
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 190
XYLENES
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr P. Lundberg (National Institute of Working
life, Solna, Sweden), Mr P.D. Howe and Dr S. Dobson (Institute of
Terrestrial Ecology, Monk's Wood, United Kingdom) and Mr M.J. Crookes
(Building Research Establishment, Watford, United Kingdom).
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1997
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme (UNEP), the
International Labour Organisation (ILO), and the World Health
Organization (WHO). The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include the
development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally comparable
results, and the development of manpower in the field of toxicology.
Other activities carried out by the IPCS include the development of
know-how for coping with chemical accidents, coordination of
laboratory testing and epidemiological studies, and promotion of
research on the mechanisms of the biological action of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Xylenes.
(Environmental health criteria ; 190)
1.Xylenes - adverse effects 2.Xylenes - toxicity
3.Environmental exposure I.Series
ISBN 92 4 157190 X (NLM Classification: QD 341.H9)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1997
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR XYLENES
Preamble
1. SUMMARY
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. In air
2.4.2. In water
2.4.3. In biological media
2.4.3.1 In blood
2.4.3.2 In urine
2.4.3.3 In exhaled air
2.4.3.4 In human milk
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production processes
3.2. Production levels
3.3. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Volatilization
4.1.2. Rain-out
4.1.3. Adsorption
4.2. Transformation
4.2.1. Biodegradation
4.2.1.1 Aerobic degradation
4.2.1.2 Anaerobic degradation
4.2.2. Abiotic degradation
4.2.2.1 Photolysis
4.2.2.2 Atmospheric oxidation
4.2.2.3 Hydrolysis
4.2.3. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Ambient air
5.1.2. Water and sediment
5.1.2.1 Surface water
5.1.2.2 Groundwater
5.1.2.3 Precipitation
5.1.2.4 Leachate
5.1.2.5 Sediment
5.1.3. Soil
5.1.4. Biota
5.2. General population exposure
5.2.1. Source of exposure
5.2.1.1 Air
5.2.1.2 Food
5.2.1.3 Drinking-water
5.2.1.4 Other source of exposure
5.2.2. Xylene levels in human biological samples
5.3. Occupational exposure during manufacture, formulation or use
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption
6.1.1. In humans
6.1.2. In laboratory animals
6.2. Distribution
6.2.1. In humans
6.2.2. In laboratory animals
6.3. Metabolic transformation
6.3.1. In humans
6.3.2. In laboratory animals
6.4. Elimination and excretion
6.4.1. In humans
6.4.2. In laboratory animals
6.5. Factors affecting toxicokinetics in humans and animals
6.6. Biological monitoring
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Inhalation studies
7.1.1.1 o-Xylene
7.1.1.2 m-Xylene
7.1.1.3 p-Xylene
7.1.1.4 Technical or undefined xylene
7.1.2. Other exposure routes
7.2. Short-term exposure
7.2.1. Inhalation studies
7.2.1.1 o-Xylene
7.2.1.2 m-Xylene
7.2.1.3 p-Xylene
7.2.1.4 Technical or undefined xylene
7.2.2. Other exposure routes
7.3. Long-term exposure
7.4. Skin and eye irritation; sensitization
7.5. Reproductive and developmental toxicology
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.8. Other effects
8. EFFECTS ON HUMANS
8.1. Acute and accidental exposure
8.2. Controlled human studies
8.3. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.2. Aquatic organisms
9.1.2.1 Algae
9.1.2.2 Higher plants
9.1.2.3 Protozoa
9.1.2.4 Invertebrates
9.1.2.5 Vertebrates
9.1.3. Terrestrial organisms
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposures
10.1.2. Effects
10.1.3. Guidance value
10.2. Evaluation of effects on the environment
10.2.1. Exposure
10.2.2. Effects
10.2.3. Risk evaluation
11. CONCLUSIONS
12. RECOMMENDATIONS
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Chātelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
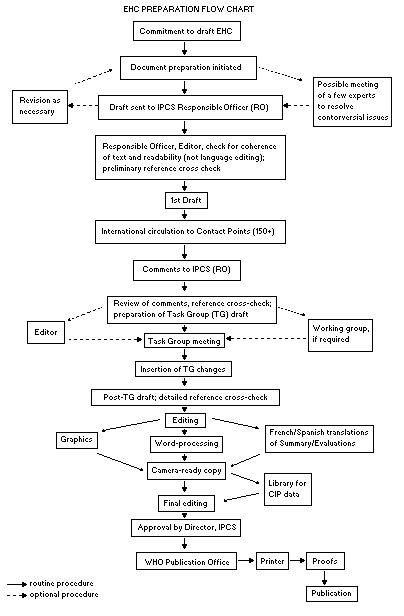 The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR XYLENES
Members
Dr E. Frantik, Centre for Industrial Hygiene and Occupational
Diseases, National Institute of Public Health, Prague, Czech
Republic
Dr U. Hass, Department of Toxicology and Biology, National Institute
of Occupational Health, Copenhagen, Denmark
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Abbots Ripton, Huntingdon Cambridgeshire, United Kingdom
(Co-Rapporteur)
Dr Young Lee, Contaminants Standards, Monitoring and Programs Branch,
Centre for Food Safety and Applied Nutrition, US Food and Drug
Administration, Washington DC, USA
Mr G. Long, Health and Welfare Canada, Environmental Health Centre,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr P. Lundberg, Department of Toxicology, National Institute for
Working Life, Solna, Sweden (Co-Rapporteur)
Dr Choon-Nam Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
Dr V. Riihimäki, Institute of Occupational Health, Helsinki, Finland
(Chairman)
Observer
Dr C.J. Bevan, Exxon Biomedical Sciences Inc., East Millstone, New
Jersey, USA
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR XYLENES
A WHO Task Group on Environmental Health Criteria for Xylenes met
in Geneva from 6 to 9 November 1995. Dr B.H. Chen, IPCS, opened the
meeting and welcomed the participants on behalf of the Director, IPCS,
and the three IPCS cooperating organizations (UNEP/ILO/WHO). The Task
Group reviewed and revised the draft criteria monograph and made an
evaluation of the risks for human health and the environment from
exposure to xylenes.
The first draft of this monograph was prepared by Dr P. Lundberg,
Mr P.D. Howe, Mr M.J. Crookes and Dr S. Dobson. The second draft was
prepared by Dr P. Lundberg and Mr P.D. Howe incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria monographs. Dr P.
Lundberg and Mr P.D. Howe contributed to the final text of the health
and environmental sections, respectively.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
ATPase adenosine triphosphatase
BCF bioconcentration factor
BTX benzene, toluene, xylene
CNS central nervous system
DMP dimethylphenol
DMSO dimethylsulfoxide
EEG electroencephalograph
FID flame-ionization detector
i.m. intramuscular
i.p. intraperitoneal
i.v. intravenous
LOAEL lowest-observed-adverse-effect level
NADPH reduced nicotinamide adenosine dinucleotide
NOAEL no-observed-adverse-effect level
PB phenobarbital
PMBA p-methylbenzyl alcohol
POCP photochemical ozone-creation potential
RMA reflex modification audiometry
s.c. subcutaneous
TCE 1,1,1-trichloroethylene
1. SUMMARY
Xylene is an aromatic hydrocarbon which exists in three isomeric
forms: ortho, meta and para. Technical grade xylene contains a
mixture of the three isomers and also some ethylbenzene. The
estimated world production in 1984 was 15.4 million tonnes. Xylene is
a colourless liquid at room temperature with an aromatic odour. The
vapour pressure lies between 0.66 and 0.86 kPa for the three isomers.
Approximately 92% of mixed xylenes is blended into petrol. It is also
used in a variety of solvent applications, particularly in the paint
and printing ink industries.
The majority of xylene released into the environment enters the
atmosphere directly. In the atmosphere the xylene isomers are readily
degraded, primarily by photooxidation. Volatilization to the
atmosphere from water is rapid for all three isomers. In soil and
water, the meta and para isomers are readily biodegraded under a wide
range of aerobic and anaerobic conditions, but the ortho isomer is
more persistent. The limited evidence available suggests that
bioaccumulation of the xylene isomers by fish and invertebrates is
low. Elimination of xylene from aquatic organisms is fairly rapid
once exposure has ceased.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3, but in suburban areas they are
around 3 µg/m3. Higher levels have been measured in urban and
industrialized areas, mean concentrations ranging up to 500 µg/m3.
However, concentrations are generally below 100 µg/m3.
Estimated daily exposure of the general population through
inhalation is 70 µg in rural areas and less than 2000 µg in urban
areas. The concentration in drinking-water ranges from not detectable
to 12 µg/litre. The data on the level in food are too limited to
estimate daily oral exposure.
Mean background concentrations of xylenes in surface water are
generally below 0.1 µg/litre. However, much higher values have been
measured in industrial areas and areas associated with the oil
industry (up to 30 µg/litre in polluted waters and up to 2000 µg/litre
near to discharge pipes). Similar background levels have been
reported for groundwater although high levels have been reported due
to localized pollution from underground storage tanks and pipes.
After inhalation exposure the retention in the lungs is about 60%
of the inhaled dose. Xylene is efficiently metabolized. More than
90% is biotransformed to methylhippuric acid, which is excreted in
urine. Xylene does not accumulate significantly in the human body.
Acute exposure to high concentrations of xylene can result in CNS
effects and irritation in humans. However, there have been no
long-term controlled human studies or epidemiological studies. The
chronic toxicity appears to be relatively low in laboratory animals.
There is suggestive evidence, however, that chronic CNS effects may
occur in animals at moderate concentrations of xylene.
Xylene appears not to be a mutagen or a carcinogen.
The critical end-point is developmental toxicity, which has been
demonstrated at an exposure level of 870 mg/m3 (200 ppm) in rats.
Based on this end-point, the recommended guidance value for xylene in
air is 0.87 mg/m3 (0.2 ppm).
The xylene isomers are of moderate to low toxicity for aquatic
organisms. For invertebrates the lowest LC50 value, based on
measured concentrations, is for o-xylene at 1 mg/litre (Daphnia
magna). The lowest LC50 values recorded for fish are 7.6 mg/litre
for o-xylene (rainbow trout; based on measured concentrations), and
7.9 and 1.7 mg/litre for m- and p-xylenes respectively (both for
striped bass; based on nominal concentrations). Limited information
is available regarding chronic exposure of aquatic organisms to
xylenes; however, rapid volatilization makes chronic exposure in
water unlikely. The acute toxicity of xylene to birds is low.
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
Xylene exists in three isomeric forms, ortho-, meta- and
para-xylene. The commercial product is a mixture of all three
isomers with m-xylene predominating, usually 60-70%. The technical
product, "mixed xylenes", contains approximately 40% m-xylene and
20% each of ethylbenzene, o-xylene and p-xylene. Small quantities
of toluene and C9 aromatic fractions may also be present (Fishbein,
1988).
Chemical formula
C8H10 C8H10 C8H10
Chemical structure
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR XYLENES
Members
Dr E. Frantik, Centre for Industrial Hygiene and Occupational
Diseases, National Institute of Public Health, Prague, Czech
Republic
Dr U. Hass, Department of Toxicology and Biology, National Institute
of Occupational Health, Copenhagen, Denmark
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Abbots Ripton, Huntingdon Cambridgeshire, United Kingdom
(Co-Rapporteur)
Dr Young Lee, Contaminants Standards, Monitoring and Programs Branch,
Centre for Food Safety and Applied Nutrition, US Food and Drug
Administration, Washington DC, USA
Mr G. Long, Health and Welfare Canada, Environmental Health Centre,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr P. Lundberg, Department of Toxicology, National Institute for
Working Life, Solna, Sweden (Co-Rapporteur)
Dr Choon-Nam Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
Dr V. Riihimäki, Institute of Occupational Health, Helsinki, Finland
(Chairman)
Observer
Dr C.J. Bevan, Exxon Biomedical Sciences Inc., East Millstone, New
Jersey, USA
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR XYLENES
A WHO Task Group on Environmental Health Criteria for Xylenes met
in Geneva from 6 to 9 November 1995. Dr B.H. Chen, IPCS, opened the
meeting and welcomed the participants on behalf of the Director, IPCS,
and the three IPCS cooperating organizations (UNEP/ILO/WHO). The Task
Group reviewed and revised the draft criteria monograph and made an
evaluation of the risks for human health and the environment from
exposure to xylenes.
The first draft of this monograph was prepared by Dr P. Lundberg,
Mr P.D. Howe, Mr M.J. Crookes and Dr S. Dobson. The second draft was
prepared by Dr P. Lundberg and Mr P.D. Howe incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria monographs. Dr P.
Lundberg and Mr P.D. Howe contributed to the final text of the health
and environmental sections, respectively.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
ATPase adenosine triphosphatase
BCF bioconcentration factor
BTX benzene, toluene, xylene
CNS central nervous system
DMP dimethylphenol
DMSO dimethylsulfoxide
EEG electroencephalograph
FID flame-ionization detector
i.m. intramuscular
i.p. intraperitoneal
i.v. intravenous
LOAEL lowest-observed-adverse-effect level
NADPH reduced nicotinamide adenosine dinucleotide
NOAEL no-observed-adverse-effect level
PB phenobarbital
PMBA p-methylbenzyl alcohol
POCP photochemical ozone-creation potential
RMA reflex modification audiometry
s.c. subcutaneous
TCE 1,1,1-trichloroethylene
1. SUMMARY
Xylene is an aromatic hydrocarbon which exists in three isomeric
forms: ortho, meta and para. Technical grade xylene contains a
mixture of the three isomers and also some ethylbenzene. The
estimated world production in 1984 was 15.4 million tonnes. Xylene is
a colourless liquid at room temperature with an aromatic odour. The
vapour pressure lies between 0.66 and 0.86 kPa for the three isomers.
Approximately 92% of mixed xylenes is blended into petrol. It is also
used in a variety of solvent applications, particularly in the paint
and printing ink industries.
The majority of xylene released into the environment enters the
atmosphere directly. In the atmosphere the xylene isomers are readily
degraded, primarily by photooxidation. Volatilization to the
atmosphere from water is rapid for all three isomers. In soil and
water, the meta and para isomers are readily biodegraded under a wide
range of aerobic and anaerobic conditions, but the ortho isomer is
more persistent. The limited evidence available suggests that
bioaccumulation of the xylene isomers by fish and invertebrates is
low. Elimination of xylene from aquatic organisms is fairly rapid
once exposure has ceased.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3, but in suburban areas they are
around 3 µg/m3. Higher levels have been measured in urban and
industrialized areas, mean concentrations ranging up to 500 µg/m3.
However, concentrations are generally below 100 µg/m3.
Estimated daily exposure of the general population through
inhalation is 70 µg in rural areas and less than 2000 µg in urban
areas. The concentration in drinking-water ranges from not detectable
to 12 µg/litre. The data on the level in food are too limited to
estimate daily oral exposure.
Mean background concentrations of xylenes in surface water are
generally below 0.1 µg/litre. However, much higher values have been
measured in industrial areas and areas associated with the oil
industry (up to 30 µg/litre in polluted waters and up to 2000 µg/litre
near to discharge pipes). Similar background levels have been
reported for groundwater although high levels have been reported due
to localized pollution from underground storage tanks and pipes.
After inhalation exposure the retention in the lungs is about 60%
of the inhaled dose. Xylene is efficiently metabolized. More than
90% is biotransformed to methylhippuric acid, which is excreted in
urine. Xylene does not accumulate significantly in the human body.
Acute exposure to high concentrations of xylene can result in CNS
effects and irritation in humans. However, there have been no
long-term controlled human studies or epidemiological studies. The
chronic toxicity appears to be relatively low in laboratory animals.
There is suggestive evidence, however, that chronic CNS effects may
occur in animals at moderate concentrations of xylene.
Xylene appears not to be a mutagen or a carcinogen.
The critical end-point is developmental toxicity, which has been
demonstrated at an exposure level of 870 mg/m3 (200 ppm) in rats.
Based on this end-point, the recommended guidance value for xylene in
air is 0.87 mg/m3 (0.2 ppm).
The xylene isomers are of moderate to low toxicity for aquatic
organisms. For invertebrates the lowest LC50 value, based on
measured concentrations, is for o-xylene at 1 mg/litre (Daphnia
magna). The lowest LC50 values recorded for fish are 7.6 mg/litre
for o-xylene (rainbow trout; based on measured concentrations), and
7.9 and 1.7 mg/litre for m- and p-xylenes respectively (both for
striped bass; based on nominal concentrations). Limited information
is available regarding chronic exposure of aquatic organisms to
xylenes; however, rapid volatilization makes chronic exposure in
water unlikely. The acute toxicity of xylene to birds is low.
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
Xylene exists in three isomeric forms, ortho-, meta- and
para-xylene. The commercial product is a mixture of all three
isomers with m-xylene predominating, usually 60-70%. The technical
product, "mixed xylenes", contains approximately 40% m-xylene and
20% each of ethylbenzene, o-xylene and p-xylene. Small quantities
of toluene and C9 aromatic fractions may also be present (Fishbein,
1988).
Chemical formula
C8H10 C8H10 C8H10
Chemical structure
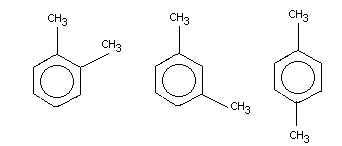 Chemical name
ortho-xylene meta-xylene para-xylene
Synonyms
1,2-dimethyl- 1,3-dimethyl- 1,4-dimethyl-
benzene benzene benzene
o-methyltoluene m-methyltoluene p-methyltoluene
1,2-xylene 1,3-xylene 1,4-xylene
o-xylol m-xylol p-xylol
ortho-xylene meta-xylene para-xylene
Relative molecular mass
106.16 106.16 106.16
CAS registry number
95-47-6 108-38-3 106-42-3
RTECS registry number
ZE 2450000 ZE 2275000 ZE 2625000
CAS registry number (mixed xylenes) 1330-20-7
RTECS registry number (mixed xylenes) ZE 210000
2.2 Physical and chemical properties
Some physical and chemical properties are given in Table 1.
Table 1. Some physical and chemical properties of xylenesa
o-Xylene m-Xylene p-Xylene
Physical state (20°C; 101.3 kPa) liquid liquid liquid
Colour colourless colourless colourless
Boiling point (°C; 101.3 kPa) 144.4 139.1 138.3
Melting point (°C; 101.3 kPa) -25.2 -47.9 13.3
Relative density (25°/4°C) 0.876 0.860 0.857
Vapour pressure (kPa at 20°C) 0.66 0.79 0.86
Flash point (°C) (closed cup) 30 25 25
Saturation % in air (101.3 kPa) 1.03 (32°C) 1.03 (28°C) 1.03 (27°C)
Explosion limits (vol-% in air) 1.0-6 1.1-7 1.1-9
Autoignition temp (°C) 465 525 525
Octanol/water partition coefficient
(log P) 3.12 3.2 3.15
Solubility in water (mg/litre) 142 146 185
a Data from Sandmeyer (1981); Verschueren (1983); ECETOC (1986); IARC, (1989);
DECOS (1991); Bell (1992)
All three isomers of xylene are soluble in organic solvents such
as ethanol, diethyl ether, acetone and benzene (ECETOC, 1986; IARC,
1989; DECOS, 1991). At room temperature the xylenes are colourless
liquids with an aromatic odour (DECOS, 1991). The odour threshold for
mixed xylene in air is approximatively 4.35 mg/m3 (1 ppm) (Carpenter
et al., 1975; Amoore & Hautala, 1983; DECOS, 1991).
2.3 Conversion factors
1 ppm = 4.35 mg/m3 at 25°C, 101.3 kPa
1 mg/m3 = 0.23 ppm at 25°C, 101.3 kPa
2.4 Analytical methods
2.4.1 In air
US NIOSH has presented a method for measuring aromatic
hydrocarbons including xylene, in air. Xylene is adsorbed to coconut
shell charcoal, eluated with carbon disulfide and determined using gas
chromatography with a flame-ionization detector (FID) (Eller, 1984).
A similar method has been described by the International Agency
for Research on Cancer (IARC) (Brown, 1988a) concerning airborne
vapours of benzene, toluene and xylenes, or mixtures thereof. The
concentration range is about 1-1000 mg/m3 (approximately 0.2-200 ppm)
in 12-litre air samples. In another method described by IARC (Brown,
1988b) hydrocarbons are adsorbed on a porous polymer, desorbed with
heat and transferred with an inert carrier gas into a gas
chromatograph equipped with a FID. For a 5-litre air sample the
concentration range is approximately 0.5-50 mg/m3 (0.1-10 ppm). With
the use of a gas chromatography and mass spectrometry (GC/MS)
technique, the detection limit can be as low as 0.2 µg/m3 (Bevan et
al., 1991).
IARC also presented a method for determinating gasoline
hydrocarbons (Brown, 1988c). The air is drawn through two adsorbent
tubes in series, containing Chemosorb 106 and charcoal, respectively.
The vapour after heat desorption is transferred to a gas chromatograph
equipped with a capillary column and FID. The method is suitable for
airborne vapours of full-range gasoline over a concentration range of
approximately 0.2-100 mg/m3 (0.04-20 ppm) in a 2.5-litre air sample.
There are commercially available badges based on passive charcoal
sampling. After extraction with carbon disulfide the xylenes can be
detected by gas chromatography (Van der Wal & Moerkerken, 1984;
Triebig & Schaller, 1986). Xylene can also be detected with infrared
analysers with a minimum concentration of 9.6 mg/m3 (2.2 ppm) at a
wavelength of 13.1 µm and a pathlength of 20.25 m. This method is
only suitable when no other compounds that absorb in the same region
are present (DECOS, 1991).
Earlier methods for determinating xylenes have been reviewed by
Fishbein et al. (1988).
2.4.2 In water
A head-space technique coupled to capillary column gas
chromatography has been described by Drozd & Novak (1978). The
detection limit is at the ppb level. The detection limits could be
lowered if the xylenes were extracted from the water by an air stream
and condensed in a refrigerated column. In another method, the sample
is extracted with hexane or heated in a water bath at 25°C for 1 h.
Aliquots are then determined by gas chromatography with FID or mass
spectometry. The detection limit is 1 µg/litre (Otson & Williams,
1981; Otson et al., 1982). Recent studies have suggested that with
the use of GC/MS the detection limits for xylene in water can be in
the range of 0.001 to 0.01 µg/litre (Kenrick et al., 1985; MAFF,
1991).
2.4.3 In biological media
A review of biological monitoring of exposure to xylene has been
produced; methods include measuring methylhippuric acid in urine,
xylene in blood and xylene in expired air (Lauwerys & Buchet, 1988).
2.4.3.1 In blood
A method using capillary head-space gas chromatography with FID
has been developed for the simultaneous determination of xylene and
other aromatic hydrocarbons, such as benzene, xylenes and ethyl-
benzene, in blood. The limit of detection is 5 µg/litre and the
response is linear between 5 and 4000 µg/litre of blood.
A head-space gas chromatographic method for determinating xylenes
in blood has also been described (Engström & Riihimäki, 1988a). This
method is suitable for the determination of xylene isomers in blood
specimens. The detection limit is 53 µg/litre. Ethylbenzene, which
normally accompanies xylenes in technical xylene, does not interfere
with the determination of xylene.
2.4.3.2 In urine
In humans, xylene is metabolized to methylhippuric acids (see
section 6.3), which are not normally present in the urine of
non-exposed people. The urine methylhippuric acid level has been
measured by gas chromatography (Engström & Bjurström, 1978),
colorimetry (Ogata & Hobara, 1979), thin-layer chromatography (Bieniek
et al., 1982) and high performance liquid chromatography (Ogata &
Taguchi, 1986).
A suitable gas chromatographic method for the determination of
methylhippuric acids in urine has been presented by Engström &
Riihimäki (1988b). The range of application is 10-2120 mg/litre. No
interference from the normal constituents of urine is seen in the
specified range. Another method using HPLC allows methylhippuric
acids, phenyl glyoxylic acid and mandelic acid to be determined
together with hippuric acid in one run (Angerer, 1988b). The limit of
detection is 50 mg/litre of urine and the response is linear up to
2000 mg/litre. The aromatic carboxylic acids excreted in urine do not
interfere with the hippuric acid determination.
2.4.3.3 In exhaled air
There is a method for determinating benzene, toluene and xylene
in breath samples by gas chromatography/mass spectrometry (Pellizzari
et al., 1988). The detection limit for xylene is 0.5 µg/m3 and the
quantification limit is 2.5 µg/m3. No interference has been
observed. The linear range for quantification using fused silica
capillaries on a gas chromatograph/mass spectrometer/computer is
generally three orders of magnitude.
2.4.3.4 In human milk
A purge and trap technique using gas chromatography and electron
impact mass spectrometry has been developed by Pellizari et al. (1982)
for the detection of xylene and various volatile compounds in human
milk.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Production processes
Before 1940 virtually all of the aromatic solvents, including
xylene, were produced from coal. Thereafter production of xylene from
petroleum started. Most mixed xylene is currently produced by
catalytic reforming of petroleum. It is also obtained from pyrolysis
gasoline as a by-product of olefin manufacture during the cracking of
hydrocarbons. Small amounts of mixed xylenes are also obtained from
coal-derived coke-oven light oil and from disproportionation of
toluene (Fishbein, 1988).
There are some differences in the composition of commercial
xylenes produced from petroleum and from coal-tar. The general
composition of xylenes from petroleum is 44% m-xylene, 20%
o-xylene, 20% p-xylene and 15% ethylbenzene. The xylenes from
coal-tar consists of 45-70% m-xylene, 23% p-xylene, 10-15%
o-xylene and 6-10% ethylbenzene. Commercial xylene may also contain
small amounts of toluene, trimethylbenzene (pseu documene), phenol,
thiophene, pyridine and non-aromatic hydrocarbons and has frequently
been contaminated with benzene (WHO, 1981; Fishbein, 1988).
3.2 Production levels
Approximately 3.9 million tonnes of mixed xylenes were isolated
in the USA in 1978. The non-isolated mixed xylenes (containing
benzene and toluene) are blended into gasoline, while the isolated
mixed xylenes are used primarily for the production of the individual
isomers and for solvent applications (Fishbein, 1988).
World production of p-xylene in 1983 was 3.9 million tonnes of
which the USA accounted for 48%, Europe 23% and Japan 16%. The world
production of o-xylene in 1983 was 1.3 million tonnes of which
western Europe produced 30% and the USA 18%. Eastern Europe was the
other large producer of o-xylene (Fishbein, 1988).
The approximate world production of o-, p- and mixed xylenes in
1984 was 15.4 million tonnes (ECETOC, 1986). The production of mixed
xylenes in 1984 in the USA was 2.78 million tonnes and that of
p-xylene was 1.94 million tonnes (Fishbein, 1988). During the same
year the production of o-xylene was 316 000 tonnes. The production
of mixed xylene and p-xylene in 1994 was 4.1 and 2.8 million tonnes,
respectively (Kirschner, 1995). The production of xylenes in some
western European countries in 1984 was estimated to be: France 85 000
tonnes, Italy 395 000 tonnes and Federal Republic of Germany 455 000
tonnes. In 1987 the figures were: (in thousands of tonnes) Canada
345, France 129, Federal Republic of Germany 501, India 28, Italy
491, Japan 1767, Republic of Korea 552, Mexico 381 and USA 2772 (IARC,
1989).
3.3 Uses
Approximately 92% of the mixed xylenes produced is blended into
gasoline. The remainder is used in a variety of solvent applications
as well as to produce the individual isomers of xylene. Xylenes are
used as solvents, particularly in the paint and printing ink
industries. The single largest end-use of mixed xylenes is in the
production of the p-xylene isomer. The major derivatives produced
from p-xylene are dimethylterephthalate and terephthalic acid used
in the production of polyester fibre, film and fabricated items
(ECETOC, 1986; Fishbein, 1988).
The o-xylene is almost exclusively used to produce phthalic
anhydride for phthalate plasticizers, and m-xylene is used for the
production of isophthalic acid, an intermediate in the manufacture of
polyester resins (ECETOC, 1986; Fishbein, 1988).
Mixed xylenes are also used in the manufacture of perfumes,
pesticide formulations, pharmaceuticals and adhesives, and in the
painting, printing, rubber, plastics and leather industries (IARC,
1989).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The majority of xylene released into the environment enters the
atmosphere directly. This results mainly from its use as a solvent
and its release in motor vehicle exhausts. A small proportion is also
likely to enter water and soil due to oil/petrol spillages etc. Once
in the environment a number of physical processes can affect its
distribution. Jori et al. (1986) calculated that more than 99% of the
xylene released ultimately partitions to the atmosphere; the
calculations were based on the Mackay fugacity model (Mackay et al.,
1985).
4.1.1 Volatilization
Owing to the relatively high vapour pressure and low water
solubility of xylenes, volatilization from water bodies to the
atmosphere is likely to be an important distribution process. The
half-life for evaporation of o-xylene from water bodies at a depth
of 1 metre has been estimated to be around 5.6 h (Mackay & Leinonen,
1975). It is expected that both m-xylene and p-xylene will behave
similarly.
No information has been reported on the volatilization rate of
xylenes from soils, but it is expected to be a fairly rapid process,
at least from near to the surface, owing to the reasonably high
volatility of xylenes.
4.1.2 Rain-out
Xylenes are only slightly soluble in water (see section 2). This
means that only a very small proportion of xylene in the atmosphere is
likely to be removed by precipitation (rain-out). This is supported
by the fact that xylenes have been detected in rainwater samples at
only very low levels (2 ng m-xylene/litre and 9 ng p-xylene/litre;
see section 5.1.2.3). It is also possible that a small amount of
xylene in soil may leach out into aquatic systems.
4.1.3 Adsorption
Xylenes are likely to be adsorbed to a small extent onto both
aquatic sediments and soil, based on their partition coefficients.
However, adsorption is dependent on such factors as the organic carbon
content and the water content.
Sediment-water partition coefficients of 8.9 for o-xylene and
10.5 for p-xylene have been measured. These were for surface
sediment from the Tamar Estuary, United Kingdom, which has an organic
carbon content of 4.02% (Vowles & Mantoura, 1987).
m-Xylene has been shown to adsorb onto soil to a small extent.
Using three soils with organic carbon contents ranging between 0.2 and
3.7%, soil organic carbon-water partition coefficient (Koc) values of
129, 158 and 289 (2.1, 2.2 and 2.5 log values) were measured (Seip et
al., 1986). A similar Koc of 219 has been quoted for o-xylene
(Pussemier et al., 1990).
p-Xylene has been shown to adsorb onto minerals and soils to a
small extent from the vapour phase (Rhue et al., 1988).
In the absence of water, soils and clay minerals exhibit a large
capacity to adsorb p-xylene, owing primarily to adsorption on
mineral surfaces. However, such dry conditions are rarely encountered
in the environment and may only exist at the soil surface or in arid
climates. When the relative humidity is increased to 67% or 90%, the
sorption of p-xylene vapour decreases significantly (Pennell et al.,
1992).
4.2 Transformation
4.2.1 Biodegradation
In soil and water, o- and p-xylene are readily biodegraded
under a wide range of aerobic and anaerobic conditions, but o-xylene
is much more persistent under similar conditions.
4.2.1.1 Aerobic degradation
Bacteria of the genus Pseudomonas have been shown to be capable of
growing using either m-xylene or p-xylene as the sole carbon
source (Davis et al., 1967; Omori et al., 1967; Omori & Yamada, 1970;
Davey & Gibson, 1974). The main initial metabolites appear to be
m-toluic acid from m-xylene and p-toluic acid from p-xylene.
Similarly, cultures of three strains of Nocardia have been shown to
metabolize p-xylene to p-toluic acid and 2,3-dihydroxy- p-toluic
acid (Raymond et al., 1969).
In contrast to this, many of the bacteria that have been shown to
be capable of growing on either m-xylene or p-xylene as sole
carbon source do not appear to be capable of growing on o-xylene as
sole carbon source (Omori et al., 1967; Davey & Gibson, 1974).
o-Xylene has been shown to undergo biodegradation in the
presence of other carbon sources. Using hexadecane as growth
substrate, o-xylene was co-oxidized to o-toluic acid by Nocardia.
A similar oxidation was observed with Pseudomonas using hexane as
the growth substrate (Jamison et al., 1976).
The biodegradation of xylenes by the autochthonous microflora in
groundwater in the presence of the water soluble fraction of gas oil
has been demonstrated by Kappeler & Wuhrmann (1978a, 1978b). After a
lag period of 3 to 4 days, individual hydrocarbon concentrations were
found to decrease at a measurable rate. The removal of m-xylene and
p-xylene was complete after 7 days. o-Xylene was shown to degrade
at a significantly slower rate than the meta and para isomers, removal
being complete after 11-12 days. In each case, the first step in the
degradation appears to be oxidation to the corresponding methylbenzyl
alcohol.
Both m-xylene and p-xylene have been shown to be readily
degraded within 13 days using a microbial inoculum from an activated
sludge wastewater treatment plant. The initial concentration of
xylene was 100 mg/litre and 30 mg/litre of sludge biomass was used.
Degradation of xylene was monitored by comparing the oxygen uptake of
the system with that of controls (Tabak et al., 1989).
The degradation of mixtures of benzene, toluene and p-xylene
has been studied using pure cultures of either Pseudomonas sp.
strain CFS-215 or Arthrobacter sp. strain HCB, or a mixed culture
indigenous to a shallow sandy aquifer. In the mixed culture, the
presence of p-xylene was found to increase the lag period before the
degradation of benzene and toluene commenced, and also appeared to
decrease the rate of toluene degradation compared to the rate obtained
without added p-xylene. Degradation of p-xylene occurred in the
mixed culture, although a long lag period was observed before
degradation commenced. When toluene was also present in the culture,
the lag period for the degradation of p-xylene was reduced and the
degradation rate was increased, but after all the toluene had been
degraded, the p-xylene degradation rate again slowed. In the
experiments with Pseudomonas sp., the degradation of p-xylene was
slow; no degradation was observed in the first 3 weeks when p-xylene
alone was present. Again, the degradation rate of p-xylene was
found to increase when toluene was also being degraded. Also, the
presence of p-xylene again increased the lag period for benzene and
toluene degradation. In the experiments with Arthrobacter sp.,
degradation of p-xylene was found to occur only in the presence of
benzene and at a slow rate (Alvarez & Vogel, 1991).
The biodegradation of o-xylene and m-xylene has been studied
in three core samples of subsurface soil: uncontaminated soil, soil
that had previously been contaminated with unleaded gasoline and soil
from an area that had previously undergone biostimulation using
hydrogen peroxide. m-Xylene was rapidly degraded in all three core
types, although the rate was faster in the previously biostimulated
sample due to a higher bacterial cell count ( m-xylene disappeared to
below the analytical detection limit within 3 weeks in the previously
biostimulated samples, whereas some remained after 3 weeks in the
previously contaminated samples). o-Xylene was found to be
recalcitrant in all of the samples (Thomas et al., 1990).
p-Xylene and o-xylene were shown to be degraded in aquifer
material collected from the contaminant plume after a large gasoline
spill. The degradation occurred fastest in material from the aerobic
degrading zone of the plume, but also occurred rapidly in
uncontaminated material (Wilson et al., 1990).
In a study using laboratory aquifer columns that simulated
saturated-flow conditions typical of a river/groundwater infiltration
system, all three xylene isomers were shown to undergo degradation
under aerobic conditions. Both m-xylene and p-xylene were
degraded to concentrations below the analytical limit of detection
within 17 days. The rate of transformation was significantly lower
for o-xylene but degradation still occurred readily (Kuhn et al.,
1985).
The rate of biodegradation of benzene, toluene and xylene (BTX)
in groundwater/soil slurries has been shown to be highly dependent on
the dissolved oxygen concentration (Chiang et al., 1989). At a
dissolved oxygen concentration of between 2 and 8 mg/litre, BTX
(initial concentrations between 120 and 16000 µg/litre) was 80-100%
degraded in 30-40 days with a half-life of 5-20 days. When the
dissolved oxygen concentration was 1 or 2 mg/litre, the BTX was
incompletely degraded (20-60%) in 30-40 days. Little or no
degradation was observed at dissolved oxygen concentrations of 0, 0.1
and 0.5 mg/litre.
The xylenes have been shown to be 100% degraded after 192 h
incubation at 13°C with natural flora in groundwater in the presence
of other components of high-octane gasoline (Jamison et al., 1976).
4.2.1.2 Anaerobic degradation
o-Xylene, along with other alkylbenzene compounds, has been
shown to undergo degradation under anaerobic methanogenic conditions.
No significant degradation of o-xylene occurred over the first 20
weeks, but after 40 weeks the concentration was reduced to 22% of the
original. Less than 1% remained after 120 weeks (Wilson et al.,
1986).
In anoxic suspensions of Pseudomonas sp. strain T cells grown
anaerobically with toluene, m-xylene and p-xylene were partially
oxidized to 3- and 4-methylbenzoate, respectively. o-Xylene was not
oxidized to 2-methylbenzoate. Suspensions of strain T cells grown
anaerobically with m-xylene and incubated with m-xylene at 5°C
accumulated 3-methylbenzaldehyde (3.5 µM after 20 min) and
3-methylbenzoate (5 µM after 20 min). After further incubation at
room temperature, the three aromatic compounds were completely
oxidized within 3 h (Seyfried et al., 1994).
Experiments have been carried out using aquifer material from a
site containing areas that were either contaminated or uncontaminated
with JP-4 jet fuel. Both mixed xylene and the individual isomers were
incubated with the aquifer material at 12°C under a nitrogen
atmosphere. Both o-xylene and m-xylene were slowly degraded in
the uncontaminated aquifer material when added individually, although
m-xylene (at 16 mg/litre) also appeared to inhibit the basal rate of
denitrification. Using mixed xylenes, a lag period of 30 days was
required before biodegradation commenced in the uncontaminated
material. m-Xylene and p-xylene were degraded to below the
analytical limit of detection within the next 26 days, but the
degradation of o-xylene was found to be much slower. In the
contaminated aquifer material, much longer lag periods and decreased
rates of biodegradation were observed, o-xylene not being
significantly degraded over a 6-month period (Hutchins et al., 1991a).
In further laboratory experiments using a mixture of benzene and
alkylbenzenes, both o-xylene and m-xylene were found to be
degraded under nitrate-reducing and nitrous oxide-reducing conditions,
but degradation of o-xylene was found to cease once the other
alkylbenzenes had been degraded (Hutchins, 1991). In field
experiments in the same aquifer, m-xylene and p-xylene were shown
to be degraded under denitrifying conditions when nitrate was injected
into the aquifer, but no evidence of biodegradation of o-xylene was
found (Hutchins et al., 1991b).
The three xylene isomers have been shown to be completely
mineralized by aquifer-derived microorganisms under sulfate-reducing
conditions. The source of the inoculum was a gasoline-contaminated
sediment. All microcosms were initially fed a mixture of benzene,
toluene, ethylbenzene, o-xylene and p-xylene (about 5 mg/litre of
each component). p-Xylene was found to be > 80% degraded within 72
days and o-xylene was > 80% degraded within 104 days. After this
initial adaptation period, o-xylene, m-xylene and p-xylene were
rapidly degraded by the system without any lag period ( m-xylene
co-elutes with p-xylene and, therefore, m-xylene was not added
initially) (Edwards et al., 1992).
Edwards & Grbic-Galic (1994) reported that o-xylene is
completely mineralized by aquifer-derived microorganisms under
anaerobic conditions. However, an adaptation period of 200 to 255
days was required before the onset of degradation. Anaerobic
degradation was found to be inhibited by the presence of some natural
organic substrates and co-contaminants.
p-Xylene and o-xylene have been shown to be degraded in
anaerobic aquifer material collected from the contaminant plume after
a large gasoline spill (Wilson et al., 1990).
All three xylene isomers have been shown to undergo degradation
under anaerobic denitrifying conditions. The rate was much lower for
o-xylene than for the other isomers. Long lag periods were observed
in all cases before degradation commenced (Kuhn et al., 1985).
Degradation of o-xylene under anaerobic conditions has been
hypothesized to explain the distribution of o-xylene in a landfill
leachate plume (Reinhard et al., 1984). m-Xylene has been shown to
be rapidly mineralized to carbon dioxide in laboratory aquifer columns
operated under continuous flow conditions with nitrite as an electron
acceptor. The degradation occurred simultaneously with the reduction
of nitrite. In contrast to this, the concentrations of o-xylene and
p-xylene were only slightly reduced in the experiment. The author
noted, however, that the experiments were carried out over a 6-day
period after the addition of the new substrate and therefore may not
have allowed a build-up of other microorganisms capable of degrading
these substrates (Kuhn et al., 1988).
The biodegradation of BTX has been shown to occur under
anaerobic, denitrifying conditions using shallow aquifer material that
had previously been exposed to BTX. o-Xylene and m-xylene were
found to be degraded to 15% and 12%, respectively, of the initial
concentration (3 mg/litre) after 62 days with added nitrate (Major et
al., 1988). Much less degradation occurred under anaerobic conditions
in the absence of added nitrate (73% o-xylene remained after 62 days
and 59% m-xylene remained after 62 days). These losses were not
considered to be significant when compared with sterile controls.
Up to 0.4 mM (42.5 mg/litre) m-xylene was found to be rapidly
mineralized in a laboratory aquifer column operated in the absence of
molecular oxygen with nitrate as an electron acceptor. Quantitative
(80%) oxidation of m-xylene to carbon dioxide occurred with
concomitant reduction of nitrate. The column was inoculated with
denitrifying river sediment that had been continuously fed m-xylene
for several months (Zeyer et al., 1986).
4.2.2 Abiotic degradation
The xylene isomers are readily degraded in the atmosphere,
photooxidation being the most important degradation process.
4.2.2.1 Photolysis
Xylenes do not absorb UV-visible radiation appreciably at
wavelengths longer than 290 nm. This means that they are unlikely to
be directly photolysed in the troposphere or in solution, as the ozone
layer absorbs wavelengths shorter than 290 nm. Experiments using
xylenes adsorbed on silica gel have shown that the photomineralization
rates for all three isomers are low using radiation with a wavelength
longer than 290 nm (Gab et al., 1977).
4.2.2.2 Atmospheric oxidation
Atmospheric oxidation of xylenes is rapid and proceeds via
free-radical chain processes. The most important oxidant is the
hydroxyl radical, but xylenes will also react with other species found
in the atmosphere, such as alkoxy radicals, peroxy radicals, ozone and
nitrogen oxides. The most likely reaction pathways occurring in the
atmosphere are hydroxyl radical addition to the aromatic ring and
hydrogen abstraction from the alkyl groups by hydroxyl radicals (Gery
et al., 1987), although reaction with nitrate radicals may become
important at night (Grosjean, 1990).
Estimates for the lifetime of xylenes in the atmosphere have been
made from smog chamber experiments and from knowledge of the rate
constant for reaction with hydroxyl radicals. Atkinson (1985)
reviewed the available hydroxyl radical reaction rate constant data
and recommended kOH values at 25°C of 1.47 × 10-11 cm3 × molecule-1
× s-1 for reaction with o-xylene, 2.45 × 10-11 cm3 × molecule-1 ×
s-1 for reaction with m-xylene and 1.52 × 10-11 cm3 × molecule-1 ×
s-1 for reaction with p-xylene.
Based on hydroxyl radical reaction rate constant data,
atmospheric lifetimes of 2.6 h for o-xylene, 1.5 h for m-xylene
and 2.4 h for p-xylene have been calculated in south-east England
(Brice & Derwent, 1978).
Lifetimes in the boundary layer of the atmosphere have been
calculated by Singh et al. (1986). Using hydroxyl radical reaction
rate constants, lifetimes of 9 sunlight hours for o-xylene, 5
sunlight hours for m-xylene and 10 sunlight hours for p-xylene
were estimated. Singh et al. (1983) estimated that around 71.3% loss
of o-xylene, 87% loss of m-xylene and 67% loss of p-xylene would
occur per day (12 sunlight hours) as a result of reaction with
hydroxyl radicals.
An important point to consider with this data is that the
calculated lifetime depends on several factors, including temperature,
and also the actual concentration of hydroxyl radicals. It is known
that the concentration of hydroxyl radicals depends greatly on the
amount of sunlight available. Thus typical figures are around 2 ×
106 molecules/cm3 in summer months, falling by approximately a
factor of 2 in the winter months (Singh et al., 1986). At night the
concentration of hydroxyl radicals is negligible. Even so, it can be
seen that xylenes are removed from the atmosphere quite readily by
reaction with hydroxyl radicals.
It is possible that xylenes will be removed from aquatic systems
by similar types of reactions, as hydroxyl radicals are known to exist
in aquatic systems (Mansour et al., 1985).
The reaction of xylene isomers with NO3 radicals has been
studied. The second-order reaction rate constants measured were:
o-xylene, k = 3.74 × 10-16 cm3 × molecule-1 × s-1; m-xylene,
k = 2.49 × 10-16 cm3 × molecule-1 × s-1; and p-xylene, k = 4.49 ×
10-16 cm3 × molecule-1 × s-1. NO3 radicals have been measured in
the lower troposphere during night time hours but photodecomposition
occurs during daylight at a wavelength of 600 nm. Typical
concentrations of NO3 radicals found during the night are 2.4 × 108
molecules/cm3 in a clean atmosphere and 2 × 109 molecules/cm3 in a
moderately polluted atmosphere (Sabljic & Güsten, 1990). Using these
concentrations, the following half-lives for the reaction of xylene
with NO3 radicals at night have been estimated: o-xylene, 15-89
days; m-xylene, 23-194 days; and p-xylene, 13-107 days. These
half-lives are much longer than those for the daylight reaction with
hydroxyl radicals, but indicate that removal of xylenes from the
atmosphere could still occur at night by this route, especially in
polluted atmospheres.
The xylenes are sufficiently susceptible to photochemical
oxidation in the lower atmosphere that they may contribute to
tropospheric ozone formation. Derwent & Jenkin (1990) calculated
POCPs (Photochemical Ozone Creation Potentials) for xylenes of 41
( o-xylene), 78 ( m-xylene) and 63 ( p-xylene). The POCP values
reflect the ability of a substance to form low-level ozone as a result
of its atmospheric degradation reactions, the POCP values being
calculated relative to ethylene (a chemical that is thought to be
important in low-level ozone formation and is given a POCP of 100) on
a unit mass emission basis.
4.2.2.3 Hydrolysis
It is considered unlikely that xylenes will hydrolyse under the
conditions found in the natural environment.
4.2.3 Bioaccumulation
Octanol-water partition coefficients of 3.12, 3.20 and 3.15
(log values) have been determined for o-xylene, m-xylene
and p-xylene, respectively. These values indicate that slight
bioaccumulation could take place in the environment. Using these
values, bioconcentration factors (BCFs) of 138 (2.14) for o-xylene,
158.5 (2.20) for m-xylene and 144.5 (2.16) for p-xylene (log
values are given in parentheses) can be estimated using the formula of
Veith et al. (1980).
Bioconcentration factors (BCFs) of 21.4 (1.33 log value) for
o-xylene and 23.6 (1.37 log value) for combined m-xylene and
p-xylene have been measured in the eel (Anguilla japonica). The
half-life for elimination of m-xylene and p-xylene from the flesh
after exposure had ceased was 2.6 days (Ogata & Miyake, 1979).
Bioconcentration factors have been measured for all three isomers
in the goldfish. The reported BCFs were 14.1 (1.15) for o-xylene,
14.8 (1.17) for m-xylene and 14.8 (1.17) for p-xylene (log values
are in parentheses) (Ogata et al., 1984).
After exposure to the water-soluble fraction of Cook Inlet crude
oil ( o-xylene concentration 0.14 mg/litre; m-xylene concentration
0.15 mg/litre) for 8 days, concentrations of 0.87 mg/kg o-xylene
and 0.90 mg/kg m-xylene were found in the Manila clam (Tapes
semidecussata). The concentrations in the clam were found to
decrease rapidly during the first 7 days after exposure ceased (Nunes
& Benville, 1979).
A BCF of 9 for mixed xylenes was measured in both the thorax and
abdomen of the adult spot shrimp (Pandelus platyceros) when it was
exposed to a water-soluble fraction of Prudhoe Bay crude oil (Sanborn
& Malins, 1980).
The low BCFs indicate that biomagnification of xylenes through
the aquatic food chain is unlikely.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Owing to analytical difficulties it is often not possible to
quantify m-xylene and p-xylene individually in environmental
samples. As a result, a large proportion of the measured levels of
these two isomers refer to the combined total of the two.
5.1.1 Ambient Air
Measured levels of o-xylene and the combined m-xylene and
p-xylene levels are shown in Table 2. Levels of xylene for indoor
air are given in section 5.2.
Typically, background levels of all three xylene isomers are
around 1 µg/m3. Higher levels have been measured in urban areas,
showing that vehicle emissions are a significant source of xylenes.
Six studies giving measured levels for m-xylene and p-xylene
as individual compounds have been reported. One study gave measured
levels in Florida, USA, of < 0.09 to 5.5 µg/m3 for m-xylene and
< 0.09 to 2.4 µg/m3 for p-xylene (Lonneman et al., 1978). Another
gave levels of 0.019 to 2.66 µg/m3 for m-xylene and 0.018 to 1.19
µg/m3 for p-xylene in the Black Forest, Germany (Jüttner, 1988a),
while another reported levels of 92.6 µg/m3 for m-xylene and 39.7
µg/m3 for p-xylene in Zurich, Switzerland (Grob & Grob, 1971).
Levels of 11.94-25.74 µg/m3 m-xylene and 5.33-11.14 µg/m3
p-xylene were reported for two cities in Taiwan (Hung & Liao, 1991).
Average values of 1.5-6.2 µg/m3 m-xylene and 0.44-2.6 µg/m3
p-xylene were found in the Netherlands, with maximum values of 11-70
µg/m3 m-xylene and 4.9-15.9 µg/m3 p-xylene (Guicherit &
Schulting, 1985). Kawata & Fujieda (1993) monitored xylene
concentrations in the air of Niigata, Japan, in 1991 and 1992. At an
urban location, mean m-xylene and p-xylene concentrations were 4.8
and 2 µg/m3, respectively. The m-xylene and p-xylene
concentrations at a rural location were 1.8 and 0.7 µg/m3,
respectively.
Svanberg et al. (1995) measured xylene levels in the air of 17
Swedish towns during the winters of 1992-1993 and 1993-1994. Mean
concentrations ranged from 17 to 47 µg/m3 and from 11 to 41 µg/m3
for m/p-xylene for the two winters, respectively, and from 18 to 53
µg/m3 and 12 to 44 µg/m3 for o-xylene.
High levels of total xylene have been measured in air samples
from within landfill sites in the United Kingdom (Young & Parker,
1983). Levels of between 36 and 77 mg/m3 were reported in domestic
landfills, with higher levels (actual levels not reported) being found
in some industrial waste landfills.
Table 2. Mean measured levels of o-xylene and m/p-xylenes in ambient air
Location o-Xylene m/p-Xylene Reference
levels levels
(µg/m3) (µg/m3)
Sydney, Australia 6.63 17.2 Nelson & Quigley (1982)
Sweden
near car factory 38 264 Petersson (1982)
1 km from the factory 11.6 85.1
background 0.21 0.58
Stockholm, Sweden
busy streets 251 and 91 Jonsson et al. (1985)
calm streets 28.3 and
15.9
The Netherlands 0.88 - 3.1 Guicherit & Schulting
max (1985)
7.5 - 22.5
Black Forest, Germany 0.024-1.77 Jüttner (1988a)
Hamburg, Germany
12 sites 4.5-15.2 Bruckmann et al. (1988)
Los Angeles, USA 28.7 Altshuller et al. (1971)
USA
car painting planta 52 158 Sexton & Westberg (1980)
9 miles from the plant 4.5 14.5
background 0.5 2.5
USA, 10 cities 2.48-8.35 4.11-19.95 Singh et al. (1983)
USA, 5 cities ND-5.24b 1.03-14.64 Sheldon et al. (1988)
Table 2. (Cont'd)
Location o-Xylene m/p-Xylene Reference
levels levels
(µg/m3) (µg/m3)
Raleigh, USA, near to 1.9-7.6 5-19.9 Chan et al. (1991b)
roads
USA, 6 cities 2.78-25.25 1.9-13.1 Singh et al. (1986)
USA
urban 5.2 1.2 Brodzinsky & Singh (1983)
rural 0.41 0.38
Taiwan
2 cities 7.14 and 15.17 Hung & Liao (1991)
Niigata, Japan
urban 2 Kawata & Fujida (1993)
rural 0.83
Finland
industrial 207 568 Kroneld (1989)
urban 0.143 0.392
Grenoble, France
winter 1.9 22.9 Foster et al. (1991)
summer 2.4 28.7
Vienna, Austria
streets 24.0 50.8 Lanzerstorfer & Puxbaum (1990)
suburbs 6.2 10.4
backgrounds 1.9 3.9
Table 2. (Cont'd)
Location o-Xylene m/p-Xylene Reference
levels levels
(µg/m3) (µg/m3)
United Kingdom
urban 5.43 12.2 Clark et al. (1984)
rural 0.75 2.2
Southampton, United Kingdom
urban 12 27 Bevan et al. (1991)
busy roads 33 69
common land 5 6
Harwell, United Kingdom 2.4 3.9 Jones (1988)
max 15.8 max 34.4
a Based on individual samples
b ND not detectable (detection limits not states)
5.1.2 Water and sediment
5.1.2.1 Surface water
Levels of the individual xylene isomers measured in surface water
are shown in Table 3. Typically, background levels of xylenes in
surface waters are low (< 0.1 µg/litre). Much higher levels have
been measured in some industrial areas and areas associated with the
oil industry. Wiesenburg et al. (1981) measured xylenes in brine from
an oil production platform in the Gulf of Mexico. Two samples
contained 480 and 1800 µg/litre of m/p-xylene and 500 and 1900
µg/litre of o-xylene. Samples were taken from an underwater vent
plume from offshore oil production operations in the same region.
Xylene concentrations in the surface water were 0.270 µg/litre for
m/p-xylene and 0.06 µg/ litre for o-xylene. Water from the
discharge pipe contained 2060 µg/litre of m/p-xylene and 1510
µg/litre of o-xylene.
It has been reported that motor boats could be a significant
source of xylenes in surface water. Measurements were carried out in
an entrance canal to a harbour on Lake Constance both before (early
morning) and during boat movement on the lake. Levels recorded before
boat movements were o-xylene 18 ng/litre, m-xylene 17 ng/litre and
p-xylene 39 ng/litre. Levels recorded during the rest of the day
were o-xylene 57-481 ng/litre, m-xylene 76-750 ng/litre and
p-xylene 62-416 ng/litre. In general, the levels of xylene
increased as the number of boats passing the sampling point increased
(Jüttner, 1988b).
Xylenes were surveyed in surface water in Japan in 1977, 1985 and
1986. No xylene isomers were detected in 1977 (detection limit = 2
µg/litre). In 1985 one out of 21 samples contained xylene at
concentrations of 0.021, 0.042 and 0.037 µg/litre for o-, m- and
p-xylene, respectively (detection limit = 0.02 µg/litre). In 1986,
the concentrations of o- and m-xylene ranged from 0.04 to 1.2
µg/litre in 12 out of 137 samples and 15 out of 126 samples, for the
two isomers respectively. p-Xylene was detected in 4 out of 122
samples at concentrations ranging from 0.06 to 0.48 µg/litre
(detection limit = 0.03 µg/litre) (EAJ, 1993).
5.1.2.2 Groundwater
Table 3 shows levels of xylene measured in groundwater.
Typically, background levels of xylenes in aquifers are low (< 0.1
µg/litre). High levels have been reported in contaminated aquifers.
The migration of petroleum products from leaking underground storage
tanks and pipelines poses a groundwater contamination problem.
Gasoline-contaminated groundwater in Los Angeles, USA, contained
xylene at a concentration of 153 µg/litre (Karlson & Frankenburger,
1989).
Very high levels of o-xylene (4001 µg/litre) and m/ p-xylene
(5385 µg/litre) have been measured in a polluted aquifer in Italy.
Water was taken from a well at a depth of 30 m and the pollution was
thought to be due to leakage from underground solvent storage tanks
(Botta et al., 1984).
5.1.2.3 Precipitation
Kawamura & Kaplan (1983) measured xylene in rainwater in Los
Angeles, USA, during 1982. An m-xylene concentration of 0.002
µg/litre and a p-xylene concentration of 0.009 µg/litre were
reported.
Table 3. Levels of xylene in water
Location Isomera Level (µg/litre)b References
Surface water
River Lee, UK T detected at a level Waggot (1981)
of > 0.1
River Besós, Spain m/p 24 Gomez-Belinchon et al.
(polluted) ortho 8.1 (1991)
River Llobregat, Spain m/p 4.7 Gomez-Belinchon et al.
(polluted) ortho 0.83 (1991)
Seawater
Off River Humber, UK T < 0.001-29.0 MAFF (1991)
Dredged spoil disposal site T < 0.001-0.330
Sewage sludge disposal site T < 0.001
North Sea, off UK coast T < 0.01-0.250 Hurford et al. (1990)
River Tees estuary, UK m/p < 0.05-1.1 Harland et al. (1985)
ortho < 0.05-1.1
Coastal site, USA m/p 0.0045-0.066 Gschwend et al. (1982)
ortho 0.0018-0.025
Barcelona, Spain m/p 0.015-0.072 Gomez-Belinchon et al.
ortho 0.004-0.210 (1991)
Gulf of Mexico: T 0.002-0.056 McDonald et al. (1988)
river mouth
Gulf of Mexico: 0.001
chemical outfall
Inner harbour of the T 0.04-0.2 McFall et al. (1985)
navigation canal of
Lake Pontchartrain, USA
Table 3. (Cont'd)
Location Isomera Level (µg/litre)b References
Wastewater
Effluent samples from a T 1.82 Kennicut II et al. (1984)
Barceloneta waste
treatment facility, Puerto
Rico (mainly pharmaceutical
in origin)
Wastewater treatment para influent = 4.40 Michael et al. (1991)
plant, Great Lakes Basin effluent = < 1
Groundwater
British aquifers para occasionally Kenrick et al. (1985)
(uncontaminated sites detected at
thought to represent 0.001-0.02
background levels) ortho detected in 19
out of 32 samples.
highest = 0.02
mean = 0.011
Groundwater, near para ND-0.5c Barker et al. (1988)
landfill site, Hamilton, ortho 0.03-0.5
Ontario, Canada
Edwards aquifer, Texas, T up to 0.08 Buszka et al. (1990)
USA
Groundwater near m/p NDd-50 Slain & Baker (1990)
bituminous layers of ortho NDd-21
shale in rock, near a
sanitary landfill site,
Ontario, Canada
Table 3. (Cont'd)
Location Isomera Level (µg/litre)b References
Groundwater near an m/p 240-830 Stuermer et al. (1982)
underground coal ortho 260-590
gasification site in (background was
the USA below the limit of
detection of
0.5 µg/litre)
Groundwater, New Jersey/ T 59-300 Rao et al. (1985)
New York, USA
a m/p = combined m-xylene and p-xylene; T = total xylene
b ND = not detected
c detection limit not stated
d detection limit = 2 µg/litre
5.1.2.4 Leachate
Barker et al. (1988) measured o- and p-xylene in the leachate
from a landfill in Hamilton, Ontario, Canada. Xylene concentrations
ranged from 30.8 to 123 µg/litre for the ortho isomer and from 12.5 to
191 µg/litre for the para isomer. Leachate from a landfill in
Minnesota, USA, contained m-xylene concentrations ranging from 21 to
150 µg/litre and o/p-xylene concentrations ranging from 12 to 170
µg/litre. Both xylene isomers were presented in all six samples
collected (Sabel & Clark, 1984).
5.1.2.5 Sediment
Tynan et al. (1991) measured levels of xylene in sediment samples
taken in Wales, United Kingdom, of up to 23.4 µg/kg and 21.2 µg/kg for
o-xylene and p-xylene, respectively. Harland et al. (1985)
reported o-xylene levels of 0.6-3.9 µg/kg and combined m/p-xylene
levels of 3.4-250 µg/kg in sediment from the River Tees estuary,
England.
Xylenes were surveyed in sediment in Japan in 1977, 1985 and
1986. No xylene isomers were detected in 1977 (detection limit = 4
µg/kg). In 1985 one out of 21 samples contained o- or m-xylene at
concentrations of 1.1 and 2 µg/kg respectively; no p-xylene was
detected (detection limits = 0.6 µg/kg for o-xylene, 1 µg/kg for
m-xylene and 2 µg/kg for p-xylene). In 1986 o-, m- and
p-xylene concentrations ranged from 0.5 to 7 µg/kg (detected in 24
out of 111 samples), 0.5 to 15 µg/kg (detected in 33 out of 118
samples) and 0.5 to 3.8 µg/kg (detected in 12 out of 105 samples), for
the three isomers respectively (detection limit = 0.5 µg/kg) (EAJ,
1993).
5.1.3 Soil
Levels of 0.15 g/kg ( o-xylene) and 0.4 g/kg ( m- and
p-xylene) have been measured at a depth of 75-250 cm in soil
from a gasoline station. The soil was thought to be contaminated
as a result of leakage from an underground storage tank (Morgan &
Watkinson, 1990).
5.1.4 Biota
Xylenes have been measured at levels of 16 µg/kg wet weight in
oysters from Lake Pontchartrain, Louisiana, USA (Ferrario et al.,
1985). Levels of combined m- and p-xylene have been measured in
fish and shellfish from three estuarine sites in the USA (Reinert et
al., 1983). The levels found were: silverside (Menidia menidia) 100
and 180 µg/kg, ribbed mussel (Modiolus demissus) 100 µg/kg, and
grass shrimp (Palaemonetes pugio) 200 µg/kg.
Xylenes were monitored in fish during 1986 in Japan. o-Xylene
concentrations ranged from 0.8 to 5 µg/kg in 41 out of 137 samples,
m-xylene concentrations ranged from 0.86 to 9.2 µg/kg in 45 out of
124 samples and p-xylene concentrations ranged from 0.8 to 3 µg/kg
in 28 out of 127 samples (detection limit = 0.8 µg/kg for all three
isomers) (EAJ, 1993).
5.2 General population exposure
5.2.1 Source of Exposure
5.2.1.1 Air
Otson et al. (1993) pooled aliquots of individual air sample
extracts from 757 randomly selected Canadian residences. The
composite sample contained o-, m- and p-xylene concentrations of
8, 7 and 6 µg/m3, respectively. Fellin & Otson (1993) studied the
seasonal trends of xylene concentrations in the indoor air of 754
randomly selected Canadian homes. Lowest mean concentrations were
found in the summer and the highest in the autumn. Mean
concentrations ranged from 3.73 to 9.12 µg/m3 for p-xylene, from
6.81 to 26.03 µg/m3 for m-xylene and from 3.03 to 8.19 µg/m3 for
o-xylene. Lioy et al. (1991) monitored indoor and outdoor air at
three homes in New Jersey, USA during 1987. Indoor concentrations
ranged from 6.0 to 20.5 µg/m3 for o-xylene and from 15.2 to 57.5
µg/m3 for p-xylene. Outdoor concentrations ranged from 1.6 to 12.7
µg/m3 for o-xylene and from 4.6 to 36.9 µg/m3 for p-xylene.
Weschler et al. (1990) monitored xylene concentrations in the air
of a building with a history of occupant health and comfort
complaints. o-Xylene concentrations ranged from 2.1 to 9 µg/m3 and
m/p-xylene concentrations ranged from 3.9 to 25 µg/m3. The highest
xylene concentrations were associated with the lift shaft.
The California Total Exposure Assessment Methodology (TEAM) study
conducted in 1984 monitored xylenes in outdoor air, personal air and
breath samples for 188 people in Los Angeles County (urban) and
Contra Costa County (rural area). The 12-h arithmetic means of the
xylene concentrations are summarized in Table 4. A second TEAM study
was carried out in 1987 with 51 residents of Los Angeles, California.
The 24-h arithmetic means of the xylene concentrations in this study
are also summarized in Table 4. Daisey et al. (1994) reported the
concentrations of xylenes in the air in 12 office buildings in
California. The concentration of o-xylene ranged from 1.3 to 6.1
µg/m3 (0.30 to 1.40 ppb) and that of m/p-xylene from 4.0 to 20.0
µg/m3 (0.93 to 4.60 ppb).
Levels measured during winter were higher than summer levels for
all types of air. Smoking was determined to be the major determinant
for the presence of xylene in breath and personal air; concentrations
in the breath of smokers were more than double those of nonsmokers.
At petrol stations, exposure to vehicle exhaust and the type of
employment contributed significantly to increased concentrations of
xylene in breath and personal air (Wallace et al., 1988). Higgins et
al. (1983) reported that the gas-phase delivery of p-xylene in
ultra-low tar delivery cigarette smoke ranged from < 0.01 to 8
µg/cigarette, while the ranges for m- and o-xylene were < 0.01 to
20 µg/cigarette and < 0.005 to 10 µg/cigarette, respectively.
Table 4. Mean xylene concentrations in personal air, outdoor air and breath samples
(Wallace et al., 1988, 1991)
Location Date Sample type m/p-Xylene o-Xylene
(µg/m3) (µg/m3)
Los Angelesa February 1984 personal airc 28 13
outdoor air 24 11
breath 3.5 1.0
Los Angelesa June 1984 personal air 24 7.2
outdoor air 9.4 2.7
breath 2.8 0.7
Contra Costaa June 1984 personal air 11 4.4
outdoor air 2.2 0.7
breath 2.5 0.6
Los Angelesb February 1987 personal air 43 16
indoor aird 30 12
outdoor aire 18 6.5
breath (median value) 2.5 0.8
Los Angelesb July 1987 personal air 27 9.2
indoor aird 12 4.3
outdoor aire 7.4 2.8
breath (median value) 0.7 0.25
a 12-h arithmetic means of xylene concentration
b 24-h arithmetic means of xylene concentration
c Air sample collected at the breathing level of the subjects
d samples collected in living room-kitchen area
e samples collected in backyards of homes
Weisel et al. (1992) analysed air within automobiles whilst
idling, driving on a suburban route in New Jersey and commuting into
New York City. During a 30-min idling period, mean m/ p-xylene
concentrations ranged from 1.3 to 42 µg/m3 and o-xylene
concentrations ranged from 0.5 to 18 µg/m3. The highest values were
recorded during the summer and the lowest during the winter. The mean
m/ p-xylene concentrations for the suburban route were 23 and 16
µg/m3 for low and high ventilation, respectively; for o-xylene mean
concentrations were 8.6 and 7.5 µg/m3. Xylene concentrations of 23
µg/m3 for m/p-xylene and 8.8 µg/m3 for o-xylene were recorded
whilst commuting into New York City; mean xylene levels of 37 µg/m3
( m/ p-xylene) and 12 µg/m3 ( o-xylene) were measured whilst
travelling through a tunnel. Chan et al. (1991) studied exposure of
commuters in Boston, USA, to xylenes and found that the highest
exposures were associated with commuting by car ( m/ p-xylene = 20.9
µg/m3; o-xylene = 7.3 µg/m3).
The levels of xylenes in samples of air in the vicinity of petrol
pumps in five Canadian cities were monitored between June and August
1985 and between January and March 1986. Measured mean concentrations
of all the isomers of xylene in the immediate vicinity of self-service
pumps were 0.716 mg/m3 in the winter and 0.973 mg/m3 in the summer,
and ranged from 0.678 to 3.77 mg/m3 and 0.001 to 6.9 mg/m3,
respectively (PACE 1987; 1989). p-Xylene represented more than 70%
of the mean concentrations for all isomers.
van Wijnen et al. (1995) monitored ambient air for traffic-related
pollutants in Amsterdam. Maximum mean time-weighted concentrations
were as high as 193 µg/m3 for car drivers, 46 µg/m3 for cyclists
and 41 µg/m3 for pedestrians.
A mean total xylene level of 65 µg/m3 (15.06 ppb) in ambient air
was measured in Turin, Italy, throughout 1991 (Gilli et al., 1994).
Within a 10-day sampling period, mean concentrations of 85 and 57
µg/m3 (19.50 and 13.13 ppb) were measured in indoor air for day-
and night-time sampling, respectively. The corresponding mean
concentrations for outdoor air were 82 and 54 µg/m3 (18.82 and 12.31
ppb) for day- and night-time sampling respectively. The mean personal
exposure of the volunteers was 84 µg/m3 (19.30 ppb).
Monitoring for xylene has been carried out at filling stations
in Rome, Italy (Lagorio et al., 1993). The range of measured
concentrations from 703 personal samples among 111 workers was 0.003
to 15.37 mg/m3 (mean: 0.32 mg/m3).
Bostrom et al. (1994) calculated the average exposure dose for
xylenes (o, m and p) to be 11 µg/m3, based on the relationship
between nitrogen oxides (NOx) and xylenes, and a mean exposure for
the Swedish population of 23 µg/m3 for nitrogen oxides.
The exposure of students commuting to school in Taipei City,
Taiwan, in 1992 has been reported by Chan et al. (1993). Students
commuting by bus were exposed to 222.8 µg/m3 of o-xylene and 418.1
µg/m3 of m/p-xylene. Students commuting by motorcycle were exposed
to 524.5 µg/m3 of o-xylene and 926.9 µg/m3 of m/p-xylene. The
air in school classrooms was also monitored; the mean concentration
of o-xylene was 26.3 µg/m3 and that of m/p-xylene was 46.4 µg/m3.
5.2.1.2 Food
o-Xylene has been detected at levels up to 25 µg/kg (mean level
9 µg/kg) in seven samples of dried beans, at a level of 8 µg/kg in
split peas and at a level of 3 µg/kg in lentils from the USA (Lovegren
et al., 1979). Xylenes have been identified but not quantified in
various other food items including cheese from Italy (Meinhart &
Schreier, 1986), dry red beans from the USA (Buttery et al., 1975),
winged beans and soybeans from the Philippines (del Rosario et al.,
1984) and tomatoes and tomato products from Japan (Chung et al.,
1983). All three isomers were detected in the volatile compounds from
roasted turkeys fed on a basal diet supplemented with tuna oil
(Crawford & Kretsch, 1976).
5.2.1.3 Drinking-water
All three xylene isomers were detected in all of 14 samples of
United Kingdom drinking-water derived from rivers, lowland reservoirs
and groundwater (detection limit not stated) (Fielding et al., 1981).
Xylenes have been shown to pass through a drinking-water treatment
plant unaltered in concentration (Dowty et al., 1975).
Otson et al. (1982) monitored 30 Canadian potable water treatment
facilities. Mean total xylene concentrations in both raw and treated
water were less than the detection limit in this study (1 µg/litre).
Maximum values were less than 1 µg/litre for raw water and 8 µg/litre
for treated water.
When Williams et al. (1982) sampled 12 Great Lakes (Canada)
municipal drinking-water supplies, o- and m-xylenes ( m-isomers)
were not detected at five of the sites and in 50% of the 22 samples.
Detectable concentrations ranged from 1.1 to 12 µg/litre.
The concentration of m- and p-xylene in tap water, Toronto,
Canada, was reported to be 0.06 µg/litre (City of Toronto, 1990). The
concentrations of xylene in seven samples of bottled water ranged from
less than the detection limit to 0.07 µg/litre.
5.2.1.4 Other source of exposure
The US EPA (Sack et al., 1992) carried out analyses of 1159
household products. The results of the analyses, according to product
category, are presented in Table 5.
5.2.2 Xylene levels in human biological samples
Xylenes have been detected in human blood at levels of between
0.5 and 160 µg/litre (mean = 5.2 µg/litre) (Antoine et al., 1986).
The level was found to be significantly elevated in 7 out of 250
people sampled. m-Xylene has been determined in human whole blood
at levels of 10-20 ng/litre (Cramer et al., 1988).
Table 5. Mean xylene concentrations in various products
m-Xylene o/p-Xylene
Product category Number of Products containing Mean concentration Products containing Mean concentration
products tested analyte (%) (% w/w) analyte (%) (% w/w)
Automotive 167 26.7 10.6 10.0 31.0
Household cleaners and 111 33.3 1.4 - -
polishers
Paint-related products 463 60.3 4.2 58.2 2.8
Fabric and leather 91 - - 33.3 0.1
treatments
Cleaners from electronic 69 - - - -
equipment
Oils, greases, lubricants 111 9.3 0.2 11.9 0.2
Adhesive-related products 76 9.1 0.2 9.1 0.2
Miscellaneous: 71 - - - -
specialized cleaners, rust
remover, correction fluid
Ashley et al. (1994) monitored blood samples from more than 600
people in the USA third national health and nutrition examination
survey. None of the subjects were occupationally exposed to xylenes.
Mean concentrations were 0.37 µg/litre for m/ p-xylene and 0.14
µg/litre for o-xylene.
Fustinoni et al. (1995) reported that the mean blood
concentration of m/p-xylene in non-smoking traffic wardens in Milan,
Italy, was 853 ng/litre before the shift and 683 ng/litre at the end
of the shift. The level in non-smokers of the clerical environment
from the same area was 809 and 629 ng/litre, respectively.
Xylenes were detected, but not quantified, in 8 out of 12 samples
of breast milk (Pellizzari et al., 1982).
Xylenes have been detected in the axilla odour from humans
(Labows et al., 1979). Placental transfer of xylene has been shown to
occur (Dowty & Laseter, 1976).
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure to xylenes alone is rare. There is usually
simultaneous exposure to other compounds, often organic solvents. In
one study, however, 10 female laboratory workers had been exposed to
xylene (vapour as well as liquid) for about 4 h daily for up to 16
years. Exposure levels, determined by only one measurement in the
breathing zone and by only one in the workroom air, were 139 mg/m3
(32 ppm) and 62 mg/m3 (14 ppm), respectively (Proust et al., 1986).
A number of studies have been performed on workers occupationally
exposed to solvent mixtures including xylenes (e.g. Seppäläinen et
al., 1978; Elofsson et al., 1980; Husman, 1980; Lindström et al.,
1982; Valciukas et al., 1985; Maizlish et al., 1987; Van Vliet et al.,
1987).
In a study on spray varnishers (Angerer & Wulf, 1985) 35 male
workers were exposed to 2.2-14.8 mg/m3 (0.5-3.4 ppm) o-xylene,
13.9-50.1 mg/m3 (3.2-11.7 ppm) m-xylene, 3.9-18.7 mg/m3 (0.9-4.3
ppm) p-xylene, 1.4-7.5 ppm ethylbenzene, < 1.5 ppm toluene, <1.2
ppm n-butanol, < 35.5 ppm 1,1,1-trichloroethane and several C9
aromatic compounds.
Mean 8-h xylene concentrations of 21.7 and 27.8 mg/m3 (5 and 6.4
ppm) were measured in a lacquer/resin spraying operation in a
woodworking facility in the USA (Fairfax, 1995).
In a study on workers engaged in dip-coating of metal parts the
mean xylene vapour concentration was 16.5 mg/m3 (3.8 ppm). The
concentration of the isomers were 3.5 mg/m3 (0.8 ppm) o-xylene, 9.1
mg/m3 (2.1 ppm) m-xylene and 3.9 mg/m3 (0.9 ppm) p-xylene (Kawai
et al., 1991a).
In a study on workers employed in printing, painting or the
manufacture of plastic coated wires, there was an exposure to mixtures
of toluene and xylenes. Personal air samples were collected and
analysed for toluene and the three isomers of xylene. Maximum
exposures to xylenes were > 435 mg/m3 (100 ppm) with a time-weighted
average of 17.4 mg/m3 (4 ppm). About half of the xylene was
m-xylene (Huang et al. 1994).
Concentrations of xylenes ranging from 0.4 to 7.0 mg/m3 (0.1 to
1.6 ppm) were measured during full-shift personal exposure monitoring
at an axle painting operation in Newark, Ohio, USA (NIOSH, 1991).
Breathing zone samples were collected from workers in the liquid
inks and paste inks departments of a small printing ink manufacturing
factory. The mean full shift concentration of xylene in the liquid
inks department was 314 mg/m3 and in the paste inks department 24
mg/m3. Other solvents in the factory were toluene, ethyl acetate,
ethanol, isopropanol and n-hexane (Lewis, 1994).
During paint operations in an aeronautical factory xylene
concentrations (sum of the three isomers) of 14.3 to 167.0 mg/m3 were
measured by personal air monitoring. Other solvents present were
methyl ethyl ketone, ethyl acetate, n-butyl alcohol, methyl isobutyl
ketone, toluene, n-butyl acetate, ethylbenzene, ethylene glycol and
monoethylether acetate (Vincent et al., 1994). Similarly,
concentrations of xylene (isomer not specified) among printers have
been measured to be between 5.6 and 91.3 mg/m3 (1.3 and 21 ppm).
Other solvents were acetone, ethyl acetate, methyl ethyl ketone, white
spirit, toluene and trichloroethylene (Nasterlack et al., 1994).
Levels of xylene to which workers have been exposed in
histological laboratories, measured as 8-h time-weighted average, are
from about 10.9 mg/m3 (2.5 ppm) to over 304.5 mg/m3 (70 ppm)
(Angerer & Lehnert, 1979; Kilburn et al, 1985; IARC, 1989). In a
hospital laboratory, levels of up to 1740 mg/m3 (400 ppm) have been
measured (Klaucke et al, 1982). In lithographical processes in Poland
the mean value in 1968 was 119 mg/m3 and ten years later 130 mg/m3
(Moszczynski & Lisiewicz, 1985), and in a chemical plant in Hungary
the mean concentration in air was 47-56 mg/m3 (Pap & Varga, 1987).
In a NIOSH study involving conducted environmental monitoring of
xylene in various worksites, the concentrations where workers were
exposed to gasoline and exhaustion emissions ranged from < 0.08 to
68.3 mg/m3 (0.02 to 15.7 ppm) (NIOSH, 1993a). The concentration in
the air in the cab ranged up to 8.7 mg/m3 (2 ppm) (NIOSH, 1993b).
The personal breathing zone samples of an indoor parking garage, a
medical taxi cab, an automobile dealership/repair shop and a state
highway maintenance garage in New York State ranged from < 0.08 to
1.87 mg/m3 (0.02 to 0.43 ppm) (NIOSH, 1993c). The concentration in
personal breathing zone samples from workers in automotive maintenance
facilities/dealers, repair shops) in Stamford, Connecticut, USA,
ranged from < 0.08 to 1.39 mg/m3 (0.02-0.32 ppm) (NIOSH, 1993d).
The highest full-shift exposure at an oil refinery in Colombia was
22.2 mg/m3 (5.1 ppm), and all short-term exposures were below the
quantifiable concentration of 1.3 mg/m3 (0.3 ppm) (NIOSH, 1994).
Kawai et al. (1991b) monitored the exposure of tank truck drivers
to xylenes in Japan. Maximum concentrations were 26.5 to 94.8 mg/m3
(6.1 to 21.8 ppm) during loading and 3.9 to 5.96 mg/m3 (0.90 to 1.37
ppm) during a delivery trip.
Fustinoni et al. (1995) measured the exposure of traffic wardens
to xylenes in Milan, Italy, in 1994. During a 10-day period, the
concentration of o-xylene was 48 µg/m3 and of m/p-xylene was 108
µg/m3. Concurrent monitoring of policemen in clerical environments
was also carried out; the concentration of o-xylene in indoor air
was 32 µg/m3 and of m/p-xylene was 53 µg/m3.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
6.1.1 In humans
In humans, absorption of xylenes has been investigated following
inhalation of the vapour or dermal application of the liquid.
Pulmonary retention of m-xylene became relatively constant at
about 60% after the first 5-10 min of exposure to 430 mg/m3 (100 ppm)
(Riihimäki et al., 1979a). The determination was made by measurement
of atmospheric and exhaled concentrations. In a previous study
(Sedivec & Flek, 1976) a pulmonary retention of 62-64% was reported
for each xylene isomer at exposure levels of 196-391 mg/m3 (45-90
ppm) for up to 7 h.
A relatively constant retention, average 59%, was reported in
individuals exposed to varying m-xylene concentrations in the range
of 304-957 mg/m3 (70-220 ppm), both at rest and while undergoing
intermittent physical exercise (Riihimäki et al., 1979b). A slight
reduction in retention was noted when resting individuals subsequently
underwent moderately heavy physical exercise. Increased pulmonary
ventilation during exercise was found to be associated with a
corresponding increase in total uptake of xylenes (Riihimäki et al.,
1979b; Åstrand et al., 1978).
In resting individuals exposed to 870 mg/m3 (200 ppm) xylene
(8.8% o-xylene, 49.4% m-xylene, 1.4% p-xylene, and 40.4%
ethylbenzene) the alveolar air level was about 15% of that in inspired
air, while 36% was recorded for individuals undergoing heavy exercise
(Åstrand et al., 1978). The ratio of m/p-xylene to ethylbenzene was
found to be similar in alveolar air and inspired air. This indicates
similar rates of pulmonary absorption for xylenes and ethylbenzene.
In this study, o-xylene was not measured (Engström & Bjurström,
1978).
During inhalation exposure in resting subjects, a levelling of
the blood xylene concentration began after about 15 min of exposure to
435-870 mg/m3 (100-200 ppm) xylene. Light exercise increased the
blood level of xylene and indications of plateauing were noted after
about 2 h (Åstrand et al., 1978). In another study, an exposure to
435-1261 mg/m3 (100-290 ppm) m-xylene revealed a rapid rise in
xylene blood levels during the first hour. Repeated exposure to 430
mg/m3 for 4.5 days (6 h/day) gave rising pre-exposure morning blood
levels, indicating some accumulation of m-xylene (Riihimäki et al.,
1979a; Riihimäki et al., 1982a,b).
Dermal absorption of xylenes has been studied after exposure to
the vapour or the liquid. Liquid xylenes (about 0.2 ml of the
individual isomers) were applied to the forearm. Uptake into the skin
was calculated by measuring the remained material after 5-15 min
(Dutkiewicz & Tyras, 1968). Uptake values of 50-160 µg/cm2 per min
were reported. However, it should be noted that not all the xylene
taken up necessarily penetrated the skin and was absorbed. In more
recent studies, dermal absorption has been studied following hand
immersion in liquid m-xylene for 15-20 min. Absorption, estimated
from the urinary level of the metabolite m-methylhippuric acid, was
recorded to be about 2 µg/cm2 per min in eight volunteers (Engström
et al., 1977). The amount absorbed (about 35 mg) through both hands
was estimated to be equal to the amount absorbed through inhalation of
435 mg/m3 (100 ppm) during the same time. Another group (Lauwerys et
al., 1978) obtained a similar value for dermal absorption (2.45
µg/cm2 per min) based on urinary levels of m-methylhippuric acid
and m-xylene levels in exhaled breath.
Dermal exposure of m-xylene vapour has been investigated in
volunteers exposed to 1305 mg/m3 (300 ppm) (two men) or 2610 mg/m3
(600 ppm) (three men) for 3.5 h. Inhalation was excluded by means of
a full facepiece supplied-air respirator with overpressure inside the
mask. The volunteers were dressed in pyjamas and performed exercise to
raise the skin temperature and perspiration (Riihimäki & Pfäffli,
1978). Dermal absorption appeared to be directly dependent on vapour
concentration. At 2610 mg/m3 the absorption was calculated to be
approximately 0.01 µg/cm2 per min. In a further experiment, three
subjects were exposed to 87 mg/m3 (20 ppm) without respirator. In
this case both pulmonary and dermal absorption could occur. The total
absorption of m-xylene in this experiment was calculated to be of
the same order of magnitude as after dermal-only exposure to 2610
mg/m3.
6.1.2 In laboratory animals
Absorption of xylene was recorded following whole-body exposure
of mice to ring-labelled 14C- m-xylene vapour for 10 min. Based on
autoradiograms the absorption was primarily through respiration
(Bergman, 1979; Bergman, 1983). All xylenes are well-absorbed orally
by rats, based on urinary metabolites. Peak blood levels were reported
4 h after an oral dose of 0.5-4 g m-xylene/kg body weight or 1.1 g
p-xylene/kg body weight (Gut & Flek, 1981).
The rate of absorption of liquid o-xylene across excised rat
skin has been calculated to be 0.103 µg/cm2 per min at steady state
(Tsuruta, 1982).
6.2 Distribution
6.2.1 In humans
Little information is available on the distribution of xylenes in
humans. A peritoneal fat/air partition coefficient of 3605 has been
determined for m-xylene in vitro (Sato et al., 1974). The times
required to reach equilibrium in tissues have been calculated from
physiological parameters. It is estimated to be a few minutes for
well-perfused parenchymal organs, a few hours for muscles and several
days for adipose tissue (Riihimäki & Savolainen, 1980).
Postmortem analysis on a woman who had swallowed xylene 4 days
prior to death, revealed xylene to be present in all tissues
investigated (Takatori et al., 1982). The ratios of the three isomers
(ortho: meta: para) were 3:5:2 in the stomach content, 3:6:1 in blood
and 4:4:2 in adipose tissue. In the brain, liver, spleen, kidney and
myocardium, however, the o-xylene accounted for about 80%.
When volunteers were exposed to 435 to 870 mg/m3 (100 to 200
ppm) mixed xylenes for 2 h, the ratio of m/p-xylene to ethylbenzene
was similar in subcutaneous fat and inspired air up to 22 h
post-exposure (Engström & Bjurström, 1978). In another study
volunteers were exposed to 391-870 mg/m3 (90-200 ppm) m-xylene 6 h
per day 5 days per week (plus an additional day after the weekend)
(Engström & Riihimäki, 1979). The proportion of absorbed m-xylene
distributed to subcutaneous fat was calculated to be about 4% in
resting individuals and 8% in those undergoing exercise.
Two adult male and two adult female volunteers were exposed by
inhalation to < 108-217 mg/m3 (25-50 ppm) of m-xylene (Laparé,
1993). Doubling the exposure concentration led to a proportional
increase in the concentrations of unchanged solvents in alveolar air
and blood at the end of a 7-h exposure period. Cumulative urinary
excretion of the metabolites exhibited a nearly proportional increase.
It is also suggested that alveolar air solvent concentration is a
reliable index of exposure to m-xylene.
6.2.2 In laboratory animals
The distribution of xylene has been studied in male rats exposed
to about 217 mg/m3 (50 ppm) 14C-labelled p-xylene for 8 h
(Carlsson, 1981). The highest concentrations were present in the
kidneys (up to about 1000 nmol/g tissue) and subcutaneous fat (up to
more than 250 nmol/g tissue). Higher concentrations than in blood
were also found in the ischiatic nerve. Lower concentrations than in
blood were found in the cerebrum, cerebellum, muscle and spleen.
Elimination half-times from fat were estimated to be 2-7 h. In
pregnant rats, o-xylene has been shown to cross the placenta. The
concentrations in fetal blood were 25-30% of that in maternal blood
after a 2-h exposure (Ungvary et al., 1980). o-Xylene was also
detected in amniotic fluid.
The concentration of m-xylene in perirenal fat and cerebrum was
positively correlated to exposure levels in rats exposed to 217 to
3262 mg/m3 (50 to 750 ppm) m-xylene 6 h/day, 5 days/week for 1-2
weeks (Savolainen & Pfäffli, 1980; Elovaara et al., 1982).
Accumulation in perirenal fat has also been shown in rats exposed to
1305 mg/m3 (300 ppm) xylene (80% m-xylene, 12% p-xylene) 6 h/day,
5 days/week for 1-2 weeks (Savolainen et al., 1979a,b). In a similar
study with 1305 mg/m3 m-xylene, no accumulation in perirenal fat or
brain tissue was recorded after one week (Elovaara et al., 1982).
When rats were exposed to 1305 mg/m3 xylene (19.2% o-xylene, 43.0%
m-xylene, 19.5% p-xylene, 18.3% ethylbenzene) during 6 h/day, 5
days/week for 18 weeks, a progressive increase of xylene levels in
perirenal fat was demonstrated over the first 2 weeks followed by a
decline (Elovaara et al., 1980). The decline was attributed to xylene
inducing its own metabolism. Similar results were obtained in a study
where rats were exposed to 1305 mg/m3 xylene (85% m-xylene, 15%
o-xylene) (Savolainen et al., 1979a,b).
The tissue distribution of 14C-labelled xylenes has been studied
in mice by low-temperature whole body autoradiography (Bergman, 1979;
Bergman, 1983; Ghantous & Danielsson, 1986). When male mice were
exposed to about 1435 mg/m3 (330 ppm) m-xylene for 10 min, high
levels of radioactivity was found immediately post-exposure in body
fat, bone-marrow, brain (white matter), spinal cord, spinal nerves,
liver and kidney. Radioactivity in the nervous system and fatty
tissues was due to xylene alone and was present for 1 and 8 h,
respectively. High levels of xylene metabolites were recorded in
blood, lung, liver and kidney for up to 8-h post-exposure and in
intestinal contents, bronchi and nasal mucosa up to 24 h (Bergman,
1979; Bergman, 1983).
Autoradiography of male mice following 10 min inhalation of
radioactively labelled p-xylene revealed an accumulation of
non-volatile metabolites in the nasal mucosa and the olfactory bulb of
the brain. It was assumed that the activity represented aromatic
acids (methyl hippuric acid and toluic acid) (Ghantous et al., 1990).
The same technique has been used to study distribution of
radioactivity after exposure of pregnant mice to about 8700 mg/m3
(2000 ppm) 14C-labelled p-xylene for 10 min (Ghantous & Danielsson,
1986). High concentrations of xylene were recorded in the adult brain
and lung with lesser amounts in kidney and liver. At all stages of
gestation studied (days 11, 14, 17) p-xylene appeared to pass
immediately from dam to embryo/fetus. The concentration in fetus,
however, was low; 2% of that in maternal brain. Xylene was evenly
distributed in the fetus following exposure on day 11. After exposure
on day 17 the xylene was located primarily in the liver (Ghantous &
Danielsson, 1986).
In a study where rabbits were exposed to xylene (27% o-xylene,
52% m-xylene, 21% p-xylene) for several months, the concentration
was reported to be higher than in blood in the adrenals, bone-marrow
and spleen (Fabre et al., 1960). Due to the limited nature of the
study it is impossible to draw any firm conclusions.
Partition coefficients have been determined in vitro for
m-xylene using tissue homogenates and blood (Sato et al., 1974).
The following blood/air values were reported: 20 (pig blood), 21
(rabbit plasma) and 37 (rabbit blood). Tissue/blood partition
coefficients reported were 1.6-2.1 (muscle, kidney, heart and lung),
3.0-3.3 (liver and brain), 42 (bone-marrow) and 146 (peritoneal fat).
The relatively low value for brain tissue has been attributed to the
content of phospholipids in which xylenes are less soluble than in
neutral fat (Riihimäki & Savolainen, 1980).
6.3 Metabolic transformation
6.3.1 In humans
Fig. 1 shows schematically the metabolic pathways for xylene
( m-xylene is used as an example) in humans.
Metabolism of xylenes by humans consists primarily of side-chain
oxidation to form methylbenzoic acid (Sedivec & Flek, 1976; Riihimäki
et al., 1979a; Riihimäki et al., 1979b). Methylbenzoic acid is
conjugated principally with glycine and excreted in urine as
methylhippuric acid. It has been estimated that glycine conjugation
would be saturated in humans exposed to about 1174 mg/m3 (270 ppm)
xylene while working and to about 3393 mg/m3 (780 ppm) while resting
(Riihimäki, 1979a). A small amount of the glucuronide ester of
methylbenzoic acid and trace levels of methylbenzyl alcohol have been
detected in human urine (Ogata et al., 1980; Engström et al., 1984;
Campbell et al., 1988).
Hydroxylation of the aromatic ring with the formation of
dimethylphenols seems to be a minor pathway in humans. The following
dimethylphenol isomers have been identified in human urine: 2,3- and
3,4-dimethylphenol (with o-xylene), 2,4-dimethylphenol (with
m-xylene) and 2,5-dimethylphenol (with p-xylene) (Sedivec & Flek,
1976; Engström et al., 1984).
Chemical name
ortho-xylene meta-xylene para-xylene
Synonyms
1,2-dimethyl- 1,3-dimethyl- 1,4-dimethyl-
benzene benzene benzene
o-methyltoluene m-methyltoluene p-methyltoluene
1,2-xylene 1,3-xylene 1,4-xylene
o-xylol m-xylol p-xylol
ortho-xylene meta-xylene para-xylene
Relative molecular mass
106.16 106.16 106.16
CAS registry number
95-47-6 108-38-3 106-42-3
RTECS registry number
ZE 2450000 ZE 2275000 ZE 2625000
CAS registry number (mixed xylenes) 1330-20-7
RTECS registry number (mixed xylenes) ZE 210000
2.2 Physical and chemical properties
Some physical and chemical properties are given in Table 1.
Table 1. Some physical and chemical properties of xylenesa
o-Xylene m-Xylene p-Xylene
Physical state (20°C; 101.3 kPa) liquid liquid liquid
Colour colourless colourless colourless
Boiling point (°C; 101.3 kPa) 144.4 139.1 138.3
Melting point (°C; 101.3 kPa) -25.2 -47.9 13.3
Relative density (25°/4°C) 0.876 0.860 0.857
Vapour pressure (kPa at 20°C) 0.66 0.79 0.86
Flash point (°C) (closed cup) 30 25 25
Saturation % in air (101.3 kPa) 1.03 (32°C) 1.03 (28°C) 1.03 (27°C)
Explosion limits (vol-% in air) 1.0-6 1.1-7 1.1-9
Autoignition temp (°C) 465 525 525
Octanol/water partition coefficient
(log P) 3.12 3.2 3.15
Solubility in water (mg/litre) 142 146 185
a Data from Sandmeyer (1981); Verschueren (1983); ECETOC (1986); IARC, (1989);
DECOS (1991); Bell (1992)
All three isomers of xylene are soluble in organic solvents such
as ethanol, diethyl ether, acetone and benzene (ECETOC, 1986; IARC,
1989; DECOS, 1991). At room temperature the xylenes are colourless
liquids with an aromatic odour (DECOS, 1991). The odour threshold for
mixed xylene in air is approximatively 4.35 mg/m3 (1 ppm) (Carpenter
et al., 1975; Amoore & Hautala, 1983; DECOS, 1991).
2.3 Conversion factors
1 ppm = 4.35 mg/m3 at 25°C, 101.3 kPa
1 mg/m3 = 0.23 ppm at 25°C, 101.3 kPa
2.4 Analytical methods
2.4.1 In air
US NIOSH has presented a method for measuring aromatic
hydrocarbons including xylene, in air. Xylene is adsorbed to coconut
shell charcoal, eluated with carbon disulfide and determined using gas
chromatography with a flame-ionization detector (FID) (Eller, 1984).
A similar method has been described by the International Agency
for Research on Cancer (IARC) (Brown, 1988a) concerning airborne
vapours of benzene, toluene and xylenes, or mixtures thereof. The
concentration range is about 1-1000 mg/m3 (approximately 0.2-200 ppm)
in 12-litre air samples. In another method described by IARC (Brown,
1988b) hydrocarbons are adsorbed on a porous polymer, desorbed with
heat and transferred with an inert carrier gas into a gas
chromatograph equipped with a FID. For a 5-litre air sample the
concentration range is approximately 0.5-50 mg/m3 (0.1-10 ppm). With
the use of a gas chromatography and mass spectrometry (GC/MS)
technique, the detection limit can be as low as 0.2 µg/m3 (Bevan et
al., 1991).
IARC also presented a method for determinating gasoline
hydrocarbons (Brown, 1988c). The air is drawn through two adsorbent
tubes in series, containing Chemosorb 106 and charcoal, respectively.
The vapour after heat desorption is transferred to a gas chromatograph
equipped with a capillary column and FID. The method is suitable for
airborne vapours of full-range gasoline over a concentration range of
approximately 0.2-100 mg/m3 (0.04-20 ppm) in a 2.5-litre air sample.
There are commercially available badges based on passive charcoal
sampling. After extraction with carbon disulfide the xylenes can be
detected by gas chromatography (Van der Wal & Moerkerken, 1984;
Triebig & Schaller, 1986). Xylene can also be detected with infrared
analysers with a minimum concentration of 9.6 mg/m3 (2.2 ppm) at a
wavelength of 13.1 µm and a pathlength of 20.25 m. This method is
only suitable when no other compounds that absorb in the same region
are present (DECOS, 1991).
Earlier methods for determinating xylenes have been reviewed by
Fishbein et al. (1988).
2.4.2 In water
A head-space technique coupled to capillary column gas
chromatography has been described by Drozd & Novak (1978). The
detection limit is at the ppb level. The detection limits could be
lowered if the xylenes were extracted from the water by an air stream
and condensed in a refrigerated column. In another method, the sample
is extracted with hexane or heated in a water bath at 25°C for 1 h.
Aliquots are then determined by gas chromatography with FID or mass
spectometry. The detection limit is 1 µg/litre (Otson & Williams,
1981; Otson et al., 1982). Recent studies have suggested that with
the use of GC/MS the detection limits for xylene in water can be in
the range of 0.001 to 0.01 µg/litre (Kenrick et al., 1985; MAFF,
1991).
2.4.3 In biological media
A review of biological monitoring of exposure to xylene has been
produced; methods include measuring methylhippuric acid in urine,
xylene in blood and xylene in expired air (Lauwerys & Buchet, 1988).
2.4.3.1 In blood
A method using capillary head-space gas chromatography with FID
has been developed for the simultaneous determination of xylene and
other aromatic hydrocarbons, such as benzene, xylenes and ethyl-
benzene, in blood. The limit of detection is 5 µg/litre and the
response is linear between 5 and 4000 µg/litre of blood.
A head-space gas chromatographic method for determinating xylenes
in blood has also been described (Engström & Riihimäki, 1988a). This
method is suitable for the determination of xylene isomers in blood
specimens. The detection limit is 53 µg/litre. Ethylbenzene, which
normally accompanies xylenes in technical xylene, does not interfere
with the determination of xylene.
2.4.3.2 In urine
In humans, xylene is metabolized to methylhippuric acids (see
section 6.3), which are not normally present in the urine of
non-exposed people. The urine methylhippuric acid level has been
measured by gas chromatography (Engström & Bjurström, 1978),
colorimetry (Ogata & Hobara, 1979), thin-layer chromatography (Bieniek
et al., 1982) and high performance liquid chromatography (Ogata &
Taguchi, 1986).
A suitable gas chromatographic method for the determination of
methylhippuric acids in urine has been presented by Engström &
Riihimäki (1988b). The range of application is 10-2120 mg/litre. No
interference from the normal constituents of urine is seen in the
specified range. Another method using HPLC allows methylhippuric
acids, phenyl glyoxylic acid and mandelic acid to be determined
together with hippuric acid in one run (Angerer, 1988b). The limit of
detection is 50 mg/litre of urine and the response is linear up to
2000 mg/litre. The aromatic carboxylic acids excreted in urine do not
interfere with the hippuric acid determination.
2.4.3.3 In exhaled air
There is a method for determinating benzene, toluene and xylene
in breath samples by gas chromatography/mass spectrometry (Pellizzari
et al., 1988). The detection limit for xylene is 0.5 µg/m3 and the
quantification limit is 2.5 µg/m3. No interference has been
observed. The linear range for quantification using fused silica
capillaries on a gas chromatograph/mass spectrometer/computer is
generally three orders of magnitude.
2.4.3.4 In human milk
A purge and trap technique using gas chromatography and electron
impact mass spectrometry has been developed by Pellizari et al. (1982)
for the detection of xylene and various volatile compounds in human
milk.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Production processes
Before 1940 virtually all of the aromatic solvents, including
xylene, were produced from coal. Thereafter production of xylene from
petroleum started. Most mixed xylene is currently produced by
catalytic reforming of petroleum. It is also obtained from pyrolysis
gasoline as a by-product of olefin manufacture during the cracking of
hydrocarbons. Small amounts of mixed xylenes are also obtained from
coal-derived coke-oven light oil and from disproportionation of
toluene (Fishbein, 1988).
There are some differences in the composition of commercial
xylenes produced from petroleum and from coal-tar. The general
composition of xylenes from petroleum is 44% m-xylene, 20%
o-xylene, 20% p-xylene and 15% ethylbenzene. The xylenes from
coal-tar consists of 45-70% m-xylene, 23% p-xylene, 10-15%
o-xylene and 6-10% ethylbenzene. Commercial xylene may also contain
small amounts of toluene, trimethylbenzene (pseu documene), phenol,
thiophene, pyridine and non-aromatic hydrocarbons and has frequently
been contaminated with benzene (WHO, 1981; Fishbein, 1988).
3.2 Production levels
Approximately 3.9 million tonnes of mixed xylenes were isolated
in the USA in 1978. The non-isolated mixed xylenes (containing
benzene and toluene) are blended into gasoline, while the isolated
mixed xylenes are used primarily for the production of the individual
isomers and for solvent applications (Fishbein, 1988).
World production of p-xylene in 1983 was 3.9 million tonnes of
which the USA accounted for 48%, Europe 23% and Japan 16%. The world
production of o-xylene in 1983 was 1.3 million tonnes of which
western Europe produced 30% and the USA 18%. Eastern Europe was the
other large producer of o-xylene (Fishbein, 1988).
The approximate world production of o-, p- and mixed xylenes in
1984 was 15.4 million tonnes (ECETOC, 1986). The production of mixed
xylenes in 1984 in the USA was 2.78 million tonnes and that of
p-xylene was 1.94 million tonnes (Fishbein, 1988). During the same
year the production of o-xylene was 316 000 tonnes. The production
of mixed xylene and p-xylene in 1994 was 4.1 and 2.8 million tonnes,
respectively (Kirschner, 1995). The production of xylenes in some
western European countries in 1984 was estimated to be: France 85 000
tonnes, Italy 395 000 tonnes and Federal Republic of Germany 455 000
tonnes. In 1987 the figures were: (in thousands of tonnes) Canada
345, France 129, Federal Republic of Germany 501, India 28, Italy
491, Japan 1767, Republic of Korea 552, Mexico 381 and USA 2772 (IARC,
1989).
3.3 Uses
Approximately 92% of the mixed xylenes produced is blended into
gasoline. The remainder is used in a variety of solvent applications
as well as to produce the individual isomers of xylene. Xylenes are
used as solvents, particularly in the paint and printing ink
industries. The single largest end-use of mixed xylenes is in the
production of the p-xylene isomer. The major derivatives produced
from p-xylene are dimethylterephthalate and terephthalic acid used
in the production of polyester fibre, film and fabricated items
(ECETOC, 1986; Fishbein, 1988).
The o-xylene is almost exclusively used to produce phthalic
anhydride for phthalate plasticizers, and m-xylene is used for the
production of isophthalic acid, an intermediate in the manufacture of
polyester resins (ECETOC, 1986; Fishbein, 1988).
Mixed xylenes are also used in the manufacture of perfumes,
pesticide formulations, pharmaceuticals and adhesives, and in the
painting, printing, rubber, plastics and leather industries (IARC,
1989).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The majority of xylene released into the environment enters the
atmosphere directly. This results mainly from its use as a solvent
and its release in motor vehicle exhausts. A small proportion is also
likely to enter water and soil due to oil/petrol spillages etc. Once
in the environment a number of physical processes can affect its
distribution. Jori et al. (1986) calculated that more than 99% of the
xylene released ultimately partitions to the atmosphere; the
calculations were based on the Mackay fugacity model (Mackay et al.,
1985).
4.1.1 Volatilization
Owing to the relatively high vapour pressure and low water
solubility of xylenes, volatilization from water bodies to the
atmosphere is likely to be an important distribution process. The
half-life for evaporation of o-xylene from water bodies at a depth
of 1 metre has been estimated to be around 5.6 h (Mackay & Leinonen,
1975). It is expected that both m-xylene and p-xylene will behave
similarly.
No information has been reported on the volatilization rate of
xylenes from soils, but it is expected to be a fairly rapid process,
at least from near to the surface, owing to the reasonably high
volatility of xylenes.
4.1.2 Rain-out
Xylenes are only slightly soluble in water (see section 2). This
means that only a very small proportion of xylene in the atmosphere is
likely to be removed by precipitation (rain-out). This is supported
by the fact that xylenes have been detected in rainwater samples at
only very low levels (2 ng m-xylene/litre and 9 ng p-xylene/litre;
see section 5.1.2.3). It is also possible that a small amount of
xylene in soil may leach out into aquatic systems.
4.1.3 Adsorption
Xylenes are likely to be adsorbed to a small extent onto both
aquatic sediments and soil, based on their partition coefficients.
However, adsorption is dependent on such factors as the organic carbon
content and the water content.
Sediment-water partition coefficients of 8.9 for o-xylene and
10.5 for p-xylene have been measured. These were for surface
sediment from the Tamar Estuary, United Kingdom, which has an organic
carbon content of 4.02% (Vowles & Mantoura, 1987).
m-Xylene has been shown to adsorb onto soil to a small extent.
Using three soils with organic carbon contents ranging between 0.2 and
3.7%, soil organic carbon-water partition coefficient (Koc) values of
129, 158 and 289 (2.1, 2.2 and 2.5 log values) were measured (Seip et
al., 1986). A similar Koc of 219 has been quoted for o-xylene
(Pussemier et al., 1990).
p-Xylene has been shown to adsorb onto minerals and soils to a
small extent from the vapour phase (Rhue et al., 1988).
In the absence of water, soils and clay minerals exhibit a large
capacity to adsorb p-xylene, owing primarily to adsorption on
mineral surfaces. However, such dry conditions are rarely encountered
in the environment and may only exist at the soil surface or in arid
climates. When the relative humidity is increased to 67% or 90%, the
sorption of p-xylene vapour decreases significantly (Pennell et al.,
1992).
4.2 Transformation
4.2.1 Biodegradation
In soil and water, o- and p-xylene are readily biodegraded
under a wide range of aerobic and anaerobic conditions, but o-xylene
is much more persistent under similar conditions.
4.2.1.1 Aerobic degradation
Bacteria of the genus Pseudomonas have been shown to be capable of
growing using either m-xylene or p-xylene as the sole carbon
source (Davis et al., 1967; Omori et al., 1967; Omori & Yamada, 1970;
Davey & Gibson, 1974). The main initial metabolites appear to be
m-toluic acid from m-xylene and p-toluic acid from p-xylene.
Similarly, cultures of three strains of Nocardia have been shown to
metabolize p-xylene to p-toluic acid and 2,3-dihydroxy- p-toluic
acid (Raymond et al., 1969).
In contrast to this, many of the bacteria that have been shown to
be capable of growing on either m-xylene or p-xylene as sole
carbon source do not appear to be capable of growing on o-xylene as
sole carbon source (Omori et al., 1967; Davey & Gibson, 1974).
o-Xylene has been shown to undergo biodegradation in the
presence of other carbon sources. Using hexadecane as growth
substrate, o-xylene was co-oxidized to o-toluic acid by Nocardia.
A similar oxidation was observed with Pseudomonas using hexane as
the growth substrate (Jamison et al., 1976).
The biodegradation of xylenes by the autochthonous microflora in
groundwater in the presence of the water soluble fraction of gas oil
has been demonstrated by Kappeler & Wuhrmann (1978a, 1978b). After a
lag period of 3 to 4 days, individual hydrocarbon concentrations were
found to decrease at a measurable rate. The removal of m-xylene and
p-xylene was complete after 7 days. o-Xylene was shown to degrade
at a significantly slower rate than the meta and para isomers, removal
being complete after 11-12 days. In each case, the first step in the
degradation appears to be oxidation to the corresponding methylbenzyl
alcohol.
Both m-xylene and p-xylene have been shown to be readily
degraded within 13 days using a microbial inoculum from an activated
sludge wastewater treatment plant. The initial concentration of
xylene was 100 mg/litre and 30 mg/litre of sludge biomass was used.
Degradation of xylene was monitored by comparing the oxygen uptake of
the system with that of controls (Tabak et al., 1989).
The degradation of mixtures of benzene, toluene and p-xylene
has been studied using pure cultures of either Pseudomonas sp.
strain CFS-215 or Arthrobacter sp. strain HCB, or a mixed culture
indigenous to a shallow sandy aquifer. In the mixed culture, the
presence of p-xylene was found to increase the lag period before the
degradation of benzene and toluene commenced, and also appeared to
decrease the rate of toluene degradation compared to the rate obtained
without added p-xylene. Degradation of p-xylene occurred in the
mixed culture, although a long lag period was observed before
degradation commenced. When toluene was also present in the culture,
the lag period for the degradation of p-xylene was reduced and the
degradation rate was increased, but after all the toluene had been
degraded, the p-xylene degradation rate again slowed. In the
experiments with Pseudomonas sp., the degradation of p-xylene was
slow; no degradation was observed in the first 3 weeks when p-xylene
alone was present. Again, the degradation rate of p-xylene was
found to increase when toluene was also being degraded. Also, the
presence of p-xylene again increased the lag period for benzene and
toluene degradation. In the experiments with Arthrobacter sp.,
degradation of p-xylene was found to occur only in the presence of
benzene and at a slow rate (Alvarez & Vogel, 1991).
The biodegradation of o-xylene and m-xylene has been studied
in three core samples of subsurface soil: uncontaminated soil, soil
that had previously been contaminated with unleaded gasoline and soil
from an area that had previously undergone biostimulation using
hydrogen peroxide. m-Xylene was rapidly degraded in all three core
types, although the rate was faster in the previously biostimulated
sample due to a higher bacterial cell count ( m-xylene disappeared to
below the analytical detection limit within 3 weeks in the previously
biostimulated samples, whereas some remained after 3 weeks in the
previously contaminated samples). o-Xylene was found to be
recalcitrant in all of the samples (Thomas et al., 1990).
p-Xylene and o-xylene were shown to be degraded in aquifer
material collected from the contaminant plume after a large gasoline
spill. The degradation occurred fastest in material from the aerobic
degrading zone of the plume, but also occurred rapidly in
uncontaminated material (Wilson et al., 1990).
In a study using laboratory aquifer columns that simulated
saturated-flow conditions typical of a river/groundwater infiltration
system, all three xylene isomers were shown to undergo degradation
under aerobic conditions. Both m-xylene and p-xylene were
degraded to concentrations below the analytical limit of detection
within 17 days. The rate of transformation was significantly lower
for o-xylene but degradation still occurred readily (Kuhn et al.,
1985).
The rate of biodegradation of benzene, toluene and xylene (BTX)
in groundwater/soil slurries has been shown to be highly dependent on
the dissolved oxygen concentration (Chiang et al., 1989). At a
dissolved oxygen concentration of between 2 and 8 mg/litre, BTX
(initial concentrations between 120 and 16000 µg/litre) was 80-100%
degraded in 30-40 days with a half-life of 5-20 days. When the
dissolved oxygen concentration was 1 or 2 mg/litre, the BTX was
incompletely degraded (20-60%) in 30-40 days. Little or no
degradation was observed at dissolved oxygen concentrations of 0, 0.1
and 0.5 mg/litre.
The xylenes have been shown to be 100% degraded after 192 h
incubation at 13°C with natural flora in groundwater in the presence
of other components of high-octane gasoline (Jamison et al., 1976).
4.2.1.2 Anaerobic degradation
o-Xylene, along with other alkylbenzene compounds, has been
shown to undergo degradation under anaerobic methanogenic conditions.
No significant degradation of o-xylene occurred over the first 20
weeks, but after 40 weeks the concentration was reduced to 22% of the
original. Less than 1% remained after 120 weeks (Wilson et al.,
1986).
In anoxic suspensions of Pseudomonas sp. strain T cells grown
anaerobically with toluene, m-xylene and p-xylene were partially
oxidized to 3- and 4-methylbenzoate, respectively. o-Xylene was not
oxidized to 2-methylbenzoate. Suspensions of strain T cells grown
anaerobically with m-xylene and incubated with m-xylene at 5°C
accumulated 3-methylbenzaldehyde (3.5 µM after 20 min) and
3-methylbenzoate (5 µM after 20 min). After further incubation at
room temperature, the three aromatic compounds were completely
oxidized within 3 h (Seyfried et al., 1994).
Experiments have been carried out using aquifer material from a
site containing areas that were either contaminated or uncontaminated
with JP-4 jet fuel. Both mixed xylene and the individual isomers were
incubated with the aquifer material at 12°C under a nitrogen
atmosphere. Both o-xylene and m-xylene were slowly degraded in
the uncontaminated aquifer material when added individually, although
m-xylene (at 16 mg/litre) also appeared to inhibit the basal rate of
denitrification. Using mixed xylenes, a lag period of 30 days was
required before biodegradation commenced in the uncontaminated
material. m-Xylene and p-xylene were degraded to below the
analytical limit of detection within the next 26 days, but the
degradation of o-xylene was found to be much slower. In the
contaminated aquifer material, much longer lag periods and decreased
rates of biodegradation were observed, o-xylene not being
significantly degraded over a 6-month period (Hutchins et al., 1991a).
In further laboratory experiments using a mixture of benzene and
alkylbenzenes, both o-xylene and m-xylene were found to be
degraded under nitrate-reducing and nitrous oxide-reducing conditions,
but degradation of o-xylene was found to cease once the other
alkylbenzenes had been degraded (Hutchins, 1991). In field
experiments in the same aquifer, m-xylene and p-xylene were shown
to be degraded under denitrifying conditions when nitrate was injected
into the aquifer, but no evidence of biodegradation of o-xylene was
found (Hutchins et al., 1991b).
The three xylene isomers have been shown to be completely
mineralized by aquifer-derived microorganisms under sulfate-reducing
conditions. The source of the inoculum was a gasoline-contaminated
sediment. All microcosms were initially fed a mixture of benzene,
toluene, ethylbenzene, o-xylene and p-xylene (about 5 mg/litre of
each component). p-Xylene was found to be > 80% degraded within 72
days and o-xylene was > 80% degraded within 104 days. After this
initial adaptation period, o-xylene, m-xylene and p-xylene were
rapidly degraded by the system without any lag period ( m-xylene
co-elutes with p-xylene and, therefore, m-xylene was not added
initially) (Edwards et al., 1992).
Edwards & Grbic-Galic (1994) reported that o-xylene is
completely mineralized by aquifer-derived microorganisms under
anaerobic conditions. However, an adaptation period of 200 to 255
days was required before the onset of degradation. Anaerobic
degradation was found to be inhibited by the presence of some natural
organic substrates and co-contaminants.
p-Xylene and o-xylene have been shown to be degraded in
anaerobic aquifer material collected from the contaminant plume after
a large gasoline spill (Wilson et al., 1990).
All three xylene isomers have been shown to undergo degradation
under anaerobic denitrifying conditions. The rate was much lower for
o-xylene than for the other isomers. Long lag periods were observed
in all cases before degradation commenced (Kuhn et al., 1985).
Degradation of o-xylene under anaerobic conditions has been
hypothesized to explain the distribution of o-xylene in a landfill
leachate plume (Reinhard et al., 1984). m-Xylene has been shown to
be rapidly mineralized to carbon dioxide in laboratory aquifer columns
operated under continuous flow conditions with nitrite as an electron
acceptor. The degradation occurred simultaneously with the reduction
of nitrite. In contrast to this, the concentrations of o-xylene and
p-xylene were only slightly reduced in the experiment. The author
noted, however, that the experiments were carried out over a 6-day
period after the addition of the new substrate and therefore may not
have allowed a build-up of other microorganisms capable of degrading
these substrates (Kuhn et al., 1988).
The biodegradation of BTX has been shown to occur under
anaerobic, denitrifying conditions using shallow aquifer material that
had previously been exposed to BTX. o-Xylene and m-xylene were
found to be degraded to 15% and 12%, respectively, of the initial
concentration (3 mg/litre) after 62 days with added nitrate (Major et
al., 1988). Much less degradation occurred under anaerobic conditions
in the absence of added nitrate (73% o-xylene remained after 62 days
and 59% m-xylene remained after 62 days). These losses were not
considered to be significant when compared with sterile controls.
Up to 0.4 mM (42.5 mg/litre) m-xylene was found to be rapidly
mineralized in a laboratory aquifer column operated in the absence of
molecular oxygen with nitrate as an electron acceptor. Quantitative
(80%) oxidation of m-xylene to carbon dioxide occurred with
concomitant reduction of nitrate. The column was inoculated with
denitrifying river sediment that had been continuously fed m-xylene
for several months (Zeyer et al., 1986).
4.2.2 Abiotic degradation
The xylene isomers are readily degraded in the atmosphere,
photooxidation being the most important degradation process.
4.2.2.1 Photolysis
Xylenes do not absorb UV-visible radiation appreciably at
wavelengths longer than 290 nm. This means that they are unlikely to
be directly photolysed in the troposphere or in solution, as the ozone
layer absorbs wavelengths shorter than 290 nm. Experiments using
xylenes adsorbed on silica gel have shown that the photomineralization
rates for all three isomers are low using radiation with a wavelength
longer than 290 nm (Gab et al., 1977).
4.2.2.2 Atmospheric oxidation
Atmospheric oxidation of xylenes is rapid and proceeds via
free-radical chain processes. The most important oxidant is the
hydroxyl radical, but xylenes will also react with other species found
in the atmosphere, such as alkoxy radicals, peroxy radicals, ozone and
nitrogen oxides. The most likely reaction pathways occurring in the
atmosphere are hydroxyl radical addition to the aromatic ring and
hydrogen abstraction from the alkyl groups by hydroxyl radicals (Gery
et al., 1987), although reaction with nitrate radicals may become
important at night (Grosjean, 1990).
Estimates for the lifetime of xylenes in the atmosphere have been
made from smog chamber experiments and from knowledge of the rate
constant for reaction with hydroxyl radicals. Atkinson (1985)
reviewed the available hydroxyl radical reaction rate constant data
and recommended kOH values at 25°C of 1.47 × 10-11 cm3 × molecule-1
× s-1 for reaction with o-xylene, 2.45 × 10-11 cm3 × molecule-1 ×
s-1 for reaction with m-xylene and 1.52 × 10-11 cm3 × molecule-1 ×
s-1 for reaction with p-xylene.
Based on hydroxyl radical reaction rate constant data,
atmospheric lifetimes of 2.6 h for o-xylene, 1.5 h for m-xylene
and 2.4 h for p-xylene have been calculated in south-east England
(Brice & Derwent, 1978).
Lifetimes in the boundary layer of the atmosphere have been
calculated by Singh et al. (1986). Using hydroxyl radical reaction
rate constants, lifetimes of 9 sunlight hours for o-xylene, 5
sunlight hours for m-xylene and 10 sunlight hours for p-xylene
were estimated. Singh et al. (1983) estimated that around 71.3% loss
of o-xylene, 87% loss of m-xylene and 67% loss of p-xylene would
occur per day (12 sunlight hours) as a result of reaction with
hydroxyl radicals.
An important point to consider with this data is that the
calculated lifetime depends on several factors, including temperature,
and also the actual concentration of hydroxyl radicals. It is known
that the concentration of hydroxyl radicals depends greatly on the
amount of sunlight available. Thus typical figures are around 2 ×
106 molecules/cm3 in summer months, falling by approximately a
factor of 2 in the winter months (Singh et al., 1986). At night the
concentration of hydroxyl radicals is negligible. Even so, it can be
seen that xylenes are removed from the atmosphere quite readily by
reaction with hydroxyl radicals.
It is possible that xylenes will be removed from aquatic systems
by similar types of reactions, as hydroxyl radicals are known to exist
in aquatic systems (Mansour et al., 1985).
The reaction of xylene isomers with NO3 radicals has been
studied. The second-order reaction rate constants measured were:
o-xylene, k = 3.74 × 10-16 cm3 × molecule-1 × s-1; m-xylene,
k = 2.49 × 10-16 cm3 × molecule-1 × s-1; and p-xylene, k = 4.49 ×
10-16 cm3 × molecule-1 × s-1. NO3 radicals have been measured in
the lower troposphere during night time hours but photodecomposition
occurs during daylight at a wavelength of 600 nm. Typical
concentrations of NO3 radicals found during the night are 2.4 × 108
molecules/cm3 in a clean atmosphere and 2 × 109 molecules/cm3 in a
moderately polluted atmosphere (Sabljic & Güsten, 1990). Using these
concentrations, the following half-lives for the reaction of xylene
with NO3 radicals at night have been estimated: o-xylene, 15-89
days; m-xylene, 23-194 days; and p-xylene, 13-107 days. These
half-lives are much longer than those for the daylight reaction with
hydroxyl radicals, but indicate that removal of xylenes from the
atmosphere could still occur at night by this route, especially in
polluted atmospheres.
The xylenes are sufficiently susceptible to photochemical
oxidation in the lower atmosphere that they may contribute to
tropospheric ozone formation. Derwent & Jenkin (1990) calculated
POCPs (Photochemical Ozone Creation Potentials) for xylenes of 41
( o-xylene), 78 ( m-xylene) and 63 ( p-xylene). The POCP values
reflect the ability of a substance to form low-level ozone as a result
of its atmospheric degradation reactions, the POCP values being
calculated relative to ethylene (a chemical that is thought to be
important in low-level ozone formation and is given a POCP of 100) on
a unit mass emission basis.
4.2.2.3 Hydrolysis
It is considered unlikely that xylenes will hydrolyse under the
conditions found in the natural environment.
4.2.3 Bioaccumulation
Octanol-water partition coefficients of 3.12, 3.20 and 3.15
(log values) have been determined for o-xylene, m-xylene
and p-xylene, respectively. These values indicate that slight
bioaccumulation could take place in the environment. Using these
values, bioconcentration factors (BCFs) of 138 (2.14) for o-xylene,
158.5 (2.20) for m-xylene and 144.5 (2.16) for p-xylene (log
values are given in parentheses) can be estimated using the formula of
Veith et al. (1980).
Bioconcentration factors (BCFs) of 21.4 (1.33 log value) for
o-xylene and 23.6 (1.37 log value) for combined m-xylene and
p-xylene have been measured in the eel (Anguilla japonica). The
half-life for elimination of m-xylene and p-xylene from the flesh
after exposure had ceased was 2.6 days (Ogata & Miyake, 1979).
Bioconcentration factors have been measured for all three isomers
in the goldfish. The reported BCFs were 14.1 (1.15) for o-xylene,
14.8 (1.17) for m-xylene and 14.8 (1.17) for p-xylene (log values
are in parentheses) (Ogata et al., 1984).
After exposure to the water-soluble fraction of Cook Inlet crude
oil ( o-xylene concentration 0.14 mg/litre; m-xylene concentration
0.15 mg/litre) for 8 days, concentrations of 0.87 mg/kg o-xylene
and 0.90 mg/kg m-xylene were found in the Manila clam (Tapes
semidecussata). The concentrations in the clam were found to
decrease rapidly during the first 7 days after exposure ceased (Nunes
& Benville, 1979).
A BCF of 9 for mixed xylenes was measured in both the thorax and
abdomen of the adult spot shrimp (Pandelus platyceros) when it was
exposed to a water-soluble fraction of Prudhoe Bay crude oil (Sanborn
& Malins, 1980).
The low BCFs indicate that biomagnification of xylenes through
the aquatic food chain is unlikely.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Owing to analytical difficulties it is often not possible to
quantify m-xylene and p-xylene individually in environmental
samples. As a result, a large proportion of the measured levels of
these two isomers refer to the combined total of the two.
5.1.1 Ambient Air
Measured levels of o-xylene and the combined m-xylene and
p-xylene levels are shown in Table 2. Levels of xylene for indoor
air are given in section 5.2.
Typically, background levels of all three xylene isomers are
around 1 µg/m3. Higher levels have been measured in urban areas,
showing that vehicle emissions are a significant source of xylenes.
Six studies giving measured levels for m-xylene and p-xylene
as individual compounds have been reported. One study gave measured
levels in Florida, USA, of < 0.09 to 5.5 µg/m3 for m-xylene and
< 0.09 to 2.4 µg/m3 for p-xylene (Lonneman et al., 1978). Another
gave levels of 0.019 to 2.66 µg/m3 for m-xylene and 0.018 to 1.19
µg/m3 for p-xylene in the Black Forest, Germany (Jüttner, 1988a),
while another reported levels of 92.6 µg/m3 for m-xylene and 39.7
µg/m3 for p-xylene in Zurich, Switzerland (Grob & Grob, 1971).
Levels of 11.94-25.74 µg/m3 m-xylene and 5.33-11.14 µg/m3
p-xylene were reported for two cities in Taiwan (Hung & Liao, 1991).
Average values of 1.5-6.2 µg/m3 m-xylene and 0.44-2.6 µg/m3
p-xylene were found in the Netherlands, with maximum values of 11-70
µg/m3 m-xylene and 4.9-15.9 µg/m3 p-xylene (Guicherit &
Schulting, 1985). Kawata & Fujieda (1993) monitored xylene
concentrations in the air of Niigata, Japan, in 1991 and 1992. At an
urban location, mean m-xylene and p-xylene concentrations were 4.8
and 2 µg/m3, respectively. The m-xylene and p-xylene
concentrations at a rural location were 1.8 and 0.7 µg/m3,
respectively.
Svanberg et al. (1995) measured xylene levels in the air of 17
Swedish towns during the winters of 1992-1993 and 1993-1994. Mean
concentrations ranged from 17 to 47 µg/m3 and from 11 to 41 µg/m3
for m/p-xylene for the two winters, respectively, and from 18 to 53
µg/m3 and 12 to 44 µg/m3 for o-xylene.
High levels of total xylene have been measured in air samples
from within landfill sites in the United Kingdom (Young & Parker,
1983). Levels of between 36 and 77 mg/m3 were reported in domestic
landfills, with higher levels (actual levels not reported) being found
in some industrial waste landfills.
Table 2. Mean measured levels of o-xylene and m/p-xylenes in ambient air
Location o-Xylene m/p-Xylene Reference
levels levels
(µg/m3) (µg/m3)
Sydney, Australia 6.63 17.2 Nelson & Quigley (1982)
Sweden
near car factory 38 264 Petersson (1982)
1 km from the factory 11.6 85.1
background 0.21 0.58
Stockholm, Sweden
busy streets 251 and 91 Jonsson et al. (1985)
calm streets 28.3 and
15.9
The Netherlands 0.88 - 3.1 Guicherit & Schulting
max (1985)
7.5 - 22.5
Black Forest, Germany 0.024-1.77 Jüttner (1988a)
Hamburg, Germany
12 sites 4.5-15.2 Bruckmann et al. (1988)
Los Angeles, USA 28.7 Altshuller et al. (1971)
USA
car painting planta 52 158 Sexton & Westberg (1980)
9 miles from the plant 4.5 14.5
background 0.5 2.5
USA, 10 cities 2.48-8.35 4.11-19.95 Singh et al. (1983)
USA, 5 cities ND-5.24b 1.03-14.64 Sheldon et al. (1988)
Table 2. (Cont'd)
Location o-Xylene m/p-Xylene Reference
levels levels
(µg/m3) (µg/m3)
Raleigh, USA, near to 1.9-7.6 5-19.9 Chan et al. (1991b)
roads
USA, 6 cities 2.78-25.25 1.9-13.1 Singh et al. (1986)
USA
urban 5.2 1.2 Brodzinsky & Singh (1983)
rural 0.41 0.38
Taiwan
2 cities 7.14 and 15.17 Hung & Liao (1991)
Niigata, Japan
urban 2 Kawata & Fujida (1993)
rural 0.83
Finland
industrial 207 568 Kroneld (1989)
urban 0.143 0.392
Grenoble, France
winter 1.9 22.9 Foster et al. (1991)
summer 2.4 28.7
Vienna, Austria
streets 24.0 50.8 Lanzerstorfer & Puxbaum (1990)
suburbs 6.2 10.4
backgrounds 1.9 3.9
Table 2. (Cont'd)
Location o-Xylene m/p-Xylene Reference
levels levels
(µg/m3) (µg/m3)
United Kingdom
urban 5.43 12.2 Clark et al. (1984)
rural 0.75 2.2
Southampton, United Kingdom
urban 12 27 Bevan et al. (1991)
busy roads 33 69
common land 5 6
Harwell, United Kingdom 2.4 3.9 Jones (1988)
max 15.8 max 34.4
a Based on individual samples
b ND not detectable (detection limits not states)
5.1.2 Water and sediment
5.1.2.1 Surface water
Levels of the individual xylene isomers measured in surface water
are shown in Table 3. Typically, background levels of xylenes in
surface waters are low (< 0.1 µg/litre). Much higher levels have
been measured in some industrial areas and areas associated with the
oil industry. Wiesenburg et al. (1981) measured xylenes in brine from
an oil production platform in the Gulf of Mexico. Two samples
contained 480 and 1800 µg/litre of m/p-xylene and 500 and 1900
µg/litre of o-xylene. Samples were taken from an underwater vent
plume from offshore oil production operations in the same region.
Xylene concentrations in the surface water were 0.270 µg/litre for
m/p-xylene and 0.06 µg/ litre for o-xylene. Water from the
discharge pipe contained 2060 µg/litre of m/p-xylene and 1510
µg/litre of o-xylene.
It has been reported that motor boats could be a significant
source of xylenes in surface water. Measurements were carried out in
an entrance canal to a harbour on Lake Constance both before (early
morning) and during boat movement on the lake. Levels recorded before
boat movements were o-xylene 18 ng/litre, m-xylene 17 ng/litre and
p-xylene 39 ng/litre. Levels recorded during the rest of the day
were o-xylene 57-481 ng/litre, m-xylene 76-750 ng/litre and
p-xylene 62-416 ng/litre. In general, the levels of xylene
increased as the number of boats passing the sampling point increased
(Jüttner, 1988b).
Xylenes were surveyed in surface water in Japan in 1977, 1985 and
1986. No xylene isomers were detected in 1977 (detection limit = 2
µg/litre). In 1985 one out of 21 samples contained xylene at
concentrations of 0.021, 0.042 and 0.037 µg/litre for o-, m- and
p-xylene, respectively (detection limit = 0.02 µg/litre). In 1986,
the concentrations of o- and m-xylene ranged from 0.04 to 1.2
µg/litre in 12 out of 137 samples and 15 out of 126 samples, for the
two isomers respectively. p-Xylene was detected in 4 out of 122
samples at concentrations ranging from 0.06 to 0.48 µg/litre
(detection limit = 0.03 µg/litre) (EAJ, 1993).
5.1.2.2 Groundwater
Table 3 shows levels of xylene measured in groundwater.
Typically, background levels of xylenes in aquifers are low (< 0.1
µg/litre). High levels have been reported in contaminated aquifers.
The migration of petroleum products from leaking underground storage
tanks and pipelines poses a groundwater contamination problem.
Gasoline-contaminated groundwater in Los Angeles, USA, contained
xylene at a concentration of 153 µg/litre (Karlson & Frankenburger,
1989).
Very high levels of o-xylene (4001 µg/litre) and m/ p-xylene
(5385 µg/litre) have been measured in a polluted aquifer in Italy.
Water was taken from a well at a depth of 30 m and the pollution was
thought to be due to leakage from underground solvent storage tanks
(Botta et al., 1984).
5.1.2.3 Precipitation
Kawamura & Kaplan (1983) measured xylene in rainwater in Los
Angeles, USA, during 1982. An m-xylene concentration of 0.002
µg/litre and a p-xylene concentration of 0.009 µg/litre were
reported.
Table 3. Levels of xylene in water
Location Isomera Level (µg/litre)b References
Surface water
River Lee, UK T detected at a level Waggot (1981)
of > 0.1
River Besós, Spain m/p 24 Gomez-Belinchon et al.
(polluted) ortho 8.1 (1991)
River Llobregat, Spain m/p 4.7 Gomez-Belinchon et al.
(polluted) ortho 0.83 (1991)
Seawater
Off River Humber, UK T < 0.001-29.0 MAFF (1991)
Dredged spoil disposal site T < 0.001-0.330
Sewage sludge disposal site T < 0.001
North Sea, off UK coast T < 0.01-0.250 Hurford et al. (1990)
River Tees estuary, UK m/p < 0.05-1.1 Harland et al. (1985)
ortho < 0.05-1.1
Coastal site, USA m/p 0.0045-0.066 Gschwend et al. (1982)
ortho 0.0018-0.025
Barcelona, Spain m/p 0.015-0.072 Gomez-Belinchon et al.
ortho 0.004-0.210 (1991)
Gulf of Mexico: T 0.002-0.056 McDonald et al. (1988)
river mouth
Gulf of Mexico: 0.001
chemical outfall
Inner harbour of the T 0.04-0.2 McFall et al. (1985)
navigation canal of
Lake Pontchartrain, USA
Table 3. (Cont'd)
Location Isomera Level (µg/litre)b References
Wastewater
Effluent samples from a T 1.82 Kennicut II et al. (1984)
Barceloneta waste
treatment facility, Puerto
Rico (mainly pharmaceutical
in origin)
Wastewater treatment para influent = 4.40 Michael et al. (1991)
plant, Great Lakes Basin effluent = < 1
Groundwater
British aquifers para occasionally Kenrick et al. (1985)
(uncontaminated sites detected at
thought to represent 0.001-0.02
background levels) ortho detected in 19
out of 32 samples.
highest = 0.02
mean = 0.011
Groundwater, near para ND-0.5c Barker et al. (1988)
landfill site, Hamilton, ortho 0.03-0.5
Ontario, Canada
Edwards aquifer, Texas, T up to 0.08 Buszka et al. (1990)
USA
Groundwater near m/p NDd-50 Slain & Baker (1990)
bituminous layers of ortho NDd-21
shale in rock, near a
sanitary landfill site,
Ontario, Canada
Table 3. (Cont'd)
Location Isomera Level (µg/litre)b References
Groundwater near an m/p 240-830 Stuermer et al. (1982)
underground coal ortho 260-590
gasification site in (background was
the USA below the limit of
detection of
0.5 µg/litre)
Groundwater, New Jersey/ T 59-300 Rao et al. (1985)
New York, USA
a m/p = combined m-xylene and p-xylene; T = total xylene
b ND = not detected
c detection limit not stated
d detection limit = 2 µg/litre
5.1.2.4 Leachate
Barker et al. (1988) measured o- and p-xylene in the leachate
from a landfill in Hamilton, Ontario, Canada. Xylene concentrations
ranged from 30.8 to 123 µg/litre for the ortho isomer and from 12.5 to
191 µg/litre for the para isomer. Leachate from a landfill in
Minnesota, USA, contained m-xylene concentrations ranging from 21 to
150 µg/litre and o/p-xylene concentrations ranging from 12 to 170
µg/litre. Both xylene isomers were presented in all six samples
collected (Sabel & Clark, 1984).
5.1.2.5 Sediment
Tynan et al. (1991) measured levels of xylene in sediment samples
taken in Wales, United Kingdom, of up to 23.4 µg/kg and 21.2 µg/kg for
o-xylene and p-xylene, respectively. Harland et al. (1985)
reported o-xylene levels of 0.6-3.9 µg/kg and combined m/p-xylene
levels of 3.4-250 µg/kg in sediment from the River Tees estuary,
England.
Xylenes were surveyed in sediment in Japan in 1977, 1985 and
1986. No xylene isomers were detected in 1977 (detection limit = 4
µg/kg). In 1985 one out of 21 samples contained o- or m-xylene at
concentrations of 1.1 and 2 µg/kg respectively; no p-xylene was
detected (detection limits = 0.6 µg/kg for o-xylene, 1 µg/kg for
m-xylene and 2 µg/kg for p-xylene). In 1986 o-, m- and
p-xylene concentrations ranged from 0.5 to 7 µg/kg (detected in 24
out of 111 samples), 0.5 to 15 µg/kg (detected in 33 out of 118
samples) and 0.5 to 3.8 µg/kg (detected in 12 out of 105 samples), for
the three isomers respectively (detection limit = 0.5 µg/kg) (EAJ,
1993).
5.1.3 Soil
Levels of 0.15 g/kg ( o-xylene) and 0.4 g/kg ( m- and
p-xylene) have been measured at a depth of 75-250 cm in soil
from a gasoline station. The soil was thought to be contaminated
as a result of leakage from an underground storage tank (Morgan &
Watkinson, 1990).
5.1.4 Biota
Xylenes have been measured at levels of 16 µg/kg wet weight in
oysters from Lake Pontchartrain, Louisiana, USA (Ferrario et al.,
1985). Levels of combined m- and p-xylene have been measured in
fish and shellfish from three estuarine sites in the USA (Reinert et
al., 1983). The levels found were: silverside (Menidia menidia) 100
and 180 µg/kg, ribbed mussel (Modiolus demissus) 100 µg/kg, and
grass shrimp (Palaemonetes pugio) 200 µg/kg.
Xylenes were monitored in fish during 1986 in Japan. o-Xylene
concentrations ranged from 0.8 to 5 µg/kg in 41 out of 137 samples,
m-xylene concentrations ranged from 0.86 to 9.2 µg/kg in 45 out of
124 samples and p-xylene concentrations ranged from 0.8 to 3 µg/kg
in 28 out of 127 samples (detection limit = 0.8 µg/kg for all three
isomers) (EAJ, 1993).
5.2 General population exposure
5.2.1 Source of Exposure
5.2.1.1 Air
Otson et al. (1993) pooled aliquots of individual air sample
extracts from 757 randomly selected Canadian residences. The
composite sample contained o-, m- and p-xylene concentrations of
8, 7 and 6 µg/m3, respectively. Fellin & Otson (1993) studied the
seasonal trends of xylene concentrations in the indoor air of 754
randomly selected Canadian homes. Lowest mean concentrations were
found in the summer and the highest in the autumn. Mean
concentrations ranged from 3.73 to 9.12 µg/m3 for p-xylene, from
6.81 to 26.03 µg/m3 for m-xylene and from 3.03 to 8.19 µg/m3 for
o-xylene. Lioy et al. (1991) monitored indoor and outdoor air at
three homes in New Jersey, USA during 1987. Indoor concentrations
ranged from 6.0 to 20.5 µg/m3 for o-xylene and from 15.2 to 57.5
µg/m3 for p-xylene. Outdoor concentrations ranged from 1.6 to 12.7
µg/m3 for o-xylene and from 4.6 to 36.9 µg/m3 for p-xylene.
Weschler et al. (1990) monitored xylene concentrations in the air
of a building with a history of occupant health and comfort
complaints. o-Xylene concentrations ranged from 2.1 to 9 µg/m3 and
m/p-xylene concentrations ranged from 3.9 to 25 µg/m3. The highest
xylene concentrations were associated with the lift shaft.
The California Total Exposure Assessment Methodology (TEAM) study
conducted in 1984 monitored xylenes in outdoor air, personal air and
breath samples for 188 people in Los Angeles County (urban) and
Contra Costa County (rural area). The 12-h arithmetic means of the
xylene concentrations are summarized in Table 4. A second TEAM study
was carried out in 1987 with 51 residents of Los Angeles, California.
The 24-h arithmetic means of the xylene concentrations in this study
are also summarized in Table 4. Daisey et al. (1994) reported the
concentrations of xylenes in the air in 12 office buildings in
California. The concentration of o-xylene ranged from 1.3 to 6.1
µg/m3 (0.30 to 1.40 ppb) and that of m/p-xylene from 4.0 to 20.0
µg/m3 (0.93 to 4.60 ppb).
Levels measured during winter were higher than summer levels for
all types of air. Smoking was determined to be the major determinant
for the presence of xylene in breath and personal air; concentrations
in the breath of smokers were more than double those of nonsmokers.
At petrol stations, exposure to vehicle exhaust and the type of
employment contributed significantly to increased concentrations of
xylene in breath and personal air (Wallace et al., 1988). Higgins et
al. (1983) reported that the gas-phase delivery of p-xylene in
ultra-low tar delivery cigarette smoke ranged from < 0.01 to 8
µg/cigarette, while the ranges for m- and o-xylene were < 0.01 to
20 µg/cigarette and < 0.005 to 10 µg/cigarette, respectively.
Table 4. Mean xylene concentrations in personal air, outdoor air and breath samples
(Wallace et al., 1988, 1991)
Location Date Sample type m/p-Xylene o-Xylene
(µg/m3) (µg/m3)
Los Angelesa February 1984 personal airc 28 13
outdoor air 24 11
breath 3.5 1.0
Los Angelesa June 1984 personal air 24 7.2
outdoor air 9.4 2.7
breath 2.8 0.7
Contra Costaa June 1984 personal air 11 4.4
outdoor air 2.2 0.7
breath 2.5 0.6
Los Angelesb February 1987 personal air 43 16
indoor aird 30 12
outdoor aire 18 6.5
breath (median value) 2.5 0.8
Los Angelesb July 1987 personal air 27 9.2
indoor aird 12 4.3
outdoor aire 7.4 2.8
breath (median value) 0.7 0.25
a 12-h arithmetic means of xylene concentration
b 24-h arithmetic means of xylene concentration
c Air sample collected at the breathing level of the subjects
d samples collected in living room-kitchen area
e samples collected in backyards of homes
Weisel et al. (1992) analysed air within automobiles whilst
idling, driving on a suburban route in New Jersey and commuting into
New York City. During a 30-min idling period, mean m/ p-xylene
concentrations ranged from 1.3 to 42 µg/m3 and o-xylene
concentrations ranged from 0.5 to 18 µg/m3. The highest values were
recorded during the summer and the lowest during the winter. The mean
m/ p-xylene concentrations for the suburban route were 23 and 16
µg/m3 for low and high ventilation, respectively; for o-xylene mean
concentrations were 8.6 and 7.5 µg/m3. Xylene concentrations of 23
µg/m3 for m/p-xylene and 8.8 µg/m3 for o-xylene were recorded
whilst commuting into New York City; mean xylene levels of 37 µg/m3
( m/ p-xylene) and 12 µg/m3 ( o-xylene) were measured whilst
travelling through a tunnel. Chan et al. (1991) studied exposure of
commuters in Boston, USA, to xylenes and found that the highest
exposures were associated with commuting by car ( m/ p-xylene = 20.9
µg/m3; o-xylene = 7.3 µg/m3).
The levels of xylenes in samples of air in the vicinity of petrol
pumps in five Canadian cities were monitored between June and August
1985 and between January and March 1986. Measured mean concentrations
of all the isomers of xylene in the immediate vicinity of self-service
pumps were 0.716 mg/m3 in the winter and 0.973 mg/m3 in the summer,
and ranged from 0.678 to 3.77 mg/m3 and 0.001 to 6.9 mg/m3,
respectively (PACE 1987; 1989). p-Xylene represented more than 70%
of the mean concentrations for all isomers.
van Wijnen et al. (1995) monitored ambient air for traffic-related
pollutants in Amsterdam. Maximum mean time-weighted concentrations
were as high as 193 µg/m3 for car drivers, 46 µg/m3 for cyclists
and 41 µg/m3 for pedestrians.
A mean total xylene level of 65 µg/m3 (15.06 ppb) in ambient air
was measured in Turin, Italy, throughout 1991 (Gilli et al., 1994).
Within a 10-day sampling period, mean concentrations of 85 and 57
µg/m3 (19.50 and 13.13 ppb) were measured in indoor air for day-
and night-time sampling, respectively. The corresponding mean
concentrations for outdoor air were 82 and 54 µg/m3 (18.82 and 12.31
ppb) for day- and night-time sampling respectively. The mean personal
exposure of the volunteers was 84 µg/m3 (19.30 ppb).
Monitoring for xylene has been carried out at filling stations
in Rome, Italy (Lagorio et al., 1993). The range of measured
concentrations from 703 personal samples among 111 workers was 0.003
to 15.37 mg/m3 (mean: 0.32 mg/m3).
Bostrom et al. (1994) calculated the average exposure dose for
xylenes (o, m and p) to be 11 µg/m3, based on the relationship
between nitrogen oxides (NOx) and xylenes, and a mean exposure for
the Swedish population of 23 µg/m3 for nitrogen oxides.
The exposure of students commuting to school in Taipei City,
Taiwan, in 1992 has been reported by Chan et al. (1993). Students
commuting by bus were exposed to 222.8 µg/m3 of o-xylene and 418.1
µg/m3 of m/p-xylene. Students commuting by motorcycle were exposed
to 524.5 µg/m3 of o-xylene and 926.9 µg/m3 of m/p-xylene. The
air in school classrooms was also monitored; the mean concentration
of o-xylene was 26.3 µg/m3 and that of m/p-xylene was 46.4 µg/m3.
5.2.1.2 Food
o-Xylene has been detected at levels up to 25 µg/kg (mean level
9 µg/kg) in seven samples of dried beans, at a level of 8 µg/kg in
split peas and at a level of 3 µg/kg in lentils from the USA (Lovegren
et al., 1979). Xylenes have been identified but not quantified in
various other food items including cheese from Italy (Meinhart &
Schreier, 1986), dry red beans from the USA (Buttery et al., 1975),
winged beans and soybeans from the Philippines (del Rosario et al.,
1984) and tomatoes and tomato products from Japan (Chung et al.,
1983). All three isomers were detected in the volatile compounds from
roasted turkeys fed on a basal diet supplemented with tuna oil
(Crawford & Kretsch, 1976).
5.2.1.3 Drinking-water
All three xylene isomers were detected in all of 14 samples of
United Kingdom drinking-water derived from rivers, lowland reservoirs
and groundwater (detection limit not stated) (Fielding et al., 1981).
Xylenes have been shown to pass through a drinking-water treatment
plant unaltered in concentration (Dowty et al., 1975).
Otson et al. (1982) monitored 30 Canadian potable water treatment
facilities. Mean total xylene concentrations in both raw and treated
water were less than the detection limit in this study (1 µg/litre).
Maximum values were less than 1 µg/litre for raw water and 8 µg/litre
for treated water.
When Williams et al. (1982) sampled 12 Great Lakes (Canada)
municipal drinking-water supplies, o- and m-xylenes ( m-isomers)
were not detected at five of the sites and in 50% of the 22 samples.
Detectable concentrations ranged from 1.1 to 12 µg/litre.
The concentration of m- and p-xylene in tap water, Toronto,
Canada, was reported to be 0.06 µg/litre (City of Toronto, 1990). The
concentrations of xylene in seven samples of bottled water ranged from
less than the detection limit to 0.07 µg/litre.
5.2.1.4 Other source of exposure
The US EPA (Sack et al., 1992) carried out analyses of 1159
household products. The results of the analyses, according to product
category, are presented in Table 5.
5.2.2 Xylene levels in human biological samples
Xylenes have been detected in human blood at levels of between
0.5 and 160 µg/litre (mean = 5.2 µg/litre) (Antoine et al., 1986).
The level was found to be significantly elevated in 7 out of 250
people sampled. m-Xylene has been determined in human whole blood
at levels of 10-20 ng/litre (Cramer et al., 1988).
Table 5. Mean xylene concentrations in various products
m-Xylene o/p-Xylene
Product category Number of Products containing Mean concentration Products containing Mean concentration
products tested analyte (%) (% w/w) analyte (%) (% w/w)
Automotive 167 26.7 10.6 10.0 31.0
Household cleaners and 111 33.3 1.4 - -
polishers
Paint-related products 463 60.3 4.2 58.2 2.8
Fabric and leather 91 - - 33.3 0.1
treatments
Cleaners from electronic 69 - - - -
equipment
Oils, greases, lubricants 111 9.3 0.2 11.9 0.2
Adhesive-related products 76 9.1 0.2 9.1 0.2
Miscellaneous: 71 - - - -
specialized cleaners, rust
remover, correction fluid
Ashley et al. (1994) monitored blood samples from more than 600
people in the USA third national health and nutrition examination
survey. None of the subjects were occupationally exposed to xylenes.
Mean concentrations were 0.37 µg/litre for m/ p-xylene and 0.14
µg/litre for o-xylene.
Fustinoni et al. (1995) reported that the mean blood
concentration of m/p-xylene in non-smoking traffic wardens in Milan,
Italy, was 853 ng/litre before the shift and 683 ng/litre at the end
of the shift. The level in non-smokers of the clerical environment
from the same area was 809 and 629 ng/litre, respectively.
Xylenes were detected, but not quantified, in 8 out of 12 samples
of breast milk (Pellizzari et al., 1982).
Xylenes have been detected in the axilla odour from humans
(Labows et al., 1979). Placental transfer of xylene has been shown to
occur (Dowty & Laseter, 1976).
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure to xylenes alone is rare. There is usually
simultaneous exposure to other compounds, often organic solvents. In
one study, however, 10 female laboratory workers had been exposed to
xylene (vapour as well as liquid) for about 4 h daily for up to 16
years. Exposure levels, determined by only one measurement in the
breathing zone and by only one in the workroom air, were 139 mg/m3
(32 ppm) and 62 mg/m3 (14 ppm), respectively (Proust et al., 1986).
A number of studies have been performed on workers occupationally
exposed to solvent mixtures including xylenes (e.g. Seppäläinen et
al., 1978; Elofsson et al., 1980; Husman, 1980; Lindström et al.,
1982; Valciukas et al., 1985; Maizlish et al., 1987; Van Vliet et al.,
1987).
In a study on spray varnishers (Angerer & Wulf, 1985) 35 male
workers were exposed to 2.2-14.8 mg/m3 (0.5-3.4 ppm) o-xylene,
13.9-50.1 mg/m3 (3.2-11.7 ppm) m-xylene, 3.9-18.7 mg/m3 (0.9-4.3
ppm) p-xylene, 1.4-7.5 ppm ethylbenzene, < 1.5 ppm toluene, <1.2
ppm n-butanol, < 35.5 ppm 1,1,1-trichloroethane and several C9
aromatic compounds.
Mean 8-h xylene concentrations of 21.7 and 27.8 mg/m3 (5 and 6.4
ppm) were measured in a lacquer/resin spraying operation in a
woodworking facility in the USA (Fairfax, 1995).
In a study on workers engaged in dip-coating of metal parts the
mean xylene vapour concentration was 16.5 mg/m3 (3.8 ppm). The
concentration of the isomers were 3.5 mg/m3 (0.8 ppm) o-xylene, 9.1
mg/m3 (2.1 ppm) m-xylene and 3.9 mg/m3 (0.9 ppm) p-xylene (Kawai
et al., 1991a).
In a study on workers employed in printing, painting or the
manufacture of plastic coated wires, there was an exposure to mixtures
of toluene and xylenes. Personal air samples were collected and
analysed for toluene and the three isomers of xylene. Maximum
exposures to xylenes were > 435 mg/m3 (100 ppm) with a time-weighted
average of 17.4 mg/m3 (4 ppm). About half of the xylene was
m-xylene (Huang et al. 1994).
Concentrations of xylenes ranging from 0.4 to 7.0 mg/m3 (0.1 to
1.6 ppm) were measured during full-shift personal exposure monitoring
at an axle painting operation in Newark, Ohio, USA (NIOSH, 1991).
Breathing zone samples were collected from workers in the liquid
inks and paste inks departments of a small printing ink manufacturing
factory. The mean full shift concentration of xylene in the liquid
inks department was 314 mg/m3 and in the paste inks department 24
mg/m3. Other solvents in the factory were toluene, ethyl acetate,
ethanol, isopropanol and n-hexane (Lewis, 1994).
During paint operations in an aeronautical factory xylene
concentrations (sum of the three isomers) of 14.3 to 167.0 mg/m3 were
measured by personal air monitoring. Other solvents present were
methyl ethyl ketone, ethyl acetate, n-butyl alcohol, methyl isobutyl
ketone, toluene, n-butyl acetate, ethylbenzene, ethylene glycol and
monoethylether acetate (Vincent et al., 1994). Similarly,
concentrations of xylene (isomer not specified) among printers have
been measured to be between 5.6 and 91.3 mg/m3 (1.3 and 21 ppm).
Other solvents were acetone, ethyl acetate, methyl ethyl ketone, white
spirit, toluene and trichloroethylene (Nasterlack et al., 1994).
Levels of xylene to which workers have been exposed in
histological laboratories, measured as 8-h time-weighted average, are
from about 10.9 mg/m3 (2.5 ppm) to over 304.5 mg/m3 (70 ppm)
(Angerer & Lehnert, 1979; Kilburn et al, 1985; IARC, 1989). In a
hospital laboratory, levels of up to 1740 mg/m3 (400 ppm) have been
measured (Klaucke et al, 1982). In lithographical processes in Poland
the mean value in 1968 was 119 mg/m3 and ten years later 130 mg/m3
(Moszczynski & Lisiewicz, 1985), and in a chemical plant in Hungary
the mean concentration in air was 47-56 mg/m3 (Pap & Varga, 1987).
In a NIOSH study involving conducted environmental monitoring of
xylene in various worksites, the concentrations where workers were
exposed to gasoline and exhaustion emissions ranged from < 0.08 to
68.3 mg/m3 (0.02 to 15.7 ppm) (NIOSH, 1993a). The concentration in
the air in the cab ranged up to 8.7 mg/m3 (2 ppm) (NIOSH, 1993b).
The personal breathing zone samples of an indoor parking garage, a
medical taxi cab, an automobile dealership/repair shop and a state
highway maintenance garage in New York State ranged from < 0.08 to
1.87 mg/m3 (0.02 to 0.43 ppm) (NIOSH, 1993c). The concentration in
personal breathing zone samples from workers in automotive maintenance
facilities/dealers, repair shops) in Stamford, Connecticut, USA,
ranged from < 0.08 to 1.39 mg/m3 (0.02-0.32 ppm) (NIOSH, 1993d).
The highest full-shift exposure at an oil refinery in Colombia was
22.2 mg/m3 (5.1 ppm), and all short-term exposures were below the
quantifiable concentration of 1.3 mg/m3 (0.3 ppm) (NIOSH, 1994).
Kawai et al. (1991b) monitored the exposure of tank truck drivers
to xylenes in Japan. Maximum concentrations were 26.5 to 94.8 mg/m3
(6.1 to 21.8 ppm) during loading and 3.9 to 5.96 mg/m3 (0.90 to 1.37
ppm) during a delivery trip.
Fustinoni et al. (1995) measured the exposure of traffic wardens
to xylenes in Milan, Italy, in 1994. During a 10-day period, the
concentration of o-xylene was 48 µg/m3 and of m/p-xylene was 108
µg/m3. Concurrent monitoring of policemen in clerical environments
was also carried out; the concentration of o-xylene in indoor air
was 32 µg/m3 and of m/p-xylene was 53 µg/m3.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
6.1.1 In humans
In humans, absorption of xylenes has been investigated following
inhalation of the vapour or dermal application of the liquid.
Pulmonary retention of m-xylene became relatively constant at
about 60% after the first 5-10 min of exposure to 430 mg/m3 (100 ppm)
(Riihimäki et al., 1979a). The determination was made by measurement
of atmospheric and exhaled concentrations. In a previous study
(Sedivec & Flek, 1976) a pulmonary retention of 62-64% was reported
for each xylene isomer at exposure levels of 196-391 mg/m3 (45-90
ppm) for up to 7 h.
A relatively constant retention, average 59%, was reported in
individuals exposed to varying m-xylene concentrations in the range
of 304-957 mg/m3 (70-220 ppm), both at rest and while undergoing
intermittent physical exercise (Riihimäki et al., 1979b). A slight
reduction in retention was noted when resting individuals subsequently
underwent moderately heavy physical exercise. Increased pulmonary
ventilation during exercise was found to be associated with a
corresponding increase in total uptake of xylenes (Riihimäki et al.,
1979b; Åstrand et al., 1978).
In resting individuals exposed to 870 mg/m3 (200 ppm) xylene
(8.8% o-xylene, 49.4% m-xylene, 1.4% p-xylene, and 40.4%
ethylbenzene) the alveolar air level was about 15% of that in inspired
air, while 36% was recorded for individuals undergoing heavy exercise
(Åstrand et al., 1978). The ratio of m/p-xylene to ethylbenzene was
found to be similar in alveolar air and inspired air. This indicates
similar rates of pulmonary absorption for xylenes and ethylbenzene.
In this study, o-xylene was not measured (Engström & Bjurström,
1978).
During inhalation exposure in resting subjects, a levelling of
the blood xylene concentration began after about 15 min of exposure to
435-870 mg/m3 (100-200 ppm) xylene. Light exercise increased the
blood level of xylene and indications of plateauing were noted after
about 2 h (Åstrand et al., 1978). In another study, an exposure to
435-1261 mg/m3 (100-290 ppm) m-xylene revealed a rapid rise in
xylene blood levels during the first hour. Repeated exposure to 430
mg/m3 for 4.5 days (6 h/day) gave rising pre-exposure morning blood
levels, indicating some accumulation of m-xylene (Riihimäki et al.,
1979a; Riihimäki et al., 1982a,b).
Dermal absorption of xylenes has been studied after exposure to
the vapour or the liquid. Liquid xylenes (about 0.2 ml of the
individual isomers) were applied to the forearm. Uptake into the skin
was calculated by measuring the remained material after 5-15 min
(Dutkiewicz & Tyras, 1968). Uptake values of 50-160 µg/cm2 per min
were reported. However, it should be noted that not all the xylene
taken up necessarily penetrated the skin and was absorbed. In more
recent studies, dermal absorption has been studied following hand
immersion in liquid m-xylene for 15-20 min. Absorption, estimated
from the urinary level of the metabolite m-methylhippuric acid, was
recorded to be about 2 µg/cm2 per min in eight volunteers (Engström
et al., 1977). The amount absorbed (about 35 mg) through both hands
was estimated to be equal to the amount absorbed through inhalation of
435 mg/m3 (100 ppm) during the same time. Another group (Lauwerys et
al., 1978) obtained a similar value for dermal absorption (2.45
µg/cm2 per min) based on urinary levels of m-methylhippuric acid
and m-xylene levels in exhaled breath.
Dermal exposure of m-xylene vapour has been investigated in
volunteers exposed to 1305 mg/m3 (300 ppm) (two men) or 2610 mg/m3
(600 ppm) (three men) for 3.5 h. Inhalation was excluded by means of
a full facepiece supplied-air respirator with overpressure inside the
mask. The volunteers were dressed in pyjamas and performed exercise to
raise the skin temperature and perspiration (Riihimäki & Pfäffli,
1978). Dermal absorption appeared to be directly dependent on vapour
concentration. At 2610 mg/m3 the absorption was calculated to be
approximately 0.01 µg/cm2 per min. In a further experiment, three
subjects were exposed to 87 mg/m3 (20 ppm) without respirator. In
this case both pulmonary and dermal absorption could occur. The total
absorption of m-xylene in this experiment was calculated to be of
the same order of magnitude as after dermal-only exposure to 2610
mg/m3.
6.1.2 In laboratory animals
Absorption of xylene was recorded following whole-body exposure
of mice to ring-labelled 14C- m-xylene vapour for 10 min. Based on
autoradiograms the absorption was primarily through respiration
(Bergman, 1979; Bergman, 1983). All xylenes are well-absorbed orally
by rats, based on urinary metabolites. Peak blood levels were reported
4 h after an oral dose of 0.5-4 g m-xylene/kg body weight or 1.1 g
p-xylene/kg body weight (Gut & Flek, 1981).
The rate of absorption of liquid o-xylene across excised rat
skin has been calculated to be 0.103 µg/cm2 per min at steady state
(Tsuruta, 1982).
6.2 Distribution
6.2.1 In humans
Little information is available on the distribution of xylenes in
humans. A peritoneal fat/air partition coefficient of 3605 has been
determined for m-xylene in vitro (Sato et al., 1974). The times
required to reach equilibrium in tissues have been calculated from
physiological parameters. It is estimated to be a few minutes for
well-perfused parenchymal organs, a few hours for muscles and several
days for adipose tissue (Riihimäki & Savolainen, 1980).
Postmortem analysis on a woman who had swallowed xylene 4 days
prior to death, revealed xylene to be present in all tissues
investigated (Takatori et al., 1982). The ratios of the three isomers
(ortho: meta: para) were 3:5:2 in the stomach content, 3:6:1 in blood
and 4:4:2 in adipose tissue. In the brain, liver, spleen, kidney and
myocardium, however, the o-xylene accounted for about 80%.
When volunteers were exposed to 435 to 870 mg/m3 (100 to 200
ppm) mixed xylenes for 2 h, the ratio of m/p-xylene to ethylbenzene
was similar in subcutaneous fat and inspired air up to 22 h
post-exposure (Engström & Bjurström, 1978). In another study
volunteers were exposed to 391-870 mg/m3 (90-200 ppm) m-xylene 6 h
per day 5 days per week (plus an additional day after the weekend)
(Engström & Riihimäki, 1979). The proportion of absorbed m-xylene
distributed to subcutaneous fat was calculated to be about 4% in
resting individuals and 8% in those undergoing exercise.
Two adult male and two adult female volunteers were exposed by
inhalation to < 108-217 mg/m3 (25-50 ppm) of m-xylene (Laparé,
1993). Doubling the exposure concentration led to a proportional
increase in the concentrations of unchanged solvents in alveolar air
and blood at the end of a 7-h exposure period. Cumulative urinary
excretion of the metabolites exhibited a nearly proportional increase.
It is also suggested that alveolar air solvent concentration is a
reliable index of exposure to m-xylene.
6.2.2 In laboratory animals
The distribution of xylene has been studied in male rats exposed
to about 217 mg/m3 (50 ppm) 14C-labelled p-xylene for 8 h
(Carlsson, 1981). The highest concentrations were present in the
kidneys (up to about 1000 nmol/g tissue) and subcutaneous fat (up to
more than 250 nmol/g tissue). Higher concentrations than in blood
were also found in the ischiatic nerve. Lower concentrations than in
blood were found in the cerebrum, cerebellum, muscle and spleen.
Elimination half-times from fat were estimated to be 2-7 h. In
pregnant rats, o-xylene has been shown to cross the placenta. The
concentrations in fetal blood were 25-30% of that in maternal blood
after a 2-h exposure (Ungvary et al., 1980). o-Xylene was also
detected in amniotic fluid.
The concentration of m-xylene in perirenal fat and cerebrum was
positively correlated to exposure levels in rats exposed to 217 to
3262 mg/m3 (50 to 750 ppm) m-xylene 6 h/day, 5 days/week for 1-2
weeks (Savolainen & Pfäffli, 1980; Elovaara et al., 1982).
Accumulation in perirenal fat has also been shown in rats exposed to
1305 mg/m3 (300 ppm) xylene (80% m-xylene, 12% p-xylene) 6 h/day,
5 days/week for 1-2 weeks (Savolainen et al., 1979a,b). In a similar
study with 1305 mg/m3 m-xylene, no accumulation in perirenal fat or
brain tissue was recorded after one week (Elovaara et al., 1982).
When rats were exposed to 1305 mg/m3 xylene (19.2% o-xylene, 43.0%
m-xylene, 19.5% p-xylene, 18.3% ethylbenzene) during 6 h/day, 5
days/week for 18 weeks, a progressive increase of xylene levels in
perirenal fat was demonstrated over the first 2 weeks followed by a
decline (Elovaara et al., 1980). The decline was attributed to xylene
inducing its own metabolism. Similar results were obtained in a study
where rats were exposed to 1305 mg/m3 xylene (85% m-xylene, 15%
o-xylene) (Savolainen et al., 1979a,b).
The tissue distribution of 14C-labelled xylenes has been studied
in mice by low-temperature whole body autoradiography (Bergman, 1979;
Bergman, 1983; Ghantous & Danielsson, 1986). When male mice were
exposed to about 1435 mg/m3 (330 ppm) m-xylene for 10 min, high
levels of radioactivity was found immediately post-exposure in body
fat, bone-marrow, brain (white matter), spinal cord, spinal nerves,
liver and kidney. Radioactivity in the nervous system and fatty
tissues was due to xylene alone and was present for 1 and 8 h,
respectively. High levels of xylene metabolites were recorded in
blood, lung, liver and kidney for up to 8-h post-exposure and in
intestinal contents, bronchi and nasal mucosa up to 24 h (Bergman,
1979; Bergman, 1983).
Autoradiography of male mice following 10 min inhalation of
radioactively labelled p-xylene revealed an accumulation of
non-volatile metabolites in the nasal mucosa and the olfactory bulb of
the brain. It was assumed that the activity represented aromatic
acids (methyl hippuric acid and toluic acid) (Ghantous et al., 1990).
The same technique has been used to study distribution of
radioactivity after exposure of pregnant mice to about 8700 mg/m3
(2000 ppm) 14C-labelled p-xylene for 10 min (Ghantous & Danielsson,
1986). High concentrations of xylene were recorded in the adult brain
and lung with lesser amounts in kidney and liver. At all stages of
gestation studied (days 11, 14, 17) p-xylene appeared to pass
immediately from dam to embryo/fetus. The concentration in fetus,
however, was low; 2% of that in maternal brain. Xylene was evenly
distributed in the fetus following exposure on day 11. After exposure
on day 17 the xylene was located primarily in the liver (Ghantous &
Danielsson, 1986).
In a study where rabbits were exposed to xylene (27% o-xylene,
52% m-xylene, 21% p-xylene) for several months, the concentration
was reported to be higher than in blood in the adrenals, bone-marrow
and spleen (Fabre et al., 1960). Due to the limited nature of the
study it is impossible to draw any firm conclusions.
Partition coefficients have been determined in vitro for
m-xylene using tissue homogenates and blood (Sato et al., 1974).
The following blood/air values were reported: 20 (pig blood), 21
(rabbit plasma) and 37 (rabbit blood). Tissue/blood partition
coefficients reported were 1.6-2.1 (muscle, kidney, heart and lung),
3.0-3.3 (liver and brain), 42 (bone-marrow) and 146 (peritoneal fat).
The relatively low value for brain tissue has been attributed to the
content of phospholipids in which xylenes are less soluble than in
neutral fat (Riihimäki & Savolainen, 1980).
6.3 Metabolic transformation
6.3.1 In humans
Fig. 1 shows schematically the metabolic pathways for xylene
( m-xylene is used as an example) in humans.
Metabolism of xylenes by humans consists primarily of side-chain
oxidation to form methylbenzoic acid (Sedivec & Flek, 1976; Riihimäki
et al., 1979a; Riihimäki et al., 1979b). Methylbenzoic acid is
conjugated principally with glycine and excreted in urine as
methylhippuric acid. It has been estimated that glycine conjugation
would be saturated in humans exposed to about 1174 mg/m3 (270 ppm)
xylene while working and to about 3393 mg/m3 (780 ppm) while resting
(Riihimäki, 1979a). A small amount of the glucuronide ester of
methylbenzoic acid and trace levels of methylbenzyl alcohol have been
detected in human urine (Ogata et al., 1980; Engström et al., 1984;
Campbell et al., 1988).
Hydroxylation of the aromatic ring with the formation of
dimethylphenols seems to be a minor pathway in humans. The following
dimethylphenol isomers have been identified in human urine: 2,3- and
3,4-dimethylphenol (with o-xylene), 2,4-dimethylphenol (with
m-xylene) and 2,5-dimethylphenol (with p-xylene) (Sedivec & Flek,
1976; Engström et al., 1984).
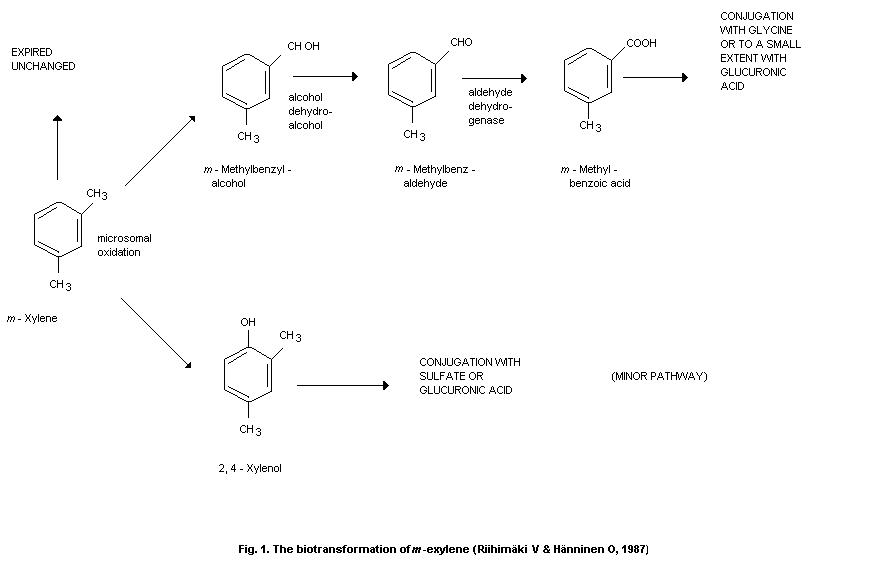 6.3.2 In laboratory animals
Most studies on metabolism of xylenes have been performed on rat.
The principal pathway involves side-chain oxidation to methylbenzoic
acid via methylbenzyl alcohol and methylbenzyl aldehyde.
Methylbenzoic acid is then conjugated with glycine or glucuronic acid
(Sugihara & Ogata, 1978; Ogata et al., 1980; Elovaara et al., 1984).
Conjugation with glycine to form methylhippuric acid predominates for
m- and p-xylene (Sugihara, 1979; Ogata & Fujii, 1979; Elovaara et
al., 1984). In the case of o-xylene, glucuronide formation has been
reported to predominate (Ogata et al., 1980).
A separate minor pathway resulting in urinary excretion of
thioethers has been studied (Van Doorn et al., 1980; Van Doorn et al.,
1981). This pathway appears to be more important for o-xylene than
for the other isomers. Hydroxylation of the aromatic ring with the
formation of dimethylphenols has been reported to be another minor
metabolic pathway in rats (Bakke & Scheline, 1970; Elovaara et al.,
1984).
Methylbenzoic acid and dimethylphenols are present in urine of
guinea-pigs and rabbits exposed to xylene isomers (Fabre et al.,
1960). After oral dosing of rabbits with xylenes the main metabolite
was methylbenzoic acid (Bray et al., 1949). The acid was considered
to be mostly present as the glycine conjugate, methylhippuric acid.
Studies with isolated perfused livers and lungs from rabbit
indicate differences in the metabolic pathways between these two
organs (Smith et al., 1982). In the liver p-methylhippuric acid was
the major metabolite detected. In the lung p-methylbenzyl alcohol
and p-methylbenzoic acid were the main metabolites detected. There
was formation of 2,5-dimethylphenol in the lungs but not in the liver.
Metabolism of m- and p-xylenes to m- and p-methylbenzyl alcohols
has been found to be greater with hepatic than with pulmonary
microsomes (Harper, 1975; Toftgård et al., 1986). Further metabolism
to methylbenzoic acid occurred in the presence of hepatic but not
pulmonary cytosolic fraction. Daily exposures to xylene increased the
activities of liver microsomal enzymes and concentrations of
cytochrome P450 (Elovaara et al., 1982; Pathiratne et al., 1986).
Metabolism of m-xylene by cerebral microsomal preparations was
reported to be very slow (Elovaara et al., 1982).
No definitive studies have been reported showing which microsomal
P-450 enzymes are involved in xylene metabolism. However when rats
were given m-xylene (1.0-1.4 ml/kg body weight) by gastric
intubation once daily for 3 consecutive days and killed 24 h after the
last treatment, xylene caused an induction of CYP2B and CYP2E1 in
liver microsomes (Raunio et al., 1990). Exposure of male Wistar rats
to each of the xylene isomers by inhalation at a concentration level
of 4000 mg/m3 for 20 h/day over 4 days similarly induced hepatic
CYP2B1, while CYP2E1 was reduced, as estimated by Western blots (Gut
et al., 1993).
6.4 Elimination and excretion
6.4.1 In humans
Absorbed xylenes are excreted mainly as metabolites in urine.
Small amounts are excreted unchanged in exhaled air. Excretion in
faeces appears to be unimportant. The rate of clearance of p-xylene
from blood has been calculated to be 2.6 litres/kg per hour at 87
mg/m3 (20 ppm) and 1.6 litres/kg per hour at 304 mg/m3 (70 ppm)
(Wallén et al., 1985).
When volunteers were exposed to a constant concentration of
about 391-870 mg/m3 (90-200 ppm) m-xylene over 5 days, at least 97%
was calculated to be excreted as m-methylbenzoic acid conjugates.
2,4-Dimethylphenol conjugates accounted for 1-2% of the metabolites
(Riihimäki et al., 1979a; Riihimäki et al., 1979b). When volunteers
were exposed to about 195 mg/m3 (45 ppm) of o-, m- or p-xylene
for 8 h, about 95-99% of the dose was excreted as methylhippuric acid
in urine. Dimethylphenol excretion was estimated to be 0.1 to 2% of
the dose absorbed (Sedivec & Flek, 1976). About 90% of the absorbed
dose of m-xylene was excreted as methylhippuric acid after exposure
to 435 mg/m3 (100 ppm) for 4 h (Lauwerys et al., 1978; Campbell et
al., 1988). On the other hand, after exposure to 600 mg/m3 (138 ppm)
of o-xylene, only 46% was excreted in urine as methylhippuric acid
and only trace amounts of the o-methylbenzoyl glucuronide were
detected (Ogata et al., 1980).
In a study of 121 male workers engaged in dip-coating of metal
parts, the mean concentration was 3.48 mg/m3 (0.8 ppm) o-xylene,
9.1 mg/m3 (2.1 ppm) m-xylene and 3.91 mg/m3 (0.9 ppm) p-xylene.
The workers were also exposed to 0.8 ppm toluene and 0.9 ppm
ethylbenzene. At the end of the 8 h-shift urine samples were
collected and methylhippuric acid was determined. There was a linear
relationship between the intensity of exposure to xylenes and the
concentration of methylhippuric acid in urine. The methylhippuric
acid concentration as a function of increasing xylene concentration
was 17.8 mg/litre per ppm (Kawai et al., 1991a).
In workers occupationally exposed to an average of 17.4 mg/m3
(4 ppm) xylene (combination of all three isomers), with a maximum of
more than 430 mg/m3 (100 ppm), the urinary excretion of
methylhippuric acid was linearly correlated with the air exposure
(Huang et al., 1994).
The urinary methylhippuric acid excretion of xylene-exposed
painters at the end of the working week showed two distinct phase of
excretion. Half-times for urinary excretion of methylhippuric acid
were estimated to be 3.6 h for the first 10 h and 30.1 h for the next
2 days after exposure (Engström et al., 1978). A positive association
between the degree of obesity and the length of half-time was seen.
In volunteers exposed to m-xylene the urinary excretion of
m-methylbenzoic acid was described as triphasic with half-times of
1-2, 10 and 20 h (Riihimäki et al., 1979a). The observed elimination
half-time in the subcutaneous adipose tissue was about 58 h (Engström
& Riikimäki, 1979).
After oral administration of o-xylene (39 mg/kg body weight)
maximum urinary levels of glycine and glucuronide conjugates of
o-methylbenzoic acid were reported to be 33.1 and 1.0% of the
administered dose, respectively. Similar values were obtained after
an oral dose of 78 mg/kg body weight (Ogata et al., 1979; Ogata et
al., 1980).
About 4-5% of the dose absorbed in the lungs is exhaled unchanged
after exposure to 870 mg/m3 (200 ppm) xylene (Sedivec & Flek, 1976,
Åstrand et al., 1978, Riihimäki et al., 1979a, Riihimäki et al.,
1979b). Elimination in exhaled breath is reported to follow a similar
triphasic profile to that for urinary excretion of methylbenzoic acid
conjugates (Riihimäki et al, 1979). An initial half-time of about one
hour was obtained in a study by Campbell et al., 1988.
6.4.2 In laboratory animals
Exhalation of unchanged m-xylene has been described in one
study on rats. Exhalation was greatest 4 h after an intraperitoneal
injection, and 13% of the dose was exhaled unchanged within 10 h
(Sugihara, 1979). In mice 3.4% of the dose was exhaled within 8 h
(Bergman, 1979; Bergman, 1983).
In rats a total of 46% of the m-xylene dose (5 mmol/kg body
weight) was excreted as m-methylbenzoic acid within 24 h, and
phenobarbital (PB) treatment increased it to 70% of the dose. PB
treatment increased the elimination of m-methylbenzoic acid after
oral administration about 4-fold in the first 3 h, more then 2-fold in
the first 12 h, and 1.5-fold within 24 h compared to untreated but
m-xylene-exposed rats (Gut & Flek, 1981).
Wistar rats were pretreated with PB for 3 days (80 mg/kg body
weight per day) and then given m-xylene orally or intraperitoneally
at a small (0.081 mmol/kg) or a large (0.81 mmol/kg) dose or by
inhalation (6 h) at a low (174 mg/m3, 40 ppm) or high (1740 mg/m3,
400 ppm) concentration. PB treatment had a significant effect on the
metabolism of inhaled m-xylene (decreased blood m-xylene and
increased urinary excretion of m-methylhippuric acid), but only at
the high dose. The PB-induced enzyme induction had an effect at both
dose levels on the metabolism of orally administered m-xylene. The
effect on intraperitoneally administered m-xylene was more similar
to that of inhaled than that of orally administered m-xylene (Kaneko
et al., 1995).
After an intraperitoneal injection of 87-348 mg/kg body weight
m-xylene to rats, 53-75% of the dose was excreted as m-methyl-
hippuric acid in urine during 24 h (Ogata & Fujii, 1979). After
an intraperitoneal dose of 319 mg/kg body weight the proportion
excreted as mercapturic acids was calculated to be 10% for o-xylene
and 0.6-1.3% for m- and p-xylene (Van Doorn et al., 1980).
Male rats were exposed to soil-adsorbed (sandy soil or clay soil)
or pure 225 µl of m-xylene containing 20 µCi of m-[14C]-xylene
through the skin (Skowronski et al., 1990). The major route of
excretion in the pure and sandy groups was via expired air followed by
urine. However, in the presence of clay soil, the percentage of the
initial dose in expired air was similar to that in urine. In the
presence of clay soil, an increase in m-xylene was observed in
adipose tissue. Methyl hippuric acid was the main urinary metabolite.
Turkall et al. (1992) reported the bioavailability of
soil-adsorbed m-xylene in male and female rats. The rats were
gavaged with an aqueous suspension of 5% of gum acacia containing 150
µl of m-xylene with 5 µCi m-[14C]-xylene alone or adsorbed to
sandy or clay soil. While ingested soil contaminated with m-xylene
produced a higher bioavailability than the chemical alone in females,
no effects of soil was observed in males. No differences in the
bioavailability of m-xylene alone were observed between the sexes.
m-Xylene was primarily metabolized and excreted in urine,
methylhippuric acid being the main urinary metabolite in all groups.
6.5 Factors affecting toxicokinetics in humans and animals
In humans, co-exposure of m-xylene and ethylbenzene and
consumption of ethanol or aspirin (acetylsalicylic acid) prior to
inhalation has been shown to reduce urinary excretion of one or more
xylene metabolites, including methylhippuric acid (Riihimäki et al.,
1982a,b; Engström et al., 1984; Campbell et al., 1988). In the case
of ethanol consumption an increase in concentration of m-xylene in
blood was described (Riihimäki et al., 1982a,b). Co-exposure to
toluene decreased the ratio of the concentration of p-xylene in
venous blood to that in exhaled air (Wallén et al., 1985). The dermal
absorption of liquid m-xylene was reduced in the presence of
isobutanol (Riihimäki, 1979a).
Volunteers given ethanol on two evenings (total dose 137 g)
preceding exposure by inhalation to either 435 or 1740 mg/m3 (100 or
400 ppm) m-xylene for 2 h enhanced the metabolism of m-xylene but
only at 1740 mg/m3 (Tardif et al., 1994). Ethanol pretreatment
decreased the concentration of m-xylene in blood and alveolar air
during and after exposure and increased urinary excretion of
m-methylhippuric acid at the end of exposure to 1740 mg/m3.
Five male volunteers were exposed for 7 h/day over 3 consecutive
days to 50 ppm toluene, 174 mg/m3 (40 ppm) xylene (15% o-xylene,
25% m-xylene and 60% p-xylene) or a combination of both. To study
high-level exposure four men were exposed for 4 h to 95 ppm toluene or
348 mg/m3 (80 ppm) xylene or a combination of both. Mixed exposure
(low-level) did not alter the concentration of the solvents in blood
or exhaled air, nor did it modify the excretion of urinary
metabolities. High-level mixed exposure, however, increased the
concentration of solvents in blood and exhaled air and caused a delay
in the urinary excretion of hippuric acid (Tardif et al., 1991).
Simultaneous exposure by inhalation to toluene and xylene (15%
o-xylene, 60% m-xylene and 25% p-xylene) has been studied in
Sprague-Dawley rats. Exposure time was 5 h and the concentrations
were 75 ppm toluene plus 979 mg/m3 (225 ppm) xylene, 150 ppm toluene
plus 652 mg/m3 (150 ppm) xylene or 225 ppm toluene plus 326 mg/m3
(75 ppm) xylene. Compared to exposure to a single solvent,
simultaneous exposure resulted in lower amounts of excreted urinary
hippuric and methylhippuric acids over 24 h. Increased concentrations
of solvents in blood and brain were found immediately post-exposure.
Simultaneous exposure also enhanced the pulmonary elimination of both
solvents (Tardif et al., 1992).
From a toxicokinetic modelling study it was concluded that there
is competitive metabolic inhibition between m-xylene and toluene in
the rat (Tardif et al., 1993a). The interaction is likely to be
observed when exposure exceeds 50 ppm of each solvent (Tardif et al.,
1993b).
Inhalation exposure of rats for 4 h to 2436 mg/m3 (560 ppm)
m-xylene or 320 ppm of ethylbenzene alone, or in combination,
indicated that in the combined exposure blood and brain m-xylene
concentrations increased by 52 and 40%, respectively, whereas there
was no corresponding effect on ethylbenzene concentrations (Frantik &
Vodickova, 1995).
In a study of metabolic interaction, Wistar rats were exposed
for 6 h to 1305 mg/m3 (300 ppm) m-xylene, 600 ppm methyl ethyl
ketone (MEK) or a mixture of both. After mixed exposure the
cytochrome P-450-dependent monooxygenase activities were additively or
synergistically induced. In the presence of MEK the overall
metabolism of m-xylene was inhibited, as shown by an increase in
xylene concentration in blood and fat and a decrease in the 24-h
excretion of xylene metabolites (Liira et al., 1991).
Male Wistar rats were exposed by inhalation for 4 h to 1000
mg/m3 (230 ppm) of o-xylene or 1700 ppm of acetone alone, or in
combination. In the combination exposure, blood xylene immediately
after the exposure was increased by 40% whereas the blood acetone
level was decreased by 15%. In a corresponding study, H-strain mice
were exposed for 2 h to 1392 mg/m3 (320 ppm) of o-xylene or 6655
mg/m3 (1530 ppm) of acetone alone or in combination. The combination
exposure was accompanied by a 33% increase of the blood xylene
concentration whereas the blood acetone level was decreased by 18%
(Vodickova et al., 1995).
6.6 Biological monitoring
More than 90% of xylene is transformed in human metabolism to
methylhippuric acid and excreted in urine. Urinary methylhippuric
acid has proved a robust measure of the amount of xylene taken up in
the body over some preceding hours. The measurement has been
routinely used in some countries for assessment of individual exposure
to xylene in the occupational setting. About 1.5-2 g methylhippuric
acid per g creatinine in the post-shift urine sample corresponds to
exposure to 435 mg/m3 (100 ppm) xylene over a full workday (Lauwerys
& Buchet, 1988). Different workloads cause variation in the excreted
amounts of methylhippuric acid at a given exposure level since the
lung uptake of xylene is directly proportional to pulmonary
ventilation. Biotransformation of xylene to methylhippuric acid is
inhibited in the presence of ethanol and acetylsalicylic acid (see
section 6.5); these interfering factors need to be controlled when the
method is applied.
The analysis of xylene in blood and exhaled air can be used for
assessment of exposure (Lauwerys & Buchet, 1988). These methods may
be particularly suitable for estimating xylene body burden caused by
the low and relatively stable background exposure in the general
population, or, in case of accidental exposure, for measuring levels
for a clinical toxicological evaluation.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Inhalation studies
7.1.1.1 o-Xylene
The LC50 value for o-xylene in Sprague-Dawley rats (12
males/group) was calculated to be 4330 ppm (95% confidence limits
4247-4432 ppm) for a 6-h exposure. The reported signs of intoxication
were hypotonia and somnolence. Autopsy on surviving animals 14 days
later revealed no macroscopic lesions of lung, liver or kidneys
(Bonnet et al., 1982). Under similar conditions the LC50 value for
mice (OF-1) was 4595 ppm (4468-4744 ppm) (Bonnet et al., 1979).
Rats (probably Wistar, sex not stated, 10 animals/group) were
exposed to 1531, 3062 or 6125 ppm for 24 h. No deaths occurred at
1531 ppm, one death at 3062 ppm and 8 deaths at 6125 ppm (Cameron et
al., 1938). In the same study one group was exposed to 12 250 ppm
o-xylene for 12 h. Two deaths occurred, one after 2 h. Autopsy of
those animals that died did not reveal any macroscopic or microscopic
lesions in the organs studied (Cameron et al., 1938). In the same
study mice (10 animals/group) were exposed to the same concentrations
for the same period of time as the rats. There were no deaths at 1531
ppm, four deaths at 3062 ppm and nine deaths at 6125 ppm. In the
group exposed to 12 250 ppm for 12 h there were two deaths, one after
9 h. The organs of animals that died after exposure showed no
characteristic changes (Cameron et al., 1938).
In a study of the effect on prenarcotic motor behaviour, groups
of eight male rats (CFY) were exposed to o-xylene (dose not stated)
for 1, 2, 3 or 4 h. No significant effects on group motor activity
were observed. Narcosis occurred at higher concentrations with a
threshold of 2180 ppm for a 4-h exposure (Molnar et al., 1986). Mice
(strain, sex and numbers not stated) were exposed for 2 h to
o-xylene in order to determine the minimum concentration needed for
an animal to fall on its side and die. The minimum concentration for
falling was 3400-4600 ppm and for death 6900 ppm (Lazarew, 1929).
In a study of subnarcotic effects of solvents in Wistar rats and
H-strain mice, using electrically evoked seizures, the lowest
effective concentration of o-xylene was 170 ppm. The criterion used
was a significant suppression by 10% of the generation and maintenance
of the seizure discharge after 4 h (rats) or 2 h (mice) of inhalation
(Frantik et al., 1994). A 30% suppression was induced by o-xylene
at a concentration of 390 ppm, corresponding to a blood concentration
of 62 µmol/litre (Frantik et al., 1993).
In order to determine the RD50 value (exposure level reducing
the respiratory rate by 50%), groups of male mice (OF-1) were exposed
to o-xylene. The RD50 value was calculated to be 1467 ppm
(1406-1530 ppm). The onset of response was rapid and the maximum
decrease in respiratory rate was reached within a few minutes (De
Ceaurriz et al., 1981).
In a study of conditioned behaviour in mice, an increased
response rate was observed after a 30-min exposure to 1400-2000 ppm
o-xylene. At higher concentrations there was a decrease in response
rate with an EC50 of 5179 ppm. The biphasic response indicates that
there was excitation of the central nervous system at low
concentrations and depression at higher concentrations (Moser et al.,
1985).
In a study to observe effects in the "behavioural despair"
swimming test, groups of 10 mice (OF-1) were exposed to 0, 1010, 1101,
1207 or 1234 ppm o-xylene for 4 h. The ID50 (50% decrease in
immobility) value was calculated to be 1127 ppm (1068-1182 ppm) (De
Ceaurriz et al., 1983). The study demonstrated subnarcotic effects by
o-xylene on the CNS.
7.1.1.2 m-Xylene
After a 6-h exposure to m-xylene the LC50 in rats and mice was
reported to be 5984 ppm (5796-6181 ppm) and 5267 ppm (5025-5490 ppm),
respectively (Bonnet et al., 1979; Bonnet et al., 1982). The signs of
toxicity consisted of hypotonia, somnolence, narcosis, and clonic
spasms leading to death due to respiratory failure (Lazarew, 1929;
Cameron et al., 1938, Bonnet et al., 1982; Moser et al., 1985; Molnar
et al., 1986).
In the comparative study by Frantik et al., (1994) described in
section 7.1.1.1, the lowest effective air concentration for m-xylene
was 210 ppm.
A biphasic CNS response occurred at similar exposure levels to
those seen for o-xylene (section 7.1.1.1) (Moser et al., 1985). The
narcotic threshold in rats of about 2100 ppm m-xylene determined in
prenarcotic behaviour studies was similar to that observed for
o-xylene (Molnar et al., 1986). During a 4-h exposure to 8000 ppm
m-xylene, 10 out of 12 rats (Carwoth-Wistar) died (Smyth et al.,
1962).
When groups of rats (Wistar; 6 males/group) were exposed for 24 h
to 0, 75, 150 or 300 ppm m-xylene, there was a significant decrease
in cytochrome P-450 concentrations at all doses, and a dose-related
decrease in 7-ethoxycoumarin-O-deethylase activity in the lung. No
abnormalities of the lungs, as determined by scanning electron
microscopy, were seen in two animals exposed to 300 ppm (Elovaara et
al., 1987).
When male Wistar rats were exposed for 4 h to a 1:1 mixture of
m-xylene and n-butyl alcohol or to the single substances at
500-4000 ppm, there were disturbances of rotarod performance. The
medial effective concentrations (EC50) were calculated to be 3080 ppm
(mixture), 6530 ppm ( n-butyl alcohol) and 1980 ppm (xylene)
respectively. The combined exposure gave less than additive effects
(Korsak et al., 1993).
A concentration-dependent decrease in respiratory rate in mice
was demonstrated in a study where mice were exposed to 500-4000 ppm of
n-butyl alcohol, m-xylene or a 1:1 mixture of both. The RD50 was
calculated to be 3010 ppm, 1360 ppm and 3140 ppm, respectively. The
combined exposure gave less than additive effects (Korsak et al.,
1993).
The acute neurobehavioural effect of m-xylene was evaluated
after 20 min inhalation exposure using a functional observations
battery in mice. In the concentration range of 2000-8000 ppm, these
effects included changes in posture, decreased arousal and rearing,
increased ease of handling, disturbances of gait, mobility and
righting reflex, decreased forelimb grip strength, increased landing
foot splay and impaired psychomotor coordination. The response to
various sensory stimuli was also decreased. These acute effects were
short-lived, recovery beginning within minutes of removal from the
exposure chamber (Tegeris & Balster, 1994).
7.1.1.3 p-Xylene
A 4-h LC50 value in rats (female Sprague-Dawley) of 4740 ppm
p-xylene has been reported (Drew & Fouts, 1974). The corresponding
6-h value was 4591 ppm (4353-5049 ppm) (Bonnet et al., 1982), and for
mice (OF-1) was 3907 ppm (3747-4015 ppm) (Bonnet et al., 1979). The
signs of toxicity were similar to those reported for the other two
isomers.
Like the other two isomers, exposure to p-xylene gave a
biphasic CNS response (Moser et al., 1985). In another study (Molnar
et al., 1986), marked activation and tremor were observed at
concentrations between 400 and 1500 ppm p-xylene in rats. The
narcotic threshold was 1940 ppm.
When Sprague-Dawley rats (16 females/group) were exposed for 4 h
to 0, 1000, 1500 or 2000 ppm p-xylene, a dose-dependent increase in
serum enzymes activites was observed. This was taken as a sign of
hepatocellular and hepatobiliary damage (Patel et al., 1979). In
other studies at higher exposure levels, no microscopic hepatic
lesions were seen in rats or mice (Cameron et al., 1938; Furnas &
Hine, 1958; Bonnet et al., 1982).
When Long Evans rats were exposed for 4 h to 0, 800 or 1600 ppm
p-xylene, the flash-evoked potential of the visual system was
reduced at the highest exposure level (Dyer et al., 1988). The
authors suggested that this may have been secondary to changes in
arousal or excitability. In a study of the effect on learning tasks
and motor activity, rats (Long Evans) were exposed to 0 or 1600 ppm
p-xylene for 4 h. Signs of toxicity (unsteadiness and fine tremor)
disappeared 30 min post-exposure. The results indicate an effect on
motor control rather than on cognitive capacity (Bushnell, 1989).
In the comparative study by Frantik et al. (1994) described above
(section 7.1.1.1), where rats and mice were exposed, the lowest
effective air concentration for p-xylene was 220 ppm.
After a 4-h exposure to 1000 ppm p-xylene there was a
decrease in pulmonary cytochrome P-450 in rabbits and a decrease in
NADPH-cytochrome c-reductase and mixed-function oxidase activity in
rats (Patel et al., 1976; Patel et al., 1978).
When Sprague-Dawley rats were exposed to 3400 ppm p-xylene for
4 h and killed 12 h later, an induction of cytochrome P-450 activities
(CYP2B) in liver microsomes was observed. On the other hand, the
pulmonary cytochrome P-450 activities were inhibited (Day et al.,
1992).
In rats (Sprague-Dawley) p-xylene (2800 ppm for 4 h) has been
shown to potentiate hepatotoxicity induced by bromobenzene, while the
effect of bromobenzene on pneumotoxicity was unaffected by p-xylene,
indicating differences in xylene metabolism between the liver and the
lung (Day et al., 1992).
7.1.1.4 Technical or undefined xylene
In rats LC50 values of 6350, 6700 and 10 950 ppm have been
reported after 4-h exposure (Hine & Zuidema, 1970; Carpenter et al.,
1975; Lundberg et al., 1983). Deaths occurred during the exposure
period. No LC50 values for mice have been found, although
experiments have been carried out at up to 7000 ppm for 30 min (Moser
et al., 1985). Signs of toxicity were the same as those produced by
the individual isomers.
No signs of toxicity were reported when rats and dogs were
exposed for 4 h to 580 and 530 ppm, respectively. The composition was
7.63% o-xylene, 65.01% m-xylene, 7.84% p-xylene and 19.27%
ethylbenzene (Carpenter et al., 1975). The same type of response at
similar exposure levels as for the three individual isomers was
observed in mice in a conditioned behavioural study (Moser et al.,
1985). In rats effects of xylene were studied on an operant behaviour
maintained by a fixed-ratio liquid reinforced schedule. A decrease
in the reinforcement rate was seen after exposure to 113 ppm for 2 h
(Ghosh et al., 1987; Ghosh & Pradhan, 1987).
When rats (Fischer F-344) were exposed to 1450 ppm xylene for 8
h, a slight increase in the auditory response threshold at 20 kHz was
noted (Pryor et al., 1987). The xylene composition was 10%
o-xylene, 80% m-xylene and 10% p-xylene.
In mice (Swiss-Webster) exposed to 1300 ppm xylene for one
minute, a decrease in respiratory rate as an indication of respiratory
tract irritation was seen (Carpenter et al., 1975). This effect was
not seen at an exposure level of 460 ppm. No histological
abnormalities were seen on histological examination of livers from
rats exposed to 5480 ppm for 4 h, nor were there any changes in serum
activities of an indicator enzyme (sorbitol dehydrogenase) for
hepatotoxicity after exposure to > 340 ppm (Lundberg et al.,
1986). No histological abnormalities were seen in cat livers after
exposure to about 9500 ppm for up to 2 h (Carpenter et al., 1975). In
dogs (Beagle) 1200 ppm xylene for 4 h caused lacrimation but there was
no noticeable effect at 530 ppm (Carpenter et al., 1975).
When groups of cats (five animals/group) were exposed for 5740,
6900 or 9200 ppm for up to 6 h, there was a concentration-dependent
decrease in time to onset of staggering and mild narcosis. There was,
however, a large individual variation. Deep narcosis was seen in four
animals at 9200 ppm xylene (Engelhardt & Estler, 1935).
7.1.2 Other exposure routes
The oral LD50 values in rat have been reported to be 3608 mg/kg
body weight for o-xylene, 5011 mg/kg body weight for m-xylene and
4029 mg body weight for p-xylene (Smyth et al., 1962). For various
mixtures of xylenes oral LD50 values in rat have been reported to be
between 3523 and 8700 mg/kg body weight (Wolf et al., 1956; Hine &
Zuidema, 1970; NTP, 1986). In mice the corresponding values were
reported to be 5627 mg/kg body weight for male and 5251 mg/kg body
weight for females (NTP, 1986). Signs of toxicity at lethal doses
were CNS depression and congestion of cells in liver, kidney and
spleen, seen by histological examination.
A dermal LD50 value for rabbits (New Zealand White) of 12 180
mg/kg has been reported for a 24-h exposure to m-xylene (Smyth et
al., 1962). From studies with intraperitoneal (i.p.), intravenous
(i.v.), subcutaneous (s.c.) and intramuscular (i.m.) administration in
rats and mice the acute toxicity of xylene is low (Bell et al., 1992).
All three isomers have been found to interfere with the
modulation of the vestibular-oculomotor pathways in the rat. The
blood threshold levels for this effect were 170-200 µg/ml following
administration by the i.v. route (Tham et al., 1984). Similar effects
have also been reported for m-xylene in rabbit. The
vestibular-oculomotor effects were seen at blood levels of 30 µg/ml
and some deaths due to respiratory stress were recorded at 100 µg/ml
(Larsby et al., 1976; Aschan et al., 1977; Ödkvist et al., 1979;
Ödkvist et al., 1980).
A dose-dependent depletion of hepatic glutathione, following i.p.
administration, has been demonstrated in rats. With o-xylene the
effect was seen from 50 mg/kg and with the other two isomers from 425
mg/kg (Van Doorn et al., 1980). A decrease in pulmonary cytochrome
P-450 levels has been observed with the three isomers, when
administered (i.p.) at 531 mg/kg (Pyykkö et al., 1987).
The CYP2B1 and CYP1A1 isoenzyme activities, benzyloxy-resorufin
O-deethylation and ethoxyresorufin O-deethylation, respectively,
were studied in nasal, pulmonary and hepatic tissues of rats injected
intraperitoneally with m-xylene. Tissues taken at 2, 12 and 24 h
after injection showed inhibition of these activities in both nasal
and pulmonary microsomes, but increased activities in hepatic
microsomes (Blanchard & Morris, 1994).
The effects of intraperitoneal administration of o-xylene
(1g/kg body weight) on: (a) rat hepatic and pulmonary mixed-function
oxidase content and activity; and (b) microsomal membrane structural
parameters were studied 1, 3, 6 and 12 h after administration (Park et
al., 1994). The pulmonary cytochrome P-450 content and aryl
hydrocarbon hydroxylase activity were decreased, a maximal inhibition
occurring 3 h after dosing. Reduced pulmonary activity for both
ethoxyresorufin O-dealkylation and benzyloxyresorufin
O-dealkylation was noted. In contrast, increased hepatic cytochrome
P-450 content was noted, with slightly increased ethaxyresorufin
O-dealkylation and markedly increased benzyloxyresorufin
O-dealkylation. An increase in pulmonary microsomal phospholipid
content and cholesterol content was noted even 1 h after dosing. In
liver the phospholipid content increased although there was no change
in cholesterol content; this suggested an increase in membrane
fluidity.
Sprague-Dawley rats were given m-xylene (1 g/kg body weight)
intraperitoneally and killed one hour after treatment. Microsomes
from the lung were then prepared. Compared to controls, m-xylene
administration decreased the CYP2B1 activity but did not alter the
CYP1A1 activity or epoxide hydrolase activity. In total, m-xylene
administration resulted in an inhibition of benzo (a)pyrene
detoxication and increased production of toxic metabolites in the
pulmonary microsomal preparations (Stickney et al., 1991).
Xylenes have been shown to inhibit the hypotonic haemolysis of
erythrocytes in vitro at low concentrations. The EC50 values were
29, 39 and 44 µg/ml for o-xylene, m-xylene and p-xylene,
respectively (Holmberg et al., 1974).
7.2 Short-term exposure
7.2.1 Inhalation studies
7.2.1.1 o-Xylene
In a study on the noradrenaline and dopamine levels in various
parts of the forebrain and hypothalamus, Sprague-Dawley rats (six
males/group) were exposed to 0 or 2000 ppm o-xylene 6 h/day for 3
days. The animals were killed within 18 h after final exposure. There
was a significant increase in catecholamine levels and turnover in
various parts of the hypothalamus and a decrease in the dopamine
turnover in the forebrain of exposed animals (Andersson et al., 1981).
In order to study the effects on cytochrome P-450 and enzyme
activities Sprague-Dawley rats (four males/group) were exposed to 0 or
2000 ppm o-xylene, 6 h/day for 3 days. There was a significant
increase in relative liver weight and cytochrome P-450 content in
exposed animals. Furthermore, there were increases in some liver
enzyme activities. In lungs, there was a decrease in cytochrome P-450
activity (Toftgård & Nilsen., 1982).
When guinea-pigs (15 per group) were exposed to 0 or 780 ppm
o-xylene, 8 h/day, 5 days/week for 6 weeks, there was a marked
decrease in body weight gain in exposed animals. No effects on the
liver, kidney, heart, spleen or lung were observed upon histological
examination (Jenkins et al., 1970). In the same study beagle dogs
were exposed for the same period of time. One dog out of two
experienced tremors throughout the exposure period. No other signs of
toxicity were reported (Jenkins et al., 1970). Squirrel monkeys were
also exposed in the same manner. One monkey out of three died on day
seven. No other signs of toxicity were reported (Jenkins et al.,
1970).
7.2.1.2 m-Xylene
In a study of levels of noradrenaline and dopamine in various
parts of the forebrain and hypothalamus, Sprague-Dawley rats (six
males/group) were exposed to 0 or 2000 ppm m-xylene, 6 h/day for 3
days. The animals were killed within 18 h after the last exposure. A
significant increase in catecholamine levels and turnover was observed
in various parts of the hypothalamus of exposed animals. There was no
effect on dopamine levels (Andersson et al., 1981).
In another study, Sprague-Dawley rats (four males/group) were
exposed to 0 or 2000 ppm m-xylene for 3 days. The effect on
cytochrome P-450 and enzyme activities in liver, kidney and lung were
studied. In exposed animals there was a significant increase in
relative liver weight, in cytochrome P-450 content in liver and
kidney, and in some enzyme activities in these two organs. The
content of pulmonary cytochrome P-450 was decreased (Toftgård &
Nilsen, 1982).
In a study of the effect on xenobiotic metabolism in Wistar rats,
10 males per group were exposed to 0, 50, 400 or 750 ppm m-xylene, 6
h/day, 5 days/week for one week. At the two highest doses there
was a significant increase in hepatic microsomal protein and
NADPH-cytochrome c reductase levels, and a decrease in hepatic
glutathione levels. There was no effect on hepatic cytochrome P-450
levels or on renal glutathione levels. At all dose levels there was a
significant increase in renal cytochrome P-450 and some enzyme
activities. When the animals were exposed to the same regimen for 2
weeks similar results were obtained. There were no abnormalities upon
histological examination of the liver (Elovaara, 1982). When Wistar
rats (20 males/group) were exposed to 0 or 300 ppm m-xylene 6 h/day,
5 days/week for one week there was, in exposed groups, a significant
increase in hepatic and renal 7-ethoxycoumarin O-deethylase activity
and in renal UDP-glucuronyl transferase. When exposure time was 2
weeks there was also a significant increase in hepatic cytochrome
P-450 and NADPH-cytochrome c reductase activities (Elovaara et al.,
1982).
In order to study the effect on lung cytochrome P-450 in rats,
six male Wistar rats/group were exposed to 0 or 300 ppm m-xylene 7
h/day, 4 days/week for 5 weeks. The only effects seen were a
significant decrease in cytochrome P-450 content and 7-ethoxycoumarin
O-deethylase activity (Elovaara et al., 1987). When the effects on
cerebral biochemistry was studied, groups of Wistar rats (15
males/group) were exposed to 0, 50, 400 or 750 ppm m-xylene 6 h/day,
5 days/week for 1 or 2 weeks. The only effect seen after one week of
exposure was a significant decrease in glutathione levels at all
concentrations. After 2 weeks there were also a dose-dependent
decrease in superoxide dismutase activity, a significant increase in
NADPH-diaphorase activity at all concentrations and a significant
increase in azoreductase activity at the two highest concentrations
(Savolainen & Pfäffli, 1980).
7.2.1.3 p-Xylene
In a study of levels of noradrenaline and dopamine in the
forebrain and hypothalamus, Sprague-Dawley rats (six males/group) were
exposed to 0 or 2000 ppm p-xylene 6 h/day for 3 days. The animals
were killed 16-18 h after the last exposure. In exposed animals there
was a significant increase in catecholamine levels and turnover in
various parts of the hypothalamus. There was no effect on dopamine
levels or turnover in the forebrain (Andersson et al., 1981).
In order to study the effect on cytochrome P-450 and enzyme
activities in the liver, kidney and lung, Sprague-Dawley rats (four
males/group) were exposed to 0 or 2000 ppm 6 h/day for 3 days. In
exposed animals there were significant increases in relative liver
weight, in hepatic cytochrome P-450 content, in NADPH-cytochrome c
reductase activity in the liver and kidney, and in 7-ethoxyresorufin
O-deethylase activity in the kidney. There was also a decrease in
pulmonary cytochrome P-450 content (Toftgård & Nilsen, 1982). To
study the lung microsomal activity, rabbits (New Zealand White; four
males/group) were exposed to 0 or 1000 ppm p-xylene 4 h/day for 2
days. In exposed animals there was a significant decrease in
microsomal cytochrome P-450 concentration and in NADPH-cytochrome c
reductase activity (Patel et al., 1978). Inhibition of CYP2B1 has
been observed in the lungs of rats dosed with p-xylene (Verschoyle
et al. 1993).
Rats exposed to 300 ppm p-xylene, 6 h/day for 1, 3 or 5 days
exhibited alterations in pulmonary microsomal membrane structural and
metabolic parameters (Silverman & Schatz, 1991). Following 1 day of
exposure, conjugated diene levels were elevated while total
phospholipid levels, cytochromes P-450 content, benzyloxyresorufin
O-dealkylase activity and 2-aminofluorene N-hydroxylase activity
were decreased. Core membrane fluidity was increased following 3 days
of exposure. After 5 days of exposure all parameters returned to
control levels with the exception of aryl hydrocarbon hydroxylase
activity, which was increased by 41%. Extracellular surfactant levels
were also decreased after 1 and 3 days of exposure but returned to
control values after 5 days. The increase in aryl hydrocarbon
hydroxylase activity after 5 days of exposure could have important
consequences on the metabolism of co-administered xenobiotics.
Male Fischer-344 rats exposed to 0 or 1600 ppm p-xylene by
inhalation, 6 h/day, for 1 or 3 days did not produce overt
hepatotoxicity but resulted in a significant increase in the
concentration of hepatic cytochrome P-450 (Simmons et al., 1991).
However, the concentration of hepatic cytochrome P-450 had returned to
control levels within 2 to 3 days after exposure.
p-Xylene has been shown to decrease axonal transport of
proteins and glycoproteins in rats (Long-Evans) exposed by inhalation
to 1600 ppm for 6 h/day, 5 days/week, for 8 days. When ethanol (10%
in drinking-water) was given during 6 days prior to inhalation of
p-xylene, the treatment prevented the decreased axonal transport.
Ethanol per se did not decrease the axonal transport (Padilla et
al., 1992).
When 10 Wistar rats and 10 mice (strain not given) were exposed
to 1226 ppm p-xylene 8 h/day for 14 days, no animals died. No other
results were reported (Cameron et al., 1938).
When mice were exposed to 1200 ppm p-xylene 6 h/day for 4 days
and infected with a sublethal dose of murine cytomegalovirus, 34%
mortality occurred, whereas no deaths occurred among uninfected,
p-xylene-exposed mice or infected, air-exposed mice (Selgrade et
al., 1993). Although p-xylene potentiated liver damage caused by
the virus, the magnitude of serum enzyme activities indicated that
this damage was not the probable cause of death. Enhanced mortality
was related to enhanced xylene toxicity due to suppression of
cytochrome P-450, although additive or synergistic damage to tissues
other than liver could not be ruled out. There was no indication that
p-xylene had caused immune suppression.
7.2.1.4 Technical or undefined xylene
When rats (strain not defined) were exposed to 620, 980 or 1600
ppm xylene 18-20 h/day for 7 days, instability, incordination and
narcosis were observed at the two highest concentrations. Signs of
mucous membrane irritation occurred, and congestion and cloudy
swelling of kidneys was reported at 980 ppm (Batchelor, 1927).
Similar results were reported in another study (Winslow, 1927).
Groups of Harlan-Wistar rats (25 males/group) were exposed to 0,
180, 460 or 810 ppm xylene 6 h/day, 5 days/week for 13 weeks. The
xylene consisted of 7.63% o-xylene, 65.01% m-xylene, 7.84%
p-xylene and 19.27% ethylbenzene. At 3, 7 and 13 weeks 3, 3 and 4
animals, respectively, were killed. No treatment-related
histopathology was seen (Carpenter et al., 1975).
In a study of levels of noradrenaline and dopamine in the
forebrain and hypothalamus of rats, the xylene mixture used was 2.0%
o-xylene, 64.5% m-xylene, 10.0% p-xylene and 23.0% ethylbenzene.
Sprague-Dawley rats (6 males/group) were exposed to 0 or 2000 ppm
xylene 6 h/day for 3 days. The animals were killed 16-18 h after the
last exposure. There was a significant increase in catecholamine
levels and turnover in the hypothalamus and a significant increase in
dopamine levels and turnover in the forebrain (Andersson et al.,
1981).
In order to study the effect on levels of neurotransmitters in
the rat brain, five to six Sprague-Dawley rats/group were exposed to
0, 200, 400 or 800 ppm xylene (mixture not defined) for 30 days.
Acetylcholine levels in the striatum were decreased at > 400 ppm.
Noradrenaline levels in the hypothalamus were increased significantly
at the highest dose. From 400 ppm the cAMP levels were decreased in
the striatum, and at 800 ppm the glutamine levels in the midbrain were
increased. At all concentrations glycine and GABA levels in the
midbrain were increased (Honma et al., 1983).
When 12 male Fischer F-344 rats/group were exposed to 0 or 1450
ppm xylene 8 h/day for 3 days, there was, in exposed animals, an
increase in the auditory response threshold at 12 and 20 kHz.
Rats (Long Evans) were exposed to 2500 ppm of mixed xylenes 6
h/day for 5 days. Testing of auditory function was conducted 5 to 8
weeks after exposure using reflex modification audiometry (RMA). The
results indicated increased RMA thresholds for the mid-frequency tones
(e.g., 8, 16 and 24 kHz) but not for higher or lower tones (Crofton et
al., 1994).
In another study Sprague-Dawley rats (four males/group) were
exposed to 0 or 630 ppm xylene 6 h/day, 5 days/week for 4 weeks. The
animals were killed the morning after the last exposure. In exposed
animals there was a significant decrease in body weight gain, while
the absolute and relative liver weights were increased. There was an
increase in hepatic cytochrome P-450 and the xylene was shown to act
as a phenobarbital-like inducer of cytochrome P-450. The xylene used
was 2.0% o-xylene, 64.5% m-xylene, 10.0% p-xylene and 23.0%
ethylbenzene (Toftgård et al., 1981).
When male Sprague-Dawley rats (8-12 animals/group) were exposed
to 0, 75, 250, 500, 1000 or 2000 ppm xylene 6 h/day for 3 days there
was a dose-dependent increase in the concentration of liver microsomal
cytochrome P-450. When animals were exposed to the two highest
concentrations for 5 days, there was a dose-dependent increase in the
surface area of smooth endoplasmic reticulum but not in rough
endoplasmic reticulum. Based on their results the authors concluded
that xylene causes phenobarbital-type induction in the liver. The
xylene used was the same as that in the previous paragraph (Toftgård
et al., 1983).
The same type of xylene was also used in a study where four male
Sprague-Dawley rats/group were exposed to 0 or 2000 ppm xylene 6 h/day
for 3 days. In exposed animals there were significant increases in
hepatic and renal cytochrome P-450 content, in NADPH-cytochrome c
reductase activities and 7-ethoxyresorufin O-deethylase activities
in liver and kidney. The pulmonary cytochrome P-450 content was
decreased. Increased enzyme activity in liver and kidney and decreased
activity in the lung were thus observed (Toftgård & Nilsen, 1982).
Groups of male Wistar rats (20 animals/group) were exposed to 0
or 300 ppm xylene 6 h/day, 5 days/week for up to two weeks. In each
group 10 animals had 15% v/v ethanol in the drinking-water. There was
a significant decrease in motor activity in exposed animals. In
addition, there was a significant increase in brain DT-diaphorase
activity, in acid proteinase and in hepatic and renal 7-ethoxycoumarin
O-deethylase activities. Concurrent dosing with ethanol had a
marked synergistic effect on hepatic and renal 7-ethoxycoumarin
O-deethylase activities. The xylene used was 80% m-xylene and 12%
p-xylene (Savolainen et al., 1979a,b).
In a study of the effect of simultaneous exposure to xylene
(undefined) and noise on metabolic activity in the myocardium, male
rats (strain not specified) were exposed to 0 or 69 ppm xylene 4 h per
day, 5 days per week for 6 weeks, and simultaneously to a noise level
of 0, 46, 85 or 95 dB. There were changes in some enzyme activities,
but the brief reporting makes the study impossible to evaluate
(Ivanovich et al., 1985).
When Beagle dogs (four males per group) were exposed to 0, 180,
460 or 810 ppm xylene 6 h per day, 5 days per week for 13 weeks, no
treatment-related effects were reported. The xylene used was 7.63%
o-xylene, 65.01% m-xylene, 7.84% p-xylene and 19.27% ethylbenzene
(Carpenter et al., 1975).
7.2.2 Other exposure routes
Decreased body weight compared to controls was noted in rats
(Sprague-Dawley) administered orally 1062 mg m-xylene/kg body weight
for 3 days (Pyykkö, 1980), and reduced terminal body weight was
observed in rats (Fischer F-344) administered 500 mg m-xylene/kg
body weight, 5 days per week, for 4 weeks (Halder et al., 1985).
Three days administration of 1062 mg m-xylene/kg body weight
resulted in significant increases in liver weight, liver cytochrome
P-450 and cytochrome b5 levels and NADPH cytochrome c reductase and
MFO enzymes activities. The same range of increases was also seen in
the kidney (Pyykkö, 1980). In another study, however, there were no
effects on kidney weight and no treatment-related abnormalities
histopathologically in Fischer rats given 500 or 2000 mg/kg body
weight 5 days per week for 4 weeks (Dési et al., 1967).
Upon oral administration of 0, 125, 250, 500, 1000 or 2000 mg
xylene per kg body weight to rats (F-344/N), in a 14-day study, a high
mortality was seen in the highest dose group. The xylene used was
9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. The body weight gain was reduced in males at > 250
mg/kg body weight and in females at 125 mg/kg body weight and
> 1000 mg/kg body weight. There were no treatment-related
abnormalities at gross necropsy (NTP, 1986). In the same study xylene
was administered at 0, 62.5, 125, 250, 500 or 1000 mg per kg body
weight, 5 days per week, for 13 weeks. No treatment-related
abnormalities were seen during gross necropsy or histopathological
examination (NTP, 1986).
Daily administration (s.c.) of xylene (undefined) for up to 4
weeks to rats (strain not given) resulted in significant mortality at
870 mg/kg body weight but not at 435 or 174 mg/kg body weight.
Repeated administration of 435 mg/kg body weight resulted in decreased
learning rate (Dési et al., 1967). When Sprague-Dawley rats were
given 0 or 3123 mg/kg body weight for 3 days, there was a significant
increase in liver weight, cytochrome P-450 levels, NADPH cytochrome c
reductase and activity of MFO enzymes. The xylene used was 30%
o-xylene, 55% m-xylene and 15% p-xylene (Pathiratne et al.,
1986).
In order to study further the effects of p-xylene, rats were
given p-methylbenzyl alcohol (PMBA) or 2,5-dimethylphenol (DMP)
(300 mg/kg body weight and 150 mg/kg body weight, respectively)
intraperitoneally once a day for 3 days. It was concluded that of the
two metabolites, PMBA may have a significant role in the inhibition of
pulmonary cytochrome P-450 caused by p-xylene (Day & Carlson, 1992).
Male Sprague-Dawley rats (n=10) were given xylene (isomer not
stated) intraperitoneally, as a single dose per day, for 3 consecutive
days. The dose given was equal to half the LD50 (1.6 ml/kg body
weight per day). Only slight somnolence was observed. After the last
dosing the animals were killed and aminopeptidase activities in
several regions of the brain were measured. The activities were
largely unaffected, compared to those of controls (De Gandarias et
al., 1993).
Brain cell cultures enriched in astroglial cells were prepared
from neonatal Sprague-Dawley rats. The cultures were exposed for 1 h
to 3, 6 or 9 mmol o-xylene/litre. The ATPase activity was reduced
in a dose-dependent manner (Naskali et al., 1994).
7.3 Long-term exposure
A short summary of long-term studies is presented in Table 6.
In Wistar rats exposed to 1000 ppm m-xylene for 6 h/day,
5 days/week for 3 months or to 100 ppm for 6 months, slight
ultrastructural changes (proliferation of smooth endoplasmic
reticulum) were found in hepatocytes. When rats were exposed to a 1:1
combination of m-xylene and toluene (500 plus 500 ppm or 50 plus 50
ppm), the changes were a combination of those of each single solvent
(Rydzynski et al., 1992). The combined exposure at both exposure
levels gave more pronounced disturbances in a rotarod performance test
and decrease in spontaneous motor activity compared to single solvent
exposure. In animals exposed to 500 plus 500 ppm for 3 months a
decrease in red blood cell count and an increase in rod neutrophil
cell count were observed (Korsak et al., 1992).
In a six-week study, groups of rats (Sprague-Dawley or
Long-Evans; 15 animals/group) were exposed to 0 or 780 ppm o-xylene
(8 h/day, 5 days/week) or to 78 ppm continously for 90 days. There
was no effect on body weight gain, leukocyte count, haemoglobin level
or heamatocrit. Histological examination of the liver, kidney, heart,
spleen and lung revealed no effects (Jenkins et al., 1970).
Table 6. Effects of xylenes in long-term studies
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Inhalation exposure
o-Xylenea rat, dog, 13 weeks 780 - Jenkins et al., 1970
guinea-pig
o-Xylenea monkey 13 weeks + 78 - Jenkins et al., 1970
m-Xylenea rat 3 months - 1000 liver cell changes (i.e. inc. smooth Rydzynski et al., 1992
endoplasmic reticulum, lysosomes)
m-Xylenea rat 6 months - 100 liver cell changes (i.e. inc. smooth Rydzynski et al., 1992
endoplasmic reticulum, lysosomes)
m-Xylenea rat 3 months - 100 dec. lymphocyte/monocyte count; Korsak et al., 1992
dec. rotorod, performance
m-Xylenea rat 6 months - 100 dec. rotorod, performance Korsak et al., 1992
dec. spontaneous motor activity
Xylenes rat 3 months 50 100 dec. rotorod performance Korsak et al., 1994
dec. spontaneous motor activity
Xylenes rat 6 months 346 923 liver effects (inc. liver weight inc. Ungvary, 1990
smooth endoplasmic reticulum, inc.
P-450 activity)
Table 6. (Cont'd)
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Inhalation exposure
Xylenes rat 13 weeks 810 - Carpenter et al., 1975
Xylenes dog 13 weeks 810 - Carpenter et al., 1975
Xylenes rat 6 weeks - 800 ototoxicity Pryor et al., 1987
(14h/day)
Xylenes rat 61 days - 1000 ototoxicity Nylen & Hagman, 1994
(18 h/day)
Xylenes rat 18 weeks - 300 liver microsomes activity Elovaara et al., 1980
Xylenes rat 18 weeks - 300 brain superoxide dismutase activity Savolainen et al., 1979
Xylenes rat 13 weeks + - 320 brain lipid composition changes Kyrklund et al., 1987
Xylenes gerbil 3 months 160 320 change in astroglial cell marker Rosengren et al., 1986
proteins
Table 6. (Cont'd)
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Oral exposure
Xylenes rat 13 weeks 1000 - NTP, 1986
Xylenes mouse 13 weeks 2000 - NTP, 1986
Xylenes rat 13 weeks - 150 increased liver weights (males); Condie et al., 1988
hyaline droplet nephropathy (males)
Xylenes rat 2 years 250 500 mortality NTP, 1986
Xylenes mouse 2 years 500 - NTP, 1986
a Only one dose was used
b Continous exposure
The effects of combined exposure to m-xylene and n-butyl
alcohol have been studied in rats exposed to the individual solvents
at 50 and 100 ppm and their 1:1 mixture at 50 plus 50 ppm and 100 plus
100 ppm, 6 h/day, 5 days/week for 3 months (Korsak et al., 1994). The
results indicate less than an additive toxic effect (motor
coordination disturbances) of combined exposure to m-xylene and
n-butyl alcohol. For xylene alone an effect was seen at 100 ppm but
not at 50 ppm.
Rats (CFY) were exposed to xylene (10% o-xylene, 50%
m-xylene, 20% p-xylene and 20% ethylbenzene) for 8 h/day up to 6
months at concentrations of 600, 1500 or 4000 mg/m3. No macroscopic
changes were seen but the relative liver weight was increased at 4000
mg/m3. At 4000 mg/m3 hypertrophy of the centrilobular zone in
the liver, including a change in the amount of smooth and rough
endoplasmic reticulum, was seen. As a result of xylene exposure the
hexobarbital sleeping time decreased. Liver enzymatic activities were
increased during the first 6 weeks. After the 4-week exposure-free
period the changes described above could no longer be seen. The same
type of changes could also be seen when xylene was applied orally,
subcutaneously or intraperitoneally for a short period of time (4-7
day). Essentially the same changes as those described for rat liver
were also seen in livers from mice and rabbits (Ungvary, 1990).
Groups of 50 male and 50 female Fischer-344/N rats received 0,
250 or 500 mg xylene/kg body weight. The xylene used was 9.1%
o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0% ethylbenzene.
The doses were given (in corn oil) by stomach tube on 5 days per week
for 103 weeks. The animals were killed within 14 days after the last
dosing. Body weights of high-dose (500 mg/kg body weight) males were
5 to 8% lower than those of vehicle controls. Results for low-dose
males and both female groups were comparable to those of controls.
Gross observation and histopathological results showed no incidences
of non-neoplastic effects in dosed groups, related to the
administration of xylene, at any sites (NTP, 1986; Huff et al., 1988).
In the same study groups of 50 male and 50 female B6C5F1 mice
received 0, 500 or 1000 mg xylene/kg body weight. The same type of
technical xylene was used. The doses were given, in corn oil, by
stomach tube 5 days per week for 103 weeks, after which the animals
were killed within 14 days. No significant difference in mean body
weights or survival was observed between treated animals and controls.
Gross observation and histopathological results indicated that at no
site were the incidences of non-neoplastic effects in dosed groups
related to the administration of xylene (see also section 7.7).
Male Fischer-344 rats were exposed to 0, 800, 1000 or 1200 ppm
xylene 14 h/day, 7 days/week for 6 weeks. At the highest dose level
there was a slight impairment of auditory (but not of visual or
somatosensory) conditioned avoidance response. All animals had
increased auditory response thresholds compared to controls at the
same frequencies. At 1000 and 800 ppm the thresholds were elevated at
16 kHz and 8 kHz and at 1200 ppm the brainstem auditory-evoked
response thresholds were elevated at 4, 8 and 16 kHz tone frequency.
The xylene used in these two studies was 10% o-xylene, 80%
m-xylene and 10% p-xylene (Pryor et al., 1987).
Wistar rats (60 males/group) were exposed to 0 or 300 ppm xylene
6 h per day, 5 days per week, for up to 18 weeks, and 50% of the
animals had 15-20% ethanol in the drinking water. The xylene used was
19.2% o-xylene, 43.0% m-xylene, 19.5% p-xylene and 18.3%
ethylbenzene. It was concluded that concurrent ethanol intake
increased hepatic and renal microsomal enzyme activities. Stearosis
in the liver was more marked in co-exposed animals than in animals
exposed only to ethanol. No treatment-related abnormalities were
observed during histopathological examination of animals exposed only
to xylene (Elovaara et al., 1980).
Exposure to 1000 ppm xylene (not defined) 18 h per day, 7 days
per week, for 61 days caused a slight loss of auditory sensitivity in
Sprague-Dawley rats. Co-exposure to n-hexane (1000 ppm) caused
a persistent loss of auditory sensitivity, which was less than
additively enhanced. Xylene inhibited n-hexane-induced impulse
velocity reduction in peripheral nerves (Nylén & Hagman, 1994).
In a study to investigate the effect on brain lipid composition,
Sprague-Dawley rats were exposed to 0 or 320 ppm xylene continuously
for 30 or 90 days. The xylene (undefined) produced only limited
transient changes (Kyrklund et al., 1987). In another study to
investigate the effect on brain with and without co-exposure to
ethanol, Wistar rats (20 males/group) were exposed to 0 or 300 ppm
xylene 6 h per day, 5 days per week, for up to 18 weeks. In each
group were 10 animals also exposed to 15% v/v ethanol in the
drinking-water. There was a significant increase in cerebral
microsomal superoxide dismutase activity by week 18, but no effect on
cerebral protein or RNA levels. Co-exposure to ethanol reduced the
effect caused by xylene exposure. The xylene used was 7.5%
o-xylene, 85.0% m-xylene and 7.5% p-xylene (Savolainen et al.,
1979a,b).
In a study of the effect on spinal cord axon membrane, Wistar
rats (five animals per group) were exposed to 0 or 300 ppm xylene
(undefined) 6 h per day, 5 days per week, for 18 weeks. In exposed
animals there was a decrease in the amount of membrane lipid per mg of
protein but no change in the cholesterol/lipid phosphorus ratio.
Ethanol (15% v/v) in the drinking-water enhanced the decrease and also
decreased the cholesterol/lipid phosphorus ratio (Savolainen &
Seppäläinen, 1979).
In a neurotoxicity study, changes in two astroglial cell marker
proteins (S-100 and GFA) and DNA were measured. Mongolian gerbils
(four of each sex per group) were exposed to 0, 160 or 320 ppm xylene
continously (24 h/day) for 3 months, followed by a 4-month
exposure-free period. The xylene used was 18% o-xylene, 70%
m-xylene, 12% p-xylene and < 3% ethylbenzene. The exposure to
xylene resulted in brain damage which was manifest as an increase in
astroglial cells. Effects were seen in particular parts of the brain
at both exposure levels but were only significant at 320 ppm
(Rosengren et al., 1986).
Ageing Long-Evans rats fed 200 ppm o-xylene for up to 6 months
showed formation of vacuolar structures in hepatocytes when examined
ultrastructurally (Bowers & Cannon, 1982).
Groups of 10 male and 10 female Sprague-Dawley rats were exposed
to mixed xylenes by gavage (in corn oil) for 90 consecutive days at
dose levels of 150, 750 and 1500 mg/kg body weight per day (Condie et
al., 1988). The most significant findings were increased relative
liver and kidney weights. Histopathology evaluation of liver and
kidney tissues revealed an increased incidence of minimal chronic
renal disease in females only. Treatment-related hepatic
histopathological changes were not detected in either sex. Hyaline
droplet formation was observed in male rats in all treated groups. In
females, there was an increased incidence of kidney effects, which
were thought to represent onset of progressive nephropathy (Condie et
al., 1988).
Mice (B6C3F1) were given orally 0, 250, 500, 1000 or 2000 mg
xylene per kg body weight for 14 days or 0, 125, 250, 500, 1000 or
2000 mg per kg body weight, 5 days per week, for 13 weeks. The xylene
used was 9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. Mean body weight gain was reduced in males at > 250
mg/kg body weight. No treatment-related abnormalities were observed
at gross necropsy or histopathological examination (NTP, 1986).
7.4 Skin and eye irritation; sensitization
Erythema and oedema were induced following a single application
(amount not stated) of xylene (not defined) to rabbit or guinea-pig
skin. There was a rapid onset and epithelial desquamation, with some
evidence of necrosis occurring after several days (Rigdon, 1940;
Steele & Wilhelm, 1966). After 24 h of exposure to 0.5 ml xylene
(undefined) under semi-occlusive conditions, irritation of rabbit skin
was observed. The irritation was graded as moderate by the authors
(Hine & Zuidema, 1970).
Application of 0.5 ml p-xylene, under a Teflon chamber, to
rabbit skin for 4 h produced a response which would be classified as
irritant using European Union criteria (Jacobs et al., 1987). In
another study, following 10 to 20 applications of xylene (not defined)
to open or semi-occluded rabbit skin for up to four weeks, a moderate
to marked irritation and moderate necrosis was reported, as was
blistering of the skin under the semi-occlusive dressing (Wolf et al.,
1956).
Application of approximately 0.05 to 0.5 ml of liquid xylenes
(individual isomers or undefined composition) to the rabbit eye was
reported to cause immediate discomfort and blepharospasm followed by
slight conjunctival irritation and very slight, transient corneal
necrosis (Wolf et al., 1956). Application of 0.1 ml liquid xylene
(not defined) was mildly irritant to rabbit eye. The lesions were not
described (Kennah et al., 1989).
No skin sensitization studies have been reported.
7.5 Reproductive and developmental toxicity
A summary of reproductive and developmental toxicity studies is
shown in Table 7.
Groups of female rats (CFY) were exposed to 0, 34, 345 or 690 ppm
o-xylene 24 h/day from day 7 to day 14 of gestation. The dams were
killed on day 21. Some ultrastructural changes in the liver and
decreased weight gain during the exposure period were observed in the
dams exposed to 345 or 690 ppm. At these concentrations lower fetal
body weight was observed, and at the highest dose level delayed
skeletal ossification was noted. There was no evidence of
malformations. Similar results were obtained when the animals were
exposed to m-xylene. With p-xylene there were signs of delayed
skeletal ossification at exposure levels showing no maternal toxicity
(at 34 and 345 ppm). At 690 ppm, increased postimplantation loss of
fetuses and more retarded fetuses were observed (Ungvary et al.,
1980).
Table 7. Reproductive and developmental effects (inhalation studies)
Compound Species Exposure Duration NOEL LOEL Endpoint Reference
o, m, p-Xylene rat 0, or 1000 24 h/day; Fused sternebrae, Hudak & Ungvary
mg/m3 days 9-14 extra ribs (1978)
Xylene rat 0, 10, 50 or 6 h/day; Maternal toxicity not Mirkova et al.
(not specified) 500 mg/m3 days 1-21 addressed (1983)
Xylene rat 0, 250, 1900 24 h/day; 250 mg/m3 Skeletal retardation Ungvary & Tatrai
(not specified) or 3400 days 7-15 (mothers and (1985)
mg/m3 offspring)
o-xylene rat 0, 150, 1500 24 h/day; 150 mg/m3 150 mg/m3 Decreased fetal weight, Ungvary et al.
or 3000 days 7-14 offspring (dams) skeletal retardation (1980)
mg/m3 1500 mg/m3
(offspring)
m-xylene rat 0, 150, 1500 24 h/day; 1500 mg/m3 3000 mg/m3 Maternal: reduced food Ungvary et al.
or 3000 days 7-14 (dams and (dams and consumption, reduced (1980)
mg/m3 offspring) offspring) weight gain
Offspring: reduced fetal
weight
p-xylene rat 0, 150, 1500 24 h/day; 150 mg/m3 Reduced mean litter Ungvary et al.
or 3000 day 7-15 (dams and size; reduced fetal (1980)
mg/m3 offspring) weight; skeletal
retardation
p-xylene rat 0 or 3000 10th day, Decreased fetal weight Ungvary et al.
mg/m3 24 h (1981)
Table 7. (Cont'd)
Compound Species Exposure Duration NOEL LOEL Endpoint Reference
p-xylene rat 0, 3500 or 6 h/day; 7000 7000 mg/m3 Maternal: reduced Rosen et al.
7000 mg/m3 days 7-16 mg/m3 (dams) weight gain (1986)
(offspring),
3500
mg/m3
(dams)
Xylene rat 0 or 600 24 h/day; Decreased maternal Ungvary (1985)
(not defined) mg/m3 day 7-15 weight gain.
Delayed fetal
development; extra ribs
Xylene rat 870 mg/m3 6 h/day; No maternal toxicity; Hass & Jakobsen
(not defined) days 4-20 delayed ossification (1993)
o, m, p-Xylene rat 2175 mg/m3 6 h/day; Delayed righting reflex; Hass et al. (1995)
days 7-20 reduced absolute brain
weight; impaired
neuromotor ability
Xylene mouse 0, 500 or 24 h/day; 500 mg/m3 1000 mg/m3 Skeletal retardation and Ungvary & Tatrai
(not specified) 1000 mg/m3 days 6-15 offspring (offspring) increased incidence of (1985)
weight-retarded fetuses
Xylene rabbit 0, 500 or 24 h/day; 500 mg/m3 Maternal: decreased Ungvary & Tatrai
(not specified) 1000 mg/m3 days 7-20 (offspring) weight gain (1985)
1000 mg/m3 Offspring: delayed
(dams) skeletal development
Rats (CFY) were exposed to 0.58, 437 or 782 ppm 24 h/day on days
7 to 15 of gestation and the dams were killed on day 21. Data
concerning maternal toxicity was not given. There was delayed
skeletal ossification at all dose levels, while decreased fetal
bodyweight, increased postimplantation loss and increased frequency of
skeletal variants (extra ribs) were observed at 782 ppm. Mice (CFLP)
were exposed to 0 or 115 ppm o-xylene for 4 h, 3 times per day on
day 6 to day 15 of gestation, and the dams were killed on day 18.
Data concerning maternal toxicity were not reported. There was
evidence of delayed weight gain and skeletal ossification in the
fetuses of exposed animals. Similar results were obtained with
m-xylene and p-xylene (Ungvary & Tatrai, 1985). When rabbits (New
Zealand White) were exposed to 0 or 115 ppm o-xylene 24 h/day from
day 7 to day 20 of gestation, no maternal toxicity or incidence of
delayed development was observed in the exposed group. Similar
results were observed with m-xylene, but an increased incidence of
post-implantation loss was observed. Exposure to 115 ppm p-xylene
gave the same results as with o-xylene, but exposure to 230 ppm
p-xylene 24 h/day resulted in no live fetuses (one dam died, three
aborted and in four there was total resorption or fetal death in
utero) (Ungvary & Tatrai, 1985).
In a study to validate a developmental toxicity screen, mice
(ICR/SIM) were exposed orally to 0 or 2000 mg m-xylene/kg body
weight from day 8 to day 12 of gestation. No effects were seen on
mothers or young (Seidenberg et al., 1986). In another study
Sprague-Dawley rats were exposed to 0, 800 or 1600 ppm p-xylene, 6 h
per day from day 7 to day 16 of gestation. The dams were allowed to
deliver their young. In the highest dose group the maternal weight
gain was significantly reduced. Exposure to p-xylene had no effects
on postnatal viability, offspring growth or function of the nervous
system (activity level and acoustic startle response) at any of the
doses tested (Rosen et al., 1986).
In an attempt to study the effect of xylene on sex steroids
during pregnancy, rats (CFY) were exposed to 0 or 681 ppm p-xylene
for 24 h on day 10 of gestation or continuously on days 9 and 10 of
gestation. The animals were killed on day 11. Data on maternal
toxicity were not reported. Sex hormone levels in the uterine and
femoral veins were decreased in the exposed group. The authors
(Ungvary et al., 1981) suggested that this may play a role in the
embryotoxicity.
Rats (CFY) were exposed to 0 or 230 ppm xylene for 24 h/day from
day 9 to day 14 of gestation, and the dams were killed on day 21. The
xylene used was 10% o-xylene, 50% m-xylene, 20% p-xylene and 20%
ethylbenzene. No maternal effects were seen in exposed animals.
There was an increased incidence of skeletal variants such as extra
ribs and fused sternebrae. Three malformations were found (2
agnathia, 1 fissura sterni), but there was no significant increase in
the frequency of malformations (Hudak & Ungvary, 1978).
In another study rats (CFY) were exposed to 0 or 138 ppm xylene
(not defined) 24 h/day from day 7 to day 15 of gestation. The dams
were killed on day 21. In the exposed group, decreased maternal
weight gain, delayed fetal development and increased incidence of
skeletal variants (extra ribs) were observed, but there was no
evidence of malformations (Ungvary, 1985).
In a report issued by the American Petroleum Institute in 1983,
described by Bell et al. (1992), Sprague-Dawley rats were exposed to a
xylene mixture of 20.4% o-xylene, 44.2% m-xylene, 20.3% p-xylene
and 12.8% ethylbenzene. There were 30 males and 60 females in the
control group, while 10 males and 20 females per group were exposed to
60 or 250 ppm xylene and 20 males and 40 females were exposed to 500
ppm xylene. Exposures were 6 h per day, 7 days per week, for a
131-day pre-mating period and a 20-day mating period. Mated females
were also exposed during days 1-20 of gestation and days 5-20 of
lactation. For males there were no effects on body weight gain, but
for females the mean body weight gain was significantly greater than
that of controls in the 60 and 250 ppm groups during the mating
period. This was not considered indicative of an adverse effect of
treatment. Mating indices were significantly lower than control
values at 250 ppm (both sexes treated) and at 500 ppm (females treated
only), but mating indices were comparable to control values at 500 ppm
when both sexes were treated or when males alone were treated. There
were no treatment-related effects on mean duration of gestation, mean
litter size or pup survival. The mean pup weight in the group where
both parents had been exposed to 500 ppm xylene was significantly
lower than for controls. In the teratogenicity part of the study
there was no evidence of an increased incidence of malformations in
exposed groups. The mean fetal weights for the 500 ppm group were
lower than the control value, but, the difference was statistically
significant for only the female fetuses.
Groups of Wistar rats were exposed to 0, 2, 11 or 114 ppm xylene
(mixed, not defined) for 6 h per day from day 1 to day 21 of
gestation. The maternal toxicity was not reported. The incidence of
post-implantation loss and fetal death was significantly increased in
the 11 ppm and 114 ppm groups. At the highest dose an increase in
some malformations was noted but no incidence was given. The results
of the study cannot be evaluated because of insufficient reporting of
exposure conditions and results (Mirkova et al., 1983). In an attempt
to repeat this study, groups of 36 Wistar rats were exposed to 200 ppm
technical xylene 6 h per day on days 4 to 20 of gestation. There were
no signs of maternal toxicity. No exposure-related differences were
found except for delayed ossification of maxillary bone. The
xylene-exposed pups had a slightly higher body weight and impaired
performance in a motor ability test, which was most marked in female
offspring (Hass & Jakobsen, 1993).
In a follow-up study (Hass et al., 1995), Wistar rats were
exposed to 500 ppm technical xylene (19% o-xylene, 45% m-xylene,
20% p-xylene and 15% ethylbenzene) for 6 h per day on gestation days
7-20. The dose level was selected so as not to induce maternal
toxicity or decrease the viability of offspring. There were 15
exposed litters and 13 control litters. A delay in the development of
the air righting reflex, a lower absolute brain weight and impaired
performance in behavioural tests for neuromotor abilities, learning
and memory were found in the offspring of the exposed rats.
Generally, the effects were most marked in the female offspring. The
alterations were long-lasting, as they were still apparent in adult
rats at the age of 4 months.
A composition of 9.1% o-xylene, 60.2% m-xylene, 13.6%
p-xylene and 17.0% ethylbenzene was given orally to CD-1 mice. The
doses were 0, 520, 1030, 2060, 2580, 3100 or 4130 mg per kg body
weight daily from day 6 to day 15 of gestation. All animals in the
highest dose group died and so did 50% of the animals in the 3100
mg/kg group. In this group there was a significant increase in the
incidence of dams with complete resorptions. There was a significant
increase in the incidence of cleft palate at > 2060 mg/kg, as well
as decreased mean fetal weight (Marks et al., 1982).
In order to study the teratogenic and embryotoxic effects of
xylene (60% p-xylene, 22% o-xylene, 18% ethylbenzene; no
m-xylene) embryos of Sprague-Dawley rats were explanted on day 9.5
of gestation and cultured in rat serum to which xylene (0.1, 0.5 or
1.0 ml/litre) dissolved in DMSO was added. The embryos were cultured
for 48 h. There were no observable teratogenic effects in terms of
malformations. However, dose-dependent embryotoxicity in terms of
retardation on growth and development was observed (Brow-Woodman et
al., 1991). In a similar study (Brown-Woodman et al., 1994) rat
embryos were incubated in vitro with up to 2.7 µmol xylene/ml for
40 h. Concentrations of > 1.89 µmol/ml retarded embryo growth and
development. The no-observed-effect level (NOEL) was 1.08 µmol/ml.
No gross morphological malformations were observed. Combined exposure
to toluene, xylene and benzene caused additive embryotoxic effects
with no evidence of synergistic action (Brown-Woodman et al., 1994).
When Sprague-Dawley rats were exposed to 1000 ppm xylene (not
defined) 18 h per day, 7 days per week, for 61 days, testicular
atrophy or loss of nerve growth factor-immunoreactive germ cell line
was not observed. Xylene also was found to protect from
n-hexane-induced testicular atrophy (Nylén et al., 1989).
Wistar rats were exposed for 7 days to xylene (isomer not stated)
twice a day until disappearance of righting reflex (xylene
concentration not given). Anaesthesia was achieved in about 10 min.
On day 8 the rats were killed. A decrease in body weight and weights
of testes and accessory reproductive organs, as well as reduced acid
phosphate activity in the prostate and reduced plasma testosterone
levels, was observed in xylene-exposed animals. There was also a
decrease in spermatozoan count in the epididymis (Yamada, 1993).
7.6 Mutagenicity and related end-points
Technical grade xylene did not produce differential killing in
DNA-repair-proficient compared to repair-deficient strains of
Bacillus subtilis rec+/- (McCarroll et al., 1981a) or Escherichia
coli (McCarroll et al., 1981b). Xylene (type not specified) did not
induce SOS activity in Salmonella typhimurium TA1535/pSK 1002
(Nakamura et al., 1987). For E. coli WP2 uvr A p-xylene was not
mutagenic in the presence or absence of an exogenous metabolic system
from PCB-induced rat liver (Shimizu et al., 1985). None of the
isomers nor unspecified xylene was mutagenic to S. typhimurium
TA1535, TA1537, TA98, TA100, UTH 8413 or UTH8414 in the presence or
absence of a metabolic system from uninduced or Arochlor-induced rat
and hamster livers (Lebowitz et al., 1979; Bos et al., 1981; Haworth
et al., 1983; Connor et al., 1985; Shimizu et al., 1985; Zeiger et
al., 1987).
Exposure to technical grade xylene containing 18.3% ethylbenzene
caused recessive lethal mutations in Drosophila melanogaster but not
exposure to m-xylene, or o-xylene (Donner et al., 1980). The same
report stated that exposure to rats for 300 ppm, 6 h per day, 5 days
per week for 9, 14 and 18 weeks did not induce chromosomal aberrations
in bone-marrow cells (Donner et al., 1980). Xylene (unspecified) did
not induce mutations in mouse lymphoma L5178Y TK+/- cells in vitro
or chromosomal aberrations in rat bone marrow cells (Lebowitz et al.,
1979). Xylene (unspecified) did not induce sister chromatid exchange
or chromosomal aberrations in human lymphocytes in vitro. No
exogenous metabolic system was used in this study (Gerner-Smidt &
Friedrich, 1978).
None of the isomers induced micronuclei in the bone marrow of
male NMRI mice after two i.p. administrations of 105-650 mg/kg body
weight at a 24-h interval, but they did, however, enhance the
induction of micronuclei by toluene (Mohtashamipur et al., 1985).
When Sprague-Dawley rats were given 440 or 1320 mg o-xylene/kg
body weight intraperitoneally, a significant increase in the
percentage of abnormal sperm was reported when the animals were housed
at 24-30°C (but not at 20-24°C). The authors (Washington et al.,
1983) interpreted this as a synergistic effect between o-xylene and
temperature.
7.7 Carcinogenicity
Group of Sprague-Dawley rats, 40 of each sex, received 500 mg
mixed xylenes (composition not specified) per kg body weight in olive
oil by stomach tube on 4 to 5 days per week for 104 weeks. Fifty
animals of each sex received olive oil only. The animals were
maintained until natural death. All animals had died by week 141. At
that time thymomas were reported in 1/34 treated males and 0/36
treated females (compared to 0/45 and 0/49, respectively, in
controls). Other haemolymphoreticular tumours (not specified) were
reported in 4/34 treated males and 3/36 treated females (compared to
3/45 and 1/49, respectively, in controls). The authors (Maltoni et
al., 1983; Maltoni et al., 1985) reported an increase in the total
number of animals with malignant tumours (type not specified) at 141
weeks, e.g., as 13/38 in treated males and 22/40 in treated females
(compared to 11/45 and 10/49, respectively, in control animals).
Combining all tumours is, however, not an acceptable basis for
analysis particularly in aged animals. No data were provided to allow
an analysis on an individual tumour-type basis.
In a carcinogenicity study, groups of 50 B6C3F1 mice of each sex
were given 0, 500 or 1000 mg xylene/kg body weight in corn oil by
stomach tube on 5 days per week for 103 weeks. The xylene used was
9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. The surviving animals were killed within 2 weeks after
the last administration. Survival at the termination of the study for
males was: 27 controls, 35 low-dose and 36 high-dose; and for females
was: 36 controls, 35 low-dose and 31 high-dose. No treatment-related
increase in the incidence of any tumour was seen in either sex (NTP,
1986; Huff et al., 1988).
The NTP also performed a carcinogenicity study on Fischer-344
rats with the same type of technical xylene. Groups of 50 rats of
each sex were given 0, 250 or 500 mg xylene/kg body weight in corn oil
by stomach tube 5 days per week for 103 weeks. The surviving animals
were killed within 2 weeks following the last administration. At the
termination of the experiment, the survival for males was: 36
controls, 25 low-dose and 20 high-dose animals, and for females was:
38 controls, 33 low-dose and 35 high-dose. For the males survival
appeared to be dose-related, but many of the early deaths were related
to gavage trauma or corn oil-xylene aspiration (3/14, 8/25, 11/30).
The incidences of tumours in treated animals of either sex were not
significantly higher than in the control group (NTP, 1986; Huff et
al., 1988).
Two studies have investigated whether exposure to xylenes alters
the incidence of experimentally induced skin neoplasia in mice (Pound
& Withers, 1963; Pound, 1970). The reporting does not allow any firm
conclusions.
7.8 Other effects
No effects were observed upon in vitro exposure of human
lymphocytes at concentrations up to 2 mM xylene for 72 h. However, at
higher concentrations, cell mortality only was significantly increased
(Richer et al., 1993).
8. EFFECTS ON HUMANS
8.1 Acute and accidental exposure
Acute poisoning and deaths have been reported after overexposure
or oral ingestion of substantial amounts of xylene. The exposure
level required for loss of consciousness has been estimated to be
10 000 ppm (Morley et al., 1970). At autopsy pulmonary congestion and
oedema have been observed after inhalation or oral intake (Morley et
al., 1970; Abu Al Ragheb et al., 1986). Among survivors coma, EEG
changes, amnesia, mental confusion and ocular nystagmus have been
reported. Evidence of gastrointestinal and respiratory symptoms as
well as impaired renal and hepatic function have also been observed
(Ghislandi & Fabiani, 1957; Recchia et al., 1985; Bakinson & Jones,
1985). After exposure to about 700 ppm (calculated) for up to one
hour, headache, nausea, irritation of the eyes, nose and throat,
dizziness, vertigo and vomiting have been reported (Klaucke et al.,
1982). Recovery seems to be complete in most non-fatal cases although
dizziness and vision problems have been observed 24 h after ingestion
of xylene (quantity unknown) (Recchia et al., 1985). In a suicidal
attempt a 30-year-old man injected 8 ml of xylene intravenously.
After 10 min he developed a life-threatening acute pulmonary failure,
but survived through medical treatment (Sevcik et al., 1992).
8.2 Controlled human studies
A number of volunteer studies have been performed predominantly
at the Finnish Institute of Occupational Health. Effects on the
sensory motor and information process functions of the central nervous
system (CNS) have been investigated. Usually m-xylene has been
used, but p-xylene and mixed xylenes have also been studied
(Savolainen & Linnavuo, 1979; Savolainen, 1980; Savolainen et al.,
1980; Seppäläinen et al., 1981; Savolainen & Riihimäki, 1981;
Savolainen et al., 1981; Savolainen et al., 1982a, Savolainen et al.,
1982b; Savolainen et al., 1984; Savolainen et al., 1985a, Savolainen
et al., 1985b). In these studies no significant effects on vestibular
or visual function, reaction times, coordination or peripheral senses
were observed during a 4-h exposure to a constant concentration of up
to 160 ppm. Slight impairment of vestibular and visual function and
reaction time was noted at exposure levels from 200 to 300 ppm. There
was adaption to the impairment over five successive daily exposures.
In another study (Anshelm Olson et al., 1985), volunteers (n=16) were
exposed for 4 h to p-xylene alone (300 mg/m3: 70 ppm) or in
combination with toluene (200 mg/m3 toluene plus 100 mg/m3
p-xylene). Heart rate, subjective symptoms, simple reaction time,
choice reaction time and short-term memory were unaffected by
exposure.
In another study nine male volunteers were exposed for 4 h to 200
ppm (TWA) m-xylene. Short-term peak exposures were up to 400 ppm.
The effects of xylene on electroencephalography (EEG) were minor and
no deleterious effects were noted (Seppäläinen et al., 1991).
Healthy male subjects were exposed to technical xylene,
containing 40% ethylbenzene, for 2 h with or without a working load of
100 watts. The air concentration was 435 or 1300 mg/m3. During work
at the higher exposure level evidence of performance decrement was
observed in three of the five performance tests: reaction time
addition test (p < 0.05), short-term memory (p < 0.05) and choice
reaction time (p < 0.10) (Gamberale et al., 1978).
Dizziness was reported by four of six volunteers exposed to 690
ppm p-xylene for 15 min (Carpenter et al., 1975). Nine volunteers
were exposed for about 4 h to either a constant or a fluctuating
pattern of m-xylene with a time-weighted average exposure
concentration of 200 ppm in both cases (Laine et al., 1993).
Prolonged simple visual and auditive choice reaction times were
observed after exposure to the peaks of 400 ppm m-xylene. Exposure
to m-xylene at a constant level of 200 ppm did not affect the ratio
of "active" to "quiet" sleep during the following night, but decreased
slightly the number of body movements in bed.
Studies on coexposure to m-xylene and ethanol (Savolainen,
1980; Seppäläinen et al., 1981; Savolainen & Riihimäki, 1981;
Riihimäki et al., 1982a,b; Riihimäki et al., 1982a,b), m-xylene and
1,1,1-trichloroethane (TCE) (Savolainen et al., 1981; Savolainen et
al., 1982) or p-xylene and toluene (Anshelm-Olson et al., 1985) have
been performed. Exposure to 145-150 ppm xylene and 0.8 g ethanol/kg
body weight had an additive disturbing effect on vestibular function.
At higher xylene concentrations (275-290 ppm) there was evidence of
functional tolerance, and xylene appeared to antagonize the effect of
ethanol on vestibular function. In a study where volunteers were
exposed to 200 ppm m-xylene, minor effects on vestibular and visual
functions and reaction time were reported. Simultaneous exposure to
400 ppm TCE had no further effect. Combined exposure to 60 ppm
p-xylene and 30 ppm toluene had no effect on reaction time,
short-term memory or heart rate.
Ten male volunteers were exposed to 100 ppm xylene (not
specified) or 100 ppm toluene or a mixture of 50 ppm of each.
Exposure time was 4 h and each person participated in four exposure
sessions. Changes in CNS functions were tested by nine psychological
tests. Xylene had the most adverse effect on simple reaction time and
choice reaction time, while the combined exposure gave weaker effects
than xylene alone but stronger than toluene alone (Dudek et al.,
1990).
A summary of acute effects from inhalation exposure is presented
in Table 8.
In studies on skin irritation, both hands of subjects were
immersed in pure m-xylene (Engström et al., 1977; Lauwerys et al.,
1978; Riihimäki, 1979a). A burning sensation was soon noticed and an
erythematous reaction was observed in the exposed skin. Of six
subjects tested, four reported eye irritation after exposure to 460 or
690 ppm xylene for 15 min. The xylene contained 7.6% o-xylene,
65.0% m-xylene, 7.8% p-xylene and 19.3% ethylbenzene. One subject
reported eye irritation at 230 ppm but none at 110 ppm (Carpenter et
al., 1975). In another study, no irritation in the eyes, nose or
throat was reported after exposure to 98, 196 or 392 ppm mixed xylenes
for 30 min (Hastings et al., 1984). In an older study exposure to 200
ppm xylene (undefined) for 3-5 min caused irritation of the eyes, nose
and throat (Nelson et al., 1943).
The skin sensitization potential was investigated using a
non-adjuvant maximization test. The xylene used was not defined.
None of 24 subjects tested showed evidence of sensitization (Kligman,
1966).
Five adult, healthy, white men were exposed for 7 h per day for 3
days to 40 ppm xylene. This exposure was repeated three times at
intervals of 2 weeks. Blood samples were taken before and after each
exposure. No significant effects were observed on sister-chromatid
exchange frequency, cell cycle time or cell mortality in lymphocytes
(Richer et al., 1993).
8.3 Occupational exposure
In workers exposed to xylene or solvent mixtures containing large
amounts of xylene, subjective symptoms have been reported (Joyner &
Leak Pegues, 1961; Glass, 1961; Hipolito, 1980; Kilburn et al., 1983;
Kilburn et al., 1985). Depression, fatigue, headache, anxiety,
feeling of drunkenness and sleep disorders were the most common
symptoms reported, but exposure levels and duration were often missing
in these reports.
Workers occupationally exposed to solvent mixtures including
xylene have been reported to have neurophysiological and psychological
disorders (Lindström, 1973; Seppäläinen et al., 1978; Elofsson et al.,
1980; Husman, 1980; Husman & Karli, 1980; Arlien-Soborg et al., 1981;
Lindström et al., 1982; Valciukas et al., 1985; Maizlish et al., 1987;
Van Vliet et al., 1987; Ruijten et al., 1994). In these studies there
was no exposure to xylene alone. Xylene was not the main solvent in
the mixture. No conclusions concerning effects of xylenes as such can
be drawn from these studies.
Table 8. Single inhalation exposure to xylene in humans
Exposure Time Effect Reference
concentration
(mg/m3)
3 000 1 h Dizziness, irritation Klaucke et al. (1982)
3 000 15 min Dizziness Carpenter et al. (1975)
1300a 2 h Performance decrement Gamberale et al. (1978)
900b 4 h Prolonged reaction times Laine et al. (1993)
900 4 h Impairment of vestibular and visual Savolainen et al.
function and prolonged reaction time (1979, 1981, 1982, 1985)
900 4 h Minor effect on EEG Seppäläinen et al. (1991)
600 4 h No effect on reaction time Savolainen et al. (1980, 1981)
450 4 h Prolonged reaction time Dudek et al. (1990)
300 4 h No effects in psychophysiological test Anshelm - Olson et al. (1985)
a during exercise
b peak values of 1800 mg/m3
Liver effects have been reported in some studies on workers
occupationally exposed to solvents containing xylene (Dössing et al.,
1981; Edling, 1982; Sotaniemi et al., 1982; Dössing et al., 1983;
Fischbein et al., 1983, Lundberg et al., 1994) but these were not
confirmed in other studies (Craveri et al., 1982; Kurppa & Husman,
1982; Lundberg & Håkansson, 1985). Recent findings seem to support
the concept that the hepatotoxicity of xylene is low (Riihimåki &
Hännien, 1987).
Some case studies and epidemiological studies have addressed a
possible association between occupational exposure to hydrocarbons
(including xylene) and proliferative glomerulonephritis (Beirne &
Brennan, 1972; Zimmerman et al., 1975; Lagrue, 1976). Exposures
have, however, been so diverse that the role of xylene is impossible
to assess. A Swedish group of researchers reported a higher
concentration of albumin, erythrocytes and leukocytes in the urine of
workers exposed predominantly to xylenes and toluene than among
controls (Askergren et al., 1981a,b,c; Askergren, 1981). Franchini et
al. (1983) found that painters exposed to toluene and xylenes at
relatively low concentrations had a higher excretion of kidney tubular
enzymes in their urine than the controls. They suggested that the
mixed solvents may exert a slight adverse effect on the kidney
tubules.
One case of contact urticaria due to occupational exposure to
airborne xylene has been reported. The level of xylene in air
exceeded 100 ppm. Direct skin contact with the solvent appeared to
have been negligible. The contact urticaria seemed likely to be an
immunological type (Palmer & Rycroft, 1993).
Thirty-five male spray varnishers were exposed to 0.5-3.4 ppm
o-xylene, 3.2-11.7 ppm m-xylene, 0.9-4.3 ppm p-xylene, 1.4-7.5
ppm ethylbenzene, < 1.5 ppm toluene, < 1.2 ppm n-butanol, < 35.5
ppm 1,1,1-trichloroethane and several C9 aromatics. In addition, some
of the lacquers contained lead pigments. The mean peripheral
erythrocyte counts and haemoglobin levels were decreased in the
exposed men compared to controls. Whether these effects were due to
xylene or the solvent mixture is uncertain (Angerer & Wulf, 1985).
The health effects on 175 factory workers in China exposed to
xylene vapour with a mixture of three isomers at a concentration of up
to 175 ppm (with time-weighted average geometric mean concentration of
14 ppm and arithmetic mean of 21 ppm) were studied. There was an
increased prevalence of subjective symptoms in the exposed workers;
these were apparently related to effects on the central nervous system
and to local effects on the eye, nose and throat (Uchida et al.,
1993). In workers exposed to a maximum concentration of 103 ppm
xylene (geometric mean 4 ppm) and 203 ppm toluene (geometric mean 3
ppm), the prevalence of some subjective CNS-related symptoms was
higher than in the controls (Chen et al., 1994). No effects on
haematology or serum biochemistry with respect to liver and kidney
functions were observed in these two studies.
Sister-chromatid exchanges (SCE) in peripheral lymphocyte
cultures have been studied in two groups of 23 workers who had been
exposed for between 4 months and 23 years to mixed xylenes (including
ethylbenzene). The exposure levels for the two groups were 11 and 13
ppm, respectively. No differences in SCE frequency was seen (Pap &
Varga., 1987). A few other reports have described increased incidence
of chromosomal aberrations or effects on sister-chromatid exchange
frequencies (Funes-Cravioto et al., 1977; Haglund et al., 1980). In
both these studies the exposure to xylene (not defined) was
accompanied by exposure to other solvents, including benzene.
No data on carcinogenic effects resulting from exposure to
xylenes have been found in the literature.
In an investigation into the effect on reproduction, the outcome
of pregnancy was studied among university laboratory employees exposed
to xylene (not defined) during the first trimester of pregnancy.
Exposure levels were not given. There was no difference in
miscarriage rate when compared to controls not exposed to solvents
(Axelsson et al., 1984).
A similar case control study on associations between laboratory
work and pregnancy outcome revealed that use of xylene for 3 or more
days a week during the first trimester was significantly associated
with an elevated risk of spontaneous abortion. The authors pointed
out, however, that laboratory workers were often exposed to several
solvents and chemicals simultaneously; only two cases and two controls
were exposed to xylene alone (Taskinen et al., 1994). About half of
the women exposed to xylene worked in pathology/histology laboratories
where there was concomitant exposure to formaldehyde vapour.
Formaldehyde (formalin) also appeared to be a significant risk factor
for spontaneous abortion. Exposure to xylene was also noted to be
associated with an increase in birth weight (Taskinen et al., 1994).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Bringmann & Kuhn (1977, 1978) exposed the bacterium Pseudomonas
putida for 16 h and the blue-green alga Microcystis aeroginosa for
8 days to xylene. They found a reduction in cell multiplication at
concentrations of >200 mg/litre.
Walton et al. (1989) studied the effect of p-xylene on the
microbial respiration of two soil types, a silt loam (1.49% organic
carbon) and a sandy loam (0.66% organic carbon). The chemical was
applied at a rate of 1000 µg/g (dry weight). Microbial respiration,
as measured by CO2 efflux, of the silt loam was unaffected. In the
sandy loam, the CO2 efflux initially decreased and then increased,
but returned to control levels within the 6-day exposure period.
All three isomers have been shown to inhibit the respiration of
sewage sludge utilizing biogenic substrates. Two screening tests were
used, RIKA (respiration inhibition kinetic analysis) and OECD 209.
The concentration of each compound used was at the limit of the
solubility in the medium (approx. 175-198 mg/litre). Inhibitions in
the respiration rate of 100% were found for all three isomers in the
RIKA screening test, and inhibitions of 22% ( o-xylene) and 43%
( p-xylene) were measured in the OECD 209 screening test. m-Xylene
was only tested at a concentration of 0.3 mg/litre in the OECD 209
screening test, which resulted in 5% inhibition (Volskay & Grady,
1990).
The toxicity of xylene to three species of environmental bacteria
has been determined using assays in sealed serum bottles to prevent
loss of chemical by volatilization. Activity of the bacteria was
measured by either gas production over 48 h (methanogens), oxygen
consumption over 15 h (aerobic heterotrophs) or ammonia use over 24 h
(Nitrosomonas). IC50 values (the concentration required to inhibit
the bacterial activity by 50%, as compared with controls) for xylene
were 250 mg/litre for methanogens, 1100 mg/litre for aerobic
heterotrophs and 100 mg/litre for Nitrosomonas (Blum & Speece,
1991).
9.1.2 Aquatic organisms
Xylene isomers are highly volatile and disappear rapidly from
solution. For example, Mackay & Wolkoff (1973) found that in agitated
water, 1m deep and with a 1m2 surface for evaporation, the half-life
for o-xylene was 39 min. When Benville & Korn (1977) monitored the
loss of xylene from solution during LC50 tests, average percentage
losses for the four time intervals studied (24, 48, 72 and 96 h) were
29, 61, 84 and > 99% respectively. These losses mean that the
exposure can be difficult to determine. For example, many of the 24-h
and 96-h LC50 values are the same or similar, suggesting that most of
the xylene had been lost during the test. Galassi et al. (1988),
using a closed static system, found mean measured concentrations of
xylene and other aromatics to fluctuate by only 10% during the test
period of up to 96 h. Care must therefore be taken when interpreting
data from open static tests over longer periods than 24 h, especially
those based on nominal concentrations. Overall it can be stated that
xylene has moderate to low acute toxicity for aquatic organisms.
9.1.2.1 Algae
Bringmann & Kühn (1977) exposed the green alga Scenedesmus
quadricauda for 8 days to xylene. They found reduction in cell
multiplication at concentrations of > 200 mg/litre. Brooks et al.
(1977) studied the effect of xylene on photosynthesis in a mixed ocean
culture of phytoplankton. They found that exposure to a concentration
of 3 mg/litre xylene for 8 h caused a 50% reduction in photosynthesis.
Galassi et al. (1988) calculated the 72-h EC50 for growth
inhibition in the alga Selenastrum capricornutum to be 4.7, 4.9 and
3.2 mg/litre for the ortho, meta and para isomers, respectively.
Similar results were obtained by Herman et al. (1990). Using the same
species of algae, they obtained 8 day EC50 values for growth
inhibition of 4.2, 3.9 and 4.4 mg/litre for the ortho, meta and para
isomers, respectively. Sheedy et al. (1991) reported a 14-day EC50
for growth inhibition of Selenastrum capricornutum of 72 mg/litre
for xylene (composition not stated).
Hutchinson et al. (1980) exposed the algae Chlamydomonas
angulosa and Chlorella vulgaris to p-xylene for 3 h. EC50
values for inhibition of photosynthesis (measured using 14CO2
uptake) were 45.7 and 105.1 mg/litre for the two species,
respectively. Kauss et al. (1973) exposed the green alga Chlorella
vulgaris to o-xylene and studied growth over a 10-day period in an
open system. At nominal concentrations of between 25 and 100
mg/litre, a short-term toxic effect was observed. However, the algal
culture recovered within 2 days. The authors pointed out that
recovery was probably due to volatilization of the chemicals. A
near-saturation concentration of 171 mg o-xylene/litre proved to be
acutely toxic and the alga did not recover. Concentrations of 25 and
50 mg o-xylene/litre progressively increased the lag period between
initial inoculation and growth. An o-xylene concentration of 100
mg/litre delayed the onset of growth for 4 days and a near-saturation
concentration of 171 mg/litre caused complete inhibition of growth
during the 10-day exposure period (Kauss & Hutchinson, 1975).
Dunstan et al. (1975) exposed marine microalgae, the diatom
Skeletonema costatum, the dinoflagellate Amphidinium carterae, the
coccolithophorid Cricosphaera and the green flagellate Dunaliella
tertiolecta, to xylene for 3 days. A xylene concentration of 10
mg/litre inhibited the growth of all species. Inhibition was most
marked in A carterae and S. costatum.
9.1.2.2 Higher plants
Frank et al. (1961) exposed the angiosperm pondweeds Elodea
canadensis, Potamogeton nodosus and P. pectinatus to xylene
(plus 2% emulsifying agent) under static, open conditions for a period
of four weeks. A concentration of 100 mg/litre was found to be toxic
(8.6 on an injury scale of 0 to 10) but 5 mg/litre was not. Both 300
and 600 mg/litre were similarly toxic after 30-min exposures in
flowing water.
9.1.2.3 Protozoa
Bringmann et al. (1980) exposed the flagellate protozoan
Chilomonas paramaecium to xylene for 48 h and found an initial
reduction (5%) in cell multiplication at concentrations of > 80
mg/litre. Rogerson et al. (1983) exposed the ciliate protozoan
Colpidium colpoda to xylene in an "open" system consisting of a
covered watchglass with an air space. Toxicity thresholds were 1.75
mg/litre (16.5 mmol/m3) for o-xylene and 162 mg/litre (1530
mmol/m3) for m-xylene; no threshold was calculated for p-xylene.
The same authors exposed the protozoan Tetrahymena ellioti to xylene
isomers in a closed system with no air space. Toxicity thresholds for
o-, p- and m-xylene were 18.5, 16.9 and 55.7 mg/litre (174, 159
and 525 mmol/m3), respectively.
9.1.2.4 Invertebrates
The LC50 values of xylene to aquatic invertebrates are
summarized in Table 9.
Le Gore (1974) exposed Pacific oyster larvae (Crassostrea
gigas) to o- and p-xylene for 48 h. LC50 values were 0.17 and
0.58 mg/litre for the two isomers, respectively, suggesting that this
organism is one of the more sensitive invertebrates to xylene
exposure. However, no experimental details were given for these
toxicity tests, which makes them difficult to interpret.
Table 9. LC50 values of xylenes to aquatic invertebrates
Organisms Size/ Static/ Open/ Temp. Hardness Isomer Duration Concentration Reference
life-stage flowa closeda (°C) (mg/litre)c (mg/litre)d
Estuarine and marine invertebrates
Pacific oyster larvae ortho 48 h 0.17 Le Gore (1974)
(Crassostrea larvae para 48 h 0.58 Le Gore (1974)
gigas)
Grass shrimp static c 21 15s 96 h 7.4 Neff et al. (1976)
(Palaemonetes static c 21 15s 24 h 14.0 Tatem et al. (1978)
pugio) 96 h 7.4 Tatem et al. (1978)
Bay shrimp adult static o 16 25s ortho 24 h 4.7 n Benville & Korn (1977)
(Crago adult static o 16 25s ortho 96 h 1.1 n Benville & Korn (1977)
franciscorum) adult static o 16 25s meta 24 h 4.1 n Benville & Korn (1977)
adult static o 16 25s meta 96 h 3.2 n Benville & Korn (1977)
adult static o 16 25s para 24 h 1.7 n Benville & Korn (1977)
adult static o 16 25s para 96 h 1.7 n Benville & Korn (1977)
Dungeness crab zoeae static+ 13 30s ortho 48 h 38 n Caldwell et al. (1977)
(Cancer zoeae static+ 13 30s ortho 96 h 6 n Caldwell et al. (1977)
magister) zoeae static+ 13 30s meta 48 h 33 n Caldwell et al. (1977)
zoeae static+ 13 30s meta 96 h 12 n Caldwell et al. (1977)
Table 9. (Cont'd)
Organisms Size/ Static/ Open/ Temp. Hardness Isomer Duration Concentration Reference
life-stage flowa closeda (°C) (mg/litre)c (mg/litre)d
Freshwater invertebrates
Water flea static o 24 h >100<1000 n Dowden & Bennett (1965)
(Daphnia magna) static 24 h 165 n Brigmann & Kühn (1982)
static c ortho 24 h 1 m Galassi et al. (1988)
static c meta 24 h 4.7 m Galassi et al. (1988)
static c para 24 h 3.6 m Galassi et al. (1988)
4-6 days static c ortho 48 h 3.2 n Bobra et al. (1983)
4-6 days static c meta 48 h 9.6 n Bobra et al. (1983)
4-6 days static c para 48 h 8.5 n Bobra et al. (1983)
< 24 h flow o 17 44.7 ortho 48 h 3.82 m Holcombe et al. (1987)
Mosquito larvae static o 24-26 24 h 13.9 Berry & Brammer (1977)
(Aedes aegypti)
Snail
(Aplexa adult flow o 17 44.7 ortho 96 h >22.4 m Holcombe et al. (1987)
hypnorum)
a static = static conditions (water unchanged for duration of test); b o = open; c = closed
static+ = semi-static conditions (water renewed at 24 hour intervals); c s = salinity (%)
flow = flow-through conditions (xylene concentration in water continuously maintained) d m = measured; n = nominal
Berry & Brammer (1977) exposed mosquito larvae (Aedes aegypti)
to xylene under static, open conditions at 25°C. Larvae were exposed
for 24 h, since no detectable levels of any water-soluble component
remained after that period. A non-lethal concentration of 7.92
mg/xylene litre was reported. However, the authors found that
bioassays using different volumes of solution and different sized
containers demonstrated the significant effect that surface area,
volume and depth can have on the results of experiments with volatile
hydrocarbons such as xylenes.
Falk-Petersen et al. (1985) exposed sea urchin (Strongy
locentrotus droebachiensis) eggs to o-xylene from fertilization
and monitored deaths, pathology, inhibition of cleavage and
differentiation, and pigment effects. Eggs were maintained in test
beakers covered with aluminium foil. They calculated a 96-h EC50,
based on all these parameters, of 4.1 mg/litre.
Freshwater mussels (Dreissena polymorpha) were exposed to
various concentrations of xylene, and the behaviour of the mussels in
terms of shell valve movements was monitored. At the start of the
experiment, all valves were gaping continuously. After a certain
period of toxicant addition, a gradual increase in valve closure
period was observed. Finally, total closure of shell valves was
observed. Effects were first seen at a xylene concentration of
11.9-19.4 mg/litre (nominal) (Slooff et al., 1983).
9.1.2.5 Vertebrates
The LC50 values of xylene to fish are summarized in Table 10.
Morrow et al. (1975) found that 100 mg xylene/litre killed 100%
of young coho salmon (Oncorhynchus kisutch) within 24 h under
static, closed conditions. Concentrations of 1 and 10 mg/litre did
not cause significant mortality within the 96-h exposure period.
Toxicity included rapid, violent and erratic swimming, "coughing",
loss of equilibrium and death.
Rainbow trout (Oncorhynchus mykiss) significantly avoided
xylene (plus 2% emulsifying agent) at a nominal concentration of 0.1
mg/litre during a 1-h test. Fish exposed to 0.001 mg/litre did not
show significant avoidance and those exposed to 0.01 mg/litre were
significantly attracted to the xylene (Folmar, 1976). Maynard & Weber
(1981) found that juvenile coho salmon were able to significantly
avoid o-xylene concentrations of > 0.2 mg/litre water.
Slooff (1979) exposed rainbow trout Oncorhynchus mykiss to
various concentrations of xylene in a flow-through, closed system and
monitored any effects of the chemical on their breathing. The lowest
concentration at which a toxic condition developed within 24 h after
toxicant administration was 2 mg/litre.
Table 10. LC50 values of xylenes to fish
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Freshwater fish
Fathead minnow 1-2 g static o 25 20 24 h 28.8 n Pickering & Henderson
(Pimephales 1-2 g static o 25 20 48 h 27.7 n (1966)
promelas) 1-2 g static o 25 20 96 h 26.7 n
1-2 g static o 25 360 24 h & 96 h 28.7 n
0.3 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Bluegill 1-2 g static o 25 20 24 h 24 n Pickering & Henderson
(Lepomis 1-2 g static o 25 20 48 h 24 n (1966)
macrochirus) 1-2 g static o 25 20 96 h 20.9 n
1.1 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Goldfish 1-2 g static o 25 20 24 h & 96 h 36.8 n Pickering & Henderson
(Carassius (1966)
auratus)
20-80 g flow 17-19 80 24 h 30.6 m Weber et al. (1975)
20-80 g flow 17-19 80 96 h 16.9 m
3.3 g static o 19-21 ortho 24 h 13 m Bridie et al. (1979)
3.3 g static o 19-21 meta 24 h 16 m
3.3 g static o 19-21 para 24 h 18 m
2.5 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Table 10. (Cont'd)
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Guppy 0.1-0.2 g static o 25 20 24 h & 96 h 34.7 n Pickering & Henderson
(Poecilia (1966)
reticulata)
static meta 14 days 305 Könemann (1981)
static ortho 7 days 291
static para 7 days 285
static c 20-22 ortho 96 h 12 m Galassi et al. (1988)
static c 20-22 meta 96 h 12.9 m
static c 20-22 para 96 h 8.8 m
Rainbow trout static c 11-13 ortho 96 h 7.6 m Galassi et al. (1988)
(Oncorhynchus static c 11-13 meta 96 h 8.4 m
mykiss) static c 11-13 para 96 h 2.6 m
flow o 9-13 89.5 96 h 10e Folmar (1976)
13.1 g flow o 17 44.7 ortho 96 h 8.05 m Holcombe et al. (1987)
0.9 g flow o 12 40-48 technical 24 h & 96 h 13.5 Walsh et al. (1977)
Zebra fish flow c 48 h 20 Sloff (1979)
(Brachydanio
rerio)
Table 10. (Cont'd)
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Golden orfe 1.2-1.8 g static 48 h 86f Juhnke & Lüdemann
(Leuciscus 1.2-1.8 g static 48 h 308f (1978)
idus
melanotus)
White sucker 2.4 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
(Catostomus
commersoni)
Marine fish
Coho salmon young static c 8 30s 24 h > 10 < 100 Morrow et al. (1975)
(Oncorhynchus
kisutch)
Striped bass 6 g static o 16 25s ortho 24 h & 96 h 9.7 n Benville & Korn (1977)
(Morone 6 g static o 16 25s meta 24 h & 96 h 7.9 n
saxatilis) 6 g static o 16 25s para 24 h & 96 h 1.7 n
a static = static conditions (water unchanged for duration of test);
flow = flow-through conditions (xylene concentration in water continuously maintained)
b o = open; c = closed
c s = salinity (°/oo)
d m = measured; n = nominal
e included 2% emulsifying agent
f results from two different laboratories
When Walsh et al. (1977) exposed rainbow trout (average weight
165 ± 86 g) to xylene concentrations of 0.31, 0.65 and 1.1 mg/litre in
artificial streams for 56 days, no adverse effects on the fish were
noted at any concentration. Using the same artificial streams, all
rainbow trout exposed to xylene concentrations of 14.2 or 22.5
mg/litre for 2 h died. Fish exposed to xylene concentrations of 3.2
and 6.2 mg/litre for 2 h showed symptoms similar to anaesthesia.
Kjorsvic et al. (1982) exposed cod eggs ( Gadus morhus L.) to
xylene isomers in covered glass dishes and monitored the effects both
during fertilization and during early cleavage of fertilised eggs.
Both m-xylene and p-xylene induced significant decreases in the
fertilization rate at concentrations above 10 mg/litre. o-Xylene
had no significant effect on the fertilization rate at concentrations
of 16-35 mg/litre. Fertilized eggs were exposed to xylene for 3 or 6
h before first cleavage. No significant difference was observed
between the individual xylene isomers or between the two exposure
periods. Effects on the early cleavage pattern were significant for
xylene concentrations between 2 and 7 mg/litre. The effects seen
included inhibition of formation of the cleavage furrow. Small cells
or a total absence of cleavage occurred on exposure to all isomers at
concentrations of 16-35 mg/litre, while incomplete or uneven cleavage
was found at exposures of 8-15 mg/litre.
Black et al. (1982) exposed embryo-larval stages of the leopard
frog Rana pipiens and rainbow trout (Oncorhynchus mykiss) to
m-xylene from 30 min after fertilization to 4 days after hatching in
a closed, flow-through system (hatching times were 5 days for the frog
and 23 days for the trout). LC50 values of 3.53 and 3.77 mg/litre
were calculated for the two species, respectively.
9.1.3 Terrestrial organisms
Hill & Camardese (1986) exposed Japanese quail (Coturnix
coturnix japonica) to xylene in 5-day dietary toxicity tests. The
LC50 was found to be greater than 20 000 mg/kg diet. No overt signs
of toxicity occurred at 5000 mg/kg.
No studies on terrestrial plants, terrestrial invertebrates or
field effects of xylenes have been reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposures
In the general environment humans are exposed to xylenes mainly
by inhalation. Absorption through the skin may also occur in the
working environment. The retention in the lungs is about 60% of the
inhaled dose. Xylene is efficiently metabolized. More than 90% is
biotransformed to methylhippuric acid, which is excreted in urine.
Xylene does not accumulate significantly in the human body.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3 with levels of 3 µg/m3 in suburban
areas. Higher levels have been measured in urban and industrialized
areas with mean concentrations ranging up to 500 µg/m3; however,
concentrations are generally below 100 µg/m3.
The estimated daily exposure of the general population through
inhalation is 70 µg in rural areas and less than 2000 µg in urban
areas. The concentration in drinking-water ranges from not detectable
to 12 µg/litre. Data on the levels in food are too limited to
estimate daily oral exposure.
10.1.2 Effects
Based on experimental human studies, xylene may have an acute
impairing effect on the sensory-motor and information-processing
functions of the central nervous system (CNS). Exposure to 435-870
mg/m3 (100-200 ppm) of xylene over 4 h caused slight impairments in
reaction time performance and vestibular functions. There was
adaptation at 200 ppm m-xylene to the impairment over five
successive days. The LOAEL of xylene for acute CNS effects, based on
one study, is 470 mg/m3 (108 ppm). It should be noted, however,
that other identical studies found significant effects only at
concentrations of 870 mg/m3 (200 ppm) or more. According to a study
by a different research group, exposure to 304 mg/m3 (70 ppm) p-
xylene for 4 h did not cause impairment of corresponding psycho-
physiological functions; 304 mg/m3 (70 ppm) xylene can therefore
be regarded as the NOAEL for acute CNS effects.
Xylene vapour becomes irritating at relatively high levels.
Among six volunteer subjects, four reported eye irritation after
exposure to 2000 or 3000 mg/m3 (460 or 690 ppm) xylene for 15 min
while one subject reported eye irritation at 1000 mg/m3 (230 ppm) and
none at 478 mg/m3 (110 ppm). According to another study, no
irritation of the eyes, nose or throat was reported after exposure to
423, 852 or 1705 mg/m3 (98, 196 or 392 ppm) mixed xylenes for 30 min.
These human findings are consistent with mouse studies showing that
strong irritancy (respiratory rate decrease by 50%) occurs at about
5960 mg/m3 (1370 ppm) xylene. The odour threshold for xylene is
about 1 ppm.
Subjective symptoms have been reported among workers exposed to
solvent mixtures containing large amounts of xylene. Long-term
exposure to xylene is suspected to affect the nervous system adversely
because chronic toxic encephalopathy and milder functional
disturbances of the brain have sometimes been found among exposed
painters and other workers. Likewise, slight changes in kidney
tubular function may occur. The specific role of xylene in these
effects cannot, however, be ascertained.
When human data are sparse, especially data from chronic studies,
animal data are used as a substitute. An assessment of the risk to
human health of exposure to xylene must rely on animal studies.
Apparently irreversible effects on the CNS were found 4 months
after a 3-month inhalation exposure (24 h per day) of Mongolian
gerbils to xylene at concentrations of 696 or 1392 mg/m3 (160 or 320
ppm). At the lower level the effects were not statistically
significant in any individual part of the brain but the changes were
all of the same nature. The study disclosed an increased
concentration of astroglial proteins in most brain regions studied,
which may indicate that glial proliferation is characteristic to
various neurodegenerative and neurotoxic states. In the light of
similar findings in animals exposed to other solvents (e.g.,
trichloroethylene, ethanol and tetrachloroethylene), the results are
estimated to be an important piece of evidence for potential
xylene-induced neurotoxicity at > 696 mg/m3 (160 ppm) (the LOAEL).
Functional changes, similar to acute effects on nervous functions,
were described after exposure to 435 mg/m3 (100 ppm), but they cannot
be discriminated from the acute effects of the last exposure. The
exposure level is consistent with the levels giving acute effects in
humans, as stated above.
No adequate studies of reproduction and development toxicity in
humans exposed to xylene alone have been published. Placental
transfer of xylene has been shown in humans and in experimental
animals.
Teratogenicity studies in pregnant animals exposed to technical
xylene or xylene isomers during organogenesis indicate that xylene may
cause reduced fetal weight and delayed ossification, but not
malformations, at dose levels causing no or only slight maternal
toxicity. LOAEL values of 500-2175 mg/m3 (115-500 ppm) have been
reported, depending on the length of the daily exposure periods (6-24
h/day). Signs of delayed ossification in the absence of lower fetal
body weight have been reported at lower dose levels. However, these
findings cannot be properly evaluated owing to incomplete description
of the criteria for assessing ossification. A NOAEL for delayed fetal
development cannot therefore be established.
In a study of postnatal development in rat offspring prenatally
exposed to 870 or 2175 mg/m3 (200 or 500 ppm) technical xylene,
behavioural impairments indicating effects on the development of the
central nervous system were detected. There was no maternal toxicity,
and the effects at 2175 mg/m3 (500 ppm) were long-lasting as they
were apparent in adult offspring. As 870 mg/m3 (200 ppm) was the
lowest dose level investigated for this effect a NOAEL could not be
established.
In several short-term and long-term animal studies, effects on
the activities of various metabolic enzymes in different organs have
been observed. Exposure to 217 mg/m3 (50 ppm) m-xylene 6 h/day for
5 days induced renal cytochrome P-450. At 1305 mg/m3 (300 ppm)
xylene (6 h/day for 14 days) an increase in hepatic and renal enzyme
activities was observed. At > 1740 mg/m3 (400 ppm) increases in
hepatic and renal metabolic enzyme levels, as well as increased
relative liver and kidney weights, have been reported. At the same
exposure levels the pulmonary P-450 content and pulmonary enzyme
activities were decreased. When rats were orally given 150 mg
xylene/kg body weight per day for 90 days, increased relative liver
weights were seen.
These changes in metabolic enzyme activities and increased
relative liver weight could be taken as an indication of metabolic
adaptation rather than toxicity.
When rats were exposed to 3480 mg/m3 (800 ppm) xylene, 14 h/day,
7 day/week for 6 weeks, an increased auditory response threshold was
reported. Thus, 3480 mg/m3 (800 ppm) is the LOAEL for ototoxicity.
For this effect no NOAEL could be established as 3480 mg/m3 (800 ppm)
was the lowest dose level used.
Xylene appears not to be a mutagen or a carcinogen.
10.1.3 Guidance value
The definition and aim of guidance values for the general
population have been described by IPCS (1994).
Although some differences in action between the three isomers
exist, there is no clear evidence that they (or mixture of them) have
totally different effects.
There have been no long-term controlled human studies or
epidemiological studies from which a guidance value may be calculated.
Epidemiological data from the occupational setting do not allow
an estimation of xylene-specific chronic nervous system effects,
neither can the neuropsychological impairment seen among
solvent-exposed workers be attributed to any specific level of xylene
in air.
On the basis of human volunteer studies (Anshelm Olson, 1985),
one may conclude that the NOAEL for acute CNS effects in humans is
about 304 mg/m3 (70 ppm) for a 4-h exposure. The use of an
uncertainty factor of 10 for intraspecies variability (the study
subjects were healthy male research workers) and an additional factor
of 6 (4-h exposure versus 24-h general population exposure) leads to a
guidance value of 4.8 mg/m3 (1.1 ppm). It should be noted, however,
that the acute CNS effect observed probably has no predictive value
for chronic CNS toxicity by xylene. The guidance value of 4.8 mg/m3
(1.1 ppm) is close to the odour threshold of xylene. It should be
noted that a subset of the human population may be sensitive enough to
experience the odour as annoying. The Task Group considered that
there was no need to add another uncertainty factor for the lack of
data from chronic exposure.
On the basis of animal studies on developmental toxicity, one may
conclude that the LOAEL for reduced fetal body weight is 500 mg/m3
(115 ppm) (Ungvary & Tatrai, 1985) and that for developmental
neurotoxicity is 870 mg/m3 (200 ppm) (Hass & Jakobsen, 1993).
Developmental neurotoxicity is a serious effect that may be
long-lasting and is therefore considered the critical effect. An
uncertainty factor of 10 for use of a LOAEL rather than a NOAEL seems
justified based on the evidence of lower fetal body weight at 500
mg/m3 (115 ppm) and limited evidence of delayed ossification at even
lower exposure levels. The use of additional factors of 10 for
interspecies variation and 10 for inter-individual variation leads to
a guidance value of 0.87 mg/m3 (0.2 ppm).
Using the hearing loss (Pryor et al., 1987) detected in animals
after exposure to 3480 mg/m3 (800 ppm) xylene for 6 weeks as a
starting point, an uncertainty factor of 10 for use of a LOAEL rather
than a NOAEL, a factor of 10 for interspecies variation and an
additional factor of 10 for inter-individual variation results in a
guidance value of 3.48 mg/m3 (0.8 ppm).
A neurotoxicity study in animals exposed continuously for 3
months to 696 or 1392 mg/m3 (160 or 320 ppm) xylene (Rosengren et
al., 1986) provided suggestive biochemical evidence of an apparently
irreversible adverse effect on the nerve cells of the brain even at
the lower level. Although there may be uncertainty concerning the
biological significance and interpretation of the findings, the Task
Group considered them potentially important and recommended further
confirmatory studies. With respect to animal-human extrapolation and
relatively short exposure, the estimate of the critical level for
life-long exposure in humans is 1.6 ppm. A guidance value of 0.87
mg/m3 (0.2 ppm) covers another uncertainty factor of approximate 10
in this respect.
Based on the above considerations, the Task Group recommended
0.87 mg/m3 (0.2 ppm) as a guidance value for the general population.
This value was derived from the LOAEL reported for developmental
neurotoxic effects in laboratory animals.
10.2 Evaluation of effects on the environment
10.2.1 Exposure
The majority of xylene released into the environment will enter
the atmosphere directly. In the atmosphere the xylene isomers are
readily degraded. Volatilization to the atmosphere from water is
rapid for all three isomers. Although the meta and para isomers are
readily biodegraded, in soil and water the ortho isomer is more
persistent. Bioaccumulation of xylene isomers by aquatic organisms is
low.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3. Mean background concentrations of
xylenes in surface waters are generally below 0.1 µg/litre. However,
higher values have been measured in industrial areas. In areas
associated with the oil industry even higher levels have been reported
but only associated with discharge pipes. Similar background levels
have been reported for groundwater, although localized pollution can
lead to higher levels.
10.2.2 Effects
The xylene isomers are of moderate to low toxicity to aquatic
organisms. The variation between each individual isomer with respect
to aquatic toxicity is generally small. The lowest LC50 value, based
on measured concentrations, is for a 24-h exposure of Daphnia magna
to 1 mg o-xylene/litre.
There is limited information regarding chronic exposure of
aquatic organisms to xylenes and none of the observed effect levels
were lower than those summarized under the acute studies.
The acute toxicity of xylene to birds is low.
10.2.3 Risk evaluation
Xylenes are rapidly degraded in the environment. However, the
photooxidation reactions of the xylene isomers in the atmosphere may
contribute to photochemical smog.
High levels of xylenes have been reported in groundwater
associated with localized pollution from underground tanks and pipes,
but the environmental significance of such values is difficult to
assess.
For the aquatic environment, the most sensitive toxicity test,
based on measured concentrations, yielded an LC50 for Daphnia
magna of 1 mg/litre ( o-xylene). This value is more than 10 000
times higher than mean background concentrations in surface water,
which are generally less than 0.1 µg/litre for each isomer. The
lowest LC50 is still over 30 times higher than the highest single
measured concentration of total xylenes in the most polluted area.
The exposure/toxicity ratio will be much higher for mean
concentrations in polluted areas.
On the basis of rapid volatilization and degradation of xylenes
and their low to moderate toxicity, the overall risk to the aquatic
environment can therefore be considered low. It should be noted,
however, that very much higher levels have been measured around
discharges from oil production sites, and higher levels are also
possible if spillage occurs.
The likely route of exposure for birds in the environment is via
food such as fish. The only acute toxicity test on birds was carried
out on 14-day old Japanese quail and gave a 5-day LC50 of > 5000
mg/kg diet. Based on food consumption and body weight, an LD50 for
the quail of > 1746 mg/kg body weight can be calculated. Using this
data an estimated LC50 for a fish-eating bird (kingfisher) based on
body weight and food consumption can be calculated.
LC50 (mg/kg dry weight diet) =
Test species LD50 (mg/kg) × body wt (kg)
food consumption (kg)
The estimated LC50 for the fish-eating bird is > 7990 mg/kg
diet. The highest water concentration (30 µg/litre) multiplied by
the highest theoretical bioconcentration factor (158) gives a worst
case residue level in fish of 4.7 mg/kg. It should be noted that the
bioconcentration factor does not take into account degradation or
metabolism. Comparing this value to the estimated LC50 value gives a
Toxicity Exposure Ratio (TER) of > 1700. Therefore, the risk to
fish-eating birds is very low.
11. CONCLUSIONS
Humans are exposed to xylene mainly by inhalation. This compound
does not accumulate significantly in the human body. Acute exposure
to high concentrations can result in CNS effects in human. There have
been no long-term controlled studies or epidemiological studies with
exposure to xylene alone. The chronic toxicity appears to be
relatively low in laboratory animals. There is suggestive evidence,
however, that chronic CNS effects may occur in animals at moderate
concentrations of xylene. Xylene appears not to be a mutagen or a
carcinogen. The critical end-point is developmental toxicity. Based
on this end-point, the recommended guidance value for xylene in air
for the general population is 0.87 mg/m3 (0.2 ppm). This value is
higher than the concentrations to which the general population is
exposed.
The xylene isomers are non-persistent chemicals, being readily
degraded in the atmosphere, soil and water. It should be noted that
o-xylene appears to biodegrade only in the presence of other carbon
sources and at a reduced rate compared to the other isomers. The
photooxidation reactions of the xylene isomers in the atmosphere may
contribute to photochemical smog.
It can be concluded that xylenes are unlikely to cause problems
in aquatic ecosystems except near to localized industrial discharges
and spillage incidents. The risk to birds from xylene exposure is
low.
12. FUTURE RESEARCH
There is little information on the long-term effects of xylene in
humans and, specifically, no dose-response or dose-effect data are
available. Epidemiological studies of populations occupationally
exposed to xylene should be encouraged. In this context the use of
xylene metabolites in urine as a marker of exposure can be of special
value because the method determines the internal doses that
individuals receive via all routes of exposure. Because xylene has
acute effects on CNS, epidemiological studies should address the CNS
as a potential target organ. Moreover, since ethylbenzene is almost
invariably one of the components in solvent mixtures at the workplace,
study designs that address possible interactions between xylene and
other solvent components are desirable.
Animal studies are needed to address biochemical, functional and
morphological evidence of chronic neurotoxicity and potential effects
on fertility. In addition, there is a need for further studies on
developmental toxicity to assess the dose-response relationship and to
estimate the NOAEL, especially for developmental neurotoxicity.
Further studies are needed to determine the effect on hearing
impairment in order to determine the NOAEL. The relationship between
the dose level and the length of the exposure period should also be
investigated. The effect of exposure to xylene together with noise
should be studied.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC) has
evaluated the carcinogenicity of xylene. There was inadequate
evidence for the carcinogenicity of xylene in humans as well as in
experimental animals. The overall evaluation was that xylene is not
classifiable as to its carcinogenicity in humans (IARC, 1989).
The European Commission (1991-1992) recommended an 8-h
time-weighted average occupational exposure limit for xylene of 217
mg/m3 (50 ppm). To limit peaks of exposure that could result in
irritation, a short-term exposure level (STEL) of 435 mg/m3 (100 ppm)
was recommended.
REFERENCES
Abu Al Ragheb S, Salhab AS, & Amr SS (1986) Suicide by xylene
ingestion: A case report and review of literature. Am J Forensic Med
Pathol, 7: 327-329.
Altshuller AP, Lonneman WA, Sutterfield FD, & Kopczyncki SL (1971)
Hydrocarbon composition of the atmosphere of the Los Angeles basin,
1967. Environ Sci Technol, 5: 39.
Alvarez PJJ & Vogel TM (1991) Substrate interactions of benzene,
toluene, and para-xylene during microbial degradation by pure cultures
and mixed culture aquifer slurries. Appl Environ Microbiol,
57: 2981-2985.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3: 272-290.
Andersson K, Fuxe K, Nilsen OG, Toftgård R, Eneroth P, & Gustafsson
J-Å (1981) Production of discrete changes in dopamine and
noradrenaline levels and turnover in various parts of the rat brain
following exposure to xylene, ortho-, and para-xylene, and
ethylbenzene. Toxicol Appl Pharmacol, 60: 535-548.
Angerer J (1988a) Determination of toluene in blood by head-space gas
chromatography.
Angerer J (1988b) Determination of hippuric acid in urine by
high-pressure liquid chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 301-306 (IARC
Scientific Publications No. 85).
Angerer J & Lehnert G (1979) Occupational chronic exposure to organic
solvents. VIII. Phenolic compounds - metabolites of alkylbenzenes in
man: Simultaneous exposure to ethylbenzene and xylenes. Int Arch Occup
Environ Health, 43: 145-150.
Angerer J & Wulf H (1985) Occupational exposure to organic solvents.
XI. Alkylbenzene exposure of varnish workers: Effects on hematopoetic
system. Int Arch Occup Environ Health, 56: 307-321.
Anshelm Olson B, Gamberale F, & Iregren A (1985) Coexposure to toluene
and p-xylene in man: central nervous functions. Br J Ind Med,
42: 117-122.
Antoine SR, Deleon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36: 364-371.
Arlien-Soborg P, Zilstorff K, Grandjean B, & Milling Pedersen L (1981)
Vestibular dysfunction in occupational chronic solvent intoxication.
Clin Otolaryngol, 6: 285-290.
Aschan G, Bunnfors I, Hydén D, Larsby B, Ödkvist LM, & Tham R (1977)
Xylene exposure. Electronystagmographic and gas chromatographic
studies in rabbits. Acta Otolaryngol, 84: 370-376.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, & Wooten JV (1994) Blood
concentrations of volatile organic compounds in a nonoccupationally
exposed US population and in groups with suspected exposure. Clin
Chem, 40(7): 1401-1404.
Askergren A (1981) Studies on kidney function in subjects exposed to
organic solvents. III. Excretion of cells in the urine. Acta Med
Scand, 210: 103-106.
Askergren A, Allgén L-G, & Bergström J (1981a) Studies on kidney
function in subjects exposed to organic solvents. II. The effect of
desmopressin in a concentration test and the effect of exposure to
organic solvents on renal concentrating ability. Acta Med Scand,
209: 485-488.
Askergren A, Allgén L-G, Karlsson C, Lundberg I, & Nyberg E (1981b)
Studies on kidney function in subjects exposed to organic solvents. I.
Excretion of albumin and ß-2-microglobulin in the urine. Acta Med
Scand, 209: 479-483.
Askergren A, Brandt R, Gullquist R, Silk B, & Strandell T (1981c)
Studies on kidney function in subjects exposed to organic solvents.
IV. Effect on 51Cr-EDTA clearance. Acta Med Scand, 210: 373-376.
Åstrand I, Engström J, & Övrum P (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man. Scand J
Work Environ Health, 4: 185-194.
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions
of the hydroxyl radical with organic compounds under atmospheric
conditions. Chem Rev, 85: 69-201.
Axelsson G, Lütz C, & Rylander R (1984) Exposure to solvents and
outcome of pregnancy in university laboratory employees. Br J Ind Med,
41: 305-312.
Bakinson MA & Jones RD (1985) Gassings due to methylene chloride,
xylene, toluene, and styrene reported to Her Majesty's Factory
Inspectorate 1961-80. Br J Ind Med, 42: 184-190.
Bakke OM & Scheline RR (1970) Hydroxylation of aromatic hydrocarbons
in the rat. Toxicol Appl Pharmacol, 16: 691-700.
Barker JF, Barbash JE, & Labonte M (1988) Groundwater contamination at
a landfill sited on fractured carbonate and shale. J Contam Hydrol,
3: 1-25.
Batchelor JJ (1927) The relative toxicity of benzol and its higher
homologues. Am J Hyg, 7: 276-298.
Beirne GJ & Brennan JT (1972) Glomerulonephritis associated with
hydrocarbon solvents. Mediated by antiglomerular basement membrane
antibody. Arch Environ Health, 25: 365-369.
Bell GM, Gordon ACH, Lee P, Doig A, MacDonald MK, Thomson D, Anderton
JL, & Robson JS (1985) Proliferative glumerulonephritis and exposure
to organic solvents. Nephron, 40: 161-165.
Bell GM, Shillaker RO, Padgham MDJ, & Standring P (1992) Xylenes.
London, Health and Safety Executive, 166 pp (Toxicity Review 26).
Benville PE & Korn S (1977) The acute toxicity of six monocyclic
aromatic crude oil components to striped bass (Morone saxatilis) and
bay shrimp (Crago franciscorum). Calif Fish Gam, 63: 204-209.
Bergman K (1979) Whole-body autoradiography and allied tracer
techniques in distribution and elimination studies of some organic
solvents. Scand J Work Environ Health, 5(suppl 1): 1-263.
Bergman K (1983) Application and results of whole-body autoradiography
in distribution studies of organic solvents. CRC Crit Rev Toxicol,
12: 59-118.
Berry WO & Brammer JD (1977) Toxicity of water-soluble gasoline
fractions to fourth-instar larvae of the mosquito Aedes aegypti L.
Environ Pollut, 13: 229-234.
Bevan MAJ, Proctor CJ, Baker-Rogers J, & Warren ND (1991) Exposure to
carbon monoxide, respirable suspended particulates and volatile
organic compounds while commuting by bicycle. Environ Sci Techol,
25: 788-791.
Bieniek G, Palys E, & Wilczok T (1982) TLC separation of hippuric,
mandelic, and phenylglyoxylic acids from urine after mixed exposure to
toluene and styrene. Br J Ind Med, 39: 187-190.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, University of
Kentucky, Water Resources Research Institute, 61 pp (Research Report
No. 133).
Blanchard KT & Morris JB (1994) Effects of m-xylene on rat nasal
cytochrome P450 mixed function oxidase activities. Toxicol Lett,
70: 253-259.
Blum DJW & Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. J Water Pollut Control Fed, 63: 198-207.
Bobra AM, Shiu WY, & Mackay D (1983) A predictive correlation for the
acute toxicity of hydrocarbons and chlorinated hydrocarbons to the
water flea (Daphnia magna). Chemosphere, 12: 1121-1129.
Bonnet P, Raoult G, & Gradiski D (1979) Concentrations léthales50 des
principaux hydrocarbures aromatiques. Arch Mal Prof, 40: 805-810.
Bonnet P, Morele Y, Raoult G, Zissu D, & Gradiski D (1982)
Détermination de la concentration léthale50 des principaux
hydrocarbures aromatiques chez le rat. Arch Mal Prof, 43: 261-265.
Bos RP, Brouns RME, van Doorn R, Theuws JLG, & Henderson PT (1981)
Non-mutagenicity of toluene, o-, m- and p-xylene, o-methyl-
benzylalcohol and o-methylbenzylsulfate in the Ames assay.
Mutat Res, 88: 273-279.
Boström E-C, Almén J, Steen B, & Westerholm R (1994) Human exposure to
urban air pollution. Environ Health Perspect, 102(suppl 4): 39-47.
Botta D, Pirri LC, & Mantica E (1984) Ground water pollution by
organic solvents and their microbial degradation products. In:
Analytical Organic Micropollutants in Water. Luxembourg, Commission of
the European Communities, pp 261-275 (Report EUR 8518).
Bowers DE Jr, Cannon MS, & Jones DH (1982) Ultrastructural changes in
livers of young and aging rats exposed to methylated benzenes. Am J
Vet Res, 43: 676-683.
Bray HG, Humphris BG, & Thorpe WV (1949) Metabolism of derivatives of
toluene. 3. o-, m- and p-xylenes. Biochem J, 45: 241-244.
Brice KA & Derwent RG (1978) Emission inventory for hydrocarbons in
the United Kingdom. Atmos Environ, 12: 2045-2054.
Bridie AL, Wolff CJM, & Winter M (1979) The acute toxicity of some
petrochemicals to goldfish. Water Res, 13: 623-626.
Bringmann G & Kühn R (1977) [Toxic threshold values for compounds
hazardous to the aquatic environment on bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda) using cell
multiplication test.] Z Wasser Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kühn R (1978) [Toxic threshold values for compounds
hazardous to the aquatic environment on blue algae (Microcystic
aeruginosa) and green algae (Scenedesmus quadricauda) using cell
multiplication test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1982) [Results of the toxic effects of compounds
hazardous to the aquatic environment on Daphnia magna using an
improved standardized test.] Z Wasser Abwasser Forsch, 15: 1-6 (in
German).
Bringmann G, Kühn R, & Winter A (1980) [Determination of biocidal
effects of compounds hazardous to the aquatic environment for
Protozoa: III. Saprozoic flagelates.] Z Wasser Abwasser Forsch,
13: 170-173 (in German).
Brodzinsky R & Singh HB (1983) Volatile organic chemicals in the
atmosphere: An assessment of available data. Washington, DC, US
Environmental Protection Agency (EPA/DF-83/005A).
Brooks JM, Fryxell GA, Reid DF, & Sackett WM (1977) Gulf underwater
flare experiment (GULFEX): Effects of hydrocarbons on phytoplankton
In: Pollutant effects on marine organisms. Proceedings of a Workshop,
College station, Texas, USA, 16-19 May 1976, pp 45-75.
Brown RH (1988a) Determination of benzene, toluene and xylene in
industrial air by charcoal tube, solvent desorption and gas
chromatography. In: Fishbein L & O'Neill IK ed. Environmental
carcinogens: Methods of analysis and exposure measurement. Volume
10: Benzene and alkylated benzenes. Lyon, International Agency for
Research on Cancer, pp 225-233 (IARC Scientific Publications No. 85).
Brown RH (1988b) Determination of benzene, toluene and xylene in
industrial air by porous polymer adsorption tube, thermal desorption
and gas chromatography. In: Fishbein L & O'Neill IK ed. Environmental
carcinogens: Methods of analysis and exposure measurement. Volume
10: Benzene and alkylated benzenes. Lyon, International Agency for
Research on Cancer, pp 235-242 (IARC Scientific Publications No. 85).
Brown RH (1988c) Determination of gasoline hydrocarbons in industrial
air by two-stage (chromosorb/charcoal) adsorption, thermal desorption
and capillary gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 243-251 (IARC
Scientific Publications No. 85).
Brown-Woodman PDC, Webster WS, Picker K, & Ritchie HE (1991)
Embryotoxicity of xylene and toluene: an in study. Ind Health,
29: 139-152.
Brown-Woodman PDC, Webster WS, Picker K, & Huq F (1994) In
assessment of individual and interactive effects of aromatic
hydrocarbons on embryonic development of the rat. Reprod Toxicol,
8: 121-135.
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other trace
organic compounds in urban air. Chemosphere, 17: 2363-2380.
Bushnell PJ (1989) Behavioral effects of acute p-xylene inhalation
in rats: Autoshaping, motor activity, and reversal learning.
Neurotoxicol Teratol, 10: 569-577.
Bushnell PJ & Peele DB (1988) Conditioned flavor aversion induced by
inhaled p-xylene in rats. Neurotoxicol Teratol, 10: 273-277.
Buszka PM, Zaugg SD, & Werner MG (1990) Determination of trace
concentrations of volatile organic compounds in ground water using
closed-loop stripping, Edwards Aquifer, Texas. Bull Environ Contam
Toxicol, 45: 507-515.
Buttery RG, Seifert RM, & Ling LC (1975) Characterization of some
volatile constituents of dry red beans. J Agric Food Chem,
23: 516-519.
Caldwell RS, Caldarone EM, & Mallon MH (1977) Effects of a
seawater-soluble fraction of Cook Inlet crude oil and its major
aromatic components on larval stages of the Dungeness crab, Cancer
magister Dana. In: Wolfe DA ed. Fate and effects of petroleum
hydrocarbons in marine ecosystems and organisms. Oxford, New York,
Pergamon Press, pp 210-220.
Cameron GR, Paterson JLH, de Saram GSW, & Thomas JC (1938) The
toxicity of some methyl derivatives of benzene with special reference
to pseudocumene and heavy coal tar naphta. J Pathol Bacteriol,
46: 95-107.
Campbell L, Wilson HK, Samuel AM, & Gompertz D (1988) Interactions of
m-xylene and aspirin metabolism in man. Br J Ind Med, 45: 127-132.
Carlsson A (1981) Distribution and elimination of 14C-xylene in rat.
Scand J Work Environ Health, 7: 51-55.
Carpenter CP, Kinkead ER, Geary DL Jr, Sullivan LJ, & King JM (1975)
Petroleum hydrocarbon toxicity studies. V. Animal and human response
to vapors of mixed xylenes. Toxicol Appl Pharmacol, 33: 543-558.
Chan C-C, Özkaynak H, Spengler JD, & Sheldon L (1991a) Driver exposure
to volatile organic compounds, CO, ozone and NO2 under different
driving conditions. Environ Sci Techol, 25: 964-972.
Chan C-C, Spengler JD, Ozkaynak H, & Lefkopoulou M (1991b) Commuter
exposures to VOCs in Boston, Massachusetts. J Air Waste Manage Assoc,
41: 1594-1600.
Chan C-C, Lin S-H, & Her GR (1993) Student's exposure to volatile
organic compounds while commuting by motorcycle and bus in Taipei
City. J Air Waste Manage Assoc, 43(9): 1231-1238.
Chen Z, Liu S-J, Cai S-X, Yao Y-M, Yin H, Ukai H, Uchida Y, Nakatsuka
H, Watanabe T, & Ikeda M (1994) Exposure of workers to a mixture of
toluene and xylenes. II. Effects. Occup Environ Med, 51: 47-49.
Chiang CY, Salanito JP, Chai EY, Colthart JD, & Klein CL (1989)
Aerobic biodegradation of benzene, toluene and xylene in a sandy
aquifer - Data analysis and computer modelling. Ground Water,
27: 823-834.
Chung TY, Hayase F, & Kato H (1983) Volatile components of ripe
tomatoes and their juices, purees and pastes. Agric Biol Chem,
47: 343-351.
City of Toronto (1990) The quality of drinking water in Toronto: A
review of tap water, bottled water and water treated by a point-of-use
device. Toronto, Canada, Department of Public Health.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural and motorway locations.
Environ Pollut, B7: 141-158.
Condie LW, Hill JR, & Borzelleca JF (1988) Oral toxicology studies
with xylene isomers and mixed xylenes. Drug Chem Toxicol, 11: 329-354.
Connor TH, Theiss JC, Hanna HA, Monteith DK, & Matney TS (1985)
Genotoxicity of organic chemicals frequently found in the air of
mobile homes. Toxicol Lett, 25: 33-40.
Cragg ST, Clarke EA, Daly IW, Miller RR, Terrill JB, & Ouellette RE
(1989) Subchronic inhalation toxicity of ethylbenzene in mice, rats,
and rabbits. Fundam Appl Toxicol, 13: 399-408.
Cramer PH, Boggess KE, Hosenfeld JM, Remmers JC, Breen JJ, Robinson
PE, & Stroup C (1988) Determination of organic chemicals in human
whole blood: Preliminary method development for volatile organics.
Bull Environ Contam Toxicol, 40: 612-681.
Craveri A, Tornaghi G, Ciaci D, Vitali L, Carucci R, De Luca P, &
Donato MF (1982) [Serum bile salts in the hepatological screening of
subjects exposed to aromatic hydrocarbons.] Giorn Clin Med,
63: 322-329 (in Italian).
Crawford L & Kretsch MJ (1976) GC-MS identification of the volatile
compounds extracted from roasted turkeys fed a basal diet supplemented
with tuna oil: Some comments on fishy flavour. J Food Sci,
41: 1470-1478.
Crofton KM, Lassiter TL, & Rebert CS (1994) Solvent-induced
ototoxicity in rats: an atypical selective mid-frequency hearing
deficit. Hear Res, 80: 25-30.
Daisey JM, Hodgson AT, Fisk WJ, Mendell MJ, & Ten Brinke J (1994)
Volatile organic compounds in twelve California office buildings:
classes, concentrations and sources. Atmos Environ, 28(22): 3557-3562.
Davey JF & Gibson DT (1974) Bacterial metabolism of para- and
meta-xylene: Oxidation of a methyl substituent. J Bacteriol,
119: 923-929.
Davis RS, Hossler FE, & Stone RW (1967) Oxidation of m- and p-
xylenes by Pseudomonas. Bateriol Proc, P162: 129.
Day BJ & Carlson GP (1992) Effect of p-xylene metabolites,
p-methylbenzyl alcohol and 2,5-dimethylphenol on rat hepatic and
pulmonary microsomal metabolism. Res Commun Chem Pathol Pharmacol,
76: 117-120.
Day BJ, DeNicola DB, Marcus CB, & Carlson GP (1992) Effect of
p-xylene inhalation on the bioactivation of bromobenzene in rat lung
and liver. Fundam Appl Toxicol, 19: 50-56.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
De Ceaurriz J, Desiles JP, Bonnet P, Marignac B, Muller J, & Guenier
JP (1983) Concentration-dependent behavioral changes in mice following
short-term inhalation exposure to various industrial solvents. Toxicol
Appl Pharmacol, 67: 383-389.
DECOS (1991) Health-based recommended occupational exposure limit for
xylene. The Hague, Directorate-General of Labour.
De Gandarias JM, Echevarria E, Irazusta J, Gil J, & Casis L (1993)
Brain aminopeptidase activity after subacute xylene exposure.
Neurotoxicol Teratol, 15: 51-53.
del Rosario R, de Lumen BO, Habu T, Flath RA, Mon TR, & Teranishi R
(1984) Comparison of headspace volatiles from winged beans and
soybeans. J Agric Food Chem, 32: 1011-1015.
Derwent RG & Jenkin ME (1990) Hydrocarbon involvement in photochemical
ozone formation in Europe. Oxfordshire, Harwell Laboratory, AEA
Environment and Energy, Modelling and Assessments Group (Report AERE
R13736).
Dési I, Kovacs F, Zahumenszky Z, & Balogh A (1967) Maze learning in
rats exposed to xylene intoxication. Psychopharmacologia (Berl),
11: 224-230.
Divincenzo GD & Krasavage WJ (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic solvents.
Am Ind Hyg Assoc J, 35: 21-29.
Donner M, Mäki-Paakkanen J, Norppa H, Sorsa M, & Vainio H (1980)
Genetic toxicology of xylenes. Mutat Res, 74: 171-172.
Dössing M, Arlien Söborg P, Milling Pedersen L, & Ranek L (1981)
[Liver damage following long-term occupational exposure to organic
solvents.] Ugeskr Laeg, 143: 2297-2301 (in Danish with English
summary).
Dössing M, Arlien Söborg P, Milling Pedersen L, & Ranek L (1983) Liver
damage associated with occupational exposure to organic solvents in
house painters. Eur J Clin Invest, 13: 151-157.
Dowden BF & Bennett HJ (1965) Toxicity of selected chemicals to
certain animals. J Water Pollut Control Fed, 37: 1308-1316.
Dowty BJ & Laseter JL (1976) The transplacental migration and
accummulation in blood of volatile organic constituents. Ped Res,
10: 696-701.
Dowty BJ, Carlisle DR, & Laseter JL (1975) New Orleans drinking water
sources tested by gas chromatography-mass spectrometry. Environ Sci
Techol, 9: 762-765.
Drew RT & Fouts JR (1974) The effects of inducers on acute p-xylene
toxicity. Toxicol Appl Pharmacol, 29: 111-112.
Drozd J & Novak J (1978) Quantitative and qualitative head-space gas
analysis of parts per billion amounts of hydrocarbons in water: A
study of model systems by capillary-column gas chromatography with
splitless sample injection. J Chromatogr, 158: 471-482.
Dudek B, Gralewicz K, Jakubowski M, Kostrzewski P, & Sokal J (1990)
Neurobehavioral effects of experimental exposure to toluene, xylene
and their mixture. Pol J Occup Med, 3: 109-116.
Dunstan WM, Atkinson LP, & Natoli J (1975) Stimulation and inhibition
of phytoplankton growth by low molecular weight hydrocarbons. Mar
Biol, 31: 305-310.
Dutkiewicz T & Tyras H (1968) Skin absorption of toluene, styrene, and
xylene by man. Br J Ind Med, 25: 243.
Dyer RS, Bercegeay MS, & Mayo LM (1988) Acute exposures to p-xylene
and toluene alter visual information processing. Neurotoxicol Teratol,
10: 147-153.
EAJ (Environment Agency, Japan) (1993) Chemicals in the environment.
Tokyo, Ministry of Health and Welfare, International Affairs Division.
ECETOC (1986) Joint assessment of commodity chemicals No. 6: Xylenes.
Brussels, European Chemical Industry Ecology and Toxicology Centre.
Edling C (1982) Interaction between drugs and solvents as a cause of
fatty change in the liver? Br J Ind Med, 39: 198-199.
Edwards EA & Grbic-Galic D (1994) Anaerobic degradation of toluene and
o-xylene by a methanogenic consortium. Appl Environ Microbiol,
60(1): 313-322.
Edwards EA, Willis LE, Reinhard M, & Grbic-Galic D (1992) Anaerobic
degradation of toluene and xylene by aquifer microorganisms under
sulfate-reducing conditions. Appl Environ Microbiol, 58: 794-800.
Eller PM (1984) NIOSH manual of analytical methods: Method 1501, 3rd
ed. Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 2.
Elofsson S-A, Gamberale F, Hindmarsh T, Iregren A, Isaksson A,
Johnsson I, Knave B, Lydahl E, Mindus P, Persson HE, Philipson B,
Steby M, Struwe G, Söderman E, Wennberg A, & Widén L (1980) Exposure
to organic solvents: A cross-sectional epidemiologic investigation on
occupationally exposed car and industrial spray painters with special
reference to the nervous system. Scand J Work Environ Health,
6: 239-273.
Elovaara E (1982) Dose-related effects of m-xylene inhalation on the
xenobiotic metabolism of the rat. Xenobiotica, 12: 345-352.
Elovaara E, Collan Y, Pfäffli P, & Vainio H (1980) The combined
toxicity of technical grade xylene and ethanol in the rat.
Xenobiotica, 10: 435-445.
Elovaara E, Pfäffli P, Savolainen H, & Vainio H (1982) Marginal role
of impaired aldehyde metabolism in m-xylene vapour-induced
biochemical effects in the rat. J Appl Toxicol, 2: 27-32.
Elovaara E, Engström K, & Vainio H (1984) Metabolism and disposition
of simultaneously inhaled m-xylene and ethylbenzene in the rat.
Toxicol Appl Pharmacol, 75: 466-478.
Elovaara E, Zitting A, Nickels J, & Aitio A (1987) m-Xylene
inhalation destroys cytochrome P-450 in rat lung at low exposure. Arch
Toxicol, 61: 21-26.
Engelhardt WE & Estler W (1935) [Experiments on the acute narcotic
effects of aliphatic and aromatic hydrocarbons: The effect of single
inhalation of gasoline, benzene, toluene, xylene in the rabbit and the
cat.] Ark Hyg, 114: 249-260 (in German).
Engström J & Bjurström R (1978) Exposure to xylene and ethylbenzene.
II. Concentration in subcutaneous adipose tissue. Scand J Work Environ
Health, 4: 195-203.
Engström J & Riihimäki V (1979) Distribution of m-xylene to
subcutaneous adipose tissue in short-term experimental human exposure.
Scand J Work Environ Health, 5: 126-134.
Engström K & Riihimäki V (1988a) Determination of xylenes in blood by
head-space gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 307-311 (IARC
Scientific Publications No. 85).
Engström K & Riihimäki V (1988b) Determination of methylhippuric acids
in urine by gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 313-318 (IARC
Scientific Publications No. 85).
Engström K, Husman K, & Riihimäki V (1977) Percutaneous absorption of
m-xylene in man. Int Arch Occup Environ Health, 39: 181-189.
Engström K, Husman K, Pfäffli P, & Riihimäki V (1978) Evaluation of
occupational exposure to xylene by blood, exhaled air and urine
analysis. Scand J Work Environ Health, 4: 114-121.
Engström K, Riihimäki V, & Laine A (1984) Urinary disposition of
ethylbenzene and m-xylene in man following separate and combined
exposure. Int Arch Occup Environ Health, 54: 355-363.
European Commission (1991-1992) Occupational exposure limits.
Recommendations of the Scientific Expert Group 1991-1992. Luxembourg,
European Commission, pp 53-55 (Report EUR 15091 EN).
Fabre R, Truhaut R, & Laham S (1960) Recherches toxicologiques sur les
solvants de remplacement du benzčne. IV. Etude des xylčnes. Arch Mal
Prof, 21: 301-313.
Fairfax R (1995) Solvent, wood dust, and noise exposure in a decoy
shop. Appl Occup Environ Hyg, 10(5): 446-448.
Falk-Petersen I-B, Kjrsvic E, Lonning S, Naley AM, & Sydnes LK (1985)
Toxic effects of hydroxylated aromatic hydrocarbons on marine embryos.
Sarsia, 70: 11-16.
Fellin P & Otson R (1993) Seasonal trends of volatile organic
compounds (VOCs) in Canadian homes. In: Indoor air '93. Proceedings of
the 6th International Conference on Indoor Air Quality and Climate,
Helsinki, Finland, 4-8 July 1993 - Volume 2: Chemicals in indoor air,
materials emissions, pp 117-122.
Ferrario JB, Lawler GC, Deleon IR, & Laseter JL (1985) Volatile
organic pollutants in biota and sediments of Lake Pontchartrain. Bull
Environ Contam Toxicol, 34: 246-255.
Fielding M, Gibson TM, James HA, McLoughlin K, & Steel CP (1981)
Organic micropollutants in drinking water. Medmenham, Marlow, United
Kingdom, Water Research Centre (Technical Report TR-159).
Fishbein L (1988) Xylenes: Uses, occurrence and exposure. In: Fishbein
L & O'Neill IK ed. Environmental carcinogens: Methods of analysis and
exposure measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 109-120 (IARC
Scientific Publications No. 85).
Fischbein A, Ross RR, & Lerman Y (1983) Industrial solvents and the
liver. Lancet, 1: 129.
Fishbein L, O'Neill IK, & Tossavainen A (1988) Past and present
exposure determination techniques for benzene, toluene and xylene. In:
Fishbein L & O'Neill IK ed. Environmental carcinogens: Methods of
analysis and exposure measurement. Volume 10: Benzene and alkylated
benzenes. Lyon, International Agency for Research on Cancer,
pp 123-148 (IARC Scientific Publications No. 85).
Folmar LC (1976) Overt avoidance reaction of rainbow trout fry to nine
herbicides. Bull Environ Contam Toxicol, 15: 509-514.
Foster P, Laffond M, Baussand P, Jacob V, & Denis I (1991) VOC
measurements in the Grenoble area and study of the benzaldehyde
behaviour in simulation chamber. Pollut Atmos, 1991: 175-191.
Franchini I, Cavatorta A, Falzoi M, Lucertini S, & Mutti A (1983)
Early indicators of renal damage in workers exposed to organic
solvents. Int Arch Occup Environ Health, 52: 1-9.
Frank PA, Otto NE, & Bartley TR (1961) Techniques for evaluating
aquatic weed herbicides. Weeds, 9: 515-521.
Frantik E, Hornychova M, & Vodickova L (1993) Equipotential
subnarcotic concentrations of solvents in air, in blood, and at the
target structure. Homeostasis, 34: 143-147.
Frantik E, Hornychova M, & Horvath M (1994) Relative acute
neurotoxicity of solvents: Isoeffective air concentrations of 48
compounds evaluated in rats and mice. Environ Res, 66: 173-185.
Frantik E & Vodickova L (1995) Combined effects of binary solvent
mixtures. Cent Eur J Occup Environ Med, 1: 31-37.
Funes-Cravioto F, Kolmodin-Hedman B, Lindsten J, Nordenskjöld M,
Zapata-Gayon C, Lambert B, Norberg E, Olin R, & Swensson Å (1977)
Chromosome aberrations and sister-chromatid exchange in workers in
chemical laboratories and a rotoprinting factory and in children of
women laboratory workers. Lancet, 2: 322-325.
Furnas DW & Hine CH (1958) Neurotoxicity of some selected hydro-
carbons. Arch Ind Health Occup Med, 18: 9-15.
Fustinoni S, Buratti M, Giampiccolo R, & Colombi A (1995) Biological
and environmental monitoring of exposure to airborne benzene and other
aromatic hydrocarbons in Milan traffic wardens. Toxicol Lett,
77(13): 387-392.
Gab S, Schmizer J, Tham H, Parlar H, & Korte F (1977) Photo-
mineralisation rate of organic compounds adsorbed on particulate
matter. Nature (Lond), 270: 331-333
Galassi S, Mingazzini M, Vigano L, Cesareo D, & Tosato ML (1988)
Approaches to modelling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicol Environ Saf, 16: 158-169.
Gamberale F, Annwall G, & Hultengren M (1978) Exposure to xylene and
ethylbenzene. III. Effects on central nervous functions. Scand J Work
Environ Health, 4: 204-211.
Gerner-Smidt P & Friedrich U (1978) The mutagenic effect of benzene,
toluene and xylene studied by the SCE technique. Mutat Res,
58: 313-316.
Gery MW, Fox DL, Kaems RM, & Stockburger L (1987) Investigation of
hydroxyl radical reactions with o-xylene and m-xylene in a
continuous stirred tank reactor. Environ Sci Techol, 21: 339-348.
Ghantous H & Danielsson BG (1986) Placental transfer and distribution
of toluene, xylene and benzene, and their metabolites during gestation
in mice. Biol Res Pregnancy, 7: 98-105.
Ghantous H, Dencker L, Gabrielsson J, Danielsson BRG, & Bergman K
(1990) Accumulation and turnover of metabolites of toluene and xylene
in nasal mucosa and olfactory bulb in the mouse. Pharmacol Toxicol,
66: 87-92.
Ghislandi E & Fabiani A (1957) [Liver damage due to accidental
ingestion of nitrocellulose paints.] Med Lav, 48: 577-579 (in Italian
with English summary).
Ghosh TK & Pradhan SN (1987) Comparison of effects of xylene and
toluene inhalation on fixed-ratio liquid-reinforced behavior in rats.
Res Commun Psychol Psychiatr Behav, 12: 305-312.
Ghosh TK, Copeland RL Jr, Parui RN, Mookherjee S, & Pradhan SN (1987)
Effect of xylene inhalation on fixed-ratio responding in rats.
Pharmacol Biochem Behav, 27: 653-657.
Gilli G, Scursatone E, & Bono R (1994) Benzene, toluene and xylenes in
air, geographical distribution in Piedmont region (Italy) and personal
exposure. Sci Total Environ, 148(1): 49-56.
Glass WI (1961) Annotation: A case of suspected xylol poisoning. N Z
Med J, 60: 113.
Gomez-Belinchon JI, Grimalt JO, & Abaigés J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water Res, 25: 577-589.
Grob K & Grob G (1971) Gas-liquid chromatographic - mass spectrometric
investigation of C6-C20 organic compounds in an urban atmosphere: An
application of ultra trace analysis on capillary columns.
J Chromatogr, 62: 1-13.
Grosjean D (1990) Atmospheric chemistry of toxic contaminants. 1.
Reaction rates and atmospheric persistence. J Air Waste Manage Assoc,
40: 1397-1402.
Gschwend PM, Zafiriou OC, Mantoura RFC, Schwarzenbach RP, & Gagosian
RB (1982) Volatile organic compounds at a coastal site. I. Seasonal
variations. Environ Sci Techol, 1: 31-38.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gut I & Flek J (1981) [Effect of microsomal enzyme induction by
phenobarbital on the metabolism of benzene, fluorobenzene, m-xylene
and p-xylene.] Prakov Lek, 33: 124-127 (in Czech with English
summary).
Gut I, Terelius Y, Frantik E, Linhart I, Soucek P, Filipcova B, &
Kluchova H (1993) Exposure to various benzene derivatives differently
induces cytochromes P450 2B1 and P450 2E1 in rat liver. Arch Toxicol,
67: 237-243.
Haglund U, Lundberg I, & Zech L (1980) Chromosome aberrations and
sister chromatid exchanges in Swedish paint industry workers. Scand J
Work Environ Health, 6: 291-298.
Halder CA, Holdsworth CE, Cockrell BY, & Piccirillo VJ (1985)
Hydrocarbon nephropathy in male rats: Identification of the
nephrotoxic components of unleaded gasoline. Toxicol Ind Health,
1: 67-87.
Harland BJ, Whitby FJ, & Comber MHI (1985) Measurement of volatile
aromatic hydrocarbons in an industrial estuary. Int J Environ Anal
Chem, 20: 295-312.
Harper C (1975) p-Xylene metabolism by rat pulmonary and hepatic
microsomes. Fed Proc, 34: 785.
Hass U & Jakobsen BM (1993) Prenatal toxicity of xylene inhalation in
the rat: A teratogenicity and postnatal study. Pharmacol Toxicol,
73: 20-23.
Hass U, Lund SP, Simonsen L, & Schaich Fries A (1995) Effects of
prenatal exposure to xylene on postnatal development and behavior in
rats. Neurotoxicol Teratol, 17: 341-349.
Hastings L, Cooper GP, & Burg W (1984) Human sensory response to
selected petroleum hydrocarbons. Adv Med Environ Toxicol, 6: 255-270.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 5(suppl 1): 3-142.
Hawthorne SB & Sievers RE (1984) Emission of organic air pollutants
from shale oil waste waters. Environ Sci Techol, 18: 483-490.
Herman DC, Inniss WE, & Mayfield CI (1990) Impact of volatile aromatic
hydrocarbons on growth of the freshwater alga Selenastrum
capricornutum. Aquat Toxicol, 18: 87-100.
Higgins CE, Griest WH, & Olerich G (1983) Application of tenax
trapping to analysis of gas phase organic compounds in ultra-low tar
cigarette smoke. J Assoc Off Anal Chem, 66(5): 1074-1083.
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington, DC,
US Department of the Interior, Fish and Wildlife Service, 138 pp (Fish
and Wildlife Technical Report No. 2).
Hine CH & Zuidema HH (1970) The toxicological properties of hydro-
carbon solvents. Ind Med, 38: 215-220.
Hipolito RN (1980) Xylene poisoning in laboratory workers: Case
reports and discussion. Lab Med, 11: 593-595.
Holcombe GW, Phipps GL, Sulaiman AH, & Hoffman AD (1987) Simultaneous
multiple species testing: Acute toxicity of 13 chemicals to 12 diverse
freshwater amphibian, fish, and invertebrate families. Arch Environ
Contam Toxicol, 16: 697-710.
Holmberg B, Jakobson I, & Malmfors T (1974) The effect of organic
solvents on erythrocytes during hypotonic hemolysis. Environ Res,
7: 193-205.
Honma T, Sudo A, Miyagawa M, Sato M, & Hasegawa H (1983) Significant
changes in the amounts of neurotransmitter and related substances in
rat brain induced by subacute exposure to low levels of toluene and
xylene. Ind Health, 21: 143-151.
Huang M-Y, Jin C, Liu Y-T, Li B-H, Qu QS, Ushida Y, Inoue O, Nakatsuka
M, Watanabe T, & Ikeda M (1994) Exposure of workers to a mixture of
toluene and xylenes. I. Metabolism. Occup Environ Med, 51: 42-46.
Hudak A & Ungvary G (1978) Embryotoxic effects of benzene and its
methyl derivatives: Toluene, xylene. Toxicology, 11: 55-63.
Huff JE, Eastin W, Roycroft J, Eustis SL, & Haseman JK (1988)
Carcinogenesis studies of benzene, methyl benzene, and dimethyl
benzenes. Ann NY Acad Sci, 534: 427-440.
Hung I-F & Liao M-H (1991) Aromatic hydrocarbons in indoor air. J
Environ Sci Health, A26: 487-492.
Hurford N, Law RJ, Fileman TW, Payne AP, & Colcomb-Heiliger K (1990)
Concentrations of chemicals in the North Sea due to operational
discharges from chemical tankers: Results from the second survey,
October 1988. Oil Chem Pollu, 7: 241-270.
Husman K (1980) Symptoms of car painters with long-term exposure to a
mixture of organic solvents. Scand J Work Environ Health, 6: 19-32.
Husman K & Karli P (1980) Clinical neurological findings among car
painters exposed to a mixture of organic solvents. Scand J Work
Environ Health, 6: 33-39.
Hutchins SR (1991) Biodegradation of monoaromatic hydrocarbons by
aquifer microrganisms using oxygen, nitrate or nitrous oxide as the
terminal electron acceptor. Appl Environ Microbiol, 57: 2403-2407.
Hutchins SR, Sewell GW, Kovacs DA, & Smith GA (1991a) Biodegradation
of aromatic hyrocarbons by aquifer microroganisms under denitrifying
conditions. Environ Sci Techol, 25: 68-76.
Hutchins SR, Downs WC, Wilson JT, Smith GB, Kovacs DA, Fine DD,
Douglass RH, & Hendrix DJ (1991b) Effect of nitrate addition on
biorestoration of fuel-contaminated aquifer: Field demonstration.
Ground Water, 29: 571-580.
Hutchinson TC, Hellebust JA, Tam D, Mascarenhas RA, & Shiu WY (1980)
The correlation of the toxicity to algae of hydrocarbons and
halogenated hydrocarbons with their physical-chemical properties.
Environ Sci Res, 16: 577-586.
IARC (1989) Xylene. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research on
Cancer, pp 125-156 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 47).
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: Assessing human health risks of chemicals:
Derivation of guidance values for health-based exposure limits. World
Health Organization, Geneva, 73 pp.
Ivanovich E, Antov G, Goranova L, Spasovski M, Bainova A, & Vasileva Z
(1985) Combined effect of some physical and chemical factors. J Hyg
Epidemiol Microbiol Immunol, 28: 105-110.
Jacobs G, Martens M, & Mosselmans G (1987) Proposal of limit
concentrations for skin irritation within the context of a new EEC
directive on the classification and labelling of preparations. Regul
Toxicol Pharmacol, 7: 370-378.
Jamison VW, Raymond RL, & Hudson JD (1976) Biodegradation of
high-octane gasoline. In: Proceedings of the 3rd International
Biodegradation Symposium. Allied Science Publishers, pp 187-196.
Jenkins LJ Jr, Jones RA, & Siegel J (1970) Long-term inhalation
screening studies of benzene, toluene, o-xylene, and cumene on
experimental animals. Toxicol Appl Pharmacol, 16: 818-823.
Jones BMR (1988) The measurement of ambient hydrocarbon concentrations
in the atmosphere at Harwell during the period April 1986 to March
1987. Oxfordshire, Harwell Laboratoty (AERE Report R13174).
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and vehicle emissions. Environ Int, 11: 383-392.
Jori A, Calamari D, Di Domenico A, Galli CL, Galli E, Marinovich M, &
Silano V (1986) Ecotoxicological profile of xylenes. Ecotoxicol
Environ Saf, 11: 44-80.
Joyner RE & Leak Pegues W (1961) A health hazard associated with epoxy
resin-concrete dust. J Occup Med, 3: 211-214.
Juhnke I & Lüdemann D (1978) [Results of acute fish toxicity of 200
chemical compounds using the golden orfe tests.] Z Wasser Abwasser
Forsch, 11: 161-164 (in German).
Jüttner F (1988a) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in the forest air of the Southern
Black Forest. Chemosphere, 17: 309-317.
Jüttner F (1988b) Motor-boat-derived volatile organic compounds (VOC)
in lakewater. Z Wasser Abwasser Forsch, 21: 36-39.
Kaneko T, Wang P-Y, Tsukada H, & Sato A (1995) m-Xylene
toxicokinetics in phenobarbital-treated rats: comparison among
inhalation exposure, oral administration, and intraperitoneal
administration. Toxicol Appl Pharmacol, 131: 13-20.
Kappeler Th & Wuhrmann K (1978a) Microbial degradation of the water
soluble fraction of gas oil. I. Water Res, 12: 327-333.
Kappeler Th & Wuhrmann K (1978b) Microbial degradation of the water
soluble fraction of gas oil. II. Bioassays with pure strains. Water
Res, 12: 335-342.
Karlson U & Frankenberger WT (1989) Microbial degradation of benzene
and toluene in groundwater. Bull Environ Contam Toxicol, 43: 505-510.
Kauss PB & Hutchinson TC (1975) The effects of water-soluble petroleum
components on the growth of Chlorella vulgaris Beijerinck. Environ
Pollut, 9: 157-174.
Kauss PB, Hutchinson TC, Soto C, Hellebust J, & Griffiths M (1973) The
toxicity of crude oil and its components to freshwater algae. In:
Prevention and control of spills. Washington, DC, American Petroleum
Institute, pp 703-714.
Kawai T, Mizunuma K, Yasugi T, Horiguchi S, Uchida Y, Iwami O, Iguchi
H, & Ikeda M (1991a) Urinary methylhippuric acid isomer levels after
occupational exposure to a xylene mixture. Int Arch Occup Environ
Health, 63: 69-75.
Kawai T, Yamaoka K, Uchida Y, & Ikeda M (1991b) Exposure to vapors of
benzene and other aromatic solvents in tank truck loading and
delivery. Bull Environ Contam Toxicol, 46(1): 1-8.
Kawamura K & Kaplan IR (1983) Organic compounds in the rain water of
Los Angeles. Environ Sci Technol, 17: 497-501.
Kawata K & Fujieda Y (1993) [Volatile aromatic hydrocarbons in ambient
air.] Jpn J Toxicol Environ Health, 39(1): 37-43 (in Japanese).
Kennah HE, Hignet S, Laux PE, Dorko JD, & Barrow CS (1989) An
objective procedure for quantitating eye irritation based upon changes
of corneal thickness. Fundam Appl Toxicol, 12: 258-268.
Kennicutt MC II, Brooks JM, & McDonald TJ (1984) Volatile organic
inputs from an ocean outfall near Barceloneta, Puerto Rico.
Chemosphere, 13: 535-548.
Kenrick MAP, Clark L, Baxter KM, Fleet M, James HA, Gibson TM, &
Turrell MB (1985) Trace organics in British aquifers: a baseline
survey. Medmenham, Marlow, United Kingdom, Water Research Centre
(Technical Report TR223).
Kilburn KH, Warshaw R, Boylen CT, Balmes J, Johnson S, Seidman B, &
DeFlorio G (1983) Toxic effects of formaldehyde and solvents in
histology technicians: A preliminary report. J Histotechnol, 6: 73-76.
Kilburn KH, Seidman BC, & Warshaw R (1985) Neurobehavioral and
respiratory symptoms of formaldehyde and xylene exposure in histology
technicians. Arch Environ Health, 40: 229-233.
Kirschner E (1995) Production of top 50 chemicals increased sub-
stantially in 1994. Chem Eng News, April: 16-20.
Kjorsvic E, Saethre LJ, & Lonning S (1982) Effects of short-term
exposure to xylenes on the early cleavage stages of cod eggs ( Gadus
morhus L.). Sarsia, 67: 299-308.
Klaucke DN, Johansen M, & Vogt RL (1982) An outbreak of xylene
intoxication in a hospital. Am J Ind Med, 3: 173-178.
Kligman AM (1966) The identification of contact allergens by human
assay. III. The maximization test: A procedure for screening and
rating contact sensitizers. J Invest Dermatol, 47: 393-409.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies: Relationship for 50 industrial pollutants.
Toxicology, 19: 229-238.
Korsak Z, Sokal JA, & Gorny R (1992) Toxic effect of combined exposure
to toluene and m-xylene in animals. III. Subchronic inhalation
study. Pol J Occup Med Environ Health, 5: 27-33.
Korsak Z, Swiercz R, & Jedrychowski R (1993) Effects of acute combined
exposure to n-butyl alcohol and m-xylene. Pol J Occup Med Environ
Health, 6: 35-41.
Korsak Z, Wisniewska-Knypl J, & Swiercz R (1994) Toxic effects of
subchronic combined exposure to n-butyl alcohol and m-xylene in
rats. Int J Occup Med Environ Health, 7: 155-166.
Kroneld R (1989) Volatile pollutants in suburban and industrial air.
Bull Environ Contam Toxicol, 42: 868-872.
Kuhn EP, Colberg PJ, Schnoor JL, Wanner O, Zehnder AJB, &
Schwarzenbach RP (1985) Microbial transformations of substituted
benzenes during infiltration of river water to ground water:
Laboratory column studies. Environ Sci Techol, 19: 961-968.
Kuhn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Kurppa K & Husman K (1982) Car painters' exposure to a mixture of
organic solvents. Serum activities of liver enzymes. Scand J Work
Environ Health, 8: 137-140.
Kyrklund T, Kjellstrand P, & Haglid K (1987) Brain lipid changes in
rats exposed to xylene and toluene. Toxicology, 45: 123-133.
Labows J, Preti G, Loezle E, Leyden J, & Kligman A (1979) Analysis of
human axillary volatiles: Compounds of exogenous origin. J Chromatogr,
163: 294-299.
Lagorio S, Forastiere F, Iavarone I, Vanacore N, Fuselli S, & Carere A
(1993) Exposure assessment in a historical cohort of filling station
attendants. Int J Epidemiol, 22(6-suppl 2): S51-S56.
Lagrue G (1976) Hydrocarbon exposure and chronic glomerulonephritis.
Lancet, 1: 1191.
Laine A, Savolainen K, Riihimäki V, Matikainen E, Salmi T, & Juntunen
J (1993) Acute effects of m-xylene inhalation on body sway, reaction
times, and sleep in man. Int Arch Occup Environ Health, 65: 179-188.
Lanzerstofer CH & Puxbaum H (1990) Volatile hydrocarbons in and around
Vienna, Austria. Water Air Soil Pollut, 51: 345-355.
Laparé S, Tardif R, & Brodeur J (1993) Effect of various exposure
scenarios on the biological monitoring of organic solvents in alveolar
air. I. Toluene and m-xylene. Int Arch Occup Environ Health,
64: 569-580.
Larsby B, Ödkvist LM, Hydén D, & Liedgren SRC (1976) Disturbancies of
the vestibular system by toxic agents. Acta Physiol Scand,
440(suppl): 108.
Lauwerys R & Buchet JP (1988) Biological monitoring of exposure to
benzene, toluene and xylene. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 205-222 (IARC
Scientific Publications No. 85).
Lauwerys RR, Dath T, Lachapelle J-M, Buchet J-P, & Roels H (1978) The
influence of two barrier creams on the percutaneous absorption of
m-xylene in man. J Occup Med, 20: 17-20.
Lazarew NW (1929) [On the toxicity of various organic compounds
vapors.] Arch Exp Pathol Pharmacol, 143: 223-233 (in German).
Lebowitz H, Brusick D, Matheson D, Jagannath DR, Reed M, Goode S, &
Roy G (1979) Commonly used fuels and solvents evaluated in a battery
of short-term bioassays. Environ Mutagen, 1: 172-173.
Le Gore RS (1974) The effects of Alaskan crude oil and selected
hydrocarbon compounds on embryonic development of the Pacific oyster,
Crassostrea gigas. Diss Abstr B, 35: 3168.
Lewis P (1994) Report of airborne solvent concentrations in a printing
ink manufacturing plant in Cape Town, Republic of South Africa. Appl
Occup Environ Hyg, 9: 147-151.
Liira J, Elovaara E, Raunio H, Riihimäki V, & Engström K (1991)
Metabolic interaction and diposition of methyl ethyl ketone and
m-xylene in rats at single and repeated inhalation exposure.
Xenobiotica, 21: 53-63.
Lindström K (1973) Psychological performances of workers exposed to
various solvents. Work Environ Health, 10: 151-155.
Lindström K, Antti-Poika M, Tola S, & Hyytiäinen A (1982)
Psychological prognosis of diagnosed chronic organic solvent
intoxication. Neurobehav Toxicol Teratol, 4: 581-588.
Lioy PJ, Wallace L, & Pellizzari E (1991) Indoor/outdoor, and personal
monitor and breath analysis relationships for selected volatile
organic compounds measured at three homes during New Jersey
TEAM - 1987. J Exp Anal Environ Epidemiol, 1(1): 45-61.
Lonneman WR, Seila RL, & Bufalini JJ (1978) Ambient air hydrocarbons
in Florida. Environ Sci Technol, 12: 459-463.
Lovegren NV, Fisher GS, Legendre MG, & Schuller WH (1979) Volatile
constituents of dried legumes. J Agric Food Chem, 27: 851-853.
Lundberg I & Håkansson M (1985) Normal serum activities of liver
enzymes in Swedish paint industry workers with heavy exposure to
organic solvents. Br J Ind Med, 42: 596-600.
Lundberg I, Håkansson M, Gustavsson P, Kronevi T, & Lidums V (1983)
[Relative hepatotoxic effect of industrial solvents.] Solna, Sweden,
National Institute of Occupational Health, pp 1-42 (Work and Health
1983:22) (in Swedish with English summary).
Lundberg I, Ekdahl M, Kronevi T, Lidums V, & Lundberg S (1986)
Relative hepatotoxicity of some industrial solvents after
intraperitoneal injection or inhalation exposure in rats. Environ Res,
40: 411-420.
Lundberg I, Nise G, Hedenborg G, Högberg M, & Vesterberg O (1994)
Liver function tests and urinary albumin in house painters with
previous heavy exposure to organic solvents. Occup Environ Med,
51: 347-353.
Lyman WJ, Reehl WF, & Rosenblatt DH (1981) Handbook of chemical
property estimation methods. New York, Mc-Graw Hill Book Company.
McCarroll NE, Keech BH, & Piper CE (1981a) A microsuspension
adaptation of the Bacillus subtilis "rec" assay. Environ Mutagen,
3: 607-616.
McCarroll NE, Piper CE, & Keech BH (1981b) An E. coli
microsuspension assay for the detection of DNA damage induced by
direct-acting agents and promutagens. Environ Mutagen, 3: 429-444.
McDonald TJ, Kennicutt MC II, & Brooks JM (1988) Volatile organic
compounds at a coastal site. Chemosphere, 17: 123-136.
McFall JA, Antoine SR, & Deleon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14: 1253.
Mackay D & Leinonen PJ (1975) Rate of evaporation of low solubility
contaminants from water bodies to the atmosphere. Environ Sci Technol,
9: 1178-1180.
Mackay D & Wolkoff AW (1973) Rate of evaporation of low solubility
contaminants from water bodies to atmosphere. Environ Sci Technol,
7: 611-614.
Mackay D, Paterson S, Cheung B, & Neely WB (1985) Evaluating the
environmental behaviour of chemicals using a level III fugacity model.
Chemosphere, 14: 335-374.
MAFF (1991) Monitoring and surveillance of non-radioactive
contaminants in the aquatic environment and activities regulating the
disposal of wastes at sea, 1988-89. Lowestoft, United Kingdom,
Ministry of Agriculture, Fisheries and Food, Directorate of Fisheries
Research (Aquatic Environment Monitoring Report No. 26).
Maizlish NA, Fine LJ, Albers JW, Whitehead L, & Langolf GD (1987) A
neurological evaluation of workers exposed to mixtures of organic
solvents. Br J Ind Med, 44: 14-25.
Major DW, Mayfield CI, & Barker JF (1988) Biotransformation of benzene
by denitrification in aquifer sand. Ground Water, 26: 8-14.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1983) Benzene as an
experimental carcinogen: up-to-date evidence. Acta Oncol, 4: 141-164.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
Current results and ongoing research. Am J Ind Med, 7: 415-446.
Mansour M, Moza PN, Barlas H, & Parlar H (1985) [A contribution to the
topic of photo-stability of environmental organic compounds in the
presence of hydrogen peroxide in aquatic systems.] Chemosphere,
14: 1469-1474 (in German).
Marks TA, Ledoux TA, & Moore JA (1982) Teratogenicity of a commercial
xylene mixture in the mouse. J Toxicol Environ Health, 9: 97-105.
Maynard DJ & Weber DD (1981) Avoidance reactions of juvenile coho
salmon (Oncorhynchus kisutch) to monocyclic aromatics. Can J Fish
Aquat Sci, 38: 772-778.
Meinhart E & Schreier P (1986) Study of flavour compounds from
Parmigiano Reggiano cheese. Milchwissenschaft, 41: 689-691.
Michael LC, Pellizzari ED, & Norwood DL (1991) Application of the
master analytical scheme to the determination of volatile organics in
wastewater influents and effluents. Environ Sci Technol, 25: 150-155.
Mirkova E, Zaikov C, Antov G, Mikhailova A, Khinkova L, & Benchev I
(1983) Prenatal toxicity of xylene. J Hyg Epidemiol Microbiol Immunol,
27: 337-343.
Mohtashamipur E, Norpoth K, Woelke U, & Huber P (1985) Effects of
ethylbenzene, toluene, and xylene on the induction of micronuclei in
bone marrow polychromatic erythrocytes of mice. Arch Toxicol,
58: 106-109.
Molnar J, Paksy KA, & Naray M (1986) Changes in the rat's motor
behaviour during 4-hr inhalation exposure to prenarcotic
concentrations of benzene and its derivatives. Acta Physiol Hungarica
67: 349-354.
Morgan P & Watkinson RJ (1990) Assessment of the potential for in
situ biotreatment of hydrocarbon-contaminated soils. Water Sci
Technol, 22: 63-68.
Morley R, Eccleston DW, Douglas CP, Greville WEJ, Scott DJ, & Anderson
J (1970) Xylene poisoning: A report on one fatal case and two cases of
recovery after prolonged unconsciousness. Br Med J, 3: 442-443.
Morrow JE, Gritz RL, & Kirton MP (1975) Effects of some components of
crude oil on young coho salmon. Copeia, 2: 326-331.
Moser VC, Coggeshall EM, & Balster RL (1985) Effects of xylene isomers
on operant responding and motor performance in mice. Toxicol Appl
Pharmacol, 80: 293-298.
Moszczynski P & Lisiewicz J (1985) Occupational exposure to benzene,
toluene and xylene and the lymphocyte lysosomal N-acetyl-beta-
D-glucosaminidase. Ind Health, 23: 47-51.
Nakamura S, Oda Y, Shimada T, Oki I, & Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutat
Res, 192: 239-246.
Naskali L, Oksanen H, & Tähti H (1994) Astrocytes as targets for CNS
effects of organic solvents in. Neurotoxicology, 15: 609-612.
Nasterlack M, Triebig G, & Stelzer O (1994) Hepatotoxic effects of
solvent exposure around permissible limits and alcohol consumption in
printers over a 4-year period. Int Arch Occup Environ Health, 66:
161-165.
Neff JM, Anderson JW, Cox BA, Laughlin RB, Rossi SS, & Tatem HE (1976)
Effects of petroleum on survival, respiration and growth of marine
animals. In: Sources, effects and sinks of hydrocarbons. Washington,
DC, American Institute of Biological Science, pp 515-523.
Nelson PF & Quigley SM (1982) Non-methane hydrocarbons in the
atmosphere of Sydney, Australia. Environ Sci Techol, 16: 650-655.
Nelson KW, Ege JF Jr, Ross M, Woodman LE, & Silverman L (1943) Sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
25: 282-285.
NIOSH (1991) Health hazard evaluation report: Rockwell International,
Newark, Ohio. Cincinnati, Ohio, National Institute for Occupational
Safety and Health (Report HETA 90-368-2137; PB92-131929).
NIOSH (1993a) Health hazard evaluation report: National Centers for
Environmental Health, Fairbanks, Alaska. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA 93-606-2336;
PB94-133667).
NIOSH (1993b) Health hazard evaluation report: Dallas and Mavis
Forwarding Co., Seattle, Washington. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA
91-0103-2351; PB94-131836).
NIOSH (1993c) Health hazard evaluation report: National Center for
Environmental Health, Albany, New York. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA
93-0884-2344; PB94-129020).
NIOSH (1993d) Health hazard evaluation report: National Centers for
Environmental Health, Stamford, Connecticut. Cincinnati, Ohio,
National Institute for Occupational Safety and Health (Report HETA
93-802-2338; PB94-134343).
NIOSH (1994) Health hazard evaluation report: Pan American Health
Organization, Bogota, Colombia (South America). Cincinnati, Ohio,
National Institute for Occupational Safety and Health (Report HETA
94-0253-2451; PB95-147062).
NTP (1986) Technical report on the toxicology and carcinogenesis
studies of xylenes (mixed) in F344/N rats and B6C3F1 mice. Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program (NIH Publication No. 87-2583).
Nunes P & Benville PE (1979) Uptake and depuration of petroleum
hydrocarbons in the Manila Clam, Tapes semidecussata Reeve. Bull
Environ Contam Toxicol, 21: 719-726.
Nylén P & Hagman M (1994) Function of the auditory and visual systems,
and of peripheral nerves in rats after long-term combined exposure to
n-hexane and methylated benzene derivatives. II. Xylene. Pharmacol
Toxicol, 74: 124-129.
Nylén P, Ebendal T, Eriksdotter-Nilsson M, Hansson T, Henschen A,
Johnson A-C, Kronevi T, Kvist U, Sjöstrand NO, Höglund G, & Olson L
(1989) Testicular atrophy and loss of nerve growth factor-
immunoreactive germ cell line in rats exposed to n-hexane and
a protective effect of simultaneous exposure to toluene or xylene.
Arch Toxicol, 63: 296-307.
Ogata M & Fujii T (1979) Urinary excretion of hippuric acid and
m-methylhippuric acid after administration of toluene and m-xylene
mixture to rats. Int Arch Occup Environ Health, 43: 45-51.
Ogata M & Hobara T (1979) A new direct method for colorimetric
determination of hippuric acid and methylhippuric acid as indices of
toluene and m-xylene, and its application to workers using thinner.
Ind Health, 17: 61-72.
Ogata M & Miyake Y (1979) Disappearance of aromatic hydrocarbons and
organic sulphur compounds from fish reared in crude oil suspensions.
Water Res, 13: 75-78.
Ogata M, Yamasaki Y, Meguro T, Sugihara R, & Shimada Y (1979)
Quantitation of urinary o-xylene metabolites in rats and human
beings by high performance liquid chromatography. Ind Health,
17: 123-125.
Ogata M, Yamazaki Y, Sugihara R, Shimada Y, & Meguro T (1980)
Quantitation of urinary o-xylene metabolites of rats and human
beings by high performance liquid chromatography. Int Arch Occup
Environ Health, 46: 127-139.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
Omori T & Yamada K (1970) Studies on the utilisation of hydrocarbons
by microorganisms. Part XVI. Detection of metabolic intermediates of
xylene and pseudocumene. Agric Biol Chem, 34: 659-663.
Omori T, Horiguchi S, & Yamada K (1967) Studies on the utilisation of
hydrocarbons by microorganisms. Part X. Screening of aromatic
hydrocarbon assimilating microorganisms and p-toluic acid formation
from p-xylene. Agric Biol Chem, 31: 1337-1342.
Otson R & Williams DT (1981) Evaluation of a liquid-liquid extraction
technique for water pollutants. J Chromatogr, 212: 187-197.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities.
J Assoc Off Anal Chem, 65: 1370-1374.
Otson R, Fellin P, & Tran Q (1993) Investigation of VOCs in Canadian
residences. In: Indoor air '93. Proceedings of the 6th International
Conference on Indoor Air Quality and Climate, Helsinki, Finland, 4-8
July 1993 - Volume 2: Chemicals in indoor air, materials emissions,
pp 141-146.
Ödkvist LM, Larsby B, Tham R, & Aschan G (1979) On the mechanism
of vestibular disturbances caused by industrial solvents. Adv
Oto-rhino-laryngol, 25: 167-172.
Ödkvist LM, Larsby B, Fredrickson JMF, Liedgren SRC, & Tham R (1980)
Vestibular and oculomotor disturbances caused by industrial solvents.
J Otolaryngol, 9: 53-59.
PACE (1987) A study of exposure to motor gasoline hydrocarbon vapours
at service stations: Phase II - Summer Study. Petroleum Association
for Conservation of the Canadian Environment (PACE Report No. 87-5).
PACE (1989) A study of exposure to motor gasoline hydrocarbon vapours
at service stations: Phase III - Winter Study. Petroleum Association
for Conservation of the Canadian Environment (PACE Report No. 89-3).
Padilla S, Lyerly DL, & Pope CN (1992) Subacute ethanol consumption
reverses p-xylene-induced decreases in axonal transport. Toxicology,
75: 159-167.
Palmer KT & Rycroft RJG (1993) Occupational airborne contact urticaria
due to xylene. Contact Dermatitis, 28: 44.
Pap M & Varga C (1987) Sister-chromatid exchanges in peripheral
lymphocytes of workers occupationally exposed to xylenes. Mutat Res,
187: 223-225.
Park SH, AuCoin TA, Silverman DM, & Schatz RA (1994) Time-dependent
effects of o-xylene on rat lung and liver microsomal membrane
structure and function. J Toxicol Environ Health, 43: 469-481.
Patel JM, Harper C, & Drew RT (1976) Inactivation of pulmonary
cytochrome P-450 by p-xylene intoxication. Pharmacologist, 18: 210.
Patel JM, Harper C, & Drew RT (1978) The biotransformation of
p-xylene to a toxic aldehyde. Drug Metab Dispos, 6: 368-374.
Patel JM, Harper C, Gupta BN, & Drew RT (1979) Changes in serum
enzymes after inhalation exposure of p-xylene. Bull Environ Contam
Toxicol, 21: 17-24.
Pathiratne A, Puyear RL, & Brammer JD (1986) A comparative study of
the effects of benzene, toluene, and xylenes on their in metabolism
and drug-metabolizing enzymes in rat liver. Toxicol Appl Pharmacol,
82: 272-280.
Pellizzari EC, Hartwell TD, Harris BSH III, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mothers milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pellizzari ED, Zweidinger RA, & Sheldon LS (1988) Determination
of benzene, toluene and xylene in breath samples by gas
chromatography/mass spectrometry. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 267-279 (IARC
Scientific Publications No. 85).
Pennell KD, Rhue RD, Rao PSC, & Johnston CT (1992) Vapor-phase
sorption of p-xylene and water on soils and clay minerals. Environ
Sci Technol, 26: 756-763.
Perry R & Twibell JD (1973) A time based elution technique for the
estimation of specific hydrocarbons in air. Atmos Environ, 7: 929.
Petersson G (1982) Ambient hydrocarbons from motor-car assembly plants
in Scandinavia. Environ Pollut, B4: 207-217.
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38: 1419-1429.
Pound AW (1970) Induced cell proliferation and the initiation of skin
tumour formation in mice by ultraviolet light. Pathology, 2: 269-275.
Pound AW & Withers HR (1963) The influence of some irritant chemicals
and scarification on tumour initiation by urethane in mice. Br J
Cancer, 17: 460-470.
Proust B, Hannequin D, Caillard JF, Prudent P, Hemet J, & Samson N
(1986) Le psychosyndrome aux solvants: Résultats des tests
psychométriques chez dix laborantines exposées. Arch Mal Prof,
47: 305-310.
Pryor GT, Robert CS, & Howd RA (1987) Hearing loss in rats caused by
inhalation of mixed xylenes and styrene. J Appl Toxicol, 7: 55-61.
Pussemier L, Szabó G, & Bulman RA (1990) Prediction of the soil
adsorption coefficient Koc for aromatic pollutants. Chemosphere,
21: 1199-1212.
Pyykkö K (1980) Effects of methylbenzenes on microsomal enzymes in rat
liver, kidney and lung. Biochim Biophys Acta, 633: 1-9.
Pyykkö K (1986) Inhibition of microsomal monooxygenases in by
aromatic hydrocarbons. Arch Toxicol, 9(suppl): 371-373.
Pyykkö K, Paavilainen S, Metsä-Ketelä T, & Laustiola K (1987) The
increasing and decreasing effects of aromatic hydrocarbon solvents on
pulmonary and hepatic cytochrome P-450 in the rat. Pharmacol Toxicol,
60: 288-293.
Rao PSC, Hornsby AG, & Jessup RE (1985) Indices for ranking the
potential for pesticide contamination of ground water. Soil Crop Sci
Soc Proc, 44: 1-8.
Raunio H, Liira J, Elovaara E, Riihimäki V, & Pelkonen O (1990)
Cytochrome P450 isozyme induction by methyl ethyl ketone and
m-xylene in rat liver. Toxicol Appl Pharmacol, 103: 175-179.
Raymond RL, Jamison VW, & Hudson JO (1969) Microbial hydrocarbon
co-oxidation. II. Use of ion-exchange resins. Appl Microbiol,
17: 512-515.
Recchia G, Perbellini L, Prati GF, Dean P, & Ancona G (1985) [Coma due
to accidental ingestion of xylene. Treatment with charcoal
hemoperfusion.] Med Lav, 76: 67-73 (in Italian with English summary).
Reinert KH, Hunter JV, & Sabatino T (1983) Dynamic heated headspace
analysis of volatile organic compounds present in fish tissue samples.
J Agric Food Chem, 31: 1057-1060.
Reinhard M, Goodman NL, & Barker JF (1984) Occurrence and distribution
of organic chemicals in two landfill leachate plumes. Environ Sci
Technol, 18: 953-961.
Rhue RD, Rao PSC, & Smith RE (1988) Vapour-phase adsorption of
alkylbenzenes and water on soils and clays. Chemosphere, 17: 727-741.
Richer CL, Chakrabarti S, Senecal-Quevillon M, Duhr MA, Zhang XX, &
Tardif R (1993) Cytogenic effects of low-level exposure to toluene,
xylene and their mixture on human blood lymphocytes. Int Arch Occup
Environ Health, 64: 81-85.
Rigdon RH (1940) Capillary permeability in areas of inflammation
produced by xylene. Arch Surg, 41: 101-109.
Riihimäki V (1979a) Conjugation and urinary excretion of toluene and
m-xylene in man. Scand J Work Environ Health, 5: 135-142.
Riihimäki V (1979b) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand J Work
Environ Health, 5: 143-150.
Riihimäki V & Hänninen O (1987) Xylenes. In: Snyder R. ed. Ethel
Browning's toxicity and metabolims of industrial solvents, 2nd ed.
Amsterdam, Oxford, New York, Elsevier Science Publishers, vol 1, pp
64-84.
Riihimäki V & Pfäffli P (1978) Percutaneous absorption of solvent
vapors in man. Scand J Work Environ Health, 4: 73-85.
Riihimäki V & Savolainen K (1980) Human exposure to m-xylene,
kinetics and acute effects on the central nervous system. Ann Occup
Hyg, 23: 411-422.
Riihimäki V, Pfäffli P, & Savolainen K (1979a) Kinetics of m-xylene
in man: Influence of intermittent physical exercise and changing
environmental concentrations on kinetics. Scand J Work Environ Health,
5: 232-248.
Riihimäki V, Pfäffli P, Savolainen K, & Pekari K (1979b) Kinetics of
m-xylene in man: General features of absorption, distribution,
biotransformation and excretion in repetitive inhalation exposure.
Scand J Work Environ Health, 5: 217-231.
Riihimäki V, Laine A, Savolainen K, & Sippel H (1982a) Acute solvent-
ethanol interactions with special reference to xylene. Scand J
Work Environ Health, 8: 77-79.
Riihimäki V, Savolainen K, Pfäffli P, Pekari K, Sippel HW, & Laine A
(1982b) Metabolic interaction between m-xylene and ethanol. Arch
Toxicol, 49: 253-263.
Roberts AE, Bogdanffy MS, Brown DR, & Schatz RA (1986) Lung metabolism
of benzo(a)pyrene in rats treated with p-xylene and/or ethanol. J
Toxicol Environ Health, 18: 257-266.
Rogerson A, Shiu WY, Huang GL, Mackay D, & Berger J (1983)
Determination and interpretation of hydrocarbon toxicity to ciliate
protozoa. Aquat Toxicol, 3: 215-228.
Rosen MB, Crofton KM, & Chernoff N (1986) Postnatal evaluation of
prenatal exposure to p-xylene in the rat. Toxicol Lett, 34: 223-229.
Rosengren LE, Kjellstrand P, Aurell A, & Haglid KG (1986) Irreversible
effects of xylene on the brain after long term exposure: A
quantitative study of DNA and the glial cell marker proteins S-100 and
GFA. Neurotoxicology, 7: 121-136.
Ruijten MW, Hooisma J, Brons JT, Habets CE, Emmen HH, & Muijser H
(1994) Neurobehavioral effects of long-term exposure to xylene and
mixed organic solvents in shipyard spray painters. Neurotoxicology,
15: 613-620.
Rydzynski K, Korsak Z, Jedlinska U, & Sokal JA (1992) The toxic
effects of combined exposure to toluene and m-xylene in animals. IV.
Liver ultrastructure after subchronic inhalatory exposure. Pol J Occup
Med Environ Health, 5: 35-42.
Sabel GV & Clark TP (1984) Volatile organic compounds as indicators of
municipal solid waste leachate contamination. Waste Manage Res,
2: 119-130.
Sabljic A & Güsten H (1990) Predicting the night-time NO3 radical
reactivity in the troposphere. Atmos Environ, 24A: 73-78.
Sack TM, Steele DH, Hammerstrom K, & Remmers K (1992) A survey of
household products for volatile organic compounds. Atmos Environ,
26A(6): 1063-1070.
Sanborn HR & Malins DC (1980) The disposition of aromatic hydrocarbons
in adult spot shrimp (Pandalus platyceros) and the formation of
metabolites of naphthalene in adult and larval spot shrimp.
Xenobiotica, 10: 193-200.
Sato A, Fujiwara Y, & Nakajima T (1974) Solubility of benzene, toluene
and m-xylene in various body fluids and tissues of rabbits. Jpn J
Ind Health, 16: 30-31.
Sauer TC (1981) Volatile liquid hydrocarbon characterisation of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environ Sci Technol, 15: 917-923.
Savolainen K (1980) Combined effects of xylene and alcohol on the
central nervous system. Acta Pharmacol Toxicol, 46: 366-372.
Savolainen H & Pfäffli P (1980) Dose-dependent neurochemical changes
during short-term inhalation exposure to m-xylene. Arch Toxicol,
45: 117-122.
Savolainen K & Linnavuo M (1979) Effects of m-xylene on human
equilibrium measured with a quantitative method. Acta Pharmacol
Toxicol, 44: 315-318.
Savolainen K & Riihimäki V (1981) Xylene and alcohol involvement of
the human equilibrium system. Acta Pharmacol Toxicol, 49: 447-451.
Savolainen H & Seppäläinen AM (1979) Biochemical and physiological
effects of organic solvents on rat axon membranes isolated by a new
technique. Neurotoxicology, 1: 467-477.
Savolainen H, Pfäffli P, Helojoki M, & Tengén M (1979a) Neurochemical
and behavioural effects of long-term intermittent inhalation of xylene
vapour and simultaneous ethanol intake. Acta Pharmacol Toxicol,
44: 200-207.
Savolainen H, Vainio H, Helojoki M, & Elovaara E (1979b) Biochemical
and toxicological effects of short-term intermittent xylene inhalation
exposure and combined ethanol intake. Arch Toxicol, 41: 195-205.
Savolainen K, Riihimäki V, Vaheri E, & Linnoila M (1980) Effects of
xylene and alcohol on vestibular and visual functions in man. Scand J
Work Environ Health, 6: 94-103
Savolainen K, Riihimäki V, Laine A, & Kekoni J (1981) Short-term
exposure of human subjects to m-xylene and 1,1,1-trichloroethane.
Int Arch Occup Environ Health, 49: 89-98.
Savolainen K, Riihimäki V, & Laine A (1982a) Biphasic effects of
inhaled solvents on human equilibrium. Acta Pharmacol Toxicol,
51: 237-242.
Savolainen K, Riihimäki V, Laine A, & Kekoni J (1982b) Short-term
exposure of human subjects to m-xylene and 1,1,1-trichloroethane.
Arch Toxicol, 5(suppl): 96-99.
Savolainen K, Kekoni J, Riihimäki V, & Laine A (1984) Immediate
effects of m-xylene on the human central nervous system. Arch
Toxicol, 7(suppl): 412-417.
Savolainen K, Riihimäki V, Luukkonen R, & Muona O (1985a) Changes in
the sense of balance correlate with concentrations of m-xylene in
venous blood. Br J Ind Med, 42: 765-769.
Savolainen K, Riihimäki V, Muona O, Kekoni J, Luukkonen R, & Laine A
(1985b) Conversely exposure-related effects between atmospheric
m-xylene concentrations and human body sense of balance. Acta
Pharmacol Toxicol, 57: 67-71.
Sedivec V & Flek J (1976) The absorption, metabolism, and excretion of
xylenes in man. Int Arch Occup Environ Health, 37: 205-217.
Seidenberg JM, Anderson DG, & Becker RA (1986) Validation of an in
vivo developmental toxicity screen in the mouse. Teratogen
Carcinogen Mutagen, 6: 361-374.
Seip HM, Alstad J, Carlberg GE, Martinsen K, & Skaane R (1986)
Measurement of mobility of organic compounds in soil. Sci Total
Environ, 50: 87-101.
Selgrade MK, Daniels MJ, Jaskot RH, Robinson BL, & Albis JW (1993)
Enhanced mortality and liver damage in virus-infected mice exposed to
p-xylene. J Toxicol Environ Health, 40: 129-144.
Seppäläinen AM, Husman K, & Mårtenson C (1978) Neurophysiological
effects of long-term exposure to a mixture of organic solvents. Scand
J Work Environ Health, 4: 304-314.
Seppäläinen AM, Savolainen K, & Kovala T (1981) Changes induced by
xylene and alcohol in human evoked potentials. Electroencephalogr Clin
Neurophysiol, 51: 148-155.
Seppäläinen AM, Laine A, Salmi T, Verkkala E, Riihimäki V, & Luukkonen
R (1991) Electroencephalographic findings during experimental human
exposure to m-xylene. Arch Environ Health, 46: 16-23.
Sevcik P, Hep A, & Peslova M (1992) Intravenous xylene poisoning.
Intensive Care Med, 18: 377-378.
Sexton K & Westberg H (1980) Ambient hydrocarbon and ozone
measurements downwind of a large automotive painting plant. Environ
Sci Technol, 14: 329-332.
Seyfried B, Glod G, Schocher R, Tschech A, & Zeyer J (1994) Initial
reactions in the anaerobic oxidation of toluene and m-xylene by
denitrifying bacteria. Appl Environ Microbiol, 60(11): 4047-4052.
Sheedy BR, Lazorchak JM, Grunwald DJ, Pickering QH, Pilli A, Hall D, &
Webb R (1991) Effects of pollution on freshwater organisms. Res J
Water Pollut Control Fed, 63: 619-696.
Sheldon LS, Zelon H, Sickles J, Eaton C, Hartwell T, & Jugners R
(1988) Monitoring in and around public access buildings. Proceedings
of the 81st Annual Meeting of the Air Pollution Control Association,
1988. Pittsburgh, Pennsylvania, Air Pollution Control Association,
9 pp (Paper 88/109.8).
Shimizu H, Suzuki Y, Takemura N, Goto S, & Matsushita H (1985) The
results of microbial mutation test for forty-three industrial
chemicals. Jpn J Ind Health, 27: 400-419.
Silverman DM & Schatz RA (1991) Pulmonary microsomal alterations
following short-term low level inhalation of p-xylene in rats.
Toxicology, 65: 271-281.
Simmons JE, Allis JW, Grose EC, Seely JC, Robinson BL, & Berman E
(1991) Assessment of the hepatotoxicity of acute and short-term
exposure to inhaled p-xylene in F-344 rats. J Toxicol Environ
Health, 32: 295-306.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1983) Measurements of
hazardous organic chemicals in the ambient atmosphere. Washington, DC,
US Environmental Protection Agency (EPA-600/3-83-002).
Singh HB, Ferek RJ, Salas LJ, & Nitz KC (1986) Toxic chemicals in the
environment: A program of field measurements. Washington, DC, US
Environmental Protection Agency (EPA-600/3-86/047).
Skowronski GA, Turkall RM, Kadry ARM, & Abdel-Rahman MS (1990) Effects
of soil on the dermal bioavailability of m-xylene in male rats.
Environ Res, 51: 182-193.
Slaine DD & Barker JF (1990) The detection of naturally occurring BTX
during a hydrogeologic investigation. Ground Water Monit Rev,
10: 89-94.
Slooff W (1979) Detection limits of a biological monitoring system
based on fish respiration. Bull Environ Contam Toxicol, 23: 517-523.
Slooff W, de Zwart D, & Marquenie JM (1983) Detection limits of a
biological monitoring system for chemical water pollution based on
mussel activity. Bull Environ Contam Toxicol, 30: 400-405.
Smith BR, Plummer JL, Wolf CR, Philpot RM, & Bend JR (1982) p-Xylene
metabolism by rabbit lung and liver and its relationship to the
selective destruction of pulmonary cytochrome P-450. J Pharmacol Exp
Ther, 223: 736-742.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: List VI. Am Ind Hyg Assoc J, 23: 95-107.
Snyder R (1987) Ethyl Browning's toxicity and metabolism of industrial
solvents - Volume 1; Hydrocarbons. Amsterdam, Oxford, New York,
Elsevier Science Publishers.
Sotaniemi EA, Sutinen S, Sutinen S, Arranto AJ, & Pelkonen RO (1982)
Liver injury in subjects occupationally exposed to chemicals in low
doses. Acta Med Scand, 212: 207-215.
Steele RH & Wilhelm DL (1966) The inflammatory reaction in chemical
injury. I. Increased vascular permeability and erythema induced by
various chemicals. J Exp Pathol, 47: 612-623.
Stickney JA, Silverman DM, & Schatz RA (1991) Role of isozyme-specific
inhibition of cytochrome P450IIB1 activity in m-xylene-induced
alterations in rat pulmonary benzo(a)pyrene metabolism. Xenobiotica,
211: 641-649.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near an underground coal gasification site in northeastern
Wyoming. Environ Sci Technol, 16: 582-587.
Sugihara R (1979) High-performance liquid chromatography studies of
toluene and xylene poisoning. Part II. Respiratory and urinary
excretion of toluene and m-xylene following intraperitoneal
administration to rats. Acta Med Okayama, 91: 1433-1440.
Sugihara R & Ogata M (1978) Quantitation of urinary m- and
p-methylhippuric acids as indices of m- and p-xylene exposure.
Int Arch Occup Environ Health, 41: 281-286.
Svanberg PA, Grennfeldt P, & Lindskog A (1995) The Swedish urban
network - Air quality measurement program. Stockholm, Sweden,
Environmental Research Institute (Unpublished report).
Tabak HH, Desai S, & Govind R (1989) Determination of biodegradability
kinetics of RCRA compounds using respirometry for structure-activity
relationships. Proceedings of the 44th Industrial Waste Conference,
1989, pp 405-423.
Takatori T, Terazawa K, Nakano K, & Inoue K (1982) An autopsy case of
alkylbenzene poisoning and its clinical finding. Nippon Hoigaku
Zasshi, 36: 654-661.
Tardif R, Laparé S, Plaa GL, & Brodeur J (1991) Effect of simultaneous
exposure to toluene and xylene on their respective biological exposure
indices in humans. Int Arch Occup Environ Health, 63: 279-284.
Tardif R, Plaa GL, & Brodeur J (1992) Influence of various mixtures of
inhaled toluene and xylene on the biological monitoring of exposure to
these solvents in rats. Can J Physiol Pharmacol, 70: 385-939.
Tardif R, Laparé S, Krishnan K, & Brodeur J (1993a) Physiologically
based modelling of the toxicokinetic interaction between toluene and
m-xylene in rat. Toxicol Appl Pharmacol, 120: 266-273.
Tardif R, Laparé S, Krishnan K, & Brodeur J (1993b) A descriptive and
mechanistic study of the interaction between toluene and xylene in
humans. Int Arch Occup Environ Health, 65: S135-S137.
Tardif R, Sato A, Laparé S, & Brodeur J (1994) Ethanol induced
modification of m-xylene toxicokinetics in humans. Occup Environ
Med, 51: 187-191.
Taskinen H, Kyyrönen P, Hemminki K, Hoikkala M, Lajunen K, & Lindbohm
M-L (1994) Laboratory work and pregnancy outcome. J Occup Med,
36: 311-319.
Tatem HE, Cox BA, & Anderson JW (1978) The toxicity of oils and
petroleum hydrocarbons to estuarine crustaceans. Estuar Coast Mar Sci,
6: 365-373.
Tegeris JS & Balster RL (1994) A comparison of the acute behavioral
effects of alkylbenzenes using a functional observational battery in
mice. Fundam Appl Toxicol, 22: 240-250.
Tham R, Bunnfors I, Eriksson B, Larsby B, Lindgren S, & Ödkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thomas JM, Gordy VR, Fiorenza S, & Ward CH (1990) Biodegradation of
BTEX in subsurface materials contaminated with gasoline: Granger,
Indiana. Water Sci Technol, 22: 53-62.
Toftgård R & Nilsen OG (1982) Effects of xylene and xylene isomers on
cytochrome P-450 and in enzymatic activities in rat liver, kidney
and lung. Toxicology, 23: 197-212.
Toftgård R, Nilsen OG, & Gustafsson J-Å (1981) Changes in rat liver
microsomal cytochrome P-450 and enzymatic activities after the
inhalation of n-hexane, xylene, methyl ethyl ketone and
methylchloroform for four weeks. Scand J Work Environ Health,
7: 31-37.
Toftgård R, Nilsen OG, Glaumann H, & Gustafsson J-Å (1983) Induction
of cytochrome P-450 in the rat liver after exposure to xylenes,
dose-response relationship and dependence on endocrine factors.
Toxicology, 27: 119-137.
Toftgård R, Haaparanta T, & Halpert J (1986) Rat lung and liver
cytochrome P-450 isozymes involved in the hydroxylation of m-xylene.
Toxicology, 39: 225-231.
Triebig G & Schaller K-H (1986) Air monitoring of solvent exposed
workers with passive samplers in comparison to "biological monitoring
(BM)". Toxicol Environ Chem, 12: 285-312.
Tsuruta H (1982) Percutaneous absorption of organic solvents. III. On
the penetration rates of hydrophobic solvents through the excised rat
skin. Ind Health, 20: 335-345.
Turkall RM, Skowronski GA, Kadry ARM, & Abdel-Rahman MS (1992) Sex
differences in the bioavailability of soil-adsorbed m-xylene in
orally exposed rats. Toxicol Lett, 63: 57-67.
Tynan P, Watts CD, Sowray A, & Hammond I (1991) The transport and fate
of organic pollutants in rivers. II. Field measurements and modelling
for styrene, xylenes, dichlorobenzenes and 4-phenyldodecane. In:
Proceedings of the 6th European Symposium on Aquatic Environment,
1990, pp 20-37.
Uchida Y, Nakatsuka H, Ukai H, Watanabe T, Liu Y-T, Huang M-Y, Wang
Y-L, Zhu F-Z, Yin H, & Ikeda M (1993) Symptoms and signs in workers
exposed predominantly to xylenes. Int Arch Occup Environ Health,
64: 597-605.
Ungvary G (1985) The possible contribution of industrial chemicals
(organic solvents) to the incidence of congenital defects caused by
teratogenic drugs and consumer goods - an experimental study. Progr
Clin Biomed Res, 163B: 295-300.
Ungvary G (1990) The effect of xylene exposure on the liver. Acta
Morphol Hung, 38: 245-258.
Ungvary G & Tatrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats and rabbits. Arch Toxicol,
8(suppl): 425-430.
Ungvary G, Tatrai E, Hudak A, Barcza G, & Lorincz M (1980) Studies on
the embryotoxic effects of ortho-, meta- and para-xylene.
Toxicology, 18: 61-74.
Ungvary G, Varga B, Horvath E, Tatrai E, & Folly G (1981) Study on the
role of maternal sex steroid production and metabolism in the
embryotoxicity of para-xylene. Toxicology, 19: 263-268.
Valciukas JA, Lilis R, Singer RM, Glickman L, & Nicholson WJ (1985)
Neurobehavioral changes among shipyard painters exposed to solvents.
Arch Environ Health, 40: 47-52.
van der Wal JF & Moerkerken A (1984) The performance of passive
diffusion monitors for organic vapours for personal sampling of
painters. Ann Occup Hyg, 28: 39-47.
van Doorn R, Bos P, Brouns RME, Leijdekkers C-M, & Henderson PT (1980)
Effect of toluene and xylenes on liver glutathione and their urinary
excretion as mercapturic acids in the rat. Arch Toxicol, 43: 293-304.
van Doorn R, Leijdekkers C-M, Bos RP, Brouns ME, & Henderson PT (1981)
Alcohol and sulphate intermediates in the metabolism of toluene and
xylenes to mercapturic acids. J Appl Toxicol, 1: 236-242.
van Vliet C, Swaen GMH, Slangen JJM, de Boorder T, & Sturmans F (1987)
The organic solvent syndrome: A comparison of cases with neuro-
psychiatric disorders among painters and construction workers.
Int Arch Occup Environ Health, 59: 493-501.
van Wijnen JH, Verhoeff AP, Jans HWA, & van Bruggen M (1995) The
exposure of cyclists, car drivers and pedestrians to traffic-related
air pollutants. Int Arch Occup Environ Health, 67(3): 187-193.
Veith GD, Macek KJ, Petrocelli SR, & Carroll J (1980) An evaluation of
using partition coefficients and water solubility to estimate
bioconcentration factors for organic chemicals in fish. In: Eaton JG,
Parrish PR, & Hendricks AC ed. Aquatic toxicology. Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp 116-129
(ASTM STP 707).
Verschoyle RD, Dinsdale D, & Wolf CR (1993) Inhibition and induction
of cytochrome P450 isoenzymes in rat lung. J Pharmacol Exp Ther,
265: 386-391.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., pp 1188-1194.
Vincent R, Poirot P, Subra I, Rieger B, & Cicolella A (1994)
Occupational exposure to organic solvents during paint dripping and
painting operations in the aeronautical industry. Int Arch Occup
Environ Health, 65: 377-380.
Vodickova L, Frantik E, & Vodickova A (1995) Neutrotrophic effects and
blood levels of solvents at combined exposures: Binary mixtures of
toluene, o-xylene and acetone in rats and mice. Cent Eur J Public
Health, 3: 57-64.
Volskay VT & Grady CPL (1990) Respiration inhibition kinetic analysis.
Water Res, 24: 863-874.
Vowles PD & Mantoura RFC (1987) Sediment-water partition coefficients
and HPLC retention factors for aromatic hydrocarbons. Chemosphere,
16: 109-116.
Waggott A (1981) Trace organic substances in the River Lee. In:
Chemistry in water reuse. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., vol 2, pp 55-99.
Wallace LA, Pellizzari ED, Hartwell TD, Whitmore R, Zelon H, Perritt
R, & Sheldon L (1988) The California TEAM study: Breath concentrations
and personal exposures to 26 volatile compounds in air and drinking
water of 188 residents of Los Angeles, Antioch, and Pittsburg, CA.
Atmos Environ, 22: 2141-2163.
Wallace L, Nelson W, Ziegenfus R, Pellizzari E, Michael L, Whitmore R,
Zelon H, Hartwell T, Perritt R, & Westerdahl D (1991) The Los Angeles
TEAM study: Personal exposures, indoor-outdoor air concentrations, and
breath concentrations of 25 volatile organic compounds. J Exp Anal
Environ Epidemiol, 1(2): 157-192.
Wallén M, Holm S, & Byfält Nordqvist M (1985) Coexposure to toluene
and p-xylene in man: Uptake and elimination. Br J Ind Med,
42: 111-116.
Walsh DF, Armstrong JG, Bariley TR, Salmon HA, & Frank PA (1977)
Residues of emulsified xylene in aquatic weed control and their impact
on rainbow trout. Springfield, Virginia, National Technical
Information Services (NTIS) (PB-267270).
Walton BT, Anderson TA, Hendricks MS, & Talmage SS (1989) Physico-
chemical properties as predictors of organic chemical effects
on soil microbial respiration. Environ Toxicol Chem, 8: 53-63.
Washington WJ, Murthy RC, Doye A, Eugene K, Brown D, & Bradley J
(1983) Induction of morphologically abnormal sperm in rats exposed to
o-xylene. Arch Androl, 11: 233-237.
Weber WJ, Cole DE, & Posner JC (1975) Analysis of emissions from
outboard two cycle marine engines. Cincinnati, Ohio, US Environmental
Protection Agency, National Environmental Research Centre, Office of
Research and Development, 249 pp (EPA 670/2-75-06; PB-242174).
Weisel CP, Lawryk NJ, & Lioy PJ (1992) Exposure to emissions from
gasoline within automobile cabins. J Exp Anal Environ Epidemiol,
2(1): 79-96.
Weschler CJ, Shields HC, & Rainer D (1990) Concentrations of volatile
organic compounds at a building with health and comfort complaints. Am
Ind Hyg Assoc J, 51(5): 261-268.
WHO (1981) Xylene. In: Recommended health-based limits in occupational
exposure to selected organic solvents. Geneva, World Health
Organization, pp 25-38 (WHO Technical Report Series No. 664).
Wiesenburg DA, Bodennec G, & Brooks JM (1981) Volatile liquid
hydrocarbons around a production platform in the Northwest Gulf of
Mexico. Bull Environ Contam Toxicol, 27: 167-174.
Williams DT, Nestmann ER, LeBel GL, Benoit FM, & Otson R (1982)
Determination of mutagenic potential and organic contaminants of Great
Lakes drinking water. Chemosphere, 11(3): 263-276.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformations of selected
alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic
aquifer material: A microcosm study. Environ Sci Technol,
20: 997-1002.
Wilson BH, Wilson JT, Kampbell DH, Bledsoe BE, & Armstrong JM (1990)
Biotransformation of monoaromatic and chlorinated hydrocarbons at an
aviation gasoline spill site. Geomicrobiol J, 8: 225-240.
Winslow C-EA (1927) Summary of the National Safety Council study of
benzol poisoning. J Ind Hyg, 9: 61-74.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene:
Experiments on laboratory animals. Arch Ind Health, 14: 387-398.
Yamada K (1993) Influence of lacquer thinner and some organic solvents
on reproductive and accessory reproductive organs in the male rat.
Biol Pharm Bull, 16: 425-427.
Young PJ & Parker A (1983) The identification and possible
environmental impact of trace gases and vapours in landfill gas. Waste
Manage Res, 1: 213-226.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests. III. Results from the testing of
255 chemicals. Environ Mutagen, 9(suppl): 1-110.
Zeyer J, Kuhn EP, & Schwarzenback RP (1986) Rapid microbial
mineralisation of toluene and 1,3-dimethylbenzene in the absence of
molecular oxygen. Appl Environ Microbiol, 52: 944-947.
Zimmerman SW, Groehler K, & Beirne GJ (1975) Hydrocarbon exposure and
chronic glomerulonephritis. Lancet, 2: 199-201.
RESUME
Le xylčne est un hydrocarbure aromatique qui existe sous trois
formes: les isomčres ortho, méta et para. Le xylčne de qualité
technique est un mélange des trois isomčres qui contient en outre un
peu d'éthylbenzčne. En 1984, la production mondiale de xylčne était
estimée ą 15,4 millions de tonnes. A la température ambiante, le
xylčne se présente sous la forme d'un liquide incolore d'odeur
aromatique. La tension de vapeur est comprise entre 0,66 et 0,86 kPa
pour les trois isomčres. Environ 92% des mélanges de xylčnes sont
incorporés ą l'essence. On les utilise aussi comme solvants, en
particulier dans les peintures et les encres d'imprimerie.
La majeure partie du xylčne libéré dans l'environnement passe
directement dans l'atmosphčre. Les trois isomčres y sont rapidement
décomposés, principalement par photooxydation. Ils se volatilisent
tous les trois rapidement ą partir de l'eau. Dans le sol et dans
l'eau, les isomčres méta et para subissent une biodégradation aisée
dans des conditions variées d'aérobiose et d'anaérobiose; en revanche,
l'isomčre ortho est plus persistant. Les données limitées dont on
dispose indiquent que les xylčnes isomčres s'accumulent peu chez les
poissons et les invertébrés. Une fois que l'exposition a cessé, ils
sont assez rapidement éliminés par les organismes aquatiques.
Les concentrations moyennes de fond des trois xylčnes dans l'air
ambiant se situent autour de la valeur caractéristique de 1 µg/m3,
mais dans les banlieues elles atteignent 3 µg/m3 environ. On a
mesuré des valeurs plus élevées en zone urbaine et industrielle, les
moyennes allant cette fois jusqu'ą 500 µg/m3. Toutefois, la
concentration est en général inférieure ą 100 µg/m3.
On estime que l'exposition journaličre de la population par la
voie respiratoire est de 70 µg en milieu rural et de 2 000 µg en
milieu urbain. Dans l'eau de boisson, la concentration varie de zéro
ą 12 µg/litre. Les données concernant la concentration dans les
denrées alimentaires sont trop limitées pour que l'on puisse évaluer
l'exposition journaličre par voie orale.
Dans les eaux superficielles, la concentration moyenne de fond
des xylčnes est généralement inférieure ą 0,1 µg/litre. Cependant, on
a mesuré des valeurs beaucoup plus élevées dans des zones
industrielles et plus particuličrement celles oł sont implantées des
industries pétroličres (jusqu'ą 30 µg/litre dans les eaux polluées et
jusqu'ą 2 000 µg/litre ą proximité des conduites de décharge). Des
valeurs analogues ont été observées dans les eaux souterraines, ces
valeurs élevées pouvant źtre attribuées dans certains cas ą une
pollution locale par des réservoirs et des canalisations enterrées.
Aprčs exposition par la voie respiratoire, la dose inhalée est
retenue ą 60% environ dans les poumons. La métabolisation est
efficace puisque le xylčne est transformé ą 90% en acide
méthylhippurique, lequel est ensuite excrété dans les urines. Le
xylčne ne s'accumule pas en quantité importante dans l'organisme
humain.
Chez l'homme, une exposition aiguė ą du xylčne sous forte
concentration peut avoir des effets sur le systčme nerveux central et
provoquer une irritation. Ces effets n'ont toutefois pas donné lieu ą
des études contrōlées ni ą des études épidémiologiques ą long terme.
Chez les animaux de laboratoire, la toxicité chronique se révčle
faible. On a cependant de bonnes raisons de penser que sous
concentration modérée, le xylčne pourrait avoir des effets sur le SNC
chez l'animal.
Le xylčne ne s'est révélé ni mutagčne ni cancérogčne.
Le point d'aboutissement toxicologique essentiel concerne l'effet
nocif que le xylčne exerce sur le développment. On l'a mis en
évidence chez le rat ą partir d'une concentration de 870 mg/m3 (200
ppm). Compte tenu de cela, la valeur-guide recommandée pour la
concentration maximale de xylčne dans l'air a été fixée ą 0,87 mg/m3
(0,2 ppm).
Les xylčnes isomčres sont faiblement ą modérément toxiques pour
les organismes aquatiques. Chez les invertébrés, c'est l' o-xylčne
qui a la Cl50 la plus faible (1 mg/litre pour Daphnia magna). Chez
les poissons, la CL50 la plus faible est également celle de
l' o-xylčne (7,6 mg/litre chez la truite arc-en-ciel, par mesure de
concentration). Des valeurs de 7,9 et 1,7 mg/litre ont été obtenues,
respectivement pour le m- et le p-xylčne, dans le cas de la perche
commune (d'aprčs la concentration nominale). On ne dispose que de
données limitées au sujet de l'exposition chronique des organismes
aquatiques aux xylčnes, mais, quoi qu'il en soit, la volatilisation
rapide de ces composés la rend peu probable. Le xylčne ne présente
qu'une faible toxicité aiguė pour les oiseaux.
RESUMEN
El xileno es un hidrocarburo aromįtico del que hay tres formas
isoméricas: orto, meta y para. El xileno de calidad técnica contiene
una mezcla de los tres isómeros y algo de etilbenceno. Se estima que
la producción mundial fue de 15,4 millones de tone-ladas en 1984. La
presión de vapor estį comprendida entre 0,66 y 0,86 kPa para los tres
isómeros. Aproximadamente un 92% de las mezclas de xilenos se combinan
con el petróleo. El producto se emplea también en diversos
disolventes, en particular en las industrias de fabricación de
pinturas y de tintas de imprenta.
La mayor parte del xileno liberado en el medio ambiente pasa
directamente a la atmósfera. En ésta los isómeros de xileno se
degradan con facilidad, principalmente por fotooxidación. Los tres
isómeros se volatilizan rįpidamente en la atmósfera a partir del agua.
En el suelo y el agua los isómeros meta y para se biodegradan
fįcilmente en una amplia variedad de condiciones aerobias y
anaerobias, pero el isómero orto es mįs persistente. Las limitadas
pruebas disponibles parecen indicar que la bioacumulación de los
isómeros de xileno por los peces y los invertebrados es baja. La
eliminación del xileno de los organismos acuįticos es bastante rįpida
a partir del momento en que se interrumpe la exposición.
Normalmente los niveles basales medios de los tres isómeros de
xileno en el aire ambiente son de aproximadamente 1 µg/m3, pero en
zonas suburbanas se hallan en torno a 3 µg/m3. Se han detectado
concentraciones mayores en zonas urbanas e industrializadas, con
niveles medios de hasta 500 µg/m3. No obstante, las concentraciones
son por lo general inferiores a 100 µg/m3.
La exposición diaria por inhalación estimada en la población
general es de 70 µg en zonas rurales y de menos de 2000 µg en zonas
urbanas. La concentración en el agua potable estį comprendida entre
valores indetectables y 12 µg/litro. Los datos disponibles sobre la
concentración en los alimentos son insuficientes para poder estimar la
exposición oral diaria.
Las concentraciones basales medias de xilenos en aguas
superficiales son generalmente inferiores a 0.1 µg/litro. Sin embargo
se han hallado valores mucho mįs altos en zonas industriales y en
zonas vinculadas a la industria petrolera (hasta 30 µg/litro en aguas
contaminadas y hasta 2000 µg/litro en las proximidades de tuberķas de
desagüe). Se ha informado del hallazgo de niveles basales similares
en aguas subterrįneas, aunque se han detectado también concentraciones
elevadas, atribuidas a contaminación localizada a partir de tanques de
almacenamiento y tuberķas subterrįneos.
Tras la exposición por inhalación la retención pulmonar es de un
60% de la dosis inhalada. El xileno es metabolizado eficientemente.
Mįs del 90% se biotransforma en įcido metilhipśrico, que se excreta
por la orina. El xileno no se acumula de forma significativa en el
organismo humano.
La exposición aguda a altas concentraciones de xileno puede
afectar al SNC y causar irritación en el hombre. Sin embargo, no se
han llevado a cabo ni estudios controlados a largo plazo en el ser
humano ni estudios epidemiológicos. La toxicidad crónica parece
relativamente baja en animales de laboratorio. Hay indicios, no
obstante, de que concentraciones moderadas de xileno pueden tener
efectos crónicos sobre el SNC en animales.
El xileno no parece tener efectos mutįgenos ni carcinógenos.
El parįmetro crķtico es la toxicidad para el desarrollo,
demostrada a niveles de exposición de 870 mg/m3 (200 ppm) en la rata.
Teniendo en cuenta este parįmetro, la concentración indicativa
recomendada para el xileno en el aire es de 0.87 mg/m3 (0,2 ppm).
Los isómeros de xileno poseen una toxicidad entre moderada y baja
para los organismos acuįticos. En invertebrados la CL50 mįs baja,
calculada a partir de las concentraciones medidas, es de 1 mg/litro
para el o-xileno (Daphnia magna). Los valores mįs bajos de CL50
detectados en peces son de 7,6 mg/litro para el o-xileno (trucha
arco iris; segśn las concentraciones medidas), y de 7,9 y 1,7 mg/litro
para los m- y p-xilenos respectivamente (ambos para la lubina
estriada; segśn las concentraciones nominales). La información
disponible respecto a la exposición crónica de organismos acuįticos a
los xilenos es limitada; no obstante, su rįpida volatilización hace
improbable la exposición crónica en el agua. La toxicidad aguda del
xileno para las aves es baja.
6.3.2 In laboratory animals
Most studies on metabolism of xylenes have been performed on rat.
The principal pathway involves side-chain oxidation to methylbenzoic
acid via methylbenzyl alcohol and methylbenzyl aldehyde.
Methylbenzoic acid is then conjugated with glycine or glucuronic acid
(Sugihara & Ogata, 1978; Ogata et al., 1980; Elovaara et al., 1984).
Conjugation with glycine to form methylhippuric acid predominates for
m- and p-xylene (Sugihara, 1979; Ogata & Fujii, 1979; Elovaara et
al., 1984). In the case of o-xylene, glucuronide formation has been
reported to predominate (Ogata et al., 1980).
A separate minor pathway resulting in urinary excretion of
thioethers has been studied (Van Doorn et al., 1980; Van Doorn et al.,
1981). This pathway appears to be more important for o-xylene than
for the other isomers. Hydroxylation of the aromatic ring with the
formation of dimethylphenols has been reported to be another minor
metabolic pathway in rats (Bakke & Scheline, 1970; Elovaara et al.,
1984).
Methylbenzoic acid and dimethylphenols are present in urine of
guinea-pigs and rabbits exposed to xylene isomers (Fabre et al.,
1960). After oral dosing of rabbits with xylenes the main metabolite
was methylbenzoic acid (Bray et al., 1949). The acid was considered
to be mostly present as the glycine conjugate, methylhippuric acid.
Studies with isolated perfused livers and lungs from rabbit
indicate differences in the metabolic pathways between these two
organs (Smith et al., 1982). In the liver p-methylhippuric acid was
the major metabolite detected. In the lung p-methylbenzyl alcohol
and p-methylbenzoic acid were the main metabolites detected. There
was formation of 2,5-dimethylphenol in the lungs but not in the liver.
Metabolism of m- and p-xylenes to m- and p-methylbenzyl alcohols
has been found to be greater with hepatic than with pulmonary
microsomes (Harper, 1975; Toftgård et al., 1986). Further metabolism
to methylbenzoic acid occurred in the presence of hepatic but not
pulmonary cytosolic fraction. Daily exposures to xylene increased the
activities of liver microsomal enzymes and concentrations of
cytochrome P450 (Elovaara et al., 1982; Pathiratne et al., 1986).
Metabolism of m-xylene by cerebral microsomal preparations was
reported to be very slow (Elovaara et al., 1982).
No definitive studies have been reported showing which microsomal
P-450 enzymes are involved in xylene metabolism. However when rats
were given m-xylene (1.0-1.4 ml/kg body weight) by gastric
intubation once daily for 3 consecutive days and killed 24 h after the
last treatment, xylene caused an induction of CYP2B and CYP2E1 in
liver microsomes (Raunio et al., 1990). Exposure of male Wistar rats
to each of the xylene isomers by inhalation at a concentration level
of 4000 mg/m3 for 20 h/day over 4 days similarly induced hepatic
CYP2B1, while CYP2E1 was reduced, as estimated by Western blots (Gut
et al., 1993).
6.4 Elimination and excretion
6.4.1 In humans
Absorbed xylenes are excreted mainly as metabolites in urine.
Small amounts are excreted unchanged in exhaled air. Excretion in
faeces appears to be unimportant. The rate of clearance of p-xylene
from blood has been calculated to be 2.6 litres/kg per hour at 87
mg/m3 (20 ppm) and 1.6 litres/kg per hour at 304 mg/m3 (70 ppm)
(Wallén et al., 1985).
When volunteers were exposed to a constant concentration of
about 391-870 mg/m3 (90-200 ppm) m-xylene over 5 days, at least 97%
was calculated to be excreted as m-methylbenzoic acid conjugates.
2,4-Dimethylphenol conjugates accounted for 1-2% of the metabolites
(Riihimäki et al., 1979a; Riihimäki et al., 1979b). When volunteers
were exposed to about 195 mg/m3 (45 ppm) of o-, m- or p-xylene
for 8 h, about 95-99% of the dose was excreted as methylhippuric acid
in urine. Dimethylphenol excretion was estimated to be 0.1 to 2% of
the dose absorbed (Sedivec & Flek, 1976). About 90% of the absorbed
dose of m-xylene was excreted as methylhippuric acid after exposure
to 435 mg/m3 (100 ppm) for 4 h (Lauwerys et al., 1978; Campbell et
al., 1988). On the other hand, after exposure to 600 mg/m3 (138 ppm)
of o-xylene, only 46% was excreted in urine as methylhippuric acid
and only trace amounts of the o-methylbenzoyl glucuronide were
detected (Ogata et al., 1980).
In a study of 121 male workers engaged in dip-coating of metal
parts, the mean concentration was 3.48 mg/m3 (0.8 ppm) o-xylene,
9.1 mg/m3 (2.1 ppm) m-xylene and 3.91 mg/m3 (0.9 ppm) p-xylene.
The workers were also exposed to 0.8 ppm toluene and 0.9 ppm
ethylbenzene. At the end of the 8 h-shift urine samples were
collected and methylhippuric acid was determined. There was a linear
relationship between the intensity of exposure to xylenes and the
concentration of methylhippuric acid in urine. The methylhippuric
acid concentration as a function of increasing xylene concentration
was 17.8 mg/litre per ppm (Kawai et al., 1991a).
In workers occupationally exposed to an average of 17.4 mg/m3
(4 ppm) xylene (combination of all three isomers), with a maximum of
more than 430 mg/m3 (100 ppm), the urinary excretion of
methylhippuric acid was linearly correlated with the air exposure
(Huang et al., 1994).
The urinary methylhippuric acid excretion of xylene-exposed
painters at the end of the working week showed two distinct phase of
excretion. Half-times for urinary excretion of methylhippuric acid
were estimated to be 3.6 h for the first 10 h and 30.1 h for the next
2 days after exposure (Engström et al., 1978). A positive association
between the degree of obesity and the length of half-time was seen.
In volunteers exposed to m-xylene the urinary excretion of
m-methylbenzoic acid was described as triphasic with half-times of
1-2, 10 and 20 h (Riihimäki et al., 1979a). The observed elimination
half-time in the subcutaneous adipose tissue was about 58 h (Engström
& Riikimäki, 1979).
After oral administration of o-xylene (39 mg/kg body weight)
maximum urinary levels of glycine and glucuronide conjugates of
o-methylbenzoic acid were reported to be 33.1 and 1.0% of the
administered dose, respectively. Similar values were obtained after
an oral dose of 78 mg/kg body weight (Ogata et al., 1979; Ogata et
al., 1980).
About 4-5% of the dose absorbed in the lungs is exhaled unchanged
after exposure to 870 mg/m3 (200 ppm) xylene (Sedivec & Flek, 1976,
Åstrand et al., 1978, Riihimäki et al., 1979a, Riihimäki et al.,
1979b). Elimination in exhaled breath is reported to follow a similar
triphasic profile to that for urinary excretion of methylbenzoic acid
conjugates (Riihimäki et al, 1979). An initial half-time of about one
hour was obtained in a study by Campbell et al., 1988.
6.4.2 In laboratory animals
Exhalation of unchanged m-xylene has been described in one
study on rats. Exhalation was greatest 4 h after an intraperitoneal
injection, and 13% of the dose was exhaled unchanged within 10 h
(Sugihara, 1979). In mice 3.4% of the dose was exhaled within 8 h
(Bergman, 1979; Bergman, 1983).
In rats a total of 46% of the m-xylene dose (5 mmol/kg body
weight) was excreted as m-methylbenzoic acid within 24 h, and
phenobarbital (PB) treatment increased it to 70% of the dose. PB
treatment increased the elimination of m-methylbenzoic acid after
oral administration about 4-fold in the first 3 h, more then 2-fold in
the first 12 h, and 1.5-fold within 24 h compared to untreated but
m-xylene-exposed rats (Gut & Flek, 1981).
Wistar rats were pretreated with PB for 3 days (80 mg/kg body
weight per day) and then given m-xylene orally or intraperitoneally
at a small (0.081 mmol/kg) or a large (0.81 mmol/kg) dose or by
inhalation (6 h) at a low (174 mg/m3, 40 ppm) or high (1740 mg/m3,
400 ppm) concentration. PB treatment had a significant effect on the
metabolism of inhaled m-xylene (decreased blood m-xylene and
increased urinary excretion of m-methylhippuric acid), but only at
the high dose. The PB-induced enzyme induction had an effect at both
dose levels on the metabolism of orally administered m-xylene. The
effect on intraperitoneally administered m-xylene was more similar
to that of inhaled than that of orally administered m-xylene (Kaneko
et al., 1995).
After an intraperitoneal injection of 87-348 mg/kg body weight
m-xylene to rats, 53-75% of the dose was excreted as m-methyl-
hippuric acid in urine during 24 h (Ogata & Fujii, 1979). After
an intraperitoneal dose of 319 mg/kg body weight the proportion
excreted as mercapturic acids was calculated to be 10% for o-xylene
and 0.6-1.3% for m- and p-xylene (Van Doorn et al., 1980).
Male rats were exposed to soil-adsorbed (sandy soil or clay soil)
or pure 225 µl of m-xylene containing 20 µCi of m-[14C]-xylene
through the skin (Skowronski et al., 1990). The major route of
excretion in the pure and sandy groups was via expired air followed by
urine. However, in the presence of clay soil, the percentage of the
initial dose in expired air was similar to that in urine. In the
presence of clay soil, an increase in m-xylene was observed in
adipose tissue. Methyl hippuric acid was the main urinary metabolite.
Turkall et al. (1992) reported the bioavailability of
soil-adsorbed m-xylene in male and female rats. The rats were
gavaged with an aqueous suspension of 5% of gum acacia containing 150
µl of m-xylene with 5 µCi m-[14C]-xylene alone or adsorbed to
sandy or clay soil. While ingested soil contaminated with m-xylene
produced a higher bioavailability than the chemical alone in females,
no effects of soil was observed in males. No differences in the
bioavailability of m-xylene alone were observed between the sexes.
m-Xylene was primarily metabolized and excreted in urine,
methylhippuric acid being the main urinary metabolite in all groups.
6.5 Factors affecting toxicokinetics in humans and animals
In humans, co-exposure of m-xylene and ethylbenzene and
consumption of ethanol or aspirin (acetylsalicylic acid) prior to
inhalation has been shown to reduce urinary excretion of one or more
xylene metabolites, including methylhippuric acid (Riihimäki et al.,
1982a,b; Engström et al., 1984; Campbell et al., 1988). In the case
of ethanol consumption an increase in concentration of m-xylene in
blood was described (Riihimäki et al., 1982a,b). Co-exposure to
toluene decreased the ratio of the concentration of p-xylene in
venous blood to that in exhaled air (Wallén et al., 1985). The dermal
absorption of liquid m-xylene was reduced in the presence of
isobutanol (Riihimäki, 1979a).
Volunteers given ethanol on two evenings (total dose 137 g)
preceding exposure by inhalation to either 435 or 1740 mg/m3 (100 or
400 ppm) m-xylene for 2 h enhanced the metabolism of m-xylene but
only at 1740 mg/m3 (Tardif et al., 1994). Ethanol pretreatment
decreased the concentration of m-xylene in blood and alveolar air
during and after exposure and increased urinary excretion of
m-methylhippuric acid at the end of exposure to 1740 mg/m3.
Five male volunteers were exposed for 7 h/day over 3 consecutive
days to 50 ppm toluene, 174 mg/m3 (40 ppm) xylene (15% o-xylene,
25% m-xylene and 60% p-xylene) or a combination of both. To study
high-level exposure four men were exposed for 4 h to 95 ppm toluene or
348 mg/m3 (80 ppm) xylene or a combination of both. Mixed exposure
(low-level) did not alter the concentration of the solvents in blood
or exhaled air, nor did it modify the excretion of urinary
metabolities. High-level mixed exposure, however, increased the
concentration of solvents in blood and exhaled air and caused a delay
in the urinary excretion of hippuric acid (Tardif et al., 1991).
Simultaneous exposure by inhalation to toluene and xylene (15%
o-xylene, 60% m-xylene and 25% p-xylene) has been studied in
Sprague-Dawley rats. Exposure time was 5 h and the concentrations
were 75 ppm toluene plus 979 mg/m3 (225 ppm) xylene, 150 ppm toluene
plus 652 mg/m3 (150 ppm) xylene or 225 ppm toluene plus 326 mg/m3
(75 ppm) xylene. Compared to exposure to a single solvent,
simultaneous exposure resulted in lower amounts of excreted urinary
hippuric and methylhippuric acids over 24 h. Increased concentrations
of solvents in blood and brain were found immediately post-exposure.
Simultaneous exposure also enhanced the pulmonary elimination of both
solvents (Tardif et al., 1992).
From a toxicokinetic modelling study it was concluded that there
is competitive metabolic inhibition between m-xylene and toluene in
the rat (Tardif et al., 1993a). The interaction is likely to be
observed when exposure exceeds 50 ppm of each solvent (Tardif et al.,
1993b).
Inhalation exposure of rats for 4 h to 2436 mg/m3 (560 ppm)
m-xylene or 320 ppm of ethylbenzene alone, or in combination,
indicated that in the combined exposure blood and brain m-xylene
concentrations increased by 52 and 40%, respectively, whereas there
was no corresponding effect on ethylbenzene concentrations (Frantik &
Vodickova, 1995).
In a study of metabolic interaction, Wistar rats were exposed
for 6 h to 1305 mg/m3 (300 ppm) m-xylene, 600 ppm methyl ethyl
ketone (MEK) or a mixture of both. After mixed exposure the
cytochrome P-450-dependent monooxygenase activities were additively or
synergistically induced. In the presence of MEK the overall
metabolism of m-xylene was inhibited, as shown by an increase in
xylene concentration in blood and fat and a decrease in the 24-h
excretion of xylene metabolites (Liira et al., 1991).
Male Wistar rats were exposed by inhalation for 4 h to 1000
mg/m3 (230 ppm) of o-xylene or 1700 ppm of acetone alone, or in
combination. In the combination exposure, blood xylene immediately
after the exposure was increased by 40% whereas the blood acetone
level was decreased by 15%. In a corresponding study, H-strain mice
were exposed for 2 h to 1392 mg/m3 (320 ppm) of o-xylene or 6655
mg/m3 (1530 ppm) of acetone alone or in combination. The combination
exposure was accompanied by a 33% increase of the blood xylene
concentration whereas the blood acetone level was decreased by 18%
(Vodickova et al., 1995).
6.6 Biological monitoring
More than 90% of xylene is transformed in human metabolism to
methylhippuric acid and excreted in urine. Urinary methylhippuric
acid has proved a robust measure of the amount of xylene taken up in
the body over some preceding hours. The measurement has been
routinely used in some countries for assessment of individual exposure
to xylene in the occupational setting. About 1.5-2 g methylhippuric
acid per g creatinine in the post-shift urine sample corresponds to
exposure to 435 mg/m3 (100 ppm) xylene over a full workday (Lauwerys
& Buchet, 1988). Different workloads cause variation in the excreted
amounts of methylhippuric acid at a given exposure level since the
lung uptake of xylene is directly proportional to pulmonary
ventilation. Biotransformation of xylene to methylhippuric acid is
inhibited in the presence of ethanol and acetylsalicylic acid (see
section 6.5); these interfering factors need to be controlled when the
method is applied.
The analysis of xylene in blood and exhaled air can be used for
assessment of exposure (Lauwerys & Buchet, 1988). These methods may
be particularly suitable for estimating xylene body burden caused by
the low and relatively stable background exposure in the general
population, or, in case of accidental exposure, for measuring levels
for a clinical toxicological evaluation.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Inhalation studies
7.1.1.1 o-Xylene
The LC50 value for o-xylene in Sprague-Dawley rats (12
males/group) was calculated to be 4330 ppm (95% confidence limits
4247-4432 ppm) for a 6-h exposure. The reported signs of intoxication
were hypotonia and somnolence. Autopsy on surviving animals 14 days
later revealed no macroscopic lesions of lung, liver or kidneys
(Bonnet et al., 1982). Under similar conditions the LC50 value for
mice (OF-1) was 4595 ppm (4468-4744 ppm) (Bonnet et al., 1979).
Rats (probably Wistar, sex not stated, 10 animals/group) were
exposed to 1531, 3062 or 6125 ppm for 24 h. No deaths occurred at
1531 ppm, one death at 3062 ppm and 8 deaths at 6125 ppm (Cameron et
al., 1938). In the same study one group was exposed to 12 250 ppm
o-xylene for 12 h. Two deaths occurred, one after 2 h. Autopsy of
those animals that died did not reveal any macroscopic or microscopic
lesions in the organs studied (Cameron et al., 1938). In the same
study mice (10 animals/group) were exposed to the same concentrations
for the same period of time as the rats. There were no deaths at 1531
ppm, four deaths at 3062 ppm and nine deaths at 6125 ppm. In the
group exposed to 12 250 ppm for 12 h there were two deaths, one after
9 h. The organs of animals that died after exposure showed no
characteristic changes (Cameron et al., 1938).
In a study of the effect on prenarcotic motor behaviour, groups
of eight male rats (CFY) were exposed to o-xylene (dose not stated)
for 1, 2, 3 or 4 h. No significant effects on group motor activity
were observed. Narcosis occurred at higher concentrations with a
threshold of 2180 ppm for a 4-h exposure (Molnar et al., 1986). Mice
(strain, sex and numbers not stated) were exposed for 2 h to
o-xylene in order to determine the minimum concentration needed for
an animal to fall on its side and die. The minimum concentration for
falling was 3400-4600 ppm and for death 6900 ppm (Lazarew, 1929).
In a study of subnarcotic effects of solvents in Wistar rats and
H-strain mice, using electrically evoked seizures, the lowest
effective concentration of o-xylene was 170 ppm. The criterion used
was a significant suppression by 10% of the generation and maintenance
of the seizure discharge after 4 h (rats) or 2 h (mice) of inhalation
(Frantik et al., 1994). A 30% suppression was induced by o-xylene
at a concentration of 390 ppm, corresponding to a blood concentration
of 62 µmol/litre (Frantik et al., 1993).
In order to determine the RD50 value (exposure level reducing
the respiratory rate by 50%), groups of male mice (OF-1) were exposed
to o-xylene. The RD50 value was calculated to be 1467 ppm
(1406-1530 ppm). The onset of response was rapid and the maximum
decrease in respiratory rate was reached within a few minutes (De
Ceaurriz et al., 1981).
In a study of conditioned behaviour in mice, an increased
response rate was observed after a 30-min exposure to 1400-2000 ppm
o-xylene. At higher concentrations there was a decrease in response
rate with an EC50 of 5179 ppm. The biphasic response indicates that
there was excitation of the central nervous system at low
concentrations and depression at higher concentrations (Moser et al.,
1985).
In a study to observe effects in the "behavioural despair"
swimming test, groups of 10 mice (OF-1) were exposed to 0, 1010, 1101,
1207 or 1234 ppm o-xylene for 4 h. The ID50 (50% decrease in
immobility) value was calculated to be 1127 ppm (1068-1182 ppm) (De
Ceaurriz et al., 1983). The study demonstrated subnarcotic effects by
o-xylene on the CNS.
7.1.1.2 m-Xylene
After a 6-h exposure to m-xylene the LC50 in rats and mice was
reported to be 5984 ppm (5796-6181 ppm) and 5267 ppm (5025-5490 ppm),
respectively (Bonnet et al., 1979; Bonnet et al., 1982). The signs of
toxicity consisted of hypotonia, somnolence, narcosis, and clonic
spasms leading to death due to respiratory failure (Lazarew, 1929;
Cameron et al., 1938, Bonnet et al., 1982; Moser et al., 1985; Molnar
et al., 1986).
In the comparative study by Frantik et al., (1994) described in
section 7.1.1.1, the lowest effective air concentration for m-xylene
was 210 ppm.
A biphasic CNS response occurred at similar exposure levels to
those seen for o-xylene (section 7.1.1.1) (Moser et al., 1985). The
narcotic threshold in rats of about 2100 ppm m-xylene determined in
prenarcotic behaviour studies was similar to that observed for
o-xylene (Molnar et al., 1986). During a 4-h exposure to 8000 ppm
m-xylene, 10 out of 12 rats (Carwoth-Wistar) died (Smyth et al.,
1962).
When groups of rats (Wistar; 6 males/group) were exposed for 24 h
to 0, 75, 150 or 300 ppm m-xylene, there was a significant decrease
in cytochrome P-450 concentrations at all doses, and a dose-related
decrease in 7-ethoxycoumarin-O-deethylase activity in the lung. No
abnormalities of the lungs, as determined by scanning electron
microscopy, were seen in two animals exposed to 300 ppm (Elovaara et
al., 1987).
When male Wistar rats were exposed for 4 h to a 1:1 mixture of
m-xylene and n-butyl alcohol or to the single substances at
500-4000 ppm, there were disturbances of rotarod performance. The
medial effective concentrations (EC50) were calculated to be 3080 ppm
(mixture), 6530 ppm ( n-butyl alcohol) and 1980 ppm (xylene)
respectively. The combined exposure gave less than additive effects
(Korsak et al., 1993).
A concentration-dependent decrease in respiratory rate in mice
was demonstrated in a study where mice were exposed to 500-4000 ppm of
n-butyl alcohol, m-xylene or a 1:1 mixture of both. The RD50 was
calculated to be 3010 ppm, 1360 ppm and 3140 ppm, respectively. The
combined exposure gave less than additive effects (Korsak et al.,
1993).
The acute neurobehavioural effect of m-xylene was evaluated
after 20 min inhalation exposure using a functional observations
battery in mice. In the concentration range of 2000-8000 ppm, these
effects included changes in posture, decreased arousal and rearing,
increased ease of handling, disturbances of gait, mobility and
righting reflex, decreased forelimb grip strength, increased landing
foot splay and impaired psychomotor coordination. The response to
various sensory stimuli was also decreased. These acute effects were
short-lived, recovery beginning within minutes of removal from the
exposure chamber (Tegeris & Balster, 1994).
7.1.1.3 p-Xylene
A 4-h LC50 value in rats (female Sprague-Dawley) of 4740 ppm
p-xylene has been reported (Drew & Fouts, 1974). The corresponding
6-h value was 4591 ppm (4353-5049 ppm) (Bonnet et al., 1982), and for
mice (OF-1) was 3907 ppm (3747-4015 ppm) (Bonnet et al., 1979). The
signs of toxicity were similar to those reported for the other two
isomers.
Like the other two isomers, exposure to p-xylene gave a
biphasic CNS response (Moser et al., 1985). In another study (Molnar
et al., 1986), marked activation and tremor were observed at
concentrations between 400 and 1500 ppm p-xylene in rats. The
narcotic threshold was 1940 ppm.
When Sprague-Dawley rats (16 females/group) were exposed for 4 h
to 0, 1000, 1500 or 2000 ppm p-xylene, a dose-dependent increase in
serum enzymes activites was observed. This was taken as a sign of
hepatocellular and hepatobiliary damage (Patel et al., 1979). In
other studies at higher exposure levels, no microscopic hepatic
lesions were seen in rats or mice (Cameron et al., 1938; Furnas &
Hine, 1958; Bonnet et al., 1982).
When Long Evans rats were exposed for 4 h to 0, 800 or 1600 ppm
p-xylene, the flash-evoked potential of the visual system was
reduced at the highest exposure level (Dyer et al., 1988). The
authors suggested that this may have been secondary to changes in
arousal or excitability. In a study of the effect on learning tasks
and motor activity, rats (Long Evans) were exposed to 0 or 1600 ppm
p-xylene for 4 h. Signs of toxicity (unsteadiness and fine tremor)
disappeared 30 min post-exposure. The results indicate an effect on
motor control rather than on cognitive capacity (Bushnell, 1989).
In the comparative study by Frantik et al. (1994) described above
(section 7.1.1.1), where rats and mice were exposed, the lowest
effective air concentration for p-xylene was 220 ppm.
After a 4-h exposure to 1000 ppm p-xylene there was a
decrease in pulmonary cytochrome P-450 in rabbits and a decrease in
NADPH-cytochrome c-reductase and mixed-function oxidase activity in
rats (Patel et al., 1976; Patel et al., 1978).
When Sprague-Dawley rats were exposed to 3400 ppm p-xylene for
4 h and killed 12 h later, an induction of cytochrome P-450 activities
(CYP2B) in liver microsomes was observed. On the other hand, the
pulmonary cytochrome P-450 activities were inhibited (Day et al.,
1992).
In rats (Sprague-Dawley) p-xylene (2800 ppm for 4 h) has been
shown to potentiate hepatotoxicity induced by bromobenzene, while the
effect of bromobenzene on pneumotoxicity was unaffected by p-xylene,
indicating differences in xylene metabolism between the liver and the
lung (Day et al., 1992).
7.1.1.4 Technical or undefined xylene
In rats LC50 values of 6350, 6700 and 10 950 ppm have been
reported after 4-h exposure (Hine & Zuidema, 1970; Carpenter et al.,
1975; Lundberg et al., 1983). Deaths occurred during the exposure
period. No LC50 values for mice have been found, although
experiments have been carried out at up to 7000 ppm for 30 min (Moser
et al., 1985). Signs of toxicity were the same as those produced by
the individual isomers.
No signs of toxicity were reported when rats and dogs were
exposed for 4 h to 580 and 530 ppm, respectively. The composition was
7.63% o-xylene, 65.01% m-xylene, 7.84% p-xylene and 19.27%
ethylbenzene (Carpenter et al., 1975). The same type of response at
similar exposure levels as for the three individual isomers was
observed in mice in a conditioned behavioural study (Moser et al.,
1985). In rats effects of xylene were studied on an operant behaviour
maintained by a fixed-ratio liquid reinforced schedule. A decrease
in the reinforcement rate was seen after exposure to 113 ppm for 2 h
(Ghosh et al., 1987; Ghosh & Pradhan, 1987).
When rats (Fischer F-344) were exposed to 1450 ppm xylene for 8
h, a slight increase in the auditory response threshold at 20 kHz was
noted (Pryor et al., 1987). The xylene composition was 10%
o-xylene, 80% m-xylene and 10% p-xylene.
In mice (Swiss-Webster) exposed to 1300 ppm xylene for one
minute, a decrease in respiratory rate as an indication of respiratory
tract irritation was seen (Carpenter et al., 1975). This effect was
not seen at an exposure level of 460 ppm. No histological
abnormalities were seen on histological examination of livers from
rats exposed to 5480 ppm for 4 h, nor were there any changes in serum
activities of an indicator enzyme (sorbitol dehydrogenase) for
hepatotoxicity after exposure to > 340 ppm (Lundberg et al.,
1986). No histological abnormalities were seen in cat livers after
exposure to about 9500 ppm for up to 2 h (Carpenter et al., 1975). In
dogs (Beagle) 1200 ppm xylene for 4 h caused lacrimation but there was
no noticeable effect at 530 ppm (Carpenter et al., 1975).
When groups of cats (five animals/group) were exposed for 5740,
6900 or 9200 ppm for up to 6 h, there was a concentration-dependent
decrease in time to onset of staggering and mild narcosis. There was,
however, a large individual variation. Deep narcosis was seen in four
animals at 9200 ppm xylene (Engelhardt & Estler, 1935).
7.1.2 Other exposure routes
The oral LD50 values in rat have been reported to be 3608 mg/kg
body weight for o-xylene, 5011 mg/kg body weight for m-xylene and
4029 mg body weight for p-xylene (Smyth et al., 1962). For various
mixtures of xylenes oral LD50 values in rat have been reported to be
between 3523 and 8700 mg/kg body weight (Wolf et al., 1956; Hine &
Zuidema, 1970; NTP, 1986). In mice the corresponding values were
reported to be 5627 mg/kg body weight for male and 5251 mg/kg body
weight for females (NTP, 1986). Signs of toxicity at lethal doses
were CNS depression and congestion of cells in liver, kidney and
spleen, seen by histological examination.
A dermal LD50 value for rabbits (New Zealand White) of 12 180
mg/kg has been reported for a 24-h exposure to m-xylene (Smyth et
al., 1962). From studies with intraperitoneal (i.p.), intravenous
(i.v.), subcutaneous (s.c.) and intramuscular (i.m.) administration in
rats and mice the acute toxicity of xylene is low (Bell et al., 1992).
All three isomers have been found to interfere with the
modulation of the vestibular-oculomotor pathways in the rat. The
blood threshold levels for this effect were 170-200 µg/ml following
administration by the i.v. route (Tham et al., 1984). Similar effects
have also been reported for m-xylene in rabbit. The
vestibular-oculomotor effects were seen at blood levels of 30 µg/ml
and some deaths due to respiratory stress were recorded at 100 µg/ml
(Larsby et al., 1976; Aschan et al., 1977; Ödkvist et al., 1979;
Ödkvist et al., 1980).
A dose-dependent depletion of hepatic glutathione, following i.p.
administration, has been demonstrated in rats. With o-xylene the
effect was seen from 50 mg/kg and with the other two isomers from 425
mg/kg (Van Doorn et al., 1980). A decrease in pulmonary cytochrome
P-450 levels has been observed with the three isomers, when
administered (i.p.) at 531 mg/kg (Pyykkö et al., 1987).
The CYP2B1 and CYP1A1 isoenzyme activities, benzyloxy-resorufin
O-deethylation and ethoxyresorufin O-deethylation, respectively,
were studied in nasal, pulmonary and hepatic tissues of rats injected
intraperitoneally with m-xylene. Tissues taken at 2, 12 and 24 h
after injection showed inhibition of these activities in both nasal
and pulmonary microsomes, but increased activities in hepatic
microsomes (Blanchard & Morris, 1994).
The effects of intraperitoneal administration of o-xylene
(1g/kg body weight) on: (a) rat hepatic and pulmonary mixed-function
oxidase content and activity; and (b) microsomal membrane structural
parameters were studied 1, 3, 6 and 12 h after administration (Park et
al., 1994). The pulmonary cytochrome P-450 content and aryl
hydrocarbon hydroxylase activity were decreased, a maximal inhibition
occurring 3 h after dosing. Reduced pulmonary activity for both
ethoxyresorufin O-dealkylation and benzyloxyresorufin
O-dealkylation was noted. In contrast, increased hepatic cytochrome
P-450 content was noted, with slightly increased ethaxyresorufin
O-dealkylation and markedly increased benzyloxyresorufin
O-dealkylation. An increase in pulmonary microsomal phospholipid
content and cholesterol content was noted even 1 h after dosing. In
liver the phospholipid content increased although there was no change
in cholesterol content; this suggested an increase in membrane
fluidity.
Sprague-Dawley rats were given m-xylene (1 g/kg body weight)
intraperitoneally and killed one hour after treatment. Microsomes
from the lung were then prepared. Compared to controls, m-xylene
administration decreased the CYP2B1 activity but did not alter the
CYP1A1 activity or epoxide hydrolase activity. In total, m-xylene
administration resulted in an inhibition of benzo (a)pyrene
detoxication and increased production of toxic metabolites in the
pulmonary microsomal preparations (Stickney et al., 1991).
Xylenes have been shown to inhibit the hypotonic haemolysis of
erythrocytes in vitro at low concentrations. The EC50 values were
29, 39 and 44 µg/ml for o-xylene, m-xylene and p-xylene,
respectively (Holmberg et al., 1974).
7.2 Short-term exposure
7.2.1 Inhalation studies
7.2.1.1 o-Xylene
In a study on the noradrenaline and dopamine levels in various
parts of the forebrain and hypothalamus, Sprague-Dawley rats (six
males/group) were exposed to 0 or 2000 ppm o-xylene 6 h/day for 3
days. The animals were killed within 18 h after final exposure. There
was a significant increase in catecholamine levels and turnover in
various parts of the hypothalamus and a decrease in the dopamine
turnover in the forebrain of exposed animals (Andersson et al., 1981).
In order to study the effects on cytochrome P-450 and enzyme
activities Sprague-Dawley rats (four males/group) were exposed to 0 or
2000 ppm o-xylene, 6 h/day for 3 days. There was a significant
increase in relative liver weight and cytochrome P-450 content in
exposed animals. Furthermore, there were increases in some liver
enzyme activities. In lungs, there was a decrease in cytochrome P-450
activity (Toftgård & Nilsen., 1982).
When guinea-pigs (15 per group) were exposed to 0 or 780 ppm
o-xylene, 8 h/day, 5 days/week for 6 weeks, there was a marked
decrease in body weight gain in exposed animals. No effects on the
liver, kidney, heart, spleen or lung were observed upon histological
examination (Jenkins et al., 1970). In the same study beagle dogs
were exposed for the same period of time. One dog out of two
experienced tremors throughout the exposure period. No other signs of
toxicity were reported (Jenkins et al., 1970). Squirrel monkeys were
also exposed in the same manner. One monkey out of three died on day
seven. No other signs of toxicity were reported (Jenkins et al.,
1970).
7.2.1.2 m-Xylene
In a study of levels of noradrenaline and dopamine in various
parts of the forebrain and hypothalamus, Sprague-Dawley rats (six
males/group) were exposed to 0 or 2000 ppm m-xylene, 6 h/day for 3
days. The animals were killed within 18 h after the last exposure. A
significant increase in catecholamine levels and turnover was observed
in various parts of the hypothalamus of exposed animals. There was no
effect on dopamine levels (Andersson et al., 1981).
In another study, Sprague-Dawley rats (four males/group) were
exposed to 0 or 2000 ppm m-xylene for 3 days. The effect on
cytochrome P-450 and enzyme activities in liver, kidney and lung were
studied. In exposed animals there was a significant increase in
relative liver weight, in cytochrome P-450 content in liver and
kidney, and in some enzyme activities in these two organs. The
content of pulmonary cytochrome P-450 was decreased (Toftgård &
Nilsen, 1982).
In a study of the effect on xenobiotic metabolism in Wistar rats,
10 males per group were exposed to 0, 50, 400 or 750 ppm m-xylene, 6
h/day, 5 days/week for one week. At the two highest doses there
was a significant increase in hepatic microsomal protein and
NADPH-cytochrome c reductase levels, and a decrease in hepatic
glutathione levels. There was no effect on hepatic cytochrome P-450
levels or on renal glutathione levels. At all dose levels there was a
significant increase in renal cytochrome P-450 and some enzyme
activities. When the animals were exposed to the same regimen for 2
weeks similar results were obtained. There were no abnormalities upon
histological examination of the liver (Elovaara, 1982). When Wistar
rats (20 males/group) were exposed to 0 or 300 ppm m-xylene 6 h/day,
5 days/week for one week there was, in exposed groups, a significant
increase in hepatic and renal 7-ethoxycoumarin O-deethylase activity
and in renal UDP-glucuronyl transferase. When exposure time was 2
weeks there was also a significant increase in hepatic cytochrome
P-450 and NADPH-cytochrome c reductase activities (Elovaara et al.,
1982).
In order to study the effect on lung cytochrome P-450 in rats,
six male Wistar rats/group were exposed to 0 or 300 ppm m-xylene 7
h/day, 4 days/week for 5 weeks. The only effects seen were a
significant decrease in cytochrome P-450 content and 7-ethoxycoumarin
O-deethylase activity (Elovaara et al., 1987). When the effects on
cerebral biochemistry was studied, groups of Wistar rats (15
males/group) were exposed to 0, 50, 400 or 750 ppm m-xylene 6 h/day,
5 days/week for 1 or 2 weeks. The only effect seen after one week of
exposure was a significant decrease in glutathione levels at all
concentrations. After 2 weeks there were also a dose-dependent
decrease in superoxide dismutase activity, a significant increase in
NADPH-diaphorase activity at all concentrations and a significant
increase in azoreductase activity at the two highest concentrations
(Savolainen & Pfäffli, 1980).
7.2.1.3 p-Xylene
In a study of levels of noradrenaline and dopamine in the
forebrain and hypothalamus, Sprague-Dawley rats (six males/group) were
exposed to 0 or 2000 ppm p-xylene 6 h/day for 3 days. The animals
were killed 16-18 h after the last exposure. In exposed animals there
was a significant increase in catecholamine levels and turnover in
various parts of the hypothalamus. There was no effect on dopamine
levels or turnover in the forebrain (Andersson et al., 1981).
In order to study the effect on cytochrome P-450 and enzyme
activities in the liver, kidney and lung, Sprague-Dawley rats (four
males/group) were exposed to 0 or 2000 ppm 6 h/day for 3 days. In
exposed animals there were significant increases in relative liver
weight, in hepatic cytochrome P-450 content, in NADPH-cytochrome c
reductase activity in the liver and kidney, and in 7-ethoxyresorufin
O-deethylase activity in the kidney. There was also a decrease in
pulmonary cytochrome P-450 content (Toftgård & Nilsen, 1982). To
study the lung microsomal activity, rabbits (New Zealand White; four
males/group) were exposed to 0 or 1000 ppm p-xylene 4 h/day for 2
days. In exposed animals there was a significant decrease in
microsomal cytochrome P-450 concentration and in NADPH-cytochrome c
reductase activity (Patel et al., 1978). Inhibition of CYP2B1 has
been observed in the lungs of rats dosed with p-xylene (Verschoyle
et al. 1993).
Rats exposed to 300 ppm p-xylene, 6 h/day for 1, 3 or 5 days
exhibited alterations in pulmonary microsomal membrane structural and
metabolic parameters (Silverman & Schatz, 1991). Following 1 day of
exposure, conjugated diene levels were elevated while total
phospholipid levels, cytochromes P-450 content, benzyloxyresorufin
O-dealkylase activity and 2-aminofluorene N-hydroxylase activity
were decreased. Core membrane fluidity was increased following 3 days
of exposure. After 5 days of exposure all parameters returned to
control levels with the exception of aryl hydrocarbon hydroxylase
activity, which was increased by 41%. Extracellular surfactant levels
were also decreased after 1 and 3 days of exposure but returned to
control values after 5 days. The increase in aryl hydrocarbon
hydroxylase activity after 5 days of exposure could have important
consequences on the metabolism of co-administered xenobiotics.
Male Fischer-344 rats exposed to 0 or 1600 ppm p-xylene by
inhalation, 6 h/day, for 1 or 3 days did not produce overt
hepatotoxicity but resulted in a significant increase in the
concentration of hepatic cytochrome P-450 (Simmons et al., 1991).
However, the concentration of hepatic cytochrome P-450 had returned to
control levels within 2 to 3 days after exposure.
p-Xylene has been shown to decrease axonal transport of
proteins and glycoproteins in rats (Long-Evans) exposed by inhalation
to 1600 ppm for 6 h/day, 5 days/week, for 8 days. When ethanol (10%
in drinking-water) was given during 6 days prior to inhalation of
p-xylene, the treatment prevented the decreased axonal transport.
Ethanol per se did not decrease the axonal transport (Padilla et
al., 1992).
When 10 Wistar rats and 10 mice (strain not given) were exposed
to 1226 ppm p-xylene 8 h/day for 14 days, no animals died. No other
results were reported (Cameron et al., 1938).
When mice were exposed to 1200 ppm p-xylene 6 h/day for 4 days
and infected with a sublethal dose of murine cytomegalovirus, 34%
mortality occurred, whereas no deaths occurred among uninfected,
p-xylene-exposed mice or infected, air-exposed mice (Selgrade et
al., 1993). Although p-xylene potentiated liver damage caused by
the virus, the magnitude of serum enzyme activities indicated that
this damage was not the probable cause of death. Enhanced mortality
was related to enhanced xylene toxicity due to suppression of
cytochrome P-450, although additive or synergistic damage to tissues
other than liver could not be ruled out. There was no indication that
p-xylene had caused immune suppression.
7.2.1.4 Technical or undefined xylene
When rats (strain not defined) were exposed to 620, 980 or 1600
ppm xylene 18-20 h/day for 7 days, instability, incordination and
narcosis were observed at the two highest concentrations. Signs of
mucous membrane irritation occurred, and congestion and cloudy
swelling of kidneys was reported at 980 ppm (Batchelor, 1927).
Similar results were reported in another study (Winslow, 1927).
Groups of Harlan-Wistar rats (25 males/group) were exposed to 0,
180, 460 or 810 ppm xylene 6 h/day, 5 days/week for 13 weeks. The
xylene consisted of 7.63% o-xylene, 65.01% m-xylene, 7.84%
p-xylene and 19.27% ethylbenzene. At 3, 7 and 13 weeks 3, 3 and 4
animals, respectively, were killed. No treatment-related
histopathology was seen (Carpenter et al., 1975).
In a study of levels of noradrenaline and dopamine in the
forebrain and hypothalamus of rats, the xylene mixture used was 2.0%
o-xylene, 64.5% m-xylene, 10.0% p-xylene and 23.0% ethylbenzene.
Sprague-Dawley rats (6 males/group) were exposed to 0 or 2000 ppm
xylene 6 h/day for 3 days. The animals were killed 16-18 h after the
last exposure. There was a significant increase in catecholamine
levels and turnover in the hypothalamus and a significant increase in
dopamine levels and turnover in the forebrain (Andersson et al.,
1981).
In order to study the effect on levels of neurotransmitters in
the rat brain, five to six Sprague-Dawley rats/group were exposed to
0, 200, 400 or 800 ppm xylene (mixture not defined) for 30 days.
Acetylcholine levels in the striatum were decreased at > 400 ppm.
Noradrenaline levels in the hypothalamus were increased significantly
at the highest dose. From 400 ppm the cAMP levels were decreased in
the striatum, and at 800 ppm the glutamine levels in the midbrain were
increased. At all concentrations glycine and GABA levels in the
midbrain were increased (Honma et al., 1983).
When 12 male Fischer F-344 rats/group were exposed to 0 or 1450
ppm xylene 8 h/day for 3 days, there was, in exposed animals, an
increase in the auditory response threshold at 12 and 20 kHz.
Rats (Long Evans) were exposed to 2500 ppm of mixed xylenes 6
h/day for 5 days. Testing of auditory function was conducted 5 to 8
weeks after exposure using reflex modification audiometry (RMA). The
results indicated increased RMA thresholds for the mid-frequency tones
(e.g., 8, 16 and 24 kHz) but not for higher or lower tones (Crofton et
al., 1994).
In another study Sprague-Dawley rats (four males/group) were
exposed to 0 or 630 ppm xylene 6 h/day, 5 days/week for 4 weeks. The
animals were killed the morning after the last exposure. In exposed
animals there was a significant decrease in body weight gain, while
the absolute and relative liver weights were increased. There was an
increase in hepatic cytochrome P-450 and the xylene was shown to act
as a phenobarbital-like inducer of cytochrome P-450. The xylene used
was 2.0% o-xylene, 64.5% m-xylene, 10.0% p-xylene and 23.0%
ethylbenzene (Toftgård et al., 1981).
When male Sprague-Dawley rats (8-12 animals/group) were exposed
to 0, 75, 250, 500, 1000 or 2000 ppm xylene 6 h/day for 3 days there
was a dose-dependent increase in the concentration of liver microsomal
cytochrome P-450. When animals were exposed to the two highest
concentrations for 5 days, there was a dose-dependent increase in the
surface area of smooth endoplasmic reticulum but not in rough
endoplasmic reticulum. Based on their results the authors concluded
that xylene causes phenobarbital-type induction in the liver. The
xylene used was the same as that in the previous paragraph (Toftgård
et al., 1983).
The same type of xylene was also used in a study where four male
Sprague-Dawley rats/group were exposed to 0 or 2000 ppm xylene 6 h/day
for 3 days. In exposed animals there were significant increases in
hepatic and renal cytochrome P-450 content, in NADPH-cytochrome c
reductase activities and 7-ethoxyresorufin O-deethylase activities
in liver and kidney. The pulmonary cytochrome P-450 content was
decreased. Increased enzyme activity in liver and kidney and decreased
activity in the lung were thus observed (Toftgård & Nilsen, 1982).
Groups of male Wistar rats (20 animals/group) were exposed to 0
or 300 ppm xylene 6 h/day, 5 days/week for up to two weeks. In each
group 10 animals had 15% v/v ethanol in the drinking-water. There was
a significant decrease in motor activity in exposed animals. In
addition, there was a significant increase in brain DT-diaphorase
activity, in acid proteinase and in hepatic and renal 7-ethoxycoumarin
O-deethylase activities. Concurrent dosing with ethanol had a
marked synergistic effect on hepatic and renal 7-ethoxycoumarin
O-deethylase activities. The xylene used was 80% m-xylene and 12%
p-xylene (Savolainen et al., 1979a,b).
In a study of the effect of simultaneous exposure to xylene
(undefined) and noise on metabolic activity in the myocardium, male
rats (strain not specified) were exposed to 0 or 69 ppm xylene 4 h per
day, 5 days per week for 6 weeks, and simultaneously to a noise level
of 0, 46, 85 or 95 dB. There were changes in some enzyme activities,
but the brief reporting makes the study impossible to evaluate
(Ivanovich et al., 1985).
When Beagle dogs (four males per group) were exposed to 0, 180,
460 or 810 ppm xylene 6 h per day, 5 days per week for 13 weeks, no
treatment-related effects were reported. The xylene used was 7.63%
o-xylene, 65.01% m-xylene, 7.84% p-xylene and 19.27% ethylbenzene
(Carpenter et al., 1975).
7.2.2 Other exposure routes
Decreased body weight compared to controls was noted in rats
(Sprague-Dawley) administered orally 1062 mg m-xylene/kg body weight
for 3 days (Pyykkö, 1980), and reduced terminal body weight was
observed in rats (Fischer F-344) administered 500 mg m-xylene/kg
body weight, 5 days per week, for 4 weeks (Halder et al., 1985).
Three days administration of 1062 mg m-xylene/kg body weight
resulted in significant increases in liver weight, liver cytochrome
P-450 and cytochrome b5 levels and NADPH cytochrome c reductase and
MFO enzymes activities. The same range of increases was also seen in
the kidney (Pyykkö, 1980). In another study, however, there were no
effects on kidney weight and no treatment-related abnormalities
histopathologically in Fischer rats given 500 or 2000 mg/kg body
weight 5 days per week for 4 weeks (Dési et al., 1967).
Upon oral administration of 0, 125, 250, 500, 1000 or 2000 mg
xylene per kg body weight to rats (F-344/N), in a 14-day study, a high
mortality was seen in the highest dose group. The xylene used was
9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. The body weight gain was reduced in males at > 250
mg/kg body weight and in females at 125 mg/kg body weight and
> 1000 mg/kg body weight. There were no treatment-related
abnormalities at gross necropsy (NTP, 1986). In the same study xylene
was administered at 0, 62.5, 125, 250, 500 or 1000 mg per kg body
weight, 5 days per week, for 13 weeks. No treatment-related
abnormalities were seen during gross necropsy or histopathological
examination (NTP, 1986).
Daily administration (s.c.) of xylene (undefined) for up to 4
weeks to rats (strain not given) resulted in significant mortality at
870 mg/kg body weight but not at 435 or 174 mg/kg body weight.
Repeated administration of 435 mg/kg body weight resulted in decreased
learning rate (Dési et al., 1967). When Sprague-Dawley rats were
given 0 or 3123 mg/kg body weight for 3 days, there was a significant
increase in liver weight, cytochrome P-450 levels, NADPH cytochrome c
reductase and activity of MFO enzymes. The xylene used was 30%
o-xylene, 55% m-xylene and 15% p-xylene (Pathiratne et al.,
1986).
In order to study further the effects of p-xylene, rats were
given p-methylbenzyl alcohol (PMBA) or 2,5-dimethylphenol (DMP)
(300 mg/kg body weight and 150 mg/kg body weight, respectively)
intraperitoneally once a day for 3 days. It was concluded that of the
two metabolites, PMBA may have a significant role in the inhibition of
pulmonary cytochrome P-450 caused by p-xylene (Day & Carlson, 1992).
Male Sprague-Dawley rats (n=10) were given xylene (isomer not
stated) intraperitoneally, as a single dose per day, for 3 consecutive
days. The dose given was equal to half the LD50 (1.6 ml/kg body
weight per day). Only slight somnolence was observed. After the last
dosing the animals were killed and aminopeptidase activities in
several regions of the brain were measured. The activities were
largely unaffected, compared to those of controls (De Gandarias et
al., 1993).
Brain cell cultures enriched in astroglial cells were prepared
from neonatal Sprague-Dawley rats. The cultures were exposed for 1 h
to 3, 6 or 9 mmol o-xylene/litre. The ATPase activity was reduced
in a dose-dependent manner (Naskali et al., 1994).
7.3 Long-term exposure
A short summary of long-term studies is presented in Table 6.
In Wistar rats exposed to 1000 ppm m-xylene for 6 h/day,
5 days/week for 3 months or to 100 ppm for 6 months, slight
ultrastructural changes (proliferation of smooth endoplasmic
reticulum) were found in hepatocytes. When rats were exposed to a 1:1
combination of m-xylene and toluene (500 plus 500 ppm or 50 plus 50
ppm), the changes were a combination of those of each single solvent
(Rydzynski et al., 1992). The combined exposure at both exposure
levels gave more pronounced disturbances in a rotarod performance test
and decrease in spontaneous motor activity compared to single solvent
exposure. In animals exposed to 500 plus 500 ppm for 3 months a
decrease in red blood cell count and an increase in rod neutrophil
cell count were observed (Korsak et al., 1992).
In a six-week study, groups of rats (Sprague-Dawley or
Long-Evans; 15 animals/group) were exposed to 0 or 780 ppm o-xylene
(8 h/day, 5 days/week) or to 78 ppm continously for 90 days. There
was no effect on body weight gain, leukocyte count, haemoglobin level
or heamatocrit. Histological examination of the liver, kidney, heart,
spleen and lung revealed no effects (Jenkins et al., 1970).
Table 6. Effects of xylenes in long-term studies
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Inhalation exposure
o-Xylenea rat, dog, 13 weeks 780 - Jenkins et al., 1970
guinea-pig
o-Xylenea monkey 13 weeks + 78 - Jenkins et al., 1970
m-Xylenea rat 3 months - 1000 liver cell changes (i.e. inc. smooth Rydzynski et al., 1992
endoplasmic reticulum, lysosomes)
m-Xylenea rat 6 months - 100 liver cell changes (i.e. inc. smooth Rydzynski et al., 1992
endoplasmic reticulum, lysosomes)
m-Xylenea rat 3 months - 100 dec. lymphocyte/monocyte count; Korsak et al., 1992
dec. rotorod, performance
m-Xylenea rat 6 months - 100 dec. rotorod, performance Korsak et al., 1992
dec. spontaneous motor activity
Xylenes rat 3 months 50 100 dec. rotorod performance Korsak et al., 1994
dec. spontaneous motor activity
Xylenes rat 6 months 346 923 liver effects (inc. liver weight inc. Ungvary, 1990
smooth endoplasmic reticulum, inc.
P-450 activity)
Table 6. (Cont'd)
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Inhalation exposure
Xylenes rat 13 weeks 810 - Carpenter et al., 1975
Xylenes dog 13 weeks 810 - Carpenter et al., 1975
Xylenes rat 6 weeks - 800 ototoxicity Pryor et al., 1987
(14h/day)
Xylenes rat 61 days - 1000 ototoxicity Nylen & Hagman, 1994
(18 h/day)
Xylenes rat 18 weeks - 300 liver microsomes activity Elovaara et al., 1980
Xylenes rat 18 weeks - 300 brain superoxide dismutase activity Savolainen et al., 1979
Xylenes rat 13 weeks + - 320 brain lipid composition changes Kyrklund et al., 1987
Xylenes gerbil 3 months 160 320 change in astroglial cell marker Rosengren et al., 1986
proteins
Table 6. (Cont'd)
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Oral exposure
Xylenes rat 13 weeks 1000 - NTP, 1986
Xylenes mouse 13 weeks 2000 - NTP, 1986
Xylenes rat 13 weeks - 150 increased liver weights (males); Condie et al., 1988
hyaline droplet nephropathy (males)
Xylenes rat 2 years 250 500 mortality NTP, 1986
Xylenes mouse 2 years 500 - NTP, 1986
a Only one dose was used
b Continous exposure
The effects of combined exposure to m-xylene and n-butyl
alcohol have been studied in rats exposed to the individual solvents
at 50 and 100 ppm and their 1:1 mixture at 50 plus 50 ppm and 100 plus
100 ppm, 6 h/day, 5 days/week for 3 months (Korsak et al., 1994). The
results indicate less than an additive toxic effect (motor
coordination disturbances) of combined exposure to m-xylene and
n-butyl alcohol. For xylene alone an effect was seen at 100 ppm but
not at 50 ppm.
Rats (CFY) were exposed to xylene (10% o-xylene, 50%
m-xylene, 20% p-xylene and 20% ethylbenzene) for 8 h/day up to 6
months at concentrations of 600, 1500 or 4000 mg/m3. No macroscopic
changes were seen but the relative liver weight was increased at 4000
mg/m3. At 4000 mg/m3 hypertrophy of the centrilobular zone in
the liver, including a change in the amount of smooth and rough
endoplasmic reticulum, was seen. As a result of xylene exposure the
hexobarbital sleeping time decreased. Liver enzymatic activities were
increased during the first 6 weeks. After the 4-week exposure-free
period the changes described above could no longer be seen. The same
type of changes could also be seen when xylene was applied orally,
subcutaneously or intraperitoneally for a short period of time (4-7
day). Essentially the same changes as those described for rat liver
were also seen in livers from mice and rabbits (Ungvary, 1990).
Groups of 50 male and 50 female Fischer-344/N rats received 0,
250 or 500 mg xylene/kg body weight. The xylene used was 9.1%
o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0% ethylbenzene.
The doses were given (in corn oil) by stomach tube on 5 days per week
for 103 weeks. The animals were killed within 14 days after the last
dosing. Body weights of high-dose (500 mg/kg body weight) males were
5 to 8% lower than those of vehicle controls. Results for low-dose
males and both female groups were comparable to those of controls.
Gross observation and histopathological results showed no incidences
of non-neoplastic effects in dosed groups, related to the
administration of xylene, at any sites (NTP, 1986; Huff et al., 1988).
In the same study groups of 50 male and 50 female B6C5F1 mice
received 0, 500 or 1000 mg xylene/kg body weight. The same type of
technical xylene was used. The doses were given, in corn oil, by
stomach tube 5 days per week for 103 weeks, after which the animals
were killed within 14 days. No significant difference in mean body
weights or survival was observed between treated animals and controls.
Gross observation and histopathological results indicated that at no
site were the incidences of non-neoplastic effects in dosed groups
related to the administration of xylene (see also section 7.7).
Male Fischer-344 rats were exposed to 0, 800, 1000 or 1200 ppm
xylene 14 h/day, 7 days/week for 6 weeks. At the highest dose level
there was a slight impairment of auditory (but not of visual or
somatosensory) conditioned avoidance response. All animals had
increased auditory response thresholds compared to controls at the
same frequencies. At 1000 and 800 ppm the thresholds were elevated at
16 kHz and 8 kHz and at 1200 ppm the brainstem auditory-evoked
response thresholds were elevated at 4, 8 and 16 kHz tone frequency.
The xylene used in these two studies was 10% o-xylene, 80%
m-xylene and 10% p-xylene (Pryor et al., 1987).
Wistar rats (60 males/group) were exposed to 0 or 300 ppm xylene
6 h per day, 5 days per week, for up to 18 weeks, and 50% of the
animals had 15-20% ethanol in the drinking water. The xylene used was
19.2% o-xylene, 43.0% m-xylene, 19.5% p-xylene and 18.3%
ethylbenzene. It was concluded that concurrent ethanol intake
increased hepatic and renal microsomal enzyme activities. Stearosis
in the liver was more marked in co-exposed animals than in animals
exposed only to ethanol. No treatment-related abnormalities were
observed during histopathological examination of animals exposed only
to xylene (Elovaara et al., 1980).
Exposure to 1000 ppm xylene (not defined) 18 h per day, 7 days
per week, for 61 days caused a slight loss of auditory sensitivity in
Sprague-Dawley rats. Co-exposure to n-hexane (1000 ppm) caused
a persistent loss of auditory sensitivity, which was less than
additively enhanced. Xylene inhibited n-hexane-induced impulse
velocity reduction in peripheral nerves (Nylén & Hagman, 1994).
In a study to investigate the effect on brain lipid composition,
Sprague-Dawley rats were exposed to 0 or 320 ppm xylene continuously
for 30 or 90 days. The xylene (undefined) produced only limited
transient changes (Kyrklund et al., 1987). In another study to
investigate the effect on brain with and without co-exposure to
ethanol, Wistar rats (20 males/group) were exposed to 0 or 300 ppm
xylene 6 h per day, 5 days per week, for up to 18 weeks. In each
group were 10 animals also exposed to 15% v/v ethanol in the
drinking-water. There was a significant increase in cerebral
microsomal superoxide dismutase activity by week 18, but no effect on
cerebral protein or RNA levels. Co-exposure to ethanol reduced the
effect caused by xylene exposure. The xylene used was 7.5%
o-xylene, 85.0% m-xylene and 7.5% p-xylene (Savolainen et al.,
1979a,b).
In a study of the effect on spinal cord axon membrane, Wistar
rats (five animals per group) were exposed to 0 or 300 ppm xylene
(undefined) 6 h per day, 5 days per week, for 18 weeks. In exposed
animals there was a decrease in the amount of membrane lipid per mg of
protein but no change in the cholesterol/lipid phosphorus ratio.
Ethanol (15% v/v) in the drinking-water enhanced the decrease and also
decreased the cholesterol/lipid phosphorus ratio (Savolainen &
Seppäläinen, 1979).
In a neurotoxicity study, changes in two astroglial cell marker
proteins (S-100 and GFA) and DNA were measured. Mongolian gerbils
(four of each sex per group) were exposed to 0, 160 or 320 ppm xylene
continously (24 h/day) for 3 months, followed by a 4-month
exposure-free period. The xylene used was 18% o-xylene, 70%
m-xylene, 12% p-xylene and < 3% ethylbenzene. The exposure to
xylene resulted in brain damage which was manifest as an increase in
astroglial cells. Effects were seen in particular parts of the brain
at both exposure levels but were only significant at 320 ppm
(Rosengren et al., 1986).
Ageing Long-Evans rats fed 200 ppm o-xylene for up to 6 months
showed formation of vacuolar structures in hepatocytes when examined
ultrastructurally (Bowers & Cannon, 1982).
Groups of 10 male and 10 female Sprague-Dawley rats were exposed
to mixed xylenes by gavage (in corn oil) for 90 consecutive days at
dose levels of 150, 750 and 1500 mg/kg body weight per day (Condie et
al., 1988). The most significant findings were increased relative
liver and kidney weights. Histopathology evaluation of liver and
kidney tissues revealed an increased incidence of minimal chronic
renal disease in females only. Treatment-related hepatic
histopathological changes were not detected in either sex. Hyaline
droplet formation was observed in male rats in all treated groups. In
females, there was an increased incidence of kidney effects, which
were thought to represent onset of progressive nephropathy (Condie et
al., 1988).
Mice (B6C3F1) were given orally 0, 250, 500, 1000 or 2000 mg
xylene per kg body weight for 14 days or 0, 125, 250, 500, 1000 or
2000 mg per kg body weight, 5 days per week, for 13 weeks. The xylene
used was 9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. Mean body weight gain was reduced in males at > 250
mg/kg body weight. No treatment-related abnormalities were observed
at gross necropsy or histopathological examination (NTP, 1986).
7.4 Skin and eye irritation; sensitization
Erythema and oedema were induced following a single application
(amount not stated) of xylene (not defined) to rabbit or guinea-pig
skin. There was a rapid onset and epithelial desquamation, with some
evidence of necrosis occurring after several days (Rigdon, 1940;
Steele & Wilhelm, 1966). After 24 h of exposure to 0.5 ml xylene
(undefined) under semi-occlusive conditions, irritation of rabbit skin
was observed. The irritation was graded as moderate by the authors
(Hine & Zuidema, 1970).
Application of 0.5 ml p-xylene, under a Teflon chamber, to
rabbit skin for 4 h produced a response which would be classified as
irritant using European Union criteria (Jacobs et al., 1987). In
another study, following 10 to 20 applications of xylene (not defined)
to open or semi-occluded rabbit skin for up to four weeks, a moderate
to marked irritation and moderate necrosis was reported, as was
blistering of the skin under the semi-occlusive dressing (Wolf et al.,
1956).
Application of approximately 0.05 to 0.5 ml of liquid xylenes
(individual isomers or undefined composition) to the rabbit eye was
reported to cause immediate discomfort and blepharospasm followed by
slight conjunctival irritation and very slight, transient corneal
necrosis (Wolf et al., 1956). Application of 0.1 ml liquid xylene
(not defined) was mildly irritant to rabbit eye. The lesions were not
described (Kennah et al., 1989).
No skin sensitization studies have been reported.
7.5 Reproductive and developmental toxicity
A summary of reproductive and developmental toxicity studies is
shown in Table 7.
Groups of female rats (CFY) were exposed to 0, 34, 345 or 690 ppm
o-xylene 24 h/day from day 7 to day 14 of gestation. The dams were
killed on day 21. Some ultrastructural changes in the liver and
decreased weight gain during the exposure period were observed in the
dams exposed to 345 or 690 ppm. At these concentrations lower fetal
body weight was observed, and at the highest dose level delayed
skeletal ossification was noted. There was no evidence of
malformations. Similar results were obtained when the animals were
exposed to m-xylene. With p-xylene there were signs of delayed
skeletal ossification at exposure levels showing no maternal toxicity
(at 34 and 345 ppm). At 690 ppm, increased postimplantation loss of
fetuses and more retarded fetuses were observed (Ungvary et al.,
1980).
Table 7. Reproductive and developmental effects (inhalation studies)
Compound Species Exposure Duration NOEL LOEL Endpoint Reference
o, m, p-Xylene rat 0, or 1000 24 h/day; Fused sternebrae, Hudak & Ungvary
mg/m3 days 9-14 extra ribs (1978)
Xylene rat 0, 10, 50 or 6 h/day; Maternal toxicity not Mirkova et al.
(not specified) 500 mg/m3 days 1-21 addressed (1983)
Xylene rat 0, 250, 1900 24 h/day; 250 mg/m3 Skeletal retardation Ungvary & Tatrai
(not specified) or 3400 days 7-15 (mothers and (1985)
mg/m3 offspring)
o-xylene rat 0, 150, 1500 24 h/day; 150 mg/m3 150 mg/m3 Decreased fetal weight, Ungvary et al.
or 3000 days 7-14 offspring (dams) skeletal retardation (1980)
mg/m3 1500 mg/m3
(offspring)
m-xylene rat 0, 150, 1500 24 h/day; 1500 mg/m3 3000 mg/m3 Maternal: reduced food Ungvary et al.
or 3000 days 7-14 (dams and (dams and consumption, reduced (1980)
mg/m3 offspring) offspring) weight gain
Offspring: reduced fetal
weight
p-xylene rat 0, 150, 1500 24 h/day; 150 mg/m3 Reduced mean litter Ungvary et al.
or 3000 day 7-15 (dams and size; reduced fetal (1980)
mg/m3 offspring) weight; skeletal
retardation
p-xylene rat 0 or 3000 10th day, Decreased fetal weight Ungvary et al.
mg/m3 24 h (1981)
Table 7. (Cont'd)
Compound Species Exposure Duration NOEL LOEL Endpoint Reference
p-xylene rat 0, 3500 or 6 h/day; 7000 7000 mg/m3 Maternal: reduced Rosen et al.
7000 mg/m3 days 7-16 mg/m3 (dams) weight gain (1986)
(offspring),
3500
mg/m3
(dams)
Xylene rat 0 or 600 24 h/day; Decreased maternal Ungvary (1985)
(not defined) mg/m3 day 7-15 weight gain.
Delayed fetal
development; extra ribs
Xylene rat 870 mg/m3 6 h/day; No maternal toxicity; Hass & Jakobsen
(not defined) days 4-20 delayed ossification (1993)
o, m, p-Xylene rat 2175 mg/m3 6 h/day; Delayed righting reflex; Hass et al. (1995)
days 7-20 reduced absolute brain
weight; impaired
neuromotor ability
Xylene mouse 0, 500 or 24 h/day; 500 mg/m3 1000 mg/m3 Skeletal retardation and Ungvary & Tatrai
(not specified) 1000 mg/m3 days 6-15 offspring (offspring) increased incidence of (1985)
weight-retarded fetuses
Xylene rabbit 0, 500 or 24 h/day; 500 mg/m3 Maternal: decreased Ungvary & Tatrai
(not specified) 1000 mg/m3 days 7-20 (offspring) weight gain (1985)
1000 mg/m3 Offspring: delayed
(dams) skeletal development
Rats (CFY) were exposed to 0.58, 437 or 782 ppm 24 h/day on days
7 to 15 of gestation and the dams were killed on day 21. Data
concerning maternal toxicity was not given. There was delayed
skeletal ossification at all dose levels, while decreased fetal
bodyweight, increased postimplantation loss and increased frequency of
skeletal variants (extra ribs) were observed at 782 ppm. Mice (CFLP)
were exposed to 0 or 115 ppm o-xylene for 4 h, 3 times per day on
day 6 to day 15 of gestation, and the dams were killed on day 18.
Data concerning maternal toxicity were not reported. There was
evidence of delayed weight gain and skeletal ossification in the
fetuses of exposed animals. Similar results were obtained with
m-xylene and p-xylene (Ungvary & Tatrai, 1985). When rabbits (New
Zealand White) were exposed to 0 or 115 ppm o-xylene 24 h/day from
day 7 to day 20 of gestation, no maternal toxicity or incidence of
delayed development was observed in the exposed group. Similar
results were observed with m-xylene, but an increased incidence of
post-implantation loss was observed. Exposure to 115 ppm p-xylene
gave the same results as with o-xylene, but exposure to 230 ppm
p-xylene 24 h/day resulted in no live fetuses (one dam died, three
aborted and in four there was total resorption or fetal death in
utero) (Ungvary & Tatrai, 1985).
In a study to validate a developmental toxicity screen, mice
(ICR/SIM) were exposed orally to 0 or 2000 mg m-xylene/kg body
weight from day 8 to day 12 of gestation. No effects were seen on
mothers or young (Seidenberg et al., 1986). In another study
Sprague-Dawley rats were exposed to 0, 800 or 1600 ppm p-xylene, 6 h
per day from day 7 to day 16 of gestation. The dams were allowed to
deliver their young. In the highest dose group the maternal weight
gain was significantly reduced. Exposure to p-xylene had no effects
on postnatal viability, offspring growth or function of the nervous
system (activity level and acoustic startle response) at any of the
doses tested (Rosen et al., 1986).
In an attempt to study the effect of xylene on sex steroids
during pregnancy, rats (CFY) were exposed to 0 or 681 ppm p-xylene
for 24 h on day 10 of gestation or continuously on days 9 and 10 of
gestation. The animals were killed on day 11. Data on maternal
toxicity were not reported. Sex hormone levels in the uterine and
femoral veins were decreased in the exposed group. The authors
(Ungvary et al., 1981) suggested that this may play a role in the
embryotoxicity.
Rats (CFY) were exposed to 0 or 230 ppm xylene for 24 h/day from
day 9 to day 14 of gestation, and the dams were killed on day 21. The
xylene used was 10% o-xylene, 50% m-xylene, 20% p-xylene and 20%
ethylbenzene. No maternal effects were seen in exposed animals.
There was an increased incidence of skeletal variants such as extra
ribs and fused sternebrae. Three malformations were found (2
agnathia, 1 fissura sterni), but there was no significant increase in
the frequency of malformations (Hudak & Ungvary, 1978).
In another study rats (CFY) were exposed to 0 or 138 ppm xylene
(not defined) 24 h/day from day 7 to day 15 of gestation. The dams
were killed on day 21. In the exposed group, decreased maternal
weight gain, delayed fetal development and increased incidence of
skeletal variants (extra ribs) were observed, but there was no
evidence of malformations (Ungvary, 1985).
In a report issued by the American Petroleum Institute in 1983,
described by Bell et al. (1992), Sprague-Dawley rats were exposed to a
xylene mixture of 20.4% o-xylene, 44.2% m-xylene, 20.3% p-xylene
and 12.8% ethylbenzene. There were 30 males and 60 females in the
control group, while 10 males and 20 females per group were exposed to
60 or 250 ppm xylene and 20 males and 40 females were exposed to 500
ppm xylene. Exposures were 6 h per day, 7 days per week, for a
131-day pre-mating period and a 20-day mating period. Mated females
were also exposed during days 1-20 of gestation and days 5-20 of
lactation. For males there were no effects on body weight gain, but
for females the mean body weight gain was significantly greater than
that of controls in the 60 and 250 ppm groups during the mating
period. This was not considered indicative of an adverse effect of
treatment. Mating indices were significantly lower than control
values at 250 ppm (both sexes treated) and at 500 ppm (females treated
only), but mating indices were comparable to control values at 500 ppm
when both sexes were treated or when males alone were treated. There
were no treatment-related effects on mean duration of gestation, mean
litter size or pup survival. The mean pup weight in the group where
both parents had been exposed to 500 ppm xylene was significantly
lower than for controls. In the teratogenicity part of the study
there was no evidence of an increased incidence of malformations in
exposed groups. The mean fetal weights for the 500 ppm group were
lower than the control value, but, the difference was statistically
significant for only the female fetuses.
Groups of Wistar rats were exposed to 0, 2, 11 or 114 ppm xylene
(mixed, not defined) for 6 h per day from day 1 to day 21 of
gestation. The maternal toxicity was not reported. The incidence of
post-implantation loss and fetal death was significantly increased in
the 11 ppm and 114 ppm groups. At the highest dose an increase in
some malformations was noted but no incidence was given. The results
of the study cannot be evaluated because of insufficient reporting of
exposure conditions and results (Mirkova et al., 1983). In an attempt
to repeat this study, groups of 36 Wistar rats were exposed to 200 ppm
technical xylene 6 h per day on days 4 to 20 of gestation. There were
no signs of maternal toxicity. No exposure-related differences were
found except for delayed ossification of maxillary bone. The
xylene-exposed pups had a slightly higher body weight and impaired
performance in a motor ability test, which was most marked in female
offspring (Hass & Jakobsen, 1993).
In a follow-up study (Hass et al., 1995), Wistar rats were
exposed to 500 ppm technical xylene (19% o-xylene, 45% m-xylene,
20% p-xylene and 15% ethylbenzene) for 6 h per day on gestation days
7-20. The dose level was selected so as not to induce maternal
toxicity or decrease the viability of offspring. There were 15
exposed litters and 13 control litters. A delay in the development of
the air righting reflex, a lower absolute brain weight and impaired
performance in behavioural tests for neuromotor abilities, learning
and memory were found in the offspring of the exposed rats.
Generally, the effects were most marked in the female offspring. The
alterations were long-lasting, as they were still apparent in adult
rats at the age of 4 months.
A composition of 9.1% o-xylene, 60.2% m-xylene, 13.6%
p-xylene and 17.0% ethylbenzene was given orally to CD-1 mice. The
doses were 0, 520, 1030, 2060, 2580, 3100 or 4130 mg per kg body
weight daily from day 6 to day 15 of gestation. All animals in the
highest dose group died and so did 50% of the animals in the 3100
mg/kg group. In this group there was a significant increase in the
incidence of dams with complete resorptions. There was a significant
increase in the incidence of cleft palate at > 2060 mg/kg, as well
as decreased mean fetal weight (Marks et al., 1982).
In order to study the teratogenic and embryotoxic effects of
xylene (60% p-xylene, 22% o-xylene, 18% ethylbenzene; no
m-xylene) embryos of Sprague-Dawley rats were explanted on day 9.5
of gestation and cultured in rat serum to which xylene (0.1, 0.5 or
1.0 ml/litre) dissolved in DMSO was added. The embryos were cultured
for 48 h. There were no observable teratogenic effects in terms of
malformations. However, dose-dependent embryotoxicity in terms of
retardation on growth and development was observed (Brow-Woodman et
al., 1991). In a similar study (Brown-Woodman et al., 1994) rat
embryos were incubated in vitro with up to 2.7 µmol xylene/ml for
40 h. Concentrations of > 1.89 µmol/ml retarded embryo growth and
development. The no-observed-effect level (NOEL) was 1.08 µmol/ml.
No gross morphological malformations were observed. Combined exposure
to toluene, xylene and benzene caused additive embryotoxic effects
with no evidence of synergistic action (Brown-Woodman et al., 1994).
When Sprague-Dawley rats were exposed to 1000 ppm xylene (not
defined) 18 h per day, 7 days per week, for 61 days, testicular
atrophy or loss of nerve growth factor-immunoreactive germ cell line
was not observed. Xylene also was found to protect from
n-hexane-induced testicular atrophy (Nylén et al., 1989).
Wistar rats were exposed for 7 days to xylene (isomer not stated)
twice a day until disappearance of righting reflex (xylene
concentration not given). Anaesthesia was achieved in about 10 min.
On day 8 the rats were killed. A decrease in body weight and weights
of testes and accessory reproductive organs, as well as reduced acid
phosphate activity in the prostate and reduced plasma testosterone
levels, was observed in xylene-exposed animals. There was also a
decrease in spermatozoan count in the epididymis (Yamada, 1993).
7.6 Mutagenicity and related end-points
Technical grade xylene did not produce differential killing in
DNA-repair-proficient compared to repair-deficient strains of
Bacillus subtilis rec+/- (McCarroll et al., 1981a) or Escherichia
coli (McCarroll et al., 1981b). Xylene (type not specified) did not
induce SOS activity in Salmonella typhimurium TA1535/pSK 1002
(Nakamura et al., 1987). For E. coli WP2 uvr A p-xylene was not
mutagenic in the presence or absence of an exogenous metabolic system
from PCB-induced rat liver (Shimizu et al., 1985). None of the
isomers nor unspecified xylene was mutagenic to S. typhimurium
TA1535, TA1537, TA98, TA100, UTH 8413 or UTH8414 in the presence or
absence of a metabolic system from uninduced or Arochlor-induced rat
and hamster livers (Lebowitz et al., 1979; Bos et al., 1981; Haworth
et al., 1983; Connor et al., 1985; Shimizu et al., 1985; Zeiger et
al., 1987).
Exposure to technical grade xylene containing 18.3% ethylbenzene
caused recessive lethal mutations in Drosophila melanogaster but not
exposure to m-xylene, or o-xylene (Donner et al., 1980). The same
report stated that exposure to rats for 300 ppm, 6 h per day, 5 days
per week for 9, 14 and 18 weeks did not induce chromosomal aberrations
in bone-marrow cells (Donner et al., 1980). Xylene (unspecified) did
not induce mutations in mouse lymphoma L5178Y TK+/- cells in vitro
or chromosomal aberrations in rat bone marrow cells (Lebowitz et al.,
1979). Xylene (unspecified) did not induce sister chromatid exchange
or chromosomal aberrations in human lymphocytes in vitro. No
exogenous metabolic system was used in this study (Gerner-Smidt &
Friedrich, 1978).
None of the isomers induced micronuclei in the bone marrow of
male NMRI mice after two i.p. administrations of 105-650 mg/kg body
weight at a 24-h interval, but they did, however, enhance the
induction of micronuclei by toluene (Mohtashamipur et al., 1985).
When Sprague-Dawley rats were given 440 or 1320 mg o-xylene/kg
body weight intraperitoneally, a significant increase in the
percentage of abnormal sperm was reported when the animals were housed
at 24-30°C (but not at 20-24°C). The authors (Washington et al.,
1983) interpreted this as a synergistic effect between o-xylene and
temperature.
7.7 Carcinogenicity
Group of Sprague-Dawley rats, 40 of each sex, received 500 mg
mixed xylenes (composition not specified) per kg body weight in olive
oil by stomach tube on 4 to 5 days per week for 104 weeks. Fifty
animals of each sex received olive oil only. The animals were
maintained until natural death. All animals had died by week 141. At
that time thymomas were reported in 1/34 treated males and 0/36
treated females (compared to 0/45 and 0/49, respectively, in
controls). Other haemolymphoreticular tumours (not specified) were
reported in 4/34 treated males and 3/36 treated females (compared to
3/45 and 1/49, respectively, in controls). The authors (Maltoni et
al., 1983; Maltoni et al., 1985) reported an increase in the total
number of animals with malignant tumours (type not specified) at 141
weeks, e.g., as 13/38 in treated males and 22/40 in treated females
(compared to 11/45 and 10/49, respectively, in control animals).
Combining all tumours is, however, not an acceptable basis for
analysis particularly in aged animals. No data were provided to allow
an analysis on an individual tumour-type basis.
In a carcinogenicity study, groups of 50 B6C3F1 mice of each sex
were given 0, 500 or 1000 mg xylene/kg body weight in corn oil by
stomach tube on 5 days per week for 103 weeks. The xylene used was
9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. The surviving animals were killed within 2 weeks after
the last administration. Survival at the termination of the study for
males was: 27 controls, 35 low-dose and 36 high-dose; and for females
was: 36 controls, 35 low-dose and 31 high-dose. No treatment-related
increase in the incidence of any tumour was seen in either sex (NTP,
1986; Huff et al., 1988).
The NTP also performed a carcinogenicity study on Fischer-344
rats with the same type of technical xylene. Groups of 50 rats of
each sex were given 0, 250 or 500 mg xylene/kg body weight in corn oil
by stomach tube 5 days per week for 103 weeks. The surviving animals
were killed within 2 weeks following the last administration. At the
termination of the experiment, the survival for males was: 36
controls, 25 low-dose and 20 high-dose animals, and for females was:
38 controls, 33 low-dose and 35 high-dose. For the males survival
appeared to be dose-related, but many of the early deaths were related
to gavage trauma or corn oil-xylene aspiration (3/14, 8/25, 11/30).
The incidences of tumours in treated animals of either sex were not
significantly higher than in the control group (NTP, 1986; Huff et
al., 1988).
Two studies have investigated whether exposure to xylenes alters
the incidence of experimentally induced skin neoplasia in mice (Pound
& Withers, 1963; Pound, 1970). The reporting does not allow any firm
conclusions.
7.8 Other effects
No effects were observed upon in vitro exposure of human
lymphocytes at concentrations up to 2 mM xylene for 72 h. However, at
higher concentrations, cell mortality only was significantly increased
(Richer et al., 1993).
8. EFFECTS ON HUMANS
8.1 Acute and accidental exposure
Acute poisoning and deaths have been reported after overexposure
or oral ingestion of substantial amounts of xylene. The exposure
level required for loss of consciousness has been estimated to be
10 000 ppm (Morley et al., 1970). At autopsy pulmonary congestion and
oedema have been observed after inhalation or oral intake (Morley et
al., 1970; Abu Al Ragheb et al., 1986). Among survivors coma, EEG
changes, amnesia, mental confusion and ocular nystagmus have been
reported. Evidence of gastrointestinal and respiratory symptoms as
well as impaired renal and hepatic function have also been observed
(Ghislandi & Fabiani, 1957; Recchia et al., 1985; Bakinson & Jones,
1985). After exposure to about 700 ppm (calculated) for up to one
hour, headache, nausea, irritation of the eyes, nose and throat,
dizziness, vertigo and vomiting have been reported (Klaucke et al.,
1982). Recovery seems to be complete in most non-fatal cases although
dizziness and vision problems have been observed 24 h after ingestion
of xylene (quantity unknown) (Recchia et al., 1985). In a suicidal
attempt a 30-year-old man injected 8 ml of xylene intravenously.
After 10 min he developed a life-threatening acute pulmonary failure,
but survived through medical treatment (Sevcik et al., 1992).
8.2 Controlled human studies
A number of volunteer studies have been performed predominantly
at the Finnish Institute of Occupational Health. Effects on the
sensory motor and information process functions of the central nervous
system (CNS) have been investigated. Usually m-xylene has been
used, but p-xylene and mixed xylenes have also been studied
(Savolainen & Linnavuo, 1979; Savolainen, 1980; Savolainen et al.,
1980; Seppäläinen et al., 1981; Savolainen & Riihimäki, 1981;
Savolainen et al., 1981; Savolainen et al., 1982a, Savolainen et al.,
1982b; Savolainen et al., 1984; Savolainen et al., 1985a, Savolainen
et al., 1985b). In these studies no significant effects on vestibular
or visual function, reaction times, coordination or peripheral senses
were observed during a 4-h exposure to a constant concentration of up
to 160 ppm. Slight impairment of vestibular and visual function and
reaction time was noted at exposure levels from 200 to 300 ppm. There
was adaption to the impairment over five successive daily exposures.
In another study (Anshelm Olson et al., 1985), volunteers (n=16) were
exposed for 4 h to p-xylene alone (300 mg/m3: 70 ppm) or in
combination with toluene (200 mg/m3 toluene plus 100 mg/m3
p-xylene). Heart rate, subjective symptoms, simple reaction time,
choice reaction time and short-term memory were unaffected by
exposure.
In another study nine male volunteers were exposed for 4 h to 200
ppm (TWA) m-xylene. Short-term peak exposures were up to 400 ppm.
The effects of xylene on electroencephalography (EEG) were minor and
no deleterious effects were noted (Seppäläinen et al., 1991).
Healthy male subjects were exposed to technical xylene,
containing 40% ethylbenzene, for 2 h with or without a working load of
100 watts. The air concentration was 435 or 1300 mg/m3. During work
at the higher exposure level evidence of performance decrement was
observed in three of the five performance tests: reaction time
addition test (p < 0.05), short-term memory (p < 0.05) and choice
reaction time (p < 0.10) (Gamberale et al., 1978).
Dizziness was reported by four of six volunteers exposed to 690
ppm p-xylene for 15 min (Carpenter et al., 1975). Nine volunteers
were exposed for about 4 h to either a constant or a fluctuating
pattern of m-xylene with a time-weighted average exposure
concentration of 200 ppm in both cases (Laine et al., 1993).
Prolonged simple visual and auditive choice reaction times were
observed after exposure to the peaks of 400 ppm m-xylene. Exposure
to m-xylene at a constant level of 200 ppm did not affect the ratio
of "active" to "quiet" sleep during the following night, but decreased
slightly the number of body movements in bed.
Studies on coexposure to m-xylene and ethanol (Savolainen,
1980; Seppäläinen et al., 1981; Savolainen & Riihimäki, 1981;
Riihimäki et al., 1982a,b; Riihimäki et al., 1982a,b), m-xylene and
1,1,1-trichloroethane (TCE) (Savolainen et al., 1981; Savolainen et
al., 1982) or p-xylene and toluene (Anshelm-Olson et al., 1985) have
been performed. Exposure to 145-150 ppm xylene and 0.8 g ethanol/kg
body weight had an additive disturbing effect on vestibular function.
At higher xylene concentrations (275-290 ppm) there was evidence of
functional tolerance, and xylene appeared to antagonize the effect of
ethanol on vestibular function. In a study where volunteers were
exposed to 200 ppm m-xylene, minor effects on vestibular and visual
functions and reaction time were reported. Simultaneous exposure to
400 ppm TCE had no further effect. Combined exposure to 60 ppm
p-xylene and 30 ppm toluene had no effect on reaction time,
short-term memory or heart rate.
Ten male volunteers were exposed to 100 ppm xylene (not
specified) or 100 ppm toluene or a mixture of 50 ppm of each.
Exposure time was 4 h and each person participated in four exposure
sessions. Changes in CNS functions were tested by nine psychological
tests. Xylene had the most adverse effect on simple reaction time and
choice reaction time, while the combined exposure gave weaker effects
than xylene alone but stronger than toluene alone (Dudek et al.,
1990).
A summary of acute effects from inhalation exposure is presented
in Table 8.
In studies on skin irritation, both hands of subjects were
immersed in pure m-xylene (Engström et al., 1977; Lauwerys et al.,
1978; Riihimäki, 1979a). A burning sensation was soon noticed and an
erythematous reaction was observed in the exposed skin. Of six
subjects tested, four reported eye irritation after exposure to 460 or
690 ppm xylene for 15 min. The xylene contained 7.6% o-xylene,
65.0% m-xylene, 7.8% p-xylene and 19.3% ethylbenzene. One subject
reported eye irritation at 230 ppm but none at 110 ppm (Carpenter et
al., 1975). In another study, no irritation in the eyes, nose or
throat was reported after exposure to 98, 196 or 392 ppm mixed xylenes
for 30 min (Hastings et al., 1984). In an older study exposure to 200
ppm xylene (undefined) for 3-5 min caused irritation of the eyes, nose
and throat (Nelson et al., 1943).
The skin sensitization potential was investigated using a
non-adjuvant maximization test. The xylene used was not defined.
None of 24 subjects tested showed evidence of sensitization (Kligman,
1966).
Five adult, healthy, white men were exposed for 7 h per day for 3
days to 40 ppm xylene. This exposure was repeated three times at
intervals of 2 weeks. Blood samples were taken before and after each
exposure. No significant effects were observed on sister-chromatid
exchange frequency, cell cycle time or cell mortality in lymphocytes
(Richer et al., 1993).
8.3 Occupational exposure
In workers exposed to xylene or solvent mixtures containing large
amounts of xylene, subjective symptoms have been reported (Joyner &
Leak Pegues, 1961; Glass, 1961; Hipolito, 1980; Kilburn et al., 1983;
Kilburn et al., 1985). Depression, fatigue, headache, anxiety,
feeling of drunkenness and sleep disorders were the most common
symptoms reported, but exposure levels and duration were often missing
in these reports.
Workers occupationally exposed to solvent mixtures including
xylene have been reported to have neurophysiological and psychological
disorders (Lindström, 1973; Seppäläinen et al., 1978; Elofsson et al.,
1980; Husman, 1980; Husman & Karli, 1980; Arlien-Soborg et al., 1981;
Lindström et al., 1982; Valciukas et al., 1985; Maizlish et al., 1987;
Van Vliet et al., 1987; Ruijten et al., 1994). In these studies there
was no exposure to xylene alone. Xylene was not the main solvent in
the mixture. No conclusions concerning effects of xylenes as such can
be drawn from these studies.
Table 8. Single inhalation exposure to xylene in humans
Exposure Time Effect Reference
concentration
(mg/m3)
3 000 1 h Dizziness, irritation Klaucke et al. (1982)
3 000 15 min Dizziness Carpenter et al. (1975)
1300a 2 h Performance decrement Gamberale et al. (1978)
900b 4 h Prolonged reaction times Laine et al. (1993)
900 4 h Impairment of vestibular and visual Savolainen et al.
function and prolonged reaction time (1979, 1981, 1982, 1985)
900 4 h Minor effect on EEG Seppäläinen et al. (1991)
600 4 h No effect on reaction time Savolainen et al. (1980, 1981)
450 4 h Prolonged reaction time Dudek et al. (1990)
300 4 h No effects in psychophysiological test Anshelm - Olson et al. (1985)
a during exercise
b peak values of 1800 mg/m3
Liver effects have been reported in some studies on workers
occupationally exposed to solvents containing xylene (Dössing et al.,
1981; Edling, 1982; Sotaniemi et al., 1982; Dössing et al., 1983;
Fischbein et al., 1983, Lundberg et al., 1994) but these were not
confirmed in other studies (Craveri et al., 1982; Kurppa & Husman,
1982; Lundberg & Håkansson, 1985). Recent findings seem to support
the concept that the hepatotoxicity of xylene is low (Riihimåki &
Hännien, 1987).
Some case studies and epidemiological studies have addressed a
possible association between occupational exposure to hydrocarbons
(including xylene) and proliferative glomerulonephritis (Beirne &
Brennan, 1972; Zimmerman et al., 1975; Lagrue, 1976). Exposures
have, however, been so diverse that the role of xylene is impossible
to assess. A Swedish group of researchers reported a higher
concentration of albumin, erythrocytes and leukocytes in the urine of
workers exposed predominantly to xylenes and toluene than among
controls (Askergren et al., 1981a,b,c; Askergren, 1981). Franchini et
al. (1983) found that painters exposed to toluene and xylenes at
relatively low concentrations had a higher excretion of kidney tubular
enzymes in their urine than the controls. They suggested that the
mixed solvents may exert a slight adverse effect on the kidney
tubules.
One case of contact urticaria due to occupational exposure to
airborne xylene has been reported. The level of xylene in air
exceeded 100 ppm. Direct skin contact with the solvent appeared to
have been negligible. The contact urticaria seemed likely to be an
immunological type (Palmer & Rycroft, 1993).
Thirty-five male spray varnishers were exposed to 0.5-3.4 ppm
o-xylene, 3.2-11.7 ppm m-xylene, 0.9-4.3 ppm p-xylene, 1.4-7.5
ppm ethylbenzene, < 1.5 ppm toluene, < 1.2 ppm n-butanol, < 35.5
ppm 1,1,1-trichloroethane and several C9 aromatics. In addition, some
of the lacquers contained lead pigments. The mean peripheral
erythrocyte counts and haemoglobin levels were decreased in the
exposed men compared to controls. Whether these effects were due to
xylene or the solvent mixture is uncertain (Angerer & Wulf, 1985).
The health effects on 175 factory workers in China exposed to
xylene vapour with a mixture of three isomers at a concentration of up
to 175 ppm (with time-weighted average geometric mean concentration of
14 ppm and arithmetic mean of 21 ppm) were studied. There was an
increased prevalence of subjective symptoms in the exposed workers;
these were apparently related to effects on the central nervous system
and to local effects on the eye, nose and throat (Uchida et al.,
1993). In workers exposed to a maximum concentration of 103 ppm
xylene (geometric mean 4 ppm) and 203 ppm toluene (geometric mean 3
ppm), the prevalence of some subjective CNS-related symptoms was
higher than in the controls (Chen et al., 1994). No effects on
haematology or serum biochemistry with respect to liver and kidney
functions were observed in these two studies.
Sister-chromatid exchanges (SCE) in peripheral lymphocyte
cultures have been studied in two groups of 23 workers who had been
exposed for between 4 months and 23 years to mixed xylenes (including
ethylbenzene). The exposure levels for the two groups were 11 and 13
ppm, respectively. No differences in SCE frequency was seen (Pap &
Varga., 1987). A few other reports have described increased incidence
of chromosomal aberrations or effects on sister-chromatid exchange
frequencies (Funes-Cravioto et al., 1977; Haglund et al., 1980). In
both these studies the exposure to xylene (not defined) was
accompanied by exposure to other solvents, including benzene.
No data on carcinogenic effects resulting from exposure to
xylenes have been found in the literature.
In an investigation into the effect on reproduction, the outcome
of pregnancy was studied among university laboratory employees exposed
to xylene (not defined) during the first trimester of pregnancy.
Exposure levels were not given. There was no difference in
miscarriage rate when compared to controls not exposed to solvents
(Axelsson et al., 1984).
A similar case control study on associations between laboratory
work and pregnancy outcome revealed that use of xylene for 3 or more
days a week during the first trimester was significantly associated
with an elevated risk of spontaneous abortion. The authors pointed
out, however, that laboratory workers were often exposed to several
solvents and chemicals simultaneously; only two cases and two controls
were exposed to xylene alone (Taskinen et al., 1994). About half of
the women exposed to xylene worked in pathology/histology laboratories
where there was concomitant exposure to formaldehyde vapour.
Formaldehyde (formalin) also appeared to be a significant risk factor
for spontaneous abortion. Exposure to xylene was also noted to be
associated with an increase in birth weight (Taskinen et al., 1994).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Bringmann & Kuhn (1977, 1978) exposed the bacterium Pseudomonas
putida for 16 h and the blue-green alga Microcystis aeroginosa for
8 days to xylene. They found a reduction in cell multiplication at
concentrations of >200 mg/litre.
Walton et al. (1989) studied the effect of p-xylene on the
microbial respiration of two soil types, a silt loam (1.49% organic
carbon) and a sandy loam (0.66% organic carbon). The chemical was
applied at a rate of 1000 µg/g (dry weight). Microbial respiration,
as measured by CO2 efflux, of the silt loam was unaffected. In the
sandy loam, the CO2 efflux initially decreased and then increased,
but returned to control levels within the 6-day exposure period.
All three isomers have been shown to inhibit the respiration of
sewage sludge utilizing biogenic substrates. Two screening tests were
used, RIKA (respiration inhibition kinetic analysis) and OECD 209.
The concentration of each compound used was at the limit of the
solubility in the medium (approx. 175-198 mg/litre). Inhibitions in
the respiration rate of 100% were found for all three isomers in the
RIKA screening test, and inhibitions of 22% ( o-xylene) and 43%
( p-xylene) were measured in the OECD 209 screening test. m-Xylene
was only tested at a concentration of 0.3 mg/litre in the OECD 209
screening test, which resulted in 5% inhibition (Volskay & Grady,
1990).
The toxicity of xylene to three species of environmental bacteria
has been determined using assays in sealed serum bottles to prevent
loss of chemical by volatilization. Activity of the bacteria was
measured by either gas production over 48 h (methanogens), oxygen
consumption over 15 h (aerobic heterotrophs) or ammonia use over 24 h
(Nitrosomonas). IC50 values (the concentration required to inhibit
the bacterial activity by 50%, as compared with controls) for xylene
were 250 mg/litre for methanogens, 1100 mg/litre for aerobic
heterotrophs and 100 mg/litre for Nitrosomonas (Blum & Speece,
1991).
9.1.2 Aquatic organisms
Xylene isomers are highly volatile and disappear rapidly from
solution. For example, Mackay & Wolkoff (1973) found that in agitated
water, 1m deep and with a 1m2 surface for evaporation, the half-life
for o-xylene was 39 min. When Benville & Korn (1977) monitored the
loss of xylene from solution during LC50 tests, average percentage
losses for the four time intervals studied (24, 48, 72 and 96 h) were
29, 61, 84 and > 99% respectively. These losses mean that the
exposure can be difficult to determine. For example, many of the 24-h
and 96-h LC50 values are the same or similar, suggesting that most of
the xylene had been lost during the test. Galassi et al. (1988),
using a closed static system, found mean measured concentrations of
xylene and other aromatics to fluctuate by only 10% during the test
period of up to 96 h. Care must therefore be taken when interpreting
data from open static tests over longer periods than 24 h, especially
those based on nominal concentrations. Overall it can be stated that
xylene has moderate to low acute toxicity for aquatic organisms.
9.1.2.1 Algae
Bringmann & Kühn (1977) exposed the green alga Scenedesmus
quadricauda for 8 days to xylene. They found reduction in cell
multiplication at concentrations of > 200 mg/litre. Brooks et al.
(1977) studied the effect of xylene on photosynthesis in a mixed ocean
culture of phytoplankton. They found that exposure to a concentration
of 3 mg/litre xylene for 8 h caused a 50% reduction in photosynthesis.
Galassi et al. (1988) calculated the 72-h EC50 for growth
inhibition in the alga Selenastrum capricornutum to be 4.7, 4.9 and
3.2 mg/litre for the ortho, meta and para isomers, respectively.
Similar results were obtained by Herman et al. (1990). Using the same
species of algae, they obtained 8 day EC50 values for growth
inhibition of 4.2, 3.9 and 4.4 mg/litre for the ortho, meta and para
isomers, respectively. Sheedy et al. (1991) reported a 14-day EC50
for growth inhibition of Selenastrum capricornutum of 72 mg/litre
for xylene (composition not stated).
Hutchinson et al. (1980) exposed the algae Chlamydomonas
angulosa and Chlorella vulgaris to p-xylene for 3 h. EC50
values for inhibition of photosynthesis (measured using 14CO2
uptake) were 45.7 and 105.1 mg/litre for the two species,
respectively. Kauss et al. (1973) exposed the green alga Chlorella
vulgaris to o-xylene and studied growth over a 10-day period in an
open system. At nominal concentrations of between 25 and 100
mg/litre, a short-term toxic effect was observed. However, the algal
culture recovered within 2 days. The authors pointed out that
recovery was probably due to volatilization of the chemicals. A
near-saturation concentration of 171 mg o-xylene/litre proved to be
acutely toxic and the alga did not recover. Concentrations of 25 and
50 mg o-xylene/litre progressively increased the lag period between
initial inoculation and growth. An o-xylene concentration of 100
mg/litre delayed the onset of growth for 4 days and a near-saturation
concentration of 171 mg/litre caused complete inhibition of growth
during the 10-day exposure period (Kauss & Hutchinson, 1975).
Dunstan et al. (1975) exposed marine microalgae, the diatom
Skeletonema costatum, the dinoflagellate Amphidinium carterae, the
coccolithophorid Cricosphaera and the green flagellate Dunaliella
tertiolecta, to xylene for 3 days. A xylene concentration of 10
mg/litre inhibited the growth of all species. Inhibition was most
marked in A carterae and S. costatum.
9.1.2.2 Higher plants
Frank et al. (1961) exposed the angiosperm pondweeds Elodea
canadensis, Potamogeton nodosus and P. pectinatus to xylene
(plus 2% emulsifying agent) under static, open conditions for a period
of four weeks. A concentration of 100 mg/litre was found to be toxic
(8.6 on an injury scale of 0 to 10) but 5 mg/litre was not. Both 300
and 600 mg/litre were similarly toxic after 30-min exposures in
flowing water.
9.1.2.3 Protozoa
Bringmann et al. (1980) exposed the flagellate protozoan
Chilomonas paramaecium to xylene for 48 h and found an initial
reduction (5%) in cell multiplication at concentrations of > 80
mg/litre. Rogerson et al. (1983) exposed the ciliate protozoan
Colpidium colpoda to xylene in an "open" system consisting of a
covered watchglass with an air space. Toxicity thresholds were 1.75
mg/litre (16.5 mmol/m3) for o-xylene and 162 mg/litre (1530
mmol/m3) for m-xylene; no threshold was calculated for p-xylene.
The same authors exposed the protozoan Tetrahymena ellioti to xylene
isomers in a closed system with no air space. Toxicity thresholds for
o-, p- and m-xylene were 18.5, 16.9 and 55.7 mg/litre (174, 159
and 525 mmol/m3), respectively.
9.1.2.4 Invertebrates
The LC50 values of xylene to aquatic invertebrates are
summarized in Table 9.
Le Gore (1974) exposed Pacific oyster larvae (Crassostrea
gigas) to o- and p-xylene for 48 h. LC50 values were 0.17 and
0.58 mg/litre for the two isomers, respectively, suggesting that this
organism is one of the more sensitive invertebrates to xylene
exposure. However, no experimental details were given for these
toxicity tests, which makes them difficult to interpret.
Table 9. LC50 values of xylenes to aquatic invertebrates
Organisms Size/ Static/ Open/ Temp. Hardness Isomer Duration Concentration Reference
life-stage flowa closeda (°C) (mg/litre)c (mg/litre)d
Estuarine and marine invertebrates
Pacific oyster larvae ortho 48 h 0.17 Le Gore (1974)
(Crassostrea larvae para 48 h 0.58 Le Gore (1974)
gigas)
Grass shrimp static c 21 15s 96 h 7.4 Neff et al. (1976)
(Palaemonetes static c 21 15s 24 h 14.0 Tatem et al. (1978)
pugio) 96 h 7.4 Tatem et al. (1978)
Bay shrimp adult static o 16 25s ortho 24 h 4.7 n Benville & Korn (1977)
(Crago adult static o 16 25s ortho 96 h 1.1 n Benville & Korn (1977)
franciscorum) adult static o 16 25s meta 24 h 4.1 n Benville & Korn (1977)
adult static o 16 25s meta 96 h 3.2 n Benville & Korn (1977)
adult static o 16 25s para 24 h 1.7 n Benville & Korn (1977)
adult static o 16 25s para 96 h 1.7 n Benville & Korn (1977)
Dungeness crab zoeae static+ 13 30s ortho 48 h 38 n Caldwell et al. (1977)
(Cancer zoeae static+ 13 30s ortho 96 h 6 n Caldwell et al. (1977)
magister) zoeae static+ 13 30s meta 48 h 33 n Caldwell et al. (1977)
zoeae static+ 13 30s meta 96 h 12 n Caldwell et al. (1977)
Table 9. (Cont'd)
Organisms Size/ Static/ Open/ Temp. Hardness Isomer Duration Concentration Reference
life-stage flowa closeda (°C) (mg/litre)c (mg/litre)d
Freshwater invertebrates
Water flea static o 24 h >100<1000 n Dowden & Bennett (1965)
(Daphnia magna) static 24 h 165 n Brigmann & Kühn (1982)
static c ortho 24 h 1 m Galassi et al. (1988)
static c meta 24 h 4.7 m Galassi et al. (1988)
static c para 24 h 3.6 m Galassi et al. (1988)
4-6 days static c ortho 48 h 3.2 n Bobra et al. (1983)
4-6 days static c meta 48 h 9.6 n Bobra et al. (1983)
4-6 days static c para 48 h 8.5 n Bobra et al. (1983)
< 24 h flow o 17 44.7 ortho 48 h 3.82 m Holcombe et al. (1987)
Mosquito larvae static o 24-26 24 h 13.9 Berry & Brammer (1977)
(Aedes aegypti)
Snail
(Aplexa adult flow o 17 44.7 ortho 96 h >22.4 m Holcombe et al. (1987)
hypnorum)
a static = static conditions (water unchanged for duration of test); b o = open; c = closed
static+ = semi-static conditions (water renewed at 24 hour intervals); c s = salinity (%)
flow = flow-through conditions (xylene concentration in water continuously maintained) d m = measured; n = nominal
Berry & Brammer (1977) exposed mosquito larvae (Aedes aegypti)
to xylene under static, open conditions at 25°C. Larvae were exposed
for 24 h, since no detectable levels of any water-soluble component
remained after that period. A non-lethal concentration of 7.92
mg/xylene litre was reported. However, the authors found that
bioassays using different volumes of solution and different sized
containers demonstrated the significant effect that surface area,
volume and depth can have on the results of experiments with volatile
hydrocarbons such as xylenes.
Falk-Petersen et al. (1985) exposed sea urchin (Strongy
locentrotus droebachiensis) eggs to o-xylene from fertilization
and monitored deaths, pathology, inhibition of cleavage and
differentiation, and pigment effects. Eggs were maintained in test
beakers covered with aluminium foil. They calculated a 96-h EC50,
based on all these parameters, of 4.1 mg/litre.
Freshwater mussels (Dreissena polymorpha) were exposed to
various concentrations of xylene, and the behaviour of the mussels in
terms of shell valve movements was monitored. At the start of the
experiment, all valves were gaping continuously. After a certain
period of toxicant addition, a gradual increase in valve closure
period was observed. Finally, total closure of shell valves was
observed. Effects were first seen at a xylene concentration of
11.9-19.4 mg/litre (nominal) (Slooff et al., 1983).
9.1.2.5 Vertebrates
The LC50 values of xylene to fish are summarized in Table 10.
Morrow et al. (1975) found that 100 mg xylene/litre killed 100%
of young coho salmon (Oncorhynchus kisutch) within 24 h under
static, closed conditions. Concentrations of 1 and 10 mg/litre did
not cause significant mortality within the 96-h exposure period.
Toxicity included rapid, violent and erratic swimming, "coughing",
loss of equilibrium and death.
Rainbow trout (Oncorhynchus mykiss) significantly avoided
xylene (plus 2% emulsifying agent) at a nominal concentration of 0.1
mg/litre during a 1-h test. Fish exposed to 0.001 mg/litre did not
show significant avoidance and those exposed to 0.01 mg/litre were
significantly attracted to the xylene (Folmar, 1976). Maynard & Weber
(1981) found that juvenile coho salmon were able to significantly
avoid o-xylene concentrations of > 0.2 mg/litre water.
Slooff (1979) exposed rainbow trout Oncorhynchus mykiss to
various concentrations of xylene in a flow-through, closed system and
monitored any effects of the chemical on their breathing. The lowest
concentration at which a toxic condition developed within 24 h after
toxicant administration was 2 mg/litre.
Table 10. LC50 values of xylenes to fish
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Freshwater fish
Fathead minnow 1-2 g static o 25 20 24 h 28.8 n Pickering & Henderson
(Pimephales 1-2 g static o 25 20 48 h 27.7 n (1966)
promelas) 1-2 g static o 25 20 96 h 26.7 n
1-2 g static o 25 360 24 h & 96 h 28.7 n
0.3 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Bluegill 1-2 g static o 25 20 24 h 24 n Pickering & Henderson
(Lepomis 1-2 g static o 25 20 48 h 24 n (1966)
macrochirus) 1-2 g static o 25 20 96 h 20.9 n
1.1 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Goldfish 1-2 g static o 25 20 24 h & 96 h 36.8 n Pickering & Henderson
(Carassius (1966)
auratus)
20-80 g flow 17-19 80 24 h 30.6 m Weber et al. (1975)
20-80 g flow 17-19 80 96 h 16.9 m
3.3 g static o 19-21 ortho 24 h 13 m Bridie et al. (1979)
3.3 g static o 19-21 meta 24 h 16 m
3.3 g static o 19-21 para 24 h 18 m
2.5 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Table 10. (Cont'd)
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Guppy 0.1-0.2 g static o 25 20 24 h & 96 h 34.7 n Pickering & Henderson
(Poecilia (1966)
reticulata)
static meta 14 days 305 Könemann (1981)
static ortho 7 days 291
static para 7 days 285
static c 20-22 ortho 96 h 12 m Galassi et al. (1988)
static c 20-22 meta 96 h 12.9 m
static c 20-22 para 96 h 8.8 m
Rainbow trout static c 11-13 ortho 96 h 7.6 m Galassi et al. (1988)
(Oncorhynchus static c 11-13 meta 96 h 8.4 m
mykiss) static c 11-13 para 96 h 2.6 m
flow o 9-13 89.5 96 h 10e Folmar (1976)
13.1 g flow o 17 44.7 ortho 96 h 8.05 m Holcombe et al. (1987)
0.9 g flow o 12 40-48 technical 24 h & 96 h 13.5 Walsh et al. (1977)
Zebra fish flow c 48 h 20 Sloff (1979)
(Brachydanio
rerio)
Table 10. (Cont'd)
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Golden orfe 1.2-1.8 g static 48 h 86f Juhnke & Lüdemann
(Leuciscus 1.2-1.8 g static 48 h 308f (1978)
idus
melanotus)
White sucker 2.4 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
(Catostomus
commersoni)
Marine fish
Coho salmon young static c 8 30s 24 h > 10 < 100 Morrow et al. (1975)
(Oncorhynchus
kisutch)
Striped bass 6 g static o 16 25s ortho 24 h & 96 h 9.7 n Benville & Korn (1977)
(Morone 6 g static o 16 25s meta 24 h & 96 h 7.9 n
saxatilis) 6 g static o 16 25s para 24 h & 96 h 1.7 n
a static = static conditions (water unchanged for duration of test);
flow = flow-through conditions (xylene concentration in water continuously maintained)
b o = open; c = closed
c s = salinity (°/oo)
d m = measured; n = nominal
e included 2% emulsifying agent
f results from two different laboratories
When Walsh et al. (1977) exposed rainbow trout (average weight
165 ± 86 g) to xylene concentrations of 0.31, 0.65 and 1.1 mg/litre in
artificial streams for 56 days, no adverse effects on the fish were
noted at any concentration. Using the same artificial streams, all
rainbow trout exposed to xylene concentrations of 14.2 or 22.5
mg/litre for 2 h died. Fish exposed to xylene concentrations of 3.2
and 6.2 mg/litre for 2 h showed symptoms similar to anaesthesia.
Kjorsvic et al. (1982) exposed cod eggs ( Gadus morhus L.) to
xylene isomers in covered glass dishes and monitored the effects both
during fertilization and during early cleavage of fertilised eggs.
Both m-xylene and p-xylene induced significant decreases in the
fertilization rate at concentrations above 10 mg/litre. o-Xylene
had no significant effect on the fertilization rate at concentrations
of 16-35 mg/litre. Fertilized eggs were exposed to xylene for 3 or 6
h before first cleavage. No significant difference was observed
between the individual xylene isomers or between the two exposure
periods. Effects on the early cleavage pattern were significant for
xylene concentrations between 2 and 7 mg/litre. The effects seen
included inhibition of formation of the cleavage furrow. Small cells
or a total absence of cleavage occurred on exposure to all isomers at
concentrations of 16-35 mg/litre, while incomplete or uneven cleavage
was found at exposures of 8-15 mg/litre.
Black et al. (1982) exposed embryo-larval stages of the leopard
frog Rana pipiens and rainbow trout (Oncorhynchus mykiss) to
m-xylene from 30 min after fertilization to 4 days after hatching in
a closed, flow-through system (hatching times were 5 days for the frog
and 23 days for the trout). LC50 values of 3.53 and 3.77 mg/litre
were calculated for the two species, respectively.
9.1.3 Terrestrial organisms
Hill & Camardese (1986) exposed Japanese quail (Coturnix
coturnix japonica) to xylene in 5-day dietary toxicity tests. The
LC50 was found to be greater than 20 000 mg/kg diet. No overt signs
of toxicity occurred at 5000 mg/kg.
No studies on terrestrial plants, terrestrial invertebrates or
field effects of xylenes have been reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposures
In the general environment humans are exposed to xylenes mainly
by inhalation. Absorption through the skin may also occur in the
working environment. The retention in the lungs is about 60% of the
inhaled dose. Xylene is efficiently metabolized. More than 90% is
biotransformed to methylhippuric acid, which is excreted in urine.
Xylene does not accumulate significantly in the human body.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3 with levels of 3 µg/m3 in suburban
areas. Higher levels have been measured in urban and industrialized
areas with mean concentrations ranging up to 500 µg/m3; however,
concentrations are generally below 100 µg/m3.
The estimated daily exposure of the general population through
inhalation is 70 µg in rural areas and less than 2000 µg in urban
areas. The concentration in drinking-water ranges from not detectable
to 12 µg/litre. Data on the levels in food are too limited to
estimate daily oral exposure.
10.1.2 Effects
Based on experimental human studies, xylene may have an acute
impairing effect on the sensory-motor and information-processing
functions of the central nervous system (CNS). Exposure to 435-870
mg/m3 (100-200 ppm) of xylene over 4 h caused slight impairments in
reaction time performance and vestibular functions. There was
adaptation at 200 ppm m-xylene to the impairment over five
successive days. The LOAEL of xylene for acute CNS effects, based on
one study, is 470 mg/m3 (108 ppm). It should be noted, however,
that other identical studies found significant effects only at
concentrations of 870 mg/m3 (200 ppm) or more. According to a study
by a different research group, exposure to 304 mg/m3 (70 ppm) p-
xylene for 4 h did not cause impairment of corresponding psycho-
physiological functions; 304 mg/m3 (70 ppm) xylene can therefore
be regarded as the NOAEL for acute CNS effects.
Xylene vapour becomes irritating at relatively high levels.
Among six volunteer subjects, four reported eye irritation after
exposure to 2000 or 3000 mg/m3 (460 or 690 ppm) xylene for 15 min
while one subject reported eye irritation at 1000 mg/m3 (230 ppm) and
none at 478 mg/m3 (110 ppm). According to another study, no
irritation of the eyes, nose or throat was reported after exposure to
423, 852 or 1705 mg/m3 (98, 196 or 392 ppm) mixed xylenes for 30 min.
These human findings are consistent with mouse studies showing that
strong irritancy (respiratory rate decrease by 50%) occurs at about
5960 mg/m3 (1370 ppm) xylene. The odour threshold for xylene is
about 1 ppm.
Subjective symptoms have been reported among workers exposed to
solvent mixtures containing large amounts of xylene. Long-term
exposure to xylene is suspected to affect the nervous system adversely
because chronic toxic encephalopathy and milder functional
disturbances of the brain have sometimes been found among exposed
painters and other workers. Likewise, slight changes in kidney
tubular function may occur. The specific role of xylene in these
effects cannot, however, be ascertained.
When human data are sparse, especially data from chronic studies,
animal data are used as a substitute. An assessment of the risk to
human health of exposure to xylene must rely on animal studies.
Apparently irreversible effects on the CNS were found 4 months
after a 3-month inhalation exposure (24 h per day) of Mongolian
gerbils to xylene at concentrations of 696 or 1392 mg/m3 (160 or 320
ppm). At the lower level the effects were not statistically
significant in any individual part of the brain but the changes were
all of the same nature. The study disclosed an increased
concentration of astroglial proteins in most brain regions studied,
which may indicate that glial proliferation is characteristic to
various neurodegenerative and neurotoxic states. In the light of
similar findings in animals exposed to other solvents (e.g.,
trichloroethylene, ethanol and tetrachloroethylene), the results are
estimated to be an important piece of evidence for potential
xylene-induced neurotoxicity at > 696 mg/m3 (160 ppm) (the LOAEL).
Functional changes, similar to acute effects on nervous functions,
were described after exposure to 435 mg/m3 (100 ppm), but they cannot
be discriminated from the acute effects of the last exposure. The
exposure level is consistent with the levels giving acute effects in
humans, as stated above.
No adequate studies of reproduction and development toxicity in
humans exposed to xylene alone have been published. Placental
transfer of xylene has been shown in humans and in experimental
animals.
Teratogenicity studies in pregnant animals exposed to technical
xylene or xylene isomers during organogenesis indicate that xylene may
cause reduced fetal weight and delayed ossification, but not
malformations, at dose levels causing no or only slight maternal
toxicity. LOAEL values of 500-2175 mg/m3 (115-500 ppm) have been
reported, depending on the length of the daily exposure periods (6-24
h/day). Signs of delayed ossification in the absence of lower fetal
body weight have been reported at lower dose levels. However, these
findings cannot be properly evaluated owing to incomplete description
of the criteria for assessing ossification. A NOAEL for delayed fetal
development cannot therefore be established.
In a study of postnatal development in rat offspring prenatally
exposed to 870 or 2175 mg/m3 (200 or 500 ppm) technical xylene,
behavioural impairments indicating effects on the development of the
central nervous system were detected. There was no maternal toxicity,
and the effects at 2175 mg/m3 (500 ppm) were long-lasting as they
were apparent in adult offspring. As 870 mg/m3 (200 ppm) was the
lowest dose level investigated for this effect a NOAEL could not be
established.
In several short-term and long-term animal studies, effects on
the activities of various metabolic enzymes in different organs have
been observed. Exposure to 217 mg/m3 (50 ppm) m-xylene 6 h/day for
5 days induced renal cytochrome P-450. At 1305 mg/m3 (300 ppm)
xylene (6 h/day for 14 days) an increase in hepatic and renal enzyme
activities was observed. At > 1740 mg/m3 (400 ppm) increases in
hepatic and renal metabolic enzyme levels, as well as increased
relative liver and kidney weights, have been reported. At the same
exposure levels the pulmonary P-450 content and pulmonary enzyme
activities were decreased. When rats were orally given 150 mg
xylene/kg body weight per day for 90 days, increased relative liver
weights were seen.
These changes in metabolic enzyme activities and increased
relative liver weight could be taken as an indication of metabolic
adaptation rather than toxicity.
When rats were exposed to 3480 mg/m3 (800 ppm) xylene, 14 h/day,
7 day/week for 6 weeks, an increased auditory response threshold was
reported. Thus, 3480 mg/m3 (800 ppm) is the LOAEL for ototoxicity.
For this effect no NOAEL could be established as 3480 mg/m3 (800 ppm)
was the lowest dose level used.
Xylene appears not to be a mutagen or a carcinogen.
10.1.3 Guidance value
The definition and aim of guidance values for the general
population have been described by IPCS (1994).
Although some differences in action between the three isomers
exist, there is no clear evidence that they (or mixture of them) have
totally different effects.
There have been no long-term controlled human studies or
epidemiological studies from which a guidance value may be calculated.
Epidemiological data from the occupational setting do not allow
an estimation of xylene-specific chronic nervous system effects,
neither can the neuropsychological impairment seen among
solvent-exposed workers be attributed to any specific level of xylene
in air.
On the basis of human volunteer studies (Anshelm Olson, 1985),
one may conclude that the NOAEL for acute CNS effects in humans is
about 304 mg/m3 (70 ppm) for a 4-h exposure. The use of an
uncertainty factor of 10 for intraspecies variability (the study
subjects were healthy male research workers) and an additional factor
of 6 (4-h exposure versus 24-h general population exposure) leads to a
guidance value of 4.8 mg/m3 (1.1 ppm). It should be noted, however,
that the acute CNS effect observed probably has no predictive value
for chronic CNS toxicity by xylene. The guidance value of 4.8 mg/m3
(1.1 ppm) is close to the odour threshold of xylene. It should be
noted that a subset of the human population may be sensitive enough to
experience the odour as annoying. The Task Group considered that
there was no need to add another uncertainty factor for the lack of
data from chronic exposure.
On the basis of animal studies on developmental toxicity, one may
conclude that the LOAEL for reduced fetal body weight is 500 mg/m3
(115 ppm) (Ungvary & Tatrai, 1985) and that for developmental
neurotoxicity is 870 mg/m3 (200 ppm) (Hass & Jakobsen, 1993).
Developmental neurotoxicity is a serious effect that may be
long-lasting and is therefore considered the critical effect. An
uncertainty factor of 10 for use of a LOAEL rather than a NOAEL seems
justified based on the evidence of lower fetal body weight at 500
mg/m3 (115 ppm) and limited evidence of delayed ossification at even
lower exposure levels. The use of additional factors of 10 for
interspecies variation and 10 for inter-individual variation leads to
a guidance value of 0.87 mg/m3 (0.2 ppm).
Using the hearing loss (Pryor et al., 1987) detected in animals
after exposure to 3480 mg/m3 (800 ppm) xylene for 6 weeks as a
starting point, an uncertainty factor of 10 for use of a LOAEL rather
than a NOAEL, a factor of 10 for interspecies variation and an
additional factor of 10 for inter-individual variation results in a
guidance value of 3.48 mg/m3 (0.8 ppm).
A neurotoxicity study in animals exposed continuously for 3
months to 696 or 1392 mg/m3 (160 or 320 ppm) xylene (Rosengren et
al., 1986) provided suggestive biochemical evidence of an apparently
irreversible adverse effect on the nerve cells of the brain even at
the lower level. Although there may be uncertainty concerning the
biological significance and interpretation of the findings, the Task
Group considered them potentially important and recommended further
confirmatory studies. With respect to animal-human extrapolation and
relatively short exposure, the estimate of the critical level for
life-long exposure in humans is 1.6 ppm. A guidance value of 0.87
mg/m3 (0.2 ppm) covers another uncertainty factor of approximate 10
in this respect.
Based on the above considerations, the Task Group recommended
0.87 mg/m3 (0.2 ppm) as a guidance value for the general population.
This value was derived from the LOAEL reported for developmental
neurotoxic effects in laboratory animals.
10.2 Evaluation of effects on the environment
10.2.1 Exposure
The majority of xylene released into the environment will enter
the atmosphere directly. In the atmosphere the xylene isomers are
readily degraded. Volatilization to the atmosphere from water is
rapid for all three isomers. Although the meta and para isomers are
readily biodegraded, in soil and water the ortho isomer is more
persistent. Bioaccumulation of xylene isomers by aquatic organisms is
low.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3. Mean background concentrations of
xylenes in surface waters are generally below 0.1 µg/litre. However,
higher values have been measured in industrial areas. In areas
associated with the oil industry even higher levels have been reported
but only associated with discharge pipes. Similar background levels
have been reported for groundwater, although localized pollution can
lead to higher levels.
10.2.2 Effects
The xylene isomers are of moderate to low toxicity to aquatic
organisms. The variation between each individual isomer with respect
to aquatic toxicity is generally small. The lowest LC50 value, based
on measured concentrations, is for a 24-h exposure of Daphnia magna
to 1 mg o-xylene/litre.
There is limited information regarding chronic exposure of
aquatic organisms to xylenes and none of the observed effect levels
were lower than those summarized under the acute studies.
The acute toxicity of xylene to birds is low.
10.2.3 Risk evaluation
Xylenes are rapidly degraded in the environment. However, the
photooxidation reactions of the xylene isomers in the atmosphere may
contribute to photochemical smog.
High levels of xylenes have been reported in groundwater
associated with localized pollution from underground tanks and pipes,
but the environmental significance of such values is difficult to
assess.
For the aquatic environment, the most sensitive toxicity test,
based on measured concentrations, yielded an LC50 for Daphnia
magna of 1 mg/litre ( o-xylene). This value is more than 10 000
times higher than mean background concentrations in surface water,
which are generally less than 0.1 µg/litre for each isomer. The
lowest LC50 is still over 30 times higher than the highest single
measured concentration of total xylenes in the most polluted area.
The exposure/toxicity ratio will be much higher for mean
concentrations in polluted areas.
On the basis of rapid volatilization and degradation of xylenes
and their low to moderate toxicity, the overall risk to the aquatic
environment can therefore be considered low. It should be noted,
however, that very much higher levels have been measured around
discharges from oil production sites, and higher levels are also
possible if spillage occurs.
The likely route of exposure for birds in the environment is via
food such as fish. The only acute toxicity test on birds was carried
out on 14-day old Japanese quail and gave a 5-day LC50 of > 5000
mg/kg diet. Based on food consumption and body weight, an LD50 for
the quail of > 1746 mg/kg body weight can be calculated. Using this
data an estimated LC50 for a fish-eating bird (kingfisher) based on
body weight and food consumption can be calculated.
LC50 (mg/kg dry weight diet) =
Test species LD50 (mg/kg) × body wt (kg)
food consumption (kg)
The estimated LC50 for the fish-eating bird is > 7990 mg/kg
diet. The highest water concentration (30 µg/litre) multiplied by
the highest theoretical bioconcentration factor (158) gives a worst
case residue level in fish of 4.7 mg/kg. It should be noted that the
bioconcentration factor does not take into account degradation or
metabolism. Comparing this value to the estimated LC50 value gives a
Toxicity Exposure Ratio (TER) of > 1700. Therefore, the risk to
fish-eating birds is very low.
11. CONCLUSIONS
Humans are exposed to xylene mainly by inhalation. This compound
does not accumulate significantly in the human body. Acute exposure
to high concentrations can result in CNS effects in human. There have
been no long-term controlled studies or epidemiological studies with
exposure to xylene alone. The chronic toxicity appears to be
relatively low in laboratory animals. There is suggestive evidence,
however, that chronic CNS effects may occur in animals at moderate
concentrations of xylene. Xylene appears not to be a mutagen or a
carcinogen. The critical end-point is developmental toxicity. Based
on this end-point, the recommended guidance value for xylene in air
for the general population is 0.87 mg/m3 (0.2 ppm). This value is
higher than the concentrations to which the general population is
exposed.
The xylene isomers are non-persistent chemicals, being readily
degraded in the atmosphere, soil and water. It should be noted that
o-xylene appears to biodegrade only in the presence of other carbon
sources and at a reduced rate compared to the other isomers. The
photooxidation reactions of the xylene isomers in the atmosphere may
contribute to photochemical smog.
It can be concluded that xylenes are unlikely to cause problems
in aquatic ecosystems except near to localized industrial discharges
and spillage incidents. The risk to birds from xylene exposure is
low.
12. FUTURE RESEARCH
There is little information on the long-term effects of xylene in
humans and, specifically, no dose-response or dose-effect data are
available. Epidemiological studies of populations occupationally
exposed to xylene should be encouraged. In this context the use of
xylene metabolites in urine as a marker of exposure can be of special
value because the method determines the internal doses that
individuals receive via all routes of exposure. Because xylene has
acute effects on CNS, epidemiological studies should address the CNS
as a potential target organ. Moreover, since ethylbenzene is almost
invariably one of the components in solvent mixtures at the workplace,
study designs that address possible interactions between xylene and
other solvent components are desirable.
Animal studies are needed to address biochemical, functional and
morphological evidence of chronic neurotoxicity and potential effects
on fertility. In addition, there is a need for further studies on
developmental toxicity to assess the dose-response relationship and to
estimate the NOAEL, especially for developmental neurotoxicity.
Further studies are needed to determine the effect on hearing
impairment in order to determine the NOAEL. The relationship between
the dose level and the length of the exposure period should also be
investigated. The effect of exposure to xylene together with noise
should be studied.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC) has
evaluated the carcinogenicity of xylene. There was inadequate
evidence for the carcinogenicity of xylene in humans as well as in
experimental animals. The overall evaluation was that xylene is not
classifiable as to its carcinogenicity in humans (IARC, 1989).
The European Commission (1991-1992) recommended an 8-h
time-weighted average occupational exposure limit for xylene of 217
mg/m3 (50 ppm). To limit peaks of exposure that could result in
irritation, a short-term exposure level (STEL) of 435 mg/m3 (100 ppm)
was recommended.
REFERENCES
Abu Al Ragheb S, Salhab AS, & Amr SS (1986) Suicide by xylene
ingestion: A case report and review of literature. Am J Forensic Med
Pathol, 7: 327-329.
Altshuller AP, Lonneman WA, Sutterfield FD, & Kopczyncki SL (1971)
Hydrocarbon composition of the atmosphere of the Los Angeles basin,
1967. Environ Sci Technol, 5: 39.
Alvarez PJJ & Vogel TM (1991) Substrate interactions of benzene,
toluene, and para-xylene during microbial degradation by pure cultures
and mixed culture aquifer slurries. Appl Environ Microbiol,
57: 2981-2985.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3: 272-290.
Andersson K, Fuxe K, Nilsen OG, Toftgård R, Eneroth P, & Gustafsson
J-Å (1981) Production of discrete changes in dopamine and
noradrenaline levels and turnover in various parts of the rat brain
following exposure to xylene, ortho-, and para-xylene, and
ethylbenzene. Toxicol Appl Pharmacol, 60: 535-548.
Angerer J (1988a) Determination of toluene in blood by head-space gas
chromatography.
Angerer J (1988b) Determination of hippuric acid in urine by
high-pressure liquid chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 301-306 (IARC
Scientific Publications No. 85).
Angerer J & Lehnert G (1979) Occupational chronic exposure to organic
solvents. VIII. Phenolic compounds - metabolites of alkylbenzenes in
man: Simultaneous exposure to ethylbenzene and xylenes. Int Arch Occup
Environ Health, 43: 145-150.
Angerer J & Wulf H (1985) Occupational exposure to organic solvents.
XI. Alkylbenzene exposure of varnish workers: Effects on hematopoetic
system. Int Arch Occup Environ Health, 56: 307-321.
Anshelm Olson B, Gamberale F, & Iregren A (1985) Coexposure to toluene
and p-xylene in man: central nervous functions. Br J Ind Med,
42: 117-122.
Antoine SR, Deleon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36: 364-371.
Arlien-Soborg P, Zilstorff K, Grandjean B, & Milling Pedersen L (1981)
Vestibular dysfunction in occupational chronic solvent intoxication.
Clin Otolaryngol, 6: 285-290.
Aschan G, Bunnfors I, Hydén D, Larsby B, Ödkvist LM, & Tham R (1977)
Xylene exposure. Electronystagmographic and gas chromatographic
studies in rabbits. Acta Otolaryngol, 84: 370-376.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, & Wooten JV (1994) Blood
concentrations of volatile organic compounds in a nonoccupationally
exposed US population and in groups with suspected exposure. Clin
Chem, 40(7): 1401-1404.
Askergren A (1981) Studies on kidney function in subjects exposed to
organic solvents. III. Excretion of cells in the urine. Acta Med
Scand, 210: 103-106.
Askergren A, Allgén L-G, & Bergström J (1981a) Studies on kidney
function in subjects exposed to organic solvents. II. The effect of
desmopressin in a concentration test and the effect of exposure to
organic solvents on renal concentrating ability. Acta Med Scand,
209: 485-488.
Askergren A, Allgén L-G, Karlsson C, Lundberg I, & Nyberg E (1981b)
Studies on kidney function in subjects exposed to organic solvents. I.
Excretion of albumin and ß-2-microglobulin in the urine. Acta Med
Scand, 209: 479-483.
Askergren A, Brandt R, Gullquist R, Silk B, & Strandell T (1981c)
Studies on kidney function in subjects exposed to organic solvents.
IV. Effect on 51Cr-EDTA clearance. Acta Med Scand, 210: 373-376.
Åstrand I, Engström J, & Övrum P (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man. Scand J
Work Environ Health, 4: 185-194.
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions
of the hydroxyl radical with organic compounds under atmospheric
conditions. Chem Rev, 85: 69-201.
Axelsson G, Lütz C, & Rylander R (1984) Exposure to solvents and
outcome of pregnancy in university laboratory employees. Br J Ind Med,
41: 305-312.
Bakinson MA & Jones RD (1985) Gassings due to methylene chloride,
xylene, toluene, and styrene reported to Her Majesty's Factory
Inspectorate 1961-80. Br J Ind Med, 42: 184-190.
Bakke OM & Scheline RR (1970) Hydroxylation of aromatic hydrocarbons
in the rat. Toxicol Appl Pharmacol, 16: 691-700.
Barker JF, Barbash JE, & Labonte M (1988) Groundwater contamination at
a landfill sited on fractured carbonate and shale. J Contam Hydrol,
3: 1-25.
Batchelor JJ (1927) The relative toxicity of benzol and its higher
homologues. Am J Hyg, 7: 276-298.
Beirne GJ & Brennan JT (1972) Glomerulonephritis associated with
hydrocarbon solvents. Mediated by antiglomerular basement membrane
antibody. Arch Environ Health, 25: 365-369.
Bell GM, Gordon ACH, Lee P, Doig A, MacDonald MK, Thomson D, Anderton
JL, & Robson JS (1985) Proliferative glumerulonephritis and exposure
to organic solvents. Nephron, 40: 161-165.
Bell GM, Shillaker RO, Padgham MDJ, & Standring P (1992) Xylenes.
London, Health and Safety Executive, 166 pp (Toxicity Review 26).
Benville PE & Korn S (1977) The acute toxicity of six monocyclic
aromatic crude oil components to striped bass (Morone saxatilis) and
bay shrimp (Crago franciscorum). Calif Fish Gam, 63: 204-209.
Bergman K (1979) Whole-body autoradiography and allied tracer
techniques in distribution and elimination studies of some organic
solvents. Scand J Work Environ Health, 5(suppl 1): 1-263.
Bergman K (1983) Application and results of whole-body autoradiography
in distribution studies of organic solvents. CRC Crit Rev Toxicol,
12: 59-118.
Berry WO & Brammer JD (1977) Toxicity of water-soluble gasoline
fractions to fourth-instar larvae of the mosquito Aedes aegypti L.
Environ Pollut, 13: 229-234.
Bevan MAJ, Proctor CJ, Baker-Rogers J, & Warren ND (1991) Exposure to
carbon monoxide, respirable suspended particulates and volatile
organic compounds while commuting by bicycle. Environ Sci Techol,
25: 788-791.
Bieniek G, Palys E, & Wilczok T (1982) TLC separation of hippuric,
mandelic, and phenylglyoxylic acids from urine after mixed exposure to
toluene and styrene. Br J Ind Med, 39: 187-190.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, University of
Kentucky, Water Resources Research Institute, 61 pp (Research Report
No. 133).
Blanchard KT & Morris JB (1994) Effects of m-xylene on rat nasal
cytochrome P450 mixed function oxidase activities. Toxicol Lett,
70: 253-259.
Blum DJW & Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. J Water Pollut Control Fed, 63: 198-207.
Bobra AM, Shiu WY, & Mackay D (1983) A predictive correlation for the
acute toxicity of hydrocarbons and chlorinated hydrocarbons to the
water flea (Daphnia magna). Chemosphere, 12: 1121-1129.
Bonnet P, Raoult G, & Gradiski D (1979) Concentrations léthales50 des
principaux hydrocarbures aromatiques. Arch Mal Prof, 40: 805-810.
Bonnet P, Morele Y, Raoult G, Zissu D, & Gradiski D (1982)
Détermination de la concentration léthale50 des principaux
hydrocarbures aromatiques chez le rat. Arch Mal Prof, 43: 261-265.
Bos RP, Brouns RME, van Doorn R, Theuws JLG, & Henderson PT (1981)
Non-mutagenicity of toluene, o-, m- and p-xylene, o-methyl-
benzylalcohol and o-methylbenzylsulfate in the Ames assay.
Mutat Res, 88: 273-279.
Boström E-C, Almén J, Steen B, & Westerholm R (1994) Human exposure to
urban air pollution. Environ Health Perspect, 102(suppl 4): 39-47.
Botta D, Pirri LC, & Mantica E (1984) Ground water pollution by
organic solvents and their microbial degradation products. In:
Analytical Organic Micropollutants in Water. Luxembourg, Commission of
the European Communities, pp 261-275 (Report EUR 8518).
Bowers DE Jr, Cannon MS, & Jones DH (1982) Ultrastructural changes in
livers of young and aging rats exposed to methylated benzenes. Am J
Vet Res, 43: 676-683.
Bray HG, Humphris BG, & Thorpe WV (1949) Metabolism of derivatives of
toluene. 3. o-, m- and p-xylenes. Biochem J, 45: 241-244.
Brice KA & Derwent RG (1978) Emission inventory for hydrocarbons in
the United Kingdom. Atmos Environ, 12: 2045-2054.
Bridie AL, Wolff CJM, & Winter M (1979) The acute toxicity of some
petrochemicals to goldfish. Water Res, 13: 623-626.
Bringmann G & Kühn R (1977) [Toxic threshold values for compounds
hazardous to the aquatic environment on bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda) using cell
multiplication test.] Z Wasser Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kühn R (1978) [Toxic threshold values for compounds
hazardous to the aquatic environment on blue algae (Microcystic
aeruginosa) and green algae (Scenedesmus quadricauda) using cell
multiplication test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1982) [Results of the toxic effects of compounds
hazardous to the aquatic environment on Daphnia magna using an
improved standardized test.] Z Wasser Abwasser Forsch, 15: 1-6 (in
German).
Bringmann G, Kühn R, & Winter A (1980) [Determination of biocidal
effects of compounds hazardous to the aquatic environment for
Protozoa: III. Saprozoic flagelates.] Z Wasser Abwasser Forsch,
13: 170-173 (in German).
Brodzinsky R & Singh HB (1983) Volatile organic chemicals in the
atmosphere: An assessment of available data. Washington, DC, US
Environmental Protection Agency (EPA/DF-83/005A).
Brooks JM, Fryxell GA, Reid DF, & Sackett WM (1977) Gulf underwater
flare experiment (GULFEX): Effects of hydrocarbons on phytoplankton
In: Pollutant effects on marine organisms. Proceedings of a Workshop,
College station, Texas, USA, 16-19 May 1976, pp 45-75.
Brown RH (1988a) Determination of benzene, toluene and xylene in
industrial air by charcoal tube, solvent desorption and gas
chromatography. In: Fishbein L & O'Neill IK ed. Environmental
carcinogens: Methods of analysis and exposure measurement. Volume
10: Benzene and alkylated benzenes. Lyon, International Agency for
Research on Cancer, pp 225-233 (IARC Scientific Publications No. 85).
Brown RH (1988b) Determination of benzene, toluene and xylene in
industrial air by porous polymer adsorption tube, thermal desorption
and gas chromatography. In: Fishbein L & O'Neill IK ed. Environmental
carcinogens: Methods of analysis and exposure measurement. Volume
10: Benzene and alkylated benzenes. Lyon, International Agency for
Research on Cancer, pp 235-242 (IARC Scientific Publications No. 85).
Brown RH (1988c) Determination of gasoline hydrocarbons in industrial
air by two-stage (chromosorb/charcoal) adsorption, thermal desorption
and capillary gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 243-251 (IARC
Scientific Publications No. 85).
Brown-Woodman PDC, Webster WS, Picker K, & Ritchie HE (1991)
Embryotoxicity of xylene and toluene: an in study. Ind Health,
29: 139-152.
Brown-Woodman PDC, Webster WS, Picker K, & Huq F (1994) In
assessment of individual and interactive effects of aromatic
hydrocarbons on embryonic development of the rat. Reprod Toxicol,
8: 121-135.
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other trace
organic compounds in urban air. Chemosphere, 17: 2363-2380.
Bushnell PJ (1989) Behavioral effects of acute p-xylene inhalation
in rats: Autoshaping, motor activity, and reversal learning.
Neurotoxicol Teratol, 10: 569-577.
Bushnell PJ & Peele DB (1988) Conditioned flavor aversion induced by
inhaled p-xylene in rats. Neurotoxicol Teratol, 10: 273-277.
Buszka PM, Zaugg SD, & Werner MG (1990) Determination of trace
concentrations of volatile organic compounds in ground water using
closed-loop stripping, Edwards Aquifer, Texas. Bull Environ Contam
Toxicol, 45: 507-515.
Buttery RG, Seifert RM, & Ling LC (1975) Characterization of some
volatile constituents of dry red beans. J Agric Food Chem,
23: 516-519.
Caldwell RS, Caldarone EM, & Mallon MH (1977) Effects of a
seawater-soluble fraction of Cook Inlet crude oil and its major
aromatic components on larval stages of the Dungeness crab, Cancer
magister Dana. In: Wolfe DA ed. Fate and effects of petroleum
hydrocarbons in marine ecosystems and organisms. Oxford, New York,
Pergamon Press, pp 210-220.
Cameron GR, Paterson JLH, de Saram GSW, & Thomas JC (1938) The
toxicity of some methyl derivatives of benzene with special reference
to pseudocumene and heavy coal tar naphta. J Pathol Bacteriol,
46: 95-107.
Campbell L, Wilson HK, Samuel AM, & Gompertz D (1988) Interactions of
m-xylene and aspirin metabolism in man. Br J Ind Med, 45: 127-132.
Carlsson A (1981) Distribution and elimination of 14C-xylene in rat.
Scand J Work Environ Health, 7: 51-55.
Carpenter CP, Kinkead ER, Geary DL Jr, Sullivan LJ, & King JM (1975)
Petroleum hydrocarbon toxicity studies. V. Animal and human response
to vapors of mixed xylenes. Toxicol Appl Pharmacol, 33: 543-558.
Chan C-C, Özkaynak H, Spengler JD, & Sheldon L (1991a) Driver exposure
to volatile organic compounds, CO, ozone and NO2 under different
driving conditions. Environ Sci Techol, 25: 964-972.
Chan C-C, Spengler JD, Ozkaynak H, & Lefkopoulou M (1991b) Commuter
exposures to VOCs in Boston, Massachusetts. J Air Waste Manage Assoc,
41: 1594-1600.
Chan C-C, Lin S-H, & Her GR (1993) Student's exposure to volatile
organic compounds while commuting by motorcycle and bus in Taipei
City. J Air Waste Manage Assoc, 43(9): 1231-1238.
Chen Z, Liu S-J, Cai S-X, Yao Y-M, Yin H, Ukai H, Uchida Y, Nakatsuka
H, Watanabe T, & Ikeda M (1994) Exposure of workers to a mixture of
toluene and xylenes. II. Effects. Occup Environ Med, 51: 47-49.
Chiang CY, Salanito JP, Chai EY, Colthart JD, & Klein CL (1989)
Aerobic biodegradation of benzene, toluene and xylene in a sandy
aquifer - Data analysis and computer modelling. Ground Water,
27: 823-834.
Chung TY, Hayase F, & Kato H (1983) Volatile components of ripe
tomatoes and their juices, purees and pastes. Agric Biol Chem,
47: 343-351.
City of Toronto (1990) The quality of drinking water in Toronto: A
review of tap water, bottled water and water treated by a point-of-use
device. Toronto, Canada, Department of Public Health.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural and motorway locations.
Environ Pollut, B7: 141-158.
Condie LW, Hill JR, & Borzelleca JF (1988) Oral toxicology studies
with xylene isomers and mixed xylenes. Drug Chem Toxicol, 11: 329-354.
Connor TH, Theiss JC, Hanna HA, Monteith DK, & Matney TS (1985)
Genotoxicity of organic chemicals frequently found in the air of
mobile homes. Toxicol Lett, 25: 33-40.
Cragg ST, Clarke EA, Daly IW, Miller RR, Terrill JB, & Ouellette RE
(1989) Subchronic inhalation toxicity of ethylbenzene in mice, rats,
and rabbits. Fundam Appl Toxicol, 13: 399-408.
Cramer PH, Boggess KE, Hosenfeld JM, Remmers JC, Breen JJ, Robinson
PE, & Stroup C (1988) Determination of organic chemicals in human
whole blood: Preliminary method development for volatile organics.
Bull Environ Contam Toxicol, 40: 612-681.
Craveri A, Tornaghi G, Ciaci D, Vitali L, Carucci R, De Luca P, &
Donato MF (1982) [Serum bile salts in the hepatological screening of
subjects exposed to aromatic hydrocarbons.] Giorn Clin Med,
63: 322-329 (in Italian).
Crawford L & Kretsch MJ (1976) GC-MS identification of the volatile
compounds extracted from roasted turkeys fed a basal diet supplemented
with tuna oil: Some comments on fishy flavour. J Food Sci,
41: 1470-1478.
Crofton KM, Lassiter TL, & Rebert CS (1994) Solvent-induced
ototoxicity in rats: an atypical selective mid-frequency hearing
deficit. Hear Res, 80: 25-30.
Daisey JM, Hodgson AT, Fisk WJ, Mendell MJ, & Ten Brinke J (1994)
Volatile organic compounds in twelve California office buildings:
classes, concentrations and sources. Atmos Environ, 28(22): 3557-3562.
Davey JF & Gibson DT (1974) Bacterial metabolism of para- and
meta-xylene: Oxidation of a methyl substituent. J Bacteriol,
119: 923-929.
Davis RS, Hossler FE, & Stone RW (1967) Oxidation of m- and p-
xylenes by Pseudomonas. Bateriol Proc, P162: 129.
Day BJ & Carlson GP (1992) Effect of p-xylene metabolites,
p-methylbenzyl alcohol and 2,5-dimethylphenol on rat hepatic and
pulmonary microsomal metabolism. Res Commun Chem Pathol Pharmacol,
76: 117-120.
Day BJ, DeNicola DB, Marcus CB, & Carlson GP (1992) Effect of
p-xylene inhalation on the bioactivation of bromobenzene in rat lung
and liver. Fundam Appl Toxicol, 19: 50-56.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
De Ceaurriz J, Desiles JP, Bonnet P, Marignac B, Muller J, & Guenier
JP (1983) Concentration-dependent behavioral changes in mice following
short-term inhalation exposure to various industrial solvents. Toxicol
Appl Pharmacol, 67: 383-389.
DECOS (1991) Health-based recommended occupational exposure limit for
xylene. The Hague, Directorate-General of Labour.
De Gandarias JM, Echevarria E, Irazusta J, Gil J, & Casis L (1993)
Brain aminopeptidase activity after subacute xylene exposure.
Neurotoxicol Teratol, 15: 51-53.
del Rosario R, de Lumen BO, Habu T, Flath RA, Mon TR, & Teranishi R
(1984) Comparison of headspace volatiles from winged beans and
soybeans. J Agric Food Chem, 32: 1011-1015.
Derwent RG & Jenkin ME (1990) Hydrocarbon involvement in photochemical
ozone formation in Europe. Oxfordshire, Harwell Laboratory, AEA
Environment and Energy, Modelling and Assessments Group (Report AERE
R13736).
Dési I, Kovacs F, Zahumenszky Z, & Balogh A (1967) Maze learning in
rats exposed to xylene intoxication. Psychopharmacologia (Berl),
11: 224-230.
Divincenzo GD & Krasavage WJ (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic solvents.
Am Ind Hyg Assoc J, 35: 21-29.
Donner M, Mäki-Paakkanen J, Norppa H, Sorsa M, & Vainio H (1980)
Genetic toxicology of xylenes. Mutat Res, 74: 171-172.
Dössing M, Arlien Söborg P, Milling Pedersen L, & Ranek L (1981)
[Liver damage following long-term occupational exposure to organic
solvents.] Ugeskr Laeg, 143: 2297-2301 (in Danish with English
summary).
Dössing M, Arlien Söborg P, Milling Pedersen L, & Ranek L (1983) Liver
damage associated with occupational exposure to organic solvents in
house painters. Eur J Clin Invest, 13: 151-157.
Dowden BF & Bennett HJ (1965) Toxicity of selected chemicals to
certain animals. J Water Pollut Control Fed, 37: 1308-1316.
Dowty BJ & Laseter JL (1976) The transplacental migration and
accummulation in blood of volatile organic constituents. Ped Res,
10: 696-701.
Dowty BJ, Carlisle DR, & Laseter JL (1975) New Orleans drinking water
sources tested by gas chromatography-mass spectrometry. Environ Sci
Techol, 9: 762-765.
Drew RT & Fouts JR (1974) The effects of inducers on acute p-xylene
toxicity. Toxicol Appl Pharmacol, 29: 111-112.
Drozd J & Novak J (1978) Quantitative and qualitative head-space gas
analysis of parts per billion amounts of hydrocarbons in water: A
study of model systems by capillary-column gas chromatography with
splitless sample injection. J Chromatogr, 158: 471-482.
Dudek B, Gralewicz K, Jakubowski M, Kostrzewski P, & Sokal J (1990)
Neurobehavioral effects of experimental exposure to toluene, xylene
and their mixture. Pol J Occup Med, 3: 109-116.
Dunstan WM, Atkinson LP, & Natoli J (1975) Stimulation and inhibition
of phytoplankton growth by low molecular weight hydrocarbons. Mar
Biol, 31: 305-310.
Dutkiewicz T & Tyras H (1968) Skin absorption of toluene, styrene, and
xylene by man. Br J Ind Med, 25: 243.
Dyer RS, Bercegeay MS, & Mayo LM (1988) Acute exposures to p-xylene
and toluene alter visual information processing. Neurotoxicol Teratol,
10: 147-153.
EAJ (Environment Agency, Japan) (1993) Chemicals in the environment.
Tokyo, Ministry of Health and Welfare, International Affairs Division.
ECETOC (1986) Joint assessment of commodity chemicals No. 6: Xylenes.
Brussels, European Chemical Industry Ecology and Toxicology Centre.
Edling C (1982) Interaction between drugs and solvents as a cause of
fatty change in the liver? Br J Ind Med, 39: 198-199.
Edwards EA & Grbic-Galic D (1994) Anaerobic degradation of toluene and
o-xylene by a methanogenic consortium. Appl Environ Microbiol,
60(1): 313-322.
Edwards EA, Willis LE, Reinhard M, & Grbic-Galic D (1992) Anaerobic
degradation of toluene and xylene by aquifer microorganisms under
sulfate-reducing conditions. Appl Environ Microbiol, 58: 794-800.
Eller PM (1984) NIOSH manual of analytical methods: Method 1501, 3rd
ed. Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 2.
Elofsson S-A, Gamberale F, Hindmarsh T, Iregren A, Isaksson A,
Johnsson I, Knave B, Lydahl E, Mindus P, Persson HE, Philipson B,
Steby M, Struwe G, Söderman E, Wennberg A, & Widén L (1980) Exposure
to organic solvents: A cross-sectional epidemiologic investigation on
occupationally exposed car and industrial spray painters with special
reference to the nervous system. Scand J Work Environ Health,
6: 239-273.
Elovaara E (1982) Dose-related effects of m-xylene inhalation on the
xenobiotic metabolism of the rat. Xenobiotica, 12: 345-352.
Elovaara E, Collan Y, Pfäffli P, & Vainio H (1980) The combined
toxicity of technical grade xylene and ethanol in the rat.
Xenobiotica, 10: 435-445.
Elovaara E, Pfäffli P, Savolainen H, & Vainio H (1982) Marginal role
of impaired aldehyde metabolism in m-xylene vapour-induced
biochemical effects in the rat. J Appl Toxicol, 2: 27-32.
Elovaara E, Engström K, & Vainio H (1984) Metabolism and disposition
of simultaneously inhaled m-xylene and ethylbenzene in the rat.
Toxicol Appl Pharmacol, 75: 466-478.
Elovaara E, Zitting A, Nickels J, & Aitio A (1987) m-Xylene
inhalation destroys cytochrome P-450 in rat lung at low exposure. Arch
Toxicol, 61: 21-26.
Engelhardt WE & Estler W (1935) [Experiments on the acute narcotic
effects of aliphatic and aromatic hydrocarbons: The effect of single
inhalation of gasoline, benzene, toluene, xylene in the rabbit and the
cat.] Ark Hyg, 114: 249-260 (in German).
Engström J & Bjurström R (1978) Exposure to xylene and ethylbenzene.
II. Concentration in subcutaneous adipose tissue. Scand J Work Environ
Health, 4: 195-203.
Engström J & Riihimäki V (1979) Distribution of m-xylene to
subcutaneous adipose tissue in short-term experimental human exposure.
Scand J Work Environ Health, 5: 126-134.
Engström K & Riihimäki V (1988a) Determination of xylenes in blood by
head-space gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 307-311 (IARC
Scientific Publications No. 85).
Engström K & Riihimäki V (1988b) Determination of methylhippuric acids
in urine by gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 313-318 (IARC
Scientific Publications No. 85).
Engström K, Husman K, & Riihimäki V (1977) Percutaneous absorption of
m-xylene in man. Int Arch Occup Environ Health, 39: 181-189.
Engström K, Husman K, Pfäffli P, & Riihimäki V (1978) Evaluation of
occupational exposure to xylene by blood, exhaled air and urine
analysis. Scand J Work Environ Health, 4: 114-121.
Engström K, Riihimäki V, & Laine A (1984) Urinary disposition of
ethylbenzene and m-xylene in man following separate and combined
exposure. Int Arch Occup Environ Health, 54: 355-363.
European Commission (1991-1992) Occupational exposure limits.
Recommendations of the Scientific Expert Group 1991-1992. Luxembourg,
European Commission, pp 53-55 (Report EUR 15091 EN).
Fabre R, Truhaut R, & Laham S (1960) Recherches toxicologiques sur les
solvants de remplacement du benzčne. IV. Etude des xylčnes. Arch Mal
Prof, 21: 301-313.
Fairfax R (1995) Solvent, wood dust, and noise exposure in a decoy
shop. Appl Occup Environ Hyg, 10(5): 446-448.
Falk-Petersen I-B, Kjrsvic E, Lonning S, Naley AM, & Sydnes LK (1985)
Toxic effects of hydroxylated aromatic hydrocarbons on marine embryos.
Sarsia, 70: 11-16.
Fellin P & Otson R (1993) Seasonal trends of volatile organic
compounds (VOCs) in Canadian homes. In: Indoor air '93. Proceedings of
the 6th International Conference on Indoor Air Quality and Climate,
Helsinki, Finland, 4-8 July 1993 - Volume 2: Chemicals in indoor air,
materials emissions, pp 117-122.
Ferrario JB, Lawler GC, Deleon IR, & Laseter JL (1985) Volatile
organic pollutants in biota and sediments of Lake Pontchartrain. Bull
Environ Contam Toxicol, 34: 246-255.
Fielding M, Gibson TM, James HA, McLoughlin K, & Steel CP (1981)
Organic micropollutants in drinking water. Medmenham, Marlow, United
Kingdom, Water Research Centre (Technical Report TR-159).
Fishbein L (1988) Xylenes: Uses, occurrence and exposure. In: Fishbein
L & O'Neill IK ed. Environmental carcinogens: Methods of analysis and
exposure measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 109-120 (IARC
Scientific Publications No. 85).
Fischbein A, Ross RR, & Lerman Y (1983) Industrial solvents and the
liver. Lancet, 1: 129.
Fishbein L, O'Neill IK, & Tossavainen A (1988) Past and present
exposure determination techniques for benzene, toluene and xylene. In:
Fishbein L & O'Neill IK ed. Environmental carcinogens: Methods of
analysis and exposure measurement. Volume 10: Benzene and alkylated
benzenes. Lyon, International Agency for Research on Cancer,
pp 123-148 (IARC Scientific Publications No. 85).
Folmar LC (1976) Overt avoidance reaction of rainbow trout fry to nine
herbicides. Bull Environ Contam Toxicol, 15: 509-514.
Foster P, Laffond M, Baussand P, Jacob V, & Denis I (1991) VOC
measurements in the Grenoble area and study of the benzaldehyde
behaviour in simulation chamber. Pollut Atmos, 1991: 175-191.
Franchini I, Cavatorta A, Falzoi M, Lucertini S, & Mutti A (1983)
Early indicators of renal damage in workers exposed to organic
solvents. Int Arch Occup Environ Health, 52: 1-9.
Frank PA, Otto NE, & Bartley TR (1961) Techniques for evaluating
aquatic weed herbicides. Weeds, 9: 515-521.
Frantik E, Hornychova M, & Vodickova L (1993) Equipotential
subnarcotic concentrations of solvents in air, in blood, and at the
target structure. Homeostasis, 34: 143-147.
Frantik E, Hornychova M, & Horvath M (1994) Relative acute
neurotoxicity of solvents: Isoeffective air concentrations of 48
compounds evaluated in rats and mice. Environ Res, 66: 173-185.
Frantik E & Vodickova L (1995) Combined effects of binary solvent
mixtures. Cent Eur J Occup Environ Med, 1: 31-37.
Funes-Cravioto F, Kolmodin-Hedman B, Lindsten J, Nordenskjöld M,
Zapata-Gayon C, Lambert B, Norberg E, Olin R, & Swensson Å (1977)
Chromosome aberrations and sister-chromatid exchange in workers in
chemical laboratories and a rotoprinting factory and in children of
women laboratory workers. Lancet, 2: 322-325.
Furnas DW & Hine CH (1958) Neurotoxicity of some selected hydro-
carbons. Arch Ind Health Occup Med, 18: 9-15.
Fustinoni S, Buratti M, Giampiccolo R, & Colombi A (1995) Biological
and environmental monitoring of exposure to airborne benzene and other
aromatic hydrocarbons in Milan traffic wardens. Toxicol Lett,
77(13): 387-392.
Gab S, Schmizer J, Tham H, Parlar H, & Korte F (1977) Photo-
mineralisation rate of organic compounds adsorbed on particulate
matter. Nature (Lond), 270: 331-333
Galassi S, Mingazzini M, Vigano L, Cesareo D, & Tosato ML (1988)
Approaches to modelling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicol Environ Saf, 16: 158-169.
Gamberale F, Annwall G, & Hultengren M (1978) Exposure to xylene and
ethylbenzene. III. Effects on central nervous functions. Scand J Work
Environ Health, 4: 204-211.
Gerner-Smidt P & Friedrich U (1978) The mutagenic effect of benzene,
toluene and xylene studied by the SCE technique. Mutat Res,
58: 313-316.
Gery MW, Fox DL, Kaems RM, & Stockburger L (1987) Investigation of
hydroxyl radical reactions with o-xylene and m-xylene in a
continuous stirred tank reactor. Environ Sci Techol, 21: 339-348.
Ghantous H & Danielsson BG (1986) Placental transfer and distribution
of toluene, xylene and benzene, and their metabolites during gestation
in mice. Biol Res Pregnancy, 7: 98-105.
Ghantous H, Dencker L, Gabrielsson J, Danielsson BRG, & Bergman K
(1990) Accumulation and turnover of metabolites of toluene and xylene
in nasal mucosa and olfactory bulb in the mouse. Pharmacol Toxicol,
66: 87-92.
Ghislandi E & Fabiani A (1957) [Liver damage due to accidental
ingestion of nitrocellulose paints.] Med Lav, 48: 577-579 (in Italian
with English summary).
Ghosh TK & Pradhan SN (1987) Comparison of effects of xylene and
toluene inhalation on fixed-ratio liquid-reinforced behavior in rats.
Res Commun Psychol Psychiatr Behav, 12: 305-312.
Ghosh TK, Copeland RL Jr, Parui RN, Mookherjee S, & Pradhan SN (1987)
Effect of xylene inhalation on fixed-ratio responding in rats.
Pharmacol Biochem Behav, 27: 653-657.
Gilli G, Scursatone E, & Bono R (1994) Benzene, toluene and xylenes in
air, geographical distribution in Piedmont region (Italy) and personal
exposure. Sci Total Environ, 148(1): 49-56.
Glass WI (1961) Annotation: A case of suspected xylol poisoning. N Z
Med J, 60: 113.
Gomez-Belinchon JI, Grimalt JO, & Abaigés J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water Res, 25: 577-589.
Grob K & Grob G (1971) Gas-liquid chromatographic - mass spectrometric
investigation of C6-C20 organic compounds in an urban atmosphere: An
application of ultra trace analysis on capillary columns.
J Chromatogr, 62: 1-13.
Grosjean D (1990) Atmospheric chemistry of toxic contaminants. 1.
Reaction rates and atmospheric persistence. J Air Waste Manage Assoc,
40: 1397-1402.
Gschwend PM, Zafiriou OC, Mantoura RFC, Schwarzenbach RP, & Gagosian
RB (1982) Volatile organic compounds at a coastal site. I. Seasonal
variations. Environ Sci Techol, 1: 31-38.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gut I & Flek J (1981) [Effect of microsomal enzyme induction by
phenobarbital on the metabolism of benzene, fluorobenzene, m-xylene
and p-xylene.] Prakov Lek, 33: 124-127 (in Czech with English
summary).
Gut I, Terelius Y, Frantik E, Linhart I, Soucek P, Filipcova B, &
Kluchova H (1993) Exposure to various benzene derivatives differently
induces cytochromes P450 2B1 and P450 2E1 in rat liver. Arch Toxicol,
67: 237-243.
Haglund U, Lundberg I, & Zech L (1980) Chromosome aberrations and
sister chromatid exchanges in Swedish paint industry workers. Scand J
Work Environ Health, 6: 291-298.
Halder CA, Holdsworth CE, Cockrell BY, & Piccirillo VJ (1985)
Hydrocarbon nephropathy in male rats: Identification of the
nephrotoxic components of unleaded gasoline. Toxicol Ind Health,
1: 67-87.
Harland BJ, Whitby FJ, & Comber MHI (1985) Measurement of volatile
aromatic hydrocarbons in an industrial estuary. Int J Environ Anal
Chem, 20: 295-312.
Harper C (1975) p-Xylene metabolism by rat pulmonary and hepatic
microsomes. Fed Proc, 34: 785.
Hass U & Jakobsen BM (1993) Prenatal toxicity of xylene inhalation in
the rat: A teratogenicity and postnatal study. Pharmacol Toxicol,
73: 20-23.
Hass U, Lund SP, Simonsen L, & Schaich Fries A (1995) Effects of
prenatal exposure to xylene on postnatal development and behavior in
rats. Neurotoxicol Teratol, 17: 341-349.
Hastings L, Cooper GP, & Burg W (1984) Human sensory response to
selected petroleum hydrocarbons. Adv Med Environ Toxicol, 6: 255-270.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 5(suppl 1): 3-142.
Hawthorne SB & Sievers RE (1984) Emission of organic air pollutants
from shale oil waste waters. Environ Sci Techol, 18: 483-490.
Herman DC, Inniss WE, & Mayfield CI (1990) Impact of volatile aromatic
hydrocarbons on growth of the freshwater alga Selenastrum
capricornutum. Aquat Toxicol, 18: 87-100.
Higgins CE, Griest WH, & Olerich G (1983) Application of tenax
trapping to analysis of gas phase organic compounds in ultra-low tar
cigarette smoke. J Assoc Off Anal Chem, 66(5): 1074-1083.
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington, DC,
US Department of the Interior, Fish and Wildlife Service, 138 pp (Fish
and Wildlife Technical Report No. 2).
Hine CH & Zuidema HH (1970) The toxicological properties of hydro-
carbon solvents. Ind Med, 38: 215-220.
Hipolito RN (1980) Xylene poisoning in laboratory workers: Case
reports and discussion. Lab Med, 11: 593-595.
Holcombe GW, Phipps GL, Sulaiman AH, & Hoffman AD (1987) Simultaneous
multiple species testing: Acute toxicity of 13 chemicals to 12 diverse
freshwater amphibian, fish, and invertebrate families. Arch Environ
Contam Toxicol, 16: 697-710.
Holmberg B, Jakobson I, & Malmfors T (1974) The effect of organic
solvents on erythrocytes during hypotonic hemolysis. Environ Res,
7: 193-205.
Honma T, Sudo A, Miyagawa M, Sato M, & Hasegawa H (1983) Significant
changes in the amounts of neurotransmitter and related substances in
rat brain induced by subacute exposure to low levels of toluene and
xylene. Ind Health, 21: 143-151.
Huang M-Y, Jin C, Liu Y-T, Li B-H, Qu QS, Ushida Y, Inoue O, Nakatsuka
M, Watanabe T, & Ikeda M (1994) Exposure of workers to a mixture of
toluene and xylenes. I. Metabolism. Occup Environ Med, 51: 42-46.
Hudak A & Ungvary G (1978) Embryotoxic effects of benzene and its
methyl derivatives: Toluene, xylene. Toxicology, 11: 55-63.
Huff JE, Eastin W, Roycroft J, Eustis SL, & Haseman JK (1988)
Carcinogenesis studies of benzene, methyl benzene, and dimethyl
benzenes. Ann NY Acad Sci, 534: 427-440.
Hung I-F & Liao M-H (1991) Aromatic hydrocarbons in indoor air. J
Environ Sci Health, A26: 487-492.
Hurford N, Law RJ, Fileman TW, Payne AP, & Colcomb-Heiliger K (1990)
Concentrations of chemicals in the North Sea due to operational
discharges from chemical tankers: Results from the second survey,
October 1988. Oil Chem Pollu, 7: 241-270.
Husman K (1980) Symptoms of car painters with long-term exposure to a
mixture of organic solvents. Scand J Work Environ Health, 6: 19-32.
Husman K & Karli P (1980) Clinical neurological findings among car
painters exposed to a mixture of organic solvents. Scand J Work
Environ Health, 6: 33-39.
Hutchins SR (1991) Biodegradation of monoaromatic hydrocarbons by
aquifer microrganisms using oxygen, nitrate or nitrous oxide as the
terminal electron acceptor. Appl Environ Microbiol, 57: 2403-2407.
Hutchins SR, Sewell GW, Kovacs DA, & Smith GA (1991a) Biodegradation
of aromatic hyrocarbons by aquifer microroganisms under denitrifying
conditions. Environ Sci Techol, 25: 68-76.
Hutchins SR, Downs WC, Wilson JT, Smith GB, Kovacs DA, Fine DD,
Douglass RH, & Hendrix DJ (1991b) Effect of nitrate addition on
biorestoration of fuel-contaminated aquifer: Field demonstration.
Ground Water, 29: 571-580.
Hutchinson TC, Hellebust JA, Tam D, Mascarenhas RA, & Shiu WY (1980)
The correlation of the toxicity to algae of hydrocarbons and
halogenated hydrocarbons with their physical-chemical properties.
Environ Sci Res, 16: 577-586.
IARC (1989) Xylene. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research on
Cancer, pp 125-156 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 47).
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: Assessing human health risks of chemicals:
Derivation of guidance values for health-based exposure limits. World
Health Organization, Geneva, 73 pp.
Ivanovich E, Antov G, Goranova L, Spasovski M, Bainova A, & Vasileva Z
(1985) Combined effect of some physical and chemical factors. J Hyg
Epidemiol Microbiol Immunol, 28: 105-110.
Jacobs G, Martens M, & Mosselmans G (1987) Proposal of limit
concentrations for skin irritation within the context of a new EEC
directive on the classification and labelling of preparations. Regul
Toxicol Pharmacol, 7: 370-378.
Jamison VW, Raymond RL, & Hudson JD (1976) Biodegradation of
high-octane gasoline. In: Proceedings of the 3rd International
Biodegradation Symposium. Allied Science Publishers, pp 187-196.
Jenkins LJ Jr, Jones RA, & Siegel J (1970) Long-term inhalation
screening studies of benzene, toluene, o-xylene, and cumene on
experimental animals. Toxicol Appl Pharmacol, 16: 818-823.
Jones BMR (1988) The measurement of ambient hydrocarbon concentrations
in the atmosphere at Harwell during the period April 1986 to March
1987. Oxfordshire, Harwell Laboratoty (AERE Report R13174).
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and vehicle emissions. Environ Int, 11: 383-392.
Jori A, Calamari D, Di Domenico A, Galli CL, Galli E, Marinovich M, &
Silano V (1986) Ecotoxicological profile of xylenes. Ecotoxicol
Environ Saf, 11: 44-80.
Joyner RE & Leak Pegues W (1961) A health hazard associated with epoxy
resin-concrete dust. J Occup Med, 3: 211-214.
Juhnke I & Lüdemann D (1978) [Results of acute fish toxicity of 200
chemical compounds using the golden orfe tests.] Z Wasser Abwasser
Forsch, 11: 161-164 (in German).
Jüttner F (1988a) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in the forest air of the Southern
Black Forest. Chemosphere, 17: 309-317.
Jüttner F (1988b) Motor-boat-derived volatile organic compounds (VOC)
in lakewater. Z Wasser Abwasser Forsch, 21: 36-39.
Kaneko T, Wang P-Y, Tsukada H, & Sato A (1995) m-Xylene
toxicokinetics in phenobarbital-treated rats: comparison among
inhalation exposure, oral administration, and intraperitoneal
administration. Toxicol Appl Pharmacol, 131: 13-20.
Kappeler Th & Wuhrmann K (1978a) Microbial degradation of the water
soluble fraction of gas oil. I. Water Res, 12: 327-333.
Kappeler Th & Wuhrmann K (1978b) Microbial degradation of the water
soluble fraction of gas oil. II. Bioassays with pure strains. Water
Res, 12: 335-342.
Karlson U & Frankenberger WT (1989) Microbial degradation of benzene
and toluene in groundwater. Bull Environ Contam Toxicol, 43: 505-510.
Kauss PB & Hutchinson TC (1975) The effects of water-soluble petroleum
components on the growth of Chlorella vulgaris Beijerinck. Environ
Pollut, 9: 157-174.
Kauss PB, Hutchinson TC, Soto C, Hellebust J, & Griffiths M (1973) The
toxicity of crude oil and its components to freshwater algae. In:
Prevention and control of spills. Washington, DC, American Petroleum
Institute, pp 703-714.
Kawai T, Mizunuma K, Yasugi T, Horiguchi S, Uchida Y, Iwami O, Iguchi
H, & Ikeda M (1991a) Urinary methylhippuric acid isomer levels after
occupational exposure to a xylene mixture. Int Arch Occup Environ
Health, 63: 69-75.
Kawai T, Yamaoka K, Uchida Y, & Ikeda M (1991b) Exposure to vapors of
benzene and other aromatic solvents in tank truck loading and
delivery. Bull Environ Contam Toxicol, 46(1): 1-8.
Kawamura K & Kaplan IR (1983) Organic compounds in the rain water of
Los Angeles. Environ Sci Technol, 17: 497-501.
Kawata K & Fujieda Y (1993) [Volatile aromatic hydrocarbons in ambient
air.] Jpn J Toxicol Environ Health, 39(1): 37-43 (in Japanese).
Kennah HE, Hignet S, Laux PE, Dorko JD, & Barrow CS (1989) An
objective procedure for quantitating eye irritation based upon changes
of corneal thickness. Fundam Appl Toxicol, 12: 258-268.
Kennicutt MC II, Brooks JM, & McDonald TJ (1984) Volatile organic
inputs from an ocean outfall near Barceloneta, Puerto Rico.
Chemosphere, 13: 535-548.
Kenrick MAP, Clark L, Baxter KM, Fleet M, James HA, Gibson TM, &
Turrell MB (1985) Trace organics in British aquifers: a baseline
survey. Medmenham, Marlow, United Kingdom, Water Research Centre
(Technical Report TR223).
Kilburn KH, Warshaw R, Boylen CT, Balmes J, Johnson S, Seidman B, &
DeFlorio G (1983) Toxic effects of formaldehyde and solvents in
histology technicians: A preliminary report. J Histotechnol, 6: 73-76.
Kilburn KH, Seidman BC, & Warshaw R (1985) Neurobehavioral and
respiratory symptoms of formaldehyde and xylene exposure in histology
technicians. Arch Environ Health, 40: 229-233.
Kirschner E (1995) Production of top 50 chemicals increased sub-
stantially in 1994. Chem Eng News, April: 16-20.
Kjorsvic E, Saethre LJ, & Lonning S (1982) Effects of short-term
exposure to xylenes on the early cleavage stages of cod eggs ( Gadus
morhus L.). Sarsia, 67: 299-308.
Klaucke DN, Johansen M, & Vogt RL (1982) An outbreak of xylene
intoxication in a hospital. Am J Ind Med, 3: 173-178.
Kligman AM (1966) The identification of contact allergens by human
assay. III. The maximization test: A procedure for screening and
rating contact sensitizers. J Invest Dermatol, 47: 393-409.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies: Relationship for 50 industrial pollutants.
Toxicology, 19: 229-238.
Korsak Z, Sokal JA, & Gorny R (1992) Toxic effect of combined exposure
to toluene and m-xylene in animals. III. Subchronic inhalation
study. Pol J Occup Med Environ Health, 5: 27-33.
Korsak Z, Swiercz R, & Jedrychowski R (1993) Effects of acute combined
exposure to n-butyl alcohol and m-xylene. Pol J Occup Med Environ
Health, 6: 35-41.
Korsak Z, Wisniewska-Knypl J, & Swiercz R (1994) Toxic effects of
subchronic combined exposure to n-butyl alcohol and m-xylene in
rats. Int J Occup Med Environ Health, 7: 155-166.
Kroneld R (1989) Volatile pollutants in suburban and industrial air.
Bull Environ Contam Toxicol, 42: 868-872.
Kuhn EP, Colberg PJ, Schnoor JL, Wanner O, Zehnder AJB, &
Schwarzenbach RP (1985) Microbial transformations of substituted
benzenes during infiltration of river water to ground water:
Laboratory column studies. Environ Sci Techol, 19: 961-968.
Kuhn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Kurppa K & Husman K (1982) Car painters' exposure to a mixture of
organic solvents. Serum activities of liver enzymes. Scand J Work
Environ Health, 8: 137-140.
Kyrklund T, Kjellstrand P, & Haglid K (1987) Brain lipid changes in
rats exposed to xylene and toluene. Toxicology, 45: 123-133.
Labows J, Preti G, Loezle E, Leyden J, & Kligman A (1979) Analysis of
human axillary volatiles: Compounds of exogenous origin. J Chromatogr,
163: 294-299.
Lagorio S, Forastiere F, Iavarone I, Vanacore N, Fuselli S, & Carere A
(1993) Exposure assessment in a historical cohort of filling station
attendants. Int J Epidemiol, 22(6-suppl 2): S51-S56.
Lagrue G (1976) Hydrocarbon exposure and chronic glomerulonephritis.
Lancet, 1: 1191.
Laine A, Savolainen K, Riihimäki V, Matikainen E, Salmi T, & Juntunen
J (1993) Acute effects of m-xylene inhalation on body sway, reaction
times, and sleep in man. Int Arch Occup Environ Health, 65: 179-188.
Lanzerstofer CH & Puxbaum H (1990) Volatile hydrocarbons in and around
Vienna, Austria. Water Air Soil Pollut, 51: 345-355.
Laparé S, Tardif R, & Brodeur J (1993) Effect of various exposure
scenarios on the biological monitoring of organic solvents in alveolar
air. I. Toluene and m-xylene. Int Arch Occup Environ Health,
64: 569-580.
Larsby B, Ödkvist LM, Hydén D, & Liedgren SRC (1976) Disturbancies of
the vestibular system by toxic agents. Acta Physiol Scand,
440(suppl): 108.
Lauwerys R & Buchet JP (1988) Biological monitoring of exposure to
benzene, toluene and xylene. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 205-222 (IARC
Scientific Publications No. 85).
Lauwerys RR, Dath T, Lachapelle J-M, Buchet J-P, & Roels H (1978) The
influence of two barrier creams on the percutaneous absorption of
m-xylene in man. J Occup Med, 20: 17-20.
Lazarew NW (1929) [On the toxicity of various organic compounds
vapors.] Arch Exp Pathol Pharmacol, 143: 223-233 (in German).
Lebowitz H, Brusick D, Matheson D, Jagannath DR, Reed M, Goode S, &
Roy G (1979) Commonly used fuels and solvents evaluated in a battery
of short-term bioassays. Environ Mutagen, 1: 172-173.
Le Gore RS (1974) The effects of Alaskan crude oil and selected
hydrocarbon compounds on embryonic development of the Pacific oyster,
Crassostrea gigas. Diss Abstr B, 35: 3168.
Lewis P (1994) Report of airborne solvent concentrations in a printing
ink manufacturing plant in Cape Town, Republic of South Africa. Appl
Occup Environ Hyg, 9: 147-151.
Liira J, Elovaara E, Raunio H, Riihimäki V, & Engström K (1991)
Metabolic interaction and diposition of methyl ethyl ketone and
m-xylene in rats at single and repeated inhalation exposure.
Xenobiotica, 21: 53-63.
Lindström K (1973) Psychological performances of workers exposed to
various solvents. Work Environ Health, 10: 151-155.
Lindström K, Antti-Poika M, Tola S, & Hyytiäinen A (1982)
Psychological prognosis of diagnosed chronic organic solvent
intoxication. Neurobehav Toxicol Teratol, 4: 581-588.
Lioy PJ, Wallace L, & Pellizzari E (1991) Indoor/outdoor, and personal
monitor and breath analysis relationships for selected volatile
organic compounds measured at three homes during New Jersey
TEAM - 1987. J Exp Anal Environ Epidemiol, 1(1): 45-61.
Lonneman WR, Seila RL, & Bufalini JJ (1978) Ambient air hydrocarbons
in Florida. Environ Sci Technol, 12: 459-463.
Lovegren NV, Fisher GS, Legendre MG, & Schuller WH (1979) Volatile
constituents of dried legumes. J Agric Food Chem, 27: 851-853.
Lundberg I & Håkansson M (1985) Normal serum activities of liver
enzymes in Swedish paint industry workers with heavy exposure to
organic solvents. Br J Ind Med, 42: 596-600.
Lundberg I, Håkansson M, Gustavsson P, Kronevi T, & Lidums V (1983)
[Relative hepatotoxic effect of industrial solvents.] Solna, Sweden,
National Institute of Occupational Health, pp 1-42 (Work and Health
1983:22) (in Swedish with English summary).
Lundberg I, Ekdahl M, Kronevi T, Lidums V, & Lundberg S (1986)
Relative hepatotoxicity of some industrial solvents after
intraperitoneal injection or inhalation exposure in rats. Environ Res,
40: 411-420.
Lundberg I, Nise G, Hedenborg G, Högberg M, & Vesterberg O (1994)
Liver function tests and urinary albumin in house painters with
previous heavy exposure to organic solvents. Occup Environ Med,
51: 347-353.
Lyman WJ, Reehl WF, & Rosenblatt DH (1981) Handbook of chemical
property estimation methods. New York, Mc-Graw Hill Book Company.
McCarroll NE, Keech BH, & Piper CE (1981a) A microsuspension
adaptation of the Bacillus subtilis "rec" assay. Environ Mutagen,
3: 607-616.
McCarroll NE, Piper CE, & Keech BH (1981b) An E. coli
microsuspension assay for the detection of DNA damage induced by
direct-acting agents and promutagens. Environ Mutagen, 3: 429-444.
McDonald TJ, Kennicutt MC II, & Brooks JM (1988) Volatile organic
compounds at a coastal site. Chemosphere, 17: 123-136.
McFall JA, Antoine SR, & Deleon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14: 1253.
Mackay D & Leinonen PJ (1975) Rate of evaporation of low solubility
contaminants from water bodies to the atmosphere. Environ Sci Technol,
9: 1178-1180.
Mackay D & Wolkoff AW (1973) Rate of evaporation of low solubility
contaminants from water bodies to atmosphere. Environ Sci Technol,
7: 611-614.
Mackay D, Paterson S, Cheung B, & Neely WB (1985) Evaluating the
environmental behaviour of chemicals using a level III fugacity model.
Chemosphere, 14: 335-374.
MAFF (1991) Monitoring and surveillance of non-radioactive
contaminants in the aquatic environment and activities regulating the
disposal of wastes at sea, 1988-89. Lowestoft, United Kingdom,
Ministry of Agriculture, Fisheries and Food, Directorate of Fisheries
Research (Aquatic Environment Monitoring Report No. 26).
Maizlish NA, Fine LJ, Albers JW, Whitehead L, & Langolf GD (1987) A
neurological evaluation of workers exposed to mixtures of organic
solvents. Br J Ind Med, 44: 14-25.
Major DW, Mayfield CI, & Barker JF (1988) Biotransformation of benzene
by denitrification in aquifer sand. Ground Water, 26: 8-14.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1983) Benzene as an
experimental carcinogen: up-to-date evidence. Acta Oncol, 4: 141-164.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
Current results and ongoing research. Am J Ind Med, 7: 415-446.
Mansour M, Moza PN, Barlas H, & Parlar H (1985) [A contribution to the
topic of photo-stability of environmental organic compounds in the
presence of hydrogen peroxide in aquatic systems.] Chemosphere,
14: 1469-1474 (in German).
Marks TA, Ledoux TA, & Moore JA (1982) Teratogenicity of a commercial
xylene mixture in the mouse. J Toxicol Environ Health, 9: 97-105.
Maynard DJ & Weber DD (1981) Avoidance reactions of juvenile coho
salmon (Oncorhynchus kisutch) to monocyclic aromatics. Can J Fish
Aquat Sci, 38: 772-778.
Meinhart E & Schreier P (1986) Study of flavour compounds from
Parmigiano Reggiano cheese. Milchwissenschaft, 41: 689-691.
Michael LC, Pellizzari ED, & Norwood DL (1991) Application of the
master analytical scheme to the determination of volatile organics in
wastewater influents and effluents. Environ Sci Technol, 25: 150-155.
Mirkova E, Zaikov C, Antov G, Mikhailova A, Khinkova L, & Benchev I
(1983) Prenatal toxicity of xylene. J Hyg Epidemiol Microbiol Immunol,
27: 337-343.
Mohtashamipur E, Norpoth K, Woelke U, & Huber P (1985) Effects of
ethylbenzene, toluene, and xylene on the induction of micronuclei in
bone marrow polychromatic erythrocytes of mice. Arch Toxicol,
58: 106-109.
Molnar J, Paksy KA, & Naray M (1986) Changes in the rat's motor
behaviour during 4-hr inhalation exposure to prenarcotic
concentrations of benzene and its derivatives. Acta Physiol Hungarica
67: 349-354.
Morgan P & Watkinson RJ (1990) Assessment of the potential for in
situ biotreatment of hydrocarbon-contaminated soils. Water Sci
Technol, 22: 63-68.
Morley R, Eccleston DW, Douglas CP, Greville WEJ, Scott DJ, & Anderson
J (1970) Xylene poisoning: A report on one fatal case and two cases of
recovery after prolonged unconsciousness. Br Med J, 3: 442-443.
Morrow JE, Gritz RL, & Kirton MP (1975) Effects of some components of
crude oil on young coho salmon. Copeia, 2: 326-331.
Moser VC, Coggeshall EM, & Balster RL (1985) Effects of xylene isomers
on operant responding and motor performance in mice. Toxicol Appl
Pharmacol, 80: 293-298.
Moszczynski P & Lisiewicz J (1985) Occupational exposure to benzene,
toluene and xylene and the lymphocyte lysosomal N-acetyl-beta-
D-glucosaminidase. Ind Health, 23: 47-51.
Nakamura S, Oda Y, Shimada T, Oki I, & Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutat
Res, 192: 239-246.
Naskali L, Oksanen H, & Tähti H (1994) Astrocytes as targets for CNS
effects of organic solvents in. Neurotoxicology, 15: 609-612.
Nasterlack M, Triebig G, & Stelzer O (1994) Hepatotoxic effects of
solvent exposure around permissible limits and alcohol consumption in
printers over a 4-year period. Int Arch Occup Environ Health, 66:
161-165.
Neff JM, Anderson JW, Cox BA, Laughlin RB, Rossi SS, & Tatem HE (1976)
Effects of petroleum on survival, respiration and growth of marine
animals. In: Sources, effects and sinks of hydrocarbons. Washington,
DC, American Institute of Biological Science, pp 515-523.
Nelson PF & Quigley SM (1982) Non-methane hydrocarbons in the
atmosphere of Sydney, Australia. Environ Sci Techol, 16: 650-655.
Nelson KW, Ege JF Jr, Ross M, Woodman LE, & Silverman L (1943) Sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
25: 282-285.
NIOSH (1991) Health hazard evaluation report: Rockwell International,
Newark, Ohio. Cincinnati, Ohio, National Institute for Occupational
Safety and Health (Report HETA 90-368-2137; PB92-131929).
NIOSH (1993a) Health hazard evaluation report: National Centers for
Environmental Health, Fairbanks, Alaska. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA 93-606-2336;
PB94-133667).
NIOSH (1993b) Health hazard evaluation report: Dallas and Mavis
Forwarding Co., Seattle, Washington. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA
91-0103-2351; PB94-131836).
NIOSH (1993c) Health hazard evaluation report: National Center for
Environmental Health, Albany, New York. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA
93-0884-2344; PB94-129020).
NIOSH (1993d) Health hazard evaluation report: National Centers for
Environmental Health, Stamford, Connecticut. Cincinnati, Ohio,
National Institute for Occupational Safety and Health (Report HETA
93-802-2338; PB94-134343).
NIOSH (1994) Health hazard evaluation report: Pan American Health
Organization, Bogota, Colombia (South America). Cincinnati, Ohio,
National Institute for Occupational Safety and Health (Report HETA
94-0253-2451; PB95-147062).
NTP (1986) Technical report on the toxicology and carcinogenesis
studies of xylenes (mixed) in F344/N rats and B6C3F1 mice. Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program (NIH Publication No. 87-2583).
Nunes P & Benville PE (1979) Uptake and depuration of petroleum
hydrocarbons in the Manila Clam, Tapes semidecussata Reeve. Bull
Environ Contam Toxicol, 21: 719-726.
Nylén P & Hagman M (1994) Function of the auditory and visual systems,
and of peripheral nerves in rats after long-term combined exposure to
n-hexane and methylated benzene derivatives. II. Xylene. Pharmacol
Toxicol, 74: 124-129.
Nylén P, Ebendal T, Eriksdotter-Nilsson M, Hansson T, Henschen A,
Johnson A-C, Kronevi T, Kvist U, Sjöstrand NO, Höglund G, & Olson L
(1989) Testicular atrophy and loss of nerve growth factor-
immunoreactive germ cell line in rats exposed to n-hexane and
a protective effect of simultaneous exposure to toluene or xylene.
Arch Toxicol, 63: 296-307.
Ogata M & Fujii T (1979) Urinary excretion of hippuric acid and
m-methylhippuric acid after administration of toluene and m-xylene
mixture to rats. Int Arch Occup Environ Health, 43: 45-51.
Ogata M & Hobara T (1979) A new direct method for colorimetric
determination of hippuric acid and methylhippuric acid as indices of
toluene and m-xylene, and its application to workers using thinner.
Ind Health, 17: 61-72.
Ogata M & Miyake Y (1979) Disappearance of aromatic hydrocarbons and
organic sulphur compounds from fish reared in crude oil suspensions.
Water Res, 13: 75-78.
Ogata M, Yamasaki Y, Meguro T, Sugihara R, & Shimada Y (1979)
Quantitation of urinary o-xylene metabolites in rats and human
beings by high performance liquid chromatography. Ind Health,
17: 123-125.
Ogata M, Yamazaki Y, Sugihara R, Shimada Y, & Meguro T (1980)
Quantitation of urinary o-xylene metabolites of rats and human
beings by high performance liquid chromatography. Int Arch Occup
Environ Health, 46: 127-139.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
Omori T & Yamada K (1970) Studies on the utilisation of hydrocarbons
by microorganisms. Part XVI. Detection of metabolic intermediates of
xylene and pseudocumene. Agric Biol Chem, 34: 659-663.
Omori T, Horiguchi S, & Yamada K (1967) Studies on the utilisation of
hydrocarbons by microorganisms. Part X. Screening of aromatic
hydrocarbon assimilating microorganisms and p-toluic acid formation
from p-xylene. Agric Biol Chem, 31: 1337-1342.
Otson R & Williams DT (1981) Evaluation of a liquid-liquid extraction
technique for water pollutants. J Chromatogr, 212: 187-197.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities.
J Assoc Off Anal Chem, 65: 1370-1374.
Otson R, Fellin P, & Tran Q (1993) Investigation of VOCs in Canadian
residences. In: Indoor air '93. Proceedings of the 6th International
Conference on Indoor Air Quality and Climate, Helsinki, Finland, 4-8
July 1993 - Volume 2: Chemicals in indoor air, materials emissions,
pp 141-146.
Ödkvist LM, Larsby B, Tham R, & Aschan G (1979) On the mechanism
of vestibular disturbances caused by industrial solvents. Adv
Oto-rhino-laryngol, 25: 167-172.
Ödkvist LM, Larsby B, Fredrickson JMF, Liedgren SRC, & Tham R (1980)
Vestibular and oculomotor disturbances caused by industrial solvents.
J Otolaryngol, 9: 53-59.
PACE (1987) A study of exposure to motor gasoline hydrocarbon vapours
at service stations: Phase II - Summer Study. Petroleum Association
for Conservation of the Canadian Environment (PACE Report No. 87-5).
PACE (1989) A study of exposure to motor gasoline hydrocarbon vapours
at service stations: Phase III - Winter Study. Petroleum Association
for Conservation of the Canadian Environment (PACE Report No. 89-3).
Padilla S, Lyerly DL, & Pope CN (1992) Subacute ethanol consumption
reverses p-xylene-induced decreases in axonal transport. Toxicology,
75: 159-167.
Palmer KT & Rycroft RJG (1993) Occupational airborne contact urticaria
due to xylene. Contact Dermatitis, 28: 44.
Pap M & Varga C (1987) Sister-chromatid exchanges in peripheral
lymphocytes of workers occupationally exposed to xylenes. Mutat Res,
187: 223-225.
Park SH, AuCoin TA, Silverman DM, & Schatz RA (1994) Time-dependent
effects of o-xylene on rat lung and liver microsomal membrane
structure and function. J Toxicol Environ Health, 43: 469-481.
Patel JM, Harper C, & Drew RT (1976) Inactivation of pulmonary
cytochrome P-450 by p-xylene intoxication. Pharmacologist, 18: 210.
Patel JM, Harper C, & Drew RT (1978) The biotransformation of
p-xylene to a toxic aldehyde. Drug Metab Dispos, 6: 368-374.
Patel JM, Harper C, Gupta BN, & Drew RT (1979) Changes in serum
enzymes after inhalation exposure of p-xylene. Bull Environ Contam
Toxicol, 21: 17-24.
Pathiratne A, Puyear RL, & Brammer JD (1986) A comparative study of
the effects of benzene, toluene, and xylenes on their in metabolism
and drug-metabolizing enzymes in rat liver. Toxicol Appl Pharmacol,
82: 272-280.
Pellizzari EC, Hartwell TD, Harris BSH III, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mothers milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pellizzari ED, Zweidinger RA, & Sheldon LS (1988) Determination
of benzene, toluene and xylene in breath samples by gas
chromatography/mass spectrometry. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 267-279 (IARC
Scientific Publications No. 85).
Pennell KD, Rhue RD, Rao PSC, & Johnston CT (1992) Vapor-phase
sorption of p-xylene and water on soils and clay minerals. Environ
Sci Technol, 26: 756-763.
Perry R & Twibell JD (1973) A time based elution technique for the
estimation of specific hydrocarbons in air. Atmos Environ, 7: 929.
Petersson G (1982) Ambient hydrocarbons from motor-car assembly plants
in Scandinavia. Environ Pollut, B4: 207-217.
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38: 1419-1429.
Pound AW (1970) Induced cell proliferation and the initiation of skin
tumour formation in mice by ultraviolet light. Pathology, 2: 269-275.
Pound AW & Withers HR (1963) The influence of some irritant chemicals
and scarification on tumour initiation by urethane in mice. Br J
Cancer, 17: 460-470.
Proust B, Hannequin D, Caillard JF, Prudent P, Hemet J, & Samson N
(1986) Le psychosyndrome aux solvants: Résultats des tests
psychométriques chez dix laborantines exposées. Arch Mal Prof,
47: 305-310.
Pryor GT, Robert CS, & Howd RA (1987) Hearing loss in rats caused by
inhalation of mixed xylenes and styrene. J Appl Toxicol, 7: 55-61.
Pussemier L, Szabó G, & Bulman RA (1990) Prediction of the soil
adsorption coefficient Koc for aromatic pollutants. Chemosphere,
21: 1199-1212.
Pyykkö K (1980) Effects of methylbenzenes on microsomal enzymes in rat
liver, kidney and lung. Biochim Biophys Acta, 633: 1-9.
Pyykkö K (1986) Inhibition of microsomal monooxygenases in by
aromatic hydrocarbons. Arch Toxicol, 9(suppl): 371-373.
Pyykkö K, Paavilainen S, Metsä-Ketelä T, & Laustiola K (1987) The
increasing and decreasing effects of aromatic hydrocarbon solvents on
pulmonary and hepatic cytochrome P-450 in the rat. Pharmacol Toxicol,
60: 288-293.
Rao PSC, Hornsby AG, & Jessup RE (1985) Indices for ranking the
potential for pesticide contamination of ground water. Soil Crop Sci
Soc Proc, 44: 1-8.
Raunio H, Liira J, Elovaara E, Riihimäki V, & Pelkonen O (1990)
Cytochrome P450 isozyme induction by methyl ethyl ketone and
m-xylene in rat liver. Toxicol Appl Pharmacol, 103: 175-179.
Raymond RL, Jamison VW, & Hudson JO (1969) Microbial hydrocarbon
co-oxidation. II. Use of ion-exchange resins. Appl Microbiol,
17: 512-515.
Recchia G, Perbellini L, Prati GF, Dean P, & Ancona G (1985) [Coma due
to accidental ingestion of xylene. Treatment with charcoal
hemoperfusion.] Med Lav, 76: 67-73 (in Italian with English summary).
Reinert KH, Hunter JV, & Sabatino T (1983) Dynamic heated headspace
analysis of volatile organic compounds present in fish tissue samples.
J Agric Food Chem, 31: 1057-1060.
Reinhard M, Goodman NL, & Barker JF (1984) Occurrence and distribution
of organic chemicals in two landfill leachate plumes. Environ Sci
Technol, 18: 953-961.
Rhue RD, Rao PSC, & Smith RE (1988) Vapour-phase adsorption of
alkylbenzenes and water on soils and clays. Chemosphere, 17: 727-741.
Richer CL, Chakrabarti S, Senecal-Quevillon M, Duhr MA, Zhang XX, &
Tardif R (1993) Cytogenic effects of low-level exposure to toluene,
xylene and their mixture on human blood lymphocytes. Int Arch Occup
Environ Health, 64: 81-85.
Rigdon RH (1940) Capillary permeability in areas of inflammation
produced by xylene. Arch Surg, 41: 101-109.
Riihimäki V (1979a) Conjugation and urinary excretion of toluene and
m-xylene in man. Scand J Work Environ Health, 5: 135-142.
Riihimäki V (1979b) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand J Work
Environ Health, 5: 143-150.
Riihimäki V & Hänninen O (1987) Xylenes. In: Snyder R. ed. Ethel
Browning's toxicity and metabolims of industrial solvents, 2nd ed.
Amsterdam, Oxford, New York, Elsevier Science Publishers, vol 1, pp
64-84.
Riihimäki V & Pfäffli P (1978) Percutaneous absorption of solvent
vapors in man. Scand J Work Environ Health, 4: 73-85.
Riihimäki V & Savolainen K (1980) Human exposure to m-xylene,
kinetics and acute effects on the central nervous system. Ann Occup
Hyg, 23: 411-422.
Riihimäki V, Pfäffli P, & Savolainen K (1979a) Kinetics of m-xylene
in man: Influence of intermittent physical exercise and changing
environmental concentrations on kinetics. Scand J Work Environ Health,
5: 232-248.
Riihimäki V, Pfäffli P, Savolainen K, & Pekari K (1979b) Kinetics of
m-xylene in man: General features of absorption, distribution,
biotransformation and excretion in repetitive inhalation exposure.
Scand J Work Environ Health, 5: 217-231.
Riihimäki V, Laine A, Savolainen K, & Sippel H (1982a) Acute solvent-
ethanol interactions with special reference to xylene. Scand J
Work Environ Health, 8: 77-79.
Riihimäki V, Savolainen K, Pfäffli P, Pekari K, Sippel HW, & Laine A
(1982b) Metabolic interaction between m-xylene and ethanol. Arch
Toxicol, 49: 253-263.
Roberts AE, Bogdanffy MS, Brown DR, & Schatz RA (1986) Lung metabolism
of benzo(a)pyrene in rats treated with p-xylene and/or ethanol. J
Toxicol Environ Health, 18: 257-266.
Rogerson A, Shiu WY, Huang GL, Mackay D, & Berger J (1983)
Determination and interpretation of hydrocarbon toxicity to ciliate
protozoa. Aquat Toxicol, 3: 215-228.
Rosen MB, Crofton KM, & Chernoff N (1986) Postnatal evaluation of
prenatal exposure to p-xylene in the rat. Toxicol Lett, 34: 223-229.
Rosengren LE, Kjellstrand P, Aurell A, & Haglid KG (1986) Irreversible
effects of xylene on the brain after long term exposure: A
quantitative study of DNA and the glial cell marker proteins S-100 and
GFA. Neurotoxicology, 7: 121-136.
Ruijten MW, Hooisma J, Brons JT, Habets CE, Emmen HH, & Muijser H
(1994) Neurobehavioral effects of long-term exposure to xylene and
mixed organic solvents in shipyard spray painters. Neurotoxicology,
15: 613-620.
Rydzynski K, Korsak Z, Jedlinska U, & Sokal JA (1992) The toxic
effects of combined exposure to toluene and m-xylene in animals. IV.
Liver ultrastructure after subchronic inhalatory exposure. Pol J Occup
Med Environ Health, 5: 35-42.
Sabel GV & Clark TP (1984) Volatile organic compounds as indicators of
municipal solid waste leachate contamination. Waste Manage Res,
2: 119-130.
Sabljic A & Güsten H (1990) Predicting the night-time NO3 radical
reactivity in the troposphere. Atmos Environ, 24A: 73-78.
Sack TM, Steele DH, Hammerstrom K, & Remmers K (1992) A survey of
household products for volatile organic compounds. Atmos Environ,
26A(6): 1063-1070.
Sanborn HR & Malins DC (1980) The disposition of aromatic hydrocarbons
in adult spot shrimp (Pandalus platyceros) and the formation of
metabolites of naphthalene in adult and larval spot shrimp.
Xenobiotica, 10: 193-200.
Sato A, Fujiwara Y, & Nakajima T (1974) Solubility of benzene, toluene
and m-xylene in various body fluids and tissues of rabbits. Jpn J
Ind Health, 16: 30-31.
Sauer TC (1981) Volatile liquid hydrocarbon characterisation of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environ Sci Technol, 15: 917-923.
Savolainen K (1980) Combined effects of xylene and alcohol on the
central nervous system. Acta Pharmacol Toxicol, 46: 366-372.
Savolainen H & Pfäffli P (1980) Dose-dependent neurochemical changes
during short-term inhalation exposure to m-xylene. Arch Toxicol,
45: 117-122.
Savolainen K & Linnavuo M (1979) Effects of m-xylene on human
equilibrium measured with a quantitative method. Acta Pharmacol
Toxicol, 44: 315-318.
Savolainen K & Riihimäki V (1981) Xylene and alcohol involvement of
the human equilibrium system. Acta Pharmacol Toxicol, 49: 447-451.
Savolainen H & Seppäläinen AM (1979) Biochemical and physiological
effects of organic solvents on rat axon membranes isolated by a new
technique. Neurotoxicology, 1: 467-477.
Savolainen H, Pfäffli P, Helojoki M, & Tengén M (1979a) Neurochemical
and behavioural effects of long-term intermittent inhalation of xylene
vapour and simultaneous ethanol intake. Acta Pharmacol Toxicol,
44: 200-207.
Savolainen H, Vainio H, Helojoki M, & Elovaara E (1979b) Biochemical
and toxicological effects of short-term intermittent xylene inhalation
exposure and combined ethanol intake. Arch Toxicol, 41: 195-205.
Savolainen K, Riihimäki V, Vaheri E, & Linnoila M (1980) Effects of
xylene and alcohol on vestibular and visual functions in man. Scand J
Work Environ Health, 6: 94-103
Savolainen K, Riihimäki V, Laine A, & Kekoni J (1981) Short-term
exposure of human subjects to m-xylene and 1,1,1-trichloroethane.
Int Arch Occup Environ Health, 49: 89-98.
Savolainen K, Riihimäki V, & Laine A (1982a) Biphasic effects of
inhaled solvents on human equilibrium. Acta Pharmacol Toxicol,
51: 237-242.
Savolainen K, Riihimäki V, Laine A, & Kekoni J (1982b) Short-term
exposure of human subjects to m-xylene and 1,1,1-trichloroethane.
Arch Toxicol, 5(suppl): 96-99.
Savolainen K, Kekoni J, Riihimäki V, & Laine A (1984) Immediate
effects of m-xylene on the human central nervous system. Arch
Toxicol, 7(suppl): 412-417.
Savolainen K, Riihimäki V, Luukkonen R, & Muona O (1985a) Changes in
the sense of balance correlate with concentrations of m-xylene in
venous blood. Br J Ind Med, 42: 765-769.
Savolainen K, Riihimäki V, Muona O, Kekoni J, Luukkonen R, & Laine A
(1985b) Conversely exposure-related effects between atmospheric
m-xylene concentrations and human body sense of balance. Acta
Pharmacol Toxicol, 57: 67-71.
Sedivec V & Flek J (1976) The absorption, metabolism, and excretion of
xylenes in man. Int Arch Occup Environ Health, 37: 205-217.
Seidenberg JM, Anderson DG, & Becker RA (1986) Validation of an in
vivo developmental toxicity screen in the mouse. Teratogen
Carcinogen Mutagen, 6: 361-374.
Seip HM, Alstad J, Carlberg GE, Martinsen K, & Skaane R (1986)
Measurement of mobility of organic compounds in soil. Sci Total
Environ, 50: 87-101.
Selgrade MK, Daniels MJ, Jaskot RH, Robinson BL, & Albis JW (1993)
Enhanced mortality and liver damage in virus-infected mice exposed to
p-xylene. J Toxicol Environ Health, 40: 129-144.
Seppäläinen AM, Husman K, & Mårtenson C (1978) Neurophysiological
effects of long-term exposure to a mixture of organic solvents. Scand
J Work Environ Health, 4: 304-314.
Seppäläinen AM, Savolainen K, & Kovala T (1981) Changes induced by
xylene and alcohol in human evoked potentials. Electroencephalogr Clin
Neurophysiol, 51: 148-155.
Seppäläinen AM, Laine A, Salmi T, Verkkala E, Riihimäki V, & Luukkonen
R (1991) Electroencephalographic findings during experimental human
exposure to m-xylene. Arch Environ Health, 46: 16-23.
Sevcik P, Hep A, & Peslova M (1992) Intravenous xylene poisoning.
Intensive Care Med, 18: 377-378.
Sexton K & Westberg H (1980) Ambient hydrocarbon and ozone
measurements downwind of a large automotive painting plant. Environ
Sci Technol, 14: 329-332.
Seyfried B, Glod G, Schocher R, Tschech A, & Zeyer J (1994) Initial
reactions in the anaerobic oxidation of toluene and m-xylene by
denitrifying bacteria. Appl Environ Microbiol, 60(11): 4047-4052.
Sheedy BR, Lazorchak JM, Grunwald DJ, Pickering QH, Pilli A, Hall D, &
Webb R (1991) Effects of pollution on freshwater organisms. Res J
Water Pollut Control Fed, 63: 619-696.
Sheldon LS, Zelon H, Sickles J, Eaton C, Hartwell T, & Jugners R
(1988) Monitoring in and around public access buildings. Proceedings
of the 81st Annual Meeting of the Air Pollution Control Association,
1988. Pittsburgh, Pennsylvania, Air Pollution Control Association,
9 pp (Paper 88/109.8).
Shimizu H, Suzuki Y, Takemura N, Goto S, & Matsushita H (1985) The
results of microbial mutation test for forty-three industrial
chemicals. Jpn J Ind Health, 27: 400-419.
Silverman DM & Schatz RA (1991) Pulmonary microsomal alterations
following short-term low level inhalation of p-xylene in rats.
Toxicology, 65: 271-281.
Simmons JE, Allis JW, Grose EC, Seely JC, Robinson BL, & Berman E
(1991) Assessment of the hepatotoxicity of acute and short-term
exposure to inhaled p-xylene in F-344 rats. J Toxicol Environ
Health, 32: 295-306.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1983) Measurements of
hazardous organic chemicals in the ambient atmosphere. Washington, DC,
US Environmental Protection Agency (EPA-600/3-83-002).
Singh HB, Ferek RJ, Salas LJ, & Nitz KC (1986) Toxic chemicals in the
environment: A program of field measurements. Washington, DC, US
Environmental Protection Agency (EPA-600/3-86/047).
Skowronski GA, Turkall RM, Kadry ARM, & Abdel-Rahman MS (1990) Effects
of soil on the dermal bioavailability of m-xylene in male rats.
Environ Res, 51: 182-193.
Slaine DD & Barker JF (1990) The detection of naturally occurring BTX
during a hydrogeologic investigation. Ground Water Monit Rev,
10: 89-94.
Slooff W (1979) Detection limits of a biological monitoring system
based on fish respiration. Bull Environ Contam Toxicol, 23: 517-523.
Slooff W, de Zwart D, & Marquenie JM (1983) Detection limits of a
biological monitoring system for chemical water pollution based on
mussel activity. Bull Environ Contam Toxicol, 30: 400-405.
Smith BR, Plummer JL, Wolf CR, Philpot RM, & Bend JR (1982) p-Xylene
metabolism by rabbit lung and liver and its relationship to the
selective destruction of pulmonary cytochrome P-450. J Pharmacol Exp
Ther, 223: 736-742.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: List VI. Am Ind Hyg Assoc J, 23: 95-107.
Snyder R (1987) Ethyl Browning's toxicity and metabolism of industrial
solvents - Volume 1; Hydrocarbons. Amsterdam, Oxford, New York,
Elsevier Science Publishers.
Sotaniemi EA, Sutinen S, Sutinen S, Arranto AJ, & Pelkonen RO (1982)
Liver injury in subjects occupationally exposed to chemicals in low
doses. Acta Med Scand, 212: 207-215.
Steele RH & Wilhelm DL (1966) The inflammatory reaction in chemical
injury. I. Increased vascular permeability and erythema induced by
various chemicals. J Exp Pathol, 47: 612-623.
Stickney JA, Silverman DM, & Schatz RA (1991) Role of isozyme-specific
inhibition of cytochrome P450IIB1 activity in m-xylene-induced
alterations in rat pulmonary benzo(a)pyrene metabolism. Xenobiotica,
211: 641-649.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near an underground coal gasification site in northeastern
Wyoming. Environ Sci Technol, 16: 582-587.
Sugihara R (1979) High-performance liquid chromatography studies of
toluene and xylene poisoning. Part II. Respiratory and urinary
excretion of toluene and m-xylene following intraperitoneal
administration to rats. Acta Med Okayama, 91: 1433-1440.
Sugihara R & Ogata M (1978) Quantitation of urinary m- and
p-methylhippuric acids as indices of m- and p-xylene exposure.
Int Arch Occup Environ Health, 41: 281-286.
Svanberg PA, Grennfeldt P, & Lindskog A (1995) The Swedish urban
network - Air quality measurement program. Stockholm, Sweden,
Environmental Research Institute (Unpublished report).
Tabak HH, Desai S, & Govind R (1989) Determination of biodegradability
kinetics of RCRA compounds using respirometry for structure-activity
relationships. Proceedings of the 44th Industrial Waste Conference,
1989, pp 405-423.
Takatori T, Terazawa K, Nakano K, & Inoue K (1982) An autopsy case of
alkylbenzene poisoning and its clinical finding. Nippon Hoigaku
Zasshi, 36: 654-661.
Tardif R, Laparé S, Plaa GL, & Brodeur J (1991) Effect of simultaneous
exposure to toluene and xylene on their respective biological exposure
indices in humans. Int Arch Occup Environ Health, 63: 279-284.
Tardif R, Plaa GL, & Brodeur J (1992) Influence of various mixtures of
inhaled toluene and xylene on the biological monitoring of exposure to
these solvents in rats. Can J Physiol Pharmacol, 70: 385-939.
Tardif R, Laparé S, Krishnan K, & Brodeur J (1993a) Physiologically
based modelling of the toxicokinetic interaction between toluene and
m-xylene in rat. Toxicol Appl Pharmacol, 120: 266-273.
Tardif R, Laparé S, Krishnan K, & Brodeur J (1993b) A descriptive and
mechanistic study of the interaction between toluene and xylene in
humans. Int Arch Occup Environ Health, 65: S135-S137.
Tardif R, Sato A, Laparé S, & Brodeur J (1994) Ethanol induced
modification of m-xylene toxicokinetics in humans. Occup Environ
Med, 51: 187-191.
Taskinen H, Kyyrönen P, Hemminki K, Hoikkala M, Lajunen K, & Lindbohm
M-L (1994) Laboratory work and pregnancy outcome. J Occup Med,
36: 311-319.
Tatem HE, Cox BA, & Anderson JW (1978) The toxicity of oils and
petroleum hydrocarbons to estuarine crustaceans. Estuar Coast Mar Sci,
6: 365-373.
Tegeris JS & Balster RL (1994) A comparison of the acute behavioral
effects of alkylbenzenes using a functional observational battery in
mice. Fundam Appl Toxicol, 22: 240-250.
Tham R, Bunnfors I, Eriksson B, Larsby B, Lindgren S, & Ödkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thomas JM, Gordy VR, Fiorenza S, & Ward CH (1990) Biodegradation of
BTEX in subsurface materials contaminated with gasoline: Granger,
Indiana. Water Sci Technol, 22: 53-62.
Toftgård R & Nilsen OG (1982) Effects of xylene and xylene isomers on
cytochrome P-450 and in enzymatic activities in rat liver, kidney
and lung. Toxicology, 23: 197-212.
Toftgård R, Nilsen OG, & Gustafsson J-Å (1981) Changes in rat liver
microsomal cytochrome P-450 and enzymatic activities after the
inhalation of n-hexane, xylene, methyl ethyl ketone and
methylchloroform for four weeks. Scand J Work Environ Health,
7: 31-37.
Toftgård R, Nilsen OG, Glaumann H, & Gustafsson J-Å (1983) Induction
of cytochrome P-450 in the rat liver after exposure to xylenes,
dose-response relationship and dependence on endocrine factors.
Toxicology, 27: 119-137.
Toftgård R, Haaparanta T, & Halpert J (1986) Rat lung and liver
cytochrome P-450 isozymes involved in the hydroxylation of m-xylene.
Toxicology, 39: 225-231.
Triebig G & Schaller K-H (1986) Air monitoring of solvent exposed
workers with passive samplers in comparison to "biological monitoring
(BM)". Toxicol Environ Chem, 12: 285-312.
Tsuruta H (1982) Percutaneous absorption of organic solvents. III. On
the penetration rates of hydrophobic solvents through the excised rat
skin. Ind Health, 20: 335-345.
Turkall RM, Skowronski GA, Kadry ARM, & Abdel-Rahman MS (1992) Sex
differences in the bioavailability of soil-adsorbed m-xylene in
orally exposed rats. Toxicol Lett, 63: 57-67.
Tynan P, Watts CD, Sowray A, & Hammond I (1991) The transport and fate
of organic pollutants in rivers. II. Field measurements and modelling
for styrene, xylenes, dichlorobenzenes and 4-phenyldodecane. In:
Proceedings of the 6th European Symposium on Aquatic Environment,
1990, pp 20-37.
Uchida Y, Nakatsuka H, Ukai H, Watanabe T, Liu Y-T, Huang M-Y, Wang
Y-L, Zhu F-Z, Yin H, & Ikeda M (1993) Symptoms and signs in workers
exposed predominantly to xylenes. Int Arch Occup Environ Health,
64: 597-605.
Ungvary G (1985) The possible contribution of industrial chemicals
(organic solvents) to the incidence of congenital defects caused by
teratogenic drugs and consumer goods - an experimental study. Progr
Clin Biomed Res, 163B: 295-300.
Ungvary G (1990) The effect of xylene exposure on the liver. Acta
Morphol Hung, 38: 245-258.
Ungvary G & Tatrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats and rabbits. Arch Toxicol,
8(suppl): 425-430.
Ungvary G, Tatrai E, Hudak A, Barcza G, & Lorincz M (1980) Studies on
the embryotoxic effects of ortho-, meta- and para-xylene.
Toxicology, 18: 61-74.
Ungvary G, Varga B, Horvath E, Tatrai E, & Folly G (1981) Study on the
role of maternal sex steroid production and metabolism in the
embryotoxicity of para-xylene. Toxicology, 19: 263-268.
Valciukas JA, Lilis R, Singer RM, Glickman L, & Nicholson WJ (1985)
Neurobehavioral changes among shipyard painters exposed to solvents.
Arch Environ Health, 40: 47-52.
van der Wal JF & Moerkerken A (1984) The performance of passive
diffusion monitors for organic vapours for personal sampling of
painters. Ann Occup Hyg, 28: 39-47.
van Doorn R, Bos P, Brouns RME, Leijdekkers C-M, & Henderson PT (1980)
Effect of toluene and xylenes on liver glutathione and their urinary
excretion as mercapturic acids in the rat. Arch Toxicol, 43: 293-304.
van Doorn R, Leijdekkers C-M, Bos RP, Brouns ME, & Henderson PT (1981)
Alcohol and sulphate intermediates in the metabolism of toluene and
xylenes to mercapturic acids. J Appl Toxicol, 1: 236-242.
van Vliet C, Swaen GMH, Slangen JJM, de Boorder T, & Sturmans F (1987)
The organic solvent syndrome: A comparison of cases with neuro-
psychiatric disorders among painters and construction workers.
Int Arch Occup Environ Health, 59: 493-501.
van Wijnen JH, Verhoeff AP, Jans HWA, & van Bruggen M (1995) The
exposure of cyclists, car drivers and pedestrians to traffic-related
air pollutants. Int Arch Occup Environ Health, 67(3): 187-193.
Veith GD, Macek KJ, Petrocelli SR, & Carroll J (1980) An evaluation of
using partition coefficients and water solubility to estimate
bioconcentration factors for organic chemicals in fish. In: Eaton JG,
Parrish PR, & Hendricks AC ed. Aquatic toxicology. Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp 116-129
(ASTM STP 707).
Verschoyle RD, Dinsdale D, & Wolf CR (1993) Inhibition and induction
of cytochrome P450 isoenzymes in rat lung. J Pharmacol Exp Ther,
265: 386-391.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., pp 1188-1194.
Vincent R, Poirot P, Subra I, Rieger B, & Cicolella A (1994)
Occupational exposure to organic solvents during paint dripping and
painting operations in the aeronautical industry. Int Arch Occup
Environ Health, 65: 377-380.
Vodickova L, Frantik E, & Vodickova A (1995) Neutrotrophic effects and
blood levels of solvents at combined exposures: Binary mixtures of
toluene, o-xylene and acetone in rats and mice. Cent Eur J Public
Health, 3: 57-64.
Volskay VT & Grady CPL (1990) Respiration inhibition kinetic analysis.
Water Res, 24: 863-874.
Vowles PD & Mantoura RFC (1987) Sediment-water partition coefficients
and HPLC retention factors for aromatic hydrocarbons. Chemosphere,
16: 109-116.
Waggott A (1981) Trace organic substances in the River Lee. In:
Chemistry in water reuse. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., vol 2, pp 55-99.
Wallace LA, Pellizzari ED, Hartwell TD, Whitmore R, Zelon H, Perritt
R, & Sheldon L (1988) The California TEAM study: Breath concentrations
and personal exposures to 26 volatile compounds in air and drinking
water of 188 residents of Los Angeles, Antioch, and Pittsburg, CA.
Atmos Environ, 22: 2141-2163.
Wallace L, Nelson W, Ziegenfus R, Pellizzari E, Michael L, Whitmore R,
Zelon H, Hartwell T, Perritt R, & Westerdahl D (1991) The Los Angeles
TEAM study: Personal exposures, indoor-outdoor air concentrations, and
breath concentrations of 25 volatile organic compounds. J Exp Anal
Environ Epidemiol, 1(2): 157-192.
Wallén M, Holm S, & Byfält Nordqvist M (1985) Coexposure to toluene
and p-xylene in man: Uptake and elimination. Br J Ind Med,
42: 111-116.
Walsh DF, Armstrong JG, Bariley TR, Salmon HA, & Frank PA (1977)
Residues of emulsified xylene in aquatic weed control and their impact
on rainbow trout. Springfield, Virginia, National Technical
Information Services (NTIS) (PB-267270).
Walton BT, Anderson TA, Hendricks MS, & Talmage SS (1989) Physico-
chemical properties as predictors of organic chemical effects
on soil microbial respiration. Environ Toxicol Chem, 8: 53-63.
Washington WJ, Murthy RC, Doye A, Eugene K, Brown D, & Bradley J
(1983) Induction of morphologically abnormal sperm in rats exposed to
o-xylene. Arch Androl, 11: 233-237.
Weber WJ, Cole DE, & Posner JC (1975) Analysis of emissions from
outboard two cycle marine engines. Cincinnati, Ohio, US Environmental
Protection Agency, National Environmental Research Centre, Office of
Research and Development, 249 pp (EPA 670/2-75-06; PB-242174).
Weisel CP, Lawryk NJ, & Lioy PJ (1992) Exposure to emissions from
gasoline within automobile cabins. J Exp Anal Environ Epidemiol,
2(1): 79-96.
Weschler CJ, Shields HC, & Rainer D (1990) Concentrations of volatile
organic compounds at a building with health and comfort complaints. Am
Ind Hyg Assoc J, 51(5): 261-268.
WHO (1981) Xylene. In: Recommended health-based limits in occupational
exposure to selected organic solvents. Geneva, World Health
Organization, pp 25-38 (WHO Technical Report Series No. 664).
Wiesenburg DA, Bodennec G, & Brooks JM (1981) Volatile liquid
hydrocarbons around a production platform in the Northwest Gulf of
Mexico. Bull Environ Contam Toxicol, 27: 167-174.
Williams DT, Nestmann ER, LeBel GL, Benoit FM, & Otson R (1982)
Determination of mutagenic potential and organic contaminants of Great
Lakes drinking water. Chemosphere, 11(3): 263-276.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformations of selected
alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic
aquifer material: A microcosm study. Environ Sci Technol,
20: 997-1002.
Wilson BH, Wilson JT, Kampbell DH, Bledsoe BE, & Armstrong JM (1990)
Biotransformation of monoaromatic and chlorinated hydrocarbons at an
aviation gasoline spill site. Geomicrobiol J, 8: 225-240.
Winslow C-EA (1927) Summary of the National Safety Council study of
benzol poisoning. J Ind Hyg, 9: 61-74.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene:
Experiments on laboratory animals. Arch Ind Health, 14: 387-398.
Yamada K (1993) Influence of lacquer thinner and some organic solvents
on reproductive and accessory reproductive organs in the male rat.
Biol Pharm Bull, 16: 425-427.
Young PJ & Parker A (1983) The identification and possible
environmental impact of trace gases and vapours in landfill gas. Waste
Manage Res, 1: 213-226.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests. III. Results from the testing of
255 chemicals. Environ Mutagen, 9(suppl): 1-110.
Zeyer J, Kuhn EP, & Schwarzenback RP (1986) Rapid microbial
mineralisation of toluene and 1,3-dimethylbenzene in the absence of
molecular oxygen. Appl Environ Microbiol, 52: 944-947.
Zimmerman SW, Groehler K, & Beirne GJ (1975) Hydrocarbon exposure and
chronic glomerulonephritis. Lancet, 2: 199-201.
RESUME
Le xylčne est un hydrocarbure aromatique qui existe sous trois
formes: les isomčres ortho, méta et para. Le xylčne de qualité
technique est un mélange des trois isomčres qui contient en outre un
peu d'éthylbenzčne. En 1984, la production mondiale de xylčne était
estimée ą 15,4 millions de tonnes. A la température ambiante, le
xylčne se présente sous la forme d'un liquide incolore d'odeur
aromatique. La tension de vapeur est comprise entre 0,66 et 0,86 kPa
pour les trois isomčres. Environ 92% des mélanges de xylčnes sont
incorporés ą l'essence. On les utilise aussi comme solvants, en
particulier dans les peintures et les encres d'imprimerie.
La majeure partie du xylčne libéré dans l'environnement passe
directement dans l'atmosphčre. Les trois isomčres y sont rapidement
décomposés, principalement par photooxydation. Ils se volatilisent
tous les trois rapidement ą partir de l'eau. Dans le sol et dans
l'eau, les isomčres méta et para subissent une biodégradation aisée
dans des conditions variées d'aérobiose et d'anaérobiose; en revanche,
l'isomčre ortho est plus persistant. Les données limitées dont on
dispose indiquent que les xylčnes isomčres s'accumulent peu chez les
poissons et les invertébrés. Une fois que l'exposition a cessé, ils
sont assez rapidement éliminés par les organismes aquatiques.
Les concentrations moyennes de fond des trois xylčnes dans l'air
ambiant se situent autour de la valeur caractéristique de 1 µg/m3,
mais dans les banlieues elles atteignent 3 µg/m3 environ. On a
mesuré des valeurs plus élevées en zone urbaine et industrielle, les
moyennes allant cette fois jusqu'ą 500 µg/m3. Toutefois, la
concentration est en général inférieure ą 100 µg/m3.
On estime que l'exposition journaličre de la population par la
voie respiratoire est de 70 µg en milieu rural et de 2 000 µg en
milieu urbain. Dans l'eau de boisson, la concentration varie de zéro
ą 12 µg/litre. Les données concernant la concentration dans les
denrées alimentaires sont trop limitées pour que l'on puisse évaluer
l'exposition journaličre par voie orale.
Dans les eaux superficielles, la concentration moyenne de fond
des xylčnes est généralement inférieure ą 0,1 µg/litre. Cependant, on
a mesuré des valeurs beaucoup plus élevées dans des zones
industrielles et plus particuličrement celles oł sont implantées des
industries pétroličres (jusqu'ą 30 µg/litre dans les eaux polluées et
jusqu'ą 2 000 µg/litre ą proximité des conduites de décharge). Des
valeurs analogues ont été observées dans les eaux souterraines, ces
valeurs élevées pouvant źtre attribuées dans certains cas ą une
pollution locale par des réservoirs et des canalisations enterrées.
Aprčs exposition par la voie respiratoire, la dose inhalée est
retenue ą 60% environ dans les poumons. La métabolisation est
efficace puisque le xylčne est transformé ą 90% en acide
méthylhippurique, lequel est ensuite excrété dans les urines. Le
xylčne ne s'accumule pas en quantité importante dans l'organisme
humain.
Chez l'homme, une exposition aiguė ą du xylčne sous forte
concentration peut avoir des effets sur le systčme nerveux central et
provoquer une irritation. Ces effets n'ont toutefois pas donné lieu ą
des études contrōlées ni ą des études épidémiologiques ą long terme.
Chez les animaux de laboratoire, la toxicité chronique se révčle
faible. On a cependant de bonnes raisons de penser que sous
concentration modérée, le xylčne pourrait avoir des effets sur le SNC
chez l'animal.
Le xylčne ne s'est révélé ni mutagčne ni cancérogčne.
Le point d'aboutissement toxicologique essentiel concerne l'effet
nocif que le xylčne exerce sur le développment. On l'a mis en
évidence chez le rat ą partir d'une concentration de 870 mg/m3 (200
ppm). Compte tenu de cela, la valeur-guide recommandée pour la
concentration maximale de xylčne dans l'air a été fixée ą 0,87 mg/m3
(0,2 ppm).
Les xylčnes isomčres sont faiblement ą modérément toxiques pour
les organismes aquatiques. Chez les invertébrés, c'est l' o-xylčne
qui a la Cl50 la plus faible (1 mg/litre pour Daphnia magna). Chez
les poissons, la CL50 la plus faible est également celle de
l' o-xylčne (7,6 mg/litre chez la truite arc-en-ciel, par mesure de
concentration). Des valeurs de 7,9 et 1,7 mg/litre ont été obtenues,
respectivement pour le m- et le p-xylčne, dans le cas de la perche
commune (d'aprčs la concentration nominale). On ne dispose que de
données limitées au sujet de l'exposition chronique des organismes
aquatiques aux xylčnes, mais, quoi qu'il en soit, la volatilisation
rapide de ces composés la rend peu probable. Le xylčne ne présente
qu'une faible toxicité aiguė pour les oiseaux.
RESUMEN
El xileno es un hidrocarburo aromįtico del que hay tres formas
isoméricas: orto, meta y para. El xileno de calidad técnica contiene
una mezcla de los tres isómeros y algo de etilbenceno. Se estima que
la producción mundial fue de 15,4 millones de tone-ladas en 1984. La
presión de vapor estį comprendida entre 0,66 y 0,86 kPa para los tres
isómeros. Aproximadamente un 92% de las mezclas de xilenos se combinan
con el petróleo. El producto se emplea también en diversos
disolventes, en particular en las industrias de fabricación de
pinturas y de tintas de imprenta.
La mayor parte del xileno liberado en el medio ambiente pasa
directamente a la atmósfera. En ésta los isómeros de xileno se
degradan con facilidad, principalmente por fotooxidación. Los tres
isómeros se volatilizan rįpidamente en la atmósfera a partir del agua.
En el suelo y el agua los isómeros meta y para se biodegradan
fįcilmente en una amplia variedad de condiciones aerobias y
anaerobias, pero el isómero orto es mįs persistente. Las limitadas
pruebas disponibles parecen indicar que la bioacumulación de los
isómeros de xileno por los peces y los invertebrados es baja. La
eliminación del xileno de los organismos acuįticos es bastante rįpida
a partir del momento en que se interrumpe la exposición.
Normalmente los niveles basales medios de los tres isómeros de
xileno en el aire ambiente son de aproximadamente 1 µg/m3, pero en
zonas suburbanas se hallan en torno a 3 µg/m3. Se han detectado
concentraciones mayores en zonas urbanas e industrializadas, con
niveles medios de hasta 500 µg/m3. No obstante, las concentraciones
son por lo general inferiores a 100 µg/m3.
La exposición diaria por inhalación estimada en la población
general es de 70 µg en zonas rurales y de menos de 2000 µg en zonas
urbanas. La concentración en el agua potable estį comprendida entre
valores indetectables y 12 µg/litro. Los datos disponibles sobre la
concentración en los alimentos son insuficientes para poder estimar la
exposición oral diaria.
Las concentraciones basales medias de xilenos en aguas
superficiales son generalmente inferiores a 0.1 µg/litro. Sin embargo
se han hallado valores mucho mįs altos en zonas industriales y en
zonas vinculadas a la industria petrolera (hasta 30 µg/litro en aguas
contaminadas y hasta 2000 µg/litro en las proximidades de tuberķas de
desagüe). Se ha informado del hallazgo de niveles basales similares
en aguas subterrįneas, aunque se han detectado también concentraciones
elevadas, atribuidas a contaminación localizada a partir de tanques de
almacenamiento y tuberķas subterrįneos.
Tras la exposición por inhalación la retención pulmonar es de un
60% de la dosis inhalada. El xileno es metabolizado eficientemente.
Mįs del 90% se biotransforma en įcido metilhipśrico, que se excreta
por la orina. El xileno no se acumula de forma significativa en el
organismo humano.
La exposición aguda a altas concentraciones de xileno puede
afectar al SNC y causar irritación en el hombre. Sin embargo, no se
han llevado a cabo ni estudios controlados a largo plazo en el ser
humano ni estudios epidemiológicos. La toxicidad crónica parece
relativamente baja en animales de laboratorio. Hay indicios, no
obstante, de que concentraciones moderadas de xileno pueden tener
efectos crónicos sobre el SNC en animales.
El xileno no parece tener efectos mutįgenos ni carcinógenos.
El parįmetro crķtico es la toxicidad para el desarrollo,
demostrada a niveles de exposición de 870 mg/m3 (200 ppm) en la rata.
Teniendo en cuenta este parįmetro, la concentración indicativa
recomendada para el xileno en el aire es de 0.87 mg/m3 (0,2 ppm).
Los isómeros de xileno poseen una toxicidad entre moderada y baja
para los organismos acuįticos. En invertebrados la CL50 mįs baja,
calculada a partir de las concentraciones medidas, es de 1 mg/litro
para el o-xileno (Daphnia magna). Los valores mįs bajos de CL50
detectados en peces son de 7,6 mg/litro para el o-xileno (trucha
arco iris; segśn las concentraciones medidas), y de 7,9 y 1,7 mg/litro
para los m- y p-xilenos respectivamente (ambos para la lubina
estriada; segśn las concentraciones nominales). La información
disponible respecto a la exposición crónica de organismos acuįticos a
los xilenos es limitada; no obstante, su rįpida volatilización hace
improbable la exposición crónica en el agua. La toxicidad aguda del
xileno para las aves es baja.
 The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR XYLENES
Members
Dr E. Frantik, Centre for Industrial Hygiene and Occupational
Diseases, National Institute of Public Health, Prague, Czech
Republic
Dr U. Hass, Department of Toxicology and Biology, National Institute
of Occupational Health, Copenhagen, Denmark
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Abbots Ripton, Huntingdon Cambridgeshire, United Kingdom
(Co-Rapporteur)
Dr Young Lee, Contaminants Standards, Monitoring and Programs Branch,
Centre for Food Safety and Applied Nutrition, US Food and Drug
Administration, Washington DC, USA
Mr G. Long, Health and Welfare Canada, Environmental Health Centre,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr P. Lundberg, Department of Toxicology, National Institute for
Working Life, Solna, Sweden (Co-Rapporteur)
Dr Choon-Nam Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
Dr V. Riihimäki, Institute of Occupational Health, Helsinki, Finland
(Chairman)
Observer
Dr C.J. Bevan, Exxon Biomedical Sciences Inc., East Millstone, New
Jersey, USA
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR XYLENES
A WHO Task Group on Environmental Health Criteria for Xylenes met
in Geneva from 6 to 9 November 1995. Dr B.H. Chen, IPCS, opened the
meeting and welcomed the participants on behalf of the Director, IPCS,
and the three IPCS cooperating organizations (UNEP/ILO/WHO). The Task
Group reviewed and revised the draft criteria monograph and made an
evaluation of the risks for human health and the environment from
exposure to xylenes.
The first draft of this monograph was prepared by Dr P. Lundberg,
Mr P.D. Howe, Mr M.J. Crookes and Dr S. Dobson. The second draft was
prepared by Dr P. Lundberg and Mr P.D. Howe incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria monographs. Dr P.
Lundberg and Mr P.D. Howe contributed to the final text of the health
and environmental sections, respectively.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
ATPase adenosine triphosphatase
BCF bioconcentration factor
BTX benzene, toluene, xylene
CNS central nervous system
DMP dimethylphenol
DMSO dimethylsulfoxide
EEG electroencephalograph
FID flame-ionization detector
i.m. intramuscular
i.p. intraperitoneal
i.v. intravenous
LOAEL lowest-observed-adverse-effect level
NADPH reduced nicotinamide adenosine dinucleotide
NOAEL no-observed-adverse-effect level
PB phenobarbital
PMBA p-methylbenzyl alcohol
POCP photochemical ozone-creation potential
RMA reflex modification audiometry
s.c. subcutaneous
TCE 1,1,1-trichloroethylene
1. SUMMARY
Xylene is an aromatic hydrocarbon which exists in three isomeric
forms: ortho, meta and para. Technical grade xylene contains a
mixture of the three isomers and also some ethylbenzene. The
estimated world production in 1984 was 15.4 million tonnes. Xylene is
a colourless liquid at room temperature with an aromatic odour. The
vapour pressure lies between 0.66 and 0.86 kPa for the three isomers.
Approximately 92% of mixed xylenes is blended into petrol. It is also
used in a variety of solvent applications, particularly in the paint
and printing ink industries.
The majority of xylene released into the environment enters the
atmosphere directly. In the atmosphere the xylene isomers are readily
degraded, primarily by photooxidation. Volatilization to the
atmosphere from water is rapid for all three isomers. In soil and
water, the meta and para isomers are readily biodegraded under a wide
range of aerobic and anaerobic conditions, but the ortho isomer is
more persistent. The limited evidence available suggests that
bioaccumulation of the xylene isomers by fish and invertebrates is
low. Elimination of xylene from aquatic organisms is fairly rapid
once exposure has ceased.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3, but in suburban areas they are
around 3 µg/m3. Higher levels have been measured in urban and
industrialized areas, mean concentrations ranging up to 500 µg/m3.
However, concentrations are generally below 100 µg/m3.
Estimated daily exposure of the general population through
inhalation is 70 µg in rural areas and less than 2000 µg in urban
areas. The concentration in drinking-water ranges from not detectable
to 12 µg/litre. The data on the level in food are too limited to
estimate daily oral exposure.
Mean background concentrations of xylenes in surface water are
generally below 0.1 µg/litre. However, much higher values have been
measured in industrial areas and areas associated with the oil
industry (up to 30 µg/litre in polluted waters and up to 2000 µg/litre
near to discharge pipes). Similar background levels have been
reported for groundwater although high levels have been reported due
to localized pollution from underground storage tanks and pipes.
After inhalation exposure the retention in the lungs is about 60%
of the inhaled dose. Xylene is efficiently metabolized. More than
90% is biotransformed to methylhippuric acid, which is excreted in
urine. Xylene does not accumulate significantly in the human body.
Acute exposure to high concentrations of xylene can result in CNS
effects and irritation in humans. However, there have been no
long-term controlled human studies or epidemiological studies. The
chronic toxicity appears to be relatively low in laboratory animals.
There is suggestive evidence, however, that chronic CNS effects may
occur in animals at moderate concentrations of xylene.
Xylene appears not to be a mutagen or a carcinogen.
The critical end-point is developmental toxicity, which has been
demonstrated at an exposure level of 870 mg/m3 (200 ppm) in rats.
Based on this end-point, the recommended guidance value for xylene in
air is 0.87 mg/m3 (0.2 ppm).
The xylene isomers are of moderate to low toxicity for aquatic
organisms. For invertebrates the lowest LC50 value, based on
measured concentrations, is for o-xylene at 1 mg/litre (Daphnia
magna). The lowest LC50 values recorded for fish are 7.6 mg/litre
for o-xylene (rainbow trout; based on measured concentrations), and
7.9 and 1.7 mg/litre for m- and p-xylenes respectively (both for
striped bass; based on nominal concentrations). Limited information
is available regarding chronic exposure of aquatic organisms to
xylenes; however, rapid volatilization makes chronic exposure in
water unlikely. The acute toxicity of xylene to birds is low.
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
Xylene exists in three isomeric forms, ortho-, meta- and
para-xylene. The commercial product is a mixture of all three
isomers with m-xylene predominating, usually 60-70%. The technical
product, "mixed xylenes", contains approximately 40% m-xylene and
20% each of ethylbenzene, o-xylene and p-xylene. Small quantities
of toluene and C9 aromatic fractions may also be present (Fishbein,
1988).
Chemical formula
C8H10 C8H10 C8H10
Chemical structure
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR XYLENES
Members
Dr E. Frantik, Centre for Industrial Hygiene and Occupational
Diseases, National Institute of Public Health, Prague, Czech
Republic
Dr U. Hass, Department of Toxicology and Biology, National Institute
of Occupational Health, Copenhagen, Denmark
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Abbots Ripton, Huntingdon Cambridgeshire, United Kingdom
(Co-Rapporteur)
Dr Young Lee, Contaminants Standards, Monitoring and Programs Branch,
Centre for Food Safety and Applied Nutrition, US Food and Drug
Administration, Washington DC, USA
Mr G. Long, Health and Welfare Canada, Environmental Health Centre,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr P. Lundberg, Department of Toxicology, National Institute for
Working Life, Solna, Sweden (Co-Rapporteur)
Dr Choon-Nam Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
Dr V. Riihimäki, Institute of Occupational Health, Helsinki, Finland
(Chairman)
Observer
Dr C.J. Bevan, Exxon Biomedical Sciences Inc., East Millstone, New
Jersey, USA
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR XYLENES
A WHO Task Group on Environmental Health Criteria for Xylenes met
in Geneva from 6 to 9 November 1995. Dr B.H. Chen, IPCS, opened the
meeting and welcomed the participants on behalf of the Director, IPCS,
and the three IPCS cooperating organizations (UNEP/ILO/WHO). The Task
Group reviewed and revised the draft criteria monograph and made an
evaluation of the risks for human health and the environment from
exposure to xylenes.
The first draft of this monograph was prepared by Dr P. Lundberg,
Mr P.D. Howe, Mr M.J. Crookes and Dr S. Dobson. The second draft was
prepared by Dr P. Lundberg and Mr P.D. Howe incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria monographs. Dr P.
Lundberg and Mr P.D. Howe contributed to the final text of the health
and environmental sections, respectively.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
ATPase adenosine triphosphatase
BCF bioconcentration factor
BTX benzene, toluene, xylene
CNS central nervous system
DMP dimethylphenol
DMSO dimethylsulfoxide
EEG electroencephalograph
FID flame-ionization detector
i.m. intramuscular
i.p. intraperitoneal
i.v. intravenous
LOAEL lowest-observed-adverse-effect level
NADPH reduced nicotinamide adenosine dinucleotide
NOAEL no-observed-adverse-effect level
PB phenobarbital
PMBA p-methylbenzyl alcohol
POCP photochemical ozone-creation potential
RMA reflex modification audiometry
s.c. subcutaneous
TCE 1,1,1-trichloroethylene
1. SUMMARY
Xylene is an aromatic hydrocarbon which exists in three isomeric
forms: ortho, meta and para. Technical grade xylene contains a
mixture of the three isomers and also some ethylbenzene. The
estimated world production in 1984 was 15.4 million tonnes. Xylene is
a colourless liquid at room temperature with an aromatic odour. The
vapour pressure lies between 0.66 and 0.86 kPa for the three isomers.
Approximately 92% of mixed xylenes is blended into petrol. It is also
used in a variety of solvent applications, particularly in the paint
and printing ink industries.
The majority of xylene released into the environment enters the
atmosphere directly. In the atmosphere the xylene isomers are readily
degraded, primarily by photooxidation. Volatilization to the
atmosphere from water is rapid for all three isomers. In soil and
water, the meta and para isomers are readily biodegraded under a wide
range of aerobic and anaerobic conditions, but the ortho isomer is
more persistent. The limited evidence available suggests that
bioaccumulation of the xylene isomers by fish and invertebrates is
low. Elimination of xylene from aquatic organisms is fairly rapid
once exposure has ceased.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3, but in suburban areas they are
around 3 µg/m3. Higher levels have been measured in urban and
industrialized areas, mean concentrations ranging up to 500 µg/m3.
However, concentrations are generally below 100 µg/m3.
Estimated daily exposure of the general population through
inhalation is 70 µg in rural areas and less than 2000 µg in urban
areas. The concentration in drinking-water ranges from not detectable
to 12 µg/litre. The data on the level in food are too limited to
estimate daily oral exposure.
Mean background concentrations of xylenes in surface water are
generally below 0.1 µg/litre. However, much higher values have been
measured in industrial areas and areas associated with the oil
industry (up to 30 µg/litre in polluted waters and up to 2000 µg/litre
near to discharge pipes). Similar background levels have been
reported for groundwater although high levels have been reported due
to localized pollution from underground storage tanks and pipes.
After inhalation exposure the retention in the lungs is about 60%
of the inhaled dose. Xylene is efficiently metabolized. More than
90% is biotransformed to methylhippuric acid, which is excreted in
urine. Xylene does not accumulate significantly in the human body.
Acute exposure to high concentrations of xylene can result in CNS
effects and irritation in humans. However, there have been no
long-term controlled human studies or epidemiological studies. The
chronic toxicity appears to be relatively low in laboratory animals.
There is suggestive evidence, however, that chronic CNS effects may
occur in animals at moderate concentrations of xylene.
Xylene appears not to be a mutagen or a carcinogen.
The critical end-point is developmental toxicity, which has been
demonstrated at an exposure level of 870 mg/m3 (200 ppm) in rats.
Based on this end-point, the recommended guidance value for xylene in
air is 0.87 mg/m3 (0.2 ppm).
The xylene isomers are of moderate to low toxicity for aquatic
organisms. For invertebrates the lowest LC50 value, based on
measured concentrations, is for o-xylene at 1 mg/litre (Daphnia
magna). The lowest LC50 values recorded for fish are 7.6 mg/litre
for o-xylene (rainbow trout; based on measured concentrations), and
7.9 and 1.7 mg/litre for m- and p-xylenes respectively (both for
striped bass; based on nominal concentrations). Limited information
is available regarding chronic exposure of aquatic organisms to
xylenes; however, rapid volatilization makes chronic exposure in
water unlikely. The acute toxicity of xylene to birds is low.
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
Xylene exists in three isomeric forms, ortho-, meta- and
para-xylene. The commercial product is a mixture of all three
isomers with m-xylene predominating, usually 60-70%. The technical
product, "mixed xylenes", contains approximately 40% m-xylene and
20% each of ethylbenzene, o-xylene and p-xylene. Small quantities
of toluene and C9 aromatic fractions may also be present (Fishbein,
1988).
Chemical formula
C8H10 C8H10 C8H10
Chemical structure
 Chemical name
ortho-xylene meta-xylene para-xylene
Synonyms
1,2-dimethyl- 1,3-dimethyl- 1,4-dimethyl-
benzene benzene benzene
o-methyltoluene m-methyltoluene p-methyltoluene
1,2-xylene 1,3-xylene 1,4-xylene
o-xylol m-xylol p-xylol
ortho-xylene meta-xylene para-xylene
Relative molecular mass
106.16 106.16 106.16
CAS registry number
Chemical name
ortho-xylene meta-xylene para-xylene
Synonyms
1,2-dimethyl- 1,3-dimethyl- 1,4-dimethyl-
benzene benzene benzene
o-methyltoluene m-methyltoluene p-methyltoluene
1,2-xylene 1,3-xylene 1,4-xylene
o-xylol m-xylol p-xylol
ortho-xylene meta-xylene para-xylene
Relative molecular mass
106.16 106.16 106.16
CAS registry number
 6.3.2 In laboratory animals
Most studies on metabolism of xylenes have been performed on rat.
The principal pathway involves side-chain oxidation to methylbenzoic
acid via methylbenzyl alcohol and methylbenzyl aldehyde.
Methylbenzoic acid is then conjugated with glycine or glucuronic acid
(Sugihara & Ogata, 1978; Ogata et al., 1980; Elovaara et al., 1984).
Conjugation with glycine to form methylhippuric acid predominates for
m- and p-xylene (Sugihara, 1979; Ogata & Fujii, 1979; Elovaara et
al., 1984). In the case of o-xylene, glucuronide formation has been
reported to predominate (Ogata et al., 1980).
A separate minor pathway resulting in urinary excretion of
thioethers has been studied (Van Doorn et al., 1980; Van Doorn et al.,
1981). This pathway appears to be more important for o-xylene than
for the other isomers. Hydroxylation of the aromatic ring with the
formation of dimethylphenols has been reported to be another minor
metabolic pathway in rats (Bakke & Scheline, 1970; Elovaara et al.,
1984).
Methylbenzoic acid and dimethylphenols are present in urine of
guinea-pigs and rabbits exposed to xylene isomers (Fabre et al.,
1960). After oral dosing of rabbits with xylenes the main metabolite
was methylbenzoic acid (Bray et al., 1949). The acid was considered
to be mostly present as the glycine conjugate, methylhippuric acid.
Studies with isolated perfused livers and lungs from rabbit
indicate differences in the metabolic pathways between these two
organs (Smith et al., 1982). In the liver p-methylhippuric acid was
the major metabolite detected. In the lung p-methylbenzyl alcohol
and p-methylbenzoic acid were the main metabolites detected. There
was formation of 2,5-dimethylphenol in the lungs but not in the liver.
Metabolism of m- and p-xylenes to m- and p-methylbenzyl alcohols
has been found to be greater with hepatic than with pulmonary
microsomes (Harper, 1975; Toftgård et al., 1986). Further metabolism
to methylbenzoic acid occurred in the presence of hepatic but not
pulmonary cytosolic fraction. Daily exposures to xylene increased the
activities of liver microsomal enzymes and concentrations of
cytochrome P450 (Elovaara et al., 1982; Pathiratne et al., 1986).
Metabolism of m-xylene by cerebral microsomal preparations was
reported to be very slow (Elovaara et al., 1982).
No definitive studies have been reported showing which microsomal
P-450 enzymes are involved in xylene metabolism. However when rats
were given m-xylene (1.0-1.4 ml/kg body weight) by gastric
intubation once daily for 3 consecutive days and killed 24 h after the
last treatment, xylene caused an induction of CYP2B and CYP2E1 in
liver microsomes (Raunio et al., 1990). Exposure of male Wistar rats
to each of the xylene isomers by inhalation at a concentration level
of 4000 mg/m3 for 20 h/day over 4 days similarly induced hepatic
CYP2B1, while CYP2E1 was reduced, as estimated by Western blots (Gut
et al., 1993).
6.4 Elimination and excretion
6.4.1 In humans
Absorbed xylenes are excreted mainly as metabolites in urine.
Small amounts are excreted unchanged in exhaled air. Excretion in
faeces appears to be unimportant. The rate of clearance of p-xylene
from blood has been calculated to be 2.6 litres/kg per hour at 87
mg/m3 (20 ppm) and 1.6 litres/kg per hour at 304 mg/m3 (70 ppm)
(Wallén et al., 1985).
When volunteers were exposed to a constant concentration of
about 391-870 mg/m3 (90-200 ppm) m-xylene over 5 days, at least 97%
was calculated to be excreted as m-methylbenzoic acid conjugates.
2,4-Dimethylphenol conjugates accounted for 1-2% of the metabolites
(Riihimäki et al., 1979a; Riihimäki et al., 1979b). When volunteers
were exposed to about 195 mg/m3 (45 ppm) of o-, m- or p-xylene
for 8 h, about 95-99% of the dose was excreted as methylhippuric acid
in urine. Dimethylphenol excretion was estimated to be 0.1 to 2% of
the dose absorbed (Sedivec & Flek, 1976). About 90% of the absorbed
dose of m-xylene was excreted as methylhippuric acid after exposure
to 435 mg/m3 (100 ppm) for 4 h (Lauwerys et al., 1978; Campbell et
al., 1988). On the other hand, after exposure to 600 mg/m3 (138 ppm)
of o-xylene, only 46% was excreted in urine as methylhippuric acid
and only trace amounts of the o-methylbenzoyl glucuronide were
detected (Ogata et al., 1980).
In a study of 121 male workers engaged in dip-coating of metal
parts, the mean concentration was 3.48 mg/m3 (0.8 ppm) o-xylene,
9.1 mg/m3 (2.1 ppm) m-xylene and 3.91 mg/m3 (0.9 ppm) p-xylene.
The workers were also exposed to 0.8 ppm toluene and 0.9 ppm
ethylbenzene. At the end of the 8 h-shift urine samples were
collected and methylhippuric acid was determined. There was a linear
relationship between the intensity of exposure to xylenes and the
concentration of methylhippuric acid in urine. The methylhippuric
acid concentration as a function of increasing xylene concentration
was 17.8 mg/litre per ppm (Kawai et al., 1991a).
In workers occupationally exposed to an average of 17.4 mg/m3
(4 ppm) xylene (combination of all three isomers), with a maximum of
more than 430 mg/m3 (100 ppm), the urinary excretion of
methylhippuric acid was linearly correlated with the air exposure
(Huang et al., 1994).
The urinary methylhippuric acid excretion of xylene-exposed
painters at the end of the working week showed two distinct phase of
excretion. Half-times for urinary excretion of methylhippuric acid
were estimated to be 3.6 h for the first 10 h and 30.1 h for the next
2 days after exposure (Engström et al., 1978). A positive association
between the degree of obesity and the length of half-time was seen.
In volunteers exposed to m-xylene the urinary excretion of
m-methylbenzoic acid was described as triphasic with half-times of
1-2, 10 and 20 h (Riihimäki et al., 1979a). The observed elimination
half-time in the subcutaneous adipose tissue was about 58 h (Engström
& Riikimäki, 1979).
After oral administration of o-xylene (39 mg/kg body weight)
maximum urinary levels of glycine and glucuronide conjugates of
o-methylbenzoic acid were reported to be 33.1 and 1.0% of the
administered dose, respectively. Similar values were obtained after
an oral dose of 78 mg/kg body weight (Ogata et al., 1979; Ogata et
al., 1980).
About 4-5% of the dose absorbed in the lungs is exhaled unchanged
after exposure to 870 mg/m3 (200 ppm) xylene (Sedivec & Flek, 1976,
Åstrand et al., 1978, Riihimäki et al., 1979a, Riihimäki et al.,
1979b). Elimination in exhaled breath is reported to follow a similar
triphasic profile to that for urinary excretion of methylbenzoic acid
conjugates (Riihimäki et al, 1979). An initial half-time of about one
hour was obtained in a study by Campbell et al., 1988.
6.4.2 In laboratory animals
Exhalation of unchanged m-xylene has been described in one
study on rats. Exhalation was greatest 4 h after an intraperitoneal
injection, and 13% of the dose was exhaled unchanged within 10 h
(Sugihara, 1979). In mice 3.4% of the dose was exhaled within 8 h
(Bergman, 1979; Bergman, 1983).
In rats a total of 46% of the m-xylene dose (5 mmol/kg body
weight) was excreted as m-methylbenzoic acid within 24 h, and
phenobarbital (PB) treatment increased it to 70% of the dose. PB
treatment increased the elimination of m-methylbenzoic acid after
oral administration about 4-fold in the first 3 h, more then 2-fold in
the first 12 h, and 1.5-fold within 24 h compared to untreated but
m-xylene-exposed rats (Gut & Flek, 1981).
Wistar rats were pretreated with PB for 3 days (80 mg/kg body
weight per day) and then given m-xylene orally or intraperitoneally
at a small (0.081 mmol/kg) or a large (0.81 mmol/kg) dose or by
inhalation (6 h) at a low (174 mg/m3, 40 ppm) or high (1740 mg/m3,
400 ppm) concentration. PB treatment had a significant effect on the
metabolism of inhaled m-xylene (decreased blood m-xylene and
increased urinary excretion of m-methylhippuric acid), but only at
the high dose. The PB-induced enzyme induction had an effect at both
dose levels on the metabolism of orally administered m-xylene. The
effect on intraperitoneally administered m-xylene was more similar
to that of inhaled than that of orally administered m-xylene (Kaneko
et al., 1995).
After an intraperitoneal injection of 87-348 mg/kg body weight
m-xylene to rats, 53-75% of the dose was excreted as m-methyl-
hippuric acid in urine during 24 h (Ogata & Fujii, 1979). After
an intraperitoneal dose of 319 mg/kg body weight the proportion
excreted as mercapturic acids was calculated to be 10% for o-xylene
and 0.6-1.3% for m- and p-xylene (Van Doorn et al., 1980).
Male rats were exposed to soil-adsorbed (sandy soil or clay soil)
or pure 225 µl of m-xylene containing 20 µCi of m-[14C]-xylene
through the skin (Skowronski et al., 1990). The major route of
excretion in the pure and sandy groups was via expired air followed by
urine. However, in the presence of clay soil, the percentage of the
initial dose in expired air was similar to that in urine. In the
presence of clay soil, an increase in m-xylene was observed in
adipose tissue. Methyl hippuric acid was the main urinary metabolite.
Turkall et al. (1992) reported the bioavailability of
soil-adsorbed m-xylene in male and female rats. The rats were
gavaged with an aqueous suspension of 5% of gum acacia containing 150
µl of m-xylene with 5 µCi m-[14C]-xylene alone or adsorbed to
sandy or clay soil. While ingested soil contaminated with m-xylene
produced a higher bioavailability than the chemical alone in females,
no effects of soil was observed in males. No differences in the
bioavailability of m-xylene alone were observed between the sexes.
m-Xylene was primarily metabolized and excreted in urine,
methylhippuric acid being the main urinary metabolite in all groups.
6.5 Factors affecting toxicokinetics in humans and animals
In humans, co-exposure of m-xylene and ethylbenzene and
consumption of ethanol or aspirin (acetylsalicylic acid) prior to
inhalation has been shown to reduce urinary excretion of one or more
xylene metabolites, including methylhippuric acid (Riihimäki et al.,
1982a,b; Engström et al., 1984; Campbell et al., 1988). In the case
of ethanol consumption an increase in concentration of m-xylene in
blood was described (Riihimäki et al., 1982a,b). Co-exposure to
toluene decreased the ratio of the concentration of p-xylene in
venous blood to that in exhaled air (Wallén et al., 1985). The dermal
absorption of liquid m-xylene was reduced in the presence of
isobutanol (Riihimäki, 1979a).
Volunteers given ethanol on two evenings (total dose 137 g)
preceding exposure by inhalation to either 435 or 1740 mg/m3 (100 or
400 ppm) m-xylene for 2 h enhanced the metabolism of m-xylene but
only at 1740 mg/m3 (Tardif et al., 1994). Ethanol pretreatment
decreased the concentration of m-xylene in blood and alveolar air
during and after exposure and increased urinary excretion of
m-methylhippuric acid at the end of exposure to 1740 mg/m3.
Five male volunteers were exposed for 7 h/day over 3 consecutive
days to 50 ppm toluene, 174 mg/m3 (40 ppm) xylene (15% o-xylene,
25% m-xylene and 60% p-xylene) or a combination of both. To study
high-level exposure four men were exposed for 4 h to 95 ppm toluene or
348 mg/m3 (80 ppm) xylene or a combination of both. Mixed exposure
(low-level) did not alter the concentration of the solvents in blood
or exhaled air, nor did it modify the excretion of urinary
metabolities. High-level mixed exposure, however, increased the
concentration of solvents in blood and exhaled air and caused a delay
in the urinary excretion of hippuric acid (Tardif et al., 1991).
Simultaneous exposure by inhalation to toluene and xylene (15%
o-xylene, 60% m-xylene and 25% p-xylene) has been studied in
Sprague-Dawley rats. Exposure time was 5 h and the concentrations
were 75 ppm toluene plus 979 mg/m3 (225 ppm) xylene, 150 ppm toluene
plus 652 mg/m3 (150 ppm) xylene or 225 ppm toluene plus 326 mg/m3
(75 ppm) xylene. Compared to exposure to a single solvent,
simultaneous exposure resulted in lower amounts of excreted urinary
hippuric and methylhippuric acids over 24 h. Increased concentrations
of solvents in blood and brain were found immediately post-exposure.
Simultaneous exposure also enhanced the pulmonary elimination of both
solvents (Tardif et al., 1992).
From a toxicokinetic modelling study it was concluded that there
is competitive metabolic inhibition between m-xylene and toluene in
the rat (Tardif et al., 1993a). The interaction is likely to be
observed when exposure exceeds 50 ppm of each solvent (Tardif et al.,
1993b).
Inhalation exposure of rats for 4 h to 2436 mg/m3 (560 ppm)
m-xylene or 320 ppm of ethylbenzene alone, or in combination,
indicated that in the combined exposure blood and brain m-xylene
concentrations increased by 52 and 40%, respectively, whereas there
was no corresponding effect on ethylbenzene concentrations (Frantik &
Vodickova, 1995).
In a study of metabolic interaction, Wistar rats were exposed
for 6 h to 1305 mg/m3 (300 ppm) m-xylene, 600 ppm methyl ethyl
ketone (MEK) or a mixture of both. After mixed exposure the
cytochrome P-450-dependent monooxygenase activities were additively or
synergistically induced. In the presence of MEK the overall
metabolism of m-xylene was inhibited, as shown by an increase in
xylene concentration in blood and fat and a decrease in the 24-h
excretion of xylene metabolites (Liira et al., 1991).
Male Wistar rats were exposed by inhalation for 4 h to 1000
mg/m3 (230 ppm) of o-xylene or 1700 ppm of acetone alone, or in
combination. In the combination exposure, blood xylene immediately
after the exposure was increased by 40% whereas the blood acetone
level was decreased by 15%. In a corresponding study, H-strain mice
were exposed for 2 h to 1392 mg/m3 (320 ppm) of o-xylene or 6655
mg/m3 (1530 ppm) of acetone alone or in combination. The combination
exposure was accompanied by a 33% increase of the blood xylene
concentration whereas the blood acetone level was decreased by 18%
(Vodickova et al., 1995).
6.6 Biological monitoring
More than 90% of xylene is transformed in human metabolism to
methylhippuric acid and excreted in urine. Urinary methylhippuric
acid has proved a robust measure of the amount of xylene taken up in
the body over some preceding hours. The measurement has been
routinely used in some countries for assessment of individual exposure
to xylene in the occupational setting. About 1.5-2 g methylhippuric
acid per g creatinine in the post-shift urine sample corresponds to
exposure to 435 mg/m3 (100 ppm) xylene over a full workday (Lauwerys
& Buchet, 1988). Different workloads cause variation in the excreted
amounts of methylhippuric acid at a given exposure level since the
lung uptake of xylene is directly proportional to pulmonary
ventilation. Biotransformation of xylene to methylhippuric acid is
inhibited in the presence of ethanol and acetylsalicylic acid (see
section 6.5); these interfering factors need to be controlled when the
method is applied.
The analysis of xylene in blood and exhaled air can be used for
assessment of exposure (Lauwerys & Buchet, 1988). These methods may
be particularly suitable for estimating xylene body burden caused by
the low and relatively stable background exposure in the general
population, or, in case of accidental exposure, for measuring levels
for a clinical toxicological evaluation.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Inhalation studies
7.1.1.1 o-Xylene
The LC50 value for o-xylene in Sprague-Dawley rats (12
males/group) was calculated to be 4330 ppm (95% confidence limits
4247-4432 ppm) for a 6-h exposure. The reported signs of intoxication
were hypotonia and somnolence. Autopsy on surviving animals 14 days
later revealed no macroscopic lesions of lung, liver or kidneys
(Bonnet et al., 1982). Under similar conditions the LC50 value for
mice (OF-1) was 4595 ppm (4468-4744 ppm) (Bonnet et al., 1979).
Rats (probably Wistar, sex not stated, 10 animals/group) were
exposed to 1531, 3062 or 6125 ppm for 24 h. No deaths occurred at
1531 ppm, one death at 3062 ppm and 8 deaths at 6125 ppm (Cameron et
al., 1938). In the same study one group was exposed to 12 250 ppm
o-xylene for 12 h. Two deaths occurred, one after 2 h. Autopsy of
those animals that died did not reveal any macroscopic or microscopic
lesions in the organs studied (Cameron et al., 1938). In the same
study mice (10 animals/group) were exposed to the same concentrations
for the same period of time as the rats. There were no deaths at 1531
ppm, four deaths at 3062 ppm and nine deaths at 6125 ppm. In the
group exposed to 12 250 ppm for 12 h there were two deaths, one after
9 h. The organs of animals that died after exposure showed no
characteristic changes (Cameron et al., 1938).
In a study of the effect on prenarcotic motor behaviour, groups
of eight male rats (CFY) were exposed to o-xylene (dose not stated)
for 1, 2, 3 or 4 h. No significant effects on group motor activity
were observed. Narcosis occurred at higher concentrations with a
threshold of 2180 ppm for a 4-h exposure (Molnar et al., 1986). Mice
(strain, sex and numbers not stated) were exposed for 2 h to
o-xylene in order to determine the minimum concentration needed for
an animal to fall on its side and die. The minimum concentration for
falling was 3400-4600 ppm and for death 6900 ppm (Lazarew, 1929).
In a study of subnarcotic effects of solvents in Wistar rats and
H-strain mice, using electrically evoked seizures, the lowest
effective concentration of o-xylene was 170 ppm. The criterion used
was a significant suppression by 10% of the generation and maintenance
of the seizure discharge after 4 h (rats) or 2 h (mice) of inhalation
(Frantik et al., 1994). A 30% suppression was induced by o-xylene
at a concentration of 390 ppm, corresponding to a blood concentration
of 62 µmol/litre (Frantik et al., 1993).
In order to determine the RD50 value (exposure level reducing
the respiratory rate by 50%), groups of male mice (OF-1) were exposed
to o-xylene. The RD50 value was calculated to be 1467 ppm
(1406-1530 ppm). The onset of response was rapid and the maximum
decrease in respiratory rate was reached within a few minutes (De
Ceaurriz et al., 1981).
In a study of conditioned behaviour in mice, an increased
response rate was observed after a 30-min exposure to 1400-2000 ppm
o-xylene. At higher concentrations there was a decrease in response
rate with an EC50 of 5179 ppm. The biphasic response indicates that
there was excitation of the central nervous system at low
concentrations and depression at higher concentrations (Moser et al.,
1985).
In a study to observe effects in the "behavioural despair"
swimming test, groups of 10 mice (OF-1) were exposed to 0, 1010, 1101,
1207 or 1234 ppm o-xylene for 4 h. The ID50 (50% decrease in
immobility) value was calculated to be 1127 ppm (1068-1182 ppm) (De
Ceaurriz et al., 1983). The study demonstrated subnarcotic effects by
o-xylene on the CNS.
7.1.1.2 m-Xylene
After a 6-h exposure to m-xylene the LC50 in rats and mice was
reported to be 5984 ppm (5796-6181 ppm) and 5267 ppm (5025-5490 ppm),
respectively (Bonnet et al., 1979; Bonnet et al., 1982). The signs of
toxicity consisted of hypotonia, somnolence, narcosis, and clonic
spasms leading to death due to respiratory failure (Lazarew, 1929;
Cameron et al., 1938, Bonnet et al., 1982; Moser et al., 1985; Molnar
et al., 1986).
In the comparative study by Frantik et al., (1994) described in
section 7.1.1.1, the lowest effective air concentration for m-xylene
was 210 ppm.
A biphasic CNS response occurred at similar exposure levels to
those seen for o-xylene (section 7.1.1.1) (Moser et al., 1985). The
narcotic threshold in rats of about 2100 ppm m-xylene determined in
prenarcotic behaviour studies was similar to that observed for
o-xylene (Molnar et al., 1986). During a 4-h exposure to 8000 ppm
m-xylene, 10 out of 12 rats (Carwoth-Wistar) died (Smyth et al.,
1962).
When groups of rats (Wistar; 6 males/group) were exposed for 24 h
to 0, 75, 150 or 300 ppm m-xylene, there was a significant decrease
in cytochrome P-450 concentrations at all doses, and a dose-related
decrease in 7-ethoxycoumarin-O-deethylase activity in the lung. No
abnormalities of the lungs, as determined by scanning electron
microscopy, were seen in two animals exposed to 300 ppm (Elovaara et
al., 1987).
When male Wistar rats were exposed for 4 h to a 1:1 mixture of
m-xylene and n-butyl alcohol or to the single substances at
500-4000 ppm, there were disturbances of rotarod performance. The
medial effective concentrations (EC50) were calculated to be 3080 ppm
(mixture), 6530 ppm ( n-butyl alcohol) and 1980 ppm (xylene)
respectively. The combined exposure gave less than additive effects
(Korsak et al., 1993).
A concentration-dependent decrease in respiratory rate in mice
was demonstrated in a study where mice were exposed to 500-4000 ppm of
n-butyl alcohol, m-xylene or a 1:1 mixture of both. The RD50 was
calculated to be 3010 ppm, 1360 ppm and 3140 ppm, respectively. The
combined exposure gave less than additive effects (Korsak et al.,
1993).
The acute neurobehavioural effect of m-xylene was evaluated
after 20 min inhalation exposure using a functional observations
battery in mice. In the concentration range of 2000-8000 ppm, these
effects included changes in posture, decreased arousal and rearing,
increased ease of handling, disturbances of gait, mobility and
righting reflex, decreased forelimb grip strength, increased landing
foot splay and impaired psychomotor coordination. The response to
various sensory stimuli was also decreased. These acute effects were
short-lived, recovery beginning within minutes of removal from the
exposure chamber (Tegeris & Balster, 1994).
7.1.1.3 p-Xylene
A 4-h LC50 value in rats (female Sprague-Dawley) of 4740 ppm
p-xylene has been reported (Drew & Fouts, 1974). The corresponding
6-h value was 4591 ppm (4353-5049 ppm) (Bonnet et al., 1982), and for
mice (OF-1) was 3907 ppm (3747-4015 ppm) (Bonnet et al., 1979). The
signs of toxicity were similar to those reported for the other two
isomers.
Like the other two isomers, exposure to p-xylene gave a
biphasic CNS response (Moser et al., 1985). In another study (Molnar
et al., 1986), marked activation and tremor were observed at
concentrations between 400 and 1500 ppm p-xylene in rats. The
narcotic threshold was 1940 ppm.
When Sprague-Dawley rats (16 females/group) were exposed for 4 h
to 0, 1000, 1500 or 2000 ppm p-xylene, a dose-dependent increase in
serum enzymes activites was observed. This was taken as a sign of
hepatocellular and hepatobiliary damage (Patel et al., 1979). In
other studies at higher exposure levels, no microscopic hepatic
lesions were seen in rats or mice (Cameron et al., 1938; Furnas &
Hine, 1958; Bonnet et al., 1982).
When Long Evans rats were exposed for 4 h to 0, 800 or 1600 ppm
p-xylene, the flash-evoked potential of the visual system was
reduced at the highest exposure level (Dyer et al., 1988). The
authors suggested that this may have been secondary to changes in
arousal or excitability. In a study of the effect on learning tasks
and motor activity, rats (Long Evans) were exposed to 0 or 1600 ppm
p-xylene for 4 h. Signs of toxicity (unsteadiness and fine tremor)
disappeared 30 min post-exposure. The results indicate an effect on
motor control rather than on cognitive capacity (Bushnell, 1989).
In the comparative study by Frantik et al. (1994) described above
(section 7.1.1.1), where rats and mice were exposed, the lowest
effective air concentration for p-xylene was 220 ppm.
After a 4-h exposure to 1000 ppm p-xylene there was a
decrease in pulmonary cytochrome P-450 in rabbits and a decrease in
NADPH-cytochrome c-reductase and mixed-function oxidase activity in
rats (Patel et al., 1976; Patel et al., 1978).
When Sprague-Dawley rats were exposed to 3400 ppm p-xylene for
4 h and killed 12 h later, an induction of cytochrome P-450 activities
(CYP2B) in liver microsomes was observed. On the other hand, the
pulmonary cytochrome P-450 activities were inhibited (Day et al.,
1992).
In rats (Sprague-Dawley) p-xylene (2800 ppm for 4 h) has been
shown to potentiate hepatotoxicity induced by bromobenzene, while the
effect of bromobenzene on pneumotoxicity was unaffected by p-xylene,
indicating differences in xylene metabolism between the liver and the
lung (Day et al., 1992).
7.1.1.4 Technical or undefined xylene
In rats LC50 values of 6350, 6700 and 10 950 ppm have been
reported after 4-h exposure (Hine & Zuidema, 1970; Carpenter et al.,
1975; Lundberg et al., 1983). Deaths occurred during the exposure
period. No LC50 values for mice have been found, although
experiments have been carried out at up to 7000 ppm for 30 min (Moser
et al., 1985). Signs of toxicity were the same as those produced by
the individual isomers.
No signs of toxicity were reported when rats and dogs were
exposed for 4 h to 580 and 530 ppm, respectively. The composition was
7.63% o-xylene, 65.01% m-xylene, 7.84% p-xylene and 19.27%
ethylbenzene (Carpenter et al., 1975). The same type of response at
similar exposure levels as for the three individual isomers was
observed in mice in a conditioned behavioural study (Moser et al.,
1985). In rats effects of xylene were studied on an operant behaviour
maintained by a fixed-ratio liquid reinforced schedule. A decrease
in the reinforcement rate was seen after exposure to 113 ppm for 2 h
(Ghosh et al., 1987; Ghosh & Pradhan, 1987).
When rats (Fischer F-344) were exposed to 1450 ppm xylene for 8
h, a slight increase in the auditory response threshold at 20 kHz was
noted (Pryor et al., 1987). The xylene composition was 10%
o-xylene, 80% m-xylene and 10% p-xylene.
In mice (Swiss-Webster) exposed to 1300 ppm xylene for one
minute, a decrease in respiratory rate as an indication of respiratory
tract irritation was seen (Carpenter et al., 1975). This effect was
not seen at an exposure level of 460 ppm. No histological
abnormalities were seen on histological examination of livers from
rats exposed to 5480 ppm for 4 h, nor were there any changes in serum
activities of an indicator enzyme (sorbitol dehydrogenase) for
hepatotoxicity after exposure to > 340 ppm (Lundberg et al.,
1986). No histological abnormalities were seen in cat livers after
exposure to about 9500 ppm for up to 2 h (Carpenter et al., 1975). In
dogs (Beagle) 1200 ppm xylene for 4 h caused lacrimation but there was
no noticeable effect at 530 ppm (Carpenter et al., 1975).
When groups of cats (five animals/group) were exposed for 5740,
6900 or 9200 ppm for up to 6 h, there was a concentration-dependent
decrease in time to onset of staggering and mild narcosis. There was,
however, a large individual variation. Deep narcosis was seen in four
animals at 9200 ppm xylene (Engelhardt & Estler, 1935).
7.1.2 Other exposure routes
The oral LD50 values in rat have been reported to be 3608 mg/kg
body weight for o-xylene, 5011 mg/kg body weight for m-xylene and
4029 mg body weight for p-xylene (Smyth et al., 1962). For various
mixtures of xylenes oral LD50 values in rat have been reported to be
between 3523 and 8700 mg/kg body weight (Wolf et al., 1956; Hine &
Zuidema, 1970; NTP, 1986). In mice the corresponding values were
reported to be 5627 mg/kg body weight for male and 5251 mg/kg body
weight for females (NTP, 1986). Signs of toxicity at lethal doses
were CNS depression and congestion of cells in liver, kidney and
spleen, seen by histological examination.
A dermal LD50 value for rabbits (New Zealand White) of 12 180
mg/kg has been reported for a 24-h exposure to m-xylene (Smyth et
al., 1962). From studies with intraperitoneal (i.p.), intravenous
(i.v.), subcutaneous (s.c.) and intramuscular (i.m.) administration in
rats and mice the acute toxicity of xylene is low (Bell et al., 1992).
All three isomers have been found to interfere with the
modulation of the vestibular-oculomotor pathways in the rat. The
blood threshold levels for this effect were 170-200 µg/ml following
administration by the i.v. route (Tham et al., 1984). Similar effects
have also been reported for m-xylene in rabbit. The
vestibular-oculomotor effects were seen at blood levels of 30 µg/ml
and some deaths due to respiratory stress were recorded at 100 µg/ml
(Larsby et al., 1976; Aschan et al., 1977; Ödkvist et al., 1979;
Ödkvist et al., 1980).
A dose-dependent depletion of hepatic glutathione, following i.p.
administration, has been demonstrated in rats. With o-xylene the
effect was seen from 50 mg/kg and with the other two isomers from 425
mg/kg (Van Doorn et al., 1980). A decrease in pulmonary cytochrome
P-450 levels has been observed with the three isomers, when
administered (i.p.) at 531 mg/kg (Pyykkö et al., 1987).
The CYP2B1 and CYP1A1 isoenzyme activities, benzyloxy-resorufin
O-deethylation and ethoxyresorufin O-deethylation, respectively,
were studied in nasal, pulmonary and hepatic tissues of rats injected
intraperitoneally with m-xylene. Tissues taken at 2, 12 and 24 h
after injection showed inhibition of these activities in both nasal
and pulmonary microsomes, but increased activities in hepatic
microsomes (Blanchard & Morris, 1994).
The effects of intraperitoneal administration of o-xylene
(1g/kg body weight) on: (a) rat hepatic and pulmonary mixed-function
oxidase content and activity; and (b) microsomal membrane structural
parameters were studied 1, 3, 6 and 12 h after administration (Park et
al., 1994). The pulmonary cytochrome P-450 content and aryl
hydrocarbon hydroxylase activity were decreased, a maximal inhibition
occurring 3 h after dosing. Reduced pulmonary activity for both
ethoxyresorufin O-dealkylation and benzyloxyresorufin
O-dealkylation was noted. In contrast, increased hepatic cytochrome
P-450 content was noted, with slightly increased ethaxyresorufin
O-dealkylation and markedly increased benzyloxyresorufin
O-dealkylation. An increase in pulmonary microsomal phospholipid
content and cholesterol content was noted even 1 h after dosing. In
liver the phospholipid content increased although there was no change
in cholesterol content; this suggested an increase in membrane
fluidity.
Sprague-Dawley rats were given m-xylene (1 g/kg body weight)
intraperitoneally and killed one hour after treatment. Microsomes
from the lung were then prepared. Compared to controls, m-xylene
administration decreased the CYP2B1 activity but did not alter the
CYP1A1 activity or epoxide hydrolase activity. In total, m-xylene
administration resulted in an inhibition of benzo (a)pyrene
detoxication and increased production of toxic metabolites in the
pulmonary microsomal preparations (Stickney et al., 1991).
Xylenes have been shown to inhibit the hypotonic haemolysis of
erythrocytes in vitro at low concentrations. The EC50 values were
29, 39 and 44 µg/ml for o-xylene, m-xylene and p-xylene,
respectively (Holmberg et al., 1974).
7.2 Short-term exposure
7.2.1 Inhalation studies
7.2.1.1 o-Xylene
In a study on the noradrenaline and dopamine levels in various
parts of the forebrain and hypothalamus, Sprague-Dawley rats (six
males/group) were exposed to 0 or 2000 ppm o-xylene 6 h/day for 3
days. The animals were killed within 18 h after final exposure. There
was a significant increase in catecholamine levels and turnover in
various parts of the hypothalamus and a decrease in the dopamine
turnover in the forebrain of exposed animals (Andersson et al., 1981).
In order to study the effects on cytochrome P-450 and enzyme
activities Sprague-Dawley rats (four males/group) were exposed to 0 or
2000 ppm o-xylene, 6 h/day for 3 days. There was a significant
increase in relative liver weight and cytochrome P-450 content in
exposed animals. Furthermore, there were increases in some liver
enzyme activities. In lungs, there was a decrease in cytochrome P-450
activity (Toftgård & Nilsen., 1982).
When guinea-pigs (15 per group) were exposed to 0 or 780 ppm
o-xylene, 8 h/day, 5 days/week for 6 weeks, there was a marked
decrease in body weight gain in exposed animals. No effects on the
liver, kidney, heart, spleen or lung were observed upon histological
examination (Jenkins et al., 1970). In the same study beagle dogs
were exposed for the same period of time. One dog out of two
experienced tremors throughout the exposure period. No other signs of
toxicity were reported (Jenkins et al., 1970). Squirrel monkeys were
also exposed in the same manner. One monkey out of three died on day
seven. No other signs of toxicity were reported (Jenkins et al.,
1970).
7.2.1.2 m-Xylene
In a study of levels of noradrenaline and dopamine in various
parts of the forebrain and hypothalamus, Sprague-Dawley rats (six
males/group) were exposed to 0 or 2000 ppm m-xylene, 6 h/day for 3
days. The animals were killed within 18 h after the last exposure. A
significant increase in catecholamine levels and turnover was observed
in various parts of the hypothalamus of exposed animals. There was no
effect on dopamine levels (Andersson et al., 1981).
In another study, Sprague-Dawley rats (four males/group) were
exposed to 0 or 2000 ppm m-xylene for 3 days. The effect on
cytochrome P-450 and enzyme activities in liver, kidney and lung were
studied. In exposed animals there was a significant increase in
relative liver weight, in cytochrome P-450 content in liver and
kidney, and in some enzyme activities in these two organs. The
content of pulmonary cytochrome P-450 was decreased (Toftgård &
Nilsen, 1982).
In a study of the effect on xenobiotic metabolism in Wistar rats,
10 males per group were exposed to 0, 50, 400 or 750 ppm m-xylene, 6
h/day, 5 days/week for one week. At the two highest doses there
was a significant increase in hepatic microsomal protein and
NADPH-cytochrome c reductase levels, and a decrease in hepatic
glutathione levels. There was no effect on hepatic cytochrome P-450
levels or on renal glutathione levels. At all dose levels there was a
significant increase in renal cytochrome P-450 and some enzyme
activities. When the animals were exposed to the same regimen for 2
weeks similar results were obtained. There were no abnormalities upon
histological examination of the liver (Elovaara, 1982). When Wistar
rats (20 males/group) were exposed to 0 or 300 ppm m-xylene 6 h/day,
5 days/week for one week there was, in exposed groups, a significant
increase in hepatic and renal 7-ethoxycoumarin O-deethylase activity
and in renal UDP-glucuronyl transferase. When exposure time was 2
weeks there was also a significant increase in hepatic cytochrome
P-450 and NADPH-cytochrome c reductase activities (Elovaara et al.,
1982).
In order to study the effect on lung cytochrome P-450 in rats,
six male Wistar rats/group were exposed to 0 or 300 ppm m-xylene 7
h/day, 4 days/week for 5 weeks. The only effects seen were a
significant decrease in cytochrome P-450 content and 7-ethoxycoumarin
O-deethylase activity (Elovaara et al., 1987). When the effects on
cerebral biochemistry was studied, groups of Wistar rats (15
males/group) were exposed to 0, 50, 400 or 750 ppm m-xylene 6 h/day,
5 days/week for 1 or 2 weeks. The only effect seen after one week of
exposure was a significant decrease in glutathione levels at all
concentrations. After 2 weeks there were also a dose-dependent
decrease in superoxide dismutase activity, a significant increase in
NADPH-diaphorase activity at all concentrations and a significant
increase in azoreductase activity at the two highest concentrations
(Savolainen & Pfäffli, 1980).
7.2.1.3 p-Xylene
In a study of levels of noradrenaline and dopamine in the
forebrain and hypothalamus, Sprague-Dawley rats (six males/group) were
exposed to 0 or 2000 ppm p-xylene 6 h/day for 3 days. The animals
were killed 16-18 h after the last exposure. In exposed animals there
was a significant increase in catecholamine levels and turnover in
various parts of the hypothalamus. There was no effect on dopamine
levels or turnover in the forebrain (Andersson et al., 1981).
In order to study the effect on cytochrome P-450 and enzyme
activities in the liver, kidney and lung, Sprague-Dawley rats (four
males/group) were exposed to 0 or 2000 ppm 6 h/day for 3 days. In
exposed animals there were significant increases in relative liver
weight, in hepatic cytochrome P-450 content, in NADPH-cytochrome c
reductase activity in the liver and kidney, and in 7-ethoxyresorufin
O-deethylase activity in the kidney. There was also a decrease in
pulmonary cytochrome P-450 content (Toftgård & Nilsen, 1982). To
study the lung microsomal activity, rabbits (New Zealand White; four
males/group) were exposed to 0 or 1000 ppm p-xylene 4 h/day for 2
days. In exposed animals there was a significant decrease in
microsomal cytochrome P-450 concentration and in NADPH-cytochrome c
reductase activity (Patel et al., 1978). Inhibition of CYP2B1 has
been observed in the lungs of rats dosed with p-xylene (Verschoyle
et al. 1993).
Rats exposed to 300 ppm p-xylene, 6 h/day for 1, 3 or 5 days
exhibited alterations in pulmonary microsomal membrane structural and
metabolic parameters (Silverman & Schatz, 1991). Following 1 day of
exposure, conjugated diene levels were elevated while total
phospholipid levels, cytochromes P-450 content, benzyloxyresorufin
O-dealkylase activity and 2-aminofluorene N-hydroxylase activity
were decreased. Core membrane fluidity was increased following 3 days
of exposure. After 5 days of exposure all parameters returned to
control levels with the exception of aryl hydrocarbon hydroxylase
activity, which was increased by 41%. Extracellular surfactant levels
were also decreased after 1 and 3 days of exposure but returned to
control values after 5 days. The increase in aryl hydrocarbon
hydroxylase activity after 5 days of exposure could have important
consequences on the metabolism of co-administered xenobiotics.
Male Fischer-344 rats exposed to 0 or 1600 ppm p-xylene by
inhalation, 6 h/day, for 1 or 3 days did not produce overt
hepatotoxicity but resulted in a significant increase in the
concentration of hepatic cytochrome P-450 (Simmons et al., 1991).
However, the concentration of hepatic cytochrome P-450 had returned to
control levels within 2 to 3 days after exposure.
p-Xylene has been shown to decrease axonal transport of
proteins and glycoproteins in rats (Long-Evans) exposed by inhalation
to 1600 ppm for 6 h/day, 5 days/week, for 8 days. When ethanol (10%
in drinking-water) was given during 6 days prior to inhalation of
p-xylene, the treatment prevented the decreased axonal transport.
Ethanol per se did not decrease the axonal transport (Padilla et
al., 1992).
When 10 Wistar rats and 10 mice (strain not given) were exposed
to 1226 ppm p-xylene 8 h/day for 14 days, no animals died. No other
results were reported (Cameron et al., 1938).
When mice were exposed to 1200 ppm p-xylene 6 h/day for 4 days
and infected with a sublethal dose of murine cytomegalovirus, 34%
mortality occurred, whereas no deaths occurred among uninfected,
p-xylene-exposed mice or infected, air-exposed mice (Selgrade et
al., 1993). Although p-xylene potentiated liver damage caused by
the virus, the magnitude of serum enzyme activities indicated that
this damage was not the probable cause of death. Enhanced mortality
was related to enhanced xylene toxicity due to suppression of
cytochrome P-450, although additive or synergistic damage to tissues
other than liver could not be ruled out. There was no indication that
p-xylene had caused immune suppression.
7.2.1.4 Technical or undefined xylene
When rats (strain not defined) were exposed to 620, 980 or 1600
ppm xylene 18-20 h/day for 7 days, instability, incordination and
narcosis were observed at the two highest concentrations. Signs of
mucous membrane irritation occurred, and congestion and cloudy
swelling of kidneys was reported at 980 ppm (Batchelor, 1927).
Similar results were reported in another study (Winslow, 1927).
Groups of Harlan-Wistar rats (25 males/group) were exposed to 0,
180, 460 or 810 ppm xylene 6 h/day, 5 days/week for 13 weeks. The
xylene consisted of 7.63% o-xylene, 65.01% m-xylene, 7.84%
p-xylene and 19.27% ethylbenzene. At 3, 7 and 13 weeks 3, 3 and 4
animals, respectively, were killed. No treatment-related
histopathology was seen (Carpenter et al., 1975).
In a study of levels of noradrenaline and dopamine in the
forebrain and hypothalamus of rats, the xylene mixture used was 2.0%
o-xylene, 64.5% m-xylene, 10.0% p-xylene and 23.0% ethylbenzene.
Sprague-Dawley rats (6 males/group) were exposed to 0 or 2000 ppm
xylene 6 h/day for 3 days. The animals were killed 16-18 h after the
last exposure. There was a significant increase in catecholamine
levels and turnover in the hypothalamus and a significant increase in
dopamine levels and turnover in the forebrain (Andersson et al.,
1981).
In order to study the effect on levels of neurotransmitters in
the rat brain, five to six Sprague-Dawley rats/group were exposed to
0, 200, 400 or 800 ppm xylene (mixture not defined) for 30 days.
Acetylcholine levels in the striatum were decreased at > 400 ppm.
Noradrenaline levels in the hypothalamus were increased significantly
at the highest dose. From 400 ppm the cAMP levels were decreased in
the striatum, and at 800 ppm the glutamine levels in the midbrain were
increased. At all concentrations glycine and GABA levels in the
midbrain were increased (Honma et al., 1983).
When 12 male Fischer F-344 rats/group were exposed to 0 or 1450
ppm xylene 8 h/day for 3 days, there was, in exposed animals, an
increase in the auditory response threshold at 12 and 20 kHz.
Rats (Long Evans) were exposed to 2500 ppm of mixed xylenes 6
h/day for 5 days. Testing of auditory function was conducted 5 to 8
weeks after exposure using reflex modification audiometry (RMA). The
results indicated increased RMA thresholds for the mid-frequency tones
(e.g., 8, 16 and 24 kHz) but not for higher or lower tones (Crofton et
al., 1994).
In another study Sprague-Dawley rats (four males/group) were
exposed to 0 or 630 ppm xylene 6 h/day, 5 days/week for 4 weeks. The
animals were killed the morning after the last exposure. In exposed
animals there was a significant decrease in body weight gain, while
the absolute and relative liver weights were increased. There was an
increase in hepatic cytochrome P-450 and the xylene was shown to act
as a phenobarbital-like inducer of cytochrome P-450. The xylene used
was 2.0% o-xylene, 64.5% m-xylene, 10.0% p-xylene and 23.0%
ethylbenzene (Toftgård et al., 1981).
When male Sprague-Dawley rats (8-12 animals/group) were exposed
to 0, 75, 250, 500, 1000 or 2000 ppm xylene 6 h/day for 3 days there
was a dose-dependent increase in the concentration of liver microsomal
cytochrome P-450. When animals were exposed to the two highest
concentrations for 5 days, there was a dose-dependent increase in the
surface area of smooth endoplasmic reticulum but not in rough
endoplasmic reticulum. Based on their results the authors concluded
that xylene causes phenobarbital-type induction in the liver. The
xylene used was the same as that in the previous paragraph (Toftgård
et al., 1983).
The same type of xylene was also used in a study where four male
Sprague-Dawley rats/group were exposed to 0 or 2000 ppm xylene 6 h/day
for 3 days. In exposed animals there were significant increases in
hepatic and renal cytochrome P-450 content, in NADPH-cytochrome c
reductase activities and 7-ethoxyresorufin O-deethylase activities
in liver and kidney. The pulmonary cytochrome P-450 content was
decreased. Increased enzyme activity in liver and kidney and decreased
activity in the lung were thus observed (Toftgård & Nilsen, 1982).
Groups of male Wistar rats (20 animals/group) were exposed to 0
or 300 ppm xylene 6 h/day, 5 days/week for up to two weeks. In each
group 10 animals had 15% v/v ethanol in the drinking-water. There was
a significant decrease in motor activity in exposed animals. In
addition, there was a significant increase in brain DT-diaphorase
activity, in acid proteinase and in hepatic and renal 7-ethoxycoumarin
O-deethylase activities. Concurrent dosing with ethanol had a
marked synergistic effect on hepatic and renal 7-ethoxycoumarin
O-deethylase activities. The xylene used was 80% m-xylene and 12%
p-xylene (Savolainen et al., 1979a,b).
In a study of the effect of simultaneous exposure to xylene
(undefined) and noise on metabolic activity in the myocardium, male
rats (strain not specified) were exposed to 0 or 69 ppm xylene 4 h per
day, 5 days per week for 6 weeks, and simultaneously to a noise level
of 0, 46, 85 or 95 dB. There were changes in some enzyme activities,
but the brief reporting makes the study impossible to evaluate
(Ivanovich et al., 1985).
When Beagle dogs (four males per group) were exposed to 0, 180,
460 or 810 ppm xylene 6 h per day, 5 days per week for 13 weeks, no
treatment-related effects were reported. The xylene used was 7.63%
o-xylene, 65.01% m-xylene, 7.84% p-xylene and 19.27% ethylbenzene
(Carpenter et al., 1975).
7.2.2 Other exposure routes
Decreased body weight compared to controls was noted in rats
(Sprague-Dawley) administered orally 1062 mg m-xylene/kg body weight
for 3 days (Pyykkö, 1980), and reduced terminal body weight was
observed in rats (Fischer F-344) administered 500 mg m-xylene/kg
body weight, 5 days per week, for 4 weeks (Halder et al., 1985).
Three days administration of 1062 mg m-xylene/kg body weight
resulted in significant increases in liver weight, liver cytochrome
P-450 and cytochrome b5 levels and NADPH cytochrome c reductase and
MFO enzymes activities. The same range of increases was also seen in
the kidney (Pyykkö, 1980). In another study, however, there were no
effects on kidney weight and no treatment-related abnormalities
histopathologically in Fischer rats given 500 or 2000 mg/kg body
weight 5 days per week for 4 weeks (Dési et al., 1967).
Upon oral administration of 0, 125, 250, 500, 1000 or 2000 mg
xylene per kg body weight to rats (F-344/N), in a 14-day study, a high
mortality was seen in the highest dose group. The xylene used was
9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. The body weight gain was reduced in males at > 250
mg/kg body weight and in females at 125 mg/kg body weight and
> 1000 mg/kg body weight. There were no treatment-related
abnormalities at gross necropsy (NTP, 1986). In the same study xylene
was administered at 0, 62.5, 125, 250, 500 or 1000 mg per kg body
weight, 5 days per week, for 13 weeks. No treatment-related
abnormalities were seen during gross necropsy or histopathological
examination (NTP, 1986).
Daily administration (s.c.) of xylene (undefined) for up to 4
weeks to rats (strain not given) resulted in significant mortality at
870 mg/kg body weight but not at 435 or 174 mg/kg body weight.
Repeated administration of 435 mg/kg body weight resulted in decreased
learning rate (Dési et al., 1967). When Sprague-Dawley rats were
given 0 or 3123 mg/kg body weight for 3 days, there was a significant
increase in liver weight, cytochrome P-450 levels, NADPH cytochrome c
reductase and activity of MFO enzymes. The xylene used was 30%
o-xylene, 55% m-xylene and 15% p-xylene (Pathiratne et al.,
1986).
In order to study further the effects of p-xylene, rats were
given p-methylbenzyl alcohol (PMBA) or 2,5-dimethylphenol (DMP)
(300 mg/kg body weight and 150 mg/kg body weight, respectively)
intraperitoneally once a day for 3 days. It was concluded that of the
two metabolites, PMBA may have a significant role in the inhibition of
pulmonary cytochrome P-450 caused by p-xylene (Day & Carlson, 1992).
Male Sprague-Dawley rats (n=10) were given xylene (isomer not
stated) intraperitoneally, as a single dose per day, for 3 consecutive
days. The dose given was equal to half the LD50 (1.6 ml/kg body
weight per day). Only slight somnolence was observed. After the last
dosing the animals were killed and aminopeptidase activities in
several regions of the brain were measured. The activities were
largely unaffected, compared to those of controls (De Gandarias et
al., 1993).
Brain cell cultures enriched in astroglial cells were prepared
from neonatal Sprague-Dawley rats. The cultures were exposed for 1 h
to 3, 6 or 9 mmol o-xylene/litre. The ATPase activity was reduced
in a dose-dependent manner (Naskali et al., 1994).
7.3 Long-term exposure
A short summary of long-term studies is presented in Table 6.
In Wistar rats exposed to 1000 ppm m-xylene for 6 h/day,
5 days/week for 3 months or to 100 ppm for 6 months, slight
ultrastructural changes (proliferation of smooth endoplasmic
reticulum) were found in hepatocytes. When rats were exposed to a 1:1
combination of m-xylene and toluene (500 plus 500 ppm or 50 plus 50
ppm), the changes were a combination of those of each single solvent
(Rydzynski et al., 1992). The combined exposure at both exposure
levels gave more pronounced disturbances in a rotarod performance test
and decrease in spontaneous motor activity compared to single solvent
exposure. In animals exposed to 500 plus 500 ppm for 3 months a
decrease in red blood cell count and an increase in rod neutrophil
cell count were observed (Korsak et al., 1992).
In a six-week study, groups of rats (Sprague-Dawley or
Long-Evans; 15 animals/group) were exposed to 0 or 780 ppm o-xylene
(8 h/day, 5 days/week) or to 78 ppm continously for 90 days. There
was no effect on body weight gain, leukocyte count, haemoglobin level
or heamatocrit. Histological examination of the liver, kidney, heart,
spleen and lung revealed no effects (Jenkins et al., 1970).
Table 6. Effects of xylenes in long-term studies
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Inhalation exposure
o-Xylenea rat, dog, 13 weeks 780 - Jenkins et al., 1970
guinea-pig
o-Xylenea monkey 13 weeks + 78 - Jenkins et al., 1970
m-Xylenea rat 3 months - 1000 liver cell changes (i.e. inc. smooth Rydzynski et al., 1992
endoplasmic reticulum, lysosomes)
m-Xylenea rat 6 months - 100 liver cell changes (i.e. inc. smooth Rydzynski et al., 1992
endoplasmic reticulum, lysosomes)
m-Xylenea rat 3 months - 100 dec. lymphocyte/monocyte count; Korsak et al., 1992
dec. rotorod, performance
m-Xylenea rat 6 months - 100 dec. rotorod, performance Korsak et al., 1992
dec. spontaneous motor activity
Xylenes rat 3 months 50 100 dec. rotorod performance Korsak et al., 1994
dec. spontaneous motor activity
Xylenes rat 6 months 346 923 liver effects (inc. liver weight inc. Ungvary, 1990
smooth endoplasmic reticulum, inc.
P-450 activity)
Table 6. (Cont'd)
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Inhalation exposure
Xylenes rat 13 weeks 810 - Carpenter et al., 1975
Xylenes dog 13 weeks 810 - Carpenter et al., 1975
Xylenes rat 6 weeks - 800 ototoxicity Pryor et al., 1987
(14h/day)
Xylenes rat 61 days - 1000 ototoxicity Nylen & Hagman, 1994
(18 h/day)
Xylenes rat 18 weeks - 300 liver microsomes activity Elovaara et al., 1980
Xylenes rat 18 weeks - 300 brain superoxide dismutase activity Savolainen et al., 1979
Xylenes rat 13 weeks + - 320 brain lipid composition changes Kyrklund et al., 1987
Xylenes gerbil 3 months 160 320 change in astroglial cell marker Rosengren et al., 1986
proteins
Table 6. (Cont'd)
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Oral exposure
Xylenes rat 13 weeks 1000 - NTP, 1986
Xylenes mouse 13 weeks 2000 - NTP, 1986
Xylenes rat 13 weeks - 150 increased liver weights (males); Condie et al., 1988
hyaline droplet nephropathy (males)
Xylenes rat 2 years 250 500 mortality NTP, 1986
Xylenes mouse 2 years 500 - NTP, 1986
a Only one dose was used
b Continous exposure
The effects of combined exposure to m-xylene and n-butyl
alcohol have been studied in rats exposed to the individual solvents
at 50 and 100 ppm and their 1:1 mixture at 50 plus 50 ppm and 100 plus
100 ppm, 6 h/day, 5 days/week for 3 months (Korsak et al., 1994). The
results indicate less than an additive toxic effect (motor
coordination disturbances) of combined exposure to m-xylene and
n-butyl alcohol. For xylene alone an effect was seen at 100 ppm but
not at 50 ppm.
Rats (CFY) were exposed to xylene (10% o-xylene, 50%
m-xylene, 20% p-xylene and 20% ethylbenzene) for 8 h/day up to 6
months at concentrations of 600, 1500 or 4000 mg/m3. No macroscopic
changes were seen but the relative liver weight was increased at 4000
mg/m3. At 4000 mg/m3 hypertrophy of the centrilobular zone in
the liver, including a change in the amount of smooth and rough
endoplasmic reticulum, was seen. As a result of xylene exposure the
hexobarbital sleeping time decreased. Liver enzymatic activities were
increased during the first 6 weeks. After the 4-week exposure-free
period the changes described above could no longer be seen. The same
type of changes could also be seen when xylene was applied orally,
subcutaneously or intraperitoneally for a short period of time (4-7
day). Essentially the same changes as those described for rat liver
were also seen in livers from mice and rabbits (Ungvary, 1990).
Groups of 50 male and 50 female Fischer-344/N rats received 0,
250 or 500 mg xylene/kg body weight. The xylene used was 9.1%
o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0% ethylbenzene.
The doses were given (in corn oil) by stomach tube on 5 days per week
for 103 weeks. The animals were killed within 14 days after the last
dosing. Body weights of high-dose (500 mg/kg body weight) males were
5 to 8% lower than those of vehicle controls. Results for low-dose
males and both female groups were comparable to those of controls.
Gross observation and histopathological results showed no incidences
of non-neoplastic effects in dosed groups, related to the
administration of xylene, at any sites (NTP, 1986; Huff et al., 1988).
In the same study groups of 50 male and 50 female B6C5F1 mice
received 0, 500 or 1000 mg xylene/kg body weight. The same type of
technical xylene was used. The doses were given, in corn oil, by
stomach tube 5 days per week for 103 weeks, after which the animals
were killed within 14 days. No significant difference in mean body
weights or survival was observed between treated animals and controls.
Gross observation and histopathological results indicated that at no
site were the incidences of non-neoplastic effects in dosed groups
related to the administration of xylene (see also section 7.7).
Male Fischer-344 rats were exposed to 0, 800, 1000 or 1200 ppm
xylene 14 h/day, 7 days/week for 6 weeks. At the highest dose level
there was a slight impairment of auditory (but not of visual or
somatosensory) conditioned avoidance response. All animals had
increased auditory response thresholds compared to controls at the
same frequencies. At 1000 and 800 ppm the thresholds were elevated at
16 kHz and 8 kHz and at 1200 ppm the brainstem auditory-evoked
response thresholds were elevated at 4, 8 and 16 kHz tone frequency.
The xylene used in these two studies was 10% o-xylene, 80%
m-xylene and 10% p-xylene (Pryor et al., 1987).
Wistar rats (60 males/group) were exposed to 0 or 300 ppm xylene
6 h per day, 5 days per week, for up to 18 weeks, and 50% of the
animals had 15-20% ethanol in the drinking water. The xylene used was
19.2% o-xylene, 43.0% m-xylene, 19.5% p-xylene and 18.3%
ethylbenzene. It was concluded that concurrent ethanol intake
increased hepatic and renal microsomal enzyme activities. Stearosis
in the liver was more marked in co-exposed animals than in animals
exposed only to ethanol. No treatment-related abnormalities were
observed during histopathological examination of animals exposed only
to xylene (Elovaara et al., 1980).
Exposure to 1000 ppm xylene (not defined) 18 h per day, 7 days
per week, for 61 days caused a slight loss of auditory sensitivity in
Sprague-Dawley rats. Co-exposure to n-hexane (1000 ppm) caused
a persistent loss of auditory sensitivity, which was less than
additively enhanced. Xylene inhibited n-hexane-induced impulse
velocity reduction in peripheral nerves (Nylén & Hagman, 1994).
In a study to investigate the effect on brain lipid composition,
Sprague-Dawley rats were exposed to 0 or 320 ppm xylene continuously
for 30 or 90 days. The xylene (undefined) produced only limited
transient changes (Kyrklund et al., 1987). In another study to
investigate the effect on brain with and without co-exposure to
ethanol, Wistar rats (20 males/group) were exposed to 0 or 300 ppm
xylene 6 h per day, 5 days per week, for up to 18 weeks. In each
group were 10 animals also exposed to 15% v/v ethanol in the
drinking-water. There was a significant increase in cerebral
microsomal superoxide dismutase activity by week 18, but no effect on
cerebral protein or RNA levels. Co-exposure to ethanol reduced the
effect caused by xylene exposure. The xylene used was 7.5%
o-xylene, 85.0% m-xylene and 7.5% p-xylene (Savolainen et al.,
1979a,b).
In a study of the effect on spinal cord axon membrane, Wistar
rats (five animals per group) were exposed to 0 or 300 ppm xylene
(undefined) 6 h per day, 5 days per week, for 18 weeks. In exposed
animals there was a decrease in the amount of membrane lipid per mg of
protein but no change in the cholesterol/lipid phosphorus ratio.
Ethanol (15% v/v) in the drinking-water enhanced the decrease and also
decreased the cholesterol/lipid phosphorus ratio (Savolainen &
Seppäläinen, 1979).
In a neurotoxicity study, changes in two astroglial cell marker
proteins (S-100 and GFA) and DNA were measured. Mongolian gerbils
(four of each sex per group) were exposed to 0, 160 or 320 ppm xylene
continously (24 h/day) for 3 months, followed by a 4-month
exposure-free period. The xylene used was 18% o-xylene, 70%
m-xylene, 12% p-xylene and < 3% ethylbenzene. The exposure to
xylene resulted in brain damage which was manifest as an increase in
astroglial cells. Effects were seen in particular parts of the brain
at both exposure levels but were only significant at 320 ppm
(Rosengren et al., 1986).
Ageing Long-Evans rats fed 200 ppm o-xylene for up to 6 months
showed formation of vacuolar structures in hepatocytes when examined
ultrastructurally (Bowers & Cannon, 1982).
Groups of 10 male and 10 female Sprague-Dawley rats were exposed
to mixed xylenes by gavage (in corn oil) for 90 consecutive days at
dose levels of 150, 750 and 1500 mg/kg body weight per day (Condie et
al., 1988). The most significant findings were increased relative
liver and kidney weights. Histopathology evaluation of liver and
kidney tissues revealed an increased incidence of minimal chronic
renal disease in females only. Treatment-related hepatic
histopathological changes were not detected in either sex. Hyaline
droplet formation was observed in male rats in all treated groups. In
females, there was an increased incidence of kidney effects, which
were thought to represent onset of progressive nephropathy (Condie et
al., 1988).
Mice (B6C3F1) were given orally 0, 250, 500, 1000 or 2000 mg
xylene per kg body weight for 14 days or 0, 125, 250, 500, 1000 or
2000 mg per kg body weight, 5 days per week, for 13 weeks. The xylene
used was 9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. Mean body weight gain was reduced in males at > 250
mg/kg body weight. No treatment-related abnormalities were observed
at gross necropsy or histopathological examination (NTP, 1986).
7.4 Skin and eye irritation; sensitization
Erythema and oedema were induced following a single application
(amount not stated) of xylene (not defined) to rabbit or guinea-pig
skin. There was a rapid onset and epithelial desquamation, with some
evidence of necrosis occurring after several days (Rigdon, 1940;
Steele & Wilhelm, 1966). After 24 h of exposure to 0.5 ml xylene
(undefined) under semi-occlusive conditions, irritation of rabbit skin
was observed. The irritation was graded as moderate by the authors
(Hine & Zuidema, 1970).
Application of 0.5 ml p-xylene, under a Teflon chamber, to
rabbit skin for 4 h produced a response which would be classified as
irritant using European Union criteria (Jacobs et al., 1987). In
another study, following 10 to 20 applications of xylene (not defined)
to open or semi-occluded rabbit skin for up to four weeks, a moderate
to marked irritation and moderate necrosis was reported, as was
blistering of the skin under the semi-occlusive dressing (Wolf et al.,
1956).
Application of approximately 0.05 to 0.5 ml of liquid xylenes
(individual isomers or undefined composition) to the rabbit eye was
reported to cause immediate discomfort and blepharospasm followed by
slight conjunctival irritation and very slight, transient corneal
necrosis (Wolf et al., 1956). Application of 0.1 ml liquid xylene
(not defined) was mildly irritant to rabbit eye. The lesions were not
described (Kennah et al., 1989).
No skin sensitization studies have been reported.
7.5 Reproductive and developmental toxicity
A summary of reproductive and developmental toxicity studies is
shown in Table 7.
Groups of female rats (CFY) were exposed to 0, 34, 345 or 690 ppm
o-xylene 24 h/day from day 7 to day 14 of gestation. The dams were
killed on day 21. Some ultrastructural changes in the liver and
decreased weight gain during the exposure period were observed in the
dams exposed to 345 or 690 ppm. At these concentrations lower fetal
body weight was observed, and at the highest dose level delayed
skeletal ossification was noted. There was no evidence of
malformations. Similar results were obtained when the animals were
exposed to m-xylene. With p-xylene there were signs of delayed
skeletal ossification at exposure levels showing no maternal toxicity
(at 34 and 345 ppm). At 690 ppm, increased postimplantation loss of
fetuses and more retarded fetuses were observed (Ungvary et al.,
1980).
Table 7. Reproductive and developmental effects (inhalation studies)
Compound Species Exposure Duration NOEL LOEL Endpoint Reference
o, m, p-Xylene rat 0, or 1000 24 h/day; Fused sternebrae, Hudak & Ungvary
mg/m3 days 9-14 extra ribs (1978)
Xylene rat 0, 10, 50 or 6 h/day; Maternal toxicity not Mirkova et al.
(not specified) 500 mg/m3 days 1-21 addressed (1983)
Xylene rat 0, 250, 1900 24 h/day; 250 mg/m3 Skeletal retardation Ungvary & Tatrai
(not specified) or 3400 days 7-15 (mothers and (1985)
mg/m3 offspring)
o-xylene rat 0, 150, 1500 24 h/day; 150 mg/m3 150 mg/m3 Decreased fetal weight, Ungvary et al.
or 3000 days 7-14 offspring (dams) skeletal retardation (1980)
mg/m3 1500 mg/m3
(offspring)
m-xylene rat 0, 150, 1500 24 h/day; 1500 mg/m3 3000 mg/m3 Maternal: reduced food Ungvary et al.
or 3000 days 7-14 (dams and (dams and consumption, reduced (1980)
mg/m3 offspring) offspring) weight gain
Offspring: reduced fetal
weight
p-xylene rat 0, 150, 1500 24 h/day; 150 mg/m3 Reduced mean litter Ungvary et al.
or 3000 day 7-15 (dams and size; reduced fetal (1980)
mg/m3 offspring) weight; skeletal
retardation
p-xylene rat 0 or 3000 10th day, Decreased fetal weight Ungvary et al.
mg/m3 24 h (1981)
Table 7. (Cont'd)
Compound Species Exposure Duration NOEL LOEL Endpoint Reference
p-xylene rat 0, 3500 or 6 h/day; 7000 7000 mg/m3 Maternal: reduced Rosen et al.
7000 mg/m3 days 7-16 mg/m3 (dams) weight gain (1986)
(offspring),
3500
mg/m3
(dams)
Xylene rat 0 or 600 24 h/day; Decreased maternal Ungvary (1985)
(not defined) mg/m3 day 7-15 weight gain.
Delayed fetal
development; extra ribs
Xylene rat 870 mg/m3 6 h/day; No maternal toxicity; Hass & Jakobsen
(not defined) days 4-20 delayed ossification (1993)
o, m, p-Xylene rat 2175 mg/m3 6 h/day; Delayed righting reflex; Hass et al. (1995)
days 7-20 reduced absolute brain
weight; impaired
neuromotor ability
Xylene mouse 0, 500 or 24 h/day; 500 mg/m3 1000 mg/m3 Skeletal retardation and Ungvary & Tatrai
(not specified) 1000 mg/m3 days 6-15 offspring (offspring) increased incidence of (1985)
weight-retarded fetuses
Xylene rabbit 0, 500 or 24 h/day; 500 mg/m3 Maternal: decreased Ungvary & Tatrai
(not specified) 1000 mg/m3 days 7-20 (offspring) weight gain (1985)
1000 mg/m3 Offspring: delayed
(dams) skeletal development
Rats (CFY) were exposed to 0.58, 437 or 782 ppm 24 h/day on days
7 to 15 of gestation and the dams were killed on day 21. Data
concerning maternal toxicity was not given. There was delayed
skeletal ossification at all dose levels, while decreased fetal
bodyweight, increased postimplantation loss and increased frequency of
skeletal variants (extra ribs) were observed at 782 ppm. Mice (CFLP)
were exposed to 0 or 115 ppm o-xylene for 4 h, 3 times per day on
day 6 to day 15 of gestation, and the dams were killed on day 18.
Data concerning maternal toxicity were not reported. There was
evidence of delayed weight gain and skeletal ossification in the
fetuses of exposed animals. Similar results were obtained with
m-xylene and p-xylene (Ungvary & Tatrai, 1985). When rabbits (New
Zealand White) were exposed to 0 or 115 ppm o-xylene 24 h/day from
day 7 to day 20 of gestation, no maternal toxicity or incidence of
delayed development was observed in the exposed group. Similar
results were observed with m-xylene, but an increased incidence of
post-implantation loss was observed. Exposure to 115 ppm p-xylene
gave the same results as with o-xylene, but exposure to 230 ppm
p-xylene 24 h/day resulted in no live fetuses (one dam died, three
aborted and in four there was total resorption or fetal death in
utero) (Ungvary & Tatrai, 1985).
In a study to validate a developmental toxicity screen, mice
(ICR/SIM) were exposed orally to 0 or 2000 mg m-xylene/kg body
weight from day 8 to day 12 of gestation. No effects were seen on
mothers or young (Seidenberg et al., 1986). In another study
Sprague-Dawley rats were exposed to 0, 800 or 1600 ppm p-xylene, 6 h
per day from day 7 to day 16 of gestation. The dams were allowed to
deliver their young. In the highest dose group the maternal weight
gain was significantly reduced. Exposure to p-xylene had no effects
on postnatal viability, offspring growth or function of the nervous
system (activity level and acoustic startle response) at any of the
doses tested (Rosen et al., 1986).
In an attempt to study the effect of xylene on sex steroids
during pregnancy, rats (CFY) were exposed to 0 or 681 ppm p-xylene
for 24 h on day 10 of gestation or continuously on days 9 and 10 of
gestation. The animals were killed on day 11. Data on maternal
toxicity were not reported. Sex hormone levels in the uterine and
femoral veins were decreased in the exposed group. The authors
(Ungvary et al., 1981) suggested that this may play a role in the
embryotoxicity.
Rats (CFY) were exposed to 0 or 230 ppm xylene for 24 h/day from
day 9 to day 14 of gestation, and the dams were killed on day 21. The
xylene used was 10% o-xylene, 50% m-xylene, 20% p-xylene and 20%
ethylbenzene. No maternal effects were seen in exposed animals.
There was an increased incidence of skeletal variants such as extra
ribs and fused sternebrae. Three malformations were found (2
agnathia, 1 fissura sterni), but there was no significant increase in
the frequency of malformations (Hudak & Ungvary, 1978).
In another study rats (CFY) were exposed to 0 or 138 ppm xylene
(not defined) 24 h/day from day 7 to day 15 of gestation. The dams
were killed on day 21. In the exposed group, decreased maternal
weight gain, delayed fetal development and increased incidence of
skeletal variants (extra ribs) were observed, but there was no
evidence of malformations (Ungvary, 1985).
In a report issued by the American Petroleum Institute in 1983,
described by Bell et al. (1992), Sprague-Dawley rats were exposed to a
xylene mixture of 20.4% o-xylene, 44.2% m-xylene, 20.3% p-xylene
and 12.8% ethylbenzene. There were 30 males and 60 females in the
control group, while 10 males and 20 females per group were exposed to
60 or 250 ppm xylene and 20 males and 40 females were exposed to 500
ppm xylene. Exposures were 6 h per day, 7 days per week, for a
131-day pre-mating period and a 20-day mating period. Mated females
were also exposed during days 1-20 of gestation and days 5-20 of
lactation. For males there were no effects on body weight gain, but
for females the mean body weight gain was significantly greater than
that of controls in the 60 and 250 ppm groups during the mating
period. This was not considered indicative of an adverse effect of
treatment. Mating indices were significantly lower than control
values at 250 ppm (both sexes treated) and at 500 ppm (females treated
only), but mating indices were comparable to control values at 500 ppm
when both sexes were treated or when males alone were treated. There
were no treatment-related effects on mean duration of gestation, mean
litter size or pup survival. The mean pup weight in the group where
both parents had been exposed to 500 ppm xylene was significantly
lower than for controls. In the teratogenicity part of the study
there was no evidence of an increased incidence of malformations in
exposed groups. The mean fetal weights for the 500 ppm group were
lower than the control value, but, the difference was statistically
significant for only the female fetuses.
Groups of Wistar rats were exposed to 0, 2, 11 or 114 ppm xylene
(mixed, not defined) for 6 h per day from day 1 to day 21 of
gestation. The maternal toxicity was not reported. The incidence of
post-implantation loss and fetal death was significantly increased in
the 11 ppm and 114 ppm groups. At the highest dose an increase in
some malformations was noted but no incidence was given. The results
of the study cannot be evaluated because of insufficient reporting of
exposure conditions and results (Mirkova et al., 1983). In an attempt
to repeat this study, groups of 36 Wistar rats were exposed to 200 ppm
technical xylene 6 h per day on days 4 to 20 of gestation. There were
no signs of maternal toxicity. No exposure-related differences were
found except for delayed ossification of maxillary bone. The
xylene-exposed pups had a slightly higher body weight and impaired
performance in a motor ability test, which was most marked in female
offspring (Hass & Jakobsen, 1993).
In a follow-up study (Hass et al., 1995), Wistar rats were
exposed to 500 ppm technical xylene (19% o-xylene, 45% m-xylene,
20% p-xylene and 15% ethylbenzene) for 6 h per day on gestation days
7-20. The dose level was selected so as not to induce maternal
toxicity or decrease the viability of offspring. There were 15
exposed litters and 13 control litters. A delay in the development of
the air righting reflex, a lower absolute brain weight and impaired
performance in behavioural tests for neuromotor abilities, learning
and memory were found in the offspring of the exposed rats.
Generally, the effects were most marked in the female offspring. The
alterations were long-lasting, as they were still apparent in adult
rats at the age of 4 months.
A composition of 9.1% o-xylene, 60.2% m-xylene, 13.6%
p-xylene and 17.0% ethylbenzene was given orally to CD-1 mice. The
doses were 0, 520, 1030, 2060, 2580, 3100 or 4130 mg per kg body
weight daily from day 6 to day 15 of gestation. All animals in the
highest dose group died and so did 50% of the animals in the 3100
mg/kg group. In this group there was a significant increase in the
incidence of dams with complete resorptions. There was a significant
increase in the incidence of cleft palate at > 2060 mg/kg, as well
as decreased mean fetal weight (Marks et al., 1982).
In order to study the teratogenic and embryotoxic effects of
xylene (60% p-xylene, 22% o-xylene, 18% ethylbenzene; no
m-xylene) embryos of Sprague-Dawley rats were explanted on day 9.5
of gestation and cultured in rat serum to which xylene (0.1, 0.5 or
1.0 ml/litre) dissolved in DMSO was added. The embryos were cultured
for 48 h. There were no observable teratogenic effects in terms of
malformations. However, dose-dependent embryotoxicity in terms of
retardation on growth and development was observed (Brow-Woodman et
al., 1991). In a similar study (Brown-Woodman et al., 1994) rat
embryos were incubated in vitro with up to 2.7 µmol xylene/ml for
40 h. Concentrations of > 1.89 µmol/ml retarded embryo growth and
development. The no-observed-effect level (NOEL) was 1.08 µmol/ml.
No gross morphological malformations were observed. Combined exposure
to toluene, xylene and benzene caused additive embryotoxic effects
with no evidence of synergistic action (Brown-Woodman et al., 1994).
When Sprague-Dawley rats were exposed to 1000 ppm xylene (not
defined) 18 h per day, 7 days per week, for 61 days, testicular
atrophy or loss of nerve growth factor-immunoreactive germ cell line
was not observed. Xylene also was found to protect from
n-hexane-induced testicular atrophy (Nylén et al., 1989).
Wistar rats were exposed for 7 days to xylene (isomer not stated)
twice a day until disappearance of righting reflex (xylene
concentration not given). Anaesthesia was achieved in about 10 min.
On day 8 the rats were killed. A decrease in body weight and weights
of testes and accessory reproductive organs, as well as reduced acid
phosphate activity in the prostate and reduced plasma testosterone
levels, was observed in xylene-exposed animals. There was also a
decrease in spermatozoan count in the epididymis (Yamada, 1993).
7.6 Mutagenicity and related end-points
Technical grade xylene did not produce differential killing in
DNA-repair-proficient compared to repair-deficient strains of
Bacillus subtilis rec+/- (McCarroll et al., 1981a) or Escherichia
coli (McCarroll et al., 1981b). Xylene (type not specified) did not
induce SOS activity in Salmonella typhimurium TA1535/pSK 1002
(Nakamura et al., 1987). For E. coli WP2 uvr A p-xylene was not
mutagenic in the presence or absence of an exogenous metabolic system
from PCB-induced rat liver (Shimizu et al., 1985). None of the
isomers nor unspecified xylene was mutagenic to S. typhimurium
TA1535, TA1537, TA98, TA100, UTH 8413 or UTH8414 in the presence or
absence of a metabolic system from uninduced or Arochlor-induced rat
and hamster livers (Lebowitz et al., 1979; Bos et al., 1981; Haworth
et al., 1983; Connor et al., 1985; Shimizu et al., 1985; Zeiger et
al., 1987).
Exposure to technical grade xylene containing 18.3% ethylbenzene
caused recessive lethal mutations in Drosophila melanogaster but not
exposure to m-xylene, or o-xylene (Donner et al., 1980). The same
report stated that exposure to rats for 300 ppm, 6 h per day, 5 days
per week for 9, 14 and 18 weeks did not induce chromosomal aberrations
in bone-marrow cells (Donner et al., 1980). Xylene (unspecified) did
not induce mutations in mouse lymphoma L5178Y TK+/- cells in vitro
or chromosomal aberrations in rat bone marrow cells (Lebowitz et al.,
1979). Xylene (unspecified) did not induce sister chromatid exchange
or chromosomal aberrations in human lymphocytes in vitro. No
exogenous metabolic system was used in this study (Gerner-Smidt &
Friedrich, 1978).
None of the isomers induced micronuclei in the bone marrow of
male NMRI mice after two i.p. administrations of 105-650 mg/kg body
weight at a 24-h interval, but they did, however, enhance the
induction of micronuclei by toluene (Mohtashamipur et al., 1985).
When Sprague-Dawley rats were given 440 or 1320 mg o-xylene/kg
body weight intraperitoneally, a significant increase in the
percentage of abnormal sperm was reported when the animals were housed
at 24-30°C (but not at 20-24°C). The authors (Washington et al.,
1983) interpreted this as a synergistic effect between o-xylene and
temperature.
7.7 Carcinogenicity
Group of Sprague-Dawley rats, 40 of each sex, received 500 mg
mixed xylenes (composition not specified) per kg body weight in olive
oil by stomach tube on 4 to 5 days per week for 104 weeks. Fifty
animals of each sex received olive oil only. The animals were
maintained until natural death. All animals had died by week 141. At
that time thymomas were reported in 1/34 treated males and 0/36
treated females (compared to 0/45 and 0/49, respectively, in
controls). Other haemolymphoreticular tumours (not specified) were
reported in 4/34 treated males and 3/36 treated females (compared to
3/45 and 1/49, respectively, in controls). The authors (Maltoni et
al., 1983; Maltoni et al., 1985) reported an increase in the total
number of animals with malignant tumours (type not specified) at 141
weeks, e.g., as 13/38 in treated males and 22/40 in treated females
(compared to 11/45 and 10/49, respectively, in control animals).
Combining all tumours is, however, not an acceptable basis for
analysis particularly in aged animals. No data were provided to allow
an analysis on an individual tumour-type basis.
In a carcinogenicity study, groups of 50 B6C3F1 mice of each sex
were given 0, 500 or 1000 mg xylene/kg body weight in corn oil by
stomach tube on 5 days per week for 103 weeks. The xylene used was
9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. The surviving animals were killed within 2 weeks after
the last administration. Survival at the termination of the study for
males was: 27 controls, 35 low-dose and 36 high-dose; and for females
was: 36 controls, 35 low-dose and 31 high-dose. No treatment-related
increase in the incidence of any tumour was seen in either sex (NTP,
1986; Huff et al., 1988).
The NTP also performed a carcinogenicity study on Fischer-344
rats with the same type of technical xylene. Groups of 50 rats of
each sex were given 0, 250 or 500 mg xylene/kg body weight in corn oil
by stomach tube 5 days per week for 103 weeks. The surviving animals
were killed within 2 weeks following the last administration. At the
termination of the experiment, the survival for males was: 36
controls, 25 low-dose and 20 high-dose animals, and for females was:
38 controls, 33 low-dose and 35 high-dose. For the males survival
appeared to be dose-related, but many of the early deaths were related
to gavage trauma or corn oil-xylene aspiration (3/14, 8/25, 11/30).
The incidences of tumours in treated animals of either sex were not
significantly higher than in the control group (NTP, 1986; Huff et
al., 1988).
Two studies have investigated whether exposure to xylenes alters
the incidence of experimentally induced skin neoplasia in mice (Pound
& Withers, 1963; Pound, 1970). The reporting does not allow any firm
conclusions.
7.8 Other effects
No effects were observed upon in vitro exposure of human
lymphocytes at concentrations up to 2 mM xylene for 72 h. However, at
higher concentrations, cell mortality only was significantly increased
(Richer et al., 1993).
8. EFFECTS ON HUMANS
8.1 Acute and accidental exposure
Acute poisoning and deaths have been reported after overexposure
or oral ingestion of substantial amounts of xylene. The exposure
level required for loss of consciousness has been estimated to be
10 000 ppm (Morley et al., 1970). At autopsy pulmonary congestion and
oedema have been observed after inhalation or oral intake (Morley et
al., 1970; Abu Al Ragheb et al., 1986). Among survivors coma, EEG
changes, amnesia, mental confusion and ocular nystagmus have been
reported. Evidence of gastrointestinal and respiratory symptoms as
well as impaired renal and hepatic function have also been observed
(Ghislandi & Fabiani, 1957; Recchia et al., 1985; Bakinson & Jones,
1985). After exposure to about 700 ppm (calculated) for up to one
hour, headache, nausea, irritation of the eyes, nose and throat,
dizziness, vertigo and vomiting have been reported (Klaucke et al.,
1982). Recovery seems to be complete in most non-fatal cases although
dizziness and vision problems have been observed 24 h after ingestion
of xylene (quantity unknown) (Recchia et al., 1985). In a suicidal
attempt a 30-year-old man injected 8 ml of xylene intravenously.
After 10 min he developed a life-threatening acute pulmonary failure,
but survived through medical treatment (Sevcik et al., 1992).
8.2 Controlled human studies
A number of volunteer studies have been performed predominantly
at the Finnish Institute of Occupational Health. Effects on the
sensory motor and information process functions of the central nervous
system (CNS) have been investigated. Usually m-xylene has been
used, but p-xylene and mixed xylenes have also been studied
(Savolainen & Linnavuo, 1979; Savolainen, 1980; Savolainen et al.,
1980; Seppäläinen et al., 1981; Savolainen & Riihimäki, 1981;
Savolainen et al., 1981; Savolainen et al., 1982a, Savolainen et al.,
1982b; Savolainen et al., 1984; Savolainen et al., 1985a, Savolainen
et al., 1985b). In these studies no significant effects on vestibular
or visual function, reaction times, coordination or peripheral senses
were observed during a 4-h exposure to a constant concentration of up
to 160 ppm. Slight impairment of vestibular and visual function and
reaction time was noted at exposure levels from 200 to 300 ppm. There
was adaption to the impairment over five successive daily exposures.
In another study (Anshelm Olson et al., 1985), volunteers (n=16) were
exposed for 4 h to p-xylene alone (300 mg/m3: 70 ppm) or in
combination with toluene (200 mg/m3 toluene plus 100 mg/m3
p-xylene). Heart rate, subjective symptoms, simple reaction time,
choice reaction time and short-term memory were unaffected by
exposure.
In another study nine male volunteers were exposed for 4 h to 200
ppm (TWA) m-xylene. Short-term peak exposures were up to 400 ppm.
The effects of xylene on electroencephalography (EEG) were minor and
no deleterious effects were noted (Seppäläinen et al., 1991).
Healthy male subjects were exposed to technical xylene,
containing 40% ethylbenzene, for 2 h with or without a working load of
100 watts. The air concentration was 435 or 1300 mg/m3. During work
at the higher exposure level evidence of performance decrement was
observed in three of the five performance tests: reaction time
addition test (p < 0.05), short-term memory (p < 0.05) and choice
reaction time (p < 0.10) (Gamberale et al., 1978).
Dizziness was reported by four of six volunteers exposed to 690
ppm p-xylene for 15 min (Carpenter et al., 1975). Nine volunteers
were exposed for about 4 h to either a constant or a fluctuating
pattern of m-xylene with a time-weighted average exposure
concentration of 200 ppm in both cases (Laine et al., 1993).
Prolonged simple visual and auditive choice reaction times were
observed after exposure to the peaks of 400 ppm m-xylene. Exposure
to m-xylene at a constant level of 200 ppm did not affect the ratio
of "active" to "quiet" sleep during the following night, but decreased
slightly the number of body movements in bed.
Studies on coexposure to m-xylene and ethanol (Savolainen,
1980; Seppäläinen et al., 1981; Savolainen & Riihimäki, 1981;
Riihimäki et al., 1982a,b; Riihimäki et al., 1982a,b), m-xylene and
1,1,1-trichloroethane (TCE) (Savolainen et al., 1981; Savolainen et
al., 1982) or p-xylene and toluene (Anshelm-Olson et al., 1985) have
been performed. Exposure to 145-150 ppm xylene and 0.8 g ethanol/kg
body weight had an additive disturbing effect on vestibular function.
At higher xylene concentrations (275-290 ppm) there was evidence of
functional tolerance, and xylene appeared to antagonize the effect of
ethanol on vestibular function. In a study where volunteers were
exposed to 200 ppm m-xylene, minor effects on vestibular and visual
functions and reaction time were reported. Simultaneous exposure to
400 ppm TCE had no further effect. Combined exposure to 60 ppm
p-xylene and 30 ppm toluene had no effect on reaction time,
short-term memory or heart rate.
Ten male volunteers were exposed to 100 ppm xylene (not
specified) or 100 ppm toluene or a mixture of 50 ppm of each.
Exposure time was 4 h and each person participated in four exposure
sessions. Changes in CNS functions were tested by nine psychological
tests. Xylene had the most adverse effect on simple reaction time and
choice reaction time, while the combined exposure gave weaker effects
than xylene alone but stronger than toluene alone (Dudek et al.,
1990).
A summary of acute effects from inhalation exposure is presented
in Table 8.
In studies on skin irritation, both hands of subjects were
immersed in pure m-xylene (Engström et al., 1977; Lauwerys et al.,
1978; Riihimäki, 1979a). A burning sensation was soon noticed and an
erythematous reaction was observed in the exposed skin. Of six
subjects tested, four reported eye irritation after exposure to 460 or
690 ppm xylene for 15 min. The xylene contained 7.6% o-xylene,
65.0% m-xylene, 7.8% p-xylene and 19.3% ethylbenzene. One subject
reported eye irritation at 230 ppm but none at 110 ppm (Carpenter et
al., 1975). In another study, no irritation in the eyes, nose or
throat was reported after exposure to 98, 196 or 392 ppm mixed xylenes
for 30 min (Hastings et al., 1984). In an older study exposure to 200
ppm xylene (undefined) for 3-5 min caused irritation of the eyes, nose
and throat (Nelson et al., 1943).
The skin sensitization potential was investigated using a
non-adjuvant maximization test. The xylene used was not defined.
None of 24 subjects tested showed evidence of sensitization (Kligman,
1966).
Five adult, healthy, white men were exposed for 7 h per day for 3
days to 40 ppm xylene. This exposure was repeated three times at
intervals of 2 weeks. Blood samples were taken before and after each
exposure. No significant effects were observed on sister-chromatid
exchange frequency, cell cycle time or cell mortality in lymphocytes
(Richer et al., 1993).
8.3 Occupational exposure
In workers exposed to xylene or solvent mixtures containing large
amounts of xylene, subjective symptoms have been reported (Joyner &
Leak Pegues, 1961; Glass, 1961; Hipolito, 1980; Kilburn et al., 1983;
Kilburn et al., 1985). Depression, fatigue, headache, anxiety,
feeling of drunkenness and sleep disorders were the most common
symptoms reported, but exposure levels and duration were often missing
in these reports.
Workers occupationally exposed to solvent mixtures including
xylene have been reported to have neurophysiological and psychological
disorders (Lindström, 1973; Seppäläinen et al., 1978; Elofsson et al.,
1980; Husman, 1980; Husman & Karli, 1980; Arlien-Soborg et al., 1981;
Lindström et al., 1982; Valciukas et al., 1985; Maizlish et al., 1987;
Van Vliet et al., 1987; Ruijten et al., 1994). In these studies there
was no exposure to xylene alone. Xylene was not the main solvent in
the mixture. No conclusions concerning effects of xylenes as such can
be drawn from these studies.
Table 8. Single inhalation exposure to xylene in humans
Exposure Time Effect Reference
concentration
(mg/m3)
3 000 1 h Dizziness, irritation Klaucke et al. (1982)
3 000 15 min Dizziness Carpenter et al. (1975)
1300a 2 h Performance decrement Gamberale et al. (1978)
900b 4 h Prolonged reaction times Laine et al. (1993)
900 4 h Impairment of vestibular and visual Savolainen et al.
function and prolonged reaction time (1979, 1981, 1982, 1985)
900 4 h Minor effect on EEG Seppäläinen et al. (1991)
600 4 h No effect on reaction time Savolainen et al. (1980, 1981)
450 4 h Prolonged reaction time Dudek et al. (1990)
300 4 h No effects in psychophysiological test Anshelm - Olson et al. (1985)
a during exercise
b peak values of 1800 mg/m3
Liver effects have been reported in some studies on workers
occupationally exposed to solvents containing xylene (Dössing et al.,
1981; Edling, 1982; Sotaniemi et al., 1982; Dössing et al., 1983;
Fischbein et al., 1983, Lundberg et al., 1994) but these were not
confirmed in other studies (Craveri et al., 1982; Kurppa & Husman,
1982; Lundberg & Håkansson, 1985). Recent findings seem to support
the concept that the hepatotoxicity of xylene is low (Riihimåki &
Hännien, 1987).
Some case studies and epidemiological studies have addressed a
possible association between occupational exposure to hydrocarbons
(including xylene) and proliferative glomerulonephritis (Beirne &
Brennan, 1972; Zimmerman et al., 1975; Lagrue, 1976). Exposures
have, however, been so diverse that the role of xylene is impossible
to assess. A Swedish group of researchers reported a higher
concentration of albumin, erythrocytes and leukocytes in the urine of
workers exposed predominantly to xylenes and toluene than among
controls (Askergren et al., 1981a,b,c; Askergren, 1981). Franchini et
al. (1983) found that painters exposed to toluene and xylenes at
relatively low concentrations had a higher excretion of kidney tubular
enzymes in their urine than the controls. They suggested that the
mixed solvents may exert a slight adverse effect on the kidney
tubules.
One case of contact urticaria due to occupational exposure to
airborne xylene has been reported. The level of xylene in air
exceeded 100 ppm. Direct skin contact with the solvent appeared to
have been negligible. The contact urticaria seemed likely to be an
immunological type (Palmer & Rycroft, 1993).
Thirty-five male spray varnishers were exposed to 0.5-3.4 ppm
o-xylene, 3.2-11.7 ppm m-xylene, 0.9-4.3 ppm p-xylene, 1.4-7.5
ppm ethylbenzene, < 1.5 ppm toluene, < 1.2 ppm n-butanol, < 35.5
ppm 1,1,1-trichloroethane and several C9 aromatics. In addition, some
of the lacquers contained lead pigments. The mean peripheral
erythrocyte counts and haemoglobin levels were decreased in the
exposed men compared to controls. Whether these effects were due to
xylene or the solvent mixture is uncertain (Angerer & Wulf, 1985).
The health effects on 175 factory workers in China exposed to
xylene vapour with a mixture of three isomers at a concentration of up
to 175 ppm (with time-weighted average geometric mean concentration of
14 ppm and arithmetic mean of 21 ppm) were studied. There was an
increased prevalence of subjective symptoms in the exposed workers;
these were apparently related to effects on the central nervous system
and to local effects on the eye, nose and throat (Uchida et al.,
1993). In workers exposed to a maximum concentration of 103 ppm
xylene (geometric mean 4 ppm) and 203 ppm toluene (geometric mean 3
ppm), the prevalence of some subjective CNS-related symptoms was
higher than in the controls (Chen et al., 1994). No effects on
haematology or serum biochemistry with respect to liver and kidney
functions were observed in these two studies.
Sister-chromatid exchanges (SCE) in peripheral lymphocyte
cultures have been studied in two groups of 23 workers who had been
exposed for between 4 months and 23 years to mixed xylenes (including
ethylbenzene). The exposure levels for the two groups were 11 and 13
ppm, respectively. No differences in SCE frequency was seen (Pap &
Varga., 1987). A few other reports have described increased incidence
of chromosomal aberrations or effects on sister-chromatid exchange
frequencies (Funes-Cravioto et al., 1977; Haglund et al., 1980). In
both these studies the exposure to xylene (not defined) was
accompanied by exposure to other solvents, including benzene.
No data on carcinogenic effects resulting from exposure to
xylenes have been found in the literature.
In an investigation into the effect on reproduction, the outcome
of pregnancy was studied among university laboratory employees exposed
to xylene (not defined) during the first trimester of pregnancy.
Exposure levels were not given. There was no difference in
miscarriage rate when compared to controls not exposed to solvents
(Axelsson et al., 1984).
A similar case control study on associations between laboratory
work and pregnancy outcome revealed that use of xylene for 3 or more
days a week during the first trimester was significantly associated
with an elevated risk of spontaneous abortion. The authors pointed
out, however, that laboratory workers were often exposed to several
solvents and chemicals simultaneously; only two cases and two controls
were exposed to xylene alone (Taskinen et al., 1994). About half of
the women exposed to xylene worked in pathology/histology laboratories
where there was concomitant exposure to formaldehyde vapour.
Formaldehyde (formalin) also appeared to be a significant risk factor
for spontaneous abortion. Exposure to xylene was also noted to be
associated with an increase in birth weight (Taskinen et al., 1994).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Bringmann & Kuhn (1977, 1978) exposed the bacterium Pseudomonas
putida for 16 h and the blue-green alga Microcystis aeroginosa for
8 days to xylene. They found a reduction in cell multiplication at
concentrations of >200 mg/litre.
Walton et al. (1989) studied the effect of p-xylene on the
microbial respiration of two soil types, a silt loam (1.49% organic
carbon) and a sandy loam (0.66% organic carbon). The chemical was
applied at a rate of 1000 µg/g (dry weight). Microbial respiration,
as measured by CO2 efflux, of the silt loam was unaffected. In the
sandy loam, the CO2 efflux initially decreased and then increased,
but returned to control levels within the 6-day exposure period.
All three isomers have been shown to inhibit the respiration of
sewage sludge utilizing biogenic substrates. Two screening tests were
used, RIKA (respiration inhibition kinetic analysis) and OECD 209.
The concentration of each compound used was at the limit of the
solubility in the medium (approx. 175-198 mg/litre). Inhibitions in
the respiration rate of 100% were found for all three isomers in the
RIKA screening test, and inhibitions of 22% ( o-xylene) and 43%
( p-xylene) were measured in the OECD 209 screening test. m-Xylene
was only tested at a concentration of 0.3 mg/litre in the OECD 209
screening test, which resulted in 5% inhibition (Volskay & Grady,
1990).
The toxicity of xylene to three species of environmental bacteria
has been determined using assays in sealed serum bottles to prevent
loss of chemical by volatilization. Activity of the bacteria was
measured by either gas production over 48 h (methanogens), oxygen
consumption over 15 h (aerobic heterotrophs) or ammonia use over 24 h
(Nitrosomonas). IC50 values (the concentration required to inhibit
the bacterial activity by 50%, as compared with controls) for xylene
were 250 mg/litre for methanogens, 1100 mg/litre for aerobic
heterotrophs and 100 mg/litre for Nitrosomonas (Blum & Speece,
1991).
9.1.2 Aquatic organisms
Xylene isomers are highly volatile and disappear rapidly from
solution. For example, Mackay & Wolkoff (1973) found that in agitated
water, 1m deep and with a 1m2 surface for evaporation, the half-life
for o-xylene was 39 min. When Benville & Korn (1977) monitored the
loss of xylene from solution during LC50 tests, average percentage
losses for the four time intervals studied (24, 48, 72 and 96 h) were
29, 61, 84 and > 99% respectively. These losses mean that the
exposure can be difficult to determine. For example, many of the 24-h
and 96-h LC50 values are the same or similar, suggesting that most of
the xylene had been lost during the test. Galassi et al. (1988),
using a closed static system, found mean measured concentrations of
xylene and other aromatics to fluctuate by only 10% during the test
period of up to 96 h. Care must therefore be taken when interpreting
data from open static tests over longer periods than 24 h, especially
those based on nominal concentrations. Overall it can be stated that
xylene has moderate to low acute toxicity for aquatic organisms.
9.1.2.1 Algae
Bringmann & Kühn (1977) exposed the green alga Scenedesmus
quadricauda for 8 days to xylene. They found reduction in cell
multiplication at concentrations of > 200 mg/litre. Brooks et al.
(1977) studied the effect of xylene on photosynthesis in a mixed ocean
culture of phytoplankton. They found that exposure to a concentration
of 3 mg/litre xylene for 8 h caused a 50% reduction in photosynthesis.
Galassi et al. (1988) calculated the 72-h EC50 for growth
inhibition in the alga Selenastrum capricornutum to be 4.7, 4.9 and
3.2 mg/litre for the ortho, meta and para isomers, respectively.
Similar results were obtained by Herman et al. (1990). Using the same
species of algae, they obtained 8 day EC50 values for growth
inhibition of 4.2, 3.9 and 4.4 mg/litre for the ortho, meta and para
isomers, respectively. Sheedy et al. (1991) reported a 14-day EC50
for growth inhibition of Selenastrum capricornutum of 72 mg/litre
for xylene (composition not stated).
Hutchinson et al. (1980) exposed the algae Chlamydomonas
angulosa and Chlorella vulgaris to p-xylene for 3 h. EC50
values for inhibition of photosynthesis (measured using 14CO2
uptake) were 45.7 and 105.1 mg/litre for the two species,
respectively. Kauss et al. (1973) exposed the green alga Chlorella
vulgaris to o-xylene and studied growth over a 10-day period in an
open system. At nominal concentrations of between 25 and 100
mg/litre, a short-term toxic effect was observed. However, the algal
culture recovered within 2 days. The authors pointed out that
recovery was probably due to volatilization of the chemicals. A
near-saturation concentration of 171 mg o-xylene/litre proved to be
acutely toxic and the alga did not recover. Concentrations of 25 and
50 mg o-xylene/litre progressively increased the lag period between
initial inoculation and growth. An o-xylene concentration of 100
mg/litre delayed the onset of growth for 4 days and a near-saturation
concentration of 171 mg/litre caused complete inhibition of growth
during the 10-day exposure period (Kauss & Hutchinson, 1975).
Dunstan et al. (1975) exposed marine microalgae, the diatom
Skeletonema costatum, the dinoflagellate Amphidinium carterae, the
coccolithophorid Cricosphaera and the green flagellate Dunaliella
tertiolecta, to xylene for 3 days. A xylene concentration of 10
mg/litre inhibited the growth of all species. Inhibition was most
marked in A carterae and S. costatum.
9.1.2.2 Higher plants
Frank et al. (1961) exposed the angiosperm pondweeds Elodea
canadensis, Potamogeton nodosus and P. pectinatus to xylene
(plus 2% emulsifying agent) under static, open conditions for a period
of four weeks. A concentration of 100 mg/litre was found to be toxic
(8.6 on an injury scale of 0 to 10) but 5 mg/litre was not. Both 300
and 600 mg/litre were similarly toxic after 30-min exposures in
flowing water.
9.1.2.3 Protozoa
Bringmann et al. (1980) exposed the flagellate protozoan
Chilomonas paramaecium to xylene for 48 h and found an initial
reduction (5%) in cell multiplication at concentrations of > 80
mg/litre. Rogerson et al. (1983) exposed the ciliate protozoan
Colpidium colpoda to xylene in an "open" system consisting of a
covered watchglass with an air space. Toxicity thresholds were 1.75
mg/litre (16.5 mmol/m3) for o-xylene and 162 mg/litre (1530
mmol/m3) for m-xylene; no threshold was calculated for p-xylene.
The same authors exposed the protozoan Tetrahymena ellioti to xylene
isomers in a closed system with no air space. Toxicity thresholds for
o-, p- and m-xylene were 18.5, 16.9 and 55.7 mg/litre (174, 159
and 525 mmol/m3), respectively.
9.1.2.4 Invertebrates
The LC50 values of xylene to aquatic invertebrates are
summarized in Table 9.
Le Gore (1974) exposed Pacific oyster larvae (Crassostrea
gigas) to o- and p-xylene for 48 h. LC50 values were 0.17 and
0.58 mg/litre for the two isomers, respectively, suggesting that this
organism is one of the more sensitive invertebrates to xylene
exposure. However, no experimental details were given for these
toxicity tests, which makes them difficult to interpret.
Table 9. LC50 values of xylenes to aquatic invertebrates
Organisms Size/ Static/ Open/ Temp. Hardness Isomer Duration Concentration Reference
life-stage flowa closeda (°C) (mg/litre)c (mg/litre)d
Estuarine and marine invertebrates
Pacific oyster larvae ortho 48 h 0.17 Le Gore (1974)
(Crassostrea larvae para 48 h 0.58 Le Gore (1974)
gigas)
Grass shrimp static c 21 15s 96 h 7.4 Neff et al. (1976)
(Palaemonetes static c 21 15s 24 h 14.0 Tatem et al. (1978)
pugio) 96 h 7.4 Tatem et al. (1978)
Bay shrimp adult static o 16 25s ortho 24 h 4.7 n Benville & Korn (1977)
(Crago adult static o 16 25s ortho 96 h 1.1 n Benville & Korn (1977)
franciscorum) adult static o 16 25s meta 24 h 4.1 n Benville & Korn (1977)
adult static o 16 25s meta 96 h 3.2 n Benville & Korn (1977)
adult static o 16 25s para 24 h 1.7 n Benville & Korn (1977)
adult static o 16 25s para 96 h 1.7 n Benville & Korn (1977)
Dungeness crab zoeae static+ 13 30s ortho 48 h 38 n Caldwell et al. (1977)
(Cancer zoeae static+ 13 30s ortho 96 h 6 n Caldwell et al. (1977)
magister) zoeae static+ 13 30s meta 48 h 33 n Caldwell et al. (1977)
zoeae static+ 13 30s meta 96 h 12 n Caldwell et al. (1977)
Table 9. (Cont'd)
Organisms Size/ Static/ Open/ Temp. Hardness Isomer Duration Concentration Reference
life-stage flowa closeda (°C) (mg/litre)c (mg/litre)d
Freshwater invertebrates
Water flea static o 24 h >100<1000 n Dowden & Bennett (1965)
(Daphnia magna) static 24 h 165 n Brigmann & Kühn (1982)
static c ortho 24 h 1 m Galassi et al. (1988)
static c meta 24 h 4.7 m Galassi et al. (1988)
static c para 24 h 3.6 m Galassi et al. (1988)
4-6 days static c ortho 48 h 3.2 n Bobra et al. (1983)
4-6 days static c meta 48 h 9.6 n Bobra et al. (1983)
4-6 days static c para 48 h 8.5 n Bobra et al. (1983)
< 24 h flow o 17 44.7 ortho 48 h 3.82 m Holcombe et al. (1987)
Mosquito larvae static o 24-26 24 h 13.9 Berry & Brammer (1977)
(Aedes aegypti)
Snail
(Aplexa adult flow o 17 44.7 ortho 96 h >22.4 m Holcombe et al. (1987)
hypnorum)
a static = static conditions (water unchanged for duration of test); b o = open; c = closed
static+ = semi-static conditions (water renewed at 24 hour intervals); c s = salinity (%)
flow = flow-through conditions (xylene concentration in water continuously maintained) d m = measured; n = nominal
Berry & Brammer (1977) exposed mosquito larvae (Aedes aegypti)
to xylene under static, open conditions at 25°C. Larvae were exposed
for 24 h, since no detectable levels of any water-soluble component
remained after that period. A non-lethal concentration of 7.92
mg/xylene litre was reported. However, the authors found that
bioassays using different volumes of solution and different sized
containers demonstrated the significant effect that surface area,
volume and depth can have on the results of experiments with volatile
hydrocarbons such as xylenes.
Falk-Petersen et al. (1985) exposed sea urchin (Strongy
locentrotus droebachiensis) eggs to o-xylene from fertilization
and monitored deaths, pathology, inhibition of cleavage and
differentiation, and pigment effects. Eggs were maintained in test
beakers covered with aluminium foil. They calculated a 96-h EC50,
based on all these parameters, of 4.1 mg/litre.
Freshwater mussels (Dreissena polymorpha) were exposed to
various concentrations of xylene, and the behaviour of the mussels in
terms of shell valve movements was monitored. At the start of the
experiment, all valves were gaping continuously. After a certain
period of toxicant addition, a gradual increase in valve closure
period was observed. Finally, total closure of shell valves was
observed. Effects were first seen at a xylene concentration of
11.9-19.4 mg/litre (nominal) (Slooff et al., 1983).
9.1.2.5 Vertebrates
The LC50 values of xylene to fish are summarized in Table 10.
Morrow et al. (1975) found that 100 mg xylene/litre killed 100%
of young coho salmon (Oncorhynchus kisutch) within 24 h under
static, closed conditions. Concentrations of 1 and 10 mg/litre did
not cause significant mortality within the 96-h exposure period.
Toxicity included rapid, violent and erratic swimming, "coughing",
loss of equilibrium and death.
Rainbow trout (Oncorhynchus mykiss) significantly avoided
xylene (plus 2% emulsifying agent) at a nominal concentration of 0.1
mg/litre during a 1-h test. Fish exposed to 0.001 mg/litre did not
show significant avoidance and those exposed to 0.01 mg/litre were
significantly attracted to the xylene (Folmar, 1976). Maynard & Weber
(1981) found that juvenile coho salmon were able to significantly
avoid o-xylene concentrations of > 0.2 mg/litre water.
Slooff (1979) exposed rainbow trout Oncorhynchus mykiss to
various concentrations of xylene in a flow-through, closed system and
monitored any effects of the chemical on their breathing. The lowest
concentration at which a toxic condition developed within 24 h after
toxicant administration was 2 mg/litre.
Table 10. LC50 values of xylenes to fish
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Freshwater fish
Fathead minnow 1-2 g static o 25 20 24 h 28.8 n Pickering & Henderson
(Pimephales 1-2 g static o 25 20 48 h 27.7 n (1966)
promelas) 1-2 g static o 25 20 96 h 26.7 n
1-2 g static o 25 360 24 h & 96 h 28.7 n
0.3 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Bluegill 1-2 g static o 25 20 24 h 24 n Pickering & Henderson
(Lepomis 1-2 g static o 25 20 48 h 24 n (1966)
macrochirus) 1-2 g static o 25 20 96 h 20.9 n
1.1 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Goldfish 1-2 g static o 25 20 24 h & 96 h 36.8 n Pickering & Henderson
(Carassius (1966)
auratus)
20-80 g flow 17-19 80 24 h 30.6 m Weber et al. (1975)
20-80 g flow 17-19 80 96 h 16.9 m
3.3 g static o 19-21 ortho 24 h 13 m Bridie et al. (1979)
3.3 g static o 19-21 meta 24 h 16 m
3.3 g static o 19-21 para 24 h 18 m
2.5 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Table 10. (Cont'd)
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Guppy 0.1-0.2 g static o 25 20 24 h & 96 h 34.7 n Pickering & Henderson
(Poecilia (1966)
reticulata)
static meta 14 days 305 Könemann (1981)
static ortho 7 days 291
static para 7 days 285
static c 20-22 ortho 96 h 12 m Galassi et al. (1988)
static c 20-22 meta 96 h 12.9 m
static c 20-22 para 96 h 8.8 m
Rainbow trout static c 11-13 ortho 96 h 7.6 m Galassi et al. (1988)
(Oncorhynchus static c 11-13 meta 96 h 8.4 m
mykiss) static c 11-13 para 96 h 2.6 m
flow o 9-13 89.5 96 h 10e Folmar (1976)
13.1 g flow o 17 44.7 ortho 96 h 8.05 m Holcombe et al. (1987)
0.9 g flow o 12 40-48 technical 24 h & 96 h 13.5 Walsh et al. (1977)
Zebra fish flow c 48 h 20 Sloff (1979)
(Brachydanio
rerio)
Table 10. (Cont'd)
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Golden orfe 1.2-1.8 g static 48 h 86f Juhnke & Lüdemann
(Leuciscus 1.2-1.8 g static 48 h 308f (1978)
idus
melanotus)
White sucker 2.4 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
(Catostomus
commersoni)
Marine fish
Coho salmon young static c 8 30s 24 h > 10 < 100 Morrow et al. (1975)
(Oncorhynchus
kisutch)
Striped bass 6 g static o 16 25s ortho 24 h & 96 h 9.7 n Benville & Korn (1977)
(Morone 6 g static o 16 25s meta 24 h & 96 h 7.9 n
saxatilis) 6 g static o 16 25s para 24 h & 96 h 1.7 n
a static = static conditions (water unchanged for duration of test);
flow = flow-through conditions (xylene concentration in water continuously maintained)
b o = open; c = closed
c s = salinity (°/oo)
d m = measured; n = nominal
e included 2% emulsifying agent
f results from two different laboratories
When Walsh et al. (1977) exposed rainbow trout (average weight
165 ± 86 g) to xylene concentrations of 0.31, 0.65 and 1.1 mg/litre in
artificial streams for 56 days, no adverse effects on the fish were
noted at any concentration. Using the same artificial streams, all
rainbow trout exposed to xylene concentrations of 14.2 or 22.5
mg/litre for 2 h died. Fish exposed to xylene concentrations of 3.2
and 6.2 mg/litre for 2 h showed symptoms similar to anaesthesia.
Kjorsvic et al. (1982) exposed cod eggs ( Gadus morhus L.) to
xylene isomers in covered glass dishes and monitored the effects both
during fertilization and during early cleavage of fertilised eggs.
Both m-xylene and p-xylene induced significant decreases in the
fertilization rate at concentrations above 10 mg/litre. o-Xylene
had no significant effect on the fertilization rate at concentrations
of 16-35 mg/litre. Fertilized eggs were exposed to xylene for 3 or 6
h before first cleavage. No significant difference was observed
between the individual xylene isomers or between the two exposure
periods. Effects on the early cleavage pattern were significant for
xylene concentrations between 2 and 7 mg/litre. The effects seen
included inhibition of formation of the cleavage furrow. Small cells
or a total absence of cleavage occurred on exposure to all isomers at
concentrations of 16-35 mg/litre, while incomplete or uneven cleavage
was found at exposures of 8-15 mg/litre.
Black et al. (1982) exposed embryo-larval stages of the leopard
frog Rana pipiens and rainbow trout (Oncorhynchus mykiss) to
m-xylene from 30 min after fertilization to 4 days after hatching in
a closed, flow-through system (hatching times were 5 days for the frog
and 23 days for the trout). LC50 values of 3.53 and 3.77 mg/litre
were calculated for the two species, respectively.
9.1.3 Terrestrial organisms
Hill & Camardese (1986) exposed Japanese quail (Coturnix
coturnix japonica) to xylene in 5-day dietary toxicity tests. The
LC50 was found to be greater than 20 000 mg/kg diet. No overt signs
of toxicity occurred at 5000 mg/kg.
No studies on terrestrial plants, terrestrial invertebrates or
field effects of xylenes have been reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposures
In the general environment humans are exposed to xylenes mainly
by inhalation. Absorption through the skin may also occur in the
working environment. The retention in the lungs is about 60% of the
inhaled dose. Xylene is efficiently metabolized. More than 90% is
biotransformed to methylhippuric acid, which is excreted in urine.
Xylene does not accumulate significantly in the human body.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3 with levels of 3 µg/m3 in suburban
areas. Higher levels have been measured in urban and industrialized
areas with mean concentrations ranging up to 500 µg/m3; however,
concentrations are generally below 100 µg/m3.
The estimated daily exposure of the general population through
inhalation is 70 µg in rural areas and less than 2000 µg in urban
areas. The concentration in drinking-water ranges from not detectable
to 12 µg/litre. Data on the levels in food are too limited to
estimate daily oral exposure.
10.1.2 Effects
Based on experimental human studies, xylene may have an acute
impairing effect on the sensory-motor and information-processing
functions of the central nervous system (CNS). Exposure to 435-870
mg/m3 (100-200 ppm) of xylene over 4 h caused slight impairments in
reaction time performance and vestibular functions. There was
adaptation at 200 ppm m-xylene to the impairment over five
successive days. The LOAEL of xylene for acute CNS effects, based on
one study, is 470 mg/m3 (108 ppm). It should be noted, however,
that other identical studies found significant effects only at
concentrations of 870 mg/m3 (200 ppm) or more. According to a study
by a different research group, exposure to 304 mg/m3 (70 ppm) p-
xylene for 4 h did not cause impairment of corresponding psycho-
physiological functions; 304 mg/m3 (70 ppm) xylene can therefore
be regarded as the NOAEL for acute CNS effects.
Xylene vapour becomes irritating at relatively high levels.
Among six volunteer subjects, four reported eye irritation after
exposure to 2000 or 3000 mg/m3 (460 or 690 ppm) xylene for 15 min
while one subject reported eye irritation at 1000 mg/m3 (230 ppm) and
none at 478 mg/m3 (110 ppm). According to another study, no
irritation of the eyes, nose or throat was reported after exposure to
423, 852 or 1705 mg/m3 (98, 196 or 392 ppm) mixed xylenes for 30 min.
These human findings are consistent with mouse studies showing that
strong irritancy (respiratory rate decrease by 50%) occurs at about
5960 mg/m3 (1370 ppm) xylene. The odour threshold for xylene is
about 1 ppm.
Subjective symptoms have been reported among workers exposed to
solvent mixtures containing large amounts of xylene. Long-term
exposure to xylene is suspected to affect the nervous system adversely
because chronic toxic encephalopathy and milder functional
disturbances of the brain have sometimes been found among exposed
painters and other workers. Likewise, slight changes in kidney
tubular function may occur. The specific role of xylene in these
effects cannot, however, be ascertained.
When human data are sparse, especially data from chronic studies,
animal data are used as a substitute. An assessment of the risk to
human health of exposure to xylene must rely on animal studies.
Apparently irreversible effects on the CNS were found 4 months
after a 3-month inhalation exposure (24 h per day) of Mongolian
gerbils to xylene at concentrations of 696 or 1392 mg/m3 (160 or 320
ppm). At the lower level the effects were not statistically
significant in any individual part of the brain but the changes were
all of the same nature. The study disclosed an increased
concentration of astroglial proteins in most brain regions studied,
which may indicate that glial proliferation is characteristic to
various neurodegenerative and neurotoxic states. In the light of
similar findings in animals exposed to other solvents (e.g.,
trichloroethylene, ethanol and tetrachloroethylene), the results are
estimated to be an important piece of evidence for potential
xylene-induced neurotoxicity at > 696 mg/m3 (160 ppm) (the LOAEL).
Functional changes, similar to acute effects on nervous functions,
were described after exposure to 435 mg/m3 (100 ppm), but they cannot
be discriminated from the acute effects of the last exposure. The
exposure level is consistent with the levels giving acute effects in
humans, as stated above.
No adequate studies of reproduction and development toxicity in
humans exposed to xylene alone have been published. Placental
transfer of xylene has been shown in humans and in experimental
animals.
Teratogenicity studies in pregnant animals exposed to technical
xylene or xylene isomers during organogenesis indicate that xylene may
cause reduced fetal weight and delayed ossification, but not
malformations, at dose levels causing no or only slight maternal
toxicity. LOAEL values of 500-2175 mg/m3 (115-500 ppm) have been
reported, depending on the length of the daily exposure periods (6-24
h/day). Signs of delayed ossification in the absence of lower fetal
body weight have been reported at lower dose levels. However, these
findings cannot be properly evaluated owing to incomplete description
of the criteria for assessing ossification. A NOAEL for delayed fetal
development cannot therefore be established.
In a study of postnatal development in rat offspring prenatally
exposed to 870 or 2175 mg/m3 (200 or 500 ppm) technical xylene,
behavioural impairments indicating effects on the development of the
central nervous system were detected. There was no maternal toxicity,
and the effects at 2175 mg/m3 (500 ppm) were long-lasting as they
were apparent in adult offspring. As 870 mg/m3 (200 ppm) was the
lowest dose level investigated for this effect a NOAEL could not be
established.
In several short-term and long-term animal studies, effects on
the activities of various metabolic enzymes in different organs have
been observed. Exposure to 217 mg/m3 (50 ppm) m-xylene 6 h/day for
5 days induced renal cytochrome P-450. At 1305 mg/m3 (300 ppm)
xylene (6 h/day for 14 days) an increase in hepatic and renal enzyme
activities was observed. At > 1740 mg/m3 (400 ppm) increases in
hepatic and renal metabolic enzyme levels, as well as increased
relative liver and kidney weights, have been reported. At the same
exposure levels the pulmonary P-450 content and pulmonary enzyme
activities were decreased. When rats were orally given 150 mg
xylene/kg body weight per day for 90 days, increased relative liver
weights were seen.
These changes in metabolic enzyme activities and increased
relative liver weight could be taken as an indication of metabolic
adaptation rather than toxicity.
When rats were exposed to 3480 mg/m3 (800 ppm) xylene, 14 h/day,
7 day/week for 6 weeks, an increased auditory response threshold was
reported. Thus, 3480 mg/m3 (800 ppm) is the LOAEL for ototoxicity.
For this effect no NOAEL could be established as 3480 mg/m3 (800 ppm)
was the lowest dose level used.
Xylene appears not to be a mutagen or a carcinogen.
10.1.3 Guidance value
The definition and aim of guidance values for the general
population have been described by IPCS (1994).
Although some differences in action between the three isomers
exist, there is no clear evidence that they (or mixture of them) have
totally different effects.
There have been no long-term controlled human studies or
epidemiological studies from which a guidance value may be calculated.
Epidemiological data from the occupational setting do not allow
an estimation of xylene-specific chronic nervous system effects,
neither can the neuropsychological impairment seen among
solvent-exposed workers be attributed to any specific level of xylene
in air.
On the basis of human volunteer studies (Anshelm Olson, 1985),
one may conclude that the NOAEL for acute CNS effects in humans is
about 304 mg/m3 (70 ppm) for a 4-h exposure. The use of an
uncertainty factor of 10 for intraspecies variability (the study
subjects were healthy male research workers) and an additional factor
of 6 (4-h exposure versus 24-h general population exposure) leads to a
guidance value of 4.8 mg/m3 (1.1 ppm). It should be noted, however,
that the acute CNS effect observed probably has no predictive value
for chronic CNS toxicity by xylene. The guidance value of 4.8 mg/m3
(1.1 ppm) is close to the odour threshold of xylene. It should be
noted that a subset of the human population may be sensitive enough to
experience the odour as annoying. The Task Group considered that
there was no need to add another uncertainty factor for the lack of
data from chronic exposure.
On the basis of animal studies on developmental toxicity, one may
conclude that the LOAEL for reduced fetal body weight is 500 mg/m3
(115 ppm) (Ungvary & Tatrai, 1985) and that for developmental
neurotoxicity is 870 mg/m3 (200 ppm) (Hass & Jakobsen, 1993).
Developmental neurotoxicity is a serious effect that may be
long-lasting and is therefore considered the critical effect. An
uncertainty factor of 10 for use of a LOAEL rather than a NOAEL seems
justified based on the evidence of lower fetal body weight at 500
mg/m3 (115 ppm) and limited evidence of delayed ossification at even
lower exposure levels. The use of additional factors of 10 for
interspecies variation and 10 for inter-individual variation leads to
a guidance value of 0.87 mg/m3 (0.2 ppm).
Using the hearing loss (Pryor et al., 1987) detected in animals
after exposure to 3480 mg/m3 (800 ppm) xylene for 6 weeks as a
starting point, an uncertainty factor of 10 for use of a LOAEL rather
than a NOAEL, a factor of 10 for interspecies variation and an
additional factor of 10 for inter-individual variation results in a
guidance value of 3.48 mg/m3 (0.8 ppm).
A neurotoxicity study in animals exposed continuously for 3
months to 696 or 1392 mg/m3 (160 or 320 ppm) xylene (Rosengren et
al., 1986) provided suggestive biochemical evidence of an apparently
irreversible adverse effect on the nerve cells of the brain even at
the lower level. Although there may be uncertainty concerning the
biological significance and interpretation of the findings, the Task
Group considered them potentially important and recommended further
confirmatory studies. With respect to animal-human extrapolation and
relatively short exposure, the estimate of the critical level for
life-long exposure in humans is 1.6 ppm. A guidance value of 0.87
mg/m3 (0.2 ppm) covers another uncertainty factor of approximate 10
in this respect.
Based on the above considerations, the Task Group recommended
0.87 mg/m3 (0.2 ppm) as a guidance value for the general population.
This value was derived from the LOAEL reported for developmental
neurotoxic effects in laboratory animals.
10.2 Evaluation of effects on the environment
10.2.1 Exposure
The majority of xylene released into the environment will enter
the atmosphere directly. In the atmosphere the xylene isomers are
readily degraded. Volatilization to the atmosphere from water is
rapid for all three isomers. Although the meta and para isomers are
readily biodegraded, in soil and water the ortho isomer is more
persistent. Bioaccumulation of xylene isomers by aquatic organisms is
low.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3. Mean background concentrations of
xylenes in surface waters are generally below 0.1 µg/litre. However,
higher values have been measured in industrial areas. In areas
associated with the oil industry even higher levels have been reported
but only associated with discharge pipes. Similar background levels
have been reported for groundwater, although localized pollution can
lead to higher levels.
10.2.2 Effects
The xylene isomers are of moderate to low toxicity to aquatic
organisms. The variation between each individual isomer with respect
to aquatic toxicity is generally small. The lowest LC50 value, based
on measured concentrations, is for a 24-h exposure of Daphnia magna
to 1 mg o-xylene/litre.
There is limited information regarding chronic exposure of
aquatic organisms to xylenes and none of the observed effect levels
were lower than those summarized under the acute studies.
The acute toxicity of xylene to birds is low.
10.2.3 Risk evaluation
Xylenes are rapidly degraded in the environment. However, the
photooxidation reactions of the xylene isomers in the atmosphere may
contribute to photochemical smog.
High levels of xylenes have been reported in groundwater
associated with localized pollution from underground tanks and pipes,
but the environmental significance of such values is difficult to
assess.
For the aquatic environment, the most sensitive toxicity test,
based on measured concentrations, yielded an LC50 for Daphnia
magna of 1 mg/litre ( o-xylene). This value is more than 10 000
times higher than mean background concentrations in surface water,
which are generally less than 0.1 µg/litre for each isomer. The
lowest LC50 is still over 30 times higher than the highest single
measured concentration of total xylenes in the most polluted area.
The exposure/toxicity ratio will be much higher for mean
concentrations in polluted areas.
On the basis of rapid volatilization and degradation of xylenes
and their low to moderate toxicity, the overall risk to the aquatic
environment can therefore be considered low. It should be noted,
however, that very much higher levels have been measured around
discharges from oil production sites, and higher levels are also
possible if spillage occurs.
The likely route of exposure for birds in the environment is via
food such as fish. The only acute toxicity test on birds was carried
out on 14-day old Japanese quail and gave a 5-day LC50 of > 5000
mg/kg diet. Based on food consumption and body weight, an LD50 for
the quail of > 1746 mg/kg body weight can be calculated. Using this
data an estimated LC50 for a fish-eating bird (kingfisher) based on
body weight and food consumption can be calculated.
LC50 (mg/kg dry weight diet) =
Test species LD50 (mg/kg) × body wt (kg)
food consumption (kg)
The estimated LC50 for the fish-eating bird is > 7990 mg/kg
diet. The highest water concentration (30 µg/litre) multiplied by
the highest theoretical bioconcentration factor (158) gives a worst
case residue level in fish of 4.7 mg/kg. It should be noted that the
bioconcentration factor does not take into account degradation or
metabolism. Comparing this value to the estimated LC50 value gives a
Toxicity Exposure Ratio (TER) of > 1700. Therefore, the risk to
fish-eating birds is very low.
11. CONCLUSIONS
Humans are exposed to xylene mainly by inhalation. This compound
does not accumulate significantly in the human body. Acute exposure
to high concentrations can result in CNS effects in human. There have
been no long-term controlled studies or epidemiological studies with
exposure to xylene alone. The chronic toxicity appears to be
relatively low in laboratory animals. There is suggestive evidence,
however, that chronic CNS effects may occur in animals at moderate
concentrations of xylene. Xylene appears not to be a mutagen or a
carcinogen. The critical end-point is developmental toxicity. Based
on this end-point, the recommended guidance value for xylene in air
for the general population is 0.87 mg/m3 (0.2 ppm). This value is
higher than the concentrations to which the general population is
exposed.
The xylene isomers are non-persistent chemicals, being readily
degraded in the atmosphere, soil and water. It should be noted that
o-xylene appears to biodegrade only in the presence of other carbon
sources and at a reduced rate compared to the other isomers. The
photooxidation reactions of the xylene isomers in the atmosphere may
contribute to photochemical smog.
It can be concluded that xylenes are unlikely to cause problems
in aquatic ecosystems except near to localized industrial discharges
and spillage incidents. The risk to birds from xylene exposure is
low.
12. FUTURE RESEARCH
There is little information on the long-term effects of xylene in
humans and, specifically, no dose-response or dose-effect data are
available. Epidemiological studies of populations occupationally
exposed to xylene should be encouraged. In this context the use of
xylene metabolites in urine as a marker of exposure can be of special
value because the method determines the internal doses that
individuals receive via all routes of exposure. Because xylene has
acute effects on CNS, epidemiological studies should address the CNS
as a potential target organ. Moreover, since ethylbenzene is almost
invariably one of the components in solvent mixtures at the workplace,
study designs that address possible interactions between xylene and
other solvent components are desirable.
Animal studies are needed to address biochemical, functional and
morphological evidence of chronic neurotoxicity and potential effects
on fertility. In addition, there is a need for further studies on
developmental toxicity to assess the dose-response relationship and to
estimate the NOAEL, especially for developmental neurotoxicity.
Further studies are needed to determine the effect on hearing
impairment in order to determine the NOAEL. The relationship between
the dose level and the length of the exposure period should also be
investigated. The effect of exposure to xylene together with noise
should be studied.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC) has
evaluated the carcinogenicity of xylene. There was inadequate
evidence for the carcinogenicity of xylene in humans as well as in
experimental animals. The overall evaluation was that xylene is not
classifiable as to its carcinogenicity in humans (IARC, 1989).
The European Commission (1991-1992) recommended an 8-h
time-weighted average occupational exposure limit for xylene of 217
mg/m3 (50 ppm). To limit peaks of exposure that could result in
irritation, a short-term exposure level (STEL) of 435 mg/m3 (100 ppm)
was recommended.
REFERENCES
Abu Al Ragheb S, Salhab AS, & Amr SS (1986) Suicide by xylene
ingestion: A case report and review of literature. Am J Forensic Med
Pathol, 7: 327-329.
Altshuller AP, Lonneman WA, Sutterfield FD, & Kopczyncki SL (1971)
Hydrocarbon composition of the atmosphere of the Los Angeles basin,
1967. Environ Sci Technol, 5: 39.
Alvarez PJJ & Vogel TM (1991) Substrate interactions of benzene,
toluene, and para-xylene during microbial degradation by pure cultures
and mixed culture aquifer slurries. Appl Environ Microbiol,
57: 2981-2985.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3: 272-290.
Andersson K, Fuxe K, Nilsen OG, Toftgård R, Eneroth P, & Gustafsson
J-Å (1981) Production of discrete changes in dopamine and
noradrenaline levels and turnover in various parts of the rat brain
following exposure to xylene, ortho-, and para-xylene, and
ethylbenzene. Toxicol Appl Pharmacol, 60: 535-548.
Angerer J (1988a) Determination of toluene in blood by head-space gas
chromatography.
Angerer J (1988b) Determination of hippuric acid in urine by
high-pressure liquid chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 301-306 (IARC
Scientific Publications No. 85).
Angerer J & Lehnert G (1979) Occupational chronic exposure to organic
solvents. VIII. Phenolic compounds - metabolites of alkylbenzenes in
man: Simultaneous exposure to ethylbenzene and xylenes. Int Arch Occup
Environ Health, 43: 145-150.
Angerer J & Wulf H (1985) Occupational exposure to organic solvents.
XI. Alkylbenzene exposure of varnish workers: Effects on hematopoetic
system. Int Arch Occup Environ Health, 56: 307-321.
Anshelm Olson B, Gamberale F, & Iregren A (1985) Coexposure to toluene
and p-xylene in man: central nervous functions. Br J Ind Med,
42: 117-122.
Antoine SR, Deleon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36: 364-371.
Arlien-Soborg P, Zilstorff K, Grandjean B, & Milling Pedersen L (1981)
Vestibular dysfunction in occupational chronic solvent intoxication.
Clin Otolaryngol, 6: 285-290.
Aschan G, Bunnfors I, Hydén D, Larsby B, Ödkvist LM, & Tham R (1977)
Xylene exposure. Electronystagmographic and gas chromatographic
studies in rabbits. Acta Otolaryngol, 84: 370-376.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, & Wooten JV (1994) Blood
concentrations of volatile organic compounds in a nonoccupationally
exposed US population and in groups with suspected exposure. Clin
Chem, 40(7): 1401-1404.
Askergren A (1981) Studies on kidney function in subjects exposed to
organic solvents. III. Excretion of cells in the urine. Acta Med
Scand, 210: 103-106.
Askergren A, Allgén L-G, & Bergström J (1981a) Studies on kidney
function in subjects exposed to organic solvents. II. The effect of
desmopressin in a concentration test and the effect of exposure to
organic solvents on renal concentrating ability. Acta Med Scand,
209: 485-488.
Askergren A, Allgén L-G, Karlsson C, Lundberg I, & Nyberg E (1981b)
Studies on kidney function in subjects exposed to organic solvents. I.
Excretion of albumin and ß-2-microglobulin in the urine. Acta Med
Scand, 209: 479-483.
Askergren A, Brandt R, Gullquist R, Silk B, & Strandell T (1981c)
Studies on kidney function in subjects exposed to organic solvents.
IV. Effect on 51Cr-EDTA clearance. Acta Med Scand, 210: 373-376.
Åstrand I, Engström J, & Övrum P (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man. Scand J
Work Environ Health, 4: 185-194.
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions
of the hydroxyl radical with organic compounds under atmospheric
conditions. Chem Rev, 85: 69-201.
Axelsson G, Lütz C, & Rylander R (1984) Exposure to solvents and
outcome of pregnancy in university laboratory employees. Br J Ind Med,
41: 305-312.
Bakinson MA & Jones RD (1985) Gassings due to methylene chloride,
xylene, toluene, and styrene reported to Her Majesty's Factory
Inspectorate 1961-80. Br J Ind Med, 42: 184-190.
Bakke OM & Scheline RR (1970) Hydroxylation of aromatic hydrocarbons
in the rat. Toxicol Appl Pharmacol, 16: 691-700.
Barker JF, Barbash JE, & Labonte M (1988) Groundwater contamination at
a landfill sited on fractured carbonate and shale. J Contam Hydrol,
3: 1-25.
Batchelor JJ (1927) The relative toxicity of benzol and its higher
homologues. Am J Hyg, 7: 276-298.
Beirne GJ & Brennan JT (1972) Glomerulonephritis associated with
hydrocarbon solvents. Mediated by antiglomerular basement membrane
antibody. Arch Environ Health, 25: 365-369.
Bell GM, Gordon ACH, Lee P, Doig A, MacDonald MK, Thomson D, Anderton
JL, & Robson JS (1985) Proliferative glumerulonephritis and exposure
to organic solvents. Nephron, 40: 161-165.
Bell GM, Shillaker RO, Padgham MDJ, & Standring P (1992) Xylenes.
London, Health and Safety Executive, 166 pp (Toxicity Review 26).
Benville PE & Korn S (1977) The acute toxicity of six monocyclic
aromatic crude oil components to striped bass (Morone saxatilis) and
bay shrimp (Crago franciscorum). Calif Fish Gam, 63: 204-209.
Bergman K (1979) Whole-body autoradiography and allied tracer
techniques in distribution and elimination studies of some organic
solvents. Scand J Work Environ Health, 5(suppl 1): 1-263.
Bergman K (1983) Application and results of whole-body autoradiography
in distribution studies of organic solvents. CRC Crit Rev Toxicol,
12: 59-118.
Berry WO & Brammer JD (1977) Toxicity of water-soluble gasoline
fractions to fourth-instar larvae of the mosquito Aedes aegypti L.
Environ Pollut, 13: 229-234.
Bevan MAJ, Proctor CJ, Baker-Rogers J, & Warren ND (1991) Exposure to
carbon monoxide, respirable suspended particulates and volatile
organic compounds while commuting by bicycle. Environ Sci Techol,
25: 788-791.
Bieniek G, Palys E, & Wilczok T (1982) TLC separation of hippuric,
mandelic, and phenylglyoxylic acids from urine after mixed exposure to
toluene and styrene. Br J Ind Med, 39: 187-190.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, University of
Kentucky, Water Resources Research Institute, 61 pp (Research Report
No. 133).
Blanchard KT & Morris JB (1994) Effects of m-xylene on rat nasal
cytochrome P450 mixed function oxidase activities. Toxicol Lett,
70: 253-259.
Blum DJW & Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. J Water Pollut Control Fed, 63: 198-207.
Bobra AM, Shiu WY, & Mackay D (1983) A predictive correlation for the
acute toxicity of hydrocarbons and chlorinated hydrocarbons to the
water flea (Daphnia magna). Chemosphere, 12: 1121-1129.
Bonnet P, Raoult G, & Gradiski D (1979) Concentrations léthales50 des
principaux hydrocarbures aromatiques. Arch Mal Prof, 40: 805-810.
Bonnet P, Morele Y, Raoult G, Zissu D, & Gradiski D (1982)
Détermination de la concentration léthale50 des principaux
hydrocarbures aromatiques chez le rat. Arch Mal Prof, 43: 261-265.
Bos RP, Brouns RME, van Doorn R, Theuws JLG, & Henderson PT (1981)
Non-mutagenicity of toluene, o-, m- and p-xylene, o-methyl-
benzylalcohol and o-methylbenzylsulfate in the Ames assay.
Mutat Res, 88: 273-279.
Boström E-C, Almén J, Steen B, & Westerholm R (1994) Human exposure to
urban air pollution. Environ Health Perspect, 102(suppl 4): 39-47.
Botta D, Pirri LC, & Mantica E (1984) Ground water pollution by
organic solvents and their microbial degradation products. In:
Analytical Organic Micropollutants in Water. Luxembourg, Commission of
the European Communities, pp 261-275 (Report EUR 8518).
Bowers DE Jr, Cannon MS, & Jones DH (1982) Ultrastructural changes in
livers of young and aging rats exposed to methylated benzenes. Am J
Vet Res, 43: 676-683.
Bray HG, Humphris BG, & Thorpe WV (1949) Metabolism of derivatives of
toluene. 3. o-, m- and p-xylenes. Biochem J, 45: 241-244.
Brice KA & Derwent RG (1978) Emission inventory for hydrocarbons in
the United Kingdom. Atmos Environ, 12: 2045-2054.
Bridie AL, Wolff CJM, & Winter M (1979) The acute toxicity of some
petrochemicals to goldfish. Water Res, 13: 623-626.
Bringmann G & Kühn R (1977) [Toxic threshold values for compounds
hazardous to the aquatic environment on bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda) using cell
multiplication test.] Z Wasser Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kühn R (1978) [Toxic threshold values for compounds
hazardous to the aquatic environment on blue algae (Microcystic
aeruginosa) and green algae (Scenedesmus quadricauda) using cell
multiplication test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1982) [Results of the toxic effects of compounds
hazardous to the aquatic environment on Daphnia magna using an
improved standardized test.] Z Wasser Abwasser Forsch, 15: 1-6 (in
German).
Bringmann G, Kühn R, & Winter A (1980) [Determination of biocidal
effects of compounds hazardous to the aquatic environment for
Protozoa: III. Saprozoic flagelates.] Z Wasser Abwasser Forsch,
13: 170-173 (in German).
Brodzinsky R & Singh HB (1983) Volatile organic chemicals in the
atmosphere: An assessment of available data. Washington, DC, US
Environmental Protection Agency (EPA/DF-83/005A).
Brooks JM, Fryxell GA, Reid DF, & Sackett WM (1977) Gulf underwater
flare experiment (GULFEX): Effects of hydrocarbons on phytoplankton
In: Pollutant effects on marine organisms. Proceedings of a Workshop,
College station, Texas, USA, 16-19 May 1976, pp 45-75.
Brown RH (1988a) Determination of benzene, toluene and xylene in
industrial air by charcoal tube, solvent desorption and gas
chromatography. In: Fishbein L & O'Neill IK ed. Environmental
carcinogens: Methods of analysis and exposure measurement. Volume
10: Benzene and alkylated benzenes. Lyon, International Agency for
Research on Cancer, pp 225-233 (IARC Scientific Publications No. 85).
Brown RH (1988b) Determination of benzene, toluene and xylene in
industrial air by porous polymer adsorption tube, thermal desorption
and gas chromatography. In: Fishbein L & O'Neill IK ed. Environmental
carcinogens: Methods of analysis and exposure measurement. Volume
10: Benzene and alkylated benzenes. Lyon, International Agency for
Research on Cancer, pp 235-242 (IARC Scientific Publications No. 85).
Brown RH (1988c) Determination of gasoline hydrocarbons in industrial
air by two-stage (chromosorb/charcoal) adsorption, thermal desorption
and capillary gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 243-251 (IARC
Scientific Publications No. 85).
Brown-Woodman PDC, Webster WS, Picker K, & Ritchie HE (1991)
Embryotoxicity of xylene and toluene: an in study. Ind Health,
29: 139-152.
Brown-Woodman PDC, Webster WS, Picker K, & Huq F (1994) In
assessment of individual and interactive effects of aromatic
hydrocarbons on embryonic development of the rat. Reprod Toxicol,
8: 121-135.
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other trace
organic compounds in urban air. Chemosphere, 17: 2363-2380.
Bushnell PJ (1989) Behavioral effects of acute p-xylene inhalation
in rats: Autoshaping, motor activity, and reversal learning.
Neurotoxicol Teratol, 10: 569-577.
Bushnell PJ & Peele DB (1988) Conditioned flavor aversion induced by
inhaled p-xylene in rats. Neurotoxicol Teratol, 10: 273-277.
Buszka PM, Zaugg SD, & Werner MG (1990) Determination of trace
concentrations of volatile organic compounds in ground water using
closed-loop stripping, Edwards Aquifer, Texas. Bull Environ Contam
Toxicol, 45: 507-515.
Buttery RG, Seifert RM, & Ling LC (1975) Characterization of some
volatile constituents of dry red beans. J Agric Food Chem,
23: 516-519.
Caldwell RS, Caldarone EM, & Mallon MH (1977) Effects of a
seawater-soluble fraction of Cook Inlet crude oil and its major
aromatic components on larval stages of the Dungeness crab, Cancer
magister Dana. In: Wolfe DA ed. Fate and effects of petroleum
hydrocarbons in marine ecosystems and organisms. Oxford, New York,
Pergamon Press, pp 210-220.
Cameron GR, Paterson JLH, de Saram GSW, & Thomas JC (1938) The
toxicity of some methyl derivatives of benzene with special reference
to pseudocumene and heavy coal tar naphta. J Pathol Bacteriol,
46: 95-107.
Campbell L, Wilson HK, Samuel AM, & Gompertz D (1988) Interactions of
m-xylene and aspirin metabolism in man. Br J Ind Med, 45: 127-132.
Carlsson A (1981) Distribution and elimination of 14C-xylene in rat.
Scand J Work Environ Health, 7: 51-55.
Carpenter CP, Kinkead ER, Geary DL Jr, Sullivan LJ, & King JM (1975)
Petroleum hydrocarbon toxicity studies. V. Animal and human response
to vapors of mixed xylenes. Toxicol Appl Pharmacol, 33: 543-558.
Chan C-C, Özkaynak H, Spengler JD, & Sheldon L (1991a) Driver exposure
to volatile organic compounds, CO, ozone and NO2 under different
driving conditions. Environ Sci Techol, 25: 964-972.
Chan C-C, Spengler JD, Ozkaynak H, & Lefkopoulou M (1991b) Commuter
exposures to VOCs in Boston, Massachusetts. J Air Waste Manage Assoc,
41: 1594-1600.
Chan C-C, Lin S-H, & Her GR (1993) Student's exposure to volatile
organic compounds while commuting by motorcycle and bus in Taipei
City. J Air Waste Manage Assoc, 43(9): 1231-1238.
Chen Z, Liu S-J, Cai S-X, Yao Y-M, Yin H, Ukai H, Uchida Y, Nakatsuka
H, Watanabe T, & Ikeda M (1994) Exposure of workers to a mixture of
toluene and xylenes. II. Effects. Occup Environ Med, 51: 47-49.
Chiang CY, Salanito JP, Chai EY, Colthart JD, & Klein CL (1989)
Aerobic biodegradation of benzene, toluene and xylene in a sandy
aquifer - Data analysis and computer modelling. Ground Water,
27: 823-834.
Chung TY, Hayase F, & Kato H (1983) Volatile components of ripe
tomatoes and their juices, purees and pastes. Agric Biol Chem,
47: 343-351.
City of Toronto (1990) The quality of drinking water in Toronto: A
review of tap water, bottled water and water treated by a point-of-use
device. Toronto, Canada, Department of Public Health.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural and motorway locations.
Environ Pollut, B7: 141-158.
Condie LW, Hill JR, & Borzelleca JF (1988) Oral toxicology studies
with xylene isomers and mixed xylenes. Drug Chem Toxicol, 11: 329-354.
Connor TH, Theiss JC, Hanna HA, Monteith DK, & Matney TS (1985)
Genotoxicity of organic chemicals frequently found in the air of
mobile homes. Toxicol Lett, 25: 33-40.
Cragg ST, Clarke EA, Daly IW, Miller RR, Terrill JB, & Ouellette RE
(1989) Subchronic inhalation toxicity of ethylbenzene in mice, rats,
and rabbits. Fundam Appl Toxicol, 13: 399-408.
Cramer PH, Boggess KE, Hosenfeld JM, Remmers JC, Breen JJ, Robinson
PE, & Stroup C (1988) Determination of organic chemicals in human
whole blood: Preliminary method development for volatile organics.
Bull Environ Contam Toxicol, 40: 612-681.
Craveri A, Tornaghi G, Ciaci D, Vitali L, Carucci R, De Luca P, &
Donato MF (1982) [Serum bile salts in the hepatological screening of
subjects exposed to aromatic hydrocarbons.] Giorn Clin Med,
63: 322-329 (in Italian).
Crawford L & Kretsch MJ (1976) GC-MS identification of the volatile
compounds extracted from roasted turkeys fed a basal diet supplemented
with tuna oil: Some comments on fishy flavour. J Food Sci,
41: 1470-1478.
Crofton KM, Lassiter TL, & Rebert CS (1994) Solvent-induced
ototoxicity in rats: an atypical selective mid-frequency hearing
deficit. Hear Res, 80: 25-30.
Daisey JM, Hodgson AT, Fisk WJ, Mendell MJ, & Ten Brinke J (1994)
Volatile organic compounds in twelve California office buildings:
classes, concentrations and sources. Atmos Environ, 28(22): 3557-3562.
Davey JF & Gibson DT (1974) Bacterial metabolism of para- and
meta-xylene: Oxidation of a methyl substituent. J Bacteriol,
119: 923-929.
Davis RS, Hossler FE, & Stone RW (1967) Oxidation of m- and p-
xylenes by Pseudomonas. Bateriol Proc, P162: 129.
Day BJ & Carlson GP (1992) Effect of p-xylene metabolites,
p-methylbenzyl alcohol and 2,5-dimethylphenol on rat hepatic and
pulmonary microsomal metabolism. Res Commun Chem Pathol Pharmacol,
76: 117-120.
Day BJ, DeNicola DB, Marcus CB, & Carlson GP (1992) Effect of
p-xylene inhalation on the bioactivation of bromobenzene in rat lung
and liver. Fundam Appl Toxicol, 19: 50-56.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
De Ceaurriz J, Desiles JP, Bonnet P, Marignac B, Muller J, & Guenier
JP (1983) Concentration-dependent behavioral changes in mice following
short-term inhalation exposure to various industrial solvents. Toxicol
Appl Pharmacol, 67: 383-389.
DECOS (1991) Health-based recommended occupational exposure limit for
xylene. The Hague, Directorate-General of Labour.
De Gandarias JM, Echevarria E, Irazusta J, Gil J, & Casis L (1993)
Brain aminopeptidase activity after subacute xylene exposure.
Neurotoxicol Teratol, 15: 51-53.
del Rosario R, de Lumen BO, Habu T, Flath RA, Mon TR, & Teranishi R
(1984) Comparison of headspace volatiles from winged beans and
soybeans. J Agric Food Chem, 32: 1011-1015.
Derwent RG & Jenkin ME (1990) Hydrocarbon involvement in photochemical
ozone formation in Europe. Oxfordshire, Harwell Laboratory, AEA
Environment and Energy, Modelling and Assessments Group (Report AERE
R13736).
Dési I, Kovacs F, Zahumenszky Z, & Balogh A (1967) Maze learning in
rats exposed to xylene intoxication. Psychopharmacologia (Berl),
11: 224-230.
Divincenzo GD & Krasavage WJ (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic solvents.
Am Ind Hyg Assoc J, 35: 21-29.
Donner M, Mäki-Paakkanen J, Norppa H, Sorsa M, & Vainio H (1980)
Genetic toxicology of xylenes. Mutat Res, 74: 171-172.
Dössing M, Arlien Söborg P, Milling Pedersen L, & Ranek L (1981)
[Liver damage following long-term occupational exposure to organic
solvents.] Ugeskr Laeg, 143: 2297-2301 (in Danish with English
summary).
Dössing M, Arlien Söborg P, Milling Pedersen L, & Ranek L (1983) Liver
damage associated with occupational exposure to organic solvents in
house painters. Eur J Clin Invest, 13: 151-157.
Dowden BF & Bennett HJ (1965) Toxicity of selected chemicals to
certain animals. J Water Pollut Control Fed, 37: 1308-1316.
Dowty BJ & Laseter JL (1976) The transplacental migration and
accummulation in blood of volatile organic constituents. Ped Res,
10: 696-701.
Dowty BJ, Carlisle DR, & Laseter JL (1975) New Orleans drinking water
sources tested by gas chromatography-mass spectrometry. Environ Sci
Techol, 9: 762-765.
Drew RT & Fouts JR (1974) The effects of inducers on acute p-xylene
toxicity. Toxicol Appl Pharmacol, 29: 111-112.
Drozd J & Novak J (1978) Quantitative and qualitative head-space gas
analysis of parts per billion amounts of hydrocarbons in water: A
study of model systems by capillary-column gas chromatography with
splitless sample injection. J Chromatogr, 158: 471-482.
Dudek B, Gralewicz K, Jakubowski M, Kostrzewski P, & Sokal J (1990)
Neurobehavioral effects of experimental exposure to toluene, xylene
and their mixture. Pol J Occup Med, 3: 109-116.
Dunstan WM, Atkinson LP, & Natoli J (1975) Stimulation and inhibition
of phytoplankton growth by low molecular weight hydrocarbons. Mar
Biol, 31: 305-310.
Dutkiewicz T & Tyras H (1968) Skin absorption of toluene, styrene, and
xylene by man. Br J Ind Med, 25: 243.
Dyer RS, Bercegeay MS, & Mayo LM (1988) Acute exposures to p-xylene
and toluene alter visual information processing. Neurotoxicol Teratol,
10: 147-153.
EAJ (Environment Agency, Japan) (1993) Chemicals in the environment.
Tokyo, Ministry of Health and Welfare, International Affairs Division.
ECETOC (1986) Joint assessment of commodity chemicals No. 6: Xylenes.
Brussels, European Chemical Industry Ecology and Toxicology Centre.
Edling C (1982) Interaction between drugs and solvents as a cause of
fatty change in the liver? Br J Ind Med, 39: 198-199.
Edwards EA & Grbic-Galic D (1994) Anaerobic degradation of toluene and
o-xylene by a methanogenic consortium. Appl Environ Microbiol,
60(1): 313-322.
Edwards EA, Willis LE, Reinhard M, & Grbic-Galic D (1992) Anaerobic
degradation of toluene and xylene by aquifer microorganisms under
sulfate-reducing conditions. Appl Environ Microbiol, 58: 794-800.
Eller PM (1984) NIOSH manual of analytical methods: Method 1501, 3rd
ed. Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 2.
Elofsson S-A, Gamberale F, Hindmarsh T, Iregren A, Isaksson A,
Johnsson I, Knave B, Lydahl E, Mindus P, Persson HE, Philipson B,
Steby M, Struwe G, Söderman E, Wennberg A, & Widén L (1980) Exposure
to organic solvents: A cross-sectional epidemiologic investigation on
occupationally exposed car and industrial spray painters with special
reference to the nervous system. Scand J Work Environ Health,
6: 239-273.
Elovaara E (1982) Dose-related effects of m-xylene inhalation on the
xenobiotic metabolism of the rat. Xenobiotica, 12: 345-352.
Elovaara E, Collan Y, Pfäffli P, & Vainio H (1980) The combined
toxicity of technical grade xylene and ethanol in the rat.
Xenobiotica, 10: 435-445.
Elovaara E, Pfäffli P, Savolainen H, & Vainio H (1982) Marginal role
of impaired aldehyde metabolism in m-xylene vapour-induced
biochemical effects in the rat. J Appl Toxicol, 2: 27-32.
Elovaara E, Engström K, & Vainio H (1984) Metabolism and disposition
of simultaneously inhaled m-xylene and ethylbenzene in the rat.
Toxicol Appl Pharmacol, 75: 466-478.
Elovaara E, Zitting A, Nickels J, & Aitio A (1987) m-Xylene
inhalation destroys cytochrome P-450 in rat lung at low exposure. Arch
Toxicol, 61: 21-26.
Engelhardt WE & Estler W (1935) [Experiments on the acute narcotic
effects of aliphatic and aromatic hydrocarbons: The effect of single
inhalation of gasoline, benzene, toluene, xylene in the rabbit and the
cat.] Ark Hyg, 114: 249-260 (in German).
Engström J & Bjurström R (1978) Exposure to xylene and ethylbenzene.
II. Concentration in subcutaneous adipose tissue. Scand J Work Environ
Health, 4: 195-203.
Engström J & Riihimäki V (1979) Distribution of m-xylene to
subcutaneous adipose tissue in short-term experimental human exposure.
Scand J Work Environ Health, 5: 126-134.
Engström K & Riihimäki V (1988a) Determination of xylenes in blood by
head-space gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 307-311 (IARC
Scientific Publications No. 85).
Engström K & Riihimäki V (1988b) Determination of methylhippuric acids
in urine by gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 313-318 (IARC
Scientific Publications No. 85).
Engström K, Husman K, & Riihimäki V (1977) Percutaneous absorption of
m-xylene in man. Int Arch Occup Environ Health, 39: 181-189.
Engström K, Husman K, Pfäffli P, & Riihimäki V (1978) Evaluation of
occupational exposure to xylene by blood, exhaled air and urine
analysis. Scand J Work Environ Health, 4: 114-121.
Engström K, Riihimäki V, & Laine A (1984) Urinary disposition of
ethylbenzene and m-xylene in man following separate and combined
exposure. Int Arch Occup Environ Health, 54: 355-363.
European Commission (1991-1992) Occupational exposure limits.
Recommendations of the Scientific Expert Group 1991-1992. Luxembourg,
European Commission, pp 53-55 (Report EUR 15091 EN).
Fabre R, Truhaut R, & Laham S (1960) Recherches toxicologiques sur les
solvants de remplacement du benzčne. IV. Etude des xylčnes. Arch Mal
Prof, 21: 301-313.
Fairfax R (1995) Solvent, wood dust, and noise exposure in a decoy
shop. Appl Occup Environ Hyg, 10(5): 446-448.
Falk-Petersen I-B, Kjrsvic E, Lonning S, Naley AM, & Sydnes LK (1985)
Toxic effects of hydroxylated aromatic hydrocarbons on marine embryos.
Sarsia, 70: 11-16.
Fellin P & Otson R (1993) Seasonal trends of volatile organic
compounds (VOCs) in Canadian homes. In: Indoor air '93. Proceedings of
the 6th International Conference on Indoor Air Quality and Climate,
Helsinki, Finland, 4-8 July 1993 - Volume 2: Chemicals in indoor air,
materials emissions, pp 117-122.
Ferrario JB, Lawler GC, Deleon IR, & Laseter JL (1985) Volatile
organic pollutants in biota and sediments of Lake Pontchartrain. Bull
Environ Contam Toxicol, 34: 246-255.
Fielding M, Gibson TM, James HA, McLoughlin K, & Steel CP (1981)
Organic micropollutants in drinking water. Medmenham, Marlow, United
Kingdom, Water Research Centre (Technical Report TR-159).
Fishbein L (1988) Xylenes: Uses, occurrence and exposure. In: Fishbein
L & O'Neill IK ed. Environmental carcinogens: Methods of analysis and
exposure measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 109-120 (IARC
Scientific Publications No. 85).
Fischbein A, Ross RR, & Lerman Y (1983) Industrial solvents and the
liver. Lancet, 1: 129.
Fishbein L, O'Neill IK, & Tossavainen A (1988) Past and present
exposure determination techniques for benzene, toluene and xylene. In:
Fishbein L & O'Neill IK ed. Environmental carcinogens: Methods of
analysis and exposure measurement. Volume 10: Benzene and alkylated
benzenes. Lyon, International Agency for Research on Cancer,
pp 123-148 (IARC Scientific Publications No. 85).
Folmar LC (1976) Overt avoidance reaction of rainbow trout fry to nine
herbicides. Bull Environ Contam Toxicol, 15: 509-514.
Foster P, Laffond M, Baussand P, Jacob V, & Denis I (1991) VOC
measurements in the Grenoble area and study of the benzaldehyde
behaviour in simulation chamber. Pollut Atmos, 1991: 175-191.
Franchini I, Cavatorta A, Falzoi M, Lucertini S, & Mutti A (1983)
Early indicators of renal damage in workers exposed to organic
solvents. Int Arch Occup Environ Health, 52: 1-9.
Frank PA, Otto NE, & Bartley TR (1961) Techniques for evaluating
aquatic weed herbicides. Weeds, 9: 515-521.
Frantik E, Hornychova M, & Vodickova L (1993) Equipotential
subnarcotic concentrations of solvents in air, in blood, and at the
target structure. Homeostasis, 34: 143-147.
Frantik E, Hornychova M, & Horvath M (1994) Relative acute
neurotoxicity of solvents: Isoeffective air concentrations of 48
compounds evaluated in rats and mice. Environ Res, 66: 173-185.
Frantik E & Vodickova L (1995) Combined effects of binary solvent
mixtures. Cent Eur J Occup Environ Med, 1: 31-37.
Funes-Cravioto F, Kolmodin-Hedman B, Lindsten J, Nordenskjöld M,
Zapata-Gayon C, Lambert B, Norberg E, Olin R, & Swensson Å (1977)
Chromosome aberrations and sister-chromatid exchange in workers in
chemical laboratories and a rotoprinting factory and in children of
women laboratory workers. Lancet, 2: 322-325.
Furnas DW & Hine CH (1958) Neurotoxicity of some selected hydro-
carbons. Arch Ind Health Occup Med, 18: 9-15.
Fustinoni S, Buratti M, Giampiccolo R, & Colombi A (1995) Biological
and environmental monitoring of exposure to airborne benzene and other
aromatic hydrocarbons in Milan traffic wardens. Toxicol Lett,
77(13): 387-392.
Gab S, Schmizer J, Tham H, Parlar H, & Korte F (1977) Photo-
mineralisation rate of organic compounds adsorbed on particulate
matter. Nature (Lond), 270: 331-333
Galassi S, Mingazzini M, Vigano L, Cesareo D, & Tosato ML (1988)
Approaches to modelling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicol Environ Saf, 16: 158-169.
Gamberale F, Annwall G, & Hultengren M (1978) Exposure to xylene and
ethylbenzene. III. Effects on central nervous functions. Scand J Work
Environ Health, 4: 204-211.
Gerner-Smidt P & Friedrich U (1978) The mutagenic effect of benzene,
toluene and xylene studied by the SCE technique. Mutat Res,
58: 313-316.
Gery MW, Fox DL, Kaems RM, & Stockburger L (1987) Investigation of
hydroxyl radical reactions with o-xylene and m-xylene in a
continuous stirred tank reactor. Environ Sci Techol, 21: 339-348.
Ghantous H & Danielsson BG (1986) Placental transfer and distribution
of toluene, xylene and benzene, and their metabolites during gestation
in mice. Biol Res Pregnancy, 7: 98-105.
Ghantous H, Dencker L, Gabrielsson J, Danielsson BRG, & Bergman K
(1990) Accumulation and turnover of metabolites of toluene and xylene
in nasal mucosa and olfactory bulb in the mouse. Pharmacol Toxicol,
66: 87-92.
Ghislandi E & Fabiani A (1957) [Liver damage due to accidental
ingestion of nitrocellulose paints.] Med Lav, 48: 577-579 (in Italian
with English summary).
Ghosh TK & Pradhan SN (1987) Comparison of effects of xylene and
toluene inhalation on fixed-ratio liquid-reinforced behavior in rats.
Res Commun Psychol Psychiatr Behav, 12: 305-312.
Ghosh TK, Copeland RL Jr, Parui RN, Mookherjee S, & Pradhan SN (1987)
Effect of xylene inhalation on fixed-ratio responding in rats.
Pharmacol Biochem Behav, 27: 653-657.
Gilli G, Scursatone E, & Bono R (1994) Benzene, toluene and xylenes in
air, geographical distribution in Piedmont region (Italy) and personal
exposure. Sci Total Environ, 148(1): 49-56.
Glass WI (1961) Annotation: A case of suspected xylol poisoning. N Z
Med J, 60: 113.
Gomez-Belinchon JI, Grimalt JO, & Abaigés J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water Res, 25: 577-589.
Grob K & Grob G (1971) Gas-liquid chromatographic - mass spectrometric
investigation of C6-C20 organic compounds in an urban atmosphere: An
application of ultra trace analysis on capillary columns.
J Chromatogr, 62: 1-13.
Grosjean D (1990) Atmospheric chemistry of toxic contaminants. 1.
Reaction rates and atmospheric persistence. J Air Waste Manage Assoc,
40: 1397-1402.
Gschwend PM, Zafiriou OC, Mantoura RFC, Schwarzenbach RP, & Gagosian
RB (1982) Volatile organic compounds at a coastal site. I. Seasonal
variations. Environ Sci Techol, 1: 31-38.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gut I & Flek J (1981) [Effect of microsomal enzyme induction by
phenobarbital on the metabolism of benzene, fluorobenzene, m-xylene
and p-xylene.] Prakov Lek, 33: 124-127 (in Czech with English
summary).
Gut I, Terelius Y, Frantik E, Linhart I, Soucek P, Filipcova B, &
Kluchova H (1993) Exposure to various benzene derivatives differently
induces cytochromes P450 2B1 and P450 2E1 in rat liver. Arch Toxicol,
67: 237-243.
Haglund U, Lundberg I, & Zech L (1980) Chromosome aberrations and
sister chromatid exchanges in Swedish paint industry workers. Scand J
Work Environ Health, 6: 291-298.
Halder CA, Holdsworth CE, Cockrell BY, & Piccirillo VJ (1985)
Hydrocarbon nephropathy in male rats: Identification of the
nephrotoxic components of unleaded gasoline. Toxicol Ind Health,
1: 67-87.
Harland BJ, Whitby FJ, & Comber MHI (1985) Measurement of volatile
aromatic hydrocarbons in an industrial estuary. Int J Environ Anal
Chem, 20: 295-312.
Harper C (1975) p-Xylene metabolism by rat pulmonary and hepatic
microsomes. Fed Proc, 34: 785.
Hass U & Jakobsen BM (1993) Prenatal toxicity of xylene inhalation in
the rat: A teratogenicity and postnatal study. Pharmacol Toxicol,
73: 20-23.
Hass U, Lund SP, Simonsen L, & Schaich Fries A (1995) Effects of
prenatal exposure to xylene on postnatal development and behavior in
rats. Neurotoxicol Teratol, 17: 341-349.
Hastings L, Cooper GP, & Burg W (1984) Human sensory response to
selected petroleum hydrocarbons. Adv Med Environ Toxicol, 6: 255-270.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 5(suppl 1): 3-142.
Hawthorne SB & Sievers RE (1984) Emission of organic air pollutants
from shale oil waste waters. Environ Sci Techol, 18: 483-490.
Herman DC, Inniss WE, & Mayfield CI (1990) Impact of volatile aromatic
hydrocarbons on growth of the freshwater alga Selenastrum
capricornutum. Aquat Toxicol, 18: 87-100.
Higgins CE, Griest WH, & Olerich G (1983) Application of tenax
trapping to analysis of gas phase organic compounds in ultra-low tar
cigarette smoke. J Assoc Off Anal Chem, 66(5): 1074-1083.
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington, DC,
US Department of the Interior, Fish and Wildlife Service, 138 pp (Fish
and Wildlife Technical Report No. 2).
Hine CH & Zuidema HH (1970) The toxicological properties of hydro-
carbon solvents. Ind Med, 38: 215-220.
Hipolito RN (1980) Xylene poisoning in laboratory workers: Case
reports and discussion. Lab Med, 11: 593-595.
Holcombe GW, Phipps GL, Sulaiman AH, & Hoffman AD (1987) Simultaneous
multiple species testing: Acute toxicity of 13 chemicals to 12 diverse
freshwater amphibian, fish, and invertebrate families. Arch Environ
Contam Toxicol, 16: 697-710.
Holmberg B, Jakobson I, & Malmfors T (1974) The effect of organic
solvents on erythrocytes during hypotonic hemolysis. Environ Res,
7: 193-205.
Honma T, Sudo A, Miyagawa M, Sato M, & Hasegawa H (1983) Significant
changes in the amounts of neurotransmitter and related substances in
rat brain induced by subacute exposure to low levels of toluene and
xylene. Ind Health, 21: 143-151.
Huang M-Y, Jin C, Liu Y-T, Li B-H, Qu QS, Ushida Y, Inoue O, Nakatsuka
M, Watanabe T, & Ikeda M (1994) Exposure of workers to a mixture of
toluene and xylenes. I. Metabolism. Occup Environ Med, 51: 42-46.
Hudak A & Ungvary G (1978) Embryotoxic effects of benzene and its
methyl derivatives: Toluene, xylene. Toxicology, 11: 55-63.
Huff JE, Eastin W, Roycroft J, Eustis SL, & Haseman JK (1988)
Carcinogenesis studies of benzene, methyl benzene, and dimethyl
benzenes. Ann NY Acad Sci, 534: 427-440.
Hung I-F & Liao M-H (1991) Aromatic hydrocarbons in indoor air. J
Environ Sci Health, A26: 487-492.
Hurford N, Law RJ, Fileman TW, Payne AP, & Colcomb-Heiliger K (1990)
Concentrations of chemicals in the North Sea due to operational
discharges from chemical tankers: Results from the second survey,
October 1988. Oil Chem Pollu, 7: 241-270.
Husman K (1980) Symptoms of car painters with long-term exposure to a
mixture of organic solvents. Scand J Work Environ Health, 6: 19-32.
Husman K & Karli P (1980) Clinical neurological findings among car
painters exposed to a mixture of organic solvents. Scand J Work
Environ Health, 6: 33-39.
Hutchins SR (1991) Biodegradation of monoaromatic hydrocarbons by
aquifer microrganisms using oxygen, nitrate or nitrous oxide as the
terminal electron acceptor. Appl Environ Microbiol, 57: 2403-2407.
Hutchins SR, Sewell GW, Kovacs DA, & Smith GA (1991a) Biodegradation
of aromatic hyrocarbons by aquifer microroganisms under denitrifying
conditions. Environ Sci Techol, 25: 68-76.
Hutchins SR, Downs WC, Wilson JT, Smith GB, Kovacs DA, Fine DD,
Douglass RH, & Hendrix DJ (1991b) Effect of nitrate addition on
biorestoration of fuel-contaminated aquifer: Field demonstration.
Ground Water, 29: 571-580.
Hutchinson TC, Hellebust JA, Tam D, Mascarenhas RA, & Shiu WY (1980)
The correlation of the toxicity to algae of hydrocarbons and
halogenated hydrocarbons with their physical-chemical properties.
Environ Sci Res, 16: 577-586.
IARC (1989) Xylene. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research on
Cancer, pp 125-156 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 47).
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: Assessing human health risks of chemicals:
Derivation of guidance values for health-based exposure limits. World
Health Organization, Geneva, 73 pp.
Ivanovich E, Antov G, Goranova L, Spasovski M, Bainova A, & Vasileva Z
(1985) Combined effect of some physical and chemical factors. J Hyg
Epidemiol Microbiol Immunol, 28: 105-110.
Jacobs G, Martens M, & Mosselmans G (1987) Proposal of limit
concentrations for skin irritation within the context of a new EEC
directive on the classification and labelling of preparations. Regul
Toxicol Pharmacol, 7: 370-378.
Jamison VW, Raymond RL, & Hudson JD (1976) Biodegradation of
high-octane gasoline. In: Proceedings of the 3rd International
Biodegradation Symposium. Allied Science Publishers, pp 187-196.
Jenkins LJ Jr, Jones RA, & Siegel J (1970) Long-term inhalation
screening studies of benzene, toluene, o-xylene, and cumene on
experimental animals. Toxicol Appl Pharmacol, 16: 818-823.
Jones BMR (1988) The measurement of ambient hydrocarbon concentrations
in the atmosphere at Harwell during the period April 1986 to March
1987. Oxfordshire, Harwell Laboratoty (AERE Report R13174).
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and vehicle emissions. Environ Int, 11: 383-392.
Jori A, Calamari D, Di Domenico A, Galli CL, Galli E, Marinovich M, &
Silano V (1986) Ecotoxicological profile of xylenes. Ecotoxicol
Environ Saf, 11: 44-80.
Joyner RE & Leak Pegues W (1961) A health hazard associated with epoxy
resin-concrete dust. J Occup Med, 3: 211-214.
Juhnke I & Lüdemann D (1978) [Results of acute fish toxicity of 200
chemical compounds using the golden orfe tests.] Z Wasser Abwasser
Forsch, 11: 161-164 (in German).
Jüttner F (1988a) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in the forest air of the Southern
Black Forest. Chemosphere, 17: 309-317.
Jüttner F (1988b) Motor-boat-derived volatile organic compounds (VOC)
in lakewater. Z Wasser Abwasser Forsch, 21: 36-39.
Kaneko T, Wang P-Y, Tsukada H, & Sato A (1995) m-Xylene
toxicokinetics in phenobarbital-treated rats: comparison among
inhalation exposure, oral administration, and intraperitoneal
administration. Toxicol Appl Pharmacol, 131: 13-20.
Kappeler Th & Wuhrmann K (1978a) Microbial degradation of the water
soluble fraction of gas oil. I. Water Res, 12: 327-333.
Kappeler Th & Wuhrmann K (1978b) Microbial degradation of the water
soluble fraction of gas oil. II. Bioassays with pure strains. Water
Res, 12: 335-342.
Karlson U & Frankenberger WT (1989) Microbial degradation of benzene
and toluene in groundwater. Bull Environ Contam Toxicol, 43: 505-510.
Kauss PB & Hutchinson TC (1975) The effects of water-soluble petroleum
components on the growth of Chlorella vulgaris Beijerinck. Environ
Pollut, 9: 157-174.
Kauss PB, Hutchinson TC, Soto C, Hellebust J, & Griffiths M (1973) The
toxicity of crude oil and its components to freshwater algae. In:
Prevention and control of spills. Washington, DC, American Petroleum
Institute, pp 703-714.
Kawai T, Mizunuma K, Yasugi T, Horiguchi S, Uchida Y, Iwami O, Iguchi
H, & Ikeda M (1991a) Urinary methylhippuric acid isomer levels after
occupational exposure to a xylene mixture. Int Arch Occup Environ
Health, 63: 69-75.
Kawai T, Yamaoka K, Uchida Y, & Ikeda M (1991b) Exposure to vapors of
benzene and other aromatic solvents in tank truck loading and
delivery. Bull Environ Contam Toxicol, 46(1): 1-8.
Kawamura K & Kaplan IR (1983) Organic compounds in the rain water of
Los Angeles. Environ Sci Technol, 17: 497-501.
Kawata K & Fujieda Y (1993) [Volatile aromatic hydrocarbons in ambient
air.] Jpn J Toxicol Environ Health, 39(1): 37-43 (in Japanese).
Kennah HE, Hignet S, Laux PE, Dorko JD, & Barrow CS (1989) An
objective procedure for quantitating eye irritation based upon changes
of corneal thickness. Fundam Appl Toxicol, 12: 258-268.
Kennicutt MC II, Brooks JM, & McDonald TJ (1984) Volatile organic
inputs from an ocean outfall near Barceloneta, Puerto Rico.
Chemosphere, 13: 535-548.
Kenrick MAP, Clark L, Baxter KM, Fleet M, James HA, Gibson TM, &
Turrell MB (1985) Trace organics in British aquifers: a baseline
survey. Medmenham, Marlow, United Kingdom, Water Research Centre
(Technical Report TR223).
Kilburn KH, Warshaw R, Boylen CT, Balmes J, Johnson S, Seidman B, &
DeFlorio G (1983) Toxic effects of formaldehyde and solvents in
histology technicians: A preliminary report. J Histotechnol, 6: 73-76.
Kilburn KH, Seidman BC, & Warshaw R (1985) Neurobehavioral and
respiratory symptoms of formaldehyde and xylene exposure in histology
technicians. Arch Environ Health, 40: 229-233.
Kirschner E (1995) Production of top 50 chemicals increased sub-
stantially in 1994. Chem Eng News, April: 16-20.
Kjorsvic E, Saethre LJ, & Lonning S (1982) Effects of short-term
exposure to xylenes on the early cleavage stages of cod eggs ( Gadus
morhus L.). Sarsia, 67: 299-308.
Klaucke DN, Johansen M, & Vogt RL (1982) An outbreak of xylene
intoxication in a hospital. Am J Ind Med, 3: 173-178.
Kligman AM (1966) The identification of contact allergens by human
assay. III. The maximization test: A procedure for screening and
rating contact sensitizers. J Invest Dermatol, 47: 393-409.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies: Relationship for 50 industrial pollutants.
Toxicology, 19: 229-238.
Korsak Z, Sokal JA, & Gorny R (1992) Toxic effect of combined exposure
to toluene and m-xylene in animals. III. Subchronic inhalation
study. Pol J Occup Med Environ Health, 5: 27-33.
Korsak Z, Swiercz R, & Jedrychowski R (1993) Effects of acute combined
exposure to n-butyl alcohol and m-xylene. Pol J Occup Med Environ
Health, 6: 35-41.
Korsak Z, Wisniewska-Knypl J, & Swiercz R (1994) Toxic effects of
subchronic combined exposure to n-butyl alcohol and m-xylene in
rats. Int J Occup Med Environ Health, 7: 155-166.
Kroneld R (1989) Volatile pollutants in suburban and industrial air.
Bull Environ Contam Toxicol, 42: 868-872.
Kuhn EP, Colberg PJ, Schnoor JL, Wanner O, Zehnder AJB, &
Schwarzenbach RP (1985) Microbial transformations of substituted
benzenes during infiltration of river water to ground water:
Laboratory column studies. Environ Sci Techol, 19: 961-968.
Kuhn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Kurppa K & Husman K (1982) Car painters' exposure to a mixture of
organic solvents. Serum activities of liver enzymes. Scand J Work
Environ Health, 8: 137-140.
Kyrklund T, Kjellstrand P, & Haglid K (1987) Brain lipid changes in
rats exposed to xylene and toluene. Toxicology, 45: 123-133.
Labows J, Preti G, Loezle E, Leyden J, & Kligman A (1979) Analysis of
human axillary volatiles: Compounds of exogenous origin. J Chromatogr,
163: 294-299.
Lagorio S, Forastiere F, Iavarone I, Vanacore N, Fuselli S, & Carere A
(1993) Exposure assessment in a historical cohort of filling station
attendants. Int J Epidemiol, 22(6-suppl 2): S51-S56.
Lagrue G (1976) Hydrocarbon exposure and chronic glomerulonephritis.
Lancet, 1: 1191.
Laine A, Savolainen K, Riihimäki V, Matikainen E, Salmi T, & Juntunen
J (1993) Acute effects of m-xylene inhalation on body sway, reaction
times, and sleep in man. Int Arch Occup Environ Health, 65: 179-188.
Lanzerstofer CH & Puxbaum H (1990) Volatile hydrocarbons in and around
Vienna, Austria. Water Air Soil Pollut, 51: 345-355.
Laparé S, Tardif R, & Brodeur J (1993) Effect of various exposure
scenarios on the biological monitoring of organic solvents in alveolar
air. I. Toluene and m-xylene. Int Arch Occup Environ Health,
64: 569-580.
Larsby B, Ödkvist LM, Hydén D, & Liedgren SRC (1976) Disturbancies of
the vestibular system by toxic agents. Acta Physiol Scand,
440(suppl): 108.
Lauwerys R & Buchet JP (1988) Biological monitoring of exposure to
benzene, toluene and xylene. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 205-222 (IARC
Scientific Publications No. 85).
Lauwerys RR, Dath T, Lachapelle J-M, Buchet J-P, & Roels H (1978) The
influence of two barrier creams on the percutaneous absorption of
m-xylene in man. J Occup Med, 20: 17-20.
Lazarew NW (1929) [On the toxicity of various organic compounds
vapors.] Arch Exp Pathol Pharmacol, 143: 223-233 (in German).
Lebowitz H, Brusick D, Matheson D, Jagannath DR, Reed M, Goode S, &
Roy G (1979) Commonly used fuels and solvents evaluated in a battery
of short-term bioassays. Environ Mutagen, 1: 172-173.
Le Gore RS (1974) The effects of Alaskan crude oil and selected
hydrocarbon compounds on embryonic development of the Pacific oyster,
Crassostrea gigas. Diss Abstr B, 35: 3168.
Lewis P (1994) Report of airborne solvent concentrations in a printing
ink manufacturing plant in Cape Town, Republic of South Africa. Appl
Occup Environ Hyg, 9: 147-151.
Liira J, Elovaara E, Raunio H, Riihimäki V, & Engström K (1991)
Metabolic interaction and diposition of methyl ethyl ketone and
m-xylene in rats at single and repeated inhalation exposure.
Xenobiotica, 21: 53-63.
Lindström K (1973) Psychological performances of workers exposed to
various solvents. Work Environ Health, 10: 151-155.
Lindström K, Antti-Poika M, Tola S, & Hyytiäinen A (1982)
Psychological prognosis of diagnosed chronic organic solvent
intoxication. Neurobehav Toxicol Teratol, 4: 581-588.
Lioy PJ, Wallace L, & Pellizzari E (1991) Indoor/outdoor, and personal
monitor and breath analysis relationships for selected volatile
organic compounds measured at three homes during New Jersey
TEAM - 1987. J Exp Anal Environ Epidemiol, 1(1): 45-61.
Lonneman WR, Seila RL, & Bufalini JJ (1978) Ambient air hydrocarbons
in Florida. Environ Sci Technol, 12: 459-463.
Lovegren NV, Fisher GS, Legendre MG, & Schuller WH (1979) Volatile
constituents of dried legumes. J Agric Food Chem, 27: 851-853.
Lundberg I & Håkansson M (1985) Normal serum activities of liver
enzymes in Swedish paint industry workers with heavy exposure to
organic solvents. Br J Ind Med, 42: 596-600.
Lundberg I, Håkansson M, Gustavsson P, Kronevi T, & Lidums V (1983)
[Relative hepatotoxic effect of industrial solvents.] Solna, Sweden,
National Institute of Occupational Health, pp 1-42 (Work and Health
1983:22) (in Swedish with English summary).
Lundberg I, Ekdahl M, Kronevi T, Lidums V, & Lundberg S (1986)
Relative hepatotoxicity of some industrial solvents after
intraperitoneal injection or inhalation exposure in rats. Environ Res,
40: 411-420.
Lundberg I, Nise G, Hedenborg G, Högberg M, & Vesterberg O (1994)
Liver function tests and urinary albumin in house painters with
previous heavy exposure to organic solvents. Occup Environ Med,
51: 347-353.
Lyman WJ, Reehl WF, & Rosenblatt DH (1981) Handbook of chemical
property estimation methods. New York, Mc-Graw Hill Book Company.
McCarroll NE, Keech BH, & Piper CE (1981a) A microsuspension
adaptation of the Bacillus subtilis "rec" assay. Environ Mutagen,
3: 607-616.
McCarroll NE, Piper CE, & Keech BH (1981b) An E. coli
microsuspension assay for the detection of DNA damage induced by
direct-acting agents and promutagens. Environ Mutagen, 3: 429-444.
McDonald TJ, Kennicutt MC II, & Brooks JM (1988) Volatile organic
compounds at a coastal site. Chemosphere, 17: 123-136.
McFall JA, Antoine SR, & Deleon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14: 1253.
Mackay D & Leinonen PJ (1975) Rate of evaporation of low solubility
contaminants from water bodies to the atmosphere. Environ Sci Technol,
9: 1178-1180.
Mackay D & Wolkoff AW (1973) Rate of evaporation of low solubility
contaminants from water bodies to atmosphere. Environ Sci Technol,
7: 611-614.
Mackay D, Paterson S, Cheung B, & Neely WB (1985) Evaluating the
environmental behaviour of chemicals using a level III fugacity model.
Chemosphere, 14: 335-374.
MAFF (1991) Monitoring and surveillance of non-radioactive
contaminants in the aquatic environment and activities regulating the
disposal of wastes at sea, 1988-89. Lowestoft, United Kingdom,
Ministry of Agriculture, Fisheries and Food, Directorate of Fisheries
Research (Aquatic Environment Monitoring Report No. 26).
Maizlish NA, Fine LJ, Albers JW, Whitehead L, & Langolf GD (1987) A
neurological evaluation of workers exposed to mixtures of organic
solvents. Br J Ind Med, 44: 14-25.
Major DW, Mayfield CI, & Barker JF (1988) Biotransformation of benzene
by denitrification in aquifer sand. Ground Water, 26: 8-14.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1983) Benzene as an
experimental carcinogen: up-to-date evidence. Acta Oncol, 4: 141-164.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
Current results and ongoing research. Am J Ind Med, 7: 415-446.
Mansour M, Moza PN, Barlas H, & Parlar H (1985) [A contribution to the
topic of photo-stability of environmental organic compounds in the
presence of hydrogen peroxide in aquatic systems.] Chemosphere,
14: 1469-1474 (in German).
Marks TA, Ledoux TA, & Moore JA (1982) Teratogenicity of a commercial
xylene mixture in the mouse. J Toxicol Environ Health, 9: 97-105.
Maynard DJ & Weber DD (1981) Avoidance reactions of juvenile coho
salmon (Oncorhynchus kisutch) to monocyclic aromatics. Can J Fish
Aquat Sci, 38: 772-778.
Meinhart E & Schreier P (1986) Study of flavour compounds from
Parmigiano Reggiano cheese. Milchwissenschaft, 41: 689-691.
Michael LC, Pellizzari ED, & Norwood DL (1991) Application of the
master analytical scheme to the determination of volatile organics in
wastewater influents and effluents. Environ Sci Technol, 25: 150-155.
Mirkova E, Zaikov C, Antov G, Mikhailova A, Khinkova L, & Benchev I
(1983) Prenatal toxicity of xylene. J Hyg Epidemiol Microbiol Immunol,
27: 337-343.
Mohtashamipur E, Norpoth K, Woelke U, & Huber P (1985) Effects of
ethylbenzene, toluene, and xylene on the induction of micronuclei in
bone marrow polychromatic erythrocytes of mice. Arch Toxicol,
58: 106-109.
Molnar J, Paksy KA, & Naray M (1986) Changes in the rat's motor
behaviour during 4-hr inhalation exposure to prenarcotic
concentrations of benzene and its derivatives. Acta Physiol Hungarica
67: 349-354.
Morgan P & Watkinson RJ (1990) Assessment of the potential for in
situ biotreatment of hydrocarbon-contaminated soils. Water Sci
Technol, 22: 63-68.
Morley R, Eccleston DW, Douglas CP, Greville WEJ, Scott DJ, & Anderson
J (1970) Xylene poisoning: A report on one fatal case and two cases of
recovery after prolonged unconsciousness. Br Med J, 3: 442-443.
Morrow JE, Gritz RL, & Kirton MP (1975) Effects of some components of
crude oil on young coho salmon. Copeia, 2: 326-331.
Moser VC, Coggeshall EM, & Balster RL (1985) Effects of xylene isomers
on operant responding and motor performance in mice. Toxicol Appl
Pharmacol, 80: 293-298.
Moszczynski P & Lisiewicz J (1985) Occupational exposure to benzene,
toluene and xylene and the lymphocyte lysosomal N-acetyl-beta-
D-glucosaminidase. Ind Health, 23: 47-51.
Nakamura S, Oda Y, Shimada T, Oki I, & Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutat
Res, 192: 239-246.
Naskali L, Oksanen H, & Tähti H (1994) Astrocytes as targets for CNS
effects of organic solvents in. Neurotoxicology, 15: 609-612.
Nasterlack M, Triebig G, & Stelzer O (1994) Hepatotoxic effects of
solvent exposure around permissible limits and alcohol consumption in
printers over a 4-year period. Int Arch Occup Environ Health, 66:
161-165.
Neff JM, Anderson JW, Cox BA, Laughlin RB, Rossi SS, & Tatem HE (1976)
Effects of petroleum on survival, respiration and growth of marine
animals. In: Sources, effects and sinks of hydrocarbons. Washington,
DC, American Institute of Biological Science, pp 515-523.
Nelson PF & Quigley SM (1982) Non-methane hydrocarbons in the
atmosphere of Sydney, Australia. Environ Sci Techol, 16: 650-655.
Nelson KW, Ege JF Jr, Ross M, Woodman LE, & Silverman L (1943) Sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
25: 282-285.
NIOSH (1991) Health hazard evaluation report: Rockwell International,
Newark, Ohio. Cincinnati, Ohio, National Institute for Occupational
Safety and Health (Report HETA 90-368-2137; PB92-131929).
NIOSH (1993a) Health hazard evaluation report: National Centers for
Environmental Health, Fairbanks, Alaska. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA 93-606-2336;
PB94-133667).
NIOSH (1993b) Health hazard evaluation report: Dallas and Mavis
Forwarding Co., Seattle, Washington. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA
91-0103-2351; PB94-131836).
NIOSH (1993c) Health hazard evaluation report: National Center for
Environmental Health, Albany, New York. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA
93-0884-2344; PB94-129020).
NIOSH (1993d) Health hazard evaluation report: National Centers for
Environmental Health, Stamford, Connecticut. Cincinnati, Ohio,
National Institute for Occupational Safety and Health (Report HETA
93-802-2338; PB94-134343).
NIOSH (1994) Health hazard evaluation report: Pan American Health
Organization, Bogota, Colombia (South America). Cincinnati, Ohio,
National Institute for Occupational Safety and Health (Report HETA
94-0253-2451; PB95-147062).
NTP (1986) Technical report on the toxicology and carcinogenesis
studies of xylenes (mixed) in F344/N rats and B6C3F1 mice. Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program (NIH Publication No. 87-2583).
Nunes P & Benville PE (1979) Uptake and depuration of petroleum
hydrocarbons in the Manila Clam, Tapes semidecussata Reeve. Bull
Environ Contam Toxicol, 21: 719-726.
Nylén P & Hagman M (1994) Function of the auditory and visual systems,
and of peripheral nerves in rats after long-term combined exposure to
n-hexane and methylated benzene derivatives. II. Xylene. Pharmacol
Toxicol, 74: 124-129.
Nylén P, Ebendal T, Eriksdotter-Nilsson M, Hansson T, Henschen A,
Johnson A-C, Kronevi T, Kvist U, Sjöstrand NO, Höglund G, & Olson L
(1989) Testicular atrophy and loss of nerve growth factor-
immunoreactive germ cell line in rats exposed to n-hexane and
a protective effect of simultaneous exposure to toluene or xylene.
Arch Toxicol, 63: 296-307.
Ogata M & Fujii T (1979) Urinary excretion of hippuric acid and
m-methylhippuric acid after administration of toluene and m-xylene
mixture to rats. Int Arch Occup Environ Health, 43: 45-51.
Ogata M & Hobara T (1979) A new direct method for colorimetric
determination of hippuric acid and methylhippuric acid as indices of
toluene and m-xylene, and its application to workers using thinner.
Ind Health, 17: 61-72.
Ogata M & Miyake Y (1979) Disappearance of aromatic hydrocarbons and
organic sulphur compounds from fish reared in crude oil suspensions.
Water Res, 13: 75-78.
Ogata M, Yamasaki Y, Meguro T, Sugihara R, & Shimada Y (1979)
Quantitation of urinary o-xylene metabolites in rats and human
beings by high performance liquid chromatography. Ind Health,
17: 123-125.
Ogata M, Yamazaki Y, Sugihara R, Shimada Y, & Meguro T (1980)
Quantitation of urinary o-xylene metabolites of rats and human
beings by high performance liquid chromatography. Int Arch Occup
Environ Health, 46: 127-139.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
Omori T & Yamada K (1970) Studies on the utilisation of hydrocarbons
by microorganisms. Part XVI. Detection of metabolic intermediates of
xylene and pseudocumene. Agric Biol Chem, 34: 659-663.
Omori T, Horiguchi S, & Yamada K (1967) Studies on the utilisation of
hydrocarbons by microorganisms. Part X. Screening of aromatic
hydrocarbon assimilating microorganisms and p-toluic acid formation
from p-xylene. Agric Biol Chem, 31: 1337-1342.
Otson R & Williams DT (1981) Evaluation of a liquid-liquid extraction
technique for water pollutants. J Chromatogr, 212: 187-197.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities.
J Assoc Off Anal Chem, 65: 1370-1374.
Otson R, Fellin P, & Tran Q (1993) Investigation of VOCs in Canadian
residences. In: Indoor air '93. Proceedings of the 6th International
Conference on Indoor Air Quality and Climate, Helsinki, Finland, 4-8
July 1993 - Volume 2: Chemicals in indoor air, materials emissions,
pp 141-146.
Ödkvist LM, Larsby B, Tham R, & Aschan G (1979) On the mechanism
of vestibular disturbances caused by industrial solvents. Adv
Oto-rhino-laryngol, 25: 167-172.
Ödkvist LM, Larsby B, Fredrickson JMF, Liedgren SRC, & Tham R (1980)
Vestibular and oculomotor disturbances caused by industrial solvents.
J Otolaryngol, 9: 53-59.
PACE (1987) A study of exposure to motor gasoline hydrocarbon vapours
at service stations: Phase II - Summer Study. Petroleum Association
for Conservation of the Canadian Environment (PACE Report No. 87-5).
PACE (1989) A study of exposure to motor gasoline hydrocarbon vapours
at service stations: Phase III - Winter Study. Petroleum Association
for Conservation of the Canadian Environment (PACE Report No. 89-3).
Padilla S, Lyerly DL, & Pope CN (1992) Subacute ethanol consumption
reverses p-xylene-induced decreases in axonal transport. Toxicology,
75: 159-167.
Palmer KT & Rycroft RJG (1993) Occupational airborne contact urticaria
due to xylene. Contact Dermatitis, 28: 44.
Pap M & Varga C (1987) Sister-chromatid exchanges in peripheral
lymphocytes of workers occupationally exposed to xylenes. Mutat Res,
187: 223-225.
Park SH, AuCoin TA, Silverman DM, & Schatz RA (1994) Time-dependent
effects of o-xylene on rat lung and liver microsomal membrane
structure and function. J Toxicol Environ Health, 43: 469-481.
Patel JM, Harper C, & Drew RT (1976) Inactivation of pulmonary
cytochrome P-450 by p-xylene intoxication. Pharmacologist, 18: 210.
Patel JM, Harper C, & Drew RT (1978) The biotransformation of
p-xylene to a toxic aldehyde. Drug Metab Dispos, 6: 368-374.
Patel JM, Harper C, Gupta BN, & Drew RT (1979) Changes in serum
enzymes after inhalation exposure of p-xylene. Bull Environ Contam
Toxicol, 21: 17-24.
Pathiratne A, Puyear RL, & Brammer JD (1986) A comparative study of
the effects of benzene, toluene, and xylenes on their in metabolism
and drug-metabolizing enzymes in rat liver. Toxicol Appl Pharmacol,
82: 272-280.
Pellizzari EC, Hartwell TD, Harris BSH III, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mothers milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pellizzari ED, Zweidinger RA, & Sheldon LS (1988) Determination
of benzene, toluene and xylene in breath samples by gas
chromatography/mass spectrometry. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 267-279 (IARC
Scientific Publications No. 85).
Pennell KD, Rhue RD, Rao PSC, & Johnston CT (1992) Vapor-phase
sorption of p-xylene and water on soils and clay minerals. Environ
Sci Technol, 26: 756-763.
Perry R & Twibell JD (1973) A time based elution technique for the
estimation of specific hydrocarbons in air. Atmos Environ, 7: 929.
Petersson G (1982) Ambient hydrocarbons from motor-car assembly plants
in Scandinavia. Environ Pollut, B4: 207-217.
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38: 1419-1429.
Pound AW (1970) Induced cell proliferation and the initiation of skin
tumour formation in mice by ultraviolet light. Pathology, 2: 269-275.
Pound AW & Withers HR (1963) The influence of some irritant chemicals
and scarification on tumour initiation by urethane in mice. Br J
Cancer, 17: 460-470.
Proust B, Hannequin D, Caillard JF, Prudent P, Hemet J, & Samson N
(1986) Le psychosyndrome aux solvants: Résultats des tests
psychométriques chez dix laborantines exposées. Arch Mal Prof,
47: 305-310.
Pryor GT, Robert CS, & Howd RA (1987) Hearing loss in rats caused by
inhalation of mixed xylenes and styrene. J Appl Toxicol, 7: 55-61.
Pussemier L, Szabó G, & Bulman RA (1990) Prediction of the soil
adsorption coefficient Koc for aromatic pollutants. Chemosphere,
21: 1199-1212.
Pyykkö K (1980) Effects of methylbenzenes on microsomal enzymes in rat
liver, kidney and lung. Biochim Biophys Acta, 633: 1-9.
Pyykkö K (1986) Inhibition of microsomal monooxygenases in by
aromatic hydrocarbons. Arch Toxicol, 9(suppl): 371-373.
Pyykkö K, Paavilainen S, Metsä-Ketelä T, & Laustiola K (1987) The
increasing and decreasing effects of aromatic hydrocarbon solvents on
pulmonary and hepatic cytochrome P-450 in the rat. Pharmacol Toxicol,
60: 288-293.
Rao PSC, Hornsby AG, & Jessup RE (1985) Indices for ranking the
potential for pesticide contamination of ground water. Soil Crop Sci
Soc Proc, 44: 1-8.
Raunio H, Liira J, Elovaara E, Riihimäki V, & Pelkonen O (1990)
Cytochrome P450 isozyme induction by methyl ethyl ketone and
m-xylene in rat liver. Toxicol Appl Pharmacol, 103: 175-179.
Raymond RL, Jamison VW, & Hudson JO (1969) Microbial hydrocarbon
co-oxidation. II. Use of ion-exchange resins. Appl Microbiol,
17: 512-515.
Recchia G, Perbellini L, Prati GF, Dean P, & Ancona G (1985) [Coma due
to accidental ingestion of xylene. Treatment with charcoal
hemoperfusion.] Med Lav, 76: 67-73 (in Italian with English summary).
Reinert KH, Hunter JV, & Sabatino T (1983) Dynamic heated headspace
analysis of volatile organic compounds present in fish tissue samples.
J Agric Food Chem, 31: 1057-1060.
Reinhard M, Goodman NL, & Barker JF (1984) Occurrence and distribution
of organic chemicals in two landfill leachate plumes. Environ Sci
Technol, 18: 953-961.
Rhue RD, Rao PSC, & Smith RE (1988) Vapour-phase adsorption of
alkylbenzenes and water on soils and clays. Chemosphere, 17: 727-741.
Richer CL, Chakrabarti S, Senecal-Quevillon M, Duhr MA, Zhang XX, &
Tardif R (1993) Cytogenic effects of low-level exposure to toluene,
xylene and their mixture on human blood lymphocytes. Int Arch Occup
Environ Health, 64: 81-85.
Rigdon RH (1940) Capillary permeability in areas of inflammation
produced by xylene. Arch Surg, 41: 101-109.
Riihimäki V (1979a) Conjugation and urinary excretion of toluene and
m-xylene in man. Scand J Work Environ Health, 5: 135-142.
Riihimäki V (1979b) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand J Work
Environ Health, 5: 143-150.
Riihimäki V & Hänninen O (1987) Xylenes. In: Snyder R. ed. Ethel
Browning's toxicity and metabolims of industrial solvents, 2nd ed.
Amsterdam, Oxford, New York, Elsevier Science Publishers, vol 1, pp
64-84.
Riihimäki V & Pfäffli P (1978) Percutaneous absorption of solvent
vapors in man. Scand J Work Environ Health, 4: 73-85.
Riihimäki V & Savolainen K (1980) Human exposure to m-xylene,
kinetics and acute effects on the central nervous system. Ann Occup
Hyg, 23: 411-422.
Riihimäki V, Pfäffli P, & Savolainen K (1979a) Kinetics of m-xylene
in man: Influence of intermittent physical exercise and changing
environmental concentrations on kinetics. Scand J Work Environ Health,
5: 232-248.
Riihimäki V, Pfäffli P, Savolainen K, & Pekari K (1979b) Kinetics of
m-xylene in man: General features of absorption, distribution,
biotransformation and excretion in repetitive inhalation exposure.
Scand J Work Environ Health, 5: 217-231.
Riihimäki V, Laine A, Savolainen K, & Sippel H (1982a) Acute solvent-
ethanol interactions with special reference to xylene. Scand J
Work Environ Health, 8: 77-79.
Riihimäki V, Savolainen K, Pfäffli P, Pekari K, Sippel HW, & Laine A
(1982b) Metabolic interaction between m-xylene and ethanol. Arch
Toxicol, 49: 253-263.
Roberts AE, Bogdanffy MS, Brown DR, & Schatz RA (1986) Lung metabolism
of benzo(a)pyrene in rats treated with p-xylene and/or ethanol. J
Toxicol Environ Health, 18: 257-266.
Rogerson A, Shiu WY, Huang GL, Mackay D, & Berger J (1983)
Determination and interpretation of hydrocarbon toxicity to ciliate
protozoa. Aquat Toxicol, 3: 215-228.
Rosen MB, Crofton KM, & Chernoff N (1986) Postnatal evaluation of
prenatal exposure to p-xylene in the rat. Toxicol Lett, 34: 223-229.
Rosengren LE, Kjellstrand P, Aurell A, & Haglid KG (1986) Irreversible
effects of xylene on the brain after long term exposure: A
quantitative study of DNA and the glial cell marker proteins S-100 and
GFA. Neurotoxicology, 7: 121-136.
Ruijten MW, Hooisma J, Brons JT, Habets CE, Emmen HH, & Muijser H
(1994) Neurobehavioral effects of long-term exposure to xylene and
mixed organic solvents in shipyard spray painters. Neurotoxicology,
15: 613-620.
Rydzynski K, Korsak Z, Jedlinska U, & Sokal JA (1992) The toxic
effects of combined exposure to toluene and m-xylene in animals. IV.
Liver ultrastructure after subchronic inhalatory exposure. Pol J Occup
Med Environ Health, 5: 35-42.
Sabel GV & Clark TP (1984) Volatile organic compounds as indicators of
municipal solid waste leachate contamination. Waste Manage Res,
2: 119-130.
Sabljic A & Güsten H (1990) Predicting the night-time NO3 radical
reactivity in the troposphere. Atmos Environ, 24A: 73-78.
Sack TM, Steele DH, Hammerstrom K, & Remmers K (1992) A survey of
household products for volatile organic compounds. Atmos Environ,
26A(6): 1063-1070.
Sanborn HR & Malins DC (1980) The disposition of aromatic hydrocarbons
in adult spot shrimp (Pandalus platyceros) and the formation of
metabolites of naphthalene in adult and larval spot shrimp.
Xenobiotica, 10: 193-200.
Sato A, Fujiwara Y, & Nakajima T (1974) Solubility of benzene, toluene
and m-xylene in various body fluids and tissues of rabbits. Jpn J
Ind Health, 16: 30-31.
Sauer TC (1981) Volatile liquid hydrocarbon characterisation of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environ Sci Technol, 15: 917-923.
Savolainen K (1980) Combined effects of xylene and alcohol on the
central nervous system. Acta Pharmacol Toxicol, 46: 366-372.
Savolainen H & Pfäffli P (1980) Dose-dependent neurochemical changes
during short-term inhalation exposure to m-xylene. Arch Toxicol,
45: 117-122.
Savolainen K & Linnavuo M (1979) Effects of m-xylene on human
equilibrium measured with a quantitative method. Acta Pharmacol
Toxicol, 44: 315-318.
Savolainen K & Riihimäki V (1981) Xylene and alcohol involvement of
the human equilibrium system. Acta Pharmacol Toxicol, 49: 447-451.
Savolainen H & Seppäläinen AM (1979) Biochemical and physiological
effects of organic solvents on rat axon membranes isolated by a new
technique. Neurotoxicology, 1: 467-477.
Savolainen H, Pfäffli P, Helojoki M, & Tengén M (1979a) Neurochemical
and behavioural effects of long-term intermittent inhalation of xylene
vapour and simultaneous ethanol intake. Acta Pharmacol Toxicol,
44: 200-207.
Savolainen H, Vainio H, Helojoki M, & Elovaara E (1979b) Biochemical
and toxicological effects of short-term intermittent xylene inhalation
exposure and combined ethanol intake. Arch Toxicol, 41: 195-205.
Savolainen K, Riihimäki V, Vaheri E, & Linnoila M (1980) Effects of
xylene and alcohol on vestibular and visual functions in man. Scand J
Work Environ Health, 6: 94-103
Savolainen K, Riihimäki V, Laine A, & Kekoni J (1981) Short-term
exposure of human subjects to m-xylene and 1,1,1-trichloroethane.
Int Arch Occup Environ Health, 49: 89-98.
Savolainen K, Riihimäki V, & Laine A (1982a) Biphasic effects of
inhaled solvents on human equilibrium. Acta Pharmacol Toxicol,
51: 237-242.
Savolainen K, Riihimäki V, Laine A, & Kekoni J (1982b) Short-term
exposure of human subjects to m-xylene and 1,1,1-trichloroethane.
Arch Toxicol, 5(suppl): 96-99.
Savolainen K, Kekoni J, Riihimäki V, & Laine A (1984) Immediate
effects of m-xylene on the human central nervous system. Arch
Toxicol, 7(suppl): 412-417.
Savolainen K, Riihimäki V, Luukkonen R, & Muona O (1985a) Changes in
the sense of balance correlate with concentrations of m-xylene in
venous blood. Br J Ind Med, 42: 765-769.
Savolainen K, Riihimäki V, Muona O, Kekoni J, Luukkonen R, & Laine A
(1985b) Conversely exposure-related effects between atmospheric
m-xylene concentrations and human body sense of balance. Acta
Pharmacol Toxicol, 57: 67-71.
Sedivec V & Flek J (1976) The absorption, metabolism, and excretion of
xylenes in man. Int Arch Occup Environ Health, 37: 205-217.
Seidenberg JM, Anderson DG, & Becker RA (1986) Validation of an in
vivo developmental toxicity screen in the mouse. Teratogen
Carcinogen Mutagen, 6: 361-374.
Seip HM, Alstad J, Carlberg GE, Martinsen K, & Skaane R (1986)
Measurement of mobility of organic compounds in soil. Sci Total
Environ, 50: 87-101.
Selgrade MK, Daniels MJ, Jaskot RH, Robinson BL, & Albis JW (1993)
Enhanced mortality and liver damage in virus-infected mice exposed to
p-xylene. J Toxicol Environ Health, 40: 129-144.
Seppäläinen AM, Husman K, & Mårtenson C (1978) Neurophysiological
effects of long-term exposure to a mixture of organic solvents. Scand
J Work Environ Health, 4: 304-314.
Seppäläinen AM, Savolainen K, & Kovala T (1981) Changes induced by
xylene and alcohol in human evoked potentials. Electroencephalogr Clin
Neurophysiol, 51: 148-155.
Seppäläinen AM, Laine A, Salmi T, Verkkala E, Riihimäki V, & Luukkonen
R (1991) Electroencephalographic findings during experimental human
exposure to m-xylene. Arch Environ Health, 46: 16-23.
Sevcik P, Hep A, & Peslova M (1992) Intravenous xylene poisoning.
Intensive Care Med, 18: 377-378.
Sexton K & Westberg H (1980) Ambient hydrocarbon and ozone
measurements downwind of a large automotive painting plant. Environ
Sci Technol, 14: 329-332.
Seyfried B, Glod G, Schocher R, Tschech A, & Zeyer J (1994) Initial
reactions in the anaerobic oxidation of toluene and m-xylene by
denitrifying bacteria. Appl Environ Microbiol, 60(11): 4047-4052.
Sheedy BR, Lazorchak JM, Grunwald DJ, Pickering QH, Pilli A, Hall D, &
Webb R (1991) Effects of pollution on freshwater organisms. Res J
Water Pollut Control Fed, 63: 619-696.
Sheldon LS, Zelon H, Sickles J, Eaton C, Hartwell T, & Jugners R
(1988) Monitoring in and around public access buildings. Proceedings
of the 81st Annual Meeting of the Air Pollution Control Association,
1988. Pittsburgh, Pennsylvania, Air Pollution Control Association,
9 pp (Paper 88/109.8).
Shimizu H, Suzuki Y, Takemura N, Goto S, & Matsushita H (1985) The
results of microbial mutation test for forty-three industrial
chemicals. Jpn J Ind Health, 27: 400-419.
Silverman DM & Schatz RA (1991) Pulmonary microsomal alterations
following short-term low level inhalation of p-xylene in rats.
Toxicology, 65: 271-281.
Simmons JE, Allis JW, Grose EC, Seely JC, Robinson BL, & Berman E
(1991) Assessment of the hepatotoxicity of acute and short-term
exposure to inhaled p-xylene in F-344 rats. J Toxicol Environ
Health, 32: 295-306.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1983) Measurements of
hazardous organic chemicals in the ambient atmosphere. Washington, DC,
US Environmental Protection Agency (EPA-600/3-83-002).
Singh HB, Ferek RJ, Salas LJ, & Nitz KC (1986) Toxic chemicals in the
environment: A program of field measurements. Washington, DC, US
Environmental Protection Agency (EPA-600/3-86/047).
Skowronski GA, Turkall RM, Kadry ARM, & Abdel-Rahman MS (1990) Effects
of soil on the dermal bioavailability of m-xylene in male rats.
Environ Res, 51: 182-193.
Slaine DD & Barker JF (1990) The detection of naturally occurring BTX
during a hydrogeologic investigation. Ground Water Monit Rev,
10: 89-94.
Slooff W (1979) Detection limits of a biological monitoring system
based on fish respiration. Bull Environ Contam Toxicol, 23: 517-523.
Slooff W, de Zwart D, & Marquenie JM (1983) Detection limits of a
biological monitoring system for chemical water pollution based on
mussel activity. Bull Environ Contam Toxicol, 30: 400-405.
Smith BR, Plummer JL, Wolf CR, Philpot RM, & Bend JR (1982) p-Xylene
metabolism by rabbit lung and liver and its relationship to the
selective destruction of pulmonary cytochrome P-450. J Pharmacol Exp
Ther, 223: 736-742.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: List VI. Am Ind Hyg Assoc J, 23: 95-107.
Snyder R (1987) Ethyl Browning's toxicity and metabolism of industrial
solvents - Volume 1; Hydrocarbons. Amsterdam, Oxford, New York,
Elsevier Science Publishers.
Sotaniemi EA, Sutinen S, Sutinen S, Arranto AJ, & Pelkonen RO (1982)
Liver injury in subjects occupationally exposed to chemicals in low
doses. Acta Med Scand, 212: 207-215.
Steele RH & Wilhelm DL (1966) The inflammatory reaction in chemical
injury. I. Increased vascular permeability and erythema induced by
various chemicals. J Exp Pathol, 47: 612-623.
Stickney JA, Silverman DM, & Schatz RA (1991) Role of isozyme-specific
inhibition of cytochrome P450IIB1 activity in m-xylene-induced
alterations in rat pulmonary benzo(a)pyrene metabolism. Xenobiotica,
211: 641-649.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near an underground coal gasification site in northeastern
Wyoming. Environ Sci Technol, 16: 582-587.
Sugihara R (1979) High-performance liquid chromatography studies of
toluene and xylene poisoning. Part II. Respiratory and urinary
excretion of toluene and m-xylene following intraperitoneal
administration to rats. Acta Med Okayama, 91: 1433-1440.
Sugihara R & Ogata M (1978) Quantitation of urinary m- and
p-methylhippuric acids as indices of m- and p-xylene exposure.
Int Arch Occup Environ Health, 41: 281-286.
Svanberg PA, Grennfeldt P, & Lindskog A (1995) The Swedish urban
network - Air quality measurement program. Stockholm, Sweden,
Environmental Research Institute (Unpublished report).
Tabak HH, Desai S, & Govind R (1989) Determination of biodegradability
kinetics of RCRA compounds using respirometry for structure-activity
relationships. Proceedings of the 44th Industrial Waste Conference,
1989, pp 405-423.
Takatori T, Terazawa K, Nakano K, & Inoue K (1982) An autopsy case of
alkylbenzene poisoning and its clinical finding. Nippon Hoigaku
Zasshi, 36: 654-661.
Tardif R, Laparé S, Plaa GL, & Brodeur J (1991) Effect of simultaneous
exposure to toluene and xylene on their respective biological exposure
indices in humans. Int Arch Occup Environ Health, 63: 279-284.
Tardif R, Plaa GL, & Brodeur J (1992) Influence of various mixtures of
inhaled toluene and xylene on the biological monitoring of exposure to
these solvents in rats. Can J Physiol Pharmacol, 70: 385-939.
Tardif R, Laparé S, Krishnan K, & Brodeur J (1993a) Physiologically
based modelling of the toxicokinetic interaction between toluene and
m-xylene in rat. Toxicol Appl Pharmacol, 120: 266-273.
Tardif R, Laparé S, Krishnan K, & Brodeur J (1993b) A descriptive and
mechanistic study of the interaction between toluene and xylene in
humans. Int Arch Occup Environ Health, 65: S135-S137.
Tardif R, Sato A, Laparé S, & Brodeur J (1994) Ethanol induced
modification of m-xylene toxicokinetics in humans. Occup Environ
Med, 51: 187-191.
Taskinen H, Kyyrönen P, Hemminki K, Hoikkala M, Lajunen K, & Lindbohm
M-L (1994) Laboratory work and pregnancy outcome. J Occup Med,
36: 311-319.
Tatem HE, Cox BA, & Anderson JW (1978) The toxicity of oils and
petroleum hydrocarbons to estuarine crustaceans. Estuar Coast Mar Sci,
6: 365-373.
Tegeris JS & Balster RL (1994) A comparison of the acute behavioral
effects of alkylbenzenes using a functional observational battery in
mice. Fundam Appl Toxicol, 22: 240-250.
Tham R, Bunnfors I, Eriksson B, Larsby B, Lindgren S, & Ödkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thomas JM, Gordy VR, Fiorenza S, & Ward CH (1990) Biodegradation of
BTEX in subsurface materials contaminated with gasoline: Granger,
Indiana. Water Sci Technol, 22: 53-62.
Toftgård R & Nilsen OG (1982) Effects of xylene and xylene isomers on
cytochrome P-450 and in enzymatic activities in rat liver, kidney
and lung. Toxicology, 23: 197-212.
Toftgård R, Nilsen OG, & Gustafsson J-Å (1981) Changes in rat liver
microsomal cytochrome P-450 and enzymatic activities after the
inhalation of n-hexane, xylene, methyl ethyl ketone and
methylchloroform for four weeks. Scand J Work Environ Health,
7: 31-37.
Toftgård R, Nilsen OG, Glaumann H, & Gustafsson J-Å (1983) Induction
of cytochrome P-450 in the rat liver after exposure to xylenes,
dose-response relationship and dependence on endocrine factors.
Toxicology, 27: 119-137.
Toftgård R, Haaparanta T, & Halpert J (1986) Rat lung and liver
cytochrome P-450 isozymes involved in the hydroxylation of m-xylene.
Toxicology, 39: 225-231.
Triebig G & Schaller K-H (1986) Air monitoring of solvent exposed
workers with passive samplers in comparison to "biological monitoring
(BM)". Toxicol Environ Chem, 12: 285-312.
Tsuruta H (1982) Percutaneous absorption of organic solvents. III. On
the penetration rates of hydrophobic solvents through the excised rat
skin. Ind Health, 20: 335-345.
Turkall RM, Skowronski GA, Kadry ARM, & Abdel-Rahman MS (1992) Sex
differences in the bioavailability of soil-adsorbed m-xylene in
orally exposed rats. Toxicol Lett, 63: 57-67.
Tynan P, Watts CD, Sowray A, & Hammond I (1991) The transport and fate
of organic pollutants in rivers. II. Field measurements and modelling
for styrene, xylenes, dichlorobenzenes and 4-phenyldodecane. In:
Proceedings of the 6th European Symposium on Aquatic Environment,
1990, pp 20-37.
Uchida Y, Nakatsuka H, Ukai H, Watanabe T, Liu Y-T, Huang M-Y, Wang
Y-L, Zhu F-Z, Yin H, & Ikeda M (1993) Symptoms and signs in workers
exposed predominantly to xylenes. Int Arch Occup Environ Health,
64: 597-605.
Ungvary G (1985) The possible contribution of industrial chemicals
(organic solvents) to the incidence of congenital defects caused by
teratogenic drugs and consumer goods - an experimental study. Progr
Clin Biomed Res, 163B: 295-300.
Ungvary G (1990) The effect of xylene exposure on the liver. Acta
Morphol Hung, 38: 245-258.
Ungvary G & Tatrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats and rabbits. Arch Toxicol,
8(suppl): 425-430.
Ungvary G, Tatrai E, Hudak A, Barcza G, & Lorincz M (1980) Studies on
the embryotoxic effects of ortho-, meta- and para-xylene.
Toxicology, 18: 61-74.
Ungvary G, Varga B, Horvath E, Tatrai E, & Folly G (1981) Study on the
role of maternal sex steroid production and metabolism in the
embryotoxicity of para-xylene. Toxicology, 19: 263-268.
Valciukas JA, Lilis R, Singer RM, Glickman L, & Nicholson WJ (1985)
Neurobehavioral changes among shipyard painters exposed to solvents.
Arch Environ Health, 40: 47-52.
van der Wal JF & Moerkerken A (1984) The performance of passive
diffusion monitors for organic vapours for personal sampling of
painters. Ann Occup Hyg, 28: 39-47.
van Doorn R, Bos P, Brouns RME, Leijdekkers C-M, & Henderson PT (1980)
Effect of toluene and xylenes on liver glutathione and their urinary
excretion as mercapturic acids in the rat. Arch Toxicol, 43: 293-304.
van Doorn R, Leijdekkers C-M, Bos RP, Brouns ME, & Henderson PT (1981)
Alcohol and sulphate intermediates in the metabolism of toluene and
xylenes to mercapturic acids. J Appl Toxicol, 1: 236-242.
van Vliet C, Swaen GMH, Slangen JJM, de Boorder T, & Sturmans F (1987)
The organic solvent syndrome: A comparison of cases with neuro-
psychiatric disorders among painters and construction workers.
Int Arch Occup Environ Health, 59: 493-501.
van Wijnen JH, Verhoeff AP, Jans HWA, & van Bruggen M (1995) The
exposure of cyclists, car drivers and pedestrians to traffic-related
air pollutants. Int Arch Occup Environ Health, 67(3): 187-193.
Veith GD, Macek KJ, Petrocelli SR, & Carroll J (1980) An evaluation of
using partition coefficients and water solubility to estimate
bioconcentration factors for organic chemicals in fish. In: Eaton JG,
Parrish PR, & Hendricks AC ed. Aquatic toxicology. Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp 116-129
(ASTM STP 707).
Verschoyle RD, Dinsdale D, & Wolf CR (1993) Inhibition and induction
of cytochrome P450 isoenzymes in rat lung. J Pharmacol Exp Ther,
265: 386-391.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., pp 1188-1194.
Vincent R, Poirot P, Subra I, Rieger B, & Cicolella A (1994)
Occupational exposure to organic solvents during paint dripping and
painting operations in the aeronautical industry. Int Arch Occup
Environ Health, 65: 377-380.
Vodickova L, Frantik E, & Vodickova A (1995) Neutrotrophic effects and
blood levels of solvents at combined exposures: Binary mixtures of
toluene, o-xylene and acetone in rats and mice. Cent Eur J Public
Health, 3: 57-64.
Volskay VT & Grady CPL (1990) Respiration inhibition kinetic analysis.
Water Res, 24: 863-874.
Vowles PD & Mantoura RFC (1987) Sediment-water partition coefficients
and HPLC retention factors for aromatic hydrocarbons. Chemosphere,
16: 109-116.
Waggott A (1981) Trace organic substances in the River Lee. In:
Chemistry in water reuse. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., vol 2, pp 55-99.
Wallace LA, Pellizzari ED, Hartwell TD, Whitmore R, Zelon H, Perritt
R, & Sheldon L (1988) The California TEAM study: Breath concentrations
and personal exposures to 26 volatile compounds in air and drinking
water of 188 residents of Los Angeles, Antioch, and Pittsburg, CA.
Atmos Environ, 22: 2141-2163.
Wallace L, Nelson W, Ziegenfus R, Pellizzari E, Michael L, Whitmore R,
Zelon H, Hartwell T, Perritt R, & Westerdahl D (1991) The Los Angeles
TEAM study: Personal exposures, indoor-outdoor air concentrations, and
breath concentrations of 25 volatile organic compounds. J Exp Anal
Environ Epidemiol, 1(2): 157-192.
Wallén M, Holm S, & Byfält Nordqvist M (1985) Coexposure to toluene
and p-xylene in man: Uptake and elimination. Br J Ind Med,
42: 111-116.
Walsh DF, Armstrong JG, Bariley TR, Salmon HA, & Frank PA (1977)
Residues of emulsified xylene in aquatic weed control and their impact
on rainbow trout. Springfield, Virginia, National Technical
Information Services (NTIS) (PB-267270).
Walton BT, Anderson TA, Hendricks MS, & Talmage SS (1989) Physico-
chemical properties as predictors of organic chemical effects
on soil microbial respiration. Environ Toxicol Chem, 8: 53-63.
Washington WJ, Murthy RC, Doye A, Eugene K, Brown D, & Bradley J
(1983) Induction of morphologically abnormal sperm in rats exposed to
o-xylene. Arch Androl, 11: 233-237.
Weber WJ, Cole DE, & Posner JC (1975) Analysis of emissions from
outboard two cycle marine engines. Cincinnati, Ohio, US Environmental
Protection Agency, National Environmental Research Centre, Office of
Research and Development, 249 pp (EPA 670/2-75-06; PB-242174).
Weisel CP, Lawryk NJ, & Lioy PJ (1992) Exposure to emissions from
gasoline within automobile cabins. J Exp Anal Environ Epidemiol,
2(1): 79-96.
Weschler CJ, Shields HC, & Rainer D (1990) Concentrations of volatile
organic compounds at a building with health and comfort complaints. Am
Ind Hyg Assoc J, 51(5): 261-268.
WHO (1981) Xylene. In: Recommended health-based limits in occupational
exposure to selected organic solvents. Geneva, World Health
Organization, pp 25-38 (WHO Technical Report Series No. 664).
Wiesenburg DA, Bodennec G, & Brooks JM (1981) Volatile liquid
hydrocarbons around a production platform in the Northwest Gulf of
Mexico. Bull Environ Contam Toxicol, 27: 167-174.
Williams DT, Nestmann ER, LeBel GL, Benoit FM, & Otson R (1982)
Determination of mutagenic potential and organic contaminants of Great
Lakes drinking water. Chemosphere, 11(3): 263-276.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformations of selected
alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic
aquifer material: A microcosm study. Environ Sci Technol,
20: 997-1002.
Wilson BH, Wilson JT, Kampbell DH, Bledsoe BE, & Armstrong JM (1990)
Biotransformation of monoaromatic and chlorinated hydrocarbons at an
aviation gasoline spill site. Geomicrobiol J, 8: 225-240.
Winslow C-EA (1927) Summary of the National Safety Council study of
benzol poisoning. J Ind Hyg, 9: 61-74.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene:
Experiments on laboratory animals. Arch Ind Health, 14: 387-398.
Yamada K (1993) Influence of lacquer thinner and some organic solvents
on reproductive and accessory reproductive organs in the male rat.
Biol Pharm Bull, 16: 425-427.
Young PJ & Parker A (1983) The identification and possible
environmental impact of trace gases and vapours in landfill gas. Waste
Manage Res, 1: 213-226.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests. III. Results from the testing of
255 chemicals. Environ Mutagen, 9(suppl): 1-110.
Zeyer J, Kuhn EP, & Schwarzenback RP (1986) Rapid microbial
mineralisation of toluene and 1,3-dimethylbenzene in the absence of
molecular oxygen. Appl Environ Microbiol, 52: 944-947.
Zimmerman SW, Groehler K, & Beirne GJ (1975) Hydrocarbon exposure and
chronic glomerulonephritis. Lancet, 2: 199-201.
RESUME
Le xylčne est un hydrocarbure aromatique qui existe sous trois
formes: les isomčres ortho, méta et para. Le xylčne de qualité
technique est un mélange des trois isomčres qui contient en outre un
peu d'éthylbenzčne. En 1984, la production mondiale de xylčne était
estimée ą 15,4 millions de tonnes. A la température ambiante, le
xylčne se présente sous la forme d'un liquide incolore d'odeur
aromatique. La tension de vapeur est comprise entre 0,66 et 0,86 kPa
pour les trois isomčres. Environ 92% des mélanges de xylčnes sont
incorporés ą l'essence. On les utilise aussi comme solvants, en
particulier dans les peintures et les encres d'imprimerie.
La majeure partie du xylčne libéré dans l'environnement passe
directement dans l'atmosphčre. Les trois isomčres y sont rapidement
décomposés, principalement par photooxydation. Ils se volatilisent
tous les trois rapidement ą partir de l'eau. Dans le sol et dans
l'eau, les isomčres méta et para subissent une biodégradation aisée
dans des conditions variées d'aérobiose et d'anaérobiose; en revanche,
l'isomčre ortho est plus persistant. Les données limitées dont on
dispose indiquent que les xylčnes isomčres s'accumulent peu chez les
poissons et les invertébrés. Une fois que l'exposition a cessé, ils
sont assez rapidement éliminés par les organismes aquatiques.
Les concentrations moyennes de fond des trois xylčnes dans l'air
ambiant se situent autour de la valeur caractéristique de 1 µg/m3,
mais dans les banlieues elles atteignent 3 µg/m3 environ. On a
mesuré des valeurs plus élevées en zone urbaine et industrielle, les
moyennes allant cette fois jusqu'ą 500 µg/m3. Toutefois, la
concentration est en général inférieure ą 100 µg/m3.
On estime que l'exposition journaličre de la population par la
voie respiratoire est de 70 µg en milieu rural et de 2 000 µg en
milieu urbain. Dans l'eau de boisson, la concentration varie de zéro
ą 12 µg/litre. Les données concernant la concentration dans les
denrées alimentaires sont trop limitées pour que l'on puisse évaluer
l'exposition journaličre par voie orale.
Dans les eaux superficielles, la concentration moyenne de fond
des xylčnes est généralement inférieure ą 0,1 µg/litre. Cependant, on
a mesuré des valeurs beaucoup plus élevées dans des zones
industrielles et plus particuličrement celles oł sont implantées des
industries pétroličres (jusqu'ą 30 µg/litre dans les eaux polluées et
jusqu'ą 2 000 µg/litre ą proximité des conduites de décharge). Des
valeurs analogues ont été observées dans les eaux souterraines, ces
valeurs élevées pouvant źtre attribuées dans certains cas ą une
pollution locale par des réservoirs et des canalisations enterrées.
Aprčs exposition par la voie respiratoire, la dose inhalée est
retenue ą 60% environ dans les poumons. La métabolisation est
efficace puisque le xylčne est transformé ą 90% en acide
méthylhippurique, lequel est ensuite excrété dans les urines. Le
xylčne ne s'accumule pas en quantité importante dans l'organisme
humain.
Chez l'homme, une exposition aiguė ą du xylčne sous forte
concentration peut avoir des effets sur le systčme nerveux central et
provoquer une irritation. Ces effets n'ont toutefois pas donné lieu ą
des études contrōlées ni ą des études épidémiologiques ą long terme.
Chez les animaux de laboratoire, la toxicité chronique se révčle
faible. On a cependant de bonnes raisons de penser que sous
concentration modérée, le xylčne pourrait avoir des effets sur le SNC
chez l'animal.
Le xylčne ne s'est révélé ni mutagčne ni cancérogčne.
Le point d'aboutissement toxicologique essentiel concerne l'effet
nocif que le xylčne exerce sur le développment. On l'a mis en
évidence chez le rat ą partir d'une concentration de 870 mg/m3 (200
ppm). Compte tenu de cela, la valeur-guide recommandée pour la
concentration maximale de xylčne dans l'air a été fixée ą 0,87 mg/m3
(0,2 ppm).
Les xylčnes isomčres sont faiblement ą modérément toxiques pour
les organismes aquatiques. Chez les invertébrés, c'est l' o-xylčne
qui a la Cl50 la plus faible (1 mg/litre pour Daphnia magna). Chez
les poissons, la CL50 la plus faible est également celle de
l' o-xylčne (7,6 mg/litre chez la truite arc-en-ciel, par mesure de
concentration). Des valeurs de 7,9 et 1,7 mg/litre ont été obtenues,
respectivement pour le m- et le p-xylčne, dans le cas de la perche
commune (d'aprčs la concentration nominale). On ne dispose que de
données limitées au sujet de l'exposition chronique des organismes
aquatiques aux xylčnes, mais, quoi qu'il en soit, la volatilisation
rapide de ces composés la rend peu probable. Le xylčne ne présente
qu'une faible toxicité aiguė pour les oiseaux.
RESUMEN
El xileno es un hidrocarburo aromįtico del que hay tres formas
isoméricas: orto, meta y para. El xileno de calidad técnica contiene
una mezcla de los tres isómeros y algo de etilbenceno. Se estima que
la producción mundial fue de 15,4 millones de tone-ladas en 1984. La
presión de vapor estį comprendida entre 0,66 y 0,86 kPa para los tres
isómeros. Aproximadamente un 92% de las mezclas de xilenos se combinan
con el petróleo. El producto se emplea también en diversos
disolventes, en particular en las industrias de fabricación de
pinturas y de tintas de imprenta.
La mayor parte del xileno liberado en el medio ambiente pasa
directamente a la atmósfera. En ésta los isómeros de xileno se
degradan con facilidad, principalmente por fotooxidación. Los tres
isómeros se volatilizan rįpidamente en la atmósfera a partir del agua.
En el suelo y el agua los isómeros meta y para se biodegradan
fįcilmente en una amplia variedad de condiciones aerobias y
anaerobias, pero el isómero orto es mįs persistente. Las limitadas
pruebas disponibles parecen indicar que la bioacumulación de los
isómeros de xileno por los peces y los invertebrados es baja. La
eliminación del xileno de los organismos acuįticos es bastante rįpida
a partir del momento en que se interrumpe la exposición.
Normalmente los niveles basales medios de los tres isómeros de
xileno en el aire ambiente son de aproximadamente 1 µg/m3, pero en
zonas suburbanas se hallan en torno a 3 µg/m3. Se han detectado
concentraciones mayores en zonas urbanas e industrializadas, con
niveles medios de hasta 500 µg/m3. No obstante, las concentraciones
son por lo general inferiores a 100 µg/m3.
La exposición diaria por inhalación estimada en la población
general es de 70 µg en zonas rurales y de menos de 2000 µg en zonas
urbanas. La concentración en el agua potable estį comprendida entre
valores indetectables y 12 µg/litro. Los datos disponibles sobre la
concentración en los alimentos son insuficientes para poder estimar la
exposición oral diaria.
Las concentraciones basales medias de xilenos en aguas
superficiales son generalmente inferiores a 0.1 µg/litro. Sin embargo
se han hallado valores mucho mįs altos en zonas industriales y en
zonas vinculadas a la industria petrolera (hasta 30 µg/litro en aguas
contaminadas y hasta 2000 µg/litro en las proximidades de tuberķas de
desagüe). Se ha informado del hallazgo de niveles basales similares
en aguas subterrįneas, aunque se han detectado también concentraciones
elevadas, atribuidas a contaminación localizada a partir de tanques de
almacenamiento y tuberķas subterrįneos.
Tras la exposición por inhalación la retención pulmonar es de un
60% de la dosis inhalada. El xileno es metabolizado eficientemente.
Mįs del 90% se biotransforma en įcido metilhipśrico, que se excreta
por la orina. El xileno no se acumula de forma significativa en el
organismo humano.
La exposición aguda a altas concentraciones de xileno puede
afectar al SNC y causar irritación en el hombre. Sin embargo, no se
han llevado a cabo ni estudios controlados a largo plazo en el ser
humano ni estudios epidemiológicos. La toxicidad crónica parece
relativamente baja en animales de laboratorio. Hay indicios, no
obstante, de que concentraciones moderadas de xileno pueden tener
efectos crónicos sobre el SNC en animales.
El xileno no parece tener efectos mutįgenos ni carcinógenos.
El parįmetro crķtico es la toxicidad para el desarrollo,
demostrada a niveles de exposición de 870 mg/m3 (200 ppm) en la rata.
Teniendo en cuenta este parįmetro, la concentración indicativa
recomendada para el xileno en el aire es de 0.87 mg/m3 (0,2 ppm).
Los isómeros de xileno poseen una toxicidad entre moderada y baja
para los organismos acuįticos. En invertebrados la CL50 mįs baja,
calculada a partir de las concentraciones medidas, es de 1 mg/litro
para el o-xileno (Daphnia magna). Los valores mįs bajos de CL50
detectados en peces son de 7,6 mg/litro para el o-xileno (trucha
arco iris; segśn las concentraciones medidas), y de 7,9 y 1,7 mg/litro
para los m- y p-xilenos respectivamente (ambos para la lubina
estriada; segśn las concentraciones nominales). La información
disponible respecto a la exposición crónica de organismos acuįticos a
los xilenos es limitada; no obstante, su rįpida volatilización hace
improbable la exposición crónica en el agua. La toxicidad aguda del
xileno para las aves es baja.
6.3.2 In laboratory animals
Most studies on metabolism of xylenes have been performed on rat.
The principal pathway involves side-chain oxidation to methylbenzoic
acid via methylbenzyl alcohol and methylbenzyl aldehyde.
Methylbenzoic acid is then conjugated with glycine or glucuronic acid
(Sugihara & Ogata, 1978; Ogata et al., 1980; Elovaara et al., 1984).
Conjugation with glycine to form methylhippuric acid predominates for
m- and p-xylene (Sugihara, 1979; Ogata & Fujii, 1979; Elovaara et
al., 1984). In the case of o-xylene, glucuronide formation has been
reported to predominate (Ogata et al., 1980).
A separate minor pathway resulting in urinary excretion of
thioethers has been studied (Van Doorn et al., 1980; Van Doorn et al.,
1981). This pathway appears to be more important for o-xylene than
for the other isomers. Hydroxylation of the aromatic ring with the
formation of dimethylphenols has been reported to be another minor
metabolic pathway in rats (Bakke & Scheline, 1970; Elovaara et al.,
1984).
Methylbenzoic acid and dimethylphenols are present in urine of
guinea-pigs and rabbits exposed to xylene isomers (Fabre et al.,
1960). After oral dosing of rabbits with xylenes the main metabolite
was methylbenzoic acid (Bray et al., 1949). The acid was considered
to be mostly present as the glycine conjugate, methylhippuric acid.
Studies with isolated perfused livers and lungs from rabbit
indicate differences in the metabolic pathways between these two
organs (Smith et al., 1982). In the liver p-methylhippuric acid was
the major metabolite detected. In the lung p-methylbenzyl alcohol
and p-methylbenzoic acid were the main metabolites detected. There
was formation of 2,5-dimethylphenol in the lungs but not in the liver.
Metabolism of m- and p-xylenes to m- and p-methylbenzyl alcohols
has been found to be greater with hepatic than with pulmonary
microsomes (Harper, 1975; Toftgård et al., 1986). Further metabolism
to methylbenzoic acid occurred in the presence of hepatic but not
pulmonary cytosolic fraction. Daily exposures to xylene increased the
activities of liver microsomal enzymes and concentrations of
cytochrome P450 (Elovaara et al., 1982; Pathiratne et al., 1986).
Metabolism of m-xylene by cerebral microsomal preparations was
reported to be very slow (Elovaara et al., 1982).
No definitive studies have been reported showing which microsomal
P-450 enzymes are involved in xylene metabolism. However when rats
were given m-xylene (1.0-1.4 ml/kg body weight) by gastric
intubation once daily for 3 consecutive days and killed 24 h after the
last treatment, xylene caused an induction of CYP2B and CYP2E1 in
liver microsomes (Raunio et al., 1990). Exposure of male Wistar rats
to each of the xylene isomers by inhalation at a concentration level
of 4000 mg/m3 for 20 h/day over 4 days similarly induced hepatic
CYP2B1, while CYP2E1 was reduced, as estimated by Western blots (Gut
et al., 1993).
6.4 Elimination and excretion
6.4.1 In humans
Absorbed xylenes are excreted mainly as metabolites in urine.
Small amounts are excreted unchanged in exhaled air. Excretion in
faeces appears to be unimportant. The rate of clearance of p-xylene
from blood has been calculated to be 2.6 litres/kg per hour at 87
mg/m3 (20 ppm) and 1.6 litres/kg per hour at 304 mg/m3 (70 ppm)
(Wallén et al., 1985).
When volunteers were exposed to a constant concentration of
about 391-870 mg/m3 (90-200 ppm) m-xylene over 5 days, at least 97%
was calculated to be excreted as m-methylbenzoic acid conjugates.
2,4-Dimethylphenol conjugates accounted for 1-2% of the metabolites
(Riihimäki et al., 1979a; Riihimäki et al., 1979b). When volunteers
were exposed to about 195 mg/m3 (45 ppm) of o-, m- or p-xylene
for 8 h, about 95-99% of the dose was excreted as methylhippuric acid
in urine. Dimethylphenol excretion was estimated to be 0.1 to 2% of
the dose absorbed (Sedivec & Flek, 1976). About 90% of the absorbed
dose of m-xylene was excreted as methylhippuric acid after exposure
to 435 mg/m3 (100 ppm) for 4 h (Lauwerys et al., 1978; Campbell et
al., 1988). On the other hand, after exposure to 600 mg/m3 (138 ppm)
of o-xylene, only 46% was excreted in urine as methylhippuric acid
and only trace amounts of the o-methylbenzoyl glucuronide were
detected (Ogata et al., 1980).
In a study of 121 male workers engaged in dip-coating of metal
parts, the mean concentration was 3.48 mg/m3 (0.8 ppm) o-xylene,
9.1 mg/m3 (2.1 ppm) m-xylene and 3.91 mg/m3 (0.9 ppm) p-xylene.
The workers were also exposed to 0.8 ppm toluene and 0.9 ppm
ethylbenzene. At the end of the 8 h-shift urine samples were
collected and methylhippuric acid was determined. There was a linear
relationship between the intensity of exposure to xylenes and the
concentration of methylhippuric acid in urine. The methylhippuric
acid concentration as a function of increasing xylene concentration
was 17.8 mg/litre per ppm (Kawai et al., 1991a).
In workers occupationally exposed to an average of 17.4 mg/m3
(4 ppm) xylene (combination of all three isomers), with a maximum of
more than 430 mg/m3 (100 ppm), the urinary excretion of
methylhippuric acid was linearly correlated with the air exposure
(Huang et al., 1994).
The urinary methylhippuric acid excretion of xylene-exposed
painters at the end of the working week showed two distinct phase of
excretion. Half-times for urinary excretion of methylhippuric acid
were estimated to be 3.6 h for the first 10 h and 30.1 h for the next
2 days after exposure (Engström et al., 1978). A positive association
between the degree of obesity and the length of half-time was seen.
In volunteers exposed to m-xylene the urinary excretion of
m-methylbenzoic acid was described as triphasic with half-times of
1-2, 10 and 20 h (Riihimäki et al., 1979a). The observed elimination
half-time in the subcutaneous adipose tissue was about 58 h (Engström
& Riikimäki, 1979).
After oral administration of o-xylene (39 mg/kg body weight)
maximum urinary levels of glycine and glucuronide conjugates of
o-methylbenzoic acid were reported to be 33.1 and 1.0% of the
administered dose, respectively. Similar values were obtained after
an oral dose of 78 mg/kg body weight (Ogata et al., 1979; Ogata et
al., 1980).
About 4-5% of the dose absorbed in the lungs is exhaled unchanged
after exposure to 870 mg/m3 (200 ppm) xylene (Sedivec & Flek, 1976,
Åstrand et al., 1978, Riihimäki et al., 1979a, Riihimäki et al.,
1979b). Elimination in exhaled breath is reported to follow a similar
triphasic profile to that for urinary excretion of methylbenzoic acid
conjugates (Riihimäki et al, 1979). An initial half-time of about one
hour was obtained in a study by Campbell et al., 1988.
6.4.2 In laboratory animals
Exhalation of unchanged m-xylene has been described in one
study on rats. Exhalation was greatest 4 h after an intraperitoneal
injection, and 13% of the dose was exhaled unchanged within 10 h
(Sugihara, 1979). In mice 3.4% of the dose was exhaled within 8 h
(Bergman, 1979; Bergman, 1983).
In rats a total of 46% of the m-xylene dose (5 mmol/kg body
weight) was excreted as m-methylbenzoic acid within 24 h, and
phenobarbital (PB) treatment increased it to 70% of the dose. PB
treatment increased the elimination of m-methylbenzoic acid after
oral administration about 4-fold in the first 3 h, more then 2-fold in
the first 12 h, and 1.5-fold within 24 h compared to untreated but
m-xylene-exposed rats (Gut & Flek, 1981).
Wistar rats were pretreated with PB for 3 days (80 mg/kg body
weight per day) and then given m-xylene orally or intraperitoneally
at a small (0.081 mmol/kg) or a large (0.81 mmol/kg) dose or by
inhalation (6 h) at a low (174 mg/m3, 40 ppm) or high (1740 mg/m3,
400 ppm) concentration. PB treatment had a significant effect on the
metabolism of inhaled m-xylene (decreased blood m-xylene and
increased urinary excretion of m-methylhippuric acid), but only at
the high dose. The PB-induced enzyme induction had an effect at both
dose levels on the metabolism of orally administered m-xylene. The
effect on intraperitoneally administered m-xylene was more similar
to that of inhaled than that of orally administered m-xylene (Kaneko
et al., 1995).
After an intraperitoneal injection of 87-348 mg/kg body weight
m-xylene to rats, 53-75% of the dose was excreted as m-methyl-
hippuric acid in urine during 24 h (Ogata & Fujii, 1979). After
an intraperitoneal dose of 319 mg/kg body weight the proportion
excreted as mercapturic acids was calculated to be 10% for o-xylene
and 0.6-1.3% for m- and p-xylene (Van Doorn et al., 1980).
Male rats were exposed to soil-adsorbed (sandy soil or clay soil)
or pure 225 µl of m-xylene containing 20 µCi of m-[14C]-xylene
through the skin (Skowronski et al., 1990). The major route of
excretion in the pure and sandy groups was via expired air followed by
urine. However, in the presence of clay soil, the percentage of the
initial dose in expired air was similar to that in urine. In the
presence of clay soil, an increase in m-xylene was observed in
adipose tissue. Methyl hippuric acid was the main urinary metabolite.
Turkall et al. (1992) reported the bioavailability of
soil-adsorbed m-xylene in male and female rats. The rats were
gavaged with an aqueous suspension of 5% of gum acacia containing 150
µl of m-xylene with 5 µCi m-[14C]-xylene alone or adsorbed to
sandy or clay soil. While ingested soil contaminated with m-xylene
produced a higher bioavailability than the chemical alone in females,
no effects of soil was observed in males. No differences in the
bioavailability of m-xylene alone were observed between the sexes.
m-Xylene was primarily metabolized and excreted in urine,
methylhippuric acid being the main urinary metabolite in all groups.
6.5 Factors affecting toxicokinetics in humans and animals
In humans, co-exposure of m-xylene and ethylbenzene and
consumption of ethanol or aspirin (acetylsalicylic acid) prior to
inhalation has been shown to reduce urinary excretion of one or more
xylene metabolites, including methylhippuric acid (Riihimäki et al.,
1982a,b; Engström et al., 1984; Campbell et al., 1988). In the case
of ethanol consumption an increase in concentration of m-xylene in
blood was described (Riihimäki et al., 1982a,b). Co-exposure to
toluene decreased the ratio of the concentration of p-xylene in
venous blood to that in exhaled air (Wallén et al., 1985). The dermal
absorption of liquid m-xylene was reduced in the presence of
isobutanol (Riihimäki, 1979a).
Volunteers given ethanol on two evenings (total dose 137 g)
preceding exposure by inhalation to either 435 or 1740 mg/m3 (100 or
400 ppm) m-xylene for 2 h enhanced the metabolism of m-xylene but
only at 1740 mg/m3 (Tardif et al., 1994). Ethanol pretreatment
decreased the concentration of m-xylene in blood and alveolar air
during and after exposure and increased urinary excretion of
m-methylhippuric acid at the end of exposure to 1740 mg/m3.
Five male volunteers were exposed for 7 h/day over 3 consecutive
days to 50 ppm toluene, 174 mg/m3 (40 ppm) xylene (15% o-xylene,
25% m-xylene and 60% p-xylene) or a combination of both. To study
high-level exposure four men were exposed for 4 h to 95 ppm toluene or
348 mg/m3 (80 ppm) xylene or a combination of both. Mixed exposure
(low-level) did not alter the concentration of the solvents in blood
or exhaled air, nor did it modify the excretion of urinary
metabolities. High-level mixed exposure, however, increased the
concentration of solvents in blood and exhaled air and caused a delay
in the urinary excretion of hippuric acid (Tardif et al., 1991).
Simultaneous exposure by inhalation to toluene and xylene (15%
o-xylene, 60% m-xylene and 25% p-xylene) has been studied in
Sprague-Dawley rats. Exposure time was 5 h and the concentrations
were 75 ppm toluene plus 979 mg/m3 (225 ppm) xylene, 150 ppm toluene
plus 652 mg/m3 (150 ppm) xylene or 225 ppm toluene plus 326 mg/m3
(75 ppm) xylene. Compared to exposure to a single solvent,
simultaneous exposure resulted in lower amounts of excreted urinary
hippuric and methylhippuric acids over 24 h. Increased concentrations
of solvents in blood and brain were found immediately post-exposure.
Simultaneous exposure also enhanced the pulmonary elimination of both
solvents (Tardif et al., 1992).
From a toxicokinetic modelling study it was concluded that there
is competitive metabolic inhibition between m-xylene and toluene in
the rat (Tardif et al., 1993a). The interaction is likely to be
observed when exposure exceeds 50 ppm of each solvent (Tardif et al.,
1993b).
Inhalation exposure of rats for 4 h to 2436 mg/m3 (560 ppm)
m-xylene or 320 ppm of ethylbenzene alone, or in combination,
indicated that in the combined exposure blood and brain m-xylene
concentrations increased by 52 and 40%, respectively, whereas there
was no corresponding effect on ethylbenzene concentrations (Frantik &
Vodickova, 1995).
In a study of metabolic interaction, Wistar rats were exposed
for 6 h to 1305 mg/m3 (300 ppm) m-xylene, 600 ppm methyl ethyl
ketone (MEK) or a mixture of both. After mixed exposure the
cytochrome P-450-dependent monooxygenase activities were additively or
synergistically induced. In the presence of MEK the overall
metabolism of m-xylene was inhibited, as shown by an increase in
xylene concentration in blood and fat and a decrease in the 24-h
excretion of xylene metabolites (Liira et al., 1991).
Male Wistar rats were exposed by inhalation for 4 h to 1000
mg/m3 (230 ppm) of o-xylene or 1700 ppm of acetone alone, or in
combination. In the combination exposure, blood xylene immediately
after the exposure was increased by 40% whereas the blood acetone
level was decreased by 15%. In a corresponding study, H-strain mice
were exposed for 2 h to 1392 mg/m3 (320 ppm) of o-xylene or 6655
mg/m3 (1530 ppm) of acetone alone or in combination. The combination
exposure was accompanied by a 33% increase of the blood xylene
concentration whereas the blood acetone level was decreased by 18%
(Vodickova et al., 1995).
6.6 Biological monitoring
More than 90% of xylene is transformed in human metabolism to
methylhippuric acid and excreted in urine. Urinary methylhippuric
acid has proved a robust measure of the amount of xylene taken up in
the body over some preceding hours. The measurement has been
routinely used in some countries for assessment of individual exposure
to xylene in the occupational setting. About 1.5-2 g methylhippuric
acid per g creatinine in the post-shift urine sample corresponds to
exposure to 435 mg/m3 (100 ppm) xylene over a full workday (Lauwerys
& Buchet, 1988). Different workloads cause variation in the excreted
amounts of methylhippuric acid at a given exposure level since the
lung uptake of xylene is directly proportional to pulmonary
ventilation. Biotransformation of xylene to methylhippuric acid is
inhibited in the presence of ethanol and acetylsalicylic acid (see
section 6.5); these interfering factors need to be controlled when the
method is applied.
The analysis of xylene in blood and exhaled air can be used for
assessment of exposure (Lauwerys & Buchet, 1988). These methods may
be particularly suitable for estimating xylene body burden caused by
the low and relatively stable background exposure in the general
population, or, in case of accidental exposure, for measuring levels
for a clinical toxicological evaluation.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Inhalation studies
7.1.1.1 o-Xylene
The LC50 value for o-xylene in Sprague-Dawley rats (12
males/group) was calculated to be 4330 ppm (95% confidence limits
4247-4432 ppm) for a 6-h exposure. The reported signs of intoxication
were hypotonia and somnolence. Autopsy on surviving animals 14 days
later revealed no macroscopic lesions of lung, liver or kidneys
(Bonnet et al., 1982). Under similar conditions the LC50 value for
mice (OF-1) was 4595 ppm (4468-4744 ppm) (Bonnet et al., 1979).
Rats (probably Wistar, sex not stated, 10 animals/group) were
exposed to 1531, 3062 or 6125 ppm for 24 h. No deaths occurred at
1531 ppm, one death at 3062 ppm and 8 deaths at 6125 ppm (Cameron et
al., 1938). In the same study one group was exposed to 12 250 ppm
o-xylene for 12 h. Two deaths occurred, one after 2 h. Autopsy of
those animals that died did not reveal any macroscopic or microscopic
lesions in the organs studied (Cameron et al., 1938). In the same
study mice (10 animals/group) were exposed to the same concentrations
for the same period of time as the rats. There were no deaths at 1531
ppm, four deaths at 3062 ppm and nine deaths at 6125 ppm. In the
group exposed to 12 250 ppm for 12 h there were two deaths, one after
9 h. The organs of animals that died after exposure showed no
characteristic changes (Cameron et al., 1938).
In a study of the effect on prenarcotic motor behaviour, groups
of eight male rats (CFY) were exposed to o-xylene (dose not stated)
for 1, 2, 3 or 4 h. No significant effects on group motor activity
were observed. Narcosis occurred at higher concentrations with a
threshold of 2180 ppm for a 4-h exposure (Molnar et al., 1986). Mice
(strain, sex and numbers not stated) were exposed for 2 h to
o-xylene in order to determine the minimum concentration needed for
an animal to fall on its side and die. The minimum concentration for
falling was 3400-4600 ppm and for death 6900 ppm (Lazarew, 1929).
In a study of subnarcotic effects of solvents in Wistar rats and
H-strain mice, using electrically evoked seizures, the lowest
effective concentration of o-xylene was 170 ppm. The criterion used
was a significant suppression by 10% of the generation and maintenance
of the seizure discharge after 4 h (rats) or 2 h (mice) of inhalation
(Frantik et al., 1994). A 30% suppression was induced by o-xylene
at a concentration of 390 ppm, corresponding to a blood concentration
of 62 µmol/litre (Frantik et al., 1993).
In order to determine the RD50 value (exposure level reducing
the respiratory rate by 50%), groups of male mice (OF-1) were exposed
to o-xylene. The RD50 value was calculated to be 1467 ppm
(1406-1530 ppm). The onset of response was rapid and the maximum
decrease in respiratory rate was reached within a few minutes (De
Ceaurriz et al., 1981).
In a study of conditioned behaviour in mice, an increased
response rate was observed after a 30-min exposure to 1400-2000 ppm
o-xylene. At higher concentrations there was a decrease in response
rate with an EC50 of 5179 ppm. The biphasic response indicates that
there was excitation of the central nervous system at low
concentrations and depression at higher concentrations (Moser et al.,
1985).
In a study to observe effects in the "behavioural despair"
swimming test, groups of 10 mice (OF-1) were exposed to 0, 1010, 1101,
1207 or 1234 ppm o-xylene for 4 h. The ID50 (50% decrease in
immobility) value was calculated to be 1127 ppm (1068-1182 ppm) (De
Ceaurriz et al., 1983). The study demonstrated subnarcotic effects by
o-xylene on the CNS.
7.1.1.2 m-Xylene
After a 6-h exposure to m-xylene the LC50 in rats and mice was
reported to be 5984 ppm (5796-6181 ppm) and 5267 ppm (5025-5490 ppm),
respectively (Bonnet et al., 1979; Bonnet et al., 1982). The signs of
toxicity consisted of hypotonia, somnolence, narcosis, and clonic
spasms leading to death due to respiratory failure (Lazarew, 1929;
Cameron et al., 1938, Bonnet et al., 1982; Moser et al., 1985; Molnar
et al., 1986).
In the comparative study by Frantik et al., (1994) described in
section 7.1.1.1, the lowest effective air concentration for m-xylene
was 210 ppm.
A biphasic CNS response occurred at similar exposure levels to
those seen for o-xylene (section 7.1.1.1) (Moser et al., 1985). The
narcotic threshold in rats of about 2100 ppm m-xylene determined in
prenarcotic behaviour studies was similar to that observed for
o-xylene (Molnar et al., 1986). During a 4-h exposure to 8000 ppm
m-xylene, 10 out of 12 rats (Carwoth-Wistar) died (Smyth et al.,
1962).
When groups of rats (Wistar; 6 males/group) were exposed for 24 h
to 0, 75, 150 or 300 ppm m-xylene, there was a significant decrease
in cytochrome P-450 concentrations at all doses, and a dose-related
decrease in 7-ethoxycoumarin-O-deethylase activity in the lung. No
abnormalities of the lungs, as determined by scanning electron
microscopy, were seen in two animals exposed to 300 ppm (Elovaara et
al., 1987).
When male Wistar rats were exposed for 4 h to a 1:1 mixture of
m-xylene and n-butyl alcohol or to the single substances at
500-4000 ppm, there were disturbances of rotarod performance. The
medial effective concentrations (EC50) were calculated to be 3080 ppm
(mixture), 6530 ppm ( n-butyl alcohol) and 1980 ppm (xylene)
respectively. The combined exposure gave less than additive effects
(Korsak et al., 1993).
A concentration-dependent decrease in respiratory rate in mice
was demonstrated in a study where mice were exposed to 500-4000 ppm of
n-butyl alcohol, m-xylene or a 1:1 mixture of both. The RD50 was
calculated to be 3010 ppm, 1360 ppm and 3140 ppm, respectively. The
combined exposure gave less than additive effects (Korsak et al.,
1993).
The acute neurobehavioural effect of m-xylene was evaluated
after 20 min inhalation exposure using a functional observations
battery in mice. In the concentration range of 2000-8000 ppm, these
effects included changes in posture, decreased arousal and rearing,
increased ease of handling, disturbances of gait, mobility and
righting reflex, decreased forelimb grip strength, increased landing
foot splay and impaired psychomotor coordination. The response to
various sensory stimuli was also decreased. These acute effects were
short-lived, recovery beginning within minutes of removal from the
exposure chamber (Tegeris & Balster, 1994).
7.1.1.3 p-Xylene
A 4-h LC50 value in rats (female Sprague-Dawley) of 4740 ppm
p-xylene has been reported (Drew & Fouts, 1974). The corresponding
6-h value was 4591 ppm (4353-5049 ppm) (Bonnet et al., 1982), and for
mice (OF-1) was 3907 ppm (3747-4015 ppm) (Bonnet et al., 1979). The
signs of toxicity were similar to those reported for the other two
isomers.
Like the other two isomers, exposure to p-xylene gave a
biphasic CNS response (Moser et al., 1985). In another study (Molnar
et al., 1986), marked activation and tremor were observed at
concentrations between 400 and 1500 ppm p-xylene in rats. The
narcotic threshold was 1940 ppm.
When Sprague-Dawley rats (16 females/group) were exposed for 4 h
to 0, 1000, 1500 or 2000 ppm p-xylene, a dose-dependent increase in
serum enzymes activites was observed. This was taken as a sign of
hepatocellular and hepatobiliary damage (Patel et al., 1979). In
other studies at higher exposure levels, no microscopic hepatic
lesions were seen in rats or mice (Cameron et al., 1938; Furnas &
Hine, 1958; Bonnet et al., 1982).
When Long Evans rats were exposed for 4 h to 0, 800 or 1600 ppm
p-xylene, the flash-evoked potential of the visual system was
reduced at the highest exposure level (Dyer et al., 1988). The
authors suggested that this may have been secondary to changes in
arousal or excitability. In a study of the effect on learning tasks
and motor activity, rats (Long Evans) were exposed to 0 or 1600 ppm
p-xylene for 4 h. Signs of toxicity (unsteadiness and fine tremor)
disappeared 30 min post-exposure. The results indicate an effect on
motor control rather than on cognitive capacity (Bushnell, 1989).
In the comparative study by Frantik et al. (1994) described above
(section 7.1.1.1), where rats and mice were exposed, the lowest
effective air concentration for p-xylene was 220 ppm.
After a 4-h exposure to 1000 ppm p-xylene there was a
decrease in pulmonary cytochrome P-450 in rabbits and a decrease in
NADPH-cytochrome c-reductase and mixed-function oxidase activity in
rats (Patel et al., 1976; Patel et al., 1978).
When Sprague-Dawley rats were exposed to 3400 ppm p-xylene for
4 h and killed 12 h later, an induction of cytochrome P-450 activities
(CYP2B) in liver microsomes was observed. On the other hand, the
pulmonary cytochrome P-450 activities were inhibited (Day et al.,
1992).
In rats (Sprague-Dawley) p-xylene (2800 ppm for 4 h) has been
shown to potentiate hepatotoxicity induced by bromobenzene, while the
effect of bromobenzene on pneumotoxicity was unaffected by p-xylene,
indicating differences in xylene metabolism between the liver and the
lung (Day et al., 1992).
7.1.1.4 Technical or undefined xylene
In rats LC50 values of 6350, 6700 and 10 950 ppm have been
reported after 4-h exposure (Hine & Zuidema, 1970; Carpenter et al.,
1975; Lundberg et al., 1983). Deaths occurred during the exposure
period. No LC50 values for mice have been found, although
experiments have been carried out at up to 7000 ppm for 30 min (Moser
et al., 1985). Signs of toxicity were the same as those produced by
the individual isomers.
No signs of toxicity were reported when rats and dogs were
exposed for 4 h to 580 and 530 ppm, respectively. The composition was
7.63% o-xylene, 65.01% m-xylene, 7.84% p-xylene and 19.27%
ethylbenzene (Carpenter et al., 1975). The same type of response at
similar exposure levels as for the three individual isomers was
observed in mice in a conditioned behavioural study (Moser et al.,
1985). In rats effects of xylene were studied on an operant behaviour
maintained by a fixed-ratio liquid reinforced schedule. A decrease
in the reinforcement rate was seen after exposure to 113 ppm for 2 h
(Ghosh et al., 1987; Ghosh & Pradhan, 1987).
When rats (Fischer F-344) were exposed to 1450 ppm xylene for 8
h, a slight increase in the auditory response threshold at 20 kHz was
noted (Pryor et al., 1987). The xylene composition was 10%
o-xylene, 80% m-xylene and 10% p-xylene.
In mice (Swiss-Webster) exposed to 1300 ppm xylene for one
minute, a decrease in respiratory rate as an indication of respiratory
tract irritation was seen (Carpenter et al., 1975). This effect was
not seen at an exposure level of 460 ppm. No histological
abnormalities were seen on histological examination of livers from
rats exposed to 5480 ppm for 4 h, nor were there any changes in serum
activities of an indicator enzyme (sorbitol dehydrogenase) for
hepatotoxicity after exposure to > 340 ppm (Lundberg et al.,
1986). No histological abnormalities were seen in cat livers after
exposure to about 9500 ppm for up to 2 h (Carpenter et al., 1975). In
dogs (Beagle) 1200 ppm xylene for 4 h caused lacrimation but there was
no noticeable effect at 530 ppm (Carpenter et al., 1975).
When groups of cats (five animals/group) were exposed for 5740,
6900 or 9200 ppm for up to 6 h, there was a concentration-dependent
decrease in time to onset of staggering and mild narcosis. There was,
however, a large individual variation. Deep narcosis was seen in four
animals at 9200 ppm xylene (Engelhardt & Estler, 1935).
7.1.2 Other exposure routes
The oral LD50 values in rat have been reported to be 3608 mg/kg
body weight for o-xylene, 5011 mg/kg body weight for m-xylene and
4029 mg body weight for p-xylene (Smyth et al., 1962). For various
mixtures of xylenes oral LD50 values in rat have been reported to be
between 3523 and 8700 mg/kg body weight (Wolf et al., 1956; Hine &
Zuidema, 1970; NTP, 1986). In mice the corresponding values were
reported to be 5627 mg/kg body weight for male and 5251 mg/kg body
weight for females (NTP, 1986). Signs of toxicity at lethal doses
were CNS depression and congestion of cells in liver, kidney and
spleen, seen by histological examination.
A dermal LD50 value for rabbits (New Zealand White) of 12 180
mg/kg has been reported for a 24-h exposure to m-xylene (Smyth et
al., 1962). From studies with intraperitoneal (i.p.), intravenous
(i.v.), subcutaneous (s.c.) and intramuscular (i.m.) administration in
rats and mice the acute toxicity of xylene is low (Bell et al., 1992).
All three isomers have been found to interfere with the
modulation of the vestibular-oculomotor pathways in the rat. The
blood threshold levels for this effect were 170-200 µg/ml following
administration by the i.v. route (Tham et al., 1984). Similar effects
have also been reported for m-xylene in rabbit. The
vestibular-oculomotor effects were seen at blood levels of 30 µg/ml
and some deaths due to respiratory stress were recorded at 100 µg/ml
(Larsby et al., 1976; Aschan et al., 1977; Ödkvist et al., 1979;
Ödkvist et al., 1980).
A dose-dependent depletion of hepatic glutathione, following i.p.
administration, has been demonstrated in rats. With o-xylene the
effect was seen from 50 mg/kg and with the other two isomers from 425
mg/kg (Van Doorn et al., 1980). A decrease in pulmonary cytochrome
P-450 levels has been observed with the three isomers, when
administered (i.p.) at 531 mg/kg (Pyykkö et al., 1987).
The CYP2B1 and CYP1A1 isoenzyme activities, benzyloxy-resorufin
O-deethylation and ethoxyresorufin O-deethylation, respectively,
were studied in nasal, pulmonary and hepatic tissues of rats injected
intraperitoneally with m-xylene. Tissues taken at 2, 12 and 24 h
after injection showed inhibition of these activities in both nasal
and pulmonary microsomes, but increased activities in hepatic
microsomes (Blanchard & Morris, 1994).
The effects of intraperitoneal administration of o-xylene
(1g/kg body weight) on: (a) rat hepatic and pulmonary mixed-function
oxidase content and activity; and (b) microsomal membrane structural
parameters were studied 1, 3, 6 and 12 h after administration (Park et
al., 1994). The pulmonary cytochrome P-450 content and aryl
hydrocarbon hydroxylase activity were decreased, a maximal inhibition
occurring 3 h after dosing. Reduced pulmonary activity for both
ethoxyresorufin O-dealkylation and benzyloxyresorufin
O-dealkylation was noted. In contrast, increased hepatic cytochrome
P-450 content was noted, with slightly increased ethaxyresorufin
O-dealkylation and markedly increased benzyloxyresorufin
O-dealkylation. An increase in pulmonary microsomal phospholipid
content and cholesterol content was noted even 1 h after dosing. In
liver the phospholipid content increased although there was no change
in cholesterol content; this suggested an increase in membrane
fluidity.
Sprague-Dawley rats were given m-xylene (1 g/kg body weight)
intraperitoneally and killed one hour after treatment. Microsomes
from the lung were then prepared. Compared to controls, m-xylene
administration decreased the CYP2B1 activity but did not alter the
CYP1A1 activity or epoxide hydrolase activity. In total, m-xylene
administration resulted in an inhibition of benzo (a)pyrene
detoxication and increased production of toxic metabolites in the
pulmonary microsomal preparations (Stickney et al., 1991).
Xylenes have been shown to inhibit the hypotonic haemolysis of
erythrocytes in vitro at low concentrations. The EC50 values were
29, 39 and 44 µg/ml for o-xylene, m-xylene and p-xylene,
respectively (Holmberg et al., 1974).
7.2 Short-term exposure
7.2.1 Inhalation studies
7.2.1.1 o-Xylene
In a study on the noradrenaline and dopamine levels in various
parts of the forebrain and hypothalamus, Sprague-Dawley rats (six
males/group) were exposed to 0 or 2000 ppm o-xylene 6 h/day for 3
days. The animals were killed within 18 h after final exposure. There
was a significant increase in catecholamine levels and turnover in
various parts of the hypothalamus and a decrease in the dopamine
turnover in the forebrain of exposed animals (Andersson et al., 1981).
In order to study the effects on cytochrome P-450 and enzyme
activities Sprague-Dawley rats (four males/group) were exposed to 0 or
2000 ppm o-xylene, 6 h/day for 3 days. There was a significant
increase in relative liver weight and cytochrome P-450 content in
exposed animals. Furthermore, there were increases in some liver
enzyme activities. In lungs, there was a decrease in cytochrome P-450
activity (Toftgård & Nilsen., 1982).
When guinea-pigs (15 per group) were exposed to 0 or 780 ppm
o-xylene, 8 h/day, 5 days/week for 6 weeks, there was a marked
decrease in body weight gain in exposed animals. No effects on the
liver, kidney, heart, spleen or lung were observed upon histological
examination (Jenkins et al., 1970). In the same study beagle dogs
were exposed for the same period of time. One dog out of two
experienced tremors throughout the exposure period. No other signs of
toxicity were reported (Jenkins et al., 1970). Squirrel monkeys were
also exposed in the same manner. One monkey out of three died on day
seven. No other signs of toxicity were reported (Jenkins et al.,
1970).
7.2.1.2 m-Xylene
In a study of levels of noradrenaline and dopamine in various
parts of the forebrain and hypothalamus, Sprague-Dawley rats (six
males/group) were exposed to 0 or 2000 ppm m-xylene, 6 h/day for 3
days. The animals were killed within 18 h after the last exposure. A
significant increase in catecholamine levels and turnover was observed
in various parts of the hypothalamus of exposed animals. There was no
effect on dopamine levels (Andersson et al., 1981).
In another study, Sprague-Dawley rats (four males/group) were
exposed to 0 or 2000 ppm m-xylene for 3 days. The effect on
cytochrome P-450 and enzyme activities in liver, kidney and lung were
studied. In exposed animals there was a significant increase in
relative liver weight, in cytochrome P-450 content in liver and
kidney, and in some enzyme activities in these two organs. The
content of pulmonary cytochrome P-450 was decreased (Toftgård &
Nilsen, 1982).
In a study of the effect on xenobiotic metabolism in Wistar rats,
10 males per group were exposed to 0, 50, 400 or 750 ppm m-xylene, 6
h/day, 5 days/week for one week. At the two highest doses there
was a significant increase in hepatic microsomal protein and
NADPH-cytochrome c reductase levels, and a decrease in hepatic
glutathione levels. There was no effect on hepatic cytochrome P-450
levels or on renal glutathione levels. At all dose levels there was a
significant increase in renal cytochrome P-450 and some enzyme
activities. When the animals were exposed to the same regimen for 2
weeks similar results were obtained. There were no abnormalities upon
histological examination of the liver (Elovaara, 1982). When Wistar
rats (20 males/group) were exposed to 0 or 300 ppm m-xylene 6 h/day,
5 days/week for one week there was, in exposed groups, a significant
increase in hepatic and renal 7-ethoxycoumarin O-deethylase activity
and in renal UDP-glucuronyl transferase. When exposure time was 2
weeks there was also a significant increase in hepatic cytochrome
P-450 and NADPH-cytochrome c reductase activities (Elovaara et al.,
1982).
In order to study the effect on lung cytochrome P-450 in rats,
six male Wistar rats/group were exposed to 0 or 300 ppm m-xylene 7
h/day, 4 days/week for 5 weeks. The only effects seen were a
significant decrease in cytochrome P-450 content and 7-ethoxycoumarin
O-deethylase activity (Elovaara et al., 1987). When the effects on
cerebral biochemistry was studied, groups of Wistar rats (15
males/group) were exposed to 0, 50, 400 or 750 ppm m-xylene 6 h/day,
5 days/week for 1 or 2 weeks. The only effect seen after one week of
exposure was a significant decrease in glutathione levels at all
concentrations. After 2 weeks there were also a dose-dependent
decrease in superoxide dismutase activity, a significant increase in
NADPH-diaphorase activity at all concentrations and a significant
increase in azoreductase activity at the two highest concentrations
(Savolainen & Pfäffli, 1980).
7.2.1.3 p-Xylene
In a study of levels of noradrenaline and dopamine in the
forebrain and hypothalamus, Sprague-Dawley rats (six males/group) were
exposed to 0 or 2000 ppm p-xylene 6 h/day for 3 days. The animals
were killed 16-18 h after the last exposure. In exposed animals there
was a significant increase in catecholamine levels and turnover in
various parts of the hypothalamus. There was no effect on dopamine
levels or turnover in the forebrain (Andersson et al., 1981).
In order to study the effect on cytochrome P-450 and enzyme
activities in the liver, kidney and lung, Sprague-Dawley rats (four
males/group) were exposed to 0 or 2000 ppm 6 h/day for 3 days. In
exposed animals there were significant increases in relative liver
weight, in hepatic cytochrome P-450 content, in NADPH-cytochrome c
reductase activity in the liver and kidney, and in 7-ethoxyresorufin
O-deethylase activity in the kidney. There was also a decrease in
pulmonary cytochrome P-450 content (Toftgård & Nilsen, 1982). To
study the lung microsomal activity, rabbits (New Zealand White; four
males/group) were exposed to 0 or 1000 ppm p-xylene 4 h/day for 2
days. In exposed animals there was a significant decrease in
microsomal cytochrome P-450 concentration and in NADPH-cytochrome c
reductase activity (Patel et al., 1978). Inhibition of CYP2B1 has
been observed in the lungs of rats dosed with p-xylene (Verschoyle
et al. 1993).
Rats exposed to 300 ppm p-xylene, 6 h/day for 1, 3 or 5 days
exhibited alterations in pulmonary microsomal membrane structural and
metabolic parameters (Silverman & Schatz, 1991). Following 1 day of
exposure, conjugated diene levels were elevated while total
phospholipid levels, cytochromes P-450 content, benzyloxyresorufin
O-dealkylase activity and 2-aminofluorene N-hydroxylase activity
were decreased. Core membrane fluidity was increased following 3 days
of exposure. After 5 days of exposure all parameters returned to
control levels with the exception of aryl hydrocarbon hydroxylase
activity, which was increased by 41%. Extracellular surfactant levels
were also decreased after 1 and 3 days of exposure but returned to
control values after 5 days. The increase in aryl hydrocarbon
hydroxylase activity after 5 days of exposure could have important
consequences on the metabolism of co-administered xenobiotics.
Male Fischer-344 rats exposed to 0 or 1600 ppm p-xylene by
inhalation, 6 h/day, for 1 or 3 days did not produce overt
hepatotoxicity but resulted in a significant increase in the
concentration of hepatic cytochrome P-450 (Simmons et al., 1991).
However, the concentration of hepatic cytochrome P-450 had returned to
control levels within 2 to 3 days after exposure.
p-Xylene has been shown to decrease axonal transport of
proteins and glycoproteins in rats (Long-Evans) exposed by inhalation
to 1600 ppm for 6 h/day, 5 days/week, for 8 days. When ethanol (10%
in drinking-water) was given during 6 days prior to inhalation of
p-xylene, the treatment prevented the decreased axonal transport.
Ethanol per se did not decrease the axonal transport (Padilla et
al., 1992).
When 10 Wistar rats and 10 mice (strain not given) were exposed
to 1226 ppm p-xylene 8 h/day for 14 days, no animals died. No other
results were reported (Cameron et al., 1938).
When mice were exposed to 1200 ppm p-xylene 6 h/day for 4 days
and infected with a sublethal dose of murine cytomegalovirus, 34%
mortality occurred, whereas no deaths occurred among uninfected,
p-xylene-exposed mice or infected, air-exposed mice (Selgrade et
al., 1993). Although p-xylene potentiated liver damage caused by
the virus, the magnitude of serum enzyme activities indicated that
this damage was not the probable cause of death. Enhanced mortality
was related to enhanced xylene toxicity due to suppression of
cytochrome P-450, although additive or synergistic damage to tissues
other than liver could not be ruled out. There was no indication that
p-xylene had caused immune suppression.
7.2.1.4 Technical or undefined xylene
When rats (strain not defined) were exposed to 620, 980 or 1600
ppm xylene 18-20 h/day for 7 days, instability, incordination and
narcosis were observed at the two highest concentrations. Signs of
mucous membrane irritation occurred, and congestion and cloudy
swelling of kidneys was reported at 980 ppm (Batchelor, 1927).
Similar results were reported in another study (Winslow, 1927).
Groups of Harlan-Wistar rats (25 males/group) were exposed to 0,
180, 460 or 810 ppm xylene 6 h/day, 5 days/week for 13 weeks. The
xylene consisted of 7.63% o-xylene, 65.01% m-xylene, 7.84%
p-xylene and 19.27% ethylbenzene. At 3, 7 and 13 weeks 3, 3 and 4
animals, respectively, were killed. No treatment-related
histopathology was seen (Carpenter et al., 1975).
In a study of levels of noradrenaline and dopamine in the
forebrain and hypothalamus of rats, the xylene mixture used was 2.0%
o-xylene, 64.5% m-xylene, 10.0% p-xylene and 23.0% ethylbenzene.
Sprague-Dawley rats (6 males/group) were exposed to 0 or 2000 ppm
xylene 6 h/day for 3 days. The animals were killed 16-18 h after the
last exposure. There was a significant increase in catecholamine
levels and turnover in the hypothalamus and a significant increase in
dopamine levels and turnover in the forebrain (Andersson et al.,
1981).
In order to study the effect on levels of neurotransmitters in
the rat brain, five to six Sprague-Dawley rats/group were exposed to
0, 200, 400 or 800 ppm xylene (mixture not defined) for 30 days.
Acetylcholine levels in the striatum were decreased at > 400 ppm.
Noradrenaline levels in the hypothalamus were increased significantly
at the highest dose. From 400 ppm the cAMP levels were decreased in
the striatum, and at 800 ppm the glutamine levels in the midbrain were
increased. At all concentrations glycine and GABA levels in the
midbrain were increased (Honma et al., 1983).
When 12 male Fischer F-344 rats/group were exposed to 0 or 1450
ppm xylene 8 h/day for 3 days, there was, in exposed animals, an
increase in the auditory response threshold at 12 and 20 kHz.
Rats (Long Evans) were exposed to 2500 ppm of mixed xylenes 6
h/day for 5 days. Testing of auditory function was conducted 5 to 8
weeks after exposure using reflex modification audiometry (RMA). The
results indicated increased RMA thresholds for the mid-frequency tones
(e.g., 8, 16 and 24 kHz) but not for higher or lower tones (Crofton et
al., 1994).
In another study Sprague-Dawley rats (four males/group) were
exposed to 0 or 630 ppm xylene 6 h/day, 5 days/week for 4 weeks. The
animals were killed the morning after the last exposure. In exposed
animals there was a significant decrease in body weight gain, while
the absolute and relative liver weights were increased. There was an
increase in hepatic cytochrome P-450 and the xylene was shown to act
as a phenobarbital-like inducer of cytochrome P-450. The xylene used
was 2.0% o-xylene, 64.5% m-xylene, 10.0% p-xylene and 23.0%
ethylbenzene (Toftgård et al., 1981).
When male Sprague-Dawley rats (8-12 animals/group) were exposed
to 0, 75, 250, 500, 1000 or 2000 ppm xylene 6 h/day for 3 days there
was a dose-dependent increase in the concentration of liver microsomal
cytochrome P-450. When animals were exposed to the two highest
concentrations for 5 days, there was a dose-dependent increase in the
surface area of smooth endoplasmic reticulum but not in rough
endoplasmic reticulum. Based on their results the authors concluded
that xylene causes phenobarbital-type induction in the liver. The
xylene used was the same as that in the previous paragraph (Toftgård
et al., 1983).
The same type of xylene was also used in a study where four male
Sprague-Dawley rats/group were exposed to 0 or 2000 ppm xylene 6 h/day
for 3 days. In exposed animals there were significant increases in
hepatic and renal cytochrome P-450 content, in NADPH-cytochrome c
reductase activities and 7-ethoxyresorufin O-deethylase activities
in liver and kidney. The pulmonary cytochrome P-450 content was
decreased. Increased enzyme activity in liver and kidney and decreased
activity in the lung were thus observed (Toftgård & Nilsen, 1982).
Groups of male Wistar rats (20 animals/group) were exposed to 0
or 300 ppm xylene 6 h/day, 5 days/week for up to two weeks. In each
group 10 animals had 15% v/v ethanol in the drinking-water. There was
a significant decrease in motor activity in exposed animals. In
addition, there was a significant increase in brain DT-diaphorase
activity, in acid proteinase and in hepatic and renal 7-ethoxycoumarin
O-deethylase activities. Concurrent dosing with ethanol had a
marked synergistic effect on hepatic and renal 7-ethoxycoumarin
O-deethylase activities. The xylene used was 80% m-xylene and 12%
p-xylene (Savolainen et al., 1979a,b).
In a study of the effect of simultaneous exposure to xylene
(undefined) and noise on metabolic activity in the myocardium, male
rats (strain not specified) were exposed to 0 or 69 ppm xylene 4 h per
day, 5 days per week for 6 weeks, and simultaneously to a noise level
of 0, 46, 85 or 95 dB. There were changes in some enzyme activities,
but the brief reporting makes the study impossible to evaluate
(Ivanovich et al., 1985).
When Beagle dogs (four males per group) were exposed to 0, 180,
460 or 810 ppm xylene 6 h per day, 5 days per week for 13 weeks, no
treatment-related effects were reported. The xylene used was 7.63%
o-xylene, 65.01% m-xylene, 7.84% p-xylene and 19.27% ethylbenzene
(Carpenter et al., 1975).
7.2.2 Other exposure routes
Decreased body weight compared to controls was noted in rats
(Sprague-Dawley) administered orally 1062 mg m-xylene/kg body weight
for 3 days (Pyykkö, 1980), and reduced terminal body weight was
observed in rats (Fischer F-344) administered 500 mg m-xylene/kg
body weight, 5 days per week, for 4 weeks (Halder et al., 1985).
Three days administration of 1062 mg m-xylene/kg body weight
resulted in significant increases in liver weight, liver cytochrome
P-450 and cytochrome b5 levels and NADPH cytochrome c reductase and
MFO enzymes activities. The same range of increases was also seen in
the kidney (Pyykkö, 1980). In another study, however, there were no
effects on kidney weight and no treatment-related abnormalities
histopathologically in Fischer rats given 500 or 2000 mg/kg body
weight 5 days per week for 4 weeks (Dési et al., 1967).
Upon oral administration of 0, 125, 250, 500, 1000 or 2000 mg
xylene per kg body weight to rats (F-344/N), in a 14-day study, a high
mortality was seen in the highest dose group. The xylene used was
9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. The body weight gain was reduced in males at > 250
mg/kg body weight and in females at 125 mg/kg body weight and
> 1000 mg/kg body weight. There were no treatment-related
abnormalities at gross necropsy (NTP, 1986). In the same study xylene
was administered at 0, 62.5, 125, 250, 500 or 1000 mg per kg body
weight, 5 days per week, for 13 weeks. No treatment-related
abnormalities were seen during gross necropsy or histopathological
examination (NTP, 1986).
Daily administration (s.c.) of xylene (undefined) for up to 4
weeks to rats (strain not given) resulted in significant mortality at
870 mg/kg body weight but not at 435 or 174 mg/kg body weight.
Repeated administration of 435 mg/kg body weight resulted in decreased
learning rate (Dési et al., 1967). When Sprague-Dawley rats were
given 0 or 3123 mg/kg body weight for 3 days, there was a significant
increase in liver weight, cytochrome P-450 levels, NADPH cytochrome c
reductase and activity of MFO enzymes. The xylene used was 30%
o-xylene, 55% m-xylene and 15% p-xylene (Pathiratne et al.,
1986).
In order to study further the effects of p-xylene, rats were
given p-methylbenzyl alcohol (PMBA) or 2,5-dimethylphenol (DMP)
(300 mg/kg body weight and 150 mg/kg body weight, respectively)
intraperitoneally once a day for 3 days. It was concluded that of the
two metabolites, PMBA may have a significant role in the inhibition of
pulmonary cytochrome P-450 caused by p-xylene (Day & Carlson, 1992).
Male Sprague-Dawley rats (n=10) were given xylene (isomer not
stated) intraperitoneally, as a single dose per day, for 3 consecutive
days. The dose given was equal to half the LD50 (1.6 ml/kg body
weight per day). Only slight somnolence was observed. After the last
dosing the animals were killed and aminopeptidase activities in
several regions of the brain were measured. The activities were
largely unaffected, compared to those of controls (De Gandarias et
al., 1993).
Brain cell cultures enriched in astroglial cells were prepared
from neonatal Sprague-Dawley rats. The cultures were exposed for 1 h
to 3, 6 or 9 mmol o-xylene/litre. The ATPase activity was reduced
in a dose-dependent manner (Naskali et al., 1994).
7.3 Long-term exposure
A short summary of long-term studies is presented in Table 6.
In Wistar rats exposed to 1000 ppm m-xylene for 6 h/day,
5 days/week for 3 months or to 100 ppm for 6 months, slight
ultrastructural changes (proliferation of smooth endoplasmic
reticulum) were found in hepatocytes. When rats were exposed to a 1:1
combination of m-xylene and toluene (500 plus 500 ppm or 50 plus 50
ppm), the changes were a combination of those of each single solvent
(Rydzynski et al., 1992). The combined exposure at both exposure
levels gave more pronounced disturbances in a rotarod performance test
and decrease in spontaneous motor activity compared to single solvent
exposure. In animals exposed to 500 plus 500 ppm for 3 months a
decrease in red blood cell count and an increase in rod neutrophil
cell count were observed (Korsak et al., 1992).
In a six-week study, groups of rats (Sprague-Dawley or
Long-Evans; 15 animals/group) were exposed to 0 or 780 ppm o-xylene
(8 h/day, 5 days/week) or to 78 ppm continously for 90 days. There
was no effect on body weight gain, leukocyte count, haemoglobin level
or heamatocrit. Histological examination of the liver, kidney, heart,
spleen and lung revealed no effects (Jenkins et al., 1970).
Table 6. Effects of xylenes in long-term studies
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Inhalation exposure
o-Xylenea rat, dog, 13 weeks 780 - Jenkins et al., 1970
guinea-pig
o-Xylenea monkey 13 weeks + 78 - Jenkins et al., 1970
m-Xylenea rat 3 months - 1000 liver cell changes (i.e. inc. smooth Rydzynski et al., 1992
endoplasmic reticulum, lysosomes)
m-Xylenea rat 6 months - 100 liver cell changes (i.e. inc. smooth Rydzynski et al., 1992
endoplasmic reticulum, lysosomes)
m-Xylenea rat 3 months - 100 dec. lymphocyte/monocyte count; Korsak et al., 1992
dec. rotorod, performance
m-Xylenea rat 6 months - 100 dec. rotorod, performance Korsak et al., 1992
dec. spontaneous motor activity
Xylenes rat 3 months 50 100 dec. rotorod performance Korsak et al., 1994
dec. spontaneous motor activity
Xylenes rat 6 months 346 923 liver effects (inc. liver weight inc. Ungvary, 1990
smooth endoplasmic reticulum, inc.
P-450 activity)
Table 6. (Cont'd)
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Inhalation exposure
Xylenes rat 13 weeks 810 - Carpenter et al., 1975
Xylenes dog 13 weeks 810 - Carpenter et al., 1975
Xylenes rat 6 weeks - 800 ototoxicity Pryor et al., 1987
(14h/day)
Xylenes rat 61 days - 1000 ototoxicity Nylen & Hagman, 1994
(18 h/day)
Xylenes rat 18 weeks - 300 liver microsomes activity Elovaara et al., 1980
Xylenes rat 18 weeks - 300 brain superoxide dismutase activity Savolainen et al., 1979
Xylenes rat 13 weeks + - 320 brain lipid composition changes Kyrklund et al., 1987
Xylenes gerbil 3 months 160 320 change in astroglial cell marker Rosengren et al., 1986
proteins
Table 6. (Cont'd)
Compound Species Exposure NOEL LOEL End-point Reference
(ppm) (ppm)
Oral exposure
Xylenes rat 13 weeks 1000 - NTP, 1986
Xylenes mouse 13 weeks 2000 - NTP, 1986
Xylenes rat 13 weeks - 150 increased liver weights (males); Condie et al., 1988
hyaline droplet nephropathy (males)
Xylenes rat 2 years 250 500 mortality NTP, 1986
Xylenes mouse 2 years 500 - NTP, 1986
a Only one dose was used
b Continous exposure
The effects of combined exposure to m-xylene and n-butyl
alcohol have been studied in rats exposed to the individual solvents
at 50 and 100 ppm and their 1:1 mixture at 50 plus 50 ppm and 100 plus
100 ppm, 6 h/day, 5 days/week for 3 months (Korsak et al., 1994). The
results indicate less than an additive toxic effect (motor
coordination disturbances) of combined exposure to m-xylene and
n-butyl alcohol. For xylene alone an effect was seen at 100 ppm but
not at 50 ppm.
Rats (CFY) were exposed to xylene (10% o-xylene, 50%
m-xylene, 20% p-xylene and 20% ethylbenzene) for 8 h/day up to 6
months at concentrations of 600, 1500 or 4000 mg/m3. No macroscopic
changes were seen but the relative liver weight was increased at 4000
mg/m3. At 4000 mg/m3 hypertrophy of the centrilobular zone in
the liver, including a change in the amount of smooth and rough
endoplasmic reticulum, was seen. As a result of xylene exposure the
hexobarbital sleeping time decreased. Liver enzymatic activities were
increased during the first 6 weeks. After the 4-week exposure-free
period the changes described above could no longer be seen. The same
type of changes could also be seen when xylene was applied orally,
subcutaneously or intraperitoneally for a short period of time (4-7
day). Essentially the same changes as those described for rat liver
were also seen in livers from mice and rabbits (Ungvary, 1990).
Groups of 50 male and 50 female Fischer-344/N rats received 0,
250 or 500 mg xylene/kg body weight. The xylene used was 9.1%
o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0% ethylbenzene.
The doses were given (in corn oil) by stomach tube on 5 days per week
for 103 weeks. The animals were killed within 14 days after the last
dosing. Body weights of high-dose (500 mg/kg body weight) males were
5 to 8% lower than those of vehicle controls. Results for low-dose
males and both female groups were comparable to those of controls.
Gross observation and histopathological results showed no incidences
of non-neoplastic effects in dosed groups, related to the
administration of xylene, at any sites (NTP, 1986; Huff et al., 1988).
In the same study groups of 50 male and 50 female B6C5F1 mice
received 0, 500 or 1000 mg xylene/kg body weight. The same type of
technical xylene was used. The doses were given, in corn oil, by
stomach tube 5 days per week for 103 weeks, after which the animals
were killed within 14 days. No significant difference in mean body
weights or survival was observed between treated animals and controls.
Gross observation and histopathological results indicated that at no
site were the incidences of non-neoplastic effects in dosed groups
related to the administration of xylene (see also section 7.7).
Male Fischer-344 rats were exposed to 0, 800, 1000 or 1200 ppm
xylene 14 h/day, 7 days/week for 6 weeks. At the highest dose level
there was a slight impairment of auditory (but not of visual or
somatosensory) conditioned avoidance response. All animals had
increased auditory response thresholds compared to controls at the
same frequencies. At 1000 and 800 ppm the thresholds were elevated at
16 kHz and 8 kHz and at 1200 ppm the brainstem auditory-evoked
response thresholds were elevated at 4, 8 and 16 kHz tone frequency.
The xylene used in these two studies was 10% o-xylene, 80%
m-xylene and 10% p-xylene (Pryor et al., 1987).
Wistar rats (60 males/group) were exposed to 0 or 300 ppm xylene
6 h per day, 5 days per week, for up to 18 weeks, and 50% of the
animals had 15-20% ethanol in the drinking water. The xylene used was
19.2% o-xylene, 43.0% m-xylene, 19.5% p-xylene and 18.3%
ethylbenzene. It was concluded that concurrent ethanol intake
increased hepatic and renal microsomal enzyme activities. Stearosis
in the liver was more marked in co-exposed animals than in animals
exposed only to ethanol. No treatment-related abnormalities were
observed during histopathological examination of animals exposed only
to xylene (Elovaara et al., 1980).
Exposure to 1000 ppm xylene (not defined) 18 h per day, 7 days
per week, for 61 days caused a slight loss of auditory sensitivity in
Sprague-Dawley rats. Co-exposure to n-hexane (1000 ppm) caused
a persistent loss of auditory sensitivity, which was less than
additively enhanced. Xylene inhibited n-hexane-induced impulse
velocity reduction in peripheral nerves (Nylén & Hagman, 1994).
In a study to investigate the effect on brain lipid composition,
Sprague-Dawley rats were exposed to 0 or 320 ppm xylene continuously
for 30 or 90 days. The xylene (undefined) produced only limited
transient changes (Kyrklund et al., 1987). In another study to
investigate the effect on brain with and without co-exposure to
ethanol, Wistar rats (20 males/group) were exposed to 0 or 300 ppm
xylene 6 h per day, 5 days per week, for up to 18 weeks. In each
group were 10 animals also exposed to 15% v/v ethanol in the
drinking-water. There was a significant increase in cerebral
microsomal superoxide dismutase activity by week 18, but no effect on
cerebral protein or RNA levels. Co-exposure to ethanol reduced the
effect caused by xylene exposure. The xylene used was 7.5%
o-xylene, 85.0% m-xylene and 7.5% p-xylene (Savolainen et al.,
1979a,b).
In a study of the effect on spinal cord axon membrane, Wistar
rats (five animals per group) were exposed to 0 or 300 ppm xylene
(undefined) 6 h per day, 5 days per week, for 18 weeks. In exposed
animals there was a decrease in the amount of membrane lipid per mg of
protein but no change in the cholesterol/lipid phosphorus ratio.
Ethanol (15% v/v) in the drinking-water enhanced the decrease and also
decreased the cholesterol/lipid phosphorus ratio (Savolainen &
Seppäläinen, 1979).
In a neurotoxicity study, changes in two astroglial cell marker
proteins (S-100 and GFA) and DNA were measured. Mongolian gerbils
(four of each sex per group) were exposed to 0, 160 or 320 ppm xylene
continously (24 h/day) for 3 months, followed by a 4-month
exposure-free period. The xylene used was 18% o-xylene, 70%
m-xylene, 12% p-xylene and < 3% ethylbenzene. The exposure to
xylene resulted in brain damage which was manifest as an increase in
astroglial cells. Effects were seen in particular parts of the brain
at both exposure levels but were only significant at 320 ppm
(Rosengren et al., 1986).
Ageing Long-Evans rats fed 200 ppm o-xylene for up to 6 months
showed formation of vacuolar structures in hepatocytes when examined
ultrastructurally (Bowers & Cannon, 1982).
Groups of 10 male and 10 female Sprague-Dawley rats were exposed
to mixed xylenes by gavage (in corn oil) for 90 consecutive days at
dose levels of 150, 750 and 1500 mg/kg body weight per day (Condie et
al., 1988). The most significant findings were increased relative
liver and kidney weights. Histopathology evaluation of liver and
kidney tissues revealed an increased incidence of minimal chronic
renal disease in females only. Treatment-related hepatic
histopathological changes were not detected in either sex. Hyaline
droplet formation was observed in male rats in all treated groups. In
females, there was an increased incidence of kidney effects, which
were thought to represent onset of progressive nephropathy (Condie et
al., 1988).
Mice (B6C3F1) were given orally 0, 250, 500, 1000 or 2000 mg
xylene per kg body weight for 14 days or 0, 125, 250, 500, 1000 or
2000 mg per kg body weight, 5 days per week, for 13 weeks. The xylene
used was 9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. Mean body weight gain was reduced in males at > 250
mg/kg body weight. No treatment-related abnormalities were observed
at gross necropsy or histopathological examination (NTP, 1986).
7.4 Skin and eye irritation; sensitization
Erythema and oedema were induced following a single application
(amount not stated) of xylene (not defined) to rabbit or guinea-pig
skin. There was a rapid onset and epithelial desquamation, with some
evidence of necrosis occurring after several days (Rigdon, 1940;
Steele & Wilhelm, 1966). After 24 h of exposure to 0.5 ml xylene
(undefined) under semi-occlusive conditions, irritation of rabbit skin
was observed. The irritation was graded as moderate by the authors
(Hine & Zuidema, 1970).
Application of 0.5 ml p-xylene, under a Teflon chamber, to
rabbit skin for 4 h produced a response which would be classified as
irritant using European Union criteria (Jacobs et al., 1987). In
another study, following 10 to 20 applications of xylene (not defined)
to open or semi-occluded rabbit skin for up to four weeks, a moderate
to marked irritation and moderate necrosis was reported, as was
blistering of the skin under the semi-occlusive dressing (Wolf et al.,
1956).
Application of approximately 0.05 to 0.5 ml of liquid xylenes
(individual isomers or undefined composition) to the rabbit eye was
reported to cause immediate discomfort and blepharospasm followed by
slight conjunctival irritation and very slight, transient corneal
necrosis (Wolf et al., 1956). Application of 0.1 ml liquid xylene
(not defined) was mildly irritant to rabbit eye. The lesions were not
described (Kennah et al., 1989).
No skin sensitization studies have been reported.
7.5 Reproductive and developmental toxicity
A summary of reproductive and developmental toxicity studies is
shown in Table 7.
Groups of female rats (CFY) were exposed to 0, 34, 345 or 690 ppm
o-xylene 24 h/day from day 7 to day 14 of gestation. The dams were
killed on day 21. Some ultrastructural changes in the liver and
decreased weight gain during the exposure period were observed in the
dams exposed to 345 or 690 ppm. At these concentrations lower fetal
body weight was observed, and at the highest dose level delayed
skeletal ossification was noted. There was no evidence of
malformations. Similar results were obtained when the animals were
exposed to m-xylene. With p-xylene there were signs of delayed
skeletal ossification at exposure levels showing no maternal toxicity
(at 34 and 345 ppm). At 690 ppm, increased postimplantation loss of
fetuses and more retarded fetuses were observed (Ungvary et al.,
1980).
Table 7. Reproductive and developmental effects (inhalation studies)
Compound Species Exposure Duration NOEL LOEL Endpoint Reference
o, m, p-Xylene rat 0, or 1000 24 h/day; Fused sternebrae, Hudak & Ungvary
mg/m3 days 9-14 extra ribs (1978)
Xylene rat 0, 10, 50 or 6 h/day; Maternal toxicity not Mirkova et al.
(not specified) 500 mg/m3 days 1-21 addressed (1983)
Xylene rat 0, 250, 1900 24 h/day; 250 mg/m3 Skeletal retardation Ungvary & Tatrai
(not specified) or 3400 days 7-15 (mothers and (1985)
mg/m3 offspring)
o-xylene rat 0, 150, 1500 24 h/day; 150 mg/m3 150 mg/m3 Decreased fetal weight, Ungvary et al.
or 3000 days 7-14 offspring (dams) skeletal retardation (1980)
mg/m3 1500 mg/m3
(offspring)
m-xylene rat 0, 150, 1500 24 h/day; 1500 mg/m3 3000 mg/m3 Maternal: reduced food Ungvary et al.
or 3000 days 7-14 (dams and (dams and consumption, reduced (1980)
mg/m3 offspring) offspring) weight gain
Offspring: reduced fetal
weight
p-xylene rat 0, 150, 1500 24 h/day; 150 mg/m3 Reduced mean litter Ungvary et al.
or 3000 day 7-15 (dams and size; reduced fetal (1980)
mg/m3 offspring) weight; skeletal
retardation
p-xylene rat 0 or 3000 10th day, Decreased fetal weight Ungvary et al.
mg/m3 24 h (1981)
Table 7. (Cont'd)
Compound Species Exposure Duration NOEL LOEL Endpoint Reference
p-xylene rat 0, 3500 or 6 h/day; 7000 7000 mg/m3 Maternal: reduced Rosen et al.
7000 mg/m3 days 7-16 mg/m3 (dams) weight gain (1986)
(offspring),
3500
mg/m3
(dams)
Xylene rat 0 or 600 24 h/day; Decreased maternal Ungvary (1985)
(not defined) mg/m3 day 7-15 weight gain.
Delayed fetal
development; extra ribs
Xylene rat 870 mg/m3 6 h/day; No maternal toxicity; Hass & Jakobsen
(not defined) days 4-20 delayed ossification (1993)
o, m, p-Xylene rat 2175 mg/m3 6 h/day; Delayed righting reflex; Hass et al. (1995)
days 7-20 reduced absolute brain
weight; impaired
neuromotor ability
Xylene mouse 0, 500 or 24 h/day; 500 mg/m3 1000 mg/m3 Skeletal retardation and Ungvary & Tatrai
(not specified) 1000 mg/m3 days 6-15 offspring (offspring) increased incidence of (1985)
weight-retarded fetuses
Xylene rabbit 0, 500 or 24 h/day; 500 mg/m3 Maternal: decreased Ungvary & Tatrai
(not specified) 1000 mg/m3 days 7-20 (offspring) weight gain (1985)
1000 mg/m3 Offspring: delayed
(dams) skeletal development
Rats (CFY) were exposed to 0.58, 437 or 782 ppm 24 h/day on days
7 to 15 of gestation and the dams were killed on day 21. Data
concerning maternal toxicity was not given. There was delayed
skeletal ossification at all dose levels, while decreased fetal
bodyweight, increased postimplantation loss and increased frequency of
skeletal variants (extra ribs) were observed at 782 ppm. Mice (CFLP)
were exposed to 0 or 115 ppm o-xylene for 4 h, 3 times per day on
day 6 to day 15 of gestation, and the dams were killed on day 18.
Data concerning maternal toxicity were not reported. There was
evidence of delayed weight gain and skeletal ossification in the
fetuses of exposed animals. Similar results were obtained with
m-xylene and p-xylene (Ungvary & Tatrai, 1985). When rabbits (New
Zealand White) were exposed to 0 or 115 ppm o-xylene 24 h/day from
day 7 to day 20 of gestation, no maternal toxicity or incidence of
delayed development was observed in the exposed group. Similar
results were observed with m-xylene, but an increased incidence of
post-implantation loss was observed. Exposure to 115 ppm p-xylene
gave the same results as with o-xylene, but exposure to 230 ppm
p-xylene 24 h/day resulted in no live fetuses (one dam died, three
aborted and in four there was total resorption or fetal death in
utero) (Ungvary & Tatrai, 1985).
In a study to validate a developmental toxicity screen, mice
(ICR/SIM) were exposed orally to 0 or 2000 mg m-xylene/kg body
weight from day 8 to day 12 of gestation. No effects were seen on
mothers or young (Seidenberg et al., 1986). In another study
Sprague-Dawley rats were exposed to 0, 800 or 1600 ppm p-xylene, 6 h
per day from day 7 to day 16 of gestation. The dams were allowed to
deliver their young. In the highest dose group the maternal weight
gain was significantly reduced. Exposure to p-xylene had no effects
on postnatal viability, offspring growth or function of the nervous
system (activity level and acoustic startle response) at any of the
doses tested (Rosen et al., 1986).
In an attempt to study the effect of xylene on sex steroids
during pregnancy, rats (CFY) were exposed to 0 or 681 ppm p-xylene
for 24 h on day 10 of gestation or continuously on days 9 and 10 of
gestation. The animals were killed on day 11. Data on maternal
toxicity were not reported. Sex hormone levels in the uterine and
femoral veins were decreased in the exposed group. The authors
(Ungvary et al., 1981) suggested that this may play a role in the
embryotoxicity.
Rats (CFY) were exposed to 0 or 230 ppm xylene for 24 h/day from
day 9 to day 14 of gestation, and the dams were killed on day 21. The
xylene used was 10% o-xylene, 50% m-xylene, 20% p-xylene and 20%
ethylbenzene. No maternal effects were seen in exposed animals.
There was an increased incidence of skeletal variants such as extra
ribs and fused sternebrae. Three malformations were found (2
agnathia, 1 fissura sterni), but there was no significant increase in
the frequency of malformations (Hudak & Ungvary, 1978).
In another study rats (CFY) were exposed to 0 or 138 ppm xylene
(not defined) 24 h/day from day 7 to day 15 of gestation. The dams
were killed on day 21. In the exposed group, decreased maternal
weight gain, delayed fetal development and increased incidence of
skeletal variants (extra ribs) were observed, but there was no
evidence of malformations (Ungvary, 1985).
In a report issued by the American Petroleum Institute in 1983,
described by Bell et al. (1992), Sprague-Dawley rats were exposed to a
xylene mixture of 20.4% o-xylene, 44.2% m-xylene, 20.3% p-xylene
and 12.8% ethylbenzene. There were 30 males and 60 females in the
control group, while 10 males and 20 females per group were exposed to
60 or 250 ppm xylene and 20 males and 40 females were exposed to 500
ppm xylene. Exposures were 6 h per day, 7 days per week, for a
131-day pre-mating period and a 20-day mating period. Mated females
were also exposed during days 1-20 of gestation and days 5-20 of
lactation. For males there were no effects on body weight gain, but
for females the mean body weight gain was significantly greater than
that of controls in the 60 and 250 ppm groups during the mating
period. This was not considered indicative of an adverse effect of
treatment. Mating indices were significantly lower than control
values at 250 ppm (both sexes treated) and at 500 ppm (females treated
only), but mating indices were comparable to control values at 500 ppm
when both sexes were treated or when males alone were treated. There
were no treatment-related effects on mean duration of gestation, mean
litter size or pup survival. The mean pup weight in the group where
both parents had been exposed to 500 ppm xylene was significantly
lower than for controls. In the teratogenicity part of the study
there was no evidence of an increased incidence of malformations in
exposed groups. The mean fetal weights for the 500 ppm group were
lower than the control value, but, the difference was statistically
significant for only the female fetuses.
Groups of Wistar rats were exposed to 0, 2, 11 or 114 ppm xylene
(mixed, not defined) for 6 h per day from day 1 to day 21 of
gestation. The maternal toxicity was not reported. The incidence of
post-implantation loss and fetal death was significantly increased in
the 11 ppm and 114 ppm groups. At the highest dose an increase in
some malformations was noted but no incidence was given. The results
of the study cannot be evaluated because of insufficient reporting of
exposure conditions and results (Mirkova et al., 1983). In an attempt
to repeat this study, groups of 36 Wistar rats were exposed to 200 ppm
technical xylene 6 h per day on days 4 to 20 of gestation. There were
no signs of maternal toxicity. No exposure-related differences were
found except for delayed ossification of maxillary bone. The
xylene-exposed pups had a slightly higher body weight and impaired
performance in a motor ability test, which was most marked in female
offspring (Hass & Jakobsen, 1993).
In a follow-up study (Hass et al., 1995), Wistar rats were
exposed to 500 ppm technical xylene (19% o-xylene, 45% m-xylene,
20% p-xylene and 15% ethylbenzene) for 6 h per day on gestation days
7-20. The dose level was selected so as not to induce maternal
toxicity or decrease the viability of offspring. There were 15
exposed litters and 13 control litters. A delay in the development of
the air righting reflex, a lower absolute brain weight and impaired
performance in behavioural tests for neuromotor abilities, learning
and memory were found in the offspring of the exposed rats.
Generally, the effects were most marked in the female offspring. The
alterations were long-lasting, as they were still apparent in adult
rats at the age of 4 months.
A composition of 9.1% o-xylene, 60.2% m-xylene, 13.6%
p-xylene and 17.0% ethylbenzene was given orally to CD-1 mice. The
doses were 0, 520, 1030, 2060, 2580, 3100 or 4130 mg per kg body
weight daily from day 6 to day 15 of gestation. All animals in the
highest dose group died and so did 50% of the animals in the 3100
mg/kg group. In this group there was a significant increase in the
incidence of dams with complete resorptions. There was a significant
increase in the incidence of cleft palate at > 2060 mg/kg, as well
as decreased mean fetal weight (Marks et al., 1982).
In order to study the teratogenic and embryotoxic effects of
xylene (60% p-xylene, 22% o-xylene, 18% ethylbenzene; no
m-xylene) embryos of Sprague-Dawley rats were explanted on day 9.5
of gestation and cultured in rat serum to which xylene (0.1, 0.5 or
1.0 ml/litre) dissolved in DMSO was added. The embryos were cultured
for 48 h. There were no observable teratogenic effects in terms of
malformations. However, dose-dependent embryotoxicity in terms of
retardation on growth and development was observed (Brow-Woodman et
al., 1991). In a similar study (Brown-Woodman et al., 1994) rat
embryos were incubated in vitro with up to 2.7 µmol xylene/ml for
40 h. Concentrations of > 1.89 µmol/ml retarded embryo growth and
development. The no-observed-effect level (NOEL) was 1.08 µmol/ml.
No gross morphological malformations were observed. Combined exposure
to toluene, xylene and benzene caused additive embryotoxic effects
with no evidence of synergistic action (Brown-Woodman et al., 1994).
When Sprague-Dawley rats were exposed to 1000 ppm xylene (not
defined) 18 h per day, 7 days per week, for 61 days, testicular
atrophy or loss of nerve growth factor-immunoreactive germ cell line
was not observed. Xylene also was found to protect from
n-hexane-induced testicular atrophy (Nylén et al., 1989).
Wistar rats were exposed for 7 days to xylene (isomer not stated)
twice a day until disappearance of righting reflex (xylene
concentration not given). Anaesthesia was achieved in about 10 min.
On day 8 the rats were killed. A decrease in body weight and weights
of testes and accessory reproductive organs, as well as reduced acid
phosphate activity in the prostate and reduced plasma testosterone
levels, was observed in xylene-exposed animals. There was also a
decrease in spermatozoan count in the epididymis (Yamada, 1993).
7.6 Mutagenicity and related end-points
Technical grade xylene did not produce differential killing in
DNA-repair-proficient compared to repair-deficient strains of
Bacillus subtilis rec+/- (McCarroll et al., 1981a) or Escherichia
coli (McCarroll et al., 1981b). Xylene (type not specified) did not
induce SOS activity in Salmonella typhimurium TA1535/pSK 1002
(Nakamura et al., 1987). For E. coli WP2 uvr A p-xylene was not
mutagenic in the presence or absence of an exogenous metabolic system
from PCB-induced rat liver (Shimizu et al., 1985). None of the
isomers nor unspecified xylene was mutagenic to S. typhimurium
TA1535, TA1537, TA98, TA100, UTH 8413 or UTH8414 in the presence or
absence of a metabolic system from uninduced or Arochlor-induced rat
and hamster livers (Lebowitz et al., 1979; Bos et al., 1981; Haworth
et al., 1983; Connor et al., 1985; Shimizu et al., 1985; Zeiger et
al., 1987).
Exposure to technical grade xylene containing 18.3% ethylbenzene
caused recessive lethal mutations in Drosophila melanogaster but not
exposure to m-xylene, or o-xylene (Donner et al., 1980). The same
report stated that exposure to rats for 300 ppm, 6 h per day, 5 days
per week for 9, 14 and 18 weeks did not induce chromosomal aberrations
in bone-marrow cells (Donner et al., 1980). Xylene (unspecified) did
not induce mutations in mouse lymphoma L5178Y TK+/- cells in vitro
or chromosomal aberrations in rat bone marrow cells (Lebowitz et al.,
1979). Xylene (unspecified) did not induce sister chromatid exchange
or chromosomal aberrations in human lymphocytes in vitro. No
exogenous metabolic system was used in this study (Gerner-Smidt &
Friedrich, 1978).
None of the isomers induced micronuclei in the bone marrow of
male NMRI mice after two i.p. administrations of 105-650 mg/kg body
weight at a 24-h interval, but they did, however, enhance the
induction of micronuclei by toluene (Mohtashamipur et al., 1985).
When Sprague-Dawley rats were given 440 or 1320 mg o-xylene/kg
body weight intraperitoneally, a significant increase in the
percentage of abnormal sperm was reported when the animals were housed
at 24-30°C (but not at 20-24°C). The authors (Washington et al.,
1983) interpreted this as a synergistic effect between o-xylene and
temperature.
7.7 Carcinogenicity
Group of Sprague-Dawley rats, 40 of each sex, received 500 mg
mixed xylenes (composition not specified) per kg body weight in olive
oil by stomach tube on 4 to 5 days per week for 104 weeks. Fifty
animals of each sex received olive oil only. The animals were
maintained until natural death. All animals had died by week 141. At
that time thymomas were reported in 1/34 treated males and 0/36
treated females (compared to 0/45 and 0/49, respectively, in
controls). Other haemolymphoreticular tumours (not specified) were
reported in 4/34 treated males and 3/36 treated females (compared to
3/45 and 1/49, respectively, in controls). The authors (Maltoni et
al., 1983; Maltoni et al., 1985) reported an increase in the total
number of animals with malignant tumours (type not specified) at 141
weeks, e.g., as 13/38 in treated males and 22/40 in treated females
(compared to 11/45 and 10/49, respectively, in control animals).
Combining all tumours is, however, not an acceptable basis for
analysis particularly in aged animals. No data were provided to allow
an analysis on an individual tumour-type basis.
In a carcinogenicity study, groups of 50 B6C3F1 mice of each sex
were given 0, 500 or 1000 mg xylene/kg body weight in corn oil by
stomach tube on 5 days per week for 103 weeks. The xylene used was
9.1% o-xylene, 60.2% m-xylene, 13.6% p-xylene and 17.0%
ethylbenzene. The surviving animals were killed within 2 weeks after
the last administration. Survival at the termination of the study for
males was: 27 controls, 35 low-dose and 36 high-dose; and for females
was: 36 controls, 35 low-dose and 31 high-dose. No treatment-related
increase in the incidence of any tumour was seen in either sex (NTP,
1986; Huff et al., 1988).
The NTP also performed a carcinogenicity study on Fischer-344
rats with the same type of technical xylene. Groups of 50 rats of
each sex were given 0, 250 or 500 mg xylene/kg body weight in corn oil
by stomach tube 5 days per week for 103 weeks. The surviving animals
were killed within 2 weeks following the last administration. At the
termination of the experiment, the survival for males was: 36
controls, 25 low-dose and 20 high-dose animals, and for females was:
38 controls, 33 low-dose and 35 high-dose. For the males survival
appeared to be dose-related, but many of the early deaths were related
to gavage trauma or corn oil-xylene aspiration (3/14, 8/25, 11/30).
The incidences of tumours in treated animals of either sex were not
significantly higher than in the control group (NTP, 1986; Huff et
al., 1988).
Two studies have investigated whether exposure to xylenes alters
the incidence of experimentally induced skin neoplasia in mice (Pound
& Withers, 1963; Pound, 1970). The reporting does not allow any firm
conclusions.
7.8 Other effects
No effects were observed upon in vitro exposure of human
lymphocytes at concentrations up to 2 mM xylene for 72 h. However, at
higher concentrations, cell mortality only was significantly increased
(Richer et al., 1993).
8. EFFECTS ON HUMANS
8.1 Acute and accidental exposure
Acute poisoning and deaths have been reported after overexposure
or oral ingestion of substantial amounts of xylene. The exposure
level required for loss of consciousness has been estimated to be
10 000 ppm (Morley et al., 1970). At autopsy pulmonary congestion and
oedema have been observed after inhalation or oral intake (Morley et
al., 1970; Abu Al Ragheb et al., 1986). Among survivors coma, EEG
changes, amnesia, mental confusion and ocular nystagmus have been
reported. Evidence of gastrointestinal and respiratory symptoms as
well as impaired renal and hepatic function have also been observed
(Ghislandi & Fabiani, 1957; Recchia et al., 1985; Bakinson & Jones,
1985). After exposure to about 700 ppm (calculated) for up to one
hour, headache, nausea, irritation of the eyes, nose and throat,
dizziness, vertigo and vomiting have been reported (Klaucke et al.,
1982). Recovery seems to be complete in most non-fatal cases although
dizziness and vision problems have been observed 24 h after ingestion
of xylene (quantity unknown) (Recchia et al., 1985). In a suicidal
attempt a 30-year-old man injected 8 ml of xylene intravenously.
After 10 min he developed a life-threatening acute pulmonary failure,
but survived through medical treatment (Sevcik et al., 1992).
8.2 Controlled human studies
A number of volunteer studies have been performed predominantly
at the Finnish Institute of Occupational Health. Effects on the
sensory motor and information process functions of the central nervous
system (CNS) have been investigated. Usually m-xylene has been
used, but p-xylene and mixed xylenes have also been studied
(Savolainen & Linnavuo, 1979; Savolainen, 1980; Savolainen et al.,
1980; Seppäläinen et al., 1981; Savolainen & Riihimäki, 1981;
Savolainen et al., 1981; Savolainen et al., 1982a, Savolainen et al.,
1982b; Savolainen et al., 1984; Savolainen et al., 1985a, Savolainen
et al., 1985b). In these studies no significant effects on vestibular
or visual function, reaction times, coordination or peripheral senses
were observed during a 4-h exposure to a constant concentration of up
to 160 ppm. Slight impairment of vestibular and visual function and
reaction time was noted at exposure levels from 200 to 300 ppm. There
was adaption to the impairment over five successive daily exposures.
In another study (Anshelm Olson et al., 1985), volunteers (n=16) were
exposed for 4 h to p-xylene alone (300 mg/m3: 70 ppm) or in
combination with toluene (200 mg/m3 toluene plus 100 mg/m3
p-xylene). Heart rate, subjective symptoms, simple reaction time,
choice reaction time and short-term memory were unaffected by
exposure.
In another study nine male volunteers were exposed for 4 h to 200
ppm (TWA) m-xylene. Short-term peak exposures were up to 400 ppm.
The effects of xylene on electroencephalography (EEG) were minor and
no deleterious effects were noted (Seppäläinen et al., 1991).
Healthy male subjects were exposed to technical xylene,
containing 40% ethylbenzene, for 2 h with or without a working load of
100 watts. The air concentration was 435 or 1300 mg/m3. During work
at the higher exposure level evidence of performance decrement was
observed in three of the five performance tests: reaction time
addition test (p < 0.05), short-term memory (p < 0.05) and choice
reaction time (p < 0.10) (Gamberale et al., 1978).
Dizziness was reported by four of six volunteers exposed to 690
ppm p-xylene for 15 min (Carpenter et al., 1975). Nine volunteers
were exposed for about 4 h to either a constant or a fluctuating
pattern of m-xylene with a time-weighted average exposure
concentration of 200 ppm in both cases (Laine et al., 1993).
Prolonged simple visual and auditive choice reaction times were
observed after exposure to the peaks of 400 ppm m-xylene. Exposure
to m-xylene at a constant level of 200 ppm did not affect the ratio
of "active" to "quiet" sleep during the following night, but decreased
slightly the number of body movements in bed.
Studies on coexposure to m-xylene and ethanol (Savolainen,
1980; Seppäläinen et al., 1981; Savolainen & Riihimäki, 1981;
Riihimäki et al., 1982a,b; Riihimäki et al., 1982a,b), m-xylene and
1,1,1-trichloroethane (TCE) (Savolainen et al., 1981; Savolainen et
al., 1982) or p-xylene and toluene (Anshelm-Olson et al., 1985) have
been performed. Exposure to 145-150 ppm xylene and 0.8 g ethanol/kg
body weight had an additive disturbing effect on vestibular function.
At higher xylene concentrations (275-290 ppm) there was evidence of
functional tolerance, and xylene appeared to antagonize the effect of
ethanol on vestibular function. In a study where volunteers were
exposed to 200 ppm m-xylene, minor effects on vestibular and visual
functions and reaction time were reported. Simultaneous exposure to
400 ppm TCE had no further effect. Combined exposure to 60 ppm
p-xylene and 30 ppm toluene had no effect on reaction time,
short-term memory or heart rate.
Ten male volunteers were exposed to 100 ppm xylene (not
specified) or 100 ppm toluene or a mixture of 50 ppm of each.
Exposure time was 4 h and each person participated in four exposure
sessions. Changes in CNS functions were tested by nine psychological
tests. Xylene had the most adverse effect on simple reaction time and
choice reaction time, while the combined exposure gave weaker effects
than xylene alone but stronger than toluene alone (Dudek et al.,
1990).
A summary of acute effects from inhalation exposure is presented
in Table 8.
In studies on skin irritation, both hands of subjects were
immersed in pure m-xylene (Engström et al., 1977; Lauwerys et al.,
1978; Riihimäki, 1979a). A burning sensation was soon noticed and an
erythematous reaction was observed in the exposed skin. Of six
subjects tested, four reported eye irritation after exposure to 460 or
690 ppm xylene for 15 min. The xylene contained 7.6% o-xylene,
65.0% m-xylene, 7.8% p-xylene and 19.3% ethylbenzene. One subject
reported eye irritation at 230 ppm but none at 110 ppm (Carpenter et
al., 1975). In another study, no irritation in the eyes, nose or
throat was reported after exposure to 98, 196 or 392 ppm mixed xylenes
for 30 min (Hastings et al., 1984). In an older study exposure to 200
ppm xylene (undefined) for 3-5 min caused irritation of the eyes, nose
and throat (Nelson et al., 1943).
The skin sensitization potential was investigated using a
non-adjuvant maximization test. The xylene used was not defined.
None of 24 subjects tested showed evidence of sensitization (Kligman,
1966).
Five adult, healthy, white men were exposed for 7 h per day for 3
days to 40 ppm xylene. This exposure was repeated three times at
intervals of 2 weeks. Blood samples were taken before and after each
exposure. No significant effects were observed on sister-chromatid
exchange frequency, cell cycle time or cell mortality in lymphocytes
(Richer et al., 1993).
8.3 Occupational exposure
In workers exposed to xylene or solvent mixtures containing large
amounts of xylene, subjective symptoms have been reported (Joyner &
Leak Pegues, 1961; Glass, 1961; Hipolito, 1980; Kilburn et al., 1983;
Kilburn et al., 1985). Depression, fatigue, headache, anxiety,
feeling of drunkenness and sleep disorders were the most common
symptoms reported, but exposure levels and duration were often missing
in these reports.
Workers occupationally exposed to solvent mixtures including
xylene have been reported to have neurophysiological and psychological
disorders (Lindström, 1973; Seppäläinen et al., 1978; Elofsson et al.,
1980; Husman, 1980; Husman & Karli, 1980; Arlien-Soborg et al., 1981;
Lindström et al., 1982; Valciukas et al., 1985; Maizlish et al., 1987;
Van Vliet et al., 1987; Ruijten et al., 1994). In these studies there
was no exposure to xylene alone. Xylene was not the main solvent in
the mixture. No conclusions concerning effects of xylenes as such can
be drawn from these studies.
Table 8. Single inhalation exposure to xylene in humans
Exposure Time Effect Reference
concentration
(mg/m3)
3 000 1 h Dizziness, irritation Klaucke et al. (1982)
3 000 15 min Dizziness Carpenter et al. (1975)
1300a 2 h Performance decrement Gamberale et al. (1978)
900b 4 h Prolonged reaction times Laine et al. (1993)
900 4 h Impairment of vestibular and visual Savolainen et al.
function and prolonged reaction time (1979, 1981, 1982, 1985)
900 4 h Minor effect on EEG Seppäläinen et al. (1991)
600 4 h No effect on reaction time Savolainen et al. (1980, 1981)
450 4 h Prolonged reaction time Dudek et al. (1990)
300 4 h No effects in psychophysiological test Anshelm - Olson et al. (1985)
a during exercise
b peak values of 1800 mg/m3
Liver effects have been reported in some studies on workers
occupationally exposed to solvents containing xylene (Dössing et al.,
1981; Edling, 1982; Sotaniemi et al., 1982; Dössing et al., 1983;
Fischbein et al., 1983, Lundberg et al., 1994) but these were not
confirmed in other studies (Craveri et al., 1982; Kurppa & Husman,
1982; Lundberg & Håkansson, 1985). Recent findings seem to support
the concept that the hepatotoxicity of xylene is low (Riihimåki &
Hännien, 1987).
Some case studies and epidemiological studies have addressed a
possible association between occupational exposure to hydrocarbons
(including xylene) and proliferative glomerulonephritis (Beirne &
Brennan, 1972; Zimmerman et al., 1975; Lagrue, 1976). Exposures
have, however, been so diverse that the role of xylene is impossible
to assess. A Swedish group of researchers reported a higher
concentration of albumin, erythrocytes and leukocytes in the urine of
workers exposed predominantly to xylenes and toluene than among
controls (Askergren et al., 1981a,b,c; Askergren, 1981). Franchini et
al. (1983) found that painters exposed to toluene and xylenes at
relatively low concentrations had a higher excretion of kidney tubular
enzymes in their urine than the controls. They suggested that the
mixed solvents may exert a slight adverse effect on the kidney
tubules.
One case of contact urticaria due to occupational exposure to
airborne xylene has been reported. The level of xylene in air
exceeded 100 ppm. Direct skin contact with the solvent appeared to
have been negligible. The contact urticaria seemed likely to be an
immunological type (Palmer & Rycroft, 1993).
Thirty-five male spray varnishers were exposed to 0.5-3.4 ppm
o-xylene, 3.2-11.7 ppm m-xylene, 0.9-4.3 ppm p-xylene, 1.4-7.5
ppm ethylbenzene, < 1.5 ppm toluene, < 1.2 ppm n-butanol, < 35.5
ppm 1,1,1-trichloroethane and several C9 aromatics. In addition, some
of the lacquers contained lead pigments. The mean peripheral
erythrocyte counts and haemoglobin levels were decreased in the
exposed men compared to controls. Whether these effects were due to
xylene or the solvent mixture is uncertain (Angerer & Wulf, 1985).
The health effects on 175 factory workers in China exposed to
xylene vapour with a mixture of three isomers at a concentration of up
to 175 ppm (with time-weighted average geometric mean concentration of
14 ppm and arithmetic mean of 21 ppm) were studied. There was an
increased prevalence of subjective symptoms in the exposed workers;
these were apparently related to effects on the central nervous system
and to local effects on the eye, nose and throat (Uchida et al.,
1993). In workers exposed to a maximum concentration of 103 ppm
xylene (geometric mean 4 ppm) and 203 ppm toluene (geometric mean 3
ppm), the prevalence of some subjective CNS-related symptoms was
higher than in the controls (Chen et al., 1994). No effects on
haematology or serum biochemistry with respect to liver and kidney
functions were observed in these two studies.
Sister-chromatid exchanges (SCE) in peripheral lymphocyte
cultures have been studied in two groups of 23 workers who had been
exposed for between 4 months and 23 years to mixed xylenes (including
ethylbenzene). The exposure levels for the two groups were 11 and 13
ppm, respectively. No differences in SCE frequency was seen (Pap &
Varga., 1987). A few other reports have described increased incidence
of chromosomal aberrations or effects on sister-chromatid exchange
frequencies (Funes-Cravioto et al., 1977; Haglund et al., 1980). In
both these studies the exposure to xylene (not defined) was
accompanied by exposure to other solvents, including benzene.
No data on carcinogenic effects resulting from exposure to
xylenes have been found in the literature.
In an investigation into the effect on reproduction, the outcome
of pregnancy was studied among university laboratory employees exposed
to xylene (not defined) during the first trimester of pregnancy.
Exposure levels were not given. There was no difference in
miscarriage rate when compared to controls not exposed to solvents
(Axelsson et al., 1984).
A similar case control study on associations between laboratory
work and pregnancy outcome revealed that use of xylene for 3 or more
days a week during the first trimester was significantly associated
with an elevated risk of spontaneous abortion. The authors pointed
out, however, that laboratory workers were often exposed to several
solvents and chemicals simultaneously; only two cases and two controls
were exposed to xylene alone (Taskinen et al., 1994). About half of
the women exposed to xylene worked in pathology/histology laboratories
where there was concomitant exposure to formaldehyde vapour.
Formaldehyde (formalin) also appeared to be a significant risk factor
for spontaneous abortion. Exposure to xylene was also noted to be
associated with an increase in birth weight (Taskinen et al., 1994).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Bringmann & Kuhn (1977, 1978) exposed the bacterium Pseudomonas
putida for 16 h and the blue-green alga Microcystis aeroginosa for
8 days to xylene. They found a reduction in cell multiplication at
concentrations of >200 mg/litre.
Walton et al. (1989) studied the effect of p-xylene on the
microbial respiration of two soil types, a silt loam (1.49% organic
carbon) and a sandy loam (0.66% organic carbon). The chemical was
applied at a rate of 1000 µg/g (dry weight). Microbial respiration,
as measured by CO2 efflux, of the silt loam was unaffected. In the
sandy loam, the CO2 efflux initially decreased and then increased,
but returned to control levels within the 6-day exposure period.
All three isomers have been shown to inhibit the respiration of
sewage sludge utilizing biogenic substrates. Two screening tests were
used, RIKA (respiration inhibition kinetic analysis) and OECD 209.
The concentration of each compound used was at the limit of the
solubility in the medium (approx. 175-198 mg/litre). Inhibitions in
the respiration rate of 100% were found for all three isomers in the
RIKA screening test, and inhibitions of 22% ( o-xylene) and 43%
( p-xylene) were measured in the OECD 209 screening test. m-Xylene
was only tested at a concentration of 0.3 mg/litre in the OECD 209
screening test, which resulted in 5% inhibition (Volskay & Grady,
1990).
The toxicity of xylene to three species of environmental bacteria
has been determined using assays in sealed serum bottles to prevent
loss of chemical by volatilization. Activity of the bacteria was
measured by either gas production over 48 h (methanogens), oxygen
consumption over 15 h (aerobic heterotrophs) or ammonia use over 24 h
(Nitrosomonas). IC50 values (the concentration required to inhibit
the bacterial activity by 50%, as compared with controls) for xylene
were 250 mg/litre for methanogens, 1100 mg/litre for aerobic
heterotrophs and 100 mg/litre for Nitrosomonas (Blum & Speece,
1991).
9.1.2 Aquatic organisms
Xylene isomers are highly volatile and disappear rapidly from
solution. For example, Mackay & Wolkoff (1973) found that in agitated
water, 1m deep and with a 1m2 surface for evaporation, the half-life
for o-xylene was 39 min. When Benville & Korn (1977) monitored the
loss of xylene from solution during LC50 tests, average percentage
losses for the four time intervals studied (24, 48, 72 and 96 h) were
29, 61, 84 and > 99% respectively. These losses mean that the
exposure can be difficult to determine. For example, many of the 24-h
and 96-h LC50 values are the same or similar, suggesting that most of
the xylene had been lost during the test. Galassi et al. (1988),
using a closed static system, found mean measured concentrations of
xylene and other aromatics to fluctuate by only 10% during the test
period of up to 96 h. Care must therefore be taken when interpreting
data from open static tests over longer periods than 24 h, especially
those based on nominal concentrations. Overall it can be stated that
xylene has moderate to low acute toxicity for aquatic organisms.
9.1.2.1 Algae
Bringmann & Kühn (1977) exposed the green alga Scenedesmus
quadricauda for 8 days to xylene. They found reduction in cell
multiplication at concentrations of > 200 mg/litre. Brooks et al.
(1977) studied the effect of xylene on photosynthesis in a mixed ocean
culture of phytoplankton. They found that exposure to a concentration
of 3 mg/litre xylene for 8 h caused a 50% reduction in photosynthesis.
Galassi et al. (1988) calculated the 72-h EC50 for growth
inhibition in the alga Selenastrum capricornutum to be 4.7, 4.9 and
3.2 mg/litre for the ortho, meta and para isomers, respectively.
Similar results were obtained by Herman et al. (1990). Using the same
species of algae, they obtained 8 day EC50 values for growth
inhibition of 4.2, 3.9 and 4.4 mg/litre for the ortho, meta and para
isomers, respectively. Sheedy et al. (1991) reported a 14-day EC50
for growth inhibition of Selenastrum capricornutum of 72 mg/litre
for xylene (composition not stated).
Hutchinson et al. (1980) exposed the algae Chlamydomonas
angulosa and Chlorella vulgaris to p-xylene for 3 h. EC50
values for inhibition of photosynthesis (measured using 14CO2
uptake) were 45.7 and 105.1 mg/litre for the two species,
respectively. Kauss et al. (1973) exposed the green alga Chlorella
vulgaris to o-xylene and studied growth over a 10-day period in an
open system. At nominal concentrations of between 25 and 100
mg/litre, a short-term toxic effect was observed. However, the algal
culture recovered within 2 days. The authors pointed out that
recovery was probably due to volatilization of the chemicals. A
near-saturation concentration of 171 mg o-xylene/litre proved to be
acutely toxic and the alga did not recover. Concentrations of 25 and
50 mg o-xylene/litre progressively increased the lag period between
initial inoculation and growth. An o-xylene concentration of 100
mg/litre delayed the onset of growth for 4 days and a near-saturation
concentration of 171 mg/litre caused complete inhibition of growth
during the 10-day exposure period (Kauss & Hutchinson, 1975).
Dunstan et al. (1975) exposed marine microalgae, the diatom
Skeletonema costatum, the dinoflagellate Amphidinium carterae, the
coccolithophorid Cricosphaera and the green flagellate Dunaliella
tertiolecta, to xylene for 3 days. A xylene concentration of 10
mg/litre inhibited the growth of all species. Inhibition was most
marked in A carterae and S. costatum.
9.1.2.2 Higher plants
Frank et al. (1961) exposed the angiosperm pondweeds Elodea
canadensis, Potamogeton nodosus and P. pectinatus to xylene
(plus 2% emulsifying agent) under static, open conditions for a period
of four weeks. A concentration of 100 mg/litre was found to be toxic
(8.6 on an injury scale of 0 to 10) but 5 mg/litre was not. Both 300
and 600 mg/litre were similarly toxic after 30-min exposures in
flowing water.
9.1.2.3 Protozoa
Bringmann et al. (1980) exposed the flagellate protozoan
Chilomonas paramaecium to xylene for 48 h and found an initial
reduction (5%) in cell multiplication at concentrations of > 80
mg/litre. Rogerson et al. (1983) exposed the ciliate protozoan
Colpidium colpoda to xylene in an "open" system consisting of a
covered watchglass with an air space. Toxicity thresholds were 1.75
mg/litre (16.5 mmol/m3) for o-xylene and 162 mg/litre (1530
mmol/m3) for m-xylene; no threshold was calculated for p-xylene.
The same authors exposed the protozoan Tetrahymena ellioti to xylene
isomers in a closed system with no air space. Toxicity thresholds for
o-, p- and m-xylene were 18.5, 16.9 and 55.7 mg/litre (174, 159
and 525 mmol/m3), respectively.
9.1.2.4 Invertebrates
The LC50 values of xylene to aquatic invertebrates are
summarized in Table 9.
Le Gore (1974) exposed Pacific oyster larvae (Crassostrea
gigas) to o- and p-xylene for 48 h. LC50 values were 0.17 and
0.58 mg/litre for the two isomers, respectively, suggesting that this
organism is one of the more sensitive invertebrates to xylene
exposure. However, no experimental details were given for these
toxicity tests, which makes them difficult to interpret.
Table 9. LC50 values of xylenes to aquatic invertebrates
Organisms Size/ Static/ Open/ Temp. Hardness Isomer Duration Concentration Reference
life-stage flowa closeda (°C) (mg/litre)c (mg/litre)d
Estuarine and marine invertebrates
Pacific oyster larvae ortho 48 h 0.17 Le Gore (1974)
(Crassostrea larvae para 48 h 0.58 Le Gore (1974)
gigas)
Grass shrimp static c 21 15s 96 h 7.4 Neff et al. (1976)
(Palaemonetes static c 21 15s 24 h 14.0 Tatem et al. (1978)
pugio) 96 h 7.4 Tatem et al. (1978)
Bay shrimp adult static o 16 25s ortho 24 h 4.7 n Benville & Korn (1977)
(Crago adult static o 16 25s ortho 96 h 1.1 n Benville & Korn (1977)
franciscorum) adult static o 16 25s meta 24 h 4.1 n Benville & Korn (1977)
adult static o 16 25s meta 96 h 3.2 n Benville & Korn (1977)
adult static o 16 25s para 24 h 1.7 n Benville & Korn (1977)
adult static o 16 25s para 96 h 1.7 n Benville & Korn (1977)
Dungeness crab zoeae static+ 13 30s ortho 48 h 38 n Caldwell et al. (1977)
(Cancer zoeae static+ 13 30s ortho 96 h 6 n Caldwell et al. (1977)
magister) zoeae static+ 13 30s meta 48 h 33 n Caldwell et al. (1977)
zoeae static+ 13 30s meta 96 h 12 n Caldwell et al. (1977)
Table 9. (Cont'd)
Organisms Size/ Static/ Open/ Temp. Hardness Isomer Duration Concentration Reference
life-stage flowa closeda (°C) (mg/litre)c (mg/litre)d
Freshwater invertebrates
Water flea static o 24 h >100<1000 n Dowden & Bennett (1965)
(Daphnia magna) static 24 h 165 n Brigmann & Kühn (1982)
static c ortho 24 h 1 m Galassi et al. (1988)
static c meta 24 h 4.7 m Galassi et al. (1988)
static c para 24 h 3.6 m Galassi et al. (1988)
4-6 days static c ortho 48 h 3.2 n Bobra et al. (1983)
4-6 days static c meta 48 h 9.6 n Bobra et al. (1983)
4-6 days static c para 48 h 8.5 n Bobra et al. (1983)
< 24 h flow o 17 44.7 ortho 48 h 3.82 m Holcombe et al. (1987)
Mosquito larvae static o 24-26 24 h 13.9 Berry & Brammer (1977)
(Aedes aegypti)
Snail
(Aplexa adult flow o 17 44.7 ortho 96 h >22.4 m Holcombe et al. (1987)
hypnorum)
a static = static conditions (water unchanged for duration of test); b o = open; c = closed
static+ = semi-static conditions (water renewed at 24 hour intervals); c s = salinity (%)
flow = flow-through conditions (xylene concentration in water continuously maintained) d m = measured; n = nominal
Berry & Brammer (1977) exposed mosquito larvae (Aedes aegypti)
to xylene under static, open conditions at 25°C. Larvae were exposed
for 24 h, since no detectable levels of any water-soluble component
remained after that period. A non-lethal concentration of 7.92
mg/xylene litre was reported. However, the authors found that
bioassays using different volumes of solution and different sized
containers demonstrated the significant effect that surface area,
volume and depth can have on the results of experiments with volatile
hydrocarbons such as xylenes.
Falk-Petersen et al. (1985) exposed sea urchin (Strongy
locentrotus droebachiensis) eggs to o-xylene from fertilization
and monitored deaths, pathology, inhibition of cleavage and
differentiation, and pigment effects. Eggs were maintained in test
beakers covered with aluminium foil. They calculated a 96-h EC50,
based on all these parameters, of 4.1 mg/litre.
Freshwater mussels (Dreissena polymorpha) were exposed to
various concentrations of xylene, and the behaviour of the mussels in
terms of shell valve movements was monitored. At the start of the
experiment, all valves were gaping continuously. After a certain
period of toxicant addition, a gradual increase in valve closure
period was observed. Finally, total closure of shell valves was
observed. Effects were first seen at a xylene concentration of
11.9-19.4 mg/litre (nominal) (Slooff et al., 1983).
9.1.2.5 Vertebrates
The LC50 values of xylene to fish are summarized in Table 10.
Morrow et al. (1975) found that 100 mg xylene/litre killed 100%
of young coho salmon (Oncorhynchus kisutch) within 24 h under
static, closed conditions. Concentrations of 1 and 10 mg/litre did
not cause significant mortality within the 96-h exposure period.
Toxicity included rapid, violent and erratic swimming, "coughing",
loss of equilibrium and death.
Rainbow trout (Oncorhynchus mykiss) significantly avoided
xylene (plus 2% emulsifying agent) at a nominal concentration of 0.1
mg/litre during a 1-h test. Fish exposed to 0.001 mg/litre did not
show significant avoidance and those exposed to 0.01 mg/litre were
significantly attracted to the xylene (Folmar, 1976). Maynard & Weber
(1981) found that juvenile coho salmon were able to significantly
avoid o-xylene concentrations of > 0.2 mg/litre water.
Slooff (1979) exposed rainbow trout Oncorhynchus mykiss to
various concentrations of xylene in a flow-through, closed system and
monitored any effects of the chemical on their breathing. The lowest
concentration at which a toxic condition developed within 24 h after
toxicant administration was 2 mg/litre.
Table 10. LC50 values of xylenes to fish
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Freshwater fish
Fathead minnow 1-2 g static o 25 20 24 h 28.8 n Pickering & Henderson
(Pimephales 1-2 g static o 25 20 48 h 27.7 n (1966)
promelas) 1-2 g static o 25 20 96 h 26.7 n
1-2 g static o 25 360 24 h & 96 h 28.7 n
0.3 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Bluegill 1-2 g static o 25 20 24 h 24 n Pickering & Henderson
(Lepomis 1-2 g static o 25 20 48 h 24 n (1966)
macrochirus) 1-2 g static o 25 20 96 h 20.9 n
1.1 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Goldfish 1-2 g static o 25 20 24 h & 96 h 36.8 n Pickering & Henderson
(Carassius (1966)
auratus)
20-80 g flow 17-19 80 24 h 30.6 m Weber et al. (1975)
20-80 g flow 17-19 80 96 h 16.9 m
3.3 g static o 19-21 ortho 24 h 13 m Bridie et al. (1979)
3.3 g static o 19-21 meta 24 h 16 m
3.3 g static o 19-21 para 24 h 18 m
2.5 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
Table 10. (Cont'd)
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Guppy 0.1-0.2 g static o 25 20 24 h & 96 h 34.7 n Pickering & Henderson
(Poecilia (1966)
reticulata)
static meta 14 days 305 Könemann (1981)
static ortho 7 days 291
static para 7 days 285
static c 20-22 ortho 96 h 12 m Galassi et al. (1988)
static c 20-22 meta 96 h 12.9 m
static c 20-22 para 96 h 8.8 m
Rainbow trout static c 11-13 ortho 96 h 7.6 m Galassi et al. (1988)
(Oncorhynchus static c 11-13 meta 96 h 8.4 m
mykiss) static c 11-13 para 96 h 2.6 m
flow o 9-13 89.5 96 h 10e Folmar (1976)
13.1 g flow o 17 44.7 ortho 96 h 8.05 m Holcombe et al. (1987)
0.9 g flow o 12 40-48 technical 24 h & 96 h 13.5 Walsh et al. (1977)
Zebra fish flow c 48 h 20 Sloff (1979)
(Brachydanio
rerio)
Table 10. (Cont'd)
Organisms Size/ Static Open/ Temp. Hardness Isomer Duration Concentration Reference
lifestage /flowa closedb °C (mg/litre)c (mg/litre)d
Golden orfe 1.2-1.8 g static 48 h 86f Juhnke & Lüdemann
(Leuciscus 1.2-1.8 g static 48 h 308f (1978)
idus
melanotus)
White sucker 2.4 g flow o 17 44.7 ortho 96 h 16.1 m Holcombe et al. (1987)
(Catostomus
commersoni)
Marine fish
Coho salmon young static c 8 30s 24 h > 10 < 100 Morrow et al. (1975)
(Oncorhynchus
kisutch)
Striped bass 6 g static o 16 25s ortho 24 h & 96 h 9.7 n Benville & Korn (1977)
(Morone 6 g static o 16 25s meta 24 h & 96 h 7.9 n
saxatilis) 6 g static o 16 25s para 24 h & 96 h 1.7 n
a static = static conditions (water unchanged for duration of test);
flow = flow-through conditions (xylene concentration in water continuously maintained)
b o = open; c = closed
c s = salinity (°/oo)
d m = measured; n = nominal
e included 2% emulsifying agent
f results from two different laboratories
When Walsh et al. (1977) exposed rainbow trout (average weight
165 ± 86 g) to xylene concentrations of 0.31, 0.65 and 1.1 mg/litre in
artificial streams for 56 days, no adverse effects on the fish were
noted at any concentration. Using the same artificial streams, all
rainbow trout exposed to xylene concentrations of 14.2 or 22.5
mg/litre for 2 h died. Fish exposed to xylene concentrations of 3.2
and 6.2 mg/litre for 2 h showed symptoms similar to anaesthesia.
Kjorsvic et al. (1982) exposed cod eggs ( Gadus morhus L.) to
xylene isomers in covered glass dishes and monitored the effects both
during fertilization and during early cleavage of fertilised eggs.
Both m-xylene and p-xylene induced significant decreases in the
fertilization rate at concentrations above 10 mg/litre. o-Xylene
had no significant effect on the fertilization rate at concentrations
of 16-35 mg/litre. Fertilized eggs were exposed to xylene for 3 or 6
h before first cleavage. No significant difference was observed
between the individual xylene isomers or between the two exposure
periods. Effects on the early cleavage pattern were significant for
xylene concentrations between 2 and 7 mg/litre. The effects seen
included inhibition of formation of the cleavage furrow. Small cells
or a total absence of cleavage occurred on exposure to all isomers at
concentrations of 16-35 mg/litre, while incomplete or uneven cleavage
was found at exposures of 8-15 mg/litre.
Black et al. (1982) exposed embryo-larval stages of the leopard
frog Rana pipiens and rainbow trout (Oncorhynchus mykiss) to
m-xylene from 30 min after fertilization to 4 days after hatching in
a closed, flow-through system (hatching times were 5 days for the frog
and 23 days for the trout). LC50 values of 3.53 and 3.77 mg/litre
were calculated for the two species, respectively.
9.1.3 Terrestrial organisms
Hill & Camardese (1986) exposed Japanese quail (Coturnix
coturnix japonica) to xylene in 5-day dietary toxicity tests. The
LC50 was found to be greater than 20 000 mg/kg diet. No overt signs
of toxicity occurred at 5000 mg/kg.
No studies on terrestrial plants, terrestrial invertebrates or
field effects of xylenes have been reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposures
In the general environment humans are exposed to xylenes mainly
by inhalation. Absorption through the skin may also occur in the
working environment. The retention in the lungs is about 60% of the
inhaled dose. Xylene is efficiently metabolized. More than 90% is
biotransformed to methylhippuric acid, which is excreted in urine.
Xylene does not accumulate significantly in the human body.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3 with levels of 3 µg/m3 in suburban
areas. Higher levels have been measured in urban and industrialized
areas with mean concentrations ranging up to 500 µg/m3; however,
concentrations are generally below 100 µg/m3.
The estimated daily exposure of the general population through
inhalation is 70 µg in rural areas and less than 2000 µg in urban
areas. The concentration in drinking-water ranges from not detectable
to 12 µg/litre. Data on the levels in food are too limited to
estimate daily oral exposure.
10.1.2 Effects
Based on experimental human studies, xylene may have an acute
impairing effect on the sensory-motor and information-processing
functions of the central nervous system (CNS). Exposure to 435-870
mg/m3 (100-200 ppm) of xylene over 4 h caused slight impairments in
reaction time performance and vestibular functions. There was
adaptation at 200 ppm m-xylene to the impairment over five
successive days. The LOAEL of xylene for acute CNS effects, based on
one study, is 470 mg/m3 (108 ppm). It should be noted, however,
that other identical studies found significant effects only at
concentrations of 870 mg/m3 (200 ppm) or more. According to a study
by a different research group, exposure to 304 mg/m3 (70 ppm) p-
xylene for 4 h did not cause impairment of corresponding psycho-
physiological functions; 304 mg/m3 (70 ppm) xylene can therefore
be regarded as the NOAEL for acute CNS effects.
Xylene vapour becomes irritating at relatively high levels.
Among six volunteer subjects, four reported eye irritation after
exposure to 2000 or 3000 mg/m3 (460 or 690 ppm) xylene for 15 min
while one subject reported eye irritation at 1000 mg/m3 (230 ppm) and
none at 478 mg/m3 (110 ppm). According to another study, no
irritation of the eyes, nose or throat was reported after exposure to
423, 852 or 1705 mg/m3 (98, 196 or 392 ppm) mixed xylenes for 30 min.
These human findings are consistent with mouse studies showing that
strong irritancy (respiratory rate decrease by 50%) occurs at about
5960 mg/m3 (1370 ppm) xylene. The odour threshold for xylene is
about 1 ppm.
Subjective symptoms have been reported among workers exposed to
solvent mixtures containing large amounts of xylene. Long-term
exposure to xylene is suspected to affect the nervous system adversely
because chronic toxic encephalopathy and milder functional
disturbances of the brain have sometimes been found among exposed
painters and other workers. Likewise, slight changes in kidney
tubular function may occur. The specific role of xylene in these
effects cannot, however, be ascertained.
When human data are sparse, especially data from chronic studies,
animal data are used as a substitute. An assessment of the risk to
human health of exposure to xylene must rely on animal studies.
Apparently irreversible effects on the CNS were found 4 months
after a 3-month inhalation exposure (24 h per day) of Mongolian
gerbils to xylene at concentrations of 696 or 1392 mg/m3 (160 or 320
ppm). At the lower level the effects were not statistically
significant in any individual part of the brain but the changes were
all of the same nature. The study disclosed an increased
concentration of astroglial proteins in most brain regions studied,
which may indicate that glial proliferation is characteristic to
various neurodegenerative and neurotoxic states. In the light of
similar findings in animals exposed to other solvents (e.g.,
trichloroethylene, ethanol and tetrachloroethylene), the results are
estimated to be an important piece of evidence for potential
xylene-induced neurotoxicity at > 696 mg/m3 (160 ppm) (the LOAEL).
Functional changes, similar to acute effects on nervous functions,
were described after exposure to 435 mg/m3 (100 ppm), but they cannot
be discriminated from the acute effects of the last exposure. The
exposure level is consistent with the levels giving acute effects in
humans, as stated above.
No adequate studies of reproduction and development toxicity in
humans exposed to xylene alone have been published. Placental
transfer of xylene has been shown in humans and in experimental
animals.
Teratogenicity studies in pregnant animals exposed to technical
xylene or xylene isomers during organogenesis indicate that xylene may
cause reduced fetal weight and delayed ossification, but not
malformations, at dose levels causing no or only slight maternal
toxicity. LOAEL values of 500-2175 mg/m3 (115-500 ppm) have been
reported, depending on the length of the daily exposure periods (6-24
h/day). Signs of delayed ossification in the absence of lower fetal
body weight have been reported at lower dose levels. However, these
findings cannot be properly evaluated owing to incomplete description
of the criteria for assessing ossification. A NOAEL for delayed fetal
development cannot therefore be established.
In a study of postnatal development in rat offspring prenatally
exposed to 870 or 2175 mg/m3 (200 or 500 ppm) technical xylene,
behavioural impairments indicating effects on the development of the
central nervous system were detected. There was no maternal toxicity,
and the effects at 2175 mg/m3 (500 ppm) were long-lasting as they
were apparent in adult offspring. As 870 mg/m3 (200 ppm) was the
lowest dose level investigated for this effect a NOAEL could not be
established.
In several short-term and long-term animal studies, effects on
the activities of various metabolic enzymes in different organs have
been observed. Exposure to 217 mg/m3 (50 ppm) m-xylene 6 h/day for
5 days induced renal cytochrome P-450. At 1305 mg/m3 (300 ppm)
xylene (6 h/day for 14 days) an increase in hepatic and renal enzyme
activities was observed. At > 1740 mg/m3 (400 ppm) increases in
hepatic and renal metabolic enzyme levels, as well as increased
relative liver and kidney weights, have been reported. At the same
exposure levels the pulmonary P-450 content and pulmonary enzyme
activities were decreased. When rats were orally given 150 mg
xylene/kg body weight per day for 90 days, increased relative liver
weights were seen.
These changes in metabolic enzyme activities and increased
relative liver weight could be taken as an indication of metabolic
adaptation rather than toxicity.
When rats were exposed to 3480 mg/m3 (800 ppm) xylene, 14 h/day,
7 day/week for 6 weeks, an increased auditory response threshold was
reported. Thus, 3480 mg/m3 (800 ppm) is the LOAEL for ototoxicity.
For this effect no NOAEL could be established as 3480 mg/m3 (800 ppm)
was the lowest dose level used.
Xylene appears not to be a mutagen or a carcinogen.
10.1.3 Guidance value
The definition and aim of guidance values for the general
population have been described by IPCS (1994).
Although some differences in action between the three isomers
exist, there is no clear evidence that they (or mixture of them) have
totally different effects.
There have been no long-term controlled human studies or
epidemiological studies from which a guidance value may be calculated.
Epidemiological data from the occupational setting do not allow
an estimation of xylene-specific chronic nervous system effects,
neither can the neuropsychological impairment seen among
solvent-exposed workers be attributed to any specific level of xylene
in air.
On the basis of human volunteer studies (Anshelm Olson, 1985),
one may conclude that the NOAEL for acute CNS effects in humans is
about 304 mg/m3 (70 ppm) for a 4-h exposure. The use of an
uncertainty factor of 10 for intraspecies variability (the study
subjects were healthy male research workers) and an additional factor
of 6 (4-h exposure versus 24-h general population exposure) leads to a
guidance value of 4.8 mg/m3 (1.1 ppm). It should be noted, however,
that the acute CNS effect observed probably has no predictive value
for chronic CNS toxicity by xylene. The guidance value of 4.8 mg/m3
(1.1 ppm) is close to the odour threshold of xylene. It should be
noted that a subset of the human population may be sensitive enough to
experience the odour as annoying. The Task Group considered that
there was no need to add another uncertainty factor for the lack of
data from chronic exposure.
On the basis of animal studies on developmental toxicity, one may
conclude that the LOAEL for reduced fetal body weight is 500 mg/m3
(115 ppm) (Ungvary & Tatrai, 1985) and that for developmental
neurotoxicity is 870 mg/m3 (200 ppm) (Hass & Jakobsen, 1993).
Developmental neurotoxicity is a serious effect that may be
long-lasting and is therefore considered the critical effect. An
uncertainty factor of 10 for use of a LOAEL rather than a NOAEL seems
justified based on the evidence of lower fetal body weight at 500
mg/m3 (115 ppm) and limited evidence of delayed ossification at even
lower exposure levels. The use of additional factors of 10 for
interspecies variation and 10 for inter-individual variation leads to
a guidance value of 0.87 mg/m3 (0.2 ppm).
Using the hearing loss (Pryor et al., 1987) detected in animals
after exposure to 3480 mg/m3 (800 ppm) xylene for 6 weeks as a
starting point, an uncertainty factor of 10 for use of a LOAEL rather
than a NOAEL, a factor of 10 for interspecies variation and an
additional factor of 10 for inter-individual variation results in a
guidance value of 3.48 mg/m3 (0.8 ppm).
A neurotoxicity study in animals exposed continuously for 3
months to 696 or 1392 mg/m3 (160 or 320 ppm) xylene (Rosengren et
al., 1986) provided suggestive biochemical evidence of an apparently
irreversible adverse effect on the nerve cells of the brain even at
the lower level. Although there may be uncertainty concerning the
biological significance and interpretation of the findings, the Task
Group considered them potentially important and recommended further
confirmatory studies. With respect to animal-human extrapolation and
relatively short exposure, the estimate of the critical level for
life-long exposure in humans is 1.6 ppm. A guidance value of 0.87
mg/m3 (0.2 ppm) covers another uncertainty factor of approximate 10
in this respect.
Based on the above considerations, the Task Group recommended
0.87 mg/m3 (0.2 ppm) as a guidance value for the general population.
This value was derived from the LOAEL reported for developmental
neurotoxic effects in laboratory animals.
10.2 Evaluation of effects on the environment
10.2.1 Exposure
The majority of xylene released into the environment will enter
the atmosphere directly. In the atmosphere the xylene isomers are
readily degraded. Volatilization to the atmosphere from water is
rapid for all three isomers. Although the meta and para isomers are
readily biodegraded, in soil and water the ortho isomer is more
persistent. Bioaccumulation of xylene isomers by aquatic organisms is
low.
Typically, mean background levels of all three xylene isomers in
ambient air are around 1 µg/m3. Mean background concentrations of
xylenes in surface waters are generally below 0.1 µg/litre. However,
higher values have been measured in industrial areas. In areas
associated with the oil industry even higher levels have been reported
but only associated with discharge pipes. Similar background levels
have been reported for groundwater, although localized pollution can
lead to higher levels.
10.2.2 Effects
The xylene isomers are of moderate to low toxicity to aquatic
organisms. The variation between each individual isomer with respect
to aquatic toxicity is generally small. The lowest LC50 value, based
on measured concentrations, is for a 24-h exposure of Daphnia magna
to 1 mg o-xylene/litre.
There is limited information regarding chronic exposure of
aquatic organisms to xylenes and none of the observed effect levels
were lower than those summarized under the acute studies.
The acute toxicity of xylene to birds is low.
10.2.3 Risk evaluation
Xylenes are rapidly degraded in the environment. However, the
photooxidation reactions of the xylene isomers in the atmosphere may
contribute to photochemical smog.
High levels of xylenes have been reported in groundwater
associated with localized pollution from underground tanks and pipes,
but the environmental significance of such values is difficult to
assess.
For the aquatic environment, the most sensitive toxicity test,
based on measured concentrations, yielded an LC50 for Daphnia
magna of 1 mg/litre ( o-xylene). This value is more than 10 000
times higher than mean background concentrations in surface water,
which are generally less than 0.1 µg/litre for each isomer. The
lowest LC50 is still over 30 times higher than the highest single
measured concentration of total xylenes in the most polluted area.
The exposure/toxicity ratio will be much higher for mean
concentrations in polluted areas.
On the basis of rapid volatilization and degradation of xylenes
and their low to moderate toxicity, the overall risk to the aquatic
environment can therefore be considered low. It should be noted,
however, that very much higher levels have been measured around
discharges from oil production sites, and higher levels are also
possible if spillage occurs.
The likely route of exposure for birds in the environment is via
food such as fish. The only acute toxicity test on birds was carried
out on 14-day old Japanese quail and gave a 5-day LC50 of > 5000
mg/kg diet. Based on food consumption and body weight, an LD50 for
the quail of > 1746 mg/kg body weight can be calculated. Using this
data an estimated LC50 for a fish-eating bird (kingfisher) based on
body weight and food consumption can be calculated.
LC50 (mg/kg dry weight diet) =
Test species LD50 (mg/kg) × body wt (kg)
food consumption (kg)
The estimated LC50 for the fish-eating bird is > 7990 mg/kg
diet. The highest water concentration (30 µg/litre) multiplied by
the highest theoretical bioconcentration factor (158) gives a worst
case residue level in fish of 4.7 mg/kg. It should be noted that the
bioconcentration factor does not take into account degradation or
metabolism. Comparing this value to the estimated LC50 value gives a
Toxicity Exposure Ratio (TER) of > 1700. Therefore, the risk to
fish-eating birds is very low.
11. CONCLUSIONS
Humans are exposed to xylene mainly by inhalation. This compound
does not accumulate significantly in the human body. Acute exposure
to high concentrations can result in CNS effects in human. There have
been no long-term controlled studies or epidemiological studies with
exposure to xylene alone. The chronic toxicity appears to be
relatively low in laboratory animals. There is suggestive evidence,
however, that chronic CNS effects may occur in animals at moderate
concentrations of xylene. Xylene appears not to be a mutagen or a
carcinogen. The critical end-point is developmental toxicity. Based
on this end-point, the recommended guidance value for xylene in air
for the general population is 0.87 mg/m3 (0.2 ppm). This value is
higher than the concentrations to which the general population is
exposed.
The xylene isomers are non-persistent chemicals, being readily
degraded in the atmosphere, soil and water. It should be noted that
o-xylene appears to biodegrade only in the presence of other carbon
sources and at a reduced rate compared to the other isomers. The
photooxidation reactions of the xylene isomers in the atmosphere may
contribute to photochemical smog.
It can be concluded that xylenes are unlikely to cause problems
in aquatic ecosystems except near to localized industrial discharges
and spillage incidents. The risk to birds from xylene exposure is
low.
12. FUTURE RESEARCH
There is little information on the long-term effects of xylene in
humans and, specifically, no dose-response or dose-effect data are
available. Epidemiological studies of populations occupationally
exposed to xylene should be encouraged. In this context the use of
xylene metabolites in urine as a marker of exposure can be of special
value because the method determines the internal doses that
individuals receive via all routes of exposure. Because xylene has
acute effects on CNS, epidemiological studies should address the CNS
as a potential target organ. Moreover, since ethylbenzene is almost
invariably one of the components in solvent mixtures at the workplace,
study designs that address possible interactions between xylene and
other solvent components are desirable.
Animal studies are needed to address biochemical, functional and
morphological evidence of chronic neurotoxicity and potential effects
on fertility. In addition, there is a need for further studies on
developmental toxicity to assess the dose-response relationship and to
estimate the NOAEL, especially for developmental neurotoxicity.
Further studies are needed to determine the effect on hearing
impairment in order to determine the NOAEL. The relationship between
the dose level and the length of the exposure period should also be
investigated. The effect of exposure to xylene together with noise
should be studied.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC) has
evaluated the carcinogenicity of xylene. There was inadequate
evidence for the carcinogenicity of xylene in humans as well as in
experimental animals. The overall evaluation was that xylene is not
classifiable as to its carcinogenicity in humans (IARC, 1989).
The European Commission (1991-1992) recommended an 8-h
time-weighted average occupational exposure limit for xylene of 217
mg/m3 (50 ppm). To limit peaks of exposure that could result in
irritation, a short-term exposure level (STEL) of 435 mg/m3 (100 ppm)
was recommended.
REFERENCES
Abu Al Ragheb S, Salhab AS, & Amr SS (1986) Suicide by xylene
ingestion: A case report and review of literature. Am J Forensic Med
Pathol, 7: 327-329.
Altshuller AP, Lonneman WA, Sutterfield FD, & Kopczyncki SL (1971)
Hydrocarbon composition of the atmosphere of the Los Angeles basin,
1967. Environ Sci Technol, 5: 39.
Alvarez PJJ & Vogel TM (1991) Substrate interactions of benzene,
toluene, and para-xylene during microbial degradation by pure cultures
and mixed culture aquifer slurries. Appl Environ Microbiol,
57: 2981-2985.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3: 272-290.
Andersson K, Fuxe K, Nilsen OG, Toftgård R, Eneroth P, & Gustafsson
J-Å (1981) Production of discrete changes in dopamine and
noradrenaline levels and turnover in various parts of the rat brain
following exposure to xylene, ortho-, and para-xylene, and
ethylbenzene. Toxicol Appl Pharmacol, 60: 535-548.
Angerer J (1988a) Determination of toluene in blood by head-space gas
chromatography.
Angerer J (1988b) Determination of hippuric acid in urine by
high-pressure liquid chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 301-306 (IARC
Scientific Publications No. 85).
Angerer J & Lehnert G (1979) Occupational chronic exposure to organic
solvents. VIII. Phenolic compounds - metabolites of alkylbenzenes in
man: Simultaneous exposure to ethylbenzene and xylenes. Int Arch Occup
Environ Health, 43: 145-150.
Angerer J & Wulf H (1985) Occupational exposure to organic solvents.
XI. Alkylbenzene exposure of varnish workers: Effects on hematopoetic
system. Int Arch Occup Environ Health, 56: 307-321.
Anshelm Olson B, Gamberale F, & Iregren A (1985) Coexposure to toluene
and p-xylene in man: central nervous functions. Br J Ind Med,
42: 117-122.
Antoine SR, Deleon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36: 364-371.
Arlien-Soborg P, Zilstorff K, Grandjean B, & Milling Pedersen L (1981)
Vestibular dysfunction in occupational chronic solvent intoxication.
Clin Otolaryngol, 6: 285-290.
Aschan G, Bunnfors I, Hydén D, Larsby B, Ödkvist LM, & Tham R (1977)
Xylene exposure. Electronystagmographic and gas chromatographic
studies in rabbits. Acta Otolaryngol, 84: 370-376.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, & Wooten JV (1994) Blood
concentrations of volatile organic compounds in a nonoccupationally
exposed US population and in groups with suspected exposure. Clin
Chem, 40(7): 1401-1404.
Askergren A (1981) Studies on kidney function in subjects exposed to
organic solvents. III. Excretion of cells in the urine. Acta Med
Scand, 210: 103-106.
Askergren A, Allgén L-G, & Bergström J (1981a) Studies on kidney
function in subjects exposed to organic solvents. II. The effect of
desmopressin in a concentration test and the effect of exposure to
organic solvents on renal concentrating ability. Acta Med Scand,
209: 485-488.
Askergren A, Allgén L-G, Karlsson C, Lundberg I, & Nyberg E (1981b)
Studies on kidney function in subjects exposed to organic solvents. I.
Excretion of albumin and ß-2-microglobulin in the urine. Acta Med
Scand, 209: 479-483.
Askergren A, Brandt R, Gullquist R, Silk B, & Strandell T (1981c)
Studies on kidney function in subjects exposed to organic solvents.
IV. Effect on 51Cr-EDTA clearance. Acta Med Scand, 210: 373-376.
Åstrand I, Engström J, & Övrum P (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man. Scand J
Work Environ Health, 4: 185-194.
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions
of the hydroxyl radical with organic compounds under atmospheric
conditions. Chem Rev, 85: 69-201.
Axelsson G, Lütz C, & Rylander R (1984) Exposure to solvents and
outcome of pregnancy in university laboratory employees. Br J Ind Med,
41: 305-312.
Bakinson MA & Jones RD (1985) Gassings due to methylene chloride,
xylene, toluene, and styrene reported to Her Majesty's Factory
Inspectorate 1961-80. Br J Ind Med, 42: 184-190.
Bakke OM & Scheline RR (1970) Hydroxylation of aromatic hydrocarbons
in the rat. Toxicol Appl Pharmacol, 16: 691-700.
Barker JF, Barbash JE, & Labonte M (1988) Groundwater contamination at
a landfill sited on fractured carbonate and shale. J Contam Hydrol,
3: 1-25.
Batchelor JJ (1927) The relative toxicity of benzol and its higher
homologues. Am J Hyg, 7: 276-298.
Beirne GJ & Brennan JT (1972) Glomerulonephritis associated with
hydrocarbon solvents. Mediated by antiglomerular basement membrane
antibody. Arch Environ Health, 25: 365-369.
Bell GM, Gordon ACH, Lee P, Doig A, MacDonald MK, Thomson D, Anderton
JL, & Robson JS (1985) Proliferative glumerulonephritis and exposure
to organic solvents. Nephron, 40: 161-165.
Bell GM, Shillaker RO, Padgham MDJ, & Standring P (1992) Xylenes.
London, Health and Safety Executive, 166 pp (Toxicity Review 26).
Benville PE & Korn S (1977) The acute toxicity of six monocyclic
aromatic crude oil components to striped bass (Morone saxatilis) and
bay shrimp (Crago franciscorum). Calif Fish Gam, 63: 204-209.
Bergman K (1979) Whole-body autoradiography and allied tracer
techniques in distribution and elimination studies of some organic
solvents. Scand J Work Environ Health, 5(suppl 1): 1-263.
Bergman K (1983) Application and results of whole-body autoradiography
in distribution studies of organic solvents. CRC Crit Rev Toxicol,
12: 59-118.
Berry WO & Brammer JD (1977) Toxicity of water-soluble gasoline
fractions to fourth-instar larvae of the mosquito Aedes aegypti L.
Environ Pollut, 13: 229-234.
Bevan MAJ, Proctor CJ, Baker-Rogers J, & Warren ND (1991) Exposure to
carbon monoxide, respirable suspended particulates and volatile
organic compounds while commuting by bicycle. Environ Sci Techol,
25: 788-791.
Bieniek G, Palys E, & Wilczok T (1982) TLC separation of hippuric,
mandelic, and phenylglyoxylic acids from urine after mixed exposure to
toluene and styrene. Br J Ind Med, 39: 187-190.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, University of
Kentucky, Water Resources Research Institute, 61 pp (Research Report
No. 133).
Blanchard KT & Morris JB (1994) Effects of m-xylene on rat nasal
cytochrome P450 mixed function oxidase activities. Toxicol Lett,
70: 253-259.
Blum DJW & Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. J Water Pollut Control Fed, 63: 198-207.
Bobra AM, Shiu WY, & Mackay D (1983) A predictive correlation for the
acute toxicity of hydrocarbons and chlorinated hydrocarbons to the
water flea (Daphnia magna). Chemosphere, 12: 1121-1129.
Bonnet P, Raoult G, & Gradiski D (1979) Concentrations léthales50 des
principaux hydrocarbures aromatiques. Arch Mal Prof, 40: 805-810.
Bonnet P, Morele Y, Raoult G, Zissu D, & Gradiski D (1982)
Détermination de la concentration léthale50 des principaux
hydrocarbures aromatiques chez le rat. Arch Mal Prof, 43: 261-265.
Bos RP, Brouns RME, van Doorn R, Theuws JLG, & Henderson PT (1981)
Non-mutagenicity of toluene, o-, m- and p-xylene, o-methyl-
benzylalcohol and o-methylbenzylsulfate in the Ames assay.
Mutat Res, 88: 273-279.
Boström E-C, Almén J, Steen B, & Westerholm R (1994) Human exposure to
urban air pollution. Environ Health Perspect, 102(suppl 4): 39-47.
Botta D, Pirri LC, & Mantica E (1984) Ground water pollution by
organic solvents and their microbial degradation products. In:
Analytical Organic Micropollutants in Water. Luxembourg, Commission of
the European Communities, pp 261-275 (Report EUR 8518).
Bowers DE Jr, Cannon MS, & Jones DH (1982) Ultrastructural changes in
livers of young and aging rats exposed to methylated benzenes. Am J
Vet Res, 43: 676-683.
Bray HG, Humphris BG, & Thorpe WV (1949) Metabolism of derivatives of
toluene. 3. o-, m- and p-xylenes. Biochem J, 45: 241-244.
Brice KA & Derwent RG (1978) Emission inventory for hydrocarbons in
the United Kingdom. Atmos Environ, 12: 2045-2054.
Bridie AL, Wolff CJM, & Winter M (1979) The acute toxicity of some
petrochemicals to goldfish. Water Res, 13: 623-626.
Bringmann G & Kühn R (1977) [Toxic threshold values for compounds
hazardous to the aquatic environment on bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda) using cell
multiplication test.] Z Wasser Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kühn R (1978) [Toxic threshold values for compounds
hazardous to the aquatic environment on blue algae (Microcystic
aeruginosa) and green algae (Scenedesmus quadricauda) using cell
multiplication test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1982) [Results of the toxic effects of compounds
hazardous to the aquatic environment on Daphnia magna using an
improved standardized test.] Z Wasser Abwasser Forsch, 15: 1-6 (in
German).
Bringmann G, Kühn R, & Winter A (1980) [Determination of biocidal
effects of compounds hazardous to the aquatic environment for
Protozoa: III. Saprozoic flagelates.] Z Wasser Abwasser Forsch,
13: 170-173 (in German).
Brodzinsky R & Singh HB (1983) Volatile organic chemicals in the
atmosphere: An assessment of available data. Washington, DC, US
Environmental Protection Agency (EPA/DF-83/005A).
Brooks JM, Fryxell GA, Reid DF, & Sackett WM (1977) Gulf underwater
flare experiment (GULFEX): Effects of hydrocarbons on phytoplankton
In: Pollutant effects on marine organisms. Proceedings of a Workshop,
College station, Texas, USA, 16-19 May 1976, pp 45-75.
Brown RH (1988a) Determination of benzene, toluene and xylene in
industrial air by charcoal tube, solvent desorption and gas
chromatography. In: Fishbein L & O'Neill IK ed. Environmental
carcinogens: Methods of analysis and exposure measurement. Volume
10: Benzene and alkylated benzenes. Lyon, International Agency for
Research on Cancer, pp 225-233 (IARC Scientific Publications No. 85).
Brown RH (1988b) Determination of benzene, toluene and xylene in
industrial air by porous polymer adsorption tube, thermal desorption
and gas chromatography. In: Fishbein L & O'Neill IK ed. Environmental
carcinogens: Methods of analysis and exposure measurement. Volume
10: Benzene and alkylated benzenes. Lyon, International Agency for
Research on Cancer, pp 235-242 (IARC Scientific Publications No. 85).
Brown RH (1988c) Determination of gasoline hydrocarbons in industrial
air by two-stage (chromosorb/charcoal) adsorption, thermal desorption
and capillary gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 243-251 (IARC
Scientific Publications No. 85).
Brown-Woodman PDC, Webster WS, Picker K, & Ritchie HE (1991)
Embryotoxicity of xylene and toluene: an in study. Ind Health,
29: 139-152.
Brown-Woodman PDC, Webster WS, Picker K, & Huq F (1994) In
assessment of individual and interactive effects of aromatic
hydrocarbons on embryonic development of the rat. Reprod Toxicol,
8: 121-135.
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other trace
organic compounds in urban air. Chemosphere, 17: 2363-2380.
Bushnell PJ (1989) Behavioral effects of acute p-xylene inhalation
in rats: Autoshaping, motor activity, and reversal learning.
Neurotoxicol Teratol, 10: 569-577.
Bushnell PJ & Peele DB (1988) Conditioned flavor aversion induced by
inhaled p-xylene in rats. Neurotoxicol Teratol, 10: 273-277.
Buszka PM, Zaugg SD, & Werner MG (1990) Determination of trace
concentrations of volatile organic compounds in ground water using
closed-loop stripping, Edwards Aquifer, Texas. Bull Environ Contam
Toxicol, 45: 507-515.
Buttery RG, Seifert RM, & Ling LC (1975) Characterization of some
volatile constituents of dry red beans. J Agric Food Chem,
23: 516-519.
Caldwell RS, Caldarone EM, & Mallon MH (1977) Effects of a
seawater-soluble fraction of Cook Inlet crude oil and its major
aromatic components on larval stages of the Dungeness crab, Cancer
magister Dana. In: Wolfe DA ed. Fate and effects of petroleum
hydrocarbons in marine ecosystems and organisms. Oxford, New York,
Pergamon Press, pp 210-220.
Cameron GR, Paterson JLH, de Saram GSW, & Thomas JC (1938) The
toxicity of some methyl derivatives of benzene with special reference
to pseudocumene and heavy coal tar naphta. J Pathol Bacteriol,
46: 95-107.
Campbell L, Wilson HK, Samuel AM, & Gompertz D (1988) Interactions of
m-xylene and aspirin metabolism in man. Br J Ind Med, 45: 127-132.
Carlsson A (1981) Distribution and elimination of 14C-xylene in rat.
Scand J Work Environ Health, 7: 51-55.
Carpenter CP, Kinkead ER, Geary DL Jr, Sullivan LJ, & King JM (1975)
Petroleum hydrocarbon toxicity studies. V. Animal and human response
to vapors of mixed xylenes. Toxicol Appl Pharmacol, 33: 543-558.
Chan C-C, Özkaynak H, Spengler JD, & Sheldon L (1991a) Driver exposure
to volatile organic compounds, CO, ozone and NO2 under different
driving conditions. Environ Sci Techol, 25: 964-972.
Chan C-C, Spengler JD, Ozkaynak H, & Lefkopoulou M (1991b) Commuter
exposures to VOCs in Boston, Massachusetts. J Air Waste Manage Assoc,
41: 1594-1600.
Chan C-C, Lin S-H, & Her GR (1993) Student's exposure to volatile
organic compounds while commuting by motorcycle and bus in Taipei
City. J Air Waste Manage Assoc, 43(9): 1231-1238.
Chen Z, Liu S-J, Cai S-X, Yao Y-M, Yin H, Ukai H, Uchida Y, Nakatsuka
H, Watanabe T, & Ikeda M (1994) Exposure of workers to a mixture of
toluene and xylenes. II. Effects. Occup Environ Med, 51: 47-49.
Chiang CY, Salanito JP, Chai EY, Colthart JD, & Klein CL (1989)
Aerobic biodegradation of benzene, toluene and xylene in a sandy
aquifer - Data analysis and computer modelling. Ground Water,
27: 823-834.
Chung TY, Hayase F, & Kato H (1983) Volatile components of ripe
tomatoes and their juices, purees and pastes. Agric Biol Chem,
47: 343-351.
City of Toronto (1990) The quality of drinking water in Toronto: A
review of tap water, bottled water and water treated by a point-of-use
device. Toronto, Canada, Department of Public Health.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural and motorway locations.
Environ Pollut, B7: 141-158.
Condie LW, Hill JR, & Borzelleca JF (1988) Oral toxicology studies
with xylene isomers and mixed xylenes. Drug Chem Toxicol, 11: 329-354.
Connor TH, Theiss JC, Hanna HA, Monteith DK, & Matney TS (1985)
Genotoxicity of organic chemicals frequently found in the air of
mobile homes. Toxicol Lett, 25: 33-40.
Cragg ST, Clarke EA, Daly IW, Miller RR, Terrill JB, & Ouellette RE
(1989) Subchronic inhalation toxicity of ethylbenzene in mice, rats,
and rabbits. Fundam Appl Toxicol, 13: 399-408.
Cramer PH, Boggess KE, Hosenfeld JM, Remmers JC, Breen JJ, Robinson
PE, & Stroup C (1988) Determination of organic chemicals in human
whole blood: Preliminary method development for volatile organics.
Bull Environ Contam Toxicol, 40: 612-681.
Craveri A, Tornaghi G, Ciaci D, Vitali L, Carucci R, De Luca P, &
Donato MF (1982) [Serum bile salts in the hepatological screening of
subjects exposed to aromatic hydrocarbons.] Giorn Clin Med,
63: 322-329 (in Italian).
Crawford L & Kretsch MJ (1976) GC-MS identification of the volatile
compounds extracted from roasted turkeys fed a basal diet supplemented
with tuna oil: Some comments on fishy flavour. J Food Sci,
41: 1470-1478.
Crofton KM, Lassiter TL, & Rebert CS (1994) Solvent-induced
ototoxicity in rats: an atypical selective mid-frequency hearing
deficit. Hear Res, 80: 25-30.
Daisey JM, Hodgson AT, Fisk WJ, Mendell MJ, & Ten Brinke J (1994)
Volatile organic compounds in twelve California office buildings:
classes, concentrations and sources. Atmos Environ, 28(22): 3557-3562.
Davey JF & Gibson DT (1974) Bacterial metabolism of para- and
meta-xylene: Oxidation of a methyl substituent. J Bacteriol,
119: 923-929.
Davis RS, Hossler FE, & Stone RW (1967) Oxidation of m- and p-
xylenes by Pseudomonas. Bateriol Proc, P162: 129.
Day BJ & Carlson GP (1992) Effect of p-xylene metabolites,
p-methylbenzyl alcohol and 2,5-dimethylphenol on rat hepatic and
pulmonary microsomal metabolism. Res Commun Chem Pathol Pharmacol,
76: 117-120.
Day BJ, DeNicola DB, Marcus CB, & Carlson GP (1992) Effect of
p-xylene inhalation on the bioactivation of bromobenzene in rat lung
and liver. Fundam Appl Toxicol, 19: 50-56.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
De Ceaurriz J, Desiles JP, Bonnet P, Marignac B, Muller J, & Guenier
JP (1983) Concentration-dependent behavioral changes in mice following
short-term inhalation exposure to various industrial solvents. Toxicol
Appl Pharmacol, 67: 383-389.
DECOS (1991) Health-based recommended occupational exposure limit for
xylene. The Hague, Directorate-General of Labour.
De Gandarias JM, Echevarria E, Irazusta J, Gil J, & Casis L (1993)
Brain aminopeptidase activity after subacute xylene exposure.
Neurotoxicol Teratol, 15: 51-53.
del Rosario R, de Lumen BO, Habu T, Flath RA, Mon TR, & Teranishi R
(1984) Comparison of headspace volatiles from winged beans and
soybeans. J Agric Food Chem, 32: 1011-1015.
Derwent RG & Jenkin ME (1990) Hydrocarbon involvement in photochemical
ozone formation in Europe. Oxfordshire, Harwell Laboratory, AEA
Environment and Energy, Modelling and Assessments Group (Report AERE
R13736).
Dési I, Kovacs F, Zahumenszky Z, & Balogh A (1967) Maze learning in
rats exposed to xylene intoxication. Psychopharmacologia (Berl),
11: 224-230.
Divincenzo GD & Krasavage WJ (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic solvents.
Am Ind Hyg Assoc J, 35: 21-29.
Donner M, Mäki-Paakkanen J, Norppa H, Sorsa M, & Vainio H (1980)
Genetic toxicology of xylenes. Mutat Res, 74: 171-172.
Dössing M, Arlien Söborg P, Milling Pedersen L, & Ranek L (1981)
[Liver damage following long-term occupational exposure to organic
solvents.] Ugeskr Laeg, 143: 2297-2301 (in Danish with English
summary).
Dössing M, Arlien Söborg P, Milling Pedersen L, & Ranek L (1983) Liver
damage associated with occupational exposure to organic solvents in
house painters. Eur J Clin Invest, 13: 151-157.
Dowden BF & Bennett HJ (1965) Toxicity of selected chemicals to
certain animals. J Water Pollut Control Fed, 37: 1308-1316.
Dowty BJ & Laseter JL (1976) The transplacental migration and
accummulation in blood of volatile organic constituents. Ped Res,
10: 696-701.
Dowty BJ, Carlisle DR, & Laseter JL (1975) New Orleans drinking water
sources tested by gas chromatography-mass spectrometry. Environ Sci
Techol, 9: 762-765.
Drew RT & Fouts JR (1974) The effects of inducers on acute p-xylene
toxicity. Toxicol Appl Pharmacol, 29: 111-112.
Drozd J & Novak J (1978) Quantitative and qualitative head-space gas
analysis of parts per billion amounts of hydrocarbons in water: A
study of model systems by capillary-column gas chromatography with
splitless sample injection. J Chromatogr, 158: 471-482.
Dudek B, Gralewicz K, Jakubowski M, Kostrzewski P, & Sokal J (1990)
Neurobehavioral effects of experimental exposure to toluene, xylene
and their mixture. Pol J Occup Med, 3: 109-116.
Dunstan WM, Atkinson LP, & Natoli J (1975) Stimulation and inhibition
of phytoplankton growth by low molecular weight hydrocarbons. Mar
Biol, 31: 305-310.
Dutkiewicz T & Tyras H (1968) Skin absorption of toluene, styrene, and
xylene by man. Br J Ind Med, 25: 243.
Dyer RS, Bercegeay MS, & Mayo LM (1988) Acute exposures to p-xylene
and toluene alter visual information processing. Neurotoxicol Teratol,
10: 147-153.
EAJ (Environment Agency, Japan) (1993) Chemicals in the environment.
Tokyo, Ministry of Health and Welfare, International Affairs Division.
ECETOC (1986) Joint assessment of commodity chemicals No. 6: Xylenes.
Brussels, European Chemical Industry Ecology and Toxicology Centre.
Edling C (1982) Interaction between drugs and solvents as a cause of
fatty change in the liver? Br J Ind Med, 39: 198-199.
Edwards EA & Grbic-Galic D (1994) Anaerobic degradation of toluene and
o-xylene by a methanogenic consortium. Appl Environ Microbiol,
60(1): 313-322.
Edwards EA, Willis LE, Reinhard M, & Grbic-Galic D (1992) Anaerobic
degradation of toluene and xylene by aquifer microorganisms under
sulfate-reducing conditions. Appl Environ Microbiol, 58: 794-800.
Eller PM (1984) NIOSH manual of analytical methods: Method 1501, 3rd
ed. Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 2.
Elofsson S-A, Gamberale F, Hindmarsh T, Iregren A, Isaksson A,
Johnsson I, Knave B, Lydahl E, Mindus P, Persson HE, Philipson B,
Steby M, Struwe G, Söderman E, Wennberg A, & Widén L (1980) Exposure
to organic solvents: A cross-sectional epidemiologic investigation on
occupationally exposed car and industrial spray painters with special
reference to the nervous system. Scand J Work Environ Health,
6: 239-273.
Elovaara E (1982) Dose-related effects of m-xylene inhalation on the
xenobiotic metabolism of the rat. Xenobiotica, 12: 345-352.
Elovaara E, Collan Y, Pfäffli P, & Vainio H (1980) The combined
toxicity of technical grade xylene and ethanol in the rat.
Xenobiotica, 10: 435-445.
Elovaara E, Pfäffli P, Savolainen H, & Vainio H (1982) Marginal role
of impaired aldehyde metabolism in m-xylene vapour-induced
biochemical effects in the rat. J Appl Toxicol, 2: 27-32.
Elovaara E, Engström K, & Vainio H (1984) Metabolism and disposition
of simultaneously inhaled m-xylene and ethylbenzene in the rat.
Toxicol Appl Pharmacol, 75: 466-478.
Elovaara E, Zitting A, Nickels J, & Aitio A (1987) m-Xylene
inhalation destroys cytochrome P-450 in rat lung at low exposure. Arch
Toxicol, 61: 21-26.
Engelhardt WE & Estler W (1935) [Experiments on the acute narcotic
effects of aliphatic and aromatic hydrocarbons: The effect of single
inhalation of gasoline, benzene, toluene, xylene in the rabbit and the
cat.] Ark Hyg, 114: 249-260 (in German).
Engström J & Bjurström R (1978) Exposure to xylene and ethylbenzene.
II. Concentration in subcutaneous adipose tissue. Scand J Work Environ
Health, 4: 195-203.
Engström J & Riihimäki V (1979) Distribution of m-xylene to
subcutaneous adipose tissue in short-term experimental human exposure.
Scand J Work Environ Health, 5: 126-134.
Engström K & Riihimäki V (1988a) Determination of xylenes in blood by
head-space gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 307-311 (IARC
Scientific Publications No. 85).
Engström K & Riihimäki V (1988b) Determination of methylhippuric acids
in urine by gas chromatography. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 313-318 (IARC
Scientific Publications No. 85).
Engström K, Husman K, & Riihimäki V (1977) Percutaneous absorption of
m-xylene in man. Int Arch Occup Environ Health, 39: 181-189.
Engström K, Husman K, Pfäffli P, & Riihimäki V (1978) Evaluation of
occupational exposure to xylene by blood, exhaled air and urine
analysis. Scand J Work Environ Health, 4: 114-121.
Engström K, Riihimäki V, & Laine A (1984) Urinary disposition of
ethylbenzene and m-xylene in man following separate and combined
exposure. Int Arch Occup Environ Health, 54: 355-363.
European Commission (1991-1992) Occupational exposure limits.
Recommendations of the Scientific Expert Group 1991-1992. Luxembourg,
European Commission, pp 53-55 (Report EUR 15091 EN).
Fabre R, Truhaut R, & Laham S (1960) Recherches toxicologiques sur les
solvants de remplacement du benzčne. IV. Etude des xylčnes. Arch Mal
Prof, 21: 301-313.
Fairfax R (1995) Solvent, wood dust, and noise exposure in a decoy
shop. Appl Occup Environ Hyg, 10(5): 446-448.
Falk-Petersen I-B, Kjrsvic E, Lonning S, Naley AM, & Sydnes LK (1985)
Toxic effects of hydroxylated aromatic hydrocarbons on marine embryos.
Sarsia, 70: 11-16.
Fellin P & Otson R (1993) Seasonal trends of volatile organic
compounds (VOCs) in Canadian homes. In: Indoor air '93. Proceedings of
the 6th International Conference on Indoor Air Quality and Climate,
Helsinki, Finland, 4-8 July 1993 - Volume 2: Chemicals in indoor air,
materials emissions, pp 117-122.
Ferrario JB, Lawler GC, Deleon IR, & Laseter JL (1985) Volatile
organic pollutants in biota and sediments of Lake Pontchartrain. Bull
Environ Contam Toxicol, 34: 246-255.
Fielding M, Gibson TM, James HA, McLoughlin K, & Steel CP (1981)
Organic micropollutants in drinking water. Medmenham, Marlow, United
Kingdom, Water Research Centre (Technical Report TR-159).
Fishbein L (1988) Xylenes: Uses, occurrence and exposure. In: Fishbein
L & O'Neill IK ed. Environmental carcinogens: Methods of analysis and
exposure measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 109-120 (IARC
Scientific Publications No. 85).
Fischbein A, Ross RR, & Lerman Y (1983) Industrial solvents and the
liver. Lancet, 1: 129.
Fishbein L, O'Neill IK, & Tossavainen A (1988) Past and present
exposure determination techniques for benzene, toluene and xylene. In:
Fishbein L & O'Neill IK ed. Environmental carcinogens: Methods of
analysis and exposure measurement. Volume 10: Benzene and alkylated
benzenes. Lyon, International Agency for Research on Cancer,
pp 123-148 (IARC Scientific Publications No. 85).
Folmar LC (1976) Overt avoidance reaction of rainbow trout fry to nine
herbicides. Bull Environ Contam Toxicol, 15: 509-514.
Foster P, Laffond M, Baussand P, Jacob V, & Denis I (1991) VOC
measurements in the Grenoble area and study of the benzaldehyde
behaviour in simulation chamber. Pollut Atmos, 1991: 175-191.
Franchini I, Cavatorta A, Falzoi M, Lucertini S, & Mutti A (1983)
Early indicators of renal damage in workers exposed to organic
solvents. Int Arch Occup Environ Health, 52: 1-9.
Frank PA, Otto NE, & Bartley TR (1961) Techniques for evaluating
aquatic weed herbicides. Weeds, 9: 515-521.
Frantik E, Hornychova M, & Vodickova L (1993) Equipotential
subnarcotic concentrations of solvents in air, in blood, and at the
target structure. Homeostasis, 34: 143-147.
Frantik E, Hornychova M, & Horvath M (1994) Relative acute
neurotoxicity of solvents: Isoeffective air concentrations of 48
compounds evaluated in rats and mice. Environ Res, 66: 173-185.
Frantik E & Vodickova L (1995) Combined effects of binary solvent
mixtures. Cent Eur J Occup Environ Med, 1: 31-37.
Funes-Cravioto F, Kolmodin-Hedman B, Lindsten J, Nordenskjöld M,
Zapata-Gayon C, Lambert B, Norberg E, Olin R, & Swensson Å (1977)
Chromosome aberrations and sister-chromatid exchange in workers in
chemical laboratories and a rotoprinting factory and in children of
women laboratory workers. Lancet, 2: 322-325.
Furnas DW & Hine CH (1958) Neurotoxicity of some selected hydro-
carbons. Arch Ind Health Occup Med, 18: 9-15.
Fustinoni S, Buratti M, Giampiccolo R, & Colombi A (1995) Biological
and environmental monitoring of exposure to airborne benzene and other
aromatic hydrocarbons in Milan traffic wardens. Toxicol Lett,
77(13): 387-392.
Gab S, Schmizer J, Tham H, Parlar H, & Korte F (1977) Photo-
mineralisation rate of organic compounds adsorbed on particulate
matter. Nature (Lond), 270: 331-333
Galassi S, Mingazzini M, Vigano L, Cesareo D, & Tosato ML (1988)
Approaches to modelling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicol Environ Saf, 16: 158-169.
Gamberale F, Annwall G, & Hultengren M (1978) Exposure to xylene and
ethylbenzene. III. Effects on central nervous functions. Scand J Work
Environ Health, 4: 204-211.
Gerner-Smidt P & Friedrich U (1978) The mutagenic effect of benzene,
toluene and xylene studied by the SCE technique. Mutat Res,
58: 313-316.
Gery MW, Fox DL, Kaems RM, & Stockburger L (1987) Investigation of
hydroxyl radical reactions with o-xylene and m-xylene in a
continuous stirred tank reactor. Environ Sci Techol, 21: 339-348.
Ghantous H & Danielsson BG (1986) Placental transfer and distribution
of toluene, xylene and benzene, and their metabolites during gestation
in mice. Biol Res Pregnancy, 7: 98-105.
Ghantous H, Dencker L, Gabrielsson J, Danielsson BRG, & Bergman K
(1990) Accumulation and turnover of metabolites of toluene and xylene
in nasal mucosa and olfactory bulb in the mouse. Pharmacol Toxicol,
66: 87-92.
Ghislandi E & Fabiani A (1957) [Liver damage due to accidental
ingestion of nitrocellulose paints.] Med Lav, 48: 577-579 (in Italian
with English summary).
Ghosh TK & Pradhan SN (1987) Comparison of effects of xylene and
toluene inhalation on fixed-ratio liquid-reinforced behavior in rats.
Res Commun Psychol Psychiatr Behav, 12: 305-312.
Ghosh TK, Copeland RL Jr, Parui RN, Mookherjee S, & Pradhan SN (1987)
Effect of xylene inhalation on fixed-ratio responding in rats.
Pharmacol Biochem Behav, 27: 653-657.
Gilli G, Scursatone E, & Bono R (1994) Benzene, toluene and xylenes in
air, geographical distribution in Piedmont region (Italy) and personal
exposure. Sci Total Environ, 148(1): 49-56.
Glass WI (1961) Annotation: A case of suspected xylol poisoning. N Z
Med J, 60: 113.
Gomez-Belinchon JI, Grimalt JO, & Abaigés J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water Res, 25: 577-589.
Grob K & Grob G (1971) Gas-liquid chromatographic - mass spectrometric
investigation of C6-C20 organic compounds in an urban atmosphere: An
application of ultra trace analysis on capillary columns.
J Chromatogr, 62: 1-13.
Grosjean D (1990) Atmospheric chemistry of toxic contaminants. 1.
Reaction rates and atmospheric persistence. J Air Waste Manage Assoc,
40: 1397-1402.
Gschwend PM, Zafiriou OC, Mantoura RFC, Schwarzenbach RP, & Gagosian
RB (1982) Volatile organic compounds at a coastal site. I. Seasonal
variations. Environ Sci Techol, 1: 31-38.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gut I & Flek J (1981) [Effect of microsomal enzyme induction by
phenobarbital on the metabolism of benzene, fluorobenzene, m-xylene
and p-xylene.] Prakov Lek, 33: 124-127 (in Czech with English
summary).
Gut I, Terelius Y, Frantik E, Linhart I, Soucek P, Filipcova B, &
Kluchova H (1993) Exposure to various benzene derivatives differently
induces cytochromes P450 2B1 and P450 2E1 in rat liver. Arch Toxicol,
67: 237-243.
Haglund U, Lundberg I, & Zech L (1980) Chromosome aberrations and
sister chromatid exchanges in Swedish paint industry workers. Scand J
Work Environ Health, 6: 291-298.
Halder CA, Holdsworth CE, Cockrell BY, & Piccirillo VJ (1985)
Hydrocarbon nephropathy in male rats: Identification of the
nephrotoxic components of unleaded gasoline. Toxicol Ind Health,
1: 67-87.
Harland BJ, Whitby FJ, & Comber MHI (1985) Measurement of volatile
aromatic hydrocarbons in an industrial estuary. Int J Environ Anal
Chem, 20: 295-312.
Harper C (1975) p-Xylene metabolism by rat pulmonary and hepatic
microsomes. Fed Proc, 34: 785.
Hass U & Jakobsen BM (1993) Prenatal toxicity of xylene inhalation in
the rat: A teratogenicity and postnatal study. Pharmacol Toxicol,
73: 20-23.
Hass U, Lund SP, Simonsen L, & Schaich Fries A (1995) Effects of
prenatal exposure to xylene on postnatal development and behavior in
rats. Neurotoxicol Teratol, 17: 341-349.
Hastings L, Cooper GP, & Burg W (1984) Human sensory response to
selected petroleum hydrocarbons. Adv Med Environ Toxicol, 6: 255-270.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 5(suppl 1): 3-142.
Hawthorne SB & Sievers RE (1984) Emission of organic air pollutants
from shale oil waste waters. Environ Sci Techol, 18: 483-490.
Herman DC, Inniss WE, & Mayfield CI (1990) Impact of volatile aromatic
hydrocarbons on growth of the freshwater alga Selenastrum
capricornutum. Aquat Toxicol, 18: 87-100.
Higgins CE, Griest WH, & Olerich G (1983) Application of tenax
trapping to analysis of gas phase organic compounds in ultra-low tar
cigarette smoke. J Assoc Off Anal Chem, 66(5): 1074-1083.
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington, DC,
US Department of the Interior, Fish and Wildlife Service, 138 pp (Fish
and Wildlife Technical Report No. 2).
Hine CH & Zuidema HH (1970) The toxicological properties of hydro-
carbon solvents. Ind Med, 38: 215-220.
Hipolito RN (1980) Xylene poisoning in laboratory workers: Case
reports and discussion. Lab Med, 11: 593-595.
Holcombe GW, Phipps GL, Sulaiman AH, & Hoffman AD (1987) Simultaneous
multiple species testing: Acute toxicity of 13 chemicals to 12 diverse
freshwater amphibian, fish, and invertebrate families. Arch Environ
Contam Toxicol, 16: 697-710.
Holmberg B, Jakobson I, & Malmfors T (1974) The effect of organic
solvents on erythrocytes during hypotonic hemolysis. Environ Res,
7: 193-205.
Honma T, Sudo A, Miyagawa M, Sato M, & Hasegawa H (1983) Significant
changes in the amounts of neurotransmitter and related substances in
rat brain induced by subacute exposure to low levels of toluene and
xylene. Ind Health, 21: 143-151.
Huang M-Y, Jin C, Liu Y-T, Li B-H, Qu QS, Ushida Y, Inoue O, Nakatsuka
M, Watanabe T, & Ikeda M (1994) Exposure of workers to a mixture of
toluene and xylenes. I. Metabolism. Occup Environ Med, 51: 42-46.
Hudak A & Ungvary G (1978) Embryotoxic effects of benzene and its
methyl derivatives: Toluene, xylene. Toxicology, 11: 55-63.
Huff JE, Eastin W, Roycroft J, Eustis SL, & Haseman JK (1988)
Carcinogenesis studies of benzene, methyl benzene, and dimethyl
benzenes. Ann NY Acad Sci, 534: 427-440.
Hung I-F & Liao M-H (1991) Aromatic hydrocarbons in indoor air. J
Environ Sci Health, A26: 487-492.
Hurford N, Law RJ, Fileman TW, Payne AP, & Colcomb-Heiliger K (1990)
Concentrations of chemicals in the North Sea due to operational
discharges from chemical tankers: Results from the second survey,
October 1988. Oil Chem Pollu, 7: 241-270.
Husman K (1980) Symptoms of car painters with long-term exposure to a
mixture of organic solvents. Scand J Work Environ Health, 6: 19-32.
Husman K & Karli P (1980) Clinical neurological findings among car
painters exposed to a mixture of organic solvents. Scand J Work
Environ Health, 6: 33-39.
Hutchins SR (1991) Biodegradation of monoaromatic hydrocarbons by
aquifer microrganisms using oxygen, nitrate or nitrous oxide as the
terminal electron acceptor. Appl Environ Microbiol, 57: 2403-2407.
Hutchins SR, Sewell GW, Kovacs DA, & Smith GA (1991a) Biodegradation
of aromatic hyrocarbons by aquifer microroganisms under denitrifying
conditions. Environ Sci Techol, 25: 68-76.
Hutchins SR, Downs WC, Wilson JT, Smith GB, Kovacs DA, Fine DD,
Douglass RH, & Hendrix DJ (1991b) Effect of nitrate addition on
biorestoration of fuel-contaminated aquifer: Field demonstration.
Ground Water, 29: 571-580.
Hutchinson TC, Hellebust JA, Tam D, Mascarenhas RA, & Shiu WY (1980)
The correlation of the toxicity to algae of hydrocarbons and
halogenated hydrocarbons with their physical-chemical properties.
Environ Sci Res, 16: 577-586.
IARC (1989) Xylene. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research on
Cancer, pp 125-156 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 47).
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: Assessing human health risks of chemicals:
Derivation of guidance values for health-based exposure limits. World
Health Organization, Geneva, 73 pp.
Ivanovich E, Antov G, Goranova L, Spasovski M, Bainova A, & Vasileva Z
(1985) Combined effect of some physical and chemical factors. J Hyg
Epidemiol Microbiol Immunol, 28: 105-110.
Jacobs G, Martens M, & Mosselmans G (1987) Proposal of limit
concentrations for skin irritation within the context of a new EEC
directive on the classification and labelling of preparations. Regul
Toxicol Pharmacol, 7: 370-378.
Jamison VW, Raymond RL, & Hudson JD (1976) Biodegradation of
high-octane gasoline. In: Proceedings of the 3rd International
Biodegradation Symposium. Allied Science Publishers, pp 187-196.
Jenkins LJ Jr, Jones RA, & Siegel J (1970) Long-term inhalation
screening studies of benzene, toluene, o-xylene, and cumene on
experimental animals. Toxicol Appl Pharmacol, 16: 818-823.
Jones BMR (1988) The measurement of ambient hydrocarbon concentrations
in the atmosphere at Harwell during the period April 1986 to March
1987. Oxfordshire, Harwell Laboratoty (AERE Report R13174).
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and vehicle emissions. Environ Int, 11: 383-392.
Jori A, Calamari D, Di Domenico A, Galli CL, Galli E, Marinovich M, &
Silano V (1986) Ecotoxicological profile of xylenes. Ecotoxicol
Environ Saf, 11: 44-80.
Joyner RE & Leak Pegues W (1961) A health hazard associated with epoxy
resin-concrete dust. J Occup Med, 3: 211-214.
Juhnke I & Lüdemann D (1978) [Results of acute fish toxicity of 200
chemical compounds using the golden orfe tests.] Z Wasser Abwasser
Forsch, 11: 161-164 (in German).
Jüttner F (1988a) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in the forest air of the Southern
Black Forest. Chemosphere, 17: 309-317.
Jüttner F (1988b) Motor-boat-derived volatile organic compounds (VOC)
in lakewater. Z Wasser Abwasser Forsch, 21: 36-39.
Kaneko T, Wang P-Y, Tsukada H, & Sato A (1995) m-Xylene
toxicokinetics in phenobarbital-treated rats: comparison among
inhalation exposure, oral administration, and intraperitoneal
administration. Toxicol Appl Pharmacol, 131: 13-20.
Kappeler Th & Wuhrmann K (1978a) Microbial degradation of the water
soluble fraction of gas oil. I. Water Res, 12: 327-333.
Kappeler Th & Wuhrmann K (1978b) Microbial degradation of the water
soluble fraction of gas oil. II. Bioassays with pure strains. Water
Res, 12: 335-342.
Karlson U & Frankenberger WT (1989) Microbial degradation of benzene
and toluene in groundwater. Bull Environ Contam Toxicol, 43: 505-510.
Kauss PB & Hutchinson TC (1975) The effects of water-soluble petroleum
components on the growth of Chlorella vulgaris Beijerinck. Environ
Pollut, 9: 157-174.
Kauss PB, Hutchinson TC, Soto C, Hellebust J, & Griffiths M (1973) The
toxicity of crude oil and its components to freshwater algae. In:
Prevention and control of spills. Washington, DC, American Petroleum
Institute, pp 703-714.
Kawai T, Mizunuma K, Yasugi T, Horiguchi S, Uchida Y, Iwami O, Iguchi
H, & Ikeda M (1991a) Urinary methylhippuric acid isomer levels after
occupational exposure to a xylene mixture. Int Arch Occup Environ
Health, 63: 69-75.
Kawai T, Yamaoka K, Uchida Y, & Ikeda M (1991b) Exposure to vapors of
benzene and other aromatic solvents in tank truck loading and
delivery. Bull Environ Contam Toxicol, 46(1): 1-8.
Kawamura K & Kaplan IR (1983) Organic compounds in the rain water of
Los Angeles. Environ Sci Technol, 17: 497-501.
Kawata K & Fujieda Y (1993) [Volatile aromatic hydrocarbons in ambient
air.] Jpn J Toxicol Environ Health, 39(1): 37-43 (in Japanese).
Kennah HE, Hignet S, Laux PE, Dorko JD, & Barrow CS (1989) An
objective procedure for quantitating eye irritation based upon changes
of corneal thickness. Fundam Appl Toxicol, 12: 258-268.
Kennicutt MC II, Brooks JM, & McDonald TJ (1984) Volatile organic
inputs from an ocean outfall near Barceloneta, Puerto Rico.
Chemosphere, 13: 535-548.
Kenrick MAP, Clark L, Baxter KM, Fleet M, James HA, Gibson TM, &
Turrell MB (1985) Trace organics in British aquifers: a baseline
survey. Medmenham, Marlow, United Kingdom, Water Research Centre
(Technical Report TR223).
Kilburn KH, Warshaw R, Boylen CT, Balmes J, Johnson S, Seidman B, &
DeFlorio G (1983) Toxic effects of formaldehyde and solvents in
histology technicians: A preliminary report. J Histotechnol, 6: 73-76.
Kilburn KH, Seidman BC, & Warshaw R (1985) Neurobehavioral and
respiratory symptoms of formaldehyde and xylene exposure in histology
technicians. Arch Environ Health, 40: 229-233.
Kirschner E (1995) Production of top 50 chemicals increased sub-
stantially in 1994. Chem Eng News, April: 16-20.
Kjorsvic E, Saethre LJ, & Lonning S (1982) Effects of short-term
exposure to xylenes on the early cleavage stages of cod eggs ( Gadus
morhus L.). Sarsia, 67: 299-308.
Klaucke DN, Johansen M, & Vogt RL (1982) An outbreak of xylene
intoxication in a hospital. Am J Ind Med, 3: 173-178.
Kligman AM (1966) The identification of contact allergens by human
assay. III. The maximization test: A procedure for screening and
rating contact sensitizers. J Invest Dermatol, 47: 393-409.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies: Relationship for 50 industrial pollutants.
Toxicology, 19: 229-238.
Korsak Z, Sokal JA, & Gorny R (1992) Toxic effect of combined exposure
to toluene and m-xylene in animals. III. Subchronic inhalation
study. Pol J Occup Med Environ Health, 5: 27-33.
Korsak Z, Swiercz R, & Jedrychowski R (1993) Effects of acute combined
exposure to n-butyl alcohol and m-xylene. Pol J Occup Med Environ
Health, 6: 35-41.
Korsak Z, Wisniewska-Knypl J, & Swiercz R (1994) Toxic effects of
subchronic combined exposure to n-butyl alcohol and m-xylene in
rats. Int J Occup Med Environ Health, 7: 155-166.
Kroneld R (1989) Volatile pollutants in suburban and industrial air.
Bull Environ Contam Toxicol, 42: 868-872.
Kuhn EP, Colberg PJ, Schnoor JL, Wanner O, Zehnder AJB, &
Schwarzenbach RP (1985) Microbial transformations of substituted
benzenes during infiltration of river water to ground water:
Laboratory column studies. Environ Sci Techol, 19: 961-968.
Kuhn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Kurppa K & Husman K (1982) Car painters' exposure to a mixture of
organic solvents. Serum activities of liver enzymes. Scand J Work
Environ Health, 8: 137-140.
Kyrklund T, Kjellstrand P, & Haglid K (1987) Brain lipid changes in
rats exposed to xylene and toluene. Toxicology, 45: 123-133.
Labows J, Preti G, Loezle E, Leyden J, & Kligman A (1979) Analysis of
human axillary volatiles: Compounds of exogenous origin. J Chromatogr,
163: 294-299.
Lagorio S, Forastiere F, Iavarone I, Vanacore N, Fuselli S, & Carere A
(1993) Exposure assessment in a historical cohort of filling station
attendants. Int J Epidemiol, 22(6-suppl 2): S51-S56.
Lagrue G (1976) Hydrocarbon exposure and chronic glomerulonephritis.
Lancet, 1: 1191.
Laine A, Savolainen K, Riihimäki V, Matikainen E, Salmi T, & Juntunen
J (1993) Acute effects of m-xylene inhalation on body sway, reaction
times, and sleep in man. Int Arch Occup Environ Health, 65: 179-188.
Lanzerstofer CH & Puxbaum H (1990) Volatile hydrocarbons in and around
Vienna, Austria. Water Air Soil Pollut, 51: 345-355.
Laparé S, Tardif R, & Brodeur J (1993) Effect of various exposure
scenarios on the biological monitoring of organic solvents in alveolar
air. I. Toluene and m-xylene. Int Arch Occup Environ Health,
64: 569-580.
Larsby B, Ödkvist LM, Hydén D, & Liedgren SRC (1976) Disturbancies of
the vestibular system by toxic agents. Acta Physiol Scand,
440(suppl): 108.
Lauwerys R & Buchet JP (1988) Biological monitoring of exposure to
benzene, toluene and xylene. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 205-222 (IARC
Scientific Publications No. 85).
Lauwerys RR, Dath T, Lachapelle J-M, Buchet J-P, & Roels H (1978) The
influence of two barrier creams on the percutaneous absorption of
m-xylene in man. J Occup Med, 20: 17-20.
Lazarew NW (1929) [On the toxicity of various organic compounds
vapors.] Arch Exp Pathol Pharmacol, 143: 223-233 (in German).
Lebowitz H, Brusick D, Matheson D, Jagannath DR, Reed M, Goode S, &
Roy G (1979) Commonly used fuels and solvents evaluated in a battery
of short-term bioassays. Environ Mutagen, 1: 172-173.
Le Gore RS (1974) The effects of Alaskan crude oil and selected
hydrocarbon compounds on embryonic development of the Pacific oyster,
Crassostrea gigas. Diss Abstr B, 35: 3168.
Lewis P (1994) Report of airborne solvent concentrations in a printing
ink manufacturing plant in Cape Town, Republic of South Africa. Appl
Occup Environ Hyg, 9: 147-151.
Liira J, Elovaara E, Raunio H, Riihimäki V, & Engström K (1991)
Metabolic interaction and diposition of methyl ethyl ketone and
m-xylene in rats at single and repeated inhalation exposure.
Xenobiotica, 21: 53-63.
Lindström K (1973) Psychological performances of workers exposed to
various solvents. Work Environ Health, 10: 151-155.
Lindström K, Antti-Poika M, Tola S, & Hyytiäinen A (1982)
Psychological prognosis of diagnosed chronic organic solvent
intoxication. Neurobehav Toxicol Teratol, 4: 581-588.
Lioy PJ, Wallace L, & Pellizzari E (1991) Indoor/outdoor, and personal
monitor and breath analysis relationships for selected volatile
organic compounds measured at three homes during New Jersey
TEAM - 1987. J Exp Anal Environ Epidemiol, 1(1): 45-61.
Lonneman WR, Seila RL, & Bufalini JJ (1978) Ambient air hydrocarbons
in Florida. Environ Sci Technol, 12: 459-463.
Lovegren NV, Fisher GS, Legendre MG, & Schuller WH (1979) Volatile
constituents of dried legumes. J Agric Food Chem, 27: 851-853.
Lundberg I & Håkansson M (1985) Normal serum activities of liver
enzymes in Swedish paint industry workers with heavy exposure to
organic solvents. Br J Ind Med, 42: 596-600.
Lundberg I, Håkansson M, Gustavsson P, Kronevi T, & Lidums V (1983)
[Relative hepatotoxic effect of industrial solvents.] Solna, Sweden,
National Institute of Occupational Health, pp 1-42 (Work and Health
1983:22) (in Swedish with English summary).
Lundberg I, Ekdahl M, Kronevi T, Lidums V, & Lundberg S (1986)
Relative hepatotoxicity of some industrial solvents after
intraperitoneal injection or inhalation exposure in rats. Environ Res,
40: 411-420.
Lundberg I, Nise G, Hedenborg G, Högberg M, & Vesterberg O (1994)
Liver function tests and urinary albumin in house painters with
previous heavy exposure to organic solvents. Occup Environ Med,
51: 347-353.
Lyman WJ, Reehl WF, & Rosenblatt DH (1981) Handbook of chemical
property estimation methods. New York, Mc-Graw Hill Book Company.
McCarroll NE, Keech BH, & Piper CE (1981a) A microsuspension
adaptation of the Bacillus subtilis "rec" assay. Environ Mutagen,
3: 607-616.
McCarroll NE, Piper CE, & Keech BH (1981b) An E. coli
microsuspension assay for the detection of DNA damage induced by
direct-acting agents and promutagens. Environ Mutagen, 3: 429-444.
McDonald TJ, Kennicutt MC II, & Brooks JM (1988) Volatile organic
compounds at a coastal site. Chemosphere, 17: 123-136.
McFall JA, Antoine SR, & Deleon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14: 1253.
Mackay D & Leinonen PJ (1975) Rate of evaporation of low solubility
contaminants from water bodies to the atmosphere. Environ Sci Technol,
9: 1178-1180.
Mackay D & Wolkoff AW (1973) Rate of evaporation of low solubility
contaminants from water bodies to atmosphere. Environ Sci Technol,
7: 611-614.
Mackay D, Paterson S, Cheung B, & Neely WB (1985) Evaluating the
environmental behaviour of chemicals using a level III fugacity model.
Chemosphere, 14: 335-374.
MAFF (1991) Monitoring and surveillance of non-radioactive
contaminants in the aquatic environment and activities regulating the
disposal of wastes at sea, 1988-89. Lowestoft, United Kingdom,
Ministry of Agriculture, Fisheries and Food, Directorate of Fisheries
Research (Aquatic Environment Monitoring Report No. 26).
Maizlish NA, Fine LJ, Albers JW, Whitehead L, & Langolf GD (1987) A
neurological evaluation of workers exposed to mixtures of organic
solvents. Br J Ind Med, 44: 14-25.
Major DW, Mayfield CI, & Barker JF (1988) Biotransformation of benzene
by denitrification in aquifer sand. Ground Water, 26: 8-14.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1983) Benzene as an
experimental carcinogen: up-to-date evidence. Acta Oncol, 4: 141-164.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
Current results and ongoing research. Am J Ind Med, 7: 415-446.
Mansour M, Moza PN, Barlas H, & Parlar H (1985) [A contribution to the
topic of photo-stability of environmental organic compounds in the
presence of hydrogen peroxide in aquatic systems.] Chemosphere,
14: 1469-1474 (in German).
Marks TA, Ledoux TA, & Moore JA (1982) Teratogenicity of a commercial
xylene mixture in the mouse. J Toxicol Environ Health, 9: 97-105.
Maynard DJ & Weber DD (1981) Avoidance reactions of juvenile coho
salmon (Oncorhynchus kisutch) to monocyclic aromatics. Can J Fish
Aquat Sci, 38: 772-778.
Meinhart E & Schreier P (1986) Study of flavour compounds from
Parmigiano Reggiano cheese. Milchwissenschaft, 41: 689-691.
Michael LC, Pellizzari ED, & Norwood DL (1991) Application of the
master analytical scheme to the determination of volatile organics in
wastewater influents and effluents. Environ Sci Technol, 25: 150-155.
Mirkova E, Zaikov C, Antov G, Mikhailova A, Khinkova L, & Benchev I
(1983) Prenatal toxicity of xylene. J Hyg Epidemiol Microbiol Immunol,
27: 337-343.
Mohtashamipur E, Norpoth K, Woelke U, & Huber P (1985) Effects of
ethylbenzene, toluene, and xylene on the induction of micronuclei in
bone marrow polychromatic erythrocytes of mice. Arch Toxicol,
58: 106-109.
Molnar J, Paksy KA, & Naray M (1986) Changes in the rat's motor
behaviour during 4-hr inhalation exposure to prenarcotic
concentrations of benzene and its derivatives. Acta Physiol Hungarica
67: 349-354.
Morgan P & Watkinson RJ (1990) Assessment of the potential for in
situ biotreatment of hydrocarbon-contaminated soils. Water Sci
Technol, 22: 63-68.
Morley R, Eccleston DW, Douglas CP, Greville WEJ, Scott DJ, & Anderson
J (1970) Xylene poisoning: A report on one fatal case and two cases of
recovery after prolonged unconsciousness. Br Med J, 3: 442-443.
Morrow JE, Gritz RL, & Kirton MP (1975) Effects of some components of
crude oil on young coho salmon. Copeia, 2: 326-331.
Moser VC, Coggeshall EM, & Balster RL (1985) Effects of xylene isomers
on operant responding and motor performance in mice. Toxicol Appl
Pharmacol, 80: 293-298.
Moszczynski P & Lisiewicz J (1985) Occupational exposure to benzene,
toluene and xylene and the lymphocyte lysosomal N-acetyl-beta-
D-glucosaminidase. Ind Health, 23: 47-51.
Nakamura S, Oda Y, Shimada T, Oki I, & Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutat
Res, 192: 239-246.
Naskali L, Oksanen H, & Tähti H (1994) Astrocytes as targets for CNS
effects of organic solvents in. Neurotoxicology, 15: 609-612.
Nasterlack M, Triebig G, & Stelzer O (1994) Hepatotoxic effects of
solvent exposure around permissible limits and alcohol consumption in
printers over a 4-year period. Int Arch Occup Environ Health, 66:
161-165.
Neff JM, Anderson JW, Cox BA, Laughlin RB, Rossi SS, & Tatem HE (1976)
Effects of petroleum on survival, respiration and growth of marine
animals. In: Sources, effects and sinks of hydrocarbons. Washington,
DC, American Institute of Biological Science, pp 515-523.
Nelson PF & Quigley SM (1982) Non-methane hydrocarbons in the
atmosphere of Sydney, Australia. Environ Sci Techol, 16: 650-655.
Nelson KW, Ege JF Jr, Ross M, Woodman LE, & Silverman L (1943) Sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
25: 282-285.
NIOSH (1991) Health hazard evaluation report: Rockwell International,
Newark, Ohio. Cincinnati, Ohio, National Institute for Occupational
Safety and Health (Report HETA 90-368-2137; PB92-131929).
NIOSH (1993a) Health hazard evaluation report: National Centers for
Environmental Health, Fairbanks, Alaska. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA 93-606-2336;
PB94-133667).
NIOSH (1993b) Health hazard evaluation report: Dallas and Mavis
Forwarding Co., Seattle, Washington. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA
91-0103-2351; PB94-131836).
NIOSH (1993c) Health hazard evaluation report: National Center for
Environmental Health, Albany, New York. Cincinnati, Ohio, National
Institute for Occupational Safety and Health (Report HETA
93-0884-2344; PB94-129020).
NIOSH (1993d) Health hazard evaluation report: National Centers for
Environmental Health, Stamford, Connecticut. Cincinnati, Ohio,
National Institute for Occupational Safety and Health (Report HETA
93-802-2338; PB94-134343).
NIOSH (1994) Health hazard evaluation report: Pan American Health
Organization, Bogota, Colombia (South America). Cincinnati, Ohio,
National Institute for Occupational Safety and Health (Report HETA
94-0253-2451; PB95-147062).
NTP (1986) Technical report on the toxicology and carcinogenesis
studies of xylenes (mixed) in F344/N rats and B6C3F1 mice. Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program (NIH Publication No. 87-2583).
Nunes P & Benville PE (1979) Uptake and depuration of petroleum
hydrocarbons in the Manila Clam, Tapes semidecussata Reeve. Bull
Environ Contam Toxicol, 21: 719-726.
Nylén P & Hagman M (1994) Function of the auditory and visual systems,
and of peripheral nerves in rats after long-term combined exposure to
n-hexane and methylated benzene derivatives. II. Xylene. Pharmacol
Toxicol, 74: 124-129.
Nylén P, Ebendal T, Eriksdotter-Nilsson M, Hansson T, Henschen A,
Johnson A-C, Kronevi T, Kvist U, Sjöstrand NO, Höglund G, & Olson L
(1989) Testicular atrophy and loss of nerve growth factor-
immunoreactive germ cell line in rats exposed to n-hexane and
a protective effect of simultaneous exposure to toluene or xylene.
Arch Toxicol, 63: 296-307.
Ogata M & Fujii T (1979) Urinary excretion of hippuric acid and
m-methylhippuric acid after administration of toluene and m-xylene
mixture to rats. Int Arch Occup Environ Health, 43: 45-51.
Ogata M & Hobara T (1979) A new direct method for colorimetric
determination of hippuric acid and methylhippuric acid as indices of
toluene and m-xylene, and its application to workers using thinner.
Ind Health, 17: 61-72.
Ogata M & Miyake Y (1979) Disappearance of aromatic hydrocarbons and
organic sulphur compounds from fish reared in crude oil suspensions.
Water Res, 13: 75-78.
Ogata M, Yamasaki Y, Meguro T, Sugihara R, & Shimada Y (1979)
Quantitation of urinary o-xylene metabolites in rats and human
beings by high performance liquid chromatography. Ind Health,
17: 123-125.
Ogata M, Yamazaki Y, Sugihara R, Shimada Y, & Meguro T (1980)
Quantitation of urinary o-xylene metabolites of rats and human
beings by high performance liquid chromatography. Int Arch Occup
Environ Health, 46: 127-139.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
Omori T & Yamada K (1970) Studies on the utilisation of hydrocarbons
by microorganisms. Part XVI. Detection of metabolic intermediates of
xylene and pseudocumene. Agric Biol Chem, 34: 659-663.
Omori T, Horiguchi S, & Yamada K (1967) Studies on the utilisation of
hydrocarbons by microorganisms. Part X. Screening of aromatic
hydrocarbon assimilating microorganisms and p-toluic acid formation
from p-xylene. Agric Biol Chem, 31: 1337-1342.
Otson R & Williams DT (1981) Evaluation of a liquid-liquid extraction
technique for water pollutants. J Chromatogr, 212: 187-197.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities.
J Assoc Off Anal Chem, 65: 1370-1374.
Otson R, Fellin P, & Tran Q (1993) Investigation of VOCs in Canadian
residences. In: Indoor air '93. Proceedings of the 6th International
Conference on Indoor Air Quality and Climate, Helsinki, Finland, 4-8
July 1993 - Volume 2: Chemicals in indoor air, materials emissions,
pp 141-146.
Ödkvist LM, Larsby B, Tham R, & Aschan G (1979) On the mechanism
of vestibular disturbances caused by industrial solvents. Adv
Oto-rhino-laryngol, 25: 167-172.
Ödkvist LM, Larsby B, Fredrickson JMF, Liedgren SRC, & Tham R (1980)
Vestibular and oculomotor disturbances caused by industrial solvents.
J Otolaryngol, 9: 53-59.
PACE (1987) A study of exposure to motor gasoline hydrocarbon vapours
at service stations: Phase II - Summer Study. Petroleum Association
for Conservation of the Canadian Environment (PACE Report No. 87-5).
PACE (1989) A study of exposure to motor gasoline hydrocarbon vapours
at service stations: Phase III - Winter Study. Petroleum Association
for Conservation of the Canadian Environment (PACE Report No. 89-3).
Padilla S, Lyerly DL, & Pope CN (1992) Subacute ethanol consumption
reverses p-xylene-induced decreases in axonal transport. Toxicology,
75: 159-167.
Palmer KT & Rycroft RJG (1993) Occupational airborne contact urticaria
due to xylene. Contact Dermatitis, 28: 44.
Pap M & Varga C (1987) Sister-chromatid exchanges in peripheral
lymphocytes of workers occupationally exposed to xylenes. Mutat Res,
187: 223-225.
Park SH, AuCoin TA, Silverman DM, & Schatz RA (1994) Time-dependent
effects of o-xylene on rat lung and liver microsomal membrane
structure and function. J Toxicol Environ Health, 43: 469-481.
Patel JM, Harper C, & Drew RT (1976) Inactivation of pulmonary
cytochrome P-450 by p-xylene intoxication. Pharmacologist, 18: 210.
Patel JM, Harper C, & Drew RT (1978) The biotransformation of
p-xylene to a toxic aldehyde. Drug Metab Dispos, 6: 368-374.
Patel JM, Harper C, Gupta BN, & Drew RT (1979) Changes in serum
enzymes after inhalation exposure of p-xylene. Bull Environ Contam
Toxicol, 21: 17-24.
Pathiratne A, Puyear RL, & Brammer JD (1986) A comparative study of
the effects of benzene, toluene, and xylenes on their in metabolism
and drug-metabolizing enzymes in rat liver. Toxicol Appl Pharmacol,
82: 272-280.
Pellizzari EC, Hartwell TD, Harris BSH III, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mothers milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pellizzari ED, Zweidinger RA, & Sheldon LS (1988) Determination
of benzene, toluene and xylene in breath samples by gas
chromatography/mass spectrometry. In: Fishbein L & O'Neill IK ed.
Environmental carcinogens: Methods of analysis and exposure
measurement. Volume 10: Benzene and alkylated benzenes. Lyon,
International Agency for Research on Cancer, pp 267-279 (IARC
Scientific Publications No. 85).
Pennell KD, Rhue RD, Rao PSC, & Johnston CT (1992) Vapor-phase
sorption of p-xylene and water on soils and clay minerals. Environ
Sci Technol, 26: 756-763.
Perry R & Twibell JD (1973) A time based elution technique for the
estimation of specific hydrocarbons in air. Atmos Environ, 7: 929.
Petersson G (1982) Ambient hydrocarbons from motor-car assembly plants
in Scandinavia. Environ Pollut, B4: 207-217.
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38: 1419-1429.
Pound AW (1970) Induced cell proliferation and the initiation of skin
tumour formation in mice by ultraviolet light. Pathology, 2: 269-275.
Pound AW & Withers HR (1963) The influence of some irritant chemicals
and scarification on tumour initiation by urethane in mice. Br J
Cancer, 17: 460-470.
Proust B, Hannequin D, Caillard JF, Prudent P, Hemet J, & Samson N
(1986) Le psychosyndrome aux solvants: Résultats des tests
psychométriques chez dix laborantines exposées. Arch Mal Prof,
47: 305-310.
Pryor GT, Robert CS, & Howd RA (1987) Hearing loss in rats caused by
inhalation of mixed xylenes and styrene. J Appl Toxicol, 7: 55-61.
Pussemier L, Szabó G, & Bulman RA (1990) Prediction of the soil
adsorption coefficient Koc for aromatic pollutants. Chemosphere,
21: 1199-1212.
Pyykkö K (1980) Effects of methylbenzenes on microsomal enzymes in rat
liver, kidney and lung. Biochim Biophys Acta, 633: 1-9.
Pyykkö K (1986) Inhibition of microsomal monooxygenases in by
aromatic hydrocarbons. Arch Toxicol, 9(suppl): 371-373.
Pyykkö K, Paavilainen S, Metsä-Ketelä T, & Laustiola K (1987) The
increasing and decreasing effects of aromatic hydrocarbon solvents on
pulmonary and hepatic cytochrome P-450 in the rat. Pharmacol Toxicol,
60: 288-293.
Rao PSC, Hornsby AG, & Jessup RE (1985) Indices for ranking the
potential for pesticide contamination of ground water. Soil Crop Sci
Soc Proc, 44: 1-8.
Raunio H, Liira J, Elovaara E, Riihimäki V, & Pelkonen O (1990)
Cytochrome P450 isozyme induction by methyl ethyl ketone and
m-xylene in rat liver. Toxicol Appl Pharmacol, 103: 175-179.
Raymond RL, Jamison VW, & Hudson JO (1969) Microbial hydrocarbon
co-oxidation. II. Use of ion-exchange resins. Appl Microbiol,
17: 512-515.
Recchia G, Perbellini L, Prati GF, Dean P, & Ancona G (1985) [Coma due
to accidental ingestion of xylene. Treatment with charcoal
hemoperfusion.] Med Lav, 76: 67-73 (in Italian with English summary).
Reinert KH, Hunter JV, & Sabatino T (1983) Dynamic heated headspace
analysis of volatile organic compounds present in fish tissue samples.
J Agric Food Chem, 31: 1057-1060.
Reinhard M, Goodman NL, & Barker JF (1984) Occurrence and distribution
of organic chemicals in two landfill leachate plumes. Environ Sci
Technol, 18: 953-961.
Rhue RD, Rao PSC, & Smith RE (1988) Vapour-phase adsorption of
alkylbenzenes and water on soils and clays. Chemosphere, 17: 727-741.
Richer CL, Chakrabarti S, Senecal-Quevillon M, Duhr MA, Zhang XX, &
Tardif R (1993) Cytogenic effects of low-level exposure to toluene,
xylene and their mixture on human blood lymphocytes. Int Arch Occup
Environ Health, 64: 81-85.
Rigdon RH (1940) Capillary permeability in areas of inflammation
produced by xylene. Arch Surg, 41: 101-109.
Riihimäki V (1979a) Conjugation and urinary excretion of toluene and
m-xylene in man. Scand J Work Environ Health, 5: 135-142.
Riihimäki V (1979b) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand J Work
Environ Health, 5: 143-150.
Riihimäki V & Hänninen O (1987) Xylenes. In: Snyder R. ed. Ethel
Browning's toxicity and metabolims of industrial solvents, 2nd ed.
Amsterdam, Oxford, New York, Elsevier Science Publishers, vol 1, pp
64-84.
Riihimäki V & Pfäffli P (1978) Percutaneous absorption of solvent
vapors in man. Scand J Work Environ Health, 4: 73-85.
Riihimäki V & Savolainen K (1980) Human exposure to m-xylene,
kinetics and acute effects on the central nervous system. Ann Occup
Hyg, 23: 411-422.
Riihimäki V, Pfäffli P, & Savolainen K (1979a) Kinetics of m-xylene
in man: Influence of intermittent physical exercise and changing
environmental concentrations on kinetics. Scand J Work Environ Health,
5: 232-248.
Riihimäki V, Pfäffli P, Savolainen K, & Pekari K (1979b) Kinetics of
m-xylene in man: General features of absorption, distribution,
biotransformation and excretion in repetitive inhalation exposure.
Scand J Work Environ Health, 5: 217-231.
Riihimäki V, Laine A, Savolainen K, & Sippel H (1982a) Acute solvent-
ethanol interactions with special reference to xylene. Scand J
Work Environ Health, 8: 77-79.
Riihimäki V, Savolainen K, Pfäffli P, Pekari K, Sippel HW, & Laine A
(1982b) Metabolic interaction between m-xylene and ethanol. Arch
Toxicol, 49: 253-263.
Roberts AE, Bogdanffy MS, Brown DR, & Schatz RA (1986) Lung metabolism
of benzo(a)pyrene in rats treated with p-xylene and/or ethanol. J
Toxicol Environ Health, 18: 257-266.
Rogerson A, Shiu WY, Huang GL, Mackay D, & Berger J (1983)
Determination and interpretation of hydrocarbon toxicity to ciliate
protozoa. Aquat Toxicol, 3: 215-228.
Rosen MB, Crofton KM, & Chernoff N (1986) Postnatal evaluation of
prenatal exposure to p-xylene in the rat. Toxicol Lett, 34: 223-229.
Rosengren LE, Kjellstrand P, Aurell A, & Haglid KG (1986) Irreversible
effects of xylene on the brain after long term exposure: A
quantitative study of DNA and the glial cell marker proteins S-100 and
GFA. Neurotoxicology, 7: 121-136.
Ruijten MW, Hooisma J, Brons JT, Habets CE, Emmen HH, & Muijser H
(1994) Neurobehavioral effects of long-term exposure to xylene and
mixed organic solvents in shipyard spray painters. Neurotoxicology,
15: 613-620.
Rydzynski K, Korsak Z, Jedlinska U, & Sokal JA (1992) The toxic
effects of combined exposure to toluene and m-xylene in animals. IV.
Liver ultrastructure after subchronic inhalatory exposure. Pol J Occup
Med Environ Health, 5: 35-42.
Sabel GV & Clark TP (1984) Volatile organic compounds as indicators of
municipal solid waste leachate contamination. Waste Manage Res,
2: 119-130.
Sabljic A & Güsten H (1990) Predicting the night-time NO3 radical
reactivity in the troposphere. Atmos Environ, 24A: 73-78.
Sack TM, Steele DH, Hammerstrom K, & Remmers K (1992) A survey of
household products for volatile organic compounds. Atmos Environ,
26A(6): 1063-1070.
Sanborn HR & Malins DC (1980) The disposition of aromatic hydrocarbons
in adult spot shrimp (Pandalus platyceros) and the formation of
metabolites of naphthalene in adult and larval spot shrimp.
Xenobiotica, 10: 193-200.
Sato A, Fujiwara Y, & Nakajima T (1974) Solubility of benzene, toluene
and m-xylene in various body fluids and tissues of rabbits. Jpn J
Ind Health, 16: 30-31.
Sauer TC (1981) Volatile liquid hydrocarbon characterisation of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environ Sci Technol, 15: 917-923.
Savolainen K (1980) Combined effects of xylene and alcohol on the
central nervous system. Acta Pharmacol Toxicol, 46: 366-372.
Savolainen H & Pfäffli P (1980) Dose-dependent neurochemical changes
during short-term inhalation exposure to m-xylene. Arch Toxicol,
45: 117-122.
Savolainen K & Linnavuo M (1979) Effects of m-xylene on human
equilibrium measured with a quantitative method. Acta Pharmacol
Toxicol, 44: 315-318.
Savolainen K & Riihimäki V (1981) Xylene and alcohol involvement of
the human equilibrium system. Acta Pharmacol Toxicol, 49: 447-451.
Savolainen H & Seppäläinen AM (1979) Biochemical and physiological
effects of organic solvents on rat axon membranes isolated by a new
technique. Neurotoxicology, 1: 467-477.
Savolainen H, Pfäffli P, Helojoki M, & Tengén M (1979a) Neurochemical
and behavioural effects of long-term intermittent inhalation of xylene
vapour and simultaneous ethanol intake. Acta Pharmacol Toxicol,
44: 200-207.
Savolainen H, Vainio H, Helojoki M, & Elovaara E (1979b) Biochemical
and toxicological effects of short-term intermittent xylene inhalation
exposure and combined ethanol intake. Arch Toxicol, 41: 195-205.
Savolainen K, Riihimäki V, Vaheri E, & Linnoila M (1980) Effects of
xylene and alcohol on vestibular and visual functions in man. Scand J
Work Environ Health, 6: 94-103
Savolainen K, Riihimäki V, Laine A, & Kekoni J (1981) Short-term
exposure of human subjects to m-xylene and 1,1,1-trichloroethane.
Int Arch Occup Environ Health, 49: 89-98.
Savolainen K, Riihimäki V, & Laine A (1982a) Biphasic effects of
inhaled solvents on human equilibrium. Acta Pharmacol Toxicol,
51: 237-242.
Savolainen K, Riihimäki V, Laine A, & Kekoni J (1982b) Short-term
exposure of human subjects to m-xylene and 1,1,1-trichloroethane.
Arch Toxicol, 5(suppl): 96-99.
Savolainen K, Kekoni J, Riihimäki V, & Laine A (1984) Immediate
effects of m-xylene on the human central nervous system. Arch
Toxicol, 7(suppl): 412-417.
Savolainen K, Riihimäki V, Luukkonen R, & Muona O (1985a) Changes in
the sense of balance correlate with concentrations of m-xylene in
venous blood. Br J Ind Med, 42: 765-769.
Savolainen K, Riihimäki V, Muona O, Kekoni J, Luukkonen R, & Laine A
(1985b) Conversely exposure-related effects between atmospheric
m-xylene concentrations and human body sense of balance. Acta
Pharmacol Toxicol, 57: 67-71.
Sedivec V & Flek J (1976) The absorption, metabolism, and excretion of
xylenes in man. Int Arch Occup Environ Health, 37: 205-217.
Seidenberg JM, Anderson DG, & Becker RA (1986) Validation of an in
vivo developmental toxicity screen in the mouse. Teratogen
Carcinogen Mutagen, 6: 361-374.
Seip HM, Alstad J, Carlberg GE, Martinsen K, & Skaane R (1986)
Measurement of mobility of organic compounds in soil. Sci Total
Environ, 50: 87-101.
Selgrade MK, Daniels MJ, Jaskot RH, Robinson BL, & Albis JW (1993)
Enhanced mortality and liver damage in virus-infected mice exposed to
p-xylene. J Toxicol Environ Health, 40: 129-144.
Seppäläinen AM, Husman K, & Mårtenson C (1978) Neurophysiological
effects of long-term exposure to a mixture of organic solvents. Scand
J Work Environ Health, 4: 304-314.
Seppäläinen AM, Savolainen K, & Kovala T (1981) Changes induced by
xylene and alcohol in human evoked potentials. Electroencephalogr Clin
Neurophysiol, 51: 148-155.
Seppäläinen AM, Laine A, Salmi T, Verkkala E, Riihimäki V, & Luukkonen
R (1991) Electroencephalographic findings during experimental human
exposure to m-xylene. Arch Environ Health, 46: 16-23.
Sevcik P, Hep A, & Peslova M (1992) Intravenous xylene poisoning.
Intensive Care Med, 18: 377-378.
Sexton K & Westberg H (1980) Ambient hydrocarbon and ozone
measurements downwind of a large automotive painting plant. Environ
Sci Technol, 14: 329-332.
Seyfried B, Glod G, Schocher R, Tschech A, & Zeyer J (1994) Initial
reactions in the anaerobic oxidation of toluene and m-xylene by
denitrifying bacteria. Appl Environ Microbiol, 60(11): 4047-4052.
Sheedy BR, Lazorchak JM, Grunwald DJ, Pickering QH, Pilli A, Hall D, &
Webb R (1991) Effects of pollution on freshwater organisms. Res J
Water Pollut Control Fed, 63: 619-696.
Sheldon LS, Zelon H, Sickles J, Eaton C, Hartwell T, & Jugners R
(1988) Monitoring in and around public access buildings. Proceedings
of the 81st Annual Meeting of the Air Pollution Control Association,
1988. Pittsburgh, Pennsylvania, Air Pollution Control Association,
9 pp (Paper 88/109.8).
Shimizu H, Suzuki Y, Takemura N, Goto S, & Matsushita H (1985) The
results of microbial mutation test for forty-three industrial
chemicals. Jpn J Ind Health, 27: 400-419.
Silverman DM & Schatz RA (1991) Pulmonary microsomal alterations
following short-term low level inhalation of p-xylene in rats.
Toxicology, 65: 271-281.
Simmons JE, Allis JW, Grose EC, Seely JC, Robinson BL, & Berman E
(1991) Assessment of the hepatotoxicity of acute and short-term
exposure to inhaled p-xylene in F-344 rats. J Toxicol Environ
Health, 32: 295-306.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1983) Measurements of
hazardous organic chemicals in the ambient atmosphere. Washington, DC,
US Environmental Protection Agency (EPA-600/3-83-002).
Singh HB, Ferek RJ, Salas LJ, & Nitz KC (1986) Toxic chemicals in the
environment: A program of field measurements. Washington, DC, US
Environmental Protection Agency (EPA-600/3-86/047).
Skowronski GA, Turkall RM, Kadry ARM, & Abdel-Rahman MS (1990) Effects
of soil on the dermal bioavailability of m-xylene in male rats.
Environ Res, 51: 182-193.
Slaine DD & Barker JF (1990) The detection of naturally occurring BTX
during a hydrogeologic investigation. Ground Water Monit Rev,
10: 89-94.
Slooff W (1979) Detection limits of a biological monitoring system
based on fish respiration. Bull Environ Contam Toxicol, 23: 517-523.
Slooff W, de Zwart D, & Marquenie JM (1983) Detection limits of a
biological monitoring system for chemical water pollution based on
mussel activity. Bull Environ Contam Toxicol, 30: 400-405.
Smith BR, Plummer JL, Wolf CR, Philpot RM, & Bend JR (1982) p-Xylene
metabolism by rabbit lung and liver and its relationship to the
selective destruction of pulmonary cytochrome P-450. J Pharmacol Exp
Ther, 223: 736-742.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: List VI. Am Ind Hyg Assoc J, 23: 95-107.
Snyder R (1987) Ethyl Browning's toxicity and metabolism of industrial
solvents - Volume 1; Hydrocarbons. Amsterdam, Oxford, New York,
Elsevier Science Publishers.
Sotaniemi EA, Sutinen S, Sutinen S, Arranto AJ, & Pelkonen RO (1982)
Liver injury in subjects occupationally exposed to chemicals in low
doses. Acta Med Scand, 212: 207-215.
Steele RH & Wilhelm DL (1966) The inflammatory reaction in chemical
injury. I. Increased vascular permeability and erythema induced by
various chemicals. J Exp Pathol, 47: 612-623.
Stickney JA, Silverman DM, & Schatz RA (1991) Role of isozyme-specific
inhibition of cytochrome P450IIB1 activity in m-xylene-induced
alterations in rat pulmonary benzo(a)pyrene metabolism. Xenobiotica,
211: 641-649.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near an underground coal gasification site in northeastern
Wyoming. Environ Sci Technol, 16: 582-587.
Sugihara R (1979) High-performance liquid chromatography studies of
toluene and xylene poisoning. Part II. Respiratory and urinary
excretion of toluene and m-xylene following intraperitoneal
administration to rats. Acta Med Okayama, 91: 1433-1440.
Sugihara R & Ogata M (1978) Quantitation of urinary m- and
p-methylhippuric acids as indices of m- and p-xylene exposure.
Int Arch Occup Environ Health, 41: 281-286.
Svanberg PA, Grennfeldt P, & Lindskog A (1995) The Swedish urban
network - Air quality measurement program. Stockholm, Sweden,
Environmental Research Institute (Unpublished report).
Tabak HH, Desai S, & Govind R (1989) Determination of biodegradability
kinetics of RCRA compounds using respirometry for structure-activity
relationships. Proceedings of the 44th Industrial Waste Conference,
1989, pp 405-423.
Takatori T, Terazawa K, Nakano K, & Inoue K (1982) An autopsy case of
alkylbenzene poisoning and its clinical finding. Nippon Hoigaku
Zasshi, 36: 654-661.
Tardif R, Laparé S, Plaa GL, & Brodeur J (1991) Effect of simultaneous
exposure to toluene and xylene on their respective biological exposure
indices in humans. Int Arch Occup Environ Health, 63: 279-284.
Tardif R, Plaa GL, & Brodeur J (1992) Influence of various mixtures of
inhaled toluene and xylene on the biological monitoring of exposure to
these solvents in rats. Can J Physiol Pharmacol, 70: 385-939.
Tardif R, Laparé S, Krishnan K, & Brodeur J (1993a) Physiologically
based modelling of the toxicokinetic interaction between toluene and
m-xylene in rat. Toxicol Appl Pharmacol, 120: 266-273.
Tardif R, Laparé S, Krishnan K, & Brodeur J (1993b) A descriptive and
mechanistic study of the interaction between toluene and xylene in
humans. Int Arch Occup Environ Health, 65: S135-S137.
Tardif R, Sato A, Laparé S, & Brodeur J (1994) Ethanol induced
modification of m-xylene toxicokinetics in humans. Occup Environ
Med, 51: 187-191.
Taskinen H, Kyyrönen P, Hemminki K, Hoikkala M, Lajunen K, & Lindbohm
M-L (1994) Laboratory work and pregnancy outcome. J Occup Med,
36: 311-319.
Tatem HE, Cox BA, & Anderson JW (1978) The toxicity of oils and
petroleum hydrocarbons to estuarine crustaceans. Estuar Coast Mar Sci,
6: 365-373.
Tegeris JS & Balster RL (1994) A comparison of the acute behavioral
effects of alkylbenzenes using a functional observational battery in
mice. Fundam Appl Toxicol, 22: 240-250.
Tham R, Bunnfors I, Eriksson B, Larsby B, Lindgren S, & Ödkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thomas JM, Gordy VR, Fiorenza S, & Ward CH (1990) Biodegradation of
BTEX in subsurface materials contaminated with gasoline: Granger,
Indiana. Water Sci Technol, 22: 53-62.
Toftgård R & Nilsen OG (1982) Effects of xylene and xylene isomers on
cytochrome P-450 and in enzymatic activities in rat liver, kidney
and lung. Toxicology, 23: 197-212.
Toftgård R, Nilsen OG, & Gustafsson J-Å (1981) Changes in rat liver
microsomal cytochrome P-450 and enzymatic activities after the
inhalation of n-hexane, xylene, methyl ethyl ketone and
methylchloroform for four weeks. Scand J Work Environ Health,
7: 31-37.
Toftgård R, Nilsen OG, Glaumann H, & Gustafsson J-Å (1983) Induction
of cytochrome P-450 in the rat liver after exposure to xylenes,
dose-response relationship and dependence on endocrine factors.
Toxicology, 27: 119-137.
Toftgård R, Haaparanta T, & Halpert J (1986) Rat lung and liver
cytochrome P-450 isozymes involved in the hydroxylation of m-xylene.
Toxicology, 39: 225-231.
Triebig G & Schaller K-H (1986) Air monitoring of solvent exposed
workers with passive samplers in comparison to "biological monitoring
(BM)". Toxicol Environ Chem, 12: 285-312.
Tsuruta H (1982) Percutaneous absorption of organic solvents. III. On
the penetration rates of hydrophobic solvents through the excised rat
skin. Ind Health, 20: 335-345.
Turkall RM, Skowronski GA, Kadry ARM, & Abdel-Rahman MS (1992) Sex
differences in the bioavailability of soil-adsorbed m-xylene in
orally exposed rats. Toxicol Lett, 63: 57-67.
Tynan P, Watts CD, Sowray A, & Hammond I (1991) The transport and fate
of organic pollutants in rivers. II. Field measurements and modelling
for styrene, xylenes, dichlorobenzenes and 4-phenyldodecane. In:
Proceedings of the 6th European Symposium on Aquatic Environment,
1990, pp 20-37.
Uchida Y, Nakatsuka H, Ukai H, Watanabe T, Liu Y-T, Huang M-Y, Wang
Y-L, Zhu F-Z, Yin H, & Ikeda M (1993) Symptoms and signs in workers
exposed predominantly to xylenes. Int Arch Occup Environ Health,
64: 597-605.
Ungvary G (1985) The possible contribution of industrial chemicals
(organic solvents) to the incidence of congenital defects caused by
teratogenic drugs and consumer goods - an experimental study. Progr
Clin Biomed Res, 163B: 295-300.
Ungvary G (1990) The effect of xylene exposure on the liver. Acta
Morphol Hung, 38: 245-258.
Ungvary G & Tatrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats and rabbits. Arch Toxicol,
8(suppl): 425-430.
Ungvary G, Tatrai E, Hudak A, Barcza G, & Lorincz M (1980) Studies on
the embryotoxic effects of ortho-, meta- and para-xylene.
Toxicology, 18: 61-74.
Ungvary G, Varga B, Horvath E, Tatrai E, & Folly G (1981) Study on the
role of maternal sex steroid production and metabolism in the
embryotoxicity of para-xylene. Toxicology, 19: 263-268.
Valciukas JA, Lilis R, Singer RM, Glickman L, & Nicholson WJ (1985)
Neurobehavioral changes among shipyard painters exposed to solvents.
Arch Environ Health, 40: 47-52.
van der Wal JF & Moerkerken A (1984) The performance of passive
diffusion monitors for organic vapours for personal sampling of
painters. Ann Occup Hyg, 28: 39-47.
van Doorn R, Bos P, Brouns RME, Leijdekkers C-M, & Henderson PT (1980)
Effect of toluene and xylenes on liver glutathione and their urinary
excretion as mercapturic acids in the rat. Arch Toxicol, 43: 293-304.
van Doorn R, Leijdekkers C-M, Bos RP, Brouns ME, & Henderson PT (1981)
Alcohol and sulphate intermediates in the metabolism of toluene and
xylenes to mercapturic acids. J Appl Toxicol, 1: 236-242.
van Vliet C, Swaen GMH, Slangen JJM, de Boorder T, & Sturmans F (1987)
The organic solvent syndrome: A comparison of cases with neuro-
psychiatric disorders among painters and construction workers.
Int Arch Occup Environ Health, 59: 493-501.
van Wijnen JH, Verhoeff AP, Jans HWA, & van Bruggen M (1995) The
exposure of cyclists, car drivers and pedestrians to traffic-related
air pollutants. Int Arch Occup Environ Health, 67(3): 187-193.
Veith GD, Macek KJ, Petrocelli SR, & Carroll J (1980) An evaluation of
using partition coefficients and water solubility to estimate
bioconcentration factors for organic chemicals in fish. In: Eaton JG,
Parrish PR, & Hendricks AC ed. Aquatic toxicology. Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp 116-129
(ASTM STP 707).
Verschoyle RD, Dinsdale D, & Wolf CR (1993) Inhibition and induction
of cytochrome P450 isoenzymes in rat lung. J Pharmacol Exp Ther,
265: 386-391.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., pp 1188-1194.
Vincent R, Poirot P, Subra I, Rieger B, & Cicolella A (1994)
Occupational exposure to organic solvents during paint dripping and
painting operations in the aeronautical industry. Int Arch Occup
Environ Health, 65: 377-380.
Vodickova L, Frantik E, & Vodickova A (1995) Neutrotrophic effects and
blood levels of solvents at combined exposures: Binary mixtures of
toluene, o-xylene and acetone in rats and mice. Cent Eur J Public
Health, 3: 57-64.
Volskay VT & Grady CPL (1990) Respiration inhibition kinetic analysis.
Water Res, 24: 863-874.
Vowles PD & Mantoura RFC (1987) Sediment-water partition coefficients
and HPLC retention factors for aromatic hydrocarbons. Chemosphere,
16: 109-116.
Waggott A (1981) Trace organic substances in the River Lee. In:
Chemistry in water reuse. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., vol 2, pp 55-99.
Wallace LA, Pellizzari ED, Hartwell TD, Whitmore R, Zelon H, Perritt
R, & Sheldon L (1988) The California TEAM study: Breath concentrations
and personal exposures to 26 volatile compounds in air and drinking
water of 188 residents of Los Angeles, Antioch, and Pittsburg, CA.
Atmos Environ, 22: 2141-2163.
Wallace L, Nelson W, Ziegenfus R, Pellizzari E, Michael L, Whitmore R,
Zelon H, Hartwell T, Perritt R, & Westerdahl D (1991) The Los Angeles
TEAM study: Personal exposures, indoor-outdoor air concentrations, and
breath concentrations of 25 volatile organic compounds. J Exp Anal
Environ Epidemiol, 1(2): 157-192.
Wallén M, Holm S, & Byfält Nordqvist M (1985) Coexposure to toluene
and p-xylene in man: Uptake and elimination. Br J Ind Med,
42: 111-116.
Walsh DF, Armstrong JG, Bariley TR, Salmon HA, & Frank PA (1977)
Residues of emulsified xylene in aquatic weed control and their impact
on rainbow trout. Springfield, Virginia, National Technical
Information Services (NTIS) (PB-267270).
Walton BT, Anderson TA, Hendricks MS, & Talmage SS (1989) Physico-
chemical properties as predictors of organic chemical effects
on soil microbial respiration. Environ Toxicol Chem, 8: 53-63.
Washington WJ, Murthy RC, Doye A, Eugene K, Brown D, & Bradley J
(1983) Induction of morphologically abnormal sperm in rats exposed to
o-xylene. Arch Androl, 11: 233-237.
Weber WJ, Cole DE, & Posner JC (1975) Analysis of emissions from
outboard two cycle marine engines. Cincinnati, Ohio, US Environmental
Protection Agency, National Environmental Research Centre, Office of
Research and Development, 249 pp (EPA 670/2-75-06; PB-242174).
Weisel CP, Lawryk NJ, & Lioy PJ (1992) Exposure to emissions from
gasoline within automobile cabins. J Exp Anal Environ Epidemiol,
2(1): 79-96.
Weschler CJ, Shields HC, & Rainer D (1990) Concentrations of volatile
organic compounds at a building with health and comfort complaints. Am
Ind Hyg Assoc J, 51(5): 261-268.
WHO (1981) Xylene. In: Recommended health-based limits in occupational
exposure to selected organic solvents. Geneva, World Health
Organization, pp 25-38 (WHO Technical Report Series No. 664).
Wiesenburg DA, Bodennec G, & Brooks JM (1981) Volatile liquid
hydrocarbons around a production platform in the Northwest Gulf of
Mexico. Bull Environ Contam Toxicol, 27: 167-174.
Williams DT, Nestmann ER, LeBel GL, Benoit FM, & Otson R (1982)
Determination of mutagenic potential and organic contaminants of Great
Lakes drinking water. Chemosphere, 11(3): 263-276.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformations of selected
alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic
aquifer material: A microcosm study. Environ Sci Technol,
20: 997-1002.
Wilson BH, Wilson JT, Kampbell DH, Bledsoe BE, & Armstrong JM (1990)
Biotransformation of monoaromatic and chlorinated hydrocarbons at an
aviation gasoline spill site. Geomicrobiol J, 8: 225-240.
Winslow C-EA (1927) Summary of the National Safety Council study of
benzol poisoning. J Ind Hyg, 9: 61-74.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene:
Experiments on laboratory animals. Arch Ind Health, 14: 387-398.
Yamada K (1993) Influence of lacquer thinner and some organic solvents
on reproductive and accessory reproductive organs in the male rat.
Biol Pharm Bull, 16: 425-427.
Young PJ & Parker A (1983) The identification and possible
environmental impact of trace gases and vapours in landfill gas. Waste
Manage Res, 1: 213-226.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests. III. Results from the testing of
255 chemicals. Environ Mutagen, 9(suppl): 1-110.
Zeyer J, Kuhn EP, & Schwarzenback RP (1986) Rapid microbial
mineralisation of toluene and 1,3-dimethylbenzene in the absence of
molecular oxygen. Appl Environ Microbiol, 52: 944-947.
Zimmerman SW, Groehler K, & Beirne GJ (1975) Hydrocarbon exposure and
chronic glomerulonephritis. Lancet, 2: 199-201.
RESUME
Le xylčne est un hydrocarbure aromatique qui existe sous trois
formes: les isomčres ortho, méta et para. Le xylčne de qualité
technique est un mélange des trois isomčres qui contient en outre un
peu d'éthylbenzčne. En 1984, la production mondiale de xylčne était
estimée ą 15,4 millions de tonnes. A la température ambiante, le
xylčne se présente sous la forme d'un liquide incolore d'odeur
aromatique. La tension de vapeur est comprise entre 0,66 et 0,86 kPa
pour les trois isomčres. Environ 92% des mélanges de xylčnes sont
incorporés ą l'essence. On les utilise aussi comme solvants, en
particulier dans les peintures et les encres d'imprimerie.
La majeure partie du xylčne libéré dans l'environnement passe
directement dans l'atmosphčre. Les trois isomčres y sont rapidement
décomposés, principalement par photooxydation. Ils se volatilisent
tous les trois rapidement ą partir de l'eau. Dans le sol et dans
l'eau, les isomčres méta et para subissent une biodégradation aisée
dans des conditions variées d'aérobiose et d'anaérobiose; en revanche,
l'isomčre ortho est plus persistant. Les données limitées dont on
dispose indiquent que les xylčnes isomčres s'accumulent peu chez les
poissons et les invertébrés. Une fois que l'exposition a cessé, ils
sont assez rapidement éliminés par les organismes aquatiques.
Les concentrations moyennes de fond des trois xylčnes dans l'air
ambiant se situent autour de la valeur caractéristique de 1 µg/m3,
mais dans les banlieues elles atteignent 3 µg/m3 environ. On a
mesuré des valeurs plus élevées en zone urbaine et industrielle, les
moyennes allant cette fois jusqu'ą 500 µg/m3. Toutefois, la
concentration est en général inférieure ą 100 µg/m3.
On estime que l'exposition journaličre de la population par la
voie respiratoire est de 70 µg en milieu rural et de 2 000 µg en
milieu urbain. Dans l'eau de boisson, la concentration varie de zéro
ą 12 µg/litre. Les données concernant la concentration dans les
denrées alimentaires sont trop limitées pour que l'on puisse évaluer
l'exposition journaličre par voie orale.
Dans les eaux superficielles, la concentration moyenne de fond
des xylčnes est généralement inférieure ą 0,1 µg/litre. Cependant, on
a mesuré des valeurs beaucoup plus élevées dans des zones
industrielles et plus particuličrement celles oł sont implantées des
industries pétroličres (jusqu'ą 30 µg/litre dans les eaux polluées et
jusqu'ą 2 000 µg/litre ą proximité des conduites de décharge). Des
valeurs analogues ont été observées dans les eaux souterraines, ces
valeurs élevées pouvant źtre attribuées dans certains cas ą une
pollution locale par des réservoirs et des canalisations enterrées.
Aprčs exposition par la voie respiratoire, la dose inhalée est
retenue ą 60% environ dans les poumons. La métabolisation est
efficace puisque le xylčne est transformé ą 90% en acide
méthylhippurique, lequel est ensuite excrété dans les urines. Le
xylčne ne s'accumule pas en quantité importante dans l'organisme
humain.
Chez l'homme, une exposition aiguė ą du xylčne sous forte
concentration peut avoir des effets sur le systčme nerveux central et
provoquer une irritation. Ces effets n'ont toutefois pas donné lieu ą
des études contrōlées ni ą des études épidémiologiques ą long terme.
Chez les animaux de laboratoire, la toxicité chronique se révčle
faible. On a cependant de bonnes raisons de penser que sous
concentration modérée, le xylčne pourrait avoir des effets sur le SNC
chez l'animal.
Le xylčne ne s'est révélé ni mutagčne ni cancérogčne.
Le point d'aboutissement toxicologique essentiel concerne l'effet
nocif que le xylčne exerce sur le développment. On l'a mis en
évidence chez le rat ą partir d'une concentration de 870 mg/m3 (200
ppm). Compte tenu de cela, la valeur-guide recommandée pour la
concentration maximale de xylčne dans l'air a été fixée ą 0,87 mg/m3
(0,2 ppm).
Les xylčnes isomčres sont faiblement ą modérément toxiques pour
les organismes aquatiques. Chez les invertébrés, c'est l' o-xylčne
qui a la Cl50 la plus faible (1 mg/litre pour Daphnia magna). Chez
les poissons, la CL50 la plus faible est également celle de
l' o-xylčne (7,6 mg/litre chez la truite arc-en-ciel, par mesure de
concentration). Des valeurs de 7,9 et 1,7 mg/litre ont été obtenues,
respectivement pour le m- et le p-xylčne, dans le cas de la perche
commune (d'aprčs la concentration nominale). On ne dispose que de
données limitées au sujet de l'exposition chronique des organismes
aquatiques aux xylčnes, mais, quoi qu'il en soit, la volatilisation
rapide de ces composés la rend peu probable. Le xylčne ne présente
qu'une faible toxicité aiguė pour les oiseaux.
RESUMEN
El xileno es un hidrocarburo aromįtico del que hay tres formas
isoméricas: orto, meta y para. El xileno de calidad técnica contiene
una mezcla de los tres isómeros y algo de etilbenceno. Se estima que
la producción mundial fue de 15,4 millones de tone-ladas en 1984. La
presión de vapor estį comprendida entre 0,66 y 0,86 kPa para los tres
isómeros. Aproximadamente un 92% de las mezclas de xilenos se combinan
con el petróleo. El producto se emplea también en diversos
disolventes, en particular en las industrias de fabricación de
pinturas y de tintas de imprenta.
La mayor parte del xileno liberado en el medio ambiente pasa
directamente a la atmósfera. En ésta los isómeros de xileno se
degradan con facilidad, principalmente por fotooxidación. Los tres
isómeros se volatilizan rįpidamente en la atmósfera a partir del agua.
En el suelo y el agua los isómeros meta y para se biodegradan
fįcilmente en una amplia variedad de condiciones aerobias y
anaerobias, pero el isómero orto es mįs persistente. Las limitadas
pruebas disponibles parecen indicar que la bioacumulación de los
isómeros de xileno por los peces y los invertebrados es baja. La
eliminación del xileno de los organismos acuįticos es bastante rįpida
a partir del momento en que se interrumpe la exposición.
Normalmente los niveles basales medios de los tres isómeros de
xileno en el aire ambiente son de aproximadamente 1 µg/m3, pero en
zonas suburbanas se hallan en torno a 3 µg/m3. Se han detectado
concentraciones mayores en zonas urbanas e industrializadas, con
niveles medios de hasta 500 µg/m3. No obstante, las concentraciones
son por lo general inferiores a 100 µg/m3.
La exposición diaria por inhalación estimada en la población
general es de 70 µg en zonas rurales y de menos de 2000 µg en zonas
urbanas. La concentración en el agua potable estį comprendida entre
valores indetectables y 12 µg/litro. Los datos disponibles sobre la
concentración en los alimentos son insuficientes para poder estimar la
exposición oral diaria.
Las concentraciones basales medias de xilenos en aguas
superficiales son generalmente inferiores a 0.1 µg/litro. Sin embargo
se han hallado valores mucho mįs altos en zonas industriales y en
zonas vinculadas a la industria petrolera (hasta 30 µg/litro en aguas
contaminadas y hasta 2000 µg/litro en las proximidades de tuberķas de
desagüe). Se ha informado del hallazgo de niveles basales similares
en aguas subterrįneas, aunque se han detectado también concentraciones
elevadas, atribuidas a contaminación localizada a partir de tanques de
almacenamiento y tuberķas subterrįneos.
Tras la exposición por inhalación la retención pulmonar es de un
60% de la dosis inhalada. El xileno es metabolizado eficientemente.
Mįs del 90% se biotransforma en įcido metilhipśrico, que se excreta
por la orina. El xileno no se acumula de forma significativa en el
organismo humano.
La exposición aguda a altas concentraciones de xileno puede
afectar al SNC y causar irritación en el hombre. Sin embargo, no se
han llevado a cabo ni estudios controlados a largo plazo en el ser
humano ni estudios epidemiológicos. La toxicidad crónica parece
relativamente baja en animales de laboratorio. Hay indicios, no
obstante, de que concentraciones moderadas de xileno pueden tener
efectos crónicos sobre el SNC en animales.
El xileno no parece tener efectos mutįgenos ni carcinógenos.
El parįmetro crķtico es la toxicidad para el desarrollo,
demostrada a niveles de exposición de 870 mg/m3 (200 ppm) en la rata.
Teniendo en cuenta este parįmetro, la concentración indicativa
recomendada para el xileno en el aire es de 0.87 mg/m3 (0,2 ppm).
Los isómeros de xileno poseen una toxicidad entre moderada y baja
para los organismos acuįticos. En invertebrados la CL50 mįs baja,
calculada a partir de las concentraciones medidas, es de 1 mg/litro
para el o-xileno (Daphnia magna). Los valores mįs bajos de CL50
detectados en peces son de 7,6 mg/litro para el o-xileno (trucha
arco iris; segśn las concentraciones medidas), y de 7,9 y 1,7 mg/litro
para los m- y p-xilenos respectivamente (ambos para la lubina
estriada; segśn las concentraciones nominales). La información
disponible respecto a la exposición crónica de organismos acuįticos a
los xilenos es limitada; no obstante, su rįpida volatilización hace
improbable la exposición crónica en el agua. La toxicidad aguda del
xileno para las aves es baja.
