
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 39
PARAQUAT AND DIQUAT
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1984
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154099 4
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1984
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR PARAQUAT AND DIQUAT
PARAQUAT
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General properties
1.1.2. Environmental distribution and
transformation - environmental effects
1.1.3. Kinetics and metabolism
1.1.4. Effects on experimental animals
1.1.5. Effects on man
1.2. Recommendations
1.2.1. General
1.2.2. Prevention and treatment
1.2.3. Experimental work
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES IN THE ENVIRONMENT
3.1. Introduction
3.1.1. Industrial technology
3.1.2. Impurities
3.2. Production and use
3.3. Mechanism of the herbicidal effect
4. ENVIRONMENTAL DISTRIBUTION AND TRANSPORTATION
4.1. Photochemical degradation
4.1.1. Photochemical degradation on plant
surfaces
4.1.2. Photochemical degradation of paraquat on
soil and other mineral surfaces
4.2. Microbial degradation
4.3. Environmental adsorption and transformation
4.3.1. Soil
4.3.2. Water
4.3.3. Air
4.3.4. Plants
4.3.5. Animals
5. BIOLOGICAL ACTIVITY OF RESIDUES
5.1. Soil organisms
5.2. Effect of residues on crop yields
5.3. Effects on fish and aquatic organisms
5.4. Effects on birds
6. KINETICS AND METABOLISM
6.1. Animal studies
6.1.1. Absorption
6.1.1.1 Oral absorption
6.1.1.2 Pulmonary absorption
6.1.1.3 Dermal absorption
6.1.2. Distribution
6.1.3. Metabolic transformation and excretion
6.2. Observations on human beings
6.2.1. Observations on paraquat poisoning after
ingestion: non-fatal cases
6.2.2. Observations on paraquat poisoning after
ingestion: fatal cases
6.2.3. Significance of paraquat concentrations in
cases of paraquat poisoning
6.3. Biochemical mechanisms
7. EFFECTS ON ANIMALS
7.1. Effects on experimental animals
7.1.1. Respiratory system
7.1.1.1 Pathomorphological lung studies
7.1.1.2 Species differences in lung injury
7.1.1.3 Functional lung studies
7.1.2. Renal system
7.1.3. Gastrointestinal tract and liver
7.1.4. Skin and eyes
7.1.5. Other systems
7.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
7.1.6.1 Effects on reproduction
7.1.6.2 Embryotoxicity and teratogenicity
7.1.7. Mutagenicity
7.1.8. Carcinogenicity
7.2. Effects on farm animals
7.3. Dose-effect of paraquat
7.4. Methods for decreasing paraquat toxicity
7.5. Relation between age, sex, and toxicity
8. EFFECTS ON MAN
8.1. Accidental and suicidal poisoning
8.1.1. Case reports
8.1.2. Distribution of cases of paraquat poisoning
8.1.3. Route of entry
8.1.4. Formulations
8.1.5. Dose
8.1.6. Clinical and pathomorphological data
relating to fatal paraquat poisoning
8.1.6.1 Respiratory system
8.1.6.2 Renal system
8.1.6.3 Gastrointestinal system, the
liver, and the pancreas
8.1.6.4 Cardiovascular system
8.1.6.5 Central nervous system
8.1.6.6 Adrenal glands
8.1.6.7 Pregnancy
8.1.7. Recovery from paraquat poisoning
8.2. Occupational exposure
8.2.1. Epidemiological studies and case reports
8.2.1.1 Spraying personnel
8.2.1.2 Formulation workers
8.2.2. Cases of occupational poisoning and local caustic effects
8.2.2.1 Oral ingestion
8.2.2.2 Dermal absorption
8.2.2.3 Local skin and nail effects
8.2.2.4 Ocular damage
8.2.2.5 Inhalation
8.3. Use of marijuana contaminated by paraquat
8.4. Guidelines for the treatment of paraquat poisoning
9. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
9.1. Exposure
9.2. Poisoning by paraquat
9.2.1. Suicidal ingestion
9.2.2. Accidental poisoning
9.2.3. Occupational poisoning
9.3. Occupational exposure
9.4. Effects
9.4.1. Paraquat toxicity in animals
9.4.2. Paraquat determination in biological fluids and tissues
9.5. Earlier evaluations by international bodies
9.6. Conclusions
REFERENCES
DIQUAT
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General properties
1.1.2. Environmental distribution and
transformation - environmental effects
1.1.3. Kinetics and metabolism
1.1.4. Effects on animals
1.1.5. Effects on man
1.2. Recommendations
1.2.1. General
1.2.2. Prevention and treatment
1.2.3. Experimental work
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and chemical properties
2.2. Analytical procedures
3. SOURCES IN THE ENVIRONMENT
3.1. Production and uses
4. ENVIRONMENTAL DISTRIBUTION, LEVELS, AND EXPOSURE
4.1. Photochemical and microbial degradation of diquat
4.1.1. Photochemical degradation
4.1.2. Microbial degradation
4.2. Diquat adsorption, residue levels, and
exposure in soil
4.2.1. Diquat adsorption on soil particles
4.2.2. Residue levels of diquat in soils
4.2.3. Effect of residual diquat on soil biological activity,
and on plants and crop yields
4.3. Diquat transformation, residue levels, and
effects on aquatic organisms and crops
4.3.1. Transformation and residue levels of diquat in water
4.3.2. Effects of residual diquat on aquatic
organisms and crops
4.4. Diquat exposure and residue levels in plants and animals
4.4.1. Plants
4.4.2. Animals
4.5. Diquat levels in air and exposure of workers
5. KINETICS AND METABOLISM
5.1. Animal studies
5.1.1. Absorption
5.1.2. Distribution
5.1.3. Metabolic transformation and excretion
5.2. Observations on man
6. EFFECTS ON ANIMALS
6.1. Effects on experimental animals
6.1.1. Gastrointestinal system and liver
6.1.2. Renal system
6.1.3. Eyes and skin
6.1.4. Respiratory system
6.1.5. Nervous system
6.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
6.1.6.1 Effects on reproduction
6.1.6.2 Embryotoxicity and teratogenicity
6.1.7. Mutagencity
6.1.8. Carcinogenicity
6.2. Effects on farm animals
6.3. Dose-effect of diquat
7. EFFECTS ON MAN
7.1. Case reports
7.2. Effects on agricultural operators
7.3. First aid and medical treatment
8. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
8.1. Exposure
8.1.1. Relative contributions of soil, water, air, and food
sources to total diquat uptake
8.1.2. General population exposure
8.1.3. Occupational exposure
8.2. Effects
8.2.1. Diquat toxicity in animals
8.3. Earlier evaluations of diquat by international bodies
8.4. Conclusions
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication, mistakes might have occurred and are
likely to occur in the future. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors found to the Manager of the
International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the
WHO Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event
of updating and re-evaluation of the conclusions contained in the
criteria documents.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
PARAQUAT AND DIQUAT
Members
Dr D.A. Akintonwa, Department of Biochemistry, College of
Medical Sciences, University of Calabar, Calabar, Nigeria
Dr A. Bainova, Institute of Hygiene and Occupational Health,
Medical Academy, Sofia, Bulgaria (Rapporteur)
Dr J. Bus, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USA
Dr R. Davies, Pialba, Queensland, Australia (Chairman)
Dr G.R. FitzGerald, Ardkeen Hospital, Waterford, Ireland
Dr S.K. Kashyap, National Institute of Occupational Health
(Indian Council of Medical Research), Meghaninagar,
Ahmedabad, India
Dr L.L. Smith, ICI Central Toxicology Laboratory, Alderley
Park, Macclesfield, Cheshire, England
Representatives of Other Organizations
Dr M.A. Arellano-Parra, Latin-American Association of Poison
Control Centres
Mme M.Th. van der Venne, Commission of the European
Communities, Health and Safety Directorate, Luxembourg
Observer
Mr G. Willis, International Group of National Associations of
Agrochemical Manufacturers (GIFAP)
Secretariat
Dr J. Cabral, International Agency for Research on Cancer,
Lyons, France
Dr M. Gilbert, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr K.W. Jager, Division of Environmental Health, International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland (Secretary)
Ms A. Sundén, Internation Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Secretariat (contd.)
Dr M. Vandekar, Division of Vector Biology and Control,
Pesticides Development and Safe Use, World Health
Organization, Geneva, Switzerland
Dr C. Xintaras, Division of Noncommunicable Diseases, Office
of Occupational Health, World Health Organization, Geneva,
Switzerland
ENVIRONMENTAL HEALTH CRITERIA FOR PARAQUAT AND DIQUAT
Following the recommendations of the United Nations Conference
on the Human Environment held in Stockholm in 1972, and in response
to a number of World Health Resolutions (WHA23.60, WHA24.47,
WHA25.58, WHA26.68), and the recommendation of the Governing
Council of the United Nations Environment Programme, (UNEP/GC/10,
3 July 1973), a programme on the integrated assessment of the
health effects of environmental pollution was initiated in 1973.
The programme, known as the WHO Environmental Health Criteria
Programme, has been implemented with the support of the Environment
Fund of the United Nations Environment Programme. In 1980, the
Environmental Health Criteria Programme was incorporated into the
International Programme on Chemical Safety (IPCS). The result of
the Environmental Health Criteria Programme is a series of criteria
documents.
A WHO Task Group on Environmental Health Criteria for Paraquat
and Diquat was held in Geneva from 5 - 10 December 1983.
Dr M. Mercier opened the meeting on behalf of the Director-General.
The Task Group reviewed and revised the draft criteria document and
made an evaluation of the health risks of exposure to paraquat and
diquat.
The draft documents were prepared by Dr A. Bainova of Bulgaria.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
PARAQUAT
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General properties
Paraquat (1,1'dimethyl, 4,4'bipyridyl) is a non selective
contact herbicide. It is produced in several countries including
China, Province of Taiwan, Italy, Japan, the United Kingdom, and
the USA, and it is used world-wide in approximately 130 countries.
If not manufactured under strictly controlled conditions, it can
contain impurities that are more toxic than the parent compound.
It is almost exclusively used as a dichloride salt and is usually
formulated to contain surfactant wetters.
Both its herbicidal and toxicological properties are dependent
on the ability of the parent cation to undergo a single electron
addition to form a free radical which reacts with molecular oxygen
to reform the cation and concomitantly produce a superoxide anion.
This oxygen radical may directly or indirectly cause cell death.
Paraquat can be detected because of its ability to form a
radical. Numerous analytical procedures are available.
1.1.2. Environmental distribution and transformation -
environmental effects
Paraquat deposits on plant surfaces undergo photochemical
degradation to compounds that have a lower order of toxicity than
the parent compound.
On reaching the soil, paraquat becomes rapidly and strongly
adsorbed to the clay minerals present. This process inactivates
the herbicidal activity of the compound. While free paraquat is
degraded by a range of soil microorganisms, degradation of
strongly-adsorbed paraquat is relatively slow. In long-term field
studies, degradation rates were 5 - 10% per year. Strongly-bound
paraquat has no adverse effects on soil microfauna or soil
microbial processes.
Paraquat residues disappear rapidly from water by adsorption on
aquatic weeds and by strong adsorption to the bottom mud. The
toxicity of paraquat for fish is low, and the compound is not
cumulative. Normal applications of paraquat for aquatic weed
control are not harmful to aquatic organisms. However, care should
be taken when applying paraquat to water containing heavy weed
growth to treat only a part of the growth, since oxygen consumed by
subsequent weed decay may decrease dissolved oxygen levels to an
extent that may be dangerous for fish. Treated water should not be
used for overhead irrigation for 10 days following treatment.
Paraquat is not volatile and following spraying the
concentrations of airborne paraquat have been shown to be very low.
Under normal working conditions, the exposure of workers in
spraying and harvesting operations remains far below present TLVs
and the exposure of passers-by or of persons living downwind of
such operations is lower still.
Normal paraquat usage has been shown not to have any harmful
effects on birds.
Finite paraquat residues are to be expected only when a crop is
sprayed directly. Cattle allowed to graze on pasture 4 h after
spraying at normal application rates did not suffer any toxic
effects. Consequent residues in products of animal origin are very
low.
1.1.3. Kinetics and metabolism
Although toxic amounts of paraquat may be absorbed after oral
ingestion, the greater part of the ingested paraquat is eliminated
unchanged in the faeces. Paraquat can also be absorbed through the
skin, particularly if it is damaged. The mechanisms of the toxic
effects of paraquat are largely the result of a metabolically
catalyzed single-electron reduction-oxidation reaction, resulting
in depletion of cellular NADPH and the generation of potentially
toxic forms of oxygen such as the superoxide radical.
Absorbed paraquat is distributed via the bloodstream to
practically all organs and tissues of the body, but no prolonged
storage takes place in any tissue. The lung selectively
accumulates paraquat from the plasma by an energy-dependent
process. Consequently, this organ contains higher concentrations
than other tissues. Since the removal of absorbed paraquat occurs
mainly via the kidneys, an early onset of renal failure following
uptake of toxic doses will have a marked effect on paraquat
elimination and distribution and on its accumulation in the lung.
1.1.4. Effects on experimental animals
A characteristic dose-related lung injury can be induced in the
rat, mouse, dog and monkey, but not in the rabbit, guinea-pig and
hamster. The pulmonary toxicity is characterized by initial
development of pulmonary oedema and damage to the alveolar
epithelium, which may progress to fibrosis. Exposure to high doses
of paraquat may also cause less severe toxicity to other organs,
primarily the liver and kidney. Minor toxic effects have been
noted only at high doses in the nervous, cardiovascular, blood,
adrenal and male reproductive systems.
Paraquat has not been found to be teratogenic or carcinogenic
in long-term studies on rats and mice. In vitro mutagenicity
studies have been inconclusive although generally suggestive of
weak potential activity, while in vivo studies were negative.
1.1.5. Effects on man
Occupational exposure to paraquat does not pose a health risk
if the recommendations for use are followed and there is adherence
to safe working practices. This has been shown in several studies
evaluating the potential risk either short- or long-term. However,
nail damage, epistaxis, and delayed skin damage have been described
and may generally be taken as an indication that work practices
should be reviewed.
In the small number of reported cases of paraquat poisoning
allegedly resulting from occupational exposure, the cause can be
identified as one or a combination of a number of factors, viz
contamination of the skin with concentrated products, use of
inadequately diluted solutions, use of faulty equipment, misuse of
equipment (e.g., blowing blocked spray jets) or failure to take
action in the event of contamination of skin or clothing. Eye and
skin damage can follow splashes with the concentrate.
A large number of cases of suicidal or accidental poisoning
from paraquat has been reported. With the exception of a few
unusual cases in which the liquid concentrate was improperly used
to treat body lice, poisoning has followed its ingestion or, in a
few cases, ingestion of the granular formulation.
Two types of fatal poisoning can be distinguished: acute
fulminant poisoning leading to death within a few days, and a more
protracted form that may last for several weeks, resulting in fatal
pulmonary fibrosis. Depending on the severity of the poisoning,
there may be involvement of kidneys, liver, and other organs.
Extensive damage to the oropharynx and the oesophagus are usually
seen in cases of ingestion of liquid concentrate.
After ingestion, speed is imperative in commencing emergency
treatment and it should be noted that this can take place before
arrival of the patient at hospital.
The response to treatment of paraquat poisoning is very
disappointing and the mortality rate remains high. In less severe
cases, without lung damage, recovery has always been complete.
The possibility of recovery clearly depends on the dose of
paraquat taken and the time interval between ingestion and the
commencement of emergency treatment.
1.2. Recommendations
1.2.1. General
Where practical and reasonable, the availability and use of the
20% liquid product should be limited to bona fide agriculturalists,
horticulturalists, and professional users who work with trained
personnel, properly maintained equipment, and adequate supervision.
Every effort should be made to prevent the practice of
decanting or rebottling of the product into improperly labelled
containers.
Further research should be carried out in order to achieve a
safer commercial product and a reduced incidence of fatalities.
National Registers of cases of poisoning should be maintained
for all classes of chemicals - including paraquat. The information
so obtained should be made available to International bodies such
as WHO.
1.2.2. Prevention and treatment
Attention should be drawn to the fact that persons with skin
lesions (either pre-existing or following contamination with
paraquat) should not be permitted to take any part in spraying
procedures until the skin condition has resolved.
It must be stressed that treatment of persons with paraquat
poisoning should be instituted as early as possible. The likelihood
of recovery from a fatal dose is greatest when therapy begins
within 5 - 6 h of poisoning.
1.2.3. Experimental work
Further research should be undertaken on the mechanism of
retention of paraquat in, amongst others, the lung and also on the
concomitant damage caused at the molecular level.
Information was presented to the Task Group showing that
saturation of the cation exchange capacity of soils is not observed
under field conditions. This indicates that residual phytotoxicity
from directly available paraquat is unlikely. It is recommended
that such information be published.
Existing mutagenicity and carcinogenicity studies, although
generally suggesting that paraquat is unlikely to produce genotoxic
effects in man, require more detailed information.
The group has been informed that new long-term toxicity and
carcinogenicity assays have been completed recently and recommends
that the results be made available in the public literature.
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
Paraquat is a non-selective contact bipyridylium herbicide.
The term has been applied to 2 technical products: 1,1'-dimethyl-
4,4'-bipyridylium dichloride (C12H14N2Cl2) or 1,1'-dimethyl-4,4'-
bipyridylium dimethylsulfate (C12H14N2[CH3SO4]2).
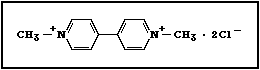
2.2. Physical and Chemical Properties
Pure paraquat salts are white and the technical products
yellow. They are crystalline, odourless, hygroscopic powders with
a relative molecular mass of 257.2 for paraquat dichloride and
408.5 for paraquat dimethylsulfate. The relative molecular mass of
the paraquat ion is 186.2 (Summers, 1980). Some of the other
physical properties of paraquat dichloride, the salt most used for
herbicide formulations, are listed in Table 1.
Table 1. Physical properties of paraquata
-------------------------------------------------------------------
Specific gravity at 20 °C 1.240 - 1.260
Melting point 175 - 180 °C
Boiling point approximately 300 °C
with decomposition
Solubility in water at 20 °C 700 g/litre
pH of liquid formulation 6.5 - 7.5
Vapour pressure not measurable
-------------------------------------------------------------------
a From: Worthing (1979).
Paraquat is slightly soluble in alcohol and practically
insoluble in organic solvents (Haley, 1979). The chemical
structure of paraquat (1,1'-dimethyl-4,4'-bipyridylium dichloride)
is:
 Paraquat is non-explosive and non-flammable in aqueous
formulations. It is corrosive to metals and incompatible with
alkylarylsulfonate wetting agents. It is stable in acid or neutral
solutions but is readily hydrolysed by alkali.
Paraquat readily undergoes a single-electron reduction to the
cation radical. The redox potential for this reaction is 446 mv.
This chemical property led to its use as a redox indicator dye
(methyl viologen) as early as 1933 (Summers, 1980).
2.3. Analytical Methods
The analytical methods for paraquat determination have been
reviewed by Haley (1979) and Summers (1980). Current procedures in
common use are listed in Table 2. Spectrophotometric
determinations involve the reaction of paraquat with 1% aqueous
sodium dithionite in 0.1 N sodium hydroxide. The absorbance of the
resulting blue cation measured at 600 nm can be used as a measure
of the paraquat concentration. Diquat does not interfere because
its radical cation is green in colour. For residue level
determinations (e.g., sub mg/kg levels) the higher intensity
absorption at 396 nm for the paraquat radical and the 379 nm for
the diquat radical are more commonly used. Calderbank & Yuen
(1965) developed a column chromatographic spectrophotometric method
that was successfully applied for soil, biological tissues, and
food. The sensitivity was 0.01 mg/kg. Gas chromatographic and
high-pressure liquid chromatographic analyses were used
satisfactorily. High-pressure liquid chromatography with
ultraviolet detection was proposed by Pryde & Darby (1975) for
determining the paraquat content of urine with a sensitivity of 100
µg/litre.
A comparison of thin-layer chromatography with the
spectrophotometric methods for determining paraquat in human
tissues showed that the former method gave less favourable results,
because of the presence of large amounts of interfering substances
from the tissues (Tsunenari et al., 1975; Haley, 1979).
Spectrophotometric determination of paraquat, after alkaline
reduction with sodium dithionite, has been published (Leary, 1978)
for soil, and plant and biological tissues, the sensitivity limit
being 0.01 mg/kg when a 50 g sample was used.
In a comparison of colorimetric, gas-liquid chromatographic
techniques and radioimmunoassay (Levitt, 1979; Stewart et al.,
1979), it was shown that the latter was a rapid method with
satisfactory sensitivity for determining paraquat in serum, urine,
and organ tissues from poisoned patients. The variation in
detection limits in paraquat determinations in soil, water, and
plant and animal material is related to the size of the sample
obtained, its purity, and the extraction of the paraquat ion from
the material tested.
(a) Soil
Analytical methods include spectrophotometry (Calderbank &
Yuen, 1965; Leary, 1978) and gas chromatography (Khan, 1974; Payne
et al., 1974).
Table 2. Analytical methods for paraquat
----------------------------------------------------------------------------------------
Matrix Analytical procedure Detection Reference
limitsa
----------------------------------------------------------------------------------------
Soil spectrophotometry 0.01 mg/kg Calderbank & Yuen (1965)
spectrophotometry - Leary (1978)
spectrophotometry 0.5 mg/kg Pope & Benner (1974)
gas chromatography 0.01 mg/kg Khan (1974)
gas chromatography 0.01 mg/kg Payne et al. (1974)
Water spectrophotometry 0.01 mg/litre Calderbank & Yuen (1965)
gas chromatography 0.01 mg/litre Soderquist & Crosby (1972)
gas chromatography 0.01 mg/litre Khan (1974)
gas chromatography 0.01 mg/litre Payne et al. (1974)
gas chromatography 10 mg/litre Ukai et al. (1977)
spectrophotometry - Pope & Benner (1974)
Air spectrophotometry 0.01 mg/m3 Calderbank & Yuen (1965)
gas chromatography 0.5 ng/m3 Seiber & Woodrow (1981)
Biological tissues spectrophotometry 0.01 µg/ml Calderbank & Yuen (1965)
spectrophotometry 0.01 µg/ml Berry & Grove (1971)
spectrophotometry 0.01 µg/ml Beyer (1970)
gas chromatography 0.03 µg/ml van Dijk et al. (1977)
gas chromatography/mass 0.025 µg/ml Draffon et al. (1977)
spectrophotometry
radioimmuno assay 0.12 µg/ml Levitt (1979)
radioimmuno assay 0.10 µg/ml Proudfoot et al. (1979)
Plants spectrophotometry 0.01 mg/kg Calderbank & Yuen (1965)
spectrophotometry 0.01 - 1 mg/kg Dickes (1979)
gas chromatography 0.01 - 1 mg/kg Paschal et al. (1979)
gas chromatography - Harrington (1979)
----------------------------------------------------------------------------------------
a The figures refer to the detection limits in the assay solutions.
(b) Water
The concentration of paraquat in water has been determined by
treating the lesser duckweed (Lemna minor) with the test sample
and comparing the time taken to produce chlorosis with known
concentrations. This procedure has been used to determine
herbicide residues in ponds and streams with a sensitivity of 0.075
mg/litre. Determination of chlorosis in Phaseolus vulgaris or
Lemna polyrhiza was classified as more sensitive than the chemical
analyses (Haley, 1979).
A change in cell-membrane permeability, as indicated by the
leakage of electrolytes from treated fronds of Lemna minor, was
used by O'Brien & Prendeville (1978) to detect paraquat in water.
The minimum detectable concentrations ranged from 1.8 - 1.7 µg of
paraquat cation/ml, after 3 h of treatment, to 180 and 17 ng/ml
after 72 h of exposure to light.
Ukai et al. (1977) found a gas chromatographic method suitable
for paraquat determination with a sensitivity of 10 - 90 µg/ml
water, using 4-anisidine as the internal standard. Pope & Benner
(1974) have also used a spectrophotometric method.
(c) Air-working environment
Sprayed or dusted, paraquat is absorbed on filter/sorbent
systems. The absorbed paraquat is dissolved and determined
spectrophotometrically using one of the classical methods
(Calderbank & Yuen, 1965; Staiff et al., 1975; Anderson et al.,
1981). Carlstrom (1971) applied a colorimetric method for
analysing paraquat formulations. Seiber & Woodrow (1981) developed
a nitrogen-selective gas chromatographic method for paraquat
determination in airborne particulate matter.
(d) Plants
The method of Calderbank & Yuen (1965) is considered to be the
best procedure for determining paraquat in crops, treated plants,
and food. The limit of the spectrophotometric analysis ranged from
0.01 - 0.1 mg/kg, depending on the crop. A gas chromatographic
method for paraquat residues in food was suggested by Dickes
(1979). A procedure based on gas-liquid chromatography (Paschal et
al., 1979) provided linear working curves over a paraquat
concentration range of 0 - 20 µg/g, determined by extraction from
1 g samples of sunflower seeds. The method has been proposed for
herbicide analyses in plant materials. A vapour-phase
chromatographic technique, used for determining paraquat in wood
(Harrington, 1979), is based on the liberation of methyl chloride
after pyrolysis.
(e) Biological material
A spectrophotometric method, applied for determining paraquat
residues in milk (ICI, 1972), had a detection limit of 0.01
mg/litre sample. Analyses of the plasma (serum) and urine of
subjects poisoned by paraquat are important for diagnosis and
prognosis. Tompsett (1970) described a method for analysing
biological samples from patients suffering from accidental oral
intoxication. Paraquat extracted from human blood, urine, and
faeces was separated on a strong acid cation-exchange resin (Beyer,
1970), reacted with sodium dithionite, and determined
spectrophotmetrically at 391 nm. The method had a sensitivity of
0.01 µg ion/ml in a 250 ml aliquot of urine. A similar procedure,
published by Pickova (1978), for estimating paraquat levels in the
urine of patients had a sensitivity of 30 µg in a sample of 50 -
500 ml. Gas chromatographic methods were successfully used (Dijk,
van et al., 1977; Draffon et al., 1977).
A radioimmunoassay using 3H-labelled paraquat was found to
be a sensitive method for analysing plasma, urine, and biological
tissues (ICI, 1979). Antibodies to paraquat were prepared in
rabbits and tested for sensitivity by a charcoal separation
technique (Levitt, 1979). The results showed that the antibodies
were specific for the herbicide. A comparison of radioimmunoassay
and gas liquid chromatographic techniques (Levitt, 1979; Proudfoot
et al., 1979) showed the high sensitivity of this method. The
total assay time was no more than 30 min. A series of 50 serum
specimens from persons poisoned with paraquat were tested by
radioimmunoassay and colorimetric analysis (Stewart et al., 1979);
the results from both methods corresponded closely.
Tsunenari et al. (1975) used 7 analytical methods for
determining paraquat with a view to diagnosing accidental,
suicidal, or homicidal poisoning. Colorimetry, with dithionite
thin-layer chromatography, was used for the qualitative assay of
paraquat in biological tissues, while ion-exchange resin column
chromatography, with colorimetry or gas chromatography, was used
for the quantitative assay. Tsunenari et al. (1981) also studied
the influence of putrefaction on paraquat determinations in autopsy
materials. Detection was possible, even in tissues in advanced
stages of decomposition.
3. SOURCES IN THE ENVIRONMENT
3.1. Introduction
3.1.1. Industrial technology
Paraquat does not occur naturally. It was originally
synthesized by Weidel & Russo as reported in 1882 (Summers, 1980).
Its herbicidal properties were discovered only in 1955. The
compound is produced by coupling pyridine in the presence of sodium
in anhydrous ammonia and quaternizing the 4,4'-bipyridyl with
methyl chloride (Fig. 1).
Paraquat is non-explosive and non-flammable in aqueous
formulations. It is corrosive to metals and incompatible with
alkylarylsulfonate wetting agents. It is stable in acid or neutral
solutions but is readily hydrolysed by alkali.
Paraquat readily undergoes a single-electron reduction to the
cation radical. The redox potential for this reaction is 446 mv.
This chemical property led to its use as a redox indicator dye
(methyl viologen) as early as 1933 (Summers, 1980).
2.3. Analytical Methods
The analytical methods for paraquat determination have been
reviewed by Haley (1979) and Summers (1980). Current procedures in
common use are listed in Table 2. Spectrophotometric
determinations involve the reaction of paraquat with 1% aqueous
sodium dithionite in 0.1 N sodium hydroxide. The absorbance of the
resulting blue cation measured at 600 nm can be used as a measure
of the paraquat concentration. Diquat does not interfere because
its radical cation is green in colour. For residue level
determinations (e.g., sub mg/kg levels) the higher intensity
absorption at 396 nm for the paraquat radical and the 379 nm for
the diquat radical are more commonly used. Calderbank & Yuen
(1965) developed a column chromatographic spectrophotometric method
that was successfully applied for soil, biological tissues, and
food. The sensitivity was 0.01 mg/kg. Gas chromatographic and
high-pressure liquid chromatographic analyses were used
satisfactorily. High-pressure liquid chromatography with
ultraviolet detection was proposed by Pryde & Darby (1975) for
determining the paraquat content of urine with a sensitivity of 100
µg/litre.
A comparison of thin-layer chromatography with the
spectrophotometric methods for determining paraquat in human
tissues showed that the former method gave less favourable results,
because of the presence of large amounts of interfering substances
from the tissues (Tsunenari et al., 1975; Haley, 1979).
Spectrophotometric determination of paraquat, after alkaline
reduction with sodium dithionite, has been published (Leary, 1978)
for soil, and plant and biological tissues, the sensitivity limit
being 0.01 mg/kg when a 50 g sample was used.
In a comparison of colorimetric, gas-liquid chromatographic
techniques and radioimmunoassay (Levitt, 1979; Stewart et al.,
1979), it was shown that the latter was a rapid method with
satisfactory sensitivity for determining paraquat in serum, urine,
and organ tissues from poisoned patients. The variation in
detection limits in paraquat determinations in soil, water, and
plant and animal material is related to the size of the sample
obtained, its purity, and the extraction of the paraquat ion from
the material tested.
(a) Soil
Analytical methods include spectrophotometry (Calderbank &
Yuen, 1965; Leary, 1978) and gas chromatography (Khan, 1974; Payne
et al., 1974).
Table 2. Analytical methods for paraquat
----------------------------------------------------------------------------------------
Matrix Analytical procedure Detection Reference
limitsa
----------------------------------------------------------------------------------------
Soil spectrophotometry 0.01 mg/kg Calderbank & Yuen (1965)
spectrophotometry - Leary (1978)
spectrophotometry 0.5 mg/kg Pope & Benner (1974)
gas chromatography 0.01 mg/kg Khan (1974)
gas chromatography 0.01 mg/kg Payne et al. (1974)
Water spectrophotometry 0.01 mg/litre Calderbank & Yuen (1965)
gas chromatography 0.01 mg/litre Soderquist & Crosby (1972)
gas chromatography 0.01 mg/litre Khan (1974)
gas chromatography 0.01 mg/litre Payne et al. (1974)
gas chromatography 10 mg/litre Ukai et al. (1977)
spectrophotometry - Pope & Benner (1974)
Air spectrophotometry 0.01 mg/m3 Calderbank & Yuen (1965)
gas chromatography 0.5 ng/m3 Seiber & Woodrow (1981)
Biological tissues spectrophotometry 0.01 µg/ml Calderbank & Yuen (1965)
spectrophotometry 0.01 µg/ml Berry & Grove (1971)
spectrophotometry 0.01 µg/ml Beyer (1970)
gas chromatography 0.03 µg/ml van Dijk et al. (1977)
gas chromatography/mass 0.025 µg/ml Draffon et al. (1977)
spectrophotometry
radioimmuno assay 0.12 µg/ml Levitt (1979)
radioimmuno assay 0.10 µg/ml Proudfoot et al. (1979)
Plants spectrophotometry 0.01 mg/kg Calderbank & Yuen (1965)
spectrophotometry 0.01 - 1 mg/kg Dickes (1979)
gas chromatography 0.01 - 1 mg/kg Paschal et al. (1979)
gas chromatography - Harrington (1979)
----------------------------------------------------------------------------------------
a The figures refer to the detection limits in the assay solutions.
(b) Water
The concentration of paraquat in water has been determined by
treating the lesser duckweed (Lemna minor) with the test sample
and comparing the time taken to produce chlorosis with known
concentrations. This procedure has been used to determine
herbicide residues in ponds and streams with a sensitivity of 0.075
mg/litre. Determination of chlorosis in Phaseolus vulgaris or
Lemna polyrhiza was classified as more sensitive than the chemical
analyses (Haley, 1979).
A change in cell-membrane permeability, as indicated by the
leakage of electrolytes from treated fronds of Lemna minor, was
used by O'Brien & Prendeville (1978) to detect paraquat in water.
The minimum detectable concentrations ranged from 1.8 - 1.7 µg of
paraquat cation/ml, after 3 h of treatment, to 180 and 17 ng/ml
after 72 h of exposure to light.
Ukai et al. (1977) found a gas chromatographic method suitable
for paraquat determination with a sensitivity of 10 - 90 µg/ml
water, using 4-anisidine as the internal standard. Pope & Benner
(1974) have also used a spectrophotometric method.
(c) Air-working environment
Sprayed or dusted, paraquat is absorbed on filter/sorbent
systems. The absorbed paraquat is dissolved and determined
spectrophotometrically using one of the classical methods
(Calderbank & Yuen, 1965; Staiff et al., 1975; Anderson et al.,
1981). Carlstrom (1971) applied a colorimetric method for
analysing paraquat formulations. Seiber & Woodrow (1981) developed
a nitrogen-selective gas chromatographic method for paraquat
determination in airborne particulate matter.
(d) Plants
The method of Calderbank & Yuen (1965) is considered to be the
best procedure for determining paraquat in crops, treated plants,
and food. The limit of the spectrophotometric analysis ranged from
0.01 - 0.1 mg/kg, depending on the crop. A gas chromatographic
method for paraquat residues in food was suggested by Dickes
(1979). A procedure based on gas-liquid chromatography (Paschal et
al., 1979) provided linear working curves over a paraquat
concentration range of 0 - 20 µg/g, determined by extraction from
1 g samples of sunflower seeds. The method has been proposed for
herbicide analyses in plant materials. A vapour-phase
chromatographic technique, used for determining paraquat in wood
(Harrington, 1979), is based on the liberation of methyl chloride
after pyrolysis.
(e) Biological material
A spectrophotometric method, applied for determining paraquat
residues in milk (ICI, 1972), had a detection limit of 0.01
mg/litre sample. Analyses of the plasma (serum) and urine of
subjects poisoned by paraquat are important for diagnosis and
prognosis. Tompsett (1970) described a method for analysing
biological samples from patients suffering from accidental oral
intoxication. Paraquat extracted from human blood, urine, and
faeces was separated on a strong acid cation-exchange resin (Beyer,
1970), reacted with sodium dithionite, and determined
spectrophotmetrically at 391 nm. The method had a sensitivity of
0.01 µg ion/ml in a 250 ml aliquot of urine. A similar procedure,
published by Pickova (1978), for estimating paraquat levels in the
urine of patients had a sensitivity of 30 µg in a sample of 50 -
500 ml. Gas chromatographic methods were successfully used (Dijk,
van et al., 1977; Draffon et al., 1977).
A radioimmunoassay using 3H-labelled paraquat was found to
be a sensitive method for analysing plasma, urine, and biological
tissues (ICI, 1979). Antibodies to paraquat were prepared in
rabbits and tested for sensitivity by a charcoal separation
technique (Levitt, 1979). The results showed that the antibodies
were specific for the herbicide. A comparison of radioimmunoassay
and gas liquid chromatographic techniques (Levitt, 1979; Proudfoot
et al., 1979) showed the high sensitivity of this method. The
total assay time was no more than 30 min. A series of 50 serum
specimens from persons poisoned with paraquat were tested by
radioimmunoassay and colorimetric analysis (Stewart et al., 1979);
the results from both methods corresponded closely.
Tsunenari et al. (1975) used 7 analytical methods for
determining paraquat with a view to diagnosing accidental,
suicidal, or homicidal poisoning. Colorimetry, with dithionite
thin-layer chromatography, was used for the qualitative assay of
paraquat in biological tissues, while ion-exchange resin column
chromatography, with colorimetry or gas chromatography, was used
for the quantitative assay. Tsunenari et al. (1981) also studied
the influence of putrefaction on paraquat determinations in autopsy
materials. Detection was possible, even in tissues in advanced
stages of decomposition.
3. SOURCES IN THE ENVIRONMENT
3.1. Introduction
3.1.1. Industrial technology
Paraquat does not occur naturally. It was originally
synthesized by Weidel & Russo as reported in 1882 (Summers, 1980).
Its herbicidal properties were discovered only in 1955. The
compound is produced by coupling pyridine in the presence of sodium
in anhydrous ammonia and quaternizing the 4,4'-bipyridyl with
methyl chloride (Fig. 1).
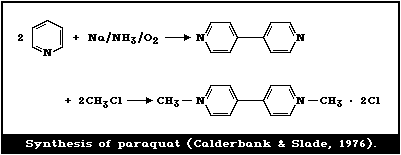 When bipyridyl is refluxed with methyl iodide, the iodide salt
is obtained. Haley (1979) and Summers (1980) thoroughly reviewed
the published methods for paraquat synthesis, and for the
separation and purification of bipyridylium salts. The yields
obtainable vary from 20% to 96% of pure product.
The first commercial paraquat formulation approved for
agricultural use was Gramoxone(R).
3.1.2. Impurities
Aqueous solutions of paraquat used as herbicides must
correspond to the FAO Specification Code 56/13/S/6 (FAO, 1973).
This requires a description of the active ingredient in the
formulation, of the impurities, of the physical and chemical
properties, and of the methods for determining the components.
The only impurity permitted in paraquat is free 4,4'-bipyridyl
at a maximum level of 0.25% of the paraquat content.
3.2. Production and Use
Paraquat is produced in several countries, including China,
Province of Taiwan, Italy, the United Kingdom, and the USA.
Formulations of the active ingredients (mainly paraquat dichloride)
are used in more than 130 countries world-wide. Paraquat
dimethylphosphate is used in the USSR. Since its introduction for
agricultural use in 1962, paraquat has been widely used for weed
control and as a dessicant. In many countries, paraquat is
formulated locally, only the technical active ingredient being
imported. Records of world production of paraquat are not
available.
Technical paraquat dichloride has been formulated in liquid
concentrates or granules. Water-soluble granules containing
paraquat (25 g/kg) and diquat (25 g/kg) are used for weed control
in private gardens. Paraquat is sold under a variety of trade
names which are summarized in Table 3.
Gramoxone(R) is a dark aqueous solution containing a paraquat
dichloride concentration of 200 ± 10 g/litre. Its specific gravity
at 20 °C is 1.1 and the crystallization point is -5 °C to 10 °C.
It is not flammable and, in its original polyethylene containers,
is stable for a long time under normal atmospheric conditions. The
formulation is incompatible with anionic surface active agents and
decomposes in ultraviolet radiation. Gramoxone(R) rapidly
corrodes aluminium; zinc, iron, and tinplate are more resistant.
Paraquat is a total contact herbicide used to control broad-
leaved and grassy weeds. It should be sprayed when the weeds are
young and less than 30 cm high. It kills all green tissues, but
does not harm the mature bark. Paraquat is used for plantation
crops (banana, cocoa-palm, coffee, oil-palm, rubber, etc.) and for
citrus fruits, apples, plums, vines, and tea. On certain crops
(potato, pineapple, sugar-cane, sunflower), it is used as a
dessicant; it is also used as a cotton defoliant. It is applied
around the trees in orchards and between the rows of crops.
Uncropped land on industrial sites, railways, roadsides, etc.
can be cleared of weeds by applying paraquat at higher
concentrations.
Gramoxone S(R) is largely applied for aquatic weed control.
Application rates usually range from 250 g - 1500 g/ha (1.1 -
7.1 1itre of Gramoxone(R), but, for grass and stubble clearing, up
to 2200 g of the herbicide are used per ha. The working dilutions
vary from 1 - 5 g per litre paraquat in water. It is applied by
ground sprayers (not mist-blowers) in 200 - 500 litres solution/ha.
3.3. Mechanism of the Herbicidal Effect
The herbicidal activity of paraquat is dependent on the parent
molecule undergoing a single-electron redox cycling reaction.
Paraquat is reduced to the paraquat radical, which, in the presence
of molecular oxygen, is immediately reoxidized forming the parent
molecule and superoxide radicals (O2-) (Conning et al., 1969). As
early as 1960, Mees had shown that oxygen was necessary for the
herbicidal activity of paraquat, suggesting the importance of the
redox cycling and O2- formation in mediating toxicity. Paraquat
was not toxic to plant leaves incubated under anaerobic conditions,
despite the continuation of photosynthetic reactions capable of
forming paraquat radicals. Exposure of the anaerobic incubates to
air, however, resulted in immediate onset of toxicity. Dodge
(1971) subsequently confirmed that isolated plant chloroplasts
could form the paraquat radical under anaerobic conditions. The
possibility that O2- generation may be an essential component of
the herbicidal activity was further supported in a study by
Youngman & Dodge (1979). These investigators observed that the
phytotoxicity of paraquat in plant cotyledons was decreased by a
copper chelate of D-penicillamine. The chelate possessed activity
similar to the enzyme superoxide dismutase (EC 1.15.1.1)
(Lengfelder & Elstner, 1978), an enzyme that detoxifies O2-
(McCord & Fridovich, 1969).
The generation of O2- may lead to many potentially
cytotoxic reactions, including the membrane-damaging process of
lipid peroxidation (Bus & Gibson, 1979). When plant leaves were
incubated with paraquat, there was rapid stimulation of the
formation of malondialdehyde, which is an indicator of lipid
peroxidation (Dodge, 1971).
Table 3. Paraquat trade namesa
--------------------------------------------------------------------------------------------------------
Products Countries Paraquat content (W/V for
liquids, W/W for solids)
--------------------------------------------------------------------------------------------------------
Dextrone X United Kingdom 20%
Dexuron United Kingdom 10%, also contains diuron
Duanti Germany, Federal Republic of 2.5%, also contains diquat
Dukatalon Israel 9%, also contains diquat
Esgram United Kingdom 20%
Frankol Prompt Germany, Federal Republic of 10%, also contains diuron
Gramazin Italy 10%, also contains simazine
Gramixel Germany, Federal Republic of 10%, also contains diuron
Gramanol United Kingdom, Ireland, 14%, also contains monolinuron
Belgium, Greece, Middle East
Gramoxone worldwide 20%
Gramoxone S worldwide 20%
Gramoxone W discontinued 20%
Gramoxone ZU The Netherlands, Belgium 20%
Gramuron Africa, Italy 10%, also contains diuron
Katalon Israel 20%
Ortho Paraquat CL USA 24.6% (2 lb/US gal)
Ortho Spot Weed & Grass Killer USA 0.2% (Solid Stream Aerosol)
Orvar United Kingdom 5%
Paracol Malaysia, Indonesia, Philippines 10%, also contains diquat
Chile, Peru
Paradi Australia 10%, also contains diquat
Pathclear United Kingdom, New Zealand 2.5%, also contains diquat,
3 aminotriazole and simazine
Preeglone Denmark, Norway 2.5%, also contains diquat
Preeglone Belgium, France, Spain 12%, also contains diquat
Preeglone Extra New Zealand 9%, also contains diquat
Priglone France, Switzerland 12%, also contains diquat
Seythe United Kingdom 20%
Spray Seed Australia 10%, also contains diquat
Terraklene United Kingdom, Ireland, Denmark, 10%, also contains simazine
France, Switzerland
Tota-Col Wide range of countries 10%, also contains diuron
Tryquat Australia 10%, also contains diquat
Weedol The Netherlands, Ireland, United Kingdom 2.5%, also contains diquat
Weedrite Canada 2.5%
Weedrite Aerosol Canada 0.44%
--------------------------------------------------------------------------------------------------------
a From: Fletcher (1975).
4. ENVIRONMENTAL DISTRIBUTION AND TRANSPORTATION
4.1. Photochemical Degradation
4.1.1. Photochemical degradation on plant surfaces
In agricultural practice, much of the paraquat sprayed is
initially deposited on plant surfaces. Slade (1965, 1966) applied
paraquat dichloride droplets to maize, tomato, and broad-bean
plants. Determinations carried out at intervals of 100 days showed
that degradation was caused by photochemical decomposition on the
leaf surfaces but not by metabolism. Degradation products isolated
from plants sprayed with 14C-paraquat dichloride included 4-
carboxyl-1-methyl-14C-pyridylium chloride and methylamine-14C-
hydrochloride. No 14C02 was detected as a photochemical
decomposition product. The photochemical degradation of paraquat
dichloride continued after the plants were dead (Fig. 2). Paraquat
photodegradation products were not translocated from the dessicated
leaves of the plants, nor were they found in the crops (cereals and
fruits), when weeds were treated with paraquat during 3 - 4
successive seasons (Calderbank, 1966).
When bipyridyl is refluxed with methyl iodide, the iodide salt
is obtained. Haley (1979) and Summers (1980) thoroughly reviewed
the published methods for paraquat synthesis, and for the
separation and purification of bipyridylium salts. The yields
obtainable vary from 20% to 96% of pure product.
The first commercial paraquat formulation approved for
agricultural use was Gramoxone(R).
3.1.2. Impurities
Aqueous solutions of paraquat used as herbicides must
correspond to the FAO Specification Code 56/13/S/6 (FAO, 1973).
This requires a description of the active ingredient in the
formulation, of the impurities, of the physical and chemical
properties, and of the methods for determining the components.
The only impurity permitted in paraquat is free 4,4'-bipyridyl
at a maximum level of 0.25% of the paraquat content.
3.2. Production and Use
Paraquat is produced in several countries, including China,
Province of Taiwan, Italy, the United Kingdom, and the USA.
Formulations of the active ingredients (mainly paraquat dichloride)
are used in more than 130 countries world-wide. Paraquat
dimethylphosphate is used in the USSR. Since its introduction for
agricultural use in 1962, paraquat has been widely used for weed
control and as a dessicant. In many countries, paraquat is
formulated locally, only the technical active ingredient being
imported. Records of world production of paraquat are not
available.
Technical paraquat dichloride has been formulated in liquid
concentrates or granules. Water-soluble granules containing
paraquat (25 g/kg) and diquat (25 g/kg) are used for weed control
in private gardens. Paraquat is sold under a variety of trade
names which are summarized in Table 3.
Gramoxone(R) is a dark aqueous solution containing a paraquat
dichloride concentration of 200 ± 10 g/litre. Its specific gravity
at 20 °C is 1.1 and the crystallization point is -5 °C to 10 °C.
It is not flammable and, in its original polyethylene containers,
is stable for a long time under normal atmospheric conditions. The
formulation is incompatible with anionic surface active agents and
decomposes in ultraviolet radiation. Gramoxone(R) rapidly
corrodes aluminium; zinc, iron, and tinplate are more resistant.
Paraquat is a total contact herbicide used to control broad-
leaved and grassy weeds. It should be sprayed when the weeds are
young and less than 30 cm high. It kills all green tissues, but
does not harm the mature bark. Paraquat is used for plantation
crops (banana, cocoa-palm, coffee, oil-palm, rubber, etc.) and for
citrus fruits, apples, plums, vines, and tea. On certain crops
(potato, pineapple, sugar-cane, sunflower), it is used as a
dessicant; it is also used as a cotton defoliant. It is applied
around the trees in orchards and between the rows of crops.
Uncropped land on industrial sites, railways, roadsides, etc.
can be cleared of weeds by applying paraquat at higher
concentrations.
Gramoxone S(R) is largely applied for aquatic weed control.
Application rates usually range from 250 g - 1500 g/ha (1.1 -
7.1 1itre of Gramoxone(R), but, for grass and stubble clearing, up
to 2200 g of the herbicide are used per ha. The working dilutions
vary from 1 - 5 g per litre paraquat in water. It is applied by
ground sprayers (not mist-blowers) in 200 - 500 litres solution/ha.
3.3. Mechanism of the Herbicidal Effect
The herbicidal activity of paraquat is dependent on the parent
molecule undergoing a single-electron redox cycling reaction.
Paraquat is reduced to the paraquat radical, which, in the presence
of molecular oxygen, is immediately reoxidized forming the parent
molecule and superoxide radicals (O2-) (Conning et al., 1969). As
early as 1960, Mees had shown that oxygen was necessary for the
herbicidal activity of paraquat, suggesting the importance of the
redox cycling and O2- formation in mediating toxicity. Paraquat
was not toxic to plant leaves incubated under anaerobic conditions,
despite the continuation of photosynthetic reactions capable of
forming paraquat radicals. Exposure of the anaerobic incubates to
air, however, resulted in immediate onset of toxicity. Dodge
(1971) subsequently confirmed that isolated plant chloroplasts
could form the paraquat radical under anaerobic conditions. The
possibility that O2- generation may be an essential component of
the herbicidal activity was further supported in a study by
Youngman & Dodge (1979). These investigators observed that the
phytotoxicity of paraquat in plant cotyledons was decreased by a
copper chelate of D-penicillamine. The chelate possessed activity
similar to the enzyme superoxide dismutase (EC 1.15.1.1)
(Lengfelder & Elstner, 1978), an enzyme that detoxifies O2-
(McCord & Fridovich, 1969).
The generation of O2- may lead to many potentially
cytotoxic reactions, including the membrane-damaging process of
lipid peroxidation (Bus & Gibson, 1979). When plant leaves were
incubated with paraquat, there was rapid stimulation of the
formation of malondialdehyde, which is an indicator of lipid
peroxidation (Dodge, 1971).
Table 3. Paraquat trade namesa
--------------------------------------------------------------------------------------------------------
Products Countries Paraquat content (W/V for
liquids, W/W for solids)
--------------------------------------------------------------------------------------------------------
Dextrone X United Kingdom 20%
Dexuron United Kingdom 10%, also contains diuron
Duanti Germany, Federal Republic of 2.5%, also contains diquat
Dukatalon Israel 9%, also contains diquat
Esgram United Kingdom 20%
Frankol Prompt Germany, Federal Republic of 10%, also contains diuron
Gramazin Italy 10%, also contains simazine
Gramixel Germany, Federal Republic of 10%, also contains diuron
Gramanol United Kingdom, Ireland, 14%, also contains monolinuron
Belgium, Greece, Middle East
Gramoxone worldwide 20%
Gramoxone S worldwide 20%
Gramoxone W discontinued 20%
Gramoxone ZU The Netherlands, Belgium 20%
Gramuron Africa, Italy 10%, also contains diuron
Katalon Israel 20%
Ortho Paraquat CL USA 24.6% (2 lb/US gal)
Ortho Spot Weed & Grass Killer USA 0.2% (Solid Stream Aerosol)
Orvar United Kingdom 5%
Paracol Malaysia, Indonesia, Philippines 10%, also contains diquat
Chile, Peru
Paradi Australia 10%, also contains diquat
Pathclear United Kingdom, New Zealand 2.5%, also contains diquat,
3 aminotriazole and simazine
Preeglone Denmark, Norway 2.5%, also contains diquat
Preeglone Belgium, France, Spain 12%, also contains diquat
Preeglone Extra New Zealand 9%, also contains diquat
Priglone France, Switzerland 12%, also contains diquat
Seythe United Kingdom 20%
Spray Seed Australia 10%, also contains diquat
Terraklene United Kingdom, Ireland, Denmark, 10%, also contains simazine
France, Switzerland
Tota-Col Wide range of countries 10%, also contains diuron
Tryquat Australia 10%, also contains diquat
Weedol The Netherlands, Ireland, United Kingdom 2.5%, also contains diquat
Weedrite Canada 2.5%
Weedrite Aerosol Canada 0.44%
--------------------------------------------------------------------------------------------------------
a From: Fletcher (1975).
4. ENVIRONMENTAL DISTRIBUTION AND TRANSPORTATION
4.1. Photochemical Degradation
4.1.1. Photochemical degradation on plant surfaces
In agricultural practice, much of the paraquat sprayed is
initially deposited on plant surfaces. Slade (1965, 1966) applied
paraquat dichloride droplets to maize, tomato, and broad-bean
plants. Determinations carried out at intervals of 100 days showed
that degradation was caused by photochemical decomposition on the
leaf surfaces but not by metabolism. Degradation products isolated
from plants sprayed with 14C-paraquat dichloride included 4-
carboxyl-1-methyl-14C-pyridylium chloride and methylamine-14C-
hydrochloride. No 14C02 was detected as a photochemical
decomposition product. The photochemical degradation of paraquat
dichloride continued after the plants were dead (Fig. 2). Paraquat
photodegradation products were not translocated from the dessicated
leaves of the plants, nor were they found in the crops (cereals and
fruits), when weeds were treated with paraquat during 3 - 4
successive seasons (Calderbank, 1966).
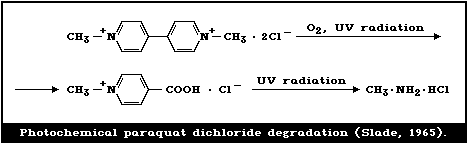 The rate of decomposition was related to the intensity of UV
radiation between 285 and 310 mµ present in daylight. In strong
sunlight, about 2/3 of the applied herbicide decomposed within a
3-week period. Vegetation directly sprayed with paraquat (1.12
kg/ha) was analysed at intervals up to 4 months. The residues
varied from 5 - 200 mg/kg. The 4-carboxyl-1-methylpyridynium
chloride ranged from 0.02 - 5 mg/kg (about 7% of the paraquat
residues determined on dry leaves). The toxicity of 4-carboxyl-1-
methylpyridylium for mammals was low, the acute oral LD50 in rats
being more than 5000 mg/kg body weight (FAO/WHO, 1971).
The degradation product from the photochemical destruction of
paraquat dimethylsulfate was N-methyl-isonicotinic acid
methylsulfate (Fig. 3).
A 90-day feeding test (Broadhurst et al., 1966) on rats
revealed that levels of 20 000 - 5000 mg/kg of the N-methyl-
isonicotinic acid methylsulfate were not toxic.
The rate of decomposition was related to the intensity of UV
radiation between 285 and 310 mµ present in daylight. In strong
sunlight, about 2/3 of the applied herbicide decomposed within a
3-week period. Vegetation directly sprayed with paraquat (1.12
kg/ha) was analysed at intervals up to 4 months. The residues
varied from 5 - 200 mg/kg. The 4-carboxyl-1-methylpyridynium
chloride ranged from 0.02 - 5 mg/kg (about 7% of the paraquat
residues determined on dry leaves). The toxicity of 4-carboxyl-1-
methylpyridylium for mammals was low, the acute oral LD50 in rats
being more than 5000 mg/kg body weight (FAO/WHO, 1971).
The degradation product from the photochemical destruction of
paraquat dimethylsulfate was N-methyl-isonicotinic acid
methylsulfate (Fig. 3).
A 90-day feeding test (Broadhurst et al., 1966) on rats
revealed that levels of 20 000 - 5000 mg/kg of the N-methyl-
isonicotinic acid methylsulfate were not toxic.
 4.1.2. Photochemical degradation of paraquat on soil and other
mineral surfaces
Slade (1966) showed that there was a breakdown, similar to that
on plant surfaces, if spots of paraquat on silica gel were exposed
to direct sunlight. When 14C-paraquat dichloride was sprayed on
the bare soil surface of a field during a hot sunny period, traces
of 4-carboxy-1-methylpyridynium chloride were detected in the top
inch of soil for the first few weeks afterwards (Calderbank &
Slade, 1976). Radioassay showed that the total soil residue did
not markedly decrease during a 6 - 18 month period, so that, in
agricultural practice, UV degradation of herbicide reaching the
soil should be regarded as insignificant.
The principal intermediates of photochemical paraquat
degradation on plants or soil surfaces are of low toxicity.
They decompose easily and are not expected to produce adverse
environmental effects.
4.2. Microbial Degradation
Microbial paraquat degradation has been thoroughly reviewed by
Haley (1979). Baldwin et al. (1966) identified many soil
microorganisms capable of degrading paraquat. The herbicide was
decomposed by Corynebacterium fascians, Clostridium pasteurianum,
and Lipomyces starkeyi. Several other microorganisms were found to
degrade paraquat (Smith et al., 1976; Tchipilska, 1980) but
Lipomyces starkeyi proved to be the most active (Burns & Audus,
1970). Burns & Audus (1970) concluded that microbiological
degradation was possible only for a short time following the
application of paraquat to soil. Once adsorbed on to clay
materials, the paraquat was inaccessible to microorganisms.
Microbial degradation of paraquat in the field is therefore
relatively slow.
Studies of 4-carboxyl-1-methylpyridylium chloride in soil have
demonstrated that the radiolabelled product readily decomposes to
form several chemical substances, including carbon dioxide. No
significant residues of the compound have been determined in plants
as a result of uptake from the soil. Wright & Cain (1970) isolated
Achromobacter D from the soil; this utilized the 4-carboxyl-1-
methylpyridylium chloride and the methylamine originating from
the N-methyl group of the molecule. The NADH and the oxygen
requirement indicated the possibility of direct oxidative
fission of a partly reduced ring to form dialdehyde, which was
then hydrolysed to formate, methylamine, and succinic dialdehyde.
The end-products of the microbial ring degradation were formate,
succinate, and carbon dioxide.
4.3. Environmental Adsorption and Transformation
4.3.1. Soil
The property of paraquat that is most important in nullifying
its impact on the environment is its rapid and complete binding to
clay soils. Desorption of the herbicide from soil particles, for
the purpose of chemical analysis, requires destruction of the
mineral particles by refluxing with strong sulfuric acid. The
strong adsorption to clay has been attributed to the flat and
highly polarizable nature of the paraquat ion (Coats et al., 1966;
Knight & Denny, 1970). Weber et al. (1965) reported that the
adsorption appeared to be one of ion exchange and was very rapid,
the rate of adsorption depending on the rate at which the paraquat
ion contacted the adsorbing particles.
In highly organic soils, the weaker adsorption sites of soil
organic matter delay the redistribution of paraquat without
inactivating it herbicidally. In this connection, Khan (1980)
reported tests showing a remarkable affinity of humic substances in
the soil for the paraquat ion. These humic substances enhance the
degradation of pesticides via non-biological pathways.
It has been demonstrated that on soil containing 98% organic
matter, the herbicidal effects of 1.12 and 2.24 kg of paraquat/ha
persisted for 16 - 29 days, but such soils are not widespread
naturally. Burns & Audus (1970) studied the migration of paraquat
from soil organic matter to clay mineral particles. The transfer
of the paraquat from the organic to the inorganic fraction, through
a membrane, was 90% complete within 6 h. The remaining 10% took
about 2 days to be transferred. No paraquat was detected in the
organic fraction after 4 days. At high paraquat concentrations
(more than 20 mg/kg in equilibrium solution), the total adsorption
capacity was greater than normal in soils with high organic
content, as opposed to those with low organic content.
Mithyanta & Perur (1975) studied samples of 4 different soils
treated with paraquat in different experimental schemes. After 24
h, the soils were extracted with a water solution of ammonium
chloride. The percentages of paraquat, extractable with water,
ranged from 4.8 - 66.9%, depending on the type of soil and the
conditions. Data on the persistence of paraquat in the soil have
also been compiled by Coats et al. (1966), Knight & Tomlinson
(1967), Knight & Denny (1970), and Burns & Audus (1970).
As summarised in section 4.2, free paraquat is degraded by a
range of microorganisms, but degradation of strongly adsorbed
paraquat is relatively slow. In plot studies, degradation was very
slow or non-detectable (Riley et al. 1976). However, in long-term
field studies, degradation rates were 5 - 10% per year. This is
greater than the rate required to prevent saturation of the
deactivation capacity of soils.
In a long-term trial on a loamy soil, plots were treated with
0, 90, 198, and 720 kg paraquat/ha, which was incorporated to a
depth of 15 cm. These rates were equivalent to 0, 50, 110, 400% of
the soils strong absorption capacity (Gowman et al., 1980;
Wilkinson, 1980; Riley, 1981). Over the 7 years, paraquat residues
declined by 5% per year (sig P = 0.05) on the 90 kg/ha plots and
by 7% per year (sig P = 0.01 on the 198 and 720 kg/ha plots. The
rate of decline on the 198 and 720 kg/ha plots was significantly
greater ( P = 0.01) than on the 90 kg/ha plots.
In another long-term trial on a sandy loam, plots were treated
annually with 4.4 kg/ha for 12 years (Hance et al, 1980). The rate
of loss of paraquat soil residues was about 10% per year and the
soil residues tended to plateau when the rate of application
equalled the rate of degradation. Data for the last 4 years (total
16 years) has confirmed the early results (Hance, unpublished
data).
Some paraquat could be recovered from its tightly bound form by
chemical destruction of the soil from field plots, several years
after application. The limit of paraquat adsorption, at which
further treatment would result in phytotoxic activity, was
considered to be important. Strong adsorption capacity was defined
as the measure of paraquat that can be adsorbed by the soil without
entailing phytotoxic effects, and this capacity was determined in
several kinds of soil with various clay and organic contents
(Knight & Tomlinson, 1967). Mechanical analyses, pH, and organic
matter content were also determined. Independently of the soils
studied, it was found that, by applying 1 kg/ha per year, it would
take from 30 - 1440 years to saturate the top 15 cm of soil at
strong adsorption sites. The conditions of study precluded any
form of paraquat degradation or metabolism in the soil. Riley et
al. (1976) reviewed the hazard of continuous application of 0.1 -
2 kg paraquat ion/ha, assuming soil contamination by 10 - 100% of
the amount applied. Bound paraquat soil residues were not adsorbed
by living organisms. Paraquat residues did not induce any effects
on microarthropods or microorganisms. Continued application of
the herbicide in different soils has been investigated by Pestemer
et al. (1979). The ED50 valuesa for phytotoxic action on lettuce
ranged from 0.01 mg/litre paraquat solution in agar-agar to 98 -
1930 mg/litre in different soils, depending on their constituents,
and 31 - 57.6 mg paraquat residues/kg have been determined in the
soil samples. There is evidence (Hance et al., 1980) that
strongly-bound paraquat residues were degraded in soil by microbial
activity at a rate of 5 - 10% per annum. A correlation was reported
between the paraquat residues, the number of treatments, doses, and
depth of soil sampling.
-------------------------------------------------------------------
a ED50 = median effective dose.
Although, as mentioned, adsorption on clay is important,
extremely sandy soils can adsorb and inactivate significant
quantities of the herbicide, as illustrated by studies on a South
African vineyard soil that contained only 1% clay (Riley et al.,
1976). Over an 8-year period, more than 20 applications (total
15.6 kg paraquat/ha) resulted in saturation of about 20% of the
soil-paraquat-strong-adsorption capacity in the top 2.5 cm. The
paraquat residues were not phytotoxic in the field or in greenhouse
tests on different plants. No paraquat residues were detected
(<0.05, <0.03, <0.03 mg/kg) in leaves, grapes, and twigs,
respectively.
Very low concentrations of free paraquat would be detected
easily by their phytotoxicity. Five trials at 4 sites were
conducted by Newman & Wilkinson (1971). In 4 of the trials, single
applications of paraquat at 112 kg/ha were made at sites subjected
to normal agricultural practice. At this unrealistic, extremely
high rate, short-duration residual phytotoxicity was observed. On
undisturbed plots of mineral soils, seedlings did not appear for
several months; on organic soils, the time lag was even longer.
After cultivation, there was no further indication of
phytotoxicity. In the 5th trial, a total of 565 kg/ha was applied
in 5 doses over 4 1/2 years. The plot then remained undisturbed,
apart from periodic cultivation of the top 20 mm to prepare a
seedbed. It was at this site that phytotoxicity to ryegrass
seedlings was detected, and free paraquat was determined in the
surface soil using the Lemna minor bioassay. Phytotoxicity was
confined to the surface layer of the soil. The free paraquat that
had leached out of the top 2.5 cm had been adsorbed in the deeper
soil layers, and this was confirmed by the absence of residual
phytotoxicity when the site was more deeply cultivated.
However, the extreme situations seen in high-dosage trials are
not encountered in practice and only serve to show the possible
consequences for the environment of a gross overdose of the
herbicide. Thus, when paraquat is used in normal application
doses, no adverse environmental effects can be expected.
Accidental spillage is probably the most likely cause of high
levels of residual paraquat. The 200 g of paraquat contained in 1
litre of Gramoxone(R) would be completely inactivated by the
addition of 10 kg of bentonite, for inactivation can be effected
either by cultivation and mixing other soil with the contaminated
layer or by adding clay minerals. Simulated spills of paraquat
have also been treated with sodium borohydride or alkali (Staiff et
al., 1981); within 1 day the paraquat in the soil had been
effectively degraded.
4.3.2. Water
The ecological effects of paraquat in water have been
studied in relation to its use as an aquatic herbicide at a
normal concentration of 1 mg/litre (Newman & Way, 1966; Grzenda et
al., 1966). Following this use, the concentration present in water
decreased to about half of the initial 1 mg/litre level within
36 h, and, in less than 2 weeks, the concentration was below
0.01 mg/litre. Weed-sample analysis, 4 days after paraquat
application, showed a residue of approximately 25 mg/kg, suggesting
that absorption by the weed was mainly responsible for paraquat
removal. Mud-residue analysis 5 1/2 months after treatment showed
that 36% of the applied paraquat remained in the mud, and 70% of
that was found in the top 2.5 cm. In the mud, paraquat had been
adsorbed on to the mineral material. Since bottom mud often has
organic components, the residues may be more accessible to
bacterial degradation. Compared to other products, paraquat
appears to be the herbicide of choice for future use in water
supplies because of its rapid disappearance from water (6 - 14 days
after treatment) (Grzenda et al., 1966). The residues were not
desorbed from the bottom sediments, and mud taken from the bottom
of a paraquat-treated lake carrying inactivated residues, showed no
toxic effects on barley seedlings that germinated on it (Way et
al., 1971).
Wauchope (1979) discussed the fate of pesticides in water
draining from fields after rain. For most formulations, a total
loss of 1.5%, or less, of the amount applied was the rule, except
when severe rainfall occurred within 1 - 2 months following
treatment. Nearly all the pesticides examined were lost by runoff;
only those binding strongly to clay particles, such as paraquat,
were carried off in the sediment phase of runoff. The lack of
paraquat runoff loss has also been discussed by Smith et al.
(1978).
Grover et al. (1980) compared the efficiency of various
herbicidal treatments for weed control in a series of irrigation
ditches. At the relatively low dose of 2.2 kg/ha, paraquat
resulted in aquatic weed suppression from 1973 to 1976, and this
made for satisfactory water flow without environmental
contamination. Water that contains small amounts of paraquat
residues loses them rapidly on contact with soil, the adsorption
process being irreversible (Knight & Tomlinson, 1967; Calderbank,
1972). Thus treated water may be used quite safely for channel
irrigation, if an interval of 10 days is observed between treatment
of the water and its use, because the paraquat will be unavailable
to the plant roots. Caution should, however, be exercised in
prolonged crop irrigation until the residue is well below 0.1
mg/litre, although phytotoxic damage is unlikely at even 0.5 mg
paraquat/litre (Calderbank, 1972).
Coats et al. (1966) treated 0.1 ha experimental ponds with
paraquat to obtain a concentration of 0.4 mg/litre. The soil in
one of the ponds was stirred twice after 24 h. Analysis of the
water over several weeks revealed a decrease from 0.4 mg/litre to
0.01 mg/litre after several weeks, but when the soil of the pond
was stirred, the paraquat concentration fell from 0.75 mg/litre
to <0.01 mg/litre after 8 - 12 days. In static water experiments,
the concentration of 0.5 - 1 mg/litre fell rapidly to about 0.1
mg/litre within 4 - 7 days of treatment in 4 trials performed by
Calderbank (1972). These reductions in the paraquat concentration
were due to its rapid adsorption and concentration in aquatic
plants. Decaying weeds transported it to the bottom mud (Table 4)
where it was not released back into the water (Way et al., 1971).
Earnest (1971) treated a pond with paraquat at an initial
concentration of 1.14 mg/litre. No residues were detected in the
water after 16 days (limit of detection 0.01 mg/litre); in the mud
the concentration was 1.13 mg/kg after 3 h and 3.25 mg/kg after 99
days. These data were confirmed by Grover et al. (1980).
Grover et al. (1980) studied irrigation water from ditches.
Three days after treatment with 2.2 kg paraquat/ha, the
concentrations in the water used to flood the treated ditches were
less than 0.01 mg/litre, and paraquat residues in the ditch water
ranged from 0.002 - 0.034 mg/litre in samples taken 3 - 5 days
after foliar applications.
Table 4. Residues of paraquat in water, weed, and bottom muda
-------------------------------------------------------------------
Days after treatment
-------------------------------------------------------------------
1 4 16 32 175 420
-------------------------------------------------------------------
Trial 1 water (mg/litre) 0.31 0.12 ND
weed (mg/kg) 13.70 25.80 21.0 0.55
mud (mg/kg) 3.70 - - - 57.1 20.1
Trial 2 water (mg/litre) 0.37 ND ND ND - -
weed (mg/kg) 25.50 40.0 37.8 27.8 - -
mud (mg/kg) ND 0.97 0.23 0.32 6.6 0.96
-------------------------------------------------------------------
a From: Way et al. (1971).
ND - not detectable.
4.3.3. Air
Paraquat is not volatile. Dry deposits of 14C-paraquat
chloride exposed at room temperature showed no measurable loss in
64 days (Coats et al., 1966). Exposure to paraquat in the air is
not important in spraying and harvesting operations; the skin is
the principal route of occupational exposure (Chester & Woollen,
1982; Staiff et al., 1975).
Air concentrations of paraquat were measured on summer days by
Makovskii (1972) using the method of Calderbank & Yuen (1965).
About 1 - 1.3 kg paraquat/ha had been applied as a herbicide or
desiccant in 0.25 - 0.35% water solutions. The paraquat aerosol
concentrations varied according to spraying method and work-place
(Table 5). Using the same analytical method, Staiff et al. (1975)
examined 35 sites after paraquat application with tractor-mounted
field sprayers or hand-pressure garden dispensers. The working
solutions contained 0.15% paraquat for field use, and 0.44% for
garden use. The respiratory exposure of field and garden operators
was below the limit of detection (<0.001 mg paraquat/h).
Mature cotton fields (Seiber & Woodrow, 1981) were sprayed with
paraquat, the dose being 0.94 kg/ha. The air paraquat
concentrations measured downwind decreased regularly from the
extrapolated interval-average values of 4.31 and 10.7 µg/m3 1
metre downwind of the 2 fields to <50 ng/m3 at 400 metres away in
the same direction. Forty-five percent of the aerosol particles
had diameters ranging from 0.01 to 4 µm. The remaining 55% had a
median diameter of 12 µm. Downwind samples taken 2 - 4 h after
spraying contained 1 - 10% of the amount dispersed, but, after
5 - 7 h, no paraquat was detectable in the air.
Table 5. Paraquat total airborne concentrations (mg/m3) in
working areasa
-------------------------------------------------------------------
Place of Number of Mean
sampling samples concentrations
± SE
-------------------------------------------------------------------
Working area sprayer loading 28 0.13 ± 0.03
tractor cabin 16 0.37 ± 0.07
(in direction of wind)
tractor cabin 16 0.55 ± 0.01
(against the wind)
manual spraying 16 0.18 ± 0.04
Treated field after 5 min 16 0.05 ± 0.01
after 10 min 32 < 0.01
after 20 min 16 0
Distance from 200 m 8 0.08 ± 0.01
treated field 400 m 8 0.04 ± 0.01
-------------------------------------------------------------------
a From: Makovskii (1972).
A study of Malaysian plantation workers, occupationally exposed
to paraquat, revealed a mean total airborne exposure of 0.97 mg/m3
for spray operators. This exposure is less than present TLVs
(Chester & Woollen, 1982). Wojeck et al. (1983) reported that
after spraying paraquat in fields of tomatoes and citrus, the total
airborne exposure ranged from 0 - 0.070 mg/h. It was less than
0.1% of the total body exposure (12.16 - 168.59 mg/h) in all
trials.
During mechanical harvesting of cotton dessicated by paraquat,
the maximum levels in airborne dust were found to be 1245 ng/m3
outside the cabin of the tractor and 516 ng/m3 inside the open
cabin. With the cabin door closed, the concentration was only 13.7
ng/m3. The trapped particulate matter consisted of dessicated
plant material and soil dust. A cascade impactor analysis
established that 57% of the paraquat had a median particle diameter
of 4 µm, 23%, 12 µm, and 11%, 3 µm. Cotton harvesting generated
parti- culate concentrations in the field comparable to those
immediately downwind of the field during spraying. Bearing in mind
the highest paraquat air concentration in the harvest-time air
(0.0012 mg/m3), a harvester operator's maximum exposure through
inhalation was calculated to be 0.01 mg/8 h/day (Seiber & Woodrow,
1981).
Bulgaria has established a maximum allowable concentration
(MAC) of 0.01 mg paraquat/m3 (1972), the Federal Republic of Germany
0.1 mg/m3 (1982), Hungary 0.02 mg/m3 (1978), and the USA a TLV of
0.1 mg/m3 (1982).
4.3.4. Plants
Paraquat residues on plants have been reviewed several times by
the Joint Meeting on Pesticide Residues (JMPR) (FAO/WHO, 1971,
1973, 1983). The residues found after paraquat was used as a
desiccant are summarized in Tables 6 and 7 (Calderbank, 1968).
Table 6. Paraquat residues (mg/kg) in cotton 10 days after
dessication at 0.55 kg/haa
-------------------------------------------------------------------
Fraction analysed Paraquat found
-------------------------------------------------------------------
Cotton as picked, including trash and balls 2.00
Ginned seed 0.18
Mechanically reginned seed 0.08
Acid-delinated seed 0.05
Lint cotton 3.00
Trash 3.70
Hulls 0.13
Crude oil ND
Meal 0.02
-------------------------------------------------------------------
a From: Calderbank (1968).
Coats et al. (1966) reported that 14C-paraquat applied to wheat
as a 1% solution was translocated in the plants, including the
roots. Slade (1966) studied the degradation of 14C-paraquat
dichloride and its photochemical degradation products in plants.
Maximum loss occurred in tomato, broad-bean, and maize when the
paraquat remained on the leaf surfaces during sunny days.
In potatoes treated with paraquat as a desiccant, Makovskii
(1972) found a residue of 0.05 mg/kg, and there was no change after
the potatoes had been boiled. No residues (limit of detection 0.01
mg/kg) were found in fruits (apples, citrus fruits, plums, pears),
tea, and cereals. In tests on sunflower seeds treated with 0.25 or
0.5 kg paraquat/ha, residues of up to 0.9 mg/kg were found in the
whole seed, up to 1.2 mg/kg in sunflower meal, and no residue in
the oil (Anonymous, 1979). Therefore, the use of sunflower meal in
the diet of hens, dairy cattle, and other livestock would not
result in paraquat levels exceeding current standards.
Table 7. Paraquat residues (mg/kg) in food crops 3 - 21 days
after dessicationa
-------------------------------------------------------------------
Crop Rate of application Paraquat found
(lb/acre)
-------------------------------------------------------------------
Barley 0.50 - 1.00 3 - 10
Wheat 0.50 - 1.00 1 - 2.5
Maize 0.50 - 1.20 ND - 0.2
Rice (with husk) 0.15 - 0.54 0.7 - 22
Rice (de-husked or polished) 0.15 - 0.54 ND - 0.2
Peas, beans, sunflower seed 0.35 - 1.20 ND - 0.2
Sorghum seed 0.25 - 1.00 0.1 - 0.4
Cotton (as picked) 0.50 - 1.00 2 - 3
Potatoes 0.50 - 1.50 0.02 - 0.13
Onions 0.50 - 2.00 ND - 0.05
Sugar cane juice 0.50 - 2.00 ND
Seed oils (sunflower, rape, up to 1.20 ND
sesame, cotton)
-------------------------------------------------------------------
a From: Calderbank (1968).
Seiber et al. (1979) determined the paraquat residues in
treated cotton (the foliage and bolls of the live plant, the lint
and seed of harvested cotton, the gin waste and the lint and
non-lint components). Gin waste residues were surveyed during
5 months of open storage. The paraquat dose had been 0.21 and
2.0 kg/ha. The results obtained are summarized in Table 8. The
minimal degradation of paraquat in the plants studied was confirmed
by Hills et al. (1981).
Significant paraquat residues are to be expected only when a
crop is directly sprayed.
After spraying fields of marijuana with paraquat for the
purpose of eradication, residues of paraquat were detected in
marijuana (Smith, 1978; Patrick, 1980). Of the 54 samples
collected in 1976, 7.4% were positive and of 46 samples collected
in 1977, 19.6% were positive.
Table 8. Paraquat residues (mg/kg) in cotton plantsa
-------------------------------------------------------------------
Material Days after Leaves Lint Non-lint Seeds
treatment
-------------------------------------------------------------------
Standing cotton plants 2 13.1 22.10 0.06
6 8.2 3.80 0.06
Harvested seed cotton 18 7.15 0.25
stored in field 49 4.85 0.18
Gin waste 49 2.7 9.3
119 5.3 10.1
171 5.8 9.7
-------------------------------------------------------------------
a From: Seiber et al. (1979).
4.3.5. Animals
The effects and fate of 14C-paraquat orally-administered to
cattle at 8 mg/kg body weight were studied by Stevens & Walley
(1966). Seven days after this single dose, 0.03 - 0.08 g/litre had
been excreted in the milk and 2.4 g/litre in the urine of the cows.
The total paraquat excretion in the milk was only 0.01% of the
ingested dose. In cows given daily oral doses of 8 mg paraquat/kg
for 3 weeks, residues of less than 0.01 mg/litre were detected in
the milk (FAO/WHO, 1977). Cattle did not suffer any toxic effects
over a 4-week period when turned loose on pasture immediately after
it had been sprayed with 1.12 kg paraquat/ha (Calderbank et al.
1968). During the first 2 weeks of grazing on the dried herbage,
it was estimated that the cattle ingested approximately half of
their acute oral LD50 (36 - 54 mg/kg body weight) every day.
Paraquat levels in the herbage ranged from about 400 mg/kg 1 day
after spraying, to about 200 mg/kg 14 days after treatment;
14 - 35 days after spraying the levels were 135 -214 mg/kg. The
4-carboxyl-1-methylpyridylium chloride content during the trial
period was 5.1 - 3.4 mg/kg. By the 4th week of the study, paraquat
levels in the urine were 0.01 - 0.19 mg/litre and in the faeces,
0.9 - 42 mg/kg. Only on the first day after spraying were paraquat
residues (0.02 mg/litre) found in the milk of 2 cows; no residues
were found (< 0.005 mg/litre) thereafter. The only organs of a
slaughtered animal that contained paraquat were the kidney
(0.03 mg/kg) and the stomach (0.05 mg/kg).
The fate of paraquat in large animals is addressed far more
completely in the Evaluations of the 1976 Joint Meeting on
Pesticide Residues (JMPR) (FAO/WHO, 1977).
Rabbits were fed with lucerne treated with normal-use levels of
paraquat (Lavaur et al., 1979). Immediately after spraying, the
paraquat residues were 272 mg/kg (dry weight of lucerne). After
24 h and 48 h, they were 114 mg/kg and 62 mg/kg, respectively. No
systemic toxicity symptoms or gastrointestinal damage were observed
in the treated rabbits.
When hens were given paraquat at 40 mg/litre in their drinking-
water for 14 days, the amount of paraquat found in the eggs rose to
0.1 mg/kg, but fell to less than 0.005 mg/kg, 6 days after
cessation of treatment (Fletcher, 1967). Eggs from hens eating
grain containing paraquat at a concentration of 10 mg/kg contained
residues below 0.025 mg/kg.
5. BIOLOGICAL ACTIVITY OF RESIDUES
5.1. Soil Organisms
Haley (1979) reviewed the effects of paraquat on soil
microorganisms and fungi, while Tu & Bollen (1968), Curry (1970),
Radaelli & Martelli (1971), Roslycky (1977), and Smith et al.
(1981a) studied the effects of paraquat on the size and composition
of the microbial soil populations, total microbial respiration in
the soil, the rate of organic matter degradation, and the number of
soil microorganisms. None of these authors found any adverse
ecological effects from normal and excessive (up to 32 times the
normal dose) paraquat treatment, although in some cases
nitrification was temporarily suppressed or activated, and some
bimodal microbiological effects were observed with intermediate
herbicide concentrations (Tu & Bollen, 1968; Tchipilska, 1980).
At normal doses, paraquat had no adverse effect on
endomycorrhiza formation and function (Smith et al., 1981a), on
total populations of bacteria, actynomyces, fungi (Roslycky, 1977;
Haley, 1979; Tchipilska, 1980; Smith et al., 1981a), or on 24
different species of soil fauna taken from 2 plots at a depth of
3.8 cm (Curry, 1970).
Curry (1970), and Riley et al. (1976) made extensive
studies of the effects of normal and high doses of paraquat on
microarthropod and earthworm populations at sites at different
stages of cultivation. The herbicide was neither harmful nor
repellant to earthworms, nor was there any evidence of a toxic
effect or of paraquat accumulation in any species examined. When
the residues in the top 2.5 cm of soil reached 20 mg/kg, the
highest concentration determined in Allolbophora caliginosa, living
near the surface, was 3.2 mg/kg (live weight). Worms from highly-
dosed plots eliminated paraquat residues within 36 h, when placed
in clean soil.
5.2. Effects of Residues on Crop Yields
The absence of adverse effects from residual paraquat on the
growth and yield of crops grown in paraquat-treated soils has been
demonstrated by Knight & Tomlinson (1967), Damanakis et al. (1970),
Newman & Wilkinson (1971), and Riley et al. (1976). It is known
that the paraquat-inactivation capacity of soils varies widely.
Paraquat has been tested on soils of low adsorption capacity, it
has been used repeatedly on the same soil (section 4.3.1) and has
been tested at extremely high concentrations. The absence of any
reports or observations of long-term phytotoxic effects confirms
the data obtained in greenhouse and laboratory studies.
5.3. Effects on Fish and Aquatic Organisms
Despite variation in LC50s for fish (67 - 110 mg/litre after
24 h, 38 - 62 mg/litre after 48 h, more than 25 - 32 mg/litre after
96 h), the herbicide has proved to have a wide margin of safety for
warm- and cold-water fish species (Calderbank, 1972). The toxicity
of paraquat for fish varies with the species, the size of the
fish, and the softness or hardness of the water. A large number
of aquatic species have shown a 100% survival at 96 mg/litre over
96 h, though the decreased oxygen concentration following decay of
weeds, may be dangerous in extreme situations. Rainbow trout
tolerated 1 mg paraquat/litre water in prolonged toxicity tests
and only a 30% mortality was recorded after 16 days of repeated
exposure (Calderbank & Slade, 1976). At the end of the test, 0.54
mg paraquat/kg was found in the rainbow trout. In a 7-day exposure
test at 1 mg paraquat/litre, the herbicide was detected in the gut
(0.41 mg/kg) and liver (0.35 mg/kg), but not in the meat of the
fish (< 0.025 mg/kg). Water snails collected from 2 ditches,
12 weeks after treatment of the waters with 1 mg/litre were found
to contain 0.43 mg herbicide/kg. Fish (major carp fingerlings)
exposed to paraquat in the presence of weeds were more susceptible
than those in weed-free environments (Singh & Yadav, 1978), owing
to the changed oxygen content of the water. Where there is heavy
weed growth, the oxygen taken up by weed decay may dangerously
reduce the oxygen available for aquatic organisms. To avoid this,
as far as possible, paraquat should be applied before weed growth
becomes dense and only to one part of the water-course or lake at
a time (FAO/WHO, 1973).
5.4. Effects on Birds
Paraquat is less toxic for birds than for mammals. The acute
oral LD50 for the hen is 262 - 380 mg/kg body weight (Table 11).
The acute oral and 24-h percutaneous (applied to feet) LD50 for
mallards are 200 and 600 mg/kg body weight, respectively (Hudson et
al., 1979). For duck, pheasants, and quail, LC50 values of
paraquat when mixed in the diet are 1000 mg/kg of food or more
(Summers, 1980); residues on sprayed vegetation would not therefore
be expected to present a hazard for birds.
When paraquat was sprayed directly on to pheasants' eggs before
incubation, treatment rates up to 2 kg paraquat/ha did not have any
effect on egg hatchability or on the birds' reproductive organs
(Newman & Edwards, 1980). In a similar study with Japanese quail
eggs, sprays containing paraquat levels of up to 3 kg/ha did not
have any effect on hatchability or development of reproductive
organs (Edwards et al., 1979). Thus, normal spray rates should not
induce any adverse effects, even if paraquat is sprayed directly on
eggs.
Bird populations have been monitored in detail, over a 5-year
period, on a farm in the United Kingdom where paraquat use was much
higher than normal; the average application to the whole arable
area was 0.6 kg/ha per year. The paraquat was applied beneath
hedgerows and along fence lines. The farm maintained an excellent
wild bird population (40 species), including ground-nesting birds
(Edwards, 1979). Most species were at a similar or greater density
than the national average in the United Kingdom.
The Ministry of Agriculture, Fisheries, and Food in the United
Kingdom has carried out detailed investigations on mammalian and
avian deaths that could have been caused by pesticides. For the
period 1971 - 81, the normal use of pesticide was not found to have
caused any significant adverse effects on mammals and birds (MAFF,
1980a, 1981). The Ministry concluded, "It is widely believed that
the use and misuse of paraquat is responsible for a considerable
number of wildlife casualties. There is no evidence from the
investigations to support this allegation...." (MAFF, 1980b).
6. KINETICS AND METABOLISM
6.1. Animal Studies
6.1.1. Absorption
6.1.1.1. Oral absorption
Daniel & Gage (1966) studied the absorption of 14C-paraquat
following oral and subcutaneous single-dose administration to rats.
About 76 - 90% of the oral doses were found in the faeces, and
11 - 20% in the urine; most of the subcutaneous dose (73 - 88%)
was found in the urine and only 2 - 14.2% in the faeces. This,
together with the absence of marked biliary excretion, was evidence
that paraquat was poorly absorbed from the gut. This low rate of
absorption was confirmed by Litchfield et al. (1973) and Conning et
al. (1969). Rats, guinea-pigs, and monkeys orally administered
LD50 doses of 14C-paraquat had low peak serum concentrations
(2.1 - 4.8 mg/litre) (Murray & Gibson, 1974). The radioactivity
levels reached a maximum 30 - 60 min after administration and then
remained relatively constant for 32 h. A dose of 126 mg/kg body
weight resulted in a rat serum level of 4.8 - 4.7 mg/litre.
In fasting dogs, low oral doses of paraquat were rapidly but
incompletely absorbed, the peak plasma concentration being attained
75 min after dosing (Bennett et al., 1976). After an oral dose of
0.12 mg/kg body weight, 46 - 66% was absorbed in 6 h. For doses of
2 - 5 mg/kg, only 22 - 38% and 25 - 28% of the dose was absorbed,
respectively. Dose-dependent data from dogs and whole-body
autoradiography suggest that absorption is facilitated in the small
intestine. Some non-ionic surfactants (0.001%) increased 14C-
paraquat transport through isolated gastric mucosa models, but
histological evaluation suggested that this was due to damage of
the epithelial cell membranes (Walters et al., 1981).
6.1.1.2. Pulmonary absorption
Absorption of paraquat following instillation and inhalation in
the lung has been described in several studies (Gage, 1968a;
Kimbrough & Gaines, 1970; Seidenfeld et al., 1978; Popenoe, 1979).
The uptake of 14C-paraquat after an intratracheal injection of
1.86 nmol/lung was investigated in the isolated perfused rat lung
by Charles et al. (1978). The efflux of 14C-paraquat was diphasic
with a rapid phase half-life of 2.65 min and a slow phase half-life
of 356 min. It was suggested that the slow phase represented a
storage pool, possibly responsible for the pulmonary toxicity of
paraquat. Various doses of 3H-paraquat (10-5 - 10-12 g) in 0.1 ml
saline were introduced directly into the left bronchus of rats
(Wyatt et al., 1981). Fifteen min after instilling 10-8 of 3H-
paraquat, 90% of the ion could be accounted for in the tissues and
urine, 50% being present in the lung. With doses at or greater
than 10-5 g, pathological changes were seen in the lung, similar to
those seen after systemic poisoning. Zavala & Rhodes (1978)
reported that the lung of the rabbit was highly sensitive to
paraquat intrabronchial instillation in doses ranging from
0.1 g - 1 pg; moderately sensitive to intraveneously administered
paraquat (25 mg/kg body weight); resistant to the herbicide when
given intraperitoneally or subcutaneously (25 mg/kg body weight).
6.1.1.3. Dermal absorption
Paraquat absorption through animal and human skin has been
studied using an in vitro technique (Walker et al., 1983). Human
skin was shown to be impermeable to paraquat, having a very low
permeability constant of 0.73. Furthermore, human skin was found
to be at least 40 times less permeable than animal skins tested
(including rat, rabbit, and guinea-pig). There are no in vivo
studies on the rate of absorption of paraquat through the skin.
However, observations of dose-related dermal toxicity in
experimental animals and human percutaneous poisoning have provided
some qualitative information concerning the dermal absorption of
paraquat (further discussed in section 8.2.2.2).
6.1.2. Distribution
Since the most characteristic feature of paraquat toxicity is
lung damage, it is important to stress the high concentrations and
retention of paraquat in the lung tissues, relative to other
tissues, following oral, intravenous, intraperitoneal,
subcutaneous, and intrabronchial routes of administration in rats,
guinea-pigs, and monkeys (Sharp et al., 1972; Ilett et al., 1974;
Murray & Gibson, 1974; Kurisaki & Sato, 1979; Waddell & Marlowe,
1980). An association between paraquat concentrations in the lung
and degree of toxicity or lung injury has been reported (Sharp et
al., 1972; Ilett et al., 1974; Waddell & Marlowe, 1980; Wyatt et
al., 1981). Some of their data are summarized in Tables 9 and 10.
Table 9. Paraquat distribution in tissues
---------------------------------------------------------------------------
Route of entry Dose Species Time after Tissue Concentration
treatment
---------------------------------------------------------------------------
1. Intrabronchial 10 ng rat 60 min plasma 0.0092 µg/litre
lung 5.2 ng
kidney 0.052 ng
liver -
heart -
brain -
2. Intravenous 20 mg/kg rat 24 h plasma 0.7 mg/litre
lung 8.0 mg/kg
kidney 1.45 mg/kg
liver 0.48 µg/g
heart 0.75 mg/kg
brain -
---------------------------------------------------------------------------
Table 9. (contd.)
---------------------------------------------------------------------------
Route of entry Dose Species Time after Tissue Concentration
treatment
---------------------------------------------------------------------------
3. Intravenous 20 mg/kg rat 24 h plasma ND
lung 11.36 µm/kg
kidney 1.93 µmol/kg
liver 0.90 µmol/kg
heart 1.13 µmol/kg
brain 0.87 µmol/kg
20 mg/kg rabbit 24 h plasma 0.28 µmol/litre
lung 7.9 nm/g
kidney 5.25 µmol/kg
liver 1.59 µmol/kg
heart 1.52 µmol/kg
brain 0.49 µmol/kg
4. Intraperitoneal 15 mg/kg rat 24 h plasma 0.32 µmol/litre
lung 26.28 µm/kg
kidney 10.4 µmol/kg
liver 5.04 µmol/kg
heart 4.59 nmol/g
brain 1.22 µmol/kg
5. Oral 126 mg/kg rat 16 h plasma 0.90 mg/litre
lung 5.0 mg/kg
kidney 7.00 mg/kg
liver 2.1 mg/kg
heart 2.7 mg/kg
brain -
22 mg/kg guinea- 16 h plasma 0.03 mg/litre
pig lung 1.29 mg/kg
kidney 1.99 mg/kg
liver 0.08 mg/kg
heart 0.31 mg/kg
brain -
---------------------------------------------------------------------------
1. From: Wyatt et al. (1981).
2. From: Sharp et al. (1972).
3. From: Ilett et al. (1974).
4. From: Maling et al. (1978).
5. From: Murray & Gibson (1974).
Table 10. Paraquat distribution in tissues (in mg/kg (mean) tissue)
----------------------------------------------------------------------------
Route of Entry Dose Species Time Lung Kidney Liver Heart Plasma
(mg/kg after
body dosing
weight)
----------------------------------------------------------------------------
1. Oral 126 rat 1 h 3.3 27.5 2.0 1.8 4.7
4 h 3.7 4.5 4.4 0.9 0.8
32 h 13.6 9.4 5.7 2.8 1.1
64 h 1.7 1.0 7.7 0.2 0.1
2. Intravenous 20 rat 1 h 9.0 25.0 5.0 - 6.0
4 h 8.0 6.0 2.0 - 0.3
24 h 6.0 1.0 0.4 - 0.07
2 days 4.0 0.8 0.3 - 0.05
----------------------------------------------------------------------------
1. From: Murray & Gibson (1974).
2. From: Sharp et al. (1972).
Toxic doses of paraquat were administered orally and iv to rats
(Sharp et al., 1972). Paraquat concentrations in the whole blood
were the same as those in the plasma. The distribution of the
herbicide in various tissues was then followed for 10 - 18 days.
The lung had the greatest retention and consequently contained the
highest concentration 4 h after dosing. Four to 10 days after
dosing, the paraquat concentration in the lung was 30 - 80 times
higher than that in the plasma. The high lung-tissue
concentrations of paraquat were confirmed by Ilett et al. (1974)
for rats and rabbits after iv injection of 20 mg 14C-paraquat/kg
body weight. Although the herbicide showed a selective
localization in rabbit lung, the concentration decreased far more
rapidly in rabbit lung than in rat lung. The rabbit did not show
any histological or biochemical signs of lung damage, and no
evidence of covalent binding of paraquat in lung tissue was found
by Ilett et al. (1974). After thorough washing of tissue
precipitate with dilute trichloroacetic acid, only insignificant
amounts of 14C-paraquat were detected in protein from the brain,
heart, kidney, liver, lung, and plasma.
Autoradiographic studies using 14C-paraquat have been carried
out on mice and rats (Litchfield et al., 1973). Paraquat was
observed in nearly all organs 10 min after intravenous injection of
20 mg/kg body weight. Waddell & Marlowe (1980) obtained similar
autoradiographic results in mice, after intravenous injection of
288 - 338 µg 3H-paraquat dichloride/kg body weight. Cellular
resolution autoradiography showed that paraquat was confined almost
entirely to cells having the distribution of alveolar Type II
cells. These cells are known to be susceptible to the toxicity of
paraquat (Kimbrough & Gaines, 1970). Waddell & Marlowe (1980)
suggested that it was unlikely that the radioactivity was bound to
cellular constituents.
No paraquat was detected in rat kidney, brain, liver, or lung
when paraquat was administered in the diet at a concentration of
50 mg/kg for a period of 8 weeks. At 120 mg/kg, it was found in
low concentrations in the lung, kidney, gastrointestinal system,
and brain (Litchfield et al., 1973). At 250 mg/kg, it was detected
in the tissues within 2 weeks. No sex differences or any clear
pattern of accumulation were noted throughout the 8-week study.
Within 1 week of return to a normal diet, no paraquat was detected
in any tissue examined. Histological changes were observed in all
lungs of animals fed paraquat at 250 mg/kg diet.
Rose et al. (1974a) demonstrated an energy-dependent
accumulation of paraquat in slices of rat lung that obeyed
saturation kinetics. The same investigators also examined the
ability of paraquat to accumulate in tissue slices from other
organs in vitro (Rose & Smith, 1977). The herbicide in brain,
adrenal gland, and kidney slices accumulated; however, the uptake
was less than 10% of that observed in the lung slices. The authors
established the uptake of paraquat by the lung in various species
(rat, rabbit, dog, monkey, man). The human lung accumulated
paraquat as strongly as that of the rat and there was a
relationship between the concentration of paraquat in the different
lung areas and the development of microscopic lung lesions. It has
been demonstrated that the rate of paraquat efflux from lung tissue
is less than its rate of accumulation in the lung slices (Smith et
al., 1981). Efflux from lung slices, prepared from rats dosed iv
with the herbicide, was found to be biphasic. There was a fast
component (half-life 20 min), followed by a first-order slow
component characterized by a half-life of 17 h. The half-life in
vitro was similar to that seen in vivo following iv
administration to rats.
6.1.3. Metabolic transformation and excretion
Paraquat participates to a considerable extent in cyclic
reduction-oxidation reactions. After undergoing a single electron
reduction in tissues, the resultant free radical is readily
oxidized by molecular oxygen to the parent compound (section 6.3).
This leads to an overall excretion of essentially unchanged
paraquat in the urine after oral administration to rats (Murray &
Gibson, 1974).
Daniel & Gage (1966) reported that paraquat was metabolized by
gut microflora following oral dosing of rats. This observation was
not confirmed in subsequent studies (Murray & Gibson, 1974) and was
later attributed to a problem with the method (FAO/WHO, 1977).
Urinary concentrations of paraquat following oral
administration are relatively low (Daniel & Gage, 1966: Murray &
Gibson, 1974; Sharp et al., 1972; Maling et al., 1978) and are thus
used to estimate its elimination from the body.
Sharp et al. (1972) reported a biphasic elimination of paraquat
from the plasma of rats after iv injection. The initial rapid
phase had a 20 - 30 min half-life, and the slower phase a half-life
of 56 h. Murray & Gibson (1974) also showed prolonged paraquat
elimination after oral administration to rats, guinea-pigs, and
monkeys. The urinary and faecal routes were equally important in
all species studied. The faecal content was due mainly to
elimination of unabsorbed paraquat. Prolonged elimination of
paraquat in all animals tested indicated retention of the herbicide
in the body.
Following iv administration to rats, about 75 - 79% of the dose
was excreted in the urine within 6 h (Maling et al., 1978). The
plasma disappearance of an iv dose of paraquat of 5 mg/kg was
fitted to a 3-compartment model. Total body clearance was
estimated to be 8.39 ± 0.54 ml/kg per min (Maling et al., 1978).
The relatively high concentration of paraquat in the duodenal and
jejunal walls suggested biliary secretion of the herbicide, and the
authors' hypothesis was supported by the observation of radio-
activity in the intestines of mice in whole-body autoradiographic
studies (Waddell & Marlowe, 1980).
Since absorbed paraquat is mainly removed via the kidneys, the
early onset of renal failure will have a marked effect on paraquat
elimination and distribution, including accumulation in the lung.
Hawksworth et al. (1981) used the dog as a model to evaluate the
influence of paraquat-induced renal failure on the kinetics of
paraquat elimination. After iv injection of a trace dose of 14C-
paraquat (30 - 50 µg/kg body weight) in dogs, the kinetics of
distribution was described by a 3-compartment model. To obtain a
good fit of the curve, it was necessary to sample the central
(plasma) compartment for at least 24 h after dosing. Simulation of
paraquat levels in the peripheral compartments suggested the
existence of a compartment with rapid uptake and removal (kidney)
and another with slow uptake (lung). The renal clearance of
paraquat approximated total body clearance indicating that paraquat
elimination occurs through renal excretion. The urinary excretion
rate of an iv dose was rapid, approximately 80 - 90% of the dose
being eliminated during the first 6 h. Intravenous injection of a
large toxic dose of paraquat (20 mg/kg body weight), however,
brought about a marked decrease in renal clearance, from 73 ml/min
to 18 ml/min after 2 1/2 h and 2 ml/min after 6 h. This data
suggested that damaged renal tubules could contribute to paraquat
accumulation in the lung.
6.2. Observations on Human Beings
6.2.1. Observations on paraquat poisoning after ingestion:
non-fatal cases
Tompsett (1970) reported a case of ingestion of 45 g of Weedol
(2.5% paraquat). On hospital admission, the gastric aspirate
contained 0.215 g paraquat/litre and the urine 0.148 g/litre.
After 2 - 4 h, paraquat concentrations dropped to 5.1 mg/litre in
the urine and 0.4 mg/litre in the serum but, 16 - 24 h after
admission, the urinary level was 0.95 mg/litre, while no paraquat
was detectable in the serum. Paraquat was also detected in the
urine for up to 15 days after poisoning, while at the same time
serum concentrations were below the detectable limits in chemical
analysis (Fletcher, 1975).
The cumulative elimination of paraquat in the faeces and urine
of a patient was followed for 7 days by van Dijk et al. (1975).
Faecal elimination increased from 340 mg the first day to 530 mg
after 7 days, while cumulative urinary excretion reached 60 mg the
lst day and increased to 75 mg after 7 days. It was calculated
that only 87 mg of paraquat had been absorbed from a total
ingestion of about 637 mg, determined in the urine, dialysate, and
faeces. In this patient, less than 14% of the ingested paraquat
was absorbed through the gastrointestinal system.
6.2.2. Observations on paraquat poisoning after ingestion:
fatal cases
It is well established that paraquat lung disease resulting in
death is usually preceded or accompanied by renal insufficiency.
This contributes to the retention of paraquat in body tissues.
Nevertheless, Fairshter et al. (1979) detected only small
concentrations (below 0.09 mg/kg) of paraquat in several organs of
patients who died 3 weeks after ingestion.
The detection of 27 mg paraquat/litre in the bile of a woman
after autopsy suggested that some faecal paraquat might be
attributable to biliary excretion (Dijk et al., 1975).
6.2.3. Significance of paraquat concentrations in cases of
paraquat poisoning
Not only oral ingestion, but also dermal absorption of paraquat
after occupational overexposure, resulted in measurable urinary
levels of paraquat. The determination of paraquat in urine and
serum is an important biological exposure test for the diagnosis
and the prognosis in cases of human poisoning.
Wright et al. (1978) followed the urinary excretion of paraquat
in 16 patients (7 of whom died). The total amount of paraquat
excreted ranged from 0.6 mg to 386 mg. The excretion rate
decreased rapidly during the 48 h following ingestion, though less
rapidly in the patients who eventually died. All patients
excreting 1 mg of paraquat or more per hour, for 8 h or more after
ingestion, died.
Plasma-paraquat concentrations were measured by gas
chromatography, radioimmunoassay, and colorimetric methods in 79
patients with paraquat poisoning (Proudfoot & Stewart, 1979). At
any given time after ingestion (within a limit of 35 h), plasma
concentrations were significantly higher in the patients who died
(Fig. 4). Patients whose plasma concentrations were not higher
than 2.0, 0.6, 0.3, 0.16, and 0.10 mg paraquat/litre at
respectively, 4 h, 6 h, 10 h, 16 h, and 24 h after the poisoning,
were likely to survive. When plasma levels exceeded 0.3 mg/litre
15 h after ingestion, a fatal outcome could be expected, despite
treatment. These conclusions were supported by the studies
performed on 28 patients by Bismuth et al. (1982).
4.1.2. Photochemical degradation of paraquat on soil and other
mineral surfaces
Slade (1966) showed that there was a breakdown, similar to that
on plant surfaces, if spots of paraquat on silica gel were exposed
to direct sunlight. When 14C-paraquat dichloride was sprayed on
the bare soil surface of a field during a hot sunny period, traces
of 4-carboxy-1-methylpyridynium chloride were detected in the top
inch of soil for the first few weeks afterwards (Calderbank &
Slade, 1976). Radioassay showed that the total soil residue did
not markedly decrease during a 6 - 18 month period, so that, in
agricultural practice, UV degradation of herbicide reaching the
soil should be regarded as insignificant.
The principal intermediates of photochemical paraquat
degradation on plants or soil surfaces are of low toxicity.
They decompose easily and are not expected to produce adverse
environmental effects.
4.2. Microbial Degradation
Microbial paraquat degradation has been thoroughly reviewed by
Haley (1979). Baldwin et al. (1966) identified many soil
microorganisms capable of degrading paraquat. The herbicide was
decomposed by Corynebacterium fascians, Clostridium pasteurianum,
and Lipomyces starkeyi. Several other microorganisms were found to
degrade paraquat (Smith et al., 1976; Tchipilska, 1980) but
Lipomyces starkeyi proved to be the most active (Burns & Audus,
1970). Burns & Audus (1970) concluded that microbiological
degradation was possible only for a short time following the
application of paraquat to soil. Once adsorbed on to clay
materials, the paraquat was inaccessible to microorganisms.
Microbial degradation of paraquat in the field is therefore
relatively slow.
Studies of 4-carboxyl-1-methylpyridylium chloride in soil have
demonstrated that the radiolabelled product readily decomposes to
form several chemical substances, including carbon dioxide. No
significant residues of the compound have been determined in plants
as a result of uptake from the soil. Wright & Cain (1970) isolated
Achromobacter D from the soil; this utilized the 4-carboxyl-1-
methylpyridylium chloride and the methylamine originating from
the N-methyl group of the molecule. The NADH and the oxygen
requirement indicated the possibility of direct oxidative
fission of a partly reduced ring to form dialdehyde, which was
then hydrolysed to formate, methylamine, and succinic dialdehyde.
The end-products of the microbial ring degradation were formate,
succinate, and carbon dioxide.
4.3. Environmental Adsorption and Transformation
4.3.1. Soil
The property of paraquat that is most important in nullifying
its impact on the environment is its rapid and complete binding to
clay soils. Desorption of the herbicide from soil particles, for
the purpose of chemical analysis, requires destruction of the
mineral particles by refluxing with strong sulfuric acid. The
strong adsorption to clay has been attributed to the flat and
highly polarizable nature of the paraquat ion (Coats et al., 1966;
Knight & Denny, 1970). Weber et al. (1965) reported that the
adsorption appeared to be one of ion exchange and was very rapid,
the rate of adsorption depending on the rate at which the paraquat
ion contacted the adsorbing particles.
In highly organic soils, the weaker adsorption sites of soil
organic matter delay the redistribution of paraquat without
inactivating it herbicidally. In this connection, Khan (1980)
reported tests showing a remarkable affinity of humic substances in
the soil for the paraquat ion. These humic substances enhance the
degradation of pesticides via non-biological pathways.
It has been demonstrated that on soil containing 98% organic
matter, the herbicidal effects of 1.12 and 2.24 kg of paraquat/ha
persisted for 16 - 29 days, but such soils are not widespread
naturally. Burns & Audus (1970) studied the migration of paraquat
from soil organic matter to clay mineral particles. The transfer
of the paraquat from the organic to the inorganic fraction, through
a membrane, was 90% complete within 6 h. The remaining 10% took
about 2 days to be transferred. No paraquat was detected in the
organic fraction after 4 days. At high paraquat concentrations
(more than 20 mg/kg in equilibrium solution), the total adsorption
capacity was greater than normal in soils with high organic
content, as opposed to those with low organic content.
Mithyanta & Perur (1975) studied samples of 4 different soils
treated with paraquat in different experimental schemes. After 24
h, the soils were extracted with a water solution of ammonium
chloride. The percentages of paraquat, extractable with water,
ranged from 4.8 - 66.9%, depending on the type of soil and the
conditions. Data on the persistence of paraquat in the soil have
also been compiled by Coats et al. (1966), Knight & Tomlinson
(1967), Knight & Denny (1970), and Burns & Audus (1970).
As summarised in section 4.2, free paraquat is degraded by a
range of microorganisms, but degradation of strongly adsorbed
paraquat is relatively slow. In plot studies, degradation was very
slow or non-detectable (Riley et al. 1976). However, in long-term
field studies, degradation rates were 5 - 10% per year. This is
greater than the rate required to prevent saturation of the
deactivation capacity of soils.
In a long-term trial on a loamy soil, plots were treated with
0, 90, 198, and 720 kg paraquat/ha, which was incorporated to a
depth of 15 cm. These rates were equivalent to 0, 50, 110, 400% of
the soils strong absorption capacity (Gowman et al., 1980;
Wilkinson, 1980; Riley, 1981). Over the 7 years, paraquat residues
declined by 5% per year (sig P = 0.05) on the 90 kg/ha plots and
by 7% per year (sig P = 0.01 on the 198 and 720 kg/ha plots. The
rate of decline on the 198 and 720 kg/ha plots was significantly
greater ( P = 0.01) than on the 90 kg/ha plots.
In another long-term trial on a sandy loam, plots were treated
annually with 4.4 kg/ha for 12 years (Hance et al, 1980). The rate
of loss of paraquat soil residues was about 10% per year and the
soil residues tended to plateau when the rate of application
equalled the rate of degradation. Data for the last 4 years (total
16 years) has confirmed the early results (Hance, unpublished
data).
Some paraquat could be recovered from its tightly bound form by
chemical destruction of the soil from field plots, several years
after application. The limit of paraquat adsorption, at which
further treatment would result in phytotoxic activity, was
considered to be important. Strong adsorption capacity was defined
as the measure of paraquat that can be adsorbed by the soil without
entailing phytotoxic effects, and this capacity was determined in
several kinds of soil with various clay and organic contents
(Knight & Tomlinson, 1967). Mechanical analyses, pH, and organic
matter content were also determined. Independently of the soils
studied, it was found that, by applying 1 kg/ha per year, it would
take from 30 - 1440 years to saturate the top 15 cm of soil at
strong adsorption sites. The conditions of study precluded any
form of paraquat degradation or metabolism in the soil. Riley et
al. (1976) reviewed the hazard of continuous application of 0.1 -
2 kg paraquat ion/ha, assuming soil contamination by 10 - 100% of
the amount applied. Bound paraquat soil residues were not adsorbed
by living organisms. Paraquat residues did not induce any effects
on microarthropods or microorganisms. Continued application of
the herbicide in different soils has been investigated by Pestemer
et al. (1979). The ED50 valuesa for phytotoxic action on lettuce
ranged from 0.01 mg/litre paraquat solution in agar-agar to 98 -
1930 mg/litre in different soils, depending on their constituents,
and 31 - 57.6 mg paraquat residues/kg have been determined in the
soil samples. There is evidence (Hance et al., 1980) that
strongly-bound paraquat residues were degraded in soil by microbial
activity at a rate of 5 - 10% per annum. A correlation was reported
between the paraquat residues, the number of treatments, doses, and
depth of soil sampling.
-------------------------------------------------------------------
a ED50 = median effective dose.
Although, as mentioned, adsorption on clay is important,
extremely sandy soils can adsorb and inactivate significant
quantities of the herbicide, as illustrated by studies on a South
African vineyard soil that contained only 1% clay (Riley et al.,
1976). Over an 8-year period, more than 20 applications (total
15.6 kg paraquat/ha) resulted in saturation of about 20% of the
soil-paraquat-strong-adsorption capacity in the top 2.5 cm. The
paraquat residues were not phytotoxic in the field or in greenhouse
tests on different plants. No paraquat residues were detected
(<0.05, <0.03, <0.03 mg/kg) in leaves, grapes, and twigs,
respectively.
Very low concentrations of free paraquat would be detected
easily by their phytotoxicity. Five trials at 4 sites were
conducted by Newman & Wilkinson (1971). In 4 of the trials, single
applications of paraquat at 112 kg/ha were made at sites subjected
to normal agricultural practice. At this unrealistic, extremely
high rate, short-duration residual phytotoxicity was observed. On
undisturbed plots of mineral soils, seedlings did not appear for
several months; on organic soils, the time lag was even longer.
After cultivation, there was no further indication of
phytotoxicity. In the 5th trial, a total of 565 kg/ha was applied
in 5 doses over 4 1/2 years. The plot then remained undisturbed,
apart from periodic cultivation of the top 20 mm to prepare a
seedbed. It was at this site that phytotoxicity to ryegrass
seedlings was detected, and free paraquat was determined in the
surface soil using the Lemna minor bioassay. Phytotoxicity was
confined to the surface layer of the soil. The free paraquat that
had leached out of the top 2.5 cm had been adsorbed in the deeper
soil layers, and this was confirmed by the absence of residual
phytotoxicity when the site was more deeply cultivated.
However, the extreme situations seen in high-dosage trials are
not encountered in practice and only serve to show the possible
consequences for the environment of a gross overdose of the
herbicide. Thus, when paraquat is used in normal application
doses, no adverse environmental effects can be expected.
Accidental spillage is probably the most likely cause of high
levels of residual paraquat. The 200 g of paraquat contained in 1
litre of Gramoxone(R) would be completely inactivated by the
addition of 10 kg of bentonite, for inactivation can be effected
either by cultivation and mixing other soil with the contaminated
layer or by adding clay minerals. Simulated spills of paraquat
have also been treated with sodium borohydride or alkali (Staiff et
al., 1981); within 1 day the paraquat in the soil had been
effectively degraded.
4.3.2. Water
The ecological effects of paraquat in water have been
studied in relation to its use as an aquatic herbicide at a
normal concentration of 1 mg/litre (Newman & Way, 1966; Grzenda et
al., 1966). Following this use, the concentration present in water
decreased to about half of the initial 1 mg/litre level within
36 h, and, in less than 2 weeks, the concentration was below
0.01 mg/litre. Weed-sample analysis, 4 days after paraquat
application, showed a residue of approximately 25 mg/kg, suggesting
that absorption by the weed was mainly responsible for paraquat
removal. Mud-residue analysis 5 1/2 months after treatment showed
that 36% of the applied paraquat remained in the mud, and 70% of
that was found in the top 2.5 cm. In the mud, paraquat had been
adsorbed on to the mineral material. Since bottom mud often has
organic components, the residues may be more accessible to
bacterial degradation. Compared to other products, paraquat
appears to be the herbicide of choice for future use in water
supplies because of its rapid disappearance from water (6 - 14 days
after treatment) (Grzenda et al., 1966). The residues were not
desorbed from the bottom sediments, and mud taken from the bottom
of a paraquat-treated lake carrying inactivated residues, showed no
toxic effects on barley seedlings that germinated on it (Way et
al., 1971).
Wauchope (1979) discussed the fate of pesticides in water
draining from fields after rain. For most formulations, a total
loss of 1.5%, or less, of the amount applied was the rule, except
when severe rainfall occurred within 1 - 2 months following
treatment. Nearly all the pesticides examined were lost by runoff;
only those binding strongly to clay particles, such as paraquat,
were carried off in the sediment phase of runoff. The lack of
paraquat runoff loss has also been discussed by Smith et al.
(1978).
Grover et al. (1980) compared the efficiency of various
herbicidal treatments for weed control in a series of irrigation
ditches. At the relatively low dose of 2.2 kg/ha, paraquat
resulted in aquatic weed suppression from 1973 to 1976, and this
made for satisfactory water flow without environmental
contamination. Water that contains small amounts of paraquat
residues loses them rapidly on contact with soil, the adsorption
process being irreversible (Knight & Tomlinson, 1967; Calderbank,
1972). Thus treated water may be used quite safely for channel
irrigation, if an interval of 10 days is observed between treatment
of the water and its use, because the paraquat will be unavailable
to the plant roots. Caution should, however, be exercised in
prolonged crop irrigation until the residue is well below 0.1
mg/litre, although phytotoxic damage is unlikely at even 0.5 mg
paraquat/litre (Calderbank, 1972).
Coats et al. (1966) treated 0.1 ha experimental ponds with
paraquat to obtain a concentration of 0.4 mg/litre. The soil in
one of the ponds was stirred twice after 24 h. Analysis of the
water over several weeks revealed a decrease from 0.4 mg/litre to
0.01 mg/litre after several weeks, but when the soil of the pond
was stirred, the paraquat concentration fell from 0.75 mg/litre
to <0.01 mg/litre after 8 - 12 days. In static water experiments,
the concentration of 0.5 - 1 mg/litre fell rapidly to about 0.1
mg/litre within 4 - 7 days of treatment in 4 trials performed by
Calderbank (1972). These reductions in the paraquat concentration
were due to its rapid adsorption and concentration in aquatic
plants. Decaying weeds transported it to the bottom mud (Table 4)
where it was not released back into the water (Way et al., 1971).
Earnest (1971) treated a pond with paraquat at an initial
concentration of 1.14 mg/litre. No residues were detected in the
water after 16 days (limit of detection 0.01 mg/litre); in the mud
the concentration was 1.13 mg/kg after 3 h and 3.25 mg/kg after 99
days. These data were confirmed by Grover et al. (1980).
Grover et al. (1980) studied irrigation water from ditches.
Three days after treatment with 2.2 kg paraquat/ha, the
concentrations in the water used to flood the treated ditches were
less than 0.01 mg/litre, and paraquat residues in the ditch water
ranged from 0.002 - 0.034 mg/litre in samples taken 3 - 5 days
after foliar applications.
Table 4. Residues of paraquat in water, weed, and bottom muda
-------------------------------------------------------------------
Days after treatment
-------------------------------------------------------------------
1 4 16 32 175 420
-------------------------------------------------------------------
Trial 1 water (mg/litre) 0.31 0.12 ND
weed (mg/kg) 13.70 25.80 21.0 0.55
mud (mg/kg) 3.70 - - - 57.1 20.1
Trial 2 water (mg/litre) 0.37 ND ND ND - -
weed (mg/kg) 25.50 40.0 37.8 27.8 - -
mud (mg/kg) ND 0.97 0.23 0.32 6.6 0.96
-------------------------------------------------------------------
a From: Way et al. (1971).
ND - not detectable.
4.3.3. Air
Paraquat is not volatile. Dry deposits of 14C-paraquat
chloride exposed at room temperature showed no measurable loss in
64 days (Coats et al., 1966). Exposure to paraquat in the air is
not important in spraying and harvesting operations; the skin is
the principal route of occupational exposure (Chester & Woollen,
1982; Staiff et al., 1975).
Air concentrations of paraquat were measured on summer days by
Makovskii (1972) using the method of Calderbank & Yuen (1965).
About 1 - 1.3 kg paraquat/ha had been applied as a herbicide or
desiccant in 0.25 - 0.35% water solutions. The paraquat aerosol
concentrations varied according to spraying method and work-place
(Table 5). Using the same analytical method, Staiff et al. (1975)
examined 35 sites after paraquat application with tractor-mounted
field sprayers or hand-pressure garden dispensers. The working
solutions contained 0.15% paraquat for field use, and 0.44% for
garden use. The respiratory exposure of field and garden operators
was below the limit of detection (<0.001 mg paraquat/h).
Mature cotton fields (Seiber & Woodrow, 1981) were sprayed with
paraquat, the dose being 0.94 kg/ha. The air paraquat
concentrations measured downwind decreased regularly from the
extrapolated interval-average values of 4.31 and 10.7 µg/m3 1
metre downwind of the 2 fields to <50 ng/m3 at 400 metres away in
the same direction. Forty-five percent of the aerosol particles
had diameters ranging from 0.01 to 4 µm. The remaining 55% had a
median diameter of 12 µm. Downwind samples taken 2 - 4 h after
spraying contained 1 - 10% of the amount dispersed, but, after
5 - 7 h, no paraquat was detectable in the air.
Table 5. Paraquat total airborne concentrations (mg/m3) in
working areasa
-------------------------------------------------------------------
Place of Number of Mean
sampling samples concentrations
± SE
-------------------------------------------------------------------
Working area sprayer loading 28 0.13 ± 0.03
tractor cabin 16 0.37 ± 0.07
(in direction of wind)
tractor cabin 16 0.55 ± 0.01
(against the wind)
manual spraying 16 0.18 ± 0.04
Treated field after 5 min 16 0.05 ± 0.01
after 10 min 32 < 0.01
after 20 min 16 0
Distance from 200 m 8 0.08 ± 0.01
treated field 400 m 8 0.04 ± 0.01
-------------------------------------------------------------------
a From: Makovskii (1972).
A study of Malaysian plantation workers, occupationally exposed
to paraquat, revealed a mean total airborne exposure of 0.97 mg/m3
for spray operators. This exposure is less than present TLVs
(Chester & Woollen, 1982). Wojeck et al. (1983) reported that
after spraying paraquat in fields of tomatoes and citrus, the total
airborne exposure ranged from 0 - 0.070 mg/h. It was less than
0.1% of the total body exposure (12.16 - 168.59 mg/h) in all
trials.
During mechanical harvesting of cotton dessicated by paraquat,
the maximum levels in airborne dust were found to be 1245 ng/m3
outside the cabin of the tractor and 516 ng/m3 inside the open
cabin. With the cabin door closed, the concentration was only 13.7
ng/m3. The trapped particulate matter consisted of dessicated
plant material and soil dust. A cascade impactor analysis
established that 57% of the paraquat had a median particle diameter
of 4 µm, 23%, 12 µm, and 11%, 3 µm. Cotton harvesting generated
parti- culate concentrations in the field comparable to those
immediately downwind of the field during spraying. Bearing in mind
the highest paraquat air concentration in the harvest-time air
(0.0012 mg/m3), a harvester operator's maximum exposure through
inhalation was calculated to be 0.01 mg/8 h/day (Seiber & Woodrow,
1981).
Bulgaria has established a maximum allowable concentration
(MAC) of 0.01 mg paraquat/m3 (1972), the Federal Republic of Germany
0.1 mg/m3 (1982), Hungary 0.02 mg/m3 (1978), and the USA a TLV of
0.1 mg/m3 (1982).
4.3.4. Plants
Paraquat residues on plants have been reviewed several times by
the Joint Meeting on Pesticide Residues (JMPR) (FAO/WHO, 1971,
1973, 1983). The residues found after paraquat was used as a
desiccant are summarized in Tables 6 and 7 (Calderbank, 1968).
Table 6. Paraquat residues (mg/kg) in cotton 10 days after
dessication at 0.55 kg/haa
-------------------------------------------------------------------
Fraction analysed Paraquat found
-------------------------------------------------------------------
Cotton as picked, including trash and balls 2.00
Ginned seed 0.18
Mechanically reginned seed 0.08
Acid-delinated seed 0.05
Lint cotton 3.00
Trash 3.70
Hulls 0.13
Crude oil ND
Meal 0.02
-------------------------------------------------------------------
a From: Calderbank (1968).
Coats et al. (1966) reported that 14C-paraquat applied to wheat
as a 1% solution was translocated in the plants, including the
roots. Slade (1966) studied the degradation of 14C-paraquat
dichloride and its photochemical degradation products in plants.
Maximum loss occurred in tomato, broad-bean, and maize when the
paraquat remained on the leaf surfaces during sunny days.
In potatoes treated with paraquat as a desiccant, Makovskii
(1972) found a residue of 0.05 mg/kg, and there was no change after
the potatoes had been boiled. No residues (limit of detection 0.01
mg/kg) were found in fruits (apples, citrus fruits, plums, pears),
tea, and cereals. In tests on sunflower seeds treated with 0.25 or
0.5 kg paraquat/ha, residues of up to 0.9 mg/kg were found in the
whole seed, up to 1.2 mg/kg in sunflower meal, and no residue in
the oil (Anonymous, 1979). Therefore, the use of sunflower meal in
the diet of hens, dairy cattle, and other livestock would not
result in paraquat levels exceeding current standards.
Table 7. Paraquat residues (mg/kg) in food crops 3 - 21 days
after dessicationa
-------------------------------------------------------------------
Crop Rate of application Paraquat found
(lb/acre)
-------------------------------------------------------------------
Barley 0.50 - 1.00 3 - 10
Wheat 0.50 - 1.00 1 - 2.5
Maize 0.50 - 1.20 ND - 0.2
Rice (with husk) 0.15 - 0.54 0.7 - 22
Rice (de-husked or polished) 0.15 - 0.54 ND - 0.2
Peas, beans, sunflower seed 0.35 - 1.20 ND - 0.2
Sorghum seed 0.25 - 1.00 0.1 - 0.4
Cotton (as picked) 0.50 - 1.00 2 - 3
Potatoes 0.50 - 1.50 0.02 - 0.13
Onions 0.50 - 2.00 ND - 0.05
Sugar cane juice 0.50 - 2.00 ND
Seed oils (sunflower, rape, up to 1.20 ND
sesame, cotton)
-------------------------------------------------------------------
a From: Calderbank (1968).
Seiber et al. (1979) determined the paraquat residues in
treated cotton (the foliage and bolls of the live plant, the lint
and seed of harvested cotton, the gin waste and the lint and
non-lint components). Gin waste residues were surveyed during
5 months of open storage. The paraquat dose had been 0.21 and
2.0 kg/ha. The results obtained are summarized in Table 8. The
minimal degradation of paraquat in the plants studied was confirmed
by Hills et al. (1981).
Significant paraquat residues are to be expected only when a
crop is directly sprayed.
After spraying fields of marijuana with paraquat for the
purpose of eradication, residues of paraquat were detected in
marijuana (Smith, 1978; Patrick, 1980). Of the 54 samples
collected in 1976, 7.4% were positive and of 46 samples collected
in 1977, 19.6% were positive.
Table 8. Paraquat residues (mg/kg) in cotton plantsa
-------------------------------------------------------------------
Material Days after Leaves Lint Non-lint Seeds
treatment
-------------------------------------------------------------------
Standing cotton plants 2 13.1 22.10 0.06
6 8.2 3.80 0.06
Harvested seed cotton 18 7.15 0.25
stored in field 49 4.85 0.18
Gin waste 49 2.7 9.3
119 5.3 10.1
171 5.8 9.7
-------------------------------------------------------------------
a From: Seiber et al. (1979).
4.3.5. Animals
The effects and fate of 14C-paraquat orally-administered to
cattle at 8 mg/kg body weight were studied by Stevens & Walley
(1966). Seven days after this single dose, 0.03 - 0.08 g/litre had
been excreted in the milk and 2.4 g/litre in the urine of the cows.
The total paraquat excretion in the milk was only 0.01% of the
ingested dose. In cows given daily oral doses of 8 mg paraquat/kg
for 3 weeks, residues of less than 0.01 mg/litre were detected in
the milk (FAO/WHO, 1977). Cattle did not suffer any toxic effects
over a 4-week period when turned loose on pasture immediately after
it had been sprayed with 1.12 kg paraquat/ha (Calderbank et al.
1968). During the first 2 weeks of grazing on the dried herbage,
it was estimated that the cattle ingested approximately half of
their acute oral LD50 (36 - 54 mg/kg body weight) every day.
Paraquat levels in the herbage ranged from about 400 mg/kg 1 day
after spraying, to about 200 mg/kg 14 days after treatment;
14 - 35 days after spraying the levels were 135 -214 mg/kg. The
4-carboxyl-1-methylpyridylium chloride content during the trial
period was 5.1 - 3.4 mg/kg. By the 4th week of the study, paraquat
levels in the urine were 0.01 - 0.19 mg/litre and in the faeces,
0.9 - 42 mg/kg. Only on the first day after spraying were paraquat
residues (0.02 mg/litre) found in the milk of 2 cows; no residues
were found (< 0.005 mg/litre) thereafter. The only organs of a
slaughtered animal that contained paraquat were the kidney
(0.03 mg/kg) and the stomach (0.05 mg/kg).
The fate of paraquat in large animals is addressed far more
completely in the Evaluations of the 1976 Joint Meeting on
Pesticide Residues (JMPR) (FAO/WHO, 1977).
Rabbits were fed with lucerne treated with normal-use levels of
paraquat (Lavaur et al., 1979). Immediately after spraying, the
paraquat residues were 272 mg/kg (dry weight of lucerne). After
24 h and 48 h, they were 114 mg/kg and 62 mg/kg, respectively. No
systemic toxicity symptoms or gastrointestinal damage were observed
in the treated rabbits.
When hens were given paraquat at 40 mg/litre in their drinking-
water for 14 days, the amount of paraquat found in the eggs rose to
0.1 mg/kg, but fell to less than 0.005 mg/kg, 6 days after
cessation of treatment (Fletcher, 1967). Eggs from hens eating
grain containing paraquat at a concentration of 10 mg/kg contained
residues below 0.025 mg/kg.
5. BIOLOGICAL ACTIVITY OF RESIDUES
5.1. Soil Organisms
Haley (1979) reviewed the effects of paraquat on soil
microorganisms and fungi, while Tu & Bollen (1968), Curry (1970),
Radaelli & Martelli (1971), Roslycky (1977), and Smith et al.
(1981a) studied the effects of paraquat on the size and composition
of the microbial soil populations, total microbial respiration in
the soil, the rate of organic matter degradation, and the number of
soil microorganisms. None of these authors found any adverse
ecological effects from normal and excessive (up to 32 times the
normal dose) paraquat treatment, although in some cases
nitrification was temporarily suppressed or activated, and some
bimodal microbiological effects were observed with intermediate
herbicide concentrations (Tu & Bollen, 1968; Tchipilska, 1980).
At normal doses, paraquat had no adverse effect on
endomycorrhiza formation and function (Smith et al., 1981a), on
total populations of bacteria, actynomyces, fungi (Roslycky, 1977;
Haley, 1979; Tchipilska, 1980; Smith et al., 1981a), or on 24
different species of soil fauna taken from 2 plots at a depth of
3.8 cm (Curry, 1970).
Curry (1970), and Riley et al. (1976) made extensive
studies of the effects of normal and high doses of paraquat on
microarthropod and earthworm populations at sites at different
stages of cultivation. The herbicide was neither harmful nor
repellant to earthworms, nor was there any evidence of a toxic
effect or of paraquat accumulation in any species examined. When
the residues in the top 2.5 cm of soil reached 20 mg/kg, the
highest concentration determined in Allolbophora caliginosa, living
near the surface, was 3.2 mg/kg (live weight). Worms from highly-
dosed plots eliminated paraquat residues within 36 h, when placed
in clean soil.
5.2. Effects of Residues on Crop Yields
The absence of adverse effects from residual paraquat on the
growth and yield of crops grown in paraquat-treated soils has been
demonstrated by Knight & Tomlinson (1967), Damanakis et al. (1970),
Newman & Wilkinson (1971), and Riley et al. (1976). It is known
that the paraquat-inactivation capacity of soils varies widely.
Paraquat has been tested on soils of low adsorption capacity, it
has been used repeatedly on the same soil (section 4.3.1) and has
been tested at extremely high concentrations. The absence of any
reports or observations of long-term phytotoxic effects confirms
the data obtained in greenhouse and laboratory studies.
5.3. Effects on Fish and Aquatic Organisms
Despite variation in LC50s for fish (67 - 110 mg/litre after
24 h, 38 - 62 mg/litre after 48 h, more than 25 - 32 mg/litre after
96 h), the herbicide has proved to have a wide margin of safety for
warm- and cold-water fish species (Calderbank, 1972). The toxicity
of paraquat for fish varies with the species, the size of the
fish, and the softness or hardness of the water. A large number
of aquatic species have shown a 100% survival at 96 mg/litre over
96 h, though the decreased oxygen concentration following decay of
weeds, may be dangerous in extreme situations. Rainbow trout
tolerated 1 mg paraquat/litre water in prolonged toxicity tests
and only a 30% mortality was recorded after 16 days of repeated
exposure (Calderbank & Slade, 1976). At the end of the test, 0.54
mg paraquat/kg was found in the rainbow trout. In a 7-day exposure
test at 1 mg paraquat/litre, the herbicide was detected in the gut
(0.41 mg/kg) and liver (0.35 mg/kg), but not in the meat of the
fish (< 0.025 mg/kg). Water snails collected from 2 ditches,
12 weeks after treatment of the waters with 1 mg/litre were found
to contain 0.43 mg herbicide/kg. Fish (major carp fingerlings)
exposed to paraquat in the presence of weeds were more susceptible
than those in weed-free environments (Singh & Yadav, 1978), owing
to the changed oxygen content of the water. Where there is heavy
weed growth, the oxygen taken up by weed decay may dangerously
reduce the oxygen available for aquatic organisms. To avoid this,
as far as possible, paraquat should be applied before weed growth
becomes dense and only to one part of the water-course or lake at
a time (FAO/WHO, 1973).
5.4. Effects on Birds
Paraquat is less toxic for birds than for mammals. The acute
oral LD50 for the hen is 262 - 380 mg/kg body weight (Table 11).
The acute oral and 24-h percutaneous (applied to feet) LD50 for
mallards are 200 and 600 mg/kg body weight, respectively (Hudson et
al., 1979). For duck, pheasants, and quail, LC50 values of
paraquat when mixed in the diet are 1000 mg/kg of food or more
(Summers, 1980); residues on sprayed vegetation would not therefore
be expected to present a hazard for birds.
When paraquat was sprayed directly on to pheasants' eggs before
incubation, treatment rates up to 2 kg paraquat/ha did not have any
effect on egg hatchability or on the birds' reproductive organs
(Newman & Edwards, 1980). In a similar study with Japanese quail
eggs, sprays containing paraquat levels of up to 3 kg/ha did not
have any effect on hatchability or development of reproductive
organs (Edwards et al., 1979). Thus, normal spray rates should not
induce any adverse effects, even if paraquat is sprayed directly on
eggs.
Bird populations have been monitored in detail, over a 5-year
period, on a farm in the United Kingdom where paraquat use was much
higher than normal; the average application to the whole arable
area was 0.6 kg/ha per year. The paraquat was applied beneath
hedgerows and along fence lines. The farm maintained an excellent
wild bird population (40 species), including ground-nesting birds
(Edwards, 1979). Most species were at a similar or greater density
than the national average in the United Kingdom.
The Ministry of Agriculture, Fisheries, and Food in the United
Kingdom has carried out detailed investigations on mammalian and
avian deaths that could have been caused by pesticides. For the
period 1971 - 81, the normal use of pesticide was not found to have
caused any significant adverse effects on mammals and birds (MAFF,
1980a, 1981). The Ministry concluded, "It is widely believed that
the use and misuse of paraquat is responsible for a considerable
number of wildlife casualties. There is no evidence from the
investigations to support this allegation...." (MAFF, 1980b).
6. KINETICS AND METABOLISM
6.1. Animal Studies
6.1.1. Absorption
6.1.1.1. Oral absorption
Daniel & Gage (1966) studied the absorption of 14C-paraquat
following oral and subcutaneous single-dose administration to rats.
About 76 - 90% of the oral doses were found in the faeces, and
11 - 20% in the urine; most of the subcutaneous dose (73 - 88%)
was found in the urine and only 2 - 14.2% in the faeces. This,
together with the absence of marked biliary excretion, was evidence
that paraquat was poorly absorbed from the gut. This low rate of
absorption was confirmed by Litchfield et al. (1973) and Conning et
al. (1969). Rats, guinea-pigs, and monkeys orally administered
LD50 doses of 14C-paraquat had low peak serum concentrations
(2.1 - 4.8 mg/litre) (Murray & Gibson, 1974). The radioactivity
levels reached a maximum 30 - 60 min after administration and then
remained relatively constant for 32 h. A dose of 126 mg/kg body
weight resulted in a rat serum level of 4.8 - 4.7 mg/litre.
In fasting dogs, low oral doses of paraquat were rapidly but
incompletely absorbed, the peak plasma concentration being attained
75 min after dosing (Bennett et al., 1976). After an oral dose of
0.12 mg/kg body weight, 46 - 66% was absorbed in 6 h. For doses of
2 - 5 mg/kg, only 22 - 38% and 25 - 28% of the dose was absorbed,
respectively. Dose-dependent data from dogs and whole-body
autoradiography suggest that absorption is facilitated in the small
intestine. Some non-ionic surfactants (0.001%) increased 14C-
paraquat transport through isolated gastric mucosa models, but
histological evaluation suggested that this was due to damage of
the epithelial cell membranes (Walters et al., 1981).
6.1.1.2. Pulmonary absorption
Absorption of paraquat following instillation and inhalation in
the lung has been described in several studies (Gage, 1968a;
Kimbrough & Gaines, 1970; Seidenfeld et al., 1978; Popenoe, 1979).
The uptake of 14C-paraquat after an intratracheal injection of
1.86 nmol/lung was investigated in the isolated perfused rat lung
by Charles et al. (1978). The efflux of 14C-paraquat was diphasic
with a rapid phase half-life of 2.65 min and a slow phase half-life
of 356 min. It was suggested that the slow phase represented a
storage pool, possibly responsible for the pulmonary toxicity of
paraquat. Various doses of 3H-paraquat (10-5 - 10-12 g) in 0.1 ml
saline were introduced directly into the left bronchus of rats
(Wyatt et al., 1981). Fifteen min after instilling 10-8 of 3H-
paraquat, 90% of the ion could be accounted for in the tissues and
urine, 50% being present in the lung. With doses at or greater
than 10-5 g, pathological changes were seen in the lung, similar to
those seen after systemic poisoning. Zavala & Rhodes (1978)
reported that the lung of the rabbit was highly sensitive to
paraquat intrabronchial instillation in doses ranging from
0.1 g - 1 pg; moderately sensitive to intraveneously administered
paraquat (25 mg/kg body weight); resistant to the herbicide when
given intraperitoneally or subcutaneously (25 mg/kg body weight).
6.1.1.3. Dermal absorption
Paraquat absorption through animal and human skin has been
studied using an in vitro technique (Walker et al., 1983). Human
skin was shown to be impermeable to paraquat, having a very low
permeability constant of 0.73. Furthermore, human skin was found
to be at least 40 times less permeable than animal skins tested
(including rat, rabbit, and guinea-pig). There are no in vivo
studies on the rate of absorption of paraquat through the skin.
However, observations of dose-related dermal toxicity in
experimental animals and human percutaneous poisoning have provided
some qualitative information concerning the dermal absorption of
paraquat (further discussed in section 8.2.2.2).
6.1.2. Distribution
Since the most characteristic feature of paraquat toxicity is
lung damage, it is important to stress the high concentrations and
retention of paraquat in the lung tissues, relative to other
tissues, following oral, intravenous, intraperitoneal,
subcutaneous, and intrabronchial routes of administration in rats,
guinea-pigs, and monkeys (Sharp et al., 1972; Ilett et al., 1974;
Murray & Gibson, 1974; Kurisaki & Sato, 1979; Waddell & Marlowe,
1980). An association between paraquat concentrations in the lung
and degree of toxicity or lung injury has been reported (Sharp et
al., 1972; Ilett et al., 1974; Waddell & Marlowe, 1980; Wyatt et
al., 1981). Some of their data are summarized in Tables 9 and 10.
Table 9. Paraquat distribution in tissues
---------------------------------------------------------------------------
Route of entry Dose Species Time after Tissue Concentration
treatment
---------------------------------------------------------------------------
1. Intrabronchial 10 ng rat 60 min plasma 0.0092 µg/litre
lung 5.2 ng
kidney 0.052 ng
liver -
heart -
brain -
2. Intravenous 20 mg/kg rat 24 h plasma 0.7 mg/litre
lung 8.0 mg/kg
kidney 1.45 mg/kg
liver 0.48 µg/g
heart 0.75 mg/kg
brain -
---------------------------------------------------------------------------
Table 9. (contd.)
---------------------------------------------------------------------------
Route of entry Dose Species Time after Tissue Concentration
treatment
---------------------------------------------------------------------------
3. Intravenous 20 mg/kg rat 24 h plasma ND
lung 11.36 µm/kg
kidney 1.93 µmol/kg
liver 0.90 µmol/kg
heart 1.13 µmol/kg
brain 0.87 µmol/kg
20 mg/kg rabbit 24 h plasma 0.28 µmol/litre
lung 7.9 nm/g
kidney 5.25 µmol/kg
liver 1.59 µmol/kg
heart 1.52 µmol/kg
brain 0.49 µmol/kg
4. Intraperitoneal 15 mg/kg rat 24 h plasma 0.32 µmol/litre
lung 26.28 µm/kg
kidney 10.4 µmol/kg
liver 5.04 µmol/kg
heart 4.59 nmol/g
brain 1.22 µmol/kg
5. Oral 126 mg/kg rat 16 h plasma 0.90 mg/litre
lung 5.0 mg/kg
kidney 7.00 mg/kg
liver 2.1 mg/kg
heart 2.7 mg/kg
brain -
22 mg/kg guinea- 16 h plasma 0.03 mg/litre
pig lung 1.29 mg/kg
kidney 1.99 mg/kg
liver 0.08 mg/kg
heart 0.31 mg/kg
brain -
---------------------------------------------------------------------------
1. From: Wyatt et al. (1981).
2. From: Sharp et al. (1972).
3. From: Ilett et al. (1974).
4. From: Maling et al. (1978).
5. From: Murray & Gibson (1974).
Table 10. Paraquat distribution in tissues (in mg/kg (mean) tissue)
----------------------------------------------------------------------------
Route of Entry Dose Species Time Lung Kidney Liver Heart Plasma
(mg/kg after
body dosing
weight)
----------------------------------------------------------------------------
1. Oral 126 rat 1 h 3.3 27.5 2.0 1.8 4.7
4 h 3.7 4.5 4.4 0.9 0.8
32 h 13.6 9.4 5.7 2.8 1.1
64 h 1.7 1.0 7.7 0.2 0.1
2. Intravenous 20 rat 1 h 9.0 25.0 5.0 - 6.0
4 h 8.0 6.0 2.0 - 0.3
24 h 6.0 1.0 0.4 - 0.07
2 days 4.0 0.8 0.3 - 0.05
----------------------------------------------------------------------------
1. From: Murray & Gibson (1974).
2. From: Sharp et al. (1972).
Toxic doses of paraquat were administered orally and iv to rats
(Sharp et al., 1972). Paraquat concentrations in the whole blood
were the same as those in the plasma. The distribution of the
herbicide in various tissues was then followed for 10 - 18 days.
The lung had the greatest retention and consequently contained the
highest concentration 4 h after dosing. Four to 10 days after
dosing, the paraquat concentration in the lung was 30 - 80 times
higher than that in the plasma. The high lung-tissue
concentrations of paraquat were confirmed by Ilett et al. (1974)
for rats and rabbits after iv injection of 20 mg 14C-paraquat/kg
body weight. Although the herbicide showed a selective
localization in rabbit lung, the concentration decreased far more
rapidly in rabbit lung than in rat lung. The rabbit did not show
any histological or biochemical signs of lung damage, and no
evidence of covalent binding of paraquat in lung tissue was found
by Ilett et al. (1974). After thorough washing of tissue
precipitate with dilute trichloroacetic acid, only insignificant
amounts of 14C-paraquat were detected in protein from the brain,
heart, kidney, liver, lung, and plasma.
Autoradiographic studies using 14C-paraquat have been carried
out on mice and rats (Litchfield et al., 1973). Paraquat was
observed in nearly all organs 10 min after intravenous injection of
20 mg/kg body weight. Waddell & Marlowe (1980) obtained similar
autoradiographic results in mice, after intravenous injection of
288 - 338 µg 3H-paraquat dichloride/kg body weight. Cellular
resolution autoradiography showed that paraquat was confined almost
entirely to cells having the distribution of alveolar Type II
cells. These cells are known to be susceptible to the toxicity of
paraquat (Kimbrough & Gaines, 1970). Waddell & Marlowe (1980)
suggested that it was unlikely that the radioactivity was bound to
cellular constituents.
No paraquat was detected in rat kidney, brain, liver, or lung
when paraquat was administered in the diet at a concentration of
50 mg/kg for a period of 8 weeks. At 120 mg/kg, it was found in
low concentrations in the lung, kidney, gastrointestinal system,
and brain (Litchfield et al., 1973). At 250 mg/kg, it was detected
in the tissues within 2 weeks. No sex differences or any clear
pattern of accumulation were noted throughout the 8-week study.
Within 1 week of return to a normal diet, no paraquat was detected
in any tissue examined. Histological changes were observed in all
lungs of animals fed paraquat at 250 mg/kg diet.
Rose et al. (1974a) demonstrated an energy-dependent
accumulation of paraquat in slices of rat lung that obeyed
saturation kinetics. The same investigators also examined the
ability of paraquat to accumulate in tissue slices from other
organs in vitro (Rose & Smith, 1977). The herbicide in brain,
adrenal gland, and kidney slices accumulated; however, the uptake
was less than 10% of that observed in the lung slices. The authors
established the uptake of paraquat by the lung in various species
(rat, rabbit, dog, monkey, man). The human lung accumulated
paraquat as strongly as that of the rat and there was a
relationship between the concentration of paraquat in the different
lung areas and the development of microscopic lung lesions. It has
been demonstrated that the rate of paraquat efflux from lung tissue
is less than its rate of accumulation in the lung slices (Smith et
al., 1981). Efflux from lung slices, prepared from rats dosed iv
with the herbicide, was found to be biphasic. There was a fast
component (half-life 20 min), followed by a first-order slow
component characterized by a half-life of 17 h. The half-life in
vitro was similar to that seen in vivo following iv
administration to rats.
6.1.3. Metabolic transformation and excretion
Paraquat participates to a considerable extent in cyclic
reduction-oxidation reactions. After undergoing a single electron
reduction in tissues, the resultant free radical is readily
oxidized by molecular oxygen to the parent compound (section 6.3).
This leads to an overall excretion of essentially unchanged
paraquat in the urine after oral administration to rats (Murray &
Gibson, 1974).
Daniel & Gage (1966) reported that paraquat was metabolized by
gut microflora following oral dosing of rats. This observation was
not confirmed in subsequent studies (Murray & Gibson, 1974) and was
later attributed to a problem with the method (FAO/WHO, 1977).
Urinary concentrations of paraquat following oral
administration are relatively low (Daniel & Gage, 1966: Murray &
Gibson, 1974; Sharp et al., 1972; Maling et al., 1978) and are thus
used to estimate its elimination from the body.
Sharp et al. (1972) reported a biphasic elimination of paraquat
from the plasma of rats after iv injection. The initial rapid
phase had a 20 - 30 min half-life, and the slower phase a half-life
of 56 h. Murray & Gibson (1974) also showed prolonged paraquat
elimination after oral administration to rats, guinea-pigs, and
monkeys. The urinary and faecal routes were equally important in
all species studied. The faecal content was due mainly to
elimination of unabsorbed paraquat. Prolonged elimination of
paraquat in all animals tested indicated retention of the herbicide
in the body.
Following iv administration to rats, about 75 - 79% of the dose
was excreted in the urine within 6 h (Maling et al., 1978). The
plasma disappearance of an iv dose of paraquat of 5 mg/kg was
fitted to a 3-compartment model. Total body clearance was
estimated to be 8.39 ± 0.54 ml/kg per min (Maling et al., 1978).
The relatively high concentration of paraquat in the duodenal and
jejunal walls suggested biliary secretion of the herbicide, and the
authors' hypothesis was supported by the observation of radio-
activity in the intestines of mice in whole-body autoradiographic
studies (Waddell & Marlowe, 1980).
Since absorbed paraquat is mainly removed via the kidneys, the
early onset of renal failure will have a marked effect on paraquat
elimination and distribution, including accumulation in the lung.
Hawksworth et al. (1981) used the dog as a model to evaluate the
influence of paraquat-induced renal failure on the kinetics of
paraquat elimination. After iv injection of a trace dose of 14C-
paraquat (30 - 50 µg/kg body weight) in dogs, the kinetics of
distribution was described by a 3-compartment model. To obtain a
good fit of the curve, it was necessary to sample the central
(plasma) compartment for at least 24 h after dosing. Simulation of
paraquat levels in the peripheral compartments suggested the
existence of a compartment with rapid uptake and removal (kidney)
and another with slow uptake (lung). The renal clearance of
paraquat approximated total body clearance indicating that paraquat
elimination occurs through renal excretion. The urinary excretion
rate of an iv dose was rapid, approximately 80 - 90% of the dose
being eliminated during the first 6 h. Intravenous injection of a
large toxic dose of paraquat (20 mg/kg body weight), however,
brought about a marked decrease in renal clearance, from 73 ml/min
to 18 ml/min after 2 1/2 h and 2 ml/min after 6 h. This data
suggested that damaged renal tubules could contribute to paraquat
accumulation in the lung.
6.2. Observations on Human Beings
6.2.1. Observations on paraquat poisoning after ingestion:
non-fatal cases
Tompsett (1970) reported a case of ingestion of 45 g of Weedol
(2.5% paraquat). On hospital admission, the gastric aspirate
contained 0.215 g paraquat/litre and the urine 0.148 g/litre.
After 2 - 4 h, paraquat concentrations dropped to 5.1 mg/litre in
the urine and 0.4 mg/litre in the serum but, 16 - 24 h after
admission, the urinary level was 0.95 mg/litre, while no paraquat
was detectable in the serum. Paraquat was also detected in the
urine for up to 15 days after poisoning, while at the same time
serum concentrations were below the detectable limits in chemical
analysis (Fletcher, 1975).
The cumulative elimination of paraquat in the faeces and urine
of a patient was followed for 7 days by van Dijk et al. (1975).
Faecal elimination increased from 340 mg the first day to 530 mg
after 7 days, while cumulative urinary excretion reached 60 mg the
lst day and increased to 75 mg after 7 days. It was calculated
that only 87 mg of paraquat had been absorbed from a total
ingestion of about 637 mg, determined in the urine, dialysate, and
faeces. In this patient, less than 14% of the ingested paraquat
was absorbed through the gastrointestinal system.
6.2.2. Observations on paraquat poisoning after ingestion:
fatal cases
It is well established that paraquat lung disease resulting in
death is usually preceded or accompanied by renal insufficiency.
This contributes to the retention of paraquat in body tissues.
Nevertheless, Fairshter et al. (1979) detected only small
concentrations (below 0.09 mg/kg) of paraquat in several organs of
patients who died 3 weeks after ingestion.
The detection of 27 mg paraquat/litre in the bile of a woman
after autopsy suggested that some faecal paraquat might be
attributable to biliary excretion (Dijk et al., 1975).
6.2.3. Significance of paraquat concentrations in cases of
paraquat poisoning
Not only oral ingestion, but also dermal absorption of paraquat
after occupational overexposure, resulted in measurable urinary
levels of paraquat. The determination of paraquat in urine and
serum is an important biological exposure test for the diagnosis
and the prognosis in cases of human poisoning.
Wright et al. (1978) followed the urinary excretion of paraquat
in 16 patients (7 of whom died). The total amount of paraquat
excreted ranged from 0.6 mg to 386 mg. The excretion rate
decreased rapidly during the 48 h following ingestion, though less
rapidly in the patients who eventually died. All patients
excreting 1 mg of paraquat or more per hour, for 8 h or more after
ingestion, died.
Plasma-paraquat concentrations were measured by gas
chromatography, radioimmunoassay, and colorimetric methods in 79
patients with paraquat poisoning (Proudfoot & Stewart, 1979). At
any given time after ingestion (within a limit of 35 h), plasma
concentrations were significantly higher in the patients who died
(Fig. 4). Patients whose plasma concentrations were not higher
than 2.0, 0.6, 0.3, 0.16, and 0.10 mg paraquat/litre at
respectively, 4 h, 6 h, 10 h, 16 h, and 24 h after the poisoning,
were likely to survive. When plasma levels exceeded 0.3 mg/litre
15 h after ingestion, a fatal outcome could be expected, despite
treatment. These conclusions were supported by the studies
performed on 28 patients by Bismuth et al. (1982).
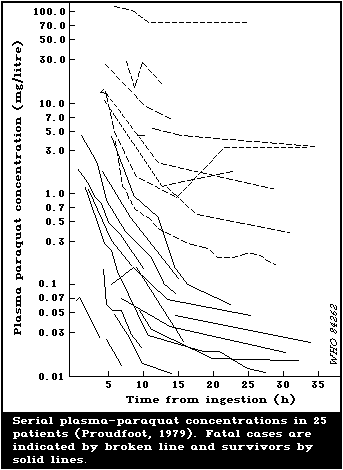 6.3. Biochemical Mechanisms
The mechanism of the toxic action of paraquat has been
extensively investigated. Several reviews or monographs have
summarized the biochemical mechanism of paraquat toxicity in
plants (Calderbank, 1968), bacteria (Fridovich & Hassan,
1979), and animals (Bus et al., 1976; Autor, 1977; Smith et
al., 1979; Bus & Gibson, in press).
Paraquat has long been known to participate in cyclic
reduction-oxidation reactions in biological systems. The compound
readily undergoes a single electron reduction in tissues, forming a
free radical. In an aerobic environment, however, a free radical
is immediately oxidized by molecular oxygen, generating the
superoxide radical (O2-). The reoxidized paraquat is capable of
accepting another electron and continuing the electron transfer
reactions in a catalytic manner (Fig. 5). Research into the
mechanism of paraquat toxicity has identified at least 2 partially
toxic consequences of the redox cycling reaction: a) generation of
O2-, and b) oxidation of cellular NADPH, which is the major source
of reducing equivalence for the intracellular reduction of
paraquat. Generation of O2- can lead to the formation of more
toxic forms of reduced oxygen, hydrogen peroxide (H2O2) and
hydroxyl radicals (OH-). Hydroxyl radicals have been implicated in
the initiation of the membrane-damaging by lipid peroxidation,
depolymerization of hyaluronic acid, inactivation of proteins and
damage to DNA (Hassan & Fridovich, 1980). Depletion of NADPH, on
the other hand, may disrupt important NADPH-requiring biochemical
processes such as fatty acid synthesis (Smith et al., 1979).
6.3. Biochemical Mechanisms
The mechanism of the toxic action of paraquat has been
extensively investigated. Several reviews or monographs have
summarized the biochemical mechanism of paraquat toxicity in
plants (Calderbank, 1968), bacteria (Fridovich & Hassan,
1979), and animals (Bus et al., 1976; Autor, 1977; Smith et
al., 1979; Bus & Gibson, in press).
Paraquat has long been known to participate in cyclic
reduction-oxidation reactions in biological systems. The compound
readily undergoes a single electron reduction in tissues, forming a
free radical. In an aerobic environment, however, a free radical
is immediately oxidized by molecular oxygen, generating the
superoxide radical (O2-). The reoxidized paraquat is capable of
accepting another electron and continuing the electron transfer
reactions in a catalytic manner (Fig. 5). Research into the
mechanism of paraquat toxicity has identified at least 2 partially
toxic consequences of the redox cycling reaction: a) generation of
O2-, and b) oxidation of cellular NADPH, which is the major source
of reducing equivalence for the intracellular reduction of
paraquat. Generation of O2- can lead to the formation of more
toxic forms of reduced oxygen, hydrogen peroxide (H2O2) and
hydroxyl radicals (OH-). Hydroxyl radicals have been implicated in
the initiation of the membrane-damaging by lipid peroxidation,
depolymerization of hyaluronic acid, inactivation of proteins and
damage to DNA (Hassan & Fridovich, 1980). Depletion of NADPH, on
the other hand, may disrupt important NADPH-requiring biochemical
processes such as fatty acid synthesis (Smith et al., 1979).
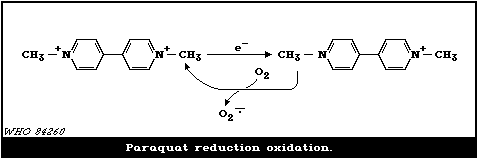 The importance of molecular oxygen and the potential role of O2-
generation in mediating have been implicated in studies on plants
(section 3.3), bacteria, and in in vitro and in vivo mammalian
systems. In cultures of Escherichia coli, Hassan & Fridovich
(1977, 1978, 1979) demonstrated that paraquat stimulated cyanide-
resistant respiration, which could be almost entirely accounted for
by an NADPH-dependent formation of O2-. The possibility that
formation of O2- might be responsible for the toxicity of paraquat
in bacteria was supported by observations that bacteria containing
elevated activities of superoxide dismutase, an enzyme that
detoxifies O2-, were resistant to paraquat toxicity (Hassan &
Fridovich, 1977, 1978; Moody & Hassan, 1982).
In vitro studies on preparations of lung and liver from various
animal species have supported the hypothesis that paraquat redox
cycling and associated O2- and H2O2 generation also occur in
mammalian systems (Gage, 1968b; Ilett et al., 1974; Montgomery,
1976, 1977; Steffen & Netter, 1979; Talcott et al., 1979). Bus et
al. (1974) reported that the single electron reduction of paraquat
in mammalian systems was catalysed by microsomal cytochrome P-450
reductase and NADPH. The observation that the in vivo toxicity of
paraquat in animals is markedly potentiated by exposure to elevated
oxygen tensions further supported the potential role for molecular
oxygen in mediating toxicity (Fisher et al., 1973b; Autor, 1974;
Bus & Gibson, l975; Witschi et al., 1977; Kehrer et al., 1979;
Keeling et al., 1981).
The results of in vivo studies conducted by Bus et al. (1974)
suggested that stimulation of lipid peroxidation, which was
dependent on paraquat redox cycling and associated O2- generation,
might be an important toxic mechanism in mammalian systems.
Consistent with this hypothesis, animals fed diets deficient in
selenium or vitamin E, in order to diminish cellular antioxidant
defences, were significantly more sensitive to paraquat toxicity
than control animals (Bus et al., 1975; Omaye et al., 1978). In
contrast to these studies, a number of studies have shown that
paraquat inhibited in vitro microsomal lipid peroxidation (Ilett
et al., 1974; Montgomery & Niewoehner, 1979; Steffen & Netter,
1979; Kornburst & Mavis, 1980). Subsequent studies have indicated,
however, that paraquat would stimulate microsomal lipid
peroxidation when an adequate supply of electrons (NADPH) and in
vitro oxygen tensions were maintained (Trush et al., 1981, 1982).
Despite the evidence described above, the hypothesis that lipid
peroxidation is the underlying toxic mechanism functioning in vivo
has not been conclusively demonstrated. Direct quantification of
paraquat-induced lipid peroxidation damage in vivo by analysis of
tissue malondialdehyde levels or ethane exhalation, both markers of
peroxidation injury, has been largely unsuccessful (Reddy et al.,
1977; Shu et al., 1979; Steffen et al., 1980). Furthermore,
attempts to counteract paraquat toxicity by administration of
various antioxidants have also been unsuccessful (Fairshter, 1981).
Superoxide radicals generated in paraquat redox cycling may
induce biochemical changes other than the initiation of
peroxidation reactions. Ross et al. (1979) demonstrated that
paraquat increased DNA strand breaks in cultured mouse
lymphoblasts. Paraquat was also reported to induce a superoxide-
dependent stimulation of guanylate cyclase (EC 4.6.1.2) activity in
rat liver (Viseley et al., 1979) and guinea-pig lung (Giri &
Krishna, 1980). These investigators postulated that increased
cyclic GMP might stimulate the pulmonary fibroproliferative changes
characteristic of paraquat toxicity (section 7.1.1.1). In other
studies, paraquat has also been found to increase collagen
synthesis in rat lung (Hollinger & Chvapel, 1977; Greenberg et al.,
1978; Thompson & Patrick, 1978; Hussain & Bhatnagar, 1979).
Redox cycling of paraquat has also been proposed to lead to
increased oxidation of cellular NADPH (Brigelius et al., 1981;
Keeling et al., 1982). The activity of pentose shunt enzymes in
the lung rapidly increased in rats administered paraquat, which
suggested an increased demand for NADPH (Fisher et al., 1975; Rose
et al., 1976). The observation that paraquat decreased fatty-acid
synthesis in lung slices (Smith et al., 1979) further supported
this hypothesis, since fatty acid synthesis requires NADPH. Direct
analysis of NADPH in the lung has confirmed that paraquat treatment
decreased the NADPH content in rat lung (Witschi et al., 1977;
Smith et al., 1979). These observations led Smith et al. (1979) to
propose that oxidation of NADPH might not only interrupt vital
physiological processes, such as fatty-acid synthesis, but also
render tissues more susceptible to lipid peroxidation by decreasing
the equivalents (NADPH) necessary for the function of the
antioxidant enzyme glutathione peroxidase (EC 1.11.1.9) (Fig. 6).
The importance of molecular oxygen and the potential role of O2-
generation in mediating have been implicated in studies on plants
(section 3.3), bacteria, and in in vitro and in vivo mammalian
systems. In cultures of Escherichia coli, Hassan & Fridovich
(1977, 1978, 1979) demonstrated that paraquat stimulated cyanide-
resistant respiration, which could be almost entirely accounted for
by an NADPH-dependent formation of O2-. The possibility that
formation of O2- might be responsible for the toxicity of paraquat
in bacteria was supported by observations that bacteria containing
elevated activities of superoxide dismutase, an enzyme that
detoxifies O2-, were resistant to paraquat toxicity (Hassan &
Fridovich, 1977, 1978; Moody & Hassan, 1982).
In vitro studies on preparations of lung and liver from various
animal species have supported the hypothesis that paraquat redox
cycling and associated O2- and H2O2 generation also occur in
mammalian systems (Gage, 1968b; Ilett et al., 1974; Montgomery,
1976, 1977; Steffen & Netter, 1979; Talcott et al., 1979). Bus et
al. (1974) reported that the single electron reduction of paraquat
in mammalian systems was catalysed by microsomal cytochrome P-450
reductase and NADPH. The observation that the in vivo toxicity of
paraquat in animals is markedly potentiated by exposure to elevated
oxygen tensions further supported the potential role for molecular
oxygen in mediating toxicity (Fisher et al., 1973b; Autor, 1974;
Bus & Gibson, l975; Witschi et al., 1977; Kehrer et al., 1979;
Keeling et al., 1981).
The results of in vivo studies conducted by Bus et al. (1974)
suggested that stimulation of lipid peroxidation, which was
dependent on paraquat redox cycling and associated O2- generation,
might be an important toxic mechanism in mammalian systems.
Consistent with this hypothesis, animals fed diets deficient in
selenium or vitamin E, in order to diminish cellular antioxidant
defences, were significantly more sensitive to paraquat toxicity
than control animals (Bus et al., 1975; Omaye et al., 1978). In
contrast to these studies, a number of studies have shown that
paraquat inhibited in vitro microsomal lipid peroxidation (Ilett
et al., 1974; Montgomery & Niewoehner, 1979; Steffen & Netter,
1979; Kornburst & Mavis, 1980). Subsequent studies have indicated,
however, that paraquat would stimulate microsomal lipid
peroxidation when an adequate supply of electrons (NADPH) and in
vitro oxygen tensions were maintained (Trush et al., 1981, 1982).
Despite the evidence described above, the hypothesis that lipid
peroxidation is the underlying toxic mechanism functioning in vivo
has not been conclusively demonstrated. Direct quantification of
paraquat-induced lipid peroxidation damage in vivo by analysis of
tissue malondialdehyde levels or ethane exhalation, both markers of
peroxidation injury, has been largely unsuccessful (Reddy et al.,
1977; Shu et al., 1979; Steffen et al., 1980). Furthermore,
attempts to counteract paraquat toxicity by administration of
various antioxidants have also been unsuccessful (Fairshter, 1981).
Superoxide radicals generated in paraquat redox cycling may
induce biochemical changes other than the initiation of
peroxidation reactions. Ross et al. (1979) demonstrated that
paraquat increased DNA strand breaks in cultured mouse
lymphoblasts. Paraquat was also reported to induce a superoxide-
dependent stimulation of guanylate cyclase (EC 4.6.1.2) activity in
rat liver (Viseley et al., 1979) and guinea-pig lung (Giri &
Krishna, 1980). These investigators postulated that increased
cyclic GMP might stimulate the pulmonary fibroproliferative changes
characteristic of paraquat toxicity (section 7.1.1.1). In other
studies, paraquat has also been found to increase collagen
synthesis in rat lung (Hollinger & Chvapel, 1977; Greenberg et al.,
1978; Thompson & Patrick, 1978; Hussain & Bhatnagar, 1979).
Redox cycling of paraquat has also been proposed to lead to
increased oxidation of cellular NADPH (Brigelius et al., 1981;
Keeling et al., 1982). The activity of pentose shunt enzymes in
the lung rapidly increased in rats administered paraquat, which
suggested an increased demand for NADPH (Fisher et al., 1975; Rose
et al., 1976). The observation that paraquat decreased fatty-acid
synthesis in lung slices (Smith et al., 1979) further supported
this hypothesis, since fatty acid synthesis requires NADPH. Direct
analysis of NADPH in the lung has confirmed that paraquat treatment
decreased the NADPH content in rat lung (Witschi et al., 1977;
Smith et al., 1979). These observations led Smith et al. (1979) to
propose that oxidation of NADPH might not only interrupt vital
physiological processes, such as fatty-acid synthesis, but also
render tissues more susceptible to lipid peroxidation by decreasing
the equivalents (NADPH) necessary for the function of the
antioxidant enzyme glutathione peroxidase (EC 1.11.1.9) (Fig. 6).
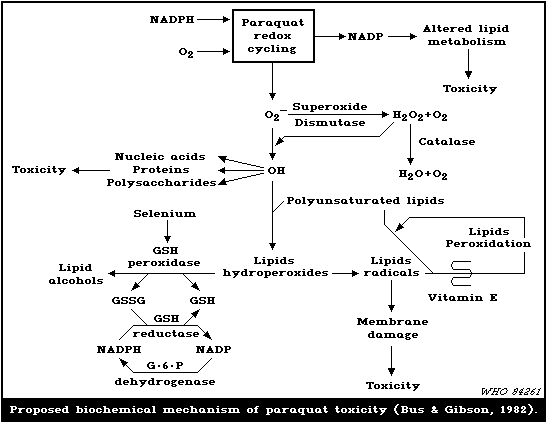 7. EFFECTS ON ANIMALS
7.1. Effects on Experimental Animals
7.1.1. Respiratory system
Toxicity studies in rats, mice, dogs, and monkeys (Clark et
al., 1966; Kimbrough & Gaines, 1970; Murray & Gibson, 1972;
Makovskii, 1972; Kelly et al., 1978) demonstrated that paraquat had
a specific effect on the lung (Table 11). Administration by every
route of entry tested whether parenteral (Fisher et al., 1973a;
Robertson, 1973; Hunsdorfer & Rose, 1980), oral (Clark et al.,
1966; Bainova, 1969a; Kimbrough, 1974; Tsutsui et al., 1976;
Dikshith et al., 1979), dermal (Howe & Wright, 1965; Bainova,
1969b; McElligott, 1972), or inhalatory (Gage, 1968b; Bainova,
1971; Makovskii, 1972; Seidenfeld et al., 1978) resulted in
irreversible changes in the lung.
Clark et al. (1966) reported that, in rats, in the earlier
stages after a single toxic oral dose of paraquat, breathing was
gasping or deep and fast, but some days after a single or repeated
toxic doses, the respiration became increasingly laboured, and the
hairs around the mouth and nares were soiled with a brownish
liquid. The extensive alveolar oedema observed in severe
intoxication was responsible for the development of hypoxia,
cyanosis, and dyspnoea. The progressive development of pulmonary
fibrosis was accompanied by difficulty in breathing, gasping, and
hyperpnoea (Smith et al., 1973).
Exposure of rats to high concentrations of respirable paraquat
aerosols was accompanied by shallow respiration. Within 2 - 3 h,
the test animals became dyspnoeic, cyanotic, and inactive, and
there were signs of local eye and nose irritation (Gage, 1968a).
7.1.1.1. Pathomorphological lung studies
Macroscopic examination of the lungs revealed that lesions and
their severity were dependent on the dose of paraquat and the time
between exposure and sacrifice (or death). The wet weight of the
lung increased after a single treatment, owing to oedema and
haemorrhage. The pathogenesis of the paraquat lung lesion has been
well characterized, and has been reviewed by Smith & Heath (1976).
The acute pulmonary toxicity of paraquat in animals has been
described as occurring in two phases (Smith & Heath, 1976). In the
initial "destructive" phase, alveolar epithelial cells were
extensively damaged and their subsequent disintegration often
resulted in a completely denuded alveolar basement membrane.
Table 11. Effects on experimental animals of repeated oral, dermal, or inhalation exposure to paraquat
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rat diet - 125 mg/kg 2 years no toxic effects Howe & Wright (1965)
Dog diet - 50 mg/kg no toxic effects
Rat diet - 0.25 mg/kg 27 days death; histological changes in the lung Clark et al. (1966)
Rat diet - 300, 400, 90 days cumulative toxic effects; chronicity Kimbrough & Gaines
500, 600, 700 mg/kg factor (Hayes) 5.2; histological (1970)
changes in the lung
Rat oral - 4, 9, 25 30 days inhibition of ChE activity, increasing Bainova (1969, 1975)
mg/kg body weight GPT activity in the serum; biochemical
per day and histological changes in the lung,
kidney, liver
Rat oral - 1.3, 2.6 4 1/2 months increased GPT and G-6-P-isomease Bainova (1969, 1975)
mg/kg body weight activities in the serum; biochemical
per day and histological changes in lung,
kidney, liver
Rat oral - 3.3, 1.3, 1 year the higher doses were toxic for both Makovskii (1972)
0.13 mg/kg body species tested; no-observed-adverse-
weight per day effect levels:
Guinea- oral - 1.0, 0.4, for rat 0.13, guinea-pig 0.04 mg/kg
pig 0.04 mg/kg body body weight per day
weight per day
Rat diet - 20 - 30 30 days histological and electron-optical lung Kimbrough (1974)
mg/kg body weight changes
per day
Mouse diet - 25, 50, 70 80 weeks death; dose-dependent clinical and FAO/WHO (1973)
mg/kg histological changes in the lung,
liver, kidney, and other organs tested
Rat oral - 25, 50, 1 - 5 days body weight loss; increased serum LDH, Tsutsui et al.
100 mg/kg body GOT activity; no haematological (1976)
weight per day changes; histological changes in the
lung, kidney, liver, myocardium
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rat drinking-water - 2 years mortality increased; histological Bainova & Vulcheva
1.3, 2.6 mg/litre changes in the lung, but only minimal (1977)
at the lowest level
Rabbit dermal - 2.8, 4.5, 20 days skin irritation; mortality and toxic Clark et al. (1966)
7, 14 mg/kg body effects at 7 & 14 mg/kg/day. LD50
weight per day mg/kg/day; no-observed-adverse-effect
level 2.8 mg/kg/day
Rat dermal - 2, 5, 15, 21 days skin irritation; mortality and toxic Bainova (1969a)
30, 45 mg/kg body effects at 5 - 45 mg/kg/day;
weight per day histological changes in the lung,
kidney, liver, myocardium; LD50
15 mg/kg/day; no-observed-adverse-
effect level 2 mg/kg/day
Rabbit dermal - from 1.56 20 days skin irritation; mortality and toxic McElligott (1972)
- 50 mg/kg per day effects at 3.13 - 192 mg/kg/day; LD50
(with occlusion) 4.5 mg/kg/day with occlusion and 24
from 2.4 - 192 mg/kg mg/kg/day without occlusion; No-
body weight per day observed-adverse-effect levels: 1.56
(without occlusion) and 2.4 mg/kg/day with and without
occlusion
Rat inhalationa - 4 days at higher concentrations (0.40 & 0.75 Gage (1968)
0.75 mg/m3 mg/m3) histological changes in the
0.4, 0.1, 0.06 mg/m3 15 days lung; no-observed-adverse-effect levels
0.003 mg/m3 60 days from 0.003 - 0.06 mg/m3 6 h daily;
6 h daily TLV - 0.1 mg/m3 paraquat aerosol
Rat inhalationa - 4 1/2 months biochemical, histochemical, and Bainova et al.
1.1, 0.05 mg/m3 histological changes in the lung at (1972)
6 h daily 1.1 mg/m3 no-observed-adverse-effect
level below 0.05 mg/m3 paraquat aerosol
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rabbit inhalationa - 3 months no clinical, functional and Seidenfeld et al.
10 mg paraquat in histological changes in the lung; no (1978)
100 ml water for the toxic effects
aerosol 2 h daily
---------------------------------------------------------------------------------------------------------
a Respirable paraquat aerosol.
Pulmonary oedema was also a characteristic of the destructive
phase, and was frequently of sufficient severity to result in the
death of the animals. Animals surviving the initial destructive
phase, which occurred in the first 1 - 4 days after acute paraquat
overexposure, progressed to what has been termed the
"proliferative" phase. In this phase, the lung was infiltrated
with profibroblastic cells that rapidly differentiated into
fibroblasts which, in some cases, progressed to fibrosis. The
histopathological outcome of the second phase may be influenced by
the treatment regimen, however. Administration of repeated low
doses of paraquat, which less severely damaged the alveolar
epithelial cells, could also induce a hyperplasia of the Type II
cells. This response may represent an attempt by the lung to
repair the damaged epithelium.
Following a single high dose of paraquat to animals, the
earliest ultrastructural changes were observed in the Type I
alveolar epithelial cells, approximately 4 - 6 h after treatment,
and were usually characterized by cellular and mitochondrial
swelling, increased numbers of mitochondria, and the appearance of
dark granules in the cytoplasm. When a high dose (approximately
LD50 or greater) was given, the lesions in the Type I cells often
progressed to the point of complete cellular disintegration leaving
areas of exposed basement membrane (Kimbrough & Gaines, 1970; Smith
et al., 1973; Smith & Heath, 1974; Vijeyaratnam & Corrin, 1971;
Klika et al., 1980).
In contrast to the effects on Type I pneumocytes, however, the
capillary endothelial cells were remarkably resistant to the toxic
effects of paraquat (Sykes et al., 1977).
Ultrastructural lesions in the alveolar Type II pneumocytes
were also observed shortly after single dose paraquat exposure,
although, generally, these lesions were not apparent until after
the first lesions were seen in the Type I cells (Kimbrough &
Gaines, 1970). Swollen mitochondria and damage to the lamellar
bodies usually occurred between 8 and 24 h after a high dose of
paraquat (Robertson, 1973; Robertson et al., 1976). Progressive
deterioration of the Type II cells continued, resulting in
completely denuded alveolar basement membranes and debris-filled
alveolar spaces (Vijeyaratnam & Corrin, 1971). Infiltration and
proliferation of fibroblasts may produce fibrosis that obliterates
the alveolar structure (Smith & Heath 1974).
Vijeyaratnam & Corrin (1971) observed that less severely
affected parts of the lung appeared to undergo epithelial
regeneration, 7 - 14 days after a single dose of paraquat.
Electron microscopic examination revealed the alveoli to be
lined with cuboidal epithelial cells that closely resembled
Type II pneumocytes except for a general lack of lamellar bodies.
Similar phenomena have also been noted by other investigators who
administered paraquat in the diet (Kimbrough & Linder, 1973) or as
repetitive intraperitoneal administrations (Smith et al., 1974).
Thus, in animals where the paraquat dose was sufficient to kill
only the Type I pneumocytes, the surviving Type II cells repaired
the damaged epithelium by proliferating and subsequently
differentiating into Type I epithelial cells. Inhaled paraquat in
aerosol produced initial necrosis and sloughing of the epithelia
and type 2 pneumocyte hyperplasia, fibroblast proliferation, and
increased synthesis of collagen in mice (Popenoe, 1979).
Histochemical alterations have been noted in rats exposed
through inhalation to 1.9 and 1.1 mg/m3 paraquat respirable
aerosol, 6 h/day, 6 days/week, for 4 1/2 months. The histoenzyme
activity of NAD lactate dehydrogenase-diaphorase, beta-
glucuronidase (EC 3.2.1.31), and acid phosphatase (EC 3.1.3.2) was
enhanced in the epithelial cells and in areas of pneumonitis
(Bainova et al., 1972). The changes were concentration-related,
although the activity of succinate dehydrogenase (EC 1.3.99.1) and
aspartate esterase appeared to be less pronounced in comparison
with the controls (Bainova et al., 1972).
7.1.1.2. Species differences in lung injury
Butler & Kleinerman (1971) injected rabbits intraperitoneally
with total doses of from 2 - 100 mg/kg body weight. Thymus atrophy
was observed, but most lungs showed only occasional and small
histological deviations that were poorly correlated with the
clinical signs of paraquat intoxication. The study confirmed the
resistance of the rabbit to paraquat-induced lung lesions (Clark et
al., 1966), and no evidence of any kind of pulmonary disease was
found; nor could significant lung injury be established in rabbits
after 30 days ingestion of 11 mg paraquat/kg in distilled water
(Dikshith et al., 1979). However, some animals showed pulmonary
fibrosis and emphysema, and a few changes were present in all
parenchymatous organs (Mehani, 1972; Zavale & Rhodes, 1978;
Dikshith et al., 1979). The rabbit also proved to be less
sensitive, than the rat, after inhalation exposure (Gage 1968a;
Seidenfeld et al., 1978).
According to Murray & Gibson (1972), and Hundsdorfer & Rose
(1980), guinea-pigs treated orally or sc did not develop the same
type of progressive pulmonary fibrosis as paraquat-intoxicated
rats. In hamsters, a single administration did not induce lung
damage, but prolonged exposure resulted in lung fibrosis (Butler,
1975).
In conclusion, for lung toxicity studies, a characteristic
dose-related pulmonary fibrosis can be induced in the rat, mouse,
dog, and monkey (Murray & Gibson, 1972) but not in the rabbit,
guinea-pig, or hamster.
7.1.1.3. Lung function studies
Rabbits exposed to an aerosol of 200 mg paraquat in 100 ml
distilled water (Seidenfeld et al., 1978) survived more than 3
exposures but showed significantly reduced arterial oxygen tension
and an increased alveolar arterial O2 gradient; specific compliance
decreased and functional residual capacity and breathing frequency
increased. Lam et al. (1980) administered paraquat at 27 mg/kg
body weight ip to rats and 0.5 mg/kg body weight intratracheally.
After 12 h, decreases were observed in total lung capacity,
functional residual capacity, vital capacity, residual volume, and
alveolar volume. These deviations persisted for 72 h. Oral
administration of paraquat at doses ranging from 1 mg/kg body
weight - 13.5 mg/kg body weight to rats resulted in functional lung
changes after 24 h.
Thus clinical, functional, and pathomorphological studies after
single and repeated exposure demonstrated that the spectrum of
paraquat lung disease depended on the magnitude of the dose and the
manner of administration (Seidenfeld et al., 1978; Restuccia et
al., 1974).
7.1.2. Renal system
In paraquat toxicity, kidney damage often precedes signs of
respiratory distress (Clark et al., 1966; Butler & Kleinerman,
1971; Murray & Gibson, 1972) (Table 11). Paraquat is excreted
mainly via the urine and the concentrations of the herbicide in the
kidneys are relatively high (section 6.1). Gross pathological and
histological examinations of paraquat-poisoned rats, guinea-pigs,
rabbits, and dogs revealed vacuolation of the convoluted renal
tubules and proximal tubular necrosis (Bainova, 1969a; Murray &
Gibson, 1972; Tsutsui et al., 1976). The degeneration of the
proximal tubular cells has also been confirmed by electron-optical
studies (Fowler & Brooks, 1971; Marek et al., 1981).
Paraquat is actively secreted by the kidney base transport
system. The nephrotoxicity caused by paraquat is pronounced and
appears to be restricted to the proximal nephron (Ecker et al.,
1975: Gibson & Cagen, 1977; Lock & Ishmael, 1979; Purser & Rose,
1979).
7.1.3. Gastrointestinal tract and liver
The clinical signs of acute and chronic oral poisoning
(Kimbrough & Gaines, 1970; Murray & Gibson, 1972; Bainova, 1969a)
or of ip injection (Butler & Kleinerman, 1971) include transient
diarrhoea and body weight loss, decreased food intake, and
dehydration. Some of the animals vomited soon after paraquat
administration. Residual skin contamination after dermal toxicity
studies on rabbits (McElligott, 1972) caused severe tongue
ulceration and an unwillingness to eat. The adverse irritant
effects were minimized by continued restraint after skin
decontamination of the treated rabbits.
There have been several reports of liver damage following
exposure to high doses of paraquat (Clark et al., 1966; Bainova,
1969a; Murray & Gibson, 1972; Tsutsui et al., 1976; Gibson & Cagen,
1977, Cagen et al., 1976). Centrilobular necrosis of hepatocytes
with proliferation of the Kupfer cells and bile canals have been
described.
In general, liver damage in experimental animals has not been
severe compared with lung and kidney damage. Serum enzyme
activities (SGOT, SGPT, LAP) only increased when large amounts of
paraquat were given (Giri et al., 1979).
7.1.4. Skin and eyes
The herbicide can provoke local irritation of the skin and
eyes. Clark et al. (1966) found skin irritation in rabbits only
when paraquat was applied beneath occlusive dressings in aqueous
solutions (total dose 1.56, 5.0, and 6.25 mg ion/kg body weight).
In mice and rats, the application of 5 - 20 g paraquat/litre
solutions in single and 21-day repeated dermal toxicity tests
provoked dose-related toxic dermatitis with erythema, oedema,
desquamation, and necrosis (Bainova, 1969b). Doses from 1.56 to 50
mg/kg, in repeated 20-day studies using the occlusive technique
(McElligott, 1972) resulted in local erythema and scab formation.
The histological changes consisted of parakeratosis and occasional
intra-epidermal pustules. A delayed skin irritant action of the
herbicide was reported by Fodri et al. (1977) in guinea-pig
studies.
No skin sensitization was observed in studies on guinea-pigs
when paraquat was applied (Bainova, 1969b; Fodri et al., 1977).
The instillation of dilutions of paraquat (up to 500 g/litre)
in rabbit eye induced inflammation within 24 h and this continued
for 96 h (Clark et al., 1966). Sinow & Wei (1973) introduced 62.5,
125, 250, 500, and 1000 paraquat/litre into the rabbit eye.
Concentrations of 62.5 and 125 g/litre caused severe conjunctival
reactions; higher levels (250 - 500 g/litre) provoked iritis and
pannus, while at the 500 g/litre concentration there was corneal
opacification, iritis, and conjunctivitis. All rabbits receiving
0.2 ml of paraquat at 1000 g/litre in 1 eye or 0.2 ml of a
concentration of 500 g/litre in both eyes died within 6 days of
application (Sinow & Wei, 1973).
Both conjunctival and dermal application of different
concentrations induced systemic toxicity (Sinow & Wei, 1973; Clark
et al., 1966; Bainova, 1969b; Kimbrough & Gaines, 1970; Makovskii,
1972; McElligott, 1972), lung, kidney, and liver damage, and death.
7.1.5. Other systems
No specific functional, histological, or biochemical effects of
paraquat have been reported in other systems that have been
examined; this is of prime importance in an evaluation of its
toxicity. When lethal doses of paraquat are given to rats,
symptoms consistent with neurological disturbances have been
observed. These include decreased motor activity, lack of
coordination, ataxia and dragging of the hind limbs (Smith et al.,
1973). Also associated with near lethal or lethal doses are damage
to the myocardium (Tsutsui et al., 1974), haemolytic anaemia
(Bainova, 1969a), increased haemosiderin in the spleen (Bainova et
al., 1972) and increased concentrations of plasma corticosteroids
(Rose et al., 1974b).
7.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
7.1.6.1. Effects on reproduction
Some histological changes in the testes have been reported in a
few paraquat toxicity studies. Butler & Kleinerman (1971) found
multinuclear giant cells in rabbit testicular tubules. When
paraquat was orally administered at 4 mg/kg body weight to male
rats for 60 days and the testes were examined, there were no
significant deviations in the spermatozoa count or motility, nor
were there any biochemical changes in the several enzymes of testes
homogenates. The histoenzyme activity of lactate dehydrogenase,
succinate dehydrogenase, DPN-diaphorase, alkaline phosphatase, and
acid phosphatase in the treated animals did not differ from that of
the controls, nor did quantitative and qualitative histological
examination of the testicular tubule cells reveal any abnormality.
A 3-generation reproduction study has been carried out on rats
treated with paraquat ion at 100 mg/kg diet (FAO/WHO, 1973). There
were no significant abnormalities in fertility, fecundity, and
neonatal morbidity or mortality, nor were there any signs of
gonadotoxicity or structural or functional lesions. Pulmonary
function in the treated offspring was normal.
Clegg (1979) has reviewed animal reproduction and
carcinogenicity studies conducted in relation to the safe use of
pesticides.
7.1.6.2. Embryotoxicity and teratogenicity
Oral or ip administration of high doses of paraquat to mice and
rats on various days of gestation produced significant maternal
toxicity, evidenced by increased mortality rates (Bainova &
Vulcheva, 1974; Bus et al., 1975). Examination of the fetuses from
the higher-dose groups revealed a reduction in fetal body weights,
delayed ossification of the sternabrae, and increased resorption
rate in mice, as a result of the maternal intoxication. The
minimal embryotoxic effect seemed due in part to difficulty in
crossing the placenta, reflected by low concentrations of paraquat
in the embryo relative to maternal tissues (Bus et al., 1975). The
absence of a specific embryotoxic action of paraquat has also been
observed and reported in other studies on rats (Khera et al., 1968;
Luty et al., 1978), mice (Selypes et al., 1980), and rabbits
(FAO/WHO, 1973).
In a perinatal toxicity study, Bus & Gibson (1975) administered
paraquat at 50 or 100 mg/litre in the drinking-water to pregnant
mice beginning on day 8 of gestation, with continued treatment of
the litters up to 42 days after birth. Paraquat treatment did not
alter postnatal growth rate, although the mortality rate in the 100
mg/litre-treated mice increased to 33% during the first 7 days
after birth. It was also noted that paraquat at 100 mg/litre
significantly increased the sensitivity of the pups to oxygen
toxicity on days 1, 28, and 42 after birth.
7.1.7. Mutagenicity
Paraquat has been found to have minimal to no genotoxic
activity when evaluated in a variety of in vitro and in vivo test
systems. In studies producing weakly positive results (Moody &
Hassan, 1982; Parry 1977, 1973; Tweats, 1975; Benigni et al., 1979;
Bignami & Grebelli, 1979), which were limited to in vitro studies,
paraquat genotoxicity was accompanied by high cytotoxicity. These
results are best explained by Moody & Hassan (1982), who showed
that the mutagenicity of paraquat in bacterial test systems
( Salmonella typhimurium TA 98 and TA 100) was mediated by the
formation of superoxide. However, other investigators (Andersen et
al., 1972; Levin et al., 1982) did not find mutagenic activity in
bacterial test systems. Furthermore, paraquat was not mutagenic
when evaluated in human leukocytes and in in vivo cytogenetic
tests on mouse bone marrow (Selypes & Paldy, 1978) and dominant
lethal tests on mice (Pasi et al., 1974; Anderson et al., 1976).
7.1.8. Carcinogenicity
A carcinogenicity study was performed on mice at dietary levels
of 25, 50, and 75 mg/kg per day for 80 weeks (FAO/WHO, 1973).
There were reduced weight gains among the animals receiving
paraquat, but deaths during the study were associated with
respiratory disease. Clinical and histopathological examination
determined that paraquat was not tumorigenic in mice.
A 2-year exposure of rats to 1.3 and 2.6 mg/litre, daily, in
the drinking-water provoked histopathological changes in the lung,
liver, kidney, and myocardium. The lung lesions were dose-related;
inflammation, atelectasis, reactive proliferation of the
epithelium, pulmonary fibrosis, and pulmonary adenomatosis were
noted, but no sign of tumour growth or atypism (Bainova & Vulcheva,
1977). Nor was any increased tumour incidence reported in rats in
a 2-year study with a maximum dietary level of 250 mg/kg diet (12.5
mg/kg body weight per day) (FAO/WHO, 1971).
Bainova & Vulcheva (1977) did not discover any indication of
tumorigenicity in a 2-year study on rats receiving paraquat at 1.3
or 2.6 mg/litre in their drinking-water (Table 11).
While testing the carcinogenicity of urethane in mice, Bojan et
al. (1978) also attempted to evaluate the influence of paraquat on
urethane-induced lung tumorigenesis. It is felt that the results
of this study are not of relevance for the assessment of the
carcinogenic potential of paraquat.
7.2. Effects on Farm Animals
The effects of paraquat on farm animals has been discussed in
section 4.3.5. The LD50 doses have been established for hen,
turkey, cow, and sheep (Howe & Wright, 1965; Clark et al., 1966;
Smalley, 1973). Massive doses resulted in convulsions,
neurological symptoms, and death due to respiratory failure.
Domestic animals may ingest paraquat by feeding on a sprayed
area, as a result of spray drifting on to their pasture, by
drinking water contaminated with paraquat used as an aquatic
herbicide, or by feeding on a crop sprayed with paraquat as a
dessicant. Sheep and calves were given paraquat at concentrations
of up to 20 mg/litre drinking-water for 1 month without any obvious
ill effects (Howe & Wright, 1965; Calderbank, 1972), and a cow
dosed with 2/3 of the LD50 of 14C-paraquat gave milk containing
less than 0.1 mg/litre. Field tests demonstrated that cattle did
not suffer any toxic effects when turned loose on pasture after it
had been sprayed with paraquat at 0.45 kg/ha. The same trial
showed that horses had local lesions of the mouth and increased
mucous secretion after grazing on newly-sprayed pasture (Calderbank
et al., 1968). The hazard to stock feeding on such pasture depends
on the density of the pasture, the dose of the herbicide, and the
length of time that has elapsed since its application.
Paraquat was fed to cattle at levels in herbage of 200 - 400
mg/kg for 1 month without any apparent ill effects, and no residues
could be detected in the meat and milk (Calderbank et al., 1968).
However, all domestic animals should be kept far from freshly-
sprayed areas, and when crops are treated with paraquat, due
attention should be paid to the accepted maximum residue limits.
7.3. Dose-Effect of Paraquat
The acute LD50 values for paraquat in various species are given
in Tables 12 and 13. The acute toxicity studies of paraquat salts
(dichloride, dimethylsulfate, dimethylphosphate) have not shown
any significant differences in the acute oral and ip LD50 in rats
(Clark et al., 1966; Makovskii, 1972).
There were no significant differences in the oral LD50 values
obtained for the same species from different laboratories, but the
acute oral LD50 values among the species examined varied.
The effects of repeated paraquat exposure are summarized in
Table 11. Paraquat was administered, orally and in the diet, to
rats, mice, guinea-pigs, and dogs. The guinea-pigs appeared to be
very sensitive (Makovskii, 1972). According to Kimbrough & Gaines
(1970), Makovskii (1972), and Bainova (1975), the herbicide has a
moderate cumulative toxicity. The joint FAO/WHO meeting (1976)
decided on a no-observed-adverse-effect level of 1.5 mg/kg body
weight per day in the rat and 1.25 mg/kg body weight per day in the
dog. As can be seen from Table 11, effects at lower levels have
been observed in other studies.
Guinea-pigs, monkeys, cattle, and human subjects are more
sensitive, while rats and birds are less sensitive to paraquat
through the gastrointestinal route.
Table 12. Paraquat LD50 (mg/kg body weight) and LC50
(mg/m3) in various species
-------------------------------------------------------------------
Species/Sex Oral Dermal Inhalation LC50
LD50 LD50 respirable
paraquat aerosol
-------------------------------------------------------------------
Rat 200a 1c
Rat (F) 100e 90e 10f
Rat (M) 110e 80e 10f
Rat 126i 350g 6g
Mouse 62d
Rabbit 500a
Rabbit 236b
Rabbit 240h
Guinea-pig 40 - 80a
Guinea-pig (M) 30b
Guinea-pig 22i
Guinea-pig 42g 319g 4g
Monkey 50i
Cat 40 - 50a
Cat (F) 35b
Hen 300 - 380a
Hen 262b
Turkey 250 - 280j approximately
375j
Cow 50 - 75a
Sheep 50 - 75a
-------------------------------------------------------------------
a Howe & Wright (1965).
b Clark et al. (1966).
c Gage (1968).
d Bainova (1971).
e Kimbrough & Gaines (1970).
f Bainova & Vulcheva (1972).
g Makovskii (1972).
h McElligott (1972).
i Murray & Gibson (1972).
j Smalley (1973).
Table 13. Paraquat LD50 (mg/kg body weight) after parenteral
treatment
-------------------------------------------------------------------
Species/Sex Subcutaneous Intraperitoneal Intravenous
-------------------------------------------------------------------
Rat (F) 19a
Rat 22b
Mouse 30e 50d
Guinea-pig (F) 3a
Guinea-pig 5b
Turkey 100c 20c
-------------------------------------------------------------------
a Clark et al. (1966). d Ecker et al. (1975).
b Makovskii (1972). e Bus et al. (1975).
c Smalley (1973).
7.4. Methods for Decreasing Paraquat Toxicity
These have been studied in connection with requirements in the
case of paraquat poisoning in man. Clark (1971) showed the
efficacy of Bentonite and Fuller's earth in binding orally
administered paraquat and preventing its absorption from the
gastrointestinal tract. Staiff et al. (1973) reported the high
adsorption capacity of Amerlite. Smith et al. (1974) found
considerably reduced plasma-paraquat levels after the combined
treatment of rats with purgatives and bentonite suspension; these
rats survived a dose that normally killed 90 - 100% of the animals.
The absorption capacities of six absorbent materials were tested by
Okonek et al. (1982) who demonstrated that activated charcoal was
the most successful in absorbing ingested paraquat in rats.
Another way of decreasing paraquat absorption is to introduce
an emetic in the concentrated formulations. Kawai et al. (1980)
examined the protection this provided in fasting and non-fasting
male and female dogs that were given paraquat containing an emetic.
The amount of paraquat eliminated by vomiting was 61 - 86% of the
orally-administered dose. In the group given paraquat only, the
blood level averaged 44 mg/litre; in the group given paraquat and
emetic, it was 0.26 mg/litre.
7.5. Relation Between Age, Sex, and Toxicity
There is no evidence that paraquat is more toxic to either sex
of adult experimental animals (section 7.3) Young rats were more
resistant than older rats, and some authors have paralleled this
resistance with that of young rats to oxygen toxicity. Smith &
Rose (1977b) found a more than 40% increase in cumulative mortality
in 180 g rats compared with 50 g rats, after oral dosing with
paraquat at 680 µmol/kg body weight. According to Smith & Rose
(1977b), the difference in renal function between young and mature
rats accounted for the difference in paraquat toxicity.
8. EFFECTS ON MAN
8.1. Accidental and Suicidal Poisoning
8.1.1. Case reports
The first fatalities from acute paraquat poisoning occurred in
1964 and were reported in 1966 (Bullivant, 1966). By 1977, 600
deaths had been reported following accidental or intentional
ingestion of paraquat. The number of accidental cases of poisoning
is small relative to instances of suicide. Because of different
requirements or practices for notification or reporting of cases of
poisoning in the many countries in which paraquat is used, the
magnitude of the problem is difficult, if not impossible, to
determine. Some representative reports on acute paraquat poisoning
are summarised in Table 14.
The earlier cases of paraquat intoxication were mostly
accidental (Fennelly et al., 1968; Matthew et al., 1968; Masterson
& Roche, 1970; Malone et al., 1971). These cases seemed to have
resulted mainly from the habit of decanting the liquid formulations
into small unmarked or incorrectly labelled containers such as
beer, wine, or soft-drink bottles.
An increased ratio of suicidal to accidental poisoning has been
noted in recent years (Fletcher, 1975; Carson & Carson, 1976;
Fitzgerald et al., 1978a; Bramley & Hart, 1983). This change from
accidental to suicidal poisoning was also reflected in the enhanced
percentage of fatal cases, shorter survival times, and
significantly higher tissue and body fluid levels (Connolly et al.,
1975; McGeown, 1975; Park et al., 1975; Carson & Carson, 1976;
Howard, 1979a; Sugaya et al., 1980; Bismuth et al., 1982).
While the vast majority of poisoning cases are due to
swallowing, a small number of fatal cases of accidental paraquat
poisoning via the skin have been reported when liquid concentrates
(200 g/litre) have been applied in order to kill body lice (Ongom
et al., 1974; Binns, 1976). A few other fatal and non-fatal cases
have been reported following skin-contamination (McDonagh & Martin,
1970; Kimura et al., 1980).
Table 14. Case report data on accidental and suicidal acute paraquat poisoning
-------------------------------------------------------------------------------
Number of cases Fatal Non-fatal Fatality Reference
-------------------------------------------------------------------------------
19 12 7 63% Malone et al. (1971)
24 3 accidental 10 14 42% Connolly et al. (1975)
19 suicidal
2 homicidal
25 10 accidental 17 8 68% McGeown (1975)
31 7 accidental 18 13 58% Park et al. (1975)
21 suicidal
3 homicidal
33 7 accidental 26 7 79% Carson & Carson (1976)
19 suicidal
16 7 9 44% Wright et al. (1978)
136 77 suicidal 92 44 68% Fitzgerald et al. (1978)
10 10 0 100% Natori et al. (1979)
188 69 119 37% Higginbottom et al. (1979)
79 28 51 35% Proudfoot et al. (1979)
68 68 suicidal 41 27 66% Howard (1979)
6 6 suicidal 5 1 83% Sugaya et al. (1980)
28 12 suicidal 17 11 61% Bismuth et al. (1982)
262 95% deliberate 94 (36%) 168 (64%) 36% Bramley & Hart (1983)
intent
-------------------------------------------------------------------------------
8.1.2. Distribution of cases of paraquat poisoning
Cases of acute paraquat poisoning have been reported in:
Bulgaria (Mircev, 1976), Denmark (Pederson et al.,1981), England,
Ireland, Scotland, and the Netherlands (Fletcher, 1975), the
Federal Republic of Germany (Grundies et al., 1971; Hofman &
Frohberg, 1972; Fletcher, 1975; Fischer & Kahler, 1979), France
(Faure et al., 1973; Gervais et al., 1975; Bismuth et al., 1982;
Efthymiou, 1983), Hungary (Farago et al., 1981), Poland (Firlik,
1978), Switzerland (Schlatter, 1976), the USA (Kimbrough, 1974;
Dearden et al., 1978; Stephens et a1., 1981), and in Yugoslavia
(Vucinovic, 1978). Recently, a number of cases of paraquat
poisoning, mainly suicidal, have also been reported in Japan
(Takahashi et al., 1978; Natori et al., 1979; Tomura et al., 1979;
Kimura et al., 1980; Matsumoto et al., 1981). No attempt has been
made to make this list exhaustive, in fact the distribution is
worldwide.
8.1.3. Route of entry
By far the most frequent route of poisoning has been ingestion.
An unusual case of subcutaneous injection of 1 ml paraquat by a
mentally disturbed farmer was reported in Israel (Almog & Tal,
1967). Cases of dermal poisoning have been mentioned in section
8.1.1. There is no evidence of fatal poisoning as a result of
inhalation.
8.1.4. Formulations
Paraquat trade names are listed in Table 3. Concentrated
liquid formulations have been responsible for most (and more
severe) poisonings than granular forms, which contain less paraquat
(McGeown, 1975; Park et al., 1975; Fitzgerald & Barnville, 1978;
Wright et al., 1978; Higginbottom et al., 1979; Howard, 1979a).
8.1.5. Dose
The minimum lethal dose of paraquat is stated to be about 35
mg/kg body weight for human beings (Pederson et al., 1981; Bismuth
et al., 1982).
Symptoms of poisoning depend on the dose absorbed. It is
difficult to estimate the dose absorbed from case histories since
in many cases the patients spat out part of the paraquat
concentrate or vomited profusely after swallowing the herbicide.
Some patients have survived after apparently ingesting 50 - 100 ml
Gramoxone(R) (10 - 20 g paraquat), whereas some died after taking
as little as 2 sachets of Weedol (2.5 g paraquat) (Table 15).
Howard (1979) demonstrated the relationship between the dose of
paraquat ingested, the time elapsing between ingestion and
institution of treatment, and the ultimate outcome in 68 cases of
intentional paraquat poisoning.
8.1.6. Clinical and pathomorphological data relating to fatal
paraquat poisoning
Cases of fatal poisoning can be sub-divided into cases of:
(a) acute fulminant poisoning from a massive dose leading
to generalized systemic poisoning and death from a
combination of acute pulmonary oedema, oliguria,
hepatocellular and adrenal failure and biochemical
disturbances (death usually occurs within 1 - 4 days);
(b) less overwhelming poisoning with slower onset of
organ failure and death from pulmonary oedema,
mediastinitis, and complications of therapy (McGeown,
1975; Fitzgerald et al., 1978a); and
(c) late pulmonary fibrosis (death ensuing 4 days to
several weeks later).
Table 15. Recovery from paraquat poisoning involving lung dysfunction
---------------------------------------------------------------------------------------------------------
Dose of paraquat Major organ Notes Reference
ingested damage
---------------------------------------------------------------------------------------------------------
50 ml kidney, liver, vomiting; pains in stomach; changes in urine Grundies et al.
lung and serum (1971)
15 ml approx. lung nausea; buccal lesions; chest X-ray: poor Lloyd (1969)
aeration at lung bases
10 ml approx. kidney, lung oliguria; changed renal function; basal rales; Fisher et al.
chest X-ray: small bilateral, pleural effusions; (1971)
limited atelectasis; functional lung change
not specified kidney, liver, oliguria; serum, and urine changes; minimal Fennelly et al.
lung deviations in the respiratory function (1971)
30 ml approx. kidney, liver nausea; oliguria; ECG changes; chest X-ray: Galloway &
myocardium, lung increased vascular markings Petrie (1972)
granular kidney, liver, 14 cases with mild oral, renal, lung, and liver Fitzgerald &
paraquat lung impairment Barniville
(1978)
50 ml kidney, liver, vomiting; diarrhoea; abdominal pain; serum and urine Rose (1980)
lung changes; dyspnoea, decreased forced vital capacity;
chest X-ray: extensive perivascular changes
---------------------------------------------------------------------------------------------------------
8.1.6.1. Respiratory system
(a) Clinical data
Soon after ingestion, there is oropharyngeal pain and swelling,
followed within a few days by exudation, ulceration, and mucosal
sloughing, sometimes with pseudomembrane formation, which on
occasion leads to total sloughing of the oropharynx and oesophagus
(Malone et al., 1971). In severe poisoning, pulmonary oedema
rapidly ensues with clinical and functional deterioration until
death. Less intense, but ultimately fatal, poisoning causes
progressive pulmonary fibrosis over days or several weeks, with
gradually increasing dyspnoea and hypoxaemic pulmonary failure.
Pulmonary oedema may occur from fluid overload in oliguric
patients. Mediasteinitis and pneumothorax are occasionally seen
(Dearden et al., 1978; Kimura et al., 1980).
Pulmonary function tests reflect the underlying pathology, with
hypoxaemia, reduction in lung volume, high alveolar-arterial
gradient, and impaired gas transfer (Cooke et al., 1973,
Higginbottom et al., 1979). Chest radiographs may show bilateral
pulmonary oedema, coalescing consolidations, and later, sequential
changes of pulmonary fibrosis (Davidson & McPherson, 1972).
(b) Pathology
At autopsy, the lungs do not collapse properly and the pleural
cavity contains a small amount of fluid. In cases of lung
fibrosis, the lungs are heavy, firm, dark purple, and rubbery.
Consolidation and decreased aeration are found predominantly at the
bases. Emphysema and atelectasis are often found.
Histological studies following lung biopsy and necropsy show
pulmonary oedema, haemorrhages, and atelectasis due to pulmonary
infiltrates, loss of alveolar epithelial cells and, at a later
stage, interstitial and intra-alveolar fibrosis (Smith & Heath,
1976).
During the first 7 days of paraquat poisoning in man, loss of
alveolar epithelial cells has been seen with alterations in, or
detachment of, the type I and II cells, proliferation of
fibroblasts and polymorphous cells, loss of surfactant secretion,
and thickening of the alveolar septa by interstitial fibrosis
(Toner et al., 1970). The later findings (2 - 3 weeks) involved
pulmonary fibrosis and endothelial abnormalities. Dearden et al.
(1978) reviewed the histological and electron-microscopic findings
in human lungs. Capillary permeability seemed to be enhanced
either by vesicles forming transendothelial channels or by
disruption of endothelial cells.
8.1.6.2. Renal system
Acute oliguric renal failure is common in severely poisoned
patients. Less severe manifestations include impaired renal
function, which may disappear before the pulmonary fibrosis
progresses (Beebeejaun et al., 1971; Fisher et al., 1971; Fletcher,
1975; Natori et al., 1979; Grant et al., 1980). Other
manifestations include proteinuria, with hyaline casts, white and
red blood cells. Tubular damage is reflected in glycosuria,
aminoaciduria, and excessive leaking of phosphorus, sodium, and
uric acid (Vaziri et al., 1979).
Soft, pale, swollen kidneys with extensive tubular necrosis,
compatible with toxic injury, are found at necropsy (Beebeejaun et
al., 1971). Sometimes necrosis of the proximal tubules is found
together with extreme dilatation of the distal tubules of the
kidney (Shuzui, 1980).
8.1.6.3. Gastrointestinal system, the liver, and the pancreas
The initial symptoms after oral ingestion of paraquat are
nausea, vomiting, upper abdominal pain, and diarrhoea. Perforation
of the oesophagus is uncommon (Ackrill et al., 1978; Natori et al.
1979).
The ingestion of large doses of paraquat has resulted in severe
liver damage (Ward et al., 1976; Grant et al., 1980) with
progressive metabolic acidosis (Shuzui, 1980; Sugaya et al., 1980).
Fatty degeneration of periportal hepatocytes and sporadic cellular
necrosis in the central region of the liver lobules have been
described (Matsumoto et al., 1980). Cholestasis and portal
inflammation may occur (Matsumoto et al., 1981). Oedematous
degeneration or necrosis of both the intra-hepatic and extra-
hepatic bile ducts, and of the gall bladder, have also been noted
(Mullick et al., 1981).
Takayama et al. (1978) noted stasis of the pancreatic duct,
with increased serum amylase levels after severe paraquat
poisoning.
8.1.6.4. Cardiovascular system
Occasionally, toxic myocarditis after paraquat ingestion has
been described (Bullivant, 1966; Malone et al., 1971; Copland et
al., 1974; Grant et al., 1980).
Takahashi et al. (1978) found fibrinoidal necrosis of the small
arteries in the pancreas, kidney, and liver on days 3 - 6 following
ingestion.
8.1.6.5. Central nervous system
The ingestion of very high doses of paraquat provoked anxiety,
convulsions, ataxia, and semi-consciousness (Grant et al., 1980;
Mukada et al., 1978). Haemorrhagic leukoencephalopathy was
present throughout the central nervous system, involving almost
exclusively the white matter. Focal haemorrhage and
demyelinization were present at various stages together with
haemorrhagic meningitis.
8.1.6.6. Adrenal glands
Adrenal cortical necrosis may contribute to death in severe
paraquat poisoning and the severity of the damage appears to be
dose-related (Nagy, 1970; McGeown, 1975; Fitzgerald et al., 1977a;
Takahashi et al., 1978).
8.1.6.7. Pregnancy
A woman, who accidentally swallowed paraquat in the 28th week
of pregnancy (Fennelly et al., 1968), died 20 days later. Gross
pathological examination did not reveal any abnormalities in the
fetal organs.
A woman, in the 7th month of pregnancy, intentionally ingested
about 60 ml of technical paraquat (Takeuchi et al., 1980) and
vomited approximately half that amount. Oliguria, jaundice, and
cough with sputum production progressed; fetal heartbeat
disappeared on the l3th day and the next day the dead fetus was
delivered. The mother died on the l7th day after poisoning. The
lungs of the dead fetus were filled with the debris of amniotic
fluid; the fetus had begun intra-uterine respiration to compensate
for the insufficient oxygen supply. No symptoms of paraquat
poisoning were noted in the body of the neonate.
A case report published by Musson & Porter (1982) concerning
paraquat ingestion by a 20-week pregnant woman, confirmed the lack
of teratogenic risk in human beings. The pregnancy was allowed to
continue after the treatment of the mother. The infant was
followed up to the age of 3 years and did well clinically, with
normal laboratory tests, development, and behaviour.
8.1.7. Recovery from paraquat poisoning
In the largest series reported (68 - 188 cases) (Fitzgerald et
al., 1978a; Higginbottom et al., 1979; Howard, 1979a; Proudfoot et
al., 1979), survival rates varied from 32% to 65% (Table 14)
Factors determining recovery from paraquat poisoning, reviewed by
Fletcher (1975), McGeown (1975), Fitzgerald & Barniville (1978),
Howard (1979a), and Bismuth et al. (1982), are shown in Table 16.
Victims of paraquat poisoning, who escape major pulmonary
complications, usually recover fully within a few weeks of
ingestion. Renal, gastrointestinal, and hepatic manifestations
return to normal (Fisher et al., 1971; Beebeejaun et al., 1971;
Grundies et al., 1971; Galloways & Petrie, 1972).
Minor pulmonary functional and radiographic abnormalities may
be transient and are of doubtful relationship to paraquat lung
injury. Some patients have recovered despite major pulmonary
abnormalities (Table 15). Among 5 survivors, Schlatter (1976)
reported no signs of lung residual disorders. Fitzgerald et al.,
(1979a) followed, for at least a year, 13 survivors of paraquat
poisoning to determine the prevalence of residual pulmonary
disability. Of 11 adults, 5 (all non-smokers) did not have any
clinical, radiological, or functional evidence of pulmonary
dysfunction; 4 others (all smokers) were considered normal on
clinical and chest X-ray examination, but had a mild deficit in
pulmonary function, while the remaining 2 adults were known to have
suffered from respiratory disability before the paraquat poisoning.
Only 1 patient showed new and persistent lung infiltrates that
could be ascribed to permanent paraquat lung damage. No
abnormalities were discovered in the 2 children studied.
Table 16. Factors determining recovery from paraquat poisoning
-------------------------------------------------------------------------
No. Factor Notes
-------------------------------------------------------------------------
1. Route of entry Most paraquat poisonings have occurred following
ingestion; ingestion following a meal usually has
less serious consequences; skin contamination
with liquid concentrate formulations is dangerous;
poisoning through inhalation is usually benign
2. Dose Dose rarely known, but usually, for survivors,
less than 6 g paraquat, often, spat out or vomited
after ingestion
3. Intention High mortality rates established in suicidal or
homicidal poisoning; many more survivors reported
reported among cases of accidental poisoning
4. Formulation High mortality rate registered after ingestion of
ingested liquid concentrates; survivors have more often
than not ingested dilute or granular formulations
5. Time of starting Treatment should start as soon as possible; delay
treatment of more than 2 - 5 h reduces chances of survival;
patients hospitalized several days after paraquat
ingestion have minimal chance of recovery
6. Decreased Occurs when there is vomiting, use of emetics
gastrointestinal stomach washout, application of adsorbents (such
absorption as Fuller's Earth or bentonite), single or
repeated, and forced diarrhoea; such treatment
should be as prompt as possible; a delay of more
than 5 h adversely affects the safe and effective
elimination of paraquat; care should be taken to
avoid complications (aspiration of Fuller's Earth,
oesophagal perforation)
7. Blood paraquat Fig. 6 (section 6.2.3) demonstrates importance of
concentrations paraquat plasma concentrations for prognosis
8. Urine paraquat Patients excreting more than 1 mg paraquat/h, 8 h
concentrations or more after ingestion, unlikely to recover
9. Renal function Patients with severe renal damage or renal failure
usually die
-------------------------------------------------------------------------
Table 16. (contd.)
-------------------------------------------------------------------------
No. Factor Notes
-------------------------------------------------------------------------
10. Forced diuresis Should not be instituted when renal damage with
oliguria present; caution needed during the first
24 h
11. Haemodialysis Important if forced diuresis cannot be carried out
-------------------------------------------------------------------------
8.2. Occupational Exposure
8.2.1. Epidemiological studies and case reports
8.2.1.1. Spraying personnel
Paraquat has been in agricultural use since the early 1960s and
several surveys have been conducted on spray operators (Swan, 1969;
Hearn & Kier, 1971; Makovskii, 1972; Staiff et al., 1975; Seiber &
Woodrow, 1981; Howard, 1979b, 1980, 1982; Chester & Ward, 1981;
Howard et al., 1981; Chester & Woollen, 1982; Wojeck et al., 1983).
Some of these studies were aimed at clinically evaluating possible
adverse effects, others at estimating inhalatory and dermal
exposure. Some of the latter studies have been summarised in Table
17 from which it can be seen that:
(a) the main route of exposure of agricultural workers to
paraquat is via the skin; respiratory exposure is
negligible.
(b) The worst case of exposure (of those examined) was
via knapsack spraying.
Table 17. Comparison of dermal and inhalation exposure resulting
from various methods of application
--------------------------------------------------------------------
Method of application Dermal exposure Respiratory exposure
(mg/h) (mg/h)
--------------------------------------------------------------------
Hand-held knapsacka 66 (0.45 - 1.3) x 10-3
(12.1 - 169.8)
Vehicle mountedb 0.4 0 - 2 x 10-3
(0.1 - 3.4)
Aerialc - a) Flagman 0.1 - 2.4 0 - 47 x 10-3
b) Pilot 0.5 - 0.1 0 - 0.6 x 10-3
c) Mixer/loader 0.18 1.3 - 1.5 x 10-3
--------------------------------------------------------------------
a From: Chester & Woolen (1982).
b From: Staiff et al. (1975).
c From: Chester & Ward (1981).
In Malaysian rubber plantations, exposure is likely to be
greater than in most other situations (Swan, 1969). Weed control
is required continuously for 10 months of the year, and the
herbicide is applied by knapsack sprayers during the entire working
day, 6 days a week. The high temperature and humidity together
with the light clothing of the sprayers increase the potential risk
of dermal exposure. In 1965, a study was carried out on a team of
6 sprayers, and in 1967 on 4 teams, to estimate the efficacy of
protective measures. The operators used spray dilutions containing
paraquat at 0.5 g/litre, for 12 weeks. Attention was paid to
personal hygiene. Each man was given a thorough physical
examination, and urine samples were taken before spraying began and
at weekly intervals throughout the study. Paraquat analyses were
carried out using the method of Calderbank & Yuen (1965). Chest
X-rays were taken before the study started and at the end of the
6th and 12th weeks.
In the course of the 2 studies, a total of 528 urine samples
were examined. Paraquat was found on 131 occasions, the maximum
concentration detected being 0.32 mg/litre in the first study and
0.15 mg/litre in the second. Average urine levels of paraquat of
0.04 mg/litre were found in the 1965 study, and of 0.006 mg/litre
in the 1967 study. After spraying ceased, these levels declined
steadily to become undetectable within a week - with one exception.
It was concluded that the workers were not subjected to hazardous
levels of paraquat.
Both trials showed that about half of the men had suffered mild
irritation of the skin and eyes, but had recovered rapidly with
treatment. Two cases of scrotal dermatitis occurred in workers
wearing trousers that were continuously soaked by the spray
solution. There were also 2 cases of epistaxis. All chest
radiographs were normal.
Studies over a period of several years on 296 workers were
performed by Hearn & Keir (1971) on a Trinidad sugar estate. This
survey drew attention to nail damage following gross contamination
with paraquat at 1 - 2 g/litre that ranged in severity from
localized discoloration to nail loss. The typical distribution of
the lesions - affecting the index, middle, and ring fingers of the
working hand - suggested that they had occurred through leakage
from the knapsack sprayer, and inadequate personal hygiene. Apart
from 2 cases of contact dermatitis of the hands, no skin, eye, or
nose irritation was reported, nor were there any systemic effects.
Similar data were obtained by Makovskii (1972), who examined
several groups of workers spraying paraquat as a herbicide and
dessicant in cotton fields during the hot season. These workers
were exposed to paraquat aerosol concentrations of 0.13 - 0.55
mg/m3 air. Dermal exposure was low, not more than 0.05 - 0.08 mg
paraquat on the hands and face. There were no complaints, nor did
the clinical and laboratory examinations of the workers demonstrate
any significant deviations from the matched control groups.
In the USA (Staiff et al., 1975), the exposure of field workers
operating tractor-mounted spray equipment in orchards was
determined. About 4.6 litre paraquat liquid concentrate (291
g/litre) was used in 935 litre water per h. In addition, exposures
from yard and garden applications were studied in volunteers using
pressurized hand dispensers containing paraquat solution (4.4
g/litre). Dermal contamination was measured by adsorbent cellulose
pads attached to the worker's body or clothing, and by hand-rinsing
in water in a polyethylene bag. Special filter pads were used in
the filter cartridges of the respirators worn by the subjects under
study.
In all, 230 dermal and respiratory exposure pads, 95 samples of
hand-rinse water, and 130 urine samples, collected during and
following the spray, were analysed. This involved 35 different
paraquat application situations. The exposure of field workers was
found to range from about 0.40 mg/h (dermal) to less than 0.001
mg/h (inhalation). As for individuals spraying the yard or garden,
exposure ranged from 0.29 mg/h (dermal) to less than 0.001 mg/h
(inhalation).
In almost all cases, dermal exposure affected the hands. The
respiratory paraquat values were generally below the sensitivity
level of the analytical method. No detectable paraquat
concentrations were found in the urine samples (lower limit 0.02
mg/litre). This study confirmed the general safety of paraquat
under correct conditions of use.
The potential long-term hazard associated with the use of
paraquat has also been studied. Howard et al. (1981) studied the
health of 27 spraymen who had been exposed to paraquat for many
months per year for an average of 5.3 years, and compared them with
two unexposed control groups consisting of 24 general workers and
23 factory workers. There were a few skin lesions resulting from
poor spraying techniques and 1 case of eye injury. The workers
were given full clinical examinations and lung, liver, and kidney
function tests were carried out. There were no significant
differences in all health parameters measured between the groups,
which led the authors to suggest that the long-term use of paraquat
was not associated with harmful effects on health.
A paraquat formulation (240 g/litre) diluted 300 times by
volume with water was sprayed for 2 h on weedy ground (Kawai &
Yoshida, 1981). No irritation of the eyes and the skin was
reported. The urine of the workers who wore gauze masks contained
1.4 - 2.7 µg paraquat, 24 h after the spraying. The urine of
workers who had worn a high-performance mask did not contain
detectable levels of paraquat. During the spraying operations, the
concentration of paraquat aerosol was 11 - 33 µg/m3 air. The total
dermal exposure was about 0.22 mg. The authors discussed the need
for protective equipment to decrease skin contact with paraquat and
to avoid aerosol inhalation.
Quantitative estimates of dermal and respiratory exposure of 26
plantation workers in Malaysia (Chester & Woollen, 1982) have shown
a mean dermal dose of 1.1 mg/kg body weight per h. The highest
individual total exposure was equivalent to 2.8 mg/kg body weight
per h; the mean respiratory exposure was 0.24 - 0.97 µg paraquat/m3
air. Spray operators and carriers were exposed to an order of 1%
or less of a TLV of 0.1 mg/m3 for respirable paraquat. Urine
levels of paraquat were generally below 0.05 mg/litre.
A study was carried out on a group of 14 spray men in Thailand
using conventional high-volume knapsack sprayers and low-volume
spinning disc applicators with paraquat ion concentrations of 1.5
g/litre and 20 g/litre, respectively (Howard, 1982). Irritation of
unprotected skin was found, and this was severe in workers using
high spray concentrations (caustic burns on the feet after work
with spinning disc applicators and paraquat solution (20 g/litre)).
Urinary paraquat levels after 14 days spraying were significantly
higher (10.21 - 0.73 mg/litre) in unprotected men using both
concentrations, and there was evidence that urinary levels of
paraquat increased as the trial progressed. No evidence of
systemic toxicity was discovered among the spray men undergoing
clinical and radiographic examination 1 week after spraying ended.
The author concluded that spray concentrations in hand-held
equipment should not exceed 5 g paraquat ion/litre.
After tomato spraying in the USA, the total body exposure to
paraquat was determined to be 168.59 mg/h (Wojeck et al., 1983).
The use of enclosed tractor cabs or a high clearance tractor
reduced total body exposures to paraquat to 26.91 mg/h or 18.38
mg/h, respectively. The authors reported that the total body
exposure of tractor spray men working in two citrus locations was
proportional to the tank concentrations (paraquat dilutions of 1.1
g/litre and 0.7 g/litre were applied); exposure levels of 28.50
mg/h and 12.16 mg/h were found for workers using the higher and the
lower concentrations, respectively. In all situations studied, the
respiratory exposure was consistently a small fraction (<0.1%) of
the total body exposure. Exposure was mainly through the skin.
8.2.1.2. Formulation workers
Groups of workers exposed to formulations were examined by
Howard (l979b). The first group of 18 workers in England comprised
subjects exposed to dust and liquid paraquat formulations during a
37.5 h working week, the mean length of exposure being 5 years.
The second group also comprised 18 males, from Malaysia, exposed to
liquid concentrate formulations during a 42-h working week, the
mean length of exposure being 2.3 years. Partly protective
clothing was worn. However, in Malaysia, no gloves, rubber aprons,
or goggles were used. The medical records and the dermatological
examinations revealed acute skin rashes, nail damage, epistaxis,
blepharitis, and delayed wound healing in 12 - 66% of these
workers. Delayed caustic effects were often found among the
Malaysian formulation workers where a lower level of safety and
hygiene was apparent. Clinical examination did not reveal any
evidence of chronic contact dermatitis, hyperkeratosis, or
eczematous lesions.
8.2.2. Cases of occupational poisoning and local caustic effects
Hayes & Vaughan (1977) reviewed deaths from pesticides in the
USA. From 1956 - 1973, no deaths attributable to paraquat were
registered among agricultural workers, but in 1974, 4 fatal cases
were associated with this herbicide, although it was not clear
whether they were accidental, suicidal, or occupational. Conso
(1979) reported 17 cases of skin and eye irritation, not
accompanied by epistaxis or other signs of systemic effects, in
paraquat-exposed workers in France. Bismuth et al. (1983)
discussed a few cases of paraquat poisoning due to skin
contamination and eye irritation.
The available evidence indicates that, at the recommended
dilution rates and correctly used, systemic oral, inhalation, or
dermal effects should not be expected. Skin and eye irritation
have occurred only when protective measures were disregarded.
However, it should be emphasized that carelessness in handling
paraquat may have serious consequences. Fitzgerald et al. (1978a)
summarized the clinical findings and pathological details
concerning 13 accidents involving paraquat among agricultural
workers, 6 of which were fatal. In 5 of these cases, swallowing was
involved.
8.2.2.1. Oral ingestion
The ingestion of paraquat may occur accidentally, if liquid
concentrates are decanted into unlabelled containers near the
working areas (Kawatomi et al., 1979), and dangerous ingestion can
occur if operators suck or blow out the blocked pipes or nozzles of
spray apparatus. Of the 6 fatalities studied by Fitzgerald et al.
(1978a), 3 swallowed Gramoxone(R) after sucking the outlet of a
sprayer. In one non-fatal case, the man had sucked out a nozzle
containing diluted paraquat, while in another case, the man who had
blown into the jet, to clear it, escaped with only minor signs of
poisoning. Dilute solution blown into the face by the wind and
splashes of concentrate that get into the mouth probably explain
the resultant signs in the mouth, on the tongue, and in the throat.
Smoking with paraquat-contaminated hands has been reported to
result in a farmer's developing oropharyngeal irritation, nausea,
and muscular weakness (Mourin, 1967).
8.2.2.2. Dermal absorption
Acute dermal paraquat poisoning has been described by
Fitzgerald et al. (1978a). The use of a leaking sprayer by a
worker with severe extensive dermatitis probably resulted in fatal
absorption of paraquat through the damaged skin. Jaros (1978) has
described how the use of concentrated solutions of paraquat (50
g/litre instead of 5 g/litre), with an old leaking knapsack
sprayer, resulted in paraquat contamination of the neck, back, and
legs of a worker. After 4 h of work, he complained of a burning
sensation on the neck and scrotum. On admission to hospital 6 days
later, cough and respiratory difficulties were recorded. Three
days later the patient died of renal and respiratory failure. This
author has stressed the need for careful handling of paraquat.
Jaros et al. (1978) have discussed several other cases of paraquat
poisoning in the CSSR related to paraquat application.
Severe skin damage, followed by death due to respiratory
insufficiency, occurred in a woman (Newhouse et al., 1978), 8 weeks
after initial contact with paraquat. The toxic dermatitis started
with scratches on the arms and legs from the branches of fruit
trees. The patient had often failed to wear protective clothing or
to shower after spraying. During the 4 weeks preceding her first
admission to hospital, she developed ulcers and respiratory
complaints combined with anorexia. Damaged and broken skin was
thus exposed to paraquat. A chest X-ray and needle biopsy of the
lung revealed pulmonary lesions. Seventeen days after discharge
from hospital, without a specific diagnosis, she was re-admitted,
and died 2 weeks later with progressive lung, hepatic, and renal
dysfunction. More recently, Levin et al. (1979) described the
clinical and pathomorphological investigation of a patient who died
of hypoxia after repeated dermal exposure to paraquat (28 g/litre)
and diquat (29 g/litre) in a water-oil dilution - contrary to
accepted practice. The worker had used a leaking sprayer. A
characteristic ulcer developed at the site of paraquat contact.
There was also lung damage. Waight & Weather (1979) reported a
fatal case of dermal poisoning with paraquat after prolonged
contact with a concentrated formulation following spillage from a
bottle in the back trouser pocket. Wohlfahrt (1982) discussed the
factors related to severe paraquat poisoning due to dermal
absorption in tropical agriculture. Three fatal incidents followed
skin contamination; one victim used paraquat to treat scabies
infestation, and one to treat lice. In all cases, the skin was
blistered and ulcerated. The patients died of progressive
respiratory failure, 4 - 7 days after the accidents. However it
has been pointed out that each of these three spraymen showed skin
lesions much more severe than would be expected had recommended and
customary dilutions been used and that, in one of these cases, the
presence of mouth and throat ulceration strongly suggested that
ingestion might also have occurred (Davies, 1982).
8.2.2.3. Local skin and nail effects
Paraquat has a delayed effect on the skin. Brief contact with
liquid formulations, as well as repeated exposure to dilute
solutions, produced skin irritation, desquamation, and, finally,
necrosis at the site of contact (Ongom et al., 1974; Binns, 1976;
Newhouse et al., 1978; Waight & Wheather, 1979; Levin et al., 1979;
Horiuchi et al., 1980). Harmful dermal effects have been reported
(Howard, 1982) among spray men who worked without protective
clothes and with naked feet. The blistering and ulceration of the
skin were due to excessive contact and inadequate personal hygiene.
Horiuchi & Ando (1980) carried out patch testing on 60 patients
with contact dermatitis due to Gramoxone(R). In 8 patients
(13.3%) positive allergic reactions were established. In another
survey with 52 persons, a positive photo-patch response was
reported in 11 patients.
Nail damage has also been reported after frequent exposure to
paraquat concentrates during the formulation of the herbicide or
the preparation of working dilutions (Samman & Johnston, 1969;
Howard, l979b). Leakage from sprayers may cause nail damage only
if there is gross contamination (Hearn & Keir, 1971). Asymmetric
discoloration and softening of the nail base appears together with
an infection, that usually persists after the loss of the nail, but
a few months after cessation of paraquat exposure, the nails
re-grow satisfactorily.
8.2.2.4. Ocular damage
A number of studies have demonstrated the hazard from splashes
of concentrated paraquat that come into contact with the eye (Swan,
1969; Schlatter, 1976; Howard, l979b, 1980; Deveckova & Myalik,
1980). Apart from irritation of the eye and blepharitis, a week
later more serious ocular damage may occur such as destruction of
the bulbar and tarsal conjunctiva and of the corneal epithelium
(Cant & Lewis, 1968). Anterior uveitis was also noted. Joyce
(1969) reported a case of conjunctival necrosis after paraquat had
been splashed into the eyes during spraying in windy weather. In a
second case, there was progressive keratitis with gross corneal
opacity. Severe conjunctival injuries with keratitis and decreased
visual acuity were reported in 3 workers by Watanabe et al. (1979)
and in another by Okawada et al. (1980). The eyes were washed with
water immediately, but the damage progressed and required treatment
for more than 3 weeks.
8.2.2.5. Inhalation
The inhalation of droplets in normal paraquat spraying does not
appear to represent a significant health hazard (Howard, 1980), and
the effects of occupational inhalation have been limited to nose
bleeds, and nasal and throat irritation (Swan, 1969; Howard,
1979b). Standard spraying equipment failed to produce significant
levels of droplets in the respirable range of < 5-7 µm diameter,
and chemical analyses of paraquat aerosols or particulate matter,
sampled from working areas, have usually shown them to be well
below the TLV. However, there have been some reports (Malone et
al., 1971; Mircev, 1976; Bismuth et al., 1982) of adverse effects
as a result of inhalation exposure.
8.3. Use of Marijuana Contaminated by Paraquat
In the USA, it has been found that marijuana sprayed with
paraquat (in an attempt to destroy the plant) may become available
for smoking by drug users. Concentrations of paraquat in marijuana
of up to 461 mg/kg have been reported (Liddle et al., 1980).
Understandably, concern has been expressed that smoking this
contaminated marijuana may be more harmful than smoking marijuana
itself. The available data do not justify an absolute conclusion.
However, paraquat is known to pyrolyse at 300 °C and it has been
established (Smith 1978) that in marijuana cigarettes contaminated
with 1000 mg paraquat/kg (1 mg, assuming a 1 g cigarette), only
0.26 µg of paraquat escaped pyrolysis and was available to be
inhaled. On this basis, the amount of paraquat inhaled by a heavy
user of contaminated marijuana will be insufficient to cause
injury. In the absence of exhaustive toxicological studies, it
cannot be stated categorically that all the pyrolysis products of
paraquat do not damage the lung. However, there has been no
confirmed injury attributable to the smoking of contaminated
marijuana.
8.4. Guidelines for the treatment of paraquat poisoning
The most important measures are the immediate neutralisation of
ingested paraquat by 15% Fuller's earth, bentonite, or activated
charcoal and urgent removal of the poison by vomiting or, when
possible, gastric washout. The urgency of these measures is such
that where transfer to hospital may involve delay of an hour or
more, this emergency treatment may need to be given by a
paramedical person, e.g., a nurse or a medical assistant. The
delay should not be more than 4 - 5 h. Furthermore, Fuller's earth
should be given together with a strong purgative such as magnesium
sulfate or mannitol.
Admission to a hospital either directly or after emergency
treatment elsewhere is essential.
Where a person has swallowed a lethal dose, the most important
single determinant of survival is the early commencement of
treatment.
Depending on local facilities, patients who reach hospital
after the initial treatment will have further treatment aimed at
neutralizing paraquat in the gastrointestinal tract (Fuller's
earth, bentonite, activated charcoal) or its excretion in the
faeces (purgatives, 10% mannitol, gut lavage). In addition,
attempts to remove absorbed paraquat from the circulation
(haemoperfusion, haemodialysis) or aid its excretion by the kidney
(forced diuresis) can be instituted.
In centres where facilities for analytical procedures are
available, measurement of urinary, or ideally plasma levels of
paraquat may give guidelines for the required intensity of
treatment or likely prognosis.
Many other therapies including corticosteroids,
immunosuppressive treatment, vitamins, beta-blocking and alkylating
agents, alpha-tocopherol, superoxide dismutase and/or glutathione
peroxidase (Autor, 1974, 1977) proved to be of no significant
importance in human paraquat poisoning (Fletcher, 1975; Fairshter
et al., 1976; Schlatter, 1976; Brown et al., 1981; Bismuth et al.,
1982). The administration of oxygen should be avoided except where
vital for the patient's comfort.
It should be noted that, as with the great majority of
chemicals, there is no specific antidote.
Care must be exercised in the administration of most of these
treatments, as the following serious complications may occur:
perforation of the oesophagus during gastric intubation; serious
blood chemistry disturbance when severe diarrhoea is induced; fluid
overload during forced diuresis (McGeown, 1975).
Despite such an array of both simple and sophisticated
measures, the response to therapy in paraquat poisoning is
disappointing and the mortality rate remains high.
In cases of skin and eye contamination, irrigation with water
(preferably running water) should be commenced urgently and must be
continued uninterrupted for at least 10 min (timed by the clock).
Eye cases should always be taken for medical treatment. In cases
of skin contamination by the concentrate or extensive and/or
prolonged contamination by the diluted material (particularly where
signs of skin irritation are present) the patient must be assessed
at hospital for systemic poisoning.
9. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
9.1. Exposure
Introduction
Paraquat is a contact herbicide or dessicant that is used to
destroy weeds in various agricultural situations. It is used in
the form of an aqueous spray, which means that potential human
exposure may occur as a result of its presence in air, on plants,
in soil, or in water.
Degradation of paraquat
Photochemical degradation takes place when paraquat-treated
plants are exposed to normal daylight and continues after the
plants are dead (section 4.1.1). The products formed have been
identified and found to be of a lower order of toxicity.
Ultraviolet degradation on soil surfaces also occurs, but
photodecomposition of paraquat in the soil is insignificant in
comparison with adsorption on clay particles. Microorganisms can
degrade free paraquat rapidly, but chemical degradation of adsorbed
paraquat is relatively slow.
Soil
Paraquat is rapidly and tightly bound to clay materials in
soils. The adsorbed paraquat is biologically inactive and in
normal agricultural use no harmful metabolic or breakdown products
are to be expected (section 4.3 and 5.1). In multiple spray
trials, paraquat residues in soil varied from 22 to 58 mg/kg.
Under field conditions, the residual paraquat is slowly
re-distributed. Long-term field studies have shown degradation
rates of 5 - 10% per annum, which is sufficient to prevent
saturation of soil deactivation capacities. At normal and high
rates of application, no adverse effects are expected in the soil
microflora and other soil organisms, or on crop growth (section
4.3.1).
Water
Following the use of paraquat as an aquatic herbicide at a
normal application rate of 1 mg/litre, the concentration was found
to decrease to about one half of the initial level within 36 h and
to below 0.0l mg/litre in less than 2 weeks (section 4.3.2).
Phytotoxic damage to crops irrigated with treated water is unlikely
to occur, if an interval of 10 days is observed between treatment
of the water and its use, because of the rapid decrease of paraquat
residues in the water.
Normal application of paraquat for aquatic weed control is not
harmful for aquatic organisms. However, care should be taken in
the application of paraquat to water containing heavy weed growth,
since oxygen consumed by subsequent weed decay may decrease oxygen
levels in the water to an extent that is dangerous for fish or
other aquatic organisms.
Air
Paraquat is not volatile so inhalation of paraquat vapour is
not a problem, in practice. However, droplets of paraquat solution
can be present in the air as a consequence of aerial, knapsack, or
tractor-mounted spraying. Paraquat aerosol concentrations (total
airborne) ranged up to 0.55 mg/m3 in the work situation, depending
on the method of spraying. The amount of respirable airborne
paraquat was found to be insignificant under normal conditions of
use (section 8.2.1).
The amount of paraquat present in airborne dust was found to
range from 0.0004 to 0.001 mg/m3. The binding of paraquat to the
dust was so tight that it did not exert any toxicological effect on
rats, when given by inhalation.
Food
Examination of paraquat-treated p1ants (section 4.3.4), or of
materials from animals fed paraquat-treated crops (section 4.3.5),
revealed low residues, so that no hazard should be expected from
paraquat residues in food when used as a herbicide or as a
desiccant. Paraquat is not subject to bioconcentration (section 5)
and has not been found to accumulate in food chains.
Environmental contamination
Exposure to paraquat from spray drift may occur in windy
weather, though field studies suggest that the airborne paraquat
concentration declines markedly within a few metres of the sprayed
area (section 4.3.3). Because of the rapid and complete binding of
paraquat to clay particles in the soil, contamination of water
supplies either from field runoff or percolation through soil to
the water table is not an environmental problem (sections 4.3.1 and
4.3.2). Paraquat has also been shown not to have any harmful
effects on birds (sections 5.3 and 5.4).
9.2. Poisoning by Paraquat
Misuse of paraquat has led to many deaths throughout the world,
mainly due to the swallowing of undiluted preparations.
9.2.1. Suicidal ingestion
The majority of paraquat poisonings are due to swallowing
liquid concentrates with suicidal intent and the mortality rate is
high. Ingestion of granular paraquat is less common and usually
causes milder poisoning, though fatalities have occurred. Paraquat
has been used to commit homicide (section 8.1).
9.2.2. Accidental poisoning
Poisoning by accidental swallowing is less common than
intentional swallowing and is usually the result of storing liquid
concentrates in inappropriate containers, particularly beer or soft
drink bottles. The mortality rate is lower than in suicidal cases.
Childhood poisoning is usually accidental. Legislation on the
control of the sale of liquid concentrates has reduced accidental
ingestion in some countries (section 8.1).
A small number of fatal cases of accidental paraquat poisoning
via the skin have been reported following the application of liquid
concentrates (200 g/litre) to kill body lice.
9.2.3. Occupational Poisoning
Cases of severe poisoning following inappropriate behaviour or
accidents while handling paraquat occur. Fatal and non-fatal
ingestion of paraquat has occurred when hand-spray operators have
attempted to clear the spray outlet by sucking on the spraying
nozzle or outlet pipes. In some of the severe cases, the authors
noted their suspicion of concealed suicidal intent. Fatal
poisoning by dermal soaking with dilute paraquat has been reported
in one operator who had severe dermatitis and had been using a
leaky sprayer (section 8.2.2).
Fatal systemic poisoning may result from continuous contact
with paraquat-soaked clothing or splashes of liquid concentrate on
the skin. Splashes of liquid concentrate may lead to severe ocular
and skin damage (sections 8.2.1, 8.2.2). Spraying with
inadequately diluted paraquat (e.g., with ultra low volume
application) may result in similar problems.
9.3. Occupational Exposure
There are several studies on paraquat exposure in normal
agricultural use. Occupational exposure may be oral, dermal, or by
inhalation. The spray aerosol and dust particles are relatively
large and are mostly deposited in the upper respiratory tract
(section 8.2.1).
The potential dermal exposure of field workers (section 8.2.1)
is closely related to working conditions. Workers on tractors were
found to have a paraquat exposure of 12 - 168 mg/h while spraying
tomatoes and citrus. In other studies, field workers were dermally
exposed to paraquat at approximately 0.40 mg/h, and individuals
spraying the garden to 0.29 mg/h. In all trials, respiratory
exposure was not higher than 0.01 mg/h. Urine concentrations in
occupationally-exposed workers were often lower than 0.01 mg/litre,
but concentrations up to 0.73 mg/litre were determined after
improper paraquat application in tropical agriculture use.
Local skin effects (contact, irritative, or photoallergic
dermatitis) delayed wound healing, and nail damage has been
observed among formulation workers or among individuals handling
the herbicide improperly. Blepharitis and epistaxis may result due
to delayed irritative action of paraquat. Such incidents
illustrate the need for strict personal hygiene and rigorous
adherence to safe handling procedures.
9.4. Effects
9.4.1. Paraquat toxicity in animals
The acute lung-directed toxicity of paraquat in man has been
confirmed in numerous studies in animals. At high doses of
paraquat, minor toxic effects have been noted primarily in liver
and kidney, and in other organ systems, including nervous,
cardiovascular, blood, adrenals and male reproductive systems.
However, toxic effects have not been reported at low doses of
paraquat. Concentrated solutions of paraquat have been found to be
irritating to both skin and eyes. The FAO/WHO (1976) has
determined no-observed-adverse-effect levels of 30 mg/kg diet,
equivalent to 1.5 mg/kg body weight per day for rats and 50 mg/kg
diet, equivalent to 1.25 mg/kg body weight per day, for dogs
exposed to paraquat dichloride. Additional animal studies have
indicated that paraquat is neither teratogenic nor carcinogenic
(sections 7.1.6 and 7.1.8). In vitro mutagenicity studies have
been inconclusive, though generally suggesting weak potential
activity, while in vivo studies have given negative results
(section 7.1.7). Thus, the results of animal studies suggest that
low-level exposure to paraquat is unlikely to induce toxic effects
in man.
9.4.2. Paraquat determinations in biological fluids and tissues
Determination of paraquat levels in stomach washings, serum,
and urine is useful for the management of poisoning (section 6.2).
The urinary levels decline rapidly during the 24 h following
exposure and may remain low for some weeks. Determination of
urinary levels of paraquat may be useful in the conduct of
epidemiological studies.
9.5. Earlier Evaluations by International Bodies
The Joint Meeting on Pesticide Residues (JMPR) has reviewed
residues and toxicity data on paraquat on several occasions
(FAO/WHO 1971, 1973, 1977, 1979, 1982, 1983). In 1972, it
estimated the acceptable daily intake (ADI) for man 0 - 0.002 mg/kg
body weight, on the basis of no-observed-adverse-effect levels of
1.50 mg/kg body weight per day in the rat and 1.25 mg/kg body
weight in the dog. Because of concern relating to lung and kidney
toxicity, this ADI was changed in the 1982 meeting to a temporary
ADI of 0 - 0.001 mg paraquat dichloride/kg body weight (or 0.0007
mg paraquat ion/kg body weight). The no-observed-adverse-effect
level for the rat remained, however, at 1.5 mg/kg body weight/day
(FAO/WHO 1983).
The same JMPRs have recommended maximum residue levels
(tolerances) for paraquat in food commodities of plant and animal
origin.
The WHO/FAO (1978) in its series of "Data sheets on chemical
pesticides" issued one on paraquat. Based on a brief review of
use, exposure, and toxicity, practical advice is given on
labelling, safe-handling, transport, storage, disposal,
decontamination, selection, training and medical supervision of
workers, first aid, and medical treatment.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechcoslovakia, the
Federal Republic of Germany, India, Japan, Kenya, Mexico, Sweden,
the United Kingdom, the USA, and the USSR) and the EEC can be found
in the IRPTC (International Register of Potentially Toxic
Chemicals) Legal file (IRPTC 1983).
9.6. Conclusions
On the basis of the above findings, it can be concluded that:
General population
Residue levels of paraquat in food and drinking-water,
resulting from its normal use, are unlikely to result in a health
hazard for the general population.
This likely lack of hazard in normal usage of dilute paraquat
is in strong contrast with the potential serious hazard that may
result from handling concentrated paraquat.
Accidental paraquat poisoning results mainly from swallowing
liquid concentrate that has been decanted into unlabelled bottles
or other containers and stored inappropriately.
The number of suicides by means of paraquat is of great
concern. The total number of such suicides is unknown.
Notwithstanding the facts that the reasons for suicide may be
manifold and complex, and that paraquat is one among many means
towards that goal, the prolonged and painful way of dying from
paraquat suggests that every effort within reason should be made to
diminish the attractiveness and availability of paraquat for this
purpose.
Occupational exposure
With reasonable work practices, including safety precautions,
hygiene measures, and proper supervision, occupational exposure
during manufacture, formulation, and application will not cause
hazard. However the undiluted concentrate must be handled with
great care because improper work practices may result in
contamination of eyes and skin (with possible consequent dermal
absorption).
Spray concentrations should not exceed 5 g paraquat ion/litre
in order to avoid skin damage and absorption of the herbicide
through the skin. Its use in hand-held ultra-low volume
application should be discouraged.
Environment
Paraquat in soil binds rapidly and tightly to clay particles
and residual phytotoxicity from freely-available paraquat is
unlikely. The toxicity of the compound for birds has been shown to
be of low significance. Under normal conditions of use, paraquat
shows low toxicity to aquatic organisms although resulting
depletion of water-oxygen because of weed decay may pose a problem.
Paraquat does not seem to represent an environmental hazard.
REFERENCES
ACKRILL, P., HASLETON, P.S., & RALSTON, A.J. (1978)
Oesophageal perforation due to paraquat. Br. med. J., 1:
1252-1253.
ALMOG, C. & TAL, E. (1967) Death from paraquat after
subcutaneous injection. Br. med. J., 3: 721.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDERSON, D., McGREGOR, D.B., & PURCHASE, I.F.H. (1976)
Dominant lethal studies with diquat and paraquat in male CD-I
mice. Mutat. Res., 40: 349-358.
ANDERSON, C.C., GUNDERSON, E.C., & COULSON, D.M. (1981)
Sampling and analytical methodology for workplace chemicals
hazards. ACS Symp. Ser., 149: 3-19.
ANONYMOUS (1979) Paraquat. FAO Plant Prod. Prot. Pap.,
15(Suppl.): 181-183.
AUTOR, A.P. (1974) Reduction of paraquat toxicity by
superoxide dismutase. Life Sci., 14: 1309-1319.
AUTOR, A.P., ed. (1977) Biochemical mechanisms of paraquat
toxicity, New York, Academic Press, pp. 39-55.
BAINOVA, A. (1969a) [Chronic oral toxicity of dipyridylium
herbicides.] Hig. Zdrav., 12: 325-332 (in Bulgarian).
BAINOVA, A. (l969b) [Experimental assessment of the effect
of dipyridylium herbicides on the skin.] Letopisi HEI, 9:
25-30 (in Bulgarian).
BAINOVA, A. (1971) [Determination of the zones of acute and
chronic inhalatory toxicity after exposure to dipyridylium
herbicides Gramoxone and Reglone.] Letopisi HEI, 28: 59-62 (in
Bulgarian).
BAINOVA, A. (1975) [Cumulative action of Gramoxone and
Reglone.] In: Problemi na Higienata, Sofia, Medizinia i
Fizkultuna, Vol. 1, pp. 31-38 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1972) Experimental
substantiation of Gramoxone MAC in working areas. In: Works of
Research Institute of Hygiene & Labour Protection, Sofia,
Medizinia i Fizkultuna, Vol. 23, pp. 71-77.
BAINOVA, A. & VULCHEVA, V. (1974) [Experimental assessment
of the effect of dipyridyliums on sex glands.] In: Works of
Research Institute of Hygiene & Labour Protection, Sofia,
Medizinia i Fizkultuna, Vol. 22, pp. 111-122 (In Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1977) Lung changes after chronic
paraquat intoxication. C. R. Acad. Bulgar. Sci., 30: 1788-1790.
BAINOVA, A., ZLATEVA, M., & VULCHEVA, V. (1972) [Chronic
inhalation toxicity of dypyridylium herbicides.] Hig. Zdrav.,
15: 25-31 (in Bulgarian).
BALDWIN, B.C., BRAY, M.F., & GEOGHEGAN, M.J. (1966) The
microbial decomposition of paraquat. Biochem. J., 101: 15.
BEEBEEJAUN, A.R., BEEVERS, G., & ROGERS, W.N. (1971)
Paraquat poisoning. Prolonged excretion. Clin. Toxicol., 4:
397-407.
BENIGNI, R., BIGNAMI, A., CARERA, A., CONTI, G., CONTI, R.,
GREBELLI, E., DOGLIOTTI, E., GUALANDI, G., NOVELLETO, A., &
ORTALI, V.A. (1979) Mutational studies with diquat and
paraquat in vitro. Mutat. Res., 68: 183-193.
BENNETT, P.N., DAVIES, D.S., & HAWKSWORTH, G.M. (1976) In
vivo adsorption studies with paraquat and diquat in the dog.
Proceedings of the British Pharmaceutical Society, 15-16 July,
England, 284 pp.
BERRY, D.J. & GROVE, J. (1971) The determination of paraquat
(1,1-dimethyl-4,4-dipyridylium cation) in urine. Clin. Chim.
Acta, 34: 5-11.
BEYER, K.H. (1970) [The analytical determination and
toxicology of paraquat.] Dtsch Apoth.-Ztg, 110: 633-635 (in
German).
BIGNAMI, M. & GREBELLI, R.A. (1979) A simplified method for
the induction of 8-azaguanine resistance in S. typhimurium.
Toxicol. Lett., 3: 169-175.
BINNS, C.W. (1976) A deadly cure for lice. Papua New Guinea
med. J., 19: 105-107.
BISMUTH, C., GARNIER, R., DALLY, S., FOURNIER, P.E., &
SCHERRMANN, J.M. (1982) Prognosis and treatment of paraquat
poisoning. A review of 28 cases. J. Toxicol. clin. Toxicol.,
19: 461-474.
BISMUTH, C., DALLY, S., & PONTAL, P.-G. (1983) Prognostic
factors and therapeutic results of paraquat poisoning.
Concerning 28 cases. Arch. Mal. prof., 44(1): 38-41.
BOJAN, F., NAGY, A., & HERMAN, K. (1978) Effect of butylated
hydroxytoluene and paraquat on urethane tumorigenesis in mouse
lung. Bull. environ. Contam. Toxicol., 20: 573-576.
BRAMLEY, A. & HART, T.B. (1983) Paraquat poisoning in the
United Kingdom. Hum. Toxicol., 2(2): 417.
BRIGELIUS, R. & ANWER, M.S. (1981) Increased biliary
GSSG-secretion and loss of hepatic glutathione in isolated rat
liver after paraquat treatment. Res. Commun. chem. Pathol.
Pharmacol., 31: 493-502.
BRIGELIUS, R., HASHEM, A., & LENGFELDER, E. (1981)
Paraquat-induced alterations of phospholipids and GSSG release
in the isolated perfused rat liver and the effect of
SOD-active copper complexes. Biochem. Pharmacol., 30: 349-354.
BROWN, E., BRANDENBURGER, A., & MALING, H.M. (1980) Effects
of paraquat and related herbicides on the acetylcholinesterase
of rat lung. Biochem. Pharmacol., 29: 456-466.
BROWN, 0., HEITKAMP, U., & SONG, C. (1981) Niacin reduces
paraquat toxicity in rats. Science, 212: 1510-1512.
BULLIVANT, C.M. (1966) Accidental poisoning by paraquat.
Report of 2 cases in man. Br. med. J., 1: 1272-1273.
BURNS, R.G. & AUDUS, L.J. (1970) Distribution and breakdown
of paraquat in soil. Weed Res., 10: 49-58.
BUS, J.S. & GIBSON, J.E. (1975) Postnatal toxicity of
chronically administered paraquat in mice and interactions
with oxygen and bromobenzene. Toxicol. appl. Pharmacol., 33:
461-470.
BUS, J.S. & GIBSON, J.E. (1979) Lipid peroxidation and its
role in toxicity. Rev. Biochem. Toxicol., 1: 125-149.
BUS, J.S. & GIBSON, J.E. (in press) Paraquat: a model for
oxidant initiated injury. In: Hook, G.E.R., ed. Pulmonary
toxicology, New York, Raven Press.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1974) Superoxide and
singlet oxygen catalysed lipid peroxidation as a possible
mechanism for paraquat toxicity. Biochem. biophys. Res.
Commun., 58: 749-755.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1975) Lipid
peroxidation as a possible mechanism for paraquat toxicity.
Res. Commun. chem. Pathol. Pharmacol., 11: 31-38.
BUS, J.S., CAGEN, S.Z., OLGAARD, M., & GIBSON, J.E. (1976)
A mechanism of paraquat toxicity in mice and rats. Toxicol.
appl. Pharmacol., 35: 501-513.
BUTLER, C. (1975) Pulmonary interstitial fibrosis from
paraquat in the hamster. Arch. Pathol., 99: 503-507.
BUTLER, C. & KLEINERMAN, J. (1971) Paraquat in the rabbit.
Br. J. ind. Med., 28: 67-71.
CAGEN, S.Z. & GIBSON, J.E. (1977) Liver damage following
paraquat in selenium-deficient and diethyl maleate pretreated
mice. Toxicol. appl. Pharmacol., 40: 193-200.
CAGEN, S.Z., JANOFF, A.S., BUS, J.S., & GIBSON, J.E. (1976)
Effect of paraquat on liver function in mice. J. Pharmacol.
exp. Ther., 198: 222-228.
CALDERBANK, A. (1966) Paraquat residues in fruit. In:
Frayer, J.D., ed. Herbicides in British fruit growing,
Blackwell, Blackwell Scientific Publications, pp. 135-136.
CALDERBANK, A. (1968) The bipyridylium herbicides. Effects
on man. In: Metcalf, R.L., ed. Advances in pest control
research, New York, Interscience Publishers, Vol. 8, 224 pp.
CALDERBANK, A. (1972) Environmental considerations in the
development of diquat and paraquat as aquatic herbicides.
Outlook Agric., 7: 51-54.
CALDERBANK, A. & SLADE, P. (1976) Diquat and paraquat. In:
Kearne, P.C. & Kaufman, D.D., ed. Herbicide chemistry,
degradation and mode of action, 2nd ed., New York, Dekker,
pp. 501-540.
CALDERBANK, A. & YUEN, S.H. (1965) An ion-exchange method
for determining paraquat residues in food crops. Analyst, 90:
99-106.
CALDERBANK, A., MCKENNA, R.H., STEVENS, M.A., & WALLEY, J.K.
(1968) Grazing trials on paraquat-treated pasture. J. Sci.
Food Agric., 19: 246-250.
CANT, J.S. & LEWIS, R.H. (1968) Ocular damage due to
paraquat and diquat. Br. med. J., 2: 224.
CARLSTROM, A.A. (1971) Collaborative check for paraquat in
formulations. J. Assoc. Off. Anal. Chem., 54: 718-719.
CARMINES, E.L., CARCHMAN, R.A., & BORZELLECA, J.F. (1981)
Investigations into the mechanism of paraquat toxicity
utilizing a cell culture system. Toxicol. appl. Pharmacol.,
58: 353-362.
CARSON, D.G.L. & CARSON, E.D. (1976) The increasing use of
paraquat as a suicide agent. Forensic Sci., 7: 151-160.
CHARLES, J.M., ABOU-DONIA, M.B., & MENZEL, D.B. (1978)
Absorption of paraquat from the airways of the perfused rat
lung. Toxicology, 8: 59-67.
CHESTER, G. & WARD, R.J. (1981) Paraquat - Occupational
exposure and drift hazard evaluation during aerial application
to cotton in California, USA, London, ICI Ltd (Central
Toxicology Laboratory Report No. CTL/P.581).
CHESTER, G. & WOOLLEN, B.H. (1982) Studies of the
occupational exposure of Malaysian plantation workers to
paraquat. Br. J. ind. Med., 38: 23-33.
CLARK, D.G. (1971) Inhibition of the absorption of paraquat
from the gastrointestinal tract by adsorbents. Br. J. ind.
Med., 28: 186-188.
CLARK, D.G., McELLIGOTT, T.F., & HURST, E.W. (1966) The
toxicity of paraquat. Br. J. ind. Med., 23: 126-133.
CLEGG, D.J. (1979) Animal reproduction and carcinogenicity
studies in relation to human safety evaluation. Dev. Toxicol.
environ. Sci., 4: 45-59.
COATS, G.E., FUNDERBURK, H.H., Jr, LAWRENCE, J.M., & DAVIS,
D.E. (1966) Factors affecting persistance and inactivation
of diquat and paraquat. Weed Res., 6: 58-66.
CONNING, D.M., FLETCHER, K., & SWAN, A.A.B. (1969) Paraquat
and related bipyridyliums. Br. med. Bull., 25: 245-249.
CONNOLLY, M.E., DAVIES, D.S., DRAFFAN, G.H., BENNETT, P.N., &
DOLLERY, C.T. (1975) Clinical experience with paraquat
poisoning. In: Fletcher, K., ed. Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-11.
CONSO, F. (1979) Paraquat poisoning: experience of poison
control centers in France. Vet. hum. Toxicol., 21(Suppl.):
112-113.
COOKE, N.J., FLENLEY, D.C., & MATTHEW, H. (1973) Paraquat
poisoning. Serial studies of lung function. Q. J. Med.,
XLII(168): 683-692.
COPLAND, G.M., KOLIN, A., & SHULMAN, H.S. (1974) Fatal
pulmonary intra-alveolar fibrosis after paraquat ingestion.
New Engl. J. Med., 291: 290-292.
CURRY, J.F. (1970) The effects of different methods of new
sward establishment and the effects of the herbicides paraquat
and dalapon on the soil fauna. Pedobiologia, 10: 329-361.
DAMANAKIS, M., BRENNAN, D.S.H., FRYER, J.D., & HOLLY, K.
(1970) Absorption and mobility of paraquat on different soil
constituents. Weed Res., 10: 264-277.
DANIEL, J.M. & GAGE, J.C. (1966) Absorption and excretion of
diquat and paraquat in rats. Br. J. ind. Med., 23: 133-136.
DASTA, J.F. (1978) Paraquat poisoning: a review. Am. J.
Hosp. Pharm., 35: 1368-1372.
DASTA, J.F. (1980) Management of paraquat poisonings. Clin.
Toxicol. Consultant, 2(1): 11-20.
DAVIDSON, J.K. & MACPHERSON, P. (1972) Pulmonary changes in
paraquat poisoning. Clin. Radiol., 23: 18-25.
DAVIES, D.S., HAWKSWORTH, G.M., & BENNETT, P.N. (1977)
Paraquat poisoning. Proc. Eur. Soc. Toxicol., 18: 21-26.
DAVIES, R.E. (1982) Skin absorption of paraquat. Med. J.
Aust., 7: 222.
DEARDEN, L.C., FAIRSHTER, R.D., MCRAE, D.M., SMITH, W.R.,
GLASER, F.L., & WILSON, A.F. (1978) Pulmonary ultrastructure
of the late aspects of human paraquat poisoning. Am. J.
Pathol., 93: 667-676.
DEVECKOVA, D. & MYALIK, M. (1980) [Gramoxone ocular burns.]
Cesk. Ophtalmol., 36: 7-10 (in Czech).
DICKES, G.J. (1979) The application of gas chromatography to
food analysis. Talanta, 26: 1065-1099.
DIJK, A. VAN, MAES, R.A.A., DROST, R.H., DOUSE, J.M.C., &
HEIJST, A.N.P. VAN (1975) Paraquat poisoning in man. Arch.
Toxicol., 34: 129-136.
DIJK, A. VAN, EBBERINK, R., GROOT, G. DE, & MAES, R.A.A.
(1977) A rapid and sensitive assay for the determination of
paraquat in plasma by gas-liquid chromatography. J. anal.
Toxicol., 1: 151-154.
DIKSHITH, T.S.S., DATTA, K.K., RAIZADA, R.B., & KUSHWAH, H.S.
(1979) Effect of paraquat dichloride in male rabbits. Indian
J. exp. Biol., 17: 926-928.
D0DGE, A.D. (1971) The mode of action of the bipyridylium
herbicides, paraquat and diquat. Endeavour, 30: 130-135.
DOUZE, J.M.C., DIJK, A. VAN, GIMBERE, J.S.F., HEIJST, A.N.P.
VAN, & MAES, R.A.A. (1975) Intensive therapy after paraquat
intoxication. In: Fletcher, K., ed. Clinical aspects of
paraquat poisoning, London, ICI Ltd, pp. 34-45.
DRAFFON, G.H., CLARE, R.A., DAVIES, D.L., HAWKSWORTH, G.M.,
MURRAY, S., & DAVIES, D.S. (1977) Qualitative determination
of the herbicide paraquat in human plasma by gas
chromatographic and mass spectrometric methods.
J. Chromatogr., 139: 311-320.
EARNEST, R.D. (1971) Effect of paraquat on fish in a
Colorado farm pond. Prog. Fish Cult., 33: 27-31.
ECKER, J.L., HOOK, J.B., & GIBSON, J.E. (1975)
Nephrotoxicity of paraquat in mice. Toxicol. appl.
Pharmacol., 34: 178-186.
EDWARDS, P.J. (1979) Status of common bird population on an
intensively managed farm where paraquat has been used
extensively, London, ICI Plant Protection Division (Report No.
RJ 0037/B).
EDWARDS, P.J., NEWMAN, J.F., & WARD, R.J. (1979) Paraquat:
effect of spraying eggs on hatchability and reproductive
organs of Japanese quail, Coturnix coturnix japonica, London,
ICI Plant Protection Division (Report No. RJ 0044B).
EFTHYMIOU, M.L. (1983) L'actualité toxicologique en matičre
de lutte chimique antiparasitaire agricole, Tours, France,
Faculté de Médecine de Tours.
FAIRSHTER, R.D. (1981) Paraquat toxicity and lipid
peroxidation. Arch. intern. Med., 141: 1121-1123.
FAIRSHTER, R.D., ROSEN, S.M., SMITH, W.R., FLAUSER, F.L.,
MCRAE, D.M., & WILSON, A.F. (1976) Paraquat poisoning. New
aspects of therapy. Q.J. Med., 45: 551-565.
FAIRSHTER, R.D., DABIR-VAZIRI, N., SMITH, W.R., GLAUSER, F.L.,
& WILSON, A.F. (1979) Paraquat poisoning: an analytical
toxicologic study of 3 cases. Toxicology, 12: 259-268.
FAO (1973) Diquat and paraquat. FAO specifications for plant
protection products, Rome, Food and Agriculture Organization
of the United Nations.
FAO/WHO (1971) Paraquat. In: 1970 Evaluation of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1973) Paraquat. In: 1972 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1976) Paraquat. In: 1975 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977) Paraquat. In: 1976 Evaluation of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1979) Paraquat. In: 1978 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1982) Paraquat. In: 1981 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1983) Paraquat. In: 1982 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FARAGO, E., HIDEG, Z., & TABATS, G. (1981) [Fatal poisonings
during the last 10 years from statistics of the Institute of
Forensic Chemistry.] Népegészsegügy, 62: 376-379 (in
Hungarian).
FAURE, J., MARKA, C., FAURE, H., YACOUB, M., & CAN, G.
(1973) Données histo-pathologiques et toxicologiques d'une
intoxication mortelle par le paraquat. Méd. lég. Dommage, 6:
417-419.
FENNELLY, J.J., GALLAGHER, J.T., & CARROLL, R.J. (1968)
Paraquat poisoning in a pregnant woman. Br. med. J., 3:
722-723.
FENNELLY, J.J., FITZGERALD, M.X., & FITZGERALD, 0. (1971)
Recovery from severe paraquat poisoning following forced
diuresis and immunosuppressive therapy. J. Irish Med. Assoc.,
64: 69-71.
FIRLIK, M. (1978) [Changes in the respiratory system during
paraquat poisoning.] Med. Pr., 29: 325-328 (in Polish).
FISHER, H.K. & KAHLER, J. (1979) [Lethal poisoning by
paraquat.] Z. Rechtsmed., 84: 61-67 (in German).
FISHER, H.K., HUMPHRIES, M., & BAILS, R. (1971) Recovery
from renal and pulmonary damage. Ann. intern. Med., 75:
731-736.
FISHER, H.K., CLEMENTS, J.A., & WRIGHT, R.R. (1973a)
Pulmonary effects of the herbicide paraquat studied 3 days
after injection in rats. J. appl. Physiol., 35: 268-273.
FISHER, H.K., CLEMENTS, J.A., & WRIGHT, R.R. (1973b)
Enhancement of oxygen toxicity by the herbicide paraquat. Ann.
Rev. respir. Dis., 107: 246-252.
FISHER, H.K., CLEMENTS, J.A., TIERNEY, D.F., & WRIGHT, R.R.
(1975) Pulmonary effects of paraquat in the first day after
injection. Am. J. Physiol., 228: 1217-1223.
FITZGERALD, G.R. & BARNIVILLE, G. (1978) Poisoning by
granular paraquat. J. Irish Coll. Physicians Surg., 7: 133-136.
FITZGERALD, G.R., BARNIVILLE, G., FITZPATRICK, P., EDWARDS,
H., SILKE, B., CARMODY, M., & O'DWYER, W.F. (1977a) Adrenal
abnormalities in paraquat poisoning. Irish J. Med. Sci., 146:
421-423.
FITZGERALD, G.R., BARNIVILLE, G., SILKE, B., CARMODY, M., &
O'DWYER, W.F. (1977b) The kidney in paraquat poisoning.
In: Proceedings of the European Dialysis and Transplantation
Association, Helsinki 1977.
FITZGERALD, G.R., BARNIVILLE, G., FLANAGAN, M., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978a) The changing pattern of
paraquat poisoning: an epidemiologic study. J. Irish Med.
Assoc., 71: 103-108.
FITZGERALD, G.R., BARNIVILLE, G., BLACK, J., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978b) Paraquat poisoning in
agricultural workers. J. Irish Med. Assoc., 71: 336-342.
FITZGERALD, G.R., BARNIVILLE, G., GIBNEY, R.T.N., &
FITZGERALD, M.X. (1979a) Clinical, radiological and
pulmonary function assessment in 13 long-term survivors of
paraquat poisoning. Thorax, 34: 414-415.
FITZGERALD, G.R., BARNIVILLE, G., DICKSTEIN, K., CARMODY, M.,
& O'DWYER, W.F. (1979b) Experience with Fuller's Earth in
paraquat poisoning. J. Irish Med. Assoc., 71: 149-152.
FLETCHER, K. (1967) Production and viability of eggs from
hens treated with paraquat. Nature (Lond.), 215: 1407-1408.
FLETCHER, K. (1974) Paraquat poisoning. In: Ballantyne, B.,
ed. Proceedings of a Symposium on Forensic Toxicology,
Bristol, John Wright, pp. 86-98.
FLETCHER, K., ed. (1975) Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-89.
FLETCHER, K. & WYATT, I. (1970) The composition of lung
lipids after poisoning with paraquat. Br. J. exp. Pathol., 51:
604-609.
FODRI, Z., SIPOS, K., & BERENCSI, G. (1977) [Study of the
irritation and allergic effects of Gramoxone in guinea-pig.]
Egeszsegtudomouny, 21: 244-249 (in Hungarian).
FOWLER, B.A. & BROOKS, R.E. (1971) Effects of the herbicide
paraquat on the ultrastructure of mouse kidney. Am. J.
Pathol., 63: 505-512.
FRIDOVICH, I. & HASSAN, H.M. (1979) Paraquat and the
exacerbation of oxygen toxicity. Trends biochem. Sci., 4:
113-115.
GAGE, J.C. (1968a) Toxicity of paraquat and diquat aerosols
generated by a size-selective cyclone. Effect of particle
size distribution. Br. J. ind. Med., 25: 304-314.
GAGE, J.C. (1968b) The action of paraquat and diquat on the
respiration of liver cell fractions. Biochem. J., 109: 757-761.
GAGE, J.C. (1969) The inhalation toxicity of
paraquat-treated soil, London, ICI Ltd (Report No. IHR/252).
GALLOWAY, D.B. & PETRIE, J.C. (1972) Recovery from severe
paraquat poisoning. Postgrad. Med. J., 48: 684-686.
GEORGE, K. & GEORGE, M. (1978) Chromosome uncoiling effect
of paraquat. Indian J. exp. Biol., 16: 933-937.
GERVAIS, P., DIAMANT-BERGER, O., BESCOL-LIVERSAC, J., GUILLAM,
C., & GUYON, F. (1975) Problčmes médico-legaux et médico-
sociaux de l'intoxication aiguë par les herbicides du groupe
du paraquat. Arch. Mal. prof., 36: 19-36.
GIBSON, J.E. & CAGEN, S.Z. (1977) Paraquat-induced
functional changes in kidney and liver. In: Autor, A.P., ed.
Biochemical mechanisms of paraquat toxicity, New York,
Academic Press, pp. 117-136.
GIRI, S.N. & KRISHNA, G.A. (1980) The effect of paraquat on
guanylate cyclase activity in relation to morphological
changes in guinea-pig lungs. Lung, 157: 127-134.
GIRI, S.N., CURRY, D.L., HOLLINGER, M.A., & FREYWALD, N.
(1979) Effect of paraquat on plasma enzymes, insulin, glucose
and liver glycogen in the rat. Environ. Res., 20: 300-308.
GOWMAN, M.E., RILEY, D., & NEWBY, S.E. (1980) Paraquat and
Diquat: Longterm high rate trial, Frensham UK. Persistence
and movement in soil and glasshouse bioassay, London, ICI Ltd
(Report RJ0014B).
GRANT, H.C., LANTOS, P.L., & PARKINSON, C. (1980) Cerebral
damage in paraquat poisoning. Histopathology, 4: 185-195.
GREENBERG, D.B., LYONS, A., & LAST, J.A. (1978)
Paraquat-induced changes in the rat of collagen biosynthesis
by rat lung. J. lab. clin. Med., 92: 1033-1042.
GROVER, R., SMITH, A.E., & KORVEN, H.C. (1980) A comparison
of chemical and cultural control of weeds in irrigation
ditchbanks. Can. J. plant Sci., 60: 185-195.
GRUNDIES, H., KOLMAR, D., & BENNHOLD, I. (1971) [Paraquat
poisoning.] Dtsch Med. Wschr., 96: 588-589 (in German).
GRZENDA, A.R., NICHOLSON, H.P., & COX, W.S. (1966)
Persistance of four herbicides in pond water. J. Am. Water
Works Assoc., 58: 326-332.
HALEY, T.J. (1979) Review of the toxicology of paraquat
(1,1-dimethyl-4,4-dipyridylium chloride). Clin. Toxicol., 14:
1-46.
HANCE, R.L., BYAST, T.H., & SMITH, P.D. (1980) Apparent
decomposition of paraquat in soil. Soil Biol. Biochem., 12:
447-448.
HARRINGTON, K.J. (1979) The detection of paraquat
(dichloride) in wood by pyrolysis vapour-phase chromatography.
Wood Sci. Technol., 13: 21-28.
HASSAN, H.M. & FRIDOVICH, I. (1977) Regulation of synthesis
of superoxide dismutase in Escherichia coli. J. Biol. Chem.,
253: 7667-7672.
HASSAN, H.M. & FRIDOVICH, I. (1978) Superoxide radical and
the enhancement of oxygen toxicity of paraquat in Escherichia
coli. J. Biol. Chem., 253: 8143-8148.
HASSAN, H.M. & FRIDOVICH, I. (1979) Paraquat and Escherichia
coli. Mechanism of production of extracellular superoxide
radical. J. Biol. Chem., 254: 10846-10852.
HASSAN, H.M. & FRIDOVICH, I. (1980) Superoxide dismutases:
detoxication by a free radical. In: Jakoby, W.B., ed.
Enzymatic basis of detoxication, New York, Academic Press,
Vol. 1, pp. 311-322.
HAWKSWORTH, G.M., BENNETT, P.N., & DAVIES, D.S. (1981)
Kinetics of paraquat elimination in the dog. Toxicol. appl.
Pharmacol., 57: 139-145.
HAYES, W.J. & VAUGHAN, W.K. (1977) Mortality from pesticides
in the United States in 1973 and 1974. Toxicol. appl.
Pharmacol., 42: 235-252.
HEARN, C.E.D. & KEIR, W. (1971) Nail damage in spray
operators exposed to paraquat. Br. J. ind. Med., 28: 399-403.
HIGGINBOTTOM, T., CROME, P., PARKINSON, C., & NUNN, J.
(1979) Further clinical observations on the pulmonary effect
of paraquat ingestion. Thorax, 34: 161-165.
HILLS, D.J., CURLEY, R.G., KNUTSON, J.D., SEIBER, J.N.,
WINTERLIN, W.L., FAUSCHKOLB, R.S., PULMAN, G.S., & ELMORE,
C.L. (1981) Composting treatment for cotton gin trasfines.
Trans. ASAE, 24: 14-19.
HOFMAN, A. & FROHBERG, H. (1972) [Gramoxone intoxication in
the Federal Republic of Germany.] Dtsch Med. Wschr., 27:
1239-1303 (in German).
HOLLINGER, M.A. & CHVAPEL, M. (1977) Effect of paraquat on
rat lung prolyl hydroxylase. Res. Commun. chem. Pathol.
Pharmacol., 16: 159-162.
HORIUCHI, N. & ANDO, S. (1980) [Contact dermatitis due to
pesticides for agricultural use.] Nippon Hifuka Gakkai Zasshi,
90: 289 (in Japanese).
HORIUCHI, N., ANDO, S., & KAMBE, Y. (1980) [Dermatitis due
to pesticides for agricultural use.] Nippon Hifuka Gakkai
Zasshi, 90: 27 (in Japanese).
HOWARD, J.K. (1979a) Recent experience with paraquat
poisoning in Great Britain: a review of 68 cases. Vet. hum.
Toxicol., 21: 213-216.
HOWARD, J.K. (l979b) A clinical survey of paraquat
formulation workers. Br. J. ind. Med., 36: 220-223.
HOWARD, J.K. (1980) Paraquat: a review of worker exposure in
normal usage. J. Soc. Occup. Med., 30: 6-11.
HOWARD, J.K. (1982) Paraquat spraying. Comparative risks
from high and low volume application methods. In: Proceedings
of 10th Asian Conference on Occupational Health, Singapore,
pp. 1-7.
HOWARD, J.K., SAHOPATHY, K.N., & WHITEHEAD, P.A. (1980) An
evaluation of the long-term effects of paraquat spraying.
Toxicol. Lett. Suppl., 1(Abstr. 068).
HOWARD, J.K., SAHOPATHY, K.N., & WHITEHEAD, P.A. (1981) A
study of the health of Malaysian plantation workers with
particular reference to paraquat spraymen. Br. J. ind. Med.,
38: 110-114.
HOWE, D.J.T. & WRIGHT, N. (1965) The toxicity of paraquat
and diquat. In: Proceedings of the 18th New Zealand Weed &
Pest Control Conference, pp. 105-114.
HUDSON, R.H., HAEGELE, M.A., & TUCKER, R.K. (1979) Acute
oral and percutaneous toxicity of pesticides to mallards:
correlations with mammalian toxicity data. Toxicol. appl.
Pharmacol., 47: 451-460.
HUNDSDORFER, S. & ROSE, I. (1980) [Lung changes produced by
paraquat: respiratory distress model in animals.] Ergeb. exp.
Med., 35: 603-609 (in German).
HUSSAIN, M.Z. & BHATNAGAR, R.S. (1979) Involvement of
superoxide in the paraquat-induced enhancement of lung
collagen synthesis in organ culture. Biochem. Biophy. Res.
Commun., 89: 71-76.
ICI Ltd (1972) Determination of residues of paraquat in
milk, London, ICI Ltd (Residue Analytical Method No. 3,
PPRAM-3).
ICI Ltd (1979) Analytical method for the determination of
paraquat by radioimmunoassay, London, ICI Ltd (PHAG).
ILETT, K.F., STRIPP, B., MENARD, R.H., REID, W.D., & GILLETTE,
J.R. (1974) Studies on the mechanism of the lung toxicity of
paraquat. Comparison of tissue distribution and some
biochemical parameters in rats and rabbits. Toxicol. appl.
Pharmacol., 28: 216-226.
IRPTC (1983) IRPTC Legal files 1983, Vol. I & II, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme.
JAROS, F. (1978) Acute percutaneous paraquat poisoning.
Lancet, 1: 275.
JAROS, F., ZUFFA, L., KRATINOVA, R., SKALA, I., & DOMSOVA, A.
(1978) [Acute percutaneous Gramoxone intoxication.] Pr. Lék.,
7: 260-262 (in Czech).
JOYCE, M. (1969) Ocular damage caused by paraquat. Br. J.
Ophtalmol., 53: 688-690.
KAWAI, M. & YOSHIDA, M. (1981) [Exposure of spray operators
to paraquat.] Nippon Doshu Eisei Zasshi, 28: 353-359 (in
Japanese).
KAWAI, S., UEDA, K., KOYAMA, K., & OGASAWARA, S. (1980)
[Studies on preventive treatment of paraquat dichloride
intoxication - Part 2.] Nippon Noson Igakkai Zasshi, 29:
546-547 (in Japanese).
KAWATOMI, M., KOGA, H., YOKOYAMA, K., FUJIMATSU, S.,
MATSUMOTO, T., FUKUDA, H., & IHIZAKI, T. (1979) [A case of
autopsy of a person who died of paraquat intoxication.] Nippon
Naiko Gakkai Zasshi, 68: 1332-1333 (in Japanese).
KEELING, P.L., PRATT, I.S., ALDRIDGE, W.N., & SMITH, L.L.
(1981) The enhancement of paraquat toxicity in rats by 85%
oxygen: lethality and cell-specific lung damage. Br. J. exp.
Pathol., 62: 643-654.
KEELING, P.L., SMITH, L.L., & ALDRIDGE, W.N. (1982) The
formation of mixed disulfides in rat lung following paraquat
administration. Biochem. Biophys. Acta, 716: 249-257.
KEHRER, J.P., HASCHEK, W.M., & WITSCHI, H. (1979) The
influence of hyperoxia on the acute toxicity of paraquat and
diquat. Drug Chem. Toxicol., 2: 397-408.
KELLY, D.F., MORGAN, D.G., DARKE, P.G.G., GIBBS, C., PEARSON,
H., & WEAVER, M.Q. (1978) Pathology of acute respiratory
distress in the dog associated with paraquat poisoning.
J. comp. Pathol., 88: 275-293.
KHAN, S.U. (1974) Determination of diquat and paraquat
residues in soil by gas chromatography. J. agric. food Chem.,
22: 863-867.
KHAN, S.U. (1980) Determining the role of humic substances
in the fate of pesticides in the environment. J. environ. Sci.
Health, 15: 1071-1090.
KHERA, K.S., WHITTA, L.K., & CLEGG, D.J. (1968) Embryopathic
effects of diquat and paraquat in rats. Ind. Med. Surg., 37:
257-261.
KIMBROUGH, R.D. (1974) Toxic effect of the herbicide
paraquat. Chest, 65: 706-708.
KIMBROUGH, R.D. & GAINES, T.B. (1970) Toxicity of paraquat
in rats and its effect on rat lungs. Toxicol. appl.
Pharmacol., 17: 679-690.
KIMBROUGH, R.D. & LINDER, R.E. (1973) The ultrastructure of
the paraquat lung lesion in the rat. Environ. Res., 6: 265-273.
KIMURA, M., SUZUKI, E., & OHNISHI, H. (1980) [A case of
autopsy of an infant intoxicated by paraquat dichloride.]
Shoni ka Rinsho, 33: 732-734 (in Japanese).
KLIKA, E., KUNC, L., ANTALIKOVA, K., & KUNCOVA, M. (1980)
The morphology of the lung in the albino rat after paraquat
administration. Folia Morphol., 28: 188-191.
KNIGHT, B.A.G. & DENNY, P.J. (1970) The interaction of
paraquat with soil: adsorption by an expanding lattice clay
mineral. Weed Res., 10: 40-48.
KNIGHT, B.A.G. & TOMLINSON, T.E. (1967) The interaction of
paraquat (1,1'-dimethyl-4,4'-dipyridylium dichloride) with
mineral soils. J. soil Sci., 18: 223-243.
KORNBRUST, D.J. & MAVIS, R.D. (1980) The effect of paraquat
on microsomal lipid peroxidation in vitro and in vivo.
Toxicol. appl. Pharmacol., 53: 323-332.
KURISAKI, E. & SATO, H. (1979) [Toxicological studies on
herbicides. Intracorporeal distribution on paraquat dichloride
and diquat dibromide in rat.] Nippon Hoigaki Zasshi, 33: 656
(in Japanese).
LAM, H.F., TAKEZAWA, J., GUPTA, B.N., & STEE, E.W. van
(1980) A comparison of the effects of paraquat and diquat on
lung compliance, lung volumes and single breath. Toxicology,
18: 111-123.
LAVAUR, E., SION, G., GROLLEAU, G., & CARPENTER-LESECK, J.
(1979) Etude comparée de l'action du diquat et du paraquat
sur la muqueuse digestive de la souris, du rat, et du lapin.
Ann. Zool. Ecol. anim., 11: 159-169.
LEARY, J.B. (1978) Diquat and paraquat. In: Zweig, G. &
Sherma, J., ed. Analytical methods for pesticides and growth
regulators, New York, Academic Press, pp. 321-325.
LENGFELDER, E. & ELSTNER, E.F. (1978) Determination of the
superoxide dismutating activity of D-penicillamine copper.
Hoppe-Seyler's Z. Physiol. Chem., 359: 751-757.
LEVIN, P.J., KLAFF, L.J., ROSE, A.G., & FERGUSON, A.D.
(1979) Pulmonary effects of contact exposure to paraquat: a
clinical and experimental study. Thorax, 34: 150-160.
LEVIN, D.E., HOLLSTEIN, M., CHRISTMAN, M.F., SCHWIERS, E.A., &
AMES, B.N. (1982) A new Salmonella tester strain (TA 102)
with AT base pairs at the site of mutation detects oxidative
mutagens. Proc. Natl Acad. Sci. USA, 79: 7445-7449.
LEVITT, T. (1979) Determinations of paraquat in clinical
practice using radioimmunoassay. Proc. Anal. Div. Chem. Soc.,
16: 72-76.
LIDDLE, J.A., NEEDHAM, L.L., ROLLEN, Z.J., ROARK, B.R., &
BAYSE, D.D. (1980) Characterization of the contamination of
marijuana with paraquat. Bull. environ. Contam. Toxicol., 24:
49-53.
LITCHFIELD, M.H., DANIEL, J.W., & LONGSHAW, S. (1973) The
tissue distribution of the bipyridylium herbicides diquat and
paraquat in rats and mice. Toxicology, 1: 155-165.
LLOYD, E.L. (1969) Recovery after taking Weedol. Br. med. J.,
2(5650): 189.
LOCK, E.A. (1979) The effect of paraquat and diquat on renal
function in the rat. Toxicol. appl. Pharmacol., 48: 327-336.
LOCK, E.A. & ISHMAEL, J. (1979) The acute toxic effects of
paraquat and diquat on the rat kidney. Toxicol. appl.
Pharmacol., 50: 67-76.
LOTT, P.F., LOTT, J.W., & DOMS, D.J. (1978) The
determination of paraquat. J. chromatogr. Sci., 16: 390-395.
LUTY, S., CISAK, E., LATUSZYNSKA, J., & PRZYLEPA, E. (1978)
[Effect of paraquat on embryonic and post-embryonic
development in rat.] Bromatol. Chem. Toksykol., 11: 159-165
(in Polish).
MAFF (1980a) Agricultural Development and Advisory Service
'Pesticide science' reference book 1980, Lowestoft, England,
Ministry of Agriculture, Fisheries & Food, pp. 69-76
MAFF (1980b) Pest Infestation Control Laboratory Report
1977-1979, Lowestoft, England, Ministry of Agriculture,
Fisheries & Food, pp. 143-153.
MAFF (1981) Agricultural Development and Advisory Service
'Pesticide science' reference book 1981, Lowestoft, England,
Ministry of Agriculture, Fisheries & Food, pp. 21-27 and 55-63.
MAKOVSKII, V.N. (1972) [Toxicological and hygiene studies of
bipyridylium herbicides diquat and paraquat,] Referat,
Vinnize, USSR, pp. 1-23 (PhD degree thesis) (in Russian).
MALING, H.M., SAUL, W., WILLIAMS, M.A., BROWN, E.A.B., &
GILLETTE, J.R. (l978) Reduced body clearance as the major
mechanisms of the potentiation of beta2-adrenergic agonists of
paraquat lethality in rat. Toxicol. appl. Pharmacol., 43:
57-72.
MALONE, J.D.G., CARMODY, M., & KEOGH, B. (1971) Paraquat
poisoning. A review of nineteen cases. J. Irish Med. Assoc.,
64: 59-68.
MAREK, J., VELENSKA, Z., & HASKOVCOVA, I. (1981) [Kidney
changes in acute phase of paraquat intoxication in rats.]
Sb. Lék., 83: 100-104 (in Czech).
MASTERSON, J.G. & ROCHE, W.J. (1970) Fatal paraquat
poisoning. J. Irish Med. Assoc., 63: 261-264.
MATSUMOTO, T., MATSUMORI, H., KUWABARA, N., FUKUDA, Y., &
ARIWA, R. (1980) [A histopathological study of the liver in
paraquat poisoning. An analysis of 14 autopsy cases with
emphasis on bile duct injury.] Acta pathol. Jpn., 30: 859-870
(in Japanese).
MATSUMOTO, S., MATSUMORI, H., KUWABARA, N., FUKUDA, Y., &
ARIWA, R. (1981) [Histopathological studies on the
disturbance of bile ducts to paraquat.] Kanzo, 22: 309 (in
Japanese).
MATTHEW, H., LOGAN, A., WOODRUFF, M.F.A., & HEARD, B. (1968)
Paraquat poisoning. Lung transplantation. Br. med. J., 3:
759-763.
MCCORD, J.M. & FRIDOVICH, I. (1969) Superoxide dismutase -
an enzymic function for erythrocuprein (hemocuprein). J. Biol.
Chem., 244: 6049-6055.
MCDONAGH, B.J. & MARTIN, J. (1970) Paraquat poisoning in
children. Arch. Dis. Child., 45: 425-427.
MCELLIGOTT, T.F. (1972) The dermal toxicity of paraquat.
Differences due to techniques of application. Toxicol. appl.
Pharmacol., 21: 361-368.
MCGEOWN, M.G. (1975) Clinical aspects of paraquat poisoning.
In: Fletcher, K., ed., Clinical aspects of paraquat poisoning,
London, ICI Ltd, pp. 12-21.
MEES, G.C. (1960) Experiments on the herbicidal action of
1,1'-ethylene-2,2'dipyridylium dibromide. Ann. appl. Biol.,
48: 601-612.
MEHANI, S. (1972) The toxic effect of paraquat in rabbits
and rats. Ain Shams med. J., 23: 599-601.
MIRCEV, N. (1976) [Acute intoxication with Gramoxone
(paraquat).] Vatreshni Bolesti, 16: 99-101 (in Bulgarian).
MITHYANTHA, M.S. & PERUR, N.G. (1975) Paraquat retention by
soils. Mysore J. agric. Sci., 9: 276-282.
MONTGOMERY, M.R. (1976) Interaction of paraquat with the
pulmonary microsomal fatty acid desaturase system. Toxicol.
appl. Pharmacol., 36: 543-554.
MONTGOMERY, M.R. (1977) Paraquat toxicity and pulmonary
superoxide dismutase: an enzymic deficiency of lung
microsomes. Res. Commun. chem. Pathol. Pharmacol., 16: 155-158.
MONTGOMERY, M.R. & NIEWOEHNER, D.E. (1979) Oxidant-induced
alterations in pulmonary microsomal mixed-function oxidation.
Acute effects of paraquat and ozone. J. environ. Sci. Health,
13: 205-219.
MOODY, C.S. & HASSAN, H.M. (1982) Mutagenicity of oxygen
free radicals. Proc. Natl Acad. Sci., 79: 2855-2859.
MOURIN, K.A. (1967) Paraquat poisoning. Br. med. J., 4: 486.
MUKADA, T., SASANO, N., & SATO, K. (1978) [Autopsy findings
in a case of acute paraquat poisoning with extensive cerebral
purpura.] Tohoku J. exp. Med., 125: 253-263 (in Japanese).
MULLICK, F.G., TSHAK, K.G., MAHABIR, K., & STROMEYER, F.W.
(1981) Hepatic injury associated with paraquat toxicity in
humans. Liver, 1: 209-221.
MURRAY, R.E. & GIBSON, J.E. (1972) A comparative study of
paraquat intoxication in rats, guinea pigs and monkeys.
Exp. mol. Pathol., 17: 317-325.
MURRAY, R.E. & GIBSON, J.E. (1974) Paraquat disposition in
rats, guinea pigs and monkeys. Toxicol. appl. Pharmacol., 27:
283-291.
MUSSON, F.A. & PORTER, C.A. (1982) Effect of ingestion of
paraquat on a 20-week gestation fetus. Postgrad. Med. J., 58:
731-732.
NAGY, A. (1970) Paraquat and adrenal cortical necrosis.
Br. med. J. 2: 669.
NATORI, H., KOIKE, M., & KIRA, S. (1979) [Respiratory
failure due to the herbicide paraquat.] Gendai Iryo, 11:
1175-1182 (in Japanese).
NEWHOUSE, M., MCEVOY, D., & ROSENTHAL, D. (1978)
Percutaneous paraquat absorption. Arch. Dermatol., 114:
1516-1519.
NEWMAN, J.F. & EDWARDS, P.J. (1980) Paraquat: effect on
spraying eggs on hatachability and on the reproductive organs
of the chicks of pheasant, Phasianus colchicus, London, ICI
Ltd (ICI Plant Protection Division Report No. RJ 0090B).
NEWMAN, J.F. & WAY, J.M. (1966) Some ecological observations
on the use of paraquat and diquat as aquatic herbicides.
Proceedings of the 8th British Weed Control Conference,
Vol. 2, pp. 582-585.
NEWMAN, J.F. & WILKINSON, W.W. (1971) The effect of excess
quantities of paraquat in soil on the growth of vegetation,
London, ICI Ltd (Report No TMJ 606 A).
O'BRIEN, M.C. & PRENDEVILLE, G.N. (1978) A rapid sensitive
bioassay for determination of paraquat and diquat in water.
Weed Res., 18: 301-303.
OGATA, M., HASEGAWA, T., & UEDA, K. (1978) [Action of
paraquat dichloride and dimethyldithiocarbamate on the
mitochondrial conversion system and superoxide dismutase.]
Samgyo Igaku, 20: 551-552 (in Japanese).
OKAWADA, N., YAGASAKI, K., & KONDO, T. (1980) [Ocular
impairments due to an agricultural pesticide.] Nippon Noson
Igakkai Zasshi, 29: 550-551 (in Japanese).
OKONEK, S., WEILEMANN, L.S., MAJDANDZIC, J., SETYADHARME, H.,
REINECKE, H.J., BALDAMUS, C.A., LOHMAN, J., BONZEL, K.E., &
THON, T. (1982) Successful treatment of paraquat poisoning.
Activated charcoal per os and, continuous hemoperfusion.
J. Toxicol. clin. Toxicol., 19: 807-819.
OMAYE, S.T., REDDY, K.A., & CROSS, C.E. (1978) Enhanced lung
toxicity of paraquat in selenium-deficient rats. Toxicol.
appl. Pharmacol., 43: 237-247.
ONGOM, V.L., OWOR, R., & TONUSANGE, E.T. (1974) Paraquat
(Gramoxone) used as a pediculocide. In: Bagshore, A.F., ed.
The uses and abuses of drugs and chemicals in tropical Africa,
Nairobi, East Africa Literature Bureau, pp. 229-233.
PARK, J., PROUDFOOT, A.T., & PRESCOTT, L.F. (1975) Paraquat
poisoning. A clinical review of 31 cases. In: Fletcher, K.,
ed., Clinical aspects of paraquat poisoning, London, ICI Ltd,
pp. 46-53.
PARRY, J.M. (1973) The induction of gene conversion in yeast
by herbicide preparations. Mutat. Res., 21: 83-91.
PARRY, J.M. (1977) The use of yeast for the detection of
environmental mutagens using a fluctuation test. Mutat. Res.,
46: 165-176.
PASCHAL, D.C., NEEDHAM, L.L., ROLLEN, Z.J., & LIDDLE, J.A.
(1979) Determination of paraquat in sunflower seeds by
reversed-phase high performance liquid chromatography.
J. Chromatogr., 171: 85-90.
PASI, A., EMBREE, J.W., EISENLORD, G.H., & HINE, C.H. (1974)
Assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 26: 171-175.
PATRICK, G.B. (1980) Marijuana and the lung. Postgrad. med.
J., 67: 110-118.
PAYNE, W.L., POPE, J.D., & BENNER, J.E. (1974) Integrated
method for trifluralen, diphenamid and paraquat in soil and
runoff from agricultural land. J. agric. food Chem., 22: 79-82.
PEDERSEN, G., PEDERSEN, A., GREGERSEN, M., & KAMPE, B.
(1981) [Paraquat poisoning.] Ugeskr. Laeg., 143: 1202-1206
(in Danish).
PESTEMER, VON W., NOLTING, H.G., & LUNDEHN, Y.-R. (1979)
[Possible effects of repeated paraquat treatments on the
residue situation in the soil.] Nachrichtenbl. Dtsch
Pflanzenschutzd., 31: 166-170 (in German).
PICKOVA, J. (1978) [Determination of paraquat in the urine.]
Pr. Lék., 30: 266-267 (in Czech).
POPE, J.D. & BENNER, J.E. (1974) Colorimetric determination
of paraquat residues in soil and water. J. AOHC, 57: 202-204.
POPENOE, D. (1979) Effects of paraquat aerosol on mouse
lung. Arch. Pathol. lab. Med., 103: 331-334.
PROUDFOOT, A.T., STEWART, M.S., LEVITT, T., & WIDDOP, B.
(1979) Paraquat poisoning: significance of plasma-paraquat
concentrations. Lancet, 2(8138): 330-332.
PRYDE, A. & DARBY, F.J. (1975) The analysis of paraquat in
the urine by high speed liquid chromatography. J. Chromatogr.,
115: 107-109.
PURSER, D.A. & ROSE, M.S. (1979) The toxicity and renal
handling of paraquat in Cynomolgus monkeys. Toxicology, 15:
31-41.
RADAELLI, L. & MARTELLI, M. (1971) Residual toxicity of
paraquat absorbed on soil. Agrochimica, 15: 344-350.
REDDY, K.A., LITOV, R.E., & OMAYE, S.T. (1977) Effect of
pre-treatment with anti-inflammatory agents on paraquat
toxicity in the rat. Res. Commun. chem. Pathol. Pharmacol.,
17: 87-100.
RESTUCCIA, A., FOGLINI, A., & DE ALENTIS NANNINI, D. (1974)
Paraquat toxicity for rabbits. Vet. Ital., 25: 555-565.
RILEY, D. (1981) The fate and effect of paraquat and diquat
residues in soil. In: Proceedings for the National Spray Seed
Conference 1981, Albury, New South Wales, Australia.
RILEY, D., WILKINSON, W., & TUCKER, B.V. (1976) Biological
unavailability of bound paraquat residues in soil. In:
Kaufman, D.P., Still, G.G., Paulson, G.D., & Bandal, S.K., ed.
Bound and conjugated pesticide residues, pp. 301-353 (ACS
Symposium Series No. 29).
ROBERTSON, B. (1973) Paraquat poisoning as an experimental
model of the idiopathic respiratory distress syndrome. Bull.
Phys. Pathol. Res., 9: 1433-1452.
ROBERTSON, B., GROSSMANN, G., & IVEMARK, B. (1976) The
alveolar lining layer in experimental paraquat poisoning. Acta
pathol. microbiol. Scand. Section A, 84: 40-46.
ROSE, J. (1980) Paraquat poisoning. Lancet, 2(8200): 924.
ROSE, M.S. & SMITH, L.L. (1977) The relevance of paraquat
accumulation by tissues. In: Autor, A.P., ed., Biochemical
mechanisms of paraquat toxicity, New York, Academic Press, pp.
71-91.
ROSE, M.S., SMITH, L.L., & WYATT, I. (1974a) Evidence of
energy-dependent accumulation of paraquat in rat lung. Nature
(Lond.), 252: 314-315.
ROSE, M.S., CRABTREE, H.C., FLETCHER, K., & WYATT, I.
(1974b) Biochemical effects of diquat and paraquat.
Disturbance of the control of corticosteroid synthesis in rat
adrenal and subsequent effects on the control of live glycogen
utilization. Biochem. J., 138: 437-443.
ROSE, M.S., SMITH, L.L., & WYATT, I. (1976) The relevance of
pentose-phosphate pathway stimulation in rat lung to the
mechanisms of paraquat toxicity. Biochem. Pharmacol., 25:
1763-1767.
ROSLYCKY, E.B. (1977) Response of soil microbiota to
selected herbicide treatments. Can. J. Microbiol., 23: 426-433.
ROSS, W.E., BLOCK, E.R., & CHANG, R.Y. (1979) Paraquat-
induced DNA damage in mammalian cells. Biochem. biophys. Res.
Commun., 91: 1302-1308.
SAITO, K., PARKER, W.B., GARDNER, D.E., & MENZEL, D.B.
(1979) Paraquat accumulation by cultured lung cells.
Pharmacology, 21(Abstr. 218): 392.
SAMMAN, P.D. & JOHNSTON, E.N. (1969) Nail damage associated
with handling of paraquat and diquat. Br. med. J., 1: 818-819.
SCHLATTER, I. (1976) [Poisoning due to herbicide paraquat.]
Schweiz. Rundsch. Med., 65: 837-843 (in German).
SEIBER, J.N. & WOODROW, J.E. (1981) Sampling and analysis of
airborne residues of paraquat in treated cotton field
environments. Arch. environ. Contam. Toxicol., 10: 133-149.
SEIBER, J.N., WINTERLIN, W.L., & MCCHESNEY, B. (1979)
Residues of toxaphene, DEE, and paraquat in plant parts and
gin waste from treated cotton fields. Arch. environ. Contam.
Toxicol., 8: 125-137.
SEIDENFELD, J.J., WYKOFF, D., ZAVALA, D.C., & RICHARDSON,
J.B. (1978) Paraquat lung injury in rabbits. Br. J. ind.
Med., 35: 245-247.
SELECT COMMITTEE ON NARCOTICS ABUSE & CONTROL (1980) The use
of paraquat to eradicate illicit marihuana crops and the
health implications of paraquat-contaminated marihuana on the
US market. A report of the 96-Congress, Washington DC, US
Government Printing Office, pp. 1-97 (SCNAC-96-1-16).
SELYPES, A. & PALDY, A. (1978) The examination of the
mutagenic effect of two pesticides: Krezonit E and Gramoxone.
Proc. Hung. Ann. Meet. Biochem., 18: 77-78.
SELYPES, A., NAGYMAJTENYI, L., & BERENCSI, G. (1980)
Mutagenic and embryotoxic effects of paraquat and diquat.
Bull. environ. Contam. Toxicol., 25: 513-517.
SHARP, C.W., OTTOLENGHI, A., & POSNER, A.S. (1972)
Correlation of paraquat toxicity with tissue concentration and
weight loss of the rat. Toxicol. appl. Pharmacol., 22: 241-251.
SHU, H., TALCOTT, R.E., RICE, S.A., & WEI, E.T. (1979) Lipid
peroxidation and paraquat toxicity. Biochem. Pharmacol., 28:
327-331.
SHUZUI, T. (1980) [An autopsy case of paraquat poisoning.]
Mie Igaku, 23: 518-521 (in Japanese).
SINGH, S.P. & YADAV, N.K. (1978) Toxicity of some herbicides
to maior carp fingerlings. Indian J. Ecol., 5: 141-147.
SINOW, J. & WEI, E. (1973) Ocular toxicity of paraquat.
Bull. environ. Contam. Toxicol., 9: 163-168.
SLADE, P. (1965) Photochemical degradation of paraquat.
Nature (Lond.), 207: 515.
SLADE, P. (1966) The fate of paraquat applied to plants.
Weed Res., 6: 158-167.
SMALLEY, H.E. (1973) Toxicity and hazard of the herbicide
paraquat in turkeys. Poultry Sci., 52: 1625-1629.
SMITH, R.J. (1978) Poisoned pot becomes burning issue in
high places. Science, 200: 417-418.
SMITH, P. & HEATH, D. (1974) The ultrastructure and time
sequence of the early stages of paraquat lung in rats.
J. Pathol., 114: 177-184.
SMITH, P. & HEATH, D. (1976) Paraquat. Clin. Rev. Toxicol.,
4: 411-445.
SMITH, L.L. & ROSE, M.S. (1977a) A comparison of the effects
of paraquat and diquat on the water content of rat lung and
the incorporation of thymidine into lung DNA. Toxicology, 8:
223-230.
SMITH, L.L. & ROSE, M.S. (1977b) Biochemical changes in
lungs exposed to paraquat. In: Autor, A.P., ed. Biochemical
mechanisms of paraquat toxicity, New York, Academic Press.
SMITH, P., HEATH, D., & RAY, J.M. (1973) The pathogenesis
and structure of paraquat-induced pulmonary fibrosis in rats.
J. Pathol., 114: 57-67.
SMITH, L.L., WRIGHT, A., WYATT, I., & ROSE, M.S. (1974)
Effective treatment for paraquat poisoning in rats and its
relevance to treatment of paraquat poisoning in man. Br. med.
J., 4: 569-571.
SMITH, S.N., LYON, A.J., & SAHID, I.B. (1976) The breakdown
of paraquat and diquat by soil fungi. New Phytol., 77: 735-740.
SMITH, C.N., LEONARD, R.A., LANGDALE, G.W., & BAILEY, G.W.
(1978) Transport of agricultural chemicals from small upland
Piedmont watersheds, Springfield, Virginia, National Technical
Information Service, p. 386 (Report PB-285, 134).
SMITH, L.L., ROSE, M.S., & WYATT, I. (1979) The pathology
and biochemistry of paraquat. In: Oxygen free radicals and
tissue damage, Amsterdam, North Holland, Excerpta Medica,
pp. 321-341 (CIBA Foundation Series 65 - new series).
SMITH, T.F., NOACK, A.J., & COSH, S.M. (1981a) The effect of
some herbicides on vesicular-arbuscular endophyte abundance in
the soil and on infection of host roots. Pestic. Sci., 12:
91-97.
SMITH, L.L., WYATT, I., & ROSE, M.S. (1981b) Factors
affecting the efflux of paraquat from rat lung slices.
Toxicology, 19: 197-207.
SODERQUIST, C.J. & CROSBY, D.G. (1972) The gas chromato-
graphic determination of paraquat in water. Bull. environ.
Contam. Toxicol., 8: 363-368.
STAIFF, D.C., IRLE, G.K., & FELSENSTEIN, W.C. (1973)
Screening of various adsorbents for protection against
paraquat poisoning. Bull. environ. Contam. Toxicol., 10:
193-199.
STAIFF, D.C., COMER, S.W., ARMSTRONG, J.F., & WOLFE, H.R.
(1975) Exposure to the herbicide paraquat. Bull. environ.
Contam. Toxicol., 14: 334-340.
STAIFF, D.C., BUTLER, L.C., & DAVIS, J.E. (1981) A fie1d
study of the chemical degradation of paraquat dichloride
following simulated spillage on soil. Bull. environ. Contam.
Toxicol., 26: 16-21.
STEFFEN, C. & NETTER, K.J. (1979) On the mechanism of
paraquat action on microsomal oxygen reduction and its
relation to lipid peroxidation. Toxicol. appl. Pharmacol., 47:
593-602.
STEFFEN, C., MULIAWAN, H., & KAPPUS, H. (1980) Lack of in
vivo lipid peroxidation in experimental paraquat poisoning.
Arch. Pharmacol., 310(3): 241-243.
STEPHENS, D.S., WALKER, D.H., SCHAFFNER, W., KAPLOWITZ, L.G.,
BRASHEAR, R.H., ROBERTS, R., & SPICKARD, W.A. (1981)
Pseudodiphtheria: prominent pharyngeal membrane associated
with fetal paraquat ingestion. Ann. intern. Med., 94: 202-204.
STEVENS, M.A. & WALLEY, J.K. (1966) Tissue and milk residues
arising from the ingestion of single doses of diquat and
paraquat by cattle. J. Sci. Food Agric., 17: 472-475.
STEWART, M.J., LEVITT, T., & JARVIE, D.R. (1979) Emergency
estimations of paraquat in plasma. A comparison of the RIA and
ion pair/colorimetric methods. Clin. Chim. Acta, 94: 253-257.
SUGAYA, H., OHE, T., UENO, T., KAWASHIMA, T., SUGITA, T.,
MAEHARA, M., HISANCHI, T., IORI, M., HARADA, H., & SHIGEMATA,
S. (1980) [Clinical discussion on six cases of paraquat
dichloride intoxication.] Nippon Naika Gakkai Zasshi, 69: 876
(in Japanese).
SUMMERS, L.A. (1980) The bipyridylium herbicides, London,
New York, Toronto, Sydney, San Francisco, Academic Press,
pp. 1-449.
SWAN, A.A.B. (1969) Exposure of spray operators to paraquat.
Br. J. ind. Med., 26: 322-329.
SYKES, B.J., PURCHASE, I.F.H., & SMITH, L.L. (1977)
Pulmonary ultrastructure after oral and intravenous dosage of
paraquat to rats. J. Pathol., 121: 233-241.
TAKAHASHI, T., YAMAMOTO, K., SAWAI, T., OKUDO, T., MUKODA, T.,
MINAGAWA, N., & SUGAYA, H. (1978) [Examination of 5
autopsies following paraquat dichloride intoxication,
especially of parenchymatous organ lesions.] Nippon
Byorigakkai Kaishi, 67: 138-139 (in Japanese).
TAKAYAMA, K., TAKEUCHI, K., SUGA, E., IWABUCHI, K., & TOMICHI,
N. (1978) [A case of autopsy following paraquat dichloride
intoxication.] Nippon Byorigakkai Kaishi, 67: 139 (in
Japanese).
TAKEUCHI, K., TAKAYAMA, K., TOMICHI, N., KAN, E., YAGAWA, K.,
& IWABUCHI, K. (1980) [Paraquat poisoning in a pregnant
woman.] Nippon Byorigakkai Kaishi, 18: 747-752 (in Japanese).
TALCOTT, R.E., SHU, H., & WEI, E.T. (1979) Dissociation of
microsomal oxygen reduction and lipid peroxidation with the
electron acceptors, paraquat and menadione. Biochem.
Pharmacol., 26: 665-671.
TCHIPILSKA, L.N. (1980) [The influence of some pesticides on
the soil microorganisms in connexion with the hygiene
evaluation of the soil,] Referat, Sofia, pp. 1-39 (PhD degree
thesis) (in Bulgarian).
THOMPSON, W.D. & PATRICK, R.S. (1978) Collagen propyl
hydroxylase levels in experimental paraquat poisoning.
Br. J. exp. Pathol., 59: 288-291.
TOMPSETT, S.L. (1970) Paraquat poisoning. Acta pharmacol.
toxicol., 28: 346-358.
TOMURA, M., ETO, K., SAGARA, K., & SATO, T. (1979) [A case
of disturbance of the liver and kidney.] Kanzo, 20: 1016 (in
Japanese).
TONER, P.G., VETTERS, J.M., SPILG, W.G.S., & HARLAND, W.A.
(1970) Fine structure of the lung lesion in a case of
paraquat poisoning. J. Pathol., 102: 182-185.
TRUSH, M.A., MIMNAUGH, E.G., GINSBURG, E., & GRAM, T.E.
(1981) In vitro stimulation by paraquat of reactive oxygen-
mediated lipid peroxidation in rat lung microsomes. Toxicol.
appl. Pharmacol., 60(2): 279-286.
TRUSH, M.A., MIMNAUGH, E.G., GINSBURG, E., & GRAM, T.E.
(1982) Studies on the in vitro interaction of mitomycin C,
nitrofurantoin, and paraquat with pulmonary microsomes.
Stimulation of reactive oxygen-dependent lipid peroxidation.
Biochem. Pharmacol., 31(21): 3335-3346.
TSUNENARI, S., MUTO, H., SASAKI, S., SUGITA, H., & KANDA, M.
(1975) [Forensic toxicological studies on herbicide
(Gramoxone).] Jpn. J. leg. Med., 29: 88-102 (in Japanese).
TSUNENARI, S., YONEMITSU, K., UCHIMURA, Y., & KANDA, M.
(1981) The influence of putrefactive changes on the
determination of paraquat in autopsy materials. Forensic Sci.
Int., 17: 51-56.
TSUTSUI, Y., NAKABAYASHI, H., SUZUKI, H., & OGURA, K. (1976)
[Studies on the toxicity of paraquat - Part I.] Nippon Noson
Igakkai Zasshi, 25: 614-621 (in Japanese).
TU, C.M. & BOLLEN, W.B. (1968) Effect of paraquat on
microbial activities in soils. Weed Res., 8: 28-37.
TWEATS, D.J. (1975) The effect of R (drug resistance)
factors on the detection of mutagens by E. coli K 12,
Brussels, Belgium, Annual Euratom Report, Biological Sciences,
316 pp.
TYBURCZYK, W., BORKOWSKA, I., CHORAGIEWICZ, H., & KLIMEK, K.
(1979) [Effect of paraquat on some biochemical tests in
rats.] Bromatol. Chem. Toksykol., 12: 283-288 (in Polish).
UKAI, S., HIROSE, K., & KAWASE, S. (1977) [Gas chromatography
of reduction products of the herbicides "diquat" and "paraquat".]
Eisei Kagaku, 23: 32-38 (in Japanese).
US CONGRESSIONAL HEARING (1979) Health implications of
paraquat-contaminated marihuana. Select Committee on Narcotics
Abuse & Control, House of Representatives. Hearing of the 96th
Congress, Washington DC, US Government Printing Office, pp.
1-103 (SCNAC-96-1-3).
VALE, J.A. (1977) Paraquat poisoning. Nurs. Times, 73:
154-155.
VAZIRI, N.D., NESS, R.L., FAIRSHTER, R.D., SMITH, W.R., &
ROSEN, S.M. (1979) Nephrotoxicity of paraquat in man. Arch.
intern. Med., 139: 172-174.
VESELEY, D.L., WATSON, B., & LEVEY, G.A. (1979) Activation
of liver guanylate cyclase by paraquat: possible role of
superoxide anion. J. Pharmacol. exp. Ther., 209: 162-164.
VIJEYARATNAM, G.S. & CORRIN, B. (1971) Experimental paraquat
poisoning: a histological and electron-optical study of the
changes in the lung. J. Pathol., 103: 123-129.
VUCINOVIC, V. (1978) [Four cases of poisoning with
paraquat.] Arch. Hig. Rada, 29: 261-265 (in Serbo-Croat).
WADDELL, W.J. & MARLOWE, C. (1980) Tissue and cellular
disposition of paraquat in mice. Toxicol. appl. Pharmacol.,
56: 127-140.
WAIGHT, J.J.J. & WHEATHER, R.H. (1979) Fatal percutaneous
paraquat poisoning. J. Am. Med. Assoc., 242: 472.
WALKER, M., DUGARD, P.H., & SCOTT, R.C. (1983) Absorption
through human and laboratory animal skins: in vitro
comparison. Acta pharm. Suec., 20(1): 52-53.
WALTERS, K.A., DUGAR, P.H., & FLORENCE, A.T. (1981)
Non-ionic surfactants and gastric mucosal transport of
paraquat. J. Pharm. Pharmacol., 33: 207-213.
WARD, C.D., STONES, D.P.A., CONNEL, H., CULLEN, D.R., &
WATKIN, J.I. (1976) Paraquat poisoning. Lancet, 1(7971):
1247.
WATANABE, I., SAKAI, K., TOYAMA, K., UENO, M., & WATANABE, M.
(1979) [On 3 cases of ocular disturbance due to Gratoxone, a
herbicide containing 24% paraquat dichloride.] Ganka Rinsho
Iho, 73: 1244-1246 (in Japanese).
WAUCHOPE, R.D. (1979) Pesticides in runoff water. Agric.
Res., 27: 11.
WAY, J.M., NEWMAN, J.F., MOORE, N.W., & KNAGGS, F.W. (1971)
Some ecological effects of the use of paraquat for the control
of weeds in small lakes. J. appl. Ecology, 8: 509-532.
WEBER, J.B., PERRY, P.W., & UPCHURCH, R.P. (1965) The
influence of temperature and time on the adsorption of
paraquat, diquat, 2,4-D and prometone by clays, charcoal and
on anion-exchange resin. Soil Sci. Am. Soc. Proc., 29: 678-688.
WHO/FAO (1978) Data sheets on pesticides No. 4, Rev.1 -
Paraquat, Geneva, World Health Organization (Unpublished
Report No.: VBC/DS/75.4 Rev.1 (8/78)).
WILKINSON, W. (1980) Paraquat and diquat: Longterm high rate
trial, Frensham UK. Management of site, effects on crops and
weeds and residues in crops, London, ICI Ltd (Report RJ0013B).
WITSCHI, H.P., KACEW, S., HIRAI, K., & COTE, M.G. (1977) In
vivo oxidation of reduced nicotine amide-adenine dinucleotide
phosphate by paraquat and diquat in rat lung. Chem. Biol.
Interactions., 19: 143-160.
WOHLFAHRT, D.J. (1982) Fatal paraquat poisoning after skin
absorption. Med. J. Aust., 1: 512-513.
WOJECK, G.A., PRICE, J.F., NIGG, A.N., & STAMPER, J.H.
(1983) Worker exposure to paraquat and diquat. Arch. environ.
Contam. Toxicol., 12: 65-70.
WORTHING, C.R. (1979) The pesticide manual, Croydon,
England, British Crop Protection Council (BCPC Publications).
WRIGHT, K.A. & CAIN, M. (1970) Microbial degradation of
4-carboxy-1-methylpyridylium chloride, a photolytic product of
paraquat. Biochem. J., 118: 52.
WRIGHT, N., YEOMAN, W.B., & HALE, K.A. (1978) Assessment of
severity of paraquat poisoning. Br. med. J., 2: 396-397.
WYATT, I., DOSS, A.W., ZAVALA, D.C., & SMITH, L.L. (1981)
Intrabronchial instillation of paraquat in rats: lung
morphology and retention study. Br. J. ind. Med., 38: 42-48.
YOSHIDA, K., TANAKA, Y., IKUNUMA, T., TERUKIMATSU, S., KUSANO,
E., ASANO, Y., & HOSODA, S. (1980) [A case report of
survival after intoxication by paraquat.] Nippon Nuika Gakkai
Zasshi, 69: 1487-1489 (in Japanese).
YOUNGMAN, R.J. & DODGE, A.D. (1979) [Mechanism of paraquat
action: inhibition of the herbicidal effect by a copper
chelate with superoxide-dismutating activity.] Z. Naturforsch.
(Teil C), 34: 1032-1035 (in German).
ZAVALA, D.C. & RHODES, M.L. (1978) An effect of paraquat on
the lungs of rabbits. Its implications in smoking contaminated
marijuana. Chest, 74: 418-420.
DIQUAT
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General properties
Diquat (1,1'ethylene, 2,2'bipyridyl) is a non-selective contact
herbicide. It is sold primarily as a 20% w/v solution in many
countries and is manufactured in the United Kingdom. It is
exclusively manufactured as a dibromide salt and is usually
formulated to contain wetters.
The herbicidal property of diquat depends on its ability to
undergo a single electron addition to form a radical that reacts
with molecular oxygen to reform diquat and concomitantly produce a
superoxide anion. This oxygen radical may directly or indirectly
cause cell death.
It is possible to detect the compound because of its ability to
form a radical. Analytical procedures are available.
1.1.2. Environmental distribution and transformation
environmental effects
Diquat undergoes rapid photochemical degradation in aqueous
solution and on surfaces. The major degradation products produced
in water have been identified and are of lower acute oral toxicity
for rats than diquat itself. The photochemical degradation of
diquat on plants is more complex than that in water. On diquat-
desiccated wheat and barley, diquat itself normally constitutes the
most important single compound. The most important photochemical
degradation products have been identified, they are of low
mammalian toxicity. No other well-defined major degradation
product is formed.
Ruminants excrete diquat and its photochemical products rapidly
and very little is transferred to milk and tissues. Consequently,
residue levels in products of animal origin are very low.
Ingestion of diquat and its photochemical products at higher levels
than would be found in practice did not induce ill effects in
ruminants.
Diquat reaching the soil becomes rapidly and strongly adsorbed
to clay minerals in soil. This process inactivates the herbicidal
activity of diquat. While free diquat is degraded by a range of
soil microorganisms, degradation of strongly adsorbed diquat is
relatively slow. In plot studies, the rate of degradation of
diquat in soil is very slow or non-detectable. However, in long-
term field studies, degradation rates of the order of 5 - 10% per
year have been shown. This is greater than the rate required in
normal practice to prevent saturation of the deactivation capacity
of agricultural/horticultural soils. Strongly-bound diquat has no
adverse effects on soil microfauna or soil microbial processes.
Diquat residues disappear rapidly from water by adsorption on
aquatic weeds and by strong adsorption on bottom mud. Diquat is of
low toxicity for fish and is not accumulated in them. Normal
applications of diquat for aquatic weed control are not harmful to
aquatic organisms. However, care should be taken in applying
diquat to water containing heavy weed growth to treat only a part
of the weed growth, since oxygen consumed by subsequent weed decay
may decrease dissolved oxygen levels to an extent that may be
dangerous for fish. Treated water should not be used for overhead
irrigation until a period of 10 days has elapsed following
treatment.
Diquat is not volatile and the concentrations of airborne
diquat during spraying have been shown to be very low.
1.1.3. Kinetics and metabolism
Diquat is poorly absorbed from the intestinal tract and skin.
Diquat monopyridone is the major metabolite of diquat in the body;
of lesser importance is diquat dipyridone. Both metabolites are
considerably less toxic than diquat itself. Depending on species
and route of administration, less than 20% of the dose is
metabolized. The gastrointestinal microflora appear to be mainly
responsible for the metabolism of diquat.
Compared with paraquat, accumulation of diquat in the lungs is
far less marked, but diquat shows a certain preference for the
kidneys. The kidneys are the major route of excretion, but a
considerable amount of diquat can also be excreted in the bile,
varying with the animal species.
1.1.4. Effects on animals
Diquat is less toxic than paraquat and does not give rise to
the specific lung disease that is so typical of paraquat poisoning.
Gastrointestinal disturbances, with vomiting, greenish diarrhoea,
and abdominal distension from the significant accumulation of water
in the lumen of the intestines, are typical of diquat poisoning,
together with progressive haemoconcentration, which may progress to
lethargy, coma, and death. At high doses, minor toxicity has been
noted in the liver, kidney, and the nervous and endocrine systems.
Diquat has induced cataracts after prolonged oral exposure
although this effect has not been reported in man. It is less
irritant to the skin, mucous membranes, and the eye than paraquat,
and is not known to be a sensitizer.
Diquat is not teratogenic or carcinogenic.
In vitro mutagenicity studies were inconclusive, though
generally suggesting only weak activity, while the results of in
vivo studies have been negative. A no-observed-adverse-effect
level of 0.75 mg diquat ion/kg body weight per day has been
established from long-term feeding studies on rats.
1.1.5. Effects on man
Occupational exposure to diquat does not pose a health risk if
the recommendations for use are followed and there is adherence to
safe working practices.
Diquat poisoning by suicidal or accidental ingestion is much
less common than paraquat poisoning. It produces a similar severe
clinical syndrome with two notable differences: (a) diarrhoea is a
prominent feature, and (b) pulmonary fibrosis has not been
described.
Accidental cases are usually due to ingestion of decanted
diquat.
The lethal dose for man appears to be approximately 6 - 12
grams of diquat dibromide. In agricultural workers, inflammation
and bleeding of the nasal mucosa have been reported, as well as
nail changes and delayed wound healing.
1.2. Recommendations
1.2.1. General
Where practical and reasonable, the availability and use of the
20% liquid product should be limited to bona fide agriculturalists,
horticulturalists, and professional users who work with trained
personnel, properly maintained equipment, and adequate supervision.
Every effort should be made to prevent the practice of
decanting or rebottling of the product into containers that have
not been properly labelled.
1.2.2. Prevention and treatment
Attention should be drawn to the fact that persons with skin
lesions (either pre-existing or following contamination with
diquat) should not be permitted to take any part in spraying
procedures until skin condition has resolved.
It must be stressed that treatment of persons with diquat
poisoning should be instituted as early as possible. The
likelihood of recovery from a fatal dose is greatest when therapy
begins within 5 - 6 h of poisoning.
1.2.3. Experimental work
Results of existing mutagenicity and carcinogenicity studies
generally suggest that diquat is unlikely to induce genotoxic
effects in man, but more detailed information is required.
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and Chemical Properties
Diquat is a non-selective contact bipyridylium herbicide and
desiccant. The herbicide is supplied mainly as an aqueous solution
of the dibromide (1,1'-ethylene-2,2'-bipyridylium dibromide,
C12H12N2 Br2), with a relative molecular mass of 184.2 based on the
cation. The commonly available analytical standard is diquat
dibromide monohydrate, which is an odourless, pale yellow,
crystalline powder. Some of the other physical properties of
diquat dibromide are listed in Table 1. It is slightly soluble in
alcohol, and practically insoluble in non-polar organic solvents
(Summers, 1980). Diquat is non-explosive and non-inflammable in
aqueous formulations.
Table 1. Physical properties of diquat dibromide
--------------------------------------------------
Specific gravity at 20 °C 1.200
Melting point 180 °C
Boiling point approximately 300 °C
with decomposition
Solubility in water at 20 °C 700 g/litre
pH of liquid formulation 6.0 - 7.0
Evaporation rate not applicable
Vapour pressure not measurable
--------------------------------------------------
Diquat is stable in neutral or acid solutions but is hydrolysed
by alkali. It is inactivated by inert clay and by anionic
surfactants. Diquat dibromide has the following chemical
structure:
7. EFFECTS ON ANIMALS
7.1. Effects on Experimental Animals
7.1.1. Respiratory system
Toxicity studies in rats, mice, dogs, and monkeys (Clark et
al., 1966; Kimbrough & Gaines, 1970; Murray & Gibson, 1972;
Makovskii, 1972; Kelly et al., 1978) demonstrated that paraquat had
a specific effect on the lung (Table 11). Administration by every
route of entry tested whether parenteral (Fisher et al., 1973a;
Robertson, 1973; Hunsdorfer & Rose, 1980), oral (Clark et al.,
1966; Bainova, 1969a; Kimbrough, 1974; Tsutsui et al., 1976;
Dikshith et al., 1979), dermal (Howe & Wright, 1965; Bainova,
1969b; McElligott, 1972), or inhalatory (Gage, 1968b; Bainova,
1971; Makovskii, 1972; Seidenfeld et al., 1978) resulted in
irreversible changes in the lung.
Clark et al. (1966) reported that, in rats, in the earlier
stages after a single toxic oral dose of paraquat, breathing was
gasping or deep and fast, but some days after a single or repeated
toxic doses, the respiration became increasingly laboured, and the
hairs around the mouth and nares were soiled with a brownish
liquid. The extensive alveolar oedema observed in severe
intoxication was responsible for the development of hypoxia,
cyanosis, and dyspnoea. The progressive development of pulmonary
fibrosis was accompanied by difficulty in breathing, gasping, and
hyperpnoea (Smith et al., 1973).
Exposure of rats to high concentrations of respirable paraquat
aerosols was accompanied by shallow respiration. Within 2 - 3 h,
the test animals became dyspnoeic, cyanotic, and inactive, and
there were signs of local eye and nose irritation (Gage, 1968a).
7.1.1.1. Pathomorphological lung studies
Macroscopic examination of the lungs revealed that lesions and
their severity were dependent on the dose of paraquat and the time
between exposure and sacrifice (or death). The wet weight of the
lung increased after a single treatment, owing to oedema and
haemorrhage. The pathogenesis of the paraquat lung lesion has been
well characterized, and has been reviewed by Smith & Heath (1976).
The acute pulmonary toxicity of paraquat in animals has been
described as occurring in two phases (Smith & Heath, 1976). In the
initial "destructive" phase, alveolar epithelial cells were
extensively damaged and their subsequent disintegration often
resulted in a completely denuded alveolar basement membrane.
Table 11. Effects on experimental animals of repeated oral, dermal, or inhalation exposure to paraquat
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rat diet - 125 mg/kg 2 years no toxic effects Howe & Wright (1965)
Dog diet - 50 mg/kg no toxic effects
Rat diet - 0.25 mg/kg 27 days death; histological changes in the lung Clark et al. (1966)
Rat diet - 300, 400, 90 days cumulative toxic effects; chronicity Kimbrough & Gaines
500, 600, 700 mg/kg factor (Hayes) 5.2; histological (1970)
changes in the lung
Rat oral - 4, 9, 25 30 days inhibition of ChE activity, increasing Bainova (1969, 1975)
mg/kg body weight GPT activity in the serum; biochemical
per day and histological changes in the lung,
kidney, liver
Rat oral - 1.3, 2.6 4 1/2 months increased GPT and G-6-P-isomease Bainova (1969, 1975)
mg/kg body weight activities in the serum; biochemical
per day and histological changes in lung,
kidney, liver
Rat oral - 3.3, 1.3, 1 year the higher doses were toxic for both Makovskii (1972)
0.13 mg/kg body species tested; no-observed-adverse-
weight per day effect levels:
Guinea- oral - 1.0, 0.4, for rat 0.13, guinea-pig 0.04 mg/kg
pig 0.04 mg/kg body body weight per day
weight per day
Rat diet - 20 - 30 30 days histological and electron-optical lung Kimbrough (1974)
mg/kg body weight changes
per day
Mouse diet - 25, 50, 70 80 weeks death; dose-dependent clinical and FAO/WHO (1973)
mg/kg histological changes in the lung,
liver, kidney, and other organs tested
Rat oral - 25, 50, 1 - 5 days body weight loss; increased serum LDH, Tsutsui et al.
100 mg/kg body GOT activity; no haematological (1976)
weight per day changes; histological changes in the
lung, kidney, liver, myocardium
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rat drinking-water - 2 years mortality increased; histological Bainova & Vulcheva
1.3, 2.6 mg/litre changes in the lung, but only minimal (1977)
at the lowest level
Rabbit dermal - 2.8, 4.5, 20 days skin irritation; mortality and toxic Clark et al. (1966)
7, 14 mg/kg body effects at 7 & 14 mg/kg/day. LD50
weight per day mg/kg/day; no-observed-adverse-effect
level 2.8 mg/kg/day
Rat dermal - 2, 5, 15, 21 days skin irritation; mortality and toxic Bainova (1969a)
30, 45 mg/kg body effects at 5 - 45 mg/kg/day;
weight per day histological changes in the lung,
kidney, liver, myocardium; LD50
15 mg/kg/day; no-observed-adverse-
effect level 2 mg/kg/day
Rabbit dermal - from 1.56 20 days skin irritation; mortality and toxic McElligott (1972)
- 50 mg/kg per day effects at 3.13 - 192 mg/kg/day; LD50
(with occlusion) 4.5 mg/kg/day with occlusion and 24
from 2.4 - 192 mg/kg mg/kg/day without occlusion; No-
body weight per day observed-adverse-effect levels: 1.56
(without occlusion) and 2.4 mg/kg/day with and without
occlusion
Rat inhalationa - 4 days at higher concentrations (0.40 & 0.75 Gage (1968)
0.75 mg/m3 mg/m3) histological changes in the
0.4, 0.1, 0.06 mg/m3 15 days lung; no-observed-adverse-effect levels
0.003 mg/m3 60 days from 0.003 - 0.06 mg/m3 6 h daily;
6 h daily TLV - 0.1 mg/m3 paraquat aerosol
Rat inhalationa - 4 1/2 months biochemical, histochemical, and Bainova et al.
1.1, 0.05 mg/m3 histological changes in the lung at (1972)
6 h daily 1.1 mg/m3 no-observed-adverse-effect
level below 0.05 mg/m3 paraquat aerosol
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rabbit inhalationa - 3 months no clinical, functional and Seidenfeld et al.
10 mg paraquat in histological changes in the lung; no (1978)
100 ml water for the toxic effects
aerosol 2 h daily
---------------------------------------------------------------------------------------------------------
a Respirable paraquat aerosol.
Pulmonary oedema was also a characteristic of the destructive
phase, and was frequently of sufficient severity to result in the
death of the animals. Animals surviving the initial destructive
phase, which occurred in the first 1 - 4 days after acute paraquat
overexposure, progressed to what has been termed the
"proliferative" phase. In this phase, the lung was infiltrated
with profibroblastic cells that rapidly differentiated into
fibroblasts which, in some cases, progressed to fibrosis. The
histopathological outcome of the second phase may be influenced by
the treatment regimen, however. Administration of repeated low
doses of paraquat, which less severely damaged the alveolar
epithelial cells, could also induce a hyperplasia of the Type II
cells. This response may represent an attempt by the lung to
repair the damaged epithelium.
Following a single high dose of paraquat to animals, the
earliest ultrastructural changes were observed in the Type I
alveolar epithelial cells, approximately 4 - 6 h after treatment,
and were usually characterized by cellular and mitochondrial
swelling, increased numbers of mitochondria, and the appearance of
dark granules in the cytoplasm. When a high dose (approximately
LD50 or greater) was given, the lesions in the Type I cells often
progressed to the point of complete cellular disintegration leaving
areas of exposed basement membrane (Kimbrough & Gaines, 1970; Smith
et al., 1973; Smith & Heath, 1974; Vijeyaratnam & Corrin, 1971;
Klika et al., 1980).
In contrast to the effects on Type I pneumocytes, however, the
capillary endothelial cells were remarkably resistant to the toxic
effects of paraquat (Sykes et al., 1977).
Ultrastructural lesions in the alveolar Type II pneumocytes
were also observed shortly after single dose paraquat exposure,
although, generally, these lesions were not apparent until after
the first lesions were seen in the Type I cells (Kimbrough &
Gaines, 1970). Swollen mitochondria and damage to the lamellar
bodies usually occurred between 8 and 24 h after a high dose of
paraquat (Robertson, 1973; Robertson et al., 1976). Progressive
deterioration of the Type II cells continued, resulting in
completely denuded alveolar basement membranes and debris-filled
alveolar spaces (Vijeyaratnam & Corrin, 1971). Infiltration and
proliferation of fibroblasts may produce fibrosis that obliterates
the alveolar structure (Smith & Heath 1974).
Vijeyaratnam & Corrin (1971) observed that less severely
affected parts of the lung appeared to undergo epithelial
regeneration, 7 - 14 days after a single dose of paraquat.
Electron microscopic examination revealed the alveoli to be
lined with cuboidal epithelial cells that closely resembled
Type II pneumocytes except for a general lack of lamellar bodies.
Similar phenomena have also been noted by other investigators who
administered paraquat in the diet (Kimbrough & Linder, 1973) or as
repetitive intraperitoneal administrations (Smith et al., 1974).
Thus, in animals where the paraquat dose was sufficient to kill
only the Type I pneumocytes, the surviving Type II cells repaired
the damaged epithelium by proliferating and subsequently
differentiating into Type I epithelial cells. Inhaled paraquat in
aerosol produced initial necrosis and sloughing of the epithelia
and type 2 pneumocyte hyperplasia, fibroblast proliferation, and
increased synthesis of collagen in mice (Popenoe, 1979).
Histochemical alterations have been noted in rats exposed
through inhalation to 1.9 and 1.1 mg/m3 paraquat respirable
aerosol, 6 h/day, 6 days/week, for 4 1/2 months. The histoenzyme
activity of NAD lactate dehydrogenase-diaphorase, beta-
glucuronidase (EC 3.2.1.31), and acid phosphatase (EC 3.1.3.2) was
enhanced in the epithelial cells and in areas of pneumonitis
(Bainova et al., 1972). The changes were concentration-related,
although the activity of succinate dehydrogenase (EC 1.3.99.1) and
aspartate esterase appeared to be less pronounced in comparison
with the controls (Bainova et al., 1972).
7.1.1.2. Species differences in lung injury
Butler & Kleinerman (1971) injected rabbits intraperitoneally
with total doses of from 2 - 100 mg/kg body weight. Thymus atrophy
was observed, but most lungs showed only occasional and small
histological deviations that were poorly correlated with the
clinical signs of paraquat intoxication. The study confirmed the
resistance of the rabbit to paraquat-induced lung lesions (Clark et
al., 1966), and no evidence of any kind of pulmonary disease was
found; nor could significant lung injury be established in rabbits
after 30 days ingestion of 11 mg paraquat/kg in distilled water
(Dikshith et al., 1979). However, some animals showed pulmonary
fibrosis and emphysema, and a few changes were present in all
parenchymatous organs (Mehani, 1972; Zavale & Rhodes, 1978;
Dikshith et al., 1979). The rabbit also proved to be less
sensitive, than the rat, after inhalation exposure (Gage 1968a;
Seidenfeld et al., 1978).
According to Murray & Gibson (1972), and Hundsdorfer & Rose
(1980), guinea-pigs treated orally or sc did not develop the same
type of progressive pulmonary fibrosis as paraquat-intoxicated
rats. In hamsters, a single administration did not induce lung
damage, but prolonged exposure resulted in lung fibrosis (Butler,
1975).
In conclusion, for lung toxicity studies, a characteristic
dose-related pulmonary fibrosis can be induced in the rat, mouse,
dog, and monkey (Murray & Gibson, 1972) but not in the rabbit,
guinea-pig, or hamster.
7.1.1.3. Lung function studies
Rabbits exposed to an aerosol of 200 mg paraquat in 100 ml
distilled water (Seidenfeld et al., 1978) survived more than 3
exposures but showed significantly reduced arterial oxygen tension
and an increased alveolar arterial O2 gradient; specific compliance
decreased and functional residual capacity and breathing frequency
increased. Lam et al. (1980) administered paraquat at 27 mg/kg
body weight ip to rats and 0.5 mg/kg body weight intratracheally.
After 12 h, decreases were observed in total lung capacity,
functional residual capacity, vital capacity, residual volume, and
alveolar volume. These deviations persisted for 72 h. Oral
administration of paraquat at doses ranging from 1 mg/kg body
weight - 13.5 mg/kg body weight to rats resulted in functional lung
changes after 24 h.
Thus clinical, functional, and pathomorphological studies after
single and repeated exposure demonstrated that the spectrum of
paraquat lung disease depended on the magnitude of the dose and the
manner of administration (Seidenfeld et al., 1978; Restuccia et
al., 1974).
7.1.2. Renal system
In paraquat toxicity, kidney damage often precedes signs of
respiratory distress (Clark et al., 1966; Butler & Kleinerman,
1971; Murray & Gibson, 1972) (Table 11). Paraquat is excreted
mainly via the urine and the concentrations of the herbicide in the
kidneys are relatively high (section 6.1). Gross pathological and
histological examinations of paraquat-poisoned rats, guinea-pigs,
rabbits, and dogs revealed vacuolation of the convoluted renal
tubules and proximal tubular necrosis (Bainova, 1969a; Murray &
Gibson, 1972; Tsutsui et al., 1976). The degeneration of the
proximal tubular cells has also been confirmed by electron-optical
studies (Fowler & Brooks, 1971; Marek et al., 1981).
Paraquat is actively secreted by the kidney base transport
system. The nephrotoxicity caused by paraquat is pronounced and
appears to be restricted to the proximal nephron (Ecker et al.,
1975: Gibson & Cagen, 1977; Lock & Ishmael, 1979; Purser & Rose,
1979).
7.1.3. Gastrointestinal tract and liver
The clinical signs of acute and chronic oral poisoning
(Kimbrough & Gaines, 1970; Murray & Gibson, 1972; Bainova, 1969a)
or of ip injection (Butler & Kleinerman, 1971) include transient
diarrhoea and body weight loss, decreased food intake, and
dehydration. Some of the animals vomited soon after paraquat
administration. Residual skin contamination after dermal toxicity
studies on rabbits (McElligott, 1972) caused severe tongue
ulceration and an unwillingness to eat. The adverse irritant
effects were minimized by continued restraint after skin
decontamination of the treated rabbits.
There have been several reports of liver damage following
exposure to high doses of paraquat (Clark et al., 1966; Bainova,
1969a; Murray & Gibson, 1972; Tsutsui et al., 1976; Gibson & Cagen,
1977, Cagen et al., 1976). Centrilobular necrosis of hepatocytes
with proliferation of the Kupfer cells and bile canals have been
described.
In general, liver damage in experimental animals has not been
severe compared with lung and kidney damage. Serum enzyme
activities (SGOT, SGPT, LAP) only increased when large amounts of
paraquat were given (Giri et al., 1979).
7.1.4. Skin and eyes
The herbicide can provoke local irritation of the skin and
eyes. Clark et al. (1966) found skin irritation in rabbits only
when paraquat was applied beneath occlusive dressings in aqueous
solutions (total dose 1.56, 5.0, and 6.25 mg ion/kg body weight).
In mice and rats, the application of 5 - 20 g paraquat/litre
solutions in single and 21-day repeated dermal toxicity tests
provoked dose-related toxic dermatitis with erythema, oedema,
desquamation, and necrosis (Bainova, 1969b). Doses from 1.56 to 50
mg/kg, in repeated 20-day studies using the occlusive technique
(McElligott, 1972) resulted in local erythema and scab formation.
The histological changes consisted of parakeratosis and occasional
intra-epidermal pustules. A delayed skin irritant action of the
herbicide was reported by Fodri et al. (1977) in guinea-pig
studies.
No skin sensitization was observed in studies on guinea-pigs
when paraquat was applied (Bainova, 1969b; Fodri et al., 1977).
The instillation of dilutions of paraquat (up to 500 g/litre)
in rabbit eye induced inflammation within 24 h and this continued
for 96 h (Clark et al., 1966). Sinow & Wei (1973) introduced 62.5,
125, 250, 500, and 1000 paraquat/litre into the rabbit eye.
Concentrations of 62.5 and 125 g/litre caused severe conjunctival
reactions; higher levels (250 - 500 g/litre) provoked iritis and
pannus, while at the 500 g/litre concentration there was corneal
opacification, iritis, and conjunctivitis. All rabbits receiving
0.2 ml of paraquat at 1000 g/litre in 1 eye or 0.2 ml of a
concentration of 500 g/litre in both eyes died within 6 days of
application (Sinow & Wei, 1973).
Both conjunctival and dermal application of different
concentrations induced systemic toxicity (Sinow & Wei, 1973; Clark
et al., 1966; Bainova, 1969b; Kimbrough & Gaines, 1970; Makovskii,
1972; McElligott, 1972), lung, kidney, and liver damage, and death.
7.1.5. Other systems
No specific functional, histological, or biochemical effects of
paraquat have been reported in other systems that have been
examined; this is of prime importance in an evaluation of its
toxicity. When lethal doses of paraquat are given to rats,
symptoms consistent with neurological disturbances have been
observed. These include decreased motor activity, lack of
coordination, ataxia and dragging of the hind limbs (Smith et al.,
1973). Also associated with near lethal or lethal doses are damage
to the myocardium (Tsutsui et al., 1974), haemolytic anaemia
(Bainova, 1969a), increased haemosiderin in the spleen (Bainova et
al., 1972) and increased concentrations of plasma corticosteroids
(Rose et al., 1974b).
7.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
7.1.6.1. Effects on reproduction
Some histological changes in the testes have been reported in a
few paraquat toxicity studies. Butler & Kleinerman (1971) found
multinuclear giant cells in rabbit testicular tubules. When
paraquat was orally administered at 4 mg/kg body weight to male
rats for 60 days and the testes were examined, there were no
significant deviations in the spermatozoa count or motility, nor
were there any biochemical changes in the several enzymes of testes
homogenates. The histoenzyme activity of lactate dehydrogenase,
succinate dehydrogenase, DPN-diaphorase, alkaline phosphatase, and
acid phosphatase in the treated animals did not differ from that of
the controls, nor did quantitative and qualitative histological
examination of the testicular tubule cells reveal any abnormality.
A 3-generation reproduction study has been carried out on rats
treated with paraquat ion at 100 mg/kg diet (FAO/WHO, 1973). There
were no significant abnormalities in fertility, fecundity, and
neonatal morbidity or mortality, nor were there any signs of
gonadotoxicity or structural or functional lesions. Pulmonary
function in the treated offspring was normal.
Clegg (1979) has reviewed animal reproduction and
carcinogenicity studies conducted in relation to the safe use of
pesticides.
7.1.6.2. Embryotoxicity and teratogenicity
Oral or ip administration of high doses of paraquat to mice and
rats on various days of gestation produced significant maternal
toxicity, evidenced by increased mortality rates (Bainova &
Vulcheva, 1974; Bus et al., 1975). Examination of the fetuses from
the higher-dose groups revealed a reduction in fetal body weights,
delayed ossification of the sternabrae, and increased resorption
rate in mice, as a result of the maternal intoxication. The
minimal embryotoxic effect seemed due in part to difficulty in
crossing the placenta, reflected by low concentrations of paraquat
in the embryo relative to maternal tissues (Bus et al., 1975). The
absence of a specific embryotoxic action of paraquat has also been
observed and reported in other studies on rats (Khera et al., 1968;
Luty et al., 1978), mice (Selypes et al., 1980), and rabbits
(FAO/WHO, 1973).
In a perinatal toxicity study, Bus & Gibson (1975) administered
paraquat at 50 or 100 mg/litre in the drinking-water to pregnant
mice beginning on day 8 of gestation, with continued treatment of
the litters up to 42 days after birth. Paraquat treatment did not
alter postnatal growth rate, although the mortality rate in the 100
mg/litre-treated mice increased to 33% during the first 7 days
after birth. It was also noted that paraquat at 100 mg/litre
significantly increased the sensitivity of the pups to oxygen
toxicity on days 1, 28, and 42 after birth.
7.1.7. Mutagenicity
Paraquat has been found to have minimal to no genotoxic
activity when evaluated in a variety of in vitro and in vivo test
systems. In studies producing weakly positive results (Moody &
Hassan, 1982; Parry 1977, 1973; Tweats, 1975; Benigni et al., 1979;
Bignami & Grebelli, 1979), which were limited to in vitro studies,
paraquat genotoxicity was accompanied by high cytotoxicity. These
results are best explained by Moody & Hassan (1982), who showed
that the mutagenicity of paraquat in bacterial test systems
( Salmonella typhimurium TA 98 and TA 100) was mediated by the
formation of superoxide. However, other investigators (Andersen et
al., 1972; Levin et al., 1982) did not find mutagenic activity in
bacterial test systems. Furthermore, paraquat was not mutagenic
when evaluated in human leukocytes and in in vivo cytogenetic
tests on mouse bone marrow (Selypes & Paldy, 1978) and dominant
lethal tests on mice (Pasi et al., 1974; Anderson et al., 1976).
7.1.8. Carcinogenicity
A carcinogenicity study was performed on mice at dietary levels
of 25, 50, and 75 mg/kg per day for 80 weeks (FAO/WHO, 1973).
There were reduced weight gains among the animals receiving
paraquat, but deaths during the study were associated with
respiratory disease. Clinical and histopathological examination
determined that paraquat was not tumorigenic in mice.
A 2-year exposure of rats to 1.3 and 2.6 mg/litre, daily, in
the drinking-water provoked histopathological changes in the lung,
liver, kidney, and myocardium. The lung lesions were dose-related;
inflammation, atelectasis, reactive proliferation of the
epithelium, pulmonary fibrosis, and pulmonary adenomatosis were
noted, but no sign of tumour growth or atypism (Bainova & Vulcheva,
1977). Nor was any increased tumour incidence reported in rats in
a 2-year study with a maximum dietary level of 250 mg/kg diet (12.5
mg/kg body weight per day) (FAO/WHO, 1971).
Bainova & Vulcheva (1977) did not discover any indication of
tumorigenicity in a 2-year study on rats receiving paraquat at 1.3
or 2.6 mg/litre in their drinking-water (Table 11).
While testing the carcinogenicity of urethane in mice, Bojan et
al. (1978) also attempted to evaluate the influence of paraquat on
urethane-induced lung tumorigenesis. It is felt that the results
of this study are not of relevance for the assessment of the
carcinogenic potential of paraquat.
7.2. Effects on Farm Animals
The effects of paraquat on farm animals has been discussed in
section 4.3.5. The LD50 doses have been established for hen,
turkey, cow, and sheep (Howe & Wright, 1965; Clark et al., 1966;
Smalley, 1973). Massive doses resulted in convulsions,
neurological symptoms, and death due to respiratory failure.
Domestic animals may ingest paraquat by feeding on a sprayed
area, as a result of spray drifting on to their pasture, by
drinking water contaminated with paraquat used as an aquatic
herbicide, or by feeding on a crop sprayed with paraquat as a
dessicant. Sheep and calves were given paraquat at concentrations
of up to 20 mg/litre drinking-water for 1 month without any obvious
ill effects (Howe & Wright, 1965; Calderbank, 1972), and a cow
dosed with 2/3 of the LD50 of 14C-paraquat gave milk containing
less than 0.1 mg/litre. Field tests demonstrated that cattle did
not suffer any toxic effects when turned loose on pasture after it
had been sprayed with paraquat at 0.45 kg/ha. The same trial
showed that horses had local lesions of the mouth and increased
mucous secretion after grazing on newly-sprayed pasture (Calderbank
et al., 1968). The hazard to stock feeding on such pasture depends
on the density of the pasture, the dose of the herbicide, and the
length of time that has elapsed since its application.
Paraquat was fed to cattle at levels in herbage of 200 - 400
mg/kg for 1 month without any apparent ill effects, and no residues
could be detected in the meat and milk (Calderbank et al., 1968).
However, all domestic animals should be kept far from freshly-
sprayed areas, and when crops are treated with paraquat, due
attention should be paid to the accepted maximum residue limits.
7.3. Dose-Effect of Paraquat
The acute LD50 values for paraquat in various species are given
in Tables 12 and 13. The acute toxicity studies of paraquat salts
(dichloride, dimethylsulfate, dimethylphosphate) have not shown
any significant differences in the acute oral and ip LD50 in rats
(Clark et al., 1966; Makovskii, 1972).
There were no significant differences in the oral LD50 values
obtained for the same species from different laboratories, but the
acute oral LD50 values among the species examined varied.
The effects of repeated paraquat exposure are summarized in
Table 11. Paraquat was administered, orally and in the diet, to
rats, mice, guinea-pigs, and dogs. The guinea-pigs appeared to be
very sensitive (Makovskii, 1972). According to Kimbrough & Gaines
(1970), Makovskii (1972), and Bainova (1975), the herbicide has a
moderate cumulative toxicity. The joint FAO/WHO meeting (1976)
decided on a no-observed-adverse-effect level of 1.5 mg/kg body
weight per day in the rat and 1.25 mg/kg body weight per day in the
dog. As can be seen from Table 11, effects at lower levels have
been observed in other studies.
Guinea-pigs, monkeys, cattle, and human subjects are more
sensitive, while rats and birds are less sensitive to paraquat
through the gastrointestinal route.
Table 12. Paraquat LD50 (mg/kg body weight) and LC50
(mg/m3) in various species
-------------------------------------------------------------------
Species/Sex Oral Dermal Inhalation LC50
LD50 LD50 respirable
paraquat aerosol
-------------------------------------------------------------------
Rat 200a 1c
Rat (F) 100e 90e 10f
Rat (M) 110e 80e 10f
Rat 126i 350g 6g
Mouse 62d
Rabbit 500a
Rabbit 236b
Rabbit 240h
Guinea-pig 40 - 80a
Guinea-pig (M) 30b
Guinea-pig 22i
Guinea-pig 42g 319g 4g
Monkey 50i
Cat 40 - 50a
Cat (F) 35b
Hen 300 - 380a
Hen 262b
Turkey 250 - 280j approximately
375j
Cow 50 - 75a
Sheep 50 - 75a
-------------------------------------------------------------------
a Howe & Wright (1965).
b Clark et al. (1966).
c Gage (1968).
d Bainova (1971).
e Kimbrough & Gaines (1970).
f Bainova & Vulcheva (1972).
g Makovskii (1972).
h McElligott (1972).
i Murray & Gibson (1972).
j Smalley (1973).
Table 13. Paraquat LD50 (mg/kg body weight) after parenteral
treatment
-------------------------------------------------------------------
Species/Sex Subcutaneous Intraperitoneal Intravenous
-------------------------------------------------------------------
Rat (F) 19a
Rat 22b
Mouse 30e 50d
Guinea-pig (F) 3a
Guinea-pig 5b
Turkey 100c 20c
-------------------------------------------------------------------
a Clark et al. (1966). d Ecker et al. (1975).
b Makovskii (1972). e Bus et al. (1975).
c Smalley (1973).
7.4. Methods for Decreasing Paraquat Toxicity
These have been studied in connection with requirements in the
case of paraquat poisoning in man. Clark (1971) showed the
efficacy of Bentonite and Fuller's earth in binding orally
administered paraquat and preventing its absorption from the
gastrointestinal tract. Staiff et al. (1973) reported the high
adsorption capacity of Amerlite. Smith et al. (1974) found
considerably reduced plasma-paraquat levels after the combined
treatment of rats with purgatives and bentonite suspension; these
rats survived a dose that normally killed 90 - 100% of the animals.
The absorption capacities of six absorbent materials were tested by
Okonek et al. (1982) who demonstrated that activated charcoal was
the most successful in absorbing ingested paraquat in rats.
Another way of decreasing paraquat absorption is to introduce
an emetic in the concentrated formulations. Kawai et al. (1980)
examined the protection this provided in fasting and non-fasting
male and female dogs that were given paraquat containing an emetic.
The amount of paraquat eliminated by vomiting was 61 - 86% of the
orally-administered dose. In the group given paraquat only, the
blood level averaged 44 mg/litre; in the group given paraquat and
emetic, it was 0.26 mg/litre.
7.5. Relation Between Age, Sex, and Toxicity
There is no evidence that paraquat is more toxic to either sex
of adult experimental animals (section 7.3) Young rats were more
resistant than older rats, and some authors have paralleled this
resistance with that of young rats to oxygen toxicity. Smith &
Rose (1977b) found a more than 40% increase in cumulative mortality
in 180 g rats compared with 50 g rats, after oral dosing with
paraquat at 680 µmol/kg body weight. According to Smith & Rose
(1977b), the difference in renal function between young and mature
rats accounted for the difference in paraquat toxicity.
8. EFFECTS ON MAN
8.1. Accidental and Suicidal Poisoning
8.1.1. Case reports
The first fatalities from acute paraquat poisoning occurred in
1964 and were reported in 1966 (Bullivant, 1966). By 1977, 600
deaths had been reported following accidental or intentional
ingestion of paraquat. The number of accidental cases of poisoning
is small relative to instances of suicide. Because of different
requirements or practices for notification or reporting of cases of
poisoning in the many countries in which paraquat is used, the
magnitude of the problem is difficult, if not impossible, to
determine. Some representative reports on acute paraquat poisoning
are summarised in Table 14.
The earlier cases of paraquat intoxication were mostly
accidental (Fennelly et al., 1968; Matthew et al., 1968; Masterson
& Roche, 1970; Malone et al., 1971). These cases seemed to have
resulted mainly from the habit of decanting the liquid formulations
into small unmarked or incorrectly labelled containers such as
beer, wine, or soft-drink bottles.
An increased ratio of suicidal to accidental poisoning has been
noted in recent years (Fletcher, 1975; Carson & Carson, 1976;
Fitzgerald et al., 1978a; Bramley & Hart, 1983). This change from
accidental to suicidal poisoning was also reflected in the enhanced
percentage of fatal cases, shorter survival times, and
significantly higher tissue and body fluid levels (Connolly et al.,
1975; McGeown, 1975; Park et al., 1975; Carson & Carson, 1976;
Howard, 1979a; Sugaya et al., 1980; Bismuth et al., 1982).
While the vast majority of poisoning cases are due to
swallowing, a small number of fatal cases of accidental paraquat
poisoning via the skin have been reported when liquid concentrates
(200 g/litre) have been applied in order to kill body lice (Ongom
et al., 1974; Binns, 1976). A few other fatal and non-fatal cases
have been reported following skin-contamination (McDonagh & Martin,
1970; Kimura et al., 1980).
Table 14. Case report data on accidental and suicidal acute paraquat poisoning
-------------------------------------------------------------------------------
Number of cases Fatal Non-fatal Fatality Reference
-------------------------------------------------------------------------------
19 12 7 63% Malone et al. (1971)
24 3 accidental 10 14 42% Connolly et al. (1975)
19 suicidal
2 homicidal
25 10 accidental 17 8 68% McGeown (1975)
31 7 accidental 18 13 58% Park et al. (1975)
21 suicidal
3 homicidal
33 7 accidental 26 7 79% Carson & Carson (1976)
19 suicidal
16 7 9 44% Wright et al. (1978)
136 77 suicidal 92 44 68% Fitzgerald et al. (1978)
10 10 0 100% Natori et al. (1979)
188 69 119 37% Higginbottom et al. (1979)
79 28 51 35% Proudfoot et al. (1979)
68 68 suicidal 41 27 66% Howard (1979)
6 6 suicidal 5 1 83% Sugaya et al. (1980)
28 12 suicidal 17 11 61% Bismuth et al. (1982)
262 95% deliberate 94 (36%) 168 (64%) 36% Bramley & Hart (1983)
intent
-------------------------------------------------------------------------------
8.1.2. Distribution of cases of paraquat poisoning
Cases of acute paraquat poisoning have been reported in:
Bulgaria (Mircev, 1976), Denmark (Pederson et al.,1981), England,
Ireland, Scotland, and the Netherlands (Fletcher, 1975), the
Federal Republic of Germany (Grundies et al., 1971; Hofman &
Frohberg, 1972; Fletcher, 1975; Fischer & Kahler, 1979), France
(Faure et al., 1973; Gervais et al., 1975; Bismuth et al., 1982;
Efthymiou, 1983), Hungary (Farago et al., 1981), Poland (Firlik,
1978), Switzerland (Schlatter, 1976), the USA (Kimbrough, 1974;
Dearden et al., 1978; Stephens et a1., 1981), and in Yugoslavia
(Vucinovic, 1978). Recently, a number of cases of paraquat
poisoning, mainly suicidal, have also been reported in Japan
(Takahashi et al., 1978; Natori et al., 1979; Tomura et al., 1979;
Kimura et al., 1980; Matsumoto et al., 1981). No attempt has been
made to make this list exhaustive, in fact the distribution is
worldwide.
8.1.3. Route of entry
By far the most frequent route of poisoning has been ingestion.
An unusual case of subcutaneous injection of 1 ml paraquat by a
mentally disturbed farmer was reported in Israel (Almog & Tal,
1967). Cases of dermal poisoning have been mentioned in section
8.1.1. There is no evidence of fatal poisoning as a result of
inhalation.
8.1.4. Formulations
Paraquat trade names are listed in Table 3. Concentrated
liquid formulations have been responsible for most (and more
severe) poisonings than granular forms, which contain less paraquat
(McGeown, 1975; Park et al., 1975; Fitzgerald & Barnville, 1978;
Wright et al., 1978; Higginbottom et al., 1979; Howard, 1979a).
8.1.5. Dose
The minimum lethal dose of paraquat is stated to be about 35
mg/kg body weight for human beings (Pederson et al., 1981; Bismuth
et al., 1982).
Symptoms of poisoning depend on the dose absorbed. It is
difficult to estimate the dose absorbed from case histories since
in many cases the patients spat out part of the paraquat
concentrate or vomited profusely after swallowing the herbicide.
Some patients have survived after apparently ingesting 50 - 100 ml
Gramoxone(R) (10 - 20 g paraquat), whereas some died after taking
as little as 2 sachets of Weedol (2.5 g paraquat) (Table 15).
Howard (1979) demonstrated the relationship between the dose of
paraquat ingested, the time elapsing between ingestion and
institution of treatment, and the ultimate outcome in 68 cases of
intentional paraquat poisoning.
8.1.6. Clinical and pathomorphological data relating to fatal
paraquat poisoning
Cases of fatal poisoning can be sub-divided into cases of:
(a) acute fulminant poisoning from a massive dose leading
to generalized systemic poisoning and death from a
combination of acute pulmonary oedema, oliguria,
hepatocellular and adrenal failure and biochemical
disturbances (death usually occurs within 1 - 4 days);
(b) less overwhelming poisoning with slower onset of
organ failure and death from pulmonary oedema,
mediastinitis, and complications of therapy (McGeown,
1975; Fitzgerald et al., 1978a); and
(c) late pulmonary fibrosis (death ensuing 4 days to
several weeks later).
Table 15. Recovery from paraquat poisoning involving lung dysfunction
---------------------------------------------------------------------------------------------------------
Dose of paraquat Major organ Notes Reference
ingested damage
---------------------------------------------------------------------------------------------------------
50 ml kidney, liver, vomiting; pains in stomach; changes in urine Grundies et al.
lung and serum (1971)
15 ml approx. lung nausea; buccal lesions; chest X-ray: poor Lloyd (1969)
aeration at lung bases
10 ml approx. kidney, lung oliguria; changed renal function; basal rales; Fisher et al.
chest X-ray: small bilateral, pleural effusions; (1971)
limited atelectasis; functional lung change
not specified kidney, liver, oliguria; serum, and urine changes; minimal Fennelly et al.
lung deviations in the respiratory function (1971)
30 ml approx. kidney, liver nausea; oliguria; ECG changes; chest X-ray: Galloway &
myocardium, lung increased vascular markings Petrie (1972)
granular kidney, liver, 14 cases with mild oral, renal, lung, and liver Fitzgerald &
paraquat lung impairment Barniville
(1978)
50 ml kidney, liver, vomiting; diarrhoea; abdominal pain; serum and urine Rose (1980)
lung changes; dyspnoea, decreased forced vital capacity;
chest X-ray: extensive perivascular changes
---------------------------------------------------------------------------------------------------------
8.1.6.1. Respiratory system
(a) Clinical data
Soon after ingestion, there is oropharyngeal pain and swelling,
followed within a few days by exudation, ulceration, and mucosal
sloughing, sometimes with pseudomembrane formation, which on
occasion leads to total sloughing of the oropharynx and oesophagus
(Malone et al., 1971). In severe poisoning, pulmonary oedema
rapidly ensues with clinical and functional deterioration until
death. Less intense, but ultimately fatal, poisoning causes
progressive pulmonary fibrosis over days or several weeks, with
gradually increasing dyspnoea and hypoxaemic pulmonary failure.
Pulmonary oedema may occur from fluid overload in oliguric
patients. Mediasteinitis and pneumothorax are occasionally seen
(Dearden et al., 1978; Kimura et al., 1980).
Pulmonary function tests reflect the underlying pathology, with
hypoxaemia, reduction in lung volume, high alveolar-arterial
gradient, and impaired gas transfer (Cooke et al., 1973,
Higginbottom et al., 1979). Chest radiographs may show bilateral
pulmonary oedema, coalescing consolidations, and later, sequential
changes of pulmonary fibrosis (Davidson & McPherson, 1972).
(b) Pathology
At autopsy, the lungs do not collapse properly and the pleural
cavity contains a small amount of fluid. In cases of lung
fibrosis, the lungs are heavy, firm, dark purple, and rubbery.
Consolidation and decreased aeration are found predominantly at the
bases. Emphysema and atelectasis are often found.
Histological studies following lung biopsy and necropsy show
pulmonary oedema, haemorrhages, and atelectasis due to pulmonary
infiltrates, loss of alveolar epithelial cells and, at a later
stage, interstitial and intra-alveolar fibrosis (Smith & Heath,
1976).
During the first 7 days of paraquat poisoning in man, loss of
alveolar epithelial cells has been seen with alterations in, or
detachment of, the type I and II cells, proliferation of
fibroblasts and polymorphous cells, loss of surfactant secretion,
and thickening of the alveolar septa by interstitial fibrosis
(Toner et al., 1970). The later findings (2 - 3 weeks) involved
pulmonary fibrosis and endothelial abnormalities. Dearden et al.
(1978) reviewed the histological and electron-microscopic findings
in human lungs. Capillary permeability seemed to be enhanced
either by vesicles forming transendothelial channels or by
disruption of endothelial cells.
8.1.6.2. Renal system
Acute oliguric renal failure is common in severely poisoned
patients. Less severe manifestations include impaired renal
function, which may disappear before the pulmonary fibrosis
progresses (Beebeejaun et al., 1971; Fisher et al., 1971; Fletcher,
1975; Natori et al., 1979; Grant et al., 1980). Other
manifestations include proteinuria, with hyaline casts, white and
red blood cells. Tubular damage is reflected in glycosuria,
aminoaciduria, and excessive leaking of phosphorus, sodium, and
uric acid (Vaziri et al., 1979).
Soft, pale, swollen kidneys with extensive tubular necrosis,
compatible with toxic injury, are found at necropsy (Beebeejaun et
al., 1971). Sometimes necrosis of the proximal tubules is found
together with extreme dilatation of the distal tubules of the
kidney (Shuzui, 1980).
8.1.6.3. Gastrointestinal system, the liver, and the pancreas
The initial symptoms after oral ingestion of paraquat are
nausea, vomiting, upper abdominal pain, and diarrhoea. Perforation
of the oesophagus is uncommon (Ackrill et al., 1978; Natori et al.
1979).
The ingestion of large doses of paraquat has resulted in severe
liver damage (Ward et al., 1976; Grant et al., 1980) with
progressive metabolic acidosis (Shuzui, 1980; Sugaya et al., 1980).
Fatty degeneration of periportal hepatocytes and sporadic cellular
necrosis in the central region of the liver lobules have been
described (Matsumoto et al., 1980). Cholestasis and portal
inflammation may occur (Matsumoto et al., 1981). Oedematous
degeneration or necrosis of both the intra-hepatic and extra-
hepatic bile ducts, and of the gall bladder, have also been noted
(Mullick et al., 1981).
Takayama et al. (1978) noted stasis of the pancreatic duct,
with increased serum amylase levels after severe paraquat
poisoning.
8.1.6.4. Cardiovascular system
Occasionally, toxic myocarditis after paraquat ingestion has
been described (Bullivant, 1966; Malone et al., 1971; Copland et
al., 1974; Grant et al., 1980).
Takahashi et al. (1978) found fibrinoidal necrosis of the small
arteries in the pancreas, kidney, and liver on days 3 - 6 following
ingestion.
8.1.6.5. Central nervous system
The ingestion of very high doses of paraquat provoked anxiety,
convulsions, ataxia, and semi-consciousness (Grant et al., 1980;
Mukada et al., 1978). Haemorrhagic leukoencephalopathy was
present throughout the central nervous system, involving almost
exclusively the white matter. Focal haemorrhage and
demyelinization were present at various stages together with
haemorrhagic meningitis.
8.1.6.6. Adrenal glands
Adrenal cortical necrosis may contribute to death in severe
paraquat poisoning and the severity of the damage appears to be
dose-related (Nagy, 1970; McGeown, 1975; Fitzgerald et al., 1977a;
Takahashi et al., 1978).
8.1.6.7. Pregnancy
A woman, who accidentally swallowed paraquat in the 28th week
of pregnancy (Fennelly et al., 1968), died 20 days later. Gross
pathological examination did not reveal any abnormalities in the
fetal organs.
A woman, in the 7th month of pregnancy, intentionally ingested
about 60 ml of technical paraquat (Takeuchi et al., 1980) and
vomited approximately half that amount. Oliguria, jaundice, and
cough with sputum production progressed; fetal heartbeat
disappeared on the l3th day and the next day the dead fetus was
delivered. The mother died on the l7th day after poisoning. The
lungs of the dead fetus were filled with the debris of amniotic
fluid; the fetus had begun intra-uterine respiration to compensate
for the insufficient oxygen supply. No symptoms of paraquat
poisoning were noted in the body of the neonate.
A case report published by Musson & Porter (1982) concerning
paraquat ingestion by a 20-week pregnant woman, confirmed the lack
of teratogenic risk in human beings. The pregnancy was allowed to
continue after the treatment of the mother. The infant was
followed up to the age of 3 years and did well clinically, with
normal laboratory tests, development, and behaviour.
8.1.7. Recovery from paraquat poisoning
In the largest series reported (68 - 188 cases) (Fitzgerald et
al., 1978a; Higginbottom et al., 1979; Howard, 1979a; Proudfoot et
al., 1979), survival rates varied from 32% to 65% (Table 14)
Factors determining recovery from paraquat poisoning, reviewed by
Fletcher (1975), McGeown (1975), Fitzgerald & Barniville (1978),
Howard (1979a), and Bismuth et al. (1982), are shown in Table 16.
Victims of paraquat poisoning, who escape major pulmonary
complications, usually recover fully within a few weeks of
ingestion. Renal, gastrointestinal, and hepatic manifestations
return to normal (Fisher et al., 1971; Beebeejaun et al., 1971;
Grundies et al., 1971; Galloways & Petrie, 1972).
Minor pulmonary functional and radiographic abnormalities may
be transient and are of doubtful relationship to paraquat lung
injury. Some patients have recovered despite major pulmonary
abnormalities (Table 15). Among 5 survivors, Schlatter (1976)
reported no signs of lung residual disorders. Fitzgerald et al.,
(1979a) followed, for at least a year, 13 survivors of paraquat
poisoning to determine the prevalence of residual pulmonary
disability. Of 11 adults, 5 (all non-smokers) did not have any
clinical, radiological, or functional evidence of pulmonary
dysfunction; 4 others (all smokers) were considered normal on
clinical and chest X-ray examination, but had a mild deficit in
pulmonary function, while the remaining 2 adults were known to have
suffered from respiratory disability before the paraquat poisoning.
Only 1 patient showed new and persistent lung infiltrates that
could be ascribed to permanent paraquat lung damage. No
abnormalities were discovered in the 2 children studied.
Table 16. Factors determining recovery from paraquat poisoning
-------------------------------------------------------------------------
No. Factor Notes
-------------------------------------------------------------------------
1. Route of entry Most paraquat poisonings have occurred following
ingestion; ingestion following a meal usually has
less serious consequences; skin contamination
with liquid concentrate formulations is dangerous;
poisoning through inhalation is usually benign
2. Dose Dose rarely known, but usually, for survivors,
less than 6 g paraquat, often, spat out or vomited
after ingestion
3. Intention High mortality rates established in suicidal or
homicidal poisoning; many more survivors reported
reported among cases of accidental poisoning
4. Formulation High mortality rate registered after ingestion of
ingested liquid concentrates; survivors have more often
than not ingested dilute or granular formulations
5. Time of starting Treatment should start as soon as possible; delay
treatment of more than 2 - 5 h reduces chances of survival;
patients hospitalized several days after paraquat
ingestion have minimal chance of recovery
6. Decreased Occurs when there is vomiting, use of emetics
gastrointestinal stomach washout, application of adsorbents (such
absorption as Fuller's Earth or bentonite), single or
repeated, and forced diarrhoea; such treatment
should be as prompt as possible; a delay of more
than 5 h adversely affects the safe and effective
elimination of paraquat; care should be taken to
avoid complications (aspiration of Fuller's Earth,
oesophagal perforation)
7. Blood paraquat Fig. 6 (section 6.2.3) demonstrates importance of
concentrations paraquat plasma concentrations for prognosis
8. Urine paraquat Patients excreting more than 1 mg paraquat/h, 8 h
concentrations or more after ingestion, unlikely to recover
9. Renal function Patients with severe renal damage or renal failure
usually die
-------------------------------------------------------------------------
Table 16. (contd.)
-------------------------------------------------------------------------
No. Factor Notes
-------------------------------------------------------------------------
10. Forced diuresis Should not be instituted when renal damage with
oliguria present; caution needed during the first
24 h
11. Haemodialysis Important if forced diuresis cannot be carried out
-------------------------------------------------------------------------
8.2. Occupational Exposure
8.2.1. Epidemiological studies and case reports
8.2.1.1. Spraying personnel
Paraquat has been in agricultural use since the early 1960s and
several surveys have been conducted on spray operators (Swan, 1969;
Hearn & Kier, 1971; Makovskii, 1972; Staiff et al., 1975; Seiber &
Woodrow, 1981; Howard, 1979b, 1980, 1982; Chester & Ward, 1981;
Howard et al., 1981; Chester & Woollen, 1982; Wojeck et al., 1983).
Some of these studies were aimed at clinically evaluating possible
adverse effects, others at estimating inhalatory and dermal
exposure. Some of the latter studies have been summarised in Table
17 from which it can be seen that:
(a) the main route of exposure of agricultural workers to
paraquat is via the skin; respiratory exposure is
negligible.
(b) The worst case of exposure (of those examined) was
via knapsack spraying.
Table 17. Comparison of dermal and inhalation exposure resulting
from various methods of application
--------------------------------------------------------------------
Method of application Dermal exposure Respiratory exposure
(mg/h) (mg/h)
--------------------------------------------------------------------
Hand-held knapsacka 66 (0.45 - 1.3) x 10-3
(12.1 - 169.8)
Vehicle mountedb 0.4 0 - 2 x 10-3
(0.1 - 3.4)
Aerialc - a) Flagman 0.1 - 2.4 0 - 47 x 10-3
b) Pilot 0.5 - 0.1 0 - 0.6 x 10-3
c) Mixer/loader 0.18 1.3 - 1.5 x 10-3
--------------------------------------------------------------------
a From: Chester & Woolen (1982).
b From: Staiff et al. (1975).
c From: Chester & Ward (1981).
In Malaysian rubber plantations, exposure is likely to be
greater than in most other situations (Swan, 1969). Weed control
is required continuously for 10 months of the year, and the
herbicide is applied by knapsack sprayers during the entire working
day, 6 days a week. The high temperature and humidity together
with the light clothing of the sprayers increase the potential risk
of dermal exposure. In 1965, a study was carried out on a team of
6 sprayers, and in 1967 on 4 teams, to estimate the efficacy of
protective measures. The operators used spray dilutions containing
paraquat at 0.5 g/litre, for 12 weeks. Attention was paid to
personal hygiene. Each man was given a thorough physical
examination, and urine samples were taken before spraying began and
at weekly intervals throughout the study. Paraquat analyses were
carried out using the method of Calderbank & Yuen (1965). Chest
X-rays were taken before the study started and at the end of the
6th and 12th weeks.
In the course of the 2 studies, a total of 528 urine samples
were examined. Paraquat was found on 131 occasions, the maximum
concentration detected being 0.32 mg/litre in the first study and
0.15 mg/litre in the second. Average urine levels of paraquat of
0.04 mg/litre were found in the 1965 study, and of 0.006 mg/litre
in the 1967 study. After spraying ceased, these levels declined
steadily to become undetectable within a week - with one exception.
It was concluded that the workers were not subjected to hazardous
levels of paraquat.
Both trials showed that about half of the men had suffered mild
irritation of the skin and eyes, but had recovered rapidly with
treatment. Two cases of scrotal dermatitis occurred in workers
wearing trousers that were continuously soaked by the spray
solution. There were also 2 cases of epistaxis. All chest
radiographs were normal.
Studies over a period of several years on 296 workers were
performed by Hearn & Keir (1971) on a Trinidad sugar estate. This
survey drew attention to nail damage following gross contamination
with paraquat at 1 - 2 g/litre that ranged in severity from
localized discoloration to nail loss. The typical distribution of
the lesions - affecting the index, middle, and ring fingers of the
working hand - suggested that they had occurred through leakage
from the knapsack sprayer, and inadequate personal hygiene. Apart
from 2 cases of contact dermatitis of the hands, no skin, eye, or
nose irritation was reported, nor were there any systemic effects.
Similar data were obtained by Makovskii (1972), who examined
several groups of workers spraying paraquat as a herbicide and
dessicant in cotton fields during the hot season. These workers
were exposed to paraquat aerosol concentrations of 0.13 - 0.55
mg/m3 air. Dermal exposure was low, not more than 0.05 - 0.08 mg
paraquat on the hands and face. There were no complaints, nor did
the clinical and laboratory examinations of the workers demonstrate
any significant deviations from the matched control groups.
In the USA (Staiff et al., 1975), the exposure of field workers
operating tractor-mounted spray equipment in orchards was
determined. About 4.6 litre paraquat liquid concentrate (291
g/litre) was used in 935 litre water per h. In addition, exposures
from yard and garden applications were studied in volunteers using
pressurized hand dispensers containing paraquat solution (4.4
g/litre). Dermal contamination was measured by adsorbent cellulose
pads attached to the worker's body or clothing, and by hand-rinsing
in water in a polyethylene bag. Special filter pads were used in
the filter cartridges of the respirators worn by the subjects under
study.
In all, 230 dermal and respiratory exposure pads, 95 samples of
hand-rinse water, and 130 urine samples, collected during and
following the spray, were analysed. This involved 35 different
paraquat application situations. The exposure of field workers was
found to range from about 0.40 mg/h (dermal) to less than 0.001
mg/h (inhalation). As for individuals spraying the yard or garden,
exposure ranged from 0.29 mg/h (dermal) to less than 0.001 mg/h
(inhalation).
In almost all cases, dermal exposure affected the hands. The
respiratory paraquat values were generally below the sensitivity
level of the analytical method. No detectable paraquat
concentrations were found in the urine samples (lower limit 0.02
mg/litre). This study confirmed the general safety of paraquat
under correct conditions of use.
The potential long-term hazard associated with the use of
paraquat has also been studied. Howard et al. (1981) studied the
health of 27 spraymen who had been exposed to paraquat for many
months per year for an average of 5.3 years, and compared them with
two unexposed control groups consisting of 24 general workers and
23 factory workers. There were a few skin lesions resulting from
poor spraying techniques and 1 case of eye injury. The workers
were given full clinical examinations and lung, liver, and kidney
function tests were carried out. There were no significant
differences in all health parameters measured between the groups,
which led the authors to suggest that the long-term use of paraquat
was not associated with harmful effects on health.
A paraquat formulation (240 g/litre) diluted 300 times by
volume with water was sprayed for 2 h on weedy ground (Kawai &
Yoshida, 1981). No irritation of the eyes and the skin was
reported. The urine of the workers who wore gauze masks contained
1.4 - 2.7 µg paraquat, 24 h after the spraying. The urine of
workers who had worn a high-performance mask did not contain
detectable levels of paraquat. During the spraying operations, the
concentration of paraquat aerosol was 11 - 33 µg/m3 air. The total
dermal exposure was about 0.22 mg. The authors discussed the need
for protective equipment to decrease skin contact with paraquat and
to avoid aerosol inhalation.
Quantitative estimates of dermal and respiratory exposure of 26
plantation workers in Malaysia (Chester & Woollen, 1982) have shown
a mean dermal dose of 1.1 mg/kg body weight per h. The highest
individual total exposure was equivalent to 2.8 mg/kg body weight
per h; the mean respiratory exposure was 0.24 - 0.97 µg paraquat/m3
air. Spray operators and carriers were exposed to an order of 1%
or less of a TLV of 0.1 mg/m3 for respirable paraquat. Urine
levels of paraquat were generally below 0.05 mg/litre.
A study was carried out on a group of 14 spray men in Thailand
using conventional high-volume knapsack sprayers and low-volume
spinning disc applicators with paraquat ion concentrations of 1.5
g/litre and 20 g/litre, respectively (Howard, 1982). Irritation of
unprotected skin was found, and this was severe in workers using
high spray concentrations (caustic burns on the feet after work
with spinning disc applicators and paraquat solution (20 g/litre)).
Urinary paraquat levels after 14 days spraying were significantly
higher (10.21 - 0.73 mg/litre) in unprotected men using both
concentrations, and there was evidence that urinary levels of
paraquat increased as the trial progressed. No evidence of
systemic toxicity was discovered among the spray men undergoing
clinical and radiographic examination 1 week after spraying ended.
The author concluded that spray concentrations in hand-held
equipment should not exceed 5 g paraquat ion/litre.
After tomato spraying in the USA, the total body exposure to
paraquat was determined to be 168.59 mg/h (Wojeck et al., 1983).
The use of enclosed tractor cabs or a high clearance tractor
reduced total body exposures to paraquat to 26.91 mg/h or 18.38
mg/h, respectively. The authors reported that the total body
exposure of tractor spray men working in two citrus locations was
proportional to the tank concentrations (paraquat dilutions of 1.1
g/litre and 0.7 g/litre were applied); exposure levels of 28.50
mg/h and 12.16 mg/h were found for workers using the higher and the
lower concentrations, respectively. In all situations studied, the
respiratory exposure was consistently a small fraction (<0.1%) of
the total body exposure. Exposure was mainly through the skin.
8.2.1.2. Formulation workers
Groups of workers exposed to formulations were examined by
Howard (l979b). The first group of 18 workers in England comprised
subjects exposed to dust and liquid paraquat formulations during a
37.5 h working week, the mean length of exposure being 5 years.
The second group also comprised 18 males, from Malaysia, exposed to
liquid concentrate formulations during a 42-h working week, the
mean length of exposure being 2.3 years. Partly protective
clothing was worn. However, in Malaysia, no gloves, rubber aprons,
or goggles were used. The medical records and the dermatological
examinations revealed acute skin rashes, nail damage, epistaxis,
blepharitis, and delayed wound healing in 12 - 66% of these
workers. Delayed caustic effects were often found among the
Malaysian formulation workers where a lower level of safety and
hygiene was apparent. Clinical examination did not reveal any
evidence of chronic contact dermatitis, hyperkeratosis, or
eczematous lesions.
8.2.2. Cases of occupational poisoning and local caustic effects
Hayes & Vaughan (1977) reviewed deaths from pesticides in the
USA. From 1956 - 1973, no deaths attributable to paraquat were
registered among agricultural workers, but in 1974, 4 fatal cases
were associated with this herbicide, although it was not clear
whether they were accidental, suicidal, or occupational. Conso
(1979) reported 17 cases of skin and eye irritation, not
accompanied by epistaxis or other signs of systemic effects, in
paraquat-exposed workers in France. Bismuth et al. (1983)
discussed a few cases of paraquat poisoning due to skin
contamination and eye irritation.
The available evidence indicates that, at the recommended
dilution rates and correctly used, systemic oral, inhalation, or
dermal effects should not be expected. Skin and eye irritation
have occurred only when protective measures were disregarded.
However, it should be emphasized that carelessness in handling
paraquat may have serious consequences. Fitzgerald et al. (1978a)
summarized the clinical findings and pathological details
concerning 13 accidents involving paraquat among agricultural
workers, 6 of which were fatal. In 5 of these cases, swallowing was
involved.
8.2.2.1. Oral ingestion
The ingestion of paraquat may occur accidentally, if liquid
concentrates are decanted into unlabelled containers near the
working areas (Kawatomi et al., 1979), and dangerous ingestion can
occur if operators suck or blow out the blocked pipes or nozzles of
spray apparatus. Of the 6 fatalities studied by Fitzgerald et al.
(1978a), 3 swallowed Gramoxone(R) after sucking the outlet of a
sprayer. In one non-fatal case, the man had sucked out a nozzle
containing diluted paraquat, while in another case, the man who had
blown into the jet, to clear it, escaped with only minor signs of
poisoning. Dilute solution blown into the face by the wind and
splashes of concentrate that get into the mouth probably explain
the resultant signs in the mouth, on the tongue, and in the throat.
Smoking with paraquat-contaminated hands has been reported to
result in a farmer's developing oropharyngeal irritation, nausea,
and muscular weakness (Mourin, 1967).
8.2.2.2. Dermal absorption
Acute dermal paraquat poisoning has been described by
Fitzgerald et al. (1978a). The use of a leaking sprayer by a
worker with severe extensive dermatitis probably resulted in fatal
absorption of paraquat through the damaged skin. Jaros (1978) has
described how the use of concentrated solutions of paraquat (50
g/litre instead of 5 g/litre), with an old leaking knapsack
sprayer, resulted in paraquat contamination of the neck, back, and
legs of a worker. After 4 h of work, he complained of a burning
sensation on the neck and scrotum. On admission to hospital 6 days
later, cough and respiratory difficulties were recorded. Three
days later the patient died of renal and respiratory failure. This
author has stressed the need for careful handling of paraquat.
Jaros et al. (1978) have discussed several other cases of paraquat
poisoning in the CSSR related to paraquat application.
Severe skin damage, followed by death due to respiratory
insufficiency, occurred in a woman (Newhouse et al., 1978), 8 weeks
after initial contact with paraquat. The toxic dermatitis started
with scratches on the arms and legs from the branches of fruit
trees. The patient had often failed to wear protective clothing or
to shower after spraying. During the 4 weeks preceding her first
admission to hospital, she developed ulcers and respiratory
complaints combined with anorexia. Damaged and broken skin was
thus exposed to paraquat. A chest X-ray and needle biopsy of the
lung revealed pulmonary lesions. Seventeen days after discharge
from hospital, without a specific diagnosis, she was re-admitted,
and died 2 weeks later with progressive lung, hepatic, and renal
dysfunction. More recently, Levin et al. (1979) described the
clinical and pathomorphological investigation of a patient who died
of hypoxia after repeated dermal exposure to paraquat (28 g/litre)
and diquat (29 g/litre) in a water-oil dilution - contrary to
accepted practice. The worker had used a leaking sprayer. A
characteristic ulcer developed at the site of paraquat contact.
There was also lung damage. Waight & Weather (1979) reported a
fatal case of dermal poisoning with paraquat after prolonged
contact with a concentrated formulation following spillage from a
bottle in the back trouser pocket. Wohlfahrt (1982) discussed the
factors related to severe paraquat poisoning due to dermal
absorption in tropical agriculture. Three fatal incidents followed
skin contamination; one victim used paraquat to treat scabies
infestation, and one to treat lice. In all cases, the skin was
blistered and ulcerated. The patients died of progressive
respiratory failure, 4 - 7 days after the accidents. However it
has been pointed out that each of these three spraymen showed skin
lesions much more severe than would be expected had recommended and
customary dilutions been used and that, in one of these cases, the
presence of mouth and throat ulceration strongly suggested that
ingestion might also have occurred (Davies, 1982).
8.2.2.3. Local skin and nail effects
Paraquat has a delayed effect on the skin. Brief contact with
liquid formulations, as well as repeated exposure to dilute
solutions, produced skin irritation, desquamation, and, finally,
necrosis at the site of contact (Ongom et al., 1974; Binns, 1976;
Newhouse et al., 1978; Waight & Wheather, 1979; Levin et al., 1979;
Horiuchi et al., 1980). Harmful dermal effects have been reported
(Howard, 1982) among spray men who worked without protective
clothes and with naked feet. The blistering and ulceration of the
skin were due to excessive contact and inadequate personal hygiene.
Horiuchi & Ando (1980) carried out patch testing on 60 patients
with contact dermatitis due to Gramoxone(R). In 8 patients
(13.3%) positive allergic reactions were established. In another
survey with 52 persons, a positive photo-patch response was
reported in 11 patients.
Nail damage has also been reported after frequent exposure to
paraquat concentrates during the formulation of the herbicide or
the preparation of working dilutions (Samman & Johnston, 1969;
Howard, l979b). Leakage from sprayers may cause nail damage only
if there is gross contamination (Hearn & Keir, 1971). Asymmetric
discoloration and softening of the nail base appears together with
an infection, that usually persists after the loss of the nail, but
a few months after cessation of paraquat exposure, the nails
re-grow satisfactorily.
8.2.2.4. Ocular damage
A number of studies have demonstrated the hazard from splashes
of concentrated paraquat that come into contact with the eye (Swan,
1969; Schlatter, 1976; Howard, l979b, 1980; Deveckova & Myalik,
1980). Apart from irritation of the eye and blepharitis, a week
later more serious ocular damage may occur such as destruction of
the bulbar and tarsal conjunctiva and of the corneal epithelium
(Cant & Lewis, 1968). Anterior uveitis was also noted. Joyce
(1969) reported a case of conjunctival necrosis after paraquat had
been splashed into the eyes during spraying in windy weather. In a
second case, there was progressive keratitis with gross corneal
opacity. Severe conjunctival injuries with keratitis and decreased
visual acuity were reported in 3 workers by Watanabe et al. (1979)
and in another by Okawada et al. (1980). The eyes were washed with
water immediately, but the damage progressed and required treatment
for more than 3 weeks.
8.2.2.5. Inhalation
The inhalation of droplets in normal paraquat spraying does not
appear to represent a significant health hazard (Howard, 1980), and
the effects of occupational inhalation have been limited to nose
bleeds, and nasal and throat irritation (Swan, 1969; Howard,
1979b). Standard spraying equipment failed to produce significant
levels of droplets in the respirable range of < 5-7 µm diameter,
and chemical analyses of paraquat aerosols or particulate matter,
sampled from working areas, have usually shown them to be well
below the TLV. However, there have been some reports (Malone et
al., 1971; Mircev, 1976; Bismuth et al., 1982) of adverse effects
as a result of inhalation exposure.
8.3. Use of Marijuana Contaminated by Paraquat
In the USA, it has been found that marijuana sprayed with
paraquat (in an attempt to destroy the plant) may become available
for smoking by drug users. Concentrations of paraquat in marijuana
of up to 461 mg/kg have been reported (Liddle et al., 1980).
Understandably, concern has been expressed that smoking this
contaminated marijuana may be more harmful than smoking marijuana
itself. The available data do not justify an absolute conclusion.
However, paraquat is known to pyrolyse at 300 °C and it has been
established (Smith 1978) that in marijuana cigarettes contaminated
with 1000 mg paraquat/kg (1 mg, assuming a 1 g cigarette), only
0.26 µg of paraquat escaped pyrolysis and was available to be
inhaled. On this basis, the amount of paraquat inhaled by a heavy
user of contaminated marijuana will be insufficient to cause
injury. In the absence of exhaustive toxicological studies, it
cannot be stated categorically that all the pyrolysis products of
paraquat do not damage the lung. However, there has been no
confirmed injury attributable to the smoking of contaminated
marijuana.
8.4. Guidelines for the treatment of paraquat poisoning
The most important measures are the immediate neutralisation of
ingested paraquat by 15% Fuller's earth, bentonite, or activated
charcoal and urgent removal of the poison by vomiting or, when
possible, gastric washout. The urgency of these measures is such
that where transfer to hospital may involve delay of an hour or
more, this emergency treatment may need to be given by a
paramedical person, e.g., a nurse or a medical assistant. The
delay should not be more than 4 - 5 h. Furthermore, Fuller's earth
should be given together with a strong purgative such as magnesium
sulfate or mannitol.
Admission to a hospital either directly or after emergency
treatment elsewhere is essential.
Where a person has swallowed a lethal dose, the most important
single determinant of survival is the early commencement of
treatment.
Depending on local facilities, patients who reach hospital
after the initial treatment will have further treatment aimed at
neutralizing paraquat in the gastrointestinal tract (Fuller's
earth, bentonite, activated charcoal) or its excretion in the
faeces (purgatives, 10% mannitol, gut lavage). In addition,
attempts to remove absorbed paraquat from the circulation
(haemoperfusion, haemodialysis) or aid its excretion by the kidney
(forced diuresis) can be instituted.
In centres where facilities for analytical procedures are
available, measurement of urinary, or ideally plasma levels of
paraquat may give guidelines for the required intensity of
treatment or likely prognosis.
Many other therapies including corticosteroids,
immunosuppressive treatment, vitamins, beta-blocking and alkylating
agents, alpha-tocopherol, superoxide dismutase and/or glutathione
peroxidase (Autor, 1974, 1977) proved to be of no significant
importance in human paraquat poisoning (Fletcher, 1975; Fairshter
et al., 1976; Schlatter, 1976; Brown et al., 1981; Bismuth et al.,
1982). The administration of oxygen should be avoided except where
vital for the patient's comfort.
It should be noted that, as with the great majority of
chemicals, there is no specific antidote.
Care must be exercised in the administration of most of these
treatments, as the following serious complications may occur:
perforation of the oesophagus during gastric intubation; serious
blood chemistry disturbance when severe diarrhoea is induced; fluid
overload during forced diuresis (McGeown, 1975).
Despite such an array of both simple and sophisticated
measures, the response to therapy in paraquat poisoning is
disappointing and the mortality rate remains high.
In cases of skin and eye contamination, irrigation with water
(preferably running water) should be commenced urgently and must be
continued uninterrupted for at least 10 min (timed by the clock).
Eye cases should always be taken for medical treatment. In cases
of skin contamination by the concentrate or extensive and/or
prolonged contamination by the diluted material (particularly where
signs of skin irritation are present) the patient must be assessed
at hospital for systemic poisoning.
9. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
9.1. Exposure
Introduction
Paraquat is a contact herbicide or dessicant that is used to
destroy weeds in various agricultural situations. It is used in
the form of an aqueous spray, which means that potential human
exposure may occur as a result of its presence in air, on plants,
in soil, or in water.
Degradation of paraquat
Photochemical degradation takes place when paraquat-treated
plants are exposed to normal daylight and continues after the
plants are dead (section 4.1.1). The products formed have been
identified and found to be of a lower order of toxicity.
Ultraviolet degradation on soil surfaces also occurs, but
photodecomposition of paraquat in the soil is insignificant in
comparison with adsorption on clay particles. Microorganisms can
degrade free paraquat rapidly, but chemical degradation of adsorbed
paraquat is relatively slow.
Soil
Paraquat is rapidly and tightly bound to clay materials in
soils. The adsorbed paraquat is biologically inactive and in
normal agricultural use no harmful metabolic or breakdown products
are to be expected (section 4.3 and 5.1). In multiple spray
trials, paraquat residues in soil varied from 22 to 58 mg/kg.
Under field conditions, the residual paraquat is slowly
re-distributed. Long-term field studies have shown degradation
rates of 5 - 10% per annum, which is sufficient to prevent
saturation of soil deactivation capacities. At normal and high
rates of application, no adverse effects are expected in the soil
microflora and other soil organisms, or on crop growth (section
4.3.1).
Water
Following the use of paraquat as an aquatic herbicide at a
normal application rate of 1 mg/litre, the concentration was found
to decrease to about one half of the initial level within 36 h and
to below 0.0l mg/litre in less than 2 weeks (section 4.3.2).
Phytotoxic damage to crops irrigated with treated water is unlikely
to occur, if an interval of 10 days is observed between treatment
of the water and its use, because of the rapid decrease of paraquat
residues in the water.
Normal application of paraquat for aquatic weed control is not
harmful for aquatic organisms. However, care should be taken in
the application of paraquat to water containing heavy weed growth,
since oxygen consumed by subsequent weed decay may decrease oxygen
levels in the water to an extent that is dangerous for fish or
other aquatic organisms.
Air
Paraquat is not volatile so inhalation of paraquat vapour is
not a problem, in practice. However, droplets of paraquat solution
can be present in the air as a consequence of aerial, knapsack, or
tractor-mounted spraying. Paraquat aerosol concentrations (total
airborne) ranged up to 0.55 mg/m3 in the work situation, depending
on the method of spraying. The amount of respirable airborne
paraquat was found to be insignificant under normal conditions of
use (section 8.2.1).
The amount of paraquat present in airborne dust was found to
range from 0.0004 to 0.001 mg/m3. The binding of paraquat to the
dust was so tight that it did not exert any toxicological effect on
rats, when given by inhalation.
Food
Examination of paraquat-treated p1ants (section 4.3.4), or of
materials from animals fed paraquat-treated crops (section 4.3.5),
revealed low residues, so that no hazard should be expected from
paraquat residues in food when used as a herbicide or as a
desiccant. Paraquat is not subject to bioconcentration (section 5)
and has not been found to accumulate in food chains.
Environmental contamination
Exposure to paraquat from spray drift may occur in windy
weather, though field studies suggest that the airborne paraquat
concentration declines markedly within a few metres of the sprayed
area (section 4.3.3). Because of the rapid and complete binding of
paraquat to clay particles in the soil, contamination of water
supplies either from field runoff or percolation through soil to
the water table is not an environmental problem (sections 4.3.1 and
4.3.2). Paraquat has also been shown not to have any harmful
effects on birds (sections 5.3 and 5.4).
9.2. Poisoning by Paraquat
Misuse of paraquat has led to many deaths throughout the world,
mainly due to the swallowing of undiluted preparations.
9.2.1. Suicidal ingestion
The majority of paraquat poisonings are due to swallowing
liquid concentrates with suicidal intent and the mortality rate is
high. Ingestion of granular paraquat is less common and usually
causes milder poisoning, though fatalities have occurred. Paraquat
has been used to commit homicide (section 8.1).
9.2.2. Accidental poisoning
Poisoning by accidental swallowing is less common than
intentional swallowing and is usually the result of storing liquid
concentrates in inappropriate containers, particularly beer or soft
drink bottles. The mortality rate is lower than in suicidal cases.
Childhood poisoning is usually accidental. Legislation on the
control of the sale of liquid concentrates has reduced accidental
ingestion in some countries (section 8.1).
A small number of fatal cases of accidental paraquat poisoning
via the skin have been reported following the application of liquid
concentrates (200 g/litre) to kill body lice.
9.2.3. Occupational Poisoning
Cases of severe poisoning following inappropriate behaviour or
accidents while handling paraquat occur. Fatal and non-fatal
ingestion of paraquat has occurred when hand-spray operators have
attempted to clear the spray outlet by sucking on the spraying
nozzle or outlet pipes. In some of the severe cases, the authors
noted their suspicion of concealed suicidal intent. Fatal
poisoning by dermal soaking with dilute paraquat has been reported
in one operator who had severe dermatitis and had been using a
leaky sprayer (section 8.2.2).
Fatal systemic poisoning may result from continuous contact
with paraquat-soaked clothing or splashes of liquid concentrate on
the skin. Splashes of liquid concentrate may lead to severe ocular
and skin damage (sections 8.2.1, 8.2.2). Spraying with
inadequately diluted paraquat (e.g., with ultra low volume
application) may result in similar problems.
9.3. Occupational Exposure
There are several studies on paraquat exposure in normal
agricultural use. Occupational exposure may be oral, dermal, or by
inhalation. The spray aerosol and dust particles are relatively
large and are mostly deposited in the upper respiratory tract
(section 8.2.1).
The potential dermal exposure of field workers (section 8.2.1)
is closely related to working conditions. Workers on tractors were
found to have a paraquat exposure of 12 - 168 mg/h while spraying
tomatoes and citrus. In other studies, field workers were dermally
exposed to paraquat at approximately 0.40 mg/h, and individuals
spraying the garden to 0.29 mg/h. In all trials, respiratory
exposure was not higher than 0.01 mg/h. Urine concentrations in
occupationally-exposed workers were often lower than 0.01 mg/litre,
but concentrations up to 0.73 mg/litre were determined after
improper paraquat application in tropical agriculture use.
Local skin effects (contact, irritative, or photoallergic
dermatitis) delayed wound healing, and nail damage has been
observed among formulation workers or among individuals handling
the herbicide improperly. Blepharitis and epistaxis may result due
to delayed irritative action of paraquat. Such incidents
illustrate the need for strict personal hygiene and rigorous
adherence to safe handling procedures.
9.4. Effects
9.4.1. Paraquat toxicity in animals
The acute lung-directed toxicity of paraquat in man has been
confirmed in numerous studies in animals. At high doses of
paraquat, minor toxic effects have been noted primarily in liver
and kidney, and in other organ systems, including nervous,
cardiovascular, blood, adrenals and male reproductive systems.
However, toxic effects have not been reported at low doses of
paraquat. Concentrated solutions of paraquat have been found to be
irritating to both skin and eyes. The FAO/WHO (1976) has
determined no-observed-adverse-effect levels of 30 mg/kg diet,
equivalent to 1.5 mg/kg body weight per day for rats and 50 mg/kg
diet, equivalent to 1.25 mg/kg body weight per day, for dogs
exposed to paraquat dichloride. Additional animal studies have
indicated that paraquat is neither teratogenic nor carcinogenic
(sections 7.1.6 and 7.1.8). In vitro mutagenicity studies have
been inconclusive, though generally suggesting weak potential
activity, while in vivo studies have given negative results
(section 7.1.7). Thus, the results of animal studies suggest that
low-level exposure to paraquat is unlikely to induce toxic effects
in man.
9.4.2. Paraquat determinations in biological fluids and tissues
Determination of paraquat levels in stomach washings, serum,
and urine is useful for the management of poisoning (section 6.2).
The urinary levels decline rapidly during the 24 h following
exposure and may remain low for some weeks. Determination of
urinary levels of paraquat may be useful in the conduct of
epidemiological studies.
9.5. Earlier Evaluations by International Bodies
The Joint Meeting on Pesticide Residues (JMPR) has reviewed
residues and toxicity data on paraquat on several occasions
(FAO/WHO 1971, 1973, 1977, 1979, 1982, 1983). In 1972, it
estimated the acceptable daily intake (ADI) for man 0 - 0.002 mg/kg
body weight, on the basis of no-observed-adverse-effect levels of
1.50 mg/kg body weight per day in the rat and 1.25 mg/kg body
weight in the dog. Because of concern relating to lung and kidney
toxicity, this ADI was changed in the 1982 meeting to a temporary
ADI of 0 - 0.001 mg paraquat dichloride/kg body weight (or 0.0007
mg paraquat ion/kg body weight). The no-observed-adverse-effect
level for the rat remained, however, at 1.5 mg/kg body weight/day
(FAO/WHO 1983).
The same JMPRs have recommended maximum residue levels
(tolerances) for paraquat in food commodities of plant and animal
origin.
The WHO/FAO (1978) in its series of "Data sheets on chemical
pesticides" issued one on paraquat. Based on a brief review of
use, exposure, and toxicity, practical advice is given on
labelling, safe-handling, transport, storage, disposal,
decontamination, selection, training and medical supervision of
workers, first aid, and medical treatment.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechcoslovakia, the
Federal Republic of Germany, India, Japan, Kenya, Mexico, Sweden,
the United Kingdom, the USA, and the USSR) and the EEC can be found
in the IRPTC (International Register of Potentially Toxic
Chemicals) Legal file (IRPTC 1983).
9.6. Conclusions
On the basis of the above findings, it can be concluded that:
General population
Residue levels of paraquat in food and drinking-water,
resulting from its normal use, are unlikely to result in a health
hazard for the general population.
This likely lack of hazard in normal usage of dilute paraquat
is in strong contrast with the potential serious hazard that may
result from handling concentrated paraquat.
Accidental paraquat poisoning results mainly from swallowing
liquid concentrate that has been decanted into unlabelled bottles
or other containers and stored inappropriately.
The number of suicides by means of paraquat is of great
concern. The total number of such suicides is unknown.
Notwithstanding the facts that the reasons for suicide may be
manifold and complex, and that paraquat is one among many means
towards that goal, the prolonged and painful way of dying from
paraquat suggests that every effort within reason should be made to
diminish the attractiveness and availability of paraquat for this
purpose.
Occupational exposure
With reasonable work practices, including safety precautions,
hygiene measures, and proper supervision, occupational exposure
during manufacture, formulation, and application will not cause
hazard. However the undiluted concentrate must be handled with
great care because improper work practices may result in
contamination of eyes and skin (with possible consequent dermal
absorption).
Spray concentrations should not exceed 5 g paraquat ion/litre
in order to avoid skin damage and absorption of the herbicide
through the skin. Its use in hand-held ultra-low volume
application should be discouraged.
Environment
Paraquat in soil binds rapidly and tightly to clay particles
and residual phytotoxicity from freely-available paraquat is
unlikely. The toxicity of the compound for birds has been shown to
be of low significance. Under normal conditions of use, paraquat
shows low toxicity to aquatic organisms although resulting
depletion of water-oxygen because of weed decay may pose a problem.
Paraquat does not seem to represent an environmental hazard.
REFERENCES
ACKRILL, P., HASLETON, P.S., & RALSTON, A.J. (1978)
Oesophageal perforation due to paraquat. Br. med. J., 1:
1252-1253.
ALMOG, C. & TAL, E. (1967) Death from paraquat after
subcutaneous injection. Br. med. J., 3: 721.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDERSON, D., McGREGOR, D.B., & PURCHASE, I.F.H. (1976)
Dominant lethal studies with diquat and paraquat in male CD-I
mice. Mutat. Res., 40: 349-358.
ANDERSON, C.C., GUNDERSON, E.C., & COULSON, D.M. (1981)
Sampling and analytical methodology for workplace chemicals
hazards. ACS Symp. Ser., 149: 3-19.
ANONYMOUS (1979) Paraquat. FAO Plant Prod. Prot. Pap.,
15(Suppl.): 181-183.
AUTOR, A.P. (1974) Reduction of paraquat toxicity by
superoxide dismutase. Life Sci., 14: 1309-1319.
AUTOR, A.P., ed. (1977) Biochemical mechanisms of paraquat
toxicity, New York, Academic Press, pp. 39-55.
BAINOVA, A. (1969a) [Chronic oral toxicity of dipyridylium
herbicides.] Hig. Zdrav., 12: 325-332 (in Bulgarian).
BAINOVA, A. (l969b) [Experimental assessment of the effect
of dipyridylium herbicides on the skin.] Letopisi HEI, 9:
25-30 (in Bulgarian).
BAINOVA, A. (1971) [Determination of the zones of acute and
chronic inhalatory toxicity after exposure to dipyridylium
herbicides Gramoxone and Reglone.] Letopisi HEI, 28: 59-62 (in
Bulgarian).
BAINOVA, A. (1975) [Cumulative action of Gramoxone and
Reglone.] In: Problemi na Higienata, Sofia, Medizinia i
Fizkultuna, Vol. 1, pp. 31-38 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1972) Experimental
substantiation of Gramoxone MAC in working areas. In: Works of
Research Institute of Hygiene & Labour Protection, Sofia,
Medizinia i Fizkultuna, Vol. 23, pp. 71-77.
BAINOVA, A. & VULCHEVA, V. (1974) [Experimental assessment
of the effect of dipyridyliums on sex glands.] In: Works of
Research Institute of Hygiene & Labour Protection, Sofia,
Medizinia i Fizkultuna, Vol. 22, pp. 111-122 (In Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1977) Lung changes after chronic
paraquat intoxication. C. R. Acad. Bulgar. Sci., 30: 1788-1790.
BAINOVA, A., ZLATEVA, M., & VULCHEVA, V. (1972) [Chronic
inhalation toxicity of dypyridylium herbicides.] Hig. Zdrav.,
15: 25-31 (in Bulgarian).
BALDWIN, B.C., BRAY, M.F., & GEOGHEGAN, M.J. (1966) The
microbial decomposition of paraquat. Biochem. J., 101: 15.
BEEBEEJAUN, A.R., BEEVERS, G., & ROGERS, W.N. (1971)
Paraquat poisoning. Prolonged excretion. Clin. Toxicol., 4:
397-407.
BENIGNI, R., BIGNAMI, A., CARERA, A., CONTI, G., CONTI, R.,
GREBELLI, E., DOGLIOTTI, E., GUALANDI, G., NOVELLETO, A., &
ORTALI, V.A. (1979) Mutational studies with diquat and
paraquat in vitro. Mutat. Res., 68: 183-193.
BENNETT, P.N., DAVIES, D.S., & HAWKSWORTH, G.M. (1976) In
vivo adsorption studies with paraquat and diquat in the dog.
Proceedings of the British Pharmaceutical Society, 15-16 July,
England, 284 pp.
BERRY, D.J. & GROVE, J. (1971) The determination of paraquat
(1,1-dimethyl-4,4-dipyridylium cation) in urine. Clin. Chim.
Acta, 34: 5-11.
BEYER, K.H. (1970) [The analytical determination and
toxicology of paraquat.] Dtsch Apoth.-Ztg, 110: 633-635 (in
German).
BIGNAMI, M. & GREBELLI, R.A. (1979) A simplified method for
the induction of 8-azaguanine resistance in S. typhimurium.
Toxicol. Lett., 3: 169-175.
BINNS, C.W. (1976) A deadly cure for lice. Papua New Guinea
med. J., 19: 105-107.
BISMUTH, C., GARNIER, R., DALLY, S., FOURNIER, P.E., &
SCHERRMANN, J.M. (1982) Prognosis and treatment of paraquat
poisoning. A review of 28 cases. J. Toxicol. clin. Toxicol.,
19: 461-474.
BISMUTH, C., DALLY, S., & PONTAL, P.-G. (1983) Prognostic
factors and therapeutic results of paraquat poisoning.
Concerning 28 cases. Arch. Mal. prof., 44(1): 38-41.
BOJAN, F., NAGY, A., & HERMAN, K. (1978) Effect of butylated
hydroxytoluene and paraquat on urethane tumorigenesis in mouse
lung. Bull. environ. Contam. Toxicol., 20: 573-576.
BRAMLEY, A. & HART, T.B. (1983) Paraquat poisoning in the
United Kingdom. Hum. Toxicol., 2(2): 417.
BRIGELIUS, R. & ANWER, M.S. (1981) Increased biliary
GSSG-secretion and loss of hepatic glutathione in isolated rat
liver after paraquat treatment. Res. Commun. chem. Pathol.
Pharmacol., 31: 493-502.
BRIGELIUS, R., HASHEM, A., & LENGFELDER, E. (1981)
Paraquat-induced alterations of phospholipids and GSSG release
in the isolated perfused rat liver and the effect of
SOD-active copper complexes. Biochem. Pharmacol., 30: 349-354.
BROWN, E., BRANDENBURGER, A., & MALING, H.M. (1980) Effects
of paraquat and related herbicides on the acetylcholinesterase
of rat lung. Biochem. Pharmacol., 29: 456-466.
BROWN, 0., HEITKAMP, U., & SONG, C. (1981) Niacin reduces
paraquat toxicity in rats. Science, 212: 1510-1512.
BULLIVANT, C.M. (1966) Accidental poisoning by paraquat.
Report of 2 cases in man. Br. med. J., 1: 1272-1273.
BURNS, R.G. & AUDUS, L.J. (1970) Distribution and breakdown
of paraquat in soil. Weed Res., 10: 49-58.
BUS, J.S. & GIBSON, J.E. (1975) Postnatal toxicity of
chronically administered paraquat in mice and interactions
with oxygen and bromobenzene. Toxicol. appl. Pharmacol., 33:
461-470.
BUS, J.S. & GIBSON, J.E. (1979) Lipid peroxidation and its
role in toxicity. Rev. Biochem. Toxicol., 1: 125-149.
BUS, J.S. & GIBSON, J.E. (in press) Paraquat: a model for
oxidant initiated injury. In: Hook, G.E.R., ed. Pulmonary
toxicology, New York, Raven Press.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1974) Superoxide and
singlet oxygen catalysed lipid peroxidation as a possible
mechanism for paraquat toxicity. Biochem. biophys. Res.
Commun., 58: 749-755.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1975) Lipid
peroxidation as a possible mechanism for paraquat toxicity.
Res. Commun. chem. Pathol. Pharmacol., 11: 31-38.
BUS, J.S., CAGEN, S.Z., OLGAARD, M., & GIBSON, J.E. (1976)
A mechanism of paraquat toxicity in mice and rats. Toxicol.
appl. Pharmacol., 35: 501-513.
BUTLER, C. (1975) Pulmonary interstitial fibrosis from
paraquat in the hamster. Arch. Pathol., 99: 503-507.
BUTLER, C. & KLEINERMAN, J. (1971) Paraquat in the rabbit.
Br. J. ind. Med., 28: 67-71.
CAGEN, S.Z. & GIBSON, J.E. (1977) Liver damage following
paraquat in selenium-deficient and diethyl maleate pretreated
mice. Toxicol. appl. Pharmacol., 40: 193-200.
CAGEN, S.Z., JANOFF, A.S., BUS, J.S., & GIBSON, J.E. (1976)
Effect of paraquat on liver function in mice. J. Pharmacol.
exp. Ther., 198: 222-228.
CALDERBANK, A. (1966) Paraquat residues in fruit. In:
Frayer, J.D., ed. Herbicides in British fruit growing,
Blackwell, Blackwell Scientific Publications, pp. 135-136.
CALDERBANK, A. (1968) The bipyridylium herbicides. Effects
on man. In: Metcalf, R.L., ed. Advances in pest control
research, New York, Interscience Publishers, Vol. 8, 224 pp.
CALDERBANK, A. (1972) Environmental considerations in the
development of diquat and paraquat as aquatic herbicides.
Outlook Agric., 7: 51-54.
CALDERBANK, A. & SLADE, P. (1976) Diquat and paraquat. In:
Kearne, P.C. & Kaufman, D.D., ed. Herbicide chemistry,
degradation and mode of action, 2nd ed., New York, Dekker,
pp. 501-540.
CALDERBANK, A. & YUEN, S.H. (1965) An ion-exchange method
for determining paraquat residues in food crops. Analyst, 90:
99-106.
CALDERBANK, A., MCKENNA, R.H., STEVENS, M.A., & WALLEY, J.K.
(1968) Grazing trials on paraquat-treated pasture. J. Sci.
Food Agric., 19: 246-250.
CANT, J.S. & LEWIS, R.H. (1968) Ocular damage due to
paraquat and diquat. Br. med. J., 2: 224.
CARLSTROM, A.A. (1971) Collaborative check for paraquat in
formulations. J. Assoc. Off. Anal. Chem., 54: 718-719.
CARMINES, E.L., CARCHMAN, R.A., & BORZELLECA, J.F. (1981)
Investigations into the mechanism of paraquat toxicity
utilizing a cell culture system. Toxicol. appl. Pharmacol.,
58: 353-362.
CARSON, D.G.L. & CARSON, E.D. (1976) The increasing use of
paraquat as a suicide agent. Forensic Sci., 7: 151-160.
CHARLES, J.M., ABOU-DONIA, M.B., & MENZEL, D.B. (1978)
Absorption of paraquat from the airways of the perfused rat
lung. Toxicology, 8: 59-67.
CHESTER, G. & WARD, R.J. (1981) Paraquat - Occupational
exposure and drift hazard evaluation during aerial application
to cotton in California, USA, London, ICI Ltd (Central
Toxicology Laboratory Report No. CTL/P.581).
CHESTER, G. & WOOLLEN, B.H. (1982) Studies of the
occupational exposure of Malaysian plantation workers to
paraquat. Br. J. ind. Med., 38: 23-33.
CLARK, D.G. (1971) Inhibition of the absorption of paraquat
from the gastrointestinal tract by adsorbents. Br. J. ind.
Med., 28: 186-188.
CLARK, D.G., McELLIGOTT, T.F., & HURST, E.W. (1966) The
toxicity of paraquat. Br. J. ind. Med., 23: 126-133.
CLEGG, D.J. (1979) Animal reproduction and carcinogenicity
studies in relation to human safety evaluation. Dev. Toxicol.
environ. Sci., 4: 45-59.
COATS, G.E., FUNDERBURK, H.H., Jr, LAWRENCE, J.M., & DAVIS,
D.E. (1966) Factors affecting persistance and inactivation
of diquat and paraquat. Weed Res., 6: 58-66.
CONNING, D.M., FLETCHER, K., & SWAN, A.A.B. (1969) Paraquat
and related bipyridyliums. Br. med. Bull., 25: 245-249.
CONNOLLY, M.E., DAVIES, D.S., DRAFFAN, G.H., BENNETT, P.N., &
DOLLERY, C.T. (1975) Clinical experience with paraquat
poisoning. In: Fletcher, K., ed. Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-11.
CONSO, F. (1979) Paraquat poisoning: experience of poison
control centers in France. Vet. hum. Toxicol., 21(Suppl.):
112-113.
COOKE, N.J., FLENLEY, D.C., & MATTHEW, H. (1973) Paraquat
poisoning. Serial studies of lung function. Q. J. Med.,
XLII(168): 683-692.
COPLAND, G.M., KOLIN, A., & SHULMAN, H.S. (1974) Fatal
pulmonary intra-alveolar fibrosis after paraquat ingestion.
New Engl. J. Med., 291: 290-292.
CURRY, J.F. (1970) The effects of different methods of new
sward establishment and the effects of the herbicides paraquat
and dalapon on the soil fauna. Pedobiologia, 10: 329-361.
DAMANAKIS, M., BRENNAN, D.S.H., FRYER, J.D., & HOLLY, K.
(1970) Absorption and mobility of paraquat on different soil
constituents. Weed Res., 10: 264-277.
DANIEL, J.M. & GAGE, J.C. (1966) Absorption and excretion of
diquat and paraquat in rats. Br. J. ind. Med., 23: 133-136.
DASTA, J.F. (1978) Paraquat poisoning: a review. Am. J.
Hosp. Pharm., 35: 1368-1372.
DASTA, J.F. (1980) Management of paraquat poisonings. Clin.
Toxicol. Consultant, 2(1): 11-20.
DAVIDSON, J.K. & MACPHERSON, P. (1972) Pulmonary changes in
paraquat poisoning. Clin. Radiol., 23: 18-25.
DAVIES, D.S., HAWKSWORTH, G.M., & BENNETT, P.N. (1977)
Paraquat poisoning. Proc. Eur. Soc. Toxicol., 18: 21-26.
DAVIES, R.E. (1982) Skin absorption of paraquat. Med. J.
Aust., 7: 222.
DEARDEN, L.C., FAIRSHTER, R.D., MCRAE, D.M., SMITH, W.R.,
GLASER, F.L., & WILSON, A.F. (1978) Pulmonary ultrastructure
of the late aspects of human paraquat poisoning. Am. J.
Pathol., 93: 667-676.
DEVECKOVA, D. & MYALIK, M. (1980) [Gramoxone ocular burns.]
Cesk. Ophtalmol., 36: 7-10 (in Czech).
DICKES, G.J. (1979) The application of gas chromatography to
food analysis. Talanta, 26: 1065-1099.
DIJK, A. VAN, MAES, R.A.A., DROST, R.H., DOUSE, J.M.C., &
HEIJST, A.N.P. VAN (1975) Paraquat poisoning in man. Arch.
Toxicol., 34: 129-136.
DIJK, A. VAN, EBBERINK, R., GROOT, G. DE, & MAES, R.A.A.
(1977) A rapid and sensitive assay for the determination of
paraquat in plasma by gas-liquid chromatography. J. anal.
Toxicol., 1: 151-154.
DIKSHITH, T.S.S., DATTA, K.K., RAIZADA, R.B., & KUSHWAH, H.S.
(1979) Effect of paraquat dichloride in male rabbits. Indian
J. exp. Biol., 17: 926-928.
D0DGE, A.D. (1971) The mode of action of the bipyridylium
herbicides, paraquat and diquat. Endeavour, 30: 130-135.
DOUZE, J.M.C., DIJK, A. VAN, GIMBERE, J.S.F., HEIJST, A.N.P.
VAN, & MAES, R.A.A. (1975) Intensive therapy after paraquat
intoxication. In: Fletcher, K., ed. Clinical aspects of
paraquat poisoning, London, ICI Ltd, pp. 34-45.
DRAFFON, G.H., CLARE, R.A., DAVIES, D.L., HAWKSWORTH, G.M.,
MURRAY, S., & DAVIES, D.S. (1977) Qualitative determination
of the herbicide paraquat in human plasma by gas
chromatographic and mass spectrometric methods.
J. Chromatogr., 139: 311-320.
EARNEST, R.D. (1971) Effect of paraquat on fish in a
Colorado farm pond. Prog. Fish Cult., 33: 27-31.
ECKER, J.L., HOOK, J.B., & GIBSON, J.E. (1975)
Nephrotoxicity of paraquat in mice. Toxicol. appl.
Pharmacol., 34: 178-186.
EDWARDS, P.J. (1979) Status of common bird population on an
intensively managed farm where paraquat has been used
extensively, London, ICI Plant Protection Division (Report No.
RJ 0037/B).
EDWARDS, P.J., NEWMAN, J.F., & WARD, R.J. (1979) Paraquat:
effect of spraying eggs on hatchability and reproductive
organs of Japanese quail, Coturnix coturnix japonica, London,
ICI Plant Protection Division (Report No. RJ 0044B).
EFTHYMIOU, M.L. (1983) L'actualité toxicologique en matičre
de lutte chimique antiparasitaire agricole, Tours, France,
Faculté de Médecine de Tours.
FAIRSHTER, R.D. (1981) Paraquat toxicity and lipid
peroxidation. Arch. intern. Med., 141: 1121-1123.
FAIRSHTER, R.D., ROSEN, S.M., SMITH, W.R., FLAUSER, F.L.,
MCRAE, D.M., & WILSON, A.F. (1976) Paraquat poisoning. New
aspects of therapy. Q.J. Med., 45: 551-565.
FAIRSHTER, R.D., DABIR-VAZIRI, N., SMITH, W.R., GLAUSER, F.L.,
& WILSON, A.F. (1979) Paraquat poisoning: an analytical
toxicologic study of 3 cases. Toxicology, 12: 259-268.
FAO (1973) Diquat and paraquat. FAO specifications for plant
protection products, Rome, Food and Agriculture Organization
of the United Nations.
FAO/WHO (1971) Paraquat. In: 1970 Evaluation of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1973) Paraquat. In: 1972 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1976) Paraquat. In: 1975 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977) Paraquat. In: 1976 Evaluation of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1979) Paraquat. In: 1978 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1982) Paraquat. In: 1981 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1983) Paraquat. In: 1982 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FARAGO, E., HIDEG, Z., & TABATS, G. (1981) [Fatal poisonings
during the last 10 years from statistics of the Institute of
Forensic Chemistry.] Népegészsegügy, 62: 376-379 (in
Hungarian).
FAURE, J., MARKA, C., FAURE, H., YACOUB, M., & CAN, G.
(1973) Données histo-pathologiques et toxicologiques d'une
intoxication mortelle par le paraquat. Méd. lég. Dommage, 6:
417-419.
FENNELLY, J.J., GALLAGHER, J.T., & CARROLL, R.J. (1968)
Paraquat poisoning in a pregnant woman. Br. med. J., 3:
722-723.
FENNELLY, J.J., FITZGERALD, M.X., & FITZGERALD, 0. (1971)
Recovery from severe paraquat poisoning following forced
diuresis and immunosuppressive therapy. J. Irish Med. Assoc.,
64: 69-71.
FIRLIK, M. (1978) [Changes in the respiratory system during
paraquat poisoning.] Med. Pr., 29: 325-328 (in Polish).
FISHER, H.K. & KAHLER, J. (1979) [Lethal poisoning by
paraquat.] Z. Rechtsmed., 84: 61-67 (in German).
FISHER, H.K., HUMPHRIES, M., & BAILS, R. (1971) Recovery
from renal and pulmonary damage. Ann. intern. Med., 75:
731-736.
FISHER, H.K., CLEMENTS, J.A., & WRIGHT, R.R. (1973a)
Pulmonary effects of the herbicide paraquat studied 3 days
after injection in rats. J. appl. Physiol., 35: 268-273.
FISHER, H.K., CLEMENTS, J.A., & WRIGHT, R.R. (1973b)
Enhancement of oxygen toxicity by the herbicide paraquat. Ann.
Rev. respir. Dis., 107: 246-252.
FISHER, H.K., CLEMENTS, J.A., TIERNEY, D.F., & WRIGHT, R.R.
(1975) Pulmonary effects of paraquat in the first day after
injection. Am. J. Physiol., 228: 1217-1223.
FITZGERALD, G.R. & BARNIVILLE, G. (1978) Poisoning by
granular paraquat. J. Irish Coll. Physicians Surg., 7: 133-136.
FITZGERALD, G.R., BARNIVILLE, G., FITZPATRICK, P., EDWARDS,
H., SILKE, B., CARMODY, M., & O'DWYER, W.F. (1977a) Adrenal
abnormalities in paraquat poisoning. Irish J. Med. Sci., 146:
421-423.
FITZGERALD, G.R., BARNIVILLE, G., SILKE, B., CARMODY, M., &
O'DWYER, W.F. (1977b) The kidney in paraquat poisoning.
In: Proceedings of the European Dialysis and Transplantation
Association, Helsinki 1977.
FITZGERALD, G.R., BARNIVILLE, G., FLANAGAN, M., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978a) The changing pattern of
paraquat poisoning: an epidemiologic study. J. Irish Med.
Assoc., 71: 103-108.
FITZGERALD, G.R., BARNIVILLE, G., BLACK, J., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978b) Paraquat poisoning in
agricultural workers. J. Irish Med. Assoc., 71: 336-342.
FITZGERALD, G.R., BARNIVILLE, G., GIBNEY, R.T.N., &
FITZGERALD, M.X. (1979a) Clinical, radiological and
pulmonary function assessment in 13 long-term survivors of
paraquat poisoning. Thorax, 34: 414-415.
FITZGERALD, G.R., BARNIVILLE, G., DICKSTEIN, K., CARMODY, M.,
& O'DWYER, W.F. (1979b) Experience with Fuller's Earth in
paraquat poisoning. J. Irish Med. Assoc., 71: 149-152.
FLETCHER, K. (1967) Production and viability of eggs from
hens treated with paraquat. Nature (Lond.), 215: 1407-1408.
FLETCHER, K. (1974) Paraquat poisoning. In: Ballantyne, B.,
ed. Proceedings of a Symposium on Forensic Toxicology,
Bristol, John Wright, pp. 86-98.
FLETCHER, K., ed. (1975) Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-89.
FLETCHER, K. & WYATT, I. (1970) The composition of lung
lipids after poisoning with paraquat. Br. J. exp. Pathol., 51:
604-609.
FODRI, Z., SIPOS, K., & BERENCSI, G. (1977) [Study of the
irritation and allergic effects of Gramoxone in guinea-pig.]
Egeszsegtudomouny, 21: 244-249 (in Hungarian).
FOWLER, B.A. & BROOKS, R.E. (1971) Effects of the herbicide
paraquat on the ultrastructure of mouse kidney. Am. J.
Pathol., 63: 505-512.
FRIDOVICH, I. & HASSAN, H.M. (1979) Paraquat and the
exacerbation of oxygen toxicity. Trends biochem. Sci., 4:
113-115.
GAGE, J.C. (1968a) Toxicity of paraquat and diquat aerosols
generated by a size-selective cyclone. Effect of particle
size distribution. Br. J. ind. Med., 25: 304-314.
GAGE, J.C. (1968b) The action of paraquat and diquat on the
respiration of liver cell fractions. Biochem. J., 109: 757-761.
GAGE, J.C. (1969) The inhalation toxicity of
paraquat-treated soil, London, ICI Ltd (Report No. IHR/252).
GALLOWAY, D.B. & PETRIE, J.C. (1972) Recovery from severe
paraquat poisoning. Postgrad. Med. J., 48: 684-686.
GEORGE, K. & GEORGE, M. (1978) Chromosome uncoiling effect
of paraquat. Indian J. exp. Biol., 16: 933-937.
GERVAIS, P., DIAMANT-BERGER, O., BESCOL-LIVERSAC, J., GUILLAM,
C., & GUYON, F. (1975) Problčmes médico-legaux et médico-
sociaux de l'intoxication aiguë par les herbicides du groupe
du paraquat. Arch. Mal. prof., 36: 19-36.
GIBSON, J.E. & CAGEN, S.Z. (1977) Paraquat-induced
functional changes in kidney and liver. In: Autor, A.P., ed.
Biochemical mechanisms of paraquat toxicity, New York,
Academic Press, pp. 117-136.
GIRI, S.N. & KRISHNA, G.A. (1980) The effect of paraquat on
guanylate cyclase activity in relation to morphological
changes in guinea-pig lungs. Lung, 157: 127-134.
GIRI, S.N., CURRY, D.L., HOLLINGER, M.A., & FREYWALD, N.
(1979) Effect of paraquat on plasma enzymes, insulin, glucose
and liver glycogen in the rat. Environ. Res., 20: 300-308.
GOWMAN, M.E., RILEY, D., & NEWBY, S.E. (1980) Paraquat and
Diquat: Longterm high rate trial, Frensham UK. Persistence
and movement in soil and glasshouse bioassay, London, ICI Ltd
(Report RJ0014B).
GRANT, H.C., LANTOS, P.L., & PARKINSON, C. (1980) Cerebral
damage in paraquat poisoning. Histopathology, 4: 185-195.
GREENBERG, D.B., LYONS, A., & LAST, J.A. (1978)
Paraquat-induced changes in the rat of collagen biosynthesis
by rat lung. J. lab. clin. Med., 92: 1033-1042.
GROVER, R., SMITH, A.E., & KORVEN, H.C. (1980) A comparison
of chemical and cultural control of weeds in irrigation
ditchbanks. Can. J. plant Sci., 60: 185-195.
GRUNDIES, H., KOLMAR, D., & BENNHOLD, I. (1971) [Paraquat
poisoning.] Dtsch Med. Wschr., 96: 588-589 (in German).
GRZENDA, A.R., NICHOLSON, H.P., & COX, W.S. (1966)
Persistance of four herbicides in pond water. J. Am. Water
Works Assoc., 58: 326-332.
HALEY, T.J. (1979) Review of the toxicology of paraquat
(1,1-dimethyl-4,4-dipyridylium chloride). Clin. Toxicol., 14:
1-46.
HANCE, R.L., BYAST, T.H., & SMITH, P.D. (1980) Apparent
decomposition of paraquat in soil. Soil Biol. Biochem., 12:
447-448.
HARRINGTON, K.J. (1979) The detection of paraquat
(dichloride) in wood by pyrolysis vapour-phase chromatography.
Wood Sci. Technol., 13: 21-28.
HASSAN, H.M. & FRIDOVICH, I. (1977) Regulation of synthesis
of superoxide dismutase in Escherichia coli. J. Biol. Chem.,
253: 7667-7672.
HASSAN, H.M. & FRIDOVICH, I. (1978) Superoxide radical and
the enhancement of oxygen toxicity of paraquat in Escherichia
coli. J. Biol. Chem., 253: 8143-8148.
HASSAN, H.M. & FRIDOVICH, I. (1979) Paraquat and Escherichia
coli. Mechanism of production of extracellular superoxide
radical. J. Biol. Chem., 254: 10846-10852.
HASSAN, H.M. & FRIDOVICH, I. (1980) Superoxide dismutases:
detoxication by a free radical. In: Jakoby, W.B., ed.
Enzymatic basis of detoxication, New York, Academic Press,
Vol. 1, pp. 311-322.
HAWKSWORTH, G.M., BENNETT, P.N., & DAVIES, D.S. (1981)
Kinetics of paraquat elimination in the dog. Toxicol. appl.
Pharmacol., 57: 139-145.
HAYES, W.J. & VAUGHAN, W.K. (1977) Mortality from pesticides
in the United States in 1973 and 1974. Toxicol. appl.
Pharmacol., 42: 235-252.
HEARN, C.E.D. & KEIR, W. (1971) Nail damage in spray
operators exposed to paraquat. Br. J. ind. Med., 28: 399-403.
HIGGINBOTTOM, T., CROME, P., PARKINSON, C., & NUNN, J.
(1979) Further clinical observations on the pulmonary effect
of paraquat ingestion. Thorax, 34: 161-165.
HILLS, D.J., CURLEY, R.G., KNUTSON, J.D., SEIBER, J.N.,
WINTERLIN, W.L., FAUSCHKOLB, R.S., PULMAN, G.S., & ELMORE,
C.L. (1981) Composting treatment for cotton gin trasfines.
Trans. ASAE, 24: 14-19.
HOFMAN, A. & FROHBERG, H. (1972) [Gramoxone intoxication in
the Federal Republic of Germany.] Dtsch Med. Wschr., 27:
1239-1303 (in German).
HOLLINGER, M.A. & CHVAPEL, M. (1977) Effect of paraquat on
rat lung prolyl hydroxylase. Res. Commun. chem. Pathol.
Pharmacol., 16: 159-162.
HORIUCHI, N. & ANDO, S. (1980) [Contact dermatitis due to
pesticides for agricultural use.] Nippon Hifuka Gakkai Zasshi,
90: 289 (in Japanese).
HORIUCHI, N., ANDO, S., & KAMBE, Y. (1980) [Dermatitis due
to pesticides for agricultural use.] Nippon Hifuka Gakkai
Zasshi, 90: 27 (in Japanese).
HOWARD, J.K. (1979a) Recent experience with paraquat
poisoning in Great Britain: a review of 68 cases. Vet. hum.
Toxicol., 21: 213-216.
HOWARD, J.K. (l979b) A clinical survey of paraquat
formulation workers. Br. J. ind. Med., 36: 220-223.
HOWARD, J.K. (1980) Paraquat: a review of worker exposure in
normal usage. J. Soc. Occup. Med., 30: 6-11.
HOWARD, J.K. (1982) Paraquat spraying. Comparative risks
from high and low volume application methods. In: Proceedings
of 10th Asian Conference on Occupational Health, Singapore,
pp. 1-7.
HOWARD, J.K., SAHOPATHY, K.N., & WHITEHEAD, P.A. (1980) An
evaluation of the long-term effects of paraquat spraying.
Toxicol. Lett. Suppl., 1(Abstr. 068).
HOWARD, J.K., SAHOPATHY, K.N., & WHITEHEAD, P.A. (1981) A
study of the health of Malaysian plantation workers with
particular reference to paraquat spraymen. Br. J. ind. Med.,
38: 110-114.
HOWE, D.J.T. & WRIGHT, N. (1965) The toxicity of paraquat
and diquat. In: Proceedings of the 18th New Zealand Weed &
Pest Control Conference, pp. 105-114.
HUDSON, R.H., HAEGELE, M.A., & TUCKER, R.K. (1979) Acute
oral and percutaneous toxicity of pesticides to mallards:
correlations with mammalian toxicity data. Toxicol. appl.
Pharmacol., 47: 451-460.
HUNDSDORFER, S. & ROSE, I. (1980) [Lung changes produced by
paraquat: respiratory distress model in animals.] Ergeb. exp.
Med., 35: 603-609 (in German).
HUSSAIN, M.Z. & BHATNAGAR, R.S. (1979) Involvement of
superoxide in the paraquat-induced enhancement of lung
collagen synthesis in organ culture. Biochem. Biophy. Res.
Commun., 89: 71-76.
ICI Ltd (1972) Determination of residues of paraquat in
milk, London, ICI Ltd (Residue Analytical Method No. 3,
PPRAM-3).
ICI Ltd (1979) Analytical method for the determination of
paraquat by radioimmunoassay, London, ICI Ltd (PHAG).
ILETT, K.F., STRIPP, B., MENARD, R.H., REID, W.D., & GILLETTE,
J.R. (1974) Studies on the mechanism of the lung toxicity of
paraquat. Comparison of tissue distribution and some
biochemical parameters in rats and rabbits. Toxicol. appl.
Pharmacol., 28: 216-226.
IRPTC (1983) IRPTC Legal files 1983, Vol. I & II, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme.
JAROS, F. (1978) Acute percutaneous paraquat poisoning.
Lancet, 1: 275.
JAROS, F., ZUFFA, L., KRATINOVA, R., SKALA, I., & DOMSOVA, A.
(1978) [Acute percutaneous Gramoxone intoxication.] Pr. Lék.,
7: 260-262 (in Czech).
JOYCE, M. (1969) Ocular damage caused by paraquat. Br. J.
Ophtalmol., 53: 688-690.
KAWAI, M. & YOSHIDA, M. (1981) [Exposure of spray operators
to paraquat.] Nippon Doshu Eisei Zasshi, 28: 353-359 (in
Japanese).
KAWAI, S., UEDA, K., KOYAMA, K., & OGASAWARA, S. (1980)
[Studies on preventive treatment of paraquat dichloride
intoxication - Part 2.] Nippon Noson Igakkai Zasshi, 29:
546-547 (in Japanese).
KAWATOMI, M., KOGA, H., YOKOYAMA, K., FUJIMATSU, S.,
MATSUMOTO, T., FUKUDA, H., & IHIZAKI, T. (1979) [A case of
autopsy of a person who died of paraquat intoxication.] Nippon
Naiko Gakkai Zasshi, 68: 1332-1333 (in Japanese).
KEELING, P.L., PRATT, I.S., ALDRIDGE, W.N., & SMITH, L.L.
(1981) The enhancement of paraquat toxicity in rats by 85%
oxygen: lethality and cell-specific lung damage. Br. J. exp.
Pathol., 62: 643-654.
KEELING, P.L., SMITH, L.L., & ALDRIDGE, W.N. (1982) The
formation of mixed disulfides in rat lung following paraquat
administration. Biochem. Biophys. Acta, 716: 249-257.
KEHRER, J.P., HASCHEK, W.M., & WITSCHI, H. (1979) The
influence of hyperoxia on the acute toxicity of paraquat and
diquat. Drug Chem. Toxicol., 2: 397-408.
KELLY, D.F., MORGAN, D.G., DARKE, P.G.G., GIBBS, C., PEARSON,
H., & WEAVER, M.Q. (1978) Pathology of acute respiratory
distress in the dog associated with paraquat poisoning.
J. comp. Pathol., 88: 275-293.
KHAN, S.U. (1974) Determination of diquat and paraquat
residues in soil by gas chromatography. J. agric. food Chem.,
22: 863-867.
KHAN, S.U. (1980) Determining the role of humic substances
in the fate of pesticides in the environment. J. environ. Sci.
Health, 15: 1071-1090.
KHERA, K.S., WHITTA, L.K., & CLEGG, D.J. (1968) Embryopathic
effects of diquat and paraquat in rats. Ind. Med. Surg., 37:
257-261.
KIMBROUGH, R.D. (1974) Toxic effect of the herbicide
paraquat. Chest, 65: 706-708.
KIMBROUGH, R.D. & GAINES, T.B. (1970) Toxicity of paraquat
in rats and its effect on rat lungs. Toxicol. appl.
Pharmacol., 17: 679-690.
KIMBROUGH, R.D. & LINDER, R.E. (1973) The ultrastructure of
the paraquat lung lesion in the rat. Environ. Res., 6: 265-273.
KIMURA, M., SUZUKI, E., & OHNISHI, H. (1980) [A case of
autopsy of an infant intoxicated by paraquat dichloride.]
Shoni ka Rinsho, 33: 732-734 (in Japanese).
KLIKA, E., KUNC, L., ANTALIKOVA, K., & KUNCOVA, M. (1980)
The morphology of the lung in the albino rat after paraquat
administration. Folia Morphol., 28: 188-191.
KNIGHT, B.A.G. & DENNY, P.J. (1970) The interaction of
paraquat with soil: adsorption by an expanding lattice clay
mineral. Weed Res., 10: 40-48.
KNIGHT, B.A.G. & TOMLINSON, T.E. (1967) The interaction of
paraquat (1,1'-dimethyl-4,4'-dipyridylium dichloride) with
mineral soils. J. soil Sci., 18: 223-243.
KORNBRUST, D.J. & MAVIS, R.D. (1980) The effect of paraquat
on microsomal lipid peroxidation in vitro and in vivo.
Toxicol. appl. Pharmacol., 53: 323-332.
KURISAKI, E. & SATO, H. (1979) [Toxicological studies on
herbicides. Intracorporeal distribution on paraquat dichloride
and diquat dibromide in rat.] Nippon Hoigaki Zasshi, 33: 656
(in Japanese).
LAM, H.F., TAKEZAWA, J., GUPTA, B.N., & STEE, E.W. van
(1980) A comparison of the effects of paraquat and diquat on
lung compliance, lung volumes and single breath. Toxicology,
18: 111-123.
LAVAUR, E., SION, G., GROLLEAU, G., & CARPENTER-LESECK, J.
(1979) Etude comparée de l'action du diquat et du paraquat
sur la muqueuse digestive de la souris, du rat, et du lapin.
Ann. Zool. Ecol. anim., 11: 159-169.
LEARY, J.B. (1978) Diquat and paraquat. In: Zweig, G. &
Sherma, J., ed. Analytical methods for pesticides and growth
regulators, New York, Academic Press, pp. 321-325.
LENGFELDER, E. & ELSTNER, E.F. (1978) Determination of the
superoxide dismutating activity of D-penicillamine copper.
Hoppe-Seyler's Z. Physiol. Chem., 359: 751-757.
LEVIN, P.J., KLAFF, L.J., ROSE, A.G., & FERGUSON, A.D.
(1979) Pulmonary effects of contact exposure to paraquat: a
clinical and experimental study. Thorax, 34: 150-160.
LEVIN, D.E., HOLLSTEIN, M., CHRISTMAN, M.F., SCHWIERS, E.A., &
AMES, B.N. (1982) A new Salmonella tester strain (TA 102)
with AT base pairs at the site of mutation detects oxidative
mutagens. Proc. Natl Acad. Sci. USA, 79: 7445-7449.
LEVITT, T. (1979) Determinations of paraquat in clinical
practice using radioimmunoassay. Proc. Anal. Div. Chem. Soc.,
16: 72-76.
LIDDLE, J.A., NEEDHAM, L.L., ROLLEN, Z.J., ROARK, B.R., &
BAYSE, D.D. (1980) Characterization of the contamination of
marijuana with paraquat. Bull. environ. Contam. Toxicol., 24:
49-53.
LITCHFIELD, M.H., DANIEL, J.W., & LONGSHAW, S. (1973) The
tissue distribution of the bipyridylium herbicides diquat and
paraquat in rats and mice. Toxicology, 1: 155-165.
LLOYD, E.L. (1969) Recovery after taking Weedol. Br. med. J.,
2(5650): 189.
LOCK, E.A. (1979) The effect of paraquat and diquat on renal
function in the rat. Toxicol. appl. Pharmacol., 48: 327-336.
LOCK, E.A. & ISHMAEL, J. (1979) The acute toxic effects of
paraquat and diquat on the rat kidney. Toxicol. appl.
Pharmacol., 50: 67-76.
LOTT, P.F., LOTT, J.W., & DOMS, D.J. (1978) The
determination of paraquat. J. chromatogr. Sci., 16: 390-395.
LUTY, S., CISAK, E., LATUSZYNSKA, J., & PRZYLEPA, E. (1978)
[Effect of paraquat on embryonic and post-embryonic
development in rat.] Bromatol. Chem. Toksykol., 11: 159-165
(in Polish).
MAFF (1980a) Agricultural Development and Advisory Service
'Pesticide science' reference book 1980, Lowestoft, England,
Ministry of Agriculture, Fisheries & Food, pp. 69-76
MAFF (1980b) Pest Infestation Control Laboratory Report
1977-1979, Lowestoft, England, Ministry of Agriculture,
Fisheries & Food, pp. 143-153.
MAFF (1981) Agricultural Development and Advisory Service
'Pesticide science' reference book 1981, Lowestoft, England,
Ministry of Agriculture, Fisheries & Food, pp. 21-27 and 55-63.
MAKOVSKII, V.N. (1972) [Toxicological and hygiene studies of
bipyridylium herbicides diquat and paraquat,] Referat,
Vinnize, USSR, pp. 1-23 (PhD degree thesis) (in Russian).
MALING, H.M., SAUL, W., WILLIAMS, M.A., BROWN, E.A.B., &
GILLETTE, J.R. (l978) Reduced body clearance as the major
mechanisms of the potentiation of beta2-adrenergic agonists of
paraquat lethality in rat. Toxicol. appl. Pharmacol., 43:
57-72.
MALONE, J.D.G., CARMODY, M., & KEOGH, B. (1971) Paraquat
poisoning. A review of nineteen cases. J. Irish Med. Assoc.,
64: 59-68.
MAREK, J., VELENSKA, Z., & HASKOVCOVA, I. (1981) [Kidney
changes in acute phase of paraquat intoxication in rats.]
Sb. Lék., 83: 100-104 (in Czech).
MASTERSON, J.G. & ROCHE, W.J. (1970) Fatal paraquat
poisoning. J. Irish Med. Assoc., 63: 261-264.
MATSUMOTO, T., MATSUMORI, H., KUWABARA, N., FUKUDA, Y., &
ARIWA, R. (1980) [A histopathological study of the liver in
paraquat poisoning. An analysis of 14 autopsy cases with
emphasis on bile duct injury.] Acta pathol. Jpn., 30: 859-870
(in Japanese).
MATSUMOTO, S., MATSUMORI, H., KUWABARA, N., FUKUDA, Y., &
ARIWA, R. (1981) [Histopathological studies on the
disturbance of bile ducts to paraquat.] Kanzo, 22: 309 (in
Japanese).
MATTHEW, H., LOGAN, A., WOODRUFF, M.F.A., & HEARD, B. (1968)
Paraquat poisoning. Lung transplantation. Br. med. J., 3:
759-763.
MCCORD, J.M. & FRIDOVICH, I. (1969) Superoxide dismutase -
an enzymic function for erythrocuprein (hemocuprein). J. Biol.
Chem., 244: 6049-6055.
MCDONAGH, B.J. & MARTIN, J. (1970) Paraquat poisoning in
children. Arch. Dis. Child., 45: 425-427.
MCELLIGOTT, T.F. (1972) The dermal toxicity of paraquat.
Differences due to techniques of application. Toxicol. appl.
Pharmacol., 21: 361-368.
MCGEOWN, M.G. (1975) Clinical aspects of paraquat poisoning.
In: Fletcher, K., ed., Clinical aspects of paraquat poisoning,
London, ICI Ltd, pp. 12-21.
MEES, G.C. (1960) Experiments on the herbicidal action of
1,1'-ethylene-2,2'dipyridylium dibromide. Ann. appl. Biol.,
48: 601-612.
MEHANI, S. (1972) The toxic effect of paraquat in rabbits
and rats. Ain Shams med. J., 23: 599-601.
MIRCEV, N. (1976) [Acute intoxication with Gramoxone
(paraquat).] Vatreshni Bolesti, 16: 99-101 (in Bulgarian).
MITHYANTHA, M.S. & PERUR, N.G. (1975) Paraquat retention by
soils. Mysore J. agric. Sci., 9: 276-282.
MONTGOMERY, M.R. (1976) Interaction of paraquat with the
pulmonary microsomal fatty acid desaturase system. Toxicol.
appl. Pharmacol., 36: 543-554.
MONTGOMERY, M.R. (1977) Paraquat toxicity and pulmonary
superoxide dismutase: an enzymic deficiency of lung
microsomes. Res. Commun. chem. Pathol. Pharmacol., 16: 155-158.
MONTGOMERY, M.R. & NIEWOEHNER, D.E. (1979) Oxidant-induced
alterations in pulmonary microsomal mixed-function oxidation.
Acute effects of paraquat and ozone. J. environ. Sci. Health,
13: 205-219.
MOODY, C.S. & HASSAN, H.M. (1982) Mutagenicity of oxygen
free radicals. Proc. Natl Acad. Sci., 79: 2855-2859.
MOURIN, K.A. (1967) Paraquat poisoning. Br. med. J., 4: 486.
MUKADA, T., SASANO, N., & SATO, K. (1978) [Autopsy findings
in a case of acute paraquat poisoning with extensive cerebral
purpura.] Tohoku J. exp. Med., 125: 253-263 (in Japanese).
MULLICK, F.G., TSHAK, K.G., MAHABIR, K., & STROMEYER, F.W.
(1981) Hepatic injury associated with paraquat toxicity in
humans. Liver, 1: 209-221.
MURRAY, R.E. & GIBSON, J.E. (1972) A comparative study of
paraquat intoxication in rats, guinea pigs and monkeys.
Exp. mol. Pathol., 17: 317-325.
MURRAY, R.E. & GIBSON, J.E. (1974) Paraquat disposition in
rats, guinea pigs and monkeys. Toxicol. appl. Pharmacol., 27:
283-291.
MUSSON, F.A. & PORTER, C.A. (1982) Effect of ingestion of
paraquat on a 20-week gestation fetus. Postgrad. Med. J., 58:
731-732.
NAGY, A. (1970) Paraquat and adrenal cortical necrosis.
Br. med. J. 2: 669.
NATORI, H., KOIKE, M., & KIRA, S. (1979) [Respiratory
failure due to the herbicide paraquat.] Gendai Iryo, 11:
1175-1182 (in Japanese).
NEWHOUSE, M., MCEVOY, D., & ROSENTHAL, D. (1978)
Percutaneous paraquat absorption. Arch. Dermatol., 114:
1516-1519.
NEWMAN, J.F. & EDWARDS, P.J. (1980) Paraquat: effect on
spraying eggs on hatachability and on the reproductive organs
of the chicks of pheasant, Phasianus colchicus, London, ICI
Ltd (ICI Plant Protection Division Report No. RJ 0090B).
NEWMAN, J.F. & WAY, J.M. (1966) Some ecological observations
on the use of paraquat and diquat as aquatic herbicides.
Proceedings of the 8th British Weed Control Conference,
Vol. 2, pp. 582-585.
NEWMAN, J.F. & WILKINSON, W.W. (1971) The effect of excess
quantities of paraquat in soil on the growth of vegetation,
London, ICI Ltd (Report No TMJ 606 A).
O'BRIEN, M.C. & PRENDEVILLE, G.N. (1978) A rapid sensitive
bioassay for determination of paraquat and diquat in water.
Weed Res., 18: 301-303.
OGATA, M., HASEGAWA, T., & UEDA, K. (1978) [Action of
paraquat dichloride and dimethyldithiocarbamate on the
mitochondrial conversion system and superoxide dismutase.]
Samgyo Igaku, 20: 551-552 (in Japanese).
OKAWADA, N., YAGASAKI, K., & KONDO, T. (1980) [Ocular
impairments due to an agricultural pesticide.] Nippon Noson
Igakkai Zasshi, 29: 550-551 (in Japanese).
OKONEK, S., WEILEMANN, L.S., MAJDANDZIC, J., SETYADHARME, H.,
REINECKE, H.J., BALDAMUS, C.A., LOHMAN, J., BONZEL, K.E., &
THON, T. (1982) Successful treatment of paraquat poisoning.
Activated charcoal per os and, continuous hemoperfusion.
J. Toxicol. clin. Toxicol., 19: 807-819.
OMAYE, S.T., REDDY, K.A., & CROSS, C.E. (1978) Enhanced lung
toxicity of paraquat in selenium-deficient rats. Toxicol.
appl. Pharmacol., 43: 237-247.
ONGOM, V.L., OWOR, R., & TONUSANGE, E.T. (1974) Paraquat
(Gramoxone) used as a pediculocide. In: Bagshore, A.F., ed.
The uses and abuses of drugs and chemicals in tropical Africa,
Nairobi, East Africa Literature Bureau, pp. 229-233.
PARK, J., PROUDFOOT, A.T., & PRESCOTT, L.F. (1975) Paraquat
poisoning. A clinical review of 31 cases. In: Fletcher, K.,
ed., Clinical aspects of paraquat poisoning, London, ICI Ltd,
pp. 46-53.
PARRY, J.M. (1973) The induction of gene conversion in yeast
by herbicide preparations. Mutat. Res., 21: 83-91.
PARRY, J.M. (1977) The use of yeast for the detection of
environmental mutagens using a fluctuation test. Mutat. Res.,
46: 165-176.
PASCHAL, D.C., NEEDHAM, L.L., ROLLEN, Z.J., & LIDDLE, J.A.
(1979) Determination of paraquat in sunflower seeds by
reversed-phase high performance liquid chromatography.
J. Chromatogr., 171: 85-90.
PASI, A., EMBREE, J.W., EISENLORD, G.H., & HINE, C.H. (1974)
Assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 26: 171-175.
PATRICK, G.B. (1980) Marijuana and the lung. Postgrad. med.
J., 67: 110-118.
PAYNE, W.L., POPE, J.D., & BENNER, J.E. (1974) Integrated
method for trifluralen, diphenamid and paraquat in soil and
runoff from agricultural land. J. agric. food Chem., 22: 79-82.
PEDERSEN, G., PEDERSEN, A., GREGERSEN, M., & KAMPE, B.
(1981) [Paraquat poisoning.] Ugeskr. Laeg., 143: 1202-1206
(in Danish).
PESTEMER, VON W., NOLTING, H.G., & LUNDEHN, Y.-R. (1979)
[Possible effects of repeated paraquat treatments on the
residue situation in the soil.] Nachrichtenbl. Dtsch
Pflanzenschutzd., 31: 166-170 (in German).
PICKOVA, J. (1978) [Determination of paraquat in the urine.]
Pr. Lék., 30: 266-267 (in Czech).
POPE, J.D. & BENNER, J.E. (1974) Colorimetric determination
of paraquat residues in soil and water. J. AOHC, 57: 202-204.
POPENOE, D. (1979) Effects of paraquat aerosol on mouse
lung. Arch. Pathol. lab. Med., 103: 331-334.
PROUDFOOT, A.T., STEWART, M.S., LEVITT, T., & WIDDOP, B.
(1979) Paraquat poisoning: significance of plasma-paraquat
concentrations. Lancet, 2(8138): 330-332.
PRYDE, A. & DARBY, F.J. (1975) The analysis of paraquat in
the urine by high speed liquid chromatography. J. Chromatogr.,
115: 107-109.
PURSER, D.A. & ROSE, M.S. (1979) The toxicity and renal
handling of paraquat in Cynomolgus monkeys. Toxicology, 15:
31-41.
RADAELLI, L. & MARTELLI, M. (1971) Residual toxicity of
paraquat absorbed on soil. Agrochimica, 15: 344-350.
REDDY, K.A., LITOV, R.E., & OMAYE, S.T. (1977) Effect of
pre-treatment with anti-inflammatory agents on paraquat
toxicity in the rat. Res. Commun. chem. Pathol. Pharmacol.,
17: 87-100.
RESTUCCIA, A., FOGLINI, A., & DE ALENTIS NANNINI, D. (1974)
Paraquat toxicity for rabbits. Vet. Ital., 25: 555-565.
RILEY, D. (1981) The fate and effect of paraquat and diquat
residues in soil. In: Proceedings for the National Spray Seed
Conference 1981, Albury, New South Wales, Australia.
RILEY, D., WILKINSON, W., & TUCKER, B.V. (1976) Biological
unavailability of bound paraquat residues in soil. In:
Kaufman, D.P., Still, G.G., Paulson, G.D., & Bandal, S.K., ed.
Bound and conjugated pesticide residues, pp. 301-353 (ACS
Symposium Series No. 29).
ROBERTSON, B. (1973) Paraquat poisoning as an experimental
model of the idiopathic respiratory distress syndrome. Bull.
Phys. Pathol. Res., 9: 1433-1452.
ROBERTSON, B., GROSSMANN, G., & IVEMARK, B. (1976) The
alveolar lining layer in experimental paraquat poisoning. Acta
pathol. microbiol. Scand. Section A, 84: 40-46.
ROSE, J. (1980) Paraquat poisoning. Lancet, 2(8200): 924.
ROSE, M.S. & SMITH, L.L. (1977) The relevance of paraquat
accumulation by tissues. In: Autor, A.P., ed., Biochemical
mechanisms of paraquat toxicity, New York, Academic Press, pp.
71-91.
ROSE, M.S., SMITH, L.L., & WYATT, I. (1974a) Evidence of
energy-dependent accumulation of paraquat in rat lung. Nature
(Lond.), 252: 314-315.
ROSE, M.S., CRABTREE, H.C., FLETCHER, K., & WYATT, I.
(1974b) Biochemical effects of diquat and paraquat.
Disturbance of the control of corticosteroid synthesis in rat
adrenal and subsequent effects on the control of live glycogen
utilization. Biochem. J., 138: 437-443.
ROSE, M.S., SMITH, L.L., & WYATT, I. (1976) The relevance of
pentose-phosphate pathway stimulation in rat lung to the
mechanisms of paraquat toxicity. Biochem. Pharmacol., 25:
1763-1767.
ROSLYCKY, E.B. (1977) Response of soil microbiota to
selected herbicide treatments. Can. J. Microbiol., 23: 426-433.
ROSS, W.E., BLOCK, E.R., & CHANG, R.Y. (1979) Paraquat-
induced DNA damage in mammalian cells. Biochem. biophys. Res.
Commun., 91: 1302-1308.
SAITO, K., PARKER, W.B., GARDNER, D.E., & MENZEL, D.B.
(1979) Paraquat accumulation by cultured lung cells.
Pharmacology, 21(Abstr. 218): 392.
SAMMAN, P.D. & JOHNSTON, E.N. (1969) Nail damage associated
with handling of paraquat and diquat. Br. med. J., 1: 818-819.
SCHLATTER, I. (1976) [Poisoning due to herbicide paraquat.]
Schweiz. Rundsch. Med., 65: 837-843 (in German).
SEIBER, J.N. & WOODROW, J.E. (1981) Sampling and analysis of
airborne residues of paraquat in treated cotton field
environments. Arch. environ. Contam. Toxicol., 10: 133-149.
SEIBER, J.N., WINTERLIN, W.L., & MCCHESNEY, B. (1979)
Residues of toxaphene, DEE, and paraquat in plant parts and
gin waste from treated cotton fields. Arch. environ. Contam.
Toxicol., 8: 125-137.
SEIDENFELD, J.J., WYKOFF, D., ZAVALA, D.C., & RICHARDSON,
J.B. (1978) Paraquat lung injury in rabbits. Br. J. ind.
Med., 35: 245-247.
SELECT COMMITTEE ON NARCOTICS ABUSE & CONTROL (1980) The use
of paraquat to eradicate illicit marihuana crops and the
health implications of paraquat-contaminated marihuana on the
US market. A report of the 96-Congress, Washington DC, US
Government Printing Office, pp. 1-97 (SCNAC-96-1-16).
SELYPES, A. & PALDY, A. (1978) The examination of the
mutagenic effect of two pesticides: Krezonit E and Gramoxone.
Proc. Hung. Ann. Meet. Biochem., 18: 77-78.
SELYPES, A., NAGYMAJTENYI, L., & BERENCSI, G. (1980)
Mutagenic and embryotoxic effects of paraquat and diquat.
Bull. environ. Contam. Toxicol., 25: 513-517.
SHARP, C.W., OTTOLENGHI, A., & POSNER, A.S. (1972)
Correlation of paraquat toxicity with tissue concentration and
weight loss of the rat. Toxicol. appl. Pharmacol., 22: 241-251.
SHU, H., TALCOTT, R.E., RICE, S.A., & WEI, E.T. (1979) Lipid
peroxidation and paraquat toxicity. Biochem. Pharmacol., 28:
327-331.
SHUZUI, T. (1980) [An autopsy case of paraquat poisoning.]
Mie Igaku, 23: 518-521 (in Japanese).
SINGH, S.P. & YADAV, N.K. (1978) Toxicity of some herbicides
to maior carp fingerlings. Indian J. Ecol., 5: 141-147.
SINOW, J. & WEI, E. (1973) Ocular toxicity of paraquat.
Bull. environ. Contam. Toxicol., 9: 163-168.
SLADE, P. (1965) Photochemical degradation of paraquat.
Nature (Lond.), 207: 515.
SLADE, P. (1966) The fate of paraquat applied to plants.
Weed Res., 6: 158-167.
SMALLEY, H.E. (1973) Toxicity and hazard of the herbicide
paraquat in turkeys. Poultry Sci., 52: 1625-1629.
SMITH, R.J. (1978) Poisoned pot becomes burning issue in
high places. Science, 200: 417-418.
SMITH, P. & HEATH, D. (1974) The ultrastructure and time
sequence of the early stages of paraquat lung in rats.
J. Pathol., 114: 177-184.
SMITH, P. & HEATH, D. (1976) Paraquat. Clin. Rev. Toxicol.,
4: 411-445.
SMITH, L.L. & ROSE, M.S. (1977a) A comparison of the effects
of paraquat and diquat on the water content of rat lung and
the incorporation of thymidine into lung DNA. Toxicology, 8:
223-230.
SMITH, L.L. & ROSE, M.S. (1977b) Biochemical changes in
lungs exposed to paraquat. In: Autor, A.P., ed. Biochemical
mechanisms of paraquat toxicity, New York, Academic Press.
SMITH, P., HEATH, D., & RAY, J.M. (1973) The pathogenesis
and structure of paraquat-induced pulmonary fibrosis in rats.
J. Pathol., 114: 57-67.
SMITH, L.L., WRIGHT, A., WYATT, I., & ROSE, M.S. (1974)
Effective treatment for paraquat poisoning in rats and its
relevance to treatment of paraquat poisoning in man. Br. med.
J., 4: 569-571.
SMITH, S.N., LYON, A.J., & SAHID, I.B. (1976) The breakdown
of paraquat and diquat by soil fungi. New Phytol., 77: 735-740.
SMITH, C.N., LEONARD, R.A., LANGDALE, G.W., & BAILEY, G.W.
(1978) Transport of agricultural chemicals from small upland
Piedmont watersheds, Springfield, Virginia, National Technical
Information Service, p. 386 (Report PB-285, 134).
SMITH, L.L., ROSE, M.S., & WYATT, I. (1979) The pathology
and biochemistry of paraquat. In: Oxygen free radicals and
tissue damage, Amsterdam, North Holland, Excerpta Medica,
pp. 321-341 (CIBA Foundation Series 65 - new series).
SMITH, T.F., NOACK, A.J., & COSH, S.M. (1981a) The effect of
some herbicides on vesicular-arbuscular endophyte abundance in
the soil and on infection of host roots. Pestic. Sci., 12:
91-97.
SMITH, L.L., WYATT, I., & ROSE, M.S. (1981b) Factors
affecting the efflux of paraquat from rat lung slices.
Toxicology, 19: 197-207.
SODERQUIST, C.J. & CROSBY, D.G. (1972) The gas chromato-
graphic determination of paraquat in water. Bull. environ.
Contam. Toxicol., 8: 363-368.
STAIFF, D.C., IRLE, G.K., & FELSENSTEIN, W.C. (1973)
Screening of various adsorbents for protection against
paraquat poisoning. Bull. environ. Contam. Toxicol., 10:
193-199.
STAIFF, D.C., COMER, S.W., ARMSTRONG, J.F., & WOLFE, H.R.
(1975) Exposure to the herbicide paraquat. Bull. environ.
Contam. Toxicol., 14: 334-340.
STAIFF, D.C., BUTLER, L.C., & DAVIS, J.E. (1981) A fie1d
study of the chemical degradation of paraquat dichloride
following simulated spillage on soil. Bull. environ. Contam.
Toxicol., 26: 16-21.
STEFFEN, C. & NETTER, K.J. (1979) On the mechanism of
paraquat action on microsomal oxygen reduction and its
relation to lipid peroxidation. Toxicol. appl. Pharmacol., 47:
593-602.
STEFFEN, C., MULIAWAN, H., & KAPPUS, H. (1980) Lack of in
vivo lipid peroxidation in experimental paraquat poisoning.
Arch. Pharmacol., 310(3): 241-243.
STEPHENS, D.S., WALKER, D.H., SCHAFFNER, W., KAPLOWITZ, L.G.,
BRASHEAR, R.H., ROBERTS, R., & SPICKARD, W.A. (1981)
Pseudodiphtheria: prominent pharyngeal membrane associated
with fetal paraquat ingestion. Ann. intern. Med., 94: 202-204.
STEVENS, M.A. & WALLEY, J.K. (1966) Tissue and milk residues
arising from the ingestion of single doses of diquat and
paraquat by cattle. J. Sci. Food Agric., 17: 472-475.
STEWART, M.J., LEVITT, T., & JARVIE, D.R. (1979) Emergency
estimations of paraquat in plasma. A comparison of the RIA and
ion pair/colorimetric methods. Clin. Chim. Acta, 94: 253-257.
SUGAYA, H., OHE, T., UENO, T., KAWASHIMA, T., SUGITA, T.,
MAEHARA, M., HISANCHI, T., IORI, M., HARADA, H., & SHIGEMATA,
S. (1980) [Clinical discussion on six cases of paraquat
dichloride intoxication.] Nippon Naika Gakkai Zasshi, 69: 876
(in Japanese).
SUMMERS, L.A. (1980) The bipyridylium herbicides, London,
New York, Toronto, Sydney, San Francisco, Academic Press,
pp. 1-449.
SWAN, A.A.B. (1969) Exposure of spray operators to paraquat.
Br. J. ind. Med., 26: 322-329.
SYKES, B.J., PURCHASE, I.F.H., & SMITH, L.L. (1977)
Pulmonary ultrastructure after oral and intravenous dosage of
paraquat to rats. J. Pathol., 121: 233-241.
TAKAHASHI, T., YAMAMOTO, K., SAWAI, T., OKUDO, T., MUKODA, T.,
MINAGAWA, N., & SUGAYA, H. (1978) [Examination of 5
autopsies following paraquat dichloride intoxication,
especially of parenchymatous organ lesions.] Nippon
Byorigakkai Kaishi, 67: 138-139 (in Japanese).
TAKAYAMA, K., TAKEUCHI, K., SUGA, E., IWABUCHI, K., & TOMICHI,
N. (1978) [A case of autopsy following paraquat dichloride
intoxication.] Nippon Byorigakkai Kaishi, 67: 139 (in
Japanese).
TAKEUCHI, K., TAKAYAMA, K., TOMICHI, N., KAN, E., YAGAWA, K.,
& IWABUCHI, K. (1980) [Paraquat poisoning in a pregnant
woman.] Nippon Byorigakkai Kaishi, 18: 747-752 (in Japanese).
TALCOTT, R.E., SHU, H., & WEI, E.T. (1979) Dissociation of
microsomal oxygen reduction and lipid peroxidation with the
electron acceptors, paraquat and menadione. Biochem.
Pharmacol., 26: 665-671.
TCHIPILSKA, L.N. (1980) [The influence of some pesticides on
the soil microorganisms in connexion with the hygiene
evaluation of the soil,] Referat, Sofia, pp. 1-39 (PhD degree
thesis) (in Bulgarian).
THOMPSON, W.D. & PATRICK, R.S. (1978) Collagen propyl
hydroxylase levels in experimental paraquat poisoning.
Br. J. exp. Pathol., 59: 288-291.
TOMPSETT, S.L. (1970) Paraquat poisoning. Acta pharmacol.
toxicol., 28: 346-358.
TOMURA, M., ETO, K., SAGARA, K., & SATO, T. (1979) [A case
of disturbance of the liver and kidney.] Kanzo, 20: 1016 (in
Japanese).
TONER, P.G., VETTERS, J.M., SPILG, W.G.S., & HARLAND, W.A.
(1970) Fine structure of the lung lesion in a case of
paraquat poisoning. J. Pathol., 102: 182-185.
TRUSH, M.A., MIMNAUGH, E.G., GINSBURG, E., & GRAM, T.E.
(1981) In vitro stimulation by paraquat of reactive oxygen-
mediated lipid peroxidation in rat lung microsomes. Toxicol.
appl. Pharmacol., 60(2): 279-286.
TRUSH, M.A., MIMNAUGH, E.G., GINSBURG, E., & GRAM, T.E.
(1982) Studies on the in vitro interaction of mitomycin C,
nitrofurantoin, and paraquat with pulmonary microsomes.
Stimulation of reactive oxygen-dependent lipid peroxidation.
Biochem. Pharmacol., 31(21): 3335-3346.
TSUNENARI, S., MUTO, H., SASAKI, S., SUGITA, H., & KANDA, M.
(1975) [Forensic toxicological studies on herbicide
(Gramoxone).] Jpn. J. leg. Med., 29: 88-102 (in Japanese).
TSUNENARI, S., YONEMITSU, K., UCHIMURA, Y., & KANDA, M.
(1981) The influence of putrefactive changes on the
determination of paraquat in autopsy materials. Forensic Sci.
Int., 17: 51-56.
TSUTSUI, Y., NAKABAYASHI, H., SUZUKI, H., & OGURA, K. (1976)
[Studies on the toxicity of paraquat - Part I.] Nippon Noson
Igakkai Zasshi, 25: 614-621 (in Japanese).
TU, C.M. & BOLLEN, W.B. (1968) Effect of paraquat on
microbial activities in soils. Weed Res., 8: 28-37.
TWEATS, D.J. (1975) The effect of R (drug resistance)
factors on the detection of mutagens by E. coli K 12,
Brussels, Belgium, Annual Euratom Report, Biological Sciences,
316 pp.
TYBURCZYK, W., BORKOWSKA, I., CHORAGIEWICZ, H., & KLIMEK, K.
(1979) [Effect of paraquat on some biochemical tests in
rats.] Bromatol. Chem. Toksykol., 12: 283-288 (in Polish).
UKAI, S., HIROSE, K., & KAWASE, S. (1977) [Gas chromatography
of reduction products of the herbicides "diquat" and "paraquat".]
Eisei Kagaku, 23: 32-38 (in Japanese).
US CONGRESSIONAL HEARING (1979) Health implications of
paraquat-contaminated marihuana. Select Committee on Narcotics
Abuse & Control, House of Representatives. Hearing of the 96th
Congress, Washington DC, US Government Printing Office, pp.
1-103 (SCNAC-96-1-3).
VALE, J.A. (1977) Paraquat poisoning. Nurs. Times, 73:
154-155.
VAZIRI, N.D., NESS, R.L., FAIRSHTER, R.D., SMITH, W.R., &
ROSEN, S.M. (1979) Nephrotoxicity of paraquat in man. Arch.
intern. Med., 139: 172-174.
VESELEY, D.L., WATSON, B., & LEVEY, G.A. (1979) Activation
of liver guanylate cyclase by paraquat: possible role of
superoxide anion. J. Pharmacol. exp. Ther., 209: 162-164.
VIJEYARATNAM, G.S. & CORRIN, B. (1971) Experimental paraquat
poisoning: a histological and electron-optical study of the
changes in the lung. J. Pathol., 103: 123-129.
VUCINOVIC, V. (1978) [Four cases of poisoning with
paraquat.] Arch. Hig. Rada, 29: 261-265 (in Serbo-Croat).
WADDELL, W.J. & MARLOWE, C. (1980) Tissue and cellular
disposition of paraquat in mice. Toxicol. appl. Pharmacol.,
56: 127-140.
WAIGHT, J.J.J. & WHEATHER, R.H. (1979) Fatal percutaneous
paraquat poisoning. J. Am. Med. Assoc., 242: 472.
WALKER, M., DUGARD, P.H., & SCOTT, R.C. (1983) Absorption
through human and laboratory animal skins: in vitro
comparison. Acta pharm. Suec., 20(1): 52-53.
WALTERS, K.A., DUGAR, P.H., & FLORENCE, A.T. (1981)
Non-ionic surfactants and gastric mucosal transport of
paraquat. J. Pharm. Pharmacol., 33: 207-213.
WARD, C.D., STONES, D.P.A., CONNEL, H., CULLEN, D.R., &
WATKIN, J.I. (1976) Paraquat poisoning. Lancet, 1(7971):
1247.
WATANABE, I., SAKAI, K., TOYAMA, K., UENO, M., & WATANABE, M.
(1979) [On 3 cases of ocular disturbance due to Gratoxone, a
herbicide containing 24% paraquat dichloride.] Ganka Rinsho
Iho, 73: 1244-1246 (in Japanese).
WAUCHOPE, R.D. (1979) Pesticides in runoff water. Agric.
Res., 27: 11.
WAY, J.M., NEWMAN, J.F., MOORE, N.W., & KNAGGS, F.W. (1971)
Some ecological effects of the use of paraquat for the control
of weeds in small lakes. J. appl. Ecology, 8: 509-532.
WEBER, J.B., PERRY, P.W., & UPCHURCH, R.P. (1965) The
influence of temperature and time on the adsorption of
paraquat, diquat, 2,4-D and prometone by clays, charcoal and
on anion-exchange resin. Soil Sci. Am. Soc. Proc., 29: 678-688.
WHO/FAO (1978) Data sheets on pesticides No. 4, Rev.1 -
Paraquat, Geneva, World Health Organization (Unpublished
Report No.: VBC/DS/75.4 Rev.1 (8/78)).
WILKINSON, W. (1980) Paraquat and diquat: Longterm high rate
trial, Frensham UK. Management of site, effects on crops and
weeds and residues in crops, London, ICI Ltd (Report RJ0013B).
WITSCHI, H.P., KACEW, S., HIRAI, K., & COTE, M.G. (1977) In
vivo oxidation of reduced nicotine amide-adenine dinucleotide
phosphate by paraquat and diquat in rat lung. Chem. Biol.
Interactions., 19: 143-160.
WOHLFAHRT, D.J. (1982) Fatal paraquat poisoning after skin
absorption. Med. J. Aust., 1: 512-513.
WOJECK, G.A., PRICE, J.F., NIGG, A.N., & STAMPER, J.H.
(1983) Worker exposure to paraquat and diquat. Arch. environ.
Contam. Toxicol., 12: 65-70.
WORTHING, C.R. (1979) The pesticide manual, Croydon,
England, British Crop Protection Council (BCPC Publications).
WRIGHT, K.A. & CAIN, M. (1970) Microbial degradation of
4-carboxy-1-methylpyridylium chloride, a photolytic product of
paraquat. Biochem. J., 118: 52.
WRIGHT, N., YEOMAN, W.B., & HALE, K.A. (1978) Assessment of
severity of paraquat poisoning. Br. med. J., 2: 396-397.
WYATT, I., DOSS, A.W., ZAVALA, D.C., & SMITH, L.L. (1981)
Intrabronchial instillation of paraquat in rats: lung
morphology and retention study. Br. J. ind. Med., 38: 42-48.
YOSHIDA, K., TANAKA, Y., IKUNUMA, T., TERUKIMATSU, S., KUSANO,
E., ASANO, Y., & HOSODA, S. (1980) [A case report of
survival after intoxication by paraquat.] Nippon Nuika Gakkai
Zasshi, 69: 1487-1489 (in Japanese).
YOUNGMAN, R.J. & DODGE, A.D. (1979) [Mechanism of paraquat
action: inhibition of the herbicidal effect by a copper
chelate with superoxide-dismutating activity.] Z. Naturforsch.
(Teil C), 34: 1032-1035 (in German).
ZAVALA, D.C. & RHODES, M.L. (1978) An effect of paraquat on
the lungs of rabbits. Its implications in smoking contaminated
marijuana. Chest, 74: 418-420.
DIQUAT
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General properties
Diquat (1,1'ethylene, 2,2'bipyridyl) is a non-selective contact
herbicide. It is sold primarily as a 20% w/v solution in many
countries and is manufactured in the United Kingdom. It is
exclusively manufactured as a dibromide salt and is usually
formulated to contain wetters.
The herbicidal property of diquat depends on its ability to
undergo a single electron addition to form a radical that reacts
with molecular oxygen to reform diquat and concomitantly produce a
superoxide anion. This oxygen radical may directly or indirectly
cause cell death.
It is possible to detect the compound because of its ability to
form a radical. Analytical procedures are available.
1.1.2. Environmental distribution and transformation
environmental effects
Diquat undergoes rapid photochemical degradation in aqueous
solution and on surfaces. The major degradation products produced
in water have been identified and are of lower acute oral toxicity
for rats than diquat itself. The photochemical degradation of
diquat on plants is more complex than that in water. On diquat-
desiccated wheat and barley, diquat itself normally constitutes the
most important single compound. The most important photochemical
degradation products have been identified, they are of low
mammalian toxicity. No other well-defined major degradation
product is formed.
Ruminants excrete diquat and its photochemical products rapidly
and very little is transferred to milk and tissues. Consequently,
residue levels in products of animal origin are very low.
Ingestion of diquat and its photochemical products at higher levels
than would be found in practice did not induce ill effects in
ruminants.
Diquat reaching the soil becomes rapidly and strongly adsorbed
to clay minerals in soil. This process inactivates the herbicidal
activity of diquat. While free diquat is degraded by a range of
soil microorganisms, degradation of strongly adsorbed diquat is
relatively slow. In plot studies, the rate of degradation of
diquat in soil is very slow or non-detectable. However, in long-
term field studies, degradation rates of the order of 5 - 10% per
year have been shown. This is greater than the rate required in
normal practice to prevent saturation of the deactivation capacity
of agricultural/horticultural soils. Strongly-bound diquat has no
adverse effects on soil microfauna or soil microbial processes.
Diquat residues disappear rapidly from water by adsorption on
aquatic weeds and by strong adsorption on bottom mud. Diquat is of
low toxicity for fish and is not accumulated in them. Normal
applications of diquat for aquatic weed control are not harmful to
aquatic organisms. However, care should be taken in applying
diquat to water containing heavy weed growth to treat only a part
of the weed growth, since oxygen consumed by subsequent weed decay
may decrease dissolved oxygen levels to an extent that may be
dangerous for fish. Treated water should not be used for overhead
irrigation until a period of 10 days has elapsed following
treatment.
Diquat is not volatile and the concentrations of airborne
diquat during spraying have been shown to be very low.
1.1.3. Kinetics and metabolism
Diquat is poorly absorbed from the intestinal tract and skin.
Diquat monopyridone is the major metabolite of diquat in the body;
of lesser importance is diquat dipyridone. Both metabolites are
considerably less toxic than diquat itself. Depending on species
and route of administration, less than 20% of the dose is
metabolized. The gastrointestinal microflora appear to be mainly
responsible for the metabolism of diquat.
Compared with paraquat, accumulation of diquat in the lungs is
far less marked, but diquat shows a certain preference for the
kidneys. The kidneys are the major route of excretion, but a
considerable amount of diquat can also be excreted in the bile,
varying with the animal species.
1.1.4. Effects on animals
Diquat is less toxic than paraquat and does not give rise to
the specific lung disease that is so typical of paraquat poisoning.
Gastrointestinal disturbances, with vomiting, greenish diarrhoea,
and abdominal distension from the significant accumulation of water
in the lumen of the intestines, are typical of diquat poisoning,
together with progressive haemoconcentration, which may progress to
lethargy, coma, and death. At high doses, minor toxicity has been
noted in the liver, kidney, and the nervous and endocrine systems.
Diquat has induced cataracts after prolonged oral exposure
although this effect has not been reported in man. It is less
irritant to the skin, mucous membranes, and the eye than paraquat,
and is not known to be a sensitizer.
Diquat is not teratogenic or carcinogenic.
In vitro mutagenicity studies were inconclusive, though
generally suggesting only weak activity, while the results of in
vivo studies have been negative. A no-observed-adverse-effect
level of 0.75 mg diquat ion/kg body weight per day has been
established from long-term feeding studies on rats.
1.1.5. Effects on man
Occupational exposure to diquat does not pose a health risk if
the recommendations for use are followed and there is adherence to
safe working practices.
Diquat poisoning by suicidal or accidental ingestion is much
less common than paraquat poisoning. It produces a similar severe
clinical syndrome with two notable differences: (a) diarrhoea is a
prominent feature, and (b) pulmonary fibrosis has not been
described.
Accidental cases are usually due to ingestion of decanted
diquat.
The lethal dose for man appears to be approximately 6 - 12
grams of diquat dibromide. In agricultural workers, inflammation
and bleeding of the nasal mucosa have been reported, as well as
nail changes and delayed wound healing.
1.2. Recommendations
1.2.1. General
Where practical and reasonable, the availability and use of the
20% liquid product should be limited to bona fide agriculturalists,
horticulturalists, and professional users who work with trained
personnel, properly maintained equipment, and adequate supervision.
Every effort should be made to prevent the practice of
decanting or rebottling of the product into containers that have
not been properly labelled.
1.2.2. Prevention and treatment
Attention should be drawn to the fact that persons with skin
lesions (either pre-existing or following contamination with
diquat) should not be permitted to take any part in spraying
procedures until skin condition has resolved.
It must be stressed that treatment of persons with diquat
poisoning should be instituted as early as possible. The
likelihood of recovery from a fatal dose is greatest when therapy
begins within 5 - 6 h of poisoning.
1.2.3. Experimental work
Results of existing mutagenicity and carcinogenicity studies
generally suggest that diquat is unlikely to induce genotoxic
effects in man, but more detailed information is required.
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and Chemical Properties
Diquat is a non-selective contact bipyridylium herbicide and
desiccant. The herbicide is supplied mainly as an aqueous solution
of the dibromide (1,1'-ethylene-2,2'-bipyridylium dibromide,
C12H12N2 Br2), with a relative molecular mass of 184.2 based on the
cation. The commonly available analytical standard is diquat
dibromide monohydrate, which is an odourless, pale yellow,
crystalline powder. Some of the other physical properties of
diquat dibromide are listed in Table 1. It is slightly soluble in
alcohol, and practically insoluble in non-polar organic solvents
(Summers, 1980). Diquat is non-explosive and non-inflammable in
aqueous formulations.
Table 1. Physical properties of diquat dibromide
--------------------------------------------------
Specific gravity at 20 °C 1.200
Melting point 180 °C
Boiling point approximately 300 °C
with decomposition
Solubility in water at 20 °C 700 g/litre
pH of liquid formulation 6.0 - 7.0
Evaporation rate not applicable
Vapour pressure not measurable
--------------------------------------------------
Diquat is stable in neutral or acid solutions but is hydrolysed
by alkali. It is inactivated by inert clay and by anionic
surfactants. Diquat dibromide has the following chemical
structure:
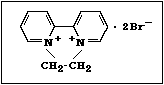 Diquat is generally marketed as an aqueous solution of the
dibromide salt Reglone(R) (200 g ion/1itre). It is a dark
reddish-brown liquid containing wetting agents that remains stable
in the original polyethylene containers, for a long time, under
normal atmospheric conditions.
Water-soluble granules containing 2.5% diquat and 2.5% paraquat
are used in home gardens. Diquat is sold under several different
trade names: Deiquat, Aquacide, Dextrone, Reglox, Weedtrim-D
(Vanholder et al., 1981). Fletcher (1975) listed the commercial
forms of diquat, many of which are combinations containing paraquat
or other herbicides.
2.2. Analytical Procedures
The detection of diquat depends on its reduction to the free
radical with sodium dithionite (Summers, 1980). Calderbank & Yuen
(1966) developed a column chromatographic procedure for
colorimetric diquat determinations in food and biological tissues.
The sensitivity of the method varied down to 0.01 mg/kg. An
immunological assay of diquat was published by Williams et al.
(1976). The minimum detectable quantity of diquat was 60 pg/ml.
Pyl & Giebelmann (1978) proposed a thin-layer chromatographic
method for diquat determinations with a detection threshold of
0.5 - 1 µg diquat.
Soil
Diquat residues in soil have been determined using
spectrophotometric analysis (ICI, 1972), the detection limit being
approximately 0.1 mg/kg, depending on the sample. An extraction
technique for the spectrophotometric measurement of diquat has been
published by Leary (1978).
Water
Diquat residues in water have been determined
spectrophotometrically with a limit of detection < 0.001 -0.01
mg/litre (ICI, 1972a). Benecke (1977) used the inhibition of algal
trichome movements by diquat involving photoelectric detection of
their inhibition. A concentration of 1 µg diquat in the test
sample was satisfactorily detected. A Lemna minor bioassay was
reported by O'Brien & Prendeville (1978) for diquat determination
in water. The minimum diquat concentration that could be detected
ranged from 1.8 µg/ml after 3 h of treatment to 0.00018 µg/ml after
72 h of treatment.
Plants and food
The method of Calderbank & Yuen (1966) has been used for
determining diquat in crops and animal tissues with detection
limits of 0.1 mg/kg to 0.01 mg/kg, depending on the sample (ICI,
1972b). Leary (1978) developed a spectrophotometric procedure for
diquat determination in crops and animal tissues (but not for whole
blood). The detection limit was 0.01 mg/kg when a 50 g sample was
taken.
A gas-chromatographic method for determining diquat residues
was published by King (1978). The detection limit was 0.01 mg/kg.
The application of gas chromatography in the analysis of food for
diquat has been discussed by Dickes (1979).
Biological tissues
The analytical method for diquat residues in milk is
spectrophotometry (ICI, 1972a), with a detection limit of 0.01
mg/litre. Tompsett (1970) reported a cation exchange technique for
colorimetric diquat determination in biological fluids and tissues
of patients with diquat poisoning. This technique is similar to
those applied for paraquat determination but more time-consuming.
A spectrophotometric procedure for diquat determination in serum,
urine, and biological tissues has been published by Leary (1978).
Gas-chromatographic analysis of herbicides containing diquat
dibromide and paraquat dichloride in forensic toxicology was
proposed by Ukai et al. (1977). The procedure was found to be well
suited for assaying diquat and paraquat simultaneously at 10 - 90
mg/litre.
3. SOURCES IN THE ENVIRONMENT
3.1. Production and Uses
Diquat is manufactured in the United Kingdom and does not occur
naturally. It is produced by the oxidative coupling of 2 molecules
of pyridine over a heated Raney nickel catalyst to 2,2'-bipyridyl.
It is then reacted with ethylene dibromide in water to give diquat.
Formulations of diquat dibromide are used in more than 100
countries all over the world, mainly as a desiccant but also as a
herbicide. In many countries, diquat is formulated locally on the
basis of the imported active ingredient. Data on world production
and uses are not available.
It is used to control both broad-leaved weeds among crops and
submerged and floating weeds in water bodies, for potato haulm
destruction, and for seed crop desiccation (rice, sunflower, etc.).
Application rates are usually of the order of 0.56 - 0.84 kg/ha for
potato haulm destruction, 0.42 -1.96 kg/ha for seed crop
desiccation, pre-harvest rice desiccation, and pre-crop weed
control (beans, beetroots, cabbages, onions, etc.), 0.42 - 1.12
kg/ha for aquatic weed control, and 0.28 - 0.84 kg/ha for pre-plant
weed control. Working dilutions vary between 1 and 5 g/litre water.
It is applied by ground sprayers (not mist-blowers) in 200 - 500
litres of the solution per hectare and in some countries aerially
in 40 - 50 litres of solution per ha.
Conning et al. (1969) summarized the mechanism of the
herbicidal effect of diquat. Light and oxygen are required for the
damage, which affects only the green parts of the plant. The
blockage of photosynthesis is due to disturbed photosynthetic
electron transport resulting from a single-electron redox cycling
reaction, as described for paraquat (Paraquat, section 3.3).
<4. ENVIRONMENTAL DISTRIBUTION, LEVELS, AND EXPOSURE
4.1. Photochemical and Microbial Degradation of Diquat
4.1.1. Photochemical degradation
In agricultural practice, most of the diquat spray is initially
deposited on plant surfaces and part of it on the soil surface.
According to Black et al. (1966), photochemical degradation is
responsible for the rapid decrease in the concentration of diquat
following the spraying of herbage. Application of 0.284 kg/ha
resulted in 12 - 48 mg diquat/kg dry herbage on the first day,
2.5 - 10.9 mg/kg after 3 - 4 days, and 1.0 - 5.7 mg/kg, 7 days
after treatment. Photochemical degradation appears to occur more
rapidly in the case of diquat than in the case of paraquat. The
light absorption maximum for diquat occurs at a longer wavelength
(310 nm) than for paraquat (256 nm), and this partly explains the
high rate of photochemical decomposition in the case of diquat.
The major degradation products have been identified; they appear to
be of low oral toxicity for rats and seem unlikely to produce
adverse environmental effects (Black et al., 1966). Cavell (1979)
monitored the photochemical degradation of 14C-diquat in aqueous
solutions aerated for 40 h. Decomposition of diquat continued
after the plants were dead and the degradation products were not
translocated from the desiccated leaves of the plants. Diquat
photochemical degradation products (Cavell, 1979) are shown in Fig.
1A.
Diquat is generally marketed as an aqueous solution of the
dibromide salt Reglone(R) (200 g ion/1itre). It is a dark
reddish-brown liquid containing wetting agents that remains stable
in the original polyethylene containers, for a long time, under
normal atmospheric conditions.
Water-soluble granules containing 2.5% diquat and 2.5% paraquat
are used in home gardens. Diquat is sold under several different
trade names: Deiquat, Aquacide, Dextrone, Reglox, Weedtrim-D
(Vanholder et al., 1981). Fletcher (1975) listed the commercial
forms of diquat, many of which are combinations containing paraquat
or other herbicides.
2.2. Analytical Procedures
The detection of diquat depends on its reduction to the free
radical with sodium dithionite (Summers, 1980). Calderbank & Yuen
(1966) developed a column chromatographic procedure for
colorimetric diquat determinations in food and biological tissues.
The sensitivity of the method varied down to 0.01 mg/kg. An
immunological assay of diquat was published by Williams et al.
(1976). The minimum detectable quantity of diquat was 60 pg/ml.
Pyl & Giebelmann (1978) proposed a thin-layer chromatographic
method for diquat determinations with a detection threshold of
0.5 - 1 µg diquat.
Soil
Diquat residues in soil have been determined using
spectrophotometric analysis (ICI, 1972), the detection limit being
approximately 0.1 mg/kg, depending on the sample. An extraction
technique for the spectrophotometric measurement of diquat has been
published by Leary (1978).
Water
Diquat residues in water have been determined
spectrophotometrically with a limit of detection < 0.001 -0.01
mg/litre (ICI, 1972a). Benecke (1977) used the inhibition of algal
trichome movements by diquat involving photoelectric detection of
their inhibition. A concentration of 1 µg diquat in the test
sample was satisfactorily detected. A Lemna minor bioassay was
reported by O'Brien & Prendeville (1978) for diquat determination
in water. The minimum diquat concentration that could be detected
ranged from 1.8 µg/ml after 3 h of treatment to 0.00018 µg/ml after
72 h of treatment.
Plants and food
The method of Calderbank & Yuen (1966) has been used for
determining diquat in crops and animal tissues with detection
limits of 0.1 mg/kg to 0.01 mg/kg, depending on the sample (ICI,
1972b). Leary (1978) developed a spectrophotometric procedure for
diquat determination in crops and animal tissues (but not for whole
blood). The detection limit was 0.01 mg/kg when a 50 g sample was
taken.
A gas-chromatographic method for determining diquat residues
was published by King (1978). The detection limit was 0.01 mg/kg.
The application of gas chromatography in the analysis of food for
diquat has been discussed by Dickes (1979).
Biological tissues
The analytical method for diquat residues in milk is
spectrophotometry (ICI, 1972a), with a detection limit of 0.01
mg/litre. Tompsett (1970) reported a cation exchange technique for
colorimetric diquat determination in biological fluids and tissues
of patients with diquat poisoning. This technique is similar to
those applied for paraquat determination but more time-consuming.
A spectrophotometric procedure for diquat determination in serum,
urine, and biological tissues has been published by Leary (1978).
Gas-chromatographic analysis of herbicides containing diquat
dibromide and paraquat dichloride in forensic toxicology was
proposed by Ukai et al. (1977). The procedure was found to be well
suited for assaying diquat and paraquat simultaneously at 10 - 90
mg/litre.
3. SOURCES IN THE ENVIRONMENT
3.1. Production and Uses
Diquat is manufactured in the United Kingdom and does not occur
naturally. It is produced by the oxidative coupling of 2 molecules
of pyridine over a heated Raney nickel catalyst to 2,2'-bipyridyl.
It is then reacted with ethylene dibromide in water to give diquat.
Formulations of diquat dibromide are used in more than 100
countries all over the world, mainly as a desiccant but also as a
herbicide. In many countries, diquat is formulated locally on the
basis of the imported active ingredient. Data on world production
and uses are not available.
It is used to control both broad-leaved weeds among crops and
submerged and floating weeds in water bodies, for potato haulm
destruction, and for seed crop desiccation (rice, sunflower, etc.).
Application rates are usually of the order of 0.56 - 0.84 kg/ha for
potato haulm destruction, 0.42 -1.96 kg/ha for seed crop
desiccation, pre-harvest rice desiccation, and pre-crop weed
control (beans, beetroots, cabbages, onions, etc.), 0.42 - 1.12
kg/ha for aquatic weed control, and 0.28 - 0.84 kg/ha for pre-plant
weed control. Working dilutions vary between 1 and 5 g/litre water.
It is applied by ground sprayers (not mist-blowers) in 200 - 500
litres of the solution per hectare and in some countries aerially
in 40 - 50 litres of solution per ha.
Conning et al. (1969) summarized the mechanism of the
herbicidal effect of diquat. Light and oxygen are required for the
damage, which affects only the green parts of the plant. The
blockage of photosynthesis is due to disturbed photosynthetic
electron transport resulting from a single-electron redox cycling
reaction, as described for paraquat (Paraquat, section 3.3).
<4. ENVIRONMENTAL DISTRIBUTION, LEVELS, AND EXPOSURE
4.1. Photochemical and Microbial Degradation of Diquat
4.1.1. Photochemical degradation
In agricultural practice, most of the diquat spray is initially
deposited on plant surfaces and part of it on the soil surface.
According to Black et al. (1966), photochemical degradation is
responsible for the rapid decrease in the concentration of diquat
following the spraying of herbage. Application of 0.284 kg/ha
resulted in 12 - 48 mg diquat/kg dry herbage on the first day,
2.5 - 10.9 mg/kg after 3 - 4 days, and 1.0 - 5.7 mg/kg, 7 days
after treatment. Photochemical degradation appears to occur more
rapidly in the case of diquat than in the case of paraquat. The
light absorption maximum for diquat occurs at a longer wavelength
(310 nm) than for paraquat (256 nm), and this partly explains the
high rate of photochemical decomposition in the case of diquat.
The major degradation products have been identified; they appear to
be of low oral toxicity for rats and seem unlikely to produce
adverse environmental effects (Black et al., 1966). Cavell (1979)
monitored the photochemical degradation of 14C-diquat in aqueous
solutions aerated for 40 h. Decomposition of diquat continued
after the plants were dead and the degradation products were not
translocated from the desiccated leaves of the plants. Diquat
photochemical degradation products (Cavell, 1979) are shown in Fig.
1A.
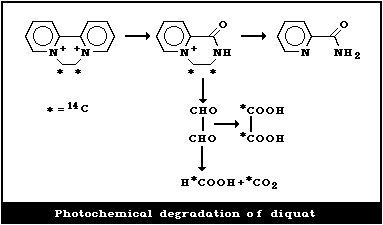 4.1.2. Microbial degradation
Photochemical degradation of diquat on plants is quicker than
microbial degradation in soil. Microbial degradation of strongly-
bound diquat in soil is slow, but is faster in culture. The
degradation of diquat by soil fungi was studied by Smith et al.
(1976). The degradation of 14C-diquat to 14CO2 by Aspergillus
niger was tested by 4 different fungal test systems. High
intracellular herbicidal levels and inability to grow in the
presence of low diquat concentrations in the media characterized
the species unable to decompose diquat. Under laboratory
conditions, diquat degradation by Pseudomonas started after 3 days
(Tchipilska, 1980). Under field conditions, degradation started
after 10 days, and was related to the ambient temperature, and the
aeration and type of soil.
The fact that no significant hazard has been observed for
ruminants from diquat-treated herbage, or for the general
population from crops and water, is explained by the rapid
photochemical degradation of diquat.
4.2. Diquat Adsorption, Residue Levels, and Exposure in Soil
4.2.1. Diquat adsorption on soil particles
Diquat binds readily to clay particles in the soil. The rate
of adsorption depends on the degree of contact of diquat with
adsorbent minerals, the type of soil, and the initial herbicide
concentrations tested. Weber et al. (1965) studied the effects of
temperature and exposure time on diquat adsorption by
montmorillonite, kaolinite, charcoal, and an anionexchange resin in
pH 6.0 phosphate buffer. Diquat was preferably adsorbed on the
clay particles by a process of ion exchange. Adsorption was
limited by the cation-exchange capacity of the test systems
examined. Coats et al. (1966) showed the adsorption capacity of
kaolinite to be about 2 g/kg and that of bentonite 80 - 100 g/kg.
A diquat soil concentration of 0.1 mg did not produce any
significant reduction in the dry weight of wheat grown in the soil
(Coats et al., 1966). The diquat appeared to be too tightly
adsorbed to the surface and between the lattices of bentonite to be
available to the wheat plant, at a soil treatment rate of 50 g/kg.
Data for diquat adsorption on sandy soils (Tucker et al., 1967)
showed that the herbicide was bound to different extents, according
to the structure of the soil particles.
4.2.2. Residue levels of diquat in soils
Makovskii (1972) reported on diquat residues in soils from
different plots, treated every year for a period of 7 years. There
were 3 - 4 treatments per season, at approximately 27.5 kg
diquat/ha. Samples were taken at 0 - 10 cm, 10 - 20 cm, and 20 -
30 cm depths in the soil; total diquat residues were shown to be
about 5.4 mg/kg soil, the mean values being 3.9 mg/kg, 1.3 mg/kg,
and 0.2 mg/kg in the respective soil layers. No diquat residues
were discovered in plants and citrus fruits sampled at different
times from the treated plots. In other studies, soil was analysed
for diquat residues on the 1st, 8th, and 15th days after applying
Reglone(R) at 0.8 litre/ha and 0.4 litre/ha (Tchipilska, 1980). On
the 1st day, residues of 0.400 mg/kg and 0.126 mg/kg were detected;
on the 8th and l5th days residues in the treated plots were lower
than 0.1 mg/kg.
As summarized in section 4.1.2, free diquat is degraded by a
range of microorganisms. While degradation of strongly-absorbed
diquat is relatively slow, results of long-term field studies have
nevertheless shown degradation rates of the order of 5 - 10% per
year. This is greater than the rate required to prevent saturation
of the deactivation capacity of soils.
In a long-term trial on a loamy soil, plots were treated with
0, 90, 198, and 720 kg diquat/ha, which was incorporated to a depth
of 15 cm. These rates were equivalent to 0, 50, 110, and 400% of
the soils strong absorption capacity (Gowman et al., 1980;
Wilkinson, 1980; Riley 1981). Over the 7 years, diquat residues
declined by 5% per year (sig P = 0.05) on the 90 kg/ha plots and
by 7% per year (sig P = 0.01) on the 198 and 720 kg/ha plots. The
rate of decline on the 198 and 720 kg/ha plots were significantly
greater ( P = .01) than on the 90 kg/ha plots.
4.2.3. Effect of residual diquat on soil biological activity, on
plants, and crop yields
A literature review and an extensive study of the effects of
different concentrations of diquat on microorganisms (saprophyte
and pathogenic microflora, and fungi) were carried out by
Tchipilska (1980). Staphylococcus aureus growth was inhibited
while Scenedesmus acutus was stimulated. Smith et al. (1981)
examined the effects of diquat applied at 0.5 - 32 times the
concentration recommended in agricultural practice on vesicular
arbuscular endophyte spore abundance in the soil and on the
infection of wheat roots. No measurable deviations in
endomycorrhiza formation and function were noted at normal
application rates. Loss of potassium and phosphate from fungi was
recorded at higher concentrations of diquat.
Coats et al. (1966) studied the uptake and translocation of
14C-diquat from soil into wheat. No metabolites were found in the
plants.
Diquat does not appear to have any significant influence on the
normal microbial activity that is important for soil fertility.
Nor is there any evidence that the recommended application rates
for diquat lead to residual effects on crop growth. Moreover,
tightly adsorbed diquat in soil is not reactivated into a
biologically active form, so that, in practice, accidental spillage
is probably the only cause of local high phytotoxic levels of
residual herbicide.
4.3. Diquat Transformation, Residue Levels, and in Effects on
Aquatic Organisms and Crops
4.3.1. Transformation and residue levels of diquat in water
In static water, initial diquat concentrations of 0.5 - 1.0
mg/litre fell rapidly to 0.1 - 0.3 mg/litre after 4-7 days
(Calderbank, 1972; Calderbank & Slade, 1976). In field
experiments, initial concentrations of 1.0, 0.8, and 0.5 mg/litre
decreased to 0.03 - 0.003 mg/litre after 7 - 14 days. This rapid
loss of diquat from treated waters was due to rapid uptake by
aquatic weeds. Two weed species (Myriophyllum spicatum and
Callitriche stagnalis) were immersed in water containing 1.0 mg
diquat/litre. The concentration of the herbicide decreased rapidly
to 0.14 - 0.03 mg/litre during a period of 6-14 days after
treatment. At the end of the experiment, the residue levels in
the weeds ranged from 6.2 - 17.4 mg/kg. In addition to uptake
by weeds, loss of diquat from treated waters was due to
photodegradation at the water surface and adsorption by bottom mud.
In field experiments carried out in 1010 m2 ponds with an initial
concentration of diquat of 2 mg/litre water, there were no residues
of diquat in the water after 8 days (Calderbank, 1972; Calderbank &
Slade, 1976).
In pond water that had been treated with diquat at 2.5 mg/litre
(Grzenda et al., 1966), residues of 0.01 - 0.08 mg/litre were found,
7-9 days after applying the herbicide, and no residues could be
determined after 14-30 days. The authors concluded that, compared
with other herbicides, diquat appeared to have the greatest
potential for use in sources of potable water.
The data obtained from studies in ponds, large and small lakes,
canals, and reservoirs demonstrate the fast disappearance of diquat
from treated waters (Calderbank, 1972). Absorption by aquatic
weeds explains the high efficacy of the herbicide. Decomposition
of the dead weeds is rapid, and diquat is not released from the
bottom mud back into the water. Applications of paraquat and
diquat each at a dose level of 1.1 kg/ha (Grover et al., 1980)
proved very effective for the control of weeds in irrigation
ditches, and the residual levels of both herbicides decreased
rapidly.
4.3.2. Effects of residual diquat on aquatic organisms and crops
The toxicity of diquat for fish varied with the species, the
size of the fish, and the softness or hardness of the water. The
LC50 values range from 12 to 90 mg/litre (24 h), 6 to 44 mg/litre
(48 h), and 4 to 36 mg/litre (96 h) (Calderbank, 1972). Reviews of
the effects of diquat on fish, aquatic invertebrates,
microbiological organisms in the soil of lakes, and phytoplankton
demonstrate that the herbicide, applied at the rates used for
aquatic weed control, did not affect estuarine fauna, oysters,
shrimps, water insects, or fish-food organisms (Calderbank, 1972;
Atkinson, 1973). At concentrations of 1 - 100 mg/litre, diquat
appeared to be less toxic for carp fingerlings than paraquat,
diuron, simazine, and dalapon (Singh & Yadev, 1978). Reish et al.
(1979) reviewed the effects of diquat on marine organisms; no
bioaccumulation by estuarine and marine organisms was found. The
toxicity of diquat for fish is low, and the main risk for aquatic
organisms and fish from its use as an aquatic weed killer is the
decreased oxygen concentration following the decay of weeds.
Trout exposed to 1 mg diquat/litre for 7 days contained
residues of 0.3 - 0.4 mg/kg in the gut, liver, and kidney, and of
0.1 - 0.3 mg/kg in the skin and gills. Residues were below the
limit of detection in muscle, spleen, and heart (Calderbank, 1972).
Trout exposed to 1 mg diquat/litre for 16 days contained residues
of 0.5 - 0.6 mg/kg, which disappeared when the fish were returned
to fresh water.
Because of irreversible adsorption, low residues in water will
be lost on contact with soil. The herbicide is thus unavailable to
plant roots. However, in overhead irrigation experiments, the use
of water containing diquat at 0.1 - 0.5 mg/litre (Calderbank, 1972)
resulted in diquat residues in the crops (tomato, lettuce, sugar
beet) ranging from less than 0.01 mg/kg to 0.04 - 0.07 mg/kg.
Thus, before using herbicide-treated waters for overhead plant
irrigation, it is advisable to allow 10 days for the diquat aquatic
residues to drop to acceptable levels.
The maximum diquat residues in water ultimately to be used for
drinking were 0.03 - 0.01 mg/litre, at the points of entry into the
public distribution system, 2-4 days after treatment; no residues
were detectable on the 10th day after applying diquat as an aquatic
herbicide. More often than not, residue levels were below the
detection limits of the analytical methods used.
4.4. Diquat Exposure and Residue Levels in Plants and Animals
4.4.1. Plants
Diquat is largely used as a desiccant in silage production. At
the recommended rates of 1.5 - 3.0 litre Reglone(R)/ha, diquat
residues were very low (Riley & Gratton, 1974). Following pre-
harvest desiccation of fodder crops, they ranged from below 0.05
mg/kg to 50 mg/kg, most of the levels determined being below 25 mg
diquat/kg (FAO/WHO, 1971, 1973). Diquat residues in the treated
herbage, sampled at different intervals after spraying with 0.258 -
0.515 mg/ha, were relatively high after 1 day (12 - 65 mg/kg), but
after 7 days had markedly decreased (1.0 - 6.5 mg/kg) (Black et
al., 1966). The levels of diquat found in silage during a 4-year
trial, with application rates of 0.190, 0.258, and 0.540 mg/ha,
varied from 1.4, 3.6, 9.3, and 13.3 to 26.8 mg/kg. The differences
were due to the atmospheric conditions at the time of desiccation
and the consequent degree of photochemical degradation of the
diquat. For this reason, diquat residues in treated herbage should
be expected to vary by an order of magnitude (10 times).
Pre-harvest desiccation of rape-seed with diquat did not result
in any detectable residues in the extracted oil and only low
residues (0.3 - 2 mg/kg) in the meal cake. Rape plants were
sprayed with 14C-diquat at 0.3 - 1.1 kg/ha, 3-14 days before
harvesting. There were no detectable residues of diquat or of its
photodegradation products in the rape-seed oil when the seeds were
harvested 7 days after desiccation, and very low diquat residues
(0.02 - 0.003 mg/kg) were determined when the seeds were harvested
14 days after treatment with diquat. The diquat residues in the
meal cake varied from 1.49 to 10.2 mg/kg, 14 days after treatment,
a large proportion being unchanged diquat (FAO/WHO, 1973).
Dembinski et al. (1971) reported diquat residues of 2 mg/kg in
sunflower seeds desiccated with Reglone(R).
Makovskii (1972) reported the diquat residue levels in weeds
treated with Reglone(R). After applications of Reglone (R) at
0.5, 1.0, and 1.3 litre/ha, the residues in dry weeds, ranged from
34 to 74 mg/kg 1 h later; from 15 to 26 mg/kg after 1 day; from
undetectable to 10 mg/kg after 4 days; from 2.8 to 3.5 mg/kg after
2 weeks; from 1.9 to 2.3 mg/kg after 4 weeks; and from undetectable
to 1.7 mg/kg after 6 weeks. The degradation of diquat in plants
was more rapid than the degradation of paraquat. The residues in
potatoes did not exceed 0.08 mg/kg, when diquat was used to destroy
potato haulm, and levels in fruits (apples, pears, plums, citrus),
tea, and cereals were undetectable (< 0.01 mg/kg), when diquat was
applied as a herbicide for weed control. Samples of potatoes
purchased from shops (Andersson & Josefsson, 1982) were analysed
for diquat residues. Residues in the range of 0.004 - 0.039 mg/kg
were found in 20 of 23 samples obtained from commercial growers.
None of the samples contained more than the residue tolerance of
0.1 mg/kg accepted for potatoes in Sweden.
Residue levels of diquat have been discussed in more detail by
the Joint Meeting on Pesticides Residues (FAO/WHO, 1971, 1973).
Residue levels of diquat in plants were summarized and published by
FAO/WHO (1977a). Some of these data are given in Table 2.
Data on diquat residues in desiccated wheat collected from 6
countries showed a mean of 0.5 mg/kg (FAO, 1979).
Table 2. Diquat residues in plantsa
------------------------------------------------------------
Plants Dose of diquat Mean value of
(kg/ha) residues (mg/kg)
------------------------------------------------------------
Wheat (grain, flour) 0.6 - 1.0 0.61, 0.22
Rice (with husk, polished) 0.2 - 0.4 0.89, 0.07
Sorghum (grain) 0.4 - 0.6 0.81
Cotton (grain) 0.4 - 1.0 0.37
Potato 0.6 - 1.0 0.03
Beans 0.3 - 1.0 0.10
Peas 0.3 - 1.0 0.05
Sugar beet (juice) 0.3 - 0.8 < 0.01
------------------------------------------------------------
a From: FAO/WHO (1977a).
4.4.2. Animals
Sheep and cattle fed silage containing diquat residues of up to
13 mg/kg were studied by Black et al. (1966). The total diquat
excreted in the urine was 0.19 - 0.65 mg over an 8-day period. No
diquat residues were detected in the brain, liver, and kidney of
sheep, or in the meat or organs of cattle fed diquat-treated silage
for one month. Milk collected on alternate days for 2 weeks was
free of diquat residues (< 0.003 mg/litre).
Feeding trials with sunflower seed containing approximately
0.20 mg diquat/kg were reported by Dembinski et al. (1971).
Although the amount of diquat consumed by the cattle over 257 days
ranged from 11.2 mg to 184.2 mg, no residues were found in any of
the milk samples analysed. Wethers fed ground sunflower seed
containing approximately 0.20 mg diquat/kg for 141 days were
estimated to have consumed a total of 14.1 mg diquat per sheep. No
residues were found in brain, liver, or kidney, nor were there any
residues in the meat, lungs, and kidney of steers treated with
diquat-desiccated sunflower forage. In long-term feeding trials
with silage, desiccated grass, lucerne, clover hay, barley straw,
and sunflower seeds containing diquat residues ranging from 0.2 to
50 mg/kg, the residues in milk and meat were determined to be less
than 0.007 mg/litre and less than 0.0006 mg/kg, respectively
(FAO/WHO, 1971, 1973, 1977a,b). Calderbank (1972) reviewed the
effects on farm animals of diquat in the drinking-water and on
herbage; there were no adverse effects on cattle and sheep and only
very low residue levels in milk, meat, and the organs analysed.
Lavaur et al. (1979) studied the effect of treated lucerne on
rabbits. Immediately after spraying, a concentration of 211 mg
diquat/kg dry weight was determined in the lucerne. After 24 h and
48 h, diquat residues were 97 mg/kg and 25 mg/kg, respectively. No
signs of poisoning or gastrointestinal damage were found in the
rabbits fed with different levels of diquat residues in the
lucerne. However, in some circumstances, lack of careful
organization may result in adverse effects of diquat on animals.
Intoxication of sheep, cattle, and swine has been reported (Schultz
et al., 1976) after the aerial application of Reglone(R) as a
rapeseed desiccant. The clinical course and the causes of the
accident stressed the need for proper diquat application by air.
For a more detailed discussion of the fate of diquat residues
in exposed animals, refer to FAO/WHO (1977a,b).
4.5. Diquat Levels in Air and Exposure of Workers
Experiments with 14C-diquat demonstrated that it was not
volatile (Coats et al., 1966). Diquat levels in air after spraying
with aerosols were determined by Makovskii (1972), using the method
of Calderbank & Yuen (1966). The application rates were 1.0 - 1.3
kg diquat/ha in working dilutions of 2.5 g and 3.3 g active
ingredient/litre, the highest diquat concentrations being found in
the tractor cabin when the door was open and spraying was in
progress in the direction of the wind (Table 3). The diquat
concentrations in air decreased rapidly 10 - 20 min after
completion of the treatment.
Wojeck et al. (1983) reported that diquat was determined in air
samples taken near the breathing zone of workers during its
application for aquatic weed control. The respiratory exposure
levels were below the limits of quantitation of the chemical
analysis.
In Bulgaria and the USSR, the proposed MAC (maximum allowable
concentration) for diquat is 0.1 mg/m3 aerosol. The TLV for diquat
in workroom air in the United Kingdom and the USA is 0.5 mg
diquat/m3 (1982), a level that will not be reached under normal
conditions of application.
Table 3. Total airborne diquat concentrations in the air of working areasa
---------------------------------------------------------------------------
Place of sampling Number of Mean concentrations
samples (mg/m3 ± SE)
---------------------------------------------------------------------------
Working area sprayer loading 20 0.12 ± 0.03
tractor cabin 8 0.56 ± 0.10
(in direction of wind)
tractor cabin 8 0.17 ± 0.04
(against the wind)
manual spraying 16 0.25 ± 0.04
Treated field after 5 min 8 0.20 ± 0.03
after 10 min 24 0.06 ± 0.01
after 20 min 8 ND
Distance from 200 m 8 0.09 ± 0.01
treated field 400 m 8 ND
---------------------------------------------------------------------------
a From: Makovskii (1972).
5. KINETICS AND METABOLISM
5.1. Animal Studies
5.1.1. Absorption
Oral absorption
Daniel & Gage (1966) studied the absorption of 14C-diquat
dibromide and 14C-diquat dichloride following oral and subcutaneous
single-dose administration to rats. About 90 - 97% of the oral
diquat dibromide and 84 - 90% of the diquat dichloride were found
in the faeces and 4 - 11% of both diquat salts in the urine.
Following subcutaneous injection of 14C-diquat (10 mg/kg body
weight) in rats, 87% of the administered dose was excreted in the
urine and 5% in the faeces within 4 days. The urine contained
mainly unchanged diquat (75% of the dose) together with diquat
monopyridone (about 3% of the dose) and diquat dipyridone (about 6%
of the dose) (FAO/WHO, 1978).
The poor absorption of diquat from the gastrointestinal tract
was confirmed by Litchfield et al. (1973) in the rat, and by Black
et al. (1966), Stevens & Walley (1966), and Dembinski et al. (1971)
in farm animals.
Pulmonary absorption
The uptake of 14C-diquat by perfused rat lung, following
intratracheal injection, was examined by Charles et al. (1978) and
Charles & Menzel (1979). Removal of 14C-diquat from the airways
was rapid, initially, but slowed down with time. The results
indicate 2 phases of absorption and removal of diquat from the
airways in the rat.
Dermal absorption
There are no data on the rate of diquat absorption through the
skin. Studies on the dose-related percutaneous toxicity of diquat
suggest that it may be dermally absorbed.
5.1.2. Distribution
Although paraquat and diquat have similar chemical, physical,
and herbicidal properties, only paraquat has been shown to damage
the lung. According to Sharp et al. (1972), diquat concentrations
in lung and muscle were much lower than the levels attained with
equal 20 mg/kg body weight iv doses of paraquat. Table 4 shows the
distribution of both in the main internal organs.
Table 4. Ratio of concentration of paraquat/diquat in
the tissues of the rata
--------------------------------------------------------
Organ Days after intravenous administration
1 3 5 7 10
--------------------------------------------------------
Lung 8 33 12 10 20
Muscle 2 13 10 7 16
Kidney 0.9 0.9 0.9 0.3 0.25
Liver 0.4 0.7 0.7 0.5 0.2
--------------------------------------------------------
a From: Sharp et al. (1972).
Diquat concentrations were higher in the kidney and the liver
but significantly lower in the lung (Table 4). In addition, the
concentrations of paraquat were 2-8 times higher than those of
diquat in the heart, adrenal glands, spleen, stomach, ileum,
testes, and thymus. Plasma levels were similar for both
bipyridylium herbicides.
Litchfield et al. (1973) injected 14C-diquat cation at 50 mg/kg
body weight iv into mice. Whole-body autoradiographs were prepared
after 10 min, 1 h, 24 h, and 72 h. Radioactivity was selectively
located in the gall bladder and was also present in cartilaginous
tissue, liver, and the gastrointestinal tract. Low radioactivity
was found in the brain and spinal cord. One h after dosing, the
amount in the urine and intestinal epithelium had increased. After
24 h, the excretion of diquat was virtually complete, although
radioactivity continued to be detected in the small and large
intestine and the bladder.
Litchfield et al. (1973) also determined diquat levels in
various tissues of male and female rats fed a diet containing
diquat dibromide monohydrate at 250 mg/kg for 2, 4, and 8 weeks.
High levels (0.18 - 1.17 mg/kg) were found in the kidney and the
large intestine; levels in the lung ranged from < 0.05 to 0.53
mg/kg; those in the liver from 0.07 - 0.22 mg/kg, while levels in
the brain, muscle, and blood were very low. At all stages of the
study, diquat lung levels were lower than those for paraquat, the
average paraquat content in the lung (at a dose of 250 mg/kg diet)
over the 8-week period being 1.7 mg/kg and the average diquat
level, 0.2 mg/kg. No sex differences were found. Within 1 week of
return to a normal diet, diquat was below the detectable limit in
all tissues examined.
Rats given paraquat or diquat orally at 680 µmol/kg had high
kidney levels of diquat throughout the 30 h period after dosing
(Rose & Smith, 1977, 1977a). There was no significant time-
dependent increase in diquat levels in the lung, liver, brain,
adrenal glands, muscle, and plasma. These results confirmed that,
following oral dosing, the lung does not accumulate diquat. Rose &
Smith (1977) also incubated rat lung slices in 10-5M paraquat and
diquat. In contrast to paraquat, diquat did not accumulate in the
lung slices, and the compound did not accumulate significantly in
any tissue slices with the exception of those from the kidney.
These observations were confirmed by Lock (1979).
Matsuura et al. (1978) studied the distribution of orally
administered LD50 doses of diquat and paraquat in rats. Two and
24 h after dosing, there were higher concentrations of diquat in
kidney, liver, and lung than in brain, heart, the gastrointestinal
system, and blood. At equitoxic doses, levels of diquat in the
lung appeared to be lower than those of paraquat. In a similar
distribution study of the LD50 and 0.5 LD50 doses of diquat and
paraquat, Kurisaki & Sato (1979) determined the tissue
concentrations from 2 to 48 h and from 2 to 9 days after treatment.
Distribution in the lung, heart, brain, liver, and kidney of the
rats agreed with previously published data.
The results of the above studies demonstrate that diquat does
not persist as long as paraquat in the body of the rat and that it
does not accumulate in the lung.
5.1.3. Metabolic transformation and excretion
Daniel & Gage (1966) reported that the amount of 14C-diquat
excreted in rat bile during the 24 h following oral doses of 1.2 -
64 mg/kg body weight represented 1.1 - 4.8% of the dose. Small
amounts were detected in the urine, but about 70% of the diquat was
present in the faeces. In other studies (FAO/WHO, 1978), the rate
of diquat metabolism in the rat was considerably lower than
previously reported by Daniel & Gage (1966). The biliary, urinary,
and faecal excretion of 14C-labelled bipyridylium herbicides was
studied by Hughes et al. (1973) in the rat, guinea-pig, and rabbit.
14C-diquat dichloride was injected ip at dose levels of 40 µmol/kg
body weight in the rat, 13 µmol/kg in guinea-pig, and 14 µmol/kg in
the rabbit. Most of the injected diquat (82% - rat, 64% -rabbit)
was found in the urine. Rabbits metabolized 18% of the dose,
guinea-pigs 5%, and rats less than 1%. The metabolites were
similar for the 3 species. The rat excreted approximately 1.4% of
the dose in the bile, the guinea-pig 4.8%, and the rabbit 2.9%.
Stevens & Walley (1966) treated cattle orally with 14C-diquat
dibromide in doses of 4, 8, and 20 mg/kg body weight. The
radioactivity levels in the milk of the cows indicated that 0.04 -
0.15% of the ingested dose was excreted in this way. Very low
levels of diquat (0.01 mg/kg) were present in muscle tissue, 2 - 8
days after dosing. A bull calf was dosed orally with 14C-diquat
dibromide at 10 mg/kg. About 2.6% of the 10 mg/kg dose was excreted
in the urine, but the major part of the dose was excreted via the
faeces. In the calf, 24 h after dosing, the residues were 0.66
mg/kg in kidney, 0.20 mg/kg in heart and skin, 0.19 mg/kg in liver,
0.03 mg/kg in lung, testes, and serum, and 0.006 mg/kg in muscle.
Studies on rats dosed orally with 14C-diquat at 45 mg/kg body
weight or subcutaneously (sc) with 10 mg/kg body weight were
reported by FAO/WHO (1978). Rats given the oral dose excreted 6%
and 89% in the urine and faeces, respectively, within 4 days and
mainly within the first 2 days. Unchanged diquat was the major
component in both urine (5% of the dose) and faeces (about 57% of
the dose). About 5% of the oral dose was excreted as diquat
monopyridone, mainly in the faeces, while diquat dipyridone
appeared to be the major urinary metabolite. Following sc
injection, rats eliminated 87% of the dose in the urine and 5% of
the dose in the faeces within 4 days. The urine contained 75% of
the dose as diquat, about 3% as diquat monopyridone, and about 6%
as diquat dipyridone. In vitro studies have shown that the caecal
microflora of the rat can metabolize about 10% of the diquat added
in a 24-h incubation period, with the formation of some diquat
monopyridone. This observation, together with the paucity of
metabolites following ip injection, suggests that diquat is
metabolized by the gastrointestinal tract bacteria.
The oral LD50 of diquat monopyridone in the rat was more than
4000 mg/kg body weight. Oral administration of diquat monopyridone
at 1000 mg/kg body weight per day for 2 weeks did not induce any
clinical, haematological, biochemical, or histopathological
deviations in the rat. In other studies, no adverse effects were
noted after sc injection of diquat monopyridone or diquat
dipyridone in rats, but 9 animals out of a group of 10 injected
with the equivalent dose (16 mg/kg body weight) of diquat were dead
by the l4th day following dosing (FAO/WHO, 1978).
5.2. Observations on Man
Feldman & Maibach (1974) studied the dermal penetration of
twelve 14C-labelled insecticides and herbicides. Diquat showed a
very low rate of dermal absorption in man. No other studies on the
kinetics of diquat in volunteers have been published, but
observations are available on accidental and suicidal ingestion
(section 7). Toxicological analysisk, at the time of admission, of
the serum of a patient, who had ingested 20 ml Reglone(R), showed
a diquat level of 0.4 mg/litre (Vanholder et al., 1981). At
postmortem examination on the 5th day after ingestion,
approximately 0.20 mg diquat/kg was determined in liver, kidneys,
muscle, and eye liquid.
6. EFFECTS ON ANIMALS
6.1. Effects on Experimental Animals
6.1.1. Gastrointestinal system and liver
Investigation of the clinical signs of acute oral intoxication
by diquat (Verbetskii & Pushkar, 1968; Clark & Hurst, 1970;
Crabtree et al., 1977; Cobb & Grimshaw, 1979) have established
gastrointestinal disturbance as the major syndrome of poisoning and
as a cause of death. In both rats and guinea-pigs, the clinical
signs of acute oral poisoning (Verbetskii & Pushkar, 1968) were
dose-dependent. At doses greater than the LD50, signs of poisoning
appeared after 6 - 12 h; at lower levels, the signs were less
obvious and appeared after 1 - 2 days. Most deaths occurred on the
3rd - 9th day after oral administration. The animals lost 7 - 35%
of their initial body weight. During the first 24 h following the
oral dosing of rats with 900 µmol diquat/kg body weight (LD50), a
reduction in water intake was noted (Crabtree et al., 1977). The
animals were subdued, showed pilo-erection and loss of appetite.
At 24 h, they excreted mucoidal, ropy faeces of a characteristic
greenish-yellow or grass-green colour, this colour being due to the
reduction of diquat by intestinal bacterial metabolism. This
colour can be reproduced in vitro with fresh intestinal contents
and actively growing bacterial isolates from them (Clark & Hurst,
1970).
A significant dose-dependent accumulation of water in the lumen
of the intestines and progressive haemoconcentration were reported
(Crabtree et al., 1977) following acute diquat intoxication in
rats. It was concluded that diquat had an adverse effect on water
distribution in the body. Rapid fluid excretion following oral
diquat poisoning suggested a direct action on the stomach and
intestinal mucosa. Monkeys dosed orally with diquat ion at 100,
200, 300, and 400 mg/kg body weight (Cobb & Grimshaw, 1979) vomited
within 2 h and showed diarrhoea within 12 h of dosing. The most
severely affected became lethargic and comatose, and finally
collapsed and died, 12 - 84 h after dosing. An increased number of
polymorphonuclear leukocytes as well as increased levels of serum
urea, plasma glucose, and serum GOT and GPT activities were
determined in monkeys that died during the study. Histological
examination revealed a distended gastrointestinal tract and a
swollen caecum; the mucosa of the stomach was ulcerated and the
small and large intestines congested. Large areas of the stomach
and intestines showed necrosis and exfoliation of the epithelium
from the mucosa. The submucosa was infiltrated with lymphocytes,
and polymorphonuclear and mononuclear cells. These changes were
most severe in the intestinal villi. The death of the monkeys was
due to destruction of the epithelial lining of the gastrointestinal
tract in combination with kidney damage.
Liver
The liver was not severely affected in acute and repeated
diquat poisoning of experimental animals. High doses sometimes
resulted in histological lesions (Verbetskii & Pushkar, 1968;
Bainova, 1975), but signs of toxic hepatitis were not described.
Gage (1968a) reported stimulated NADPH oxidase activity in rat
liver microsomes in vitro after exposure to diquat.
6.1.2. Renal system
The major route of diquat elimination is through the kidneys.
High doses of diquat provoke histological and biochemical changes
in the kidneys, but the most severe damage occured in relation to
renal excretion function (Lock & Ishmael, 1979).
Kidney damage following acute and repeated diquat poisoning was
reported by Verbetskii & Pushkar (1968), Bainova (1969), Cobb &
Grimshaw (1979), Lock (1979), and Lock & Ishmael (1979). Rats,
guinea-pigs, and monkeys were investigated after oral poisoning
with the herbicide. Diquat, orally administered at 680 µmol/kg to
rats, induced a significant increase in diuresis, proteinuria, and
glucosuria after 6-24 h. Biochemical tests in vitro revealed a
decrease in N'-methylnicotinamide, but not 4-aminohippurate,
accumulation by renal cortical slices suggesting competition for
the base transport system. Stimulation of the pentose phosphate
pathway and inhibition of fatty acid synthesis were found when
diquat was added to renal cortical slices in vitro. No such
changes were noted when the renal cortical slices were prepared
from rats previously treated with diquat (Lock, 1979).
Lock (1979) also investigated the changes in several variables
and the clearance of diquat by the rat kidney after oral
administration of toxic doses (680 and 900 µmol/kg body weight).
Diquat was not bound to the proteins of the rat plasma. Active
renal secretion was confirmed by the fact that diquat was cleared
by the kidney at a slightly higher rate than inulin. In rats
treated orally with diquat at 540 µmol/kg body weight, renal
clearance decreased after 24 h. However, the reduction in renal
function induced by diquat (Lock 1979) was considered to be
secondary and due to water redistribution caused by acute
poisoning.
Histopathological changes have been reported in the kidneys of
animals poisoned with high doses of the herbicide (Verbetskii &
Pushkar, 1968; Cobb & Grimshaw, 1979; Lock & Ishmael, 1979). The
renal papillae were hyperaemic, degeneration and necrosis of the
epithelium of the proximal and distal convoluted tubules were
noted, the epithelial cells were exfoliated, and the nuclei
pycnotic.
6.1.3. Eyes and skin
Eye irritation
The local irritation caused by diquat is less pronounced than
that caused by paraquat. One drop of 20% solution gave rise to
slight conjunctival irritation of the rabbit eye, which persisted
for 2 days (Clark & Hurst, 1970). A 40% diquat solution induced
moderate conjunctival irritation.
Eye cataract
Both rats and dogs fed diets containing diquat developed
cataracts (Howe & Wright, 1965). However, rats fed 7.5 mg
diquat/kg diet over a life-span did not develop cataracts, while 70
mg diquat/kg diet appeared to be the no-observed-adverse-effect
level for dogs. According to Clark & Hurst (1970), rats on diets
containing 50 mg diquat/kg or more developed cataracts in the
course of the study. In another group fed a diet containing 1 g
diquat/kg, eye opacities were discovered within 6 months, while a
few animals on diets of 100 mg/kg and 50 mg/kg showed slight
opacities at the end of the study period. A 2-year test with a
diet containing diquat at 10 mg/kg did not induce cataracts in
rats.
Bilateral cataracts were discovered in all dogs 10 - 11 months
following oral administration of diquat at 15 mg/kg body weight per
day. The dose of 5 mg/kg body weight per day induced eye opacities
after 17 months, and doses of 1.7, 0.8, and 0.4 mg/kg body weight
per day were ineffective after 3 - 4 years of treatment.
A 2-year feeding study was carried out with diquat levels of
15, 25, and 75 mg/kg in the diet of rats. Only the 25 and 75 mg/kg
levels caused cataracts (FAO/WHO, 1978).
Pirie & Rees (1970) confirmed that rats fed diquat dibromide at
0.5 - 0.75 g/kg in the diet developed cataracts. In vivo
observations showed that, invariably, the first change seen was an
opacity in the posterior cortex, immediately under the posterior
capsule of the lens. The next stage was a defined nuclear cataract
that could be seen with the naked eye. Finally, shrinkage and
complete opacity occurred. This histological study revealed that
the first posterior cortical opacity was formed from damaged
epithelial cells. The level of diquat in the blood of these rats
was less than 2.2 µM. No diquat accumulation was registered in the
lens of these rats. The mechanism of the specific cataractogenic
action of diquat is not clear, although in vitro studies
demonstrated that reduction of diquat by the lens was enzymatically
catalysed by glutathione reductase (EC 1.6.4.2) with NADPH as the
source of reducing equivalents. The loss of ascorbic acid from the
lens and the ocular fluids of treated rats was proposed as a factor
for maintaining the normal glutathione level in the rat lens.
Local skin effects
Single diquat applications on the skin of mice (Bainova, l969a)
and rabbits (Clark & Hurst, 1970) did not cause any local
irritation. Daily applications of 1% diquat solution in water to
the skin of rats provoked slight erythema at the site of contact
during the first 10 days, while daily applications of diquat at 20
mg/kg body weight to the skin of rabbits caused mild erythema,
thickening of the skin, and some scabbing (Clark & Hurst, 1970).
Diquat has not been found to be a sensitizer (Bainova, (l969a).
6.1.4. Respiratory system
The effect of diquat on the respiratory system has been studied
after parenteral (Hawkins et al., 1979; Lam et al., 1980), oral
(Verbetskii & Pushkar, 1968; Bainova, 1969; Bainova & Vulcheva,
1978), intratracheal (Lam et al., 1980), and inhalation exposure
(Gage, 1968; Bainova et al., 1972). Unlike paraquat, no specific
effects on the lung were reported, though difficulties in breathing
occurred after severe acute poisoning of the animals with diquat.
6.1.5. Nervous system
General depression and lethargy were most commonly seen
following the administration of high doses of diquat to guinea-pigs
and rats (Verbetskii & Pushkar, 1968; Clark & Hurst, 1970; Crabtree
et al., 1977), and to monkeys (Cobb & Grimshaw, 1979).
6.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
6.1.6.1. Effects on reproduction
Male rats were dosed orally with diquat dibromide at 6.5 mg/kg
body weight per day, for 60 days, and the testes were then examined
biochemically and histologically (Bainova & Vulcheva, 1974). There
were no significant changes in the sperm count, sperm motility, the
testicular tubules, the basal cells, or in the activity of several
enzymes.
A 2-generation study on rats was carried out with dietary
levels of 125 and 500 mg diquat/kg. The 500 mg/kg dose resulted in
reduced body weight for F1a, F1b, F2a, and F2b, and increased
cataracts in F1b and F2b after 91-280 days of exposure. The 125
mg/kg dose resulted in decreased body weight in F1b and F2b, but no
lens opacities were noted (FAO/WHO, 1973).
6.1.6.2. Embryotoxicity and teratogenicity
Diquat was reported to have induced deviations in the prenatal
development of rats (Khera et al., 1968). Bus et al. (1975)
studied the fetal toxicity and teratogenicity of diquat in rats by
administering 15 mg/kg body weight iv on days 7-21 of gestation.
This resulted in 57% fetal resorption compared with 7.6% for
paraquat. The incidence of maternal deaths was essentially the
same. When 14C-diquat and 14C-paraquat were administered to rats,
iv, in a dose of 15 mg/kg body weight on days 13, 16, and 21 of
gestation, paraquat increased radioactivity in fetal lung whereas
diquat appeared to have a stronger embryotoxic action than
paraquat. In the review published in 1979 by FAO/WHO, it was
reported that diquat dibromide monohydrate, administered orally to
pregnant rabbits at doses of 1.25, 2.5, and 5.0 mg/kg had no
adverse effect on the fetuses. In groups of pregnant rats kept on
diets containing 125 and 500 mg diquat cation/kg throughout
gestation, reduced body weight was noted only in the fetuses of
mothers from the 500 mg/kg group. A slightly increased incidence
of subcutaneous haemorrhages was also noted.
Teratogenicity studies in mice have been reported by Selypes et
al. (1980). Single ip doses of diquat at 2.7 and 11 mg/kg body
weight were injected on days 9, 10, 11, and 12 of gestation. The
number of dead fetuses, as well as post-implantational lethality,
increased significantly: average embryo weight was lower and,
though no congenital malformations were noted, there were signs of
skeletal retardation such as large fontanelles, wider cerebral
sutures, flat-shaped ventral nuclei of the vertebrae, and delayed
ossification in the sternum and phalanges. The embryotoxic effect
in mice of high doses of diquat was thus confirmed, but no
chromosomal aberrations were noted in the liver cells of the
embryos from diquat-treated female mice.
6.1.7. Mutagenicity
Studies on the genotoxic potential of diquat are rather
contradictory. Diquat was negative in the Ames test, with and
without metabolic activation (Anderson et al., 1972; Benigni et
al., 1979; Levin et al., 1982). Dominant lethal assays in mice
performed by various authors with several doses of the herbicide
gave negative results (Pasi et al., 1974; Pasi & Embree, 1975;
Anderson et al., 1976). Selypes et al. (1980) injected mice ip
with 22 mg/kg (LD50) diquat, while another group of mice was dosed
orally with 90 mg/kg (0.5 LD50). After 24 and 38 h, preparations of
bone marrow were examined for chromosome aberrations; no
statistically significant changes were determined.
On the other hand, diquat was found to induce slight gene
conversion in Saccharomyces cerevisiae (Siebert & Lemperle, 1974).
Ahmed et al. (1977) reported that diquat induced DNA changes in
cultured SV-40-transformed human cells, with and without metabolic
activation, and the induction of 8-azaguanine resistance in the
Salmonella typhimurium assay was positive (Benigni et al., 1979;
Bignami & Crebelli, 1979). Benigni et al. (1979) also found that
diquat was positive in an S. typhimurium repair test. It was
further reported by these authors that diquat induced gene
mutations in Aspergillus nidulans, and increased unscheduled DNA
synthesis in human epithelial-like cells. They commented that
diquat may have an effect on a number of different genetic
endpoints.
6.1.8. Carcinogenicity
In 2-year feeding studies on rats (Clark & Hurst, 1970), diquat
at levels of up to 720 mg/kg diet did not induce tumours. The
daily ingestion of 2 and 4 mg diquat per kg body weight in water
for a period of 2 years did not have any significant effects on the
health and mortality rate in rats (Bainova & Vulcheva, 1978). Some
histological changes related to chronic interstitial infiltration
and pulmonary adenomatosis in the lungs were found, especially
after the higher dose, but there were no indications of malignancy.
6.2. Effects on Farm Animals
The effects of diquat on farm animals was studied in relation
to its application as an aquatic herbicide and desiccant (Howe &
Wright, 1965; Black et al., 1966; Stevens & Walley, 1966) (section
4.4). Little variation in diquat toxicity in the various animal
species was found, but cattle appeared to be the most sensitive
(LD50 for cattle approximately 30 mg/kg, LD50 for rat 230 mg/kg).
Single oral doses up to 8 mg/kg produced no signs of toxicity in
cows (Stevens & Walley, 1966), and the continuous exposure of
animals via the forage to doses ranging from 0.2 to 330 mg/kg in
the diet (Calderbank, 1972) did not induce any clinical or
pathological changes in farm animals.
Calderbank (1972) recommended that domestic animals should not
be allowed to enter fields newly treated with diquat, nor be given
water recently treated with the herbicide. When edible crops are
treated with diquat, as desiccant, at least 4 days should elapse
before the crops are fed to stock, and when diquat is used for
aquatic weed control, at least 7 days should elapse before the
treated water is used for field irrigation. Recommended levels for
weed control must be observed (Calderbank, 1972).
Sheep given doses of 1, 5, 10, and 20 mg diquat/kg per day in
their drinking-water for 1 month and calves similarly exposed to 5
and 20 mg diquat/kg per day did not show any adverse toxicological
effects as evidenced by growth, food consumption, and observation.
6.3. Dose-Effect of Diquat
The acute LD50 values of diquat in various species were
published by Howe & Wright (1965) and Clark & Hurst (1970). The
acute toxicity of diquat salts (Table 5) does not differ
significantly and is similar for both sexes.
Table 6 summarizes the acute oral, dermal, and inhalation LD50
and LC50 values of diquat in various experimental and domestic
animals. There are no marked species differences but cattle,
guinea-pigs, and monkeys appear to be the most sensitive species.
The few cases of acute diquat poisoning in man have not furnished
sufficient data to determine the lethal dose for man.
The dose-effect relationship of repeated diquat exposure, from
various studies, is summarized in Table 7. Rats, guinea-pigs and
dogs were subjected to ora1 and dietary administration of diquat.
Guinea-pigs appeared to be rather sensitive (Makovskii, 1972), but
the herbicide did not induce cumulative toxic effects (Bainova,
1969, 1975; Makovskii, 1972), because of its relatively rapid
elimination from the organism and the absence of deposits in the
tissues.
Table 5. LD50 (mg/kg) of diquat salts in rats
-----------------------------------------------
Diquat Route of entry Sex LD50
(mg/kg)
-----------------------------------------------
Diquat dibromide oral 215b
Diquat dibromide oral 210b
Diquat dibromide subcutaneous F 11a
Diquat dichloride subcutaneous F 10a
Diquat dichloride subcutaneous M 11a
Diquat dibromide subcutaneous 22b
-----------------------------------------------
a From: Clark & Hurst (1970).
b From: Makovskii (1972).
Table 6. Diquat LD50 (mg/kg) and LC50 (mg/m3)
in various species
----------------------------------------------
Species Oral Dermal Inhalationa
(mg/kg) (mg/kg) (mg/m3)
----------------------------------------------
Rat 400b 650f 35f
Rat 281c 83h
Rat 231e
Rat 215f
Rat 130g
Mouse 170b 430d
Mouse 125e
Rabbit 190b
Rabbit 101e > 400e
Guinea-pig 123c 400f 38f
Guinea-pig approx. 100e
Guinea-pig 100f
Hen 400 - 800b
Hen 200 - 400e
Dog > 200b
Dog 100 - 200e
Cow approx. 30f
Cow 30e
Monkey 100 - 300i
----------------------------------------------
a Respirable diquat aerosol.
b From: Howe & Wright (1965).
c From: Verbetskii & Pushkar (1968).
d From: Bainova (1969a).
e From: Clark & Hurst (1970).
f From: Makovskii (1972).
g From: Bainova (1975).
h From: Bainova & Vulcheva (1977).
i From: Cobb & Grimshaw (1979).
Table 7. Effect of repeated oral, dermal, and inhalation exposure to diquat in experimental animals
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Results obtained Reference
---------------------------------------------------------------------------------------------------------
Rat 87.5, 175, and 350 mg 2 years cataract at all dietary levels FAO/WHO (1971)
diquat ion/kg of diet
Rat 7.2, 36, 72, 180, 2 years no deaths; reduced growth in males at FAO/WHO (1971)
360, and 720 mg diquat highest dietary level; cataract at
ion/kg diet dietary levels of 36 mg diquat ion/kg
diet and above; no cataract at 7.2 mg/kg
Rat 15, 25, and 75 mg 2 years no deaths; "no effect" level for FAO/WHO (1978)
diquat ion/kg diet cataractogenesis 15 mg diquat ion/kg
diet
Rat oral - 6.5, 13, and 40 30 days dose-related biochemical and Bainova (1969,
mg/kg body weight per histological changes in kidney, liver, 1975)
day 2.1 and 4.3 mg/kg 4 1/2 months gastrointestinal system, and lung; no
body weight per day haematological changes; increased
G-6-P-isomerase serum activity;
histological changes at 4.3 mg/kg
body weight per day
Rat oral - 0.2, 2.1, and 1 year the higher doses were toxic for the Makovskii (1972)
5.3 mg/kg body weight 2 species; no-observed-effect levels
per day
Guinea- day 0.1, 1.0, and 2.5 0.2 and 0.1 mg/kg body weight per day
pig mg/kg body weight per for rat and guinea-pig
day
Dog 10, 20, 50, 140, and up to 4 no cataracts at dietary levels up to and FAO/WHO (1971)
420 mg diquat ion/kg years including 50 mg/kg; cataract at 2 higher
of diet dietary levels; no mortality; no effects
on growth
Rat oral - 2 and 4 mg/kg 1 and 2 no increase in mortality rates; Bainova &
per day years histological changes in lungs after Vulcheva (1978)
treatment with 4 mg/kg per day in
drinking-water; minimal effective dose
2 mg/kg per day
---------------------------------------------------------------------------------------------------------
Table 7. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Results obtained Reference
---------------------------------------------------------------------------------------------------------
rat dermal - 5, 10, 20 20 days slight skin irrritation, death and toxic Bainova (1969a)
60, and 120 mg/kg effects at 10 - 120 mg/kg per day;
per day dilation of the gastrointestinal system
at toxic levels; histological changes in
kidney, gastrointestinal system, liver,
and lung at toxic levels, LD50 35 mg/kg
per day without occlusion; no-observed-
effect dose 5 mg/kg per day
Rabbit dermal - 20 and 40 20 days mild skin irritation; toxic effects at Clark & Hurst
at 40 mg/kg per day; no clinical signs (1970)
of toxicity at 20 mg/kg per day; LD50
between 20 and 40 mg/kg per day
Rat inhalationa - 0.50, 15 days clinical signs of irritation and Gage (1968)
1.60, and 2.0 mg/m3 histological changes in lungs at 2mg/m3;
6 h daily no clinical haematological, and
histological deviations at 0.50 mg/m3;
minimum effective concentration 1.0
mg/m3 diquat aerosol
Rat inhalationa - 0.32 4 1/2 months biochemical and histological changes in Bainova (1972)
and 1.90 mg/m3, lungs at 1.90 mg/m3; minimal effective
6 h daily concentration 0.32 mg/m3 diquat aerosol
Rat inhalationa - 0.4, 4 months clinical signs of irritation and toxic Makovskii
0.7, and 1.9 mg/m3, effects at 1.9 mg/m3; 0.7 mg/m3 produced (1972)
4 h daily changes in some rats; minimal effective
concentration 0.4 mg/m3 diquat aerosol
---------------------------------------------------------------------------------------------------------
a Respirable diquat aerosol.
7. EFFECTS ON MAN
7.1. Case Reports
Several cases of acute diquat poisoning among the general
population have been reported in the literature. Fitzgerald et al.
(1978) found 5 cases from 1967 to 1977 in Ireland. Vanholder et
al., (1981) summarized the clinical outcome and the treatment of 11
patients with diquat poisoning (6 fatal and 5 non-fatal).
(a) Suicidal diquat poisoning
Schönborn et al. (1971) reported the fatal case of a man who
drank 2 - 3 mouthfuls of Reglone(R) (estimated 15 - 22 g diquat)
with the intention of committing suicide. Severe vomiting occurred
after 2 h and, 2 h later, watery diarrhoea, the stools having a
peculiar yellow-greenish colour. During the next 6 h, the patient
lost about 3.5 litres of liquid through faeces and 4 litres of
liquid through vomiting. The urine was very concentrated, the
haematocrit was 55%. Serum enzyme activity showed toxic liver
damage, and proteinuria and metabolic acidosis were registered. On
the 2nd day, there were ulcers and severe oropharyngeal
inflammation, on the 3rd day, increasing restlessness, optical
hallucinations, and delirium and stridulous breathing developed.
During the 4th-6th days, anuria, raised body temperature,
generalized convulsions, and coma were registered, and the patient
died on the 7th day of cardiac insufficiency and thrombocytopenia.
The autopsy revealed extensive necrosis of the pharynx and
oesophagus, and petechial bleeding and erosions in the
gastrointestinal tract; pulmonary oedema with haemorrhages, hyaline
membrane production, and bronchopneumonic foci were noted in the
lungs; fatty degeneration was found in the liver and heart, and
severe degeneration of the tubulus epithelium with necrosis in the
kidneys, while the signs of circulatory failure with oedema and
haemorrhagic diapedesis of the brain explained the central nervous
system effects. The diquat concentrations measured on the 1st day
after ingestion were 1.85 mg/litre in the urine and 0.47 mg/litre
in the blood. Higher diquat levels were determined post mortem in
the kidneys, spleen, and lungs (1.19, 1.04, and 0.56 mg/kg,
respectively).
In a second case of suicide, the subject had taken unknown
quantities of Reglone(R) during a period of 3 days (Okonek &
Hofmann, 1975). One day after the second ingestion, she was
admitted to hospital - shocked, sleepy, anuric, with haemorrhagic
mucosal necrosis in the mouth, throat, and eosophagus. Four h
after admission to hospital, the diquat serum level was 1.038
mg/litre. This decreased to 0.30 mg/litre following dialysis.
Death from cardiovascular collapse ensued 46 h after admission.
Vanholder et al. (1981) concluded, from their review of 11
cases, that the lethal dose of Reglone(R) is 30 - 60 ml or
approximately 6 - 12 g diquat dibromide.
An unusual case of diquat poisoning was described by Narita et
al. (1978). A clerk, after drinking heavily, swallowed about 200
ml 30% diquat dibromide formulation. Vomiting was accompanied by
great thirst, severe irritation of the mouth, diarrhoea, and a
temperature of 39 °C. After 24 h, the patient became anuric and
developed acute renal failure; he was comatose and inarticulate,
and had meiosis and unclear light reflexes. He died from dyspnoea
38 1/2 h after ingestion of diquat. Autopsy revealed renal failure
with tubular necrosis, lung haemorrhages, haemorrhagic ulcers, and
erosions in the stomach, and severe congestion of the lungs,
kidneys, liver, gastrointestinal system, and adrenal glands. High
diquat residues were determined in the kidneys, liver, lungs, and
intestines. Vanholder et al. (1981) reported 2 cases of Reglone(R)
ingestion (50 ml and 20 ml) in suicide attempts. Because of
vomiting and diarrhoea, they were admitted to local hospitals, but
no specific treatment was given and the patients were released in
satisfactory clinical condition. However, because of the
development of progressive oliguria several h later, the patients
returned to the hospital. The diquat serum levels were found to be
4.5 and 0.4 mg/litre, respectively. The patients died 1 and 5 days
after the ingestion of diquat.
(b) Accidental diquat poisoning
Oreopoulos & McEvoy (1969) described a patient who accidentally
took a mouthful of Reglone(R) from a soft drink bottle. He spat
out part of it. After 8-10 h, he had diarrhoea and 2 ulcers in the
mouth, but there was no clinical evidence of respiratory, renal, or
central nervous system effects on examination in hospital, and all
laboratory and biochemical examinations were within the normal
physiological limits. The patient continued to excrete diquat in
the urine for 11 days after ingestion. He underwent forced
diuresis and left the hospital in good condition.
Another case of acute poisoning following the accidental
ingestion of less than a mouthful of diquat was reported by Fel et
al. (1976). Nausea, vomiting, and diarrhoea were the first
effects. The patient then developed uraemia, oliguria, and anuria
despite forced diuresis for 2 - 3 days after the accident.
Haemodialysis proved more successful. Bilateral pneumonia was
noted during the 2nd week, but was cured with antibiotics, and the
patient was discharged on the 26th day in good health.
7.2. Effects on Agricultural Operators
A few studies have been performed on workers spraying diquat.
Air concentrations of diquat aerosol were measured by Makovski
(1972) (Table 3). The dermal exposure of the spraymen ranged from
0.05 mg to 0.08 mg on the face and hands after 2 - 3 h of daily
work. The spraymen did not have any complaints, and the clinical
and laboratory examinations did not reveal any significant
differences in comparison with control groups. Wojeck et al.
(1983) studied the exposure of workers applying 1.76% diquat by
hand-operated spray against water hyacinths or using direct
injection of 4.41% spray mixture into the water for hydrilla
control. The spray crews applied diquat 2 - 5 h daily for 4 days
weekly. The inhalatory exposure was found to be < 0.01 mg/h. The
dermal exposure of the spraymen and the airboat drivers were
estimated to be 1.82 and 0.20 mg diquat/h, during the treatment of
water hyacinths. The dermal exposures of the spraymen and the
mixer of diquat for the treatment of water hydrilla were 0.17 and
0.47 mg/h, respectively. The results of urine analysis of all
workers involved in the study were negative (< 0.047 mg/litre).
The dermal exposure to diquat was closely related to the
concentrations used in the working solutions.
Inflammation and bleeding of the nasal mucosa were observed in
people handling crystalline diquat powder in the laboratory or
under field conditions (Clark & Hurst, 1970). Epistaxis during
agricultural diquat application is related to the inhalation of
droplets or splashes from the careless mixing of liquid
concentrates. A worker who spent some considerable time in an
aerosol spray drift developed irritation of the upper respiratory
tract.
According to Clark & Hurst (1970), if a 20% diquat solution
comes into contact with the nail base, nail growth disturbances may
result, and discoloured spots, white bands, and shedding of the
nail were seen after prolonged contact with concentrated diquat.
The nail re-grew normally once exposure was discontinued. No
adverse effects on the nails were observed following the use of
diluted diquat spray solutions in agriculture. Concentrated diquat
formulations have also been reported to delay the healing of
superficial cuts on the hands of spray workers.
Cataracts have never been observed in man following exposure to
diquat (FAO/WHO, 1978; Hayes, 1982).
7.3. First Aid and Medical Treatment
These are essentially the same as those given for paraquat
(section 8.4). See also WHO/FAO (1979).
8. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
8.1. Exposure
8.1.1. Relative contributions of soil, water, air, and food sources
to total diquat uptake
Introduction
Diquat is a contact herbicide and dessicant that is used to
destroy weeds in various agricultural situations. It is used in
the form of an aqueous spray, which means that the potential
exposure of man may occur as a result of its presence in air, on
plants, in soil, or in water.
Degradation of Diquat
Photochemical degradation takes place, when diquat treated
plants are exposed to normal daylight, and continues after plants
are dead. The products formed are of lower toxicity than diquat.
The rapidity of photochemical degradation on plant and soil
surfaces minimizes the hazard of diquat for the environment.
Soil
Diquat is rapidly and tightly bound to clay particles in the
soil, and is thereafter inert. In normal agricultural use, no
toxic breakdown products are to be expected in the soil (section
4.2) where diquat is less persistent than paraquat. Total diquat
residues in the soil after repeated spraying ranged from 0.2 to 3.9
mg/kg. On the 15th day after a single application of diquat,
residues were less than 0.1 mg/kg in field studies. Even at high
rates of application, no specific adverse effects are found on soil
microorganisms, fungi, or invertebrates, and no phytotoxic effects
have been reported on crops.
Water
Following its use as an aquatic herbicide at normal application
rates, diquat residues in water have been found to decrease rapidly
to essentially undetectable levels within 7-14 days (section 4.3).
Toxic effects on fish and other living organisms in the water are
unlikely, because diquat is rapidly photodegraded, absorbed by
aquatic weeds, or adsorbed to soil particles at the bottom.
However, caution should be taken in the application of diquat to
water containing heavy weed growth, since oxygen consumed by
subsequent weed decay may decrease the oxygen content of the water
to such an extent that it is dangerous for fish or other aquatic
organisms. No phytotoxic damage should occur on crops irrigated
with diquat-treated water, if at least 10 days is allowed to elapse
between treatment and irrigation.
Air
Diquat is not volatile. Inhalation exposure can occur via
spray aerosols or contaminated dust but, if correctly applied,
diquat should not give rise to significant inhalation exposure of
the sprayers (section 4.5). Total airborne aerosol concentrations
of diquat in the air in working areas ranged from 0.06 to 0.56
mg/m3, depending on the method of application and the period of
time after the spraying.
Food
Extensive studies on forage desiccated with diquat have
demonstrated that the residues are very low within some days of the
application of the desiccant. Diquat residues in the treated
herbage following pre-harvest desiccation ranged from 0.02 to 25
mg/kg at different intervals after spraying. Trials in which such
forage was fed to cattle and sheep have demonstrated insignificant
residue levels in the milk, meat, and internal organs (section
4.4). Residues found in vegetables, fruits, and cereals have been
low. There is no bioaccumulation.
8.1.2. General population exposure
Inhalation exposure of the general population to diquat may
occur from spray drift off the treated fields, but this is thought
to be insignificant. There are no published data on total diquat
intake among the general population but this again is expected to
be insignificant on the basis of known residue levels. Studies on
its environmental distribution point to a low environmental hazard.
Due to diquat's rapid and complete binding to clay minerals in
soil, contamination of water supplies either from field runoff or
percolation through soil to the water table is not expected
(section 4.2).
Few cases of diquat poisoning have been reported (section 7.1).
Most cases are due to the intentional ingestion of concentrated
formulations, but accidental ingestion has occurred. The decanting
of liquid concentrate formulations into beer, wine, or soft drink
bottles, and subsequent inappropriate storage, is very dangerous.
The acute lethal dose of diquat dibromide is considered to be
6 - 12 g for man. Recovery from diquat poisoning depends on the
cause of ingestion, the dose absorbed, the renal damage, and prompt
initiation of therapy. No long-term adverse effects have been
reported in those who have survived acute diquat poisoning.
8.1.3. Occupational exposure
There may be inhalation, dermal, and to some extent oral
occupational exposure. Spray aerosols and dust particles settle in
the upper respiratory tract. Diquat aerosol concentrations range
from 0.06 to 0.56 mg/m3, according to the spraying method. At a
distance of 200 - 400 m from the treated field, they decrease to
0.09 mg/m3 and less than 0.01 mg/m3. Inhalation exposure was found
to be very low in comparison with dermal (0.17 - 1.82 mg/h)
exposure to diquat during application for aquatic weed control.
Skin irritation, epistaxis, nail damage, and delayed wound healing
have been reported. However, no data on severe or fatal cases of
occupational intoxication, acute ocular damage, or occupational
contact dermatitis caused by diquat were found in the literature.
8.2. Effects
8.2.1. Diquat toxicity in animals
Diquat is less toxic than paraquat and does not cause the
specific lung disease so typical of paraquat exposure.
The primary toxic effect of diquat in animals is
gastrointestinal damage resulting in diarrhoea with consequent
dehydration. After high doses of diquat, minor toxic effects have
been noted in the liver, kidney, and the nervous and endocrine
systems. High concentrations of diquat are irritating to the skin,
although less so than paraquat. Development of eye cataracts has
been reported in rats and dogs following long-term treatment with
diquat (section 6.1.3). This observation has not been reported in
man. Diquat is embryotoxic but it has not been found to be
teratogenic in rats and mice or carcinogenic in long-term feeding
studies on rats given diquat at levels up to 720 mg/kg diet
(sections 6.1.7 and 6.1.8). In vitro mutagenicity studies have
been inconclusive, although generally suggesting weak activity,
while the results of in vivo studies have been negative (section
6.1.8). Thus, the results of animal studies suggest that low-level
exposure to diquat is unlikely to induce toxic effects in man. The
no-observed-effect level in rats has been estimated to be 0.75 mg
diquat ion/kg body weight per day (FAO/WHO, 1978).
8.3. Earlier Evaluations of Diquat by International Bodies
The Joint Meeting on Pesticide Residues (JMPR) reviewed and
published residue and toxicity data on diquat in 1970, 1972, 1976,
1977, 1978 (FAO/WHO 1971, 1973, 1977a,b, 1978, 1979). In 1977, it
estimated the acceptable daily intake (ADI) for man as 0 - 0.008
mg/kg body weight expressed as diquat ion (FAO/WHO 1978).
The same JMPRs have recommended maximum residue levels
(tolerances) for diquat in food commodities of plant and animal
origin.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC are available
from the IRPTC (International Register for Potentially Toxic
Chemicals) legal file (IRPTC 1983).
A data sheet on diquat has been prepared by WHO/FAO (1979) in a
series of "Data sheets on chemical pesticides". Based on a brief
review of use, exposure, and toxicity, practical advice is given on
labelling, safe-handling, transport, storage, disposal,
decontamination, selection, training and medical supervision of
workers, first aid, and medical treatment.
8.4. Conclusions
On the basis of the above findings, it can be concluded that:
General population
Residue levels of diquat in food and drinking-water, resulting
from its normal use, are unlikely to result in a health hazard for
the general population.
Diquat has caused some fatalities following suicidal ingestion.
Occasional accidental fatalities have followed ingestion of
decanted diquat. Ill-effects similar to those caused by paraquat
occur, but the characteristic fibrosis of the lungs is not a
feature.
Occupational exposure
With reasonable work practices including safety precautions,
hygiene measures, and proper supervision, occupational exposure
during the manufacture, formulation, and application of diquat will
not cause a hazard. However, the undiluted concentrate must be
handled with great care, because contamination of eyes and skin
(with possible consequent dermal absorption) can result from
improper work practices.
Environment
Diquat in soil binds rapidly and tightly to clay particles and
residual phytotoxicity from freely available diquat is unlikely.
Under normal conditions of use, the toxicity of diquat for aquatic
organisms is low, though resulting depletion of water oxygen due to
weed decay may pose a problem. Diquat does not seem to represent
an environmental hazard.
REFERENCES
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide-
induced DNA damage and its repair in cultured human cells.
Mutat. Res., 42: 161-164.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDERSON, D., MCGREGOR, D.B., & PURCHASE, I.F.H. (1976)
Dominant lethal studies with diquat and paraquat in male CD-1
mice. Mutat. Res., 40: 349-358.
ANDERSSON, A. & JOSEFSSON, E. (1982) Residues of diquat in
potatoes. Var Föda, 34(Suppl. 3): 223-225.
ATKINSON, G. (1973) Effects of diquat on the microbiological
organisms in the soil of lakes. In: Proceedings of the
Pollution Residue Conference, Wairakei, New Zealand,
pp. 529-538.
BAINOVA, A. (1969a) [Chronic oral toxicity of bipyridylium
herbicides.] Hig. Zdrav., 12: 325-332 (in Bulgarian).
BAINOVA, A. (l969b) [Experimental assessment of the effect
of dipyridylium herbicides on the skin.] Letopisi HEI, 9:
25-30 (in Bulgarian).
BAINOVA, A. (1975) [Cumulative action of Gramoxone and
Reglone.] In: Problemi na higienata, Sofia, Medizina i
Fizkultura, Vol. 1, pp. 31-38 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1974) [Experimental assessment
of the effect of dipyridyliums on sex glands.] In: Works of
the Research Institute of Hygiene and Laboratory Protection,
Sofia, Medizina i Fizkultura, Vol. 22, pp. 111-122 (in
Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1977) [Experimental
substantiation of Reglone MAC in the working environment.] In:
Problemi na higienata, Sofia, Medizina i Fizkultura, Vol. III,
pp. 11-17 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1978) Chronic action of diquat
on the lungs. C. R. Acad. Bulgar Sci., 31: 1369-1372.
BAINOVA A., ZLATEVA, M., & VULCHEVA, V. (1972) [Chronic
inhalation toxicity of bipyridylium herbicides.] Hig. Zdrav.,
15: 25-31 (in Bulgarian).
BENECKE, G. (1977) [Automatic evaluation of an algal
bioassay-inhibition of the movement of a blue-green alga
(Phormidium sp.) by the herbicide diquat.] Z. Wasser-
Abwasserforsch., 10: 195-197 (in German).
BENIGNI, R., BIGNAMI, M., CARERE, A., CONTI, G., CONTI, L.,
CREBELLI, R., DOGLIOTTI, E., GUALANDI, G., NOVELLETTO, A., &
ORTALI, V.A. (1979) Mutational studies with diquat and
paraquat in vitro. Mutat. Res., 68: 183-193.
BIGNAMI, M. & CREBELLI, R. (1979) A simplified method for
the induction of S-azaguanine resistance in S. typhimurium.
Toxicol. Lett., 3: 169-175.
BLACK, W.J.M., CALDERBANK, A., DOUGLAS, G., & McKENNA, R.H.
(1966) Residues in herbage and silage and feeding experiments
following the use of diquat as a desiccant. J. Sci. Food
Agric., 17: 506-509.
BUS, J.S., PREACHE, M.M., CAGEN, S.Z., POSNER, H.S., ELIASON,
B.C., SHARP C.W., & GIBSON, J.E. (1975) Fetal toxicity and
distribution of paraquat and diquat in mice and rats. Toxicol.
appl. Pharmacol., 33: 450-460.
CALDERBANK, A. (1972) Experimental considerations in the
development of diquat and paraquat as aquatic herbicides.
Outlook Agric., 7: 51-54.
CALDERBANK, A. & SLADE, P. (1976) Diquat and paraquat. In:
Kearne, P.C. & Kaufman, D. D. ed. Herbicide chemistry,
degradation and mode of action, 2nd ed., New York, Dekker,
pp. 501-540.
CALDERBANK, A. & YUEN, H. (1966) An improved method for
determining residues of diquat. Analyst, 91: 625-629.
CAVELL, B.D. (1979) Methods used in the study of the
photochemical degradation of pesticides. Pestic. Sci., 10:
177-180.
CHARLES, J.M. & MENZEL, D.B. (1979) Influence of atmospheric
particles on pulmonary absorption phenomenon. ACS Symp. Ser.,
174 (CH 15): 287-301.
CHARLES, J.M., ABOU-DONIA, M.B., & MENZEL, D.B. (1978)
Absorption of paraquat and diquat from the airways of perfused
rat lung. Toxicology, 8: 59-67.
CLARK, D.G. & HURST, E.W. (1970) The toxicity of diquat.
Br. J. ind. Med., 27: 51-55.
COATS, G.E., FUNDERBURK, H.H., LAWRENCE, J.M., & DAVIS, D.E.
(1966) Factors affecting persistence and inactivation of
diquat and paraquat. Weed Res., 6: 58-66.
COBB, L.M. & GRIMSHAW, P. (1979) Acute toxicity of oral
diquat (1,1'-ethylene-2'2'-bipyridylium) in Cynomolgus
monkeys. Toxicol. appl. Pharmacol., 51: 277-282.
CONNING, D.M., FLETCHER, K., & SWAN, A.A.B. (1969) Paraquat
and related bipyridyls. Br. med. Bull., 25: 245-249.
CRABTREE, H.C., LOCK, E.A., & ROSE, M.S. (1977) Effects of
diquat on the gastrointestinal tract of rats. Toxicol. appl.
Pharmacol., 41: 585-595.
DANIEL, J.W. & GAGE, J.C. (1966) Absorption and excretion of
diquat and paraquat in rats. Br. J. ind. Med., 23: 133-136.
DEMBINSKI, F., PONIKIEWSKA, T., & TRZEBNY, W. (1971) [Ground
seed of sunflower desiccated with Reglone as fodder for
ruminants.] Pamiet. Pulawski Pr., 49: 205-211 (in Polish).
DICKES, G.J. (1979) The application of gas chromatography to
food analysis. Talanta, 26: 1065-1099.
FAO/WHO (1971) Diquat. In: 1970 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1973) Diquat. In: 1972 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977a) Diquat. In: 1976 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977b) Pesticide residues in food, Rome, Food and
Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, 10).
FAO/WHO (1978) Diquat. In: 1977 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1979) Diquat. In: 1978 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO (1979) Diquat, Rome, Food and Agriculture Organization
of the United Nations, pp. 101-115 (FAO Plant Production and
Protection Paper No. 15, Supplement).
FEL, P., ZALA, I., IZULE, E., & VARGA, L. (1976)
[Haemodialysis in diquat poisoning.] Orv. Metilap, 117:
1773-1774 (in Hungarian).
FELDMAN, K.J. & MAIBACH, H.I. (1974) Percutaneous
penetration of some pesticides and herbicides in man. Toxicol.
appl. Pharmacol., 28: 126-132.
FITZGERALD, G.R., BARNIVILLE, G., FLANAGAN, M., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978) The changing pattern of
paraquat poisoning: an epidemiologic study. J. Irish Med.
Assoc., 71: 103-108.
FLETCHER, K., ed. (1975) Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-89
GAGE, J.C. (1968a) Toxicity of paraquat and diquat aerosols
generated by a size-selective cyclone: effect of particle size
distribution. Br. J. ind. Med., 25: 304-314.
GAGE, J.C. (1968b) The action of paraquat and diquat on the
respiration of the liver cell fractions. Biochem. J., 1O9:
757-761.
GOWMAN, M.E., REILY, D., & NEWBY, S.E. (1980) Paraquat and
Diquat: Longterm high rate trial, Frensham, United Kingdom. 2.
Persistence and movement in soil and glasshouse bioassay,
London, ICI Ltd (Report RJ0014B).
GROVER, R., SMITH, A.E., & KORVEN, H.C. (1980) A comparison
of chemical and cultural control of weeds in irrigation
ditchbanks. Can. J. Plant Sci., 60: 185-195.
GRZENDA, A.R., NICHOLSON, H.P., & COX, W.S. (1966)
Persistence of four herbicides in pond water. J. Am. Water
Works Assoc., 3: 326-332.
HAWKINS, S.F., MEDINA, M.A., & STAVINOHA, W. (1979) The
acute in vivo effect of paraquat and diquat on intermediary
metabolism in mouse lung. Proc. Fed. Am. Soc. Exp. Biol.,
38(3, Pt I): 582.
HAYES, W.J., Jr (1982) Pesticides studies in man, Baltimore,
London, Williams & Wilkins, 561 pp.
HOWE, D.J.T. & WRIGHT, N. (1965) The toxicity of paraquat
and diquat. In: Proceedings of the 18th New Zealand Weed &
Pest Control Conference, pp. 105-114.
HUGHES, R.D., MILLBURN, P., & WILLIAMS, R.T. (1973) Biliary
excretion of some diquaternary ammonium cations in the rat,
guinea-pig and rabbit. Biochem. J., 136: 979-984.
ICI (1972a) Determination of residues of diquat in soil -
residue analytical method No. 6, London, ICI Ltd (Report No.
PPRAM-6).
ICI (l972b) Determination of residues of diquat in milk and
water - residue analytical method No.7, London, ICI Ltd
(Report No. PPRAM-7).
ICI (l972c) Determination of residues of diquat in crops and
animal tissues - residue analytical method No. 5, London, ICI
Ltd (Report No. PPRAM-5).
IRPTC (1983) IRPTC Legal files 1983 Vol. I & II, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme.
KHERA, K.S., WHITTA, L.L., & CLEGG, D.J. (1968) Embryopathic
effects of diquat and paraquat in rats. Ind. Med. Surg., 37:
257-261.
KING, R.R. (1978) Gas-chromatographic determination of
diquat residues in potato tubers. J. agric. food Chem., 26:
1460-1463.
KURISAKI, E. & SATO, H. (1979) [Toxicological studies on
herbicides: intracorporal distribution of paraquat dichloride
and diquat dibromide in rat.] Nippon Hoigaku Zasshi, 33: 656
(in Japanese).
LAM, H.F., TAKAZAWA, J., GUPTA, B.N., & STEE, E.W. VAN
(1980) A comparison of the effects of paraquat and diquat on
lung compliance, lung volume and single-breath diffusing
capacity in the rat. Toxicology, 18: 111-123.
LAVAUR, E., SION, G., GROLLEAU, G., & CARPENTER-LESECK, J.
(1979) Etude comparée de l'action du diquat et du paraquat
sur la muqueuse digestive de la souris, du rat et du lapin.
Ann. Zool. Ecol. anim., 11: 159-169.
LEARY, J.B. (1978) Diquat and paraquat. In: Zweig, G. &
Sharma, J., ed. Analytical methods for pesticides and plant
growth regulators, New York, Academic Press, pp. 321-325.
LEVIN, D.E., HOLLSTEIN, M., CHRISTMAN, M.F., SCHWIERS, E.A., &
AMES, B.N. (1982) A new Salmonella tester strain (TA 102)
with AT base pairs at the site of mutation detects oxidative
mutagens. Proc. Natl Acad. Sci. US, 79: 7445-7449.
LITCHFIELD, N.H., DANIEL, J.W., & LONGSHAW, S. (1973) The
tissue distribution of the bipyridylium herbicides diquat and
paraquat in rats and mice. Toxicology, 1: 155-165.
LOCK, E.A. (1979) The effect of paraquat and diquat on renal
function in the rat. Toxicol. appl. Pharmacol., 48: 327-336.
LOCK, E.A. & ISHMAEL, J. (1979) The acute toxic effects of
paraquat and diquat on the rat kidney. Toxicol. appl.
Pharmacol., 50: 67-76.
MAKOVSKII, V.N. (1972) [Toxicological and hygiene studies of
the bipyridylium herbicides diquat and paraquat,] Referat,
Vinniza, USSR, pp. 1-23 (PhD thesis) (in Russian).
MATSUURA, N., TAKINAMI, M., KURISAKI, E., & SATOO, H. (1978)
[Distribution of paraquat dichloride and diquat dibromide in
the living body.] Fukushima Igakkai Zasshi, 28: 212 (in
Japanese).
NARITA, S., MATOJUKU, M., SATO, J., & MORI, H. (1978)
[Autopsy in acute suicidal poisoning with diquat dibromide.]
Nippon Igakkai Zasshi, 27: 454-455 (in Japanese).
O'BRIEN, M.C. & PRENDEVILLE, G.N. (1978) A rapid sensitive
bioassay for determination of paraquat and diquat in water.
Weed Res., 18: 301-303.
OKONEK, S. & HOFMANN, A. (1975) On the question of
extracorporeal haemodialysis for diquat intoxication. Arch.
Toxicol., 33: 251-257.
OREOPOULOS, D.G. & MCEVOY, J. (1969) Diquat poisoning.
Postgrad. med. J., 45: 635-637.
PASI, A. & EMBREE, J.W. (1975) Further comments on the
assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 31: 125-126.
PASI, A., EMBREE, J.W., EISENLORD, G.A., & HINE, C.H. (1974)
Assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 26: 171-175.
PIRIE, A. & REES, J.R. (1970) Diquat cataract in the rat.
Exp. Eye Res., 9: 198-203.
PYL, W. & GIEBELMANN, R. (1978) [Detection of chlorocholine
chloride, diquat and paraquat.] Arch. vet. Med., 32: 601-602
(in German).
REISH, D., ROSSI, S.S., MEARNS, A.J., OSHIDA, P.S., & WILKES,
F.G. (1979) Marine and estuarine pollution. J. Water Pollut.
Control Fed., 51: 1477-1517.
RILEY, D. (1981) The fate and effect and diquat residues in
soil. In: Proceedings of the National Spray Seed Conference
1981, Albury, New South Wales, Australia.
RILEY, D. & GRATTON, R.P. (1974) Unavailability to plants of
diquat residues in soils. 10th Tr. Muzhdunar. Kongr.
Pochvoved., USSR, 3: 193-203.
ROSE, M.S. & SMITH, L.L. (1977a) The relevance of paraquat
accumulation by tissues. In: Autor, P.A., ed. Biochemical
mechanisms of paraquat toxicity, New York, Academic Press,
pp. 71-91.
ROSE, M.S. & SMITH, L.L. (l977b) Tissue uptake of paraquat
and diquat. Gen. Pharmacol., 8: 173-176.
SCHOENBORN, H., SCHUSTER, H.P., & KOESSLING, F.K. (1971)
[Clinical and muphological findings in an acute oral
intoxication with diquat Reglon.] Arch. Toxicol., 27: 204-216
(in German).
SCHULTZ, VON O., KIRCHNER, K., MUELLER, P., & ROTHE, R.
(1976) [Reglone (Diquat) cause of intoxication of sheep,
cattle, and swine.] Monatsh. Veterinärmed., 31: 647-649 (in
German).
SELYPES, A., NAGYMAJTENYI, L., & BERENCSI, G. (1980)
Mutagenic and embryotoxic effects of paraquat and diquat.
Bull. environ. Contam. Toxicol., 25: 513-517.
SHARP, C.W., OTTLENGHI, A., & POSNER, H.S. (1972)
Correlation of paraquat toxicity with tissue concentrations
and weight loss of the rat. Toxicol. appl. Pharmacol., 22:
241-251.
SIEBERT, D. & LEMPERLE, E. (1974) Genetic effects of
herbicides: induction of mitotic gene conversation in
Saccharomyces cervisiae. Mutat. Res., 22: 1ll-120.
SINGH, S.P. & YADAV, N.K. (1978) Toxicity of some herbicides
to maior carp fingerlings. Indian J. Ecol., 5: 141-147.
SMITH, S.N., LYON, A.J., & SAHID, I.B. (1976) The breakdown
of paraquat and diquat by soil fungi. New Phytol., 77: 735-740.
SMITH, T.P., NOACK, A., & COSH, S.M. (1981) The effect of
some herbicides on vesicular-arbuscular endophyte abundance in
the soil and on infection of host roots. Pestic. Sci., 12:
91-97.
STEVENS, M.A. & WALLEY, J.K. (1966) Tissue and milk residues
arising from the ingestion of single doses of diquat and
paraquat by cattle. J. Sci. Food Agric., 17: 472-475.
SUMMERS, L.A. (1980) The bipyridilium herbicides, London,
New York, Toronto, San Francisco, Academic Press, pp. 1-449.
TSCHIPILSKA, L.N. (1980) [The influence of some pesticides
on the soil microorganisms in connection with the hygiene
evaluation of the soil,] Referat, Sofia, Bulgaria, pp. 1-39
(PhD thesis) (in Bulgarian).
TOMPSETT, S.L. (1970) Paraquat poisoning. Acta pharmacol.
toxicol., 28: 346-358.
TUCKER, V.B., PACK, D.E., & OSPENSON, J.N. (1967) Adsorption
of bipyridylium herbicides in soil. J. agric. food Chem., 15:
1005-1008.
UKAI, S., HIROSE, K., & KAWASE, S. (1977) [Forensic chemical
studies on drugs and chemicals. III. Gas chromatography of
reduction products of the herbicides diquat and paraquat.]
Eisei Kagaku, 23: 32-38 (in Japanese).
VANHOLDER, R., COLARDYN, F., RENCK, DE J., PRAET, M., LAMEIRE,
N., & RINGOIR, S. (1981) Diquat intoxication. Report of two
cases and review of the literature. Am. J. Med., 70: 1267-1271.
VERBETSKII, V.E. & PUSHKAR, M.S. (1968) [Pathological
changes in the organs of animals on acute poisoning with the
herbicide diquat (Reglone).] In: Some questions concerning
human and animal morphology, Medizina, Odessa, pp. 51-52 (in
Russian).
WEBER, J.B., PERRY, P.W., & UPCHURCH, R.P. (1965) The
influence of temperature and time on the adsorption of
paraquat, diquat, 2,4-D and prometone by clays, charcoal and
anion exchange resin. In: Soil Science Society Proceedings,
pp. 678-688.
WHO/FAO (1979) Data sheets on pesticides: Diquat, Geneva,
World Health Organization, pp. 1-7 (Unpublished Report No.
VBC/DS/79-40).
WILKINSON, W. (1980) Paraquat and Diquat: Longterm high rate
trial, Frensham, United Kingdom. 1. Management of site,
effects on crops and weeds and residues in crops, London, ICI
Ltd (Report No. RJ0013B).
WILLIAMS, C.B., LEVISON, S.A., & DANDLIKER, W.B. (1976)
Application of immunological techniques to the detection of
organic contaminants of environmental concern. In: Proceedings
of the University of Montana Annual Conference on Trace
Substances & Environmental Health, No. 10, pp. 317-322.
WOJECK, G.A., PRICE, J.F., NIGG, H.N., & STAMPER, J.H.
(1983) Worker exposure to paraquat and diquat. Arch. environ.
Contam. Toxicol., 12: 65-70.
4.1.2. Microbial degradation
Photochemical degradation of diquat on plants is quicker than
microbial degradation in soil. Microbial degradation of strongly-
bound diquat in soil is slow, but is faster in culture. The
degradation of diquat by soil fungi was studied by Smith et al.
(1976). The degradation of 14C-diquat to 14CO2 by Aspergillus
niger was tested by 4 different fungal test systems. High
intracellular herbicidal levels and inability to grow in the
presence of low diquat concentrations in the media characterized
the species unable to decompose diquat. Under laboratory
conditions, diquat degradation by Pseudomonas started after 3 days
(Tchipilska, 1980). Under field conditions, degradation started
after 10 days, and was related to the ambient temperature, and the
aeration and type of soil.
The fact that no significant hazard has been observed for
ruminants from diquat-treated herbage, or for the general
population from crops and water, is explained by the rapid
photochemical degradation of diquat.
4.2. Diquat Adsorption, Residue Levels, and Exposure in Soil
4.2.1. Diquat adsorption on soil particles
Diquat binds readily to clay particles in the soil. The rate
of adsorption depends on the degree of contact of diquat with
adsorbent minerals, the type of soil, and the initial herbicide
concentrations tested. Weber et al. (1965) studied the effects of
temperature and exposure time on diquat adsorption by
montmorillonite, kaolinite, charcoal, and an anionexchange resin in
pH 6.0 phosphate buffer. Diquat was preferably adsorbed on the
clay particles by a process of ion exchange. Adsorption was
limited by the cation-exchange capacity of the test systems
examined. Coats et al. (1966) showed the adsorption capacity of
kaolinite to be about 2 g/kg and that of bentonite 80 - 100 g/kg.
A diquat soil concentration of 0.1 mg did not produce any
significant reduction in the dry weight of wheat grown in the soil
(Coats et al., 1966). The diquat appeared to be too tightly
adsorbed to the surface and between the lattices of bentonite to be
available to the wheat plant, at a soil treatment rate of 50 g/kg.
Data for diquat adsorption on sandy soils (Tucker et al., 1967)
showed that the herbicide was bound to different extents, according
to the structure of the soil particles.
4.2.2. Residue levels of diquat in soils
Makovskii (1972) reported on diquat residues in soils from
different plots, treated every year for a period of 7 years. There
were 3 - 4 treatments per season, at approximately 27.5 kg
diquat/ha. Samples were taken at 0 - 10 cm, 10 - 20 cm, and 20 -
30 cm depths in the soil; total diquat residues were shown to be
about 5.4 mg/kg soil, the mean values being 3.9 mg/kg, 1.3 mg/kg,
and 0.2 mg/kg in the respective soil layers. No diquat residues
were discovered in plants and citrus fruits sampled at different
times from the treated plots. In other studies, soil was analysed
for diquat residues on the 1st, 8th, and 15th days after applying
Reglone(R) at 0.8 litre/ha and 0.4 litre/ha (Tchipilska, 1980). On
the 1st day, residues of 0.400 mg/kg and 0.126 mg/kg were detected;
on the 8th and l5th days residues in the treated plots were lower
than 0.1 mg/kg.
As summarized in section 4.1.2, free diquat is degraded by a
range of microorganisms. While degradation of strongly-absorbed
diquat is relatively slow, results of long-term field studies have
nevertheless shown degradation rates of the order of 5 - 10% per
year. This is greater than the rate required to prevent saturation
of the deactivation capacity of soils.
In a long-term trial on a loamy soil, plots were treated with
0, 90, 198, and 720 kg diquat/ha, which was incorporated to a depth
of 15 cm. These rates were equivalent to 0, 50, 110, and 400% of
the soils strong absorption capacity (Gowman et al., 1980;
Wilkinson, 1980; Riley 1981). Over the 7 years, diquat residues
declined by 5% per year (sig P = 0.05) on the 90 kg/ha plots and
by 7% per year (sig P = 0.01) on the 198 and 720 kg/ha plots. The
rate of decline on the 198 and 720 kg/ha plots were significantly
greater ( P = .01) than on the 90 kg/ha plots.
4.2.3. Effect of residual diquat on soil biological activity, on
plants, and crop yields
A literature review and an extensive study of the effects of
different concentrations of diquat on microorganisms (saprophyte
and pathogenic microflora, and fungi) were carried out by
Tchipilska (1980). Staphylococcus aureus growth was inhibited
while Scenedesmus acutus was stimulated. Smith et al. (1981)
examined the effects of diquat applied at 0.5 - 32 times the
concentration recommended in agricultural practice on vesicular
arbuscular endophyte spore abundance in the soil and on the
infection of wheat roots. No measurable deviations in
endomycorrhiza formation and function were noted at normal
application rates. Loss of potassium and phosphate from fungi was
recorded at higher concentrations of diquat.
Coats et al. (1966) studied the uptake and translocation of
14C-diquat from soil into wheat. No metabolites were found in the
plants.
Diquat does not appear to have any significant influence on the
normal microbial activity that is important for soil fertility.
Nor is there any evidence that the recommended application rates
for diquat lead to residual effects on crop growth. Moreover,
tightly adsorbed diquat in soil is not reactivated into a
biologically active form, so that, in practice, accidental spillage
is probably the only cause of local high phytotoxic levels of
residual herbicide.
4.3. Diquat Transformation, Residue Levels, and in Effects on
Aquatic Organisms and Crops
4.3.1. Transformation and residue levels of diquat in water
In static water, initial diquat concentrations of 0.5 - 1.0
mg/litre fell rapidly to 0.1 - 0.3 mg/litre after 4-7 days
(Calderbank, 1972; Calderbank & Slade, 1976). In field
experiments, initial concentrations of 1.0, 0.8, and 0.5 mg/litre
decreased to 0.03 - 0.003 mg/litre after 7 - 14 days. This rapid
loss of diquat from treated waters was due to rapid uptake by
aquatic weeds. Two weed species (Myriophyllum spicatum and
Callitriche stagnalis) were immersed in water containing 1.0 mg
diquat/litre. The concentration of the herbicide decreased rapidly
to 0.14 - 0.03 mg/litre during a period of 6-14 days after
treatment. At the end of the experiment, the residue levels in
the weeds ranged from 6.2 - 17.4 mg/kg. In addition to uptake
by weeds, loss of diquat from treated waters was due to
photodegradation at the water surface and adsorption by bottom mud.
In field experiments carried out in 1010 m2 ponds with an initial
concentration of diquat of 2 mg/litre water, there were no residues
of diquat in the water after 8 days (Calderbank, 1972; Calderbank &
Slade, 1976).
In pond water that had been treated with diquat at 2.5 mg/litre
(Grzenda et al., 1966), residues of 0.01 - 0.08 mg/litre were found,
7-9 days after applying the herbicide, and no residues could be
determined after 14-30 days. The authors concluded that, compared
with other herbicides, diquat appeared to have the greatest
potential for use in sources of potable water.
The data obtained from studies in ponds, large and small lakes,
canals, and reservoirs demonstrate the fast disappearance of diquat
from treated waters (Calderbank, 1972). Absorption by aquatic
weeds explains the high efficacy of the herbicide. Decomposition
of the dead weeds is rapid, and diquat is not released from the
bottom mud back into the water. Applications of paraquat and
diquat each at a dose level of 1.1 kg/ha (Grover et al., 1980)
proved very effective for the control of weeds in irrigation
ditches, and the residual levels of both herbicides decreased
rapidly.
4.3.2. Effects of residual diquat on aquatic organisms and crops
The toxicity of diquat for fish varied with the species, the
size of the fish, and the softness or hardness of the water. The
LC50 values range from 12 to 90 mg/litre (24 h), 6 to 44 mg/litre
(48 h), and 4 to 36 mg/litre (96 h) (Calderbank, 1972). Reviews of
the effects of diquat on fish, aquatic invertebrates,
microbiological organisms in the soil of lakes, and phytoplankton
demonstrate that the herbicide, applied at the rates used for
aquatic weed control, did not affect estuarine fauna, oysters,
shrimps, water insects, or fish-food organisms (Calderbank, 1972;
Atkinson, 1973). At concentrations of 1 - 100 mg/litre, diquat
appeared to be less toxic for carp fingerlings than paraquat,
diuron, simazine, and dalapon (Singh & Yadev, 1978). Reish et al.
(1979) reviewed the effects of diquat on marine organisms; no
bioaccumulation by estuarine and marine organisms was found. The
toxicity of diquat for fish is low, and the main risk for aquatic
organisms and fish from its use as an aquatic weed killer is the
decreased oxygen concentration following the decay of weeds.
Trout exposed to 1 mg diquat/litre for 7 days contained
residues of 0.3 - 0.4 mg/kg in the gut, liver, and kidney, and of
0.1 - 0.3 mg/kg in the skin and gills. Residues were below the
limit of detection in muscle, spleen, and heart (Calderbank, 1972).
Trout exposed to 1 mg diquat/litre for 16 days contained residues
of 0.5 - 0.6 mg/kg, which disappeared when the fish were returned
to fresh water.
Because of irreversible adsorption, low residues in water will
be lost on contact with soil. The herbicide is thus unavailable to
plant roots. However, in overhead irrigation experiments, the use
of water containing diquat at 0.1 - 0.5 mg/litre (Calderbank, 1972)
resulted in diquat residues in the crops (tomato, lettuce, sugar
beet) ranging from less than 0.01 mg/kg to 0.04 - 0.07 mg/kg.
Thus, before using herbicide-treated waters for overhead plant
irrigation, it is advisable to allow 10 days for the diquat aquatic
residues to drop to acceptable levels.
The maximum diquat residues in water ultimately to be used for
drinking were 0.03 - 0.01 mg/litre, at the points of entry into the
public distribution system, 2-4 days after treatment; no residues
were detectable on the 10th day after applying diquat as an aquatic
herbicide. More often than not, residue levels were below the
detection limits of the analytical methods used.
4.4. Diquat Exposure and Residue Levels in Plants and Animals
4.4.1. Plants
Diquat is largely used as a desiccant in silage production. At
the recommended rates of 1.5 - 3.0 litre Reglone(R)/ha, diquat
residues were very low (Riley & Gratton, 1974). Following pre-
harvest desiccation of fodder crops, they ranged from below 0.05
mg/kg to 50 mg/kg, most of the levels determined being below 25 mg
diquat/kg (FAO/WHO, 1971, 1973). Diquat residues in the treated
herbage, sampled at different intervals after spraying with 0.258 -
0.515 mg/ha, were relatively high after 1 day (12 - 65 mg/kg), but
after 7 days had markedly decreased (1.0 - 6.5 mg/kg) (Black et
al., 1966). The levels of diquat found in silage during a 4-year
trial, with application rates of 0.190, 0.258, and 0.540 mg/ha,
varied from 1.4, 3.6, 9.3, and 13.3 to 26.8 mg/kg. The differences
were due to the atmospheric conditions at the time of desiccation
and the consequent degree of photochemical degradation of the
diquat. For this reason, diquat residues in treated herbage should
be expected to vary by an order of magnitude (10 times).
Pre-harvest desiccation of rape-seed with diquat did not result
in any detectable residues in the extracted oil and only low
residues (0.3 - 2 mg/kg) in the meal cake. Rape plants were
sprayed with 14C-diquat at 0.3 - 1.1 kg/ha, 3-14 days before
harvesting. There were no detectable residues of diquat or of its
photodegradation products in the rape-seed oil when the seeds were
harvested 7 days after desiccation, and very low diquat residues
(0.02 - 0.003 mg/kg) were determined when the seeds were harvested
14 days after treatment with diquat. The diquat residues in the
meal cake varied from 1.49 to 10.2 mg/kg, 14 days after treatment,
a large proportion being unchanged diquat (FAO/WHO, 1973).
Dembinski et al. (1971) reported diquat residues of 2 mg/kg in
sunflower seeds desiccated with Reglone(R).
Makovskii (1972) reported the diquat residue levels in weeds
treated with Reglone(R). After applications of Reglone (R) at
0.5, 1.0, and 1.3 litre/ha, the residues in dry weeds, ranged from
34 to 74 mg/kg 1 h later; from 15 to 26 mg/kg after 1 day; from
undetectable to 10 mg/kg after 4 days; from 2.8 to 3.5 mg/kg after
2 weeks; from 1.9 to 2.3 mg/kg after 4 weeks; and from undetectable
to 1.7 mg/kg after 6 weeks. The degradation of diquat in plants
was more rapid than the degradation of paraquat. The residues in
potatoes did not exceed 0.08 mg/kg, when diquat was used to destroy
potato haulm, and levels in fruits (apples, pears, plums, citrus),
tea, and cereals were undetectable (< 0.01 mg/kg), when diquat was
applied as a herbicide for weed control. Samples of potatoes
purchased from shops (Andersson & Josefsson, 1982) were analysed
for diquat residues. Residues in the range of 0.004 - 0.039 mg/kg
were found in 20 of 23 samples obtained from commercial growers.
None of the samples contained more than the residue tolerance of
0.1 mg/kg accepted for potatoes in Sweden.
Residue levels of diquat have been discussed in more detail by
the Joint Meeting on Pesticides Residues (FAO/WHO, 1971, 1973).
Residue levels of diquat in plants were summarized and published by
FAO/WHO (1977a). Some of these data are given in Table 2.
Data on diquat residues in desiccated wheat collected from 6
countries showed a mean of 0.5 mg/kg (FAO, 1979).
Table 2. Diquat residues in plantsa
------------------------------------------------------------
Plants Dose of diquat Mean value of
(kg/ha) residues (mg/kg)
------------------------------------------------------------
Wheat (grain, flour) 0.6 - 1.0 0.61, 0.22
Rice (with husk, polished) 0.2 - 0.4 0.89, 0.07
Sorghum (grain) 0.4 - 0.6 0.81
Cotton (grain) 0.4 - 1.0 0.37
Potato 0.6 - 1.0 0.03
Beans 0.3 - 1.0 0.10
Peas 0.3 - 1.0 0.05
Sugar beet (juice) 0.3 - 0.8 < 0.01
------------------------------------------------------------
a From: FAO/WHO (1977a).
4.4.2. Animals
Sheep and cattle fed silage containing diquat residues of up to
13 mg/kg were studied by Black et al. (1966). The total diquat
excreted in the urine was 0.19 - 0.65 mg over an 8-day period. No
diquat residues were detected in the brain, liver, and kidney of
sheep, or in the meat or organs of cattle fed diquat-treated silage
for one month. Milk collected on alternate days for 2 weeks was
free of diquat residues (< 0.003 mg/litre).
Feeding trials with sunflower seed containing approximately
0.20 mg diquat/kg were reported by Dembinski et al. (1971).
Although the amount of diquat consumed by the cattle over 257 days
ranged from 11.2 mg to 184.2 mg, no residues were found in any of
the milk samples analysed. Wethers fed ground sunflower seed
containing approximately 0.20 mg diquat/kg for 141 days were
estimated to have consumed a total of 14.1 mg diquat per sheep. No
residues were found in brain, liver, or kidney, nor were there any
residues in the meat, lungs, and kidney of steers treated with
diquat-desiccated sunflower forage. In long-term feeding trials
with silage, desiccated grass, lucerne, clover hay, barley straw,
and sunflower seeds containing diquat residues ranging from 0.2 to
50 mg/kg, the residues in milk and meat were determined to be less
than 0.007 mg/litre and less than 0.0006 mg/kg, respectively
(FAO/WHO, 1971, 1973, 1977a,b). Calderbank (1972) reviewed the
effects on farm animals of diquat in the drinking-water and on
herbage; there were no adverse effects on cattle and sheep and only
very low residue levels in milk, meat, and the organs analysed.
Lavaur et al. (1979) studied the effect of treated lucerne on
rabbits. Immediately after spraying, a concentration of 211 mg
diquat/kg dry weight was determined in the lucerne. After 24 h and
48 h, diquat residues were 97 mg/kg and 25 mg/kg, respectively. No
signs of poisoning or gastrointestinal damage were found in the
rabbits fed with different levels of diquat residues in the
lucerne. However, in some circumstances, lack of careful
organization may result in adverse effects of diquat on animals.
Intoxication of sheep, cattle, and swine has been reported (Schultz
et al., 1976) after the aerial application of Reglone(R) as a
rapeseed desiccant. The clinical course and the causes of the
accident stressed the need for proper diquat application by air.
For a more detailed discussion of the fate of diquat residues
in exposed animals, refer to FAO/WHO (1977a,b).
4.5. Diquat Levels in Air and Exposure of Workers
Experiments with 14C-diquat demonstrated that it was not
volatile (Coats et al., 1966). Diquat levels in air after spraying
with aerosols were determined by Makovskii (1972), using the method
of Calderbank & Yuen (1966). The application rates were 1.0 - 1.3
kg diquat/ha in working dilutions of 2.5 g and 3.3 g active
ingredient/litre, the highest diquat concentrations being found in
the tractor cabin when the door was open and spraying was in
progress in the direction of the wind (Table 3). The diquat
concentrations in air decreased rapidly 10 - 20 min after
completion of the treatment.
Wojeck et al. (1983) reported that diquat was determined in air
samples taken near the breathing zone of workers during its
application for aquatic weed control. The respiratory exposure
levels were below the limits of quantitation of the chemical
analysis.
In Bulgaria and the USSR, the proposed MAC (maximum allowable
concentration) for diquat is 0.1 mg/m3 aerosol. The TLV for diquat
in workroom air in the United Kingdom and the USA is 0.5 mg
diquat/m3 (1982), a level that will not be reached under normal
conditions of application.
Table 3. Total airborne diquat concentrations in the air of working areasa
---------------------------------------------------------------------------
Place of sampling Number of Mean concentrations
samples (mg/m3 ± SE)
---------------------------------------------------------------------------
Working area sprayer loading 20 0.12 ± 0.03
tractor cabin 8 0.56 ± 0.10
(in direction of wind)
tractor cabin 8 0.17 ± 0.04
(against the wind)
manual spraying 16 0.25 ± 0.04
Treated field after 5 min 8 0.20 ± 0.03
after 10 min 24 0.06 ± 0.01
after 20 min 8 ND
Distance from 200 m 8 0.09 ± 0.01
treated field 400 m 8 ND
---------------------------------------------------------------------------
a From: Makovskii (1972).
5. KINETICS AND METABOLISM
5.1. Animal Studies
5.1.1. Absorption
Oral absorption
Daniel & Gage (1966) studied the absorption of 14C-diquat
dibromide and 14C-diquat dichloride following oral and subcutaneous
single-dose administration to rats. About 90 - 97% of the oral
diquat dibromide and 84 - 90% of the diquat dichloride were found
in the faeces and 4 - 11% of both diquat salts in the urine.
Following subcutaneous injection of 14C-diquat (10 mg/kg body
weight) in rats, 87% of the administered dose was excreted in the
urine and 5% in the faeces within 4 days. The urine contained
mainly unchanged diquat (75% of the dose) together with diquat
monopyridone (about 3% of the dose) and diquat dipyridone (about 6%
of the dose) (FAO/WHO, 1978).
The poor absorption of diquat from the gastrointestinal tract
was confirmed by Litchfield et al. (1973) in the rat, and by Black
et al. (1966), Stevens & Walley (1966), and Dembinski et al. (1971)
in farm animals.
Pulmonary absorption
The uptake of 14C-diquat by perfused rat lung, following
intratracheal injection, was examined by Charles et al. (1978) and
Charles & Menzel (1979). Removal of 14C-diquat from the airways
was rapid, initially, but slowed down with time. The results
indicate 2 phases of absorption and removal of diquat from the
airways in the rat.
Dermal absorption
There are no data on the rate of diquat absorption through the
skin. Studies on the dose-related percutaneous toxicity of diquat
suggest that it may be dermally absorbed.
5.1.2. Distribution
Although paraquat and diquat have similar chemical, physical,
and herbicidal properties, only paraquat has been shown to damage
the lung. According to Sharp et al. (1972), diquat concentrations
in lung and muscle were much lower than the levels attained with
equal 20 mg/kg body weight iv doses of paraquat. Table 4 shows the
distribution of both in the main internal organs.
Table 4. Ratio of concentration of paraquat/diquat in
the tissues of the rata
--------------------------------------------------------
Organ Days after intravenous administration
1 3 5 7 10
--------------------------------------------------------
Lung 8 33 12 10 20
Muscle 2 13 10 7 16
Kidney 0.9 0.9 0.9 0.3 0.25
Liver 0.4 0.7 0.7 0.5 0.2
--------------------------------------------------------
a From: Sharp et al. (1972).
Diquat concentrations were higher in the kidney and the liver
but significantly lower in the lung (Table 4). In addition, the
concentrations of paraquat were 2-8 times higher than those of
diquat in the heart, adrenal glands, spleen, stomach, ileum,
testes, and thymus. Plasma levels were similar for both
bipyridylium herbicides.
Litchfield et al. (1973) injected 14C-diquat cation at 50 mg/kg
body weight iv into mice. Whole-body autoradiographs were prepared
after 10 min, 1 h, 24 h, and 72 h. Radioactivity was selectively
located in the gall bladder and was also present in cartilaginous
tissue, liver, and the gastrointestinal tract. Low radioactivity
was found in the brain and spinal cord. One h after dosing, the
amount in the urine and intestinal epithelium had increased. After
24 h, the excretion of diquat was virtually complete, although
radioactivity continued to be detected in the small and large
intestine and the bladder.
Litchfield et al. (1973) also determined diquat levels in
various tissues of male and female rats fed a diet containing
diquat dibromide monohydrate at 250 mg/kg for 2, 4, and 8 weeks.
High levels (0.18 - 1.17 mg/kg) were found in the kidney and the
large intestine; levels in the lung ranged from < 0.05 to 0.53
mg/kg; those in the liver from 0.07 - 0.22 mg/kg, while levels in
the brain, muscle, and blood were very low. At all stages of the
study, diquat lung levels were lower than those for paraquat, the
average paraquat content in the lung (at a dose of 250 mg/kg diet)
over the 8-week period being 1.7 mg/kg and the average diquat
level, 0.2 mg/kg. No sex differences were found. Within 1 week of
return to a normal diet, diquat was below the detectable limit in
all tissues examined.
Rats given paraquat or diquat orally at 680 µmol/kg had high
kidney levels of diquat throughout the 30 h period after dosing
(Rose & Smith, 1977, 1977a). There was no significant time-
dependent increase in diquat levels in the lung, liver, brain,
adrenal glands, muscle, and plasma. These results confirmed that,
following oral dosing, the lung does not accumulate diquat. Rose &
Smith (1977) also incubated rat lung slices in 10-5M paraquat and
diquat. In contrast to paraquat, diquat did not accumulate in the
lung slices, and the compound did not accumulate significantly in
any tissue slices with the exception of those from the kidney.
These observations were confirmed by Lock (1979).
Matsuura et al. (1978) studied the distribution of orally
administered LD50 doses of diquat and paraquat in rats. Two and
24 h after dosing, there were higher concentrations of diquat in
kidney, liver, and lung than in brain, heart, the gastrointestinal
system, and blood. At equitoxic doses, levels of diquat in the
lung appeared to be lower than those of paraquat. In a similar
distribution study of the LD50 and 0.5 LD50 doses of diquat and
paraquat, Kurisaki & Sato (1979) determined the tissue
concentrations from 2 to 48 h and from 2 to 9 days after treatment.
Distribution in the lung, heart, brain, liver, and kidney of the
rats agreed with previously published data.
The results of the above studies demonstrate that diquat does
not persist as long as paraquat in the body of the rat and that it
does not accumulate in the lung.
5.1.3. Metabolic transformation and excretion
Daniel & Gage (1966) reported that the amount of 14C-diquat
excreted in rat bile during the 24 h following oral doses of 1.2 -
64 mg/kg body weight represented 1.1 - 4.8% of the dose. Small
amounts were detected in the urine, but about 70% of the diquat was
present in the faeces. In other studies (FAO/WHO, 1978), the rate
of diquat metabolism in the rat was considerably lower than
previously reported by Daniel & Gage (1966). The biliary, urinary,
and faecal excretion of 14C-labelled bipyridylium herbicides was
studied by Hughes et al. (1973) in the rat, guinea-pig, and rabbit.
14C-diquat dichloride was injected ip at dose levels of 40 µmol/kg
body weight in the rat, 13 µmol/kg in guinea-pig, and 14 µmol/kg in
the rabbit. Most of the injected diquat (82% - rat, 64% -rabbit)
was found in the urine. Rabbits metabolized 18% of the dose,
guinea-pigs 5%, and rats less than 1%. The metabolites were
similar for the 3 species. The rat excreted approximately 1.4% of
the dose in the bile, the guinea-pig 4.8%, and the rabbit 2.9%.
Stevens & Walley (1966) treated cattle orally with 14C-diquat
dibromide in doses of 4, 8, and 20 mg/kg body weight. The
radioactivity levels in the milk of the cows indicated that 0.04 -
0.15% of the ingested dose was excreted in this way. Very low
levels of diquat (0.01 mg/kg) were present in muscle tissue, 2 - 8
days after dosing. A bull calf was dosed orally with 14C-diquat
dibromide at 10 mg/kg. About 2.6% of the 10 mg/kg dose was excreted
in the urine, but the major part of the dose was excreted via the
faeces. In the calf, 24 h after dosing, the residues were 0.66
mg/kg in kidney, 0.20 mg/kg in heart and skin, 0.19 mg/kg in liver,
0.03 mg/kg in lung, testes, and serum, and 0.006 mg/kg in muscle.
Studies on rats dosed orally with 14C-diquat at 45 mg/kg body
weight or subcutaneously (sc) with 10 mg/kg body weight were
reported by FAO/WHO (1978). Rats given the oral dose excreted 6%
and 89% in the urine and faeces, respectively, within 4 days and
mainly within the first 2 days. Unchanged diquat was the major
component in both urine (5% of the dose) and faeces (about 57% of
the dose). About 5% of the oral dose was excreted as diquat
monopyridone, mainly in the faeces, while diquat dipyridone
appeared to be the major urinary metabolite. Following sc
injection, rats eliminated 87% of the dose in the urine and 5% of
the dose in the faeces within 4 days. The urine contained 75% of
the dose as diquat, about 3% as diquat monopyridone, and about 6%
as diquat dipyridone. In vitro studies have shown that the caecal
microflora of the rat can metabolize about 10% of the diquat added
in a 24-h incubation period, with the formation of some diquat
monopyridone. This observation, together with the paucity of
metabolites following ip injection, suggests that diquat is
metabolized by the gastrointestinal tract bacteria.
The oral LD50 of diquat monopyridone in the rat was more than
4000 mg/kg body weight. Oral administration of diquat monopyridone
at 1000 mg/kg body weight per day for 2 weeks did not induce any
clinical, haematological, biochemical, or histopathological
deviations in the rat. In other studies, no adverse effects were
noted after sc injection of diquat monopyridone or diquat
dipyridone in rats, but 9 animals out of a group of 10 injected
with the equivalent dose (16 mg/kg body weight) of diquat were dead
by the l4th day following dosing (FAO/WHO, 1978).
5.2. Observations on Man
Feldman & Maibach (1974) studied the dermal penetration of
twelve 14C-labelled insecticides and herbicides. Diquat showed a
very low rate of dermal absorption in man. No other studies on the
kinetics of diquat in volunteers have been published, but
observations are available on accidental and suicidal ingestion
(section 7). Toxicological analysisk, at the time of admission, of
the serum of a patient, who had ingested 20 ml Reglone(R), showed
a diquat level of 0.4 mg/litre (Vanholder et al., 1981). At
postmortem examination on the 5th day after ingestion,
approximately 0.20 mg diquat/kg was determined in liver, kidneys,
muscle, and eye liquid.
6. EFFECTS ON ANIMALS
6.1. Effects on Experimental Animals
6.1.1. Gastrointestinal system and liver
Investigation of the clinical signs of acute oral intoxication
by diquat (Verbetskii & Pushkar, 1968; Clark & Hurst, 1970;
Crabtree et al., 1977; Cobb & Grimshaw, 1979) have established
gastrointestinal disturbance as the major syndrome of poisoning and
as a cause of death. In both rats and guinea-pigs, the clinical
signs of acute oral poisoning (Verbetskii & Pushkar, 1968) were
dose-dependent. At doses greater than the LD50, signs of poisoning
appeared after 6 - 12 h; at lower levels, the signs were less
obvious and appeared after 1 - 2 days. Most deaths occurred on the
3rd - 9th day after oral administration. The animals lost 7 - 35%
of their initial body weight. During the first 24 h following the
oral dosing of rats with 900 µmol diquat/kg body weight (LD50), a
reduction in water intake was noted (Crabtree et al., 1977). The
animals were subdued, showed pilo-erection and loss of appetite.
At 24 h, they excreted mucoidal, ropy faeces of a characteristic
greenish-yellow or grass-green colour, this colour being due to the
reduction of diquat by intestinal bacterial metabolism. This
colour can be reproduced in vitro with fresh intestinal contents
and actively growing bacterial isolates from them (Clark & Hurst,
1970).
A significant dose-dependent accumulation of water in the lumen
of the intestines and progressive haemoconcentration were reported
(Crabtree et al., 1977) following acute diquat intoxication in
rats. It was concluded that diquat had an adverse effect on water
distribution in the body. Rapid fluid excretion following oral
diquat poisoning suggested a direct action on the stomach and
intestinal mucosa. Monkeys dosed orally with diquat ion at 100,
200, 300, and 400 mg/kg body weight (Cobb & Grimshaw, 1979) vomited
within 2 h and showed diarrhoea within 12 h of dosing. The most
severely affected became lethargic and comatose, and finally
collapsed and died, 12 - 84 h after dosing. An increased number of
polymorphonuclear leukocytes as well as increased levels of serum
urea, plasma glucose, and serum GOT and GPT activities were
determined in monkeys that died during the study. Histological
examination revealed a distended gastrointestinal tract and a
swollen caecum; the mucosa of the stomach was ulcerated and the
small and large intestines congested. Large areas of the stomach
and intestines showed necrosis and exfoliation of the epithelium
from the mucosa. The submucosa was infiltrated with lymphocytes,
and polymorphonuclear and mononuclear cells. These changes were
most severe in the intestinal villi. The death of the monkeys was
due to destruction of the epithelial lining of the gastrointestinal
tract in combination with kidney damage.
Liver
The liver was not severely affected in acute and repeated
diquat poisoning of experimental animals. High doses sometimes
resulted in histological lesions (Verbetskii & Pushkar, 1968;
Bainova, 1975), but signs of toxic hepatitis were not described.
Gage (1968a) reported stimulated NADPH oxidase activity in rat
liver microsomes in vitro after exposure to diquat.
6.1.2. Renal system
The major route of diquat elimination is through the kidneys.
High doses of diquat provoke histological and biochemical changes
in the kidneys, but the most severe damage occured in relation to
renal excretion function (Lock & Ishmael, 1979).
Kidney damage following acute and repeated diquat poisoning was
reported by Verbetskii & Pushkar (1968), Bainova (1969), Cobb &
Grimshaw (1979), Lock (1979), and Lock & Ishmael (1979). Rats,
guinea-pigs, and monkeys were investigated after oral poisoning
with the herbicide. Diquat, orally administered at 680 µmol/kg to
rats, induced a significant increase in diuresis, proteinuria, and
glucosuria after 6-24 h. Biochemical tests in vitro revealed a
decrease in N'-methylnicotinamide, but not 4-aminohippurate,
accumulation by renal cortical slices suggesting competition for
the base transport system. Stimulation of the pentose phosphate
pathway and inhibition of fatty acid synthesis were found when
diquat was added to renal cortical slices in vitro. No such
changes were noted when the renal cortical slices were prepared
from rats previously treated with diquat (Lock, 1979).
Lock (1979) also investigated the changes in several variables
and the clearance of diquat by the rat kidney after oral
administration of toxic doses (680 and 900 µmol/kg body weight).
Diquat was not bound to the proteins of the rat plasma. Active
renal secretion was confirmed by the fact that diquat was cleared
by the kidney at a slightly higher rate than inulin. In rats
treated orally with diquat at 540 µmol/kg body weight, renal
clearance decreased after 24 h. However, the reduction in renal
function induced by diquat (Lock 1979) was considered to be
secondary and due to water redistribution caused by acute
poisoning.
Histopathological changes have been reported in the kidneys of
animals poisoned with high doses of the herbicide (Verbetskii &
Pushkar, 1968; Cobb & Grimshaw, 1979; Lock & Ishmael, 1979). The
renal papillae were hyperaemic, degeneration and necrosis of the
epithelium of the proximal and distal convoluted tubules were
noted, the epithelial cells were exfoliated, and the nuclei
pycnotic.
6.1.3. Eyes and skin
Eye irritation
The local irritation caused by diquat is less pronounced than
that caused by paraquat. One drop of 20% solution gave rise to
slight conjunctival irritation of the rabbit eye, which persisted
for 2 days (Clark & Hurst, 1970). A 40% diquat solution induced
moderate conjunctival irritation.
Eye cataract
Both rats and dogs fed diets containing diquat developed
cataracts (Howe & Wright, 1965). However, rats fed 7.5 mg
diquat/kg diet over a life-span did not develop cataracts, while 70
mg diquat/kg diet appeared to be the no-observed-adverse-effect
level for dogs. According to Clark & Hurst (1970), rats on diets
containing 50 mg diquat/kg or more developed cataracts in the
course of the study. In another group fed a diet containing 1 g
diquat/kg, eye opacities were discovered within 6 months, while a
few animals on diets of 100 mg/kg and 50 mg/kg showed slight
opacities at the end of the study period. A 2-year test with a
diet containing diquat at 10 mg/kg did not induce cataracts in
rats.
Bilateral cataracts were discovered in all dogs 10 - 11 months
following oral administration of diquat at 15 mg/kg body weight per
day. The dose of 5 mg/kg body weight per day induced eye opacities
after 17 months, and doses of 1.7, 0.8, and 0.4 mg/kg body weight
per day were ineffective after 3 - 4 years of treatment.
A 2-year feeding study was carried out with diquat levels of
15, 25, and 75 mg/kg in the diet of rats. Only the 25 and 75 mg/kg
levels caused cataracts (FAO/WHO, 1978).
Pirie & Rees (1970) confirmed that rats fed diquat dibromide at
0.5 - 0.75 g/kg in the diet developed cataracts. In vivo
observations showed that, invariably, the first change seen was an
opacity in the posterior cortex, immediately under the posterior
capsule of the lens. The next stage was a defined nuclear cataract
that could be seen with the naked eye. Finally, shrinkage and
complete opacity occurred. This histological study revealed that
the first posterior cortical opacity was formed from damaged
epithelial cells. The level of diquat in the blood of these rats
was less than 2.2 µM. No diquat accumulation was registered in the
lens of these rats. The mechanism of the specific cataractogenic
action of diquat is not clear, although in vitro studies
demonstrated that reduction of diquat by the lens was enzymatically
catalysed by glutathione reductase (EC 1.6.4.2) with NADPH as the
source of reducing equivalents. The loss of ascorbic acid from the
lens and the ocular fluids of treated rats was proposed as a factor
for maintaining the normal glutathione level in the rat lens.
Local skin effects
Single diquat applications on the skin of mice (Bainova, l969a)
and rabbits (Clark & Hurst, 1970) did not cause any local
irritation. Daily applications of 1% diquat solution in water to
the skin of rats provoked slight erythema at the site of contact
during the first 10 days, while daily applications of diquat at 20
mg/kg body weight to the skin of rabbits caused mild erythema,
thickening of the skin, and some scabbing (Clark & Hurst, 1970).
Diquat has not been found to be a sensitizer (Bainova, (l969a).
6.1.4. Respiratory system
The effect of diquat on the respiratory system has been studied
after parenteral (Hawkins et al., 1979; Lam et al., 1980), oral
(Verbetskii & Pushkar, 1968; Bainova, 1969; Bainova & Vulcheva,
1978), intratracheal (Lam et al., 1980), and inhalation exposure
(Gage, 1968; Bainova et al., 1972). Unlike paraquat, no specific
effects on the lung were reported, though difficulties in breathing
occurred after severe acute poisoning of the animals with diquat.
6.1.5. Nervous system
General depression and lethargy were most commonly seen
following the administration of high doses of diquat to guinea-pigs
and rats (Verbetskii & Pushkar, 1968; Clark & Hurst, 1970; Crabtree
et al., 1977), and to monkeys (Cobb & Grimshaw, 1979).
6.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
6.1.6.1. Effects on reproduction
Male rats were dosed orally with diquat dibromide at 6.5 mg/kg
body weight per day, for 60 days, and the testes were then examined
biochemically and histologically (Bainova & Vulcheva, 1974). There
were no significant changes in the sperm count, sperm motility, the
testicular tubules, the basal cells, or in the activity of several
enzymes.
A 2-generation study on rats was carried out with dietary
levels of 125 and 500 mg diquat/kg. The 500 mg/kg dose resulted in
reduced body weight for F1a, F1b, F2a, and F2b, and increased
cataracts in F1b and F2b after 91-280 days of exposure. The 125
mg/kg dose resulted in decreased body weight in F1b and F2b, but no
lens opacities were noted (FAO/WHO, 1973).
6.1.6.2. Embryotoxicity and teratogenicity
Diquat was reported to have induced deviations in the prenatal
development of rats (Khera et al., 1968). Bus et al. (1975)
studied the fetal toxicity and teratogenicity of diquat in rats by
administering 15 mg/kg body weight iv on days 7-21 of gestation.
This resulted in 57% fetal resorption compared with 7.6% for
paraquat. The incidence of maternal deaths was essentially the
same. When 14C-diquat and 14C-paraquat were administered to rats,
iv, in a dose of 15 mg/kg body weight on days 13, 16, and 21 of
gestation, paraquat increased radioactivity in fetal lung whereas
diquat appeared to have a stronger embryotoxic action than
paraquat. In the review published in 1979 by FAO/WHO, it was
reported that diquat dibromide monohydrate, administered orally to
pregnant rabbits at doses of 1.25, 2.5, and 5.0 mg/kg had no
adverse effect on the fetuses. In groups of pregnant rats kept on
diets containing 125 and 500 mg diquat cation/kg throughout
gestation, reduced body weight was noted only in the fetuses of
mothers from the 500 mg/kg group. A slightly increased incidence
of subcutaneous haemorrhages was also noted.
Teratogenicity studies in mice have been reported by Selypes et
al. (1980). Single ip doses of diquat at 2.7 and 11 mg/kg body
weight were injected on days 9, 10, 11, and 12 of gestation. The
number of dead fetuses, as well as post-implantational lethality,
increased significantly: average embryo weight was lower and,
though no congenital malformations were noted, there were signs of
skeletal retardation such as large fontanelles, wider cerebral
sutures, flat-shaped ventral nuclei of the vertebrae, and delayed
ossification in the sternum and phalanges. The embryotoxic effect
in mice of high doses of diquat was thus confirmed, but no
chromosomal aberrations were noted in the liver cells of the
embryos from diquat-treated female mice.
6.1.7. Mutagenicity
Studies on the genotoxic potential of diquat are rather
contradictory. Diquat was negative in the Ames test, with and
without metabolic activation (Anderson et al., 1972; Benigni et
al., 1979; Levin et al., 1982). Dominant lethal assays in mice
performed by various authors with several doses of the herbicide
gave negative results (Pasi et al., 1974; Pasi & Embree, 1975;
Anderson et al., 1976). Selypes et al. (1980) injected mice ip
with 22 mg/kg (LD50) diquat, while another group of mice was dosed
orally with 90 mg/kg (0.5 LD50). After 24 and 38 h, preparations of
bone marrow were examined for chromosome aberrations; no
statistically significant changes were determined.
On the other hand, diquat was found to induce slight gene
conversion in Saccharomyces cerevisiae (Siebert & Lemperle, 1974).
Ahmed et al. (1977) reported that diquat induced DNA changes in
cultured SV-40-transformed human cells, with and without metabolic
activation, and the induction of 8-azaguanine resistance in the
Salmonella typhimurium assay was positive (Benigni et al., 1979;
Bignami & Crebelli, 1979). Benigni et al. (1979) also found that
diquat was positive in an S. typhimurium repair test. It was
further reported by these authors that diquat induced gene
mutations in Aspergillus nidulans, and increased unscheduled DNA
synthesis in human epithelial-like cells. They commented that
diquat may have an effect on a number of different genetic
endpoints.
6.1.8. Carcinogenicity
In 2-year feeding studies on rats (Clark & Hurst, 1970), diquat
at levels of up to 720 mg/kg diet did not induce tumours. The
daily ingestion of 2 and 4 mg diquat per kg body weight in water
for a period of 2 years did not have any significant effects on the
health and mortality rate in rats (Bainova & Vulcheva, 1978). Some
histological changes related to chronic interstitial infiltration
and pulmonary adenomatosis in the lungs were found, especially
after the higher dose, but there were no indications of malignancy.
6.2. Effects on Farm Animals
The effects of diquat on farm animals was studied in relation
to its application as an aquatic herbicide and desiccant (Howe &
Wright, 1965; Black et al., 1966; Stevens & Walley, 1966) (section
4.4). Little variation in diquat toxicity in the various animal
species was found, but cattle appeared to be the most sensitive
(LD50 for cattle approximately 30 mg/kg, LD50 for rat 230 mg/kg).
Single oral doses up to 8 mg/kg produced no signs of toxicity in
cows (Stevens & Walley, 1966), and the continuous exposure of
animals via the forage to doses ranging from 0.2 to 330 mg/kg in
the diet (Calderbank, 1972) did not induce any clinical or
pathological changes in farm animals.
Calderbank (1972) recommended that domestic animals should not
be allowed to enter fields newly treated with diquat, nor be given
water recently treated with the herbicide. When edible crops are
treated with diquat, as desiccant, at least 4 days should elapse
before the crops are fed to stock, and when diquat is used for
aquatic weed control, at least 7 days should elapse before the
treated water is used for field irrigation. Recommended levels for
weed control must be observed (Calderbank, 1972).
Sheep given doses of 1, 5, 10, and 20 mg diquat/kg per day in
their drinking-water for 1 month and calves similarly exposed to 5
and 20 mg diquat/kg per day did not show any adverse toxicological
effects as evidenced by growth, food consumption, and observation.
6.3. Dose-Effect of Diquat
The acute LD50 values of diquat in various species were
published by Howe & Wright (1965) and Clark & Hurst (1970). The
acute toxicity of diquat salts (Table 5) does not differ
significantly and is similar for both sexes.
Table 6 summarizes the acute oral, dermal, and inhalation LD50
and LC50 values of diquat in various experimental and domestic
animals. There are no marked species differences but cattle,
guinea-pigs, and monkeys appear to be the most sensitive species.
The few cases of acute diquat poisoning in man have not furnished
sufficient data to determine the lethal dose for man.
The dose-effect relationship of repeated diquat exposure, from
various studies, is summarized in Table 7. Rats, guinea-pigs and
dogs were subjected to ora1 and dietary administration of diquat.
Guinea-pigs appeared to be rather sensitive (Makovskii, 1972), but
the herbicide did not induce cumulative toxic effects (Bainova,
1969, 1975; Makovskii, 1972), because of its relatively rapid
elimination from the organism and the absence of deposits in the
tissues.
Table 5. LD50 (mg/kg) of diquat salts in rats
-----------------------------------------------
Diquat Route of entry Sex LD50
(mg/kg)
-----------------------------------------------
Diquat dibromide oral 215b
Diquat dibromide oral 210b
Diquat dibromide subcutaneous F 11a
Diquat dichloride subcutaneous F 10a
Diquat dichloride subcutaneous M 11a
Diquat dibromide subcutaneous 22b
-----------------------------------------------
a From: Clark & Hurst (1970).
b From: Makovskii (1972).
Table 6. Diquat LD50 (mg/kg) and LC50 (mg/m3)
in various species
----------------------------------------------
Species Oral Dermal Inhalationa
(mg/kg) (mg/kg) (mg/m3)
----------------------------------------------
Rat 400b 650f 35f
Rat 281c 83h
Rat 231e
Rat 215f
Rat 130g
Mouse 170b 430d
Mouse 125e
Rabbit 190b
Rabbit 101e > 400e
Guinea-pig 123c 400f 38f
Guinea-pig approx. 100e
Guinea-pig 100f
Hen 400 - 800b
Hen 200 - 400e
Dog > 200b
Dog 100 - 200e
Cow approx. 30f
Cow 30e
Monkey 100 - 300i
----------------------------------------------
a Respirable diquat aerosol.
b From: Howe & Wright (1965).
c From: Verbetskii & Pushkar (1968).
d From: Bainova (1969a).
e From: Clark & Hurst (1970).
f From: Makovskii (1972).
g From: Bainova (1975).
h From: Bainova & Vulcheva (1977).
i From: Cobb & Grimshaw (1979).
Table 7. Effect of repeated oral, dermal, and inhalation exposure to diquat in experimental animals
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Results obtained Reference
---------------------------------------------------------------------------------------------------------
Rat 87.5, 175, and 350 mg 2 years cataract at all dietary levels FAO/WHO (1971)
diquat ion/kg of diet
Rat 7.2, 36, 72, 180, 2 years no deaths; reduced growth in males at FAO/WHO (1971)
360, and 720 mg diquat highest dietary level; cataract at
ion/kg diet dietary levels of 36 mg diquat ion/kg
diet and above; no cataract at 7.2 mg/kg
Rat 15, 25, and 75 mg 2 years no deaths; "no effect" level for FAO/WHO (1978)
diquat ion/kg diet cataractogenesis 15 mg diquat ion/kg
diet
Rat oral - 6.5, 13, and 40 30 days dose-related biochemical and Bainova (1969,
mg/kg body weight per histological changes in kidney, liver, 1975)
day 2.1 and 4.3 mg/kg 4 1/2 months gastrointestinal system, and lung; no
body weight per day haematological changes; increased
G-6-P-isomerase serum activity;
histological changes at 4.3 mg/kg
body weight per day
Rat oral - 0.2, 2.1, and 1 year the higher doses were toxic for the Makovskii (1972)
5.3 mg/kg body weight 2 species; no-observed-effect levels
per day
Guinea- day 0.1, 1.0, and 2.5 0.2 and 0.1 mg/kg body weight per day
pig mg/kg body weight per for rat and guinea-pig
day
Dog 10, 20, 50, 140, and up to 4 no cataracts at dietary levels up to and FAO/WHO (1971)
420 mg diquat ion/kg years including 50 mg/kg; cataract at 2 higher
of diet dietary levels; no mortality; no effects
on growth
Rat oral - 2 and 4 mg/kg 1 and 2 no increase in mortality rates; Bainova &
per day years histological changes in lungs after Vulcheva (1978)
treatment with 4 mg/kg per day in
drinking-water; minimal effective dose
2 mg/kg per day
---------------------------------------------------------------------------------------------------------
Table 7. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Results obtained Reference
---------------------------------------------------------------------------------------------------------
rat dermal - 5, 10, 20 20 days slight skin irrritation, death and toxic Bainova (1969a)
60, and 120 mg/kg effects at 10 - 120 mg/kg per day;
per day dilation of the gastrointestinal system
at toxic levels; histological changes in
kidney, gastrointestinal system, liver,
and lung at toxic levels, LD50 35 mg/kg
per day without occlusion; no-observed-
effect dose 5 mg/kg per day
Rabbit dermal - 20 and 40 20 days mild skin irritation; toxic effects at Clark & Hurst
at 40 mg/kg per day; no clinical signs (1970)
of toxicity at 20 mg/kg per day; LD50
between 20 and 40 mg/kg per day
Rat inhalationa - 0.50, 15 days clinical signs of irritation and Gage (1968)
1.60, and 2.0 mg/m3 histological changes in lungs at 2mg/m3;
6 h daily no clinical haematological, and
histological deviations at 0.50 mg/m3;
minimum effective concentration 1.0
mg/m3 diquat aerosol
Rat inhalationa - 0.32 4 1/2 months biochemical and histological changes in Bainova (1972)
and 1.90 mg/m3, lungs at 1.90 mg/m3; minimal effective
6 h daily concentration 0.32 mg/m3 diquat aerosol
Rat inhalationa - 0.4, 4 months clinical signs of irritation and toxic Makovskii
0.7, and 1.9 mg/m3, effects at 1.9 mg/m3; 0.7 mg/m3 produced (1972)
4 h daily changes in some rats; minimal effective
concentration 0.4 mg/m3 diquat aerosol
---------------------------------------------------------------------------------------------------------
a Respirable diquat aerosol.
7. EFFECTS ON MAN
7.1. Case Reports
Several cases of acute diquat poisoning among the general
population have been reported in the literature. Fitzgerald et al.
(1978) found 5 cases from 1967 to 1977 in Ireland. Vanholder et
al., (1981) summarized the clinical outcome and the treatment of 11
patients with diquat poisoning (6 fatal and 5 non-fatal).
(a) Suicidal diquat poisoning
Schönborn et al. (1971) reported the fatal case of a man who
drank 2 - 3 mouthfuls of Reglone(R) (estimated 15 - 22 g diquat)
with the intention of committing suicide. Severe vomiting occurred
after 2 h and, 2 h later, watery diarrhoea, the stools having a
peculiar yellow-greenish colour. During the next 6 h, the patient
lost about 3.5 litres of liquid through faeces and 4 litres of
liquid through vomiting. The urine was very concentrated, the
haematocrit was 55%. Serum enzyme activity showed toxic liver
damage, and proteinuria and metabolic acidosis were registered. On
the 2nd day, there were ulcers and severe oropharyngeal
inflammation, on the 3rd day, increasing restlessness, optical
hallucinations, and delirium and stridulous breathing developed.
During the 4th-6th days, anuria, raised body temperature,
generalized convulsions, and coma were registered, and the patient
died on the 7th day of cardiac insufficiency and thrombocytopenia.
The autopsy revealed extensive necrosis of the pharynx and
oesophagus, and petechial bleeding and erosions in the
gastrointestinal tract; pulmonary oedema with haemorrhages, hyaline
membrane production, and bronchopneumonic foci were noted in the
lungs; fatty degeneration was found in the liver and heart, and
severe degeneration of the tubulus epithelium with necrosis in the
kidneys, while the signs of circulatory failure with oedema and
haemorrhagic diapedesis of the brain explained the central nervous
system effects. The diquat concentrations measured on the 1st day
after ingestion were 1.85 mg/litre in the urine and 0.47 mg/litre
in the blood. Higher diquat levels were determined post mortem in
the kidneys, spleen, and lungs (1.19, 1.04, and 0.56 mg/kg,
respectively).
In a second case of suicide, the subject had taken unknown
quantities of Reglone(R) during a period of 3 days (Okonek &
Hofmann, 1975). One day after the second ingestion, she was
admitted to hospital - shocked, sleepy, anuric, with haemorrhagic
mucosal necrosis in the mouth, throat, and eosophagus. Four h
after admission to hospital, the diquat serum level was 1.038
mg/litre. This decreased to 0.30 mg/litre following dialysis.
Death from cardiovascular collapse ensued 46 h after admission.
Vanholder et al. (1981) concluded, from their review of 11
cases, that the lethal dose of Reglone(R) is 30 - 60 ml or
approximately 6 - 12 g diquat dibromide.
An unusual case of diquat poisoning was described by Narita et
al. (1978). A clerk, after drinking heavily, swallowed about 200
ml 30% diquat dibromide formulation. Vomiting was accompanied by
great thirst, severe irritation of the mouth, diarrhoea, and a
temperature of 39 °C. After 24 h, the patient became anuric and
developed acute renal failure; he was comatose and inarticulate,
and had meiosis and unclear light reflexes. He died from dyspnoea
38 1/2 h after ingestion of diquat. Autopsy revealed renal failure
with tubular necrosis, lung haemorrhages, haemorrhagic ulcers, and
erosions in the stomach, and severe congestion of the lungs,
kidneys, liver, gastrointestinal system, and adrenal glands. High
diquat residues were determined in the kidneys, liver, lungs, and
intestines. Vanholder et al. (1981) reported 2 cases of Reglone(R)
ingestion (50 ml and 20 ml) in suicide attempts. Because of
vomiting and diarrhoea, they were admitted to local hospitals, but
no specific treatment was given and the patients were released in
satisfactory clinical condition. However, because of the
development of progressive oliguria several h later, the patients
returned to the hospital. The diquat serum levels were found to be
4.5 and 0.4 mg/litre, respectively. The patients died 1 and 5 days
after the ingestion of diquat.
(b) Accidental diquat poisoning
Oreopoulos & McEvoy (1969) described a patient who accidentally
took a mouthful of Reglone(R) from a soft drink bottle. He spat
out part of it. After 8-10 h, he had diarrhoea and 2 ulcers in the
mouth, but there was no clinical evidence of respiratory, renal, or
central nervous system effects on examination in hospital, and all
laboratory and biochemical examinations were within the normal
physiological limits. The patient continued to excrete diquat in
the urine for 11 days after ingestion. He underwent forced
diuresis and left the hospital in good condition.
Another case of acute poisoning following the accidental
ingestion of less than a mouthful of diquat was reported by Fel et
al. (1976). Nausea, vomiting, and diarrhoea were the first
effects. The patient then developed uraemia, oliguria, and anuria
despite forced diuresis for 2 - 3 days after the accident.
Haemodialysis proved more successful. Bilateral pneumonia was
noted during the 2nd week, but was cured with antibiotics, and the
patient was discharged on the 26th day in good health.
7.2. Effects on Agricultural Operators
A few studies have been performed on workers spraying diquat.
Air concentrations of diquat aerosol were measured by Makovski
(1972) (Table 3). The dermal exposure of the spraymen ranged from
0.05 mg to 0.08 mg on the face and hands after 2 - 3 h of daily
work. The spraymen did not have any complaints, and the clinical
and laboratory examinations did not reveal any significant
differences in comparison with control groups. Wojeck et al.
(1983) studied the exposure of workers applying 1.76% diquat by
hand-operated spray against water hyacinths or using direct
injection of 4.41% spray mixture into the water for hydrilla
control. The spray crews applied diquat 2 - 5 h daily for 4 days
weekly. The inhalatory exposure was found to be < 0.01 mg/h. The
dermal exposure of the spraymen and the airboat drivers were
estimated to be 1.82 and 0.20 mg diquat/h, during the treatment of
water hyacinths. The dermal exposures of the spraymen and the
mixer of diquat for the treatment of water hydrilla were 0.17 and
0.47 mg/h, respectively. The results of urine analysis of all
workers involved in the study were negative (< 0.047 mg/litre).
The dermal exposure to diquat was closely related to the
concentrations used in the working solutions.
Inflammation and bleeding of the nasal mucosa were observed in
people handling crystalline diquat powder in the laboratory or
under field conditions (Clark & Hurst, 1970). Epistaxis during
agricultural diquat application is related to the inhalation of
droplets or splashes from the careless mixing of liquid
concentrates. A worker who spent some considerable time in an
aerosol spray drift developed irritation of the upper respiratory
tract.
According to Clark & Hurst (1970), if a 20% diquat solution
comes into contact with the nail base, nail growth disturbances may
result, and discoloured spots, white bands, and shedding of the
nail were seen after prolonged contact with concentrated diquat.
The nail re-grew normally once exposure was discontinued. No
adverse effects on the nails were observed following the use of
diluted diquat spray solutions in agriculture. Concentrated diquat
formulations have also been reported to delay the healing of
superficial cuts on the hands of spray workers.
Cataracts have never been observed in man following exposure to
diquat (FAO/WHO, 1978; Hayes, 1982).
7.3. First Aid and Medical Treatment
These are essentially the same as those given for paraquat
(section 8.4). See also WHO/FAO (1979).
8. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
8.1. Exposure
8.1.1. Relative contributions of soil, water, air, and food sources
to total diquat uptake
Introduction
Diquat is a contact herbicide and dessicant that is used to
destroy weeds in various agricultural situations. It is used in
the form of an aqueous spray, which means that the potential
exposure of man may occur as a result of its presence in air, on
plants, in soil, or in water.
Degradation of Diquat
Photochemical degradation takes place, when diquat treated
plants are exposed to normal daylight, and continues after plants
are dead. The products formed are of lower toxicity than diquat.
The rapidity of photochemical degradation on plant and soil
surfaces minimizes the hazard of diquat for the environment.
Soil
Diquat is rapidly and tightly bound to clay particles in the
soil, and is thereafter inert. In normal agricultural use, no
toxic breakdown products are to be expected in the soil (section
4.2) where diquat is less persistent than paraquat. Total diquat
residues in the soil after repeated spraying ranged from 0.2 to 3.9
mg/kg. On the 15th day after a single application of diquat,
residues were less than 0.1 mg/kg in field studies. Even at high
rates of application, no specific adverse effects are found on soil
microorganisms, fungi, or invertebrates, and no phytotoxic effects
have been reported on crops.
Water
Following its use as an aquatic herbicide at normal application
rates, diquat residues in water have been found to decrease rapidly
to essentially undetectable levels within 7-14 days (section 4.3).
Toxic effects on fish and other living organisms in the water are
unlikely, because diquat is rapidly photodegraded, absorbed by
aquatic weeds, or adsorbed to soil particles at the bottom.
However, caution should be taken in the application of diquat to
water containing heavy weed growth, since oxygen consumed by
subsequent weed decay may decrease the oxygen content of the water
to such an extent that it is dangerous for fish or other aquatic
organisms. No phytotoxic damage should occur on crops irrigated
with diquat-treated water, if at least 10 days is allowed to elapse
between treatment and irrigation.
Air
Diquat is not volatile. Inhalation exposure can occur via
spray aerosols or contaminated dust but, if correctly applied,
diquat should not give rise to significant inhalation exposure of
the sprayers (section 4.5). Total airborne aerosol concentrations
of diquat in the air in working areas ranged from 0.06 to 0.56
mg/m3, depending on the method of application and the period of
time after the spraying.
Food
Extensive studies on forage desiccated with diquat have
demonstrated that the residues are very low within some days of the
application of the desiccant. Diquat residues in the treated
herbage following pre-harvest desiccation ranged from 0.02 to 25
mg/kg at different intervals after spraying. Trials in which such
forage was fed to cattle and sheep have demonstrated insignificant
residue levels in the milk, meat, and internal organs (section
4.4). Residues found in vegetables, fruits, and cereals have been
low. There is no bioaccumulation.
8.1.2. General population exposure
Inhalation exposure of the general population to diquat may
occur from spray drift off the treated fields, but this is thought
to be insignificant. There are no published data on total diquat
intake among the general population but this again is expected to
be insignificant on the basis of known residue levels. Studies on
its environmental distribution point to a low environmental hazard.
Due to diquat's rapid and complete binding to clay minerals in
soil, contamination of water supplies either from field runoff or
percolation through soil to the water table is not expected
(section 4.2).
Few cases of diquat poisoning have been reported (section 7.1).
Most cases are due to the intentional ingestion of concentrated
formulations, but accidental ingestion has occurred. The decanting
of liquid concentrate formulations into beer, wine, or soft drink
bottles, and subsequent inappropriate storage, is very dangerous.
The acute lethal dose of diquat dibromide is considered to be
6 - 12 g for man. Recovery from diquat poisoning depends on the
cause of ingestion, the dose absorbed, the renal damage, and prompt
initiation of therapy. No long-term adverse effects have been
reported in those who have survived acute diquat poisoning.
8.1.3. Occupational exposure
There may be inhalation, dermal, and to some extent oral
occupational exposure. Spray aerosols and dust particles settle in
the upper respiratory tract. Diquat aerosol concentrations range
from 0.06 to 0.56 mg/m3, according to the spraying method. At a
distance of 200 - 400 m from the treated field, they decrease to
0.09 mg/m3 and less than 0.01 mg/m3. Inhalation exposure was found
to be very low in comparison with dermal (0.17 - 1.82 mg/h)
exposure to diquat during application for aquatic weed control.
Skin irritation, epistaxis, nail damage, and delayed wound healing
have been reported. However, no data on severe or fatal cases of
occupational intoxication, acute ocular damage, or occupational
contact dermatitis caused by diquat were found in the literature.
8.2. Effects
8.2.1. Diquat toxicity in animals
Diquat is less toxic than paraquat and does not cause the
specific lung disease so typical of paraquat exposure.
The primary toxic effect of diquat in animals is
gastrointestinal damage resulting in diarrhoea with consequent
dehydration. After high doses of diquat, minor toxic effects have
been noted in the liver, kidney, and the nervous and endocrine
systems. High concentrations of diquat are irritating to the skin,
although less so than paraquat. Development of eye cataracts has
been reported in rats and dogs following long-term treatment with
diquat (section 6.1.3). This observation has not been reported in
man. Diquat is embryotoxic but it has not been found to be
teratogenic in rats and mice or carcinogenic in long-term feeding
studies on rats given diquat at levels up to 720 mg/kg diet
(sections 6.1.7 and 6.1.8). In vitro mutagenicity studies have
been inconclusive, although generally suggesting weak activity,
while the results of in vivo studies have been negative (section
6.1.8). Thus, the results of animal studies suggest that low-level
exposure to diquat is unlikely to induce toxic effects in man. The
no-observed-effect level in rats has been estimated to be 0.75 mg
diquat ion/kg body weight per day (FAO/WHO, 1978).
8.3. Earlier Evaluations of Diquat by International Bodies
The Joint Meeting on Pesticide Residues (JMPR) reviewed and
published residue and toxicity data on diquat in 1970, 1972, 1976,
1977, 1978 (FAO/WHO 1971, 1973, 1977a,b, 1978, 1979). In 1977, it
estimated the acceptable daily intake (ADI) for man as 0 - 0.008
mg/kg body weight expressed as diquat ion (FAO/WHO 1978).
The same JMPRs have recommended maximum residue levels
(tolerances) for diquat in food commodities of plant and animal
origin.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC are available
from the IRPTC (International Register for Potentially Toxic
Chemicals) legal file (IRPTC 1983).
A data sheet on diquat has been prepared by WHO/FAO (1979) in a
series of "Data sheets on chemical pesticides". Based on a brief
review of use, exposure, and toxicity, practical advice is given on
labelling, safe-handling, transport, storage, disposal,
decontamination, selection, training and medical supervision of
workers, first aid, and medical treatment.
8.4. Conclusions
On the basis of the above findings, it can be concluded that:
General population
Residue levels of diquat in food and drinking-water, resulting
from its normal use, are unlikely to result in a health hazard for
the general population.
Diquat has caused some fatalities following suicidal ingestion.
Occasional accidental fatalities have followed ingestion of
decanted diquat. Ill-effects similar to those caused by paraquat
occur, but the characteristic fibrosis of the lungs is not a
feature.
Occupational exposure
With reasonable work practices including safety precautions,
hygiene measures, and proper supervision, occupational exposure
during the manufacture, formulation, and application of diquat will
not cause a hazard. However, the undiluted concentrate must be
handled with great care, because contamination of eyes and skin
(with possible consequent dermal absorption) can result from
improper work practices.
Environment
Diquat in soil binds rapidly and tightly to clay particles and
residual phytotoxicity from freely available diquat is unlikely.
Under normal conditions of use, the toxicity of diquat for aquatic
organisms is low, though resulting depletion of water oxygen due to
weed decay may pose a problem. Diquat does not seem to represent
an environmental hazard.
REFERENCES
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide-
induced DNA damage and its repair in cultured human cells.
Mutat. Res., 42: 161-164.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDERSON, D., MCGREGOR, D.B., & PURCHASE, I.F.H. (1976)
Dominant lethal studies with diquat and paraquat in male CD-1
mice. Mutat. Res., 40: 349-358.
ANDERSSON, A. & JOSEFSSON, E. (1982) Residues of diquat in
potatoes. Var Föda, 34(Suppl. 3): 223-225.
ATKINSON, G. (1973) Effects of diquat on the microbiological
organisms in the soil of lakes. In: Proceedings of the
Pollution Residue Conference, Wairakei, New Zealand,
pp. 529-538.
BAINOVA, A. (1969a) [Chronic oral toxicity of bipyridylium
herbicides.] Hig. Zdrav., 12: 325-332 (in Bulgarian).
BAINOVA, A. (l969b) [Experimental assessment of the effect
of dipyridylium herbicides on the skin.] Letopisi HEI, 9:
25-30 (in Bulgarian).
BAINOVA, A. (1975) [Cumulative action of Gramoxone and
Reglone.] In: Problemi na higienata, Sofia, Medizina i
Fizkultura, Vol. 1, pp. 31-38 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1974) [Experimental assessment
of the effect of dipyridyliums on sex glands.] In: Works of
the Research Institute of Hygiene and Laboratory Protection,
Sofia, Medizina i Fizkultura, Vol. 22, pp. 111-122 (in
Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1977) [Experimental
substantiation of Reglone MAC in the working environment.] In:
Problemi na higienata, Sofia, Medizina i Fizkultura, Vol. III,
pp. 11-17 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1978) Chronic action of diquat
on the lungs. C. R. Acad. Bulgar Sci., 31: 1369-1372.
BAINOVA A., ZLATEVA, M., & VULCHEVA, V. (1972) [Chronic
inhalation toxicity of bipyridylium herbicides.] Hig. Zdrav.,
15: 25-31 (in Bulgarian).
BENECKE, G. (1977) [Automatic evaluation of an algal
bioassay-inhibition of the movement of a blue-green alga
(Phormidium sp.) by the herbicide diquat.] Z. Wasser-
Abwasserforsch., 10: 195-197 (in German).
BENIGNI, R., BIGNAMI, M., CARERE, A., CONTI, G., CONTI, L.,
CREBELLI, R., DOGLIOTTI, E., GUALANDI, G., NOVELLETTO, A., &
ORTALI, V.A. (1979) Mutational studies with diquat and
paraquat in vitro. Mutat. Res., 68: 183-193.
BIGNAMI, M. & CREBELLI, R. (1979) A simplified method for
the induction of S-azaguanine resistance in S. typhimurium.
Toxicol. Lett., 3: 169-175.
BLACK, W.J.M., CALDERBANK, A., DOUGLAS, G., & McKENNA, R.H.
(1966) Residues in herbage and silage and feeding experiments
following the use of diquat as a desiccant. J. Sci. Food
Agric., 17: 506-509.
BUS, J.S., PREACHE, M.M., CAGEN, S.Z., POSNER, H.S., ELIASON,
B.C., SHARP C.W., & GIBSON, J.E. (1975) Fetal toxicity and
distribution of paraquat and diquat in mice and rats. Toxicol.
appl. Pharmacol., 33: 450-460.
CALDERBANK, A. (1972) Experimental considerations in the
development of diquat and paraquat as aquatic herbicides.
Outlook Agric., 7: 51-54.
CALDERBANK, A. & SLADE, P. (1976) Diquat and paraquat. In:
Kearne, P.C. & Kaufman, D. D. ed. Herbicide chemistry,
degradation and mode of action, 2nd ed., New York, Dekker,
pp. 501-540.
CALDERBANK, A. & YUEN, H. (1966) An improved method for
determining residues of diquat. Analyst, 91: 625-629.
CAVELL, B.D. (1979) Methods used in the study of the
photochemical degradation of pesticides. Pestic. Sci., 10:
177-180.
CHARLES, J.M. & MENZEL, D.B. (1979) Influence of atmospheric
particles on pulmonary absorption phenomenon. ACS Symp. Ser.,
174 (CH 15): 287-301.
CHARLES, J.M., ABOU-DONIA, M.B., & MENZEL, D.B. (1978)
Absorption of paraquat and diquat from the airways of perfused
rat lung. Toxicology, 8: 59-67.
CLARK, D.G. & HURST, E.W. (1970) The toxicity of diquat.
Br. J. ind. Med., 27: 51-55.
COATS, G.E., FUNDERBURK, H.H., LAWRENCE, J.M., & DAVIS, D.E.
(1966) Factors affecting persistence and inactivation of
diquat and paraquat. Weed Res., 6: 58-66.
COBB, L.M. & GRIMSHAW, P. (1979) Acute toxicity of oral
diquat (1,1'-ethylene-2'2'-bipyridylium) in Cynomolgus
monkeys. Toxicol. appl. Pharmacol., 51: 277-282.
CONNING, D.M., FLETCHER, K., & SWAN, A.A.B. (1969) Paraquat
and related bipyridyls. Br. med. Bull., 25: 245-249.
CRABTREE, H.C., LOCK, E.A., & ROSE, M.S. (1977) Effects of
diquat on the gastrointestinal tract of rats. Toxicol. appl.
Pharmacol., 41: 585-595.
DANIEL, J.W. & GAGE, J.C. (1966) Absorption and excretion of
diquat and paraquat in rats. Br. J. ind. Med., 23: 133-136.
DEMBINSKI, F., PONIKIEWSKA, T., & TRZEBNY, W. (1971) [Ground
seed of sunflower desiccated with Reglone as fodder for
ruminants.] Pamiet. Pulawski Pr., 49: 205-211 (in Polish).
DICKES, G.J. (1979) The application of gas chromatography to
food analysis. Talanta, 26: 1065-1099.
FAO/WHO (1971) Diquat. In: 1970 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1973) Diquat. In: 1972 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977a) Diquat. In: 1976 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977b) Pesticide residues in food, Rome, Food and
Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, 10).
FAO/WHO (1978) Diquat. In: 1977 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1979) Diquat. In: 1978 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO (1979) Diquat, Rome, Food and Agriculture Organization
of the United Nations, pp. 101-115 (FAO Plant Production and
Protection Paper No. 15, Supplement).
FEL, P., ZALA, I., IZULE, E., & VARGA, L. (1976)
[Haemodialysis in diquat poisoning.] Orv. Metilap, 117:
1773-1774 (in Hungarian).
FELDMAN, K.J. & MAIBACH, H.I. (1974) Percutaneous
penetration of some pesticides and herbicides in man. Toxicol.
appl. Pharmacol., 28: 126-132.
FITZGERALD, G.R., BARNIVILLE, G., FLANAGAN, M., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978) The changing pattern of
paraquat poisoning: an epidemiologic study. J. Irish Med.
Assoc., 71: 103-108.
FLETCHER, K., ed. (1975) Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-89
GAGE, J.C. (1968a) Toxicity of paraquat and diquat aerosols
generated by a size-selective cyclone: effect of particle size
distribution. Br. J. ind. Med., 25: 304-314.
GAGE, J.C. (1968b) The action of paraquat and diquat on the
respiration of the liver cell fractions. Biochem. J., 1O9:
757-761.
GOWMAN, M.E., REILY, D., & NEWBY, S.E. (1980) Paraquat and
Diquat: Longterm high rate trial, Frensham, United Kingdom. 2.
Persistence and movement in soil and glasshouse bioassay,
London, ICI Ltd (Report RJ0014B).
GROVER, R., SMITH, A.E., & KORVEN, H.C. (1980) A comparison
of chemical and cultural control of weeds in irrigation
ditchbanks. Can. J. Plant Sci., 60: 185-195.
GRZENDA, A.R., NICHOLSON, H.P., & COX, W.S. (1966)
Persistence of four herbicides in pond water. J. Am. Water
Works Assoc., 3: 326-332.
HAWKINS, S.F., MEDINA, M.A., & STAVINOHA, W. (1979) The
acute in vivo effect of paraquat and diquat on intermediary
metabolism in mouse lung. Proc. Fed. Am. Soc. Exp. Biol.,
38(3, Pt I): 582.
HAYES, W.J., Jr (1982) Pesticides studies in man, Baltimore,
London, Williams & Wilkins, 561 pp.
HOWE, D.J.T. & WRIGHT, N. (1965) The toxicity of paraquat
and diquat. In: Proceedings of the 18th New Zealand Weed &
Pest Control Conference, pp. 105-114.
HUGHES, R.D., MILLBURN, P., & WILLIAMS, R.T. (1973) Biliary
excretion of some diquaternary ammonium cations in the rat,
guinea-pig and rabbit. Biochem. J., 136: 979-984.
ICI (1972a) Determination of residues of diquat in soil -
residue analytical method No. 6, London, ICI Ltd (Report No.
PPRAM-6).
ICI (l972b) Determination of residues of diquat in milk and
water - residue analytical method No.7, London, ICI Ltd
(Report No. PPRAM-7).
ICI (l972c) Determination of residues of diquat in crops and
animal tissues - residue analytical method No. 5, London, ICI
Ltd (Report No. PPRAM-5).
IRPTC (1983) IRPTC Legal files 1983 Vol. I & II, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme.
KHERA, K.S., WHITTA, L.L., & CLEGG, D.J. (1968) Embryopathic
effects of diquat and paraquat in rats. Ind. Med. Surg., 37:
257-261.
KING, R.R. (1978) Gas-chromatographic determination of
diquat residues in potato tubers. J. agric. food Chem., 26:
1460-1463.
KURISAKI, E. & SATO, H. (1979) [Toxicological studies on
herbicides: intracorporal distribution of paraquat dichloride
and diquat dibromide in rat.] Nippon Hoigaku Zasshi, 33: 656
(in Japanese).
LAM, H.F., TAKAZAWA, J., GUPTA, B.N., & STEE, E.W. VAN
(1980) A comparison of the effects of paraquat and diquat on
lung compliance, lung volume and single-breath diffusing
capacity in the rat. Toxicology, 18: 111-123.
LAVAUR, E., SION, G., GROLLEAU, G., & CARPENTER-LESECK, J.
(1979) Etude comparée de l'action du diquat et du paraquat
sur la muqueuse digestive de la souris, du rat et du lapin.
Ann. Zool. Ecol. anim., 11: 159-169.
LEARY, J.B. (1978) Diquat and paraquat. In: Zweig, G. &
Sharma, J., ed. Analytical methods for pesticides and plant
growth regulators, New York, Academic Press, pp. 321-325.
LEVIN, D.E., HOLLSTEIN, M., CHRISTMAN, M.F., SCHWIERS, E.A., &
AMES, B.N. (1982) A new Salmonella tester strain (TA 102)
with AT base pairs at the site of mutation detects oxidative
mutagens. Proc. Natl Acad. Sci. US, 79: 7445-7449.
LITCHFIELD, N.H., DANIEL, J.W., & LONGSHAW, S. (1973) The
tissue distribution of the bipyridylium herbicides diquat and
paraquat in rats and mice. Toxicology, 1: 155-165.
LOCK, E.A. (1979) The effect of paraquat and diquat on renal
function in the rat. Toxicol. appl. Pharmacol., 48: 327-336.
LOCK, E.A. & ISHMAEL, J. (1979) The acute toxic effects of
paraquat and diquat on the rat kidney. Toxicol. appl.
Pharmacol., 50: 67-76.
MAKOVSKII, V.N. (1972) [Toxicological and hygiene studies of
the bipyridylium herbicides diquat and paraquat,] Referat,
Vinniza, USSR, pp. 1-23 (PhD thesis) (in Russian).
MATSUURA, N., TAKINAMI, M., KURISAKI, E., & SATOO, H. (1978)
[Distribution of paraquat dichloride and diquat dibromide in
the living body.] Fukushima Igakkai Zasshi, 28: 212 (in
Japanese).
NARITA, S., MATOJUKU, M., SATO, J., & MORI, H. (1978)
[Autopsy in acute suicidal poisoning with diquat dibromide.]
Nippon Igakkai Zasshi, 27: 454-455 (in Japanese).
O'BRIEN, M.C. & PRENDEVILLE, G.N. (1978) A rapid sensitive
bioassay for determination of paraquat and diquat in water.
Weed Res., 18: 301-303.
OKONEK, S. & HOFMANN, A. (1975) On the question of
extracorporeal haemodialysis for diquat intoxication. Arch.
Toxicol., 33: 251-257.
OREOPOULOS, D.G. & MCEVOY, J. (1969) Diquat poisoning.
Postgrad. med. J., 45: 635-637.
PASI, A. & EMBREE, J.W. (1975) Further comments on the
assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 31: 125-126.
PASI, A., EMBREE, J.W., EISENLORD, G.A., & HINE, C.H. (1974)
Assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 26: 171-175.
PIRIE, A. & REES, J.R. (1970) Diquat cataract in the rat.
Exp. Eye Res., 9: 198-203.
PYL, W. & GIEBELMANN, R. (1978) [Detection of chlorocholine
chloride, diquat and paraquat.] Arch. vet. Med., 32: 601-602
(in German).
REISH, D., ROSSI, S.S., MEARNS, A.J., OSHIDA, P.S., & WILKES,
F.G. (1979) Marine and estuarine pollution. J. Water Pollut.
Control Fed., 51: 1477-1517.
RILEY, D. (1981) The fate and effect and diquat residues in
soil. In: Proceedings of the National Spray Seed Conference
1981, Albury, New South Wales, Australia.
RILEY, D. & GRATTON, R.P. (1974) Unavailability to plants of
diquat residues in soils. 10th Tr. Muzhdunar. Kongr.
Pochvoved., USSR, 3: 193-203.
ROSE, M.S. & SMITH, L.L. (1977a) The relevance of paraquat
accumulation by tissues. In: Autor, P.A., ed. Biochemical
mechanisms of paraquat toxicity, New York, Academic Press,
pp. 71-91.
ROSE, M.S. & SMITH, L.L. (l977b) Tissue uptake of paraquat
and diquat. Gen. Pharmacol., 8: 173-176.
SCHOENBORN, H., SCHUSTER, H.P., & KOESSLING, F.K. (1971)
[Clinical and muphological findings in an acute oral
intoxication with diquat Reglon.] Arch. Toxicol., 27: 204-216
(in German).
SCHULTZ, VON O., KIRCHNER, K., MUELLER, P., & ROTHE, R.
(1976) [Reglone (Diquat) cause of intoxication of sheep,
cattle, and swine.] Monatsh. Veterinärmed., 31: 647-649 (in
German).
SELYPES, A., NAGYMAJTENYI, L., & BERENCSI, G. (1980)
Mutagenic and embryotoxic effects of paraquat and diquat.
Bull. environ. Contam. Toxicol., 25: 513-517.
SHARP, C.W., OTTLENGHI, A., & POSNER, H.S. (1972)
Correlation of paraquat toxicity with tissue concentrations
and weight loss of the rat. Toxicol. appl. Pharmacol., 22:
241-251.
SIEBERT, D. & LEMPERLE, E. (1974) Genetic effects of
herbicides: induction of mitotic gene conversation in
Saccharomyces cervisiae. Mutat. Res., 22: 1ll-120.
SINGH, S.P. & YADAV, N.K. (1978) Toxicity of some herbicides
to maior carp fingerlings. Indian J. Ecol., 5: 141-147.
SMITH, S.N., LYON, A.J., & SAHID, I.B. (1976) The breakdown
of paraquat and diquat by soil fungi. New Phytol., 77: 735-740.
SMITH, T.P., NOACK, A., & COSH, S.M. (1981) The effect of
some herbicides on vesicular-arbuscular endophyte abundance in
the soil and on infection of host roots. Pestic. Sci., 12:
91-97.
STEVENS, M.A. & WALLEY, J.K. (1966) Tissue and milk residues
arising from the ingestion of single doses of diquat and
paraquat by cattle. J. Sci. Food Agric., 17: 472-475.
SUMMERS, L.A. (1980) The bipyridilium herbicides, London,
New York, Toronto, San Francisco, Academic Press, pp. 1-449.
TSCHIPILSKA, L.N. (1980) [The influence of some pesticides
on the soil microorganisms in connection with the hygiene
evaluation of the soil,] Referat, Sofia, Bulgaria, pp. 1-39
(PhD thesis) (in Bulgarian).
TOMPSETT, S.L. (1970) Paraquat poisoning. Acta pharmacol.
toxicol., 28: 346-358.
TUCKER, V.B., PACK, D.E., & OSPENSON, J.N. (1967) Adsorption
of bipyridylium herbicides in soil. J. agric. food Chem., 15:
1005-1008.
UKAI, S., HIROSE, K., & KAWASE, S. (1977) [Forensic chemical
studies on drugs and chemicals. III. Gas chromatography of
reduction products of the herbicides diquat and paraquat.]
Eisei Kagaku, 23: 32-38 (in Japanese).
VANHOLDER, R., COLARDYN, F., RENCK, DE J., PRAET, M., LAMEIRE,
N., & RINGOIR, S. (1981) Diquat intoxication. Report of two
cases and review of the literature. Am. J. Med., 70: 1267-1271.
VERBETSKII, V.E. & PUSHKAR, M.S. (1968) [Pathological
changes in the organs of animals on acute poisoning with the
herbicide diquat (Reglone).] In: Some questions concerning
human and animal morphology, Medizina, Odessa, pp. 51-52 (in
Russian).
WEBER, J.B., PERRY, P.W., & UPCHURCH, R.P. (1965) The
influence of temperature and time on the adsorption of
paraquat, diquat, 2,4-D and prometone by clays, charcoal and
anion exchange resin. In: Soil Science Society Proceedings,
pp. 678-688.
WHO/FAO (1979) Data sheets on pesticides: Diquat, Geneva,
World Health Organization, pp. 1-7 (Unpublished Report No.
VBC/DS/79-40).
WILKINSON, W. (1980) Paraquat and Diquat: Longterm high rate
trial, Frensham, United Kingdom. 1. Management of site,
effects on crops and weeds and residues in crops, London, ICI
Ltd (Report No. RJ0013B).
WILLIAMS, C.B., LEVISON, S.A., & DANDLIKER, W.B. (1976)
Application of immunological techniques to the detection of
organic contaminants of environmental concern. In: Proceedings
of the University of Montana Annual Conference on Trace
Substances & Environmental Health, No. 10, pp. 317-322.
WOJECK, G.A., PRICE, J.F., NIGG, H.N., & STAMPER, J.H.
(1983) Worker exposure to paraquat and diquat. Arch. environ.
Contam. Toxicol., 12: 65-70.
 Paraquat is non-explosive and non-flammable in aqueous
formulations. It is corrosive to metals and incompatible with
alkylarylsulfonate wetting agents. It is stable in acid or neutral
solutions but is readily hydrolysed by alkali.
Paraquat readily undergoes a single-electron reduction to the
cation radical. The redox potential for this reaction is 446 mv.
This chemical property led to its use as a redox indicator dye
(methyl viologen) as early as 1933 (Summers, 1980).
2.3. Analytical Methods
The analytical methods for paraquat determination have been
reviewed by Haley (1979) and Summers (1980). Current procedures in
common use are listed in Table 2. Spectrophotometric
determinations involve the reaction of paraquat with 1% aqueous
sodium dithionite in 0.1 N sodium hydroxide. The absorbance of the
resulting blue cation measured at 600 nm can be used as a measure
of the paraquat concentration. Diquat does not interfere because
its radical cation is green in colour. For residue level
determinations (e.g., sub mg/kg levels) the higher intensity
absorption at 396 nm for the paraquat radical and the 379 nm for
the diquat radical are more commonly used. Calderbank & Yuen
(1965) developed a column chromatographic spectrophotometric method
that was successfully applied for soil, biological tissues, and
food. The sensitivity was 0.01 mg/kg. Gas chromatographic and
high-pressure liquid chromatographic analyses were used
satisfactorily. High-pressure liquid chromatography with
ultraviolet detection was proposed by Pryde & Darby (1975) for
determining the paraquat content of urine with a sensitivity of 100
µg/litre.
A comparison of thin-layer chromatography with the
spectrophotometric methods for determining paraquat in human
tissues showed that the former method gave less favourable results,
because of the presence of large amounts of interfering substances
from the tissues (Tsunenari et al., 1975; Haley, 1979).
Spectrophotometric determination of paraquat, after alkaline
reduction with sodium dithionite, has been published (Leary, 1978)
for soil, and plant and biological tissues, the sensitivity limit
being 0.01 mg/kg when a 50 g sample was used.
In a comparison of colorimetric, gas-liquid chromatographic
techniques and radioimmunoassay (Levitt, 1979; Stewart et al.,
1979), it was shown that the latter was a rapid method with
satisfactory sensitivity for determining paraquat in serum, urine,
and organ tissues from poisoned patients. The variation in
detection limits in paraquat determinations in soil, water, and
plant and animal material is related to the size of the sample
obtained, its purity, and the extraction of the paraquat ion from
the material tested.
(a) Soil
Analytical methods include spectrophotometry (Calderbank &
Yuen, 1965; Leary, 1978) and gas chromatography (Khan, 1974; Payne
et al., 1974).
Table 2. Analytical methods for paraquat
----------------------------------------------------------------------------------------
Matrix Analytical procedure Detection Reference
limitsa
----------------------------------------------------------------------------------------
Soil spectrophotometry 0.01 mg/kg Calderbank & Yuen (1965)
spectrophotometry - Leary (1978)
spectrophotometry 0.5 mg/kg Pope & Benner (1974)
gas chromatography 0.01 mg/kg Khan (1974)
gas chromatography 0.01 mg/kg Payne et al. (1974)
Water spectrophotometry 0.01 mg/litre Calderbank & Yuen (1965)
gas chromatography 0.01 mg/litre Soderquist & Crosby (1972)
gas chromatography 0.01 mg/litre Khan (1974)
gas chromatography 0.01 mg/litre Payne et al. (1974)
gas chromatography 10 mg/litre Ukai et al. (1977)
spectrophotometry - Pope & Benner (1974)
Air spectrophotometry 0.01 mg/m3 Calderbank & Yuen (1965)
gas chromatography 0.5 ng/m3 Seiber & Woodrow (1981)
Biological tissues spectrophotometry 0.01 µg/ml Calderbank & Yuen (1965)
spectrophotometry 0.01 µg/ml Berry & Grove (1971)
spectrophotometry 0.01 µg/ml Beyer (1970)
gas chromatography 0.03 µg/ml van Dijk et al. (1977)
gas chromatography/mass 0.025 µg/ml Draffon et al. (1977)
spectrophotometry
radioimmuno assay 0.12 µg/ml Levitt (1979)
radioimmuno assay 0.10 µg/ml Proudfoot et al. (1979)
Plants spectrophotometry 0.01 mg/kg Calderbank & Yuen (1965)
spectrophotometry 0.01 - 1 mg/kg Dickes (1979)
gas chromatography 0.01 - 1 mg/kg Paschal et al. (1979)
gas chromatography - Harrington (1979)
----------------------------------------------------------------------------------------
a The figures refer to the detection limits in the assay solutions.
(b) Water
The concentration of paraquat in water has been determined by
treating the lesser duckweed (Lemna minor) with the test sample
and comparing the time taken to produce chlorosis with known
concentrations. This procedure has been used to determine
herbicide residues in ponds and streams with a sensitivity of 0.075
mg/litre. Determination of chlorosis in Phaseolus vulgaris or
Lemna polyrhiza was classified as more sensitive than the chemical
analyses (Haley, 1979).
A change in cell-membrane permeability, as indicated by the
leakage of electrolytes from treated fronds of Lemna minor, was
used by O'Brien & Prendeville (1978) to detect paraquat in water.
The minimum detectable concentrations ranged from 1.8 - 1.7 µg of
paraquat cation/ml, after 3 h of treatment, to 180 and 17 ng/ml
after 72 h of exposure to light.
Ukai et al. (1977) found a gas chromatographic method suitable
for paraquat determination with a sensitivity of 10 - 90 µg/ml
water, using 4-anisidine as the internal standard. Pope & Benner
(1974) have also used a spectrophotometric method.
(c) Air-working environment
Sprayed or dusted, paraquat is absorbed on filter/sorbent
systems. The absorbed paraquat is dissolved and determined
spectrophotometrically using one of the classical methods
(Calderbank & Yuen, 1965; Staiff et al., 1975; Anderson et al.,
1981). Carlstrom (1971) applied a colorimetric method for
analysing paraquat formulations. Seiber & Woodrow (1981) developed
a nitrogen-selective gas chromatographic method for paraquat
determination in airborne particulate matter.
(d) Plants
The method of Calderbank & Yuen (1965) is considered to be the
best procedure for determining paraquat in crops, treated plants,
and food. The limit of the spectrophotometric analysis ranged from
0.01 - 0.1 mg/kg, depending on the crop. A gas chromatographic
method for paraquat residues in food was suggested by Dickes
(1979). A procedure based on gas-liquid chromatography (Paschal et
al., 1979) provided linear working curves over a paraquat
concentration range of 0 - 20 µg/g, determined by extraction from
1 g samples of sunflower seeds. The method has been proposed for
herbicide analyses in plant materials. A vapour-phase
chromatographic technique, used for determining paraquat in wood
(Harrington, 1979), is based on the liberation of methyl chloride
after pyrolysis.
(e) Biological material
A spectrophotometric method, applied for determining paraquat
residues in milk (ICI, 1972), had a detection limit of 0.01
mg/litre sample. Analyses of the plasma (serum) and urine of
subjects poisoned by paraquat are important for diagnosis and
prognosis. Tompsett (1970) described a method for analysing
biological samples from patients suffering from accidental oral
intoxication. Paraquat extracted from human blood, urine, and
faeces was separated on a strong acid cation-exchange resin (Beyer,
1970), reacted with sodium dithionite, and determined
spectrophotmetrically at 391 nm. The method had a sensitivity of
0.01 µg ion/ml in a 250 ml aliquot of urine. A similar procedure,
published by Pickova (1978), for estimating paraquat levels in the
urine of patients had a sensitivity of 30 µg in a sample of 50 -
500 ml. Gas chromatographic methods were successfully used (Dijk,
van et al., 1977; Draffon et al., 1977).
A radioimmunoassay using 3H-labelled paraquat was found to
be a sensitive method for analysing plasma, urine, and biological
tissues (ICI, 1979). Antibodies to paraquat were prepared in
rabbits and tested for sensitivity by a charcoal separation
technique (Levitt, 1979). The results showed that the antibodies
were specific for the herbicide. A comparison of radioimmunoassay
and gas liquid chromatographic techniques (Levitt, 1979; Proudfoot
et al., 1979) showed the high sensitivity of this method. The
total assay time was no more than 30 min. A series of 50 serum
specimens from persons poisoned with paraquat were tested by
radioimmunoassay and colorimetric analysis (Stewart et al., 1979);
the results from both methods corresponded closely.
Tsunenari et al. (1975) used 7 analytical methods for
determining paraquat with a view to diagnosing accidental,
suicidal, or homicidal poisoning. Colorimetry, with dithionite
thin-layer chromatography, was used for the qualitative assay of
paraquat in biological tissues, while ion-exchange resin column
chromatography, with colorimetry or gas chromatography, was used
for the quantitative assay. Tsunenari et al. (1981) also studied
the influence of putrefaction on paraquat determinations in autopsy
materials. Detection was possible, even in tissues in advanced
stages of decomposition.
3. SOURCES IN THE ENVIRONMENT
3.1. Introduction
3.1.1. Industrial technology
Paraquat does not occur naturally. It was originally
synthesized by Weidel & Russo as reported in 1882 (Summers, 1980).
Its herbicidal properties were discovered only in 1955. The
compound is produced by coupling pyridine in the presence of sodium
in anhydrous ammonia and quaternizing the 4,4'-bipyridyl with
methyl chloride (Fig. 1).
Paraquat is non-explosive and non-flammable in aqueous
formulations. It is corrosive to metals and incompatible with
alkylarylsulfonate wetting agents. It is stable in acid or neutral
solutions but is readily hydrolysed by alkali.
Paraquat readily undergoes a single-electron reduction to the
cation radical. The redox potential for this reaction is 446 mv.
This chemical property led to its use as a redox indicator dye
(methyl viologen) as early as 1933 (Summers, 1980).
2.3. Analytical Methods
The analytical methods for paraquat determination have been
reviewed by Haley (1979) and Summers (1980). Current procedures in
common use are listed in Table 2. Spectrophotometric
determinations involve the reaction of paraquat with 1% aqueous
sodium dithionite in 0.1 N sodium hydroxide. The absorbance of the
resulting blue cation measured at 600 nm can be used as a measure
of the paraquat concentration. Diquat does not interfere because
its radical cation is green in colour. For residue level
determinations (e.g., sub mg/kg levels) the higher intensity
absorption at 396 nm for the paraquat radical and the 379 nm for
the diquat radical are more commonly used. Calderbank & Yuen
(1965) developed a column chromatographic spectrophotometric method
that was successfully applied for soil, biological tissues, and
food. The sensitivity was 0.01 mg/kg. Gas chromatographic and
high-pressure liquid chromatographic analyses were used
satisfactorily. High-pressure liquid chromatography with
ultraviolet detection was proposed by Pryde & Darby (1975) for
determining the paraquat content of urine with a sensitivity of 100
µg/litre.
A comparison of thin-layer chromatography with the
spectrophotometric methods for determining paraquat in human
tissues showed that the former method gave less favourable results,
because of the presence of large amounts of interfering substances
from the tissues (Tsunenari et al., 1975; Haley, 1979).
Spectrophotometric determination of paraquat, after alkaline
reduction with sodium dithionite, has been published (Leary, 1978)
for soil, and plant and biological tissues, the sensitivity limit
being 0.01 mg/kg when a 50 g sample was used.
In a comparison of colorimetric, gas-liquid chromatographic
techniques and radioimmunoassay (Levitt, 1979; Stewart et al.,
1979), it was shown that the latter was a rapid method with
satisfactory sensitivity for determining paraquat in serum, urine,
and organ tissues from poisoned patients. The variation in
detection limits in paraquat determinations in soil, water, and
plant and animal material is related to the size of the sample
obtained, its purity, and the extraction of the paraquat ion from
the material tested.
(a) Soil
Analytical methods include spectrophotometry (Calderbank &
Yuen, 1965; Leary, 1978) and gas chromatography (Khan, 1974; Payne
et al., 1974).
Table 2. Analytical methods for paraquat
----------------------------------------------------------------------------------------
Matrix Analytical procedure Detection Reference
limitsa
----------------------------------------------------------------------------------------
Soil spectrophotometry 0.01 mg/kg Calderbank & Yuen (1965)
spectrophotometry - Leary (1978)
spectrophotometry 0.5 mg/kg Pope & Benner (1974)
gas chromatography 0.01 mg/kg Khan (1974)
gas chromatography 0.01 mg/kg Payne et al. (1974)
Water spectrophotometry 0.01 mg/litre Calderbank & Yuen (1965)
gas chromatography 0.01 mg/litre Soderquist & Crosby (1972)
gas chromatography 0.01 mg/litre Khan (1974)
gas chromatography 0.01 mg/litre Payne et al. (1974)
gas chromatography 10 mg/litre Ukai et al. (1977)
spectrophotometry - Pope & Benner (1974)
Air spectrophotometry 0.01 mg/m3 Calderbank & Yuen (1965)
gas chromatography 0.5 ng/m3 Seiber & Woodrow (1981)
Biological tissues spectrophotometry 0.01 µg/ml Calderbank & Yuen (1965)
spectrophotometry 0.01 µg/ml Berry & Grove (1971)
spectrophotometry 0.01 µg/ml Beyer (1970)
gas chromatography 0.03 µg/ml van Dijk et al. (1977)
gas chromatography/mass 0.025 µg/ml Draffon et al. (1977)
spectrophotometry
radioimmuno assay 0.12 µg/ml Levitt (1979)
radioimmuno assay 0.10 µg/ml Proudfoot et al. (1979)
Plants spectrophotometry 0.01 mg/kg Calderbank & Yuen (1965)
spectrophotometry 0.01 - 1 mg/kg Dickes (1979)
gas chromatography 0.01 - 1 mg/kg Paschal et al. (1979)
gas chromatography - Harrington (1979)
----------------------------------------------------------------------------------------
a The figures refer to the detection limits in the assay solutions.
(b) Water
The concentration of paraquat in water has been determined by
treating the lesser duckweed (Lemna minor) with the test sample
and comparing the time taken to produce chlorosis with known
concentrations. This procedure has been used to determine
herbicide residues in ponds and streams with a sensitivity of 0.075
mg/litre. Determination of chlorosis in Phaseolus vulgaris or
Lemna polyrhiza was classified as more sensitive than the chemical
analyses (Haley, 1979).
A change in cell-membrane permeability, as indicated by the
leakage of electrolytes from treated fronds of Lemna minor, was
used by O'Brien & Prendeville (1978) to detect paraquat in water.
The minimum detectable concentrations ranged from 1.8 - 1.7 µg of
paraquat cation/ml, after 3 h of treatment, to 180 and 17 ng/ml
after 72 h of exposure to light.
Ukai et al. (1977) found a gas chromatographic method suitable
for paraquat determination with a sensitivity of 10 - 90 µg/ml
water, using 4-anisidine as the internal standard. Pope & Benner
(1974) have also used a spectrophotometric method.
(c) Air-working environment
Sprayed or dusted, paraquat is absorbed on filter/sorbent
systems. The absorbed paraquat is dissolved and determined
spectrophotometrically using one of the classical methods
(Calderbank & Yuen, 1965; Staiff et al., 1975; Anderson et al.,
1981). Carlstrom (1971) applied a colorimetric method for
analysing paraquat formulations. Seiber & Woodrow (1981) developed
a nitrogen-selective gas chromatographic method for paraquat
determination in airborne particulate matter.
(d) Plants
The method of Calderbank & Yuen (1965) is considered to be the
best procedure for determining paraquat in crops, treated plants,
and food. The limit of the spectrophotometric analysis ranged from
0.01 - 0.1 mg/kg, depending on the crop. A gas chromatographic
method for paraquat residues in food was suggested by Dickes
(1979). A procedure based on gas-liquid chromatography (Paschal et
al., 1979) provided linear working curves over a paraquat
concentration range of 0 - 20 µg/g, determined by extraction from
1 g samples of sunflower seeds. The method has been proposed for
herbicide analyses in plant materials. A vapour-phase
chromatographic technique, used for determining paraquat in wood
(Harrington, 1979), is based on the liberation of methyl chloride
after pyrolysis.
(e) Biological material
A spectrophotometric method, applied for determining paraquat
residues in milk (ICI, 1972), had a detection limit of 0.01
mg/litre sample. Analyses of the plasma (serum) and urine of
subjects poisoned by paraquat are important for diagnosis and
prognosis. Tompsett (1970) described a method for analysing
biological samples from patients suffering from accidental oral
intoxication. Paraquat extracted from human blood, urine, and
faeces was separated on a strong acid cation-exchange resin (Beyer,
1970), reacted with sodium dithionite, and determined
spectrophotmetrically at 391 nm. The method had a sensitivity of
0.01 µg ion/ml in a 250 ml aliquot of urine. A similar procedure,
published by Pickova (1978), for estimating paraquat levels in the
urine of patients had a sensitivity of 30 µg in a sample of 50 -
500 ml. Gas chromatographic methods were successfully used (Dijk,
van et al., 1977; Draffon et al., 1977).
A radioimmunoassay using 3H-labelled paraquat was found to
be a sensitive method for analysing plasma, urine, and biological
tissues (ICI, 1979). Antibodies to paraquat were prepared in
rabbits and tested for sensitivity by a charcoal separation
technique (Levitt, 1979). The results showed that the antibodies
were specific for the herbicide. A comparison of radioimmunoassay
and gas liquid chromatographic techniques (Levitt, 1979; Proudfoot
et al., 1979) showed the high sensitivity of this method. The
total assay time was no more than 30 min. A series of 50 serum
specimens from persons poisoned with paraquat were tested by
radioimmunoassay and colorimetric analysis (Stewart et al., 1979);
the results from both methods corresponded closely.
Tsunenari et al. (1975) used 7 analytical methods for
determining paraquat with a view to diagnosing accidental,
suicidal, or homicidal poisoning. Colorimetry, with dithionite
thin-layer chromatography, was used for the qualitative assay of
paraquat in biological tissues, while ion-exchange resin column
chromatography, with colorimetry or gas chromatography, was used
for the quantitative assay. Tsunenari et al. (1981) also studied
the influence of putrefaction on paraquat determinations in autopsy
materials. Detection was possible, even in tissues in advanced
stages of decomposition.
3. SOURCES IN THE ENVIRONMENT
3.1. Introduction
3.1.1. Industrial technology
Paraquat does not occur naturally. It was originally
synthesized by Weidel & Russo as reported in 1882 (Summers, 1980).
Its herbicidal properties were discovered only in 1955. The
compound is produced by coupling pyridine in the presence of sodium
in anhydrous ammonia and quaternizing the 4,4'-bipyridyl with
methyl chloride (Fig. 1).
 When bipyridyl is refluxed with methyl iodide, the iodide salt
is obtained. Haley (1979) and Summers (1980) thoroughly reviewed
the published methods for paraquat synthesis, and for the
separation and purification of bipyridylium salts. The yields
obtainable vary from 20% to 96% of pure product.
The first commercial paraquat formulation approved for
agricultural use was Gramoxone(R).
3.1.2. Impurities
Aqueous solutions of paraquat used as herbicides must
correspond to the FAO Specification Code 56/13/S/6 (FAO, 1973).
This requires a description of the active ingredient in the
formulation, of the impurities, of the physical and chemical
properties, and of the methods for determining the components.
The only impurity permitted in paraquat is free 4,4'-bipyridyl
at a maximum level of 0.25% of the paraquat content.
3.2. Production and Use
Paraquat is produced in several countries, including China,
Province of Taiwan, Italy, the United Kingdom, and the USA.
Formulations of the active ingredients (mainly paraquat dichloride)
are used in more than 130 countries world-wide. Paraquat
dimethylphosphate is used in the USSR. Since its introduction for
agricultural use in 1962, paraquat has been widely used for weed
control and as a dessicant. In many countries, paraquat is
formulated locally, only the technical active ingredient being
imported. Records of world production of paraquat are not
available.
Technical paraquat dichloride has been formulated in liquid
concentrates or granules. Water-soluble granules containing
paraquat (25 g/kg) and diquat (25 g/kg) are used for weed control
in private gardens. Paraquat is sold under a variety of trade
names which are summarized in Table 3.
Gramoxone(R) is a dark aqueous solution containing a paraquat
dichloride concentration of 200 ± 10 g/litre. Its specific gravity
at 20 °C is 1.1 and the crystallization point is -5 °C to 10 °C.
It is not flammable and, in its original polyethylene containers,
is stable for a long time under normal atmospheric conditions. The
formulation is incompatible with anionic surface active agents and
decomposes in ultraviolet radiation. Gramoxone(R) rapidly
corrodes aluminium; zinc, iron, and tinplate are more resistant.
Paraquat is a total contact herbicide used to control broad-
leaved and grassy weeds. It should be sprayed when the weeds are
young and less than 30 cm high. It kills all green tissues, but
does not harm the mature bark. Paraquat is used for plantation
crops (banana, cocoa-palm, coffee, oil-palm, rubber, etc.) and for
citrus fruits, apples, plums, vines, and tea. On certain crops
(potato, pineapple, sugar-cane, sunflower), it is used as a
dessicant; it is also used as a cotton defoliant. It is applied
around the trees in orchards and between the rows of crops.
Uncropped land on industrial sites, railways, roadsides, etc.
can be cleared of weeds by applying paraquat at higher
concentrations.
Gramoxone S(R) is largely applied for aquatic weed control.
Application rates usually range from 250 g - 1500 g/ha (1.1 -
7.1 1itre of Gramoxone(R), but, for grass and stubble clearing, up
to 2200 g of the herbicide are used per ha. The working dilutions
vary from 1 - 5 g per litre paraquat in water. It is applied by
ground sprayers (not mist-blowers) in 200 - 500 litres solution/ha.
3.3. Mechanism of the Herbicidal Effect
The herbicidal activity of paraquat is dependent on the parent
molecule undergoing a single-electron redox cycling reaction.
Paraquat is reduced to the paraquat radical, which, in the presence
of molecular oxygen, is immediately reoxidized forming the parent
molecule and superoxide radicals (O2-) (Conning et al., 1969). As
early as 1960, Mees had shown that oxygen was necessary for the
herbicidal activity of paraquat, suggesting the importance of the
redox cycling and O2- formation in mediating toxicity. Paraquat
was not toxic to plant leaves incubated under anaerobic conditions,
despite the continuation of photosynthetic reactions capable of
forming paraquat radicals. Exposure of the anaerobic incubates to
air, however, resulted in immediate onset of toxicity. Dodge
(1971) subsequently confirmed that isolated plant chloroplasts
could form the paraquat radical under anaerobic conditions. The
possibility that O2- generation may be an essential component of
the herbicidal activity was further supported in a study by
Youngman & Dodge (1979). These investigators observed that the
phytotoxicity of paraquat in plant cotyledons was decreased by a
copper chelate of D-penicillamine. The chelate possessed activity
similar to the enzyme superoxide dismutase (EC 1.15.1.1)
(Lengfelder & Elstner, 1978), an enzyme that detoxifies O2-
(McCord & Fridovich, 1969).
The generation of O2- may lead to many potentially
cytotoxic reactions, including the membrane-damaging process of
lipid peroxidation (Bus & Gibson, 1979). When plant leaves were
incubated with paraquat, there was rapid stimulation of the
formation of malondialdehyde, which is an indicator of lipid
peroxidation (Dodge, 1971).
Table 3. Paraquat trade namesa
--------------------------------------------------------------------------------------------------------
Products Countries Paraquat content (W/V for
liquids, W/W for solids)
--------------------------------------------------------------------------------------------------------
Dextrone X United Kingdom 20%
Dexuron United Kingdom 10%, also contains diuron
Duanti Germany, Federal Republic of 2.5%, also contains diquat
Dukatalon Israel 9%, also contains diquat
Esgram United Kingdom 20%
Frankol Prompt Germany, Federal Republic of 10%, also contains diuron
Gramazin Italy 10%, also contains simazine
Gramixel Germany, Federal Republic of 10%, also contains diuron
Gramanol United Kingdom, Ireland, 14%, also contains monolinuron
Belgium, Greece, Middle East
Gramoxone worldwide 20%
Gramoxone S worldwide 20%
Gramoxone W discontinued 20%
Gramoxone ZU The Netherlands, Belgium 20%
Gramuron Africa, Italy 10%, also contains diuron
Katalon Israel 20%
Ortho Paraquat CL USA 24.6% (2 lb/US gal)
Ortho Spot Weed & Grass Killer USA 0.2% (Solid Stream Aerosol)
Orvar United Kingdom 5%
Paracol Malaysia, Indonesia, Philippines 10%, also contains diquat
Chile, Peru
Paradi Australia 10%, also contains diquat
Pathclear United Kingdom, New Zealand 2.5%, also contains diquat,
3 aminotriazole and simazine
Preeglone Denmark, Norway 2.5%, also contains diquat
Preeglone Belgium, France, Spain 12%, also contains diquat
Preeglone Extra New Zealand 9%, also contains diquat
Priglone France, Switzerland 12%, also contains diquat
Seythe United Kingdom 20%
Spray Seed Australia 10%, also contains diquat
Terraklene United Kingdom, Ireland, Denmark, 10%, also contains simazine
France, Switzerland
Tota-Col Wide range of countries 10%, also contains diuron
Tryquat Australia 10%, also contains diquat
Weedol The Netherlands, Ireland, United Kingdom 2.5%, also contains diquat
Weedrite Canada 2.5%
Weedrite Aerosol Canada 0.44%
--------------------------------------------------------------------------------------------------------
a From: Fletcher (1975).
4. ENVIRONMENTAL DISTRIBUTION AND TRANSPORTATION
4.1. Photochemical Degradation
4.1.1. Photochemical degradation on plant surfaces
In agricultural practice, much of the paraquat sprayed is
initially deposited on plant surfaces. Slade (1965, 1966) applied
paraquat dichloride droplets to maize, tomato, and broad-bean
plants. Determinations carried out at intervals of 100 days showed
that degradation was caused by photochemical decomposition on the
leaf surfaces but not by metabolism. Degradation products isolated
from plants sprayed with 14C-paraquat dichloride included 4-
carboxyl-1-methyl-14C-pyridylium chloride and methylamine-14C-
hydrochloride. No 14C02 was detected as a photochemical
decomposition product. The photochemical degradation of paraquat
dichloride continued after the plants were dead (Fig. 2). Paraquat
photodegradation products were not translocated from the dessicated
leaves of the plants, nor were they found in the crops (cereals and
fruits), when weeds were treated with paraquat during 3 - 4
successive seasons (Calderbank, 1966).
When bipyridyl is refluxed with methyl iodide, the iodide salt
is obtained. Haley (1979) and Summers (1980) thoroughly reviewed
the published methods for paraquat synthesis, and for the
separation and purification of bipyridylium salts. The yields
obtainable vary from 20% to 96% of pure product.
The first commercial paraquat formulation approved for
agricultural use was Gramoxone(R).
3.1.2. Impurities
Aqueous solutions of paraquat used as herbicides must
correspond to the FAO Specification Code 56/13/S/6 (FAO, 1973).
This requires a description of the active ingredient in the
formulation, of the impurities, of the physical and chemical
properties, and of the methods for determining the components.
The only impurity permitted in paraquat is free 4,4'-bipyridyl
at a maximum level of 0.25% of the paraquat content.
3.2. Production and Use
Paraquat is produced in several countries, including China,
Province of Taiwan, Italy, the United Kingdom, and the USA.
Formulations of the active ingredients (mainly paraquat dichloride)
are used in more than 130 countries world-wide. Paraquat
dimethylphosphate is used in the USSR. Since its introduction for
agricultural use in 1962, paraquat has been widely used for weed
control and as a dessicant. In many countries, paraquat is
formulated locally, only the technical active ingredient being
imported. Records of world production of paraquat are not
available.
Technical paraquat dichloride has been formulated in liquid
concentrates or granules. Water-soluble granules containing
paraquat (25 g/kg) and diquat (25 g/kg) are used for weed control
in private gardens. Paraquat is sold under a variety of trade
names which are summarized in Table 3.
Gramoxone(R) is a dark aqueous solution containing a paraquat
dichloride concentration of 200 ± 10 g/litre. Its specific gravity
at 20 °C is 1.1 and the crystallization point is -5 °C to 10 °C.
It is not flammable and, in its original polyethylene containers,
is stable for a long time under normal atmospheric conditions. The
formulation is incompatible with anionic surface active agents and
decomposes in ultraviolet radiation. Gramoxone(R) rapidly
corrodes aluminium; zinc, iron, and tinplate are more resistant.
Paraquat is a total contact herbicide used to control broad-
leaved and grassy weeds. It should be sprayed when the weeds are
young and less than 30 cm high. It kills all green tissues, but
does not harm the mature bark. Paraquat is used for plantation
crops (banana, cocoa-palm, coffee, oil-palm, rubber, etc.) and for
citrus fruits, apples, plums, vines, and tea. On certain crops
(potato, pineapple, sugar-cane, sunflower), it is used as a
dessicant; it is also used as a cotton defoliant. It is applied
around the trees in orchards and between the rows of crops.
Uncropped land on industrial sites, railways, roadsides, etc.
can be cleared of weeds by applying paraquat at higher
concentrations.
Gramoxone S(R) is largely applied for aquatic weed control.
Application rates usually range from 250 g - 1500 g/ha (1.1 -
7.1 1itre of Gramoxone(R), but, for grass and stubble clearing, up
to 2200 g of the herbicide are used per ha. The working dilutions
vary from 1 - 5 g per litre paraquat in water. It is applied by
ground sprayers (not mist-blowers) in 200 - 500 litres solution/ha.
3.3. Mechanism of the Herbicidal Effect
The herbicidal activity of paraquat is dependent on the parent
molecule undergoing a single-electron redox cycling reaction.
Paraquat is reduced to the paraquat radical, which, in the presence
of molecular oxygen, is immediately reoxidized forming the parent
molecule and superoxide radicals (O2-) (Conning et al., 1969). As
early as 1960, Mees had shown that oxygen was necessary for the
herbicidal activity of paraquat, suggesting the importance of the
redox cycling and O2- formation in mediating toxicity. Paraquat
was not toxic to plant leaves incubated under anaerobic conditions,
despite the continuation of photosynthetic reactions capable of
forming paraquat radicals. Exposure of the anaerobic incubates to
air, however, resulted in immediate onset of toxicity. Dodge
(1971) subsequently confirmed that isolated plant chloroplasts
could form the paraquat radical under anaerobic conditions. The
possibility that O2- generation may be an essential component of
the herbicidal activity was further supported in a study by
Youngman & Dodge (1979). These investigators observed that the
phytotoxicity of paraquat in plant cotyledons was decreased by a
copper chelate of D-penicillamine. The chelate possessed activity
similar to the enzyme superoxide dismutase (EC 1.15.1.1)
(Lengfelder & Elstner, 1978), an enzyme that detoxifies O2-
(McCord & Fridovich, 1969).
The generation of O2- may lead to many potentially
cytotoxic reactions, including the membrane-damaging process of
lipid peroxidation (Bus & Gibson, 1979). When plant leaves were
incubated with paraquat, there was rapid stimulation of the
formation of malondialdehyde, which is an indicator of lipid
peroxidation (Dodge, 1971).
Table 3. Paraquat trade namesa
--------------------------------------------------------------------------------------------------------
Products Countries Paraquat content (W/V for
liquids, W/W for solids)
--------------------------------------------------------------------------------------------------------
Dextrone X United Kingdom 20%
Dexuron United Kingdom 10%, also contains diuron
Duanti Germany, Federal Republic of 2.5%, also contains diquat
Dukatalon Israel 9%, also contains diquat
Esgram United Kingdom 20%
Frankol Prompt Germany, Federal Republic of 10%, also contains diuron
Gramazin Italy 10%, also contains simazine
Gramixel Germany, Federal Republic of 10%, also contains diuron
Gramanol United Kingdom, Ireland, 14%, also contains monolinuron
Belgium, Greece, Middle East
Gramoxone worldwide 20%
Gramoxone S worldwide 20%
Gramoxone W discontinued 20%
Gramoxone ZU The Netherlands, Belgium 20%
Gramuron Africa, Italy 10%, also contains diuron
Katalon Israel 20%
Ortho Paraquat CL USA 24.6% (2 lb/US gal)
Ortho Spot Weed & Grass Killer USA 0.2% (Solid Stream Aerosol)
Orvar United Kingdom 5%
Paracol Malaysia, Indonesia, Philippines 10%, also contains diquat
Chile, Peru
Paradi Australia 10%, also contains diquat
Pathclear United Kingdom, New Zealand 2.5%, also contains diquat,
3 aminotriazole and simazine
Preeglone Denmark, Norway 2.5%, also contains diquat
Preeglone Belgium, France, Spain 12%, also contains diquat
Preeglone Extra New Zealand 9%, also contains diquat
Priglone France, Switzerland 12%, also contains diquat
Seythe United Kingdom 20%
Spray Seed Australia 10%, also contains diquat
Terraklene United Kingdom, Ireland, Denmark, 10%, also contains simazine
France, Switzerland
Tota-Col Wide range of countries 10%, also contains diuron
Tryquat Australia 10%, also contains diquat
Weedol The Netherlands, Ireland, United Kingdom 2.5%, also contains diquat
Weedrite Canada 2.5%
Weedrite Aerosol Canada 0.44%
--------------------------------------------------------------------------------------------------------
a From: Fletcher (1975).
4. ENVIRONMENTAL DISTRIBUTION AND TRANSPORTATION
4.1. Photochemical Degradation
4.1.1. Photochemical degradation on plant surfaces
In agricultural practice, much of the paraquat sprayed is
initially deposited on plant surfaces. Slade (1965, 1966) applied
paraquat dichloride droplets to maize, tomato, and broad-bean
plants. Determinations carried out at intervals of 100 days showed
that degradation was caused by photochemical decomposition on the
leaf surfaces but not by metabolism. Degradation products isolated
from plants sprayed with 14C-paraquat dichloride included 4-
carboxyl-1-methyl-14C-pyridylium chloride and methylamine-14C-
hydrochloride. No 14C02 was detected as a photochemical
decomposition product. The photochemical degradation of paraquat
dichloride continued after the plants were dead (Fig. 2). Paraquat
photodegradation products were not translocated from the dessicated
leaves of the plants, nor were they found in the crops (cereals and
fruits), when weeds were treated with paraquat during 3 - 4
successive seasons (Calderbank, 1966).
 The rate of decomposition was related to the intensity of UV
radiation between 285 and 310 mµ present in daylight. In strong
sunlight, about 2/3 of the applied herbicide decomposed within a
3-week period. Vegetation directly sprayed with paraquat (1.12
kg/ha) was analysed at intervals up to 4 months. The residues
varied from 5 - 200 mg/kg. The 4-carboxyl-1-methylpyridynium
chloride ranged from 0.02 - 5 mg/kg (about 7% of the paraquat
residues determined on dry leaves). The toxicity of 4-carboxyl-1-
methylpyridylium for mammals was low, the acute oral LD50 in rats
being more than 5000 mg/kg body weight (FAO/WHO, 1971).
The degradation product from the photochemical destruction of
paraquat dimethylsulfate was N-methyl-isonicotinic acid
methylsulfate (Fig. 3).
A 90-day feeding test (Broadhurst et al., 1966) on rats
revealed that levels of 20 000 - 5000 mg/kg of the N-methyl-
isonicotinic acid methylsulfate were not toxic.
The rate of decomposition was related to the intensity of UV
radiation between 285 and 310 mµ present in daylight. In strong
sunlight, about 2/3 of the applied herbicide decomposed within a
3-week period. Vegetation directly sprayed with paraquat (1.12
kg/ha) was analysed at intervals up to 4 months. The residues
varied from 5 - 200 mg/kg. The 4-carboxyl-1-methylpyridynium
chloride ranged from 0.02 - 5 mg/kg (about 7% of the paraquat
residues determined on dry leaves). The toxicity of 4-carboxyl-1-
methylpyridylium for mammals was low, the acute oral LD50 in rats
being more than 5000 mg/kg body weight (FAO/WHO, 1971).
The degradation product from the photochemical destruction of
paraquat dimethylsulfate was N-methyl-isonicotinic acid
methylsulfate (Fig. 3).
A 90-day feeding test (Broadhurst et al., 1966) on rats
revealed that levels of 20 000 - 5000 mg/kg of the N-methyl-
isonicotinic acid methylsulfate were not toxic.
 4.1.2. Photochemical degradation of paraquat on soil and other
mineral surfaces
Slade (1966) showed that there was a breakdown, similar to that
on plant surfaces, if spots of paraquat on silica gel were exposed
to direct sunlight. When 14C-paraquat dichloride was sprayed on
the bare soil surface of a field during a hot sunny period, traces
of 4-carboxy-1-methylpyridynium chloride were detected in the top
inch of soil for the first few weeks afterwards (Calderbank &
Slade, 1976). Radioassay showed that the total soil residue did
not markedly decrease during a 6 - 18 month period, so that, in
agricultural practice, UV degradation of herbicide reaching the
soil should be regarded as insignificant.
The principal intermediates of photochemical paraquat
degradation on plants or soil surfaces are of low toxicity.
They decompose easily and are not expected to produce adverse
environmental effects.
4.2. Microbial Degradation
Microbial paraquat degradation has been thoroughly reviewed by
Haley (1979). Baldwin et al. (1966) identified many soil
microorganisms capable of degrading paraquat. The herbicide was
decomposed by Corynebacterium fascians, Clostridium pasteurianum,
and Lipomyces starkeyi. Several other microorganisms were found to
degrade paraquat (Smith et al., 1976; Tchipilska, 1980) but
Lipomyces starkeyi proved to be the most active (Burns & Audus,
1970). Burns & Audus (1970) concluded that microbiological
degradation was possible only for a short time following the
application of paraquat to soil. Once adsorbed on to clay
materials, the paraquat was inaccessible to microorganisms.
Microbial degradation of paraquat in the field is therefore
relatively slow.
Studies of 4-carboxyl-1-methylpyridylium chloride in soil have
demonstrated that the radiolabelled product readily decomposes to
form several chemical substances, including carbon dioxide. No
significant residues of the compound have been determined in plants
as a result of uptake from the soil. Wright & Cain (1970) isolated
Achromobacter D from the soil; this utilized the 4-carboxyl-1-
methylpyridylium chloride and the methylamine originating from
the N-methyl group of the molecule. The NADH and the oxygen
requirement indicated the possibility of direct oxidative
fission of a partly reduced ring to form dialdehyde, which was
then hydrolysed to formate, methylamine, and succinic dialdehyde.
The end-products of the microbial ring degradation were formate,
succinate, and carbon dioxide.
4.3. Environmental Adsorption and Transformation
4.3.1. Soil
The property of paraquat that is most important in nullifying
its impact on the environment is its rapid and complete binding to
clay soils. Desorption of the herbicide from soil particles, for
the purpose of chemical analysis, requires destruction of the
mineral particles by refluxing with strong sulfuric acid. The
strong adsorption to clay has been attributed to the flat and
highly polarizable nature of the paraquat ion (Coats et al., 1966;
Knight & Denny, 1970). Weber et al. (1965) reported that the
adsorption appeared to be one of ion exchange and was very rapid,
the rate of adsorption depending on the rate at which the paraquat
ion contacted the adsorbing particles.
In highly organic soils, the weaker adsorption sites of soil
organic matter delay the redistribution of paraquat without
inactivating it herbicidally. In this connection, Khan (1980)
reported tests showing a remarkable affinity of humic substances in
the soil for the paraquat ion. These humic substances enhance the
degradation of pesticides via non-biological pathways.
It has been demonstrated that on soil containing 98% organic
matter, the herbicidal effects of 1.12 and 2.24 kg of paraquat/ha
persisted for 16 - 29 days, but such soils are not widespread
naturally. Burns & Audus (1970) studied the migration of paraquat
from soil organic matter to clay mineral particles. The transfer
of the paraquat from the organic to the inorganic fraction, through
a membrane, was 90% complete within 6 h. The remaining 10% took
about 2 days to be transferred. No paraquat was detected in the
organic fraction after 4 days. At high paraquat concentrations
(more than 20 mg/kg in equilibrium solution), the total adsorption
capacity was greater than normal in soils with high organic
content, as opposed to those with low organic content.
Mithyanta & Perur (1975) studied samples of 4 different soils
treated with paraquat in different experimental schemes. After 24
h, the soils were extracted with a water solution of ammonium
chloride. The percentages of paraquat, extractable with water,
ranged from 4.8 - 66.9%, depending on the type of soil and the
conditions. Data on the persistence of paraquat in the soil have
also been compiled by Coats et al. (1966), Knight & Tomlinson
(1967), Knight & Denny (1970), and Burns & Audus (1970).
As summarised in section 4.2, free paraquat is degraded by a
range of microorganisms, but degradation of strongly adsorbed
paraquat is relatively slow. In plot studies, degradation was very
slow or non-detectable (Riley et al. 1976). However, in long-term
field studies, degradation rates were 5 - 10% per year. This is
greater than the rate required to prevent saturation of the
deactivation capacity of soils.
In a long-term trial on a loamy soil, plots were treated with
0, 90, 198, and 720 kg paraquat/ha, which was incorporated to a
depth of 15 cm. These rates were equivalent to 0, 50, 110, 400% of
the soils strong absorption capacity (Gowman et al., 1980;
Wilkinson, 1980; Riley, 1981). Over the 7 years, paraquat residues
declined by 5% per year (sig P = 0.05) on the 90 kg/ha plots and
by 7% per year (sig P = 0.01 on the 198 and 720 kg/ha plots. The
rate of decline on the 198 and 720 kg/ha plots was significantly
greater ( P = 0.01) than on the 90 kg/ha plots.
In another long-term trial on a sandy loam, plots were treated
annually with 4.4 kg/ha for 12 years (Hance et al, 1980). The rate
of loss of paraquat soil residues was about 10% per year and the
soil residues tended to plateau when the rate of application
equalled the rate of degradation. Data for the last 4 years (total
16 years) has confirmed the early results (Hance, unpublished
data).
Some paraquat could be recovered from its tightly bound form by
chemical destruction of the soil from field plots, several years
after application. The limit of paraquat adsorption, at which
further treatment would result in phytotoxic activity, was
considered to be important. Strong adsorption capacity was defined
as the measure of paraquat that can be adsorbed by the soil without
entailing phytotoxic effects, and this capacity was determined in
several kinds of soil with various clay and organic contents
(Knight & Tomlinson, 1967). Mechanical analyses, pH, and organic
matter content were also determined. Independently of the soils
studied, it was found that, by applying 1 kg/ha per year, it would
take from 30 - 1440 years to saturate the top 15 cm of soil at
strong adsorption sites. The conditions of study precluded any
form of paraquat degradation or metabolism in the soil. Riley et
al. (1976) reviewed the hazard of continuous application of 0.1 -
2 kg paraquat ion/ha, assuming soil contamination by 10 - 100% of
the amount applied. Bound paraquat soil residues were not adsorbed
by living organisms. Paraquat residues did not induce any effects
on microarthropods or microorganisms. Continued application of
the herbicide in different soils has been investigated by Pestemer
et al. (1979). The ED50 valuesa for phytotoxic action on lettuce
ranged from 0.01 mg/litre paraquat solution in agar-agar to 98 -
1930 mg/litre in different soils, depending on their constituents,
and 31 - 57.6 mg paraquat residues/kg have been determined in the
soil samples. There is evidence (Hance et al., 1980) that
strongly-bound paraquat residues were degraded in soil by microbial
activity at a rate of 5 - 10% per annum. A correlation was reported
between the paraquat residues, the number of treatments, doses, and
depth of soil sampling.
-------------------------------------------------------------------
a ED50 = median effective dose.
Although, as mentioned, adsorption on clay is important,
extremely sandy soils can adsorb and inactivate significant
quantities of the herbicide, as illustrated by studies on a South
African vineyard soil that contained only 1% clay (Riley et al.,
1976). Over an 8-year period, more than 20 applications (total
15.6 kg paraquat/ha) resulted in saturation of about 20% of the
soil-paraquat-strong-adsorption capacity in the top 2.5 cm. The
paraquat residues were not phytotoxic in the field or in greenhouse
tests on different plants. No paraquat residues were detected
(<0.05, <0.03, <0.03 mg/kg) in leaves, grapes, and twigs,
respectively.
Very low concentrations of free paraquat would be detected
easily by their phytotoxicity. Five trials at 4 sites were
conducted by Newman & Wilkinson (1971). In 4 of the trials, single
applications of paraquat at 112 kg/ha were made at sites subjected
to normal agricultural practice. At this unrealistic, extremely
high rate, short-duration residual phytotoxicity was observed. On
undisturbed plots of mineral soils, seedlings did not appear for
several months; on organic soils, the time lag was even longer.
After cultivation, there was no further indication of
phytotoxicity. In the 5th trial, a total of 565 kg/ha was applied
in 5 doses over 4 1/2 years. The plot then remained undisturbed,
apart from periodic cultivation of the top 20 mm to prepare a
seedbed. It was at this site that phytotoxicity to ryegrass
seedlings was detected, and free paraquat was determined in the
surface soil using the Lemna minor bioassay. Phytotoxicity was
confined to the surface layer of the soil. The free paraquat that
had leached out of the top 2.5 cm had been adsorbed in the deeper
soil layers, and this was confirmed by the absence of residual
phytotoxicity when the site was more deeply cultivated.
However, the extreme situations seen in high-dosage trials are
not encountered in practice and only serve to show the possible
consequences for the environment of a gross overdose of the
herbicide. Thus, when paraquat is used in normal application
doses, no adverse environmental effects can be expected.
Accidental spillage is probably the most likely cause of high
levels of residual paraquat. The 200 g of paraquat contained in 1
litre of Gramoxone(R) would be completely inactivated by the
addition of 10 kg of bentonite, for inactivation can be effected
either by cultivation and mixing other soil with the contaminated
layer or by adding clay minerals. Simulated spills of paraquat
have also been treated with sodium borohydride or alkali (Staiff et
al., 1981); within 1 day the paraquat in the soil had been
effectively degraded.
4.3.2. Water
The ecological effects of paraquat in water have been
studied in relation to its use as an aquatic herbicide at a
normal concentration of 1 mg/litre (Newman & Way, 1966; Grzenda et
al., 1966). Following this use, the concentration present in water
decreased to about half of the initial 1 mg/litre level within
36 h, and, in less than 2 weeks, the concentration was below
0.01 mg/litre. Weed-sample analysis, 4 days after paraquat
application, showed a residue of approximately 25 mg/kg, suggesting
that absorption by the weed was mainly responsible for paraquat
removal. Mud-residue analysis 5 1/2 months after treatment showed
that 36% of the applied paraquat remained in the mud, and 70% of
that was found in the top 2.5 cm. In the mud, paraquat had been
adsorbed on to the mineral material. Since bottom mud often has
organic components, the residues may be more accessible to
bacterial degradation. Compared to other products, paraquat
appears to be the herbicide of choice for future use in water
supplies because of its rapid disappearance from water (6 - 14 days
after treatment) (Grzenda et al., 1966). The residues were not
desorbed from the bottom sediments, and mud taken from the bottom
of a paraquat-treated lake carrying inactivated residues, showed no
toxic effects on barley seedlings that germinated on it (Way et
al., 1971).
Wauchope (1979) discussed the fate of pesticides in water
draining from fields after rain. For most formulations, a total
loss of 1.5%, or less, of the amount applied was the rule, except
when severe rainfall occurred within 1 - 2 months following
treatment. Nearly all the pesticides examined were lost by runoff;
only those binding strongly to clay particles, such as paraquat,
were carried off in the sediment phase of runoff. The lack of
paraquat runoff loss has also been discussed by Smith et al.
(1978).
Grover et al. (1980) compared the efficiency of various
herbicidal treatments for weed control in a series of irrigation
ditches. At the relatively low dose of 2.2 kg/ha, paraquat
resulted in aquatic weed suppression from 1973 to 1976, and this
made for satisfactory water flow without environmental
contamination. Water that contains small amounts of paraquat
residues loses them rapidly on contact with soil, the adsorption
process being irreversible (Knight & Tomlinson, 1967; Calderbank,
1972). Thus treated water may be used quite safely for channel
irrigation, if an interval of 10 days is observed between treatment
of the water and its use, because the paraquat will be unavailable
to the plant roots. Caution should, however, be exercised in
prolonged crop irrigation until the residue is well below 0.1
mg/litre, although phytotoxic damage is unlikely at even 0.5 mg
paraquat/litre (Calderbank, 1972).
Coats et al. (1966) treated 0.1 ha experimental ponds with
paraquat to obtain a concentration of 0.4 mg/litre. The soil in
one of the ponds was stirred twice after 24 h. Analysis of the
water over several weeks revealed a decrease from 0.4 mg/litre to
0.01 mg/litre after several weeks, but when the soil of the pond
was stirred, the paraquat concentration fell from 0.75 mg/litre
to <0.01 mg/litre after 8 - 12 days. In static water experiments,
the concentration of 0.5 - 1 mg/litre fell rapidly to about 0.1
mg/litre within 4 - 7 days of treatment in 4 trials performed by
Calderbank (1972). These reductions in the paraquat concentration
were due to its rapid adsorption and concentration in aquatic
plants. Decaying weeds transported it to the bottom mud (Table 4)
where it was not released back into the water (Way et al., 1971).
Earnest (1971) treated a pond with paraquat at an initial
concentration of 1.14 mg/litre. No residues were detected in the
water after 16 days (limit of detection 0.01 mg/litre); in the mud
the concentration was 1.13 mg/kg after 3 h and 3.25 mg/kg after 99
days. These data were confirmed by Grover et al. (1980).
Grover et al. (1980) studied irrigation water from ditches.
Three days after treatment with 2.2 kg paraquat/ha, the
concentrations in the water used to flood the treated ditches were
less than 0.01 mg/litre, and paraquat residues in the ditch water
ranged from 0.002 - 0.034 mg/litre in samples taken 3 - 5 days
after foliar applications.
Table 4. Residues of paraquat in water, weed, and bottom muda
-------------------------------------------------------------------
Days after treatment
-------------------------------------------------------------------
1 4 16 32 175 420
-------------------------------------------------------------------
Trial 1 water (mg/litre) 0.31 0.12 ND
weed (mg/kg) 13.70 25.80 21.0 0.55
mud (mg/kg) 3.70 - - - 57.1 20.1
Trial 2 water (mg/litre) 0.37 ND ND ND - -
weed (mg/kg) 25.50 40.0 37.8 27.8 - -
mud (mg/kg) ND 0.97 0.23 0.32 6.6 0.96
-------------------------------------------------------------------
a From: Way et al. (1971).
ND - not detectable.
4.3.3. Air
Paraquat is not volatile. Dry deposits of 14C-paraquat
chloride exposed at room temperature showed no measurable loss in
64 days (Coats et al., 1966). Exposure to paraquat in the air is
not important in spraying and harvesting operations; the skin is
the principal route of occupational exposure (Chester & Woollen,
1982; Staiff et al., 1975).
Air concentrations of paraquat were measured on summer days by
Makovskii (1972) using the method of Calderbank & Yuen (1965).
About 1 - 1.3 kg paraquat/ha had been applied as a herbicide or
desiccant in 0.25 - 0.35% water solutions. The paraquat aerosol
concentrations varied according to spraying method and work-place
(Table 5). Using the same analytical method, Staiff et al. (1975)
examined 35 sites after paraquat application with tractor-mounted
field sprayers or hand-pressure garden dispensers. The working
solutions contained 0.15% paraquat for field use, and 0.44% for
garden use. The respiratory exposure of field and garden operators
was below the limit of detection (<0.001 mg paraquat/h).
Mature cotton fields (Seiber & Woodrow, 1981) were sprayed with
paraquat, the dose being 0.94 kg/ha. The air paraquat
concentrations measured downwind decreased regularly from the
extrapolated interval-average values of 4.31 and 10.7 µg/m3 1
metre downwind of the 2 fields to <50 ng/m3 at 400 metres away in
the same direction. Forty-five percent of the aerosol particles
had diameters ranging from 0.01 to 4 µm. The remaining 55% had a
median diameter of 12 µm. Downwind samples taken 2 - 4 h after
spraying contained 1 - 10% of the amount dispersed, but, after
5 - 7 h, no paraquat was detectable in the air.
Table 5. Paraquat total airborne concentrations (mg/m3) in
working areasa
-------------------------------------------------------------------
Place of Number of Mean
sampling samples concentrations
± SE
-------------------------------------------------------------------
Working area sprayer loading 28 0.13 ± 0.03
tractor cabin 16 0.37 ± 0.07
(in direction of wind)
tractor cabin 16 0.55 ± 0.01
(against the wind)
manual spraying 16 0.18 ± 0.04
Treated field after 5 min 16 0.05 ± 0.01
after 10 min 32 < 0.01
after 20 min 16 0
Distance from 200 m 8 0.08 ± 0.01
treated field 400 m 8 0.04 ± 0.01
-------------------------------------------------------------------
a From: Makovskii (1972).
A study of Malaysian plantation workers, occupationally exposed
to paraquat, revealed a mean total airborne exposure of 0.97 mg/m3
for spray operators. This exposure is less than present TLVs
(Chester & Woollen, 1982). Wojeck et al. (1983) reported that
after spraying paraquat in fields of tomatoes and citrus, the total
airborne exposure ranged from 0 - 0.070 mg/h. It was less than
0.1% of the total body exposure (12.16 - 168.59 mg/h) in all
trials.
During mechanical harvesting of cotton dessicated by paraquat,
the maximum levels in airborne dust were found to be 1245 ng/m3
outside the cabin of the tractor and 516 ng/m3 inside the open
cabin. With the cabin door closed, the concentration was only 13.7
ng/m3. The trapped particulate matter consisted of dessicated
plant material and soil dust. A cascade impactor analysis
established that 57% of the paraquat had a median particle diameter
of 4 µm, 23%, 12 µm, and 11%, 3 µm. Cotton harvesting generated
parti- culate concentrations in the field comparable to those
immediately downwind of the field during spraying. Bearing in mind
the highest paraquat air concentration in the harvest-time air
(0.0012 mg/m3), a harvester operator's maximum exposure through
inhalation was calculated to be 0.01 mg/8 h/day (Seiber & Woodrow,
1981).
Bulgaria has established a maximum allowable concentration
(MAC) of 0.01 mg paraquat/m3 (1972), the Federal Republic of Germany
0.1 mg/m3 (1982), Hungary 0.02 mg/m3 (1978), and the USA a TLV of
0.1 mg/m3 (1982).
4.3.4. Plants
Paraquat residues on plants have been reviewed several times by
the Joint Meeting on Pesticide Residues (JMPR) (FAO/WHO, 1971,
1973, 1983). The residues found after paraquat was used as a
desiccant are summarized in Tables 6 and 7 (Calderbank, 1968).
Table 6. Paraquat residues (mg/kg) in cotton 10 days after
dessication at 0.55 kg/haa
-------------------------------------------------------------------
Fraction analysed Paraquat found
-------------------------------------------------------------------
Cotton as picked, including trash and balls 2.00
Ginned seed 0.18
Mechanically reginned seed 0.08
Acid-delinated seed 0.05
Lint cotton 3.00
Trash 3.70
Hulls 0.13
Crude oil ND
Meal 0.02
-------------------------------------------------------------------
a From: Calderbank (1968).
Coats et al. (1966) reported that 14C-paraquat applied to wheat
as a 1% solution was translocated in the plants, including the
roots. Slade (1966) studied the degradation of 14C-paraquat
dichloride and its photochemical degradation products in plants.
Maximum loss occurred in tomato, broad-bean, and maize when the
paraquat remained on the leaf surfaces during sunny days.
In potatoes treated with paraquat as a desiccant, Makovskii
(1972) found a residue of 0.05 mg/kg, and there was no change after
the potatoes had been boiled. No residues (limit of detection 0.01
mg/kg) were found in fruits (apples, citrus fruits, plums, pears),
tea, and cereals. In tests on sunflower seeds treated with 0.25 or
0.5 kg paraquat/ha, residues of up to 0.9 mg/kg were found in the
whole seed, up to 1.2 mg/kg in sunflower meal, and no residue in
the oil (Anonymous, 1979). Therefore, the use of sunflower meal in
the diet of hens, dairy cattle, and other livestock would not
result in paraquat levels exceeding current standards.
Table 7. Paraquat residues (mg/kg) in food crops 3 - 21 days
after dessicationa
-------------------------------------------------------------------
Crop Rate of application Paraquat found
(lb/acre)
-------------------------------------------------------------------
Barley 0.50 - 1.00 3 - 10
Wheat 0.50 - 1.00 1 - 2.5
Maize 0.50 - 1.20 ND - 0.2
Rice (with husk) 0.15 - 0.54 0.7 - 22
Rice (de-husked or polished) 0.15 - 0.54 ND - 0.2
Peas, beans, sunflower seed 0.35 - 1.20 ND - 0.2
Sorghum seed 0.25 - 1.00 0.1 - 0.4
Cotton (as picked) 0.50 - 1.00 2 - 3
Potatoes 0.50 - 1.50 0.02 - 0.13
Onions 0.50 - 2.00 ND - 0.05
Sugar cane juice 0.50 - 2.00 ND
Seed oils (sunflower, rape, up to 1.20 ND
sesame, cotton)
-------------------------------------------------------------------
a From: Calderbank (1968).
Seiber et al. (1979) determined the paraquat residues in
treated cotton (the foliage and bolls of the live plant, the lint
and seed of harvested cotton, the gin waste and the lint and
non-lint components). Gin waste residues were surveyed during
5 months of open storage. The paraquat dose had been 0.21 and
2.0 kg/ha. The results obtained are summarized in Table 8. The
minimal degradation of paraquat in the plants studied was confirmed
by Hills et al. (1981).
Significant paraquat residues are to be expected only when a
crop is directly sprayed.
After spraying fields of marijuana with paraquat for the
purpose of eradication, residues of paraquat were detected in
marijuana (Smith, 1978; Patrick, 1980). Of the 54 samples
collected in 1976, 7.4% were positive and of 46 samples collected
in 1977, 19.6% were positive.
Table 8. Paraquat residues (mg/kg) in cotton plantsa
-------------------------------------------------------------------
Material Days after Leaves Lint Non-lint Seeds
treatment
-------------------------------------------------------------------
Standing cotton plants 2 13.1 22.10 0.06
6 8.2 3.80 0.06
Harvested seed cotton 18 7.15 0.25
stored in field 49 4.85 0.18
Gin waste 49 2.7 9.3
119 5.3 10.1
171 5.8 9.7
-------------------------------------------------------------------
a From: Seiber et al. (1979).
4.3.5. Animals
The effects and fate of 14C-paraquat orally-administered to
cattle at 8 mg/kg body weight were studied by Stevens & Walley
(1966). Seven days after this single dose, 0.03 - 0.08 g/litre had
been excreted in the milk and 2.4 g/litre in the urine of the cows.
The total paraquat excretion in the milk was only 0.01% of the
ingested dose. In cows given daily oral doses of 8 mg paraquat/kg
for 3 weeks, residues of less than 0.01 mg/litre were detected in
the milk (FAO/WHO, 1977). Cattle did not suffer any toxic effects
over a 4-week period when turned loose on pasture immediately after
it had been sprayed with 1.12 kg paraquat/ha (Calderbank et al.
1968). During the first 2 weeks of grazing on the dried herbage,
it was estimated that the cattle ingested approximately half of
their acute oral LD50 (36 - 54 mg/kg body weight) every day.
Paraquat levels in the herbage ranged from about 400 mg/kg 1 day
after spraying, to about 200 mg/kg 14 days after treatment;
14 - 35 days after spraying the levels were 135 -214 mg/kg. The
4-carboxyl-1-methylpyridylium chloride content during the trial
period was 5.1 - 3.4 mg/kg. By the 4th week of the study, paraquat
levels in the urine were 0.01 - 0.19 mg/litre and in the faeces,
0.9 - 42 mg/kg. Only on the first day after spraying were paraquat
residues (0.02 mg/litre) found in the milk of 2 cows; no residues
were found (< 0.005 mg/litre) thereafter. The only organs of a
slaughtered animal that contained paraquat were the kidney
(0.03 mg/kg) and the stomach (0.05 mg/kg).
The fate of paraquat in large animals is addressed far more
completely in the Evaluations of the 1976 Joint Meeting on
Pesticide Residues (JMPR) (FAO/WHO, 1977).
Rabbits were fed with lucerne treated with normal-use levels of
paraquat (Lavaur et al., 1979). Immediately after spraying, the
paraquat residues were 272 mg/kg (dry weight of lucerne). After
24 h and 48 h, they were 114 mg/kg and 62 mg/kg, respectively. No
systemic toxicity symptoms or gastrointestinal damage were observed
in the treated rabbits.
When hens were given paraquat at 40 mg/litre in their drinking-
water for 14 days, the amount of paraquat found in the eggs rose to
0.1 mg/kg, but fell to less than 0.005 mg/kg, 6 days after
cessation of treatment (Fletcher, 1967). Eggs from hens eating
grain containing paraquat at a concentration of 10 mg/kg contained
residues below 0.025 mg/kg.
5. BIOLOGICAL ACTIVITY OF RESIDUES
5.1. Soil Organisms
Haley (1979) reviewed the effects of paraquat on soil
microorganisms and fungi, while Tu & Bollen (1968), Curry (1970),
Radaelli & Martelli (1971), Roslycky (1977), and Smith et al.
(1981a) studied the effects of paraquat on the size and composition
of the microbial soil populations, total microbial respiration in
the soil, the rate of organic matter degradation, and the number of
soil microorganisms. None of these authors found any adverse
ecological effects from normal and excessive (up to 32 times the
normal dose) paraquat treatment, although in some cases
nitrification was temporarily suppressed or activated, and some
bimodal microbiological effects were observed with intermediate
herbicide concentrations (Tu & Bollen, 1968; Tchipilska, 1980).
At normal doses, paraquat had no adverse effect on
endomycorrhiza formation and function (Smith et al., 1981a), on
total populations of bacteria, actynomyces, fungi (Roslycky, 1977;
Haley, 1979; Tchipilska, 1980; Smith et al., 1981a), or on 24
different species of soil fauna taken from 2 plots at a depth of
3.8 cm (Curry, 1970).
Curry (1970), and Riley et al. (1976) made extensive
studies of the effects of normal and high doses of paraquat on
microarthropod and earthworm populations at sites at different
stages of cultivation. The herbicide was neither harmful nor
repellant to earthworms, nor was there any evidence of a toxic
effect or of paraquat accumulation in any species examined. When
the residues in the top 2.5 cm of soil reached 20 mg/kg, the
highest concentration determined in Allolbophora caliginosa, living
near the surface, was 3.2 mg/kg (live weight). Worms from highly-
dosed plots eliminated paraquat residues within 36 h, when placed
in clean soil.
5.2. Effects of Residues on Crop Yields
The absence of adverse effects from residual paraquat on the
growth and yield of crops grown in paraquat-treated soils has been
demonstrated by Knight & Tomlinson (1967), Damanakis et al. (1970),
Newman & Wilkinson (1971), and Riley et al. (1976). It is known
that the paraquat-inactivation capacity of soils varies widely.
Paraquat has been tested on soils of low adsorption capacity, it
has been used repeatedly on the same soil (section 4.3.1) and has
been tested at extremely high concentrations. The absence of any
reports or observations of long-term phytotoxic effects confirms
the data obtained in greenhouse and laboratory studies.
5.3. Effects on Fish and Aquatic Organisms
Despite variation in LC50s for fish (67 - 110 mg/litre after
24 h, 38 - 62 mg/litre after 48 h, more than 25 - 32 mg/litre after
96 h), the herbicide has proved to have a wide margin of safety for
warm- and cold-water fish species (Calderbank, 1972). The toxicity
of paraquat for fish varies with the species, the size of the
fish, and the softness or hardness of the water. A large number
of aquatic species have shown a 100% survival at 96 mg/litre over
96 h, though the decreased oxygen concentration following decay of
weeds, may be dangerous in extreme situations. Rainbow trout
tolerated 1 mg paraquat/litre water in prolonged toxicity tests
and only a 30% mortality was recorded after 16 days of repeated
exposure (Calderbank & Slade, 1976). At the end of the test, 0.54
mg paraquat/kg was found in the rainbow trout. In a 7-day exposure
test at 1 mg paraquat/litre, the herbicide was detected in the gut
(0.41 mg/kg) and liver (0.35 mg/kg), but not in the meat of the
fish (< 0.025 mg/kg). Water snails collected from 2 ditches,
12 weeks after treatment of the waters with 1 mg/litre were found
to contain 0.43 mg herbicide/kg. Fish (major carp fingerlings)
exposed to paraquat in the presence of weeds were more susceptible
than those in weed-free environments (Singh & Yadav, 1978), owing
to the changed oxygen content of the water. Where there is heavy
weed growth, the oxygen taken up by weed decay may dangerously
reduce the oxygen available for aquatic organisms. To avoid this,
as far as possible, paraquat should be applied before weed growth
becomes dense and only to one part of the water-course or lake at
a time (FAO/WHO, 1973).
5.4. Effects on Birds
Paraquat is less toxic for birds than for mammals. The acute
oral LD50 for the hen is 262 - 380 mg/kg body weight (Table 11).
The acute oral and 24-h percutaneous (applied to feet) LD50 for
mallards are 200 and 600 mg/kg body weight, respectively (Hudson et
al., 1979). For duck, pheasants, and quail, LC50 values of
paraquat when mixed in the diet are 1000 mg/kg of food or more
(Summers, 1980); residues on sprayed vegetation would not therefore
be expected to present a hazard for birds.
When paraquat was sprayed directly on to pheasants' eggs before
incubation, treatment rates up to 2 kg paraquat/ha did not have any
effect on egg hatchability or on the birds' reproductive organs
(Newman & Edwards, 1980). In a similar study with Japanese quail
eggs, sprays containing paraquat levels of up to 3 kg/ha did not
have any effect on hatchability or development of reproductive
organs (Edwards et al., 1979). Thus, normal spray rates should not
induce any adverse effects, even if paraquat is sprayed directly on
eggs.
Bird populations have been monitored in detail, over a 5-year
period, on a farm in the United Kingdom where paraquat use was much
higher than normal; the average application to the whole arable
area was 0.6 kg/ha per year. The paraquat was applied beneath
hedgerows and along fence lines. The farm maintained an excellent
wild bird population (40 species), including ground-nesting birds
(Edwards, 1979). Most species were at a similar or greater density
than the national average in the United Kingdom.
The Ministry of Agriculture, Fisheries, and Food in the United
Kingdom has carried out detailed investigations on mammalian and
avian deaths that could have been caused by pesticides. For the
period 1971 - 81, the normal use of pesticide was not found to have
caused any significant adverse effects on mammals and birds (MAFF,
1980a, 1981). The Ministry concluded, "It is widely believed that
the use and misuse of paraquat is responsible for a considerable
number of wildlife casualties. There is no evidence from the
investigations to support this allegation...." (MAFF, 1980b).
6. KINETICS AND METABOLISM
6.1. Animal Studies
6.1.1. Absorption
6.1.1.1. Oral absorption
Daniel & Gage (1966) studied the absorption of 14C-paraquat
following oral and subcutaneous single-dose administration to rats.
About 76 - 90% of the oral doses were found in the faeces, and
11 - 20% in the urine; most of the subcutaneous dose (73 - 88%)
was found in the urine and only 2 - 14.2% in the faeces. This,
together with the absence of marked biliary excretion, was evidence
that paraquat was poorly absorbed from the gut. This low rate of
absorption was confirmed by Litchfield et al. (1973) and Conning et
al. (1969). Rats, guinea-pigs, and monkeys orally administered
LD50 doses of 14C-paraquat had low peak serum concentrations
(2.1 - 4.8 mg/litre) (Murray & Gibson, 1974). The radioactivity
levels reached a maximum 30 - 60 min after administration and then
remained relatively constant for 32 h. A dose of 126 mg/kg body
weight resulted in a rat serum level of 4.8 - 4.7 mg/litre.
In fasting dogs, low oral doses of paraquat were rapidly but
incompletely absorbed, the peak plasma concentration being attained
75 min after dosing (Bennett et al., 1976). After an oral dose of
0.12 mg/kg body weight, 46 - 66% was absorbed in 6 h. For doses of
2 - 5 mg/kg, only 22 - 38% and 25 - 28% of the dose was absorbed,
respectively. Dose-dependent data from dogs and whole-body
autoradiography suggest that absorption is facilitated in the small
intestine. Some non-ionic surfactants (0.001%) increased 14C-
paraquat transport through isolated gastric mucosa models, but
histological evaluation suggested that this was due to damage of
the epithelial cell membranes (Walters et al., 1981).
6.1.1.2. Pulmonary absorption
Absorption of paraquat following instillation and inhalation in
the lung has been described in several studies (Gage, 1968a;
Kimbrough & Gaines, 1970; Seidenfeld et al., 1978; Popenoe, 1979).
The uptake of 14C-paraquat after an intratracheal injection of
1.86 nmol/lung was investigated in the isolated perfused rat lung
by Charles et al. (1978). The efflux of 14C-paraquat was diphasic
with a rapid phase half-life of 2.65 min and a slow phase half-life
of 356 min. It was suggested that the slow phase represented a
storage pool, possibly responsible for the pulmonary toxicity of
paraquat. Various doses of 3H-paraquat (10-5 - 10-12 g) in 0.1 ml
saline were introduced directly into the left bronchus of rats
(Wyatt et al., 1981). Fifteen min after instilling 10-8 of 3H-
paraquat, 90% of the ion could be accounted for in the tissues and
urine, 50% being present in the lung. With doses at or greater
than 10-5 g, pathological changes were seen in the lung, similar to
those seen after systemic poisoning. Zavala & Rhodes (1978)
reported that the lung of the rabbit was highly sensitive to
paraquat intrabronchial instillation in doses ranging from
0.1 g - 1 pg; moderately sensitive to intraveneously administered
paraquat (25 mg/kg body weight); resistant to the herbicide when
given intraperitoneally or subcutaneously (25 mg/kg body weight).
6.1.1.3. Dermal absorption
Paraquat absorption through animal and human skin has been
studied using an in vitro technique (Walker et al., 1983). Human
skin was shown to be impermeable to paraquat, having a very low
permeability constant of 0.73. Furthermore, human skin was found
to be at least 40 times less permeable than animal skins tested
(including rat, rabbit, and guinea-pig). There are no in vivo
studies on the rate of absorption of paraquat through the skin.
However, observations of dose-related dermal toxicity in
experimental animals and human percutaneous poisoning have provided
some qualitative information concerning the dermal absorption of
paraquat (further discussed in section 8.2.2.2).
6.1.2. Distribution
Since the most characteristic feature of paraquat toxicity is
lung damage, it is important to stress the high concentrations and
retention of paraquat in the lung tissues, relative to other
tissues, following oral, intravenous, intraperitoneal,
subcutaneous, and intrabronchial routes of administration in rats,
guinea-pigs, and monkeys (Sharp et al., 1972; Ilett et al., 1974;
Murray & Gibson, 1974; Kurisaki & Sato, 1979; Waddell & Marlowe,
1980). An association between paraquat concentrations in the lung
and degree of toxicity or lung injury has been reported (Sharp et
al., 1972; Ilett et al., 1974; Waddell & Marlowe, 1980; Wyatt et
al., 1981). Some of their data are summarized in Tables 9 and 10.
Table 9. Paraquat distribution in tissues
---------------------------------------------------------------------------
Route of entry Dose Species Time after Tissue Concentration
treatment
---------------------------------------------------------------------------
1. Intrabronchial 10 ng rat 60 min plasma 0.0092 µg/litre
lung 5.2 ng
kidney 0.052 ng
liver -
heart -
brain -
2. Intravenous 20 mg/kg rat 24 h plasma 0.7 mg/litre
lung 8.0 mg/kg
kidney 1.45 mg/kg
liver 0.48 µg/g
heart 0.75 mg/kg
brain -
---------------------------------------------------------------------------
Table 9. (contd.)
---------------------------------------------------------------------------
Route of entry Dose Species Time after Tissue Concentration
treatment
---------------------------------------------------------------------------
3. Intravenous 20 mg/kg rat 24 h plasma ND
lung 11.36 µm/kg
kidney 1.93 µmol/kg
liver 0.90 µmol/kg
heart 1.13 µmol/kg
brain 0.87 µmol/kg
20 mg/kg rabbit 24 h plasma 0.28 µmol/litre
lung 7.9 nm/g
kidney 5.25 µmol/kg
liver 1.59 µmol/kg
heart 1.52 µmol/kg
brain 0.49 µmol/kg
4. Intraperitoneal 15 mg/kg rat 24 h plasma 0.32 µmol/litre
lung 26.28 µm/kg
kidney 10.4 µmol/kg
liver 5.04 µmol/kg
heart 4.59 nmol/g
brain 1.22 µmol/kg
5. Oral 126 mg/kg rat 16 h plasma 0.90 mg/litre
lung 5.0 mg/kg
kidney 7.00 mg/kg
liver 2.1 mg/kg
heart 2.7 mg/kg
brain -
22 mg/kg guinea- 16 h plasma 0.03 mg/litre
pig lung 1.29 mg/kg
kidney 1.99 mg/kg
liver 0.08 mg/kg
heart 0.31 mg/kg
brain -
---------------------------------------------------------------------------
1. From: Wyatt et al. (1981).
2. From: Sharp et al. (1972).
3. From: Ilett et al. (1974).
4. From: Maling et al. (1978).
5. From: Murray & Gibson (1974).
Table 10. Paraquat distribution in tissues (in mg/kg (mean) tissue)
----------------------------------------------------------------------------
Route of Entry Dose Species Time Lung Kidney Liver Heart Plasma
(mg/kg after
body dosing
weight)
----------------------------------------------------------------------------
1. Oral 126 rat 1 h 3.3 27.5 2.0 1.8 4.7
4 h 3.7 4.5 4.4 0.9 0.8
32 h 13.6 9.4 5.7 2.8 1.1
64 h 1.7 1.0 7.7 0.2 0.1
2. Intravenous 20 rat 1 h 9.0 25.0 5.0 - 6.0
4 h 8.0 6.0 2.0 - 0.3
24 h 6.0 1.0 0.4 - 0.07
2 days 4.0 0.8 0.3 - 0.05
----------------------------------------------------------------------------
1. From: Murray & Gibson (1974).
2. From: Sharp et al. (1972).
Toxic doses of paraquat were administered orally and iv to rats
(Sharp et al., 1972). Paraquat concentrations in the whole blood
were the same as those in the plasma. The distribution of the
herbicide in various tissues was then followed for 10 - 18 days.
The lung had the greatest retention and consequently contained the
highest concentration 4 h after dosing. Four to 10 days after
dosing, the paraquat concentration in the lung was 30 - 80 times
higher than that in the plasma. The high lung-tissue
concentrations of paraquat were confirmed by Ilett et al. (1974)
for rats and rabbits after iv injection of 20 mg 14C-paraquat/kg
body weight. Although the herbicide showed a selective
localization in rabbit lung, the concentration decreased far more
rapidly in rabbit lung than in rat lung. The rabbit did not show
any histological or biochemical signs of lung damage, and no
evidence of covalent binding of paraquat in lung tissue was found
by Ilett et al. (1974). After thorough washing of tissue
precipitate with dilute trichloroacetic acid, only insignificant
amounts of 14C-paraquat were detected in protein from the brain,
heart, kidney, liver, lung, and plasma.
Autoradiographic studies using 14C-paraquat have been carried
out on mice and rats (Litchfield et al., 1973). Paraquat was
observed in nearly all organs 10 min after intravenous injection of
20 mg/kg body weight. Waddell & Marlowe (1980) obtained similar
autoradiographic results in mice, after intravenous injection of
288 - 338 µg 3H-paraquat dichloride/kg body weight. Cellular
resolution autoradiography showed that paraquat was confined almost
entirely to cells having the distribution of alveolar Type II
cells. These cells are known to be susceptible to the toxicity of
paraquat (Kimbrough & Gaines, 1970). Waddell & Marlowe (1980)
suggested that it was unlikely that the radioactivity was bound to
cellular constituents.
No paraquat was detected in rat kidney, brain, liver, or lung
when paraquat was administered in the diet at a concentration of
50 mg/kg for a period of 8 weeks. At 120 mg/kg, it was found in
low concentrations in the lung, kidney, gastrointestinal system,
and brain (Litchfield et al., 1973). At 250 mg/kg, it was detected
in the tissues within 2 weeks. No sex differences or any clear
pattern of accumulation were noted throughout the 8-week study.
Within 1 week of return to a normal diet, no paraquat was detected
in any tissue examined. Histological changes were observed in all
lungs of animals fed paraquat at 250 mg/kg diet.
Rose et al. (1974a) demonstrated an energy-dependent
accumulation of paraquat in slices of rat lung that obeyed
saturation kinetics. The same investigators also examined the
ability of paraquat to accumulate in tissue slices from other
organs in vitro (Rose & Smith, 1977). The herbicide in brain,
adrenal gland, and kidney slices accumulated; however, the uptake
was less than 10% of that observed in the lung slices. The authors
established the uptake of paraquat by the lung in various species
(rat, rabbit, dog, monkey, man). The human lung accumulated
paraquat as strongly as that of the rat and there was a
relationship between the concentration of paraquat in the different
lung areas and the development of microscopic lung lesions. It has
been demonstrated that the rate of paraquat efflux from lung tissue
is less than its rate of accumulation in the lung slices (Smith et
al., 1981). Efflux from lung slices, prepared from rats dosed iv
with the herbicide, was found to be biphasic. There was a fast
component (half-life 20 min), followed by a first-order slow
component characterized by a half-life of 17 h. The half-life in
vitro was similar to that seen in vivo following iv
administration to rats.
6.1.3. Metabolic transformation and excretion
Paraquat participates to a considerable extent in cyclic
reduction-oxidation reactions. After undergoing a single electron
reduction in tissues, the resultant free radical is readily
oxidized by molecular oxygen to the parent compound (section 6.3).
This leads to an overall excretion of essentially unchanged
paraquat in the urine after oral administration to rats (Murray &
Gibson, 1974).
Daniel & Gage (1966) reported that paraquat was metabolized by
gut microflora following oral dosing of rats. This observation was
not confirmed in subsequent studies (Murray & Gibson, 1974) and was
later attributed to a problem with the method (FAO/WHO, 1977).
Urinary concentrations of paraquat following oral
administration are relatively low (Daniel & Gage, 1966: Murray &
Gibson, 1974; Sharp et al., 1972; Maling et al., 1978) and are thus
used to estimate its elimination from the body.
Sharp et al. (1972) reported a biphasic elimination of paraquat
from the plasma of rats after iv injection. The initial rapid
phase had a 20 - 30 min half-life, and the slower phase a half-life
of 56 h. Murray & Gibson (1974) also showed prolonged paraquat
elimination after oral administration to rats, guinea-pigs, and
monkeys. The urinary and faecal routes were equally important in
all species studied. The faecal content was due mainly to
elimination of unabsorbed paraquat. Prolonged elimination of
paraquat in all animals tested indicated retention of the herbicide
in the body.
Following iv administration to rats, about 75 - 79% of the dose
was excreted in the urine within 6 h (Maling et al., 1978). The
plasma disappearance of an iv dose of paraquat of 5 mg/kg was
fitted to a 3-compartment model. Total body clearance was
estimated to be 8.39 ± 0.54 ml/kg per min (Maling et al., 1978).
The relatively high concentration of paraquat in the duodenal and
jejunal walls suggested biliary secretion of the herbicide, and the
authors' hypothesis was supported by the observation of radio-
activity in the intestines of mice in whole-body autoradiographic
studies (Waddell & Marlowe, 1980).
Since absorbed paraquat is mainly removed via the kidneys, the
early onset of renal failure will have a marked effect on paraquat
elimination and distribution, including accumulation in the lung.
Hawksworth et al. (1981) used the dog as a model to evaluate the
influence of paraquat-induced renal failure on the kinetics of
paraquat elimination. After iv injection of a trace dose of 14C-
paraquat (30 - 50 µg/kg body weight) in dogs, the kinetics of
distribution was described by a 3-compartment model. To obtain a
good fit of the curve, it was necessary to sample the central
(plasma) compartment for at least 24 h after dosing. Simulation of
paraquat levels in the peripheral compartments suggested the
existence of a compartment with rapid uptake and removal (kidney)
and another with slow uptake (lung). The renal clearance of
paraquat approximated total body clearance indicating that paraquat
elimination occurs through renal excretion. The urinary excretion
rate of an iv dose was rapid, approximately 80 - 90% of the dose
being eliminated during the first 6 h. Intravenous injection of a
large toxic dose of paraquat (20 mg/kg body weight), however,
brought about a marked decrease in renal clearance, from 73 ml/min
to 18 ml/min after 2 1/2 h and 2 ml/min after 6 h. This data
suggested that damaged renal tubules could contribute to paraquat
accumulation in the lung.
6.2. Observations on Human Beings
6.2.1. Observations on paraquat poisoning after ingestion:
non-fatal cases
Tompsett (1970) reported a case of ingestion of 45 g of Weedol
(2.5% paraquat). On hospital admission, the gastric aspirate
contained 0.215 g paraquat/litre and the urine 0.148 g/litre.
After 2 - 4 h, paraquat concentrations dropped to 5.1 mg/litre in
the urine and 0.4 mg/litre in the serum but, 16 - 24 h after
admission, the urinary level was 0.95 mg/litre, while no paraquat
was detectable in the serum. Paraquat was also detected in the
urine for up to 15 days after poisoning, while at the same time
serum concentrations were below the detectable limits in chemical
analysis (Fletcher, 1975).
The cumulative elimination of paraquat in the faeces and urine
of a patient was followed for 7 days by van Dijk et al. (1975).
Faecal elimination increased from 340 mg the first day to 530 mg
after 7 days, while cumulative urinary excretion reached 60 mg the
lst day and increased to 75 mg after 7 days. It was calculated
that only 87 mg of paraquat had been absorbed from a total
ingestion of about 637 mg, determined in the urine, dialysate, and
faeces. In this patient, less than 14% of the ingested paraquat
was absorbed through the gastrointestinal system.
6.2.2. Observations on paraquat poisoning after ingestion:
fatal cases
It is well established that paraquat lung disease resulting in
death is usually preceded or accompanied by renal insufficiency.
This contributes to the retention of paraquat in body tissues.
Nevertheless, Fairshter et al. (1979) detected only small
concentrations (below 0.09 mg/kg) of paraquat in several organs of
patients who died 3 weeks after ingestion.
The detection of 27 mg paraquat/litre in the bile of a woman
after autopsy suggested that some faecal paraquat might be
attributable to biliary excretion (Dijk et al., 1975).
6.2.3. Significance of paraquat concentrations in cases of
paraquat poisoning
Not only oral ingestion, but also dermal absorption of paraquat
after occupational overexposure, resulted in measurable urinary
levels of paraquat. The determination of paraquat in urine and
serum is an important biological exposure test for the diagnosis
and the prognosis in cases of human poisoning.
Wright et al. (1978) followed the urinary excretion of paraquat
in 16 patients (7 of whom died). The total amount of paraquat
excreted ranged from 0.6 mg to 386 mg. The excretion rate
decreased rapidly during the 48 h following ingestion, though less
rapidly in the patients who eventually died. All patients
excreting 1 mg of paraquat or more per hour, for 8 h or more after
ingestion, died.
Plasma-paraquat concentrations were measured by gas
chromatography, radioimmunoassay, and colorimetric methods in 79
patients with paraquat poisoning (Proudfoot & Stewart, 1979). At
any given time after ingestion (within a limit of 35 h), plasma
concentrations were significantly higher in the patients who died
(Fig. 4). Patients whose plasma concentrations were not higher
than 2.0, 0.6, 0.3, 0.16, and 0.10 mg paraquat/litre at
respectively, 4 h, 6 h, 10 h, 16 h, and 24 h after the poisoning,
were likely to survive. When plasma levels exceeded 0.3 mg/litre
15 h after ingestion, a fatal outcome could be expected, despite
treatment. These conclusions were supported by the studies
performed on 28 patients by Bismuth et al. (1982).
4.1.2. Photochemical degradation of paraquat on soil and other
mineral surfaces
Slade (1966) showed that there was a breakdown, similar to that
on plant surfaces, if spots of paraquat on silica gel were exposed
to direct sunlight. When 14C-paraquat dichloride was sprayed on
the bare soil surface of a field during a hot sunny period, traces
of 4-carboxy-1-methylpyridynium chloride were detected in the top
inch of soil for the first few weeks afterwards (Calderbank &
Slade, 1976). Radioassay showed that the total soil residue did
not markedly decrease during a 6 - 18 month period, so that, in
agricultural practice, UV degradation of herbicide reaching the
soil should be regarded as insignificant.
The principal intermediates of photochemical paraquat
degradation on plants or soil surfaces are of low toxicity.
They decompose easily and are not expected to produce adverse
environmental effects.
4.2. Microbial Degradation
Microbial paraquat degradation has been thoroughly reviewed by
Haley (1979). Baldwin et al. (1966) identified many soil
microorganisms capable of degrading paraquat. The herbicide was
decomposed by Corynebacterium fascians, Clostridium pasteurianum,
and Lipomyces starkeyi. Several other microorganisms were found to
degrade paraquat (Smith et al., 1976; Tchipilska, 1980) but
Lipomyces starkeyi proved to be the most active (Burns & Audus,
1970). Burns & Audus (1970) concluded that microbiological
degradation was possible only for a short time following the
application of paraquat to soil. Once adsorbed on to clay
materials, the paraquat was inaccessible to microorganisms.
Microbial degradation of paraquat in the field is therefore
relatively slow.
Studies of 4-carboxyl-1-methylpyridylium chloride in soil have
demonstrated that the radiolabelled product readily decomposes to
form several chemical substances, including carbon dioxide. No
significant residues of the compound have been determined in plants
as a result of uptake from the soil. Wright & Cain (1970) isolated
Achromobacter D from the soil; this utilized the 4-carboxyl-1-
methylpyridylium chloride and the methylamine originating from
the N-methyl group of the molecule. The NADH and the oxygen
requirement indicated the possibility of direct oxidative
fission of a partly reduced ring to form dialdehyde, which was
then hydrolysed to formate, methylamine, and succinic dialdehyde.
The end-products of the microbial ring degradation were formate,
succinate, and carbon dioxide.
4.3. Environmental Adsorption and Transformation
4.3.1. Soil
The property of paraquat that is most important in nullifying
its impact on the environment is its rapid and complete binding to
clay soils. Desorption of the herbicide from soil particles, for
the purpose of chemical analysis, requires destruction of the
mineral particles by refluxing with strong sulfuric acid. The
strong adsorption to clay has been attributed to the flat and
highly polarizable nature of the paraquat ion (Coats et al., 1966;
Knight & Denny, 1970). Weber et al. (1965) reported that the
adsorption appeared to be one of ion exchange and was very rapid,
the rate of adsorption depending on the rate at which the paraquat
ion contacted the adsorbing particles.
In highly organic soils, the weaker adsorption sites of soil
organic matter delay the redistribution of paraquat without
inactivating it herbicidally. In this connection, Khan (1980)
reported tests showing a remarkable affinity of humic substances in
the soil for the paraquat ion. These humic substances enhance the
degradation of pesticides via non-biological pathways.
It has been demonstrated that on soil containing 98% organic
matter, the herbicidal effects of 1.12 and 2.24 kg of paraquat/ha
persisted for 16 - 29 days, but such soils are not widespread
naturally. Burns & Audus (1970) studied the migration of paraquat
from soil organic matter to clay mineral particles. The transfer
of the paraquat from the organic to the inorganic fraction, through
a membrane, was 90% complete within 6 h. The remaining 10% took
about 2 days to be transferred. No paraquat was detected in the
organic fraction after 4 days. At high paraquat concentrations
(more than 20 mg/kg in equilibrium solution), the total adsorption
capacity was greater than normal in soils with high organic
content, as opposed to those with low organic content.
Mithyanta & Perur (1975) studied samples of 4 different soils
treated with paraquat in different experimental schemes. After 24
h, the soils were extracted with a water solution of ammonium
chloride. The percentages of paraquat, extractable with water,
ranged from 4.8 - 66.9%, depending on the type of soil and the
conditions. Data on the persistence of paraquat in the soil have
also been compiled by Coats et al. (1966), Knight & Tomlinson
(1967), Knight & Denny (1970), and Burns & Audus (1970).
As summarised in section 4.2, free paraquat is degraded by a
range of microorganisms, but degradation of strongly adsorbed
paraquat is relatively slow. In plot studies, degradation was very
slow or non-detectable (Riley et al. 1976). However, in long-term
field studies, degradation rates were 5 - 10% per year. This is
greater than the rate required to prevent saturation of the
deactivation capacity of soils.
In a long-term trial on a loamy soil, plots were treated with
0, 90, 198, and 720 kg paraquat/ha, which was incorporated to a
depth of 15 cm. These rates were equivalent to 0, 50, 110, 400% of
the soils strong absorption capacity (Gowman et al., 1980;
Wilkinson, 1980; Riley, 1981). Over the 7 years, paraquat residues
declined by 5% per year (sig P = 0.05) on the 90 kg/ha plots and
by 7% per year (sig P = 0.01 on the 198 and 720 kg/ha plots. The
rate of decline on the 198 and 720 kg/ha plots was significantly
greater ( P = 0.01) than on the 90 kg/ha plots.
In another long-term trial on a sandy loam, plots were treated
annually with 4.4 kg/ha for 12 years (Hance et al, 1980). The rate
of loss of paraquat soil residues was about 10% per year and the
soil residues tended to plateau when the rate of application
equalled the rate of degradation. Data for the last 4 years (total
16 years) has confirmed the early results (Hance, unpublished
data).
Some paraquat could be recovered from its tightly bound form by
chemical destruction of the soil from field plots, several years
after application. The limit of paraquat adsorption, at which
further treatment would result in phytotoxic activity, was
considered to be important. Strong adsorption capacity was defined
as the measure of paraquat that can be adsorbed by the soil without
entailing phytotoxic effects, and this capacity was determined in
several kinds of soil with various clay and organic contents
(Knight & Tomlinson, 1967). Mechanical analyses, pH, and organic
matter content were also determined. Independently of the soils
studied, it was found that, by applying 1 kg/ha per year, it would
take from 30 - 1440 years to saturate the top 15 cm of soil at
strong adsorption sites. The conditions of study precluded any
form of paraquat degradation or metabolism in the soil. Riley et
al. (1976) reviewed the hazard of continuous application of 0.1 -
2 kg paraquat ion/ha, assuming soil contamination by 10 - 100% of
the amount applied. Bound paraquat soil residues were not adsorbed
by living organisms. Paraquat residues did not induce any effects
on microarthropods or microorganisms. Continued application of
the herbicide in different soils has been investigated by Pestemer
et al. (1979). The ED50 valuesa for phytotoxic action on lettuce
ranged from 0.01 mg/litre paraquat solution in agar-agar to 98 -
1930 mg/litre in different soils, depending on their constituents,
and 31 - 57.6 mg paraquat residues/kg have been determined in the
soil samples. There is evidence (Hance et al., 1980) that
strongly-bound paraquat residues were degraded in soil by microbial
activity at a rate of 5 - 10% per annum. A correlation was reported
between the paraquat residues, the number of treatments, doses, and
depth of soil sampling.
-------------------------------------------------------------------
a ED50 = median effective dose.
Although, as mentioned, adsorption on clay is important,
extremely sandy soils can adsorb and inactivate significant
quantities of the herbicide, as illustrated by studies on a South
African vineyard soil that contained only 1% clay (Riley et al.,
1976). Over an 8-year period, more than 20 applications (total
15.6 kg paraquat/ha) resulted in saturation of about 20% of the
soil-paraquat-strong-adsorption capacity in the top 2.5 cm. The
paraquat residues were not phytotoxic in the field or in greenhouse
tests on different plants. No paraquat residues were detected
(<0.05, <0.03, <0.03 mg/kg) in leaves, grapes, and twigs,
respectively.
Very low concentrations of free paraquat would be detected
easily by their phytotoxicity. Five trials at 4 sites were
conducted by Newman & Wilkinson (1971). In 4 of the trials, single
applications of paraquat at 112 kg/ha were made at sites subjected
to normal agricultural practice. At this unrealistic, extremely
high rate, short-duration residual phytotoxicity was observed. On
undisturbed plots of mineral soils, seedlings did not appear for
several months; on organic soils, the time lag was even longer.
After cultivation, there was no further indication of
phytotoxicity. In the 5th trial, a total of 565 kg/ha was applied
in 5 doses over 4 1/2 years. The plot then remained undisturbed,
apart from periodic cultivation of the top 20 mm to prepare a
seedbed. It was at this site that phytotoxicity to ryegrass
seedlings was detected, and free paraquat was determined in the
surface soil using the Lemna minor bioassay. Phytotoxicity was
confined to the surface layer of the soil. The free paraquat that
had leached out of the top 2.5 cm had been adsorbed in the deeper
soil layers, and this was confirmed by the absence of residual
phytotoxicity when the site was more deeply cultivated.
However, the extreme situations seen in high-dosage trials are
not encountered in practice and only serve to show the possible
consequences for the environment of a gross overdose of the
herbicide. Thus, when paraquat is used in normal application
doses, no adverse environmental effects can be expected.
Accidental spillage is probably the most likely cause of high
levels of residual paraquat. The 200 g of paraquat contained in 1
litre of Gramoxone(R) would be completely inactivated by the
addition of 10 kg of bentonite, for inactivation can be effected
either by cultivation and mixing other soil with the contaminated
layer or by adding clay minerals. Simulated spills of paraquat
have also been treated with sodium borohydride or alkali (Staiff et
al., 1981); within 1 day the paraquat in the soil had been
effectively degraded.
4.3.2. Water
The ecological effects of paraquat in water have been
studied in relation to its use as an aquatic herbicide at a
normal concentration of 1 mg/litre (Newman & Way, 1966; Grzenda et
al., 1966). Following this use, the concentration present in water
decreased to about half of the initial 1 mg/litre level within
36 h, and, in less than 2 weeks, the concentration was below
0.01 mg/litre. Weed-sample analysis, 4 days after paraquat
application, showed a residue of approximately 25 mg/kg, suggesting
that absorption by the weed was mainly responsible for paraquat
removal. Mud-residue analysis 5 1/2 months after treatment showed
that 36% of the applied paraquat remained in the mud, and 70% of
that was found in the top 2.5 cm. In the mud, paraquat had been
adsorbed on to the mineral material. Since bottom mud often has
organic components, the residues may be more accessible to
bacterial degradation. Compared to other products, paraquat
appears to be the herbicide of choice for future use in water
supplies because of its rapid disappearance from water (6 - 14 days
after treatment) (Grzenda et al., 1966). The residues were not
desorbed from the bottom sediments, and mud taken from the bottom
of a paraquat-treated lake carrying inactivated residues, showed no
toxic effects on barley seedlings that germinated on it (Way et
al., 1971).
Wauchope (1979) discussed the fate of pesticides in water
draining from fields after rain. For most formulations, a total
loss of 1.5%, or less, of the amount applied was the rule, except
when severe rainfall occurred within 1 - 2 months following
treatment. Nearly all the pesticides examined were lost by runoff;
only those binding strongly to clay particles, such as paraquat,
were carried off in the sediment phase of runoff. The lack of
paraquat runoff loss has also been discussed by Smith et al.
(1978).
Grover et al. (1980) compared the efficiency of various
herbicidal treatments for weed control in a series of irrigation
ditches. At the relatively low dose of 2.2 kg/ha, paraquat
resulted in aquatic weed suppression from 1973 to 1976, and this
made for satisfactory water flow without environmental
contamination. Water that contains small amounts of paraquat
residues loses them rapidly on contact with soil, the adsorption
process being irreversible (Knight & Tomlinson, 1967; Calderbank,
1972). Thus treated water may be used quite safely for channel
irrigation, if an interval of 10 days is observed between treatment
of the water and its use, because the paraquat will be unavailable
to the plant roots. Caution should, however, be exercised in
prolonged crop irrigation until the residue is well below 0.1
mg/litre, although phytotoxic damage is unlikely at even 0.5 mg
paraquat/litre (Calderbank, 1972).
Coats et al. (1966) treated 0.1 ha experimental ponds with
paraquat to obtain a concentration of 0.4 mg/litre. The soil in
one of the ponds was stirred twice after 24 h. Analysis of the
water over several weeks revealed a decrease from 0.4 mg/litre to
0.01 mg/litre after several weeks, but when the soil of the pond
was stirred, the paraquat concentration fell from 0.75 mg/litre
to <0.01 mg/litre after 8 - 12 days. In static water experiments,
the concentration of 0.5 - 1 mg/litre fell rapidly to about 0.1
mg/litre within 4 - 7 days of treatment in 4 trials performed by
Calderbank (1972). These reductions in the paraquat concentration
were due to its rapid adsorption and concentration in aquatic
plants. Decaying weeds transported it to the bottom mud (Table 4)
where it was not released back into the water (Way et al., 1971).
Earnest (1971) treated a pond with paraquat at an initial
concentration of 1.14 mg/litre. No residues were detected in the
water after 16 days (limit of detection 0.01 mg/litre); in the mud
the concentration was 1.13 mg/kg after 3 h and 3.25 mg/kg after 99
days. These data were confirmed by Grover et al. (1980).
Grover et al. (1980) studied irrigation water from ditches.
Three days after treatment with 2.2 kg paraquat/ha, the
concentrations in the water used to flood the treated ditches were
less than 0.01 mg/litre, and paraquat residues in the ditch water
ranged from 0.002 - 0.034 mg/litre in samples taken 3 - 5 days
after foliar applications.
Table 4. Residues of paraquat in water, weed, and bottom muda
-------------------------------------------------------------------
Days after treatment
-------------------------------------------------------------------
1 4 16 32 175 420
-------------------------------------------------------------------
Trial 1 water (mg/litre) 0.31 0.12 ND
weed (mg/kg) 13.70 25.80 21.0 0.55
mud (mg/kg) 3.70 - - - 57.1 20.1
Trial 2 water (mg/litre) 0.37 ND ND ND - -
weed (mg/kg) 25.50 40.0 37.8 27.8 - -
mud (mg/kg) ND 0.97 0.23 0.32 6.6 0.96
-------------------------------------------------------------------
a From: Way et al. (1971).
ND - not detectable.
4.3.3. Air
Paraquat is not volatile. Dry deposits of 14C-paraquat
chloride exposed at room temperature showed no measurable loss in
64 days (Coats et al., 1966). Exposure to paraquat in the air is
not important in spraying and harvesting operations; the skin is
the principal route of occupational exposure (Chester & Woollen,
1982; Staiff et al., 1975).
Air concentrations of paraquat were measured on summer days by
Makovskii (1972) using the method of Calderbank & Yuen (1965).
About 1 - 1.3 kg paraquat/ha had been applied as a herbicide or
desiccant in 0.25 - 0.35% water solutions. The paraquat aerosol
concentrations varied according to spraying method and work-place
(Table 5). Using the same analytical method, Staiff et al. (1975)
examined 35 sites after paraquat application with tractor-mounted
field sprayers or hand-pressure garden dispensers. The working
solutions contained 0.15% paraquat for field use, and 0.44% for
garden use. The respiratory exposure of field and garden operators
was below the limit of detection (<0.001 mg paraquat/h).
Mature cotton fields (Seiber & Woodrow, 1981) were sprayed with
paraquat, the dose being 0.94 kg/ha. The air paraquat
concentrations measured downwind decreased regularly from the
extrapolated interval-average values of 4.31 and 10.7 µg/m3 1
metre downwind of the 2 fields to <50 ng/m3 at 400 metres away in
the same direction. Forty-five percent of the aerosol particles
had diameters ranging from 0.01 to 4 µm. The remaining 55% had a
median diameter of 12 µm. Downwind samples taken 2 - 4 h after
spraying contained 1 - 10% of the amount dispersed, but, after
5 - 7 h, no paraquat was detectable in the air.
Table 5. Paraquat total airborne concentrations (mg/m3) in
working areasa
-------------------------------------------------------------------
Place of Number of Mean
sampling samples concentrations
± SE
-------------------------------------------------------------------
Working area sprayer loading 28 0.13 ± 0.03
tractor cabin 16 0.37 ± 0.07
(in direction of wind)
tractor cabin 16 0.55 ± 0.01
(against the wind)
manual spraying 16 0.18 ± 0.04
Treated field after 5 min 16 0.05 ± 0.01
after 10 min 32 < 0.01
after 20 min 16 0
Distance from 200 m 8 0.08 ± 0.01
treated field 400 m 8 0.04 ± 0.01
-------------------------------------------------------------------
a From: Makovskii (1972).
A study of Malaysian plantation workers, occupationally exposed
to paraquat, revealed a mean total airborne exposure of 0.97 mg/m3
for spray operators. This exposure is less than present TLVs
(Chester & Woollen, 1982). Wojeck et al. (1983) reported that
after spraying paraquat in fields of tomatoes and citrus, the total
airborne exposure ranged from 0 - 0.070 mg/h. It was less than
0.1% of the total body exposure (12.16 - 168.59 mg/h) in all
trials.
During mechanical harvesting of cotton dessicated by paraquat,
the maximum levels in airborne dust were found to be 1245 ng/m3
outside the cabin of the tractor and 516 ng/m3 inside the open
cabin. With the cabin door closed, the concentration was only 13.7
ng/m3. The trapped particulate matter consisted of dessicated
plant material and soil dust. A cascade impactor analysis
established that 57% of the paraquat had a median particle diameter
of 4 µm, 23%, 12 µm, and 11%, 3 µm. Cotton harvesting generated
parti- culate concentrations in the field comparable to those
immediately downwind of the field during spraying. Bearing in mind
the highest paraquat air concentration in the harvest-time air
(0.0012 mg/m3), a harvester operator's maximum exposure through
inhalation was calculated to be 0.01 mg/8 h/day (Seiber & Woodrow,
1981).
Bulgaria has established a maximum allowable concentration
(MAC) of 0.01 mg paraquat/m3 (1972), the Federal Republic of Germany
0.1 mg/m3 (1982), Hungary 0.02 mg/m3 (1978), and the USA a TLV of
0.1 mg/m3 (1982).
4.3.4. Plants
Paraquat residues on plants have been reviewed several times by
the Joint Meeting on Pesticide Residues (JMPR) (FAO/WHO, 1971,
1973, 1983). The residues found after paraquat was used as a
desiccant are summarized in Tables 6 and 7 (Calderbank, 1968).
Table 6. Paraquat residues (mg/kg) in cotton 10 days after
dessication at 0.55 kg/haa
-------------------------------------------------------------------
Fraction analysed Paraquat found
-------------------------------------------------------------------
Cotton as picked, including trash and balls 2.00
Ginned seed 0.18
Mechanically reginned seed 0.08
Acid-delinated seed 0.05
Lint cotton 3.00
Trash 3.70
Hulls 0.13
Crude oil ND
Meal 0.02
-------------------------------------------------------------------
a From: Calderbank (1968).
Coats et al. (1966) reported that 14C-paraquat applied to wheat
as a 1% solution was translocated in the plants, including the
roots. Slade (1966) studied the degradation of 14C-paraquat
dichloride and its photochemical degradation products in plants.
Maximum loss occurred in tomato, broad-bean, and maize when the
paraquat remained on the leaf surfaces during sunny days.
In potatoes treated with paraquat as a desiccant, Makovskii
(1972) found a residue of 0.05 mg/kg, and there was no change after
the potatoes had been boiled. No residues (limit of detection 0.01
mg/kg) were found in fruits (apples, citrus fruits, plums, pears),
tea, and cereals. In tests on sunflower seeds treated with 0.25 or
0.5 kg paraquat/ha, residues of up to 0.9 mg/kg were found in the
whole seed, up to 1.2 mg/kg in sunflower meal, and no residue in
the oil (Anonymous, 1979). Therefore, the use of sunflower meal in
the diet of hens, dairy cattle, and other livestock would not
result in paraquat levels exceeding current standards.
Table 7. Paraquat residues (mg/kg) in food crops 3 - 21 days
after dessicationa
-------------------------------------------------------------------
Crop Rate of application Paraquat found
(lb/acre)
-------------------------------------------------------------------
Barley 0.50 - 1.00 3 - 10
Wheat 0.50 - 1.00 1 - 2.5
Maize 0.50 - 1.20 ND - 0.2
Rice (with husk) 0.15 - 0.54 0.7 - 22
Rice (de-husked or polished) 0.15 - 0.54 ND - 0.2
Peas, beans, sunflower seed 0.35 - 1.20 ND - 0.2
Sorghum seed 0.25 - 1.00 0.1 - 0.4
Cotton (as picked) 0.50 - 1.00 2 - 3
Potatoes 0.50 - 1.50 0.02 - 0.13
Onions 0.50 - 2.00 ND - 0.05
Sugar cane juice 0.50 - 2.00 ND
Seed oils (sunflower, rape, up to 1.20 ND
sesame, cotton)
-------------------------------------------------------------------
a From: Calderbank (1968).
Seiber et al. (1979) determined the paraquat residues in
treated cotton (the foliage and bolls of the live plant, the lint
and seed of harvested cotton, the gin waste and the lint and
non-lint components). Gin waste residues were surveyed during
5 months of open storage. The paraquat dose had been 0.21 and
2.0 kg/ha. The results obtained are summarized in Table 8. The
minimal degradation of paraquat in the plants studied was confirmed
by Hills et al. (1981).
Significant paraquat residues are to be expected only when a
crop is directly sprayed.
After spraying fields of marijuana with paraquat for the
purpose of eradication, residues of paraquat were detected in
marijuana (Smith, 1978; Patrick, 1980). Of the 54 samples
collected in 1976, 7.4% were positive and of 46 samples collected
in 1977, 19.6% were positive.
Table 8. Paraquat residues (mg/kg) in cotton plantsa
-------------------------------------------------------------------
Material Days after Leaves Lint Non-lint Seeds
treatment
-------------------------------------------------------------------
Standing cotton plants 2 13.1 22.10 0.06
6 8.2 3.80 0.06
Harvested seed cotton 18 7.15 0.25
stored in field 49 4.85 0.18
Gin waste 49 2.7 9.3
119 5.3 10.1
171 5.8 9.7
-------------------------------------------------------------------
a From: Seiber et al. (1979).
4.3.5. Animals
The effects and fate of 14C-paraquat orally-administered to
cattle at 8 mg/kg body weight were studied by Stevens & Walley
(1966). Seven days after this single dose, 0.03 - 0.08 g/litre had
been excreted in the milk and 2.4 g/litre in the urine of the cows.
The total paraquat excretion in the milk was only 0.01% of the
ingested dose. In cows given daily oral doses of 8 mg paraquat/kg
for 3 weeks, residues of less than 0.01 mg/litre were detected in
the milk (FAO/WHO, 1977). Cattle did not suffer any toxic effects
over a 4-week period when turned loose on pasture immediately after
it had been sprayed with 1.12 kg paraquat/ha (Calderbank et al.
1968). During the first 2 weeks of grazing on the dried herbage,
it was estimated that the cattle ingested approximately half of
their acute oral LD50 (36 - 54 mg/kg body weight) every day.
Paraquat levels in the herbage ranged from about 400 mg/kg 1 day
after spraying, to about 200 mg/kg 14 days after treatment;
14 - 35 days after spraying the levels were 135 -214 mg/kg. The
4-carboxyl-1-methylpyridylium chloride content during the trial
period was 5.1 - 3.4 mg/kg. By the 4th week of the study, paraquat
levels in the urine were 0.01 - 0.19 mg/litre and in the faeces,
0.9 - 42 mg/kg. Only on the first day after spraying were paraquat
residues (0.02 mg/litre) found in the milk of 2 cows; no residues
were found (< 0.005 mg/litre) thereafter. The only organs of a
slaughtered animal that contained paraquat were the kidney
(0.03 mg/kg) and the stomach (0.05 mg/kg).
The fate of paraquat in large animals is addressed far more
completely in the Evaluations of the 1976 Joint Meeting on
Pesticide Residues (JMPR) (FAO/WHO, 1977).
Rabbits were fed with lucerne treated with normal-use levels of
paraquat (Lavaur et al., 1979). Immediately after spraying, the
paraquat residues were 272 mg/kg (dry weight of lucerne). After
24 h and 48 h, they were 114 mg/kg and 62 mg/kg, respectively. No
systemic toxicity symptoms or gastrointestinal damage were observed
in the treated rabbits.
When hens were given paraquat at 40 mg/litre in their drinking-
water for 14 days, the amount of paraquat found in the eggs rose to
0.1 mg/kg, but fell to less than 0.005 mg/kg, 6 days after
cessation of treatment (Fletcher, 1967). Eggs from hens eating
grain containing paraquat at a concentration of 10 mg/kg contained
residues below 0.025 mg/kg.
5. BIOLOGICAL ACTIVITY OF RESIDUES
5.1. Soil Organisms
Haley (1979) reviewed the effects of paraquat on soil
microorganisms and fungi, while Tu & Bollen (1968), Curry (1970),
Radaelli & Martelli (1971), Roslycky (1977), and Smith et al.
(1981a) studied the effects of paraquat on the size and composition
of the microbial soil populations, total microbial respiration in
the soil, the rate of organic matter degradation, and the number of
soil microorganisms. None of these authors found any adverse
ecological effects from normal and excessive (up to 32 times the
normal dose) paraquat treatment, although in some cases
nitrification was temporarily suppressed or activated, and some
bimodal microbiological effects were observed with intermediate
herbicide concentrations (Tu & Bollen, 1968; Tchipilska, 1980).
At normal doses, paraquat had no adverse effect on
endomycorrhiza formation and function (Smith et al., 1981a), on
total populations of bacteria, actynomyces, fungi (Roslycky, 1977;
Haley, 1979; Tchipilska, 1980; Smith et al., 1981a), or on 24
different species of soil fauna taken from 2 plots at a depth of
3.8 cm (Curry, 1970).
Curry (1970), and Riley et al. (1976) made extensive
studies of the effects of normal and high doses of paraquat on
microarthropod and earthworm populations at sites at different
stages of cultivation. The herbicide was neither harmful nor
repellant to earthworms, nor was there any evidence of a toxic
effect or of paraquat accumulation in any species examined. When
the residues in the top 2.5 cm of soil reached 20 mg/kg, the
highest concentration determined in Allolbophora caliginosa, living
near the surface, was 3.2 mg/kg (live weight). Worms from highly-
dosed plots eliminated paraquat residues within 36 h, when placed
in clean soil.
5.2. Effects of Residues on Crop Yields
The absence of adverse effects from residual paraquat on the
growth and yield of crops grown in paraquat-treated soils has been
demonstrated by Knight & Tomlinson (1967), Damanakis et al. (1970),
Newman & Wilkinson (1971), and Riley et al. (1976). It is known
that the paraquat-inactivation capacity of soils varies widely.
Paraquat has been tested on soils of low adsorption capacity, it
has been used repeatedly on the same soil (section 4.3.1) and has
been tested at extremely high concentrations. The absence of any
reports or observations of long-term phytotoxic effects confirms
the data obtained in greenhouse and laboratory studies.
5.3. Effects on Fish and Aquatic Organisms
Despite variation in LC50s for fish (67 - 110 mg/litre after
24 h, 38 - 62 mg/litre after 48 h, more than 25 - 32 mg/litre after
96 h), the herbicide has proved to have a wide margin of safety for
warm- and cold-water fish species (Calderbank, 1972). The toxicity
of paraquat for fish varies with the species, the size of the
fish, and the softness or hardness of the water. A large number
of aquatic species have shown a 100% survival at 96 mg/litre over
96 h, though the decreased oxygen concentration following decay of
weeds, may be dangerous in extreme situations. Rainbow trout
tolerated 1 mg paraquat/litre water in prolonged toxicity tests
and only a 30% mortality was recorded after 16 days of repeated
exposure (Calderbank & Slade, 1976). At the end of the test, 0.54
mg paraquat/kg was found in the rainbow trout. In a 7-day exposure
test at 1 mg paraquat/litre, the herbicide was detected in the gut
(0.41 mg/kg) and liver (0.35 mg/kg), but not in the meat of the
fish (< 0.025 mg/kg). Water snails collected from 2 ditches,
12 weeks after treatment of the waters with 1 mg/litre were found
to contain 0.43 mg herbicide/kg. Fish (major carp fingerlings)
exposed to paraquat in the presence of weeds were more susceptible
than those in weed-free environments (Singh & Yadav, 1978), owing
to the changed oxygen content of the water. Where there is heavy
weed growth, the oxygen taken up by weed decay may dangerously
reduce the oxygen available for aquatic organisms. To avoid this,
as far as possible, paraquat should be applied before weed growth
becomes dense and only to one part of the water-course or lake at
a time (FAO/WHO, 1973).
5.4. Effects on Birds
Paraquat is less toxic for birds than for mammals. The acute
oral LD50 for the hen is 262 - 380 mg/kg body weight (Table 11).
The acute oral and 24-h percutaneous (applied to feet) LD50 for
mallards are 200 and 600 mg/kg body weight, respectively (Hudson et
al., 1979). For duck, pheasants, and quail, LC50 values of
paraquat when mixed in the diet are 1000 mg/kg of food or more
(Summers, 1980); residues on sprayed vegetation would not therefore
be expected to present a hazard for birds.
When paraquat was sprayed directly on to pheasants' eggs before
incubation, treatment rates up to 2 kg paraquat/ha did not have any
effect on egg hatchability or on the birds' reproductive organs
(Newman & Edwards, 1980). In a similar study with Japanese quail
eggs, sprays containing paraquat levels of up to 3 kg/ha did not
have any effect on hatchability or development of reproductive
organs (Edwards et al., 1979). Thus, normal spray rates should not
induce any adverse effects, even if paraquat is sprayed directly on
eggs.
Bird populations have been monitored in detail, over a 5-year
period, on a farm in the United Kingdom where paraquat use was much
higher than normal; the average application to the whole arable
area was 0.6 kg/ha per year. The paraquat was applied beneath
hedgerows and along fence lines. The farm maintained an excellent
wild bird population (40 species), including ground-nesting birds
(Edwards, 1979). Most species were at a similar or greater density
than the national average in the United Kingdom.
The Ministry of Agriculture, Fisheries, and Food in the United
Kingdom has carried out detailed investigations on mammalian and
avian deaths that could have been caused by pesticides. For the
period 1971 - 81, the normal use of pesticide was not found to have
caused any significant adverse effects on mammals and birds (MAFF,
1980a, 1981). The Ministry concluded, "It is widely believed that
the use and misuse of paraquat is responsible for a considerable
number of wildlife casualties. There is no evidence from the
investigations to support this allegation...." (MAFF, 1980b).
6. KINETICS AND METABOLISM
6.1. Animal Studies
6.1.1. Absorption
6.1.1.1. Oral absorption
Daniel & Gage (1966) studied the absorption of 14C-paraquat
following oral and subcutaneous single-dose administration to rats.
About 76 - 90% of the oral doses were found in the faeces, and
11 - 20% in the urine; most of the subcutaneous dose (73 - 88%)
was found in the urine and only 2 - 14.2% in the faeces. This,
together with the absence of marked biliary excretion, was evidence
that paraquat was poorly absorbed from the gut. This low rate of
absorption was confirmed by Litchfield et al. (1973) and Conning et
al. (1969). Rats, guinea-pigs, and monkeys orally administered
LD50 doses of 14C-paraquat had low peak serum concentrations
(2.1 - 4.8 mg/litre) (Murray & Gibson, 1974). The radioactivity
levels reached a maximum 30 - 60 min after administration and then
remained relatively constant for 32 h. A dose of 126 mg/kg body
weight resulted in a rat serum level of 4.8 - 4.7 mg/litre.
In fasting dogs, low oral doses of paraquat were rapidly but
incompletely absorbed, the peak plasma concentration being attained
75 min after dosing (Bennett et al., 1976). After an oral dose of
0.12 mg/kg body weight, 46 - 66% was absorbed in 6 h. For doses of
2 - 5 mg/kg, only 22 - 38% and 25 - 28% of the dose was absorbed,
respectively. Dose-dependent data from dogs and whole-body
autoradiography suggest that absorption is facilitated in the small
intestine. Some non-ionic surfactants (0.001%) increased 14C-
paraquat transport through isolated gastric mucosa models, but
histological evaluation suggested that this was due to damage of
the epithelial cell membranes (Walters et al., 1981).
6.1.1.2. Pulmonary absorption
Absorption of paraquat following instillation and inhalation in
the lung has been described in several studies (Gage, 1968a;
Kimbrough & Gaines, 1970; Seidenfeld et al., 1978; Popenoe, 1979).
The uptake of 14C-paraquat after an intratracheal injection of
1.86 nmol/lung was investigated in the isolated perfused rat lung
by Charles et al. (1978). The efflux of 14C-paraquat was diphasic
with a rapid phase half-life of 2.65 min and a slow phase half-life
of 356 min. It was suggested that the slow phase represented a
storage pool, possibly responsible for the pulmonary toxicity of
paraquat. Various doses of 3H-paraquat (10-5 - 10-12 g) in 0.1 ml
saline were introduced directly into the left bronchus of rats
(Wyatt et al., 1981). Fifteen min after instilling 10-8 of 3H-
paraquat, 90% of the ion could be accounted for in the tissues and
urine, 50% being present in the lung. With doses at or greater
than 10-5 g, pathological changes were seen in the lung, similar to
those seen after systemic poisoning. Zavala & Rhodes (1978)
reported that the lung of the rabbit was highly sensitive to
paraquat intrabronchial instillation in doses ranging from
0.1 g - 1 pg; moderately sensitive to intraveneously administered
paraquat (25 mg/kg body weight); resistant to the herbicide when
given intraperitoneally or subcutaneously (25 mg/kg body weight).
6.1.1.3. Dermal absorption
Paraquat absorption through animal and human skin has been
studied using an in vitro technique (Walker et al., 1983). Human
skin was shown to be impermeable to paraquat, having a very low
permeability constant of 0.73. Furthermore, human skin was found
to be at least 40 times less permeable than animal skins tested
(including rat, rabbit, and guinea-pig). There are no in vivo
studies on the rate of absorption of paraquat through the skin.
However, observations of dose-related dermal toxicity in
experimental animals and human percutaneous poisoning have provided
some qualitative information concerning the dermal absorption of
paraquat (further discussed in section 8.2.2.2).
6.1.2. Distribution
Since the most characteristic feature of paraquat toxicity is
lung damage, it is important to stress the high concentrations and
retention of paraquat in the lung tissues, relative to other
tissues, following oral, intravenous, intraperitoneal,
subcutaneous, and intrabronchial routes of administration in rats,
guinea-pigs, and monkeys (Sharp et al., 1972; Ilett et al., 1974;
Murray & Gibson, 1974; Kurisaki & Sato, 1979; Waddell & Marlowe,
1980). An association between paraquat concentrations in the lung
and degree of toxicity or lung injury has been reported (Sharp et
al., 1972; Ilett et al., 1974; Waddell & Marlowe, 1980; Wyatt et
al., 1981). Some of their data are summarized in Tables 9 and 10.
Table 9. Paraquat distribution in tissues
---------------------------------------------------------------------------
Route of entry Dose Species Time after Tissue Concentration
treatment
---------------------------------------------------------------------------
1. Intrabronchial 10 ng rat 60 min plasma 0.0092 µg/litre
lung 5.2 ng
kidney 0.052 ng
liver -
heart -
brain -
2. Intravenous 20 mg/kg rat 24 h plasma 0.7 mg/litre
lung 8.0 mg/kg
kidney 1.45 mg/kg
liver 0.48 µg/g
heart 0.75 mg/kg
brain -
---------------------------------------------------------------------------
Table 9. (contd.)
---------------------------------------------------------------------------
Route of entry Dose Species Time after Tissue Concentration
treatment
---------------------------------------------------------------------------
3. Intravenous 20 mg/kg rat 24 h plasma ND
lung 11.36 µm/kg
kidney 1.93 µmol/kg
liver 0.90 µmol/kg
heart 1.13 µmol/kg
brain 0.87 µmol/kg
20 mg/kg rabbit 24 h plasma 0.28 µmol/litre
lung 7.9 nm/g
kidney 5.25 µmol/kg
liver 1.59 µmol/kg
heart 1.52 µmol/kg
brain 0.49 µmol/kg
4. Intraperitoneal 15 mg/kg rat 24 h plasma 0.32 µmol/litre
lung 26.28 µm/kg
kidney 10.4 µmol/kg
liver 5.04 µmol/kg
heart 4.59 nmol/g
brain 1.22 µmol/kg
5. Oral 126 mg/kg rat 16 h plasma 0.90 mg/litre
lung 5.0 mg/kg
kidney 7.00 mg/kg
liver 2.1 mg/kg
heart 2.7 mg/kg
brain -
22 mg/kg guinea- 16 h plasma 0.03 mg/litre
pig lung 1.29 mg/kg
kidney 1.99 mg/kg
liver 0.08 mg/kg
heart 0.31 mg/kg
brain -
---------------------------------------------------------------------------
1. From: Wyatt et al. (1981).
2. From: Sharp et al. (1972).
3. From: Ilett et al. (1974).
4. From: Maling et al. (1978).
5. From: Murray & Gibson (1974).
Table 10. Paraquat distribution in tissues (in mg/kg (mean) tissue)
----------------------------------------------------------------------------
Route of Entry Dose Species Time Lung Kidney Liver Heart Plasma
(mg/kg after
body dosing
weight)
----------------------------------------------------------------------------
1. Oral 126 rat 1 h 3.3 27.5 2.0 1.8 4.7
4 h 3.7 4.5 4.4 0.9 0.8
32 h 13.6 9.4 5.7 2.8 1.1
64 h 1.7 1.0 7.7 0.2 0.1
2. Intravenous 20 rat 1 h 9.0 25.0 5.0 - 6.0
4 h 8.0 6.0 2.0 - 0.3
24 h 6.0 1.0 0.4 - 0.07
2 days 4.0 0.8 0.3 - 0.05
----------------------------------------------------------------------------
1. From: Murray & Gibson (1974).
2. From: Sharp et al. (1972).
Toxic doses of paraquat were administered orally and iv to rats
(Sharp et al., 1972). Paraquat concentrations in the whole blood
were the same as those in the plasma. The distribution of the
herbicide in various tissues was then followed for 10 - 18 days.
The lung had the greatest retention and consequently contained the
highest concentration 4 h after dosing. Four to 10 days after
dosing, the paraquat concentration in the lung was 30 - 80 times
higher than that in the plasma. The high lung-tissue
concentrations of paraquat were confirmed by Ilett et al. (1974)
for rats and rabbits after iv injection of 20 mg 14C-paraquat/kg
body weight. Although the herbicide showed a selective
localization in rabbit lung, the concentration decreased far more
rapidly in rabbit lung than in rat lung. The rabbit did not show
any histological or biochemical signs of lung damage, and no
evidence of covalent binding of paraquat in lung tissue was found
by Ilett et al. (1974). After thorough washing of tissue
precipitate with dilute trichloroacetic acid, only insignificant
amounts of 14C-paraquat were detected in protein from the brain,
heart, kidney, liver, lung, and plasma.
Autoradiographic studies using 14C-paraquat have been carried
out on mice and rats (Litchfield et al., 1973). Paraquat was
observed in nearly all organs 10 min after intravenous injection of
20 mg/kg body weight. Waddell & Marlowe (1980) obtained similar
autoradiographic results in mice, after intravenous injection of
288 - 338 µg 3H-paraquat dichloride/kg body weight. Cellular
resolution autoradiography showed that paraquat was confined almost
entirely to cells having the distribution of alveolar Type II
cells. These cells are known to be susceptible to the toxicity of
paraquat (Kimbrough & Gaines, 1970). Waddell & Marlowe (1980)
suggested that it was unlikely that the radioactivity was bound to
cellular constituents.
No paraquat was detected in rat kidney, brain, liver, or lung
when paraquat was administered in the diet at a concentration of
50 mg/kg for a period of 8 weeks. At 120 mg/kg, it was found in
low concentrations in the lung, kidney, gastrointestinal system,
and brain (Litchfield et al., 1973). At 250 mg/kg, it was detected
in the tissues within 2 weeks. No sex differences or any clear
pattern of accumulation were noted throughout the 8-week study.
Within 1 week of return to a normal diet, no paraquat was detected
in any tissue examined. Histological changes were observed in all
lungs of animals fed paraquat at 250 mg/kg diet.
Rose et al. (1974a) demonstrated an energy-dependent
accumulation of paraquat in slices of rat lung that obeyed
saturation kinetics. The same investigators also examined the
ability of paraquat to accumulate in tissue slices from other
organs in vitro (Rose & Smith, 1977). The herbicide in brain,
adrenal gland, and kidney slices accumulated; however, the uptake
was less than 10% of that observed in the lung slices. The authors
established the uptake of paraquat by the lung in various species
(rat, rabbit, dog, monkey, man). The human lung accumulated
paraquat as strongly as that of the rat and there was a
relationship between the concentration of paraquat in the different
lung areas and the development of microscopic lung lesions. It has
been demonstrated that the rate of paraquat efflux from lung tissue
is less than its rate of accumulation in the lung slices (Smith et
al., 1981). Efflux from lung slices, prepared from rats dosed iv
with the herbicide, was found to be biphasic. There was a fast
component (half-life 20 min), followed by a first-order slow
component characterized by a half-life of 17 h. The half-life in
vitro was similar to that seen in vivo following iv
administration to rats.
6.1.3. Metabolic transformation and excretion
Paraquat participates to a considerable extent in cyclic
reduction-oxidation reactions. After undergoing a single electron
reduction in tissues, the resultant free radical is readily
oxidized by molecular oxygen to the parent compound (section 6.3).
This leads to an overall excretion of essentially unchanged
paraquat in the urine after oral administration to rats (Murray &
Gibson, 1974).
Daniel & Gage (1966) reported that paraquat was metabolized by
gut microflora following oral dosing of rats. This observation was
not confirmed in subsequent studies (Murray & Gibson, 1974) and was
later attributed to a problem with the method (FAO/WHO, 1977).
Urinary concentrations of paraquat following oral
administration are relatively low (Daniel & Gage, 1966: Murray &
Gibson, 1974; Sharp et al., 1972; Maling et al., 1978) and are thus
used to estimate its elimination from the body.
Sharp et al. (1972) reported a biphasic elimination of paraquat
from the plasma of rats after iv injection. The initial rapid
phase had a 20 - 30 min half-life, and the slower phase a half-life
of 56 h. Murray & Gibson (1974) also showed prolonged paraquat
elimination after oral administration to rats, guinea-pigs, and
monkeys. The urinary and faecal routes were equally important in
all species studied. The faecal content was due mainly to
elimination of unabsorbed paraquat. Prolonged elimination of
paraquat in all animals tested indicated retention of the herbicide
in the body.
Following iv administration to rats, about 75 - 79% of the dose
was excreted in the urine within 6 h (Maling et al., 1978). The
plasma disappearance of an iv dose of paraquat of 5 mg/kg was
fitted to a 3-compartment model. Total body clearance was
estimated to be 8.39 ± 0.54 ml/kg per min (Maling et al., 1978).
The relatively high concentration of paraquat in the duodenal and
jejunal walls suggested biliary secretion of the herbicide, and the
authors' hypothesis was supported by the observation of radio-
activity in the intestines of mice in whole-body autoradiographic
studies (Waddell & Marlowe, 1980).
Since absorbed paraquat is mainly removed via the kidneys, the
early onset of renal failure will have a marked effect on paraquat
elimination and distribution, including accumulation in the lung.
Hawksworth et al. (1981) used the dog as a model to evaluate the
influence of paraquat-induced renal failure on the kinetics of
paraquat elimination. After iv injection of a trace dose of 14C-
paraquat (30 - 50 µg/kg body weight) in dogs, the kinetics of
distribution was described by a 3-compartment model. To obtain a
good fit of the curve, it was necessary to sample the central
(plasma) compartment for at least 24 h after dosing. Simulation of
paraquat levels in the peripheral compartments suggested the
existence of a compartment with rapid uptake and removal (kidney)
and another with slow uptake (lung). The renal clearance of
paraquat approximated total body clearance indicating that paraquat
elimination occurs through renal excretion. The urinary excretion
rate of an iv dose was rapid, approximately 80 - 90% of the dose
being eliminated during the first 6 h. Intravenous injection of a
large toxic dose of paraquat (20 mg/kg body weight), however,
brought about a marked decrease in renal clearance, from 73 ml/min
to 18 ml/min after 2 1/2 h and 2 ml/min after 6 h. This data
suggested that damaged renal tubules could contribute to paraquat
accumulation in the lung.
6.2. Observations on Human Beings
6.2.1. Observations on paraquat poisoning after ingestion:
non-fatal cases
Tompsett (1970) reported a case of ingestion of 45 g of Weedol
(2.5% paraquat). On hospital admission, the gastric aspirate
contained 0.215 g paraquat/litre and the urine 0.148 g/litre.
After 2 - 4 h, paraquat concentrations dropped to 5.1 mg/litre in
the urine and 0.4 mg/litre in the serum but, 16 - 24 h after
admission, the urinary level was 0.95 mg/litre, while no paraquat
was detectable in the serum. Paraquat was also detected in the
urine for up to 15 days after poisoning, while at the same time
serum concentrations were below the detectable limits in chemical
analysis (Fletcher, 1975).
The cumulative elimination of paraquat in the faeces and urine
of a patient was followed for 7 days by van Dijk et al. (1975).
Faecal elimination increased from 340 mg the first day to 530 mg
after 7 days, while cumulative urinary excretion reached 60 mg the
lst day and increased to 75 mg after 7 days. It was calculated
that only 87 mg of paraquat had been absorbed from a total
ingestion of about 637 mg, determined in the urine, dialysate, and
faeces. In this patient, less than 14% of the ingested paraquat
was absorbed through the gastrointestinal system.
6.2.2. Observations on paraquat poisoning after ingestion:
fatal cases
It is well established that paraquat lung disease resulting in
death is usually preceded or accompanied by renal insufficiency.
This contributes to the retention of paraquat in body tissues.
Nevertheless, Fairshter et al. (1979) detected only small
concentrations (below 0.09 mg/kg) of paraquat in several organs of
patients who died 3 weeks after ingestion.
The detection of 27 mg paraquat/litre in the bile of a woman
after autopsy suggested that some faecal paraquat might be
attributable to biliary excretion (Dijk et al., 1975).
6.2.3. Significance of paraquat concentrations in cases of
paraquat poisoning
Not only oral ingestion, but also dermal absorption of paraquat
after occupational overexposure, resulted in measurable urinary
levels of paraquat. The determination of paraquat in urine and
serum is an important biological exposure test for the diagnosis
and the prognosis in cases of human poisoning.
Wright et al. (1978) followed the urinary excretion of paraquat
in 16 patients (7 of whom died). The total amount of paraquat
excreted ranged from 0.6 mg to 386 mg. The excretion rate
decreased rapidly during the 48 h following ingestion, though less
rapidly in the patients who eventually died. All patients
excreting 1 mg of paraquat or more per hour, for 8 h or more after
ingestion, died.
Plasma-paraquat concentrations were measured by gas
chromatography, radioimmunoassay, and colorimetric methods in 79
patients with paraquat poisoning (Proudfoot & Stewart, 1979). At
any given time after ingestion (within a limit of 35 h), plasma
concentrations were significantly higher in the patients who died
(Fig. 4). Patients whose plasma concentrations were not higher
than 2.0, 0.6, 0.3, 0.16, and 0.10 mg paraquat/litre at
respectively, 4 h, 6 h, 10 h, 16 h, and 24 h after the poisoning,
were likely to survive. When plasma levels exceeded 0.3 mg/litre
15 h after ingestion, a fatal outcome could be expected, despite
treatment. These conclusions were supported by the studies
performed on 28 patients by Bismuth et al. (1982).
 6.3. Biochemical Mechanisms
The mechanism of the toxic action of paraquat has been
extensively investigated. Several reviews or monographs have
summarized the biochemical mechanism of paraquat toxicity in
plants (Calderbank, 1968), bacteria (Fridovich & Hassan,
1979), and animals (Bus et al., 1976; Autor, 1977; Smith et
al., 1979; Bus & Gibson, in press).
Paraquat has long been known to participate in cyclic
reduction-oxidation reactions in biological systems. The compound
readily undergoes a single electron reduction in tissues, forming a
free radical. In an aerobic environment, however, a free radical
is immediately oxidized by molecular oxygen, generating the
superoxide radical (O2-). The reoxidized paraquat is capable of
accepting another electron and continuing the electron transfer
reactions in a catalytic manner (Fig. 5). Research into the
mechanism of paraquat toxicity has identified at least 2 partially
toxic consequences of the redox cycling reaction: a) generation of
O2-, and b) oxidation of cellular NADPH, which is the major source
of reducing equivalence for the intracellular reduction of
paraquat. Generation of O2- can lead to the formation of more
toxic forms of reduced oxygen, hydrogen peroxide (H2O2) and
hydroxyl radicals (OH-). Hydroxyl radicals have been implicated in
the initiation of the membrane-damaging by lipid peroxidation,
depolymerization of hyaluronic acid, inactivation of proteins and
damage to DNA (Hassan & Fridovich, 1980). Depletion of NADPH, on
the other hand, may disrupt important NADPH-requiring biochemical
processes such as fatty acid synthesis (Smith et al., 1979).
6.3. Biochemical Mechanisms
The mechanism of the toxic action of paraquat has been
extensively investigated. Several reviews or monographs have
summarized the biochemical mechanism of paraquat toxicity in
plants (Calderbank, 1968), bacteria (Fridovich & Hassan,
1979), and animals (Bus et al., 1976; Autor, 1977; Smith et
al., 1979; Bus & Gibson, in press).
Paraquat has long been known to participate in cyclic
reduction-oxidation reactions in biological systems. The compound
readily undergoes a single electron reduction in tissues, forming a
free radical. In an aerobic environment, however, a free radical
is immediately oxidized by molecular oxygen, generating the
superoxide radical (O2-). The reoxidized paraquat is capable of
accepting another electron and continuing the electron transfer
reactions in a catalytic manner (Fig. 5). Research into the
mechanism of paraquat toxicity has identified at least 2 partially
toxic consequences of the redox cycling reaction: a) generation of
O2-, and b) oxidation of cellular NADPH, which is the major source
of reducing equivalence for the intracellular reduction of
paraquat. Generation of O2- can lead to the formation of more
toxic forms of reduced oxygen, hydrogen peroxide (H2O2) and
hydroxyl radicals (OH-). Hydroxyl radicals have been implicated in
the initiation of the membrane-damaging by lipid peroxidation,
depolymerization of hyaluronic acid, inactivation of proteins and
damage to DNA (Hassan & Fridovich, 1980). Depletion of NADPH, on
the other hand, may disrupt important NADPH-requiring biochemical
processes such as fatty acid synthesis (Smith et al., 1979).
 The importance of molecular oxygen and the potential role of O2-
generation in mediating have been implicated in studies on plants
(section 3.3), bacteria, and in in vitro and in vivo mammalian
systems. In cultures of Escherichia coli, Hassan & Fridovich
(1977, 1978, 1979) demonstrated that paraquat stimulated cyanide-
resistant respiration, which could be almost entirely accounted for
by an NADPH-dependent formation of O2-. The possibility that
formation of O2- might be responsible for the toxicity of paraquat
in bacteria was supported by observations that bacteria containing
elevated activities of superoxide dismutase, an enzyme that
detoxifies O2-, were resistant to paraquat toxicity (Hassan &
Fridovich, 1977, 1978; Moody & Hassan, 1982).
In vitro studies on preparations of lung and liver from various
animal species have supported the hypothesis that paraquat redox
cycling and associated O2- and H2O2 generation also occur in
mammalian systems (Gage, 1968b; Ilett et al., 1974; Montgomery,
1976, 1977; Steffen & Netter, 1979; Talcott et al., 1979). Bus et
al. (1974) reported that the single electron reduction of paraquat
in mammalian systems was catalysed by microsomal cytochrome P-450
reductase and NADPH. The observation that the in vivo toxicity of
paraquat in animals is markedly potentiated by exposure to elevated
oxygen tensions further supported the potential role for molecular
oxygen in mediating toxicity (Fisher et al., 1973b; Autor, 1974;
Bus & Gibson, l975; Witschi et al., 1977; Kehrer et al., 1979;
Keeling et al., 1981).
The results of in vivo studies conducted by Bus et al. (1974)
suggested that stimulation of lipid peroxidation, which was
dependent on paraquat redox cycling and associated O2- generation,
might be an important toxic mechanism in mammalian systems.
Consistent with this hypothesis, animals fed diets deficient in
selenium or vitamin E, in order to diminish cellular antioxidant
defences, were significantly more sensitive to paraquat toxicity
than control animals (Bus et al., 1975; Omaye et al., 1978). In
contrast to these studies, a number of studies have shown that
paraquat inhibited in vitro microsomal lipid peroxidation (Ilett
et al., 1974; Montgomery & Niewoehner, 1979; Steffen & Netter,
1979; Kornburst & Mavis, 1980). Subsequent studies have indicated,
however, that paraquat would stimulate microsomal lipid
peroxidation when an adequate supply of electrons (NADPH) and in
vitro oxygen tensions were maintained (Trush et al., 1981, 1982).
Despite the evidence described above, the hypothesis that lipid
peroxidation is the underlying toxic mechanism functioning in vivo
has not been conclusively demonstrated. Direct quantification of
paraquat-induced lipid peroxidation damage in vivo by analysis of
tissue malondialdehyde levels or ethane exhalation, both markers of
peroxidation injury, has been largely unsuccessful (Reddy et al.,
1977; Shu et al., 1979; Steffen et al., 1980). Furthermore,
attempts to counteract paraquat toxicity by administration of
various antioxidants have also been unsuccessful (Fairshter, 1981).
Superoxide radicals generated in paraquat redox cycling may
induce biochemical changes other than the initiation of
peroxidation reactions. Ross et al. (1979) demonstrated that
paraquat increased DNA strand breaks in cultured mouse
lymphoblasts. Paraquat was also reported to induce a superoxide-
dependent stimulation of guanylate cyclase (EC 4.6.1.2) activity in
rat liver (Viseley et al., 1979) and guinea-pig lung (Giri &
Krishna, 1980). These investigators postulated that increased
cyclic GMP might stimulate the pulmonary fibroproliferative changes
characteristic of paraquat toxicity (section 7.1.1.1). In other
studies, paraquat has also been found to increase collagen
synthesis in rat lung (Hollinger & Chvapel, 1977; Greenberg et al.,
1978; Thompson & Patrick, 1978; Hussain & Bhatnagar, 1979).
Redox cycling of paraquat has also been proposed to lead to
increased oxidation of cellular NADPH (Brigelius et al., 1981;
Keeling et al., 1982). The activity of pentose shunt enzymes in
the lung rapidly increased in rats administered paraquat, which
suggested an increased demand for NADPH (Fisher et al., 1975; Rose
et al., 1976). The observation that paraquat decreased fatty-acid
synthesis in lung slices (Smith et al., 1979) further supported
this hypothesis, since fatty acid synthesis requires NADPH. Direct
analysis of NADPH in the lung has confirmed that paraquat treatment
decreased the NADPH content in rat lung (Witschi et al., 1977;
Smith et al., 1979). These observations led Smith et al. (1979) to
propose that oxidation of NADPH might not only interrupt vital
physiological processes, such as fatty-acid synthesis, but also
render tissues more susceptible to lipid peroxidation by decreasing
the equivalents (NADPH) necessary for the function of the
antioxidant enzyme glutathione peroxidase (EC 1.11.1.9) (Fig. 6).
The importance of molecular oxygen and the potential role of O2-
generation in mediating have been implicated in studies on plants
(section 3.3), bacteria, and in in vitro and in vivo mammalian
systems. In cultures of Escherichia coli, Hassan & Fridovich
(1977, 1978, 1979) demonstrated that paraquat stimulated cyanide-
resistant respiration, which could be almost entirely accounted for
by an NADPH-dependent formation of O2-. The possibility that
formation of O2- might be responsible for the toxicity of paraquat
in bacteria was supported by observations that bacteria containing
elevated activities of superoxide dismutase, an enzyme that
detoxifies O2-, were resistant to paraquat toxicity (Hassan &
Fridovich, 1977, 1978; Moody & Hassan, 1982).
In vitro studies on preparations of lung and liver from various
animal species have supported the hypothesis that paraquat redox
cycling and associated O2- and H2O2 generation also occur in
mammalian systems (Gage, 1968b; Ilett et al., 1974; Montgomery,
1976, 1977; Steffen & Netter, 1979; Talcott et al., 1979). Bus et
al. (1974) reported that the single electron reduction of paraquat
in mammalian systems was catalysed by microsomal cytochrome P-450
reductase and NADPH. The observation that the in vivo toxicity of
paraquat in animals is markedly potentiated by exposure to elevated
oxygen tensions further supported the potential role for molecular
oxygen in mediating toxicity (Fisher et al., 1973b; Autor, 1974;
Bus & Gibson, l975; Witschi et al., 1977; Kehrer et al., 1979;
Keeling et al., 1981).
The results of in vivo studies conducted by Bus et al. (1974)
suggested that stimulation of lipid peroxidation, which was
dependent on paraquat redox cycling and associated O2- generation,
might be an important toxic mechanism in mammalian systems.
Consistent with this hypothesis, animals fed diets deficient in
selenium or vitamin E, in order to diminish cellular antioxidant
defences, were significantly more sensitive to paraquat toxicity
than control animals (Bus et al., 1975; Omaye et al., 1978). In
contrast to these studies, a number of studies have shown that
paraquat inhibited in vitro microsomal lipid peroxidation (Ilett
et al., 1974; Montgomery & Niewoehner, 1979; Steffen & Netter,
1979; Kornburst & Mavis, 1980). Subsequent studies have indicated,
however, that paraquat would stimulate microsomal lipid
peroxidation when an adequate supply of electrons (NADPH) and in
vitro oxygen tensions were maintained (Trush et al., 1981, 1982).
Despite the evidence described above, the hypothesis that lipid
peroxidation is the underlying toxic mechanism functioning in vivo
has not been conclusively demonstrated. Direct quantification of
paraquat-induced lipid peroxidation damage in vivo by analysis of
tissue malondialdehyde levels or ethane exhalation, both markers of
peroxidation injury, has been largely unsuccessful (Reddy et al.,
1977; Shu et al., 1979; Steffen et al., 1980). Furthermore,
attempts to counteract paraquat toxicity by administration of
various antioxidants have also been unsuccessful (Fairshter, 1981).
Superoxide radicals generated in paraquat redox cycling may
induce biochemical changes other than the initiation of
peroxidation reactions. Ross et al. (1979) demonstrated that
paraquat increased DNA strand breaks in cultured mouse
lymphoblasts. Paraquat was also reported to induce a superoxide-
dependent stimulation of guanylate cyclase (EC 4.6.1.2) activity in
rat liver (Viseley et al., 1979) and guinea-pig lung (Giri &
Krishna, 1980). These investigators postulated that increased
cyclic GMP might stimulate the pulmonary fibroproliferative changes
characteristic of paraquat toxicity (section 7.1.1.1). In other
studies, paraquat has also been found to increase collagen
synthesis in rat lung (Hollinger & Chvapel, 1977; Greenberg et al.,
1978; Thompson & Patrick, 1978; Hussain & Bhatnagar, 1979).
Redox cycling of paraquat has also been proposed to lead to
increased oxidation of cellular NADPH (Brigelius et al., 1981;
Keeling et al., 1982). The activity of pentose shunt enzymes in
the lung rapidly increased in rats administered paraquat, which
suggested an increased demand for NADPH (Fisher et al., 1975; Rose
et al., 1976). The observation that paraquat decreased fatty-acid
synthesis in lung slices (Smith et al., 1979) further supported
this hypothesis, since fatty acid synthesis requires NADPH. Direct
analysis of NADPH in the lung has confirmed that paraquat treatment
decreased the NADPH content in rat lung (Witschi et al., 1977;
Smith et al., 1979). These observations led Smith et al. (1979) to
propose that oxidation of NADPH might not only interrupt vital
physiological processes, such as fatty-acid synthesis, but also
render tissues more susceptible to lipid peroxidation by decreasing
the equivalents (NADPH) necessary for the function of the
antioxidant enzyme glutathione peroxidase (EC 1.11.1.9) (Fig. 6).
 7. EFFECTS ON ANIMALS
7.1. Effects on Experimental Animals
7.1.1. Respiratory system
Toxicity studies in rats, mice, dogs, and monkeys (Clark et
al., 1966; Kimbrough & Gaines, 1970; Murray & Gibson, 1972;
Makovskii, 1972; Kelly et al., 1978) demonstrated that paraquat had
a specific effect on the lung (Table 11). Administration by every
route of entry tested whether parenteral (Fisher et al., 1973a;
Robertson, 1973; Hunsdorfer & Rose, 1980), oral (Clark et al.,
1966; Bainova, 1969a; Kimbrough, 1974; Tsutsui et al., 1976;
Dikshith et al., 1979), dermal (Howe & Wright, 1965; Bainova,
1969b; McElligott, 1972), or inhalatory (Gage, 1968b; Bainova,
1971; Makovskii, 1972; Seidenfeld et al., 1978) resulted in
irreversible changes in the lung.
Clark et al. (1966) reported that, in rats, in the earlier
stages after a single toxic oral dose of paraquat, breathing was
gasping or deep and fast, but some days after a single or repeated
toxic doses, the respiration became increasingly laboured, and the
hairs around the mouth and nares were soiled with a brownish
liquid. The extensive alveolar oedema observed in severe
intoxication was responsible for the development of hypoxia,
cyanosis, and dyspnoea. The progressive development of pulmonary
fibrosis was accompanied by difficulty in breathing, gasping, and
hyperpnoea (Smith et al., 1973).
Exposure of rats to high concentrations of respirable paraquat
aerosols was accompanied by shallow respiration. Within 2 - 3 h,
the test animals became dyspnoeic, cyanotic, and inactive, and
there were signs of local eye and nose irritation (Gage, 1968a).
7.1.1.1. Pathomorphological lung studies
Macroscopic examination of the lungs revealed that lesions and
their severity were dependent on the dose of paraquat and the time
between exposure and sacrifice (or death). The wet weight of the
lung increased after a single treatment, owing to oedema and
haemorrhage. The pathogenesis of the paraquat lung lesion has been
well characterized, and has been reviewed by Smith & Heath (1976).
The acute pulmonary toxicity of paraquat in animals has been
described as occurring in two phases (Smith & Heath, 1976). In the
initial "destructive" phase, alveolar epithelial cells were
extensively damaged and their subsequent disintegration often
resulted in a completely denuded alveolar basement membrane.
Table 11. Effects on experimental animals of repeated oral, dermal, or inhalation exposure to paraquat
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rat diet - 125 mg/kg 2 years no toxic effects Howe & Wright (1965)
Dog diet - 50 mg/kg no toxic effects
Rat diet - 0.25 mg/kg 27 days death; histological changes in the lung Clark et al. (1966)
Rat diet - 300, 400, 90 days cumulative toxic effects; chronicity Kimbrough & Gaines
500, 600, 700 mg/kg factor (Hayes) 5.2; histological (1970)
changes in the lung
Rat oral - 4, 9, 25 30 days inhibition of ChE activity, increasing Bainova (1969, 1975)
mg/kg body weight GPT activity in the serum; biochemical
per day and histological changes in the lung,
kidney, liver
Rat oral - 1.3, 2.6 4 1/2 months increased GPT and G-6-P-isomease Bainova (1969, 1975)
mg/kg body weight activities in the serum; biochemical
per day and histological changes in lung,
kidney, liver
Rat oral - 3.3, 1.3, 1 year the higher doses were toxic for both Makovskii (1972)
0.13 mg/kg body species tested; no-observed-adverse-
weight per day effect levels:
Guinea- oral - 1.0, 0.4, for rat 0.13, guinea-pig 0.04 mg/kg
pig 0.04 mg/kg body body weight per day
weight per day
Rat diet - 20 - 30 30 days histological and electron-optical lung Kimbrough (1974)
mg/kg body weight changes
per day
Mouse diet - 25, 50, 70 80 weeks death; dose-dependent clinical and FAO/WHO (1973)
mg/kg histological changes in the lung,
liver, kidney, and other organs tested
Rat oral - 25, 50, 1 - 5 days body weight loss; increased serum LDH, Tsutsui et al.
100 mg/kg body GOT activity; no haematological (1976)
weight per day changes; histological changes in the
lung, kidney, liver, myocardium
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rat drinking-water - 2 years mortality increased; histological Bainova & Vulcheva
1.3, 2.6 mg/litre changes in the lung, but only minimal (1977)
at the lowest level
Rabbit dermal - 2.8, 4.5, 20 days skin irritation; mortality and toxic Clark et al. (1966)
7, 14 mg/kg body effects at 7 & 14 mg/kg/day. LD50
weight per day mg/kg/day; no-observed-adverse-effect
level 2.8 mg/kg/day
Rat dermal - 2, 5, 15, 21 days skin irritation; mortality and toxic Bainova (1969a)
30, 45 mg/kg body effects at 5 - 45 mg/kg/day;
weight per day histological changes in the lung,
kidney, liver, myocardium; LD50
15 mg/kg/day; no-observed-adverse-
effect level 2 mg/kg/day
Rabbit dermal - from 1.56 20 days skin irritation; mortality and toxic McElligott (1972)
- 50 mg/kg per day effects at 3.13 - 192 mg/kg/day; LD50
(with occlusion) 4.5 mg/kg/day with occlusion and 24
from 2.4 - 192 mg/kg mg/kg/day without occlusion; No-
body weight per day observed-adverse-effect levels: 1.56
(without occlusion) and 2.4 mg/kg/day with and without
occlusion
Rat inhalationa - 4 days at higher concentrations (0.40 & 0.75 Gage (1968)
0.75 mg/m3 mg/m3) histological changes in the
0.4, 0.1, 0.06 mg/m3 15 days lung; no-observed-adverse-effect levels
0.003 mg/m3 60 days from 0.003 - 0.06 mg/m3 6 h daily;
6 h daily TLV - 0.1 mg/m3 paraquat aerosol
Rat inhalationa - 4 1/2 months biochemical, histochemical, and Bainova et al.
1.1, 0.05 mg/m3 histological changes in the lung at (1972)
6 h daily 1.1 mg/m3 no-observed-adverse-effect
level below 0.05 mg/m3 paraquat aerosol
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rabbit inhalationa - 3 months no clinical, functional and Seidenfeld et al.
10 mg paraquat in histological changes in the lung; no (1978)
100 ml water for the toxic effects
aerosol 2 h daily
---------------------------------------------------------------------------------------------------------
a Respirable paraquat aerosol.
Pulmonary oedema was also a characteristic of the destructive
phase, and was frequently of sufficient severity to result in the
death of the animals. Animals surviving the initial destructive
phase, which occurred in the first 1 - 4 days after acute paraquat
overexposure, progressed to what has been termed the
"proliferative" phase. In this phase, the lung was infiltrated
with profibroblastic cells that rapidly differentiated into
fibroblasts which, in some cases, progressed to fibrosis. The
histopathological outcome of the second phase may be influenced by
the treatment regimen, however. Administration of repeated low
doses of paraquat, which less severely damaged the alveolar
epithelial cells, could also induce a hyperplasia of the Type II
cells. This response may represent an attempt by the lung to
repair the damaged epithelium.
Following a single high dose of paraquat to animals, the
earliest ultrastructural changes were observed in the Type I
alveolar epithelial cells, approximately 4 - 6 h after treatment,
and were usually characterized by cellular and mitochondrial
swelling, increased numbers of mitochondria, and the appearance of
dark granules in the cytoplasm. When a high dose (approximately
LD50 or greater) was given, the lesions in the Type I cells often
progressed to the point of complete cellular disintegration leaving
areas of exposed basement membrane (Kimbrough & Gaines, 1970; Smith
et al., 1973; Smith & Heath, 1974; Vijeyaratnam & Corrin, 1971;
Klika et al., 1980).
In contrast to the effects on Type I pneumocytes, however, the
capillary endothelial cells were remarkably resistant to the toxic
effects of paraquat (Sykes et al., 1977).
Ultrastructural lesions in the alveolar Type II pneumocytes
were also observed shortly after single dose paraquat exposure,
although, generally, these lesions were not apparent until after
the first lesions were seen in the Type I cells (Kimbrough &
Gaines, 1970). Swollen mitochondria and damage to the lamellar
bodies usually occurred between 8 and 24 h after a high dose of
paraquat (Robertson, 1973; Robertson et al., 1976). Progressive
deterioration of the Type II cells continued, resulting in
completely denuded alveolar basement membranes and debris-filled
alveolar spaces (Vijeyaratnam & Corrin, 1971). Infiltration and
proliferation of fibroblasts may produce fibrosis that obliterates
the alveolar structure (Smith & Heath 1974).
Vijeyaratnam & Corrin (1971) observed that less severely
affected parts of the lung appeared to undergo epithelial
regeneration, 7 - 14 days after a single dose of paraquat.
Electron microscopic examination revealed the alveoli to be
lined with cuboidal epithelial cells that closely resembled
Type II pneumocytes except for a general lack of lamellar bodies.
Similar phenomena have also been noted by other investigators who
administered paraquat in the diet (Kimbrough & Linder, 1973) or as
repetitive intraperitoneal administrations (Smith et al., 1974).
Thus, in animals where the paraquat dose was sufficient to kill
only the Type I pneumocytes, the surviving Type II cells repaired
the damaged epithelium by proliferating and subsequently
differentiating into Type I epithelial cells. Inhaled paraquat in
aerosol produced initial necrosis and sloughing of the epithelia
and type 2 pneumocyte hyperplasia, fibroblast proliferation, and
increased synthesis of collagen in mice (Popenoe, 1979).
Histochemical alterations have been noted in rats exposed
through inhalation to 1.9 and 1.1 mg/m3 paraquat respirable
aerosol, 6 h/day, 6 days/week, for 4 1/2 months. The histoenzyme
activity of NAD lactate dehydrogenase-diaphorase, beta-
glucuronidase (EC 3.2.1.31), and acid phosphatase (EC 3.1.3.2) was
enhanced in the epithelial cells and in areas of pneumonitis
(Bainova et al., 1972). The changes were concentration-related,
although the activity of succinate dehydrogenase (EC 1.3.99.1) and
aspartate esterase appeared to be less pronounced in comparison
with the controls (Bainova et al., 1972).
7.1.1.2. Species differences in lung injury
Butler & Kleinerman (1971) injected rabbits intraperitoneally
with total doses of from 2 - 100 mg/kg body weight. Thymus atrophy
was observed, but most lungs showed only occasional and small
histological deviations that were poorly correlated with the
clinical signs of paraquat intoxication. The study confirmed the
resistance of the rabbit to paraquat-induced lung lesions (Clark et
al., 1966), and no evidence of any kind of pulmonary disease was
found; nor could significant lung injury be established in rabbits
after 30 days ingestion of 11 mg paraquat/kg in distilled water
(Dikshith et al., 1979). However, some animals showed pulmonary
fibrosis and emphysema, and a few changes were present in all
parenchymatous organs (Mehani, 1972; Zavale & Rhodes, 1978;
Dikshith et al., 1979). The rabbit also proved to be less
sensitive, than the rat, after inhalation exposure (Gage 1968a;
Seidenfeld et al., 1978).
According to Murray & Gibson (1972), and Hundsdorfer & Rose
(1980), guinea-pigs treated orally or sc did not develop the same
type of progressive pulmonary fibrosis as paraquat-intoxicated
rats. In hamsters, a single administration did not induce lung
damage, but prolonged exposure resulted in lung fibrosis (Butler,
1975).
In conclusion, for lung toxicity studies, a characteristic
dose-related pulmonary fibrosis can be induced in the rat, mouse,
dog, and monkey (Murray & Gibson, 1972) but not in the rabbit,
guinea-pig, or hamster.
7.1.1.3. Lung function studies
Rabbits exposed to an aerosol of 200 mg paraquat in 100 ml
distilled water (Seidenfeld et al., 1978) survived more than 3
exposures but showed significantly reduced arterial oxygen tension
and an increased alveolar arterial O2 gradient; specific compliance
decreased and functional residual capacity and breathing frequency
increased. Lam et al. (1980) administered paraquat at 27 mg/kg
body weight ip to rats and 0.5 mg/kg body weight intratracheally.
After 12 h, decreases were observed in total lung capacity,
functional residual capacity, vital capacity, residual volume, and
alveolar volume. These deviations persisted for 72 h. Oral
administration of paraquat at doses ranging from 1 mg/kg body
weight - 13.5 mg/kg body weight to rats resulted in functional lung
changes after 24 h.
Thus clinical, functional, and pathomorphological studies after
single and repeated exposure demonstrated that the spectrum of
paraquat lung disease depended on the magnitude of the dose and the
manner of administration (Seidenfeld et al., 1978; Restuccia et
al., 1974).
7.1.2. Renal system
In paraquat toxicity, kidney damage often precedes signs of
respiratory distress (Clark et al., 1966; Butler & Kleinerman,
1971; Murray & Gibson, 1972) (Table 11). Paraquat is excreted
mainly via the urine and the concentrations of the herbicide in the
kidneys are relatively high (section 6.1). Gross pathological and
histological examinations of paraquat-poisoned rats, guinea-pigs,
rabbits, and dogs revealed vacuolation of the convoluted renal
tubules and proximal tubular necrosis (Bainova, 1969a; Murray &
Gibson, 1972; Tsutsui et al., 1976). The degeneration of the
proximal tubular cells has also been confirmed by electron-optical
studies (Fowler & Brooks, 1971; Marek et al., 1981).
Paraquat is actively secreted by the kidney base transport
system. The nephrotoxicity caused by paraquat is pronounced and
appears to be restricted to the proximal nephron (Ecker et al.,
1975: Gibson & Cagen, 1977; Lock & Ishmael, 1979; Purser & Rose,
1979).
7.1.3. Gastrointestinal tract and liver
The clinical signs of acute and chronic oral poisoning
(Kimbrough & Gaines, 1970; Murray & Gibson, 1972; Bainova, 1969a)
or of ip injection (Butler & Kleinerman, 1971) include transient
diarrhoea and body weight loss, decreased food intake, and
dehydration. Some of the animals vomited soon after paraquat
administration. Residual skin contamination after dermal toxicity
studies on rabbits (McElligott, 1972) caused severe tongue
ulceration and an unwillingness to eat. The adverse irritant
effects were minimized by continued restraint after skin
decontamination of the treated rabbits.
There have been several reports of liver damage following
exposure to high doses of paraquat (Clark et al., 1966; Bainova,
1969a; Murray & Gibson, 1972; Tsutsui et al., 1976; Gibson & Cagen,
1977, Cagen et al., 1976). Centrilobular necrosis of hepatocytes
with proliferation of the Kupfer cells and bile canals have been
described.
In general, liver damage in experimental animals has not been
severe compared with lung and kidney damage. Serum enzyme
activities (SGOT, SGPT, LAP) only increased when large amounts of
paraquat were given (Giri et al., 1979).
7.1.4. Skin and eyes
The herbicide can provoke local irritation of the skin and
eyes. Clark et al. (1966) found skin irritation in rabbits only
when paraquat was applied beneath occlusive dressings in aqueous
solutions (total dose 1.56, 5.0, and 6.25 mg ion/kg body weight).
In mice and rats, the application of 5 - 20 g paraquat/litre
solutions in single and 21-day repeated dermal toxicity tests
provoked dose-related toxic dermatitis with erythema, oedema,
desquamation, and necrosis (Bainova, 1969b). Doses from 1.56 to 50
mg/kg, in repeated 20-day studies using the occlusive technique
(McElligott, 1972) resulted in local erythema and scab formation.
The histological changes consisted of parakeratosis and occasional
intra-epidermal pustules. A delayed skin irritant action of the
herbicide was reported by Fodri et al. (1977) in guinea-pig
studies.
No skin sensitization was observed in studies on guinea-pigs
when paraquat was applied (Bainova, 1969b; Fodri et al., 1977).
The instillation of dilutions of paraquat (up to 500 g/litre)
in rabbit eye induced inflammation within 24 h and this continued
for 96 h (Clark et al., 1966). Sinow & Wei (1973) introduced 62.5,
125, 250, 500, and 1000 paraquat/litre into the rabbit eye.
Concentrations of 62.5 and 125 g/litre caused severe conjunctival
reactions; higher levels (250 - 500 g/litre) provoked iritis and
pannus, while at the 500 g/litre concentration there was corneal
opacification, iritis, and conjunctivitis. All rabbits receiving
0.2 ml of paraquat at 1000 g/litre in 1 eye or 0.2 ml of a
concentration of 500 g/litre in both eyes died within 6 days of
application (Sinow & Wei, 1973).
Both conjunctival and dermal application of different
concentrations induced systemic toxicity (Sinow & Wei, 1973; Clark
et al., 1966; Bainova, 1969b; Kimbrough & Gaines, 1970; Makovskii,
1972; McElligott, 1972), lung, kidney, and liver damage, and death.
7.1.5. Other systems
No specific functional, histological, or biochemical effects of
paraquat have been reported in other systems that have been
examined; this is of prime importance in an evaluation of its
toxicity. When lethal doses of paraquat are given to rats,
symptoms consistent with neurological disturbances have been
observed. These include decreased motor activity, lack of
coordination, ataxia and dragging of the hind limbs (Smith et al.,
1973). Also associated with near lethal or lethal doses are damage
to the myocardium (Tsutsui et al., 1974), haemolytic anaemia
(Bainova, 1969a), increased haemosiderin in the spleen (Bainova et
al., 1972) and increased concentrations of plasma corticosteroids
(Rose et al., 1974b).
7.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
7.1.6.1. Effects on reproduction
Some histological changes in the testes have been reported in a
few paraquat toxicity studies. Butler & Kleinerman (1971) found
multinuclear giant cells in rabbit testicular tubules. When
paraquat was orally administered at 4 mg/kg body weight to male
rats for 60 days and the testes were examined, there were no
significant deviations in the spermatozoa count or motility, nor
were there any biochemical changes in the several enzymes of testes
homogenates. The histoenzyme activity of lactate dehydrogenase,
succinate dehydrogenase, DPN-diaphorase, alkaline phosphatase, and
acid phosphatase in the treated animals did not differ from that of
the controls, nor did quantitative and qualitative histological
examination of the testicular tubule cells reveal any abnormality.
A 3-generation reproduction study has been carried out on rats
treated with paraquat ion at 100 mg/kg diet (FAO/WHO, 1973). There
were no significant abnormalities in fertility, fecundity, and
neonatal morbidity or mortality, nor were there any signs of
gonadotoxicity or structural or functional lesions. Pulmonary
function in the treated offspring was normal.
Clegg (1979) has reviewed animal reproduction and
carcinogenicity studies conducted in relation to the safe use of
pesticides.
7.1.6.2. Embryotoxicity and teratogenicity
Oral or ip administration of high doses of paraquat to mice and
rats on various days of gestation produced significant maternal
toxicity, evidenced by increased mortality rates (Bainova &
Vulcheva, 1974; Bus et al., 1975). Examination of the fetuses from
the higher-dose groups revealed a reduction in fetal body weights,
delayed ossification of the sternabrae, and increased resorption
rate in mice, as a result of the maternal intoxication. The
minimal embryotoxic effect seemed due in part to difficulty in
crossing the placenta, reflected by low concentrations of paraquat
in the embryo relative to maternal tissues (Bus et al., 1975). The
absence of a specific embryotoxic action of paraquat has also been
observed and reported in other studies on rats (Khera et al., 1968;
Luty et al., 1978), mice (Selypes et al., 1980), and rabbits
(FAO/WHO, 1973).
In a perinatal toxicity study, Bus & Gibson (1975) administered
paraquat at 50 or 100 mg/litre in the drinking-water to pregnant
mice beginning on day 8 of gestation, with continued treatment of
the litters up to 42 days after birth. Paraquat treatment did not
alter postnatal growth rate, although the mortality rate in the 100
mg/litre-treated mice increased to 33% during the first 7 days
after birth. It was also noted that paraquat at 100 mg/litre
significantly increased the sensitivity of the pups to oxygen
toxicity on days 1, 28, and 42 after birth.
7.1.7. Mutagenicity
Paraquat has been found to have minimal to no genotoxic
activity when evaluated in a variety of in vitro and in vivo test
systems. In studies producing weakly positive results (Moody &
Hassan, 1982; Parry 1977, 1973; Tweats, 1975; Benigni et al., 1979;
Bignami & Grebelli, 1979), which were limited to in vitro studies,
paraquat genotoxicity was accompanied by high cytotoxicity. These
results are best explained by Moody & Hassan (1982), who showed
that the mutagenicity of paraquat in bacterial test systems
( Salmonella typhimurium TA 98 and TA 100) was mediated by the
formation of superoxide. However, other investigators (Andersen et
al., 1972; Levin et al., 1982) did not find mutagenic activity in
bacterial test systems. Furthermore, paraquat was not mutagenic
when evaluated in human leukocytes and in in vivo cytogenetic
tests on mouse bone marrow (Selypes & Paldy, 1978) and dominant
lethal tests on mice (Pasi et al., 1974; Anderson et al., 1976).
7.1.8. Carcinogenicity
A carcinogenicity study was performed on mice at dietary levels
of 25, 50, and 75 mg/kg per day for 80 weeks (FAO/WHO, 1973).
There were reduced weight gains among the animals receiving
paraquat, but deaths during the study were associated with
respiratory disease. Clinical and histopathological examination
determined that paraquat was not tumorigenic in mice.
A 2-year exposure of rats to 1.3 and 2.6 mg/litre, daily, in
the drinking-water provoked histopathological changes in the lung,
liver, kidney, and myocardium. The lung lesions were dose-related;
inflammation, atelectasis, reactive proliferation of the
epithelium, pulmonary fibrosis, and pulmonary adenomatosis were
noted, but no sign of tumour growth or atypism (Bainova & Vulcheva,
1977). Nor was any increased tumour incidence reported in rats in
a 2-year study with a maximum dietary level of 250 mg/kg diet (12.5
mg/kg body weight per day) (FAO/WHO, 1971).
Bainova & Vulcheva (1977) did not discover any indication of
tumorigenicity in a 2-year study on rats receiving paraquat at 1.3
or 2.6 mg/litre in their drinking-water (Table 11).
While testing the carcinogenicity of urethane in mice, Bojan et
al. (1978) also attempted to evaluate the influence of paraquat on
urethane-induced lung tumorigenesis. It is felt that the results
of this study are not of relevance for the assessment of the
carcinogenic potential of paraquat.
7.2. Effects on Farm Animals
The effects of paraquat on farm animals has been discussed in
section 4.3.5. The LD50 doses have been established for hen,
turkey, cow, and sheep (Howe & Wright, 1965; Clark et al., 1966;
Smalley, 1973). Massive doses resulted in convulsions,
neurological symptoms, and death due to respiratory failure.
Domestic animals may ingest paraquat by feeding on a sprayed
area, as a result of spray drifting on to their pasture, by
drinking water contaminated with paraquat used as an aquatic
herbicide, or by feeding on a crop sprayed with paraquat as a
dessicant. Sheep and calves were given paraquat at concentrations
of up to 20 mg/litre drinking-water for 1 month without any obvious
ill effects (Howe & Wright, 1965; Calderbank, 1972), and a cow
dosed with 2/3 of the LD50 of 14C-paraquat gave milk containing
less than 0.1 mg/litre. Field tests demonstrated that cattle did
not suffer any toxic effects when turned loose on pasture after it
had been sprayed with paraquat at 0.45 kg/ha. The same trial
showed that horses had local lesions of the mouth and increased
mucous secretion after grazing on newly-sprayed pasture (Calderbank
et al., 1968). The hazard to stock feeding on such pasture depends
on the density of the pasture, the dose of the herbicide, and the
length of time that has elapsed since its application.
Paraquat was fed to cattle at levels in herbage of 200 - 400
mg/kg for 1 month without any apparent ill effects, and no residues
could be detected in the meat and milk (Calderbank et al., 1968).
However, all domestic animals should be kept far from freshly-
sprayed areas, and when crops are treated with paraquat, due
attention should be paid to the accepted maximum residue limits.
7.3. Dose-Effect of Paraquat
The acute LD50 values for paraquat in various species are given
in Tables 12 and 13. The acute toxicity studies of paraquat salts
(dichloride, dimethylsulfate, dimethylphosphate) have not shown
any significant differences in the acute oral and ip LD50 in rats
(Clark et al., 1966; Makovskii, 1972).
There were no significant differences in the oral LD50 values
obtained for the same species from different laboratories, but the
acute oral LD50 values among the species examined varied.
The effects of repeated paraquat exposure are summarized in
Table 11. Paraquat was administered, orally and in the diet, to
rats, mice, guinea-pigs, and dogs. The guinea-pigs appeared to be
very sensitive (Makovskii, 1972). According to Kimbrough & Gaines
(1970), Makovskii (1972), and Bainova (1975), the herbicide has a
moderate cumulative toxicity. The joint FAO/WHO meeting (1976)
decided on a no-observed-adverse-effect level of 1.5 mg/kg body
weight per day in the rat and 1.25 mg/kg body weight per day in the
dog. As can be seen from Table 11, effects at lower levels have
been observed in other studies.
Guinea-pigs, monkeys, cattle, and human subjects are more
sensitive, while rats and birds are less sensitive to paraquat
through the gastrointestinal route.
Table 12. Paraquat LD50 (mg/kg body weight) and LC50
(mg/m3) in various species
-------------------------------------------------------------------
Species/Sex Oral Dermal Inhalation LC50
LD50 LD50 respirable
paraquat aerosol
-------------------------------------------------------------------
Rat 200a 1c
Rat (F) 100e 90e 10f
Rat (M) 110e 80e 10f
Rat 126i 350g 6g
Mouse 62d
Rabbit 500a
Rabbit 236b
Rabbit 240h
Guinea-pig 40 - 80a
Guinea-pig (M) 30b
Guinea-pig 22i
Guinea-pig 42g 319g 4g
Monkey 50i
Cat 40 - 50a
Cat (F) 35b
Hen 300 - 380a
Hen 262b
Turkey 250 - 280j approximately
375j
Cow 50 - 75a
Sheep 50 - 75a
-------------------------------------------------------------------
a Howe & Wright (1965).
b Clark et al. (1966).
c Gage (1968).
d Bainova (1971).
e Kimbrough & Gaines (1970).
f Bainova & Vulcheva (1972).
g Makovskii (1972).
h McElligott (1972).
i Murray & Gibson (1972).
j Smalley (1973).
Table 13. Paraquat LD50 (mg/kg body weight) after parenteral
treatment
-------------------------------------------------------------------
Species/Sex Subcutaneous Intraperitoneal Intravenous
-------------------------------------------------------------------
Rat (F) 19a
Rat 22b
Mouse 30e 50d
Guinea-pig (F) 3a
Guinea-pig 5b
Turkey 100c 20c
-------------------------------------------------------------------
a Clark et al. (1966). d Ecker et al. (1975).
b Makovskii (1972). e Bus et al. (1975).
c Smalley (1973).
7.4. Methods for Decreasing Paraquat Toxicity
These have been studied in connection with requirements in the
case of paraquat poisoning in man. Clark (1971) showed the
efficacy of Bentonite and Fuller's earth in binding orally
administered paraquat and preventing its absorption from the
gastrointestinal tract. Staiff et al. (1973) reported the high
adsorption capacity of Amerlite. Smith et al. (1974) found
considerably reduced plasma-paraquat levels after the combined
treatment of rats with purgatives and bentonite suspension; these
rats survived a dose that normally killed 90 - 100% of the animals.
The absorption capacities of six absorbent materials were tested by
Okonek et al. (1982) who demonstrated that activated charcoal was
the most successful in absorbing ingested paraquat in rats.
Another way of decreasing paraquat absorption is to introduce
an emetic in the concentrated formulations. Kawai et al. (1980)
examined the protection this provided in fasting and non-fasting
male and female dogs that were given paraquat containing an emetic.
The amount of paraquat eliminated by vomiting was 61 - 86% of the
orally-administered dose. In the group given paraquat only, the
blood level averaged 44 mg/litre; in the group given paraquat and
emetic, it was 0.26 mg/litre.
7.5. Relation Between Age, Sex, and Toxicity
There is no evidence that paraquat is more toxic to either sex
of adult experimental animals (section 7.3) Young rats were more
resistant than older rats, and some authors have paralleled this
resistance with that of young rats to oxygen toxicity. Smith &
Rose (1977b) found a more than 40% increase in cumulative mortality
in 180 g rats compared with 50 g rats, after oral dosing with
paraquat at 680 µmol/kg body weight. According to Smith & Rose
(1977b), the difference in renal function between young and mature
rats accounted for the difference in paraquat toxicity.
8. EFFECTS ON MAN
8.1. Accidental and Suicidal Poisoning
8.1.1. Case reports
The first fatalities from acute paraquat poisoning occurred in
1964 and were reported in 1966 (Bullivant, 1966). By 1977, 600
deaths had been reported following accidental or intentional
ingestion of paraquat. The number of accidental cases of poisoning
is small relative to instances of suicide. Because of different
requirements or practices for notification or reporting of cases of
poisoning in the many countries in which paraquat is used, the
magnitude of the problem is difficult, if not impossible, to
determine. Some representative reports on acute paraquat poisoning
are summarised in Table 14.
The earlier cases of paraquat intoxication were mostly
accidental (Fennelly et al., 1968; Matthew et al., 1968; Masterson
& Roche, 1970; Malone et al., 1971). These cases seemed to have
resulted mainly from the habit of decanting the liquid formulations
into small unmarked or incorrectly labelled containers such as
beer, wine, or soft-drink bottles.
An increased ratio of suicidal to accidental poisoning has been
noted in recent years (Fletcher, 1975; Carson & Carson, 1976;
Fitzgerald et al., 1978a; Bramley & Hart, 1983). This change from
accidental to suicidal poisoning was also reflected in the enhanced
percentage of fatal cases, shorter survival times, and
significantly higher tissue and body fluid levels (Connolly et al.,
1975; McGeown, 1975; Park et al., 1975; Carson & Carson, 1976;
Howard, 1979a; Sugaya et al., 1980; Bismuth et al., 1982).
While the vast majority of poisoning cases are due to
swallowing, a small number of fatal cases of accidental paraquat
poisoning via the skin have been reported when liquid concentrates
(200 g/litre) have been applied in order to kill body lice (Ongom
et al., 1974; Binns, 1976). A few other fatal and non-fatal cases
have been reported following skin-contamination (McDonagh & Martin,
1970; Kimura et al., 1980).
Table 14. Case report data on accidental and suicidal acute paraquat poisoning
-------------------------------------------------------------------------------
Number of cases Fatal Non-fatal Fatality Reference
-------------------------------------------------------------------------------
19 12 7 63% Malone et al. (1971)
24 3 accidental 10 14 42% Connolly et al. (1975)
19 suicidal
2 homicidal
25 10 accidental 17 8 68% McGeown (1975)
31 7 accidental 18 13 58% Park et al. (1975)
21 suicidal
3 homicidal
33 7 accidental 26 7 79% Carson & Carson (1976)
19 suicidal
16 7 9 44% Wright et al. (1978)
136 77 suicidal 92 44 68% Fitzgerald et al. (1978)
10 10 0 100% Natori et al. (1979)
188 69 119 37% Higginbottom et al. (1979)
79 28 51 35% Proudfoot et al. (1979)
68 68 suicidal 41 27 66% Howard (1979)
6 6 suicidal 5 1 83% Sugaya et al. (1980)
28 12 suicidal 17 11 61% Bismuth et al. (1982)
262 95% deliberate 94 (36%) 168 (64%) 36% Bramley & Hart (1983)
intent
-------------------------------------------------------------------------------
8.1.2. Distribution of cases of paraquat poisoning
Cases of acute paraquat poisoning have been reported in:
Bulgaria (Mircev, 1976), Denmark (Pederson et al.,1981), England,
Ireland, Scotland, and the Netherlands (Fletcher, 1975), the
Federal Republic of Germany (Grundies et al., 1971; Hofman &
Frohberg, 1972; Fletcher, 1975; Fischer & Kahler, 1979), France
(Faure et al., 1973; Gervais et al., 1975; Bismuth et al., 1982;
Efthymiou, 1983), Hungary (Farago et al., 1981), Poland (Firlik,
1978), Switzerland (Schlatter, 1976), the USA (Kimbrough, 1974;
Dearden et al., 1978; Stephens et a1., 1981), and in Yugoslavia
(Vucinovic, 1978). Recently, a number of cases of paraquat
poisoning, mainly suicidal, have also been reported in Japan
(Takahashi et al., 1978; Natori et al., 1979; Tomura et al., 1979;
Kimura et al., 1980; Matsumoto et al., 1981). No attempt has been
made to make this list exhaustive, in fact the distribution is
worldwide.
8.1.3. Route of entry
By far the most frequent route of poisoning has been ingestion.
An unusual case of subcutaneous injection of 1 ml paraquat by a
mentally disturbed farmer was reported in Israel (Almog & Tal,
1967). Cases of dermal poisoning have been mentioned in section
8.1.1. There is no evidence of fatal poisoning as a result of
inhalation.
8.1.4. Formulations
Paraquat trade names are listed in Table 3. Concentrated
liquid formulations have been responsible for most (and more
severe) poisonings than granular forms, which contain less paraquat
(McGeown, 1975; Park et al., 1975; Fitzgerald & Barnville, 1978;
Wright et al., 1978; Higginbottom et al., 1979; Howard, 1979a).
8.1.5. Dose
The minimum lethal dose of paraquat is stated to be about 35
mg/kg body weight for human beings (Pederson et al., 1981; Bismuth
et al., 1982).
Symptoms of poisoning depend on the dose absorbed. It is
difficult to estimate the dose absorbed from case histories since
in many cases the patients spat out part of the paraquat
concentrate or vomited profusely after swallowing the herbicide.
Some patients have survived after apparently ingesting 50 - 100 ml
Gramoxone(R) (10 - 20 g paraquat), whereas some died after taking
as little as 2 sachets of Weedol (2.5 g paraquat) (Table 15).
Howard (1979) demonstrated the relationship between the dose of
paraquat ingested, the time elapsing between ingestion and
institution of treatment, and the ultimate outcome in 68 cases of
intentional paraquat poisoning.
8.1.6. Clinical and pathomorphological data relating to fatal
paraquat poisoning
Cases of fatal poisoning can be sub-divided into cases of:
(a) acute fulminant poisoning from a massive dose leading
to generalized systemic poisoning and death from a
combination of acute pulmonary oedema, oliguria,
hepatocellular and adrenal failure and biochemical
disturbances (death usually occurs within 1 - 4 days);
(b) less overwhelming poisoning with slower onset of
organ failure and death from pulmonary oedema,
mediastinitis, and complications of therapy (McGeown,
1975; Fitzgerald et al., 1978a); and
(c) late pulmonary fibrosis (death ensuing 4 days to
several weeks later).
Table 15. Recovery from paraquat poisoning involving lung dysfunction
---------------------------------------------------------------------------------------------------------
Dose of paraquat Major organ Notes Reference
ingested damage
---------------------------------------------------------------------------------------------------------
50 ml kidney, liver, vomiting; pains in stomach; changes in urine Grundies et al.
lung and serum (1971)
15 ml approx. lung nausea; buccal lesions; chest X-ray: poor Lloyd (1969)
aeration at lung bases
10 ml approx. kidney, lung oliguria; changed renal function; basal rales; Fisher et al.
chest X-ray: small bilateral, pleural effusions; (1971)
limited atelectasis; functional lung change
not specified kidney, liver, oliguria; serum, and urine changes; minimal Fennelly et al.
lung deviations in the respiratory function (1971)
30 ml approx. kidney, liver nausea; oliguria; ECG changes; chest X-ray: Galloway &
myocardium, lung increased vascular markings Petrie (1972)
granular kidney, liver, 14 cases with mild oral, renal, lung, and liver Fitzgerald &
paraquat lung impairment Barniville
(1978)
50 ml kidney, liver, vomiting; diarrhoea; abdominal pain; serum and urine Rose (1980)
lung changes; dyspnoea, decreased forced vital capacity;
chest X-ray: extensive perivascular changes
---------------------------------------------------------------------------------------------------------
8.1.6.1. Respiratory system
(a) Clinical data
Soon after ingestion, there is oropharyngeal pain and swelling,
followed within a few days by exudation, ulceration, and mucosal
sloughing, sometimes with pseudomembrane formation, which on
occasion leads to total sloughing of the oropharynx and oesophagus
(Malone et al., 1971). In severe poisoning, pulmonary oedema
rapidly ensues with clinical and functional deterioration until
death. Less intense, but ultimately fatal, poisoning causes
progressive pulmonary fibrosis over days or several weeks, with
gradually increasing dyspnoea and hypoxaemic pulmonary failure.
Pulmonary oedema may occur from fluid overload in oliguric
patients. Mediasteinitis and pneumothorax are occasionally seen
(Dearden et al., 1978; Kimura et al., 1980).
Pulmonary function tests reflect the underlying pathology, with
hypoxaemia, reduction in lung volume, high alveolar-arterial
gradient, and impaired gas transfer (Cooke et al., 1973,
Higginbottom et al., 1979). Chest radiographs may show bilateral
pulmonary oedema, coalescing consolidations, and later, sequential
changes of pulmonary fibrosis (Davidson & McPherson, 1972).
(b) Pathology
At autopsy, the lungs do not collapse properly and the pleural
cavity contains a small amount of fluid. In cases of lung
fibrosis, the lungs are heavy, firm, dark purple, and rubbery.
Consolidation and decreased aeration are found predominantly at the
bases. Emphysema and atelectasis are often found.
Histological studies following lung biopsy and necropsy show
pulmonary oedema, haemorrhages, and atelectasis due to pulmonary
infiltrates, loss of alveolar epithelial cells and, at a later
stage, interstitial and intra-alveolar fibrosis (Smith & Heath,
1976).
During the first 7 days of paraquat poisoning in man, loss of
alveolar epithelial cells has been seen with alterations in, or
detachment of, the type I and II cells, proliferation of
fibroblasts and polymorphous cells, loss of surfactant secretion,
and thickening of the alveolar septa by interstitial fibrosis
(Toner et al., 1970). The later findings (2 - 3 weeks) involved
pulmonary fibrosis and endothelial abnormalities. Dearden et al.
(1978) reviewed the histological and electron-microscopic findings
in human lungs. Capillary permeability seemed to be enhanced
either by vesicles forming transendothelial channels or by
disruption of endothelial cells.
8.1.6.2. Renal system
Acute oliguric renal failure is common in severely poisoned
patients. Less severe manifestations include impaired renal
function, which may disappear before the pulmonary fibrosis
progresses (Beebeejaun et al., 1971; Fisher et al., 1971; Fletcher,
1975; Natori et al., 1979; Grant et al., 1980). Other
manifestations include proteinuria, with hyaline casts, white and
red blood cells. Tubular damage is reflected in glycosuria,
aminoaciduria, and excessive leaking of phosphorus, sodium, and
uric acid (Vaziri et al., 1979).
Soft, pale, swollen kidneys with extensive tubular necrosis,
compatible with toxic injury, are found at necropsy (Beebeejaun et
al., 1971). Sometimes necrosis of the proximal tubules is found
together with extreme dilatation of the distal tubules of the
kidney (Shuzui, 1980).
8.1.6.3. Gastrointestinal system, the liver, and the pancreas
The initial symptoms after oral ingestion of paraquat are
nausea, vomiting, upper abdominal pain, and diarrhoea. Perforation
of the oesophagus is uncommon (Ackrill et al., 1978; Natori et al.
1979).
The ingestion of large doses of paraquat has resulted in severe
liver damage (Ward et al., 1976; Grant et al., 1980) with
progressive metabolic acidosis (Shuzui, 1980; Sugaya et al., 1980).
Fatty degeneration of periportal hepatocytes and sporadic cellular
necrosis in the central region of the liver lobules have been
described (Matsumoto et al., 1980). Cholestasis and portal
inflammation may occur (Matsumoto et al., 1981). Oedematous
degeneration or necrosis of both the intra-hepatic and extra-
hepatic bile ducts, and of the gall bladder, have also been noted
(Mullick et al., 1981).
Takayama et al. (1978) noted stasis of the pancreatic duct,
with increased serum amylase levels after severe paraquat
poisoning.
8.1.6.4. Cardiovascular system
Occasionally, toxic myocarditis after paraquat ingestion has
been described (Bullivant, 1966; Malone et al., 1971; Copland et
al., 1974; Grant et al., 1980).
Takahashi et al. (1978) found fibrinoidal necrosis of the small
arteries in the pancreas, kidney, and liver on days 3 - 6 following
ingestion.
8.1.6.5. Central nervous system
The ingestion of very high doses of paraquat provoked anxiety,
convulsions, ataxia, and semi-consciousness (Grant et al., 1980;
Mukada et al., 1978). Haemorrhagic leukoencephalopathy was
present throughout the central nervous system, involving almost
exclusively the white matter. Focal haemorrhage and
demyelinization were present at various stages together with
haemorrhagic meningitis.
8.1.6.6. Adrenal glands
Adrenal cortical necrosis may contribute to death in severe
paraquat poisoning and the severity of the damage appears to be
dose-related (Nagy, 1970; McGeown, 1975; Fitzgerald et al., 1977a;
Takahashi et al., 1978).
8.1.6.7. Pregnancy
A woman, who accidentally swallowed paraquat in the 28th week
of pregnancy (Fennelly et al., 1968), died 20 days later. Gross
pathological examination did not reveal any abnormalities in the
fetal organs.
A woman, in the 7th month of pregnancy, intentionally ingested
about 60 ml of technical paraquat (Takeuchi et al., 1980) and
vomited approximately half that amount. Oliguria, jaundice, and
cough with sputum production progressed; fetal heartbeat
disappeared on the l3th day and the next day the dead fetus was
delivered. The mother died on the l7th day after poisoning. The
lungs of the dead fetus were filled with the debris of amniotic
fluid; the fetus had begun intra-uterine respiration to compensate
for the insufficient oxygen supply. No symptoms of paraquat
poisoning were noted in the body of the neonate.
A case report published by Musson & Porter (1982) concerning
paraquat ingestion by a 20-week pregnant woman, confirmed the lack
of teratogenic risk in human beings. The pregnancy was allowed to
continue after the treatment of the mother. The infant was
followed up to the age of 3 years and did well clinically, with
normal laboratory tests, development, and behaviour.
8.1.7. Recovery from paraquat poisoning
In the largest series reported (68 - 188 cases) (Fitzgerald et
al., 1978a; Higginbottom et al., 1979; Howard, 1979a; Proudfoot et
al., 1979), survival rates varied from 32% to 65% (Table 14)
Factors determining recovery from paraquat poisoning, reviewed by
Fletcher (1975), McGeown (1975), Fitzgerald & Barniville (1978),
Howard (1979a), and Bismuth et al. (1982), are shown in Table 16.
Victims of paraquat poisoning, who escape major pulmonary
complications, usually recover fully within a few weeks of
ingestion. Renal, gastrointestinal, and hepatic manifestations
return to normal (Fisher et al., 1971; Beebeejaun et al., 1971;
Grundies et al., 1971; Galloways & Petrie, 1972).
Minor pulmonary functional and radiographic abnormalities may
be transient and are of doubtful relationship to paraquat lung
injury. Some patients have recovered despite major pulmonary
abnormalities (Table 15). Among 5 survivors, Schlatter (1976)
reported no signs of lung residual disorders. Fitzgerald et al.,
(1979a) followed, for at least a year, 13 survivors of paraquat
poisoning to determine the prevalence of residual pulmonary
disability. Of 11 adults, 5 (all non-smokers) did not have any
clinical, radiological, or functional evidence of pulmonary
dysfunction; 4 others (all smokers) were considered normal on
clinical and chest X-ray examination, but had a mild deficit in
pulmonary function, while the remaining 2 adults were known to have
suffered from respiratory disability before the paraquat poisoning.
Only 1 patient showed new and persistent lung infiltrates that
could be ascribed to permanent paraquat lung damage. No
abnormalities were discovered in the 2 children studied.
Table 16. Factors determining recovery from paraquat poisoning
-------------------------------------------------------------------------
No. Factor Notes
-------------------------------------------------------------------------
1. Route of entry Most paraquat poisonings have occurred following
ingestion; ingestion following a meal usually has
less serious consequences; skin contamination
with liquid concentrate formulations is dangerous;
poisoning through inhalation is usually benign
2. Dose Dose rarely known, but usually, for survivors,
less than 6 g paraquat, often, spat out or vomited
after ingestion
3. Intention High mortality rates established in suicidal or
homicidal poisoning; many more survivors reported
reported among cases of accidental poisoning
4. Formulation High mortality rate registered after ingestion of
ingested liquid concentrates; survivors have more often
than not ingested dilute or granular formulations
5. Time of starting Treatment should start as soon as possible; delay
treatment of more than 2 - 5 h reduces chances of survival;
patients hospitalized several days after paraquat
ingestion have minimal chance of recovery
6. Decreased Occurs when there is vomiting, use of emetics
gastrointestinal stomach washout, application of adsorbents (such
absorption as Fuller's Earth or bentonite), single or
repeated, and forced diarrhoea; such treatment
should be as prompt as possible; a delay of more
than 5 h adversely affects the safe and effective
elimination of paraquat; care should be taken to
avoid complications (aspiration of Fuller's Earth,
oesophagal perforation)
7. Blood paraquat Fig. 6 (section 6.2.3) demonstrates importance of
concentrations paraquat plasma concentrations for prognosis
8. Urine paraquat Patients excreting more than 1 mg paraquat/h, 8 h
concentrations or more after ingestion, unlikely to recover
9. Renal function Patients with severe renal damage or renal failure
usually die
-------------------------------------------------------------------------
Table 16. (contd.)
-------------------------------------------------------------------------
No. Factor Notes
-------------------------------------------------------------------------
10. Forced diuresis Should not be instituted when renal damage with
oliguria present; caution needed during the first
24 h
11. Haemodialysis Important if forced diuresis cannot be carried out
-------------------------------------------------------------------------
8.2. Occupational Exposure
8.2.1. Epidemiological studies and case reports
8.2.1.1. Spraying personnel
Paraquat has been in agricultural use since the early 1960s and
several surveys have been conducted on spray operators (Swan, 1969;
Hearn & Kier, 1971; Makovskii, 1972; Staiff et al., 1975; Seiber &
Woodrow, 1981; Howard, 1979b, 1980, 1982; Chester & Ward, 1981;
Howard et al., 1981; Chester & Woollen, 1982; Wojeck et al., 1983).
Some of these studies were aimed at clinically evaluating possible
adverse effects, others at estimating inhalatory and dermal
exposure. Some of the latter studies have been summarised in Table
17 from which it can be seen that:
(a) the main route of exposure of agricultural workers to
paraquat is via the skin; respiratory exposure is
negligible.
(b) The worst case of exposure (of those examined) was
via knapsack spraying.
Table 17. Comparison of dermal and inhalation exposure resulting
from various methods of application
--------------------------------------------------------------------
Method of application Dermal exposure Respiratory exposure
(mg/h) (mg/h)
--------------------------------------------------------------------
Hand-held knapsacka 66 (0.45 - 1.3) x 10-3
(12.1 - 169.8)
Vehicle mountedb 0.4 0 - 2 x 10-3
(0.1 - 3.4)
Aerialc - a) Flagman 0.1 - 2.4 0 - 47 x 10-3
b) Pilot 0.5 - 0.1 0 - 0.6 x 10-3
c) Mixer/loader 0.18 1.3 - 1.5 x 10-3
--------------------------------------------------------------------
a From: Chester & Woolen (1982).
b From: Staiff et al. (1975).
c From: Chester & Ward (1981).
In Malaysian rubber plantations, exposure is likely to be
greater than in most other situations (Swan, 1969). Weed control
is required continuously for 10 months of the year, and the
herbicide is applied by knapsack sprayers during the entire working
day, 6 days a week. The high temperature and humidity together
with the light clothing of the sprayers increase the potential risk
of dermal exposure. In 1965, a study was carried out on a team of
6 sprayers, and in 1967 on 4 teams, to estimate the efficacy of
protective measures. The operators used spray dilutions containing
paraquat at 0.5 g/litre, for 12 weeks. Attention was paid to
personal hygiene. Each man was given a thorough physical
examination, and urine samples were taken before spraying began and
at weekly intervals throughout the study. Paraquat analyses were
carried out using the method of Calderbank & Yuen (1965). Chest
X-rays were taken before the study started and at the end of the
6th and 12th weeks.
In the course of the 2 studies, a total of 528 urine samples
were examined. Paraquat was found on 131 occasions, the maximum
concentration detected being 0.32 mg/litre in the first study and
0.15 mg/litre in the second. Average urine levels of paraquat of
0.04 mg/litre were found in the 1965 study, and of 0.006 mg/litre
in the 1967 study. After spraying ceased, these levels declined
steadily to become undetectable within a week - with one exception.
It was concluded that the workers were not subjected to hazardous
levels of paraquat.
Both trials showed that about half of the men had suffered mild
irritation of the skin and eyes, but had recovered rapidly with
treatment. Two cases of scrotal dermatitis occurred in workers
wearing trousers that were continuously soaked by the spray
solution. There were also 2 cases of epistaxis. All chest
radiographs were normal.
Studies over a period of several years on 296 workers were
performed by Hearn & Keir (1971) on a Trinidad sugar estate. This
survey drew attention to nail damage following gross contamination
with paraquat at 1 - 2 g/litre that ranged in severity from
localized discoloration to nail loss. The typical distribution of
the lesions - affecting the index, middle, and ring fingers of the
working hand - suggested that they had occurred through leakage
from the knapsack sprayer, and inadequate personal hygiene. Apart
from 2 cases of contact dermatitis of the hands, no skin, eye, or
nose irritation was reported, nor were there any systemic effects.
Similar data were obtained by Makovskii (1972), who examined
several groups of workers spraying paraquat as a herbicide and
dessicant in cotton fields during the hot season. These workers
were exposed to paraquat aerosol concentrations of 0.13 - 0.55
mg/m3 air. Dermal exposure was low, not more than 0.05 - 0.08 mg
paraquat on the hands and face. There were no complaints, nor did
the clinical and laboratory examinations of the workers demonstrate
any significant deviations from the matched control groups.
In the USA (Staiff et al., 1975), the exposure of field workers
operating tractor-mounted spray equipment in orchards was
determined. About 4.6 litre paraquat liquid concentrate (291
g/litre) was used in 935 litre water per h. In addition, exposures
from yard and garden applications were studied in volunteers using
pressurized hand dispensers containing paraquat solution (4.4
g/litre). Dermal contamination was measured by adsorbent cellulose
pads attached to the worker's body or clothing, and by hand-rinsing
in water in a polyethylene bag. Special filter pads were used in
the filter cartridges of the respirators worn by the subjects under
study.
In all, 230 dermal and respiratory exposure pads, 95 samples of
hand-rinse water, and 130 urine samples, collected during and
following the spray, were analysed. This involved 35 different
paraquat application situations. The exposure of field workers was
found to range from about 0.40 mg/h (dermal) to less than 0.001
mg/h (inhalation). As for individuals spraying the yard or garden,
exposure ranged from 0.29 mg/h (dermal) to less than 0.001 mg/h
(inhalation).
In almost all cases, dermal exposure affected the hands. The
respiratory paraquat values were generally below the sensitivity
level of the analytical method. No detectable paraquat
concentrations were found in the urine samples (lower limit 0.02
mg/litre). This study confirmed the general safety of paraquat
under correct conditions of use.
The potential long-term hazard associated with the use of
paraquat has also been studied. Howard et al. (1981) studied the
health of 27 spraymen who had been exposed to paraquat for many
months per year for an average of 5.3 years, and compared them with
two unexposed control groups consisting of 24 general workers and
23 factory workers. There were a few skin lesions resulting from
poor spraying techniques and 1 case of eye injury. The workers
were given full clinical examinations and lung, liver, and kidney
function tests were carried out. There were no significant
differences in all health parameters measured between the groups,
which led the authors to suggest that the long-term use of paraquat
was not associated with harmful effects on health.
A paraquat formulation (240 g/litre) diluted 300 times by
volume with water was sprayed for 2 h on weedy ground (Kawai &
Yoshida, 1981). No irritation of the eyes and the skin was
reported. The urine of the workers who wore gauze masks contained
1.4 - 2.7 µg paraquat, 24 h after the spraying. The urine of
workers who had worn a high-performance mask did not contain
detectable levels of paraquat. During the spraying operations, the
concentration of paraquat aerosol was 11 - 33 µg/m3 air. The total
dermal exposure was about 0.22 mg. The authors discussed the need
for protective equipment to decrease skin contact with paraquat and
to avoid aerosol inhalation.
Quantitative estimates of dermal and respiratory exposure of 26
plantation workers in Malaysia (Chester & Woollen, 1982) have shown
a mean dermal dose of 1.1 mg/kg body weight per h. The highest
individual total exposure was equivalent to 2.8 mg/kg body weight
per h; the mean respiratory exposure was 0.24 - 0.97 µg paraquat/m3
air. Spray operators and carriers were exposed to an order of 1%
or less of a TLV of 0.1 mg/m3 for respirable paraquat. Urine
levels of paraquat were generally below 0.05 mg/litre.
A study was carried out on a group of 14 spray men in Thailand
using conventional high-volume knapsack sprayers and low-volume
spinning disc applicators with paraquat ion concentrations of 1.5
g/litre and 20 g/litre, respectively (Howard, 1982). Irritation of
unprotected skin was found, and this was severe in workers using
high spray concentrations (caustic burns on the feet after work
with spinning disc applicators and paraquat solution (20 g/litre)).
Urinary paraquat levels after 14 days spraying were significantly
higher (10.21 - 0.73 mg/litre) in unprotected men using both
concentrations, and there was evidence that urinary levels of
paraquat increased as the trial progressed. No evidence of
systemic toxicity was discovered among the spray men undergoing
clinical and radiographic examination 1 week after spraying ended.
The author concluded that spray concentrations in hand-held
equipment should not exceed 5 g paraquat ion/litre.
After tomato spraying in the USA, the total body exposure to
paraquat was determined to be 168.59 mg/h (Wojeck et al., 1983).
The use of enclosed tractor cabs or a high clearance tractor
reduced total body exposures to paraquat to 26.91 mg/h or 18.38
mg/h, respectively. The authors reported that the total body
exposure of tractor spray men working in two citrus locations was
proportional to the tank concentrations (paraquat dilutions of 1.1
g/litre and 0.7 g/litre were applied); exposure levels of 28.50
mg/h and 12.16 mg/h were found for workers using the higher and the
lower concentrations, respectively. In all situations studied, the
respiratory exposure was consistently a small fraction (<0.1%) of
the total body exposure. Exposure was mainly through the skin.
8.2.1.2. Formulation workers
Groups of workers exposed to formulations were examined by
Howard (l979b). The first group of 18 workers in England comprised
subjects exposed to dust and liquid paraquat formulations during a
37.5 h working week, the mean length of exposure being 5 years.
The second group also comprised 18 males, from Malaysia, exposed to
liquid concentrate formulations during a 42-h working week, the
mean length of exposure being 2.3 years. Partly protective
clothing was worn. However, in Malaysia, no gloves, rubber aprons,
or goggles were used. The medical records and the dermatological
examinations revealed acute skin rashes, nail damage, epistaxis,
blepharitis, and delayed wound healing in 12 - 66% of these
workers. Delayed caustic effects were often found among the
Malaysian formulation workers where a lower level of safety and
hygiene was apparent. Clinical examination did not reveal any
evidence of chronic contact dermatitis, hyperkeratosis, or
eczematous lesions.
8.2.2. Cases of occupational poisoning and local caustic effects
Hayes & Vaughan (1977) reviewed deaths from pesticides in the
USA. From 1956 - 1973, no deaths attributable to paraquat were
registered among agricultural workers, but in 1974, 4 fatal cases
were associated with this herbicide, although it was not clear
whether they were accidental, suicidal, or occupational. Conso
(1979) reported 17 cases of skin and eye irritation, not
accompanied by epistaxis or other signs of systemic effects, in
paraquat-exposed workers in France. Bismuth et al. (1983)
discussed a few cases of paraquat poisoning due to skin
contamination and eye irritation.
The available evidence indicates that, at the recommended
dilution rates and correctly used, systemic oral, inhalation, or
dermal effects should not be expected. Skin and eye irritation
have occurred only when protective measures were disregarded.
However, it should be emphasized that carelessness in handling
paraquat may have serious consequences. Fitzgerald et al. (1978a)
summarized the clinical findings and pathological details
concerning 13 accidents involving paraquat among agricultural
workers, 6 of which were fatal. In 5 of these cases, swallowing was
involved.
8.2.2.1. Oral ingestion
The ingestion of paraquat may occur accidentally, if liquid
concentrates are decanted into unlabelled containers near the
working areas (Kawatomi et al., 1979), and dangerous ingestion can
occur if operators suck or blow out the blocked pipes or nozzles of
spray apparatus. Of the 6 fatalities studied by Fitzgerald et al.
(1978a), 3 swallowed Gramoxone(R) after sucking the outlet of a
sprayer. In one non-fatal case, the man had sucked out a nozzle
containing diluted paraquat, while in another case, the man who had
blown into the jet, to clear it, escaped with only minor signs of
poisoning. Dilute solution blown into the face by the wind and
splashes of concentrate that get into the mouth probably explain
the resultant signs in the mouth, on the tongue, and in the throat.
Smoking with paraquat-contaminated hands has been reported to
result in a farmer's developing oropharyngeal irritation, nausea,
and muscular weakness (Mourin, 1967).
8.2.2.2. Dermal absorption
Acute dermal paraquat poisoning has been described by
Fitzgerald et al. (1978a). The use of a leaking sprayer by a
worker with severe extensive dermatitis probably resulted in fatal
absorption of paraquat through the damaged skin. Jaros (1978) has
described how the use of concentrated solutions of paraquat (50
g/litre instead of 5 g/litre), with an old leaking knapsack
sprayer, resulted in paraquat contamination of the neck, back, and
legs of a worker. After 4 h of work, he complained of a burning
sensation on the neck and scrotum. On admission to hospital 6 days
later, cough and respiratory difficulties were recorded. Three
days later the patient died of renal and respiratory failure. This
author has stressed the need for careful handling of paraquat.
Jaros et al. (1978) have discussed several other cases of paraquat
poisoning in the CSSR related to paraquat application.
Severe skin damage, followed by death due to respiratory
insufficiency, occurred in a woman (Newhouse et al., 1978), 8 weeks
after initial contact with paraquat. The toxic dermatitis started
with scratches on the arms and legs from the branches of fruit
trees. The patient had often failed to wear protective clothing or
to shower after spraying. During the 4 weeks preceding her first
admission to hospital, she developed ulcers and respiratory
complaints combined with anorexia. Damaged and broken skin was
thus exposed to paraquat. A chest X-ray and needle biopsy of the
lung revealed pulmonary lesions. Seventeen days after discharge
from hospital, without a specific diagnosis, she was re-admitted,
and died 2 weeks later with progressive lung, hepatic, and renal
dysfunction. More recently, Levin et al. (1979) described the
clinical and pathomorphological investigation of a patient who died
of hypoxia after repeated dermal exposure to paraquat (28 g/litre)
and diquat (29 g/litre) in a water-oil dilution - contrary to
accepted practice. The worker had used a leaking sprayer. A
characteristic ulcer developed at the site of paraquat contact.
There was also lung damage. Waight & Weather (1979) reported a
fatal case of dermal poisoning with paraquat after prolonged
contact with a concentrated formulation following spillage from a
bottle in the back trouser pocket. Wohlfahrt (1982) discussed the
factors related to severe paraquat poisoning due to dermal
absorption in tropical agriculture. Three fatal incidents followed
skin contamination; one victim used paraquat to treat scabies
infestation, and one to treat lice. In all cases, the skin was
blistered and ulcerated. The patients died of progressive
respiratory failure, 4 - 7 days after the accidents. However it
has been pointed out that each of these three spraymen showed skin
lesions much more severe than would be expected had recommended and
customary dilutions been used and that, in one of these cases, the
presence of mouth and throat ulceration strongly suggested that
ingestion might also have occurred (Davies, 1982).
8.2.2.3. Local skin and nail effects
Paraquat has a delayed effect on the skin. Brief contact with
liquid formulations, as well as repeated exposure to dilute
solutions, produced skin irritation, desquamation, and, finally,
necrosis at the site of contact (Ongom et al., 1974; Binns, 1976;
Newhouse et al., 1978; Waight & Wheather, 1979; Levin et al., 1979;
Horiuchi et al., 1980). Harmful dermal effects have been reported
(Howard, 1982) among spray men who worked without protective
clothes and with naked feet. The blistering and ulceration of the
skin were due to excessive contact and inadequate personal hygiene.
Horiuchi & Ando (1980) carried out patch testing on 60 patients
with contact dermatitis due to Gramoxone(R). In 8 patients
(13.3%) positive allergic reactions were established. In another
survey with 52 persons, a positive photo-patch response was
reported in 11 patients.
Nail damage has also been reported after frequent exposure to
paraquat concentrates during the formulation of the herbicide or
the preparation of working dilutions (Samman & Johnston, 1969;
Howard, l979b). Leakage from sprayers may cause nail damage only
if there is gross contamination (Hearn & Keir, 1971). Asymmetric
discoloration and softening of the nail base appears together with
an infection, that usually persists after the loss of the nail, but
a few months after cessation of paraquat exposure, the nails
re-grow satisfactorily.
8.2.2.4. Ocular damage
A number of studies have demonstrated the hazard from splashes
of concentrated paraquat that come into contact with the eye (Swan,
1969; Schlatter, 1976; Howard, l979b, 1980; Deveckova & Myalik,
1980). Apart from irritation of the eye and blepharitis, a week
later more serious ocular damage may occur such as destruction of
the bulbar and tarsal conjunctiva and of the corneal epithelium
(Cant & Lewis, 1968). Anterior uveitis was also noted. Joyce
(1969) reported a case of conjunctival necrosis after paraquat had
been splashed into the eyes during spraying in windy weather. In a
second case, there was progressive keratitis with gross corneal
opacity. Severe conjunctival injuries with keratitis and decreased
visual acuity were reported in 3 workers by Watanabe et al. (1979)
and in another by Okawada et al. (1980). The eyes were washed with
water immediately, but the damage progressed and required treatment
for more than 3 weeks.
8.2.2.5. Inhalation
The inhalation of droplets in normal paraquat spraying does not
appear to represent a significant health hazard (Howard, 1980), and
the effects of occupational inhalation have been limited to nose
bleeds, and nasal and throat irritation (Swan, 1969; Howard,
1979b). Standard spraying equipment failed to produce significant
levels of droplets in the respirable range of < 5-7 µm diameter,
and chemical analyses of paraquat aerosols or particulate matter,
sampled from working areas, have usually shown them to be well
below the TLV. However, there have been some reports (Malone et
al., 1971; Mircev, 1976; Bismuth et al., 1982) of adverse effects
as a result of inhalation exposure.
8.3. Use of Marijuana Contaminated by Paraquat
In the USA, it has been found that marijuana sprayed with
paraquat (in an attempt to destroy the plant) may become available
for smoking by drug users. Concentrations of paraquat in marijuana
of up to 461 mg/kg have been reported (Liddle et al., 1980).
Understandably, concern has been expressed that smoking this
contaminated marijuana may be more harmful than smoking marijuana
itself. The available data do not justify an absolute conclusion.
However, paraquat is known to pyrolyse at 300 °C and it has been
established (Smith 1978) that in marijuana cigarettes contaminated
with 1000 mg paraquat/kg (1 mg, assuming a 1 g cigarette), only
0.26 µg of paraquat escaped pyrolysis and was available to be
inhaled. On this basis, the amount of paraquat inhaled by a heavy
user of contaminated marijuana will be insufficient to cause
injury. In the absence of exhaustive toxicological studies, it
cannot be stated categorically that all the pyrolysis products of
paraquat do not damage the lung. However, there has been no
confirmed injury attributable to the smoking of contaminated
marijuana.
8.4. Guidelines for the treatment of paraquat poisoning
The most important measures are the immediate neutralisation of
ingested paraquat by 15% Fuller's earth, bentonite, or activated
charcoal and urgent removal of the poison by vomiting or, when
possible, gastric washout. The urgency of these measures is such
that where transfer to hospital may involve delay of an hour or
more, this emergency treatment may need to be given by a
paramedical person, e.g., a nurse or a medical assistant. The
delay should not be more than 4 - 5 h. Furthermore, Fuller's earth
should be given together with a strong purgative such as magnesium
sulfate or mannitol.
Admission to a hospital either directly or after emergency
treatment elsewhere is essential.
Where a person has swallowed a lethal dose, the most important
single determinant of survival is the early commencement of
treatment.
Depending on local facilities, patients who reach hospital
after the initial treatment will have further treatment aimed at
neutralizing paraquat in the gastrointestinal tract (Fuller's
earth, bentonite, activated charcoal) or its excretion in the
faeces (purgatives, 10% mannitol, gut lavage). In addition,
attempts to remove absorbed paraquat from the circulation
(haemoperfusion, haemodialysis) or aid its excretion by the kidney
(forced diuresis) can be instituted.
In centres where facilities for analytical procedures are
available, measurement of urinary, or ideally plasma levels of
paraquat may give guidelines for the required intensity of
treatment or likely prognosis.
Many other therapies including corticosteroids,
immunosuppressive treatment, vitamins, beta-blocking and alkylating
agents, alpha-tocopherol, superoxide dismutase and/or glutathione
peroxidase (Autor, 1974, 1977) proved to be of no significant
importance in human paraquat poisoning (Fletcher, 1975; Fairshter
et al., 1976; Schlatter, 1976; Brown et al., 1981; Bismuth et al.,
1982). The administration of oxygen should be avoided except where
vital for the patient's comfort.
It should be noted that, as with the great majority of
chemicals, there is no specific antidote.
Care must be exercised in the administration of most of these
treatments, as the following serious complications may occur:
perforation of the oesophagus during gastric intubation; serious
blood chemistry disturbance when severe diarrhoea is induced; fluid
overload during forced diuresis (McGeown, 1975).
Despite such an array of both simple and sophisticated
measures, the response to therapy in paraquat poisoning is
disappointing and the mortality rate remains high.
In cases of skin and eye contamination, irrigation with water
(preferably running water) should be commenced urgently and must be
continued uninterrupted for at least 10 min (timed by the clock).
Eye cases should always be taken for medical treatment. In cases
of skin contamination by the concentrate or extensive and/or
prolonged contamination by the diluted material (particularly where
signs of skin irritation are present) the patient must be assessed
at hospital for systemic poisoning.
9. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
9.1. Exposure
Introduction
Paraquat is a contact herbicide or dessicant that is used to
destroy weeds in various agricultural situations. It is used in
the form of an aqueous spray, which means that potential human
exposure may occur as a result of its presence in air, on plants,
in soil, or in water.
Degradation of paraquat
Photochemical degradation takes place when paraquat-treated
plants are exposed to normal daylight and continues after the
plants are dead (section 4.1.1). The products formed have been
identified and found to be of a lower order of toxicity.
Ultraviolet degradation on soil surfaces also occurs, but
photodecomposition of paraquat in the soil is insignificant in
comparison with adsorption on clay particles. Microorganisms can
degrade free paraquat rapidly, but chemical degradation of adsorbed
paraquat is relatively slow.
Soil
Paraquat is rapidly and tightly bound to clay materials in
soils. The adsorbed paraquat is biologically inactive and in
normal agricultural use no harmful metabolic or breakdown products
are to be expected (section 4.3 and 5.1). In multiple spray
trials, paraquat residues in soil varied from 22 to 58 mg/kg.
Under field conditions, the residual paraquat is slowly
re-distributed. Long-term field studies have shown degradation
rates of 5 - 10% per annum, which is sufficient to prevent
saturation of soil deactivation capacities. At normal and high
rates of application, no adverse effects are expected in the soil
microflora and other soil organisms, or on crop growth (section
4.3.1).
Water
Following the use of paraquat as an aquatic herbicide at a
normal application rate of 1 mg/litre, the concentration was found
to decrease to about one half of the initial level within 36 h and
to below 0.0l mg/litre in less than 2 weeks (section 4.3.2).
Phytotoxic damage to crops irrigated with treated water is unlikely
to occur, if an interval of 10 days is observed between treatment
of the water and its use, because of the rapid decrease of paraquat
residues in the water.
Normal application of paraquat for aquatic weed control is not
harmful for aquatic organisms. However, care should be taken in
the application of paraquat to water containing heavy weed growth,
since oxygen consumed by subsequent weed decay may decrease oxygen
levels in the water to an extent that is dangerous for fish or
other aquatic organisms.
Air
Paraquat is not volatile so inhalation of paraquat vapour is
not a problem, in practice. However, droplets of paraquat solution
can be present in the air as a consequence of aerial, knapsack, or
tractor-mounted spraying. Paraquat aerosol concentrations (total
airborne) ranged up to 0.55 mg/m3 in the work situation, depending
on the method of spraying. The amount of respirable airborne
paraquat was found to be insignificant under normal conditions of
use (section 8.2.1).
The amount of paraquat present in airborne dust was found to
range from 0.0004 to 0.001 mg/m3. The binding of paraquat to the
dust was so tight that it did not exert any toxicological effect on
rats, when given by inhalation.
Food
Examination of paraquat-treated p1ants (section 4.3.4), or of
materials from animals fed paraquat-treated crops (section 4.3.5),
revealed low residues, so that no hazard should be expected from
paraquat residues in food when used as a herbicide or as a
desiccant. Paraquat is not subject to bioconcentration (section 5)
and has not been found to accumulate in food chains.
Environmental contamination
Exposure to paraquat from spray drift may occur in windy
weather, though field studies suggest that the airborne paraquat
concentration declines markedly within a few metres of the sprayed
area (section 4.3.3). Because of the rapid and complete binding of
paraquat to clay particles in the soil, contamination of water
supplies either from field runoff or percolation through soil to
the water table is not an environmental problem (sections 4.3.1 and
4.3.2). Paraquat has also been shown not to have any harmful
effects on birds (sections 5.3 and 5.4).
9.2. Poisoning by Paraquat
Misuse of paraquat has led to many deaths throughout the world,
mainly due to the swallowing of undiluted preparations.
9.2.1. Suicidal ingestion
The majority of paraquat poisonings are due to swallowing
liquid concentrates with suicidal intent and the mortality rate is
high. Ingestion of granular paraquat is less common and usually
causes milder poisoning, though fatalities have occurred. Paraquat
has been used to commit homicide (section 8.1).
9.2.2. Accidental poisoning
Poisoning by accidental swallowing is less common than
intentional swallowing and is usually the result of storing liquid
concentrates in inappropriate containers, particularly beer or soft
drink bottles. The mortality rate is lower than in suicidal cases.
Childhood poisoning is usually accidental. Legislation on the
control of the sale of liquid concentrates has reduced accidental
ingestion in some countries (section 8.1).
A small number of fatal cases of accidental paraquat poisoning
via the skin have been reported following the application of liquid
concentrates (200 g/litre) to kill body lice.
9.2.3. Occupational Poisoning
Cases of severe poisoning following inappropriate behaviour or
accidents while handling paraquat occur. Fatal and non-fatal
ingestion of paraquat has occurred when hand-spray operators have
attempted to clear the spray outlet by sucking on the spraying
nozzle or outlet pipes. In some of the severe cases, the authors
noted their suspicion of concealed suicidal intent. Fatal
poisoning by dermal soaking with dilute paraquat has been reported
in one operator who had severe dermatitis and had been using a
leaky sprayer (section 8.2.2).
Fatal systemic poisoning may result from continuous contact
with paraquat-soaked clothing or splashes of liquid concentrate on
the skin. Splashes of liquid concentrate may lead to severe ocular
and skin damage (sections 8.2.1, 8.2.2). Spraying with
inadequately diluted paraquat (e.g., with ultra low volume
application) may result in similar problems.
9.3. Occupational Exposure
There are several studies on paraquat exposure in normal
agricultural use. Occupational exposure may be oral, dermal, or by
inhalation. The spray aerosol and dust particles are relatively
large and are mostly deposited in the upper respiratory tract
(section 8.2.1).
The potential dermal exposure of field workers (section 8.2.1)
is closely related to working conditions. Workers on tractors were
found to have a paraquat exposure of 12 - 168 mg/h while spraying
tomatoes and citrus. In other studies, field workers were dermally
exposed to paraquat at approximately 0.40 mg/h, and individuals
spraying the garden to 0.29 mg/h. In all trials, respiratory
exposure was not higher than 0.01 mg/h. Urine concentrations in
occupationally-exposed workers were often lower than 0.01 mg/litre,
but concentrations up to 0.73 mg/litre were determined after
improper paraquat application in tropical agriculture use.
Local skin effects (contact, irritative, or photoallergic
dermatitis) delayed wound healing, and nail damage has been
observed among formulation workers or among individuals handling
the herbicide improperly. Blepharitis and epistaxis may result due
to delayed irritative action of paraquat. Such incidents
illustrate the need for strict personal hygiene and rigorous
adherence to safe handling procedures.
9.4. Effects
9.4.1. Paraquat toxicity in animals
The acute lung-directed toxicity of paraquat in man has been
confirmed in numerous studies in animals. At high doses of
paraquat, minor toxic effects have been noted primarily in liver
and kidney, and in other organ systems, including nervous,
cardiovascular, blood, adrenals and male reproductive systems.
However, toxic effects have not been reported at low doses of
paraquat. Concentrated solutions of paraquat have been found to be
irritating to both skin and eyes. The FAO/WHO (1976) has
determined no-observed-adverse-effect levels of 30 mg/kg diet,
equivalent to 1.5 mg/kg body weight per day for rats and 50 mg/kg
diet, equivalent to 1.25 mg/kg body weight per day, for dogs
exposed to paraquat dichloride. Additional animal studies have
indicated that paraquat is neither teratogenic nor carcinogenic
(sections 7.1.6 and 7.1.8). In vitro mutagenicity studies have
been inconclusive, though generally suggesting weak potential
activity, while in vivo studies have given negative results
(section 7.1.7). Thus, the results of animal studies suggest that
low-level exposure to paraquat is unlikely to induce toxic effects
in man.
9.4.2. Paraquat determinations in biological fluids and tissues
Determination of paraquat levels in stomach washings, serum,
and urine is useful for the management of poisoning (section 6.2).
The urinary levels decline rapidly during the 24 h following
exposure and may remain low for some weeks. Determination of
urinary levels of paraquat may be useful in the conduct of
epidemiological studies.
9.5. Earlier Evaluations by International Bodies
The Joint Meeting on Pesticide Residues (JMPR) has reviewed
residues and toxicity data on paraquat on several occasions
(FAO/WHO 1971, 1973, 1977, 1979, 1982, 1983). In 1972, it
estimated the acceptable daily intake (ADI) for man 0 - 0.002 mg/kg
body weight, on the basis of no-observed-adverse-effect levels of
1.50 mg/kg body weight per day in the rat and 1.25 mg/kg body
weight in the dog. Because of concern relating to lung and kidney
toxicity, this ADI was changed in the 1982 meeting to a temporary
ADI of 0 - 0.001 mg paraquat dichloride/kg body weight (or 0.0007
mg paraquat ion/kg body weight). The no-observed-adverse-effect
level for the rat remained, however, at 1.5 mg/kg body weight/day
(FAO/WHO 1983).
The same JMPRs have recommended maximum residue levels
(tolerances) for paraquat in food commodities of plant and animal
origin.
The WHO/FAO (1978) in its series of "Data sheets on chemical
pesticides" issued one on paraquat. Based on a brief review of
use, exposure, and toxicity, practical advice is given on
labelling, safe-handling, transport, storage, disposal,
decontamination, selection, training and medical supervision of
workers, first aid, and medical treatment.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechcoslovakia, the
Federal Republic of Germany, India, Japan, Kenya, Mexico, Sweden,
the United Kingdom, the USA, and the USSR) and the EEC can be found
in the IRPTC (International Register of Potentially Toxic
Chemicals) Legal file (IRPTC 1983).
9.6. Conclusions
On the basis of the above findings, it can be concluded that:
General population
Residue levels of paraquat in food and drinking-water,
resulting from its normal use, are unlikely to result in a health
hazard for the general population.
This likely lack of hazard in normal usage of dilute paraquat
is in strong contrast with the potential serious hazard that may
result from handling concentrated paraquat.
Accidental paraquat poisoning results mainly from swallowing
liquid concentrate that has been decanted into unlabelled bottles
or other containers and stored inappropriately.
The number of suicides by means of paraquat is of great
concern. The total number of such suicides is unknown.
Notwithstanding the facts that the reasons for suicide may be
manifold and complex, and that paraquat is one among many means
towards that goal, the prolonged and painful way of dying from
paraquat suggests that every effort within reason should be made to
diminish the attractiveness and availability of paraquat for this
purpose.
Occupational exposure
With reasonable work practices, including safety precautions,
hygiene measures, and proper supervision, occupational exposure
during manufacture, formulation, and application will not cause
hazard. However the undiluted concentrate must be handled with
great care because improper work practices may result in
contamination of eyes and skin (with possible consequent dermal
absorption).
Spray concentrations should not exceed 5 g paraquat ion/litre
in order to avoid skin damage and absorption of the herbicide
through the skin. Its use in hand-held ultra-low volume
application should be discouraged.
Environment
Paraquat in soil binds rapidly and tightly to clay particles
and residual phytotoxicity from freely-available paraquat is
unlikely. The toxicity of the compound for birds has been shown to
be of low significance. Under normal conditions of use, paraquat
shows low toxicity to aquatic organisms although resulting
depletion of water-oxygen because of weed decay may pose a problem.
Paraquat does not seem to represent an environmental hazard.
REFERENCES
ACKRILL, P., HASLETON, P.S., & RALSTON, A.J. (1978)
Oesophageal perforation due to paraquat. Br. med. J., 1:
1252-1253.
ALMOG, C. & TAL, E. (1967) Death from paraquat after
subcutaneous injection. Br. med. J., 3: 721.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDERSON, D., McGREGOR, D.B., & PURCHASE, I.F.H. (1976)
Dominant lethal studies with diquat and paraquat in male CD-I
mice. Mutat. Res., 40: 349-358.
ANDERSON, C.C., GUNDERSON, E.C., & COULSON, D.M. (1981)
Sampling and analytical methodology for workplace chemicals
hazards. ACS Symp. Ser., 149: 3-19.
ANONYMOUS (1979) Paraquat. FAO Plant Prod. Prot. Pap.,
15(Suppl.): 181-183.
AUTOR, A.P. (1974) Reduction of paraquat toxicity by
superoxide dismutase. Life Sci., 14: 1309-1319.
AUTOR, A.P., ed. (1977) Biochemical mechanisms of paraquat
toxicity, New York, Academic Press, pp. 39-55.
BAINOVA, A. (1969a) [Chronic oral toxicity of dipyridylium
herbicides.] Hig. Zdrav., 12: 325-332 (in Bulgarian).
BAINOVA, A. (l969b) [Experimental assessment of the effect
of dipyridylium herbicides on the skin.] Letopisi HEI, 9:
25-30 (in Bulgarian).
BAINOVA, A. (1971) [Determination of the zones of acute and
chronic inhalatory toxicity after exposure to dipyridylium
herbicides Gramoxone and Reglone.] Letopisi HEI, 28: 59-62 (in
Bulgarian).
BAINOVA, A. (1975) [Cumulative action of Gramoxone and
Reglone.] In: Problemi na Higienata, Sofia, Medizinia i
Fizkultuna, Vol. 1, pp. 31-38 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1972) Experimental
substantiation of Gramoxone MAC in working areas. In: Works of
Research Institute of Hygiene & Labour Protection, Sofia,
Medizinia i Fizkultuna, Vol. 23, pp. 71-77.
BAINOVA, A. & VULCHEVA, V. (1974) [Experimental assessment
of the effect of dipyridyliums on sex glands.] In: Works of
Research Institute of Hygiene & Labour Protection, Sofia,
Medizinia i Fizkultuna, Vol. 22, pp. 111-122 (In Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1977) Lung changes after chronic
paraquat intoxication. C. R. Acad. Bulgar. Sci., 30: 1788-1790.
BAINOVA, A., ZLATEVA, M., & VULCHEVA, V. (1972) [Chronic
inhalation toxicity of dypyridylium herbicides.] Hig. Zdrav.,
15: 25-31 (in Bulgarian).
BALDWIN, B.C., BRAY, M.F., & GEOGHEGAN, M.J. (1966) The
microbial decomposition of paraquat. Biochem. J., 101: 15.
BEEBEEJAUN, A.R., BEEVERS, G., & ROGERS, W.N. (1971)
Paraquat poisoning. Prolonged excretion. Clin. Toxicol., 4:
397-407.
BENIGNI, R., BIGNAMI, A., CARERA, A., CONTI, G., CONTI, R.,
GREBELLI, E., DOGLIOTTI, E., GUALANDI, G., NOVELLETO, A., &
ORTALI, V.A. (1979) Mutational studies with diquat and
paraquat in vitro. Mutat. Res., 68: 183-193.
BENNETT, P.N., DAVIES, D.S., & HAWKSWORTH, G.M. (1976) In
vivo adsorption studies with paraquat and diquat in the dog.
Proceedings of the British Pharmaceutical Society, 15-16 July,
England, 284 pp.
BERRY, D.J. & GROVE, J. (1971) The determination of paraquat
(1,1-dimethyl-4,4-dipyridylium cation) in urine. Clin. Chim.
Acta, 34: 5-11.
BEYER, K.H. (1970) [The analytical determination and
toxicology of paraquat.] Dtsch Apoth.-Ztg, 110: 633-635 (in
German).
BIGNAMI, M. & GREBELLI, R.A. (1979) A simplified method for
the induction of 8-azaguanine resistance in S. typhimurium.
Toxicol. Lett., 3: 169-175.
BINNS, C.W. (1976) A deadly cure for lice. Papua New Guinea
med. J., 19: 105-107.
BISMUTH, C., GARNIER, R., DALLY, S., FOURNIER, P.E., &
SCHERRMANN, J.M. (1982) Prognosis and treatment of paraquat
poisoning. A review of 28 cases. J. Toxicol. clin. Toxicol.,
19: 461-474.
BISMUTH, C., DALLY, S., & PONTAL, P.-G. (1983) Prognostic
factors and therapeutic results of paraquat poisoning.
Concerning 28 cases. Arch. Mal. prof., 44(1): 38-41.
BOJAN, F., NAGY, A., & HERMAN, K. (1978) Effect of butylated
hydroxytoluene and paraquat on urethane tumorigenesis in mouse
lung. Bull. environ. Contam. Toxicol., 20: 573-576.
BRAMLEY, A. & HART, T.B. (1983) Paraquat poisoning in the
United Kingdom. Hum. Toxicol., 2(2): 417.
BRIGELIUS, R. & ANWER, M.S. (1981) Increased biliary
GSSG-secretion and loss of hepatic glutathione in isolated rat
liver after paraquat treatment. Res. Commun. chem. Pathol.
Pharmacol., 31: 493-502.
BRIGELIUS, R., HASHEM, A., & LENGFELDER, E. (1981)
Paraquat-induced alterations of phospholipids and GSSG release
in the isolated perfused rat liver and the effect of
SOD-active copper complexes. Biochem. Pharmacol., 30: 349-354.
BROWN, E., BRANDENBURGER, A., & MALING, H.M. (1980) Effects
of paraquat and related herbicides on the acetylcholinesterase
of rat lung. Biochem. Pharmacol., 29: 456-466.
BROWN, 0., HEITKAMP, U., & SONG, C. (1981) Niacin reduces
paraquat toxicity in rats. Science, 212: 1510-1512.
BULLIVANT, C.M. (1966) Accidental poisoning by paraquat.
Report of 2 cases in man. Br. med. J., 1: 1272-1273.
BURNS, R.G. & AUDUS, L.J. (1970) Distribution and breakdown
of paraquat in soil. Weed Res., 10: 49-58.
BUS, J.S. & GIBSON, J.E. (1975) Postnatal toxicity of
chronically administered paraquat in mice and interactions
with oxygen and bromobenzene. Toxicol. appl. Pharmacol., 33:
461-470.
BUS, J.S. & GIBSON, J.E. (1979) Lipid peroxidation and its
role in toxicity. Rev. Biochem. Toxicol., 1: 125-149.
BUS, J.S. & GIBSON, J.E. (in press) Paraquat: a model for
oxidant initiated injury. In: Hook, G.E.R., ed. Pulmonary
toxicology, New York, Raven Press.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1974) Superoxide and
singlet oxygen catalysed lipid peroxidation as a possible
mechanism for paraquat toxicity. Biochem. biophys. Res.
Commun., 58: 749-755.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1975) Lipid
peroxidation as a possible mechanism for paraquat toxicity.
Res. Commun. chem. Pathol. Pharmacol., 11: 31-38.
BUS, J.S., CAGEN, S.Z., OLGAARD, M., & GIBSON, J.E. (1976)
A mechanism of paraquat toxicity in mice and rats. Toxicol.
appl. Pharmacol., 35: 501-513.
BUTLER, C. (1975) Pulmonary interstitial fibrosis from
paraquat in the hamster. Arch. Pathol., 99: 503-507.
BUTLER, C. & KLEINERMAN, J. (1971) Paraquat in the rabbit.
Br. J. ind. Med., 28: 67-71.
CAGEN, S.Z. & GIBSON, J.E. (1977) Liver damage following
paraquat in selenium-deficient and diethyl maleate pretreated
mice. Toxicol. appl. Pharmacol., 40: 193-200.
CAGEN, S.Z., JANOFF, A.S., BUS, J.S., & GIBSON, J.E. (1976)
Effect of paraquat on liver function in mice. J. Pharmacol.
exp. Ther., 198: 222-228.
CALDERBANK, A. (1966) Paraquat residues in fruit. In:
Frayer, J.D., ed. Herbicides in British fruit growing,
Blackwell, Blackwell Scientific Publications, pp. 135-136.
CALDERBANK, A. (1968) The bipyridylium herbicides. Effects
on man. In: Metcalf, R.L., ed. Advances in pest control
research, New York, Interscience Publishers, Vol. 8, 224 pp.
CALDERBANK, A. (1972) Environmental considerations in the
development of diquat and paraquat as aquatic herbicides.
Outlook Agric., 7: 51-54.
CALDERBANK, A. & SLADE, P. (1976) Diquat and paraquat. In:
Kearne, P.C. & Kaufman, D.D., ed. Herbicide chemistry,
degradation and mode of action, 2nd ed., New York, Dekker,
pp. 501-540.
CALDERBANK, A. & YUEN, S.H. (1965) An ion-exchange method
for determining paraquat residues in food crops. Analyst, 90:
99-106.
CALDERBANK, A., MCKENNA, R.H., STEVENS, M.A., & WALLEY, J.K.
(1968) Grazing trials on paraquat-treated pasture. J. Sci.
Food Agric., 19: 246-250.
CANT, J.S. & LEWIS, R.H. (1968) Ocular damage due to
paraquat and diquat. Br. med. J., 2: 224.
CARLSTROM, A.A. (1971) Collaborative check for paraquat in
formulations. J. Assoc. Off. Anal. Chem., 54: 718-719.
CARMINES, E.L., CARCHMAN, R.A., & BORZELLECA, J.F. (1981)
Investigations into the mechanism of paraquat toxicity
utilizing a cell culture system. Toxicol. appl. Pharmacol.,
58: 353-362.
CARSON, D.G.L. & CARSON, E.D. (1976) The increasing use of
paraquat as a suicide agent. Forensic Sci., 7: 151-160.
CHARLES, J.M., ABOU-DONIA, M.B., & MENZEL, D.B. (1978)
Absorption of paraquat from the airways of the perfused rat
lung. Toxicology, 8: 59-67.
CHESTER, G. & WARD, R.J. (1981) Paraquat - Occupational
exposure and drift hazard evaluation during aerial application
to cotton in California, USA, London, ICI Ltd (Central
Toxicology Laboratory Report No. CTL/P.581).
CHESTER, G. & WOOLLEN, B.H. (1982) Studies of the
occupational exposure of Malaysian plantation workers to
paraquat. Br. J. ind. Med., 38: 23-33.
CLARK, D.G. (1971) Inhibition of the absorption of paraquat
from the gastrointestinal tract by adsorbents. Br. J. ind.
Med., 28: 186-188.
CLARK, D.G., McELLIGOTT, T.F., & HURST, E.W. (1966) The
toxicity of paraquat. Br. J. ind. Med., 23: 126-133.
CLEGG, D.J. (1979) Animal reproduction and carcinogenicity
studies in relation to human safety evaluation. Dev. Toxicol.
environ. Sci., 4: 45-59.
COATS, G.E., FUNDERBURK, H.H., Jr, LAWRENCE, J.M., & DAVIS,
D.E. (1966) Factors affecting persistance and inactivation
of diquat and paraquat. Weed Res., 6: 58-66.
CONNING, D.M., FLETCHER, K., & SWAN, A.A.B. (1969) Paraquat
and related bipyridyliums. Br. med. Bull., 25: 245-249.
CONNOLLY, M.E., DAVIES, D.S., DRAFFAN, G.H., BENNETT, P.N., &
DOLLERY, C.T. (1975) Clinical experience with paraquat
poisoning. In: Fletcher, K., ed. Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-11.
CONSO, F. (1979) Paraquat poisoning: experience of poison
control centers in France. Vet. hum. Toxicol., 21(Suppl.):
112-113.
COOKE, N.J., FLENLEY, D.C., & MATTHEW, H. (1973) Paraquat
poisoning. Serial studies of lung function. Q. J. Med.,
XLII(168): 683-692.
COPLAND, G.M., KOLIN, A., & SHULMAN, H.S. (1974) Fatal
pulmonary intra-alveolar fibrosis after paraquat ingestion.
New Engl. J. Med., 291: 290-292.
CURRY, J.F. (1970) The effects of different methods of new
sward establishment and the effects of the herbicides paraquat
and dalapon on the soil fauna. Pedobiologia, 10: 329-361.
DAMANAKIS, M., BRENNAN, D.S.H., FRYER, J.D., & HOLLY, K.
(1970) Absorption and mobility of paraquat on different soil
constituents. Weed Res., 10: 264-277.
DANIEL, J.M. & GAGE, J.C. (1966) Absorption and excretion of
diquat and paraquat in rats. Br. J. ind. Med., 23: 133-136.
DASTA, J.F. (1978) Paraquat poisoning: a review. Am. J.
Hosp. Pharm., 35: 1368-1372.
DASTA, J.F. (1980) Management of paraquat poisonings. Clin.
Toxicol. Consultant, 2(1): 11-20.
DAVIDSON, J.K. & MACPHERSON, P. (1972) Pulmonary changes in
paraquat poisoning. Clin. Radiol., 23: 18-25.
DAVIES, D.S., HAWKSWORTH, G.M., & BENNETT, P.N. (1977)
Paraquat poisoning. Proc. Eur. Soc. Toxicol., 18: 21-26.
DAVIES, R.E. (1982) Skin absorption of paraquat. Med. J.
Aust., 7: 222.
DEARDEN, L.C., FAIRSHTER, R.D., MCRAE, D.M., SMITH, W.R.,
GLASER, F.L., & WILSON, A.F. (1978) Pulmonary ultrastructure
of the late aspects of human paraquat poisoning. Am. J.
Pathol., 93: 667-676.
DEVECKOVA, D. & MYALIK, M. (1980) [Gramoxone ocular burns.]
Cesk. Ophtalmol., 36: 7-10 (in Czech).
DICKES, G.J. (1979) The application of gas chromatography to
food analysis. Talanta, 26: 1065-1099.
DIJK, A. VAN, MAES, R.A.A., DROST, R.H., DOUSE, J.M.C., &
HEIJST, A.N.P. VAN (1975) Paraquat poisoning in man. Arch.
Toxicol., 34: 129-136.
DIJK, A. VAN, EBBERINK, R., GROOT, G. DE, & MAES, R.A.A.
(1977) A rapid and sensitive assay for the determination of
paraquat in plasma by gas-liquid chromatography. J. anal.
Toxicol., 1: 151-154.
DIKSHITH, T.S.S., DATTA, K.K., RAIZADA, R.B., & KUSHWAH, H.S.
(1979) Effect of paraquat dichloride in male rabbits. Indian
J. exp. Biol., 17: 926-928.
D0DGE, A.D. (1971) The mode of action of the bipyridylium
herbicides, paraquat and diquat. Endeavour, 30: 130-135.
DOUZE, J.M.C., DIJK, A. VAN, GIMBERE, J.S.F., HEIJST, A.N.P.
VAN, & MAES, R.A.A. (1975) Intensive therapy after paraquat
intoxication. In: Fletcher, K., ed. Clinical aspects of
paraquat poisoning, London, ICI Ltd, pp. 34-45.
DRAFFON, G.H., CLARE, R.A., DAVIES, D.L., HAWKSWORTH, G.M.,
MURRAY, S., & DAVIES, D.S. (1977) Qualitative determination
of the herbicide paraquat in human plasma by gas
chromatographic and mass spectrometric methods.
J. Chromatogr., 139: 311-320.
EARNEST, R.D. (1971) Effect of paraquat on fish in a
Colorado farm pond. Prog. Fish Cult., 33: 27-31.
ECKER, J.L., HOOK, J.B., & GIBSON, J.E. (1975)
Nephrotoxicity of paraquat in mice. Toxicol. appl.
Pharmacol., 34: 178-186.
EDWARDS, P.J. (1979) Status of common bird population on an
intensively managed farm where paraquat has been used
extensively, London, ICI Plant Protection Division (Report No.
RJ 0037/B).
EDWARDS, P.J., NEWMAN, J.F., & WARD, R.J. (1979) Paraquat:
effect of spraying eggs on hatchability and reproductive
organs of Japanese quail, Coturnix coturnix japonica, London,
ICI Plant Protection Division (Report No. RJ 0044B).
EFTHYMIOU, M.L. (1983) L'actualité toxicologique en matičre
de lutte chimique antiparasitaire agricole, Tours, France,
Faculté de Médecine de Tours.
FAIRSHTER, R.D. (1981) Paraquat toxicity and lipid
peroxidation. Arch. intern. Med., 141: 1121-1123.
FAIRSHTER, R.D., ROSEN, S.M., SMITH, W.R., FLAUSER, F.L.,
MCRAE, D.M., & WILSON, A.F. (1976) Paraquat poisoning. New
aspects of therapy. Q.J. Med., 45: 551-565.
FAIRSHTER, R.D., DABIR-VAZIRI, N., SMITH, W.R., GLAUSER, F.L.,
& WILSON, A.F. (1979) Paraquat poisoning: an analytical
toxicologic study of 3 cases. Toxicology, 12: 259-268.
FAO (1973) Diquat and paraquat. FAO specifications for plant
protection products, Rome, Food and Agriculture Organization
of the United Nations.
FAO/WHO (1971) Paraquat. In: 1970 Evaluation of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1973) Paraquat. In: 1972 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1976) Paraquat. In: 1975 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977) Paraquat. In: 1976 Evaluation of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1979) Paraquat. In: 1978 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1982) Paraquat. In: 1981 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1983) Paraquat. In: 1982 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FARAGO, E., HIDEG, Z., & TABATS, G. (1981) [Fatal poisonings
during the last 10 years from statistics of the Institute of
Forensic Chemistry.] Népegészsegügy, 62: 376-379 (in
Hungarian).
FAURE, J., MARKA, C., FAURE, H., YACOUB, M., & CAN, G.
(1973) Données histo-pathologiques et toxicologiques d'une
intoxication mortelle par le paraquat. Méd. lég. Dommage, 6:
417-419.
FENNELLY, J.J., GALLAGHER, J.T., & CARROLL, R.J. (1968)
Paraquat poisoning in a pregnant woman. Br. med. J., 3:
722-723.
FENNELLY, J.J., FITZGERALD, M.X., & FITZGERALD, 0. (1971)
Recovery from severe paraquat poisoning following forced
diuresis and immunosuppressive therapy. J. Irish Med. Assoc.,
64: 69-71.
FIRLIK, M. (1978) [Changes in the respiratory system during
paraquat poisoning.] Med. Pr., 29: 325-328 (in Polish).
FISHER, H.K. & KAHLER, J. (1979) [Lethal poisoning by
paraquat.] Z. Rechtsmed., 84: 61-67 (in German).
FISHER, H.K., HUMPHRIES, M., & BAILS, R. (1971) Recovery
from renal and pulmonary damage. Ann. intern. Med., 75:
731-736.
FISHER, H.K., CLEMENTS, J.A., & WRIGHT, R.R. (1973a)
Pulmonary effects of the herbicide paraquat studied 3 days
after injection in rats. J. appl. Physiol., 35: 268-273.
FISHER, H.K., CLEMENTS, J.A., & WRIGHT, R.R. (1973b)
Enhancement of oxygen toxicity by the herbicide paraquat. Ann.
Rev. respir. Dis., 107: 246-252.
FISHER, H.K., CLEMENTS, J.A., TIERNEY, D.F., & WRIGHT, R.R.
(1975) Pulmonary effects of paraquat in the first day after
injection. Am. J. Physiol., 228: 1217-1223.
FITZGERALD, G.R. & BARNIVILLE, G. (1978) Poisoning by
granular paraquat. J. Irish Coll. Physicians Surg., 7: 133-136.
FITZGERALD, G.R., BARNIVILLE, G., FITZPATRICK, P., EDWARDS,
H., SILKE, B., CARMODY, M., & O'DWYER, W.F. (1977a) Adrenal
abnormalities in paraquat poisoning. Irish J. Med. Sci., 146:
421-423.
FITZGERALD, G.R., BARNIVILLE, G., SILKE, B., CARMODY, M., &
O'DWYER, W.F. (1977b) The kidney in paraquat poisoning.
In: Proceedings of the European Dialysis and Transplantation
Association, Helsinki 1977.
FITZGERALD, G.R., BARNIVILLE, G., FLANAGAN, M., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978a) The changing pattern of
paraquat poisoning: an epidemiologic study. J. Irish Med.
Assoc., 71: 103-108.
FITZGERALD, G.R., BARNIVILLE, G., BLACK, J., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978b) Paraquat poisoning in
agricultural workers. J. Irish Med. Assoc., 71: 336-342.
FITZGERALD, G.R., BARNIVILLE, G., GIBNEY, R.T.N., &
FITZGERALD, M.X. (1979a) Clinical, radiological and
pulmonary function assessment in 13 long-term survivors of
paraquat poisoning. Thorax, 34: 414-415.
FITZGERALD, G.R., BARNIVILLE, G., DICKSTEIN, K., CARMODY, M.,
& O'DWYER, W.F. (1979b) Experience with Fuller's Earth in
paraquat poisoning. J. Irish Med. Assoc., 71: 149-152.
FLETCHER, K. (1967) Production and viability of eggs from
hens treated with paraquat. Nature (Lond.), 215: 1407-1408.
FLETCHER, K. (1974) Paraquat poisoning. In: Ballantyne, B.,
ed. Proceedings of a Symposium on Forensic Toxicology,
Bristol, John Wright, pp. 86-98.
FLETCHER, K., ed. (1975) Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-89.
FLETCHER, K. & WYATT, I. (1970) The composition of lung
lipids after poisoning with paraquat. Br. J. exp. Pathol., 51:
604-609.
FODRI, Z., SIPOS, K., & BERENCSI, G. (1977) [Study of the
irritation and allergic effects of Gramoxone in guinea-pig.]
Egeszsegtudomouny, 21: 244-249 (in Hungarian).
FOWLER, B.A. & BROOKS, R.E. (1971) Effects of the herbicide
paraquat on the ultrastructure of mouse kidney. Am. J.
Pathol., 63: 505-512.
FRIDOVICH, I. & HASSAN, H.M. (1979) Paraquat and the
exacerbation of oxygen toxicity. Trends biochem. Sci., 4:
113-115.
GAGE, J.C. (1968a) Toxicity of paraquat and diquat aerosols
generated by a size-selective cyclone. Effect of particle
size distribution. Br. J. ind. Med., 25: 304-314.
GAGE, J.C. (1968b) The action of paraquat and diquat on the
respiration of liver cell fractions. Biochem. J., 109: 757-761.
GAGE, J.C. (1969) The inhalation toxicity of
paraquat-treated soil, London, ICI Ltd (Report No. IHR/252).
GALLOWAY, D.B. & PETRIE, J.C. (1972) Recovery from severe
paraquat poisoning. Postgrad. Med. J., 48: 684-686.
GEORGE, K. & GEORGE, M. (1978) Chromosome uncoiling effect
of paraquat. Indian J. exp. Biol., 16: 933-937.
GERVAIS, P., DIAMANT-BERGER, O., BESCOL-LIVERSAC, J., GUILLAM,
C., & GUYON, F. (1975) Problčmes médico-legaux et médico-
sociaux de l'intoxication aiguë par les herbicides du groupe
du paraquat. Arch. Mal. prof., 36: 19-36.
GIBSON, J.E. & CAGEN, S.Z. (1977) Paraquat-induced
functional changes in kidney and liver. In: Autor, A.P., ed.
Biochemical mechanisms of paraquat toxicity, New York,
Academic Press, pp. 117-136.
GIRI, S.N. & KRISHNA, G.A. (1980) The effect of paraquat on
guanylate cyclase activity in relation to morphological
changes in guinea-pig lungs. Lung, 157: 127-134.
GIRI, S.N., CURRY, D.L., HOLLINGER, M.A., & FREYWALD, N.
(1979) Effect of paraquat on plasma enzymes, insulin, glucose
and liver glycogen in the rat. Environ. Res., 20: 300-308.
GOWMAN, M.E., RILEY, D., & NEWBY, S.E. (1980) Paraquat and
Diquat: Longterm high rate trial, Frensham UK. Persistence
and movement in soil and glasshouse bioassay, London, ICI Ltd
(Report RJ0014B).
GRANT, H.C., LANTOS, P.L., & PARKINSON, C. (1980) Cerebral
damage in paraquat poisoning. Histopathology, 4: 185-195.
GREENBERG, D.B., LYONS, A., & LAST, J.A. (1978)
Paraquat-induced changes in the rat of collagen biosynthesis
by rat lung. J. lab. clin. Med., 92: 1033-1042.
GROVER, R., SMITH, A.E., & KORVEN, H.C. (1980) A comparison
of chemical and cultural control of weeds in irrigation
ditchbanks. Can. J. plant Sci., 60: 185-195.
GRUNDIES, H., KOLMAR, D., & BENNHOLD, I. (1971) [Paraquat
poisoning.] Dtsch Med. Wschr., 96: 588-589 (in German).
GRZENDA, A.R., NICHOLSON, H.P., & COX, W.S. (1966)
Persistance of four herbicides in pond water. J. Am. Water
Works Assoc., 58: 326-332.
HALEY, T.J. (1979) Review of the toxicology of paraquat
(1,1-dimethyl-4,4-dipyridylium chloride). Clin. Toxicol., 14:
1-46.
HANCE, R.L., BYAST, T.H., & SMITH, P.D. (1980) Apparent
decomposition of paraquat in soil. Soil Biol. Biochem., 12:
447-448.
HARRINGTON, K.J. (1979) The detection of paraquat
(dichloride) in wood by pyrolysis vapour-phase chromatography.
Wood Sci. Technol., 13: 21-28.
HASSAN, H.M. & FRIDOVICH, I. (1977) Regulation of synthesis
of superoxide dismutase in Escherichia coli. J. Biol. Chem.,
253: 7667-7672.
HASSAN, H.M. & FRIDOVICH, I. (1978) Superoxide radical and
the enhancement of oxygen toxicity of paraquat in Escherichia
coli. J. Biol. Chem., 253: 8143-8148.
HASSAN, H.M. & FRIDOVICH, I. (1979) Paraquat and Escherichia
coli. Mechanism of production of extracellular superoxide
radical. J. Biol. Chem., 254: 10846-10852.
HASSAN, H.M. & FRIDOVICH, I. (1980) Superoxide dismutases:
detoxication by a free radical. In: Jakoby, W.B., ed.
Enzymatic basis of detoxication, New York, Academic Press,
Vol. 1, pp. 311-322.
HAWKSWORTH, G.M., BENNETT, P.N., & DAVIES, D.S. (1981)
Kinetics of paraquat elimination in the dog. Toxicol. appl.
Pharmacol., 57: 139-145.
HAYES, W.J. & VAUGHAN, W.K. (1977) Mortality from pesticides
in the United States in 1973 and 1974. Toxicol. appl.
Pharmacol., 42: 235-252.
HEARN, C.E.D. & KEIR, W. (1971) Nail damage in spray
operators exposed to paraquat. Br. J. ind. Med., 28: 399-403.
HIGGINBOTTOM, T., CROME, P., PARKINSON, C., & NUNN, J.
(1979) Further clinical observations on the pulmonary effect
of paraquat ingestion. Thorax, 34: 161-165.
HILLS, D.J., CURLEY, R.G., KNUTSON, J.D., SEIBER, J.N.,
WINTERLIN, W.L., FAUSCHKOLB, R.S., PULMAN, G.S., & ELMORE,
C.L. (1981) Composting treatment for cotton gin trasfines.
Trans. ASAE, 24: 14-19.
HOFMAN, A. & FROHBERG, H. (1972) [Gramoxone intoxication in
the Federal Republic of Germany.] Dtsch Med. Wschr., 27:
1239-1303 (in German).
HOLLINGER, M.A. & CHVAPEL, M. (1977) Effect of paraquat on
rat lung prolyl hydroxylase. Res. Commun. chem. Pathol.
Pharmacol., 16: 159-162.
HORIUCHI, N. & ANDO, S. (1980) [Contact dermatitis due to
pesticides for agricultural use.] Nippon Hifuka Gakkai Zasshi,
90: 289 (in Japanese).
HORIUCHI, N., ANDO, S., & KAMBE, Y. (1980) [Dermatitis due
to pesticides for agricultural use.] Nippon Hifuka Gakkai
Zasshi, 90: 27 (in Japanese).
HOWARD, J.K. (1979a) Recent experience with paraquat
poisoning in Great Britain: a review of 68 cases. Vet. hum.
Toxicol., 21: 213-216.
HOWARD, J.K. (l979b) A clinical survey of paraquat
formulation workers. Br. J. ind. Med., 36: 220-223.
HOWARD, J.K. (1980) Paraquat: a review of worker exposure in
normal usage. J. Soc. Occup. Med., 30: 6-11.
HOWARD, J.K. (1982) Paraquat spraying. Comparative risks
from high and low volume application methods. In: Proceedings
of 10th Asian Conference on Occupational Health, Singapore,
pp. 1-7.
HOWARD, J.K., SAHOPATHY, K.N., & WHITEHEAD, P.A. (1980) An
evaluation of the long-term effects of paraquat spraying.
Toxicol. Lett. Suppl., 1(Abstr. 068).
HOWARD, J.K., SAHOPATHY, K.N., & WHITEHEAD, P.A. (1981) A
study of the health of Malaysian plantation workers with
particular reference to paraquat spraymen. Br. J. ind. Med.,
38: 110-114.
HOWE, D.J.T. & WRIGHT, N. (1965) The toxicity of paraquat
and diquat. In: Proceedings of the 18th New Zealand Weed &
Pest Control Conference, pp. 105-114.
HUDSON, R.H., HAEGELE, M.A., & TUCKER, R.K. (1979) Acute
oral and percutaneous toxicity of pesticides to mallards:
correlations with mammalian toxicity data. Toxicol. appl.
Pharmacol., 47: 451-460.
HUNDSDORFER, S. & ROSE, I. (1980) [Lung changes produced by
paraquat: respiratory distress model in animals.] Ergeb. exp.
Med., 35: 603-609 (in German).
HUSSAIN, M.Z. & BHATNAGAR, R.S. (1979) Involvement of
superoxide in the paraquat-induced enhancement of lung
collagen synthesis in organ culture. Biochem. Biophy. Res.
Commun., 89: 71-76.
ICI Ltd (1972) Determination of residues of paraquat in
milk, London, ICI Ltd (Residue Analytical Method No. 3,
PPRAM-3).
ICI Ltd (1979) Analytical method for the determination of
paraquat by radioimmunoassay, London, ICI Ltd (PHAG).
ILETT, K.F., STRIPP, B., MENARD, R.H., REID, W.D., & GILLETTE,
J.R. (1974) Studies on the mechanism of the lung toxicity of
paraquat. Comparison of tissue distribution and some
biochemical parameters in rats and rabbits. Toxicol. appl.
Pharmacol., 28: 216-226.
IRPTC (1983) IRPTC Legal files 1983, Vol. I & II, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme.
JAROS, F. (1978) Acute percutaneous paraquat poisoning.
Lancet, 1: 275.
JAROS, F., ZUFFA, L., KRATINOVA, R., SKALA, I., & DOMSOVA, A.
(1978) [Acute percutaneous Gramoxone intoxication.] Pr. Lék.,
7: 260-262 (in Czech).
JOYCE, M. (1969) Ocular damage caused by paraquat. Br. J.
Ophtalmol., 53: 688-690.
KAWAI, M. & YOSHIDA, M. (1981) [Exposure of spray operators
to paraquat.] Nippon Doshu Eisei Zasshi, 28: 353-359 (in
Japanese).
KAWAI, S., UEDA, K., KOYAMA, K., & OGASAWARA, S. (1980)
[Studies on preventive treatment of paraquat dichloride
intoxication - Part 2.] Nippon Noson Igakkai Zasshi, 29:
546-547 (in Japanese).
KAWATOMI, M., KOGA, H., YOKOYAMA, K., FUJIMATSU, S.,
MATSUMOTO, T., FUKUDA, H., & IHIZAKI, T. (1979) [A case of
autopsy of a person who died of paraquat intoxication.] Nippon
Naiko Gakkai Zasshi, 68: 1332-1333 (in Japanese).
KEELING, P.L., PRATT, I.S., ALDRIDGE, W.N., & SMITH, L.L.
(1981) The enhancement of paraquat toxicity in rats by 85%
oxygen: lethality and cell-specific lung damage. Br. J. exp.
Pathol., 62: 643-654.
KEELING, P.L., SMITH, L.L., & ALDRIDGE, W.N. (1982) The
formation of mixed disulfides in rat lung following paraquat
administration. Biochem. Biophys. Acta, 716: 249-257.
KEHRER, J.P., HASCHEK, W.M., & WITSCHI, H. (1979) The
influence of hyperoxia on the acute toxicity of paraquat and
diquat. Drug Chem. Toxicol., 2: 397-408.
KELLY, D.F., MORGAN, D.G., DARKE, P.G.G., GIBBS, C., PEARSON,
H., & WEAVER, M.Q. (1978) Pathology of acute respiratory
distress in the dog associated with paraquat poisoning.
J. comp. Pathol., 88: 275-293.
KHAN, S.U. (1974) Determination of diquat and paraquat
residues in soil by gas chromatography. J. agric. food Chem.,
22: 863-867.
KHAN, S.U. (1980) Determining the role of humic substances
in the fate of pesticides in the environment. J. environ. Sci.
Health, 15: 1071-1090.
KHERA, K.S., WHITTA, L.K., & CLEGG, D.J. (1968) Embryopathic
effects of diquat and paraquat in rats. Ind. Med. Surg., 37:
257-261.
KIMBROUGH, R.D. (1974) Toxic effect of the herbicide
paraquat. Chest, 65: 706-708.
KIMBROUGH, R.D. & GAINES, T.B. (1970) Toxicity of paraquat
in rats and its effect on rat lungs. Toxicol. appl.
Pharmacol., 17: 679-690.
KIMBROUGH, R.D. & LINDER, R.E. (1973) The ultrastructure of
the paraquat lung lesion in the rat. Environ. Res., 6: 265-273.
KIMURA, M., SUZUKI, E., & OHNISHI, H. (1980) [A case of
autopsy of an infant intoxicated by paraquat dichloride.]
Shoni ka Rinsho, 33: 732-734 (in Japanese).
KLIKA, E., KUNC, L., ANTALIKOVA, K., & KUNCOVA, M. (1980)
The morphology of the lung in the albino rat after paraquat
administration. Folia Morphol., 28: 188-191.
KNIGHT, B.A.G. & DENNY, P.J. (1970) The interaction of
paraquat with soil: adsorption by an expanding lattice clay
mineral. Weed Res., 10: 40-48.
KNIGHT, B.A.G. & TOMLINSON, T.E. (1967) The interaction of
paraquat (1,1'-dimethyl-4,4'-dipyridylium dichloride) with
mineral soils. J. soil Sci., 18: 223-243.
KORNBRUST, D.J. & MAVIS, R.D. (1980) The effect of paraquat
on microsomal lipid peroxidation in vitro and in vivo.
Toxicol. appl. Pharmacol., 53: 323-332.
KURISAKI, E. & SATO, H. (1979) [Toxicological studies on
herbicides. Intracorporeal distribution on paraquat dichloride
and diquat dibromide in rat.] Nippon Hoigaki Zasshi, 33: 656
(in Japanese).
LAM, H.F., TAKEZAWA, J., GUPTA, B.N., & STEE, E.W. van
(1980) A comparison of the effects of paraquat and diquat on
lung compliance, lung volumes and single breath. Toxicology,
18: 111-123.
LAVAUR, E., SION, G., GROLLEAU, G., & CARPENTER-LESECK, J.
(1979) Etude comparée de l'action du diquat et du paraquat
sur la muqueuse digestive de la souris, du rat, et du lapin.
Ann. Zool. Ecol. anim., 11: 159-169.
LEARY, J.B. (1978) Diquat and paraquat. In: Zweig, G. &
Sherma, J., ed. Analytical methods for pesticides and growth
regulators, New York, Academic Press, pp. 321-325.
LENGFELDER, E. & ELSTNER, E.F. (1978) Determination of the
superoxide dismutating activity of D-penicillamine copper.
Hoppe-Seyler's Z. Physiol. Chem., 359: 751-757.
LEVIN, P.J., KLAFF, L.J., ROSE, A.G., & FERGUSON, A.D.
(1979) Pulmonary effects of contact exposure to paraquat: a
clinical and experimental study. Thorax, 34: 150-160.
LEVIN, D.E., HOLLSTEIN, M., CHRISTMAN, M.F., SCHWIERS, E.A., &
AMES, B.N. (1982) A new Salmonella tester strain (TA 102)
with AT base pairs at the site of mutation detects oxidative
mutagens. Proc. Natl Acad. Sci. USA, 79: 7445-7449.
LEVITT, T. (1979) Determinations of paraquat in clinical
practice using radioimmunoassay. Proc. Anal. Div. Chem. Soc.,
16: 72-76.
LIDDLE, J.A., NEEDHAM, L.L., ROLLEN, Z.J., ROARK, B.R., &
BAYSE, D.D. (1980) Characterization of the contamination of
marijuana with paraquat. Bull. environ. Contam. Toxicol., 24:
49-53.
LITCHFIELD, M.H., DANIEL, J.W., & LONGSHAW, S. (1973) The
tissue distribution of the bipyridylium herbicides diquat and
paraquat in rats and mice. Toxicology, 1: 155-165.
LLOYD, E.L. (1969) Recovery after taking Weedol. Br. med. J.,
2(5650): 189.
LOCK, E.A. (1979) The effect of paraquat and diquat on renal
function in the rat. Toxicol. appl. Pharmacol., 48: 327-336.
LOCK, E.A. & ISHMAEL, J. (1979) The acute toxic effects of
paraquat and diquat on the rat kidney. Toxicol. appl.
Pharmacol., 50: 67-76.
LOTT, P.F., LOTT, J.W., & DOMS, D.J. (1978) The
determination of paraquat. J. chromatogr. Sci., 16: 390-395.
LUTY, S., CISAK, E., LATUSZYNSKA, J., & PRZYLEPA, E. (1978)
[Effect of paraquat on embryonic and post-embryonic
development in rat.] Bromatol. Chem. Toksykol., 11: 159-165
(in Polish).
MAFF (1980a) Agricultural Development and Advisory Service
'Pesticide science' reference book 1980, Lowestoft, England,
Ministry of Agriculture, Fisheries & Food, pp. 69-76
MAFF (1980b) Pest Infestation Control Laboratory Report
1977-1979, Lowestoft, England, Ministry of Agriculture,
Fisheries & Food, pp. 143-153.
MAFF (1981) Agricultural Development and Advisory Service
'Pesticide science' reference book 1981, Lowestoft, England,
Ministry of Agriculture, Fisheries & Food, pp. 21-27 and 55-63.
MAKOVSKII, V.N. (1972) [Toxicological and hygiene studies of
bipyridylium herbicides diquat and paraquat,] Referat,
Vinnize, USSR, pp. 1-23 (PhD degree thesis) (in Russian).
MALING, H.M., SAUL, W., WILLIAMS, M.A., BROWN, E.A.B., &
GILLETTE, J.R. (l978) Reduced body clearance as the major
mechanisms of the potentiation of beta2-adrenergic agonists of
paraquat lethality in rat. Toxicol. appl. Pharmacol., 43:
57-72.
MALONE, J.D.G., CARMODY, M., & KEOGH, B. (1971) Paraquat
poisoning. A review of nineteen cases. J. Irish Med. Assoc.,
64: 59-68.
MAREK, J., VELENSKA, Z., & HASKOVCOVA, I. (1981) [Kidney
changes in acute phase of paraquat intoxication in rats.]
Sb. Lék., 83: 100-104 (in Czech).
MASTERSON, J.G. & ROCHE, W.J. (1970) Fatal paraquat
poisoning. J. Irish Med. Assoc., 63: 261-264.
MATSUMOTO, T., MATSUMORI, H., KUWABARA, N., FUKUDA, Y., &
ARIWA, R. (1980) [A histopathological study of the liver in
paraquat poisoning. An analysis of 14 autopsy cases with
emphasis on bile duct injury.] Acta pathol. Jpn., 30: 859-870
(in Japanese).
MATSUMOTO, S., MATSUMORI, H., KUWABARA, N., FUKUDA, Y., &
ARIWA, R. (1981) [Histopathological studies on the
disturbance of bile ducts to paraquat.] Kanzo, 22: 309 (in
Japanese).
MATTHEW, H., LOGAN, A., WOODRUFF, M.F.A., & HEARD, B. (1968)
Paraquat poisoning. Lung transplantation. Br. med. J., 3:
759-763.
MCCORD, J.M. & FRIDOVICH, I. (1969) Superoxide dismutase -
an enzymic function for erythrocuprein (hemocuprein). J. Biol.
Chem., 244: 6049-6055.
MCDONAGH, B.J. & MARTIN, J. (1970) Paraquat poisoning in
children. Arch. Dis. Child., 45: 425-427.
MCELLIGOTT, T.F. (1972) The dermal toxicity of paraquat.
Differences due to techniques of application. Toxicol. appl.
Pharmacol., 21: 361-368.
MCGEOWN, M.G. (1975) Clinical aspects of paraquat poisoning.
In: Fletcher, K., ed., Clinical aspects of paraquat poisoning,
London, ICI Ltd, pp. 12-21.
MEES, G.C. (1960) Experiments on the herbicidal action of
1,1'-ethylene-2,2'dipyridylium dibromide. Ann. appl. Biol.,
48: 601-612.
MEHANI, S. (1972) The toxic effect of paraquat in rabbits
and rats. Ain Shams med. J., 23: 599-601.
MIRCEV, N. (1976) [Acute intoxication with Gramoxone
(paraquat).] Vatreshni Bolesti, 16: 99-101 (in Bulgarian).
MITHYANTHA, M.S. & PERUR, N.G. (1975) Paraquat retention by
soils. Mysore J. agric. Sci., 9: 276-282.
MONTGOMERY, M.R. (1976) Interaction of paraquat with the
pulmonary microsomal fatty acid desaturase system. Toxicol.
appl. Pharmacol., 36: 543-554.
MONTGOMERY, M.R. (1977) Paraquat toxicity and pulmonary
superoxide dismutase: an enzymic deficiency of lung
microsomes. Res. Commun. chem. Pathol. Pharmacol., 16: 155-158.
MONTGOMERY, M.R. & NIEWOEHNER, D.E. (1979) Oxidant-induced
alterations in pulmonary microsomal mixed-function oxidation.
Acute effects of paraquat and ozone. J. environ. Sci. Health,
13: 205-219.
MOODY, C.S. & HASSAN, H.M. (1982) Mutagenicity of oxygen
free radicals. Proc. Natl Acad. Sci., 79: 2855-2859.
MOURIN, K.A. (1967) Paraquat poisoning. Br. med. J., 4: 486.
MUKADA, T., SASANO, N., & SATO, K. (1978) [Autopsy findings
in a case of acute paraquat poisoning with extensive cerebral
purpura.] Tohoku J. exp. Med., 125: 253-263 (in Japanese).
MULLICK, F.G., TSHAK, K.G., MAHABIR, K., & STROMEYER, F.W.
(1981) Hepatic injury associated with paraquat toxicity in
humans. Liver, 1: 209-221.
MURRAY, R.E. & GIBSON, J.E. (1972) A comparative study of
paraquat intoxication in rats, guinea pigs and monkeys.
Exp. mol. Pathol., 17: 317-325.
MURRAY, R.E. & GIBSON, J.E. (1974) Paraquat disposition in
rats, guinea pigs and monkeys. Toxicol. appl. Pharmacol., 27:
283-291.
MUSSON, F.A. & PORTER, C.A. (1982) Effect of ingestion of
paraquat on a 20-week gestation fetus. Postgrad. Med. J., 58:
731-732.
NAGY, A. (1970) Paraquat and adrenal cortical necrosis.
Br. med. J. 2: 669.
NATORI, H., KOIKE, M., & KIRA, S. (1979) [Respiratory
failure due to the herbicide paraquat.] Gendai Iryo, 11:
1175-1182 (in Japanese).
NEWHOUSE, M., MCEVOY, D., & ROSENTHAL, D. (1978)
Percutaneous paraquat absorption. Arch. Dermatol., 114:
1516-1519.
NEWMAN, J.F. & EDWARDS, P.J. (1980) Paraquat: effect on
spraying eggs on hatachability and on the reproductive organs
of the chicks of pheasant, Phasianus colchicus, London, ICI
Ltd (ICI Plant Protection Division Report No. RJ 0090B).
NEWMAN, J.F. & WAY, J.M. (1966) Some ecological observations
on the use of paraquat and diquat as aquatic herbicides.
Proceedings of the 8th British Weed Control Conference,
Vol. 2, pp. 582-585.
NEWMAN, J.F. & WILKINSON, W.W. (1971) The effect of excess
quantities of paraquat in soil on the growth of vegetation,
London, ICI Ltd (Report No TMJ 606 A).
O'BRIEN, M.C. & PRENDEVILLE, G.N. (1978) A rapid sensitive
bioassay for determination of paraquat and diquat in water.
Weed Res., 18: 301-303.
OGATA, M., HASEGAWA, T., & UEDA, K. (1978) [Action of
paraquat dichloride and dimethyldithiocarbamate on the
mitochondrial conversion system and superoxide dismutase.]
Samgyo Igaku, 20: 551-552 (in Japanese).
OKAWADA, N., YAGASAKI, K., & KONDO, T. (1980) [Ocular
impairments due to an agricultural pesticide.] Nippon Noson
Igakkai Zasshi, 29: 550-551 (in Japanese).
OKONEK, S., WEILEMANN, L.S., MAJDANDZIC, J., SETYADHARME, H.,
REINECKE, H.J., BALDAMUS, C.A., LOHMAN, J., BONZEL, K.E., &
THON, T. (1982) Successful treatment of paraquat poisoning.
Activated charcoal per os and, continuous hemoperfusion.
J. Toxicol. clin. Toxicol., 19: 807-819.
OMAYE, S.T., REDDY, K.A., & CROSS, C.E. (1978) Enhanced lung
toxicity of paraquat in selenium-deficient rats. Toxicol.
appl. Pharmacol., 43: 237-247.
ONGOM, V.L., OWOR, R., & TONUSANGE, E.T. (1974) Paraquat
(Gramoxone) used as a pediculocide. In: Bagshore, A.F., ed.
The uses and abuses of drugs and chemicals in tropical Africa,
Nairobi, East Africa Literature Bureau, pp. 229-233.
PARK, J., PROUDFOOT, A.T., & PRESCOTT, L.F. (1975) Paraquat
poisoning. A clinical review of 31 cases. In: Fletcher, K.,
ed., Clinical aspects of paraquat poisoning, London, ICI Ltd,
pp. 46-53.
PARRY, J.M. (1973) The induction of gene conversion in yeast
by herbicide preparations. Mutat. Res., 21: 83-91.
PARRY, J.M. (1977) The use of yeast for the detection of
environmental mutagens using a fluctuation test. Mutat. Res.,
46: 165-176.
PASCHAL, D.C., NEEDHAM, L.L., ROLLEN, Z.J., & LIDDLE, J.A.
(1979) Determination of paraquat in sunflower seeds by
reversed-phase high performance liquid chromatography.
J. Chromatogr., 171: 85-90.
PASI, A., EMBREE, J.W., EISENLORD, G.H., & HINE, C.H. (1974)
Assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 26: 171-175.
PATRICK, G.B. (1980) Marijuana and the lung. Postgrad. med.
J., 67: 110-118.
PAYNE, W.L., POPE, J.D., & BENNER, J.E. (1974) Integrated
method for trifluralen, diphenamid and paraquat in soil and
runoff from agricultural land. J. agric. food Chem., 22: 79-82.
PEDERSEN, G., PEDERSEN, A., GREGERSEN, M., & KAMPE, B.
(1981) [Paraquat poisoning.] Ugeskr. Laeg., 143: 1202-1206
(in Danish).
PESTEMER, VON W., NOLTING, H.G., & LUNDEHN, Y.-R. (1979)
[Possible effects of repeated paraquat treatments on the
residue situation in the soil.] Nachrichtenbl. Dtsch
Pflanzenschutzd., 31: 166-170 (in German).
PICKOVA, J. (1978) [Determination of paraquat in the urine.]
Pr. Lék., 30: 266-267 (in Czech).
POPE, J.D. & BENNER, J.E. (1974) Colorimetric determination
of paraquat residues in soil and water. J. AOHC, 57: 202-204.
POPENOE, D. (1979) Effects of paraquat aerosol on mouse
lung. Arch. Pathol. lab. Med., 103: 331-334.
PROUDFOOT, A.T., STEWART, M.S., LEVITT, T., & WIDDOP, B.
(1979) Paraquat poisoning: significance of plasma-paraquat
concentrations. Lancet, 2(8138): 330-332.
PRYDE, A. & DARBY, F.J. (1975) The analysis of paraquat in
the urine by high speed liquid chromatography. J. Chromatogr.,
115: 107-109.
PURSER, D.A. & ROSE, M.S. (1979) The toxicity and renal
handling of paraquat in Cynomolgus monkeys. Toxicology, 15:
31-41.
RADAELLI, L. & MARTELLI, M. (1971) Residual toxicity of
paraquat absorbed on soil. Agrochimica, 15: 344-350.
REDDY, K.A., LITOV, R.E., & OMAYE, S.T. (1977) Effect of
pre-treatment with anti-inflammatory agents on paraquat
toxicity in the rat. Res. Commun. chem. Pathol. Pharmacol.,
17: 87-100.
RESTUCCIA, A., FOGLINI, A., & DE ALENTIS NANNINI, D. (1974)
Paraquat toxicity for rabbits. Vet. Ital., 25: 555-565.
RILEY, D. (1981) The fate and effect of paraquat and diquat
residues in soil. In: Proceedings for the National Spray Seed
Conference 1981, Albury, New South Wales, Australia.
RILEY, D., WILKINSON, W., & TUCKER, B.V. (1976) Biological
unavailability of bound paraquat residues in soil. In:
Kaufman, D.P., Still, G.G., Paulson, G.D., & Bandal, S.K., ed.
Bound and conjugated pesticide residues, pp. 301-353 (ACS
Symposium Series No. 29).
ROBERTSON, B. (1973) Paraquat poisoning as an experimental
model of the idiopathic respiratory distress syndrome. Bull.
Phys. Pathol. Res., 9: 1433-1452.
ROBERTSON, B., GROSSMANN, G., & IVEMARK, B. (1976) The
alveolar lining layer in experimental paraquat poisoning. Acta
pathol. microbiol. Scand. Section A, 84: 40-46.
ROSE, J. (1980) Paraquat poisoning. Lancet, 2(8200): 924.
ROSE, M.S. & SMITH, L.L. (1977) The relevance of paraquat
accumulation by tissues. In: Autor, A.P., ed., Biochemical
mechanisms of paraquat toxicity, New York, Academic Press, pp.
71-91.
ROSE, M.S., SMITH, L.L., & WYATT, I. (1974a) Evidence of
energy-dependent accumulation of paraquat in rat lung. Nature
(Lond.), 252: 314-315.
ROSE, M.S., CRABTREE, H.C., FLETCHER, K., & WYATT, I.
(1974b) Biochemical effects of diquat and paraquat.
Disturbance of the control of corticosteroid synthesis in rat
adrenal and subsequent effects on the control of live glycogen
utilization. Biochem. J., 138: 437-443.
ROSE, M.S., SMITH, L.L., & WYATT, I. (1976) The relevance of
pentose-phosphate pathway stimulation in rat lung to the
mechanisms of paraquat toxicity. Biochem. Pharmacol., 25:
1763-1767.
ROSLYCKY, E.B. (1977) Response of soil microbiota to
selected herbicide treatments. Can. J. Microbiol., 23: 426-433.
ROSS, W.E., BLOCK, E.R., & CHANG, R.Y. (1979) Paraquat-
induced DNA damage in mammalian cells. Biochem. biophys. Res.
Commun., 91: 1302-1308.
SAITO, K., PARKER, W.B., GARDNER, D.E., & MENZEL, D.B.
(1979) Paraquat accumulation by cultured lung cells.
Pharmacology, 21(Abstr. 218): 392.
SAMMAN, P.D. & JOHNSTON, E.N. (1969) Nail damage associated
with handling of paraquat and diquat. Br. med. J., 1: 818-819.
SCHLATTER, I. (1976) [Poisoning due to herbicide paraquat.]
Schweiz. Rundsch. Med., 65: 837-843 (in German).
SEIBER, J.N. & WOODROW, J.E. (1981) Sampling and analysis of
airborne residues of paraquat in treated cotton field
environments. Arch. environ. Contam. Toxicol., 10: 133-149.
SEIBER, J.N., WINTERLIN, W.L., & MCCHESNEY, B. (1979)
Residues of toxaphene, DEE, and paraquat in plant parts and
gin waste from treated cotton fields. Arch. environ. Contam.
Toxicol., 8: 125-137.
SEIDENFELD, J.J., WYKOFF, D., ZAVALA, D.C., & RICHARDSON,
J.B. (1978) Paraquat lung injury in rabbits. Br. J. ind.
Med., 35: 245-247.
SELECT COMMITTEE ON NARCOTICS ABUSE & CONTROL (1980) The use
of paraquat to eradicate illicit marihuana crops and the
health implications of paraquat-contaminated marihuana on the
US market. A report of the 96-Congress, Washington DC, US
Government Printing Office, pp. 1-97 (SCNAC-96-1-16).
SELYPES, A. & PALDY, A. (1978) The examination of the
mutagenic effect of two pesticides: Krezonit E and Gramoxone.
Proc. Hung. Ann. Meet. Biochem., 18: 77-78.
SELYPES, A., NAGYMAJTENYI, L., & BERENCSI, G. (1980)
Mutagenic and embryotoxic effects of paraquat and diquat.
Bull. environ. Contam. Toxicol., 25: 513-517.
SHARP, C.W., OTTOLENGHI, A., & POSNER, A.S. (1972)
Correlation of paraquat toxicity with tissue concentration and
weight loss of the rat. Toxicol. appl. Pharmacol., 22: 241-251.
SHU, H., TALCOTT, R.E., RICE, S.A., & WEI, E.T. (1979) Lipid
peroxidation and paraquat toxicity. Biochem. Pharmacol., 28:
327-331.
SHUZUI, T. (1980) [An autopsy case of paraquat poisoning.]
Mie Igaku, 23: 518-521 (in Japanese).
SINGH, S.P. & YADAV, N.K. (1978) Toxicity of some herbicides
to maior carp fingerlings. Indian J. Ecol., 5: 141-147.
SINOW, J. & WEI, E. (1973) Ocular toxicity of paraquat.
Bull. environ. Contam. Toxicol., 9: 163-168.
SLADE, P. (1965) Photochemical degradation of paraquat.
Nature (Lond.), 207: 515.
SLADE, P. (1966) The fate of paraquat applied to plants.
Weed Res., 6: 158-167.
SMALLEY, H.E. (1973) Toxicity and hazard of the herbicide
paraquat in turkeys. Poultry Sci., 52: 1625-1629.
SMITH, R.J. (1978) Poisoned pot becomes burning issue in
high places. Science, 200: 417-418.
SMITH, P. & HEATH, D. (1974) The ultrastructure and time
sequence of the early stages of paraquat lung in rats.
J. Pathol., 114: 177-184.
SMITH, P. & HEATH, D. (1976) Paraquat. Clin. Rev. Toxicol.,
4: 411-445.
SMITH, L.L. & ROSE, M.S. (1977a) A comparison of the effects
of paraquat and diquat on the water content of rat lung and
the incorporation of thymidine into lung DNA. Toxicology, 8:
223-230.
SMITH, L.L. & ROSE, M.S. (1977b) Biochemical changes in
lungs exposed to paraquat. In: Autor, A.P., ed. Biochemical
mechanisms of paraquat toxicity, New York, Academic Press.
SMITH, P., HEATH, D., & RAY, J.M. (1973) The pathogenesis
and structure of paraquat-induced pulmonary fibrosis in rats.
J. Pathol., 114: 57-67.
SMITH, L.L., WRIGHT, A., WYATT, I., & ROSE, M.S. (1974)
Effective treatment for paraquat poisoning in rats and its
relevance to treatment of paraquat poisoning in man. Br. med.
J., 4: 569-571.
SMITH, S.N., LYON, A.J., & SAHID, I.B. (1976) The breakdown
of paraquat and diquat by soil fungi. New Phytol., 77: 735-740.
SMITH, C.N., LEONARD, R.A., LANGDALE, G.W., & BAILEY, G.W.
(1978) Transport of agricultural chemicals from small upland
Piedmont watersheds, Springfield, Virginia, National Technical
Information Service, p. 386 (Report PB-285, 134).
SMITH, L.L., ROSE, M.S., & WYATT, I. (1979) The pathology
and biochemistry of paraquat. In: Oxygen free radicals and
tissue damage, Amsterdam, North Holland, Excerpta Medica,
pp. 321-341 (CIBA Foundation Series 65 - new series).
SMITH, T.F., NOACK, A.J., & COSH, S.M. (1981a) The effect of
some herbicides on vesicular-arbuscular endophyte abundance in
the soil and on infection of host roots. Pestic. Sci., 12:
91-97.
SMITH, L.L., WYATT, I., & ROSE, M.S. (1981b) Factors
affecting the efflux of paraquat from rat lung slices.
Toxicology, 19: 197-207.
SODERQUIST, C.J. & CROSBY, D.G. (1972) The gas chromato-
graphic determination of paraquat in water. Bull. environ.
Contam. Toxicol., 8: 363-368.
STAIFF, D.C., IRLE, G.K., & FELSENSTEIN, W.C. (1973)
Screening of various adsorbents for protection against
paraquat poisoning. Bull. environ. Contam. Toxicol., 10:
193-199.
STAIFF, D.C., COMER, S.W., ARMSTRONG, J.F., & WOLFE, H.R.
(1975) Exposure to the herbicide paraquat. Bull. environ.
Contam. Toxicol., 14: 334-340.
STAIFF, D.C., BUTLER, L.C., & DAVIS, J.E. (1981) A fie1d
study of the chemical degradation of paraquat dichloride
following simulated spillage on soil. Bull. environ. Contam.
Toxicol., 26: 16-21.
STEFFEN, C. & NETTER, K.J. (1979) On the mechanism of
paraquat action on microsomal oxygen reduction and its
relation to lipid peroxidation. Toxicol. appl. Pharmacol., 47:
593-602.
STEFFEN, C., MULIAWAN, H., & KAPPUS, H. (1980) Lack of in
vivo lipid peroxidation in experimental paraquat poisoning.
Arch. Pharmacol., 310(3): 241-243.
STEPHENS, D.S., WALKER, D.H., SCHAFFNER, W., KAPLOWITZ, L.G.,
BRASHEAR, R.H., ROBERTS, R., & SPICKARD, W.A. (1981)
Pseudodiphtheria: prominent pharyngeal membrane associated
with fetal paraquat ingestion. Ann. intern. Med., 94: 202-204.
STEVENS, M.A. & WALLEY, J.K. (1966) Tissue and milk residues
arising from the ingestion of single doses of diquat and
paraquat by cattle. J. Sci. Food Agric., 17: 472-475.
STEWART, M.J., LEVITT, T., & JARVIE, D.R. (1979) Emergency
estimations of paraquat in plasma. A comparison of the RIA and
ion pair/colorimetric methods. Clin. Chim. Acta, 94: 253-257.
SUGAYA, H., OHE, T., UENO, T., KAWASHIMA, T., SUGITA, T.,
MAEHARA, M., HISANCHI, T., IORI, M., HARADA, H., & SHIGEMATA,
S. (1980) [Clinical discussion on six cases of paraquat
dichloride intoxication.] Nippon Naika Gakkai Zasshi, 69: 876
(in Japanese).
SUMMERS, L.A. (1980) The bipyridylium herbicides, London,
New York, Toronto, Sydney, San Francisco, Academic Press,
pp. 1-449.
SWAN, A.A.B. (1969) Exposure of spray operators to paraquat.
Br. J. ind. Med., 26: 322-329.
SYKES, B.J., PURCHASE, I.F.H., & SMITH, L.L. (1977)
Pulmonary ultrastructure after oral and intravenous dosage of
paraquat to rats. J. Pathol., 121: 233-241.
TAKAHASHI, T., YAMAMOTO, K., SAWAI, T., OKUDO, T., MUKODA, T.,
MINAGAWA, N., & SUGAYA, H. (1978) [Examination of 5
autopsies following paraquat dichloride intoxication,
especially of parenchymatous organ lesions.] Nippon
Byorigakkai Kaishi, 67: 138-139 (in Japanese).
TAKAYAMA, K., TAKEUCHI, K., SUGA, E., IWABUCHI, K., & TOMICHI,
N. (1978) [A case of autopsy following paraquat dichloride
intoxication.] Nippon Byorigakkai Kaishi, 67: 139 (in
Japanese).
TAKEUCHI, K., TAKAYAMA, K., TOMICHI, N., KAN, E., YAGAWA, K.,
& IWABUCHI, K. (1980) [Paraquat poisoning in a pregnant
woman.] Nippon Byorigakkai Kaishi, 18: 747-752 (in Japanese).
TALCOTT, R.E., SHU, H., & WEI, E.T. (1979) Dissociation of
microsomal oxygen reduction and lipid peroxidation with the
electron acceptors, paraquat and menadione. Biochem.
Pharmacol., 26: 665-671.
TCHIPILSKA, L.N. (1980) [The influence of some pesticides on
the soil microorganisms in connexion with the hygiene
evaluation of the soil,] Referat, Sofia, pp. 1-39 (PhD degree
thesis) (in Bulgarian).
THOMPSON, W.D. & PATRICK, R.S. (1978) Collagen propyl
hydroxylase levels in experimental paraquat poisoning.
Br. J. exp. Pathol., 59: 288-291.
TOMPSETT, S.L. (1970) Paraquat poisoning. Acta pharmacol.
toxicol., 28: 346-358.
TOMURA, M., ETO, K., SAGARA, K., & SATO, T. (1979) [A case
of disturbance of the liver and kidney.] Kanzo, 20: 1016 (in
Japanese).
TONER, P.G., VETTERS, J.M., SPILG, W.G.S., & HARLAND, W.A.
(1970) Fine structure of the lung lesion in a case of
paraquat poisoning. J. Pathol., 102: 182-185.
TRUSH, M.A., MIMNAUGH, E.G., GINSBURG, E., & GRAM, T.E.
(1981) In vitro stimulation by paraquat of reactive oxygen-
mediated lipid peroxidation in rat lung microsomes. Toxicol.
appl. Pharmacol., 60(2): 279-286.
TRUSH, M.A., MIMNAUGH, E.G., GINSBURG, E., & GRAM, T.E.
(1982) Studies on the in vitro interaction of mitomycin C,
nitrofurantoin, and paraquat with pulmonary microsomes.
Stimulation of reactive oxygen-dependent lipid peroxidation.
Biochem. Pharmacol., 31(21): 3335-3346.
TSUNENARI, S., MUTO, H., SASAKI, S., SUGITA, H., & KANDA, M.
(1975) [Forensic toxicological studies on herbicide
(Gramoxone).] Jpn. J. leg. Med., 29: 88-102 (in Japanese).
TSUNENARI, S., YONEMITSU, K., UCHIMURA, Y., & KANDA, M.
(1981) The influence of putrefactive changes on the
determination of paraquat in autopsy materials. Forensic Sci.
Int., 17: 51-56.
TSUTSUI, Y., NAKABAYASHI, H., SUZUKI, H., & OGURA, K. (1976)
[Studies on the toxicity of paraquat - Part I.] Nippon Noson
Igakkai Zasshi, 25: 614-621 (in Japanese).
TU, C.M. & BOLLEN, W.B. (1968) Effect of paraquat on
microbial activities in soils. Weed Res., 8: 28-37.
TWEATS, D.J. (1975) The effect of R (drug resistance)
factors on the detection of mutagens by E. coli K 12,
Brussels, Belgium, Annual Euratom Report, Biological Sciences,
316 pp.
TYBURCZYK, W., BORKOWSKA, I., CHORAGIEWICZ, H., & KLIMEK, K.
(1979) [Effect of paraquat on some biochemical tests in
rats.] Bromatol. Chem. Toksykol., 12: 283-288 (in Polish).
UKAI, S., HIROSE, K., & KAWASE, S. (1977) [Gas chromatography
of reduction products of the herbicides "diquat" and "paraquat".]
Eisei Kagaku, 23: 32-38 (in Japanese).
US CONGRESSIONAL HEARING (1979) Health implications of
paraquat-contaminated marihuana. Select Committee on Narcotics
Abuse & Control, House of Representatives. Hearing of the 96th
Congress, Washington DC, US Government Printing Office, pp.
1-103 (SCNAC-96-1-3).
VALE, J.A. (1977) Paraquat poisoning. Nurs. Times, 73:
154-155.
VAZIRI, N.D., NESS, R.L., FAIRSHTER, R.D., SMITH, W.R., &
ROSEN, S.M. (1979) Nephrotoxicity of paraquat in man. Arch.
intern. Med., 139: 172-174.
VESELEY, D.L., WATSON, B., & LEVEY, G.A. (1979) Activation
of liver guanylate cyclase by paraquat: possible role of
superoxide anion. J. Pharmacol. exp. Ther., 209: 162-164.
VIJEYARATNAM, G.S. & CORRIN, B. (1971) Experimental paraquat
poisoning: a histological and electron-optical study of the
changes in the lung. J. Pathol., 103: 123-129.
VUCINOVIC, V. (1978) [Four cases of poisoning with
paraquat.] Arch. Hig. Rada, 29: 261-265 (in Serbo-Croat).
WADDELL, W.J. & MARLOWE, C. (1980) Tissue and cellular
disposition of paraquat in mice. Toxicol. appl. Pharmacol.,
56: 127-140.
WAIGHT, J.J.J. & WHEATHER, R.H. (1979) Fatal percutaneous
paraquat poisoning. J. Am. Med. Assoc., 242: 472.
WALKER, M., DUGARD, P.H., & SCOTT, R.C. (1983) Absorption
through human and laboratory animal skins: in vitro
comparison. Acta pharm. Suec., 20(1): 52-53.
WALTERS, K.A., DUGAR, P.H., & FLORENCE, A.T. (1981)
Non-ionic surfactants and gastric mucosal transport of
paraquat. J. Pharm. Pharmacol., 33: 207-213.
WARD, C.D., STONES, D.P.A., CONNEL, H., CULLEN, D.R., &
WATKIN, J.I. (1976) Paraquat poisoning. Lancet, 1(7971):
1247.
WATANABE, I., SAKAI, K., TOYAMA, K., UENO, M., & WATANABE, M.
(1979) [On 3 cases of ocular disturbance due to Gratoxone, a
herbicide containing 24% paraquat dichloride.] Ganka Rinsho
Iho, 73: 1244-1246 (in Japanese).
WAUCHOPE, R.D. (1979) Pesticides in runoff water. Agric.
Res., 27: 11.
WAY, J.M., NEWMAN, J.F., MOORE, N.W., & KNAGGS, F.W. (1971)
Some ecological effects of the use of paraquat for the control
of weeds in small lakes. J. appl. Ecology, 8: 509-532.
WEBER, J.B., PERRY, P.W., & UPCHURCH, R.P. (1965) The
influence of temperature and time on the adsorption of
paraquat, diquat, 2,4-D and prometone by clays, charcoal and
on anion-exchange resin. Soil Sci. Am. Soc. Proc., 29: 678-688.
WHO/FAO (1978) Data sheets on pesticides No. 4, Rev.1 -
Paraquat, Geneva, World Health Organization (Unpublished
Report No.: VBC/DS/75.4 Rev.1 (8/78)).
WILKINSON, W. (1980) Paraquat and diquat: Longterm high rate
trial, Frensham UK. Management of site, effects on crops and
weeds and residues in crops, London, ICI Ltd (Report RJ0013B).
WITSCHI, H.P., KACEW, S., HIRAI, K., & COTE, M.G. (1977) In
vivo oxidation of reduced nicotine amide-adenine dinucleotide
phosphate by paraquat and diquat in rat lung. Chem. Biol.
Interactions., 19: 143-160.
WOHLFAHRT, D.J. (1982) Fatal paraquat poisoning after skin
absorption. Med. J. Aust., 1: 512-513.
WOJECK, G.A., PRICE, J.F., NIGG, A.N., & STAMPER, J.H.
(1983) Worker exposure to paraquat and diquat. Arch. environ.
Contam. Toxicol., 12: 65-70.
WORTHING, C.R. (1979) The pesticide manual, Croydon,
England, British Crop Protection Council (BCPC Publications).
WRIGHT, K.A. & CAIN, M. (1970) Microbial degradation of
4-carboxy-1-methylpyridylium chloride, a photolytic product of
paraquat. Biochem. J., 118: 52.
WRIGHT, N., YEOMAN, W.B., & HALE, K.A. (1978) Assessment of
severity of paraquat poisoning. Br. med. J., 2: 396-397.
WYATT, I., DOSS, A.W., ZAVALA, D.C., & SMITH, L.L. (1981)
Intrabronchial instillation of paraquat in rats: lung
morphology and retention study. Br. J. ind. Med., 38: 42-48.
YOSHIDA, K., TANAKA, Y., IKUNUMA, T., TERUKIMATSU, S., KUSANO,
E., ASANO, Y., & HOSODA, S. (1980) [A case report of
survival after intoxication by paraquat.] Nippon Nuika Gakkai
Zasshi, 69: 1487-1489 (in Japanese).
YOUNGMAN, R.J. & DODGE, A.D. (1979) [Mechanism of paraquat
action: inhibition of the herbicidal effect by a copper
chelate with superoxide-dismutating activity.] Z. Naturforsch.
(Teil C), 34: 1032-1035 (in German).
ZAVALA, D.C. & RHODES, M.L. (1978) An effect of paraquat on
the lungs of rabbits. Its implications in smoking contaminated
marijuana. Chest, 74: 418-420.
DIQUAT
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General properties
Diquat (1,1'ethylene, 2,2'bipyridyl) is a non-selective contact
herbicide. It is sold primarily as a 20% w/v solution in many
countries and is manufactured in the United Kingdom. It is
exclusively manufactured as a dibromide salt and is usually
formulated to contain wetters.
The herbicidal property of diquat depends on its ability to
undergo a single electron addition to form a radical that reacts
with molecular oxygen to reform diquat and concomitantly produce a
superoxide anion. This oxygen radical may directly or indirectly
cause cell death.
It is possible to detect the compound because of its ability to
form a radical. Analytical procedures are available.
1.1.2. Environmental distribution and transformation
environmental effects
Diquat undergoes rapid photochemical degradation in aqueous
solution and on surfaces. The major degradation products produced
in water have been identified and are of lower acute oral toxicity
for rats than diquat itself. The photochemical degradation of
diquat on plants is more complex than that in water. On diquat-
desiccated wheat and barley, diquat itself normally constitutes the
most important single compound. The most important photochemical
degradation products have been identified, they are of low
mammalian toxicity. No other well-defined major degradation
product is formed.
Ruminants excrete diquat and its photochemical products rapidly
and very little is transferred to milk and tissues. Consequently,
residue levels in products of animal origin are very low.
Ingestion of diquat and its photochemical products at higher levels
than would be found in practice did not induce ill effects in
ruminants.
Diquat reaching the soil becomes rapidly and strongly adsorbed
to clay minerals in soil. This process inactivates the herbicidal
activity of diquat. While free diquat is degraded by a range of
soil microorganisms, degradation of strongly adsorbed diquat is
relatively slow. In plot studies, the rate of degradation of
diquat in soil is very slow or non-detectable. However, in long-
term field studies, degradation rates of the order of 5 - 10% per
year have been shown. This is greater than the rate required in
normal practice to prevent saturation of the deactivation capacity
of agricultural/horticultural soils. Strongly-bound diquat has no
adverse effects on soil microfauna or soil microbial processes.
Diquat residues disappear rapidly from water by adsorption on
aquatic weeds and by strong adsorption on bottom mud. Diquat is of
low toxicity for fish and is not accumulated in them. Normal
applications of diquat for aquatic weed control are not harmful to
aquatic organisms. However, care should be taken in applying
diquat to water containing heavy weed growth to treat only a part
of the weed growth, since oxygen consumed by subsequent weed decay
may decrease dissolved oxygen levels to an extent that may be
dangerous for fish. Treated water should not be used for overhead
irrigation until a period of 10 days has elapsed following
treatment.
Diquat is not volatile and the concentrations of airborne
diquat during spraying have been shown to be very low.
1.1.3. Kinetics and metabolism
Diquat is poorly absorbed from the intestinal tract and skin.
Diquat monopyridone is the major metabolite of diquat in the body;
of lesser importance is diquat dipyridone. Both metabolites are
considerably less toxic than diquat itself. Depending on species
and route of administration, less than 20% of the dose is
metabolized. The gastrointestinal microflora appear to be mainly
responsible for the metabolism of diquat.
Compared with paraquat, accumulation of diquat in the lungs is
far less marked, but diquat shows a certain preference for the
kidneys. The kidneys are the major route of excretion, but a
considerable amount of diquat can also be excreted in the bile,
varying with the animal species.
1.1.4. Effects on animals
Diquat is less toxic than paraquat and does not give rise to
the specific lung disease that is so typical of paraquat poisoning.
Gastrointestinal disturbances, with vomiting, greenish diarrhoea,
and abdominal distension from the significant accumulation of water
in the lumen of the intestines, are typical of diquat poisoning,
together with progressive haemoconcentration, which may progress to
lethargy, coma, and death. At high doses, minor toxicity has been
noted in the liver, kidney, and the nervous and endocrine systems.
Diquat has induced cataracts after prolonged oral exposure
although this effect has not been reported in man. It is less
irritant to the skin, mucous membranes, and the eye than paraquat,
and is not known to be a sensitizer.
Diquat is not teratogenic or carcinogenic.
In vitro mutagenicity studies were inconclusive, though
generally suggesting only weak activity, while the results of in
vivo studies have been negative. A no-observed-adverse-effect
level of 0.75 mg diquat ion/kg body weight per day has been
established from long-term feeding studies on rats.
1.1.5. Effects on man
Occupational exposure to diquat does not pose a health risk if
the recommendations for use are followed and there is adherence to
safe working practices.
Diquat poisoning by suicidal or accidental ingestion is much
less common than paraquat poisoning. It produces a similar severe
clinical syndrome with two notable differences: (a) diarrhoea is a
prominent feature, and (b) pulmonary fibrosis has not been
described.
Accidental cases are usually due to ingestion of decanted
diquat.
The lethal dose for man appears to be approximately 6 - 12
grams of diquat dibromide. In agricultural workers, inflammation
and bleeding of the nasal mucosa have been reported, as well as
nail changes and delayed wound healing.
1.2. Recommendations
1.2.1. General
Where practical and reasonable, the availability and use of the
20% liquid product should be limited to bona fide agriculturalists,
horticulturalists, and professional users who work with trained
personnel, properly maintained equipment, and adequate supervision.
Every effort should be made to prevent the practice of
decanting or rebottling of the product into containers that have
not been properly labelled.
1.2.2. Prevention and treatment
Attention should be drawn to the fact that persons with skin
lesions (either pre-existing or following contamination with
diquat) should not be permitted to take any part in spraying
procedures until skin condition has resolved.
It must be stressed that treatment of persons with diquat
poisoning should be instituted as early as possible. The
likelihood of recovery from a fatal dose is greatest when therapy
begins within 5 - 6 h of poisoning.
1.2.3. Experimental work
Results of existing mutagenicity and carcinogenicity studies
generally suggest that diquat is unlikely to induce genotoxic
effects in man, but more detailed information is required.
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and Chemical Properties
Diquat is a non-selective contact bipyridylium herbicide and
desiccant. The herbicide is supplied mainly as an aqueous solution
of the dibromide (1,1'-ethylene-2,2'-bipyridylium dibromide,
C12H12N2 Br2), with a relative molecular mass of 184.2 based on the
cation. The commonly available analytical standard is diquat
dibromide monohydrate, which is an odourless, pale yellow,
crystalline powder. Some of the other physical properties of
diquat dibromide are listed in Table 1. It is slightly soluble in
alcohol, and practically insoluble in non-polar organic solvents
(Summers, 1980). Diquat is non-explosive and non-inflammable in
aqueous formulations.
Table 1. Physical properties of diquat dibromide
--------------------------------------------------
Specific gravity at 20 °C 1.200
Melting point 180 °C
Boiling point approximately 300 °C
with decomposition
Solubility in water at 20 °C 700 g/litre
pH of liquid formulation 6.0 - 7.0
Evaporation rate not applicable
Vapour pressure not measurable
--------------------------------------------------
Diquat is stable in neutral or acid solutions but is hydrolysed
by alkali. It is inactivated by inert clay and by anionic
surfactants. Diquat dibromide has the following chemical
structure:
7. EFFECTS ON ANIMALS
7.1. Effects on Experimental Animals
7.1.1. Respiratory system
Toxicity studies in rats, mice, dogs, and monkeys (Clark et
al., 1966; Kimbrough & Gaines, 1970; Murray & Gibson, 1972;
Makovskii, 1972; Kelly et al., 1978) demonstrated that paraquat had
a specific effect on the lung (Table 11). Administration by every
route of entry tested whether parenteral (Fisher et al., 1973a;
Robertson, 1973; Hunsdorfer & Rose, 1980), oral (Clark et al.,
1966; Bainova, 1969a; Kimbrough, 1974; Tsutsui et al., 1976;
Dikshith et al., 1979), dermal (Howe & Wright, 1965; Bainova,
1969b; McElligott, 1972), or inhalatory (Gage, 1968b; Bainova,
1971; Makovskii, 1972; Seidenfeld et al., 1978) resulted in
irreversible changes in the lung.
Clark et al. (1966) reported that, in rats, in the earlier
stages after a single toxic oral dose of paraquat, breathing was
gasping or deep and fast, but some days after a single or repeated
toxic doses, the respiration became increasingly laboured, and the
hairs around the mouth and nares were soiled with a brownish
liquid. The extensive alveolar oedema observed in severe
intoxication was responsible for the development of hypoxia,
cyanosis, and dyspnoea. The progressive development of pulmonary
fibrosis was accompanied by difficulty in breathing, gasping, and
hyperpnoea (Smith et al., 1973).
Exposure of rats to high concentrations of respirable paraquat
aerosols was accompanied by shallow respiration. Within 2 - 3 h,
the test animals became dyspnoeic, cyanotic, and inactive, and
there were signs of local eye and nose irritation (Gage, 1968a).
7.1.1.1. Pathomorphological lung studies
Macroscopic examination of the lungs revealed that lesions and
their severity were dependent on the dose of paraquat and the time
between exposure and sacrifice (or death). The wet weight of the
lung increased after a single treatment, owing to oedema and
haemorrhage. The pathogenesis of the paraquat lung lesion has been
well characterized, and has been reviewed by Smith & Heath (1976).
The acute pulmonary toxicity of paraquat in animals has been
described as occurring in two phases (Smith & Heath, 1976). In the
initial "destructive" phase, alveolar epithelial cells were
extensively damaged and their subsequent disintegration often
resulted in a completely denuded alveolar basement membrane.
Table 11. Effects on experimental animals of repeated oral, dermal, or inhalation exposure to paraquat
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rat diet - 125 mg/kg 2 years no toxic effects Howe & Wright (1965)
Dog diet - 50 mg/kg no toxic effects
Rat diet - 0.25 mg/kg 27 days death; histological changes in the lung Clark et al. (1966)
Rat diet - 300, 400, 90 days cumulative toxic effects; chronicity Kimbrough & Gaines
500, 600, 700 mg/kg factor (Hayes) 5.2; histological (1970)
changes in the lung
Rat oral - 4, 9, 25 30 days inhibition of ChE activity, increasing Bainova (1969, 1975)
mg/kg body weight GPT activity in the serum; biochemical
per day and histological changes in the lung,
kidney, liver
Rat oral - 1.3, 2.6 4 1/2 months increased GPT and G-6-P-isomease Bainova (1969, 1975)
mg/kg body weight activities in the serum; biochemical
per day and histological changes in lung,
kidney, liver
Rat oral - 3.3, 1.3, 1 year the higher doses were toxic for both Makovskii (1972)
0.13 mg/kg body species tested; no-observed-adverse-
weight per day effect levels:
Guinea- oral - 1.0, 0.4, for rat 0.13, guinea-pig 0.04 mg/kg
pig 0.04 mg/kg body body weight per day
weight per day
Rat diet - 20 - 30 30 days histological and electron-optical lung Kimbrough (1974)
mg/kg body weight changes
per day
Mouse diet - 25, 50, 70 80 weeks death; dose-dependent clinical and FAO/WHO (1973)
mg/kg histological changes in the lung,
liver, kidney, and other organs tested
Rat oral - 25, 50, 1 - 5 days body weight loss; increased serum LDH, Tsutsui et al.
100 mg/kg body GOT activity; no haematological (1976)
weight per day changes; histological changes in the
lung, kidney, liver, myocardium
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rat drinking-water - 2 years mortality increased; histological Bainova & Vulcheva
1.3, 2.6 mg/litre changes in the lung, but only minimal (1977)
at the lowest level
Rabbit dermal - 2.8, 4.5, 20 days skin irritation; mortality and toxic Clark et al. (1966)
7, 14 mg/kg body effects at 7 & 14 mg/kg/day. LD50
weight per day mg/kg/day; no-observed-adverse-effect
level 2.8 mg/kg/day
Rat dermal - 2, 5, 15, 21 days skin irritation; mortality and toxic Bainova (1969a)
30, 45 mg/kg body effects at 5 - 45 mg/kg/day;
weight per day histological changes in the lung,
kidney, liver, myocardium; LD50
15 mg/kg/day; no-observed-adverse-
effect level 2 mg/kg/day
Rabbit dermal - from 1.56 20 days skin irritation; mortality and toxic McElligott (1972)
- 50 mg/kg per day effects at 3.13 - 192 mg/kg/day; LD50
(with occlusion) 4.5 mg/kg/day with occlusion and 24
from 2.4 - 192 mg/kg mg/kg/day without occlusion; No-
body weight per day observed-adverse-effect levels: 1.56
(without occlusion) and 2.4 mg/kg/day with and without
occlusion
Rat inhalationa - 4 days at higher concentrations (0.40 & 0.75 Gage (1968)
0.75 mg/m3 mg/m3) histological changes in the
0.4, 0.1, 0.06 mg/m3 15 days lung; no-observed-adverse-effect levels
0.003 mg/m3 60 days from 0.003 - 0.06 mg/m3 6 h daily;
6 h daily TLV - 0.1 mg/m3 paraquat aerosol
Rat inhalationa - 4 1/2 months biochemical, histochemical, and Bainova et al.
1.1, 0.05 mg/m3 histological changes in the lung at (1972)
6 h daily 1.1 mg/m3 no-observed-adverse-effect
level below 0.05 mg/m3 paraquat aerosol
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Effects obtained Reference
---------------------------------------------------------------------------------------------------------
Rabbit inhalationa - 3 months no clinical, functional and Seidenfeld et al.
10 mg paraquat in histological changes in the lung; no (1978)
100 ml water for the toxic effects
aerosol 2 h daily
---------------------------------------------------------------------------------------------------------
a Respirable paraquat aerosol.
Pulmonary oedema was also a characteristic of the destructive
phase, and was frequently of sufficient severity to result in the
death of the animals. Animals surviving the initial destructive
phase, which occurred in the first 1 - 4 days after acute paraquat
overexposure, progressed to what has been termed the
"proliferative" phase. In this phase, the lung was infiltrated
with profibroblastic cells that rapidly differentiated into
fibroblasts which, in some cases, progressed to fibrosis. The
histopathological outcome of the second phase may be influenced by
the treatment regimen, however. Administration of repeated low
doses of paraquat, which less severely damaged the alveolar
epithelial cells, could also induce a hyperplasia of the Type II
cells. This response may represent an attempt by the lung to
repair the damaged epithelium.
Following a single high dose of paraquat to animals, the
earliest ultrastructural changes were observed in the Type I
alveolar epithelial cells, approximately 4 - 6 h after treatment,
and were usually characterized by cellular and mitochondrial
swelling, increased numbers of mitochondria, and the appearance of
dark granules in the cytoplasm. When a high dose (approximately
LD50 or greater) was given, the lesions in the Type I cells often
progressed to the point of complete cellular disintegration leaving
areas of exposed basement membrane (Kimbrough & Gaines, 1970; Smith
et al., 1973; Smith & Heath, 1974; Vijeyaratnam & Corrin, 1971;
Klika et al., 1980).
In contrast to the effects on Type I pneumocytes, however, the
capillary endothelial cells were remarkably resistant to the toxic
effects of paraquat (Sykes et al., 1977).
Ultrastructural lesions in the alveolar Type II pneumocytes
were also observed shortly after single dose paraquat exposure,
although, generally, these lesions were not apparent until after
the first lesions were seen in the Type I cells (Kimbrough &
Gaines, 1970). Swollen mitochondria and damage to the lamellar
bodies usually occurred between 8 and 24 h after a high dose of
paraquat (Robertson, 1973; Robertson et al., 1976). Progressive
deterioration of the Type II cells continued, resulting in
completely denuded alveolar basement membranes and debris-filled
alveolar spaces (Vijeyaratnam & Corrin, 1971). Infiltration and
proliferation of fibroblasts may produce fibrosis that obliterates
the alveolar structure (Smith & Heath 1974).
Vijeyaratnam & Corrin (1971) observed that less severely
affected parts of the lung appeared to undergo epithelial
regeneration, 7 - 14 days after a single dose of paraquat.
Electron microscopic examination revealed the alveoli to be
lined with cuboidal epithelial cells that closely resembled
Type II pneumocytes except for a general lack of lamellar bodies.
Similar phenomena have also been noted by other investigators who
administered paraquat in the diet (Kimbrough & Linder, 1973) or as
repetitive intraperitoneal administrations (Smith et al., 1974).
Thus, in animals where the paraquat dose was sufficient to kill
only the Type I pneumocytes, the surviving Type II cells repaired
the damaged epithelium by proliferating and subsequently
differentiating into Type I epithelial cells. Inhaled paraquat in
aerosol produced initial necrosis and sloughing of the epithelia
and type 2 pneumocyte hyperplasia, fibroblast proliferation, and
increased synthesis of collagen in mice (Popenoe, 1979).
Histochemical alterations have been noted in rats exposed
through inhalation to 1.9 and 1.1 mg/m3 paraquat respirable
aerosol, 6 h/day, 6 days/week, for 4 1/2 months. The histoenzyme
activity of NAD lactate dehydrogenase-diaphorase, beta-
glucuronidase (EC 3.2.1.31), and acid phosphatase (EC 3.1.3.2) was
enhanced in the epithelial cells and in areas of pneumonitis
(Bainova et al., 1972). The changes were concentration-related,
although the activity of succinate dehydrogenase (EC 1.3.99.1) and
aspartate esterase appeared to be less pronounced in comparison
with the controls (Bainova et al., 1972).
7.1.1.2. Species differences in lung injury
Butler & Kleinerman (1971) injected rabbits intraperitoneally
with total doses of from 2 - 100 mg/kg body weight. Thymus atrophy
was observed, but most lungs showed only occasional and small
histological deviations that were poorly correlated with the
clinical signs of paraquat intoxication. The study confirmed the
resistance of the rabbit to paraquat-induced lung lesions (Clark et
al., 1966), and no evidence of any kind of pulmonary disease was
found; nor could significant lung injury be established in rabbits
after 30 days ingestion of 11 mg paraquat/kg in distilled water
(Dikshith et al., 1979). However, some animals showed pulmonary
fibrosis and emphysema, and a few changes were present in all
parenchymatous organs (Mehani, 1972; Zavale & Rhodes, 1978;
Dikshith et al., 1979). The rabbit also proved to be less
sensitive, than the rat, after inhalation exposure (Gage 1968a;
Seidenfeld et al., 1978).
According to Murray & Gibson (1972), and Hundsdorfer & Rose
(1980), guinea-pigs treated orally or sc did not develop the same
type of progressive pulmonary fibrosis as paraquat-intoxicated
rats. In hamsters, a single administration did not induce lung
damage, but prolonged exposure resulted in lung fibrosis (Butler,
1975).
In conclusion, for lung toxicity studies, a characteristic
dose-related pulmonary fibrosis can be induced in the rat, mouse,
dog, and monkey (Murray & Gibson, 1972) but not in the rabbit,
guinea-pig, or hamster.
7.1.1.3. Lung function studies
Rabbits exposed to an aerosol of 200 mg paraquat in 100 ml
distilled water (Seidenfeld et al., 1978) survived more than 3
exposures but showed significantly reduced arterial oxygen tension
and an increased alveolar arterial O2 gradient; specific compliance
decreased and functional residual capacity and breathing frequency
increased. Lam et al. (1980) administered paraquat at 27 mg/kg
body weight ip to rats and 0.5 mg/kg body weight intratracheally.
After 12 h, decreases were observed in total lung capacity,
functional residual capacity, vital capacity, residual volume, and
alveolar volume. These deviations persisted for 72 h. Oral
administration of paraquat at doses ranging from 1 mg/kg body
weight - 13.5 mg/kg body weight to rats resulted in functional lung
changes after 24 h.
Thus clinical, functional, and pathomorphological studies after
single and repeated exposure demonstrated that the spectrum of
paraquat lung disease depended on the magnitude of the dose and the
manner of administration (Seidenfeld et al., 1978; Restuccia et
al., 1974).
7.1.2. Renal system
In paraquat toxicity, kidney damage often precedes signs of
respiratory distress (Clark et al., 1966; Butler & Kleinerman,
1971; Murray & Gibson, 1972) (Table 11). Paraquat is excreted
mainly via the urine and the concentrations of the herbicide in the
kidneys are relatively high (section 6.1). Gross pathological and
histological examinations of paraquat-poisoned rats, guinea-pigs,
rabbits, and dogs revealed vacuolation of the convoluted renal
tubules and proximal tubular necrosis (Bainova, 1969a; Murray &
Gibson, 1972; Tsutsui et al., 1976). The degeneration of the
proximal tubular cells has also been confirmed by electron-optical
studies (Fowler & Brooks, 1971; Marek et al., 1981).
Paraquat is actively secreted by the kidney base transport
system. The nephrotoxicity caused by paraquat is pronounced and
appears to be restricted to the proximal nephron (Ecker et al.,
1975: Gibson & Cagen, 1977; Lock & Ishmael, 1979; Purser & Rose,
1979).
7.1.3. Gastrointestinal tract and liver
The clinical signs of acute and chronic oral poisoning
(Kimbrough & Gaines, 1970; Murray & Gibson, 1972; Bainova, 1969a)
or of ip injection (Butler & Kleinerman, 1971) include transient
diarrhoea and body weight loss, decreased food intake, and
dehydration. Some of the animals vomited soon after paraquat
administration. Residual skin contamination after dermal toxicity
studies on rabbits (McElligott, 1972) caused severe tongue
ulceration and an unwillingness to eat. The adverse irritant
effects were minimized by continued restraint after skin
decontamination of the treated rabbits.
There have been several reports of liver damage following
exposure to high doses of paraquat (Clark et al., 1966; Bainova,
1969a; Murray & Gibson, 1972; Tsutsui et al., 1976; Gibson & Cagen,
1977, Cagen et al., 1976). Centrilobular necrosis of hepatocytes
with proliferation of the Kupfer cells and bile canals have been
described.
In general, liver damage in experimental animals has not been
severe compared with lung and kidney damage. Serum enzyme
activities (SGOT, SGPT, LAP) only increased when large amounts of
paraquat were given (Giri et al., 1979).
7.1.4. Skin and eyes
The herbicide can provoke local irritation of the skin and
eyes. Clark et al. (1966) found skin irritation in rabbits only
when paraquat was applied beneath occlusive dressings in aqueous
solutions (total dose 1.56, 5.0, and 6.25 mg ion/kg body weight).
In mice and rats, the application of 5 - 20 g paraquat/litre
solutions in single and 21-day repeated dermal toxicity tests
provoked dose-related toxic dermatitis with erythema, oedema,
desquamation, and necrosis (Bainova, 1969b). Doses from 1.56 to 50
mg/kg, in repeated 20-day studies using the occlusive technique
(McElligott, 1972) resulted in local erythema and scab formation.
The histological changes consisted of parakeratosis and occasional
intra-epidermal pustules. A delayed skin irritant action of the
herbicide was reported by Fodri et al. (1977) in guinea-pig
studies.
No skin sensitization was observed in studies on guinea-pigs
when paraquat was applied (Bainova, 1969b; Fodri et al., 1977).
The instillation of dilutions of paraquat (up to 500 g/litre)
in rabbit eye induced inflammation within 24 h and this continued
for 96 h (Clark et al., 1966). Sinow & Wei (1973) introduced 62.5,
125, 250, 500, and 1000 paraquat/litre into the rabbit eye.
Concentrations of 62.5 and 125 g/litre caused severe conjunctival
reactions; higher levels (250 - 500 g/litre) provoked iritis and
pannus, while at the 500 g/litre concentration there was corneal
opacification, iritis, and conjunctivitis. All rabbits receiving
0.2 ml of paraquat at 1000 g/litre in 1 eye or 0.2 ml of a
concentration of 500 g/litre in both eyes died within 6 days of
application (Sinow & Wei, 1973).
Both conjunctival and dermal application of different
concentrations induced systemic toxicity (Sinow & Wei, 1973; Clark
et al., 1966; Bainova, 1969b; Kimbrough & Gaines, 1970; Makovskii,
1972; McElligott, 1972), lung, kidney, and liver damage, and death.
7.1.5. Other systems
No specific functional, histological, or biochemical effects of
paraquat have been reported in other systems that have been
examined; this is of prime importance in an evaluation of its
toxicity. When lethal doses of paraquat are given to rats,
symptoms consistent with neurological disturbances have been
observed. These include decreased motor activity, lack of
coordination, ataxia and dragging of the hind limbs (Smith et al.,
1973). Also associated with near lethal or lethal doses are damage
to the myocardium (Tsutsui et al., 1974), haemolytic anaemia
(Bainova, 1969a), increased haemosiderin in the spleen (Bainova et
al., 1972) and increased concentrations of plasma corticosteroids
(Rose et al., 1974b).
7.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
7.1.6.1. Effects on reproduction
Some histological changes in the testes have been reported in a
few paraquat toxicity studies. Butler & Kleinerman (1971) found
multinuclear giant cells in rabbit testicular tubules. When
paraquat was orally administered at 4 mg/kg body weight to male
rats for 60 days and the testes were examined, there were no
significant deviations in the spermatozoa count or motility, nor
were there any biochemical changes in the several enzymes of testes
homogenates. The histoenzyme activity of lactate dehydrogenase,
succinate dehydrogenase, DPN-diaphorase, alkaline phosphatase, and
acid phosphatase in the treated animals did not differ from that of
the controls, nor did quantitative and qualitative histological
examination of the testicular tubule cells reveal any abnormality.
A 3-generation reproduction study has been carried out on rats
treated with paraquat ion at 100 mg/kg diet (FAO/WHO, 1973). There
were no significant abnormalities in fertility, fecundity, and
neonatal morbidity or mortality, nor were there any signs of
gonadotoxicity or structural or functional lesions. Pulmonary
function in the treated offspring was normal.
Clegg (1979) has reviewed animal reproduction and
carcinogenicity studies conducted in relation to the safe use of
pesticides.
7.1.6.2. Embryotoxicity and teratogenicity
Oral or ip administration of high doses of paraquat to mice and
rats on various days of gestation produced significant maternal
toxicity, evidenced by increased mortality rates (Bainova &
Vulcheva, 1974; Bus et al., 1975). Examination of the fetuses from
the higher-dose groups revealed a reduction in fetal body weights,
delayed ossification of the sternabrae, and increased resorption
rate in mice, as a result of the maternal intoxication. The
minimal embryotoxic effect seemed due in part to difficulty in
crossing the placenta, reflected by low concentrations of paraquat
in the embryo relative to maternal tissues (Bus et al., 1975). The
absence of a specific embryotoxic action of paraquat has also been
observed and reported in other studies on rats (Khera et al., 1968;
Luty et al., 1978), mice (Selypes et al., 1980), and rabbits
(FAO/WHO, 1973).
In a perinatal toxicity study, Bus & Gibson (1975) administered
paraquat at 50 or 100 mg/litre in the drinking-water to pregnant
mice beginning on day 8 of gestation, with continued treatment of
the litters up to 42 days after birth. Paraquat treatment did not
alter postnatal growth rate, although the mortality rate in the 100
mg/litre-treated mice increased to 33% during the first 7 days
after birth. It was also noted that paraquat at 100 mg/litre
significantly increased the sensitivity of the pups to oxygen
toxicity on days 1, 28, and 42 after birth.
7.1.7. Mutagenicity
Paraquat has been found to have minimal to no genotoxic
activity when evaluated in a variety of in vitro and in vivo test
systems. In studies producing weakly positive results (Moody &
Hassan, 1982; Parry 1977, 1973; Tweats, 1975; Benigni et al., 1979;
Bignami & Grebelli, 1979), which were limited to in vitro studies,
paraquat genotoxicity was accompanied by high cytotoxicity. These
results are best explained by Moody & Hassan (1982), who showed
that the mutagenicity of paraquat in bacterial test systems
( Salmonella typhimurium TA 98 and TA 100) was mediated by the
formation of superoxide. However, other investigators (Andersen et
al., 1972; Levin et al., 1982) did not find mutagenic activity in
bacterial test systems. Furthermore, paraquat was not mutagenic
when evaluated in human leukocytes and in in vivo cytogenetic
tests on mouse bone marrow (Selypes & Paldy, 1978) and dominant
lethal tests on mice (Pasi et al., 1974; Anderson et al., 1976).
7.1.8. Carcinogenicity
A carcinogenicity study was performed on mice at dietary levels
of 25, 50, and 75 mg/kg per day for 80 weeks (FAO/WHO, 1973).
There were reduced weight gains among the animals receiving
paraquat, but deaths during the study were associated with
respiratory disease. Clinical and histopathological examination
determined that paraquat was not tumorigenic in mice.
A 2-year exposure of rats to 1.3 and 2.6 mg/litre, daily, in
the drinking-water provoked histopathological changes in the lung,
liver, kidney, and myocardium. The lung lesions were dose-related;
inflammation, atelectasis, reactive proliferation of the
epithelium, pulmonary fibrosis, and pulmonary adenomatosis were
noted, but no sign of tumour growth or atypism (Bainova & Vulcheva,
1977). Nor was any increased tumour incidence reported in rats in
a 2-year study with a maximum dietary level of 250 mg/kg diet (12.5
mg/kg body weight per day) (FAO/WHO, 1971).
Bainova & Vulcheva (1977) did not discover any indication of
tumorigenicity in a 2-year study on rats receiving paraquat at 1.3
or 2.6 mg/litre in their drinking-water (Table 11).
While testing the carcinogenicity of urethane in mice, Bojan et
al. (1978) also attempted to evaluate the influence of paraquat on
urethane-induced lung tumorigenesis. It is felt that the results
of this study are not of relevance for the assessment of the
carcinogenic potential of paraquat.
7.2. Effects on Farm Animals
The effects of paraquat on farm animals has been discussed in
section 4.3.5. The LD50 doses have been established for hen,
turkey, cow, and sheep (Howe & Wright, 1965; Clark et al., 1966;
Smalley, 1973). Massive doses resulted in convulsions,
neurological symptoms, and death due to respiratory failure.
Domestic animals may ingest paraquat by feeding on a sprayed
area, as a result of spray drifting on to their pasture, by
drinking water contaminated with paraquat used as an aquatic
herbicide, or by feeding on a crop sprayed with paraquat as a
dessicant. Sheep and calves were given paraquat at concentrations
of up to 20 mg/litre drinking-water for 1 month without any obvious
ill effects (Howe & Wright, 1965; Calderbank, 1972), and a cow
dosed with 2/3 of the LD50 of 14C-paraquat gave milk containing
less than 0.1 mg/litre. Field tests demonstrated that cattle did
not suffer any toxic effects when turned loose on pasture after it
had been sprayed with paraquat at 0.45 kg/ha. The same trial
showed that horses had local lesions of the mouth and increased
mucous secretion after grazing on newly-sprayed pasture (Calderbank
et al., 1968). The hazard to stock feeding on such pasture depends
on the density of the pasture, the dose of the herbicide, and the
length of time that has elapsed since its application.
Paraquat was fed to cattle at levels in herbage of 200 - 400
mg/kg for 1 month without any apparent ill effects, and no residues
could be detected in the meat and milk (Calderbank et al., 1968).
However, all domestic animals should be kept far from freshly-
sprayed areas, and when crops are treated with paraquat, due
attention should be paid to the accepted maximum residue limits.
7.3. Dose-Effect of Paraquat
The acute LD50 values for paraquat in various species are given
in Tables 12 and 13. The acute toxicity studies of paraquat salts
(dichloride, dimethylsulfate, dimethylphosphate) have not shown
any significant differences in the acute oral and ip LD50 in rats
(Clark et al., 1966; Makovskii, 1972).
There were no significant differences in the oral LD50 values
obtained for the same species from different laboratories, but the
acute oral LD50 values among the species examined varied.
The effects of repeated paraquat exposure are summarized in
Table 11. Paraquat was administered, orally and in the diet, to
rats, mice, guinea-pigs, and dogs. The guinea-pigs appeared to be
very sensitive (Makovskii, 1972). According to Kimbrough & Gaines
(1970), Makovskii (1972), and Bainova (1975), the herbicide has a
moderate cumulative toxicity. The joint FAO/WHO meeting (1976)
decided on a no-observed-adverse-effect level of 1.5 mg/kg body
weight per day in the rat and 1.25 mg/kg body weight per day in the
dog. As can be seen from Table 11, effects at lower levels have
been observed in other studies.
Guinea-pigs, monkeys, cattle, and human subjects are more
sensitive, while rats and birds are less sensitive to paraquat
through the gastrointestinal route.
Table 12. Paraquat LD50 (mg/kg body weight) and LC50
(mg/m3) in various species
-------------------------------------------------------------------
Species/Sex Oral Dermal Inhalation LC50
LD50 LD50 respirable
paraquat aerosol
-------------------------------------------------------------------
Rat 200a 1c
Rat (F) 100e 90e 10f
Rat (M) 110e 80e 10f
Rat 126i 350g 6g
Mouse 62d
Rabbit 500a
Rabbit 236b
Rabbit 240h
Guinea-pig 40 - 80a
Guinea-pig (M) 30b
Guinea-pig 22i
Guinea-pig 42g 319g 4g
Monkey 50i
Cat 40 - 50a
Cat (F) 35b
Hen 300 - 380a
Hen 262b
Turkey 250 - 280j approximately
375j
Cow 50 - 75a
Sheep 50 - 75a
-------------------------------------------------------------------
a Howe & Wright (1965).
b Clark et al. (1966).
c Gage (1968).
d Bainova (1971).
e Kimbrough & Gaines (1970).
f Bainova & Vulcheva (1972).
g Makovskii (1972).
h McElligott (1972).
i Murray & Gibson (1972).
j Smalley (1973).
Table 13. Paraquat LD50 (mg/kg body weight) after parenteral
treatment
-------------------------------------------------------------------
Species/Sex Subcutaneous Intraperitoneal Intravenous
-------------------------------------------------------------------
Rat (F) 19a
Rat 22b
Mouse 30e 50d
Guinea-pig (F) 3a
Guinea-pig 5b
Turkey 100c 20c
-------------------------------------------------------------------
a Clark et al. (1966). d Ecker et al. (1975).
b Makovskii (1972). e Bus et al. (1975).
c Smalley (1973).
7.4. Methods for Decreasing Paraquat Toxicity
These have been studied in connection with requirements in the
case of paraquat poisoning in man. Clark (1971) showed the
efficacy of Bentonite and Fuller's earth in binding orally
administered paraquat and preventing its absorption from the
gastrointestinal tract. Staiff et al. (1973) reported the high
adsorption capacity of Amerlite. Smith et al. (1974) found
considerably reduced plasma-paraquat levels after the combined
treatment of rats with purgatives and bentonite suspension; these
rats survived a dose that normally killed 90 - 100% of the animals.
The absorption capacities of six absorbent materials were tested by
Okonek et al. (1982) who demonstrated that activated charcoal was
the most successful in absorbing ingested paraquat in rats.
Another way of decreasing paraquat absorption is to introduce
an emetic in the concentrated formulations. Kawai et al. (1980)
examined the protection this provided in fasting and non-fasting
male and female dogs that were given paraquat containing an emetic.
The amount of paraquat eliminated by vomiting was 61 - 86% of the
orally-administered dose. In the group given paraquat only, the
blood level averaged 44 mg/litre; in the group given paraquat and
emetic, it was 0.26 mg/litre.
7.5. Relation Between Age, Sex, and Toxicity
There is no evidence that paraquat is more toxic to either sex
of adult experimental animals (section 7.3) Young rats were more
resistant than older rats, and some authors have paralleled this
resistance with that of young rats to oxygen toxicity. Smith &
Rose (1977b) found a more than 40% increase in cumulative mortality
in 180 g rats compared with 50 g rats, after oral dosing with
paraquat at 680 µmol/kg body weight. According to Smith & Rose
(1977b), the difference in renal function between young and mature
rats accounted for the difference in paraquat toxicity.
8. EFFECTS ON MAN
8.1. Accidental and Suicidal Poisoning
8.1.1. Case reports
The first fatalities from acute paraquat poisoning occurred in
1964 and were reported in 1966 (Bullivant, 1966). By 1977, 600
deaths had been reported following accidental or intentional
ingestion of paraquat. The number of accidental cases of poisoning
is small relative to instances of suicide. Because of different
requirements or practices for notification or reporting of cases of
poisoning in the many countries in which paraquat is used, the
magnitude of the problem is difficult, if not impossible, to
determine. Some representative reports on acute paraquat poisoning
are summarised in Table 14.
The earlier cases of paraquat intoxication were mostly
accidental (Fennelly et al., 1968; Matthew et al., 1968; Masterson
& Roche, 1970; Malone et al., 1971). These cases seemed to have
resulted mainly from the habit of decanting the liquid formulations
into small unmarked or incorrectly labelled containers such as
beer, wine, or soft-drink bottles.
An increased ratio of suicidal to accidental poisoning has been
noted in recent years (Fletcher, 1975; Carson & Carson, 1976;
Fitzgerald et al., 1978a; Bramley & Hart, 1983). This change from
accidental to suicidal poisoning was also reflected in the enhanced
percentage of fatal cases, shorter survival times, and
significantly higher tissue and body fluid levels (Connolly et al.,
1975; McGeown, 1975; Park et al., 1975; Carson & Carson, 1976;
Howard, 1979a; Sugaya et al., 1980; Bismuth et al., 1982).
While the vast majority of poisoning cases are due to
swallowing, a small number of fatal cases of accidental paraquat
poisoning via the skin have been reported when liquid concentrates
(200 g/litre) have been applied in order to kill body lice (Ongom
et al., 1974; Binns, 1976). A few other fatal and non-fatal cases
have been reported following skin-contamination (McDonagh & Martin,
1970; Kimura et al., 1980).
Table 14. Case report data on accidental and suicidal acute paraquat poisoning
-------------------------------------------------------------------------------
Number of cases Fatal Non-fatal Fatality Reference
-------------------------------------------------------------------------------
19 12 7 63% Malone et al. (1971)
24 3 accidental 10 14 42% Connolly et al. (1975)
19 suicidal
2 homicidal
25 10 accidental 17 8 68% McGeown (1975)
31 7 accidental 18 13 58% Park et al. (1975)
21 suicidal
3 homicidal
33 7 accidental 26 7 79% Carson & Carson (1976)
19 suicidal
16 7 9 44% Wright et al. (1978)
136 77 suicidal 92 44 68% Fitzgerald et al. (1978)
10 10 0 100% Natori et al. (1979)
188 69 119 37% Higginbottom et al. (1979)
79 28 51 35% Proudfoot et al. (1979)
68 68 suicidal 41 27 66% Howard (1979)
6 6 suicidal 5 1 83% Sugaya et al. (1980)
28 12 suicidal 17 11 61% Bismuth et al. (1982)
262 95% deliberate 94 (36%) 168 (64%) 36% Bramley & Hart (1983)
intent
-------------------------------------------------------------------------------
8.1.2. Distribution of cases of paraquat poisoning
Cases of acute paraquat poisoning have been reported in:
Bulgaria (Mircev, 1976), Denmark (Pederson et al.,1981), England,
Ireland, Scotland, and the Netherlands (Fletcher, 1975), the
Federal Republic of Germany (Grundies et al., 1971; Hofman &
Frohberg, 1972; Fletcher, 1975; Fischer & Kahler, 1979), France
(Faure et al., 1973; Gervais et al., 1975; Bismuth et al., 1982;
Efthymiou, 1983), Hungary (Farago et al., 1981), Poland (Firlik,
1978), Switzerland (Schlatter, 1976), the USA (Kimbrough, 1974;
Dearden et al., 1978; Stephens et a1., 1981), and in Yugoslavia
(Vucinovic, 1978). Recently, a number of cases of paraquat
poisoning, mainly suicidal, have also been reported in Japan
(Takahashi et al., 1978; Natori et al., 1979; Tomura et al., 1979;
Kimura et al., 1980; Matsumoto et al., 1981). No attempt has been
made to make this list exhaustive, in fact the distribution is
worldwide.
8.1.3. Route of entry
By far the most frequent route of poisoning has been ingestion.
An unusual case of subcutaneous injection of 1 ml paraquat by a
mentally disturbed farmer was reported in Israel (Almog & Tal,
1967). Cases of dermal poisoning have been mentioned in section
8.1.1. There is no evidence of fatal poisoning as a result of
inhalation.
8.1.4. Formulations
Paraquat trade names are listed in Table 3. Concentrated
liquid formulations have been responsible for most (and more
severe) poisonings than granular forms, which contain less paraquat
(McGeown, 1975; Park et al., 1975; Fitzgerald & Barnville, 1978;
Wright et al., 1978; Higginbottom et al., 1979; Howard, 1979a).
8.1.5. Dose
The minimum lethal dose of paraquat is stated to be about 35
mg/kg body weight for human beings (Pederson et al., 1981; Bismuth
et al., 1982).
Symptoms of poisoning depend on the dose absorbed. It is
difficult to estimate the dose absorbed from case histories since
in many cases the patients spat out part of the paraquat
concentrate or vomited profusely after swallowing the herbicide.
Some patients have survived after apparently ingesting 50 - 100 ml
Gramoxone(R) (10 - 20 g paraquat), whereas some died after taking
as little as 2 sachets of Weedol (2.5 g paraquat) (Table 15).
Howard (1979) demonstrated the relationship between the dose of
paraquat ingested, the time elapsing between ingestion and
institution of treatment, and the ultimate outcome in 68 cases of
intentional paraquat poisoning.
8.1.6. Clinical and pathomorphological data relating to fatal
paraquat poisoning
Cases of fatal poisoning can be sub-divided into cases of:
(a) acute fulminant poisoning from a massive dose leading
to generalized systemic poisoning and death from a
combination of acute pulmonary oedema, oliguria,
hepatocellular and adrenal failure and biochemical
disturbances (death usually occurs within 1 - 4 days);
(b) less overwhelming poisoning with slower onset of
organ failure and death from pulmonary oedema,
mediastinitis, and complications of therapy (McGeown,
1975; Fitzgerald et al., 1978a); and
(c) late pulmonary fibrosis (death ensuing 4 days to
several weeks later).
Table 15. Recovery from paraquat poisoning involving lung dysfunction
---------------------------------------------------------------------------------------------------------
Dose of paraquat Major organ Notes Reference
ingested damage
---------------------------------------------------------------------------------------------------------
50 ml kidney, liver, vomiting; pains in stomach; changes in urine Grundies et al.
lung and serum (1971)
15 ml approx. lung nausea; buccal lesions; chest X-ray: poor Lloyd (1969)
aeration at lung bases
10 ml approx. kidney, lung oliguria; changed renal function; basal rales; Fisher et al.
chest X-ray: small bilateral, pleural effusions; (1971)
limited atelectasis; functional lung change
not specified kidney, liver, oliguria; serum, and urine changes; minimal Fennelly et al.
lung deviations in the respiratory function (1971)
30 ml approx. kidney, liver nausea; oliguria; ECG changes; chest X-ray: Galloway &
myocardium, lung increased vascular markings Petrie (1972)
granular kidney, liver, 14 cases with mild oral, renal, lung, and liver Fitzgerald &
paraquat lung impairment Barniville
(1978)
50 ml kidney, liver, vomiting; diarrhoea; abdominal pain; serum and urine Rose (1980)
lung changes; dyspnoea, decreased forced vital capacity;
chest X-ray: extensive perivascular changes
---------------------------------------------------------------------------------------------------------
8.1.6.1. Respiratory system
(a) Clinical data
Soon after ingestion, there is oropharyngeal pain and swelling,
followed within a few days by exudation, ulceration, and mucosal
sloughing, sometimes with pseudomembrane formation, which on
occasion leads to total sloughing of the oropharynx and oesophagus
(Malone et al., 1971). In severe poisoning, pulmonary oedema
rapidly ensues with clinical and functional deterioration until
death. Less intense, but ultimately fatal, poisoning causes
progressive pulmonary fibrosis over days or several weeks, with
gradually increasing dyspnoea and hypoxaemic pulmonary failure.
Pulmonary oedema may occur from fluid overload in oliguric
patients. Mediasteinitis and pneumothorax are occasionally seen
(Dearden et al., 1978; Kimura et al., 1980).
Pulmonary function tests reflect the underlying pathology, with
hypoxaemia, reduction in lung volume, high alveolar-arterial
gradient, and impaired gas transfer (Cooke et al., 1973,
Higginbottom et al., 1979). Chest radiographs may show bilateral
pulmonary oedema, coalescing consolidations, and later, sequential
changes of pulmonary fibrosis (Davidson & McPherson, 1972).
(b) Pathology
At autopsy, the lungs do not collapse properly and the pleural
cavity contains a small amount of fluid. In cases of lung
fibrosis, the lungs are heavy, firm, dark purple, and rubbery.
Consolidation and decreased aeration are found predominantly at the
bases. Emphysema and atelectasis are often found.
Histological studies following lung biopsy and necropsy show
pulmonary oedema, haemorrhages, and atelectasis due to pulmonary
infiltrates, loss of alveolar epithelial cells and, at a later
stage, interstitial and intra-alveolar fibrosis (Smith & Heath,
1976).
During the first 7 days of paraquat poisoning in man, loss of
alveolar epithelial cells has been seen with alterations in, or
detachment of, the type I and II cells, proliferation of
fibroblasts and polymorphous cells, loss of surfactant secretion,
and thickening of the alveolar septa by interstitial fibrosis
(Toner et al., 1970). The later findings (2 - 3 weeks) involved
pulmonary fibrosis and endothelial abnormalities. Dearden et al.
(1978) reviewed the histological and electron-microscopic findings
in human lungs. Capillary permeability seemed to be enhanced
either by vesicles forming transendothelial channels or by
disruption of endothelial cells.
8.1.6.2. Renal system
Acute oliguric renal failure is common in severely poisoned
patients. Less severe manifestations include impaired renal
function, which may disappear before the pulmonary fibrosis
progresses (Beebeejaun et al., 1971; Fisher et al., 1971; Fletcher,
1975; Natori et al., 1979; Grant et al., 1980). Other
manifestations include proteinuria, with hyaline casts, white and
red blood cells. Tubular damage is reflected in glycosuria,
aminoaciduria, and excessive leaking of phosphorus, sodium, and
uric acid (Vaziri et al., 1979).
Soft, pale, swollen kidneys with extensive tubular necrosis,
compatible with toxic injury, are found at necropsy (Beebeejaun et
al., 1971). Sometimes necrosis of the proximal tubules is found
together with extreme dilatation of the distal tubules of the
kidney (Shuzui, 1980).
8.1.6.3. Gastrointestinal system, the liver, and the pancreas
The initial symptoms after oral ingestion of paraquat are
nausea, vomiting, upper abdominal pain, and diarrhoea. Perforation
of the oesophagus is uncommon (Ackrill et al., 1978; Natori et al.
1979).
The ingestion of large doses of paraquat has resulted in severe
liver damage (Ward et al., 1976; Grant et al., 1980) with
progressive metabolic acidosis (Shuzui, 1980; Sugaya et al., 1980).
Fatty degeneration of periportal hepatocytes and sporadic cellular
necrosis in the central region of the liver lobules have been
described (Matsumoto et al., 1980). Cholestasis and portal
inflammation may occur (Matsumoto et al., 1981). Oedematous
degeneration or necrosis of both the intra-hepatic and extra-
hepatic bile ducts, and of the gall bladder, have also been noted
(Mullick et al., 1981).
Takayama et al. (1978) noted stasis of the pancreatic duct,
with increased serum amylase levels after severe paraquat
poisoning.
8.1.6.4. Cardiovascular system
Occasionally, toxic myocarditis after paraquat ingestion has
been described (Bullivant, 1966; Malone et al., 1971; Copland et
al., 1974; Grant et al., 1980).
Takahashi et al. (1978) found fibrinoidal necrosis of the small
arteries in the pancreas, kidney, and liver on days 3 - 6 following
ingestion.
8.1.6.5. Central nervous system
The ingestion of very high doses of paraquat provoked anxiety,
convulsions, ataxia, and semi-consciousness (Grant et al., 1980;
Mukada et al., 1978). Haemorrhagic leukoencephalopathy was
present throughout the central nervous system, involving almost
exclusively the white matter. Focal haemorrhage and
demyelinization were present at various stages together with
haemorrhagic meningitis.
8.1.6.6. Adrenal glands
Adrenal cortical necrosis may contribute to death in severe
paraquat poisoning and the severity of the damage appears to be
dose-related (Nagy, 1970; McGeown, 1975; Fitzgerald et al., 1977a;
Takahashi et al., 1978).
8.1.6.7. Pregnancy
A woman, who accidentally swallowed paraquat in the 28th week
of pregnancy (Fennelly et al., 1968), died 20 days later. Gross
pathological examination did not reveal any abnormalities in the
fetal organs.
A woman, in the 7th month of pregnancy, intentionally ingested
about 60 ml of technical paraquat (Takeuchi et al., 1980) and
vomited approximately half that amount. Oliguria, jaundice, and
cough with sputum production progressed; fetal heartbeat
disappeared on the l3th day and the next day the dead fetus was
delivered. The mother died on the l7th day after poisoning. The
lungs of the dead fetus were filled with the debris of amniotic
fluid; the fetus had begun intra-uterine respiration to compensate
for the insufficient oxygen supply. No symptoms of paraquat
poisoning were noted in the body of the neonate.
A case report published by Musson & Porter (1982) concerning
paraquat ingestion by a 20-week pregnant woman, confirmed the lack
of teratogenic risk in human beings. The pregnancy was allowed to
continue after the treatment of the mother. The infant was
followed up to the age of 3 years and did well clinically, with
normal laboratory tests, development, and behaviour.
8.1.7. Recovery from paraquat poisoning
In the largest series reported (68 - 188 cases) (Fitzgerald et
al., 1978a; Higginbottom et al., 1979; Howard, 1979a; Proudfoot et
al., 1979), survival rates varied from 32% to 65% (Table 14)
Factors determining recovery from paraquat poisoning, reviewed by
Fletcher (1975), McGeown (1975), Fitzgerald & Barniville (1978),
Howard (1979a), and Bismuth et al. (1982), are shown in Table 16.
Victims of paraquat poisoning, who escape major pulmonary
complications, usually recover fully within a few weeks of
ingestion. Renal, gastrointestinal, and hepatic manifestations
return to normal (Fisher et al., 1971; Beebeejaun et al., 1971;
Grundies et al., 1971; Galloways & Petrie, 1972).
Minor pulmonary functional and radiographic abnormalities may
be transient and are of doubtful relationship to paraquat lung
injury. Some patients have recovered despite major pulmonary
abnormalities (Table 15). Among 5 survivors, Schlatter (1976)
reported no signs of lung residual disorders. Fitzgerald et al.,
(1979a) followed, for at least a year, 13 survivors of paraquat
poisoning to determine the prevalence of residual pulmonary
disability. Of 11 adults, 5 (all non-smokers) did not have any
clinical, radiological, or functional evidence of pulmonary
dysfunction; 4 others (all smokers) were considered normal on
clinical and chest X-ray examination, but had a mild deficit in
pulmonary function, while the remaining 2 adults were known to have
suffered from respiratory disability before the paraquat poisoning.
Only 1 patient showed new and persistent lung infiltrates that
could be ascribed to permanent paraquat lung damage. No
abnormalities were discovered in the 2 children studied.
Table 16. Factors determining recovery from paraquat poisoning
-------------------------------------------------------------------------
No. Factor Notes
-------------------------------------------------------------------------
1. Route of entry Most paraquat poisonings have occurred following
ingestion; ingestion following a meal usually has
less serious consequences; skin contamination
with liquid concentrate formulations is dangerous;
poisoning through inhalation is usually benign
2. Dose Dose rarely known, but usually, for survivors,
less than 6 g paraquat, often, spat out or vomited
after ingestion
3. Intention High mortality rates established in suicidal or
homicidal poisoning; many more survivors reported
reported among cases of accidental poisoning
4. Formulation High mortality rate registered after ingestion of
ingested liquid concentrates; survivors have more often
than not ingested dilute or granular formulations
5. Time of starting Treatment should start as soon as possible; delay
treatment of more than 2 - 5 h reduces chances of survival;
patients hospitalized several days after paraquat
ingestion have minimal chance of recovery
6. Decreased Occurs when there is vomiting, use of emetics
gastrointestinal stomach washout, application of adsorbents (such
absorption as Fuller's Earth or bentonite), single or
repeated, and forced diarrhoea; such treatment
should be as prompt as possible; a delay of more
than 5 h adversely affects the safe and effective
elimination of paraquat; care should be taken to
avoid complications (aspiration of Fuller's Earth,
oesophagal perforation)
7. Blood paraquat Fig. 6 (section 6.2.3) demonstrates importance of
concentrations paraquat plasma concentrations for prognosis
8. Urine paraquat Patients excreting more than 1 mg paraquat/h, 8 h
concentrations or more after ingestion, unlikely to recover
9. Renal function Patients with severe renal damage or renal failure
usually die
-------------------------------------------------------------------------
Table 16. (contd.)
-------------------------------------------------------------------------
No. Factor Notes
-------------------------------------------------------------------------
10. Forced diuresis Should not be instituted when renal damage with
oliguria present; caution needed during the first
24 h
11. Haemodialysis Important if forced diuresis cannot be carried out
-------------------------------------------------------------------------
8.2. Occupational Exposure
8.2.1. Epidemiological studies and case reports
8.2.1.1. Spraying personnel
Paraquat has been in agricultural use since the early 1960s and
several surveys have been conducted on spray operators (Swan, 1969;
Hearn & Kier, 1971; Makovskii, 1972; Staiff et al., 1975; Seiber &
Woodrow, 1981; Howard, 1979b, 1980, 1982; Chester & Ward, 1981;
Howard et al., 1981; Chester & Woollen, 1982; Wojeck et al., 1983).
Some of these studies were aimed at clinically evaluating possible
adverse effects, others at estimating inhalatory and dermal
exposure. Some of the latter studies have been summarised in Table
17 from which it can be seen that:
(a) the main route of exposure of agricultural workers to
paraquat is via the skin; respiratory exposure is
negligible.
(b) The worst case of exposure (of those examined) was
via knapsack spraying.
Table 17. Comparison of dermal and inhalation exposure resulting
from various methods of application
--------------------------------------------------------------------
Method of application Dermal exposure Respiratory exposure
(mg/h) (mg/h)
--------------------------------------------------------------------
Hand-held knapsacka 66 (0.45 - 1.3) x 10-3
(12.1 - 169.8)
Vehicle mountedb 0.4 0 - 2 x 10-3
(0.1 - 3.4)
Aerialc - a) Flagman 0.1 - 2.4 0 - 47 x 10-3
b) Pilot 0.5 - 0.1 0 - 0.6 x 10-3
c) Mixer/loader 0.18 1.3 - 1.5 x 10-3
--------------------------------------------------------------------
a From: Chester & Woolen (1982).
b From: Staiff et al. (1975).
c From: Chester & Ward (1981).
In Malaysian rubber plantations, exposure is likely to be
greater than in most other situations (Swan, 1969). Weed control
is required continuously for 10 months of the year, and the
herbicide is applied by knapsack sprayers during the entire working
day, 6 days a week. The high temperature and humidity together
with the light clothing of the sprayers increase the potential risk
of dermal exposure. In 1965, a study was carried out on a team of
6 sprayers, and in 1967 on 4 teams, to estimate the efficacy of
protective measures. The operators used spray dilutions containing
paraquat at 0.5 g/litre, for 12 weeks. Attention was paid to
personal hygiene. Each man was given a thorough physical
examination, and urine samples were taken before spraying began and
at weekly intervals throughout the study. Paraquat analyses were
carried out using the method of Calderbank & Yuen (1965). Chest
X-rays were taken before the study started and at the end of the
6th and 12th weeks.
In the course of the 2 studies, a total of 528 urine samples
were examined. Paraquat was found on 131 occasions, the maximum
concentration detected being 0.32 mg/litre in the first study and
0.15 mg/litre in the second. Average urine levels of paraquat of
0.04 mg/litre were found in the 1965 study, and of 0.006 mg/litre
in the 1967 study. After spraying ceased, these levels declined
steadily to become undetectable within a week - with one exception.
It was concluded that the workers were not subjected to hazardous
levels of paraquat.
Both trials showed that about half of the men had suffered mild
irritation of the skin and eyes, but had recovered rapidly with
treatment. Two cases of scrotal dermatitis occurred in workers
wearing trousers that were continuously soaked by the spray
solution. There were also 2 cases of epistaxis. All chest
radiographs were normal.
Studies over a period of several years on 296 workers were
performed by Hearn & Keir (1971) on a Trinidad sugar estate. This
survey drew attention to nail damage following gross contamination
with paraquat at 1 - 2 g/litre that ranged in severity from
localized discoloration to nail loss. The typical distribution of
the lesions - affecting the index, middle, and ring fingers of the
working hand - suggested that they had occurred through leakage
from the knapsack sprayer, and inadequate personal hygiene. Apart
from 2 cases of contact dermatitis of the hands, no skin, eye, or
nose irritation was reported, nor were there any systemic effects.
Similar data were obtained by Makovskii (1972), who examined
several groups of workers spraying paraquat as a herbicide and
dessicant in cotton fields during the hot season. These workers
were exposed to paraquat aerosol concentrations of 0.13 - 0.55
mg/m3 air. Dermal exposure was low, not more than 0.05 - 0.08 mg
paraquat on the hands and face. There were no complaints, nor did
the clinical and laboratory examinations of the workers demonstrate
any significant deviations from the matched control groups.
In the USA (Staiff et al., 1975), the exposure of field workers
operating tractor-mounted spray equipment in orchards was
determined. About 4.6 litre paraquat liquid concentrate (291
g/litre) was used in 935 litre water per h. In addition, exposures
from yard and garden applications were studied in volunteers using
pressurized hand dispensers containing paraquat solution (4.4
g/litre). Dermal contamination was measured by adsorbent cellulose
pads attached to the worker's body or clothing, and by hand-rinsing
in water in a polyethylene bag. Special filter pads were used in
the filter cartridges of the respirators worn by the subjects under
study.
In all, 230 dermal and respiratory exposure pads, 95 samples of
hand-rinse water, and 130 urine samples, collected during and
following the spray, were analysed. This involved 35 different
paraquat application situations. The exposure of field workers was
found to range from about 0.40 mg/h (dermal) to less than 0.001
mg/h (inhalation). As for individuals spraying the yard or garden,
exposure ranged from 0.29 mg/h (dermal) to less than 0.001 mg/h
(inhalation).
In almost all cases, dermal exposure affected the hands. The
respiratory paraquat values were generally below the sensitivity
level of the analytical method. No detectable paraquat
concentrations were found in the urine samples (lower limit 0.02
mg/litre). This study confirmed the general safety of paraquat
under correct conditions of use.
The potential long-term hazard associated with the use of
paraquat has also been studied. Howard et al. (1981) studied the
health of 27 spraymen who had been exposed to paraquat for many
months per year for an average of 5.3 years, and compared them with
two unexposed control groups consisting of 24 general workers and
23 factory workers. There were a few skin lesions resulting from
poor spraying techniques and 1 case of eye injury. The workers
were given full clinical examinations and lung, liver, and kidney
function tests were carried out. There were no significant
differences in all health parameters measured between the groups,
which led the authors to suggest that the long-term use of paraquat
was not associated with harmful effects on health.
A paraquat formulation (240 g/litre) diluted 300 times by
volume with water was sprayed for 2 h on weedy ground (Kawai &
Yoshida, 1981). No irritation of the eyes and the skin was
reported. The urine of the workers who wore gauze masks contained
1.4 - 2.7 µg paraquat, 24 h after the spraying. The urine of
workers who had worn a high-performance mask did not contain
detectable levels of paraquat. During the spraying operations, the
concentration of paraquat aerosol was 11 - 33 µg/m3 air. The total
dermal exposure was about 0.22 mg. The authors discussed the need
for protective equipment to decrease skin contact with paraquat and
to avoid aerosol inhalation.
Quantitative estimates of dermal and respiratory exposure of 26
plantation workers in Malaysia (Chester & Woollen, 1982) have shown
a mean dermal dose of 1.1 mg/kg body weight per h. The highest
individual total exposure was equivalent to 2.8 mg/kg body weight
per h; the mean respiratory exposure was 0.24 - 0.97 µg paraquat/m3
air. Spray operators and carriers were exposed to an order of 1%
or less of a TLV of 0.1 mg/m3 for respirable paraquat. Urine
levels of paraquat were generally below 0.05 mg/litre.
A study was carried out on a group of 14 spray men in Thailand
using conventional high-volume knapsack sprayers and low-volume
spinning disc applicators with paraquat ion concentrations of 1.5
g/litre and 20 g/litre, respectively (Howard, 1982). Irritation of
unprotected skin was found, and this was severe in workers using
high spray concentrations (caustic burns on the feet after work
with spinning disc applicators and paraquat solution (20 g/litre)).
Urinary paraquat levels after 14 days spraying were significantly
higher (10.21 - 0.73 mg/litre) in unprotected men using both
concentrations, and there was evidence that urinary levels of
paraquat increased as the trial progressed. No evidence of
systemic toxicity was discovered among the spray men undergoing
clinical and radiographic examination 1 week after spraying ended.
The author concluded that spray concentrations in hand-held
equipment should not exceed 5 g paraquat ion/litre.
After tomato spraying in the USA, the total body exposure to
paraquat was determined to be 168.59 mg/h (Wojeck et al., 1983).
The use of enclosed tractor cabs or a high clearance tractor
reduced total body exposures to paraquat to 26.91 mg/h or 18.38
mg/h, respectively. The authors reported that the total body
exposure of tractor spray men working in two citrus locations was
proportional to the tank concentrations (paraquat dilutions of 1.1
g/litre and 0.7 g/litre were applied); exposure levels of 28.50
mg/h and 12.16 mg/h were found for workers using the higher and the
lower concentrations, respectively. In all situations studied, the
respiratory exposure was consistently a small fraction (<0.1%) of
the total body exposure. Exposure was mainly through the skin.
8.2.1.2. Formulation workers
Groups of workers exposed to formulations were examined by
Howard (l979b). The first group of 18 workers in England comprised
subjects exposed to dust and liquid paraquat formulations during a
37.5 h working week, the mean length of exposure being 5 years.
The second group also comprised 18 males, from Malaysia, exposed to
liquid concentrate formulations during a 42-h working week, the
mean length of exposure being 2.3 years. Partly protective
clothing was worn. However, in Malaysia, no gloves, rubber aprons,
or goggles were used. The medical records and the dermatological
examinations revealed acute skin rashes, nail damage, epistaxis,
blepharitis, and delayed wound healing in 12 - 66% of these
workers. Delayed caustic effects were often found among the
Malaysian formulation workers where a lower level of safety and
hygiene was apparent. Clinical examination did not reveal any
evidence of chronic contact dermatitis, hyperkeratosis, or
eczematous lesions.
8.2.2. Cases of occupational poisoning and local caustic effects
Hayes & Vaughan (1977) reviewed deaths from pesticides in the
USA. From 1956 - 1973, no deaths attributable to paraquat were
registered among agricultural workers, but in 1974, 4 fatal cases
were associated with this herbicide, although it was not clear
whether they were accidental, suicidal, or occupational. Conso
(1979) reported 17 cases of skin and eye irritation, not
accompanied by epistaxis or other signs of systemic effects, in
paraquat-exposed workers in France. Bismuth et al. (1983)
discussed a few cases of paraquat poisoning due to skin
contamination and eye irritation.
The available evidence indicates that, at the recommended
dilution rates and correctly used, systemic oral, inhalation, or
dermal effects should not be expected. Skin and eye irritation
have occurred only when protective measures were disregarded.
However, it should be emphasized that carelessness in handling
paraquat may have serious consequences. Fitzgerald et al. (1978a)
summarized the clinical findings and pathological details
concerning 13 accidents involving paraquat among agricultural
workers, 6 of which were fatal. In 5 of these cases, swallowing was
involved.
8.2.2.1. Oral ingestion
The ingestion of paraquat may occur accidentally, if liquid
concentrates are decanted into unlabelled containers near the
working areas (Kawatomi et al., 1979), and dangerous ingestion can
occur if operators suck or blow out the blocked pipes or nozzles of
spray apparatus. Of the 6 fatalities studied by Fitzgerald et al.
(1978a), 3 swallowed Gramoxone(R) after sucking the outlet of a
sprayer. In one non-fatal case, the man had sucked out a nozzle
containing diluted paraquat, while in another case, the man who had
blown into the jet, to clear it, escaped with only minor signs of
poisoning. Dilute solution blown into the face by the wind and
splashes of concentrate that get into the mouth probably explain
the resultant signs in the mouth, on the tongue, and in the throat.
Smoking with paraquat-contaminated hands has been reported to
result in a farmer's developing oropharyngeal irritation, nausea,
and muscular weakness (Mourin, 1967).
8.2.2.2. Dermal absorption
Acute dermal paraquat poisoning has been described by
Fitzgerald et al. (1978a). The use of a leaking sprayer by a
worker with severe extensive dermatitis probably resulted in fatal
absorption of paraquat through the damaged skin. Jaros (1978) has
described how the use of concentrated solutions of paraquat (50
g/litre instead of 5 g/litre), with an old leaking knapsack
sprayer, resulted in paraquat contamination of the neck, back, and
legs of a worker. After 4 h of work, he complained of a burning
sensation on the neck and scrotum. On admission to hospital 6 days
later, cough and respiratory difficulties were recorded. Three
days later the patient died of renal and respiratory failure. This
author has stressed the need for careful handling of paraquat.
Jaros et al. (1978) have discussed several other cases of paraquat
poisoning in the CSSR related to paraquat application.
Severe skin damage, followed by death due to respiratory
insufficiency, occurred in a woman (Newhouse et al., 1978), 8 weeks
after initial contact with paraquat. The toxic dermatitis started
with scratches on the arms and legs from the branches of fruit
trees. The patient had often failed to wear protective clothing or
to shower after spraying. During the 4 weeks preceding her first
admission to hospital, she developed ulcers and respiratory
complaints combined with anorexia. Damaged and broken skin was
thus exposed to paraquat. A chest X-ray and needle biopsy of the
lung revealed pulmonary lesions. Seventeen days after discharge
from hospital, without a specific diagnosis, she was re-admitted,
and died 2 weeks later with progressive lung, hepatic, and renal
dysfunction. More recently, Levin et al. (1979) described the
clinical and pathomorphological investigation of a patient who died
of hypoxia after repeated dermal exposure to paraquat (28 g/litre)
and diquat (29 g/litre) in a water-oil dilution - contrary to
accepted practice. The worker had used a leaking sprayer. A
characteristic ulcer developed at the site of paraquat contact.
There was also lung damage. Waight & Weather (1979) reported a
fatal case of dermal poisoning with paraquat after prolonged
contact with a concentrated formulation following spillage from a
bottle in the back trouser pocket. Wohlfahrt (1982) discussed the
factors related to severe paraquat poisoning due to dermal
absorption in tropical agriculture. Three fatal incidents followed
skin contamination; one victim used paraquat to treat scabies
infestation, and one to treat lice. In all cases, the skin was
blistered and ulcerated. The patients died of progressive
respiratory failure, 4 - 7 days after the accidents. However it
has been pointed out that each of these three spraymen showed skin
lesions much more severe than would be expected had recommended and
customary dilutions been used and that, in one of these cases, the
presence of mouth and throat ulceration strongly suggested that
ingestion might also have occurred (Davies, 1982).
8.2.2.3. Local skin and nail effects
Paraquat has a delayed effect on the skin. Brief contact with
liquid formulations, as well as repeated exposure to dilute
solutions, produced skin irritation, desquamation, and, finally,
necrosis at the site of contact (Ongom et al., 1974; Binns, 1976;
Newhouse et al., 1978; Waight & Wheather, 1979; Levin et al., 1979;
Horiuchi et al., 1980). Harmful dermal effects have been reported
(Howard, 1982) among spray men who worked without protective
clothes and with naked feet. The blistering and ulceration of the
skin were due to excessive contact and inadequate personal hygiene.
Horiuchi & Ando (1980) carried out patch testing on 60 patients
with contact dermatitis due to Gramoxone(R). In 8 patients
(13.3%) positive allergic reactions were established. In another
survey with 52 persons, a positive photo-patch response was
reported in 11 patients.
Nail damage has also been reported after frequent exposure to
paraquat concentrates during the formulation of the herbicide or
the preparation of working dilutions (Samman & Johnston, 1969;
Howard, l979b). Leakage from sprayers may cause nail damage only
if there is gross contamination (Hearn & Keir, 1971). Asymmetric
discoloration and softening of the nail base appears together with
an infection, that usually persists after the loss of the nail, but
a few months after cessation of paraquat exposure, the nails
re-grow satisfactorily.
8.2.2.4. Ocular damage
A number of studies have demonstrated the hazard from splashes
of concentrated paraquat that come into contact with the eye (Swan,
1969; Schlatter, 1976; Howard, l979b, 1980; Deveckova & Myalik,
1980). Apart from irritation of the eye and blepharitis, a week
later more serious ocular damage may occur such as destruction of
the bulbar and tarsal conjunctiva and of the corneal epithelium
(Cant & Lewis, 1968). Anterior uveitis was also noted. Joyce
(1969) reported a case of conjunctival necrosis after paraquat had
been splashed into the eyes during spraying in windy weather. In a
second case, there was progressive keratitis with gross corneal
opacity. Severe conjunctival injuries with keratitis and decreased
visual acuity were reported in 3 workers by Watanabe et al. (1979)
and in another by Okawada et al. (1980). The eyes were washed with
water immediately, but the damage progressed and required treatment
for more than 3 weeks.
8.2.2.5. Inhalation
The inhalation of droplets in normal paraquat spraying does not
appear to represent a significant health hazard (Howard, 1980), and
the effects of occupational inhalation have been limited to nose
bleeds, and nasal and throat irritation (Swan, 1969; Howard,
1979b). Standard spraying equipment failed to produce significant
levels of droplets in the respirable range of < 5-7 µm diameter,
and chemical analyses of paraquat aerosols or particulate matter,
sampled from working areas, have usually shown them to be well
below the TLV. However, there have been some reports (Malone et
al., 1971; Mircev, 1976; Bismuth et al., 1982) of adverse effects
as a result of inhalation exposure.
8.3. Use of Marijuana Contaminated by Paraquat
In the USA, it has been found that marijuana sprayed with
paraquat (in an attempt to destroy the plant) may become available
for smoking by drug users. Concentrations of paraquat in marijuana
of up to 461 mg/kg have been reported (Liddle et al., 1980).
Understandably, concern has been expressed that smoking this
contaminated marijuana may be more harmful than smoking marijuana
itself. The available data do not justify an absolute conclusion.
However, paraquat is known to pyrolyse at 300 °C and it has been
established (Smith 1978) that in marijuana cigarettes contaminated
with 1000 mg paraquat/kg (1 mg, assuming a 1 g cigarette), only
0.26 µg of paraquat escaped pyrolysis and was available to be
inhaled. On this basis, the amount of paraquat inhaled by a heavy
user of contaminated marijuana will be insufficient to cause
injury. In the absence of exhaustive toxicological studies, it
cannot be stated categorically that all the pyrolysis products of
paraquat do not damage the lung. However, there has been no
confirmed injury attributable to the smoking of contaminated
marijuana.
8.4. Guidelines for the treatment of paraquat poisoning
The most important measures are the immediate neutralisation of
ingested paraquat by 15% Fuller's earth, bentonite, or activated
charcoal and urgent removal of the poison by vomiting or, when
possible, gastric washout. The urgency of these measures is such
that where transfer to hospital may involve delay of an hour or
more, this emergency treatment may need to be given by a
paramedical person, e.g., a nurse or a medical assistant. The
delay should not be more than 4 - 5 h. Furthermore, Fuller's earth
should be given together with a strong purgative such as magnesium
sulfate or mannitol.
Admission to a hospital either directly or after emergency
treatment elsewhere is essential.
Where a person has swallowed a lethal dose, the most important
single determinant of survival is the early commencement of
treatment.
Depending on local facilities, patients who reach hospital
after the initial treatment will have further treatment aimed at
neutralizing paraquat in the gastrointestinal tract (Fuller's
earth, bentonite, activated charcoal) or its excretion in the
faeces (purgatives, 10% mannitol, gut lavage). In addition,
attempts to remove absorbed paraquat from the circulation
(haemoperfusion, haemodialysis) or aid its excretion by the kidney
(forced diuresis) can be instituted.
In centres where facilities for analytical procedures are
available, measurement of urinary, or ideally plasma levels of
paraquat may give guidelines for the required intensity of
treatment or likely prognosis.
Many other therapies including corticosteroids,
immunosuppressive treatment, vitamins, beta-blocking and alkylating
agents, alpha-tocopherol, superoxide dismutase and/or glutathione
peroxidase (Autor, 1974, 1977) proved to be of no significant
importance in human paraquat poisoning (Fletcher, 1975; Fairshter
et al., 1976; Schlatter, 1976; Brown et al., 1981; Bismuth et al.,
1982). The administration of oxygen should be avoided except where
vital for the patient's comfort.
It should be noted that, as with the great majority of
chemicals, there is no specific antidote.
Care must be exercised in the administration of most of these
treatments, as the following serious complications may occur:
perforation of the oesophagus during gastric intubation; serious
blood chemistry disturbance when severe diarrhoea is induced; fluid
overload during forced diuresis (McGeown, 1975).
Despite such an array of both simple and sophisticated
measures, the response to therapy in paraquat poisoning is
disappointing and the mortality rate remains high.
In cases of skin and eye contamination, irrigation with water
(preferably running water) should be commenced urgently and must be
continued uninterrupted for at least 10 min (timed by the clock).
Eye cases should always be taken for medical treatment. In cases
of skin contamination by the concentrate or extensive and/or
prolonged contamination by the diluted material (particularly where
signs of skin irritation are present) the patient must be assessed
at hospital for systemic poisoning.
9. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
9.1. Exposure
Introduction
Paraquat is a contact herbicide or dessicant that is used to
destroy weeds in various agricultural situations. It is used in
the form of an aqueous spray, which means that potential human
exposure may occur as a result of its presence in air, on plants,
in soil, or in water.
Degradation of paraquat
Photochemical degradation takes place when paraquat-treated
plants are exposed to normal daylight and continues after the
plants are dead (section 4.1.1). The products formed have been
identified and found to be of a lower order of toxicity.
Ultraviolet degradation on soil surfaces also occurs, but
photodecomposition of paraquat in the soil is insignificant in
comparison with adsorption on clay particles. Microorganisms can
degrade free paraquat rapidly, but chemical degradation of adsorbed
paraquat is relatively slow.
Soil
Paraquat is rapidly and tightly bound to clay materials in
soils. The adsorbed paraquat is biologically inactive and in
normal agricultural use no harmful metabolic or breakdown products
are to be expected (section 4.3 and 5.1). In multiple spray
trials, paraquat residues in soil varied from 22 to 58 mg/kg.
Under field conditions, the residual paraquat is slowly
re-distributed. Long-term field studies have shown degradation
rates of 5 - 10% per annum, which is sufficient to prevent
saturation of soil deactivation capacities. At normal and high
rates of application, no adverse effects are expected in the soil
microflora and other soil organisms, or on crop growth (section
4.3.1).
Water
Following the use of paraquat as an aquatic herbicide at a
normal application rate of 1 mg/litre, the concentration was found
to decrease to about one half of the initial level within 36 h and
to below 0.0l mg/litre in less than 2 weeks (section 4.3.2).
Phytotoxic damage to crops irrigated with treated water is unlikely
to occur, if an interval of 10 days is observed between treatment
of the water and its use, because of the rapid decrease of paraquat
residues in the water.
Normal application of paraquat for aquatic weed control is not
harmful for aquatic organisms. However, care should be taken in
the application of paraquat to water containing heavy weed growth,
since oxygen consumed by subsequent weed decay may decrease oxygen
levels in the water to an extent that is dangerous for fish or
other aquatic organisms.
Air
Paraquat is not volatile so inhalation of paraquat vapour is
not a problem, in practice. However, droplets of paraquat solution
can be present in the air as a consequence of aerial, knapsack, or
tractor-mounted spraying. Paraquat aerosol concentrations (total
airborne) ranged up to 0.55 mg/m3 in the work situation, depending
on the method of spraying. The amount of respirable airborne
paraquat was found to be insignificant under normal conditions of
use (section 8.2.1).
The amount of paraquat present in airborne dust was found to
range from 0.0004 to 0.001 mg/m3. The binding of paraquat to the
dust was so tight that it did not exert any toxicological effect on
rats, when given by inhalation.
Food
Examination of paraquat-treated p1ants (section 4.3.4), or of
materials from animals fed paraquat-treated crops (section 4.3.5),
revealed low residues, so that no hazard should be expected from
paraquat residues in food when used as a herbicide or as a
desiccant. Paraquat is not subject to bioconcentration (section 5)
and has not been found to accumulate in food chains.
Environmental contamination
Exposure to paraquat from spray drift may occur in windy
weather, though field studies suggest that the airborne paraquat
concentration declines markedly within a few metres of the sprayed
area (section 4.3.3). Because of the rapid and complete binding of
paraquat to clay particles in the soil, contamination of water
supplies either from field runoff or percolation through soil to
the water table is not an environmental problem (sections 4.3.1 and
4.3.2). Paraquat has also been shown not to have any harmful
effects on birds (sections 5.3 and 5.4).
9.2. Poisoning by Paraquat
Misuse of paraquat has led to many deaths throughout the world,
mainly due to the swallowing of undiluted preparations.
9.2.1. Suicidal ingestion
The majority of paraquat poisonings are due to swallowing
liquid concentrates with suicidal intent and the mortality rate is
high. Ingestion of granular paraquat is less common and usually
causes milder poisoning, though fatalities have occurred. Paraquat
has been used to commit homicide (section 8.1).
9.2.2. Accidental poisoning
Poisoning by accidental swallowing is less common than
intentional swallowing and is usually the result of storing liquid
concentrates in inappropriate containers, particularly beer or soft
drink bottles. The mortality rate is lower than in suicidal cases.
Childhood poisoning is usually accidental. Legislation on the
control of the sale of liquid concentrates has reduced accidental
ingestion in some countries (section 8.1).
A small number of fatal cases of accidental paraquat poisoning
via the skin have been reported following the application of liquid
concentrates (200 g/litre) to kill body lice.
9.2.3. Occupational Poisoning
Cases of severe poisoning following inappropriate behaviour or
accidents while handling paraquat occur. Fatal and non-fatal
ingestion of paraquat has occurred when hand-spray operators have
attempted to clear the spray outlet by sucking on the spraying
nozzle or outlet pipes. In some of the severe cases, the authors
noted their suspicion of concealed suicidal intent. Fatal
poisoning by dermal soaking with dilute paraquat has been reported
in one operator who had severe dermatitis and had been using a
leaky sprayer (section 8.2.2).
Fatal systemic poisoning may result from continuous contact
with paraquat-soaked clothing or splashes of liquid concentrate on
the skin. Splashes of liquid concentrate may lead to severe ocular
and skin damage (sections 8.2.1, 8.2.2). Spraying with
inadequately diluted paraquat (e.g., with ultra low volume
application) may result in similar problems.
9.3. Occupational Exposure
There are several studies on paraquat exposure in normal
agricultural use. Occupational exposure may be oral, dermal, or by
inhalation. The spray aerosol and dust particles are relatively
large and are mostly deposited in the upper respiratory tract
(section 8.2.1).
The potential dermal exposure of field workers (section 8.2.1)
is closely related to working conditions. Workers on tractors were
found to have a paraquat exposure of 12 - 168 mg/h while spraying
tomatoes and citrus. In other studies, field workers were dermally
exposed to paraquat at approximately 0.40 mg/h, and individuals
spraying the garden to 0.29 mg/h. In all trials, respiratory
exposure was not higher than 0.01 mg/h. Urine concentrations in
occupationally-exposed workers were often lower than 0.01 mg/litre,
but concentrations up to 0.73 mg/litre were determined after
improper paraquat application in tropical agriculture use.
Local skin effects (contact, irritative, or photoallergic
dermatitis) delayed wound healing, and nail damage has been
observed among formulation workers or among individuals handling
the herbicide improperly. Blepharitis and epistaxis may result due
to delayed irritative action of paraquat. Such incidents
illustrate the need for strict personal hygiene and rigorous
adherence to safe handling procedures.
9.4. Effects
9.4.1. Paraquat toxicity in animals
The acute lung-directed toxicity of paraquat in man has been
confirmed in numerous studies in animals. At high doses of
paraquat, minor toxic effects have been noted primarily in liver
and kidney, and in other organ systems, including nervous,
cardiovascular, blood, adrenals and male reproductive systems.
However, toxic effects have not been reported at low doses of
paraquat. Concentrated solutions of paraquat have been found to be
irritating to both skin and eyes. The FAO/WHO (1976) has
determined no-observed-adverse-effect levels of 30 mg/kg diet,
equivalent to 1.5 mg/kg body weight per day for rats and 50 mg/kg
diet, equivalent to 1.25 mg/kg body weight per day, for dogs
exposed to paraquat dichloride. Additional animal studies have
indicated that paraquat is neither teratogenic nor carcinogenic
(sections 7.1.6 and 7.1.8). In vitro mutagenicity studies have
been inconclusive, though generally suggesting weak potential
activity, while in vivo studies have given negative results
(section 7.1.7). Thus, the results of animal studies suggest that
low-level exposure to paraquat is unlikely to induce toxic effects
in man.
9.4.2. Paraquat determinations in biological fluids and tissues
Determination of paraquat levels in stomach washings, serum,
and urine is useful for the management of poisoning (section 6.2).
The urinary levels decline rapidly during the 24 h following
exposure and may remain low for some weeks. Determination of
urinary levels of paraquat may be useful in the conduct of
epidemiological studies.
9.5. Earlier Evaluations by International Bodies
The Joint Meeting on Pesticide Residues (JMPR) has reviewed
residues and toxicity data on paraquat on several occasions
(FAO/WHO 1971, 1973, 1977, 1979, 1982, 1983). In 1972, it
estimated the acceptable daily intake (ADI) for man 0 - 0.002 mg/kg
body weight, on the basis of no-observed-adverse-effect levels of
1.50 mg/kg body weight per day in the rat and 1.25 mg/kg body
weight in the dog. Because of concern relating to lung and kidney
toxicity, this ADI was changed in the 1982 meeting to a temporary
ADI of 0 - 0.001 mg paraquat dichloride/kg body weight (or 0.0007
mg paraquat ion/kg body weight). The no-observed-adverse-effect
level for the rat remained, however, at 1.5 mg/kg body weight/day
(FAO/WHO 1983).
The same JMPRs have recommended maximum residue levels
(tolerances) for paraquat in food commodities of plant and animal
origin.
The WHO/FAO (1978) in its series of "Data sheets on chemical
pesticides" issued one on paraquat. Based on a brief review of
use, exposure, and toxicity, practical advice is given on
labelling, safe-handling, transport, storage, disposal,
decontamination, selection, training and medical supervision of
workers, first aid, and medical treatment.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechcoslovakia, the
Federal Republic of Germany, India, Japan, Kenya, Mexico, Sweden,
the United Kingdom, the USA, and the USSR) and the EEC can be found
in the IRPTC (International Register of Potentially Toxic
Chemicals) Legal file (IRPTC 1983).
9.6. Conclusions
On the basis of the above findings, it can be concluded that:
General population
Residue levels of paraquat in food and drinking-water,
resulting from its normal use, are unlikely to result in a health
hazard for the general population.
This likely lack of hazard in normal usage of dilute paraquat
is in strong contrast with the potential serious hazard that may
result from handling concentrated paraquat.
Accidental paraquat poisoning results mainly from swallowing
liquid concentrate that has been decanted into unlabelled bottles
or other containers and stored inappropriately.
The number of suicides by means of paraquat is of great
concern. The total number of such suicides is unknown.
Notwithstanding the facts that the reasons for suicide may be
manifold and complex, and that paraquat is one among many means
towards that goal, the prolonged and painful way of dying from
paraquat suggests that every effort within reason should be made to
diminish the attractiveness and availability of paraquat for this
purpose.
Occupational exposure
With reasonable work practices, including safety precautions,
hygiene measures, and proper supervision, occupational exposure
during manufacture, formulation, and application will not cause
hazard. However the undiluted concentrate must be handled with
great care because improper work practices may result in
contamination of eyes and skin (with possible consequent dermal
absorption).
Spray concentrations should not exceed 5 g paraquat ion/litre
in order to avoid skin damage and absorption of the herbicide
through the skin. Its use in hand-held ultra-low volume
application should be discouraged.
Environment
Paraquat in soil binds rapidly and tightly to clay particles
and residual phytotoxicity from freely-available paraquat is
unlikely. The toxicity of the compound for birds has been shown to
be of low significance. Under normal conditions of use, paraquat
shows low toxicity to aquatic organisms although resulting
depletion of water-oxygen because of weed decay may pose a problem.
Paraquat does not seem to represent an environmental hazard.
REFERENCES
ACKRILL, P., HASLETON, P.S., & RALSTON, A.J. (1978)
Oesophageal perforation due to paraquat. Br. med. J., 1:
1252-1253.
ALMOG, C. & TAL, E. (1967) Death from paraquat after
subcutaneous injection. Br. med. J., 3: 721.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDERSON, D., McGREGOR, D.B., & PURCHASE, I.F.H. (1976)
Dominant lethal studies with diquat and paraquat in male CD-I
mice. Mutat. Res., 40: 349-358.
ANDERSON, C.C., GUNDERSON, E.C., & COULSON, D.M. (1981)
Sampling and analytical methodology for workplace chemicals
hazards. ACS Symp. Ser., 149: 3-19.
ANONYMOUS (1979) Paraquat. FAO Plant Prod. Prot. Pap.,
15(Suppl.): 181-183.
AUTOR, A.P. (1974) Reduction of paraquat toxicity by
superoxide dismutase. Life Sci., 14: 1309-1319.
AUTOR, A.P., ed. (1977) Biochemical mechanisms of paraquat
toxicity, New York, Academic Press, pp. 39-55.
BAINOVA, A. (1969a) [Chronic oral toxicity of dipyridylium
herbicides.] Hig. Zdrav., 12: 325-332 (in Bulgarian).
BAINOVA, A. (l969b) [Experimental assessment of the effect
of dipyridylium herbicides on the skin.] Letopisi HEI, 9:
25-30 (in Bulgarian).
BAINOVA, A. (1971) [Determination of the zones of acute and
chronic inhalatory toxicity after exposure to dipyridylium
herbicides Gramoxone and Reglone.] Letopisi HEI, 28: 59-62 (in
Bulgarian).
BAINOVA, A. (1975) [Cumulative action of Gramoxone and
Reglone.] In: Problemi na Higienata, Sofia, Medizinia i
Fizkultuna, Vol. 1, pp. 31-38 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1972) Experimental
substantiation of Gramoxone MAC in working areas. In: Works of
Research Institute of Hygiene & Labour Protection, Sofia,
Medizinia i Fizkultuna, Vol. 23, pp. 71-77.
BAINOVA, A. & VULCHEVA, V. (1974) [Experimental assessment
of the effect of dipyridyliums on sex glands.] In: Works of
Research Institute of Hygiene & Labour Protection, Sofia,
Medizinia i Fizkultuna, Vol. 22, pp. 111-122 (In Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1977) Lung changes after chronic
paraquat intoxication. C. R. Acad. Bulgar. Sci., 30: 1788-1790.
BAINOVA, A., ZLATEVA, M., & VULCHEVA, V. (1972) [Chronic
inhalation toxicity of dypyridylium herbicides.] Hig. Zdrav.,
15: 25-31 (in Bulgarian).
BALDWIN, B.C., BRAY, M.F., & GEOGHEGAN, M.J. (1966) The
microbial decomposition of paraquat. Biochem. J., 101: 15.
BEEBEEJAUN, A.R., BEEVERS, G., & ROGERS, W.N. (1971)
Paraquat poisoning. Prolonged excretion. Clin. Toxicol., 4:
397-407.
BENIGNI, R., BIGNAMI, A., CARERA, A., CONTI, G., CONTI, R.,
GREBELLI, E., DOGLIOTTI, E., GUALANDI, G., NOVELLETO, A., &
ORTALI, V.A. (1979) Mutational studies with diquat and
paraquat in vitro. Mutat. Res., 68: 183-193.
BENNETT, P.N., DAVIES, D.S., & HAWKSWORTH, G.M. (1976) In
vivo adsorption studies with paraquat and diquat in the dog.
Proceedings of the British Pharmaceutical Society, 15-16 July,
England, 284 pp.
BERRY, D.J. & GROVE, J. (1971) The determination of paraquat
(1,1-dimethyl-4,4-dipyridylium cation) in urine. Clin. Chim.
Acta, 34: 5-11.
BEYER, K.H. (1970) [The analytical determination and
toxicology of paraquat.] Dtsch Apoth.-Ztg, 110: 633-635 (in
German).
BIGNAMI, M. & GREBELLI, R.A. (1979) A simplified method for
the induction of 8-azaguanine resistance in S. typhimurium.
Toxicol. Lett., 3: 169-175.
BINNS, C.W. (1976) A deadly cure for lice. Papua New Guinea
med. J., 19: 105-107.
BISMUTH, C., GARNIER, R., DALLY, S., FOURNIER, P.E., &
SCHERRMANN, J.M. (1982) Prognosis and treatment of paraquat
poisoning. A review of 28 cases. J. Toxicol. clin. Toxicol.,
19: 461-474.
BISMUTH, C., DALLY, S., & PONTAL, P.-G. (1983) Prognostic
factors and therapeutic results of paraquat poisoning.
Concerning 28 cases. Arch. Mal. prof., 44(1): 38-41.
BOJAN, F., NAGY, A., & HERMAN, K. (1978) Effect of butylated
hydroxytoluene and paraquat on urethane tumorigenesis in mouse
lung. Bull. environ. Contam. Toxicol., 20: 573-576.
BRAMLEY, A. & HART, T.B. (1983) Paraquat poisoning in the
United Kingdom. Hum. Toxicol., 2(2): 417.
BRIGELIUS, R. & ANWER, M.S. (1981) Increased biliary
GSSG-secretion and loss of hepatic glutathione in isolated rat
liver after paraquat treatment. Res. Commun. chem. Pathol.
Pharmacol., 31: 493-502.
BRIGELIUS, R., HASHEM, A., & LENGFELDER, E. (1981)
Paraquat-induced alterations of phospholipids and GSSG release
in the isolated perfused rat liver and the effect of
SOD-active copper complexes. Biochem. Pharmacol., 30: 349-354.
BROWN, E., BRANDENBURGER, A., & MALING, H.M. (1980) Effects
of paraquat and related herbicides on the acetylcholinesterase
of rat lung. Biochem. Pharmacol., 29: 456-466.
BROWN, 0., HEITKAMP, U., & SONG, C. (1981) Niacin reduces
paraquat toxicity in rats. Science, 212: 1510-1512.
BULLIVANT, C.M. (1966) Accidental poisoning by paraquat.
Report of 2 cases in man. Br. med. J., 1: 1272-1273.
BURNS, R.G. & AUDUS, L.J. (1970) Distribution and breakdown
of paraquat in soil. Weed Res., 10: 49-58.
BUS, J.S. & GIBSON, J.E. (1975) Postnatal toxicity of
chronically administered paraquat in mice and interactions
with oxygen and bromobenzene. Toxicol. appl. Pharmacol., 33:
461-470.
BUS, J.S. & GIBSON, J.E. (1979) Lipid peroxidation and its
role in toxicity. Rev. Biochem. Toxicol., 1: 125-149.
BUS, J.S. & GIBSON, J.E. (in press) Paraquat: a model for
oxidant initiated injury. In: Hook, G.E.R., ed. Pulmonary
toxicology, New York, Raven Press.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1974) Superoxide and
singlet oxygen catalysed lipid peroxidation as a possible
mechanism for paraquat toxicity. Biochem. biophys. Res.
Commun., 58: 749-755.
BUS, J.S., AUST, S.D., & GIBSON, J.E. (1975) Lipid
peroxidation as a possible mechanism for paraquat toxicity.
Res. Commun. chem. Pathol. Pharmacol., 11: 31-38.
BUS, J.S., CAGEN, S.Z., OLGAARD, M., & GIBSON, J.E. (1976)
A mechanism of paraquat toxicity in mice and rats. Toxicol.
appl. Pharmacol., 35: 501-513.
BUTLER, C. (1975) Pulmonary interstitial fibrosis from
paraquat in the hamster. Arch. Pathol., 99: 503-507.
BUTLER, C. & KLEINERMAN, J. (1971) Paraquat in the rabbit.
Br. J. ind. Med., 28: 67-71.
CAGEN, S.Z. & GIBSON, J.E. (1977) Liver damage following
paraquat in selenium-deficient and diethyl maleate pretreated
mice. Toxicol. appl. Pharmacol., 40: 193-200.
CAGEN, S.Z., JANOFF, A.S., BUS, J.S., & GIBSON, J.E. (1976)
Effect of paraquat on liver function in mice. J. Pharmacol.
exp. Ther., 198: 222-228.
CALDERBANK, A. (1966) Paraquat residues in fruit. In:
Frayer, J.D., ed. Herbicides in British fruit growing,
Blackwell, Blackwell Scientific Publications, pp. 135-136.
CALDERBANK, A. (1968) The bipyridylium herbicides. Effects
on man. In: Metcalf, R.L., ed. Advances in pest control
research, New York, Interscience Publishers, Vol. 8, 224 pp.
CALDERBANK, A. (1972) Environmental considerations in the
development of diquat and paraquat as aquatic herbicides.
Outlook Agric., 7: 51-54.
CALDERBANK, A. & SLADE, P. (1976) Diquat and paraquat. In:
Kearne, P.C. & Kaufman, D.D., ed. Herbicide chemistry,
degradation and mode of action, 2nd ed., New York, Dekker,
pp. 501-540.
CALDERBANK, A. & YUEN, S.H. (1965) An ion-exchange method
for determining paraquat residues in food crops. Analyst, 90:
99-106.
CALDERBANK, A., MCKENNA, R.H., STEVENS, M.A., & WALLEY, J.K.
(1968) Grazing trials on paraquat-treated pasture. J. Sci.
Food Agric., 19: 246-250.
CANT, J.S. & LEWIS, R.H. (1968) Ocular damage due to
paraquat and diquat. Br. med. J., 2: 224.
CARLSTROM, A.A. (1971) Collaborative check for paraquat in
formulations. J. Assoc. Off. Anal. Chem., 54: 718-719.
CARMINES, E.L., CARCHMAN, R.A., & BORZELLECA, J.F. (1981)
Investigations into the mechanism of paraquat toxicity
utilizing a cell culture system. Toxicol. appl. Pharmacol.,
58: 353-362.
CARSON, D.G.L. & CARSON, E.D. (1976) The increasing use of
paraquat as a suicide agent. Forensic Sci., 7: 151-160.
CHARLES, J.M., ABOU-DONIA, M.B., & MENZEL, D.B. (1978)
Absorption of paraquat from the airways of the perfused rat
lung. Toxicology, 8: 59-67.
CHESTER, G. & WARD, R.J. (1981) Paraquat - Occupational
exposure and drift hazard evaluation during aerial application
to cotton in California, USA, London, ICI Ltd (Central
Toxicology Laboratory Report No. CTL/P.581).
CHESTER, G. & WOOLLEN, B.H. (1982) Studies of the
occupational exposure of Malaysian plantation workers to
paraquat. Br. J. ind. Med., 38: 23-33.
CLARK, D.G. (1971) Inhibition of the absorption of paraquat
from the gastrointestinal tract by adsorbents. Br. J. ind.
Med., 28: 186-188.
CLARK, D.G., McELLIGOTT, T.F., & HURST, E.W. (1966) The
toxicity of paraquat. Br. J. ind. Med., 23: 126-133.
CLEGG, D.J. (1979) Animal reproduction and carcinogenicity
studies in relation to human safety evaluation. Dev. Toxicol.
environ. Sci., 4: 45-59.
COATS, G.E., FUNDERBURK, H.H., Jr, LAWRENCE, J.M., & DAVIS,
D.E. (1966) Factors affecting persistance and inactivation
of diquat and paraquat. Weed Res., 6: 58-66.
CONNING, D.M., FLETCHER, K., & SWAN, A.A.B. (1969) Paraquat
and related bipyridyliums. Br. med. Bull., 25: 245-249.
CONNOLLY, M.E., DAVIES, D.S., DRAFFAN, G.H., BENNETT, P.N., &
DOLLERY, C.T. (1975) Clinical experience with paraquat
poisoning. In: Fletcher, K., ed. Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-11.
CONSO, F. (1979) Paraquat poisoning: experience of poison
control centers in France. Vet. hum. Toxicol., 21(Suppl.):
112-113.
COOKE, N.J., FLENLEY, D.C., & MATTHEW, H. (1973) Paraquat
poisoning. Serial studies of lung function. Q. J. Med.,
XLII(168): 683-692.
COPLAND, G.M., KOLIN, A., & SHULMAN, H.S. (1974) Fatal
pulmonary intra-alveolar fibrosis after paraquat ingestion.
New Engl. J. Med., 291: 290-292.
CURRY, J.F. (1970) The effects of different methods of new
sward establishment and the effects of the herbicides paraquat
and dalapon on the soil fauna. Pedobiologia, 10: 329-361.
DAMANAKIS, M., BRENNAN, D.S.H., FRYER, J.D., & HOLLY, K.
(1970) Absorption and mobility of paraquat on different soil
constituents. Weed Res., 10: 264-277.
DANIEL, J.M. & GAGE, J.C. (1966) Absorption and excretion of
diquat and paraquat in rats. Br. J. ind. Med., 23: 133-136.
DASTA, J.F. (1978) Paraquat poisoning: a review. Am. J.
Hosp. Pharm., 35: 1368-1372.
DASTA, J.F. (1980) Management of paraquat poisonings. Clin.
Toxicol. Consultant, 2(1): 11-20.
DAVIDSON, J.K. & MACPHERSON, P. (1972) Pulmonary changes in
paraquat poisoning. Clin. Radiol., 23: 18-25.
DAVIES, D.S., HAWKSWORTH, G.M., & BENNETT, P.N. (1977)
Paraquat poisoning. Proc. Eur. Soc. Toxicol., 18: 21-26.
DAVIES, R.E. (1982) Skin absorption of paraquat. Med. J.
Aust., 7: 222.
DEARDEN, L.C., FAIRSHTER, R.D., MCRAE, D.M., SMITH, W.R.,
GLASER, F.L., & WILSON, A.F. (1978) Pulmonary ultrastructure
of the late aspects of human paraquat poisoning. Am. J.
Pathol., 93: 667-676.
DEVECKOVA, D. & MYALIK, M. (1980) [Gramoxone ocular burns.]
Cesk. Ophtalmol., 36: 7-10 (in Czech).
DICKES, G.J. (1979) The application of gas chromatography to
food analysis. Talanta, 26: 1065-1099.
DIJK, A. VAN, MAES, R.A.A., DROST, R.H., DOUSE, J.M.C., &
HEIJST, A.N.P. VAN (1975) Paraquat poisoning in man. Arch.
Toxicol., 34: 129-136.
DIJK, A. VAN, EBBERINK, R., GROOT, G. DE, & MAES, R.A.A.
(1977) A rapid and sensitive assay for the determination of
paraquat in plasma by gas-liquid chromatography. J. anal.
Toxicol., 1: 151-154.
DIKSHITH, T.S.S., DATTA, K.K., RAIZADA, R.B., & KUSHWAH, H.S.
(1979) Effect of paraquat dichloride in male rabbits. Indian
J. exp. Biol., 17: 926-928.
D0DGE, A.D. (1971) The mode of action of the bipyridylium
herbicides, paraquat and diquat. Endeavour, 30: 130-135.
DOUZE, J.M.C., DIJK, A. VAN, GIMBERE, J.S.F., HEIJST, A.N.P.
VAN, & MAES, R.A.A. (1975) Intensive therapy after paraquat
intoxication. In: Fletcher, K., ed. Clinical aspects of
paraquat poisoning, London, ICI Ltd, pp. 34-45.
DRAFFON, G.H., CLARE, R.A., DAVIES, D.L., HAWKSWORTH, G.M.,
MURRAY, S., & DAVIES, D.S. (1977) Qualitative determination
of the herbicide paraquat in human plasma by gas
chromatographic and mass spectrometric methods.
J. Chromatogr., 139: 311-320.
EARNEST, R.D. (1971) Effect of paraquat on fish in a
Colorado farm pond. Prog. Fish Cult., 33: 27-31.
ECKER, J.L., HOOK, J.B., & GIBSON, J.E. (1975)
Nephrotoxicity of paraquat in mice. Toxicol. appl.
Pharmacol., 34: 178-186.
EDWARDS, P.J. (1979) Status of common bird population on an
intensively managed farm where paraquat has been used
extensively, London, ICI Plant Protection Division (Report No.
RJ 0037/B).
EDWARDS, P.J., NEWMAN, J.F., & WARD, R.J. (1979) Paraquat:
effect of spraying eggs on hatchability and reproductive
organs of Japanese quail, Coturnix coturnix japonica, London,
ICI Plant Protection Division (Report No. RJ 0044B).
EFTHYMIOU, M.L. (1983) L'actualité toxicologique en matičre
de lutte chimique antiparasitaire agricole, Tours, France,
Faculté de Médecine de Tours.
FAIRSHTER, R.D. (1981) Paraquat toxicity and lipid
peroxidation. Arch. intern. Med., 141: 1121-1123.
FAIRSHTER, R.D., ROSEN, S.M., SMITH, W.R., FLAUSER, F.L.,
MCRAE, D.M., & WILSON, A.F. (1976) Paraquat poisoning. New
aspects of therapy. Q.J. Med., 45: 551-565.
FAIRSHTER, R.D., DABIR-VAZIRI, N., SMITH, W.R., GLAUSER, F.L.,
& WILSON, A.F. (1979) Paraquat poisoning: an analytical
toxicologic study of 3 cases. Toxicology, 12: 259-268.
FAO (1973) Diquat and paraquat. FAO specifications for plant
protection products, Rome, Food and Agriculture Organization
of the United Nations.
FAO/WHO (1971) Paraquat. In: 1970 Evaluation of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1973) Paraquat. In: 1972 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1976) Paraquat. In: 1975 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977) Paraquat. In: 1976 Evaluation of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1979) Paraquat. In: 1978 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1982) Paraquat. In: 1981 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1983) Paraquat. In: 1982 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FARAGO, E., HIDEG, Z., & TABATS, G. (1981) [Fatal poisonings
during the last 10 years from statistics of the Institute of
Forensic Chemistry.] Népegészsegügy, 62: 376-379 (in
Hungarian).
FAURE, J., MARKA, C., FAURE, H., YACOUB, M., & CAN, G.
(1973) Données histo-pathologiques et toxicologiques d'une
intoxication mortelle par le paraquat. Méd. lég. Dommage, 6:
417-419.
FENNELLY, J.J., GALLAGHER, J.T., & CARROLL, R.J. (1968)
Paraquat poisoning in a pregnant woman. Br. med. J., 3:
722-723.
FENNELLY, J.J., FITZGERALD, M.X., & FITZGERALD, 0. (1971)
Recovery from severe paraquat poisoning following forced
diuresis and immunosuppressive therapy. J. Irish Med. Assoc.,
64: 69-71.
FIRLIK, M. (1978) [Changes in the respiratory system during
paraquat poisoning.] Med. Pr., 29: 325-328 (in Polish).
FISHER, H.K. & KAHLER, J. (1979) [Lethal poisoning by
paraquat.] Z. Rechtsmed., 84: 61-67 (in German).
FISHER, H.K., HUMPHRIES, M., & BAILS, R. (1971) Recovery
from renal and pulmonary damage. Ann. intern. Med., 75:
731-736.
FISHER, H.K., CLEMENTS, J.A., & WRIGHT, R.R. (1973a)
Pulmonary effects of the herbicide paraquat studied 3 days
after injection in rats. J. appl. Physiol., 35: 268-273.
FISHER, H.K., CLEMENTS, J.A., & WRIGHT, R.R. (1973b)
Enhancement of oxygen toxicity by the herbicide paraquat. Ann.
Rev. respir. Dis., 107: 246-252.
FISHER, H.K., CLEMENTS, J.A., TIERNEY, D.F., & WRIGHT, R.R.
(1975) Pulmonary effects of paraquat in the first day after
injection. Am. J. Physiol., 228: 1217-1223.
FITZGERALD, G.R. & BARNIVILLE, G. (1978) Poisoning by
granular paraquat. J. Irish Coll. Physicians Surg., 7: 133-136.
FITZGERALD, G.R., BARNIVILLE, G., FITZPATRICK, P., EDWARDS,
H., SILKE, B., CARMODY, M., & O'DWYER, W.F. (1977a) Adrenal
abnormalities in paraquat poisoning. Irish J. Med. Sci., 146:
421-423.
FITZGERALD, G.R., BARNIVILLE, G., SILKE, B., CARMODY, M., &
O'DWYER, W.F. (1977b) The kidney in paraquat poisoning.
In: Proceedings of the European Dialysis and Transplantation
Association, Helsinki 1977.
FITZGERALD, G.R., BARNIVILLE, G., FLANAGAN, M., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978a) The changing pattern of
paraquat poisoning: an epidemiologic study. J. Irish Med.
Assoc., 71: 103-108.
FITZGERALD, G.R., BARNIVILLE, G., BLACK, J., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978b) Paraquat poisoning in
agricultural workers. J. Irish Med. Assoc., 71: 336-342.
FITZGERALD, G.R., BARNIVILLE, G., GIBNEY, R.T.N., &
FITZGERALD, M.X. (1979a) Clinical, radiological and
pulmonary function assessment in 13 long-term survivors of
paraquat poisoning. Thorax, 34: 414-415.
FITZGERALD, G.R., BARNIVILLE, G., DICKSTEIN, K., CARMODY, M.,
& O'DWYER, W.F. (1979b) Experience with Fuller's Earth in
paraquat poisoning. J. Irish Med. Assoc., 71: 149-152.
FLETCHER, K. (1967) Production and viability of eggs from
hens treated with paraquat. Nature (Lond.), 215: 1407-1408.
FLETCHER, K. (1974) Paraquat poisoning. In: Ballantyne, B.,
ed. Proceedings of a Symposium on Forensic Toxicology,
Bristol, John Wright, pp. 86-98.
FLETCHER, K., ed. (1975) Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-89.
FLETCHER, K. & WYATT, I. (1970) The composition of lung
lipids after poisoning with paraquat. Br. J. exp. Pathol., 51:
604-609.
FODRI, Z., SIPOS, K., & BERENCSI, G. (1977) [Study of the
irritation and allergic effects of Gramoxone in guinea-pig.]
Egeszsegtudomouny, 21: 244-249 (in Hungarian).
FOWLER, B.A. & BROOKS, R.E. (1971) Effects of the herbicide
paraquat on the ultrastructure of mouse kidney. Am. J.
Pathol., 63: 505-512.
FRIDOVICH, I. & HASSAN, H.M. (1979) Paraquat and the
exacerbation of oxygen toxicity. Trends biochem. Sci., 4:
113-115.
GAGE, J.C. (1968a) Toxicity of paraquat and diquat aerosols
generated by a size-selective cyclone. Effect of particle
size distribution. Br. J. ind. Med., 25: 304-314.
GAGE, J.C. (1968b) The action of paraquat and diquat on the
respiration of liver cell fractions. Biochem. J., 109: 757-761.
GAGE, J.C. (1969) The inhalation toxicity of
paraquat-treated soil, London, ICI Ltd (Report No. IHR/252).
GALLOWAY, D.B. & PETRIE, J.C. (1972) Recovery from severe
paraquat poisoning. Postgrad. Med. J., 48: 684-686.
GEORGE, K. & GEORGE, M. (1978) Chromosome uncoiling effect
of paraquat. Indian J. exp. Biol., 16: 933-937.
GERVAIS, P., DIAMANT-BERGER, O., BESCOL-LIVERSAC, J., GUILLAM,
C., & GUYON, F. (1975) Problčmes médico-legaux et médico-
sociaux de l'intoxication aiguë par les herbicides du groupe
du paraquat. Arch. Mal. prof., 36: 19-36.
GIBSON, J.E. & CAGEN, S.Z. (1977) Paraquat-induced
functional changes in kidney and liver. In: Autor, A.P., ed.
Biochemical mechanisms of paraquat toxicity, New York,
Academic Press, pp. 117-136.
GIRI, S.N. & KRISHNA, G.A. (1980) The effect of paraquat on
guanylate cyclase activity in relation to morphological
changes in guinea-pig lungs. Lung, 157: 127-134.
GIRI, S.N., CURRY, D.L., HOLLINGER, M.A., & FREYWALD, N.
(1979) Effect of paraquat on plasma enzymes, insulin, glucose
and liver glycogen in the rat. Environ. Res., 20: 300-308.
GOWMAN, M.E., RILEY, D., & NEWBY, S.E. (1980) Paraquat and
Diquat: Longterm high rate trial, Frensham UK. Persistence
and movement in soil and glasshouse bioassay, London, ICI Ltd
(Report RJ0014B).
GRANT, H.C., LANTOS, P.L., & PARKINSON, C. (1980) Cerebral
damage in paraquat poisoning. Histopathology, 4: 185-195.
GREENBERG, D.B., LYONS, A., & LAST, J.A. (1978)
Paraquat-induced changes in the rat of collagen biosynthesis
by rat lung. J. lab. clin. Med., 92: 1033-1042.
GROVER, R., SMITH, A.E., & KORVEN, H.C. (1980) A comparison
of chemical and cultural control of weeds in irrigation
ditchbanks. Can. J. plant Sci., 60: 185-195.
GRUNDIES, H., KOLMAR, D., & BENNHOLD, I. (1971) [Paraquat
poisoning.] Dtsch Med. Wschr., 96: 588-589 (in German).
GRZENDA, A.R., NICHOLSON, H.P., & COX, W.S. (1966)
Persistance of four herbicides in pond water. J. Am. Water
Works Assoc., 58: 326-332.
HALEY, T.J. (1979) Review of the toxicology of paraquat
(1,1-dimethyl-4,4-dipyridylium chloride). Clin. Toxicol., 14:
1-46.
HANCE, R.L., BYAST, T.H., & SMITH, P.D. (1980) Apparent
decomposition of paraquat in soil. Soil Biol. Biochem., 12:
447-448.
HARRINGTON, K.J. (1979) The detection of paraquat
(dichloride) in wood by pyrolysis vapour-phase chromatography.
Wood Sci. Technol., 13: 21-28.
HASSAN, H.M. & FRIDOVICH, I. (1977) Regulation of synthesis
of superoxide dismutase in Escherichia coli. J. Biol. Chem.,
253: 7667-7672.
HASSAN, H.M. & FRIDOVICH, I. (1978) Superoxide radical and
the enhancement of oxygen toxicity of paraquat in Escherichia
coli. J. Biol. Chem., 253: 8143-8148.
HASSAN, H.M. & FRIDOVICH, I. (1979) Paraquat and Escherichia
coli. Mechanism of production of extracellular superoxide
radical. J. Biol. Chem., 254: 10846-10852.
HASSAN, H.M. & FRIDOVICH, I. (1980) Superoxide dismutases:
detoxication by a free radical. In: Jakoby, W.B., ed.
Enzymatic basis of detoxication, New York, Academic Press,
Vol. 1, pp. 311-322.
HAWKSWORTH, G.M., BENNETT, P.N., & DAVIES, D.S. (1981)
Kinetics of paraquat elimination in the dog. Toxicol. appl.
Pharmacol., 57: 139-145.
HAYES, W.J. & VAUGHAN, W.K. (1977) Mortality from pesticides
in the United States in 1973 and 1974. Toxicol. appl.
Pharmacol., 42: 235-252.
HEARN, C.E.D. & KEIR, W. (1971) Nail damage in spray
operators exposed to paraquat. Br. J. ind. Med., 28: 399-403.
HIGGINBOTTOM, T., CROME, P., PARKINSON, C., & NUNN, J.
(1979) Further clinical observations on the pulmonary effect
of paraquat ingestion. Thorax, 34: 161-165.
HILLS, D.J., CURLEY, R.G., KNUTSON, J.D., SEIBER, J.N.,
WINTERLIN, W.L., FAUSCHKOLB, R.S., PULMAN, G.S., & ELMORE,
C.L. (1981) Composting treatment for cotton gin trasfines.
Trans. ASAE, 24: 14-19.
HOFMAN, A. & FROHBERG, H. (1972) [Gramoxone intoxication in
the Federal Republic of Germany.] Dtsch Med. Wschr., 27:
1239-1303 (in German).
HOLLINGER, M.A. & CHVAPEL, M. (1977) Effect of paraquat on
rat lung prolyl hydroxylase. Res. Commun. chem. Pathol.
Pharmacol., 16: 159-162.
HORIUCHI, N. & ANDO, S. (1980) [Contact dermatitis due to
pesticides for agricultural use.] Nippon Hifuka Gakkai Zasshi,
90: 289 (in Japanese).
HORIUCHI, N., ANDO, S., & KAMBE, Y. (1980) [Dermatitis due
to pesticides for agricultural use.] Nippon Hifuka Gakkai
Zasshi, 90: 27 (in Japanese).
HOWARD, J.K. (1979a) Recent experience with paraquat
poisoning in Great Britain: a review of 68 cases. Vet. hum.
Toxicol., 21: 213-216.
HOWARD, J.K. (l979b) A clinical survey of paraquat
formulation workers. Br. J. ind. Med., 36: 220-223.
HOWARD, J.K. (1980) Paraquat: a review of worker exposure in
normal usage. J. Soc. Occup. Med., 30: 6-11.
HOWARD, J.K. (1982) Paraquat spraying. Comparative risks
from high and low volume application methods. In: Proceedings
of 10th Asian Conference on Occupational Health, Singapore,
pp. 1-7.
HOWARD, J.K., SAHOPATHY, K.N., & WHITEHEAD, P.A. (1980) An
evaluation of the long-term effects of paraquat spraying.
Toxicol. Lett. Suppl., 1(Abstr. 068).
HOWARD, J.K., SAHOPATHY, K.N., & WHITEHEAD, P.A. (1981) A
study of the health of Malaysian plantation workers with
particular reference to paraquat spraymen. Br. J. ind. Med.,
38: 110-114.
HOWE, D.J.T. & WRIGHT, N. (1965) The toxicity of paraquat
and diquat. In: Proceedings of the 18th New Zealand Weed &
Pest Control Conference, pp. 105-114.
HUDSON, R.H., HAEGELE, M.A., & TUCKER, R.K. (1979) Acute
oral and percutaneous toxicity of pesticides to mallards:
correlations with mammalian toxicity data. Toxicol. appl.
Pharmacol., 47: 451-460.
HUNDSDORFER, S. & ROSE, I. (1980) [Lung changes produced by
paraquat: respiratory distress model in animals.] Ergeb. exp.
Med., 35: 603-609 (in German).
HUSSAIN, M.Z. & BHATNAGAR, R.S. (1979) Involvement of
superoxide in the paraquat-induced enhancement of lung
collagen synthesis in organ culture. Biochem. Biophy. Res.
Commun., 89: 71-76.
ICI Ltd (1972) Determination of residues of paraquat in
milk, London, ICI Ltd (Residue Analytical Method No. 3,
PPRAM-3).
ICI Ltd (1979) Analytical method for the determination of
paraquat by radioimmunoassay, London, ICI Ltd (PHAG).
ILETT, K.F., STRIPP, B., MENARD, R.H., REID, W.D., & GILLETTE,
J.R. (1974) Studies on the mechanism of the lung toxicity of
paraquat. Comparison of tissue distribution and some
biochemical parameters in rats and rabbits. Toxicol. appl.
Pharmacol., 28: 216-226.
IRPTC (1983) IRPTC Legal files 1983, Vol. I & II, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme.
JAROS, F. (1978) Acute percutaneous paraquat poisoning.
Lancet, 1: 275.
JAROS, F., ZUFFA, L., KRATINOVA, R., SKALA, I., & DOMSOVA, A.
(1978) [Acute percutaneous Gramoxone intoxication.] Pr. Lék.,
7: 260-262 (in Czech).
JOYCE, M. (1969) Ocular damage caused by paraquat. Br. J.
Ophtalmol., 53: 688-690.
KAWAI, M. & YOSHIDA, M. (1981) [Exposure of spray operators
to paraquat.] Nippon Doshu Eisei Zasshi, 28: 353-359 (in
Japanese).
KAWAI, S., UEDA, K., KOYAMA, K., & OGASAWARA, S. (1980)
[Studies on preventive treatment of paraquat dichloride
intoxication - Part 2.] Nippon Noson Igakkai Zasshi, 29:
546-547 (in Japanese).
KAWATOMI, M., KOGA, H., YOKOYAMA, K., FUJIMATSU, S.,
MATSUMOTO, T., FUKUDA, H., & IHIZAKI, T. (1979) [A case of
autopsy of a person who died of paraquat intoxication.] Nippon
Naiko Gakkai Zasshi, 68: 1332-1333 (in Japanese).
KEELING, P.L., PRATT, I.S., ALDRIDGE, W.N., & SMITH, L.L.
(1981) The enhancement of paraquat toxicity in rats by 85%
oxygen: lethality and cell-specific lung damage. Br. J. exp.
Pathol., 62: 643-654.
KEELING, P.L., SMITH, L.L., & ALDRIDGE, W.N. (1982) The
formation of mixed disulfides in rat lung following paraquat
administration. Biochem. Biophys. Acta, 716: 249-257.
KEHRER, J.P., HASCHEK, W.M., & WITSCHI, H. (1979) The
influence of hyperoxia on the acute toxicity of paraquat and
diquat. Drug Chem. Toxicol., 2: 397-408.
KELLY, D.F., MORGAN, D.G., DARKE, P.G.G., GIBBS, C., PEARSON,
H., & WEAVER, M.Q. (1978) Pathology of acute respiratory
distress in the dog associated with paraquat poisoning.
J. comp. Pathol., 88: 275-293.
KHAN, S.U. (1974) Determination of diquat and paraquat
residues in soil by gas chromatography. J. agric. food Chem.,
22: 863-867.
KHAN, S.U. (1980) Determining the role of humic substances
in the fate of pesticides in the environment. J. environ. Sci.
Health, 15: 1071-1090.
KHERA, K.S., WHITTA, L.K., & CLEGG, D.J. (1968) Embryopathic
effects of diquat and paraquat in rats. Ind. Med. Surg., 37:
257-261.
KIMBROUGH, R.D. (1974) Toxic effect of the herbicide
paraquat. Chest, 65: 706-708.
KIMBROUGH, R.D. & GAINES, T.B. (1970) Toxicity of paraquat
in rats and its effect on rat lungs. Toxicol. appl.
Pharmacol., 17: 679-690.
KIMBROUGH, R.D. & LINDER, R.E. (1973) The ultrastructure of
the paraquat lung lesion in the rat. Environ. Res., 6: 265-273.
KIMURA, M., SUZUKI, E., & OHNISHI, H. (1980) [A case of
autopsy of an infant intoxicated by paraquat dichloride.]
Shoni ka Rinsho, 33: 732-734 (in Japanese).
KLIKA, E., KUNC, L., ANTALIKOVA, K., & KUNCOVA, M. (1980)
The morphology of the lung in the albino rat after paraquat
administration. Folia Morphol., 28: 188-191.
KNIGHT, B.A.G. & DENNY, P.J. (1970) The interaction of
paraquat with soil: adsorption by an expanding lattice clay
mineral. Weed Res., 10: 40-48.
KNIGHT, B.A.G. & TOMLINSON, T.E. (1967) The interaction of
paraquat (1,1'-dimethyl-4,4'-dipyridylium dichloride) with
mineral soils. J. soil Sci., 18: 223-243.
KORNBRUST, D.J. & MAVIS, R.D. (1980) The effect of paraquat
on microsomal lipid peroxidation in vitro and in vivo.
Toxicol. appl. Pharmacol., 53: 323-332.
KURISAKI, E. & SATO, H. (1979) [Toxicological studies on
herbicides. Intracorporeal distribution on paraquat dichloride
and diquat dibromide in rat.] Nippon Hoigaki Zasshi, 33: 656
(in Japanese).
LAM, H.F., TAKEZAWA, J., GUPTA, B.N., & STEE, E.W. van
(1980) A comparison of the effects of paraquat and diquat on
lung compliance, lung volumes and single breath. Toxicology,
18: 111-123.
LAVAUR, E., SION, G., GROLLEAU, G., & CARPENTER-LESECK, J.
(1979) Etude comparée de l'action du diquat et du paraquat
sur la muqueuse digestive de la souris, du rat, et du lapin.
Ann. Zool. Ecol. anim., 11: 159-169.
LEARY, J.B. (1978) Diquat and paraquat. In: Zweig, G. &
Sherma, J., ed. Analytical methods for pesticides and growth
regulators, New York, Academic Press, pp. 321-325.
LENGFELDER, E. & ELSTNER, E.F. (1978) Determination of the
superoxide dismutating activity of D-penicillamine copper.
Hoppe-Seyler's Z. Physiol. Chem., 359: 751-757.
LEVIN, P.J., KLAFF, L.J., ROSE, A.G., & FERGUSON, A.D.
(1979) Pulmonary effects of contact exposure to paraquat: a
clinical and experimental study. Thorax, 34: 150-160.
LEVIN, D.E., HOLLSTEIN, M., CHRISTMAN, M.F., SCHWIERS, E.A., &
AMES, B.N. (1982) A new Salmonella tester strain (TA 102)
with AT base pairs at the site of mutation detects oxidative
mutagens. Proc. Natl Acad. Sci. USA, 79: 7445-7449.
LEVITT, T. (1979) Determinations of paraquat in clinical
practice using radioimmunoassay. Proc. Anal. Div. Chem. Soc.,
16: 72-76.
LIDDLE, J.A., NEEDHAM, L.L., ROLLEN, Z.J., ROARK, B.R., &
BAYSE, D.D. (1980) Characterization of the contamination of
marijuana with paraquat. Bull. environ. Contam. Toxicol., 24:
49-53.
LITCHFIELD, M.H., DANIEL, J.W., & LONGSHAW, S. (1973) The
tissue distribution of the bipyridylium herbicides diquat and
paraquat in rats and mice. Toxicology, 1: 155-165.
LLOYD, E.L. (1969) Recovery after taking Weedol. Br. med. J.,
2(5650): 189.
LOCK, E.A. (1979) The effect of paraquat and diquat on renal
function in the rat. Toxicol. appl. Pharmacol., 48: 327-336.
LOCK, E.A. & ISHMAEL, J. (1979) The acute toxic effects of
paraquat and diquat on the rat kidney. Toxicol. appl.
Pharmacol., 50: 67-76.
LOTT, P.F., LOTT, J.W., & DOMS, D.J. (1978) The
determination of paraquat. J. chromatogr. Sci., 16: 390-395.
LUTY, S., CISAK, E., LATUSZYNSKA, J., & PRZYLEPA, E. (1978)
[Effect of paraquat on embryonic and post-embryonic
development in rat.] Bromatol. Chem. Toksykol., 11: 159-165
(in Polish).
MAFF (1980a) Agricultural Development and Advisory Service
'Pesticide science' reference book 1980, Lowestoft, England,
Ministry of Agriculture, Fisheries & Food, pp. 69-76
MAFF (1980b) Pest Infestation Control Laboratory Report
1977-1979, Lowestoft, England, Ministry of Agriculture,
Fisheries & Food, pp. 143-153.
MAFF (1981) Agricultural Development and Advisory Service
'Pesticide science' reference book 1981, Lowestoft, England,
Ministry of Agriculture, Fisheries & Food, pp. 21-27 and 55-63.
MAKOVSKII, V.N. (1972) [Toxicological and hygiene studies of
bipyridylium herbicides diquat and paraquat,] Referat,
Vinnize, USSR, pp. 1-23 (PhD degree thesis) (in Russian).
MALING, H.M., SAUL, W., WILLIAMS, M.A., BROWN, E.A.B., &
GILLETTE, J.R. (l978) Reduced body clearance as the major
mechanisms of the potentiation of beta2-adrenergic agonists of
paraquat lethality in rat. Toxicol. appl. Pharmacol., 43:
57-72.
MALONE, J.D.G., CARMODY, M., & KEOGH, B. (1971) Paraquat
poisoning. A review of nineteen cases. J. Irish Med. Assoc.,
64: 59-68.
MAREK, J., VELENSKA, Z., & HASKOVCOVA, I. (1981) [Kidney
changes in acute phase of paraquat intoxication in rats.]
Sb. Lék., 83: 100-104 (in Czech).
MASTERSON, J.G. & ROCHE, W.J. (1970) Fatal paraquat
poisoning. J. Irish Med. Assoc., 63: 261-264.
MATSUMOTO, T., MATSUMORI, H., KUWABARA, N., FUKUDA, Y., &
ARIWA, R. (1980) [A histopathological study of the liver in
paraquat poisoning. An analysis of 14 autopsy cases with
emphasis on bile duct injury.] Acta pathol. Jpn., 30: 859-870
(in Japanese).
MATSUMOTO, S., MATSUMORI, H., KUWABARA, N., FUKUDA, Y., &
ARIWA, R. (1981) [Histopathological studies on the
disturbance of bile ducts to paraquat.] Kanzo, 22: 309 (in
Japanese).
MATTHEW, H., LOGAN, A., WOODRUFF, M.F.A., & HEARD, B. (1968)
Paraquat poisoning. Lung transplantation. Br. med. J., 3:
759-763.
MCCORD, J.M. & FRIDOVICH, I. (1969) Superoxide dismutase -
an enzymic function for erythrocuprein (hemocuprein). J. Biol.
Chem., 244: 6049-6055.
MCDONAGH, B.J. & MARTIN, J. (1970) Paraquat poisoning in
children. Arch. Dis. Child., 45: 425-427.
MCELLIGOTT, T.F. (1972) The dermal toxicity of paraquat.
Differences due to techniques of application. Toxicol. appl.
Pharmacol., 21: 361-368.
MCGEOWN, M.G. (1975) Clinical aspects of paraquat poisoning.
In: Fletcher, K., ed., Clinical aspects of paraquat poisoning,
London, ICI Ltd, pp. 12-21.
MEES, G.C. (1960) Experiments on the herbicidal action of
1,1'-ethylene-2,2'dipyridylium dibromide. Ann. appl. Biol.,
48: 601-612.
MEHANI, S. (1972) The toxic effect of paraquat in rabbits
and rats. Ain Shams med. J., 23: 599-601.
MIRCEV, N. (1976) [Acute intoxication with Gramoxone
(paraquat).] Vatreshni Bolesti, 16: 99-101 (in Bulgarian).
MITHYANTHA, M.S. & PERUR, N.G. (1975) Paraquat retention by
soils. Mysore J. agric. Sci., 9: 276-282.
MONTGOMERY, M.R. (1976) Interaction of paraquat with the
pulmonary microsomal fatty acid desaturase system. Toxicol.
appl. Pharmacol., 36: 543-554.
MONTGOMERY, M.R. (1977) Paraquat toxicity and pulmonary
superoxide dismutase: an enzymic deficiency of lung
microsomes. Res. Commun. chem. Pathol. Pharmacol., 16: 155-158.
MONTGOMERY, M.R. & NIEWOEHNER, D.E. (1979) Oxidant-induced
alterations in pulmonary microsomal mixed-function oxidation.
Acute effects of paraquat and ozone. J. environ. Sci. Health,
13: 205-219.
MOODY, C.S. & HASSAN, H.M. (1982) Mutagenicity of oxygen
free radicals. Proc. Natl Acad. Sci., 79: 2855-2859.
MOURIN, K.A. (1967) Paraquat poisoning. Br. med. J., 4: 486.
MUKADA, T., SASANO, N., & SATO, K. (1978) [Autopsy findings
in a case of acute paraquat poisoning with extensive cerebral
purpura.] Tohoku J. exp. Med., 125: 253-263 (in Japanese).
MULLICK, F.G., TSHAK, K.G., MAHABIR, K., & STROMEYER, F.W.
(1981) Hepatic injury associated with paraquat toxicity in
humans. Liver, 1: 209-221.
MURRAY, R.E. & GIBSON, J.E. (1972) A comparative study of
paraquat intoxication in rats, guinea pigs and monkeys.
Exp. mol. Pathol., 17: 317-325.
MURRAY, R.E. & GIBSON, J.E. (1974) Paraquat disposition in
rats, guinea pigs and monkeys. Toxicol. appl. Pharmacol., 27:
283-291.
MUSSON, F.A. & PORTER, C.A. (1982) Effect of ingestion of
paraquat on a 20-week gestation fetus. Postgrad. Med. J., 58:
731-732.
NAGY, A. (1970) Paraquat and adrenal cortical necrosis.
Br. med. J. 2: 669.
NATORI, H., KOIKE, M., & KIRA, S. (1979) [Respiratory
failure due to the herbicide paraquat.] Gendai Iryo, 11:
1175-1182 (in Japanese).
NEWHOUSE, M., MCEVOY, D., & ROSENTHAL, D. (1978)
Percutaneous paraquat absorption. Arch. Dermatol., 114:
1516-1519.
NEWMAN, J.F. & EDWARDS, P.J. (1980) Paraquat: effect on
spraying eggs on hatachability and on the reproductive organs
of the chicks of pheasant, Phasianus colchicus, London, ICI
Ltd (ICI Plant Protection Division Report No. RJ 0090B).
NEWMAN, J.F. & WAY, J.M. (1966) Some ecological observations
on the use of paraquat and diquat as aquatic herbicides.
Proceedings of the 8th British Weed Control Conference,
Vol. 2, pp. 582-585.
NEWMAN, J.F. & WILKINSON, W.W. (1971) The effect of excess
quantities of paraquat in soil on the growth of vegetation,
London, ICI Ltd (Report No TMJ 606 A).
O'BRIEN, M.C. & PRENDEVILLE, G.N. (1978) A rapid sensitive
bioassay for determination of paraquat and diquat in water.
Weed Res., 18: 301-303.
OGATA, M., HASEGAWA, T., & UEDA, K. (1978) [Action of
paraquat dichloride and dimethyldithiocarbamate on the
mitochondrial conversion system and superoxide dismutase.]
Samgyo Igaku, 20: 551-552 (in Japanese).
OKAWADA, N., YAGASAKI, K., & KONDO, T. (1980) [Ocular
impairments due to an agricultural pesticide.] Nippon Noson
Igakkai Zasshi, 29: 550-551 (in Japanese).
OKONEK, S., WEILEMANN, L.S., MAJDANDZIC, J., SETYADHARME, H.,
REINECKE, H.J., BALDAMUS, C.A., LOHMAN, J., BONZEL, K.E., &
THON, T. (1982) Successful treatment of paraquat poisoning.
Activated charcoal per os and, continuous hemoperfusion.
J. Toxicol. clin. Toxicol., 19: 807-819.
OMAYE, S.T., REDDY, K.A., & CROSS, C.E. (1978) Enhanced lung
toxicity of paraquat in selenium-deficient rats. Toxicol.
appl. Pharmacol., 43: 237-247.
ONGOM, V.L., OWOR, R., & TONUSANGE, E.T. (1974) Paraquat
(Gramoxone) used as a pediculocide. In: Bagshore, A.F., ed.
The uses and abuses of drugs and chemicals in tropical Africa,
Nairobi, East Africa Literature Bureau, pp. 229-233.
PARK, J., PROUDFOOT, A.T., & PRESCOTT, L.F. (1975) Paraquat
poisoning. A clinical review of 31 cases. In: Fletcher, K.,
ed., Clinical aspects of paraquat poisoning, London, ICI Ltd,
pp. 46-53.
PARRY, J.M. (1973) The induction of gene conversion in yeast
by herbicide preparations. Mutat. Res., 21: 83-91.
PARRY, J.M. (1977) The use of yeast for the detection of
environmental mutagens using a fluctuation test. Mutat. Res.,
46: 165-176.
PASCHAL, D.C., NEEDHAM, L.L., ROLLEN, Z.J., & LIDDLE, J.A.
(1979) Determination of paraquat in sunflower seeds by
reversed-phase high performance liquid chromatography.
J. Chromatogr., 171: 85-90.
PASI, A., EMBREE, J.W., EISENLORD, G.H., & HINE, C.H. (1974)
Assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 26: 171-175.
PATRICK, G.B. (1980) Marijuana and the lung. Postgrad. med.
J., 67: 110-118.
PAYNE, W.L., POPE, J.D., & BENNER, J.E. (1974) Integrated
method for trifluralen, diphenamid and paraquat in soil and
runoff from agricultural land. J. agric. food Chem., 22: 79-82.
PEDERSEN, G., PEDERSEN, A., GREGERSEN, M., & KAMPE, B.
(1981) [Paraquat poisoning.] Ugeskr. Laeg., 143: 1202-1206
(in Danish).
PESTEMER, VON W., NOLTING, H.G., & LUNDEHN, Y.-R. (1979)
[Possible effects of repeated paraquat treatments on the
residue situation in the soil.] Nachrichtenbl. Dtsch
Pflanzenschutzd., 31: 166-170 (in German).
PICKOVA, J. (1978) [Determination of paraquat in the urine.]
Pr. Lék., 30: 266-267 (in Czech).
POPE, J.D. & BENNER, J.E. (1974) Colorimetric determination
of paraquat residues in soil and water. J. AOHC, 57: 202-204.
POPENOE, D. (1979) Effects of paraquat aerosol on mouse
lung. Arch. Pathol. lab. Med., 103: 331-334.
PROUDFOOT, A.T., STEWART, M.S., LEVITT, T., & WIDDOP, B.
(1979) Paraquat poisoning: significance of plasma-paraquat
concentrations. Lancet, 2(8138): 330-332.
PRYDE, A. & DARBY, F.J. (1975) The analysis of paraquat in
the urine by high speed liquid chromatography. J. Chromatogr.,
115: 107-109.
PURSER, D.A. & ROSE, M.S. (1979) The toxicity and renal
handling of paraquat in Cynomolgus monkeys. Toxicology, 15:
31-41.
RADAELLI, L. & MARTELLI, M. (1971) Residual toxicity of
paraquat absorbed on soil. Agrochimica, 15: 344-350.
REDDY, K.A., LITOV, R.E., & OMAYE, S.T. (1977) Effect of
pre-treatment with anti-inflammatory agents on paraquat
toxicity in the rat. Res. Commun. chem. Pathol. Pharmacol.,
17: 87-100.
RESTUCCIA, A., FOGLINI, A., & DE ALENTIS NANNINI, D. (1974)
Paraquat toxicity for rabbits. Vet. Ital., 25: 555-565.
RILEY, D. (1981) The fate and effect of paraquat and diquat
residues in soil. In: Proceedings for the National Spray Seed
Conference 1981, Albury, New South Wales, Australia.
RILEY, D., WILKINSON, W., & TUCKER, B.V. (1976) Biological
unavailability of bound paraquat residues in soil. In:
Kaufman, D.P., Still, G.G., Paulson, G.D., & Bandal, S.K., ed.
Bound and conjugated pesticide residues, pp. 301-353 (ACS
Symposium Series No. 29).
ROBERTSON, B. (1973) Paraquat poisoning as an experimental
model of the idiopathic respiratory distress syndrome. Bull.
Phys. Pathol. Res., 9: 1433-1452.
ROBERTSON, B., GROSSMANN, G., & IVEMARK, B. (1976) The
alveolar lining layer in experimental paraquat poisoning. Acta
pathol. microbiol. Scand. Section A, 84: 40-46.
ROSE, J. (1980) Paraquat poisoning. Lancet, 2(8200): 924.
ROSE, M.S. & SMITH, L.L. (1977) The relevance of paraquat
accumulation by tissues. In: Autor, A.P., ed., Biochemical
mechanisms of paraquat toxicity, New York, Academic Press, pp.
71-91.
ROSE, M.S., SMITH, L.L., & WYATT, I. (1974a) Evidence of
energy-dependent accumulation of paraquat in rat lung. Nature
(Lond.), 252: 314-315.
ROSE, M.S., CRABTREE, H.C., FLETCHER, K., & WYATT, I.
(1974b) Biochemical effects of diquat and paraquat.
Disturbance of the control of corticosteroid synthesis in rat
adrenal and subsequent effects on the control of live glycogen
utilization. Biochem. J., 138: 437-443.
ROSE, M.S., SMITH, L.L., & WYATT, I. (1976) The relevance of
pentose-phosphate pathway stimulation in rat lung to the
mechanisms of paraquat toxicity. Biochem. Pharmacol., 25:
1763-1767.
ROSLYCKY, E.B. (1977) Response of soil microbiota to
selected herbicide treatments. Can. J. Microbiol., 23: 426-433.
ROSS, W.E., BLOCK, E.R., & CHANG, R.Y. (1979) Paraquat-
induced DNA damage in mammalian cells. Biochem. biophys. Res.
Commun., 91: 1302-1308.
SAITO, K., PARKER, W.B., GARDNER, D.E., & MENZEL, D.B.
(1979) Paraquat accumulation by cultured lung cells.
Pharmacology, 21(Abstr. 218): 392.
SAMMAN, P.D. & JOHNSTON, E.N. (1969) Nail damage associated
with handling of paraquat and diquat. Br. med. J., 1: 818-819.
SCHLATTER, I. (1976) [Poisoning due to herbicide paraquat.]
Schweiz. Rundsch. Med., 65: 837-843 (in German).
SEIBER, J.N. & WOODROW, J.E. (1981) Sampling and analysis of
airborne residues of paraquat in treated cotton field
environments. Arch. environ. Contam. Toxicol., 10: 133-149.
SEIBER, J.N., WINTERLIN, W.L., & MCCHESNEY, B. (1979)
Residues of toxaphene, DEE, and paraquat in plant parts and
gin waste from treated cotton fields. Arch. environ. Contam.
Toxicol., 8: 125-137.
SEIDENFELD, J.J., WYKOFF, D., ZAVALA, D.C., & RICHARDSON,
J.B. (1978) Paraquat lung injury in rabbits. Br. J. ind.
Med., 35: 245-247.
SELECT COMMITTEE ON NARCOTICS ABUSE & CONTROL (1980) The use
of paraquat to eradicate illicit marihuana crops and the
health implications of paraquat-contaminated marihuana on the
US market. A report of the 96-Congress, Washington DC, US
Government Printing Office, pp. 1-97 (SCNAC-96-1-16).
SELYPES, A. & PALDY, A. (1978) The examination of the
mutagenic effect of two pesticides: Krezonit E and Gramoxone.
Proc. Hung. Ann. Meet. Biochem., 18: 77-78.
SELYPES, A., NAGYMAJTENYI, L., & BERENCSI, G. (1980)
Mutagenic and embryotoxic effects of paraquat and diquat.
Bull. environ. Contam. Toxicol., 25: 513-517.
SHARP, C.W., OTTOLENGHI, A., & POSNER, A.S. (1972)
Correlation of paraquat toxicity with tissue concentration and
weight loss of the rat. Toxicol. appl. Pharmacol., 22: 241-251.
SHU, H., TALCOTT, R.E., RICE, S.A., & WEI, E.T. (1979) Lipid
peroxidation and paraquat toxicity. Biochem. Pharmacol., 28:
327-331.
SHUZUI, T. (1980) [An autopsy case of paraquat poisoning.]
Mie Igaku, 23: 518-521 (in Japanese).
SINGH, S.P. & YADAV, N.K. (1978) Toxicity of some herbicides
to maior carp fingerlings. Indian J. Ecol., 5: 141-147.
SINOW, J. & WEI, E. (1973) Ocular toxicity of paraquat.
Bull. environ. Contam. Toxicol., 9: 163-168.
SLADE, P. (1965) Photochemical degradation of paraquat.
Nature (Lond.), 207: 515.
SLADE, P. (1966) The fate of paraquat applied to plants.
Weed Res., 6: 158-167.
SMALLEY, H.E. (1973) Toxicity and hazard of the herbicide
paraquat in turkeys. Poultry Sci., 52: 1625-1629.
SMITH, R.J. (1978) Poisoned pot becomes burning issue in
high places. Science, 200: 417-418.
SMITH, P. & HEATH, D. (1974) The ultrastructure and time
sequence of the early stages of paraquat lung in rats.
J. Pathol., 114: 177-184.
SMITH, P. & HEATH, D. (1976) Paraquat. Clin. Rev. Toxicol.,
4: 411-445.
SMITH, L.L. & ROSE, M.S. (1977a) A comparison of the effects
of paraquat and diquat on the water content of rat lung and
the incorporation of thymidine into lung DNA. Toxicology, 8:
223-230.
SMITH, L.L. & ROSE, M.S. (1977b) Biochemical changes in
lungs exposed to paraquat. In: Autor, A.P., ed. Biochemical
mechanisms of paraquat toxicity, New York, Academic Press.
SMITH, P., HEATH, D., & RAY, J.M. (1973) The pathogenesis
and structure of paraquat-induced pulmonary fibrosis in rats.
J. Pathol., 114: 57-67.
SMITH, L.L., WRIGHT, A., WYATT, I., & ROSE, M.S. (1974)
Effective treatment for paraquat poisoning in rats and its
relevance to treatment of paraquat poisoning in man. Br. med.
J., 4: 569-571.
SMITH, S.N., LYON, A.J., & SAHID, I.B. (1976) The breakdown
of paraquat and diquat by soil fungi. New Phytol., 77: 735-740.
SMITH, C.N., LEONARD, R.A., LANGDALE, G.W., & BAILEY, G.W.
(1978) Transport of agricultural chemicals from small upland
Piedmont watersheds, Springfield, Virginia, National Technical
Information Service, p. 386 (Report PB-285, 134).
SMITH, L.L., ROSE, M.S., & WYATT, I. (1979) The pathology
and biochemistry of paraquat. In: Oxygen free radicals and
tissue damage, Amsterdam, North Holland, Excerpta Medica,
pp. 321-341 (CIBA Foundation Series 65 - new series).
SMITH, T.F., NOACK, A.J., & COSH, S.M. (1981a) The effect of
some herbicides on vesicular-arbuscular endophyte abundance in
the soil and on infection of host roots. Pestic. Sci., 12:
91-97.
SMITH, L.L., WYATT, I., & ROSE, M.S. (1981b) Factors
affecting the efflux of paraquat from rat lung slices.
Toxicology, 19: 197-207.
SODERQUIST, C.J. & CROSBY, D.G. (1972) The gas chromato-
graphic determination of paraquat in water. Bull. environ.
Contam. Toxicol., 8: 363-368.
STAIFF, D.C., IRLE, G.K., & FELSENSTEIN, W.C. (1973)
Screening of various adsorbents for protection against
paraquat poisoning. Bull. environ. Contam. Toxicol., 10:
193-199.
STAIFF, D.C., COMER, S.W., ARMSTRONG, J.F., & WOLFE, H.R.
(1975) Exposure to the herbicide paraquat. Bull. environ.
Contam. Toxicol., 14: 334-340.
STAIFF, D.C., BUTLER, L.C., & DAVIS, J.E. (1981) A fie1d
study of the chemical degradation of paraquat dichloride
following simulated spillage on soil. Bull. environ. Contam.
Toxicol., 26: 16-21.
STEFFEN, C. & NETTER, K.J. (1979) On the mechanism of
paraquat action on microsomal oxygen reduction and its
relation to lipid peroxidation. Toxicol. appl. Pharmacol., 47:
593-602.
STEFFEN, C., MULIAWAN, H., & KAPPUS, H. (1980) Lack of in
vivo lipid peroxidation in experimental paraquat poisoning.
Arch. Pharmacol., 310(3): 241-243.
STEPHENS, D.S., WALKER, D.H., SCHAFFNER, W., KAPLOWITZ, L.G.,
BRASHEAR, R.H., ROBERTS, R., & SPICKARD, W.A. (1981)
Pseudodiphtheria: prominent pharyngeal membrane associated
with fetal paraquat ingestion. Ann. intern. Med., 94: 202-204.
STEVENS, M.A. & WALLEY, J.K. (1966) Tissue and milk residues
arising from the ingestion of single doses of diquat and
paraquat by cattle. J. Sci. Food Agric., 17: 472-475.
STEWART, M.J., LEVITT, T., & JARVIE, D.R. (1979) Emergency
estimations of paraquat in plasma. A comparison of the RIA and
ion pair/colorimetric methods. Clin. Chim. Acta, 94: 253-257.
SUGAYA, H., OHE, T., UENO, T., KAWASHIMA, T., SUGITA, T.,
MAEHARA, M., HISANCHI, T., IORI, M., HARADA, H., & SHIGEMATA,
S. (1980) [Clinical discussion on six cases of paraquat
dichloride intoxication.] Nippon Naika Gakkai Zasshi, 69: 876
(in Japanese).
SUMMERS, L.A. (1980) The bipyridylium herbicides, London,
New York, Toronto, Sydney, San Francisco, Academic Press,
pp. 1-449.
SWAN, A.A.B. (1969) Exposure of spray operators to paraquat.
Br. J. ind. Med., 26: 322-329.
SYKES, B.J., PURCHASE, I.F.H., & SMITH, L.L. (1977)
Pulmonary ultrastructure after oral and intravenous dosage of
paraquat to rats. J. Pathol., 121: 233-241.
TAKAHASHI, T., YAMAMOTO, K., SAWAI, T., OKUDO, T., MUKODA, T.,
MINAGAWA, N., & SUGAYA, H. (1978) [Examination of 5
autopsies following paraquat dichloride intoxication,
especially of parenchymatous organ lesions.] Nippon
Byorigakkai Kaishi, 67: 138-139 (in Japanese).
TAKAYAMA, K., TAKEUCHI, K., SUGA, E., IWABUCHI, K., & TOMICHI,
N. (1978) [A case of autopsy following paraquat dichloride
intoxication.] Nippon Byorigakkai Kaishi, 67: 139 (in
Japanese).
TAKEUCHI, K., TAKAYAMA, K., TOMICHI, N., KAN, E., YAGAWA, K.,
& IWABUCHI, K. (1980) [Paraquat poisoning in a pregnant
woman.] Nippon Byorigakkai Kaishi, 18: 747-752 (in Japanese).
TALCOTT, R.E., SHU, H., & WEI, E.T. (1979) Dissociation of
microsomal oxygen reduction and lipid peroxidation with the
electron acceptors, paraquat and menadione. Biochem.
Pharmacol., 26: 665-671.
TCHIPILSKA, L.N. (1980) [The influence of some pesticides on
the soil microorganisms in connexion with the hygiene
evaluation of the soil,] Referat, Sofia, pp. 1-39 (PhD degree
thesis) (in Bulgarian).
THOMPSON, W.D. & PATRICK, R.S. (1978) Collagen propyl
hydroxylase levels in experimental paraquat poisoning.
Br. J. exp. Pathol., 59: 288-291.
TOMPSETT, S.L. (1970) Paraquat poisoning. Acta pharmacol.
toxicol., 28: 346-358.
TOMURA, M., ETO, K., SAGARA, K., & SATO, T. (1979) [A case
of disturbance of the liver and kidney.] Kanzo, 20: 1016 (in
Japanese).
TONER, P.G., VETTERS, J.M., SPILG, W.G.S., & HARLAND, W.A.
(1970) Fine structure of the lung lesion in a case of
paraquat poisoning. J. Pathol., 102: 182-185.
TRUSH, M.A., MIMNAUGH, E.G., GINSBURG, E., & GRAM, T.E.
(1981) In vitro stimulation by paraquat of reactive oxygen-
mediated lipid peroxidation in rat lung microsomes. Toxicol.
appl. Pharmacol., 60(2): 279-286.
TRUSH, M.A., MIMNAUGH, E.G., GINSBURG, E., & GRAM, T.E.
(1982) Studies on the in vitro interaction of mitomycin C,
nitrofurantoin, and paraquat with pulmonary microsomes.
Stimulation of reactive oxygen-dependent lipid peroxidation.
Biochem. Pharmacol., 31(21): 3335-3346.
TSUNENARI, S., MUTO, H., SASAKI, S., SUGITA, H., & KANDA, M.
(1975) [Forensic toxicological studies on herbicide
(Gramoxone).] Jpn. J. leg. Med., 29: 88-102 (in Japanese).
TSUNENARI, S., YONEMITSU, K., UCHIMURA, Y., & KANDA, M.
(1981) The influence of putrefactive changes on the
determination of paraquat in autopsy materials. Forensic Sci.
Int., 17: 51-56.
TSUTSUI, Y., NAKABAYASHI, H., SUZUKI, H., & OGURA, K. (1976)
[Studies on the toxicity of paraquat - Part I.] Nippon Noson
Igakkai Zasshi, 25: 614-621 (in Japanese).
TU, C.M. & BOLLEN, W.B. (1968) Effect of paraquat on
microbial activities in soils. Weed Res., 8: 28-37.
TWEATS, D.J. (1975) The effect of R (drug resistance)
factors on the detection of mutagens by E. coli K 12,
Brussels, Belgium, Annual Euratom Report, Biological Sciences,
316 pp.
TYBURCZYK, W., BORKOWSKA, I., CHORAGIEWICZ, H., & KLIMEK, K.
(1979) [Effect of paraquat on some biochemical tests in
rats.] Bromatol. Chem. Toksykol., 12: 283-288 (in Polish).
UKAI, S., HIROSE, K., & KAWASE, S. (1977) [Gas chromatography
of reduction products of the herbicides "diquat" and "paraquat".]
Eisei Kagaku, 23: 32-38 (in Japanese).
US CONGRESSIONAL HEARING (1979) Health implications of
paraquat-contaminated marihuana. Select Committee on Narcotics
Abuse & Control, House of Representatives. Hearing of the 96th
Congress, Washington DC, US Government Printing Office, pp.
1-103 (SCNAC-96-1-3).
VALE, J.A. (1977) Paraquat poisoning. Nurs. Times, 73:
154-155.
VAZIRI, N.D., NESS, R.L., FAIRSHTER, R.D., SMITH, W.R., &
ROSEN, S.M. (1979) Nephrotoxicity of paraquat in man. Arch.
intern. Med., 139: 172-174.
VESELEY, D.L., WATSON, B., & LEVEY, G.A. (1979) Activation
of liver guanylate cyclase by paraquat: possible role of
superoxide anion. J. Pharmacol. exp. Ther., 209: 162-164.
VIJEYARATNAM, G.S. & CORRIN, B. (1971) Experimental paraquat
poisoning: a histological and electron-optical study of the
changes in the lung. J. Pathol., 103: 123-129.
VUCINOVIC, V. (1978) [Four cases of poisoning with
paraquat.] Arch. Hig. Rada, 29: 261-265 (in Serbo-Croat).
WADDELL, W.J. & MARLOWE, C. (1980) Tissue and cellular
disposition of paraquat in mice. Toxicol. appl. Pharmacol.,
56: 127-140.
WAIGHT, J.J.J. & WHEATHER, R.H. (1979) Fatal percutaneous
paraquat poisoning. J. Am. Med. Assoc., 242: 472.
WALKER, M., DUGARD, P.H., & SCOTT, R.C. (1983) Absorption
through human and laboratory animal skins: in vitro
comparison. Acta pharm. Suec., 20(1): 52-53.
WALTERS, K.A., DUGAR, P.H., & FLORENCE, A.T. (1981)
Non-ionic surfactants and gastric mucosal transport of
paraquat. J. Pharm. Pharmacol., 33: 207-213.
WARD, C.D., STONES, D.P.A., CONNEL, H., CULLEN, D.R., &
WATKIN, J.I. (1976) Paraquat poisoning. Lancet, 1(7971):
1247.
WATANABE, I., SAKAI, K., TOYAMA, K., UENO, M., & WATANABE, M.
(1979) [On 3 cases of ocular disturbance due to Gratoxone, a
herbicide containing 24% paraquat dichloride.] Ganka Rinsho
Iho, 73: 1244-1246 (in Japanese).
WAUCHOPE, R.D. (1979) Pesticides in runoff water. Agric.
Res., 27: 11.
WAY, J.M., NEWMAN, J.F., MOORE, N.W., & KNAGGS, F.W. (1971)
Some ecological effects of the use of paraquat for the control
of weeds in small lakes. J. appl. Ecology, 8: 509-532.
WEBER, J.B., PERRY, P.W., & UPCHURCH, R.P. (1965) The
influence of temperature and time on the adsorption of
paraquat, diquat, 2,4-D and prometone by clays, charcoal and
on anion-exchange resin. Soil Sci. Am. Soc. Proc., 29: 678-688.
WHO/FAO (1978) Data sheets on pesticides No. 4, Rev.1 -
Paraquat, Geneva, World Health Organization (Unpublished
Report No.: VBC/DS/75.4 Rev.1 (8/78)).
WILKINSON, W. (1980) Paraquat and diquat: Longterm high rate
trial, Frensham UK. Management of site, effects on crops and
weeds and residues in crops, London, ICI Ltd (Report RJ0013B).
WITSCHI, H.P., KACEW, S., HIRAI, K., & COTE, M.G. (1977) In
vivo oxidation of reduced nicotine amide-adenine dinucleotide
phosphate by paraquat and diquat in rat lung. Chem. Biol.
Interactions., 19: 143-160.
WOHLFAHRT, D.J. (1982) Fatal paraquat poisoning after skin
absorption. Med. J. Aust., 1: 512-513.
WOJECK, G.A., PRICE, J.F., NIGG, A.N., & STAMPER, J.H.
(1983) Worker exposure to paraquat and diquat. Arch. environ.
Contam. Toxicol., 12: 65-70.
WORTHING, C.R. (1979) The pesticide manual, Croydon,
England, British Crop Protection Council (BCPC Publications).
WRIGHT, K.A. & CAIN, M. (1970) Microbial degradation of
4-carboxy-1-methylpyridylium chloride, a photolytic product of
paraquat. Biochem. J., 118: 52.
WRIGHT, N., YEOMAN, W.B., & HALE, K.A. (1978) Assessment of
severity of paraquat poisoning. Br. med. J., 2: 396-397.
WYATT, I., DOSS, A.W., ZAVALA, D.C., & SMITH, L.L. (1981)
Intrabronchial instillation of paraquat in rats: lung
morphology and retention study. Br. J. ind. Med., 38: 42-48.
YOSHIDA, K., TANAKA, Y., IKUNUMA, T., TERUKIMATSU, S., KUSANO,
E., ASANO, Y., & HOSODA, S. (1980) [A case report of
survival after intoxication by paraquat.] Nippon Nuika Gakkai
Zasshi, 69: 1487-1489 (in Japanese).
YOUNGMAN, R.J. & DODGE, A.D. (1979) [Mechanism of paraquat
action: inhibition of the herbicidal effect by a copper
chelate with superoxide-dismutating activity.] Z. Naturforsch.
(Teil C), 34: 1032-1035 (in German).
ZAVALA, D.C. & RHODES, M.L. (1978) An effect of paraquat on
the lungs of rabbits. Its implications in smoking contaminated
marijuana. Chest, 74: 418-420.
DIQUAT
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General properties
Diquat (1,1'ethylene, 2,2'bipyridyl) is a non-selective contact
herbicide. It is sold primarily as a 20% w/v solution in many
countries and is manufactured in the United Kingdom. It is
exclusively manufactured as a dibromide salt and is usually
formulated to contain wetters.
The herbicidal property of diquat depends on its ability to
undergo a single electron addition to form a radical that reacts
with molecular oxygen to reform diquat and concomitantly produce a
superoxide anion. This oxygen radical may directly or indirectly
cause cell death.
It is possible to detect the compound because of its ability to
form a radical. Analytical procedures are available.
1.1.2. Environmental distribution and transformation
environmental effects
Diquat undergoes rapid photochemical degradation in aqueous
solution and on surfaces. The major degradation products produced
in water have been identified and are of lower acute oral toxicity
for rats than diquat itself. The photochemical degradation of
diquat on plants is more complex than that in water. On diquat-
desiccated wheat and barley, diquat itself normally constitutes the
most important single compound. The most important photochemical
degradation products have been identified, they are of low
mammalian toxicity. No other well-defined major degradation
product is formed.
Ruminants excrete diquat and its photochemical products rapidly
and very little is transferred to milk and tissues. Consequently,
residue levels in products of animal origin are very low.
Ingestion of diquat and its photochemical products at higher levels
than would be found in practice did not induce ill effects in
ruminants.
Diquat reaching the soil becomes rapidly and strongly adsorbed
to clay minerals in soil. This process inactivates the herbicidal
activity of diquat. While free diquat is degraded by a range of
soil microorganisms, degradation of strongly adsorbed diquat is
relatively slow. In plot studies, the rate of degradation of
diquat in soil is very slow or non-detectable. However, in long-
term field studies, degradation rates of the order of 5 - 10% per
year have been shown. This is greater than the rate required in
normal practice to prevent saturation of the deactivation capacity
of agricultural/horticultural soils. Strongly-bound diquat has no
adverse effects on soil microfauna or soil microbial processes.
Diquat residues disappear rapidly from water by adsorption on
aquatic weeds and by strong adsorption on bottom mud. Diquat is of
low toxicity for fish and is not accumulated in them. Normal
applications of diquat for aquatic weed control are not harmful to
aquatic organisms. However, care should be taken in applying
diquat to water containing heavy weed growth to treat only a part
of the weed growth, since oxygen consumed by subsequent weed decay
may decrease dissolved oxygen levels to an extent that may be
dangerous for fish. Treated water should not be used for overhead
irrigation until a period of 10 days has elapsed following
treatment.
Diquat is not volatile and the concentrations of airborne
diquat during spraying have been shown to be very low.
1.1.3. Kinetics and metabolism
Diquat is poorly absorbed from the intestinal tract and skin.
Diquat monopyridone is the major metabolite of diquat in the body;
of lesser importance is diquat dipyridone. Both metabolites are
considerably less toxic than diquat itself. Depending on species
and route of administration, less than 20% of the dose is
metabolized. The gastrointestinal microflora appear to be mainly
responsible for the metabolism of diquat.
Compared with paraquat, accumulation of diquat in the lungs is
far less marked, but diquat shows a certain preference for the
kidneys. The kidneys are the major route of excretion, but a
considerable amount of diquat can also be excreted in the bile,
varying with the animal species.
1.1.4. Effects on animals
Diquat is less toxic than paraquat and does not give rise to
the specific lung disease that is so typical of paraquat poisoning.
Gastrointestinal disturbances, with vomiting, greenish diarrhoea,
and abdominal distension from the significant accumulation of water
in the lumen of the intestines, are typical of diquat poisoning,
together with progressive haemoconcentration, which may progress to
lethargy, coma, and death. At high doses, minor toxicity has been
noted in the liver, kidney, and the nervous and endocrine systems.
Diquat has induced cataracts after prolonged oral exposure
although this effect has not been reported in man. It is less
irritant to the skin, mucous membranes, and the eye than paraquat,
and is not known to be a sensitizer.
Diquat is not teratogenic or carcinogenic.
In vitro mutagenicity studies were inconclusive, though
generally suggesting only weak activity, while the results of in
vivo studies have been negative. A no-observed-adverse-effect
level of 0.75 mg diquat ion/kg body weight per day has been
established from long-term feeding studies on rats.
1.1.5. Effects on man
Occupational exposure to diquat does not pose a health risk if
the recommendations for use are followed and there is adherence to
safe working practices.
Diquat poisoning by suicidal or accidental ingestion is much
less common than paraquat poisoning. It produces a similar severe
clinical syndrome with two notable differences: (a) diarrhoea is a
prominent feature, and (b) pulmonary fibrosis has not been
described.
Accidental cases are usually due to ingestion of decanted
diquat.
The lethal dose for man appears to be approximately 6 - 12
grams of diquat dibromide. In agricultural workers, inflammation
and bleeding of the nasal mucosa have been reported, as well as
nail changes and delayed wound healing.
1.2. Recommendations
1.2.1. General
Where practical and reasonable, the availability and use of the
20% liquid product should be limited to bona fide agriculturalists,
horticulturalists, and professional users who work with trained
personnel, properly maintained equipment, and adequate supervision.
Every effort should be made to prevent the practice of
decanting or rebottling of the product into containers that have
not been properly labelled.
1.2.2. Prevention and treatment
Attention should be drawn to the fact that persons with skin
lesions (either pre-existing or following contamination with
diquat) should not be permitted to take any part in spraying
procedures until skin condition has resolved.
It must be stressed that treatment of persons with diquat
poisoning should be instituted as early as possible. The
likelihood of recovery from a fatal dose is greatest when therapy
begins within 5 - 6 h of poisoning.
1.2.3. Experimental work
Results of existing mutagenicity and carcinogenicity studies
generally suggest that diquat is unlikely to induce genotoxic
effects in man, but more detailed information is required.
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and Chemical Properties
Diquat is a non-selective contact bipyridylium herbicide and
desiccant. The herbicide is supplied mainly as an aqueous solution
of the dibromide (1,1'-ethylene-2,2'-bipyridylium dibromide,
C12H12N2 Br2), with a relative molecular mass of 184.2 based on the
cation. The commonly available analytical standard is diquat
dibromide monohydrate, which is an odourless, pale yellow,
crystalline powder. Some of the other physical properties of
diquat dibromide are listed in Table 1. It is slightly soluble in
alcohol, and practically insoluble in non-polar organic solvents
(Summers, 1980). Diquat is non-explosive and non-inflammable in
aqueous formulations.
Table 1. Physical properties of diquat dibromide
--------------------------------------------------
Specific gravity at 20 °C 1.200
Melting point 180 °C
Boiling point approximately 300 °C
with decomposition
Solubility in water at 20 °C 700 g/litre
pH of liquid formulation 6.0 - 7.0
Evaporation rate not applicable
Vapour pressure not measurable
--------------------------------------------------
Diquat is stable in neutral or acid solutions but is hydrolysed
by alkali. It is inactivated by inert clay and by anionic
surfactants. Diquat dibromide has the following chemical
structure:
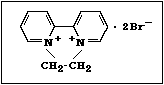 Diquat is generally marketed as an aqueous solution of the
dibromide salt Reglone(R) (200 g ion/1itre). It is a dark
reddish-brown liquid containing wetting agents that remains stable
in the original polyethylene containers, for a long time, under
normal atmospheric conditions.
Water-soluble granules containing 2.5% diquat and 2.5% paraquat
are used in home gardens. Diquat is sold under several different
trade names: Deiquat, Aquacide, Dextrone, Reglox, Weedtrim-D
(Vanholder et al., 1981). Fletcher (1975) listed the commercial
forms of diquat, many of which are combinations containing paraquat
or other herbicides.
2.2. Analytical Procedures
The detection of diquat depends on its reduction to the free
radical with sodium dithionite (Summers, 1980). Calderbank & Yuen
(1966) developed a column chromatographic procedure for
colorimetric diquat determinations in food and biological tissues.
The sensitivity of the method varied down to 0.01 mg/kg. An
immunological assay of diquat was published by Williams et al.
(1976). The minimum detectable quantity of diquat was 60 pg/ml.
Pyl & Giebelmann (1978) proposed a thin-layer chromatographic
method for diquat determinations with a detection threshold of
0.5 - 1 µg diquat.
Soil
Diquat residues in soil have been determined using
spectrophotometric analysis (ICI, 1972), the detection limit being
approximately 0.1 mg/kg, depending on the sample. An extraction
technique for the spectrophotometric measurement of diquat has been
published by Leary (1978).
Water
Diquat residues in water have been determined
spectrophotometrically with a limit of detection < 0.001 -0.01
mg/litre (ICI, 1972a). Benecke (1977) used the inhibition of algal
trichome movements by diquat involving photoelectric detection of
their inhibition. A concentration of 1 µg diquat in the test
sample was satisfactorily detected. A Lemna minor bioassay was
reported by O'Brien & Prendeville (1978) for diquat determination
in water. The minimum diquat concentration that could be detected
ranged from 1.8 µg/ml after 3 h of treatment to 0.00018 µg/ml after
72 h of treatment.
Plants and food
The method of Calderbank & Yuen (1966) has been used for
determining diquat in crops and animal tissues with detection
limits of 0.1 mg/kg to 0.01 mg/kg, depending on the sample (ICI,
1972b). Leary (1978) developed a spectrophotometric procedure for
diquat determination in crops and animal tissues (but not for whole
blood). The detection limit was 0.01 mg/kg when a 50 g sample was
taken.
A gas-chromatographic method for determining diquat residues
was published by King (1978). The detection limit was 0.01 mg/kg.
The application of gas chromatography in the analysis of food for
diquat has been discussed by Dickes (1979).
Biological tissues
The analytical method for diquat residues in milk is
spectrophotometry (ICI, 1972a), with a detection limit of 0.01
mg/litre. Tompsett (1970) reported a cation exchange technique for
colorimetric diquat determination in biological fluids and tissues
of patients with diquat poisoning. This technique is similar to
those applied for paraquat determination but more time-consuming.
A spectrophotometric procedure for diquat determination in serum,
urine, and biological tissues has been published by Leary (1978).
Gas-chromatographic analysis of herbicides containing diquat
dibromide and paraquat dichloride in forensic toxicology was
proposed by Ukai et al. (1977). The procedure was found to be well
suited for assaying diquat and paraquat simultaneously at 10 - 90
mg/litre.
3. SOURCES IN THE ENVIRONMENT
3.1. Production and Uses
Diquat is manufactured in the United Kingdom and does not occur
naturally. It is produced by the oxidative coupling of 2 molecules
of pyridine over a heated Raney nickel catalyst to 2,2'-bipyridyl.
It is then reacted with ethylene dibromide in water to give diquat.
Formulations of diquat dibromide are used in more than 100
countries all over the world, mainly as a desiccant but also as a
herbicide. In many countries, diquat is formulated locally on the
basis of the imported active ingredient. Data on world production
and uses are not available.
It is used to control both broad-leaved weeds among crops and
submerged and floating weeds in water bodies, for potato haulm
destruction, and for seed crop desiccation (rice, sunflower, etc.).
Application rates are usually of the order of 0.56 - 0.84 kg/ha for
potato haulm destruction, 0.42 -1.96 kg/ha for seed crop
desiccation, pre-harvest rice desiccation, and pre-crop weed
control (beans, beetroots, cabbages, onions, etc.), 0.42 - 1.12
kg/ha for aquatic weed control, and 0.28 - 0.84 kg/ha for pre-plant
weed control. Working dilutions vary between 1 and 5 g/litre water.
It is applied by ground sprayers (not mist-blowers) in 200 - 500
litres of the solution per hectare and in some countries aerially
in 40 - 50 litres of solution per ha.
Conning et al. (1969) summarized the mechanism of the
herbicidal effect of diquat. Light and oxygen are required for the
damage, which affects only the green parts of the plant. The
blockage of photosynthesis is due to disturbed photosynthetic
electron transport resulting from a single-electron redox cycling
reaction, as described for paraquat (Paraquat, section 3.3).
<4. ENVIRONMENTAL DISTRIBUTION, LEVELS, AND EXPOSURE
4.1. Photochemical and Microbial Degradation of Diquat
4.1.1. Photochemical degradation
In agricultural practice, most of the diquat spray is initially
deposited on plant surfaces and part of it on the soil surface.
According to Black et al. (1966), photochemical degradation is
responsible for the rapid decrease in the concentration of diquat
following the spraying of herbage. Application of 0.284 kg/ha
resulted in 12 - 48 mg diquat/kg dry herbage on the first day,
2.5 - 10.9 mg/kg after 3 - 4 days, and 1.0 - 5.7 mg/kg, 7 days
after treatment. Photochemical degradation appears to occur more
rapidly in the case of diquat than in the case of paraquat. The
light absorption maximum for diquat occurs at a longer wavelength
(310 nm) than for paraquat (256 nm), and this partly explains the
high rate of photochemical decomposition in the case of diquat.
The major degradation products have been identified; they appear to
be of low oral toxicity for rats and seem unlikely to produce
adverse environmental effects (Black et al., 1966). Cavell (1979)
monitored the photochemical degradation of 14C-diquat in aqueous
solutions aerated for 40 h. Decomposition of diquat continued
after the plants were dead and the degradation products were not
translocated from the desiccated leaves of the plants. Diquat
photochemical degradation products (Cavell, 1979) are shown in Fig.
1A.
Diquat is generally marketed as an aqueous solution of the
dibromide salt Reglone(R) (200 g ion/1itre). It is a dark
reddish-brown liquid containing wetting agents that remains stable
in the original polyethylene containers, for a long time, under
normal atmospheric conditions.
Water-soluble granules containing 2.5% diquat and 2.5% paraquat
are used in home gardens. Diquat is sold under several different
trade names: Deiquat, Aquacide, Dextrone, Reglox, Weedtrim-D
(Vanholder et al., 1981). Fletcher (1975) listed the commercial
forms of diquat, many of which are combinations containing paraquat
or other herbicides.
2.2. Analytical Procedures
The detection of diquat depends on its reduction to the free
radical with sodium dithionite (Summers, 1980). Calderbank & Yuen
(1966) developed a column chromatographic procedure for
colorimetric diquat determinations in food and biological tissues.
The sensitivity of the method varied down to 0.01 mg/kg. An
immunological assay of diquat was published by Williams et al.
(1976). The minimum detectable quantity of diquat was 60 pg/ml.
Pyl & Giebelmann (1978) proposed a thin-layer chromatographic
method for diquat determinations with a detection threshold of
0.5 - 1 µg diquat.
Soil
Diquat residues in soil have been determined using
spectrophotometric analysis (ICI, 1972), the detection limit being
approximately 0.1 mg/kg, depending on the sample. An extraction
technique for the spectrophotometric measurement of diquat has been
published by Leary (1978).
Water
Diquat residues in water have been determined
spectrophotometrically with a limit of detection < 0.001 -0.01
mg/litre (ICI, 1972a). Benecke (1977) used the inhibition of algal
trichome movements by diquat involving photoelectric detection of
their inhibition. A concentration of 1 µg diquat in the test
sample was satisfactorily detected. A Lemna minor bioassay was
reported by O'Brien & Prendeville (1978) for diquat determination
in water. The minimum diquat concentration that could be detected
ranged from 1.8 µg/ml after 3 h of treatment to 0.00018 µg/ml after
72 h of treatment.
Plants and food
The method of Calderbank & Yuen (1966) has been used for
determining diquat in crops and animal tissues with detection
limits of 0.1 mg/kg to 0.01 mg/kg, depending on the sample (ICI,
1972b). Leary (1978) developed a spectrophotometric procedure for
diquat determination in crops and animal tissues (but not for whole
blood). The detection limit was 0.01 mg/kg when a 50 g sample was
taken.
A gas-chromatographic method for determining diquat residues
was published by King (1978). The detection limit was 0.01 mg/kg.
The application of gas chromatography in the analysis of food for
diquat has been discussed by Dickes (1979).
Biological tissues
The analytical method for diquat residues in milk is
spectrophotometry (ICI, 1972a), with a detection limit of 0.01
mg/litre. Tompsett (1970) reported a cation exchange technique for
colorimetric diquat determination in biological fluids and tissues
of patients with diquat poisoning. This technique is similar to
those applied for paraquat determination but more time-consuming.
A spectrophotometric procedure for diquat determination in serum,
urine, and biological tissues has been published by Leary (1978).
Gas-chromatographic analysis of herbicides containing diquat
dibromide and paraquat dichloride in forensic toxicology was
proposed by Ukai et al. (1977). The procedure was found to be well
suited for assaying diquat and paraquat simultaneously at 10 - 90
mg/litre.
3. SOURCES IN THE ENVIRONMENT
3.1. Production and Uses
Diquat is manufactured in the United Kingdom and does not occur
naturally. It is produced by the oxidative coupling of 2 molecules
of pyridine over a heated Raney nickel catalyst to 2,2'-bipyridyl.
It is then reacted with ethylene dibromide in water to give diquat.
Formulations of diquat dibromide are used in more than 100
countries all over the world, mainly as a desiccant but also as a
herbicide. In many countries, diquat is formulated locally on the
basis of the imported active ingredient. Data on world production
and uses are not available.
It is used to control both broad-leaved weeds among crops and
submerged and floating weeds in water bodies, for potato haulm
destruction, and for seed crop desiccation (rice, sunflower, etc.).
Application rates are usually of the order of 0.56 - 0.84 kg/ha for
potato haulm destruction, 0.42 -1.96 kg/ha for seed crop
desiccation, pre-harvest rice desiccation, and pre-crop weed
control (beans, beetroots, cabbages, onions, etc.), 0.42 - 1.12
kg/ha for aquatic weed control, and 0.28 - 0.84 kg/ha for pre-plant
weed control. Working dilutions vary between 1 and 5 g/litre water.
It is applied by ground sprayers (not mist-blowers) in 200 - 500
litres of the solution per hectare and in some countries aerially
in 40 - 50 litres of solution per ha.
Conning et al. (1969) summarized the mechanism of the
herbicidal effect of diquat. Light and oxygen are required for the
damage, which affects only the green parts of the plant. The
blockage of photosynthesis is due to disturbed photosynthetic
electron transport resulting from a single-electron redox cycling
reaction, as described for paraquat (Paraquat, section 3.3).
<4. ENVIRONMENTAL DISTRIBUTION, LEVELS, AND EXPOSURE
4.1. Photochemical and Microbial Degradation of Diquat
4.1.1. Photochemical degradation
In agricultural practice, most of the diquat spray is initially
deposited on plant surfaces and part of it on the soil surface.
According to Black et al. (1966), photochemical degradation is
responsible for the rapid decrease in the concentration of diquat
following the spraying of herbage. Application of 0.284 kg/ha
resulted in 12 - 48 mg diquat/kg dry herbage on the first day,
2.5 - 10.9 mg/kg after 3 - 4 days, and 1.0 - 5.7 mg/kg, 7 days
after treatment. Photochemical degradation appears to occur more
rapidly in the case of diquat than in the case of paraquat. The
light absorption maximum for diquat occurs at a longer wavelength
(310 nm) than for paraquat (256 nm), and this partly explains the
high rate of photochemical decomposition in the case of diquat.
The major degradation products have been identified; they appear to
be of low oral toxicity for rats and seem unlikely to produce
adverse environmental effects (Black et al., 1966). Cavell (1979)
monitored the photochemical degradation of 14C-diquat in aqueous
solutions aerated for 40 h. Decomposition of diquat continued
after the plants were dead and the degradation products were not
translocated from the desiccated leaves of the plants. Diquat
photochemical degradation products (Cavell, 1979) are shown in Fig.
1A.
 4.1.2. Microbial degradation
Photochemical degradation of diquat on plants is quicker than
microbial degradation in soil. Microbial degradation of strongly-
bound diquat in soil is slow, but is faster in culture. The
degradation of diquat by soil fungi was studied by Smith et al.
(1976). The degradation of 14C-diquat to 14CO2 by Aspergillus
niger was tested by 4 different fungal test systems. High
intracellular herbicidal levels and inability to grow in the
presence of low diquat concentrations in the media characterized
the species unable to decompose diquat. Under laboratory
conditions, diquat degradation by Pseudomonas started after 3 days
(Tchipilska, 1980). Under field conditions, degradation started
after 10 days, and was related to the ambient temperature, and the
aeration and type of soil.
The fact that no significant hazard has been observed for
ruminants from diquat-treated herbage, or for the general
population from crops and water, is explained by the rapid
photochemical degradation of diquat.
4.2. Diquat Adsorption, Residue Levels, and Exposure in Soil
4.2.1. Diquat adsorption on soil particles
Diquat binds readily to clay particles in the soil. The rate
of adsorption depends on the degree of contact of diquat with
adsorbent minerals, the type of soil, and the initial herbicide
concentrations tested. Weber et al. (1965) studied the effects of
temperature and exposure time on diquat adsorption by
montmorillonite, kaolinite, charcoal, and an anionexchange resin in
pH 6.0 phosphate buffer. Diquat was preferably adsorbed on the
clay particles by a process of ion exchange. Adsorption was
limited by the cation-exchange capacity of the test systems
examined. Coats et al. (1966) showed the adsorption capacity of
kaolinite to be about 2 g/kg and that of bentonite 80 - 100 g/kg.
A diquat soil concentration of 0.1 mg did not produce any
significant reduction in the dry weight of wheat grown in the soil
(Coats et al., 1966). The diquat appeared to be too tightly
adsorbed to the surface and between the lattices of bentonite to be
available to the wheat plant, at a soil treatment rate of 50 g/kg.
Data for diquat adsorption on sandy soils (Tucker et al., 1967)
showed that the herbicide was bound to different extents, according
to the structure of the soil particles.
4.2.2. Residue levels of diquat in soils
Makovskii (1972) reported on diquat residues in soils from
different plots, treated every year for a period of 7 years. There
were 3 - 4 treatments per season, at approximately 27.5 kg
diquat/ha. Samples were taken at 0 - 10 cm, 10 - 20 cm, and 20 -
30 cm depths in the soil; total diquat residues were shown to be
about 5.4 mg/kg soil, the mean values being 3.9 mg/kg, 1.3 mg/kg,
and 0.2 mg/kg in the respective soil layers. No diquat residues
were discovered in plants and citrus fruits sampled at different
times from the treated plots. In other studies, soil was analysed
for diquat residues on the 1st, 8th, and 15th days after applying
Reglone(R) at 0.8 litre/ha and 0.4 litre/ha (Tchipilska, 1980). On
the 1st day, residues of 0.400 mg/kg and 0.126 mg/kg were detected;
on the 8th and l5th days residues in the treated plots were lower
than 0.1 mg/kg.
As summarized in section 4.1.2, free diquat is degraded by a
range of microorganisms. While degradation of strongly-absorbed
diquat is relatively slow, results of long-term field studies have
nevertheless shown degradation rates of the order of 5 - 10% per
year. This is greater than the rate required to prevent saturation
of the deactivation capacity of soils.
In a long-term trial on a loamy soil, plots were treated with
0, 90, 198, and 720 kg diquat/ha, which was incorporated to a depth
of 15 cm. These rates were equivalent to 0, 50, 110, and 400% of
the soils strong absorption capacity (Gowman et al., 1980;
Wilkinson, 1980; Riley 1981). Over the 7 years, diquat residues
declined by 5% per year (sig P = 0.05) on the 90 kg/ha plots and
by 7% per year (sig P = 0.01) on the 198 and 720 kg/ha plots. The
rate of decline on the 198 and 720 kg/ha plots were significantly
greater ( P = .01) than on the 90 kg/ha plots.
4.2.3. Effect of residual diquat on soil biological activity, on
plants, and crop yields
A literature review and an extensive study of the effects of
different concentrations of diquat on microorganisms (saprophyte
and pathogenic microflora, and fungi) were carried out by
Tchipilska (1980). Staphylococcus aureus growth was inhibited
while Scenedesmus acutus was stimulated. Smith et al. (1981)
examined the effects of diquat applied at 0.5 - 32 times the
concentration recommended in agricultural practice on vesicular
arbuscular endophyte spore abundance in the soil and on the
infection of wheat roots. No measurable deviations in
endomycorrhiza formation and function were noted at normal
application rates. Loss of potassium and phosphate from fungi was
recorded at higher concentrations of diquat.
Coats et al. (1966) studied the uptake and translocation of
14C-diquat from soil into wheat. No metabolites were found in the
plants.
Diquat does not appear to have any significant influence on the
normal microbial activity that is important for soil fertility.
Nor is there any evidence that the recommended application rates
for diquat lead to residual effects on crop growth. Moreover,
tightly adsorbed diquat in soil is not reactivated into a
biologically active form, so that, in practice, accidental spillage
is probably the only cause of local high phytotoxic levels of
residual herbicide.
4.3. Diquat Transformation, Residue Levels, and in Effects on
Aquatic Organisms and Crops
4.3.1. Transformation and residue levels of diquat in water
In static water, initial diquat concentrations of 0.5 - 1.0
mg/litre fell rapidly to 0.1 - 0.3 mg/litre after 4-7 days
(Calderbank, 1972; Calderbank & Slade, 1976). In field
experiments, initial concentrations of 1.0, 0.8, and 0.5 mg/litre
decreased to 0.03 - 0.003 mg/litre after 7 - 14 days. This rapid
loss of diquat from treated waters was due to rapid uptake by
aquatic weeds. Two weed species (Myriophyllum spicatum and
Callitriche stagnalis) were immersed in water containing 1.0 mg
diquat/litre. The concentration of the herbicide decreased rapidly
to 0.14 - 0.03 mg/litre during a period of 6-14 days after
treatment. At the end of the experiment, the residue levels in
the weeds ranged from 6.2 - 17.4 mg/kg. In addition to uptake
by weeds, loss of diquat from treated waters was due to
photodegradation at the water surface and adsorption by bottom mud.
In field experiments carried out in 1010 m2 ponds with an initial
concentration of diquat of 2 mg/litre water, there were no residues
of diquat in the water after 8 days (Calderbank, 1972; Calderbank &
Slade, 1976).
In pond water that had been treated with diquat at 2.5 mg/litre
(Grzenda et al., 1966), residues of 0.01 - 0.08 mg/litre were found,
7-9 days after applying the herbicide, and no residues could be
determined after 14-30 days. The authors concluded that, compared
with other herbicides, diquat appeared to have the greatest
potential for use in sources of potable water.
The data obtained from studies in ponds, large and small lakes,
canals, and reservoirs demonstrate the fast disappearance of diquat
from treated waters (Calderbank, 1972). Absorption by aquatic
weeds explains the high efficacy of the herbicide. Decomposition
of the dead weeds is rapid, and diquat is not released from the
bottom mud back into the water. Applications of paraquat and
diquat each at a dose level of 1.1 kg/ha (Grover et al., 1980)
proved very effective for the control of weeds in irrigation
ditches, and the residual levels of both herbicides decreased
rapidly.
4.3.2. Effects of residual diquat on aquatic organisms and crops
The toxicity of diquat for fish varied with the species, the
size of the fish, and the softness or hardness of the water. The
LC50 values range from 12 to 90 mg/litre (24 h), 6 to 44 mg/litre
(48 h), and 4 to 36 mg/litre (96 h) (Calderbank, 1972). Reviews of
the effects of diquat on fish, aquatic invertebrates,
microbiological organisms in the soil of lakes, and phytoplankton
demonstrate that the herbicide, applied at the rates used for
aquatic weed control, did not affect estuarine fauna, oysters,
shrimps, water insects, or fish-food organisms (Calderbank, 1972;
Atkinson, 1973). At concentrations of 1 - 100 mg/litre, diquat
appeared to be less toxic for carp fingerlings than paraquat,
diuron, simazine, and dalapon (Singh & Yadev, 1978). Reish et al.
(1979) reviewed the effects of diquat on marine organisms; no
bioaccumulation by estuarine and marine organisms was found. The
toxicity of diquat for fish is low, and the main risk for aquatic
organisms and fish from its use as an aquatic weed killer is the
decreased oxygen concentration following the decay of weeds.
Trout exposed to 1 mg diquat/litre for 7 days contained
residues of 0.3 - 0.4 mg/kg in the gut, liver, and kidney, and of
0.1 - 0.3 mg/kg in the skin and gills. Residues were below the
limit of detection in muscle, spleen, and heart (Calderbank, 1972).
Trout exposed to 1 mg diquat/litre for 16 days contained residues
of 0.5 - 0.6 mg/kg, which disappeared when the fish were returned
to fresh water.
Because of irreversible adsorption, low residues in water will
be lost on contact with soil. The herbicide is thus unavailable to
plant roots. However, in overhead irrigation experiments, the use
of water containing diquat at 0.1 - 0.5 mg/litre (Calderbank, 1972)
resulted in diquat residues in the crops (tomato, lettuce, sugar
beet) ranging from less than 0.01 mg/kg to 0.04 - 0.07 mg/kg.
Thus, before using herbicide-treated waters for overhead plant
irrigation, it is advisable to allow 10 days for the diquat aquatic
residues to drop to acceptable levels.
The maximum diquat residues in water ultimately to be used for
drinking were 0.03 - 0.01 mg/litre, at the points of entry into the
public distribution system, 2-4 days after treatment; no residues
were detectable on the 10th day after applying diquat as an aquatic
herbicide. More often than not, residue levels were below the
detection limits of the analytical methods used.
4.4. Diquat Exposure and Residue Levels in Plants and Animals
4.4.1. Plants
Diquat is largely used as a desiccant in silage production. At
the recommended rates of 1.5 - 3.0 litre Reglone(R)/ha, diquat
residues were very low (Riley & Gratton, 1974). Following pre-
harvest desiccation of fodder crops, they ranged from below 0.05
mg/kg to 50 mg/kg, most of the levels determined being below 25 mg
diquat/kg (FAO/WHO, 1971, 1973). Diquat residues in the treated
herbage, sampled at different intervals after spraying with 0.258 -
0.515 mg/ha, were relatively high after 1 day (12 - 65 mg/kg), but
after 7 days had markedly decreased (1.0 - 6.5 mg/kg) (Black et
al., 1966). The levels of diquat found in silage during a 4-year
trial, with application rates of 0.190, 0.258, and 0.540 mg/ha,
varied from 1.4, 3.6, 9.3, and 13.3 to 26.8 mg/kg. The differences
were due to the atmospheric conditions at the time of desiccation
and the consequent degree of photochemical degradation of the
diquat. For this reason, diquat residues in treated herbage should
be expected to vary by an order of magnitude (10 times).
Pre-harvest desiccation of rape-seed with diquat did not result
in any detectable residues in the extracted oil and only low
residues (0.3 - 2 mg/kg) in the meal cake. Rape plants were
sprayed with 14C-diquat at 0.3 - 1.1 kg/ha, 3-14 days before
harvesting. There were no detectable residues of diquat or of its
photodegradation products in the rape-seed oil when the seeds were
harvested 7 days after desiccation, and very low diquat residues
(0.02 - 0.003 mg/kg) were determined when the seeds were harvested
14 days after treatment with diquat. The diquat residues in the
meal cake varied from 1.49 to 10.2 mg/kg, 14 days after treatment,
a large proportion being unchanged diquat (FAO/WHO, 1973).
Dembinski et al. (1971) reported diquat residues of 2 mg/kg in
sunflower seeds desiccated with Reglone(R).
Makovskii (1972) reported the diquat residue levels in weeds
treated with Reglone(R). After applications of Reglone (R) at
0.5, 1.0, and 1.3 litre/ha, the residues in dry weeds, ranged from
34 to 74 mg/kg 1 h later; from 15 to 26 mg/kg after 1 day; from
undetectable to 10 mg/kg after 4 days; from 2.8 to 3.5 mg/kg after
2 weeks; from 1.9 to 2.3 mg/kg after 4 weeks; and from undetectable
to 1.7 mg/kg after 6 weeks. The degradation of diquat in plants
was more rapid than the degradation of paraquat. The residues in
potatoes did not exceed 0.08 mg/kg, when diquat was used to destroy
potato haulm, and levels in fruits (apples, pears, plums, citrus),
tea, and cereals were undetectable (< 0.01 mg/kg), when diquat was
applied as a herbicide for weed control. Samples of potatoes
purchased from shops (Andersson & Josefsson, 1982) were analysed
for diquat residues. Residues in the range of 0.004 - 0.039 mg/kg
were found in 20 of 23 samples obtained from commercial growers.
None of the samples contained more than the residue tolerance of
0.1 mg/kg accepted for potatoes in Sweden.
Residue levels of diquat have been discussed in more detail by
the Joint Meeting on Pesticides Residues (FAO/WHO, 1971, 1973).
Residue levels of diquat in plants were summarized and published by
FAO/WHO (1977a). Some of these data are given in Table 2.
Data on diquat residues in desiccated wheat collected from 6
countries showed a mean of 0.5 mg/kg (FAO, 1979).
Table 2. Diquat residues in plantsa
------------------------------------------------------------
Plants Dose of diquat Mean value of
(kg/ha) residues (mg/kg)
------------------------------------------------------------
Wheat (grain, flour) 0.6 - 1.0 0.61, 0.22
Rice (with husk, polished) 0.2 - 0.4 0.89, 0.07
Sorghum (grain) 0.4 - 0.6 0.81
Cotton (grain) 0.4 - 1.0 0.37
Potato 0.6 - 1.0 0.03
Beans 0.3 - 1.0 0.10
Peas 0.3 - 1.0 0.05
Sugar beet (juice) 0.3 - 0.8 < 0.01
------------------------------------------------------------
a From: FAO/WHO (1977a).
4.4.2. Animals
Sheep and cattle fed silage containing diquat residues of up to
13 mg/kg were studied by Black et al. (1966). The total diquat
excreted in the urine was 0.19 - 0.65 mg over an 8-day period. No
diquat residues were detected in the brain, liver, and kidney of
sheep, or in the meat or organs of cattle fed diquat-treated silage
for one month. Milk collected on alternate days for 2 weeks was
free of diquat residues (< 0.003 mg/litre).
Feeding trials with sunflower seed containing approximately
0.20 mg diquat/kg were reported by Dembinski et al. (1971).
Although the amount of diquat consumed by the cattle over 257 days
ranged from 11.2 mg to 184.2 mg, no residues were found in any of
the milk samples analysed. Wethers fed ground sunflower seed
containing approximately 0.20 mg diquat/kg for 141 days were
estimated to have consumed a total of 14.1 mg diquat per sheep. No
residues were found in brain, liver, or kidney, nor were there any
residues in the meat, lungs, and kidney of steers treated with
diquat-desiccated sunflower forage. In long-term feeding trials
with silage, desiccated grass, lucerne, clover hay, barley straw,
and sunflower seeds containing diquat residues ranging from 0.2 to
50 mg/kg, the residues in milk and meat were determined to be less
than 0.007 mg/litre and less than 0.0006 mg/kg, respectively
(FAO/WHO, 1971, 1973, 1977a,b). Calderbank (1972) reviewed the
effects on farm animals of diquat in the drinking-water and on
herbage; there were no adverse effects on cattle and sheep and only
very low residue levels in milk, meat, and the organs analysed.
Lavaur et al. (1979) studied the effect of treated lucerne on
rabbits. Immediately after spraying, a concentration of 211 mg
diquat/kg dry weight was determined in the lucerne. After 24 h and
48 h, diquat residues were 97 mg/kg and 25 mg/kg, respectively. No
signs of poisoning or gastrointestinal damage were found in the
rabbits fed with different levels of diquat residues in the
lucerne. However, in some circumstances, lack of careful
organization may result in adverse effects of diquat on animals.
Intoxication of sheep, cattle, and swine has been reported (Schultz
et al., 1976) after the aerial application of Reglone(R) as a
rapeseed desiccant. The clinical course and the causes of the
accident stressed the need for proper diquat application by air.
For a more detailed discussion of the fate of diquat residues
in exposed animals, refer to FAO/WHO (1977a,b).
4.5. Diquat Levels in Air and Exposure of Workers
Experiments with 14C-diquat demonstrated that it was not
volatile (Coats et al., 1966). Diquat levels in air after spraying
with aerosols were determined by Makovskii (1972), using the method
of Calderbank & Yuen (1966). The application rates were 1.0 - 1.3
kg diquat/ha in working dilutions of 2.5 g and 3.3 g active
ingredient/litre, the highest diquat concentrations being found in
the tractor cabin when the door was open and spraying was in
progress in the direction of the wind (Table 3). The diquat
concentrations in air decreased rapidly 10 - 20 min after
completion of the treatment.
Wojeck et al. (1983) reported that diquat was determined in air
samples taken near the breathing zone of workers during its
application for aquatic weed control. The respiratory exposure
levels were below the limits of quantitation of the chemical
analysis.
In Bulgaria and the USSR, the proposed MAC (maximum allowable
concentration) for diquat is 0.1 mg/m3 aerosol. The TLV for diquat
in workroom air in the United Kingdom and the USA is 0.5 mg
diquat/m3 (1982), a level that will not be reached under normal
conditions of application.
Table 3. Total airborne diquat concentrations in the air of working areasa
---------------------------------------------------------------------------
Place of sampling Number of Mean concentrations
samples (mg/m3 ± SE)
---------------------------------------------------------------------------
Working area sprayer loading 20 0.12 ± 0.03
tractor cabin 8 0.56 ± 0.10
(in direction of wind)
tractor cabin 8 0.17 ± 0.04
(against the wind)
manual spraying 16 0.25 ± 0.04
Treated field after 5 min 8 0.20 ± 0.03
after 10 min 24 0.06 ± 0.01
after 20 min 8 ND
Distance from 200 m 8 0.09 ± 0.01
treated field 400 m 8 ND
---------------------------------------------------------------------------
a From: Makovskii (1972).
5. KINETICS AND METABOLISM
5.1. Animal Studies
5.1.1. Absorption
Oral absorption
Daniel & Gage (1966) studied the absorption of 14C-diquat
dibromide and 14C-diquat dichloride following oral and subcutaneous
single-dose administration to rats. About 90 - 97% of the oral
diquat dibromide and 84 - 90% of the diquat dichloride were found
in the faeces and 4 - 11% of both diquat salts in the urine.
Following subcutaneous injection of 14C-diquat (10 mg/kg body
weight) in rats, 87% of the administered dose was excreted in the
urine and 5% in the faeces within 4 days. The urine contained
mainly unchanged diquat (75% of the dose) together with diquat
monopyridone (about 3% of the dose) and diquat dipyridone (about 6%
of the dose) (FAO/WHO, 1978).
The poor absorption of diquat from the gastrointestinal tract
was confirmed by Litchfield et al. (1973) in the rat, and by Black
et al. (1966), Stevens & Walley (1966), and Dembinski et al. (1971)
in farm animals.
Pulmonary absorption
The uptake of 14C-diquat by perfused rat lung, following
intratracheal injection, was examined by Charles et al. (1978) and
Charles & Menzel (1979). Removal of 14C-diquat from the airways
was rapid, initially, but slowed down with time. The results
indicate 2 phases of absorption and removal of diquat from the
airways in the rat.
Dermal absorption
There are no data on the rate of diquat absorption through the
skin. Studies on the dose-related percutaneous toxicity of diquat
suggest that it may be dermally absorbed.
5.1.2. Distribution
Although paraquat and diquat have similar chemical, physical,
and herbicidal properties, only paraquat has been shown to damage
the lung. According to Sharp et al. (1972), diquat concentrations
in lung and muscle were much lower than the levels attained with
equal 20 mg/kg body weight iv doses of paraquat. Table 4 shows the
distribution of both in the main internal organs.
Table 4. Ratio of concentration of paraquat/diquat in
the tissues of the rata
--------------------------------------------------------
Organ Days after intravenous administration
1 3 5 7 10
--------------------------------------------------------
Lung 8 33 12 10 20
Muscle 2 13 10 7 16
Kidney 0.9 0.9 0.9 0.3 0.25
Liver 0.4 0.7 0.7 0.5 0.2
--------------------------------------------------------
a From: Sharp et al. (1972).
Diquat concentrations were higher in the kidney and the liver
but significantly lower in the lung (Table 4). In addition, the
concentrations of paraquat were 2-8 times higher than those of
diquat in the heart, adrenal glands, spleen, stomach, ileum,
testes, and thymus. Plasma levels were similar for both
bipyridylium herbicides.
Litchfield et al. (1973) injected 14C-diquat cation at 50 mg/kg
body weight iv into mice. Whole-body autoradiographs were prepared
after 10 min, 1 h, 24 h, and 72 h. Radioactivity was selectively
located in the gall bladder and was also present in cartilaginous
tissue, liver, and the gastrointestinal tract. Low radioactivity
was found in the brain and spinal cord. One h after dosing, the
amount in the urine and intestinal epithelium had increased. After
24 h, the excretion of diquat was virtually complete, although
radioactivity continued to be detected in the small and large
intestine and the bladder.
Litchfield et al. (1973) also determined diquat levels in
various tissues of male and female rats fed a diet containing
diquat dibromide monohydrate at 250 mg/kg for 2, 4, and 8 weeks.
High levels (0.18 - 1.17 mg/kg) were found in the kidney and the
large intestine; levels in the lung ranged from < 0.05 to 0.53
mg/kg; those in the liver from 0.07 - 0.22 mg/kg, while levels in
the brain, muscle, and blood were very low. At all stages of the
study, diquat lung levels were lower than those for paraquat, the
average paraquat content in the lung (at a dose of 250 mg/kg diet)
over the 8-week period being 1.7 mg/kg and the average diquat
level, 0.2 mg/kg. No sex differences were found. Within 1 week of
return to a normal diet, diquat was below the detectable limit in
all tissues examined.
Rats given paraquat or diquat orally at 680 µmol/kg had high
kidney levels of diquat throughout the 30 h period after dosing
(Rose & Smith, 1977, 1977a). There was no significant time-
dependent increase in diquat levels in the lung, liver, brain,
adrenal glands, muscle, and plasma. These results confirmed that,
following oral dosing, the lung does not accumulate diquat. Rose &
Smith (1977) also incubated rat lung slices in 10-5M paraquat and
diquat. In contrast to paraquat, diquat did not accumulate in the
lung slices, and the compound did not accumulate significantly in
any tissue slices with the exception of those from the kidney.
These observations were confirmed by Lock (1979).
Matsuura et al. (1978) studied the distribution of orally
administered LD50 doses of diquat and paraquat in rats. Two and
24 h after dosing, there were higher concentrations of diquat in
kidney, liver, and lung than in brain, heart, the gastrointestinal
system, and blood. At equitoxic doses, levels of diquat in the
lung appeared to be lower than those of paraquat. In a similar
distribution study of the LD50 and 0.5 LD50 doses of diquat and
paraquat, Kurisaki & Sato (1979) determined the tissue
concentrations from 2 to 48 h and from 2 to 9 days after treatment.
Distribution in the lung, heart, brain, liver, and kidney of the
rats agreed with previously published data.
The results of the above studies demonstrate that diquat does
not persist as long as paraquat in the body of the rat and that it
does not accumulate in the lung.
5.1.3. Metabolic transformation and excretion
Daniel & Gage (1966) reported that the amount of 14C-diquat
excreted in rat bile during the 24 h following oral doses of 1.2 -
64 mg/kg body weight represented 1.1 - 4.8% of the dose. Small
amounts were detected in the urine, but about 70% of the diquat was
present in the faeces. In other studies (FAO/WHO, 1978), the rate
of diquat metabolism in the rat was considerably lower than
previously reported by Daniel & Gage (1966). The biliary, urinary,
and faecal excretion of 14C-labelled bipyridylium herbicides was
studied by Hughes et al. (1973) in the rat, guinea-pig, and rabbit.
14C-diquat dichloride was injected ip at dose levels of 40 µmol/kg
body weight in the rat, 13 µmol/kg in guinea-pig, and 14 µmol/kg in
the rabbit. Most of the injected diquat (82% - rat, 64% -rabbit)
was found in the urine. Rabbits metabolized 18% of the dose,
guinea-pigs 5%, and rats less than 1%. The metabolites were
similar for the 3 species. The rat excreted approximately 1.4% of
the dose in the bile, the guinea-pig 4.8%, and the rabbit 2.9%.
Stevens & Walley (1966) treated cattle orally with 14C-diquat
dibromide in doses of 4, 8, and 20 mg/kg body weight. The
radioactivity levels in the milk of the cows indicated that 0.04 -
0.15% of the ingested dose was excreted in this way. Very low
levels of diquat (0.01 mg/kg) were present in muscle tissue, 2 - 8
days after dosing. A bull calf was dosed orally with 14C-diquat
dibromide at 10 mg/kg. About 2.6% of the 10 mg/kg dose was excreted
in the urine, but the major part of the dose was excreted via the
faeces. In the calf, 24 h after dosing, the residues were 0.66
mg/kg in kidney, 0.20 mg/kg in heart and skin, 0.19 mg/kg in liver,
0.03 mg/kg in lung, testes, and serum, and 0.006 mg/kg in muscle.
Studies on rats dosed orally with 14C-diquat at 45 mg/kg body
weight or subcutaneously (sc) with 10 mg/kg body weight were
reported by FAO/WHO (1978). Rats given the oral dose excreted 6%
and 89% in the urine and faeces, respectively, within 4 days and
mainly within the first 2 days. Unchanged diquat was the major
component in both urine (5% of the dose) and faeces (about 57% of
the dose). About 5% of the oral dose was excreted as diquat
monopyridone, mainly in the faeces, while diquat dipyridone
appeared to be the major urinary metabolite. Following sc
injection, rats eliminated 87% of the dose in the urine and 5% of
the dose in the faeces within 4 days. The urine contained 75% of
the dose as diquat, about 3% as diquat monopyridone, and about 6%
as diquat dipyridone. In vitro studies have shown that the caecal
microflora of the rat can metabolize about 10% of the diquat added
in a 24-h incubation period, with the formation of some diquat
monopyridone. This observation, together with the paucity of
metabolites following ip injection, suggests that diquat is
metabolized by the gastrointestinal tract bacteria.
The oral LD50 of diquat monopyridone in the rat was more than
4000 mg/kg body weight. Oral administration of diquat monopyridone
at 1000 mg/kg body weight per day for 2 weeks did not induce any
clinical, haematological, biochemical, or histopathological
deviations in the rat. In other studies, no adverse effects were
noted after sc injection of diquat monopyridone or diquat
dipyridone in rats, but 9 animals out of a group of 10 injected
with the equivalent dose (16 mg/kg body weight) of diquat were dead
by the l4th day following dosing (FAO/WHO, 1978).
5.2. Observations on Man
Feldman & Maibach (1974) studied the dermal penetration of
twelve 14C-labelled insecticides and herbicides. Diquat showed a
very low rate of dermal absorption in man. No other studies on the
kinetics of diquat in volunteers have been published, but
observations are available on accidental and suicidal ingestion
(section 7). Toxicological analysisk, at the time of admission, of
the serum of a patient, who had ingested 20 ml Reglone(R), showed
a diquat level of 0.4 mg/litre (Vanholder et al., 1981). At
postmortem examination on the 5th day after ingestion,
approximately 0.20 mg diquat/kg was determined in liver, kidneys,
muscle, and eye liquid.
6. EFFECTS ON ANIMALS
6.1. Effects on Experimental Animals
6.1.1. Gastrointestinal system and liver
Investigation of the clinical signs of acute oral intoxication
by diquat (Verbetskii & Pushkar, 1968; Clark & Hurst, 1970;
Crabtree et al., 1977; Cobb & Grimshaw, 1979) have established
gastrointestinal disturbance as the major syndrome of poisoning and
as a cause of death. In both rats and guinea-pigs, the clinical
signs of acute oral poisoning (Verbetskii & Pushkar, 1968) were
dose-dependent. At doses greater than the LD50, signs of poisoning
appeared after 6 - 12 h; at lower levels, the signs were less
obvious and appeared after 1 - 2 days. Most deaths occurred on the
3rd - 9th day after oral administration. The animals lost 7 - 35%
of their initial body weight. During the first 24 h following the
oral dosing of rats with 900 µmol diquat/kg body weight (LD50), a
reduction in water intake was noted (Crabtree et al., 1977). The
animals were subdued, showed pilo-erection and loss of appetite.
At 24 h, they excreted mucoidal, ropy faeces of a characteristic
greenish-yellow or grass-green colour, this colour being due to the
reduction of diquat by intestinal bacterial metabolism. This
colour can be reproduced in vitro with fresh intestinal contents
and actively growing bacterial isolates from them (Clark & Hurst,
1970).
A significant dose-dependent accumulation of water in the lumen
of the intestines and progressive haemoconcentration were reported
(Crabtree et al., 1977) following acute diquat intoxication in
rats. It was concluded that diquat had an adverse effect on water
distribution in the body. Rapid fluid excretion following oral
diquat poisoning suggested a direct action on the stomach and
intestinal mucosa. Monkeys dosed orally with diquat ion at 100,
200, 300, and 400 mg/kg body weight (Cobb & Grimshaw, 1979) vomited
within 2 h and showed diarrhoea within 12 h of dosing. The most
severely affected became lethargic and comatose, and finally
collapsed and died, 12 - 84 h after dosing. An increased number of
polymorphonuclear leukocytes as well as increased levels of serum
urea, plasma glucose, and serum GOT and GPT activities were
determined in monkeys that died during the study. Histological
examination revealed a distended gastrointestinal tract and a
swollen caecum; the mucosa of the stomach was ulcerated and the
small and large intestines congested. Large areas of the stomach
and intestines showed necrosis and exfoliation of the epithelium
from the mucosa. The submucosa was infiltrated with lymphocytes,
and polymorphonuclear and mononuclear cells. These changes were
most severe in the intestinal villi. The death of the monkeys was
due to destruction of the epithelial lining of the gastrointestinal
tract in combination with kidney damage.
Liver
The liver was not severely affected in acute and repeated
diquat poisoning of experimental animals. High doses sometimes
resulted in histological lesions (Verbetskii & Pushkar, 1968;
Bainova, 1975), but signs of toxic hepatitis were not described.
Gage (1968a) reported stimulated NADPH oxidase activity in rat
liver microsomes in vitro after exposure to diquat.
6.1.2. Renal system
The major route of diquat elimination is through the kidneys.
High doses of diquat provoke histological and biochemical changes
in the kidneys, but the most severe damage occured in relation to
renal excretion function (Lock & Ishmael, 1979).
Kidney damage following acute and repeated diquat poisoning was
reported by Verbetskii & Pushkar (1968), Bainova (1969), Cobb &
Grimshaw (1979), Lock (1979), and Lock & Ishmael (1979). Rats,
guinea-pigs, and monkeys were investigated after oral poisoning
with the herbicide. Diquat, orally administered at 680 µmol/kg to
rats, induced a significant increase in diuresis, proteinuria, and
glucosuria after 6-24 h. Biochemical tests in vitro revealed a
decrease in N'-methylnicotinamide, but not 4-aminohippurate,
accumulation by renal cortical slices suggesting competition for
the base transport system. Stimulation of the pentose phosphate
pathway and inhibition of fatty acid synthesis were found when
diquat was added to renal cortical slices in vitro. No such
changes were noted when the renal cortical slices were prepared
from rats previously treated with diquat (Lock, 1979).
Lock (1979) also investigated the changes in several variables
and the clearance of diquat by the rat kidney after oral
administration of toxic doses (680 and 900 µmol/kg body weight).
Diquat was not bound to the proteins of the rat plasma. Active
renal secretion was confirmed by the fact that diquat was cleared
by the kidney at a slightly higher rate than inulin. In rats
treated orally with diquat at 540 µmol/kg body weight, renal
clearance decreased after 24 h. However, the reduction in renal
function induced by diquat (Lock 1979) was considered to be
secondary and due to water redistribution caused by acute
poisoning.
Histopathological changes have been reported in the kidneys of
animals poisoned with high doses of the herbicide (Verbetskii &
Pushkar, 1968; Cobb & Grimshaw, 1979; Lock & Ishmael, 1979). The
renal papillae were hyperaemic, degeneration and necrosis of the
epithelium of the proximal and distal convoluted tubules were
noted, the epithelial cells were exfoliated, and the nuclei
pycnotic.
6.1.3. Eyes and skin
Eye irritation
The local irritation caused by diquat is less pronounced than
that caused by paraquat. One drop of 20% solution gave rise to
slight conjunctival irritation of the rabbit eye, which persisted
for 2 days (Clark & Hurst, 1970). A 40% diquat solution induced
moderate conjunctival irritation.
Eye cataract
Both rats and dogs fed diets containing diquat developed
cataracts (Howe & Wright, 1965). However, rats fed 7.5 mg
diquat/kg diet over a life-span did not develop cataracts, while 70
mg diquat/kg diet appeared to be the no-observed-adverse-effect
level for dogs. According to Clark & Hurst (1970), rats on diets
containing 50 mg diquat/kg or more developed cataracts in the
course of the study. In another group fed a diet containing 1 g
diquat/kg, eye opacities were discovered within 6 months, while a
few animals on diets of 100 mg/kg and 50 mg/kg showed slight
opacities at the end of the study period. A 2-year test with a
diet containing diquat at 10 mg/kg did not induce cataracts in
rats.
Bilateral cataracts were discovered in all dogs 10 - 11 months
following oral administration of diquat at 15 mg/kg body weight per
day. The dose of 5 mg/kg body weight per day induced eye opacities
after 17 months, and doses of 1.7, 0.8, and 0.4 mg/kg body weight
per day were ineffective after 3 - 4 years of treatment.
A 2-year feeding study was carried out with diquat levels of
15, 25, and 75 mg/kg in the diet of rats. Only the 25 and 75 mg/kg
levels caused cataracts (FAO/WHO, 1978).
Pirie & Rees (1970) confirmed that rats fed diquat dibromide at
0.5 - 0.75 g/kg in the diet developed cataracts. In vivo
observations showed that, invariably, the first change seen was an
opacity in the posterior cortex, immediately under the posterior
capsule of the lens. The next stage was a defined nuclear cataract
that could be seen with the naked eye. Finally, shrinkage and
complete opacity occurred. This histological study revealed that
the first posterior cortical opacity was formed from damaged
epithelial cells. The level of diquat in the blood of these rats
was less than 2.2 µM. No diquat accumulation was registered in the
lens of these rats. The mechanism of the specific cataractogenic
action of diquat is not clear, although in vitro studies
demonstrated that reduction of diquat by the lens was enzymatically
catalysed by glutathione reductase (EC 1.6.4.2) with NADPH as the
source of reducing equivalents. The loss of ascorbic acid from the
lens and the ocular fluids of treated rats was proposed as a factor
for maintaining the normal glutathione level in the rat lens.
Local skin effects
Single diquat applications on the skin of mice (Bainova, l969a)
and rabbits (Clark & Hurst, 1970) did not cause any local
irritation. Daily applications of 1% diquat solution in water to
the skin of rats provoked slight erythema at the site of contact
during the first 10 days, while daily applications of diquat at 20
mg/kg body weight to the skin of rabbits caused mild erythema,
thickening of the skin, and some scabbing (Clark & Hurst, 1970).
Diquat has not been found to be a sensitizer (Bainova, (l969a).
6.1.4. Respiratory system
The effect of diquat on the respiratory system has been studied
after parenteral (Hawkins et al., 1979; Lam et al., 1980), oral
(Verbetskii & Pushkar, 1968; Bainova, 1969; Bainova & Vulcheva,
1978), intratracheal (Lam et al., 1980), and inhalation exposure
(Gage, 1968; Bainova et al., 1972). Unlike paraquat, no specific
effects on the lung were reported, though difficulties in breathing
occurred after severe acute poisoning of the animals with diquat.
6.1.5. Nervous system
General depression and lethargy were most commonly seen
following the administration of high doses of diquat to guinea-pigs
and rats (Verbetskii & Pushkar, 1968; Clark & Hurst, 1970; Crabtree
et al., 1977), and to monkeys (Cobb & Grimshaw, 1979).
6.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
6.1.6.1. Effects on reproduction
Male rats were dosed orally with diquat dibromide at 6.5 mg/kg
body weight per day, for 60 days, and the testes were then examined
biochemically and histologically (Bainova & Vulcheva, 1974). There
were no significant changes in the sperm count, sperm motility, the
testicular tubules, the basal cells, or in the activity of several
enzymes.
A 2-generation study on rats was carried out with dietary
levels of 125 and 500 mg diquat/kg. The 500 mg/kg dose resulted in
reduced body weight for F1a, F1b, F2a, and F2b, and increased
cataracts in F1b and F2b after 91-280 days of exposure. The 125
mg/kg dose resulted in decreased body weight in F1b and F2b, but no
lens opacities were noted (FAO/WHO, 1973).
6.1.6.2. Embryotoxicity and teratogenicity
Diquat was reported to have induced deviations in the prenatal
development of rats (Khera et al., 1968). Bus et al. (1975)
studied the fetal toxicity and teratogenicity of diquat in rats by
administering 15 mg/kg body weight iv on days 7-21 of gestation.
This resulted in 57% fetal resorption compared with 7.6% for
paraquat. The incidence of maternal deaths was essentially the
same. When 14C-diquat and 14C-paraquat were administered to rats,
iv, in a dose of 15 mg/kg body weight on days 13, 16, and 21 of
gestation, paraquat increased radioactivity in fetal lung whereas
diquat appeared to have a stronger embryotoxic action than
paraquat. In the review published in 1979 by FAO/WHO, it was
reported that diquat dibromide monohydrate, administered orally to
pregnant rabbits at doses of 1.25, 2.5, and 5.0 mg/kg had no
adverse effect on the fetuses. In groups of pregnant rats kept on
diets containing 125 and 500 mg diquat cation/kg throughout
gestation, reduced body weight was noted only in the fetuses of
mothers from the 500 mg/kg group. A slightly increased incidence
of subcutaneous haemorrhages was also noted.
Teratogenicity studies in mice have been reported by Selypes et
al. (1980). Single ip doses of diquat at 2.7 and 11 mg/kg body
weight were injected on days 9, 10, 11, and 12 of gestation. The
number of dead fetuses, as well as post-implantational lethality,
increased significantly: average embryo weight was lower and,
though no congenital malformations were noted, there were signs of
skeletal retardation such as large fontanelles, wider cerebral
sutures, flat-shaped ventral nuclei of the vertebrae, and delayed
ossification in the sternum and phalanges. The embryotoxic effect
in mice of high doses of diquat was thus confirmed, but no
chromosomal aberrations were noted in the liver cells of the
embryos from diquat-treated female mice.
6.1.7. Mutagenicity
Studies on the genotoxic potential of diquat are rather
contradictory. Diquat was negative in the Ames test, with and
without metabolic activation (Anderson et al., 1972; Benigni et
al., 1979; Levin et al., 1982). Dominant lethal assays in mice
performed by various authors with several doses of the herbicide
gave negative results (Pasi et al., 1974; Pasi & Embree, 1975;
Anderson et al., 1976). Selypes et al. (1980) injected mice ip
with 22 mg/kg (LD50) diquat, while another group of mice was dosed
orally with 90 mg/kg (0.5 LD50). After 24 and 38 h, preparations of
bone marrow were examined for chromosome aberrations; no
statistically significant changes were determined.
On the other hand, diquat was found to induce slight gene
conversion in Saccharomyces cerevisiae (Siebert & Lemperle, 1974).
Ahmed et al. (1977) reported that diquat induced DNA changes in
cultured SV-40-transformed human cells, with and without metabolic
activation, and the induction of 8-azaguanine resistance in the
Salmonella typhimurium assay was positive (Benigni et al., 1979;
Bignami & Crebelli, 1979). Benigni et al. (1979) also found that
diquat was positive in an S. typhimurium repair test. It was
further reported by these authors that diquat induced gene
mutations in Aspergillus nidulans, and increased unscheduled DNA
synthesis in human epithelial-like cells. They commented that
diquat may have an effect on a number of different genetic
endpoints.
6.1.8. Carcinogenicity
In 2-year feeding studies on rats (Clark & Hurst, 1970), diquat
at levels of up to 720 mg/kg diet did not induce tumours. The
daily ingestion of 2 and 4 mg diquat per kg body weight in water
for a period of 2 years did not have any significant effects on the
health and mortality rate in rats (Bainova & Vulcheva, 1978). Some
histological changes related to chronic interstitial infiltration
and pulmonary adenomatosis in the lungs were found, especially
after the higher dose, but there were no indications of malignancy.
6.2. Effects on Farm Animals
The effects of diquat on farm animals was studied in relation
to its application as an aquatic herbicide and desiccant (Howe &
Wright, 1965; Black et al., 1966; Stevens & Walley, 1966) (section
4.4). Little variation in diquat toxicity in the various animal
species was found, but cattle appeared to be the most sensitive
(LD50 for cattle approximately 30 mg/kg, LD50 for rat 230 mg/kg).
Single oral doses up to 8 mg/kg produced no signs of toxicity in
cows (Stevens & Walley, 1966), and the continuous exposure of
animals via the forage to doses ranging from 0.2 to 330 mg/kg in
the diet (Calderbank, 1972) did not induce any clinical or
pathological changes in farm animals.
Calderbank (1972) recommended that domestic animals should not
be allowed to enter fields newly treated with diquat, nor be given
water recently treated with the herbicide. When edible crops are
treated with diquat, as desiccant, at least 4 days should elapse
before the crops are fed to stock, and when diquat is used for
aquatic weed control, at least 7 days should elapse before the
treated water is used for field irrigation. Recommended levels for
weed control must be observed (Calderbank, 1972).
Sheep given doses of 1, 5, 10, and 20 mg diquat/kg per day in
their drinking-water for 1 month and calves similarly exposed to 5
and 20 mg diquat/kg per day did not show any adverse toxicological
effects as evidenced by growth, food consumption, and observation.
6.3. Dose-Effect of Diquat
The acute LD50 values of diquat in various species were
published by Howe & Wright (1965) and Clark & Hurst (1970). The
acute toxicity of diquat salts (Table 5) does not differ
significantly and is similar for both sexes.
Table 6 summarizes the acute oral, dermal, and inhalation LD50
and LC50 values of diquat in various experimental and domestic
animals. There are no marked species differences but cattle,
guinea-pigs, and monkeys appear to be the most sensitive species.
The few cases of acute diquat poisoning in man have not furnished
sufficient data to determine the lethal dose for man.
The dose-effect relationship of repeated diquat exposure, from
various studies, is summarized in Table 7. Rats, guinea-pigs and
dogs were subjected to ora1 and dietary administration of diquat.
Guinea-pigs appeared to be rather sensitive (Makovskii, 1972), but
the herbicide did not induce cumulative toxic effects (Bainova,
1969, 1975; Makovskii, 1972), because of its relatively rapid
elimination from the organism and the absence of deposits in the
tissues.
Table 5. LD50 (mg/kg) of diquat salts in rats
-----------------------------------------------
Diquat Route of entry Sex LD50
(mg/kg)
-----------------------------------------------
Diquat dibromide oral 215b
Diquat dibromide oral 210b
Diquat dibromide subcutaneous F 11a
Diquat dichloride subcutaneous F 10a
Diquat dichloride subcutaneous M 11a
Diquat dibromide subcutaneous 22b
-----------------------------------------------
a From: Clark & Hurst (1970).
b From: Makovskii (1972).
Table 6. Diquat LD50 (mg/kg) and LC50 (mg/m3)
in various species
----------------------------------------------
Species Oral Dermal Inhalationa
(mg/kg) (mg/kg) (mg/m3)
----------------------------------------------
Rat 400b 650f 35f
Rat 281c 83h
Rat 231e
Rat 215f
Rat 130g
Mouse 170b 430d
Mouse 125e
Rabbit 190b
Rabbit 101e > 400e
Guinea-pig 123c 400f 38f
Guinea-pig approx. 100e
Guinea-pig 100f
Hen 400 - 800b
Hen 200 - 400e
Dog > 200b
Dog 100 - 200e
Cow approx. 30f
Cow 30e
Monkey 100 - 300i
----------------------------------------------
a Respirable diquat aerosol.
b From: Howe & Wright (1965).
c From: Verbetskii & Pushkar (1968).
d From: Bainova (1969a).
e From: Clark & Hurst (1970).
f From: Makovskii (1972).
g From: Bainova (1975).
h From: Bainova & Vulcheva (1977).
i From: Cobb & Grimshaw (1979).
Table 7. Effect of repeated oral, dermal, and inhalation exposure to diquat in experimental animals
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Results obtained Reference
---------------------------------------------------------------------------------------------------------
Rat 87.5, 175, and 350 mg 2 years cataract at all dietary levels FAO/WHO (1971)
diquat ion/kg of diet
Rat 7.2, 36, 72, 180, 2 years no deaths; reduced growth in males at FAO/WHO (1971)
360, and 720 mg diquat highest dietary level; cataract at
ion/kg diet dietary levels of 36 mg diquat ion/kg
diet and above; no cataract at 7.2 mg/kg
Rat 15, 25, and 75 mg 2 years no deaths; "no effect" level for FAO/WHO (1978)
diquat ion/kg diet cataractogenesis 15 mg diquat ion/kg
diet
Rat oral - 6.5, 13, and 40 30 days dose-related biochemical and Bainova (1969,
mg/kg body weight per histological changes in kidney, liver, 1975)
day 2.1 and 4.3 mg/kg 4 1/2 months gastrointestinal system, and lung; no
body weight per day haematological changes; increased
G-6-P-isomerase serum activity;
histological changes at 4.3 mg/kg
body weight per day
Rat oral - 0.2, 2.1, and 1 year the higher doses were toxic for the Makovskii (1972)
5.3 mg/kg body weight 2 species; no-observed-effect levels
per day
Guinea- day 0.1, 1.0, and 2.5 0.2 and 0.1 mg/kg body weight per day
pig mg/kg body weight per for rat and guinea-pig
day
Dog 10, 20, 50, 140, and up to 4 no cataracts at dietary levels up to and FAO/WHO (1971)
420 mg diquat ion/kg years including 50 mg/kg; cataract at 2 higher
of diet dietary levels; no mortality; no effects
on growth
Rat oral - 2 and 4 mg/kg 1 and 2 no increase in mortality rates; Bainova &
per day years histological changes in lungs after Vulcheva (1978)
treatment with 4 mg/kg per day in
drinking-water; minimal effective dose
2 mg/kg per day
---------------------------------------------------------------------------------------------------------
Table 7. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Results obtained Reference
---------------------------------------------------------------------------------------------------------
rat dermal - 5, 10, 20 20 days slight skin irrritation, death and toxic Bainova (1969a)
60, and 120 mg/kg effects at 10 - 120 mg/kg per day;
per day dilation of the gastrointestinal system
at toxic levels; histological changes in
kidney, gastrointestinal system, liver,
and lung at toxic levels, LD50 35 mg/kg
per day without occlusion; no-observed-
effect dose 5 mg/kg per day
Rabbit dermal - 20 and 40 20 days mild skin irritation; toxic effects at Clark & Hurst
at 40 mg/kg per day; no clinical signs (1970)
of toxicity at 20 mg/kg per day; LD50
between 20 and 40 mg/kg per day
Rat inhalationa - 0.50, 15 days clinical signs of irritation and Gage (1968)
1.60, and 2.0 mg/m3 histological changes in lungs at 2mg/m3;
6 h daily no clinical haematological, and
histological deviations at 0.50 mg/m3;
minimum effective concentration 1.0
mg/m3 diquat aerosol
Rat inhalationa - 0.32 4 1/2 months biochemical and histological changes in Bainova (1972)
and 1.90 mg/m3, lungs at 1.90 mg/m3; minimal effective
6 h daily concentration 0.32 mg/m3 diquat aerosol
Rat inhalationa - 0.4, 4 months clinical signs of irritation and toxic Makovskii
0.7, and 1.9 mg/m3, effects at 1.9 mg/m3; 0.7 mg/m3 produced (1972)
4 h daily changes in some rats; minimal effective
concentration 0.4 mg/m3 diquat aerosol
---------------------------------------------------------------------------------------------------------
a Respirable diquat aerosol.
7. EFFECTS ON MAN
7.1. Case Reports
Several cases of acute diquat poisoning among the general
population have been reported in the literature. Fitzgerald et al.
(1978) found 5 cases from 1967 to 1977 in Ireland. Vanholder et
al., (1981) summarized the clinical outcome and the treatment of 11
patients with diquat poisoning (6 fatal and 5 non-fatal).
(a) Suicidal diquat poisoning
Schönborn et al. (1971) reported the fatal case of a man who
drank 2 - 3 mouthfuls of Reglone(R) (estimated 15 - 22 g diquat)
with the intention of committing suicide. Severe vomiting occurred
after 2 h and, 2 h later, watery diarrhoea, the stools having a
peculiar yellow-greenish colour. During the next 6 h, the patient
lost about 3.5 litres of liquid through faeces and 4 litres of
liquid through vomiting. The urine was very concentrated, the
haematocrit was 55%. Serum enzyme activity showed toxic liver
damage, and proteinuria and metabolic acidosis were registered. On
the 2nd day, there were ulcers and severe oropharyngeal
inflammation, on the 3rd day, increasing restlessness, optical
hallucinations, and delirium and stridulous breathing developed.
During the 4th-6th days, anuria, raised body temperature,
generalized convulsions, and coma were registered, and the patient
died on the 7th day of cardiac insufficiency and thrombocytopenia.
The autopsy revealed extensive necrosis of the pharynx and
oesophagus, and petechial bleeding and erosions in the
gastrointestinal tract; pulmonary oedema with haemorrhages, hyaline
membrane production, and bronchopneumonic foci were noted in the
lungs; fatty degeneration was found in the liver and heart, and
severe degeneration of the tubulus epithelium with necrosis in the
kidneys, while the signs of circulatory failure with oedema and
haemorrhagic diapedesis of the brain explained the central nervous
system effects. The diquat concentrations measured on the 1st day
after ingestion were 1.85 mg/litre in the urine and 0.47 mg/litre
in the blood. Higher diquat levels were determined post mortem in
the kidneys, spleen, and lungs (1.19, 1.04, and 0.56 mg/kg,
respectively).
In a second case of suicide, the subject had taken unknown
quantities of Reglone(R) during a period of 3 days (Okonek &
Hofmann, 1975). One day after the second ingestion, she was
admitted to hospital - shocked, sleepy, anuric, with haemorrhagic
mucosal necrosis in the mouth, throat, and eosophagus. Four h
after admission to hospital, the diquat serum level was 1.038
mg/litre. This decreased to 0.30 mg/litre following dialysis.
Death from cardiovascular collapse ensued 46 h after admission.
Vanholder et al. (1981) concluded, from their review of 11
cases, that the lethal dose of Reglone(R) is 30 - 60 ml or
approximately 6 - 12 g diquat dibromide.
An unusual case of diquat poisoning was described by Narita et
al. (1978). A clerk, after drinking heavily, swallowed about 200
ml 30% diquat dibromide formulation. Vomiting was accompanied by
great thirst, severe irritation of the mouth, diarrhoea, and a
temperature of 39 °C. After 24 h, the patient became anuric and
developed acute renal failure; he was comatose and inarticulate,
and had meiosis and unclear light reflexes. He died from dyspnoea
38 1/2 h after ingestion of diquat. Autopsy revealed renal failure
with tubular necrosis, lung haemorrhages, haemorrhagic ulcers, and
erosions in the stomach, and severe congestion of the lungs,
kidneys, liver, gastrointestinal system, and adrenal glands. High
diquat residues were determined in the kidneys, liver, lungs, and
intestines. Vanholder et al. (1981) reported 2 cases of Reglone(R)
ingestion (50 ml and 20 ml) in suicide attempts. Because of
vomiting and diarrhoea, they were admitted to local hospitals, but
no specific treatment was given and the patients were released in
satisfactory clinical condition. However, because of the
development of progressive oliguria several h later, the patients
returned to the hospital. The diquat serum levels were found to be
4.5 and 0.4 mg/litre, respectively. The patients died 1 and 5 days
after the ingestion of diquat.
(b) Accidental diquat poisoning
Oreopoulos & McEvoy (1969) described a patient who accidentally
took a mouthful of Reglone(R) from a soft drink bottle. He spat
out part of it. After 8-10 h, he had diarrhoea and 2 ulcers in the
mouth, but there was no clinical evidence of respiratory, renal, or
central nervous system effects on examination in hospital, and all
laboratory and biochemical examinations were within the normal
physiological limits. The patient continued to excrete diquat in
the urine for 11 days after ingestion. He underwent forced
diuresis and left the hospital in good condition.
Another case of acute poisoning following the accidental
ingestion of less than a mouthful of diquat was reported by Fel et
al. (1976). Nausea, vomiting, and diarrhoea were the first
effects. The patient then developed uraemia, oliguria, and anuria
despite forced diuresis for 2 - 3 days after the accident.
Haemodialysis proved more successful. Bilateral pneumonia was
noted during the 2nd week, but was cured with antibiotics, and the
patient was discharged on the 26th day in good health.
7.2. Effects on Agricultural Operators
A few studies have been performed on workers spraying diquat.
Air concentrations of diquat aerosol were measured by Makovski
(1972) (Table 3). The dermal exposure of the spraymen ranged from
0.05 mg to 0.08 mg on the face and hands after 2 - 3 h of daily
work. The spraymen did not have any complaints, and the clinical
and laboratory examinations did not reveal any significant
differences in comparison with control groups. Wojeck et al.
(1983) studied the exposure of workers applying 1.76% diquat by
hand-operated spray against water hyacinths or using direct
injection of 4.41% spray mixture into the water for hydrilla
control. The spray crews applied diquat 2 - 5 h daily for 4 days
weekly. The inhalatory exposure was found to be < 0.01 mg/h. The
dermal exposure of the spraymen and the airboat drivers were
estimated to be 1.82 and 0.20 mg diquat/h, during the treatment of
water hyacinths. The dermal exposures of the spraymen and the
mixer of diquat for the treatment of water hydrilla were 0.17 and
0.47 mg/h, respectively. The results of urine analysis of all
workers involved in the study were negative (< 0.047 mg/litre).
The dermal exposure to diquat was closely related to the
concentrations used in the working solutions.
Inflammation and bleeding of the nasal mucosa were observed in
people handling crystalline diquat powder in the laboratory or
under field conditions (Clark & Hurst, 1970). Epistaxis during
agricultural diquat application is related to the inhalation of
droplets or splashes from the careless mixing of liquid
concentrates. A worker who spent some considerable time in an
aerosol spray drift developed irritation of the upper respiratory
tract.
According to Clark & Hurst (1970), if a 20% diquat solution
comes into contact with the nail base, nail growth disturbances may
result, and discoloured spots, white bands, and shedding of the
nail were seen after prolonged contact with concentrated diquat.
The nail re-grew normally once exposure was discontinued. No
adverse effects on the nails were observed following the use of
diluted diquat spray solutions in agriculture. Concentrated diquat
formulations have also been reported to delay the healing of
superficial cuts on the hands of spray workers.
Cataracts have never been observed in man following exposure to
diquat (FAO/WHO, 1978; Hayes, 1982).
7.3. First Aid and Medical Treatment
These are essentially the same as those given for paraquat
(section 8.4). See also WHO/FAO (1979).
8. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
8.1. Exposure
8.1.1. Relative contributions of soil, water, air, and food sources
to total diquat uptake
Introduction
Diquat is a contact herbicide and dessicant that is used to
destroy weeds in various agricultural situations. It is used in
the form of an aqueous spray, which means that the potential
exposure of man may occur as a result of its presence in air, on
plants, in soil, or in water.
Degradation of Diquat
Photochemical degradation takes place, when diquat treated
plants are exposed to normal daylight, and continues after plants
are dead. The products formed are of lower toxicity than diquat.
The rapidity of photochemical degradation on plant and soil
surfaces minimizes the hazard of diquat for the environment.
Soil
Diquat is rapidly and tightly bound to clay particles in the
soil, and is thereafter inert. In normal agricultural use, no
toxic breakdown products are to be expected in the soil (section
4.2) where diquat is less persistent than paraquat. Total diquat
residues in the soil after repeated spraying ranged from 0.2 to 3.9
mg/kg. On the 15th day after a single application of diquat,
residues were less than 0.1 mg/kg in field studies. Even at high
rates of application, no specific adverse effects are found on soil
microorganisms, fungi, or invertebrates, and no phytotoxic effects
have been reported on crops.
Water
Following its use as an aquatic herbicide at normal application
rates, diquat residues in water have been found to decrease rapidly
to essentially undetectable levels within 7-14 days (section 4.3).
Toxic effects on fish and other living organisms in the water are
unlikely, because diquat is rapidly photodegraded, absorbed by
aquatic weeds, or adsorbed to soil particles at the bottom.
However, caution should be taken in the application of diquat to
water containing heavy weed growth, since oxygen consumed by
subsequent weed decay may decrease the oxygen content of the water
to such an extent that it is dangerous for fish or other aquatic
organisms. No phytotoxic damage should occur on crops irrigated
with diquat-treated water, if at least 10 days is allowed to elapse
between treatment and irrigation.
Air
Diquat is not volatile. Inhalation exposure can occur via
spray aerosols or contaminated dust but, if correctly applied,
diquat should not give rise to significant inhalation exposure of
the sprayers (section 4.5). Total airborne aerosol concentrations
of diquat in the air in working areas ranged from 0.06 to 0.56
mg/m3, depending on the method of application and the period of
time after the spraying.
Food
Extensive studies on forage desiccated with diquat have
demonstrated that the residues are very low within some days of the
application of the desiccant. Diquat residues in the treated
herbage following pre-harvest desiccation ranged from 0.02 to 25
mg/kg at different intervals after spraying. Trials in which such
forage was fed to cattle and sheep have demonstrated insignificant
residue levels in the milk, meat, and internal organs (section
4.4). Residues found in vegetables, fruits, and cereals have been
low. There is no bioaccumulation.
8.1.2. General population exposure
Inhalation exposure of the general population to diquat may
occur from spray drift off the treated fields, but this is thought
to be insignificant. There are no published data on total diquat
intake among the general population but this again is expected to
be insignificant on the basis of known residue levels. Studies on
its environmental distribution point to a low environmental hazard.
Due to diquat's rapid and complete binding to clay minerals in
soil, contamination of water supplies either from field runoff or
percolation through soil to the water table is not expected
(section 4.2).
Few cases of diquat poisoning have been reported (section 7.1).
Most cases are due to the intentional ingestion of concentrated
formulations, but accidental ingestion has occurred. The decanting
of liquid concentrate formulations into beer, wine, or soft drink
bottles, and subsequent inappropriate storage, is very dangerous.
The acute lethal dose of diquat dibromide is considered to be
6 - 12 g for man. Recovery from diquat poisoning depends on the
cause of ingestion, the dose absorbed, the renal damage, and prompt
initiation of therapy. No long-term adverse effects have been
reported in those who have survived acute diquat poisoning.
8.1.3. Occupational exposure
There may be inhalation, dermal, and to some extent oral
occupational exposure. Spray aerosols and dust particles settle in
the upper respiratory tract. Diquat aerosol concentrations range
from 0.06 to 0.56 mg/m3, according to the spraying method. At a
distance of 200 - 400 m from the treated field, they decrease to
0.09 mg/m3 and less than 0.01 mg/m3. Inhalation exposure was found
to be very low in comparison with dermal (0.17 - 1.82 mg/h)
exposure to diquat during application for aquatic weed control.
Skin irritation, epistaxis, nail damage, and delayed wound healing
have been reported. However, no data on severe or fatal cases of
occupational intoxication, acute ocular damage, or occupational
contact dermatitis caused by diquat were found in the literature.
8.2. Effects
8.2.1. Diquat toxicity in animals
Diquat is less toxic than paraquat and does not cause the
specific lung disease so typical of paraquat exposure.
The primary toxic effect of diquat in animals is
gastrointestinal damage resulting in diarrhoea with consequent
dehydration. After high doses of diquat, minor toxic effects have
been noted in the liver, kidney, and the nervous and endocrine
systems. High concentrations of diquat are irritating to the skin,
although less so than paraquat. Development of eye cataracts has
been reported in rats and dogs following long-term treatment with
diquat (section 6.1.3). This observation has not been reported in
man. Diquat is embryotoxic but it has not been found to be
teratogenic in rats and mice or carcinogenic in long-term feeding
studies on rats given diquat at levels up to 720 mg/kg diet
(sections 6.1.7 and 6.1.8). In vitro mutagenicity studies have
been inconclusive, although generally suggesting weak activity,
while the results of in vivo studies have been negative (section
6.1.8). Thus, the results of animal studies suggest that low-level
exposure to diquat is unlikely to induce toxic effects in man. The
no-observed-effect level in rats has been estimated to be 0.75 mg
diquat ion/kg body weight per day (FAO/WHO, 1978).
8.3. Earlier Evaluations of Diquat by International Bodies
The Joint Meeting on Pesticide Residues (JMPR) reviewed and
published residue and toxicity data on diquat in 1970, 1972, 1976,
1977, 1978 (FAO/WHO 1971, 1973, 1977a,b, 1978, 1979). In 1977, it
estimated the acceptable daily intake (ADI) for man as 0 - 0.008
mg/kg body weight expressed as diquat ion (FAO/WHO 1978).
The same JMPRs have recommended maximum residue levels
(tolerances) for diquat in food commodities of plant and animal
origin.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC are available
from the IRPTC (International Register for Potentially Toxic
Chemicals) legal file (IRPTC 1983).
A data sheet on diquat has been prepared by WHO/FAO (1979) in a
series of "Data sheets on chemical pesticides". Based on a brief
review of use, exposure, and toxicity, practical advice is given on
labelling, safe-handling, transport, storage, disposal,
decontamination, selection, training and medical supervision of
workers, first aid, and medical treatment.
8.4. Conclusions
On the basis of the above findings, it can be concluded that:
General population
Residue levels of diquat in food and drinking-water, resulting
from its normal use, are unlikely to result in a health hazard for
the general population.
Diquat has caused some fatalities following suicidal ingestion.
Occasional accidental fatalities have followed ingestion of
decanted diquat. Ill-effects similar to those caused by paraquat
occur, but the characteristic fibrosis of the lungs is not a
feature.
Occupational exposure
With reasonable work practices including safety precautions,
hygiene measures, and proper supervision, occupational exposure
during the manufacture, formulation, and application of diquat will
not cause a hazard. However, the undiluted concentrate must be
handled with great care, because contamination of eyes and skin
(with possible consequent dermal absorption) can result from
improper work practices.
Environment
Diquat in soil binds rapidly and tightly to clay particles and
residual phytotoxicity from freely available diquat is unlikely.
Under normal conditions of use, the toxicity of diquat for aquatic
organisms is low, though resulting depletion of water oxygen due to
weed decay may pose a problem. Diquat does not seem to represent
an environmental hazard.
REFERENCES
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide-
induced DNA damage and its repair in cultured human cells.
Mutat. Res., 42: 161-164.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDERSON, D., MCGREGOR, D.B., & PURCHASE, I.F.H. (1976)
Dominant lethal studies with diquat and paraquat in male CD-1
mice. Mutat. Res., 40: 349-358.
ANDERSSON, A. & JOSEFSSON, E. (1982) Residues of diquat in
potatoes. Var Föda, 34(Suppl. 3): 223-225.
ATKINSON, G. (1973) Effects of diquat on the microbiological
organisms in the soil of lakes. In: Proceedings of the
Pollution Residue Conference, Wairakei, New Zealand,
pp. 529-538.
BAINOVA, A. (1969a) [Chronic oral toxicity of bipyridylium
herbicides.] Hig. Zdrav., 12: 325-332 (in Bulgarian).
BAINOVA, A. (l969b) [Experimental assessment of the effect
of dipyridylium herbicides on the skin.] Letopisi HEI, 9:
25-30 (in Bulgarian).
BAINOVA, A. (1975) [Cumulative action of Gramoxone and
Reglone.] In: Problemi na higienata, Sofia, Medizina i
Fizkultura, Vol. 1, pp. 31-38 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1974) [Experimental assessment
of the effect of dipyridyliums on sex glands.] In: Works of
the Research Institute of Hygiene and Laboratory Protection,
Sofia, Medizina i Fizkultura, Vol. 22, pp. 111-122 (in
Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1977) [Experimental
substantiation of Reglone MAC in the working environment.] In:
Problemi na higienata, Sofia, Medizina i Fizkultura, Vol. III,
pp. 11-17 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1978) Chronic action of diquat
on the lungs. C. R. Acad. Bulgar Sci., 31: 1369-1372.
BAINOVA A., ZLATEVA, M., & VULCHEVA, V. (1972) [Chronic
inhalation toxicity of bipyridylium herbicides.] Hig. Zdrav.,
15: 25-31 (in Bulgarian).
BENECKE, G. (1977) [Automatic evaluation of an algal
bioassay-inhibition of the movement of a blue-green alga
(Phormidium sp.) by the herbicide diquat.] Z. Wasser-
Abwasserforsch., 10: 195-197 (in German).
BENIGNI, R., BIGNAMI, M., CARERE, A., CONTI, G., CONTI, L.,
CREBELLI, R., DOGLIOTTI, E., GUALANDI, G., NOVELLETTO, A., &
ORTALI, V.A. (1979) Mutational studies with diquat and
paraquat in vitro. Mutat. Res., 68: 183-193.
BIGNAMI, M. & CREBELLI, R. (1979) A simplified method for
the induction of S-azaguanine resistance in S. typhimurium.
Toxicol. Lett., 3: 169-175.
BLACK, W.J.M., CALDERBANK, A., DOUGLAS, G., & McKENNA, R.H.
(1966) Residues in herbage and silage and feeding experiments
following the use of diquat as a desiccant. J. Sci. Food
Agric., 17: 506-509.
BUS, J.S., PREACHE, M.M., CAGEN, S.Z., POSNER, H.S., ELIASON,
B.C., SHARP C.W., & GIBSON, J.E. (1975) Fetal toxicity and
distribution of paraquat and diquat in mice and rats. Toxicol.
appl. Pharmacol., 33: 450-460.
CALDERBANK, A. (1972) Experimental considerations in the
development of diquat and paraquat as aquatic herbicides.
Outlook Agric., 7: 51-54.
CALDERBANK, A. & SLADE, P. (1976) Diquat and paraquat. In:
Kearne, P.C. & Kaufman, D. D. ed. Herbicide chemistry,
degradation and mode of action, 2nd ed., New York, Dekker,
pp. 501-540.
CALDERBANK, A. & YUEN, H. (1966) An improved method for
determining residues of diquat. Analyst, 91: 625-629.
CAVELL, B.D. (1979) Methods used in the study of the
photochemical degradation of pesticides. Pestic. Sci., 10:
177-180.
CHARLES, J.M. & MENZEL, D.B. (1979) Influence of atmospheric
particles on pulmonary absorption phenomenon. ACS Symp. Ser.,
174 (CH 15): 287-301.
CHARLES, J.M., ABOU-DONIA, M.B., & MENZEL, D.B. (1978)
Absorption of paraquat and diquat from the airways of perfused
rat lung. Toxicology, 8: 59-67.
CLARK, D.G. & HURST, E.W. (1970) The toxicity of diquat.
Br. J. ind. Med., 27: 51-55.
COATS, G.E., FUNDERBURK, H.H., LAWRENCE, J.M., & DAVIS, D.E.
(1966) Factors affecting persistence and inactivation of
diquat and paraquat. Weed Res., 6: 58-66.
COBB, L.M. & GRIMSHAW, P. (1979) Acute toxicity of oral
diquat (1,1'-ethylene-2'2'-bipyridylium) in Cynomolgus
monkeys. Toxicol. appl. Pharmacol., 51: 277-282.
CONNING, D.M., FLETCHER, K., & SWAN, A.A.B. (1969) Paraquat
and related bipyridyls. Br. med. Bull., 25: 245-249.
CRABTREE, H.C., LOCK, E.A., & ROSE, M.S. (1977) Effects of
diquat on the gastrointestinal tract of rats. Toxicol. appl.
Pharmacol., 41: 585-595.
DANIEL, J.W. & GAGE, J.C. (1966) Absorption and excretion of
diquat and paraquat in rats. Br. J. ind. Med., 23: 133-136.
DEMBINSKI, F., PONIKIEWSKA, T., & TRZEBNY, W. (1971) [Ground
seed of sunflower desiccated with Reglone as fodder for
ruminants.] Pamiet. Pulawski Pr., 49: 205-211 (in Polish).
DICKES, G.J. (1979) The application of gas chromatography to
food analysis. Talanta, 26: 1065-1099.
FAO/WHO (1971) Diquat. In: 1970 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1973) Diquat. In: 1972 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977a) Diquat. In: 1976 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977b) Pesticide residues in food, Rome, Food and
Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, 10).
FAO/WHO (1978) Diquat. In: 1977 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1979) Diquat. In: 1978 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO (1979) Diquat, Rome, Food and Agriculture Organization
of the United Nations, pp. 101-115 (FAO Plant Production and
Protection Paper No. 15, Supplement).
FEL, P., ZALA, I., IZULE, E., & VARGA, L. (1976)
[Haemodialysis in diquat poisoning.] Orv. Metilap, 117:
1773-1774 (in Hungarian).
FELDMAN, K.J. & MAIBACH, H.I. (1974) Percutaneous
penetration of some pesticides and herbicides in man. Toxicol.
appl. Pharmacol., 28: 126-132.
FITZGERALD, G.R., BARNIVILLE, G., FLANAGAN, M., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978) The changing pattern of
paraquat poisoning: an epidemiologic study. J. Irish Med.
Assoc., 71: 103-108.
FLETCHER, K., ed. (1975) Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-89
GAGE, J.C. (1968a) Toxicity of paraquat and diquat aerosols
generated by a size-selective cyclone: effect of particle size
distribution. Br. J. ind. Med., 25: 304-314.
GAGE, J.C. (1968b) The action of paraquat and diquat on the
respiration of the liver cell fractions. Biochem. J., 1O9:
757-761.
GOWMAN, M.E., REILY, D., & NEWBY, S.E. (1980) Paraquat and
Diquat: Longterm high rate trial, Frensham, United Kingdom. 2.
Persistence and movement in soil and glasshouse bioassay,
London, ICI Ltd (Report RJ0014B).
GROVER, R., SMITH, A.E., & KORVEN, H.C. (1980) A comparison
of chemical and cultural control of weeds in irrigation
ditchbanks. Can. J. Plant Sci., 60: 185-195.
GRZENDA, A.R., NICHOLSON, H.P., & COX, W.S. (1966)
Persistence of four herbicides in pond water. J. Am. Water
Works Assoc., 3: 326-332.
HAWKINS, S.F., MEDINA, M.A., & STAVINOHA, W. (1979) The
acute in vivo effect of paraquat and diquat on intermediary
metabolism in mouse lung. Proc. Fed. Am. Soc. Exp. Biol.,
38(3, Pt I): 582.
HAYES, W.J., Jr (1982) Pesticides studies in man, Baltimore,
London, Williams & Wilkins, 561 pp.
HOWE, D.J.T. & WRIGHT, N. (1965) The toxicity of paraquat
and diquat. In: Proceedings of the 18th New Zealand Weed &
Pest Control Conference, pp. 105-114.
HUGHES, R.D., MILLBURN, P., & WILLIAMS, R.T. (1973) Biliary
excretion of some diquaternary ammonium cations in the rat,
guinea-pig and rabbit. Biochem. J., 136: 979-984.
ICI (1972a) Determination of residues of diquat in soil -
residue analytical method No. 6, London, ICI Ltd (Report No.
PPRAM-6).
ICI (l972b) Determination of residues of diquat in milk and
water - residue analytical method No.7, London, ICI Ltd
(Report No. PPRAM-7).
ICI (l972c) Determination of residues of diquat in crops and
animal tissues - residue analytical method No. 5, London, ICI
Ltd (Report No. PPRAM-5).
IRPTC (1983) IRPTC Legal files 1983 Vol. I & II, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme.
KHERA, K.S., WHITTA, L.L., & CLEGG, D.J. (1968) Embryopathic
effects of diquat and paraquat in rats. Ind. Med. Surg., 37:
257-261.
KING, R.R. (1978) Gas-chromatographic determination of
diquat residues in potato tubers. J. agric. food Chem., 26:
1460-1463.
KURISAKI, E. & SATO, H. (1979) [Toxicological studies on
herbicides: intracorporal distribution of paraquat dichloride
and diquat dibromide in rat.] Nippon Hoigaku Zasshi, 33: 656
(in Japanese).
LAM, H.F., TAKAZAWA, J., GUPTA, B.N., & STEE, E.W. VAN
(1980) A comparison of the effects of paraquat and diquat on
lung compliance, lung volume and single-breath diffusing
capacity in the rat. Toxicology, 18: 111-123.
LAVAUR, E., SION, G., GROLLEAU, G., & CARPENTER-LESECK, J.
(1979) Etude comparée de l'action du diquat et du paraquat
sur la muqueuse digestive de la souris, du rat et du lapin.
Ann. Zool. Ecol. anim., 11: 159-169.
LEARY, J.B. (1978) Diquat and paraquat. In: Zweig, G. &
Sharma, J., ed. Analytical methods for pesticides and plant
growth regulators, New York, Academic Press, pp. 321-325.
LEVIN, D.E., HOLLSTEIN, M., CHRISTMAN, M.F., SCHWIERS, E.A., &
AMES, B.N. (1982) A new Salmonella tester strain (TA 102)
with AT base pairs at the site of mutation detects oxidative
mutagens. Proc. Natl Acad. Sci. US, 79: 7445-7449.
LITCHFIELD, N.H., DANIEL, J.W., & LONGSHAW, S. (1973) The
tissue distribution of the bipyridylium herbicides diquat and
paraquat in rats and mice. Toxicology, 1: 155-165.
LOCK, E.A. (1979) The effect of paraquat and diquat on renal
function in the rat. Toxicol. appl. Pharmacol., 48: 327-336.
LOCK, E.A. & ISHMAEL, J. (1979) The acute toxic effects of
paraquat and diquat on the rat kidney. Toxicol. appl.
Pharmacol., 50: 67-76.
MAKOVSKII, V.N. (1972) [Toxicological and hygiene studies of
the bipyridylium herbicides diquat and paraquat,] Referat,
Vinniza, USSR, pp. 1-23 (PhD thesis) (in Russian).
MATSUURA, N., TAKINAMI, M., KURISAKI, E., & SATOO, H. (1978)
[Distribution of paraquat dichloride and diquat dibromide in
the living body.] Fukushima Igakkai Zasshi, 28: 212 (in
Japanese).
NARITA, S., MATOJUKU, M., SATO, J., & MORI, H. (1978)
[Autopsy in acute suicidal poisoning with diquat dibromide.]
Nippon Igakkai Zasshi, 27: 454-455 (in Japanese).
O'BRIEN, M.C. & PRENDEVILLE, G.N. (1978) A rapid sensitive
bioassay for determination of paraquat and diquat in water.
Weed Res., 18: 301-303.
OKONEK, S. & HOFMANN, A. (1975) On the question of
extracorporeal haemodialysis for diquat intoxication. Arch.
Toxicol., 33: 251-257.
OREOPOULOS, D.G. & MCEVOY, J. (1969) Diquat poisoning.
Postgrad. med. J., 45: 635-637.
PASI, A. & EMBREE, J.W. (1975) Further comments on the
assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 31: 125-126.
PASI, A., EMBREE, J.W., EISENLORD, G.A., & HINE, C.H. (1974)
Assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 26: 171-175.
PIRIE, A. & REES, J.R. (1970) Diquat cataract in the rat.
Exp. Eye Res., 9: 198-203.
PYL, W. & GIEBELMANN, R. (1978) [Detection of chlorocholine
chloride, diquat and paraquat.] Arch. vet. Med., 32: 601-602
(in German).
REISH, D., ROSSI, S.S., MEARNS, A.J., OSHIDA, P.S., & WILKES,
F.G. (1979) Marine and estuarine pollution. J. Water Pollut.
Control Fed., 51: 1477-1517.
RILEY, D. (1981) The fate and effect and diquat residues in
soil. In: Proceedings of the National Spray Seed Conference
1981, Albury, New South Wales, Australia.
RILEY, D. & GRATTON, R.P. (1974) Unavailability to plants of
diquat residues in soils. 10th Tr. Muzhdunar. Kongr.
Pochvoved., USSR, 3: 193-203.
ROSE, M.S. & SMITH, L.L. (1977a) The relevance of paraquat
accumulation by tissues. In: Autor, P.A., ed. Biochemical
mechanisms of paraquat toxicity, New York, Academic Press,
pp. 71-91.
ROSE, M.S. & SMITH, L.L. (l977b) Tissue uptake of paraquat
and diquat. Gen. Pharmacol., 8: 173-176.
SCHOENBORN, H., SCHUSTER, H.P., & KOESSLING, F.K. (1971)
[Clinical and muphological findings in an acute oral
intoxication with diquat Reglon.] Arch. Toxicol., 27: 204-216
(in German).
SCHULTZ, VON O., KIRCHNER, K., MUELLER, P., & ROTHE, R.
(1976) [Reglone (Diquat) cause of intoxication of sheep,
cattle, and swine.] Monatsh. Veterinärmed., 31: 647-649 (in
German).
SELYPES, A., NAGYMAJTENYI, L., & BERENCSI, G. (1980)
Mutagenic and embryotoxic effects of paraquat and diquat.
Bull. environ. Contam. Toxicol., 25: 513-517.
SHARP, C.W., OTTLENGHI, A., & POSNER, H.S. (1972)
Correlation of paraquat toxicity with tissue concentrations
and weight loss of the rat. Toxicol. appl. Pharmacol., 22:
241-251.
SIEBERT, D. & LEMPERLE, E. (1974) Genetic effects of
herbicides: induction of mitotic gene conversation in
Saccharomyces cervisiae. Mutat. Res., 22: 1ll-120.
SINGH, S.P. & YADAV, N.K. (1978) Toxicity of some herbicides
to maior carp fingerlings. Indian J. Ecol., 5: 141-147.
SMITH, S.N., LYON, A.J., & SAHID, I.B. (1976) The breakdown
of paraquat and diquat by soil fungi. New Phytol., 77: 735-740.
SMITH, T.P., NOACK, A., & COSH, S.M. (1981) The effect of
some herbicides on vesicular-arbuscular endophyte abundance in
the soil and on infection of host roots. Pestic. Sci., 12:
91-97.
STEVENS, M.A. & WALLEY, J.K. (1966) Tissue and milk residues
arising from the ingestion of single doses of diquat and
paraquat by cattle. J. Sci. Food Agric., 17: 472-475.
SUMMERS, L.A. (1980) The bipyridilium herbicides, London,
New York, Toronto, San Francisco, Academic Press, pp. 1-449.
TSCHIPILSKA, L.N. (1980) [The influence of some pesticides
on the soil microorganisms in connection with the hygiene
evaluation of the soil,] Referat, Sofia, Bulgaria, pp. 1-39
(PhD thesis) (in Bulgarian).
TOMPSETT, S.L. (1970) Paraquat poisoning. Acta pharmacol.
toxicol., 28: 346-358.
TUCKER, V.B., PACK, D.E., & OSPENSON, J.N. (1967) Adsorption
of bipyridylium herbicides in soil. J. agric. food Chem., 15:
1005-1008.
UKAI, S., HIROSE, K., & KAWASE, S. (1977) [Forensic chemical
studies on drugs and chemicals. III. Gas chromatography of
reduction products of the herbicides diquat and paraquat.]
Eisei Kagaku, 23: 32-38 (in Japanese).
VANHOLDER, R., COLARDYN, F., RENCK, DE J., PRAET, M., LAMEIRE,
N., & RINGOIR, S. (1981) Diquat intoxication. Report of two
cases and review of the literature. Am. J. Med., 70: 1267-1271.
VERBETSKII, V.E. & PUSHKAR, M.S. (1968) [Pathological
changes in the organs of animals on acute poisoning with the
herbicide diquat (Reglone).] In: Some questions concerning
human and animal morphology, Medizina, Odessa, pp. 51-52 (in
Russian).
WEBER, J.B., PERRY, P.W., & UPCHURCH, R.P. (1965) The
influence of temperature and time on the adsorption of
paraquat, diquat, 2,4-D and prometone by clays, charcoal and
anion exchange resin. In: Soil Science Society Proceedings,
pp. 678-688.
WHO/FAO (1979) Data sheets on pesticides: Diquat, Geneva,
World Health Organization, pp. 1-7 (Unpublished Report No.
VBC/DS/79-40).
WILKINSON, W. (1980) Paraquat and Diquat: Longterm high rate
trial, Frensham, United Kingdom. 1. Management of site,
effects on crops and weeds and residues in crops, London, ICI
Ltd (Report No. RJ0013B).
WILLIAMS, C.B., LEVISON, S.A., & DANDLIKER, W.B. (1976)
Application of immunological techniques to the detection of
organic contaminants of environmental concern. In: Proceedings
of the University of Montana Annual Conference on Trace
Substances & Environmental Health, No. 10, pp. 317-322.
WOJECK, G.A., PRICE, J.F., NIGG, H.N., & STAMPER, J.H.
(1983) Worker exposure to paraquat and diquat. Arch. environ.
Contam. Toxicol., 12: 65-70.
4.1.2. Microbial degradation
Photochemical degradation of diquat on plants is quicker than
microbial degradation in soil. Microbial degradation of strongly-
bound diquat in soil is slow, but is faster in culture. The
degradation of diquat by soil fungi was studied by Smith et al.
(1976). The degradation of 14C-diquat to 14CO2 by Aspergillus
niger was tested by 4 different fungal test systems. High
intracellular herbicidal levels and inability to grow in the
presence of low diquat concentrations in the media characterized
the species unable to decompose diquat. Under laboratory
conditions, diquat degradation by Pseudomonas started after 3 days
(Tchipilska, 1980). Under field conditions, degradation started
after 10 days, and was related to the ambient temperature, and the
aeration and type of soil.
The fact that no significant hazard has been observed for
ruminants from diquat-treated herbage, or for the general
population from crops and water, is explained by the rapid
photochemical degradation of diquat.
4.2. Diquat Adsorption, Residue Levels, and Exposure in Soil
4.2.1. Diquat adsorption on soil particles
Diquat binds readily to clay particles in the soil. The rate
of adsorption depends on the degree of contact of diquat with
adsorbent minerals, the type of soil, and the initial herbicide
concentrations tested. Weber et al. (1965) studied the effects of
temperature and exposure time on diquat adsorption by
montmorillonite, kaolinite, charcoal, and an anionexchange resin in
pH 6.0 phosphate buffer. Diquat was preferably adsorbed on the
clay particles by a process of ion exchange. Adsorption was
limited by the cation-exchange capacity of the test systems
examined. Coats et al. (1966) showed the adsorption capacity of
kaolinite to be about 2 g/kg and that of bentonite 80 - 100 g/kg.
A diquat soil concentration of 0.1 mg did not produce any
significant reduction in the dry weight of wheat grown in the soil
(Coats et al., 1966). The diquat appeared to be too tightly
adsorbed to the surface and between the lattices of bentonite to be
available to the wheat plant, at a soil treatment rate of 50 g/kg.
Data for diquat adsorption on sandy soils (Tucker et al., 1967)
showed that the herbicide was bound to different extents, according
to the structure of the soil particles.
4.2.2. Residue levels of diquat in soils
Makovskii (1972) reported on diquat residues in soils from
different plots, treated every year for a period of 7 years. There
were 3 - 4 treatments per season, at approximately 27.5 kg
diquat/ha. Samples were taken at 0 - 10 cm, 10 - 20 cm, and 20 -
30 cm depths in the soil; total diquat residues were shown to be
about 5.4 mg/kg soil, the mean values being 3.9 mg/kg, 1.3 mg/kg,
and 0.2 mg/kg in the respective soil layers. No diquat residues
were discovered in plants and citrus fruits sampled at different
times from the treated plots. In other studies, soil was analysed
for diquat residues on the 1st, 8th, and 15th days after applying
Reglone(R) at 0.8 litre/ha and 0.4 litre/ha (Tchipilska, 1980). On
the 1st day, residues of 0.400 mg/kg and 0.126 mg/kg were detected;
on the 8th and l5th days residues in the treated plots were lower
than 0.1 mg/kg.
As summarized in section 4.1.2, free diquat is degraded by a
range of microorganisms. While degradation of strongly-absorbed
diquat is relatively slow, results of long-term field studies have
nevertheless shown degradation rates of the order of 5 - 10% per
year. This is greater than the rate required to prevent saturation
of the deactivation capacity of soils.
In a long-term trial on a loamy soil, plots were treated with
0, 90, 198, and 720 kg diquat/ha, which was incorporated to a depth
of 15 cm. These rates were equivalent to 0, 50, 110, and 400% of
the soils strong absorption capacity (Gowman et al., 1980;
Wilkinson, 1980; Riley 1981). Over the 7 years, diquat residues
declined by 5% per year (sig P = 0.05) on the 90 kg/ha plots and
by 7% per year (sig P = 0.01) on the 198 and 720 kg/ha plots. The
rate of decline on the 198 and 720 kg/ha plots were significantly
greater ( P = .01) than on the 90 kg/ha plots.
4.2.3. Effect of residual diquat on soil biological activity, on
plants, and crop yields
A literature review and an extensive study of the effects of
different concentrations of diquat on microorganisms (saprophyte
and pathogenic microflora, and fungi) were carried out by
Tchipilska (1980). Staphylococcus aureus growth was inhibited
while Scenedesmus acutus was stimulated. Smith et al. (1981)
examined the effects of diquat applied at 0.5 - 32 times the
concentration recommended in agricultural practice on vesicular
arbuscular endophyte spore abundance in the soil and on the
infection of wheat roots. No measurable deviations in
endomycorrhiza formation and function were noted at normal
application rates. Loss of potassium and phosphate from fungi was
recorded at higher concentrations of diquat.
Coats et al. (1966) studied the uptake and translocation of
14C-diquat from soil into wheat. No metabolites were found in the
plants.
Diquat does not appear to have any significant influence on the
normal microbial activity that is important for soil fertility.
Nor is there any evidence that the recommended application rates
for diquat lead to residual effects on crop growth. Moreover,
tightly adsorbed diquat in soil is not reactivated into a
biologically active form, so that, in practice, accidental spillage
is probably the only cause of local high phytotoxic levels of
residual herbicide.
4.3. Diquat Transformation, Residue Levels, and in Effects on
Aquatic Organisms and Crops
4.3.1. Transformation and residue levels of diquat in water
In static water, initial diquat concentrations of 0.5 - 1.0
mg/litre fell rapidly to 0.1 - 0.3 mg/litre after 4-7 days
(Calderbank, 1972; Calderbank & Slade, 1976). In field
experiments, initial concentrations of 1.0, 0.8, and 0.5 mg/litre
decreased to 0.03 - 0.003 mg/litre after 7 - 14 days. This rapid
loss of diquat from treated waters was due to rapid uptake by
aquatic weeds. Two weed species (Myriophyllum spicatum and
Callitriche stagnalis) were immersed in water containing 1.0 mg
diquat/litre. The concentration of the herbicide decreased rapidly
to 0.14 - 0.03 mg/litre during a period of 6-14 days after
treatment. At the end of the experiment, the residue levels in
the weeds ranged from 6.2 - 17.4 mg/kg. In addition to uptake
by weeds, loss of diquat from treated waters was due to
photodegradation at the water surface and adsorption by bottom mud.
In field experiments carried out in 1010 m2 ponds with an initial
concentration of diquat of 2 mg/litre water, there were no residues
of diquat in the water after 8 days (Calderbank, 1972; Calderbank &
Slade, 1976).
In pond water that had been treated with diquat at 2.5 mg/litre
(Grzenda et al., 1966), residues of 0.01 - 0.08 mg/litre were found,
7-9 days after applying the herbicide, and no residues could be
determined after 14-30 days. The authors concluded that, compared
with other herbicides, diquat appeared to have the greatest
potential for use in sources of potable water.
The data obtained from studies in ponds, large and small lakes,
canals, and reservoirs demonstrate the fast disappearance of diquat
from treated waters (Calderbank, 1972). Absorption by aquatic
weeds explains the high efficacy of the herbicide. Decomposition
of the dead weeds is rapid, and diquat is not released from the
bottom mud back into the water. Applications of paraquat and
diquat each at a dose level of 1.1 kg/ha (Grover et al., 1980)
proved very effective for the control of weeds in irrigation
ditches, and the residual levels of both herbicides decreased
rapidly.
4.3.2. Effects of residual diquat on aquatic organisms and crops
The toxicity of diquat for fish varied with the species, the
size of the fish, and the softness or hardness of the water. The
LC50 values range from 12 to 90 mg/litre (24 h), 6 to 44 mg/litre
(48 h), and 4 to 36 mg/litre (96 h) (Calderbank, 1972). Reviews of
the effects of diquat on fish, aquatic invertebrates,
microbiological organisms in the soil of lakes, and phytoplankton
demonstrate that the herbicide, applied at the rates used for
aquatic weed control, did not affect estuarine fauna, oysters,
shrimps, water insects, or fish-food organisms (Calderbank, 1972;
Atkinson, 1973). At concentrations of 1 - 100 mg/litre, diquat
appeared to be less toxic for carp fingerlings than paraquat,
diuron, simazine, and dalapon (Singh & Yadev, 1978). Reish et al.
(1979) reviewed the effects of diquat on marine organisms; no
bioaccumulation by estuarine and marine organisms was found. The
toxicity of diquat for fish is low, and the main risk for aquatic
organisms and fish from its use as an aquatic weed killer is the
decreased oxygen concentration following the decay of weeds.
Trout exposed to 1 mg diquat/litre for 7 days contained
residues of 0.3 - 0.4 mg/kg in the gut, liver, and kidney, and of
0.1 - 0.3 mg/kg in the skin and gills. Residues were below the
limit of detection in muscle, spleen, and heart (Calderbank, 1972).
Trout exposed to 1 mg diquat/litre for 16 days contained residues
of 0.5 - 0.6 mg/kg, which disappeared when the fish were returned
to fresh water.
Because of irreversible adsorption, low residues in water will
be lost on contact with soil. The herbicide is thus unavailable to
plant roots. However, in overhead irrigation experiments, the use
of water containing diquat at 0.1 - 0.5 mg/litre (Calderbank, 1972)
resulted in diquat residues in the crops (tomato, lettuce, sugar
beet) ranging from less than 0.01 mg/kg to 0.04 - 0.07 mg/kg.
Thus, before using herbicide-treated waters for overhead plant
irrigation, it is advisable to allow 10 days for the diquat aquatic
residues to drop to acceptable levels.
The maximum diquat residues in water ultimately to be used for
drinking were 0.03 - 0.01 mg/litre, at the points of entry into the
public distribution system, 2-4 days after treatment; no residues
were detectable on the 10th day after applying diquat as an aquatic
herbicide. More often than not, residue levels were below the
detection limits of the analytical methods used.
4.4. Diquat Exposure and Residue Levels in Plants and Animals
4.4.1. Plants
Diquat is largely used as a desiccant in silage production. At
the recommended rates of 1.5 - 3.0 litre Reglone(R)/ha, diquat
residues were very low (Riley & Gratton, 1974). Following pre-
harvest desiccation of fodder crops, they ranged from below 0.05
mg/kg to 50 mg/kg, most of the levels determined being below 25 mg
diquat/kg (FAO/WHO, 1971, 1973). Diquat residues in the treated
herbage, sampled at different intervals after spraying with 0.258 -
0.515 mg/ha, were relatively high after 1 day (12 - 65 mg/kg), but
after 7 days had markedly decreased (1.0 - 6.5 mg/kg) (Black et
al., 1966). The levels of diquat found in silage during a 4-year
trial, with application rates of 0.190, 0.258, and 0.540 mg/ha,
varied from 1.4, 3.6, 9.3, and 13.3 to 26.8 mg/kg. The differences
were due to the atmospheric conditions at the time of desiccation
and the consequent degree of photochemical degradation of the
diquat. For this reason, diquat residues in treated herbage should
be expected to vary by an order of magnitude (10 times).
Pre-harvest desiccation of rape-seed with diquat did not result
in any detectable residues in the extracted oil and only low
residues (0.3 - 2 mg/kg) in the meal cake. Rape plants were
sprayed with 14C-diquat at 0.3 - 1.1 kg/ha, 3-14 days before
harvesting. There were no detectable residues of diquat or of its
photodegradation products in the rape-seed oil when the seeds were
harvested 7 days after desiccation, and very low diquat residues
(0.02 - 0.003 mg/kg) were determined when the seeds were harvested
14 days after treatment with diquat. The diquat residues in the
meal cake varied from 1.49 to 10.2 mg/kg, 14 days after treatment,
a large proportion being unchanged diquat (FAO/WHO, 1973).
Dembinski et al. (1971) reported diquat residues of 2 mg/kg in
sunflower seeds desiccated with Reglone(R).
Makovskii (1972) reported the diquat residue levels in weeds
treated with Reglone(R). After applications of Reglone (R) at
0.5, 1.0, and 1.3 litre/ha, the residues in dry weeds, ranged from
34 to 74 mg/kg 1 h later; from 15 to 26 mg/kg after 1 day; from
undetectable to 10 mg/kg after 4 days; from 2.8 to 3.5 mg/kg after
2 weeks; from 1.9 to 2.3 mg/kg after 4 weeks; and from undetectable
to 1.7 mg/kg after 6 weeks. The degradation of diquat in plants
was more rapid than the degradation of paraquat. The residues in
potatoes did not exceed 0.08 mg/kg, when diquat was used to destroy
potato haulm, and levels in fruits (apples, pears, plums, citrus),
tea, and cereals were undetectable (< 0.01 mg/kg), when diquat was
applied as a herbicide for weed control. Samples of potatoes
purchased from shops (Andersson & Josefsson, 1982) were analysed
for diquat residues. Residues in the range of 0.004 - 0.039 mg/kg
were found in 20 of 23 samples obtained from commercial growers.
None of the samples contained more than the residue tolerance of
0.1 mg/kg accepted for potatoes in Sweden.
Residue levels of diquat have been discussed in more detail by
the Joint Meeting on Pesticides Residues (FAO/WHO, 1971, 1973).
Residue levels of diquat in plants were summarized and published by
FAO/WHO (1977a). Some of these data are given in Table 2.
Data on diquat residues in desiccated wheat collected from 6
countries showed a mean of 0.5 mg/kg (FAO, 1979).
Table 2. Diquat residues in plantsa
------------------------------------------------------------
Plants Dose of diquat Mean value of
(kg/ha) residues (mg/kg)
------------------------------------------------------------
Wheat (grain, flour) 0.6 - 1.0 0.61, 0.22
Rice (with husk, polished) 0.2 - 0.4 0.89, 0.07
Sorghum (grain) 0.4 - 0.6 0.81
Cotton (grain) 0.4 - 1.0 0.37
Potato 0.6 - 1.0 0.03
Beans 0.3 - 1.0 0.10
Peas 0.3 - 1.0 0.05
Sugar beet (juice) 0.3 - 0.8 < 0.01
------------------------------------------------------------
a From: FAO/WHO (1977a).
4.4.2. Animals
Sheep and cattle fed silage containing diquat residues of up to
13 mg/kg were studied by Black et al. (1966). The total diquat
excreted in the urine was 0.19 - 0.65 mg over an 8-day period. No
diquat residues were detected in the brain, liver, and kidney of
sheep, or in the meat or organs of cattle fed diquat-treated silage
for one month. Milk collected on alternate days for 2 weeks was
free of diquat residues (< 0.003 mg/litre).
Feeding trials with sunflower seed containing approximately
0.20 mg diquat/kg were reported by Dembinski et al. (1971).
Although the amount of diquat consumed by the cattle over 257 days
ranged from 11.2 mg to 184.2 mg, no residues were found in any of
the milk samples analysed. Wethers fed ground sunflower seed
containing approximately 0.20 mg diquat/kg for 141 days were
estimated to have consumed a total of 14.1 mg diquat per sheep. No
residues were found in brain, liver, or kidney, nor were there any
residues in the meat, lungs, and kidney of steers treated with
diquat-desiccated sunflower forage. In long-term feeding trials
with silage, desiccated grass, lucerne, clover hay, barley straw,
and sunflower seeds containing diquat residues ranging from 0.2 to
50 mg/kg, the residues in milk and meat were determined to be less
than 0.007 mg/litre and less than 0.0006 mg/kg, respectively
(FAO/WHO, 1971, 1973, 1977a,b). Calderbank (1972) reviewed the
effects on farm animals of diquat in the drinking-water and on
herbage; there were no adverse effects on cattle and sheep and only
very low residue levels in milk, meat, and the organs analysed.
Lavaur et al. (1979) studied the effect of treated lucerne on
rabbits. Immediately after spraying, a concentration of 211 mg
diquat/kg dry weight was determined in the lucerne. After 24 h and
48 h, diquat residues were 97 mg/kg and 25 mg/kg, respectively. No
signs of poisoning or gastrointestinal damage were found in the
rabbits fed with different levels of diquat residues in the
lucerne. However, in some circumstances, lack of careful
organization may result in adverse effects of diquat on animals.
Intoxication of sheep, cattle, and swine has been reported (Schultz
et al., 1976) after the aerial application of Reglone(R) as a
rapeseed desiccant. The clinical course and the causes of the
accident stressed the need for proper diquat application by air.
For a more detailed discussion of the fate of diquat residues
in exposed animals, refer to FAO/WHO (1977a,b).
4.5. Diquat Levels in Air and Exposure of Workers
Experiments with 14C-diquat demonstrated that it was not
volatile (Coats et al., 1966). Diquat levels in air after spraying
with aerosols were determined by Makovskii (1972), using the method
of Calderbank & Yuen (1966). The application rates were 1.0 - 1.3
kg diquat/ha in working dilutions of 2.5 g and 3.3 g active
ingredient/litre, the highest diquat concentrations being found in
the tractor cabin when the door was open and spraying was in
progress in the direction of the wind (Table 3). The diquat
concentrations in air decreased rapidly 10 - 20 min after
completion of the treatment.
Wojeck et al. (1983) reported that diquat was determined in air
samples taken near the breathing zone of workers during its
application for aquatic weed control. The respiratory exposure
levels were below the limits of quantitation of the chemical
analysis.
In Bulgaria and the USSR, the proposed MAC (maximum allowable
concentration) for diquat is 0.1 mg/m3 aerosol. The TLV for diquat
in workroom air in the United Kingdom and the USA is 0.5 mg
diquat/m3 (1982), a level that will not be reached under normal
conditions of application.
Table 3. Total airborne diquat concentrations in the air of working areasa
---------------------------------------------------------------------------
Place of sampling Number of Mean concentrations
samples (mg/m3 ± SE)
---------------------------------------------------------------------------
Working area sprayer loading 20 0.12 ± 0.03
tractor cabin 8 0.56 ± 0.10
(in direction of wind)
tractor cabin 8 0.17 ± 0.04
(against the wind)
manual spraying 16 0.25 ± 0.04
Treated field after 5 min 8 0.20 ± 0.03
after 10 min 24 0.06 ± 0.01
after 20 min 8 ND
Distance from 200 m 8 0.09 ± 0.01
treated field 400 m 8 ND
---------------------------------------------------------------------------
a From: Makovskii (1972).
5. KINETICS AND METABOLISM
5.1. Animal Studies
5.1.1. Absorption
Oral absorption
Daniel & Gage (1966) studied the absorption of 14C-diquat
dibromide and 14C-diquat dichloride following oral and subcutaneous
single-dose administration to rats. About 90 - 97% of the oral
diquat dibromide and 84 - 90% of the diquat dichloride were found
in the faeces and 4 - 11% of both diquat salts in the urine.
Following subcutaneous injection of 14C-diquat (10 mg/kg body
weight) in rats, 87% of the administered dose was excreted in the
urine and 5% in the faeces within 4 days. The urine contained
mainly unchanged diquat (75% of the dose) together with diquat
monopyridone (about 3% of the dose) and diquat dipyridone (about 6%
of the dose) (FAO/WHO, 1978).
The poor absorption of diquat from the gastrointestinal tract
was confirmed by Litchfield et al. (1973) in the rat, and by Black
et al. (1966), Stevens & Walley (1966), and Dembinski et al. (1971)
in farm animals.
Pulmonary absorption
The uptake of 14C-diquat by perfused rat lung, following
intratracheal injection, was examined by Charles et al. (1978) and
Charles & Menzel (1979). Removal of 14C-diquat from the airways
was rapid, initially, but slowed down with time. The results
indicate 2 phases of absorption and removal of diquat from the
airways in the rat.
Dermal absorption
There are no data on the rate of diquat absorption through the
skin. Studies on the dose-related percutaneous toxicity of diquat
suggest that it may be dermally absorbed.
5.1.2. Distribution
Although paraquat and diquat have similar chemical, physical,
and herbicidal properties, only paraquat has been shown to damage
the lung. According to Sharp et al. (1972), diquat concentrations
in lung and muscle were much lower than the levels attained with
equal 20 mg/kg body weight iv doses of paraquat. Table 4 shows the
distribution of both in the main internal organs.
Table 4. Ratio of concentration of paraquat/diquat in
the tissues of the rata
--------------------------------------------------------
Organ Days after intravenous administration
1 3 5 7 10
--------------------------------------------------------
Lung 8 33 12 10 20
Muscle 2 13 10 7 16
Kidney 0.9 0.9 0.9 0.3 0.25
Liver 0.4 0.7 0.7 0.5 0.2
--------------------------------------------------------
a From: Sharp et al. (1972).
Diquat concentrations were higher in the kidney and the liver
but significantly lower in the lung (Table 4). In addition, the
concentrations of paraquat were 2-8 times higher than those of
diquat in the heart, adrenal glands, spleen, stomach, ileum,
testes, and thymus. Plasma levels were similar for both
bipyridylium herbicides.
Litchfield et al. (1973) injected 14C-diquat cation at 50 mg/kg
body weight iv into mice. Whole-body autoradiographs were prepared
after 10 min, 1 h, 24 h, and 72 h. Radioactivity was selectively
located in the gall bladder and was also present in cartilaginous
tissue, liver, and the gastrointestinal tract. Low radioactivity
was found in the brain and spinal cord. One h after dosing, the
amount in the urine and intestinal epithelium had increased. After
24 h, the excretion of diquat was virtually complete, although
radioactivity continued to be detected in the small and large
intestine and the bladder.
Litchfield et al. (1973) also determined diquat levels in
various tissues of male and female rats fed a diet containing
diquat dibromide monohydrate at 250 mg/kg for 2, 4, and 8 weeks.
High levels (0.18 - 1.17 mg/kg) were found in the kidney and the
large intestine; levels in the lung ranged from < 0.05 to 0.53
mg/kg; those in the liver from 0.07 - 0.22 mg/kg, while levels in
the brain, muscle, and blood were very low. At all stages of the
study, diquat lung levels were lower than those for paraquat, the
average paraquat content in the lung (at a dose of 250 mg/kg diet)
over the 8-week period being 1.7 mg/kg and the average diquat
level, 0.2 mg/kg. No sex differences were found. Within 1 week of
return to a normal diet, diquat was below the detectable limit in
all tissues examined.
Rats given paraquat or diquat orally at 680 µmol/kg had high
kidney levels of diquat throughout the 30 h period after dosing
(Rose & Smith, 1977, 1977a). There was no significant time-
dependent increase in diquat levels in the lung, liver, brain,
adrenal glands, muscle, and plasma. These results confirmed that,
following oral dosing, the lung does not accumulate diquat. Rose &
Smith (1977) also incubated rat lung slices in 10-5M paraquat and
diquat. In contrast to paraquat, diquat did not accumulate in the
lung slices, and the compound did not accumulate significantly in
any tissue slices with the exception of those from the kidney.
These observations were confirmed by Lock (1979).
Matsuura et al. (1978) studied the distribution of orally
administered LD50 doses of diquat and paraquat in rats. Two and
24 h after dosing, there were higher concentrations of diquat in
kidney, liver, and lung than in brain, heart, the gastrointestinal
system, and blood. At equitoxic doses, levels of diquat in the
lung appeared to be lower than those of paraquat. In a similar
distribution study of the LD50 and 0.5 LD50 doses of diquat and
paraquat, Kurisaki & Sato (1979) determined the tissue
concentrations from 2 to 48 h and from 2 to 9 days after treatment.
Distribution in the lung, heart, brain, liver, and kidney of the
rats agreed with previously published data.
The results of the above studies demonstrate that diquat does
not persist as long as paraquat in the body of the rat and that it
does not accumulate in the lung.
5.1.3. Metabolic transformation and excretion
Daniel & Gage (1966) reported that the amount of 14C-diquat
excreted in rat bile during the 24 h following oral doses of 1.2 -
64 mg/kg body weight represented 1.1 - 4.8% of the dose. Small
amounts were detected in the urine, but about 70% of the diquat was
present in the faeces. In other studies (FAO/WHO, 1978), the rate
of diquat metabolism in the rat was considerably lower than
previously reported by Daniel & Gage (1966). The biliary, urinary,
and faecal excretion of 14C-labelled bipyridylium herbicides was
studied by Hughes et al. (1973) in the rat, guinea-pig, and rabbit.
14C-diquat dichloride was injected ip at dose levels of 40 µmol/kg
body weight in the rat, 13 µmol/kg in guinea-pig, and 14 µmol/kg in
the rabbit. Most of the injected diquat (82% - rat, 64% -rabbit)
was found in the urine. Rabbits metabolized 18% of the dose,
guinea-pigs 5%, and rats less than 1%. The metabolites were
similar for the 3 species. The rat excreted approximately 1.4% of
the dose in the bile, the guinea-pig 4.8%, and the rabbit 2.9%.
Stevens & Walley (1966) treated cattle orally with 14C-diquat
dibromide in doses of 4, 8, and 20 mg/kg body weight. The
radioactivity levels in the milk of the cows indicated that 0.04 -
0.15% of the ingested dose was excreted in this way. Very low
levels of diquat (0.01 mg/kg) were present in muscle tissue, 2 - 8
days after dosing. A bull calf was dosed orally with 14C-diquat
dibromide at 10 mg/kg. About 2.6% of the 10 mg/kg dose was excreted
in the urine, but the major part of the dose was excreted via the
faeces. In the calf, 24 h after dosing, the residues were 0.66
mg/kg in kidney, 0.20 mg/kg in heart and skin, 0.19 mg/kg in liver,
0.03 mg/kg in lung, testes, and serum, and 0.006 mg/kg in muscle.
Studies on rats dosed orally with 14C-diquat at 45 mg/kg body
weight or subcutaneously (sc) with 10 mg/kg body weight were
reported by FAO/WHO (1978). Rats given the oral dose excreted 6%
and 89% in the urine and faeces, respectively, within 4 days and
mainly within the first 2 days. Unchanged diquat was the major
component in both urine (5% of the dose) and faeces (about 57% of
the dose). About 5% of the oral dose was excreted as diquat
monopyridone, mainly in the faeces, while diquat dipyridone
appeared to be the major urinary metabolite. Following sc
injection, rats eliminated 87% of the dose in the urine and 5% of
the dose in the faeces within 4 days. The urine contained 75% of
the dose as diquat, about 3% as diquat monopyridone, and about 6%
as diquat dipyridone. In vitro studies have shown that the caecal
microflora of the rat can metabolize about 10% of the diquat added
in a 24-h incubation period, with the formation of some diquat
monopyridone. This observation, together with the paucity of
metabolites following ip injection, suggests that diquat is
metabolized by the gastrointestinal tract bacteria.
The oral LD50 of diquat monopyridone in the rat was more than
4000 mg/kg body weight. Oral administration of diquat monopyridone
at 1000 mg/kg body weight per day for 2 weeks did not induce any
clinical, haematological, biochemical, or histopathological
deviations in the rat. In other studies, no adverse effects were
noted after sc injection of diquat monopyridone or diquat
dipyridone in rats, but 9 animals out of a group of 10 injected
with the equivalent dose (16 mg/kg body weight) of diquat were dead
by the l4th day following dosing (FAO/WHO, 1978).
5.2. Observations on Man
Feldman & Maibach (1974) studied the dermal penetration of
twelve 14C-labelled insecticides and herbicides. Diquat showed a
very low rate of dermal absorption in man. No other studies on the
kinetics of diquat in volunteers have been published, but
observations are available on accidental and suicidal ingestion
(section 7). Toxicological analysisk, at the time of admission, of
the serum of a patient, who had ingested 20 ml Reglone(R), showed
a diquat level of 0.4 mg/litre (Vanholder et al., 1981). At
postmortem examination on the 5th day after ingestion,
approximately 0.20 mg diquat/kg was determined in liver, kidneys,
muscle, and eye liquid.
6. EFFECTS ON ANIMALS
6.1. Effects on Experimental Animals
6.1.1. Gastrointestinal system and liver
Investigation of the clinical signs of acute oral intoxication
by diquat (Verbetskii & Pushkar, 1968; Clark & Hurst, 1970;
Crabtree et al., 1977; Cobb & Grimshaw, 1979) have established
gastrointestinal disturbance as the major syndrome of poisoning and
as a cause of death. In both rats and guinea-pigs, the clinical
signs of acute oral poisoning (Verbetskii & Pushkar, 1968) were
dose-dependent. At doses greater than the LD50, signs of poisoning
appeared after 6 - 12 h; at lower levels, the signs were less
obvious and appeared after 1 - 2 days. Most deaths occurred on the
3rd - 9th day after oral administration. The animals lost 7 - 35%
of their initial body weight. During the first 24 h following the
oral dosing of rats with 900 µmol diquat/kg body weight (LD50), a
reduction in water intake was noted (Crabtree et al., 1977). The
animals were subdued, showed pilo-erection and loss of appetite.
At 24 h, they excreted mucoidal, ropy faeces of a characteristic
greenish-yellow or grass-green colour, this colour being due to the
reduction of diquat by intestinal bacterial metabolism. This
colour can be reproduced in vitro with fresh intestinal contents
and actively growing bacterial isolates from them (Clark & Hurst,
1970).
A significant dose-dependent accumulation of water in the lumen
of the intestines and progressive haemoconcentration were reported
(Crabtree et al., 1977) following acute diquat intoxication in
rats. It was concluded that diquat had an adverse effect on water
distribution in the body. Rapid fluid excretion following oral
diquat poisoning suggested a direct action on the stomach and
intestinal mucosa. Monkeys dosed orally with diquat ion at 100,
200, 300, and 400 mg/kg body weight (Cobb & Grimshaw, 1979) vomited
within 2 h and showed diarrhoea within 12 h of dosing. The most
severely affected became lethargic and comatose, and finally
collapsed and died, 12 - 84 h after dosing. An increased number of
polymorphonuclear leukocytes as well as increased levels of serum
urea, plasma glucose, and serum GOT and GPT activities were
determined in monkeys that died during the study. Histological
examination revealed a distended gastrointestinal tract and a
swollen caecum; the mucosa of the stomach was ulcerated and the
small and large intestines congested. Large areas of the stomach
and intestines showed necrosis and exfoliation of the epithelium
from the mucosa. The submucosa was infiltrated with lymphocytes,
and polymorphonuclear and mononuclear cells. These changes were
most severe in the intestinal villi. The death of the monkeys was
due to destruction of the epithelial lining of the gastrointestinal
tract in combination with kidney damage.
Liver
The liver was not severely affected in acute and repeated
diquat poisoning of experimental animals. High doses sometimes
resulted in histological lesions (Verbetskii & Pushkar, 1968;
Bainova, 1975), but signs of toxic hepatitis were not described.
Gage (1968a) reported stimulated NADPH oxidase activity in rat
liver microsomes in vitro after exposure to diquat.
6.1.2. Renal system
The major route of diquat elimination is through the kidneys.
High doses of diquat provoke histological and biochemical changes
in the kidneys, but the most severe damage occured in relation to
renal excretion function (Lock & Ishmael, 1979).
Kidney damage following acute and repeated diquat poisoning was
reported by Verbetskii & Pushkar (1968), Bainova (1969), Cobb &
Grimshaw (1979), Lock (1979), and Lock & Ishmael (1979). Rats,
guinea-pigs, and monkeys were investigated after oral poisoning
with the herbicide. Diquat, orally administered at 680 µmol/kg to
rats, induced a significant increase in diuresis, proteinuria, and
glucosuria after 6-24 h. Biochemical tests in vitro revealed a
decrease in N'-methylnicotinamide, but not 4-aminohippurate,
accumulation by renal cortical slices suggesting competition for
the base transport system. Stimulation of the pentose phosphate
pathway and inhibition of fatty acid synthesis were found when
diquat was added to renal cortical slices in vitro. No such
changes were noted when the renal cortical slices were prepared
from rats previously treated with diquat (Lock, 1979).
Lock (1979) also investigated the changes in several variables
and the clearance of diquat by the rat kidney after oral
administration of toxic doses (680 and 900 µmol/kg body weight).
Diquat was not bound to the proteins of the rat plasma. Active
renal secretion was confirmed by the fact that diquat was cleared
by the kidney at a slightly higher rate than inulin. In rats
treated orally with diquat at 540 µmol/kg body weight, renal
clearance decreased after 24 h. However, the reduction in renal
function induced by diquat (Lock 1979) was considered to be
secondary and due to water redistribution caused by acute
poisoning.
Histopathological changes have been reported in the kidneys of
animals poisoned with high doses of the herbicide (Verbetskii &
Pushkar, 1968; Cobb & Grimshaw, 1979; Lock & Ishmael, 1979). The
renal papillae were hyperaemic, degeneration and necrosis of the
epithelium of the proximal and distal convoluted tubules were
noted, the epithelial cells were exfoliated, and the nuclei
pycnotic.
6.1.3. Eyes and skin
Eye irritation
The local irritation caused by diquat is less pronounced than
that caused by paraquat. One drop of 20% solution gave rise to
slight conjunctival irritation of the rabbit eye, which persisted
for 2 days (Clark & Hurst, 1970). A 40% diquat solution induced
moderate conjunctival irritation.
Eye cataract
Both rats and dogs fed diets containing diquat developed
cataracts (Howe & Wright, 1965). However, rats fed 7.5 mg
diquat/kg diet over a life-span did not develop cataracts, while 70
mg diquat/kg diet appeared to be the no-observed-adverse-effect
level for dogs. According to Clark & Hurst (1970), rats on diets
containing 50 mg diquat/kg or more developed cataracts in the
course of the study. In another group fed a diet containing 1 g
diquat/kg, eye opacities were discovered within 6 months, while a
few animals on diets of 100 mg/kg and 50 mg/kg showed slight
opacities at the end of the study period. A 2-year test with a
diet containing diquat at 10 mg/kg did not induce cataracts in
rats.
Bilateral cataracts were discovered in all dogs 10 - 11 months
following oral administration of diquat at 15 mg/kg body weight per
day. The dose of 5 mg/kg body weight per day induced eye opacities
after 17 months, and doses of 1.7, 0.8, and 0.4 mg/kg body weight
per day were ineffective after 3 - 4 years of treatment.
A 2-year feeding study was carried out with diquat levels of
15, 25, and 75 mg/kg in the diet of rats. Only the 25 and 75 mg/kg
levels caused cataracts (FAO/WHO, 1978).
Pirie & Rees (1970) confirmed that rats fed diquat dibromide at
0.5 - 0.75 g/kg in the diet developed cataracts. In vivo
observations showed that, invariably, the first change seen was an
opacity in the posterior cortex, immediately under the posterior
capsule of the lens. The next stage was a defined nuclear cataract
that could be seen with the naked eye. Finally, shrinkage and
complete opacity occurred. This histological study revealed that
the first posterior cortical opacity was formed from damaged
epithelial cells. The level of diquat in the blood of these rats
was less than 2.2 µM. No diquat accumulation was registered in the
lens of these rats. The mechanism of the specific cataractogenic
action of diquat is not clear, although in vitro studies
demonstrated that reduction of diquat by the lens was enzymatically
catalysed by glutathione reductase (EC 1.6.4.2) with NADPH as the
source of reducing equivalents. The loss of ascorbic acid from the
lens and the ocular fluids of treated rats was proposed as a factor
for maintaining the normal glutathione level in the rat lens.
Local skin effects
Single diquat applications on the skin of mice (Bainova, l969a)
and rabbits (Clark & Hurst, 1970) did not cause any local
irritation. Daily applications of 1% diquat solution in water to
the skin of rats provoked slight erythema at the site of contact
during the first 10 days, while daily applications of diquat at 20
mg/kg body weight to the skin of rabbits caused mild erythema,
thickening of the skin, and some scabbing (Clark & Hurst, 1970).
Diquat has not been found to be a sensitizer (Bainova, (l969a).
6.1.4. Respiratory system
The effect of diquat on the respiratory system has been studied
after parenteral (Hawkins et al., 1979; Lam et al., 1980), oral
(Verbetskii & Pushkar, 1968; Bainova, 1969; Bainova & Vulcheva,
1978), intratracheal (Lam et al., 1980), and inhalation exposure
(Gage, 1968; Bainova et al., 1972). Unlike paraquat, no specific
effects on the lung were reported, though difficulties in breathing
occurred after severe acute poisoning of the animals with diquat.
6.1.5. Nervous system
General depression and lethargy were most commonly seen
following the administration of high doses of diquat to guinea-pigs
and rats (Verbetskii & Pushkar, 1968; Clark & Hurst, 1970; Crabtree
et al., 1977), and to monkeys (Cobb & Grimshaw, 1979).
6.1.6. Effects on reproduction, embryotoxicity, and teratogenicity
6.1.6.1. Effects on reproduction
Male rats were dosed orally with diquat dibromide at 6.5 mg/kg
body weight per day, for 60 days, and the testes were then examined
biochemically and histologically (Bainova & Vulcheva, 1974). There
were no significant changes in the sperm count, sperm motility, the
testicular tubules, the basal cells, or in the activity of several
enzymes.
A 2-generation study on rats was carried out with dietary
levels of 125 and 500 mg diquat/kg. The 500 mg/kg dose resulted in
reduced body weight for F1a, F1b, F2a, and F2b, and increased
cataracts in F1b and F2b after 91-280 days of exposure. The 125
mg/kg dose resulted in decreased body weight in F1b and F2b, but no
lens opacities were noted (FAO/WHO, 1973).
6.1.6.2. Embryotoxicity and teratogenicity
Diquat was reported to have induced deviations in the prenatal
development of rats (Khera et al., 1968). Bus et al. (1975)
studied the fetal toxicity and teratogenicity of diquat in rats by
administering 15 mg/kg body weight iv on days 7-21 of gestation.
This resulted in 57% fetal resorption compared with 7.6% for
paraquat. The incidence of maternal deaths was essentially the
same. When 14C-diquat and 14C-paraquat were administered to rats,
iv, in a dose of 15 mg/kg body weight on days 13, 16, and 21 of
gestation, paraquat increased radioactivity in fetal lung whereas
diquat appeared to have a stronger embryotoxic action than
paraquat. In the review published in 1979 by FAO/WHO, it was
reported that diquat dibromide monohydrate, administered orally to
pregnant rabbits at doses of 1.25, 2.5, and 5.0 mg/kg had no
adverse effect on the fetuses. In groups of pregnant rats kept on
diets containing 125 and 500 mg diquat cation/kg throughout
gestation, reduced body weight was noted only in the fetuses of
mothers from the 500 mg/kg group. A slightly increased incidence
of subcutaneous haemorrhages was also noted.
Teratogenicity studies in mice have been reported by Selypes et
al. (1980). Single ip doses of diquat at 2.7 and 11 mg/kg body
weight were injected on days 9, 10, 11, and 12 of gestation. The
number of dead fetuses, as well as post-implantational lethality,
increased significantly: average embryo weight was lower and,
though no congenital malformations were noted, there were signs of
skeletal retardation such as large fontanelles, wider cerebral
sutures, flat-shaped ventral nuclei of the vertebrae, and delayed
ossification in the sternum and phalanges. The embryotoxic effect
in mice of high doses of diquat was thus confirmed, but no
chromosomal aberrations were noted in the liver cells of the
embryos from diquat-treated female mice.
6.1.7. Mutagenicity
Studies on the genotoxic potential of diquat are rather
contradictory. Diquat was negative in the Ames test, with and
without metabolic activation (Anderson et al., 1972; Benigni et
al., 1979; Levin et al., 1982). Dominant lethal assays in mice
performed by various authors with several doses of the herbicide
gave negative results (Pasi et al., 1974; Pasi & Embree, 1975;
Anderson et al., 1976). Selypes et al. (1980) injected mice ip
with 22 mg/kg (LD50) diquat, while another group of mice was dosed
orally with 90 mg/kg (0.5 LD50). After 24 and 38 h, preparations of
bone marrow were examined for chromosome aberrations; no
statistically significant changes were determined.
On the other hand, diquat was found to induce slight gene
conversion in Saccharomyces cerevisiae (Siebert & Lemperle, 1974).
Ahmed et al. (1977) reported that diquat induced DNA changes in
cultured SV-40-transformed human cells, with and without metabolic
activation, and the induction of 8-azaguanine resistance in the
Salmonella typhimurium assay was positive (Benigni et al., 1979;
Bignami & Crebelli, 1979). Benigni et al. (1979) also found that
diquat was positive in an S. typhimurium repair test. It was
further reported by these authors that diquat induced gene
mutations in Aspergillus nidulans, and increased unscheduled DNA
synthesis in human epithelial-like cells. They commented that
diquat may have an effect on a number of different genetic
endpoints.
6.1.8. Carcinogenicity
In 2-year feeding studies on rats (Clark & Hurst, 1970), diquat
at levels of up to 720 mg/kg diet did not induce tumours. The
daily ingestion of 2 and 4 mg diquat per kg body weight in water
for a period of 2 years did not have any significant effects on the
health and mortality rate in rats (Bainova & Vulcheva, 1978). Some
histological changes related to chronic interstitial infiltration
and pulmonary adenomatosis in the lungs were found, especially
after the higher dose, but there were no indications of malignancy.
6.2. Effects on Farm Animals
The effects of diquat on farm animals was studied in relation
to its application as an aquatic herbicide and desiccant (Howe &
Wright, 1965; Black et al., 1966; Stevens & Walley, 1966) (section
4.4). Little variation in diquat toxicity in the various animal
species was found, but cattle appeared to be the most sensitive
(LD50 for cattle approximately 30 mg/kg, LD50 for rat 230 mg/kg).
Single oral doses up to 8 mg/kg produced no signs of toxicity in
cows (Stevens & Walley, 1966), and the continuous exposure of
animals via the forage to doses ranging from 0.2 to 330 mg/kg in
the diet (Calderbank, 1972) did not induce any clinical or
pathological changes in farm animals.
Calderbank (1972) recommended that domestic animals should not
be allowed to enter fields newly treated with diquat, nor be given
water recently treated with the herbicide. When edible crops are
treated with diquat, as desiccant, at least 4 days should elapse
before the crops are fed to stock, and when diquat is used for
aquatic weed control, at least 7 days should elapse before the
treated water is used for field irrigation. Recommended levels for
weed control must be observed (Calderbank, 1972).
Sheep given doses of 1, 5, 10, and 20 mg diquat/kg per day in
their drinking-water for 1 month and calves similarly exposed to 5
and 20 mg diquat/kg per day did not show any adverse toxicological
effects as evidenced by growth, food consumption, and observation.
6.3. Dose-Effect of Diquat
The acute LD50 values of diquat in various species were
published by Howe & Wright (1965) and Clark & Hurst (1970). The
acute toxicity of diquat salts (Table 5) does not differ
significantly and is similar for both sexes.
Table 6 summarizes the acute oral, dermal, and inhalation LD50
and LC50 values of diquat in various experimental and domestic
animals. There are no marked species differences but cattle,
guinea-pigs, and monkeys appear to be the most sensitive species.
The few cases of acute diquat poisoning in man have not furnished
sufficient data to determine the lethal dose for man.
The dose-effect relationship of repeated diquat exposure, from
various studies, is summarized in Table 7. Rats, guinea-pigs and
dogs were subjected to ora1 and dietary administration of diquat.
Guinea-pigs appeared to be rather sensitive (Makovskii, 1972), but
the herbicide did not induce cumulative toxic effects (Bainova,
1969, 1975; Makovskii, 1972), because of its relatively rapid
elimination from the organism and the absence of deposits in the
tissues.
Table 5. LD50 (mg/kg) of diquat salts in rats
-----------------------------------------------
Diquat Route of entry Sex LD50
(mg/kg)
-----------------------------------------------
Diquat dibromide oral 215b
Diquat dibromide oral 210b
Diquat dibromide subcutaneous F 11a
Diquat dichloride subcutaneous F 10a
Diquat dichloride subcutaneous M 11a
Diquat dibromide subcutaneous 22b
-----------------------------------------------
a From: Clark & Hurst (1970).
b From: Makovskii (1972).
Table 6. Diquat LD50 (mg/kg) and LC50 (mg/m3)
in various species
----------------------------------------------
Species Oral Dermal Inhalationa
(mg/kg) (mg/kg) (mg/m3)
----------------------------------------------
Rat 400b 650f 35f
Rat 281c 83h
Rat 231e
Rat 215f
Rat 130g
Mouse 170b 430d
Mouse 125e
Rabbit 190b
Rabbit 101e > 400e
Guinea-pig 123c 400f 38f
Guinea-pig approx. 100e
Guinea-pig 100f
Hen 400 - 800b
Hen 200 - 400e
Dog > 200b
Dog 100 - 200e
Cow approx. 30f
Cow 30e
Monkey 100 - 300i
----------------------------------------------
a Respirable diquat aerosol.
b From: Howe & Wright (1965).
c From: Verbetskii & Pushkar (1968).
d From: Bainova (1969a).
e From: Clark & Hurst (1970).
f From: Makovskii (1972).
g From: Bainova (1975).
h From: Bainova & Vulcheva (1977).
i From: Cobb & Grimshaw (1979).
Table 7. Effect of repeated oral, dermal, and inhalation exposure to diquat in experimental animals
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Results obtained Reference
---------------------------------------------------------------------------------------------------------
Rat 87.5, 175, and 350 mg 2 years cataract at all dietary levels FAO/WHO (1971)
diquat ion/kg of diet
Rat 7.2, 36, 72, 180, 2 years no deaths; reduced growth in males at FAO/WHO (1971)
360, and 720 mg diquat highest dietary level; cataract at
ion/kg diet dietary levels of 36 mg diquat ion/kg
diet and above; no cataract at 7.2 mg/kg
Rat 15, 25, and 75 mg 2 years no deaths; "no effect" level for FAO/WHO (1978)
diquat ion/kg diet cataractogenesis 15 mg diquat ion/kg
diet
Rat oral - 6.5, 13, and 40 30 days dose-related biochemical and Bainova (1969,
mg/kg body weight per histological changes in kidney, liver, 1975)
day 2.1 and 4.3 mg/kg 4 1/2 months gastrointestinal system, and lung; no
body weight per day haematological changes; increased
G-6-P-isomerase serum activity;
histological changes at 4.3 mg/kg
body weight per day
Rat oral - 0.2, 2.1, and 1 year the higher doses were toxic for the Makovskii (1972)
5.3 mg/kg body weight 2 species; no-observed-effect levels
per day
Guinea- day 0.1, 1.0, and 2.5 0.2 and 0.1 mg/kg body weight per day
pig mg/kg body weight per for rat and guinea-pig
day
Dog 10, 20, 50, 140, and up to 4 no cataracts at dietary levels up to and FAO/WHO (1971)
420 mg diquat ion/kg years including 50 mg/kg; cataract at 2 higher
of diet dietary levels; no mortality; no effects
on growth
Rat oral - 2 and 4 mg/kg 1 and 2 no increase in mortality rates; Bainova &
per day years histological changes in lungs after Vulcheva (1978)
treatment with 4 mg/kg per day in
drinking-water; minimal effective dose
2 mg/kg per day
---------------------------------------------------------------------------------------------------------
Table 7. (contd.)
---------------------------------------------------------------------------------------------------------
Species Dosage Duration Results obtained Reference
---------------------------------------------------------------------------------------------------------
rat dermal - 5, 10, 20 20 days slight skin irrritation, death and toxic Bainova (1969a)
60, and 120 mg/kg effects at 10 - 120 mg/kg per day;
per day dilation of the gastrointestinal system
at toxic levels; histological changes in
kidney, gastrointestinal system, liver,
and lung at toxic levels, LD50 35 mg/kg
per day without occlusion; no-observed-
effect dose 5 mg/kg per day
Rabbit dermal - 20 and 40 20 days mild skin irritation; toxic effects at Clark & Hurst
at 40 mg/kg per day; no clinical signs (1970)
of toxicity at 20 mg/kg per day; LD50
between 20 and 40 mg/kg per day
Rat inhalationa - 0.50, 15 days clinical signs of irritation and Gage (1968)
1.60, and 2.0 mg/m3 histological changes in lungs at 2mg/m3;
6 h daily no clinical haematological, and
histological deviations at 0.50 mg/m3;
minimum effective concentration 1.0
mg/m3 diquat aerosol
Rat inhalationa - 0.32 4 1/2 months biochemical and histological changes in Bainova (1972)
and 1.90 mg/m3, lungs at 1.90 mg/m3; minimal effective
6 h daily concentration 0.32 mg/m3 diquat aerosol
Rat inhalationa - 0.4, 4 months clinical signs of irritation and toxic Makovskii
0.7, and 1.9 mg/m3, effects at 1.9 mg/m3; 0.7 mg/m3 produced (1972)
4 h daily changes in some rats; minimal effective
concentration 0.4 mg/m3 diquat aerosol
---------------------------------------------------------------------------------------------------------
a Respirable diquat aerosol.
7. EFFECTS ON MAN
7.1. Case Reports
Several cases of acute diquat poisoning among the general
population have been reported in the literature. Fitzgerald et al.
(1978) found 5 cases from 1967 to 1977 in Ireland. Vanholder et
al., (1981) summarized the clinical outcome and the treatment of 11
patients with diquat poisoning (6 fatal and 5 non-fatal).
(a) Suicidal diquat poisoning
Schönborn et al. (1971) reported the fatal case of a man who
drank 2 - 3 mouthfuls of Reglone(R) (estimated 15 - 22 g diquat)
with the intention of committing suicide. Severe vomiting occurred
after 2 h and, 2 h later, watery diarrhoea, the stools having a
peculiar yellow-greenish colour. During the next 6 h, the patient
lost about 3.5 litres of liquid through faeces and 4 litres of
liquid through vomiting. The urine was very concentrated, the
haematocrit was 55%. Serum enzyme activity showed toxic liver
damage, and proteinuria and metabolic acidosis were registered. On
the 2nd day, there were ulcers and severe oropharyngeal
inflammation, on the 3rd day, increasing restlessness, optical
hallucinations, and delirium and stridulous breathing developed.
During the 4th-6th days, anuria, raised body temperature,
generalized convulsions, and coma were registered, and the patient
died on the 7th day of cardiac insufficiency and thrombocytopenia.
The autopsy revealed extensive necrosis of the pharynx and
oesophagus, and petechial bleeding and erosions in the
gastrointestinal tract; pulmonary oedema with haemorrhages, hyaline
membrane production, and bronchopneumonic foci were noted in the
lungs; fatty degeneration was found in the liver and heart, and
severe degeneration of the tubulus epithelium with necrosis in the
kidneys, while the signs of circulatory failure with oedema and
haemorrhagic diapedesis of the brain explained the central nervous
system effects. The diquat concentrations measured on the 1st day
after ingestion were 1.85 mg/litre in the urine and 0.47 mg/litre
in the blood. Higher diquat levels were determined post mortem in
the kidneys, spleen, and lungs (1.19, 1.04, and 0.56 mg/kg,
respectively).
In a second case of suicide, the subject had taken unknown
quantities of Reglone(R) during a period of 3 days (Okonek &
Hofmann, 1975). One day after the second ingestion, she was
admitted to hospital - shocked, sleepy, anuric, with haemorrhagic
mucosal necrosis in the mouth, throat, and eosophagus. Four h
after admission to hospital, the diquat serum level was 1.038
mg/litre. This decreased to 0.30 mg/litre following dialysis.
Death from cardiovascular collapse ensued 46 h after admission.
Vanholder et al. (1981) concluded, from their review of 11
cases, that the lethal dose of Reglone(R) is 30 - 60 ml or
approximately 6 - 12 g diquat dibromide.
An unusual case of diquat poisoning was described by Narita et
al. (1978). A clerk, after drinking heavily, swallowed about 200
ml 30% diquat dibromide formulation. Vomiting was accompanied by
great thirst, severe irritation of the mouth, diarrhoea, and a
temperature of 39 °C. After 24 h, the patient became anuric and
developed acute renal failure; he was comatose and inarticulate,
and had meiosis and unclear light reflexes. He died from dyspnoea
38 1/2 h after ingestion of diquat. Autopsy revealed renal failure
with tubular necrosis, lung haemorrhages, haemorrhagic ulcers, and
erosions in the stomach, and severe congestion of the lungs,
kidneys, liver, gastrointestinal system, and adrenal glands. High
diquat residues were determined in the kidneys, liver, lungs, and
intestines. Vanholder et al. (1981) reported 2 cases of Reglone(R)
ingestion (50 ml and 20 ml) in suicide attempts. Because of
vomiting and diarrhoea, they were admitted to local hospitals, but
no specific treatment was given and the patients were released in
satisfactory clinical condition. However, because of the
development of progressive oliguria several h later, the patients
returned to the hospital. The diquat serum levels were found to be
4.5 and 0.4 mg/litre, respectively. The patients died 1 and 5 days
after the ingestion of diquat.
(b) Accidental diquat poisoning
Oreopoulos & McEvoy (1969) described a patient who accidentally
took a mouthful of Reglone(R) from a soft drink bottle. He spat
out part of it. After 8-10 h, he had diarrhoea and 2 ulcers in the
mouth, but there was no clinical evidence of respiratory, renal, or
central nervous system effects on examination in hospital, and all
laboratory and biochemical examinations were within the normal
physiological limits. The patient continued to excrete diquat in
the urine for 11 days after ingestion. He underwent forced
diuresis and left the hospital in good condition.
Another case of acute poisoning following the accidental
ingestion of less than a mouthful of diquat was reported by Fel et
al. (1976). Nausea, vomiting, and diarrhoea were the first
effects. The patient then developed uraemia, oliguria, and anuria
despite forced diuresis for 2 - 3 days after the accident.
Haemodialysis proved more successful. Bilateral pneumonia was
noted during the 2nd week, but was cured with antibiotics, and the
patient was discharged on the 26th day in good health.
7.2. Effects on Agricultural Operators
A few studies have been performed on workers spraying diquat.
Air concentrations of diquat aerosol were measured by Makovski
(1972) (Table 3). The dermal exposure of the spraymen ranged from
0.05 mg to 0.08 mg on the face and hands after 2 - 3 h of daily
work. The spraymen did not have any complaints, and the clinical
and laboratory examinations did not reveal any significant
differences in comparison with control groups. Wojeck et al.
(1983) studied the exposure of workers applying 1.76% diquat by
hand-operated spray against water hyacinths or using direct
injection of 4.41% spray mixture into the water for hydrilla
control. The spray crews applied diquat 2 - 5 h daily for 4 days
weekly. The inhalatory exposure was found to be < 0.01 mg/h. The
dermal exposure of the spraymen and the airboat drivers were
estimated to be 1.82 and 0.20 mg diquat/h, during the treatment of
water hyacinths. The dermal exposures of the spraymen and the
mixer of diquat for the treatment of water hydrilla were 0.17 and
0.47 mg/h, respectively. The results of urine analysis of all
workers involved in the study were negative (< 0.047 mg/litre).
The dermal exposure to diquat was closely related to the
concentrations used in the working solutions.
Inflammation and bleeding of the nasal mucosa were observed in
people handling crystalline diquat powder in the laboratory or
under field conditions (Clark & Hurst, 1970). Epistaxis during
agricultural diquat application is related to the inhalation of
droplets or splashes from the careless mixing of liquid
concentrates. A worker who spent some considerable time in an
aerosol spray drift developed irritation of the upper respiratory
tract.
According to Clark & Hurst (1970), if a 20% diquat solution
comes into contact with the nail base, nail growth disturbances may
result, and discoloured spots, white bands, and shedding of the
nail were seen after prolonged contact with concentrated diquat.
The nail re-grew normally once exposure was discontinued. No
adverse effects on the nails were observed following the use of
diluted diquat spray solutions in agriculture. Concentrated diquat
formulations have also been reported to delay the healing of
superficial cuts on the hands of spray workers.
Cataracts have never been observed in man following exposure to
diquat (FAO/WHO, 1978; Hayes, 1982).
7.3. First Aid and Medical Treatment
These are essentially the same as those given for paraquat
(section 8.4). See also WHO/FAO (1979).
8. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE ENVIRONMENT
8.1. Exposure
8.1.1. Relative contributions of soil, water, air, and food sources
to total diquat uptake
Introduction
Diquat is a contact herbicide and dessicant that is used to
destroy weeds in various agricultural situations. It is used in
the form of an aqueous spray, which means that the potential
exposure of man may occur as a result of its presence in air, on
plants, in soil, or in water.
Degradation of Diquat
Photochemical degradation takes place, when diquat treated
plants are exposed to normal daylight, and continues after plants
are dead. The products formed are of lower toxicity than diquat.
The rapidity of photochemical degradation on plant and soil
surfaces minimizes the hazard of diquat for the environment.
Soil
Diquat is rapidly and tightly bound to clay particles in the
soil, and is thereafter inert. In normal agricultural use, no
toxic breakdown products are to be expected in the soil (section
4.2) where diquat is less persistent than paraquat. Total diquat
residues in the soil after repeated spraying ranged from 0.2 to 3.9
mg/kg. On the 15th day after a single application of diquat,
residues were less than 0.1 mg/kg in field studies. Even at high
rates of application, no specific adverse effects are found on soil
microorganisms, fungi, or invertebrates, and no phytotoxic effects
have been reported on crops.
Water
Following its use as an aquatic herbicide at normal application
rates, diquat residues in water have been found to decrease rapidly
to essentially undetectable levels within 7-14 days (section 4.3).
Toxic effects on fish and other living organisms in the water are
unlikely, because diquat is rapidly photodegraded, absorbed by
aquatic weeds, or adsorbed to soil particles at the bottom.
However, caution should be taken in the application of diquat to
water containing heavy weed growth, since oxygen consumed by
subsequent weed decay may decrease the oxygen content of the water
to such an extent that it is dangerous for fish or other aquatic
organisms. No phytotoxic damage should occur on crops irrigated
with diquat-treated water, if at least 10 days is allowed to elapse
between treatment and irrigation.
Air
Diquat is not volatile. Inhalation exposure can occur via
spray aerosols or contaminated dust but, if correctly applied,
diquat should not give rise to significant inhalation exposure of
the sprayers (section 4.5). Total airborne aerosol concentrations
of diquat in the air in working areas ranged from 0.06 to 0.56
mg/m3, depending on the method of application and the period of
time after the spraying.
Food
Extensive studies on forage desiccated with diquat have
demonstrated that the residues are very low within some days of the
application of the desiccant. Diquat residues in the treated
herbage following pre-harvest desiccation ranged from 0.02 to 25
mg/kg at different intervals after spraying. Trials in which such
forage was fed to cattle and sheep have demonstrated insignificant
residue levels in the milk, meat, and internal organs (section
4.4). Residues found in vegetables, fruits, and cereals have been
low. There is no bioaccumulation.
8.1.2. General population exposure
Inhalation exposure of the general population to diquat may
occur from spray drift off the treated fields, but this is thought
to be insignificant. There are no published data on total diquat
intake among the general population but this again is expected to
be insignificant on the basis of known residue levels. Studies on
its environmental distribution point to a low environmental hazard.
Due to diquat's rapid and complete binding to clay minerals in
soil, contamination of water supplies either from field runoff or
percolation through soil to the water table is not expected
(section 4.2).
Few cases of diquat poisoning have been reported (section 7.1).
Most cases are due to the intentional ingestion of concentrated
formulations, but accidental ingestion has occurred. The decanting
of liquid concentrate formulations into beer, wine, or soft drink
bottles, and subsequent inappropriate storage, is very dangerous.
The acute lethal dose of diquat dibromide is considered to be
6 - 12 g for man. Recovery from diquat poisoning depends on the
cause of ingestion, the dose absorbed, the renal damage, and prompt
initiation of therapy. No long-term adverse effects have been
reported in those who have survived acute diquat poisoning.
8.1.3. Occupational exposure
There may be inhalation, dermal, and to some extent oral
occupational exposure. Spray aerosols and dust particles settle in
the upper respiratory tract. Diquat aerosol concentrations range
from 0.06 to 0.56 mg/m3, according to the spraying method. At a
distance of 200 - 400 m from the treated field, they decrease to
0.09 mg/m3 and less than 0.01 mg/m3. Inhalation exposure was found
to be very low in comparison with dermal (0.17 - 1.82 mg/h)
exposure to diquat during application for aquatic weed control.
Skin irritation, epistaxis, nail damage, and delayed wound healing
have been reported. However, no data on severe or fatal cases of
occupational intoxication, acute ocular damage, or occupational
contact dermatitis caused by diquat were found in the literature.
8.2. Effects
8.2.1. Diquat toxicity in animals
Diquat is less toxic than paraquat and does not cause the
specific lung disease so typical of paraquat exposure.
The primary toxic effect of diquat in animals is
gastrointestinal damage resulting in diarrhoea with consequent
dehydration. After high doses of diquat, minor toxic effects have
been noted in the liver, kidney, and the nervous and endocrine
systems. High concentrations of diquat are irritating to the skin,
although less so than paraquat. Development of eye cataracts has
been reported in rats and dogs following long-term treatment with
diquat (section 6.1.3). This observation has not been reported in
man. Diquat is embryotoxic but it has not been found to be
teratogenic in rats and mice or carcinogenic in long-term feeding
studies on rats given diquat at levels up to 720 mg/kg diet
(sections 6.1.7 and 6.1.8). In vitro mutagenicity studies have
been inconclusive, although generally suggesting weak activity,
while the results of in vivo studies have been negative (section
6.1.8). Thus, the results of animal studies suggest that low-level
exposure to diquat is unlikely to induce toxic effects in man. The
no-observed-effect level in rats has been estimated to be 0.75 mg
diquat ion/kg body weight per day (FAO/WHO, 1978).
8.3. Earlier Evaluations of Diquat by International Bodies
The Joint Meeting on Pesticide Residues (JMPR) reviewed and
published residue and toxicity data on diquat in 1970, 1972, 1976,
1977, 1978 (FAO/WHO 1971, 1973, 1977a,b, 1978, 1979). In 1977, it
estimated the acceptable daily intake (ADI) for man as 0 - 0.008
mg/kg body weight expressed as diquat ion (FAO/WHO 1978).
The same JMPRs have recommended maximum residue levels
(tolerances) for diquat in food commodities of plant and animal
origin.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC are available
from the IRPTC (International Register for Potentially Toxic
Chemicals) legal file (IRPTC 1983).
A data sheet on diquat has been prepared by WHO/FAO (1979) in a
series of "Data sheets on chemical pesticides". Based on a brief
review of use, exposure, and toxicity, practical advice is given on
labelling, safe-handling, transport, storage, disposal,
decontamination, selection, training and medical supervision of
workers, first aid, and medical treatment.
8.4. Conclusions
On the basis of the above findings, it can be concluded that:
General population
Residue levels of diquat in food and drinking-water, resulting
from its normal use, are unlikely to result in a health hazard for
the general population.
Diquat has caused some fatalities following suicidal ingestion.
Occasional accidental fatalities have followed ingestion of
decanted diquat. Ill-effects similar to those caused by paraquat
occur, but the characteristic fibrosis of the lungs is not a
feature.
Occupational exposure
With reasonable work practices including safety precautions,
hygiene measures, and proper supervision, occupational exposure
during the manufacture, formulation, and application of diquat will
not cause a hazard. However, the undiluted concentrate must be
handled with great care, because contamination of eyes and skin
(with possible consequent dermal absorption) can result from
improper work practices.
Environment
Diquat in soil binds rapidly and tightly to clay particles and
residual phytotoxicity from freely available diquat is unlikely.
Under normal conditions of use, the toxicity of diquat for aquatic
organisms is low, though resulting depletion of water oxygen due to
weed decay may pose a problem. Diquat does not seem to represent
an environmental hazard.
REFERENCES
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide-
induced DNA damage and its repair in cultured human cells.
Mutat. Res., 42: 161-164.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDERSON, D., MCGREGOR, D.B., & PURCHASE, I.F.H. (1976)
Dominant lethal studies with diquat and paraquat in male CD-1
mice. Mutat. Res., 40: 349-358.
ANDERSSON, A. & JOSEFSSON, E. (1982) Residues of diquat in
potatoes. Var Föda, 34(Suppl. 3): 223-225.
ATKINSON, G. (1973) Effects of diquat on the microbiological
organisms in the soil of lakes. In: Proceedings of the
Pollution Residue Conference, Wairakei, New Zealand,
pp. 529-538.
BAINOVA, A. (1969a) [Chronic oral toxicity of bipyridylium
herbicides.] Hig. Zdrav., 12: 325-332 (in Bulgarian).
BAINOVA, A. (l969b) [Experimental assessment of the effect
of dipyridylium herbicides on the skin.] Letopisi HEI, 9:
25-30 (in Bulgarian).
BAINOVA, A. (1975) [Cumulative action of Gramoxone and
Reglone.] In: Problemi na higienata, Sofia, Medizina i
Fizkultura, Vol. 1, pp. 31-38 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1974) [Experimental assessment
of the effect of dipyridyliums on sex glands.] In: Works of
the Research Institute of Hygiene and Laboratory Protection,
Sofia, Medizina i Fizkultura, Vol. 22, pp. 111-122 (in
Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1977) [Experimental
substantiation of Reglone MAC in the working environment.] In:
Problemi na higienata, Sofia, Medizina i Fizkultura, Vol. III,
pp. 11-17 (in Bulgarian).
BAINOVA, A. & VULCHEVA, V. (1978) Chronic action of diquat
on the lungs. C. R. Acad. Bulgar Sci., 31: 1369-1372.
BAINOVA A., ZLATEVA, M., & VULCHEVA, V. (1972) [Chronic
inhalation toxicity of bipyridylium herbicides.] Hig. Zdrav.,
15: 25-31 (in Bulgarian).
BENECKE, G. (1977) [Automatic evaluation of an algal
bioassay-inhibition of the movement of a blue-green alga
(Phormidium sp.) by the herbicide diquat.] Z. Wasser-
Abwasserforsch., 10: 195-197 (in German).
BENIGNI, R., BIGNAMI, M., CARERE, A., CONTI, G., CONTI, L.,
CREBELLI, R., DOGLIOTTI, E., GUALANDI, G., NOVELLETTO, A., &
ORTALI, V.A. (1979) Mutational studies with diquat and
paraquat in vitro. Mutat. Res., 68: 183-193.
BIGNAMI, M. & CREBELLI, R. (1979) A simplified method for
the induction of S-azaguanine resistance in S. typhimurium.
Toxicol. Lett., 3: 169-175.
BLACK, W.J.M., CALDERBANK, A., DOUGLAS, G., & McKENNA, R.H.
(1966) Residues in herbage and silage and feeding experiments
following the use of diquat as a desiccant. J. Sci. Food
Agric., 17: 506-509.
BUS, J.S., PREACHE, M.M., CAGEN, S.Z., POSNER, H.S., ELIASON,
B.C., SHARP C.W., & GIBSON, J.E. (1975) Fetal toxicity and
distribution of paraquat and diquat in mice and rats. Toxicol.
appl. Pharmacol., 33: 450-460.
CALDERBANK, A. (1972) Experimental considerations in the
development of diquat and paraquat as aquatic herbicides.
Outlook Agric., 7: 51-54.
CALDERBANK, A. & SLADE, P. (1976) Diquat and paraquat. In:
Kearne, P.C. & Kaufman, D. D. ed. Herbicide chemistry,
degradation and mode of action, 2nd ed., New York, Dekker,
pp. 501-540.
CALDERBANK, A. & YUEN, H. (1966) An improved method for
determining residues of diquat. Analyst, 91: 625-629.
CAVELL, B.D. (1979) Methods used in the study of the
photochemical degradation of pesticides. Pestic. Sci., 10:
177-180.
CHARLES, J.M. & MENZEL, D.B. (1979) Influence of atmospheric
particles on pulmonary absorption phenomenon. ACS Symp. Ser.,
174 (CH 15): 287-301.
CHARLES, J.M., ABOU-DONIA, M.B., & MENZEL, D.B. (1978)
Absorption of paraquat and diquat from the airways of perfused
rat lung. Toxicology, 8: 59-67.
CLARK, D.G. & HURST, E.W. (1970) The toxicity of diquat.
Br. J. ind. Med., 27: 51-55.
COATS, G.E., FUNDERBURK, H.H., LAWRENCE, J.M., & DAVIS, D.E.
(1966) Factors affecting persistence and inactivation of
diquat and paraquat. Weed Res., 6: 58-66.
COBB, L.M. & GRIMSHAW, P. (1979) Acute toxicity of oral
diquat (1,1'-ethylene-2'2'-bipyridylium) in Cynomolgus
monkeys. Toxicol. appl. Pharmacol., 51: 277-282.
CONNING, D.M., FLETCHER, K., & SWAN, A.A.B. (1969) Paraquat
and related bipyridyls. Br. med. Bull., 25: 245-249.
CRABTREE, H.C., LOCK, E.A., & ROSE, M.S. (1977) Effects of
diquat on the gastrointestinal tract of rats. Toxicol. appl.
Pharmacol., 41: 585-595.
DANIEL, J.W. & GAGE, J.C. (1966) Absorption and excretion of
diquat and paraquat in rats. Br. J. ind. Med., 23: 133-136.
DEMBINSKI, F., PONIKIEWSKA, T., & TRZEBNY, W. (1971) [Ground
seed of sunflower desiccated with Reglone as fodder for
ruminants.] Pamiet. Pulawski Pr., 49: 205-211 (in Polish).
DICKES, G.J. (1979) The application of gas chromatography to
food analysis. Talanta, 26: 1065-1099.
FAO/WHO (1971) Diquat. In: 1970 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1973) Diquat. In: 1972 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977a) Diquat. In: 1976 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1977b) Pesticide residues in food, Rome, Food and
Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, 10).
FAO/WHO (1978) Diquat. In: 1977 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1979) Diquat. In: 1978 Evaluations of some
pesticide residues in food, Rome, Food and Agriculture
Organization of the United Nations.
FAO (1979) Diquat, Rome, Food and Agriculture Organization
of the United Nations, pp. 101-115 (FAO Plant Production and
Protection Paper No. 15, Supplement).
FEL, P., ZALA, I., IZULE, E., & VARGA, L. (1976)
[Haemodialysis in diquat poisoning.] Orv. Metilap, 117:
1773-1774 (in Hungarian).
FELDMAN, K.J. & MAIBACH, H.I. (1974) Percutaneous
penetration of some pesticides and herbicides in man. Toxicol.
appl. Pharmacol., 28: 126-132.
FITZGERALD, G.R., BARNIVILLE, G., FLANAGAN, M., SILKE, B.,
CARMODY, M., & O'DWYER, W.F. (1978) The changing pattern of
paraquat poisoning: an epidemiologic study. J. Irish Med.
Assoc., 71: 103-108.
FLETCHER, K., ed. (1975) Clinical aspects of paraquat
poisoning, London, ICI Ltd, pp. 1-89
GAGE, J.C. (1968a) Toxicity of paraquat and diquat aerosols
generated by a size-selective cyclone: effect of particle size
distribution. Br. J. ind. Med., 25: 304-314.
GAGE, J.C. (1968b) The action of paraquat and diquat on the
respiration of the liver cell fractions. Biochem. J., 1O9:
757-761.
GOWMAN, M.E., REILY, D., & NEWBY, S.E. (1980) Paraquat and
Diquat: Longterm high rate trial, Frensham, United Kingdom. 2.
Persistence and movement in soil and glasshouse bioassay,
London, ICI Ltd (Report RJ0014B).
GROVER, R., SMITH, A.E., & KORVEN, H.C. (1980) A comparison
of chemical and cultural control of weeds in irrigation
ditchbanks. Can. J. Plant Sci., 60: 185-195.
GRZENDA, A.R., NICHOLSON, H.P., & COX, W.S. (1966)
Persistence of four herbicides in pond water. J. Am. Water
Works Assoc., 3: 326-332.
HAWKINS, S.F., MEDINA, M.A., & STAVINOHA, W. (1979) The
acute in vivo effect of paraquat and diquat on intermediary
metabolism in mouse lung. Proc. Fed. Am. Soc. Exp. Biol.,
38(3, Pt I): 582.
HAYES, W.J., Jr (1982) Pesticides studies in man, Baltimore,
London, Williams & Wilkins, 561 pp.
HOWE, D.J.T. & WRIGHT, N. (1965) The toxicity of paraquat
and diquat. In: Proceedings of the 18th New Zealand Weed &
Pest Control Conference, pp. 105-114.
HUGHES, R.D., MILLBURN, P., & WILLIAMS, R.T. (1973) Biliary
excretion of some diquaternary ammonium cations in the rat,
guinea-pig and rabbit. Biochem. J., 136: 979-984.
ICI (1972a) Determination of residues of diquat in soil -
residue analytical method No. 6, London, ICI Ltd (Report No.
PPRAM-6).
ICI (l972b) Determination of residues of diquat in milk and
water - residue analytical method No.7, London, ICI Ltd
(Report No. PPRAM-7).
ICI (l972c) Determination of residues of diquat in crops and
animal tissues - residue analytical method No. 5, London, ICI
Ltd (Report No. PPRAM-5).
IRPTC (1983) IRPTC Legal files 1983 Vol. I & II, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme.
KHERA, K.S., WHITTA, L.L., & CLEGG, D.J. (1968) Embryopathic
effects of diquat and paraquat in rats. Ind. Med. Surg., 37:
257-261.
KING, R.R. (1978) Gas-chromatographic determination of
diquat residues in potato tubers. J. agric. food Chem., 26:
1460-1463.
KURISAKI, E. & SATO, H. (1979) [Toxicological studies on
herbicides: intracorporal distribution of paraquat dichloride
and diquat dibromide in rat.] Nippon Hoigaku Zasshi, 33: 656
(in Japanese).
LAM, H.F., TAKAZAWA, J., GUPTA, B.N., & STEE, E.W. VAN
(1980) A comparison of the effects of paraquat and diquat on
lung compliance, lung volume and single-breath diffusing
capacity in the rat. Toxicology, 18: 111-123.
LAVAUR, E., SION, G., GROLLEAU, G., & CARPENTER-LESECK, J.
(1979) Etude comparée de l'action du diquat et du paraquat
sur la muqueuse digestive de la souris, du rat et du lapin.
Ann. Zool. Ecol. anim., 11: 159-169.
LEARY, J.B. (1978) Diquat and paraquat. In: Zweig, G. &
Sharma, J., ed. Analytical methods for pesticides and plant
growth regulators, New York, Academic Press, pp. 321-325.
LEVIN, D.E., HOLLSTEIN, M., CHRISTMAN, M.F., SCHWIERS, E.A., &
AMES, B.N. (1982) A new Salmonella tester strain (TA 102)
with AT base pairs at the site of mutation detects oxidative
mutagens. Proc. Natl Acad. Sci. US, 79: 7445-7449.
LITCHFIELD, N.H., DANIEL, J.W., & LONGSHAW, S. (1973) The
tissue distribution of the bipyridylium herbicides diquat and
paraquat in rats and mice. Toxicology, 1: 155-165.
LOCK, E.A. (1979) The effect of paraquat and diquat on renal
function in the rat. Toxicol. appl. Pharmacol., 48: 327-336.
LOCK, E.A. & ISHMAEL, J. (1979) The acute toxic effects of
paraquat and diquat on the rat kidney. Toxicol. appl.
Pharmacol., 50: 67-76.
MAKOVSKII, V.N. (1972) [Toxicological and hygiene studies of
the bipyridylium herbicides diquat and paraquat,] Referat,
Vinniza, USSR, pp. 1-23 (PhD thesis) (in Russian).
MATSUURA, N., TAKINAMI, M., KURISAKI, E., & SATOO, H. (1978)
[Distribution of paraquat dichloride and diquat dibromide in
the living body.] Fukushima Igakkai Zasshi, 28: 212 (in
Japanese).
NARITA, S., MATOJUKU, M., SATO, J., & MORI, H. (1978)
[Autopsy in acute suicidal poisoning with diquat dibromide.]
Nippon Igakkai Zasshi, 27: 454-455 (in Japanese).
O'BRIEN, M.C. & PRENDEVILLE, G.N. (1978) A rapid sensitive
bioassay for determination of paraquat and diquat in water.
Weed Res., 18: 301-303.
OKONEK, S. & HOFMANN, A. (1975) On the question of
extracorporeal haemodialysis for diquat intoxication. Arch.
Toxicol., 33: 251-257.
OREOPOULOS, D.G. & MCEVOY, J. (1969) Diquat poisoning.
Postgrad. med. J., 45: 635-637.
PASI, A. & EMBREE, J.W. (1975) Further comments on the
assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 31: 125-126.
PASI, A., EMBREE, J.W., EISENLORD, G.A., & HINE, C.H. (1974)
Assessment of the mutagenic properties of diquat and paraquat
in the murine dominant lethal test. Mutat. Res., 26: 171-175.
PIRIE, A. & REES, J.R. (1970) Diquat cataract in the rat.
Exp. Eye Res., 9: 198-203.
PYL, W. & GIEBELMANN, R. (1978) [Detection of chlorocholine
chloride, diquat and paraquat.] Arch. vet. Med., 32: 601-602
(in German).
REISH, D., ROSSI, S.S., MEARNS, A.J., OSHIDA, P.S., & WILKES,
F.G. (1979) Marine and estuarine pollution. J. Water Pollut.
Control Fed., 51: 1477-1517.
RILEY, D. (1981) The fate and effect and diquat residues in
soil. In: Proceedings of the National Spray Seed Conference
1981, Albury, New South Wales, Australia.
RILEY, D. & GRATTON, R.P. (1974) Unavailability to plants of
diquat residues in soils. 10th Tr. Muzhdunar. Kongr.
Pochvoved., USSR, 3: 193-203.
ROSE, M.S. & SMITH, L.L. (1977a) The relevance of paraquat
accumulation by tissues. In: Autor, P.A., ed. Biochemical
mechanisms of paraquat toxicity, New York, Academic Press,
pp. 71-91.
ROSE, M.S. & SMITH, L.L. (l977b) Tissue uptake of paraquat
and diquat. Gen. Pharmacol., 8: 173-176.
SCHOENBORN, H., SCHUSTER, H.P., & KOESSLING, F.K. (1971)
[Clinical and muphological findings in an acute oral
intoxication with diquat Reglon.] Arch. Toxicol., 27: 204-216
(in German).
SCHULTZ, VON O., KIRCHNER, K., MUELLER, P., & ROTHE, R.
(1976) [Reglone (Diquat) cause of intoxication of sheep,
cattle, and swine.] Monatsh. Veterinärmed., 31: 647-649 (in
German).
SELYPES, A., NAGYMAJTENYI, L., & BERENCSI, G. (1980)
Mutagenic and embryotoxic effects of paraquat and diquat.
Bull. environ. Contam. Toxicol., 25: 513-517.
SHARP, C.W., OTTLENGHI, A., & POSNER, H.S. (1972)
Correlation of paraquat toxicity with tissue concentrations
and weight loss of the rat. Toxicol. appl. Pharmacol., 22:
241-251.
SIEBERT, D. & LEMPERLE, E. (1974) Genetic effects of
herbicides: induction of mitotic gene conversation in
Saccharomyces cervisiae. Mutat. Res., 22: 1ll-120.
SINGH, S.P. & YADAV, N.K. (1978) Toxicity of some herbicides
to maior carp fingerlings. Indian J. Ecol., 5: 141-147.
SMITH, S.N., LYON, A.J., & SAHID, I.B. (1976) The breakdown
of paraquat and diquat by soil fungi. New Phytol., 77: 735-740.
SMITH, T.P., NOACK, A., & COSH, S.M. (1981) The effect of
some herbicides on vesicular-arbuscular endophyte abundance in
the soil and on infection of host roots. Pestic. Sci., 12:
91-97.
STEVENS, M.A. & WALLEY, J.K. (1966) Tissue and milk residues
arising from the ingestion of single doses of diquat and
paraquat by cattle. J. Sci. Food Agric., 17: 472-475.
SUMMERS, L.A. (1980) The bipyridilium herbicides, London,
New York, Toronto, San Francisco, Academic Press, pp. 1-449.
TSCHIPILSKA, L.N. (1980) [The influence of some pesticides
on the soil microorganisms in connection with the hygiene
evaluation of the soil,] Referat, Sofia, Bulgaria, pp. 1-39
(PhD thesis) (in Bulgarian).
TOMPSETT, S.L. (1970) Paraquat poisoning. Acta pharmacol.
toxicol., 28: 346-358.
TUCKER, V.B., PACK, D.E., & OSPENSON, J.N. (1967) Adsorption
of bipyridylium herbicides in soil. J. agric. food Chem., 15:
1005-1008.
UKAI, S., HIROSE, K., & KAWASE, S. (1977) [Forensic chemical
studies on drugs and chemicals. III. Gas chromatography of
reduction products of the herbicides diquat and paraquat.]
Eisei Kagaku, 23: 32-38 (in Japanese).
VANHOLDER, R., COLARDYN, F., RENCK, DE J., PRAET, M., LAMEIRE,
N., & RINGOIR, S. (1981) Diquat intoxication. Report of two
cases and review of the literature. Am. J. Med., 70: 1267-1271.
VERBETSKII, V.E. & PUSHKAR, M.S. (1968) [Pathological
changes in the organs of animals on acute poisoning with the
herbicide diquat (Reglone).] In: Some questions concerning
human and animal morphology, Medizina, Odessa, pp. 51-52 (in
Russian).
WEBER, J.B., PERRY, P.W., & UPCHURCH, R.P. (1965) The
influence of temperature and time on the adsorption of
paraquat, diquat, 2,4-D and prometone by clays, charcoal and
anion exchange resin. In: Soil Science Society Proceedings,
pp. 678-688.
WHO/FAO (1979) Data sheets on pesticides: Diquat, Geneva,
World Health Organization, pp. 1-7 (Unpublished Report No.
VBC/DS/79-40).
WILKINSON, W. (1980) Paraquat and Diquat: Longterm high rate
trial, Frensham, United Kingdom. 1. Management of site,
effects on crops and weeds and residues in crops, London, ICI
Ltd (Report No. RJ0013B).
WILLIAMS, C.B., LEVISON, S.A., & DANDLIKER, W.B. (1976)
Application of immunological techniques to the detection of
organic contaminants of environmental concern. In: Proceedings
of the University of Montana Annual Conference on Trace
Substances & Environmental Health, No. 10, pp. 317-322.
WOJECK, G.A., PRICE, J.F., NIGG, H.N., & STAMPER, J.H.
(1983) Worker exposure to paraquat and diquat. Arch. environ.
Contam. Toxicol., 12: 65-70.
