Hemphill, D.D., ed. Trace
substances in environmental health. IX, Columbia, Missouri,
University of Missouri Press, pp. 15-22.
SHAMBERGER, R.J., TYTKO, S.A., & WILLIS, C.E. (1976)
Antioxidants and cancer. VI. Selenium and age-adjusted human
cancer mortality. Arch. environ. Health, 31: 231-235.
SHAMBERGER, R.J., CORLETT, C.L., BEAMAN, K.D., & KASTEN, B.L.
(1979) Antioxidants reduce the mutagenic effect of malonaldehyde
and beta-propiolactone. Partix, antioxidants, and cancer.
Mutat. Res., 66: 349-355.
SHAMBERGER, R.J., WILLIS, C.E., & MCCORMACK, L.J. (1979)
Selenium and heart disease. III. Blood selenium and heart
mortality in 19 states. In: Hemphill, D.D., ed. Trace
substances in environmental health, Columbia, Missouri,
University of Missouri, pp. 59-63.
SHAPIRO, J.R. (1972) Selenium and carcinogenesis: a review.
Ann. NY Acad. Sci., 192: 215-219.
SHEARER, T.R. (1973) Lack of effect of selenium on
glycolytic and citric acid cycle intermediates in rat kidney
and liver. Proc. Soc. Exp. Biol. Med., 144: 688-691.
SHEARER, T.R. (1975) Developmental and postdevelopmental
uptake of dietary organic and inorganic selenium into the
molar teeth of rats. J. Nutr., 105: 338-347.
SHEARER, T.R. & HADJIMARKOS, D.M. (1975) Geographic distribution
of selenium in human milk. Arch. environ. Health, 30: 230-233.
SHEARER, T.R. & RIDLINGTON, J.W. (1976) Fluoride-selenium
interaction in the hard and soft tissues of the rat. J. Nutr.,
106: 451-456.
SHEARER, T.R., MCCORMACK, D.W., DESART, D.J., BRITTON, J.L., &
LOPEZ, M.T. (1980) Histological evaluation of selenium
induced cataracts. Exp. Eye Res., 31: 327-333.
SHENDRIKAR, A.D. (1974) Critical evaluation of analytical
methods for the determination of selenium in air, water, and
biological materials. Sci. total Environ., 3: 155-168.
SHENDRIKAR, A.D. & FAUDEL, G.B. (1978) Distribution of trace
metals during oil shale retorting. Environ. Sci. Technol., 12:
332-334.
SHRIFT, A. (1964) A selenium cycle in nature? Nature
(Lond.), 201: 1304-1305.
SHRIFT, A. (1973) Selenium compounds in nature and medicine.
Metabolism of selenium by plants and microorganisms. In:
Klayman, D.L. & Gunther, W.H.H., ed. Organic selenium
compounds: their chemistry and biology, New York, John Wiley
and Sons, Inc, pp. 763-814.
SHRIFT, A. & VIRUPAKSHA, T.K. (1965) Seleno-amino acids in
selenium-accumulating plants. Biochim. Biophys. Acta, 100:
67-75.
SHUM, G.T.C., FREEMAN, H.C., & UTHE, J.F. (1977) Flameless
atomic absorption spectrophotometry of selenium in fish and
food products. J. Assoc. Off. Anal. Chem., 60: 1010-1014.
SHUMAEV, V.D., MAKUSHINSKAJA, N.D., & CHIGRINA, T.A. (1976)
[Hygiene aspects of industrial waste water pollution in some
non-ferrous metal enterprises in Kazahstan by chemicals
regulated for the point of sanitary toxicological risk
characteristics.] Zolvavoohram Kazah, 4: 36-38 (in Russian).
SIMPSON, B.H. (1972a) An epidemiological study of carcinoma
of the small intestine in New Zealand sheep. N.Z. vet. J., 20:
91-97.
SIMPSON, B.H. (1972b) The possible relationship of selenium
and superphosphate to the frequencey of occurrence of
intestinal carcinomas in sheep. N.Z. vet. J., 20: 224.
SINDEEVA, N.D. (1959) [Minerology, occurrence and main
characteristics of the geochemistry of selenium and
tellurium,] Moscow, Publishing House of the Academy of
Sciences of the USSR, 257 pp (in Russian).
SIRIANNI, S.R. & HUANG, C.C. (1983) Induction of sister
chromatid exchange by various selenium compounds in Chinese
hamster cells in the presence and absence of S9 mixture.
Cancer Lett., 18: 109-116.
SIVERTSEN, T., KARLSEN, J.T., & FROSLIE, A. (1977) The
relationship of erythrocyte glutathione peroxidase to blood
selenium in swine. Acta vet. Scand., 18: 494-500.
SKORNJAKOVA, L.V., BURCHANOV, A.I., & SALECHOV, M.I. (1969)
[On the acute manifestations of selenium poisoning.] Gig. Tr.
Prof. Zabol., 11: 45-46 (in Russian).
SMITH, C.R., Jr, WEISLEDER, D., MILLER, R.W., PALMER, I.S., &
OLSON, O.E. (1980) Linustatin and neolinustatin: cyanogenic
glycosides of linseed meal that protect animals against
selenium toxicity. J. org. Chem., 45: 507-510.
SMITH, M.I. & STOHLMAN, E.F. (1940) Further observations on
the influence of dietary protein on the toxicity of selenium.
J. Pharmacol. exp. Ther., 70: 270-278.
SMITH, M.I. & WESTFALL, B.B. (1937) Further field studies on
the selenium problem in relation to public health. US Public
Health Rep., 52: 1375-1384.
SMITH, M.I., FRANKE, K.W., & WESTFALL, B.B. (1936) The
selenium problem in relation to public health. A preliminary
survey to determine the possibility of selenium intoxication
in the rural population living on seleniferous soil. US Public
Health Rep., 51: 1496-1505.
SMITH, M.I., WESTFALL, B.B., & STOHLMAN, E.F., Jr (1937) The
elmination of selenium and its distribution in the tissues. US
Public Health Rep., 52: 1171-1177.
SMITH, M.I., WESTFALL, B.B., & STOHLMAN, E.F., Jr (1938)
Studies on the fate of selenium in the organism. US Public
Health Rep., 53: 1199-1216.
SOKOLOFF, L. (1985) Endemic forms of osteoarthritis. Clin.
rheum. Dis., 11: 187-202.
SPALLHOLZ, J.E., MARTIN, J.L., GERLACH, M.L., & HEINZERLING,
R.H. (1973) Immunologic responses of mice fed diets
supplemented with selenite selenium. Proc. Soc. Exp. Biol.
Med., 143: 685-689.
SPALLHOLZ, J.E., MARTIN, J.L., GERLACH, M.L., & HEINZERLING,
R.H. (1975) Injectable selenium: effect on the primary
immune response of mice. Proc. Soc. Exp. Biol. Med., 148:
37-40.
SPALLHOLZ, J.E., COLLINS, G.E., & SCHWARZ, K. (1978) A
single test-tube method for the fluorometric micro-
determination of selenium. Bioinorg. Chem., 9: 453-459.
SPRINKER, L.H., HARR, J.R., NEWBERNE, P.M., WHANGER, P.D., &
WESWIG, P.H. (197l) Selenium deficiency lesions in rats fed
vitamin E supplemented rations. Nutr. Rep. Int., 4: 335-340.
STADTMAN, T.C. (1977) Biological function of selenium. Nutr.
Rev., 35: 161-166.
STEAD, R.J., HINKS, L.J., HODSON, M.E., REDINGTON, A.N.,
CLAYTON, B.E., & BATTEN, J.C. (1985) Selenium deficiency and
possible increased risk of carcinoma in adults with cystic
fibrosis. Lancet (October): 862-863.
STEWART, R.D.H., GRIFFITHS, N.M., THOMSON, C.D., & ROBINSON,
M.F. (1978) Quantitative selenium metabolism in normal New
Zealand women. Br. J. Nutr., 40: 45-54.
STOEWSAND, G.S., GUTENMANN, W.H., & LISK, D.J. (1978) Wheat
grown on fly ash: high selenium uptake and response when fed
to Japanese quail. J. agric. food Chem., 26: 757-759.
SU, Y. & YU, W. (1983) Nutritional bio-geochemical etiology
of Keshan disease. Chin. med. J., 96: 594-596.
SU, Y., CUI, S., GU, B., ZENG, X., & YU, W. (1982)
Experimental study of the effects of corn and vegetables from
Keshan disease endemic districts on the growth and myocardium
in rats. Acta nutr. Sinica, 4: 261-269.
SUBCOMMITTEE ON SELENIUM - COMMITTEE ON ANIMAL NUTRITION
(1983) Selenium in nutrition, revised ed., Washington DC,
Board on Agriculture, National Research Council, National
Academy of Sciences, 174 pp.
SUCHKOV, B.P. (1971) [Selenium content in major nutrients
consumed by the population of the Ukrainian SSR.] Vopr.
Pitan., 30(b): 75-77 (in Russian).
SUCHKOV, B.P. & KACAP, I.M. (1971) [Dental caries in the
population of the Chernovitsk region in relation with the
impact of microelements.] Gig. i Sanit., 3: 91-93 (in Russian).
SUCHKOV, B.P. & ZHIVECKIJ, A.V. (1973) [Selenium levels in
the blood of healthy people and people with neoplasms in a
Chernovici region republican interdepartmental collective.]
In: [Microelements in medicine,] Kiev, Zdarovie, Zdarovie
Publishing House, Vol. 4, pp. 69-73 (in Russian).
SUCHKOV, B.P., KACAP, I.M., & GULGASENKO, A.I. (1973)
[Affection of the population of the Chernovitsi region with
caries in association with selenium content in the teeth].
Stomatologia, 52: 21-23 (in Russian).
SUCHKOV, B.P., SHEVCHUK, I.A., & MARBAR, A.I. (1977)
[Histopathomorphological changes in the organs and tissues of
laboratory animals on a synthetic diet with a low content of
selenium and vitamin E.] Vopr. Pitan., 2: 33-41 (in Russian).
SUCHKOV, B.P., SHTUTMAN, C.M., & HALMURADOV, A.G. (1978)
[The biochemical role of selenium in animal organisms.]
Ukrain. Biochem., 50: 659-671 (in Russian).
SUN, S., ZHAI, F., ZHOU, L., & YANG, G. (1985) The
bioavailability of soil selenium in Keshan disease and high
selenium areas. Chinese J. end. Dis., 4: 21-28.
SUNDE, R.A. (1984) Selenoproteins. J. Am. Oil Chem. Soc.,
61: 1891-1898.
SUNDSTROM, H., KORPELA, H., VIINIKKA, L., & KAUPPILA, A.
(1984a) Serum selenium and glutathione peroxidase, and plasma
lipid peroxides in uterine, ovarian or vulvar cancer, and
their responses to antioxidants in patients with ovarian
cancer. Cancer Lett., 24: 1-10.
SUNDSTROM, H., YRJANHEIKKI, E., & KAUPPILA, A. (1984b) Serum
selenium in patients with ovarian cancer during and after
therapy. Carcinogenesis, 5(6): 731-734.
SVERDLINA, N.T & MASLENNIKOVA, V.S. (1961) [Hygienic
evaluation of working conditions in production of selenium
photocells.] Leningrad, Mater. manch sessin Leningrad NII gig.
truda prof. Zab, pp. 73-75 (in Russian).
SWEINS, A. (1983) Protective effect of selenium against
arsenic-induced chromosomal damage in cultured human lympho-
cytes. Hereditas, 98: 249-252.
SYMANSKI, H. (1950) [A case of hygrogen selenide poisoning.]
Dtsch. Med. Wochenschr., 75: 1730 (in German).
SZYDLOWSKI, F.J. & DUNMIRE, D.L. (1979) Semi-automatic
digestion and automatic analysis for selenium in animal feeds.
Anal. Chim Acta, 105: 445-449.
TAN, J., ZHENG, D., HOU, S., ZHU, W., LI, R., ZHU, Z., & WANG,
W. (in press) Selenium ecological chemico-geography and
endemic Keshan disease and Kashin-Beck disease in China. In:
Proceedings of the Third International Symposium on Selenium
in Biology and Medicine.
TANK, G. & STORVICK, C.A. (1960) Effect of naturally
occurring selenium and vanadium on dental caries. J. dent.
Res., 39: 473-488.
TAYLOR, F.B. (1963) Significance of trace elements in public
finished water supplies. J. Am. Water Works Assoc., 55:
619-623.
TEEL, R.W. (1984) A comparison of the effect of selenium on
the mutagenicity and metabolism of benzo(alpha)pyrene in rat and
hamster liver S9 activation systems. Cancer Lett., 24: 281-289.
TEEL, R.W. & KAIN, S.R. (1984) Selenium modified mutagenicity
and metabolism of benzo(alpha)pyrene in an S9-dependent system.
Mutat. Res., 127: 9-14.
THOMPSON, H.J. (1984) Selenium as an anticarcinogen. J.
agric. food Chem., 32: 422-425.
THOMPSON, J.N. & SCOTT, M.L. (1969) Role of selenium in the
nutrition of the chick. J. Nutr., 97: 335-342.
THOMPSON, J.N. & SCOTT, M.L. (1970) Impaired lipid and
vitamin E absorption related to atrophy of the pancreas in
selenium-deficient chicks. J. Nutr., 100: 797-809.
THOMPSON, J.N., ERDODY, P., & SMITH, D.C. (1975) Selenium
content of food consumed by Canadians. J. Nutr., 105: 274-277.
THOMPSON, K.C. (1975) The atomic-fluorescence determination
of antimony, arsenic, selenium and tellurium by using the
hydride generation technique. Analyst, 100: 307-310.
THOMPSON, R.H., MCMURRAY, C.H., & BLANCHFLOWER, W.J. (1976)
The levels of selenium and glutathione peroxidase activity in
blood of sheep, cows and pigs. Res. vet. Sci., 20: 229-231.
THOMSON, C.D. (1974) Recovery of large doses of selenium
given as sodium selenite with or without vitamin E. N.Z. med.
J., 80: 163-168.
THOMSON, C.D. (1985) Selenium dependent and non-selenium
dependent glutathione peroxidase in human tissues of New
Zealand residents. Biochem. Int., 10: 673-679.
THOMSON, C.D. & ROBINSON, M.F. (1980) Selenium in human
health with emphasis on those aspects peculiar to New
Zealand. Am. J. clin. Nutr., 33: 303-323.
THOMSON, C.D. & ROBINSON, M.F. (1986) Urinary and faecal
excretions and absorption of a large supplement of selenium as
selenite or as selenate. Am. J. clin. Nutr. (submitted for
publication).
THOMSON, C.D. & STEWART, R.D.H. (1973) Metabolic studies of
75Se selenomethionine and 75Se selenite in the rat. Br. J.
Nutr., 30: 139-147.
THOMSON, C.D. & STEWART, R.D.H. (1974) The metabolism of
75Se selenite in young women. Br. J. Nutr., 32: 47-57.
THOMSON, C.D., ROBINSON, B.A., STEWART, R.D.H., & ROBINSON,
M.F. (1975a) Metabolic studies of 75Se selenocystine and
75Se selenomethionine in the rat. Br. J. Nutr., 34: 501-509.
THOMSON, C.D., STEWART, R.D.H., & ROBINSON, M.F. (1975b)
Metabolic studies in rats of 75Se selenomethionine and of 75Se
incorporated in vivo into rabbit kidney. Br. J. Nutr., 33:
45-54.
THOMSON, C.D., REA, H.M., ROBINSON, M.F., & CHAPMAN, O.W.
(1977a) Low blood selenium concentrations and glutathione
peroxidase activities in elderly people. Proc. Univ. Otago
Med. Sch., 55: 18-19.
THOMSON, C.D., REA, H.M., DOESBURG, V.M., & ROBINSON, M.F.
(1977b) Selenium concentrations and glutathione peroxidase
activities in whole blood of New Zealand residents. Br. J.
Nutr., 37: 457-460.
THOMSON, C.D., BURTON, C.E., & ROBINSON, M.F. (1978) On
supplementing the selenium intake of New Zealanders. I. Short
experiments with large doses of selenite or selenomethionine.
Br. J. Nutr., 39: 579-587.
THOMSON, C.D., ROBINSON, M.F., CAMPBELL, D.R., & REA, H.M.
(1982) Effect of prolonged supplementation with daily
supplements of selenomethionine and sodium selenite on
glutathione peroxidase activity in blood of New Zealand
residents. Am. J. clin. Nutr., 36: 24-31.
THOMSON, C.D., ONG, L.K., & ROBINSON, M.F. (1985) Effect of
supplementation with high-selenium wheat bread on selenium,
glutathione peroxidase and related enzymes in blood components
of New Zealand residents. Am. J. clin. Nutr., 41: 1015-1022.
THORN, J., ROBERTSON, J., & BUSS, D.H. (1978) Trace
nutrients. Selenium in British food. Br. J. Nutr., 39: 391-396.
TINSLEY, I.J., HARR, J.R., BONE, J.F., WESWIG, P.H., &
YAMAMOTO, R.S. (1967) Selenium toxicity in rats. I. Growth
and longevity. In: Muth, O.H., Oldfield, J.E., & Weswig, P.H.,
ed. Selenium in biomedicine, Westport, Connecticut, The AVI
Publishing Co., Inc, pp. 141-152.
TSEN, C.C. & COLLIER, H.B. (1959) Selenite as a relatively
weak inhibitor of some sulphydryl enzymes. Nature (Lond.),
183: 1327-1328.
TSEN, C.C. & TAPPEL, A.L. (1958) Catalytic oxidation of
glutathione and other sulfhydryl compounds by selenite. J.
biol. Chem., 233(5): 1230-1232.
TSONGAS, T.A. & FERGUSON, S.W. (1977) Human health effects
of selenium in a rural Colorado drinking-water supply. In:
Hemphill, D.D., ed. Trace substances in environmental health.
XI, Columbia, Missouri, University of Missouri Press, pp.
30-35.
ULLREY, D.E., BRADY, P.S., WHETTER, P.A., KU, P.K., & MAGEE,
W.T. (1977) Selenium supplementation of diets for sheep and
beef cattle. J. anal. Sci., 45: 559-565.
UNDERWOOD, E.J. (1977) Trace elements in human and animal
nutrition, 4th ed., New York, Academic Press, 545 pp.
US DEPARTMENT OF HEALTH AND HUMAN SERVICES, FOOD AND DRUG
ADMINISTRATION (1984) 21 CFR Pt.573.920 Selenium. Fed. Reg.,
49: 627-628.
US FDA (1974) Final environmental impact statement rule
making on selenium in animal feeds, Washington DC, US Food and
Drug Administration, 131 pp.
US FDA (1975) Bureau of Foods Compliance Program Evaluation
Report. Total diet studies, Fiscal Year 1974, Washington DC,
US Food and Drug Administration, 32 pp.
US NAS/NRC (197l) Selenium in nutrition, Washington DC,
National Academy of Science, National Research Council,
Agricultural Board, Committee on Animal Nutrition,
Subcommittee on Selenium, 79 pp.
US NAS/NRC (1976) Selenium, Washington DC, National Academy
of Science, National Research Council, Assembly of Life
Sciences, Medical and Biological Effects of Environmental
Pollutants, 203 pp.
US NAS/NRC (1979) Zinc, Baltimore, Maryland, National
Academy of Science, National Research Council, Assembly of
Life Sciences, Committee on Medical and Biologic Effects of
Environmental Pollutants, 471 pp.
US NAS/NRC (1980) Recommended dietary allowances, Washington
DC, National Academy of Science, National Research Council,
Food and Nutrition Board, Committee on Dietary Allowances, 185
pp.
VAN DER LINDEN, R., DE CORTE, F., & HOSTE, J. (1974)
Activation analysis of biological material with ruthenium as a
multi-element comparator. Anal. Chim. Acta, 71: 263-275.
VAN KAMPEN, K.R. & JAMES, L.F. (1978) Manifestations of
intoxication by selenium-accumulating plants. In: Keeler,
R.F., Van Kampen, K.R., & James, L.F., ed. Effects of
poisonous plants on livestock, New York, Academic Press,
pp. 135-138.
VAN RIJ, A.M., ROBINSON, M.F., GODFREY, P.J., THOMSON, C.D., &
RHEA, H.M. (1978) Selenium blood levels in cancer and other
diseases in surgery. In: Hemphill, D.D., ed. Trace substances
in environmental health. XII, Columbia, Missouri, University
of Missouri Press, pp. 157-163.
VAN RIJ, A.M., THOMSON, C.D., MCKENZIE, J.M., & ROBINSON,
M.F. (1979) Selenium deficiency in total parenteral
nutrition. Am. J. clin. Nutr., 32: 2076-2085.
VAN VLEET, J.F. (1976) Induction of lesions of selenium-
vitamin E deficiency in pigs fed silver. Am. J. vet. Res., 37:
1415-1420.
VAN VLEET, J.F., MEYER, K.B., & OLANDER, H.J. (1974) Acute
selenium toxicosis induced in baby pigs by parenteral
administration of selenium-vitamin E preparations. J. Am. Vet.
Med. Assoc., 165: 543-547.
VARO, P. & KOIVISTOINEN, P. (1980) Mineral element
composition of Finnish foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn,
Zn, Mo, Co, Ni, Cr, F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb,
and ash. XII. General discussion and nutritional evaluation.
Acta agric. Scand. Suppl., 22: 165.
VARO, P. & KOIVISTOINEN, P. (1981) Annual variations in the
average selenium intake in Finland: cereal products and milk
as sources of selenium in 1979/80. Int. J. Vit. Nutr. Res.,
51: 79-84.
VERNIE, L.N. (1984) Selenium in carcinogenesis. Biochim.
Biophys. Acta, 738(4): 203-217.
VERNIE, L.N., BONT, W.S., GINJAAR, H.B., & EMMELOT, P.
(1975) Elongation factor 2 as the target of the reaction
product between sodium selenite and glutathione (GSSeSG) in
the inhibiting of amino acid incorporation in vitro. Biochim.
Biophys. Acta, 414: 283-292.
VERNIE, L.N., GINJARR, H.B., WILDERS, I.T., & BONT, W.S.
(1978) Amino acid incorporation in a cell-free system derived
from rat liver studied with the aid of selenodiglutathione.
Biochim. Biophys. Acta, 518(3): 507-517.
VIRTAMO, J., VALKEILA, E., ALFTHAN, G., PUNSAR, S., HUTTUNEN,
J.K., & KARVONEN, M. (1985) Serum selenium and the risk of
coronary heart disease and stroke. Am. J. Epidemiol., 122(2):
276-282.
VOBECKY, M., PAVLIK, L., & BENES, J. (1977) Non-destructive
neutron activation assay of submicrogram quantities of
selenium. Radiochem. Radioanal. Lett., 29(4): 159-164.
VOBECKY, M., DEDINA, J., PAVLIK, L., & VALASEK, J. (1979)
Gamma-ray interferences in the determination of selenium by
the Inaa method. Radiochem. Radioanal. Lett., 38(3): 197-204.
VOLGAREV, M.N. & TSCHERKES, L.A. (1967) Further studies in
tissue changes associated with sodium selenate. In: Muth,
O.H., Oldfield, J.E., & Weswig, P.H., ed. Selenium in
biomedicine, Westport, Connecticut, The AVI Publishing Co.,
Inc, pp. 179-184.
WAGNER, P.A., HOEKSTRA, W.G., & GANTHER, H.E. (1975)
Alleviation of silver toxicity by selenite in the rat in
relation to tissue glutathione peroxidase. Proc. Soc. Exp.
Biol. Med., 148: 1106-1110.
WAHLSTROM, R.C. & OLSON, O.E. (1959) The effect of selenium
on reproduction in swine. J. anim. Sci., 18: 141-145.
WALKER, G.W.R. & BRADLEY, A.M. (1969) Interacting effects of
sodium monohydrogenarsenate and selenocystine on crossing over
in Drosophila melanogaster. Can. J. Genet. Cytol., 11: 677-688.
WALKER, G.W.R. & TING, K.P. (1967) Effect of selenium on
recombination in barley. Can. J. Genet. Cytol., 9: 314-320.
WANG, Z., LI, C., & LI, L. (1985) An epidemiological
investigation on the selenium content of water, cereals and
hair of children in Heilongjiang province. Chinese J. end.
Dis., 4: 330-333.
WARRINGTON, P.B. (1979) Selenium. Eng. Min. J., 180: 149-150.
WASLIEN, C.I. (1976) Human intake of trace elements. In:
Prasad, A.S., ed. Trace elements in human health and disease.
II. Essential and toxic elements, New York, Academic Press,
pp. 347-370.
WATKINSON, J.H. (1967) Analytical methods for selenium in
biological material. In: Muth, O.H., Oldfield, J.E., & Weswig,
P.H., ed. Selenium in biomedicine, Westport, Connecticut, The
AVI publishing Co., Inc, pp. 97-117.
WATKINSON, J.H. (1974) The selenium status of New
Zealanders. N.Z. med. J., 80: 202-205.
WATKINSON, J.H. (1979) Semi-automated fluorimetric
determination of nanogram quantities of selenium in biological
materials. Anal. Chim. Acta, 105: 319-325.
WATKINSON, J.H. (1981) Changes of blood selenium in New
Zealand adults with time and importation of Australian wheat.
Am. J. clin. Nutr., 34: 936-942.
WATKINSON, J.H. & BROWN, M.W. (1979) New phase-separating
device and other improvements in the semi-automated
fluorimetric determination of selenium. Anal. Chim. Acta, 105:
451-454.
WEDDERBURN, J.F. (1972) Selenium and cancer. N.Z. vet. J.,
20: 56-57.
WEISSMAN, S.H., CUDDIHY, R.G., & BURKSTALLER, M.A. (1979)
Distribution and retention of inhaled selenious acid and
selenium metal aerosols in Beagle dogs. In: Hemphill, D.D.,
ed. Trace substances in environmental health. XIII, Columbia,
Missouri, University of Missouri Press, pp. 477-482.
WEISSMAN, S.H., CUDDIHY, R.G., & MEDINSKY, M.A. (1983)
Absorption, distribution, and retention of inhaled selenious
acid and selenium metal aerosols in Beagle dogs. Toxicol.
appl. Pharmacol., 67: 331-337.
WELSH, S.O. (1979) The protective effect of vitamin E and
N,N'-diphenyl- p-phenylenediamine (DPPD) against methyl mercury
toxicity in the rat. J. Nutr., 109: 1673-1681.
WELSH, S.O. & SOARES, J.H., Jr (1976) The protective effect
of vitamin E and selenium against methyl mercury toxicity in
the Japanese quail. Nutr. Rep. Int., 13: 43-51.
WELSH, S.O., HOLDEN, J.W., WOLF, W.R., & LEVANDER, O.A.
(1981) Selenium intake of Maryland residents consuming
self-selected diets. J. Am. Diet. Assoc., 79: 277-285.
WESTERMANN, D.T. & ROBBINS, C.W. (1974) Effect of S04-S
fertilization on Se concentration of alfalfa (Medicago sativa
L). Agron. J., 66: 207-208.
WESTERMARCK, T. (1977) Selenium content of tissues in
Finnish infants and adults with various diseases, and studies
on the effects of selenium supplementation in neuronal ceroid
lipofuscinosis patients. Acta pharmacol. toxicol., 41: 121-128.
WESTERMARCK, T., RAUNU, P., KIRJARINTA, M., & LAPPALAINEN, L.
(1977) Selenium content of whole blood and serum in adults
and children of different ages from different parts of
Finland. Acta pharmacol. toxicol., 40: 465-475.
WESWIG, P.H., TINSLEY, I.J., HARR, J.R., BONE, J.F., YAMAMOTO,
R.S., & FALK, H. (1966) Bioassay of selenium compounds for
carcinogenesis in rats. Final report, Corvallis, Oregon State
University, Departments of Agricultral Chemistry and
Veterinary Medicine, 131 pp.
WHANGER, P.D. (1976) Selenium versus metal toxicity in
animals. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 234-252.
WHANGER, P.D., MUTH, O.H., OLDFIELD, J.E., & WESWIG, P.H.
(1969) Influence of sulfur on incidence of white muscle
disease in lambs. J. Nutr., 97: 553-562.
WHANGER, P.D., PEDERSEN, N.D., & WESWIG, P.H. (1973)
Selenium proteins in ovine tissues. II. Spectral properties of
a 10,000 molecular weight selenium protein. Biochem. Biophys.
Res. Commun., 53: 1031-1035.
WHANGER, P.D., PEDERSON, N.D., HATFIELD, J., & WESWIG, P.H.
(1976a) Absorption of selenite and selenomethionine from
ligated digestive tract segments in rats. Proc. Soc. Exp.
Biol. Med., 153: 295-297.
WHANGER, P.D., WESWIG, P.H., SCHMITZ, J.A., & OLDFIELD, J.E.
(1976b) Effects of selenium, cadmium, mercury, tellurium,
arsenic, silver, and cobalt on White Muscle Disease in lambs
and effect of dietary forms of arsenic on its accumulation in
tissues. Nutr. Rep. Int., 14: 63-72.
WHEATCROFT, M.G., ENGLISH, J.A., & SCHLACK, C.A. (1951)
Effects of selenium on the incidence of dental caries in white
rats. J. dent. Res., 30: 523-524.
WHETTER, P.A. & ULLREY, D.E. (1978) Improved fluorometric
method for determining selenium. J. Assoc. Off. Anal. Chem.,
61: 927-930.
WHITEACRE, M.E., COMBS, G.F., & PARKER, R.S. (1983)
Peroxidative damage in nutritional pancreatic atrophy due to
selenium-deficiency in the chick. Fed. Proc. Fed. Am. Soc.
Exp. Biol., 42: 928.
WHITING, R.F., WEI, L., & STICH, H.F. (1980) Unscheduled DNA
synthesis and chromosome aberrations induced by inorganic and
organic selenium compounds in the presence of glutathione.
Mutat. Res., 78: 159-169.
WHO (1984) Guidelines for drinking water quality. Volume 2:
Health criteria and other supporting information, Geneva,
World Health Organization, 335 pp.
WILKIE, J.B. & YOUNG, M. (1970) Improvement in the 2,3-
diaminoaphthalene reagent for microfluorescent. Determination
of selenium in biological materials. J. agric. food Chem., 18:
944-945.
WILLETT, W.C., MORRIS, J.S., PRESSEL, S., TAYLOR, J.O., POLK,
B.F., STAMPFER, M.J., ROSNER, B., SCHNEIDER, K., & HAMES,
C.G. (1983) Prediagnostic serum selenium and risk of cancer.
Lancet (July): 130-134.
WILLIAMS, K.T. & BYERS, H.G. (1935) Occurrence of selenium
in the Colorado River and some of its tributaries. Ind. Eng.
Chem. Anal. Ed., 7: 431-432.
WILLIAMS, M.M.I. (1983) Selenium and glutathione peroxidase
in mature human milk. Proc. Univ. Otago Med. Sch., 61: 20-21.
WILSON, H.M. (1962) Selenium oxide poisoning. N.C. med. J.,
23: 73-75.
WILSON, P.S. & JUDSON, G.J. (1976) Glutathione peroxidase
activity in bovine and ovine erythrocytes in relation to blood
selenium concentration. Br. vet. J., 132: 428-434.
WITTING, L.A. & HORWITT, M.K. (1964) Effects of dietary
selenium, methionine, fat level, and tocopherol on rat growth.
J. Nutr., 84: 351-360.
WRIGHT, P.L. & BELL, M.C. (1966) Comparative metabolism of
selenium and tellurium in sheep and swine. Am. J. Physiol.,
211: 6-10.
WU, A.S.H., OLDFIELD, J.E., & WHANGER, P.D. (197l) Effect of
selenium, chromium and vitamin E on spermatogensis. J. anal.
Sci., 33: 273.
WU, A.S.H., OLDFIELD, J.E., WHANGER, P.D., & WESWIG, P.H.
(1973) Effect of selenium, vitamin E, and antioxidants on
testicular function in rats. Biol. Reprod., 8: 625-629.
WU, A.S.H., OLDFIELD, J.E., SHULL, L.R., & CHEEKE, P.R.
(1979) Specific effect of selenium deficiency on rat sperm.
Biol. Reprod., 20: 793-798.
XIA, X. & YU, S. (in press) Studies on the anti-carcinogenic
mechanisms of selenium - The effects of glucose oxidation and
related enzymes. Chinese J. Cancer.
YANG, G.Q., WANG, S., ZHOU, R., & SUN, S. (1983) Endemic
selenium intoxication of humans in China. Am. J. clin. Nutr.,
37: 872-881.
YANG, G., CHEN, J., WEN, Z., GE, K., ZHU, L., CHEN, X., &
CHEN, X. (1984) The role of selenium in Keshan disease. In:
Draper, H.H., ed. Advances in nutritional research, New York,
Plenum Press, pp. 203-231.
YANG, G., ZHOU, R., YIN, S., PIAO, J., ZHU, L., LIN, S., & GU,
L., MAI, R., XU, J., QIAG, F., LU, M., DENG, X., HUANG, J.,
LIN, & ZHOU, W. (1985) [Studies on the selenium requirement
of Chinese people. I. Physiological requirement, minimum
requirement, and minimum allowance.] J. Inst. Health, 14:
24-28 (in Chinese).
YANG, G.Q., ZHU, L.Z., LIU, S.J., GU, L.Z., QIAN, P.C., HUANG,
J.H., & LU, M.D. (1986) Studies of human selenium requirements
in China. In: Combs, G.F., Spallholz, J.E., Levander, O.A., &
Oldfield, J.E., ed. Selenium in biology and medicine, 3rd
Symposium, Westport, Connecticut, AVI Publishing Company.
YASUMOTO, K., IWAMI, K., YOSHIDA, M., & MITSUDA, H. (1976)
Selenium content of foods and its average daily intake in
Japan. Eiyo To Shokuryo, 29: 511-515.
YEH, Y. & JOHNSON, R.M. (1973) Vitamin E deficiency in the
rat. IV. Alteration in mitochondrial membrane and its relation
to respiratory decline. Arch. Biochem. Biophys., 159: 821-831.
YOUNG, S. & KEELER, R.F. (1962) Nutritional muscular dystrophy
in lambs. The effect of muscular activity on the symmetrical
distribution of lesions. Am. J. vet. Res., 23: 966-971.
YU, W.H. (1982) A study of nutritional and bio-geochemical
factors in the occurrence and development of Keshan disease.
Jpn Circ. J., 46: 1201-1207.
YU, S., CHU, Y., GONG, X., & HOU, C. (1985) Region variation
of cancer mortality incidence and its relation to selenium
levels in China. Ecol. trace Element Res., 7: 21-23.
YU, S., ZHU, Y., HON, C., & HUANG, C. (in press) Selective
effects of selenium on the function and structure of
mitochondria isolated from hepatoma and normal liver. In:
Proceedings of the Third International Symposium of Selenium
in Biology and Medicine.
ZABEL, N.L., HARLAND, J., GORMICAN, A.T., & GANTHER, H.E.
(1978) Selenium content of commercial formula diets. Am. J.
clin. Nutr., 31: 850-858.
ZHU, L. & LU, Z. et al. (1981) Effects in pigs fed the crops
grown in Keshan disease affected province of China. In:
Howell, J.M., Gawthone, J.M., & White, C.L., ed. Trace
Element Metabolism in Man and Animals (TEMA 4).
ZOLLER, W.H. & REAMER, D.C. (1976) Selenium in the
atmosphere. In: Proceedings of the Symposium on Selenium-
Tellurium in the Environment, Pittsburgh, Pennsylvania,
Industrial Health Foundation, pp. 54-66.
ZOLLER, W.H., GLADNEY, E.S., & DUCE, R.A. (1974) Atmospheric
concentrations and sources of trace metals at the South Pole.
Science, 183: 198-200.
