
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 186
ETHYLBENZENE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr P. Lundberg (National Institute of
Occupational Health, Sweden), Dr M. Crookes (Building Research
Establishment, United Kingdom), and Dr S. Dobson and Mr P. Howe
(Institute of Terrestrial Ecology, Monks Wood, United Kingdom)
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1996
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme (UNEP), the
International Labour Organisation (ILO), and the World Health
Organization (WHO). The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include the
development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally comparable
results, and the development of manpower in the field of toxicology.
Other activities carried out by the IPCS include the development of
know-how for coping with chemical accidents, coordination of
laboratory testing and epidemiological studies, and promotion of
research on the mechanisms of the biological action of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Ethylbenzene
(Environmental health criteria ; 186)
1.Ethylbenzene - toxicity 2.Benzene derivatives
3.Environmental exposure I.Series
ISBN 92 4 157186 1 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLBENZENE
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. Ethylbenzene in air
2.4.2. Ethylbenzene in water
2.4.3. Ethylbenzene in biological material
2.4.4. Metabolites of ethylbenzene in urine
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production processes
3.2.2. Production levels
3.2.3. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.4. Sediment
4.2. Transformation
4.2.1. Biodegradation
4.2.1.1 Aerobic degradation
4.2.1.2 Anaerobic degradation
4.2.2. Abiotic degradation
4.2.2.1 Photolysis
4.2.2.2 Photo-oxidation
4.2.2.3 Hydrolysis
4.2.3. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Surface water and sediment
5.1.3. Groundwater
5.1.4. Urban run-off, effluent and landfill leachate
5.1.5. Soil
5.1.6. Biota
5.2. General population exposure
5.2.1. Environmental sources
5.2.2. Food
5.2.3. Drinking-water
5.3. Occupational exposure during manufacture,
formulation or use
5.3.1. Biological monitoring
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption
6.1.1. Skin absorption
6.1.2. Absorption via inhalation
6.1.3. Absorption after oral intake
6.2. Distribution
6.3. Metabolic transformation
6.4. Elimination and excretion
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term exposure
7.3. Long-term exposure
7.3.1. Oral exposure
7.3.2. Inhalation exposure
7.4. Skin and eye irritation, sensitization
7.5. Reproductive toxicity, embryotoxicity
and teratogenicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.8. Other special studies
7.9. Factors modifying toxicity
8. EFFECTS ON HUMANS
8.1. Volunteer studies
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.2. Aquatic organisms
9.3. Terrestrial organisms
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
11. CONCLUSIONS
12. FURTHER RESEARCH
13. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new 14 partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
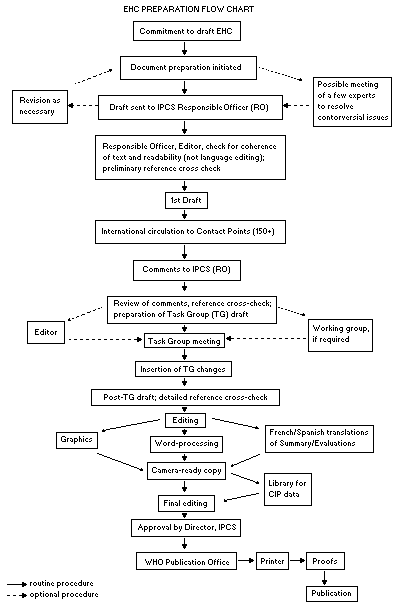 governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by
the range of expertise required for the subject of the meeting and by
the need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLBENZENE
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton, Surrey,
United Kingdom
Dr A. Bobra, Environment Canada, Orleans, Ontario, Canada
Dr K. Hatfield, Division of Standards Development and Technology
Transfer, National Institute for Occupational Safety and Health,
Cincinnati, Ohio, USA
Mr L. Heiskanen, Environmental Health Assessment and Criteria Section,
Chemical Safety Unit, Department of Human Services and Health,
Canberra, Australia
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Co-rapporteur)
Dr You-xin Liang, Department of Occupational Health, Shanghai Medical
University, Shanghai, China
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Dr P. Lundberg, Department of Toxicology, National Institute of
Occupational Health, Solna, Sweden (Co-rapporteur)
Dr Vesa Riihimaki, Institute of Occupational Health, Helsinki, Finland
Dr Leif Simonsen, National Institute of Occupational Health,
Copenhagen, Denmark
Representatives of other Organizations
Dr P. Montuschi, Institute of Pharmacology, Faculty of Medicine and
Surgery, Catholic University of the Sacred Heart, Rome, Italy
(representing the International Union of Pharmacology)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLBENZENE
A WHO Task Group on Environmental Health Criteria for
Ethylbenzene met at the British Industrial Biological Research
Association (BIBRA) Toxicology International, Carshalton, Surrey,
United Kingdom, from 27 February to 2 March 1995. Dr D. Anderson
opened the meeting and welcomed the participants on behalf of the host
institute. Dr B.H. Chen, IPCS, welcomed the participants on behalf of
the Director, IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
criteria monograph and made an evaluation of the risks for human
health and the environment from exposure to ethylbenzene.
Dr P. Lundberg, National Institute of Occupational Health,
Sweden, Dr M. Crookes, Building Research Establishment, United Kingdom
and Dr S. Dobson and Mr P. Howe, Institute of Terrestrial Ecology,
Monks Wood, United Kingdom, prepared the first draft of this
monograph. The second draft was prepared by Dr Lundberg and Mr Howe,
incorporating comments received following the circulation of the first
draft to the IPCS Contact Points for Environmental Health Criteria
monographs. Dr S. Soliman, College of Agriculture & Veterinary
Medicine, Saudi Arabia, contributed to the final text of the document.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contributions to the IPCS.
ABBREVIATIONS
FID flame ionization detection
GC gas chromatography
MS mass spectrometry
PID photoionization detection
VOC volatile organic compound
1. SUMMARY
Ethylbenzene is an aromatic hydrocarbon manufactured by
alkylation from benzene and ethylene. The estimated yearly production
in the USA is about 5 million tonnes, and in 1983 it was approximately
3 million tonnes in western Europe. Ethylbenzene is a colourless
liquid with a sweet gasoline-like odour. It is mainly used for the
production of styrene. It is also used in technical xylene as a
solvent in paints and lacquers and in the rubber and chemical
manufacturing industries. It is found in crude oils, refined
petroleum products and combustion products.
Ethylbenzene is a non-persistent chemical, being degraded
primarily by photo-oxidation and biodegradation. Volatilization to
the atmosphere is rapid. The photo-oxidation reaction of ethylbenzene
in the atmosphere may contribute to photochemical smog formation.
The log octanol-water partition coefficient is 3.13, indicating a
potential for bioaccumulation. However, the limited evidence
available shows that ethylbenzene bioconcentration factors are low for
fish and molluscs. Elimination from aquatic organisms appears to be
rapid.
Ethylbenzene levels in air at rural sites are generally less than
2 µg/m3. Mean levels of ethylbenzene ranging from 0.74 to 100 µg/m3
have been measured at urban sites. The levels of ethylbenzene found
in surface waters are generally less than 0.1 µg/litre in
non-industrial areas. In industrial and urban areas ethylbenzene
concentrations of up to 15 µg/litre have been reported. Ethylbenzene
levels in sediments are generally less than 0.5 µg/kg, although levels
between 1 and 5 µg/kg have been found in sediments from heavily
industrialized areas. Concentrations in uncontaminated groundwater are
generally less than 0.1 µg/litre, but are much higher in contaminated
groundwater.
The acute toxicity of ethylbenzene to algae, aquatic
invertebrates and fish is moderate. The lowest acute toxicity values
are 4.6 mg/litre for the alga Selenastrum capricornutum (72-h EC50
based on growth inhibition), 1.8 mg/litre for Daphnia magna (48-h
LC50) and 4.2 mg/litre for rainbow trout (96-h LC50). No information
is available regarding chronic exposure of aquatic organisms to
ethylbenzene.
There is limited information regarding the toxicity of
ethylbenzene to bacteria and earthworms. There are no data for
terrestrial plants, birds or wild mammals.
Human exposure to ethylbenzene occurs mainly by inhalation;
40-60% of inhaled ethylbenzene is retained in the lung. Ethylbenzene is
extensively metabolized, mainly to mandelic and phenylglyoxylic acids.
These urinary metabolites can be used to monitor human exposures.
Ethylbenzene has low acute and chronic toxicity for both animals
and humans. It is toxic to the central nervous system and is an
irritant of mucous membranes and the eyes. The threshold for these
effects in humans after short single exposures was estimated to be
about 430-860 mg/m3 (100-200 ppm).
Inhalation of ethylbenzene for 13 weeks by rats and mice at
concentrations up to 4300 mg/m3 (1000 ppm) did not lead to
histopathological lesions. The no-observed-effect level, based on
increased liver weight in rats, was 2150 mg/m3 (500 ppm).
Ethylbenzene is an inducer of liver microsomal enzymes. It is
not mutagenic or teratogenic in rats and rabbits. No information is
available on reproductive toxicity or carcinogenicity of ethylbenzene.
A guidance value of 22 mg/m3 (5 ppm) has been calculated from
animal studies. The estimated exposure of the general population
(even in the worst case situation) is below this guidance value.
Long-term occupational exposure to ethylbenzene concentrations
estimated to be of this order of magnitude did not cause adverse
health effects in workers.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Empirical formula: C8H10
Chemical structure:
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by
the range of expertise required for the subject of the meeting and by
the need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLBENZENE
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton, Surrey,
United Kingdom
Dr A. Bobra, Environment Canada, Orleans, Ontario, Canada
Dr K. Hatfield, Division of Standards Development and Technology
Transfer, National Institute for Occupational Safety and Health,
Cincinnati, Ohio, USA
Mr L. Heiskanen, Environmental Health Assessment and Criteria Section,
Chemical Safety Unit, Department of Human Services and Health,
Canberra, Australia
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Co-rapporteur)
Dr You-xin Liang, Department of Occupational Health, Shanghai Medical
University, Shanghai, China
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Dr P. Lundberg, Department of Toxicology, National Institute of
Occupational Health, Solna, Sweden (Co-rapporteur)
Dr Vesa Riihimaki, Institute of Occupational Health, Helsinki, Finland
Dr Leif Simonsen, National Institute of Occupational Health,
Copenhagen, Denmark
Representatives of other Organizations
Dr P. Montuschi, Institute of Pharmacology, Faculty of Medicine and
Surgery, Catholic University of the Sacred Heart, Rome, Italy
(representing the International Union of Pharmacology)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLBENZENE
A WHO Task Group on Environmental Health Criteria for
Ethylbenzene met at the British Industrial Biological Research
Association (BIBRA) Toxicology International, Carshalton, Surrey,
United Kingdom, from 27 February to 2 March 1995. Dr D. Anderson
opened the meeting and welcomed the participants on behalf of the host
institute. Dr B.H. Chen, IPCS, welcomed the participants on behalf of
the Director, IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
criteria monograph and made an evaluation of the risks for human
health and the environment from exposure to ethylbenzene.
Dr P. Lundberg, National Institute of Occupational Health,
Sweden, Dr M. Crookes, Building Research Establishment, United Kingdom
and Dr S. Dobson and Mr P. Howe, Institute of Terrestrial Ecology,
Monks Wood, United Kingdom, prepared the first draft of this
monograph. The second draft was prepared by Dr Lundberg and Mr Howe,
incorporating comments received following the circulation of the first
draft to the IPCS Contact Points for Environmental Health Criteria
monographs. Dr S. Soliman, College of Agriculture & Veterinary
Medicine, Saudi Arabia, contributed to the final text of the document.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contributions to the IPCS.
ABBREVIATIONS
FID flame ionization detection
GC gas chromatography
MS mass spectrometry
PID photoionization detection
VOC volatile organic compound
1. SUMMARY
Ethylbenzene is an aromatic hydrocarbon manufactured by
alkylation from benzene and ethylene. The estimated yearly production
in the USA is about 5 million tonnes, and in 1983 it was approximately
3 million tonnes in western Europe. Ethylbenzene is a colourless
liquid with a sweet gasoline-like odour. It is mainly used for the
production of styrene. It is also used in technical xylene as a
solvent in paints and lacquers and in the rubber and chemical
manufacturing industries. It is found in crude oils, refined
petroleum products and combustion products.
Ethylbenzene is a non-persistent chemical, being degraded
primarily by photo-oxidation and biodegradation. Volatilization to
the atmosphere is rapid. The photo-oxidation reaction of ethylbenzene
in the atmosphere may contribute to photochemical smog formation.
The log octanol-water partition coefficient is 3.13, indicating a
potential for bioaccumulation. However, the limited evidence
available shows that ethylbenzene bioconcentration factors are low for
fish and molluscs. Elimination from aquatic organisms appears to be
rapid.
Ethylbenzene levels in air at rural sites are generally less than
2 µg/m3. Mean levels of ethylbenzene ranging from 0.74 to 100 µg/m3
have been measured at urban sites. The levels of ethylbenzene found
in surface waters are generally less than 0.1 µg/litre in
non-industrial areas. In industrial and urban areas ethylbenzene
concentrations of up to 15 µg/litre have been reported. Ethylbenzene
levels in sediments are generally less than 0.5 µg/kg, although levels
between 1 and 5 µg/kg have been found in sediments from heavily
industrialized areas. Concentrations in uncontaminated groundwater are
generally less than 0.1 µg/litre, but are much higher in contaminated
groundwater.
The acute toxicity of ethylbenzene to algae, aquatic
invertebrates and fish is moderate. The lowest acute toxicity values
are 4.6 mg/litre for the alga Selenastrum capricornutum (72-h EC50
based on growth inhibition), 1.8 mg/litre for Daphnia magna (48-h
LC50) and 4.2 mg/litre for rainbow trout (96-h LC50). No information
is available regarding chronic exposure of aquatic organisms to
ethylbenzene.
There is limited information regarding the toxicity of
ethylbenzene to bacteria and earthworms. There are no data for
terrestrial plants, birds or wild mammals.
Human exposure to ethylbenzene occurs mainly by inhalation;
40-60% of inhaled ethylbenzene is retained in the lung. Ethylbenzene is
extensively metabolized, mainly to mandelic and phenylglyoxylic acids.
These urinary metabolites can be used to monitor human exposures.
Ethylbenzene has low acute and chronic toxicity for both animals
and humans. It is toxic to the central nervous system and is an
irritant of mucous membranes and the eyes. The threshold for these
effects in humans after short single exposures was estimated to be
about 430-860 mg/m3 (100-200 ppm).
Inhalation of ethylbenzene for 13 weeks by rats and mice at
concentrations up to 4300 mg/m3 (1000 ppm) did not lead to
histopathological lesions. The no-observed-effect level, based on
increased liver weight in rats, was 2150 mg/m3 (500 ppm).
Ethylbenzene is an inducer of liver microsomal enzymes. It is
not mutagenic or teratogenic in rats and rabbits. No information is
available on reproductive toxicity or carcinogenicity of ethylbenzene.
A guidance value of 22 mg/m3 (5 ppm) has been calculated from
animal studies. The estimated exposure of the general population
(even in the worst case situation) is below this guidance value.
Long-term occupational exposure to ethylbenzene concentrations
estimated to be of this order of magnitude did not cause adverse
health effects in workers.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Empirical formula: C8H10
Chemical structure:
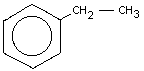 Chemical name: Ethylbenzene
Synonyms: Phenylethane, EB, Ethylbenzol
Relative molecular mass: 106.16
CAS registry number: 100-41-4
RTECS registry number: DA 07000000
EEC number: 601-023-00-4
2.2 Physical and chemical properties
Some physical and chemical properties of ethylbenzene are given
in Table 1.
The solubility of ethylbenzene in water is 152 mg/litre at 20°C
and 101.3 kPa (DEC, 1992) and 138 mg/litre at 15°C (Heilbron et al.,
1946). Ethylbenzene is soluble in ethanol, diethylether and most
other organic solvents (ECETOC, 1986; DEC, 1992). At room temperature
ethylbenzene is a colourless liquid with a sweet, gasoline-like odour
(Windholz, 1983).
The odour threshold concentration in air is about 2 mg/m3 and in
water about 0.1 mg/litre (temperature not stated) (Anon, 1987; DEC,
1992). Ethylbenzene floats on water and, because of its significant
vapour pressure and low water solubility, it will disperse in the
atmosphere (ECETOC, 1986).
Table 1. Some physical and chemical properties of ethylbenzenea
Physical state (20°C; 101.3 kPa) liquid
Colour colourless
Boiling point (°C) (101.3 kPa) 136.2
Melting point (°C) -94.95
Density (25°C; g/cm3) 0.866
Vapour pressure (kPa at 20°C) 1.24
Flash point (°C) 12.8
15
23
Refractive index (15°C, D line) 1.49857
Saturation % in air (20°C; 101.3 kPa) 1.2
Explosion limits (20°C; 101.3 kPa) 1-7.8
Log octanol/water partition coefficient (Log Kow) 3.13
Henry's Law Constant (Pa m3/mol) 887
Log Sorption Partition Coefficient (Log Koc) 1.98-3.04
Water Solubility (20°C; 101.3 kPa; mg/L) 152
a From: Heilbron et al. (1946); Sax (1979); Verschueren (1983);
Ullman (1983); Anon (1987); Weast (1988); ATSDR (1990);
Cavender (1993); DEC (1992); Mackay et al. (1992)
2.3 Conversion factors
1 ppm = 4.3 mg/m3 at 20°C and 101.3 kPa
1 mg/m3 = 0.23 ppm at 20°C and 101.3 kPa
2.4 Analytical methods
2.4.1 Ethylbenzene in air
Ethylbenzene in air can be analysed according to NIOSH (1984).
The air is sampled on a solid sorbent (coconut shell charcoal) and
desorbed with carbon disulfide. Aliquots are analysed by gas
chromatography (GC) with flame ionization detection (FID). With a
desorption volume of 0.5 ml, 2.17-8.62 mg can be measured. The
detection limit is about 0.1 mg/m3 for a 10-litre sample. Other
volatile organic solvents are possible interferences (NIOSH, 1984).
To avoid the use of carbon disulfide, sampling on montmorillonite
clays (minerals consisting of a three-layer aluminosilicate lattice)
and thermal desorption have been used (Harper & Purnell, 1990). This
method has not, however, yet been fully evaluated under actual
sampling conditions in the field.
A method using photoionization detection (PID) for GC, instead of
FID, has been described. PID is one to two orders of magnitude more
sensitive to most aromatics than is FID (Hester & Meyer, 1979). In an
evaluation of sampling and analytical methods for monitoring several
volatile organic compounds in air, sampling in a stainless steel
canister was shown to be the best. Using cryogenic pre-concentration
followed by gas-liquid chromatography (GLC) equipped with a selective
detector, ethylbenzene could be analysed at the ppb level (Jayanty,
1989).
The sensitivity of the GC/FID method for analysing ethylbenzene
and other volatile organic compounds can be improved by using wide
bore capillary columns (0.4-0.75 mm internal diameter). By this
means, ethylbenzene can be separated from the C8 isomers even in
complex mixtures (Frank et al., 1990). Several kinds of Bentones with
structures similar to that of Bentone 34 have been tested and compared
for the purpose of improving the resolution of ethylbenzene and xylene
isomers by GC. Bentone SD-3 was found to have higher selectivity
toward these close-boiling compounds than the well-known stationary
phase Bentone 34 (Zlatkis & Jiao, 1991).
Ultraviolet-spectrometry has also been used for analysis
(Yamamoto & Cook, 1968). Commercial detection tubes are available
with a detection range of 132.3-1764.0 mg/m3 (DEC, 1992) and of
4.41-220 µg (Gentry & Walsh, 1987).
2.4.2 Ethylbenzene in water
Determination of ethylbenzene in water has been performed by
using the "head-space" technique coupled to GLC, in combination with
mass spectrometry (MS) or infrared detection (Rosen et al., 1963;
Burnham et al., 1972; Kleopfer & Fairless, 1972; Grob, 1973). A
purge-and-trap method has been developed for determination of four
pollutants, including ethylbenzene, in aqueous samples. Water samples
are purged at 50°C with helium and the analytes are trapped on Tenax
GC. The trap is thermally desorbed directly into a gas chromatograph
equipped with FID. The method is laboratory validated for the range
of 20-500 ppb using a 5 g aqueous sample (Warner & Beasley, 1984).
Zhang & Pawliszyn (1993) developed a headspace solid phase
microextraction technique to determine ethylbenzene and other volatile
organic compounds (VOC) in water. The detection limit was found to be
at the ng/litre level.
2.4.3 Ethylbenzene in biological material
The head-space technique can also be used for measurements of
ethylbenzene in blood. The detection limit has been reported to be
0.01 mg/litre (Radzikowska-Kintzi & Jakubowski, 1981). The head-space
methodology must, however, be optimized specifically for blood rather
than using parameters derived from head-space experiments with aqueous
media (Dills et al., 1991).
An analytical method has been developed that enables the
determination of ethylbenzene and other volatile organic compounds in
10 ml of blood samples at the ng/litre level. The method depends on
purge-and-trap GC/MS and shows excellent reproducibility and recovery
even at ultra-trace levels (Ashley et al., 1992).
A method for analysing ethylbenzene in subcutaneous fat was
described by Wolff et al. (1977). Fat biopsies are obtained by using
a 30 cm3 silanized glass syringe and a size 16 G needle. The fat
globules are washed from the syringe and needle by saline. The fat-
saline suspension is frozen and thawed to 0°C prior to analysis. Fat
globules are weighed and aliquots of CS2 are added. The solution is
analysed by GC with dual flame ionization detectors.
A method for the determination of ethylbenzene and other
alkylbenzenes in plant foliage was developed by Keymeulen et al.
(1991). Using a gas chromatograph-quadrupole mass spectrometer in
the selected-ion monitoring mode, calibration graphs and detection
limits for these hydrocarbons were determined. Extraction was
performed with dichloromethane and the optimum extraction time was
found to be 6 h.
Murray & Lockhart (1981) prepared fish muscle for analysis by
extraction with dichloromethane and clean up on a florisil column.
Samples were analysed using GC with a FID. A detection limit of
5 µg/g was achieved with 98-102% recovery. A procedure to identify and
quantify ethylbenzene in fish samples by GC/MS using a fused-silica
capillary column (FSCC) and vacuum extraction has been developed
(Hiatt 1981, 1983; Dreisch & Munson 1983). Improved resolution and
detection limits at the ng/g level have been achieved with this
technique.
2.4.4 Metabolites of ethylbenzene in urine
One of the biomarkers of human exposure to ethylbenzene is the
urinary concentration of mandelic acid. Mandelic acid is also a
metabolite of styrene. Methods to monitor mandelic acid in urine were
initially developed in order to evaluate exposure to styrene.
In the GC method, mandelic acid is determined after extraction
from urine by diethyl ether (Engström & Rantanen, 1974; Gromiec &
Piotrowski, 1984). The detection limit for mandelic acid by this
method was 1.0 mg/litre. Gas chromato-graphic methods require
derivatization of the acid with diazomethane or silyl reagent before
analysis. This is not necessary for the HPLC or the ITP methods
(Sollenberg, 1991). Urinary samples extracted by diethyl ether can
also be determined by isotachophoresis (ITP) with a detection limit of
0.04 mmol/litre (Sollenberg, 1991). This method is comparable to a
high-performance liquid chromatographic (HPLC) method first described
in 1977, with a detection limit of 0.01 mmol/litre (Ogata et al.,
1977; Sollenberg, 1991).
Determination of another major metabolite of ethylbenzene,
phenylglyoxylic acid, in the urine of occupationally exposed people
has been carried out by HPLC methods. The limit of determination is
0.1 mg/litre (Inoue et al., 1995).
HPLC and ITP techniques can also be used for the simultaneous
determination of mandelic and phenylglyoxylic acids in the urine of
rats (Sollenberg et al., 1985).
GC methods have been developed for analysis of other metabolic
products of ethylbenzene. Simultaneous determination of several
minor metabolites in urine from man and rats (e.g., acetophenone,
1-phenylethanol, omega-hydroxyacetophenone, 4-ethylphenol,
2,4-dimethylphenol and 3-methylbenzylalcohol) can be achieved with one
method (Engström, 1984a).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Ethylbenzene is present in crude oil (Wiesenburg et al., 1981).
3.2 Anthropogenic sources
Ethylbenzene is present in refined products (Korte & Boedefeld,
1978). It is produced by incomplete combustion of natural materials,
making it a component of forest fires and cigarette smoke.
3.2.1 Production processes
About 90% of all ethylbenzene used in the chemical industry is
produced via the classic Friedel-Crafts alkylation of benzene with
ethylene using soluble aluminium chloride catalyst. These
liquid-phase processes generally involve ethyl chloride or
occasionally hydrogen chloride as a catalyst promoter. In a variation
on this method, dry benzene plus ethylene, catalyst and promoter are
fed continuously to the alkylation reactor (Lewis et al., 1983;
Fishbein, 1985).
Other procedures which have been employed to a much lesser extent
for the preparation of ethylbenzene include fractionation of petroleum
and ultra-fractionation from a mixed xylene stream (Seader, 1982;
Fishbein, 1985). It is not, however, economical to isolate
ethylbenzene from the catalytic raffinate (Fishbein, 1985; ECETOC,
1986).
An interesting approach for the preparation of ethylbenzene has
been developed by Levesque & Dao (1989). In this method the
alkylation of benzene to produce ethylbenzene was performed
successfully using an aqueous solution of ethanol of concentration
similar to a fermentation broth.
3.2.2 Production levels
In the USA, the production of ethylbenzene in 1982 and 1983 was
3.0 and 3.6 million tonnes, respectively (Webber, 1984). According to
Fishbein (1985), the annual capacity for the production of
ethylbenzene was estimated to be about 4.6 million tonnes in the USA
in 1983. In 1986, production in the USA was reported to be
approximately 4.1 million tonnes (US ITC, 1987). The estimated annual
production for ethylbenzene was 5.3 and 5.1 million tonnes for 1993
and 1992, respectively, in the USA. Ethylbenzene was the 19th in 1993
and the 18th in 1992 chemical out of the top 50 chemicals in the USA
(US Chemical Industry, 1994). In 1983 the production of ethylbenzene
in western Europe was around 3 million tonnes (ECETOC, 1986).
3.2.3 Uses
About 95% of ethylbenzene produced is employed for the production
of styrene. Ethylbenzene is a constituent (15-20%) of commercial
xylene ("mixed xylenes"), and hence used as a component of solvents,
as a diluent in paints and lacquers, and as a solvent in the rubber
and chemical manufacturing industries.
Ethylbenzene ("mixed xylenes") can also be added to motor fuels.
A typical ethylbenzene content of a reformate is about 4% (by volume)
(Fishbein, 1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The majority of ethylbenzene released into the environment passes
directly into the atmosphere or into surface water.
4.1.1 Air
It can be predicted from physico-chemical properties that, when
ethylbenzene is released into air, the major part remains in the
atmosphere and only small amounts are found in water, soil and
sediment. If it is assumed that all ethylbenzene is continuously
released to the atmosphere, the Level III generic fugacity model
consisting of homogeneous compartments of air, water, soil and
sediment predicts that over 99% of ethylbenzene would be distributed
in the atmosphere at steady state. Ethylbenzene discharged to the
atmosphere has very little potential for entering other media.
Precipitation from the atmosphere can occur. The key processes
determining overall fate are reaction in the air and advection (Mackay
et al., 1992).
Ethylbenzene has a low water solubility (152 mg/litre at 20°C)
and a relatively high vapour pressure (1.24 kPa at 20°C). This means
that only a very small proportion of ethylbenzene in the atmosphere is
likely to be removed by precipitation. This is shown by the fact that
it has been detected only at low levels in rain water samples (see
section 5, Table 3). Ethylbenzene may adsorb to atmospheric
particulates and be removed along with the particles by precipitation
or dry deposition.
4.1.2 Water
Transport and distribution of a substance in the aquatic
environment are dependent on its solubility, movement of the water,
exchanges at the air-water interfaces, adsorption to sediment and
particulate matter, and bioconcentration in aquatic organisms. The
residence time in water is also dependent upon the type of
environmental conditions encountered, such as temperature, wind speed,
currents, ice cover, etc.
For ethylbenzene, the half-life at 20°C in a river 1 m deep,
flowing at 1 m/sec and with a wind velocity of 3 m/sec, calculated
according to the method described by Thomas (1982) for high volatility
compounds, is 3.1 h. The half-lives in a marine mesocosm were 20 days
at 8-16°C in the spring, 2.1 days at 20-22°C in the summer and 13 days
at 3-7°C in the winter (Wakeham et al., 1983). Volatilization was a
dominant factor. The increased turnover time during summer was also
probably due to biodegradation. The seasonal variations between
winter and spring may have largely been due to changes in hydrodynamic
conditions as a result of changes in wind-driven turbulence.
If it is assumed that ethylbenzene is continuously released only
into the water compartment, the Level III generic fugacity model
predicts that approximately 93% of ethylbenzene would be distributed
into the water at steady state, 4.5% into the atmosphere, 2.5% into
the sediment and 1% into the soil. The key processes determining
overall fate are reaction in water and evaporation (Mackay et al.,
1992).
It has been estimated (Callahan, 1979) from a computed Henry's
Law constant of 6.44 × 10-3 atmos.m3.mol-1 that the volatility of
ethylbenzene from water will be very similar to that of toluene
(Henry's Law constant = 6.68 × 10-3 atmos.m3.mol-1). Thus,
ethylbenzene can be expected to have a half-life for volatilization
from still water at a depth of 1 m of about 5 to 6 h (Mackay &
Leinonen, 1975). A measured value of the Henry's Law constant of
8.43 × 10-3 atmos.m3.mol-1 (Mackay et al., 1979) supports this
estimate.
4.1.3 Soil
If it is assumed that ethylbenzene is only released to the soil
compartment, the Level III generic fugacity model indicates that
approximately 1% of ethylbenzene should be distributed into water,
4.9% into the atmosphere, 94.7% into the soil and >1% into the
sediment. The soil acts only as a reservoir. The soil concentration
is controlled almost entirely by the rate at which it can evaporate
(Mackay et al., 1992).
In a study by Jaynes & Boyd (1991), the sorption isotherms of
ethylbenzene and some other volatile organic compounds on organo-clays
indicated that sorption occurred by partition interactions with the
hexadecyltrimethylammonium (HDTMA)-derived organic phase.
Mineral-charge effects on sorption of ethylbenzene were evident.
Greater sorption of ethylbenzene and other alkylbenzenes by high-
charge HDTMA clays was attributed to the ability of the large basal
spacings to accommodate larger solute molecules (Jaynes & Boyd, 1991).
Several studies of soil-water partitioning for ethylbenzene have
been reported. In one study a value of 1.01 (log value) was found for
the soil-water partition coefficient (Kp) for a soil of 4.02
(± 0.06)% organic carbon content (Vowles & Mantoura, 1987). Pussemier
et al. (1990) reported a soil organic carbon-water partition
coefficient (Koc) of 2.41 (log value). Roy & Griffin (1985)
estimated log Koc values of 2.60 and 2.87 derived from equations
using solubility and Kow data, respectively. The soil organic
matter-water partition coefficient (Kom) was measured as 1.98 (log
value) for a soil containing 1.9% organic matter (Chiou et al., 1983).
Lee et al. (1989) reported log Kom values ranging from 1.73 to 1.97
for untreated soil and from 2.37 to 3.23 for soil treated with organic
cations. It is likely that ethylbenzene will be adsorbed to soil to
some extent. Roy & Griffin (1985) predicted that ethylbenzene would
have low mobility in water-saturated soil, based on the predicted Koc
values. However, Howard (1989) stated that the range of soil-water
partition coefficients suggests that ethylbenzene is adsorbed
moderately by soil and will probably leach through soil. The presence
of ethylbenzene in bank infiltrate water suggests that there is a high
probability of it leaching through soil. Other factors influencing
the movement of ethylbenzene through soil to groundwater include soil
type, soil porosity, amount of rainfall, depth of groundwater and
extent of degradation.
The competitive adsorption of ethylbenzene and water on bentonite
was studied by Rhue et al. (1989) using a technique that allowed the
amount of adsorbed water and the alkylbenzene to be measured
independently. Results indicated that ethylbenzene adsorption on the
clay was not affected by water at relative humidities near 0.23 but
was reduced significantly at values near 0.5.
Laboratory studies indicate that volatilization of ethylbenzene
occurs rapidly from sludge-treated soil (100% removal of an initial
concentration of 50 mg/kg occurred within 6 days) (Water Pollution
Control, 1989).
It has been reported that sorption isotherms of ethylbenzene and
some other nonionic organic compounds by maize (Zea mays) residues
and soil are linear. Sorption coefficients of the corn residues were
from 35 to 60 times greater than for surface soil (1.9% organic
matter), demonstrating the high sorptive capability of these residues
(Boyd et al., 1990).
Annable et al. (1993) studied the reduction of gasoline component
(including ethylbenzene) leaching potential by soil venting. Results
from columns vented for different periods of time showed vented soil
to be effective at reducing constituent concentrations in leachate
ultimately to about 1 µg/litre.
Clapp et al. (1994) compared the performance of activated sludge
(AS) and fixed-film processes with biological aerated filter (BAF)
fermenters for removal of priority pollutants, including ethylbenzene.
They found that the AS and BAF fermenters achieved comparable VOC
removal, and stripping rates were slightly higher for the AS
fermenters; degradation rates were slightly higher for the BAF
fermenters.
4.1.4 Sediment
The physical-chemical properties of ethylbenzene indicate that
only small amounts should be found in sediment.
4.2 Transformation
4.2.1 Biodegradation
4.2.1.1 Aerobic degradation
Ethylbenzene has been shown to be biodegradable in aquatic
systems. In simulations of spring and summer conditions in a coastal
bay, half-lives of 20 days and 2.1 days, respectively, were obtained
for ethylbenzene. Both volatilization and biodegradation were
responsible for removal (Wakeham et al., 1983). The reduction in
half-life in the summer was thought to represent an increase in
microbial degradation. An adaptation period was found to be important
for microbial degradation to take place. It was concluded that
microbial degradation becomes important under warm conditions, with
high biological activity, for the removal of ethylbenzene from the
aquatic environment. Howard (1991) report a half-life for aqueous
biodegradation, in an unacclimated system, of 3 to 10 days.
In an inherent biodegradability test (OECD 302 C), ethylbenzene
was degraded by 81 to 126% biological oxygen demand (BOD) in 2 weeks
(CITI, 1992). In another biodegradability test, ECETOC (1986)
reported that the BOD of ethylbenzene was determined after 6, 9 and 20
days and that biodegradation corresponding to 32, 36 and 45% of the
theoretical oxygen demand (TOD) was found. Pitter & Chudoba (1990)
reported a BOD5/TOD ratio of 0.29 for ethylbenzene.
Ethylbenzene, as part of the water-soluble fraction of gas oil,
has been shown to be degraded to 1-phenylethanol by the autochthonous
microflora of clean groundwater. After an initial lag phase of 3 to 4
days, complete disappearance of ethylbenzene (from an initial
concentration of 45 µg/litre) occurred within 12 days at 10°C
(Kappeler & Wuhrmann, 1978a,b).
Ethylbenzene was shown to be removed in core samples from an area
that had been previously contaminated with unleaded gasoline.
Complete degradation occurred within three weeks on incubation of
ethylbenzene in core samples that had previously had hydrogen peroxide
added, whereas a small amount of ethylbenzene remained after three
weeks in the previously gasoline-contaminated and uncontaminated core
samples (Thomas et al., 1990).
In static experiments, where ethylbenzene was incubated in the
dark for 7 days with settled domestic wastewater as microbial
inoculum, followed by 3 weekly subcultures from the medium,
ethylbenzene was shown to be 100% degraded within 7 days in the
initial inoculum and the three subsequent subcultures when the initial
ethylbenzene concentration was 5 mg/litre. At an initial ethylbenzene
concentration of 10 mg/litre, 69% degradation of ethylbenzene occurred
within 7 days in the initial culture, rising to 100% degradation
within 7 days in the third subculture, indicating that gradual
adaptation was needed for degradation of the higher concentration
(Tabak et al., 1981).
Soil bacteria have been shown to be capable of using ethylbenzene
as a sole carbon source. Microbial oxidative degradation has been
shown to proceed via hydroxylation of the aromatic ring to give
2,3-dihydroxy-1-ethylbenzene (Gibson et al., 1973). A similar
intermediate has been postulated in the degradation of ethylbenzene by
Pseudomonas sp. NCIB 10643 cultures. The 2,3-dihydroxy intermediate
was suggested to undergo further degradation by meta cleavage of the
aromatic ring (Smith & Ratledge, 1989).
Nocardia tartaricans has been shown to be capable of converting
ethylbenzene to 1-phenylethanol and acetophenone in a shake flask
culture using hexadecane as the source of carbon and energy (Cox &
Goldsmith, 1979).
Bestetti & Galli (1984) showed that Pseudomonas fluorescens can
utilize ethylbenzene as sole carbon source. Degradation appeared to
occur by meta cleavage of the ring, with formation of the semi-
aldehyde. Utkin et al. (1991) have also recently shown that a species
of Pseudomonas is capable of growing on ethylbenzene as the
sole source of carbon. Products observed included 1-phenylethanol,
2-phenylethanol, phenylacetate, salicylate, 2-hydroxyphenylacetate and
mandelate.
4.2.1.2 Anaerobic degradation
In a study to simulate the anaerobic degradation of landfill
leachate on aquifer material that was known to support methanogenesis,
no significant degradation of ethylbenzene was observed over the first
20 weeks of the experiment. However after 40 weeks the concentration
of ethylbenzene was found to be 26% of the original value, and after
120 weeks the concentration was < 1% of the original value (Wilson et
al., 1986).
Ethylbenzene, at a concentration of 500 mg/litre, was not found
to be metabolized by or toxic to enriched methane-producing cultures
(Chou et al., 1978).
Ethylbenzene has been shown to undergo anaerobic degradation by
aquifer microorganisms under denitrifying conditions in the presence
of nitrate. A lag period of around 30 days was observed before
biodegradation of ethylbenzene occurred, but total removal of
ethylbenzene occurred within the 56-day test period. Using aquifer
material that had been previously contaminated with jet fuel, little
degradation of ethylbenzene occurred over the 180-day test period, but
this was enhanced by addition of nitrate (Hutchins et al., 1991a,b).
Kuhn et al. (1988) also studied the degradation of ethylbenzene under
denitrifying conditions using nitrate as the sole electron acceptor.
In their experiment an aquifer column was used which was capable of
degrading m-xylene. The concentration of ethylbenzene was only
slightly reduced during passage through the column, and the authors
concluded that microbial mineralization of ethylbenzene was unlikely
under denitrifying conditions. However, they did point out that the
experiment was only carried out for 6 days. A longer experimental
period might have allowed another microbial population to grow within
the column that could have been capable of degrading ethylbenzene.
Ethylbenzene, as a component of crude oil contamination of anoxic
groundwater, has been found to be degraded in the anoxic region, but
the rate of disappearance was found to increase significantly in the
more oxygenated parts of the aquifer (Cozzarelli et al., 1990).
4.2.2 Abiotic degradation
4.2.2.1 Photolysis
Ethylbenzene does not absorb UV-visible radiation appreciably at
wavelengths longer than 290 nm. This means that it is unlikely to be
directly photolysed in the troposphere or in solution, as the earth's
ozone layer absorbs radiation at wavelengths less than 290 nm (Crookes
& Howe, 1992). Mabey et al. (1982) stated that direct photolysis of
ethylbenzene is not environmentally significant.
4.2.2.2 Photo-oxidation
Atmospheric oxidation of ethylbenzene is rapid and proceeds via
free-radical chain processes. The most important oxidant is the
hydroxyl radical, but ethylbenzene is also reactive with other species
found in the atmosphere, such as alkoxy radicals, peroxy radicals,
ozone and nitrogen oxides. Estimates for the half-life of
ethylbenzene in the atmosphere have been made from smog chamber
experiments and from knowledge of the reaction rate constant for
reaction with hydroxyl radicals. Atkinson (1985) reviewed the
available hydroxyl radical reaction rate constant data and recommended
a kOH value of 7.5 × 10-12 molecule-1.cm3.sec-1 at 25°C for
reaction with ethylbenzene.
A study by Callahan (1979) produced an atmospheric half-life of
around 15 h for ethylbenzene. Another report gave a figure of 51%
loss of ethylbenzene due to reaction with hydroxyl radicals in one day
(12 sunlight hours) (Singh et al., 1981, 1983). An atmospheric
lifetime of 14 sunlight hours has been quoted based on a value of kOH
(Singh et al., 1986). An important point when considering these data
is that the half-life calculated depends on several factors, including
temperature and also the actual concentration of hydroxyl radicals in
the atmosphere. It is known that the concentration of hydroxyl
radicals depends greatly on the amount of sunlight available; thus a
typical figure is around 2 × 106 molecules/cm3 in summer months,
falling by a factor of approximately 2 in winter months (Singh et al.,
1986). At night the concentration of hydroxyl radicals is negligible.
Even so, it can be seen that ethylbenzene is removed from the
atmosphere quite readily by reaction with hydroxyl radicals. It is
also possible that ethylbenzene will be removed from aquatic systems
by similar types of reactions, as hydroxyl radicals are known to exist
in aquatic systems.
4.2.2.3 Hydrolysis
It is considered unlikely that ethylbenzene will hydrolyse under
typical conditions found in the environment.
4.2.3 Bioaccumulation
Ethylbenzene has an octanol-water partition coefficient of 3.13
(log value), which indicates that bioaccumulation of ethylbenzene
could take place. Using this partition coefficient, an estimated
bioconcentration factor (BCF) of 2.16 (log value) can be calculated
(Bysshe, 1982).
In goldfish, a measured BCF of 1.19 (log value) has been reported
(Ogata et al., 1984). No details of exposure concentrations or length
of exposure were given.
When the manila clam (Tapes semidecussata) was exposed to
ethylbenzene at a concentration of 0.08 mg/litre in water containing
other petroleum hydrocarbons, the concentration found in the tissue
was 0.37 mg/kg after 8 days. Depuration occurred rapidly after
exposure ceased, tissue concentrations being below the limit of
detection (< 0.13 mg/kg) after 15 days (Nunes & Benville, 1979).
The low measured BCF values indicate that biomagnification of
ethylbenzene through the aquatic food chain is unlikely. No aquatic
food chain magnification was predicted from the model calculations and
empirical observations by Thomann (1989).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Measured levels of ethylbenzene in air are presented in Table 2.
Mean levels of ethylbenzene ranging from 0.74 to 100 µg/m3 have been
measured at urban sites. Industrial releases and vehicle emissions
are the principal sources of ethylbenzene. Levels found at rural
sites are generally < 2 µg/m3.
Ethylbenzene levels for indoor air are included in section 5.2.1.
5.1.2 Surface water and sediment
The levels of ethylbenzene found in surface water are shown in
Table 3. These are generally less than 0.1 µg/litre in non-industrial
areas. In industrial and urban areas ethylbenzene concentrations of
up to 15 µg/litre have been reported.
In 1985, 21 water samples and 21 bottom sediment samples were
collected at 7 sites in Japan and were analysed for the presence of
ethylbenzene. None of the water samples contained ethylbenzene; 3 of
the sediment samples from one site contained ethylbenzene
concentrations of 0.9 to 2.7 µg/kg dry weight. The detection limit
was 0.02 µg/litre for water and 0.8 µg/kg dry weight for bottom
sediment. In 1986, ethylbenzene was detected in 7 out of 133 samples
of surface water at 5 out of 46 sites (0.03-1.1 µg/litre) and in
28 out of 120 samples of bottom sediment at 15 out of 40 sites
(0.5-28 µg/kg dry weight). The detection limit was 0.03 µg/litre for
water and 0.5 µg/kg dry weight for sediment (EAJ, 1989).
Staples et al. (1985) reviewed the US EPA's STORET water quality
database and reported that median levels of ethylbenzene in ambient
surface water were less than 5.0 µg/litre between 1980 and 1982.
Ethylbenzene was detected in 10% of the 1101 samples collected during
this period. The median ethylbenzene concentration in sediment was
5.0 µg/kg dry weight, the compound being detected in 11% of the 350
samples.
In a study of the Tees Estuary, United Kingdom, levels of
ethylbenzene between 1 and 5 µg/kg were found in river sediment from a
heavily industrialized area (Whitby et al., 1982).
Table 2. Concentrations of ethylbenzene in air
Sampling source Concentration (µg/m3) References
Rural 0.23-1.6 (range of means) Petersson (1982), Clark et al. (1984b), Jüttner (1988), Lanzerstorfer
& Puxbaum (1990), Kawata & Fujeda (1993)
Urban 0.74-100 (range of means) Grob & Grob (1971), Bos et al. (1977), Louw et al. (1977), Singh et
(maximum value, 360) al. (1981; 1982; 1986), Nelson & Quigley (1982), Harkov et al. (1983),
De Bortoli et al. (1984), Clark et al. (1984a), Guicherit & Schulting
(1985), Jonsson et al. (1985), Hunt et al. (1986), Bruckmann et al.
(1988), Lanzerstorfer & Puxbaum (1990), Chan et al. (1991a), Derwent
(personal communication to the IPCS, 1991),
Industrial/residential site 22.0 (annual mean) Bruckmann et al. (1988)
near a rubber factory
Industrial site near 10.8 (annual mean) Bruckmann et al. (1988)
refineries producing
lubricating oil
Industrial site 94 (mean) Kroneld (1989)
near automotive 52 (< 1.6 km away) Sexton & Westberg (1980)
painting plant 11.5 (6.4 km away)
5 (17.6 km away)
Near to car plant 86 Petersson (1982)
27.8 (1 km away)
Road tunnels 2.1-48.2 Bos et al. (1977), Hampton et al. (1983), Dannecker et al. (1990)
Motorway 147 (mean) Thorburn & Colenutt (1979)
Table 3. Concentrations of ethylbenzene in water
Sampling source Concentration Reference
(µg/litre)a
Surface waters
Non-industrial river sites < 0.1 Waggott (1981), McFall et al. (1985), SAC (1989)
Industrial/urban river sites 1.9-15 Gomez-Belinchon et al. (1991)
(range of means)
Estuary (industrial area) ND-1.8 Whitby et al. (1982)
Seawater 0.0018-0.026 Gschwend et al. (1982), Gomez-Belinchon
(range of means) et al. (1991)
Sea near offshore oil 0.07 Sauer (1981)
platform
Rainwater 0.0006-0.009 Kawamura & Kaplan (1983), Pankow et al. (1984)
Groundwater
Uncontaminated NDb-0.07 Kenrick et al. (1985)
Contaminated 30-2000 Tester & Harker (1981), Van Duijvenbooden &
Kooper (1981), Stuermer et al. (1982), Rao et al.
(1985)
Water-table at a solvent up to 28 000 Cline & Viste (1985)
recovery facility
Table 3. (Cont'd)
Sampling source Concentration Reference
(µg/litre)a
Effluent
Effluent from wastewater/ NDc-14 Kennicutt et al. (1984); Namkung & Rittmann
sewage treatment works (1987); Feiler et al. (1979); Michael et al.
(1991); Gossett et al. (1983)
Landfill leachate 1.7-2310 Först et al. (1984); Reinhard et al. (1984);
Van Duijvenbooden & Kooper (1981); Cline &
Viste (1985)
a ND = not detected
b detection limit = 0.01 µg/litre
c detection limit not stated
A sludge characterization study for a slip containing wastewater
sludge situated in Baltimore Harbour, USA, was performed. The slip
contained an estimated 14 100 m3 of sludge, which averaged 20% solids
(by weight). Organic compounds were found to be the primary
constituents in the sludge, the highest concentrations being
represented by benzene, ethylbenzene, toluene and xylenes (Mott &
Romanow, 1991/1992).
5.1.3 Groundwater
The levels of ethylbenzene in groundwater are summarized in Table
3.
Ethylbenzene levels in uncontaminated groundwater are generally
< 0.1 µg/litre. However, much higher levels have been reported for
groundwater contaminated via waste disposal, fuel spillage and
industrial facilities. At a solvent recovery facility, ethylbenzene
concentrations of up to 28 000 µg/litre were measured.
Lesage et al. (1990) detected ethylbenzene in 3% of anoxic
groundwater samples at a concentration of 2 µg/litre. Goodenkauf &
Atkinson (1986) analysed 63 wells and detected ethylbenzene in only
one at a concentration of 0.99 µg/litre; the detection limit was
0.5 µg/litre. Ethylbenzene was found in 3 out of 466 groundwater
samples collected in the USA in 1982. The maximum concentration was
1.1 µg/litre and the detection limit 0.5 µg/litre (Cotruvo, 1985).
5.1.4 Urban run-off, effluent and landfill leachate
The levels of ethylbenzene in effluent from wastewater/sewage
treatment plants and landfill leachate are summarized in Table 3.
When Perry et al. (1979) analysed a range of industrial effluent
samples, 19 contained < 10 µg/litre, 4 contained 10-100 µg/litre and
2 contained > 100 µg/litre. Staples et al. (1985) reported that
ethylbenzene was detected in 7.4% of 1368 industrial effluent samples
collected between 1980 and 1983, the median concentration being less
than 3.0 µg/litre.
Cole et al. (1984) detected ethylbenzene in 4% of urban run-off
samples. Concentrations ranged from 1-2 µg/litre; however, no
detection limits were stated.
5.1.5 Soil
ATSDR (1990) reported that ethylbenzene was detected in 9.22%
soil samples from 1177 sites. The geometric mean of these samples was
697 µg/kg.
5.1.6 Biota
Several species of aquatic organisms have been analysed for
ethylbenzene (Table 4).
Table 4. Levels of ethylbenzene in aquatic species (Gossett et al., 1983)
Species Ethylbenzene level
µg/kg wet weight
Pacific sanddab (Citharichthys xanthostigma) (liver) <0.3
scorpion fish (Scorpaena guttata) (liver) <0.3
Dover sole (Microstomus pacificus) (liver) 0.3
white croaker (Genyonemus lineatus) (liver) 4
shrimp (muscle) <0.3
invertebrate (whole body) <0.3
NOTE: No detection limits were stated; organisms were collected from an
area near to the discharge zone of a waste treatment plant; ethylbenzene
levels of 14 µg/litre in effluent and 0.5 µg/kg (dryweight) in sediment
were measured in the area at the time of sampling.
In 1986, ethylbenzene was detected in 43 out of 138 fish samples
at 16 out of 42 sites in Japan, the concentrations ranging from 1.0 to
9.8 µg/kg wet weight. The detection limit was 1 µg/kg wet weight
(EAJ, 1989).
Staples et al. (1985) reviewed the US EPA's STORET water quality
database and reported that ethylbenzene was not detected in 97 biota
samples (detection limit, 0.025 mg/kg wet weight).
Lockhart et al. (1992) reported data on ethylbenzene levels in
freshwater fish sampled in the Canadian Arctic in 1985 and 1986. Mean
ethylbenzene concentrations ranged from 2.45 to 49.6 µg/kg in muscle
tissue and from 1.81 to 46.3 µg/kg in liver tissue for burbot. In
whitefish muscle tissue samples, mean ethylbenzene concentrations
ranged from 7.46 to 104 µg/kg.
5.2 General population exposure
5.2.1 Environmental sources
The magnitude of natural releases into the environment has not
been established.
Although ethylbenzene is ubiquitous in rural and urban
atmospheres, levels in urban areas are elevated due to vehicular and
industrial emissions. Ethylbenzene was not detectable in some rural
samples, while those taken on busy urban streets contained levels up
to 99 µg/m3 (23.1 ppb) (ATSDR, 1990).
The Environmental Protection Agency (USA) conducted a study of
ethylbenzene levels in public access buildings and found that
concentration, which was 387 µg/m3 (90 ppb) at the time construction
was completed, declined to 39 µg/m3 (9 ppb) following several months
of occupation of the building. This indicated that building materials
and/or finishings, such as paints, carpets and adhesives, were likely
sources of emissions (Pellizzari et al., 1984). Subsequent emission
studies using inhalation chambers revealed that ethylbenzene was
emitted from glued carpet at a mean level of 6.4 (± 3.2) µg/m3,
corresponding to an emission rate of 77 (± 39) ng/min per m2 (Wallace
et al., 1987c). Hodgson et al. (1991) studied the emissions of
volatile organic compounds in a new office building over a period of
14 months. Ethylbenzene levels in the building ranged from 7.0 to
11.8 µg/m3, as compared to 1.8 µg/m3 in the outdoor air. The
authors suggested that motor vehicles in the underground carpark of
the building were one of the major sources of ethylbenzene, but this
area was not specifically monitored.
Wallace et al. (1987a,b) monitored ethylbenzene in breathing-zone
air, exhaled air and ambient air samples taken from some of the home
backyards of 400 residents of an industrial/chemical manufacturing
area (the cities of Bayonne and Elizabeth, New Jersey, USA). Median
levels of ethylbenzene ranged from 4.6 to 7.1 µg/m3 for breathing-
zone air, 1.3 to 2.9 µg/m3 for exhaled air and 2.2 to 4.0 µg/m3
for backyard air. Personal air monitoring conducted at home
yielded high ethylbenzene levels, believed to be due to the presence
of the chemical in tobacco smoke. The maximum geometric mean
ethylbenzene exposure of people living in homes with smokers (13
µg/m3) was approximately 1.5 times the geometric mean of people
living in homes without smokers (8 µg/m3). Wallace et al. (1987a)
found the geometric mean level of ethylbenzene in the expired air of
smokers (n=200) to be 2 to 3 times higher than in that of non-smokers
(n=322). Wallace et al. (1987a) estimated that the total amount of
ethylbenzene in the mainstream smoke of a single cigarette, containing
16 mg of tar and nicotine, was 8 µg.
In another study, the blood concentration of ethylbenzene was
measured in 13 non-smokers and 14 cigarette smokers, all living in an
urban area. The concentration of ethylbenzene in blood ranged from
175 to 2284 ng/litre and 378 to 2697 ng/litre, respectively
(Hajimiragha et al., 1989).
Fellin & Otson (1993) monitored indoor air for ethylbenzene in
754 randomly selected Canadian residences in 1986. Mean ethylbenzene
concentrations were 6.46 µg/m3 in winter, 8.15 µg/m3 in spring, 4.35
µg/m3 in summer and 13.97 µg/m3 in autumn.
Wallace et al. (1989) carried out a study on seven volunteers who
performed 25 common activities thought to increase personal exposure
to volatile organic compounds during a 3-month period. Monitoring
personal, indoor and outdoor air levels, as well as exhaled breath,
revealed that painting and using a carburettor cleaner resulted in an
80-fold increase in ethylbenzene exposure. Combustion sources
(including cigarette smoke), gasoline vapours and consumer products
containing ethylbenzene increased exposures by up to 6 times over the
background level.
Chan et al. (1991b) studied exposure of commuters in Boston, USA
to ethylbenzene. These individuals spent 1.3 to 1.7 h per day (5% to
7% of the day) commuting and this contributed 10-20% of their total
daily ethylbenzene exposure. The results showed that the highest
exposures were associated with commuting by car (5.8 µg/m3) and that
the use of car heaters resulted in even higher in-vehicle levels of
ethylbenzene. Heater use resulted in a passenger compartment mean
ethylbenzene level of 8 µg/m3, whereas non-use resulted in 3.7
µg/m3. The authors postulated that heaters can increase influx of
both the vehicle's own exhaust and general roadway exhaust.
Coal-fired power stations have been found to emit ethylbenzene
along with other volatile organic compounds (Garcia, 1992).
Bevan et al. (1991) monitored exposure to vehicle emissions while
commuting by bicycle on urban roads in Southampton, United Kingdom and
compared it with ethylbenzene exposure for a typical suburban area.
Mean ethylbenzene levels on urban roads were 30.3 µg/m3 compared with
15.1 µg/m3 for suburban areas. The authors reported that 2 metres
from the exhaust of a stationary idling vehicle the mean ethylbenzene
level was 137 µg/m3.
Ashley et al. (1994) analysed the blood ethylbenzene
concentration of 631 non-occupationally exposed people in the USA.
The mean and median levels were 0.11 and 0.06 µg/litre, respectively,
and the detection limit was 0.02 µg/litre.
Kawai et al. (1992) evaluated urinalysis and blood analysis as
means of detecting human exposure to ethylbenzene and some other
volatile organic compounds, using 143 exposed and 20 non-exposed
workers. They found that both solvent concentration in blood and
metabolite concentration in urine correlated significantly with the
concentration of the solvent in air.
Pellizzari et al. (1982) analysed volatile organic compounds in
human milk samples taken from lactating women living in urban areas of
the USA and found ethylbenzene in all eight samples. Ethylbenzene has
also been detected in human axillary volatiles (Labows et al., 1979).
However, both these studies were based solely on qualitative scans of
the mass spectra peaks from GC/MS analysis; no detection limits were
stated.
5.2.2 Food
Ethylbenzene has been detected in several types of dried legumes.
Levels of between 0 and 11 µg/kg (mean 5 µg/kg) in beans, 13 µg/kg in
split peas and 5 µg/kg in lentils were measured (Lovegren et al.,
1979).
Although ethylbenzene has been detected in the skin of roasted
guinea hens (at a level of 2 µg/kg) by Noleau & Toulemonde (1988), the
authors did not state whether the source of the ethylbenzene was
directly from the skin or the cooking process.
5.2.3 Drinking-water
Otson et al. (1982) found that ethylbenzene levels in Canadian
treated potable water ranged from <1 to 10 µg/litre. Westrick et al.
(1984) reported that ethylbenzene was detected in 8 out of 945 samples
of finished (undefined) water from groundwater supplies. The levels
ranged from 0.74 to 12 µg/litre. Coleman et al. (1984) analysed
drinking-water from Cincinnati, USA and found an ethylbenzene level
of 0.036 µg/litre.
Durst & Laperle (1990) studied the migration of ethylbenzene from
polystyrene containers into stored deionized water. The water samples
were stored for up to 90 days at temperatures ranging from 24 to 66°C.
Migration of ethylbenzene increased with time and storage temperature.
The levels in the water samples ranged from 16 µg/litre on day 1 to 41
µg/litre at 24°C, 48 µg/litre at 38°C and 107 µg/litre at 52°C.
Ethylbenzene levels of up to 209 µg/litre were detected on day 8 at
66°C.
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure to ethylbenzene alone is rare.
Simultaneous exposure to other organic solvents usually occurs.
The following ethylbenzene exposure levels have been reported
from various occupational settings: exposure to gasoline, mean
concentration of less than 0.08 mg/m3 (Rappaport et al., 1987);
exposure to jet fuel, mean concentrations of 0.02 mg/m3 (4 h) and
0.07 mg/m3 (15 min) and maximum concentrations of 1.3 mg/m3 (4 h)
and 8.0 mg/m3 (15 min) (Holm et al., 1987); exposure in petroleum and
chemical factories, mean concentration of 7.7 mg/m3 (Inoue et al.,
1995); exposure while varnishing vehicles, average concentration of 17
mg/m3 (Angerer & Wulf, 1985); exposure during paint-rolling and
brushing, maximum concentration of 3.2 mg/m3 (Verhoeff et al.,
1988); exposure in a petroleum company and a pharmaceutical factory,
concentration range of 0.05-23 mg/m3 (Lu & Zhen, 1989).
During painting operations, ethylbenzene and some other volatile
organic compounds were detected in the working atmosphere at
concentrations ranging from 0.1 to 69.1 ppm (Vincent et al., 1994).
5.3.1 Biological monitoring
Determination of mandelic acid in urine has been recommended as a
biomarker of exposure to ethylbenzene. In studies by Bardodej &
Bardodejová (1970) and by Gromieck & Piotrowski (1984), exposure to
ethylbenzene at 430 mg/m3 (100 ppm) for 8 h resulted in 13 mmol/litre
and 7.8 mmol/litre, respectively, of mandelic acid in the
end-of-exposure urine samples. A value of 1.5 g mandelic acid per g
creatinine (about 10 mmol/litre) in the post-shift urine has been
proposed as a Biological Exposure Index (ACGIH, 1985-1986).
In specific analysis (e.g., by gas chromatography), the urinary
level of mandelic acid is negligible (less than 0.2 mmol/litre) in the
general population. However, certain drugs may be metabolized to
mandelic acid (Aitio et al., 1994).
Monitoring of personal exposure has shown that low ethylbenzene
concentrations of approximately 8.6 mg/m3 (2 ppm) correlate
significantly (correlation coefficients of 0.6-0.7) with urinary
phenylglyoxylic acid concentration, suggesting that measurements of
this acid in the urine could be used for biomonitoring (Inoue et al.,
1995).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
6.1.1 Skin absorption
One human subject was exposed for 2 h to ethylbenzene vapour at
concentrations ranging from 650 to 1300 mg/m3 in an exposure chamber.
The exposed skin accounted for 90-95% of the total skin area. Clean
breathing air was provided by means of a gas-tight respirator. The
mandelic acid concentration in urine, before, during and up to 6 h
after exposure, was within physiological limits (approximately 2.7
mg/litre). The authors concluded that the skin is not a relevant
route of entry into the body for ethylbenzene vapours (Gromiec &
Piotrowski, 1984).
The possible absorption of liquid ethylbenzene across human sin
has also been studied (Dutkiewicz & Tyras, 1967). Ethylbenzene
(0.2 ml=174 mg) was applied in a watch glass tightly fixed on the
forearm. The exposed skin area was 17.3 cm2. After 10-15 min the
contents of the watch glass space was extracted with ethanol and the
recovered amount of ethylbenzene determined spectrophotometrically.
On the basis of the quantity of ethylbenzene not recovered, the mean
absorption rate for seven people was calculated to be 28 mg/cm2 per
hour (range 22-33 mg cm2 per hour).
The penetration rate of ethylbenzene through excised rat skin has
been determined in a penetration chamber. One ml of ethylbenzene was
applied to 2.55 cm2 skin. After a 6-h application period, the
penetration rate was found to be about 0.99 nmoles/cm2 per min
(6 µg/cm2 per hour (Tsuruta, 1982).
Percutaneous absorption of ethylbenzene has been studied in
hairless mice (11 animals) (Susten et al., 1990). 14C-ring-labelled
ethylbenzene (in a volume of 5 µl) was injected into a chamber glued
onto the back skin (0.8 cm2), and the animals were housed in
metabolism cages for 4 h. During that period exhaled breath samples
were collected. At the end of 4 h, the animals were killed and the
absorbed dose was measured in the excreta and carcass. A total of
95.2 (± 1)% of the nominal dose was recovered. The absorption rate
was calculated to be 0.037 (± 0.0315) mg/cm2 per min (2.2 ± 1.9
mg/cm2 per hour).
Dermal absorption of volatile organic chemicals from aqueous
solutions has been studied in male Fischer-344 rats. For 24 h the
rats were exposed (3.1 cm2 dorsal shaved skin) to 2 ml (in a glass
exposure cell) of one-third saturated, two-thirds saturated, or a
fully saturated solution of ethylbenzene. Blood samples were obtained
at 0, 0.5, 1, 2, 4, 8, 12 and 24 h. The peak blood level (exposure to
neat ethylbenzene) was 5.6 mg/litre. The level reached a maximum
within 4 h and then either remained at about the same level for the
duration of the exposure or decreased. The blood levels were directly
related to the exposure concentrations (Morgan et al., 1991).
The data concerning skin permeability of ethylbenzene in humans
(Dutkievicz and Tyras, 1967) is not consistent with the animal data.
The reliability of the estimated fluxes of ethylbenzene through human
skin must be questioned because they are many times higher than the
measured fluxes through rat skin, whereas from studies of in vitro
percutaneous absorption it is known that rat skin is more permeable
than human skin (mean ratio about 3) for several chemicals (Barber et
al., 1992).
6.1.2 Absorption via inhalation
When volunteers (number not given) were exposed to 99, 185, 198
or 365 mg/m3 (23, 43, 46 or 85 ppm) ethylbenzene for 8 h, 64% of the
inhaled ethylbenzene was taken up by the respiratory tract (Bardodej &
Bardodejova, 1966). In another study, six volunteers were exposed
under controlled conditions for 8 h to 18, 34, 80, 150 or 200 mg/m3.
The retention of ethylbenzene in the lungs (difference in
concentration between inhaled and exhaled air) was 49% (± 5%)
independent of the exposure concentration (Gromiec & Piotrowski,
1984).
When volunteers were exposed to 430 mg/m3 or 870 mg/m3 of
"industrial xylene" (containing 40% ethylbenzene and 60% xylenes) for
2 h, about 60% was taken up, independent of concentration. If the
workload increased during exposure, the retention dropped to 50%
(Ĺstrand et al., 1978).
In a study by Chin et al. (1980a), rats (male, Harlan-Wistar)
were exposed to 14C-labelled ethylbenzene at a concentration of 1000
mg/m3 for 6 h. Assuming a ventilation rate of 100 ml/min, each rat
had an estimated intake of 36 mg ethylbenzene, of which 44% was
absorbed.
6.1.3 Absorption after oral intake
Toxicity studies in various animal species show indirectly that
ethylbenzene is absorbed after oral administration (Wolf et al., 1956,
NTP, 1992). Moreover, in one study, ethylbenzene appeared to be
rapidly and well absorbed from the gastro-intestinal tract since more
than 80% of the administered radioactively labelled compound was
recovered in urine within 48 h (Climie et al., 1983).
6.2 Distribution
When volunteers (n=12) were exposed for 2 h to 100 or 200 ppm
"industrial xylene", the amount of ethylbenzene taken up correlated
with the amount of body fat ("industrial xylene" consisted of 40.4%
ethylbenzene, 49.4% m-xylene, 8.8% o-xylene and 1.4% p-xylene).
The concentration of ethylbenzene ranged from 4 to 8 mg/kg in
subcutaneous adipose tissue 30 min after exposure. There was,
however, a negative correlation between the concentration in the
adipose tissue and the estimated relative amount of fat (Engström &
Bjurström, 1978).
The ethylbenzene concentration in the subcutaneous fat of workers
in a styrene polymerization plant was less than 0.8 mg/kg. The level
of exposure to ethylbenzene was reported to be below 17 mg/m3 (4
ppm). The 25 workers were exposed to a variety of other chemicals as
well (Wolff et al., 1977).
When rats were exposed to 1000 mg/m3 14C-ring-labelled
ethylbenzene for 6 h, 0.2% of this radioactivity was found 42 h later
in the tissues, mainly in the liver, gastrointestinal tract, fat and
the carcass (Chin et al., 1980a).
In a study by Engström et al., (1985), male Wistar rats (n = 20)
were exposed to 215, 1290 or 2580 mg ethylbenzene/m3 (50, 300 or 600
ppm) for 6 h/day, and 5 days/week for up to 16 weeks. The
concentration of ethylbenzene in perirenal fat was measured in weeks
2, 5 and 9. There were no consistent changes in ethylbenzene levels
during the course of exposure. After 16 weeks the amount of
ethylbenzene in perirenal fat was 8.5, 167.7 and 262.2 mg/kg fat at
the three exposure levels, respectively.
6.3 Metabolic transformation
Metabolic pathways of ethylbenzene based on urinary metabolites
have been proposed for humans (Fig. 1) and for rats (Fig. 2). The
main metabolic pathway is oxidation of the side chain, both in humans
and in animals. However, it has been demonstrated that there are both
qualitative and quantitative inter-species differences in the
metabolites produced (Engström et al., 1984; Engström 1984b). In
humans, the main metabolites of ethylbenzene are mandelic and
phenylglyoxylic acids. In several animal species the metabolic
transformation continues to benzoic acid, leading to excretion of
hippuric acid after conjugation with glycine. This conjugate is
generally one of the main urinary metabolites, together with mandelic
acid, in rats and dogs (Chin et al., 1980b). Hydroxylation of the
aromatic nucleus is a minor pathway. In the rabbit this phenolic
pathway accounts for less than 2% of the ethylbenzene absorbed (Kiese
& Lenk, 1974).
Chemical name: Ethylbenzene
Synonyms: Phenylethane, EB, Ethylbenzol
Relative molecular mass: 106.16
CAS registry number: 100-41-4
RTECS registry number: DA 07000000
EEC number: 601-023-00-4
2.2 Physical and chemical properties
Some physical and chemical properties of ethylbenzene are given
in Table 1.
The solubility of ethylbenzene in water is 152 mg/litre at 20°C
and 101.3 kPa (DEC, 1992) and 138 mg/litre at 15°C (Heilbron et al.,
1946). Ethylbenzene is soluble in ethanol, diethylether and most
other organic solvents (ECETOC, 1986; DEC, 1992). At room temperature
ethylbenzene is a colourless liquid with a sweet, gasoline-like odour
(Windholz, 1983).
The odour threshold concentration in air is about 2 mg/m3 and in
water about 0.1 mg/litre (temperature not stated) (Anon, 1987; DEC,
1992). Ethylbenzene floats on water and, because of its significant
vapour pressure and low water solubility, it will disperse in the
atmosphere (ECETOC, 1986).
Table 1. Some physical and chemical properties of ethylbenzenea
Physical state (20°C; 101.3 kPa) liquid
Colour colourless
Boiling point (°C) (101.3 kPa) 136.2
Melting point (°C) -94.95
Density (25°C; g/cm3) 0.866
Vapour pressure (kPa at 20°C) 1.24
Flash point (°C) 12.8
15
23
Refractive index (15°C, D line) 1.49857
Saturation % in air (20°C; 101.3 kPa) 1.2
Explosion limits (20°C; 101.3 kPa) 1-7.8
Log octanol/water partition coefficient (Log Kow) 3.13
Henry's Law Constant (Pa m3/mol) 887
Log Sorption Partition Coefficient (Log Koc) 1.98-3.04
Water Solubility (20°C; 101.3 kPa; mg/L) 152
a From: Heilbron et al. (1946); Sax (1979); Verschueren (1983);
Ullman (1983); Anon (1987); Weast (1988); ATSDR (1990);
Cavender (1993); DEC (1992); Mackay et al. (1992)
2.3 Conversion factors
1 ppm = 4.3 mg/m3 at 20°C and 101.3 kPa
1 mg/m3 = 0.23 ppm at 20°C and 101.3 kPa
2.4 Analytical methods
2.4.1 Ethylbenzene in air
Ethylbenzene in air can be analysed according to NIOSH (1984).
The air is sampled on a solid sorbent (coconut shell charcoal) and
desorbed with carbon disulfide. Aliquots are analysed by gas
chromatography (GC) with flame ionization detection (FID). With a
desorption volume of 0.5 ml, 2.17-8.62 mg can be measured. The
detection limit is about 0.1 mg/m3 for a 10-litre sample. Other
volatile organic solvents are possible interferences (NIOSH, 1984).
To avoid the use of carbon disulfide, sampling on montmorillonite
clays (minerals consisting of a three-layer aluminosilicate lattice)
and thermal desorption have been used (Harper & Purnell, 1990). This
method has not, however, yet been fully evaluated under actual
sampling conditions in the field.
A method using photoionization detection (PID) for GC, instead of
FID, has been described. PID is one to two orders of magnitude more
sensitive to most aromatics than is FID (Hester & Meyer, 1979). In an
evaluation of sampling and analytical methods for monitoring several
volatile organic compounds in air, sampling in a stainless steel
canister was shown to be the best. Using cryogenic pre-concentration
followed by gas-liquid chromatography (GLC) equipped with a selective
detector, ethylbenzene could be analysed at the ppb level (Jayanty,
1989).
The sensitivity of the GC/FID method for analysing ethylbenzene
and other volatile organic compounds can be improved by using wide
bore capillary columns (0.4-0.75 mm internal diameter). By this
means, ethylbenzene can be separated from the C8 isomers even in
complex mixtures (Frank et al., 1990). Several kinds of Bentones with
structures similar to that of Bentone 34 have been tested and compared
for the purpose of improving the resolution of ethylbenzene and xylene
isomers by GC. Bentone SD-3 was found to have higher selectivity
toward these close-boiling compounds than the well-known stationary
phase Bentone 34 (Zlatkis & Jiao, 1991).
Ultraviolet-spectrometry has also been used for analysis
(Yamamoto & Cook, 1968). Commercial detection tubes are available
with a detection range of 132.3-1764.0 mg/m3 (DEC, 1992) and of
4.41-220 µg (Gentry & Walsh, 1987).
2.4.2 Ethylbenzene in water
Determination of ethylbenzene in water has been performed by
using the "head-space" technique coupled to GLC, in combination with
mass spectrometry (MS) or infrared detection (Rosen et al., 1963;
Burnham et al., 1972; Kleopfer & Fairless, 1972; Grob, 1973). A
purge-and-trap method has been developed for determination of four
pollutants, including ethylbenzene, in aqueous samples. Water samples
are purged at 50°C with helium and the analytes are trapped on Tenax
GC. The trap is thermally desorbed directly into a gas chromatograph
equipped with FID. The method is laboratory validated for the range
of 20-500 ppb using a 5 g aqueous sample (Warner & Beasley, 1984).
Zhang & Pawliszyn (1993) developed a headspace solid phase
microextraction technique to determine ethylbenzene and other volatile
organic compounds (VOC) in water. The detection limit was found to be
at the ng/litre level.
2.4.3 Ethylbenzene in biological material
The head-space technique can also be used for measurements of
ethylbenzene in blood. The detection limit has been reported to be
0.01 mg/litre (Radzikowska-Kintzi & Jakubowski, 1981). The head-space
methodology must, however, be optimized specifically for blood rather
than using parameters derived from head-space experiments with aqueous
media (Dills et al., 1991).
An analytical method has been developed that enables the
determination of ethylbenzene and other volatile organic compounds in
10 ml of blood samples at the ng/litre level. The method depends on
purge-and-trap GC/MS and shows excellent reproducibility and recovery
even at ultra-trace levels (Ashley et al., 1992).
A method for analysing ethylbenzene in subcutaneous fat was
described by Wolff et al. (1977). Fat biopsies are obtained by using
a 30 cm3 silanized glass syringe and a size 16 G needle. The fat
globules are washed from the syringe and needle by saline. The fat-
saline suspension is frozen and thawed to 0°C prior to analysis. Fat
globules are weighed and aliquots of CS2 are added. The solution is
analysed by GC with dual flame ionization detectors.
A method for the determination of ethylbenzene and other
alkylbenzenes in plant foliage was developed by Keymeulen et al.
(1991). Using a gas chromatograph-quadrupole mass spectrometer in
the selected-ion monitoring mode, calibration graphs and detection
limits for these hydrocarbons were determined. Extraction was
performed with dichloromethane and the optimum extraction time was
found to be 6 h.
Murray & Lockhart (1981) prepared fish muscle for analysis by
extraction with dichloromethane and clean up on a florisil column.
Samples were analysed using GC with a FID. A detection limit of
5 µg/g was achieved with 98-102% recovery. A procedure to identify and
quantify ethylbenzene in fish samples by GC/MS using a fused-silica
capillary column (FSCC) and vacuum extraction has been developed
(Hiatt 1981, 1983; Dreisch & Munson 1983). Improved resolution and
detection limits at the ng/g level have been achieved with this
technique.
2.4.4 Metabolites of ethylbenzene in urine
One of the biomarkers of human exposure to ethylbenzene is the
urinary concentration of mandelic acid. Mandelic acid is also a
metabolite of styrene. Methods to monitor mandelic acid in urine were
initially developed in order to evaluate exposure to styrene.
In the GC method, mandelic acid is determined after extraction
from urine by diethyl ether (Engström & Rantanen, 1974; Gromiec &
Piotrowski, 1984). The detection limit for mandelic acid by this
method was 1.0 mg/litre. Gas chromato-graphic methods require
derivatization of the acid with diazomethane or silyl reagent before
analysis. This is not necessary for the HPLC or the ITP methods
(Sollenberg, 1991). Urinary samples extracted by diethyl ether can
also be determined by isotachophoresis (ITP) with a detection limit of
0.04 mmol/litre (Sollenberg, 1991). This method is comparable to a
high-performance liquid chromatographic (HPLC) method first described
in 1977, with a detection limit of 0.01 mmol/litre (Ogata et al.,
1977; Sollenberg, 1991).
Determination of another major metabolite of ethylbenzene,
phenylglyoxylic acid, in the urine of occupationally exposed people
has been carried out by HPLC methods. The limit of determination is
0.1 mg/litre (Inoue et al., 1995).
HPLC and ITP techniques can also be used for the simultaneous
determination of mandelic and phenylglyoxylic acids in the urine of
rats (Sollenberg et al., 1985).
GC methods have been developed for analysis of other metabolic
products of ethylbenzene. Simultaneous determination of several
minor metabolites in urine from man and rats (e.g., acetophenone,
1-phenylethanol, omega-hydroxyacetophenone, 4-ethylphenol,
2,4-dimethylphenol and 3-methylbenzylalcohol) can be achieved with one
method (Engström, 1984a).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Ethylbenzene is present in crude oil (Wiesenburg et al., 1981).
3.2 Anthropogenic sources
Ethylbenzene is present in refined products (Korte & Boedefeld,
1978). It is produced by incomplete combustion of natural materials,
making it a component of forest fires and cigarette smoke.
3.2.1 Production processes
About 90% of all ethylbenzene used in the chemical industry is
produced via the classic Friedel-Crafts alkylation of benzene with
ethylene using soluble aluminium chloride catalyst. These
liquid-phase processes generally involve ethyl chloride or
occasionally hydrogen chloride as a catalyst promoter. In a variation
on this method, dry benzene plus ethylene, catalyst and promoter are
fed continuously to the alkylation reactor (Lewis et al., 1983;
Fishbein, 1985).
Other procedures which have been employed to a much lesser extent
for the preparation of ethylbenzene include fractionation of petroleum
and ultra-fractionation from a mixed xylene stream (Seader, 1982;
Fishbein, 1985). It is not, however, economical to isolate
ethylbenzene from the catalytic raffinate (Fishbein, 1985; ECETOC,
1986).
An interesting approach for the preparation of ethylbenzene has
been developed by Levesque & Dao (1989). In this method the
alkylation of benzene to produce ethylbenzene was performed
successfully using an aqueous solution of ethanol of concentration
similar to a fermentation broth.
3.2.2 Production levels
In the USA, the production of ethylbenzene in 1982 and 1983 was
3.0 and 3.6 million tonnes, respectively (Webber, 1984). According to
Fishbein (1985), the annual capacity for the production of
ethylbenzene was estimated to be about 4.6 million tonnes in the USA
in 1983. In 1986, production in the USA was reported to be
approximately 4.1 million tonnes (US ITC, 1987). The estimated annual
production for ethylbenzene was 5.3 and 5.1 million tonnes for 1993
and 1992, respectively, in the USA. Ethylbenzene was the 19th in 1993
and the 18th in 1992 chemical out of the top 50 chemicals in the USA
(US Chemical Industry, 1994). In 1983 the production of ethylbenzene
in western Europe was around 3 million tonnes (ECETOC, 1986).
3.2.3 Uses
About 95% of ethylbenzene produced is employed for the production
of styrene. Ethylbenzene is a constituent (15-20%) of commercial
xylene ("mixed xylenes"), and hence used as a component of solvents,
as a diluent in paints and lacquers, and as a solvent in the rubber
and chemical manufacturing industries.
Ethylbenzene ("mixed xylenes") can also be added to motor fuels.
A typical ethylbenzene content of a reformate is about 4% (by volume)
(Fishbein, 1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The majority of ethylbenzene released into the environment passes
directly into the atmosphere or into surface water.
4.1.1 Air
It can be predicted from physico-chemical properties that, when
ethylbenzene is released into air, the major part remains in the
atmosphere and only small amounts are found in water, soil and
sediment. If it is assumed that all ethylbenzene is continuously
released to the atmosphere, the Level III generic fugacity model
consisting of homogeneous compartments of air, water, soil and
sediment predicts that over 99% of ethylbenzene would be distributed
in the atmosphere at steady state. Ethylbenzene discharged to the
atmosphere has very little potential for entering other media.
Precipitation from the atmosphere can occur. The key processes
determining overall fate are reaction in the air and advection (Mackay
et al., 1992).
Ethylbenzene has a low water solubility (152 mg/litre at 20°C)
and a relatively high vapour pressure (1.24 kPa at 20°C). This means
that only a very small proportion of ethylbenzene in the atmosphere is
likely to be removed by precipitation. This is shown by the fact that
it has been detected only at low levels in rain water samples (see
section 5, Table 3). Ethylbenzene may adsorb to atmospheric
particulates and be removed along with the particles by precipitation
or dry deposition.
4.1.2 Water
Transport and distribution of a substance in the aquatic
environment are dependent on its solubility, movement of the water,
exchanges at the air-water interfaces, adsorption to sediment and
particulate matter, and bioconcentration in aquatic organisms. The
residence time in water is also dependent upon the type of
environmental conditions encountered, such as temperature, wind speed,
currents, ice cover, etc.
For ethylbenzene, the half-life at 20°C in a river 1 m deep,
flowing at 1 m/sec and with a wind velocity of 3 m/sec, calculated
according to the method described by Thomas (1982) for high volatility
compounds, is 3.1 h. The half-lives in a marine mesocosm were 20 days
at 8-16°C in the spring, 2.1 days at 20-22°C in the summer and 13 days
at 3-7°C in the winter (Wakeham et al., 1983). Volatilization was a
dominant factor. The increased turnover time during summer was also
probably due to biodegradation. The seasonal variations between
winter and spring may have largely been due to changes in hydrodynamic
conditions as a result of changes in wind-driven turbulence.
If it is assumed that ethylbenzene is continuously released only
into the water compartment, the Level III generic fugacity model
predicts that approximately 93% of ethylbenzene would be distributed
into the water at steady state, 4.5% into the atmosphere, 2.5% into
the sediment and 1% into the soil. The key processes determining
overall fate are reaction in water and evaporation (Mackay et al.,
1992).
It has been estimated (Callahan, 1979) from a computed Henry's
Law constant of 6.44 × 10-3 atmos.m3.mol-1 that the volatility of
ethylbenzene from water will be very similar to that of toluene
(Henry's Law constant = 6.68 × 10-3 atmos.m3.mol-1). Thus,
ethylbenzene can be expected to have a half-life for volatilization
from still water at a depth of 1 m of about 5 to 6 h (Mackay &
Leinonen, 1975). A measured value of the Henry's Law constant of
8.43 × 10-3 atmos.m3.mol-1 (Mackay et al., 1979) supports this
estimate.
4.1.3 Soil
If it is assumed that ethylbenzene is only released to the soil
compartment, the Level III generic fugacity model indicates that
approximately 1% of ethylbenzene should be distributed into water,
4.9% into the atmosphere, 94.7% into the soil and >1% into the
sediment. The soil acts only as a reservoir. The soil concentration
is controlled almost entirely by the rate at which it can evaporate
(Mackay et al., 1992).
In a study by Jaynes & Boyd (1991), the sorption isotherms of
ethylbenzene and some other volatile organic compounds on organo-clays
indicated that sorption occurred by partition interactions with the
hexadecyltrimethylammonium (HDTMA)-derived organic phase.
Mineral-charge effects on sorption of ethylbenzene were evident.
Greater sorption of ethylbenzene and other alkylbenzenes by high-
charge HDTMA clays was attributed to the ability of the large basal
spacings to accommodate larger solute molecules (Jaynes & Boyd, 1991).
Several studies of soil-water partitioning for ethylbenzene have
been reported. In one study a value of 1.01 (log value) was found for
the soil-water partition coefficient (Kp) for a soil of 4.02
(± 0.06)% organic carbon content (Vowles & Mantoura, 1987). Pussemier
et al. (1990) reported a soil organic carbon-water partition
coefficient (Koc) of 2.41 (log value). Roy & Griffin (1985)
estimated log Koc values of 2.60 and 2.87 derived from equations
using solubility and Kow data, respectively. The soil organic
matter-water partition coefficient (Kom) was measured as 1.98 (log
value) for a soil containing 1.9% organic matter (Chiou et al., 1983).
Lee et al. (1989) reported log Kom values ranging from 1.73 to 1.97
for untreated soil and from 2.37 to 3.23 for soil treated with organic
cations. It is likely that ethylbenzene will be adsorbed to soil to
some extent. Roy & Griffin (1985) predicted that ethylbenzene would
have low mobility in water-saturated soil, based on the predicted Koc
values. However, Howard (1989) stated that the range of soil-water
partition coefficients suggests that ethylbenzene is adsorbed
moderately by soil and will probably leach through soil. The presence
of ethylbenzene in bank infiltrate water suggests that there is a high
probability of it leaching through soil. Other factors influencing
the movement of ethylbenzene through soil to groundwater include soil
type, soil porosity, amount of rainfall, depth of groundwater and
extent of degradation.
The competitive adsorption of ethylbenzene and water on bentonite
was studied by Rhue et al. (1989) using a technique that allowed the
amount of adsorbed water and the alkylbenzene to be measured
independently. Results indicated that ethylbenzene adsorption on the
clay was not affected by water at relative humidities near 0.23 but
was reduced significantly at values near 0.5.
Laboratory studies indicate that volatilization of ethylbenzene
occurs rapidly from sludge-treated soil (100% removal of an initial
concentration of 50 mg/kg occurred within 6 days) (Water Pollution
Control, 1989).
It has been reported that sorption isotherms of ethylbenzene and
some other nonionic organic compounds by maize (Zea mays) residues
and soil are linear. Sorption coefficients of the corn residues were
from 35 to 60 times greater than for surface soil (1.9% organic
matter), demonstrating the high sorptive capability of these residues
(Boyd et al., 1990).
Annable et al. (1993) studied the reduction of gasoline component
(including ethylbenzene) leaching potential by soil venting. Results
from columns vented for different periods of time showed vented soil
to be effective at reducing constituent concentrations in leachate
ultimately to about 1 µg/litre.
Clapp et al. (1994) compared the performance of activated sludge
(AS) and fixed-film processes with biological aerated filter (BAF)
fermenters for removal of priority pollutants, including ethylbenzene.
They found that the AS and BAF fermenters achieved comparable VOC
removal, and stripping rates were slightly higher for the AS
fermenters; degradation rates were slightly higher for the BAF
fermenters.
4.1.4 Sediment
The physical-chemical properties of ethylbenzene indicate that
only small amounts should be found in sediment.
4.2 Transformation
4.2.1 Biodegradation
4.2.1.1 Aerobic degradation
Ethylbenzene has been shown to be biodegradable in aquatic
systems. In simulations of spring and summer conditions in a coastal
bay, half-lives of 20 days and 2.1 days, respectively, were obtained
for ethylbenzene. Both volatilization and biodegradation were
responsible for removal (Wakeham et al., 1983). The reduction in
half-life in the summer was thought to represent an increase in
microbial degradation. An adaptation period was found to be important
for microbial degradation to take place. It was concluded that
microbial degradation becomes important under warm conditions, with
high biological activity, for the removal of ethylbenzene from the
aquatic environment. Howard (1991) report a half-life for aqueous
biodegradation, in an unacclimated system, of 3 to 10 days.
In an inherent biodegradability test (OECD 302 C), ethylbenzene
was degraded by 81 to 126% biological oxygen demand (BOD) in 2 weeks
(CITI, 1992). In another biodegradability test, ECETOC (1986)
reported that the BOD of ethylbenzene was determined after 6, 9 and 20
days and that biodegradation corresponding to 32, 36 and 45% of the
theoretical oxygen demand (TOD) was found. Pitter & Chudoba (1990)
reported a BOD5/TOD ratio of 0.29 for ethylbenzene.
Ethylbenzene, as part of the water-soluble fraction of gas oil,
has been shown to be degraded to 1-phenylethanol by the autochthonous
microflora of clean groundwater. After an initial lag phase of 3 to 4
days, complete disappearance of ethylbenzene (from an initial
concentration of 45 µg/litre) occurred within 12 days at 10°C
(Kappeler & Wuhrmann, 1978a,b).
Ethylbenzene was shown to be removed in core samples from an area
that had been previously contaminated with unleaded gasoline.
Complete degradation occurred within three weeks on incubation of
ethylbenzene in core samples that had previously had hydrogen peroxide
added, whereas a small amount of ethylbenzene remained after three
weeks in the previously gasoline-contaminated and uncontaminated core
samples (Thomas et al., 1990).
In static experiments, where ethylbenzene was incubated in the
dark for 7 days with settled domestic wastewater as microbial
inoculum, followed by 3 weekly subcultures from the medium,
ethylbenzene was shown to be 100% degraded within 7 days in the
initial inoculum and the three subsequent subcultures when the initial
ethylbenzene concentration was 5 mg/litre. At an initial ethylbenzene
concentration of 10 mg/litre, 69% degradation of ethylbenzene occurred
within 7 days in the initial culture, rising to 100% degradation
within 7 days in the third subculture, indicating that gradual
adaptation was needed for degradation of the higher concentration
(Tabak et al., 1981).
Soil bacteria have been shown to be capable of using ethylbenzene
as a sole carbon source. Microbial oxidative degradation has been
shown to proceed via hydroxylation of the aromatic ring to give
2,3-dihydroxy-1-ethylbenzene (Gibson et al., 1973). A similar
intermediate has been postulated in the degradation of ethylbenzene by
Pseudomonas sp. NCIB 10643 cultures. The 2,3-dihydroxy intermediate
was suggested to undergo further degradation by meta cleavage of the
aromatic ring (Smith & Ratledge, 1989).
Nocardia tartaricans has been shown to be capable of converting
ethylbenzene to 1-phenylethanol and acetophenone in a shake flask
culture using hexadecane as the source of carbon and energy (Cox &
Goldsmith, 1979).
Bestetti & Galli (1984) showed that Pseudomonas fluorescens can
utilize ethylbenzene as sole carbon source. Degradation appeared to
occur by meta cleavage of the ring, with formation of the semi-
aldehyde. Utkin et al. (1991) have also recently shown that a species
of Pseudomonas is capable of growing on ethylbenzene as the
sole source of carbon. Products observed included 1-phenylethanol,
2-phenylethanol, phenylacetate, salicylate, 2-hydroxyphenylacetate and
mandelate.
4.2.1.2 Anaerobic degradation
In a study to simulate the anaerobic degradation of landfill
leachate on aquifer material that was known to support methanogenesis,
no significant degradation of ethylbenzene was observed over the first
20 weeks of the experiment. However after 40 weeks the concentration
of ethylbenzene was found to be 26% of the original value, and after
120 weeks the concentration was < 1% of the original value (Wilson et
al., 1986).
Ethylbenzene, at a concentration of 500 mg/litre, was not found
to be metabolized by or toxic to enriched methane-producing cultures
(Chou et al., 1978).
Ethylbenzene has been shown to undergo anaerobic degradation by
aquifer microorganisms under denitrifying conditions in the presence
of nitrate. A lag period of around 30 days was observed before
biodegradation of ethylbenzene occurred, but total removal of
ethylbenzene occurred within the 56-day test period. Using aquifer
material that had been previously contaminated with jet fuel, little
degradation of ethylbenzene occurred over the 180-day test period, but
this was enhanced by addition of nitrate (Hutchins et al., 1991a,b).
Kuhn et al. (1988) also studied the degradation of ethylbenzene under
denitrifying conditions using nitrate as the sole electron acceptor.
In their experiment an aquifer column was used which was capable of
degrading m-xylene. The concentration of ethylbenzene was only
slightly reduced during passage through the column, and the authors
concluded that microbial mineralization of ethylbenzene was unlikely
under denitrifying conditions. However, they did point out that the
experiment was only carried out for 6 days. A longer experimental
period might have allowed another microbial population to grow within
the column that could have been capable of degrading ethylbenzene.
Ethylbenzene, as a component of crude oil contamination of anoxic
groundwater, has been found to be degraded in the anoxic region, but
the rate of disappearance was found to increase significantly in the
more oxygenated parts of the aquifer (Cozzarelli et al., 1990).
4.2.2 Abiotic degradation
4.2.2.1 Photolysis
Ethylbenzene does not absorb UV-visible radiation appreciably at
wavelengths longer than 290 nm. This means that it is unlikely to be
directly photolysed in the troposphere or in solution, as the earth's
ozone layer absorbs radiation at wavelengths less than 290 nm (Crookes
& Howe, 1992). Mabey et al. (1982) stated that direct photolysis of
ethylbenzene is not environmentally significant.
4.2.2.2 Photo-oxidation
Atmospheric oxidation of ethylbenzene is rapid and proceeds via
free-radical chain processes. The most important oxidant is the
hydroxyl radical, but ethylbenzene is also reactive with other species
found in the atmosphere, such as alkoxy radicals, peroxy radicals,
ozone and nitrogen oxides. Estimates for the half-life of
ethylbenzene in the atmosphere have been made from smog chamber
experiments and from knowledge of the reaction rate constant for
reaction with hydroxyl radicals. Atkinson (1985) reviewed the
available hydroxyl radical reaction rate constant data and recommended
a kOH value of 7.5 × 10-12 molecule-1.cm3.sec-1 at 25°C for
reaction with ethylbenzene.
A study by Callahan (1979) produced an atmospheric half-life of
around 15 h for ethylbenzene. Another report gave a figure of 51%
loss of ethylbenzene due to reaction with hydroxyl radicals in one day
(12 sunlight hours) (Singh et al., 1981, 1983). An atmospheric
lifetime of 14 sunlight hours has been quoted based on a value of kOH
(Singh et al., 1986). An important point when considering these data
is that the half-life calculated depends on several factors, including
temperature and also the actual concentration of hydroxyl radicals in
the atmosphere. It is known that the concentration of hydroxyl
radicals depends greatly on the amount of sunlight available; thus a
typical figure is around 2 × 106 molecules/cm3 in summer months,
falling by a factor of approximately 2 in winter months (Singh et al.,
1986). At night the concentration of hydroxyl radicals is negligible.
Even so, it can be seen that ethylbenzene is removed from the
atmosphere quite readily by reaction with hydroxyl radicals. It is
also possible that ethylbenzene will be removed from aquatic systems
by similar types of reactions, as hydroxyl radicals are known to exist
in aquatic systems.
4.2.2.3 Hydrolysis
It is considered unlikely that ethylbenzene will hydrolyse under
typical conditions found in the environment.
4.2.3 Bioaccumulation
Ethylbenzene has an octanol-water partition coefficient of 3.13
(log value), which indicates that bioaccumulation of ethylbenzene
could take place. Using this partition coefficient, an estimated
bioconcentration factor (BCF) of 2.16 (log value) can be calculated
(Bysshe, 1982).
In goldfish, a measured BCF of 1.19 (log value) has been reported
(Ogata et al., 1984). No details of exposure concentrations or length
of exposure were given.
When the manila clam (Tapes semidecussata) was exposed to
ethylbenzene at a concentration of 0.08 mg/litre in water containing
other petroleum hydrocarbons, the concentration found in the tissue
was 0.37 mg/kg after 8 days. Depuration occurred rapidly after
exposure ceased, tissue concentrations being below the limit of
detection (< 0.13 mg/kg) after 15 days (Nunes & Benville, 1979).
The low measured BCF values indicate that biomagnification of
ethylbenzene through the aquatic food chain is unlikely. No aquatic
food chain magnification was predicted from the model calculations and
empirical observations by Thomann (1989).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Measured levels of ethylbenzene in air are presented in Table 2.
Mean levels of ethylbenzene ranging from 0.74 to 100 µg/m3 have been
measured at urban sites. Industrial releases and vehicle emissions
are the principal sources of ethylbenzene. Levels found at rural
sites are generally < 2 µg/m3.
Ethylbenzene levels for indoor air are included in section 5.2.1.
5.1.2 Surface water and sediment
The levels of ethylbenzene found in surface water are shown in
Table 3. These are generally less than 0.1 µg/litre in non-industrial
areas. In industrial and urban areas ethylbenzene concentrations of
up to 15 µg/litre have been reported.
In 1985, 21 water samples and 21 bottom sediment samples were
collected at 7 sites in Japan and were analysed for the presence of
ethylbenzene. None of the water samples contained ethylbenzene; 3 of
the sediment samples from one site contained ethylbenzene
concentrations of 0.9 to 2.7 µg/kg dry weight. The detection limit
was 0.02 µg/litre for water and 0.8 µg/kg dry weight for bottom
sediment. In 1986, ethylbenzene was detected in 7 out of 133 samples
of surface water at 5 out of 46 sites (0.03-1.1 µg/litre) and in
28 out of 120 samples of bottom sediment at 15 out of 40 sites
(0.5-28 µg/kg dry weight). The detection limit was 0.03 µg/litre for
water and 0.5 µg/kg dry weight for sediment (EAJ, 1989).
Staples et al. (1985) reviewed the US EPA's STORET water quality
database and reported that median levels of ethylbenzene in ambient
surface water were less than 5.0 µg/litre between 1980 and 1982.
Ethylbenzene was detected in 10% of the 1101 samples collected during
this period. The median ethylbenzene concentration in sediment was
5.0 µg/kg dry weight, the compound being detected in 11% of the 350
samples.
In a study of the Tees Estuary, United Kingdom, levels of
ethylbenzene between 1 and 5 µg/kg were found in river sediment from a
heavily industrialized area (Whitby et al., 1982).
Table 2. Concentrations of ethylbenzene in air
Sampling source Concentration (µg/m3) References
Rural 0.23-1.6 (range of means) Petersson (1982), Clark et al. (1984b), Jüttner (1988), Lanzerstorfer
& Puxbaum (1990), Kawata & Fujeda (1993)
Urban 0.74-100 (range of means) Grob & Grob (1971), Bos et al. (1977), Louw et al. (1977), Singh et
(maximum value, 360) al. (1981; 1982; 1986), Nelson & Quigley (1982), Harkov et al. (1983),
De Bortoli et al. (1984), Clark et al. (1984a), Guicherit & Schulting
(1985), Jonsson et al. (1985), Hunt et al. (1986), Bruckmann et al.
(1988), Lanzerstorfer & Puxbaum (1990), Chan et al. (1991a), Derwent
(personal communication to the IPCS, 1991),
Industrial/residential site 22.0 (annual mean) Bruckmann et al. (1988)
near a rubber factory
Industrial site near 10.8 (annual mean) Bruckmann et al. (1988)
refineries producing
lubricating oil
Industrial site 94 (mean) Kroneld (1989)
near automotive 52 (< 1.6 km away) Sexton & Westberg (1980)
painting plant 11.5 (6.4 km away)
5 (17.6 km away)
Near to car plant 86 Petersson (1982)
27.8 (1 km away)
Road tunnels 2.1-48.2 Bos et al. (1977), Hampton et al. (1983), Dannecker et al. (1990)
Motorway 147 (mean) Thorburn & Colenutt (1979)
Table 3. Concentrations of ethylbenzene in water
Sampling source Concentration Reference
(µg/litre)a
Surface waters
Non-industrial river sites < 0.1 Waggott (1981), McFall et al. (1985), SAC (1989)
Industrial/urban river sites 1.9-15 Gomez-Belinchon et al. (1991)
(range of means)
Estuary (industrial area) ND-1.8 Whitby et al. (1982)
Seawater 0.0018-0.026 Gschwend et al. (1982), Gomez-Belinchon
(range of means) et al. (1991)
Sea near offshore oil 0.07 Sauer (1981)
platform
Rainwater 0.0006-0.009 Kawamura & Kaplan (1983), Pankow et al. (1984)
Groundwater
Uncontaminated NDb-0.07 Kenrick et al. (1985)
Contaminated 30-2000 Tester & Harker (1981), Van Duijvenbooden &
Kooper (1981), Stuermer et al. (1982), Rao et al.
(1985)
Water-table at a solvent up to 28 000 Cline & Viste (1985)
recovery facility
Table 3. (Cont'd)
Sampling source Concentration Reference
(µg/litre)a
Effluent
Effluent from wastewater/ NDc-14 Kennicutt et al. (1984); Namkung & Rittmann
sewage treatment works (1987); Feiler et al. (1979); Michael et al.
(1991); Gossett et al. (1983)
Landfill leachate 1.7-2310 Först et al. (1984); Reinhard et al. (1984);
Van Duijvenbooden & Kooper (1981); Cline &
Viste (1985)
a ND = not detected
b detection limit = 0.01 µg/litre
c detection limit not stated
A sludge characterization study for a slip containing wastewater
sludge situated in Baltimore Harbour, USA, was performed. The slip
contained an estimated 14 100 m3 of sludge, which averaged 20% solids
(by weight). Organic compounds were found to be the primary
constituents in the sludge, the highest concentrations being
represented by benzene, ethylbenzene, toluene and xylenes (Mott &
Romanow, 1991/1992).
5.1.3 Groundwater
The levels of ethylbenzene in groundwater are summarized in Table
3.
Ethylbenzene levels in uncontaminated groundwater are generally
< 0.1 µg/litre. However, much higher levels have been reported for
groundwater contaminated via waste disposal, fuel spillage and
industrial facilities. At a solvent recovery facility, ethylbenzene
concentrations of up to 28 000 µg/litre were measured.
Lesage et al. (1990) detected ethylbenzene in 3% of anoxic
groundwater samples at a concentration of 2 µg/litre. Goodenkauf &
Atkinson (1986) analysed 63 wells and detected ethylbenzene in only
one at a concentration of 0.99 µg/litre; the detection limit was
0.5 µg/litre. Ethylbenzene was found in 3 out of 466 groundwater
samples collected in the USA in 1982. The maximum concentration was
1.1 µg/litre and the detection limit 0.5 µg/litre (Cotruvo, 1985).
5.1.4 Urban run-off, effluent and landfill leachate
The levels of ethylbenzene in effluent from wastewater/sewage
treatment plants and landfill leachate are summarized in Table 3.
When Perry et al. (1979) analysed a range of industrial effluent
samples, 19 contained < 10 µg/litre, 4 contained 10-100 µg/litre and
2 contained > 100 µg/litre. Staples et al. (1985) reported that
ethylbenzene was detected in 7.4% of 1368 industrial effluent samples
collected between 1980 and 1983, the median concentration being less
than 3.0 µg/litre.
Cole et al. (1984) detected ethylbenzene in 4% of urban run-off
samples. Concentrations ranged from 1-2 µg/litre; however, no
detection limits were stated.
5.1.5 Soil
ATSDR (1990) reported that ethylbenzene was detected in 9.22%
soil samples from 1177 sites. The geometric mean of these samples was
697 µg/kg.
5.1.6 Biota
Several species of aquatic organisms have been analysed for
ethylbenzene (Table 4).
Table 4. Levels of ethylbenzene in aquatic species (Gossett et al., 1983)
Species Ethylbenzene level
µg/kg wet weight
Pacific sanddab (Citharichthys xanthostigma) (liver) <0.3
scorpion fish (Scorpaena guttata) (liver) <0.3
Dover sole (Microstomus pacificus) (liver) 0.3
white croaker (Genyonemus lineatus) (liver) 4
shrimp (muscle) <0.3
invertebrate (whole body) <0.3
NOTE: No detection limits were stated; organisms were collected from an
area near to the discharge zone of a waste treatment plant; ethylbenzene
levels of 14 µg/litre in effluent and 0.5 µg/kg (dryweight) in sediment
were measured in the area at the time of sampling.
In 1986, ethylbenzene was detected in 43 out of 138 fish samples
at 16 out of 42 sites in Japan, the concentrations ranging from 1.0 to
9.8 µg/kg wet weight. The detection limit was 1 µg/kg wet weight
(EAJ, 1989).
Staples et al. (1985) reviewed the US EPA's STORET water quality
database and reported that ethylbenzene was not detected in 97 biota
samples (detection limit, 0.025 mg/kg wet weight).
Lockhart et al. (1992) reported data on ethylbenzene levels in
freshwater fish sampled in the Canadian Arctic in 1985 and 1986. Mean
ethylbenzene concentrations ranged from 2.45 to 49.6 µg/kg in muscle
tissue and from 1.81 to 46.3 µg/kg in liver tissue for burbot. In
whitefish muscle tissue samples, mean ethylbenzene concentrations
ranged from 7.46 to 104 µg/kg.
5.2 General population exposure
5.2.1 Environmental sources
The magnitude of natural releases into the environment has not
been established.
Although ethylbenzene is ubiquitous in rural and urban
atmospheres, levels in urban areas are elevated due to vehicular and
industrial emissions. Ethylbenzene was not detectable in some rural
samples, while those taken on busy urban streets contained levels up
to 99 µg/m3 (23.1 ppb) (ATSDR, 1990).
The Environmental Protection Agency (USA) conducted a study of
ethylbenzene levels in public access buildings and found that
concentration, which was 387 µg/m3 (90 ppb) at the time construction
was completed, declined to 39 µg/m3 (9 ppb) following several months
of occupation of the building. This indicated that building materials
and/or finishings, such as paints, carpets and adhesives, were likely
sources of emissions (Pellizzari et al., 1984). Subsequent emission
studies using inhalation chambers revealed that ethylbenzene was
emitted from glued carpet at a mean level of 6.4 (± 3.2) µg/m3,
corresponding to an emission rate of 77 (± 39) ng/min per m2 (Wallace
et al., 1987c). Hodgson et al. (1991) studied the emissions of
volatile organic compounds in a new office building over a period of
14 months. Ethylbenzene levels in the building ranged from 7.0 to
11.8 µg/m3, as compared to 1.8 µg/m3 in the outdoor air. The
authors suggested that motor vehicles in the underground carpark of
the building were one of the major sources of ethylbenzene, but this
area was not specifically monitored.
Wallace et al. (1987a,b) monitored ethylbenzene in breathing-zone
air, exhaled air and ambient air samples taken from some of the home
backyards of 400 residents of an industrial/chemical manufacturing
area (the cities of Bayonne and Elizabeth, New Jersey, USA). Median
levels of ethylbenzene ranged from 4.6 to 7.1 µg/m3 for breathing-
zone air, 1.3 to 2.9 µg/m3 for exhaled air and 2.2 to 4.0 µg/m3
for backyard air. Personal air monitoring conducted at home
yielded high ethylbenzene levels, believed to be due to the presence
of the chemical in tobacco smoke. The maximum geometric mean
ethylbenzene exposure of people living in homes with smokers (13
µg/m3) was approximately 1.5 times the geometric mean of people
living in homes without smokers (8 µg/m3). Wallace et al. (1987a)
found the geometric mean level of ethylbenzene in the expired air of
smokers (n=200) to be 2 to 3 times higher than in that of non-smokers
(n=322). Wallace et al. (1987a) estimated that the total amount of
ethylbenzene in the mainstream smoke of a single cigarette, containing
16 mg of tar and nicotine, was 8 µg.
In another study, the blood concentration of ethylbenzene was
measured in 13 non-smokers and 14 cigarette smokers, all living in an
urban area. The concentration of ethylbenzene in blood ranged from
175 to 2284 ng/litre and 378 to 2697 ng/litre, respectively
(Hajimiragha et al., 1989).
Fellin & Otson (1993) monitored indoor air for ethylbenzene in
754 randomly selected Canadian residences in 1986. Mean ethylbenzene
concentrations were 6.46 µg/m3 in winter, 8.15 µg/m3 in spring, 4.35
µg/m3 in summer and 13.97 µg/m3 in autumn.
Wallace et al. (1989) carried out a study on seven volunteers who
performed 25 common activities thought to increase personal exposure
to volatile organic compounds during a 3-month period. Monitoring
personal, indoor and outdoor air levels, as well as exhaled breath,
revealed that painting and using a carburettor cleaner resulted in an
80-fold increase in ethylbenzene exposure. Combustion sources
(including cigarette smoke), gasoline vapours and consumer products
containing ethylbenzene increased exposures by up to 6 times over the
background level.
Chan et al. (1991b) studied exposure of commuters in Boston, USA
to ethylbenzene. These individuals spent 1.3 to 1.7 h per day (5% to
7% of the day) commuting and this contributed 10-20% of their total
daily ethylbenzene exposure. The results showed that the highest
exposures were associated with commuting by car (5.8 µg/m3) and that
the use of car heaters resulted in even higher in-vehicle levels of
ethylbenzene. Heater use resulted in a passenger compartment mean
ethylbenzene level of 8 µg/m3, whereas non-use resulted in 3.7
µg/m3. The authors postulated that heaters can increase influx of
both the vehicle's own exhaust and general roadway exhaust.
Coal-fired power stations have been found to emit ethylbenzene
along with other volatile organic compounds (Garcia, 1992).
Bevan et al. (1991) monitored exposure to vehicle emissions while
commuting by bicycle on urban roads in Southampton, United Kingdom and
compared it with ethylbenzene exposure for a typical suburban area.
Mean ethylbenzene levels on urban roads were 30.3 µg/m3 compared with
15.1 µg/m3 for suburban areas. The authors reported that 2 metres
from the exhaust of a stationary idling vehicle the mean ethylbenzene
level was 137 µg/m3.
Ashley et al. (1994) analysed the blood ethylbenzene
concentration of 631 non-occupationally exposed people in the USA.
The mean and median levels were 0.11 and 0.06 µg/litre, respectively,
and the detection limit was 0.02 µg/litre.
Kawai et al. (1992) evaluated urinalysis and blood analysis as
means of detecting human exposure to ethylbenzene and some other
volatile organic compounds, using 143 exposed and 20 non-exposed
workers. They found that both solvent concentration in blood and
metabolite concentration in urine correlated significantly with the
concentration of the solvent in air.
Pellizzari et al. (1982) analysed volatile organic compounds in
human milk samples taken from lactating women living in urban areas of
the USA and found ethylbenzene in all eight samples. Ethylbenzene has
also been detected in human axillary volatiles (Labows et al., 1979).
However, both these studies were based solely on qualitative scans of
the mass spectra peaks from GC/MS analysis; no detection limits were
stated.
5.2.2 Food
Ethylbenzene has been detected in several types of dried legumes.
Levels of between 0 and 11 µg/kg (mean 5 µg/kg) in beans, 13 µg/kg in
split peas and 5 µg/kg in lentils were measured (Lovegren et al.,
1979).
Although ethylbenzene has been detected in the skin of roasted
guinea hens (at a level of 2 µg/kg) by Noleau & Toulemonde (1988), the
authors did not state whether the source of the ethylbenzene was
directly from the skin or the cooking process.
5.2.3 Drinking-water
Otson et al. (1982) found that ethylbenzene levels in Canadian
treated potable water ranged from <1 to 10 µg/litre. Westrick et al.
(1984) reported that ethylbenzene was detected in 8 out of 945 samples
of finished (undefined) water from groundwater supplies. The levels
ranged from 0.74 to 12 µg/litre. Coleman et al. (1984) analysed
drinking-water from Cincinnati, USA and found an ethylbenzene level
of 0.036 µg/litre.
Durst & Laperle (1990) studied the migration of ethylbenzene from
polystyrene containers into stored deionized water. The water samples
were stored for up to 90 days at temperatures ranging from 24 to 66°C.
Migration of ethylbenzene increased with time and storage temperature.
The levels in the water samples ranged from 16 µg/litre on day 1 to 41
µg/litre at 24°C, 48 µg/litre at 38°C and 107 µg/litre at 52°C.
Ethylbenzene levels of up to 209 µg/litre were detected on day 8 at
66°C.
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure to ethylbenzene alone is rare.
Simultaneous exposure to other organic solvents usually occurs.
The following ethylbenzene exposure levels have been reported
from various occupational settings: exposure to gasoline, mean
concentration of less than 0.08 mg/m3 (Rappaport et al., 1987);
exposure to jet fuel, mean concentrations of 0.02 mg/m3 (4 h) and
0.07 mg/m3 (15 min) and maximum concentrations of 1.3 mg/m3 (4 h)
and 8.0 mg/m3 (15 min) (Holm et al., 1987); exposure in petroleum and
chemical factories, mean concentration of 7.7 mg/m3 (Inoue et al.,
1995); exposure while varnishing vehicles, average concentration of 17
mg/m3 (Angerer & Wulf, 1985); exposure during paint-rolling and
brushing, maximum concentration of 3.2 mg/m3 (Verhoeff et al.,
1988); exposure in a petroleum company and a pharmaceutical factory,
concentration range of 0.05-23 mg/m3 (Lu & Zhen, 1989).
During painting operations, ethylbenzene and some other volatile
organic compounds were detected in the working atmosphere at
concentrations ranging from 0.1 to 69.1 ppm (Vincent et al., 1994).
5.3.1 Biological monitoring
Determination of mandelic acid in urine has been recommended as a
biomarker of exposure to ethylbenzene. In studies by Bardodej &
Bardodejová (1970) and by Gromieck & Piotrowski (1984), exposure to
ethylbenzene at 430 mg/m3 (100 ppm) for 8 h resulted in 13 mmol/litre
and 7.8 mmol/litre, respectively, of mandelic acid in the
end-of-exposure urine samples. A value of 1.5 g mandelic acid per g
creatinine (about 10 mmol/litre) in the post-shift urine has been
proposed as a Biological Exposure Index (ACGIH, 1985-1986).
In specific analysis (e.g., by gas chromatography), the urinary
level of mandelic acid is negligible (less than 0.2 mmol/litre) in the
general population. However, certain drugs may be metabolized to
mandelic acid (Aitio et al., 1994).
Monitoring of personal exposure has shown that low ethylbenzene
concentrations of approximately 8.6 mg/m3 (2 ppm) correlate
significantly (correlation coefficients of 0.6-0.7) with urinary
phenylglyoxylic acid concentration, suggesting that measurements of
this acid in the urine could be used for biomonitoring (Inoue et al.,
1995).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
6.1.1 Skin absorption
One human subject was exposed for 2 h to ethylbenzene vapour at
concentrations ranging from 650 to 1300 mg/m3 in an exposure chamber.
The exposed skin accounted for 90-95% of the total skin area. Clean
breathing air was provided by means of a gas-tight respirator. The
mandelic acid concentration in urine, before, during and up to 6 h
after exposure, was within physiological limits (approximately 2.7
mg/litre). The authors concluded that the skin is not a relevant
route of entry into the body for ethylbenzene vapours (Gromiec &
Piotrowski, 1984).
The possible absorption of liquid ethylbenzene across human sin
has also been studied (Dutkiewicz & Tyras, 1967). Ethylbenzene
(0.2 ml=174 mg) was applied in a watch glass tightly fixed on the
forearm. The exposed skin area was 17.3 cm2. After 10-15 min the
contents of the watch glass space was extracted with ethanol and the
recovered amount of ethylbenzene determined spectrophotometrically.
On the basis of the quantity of ethylbenzene not recovered, the mean
absorption rate for seven people was calculated to be 28 mg/cm2 per
hour (range 22-33 mg cm2 per hour).
The penetration rate of ethylbenzene through excised rat skin has
been determined in a penetration chamber. One ml of ethylbenzene was
applied to 2.55 cm2 skin. After a 6-h application period, the
penetration rate was found to be about 0.99 nmoles/cm2 per min
(6 µg/cm2 per hour (Tsuruta, 1982).
Percutaneous absorption of ethylbenzene has been studied in
hairless mice (11 animals) (Susten et al., 1990). 14C-ring-labelled
ethylbenzene (in a volume of 5 µl) was injected into a chamber glued
onto the back skin (0.8 cm2), and the animals were housed in
metabolism cages for 4 h. During that period exhaled breath samples
were collected. At the end of 4 h, the animals were killed and the
absorbed dose was measured in the excreta and carcass. A total of
95.2 (± 1)% of the nominal dose was recovered. The absorption rate
was calculated to be 0.037 (± 0.0315) mg/cm2 per min (2.2 ± 1.9
mg/cm2 per hour).
Dermal absorption of volatile organic chemicals from aqueous
solutions has been studied in male Fischer-344 rats. For 24 h the
rats were exposed (3.1 cm2 dorsal shaved skin) to 2 ml (in a glass
exposure cell) of one-third saturated, two-thirds saturated, or a
fully saturated solution of ethylbenzene. Blood samples were obtained
at 0, 0.5, 1, 2, 4, 8, 12 and 24 h. The peak blood level (exposure to
neat ethylbenzene) was 5.6 mg/litre. The level reached a maximum
within 4 h and then either remained at about the same level for the
duration of the exposure or decreased. The blood levels were directly
related to the exposure concentrations (Morgan et al., 1991).
The data concerning skin permeability of ethylbenzene in humans
(Dutkievicz and Tyras, 1967) is not consistent with the animal data.
The reliability of the estimated fluxes of ethylbenzene through human
skin must be questioned because they are many times higher than the
measured fluxes through rat skin, whereas from studies of in vitro
percutaneous absorption it is known that rat skin is more permeable
than human skin (mean ratio about 3) for several chemicals (Barber et
al., 1992).
6.1.2 Absorption via inhalation
When volunteers (number not given) were exposed to 99, 185, 198
or 365 mg/m3 (23, 43, 46 or 85 ppm) ethylbenzene for 8 h, 64% of the
inhaled ethylbenzene was taken up by the respiratory tract (Bardodej &
Bardodejova, 1966). In another study, six volunteers were exposed
under controlled conditions for 8 h to 18, 34, 80, 150 or 200 mg/m3.
The retention of ethylbenzene in the lungs (difference in
concentration between inhaled and exhaled air) was 49% (± 5%)
independent of the exposure concentration (Gromiec & Piotrowski,
1984).
When volunteers were exposed to 430 mg/m3 or 870 mg/m3 of
"industrial xylene" (containing 40% ethylbenzene and 60% xylenes) for
2 h, about 60% was taken up, independent of concentration. If the
workload increased during exposure, the retention dropped to 50%
(Ĺstrand et al., 1978).
In a study by Chin et al. (1980a), rats (male, Harlan-Wistar)
were exposed to 14C-labelled ethylbenzene at a concentration of 1000
mg/m3 for 6 h. Assuming a ventilation rate of 100 ml/min, each rat
had an estimated intake of 36 mg ethylbenzene, of which 44% was
absorbed.
6.1.3 Absorption after oral intake
Toxicity studies in various animal species show indirectly that
ethylbenzene is absorbed after oral administration (Wolf et al., 1956,
NTP, 1992). Moreover, in one study, ethylbenzene appeared to be
rapidly and well absorbed from the gastro-intestinal tract since more
than 80% of the administered radioactively labelled compound was
recovered in urine within 48 h (Climie et al., 1983).
6.2 Distribution
When volunteers (n=12) were exposed for 2 h to 100 or 200 ppm
"industrial xylene", the amount of ethylbenzene taken up correlated
with the amount of body fat ("industrial xylene" consisted of 40.4%
ethylbenzene, 49.4% m-xylene, 8.8% o-xylene and 1.4% p-xylene).
The concentration of ethylbenzene ranged from 4 to 8 mg/kg in
subcutaneous adipose tissue 30 min after exposure. There was,
however, a negative correlation between the concentration in the
adipose tissue and the estimated relative amount of fat (Engström &
Bjurström, 1978).
The ethylbenzene concentration in the subcutaneous fat of workers
in a styrene polymerization plant was less than 0.8 mg/kg. The level
of exposure to ethylbenzene was reported to be below 17 mg/m3 (4
ppm). The 25 workers were exposed to a variety of other chemicals as
well (Wolff et al., 1977).
When rats were exposed to 1000 mg/m3 14C-ring-labelled
ethylbenzene for 6 h, 0.2% of this radioactivity was found 42 h later
in the tissues, mainly in the liver, gastrointestinal tract, fat and
the carcass (Chin et al., 1980a).
In a study by Engström et al., (1985), male Wistar rats (n = 20)
were exposed to 215, 1290 or 2580 mg ethylbenzene/m3 (50, 300 or 600
ppm) for 6 h/day, and 5 days/week for up to 16 weeks. The
concentration of ethylbenzene in perirenal fat was measured in weeks
2, 5 and 9. There were no consistent changes in ethylbenzene levels
during the course of exposure. After 16 weeks the amount of
ethylbenzene in perirenal fat was 8.5, 167.7 and 262.2 mg/kg fat at
the three exposure levels, respectively.
6.3 Metabolic transformation
Metabolic pathways of ethylbenzene based on urinary metabolites
have been proposed for humans (Fig. 1) and for rats (Fig. 2). The
main metabolic pathway is oxidation of the side chain, both in humans
and in animals. However, it has been demonstrated that there are both
qualitative and quantitative inter-species differences in the
metabolites produced (Engström et al., 1984; Engström 1984b). In
humans, the main metabolites of ethylbenzene are mandelic and
phenylglyoxylic acids. In several animal species the metabolic
transformation continues to benzoic acid, leading to excretion of
hippuric acid after conjugation with glycine. This conjugate is
generally one of the main urinary metabolites, together with mandelic
acid, in rats and dogs (Chin et al., 1980b). Hydroxylation of the
aromatic nucleus is a minor pathway. In the rabbit this phenolic
pathway accounts for less than 2% of the ethylbenzene absorbed (Kiese
& Lenk, 1974).
 Ethylbenzene is metabolized by the microsomal cytochrome P-450
enzyme system. Although specific isozymes have not been unequivocally
identified, enzyme induction studies suggest that CYP2B1/2, CYP1A1/2
and CYP2E1 may be involved (see section 7.8).
Four male volunteers were exposed to 655 mg/m3 (150 ppm)
ethylbenzene for 4 h and urine was collected for 24 h. Mandelic acid
(71.5%) and phenylglyoxylic acid (19.1%) were the main metabolites,
but smaller amounts of 1-phenylethanol, p-hydroxyacetophenone,
m-hydroxyacetophenone, 1-phenyl-1,2-ethandiol, 4-ethylphenol,
omega-hydroxyacetophenone and acetophenone were also found. Ring
oxidation accounted for 4.0%. Simultaneous exposure to 150 ppm
m-xylene did not alter the urinary metabolite pattern, but it
delayed excretion and decreased the amounts of metabolites excreted
(Engström et al., 1984). Mandelic acid (64%) and phenylglyoxylic aid
(25%) were found to be the main urinary excretion products in
volunteers after an 8-h exposure to 99-365 mg/m3 (23-85 ppm)
ethylbenzene (Bardodej & Bardodejová, 1970).
When two volunteers were exposed by inhalation to 430 mg/m3 (100
ppm) ethylbenzene for 4 h, most of the mandelic acid was excreted as
the R-enantiomer (Drummond et al., 1989).
In a study by Korn et al. (1992), urinary samples from workers
exposed to ethylbenzene, toluene and xylenes were analysed. The
average urinary concentration of phenylglyoxylic acid was 50.1
mg/litre. The concentration of mandelic acids was 135.2 mg/litre, of
which 127.8 mg/litre was R-mandelic acid and 7.3 mg/litre was
S-mandelic acid. The R/S ratio was independent of the air
concentration of ethylbenzene, which varied between 6.4 and 142 mg/m3
(1.5 and 33 ppm).
Four female histology laboratory assistants were exposed to a
mixture of xylenes (75%) and ethylbenzene (25%). The air concentration
of the solvent was 160-179 mg/m3, and the concentration of
ethylbenzene in blood collected at the end of the working day was
reported to be 0.5 to 0.8 mg/litre. The 24-h excretion of
2-ethylphenol in urine varied between 4.4 and 6.0 mg corresponding to
1 to 1.5% of the retained ethylbenzene (Angerer & Lehnert, 1979). The
findings by Angerer & Lehnert (1979) that ethylbenzene is metabolized
to 2-ethylphenol could not be verified by Engström et al. (1984).
Male Wistar rats (6 per group) were exposed for 6 h to
ethylbenzene at 1290 or 2580 mg/m3 (300 or 600 ppm). The urine was
collected for 48 h from the onset of exposure. Altogether, 14
different metabolites from ethylbenzene were identified. The main
metabolites were 1-phenylethanol, mandelic acid and benzoic acid, each
of which accounted for about 25%. Only 13% (low dose) and 6% (high
dose) of the estimated absorbed doses were eliminated during the 6-h
exposure. Over the 48-h period, the corresponding values were 83% and
59%, respectively. The metabolic pattern was similar, irrespective of
the exposure level (Engström, 1984b).
In another study, male Wistar rats (20 per group) were exposed to
215, 1290 or 2580 mg ethylbenzene/m3 (50, 300 or 600 ppm) for 6
h/day, 5 days/week, for up to 16 weeks. Urinary excretion of some of
the metabolites was measured in weeks 2, 5 and 9. A significant
dose-related percentage decrease of phenylglyoxylic acid and hippuric
acid plus benzoic acid was found. A corresponding increase of
1-phenylethanol and omega-hydroxyacetophenone excretion was also
noted. The total amount of metabolites in urine collected during the
24 h after onset of exposure remained, however, constant at each
exposure level throughout the study (Engström et al., 1985).
When a single oral dose of 318 mg ethylbenzene/kg body weight was
administered to rabbits, the main urinary metabolites found were
hippuric acid and methylphenylglucuronic acid, which together
represented 60-70% of the dose, while mandelic acid and phenaceturic
acid were minor metabolites (El Masry et al., 1956).
It has been established in in vivo studies that experimental
animals convert ethylbenzene mainly to the R-enantiomer of mandelic
acid (Drummond et al., 1990). In an in vitro study it was found
that ethylbenzene is hydroxylated by cytochrome P-450cam (from
Escherichera coli) almost exclusively at the secondary ethyl carbon
with about a 2:1 ratio of R:S products (Filipovic et al., 1992).
The rates of metabolism of ethylbenzene have been studied in
vitro in rabbit liver and lung. Organs from five female animals
were used, but details of the experimental procedures were not
reported. For the liver (mean weight 76 g) the rate of metabolism was
453 nmol/g tissue per 10 min (34.4 µmol per liver per 10 min or 11.7
nmol per nmol cytochrome P-450 per 10 min). The corresponding figures
for lung (mean weight 7.7 g), were 680 nmol, 5.3µmol and 200.1 nmol,
respectively. Thus, lung tissue may significantly contribute to the
body clearance of ethylbenzene in rabbits (Sato & Nakajima, 1987).
6.4 Elimination and excretion
In humans, ethylbenzene is mainly excreted in the urine as
mandelic and phenylglyoxylic acids (Bardodej & Bardodejová, 1970;
Ĺstrand et al., 1978; Engström et al., 1984; Gromiec & Piotrowski,
1984). Only up to 5% of retained ethylbenzene is estimated to be
exhaled without transformation (Ĺstrand et al., 1978). The
elimination half-lives of ethylbenzene in exhaled air and urine have
been estimated to be 0.5-3 h and 8 h, respectively (Wolff, 1976). In
human volunteers exposed to 100 or 200 ppm "industrial xylene" for
2 h, there was no decline in the concentration of xylenes plus
ethylbenzene in the gluteal subcutaneous adipose tissue between 30 min
and 22 h after exposure (Engström & Bjurström, 1978). The elimination
of mandelic acid has been found to be biphasic, with half-lives of 3.1
and 24.5 h (Gromiec & Piotrowski, 1984).
The elimination kinetics for 10 volatile organic compounds,
including ethylbenzene, has been studied in human volunteers exposed
to a variety of consumer products. Breath samples were collected
post-exposure and analysed by GC/MS. The half-lives for the 10
chemicals varied from a few hours to 1-2 days. The authors concluded
that volatile organic compounds exhibit relatively short residence
times in the body (Pellizzari et al., 1992).
Male Harlan-Wistar rats exposed to 14C-ring-labelled
ethylbenzene (1000 mg/m3) for 6 h excreted 82% of the radioactivity
in the urine, 8.2% in expired air (0.03% as CO2) and 0.7% in faeces.
After 42 h, 0.2% remained in the tissues. The remaining 8.3% could not
be accounted for (Chin et al., 1980a).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Single high exposures to ethylbenzene cause irritation of the
mucous membranes and central nervous system effects. The results from
single exposure in vivo studies are summarized in Table 5.
Table 5. Single exposure of animals to ethylbenzenea
Species Route Dose Parameter Reference
Rat oral 3.5 g/kg LD50 Wolf et al. (1956)
Rat oral 4.7 g/kg LD50 Smyth et al. (1962)
Rat inhalation 9.37 g/m3 (2180 ppm) Minimum Molnár et al. (1986)
narcotic conc.
Rat inhalation 17.2 g/m3 (4000 ppm) 1 h LC10 Smyth et al. (1962)
Rat inhalation 17.2 g/m3 (4000 ppm) 4 h LC50 Smyth et al. (1962)
Rat inhalation 34.4 g/m3 (8000 ppm) 1 h LC100 Smyth et al. (1962)
Rabbit dermal 77.4 g/kg LD50 Smyth et al. (1962)
a Additional information is given in the following reviews: DFG (1985), ECETOC (1986).
7.2 Short-term exposure
In a short-term study, six male rats (Sprague Dawley) were
exposed for 6 h/day during 3 consecutive days to 8.6 g/m3 (2000 ppm)
ethylbenzene. The animals were killed 16-18 h after the last
exposure. Small increases in dopamine and noradrenaline levels and
turnover in various parts of the hypothalamus and the median eminence
were reported. Ethylbenzene was also found to produce selective
reduction in prolactin and corticosterone secretion and selective
increase in dopamine turnover within the dopamine-cholecystokinin-8-
immuno-reactive nerve terminals of the nucleus accumbens (posterior
part) (Andersson et al., 1981).
When eight male rabbits (New Zealand) were exposed 12 h daily for
7 days to 3.22 g/m3 (750 ppm) ethylbenzene, there was a marked
(p<0.05) depletion of striatal and tuberoinfundibular dopamine. Such
an effect was also caused by intraperitoneal dosing of rabbits (eight
per group) with mandelic or phenylglyoxylic acid (4 mmol/kg per day
for 3 days) in saline (Romanelli et al., 1986).
In a 4-week inhalation study Fischer-344 rats (five of each sex
per group) were exposed to ethylbenzene for 6 h/day, 5 days per week,
at exposure levels of 0, 426, 1643 or 3363 mg/m3 (0, 99, 382 or 782
ppm). At the two highest exposure levels, sporadic lacrimation and
salivation, as well as significantly (p<0.05) increased liver
weights, were seen. At the highest exposure level, there was a small
increase in leukocyte counts and, in males, a marginal increase in
platelet counts (Cragg et al., 1989).
In the same study, mice (B6C3F1) of both sexes were similarly
exposed. At 1643 and 3363 mg ethylbenzene/m3, females showed
significantly (p<0.01) increased absolute and relative liver weights.
In males a significantly (p<0.05) increased relative liver-to-brain
weight ratio was seen. Male and female rabbits (New Zealand White)
were also used in this study. The exposure levels were 0, 1643, 3363
and 6923 mg/m3 (0, 382, 782 and 1610 ppm). At the highest exposure
level females gained weight more slowly than controls but neither sex
exhibited gross or microscopic organ changes (Cragg et al., 1989).
No changes in mortality pattern were seen in the three species.
There were no changes in clinical chemistry parameters in rats or
rabbits. Mice were not subjected to clinical chemistry or
haematological examinations due to the small volume of blood that
could be collected. For similar reasons, urinalyses were performed
for rats (no change) but not for mice. Rabbits were excluded from
urinalysis for logistical reasons. No changes in gross or microscopic
pathology were noted in any of over 30 tissues from each of the three
species when the animals were exposed at the highest concentration
(Cragg et al., 1989).
7.3 Long-term exposure
7.3.1 Oral exposure
Matched groups of 10 Wistar female rats were given daily
ethylbenzene doses of 0, 13.6, 136, 408 or 680 mg/kg by stomach tube
5 days a week for 6 months. The two highest dosages induced slight
increases in liver and kidney weights and slight cloudy swelling of
parenchymal liver cells and of the tubular epithelium in the kidney
(Wolf et al., 1956).
7.3.2 Inhalation exposure
In a 13-week National Toxicology Program study, groups of 10 rats
(F-344/N) and 10 mice (B6C3F1) of each sex were exposed for 6 h (plus
10 min to reach 90% of the target chamber concentration) per day,
5 days per week for 92 (female rats), 93 (male rats), 97 (female mice)
or 98 (male mice) days, at ethylbenzene concen-trations of 0, 430,
1075, 2150, 3225 or 4300 mg/m3 (0, 100, 250, 500, 750 or 1000 ppm).
Blood for clinical chemistry and haematological examination was
collected on study days 4 and 23 and again at week 13 from both male
and female rats. Dose-related increases in absolute liver weight were
seen in both sexes of mice exposed to the two highest dose levels, and
the relative kidney weight of female mice exposed to 4300 mg/m3 was
greater than that of the controls. Increased absolute and relative
liver and kidney weights were seen in male rats exposed to the two
highest dose levels. Increased absolute liver and kidney weights were
seen in female rats exposed to the three highest dose levels, but no
increased relative liver and kidney weights were seen. No chemically
related histopathological changes were observed in any rat or mouse
tissues. Clinical chemistry results were negative (NTP, 1992).
In another study, groups of five male rats (Wistar) were exposed
for 6 h/day, 5 days/week to ethylbenzene concentrations of 0, 215,
1290 or 2580 mg/m3 (0, 50, 300 or 600 ppm) and sacrificed after 2, 5,
9 or 16 weeks of exposure. At 2580 mg/m3 liver cells showed a slight
proliferation of smooth endoplasmic reticulum, slight degranulation
and splitting of rough endoplasmic reticulum, and enlarged
mitochondria. At the same dose level, liver microsomal protein, but
not cytochrome P-450, concentration was slightly increased. There was
also an increase in NADPH-cytochrome c reductase, 7-ethoxycoumarin-
O-deethylase and UDPG-transferase activities in the liver. In the
kidney only the two latter enzymes showed dose-related increases.
Urinary excretion of thioethers was measured to ascertain the
generation of electrophilic intermediates during ethylbenzene
metabolism. Excretion of thioethers increased in a dose-dependent
manner, with some fluctuation over the course of 7 weeks, reaching
about eight times the control level at 2580 mg ethylbenzene/m3.
However, there was no decrease in hepatic or renal levels of
glutathione (GSH), indicating that the cells were able to maintain the
intra-cellular homeostasis of GSH during exposure (Elovaara et al.,
1985).
In inhalation experiments, matched groups of 10-25 male and
female Wistar rats, 5-10 guinea-pigs, 1-2 rabbits and 1-2 rhesus
monkeys of either sex or both sexes were all exposed 7 h/day, 5
days/week, for up to 6 months. The exposure levels were 0, 1720 and
2580 mg/m3 (0, 400 and 600 ppm) for 186 days, 5375 mg/m3 (1250 ppm)
for 214 days (no monkeys) or 9460 mg/m3 (2200 ppm) for 144 days (rats
only). Slight effects were seen in rats: increased liver and kidney
weights at 1720 mg/m3; increased liver and kidney weights at 2580
mg/m3; and small histopathological changes (cloudy swelling) in liver
and kidney at 5375 and 9460 mg/m3 (Wolf et al. 1956).
In guinea-pigs and monkeys slightly increased liver weights were
noted in the 2580 mg/m3 group only. At the same exposure level,
small histopathological effects in the testes, described as
degeneration of the germinal epithelium, were seen in rabbits and
monkeys. At 5375 mg/m3 a slight growth depression was noted in
guinea-pigs. The no-observed-effect level (all four species) was
considered to be about 860 mg/m3 (200 ppm) (Wolf et al., 1956). It
should be noted, however, that Cragg et al. (1989) found no
histopathological effects in the testes of rats and rabbits exposed to
up to 3363 mg/m3 (782 ppm) for 4 weeks, and the lack of toxicity was
confirmed by NTP (1992).
7.4 Skin and eye irritation, sensitization
Inhalation for 3 min of ethylbenzene at a concentration of 4300
mg/m3 caused slight nasal irritation in guinea-pigs, and an 8-min
exposure caused eye irritation as well. At 8600 mg/m3 a 1-min
exposure was enough to cause both effects (Cavender, 1993).
Two drops of undiluted ethylbenzene placed in the eyes of rabbits
resulted in slight conjunctival irritation but no effects on the
cornea (Wolf et al., 1956). A slight conjunctival irritation with
some reversible corneal injury was reported in rabbits in a study by
Smyth et al. 1962.
Undiluted ethylbenzene has been shown to produce moderate
irritation when applied to the uncovered skin of rabbits (Smyth et
al., 1962). The application of undiluted ethylbenzene to the ear and
to the shaved abdomen of rabbits up to 20 times during a 4-week period
resulted in moderate irritation. There was erythema and oedema with
superficial necrosis and exfoliation of large patches of skin (Wolf et
al., 1956).
No animal sensitization studies have been reported.
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
In an inhalation study, rats (Wistar or Sprague-Dawley) and
rabbits (New Zealand White) were exposed to 430 or 4300 mg/m3 (100 or
1000 ppm) ethylbenzene for 6 to 7 h/day on gestation days 1 to 19
(rats) or 1 to 24 (rabbits). All pregnant animals were sacrificed on
the day before term (day 21 for rats, day 30 for rabbits). The
rabbits had a significantly (p<0.05) reduced number of live pups per
litter at both exposure levels, but the number of implantations per
litter and the number of dead or resorbed fetuses per litter did not
differ from those of the controls. Maternal toxicity in rats exposed
to 4300 mg/m3 was reflected in increased liver, kidney and spleen
weights. There was a significant (p<0.05) increase in the incidence
of extra ribs in both of the exposed rat groups (Hardin et al., 1981).
In a further study, rats (CFY) were exposed to ethylbenzene
concentrations of 600, 1200 or 2400 mg/m3 continuously (24 h/day)
from day 7 to day 15 of pregnancy. They were then killed on day 21.
Mice (CFLP) were exposed for three periods of 4 h per day to 500
mg/m3 on days 6-15 of pregnancy and killed on day 18. Rabbits (New
Zealand White) were exposed continuously on days 7-20 of gestation to
500 or 1000 mg/m3 and were killed at day 30. The maternal toxic
effects (not specified) in mice and rats were moderate and
dose-dependent. In both species ethylbenzene caused skeletal growth
retardation, extra ribs and reduced fetal growth rate at the highest
concentration. In rabbits, the highest dose concentration caused mild
maternal toxic effects (decreased weight gain) and reduction in the
number of fetuses due to abortion (Ungváry & Tátrai, 1985).
When rats (F-344/N) and mice (B6C3F1) were exposed to
ethylbenzene at concentrations of 0, 430, 2150 and 4300 mg/m3 (0,
100, 500 and 1000 ppm), 6 h per day, 5 days per week, for 13 weeks,
there were no changes in sperm or vaginal cytology (NTP, 1992).
Rat embryos were explanted on day 9 of gestation and cultured in
rat serum with added xylene (containing 18% ethylbenzene) at
concentrations up to 1.0 ml/litre serum. Dose-dependent retardation
of growth and development was seen but there were no observable
teratogenic effects (Brown-Woodman et al., 1991).
No multigeneration and reproductive studies on ethylbenzene have
been reported.
7.6 Mutagenicity and related end-points
In a National Toxicology Program study, ethylbenzene was not
mutagenic in Salmonella tests and did not induce chromosomal
aberrations or sister chromatid exchange in Chinese hamster ovary
(CHO) cells in vitro, although it did induce trifluorothymidine
resistance in mouse lymphoma cells at the highest concentration tested
(80 mg/litre). There was no increase of micronuclei in the peripheral
blood of mice exposed to ethylbenzene (NTP, 1992).
In several other studies ethylbenzene did not induce point
mutations (with or without added metabolic activation system). In
addition, it did not cause an increase in the spontaneous
recessive-lethal frequency in the Drosophila recessive-lethal test,
nor had it any chromosomal effects in vitro (Donner et al., 1980;
Florin et al., 1980; Dean et al., 1985). Ethylbenzene had a marginal
effect on sister chromatid exchange in human lymphocytes in vitro
when a high (10 mmol/litre) concentration was used (Norppa & Vainio,
1983). In addition, in the TK+/- test in mouse lymphoma cells there
was a slight effect at a high concentration (80 mg/litre) (McGregor et
al., 1988). This study is obviously the same as the one subsequently
reported by NTP (1992).
No excess of chromosomal aberrations in bone marrow cells was
seen in rats after up to 18 weeks of exposure (6 h/day, 5 days/week)
to 300 ppm of a xylene mixture containing 18.3% ethylbenzene (Donner
et al., 1980).
7.7 Carcinogenicity
In a carcinogenicity study, rats (Sprague-Dawley) were exposed to
one of several aromatic hydrocarbons, including ethylbenzene. Groups
of rats (40 of each sex) were exposed to 500 mg ethylbenzene (in olive
oil) per kg body weight by gavage, 4 or 5 days per week for 104 weeks.
Results were determined after 141 weeks. The first malignant tumour,
a nephroblastoma, was observed after 33 weeks. The total number of
malignant tumours was 31 in the 77 animals of the exposed group alive
at 33 weeks compared with an incidence of 23 of 94 animals in the
control group. The authors concluded that ethylbenzene caused an
increase in the incidence of total malignant tumours, although there
was no increase in the incidence of any specific type of tumour
(Maltoni et al., 1985). It is difficult to draw any firm conclusion
from this study because of inadequate reporting.
7.8 Other special studies
Ethylbenzene was found to have low acute cytotoxicity in vitro
on Ehrlich ascites cells (Holmberg & Malmfors, 1974).
The effects of ethylbenzene have been studied in four in vitro
test systems: decreased cell growth in Ascites sarcoma BP 8 cells;
decreased oxidative metabolism in hamster brown fat cells; cell
membrane damage of human embryonic lung fibroblasts; and inhibition of
ciliary activity in chicken embryo trachea. On a 0-9 point scale
(equivalent to 0-100%) the activity of these four systems scored 4, 6,
8 and 8, respectively (Curvall et al., 1984).
The activity of cytochrome CYP2E1 can be monitored in microsomal
preparations by p-nitrophenol hydroxylation. When rabbit (white
male New Zealand) liver microsomes were treated with up to 0.25 mM
ethylbenzene an inhibition of p-nitrophenol hydroxylation was seen
(Koop & Laethem, 1992).
In a study by Pyykkö et al. (1987), Sprague-Dawley male rats were
given ethylbenzene dissolved in corn oil intraperitoneally in a single
dose of 5 mmol/kg (530 mg/kg body weight). The rats were killed 24 h
later and the livers and lungs removed. In the liver 7-ethoxycoumarin-
0-deethylase (a marker of CYP2B1/2) was induced 4-fold; no induction
was found in the lung. Ethylbenzene also induced 7-ethoxyresorufin-
0-deethylase (a marker of CYP1A1/2) both in the liver (4-fold)
and the lung (2.5 fold). These findings are common to many aromatic
solvents.
Alterations in the levels of specific cytochrome P-450 isozymes
were measured by Western immunoblotting techniques using rabbit
anti-rat polyclonal antibodies for cytochrome CYP1A1, CYP2B1 and
CYP2E1 on rat liver microsomes. The rats, male and female Holtzman
rats, were given 10 mmoles ethylbenzene per kg body weight for 3 days
and killed 24 h after the last injection. Ethylbenzene was shown to
induce CYP2B1/2B2 to a greater extent in male rats, while cytochrome
CYP2E1 was only induced in female rats. The level of cytochrome
CYP1A1 was not affected by ethylbenzene (Sequeira et al., 1992).
In another study (Gut et al., 1993), Wistar male rats were
exposed in a dynamic inhalation apparatus to 4 mg ethylbenzene/litre
air, 20 h per day, for 4 days. The rats were then killed and liver
microsomes prepared. By use of Western immunoblotting techniques it
was shown that cytochrome CYP2B1 was induced and the cytochrome CYP2E1
levels were decreased.
In a sensory irritation test, groups of four male mice
(Swiss-Webster) were exposed for 30 min to ethylbenzene at
concentrations of 1.76, 3.7, 8.06, 17.07 or 41.45 g/m3 (410, 860,
1875, 3970 or 9640 ppm). The RD50 value, i.e. the concentration
necessary to depress the respiratory rate by 50%, was calculated to be
17.46 g/m3 (4060 ppm) (95% confidence interval 10.6-28.6 g/m3;
2480-6660 ppm). The respiratory rate decreased due to sensory
irritation of the upper respiratory tract (Damgĺrd Nielsen & Alarie,
1982). In a similar test male mice (Swiss F1) were exposed for about
5 min to different concentrations of ethylbenzene. At least four
different concentrations were used and there were six mice for each
concentration. In this study the RD50 value was calculated to be
6.16 g/m3 (1432 ppm) (De Ceaurriz et al., 1981).
7.9 Factors modifying toxicity
Rats (Sprague-Dawley) were exposed for 2 h to 774 mg/m3 (180
ppm) ethylbenzene. One of two corresponding animals received 20 mmol
ethanol/kg body weight in physiological saline intraperitoneally
before exposure to ethylbenzene. Ethanol enhanced significantly (1.4
fold) the blood levels of inhaled ethylbenzene (Römer et al., 1986).
8. EFFECTS ON HUMANS
8.1 Volunteer studies
A dermal maximization test conducted on 25 volunteers at a
concentration of 10% ethylbenzene in petrolatum produced no skin
sensitization reaction (ECETOC, 1986).
In a review of studies from the 1930s, it was stated that
exposure to 21.5 g/m3 (5000 ppm) ethylbenzene for a few seconds gives
intolerable irritation of nose, eyes and throat. A few seconds of
exposure to 4.3 g/m3 (1000 ppm) initially gives eye irritation which
diminishes after a few minutes of exposure (Damgĺrd Nielsen & Alarie,
1982).
In a study on ethylbenzene metabolism in man it was incidentally
reported that, when the exposure was above the occupational limit
value (8 h; 430 mg/m3,100 ppm), complaints of fatigue, sleepiness,
headache and irritation of the eyes and respiratory tract were
reported (Bardodej & Bardodejová, 1970).
Healthy male subjects were exposed to technical xylene
(containing 20.7% ethylbenzene) for 2 h with or without a 100-watt
workload on an ergometer cycle. The air concentration of technical
xylene was 435 or 1300 mg/m3. During work at the higher exposure
level, evidence of performance decrement was observed in three of the
five performance tests: reaction time addition test (p<0.05),
short-term memory (p<0.05) and choice reaction time (p<0.10)
(Gamberale et al., 1978).
8.2 Occupational exposure
A number of epidemiological studies have been carried out on
groups occupationally exposed to mixtures of solvents, including
ethylbenzene. In these studies it is difficult to attribute the
effects to ethylbenzene or any other single chemical present.
Ethylbenzene occurs in mixed xylenes (at up to 30%) and effects of
occupational exposure to mixed xylenes are usually presented as
effects of xylenes and not of ethylbenzene.
A cross-sectional epidemiological study showed no strong evidence
of adverse neurobehavioural effects in 105 house painters when
compared with 53 workers from various professions (non-painters). The
concentration of ethylbenzene at the workplace was up to 12.9 mg/m3
(3 ppm). Other solvents present were ethyl acetate, toluene, butyl
acetate, methyl isobutyl ketone and xylene. In two neurobehavioural
tests significant differences were found between painters and controls
(the tests were "change of personality" and "short-term memory
capacity"). In a subgroup of painters with repeated prenarcotic
symptoms at the workplace, the differences were more pronounced
(Triebig et al., 1988).
In a study involving 35 spray painters, employed for between 2
and 26 years, erythrocyte and haemoglobin levels were slightly (but
not statistically significantly) lower than those of the controls.
The concentration of ethylbenzene was 17.2 mg/m3 (4 ppm) (Angerer &
Wulf, 1985).
In a medical surveillance report of some 200 ethylbenzene-
production workers, mandelic acid concentrations in urine were
measured twice a year for 20 years. Mandelic acid concentration
in the samples never exceeded 3.25 mmol/litre (=497 mg/litre), and the
mean value was 0.20-0.30 mmol/litre. According to the authors, a
post-shift urine mandelic acid concentration of 6.25 mmol/litre is
equivalent to an air concentration of 200 mg/m3. Therefore, the
measured maximum and mean mandelic acid values were considered to be
equivalent to air ethylbenzene concentrations of about 86 and 8.6
mg/m3 (20 and 2 ppm), respectively. None of the workers examined
over the last 10 years showed any effects on the levels of
haemoglobin, leucocytes or platelets, nor did they have a changed
haematocrit or alanine aminotransferase activity (Bardodej & Círek,
1988).
Minor changes in evoked potential and nerve conduction velocity
were found in 22 workers exposed to ethylbenzene at concentrations of
0.43-17.2 mg/m3 (0.1-4 ppm) for 4-20 years. They were also exposed
to styrene (about 1.5 ppm) (Lu & Zhen, 1989).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Ethylbenzene has been shown to inhibit the respiration of sewage
sludge utilizing biogenic substrates. Two screening tests were used,
RIKA and OECD 209. The concentration of ethylbenzene used was at the
limit of solubility in the medium (approximately 150 mg/litre), and
inhibitions of the respiration rate of 30% (ACCEDE 209) and 100%
(RIKA) were observed (Volskay & Grady, 1990).
Bringmann & Kühn (1980) studied the effect of ethylbenzene on
bacteria. A toxicity threshold of 12 mg/litre for Pseudomonas putida
was obtained in a cell multiplication inhibition test.
9.2 Aquatic organisms
Numerous acute toxicity tests have been carried out on
ethylbenzene. Organisms that have been studied include protozoans
(Bringmann & Kühn, 1980), algae (US EPA, 1978; Bringmann & Kühn, 1980;
Galassi et al., 1988; Masten et al. 1994), water fleas (LeBlanc, 1980;
Bringmann & Kühn, 1982; Abernethy et al., 1986; Galassi et al., 1988;
Vigano, 1993), diatoms (US EPA, 1978; Masten et al., 1994), copepods,
grass shrimp (Potera, 1975), bay shrimps (Benville & Korn, 1977),
mysid shrimps (US EPA, 1978; Masten et al., 1994), Dungeness crabs
(Caldwell et al., 1976) and Pacific oysters (LeGore, 1974). Fish that
have been studied include rainbow trout (Mayer & Ellersieck, 1986;
Galassi et al., 1988), guppy (Pickering & Henderson, 1966; Galassi et
al., 1988), bluegill (Pickering & Henderson, 1966; Buccafusco et al.,
1981), fathead minnow, goldfish (Pickering & Henderson, 1966), channel
catfish (Mayer & Ellersieck, 1986), Atlantic silverside (Masten et
al., 1994), striped bass (Benville & Korn, 1977) and sheepshead minnow
(US EPA, 1978; Heitmuller et al., 1981).
Many of the test results are not comparable, owing to
inconsistent exposure conditions, resulting from emulsions, open
static systems and systems with large air spaces. Aquatic toxicity
results from consistent exposure conditions, which are comparable, are
shown in Table 6.
No information regarding chronic exposure of aquatic organisms to
ethylbenzene has been reported.
Table 6. Toxicity of ethylbenzene to aquatic organisms
Species Age/size Stat/flowa Temperature Salinity pH Parameterc Concentration Reference
(°C) (0/00) (mg/litre)d
Alga stat 72-h EC50 4.6 Galassi et al. (1988)
(Selenastrum stat 19-21 48-h EC50 7.2 (3.4-15.1) Masten et al. (1994)
capricornutum) stat 19-21 96-h EC50 3.6 (1.7-7.6) Masten et al. (1994)
Water flea < 24 h statb 24-h LC50 2.2 Galassi et al. (1988)
(Daphnia magna) statb 21-25 48-h LC50 2.1e Abernethy et al. (1986)
< 24 h statb 48-h LC50 1.81-2.38 Viganň (1993)
Diatom
(Skeletonema statb 19-21 48-h EC50 7.5 (5.0-11.2) Masten et al. (1994)
costatum) statb 19-21 72-h EC50 4.9 (2.4-9.8) Masten et al. (1994)
statb 19-21 96-h EC50 7.7 (5.9-10.0) Masten et al. (1994)
Mysid shrimp < 24 h flow 24-26 20 8.0 48-h LC50 >5.2 Masten et al. (1994)
(Mysidopsis < 24 h flow 24-26 20 8.0 96-h LC50 2.6 (2.0-3.3) Masten et al. (1994)
bahia)
Rainbow trout stat 96-h LC50 4.2 Galassi et al. (1988)
(Oncorhynchus
mykiss)
Guppy stat 96-h LC50 9.2 Galassi et al. (1988)
(Poecilia
reticulata)
Table 6. (Cont'd)
Species Age/size Stat/flowa Temperature Salinity pH Parameterc Concentration Reference
(°C) (0/00) (mg/litre)d
Atlantic silverside 3-15 mg flow 21-23 20 48-h LC50 6.4 (5.8-7.5) Masten et al. (1994)
(Menidia menidia) 3-15 mg flow 21-23 20 96-h LC50 5.1 (4.4-5.7) Masten et al. (1994)
a in all cases a closed system was used; stat = static conditions (water unchanged for the duration of the test);
flow = flow-through conditions (ethylbenzene concentration in water continously maintained)
b air space was eliminated
c the EC50 for algae was based on growth inhibition
d test concentrations were measured, unless stated otherwise
e nominal test concentration;
9.3 Terrestrial organisms
An LC50 value of 47 µg per cm2 of contact area was obtained for
earthworms exposed to ethylbenzene adsorbed on filter paper in glass
vials (Neuhauser et al., 1986). Callahan et al. (1994) reported a
2-day LC50 value of 4.93 µg/kg for Eisenia foetida in a contact
toxicity test.
No toxicity data on plants, birds and wild mammals have been
reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
The acute and chronic toxicities of ethylbenzene are low. The
toxic effects in humans and animals relate to depression of the
central nervous system (CNS) and to irritation of the mucous membranes
and eyes. No data concerning carcinogenic or reproductive effects
have been reported. Ethylbenzene does not have significant mutagenic
properties or teratogenic effects.
Exposure to more than 430 mg/m3 (100 ppm) causes symptoms of CNS
depression and irritation in humans.
A 20-year medical surveillance study of 200 workers showed no
indications of effects in routine blood tests. The maximum exposure,
estimated from the urinary concentration of mandelic acid, was less
than about 86 mg/m3 (20 ppm) and the mean value about 8.6 mg/m3
(2 ppm).
In a 13-week animal study, increased liver weight was the only
dose-related biological finding in male rats. This was seen at a
concentration of 3225 mg/m3 (750 ppm) or more.
The definition and aim of the use of a guidance value for the
general population have been described by IPCS (1994).
On the basis of biological significance criteria cited above, a
no-observed-effect level (NOEL) of 2150 mg/m3 (500 ppm) was defined.
The no-observed-adverse-effect level would be higher than 4300 mg/m3
(1000 ppm) (highest concentration used), since the increase in liver
weight was not associated with any histopathological findings. A NOEL
of 2150 mg/m3 (500 ppm) was used as the basis for determining the
guidance value. The following uncertainty factors were used: 10 for
interspecies variability; 5 for intraspecies variability (effect seen
in males only); and 2 for lack of chronic toxicity data. This gives a
guidance value of 22 mg/m3 (5 ppm).
From the medical surveillance study the NOEL could be estimated
to be between 8.6 mg/m3 (2 ppm) (mean value) and 86 mg/m3 (20 ppm)
(maximum value). However, since no dose-response relationship was
derived, this study is not suitable for the estimation of a guidance
value. Furthermore, no effects would be expected at this exposure
level, which is almost the same as the guidance value.
Humans are exposed to ethylbenzene principally by inhalation,
where the substance is rapidly absorbed into the body. Exposure by
skin absorption or ingestion may also occur. Ethylbenzene is not
considered to bioaccumulate.
Table 7 gives the estimated weekly ethylbenzene dose resulting
from different types of exposure. A guidance value of 22 mg/m3
(5 ppm) ethylbenzene is approximately equivalent to a weekly ethyl-
benzene dose of 2000 mg. This is 100 times higher than the worst
exposure situation of the general population.
Table 7. Estimated weekly ethylbenzene dose from different types of exposures
Type of exposure Reported concentration Weekly total amount Dose mg/week
in media inhaled, ingested
or smoked
General population
Inhalation (means)b 0.74-100 µg/m3 140 m3 0.07-10c
Ingestion (food) 13 µg/kgd 7 kg 0.1
Drinking- or groundwaterb 0.07-1.1 µg/litree 14 litres 0.01
30.0 µg/litref 0.42
Occupational
Inhalation (mean)g 10 mg/m3 300c
Inhalation (max)g 100 mg/m3 50 m3 3000c
Inhalationh 430 mg/m3 12700c
Smokers 8 µg/cigarettei 140 cigarettes/week 1.1
a Based on a breathing rate of 20 m3/day
b Values from Tables 2 and 3
c Retention 60%
d Lovegren et al. (1979) (highest reported value)
e Highest reported values of uncontaminated groundwater
f Lowest reported value of contaminated groundwater
g Bardodej & Círek (1988)
h Corresponds to occupational exposure limits in several countries
i Wallace et al. (1987a)
A 2-year carcinogenicity study has been performed by the National
Toxicology Program (USA) but the results are not yet available.
10.2 Evaluation of effects on the environment
Ethylbenzene is found in air, water, soil, sediment, biota and
groundwater. It is released primarily into air and water from various
natural and anthropogenic sources. The atmosphere is the major sink
for ethylbenzene. Ethylbenzene is rapidly photo-oxidized in the
atmosphere and this may contribute to photo-chemical smog formation.
In water, the key processes determining overall fate are
volatilization and biodegradation.
The log octanol/water partition coefficient is 3.13, indicating a
potential for bioaccumulation. However, the limited evidence
available shows that ethylbenzene bioconcentration factors are low for
fish and molluscs. Elimination from aquatic organisms appears to be
rapid. Biomagnification through the food chain is unlikely.
Mean levels of ethylbenzene in air ranging from 0.74 to 100
µg/m3 have been measured at urban sites. Industrial releases and
vehicle emissions are the principal sources of ethylbenzene. Levels
found at rural sites are generally <2 µg/m3. The levels of
ethylbenzene in surface water are generally less than 0.1 µg/litre in
non-industrial areas. In industrial and urban areas ethylbenzene
concentrations of up to 15 µg/litre have been reported. Urban
run-off, effluent and landfill leachate are sources of local
contamination. Ethylbenzene levels in sediment are generally < 0.5
µg/kg. Levels of ethylbenzene between 1 and 5 µg/kg have been found
in sediments from heavily industrialized areas. Ethylbenzene levels
in uncontaminated groundwater are generally < 0.1 µg/litre. However,
much higher levels have been reported for groundwater contaminated via
waste disposal, fuel spillage and industrial facilities.
Acute toxicity studies on aquatic organisms show ethylbenzene to
be of moderate toxicity. The lowest acute toxicity values are 4.6
mg/litre for algae (72-h EC50), 1.8 mg/litre for daphnids (48-h LC50)
and 4.2 mg/litre for fish (96-h LC50). There are no chronic toxicity
studies on aquatic organisms.
There is limited information regarding the toxicity of
ethylbenzene to bacteria and earthworms. There are no data for
terrestrial plants, birds or wild mammals.
On the basis of available data, it is concluded that ethylbenzene
is unlikely to be found at levels in the environment that will cause
adverse effects on aquatic and terrestrial ecosystems, except in cases
of spills or point-source emissions.
11. CONCLUSIONS
Ethylbenzene has low toxicity. Its vapour irritates the mucous
membranes to a limited extent and causes prenarcotic effects on the
central nervous system.
With respect to the general population, a tentative guidance
value of 22 mg/m3 (5 ppm) for ethylbenzene in inhaled air has been
derived, although information on certain important toxicity end-points
are unavailable. This value would correspond to a weekly absorbed
dose (daily ventilation of 20 m3 with 60% retention) of about 2000
mg. It is at least 200 times higher than the dose received in the
most polluted living environment reported. Hence, no harmful effects
on the general population would be expected. However, it should be
noted that the guidance value of 22 mg/m3 (5 ppm) is about 10 times
higher than the odour threshold (about 2.2 mg/m3; 0.5 ppm), and so
exposure at that level may cause annoyance. On the other hand, odour
detection may be considered to be a safeguard against excessive
exposure.
Ethylbenzene is a non-persistent chemical and is degraded
primarily by photooxidation and biodegradation. Volatilization to the
atmosphere is rapid. Photooxidation reactions of ethylbenzene may
contribute to photochemical smog formation.
Limited evidence suggests that bioaccumulation is low in aquatic
organisms. Ethylbenzene is unlikely to cause adverse effects in
aquatic or terrestrial ecosystems except in cases of spills or
point-source emissions.
12. FURTHER RESEARCH
a) To fill the data gaps that currently limit the toxicological
evaluation of ethylbenzene, an appropriate rodent carcinogenicity
study and a reproductive toxicity study are needed. (The former
study has been conducted but the study report has not yet been
published.)
b) There is little information on the long-term effects of
ethylbenzene in humans and, in particular, no dose-response or
dose-effect data are at hand. Epidemiological studies of
populations occupationally exposed to ethylbenzene should be
encouraged. In this context, the use of ethylbenzene metabolites
in urine as a marker of exposure can be of special value because
the method determines the internal doses that individuals receive
via all routes of exposure. Because ethylbenzene is a central
nervous system depressant, and because some studies suggest that
high doses of the substance or its metabolites may affect the
metabolism of some neurotransmitters in the brain, epidemiological
studies should address the central nervous system as a potential
target organ. Moreover, since ethylbenzene is almost invariably
only one of the components in solvent mixtures at the workplace,
study designs that address possible interactions between
ethylbenzene and other solvents are desirable.
c) Further mechanistic studies are needed.
13. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
A guideline value of 300 µg/litre for ethylbenzene in
drinking-water was recommended by WHO in 1993.
REFERENCES
Abernethy S, Bobra AM, Shiu WY, Wells PG, & Mackay D (1986) Acute
lethal toxicity of hydrocarbons and chlorinated hydrocarbons to two
planktonic crustaceans: The key role of organism-water partitioning.
Aquat Toxicol, 8: 163-174.
ACGIH (1985-1986) Threshold limit values and biological exposure
indices. Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
Aitio A, Riihimäki V, Liesivuori J, Järvisalo J, & Hernberg S (1994)
Biological monitoring. In: Zenz C, Dickerson OB, & Horvath EP Jr ed.
Occupational medicine, 3rd ed. Saint Louis, Missouri, C.V. Mosby &
Co., pp 132-158.
Andersson K, Fuxe K, Nilsen OG, Toftgĺrd R, Eneroth P, & Gustafsson JĹ
(1981) Production of discrete changes in dopamine and noradrenaline
levels and turnover in various parts of the rat brain following
exposure to xylene, ortho-, meta-, and para-xylene, and
ethylbenzene. Toxicol Appl Pharmacol, 60: 535-548.
Angerer J & Lehnert G (1979) Occupational chronic exposure to organic
solvents. VIII. Phenolic compounds-metabolites of alkylbenzenes in
man. Simultaneous exposure to ethylbenzene and xylenes. Int Arch Occup
Environ Health, 43: 145-150.
Angerer J & Wulf H (1985) Occupational chronic exposure to organic
solvents. XI. Alkylbenzene exposure of varnish workers: effects on
hematopoietic system. Int Arch Occup Environ Health, 56: 307-321.
Annable MD, Wallace RB, Hayden NJ, & Voice TC (1993) Reduction of
gasoline component leaching potential by soil venting. J Contam
Hydrol, 12(1-2): 151-170.
Anon (1987) Chemical review: ethylbenzene. Danger Prop Ind Mater Rep,
7: 13-35.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Holler JS, Needham LL, &
Patterson DG Jr (1992) Determining volatile organic compounds in human
blood from a large sample production by using purge and trap gas
chromatography and mass spectrometry. Anal Chem, 64(9): 1021-1029.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, & Wooten JV (1994) Blood
concentrations of volatile organic compounds in a non occupationally
exposed US population and in groups with suspected exposure. Clin
Chem, 40(7): 1401-1404.
Ĺstrand I, Engström J, & Övrum P (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man. Scand J
Work Environ Health, 4: 185-194.
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions
of the hydroxyl radical with organic compounds under atmospheric
conditions. Chem Rev, 85: 69-201.
ATSDR (1990) Toxicological profile for ethylbenzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry (ATSDR/TP/-90-15).
Barber ED, Teetsel NM, Kolberg KF, & Guest D (1992) A comparative
study of the rates of in vitro percutaneous absorption of eight
chemicals using rat and human skin. Fundam Appl Toxicol, 19: 493-497.
Bardodej Z & Bardodejová E (1970) Biotransformation of ethylbenzene,
styrene, and alpha-methylstyrene in man. Am Ind Hyg Assoc J, 31:
206-209.
Bardodej Z & Círek A (1988) Long-term study on workers occupationally
exposed to ethylbenzene. J Hyg Epidemiol Microbiol Immunol, 32: 1-5.
Benville PE Jr & Korn S (1977) The acute toxicity of six monocyclic
aromatic crude oil components to striped bass (Morone saxatilis) and
bay shrimp (Crago franciscorum). Calif Fish Game, 63: 204-209.
Bestetti G & Galli E (1984) Plasmid-coded degradation of ethylbenzene
and 1-phenylethanol in Pseudomonas fluorescens. FEMS Microbiol Lett,
21: 165-168.
Bevan MAJ, Proctor CJ, Baker-Rogers J, & Warren ND (1991) Exposure to
carbon monoxide, respirable suspended particulates and volatile
organic compounds while commuting by bicycle. Environ Sci Technol,
25: 788-791.
Bos R, Guicherit R, & Hoogeveen A (1977) Distribution of some
hydrocarbons in ambient air near Delft and the influence on the
formation of secondary air pollutants. Sci Total Environ, 7: 269-281.
Boyd SA, Xiangcan J, & Lee JF (1990) Sorption of nonionic organic
compounds by corn residues from a no-tillage field. J Environ Qual,
19(4): 734-738.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1982) [Findings concerning the harmful effect
of water pollutants on Daphnia magna in a further developed
standardized test procedure.] Z Wasser/Abwasser Forsch, 15(1): 1 (in
German).
Brown-Woodman PDC, Webster WS, Picher K, & Ritchie HE (1991)
Embryotoxicity of xylene and toluene: an in vitro study. Ind Health,
29: 139-152.
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other trace
compounds in urban air. Chemosphere, 17: 2363-2380.
Buccafusco RJ, Ells SJ, & LeBlanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Burghardt E & Jeltes R (1975) Gas chromatographic determination of
aromatic hydrocarbons in air using a semi-automatic preconcentration
method. Atmos Environ, 9: 935-940.
Burnham AK, Calder GV, Fritz JS, Junk GA, Svec HJ, & Willis R (1972)
Identification and estimation of neutral organic contaminants in
potable water. Anal Chem, 44: 139-142.
Bysshe SE (1982) Bioconcentration factors in aquatic organisms. In:
Lyan WJ, Reehl WF, & Rosenblatt DH ed. Handbook of chemical property
estimation methods - Environmental behaviour of organic compounds. New
York, McGraw-Hill Book Company, pp 5/1-5/30.
Caldwell RS, Caldarone EM, & Mallon MH (1976) Effects of a seawater-
soluble fraction of Cook Inlet crude oil and its major aromatic
components on larval stages of the Dungeness crab, Cancer
magister dana. In: Fate and effects of petroleum hydrocarbon in
marine organisms and ecosystems. Oxford, New York, Pergamon Press, pp
210-220.
Callahan CA, Shirazi MA, & Neuhauser EF (1994) Comparative toxicity of
chemicals to earthworms. Environ Toxicol Chem, 13(2): 291-298.
Callahan MA (1979) Water related environmental fate of 129 priority
pollutants. Washington, DC, US Environmental Protection Agency (EPA
440/4-79-029b).
Cavender F (1993) Aromatic hydrocarbons. In: Clayton GD & Clayton FE
ed. Patty's industrial hygiene and toxicology, 4th revis ed. New
York, John Wiley and Sons, vol 2B, pp 1267-1442.
Chan CC, Özkaynak H, Spengler JD, & Sheldon L (1991a) Driver exposure
to volatile organic compounds, CO, ozone and NO2 under different
driving conditions. Environ Sci Technol, 25: 964-972.
Chan CC, Spengler JD, Özkaynak H, & Lefkopoulou M (1991b) Commuter
exposures to VOCs in Boston, Massachusetts. J Air Waste Manage Assoc,
41(12): 1594-1600.
Chin BH, McKelvey JA, Tyler TR, Calisti LJ, Kozbelt SJ, & Sullivan LJ
(1980a) Absorption, distribution, and excretion of ethylbenzene,
ethylcyclohexane and methylethylbenzene isomers in rats. Bull Environ
Contam Toxicol, 24: 477-483.
Chin BH, McKelvey JA, Calisti LJ, Kozbelt SJ, & Sullivan LJ (1980b) A
comparison of in vivo and in vitro (tissue explant) techniques:
metabolic profile of ethylbenzene in the rat and the dog. Bull Environ
Contam Toxicol, 25: 241-245.
Chiou CT, Porter PE, & Schmedding DW (1983) Partition equilibria of
non-ionic organic compounds between soil organic matter and water.
Environ Sci Technol, 17: 227-231.
Chou WL, Speece RE, & Siddiqi RH (1978) Acclimation and degradation of
petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
CITI (1992) Biodegradation and bioaccumulation data on existing
chemicals based on the CSCL Japan. Tokyo, Chemicals Inspection and
Testing Institute, pp 3-9.
Clapp LW, Talarczyk MR, Park JK, & Boyle WC (1994) Performance
comparison between activated sludge and fixed-film processes for
priority pollutant removals. Water Environ Res, 66(2): 153-160.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984a) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural and motorway locations.
Environ Pollut, B7: 141-158.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984b) Ambient air
measurements of aromatic and halogenated hydrocarbons at urban, rural
and motorway locations. Sci Total Environ, 39: 265-279.
Climie IJG, Hutson DH, & Stoydin G (1983) The metabolism of
ethylbenzene hydroperoxide in the rat. Xenobiotica, 13: 611-618.
Cline PV & Viste DR (1985) Migration and degradation patterns of
volatile organic compounds. Waste Manage Res, 3: 351-360.
Cole RH, Frederick RE, Healy RP, & Rolan RG (1984) Preliminary
findings of the priority pollutant monitoring project of the
nationwide urban runoff program. J Water Pollut Control Fed,
56: 898-908.
Coleman WE, Munch JW, Streicher RP, Ringhand HP, & Kopfler FC (1984)
Identification and measurement of components in gasoline, kerosene and
No.2 fuel oil that partition into the aqueous phase after mixing. Arch
Environ Contam Toxicol, 13: 171-178.
Cotruvo JA (1985) Organic micropollutants in drinking water: An
overview. Sci Total Environ, 47: 7-26.
Cox DP & Goldsmith CD (1979) Microbial conversion of ethylbenzene to
1-phenylethanol and acetophenone by Nocardia tartaricans ATCC 31190.
Appl Environ Microbiol, 38: 514-520.
Cozzarelli IM, Eganhouse RP, & Baedecker MJ (1990) Transformation of
monoaromatic hydrocarbons to organic acids in anoxic groundwater
environment. Environ Geol Water Sci, 16: 135-141.
Cragg ST, Clarke EA, Daly IW, Miller RR, Terrill JB, & Ouellette RE
(1989) Subchronic inhalation toxicity of ethylbenzene in mice, rats,
and rabbits. Fundam Appl Toxicol, 13: 399-408.
Crookes MJ & Howe PD (1992) Environmental hazard assessment:
Ethylbenzene. Garston, United Kingdom, Department of the Environment,
Directorate for Air, Climate and Toxic Substances, Toxic Substances
Division, Public Building Research Establishment, 40 pp (TSD/7).
Curvall M, Enzell CR, & Pettersson B (1984) An evaluation of the
utility of four in vitro short term tests for predicting the
cytotoxicity of individual compounds derived from tobacco smoke. Cell
Biol Toxicol, 1: 173-193.
Damgĺrd Nielsen GD & Alarie Y (1982) Sensory irritation, pulmonary
irritation and respiratory stimulation by airborne benzene and
alkylbenzenes: Prediction of safe industrial exposure levels and
correlation with their thermodynamic properties. Toxicol Appl
Pharmacol, 65: 459-477.
Dannecker W, Schröder B, & Stechmann H (1990) Organic and inorganic
substances in highway tunnel exhaust air. Sci Total Environ, 93:
293-300.
Dean BJ, Brooks TM, Hodson-Walker G, & Hutson DH (1985) Genetic
toxicology testing of 41 industrial chemicals. Mutat Res, 153: 57-77.
De Bortoli M, Knöppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1984) Integrating 'real life' measurements of
organic pollution in indoor and outdoor air of homes in Northern
Italy. In: Proceedings of the 3rd International Conference on Indoor
Air Quality and Climate. Stockholm, Swedish Council for Building
Research, vol 4, pp 21-26.
DEC (1992) Health-based recommended occupational exposure limit for
ethylbenzene. The Hague, Directorate-General of Labour.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
DFG (German Research Council) (1985) [Materials hazardous to health.
Justification of MAC values in terms of toxicology and occupational
medicine], 11th ed. Weinheim, VCH Publishers (in German).
Dills RL, Kent SD, Checkoway H, & Kalman DA (1991) Quantification of
volatile solvents in blood by static headspace analysis. Talanta,
38: 365-374.
Donner M, Mäki-Paakkanen J, Norppa H, Sorsa M, & Vainio H (1980)
Genetic toxicology of xylenes. Mutat Res, 74: 171-172.
Dreisch FA & Munson TO (1983) Purge-and-trap analysis using fused
silica capillary column GC/MS. J Chromatogr Sci, 21: 111-118.
Drummond L, Caldwell J, & Wilson HK (1989) The metabolism of
ethylbenzene and styrene to mandelic acid: stereo chemical
considerations. Xenobiotica, 19: 199-207.
Drummond L, Caldwell J, & Wilson HK (1990) The stereo selectivity of
1,2-phenylethanediol and mandelic acid metabolism and disposition in
the rat. Xenobiotica, 20: 159-168.
Durst GL & Laperle EA (1990) Styrene monomer migration as monitored by
purge and trap gas chromatography and sensory analysis for polystyrene
containers. J Food Sci, 55(2): 522-524.
Dutkiewicz T & Tyras H (1967) A study of the skin absorption of
ethylbenzene in man. Br J Ind Med, 24: 330-332.
EAJ (1989) Chemicals in the environment. Tokyo, Environment Agency of
Japan.
ECETOC (1986) Joint assessment of commodity chemicals No.7:
Ethylbenzene. Brussels, European Chemical Industry Ecology and
Toxicology Centre.
El Masry AM, Smith JN, & Williams RT (1956) Studies in detoxication.
69. The metabolism of alkylbenzenes: n-propylbenzene and
n-butylbenzene with further observations on ethylbenzene. Biochem J,
64: 50-56.
Elovaara E, Engström K, Nickels J, Aitio A, & Vainio H (1985)
Biochemical and morphological effects of long-term inhalation exposure
of rats to ethylbenzene. Xenobiotica, 15: 299-308.
Engström KM (1984a) Urinalysis of minor metabolites of ethylbenzene
and m-xylene. Scand J Work Environ Health, 10: 75-81.
Engström KM (1984b) Metabolism of inhaled ethylbenzene in rats. Scand
J Work Environ Health, 10: 83-87.
Engström J & Bjurström R (1978) Exposure to xylene and ethylbenzene.
II. Concentration in subcutaneous adipose tissue. Scand J Work Environ
Health, 4: 195-203.
Engström KM & Rantanen J (1974) A new gas chromatographic method for
determination of mandelic acid in urine. Int Arch Arbeitsmed,
33: 163-167.
Engström KM, Riihimäki V, & Laine A (1984) Urinary disposition of
ethylbenzene and m-xylene in man following separate and combined
exposure. Int Arch Occup Environ Health, 54: 355-363.
Engström KM, Elovaara E, & Aitio A (1985) Metabolism of ethylbenzene
in the rat during long-term intermittent inhalation exposure.
Xenobiotica, 15: 281-286.
Feiler HD, Vernick AS, & Storch PJ (1979) Fate of priority pollutants
in POTW's. In: Proceedings of the 8th National Conference on Municipal
Sludge Management, pp 72-81.
Fellin P & Otson R (1993) Seasonal trends of volatile organic
compounds (VOCs) in Canadian homes. In: Proceedings of the 6th
Conference on Indoor Air Quality and Climate, Helsinki, 1993, vol 2,
pp 117-122.
Filipovic D, Paulsen MD, Loida PJ, Sligar SG, & Ornstein RL (1992)
Ethylbenzene hydroxylation by cytochrome P450cam. Biochem Biophys Res
Commun, 189: 488-495.
Fishbein L (1985) An overview of environmental and toxicological
aspects of aromatic hydrocarbons. IV. Ethylbenzene. Sci Total Environ,
44: 269-287.
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15: 219-232.
Först C, Stieglitz L, Roth W, & Kuhnmünch S (1984) Quantitative
analysis of volatile organic compounds in landfill leachates. Int J
Environ Anal Chem, 37: 287-293.
Frank H, Senf L, & Welsch T (1990) Some application of wide bore
capillary gas chromatography in occupational medicine. Z Gesamte Hyg
Grenzgeb, 36(12): 668-671.
Gamberale F, Annwall G, & Hultengren M (1978) Exposure to xylene and
ethylbenzene. III. Effects on central nervous functions. Scand J Work
Environ Health, 4: 204-211.
Galassi S, Mingazzini M, Vigano L, Cesareo D, & Tosato ML (1988)
Approaches to modelling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicol Environ Saf, 16: 158-169.
Garcia JP, Beyne-Masclet S, Mouvier G, & Masclet P (1992) Emissions of
volatile organic compounds by coal-fire power stations. Atmos Environ,
A26(9): 1589-1597.
Gentry SJ & Walsh PT (1987) Eight-hour TWA personal monitoring using a
diffusive sampler and short-term stain tube. Am Ind Hyg Assoc J,
48: 287-292.
Gibson DT, Gschwendt B, Yeh WK, & Kobal VM (1973) Initial reactions in
the oxidation of ethylbenzene by Pseudomonas putida. Biochemistry,
12: 1520-1528.
Gomez-Belinchon JI, Grimalt JO, & Abaigčs J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water Res, 25: 577-589.
Goodenkauf O & Atkinson JC (1986) Occurrence of volatile organic
chemicals in Nebraska ground water. Ground Water, 24: 231-233.
Gossett RW, Brown DA, & Young DR (1983) Predicting the bioaccumulation
of organic compounds in marine organisms using octanol/water partition
coefficients. Mar Pollut Bull, 14: 387-392.
Grob K (1973) Organic substances in potable water and in its
precursor. Part 1. Methods for their determination by gas-liquid
chromatography. J Chromatogr, 84: 255-273.
Grob K & Grob G (1971) Gas-liquid chromatographic-mass spectrometric
investigation of C6-C20 organic compounds in an urban atmosphere
- An application of ultra trace analysis on capillary columns. J
Chromatogr, 62: 1-13.
Gromiec JP & & Piotrowski JK (1984) Urinary mandelic acid as an
exposure test for ethylbenzene. Int Arch Occup Environ Health,
55: 61-72.
Gschwend PM, Zafiriou OC, Mantoura RFC, Schwarzenbach RP, & Gagosian
RB (1982) Volatile organic compounds at a coastal site. I. Seasonal
variations. Environ Sci Technol, 16: 31-38.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gut I, Terelius Y, Frantik E, Linhart I, Soucek P, Filipcová B, &
Klucková H (1993) Exposure to various benzene derivatives
differentially induces cytochromes P450 2B1 and P450 2E1 in rat liver.
Arch Toxicol, 67: 237-243.
Hajimiragha H, Ewers U, Brockhaus A, & Boettger A (1989) Levels of
benzene and other volatile aromatic compounds in the blood of
non-smokers and smokers. Int Arch Occup Environ Health, 61: 513-518.
Hampton CV, Pierson WR, Schuetzle D, & Harvey TM (1983) Hydrocarbon
gases emitted from vehicles on the road. 2. Determination of emission
rates from diesel and spark-ignition vehicles. Environ Sci Technol,
17: 699-708.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(Suppl 4): 66-75.
Harkov R, Kebbekus B, Bozzelli JW, & Lioy PJ (1983) Measurement of
selected volatile organic compounds at three locations in New Jersey
during the summer season. J Air Pollut Control Assoc, 33: 1177-1183.
Harper M & Purnell CJ (1990) Alkylammonium montmorillonites as
adsorbents for organic vapors from air. Environ Sci Technol,
24: 55-62.
Heilbron I, Bunbury HM, & Jones WE ed. (1946) Dictionary of organic
compounds. London, vol II, p 20.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Herman DC, Inniss WE, & Mayfield CI (1990) Impact of volatile aromatic
hydrocarbon, alone or in combination, on growth of the freshwater alga
Selenastrum capriocornutum. Aquat Toxicol, 18(2): 87-100.
Hester NE & Meyer RA (1979) A sensitive technique for measurement of
benzene and alkylbenzenes in air. Environ Sci Technol, 13: 107-109.
Hiatt MH (1981) Analysis of fish and sediment for volatile priority
pollutants. Anal Chem, 53: 1541-1543.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography-mass spectrometry. Anal Chem, 55: 506-516.
Hodgson AT, Daisey JM, & Grot RA (1991) Sources and source strengths
of volatile organic compounds in a new office building. J Air Waste
Manage Assoc, 41(11): 1461-1468.
Holm S, Norbäck D, Frenning B, & Göthe CJ (1987) Hydrocarbon exposure
from handling jet fuel of some Swedish aircraft units. Scand J Work
Environ Health, 13: 438-444.
Holmberg B & Malmfors T (1974) The cytotoxicity of some organic
solvents. Environ Res, 7: 183-192.
Howard PH (1989) Handbook of environmental fate and exposure data for
organic chemicals. Volume 1: Large production and priority pollutants.
Chelsea, Michigan, Lewis Publishers, Inc.
Howard PH (1991) Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers, Inc., pp 340-341.
Hunt WF Jr, Faoro RB, & Freas W (1986) Report of the interim data base
for state and local air toxic volatile organic chemical measurements.
Washington, DC, US Environmental Protection Agency (EPA 450/4-86-012).
Hutchins SR, Sewell GW, Kovacs DA, & Smith GA (1991a) Biodegradation
of aromatic hydrocarbons by aquifer microorganisms under denitrifying
conditions. Environ Sci Technol, 25: 68-76.
Hutchins SR, Downs WC, Wilson JT, Smith GB, Kovacs DA, Fine DD,
Douglass RH, & Hendrix DJ (1991b) Effect of nitrate addition on
biotransformation of fuel-contaminated aquifer: Field demonstration.
Ground Water, 29(4): 571-580.
Inoue O, Seiji K, Kudo S, Jin C, Cai SX, Liu SJ, Watanabe T, Nakatsuka
H, & Ikeda M (1995) Urinary phenylglyoxylic acid excretion after
exposure to ethylbenzene among exposed Chinese workers. Int J Occup
Environ Health, 1: 1-8.
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: Assessing human health risks of chemicals:
Derivation of guidance values for health-based exposure limits.
Geneva, World Health Organization, 73 pp.
Jayanty RKM (1989) Evaluation of sampling and analytical methods for
monitoring toxic organics in air. Atmos Environ, 23: 777-782.
Jaynes WF & Boyd SA (1991) Clay mineral type and organic compound
sorption by hexadecyltrine-thylammonium-exchanged clays. Soil Sci Soc
Am J, 55(1): 43-48.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and in vehicle emissions. Environ Int, 11: 383-392.
Jüttner F (1988) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in forest air of the Southern Black
Forest. Chemosphere, 17: 309-317.
Kappeler T & Wuhrmann K (1978a) Microbial degradation of the
water-soluble fraction of gas oil, I. Water Res, 12: 327-333.
Kappeler T & Wuhrmann K (1978b) Microbial degradation of the
water-soluble fraction of gas oil. II. Bioassays with pure strains.
Water Res, 12: 335-342.
Kawai T, Yasugi T, Mizunuma K, Horiguchi S. Iguchi H, Uchida Y, Iwami
O, & Ikeda M (1992) Comparative evaluation of urinalysis and blood
analysis as means of detecting exposure to organic solvents at low
concentrations. Int Arch Occup Environ Health, 64(4): 223-234.
Kawamura K & Kaplan IR (1983) Organic compounds in the rainwater of
Los Angeles. Environ Sci Technol, 17: 497-501.
Kawata K & Fujeda Y (1993) Volatile aromatic hydrocarbons in ambient
air. Jpn J Toxicol Environ Health, 39(1): 37-43.
Kennicutt MC II, Brooks JM, & McDonald TJ (1984) Volatile organic
inputs from an ocean outfall near Barceloneta, Puerto Rico.
Chemosphere, 13: 535-548.
Kenrick MAP, Clark L, Baxter KM, Fleet M, Jones HA, Gibson TM, &
Turrell MB (1985) Trace organics in British aquifers - a baseline
survey. Water Research Centre, Environment (TR No. 223).
Keymeulen R, Van-Langenhove H, & Schamp N (1991) Determination of
monocyclic aromatic hydrocarbons in plant cuticles by gas
chromatography-mass spectrometry. J Chromatogr, 541(1-2): 83-88.
Kiese M & Lenk W (1974) Hydroxyacetophenones: urinary metabolites of
ethylbenzene and acetophenone in the rabbit. Xenobiotica, 4: 337-343.
Kleopfer RD & Fairless BJ (1972) Characterization of organic
components in a municipal water supply. Environ Sci Technol,
6: 1036-1037.
Koop DR & Laethem CL (1992) Inhibition of rabbit microsomal cytochrome
P-450 2E1-dependent p-nitrophenol hydroxylation by substituted benzene
derivatives. Drug Metab Dispos, 20: 775-777.
Korn M, Gfrörer W, Herz R, Wodarz I, & Wodarz R (1992)
Stereometabolism of ethylbenzene in man: gas chromatographic
determination of urinary excreted mandelic acid enantiomers and
phenylglyoxylic acid and their relation to the height of occupational
exposure. Int Arch Occup Environ Health, 64: 75-78.
Korte F & Boedefeld E (1978) Ecotoxicological review of the global
impact of the petroleum industry and its products. Ecotoxicol Environ
Saf, 2: 55-103.
Kroneld R (1989) Volatile pollutants in suburban and industrial air.
Bull Environ Contam Toxicol, 42: 868-872.
Kuhn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Labows J, Preti G, Hoelzle E, Leyden J, & Klingman A (1979) Analysis
of human axillary volatiles: Compounds of exogenous origin. J
Chromatogr, 163: 294-299.
Lanzerstorfer CH & Puxbaum H (1990) Volatile hydrocarbons in and
around Vienna, Austria. Water Air Soil Pollut, 51: 345-355.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lee JF, Crum JR, & Boyd SA (1989) Enhanced retention of organic
contaminants by soils exchanged with organic cations. Environ Sci
Technol, 23: 1365-1372.
LeGore RS (1974) The effect of Alaskan crude oil and selected
hydrocarbon compounds on embryonic development of the pacific oyster,
Crassostrea gigas. Doctoral Thesis, University of Washington. Diss
Abstr, B35: 3168.
Lesage S, Jackson RE, Priddle MW, & Riemann PG (1990) Occurrence and
fate of organic solvent residues in anoxic groundwater at the
Gloucester Landfill, Canada. Environ Sci Technol, 24: 559-566.
Levesque P & Dao LH (1989) Alkylation of benzene using an aqueous
solution of ethanol. Appl Catal, 53(2-3): 157-167.
Lewis PJ, Hagopian C, & Koch P (1983) Styrene. In: Kirk RE & Othmer DF
ed. Encyclopedia of chemical technology, 3rd ed. New York, Wiley
Interscience, vol 21, pp 770-801.
Lockhart WL, Wagemann R, Tracey B, Sutherland D, & Thomas DJ (1992)
Presence and implications of chemical contaminants in the freshwaters
of the Canadian Arctic. Sci Total Environ, 122(1/2): 165-243
Louw CW, Richards JF, & Faure PK (1977) Determination of volatile
organic compounds in city air by gas chromatography combined with
standard addition, selective subtraction, infra red spectrometry and
mass spectrometry. Atmos Environ, 11: 703-717.
Lovegren NV, Fisher GS, Legendre MG, & Schuller WH (1979) Volatile
constituents of dried legumes. J Agric Food Chem, 27: 851-853.
Lu BQ & Zhen ZH (1989) [Health standards for ethylbenzene in the air
of workplaces.] Beijing, Chinese Academy of Preventive Medicine,
Institute of Occupational Medicine (in Chinese).
Mabey W, Smith JH, Podoll, RT, Johnson HL, Mill T, Chou TW, Gate J,
Waight-Partridge I, Jaber H, & Vandenberg D (1982) Aquatic fate
process for organic priority pollutants. Washington, DC, US
Environmental Protection Agency (EPA 440/481-14).
McFall JA, Antoine SR, & DeLeon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14: 1253-1265.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma
cell forward mutation assay: III. 72 coded chemicals. Environ Mol
Mutagen, 12: 85-154.
Mackay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to atmosphere. Environ Sci Technol,
9: 1178-1180.
Mackay D, Shiu WY, & Sutherland RP (1979) Determination of air-water
Henry's law constants for hydrophobic pollutants. Environ Sci Technol,
13: 333-337.
Mackay D, Shiu WY, & Ma KC (1992) The illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Volume 1. Monoaromatic hydrocarbons, chlorobenzenes and
polychlorinated biphenyls. Chelsea, Michigan, Lewis Publishers, Inc.
MacLeod G & Ames JM (1987) Effect of water on the production of cooked
beef aroma compounds. J Food Sci, 52: 42-45.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
Current results and ongoing research. Am J Ind Med, 7: 415-446.
Masten LW, Boeri RL & Walker JD (1994) Strategies employed to
determine the acute aquatic toxicity of ethyl benzene, a
highly-volatile, poorly water-soluble chemical. Ecotoxicol Environ
Saf, 27: 335-348.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, p 460 (Resource Publication No. 160).
Michael LC, Pellizzari ED, & Norwood DL (1991) Application of the
master analytical scheme to the determination of volatile organics in
wastewater influent and effluents. Environ Sci Technol, 25: 150-155
Molnár J, Paksy KA, & Náray M (1986) Changes in the rat's motor
behaviour during 4-hour inhalation exposure to prenarcotic
concentrations of benzene and its derivatives. Acta Physiol Hung,
67: 349-354.
Morgan DL, Cooper SW, Carlock DL, Sykora JJ, Sutton B, Mattie DR, &
McDougal JN (1991) Dermal absorption of neat and aqueous volatile
organic chemicals in the Fischer 344 rat. Environ Res, 55: 51-63.
Mott JG & Romanow S (1991/1992) Sludge characterization, removal and
dewatering. J Hazard Mater, 29(1): 127-140.
Murray DAJ & Lockhart WL (1981) Microextraction and gas chromatographic
analysis of selected petroleum hydrocarbons in water and fish tissue.
J Chromatogr, 212: 305-311.
Namkung E & Rittmann BE (1987) Estimating volatile organic compound
emissions from publicly owned treatment works. J Water Pollut Control
Fed, 59: 670-678.
Nelson PF & Quigley SM (1982) Non-methane hydrocarbons in the
atmosphere of Sydney, Australia. Environ Sci Technol, 16: 650-655.
Neuhauser EF, Loehr RC, & Malecki MR (1986) Contact and artificial
soil tests using earthworms to evaluate the impact of wastes in soil.
In: Petros JK, Lacy WJ, & Conway RA end. Hazardous and industrial
solid waste testing: Fourth symposium. Philadelphia, Pennsylvania,
American Society for Testing and Materials, pp 192-203 (ASTM STP 886).
NIOSH (1984) Method 1501. In: Manual of analytical methods, 3rd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 2.
Noleau I & Toulemonde B (1988) Volatile components of roasted guinea
hen. Lebensm.Wiss Technol, 21: 195-197.
Norppa H & Vainio H (1983) Induction of sister-chromatid exchanges by
styrene analogues in cultured human lymphocytes. Mutat Res,
116: 379-387.
NTP (1992) Toxicity studies of ethylbenzene in F344/N rats and B6C3F1
mice (inhalation studies). Research Triangle Park, North Carolina, US
Department of Health and Human Services, National Toxicology Program
(NIH Publication No. 92-3129).
Nunes P & Benville PE Jr (1979) Uptake and depuration of petroleum
hydrocarbons in the Manila clam, Tapes semidecussata Reeve. Bull
Environ Contam Toxicol, 21:719-726.
Ogata M, Sugihara R, & Kira S (1977) Quantitative determination of
urinary hippuric acid and m- or p-methylhippuric acid as indices
of toluene and m- or p-xylene exposure by high performance liquid
chromatography. Int Arch Occup Environ Health, 39: 199-206.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities. J
Assoc Off Anal Chem, 65: 1370-1374.
Pankow JF, Isabelle LM, & Asher WE (1984) Trace organic compounds in
rain. I. Sampler design and analysis by adsorption/thermal desorption
(ATD). Environ Sci Technol, 18: 310-318.
Pellizzari ED, Hartwell TD, Harris BSH, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pellizzari ED, Sheldon L, Sparacino C, Bursey J, Wallace L, & Bromberg
S (1984) Volatile organic levels in indoor air. In: Berglund B,
Lindvall T, & Sundell J ed. Indoor air, chemical characterization and
personal exposure. Stockholm, Swedish Council for Building Research,
vol 4, pp 21-26.
Pellizzari ED, Wallace LA, & Gordon SM (1992) Elimination kinetics of
volatile organics in humans using breath measurements. J Expo Anal
Environ Epidemiol, 2: 341-355.
Perry DL, Chuang CC, Jungclaus GA, & Warner JS (1979) Identification
of organic compounds in industrial effluent discharges. Washington,
DC, US Environmental Protection Agency (EPA 600/4-79-016).
Petersson G (1982) Ambient hydrocarbons from motor-car assembly plants
in Scandinavia. Environ Pollut, B4: 207-217.
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38: 1419-1429.
Pitter P & Chudoba J (1990) Biodegradability of organic substances in
the aquatic environment. Boca Raton, Florida, CRC Press, Inc., p 269.
Potera FT (1975) The effects of benzene, toluene and ethylbenzene on
several important members of the estuarine ecosystem. Lehigh
University (Ph.D. dissertation).
Pussemier L, Szabó G, & Bulman RA (1990) Prediction of the soil
adsorption coefficient Koc for aromatic pollutants. Chemosphere
21: 1199-1212.
Pyykkö K, Paavilainen S, Metsä-Ketelä T, & Laustiola K (1987) The
increasing and decreasing effects of aromatic hydrocarbon solvents on
pulmonary and hepatic cytochrome P-450 in the rat. Pharmacol Toxicol,
60: 288-293.
Radzikowska-Kintzi H & Jakubowski M (1981) Internal standardization in
the head space analysis of organic solvents in blood. Int Arch Occup
Environ Health, 49: 115-123.
Rao PSC, Hornsby AG, & Jessup RE (1985) Indices for ranking the
potential for pesticide contamination of groundwater. Soil Crop Sci
Soc Proc, 44: 1-8.
Rappaport SM, Selvin S, & Waters MA (1987) Exposures to hydrocarbon
components of gasoline in the petroleum industry. Appl Ind Hyg,
2: 148-154.
Reinhard M, Goodman NL, & Barker JF (1984) Occurrence and distribution
of organic chemicals in two landfill leachate plumes. Environ Sci
Technol, 18: 953-961.
Rhue RD, Pennell KD, Rao PS, & Reve WH (1989) Competitive adsorption
of alkylbenzene and water vapors on predominantly mineral surfaces.
Chemosphere, 18(9-10): 1971-1986.
Romanelli A, Falzoi M, Mutti A, Bergamaschi E, & Franchini I (1986)
Effects of some monocyclic aromatic solvents and their metabolites on
brain dopamine in rabbits. J Appl Toxicol, 6: 431-435.
Römer KG, Federsel RJ, & Freundt KJ (1986) Rise of inhaled toluene,
ethyl benzene, m-xylene, or mesitylene in rat blood after treatment
with ethanol. Bull Environ Contam Toxicol, 37: 874-876.
Rosen AA, Skeel RJ, & Ettinger MB (1963) Relationship of river water
odour to specific organic contaminants. J Water Pollut Control Fed,
35: 777-782.
Roy WR & Griffin RA (1985) Mobility of organic solvents in
water-saturated soil material. Environ Geol Water Sci, 7: 241-247.
SAC (1989) Analysis of potentially dangerous substances in UK
waters. Bedford, United Kingdom, SAC Scientific (DOE Reference
No. PECD-7/7/306).
Sato A & Nakajima T (1987) Pharmacokinetics of organic solvent vapors
in relation to their toxicity. Scand J Work Environ Health, 13: 81-93.
Sauer TC Jr (1981) Volatile liquid hydrocarbon characterization of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environ Sci Technol, 15: 917-923.
Sax NI (1979) Dangerous properties of industrial chemicals, 5th ed.
New York, Van Nostrand Reinhold Co.
Seader JD (1982) Separation systems synthesis. In: Kirk RE & Othmer DF
ed. Encyclopedia of chemical technology, 3rd ed. New York, Wiley
Interscience, vol 20, pp 710-711.
Sequeira DJ, Eyer CS, Cawley GF, Nick TG, & Backes WL (1992)
Ethylbenzene-mediated induction of cytochrome P450 isozymes in male
and female rats. Biochem Pharmacol, 44: 1171-1182.
Sexton K & Westberg H (1980) Ambient hydrocarbon and ozone
measurements downwind of a large automotive painting plant. Environ
Sci Technol, 14: 329-332.
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environ Sci Technol, 16: 872-880.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1983) Measurements of
hazardous organic chemicals in the ambient atmosphere. Washington, DC,
US Environmental Protection Agency (EPA 600/3-83-002).
Singh HB, Ferek RJ, Salas LJ, & Nitz KC (1986) Toxic chemicals in the
environment: A program of field measurements. Washington, DC, US
Environmental Protection Agency (EPA 600/3-86/047).
Smith MR & Ratledge C (1989) Catabolism of alkylbenzenes by
Pseudomonas sp. NCIB 10643. Appl Microbiol Biotechnol, 32: 68-75.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: List VI. Am Ind Hyg Assoc J, 23: 95-107.
Sollenberg J (1991) Analytical isotachophoresis in biological
monitoring of exposure to industrial chemicals. J Chromatogr,
545: 369-374.
Sollenberg J, Smallwood AW, & Lowry LK (1985) Determination of
mandelic and phenylglyoxylic acids in rat urine by high-performance
liquid chromatography and by isotachophoresis. J Chromatogr,
343: 175-178.
Staples CA, Werner AF, & Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environ Toxicol Chem, 4(2): 131-142.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near to an underground coal gasification site in
North-Eastern Wyoming. Environ Sci Technol, 16: 582-587.
Susten AS, Niemeier RW, & Simon SD (1990) In vivo percutaneous
absorption studies of volatile organic solvents in hairless mice. II.
Toluene, ethylbenzene and aniline. J Appl Toxicol, 10: 217-225.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tester DJ & Harker RJ (1981) Groundwater pollution investigations in
the Great Ouse basin. Water Pollut Control, 80: 614-631.
Thomann RV (1989) Bioaccumulation model of organic chemical
distribution in aquatic food chains. Environ Sci Technol,
23(6): 699-707.
Thomas JM, Gordy VR, Fiorenza S, & Ward CH (1990) Biodegradation of
BTEX in subsurface materials contaminated with gasoline: Granger,
Indiana. Water Sci Technol, 22: 53-62.
Thomas RG (1982) Volatilization. In: Lyman WJ, Reehl WF, & Rosenblatt
DH ed. Handbook of chemical properties: Estimation methods. New York,
McGraw-Hill Book Co.
Thorburn S & Colenutt BA (1979) A gas chromatographic comparison of
volatile organic compounds in urban and rural atmospheres. Int J
Environ Stud, 13: 265-271.
Triebig G, Claus D, Csuzda I, Druschky KF, Holler P, Kinzel W, Lehrl
S, Reichwein P, Weidenhammer W, Weitbrecht WU, Weltle D, Schaller KH,
& Valentin H (1988) Cross-sectional epidemiological study on
neurotoxicity of solvents in paints and lacquers. Int Arch Occup
Environ Health, 60: 233-241.
Tsuruta H (1982) Percutaneous absorption of organic solvents. III. On
the penetration rates of hydrophobic solvents through the excised rat
skin. Ind Health, 20: 335-345.
Ullman (1983) [Encyclopaedia of chemical technology.] Weinheim, Verlag
Chemie, vol 24, pp 525-544 (in German).
Ungváry G & Tátrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats, and rabbits. Arch Toxicol,
8(suppl): 425-430.
US Chemical Industry (1994) Facts and figures for the chemical
industry. Chem Eng News, July 4: 28-33.
US EPA (1978) In-depth studies on health and environmental impacts of
selected water pollutants. Washington, DC, US Environmental Protection
Agency (Contract No. 68-01-4646).
US ITC (1987) Synthetic organic chemicals: United States production
and sales, 1986. Washington, DC, US International Trade Commission.
Utkin IB, Yakimov MM, Matveeva LN, Kozlyak EI, Rogozhin IS, Solomon
ZG, & Bezborodov AM (1991) Degradation of styrene and ethylbenzene by
Pseudomonas species Y2. Microbiol Lett, 77: 237-242.
Van Duijvenbooden W & Kooper WF (1981) Effects on groundwater flow and
groundwater quality of a waste disposal site in Noordwijk, The
Netherlands. Sci Total Environ, 21: 85-92.
Verhoeff AP, Suk J, & Van Wijnen JH (1988) Residential indoor air
contamination by screen printing plants. Int Arch Occup Environ
Health, 60: 201-209.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., pp 628-630.
Viganň L (1993) Reproductive strategy of Daphnia magna and toxicity
of organic compounds. Water Res, 27: 903-909.
Vincent R, Poirot P, Sabra I, Rieger B, & Cicolella A (1994)
Occupational exposure to organic solvents during paint stripping and
painting operations in the aeronautical industry. Int Arch Occup
Environ Health, 65(6): 377-380.
Volskay VT Jr & Grady CPL Jr (1990) Respiration inhibition kinetic
analysis. Water Res, 24: 863-874.
Vowles PD & Mantoura RFC (1987) Sediment-water partition coefficients
and HPLC retention factors of aromatic hydrocarbons. Chemosphere,
16: 109-116.
Waggott A (1981) Trace organic substances in the River Lee. In:
Chemistry in water reuse. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., vol 2, pp 55-99.
Wakeham SG, Davis AC, & Karas JL (1983) Mesocosm experiments to
determine the fate and persistence of volatile organic compounds in
coastal seawater. Environ Sci Technol, 17: 611-617.
Wallace LA, Pellizzari E, Hartwell TD, Perritt R, & Ziegenfus R
(1987a) Exposures to benzene and other volatile compounds from active
and passive smoking. Arch Environ Health, 42(5): 272-279.
Wallace LA, Pellizzari E, Hartwell TD, Sparacino C, Withmore R,
Sheldon L, Zelon H, & Perritt R (1987b) The TEAM study: Personal
exposures to toxic substances in air, drinking water, and breath of
400 residents of New Jersey, North Carolina, and North Dakota. Environ
Res, 43: 290-307.
Wallace LA, Pellizzari E, Leaderer B, Zelon H, & Sheldon L (1987c)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Wallace LA, Pellizzari ED, Hartwell TD, Davis V, Michael LC, &
Whitmore RW (1989) The influence of personal activities on exposure to
volatile organic compounds. Environ Res, 50(1): 37-55.
Warner JM & Beasley RK (1984) Purge and trap chromatographic method
for the determination of acrylonitrile, chlorobenzene,
1,2-dichloroethane and ethylbenzene in aqueous samples. Anal Chem,
56: 1953-1956.
Water Pollution Control (1989) Fate of organics on land-applied
sludges. Water Pollut Control, 127: 12.
Weast RC ed (1988) Handbook of chemistry and physics, 69th ed. Boca
Raton, Florida, CRC Press, Inc.
Webber D (1984) Top 50 chemical products. Chem Eng News, May 7: 8-10.
Westrick JJ, Mello JW, & Thomas RF (1984) The groundwater supply
survey. J Am Water Works Assoc, 76: 52-59.
Whitby FJ, Harland BJ, & Comber MHI (1982) Aromatic hydrocarbons in
the Tees Estuary. London, Department of Health (DOE Contract
PECD-7/7/23).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization.
Wiesenburg DA, Bodennec G, & Brooks JM (1981) Volatile liquid
hydrocarbons around a production platform in the northwest Gulf of
Mexico. Bull Environ Contam Toxicol, 27: 167-174.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformation of selected
alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic
aquifer material: A microcosm study. Environ Sci Technol, 20:
997-1002.
Windholz M ed (1983) The Merck index: An encyclopedia of chemicals,
drugs and biologicals, 10th ed. Rahway, New Jersey, Merck & Co., Inc.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene. Arch
Ind Health, 14: 387-398.
Wolff MS (1976) Evidence for existence in human tissues of monomers
for plastics and rubber manufacture. Environ Health Perspect,
17: 183-187.
Wolff MS, Daum SM, Lorimer WV, Selikoff IJ, & Aubrey BB (1977) Styrene
and related hydrocarbons in subcutaneous fat from polymerization
workers. J Toxicol Environ Health, 2: 997-1005.
Yamamoto RK & Cook WA (1968) Determination of ethyl benzene and
styrene in air by ultraviolet spectrophotometry. Am Ind Hyg Assoc J,
29: 238-241.
Zhang Z & Pawliszyn J (1993) Headspace solid-phase microextraction.
Anal Chem, 65(14): 1843-1852.
Zlatkis A & Jiao J (1991) Evaluation of new organoclay stationary
phases for the separation of ethylbenzene and xylene isomers.
Chromatographia, 31(9-10): 457-464.
1. RESUME
L'éthylbenzčne est un hydrocarbure aromatique qui s'obtient par
une réaction d'alkylation mettant en jeu le benzčne et l'éthylčne.
Aux Etats-Unis, on estime sa production annuelle ŕ environ 5 millions
de tonnes. En 1983, l'Europe occidentale en a produit environ 3
millions de tonnes. Il se présente sous la forme d'un liquide
incolore dégageant une odeur douceâtre qui rappelle l'essence. On
l'utilise principalement pour la production de styrčne. Ajouté ŕ du
xylčne technique, il sert également de solvant pour les peintures et
les vernis et on l'emploie aussi dans l'industrie chimique et dans
celle du caoutchouc. Il est présent dans le pétrole brut, les
produits raffinés dérivés du pétrole et dans leurs produits de
combustion.
L'éthylbenzčne n'est pas persistant car il se décompose dans
l'environnement, principalement par photo-oxydation et par dégradation
biologique. Il est possible que la photo-oxydation de l'éthylbenzčne
dans l'atmosphčre contribue ŕ la formation du smog photochimique.
Le logarithme du coéfficient de partage entre l'octanol et l'eau
est égal ŕ 3,13, ce qui indique que le composé se pręte ŕ une
bioaccumulation. Cependant, les données limitées dont on dispose
montrent que le facteur de bioaccumulation de l'éthylbenzčne est
faible pour les mollusques et les poissons. Apparemment, il est vite
éliminé par les organismes aquatiques.
A la campagne, la concentration d'éthylbenzčne dans l'air est
généralement inférieure ŕ 2 µg/m3. Sur des sites urbains, on a
trouvé des teneurs moyennes allant de 0,74 ŕ 100 µg/m3. La
concentration d'éthylbenzčne présente dans les eaux superficielles
est généralement inférieure ŕ 0,1 µg/litre dans les zones non
industrialisées. En revanche, dans les zones urbaines ou
industrialisées, la concentration peut atteindre 15 µg/litre. Dans
les sédiments, la concentration est en général inférieure ŕ
0,5 µg/litre, encore que l'on ait signalé des valeurs comprises entre
1 et 5 µg/litre dans des sédiments provenant de régions fortement
industrialisées. Dans les eaux souterraines non contaminées, la
concentration est habituellement inférieure ŕ 0,1 µg/litre, mais elle
est beaucoup plus élevée dans les eaux contaminées.
L'éthylbenzčne présente une toxicité aiguë modérée pour les
algues, les invertébrés aquatiques et les poissons. La valeur de la
CE50 ŕ 72 h est de 4,6 mg/litre pour l'algue Selenastrum
capricornutum, la CL50 ŕ 48 h est de 1,8 mg/litre pour Daphnia
magna, et on a une CL50 ŕ 96 h de 4,2 mg/litre pour la truite
arc-en-ciel. On ne possčde aucune donnée sur l'exposition chronique
des organismes aquatiques ŕ l'éthylbenzčne.
En ce qui concerne les bactéries et les lombrics, les données
toxicologiques sont limitées. Il n'en n'existe aucune sur les
végétaux terrestres, les oiseaux ou les mammifčres sauvages.
Chez l'homme, l'exposition ŕ l'éthylbenzčne se produit
principalement par inhalation; 40 ŕ 60% du composé sont retenus dans
les poumons. L'éthylbenzčne est fortement métabolisé, principalement
en acides mandélique et phénylglyoxylique. On peut utiliser les
métabolites présents dans les urines pour surveiller l'exposition
humaine.
Qu'elle soit aiguë ou chronique, la toxicité de l'éthylbenzčne
est faible pour l'homme et les animaux. Il exerce des effets toxiques
sur le systčme nerveux central et il est irritant pour les muqueuses
et les yeux. Le seuil de concentration pour ces effets chez l'homme a
été estimé ŕ environ 430-860 mg/m3 (100-200 ppm) lors d'une seule
exposition de courte durée.
Des rats et des souris ŕ qui on avait fait inhaler pendant 13
semaines de l'éthylbenzčne ŕ des concentrations allant jusqu'ŕ 4300
mg/m3, n'ont présenté aucune lésion histopathologique. La dose sans
effet observable (critčre retenu: l'augmentation du poids du foie) a
été estimée ŕ 2150 mg/m3 (500 ppm).
L'éthylbenzčne stimule les enzymes des microsomes hépatiques. Il
n'est ni mutagčne ni tératogčne pour le rat ou le lapin. On ne dispose
d'aucune donnée au sujet de ses effets toxiques éventuels sur
l'appareil reproducteur ni sur son pouvoir cancérogčne.
Une valeur-guide de 22 mg/litre (5 ppm) a été calculée ŕ partir
des résultats fournis par les études sur l'animal. On estime que la
population générale est exposée ŕ des concentrations inférieures ŕ
cette valeur, męme dans les cas les plus graves. On a constaté qu'une
exposition de longue durée en milieu professionnel, ŕ des concentration
de cet ordre, n'avait aucun effet nocif sur la santé des travailleurs
exposés.
RESUMEN
El etilbenceno es un hidrocarburo aromático que se obtiene por
alkilación del benceno y del etileno. La producción estimada en los
Estados Unidos de América es de unos cinco millones de toneladas por
ańo, y en Europa occidental fue de aproximadamente tres millones de
toneladas en 1983. El etilbenceno es un líquido incoloro de olor
dulce semejante al de la gasolina. Se utiliza principalmente para la
producción de estireno. También se utiliza en el xileno técnico como
disolvente de pinturas y lacas, así como en la industria del caucho y
en la fabricación de sustancias químicas. Se encuentra en el petróleo
crudo, en los productos de petróleo refinados y en productos de
combustión.
El etilbenceno es una sustancia química no persistente, que se
degrada principalmente por fotooxidación y biodegradación. Su
volatilización en la atmósfera es rápida. La reacción de foto-
oxidación del etilbenceno en la atmósfera puede contribuir a la
formación de niebla fotoquímica.
El logaritmo del coeficiente de reparto octanol-agua es 3,13, lo
que indica posibilidad de bioacumulación. Sin embargo, los limitados
indicios disponibles muestran que los factores de bioconcentración del
etilbenceno son bajos para peces y moluscos. La eliminación por los
organismos acuáticos parece ser rápida.
Los niveles de etilbenceno en el aire en puntos rurales son
generalmente inferiores a 2 µg/m3. En puntos urbanos se han
registrado niveles medios de etilbenceno que oscilan entre 0,74 y 100
µg/m3. Los niveles de etilbenceno detectados en las aguas
superficiales son generalmente inferiores a 0,1 µg/litro en zonas no
industriales. Se han comunicado concentraciones de etilbenceno de
hasta 15 µg/litro en zonas industriales y urbanas. Los niveles de
etilbenceno en sedimentos son generalmente inferiores a 0,5 µg/kg,
aunque en sedimentos de zonas muy industrializadas se han encontrado
niveles de 1 a 5 µg/kg. Las concentraciones en aguas subterráneas no
contaminadas son generalmente inferiores a 0,1 µg/litro, pero son
mucho más elevadas en aguas subterráneas contaminadas.
La toxicidad aguda del etilbenceno para las algas, los
invertebrados acuáticos y los peces es moderada. Los valores de
toxicidad aguda más bajos son de 4,6 mg/litro para el alga
Selenastrum capricornutum (CE50 a las 72 horas, sobre la base de la
inhibición del crecimiento), 1,8 mg/litro para Daphnia magna (CL50
a las 48 horas) y 4,2 mg/litro para la trucha irisada (CL50 a las 96
horas). No se dispone de información sobre la exposición crónica de
los organismos acuáticos al etilbenceno.
Hay información limitada sobre la toxicidad del etilbenceno para
las bacterias y para las lombrices. No hay datos relativos a las
plantas terrestres, las aves y los mamíferos silvestres.
La exposición humana al etilbenceno se produce principalmente por
inhalación; el 40-60% del etilbenceno inhalado se retiene en los
pulmones. El etilbenceno se metaboliza extensamente, transformándose
sobre todo en ácidos mandélico y fenilglioxílico. Estos metabolitos
urinarios pueden utilizarse para vigilar la exposición humana.
El etilbenceno tiene una toxicidad aguda y crónica baja tanto
para los animales como para el hombre. Es tóxico para el sistema
nervioso central e irrita las mucosas y los ojos. El umbral para esos
efectos en el ser humano después de exposiciones únicas breves se
estimó en aproximadamente 430-860 mg/m3 (100-200 ppm).
La inhalación de etilbenceno por ratas y ratones durante 13
semanas en concentraciones de hasta 4300 mg/m3 (1000 ppm) no dio
lugar a lesiones histopatológicas. El nivel sin efectos observados,
sobre la base de un aumento del peso del hígado en las ratas, fue de
2150 mg/m3 (500 ppm).
El etilbenceno es un inductor de las enzimas microsómicas
hepáticas. No es mutagénico ni teratogénico en ratas y conejos. No
se dispone de información sobre la toxicidad reproductiva ni la
carcinogenicidad del etilbenceno.
Se ha calculado un valor de orientación de 22 mg/m3 (5 ppm) a
partir de estudios realizados en animales. La exposición estimada de
la población general (incluso en la peor de las situaciones) es
inferior a ese valor de orientación. La exposición ocupacional a
largo plazo a concentraciones de etilbenceno estimadas en este orden
de magnitud no ocasionaron efectos adversos en la salud de los
trabajadores.
Ethylbenzene is metabolized by the microsomal cytochrome P-450
enzyme system. Although specific isozymes have not been unequivocally
identified, enzyme induction studies suggest that CYP2B1/2, CYP1A1/2
and CYP2E1 may be involved (see section 7.8).
Four male volunteers were exposed to 655 mg/m3 (150 ppm)
ethylbenzene for 4 h and urine was collected for 24 h. Mandelic acid
(71.5%) and phenylglyoxylic acid (19.1%) were the main metabolites,
but smaller amounts of 1-phenylethanol, p-hydroxyacetophenone,
m-hydroxyacetophenone, 1-phenyl-1,2-ethandiol, 4-ethylphenol,
omega-hydroxyacetophenone and acetophenone were also found. Ring
oxidation accounted for 4.0%. Simultaneous exposure to 150 ppm
m-xylene did not alter the urinary metabolite pattern, but it
delayed excretion and decreased the amounts of metabolites excreted
(Engström et al., 1984). Mandelic acid (64%) and phenylglyoxylic aid
(25%) were found to be the main urinary excretion products in
volunteers after an 8-h exposure to 99-365 mg/m3 (23-85 ppm)
ethylbenzene (Bardodej & Bardodejová, 1970).
When two volunteers were exposed by inhalation to 430 mg/m3 (100
ppm) ethylbenzene for 4 h, most of the mandelic acid was excreted as
the R-enantiomer (Drummond et al., 1989).
In a study by Korn et al. (1992), urinary samples from workers
exposed to ethylbenzene, toluene and xylenes were analysed. The
average urinary concentration of phenylglyoxylic acid was 50.1
mg/litre. The concentration of mandelic acids was 135.2 mg/litre, of
which 127.8 mg/litre was R-mandelic acid and 7.3 mg/litre was
S-mandelic acid. The R/S ratio was independent of the air
concentration of ethylbenzene, which varied between 6.4 and 142 mg/m3
(1.5 and 33 ppm).
Four female histology laboratory assistants were exposed to a
mixture of xylenes (75%) and ethylbenzene (25%). The air concentration
of the solvent was 160-179 mg/m3, and the concentration of
ethylbenzene in blood collected at the end of the working day was
reported to be 0.5 to 0.8 mg/litre. The 24-h excretion of
2-ethylphenol in urine varied between 4.4 and 6.0 mg corresponding to
1 to 1.5% of the retained ethylbenzene (Angerer & Lehnert, 1979). The
findings by Angerer & Lehnert (1979) that ethylbenzene is metabolized
to 2-ethylphenol could not be verified by Engström et al. (1984).
Male Wistar rats (6 per group) were exposed for 6 h to
ethylbenzene at 1290 or 2580 mg/m3 (300 or 600 ppm). The urine was
collected for 48 h from the onset of exposure. Altogether, 14
different metabolites from ethylbenzene were identified. The main
metabolites were 1-phenylethanol, mandelic acid and benzoic acid, each
of which accounted for about 25%. Only 13% (low dose) and 6% (high
dose) of the estimated absorbed doses were eliminated during the 6-h
exposure. Over the 48-h period, the corresponding values were 83% and
59%, respectively. The metabolic pattern was similar, irrespective of
the exposure level (Engström, 1984b).
In another study, male Wistar rats (20 per group) were exposed to
215, 1290 or 2580 mg ethylbenzene/m3 (50, 300 or 600 ppm) for 6
h/day, 5 days/week, for up to 16 weeks. Urinary excretion of some of
the metabolites was measured in weeks 2, 5 and 9. A significant
dose-related percentage decrease of phenylglyoxylic acid and hippuric
acid plus benzoic acid was found. A corresponding increase of
1-phenylethanol and omega-hydroxyacetophenone excretion was also
noted. The total amount of metabolites in urine collected during the
24 h after onset of exposure remained, however, constant at each
exposure level throughout the study (Engström et al., 1985).
When a single oral dose of 318 mg ethylbenzene/kg body weight was
administered to rabbits, the main urinary metabolites found were
hippuric acid and methylphenylglucuronic acid, which together
represented 60-70% of the dose, while mandelic acid and phenaceturic
acid were minor metabolites (El Masry et al., 1956).
It has been established in in vivo studies that experimental
animals convert ethylbenzene mainly to the R-enantiomer of mandelic
acid (Drummond et al., 1990). In an in vitro study it was found
that ethylbenzene is hydroxylated by cytochrome P-450cam (from
Escherichera coli) almost exclusively at the secondary ethyl carbon
with about a 2:1 ratio of R:S products (Filipovic et al., 1992).
The rates of metabolism of ethylbenzene have been studied in
vitro in rabbit liver and lung. Organs from five female animals
were used, but details of the experimental procedures were not
reported. For the liver (mean weight 76 g) the rate of metabolism was
453 nmol/g tissue per 10 min (34.4 µmol per liver per 10 min or 11.7
nmol per nmol cytochrome P-450 per 10 min). The corresponding figures
for lung (mean weight 7.7 g), were 680 nmol, 5.3µmol and 200.1 nmol,
respectively. Thus, lung tissue may significantly contribute to the
body clearance of ethylbenzene in rabbits (Sato & Nakajima, 1987).
6.4 Elimination and excretion
In humans, ethylbenzene is mainly excreted in the urine as
mandelic and phenylglyoxylic acids (Bardodej & Bardodejová, 1970;
Ĺstrand et al., 1978; Engström et al., 1984; Gromiec & Piotrowski,
1984). Only up to 5% of retained ethylbenzene is estimated to be
exhaled without transformation (Ĺstrand et al., 1978). The
elimination half-lives of ethylbenzene in exhaled air and urine have
been estimated to be 0.5-3 h and 8 h, respectively (Wolff, 1976). In
human volunteers exposed to 100 or 200 ppm "industrial xylene" for
2 h, there was no decline in the concentration of xylenes plus
ethylbenzene in the gluteal subcutaneous adipose tissue between 30 min
and 22 h after exposure (Engström & Bjurström, 1978). The elimination
of mandelic acid has been found to be biphasic, with half-lives of 3.1
and 24.5 h (Gromiec & Piotrowski, 1984).
The elimination kinetics for 10 volatile organic compounds,
including ethylbenzene, has been studied in human volunteers exposed
to a variety of consumer products. Breath samples were collected
post-exposure and analysed by GC/MS. The half-lives for the 10
chemicals varied from a few hours to 1-2 days. The authors concluded
that volatile organic compounds exhibit relatively short residence
times in the body (Pellizzari et al., 1992).
Male Harlan-Wistar rats exposed to 14C-ring-labelled
ethylbenzene (1000 mg/m3) for 6 h excreted 82% of the radioactivity
in the urine, 8.2% in expired air (0.03% as CO2) and 0.7% in faeces.
After 42 h, 0.2% remained in the tissues. The remaining 8.3% could not
be accounted for (Chin et al., 1980a).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Single high exposures to ethylbenzene cause irritation of the
mucous membranes and central nervous system effects. The results from
single exposure in vivo studies are summarized in Table 5.
Table 5. Single exposure of animals to ethylbenzenea
Species Route Dose Parameter Reference
Rat oral 3.5 g/kg LD50 Wolf et al. (1956)
Rat oral 4.7 g/kg LD50 Smyth et al. (1962)
Rat inhalation 9.37 g/m3 (2180 ppm) Minimum Molnár et al. (1986)
narcotic conc.
Rat inhalation 17.2 g/m3 (4000 ppm) 1 h LC10 Smyth et al. (1962)
Rat inhalation 17.2 g/m3 (4000 ppm) 4 h LC50 Smyth et al. (1962)
Rat inhalation 34.4 g/m3 (8000 ppm) 1 h LC100 Smyth et al. (1962)
Rabbit dermal 77.4 g/kg LD50 Smyth et al. (1962)
a Additional information is given in the following reviews: DFG (1985), ECETOC (1986).
7.2 Short-term exposure
In a short-term study, six male rats (Sprague Dawley) were
exposed for 6 h/day during 3 consecutive days to 8.6 g/m3 (2000 ppm)
ethylbenzene. The animals were killed 16-18 h after the last
exposure. Small increases in dopamine and noradrenaline levels and
turnover in various parts of the hypothalamus and the median eminence
were reported. Ethylbenzene was also found to produce selective
reduction in prolactin and corticosterone secretion and selective
increase in dopamine turnover within the dopamine-cholecystokinin-8-
immuno-reactive nerve terminals of the nucleus accumbens (posterior
part) (Andersson et al., 1981).
When eight male rabbits (New Zealand) were exposed 12 h daily for
7 days to 3.22 g/m3 (750 ppm) ethylbenzene, there was a marked
(p<0.05) depletion of striatal and tuberoinfundibular dopamine. Such
an effect was also caused by intraperitoneal dosing of rabbits (eight
per group) with mandelic or phenylglyoxylic acid (4 mmol/kg per day
for 3 days) in saline (Romanelli et al., 1986).
In a 4-week inhalation study Fischer-344 rats (five of each sex
per group) were exposed to ethylbenzene for 6 h/day, 5 days per week,
at exposure levels of 0, 426, 1643 or 3363 mg/m3 (0, 99, 382 or 782
ppm). At the two highest exposure levels, sporadic lacrimation and
salivation, as well as significantly (p<0.05) increased liver
weights, were seen. At the highest exposure level, there was a small
increase in leukocyte counts and, in males, a marginal increase in
platelet counts (Cragg et al., 1989).
In the same study, mice (B6C3F1) of both sexes were similarly
exposed. At 1643 and 3363 mg ethylbenzene/m3, females showed
significantly (p<0.01) increased absolute and relative liver weights.
In males a significantly (p<0.05) increased relative liver-to-brain
weight ratio was seen. Male and female rabbits (New Zealand White)
were also used in this study. The exposure levels were 0, 1643, 3363
and 6923 mg/m3 (0, 382, 782 and 1610 ppm). At the highest exposure
level females gained weight more slowly than controls but neither sex
exhibited gross or microscopic organ changes (Cragg et al., 1989).
No changes in mortality pattern were seen in the three species.
There were no changes in clinical chemistry parameters in rats or
rabbits. Mice were not subjected to clinical chemistry or
haematological examinations due to the small volume of blood that
could be collected. For similar reasons, urinalyses were performed
for rats (no change) but not for mice. Rabbits were excluded from
urinalysis for logistical reasons. No changes in gross or microscopic
pathology were noted in any of over 30 tissues from each of the three
species when the animals were exposed at the highest concentration
(Cragg et al., 1989).
7.3 Long-term exposure
7.3.1 Oral exposure
Matched groups of 10 Wistar female rats were given daily
ethylbenzene doses of 0, 13.6, 136, 408 or 680 mg/kg by stomach tube
5 days a week for 6 months. The two highest dosages induced slight
increases in liver and kidney weights and slight cloudy swelling of
parenchymal liver cells and of the tubular epithelium in the kidney
(Wolf et al., 1956).
7.3.2 Inhalation exposure
In a 13-week National Toxicology Program study, groups of 10 rats
(F-344/N) and 10 mice (B6C3F1) of each sex were exposed for 6 h (plus
10 min to reach 90% of the target chamber concentration) per day,
5 days per week for 92 (female rats), 93 (male rats), 97 (female mice)
or 98 (male mice) days, at ethylbenzene concen-trations of 0, 430,
1075, 2150, 3225 or 4300 mg/m3 (0, 100, 250, 500, 750 or 1000 ppm).
Blood for clinical chemistry and haematological examination was
collected on study days 4 and 23 and again at week 13 from both male
and female rats. Dose-related increases in absolute liver weight were
seen in both sexes of mice exposed to the two highest dose levels, and
the relative kidney weight of female mice exposed to 4300 mg/m3 was
greater than that of the controls. Increased absolute and relative
liver and kidney weights were seen in male rats exposed to the two
highest dose levels. Increased absolute liver and kidney weights were
seen in female rats exposed to the three highest dose levels, but no
increased relative liver and kidney weights were seen. No chemically
related histopathological changes were observed in any rat or mouse
tissues. Clinical chemistry results were negative (NTP, 1992).
In another study, groups of five male rats (Wistar) were exposed
for 6 h/day, 5 days/week to ethylbenzene concentrations of 0, 215,
1290 or 2580 mg/m3 (0, 50, 300 or 600 ppm) and sacrificed after 2, 5,
9 or 16 weeks of exposure. At 2580 mg/m3 liver cells showed a slight
proliferation of smooth endoplasmic reticulum, slight degranulation
and splitting of rough endoplasmic reticulum, and enlarged
mitochondria. At the same dose level, liver microsomal protein, but
not cytochrome P-450, concentration was slightly increased. There was
also an increase in NADPH-cytochrome c reductase, 7-ethoxycoumarin-
O-deethylase and UDPG-transferase activities in the liver. In the
kidney only the two latter enzymes showed dose-related increases.
Urinary excretion of thioethers was measured to ascertain the
generation of electrophilic intermediates during ethylbenzene
metabolism. Excretion of thioethers increased in a dose-dependent
manner, with some fluctuation over the course of 7 weeks, reaching
about eight times the control level at 2580 mg ethylbenzene/m3.
However, there was no decrease in hepatic or renal levels of
glutathione (GSH), indicating that the cells were able to maintain the
intra-cellular homeostasis of GSH during exposure (Elovaara et al.,
1985).
In inhalation experiments, matched groups of 10-25 male and
female Wistar rats, 5-10 guinea-pigs, 1-2 rabbits and 1-2 rhesus
monkeys of either sex or both sexes were all exposed 7 h/day, 5
days/week, for up to 6 months. The exposure levels were 0, 1720 and
2580 mg/m3 (0, 400 and 600 ppm) for 186 days, 5375 mg/m3 (1250 ppm)
for 214 days (no monkeys) or 9460 mg/m3 (2200 ppm) for 144 days (rats
only). Slight effects were seen in rats: increased liver and kidney
weights at 1720 mg/m3; increased liver and kidney weights at 2580
mg/m3; and small histopathological changes (cloudy swelling) in liver
and kidney at 5375 and 9460 mg/m3 (Wolf et al. 1956).
In guinea-pigs and monkeys slightly increased liver weights were
noted in the 2580 mg/m3 group only. At the same exposure level,
small histopathological effects in the testes, described as
degeneration of the germinal epithelium, were seen in rabbits and
monkeys. At 5375 mg/m3 a slight growth depression was noted in
guinea-pigs. The no-observed-effect level (all four species) was
considered to be about 860 mg/m3 (200 ppm) (Wolf et al., 1956). It
should be noted, however, that Cragg et al. (1989) found no
histopathological effects in the testes of rats and rabbits exposed to
up to 3363 mg/m3 (782 ppm) for 4 weeks, and the lack of toxicity was
confirmed by NTP (1992).
7.4 Skin and eye irritation, sensitization
Inhalation for 3 min of ethylbenzene at a concentration of 4300
mg/m3 caused slight nasal irritation in guinea-pigs, and an 8-min
exposure caused eye irritation as well. At 8600 mg/m3 a 1-min
exposure was enough to cause both effects (Cavender, 1993).
Two drops of undiluted ethylbenzene placed in the eyes of rabbits
resulted in slight conjunctival irritation but no effects on the
cornea (Wolf et al., 1956). A slight conjunctival irritation with
some reversible corneal injury was reported in rabbits in a study by
Smyth et al. 1962.
Undiluted ethylbenzene has been shown to produce moderate
irritation when applied to the uncovered skin of rabbits (Smyth et
al., 1962). The application of undiluted ethylbenzene to the ear and
to the shaved abdomen of rabbits up to 20 times during a 4-week period
resulted in moderate irritation. There was erythema and oedema with
superficial necrosis and exfoliation of large patches of skin (Wolf et
al., 1956).
No animal sensitization studies have been reported.
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
In an inhalation study, rats (Wistar or Sprague-Dawley) and
rabbits (New Zealand White) were exposed to 430 or 4300 mg/m3 (100 or
1000 ppm) ethylbenzene for 6 to 7 h/day on gestation days 1 to 19
(rats) or 1 to 24 (rabbits). All pregnant animals were sacrificed on
the day before term (day 21 for rats, day 30 for rabbits). The
rabbits had a significantly (p<0.05) reduced number of live pups per
litter at both exposure levels, but the number of implantations per
litter and the number of dead or resorbed fetuses per litter did not
differ from those of the controls. Maternal toxicity in rats exposed
to 4300 mg/m3 was reflected in increased liver, kidney and spleen
weights. There was a significant (p<0.05) increase in the incidence
of extra ribs in both of the exposed rat groups (Hardin et al., 1981).
In a further study, rats (CFY) were exposed to ethylbenzene
concentrations of 600, 1200 or 2400 mg/m3 continuously (24 h/day)
from day 7 to day 15 of pregnancy. They were then killed on day 21.
Mice (CFLP) were exposed for three periods of 4 h per day to 500
mg/m3 on days 6-15 of pregnancy and killed on day 18. Rabbits (New
Zealand White) were exposed continuously on days 7-20 of gestation to
500 or 1000 mg/m3 and were killed at day 30. The maternal toxic
effects (not specified) in mice and rats were moderate and
dose-dependent. In both species ethylbenzene caused skeletal growth
retardation, extra ribs and reduced fetal growth rate at the highest
concentration. In rabbits, the highest dose concentration caused mild
maternal toxic effects (decreased weight gain) and reduction in the
number of fetuses due to abortion (Ungváry & Tátrai, 1985).
When rats (F-344/N) and mice (B6C3F1) were exposed to
ethylbenzene at concentrations of 0, 430, 2150 and 4300 mg/m3 (0,
100, 500 and 1000 ppm), 6 h per day, 5 days per week, for 13 weeks,
there were no changes in sperm or vaginal cytology (NTP, 1992).
Rat embryos were explanted on day 9 of gestation and cultured in
rat serum with added xylene (containing 18% ethylbenzene) at
concentrations up to 1.0 ml/litre serum. Dose-dependent retardation
of growth and development was seen but there were no observable
teratogenic effects (Brown-Woodman et al., 1991).
No multigeneration and reproductive studies on ethylbenzene have
been reported.
7.6 Mutagenicity and related end-points
In a National Toxicology Program study, ethylbenzene was not
mutagenic in Salmonella tests and did not induce chromosomal
aberrations or sister chromatid exchange in Chinese hamster ovary
(CHO) cells in vitro, although it did induce trifluorothymidine
resistance in mouse lymphoma cells at the highest concentration tested
(80 mg/litre). There was no increase of micronuclei in the peripheral
blood of mice exposed to ethylbenzene (NTP, 1992).
In several other studies ethylbenzene did not induce point
mutations (with or without added metabolic activation system). In
addition, it did not cause an increase in the spontaneous
recessive-lethal frequency in the Drosophila recessive-lethal test,
nor had it any chromosomal effects in vitro (Donner et al., 1980;
Florin et al., 1980; Dean et al., 1985). Ethylbenzene had a marginal
effect on sister chromatid exchange in human lymphocytes in vitro
when a high (10 mmol/litre) concentration was used (Norppa & Vainio,
1983). In addition, in the TK+/- test in mouse lymphoma cells there
was a slight effect at a high concentration (80 mg/litre) (McGregor et
al., 1988). This study is obviously the same as the one subsequently
reported by NTP (1992).
No excess of chromosomal aberrations in bone marrow cells was
seen in rats after up to 18 weeks of exposure (6 h/day, 5 days/week)
to 300 ppm of a xylene mixture containing 18.3% ethylbenzene (Donner
et al., 1980).
7.7 Carcinogenicity
In a carcinogenicity study, rats (Sprague-Dawley) were exposed to
one of several aromatic hydrocarbons, including ethylbenzene. Groups
of rats (40 of each sex) were exposed to 500 mg ethylbenzene (in olive
oil) per kg body weight by gavage, 4 or 5 days per week for 104 weeks.
Results were determined after 141 weeks. The first malignant tumour,
a nephroblastoma, was observed after 33 weeks. The total number of
malignant tumours was 31 in the 77 animals of the exposed group alive
at 33 weeks compared with an incidence of 23 of 94 animals in the
control group. The authors concluded that ethylbenzene caused an
increase in the incidence of total malignant tumours, although there
was no increase in the incidence of any specific type of tumour
(Maltoni et al., 1985). It is difficult to draw any firm conclusion
from this study because of inadequate reporting.
7.8 Other special studies
Ethylbenzene was found to have low acute cytotoxicity in vitro
on Ehrlich ascites cells (Holmberg & Malmfors, 1974).
The effects of ethylbenzene have been studied in four in vitro
test systems: decreased cell growth in Ascites sarcoma BP 8 cells;
decreased oxidative metabolism in hamster brown fat cells; cell
membrane damage of human embryonic lung fibroblasts; and inhibition of
ciliary activity in chicken embryo trachea. On a 0-9 point scale
(equivalent to 0-100%) the activity of these four systems scored 4, 6,
8 and 8, respectively (Curvall et al., 1984).
The activity of cytochrome CYP2E1 can be monitored in microsomal
preparations by p-nitrophenol hydroxylation. When rabbit (white
male New Zealand) liver microsomes were treated with up to 0.25 mM
ethylbenzene an inhibition of p-nitrophenol hydroxylation was seen
(Koop & Laethem, 1992).
In a study by Pyykkö et al. (1987), Sprague-Dawley male rats were
given ethylbenzene dissolved in corn oil intraperitoneally in a single
dose of 5 mmol/kg (530 mg/kg body weight). The rats were killed 24 h
later and the livers and lungs removed. In the liver 7-ethoxycoumarin-
0-deethylase (a marker of CYP2B1/2) was induced 4-fold; no induction
was found in the lung. Ethylbenzene also induced 7-ethoxyresorufin-
0-deethylase (a marker of CYP1A1/2) both in the liver (4-fold)
and the lung (2.5 fold). These findings are common to many aromatic
solvents.
Alterations in the levels of specific cytochrome P-450 isozymes
were measured by Western immunoblotting techniques using rabbit
anti-rat polyclonal antibodies for cytochrome CYP1A1, CYP2B1 and
CYP2E1 on rat liver microsomes. The rats, male and female Holtzman
rats, were given 10 mmoles ethylbenzene per kg body weight for 3 days
and killed 24 h after the last injection. Ethylbenzene was shown to
induce CYP2B1/2B2 to a greater extent in male rats, while cytochrome
CYP2E1 was only induced in female rats. The level of cytochrome
CYP1A1 was not affected by ethylbenzene (Sequeira et al., 1992).
In another study (Gut et al., 1993), Wistar male rats were
exposed in a dynamic inhalation apparatus to 4 mg ethylbenzene/litre
air, 20 h per day, for 4 days. The rats were then killed and liver
microsomes prepared. By use of Western immunoblotting techniques it
was shown that cytochrome CYP2B1 was induced and the cytochrome CYP2E1
levels were decreased.
In a sensory irritation test, groups of four male mice
(Swiss-Webster) were exposed for 30 min to ethylbenzene at
concentrations of 1.76, 3.7, 8.06, 17.07 or 41.45 g/m3 (410, 860,
1875, 3970 or 9640 ppm). The RD50 value, i.e. the concentration
necessary to depress the respiratory rate by 50%, was calculated to be
17.46 g/m3 (4060 ppm) (95% confidence interval 10.6-28.6 g/m3;
2480-6660 ppm). The respiratory rate decreased due to sensory
irritation of the upper respiratory tract (Damgĺrd Nielsen & Alarie,
1982). In a similar test male mice (Swiss F1) were exposed for about
5 min to different concentrations of ethylbenzene. At least four
different concentrations were used and there were six mice for each
concentration. In this study the RD50 value was calculated to be
6.16 g/m3 (1432 ppm) (De Ceaurriz et al., 1981).
7.9 Factors modifying toxicity
Rats (Sprague-Dawley) were exposed for 2 h to 774 mg/m3 (180
ppm) ethylbenzene. One of two corresponding animals received 20 mmol
ethanol/kg body weight in physiological saline intraperitoneally
before exposure to ethylbenzene. Ethanol enhanced significantly (1.4
fold) the blood levels of inhaled ethylbenzene (Römer et al., 1986).
8. EFFECTS ON HUMANS
8.1 Volunteer studies
A dermal maximization test conducted on 25 volunteers at a
concentration of 10% ethylbenzene in petrolatum produced no skin
sensitization reaction (ECETOC, 1986).
In a review of studies from the 1930s, it was stated that
exposure to 21.5 g/m3 (5000 ppm) ethylbenzene for a few seconds gives
intolerable irritation of nose, eyes and throat. A few seconds of
exposure to 4.3 g/m3 (1000 ppm) initially gives eye irritation which
diminishes after a few minutes of exposure (Damgĺrd Nielsen & Alarie,
1982).
In a study on ethylbenzene metabolism in man it was incidentally
reported that, when the exposure was above the occupational limit
value (8 h; 430 mg/m3,100 ppm), complaints of fatigue, sleepiness,
headache and irritation of the eyes and respiratory tract were
reported (Bardodej & Bardodejová, 1970).
Healthy male subjects were exposed to technical xylene
(containing 20.7% ethylbenzene) for 2 h with or without a 100-watt
workload on an ergometer cycle. The air concentration of technical
xylene was 435 or 1300 mg/m3. During work at the higher exposure
level, evidence of performance decrement was observed in three of the
five performance tests: reaction time addition test (p<0.05),
short-term memory (p<0.05) and choice reaction time (p<0.10)
(Gamberale et al., 1978).
8.2 Occupational exposure
A number of epidemiological studies have been carried out on
groups occupationally exposed to mixtures of solvents, including
ethylbenzene. In these studies it is difficult to attribute the
effects to ethylbenzene or any other single chemical present.
Ethylbenzene occurs in mixed xylenes (at up to 30%) and effects of
occupational exposure to mixed xylenes are usually presented as
effects of xylenes and not of ethylbenzene.
A cross-sectional epidemiological study showed no strong evidence
of adverse neurobehavioural effects in 105 house painters when
compared with 53 workers from various professions (non-painters). The
concentration of ethylbenzene at the workplace was up to 12.9 mg/m3
(3 ppm). Other solvents present were ethyl acetate, toluene, butyl
acetate, methyl isobutyl ketone and xylene. In two neurobehavioural
tests significant differences were found between painters and controls
(the tests were "change of personality" and "short-term memory
capacity"). In a subgroup of painters with repeated prenarcotic
symptoms at the workplace, the differences were more pronounced
(Triebig et al., 1988).
In a study involving 35 spray painters, employed for between 2
and 26 years, erythrocyte and haemoglobin levels were slightly (but
not statistically significantly) lower than those of the controls.
The concentration of ethylbenzene was 17.2 mg/m3 (4 ppm) (Angerer &
Wulf, 1985).
In a medical surveillance report of some 200 ethylbenzene-
production workers, mandelic acid concentrations in urine were
measured twice a year for 20 years. Mandelic acid concentration
in the samples never exceeded 3.25 mmol/litre (=497 mg/litre), and the
mean value was 0.20-0.30 mmol/litre. According to the authors, a
post-shift urine mandelic acid concentration of 6.25 mmol/litre is
equivalent to an air concentration of 200 mg/m3. Therefore, the
measured maximum and mean mandelic acid values were considered to be
equivalent to air ethylbenzene concentrations of about 86 and 8.6
mg/m3 (20 and 2 ppm), respectively. None of the workers examined
over the last 10 years showed any effects on the levels of
haemoglobin, leucocytes or platelets, nor did they have a changed
haematocrit or alanine aminotransferase activity (Bardodej & Círek,
1988).
Minor changes in evoked potential and nerve conduction velocity
were found in 22 workers exposed to ethylbenzene at concentrations of
0.43-17.2 mg/m3 (0.1-4 ppm) for 4-20 years. They were also exposed
to styrene (about 1.5 ppm) (Lu & Zhen, 1989).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Ethylbenzene has been shown to inhibit the respiration of sewage
sludge utilizing biogenic substrates. Two screening tests were used,
RIKA and OECD 209. The concentration of ethylbenzene used was at the
limit of solubility in the medium (approximately 150 mg/litre), and
inhibitions of the respiration rate of 30% (ACCEDE 209) and 100%
(RIKA) were observed (Volskay & Grady, 1990).
Bringmann & Kühn (1980) studied the effect of ethylbenzene on
bacteria. A toxicity threshold of 12 mg/litre for Pseudomonas putida
was obtained in a cell multiplication inhibition test.
9.2 Aquatic organisms
Numerous acute toxicity tests have been carried out on
ethylbenzene. Organisms that have been studied include protozoans
(Bringmann & Kühn, 1980), algae (US EPA, 1978; Bringmann & Kühn, 1980;
Galassi et al., 1988; Masten et al. 1994), water fleas (LeBlanc, 1980;
Bringmann & Kühn, 1982; Abernethy et al., 1986; Galassi et al., 1988;
Vigano, 1993), diatoms (US EPA, 1978; Masten et al., 1994), copepods,
grass shrimp (Potera, 1975), bay shrimps (Benville & Korn, 1977),
mysid shrimps (US EPA, 1978; Masten et al., 1994), Dungeness crabs
(Caldwell et al., 1976) and Pacific oysters (LeGore, 1974). Fish that
have been studied include rainbow trout (Mayer & Ellersieck, 1986;
Galassi et al., 1988), guppy (Pickering & Henderson, 1966; Galassi et
al., 1988), bluegill (Pickering & Henderson, 1966; Buccafusco et al.,
1981), fathead minnow, goldfish (Pickering & Henderson, 1966), channel
catfish (Mayer & Ellersieck, 1986), Atlantic silverside (Masten et
al., 1994), striped bass (Benville & Korn, 1977) and sheepshead minnow
(US EPA, 1978; Heitmuller et al., 1981).
Many of the test results are not comparable, owing to
inconsistent exposure conditions, resulting from emulsions, open
static systems and systems with large air spaces. Aquatic toxicity
results from consistent exposure conditions, which are comparable, are
shown in Table 6.
No information regarding chronic exposure of aquatic organisms to
ethylbenzene has been reported.
Table 6. Toxicity of ethylbenzene to aquatic organisms
Species Age/size Stat/flowa Temperature Salinity pH Parameterc Concentration Reference
(°C) (0/00) (mg/litre)d
Alga stat 72-h EC50 4.6 Galassi et al. (1988)
(Selenastrum stat 19-21 48-h EC50 7.2 (3.4-15.1) Masten et al. (1994)
capricornutum) stat 19-21 96-h EC50 3.6 (1.7-7.6) Masten et al. (1994)
Water flea < 24 h statb 24-h LC50 2.2 Galassi et al. (1988)
(Daphnia magna) statb 21-25 48-h LC50 2.1e Abernethy et al. (1986)
< 24 h statb 48-h LC50 1.81-2.38 Viganň (1993)
Diatom
(Skeletonema statb 19-21 48-h EC50 7.5 (5.0-11.2) Masten et al. (1994)
costatum) statb 19-21 72-h EC50 4.9 (2.4-9.8) Masten et al. (1994)
statb 19-21 96-h EC50 7.7 (5.9-10.0) Masten et al. (1994)
Mysid shrimp < 24 h flow 24-26 20 8.0 48-h LC50 >5.2 Masten et al. (1994)
(Mysidopsis < 24 h flow 24-26 20 8.0 96-h LC50 2.6 (2.0-3.3) Masten et al. (1994)
bahia)
Rainbow trout stat 96-h LC50 4.2 Galassi et al. (1988)
(Oncorhynchus
mykiss)
Guppy stat 96-h LC50 9.2 Galassi et al. (1988)
(Poecilia
reticulata)
Table 6. (Cont'd)
Species Age/size Stat/flowa Temperature Salinity pH Parameterc Concentration Reference
(°C) (0/00) (mg/litre)d
Atlantic silverside 3-15 mg flow 21-23 20 48-h LC50 6.4 (5.8-7.5) Masten et al. (1994)
(Menidia menidia) 3-15 mg flow 21-23 20 96-h LC50 5.1 (4.4-5.7) Masten et al. (1994)
a in all cases a closed system was used; stat = static conditions (water unchanged for the duration of the test);
flow = flow-through conditions (ethylbenzene concentration in water continously maintained)
b air space was eliminated
c the EC50 for algae was based on growth inhibition
d test concentrations were measured, unless stated otherwise
e nominal test concentration;
9.3 Terrestrial organisms
An LC50 value of 47 µg per cm2 of contact area was obtained for
earthworms exposed to ethylbenzene adsorbed on filter paper in glass
vials (Neuhauser et al., 1986). Callahan et al. (1994) reported a
2-day LC50 value of 4.93 µg/kg for Eisenia foetida in a contact
toxicity test.
No toxicity data on plants, birds and wild mammals have been
reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
The acute and chronic toxicities of ethylbenzene are low. The
toxic effects in humans and animals relate to depression of the
central nervous system (CNS) and to irritation of the mucous membranes
and eyes. No data concerning carcinogenic or reproductive effects
have been reported. Ethylbenzene does not have significant mutagenic
properties or teratogenic effects.
Exposure to more than 430 mg/m3 (100 ppm) causes symptoms of CNS
depression and irritation in humans.
A 20-year medical surveillance study of 200 workers showed no
indications of effects in routine blood tests. The maximum exposure,
estimated from the urinary concentration of mandelic acid, was less
than about 86 mg/m3 (20 ppm) and the mean value about 8.6 mg/m3
(2 ppm).
In a 13-week animal study, increased liver weight was the only
dose-related biological finding in male rats. This was seen at a
concentration of 3225 mg/m3 (750 ppm) or more.
The definition and aim of the use of a guidance value for the
general population have been described by IPCS (1994).
On the basis of biological significance criteria cited above, a
no-observed-effect level (NOEL) of 2150 mg/m3 (500 ppm) was defined.
The no-observed-adverse-effect level would be higher than 4300 mg/m3
(1000 ppm) (highest concentration used), since the increase in liver
weight was not associated with any histopathological findings. A NOEL
of 2150 mg/m3 (500 ppm) was used as the basis for determining the
guidance value. The following uncertainty factors were used: 10 for
interspecies variability; 5 for intraspecies variability (effect seen
in males only); and 2 for lack of chronic toxicity data. This gives a
guidance value of 22 mg/m3 (5 ppm).
From the medical surveillance study the NOEL could be estimated
to be between 8.6 mg/m3 (2 ppm) (mean value) and 86 mg/m3 (20 ppm)
(maximum value). However, since no dose-response relationship was
derived, this study is not suitable for the estimation of a guidance
value. Furthermore, no effects would be expected at this exposure
level, which is almost the same as the guidance value.
Humans are exposed to ethylbenzene principally by inhalation,
where the substance is rapidly absorbed into the body. Exposure by
skin absorption or ingestion may also occur. Ethylbenzene is not
considered to bioaccumulate.
Table 7 gives the estimated weekly ethylbenzene dose resulting
from different types of exposure. A guidance value of 22 mg/m3
(5 ppm) ethylbenzene is approximately equivalent to a weekly ethyl-
benzene dose of 2000 mg. This is 100 times higher than the worst
exposure situation of the general population.
Table 7. Estimated weekly ethylbenzene dose from different types of exposures
Type of exposure Reported concentration Weekly total amount Dose mg/week
in media inhaled, ingested
or smoked
General population
Inhalation (means)b 0.74-100 µg/m3 140 m3 0.07-10c
Ingestion (food) 13 µg/kgd 7 kg 0.1
Drinking- or groundwaterb 0.07-1.1 µg/litree 14 litres 0.01
30.0 µg/litref 0.42
Occupational
Inhalation (mean)g 10 mg/m3 300c
Inhalation (max)g 100 mg/m3 50 m3 3000c
Inhalationh 430 mg/m3 12700c
Smokers 8 µg/cigarettei 140 cigarettes/week 1.1
a Based on a breathing rate of 20 m3/day
b Values from Tables 2 and 3
c Retention 60%
d Lovegren et al. (1979) (highest reported value)
e Highest reported values of uncontaminated groundwater
f Lowest reported value of contaminated groundwater
g Bardodej & Círek (1988)
h Corresponds to occupational exposure limits in several countries
i Wallace et al. (1987a)
A 2-year carcinogenicity study has been performed by the National
Toxicology Program (USA) but the results are not yet available.
10.2 Evaluation of effects on the environment
Ethylbenzene is found in air, water, soil, sediment, biota and
groundwater. It is released primarily into air and water from various
natural and anthropogenic sources. The atmosphere is the major sink
for ethylbenzene. Ethylbenzene is rapidly photo-oxidized in the
atmosphere and this may contribute to photo-chemical smog formation.
In water, the key processes determining overall fate are
volatilization and biodegradation.
The log octanol/water partition coefficient is 3.13, indicating a
potential for bioaccumulation. However, the limited evidence
available shows that ethylbenzene bioconcentration factors are low for
fish and molluscs. Elimination from aquatic organisms appears to be
rapid. Biomagnification through the food chain is unlikely.
Mean levels of ethylbenzene in air ranging from 0.74 to 100
µg/m3 have been measured at urban sites. Industrial releases and
vehicle emissions are the principal sources of ethylbenzene. Levels
found at rural sites are generally <2 µg/m3. The levels of
ethylbenzene in surface water are generally less than 0.1 µg/litre in
non-industrial areas. In industrial and urban areas ethylbenzene
concentrations of up to 15 µg/litre have been reported. Urban
run-off, effluent and landfill leachate are sources of local
contamination. Ethylbenzene levels in sediment are generally < 0.5
µg/kg. Levels of ethylbenzene between 1 and 5 µg/kg have been found
in sediments from heavily industrialized areas. Ethylbenzene levels
in uncontaminated groundwater are generally < 0.1 µg/litre. However,
much higher levels have been reported for groundwater contaminated via
waste disposal, fuel spillage and industrial facilities.
Acute toxicity studies on aquatic organisms show ethylbenzene to
be of moderate toxicity. The lowest acute toxicity values are 4.6
mg/litre for algae (72-h EC50), 1.8 mg/litre for daphnids (48-h LC50)
and 4.2 mg/litre for fish (96-h LC50). There are no chronic toxicity
studies on aquatic organisms.
There is limited information regarding the toxicity of
ethylbenzene to bacteria and earthworms. There are no data for
terrestrial plants, birds or wild mammals.
On the basis of available data, it is concluded that ethylbenzene
is unlikely to be found at levels in the environment that will cause
adverse effects on aquatic and terrestrial ecosystems, except in cases
of spills or point-source emissions.
11. CONCLUSIONS
Ethylbenzene has low toxicity. Its vapour irritates the mucous
membranes to a limited extent and causes prenarcotic effects on the
central nervous system.
With respect to the general population, a tentative guidance
value of 22 mg/m3 (5 ppm) for ethylbenzene in inhaled air has been
derived, although information on certain important toxicity end-points
are unavailable. This value would correspond to a weekly absorbed
dose (daily ventilation of 20 m3 with 60% retention) of about 2000
mg. It is at least 200 times higher than the dose received in the
most polluted living environment reported. Hence, no harmful effects
on the general population would be expected. However, it should be
noted that the guidance value of 22 mg/m3 (5 ppm) is about 10 times
higher than the odour threshold (about 2.2 mg/m3; 0.5 ppm), and so
exposure at that level may cause annoyance. On the other hand, odour
detection may be considered to be a safeguard against excessive
exposure.
Ethylbenzene is a non-persistent chemical and is degraded
primarily by photooxidation and biodegradation. Volatilization to the
atmosphere is rapid. Photooxidation reactions of ethylbenzene may
contribute to photochemical smog formation.
Limited evidence suggests that bioaccumulation is low in aquatic
organisms. Ethylbenzene is unlikely to cause adverse effects in
aquatic or terrestrial ecosystems except in cases of spills or
point-source emissions.
12. FURTHER RESEARCH
a) To fill the data gaps that currently limit the toxicological
evaluation of ethylbenzene, an appropriate rodent carcinogenicity
study and a reproductive toxicity study are needed. (The former
study has been conducted but the study report has not yet been
published.)
b) There is little information on the long-term effects of
ethylbenzene in humans and, in particular, no dose-response or
dose-effect data are at hand. Epidemiological studies of
populations occupationally exposed to ethylbenzene should be
encouraged. In this context, the use of ethylbenzene metabolites
in urine as a marker of exposure can be of special value because
the method determines the internal doses that individuals receive
via all routes of exposure. Because ethylbenzene is a central
nervous system depressant, and because some studies suggest that
high doses of the substance or its metabolites may affect the
metabolism of some neurotransmitters in the brain, epidemiological
studies should address the central nervous system as a potential
target organ. Moreover, since ethylbenzene is almost invariably
only one of the components in solvent mixtures at the workplace,
study designs that address possible interactions between
ethylbenzene and other solvents are desirable.
c) Further mechanistic studies are needed.
13. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
A guideline value of 300 µg/litre for ethylbenzene in
drinking-water was recommended by WHO in 1993.
REFERENCES
Abernethy S, Bobra AM, Shiu WY, Wells PG, & Mackay D (1986) Acute
lethal toxicity of hydrocarbons and chlorinated hydrocarbons to two
planktonic crustaceans: The key role of organism-water partitioning.
Aquat Toxicol, 8: 163-174.
ACGIH (1985-1986) Threshold limit values and biological exposure
indices. Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
Aitio A, Riihimäki V, Liesivuori J, Järvisalo J, & Hernberg S (1994)
Biological monitoring. In: Zenz C, Dickerson OB, & Horvath EP Jr ed.
Occupational medicine, 3rd ed. Saint Louis, Missouri, C.V. Mosby &
Co., pp 132-158.
Andersson K, Fuxe K, Nilsen OG, Toftgĺrd R, Eneroth P, & Gustafsson JĹ
(1981) Production of discrete changes in dopamine and noradrenaline
levels and turnover in various parts of the rat brain following
exposure to xylene, ortho-, meta-, and para-xylene, and
ethylbenzene. Toxicol Appl Pharmacol, 60: 535-548.
Angerer J & Lehnert G (1979) Occupational chronic exposure to organic
solvents. VIII. Phenolic compounds-metabolites of alkylbenzenes in
man. Simultaneous exposure to ethylbenzene and xylenes. Int Arch Occup
Environ Health, 43: 145-150.
Angerer J & Wulf H (1985) Occupational chronic exposure to organic
solvents. XI. Alkylbenzene exposure of varnish workers: effects on
hematopoietic system. Int Arch Occup Environ Health, 56: 307-321.
Annable MD, Wallace RB, Hayden NJ, & Voice TC (1993) Reduction of
gasoline component leaching potential by soil venting. J Contam
Hydrol, 12(1-2): 151-170.
Anon (1987) Chemical review: ethylbenzene. Danger Prop Ind Mater Rep,
7: 13-35.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Holler JS, Needham LL, &
Patterson DG Jr (1992) Determining volatile organic compounds in human
blood from a large sample production by using purge and trap gas
chromatography and mass spectrometry. Anal Chem, 64(9): 1021-1029.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, & Wooten JV (1994) Blood
concentrations of volatile organic compounds in a non occupationally
exposed US population and in groups with suspected exposure. Clin
Chem, 40(7): 1401-1404.
Ĺstrand I, Engström J, & Övrum P (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man. Scand J
Work Environ Health, 4: 185-194.
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions
of the hydroxyl radical with organic compounds under atmospheric
conditions. Chem Rev, 85: 69-201.
ATSDR (1990) Toxicological profile for ethylbenzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry (ATSDR/TP/-90-15).
Barber ED, Teetsel NM, Kolberg KF, & Guest D (1992) A comparative
study of the rates of in vitro percutaneous absorption of eight
chemicals using rat and human skin. Fundam Appl Toxicol, 19: 493-497.
Bardodej Z & Bardodejová E (1970) Biotransformation of ethylbenzene,
styrene, and alpha-methylstyrene in man. Am Ind Hyg Assoc J, 31:
206-209.
Bardodej Z & Círek A (1988) Long-term study on workers occupationally
exposed to ethylbenzene. J Hyg Epidemiol Microbiol Immunol, 32: 1-5.
Benville PE Jr & Korn S (1977) The acute toxicity of six monocyclic
aromatic crude oil components to striped bass (Morone saxatilis) and
bay shrimp (Crago franciscorum). Calif Fish Game, 63: 204-209.
Bestetti G & Galli E (1984) Plasmid-coded degradation of ethylbenzene
and 1-phenylethanol in Pseudomonas fluorescens. FEMS Microbiol Lett,
21: 165-168.
Bevan MAJ, Proctor CJ, Baker-Rogers J, & Warren ND (1991) Exposure to
carbon monoxide, respirable suspended particulates and volatile
organic compounds while commuting by bicycle. Environ Sci Technol,
25: 788-791.
Bos R, Guicherit R, & Hoogeveen A (1977) Distribution of some
hydrocarbons in ambient air near Delft and the influence on the
formation of secondary air pollutants. Sci Total Environ, 7: 269-281.
Boyd SA, Xiangcan J, & Lee JF (1990) Sorption of nonionic organic
compounds by corn residues from a no-tillage field. J Environ Qual,
19(4): 734-738.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1982) [Findings concerning the harmful effect
of water pollutants on Daphnia magna in a further developed
standardized test procedure.] Z Wasser/Abwasser Forsch, 15(1): 1 (in
German).
Brown-Woodman PDC, Webster WS, Picher K, & Ritchie HE (1991)
Embryotoxicity of xylene and toluene: an in vitro study. Ind Health,
29: 139-152.
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other trace
compounds in urban air. Chemosphere, 17: 2363-2380.
Buccafusco RJ, Ells SJ, & LeBlanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Burghardt E & Jeltes R (1975) Gas chromatographic determination of
aromatic hydrocarbons in air using a semi-automatic preconcentration
method. Atmos Environ, 9: 935-940.
Burnham AK, Calder GV, Fritz JS, Junk GA, Svec HJ, & Willis R (1972)
Identification and estimation of neutral organic contaminants in
potable water. Anal Chem, 44: 139-142.
Bysshe SE (1982) Bioconcentration factors in aquatic organisms. In:
Lyan WJ, Reehl WF, & Rosenblatt DH ed. Handbook of chemical property
estimation methods - Environmental behaviour of organic compounds. New
York, McGraw-Hill Book Company, pp 5/1-5/30.
Caldwell RS, Caldarone EM, & Mallon MH (1976) Effects of a seawater-
soluble fraction of Cook Inlet crude oil and its major aromatic
components on larval stages of the Dungeness crab, Cancer
magister dana. In: Fate and effects of petroleum hydrocarbon in
marine organisms and ecosystems. Oxford, New York, Pergamon Press, pp
210-220.
Callahan CA, Shirazi MA, & Neuhauser EF (1994) Comparative toxicity of
chemicals to earthworms. Environ Toxicol Chem, 13(2): 291-298.
Callahan MA (1979) Water related environmental fate of 129 priority
pollutants. Washington, DC, US Environmental Protection Agency (EPA
440/4-79-029b).
Cavender F (1993) Aromatic hydrocarbons. In: Clayton GD & Clayton FE
ed. Patty's industrial hygiene and toxicology, 4th revis ed. New
York, John Wiley and Sons, vol 2B, pp 1267-1442.
Chan CC, Özkaynak H, Spengler JD, & Sheldon L (1991a) Driver exposure
to volatile organic compounds, CO, ozone and NO2 under different
driving conditions. Environ Sci Technol, 25: 964-972.
Chan CC, Spengler JD, Özkaynak H, & Lefkopoulou M (1991b) Commuter
exposures to VOCs in Boston, Massachusetts. J Air Waste Manage Assoc,
41(12): 1594-1600.
Chin BH, McKelvey JA, Tyler TR, Calisti LJ, Kozbelt SJ, & Sullivan LJ
(1980a) Absorption, distribution, and excretion of ethylbenzene,
ethylcyclohexane and methylethylbenzene isomers in rats. Bull Environ
Contam Toxicol, 24: 477-483.
Chin BH, McKelvey JA, Calisti LJ, Kozbelt SJ, & Sullivan LJ (1980b) A
comparison of in vivo and in vitro (tissue explant) techniques:
metabolic profile of ethylbenzene in the rat and the dog. Bull Environ
Contam Toxicol, 25: 241-245.
Chiou CT, Porter PE, & Schmedding DW (1983) Partition equilibria of
non-ionic organic compounds between soil organic matter and water.
Environ Sci Technol, 17: 227-231.
Chou WL, Speece RE, & Siddiqi RH (1978) Acclimation and degradation of
petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
CITI (1992) Biodegradation and bioaccumulation data on existing
chemicals based on the CSCL Japan. Tokyo, Chemicals Inspection and
Testing Institute, pp 3-9.
Clapp LW, Talarczyk MR, Park JK, & Boyle WC (1994) Performance
comparison between activated sludge and fixed-film processes for
priority pollutant removals. Water Environ Res, 66(2): 153-160.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984a) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural and motorway locations.
Environ Pollut, B7: 141-158.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984b) Ambient air
measurements of aromatic and halogenated hydrocarbons at urban, rural
and motorway locations. Sci Total Environ, 39: 265-279.
Climie IJG, Hutson DH, & Stoydin G (1983) The metabolism of
ethylbenzene hydroperoxide in the rat. Xenobiotica, 13: 611-618.
Cline PV & Viste DR (1985) Migration and degradation patterns of
volatile organic compounds. Waste Manage Res, 3: 351-360.
Cole RH, Frederick RE, Healy RP, & Rolan RG (1984) Preliminary
findings of the priority pollutant monitoring project of the
nationwide urban runoff program. J Water Pollut Control Fed,
56: 898-908.
Coleman WE, Munch JW, Streicher RP, Ringhand HP, & Kopfler FC (1984)
Identification and measurement of components in gasoline, kerosene and
No.2 fuel oil that partition into the aqueous phase after mixing. Arch
Environ Contam Toxicol, 13: 171-178.
Cotruvo JA (1985) Organic micropollutants in drinking water: An
overview. Sci Total Environ, 47: 7-26.
Cox DP & Goldsmith CD (1979) Microbial conversion of ethylbenzene to
1-phenylethanol and acetophenone by Nocardia tartaricans ATCC 31190.
Appl Environ Microbiol, 38: 514-520.
Cozzarelli IM, Eganhouse RP, & Baedecker MJ (1990) Transformation of
monoaromatic hydrocarbons to organic acids in anoxic groundwater
environment. Environ Geol Water Sci, 16: 135-141.
Cragg ST, Clarke EA, Daly IW, Miller RR, Terrill JB, & Ouellette RE
(1989) Subchronic inhalation toxicity of ethylbenzene in mice, rats,
and rabbits. Fundam Appl Toxicol, 13: 399-408.
Crookes MJ & Howe PD (1992) Environmental hazard assessment:
Ethylbenzene. Garston, United Kingdom, Department of the Environment,
Directorate for Air, Climate and Toxic Substances, Toxic Substances
Division, Public Building Research Establishment, 40 pp (TSD/7).
Curvall M, Enzell CR, & Pettersson B (1984) An evaluation of the
utility of four in vitro short term tests for predicting the
cytotoxicity of individual compounds derived from tobacco smoke. Cell
Biol Toxicol, 1: 173-193.
Damgĺrd Nielsen GD & Alarie Y (1982) Sensory irritation, pulmonary
irritation and respiratory stimulation by airborne benzene and
alkylbenzenes: Prediction of safe industrial exposure levels and
correlation with their thermodynamic properties. Toxicol Appl
Pharmacol, 65: 459-477.
Dannecker W, Schröder B, & Stechmann H (1990) Organic and inorganic
substances in highway tunnel exhaust air. Sci Total Environ, 93:
293-300.
Dean BJ, Brooks TM, Hodson-Walker G, & Hutson DH (1985) Genetic
toxicology testing of 41 industrial chemicals. Mutat Res, 153: 57-77.
De Bortoli M, Knöppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1984) Integrating 'real life' measurements of
organic pollution in indoor and outdoor air of homes in Northern
Italy. In: Proceedings of the 3rd International Conference on Indoor
Air Quality and Climate. Stockholm, Swedish Council for Building
Research, vol 4, pp 21-26.
DEC (1992) Health-based recommended occupational exposure limit for
ethylbenzene. The Hague, Directorate-General of Labour.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
DFG (German Research Council) (1985) [Materials hazardous to health.
Justification of MAC values in terms of toxicology and occupational
medicine], 11th ed. Weinheim, VCH Publishers (in German).
Dills RL, Kent SD, Checkoway H, & Kalman DA (1991) Quantification of
volatile solvents in blood by static headspace analysis. Talanta,
38: 365-374.
Donner M, Mäki-Paakkanen J, Norppa H, Sorsa M, & Vainio H (1980)
Genetic toxicology of xylenes. Mutat Res, 74: 171-172.
Dreisch FA & Munson TO (1983) Purge-and-trap analysis using fused
silica capillary column GC/MS. J Chromatogr Sci, 21: 111-118.
Drummond L, Caldwell J, & Wilson HK (1989) The metabolism of
ethylbenzene and styrene to mandelic acid: stereo chemical
considerations. Xenobiotica, 19: 199-207.
Drummond L, Caldwell J, & Wilson HK (1990) The stereo selectivity of
1,2-phenylethanediol and mandelic acid metabolism and disposition in
the rat. Xenobiotica, 20: 159-168.
Durst GL & Laperle EA (1990) Styrene monomer migration as monitored by
purge and trap gas chromatography and sensory analysis for polystyrene
containers. J Food Sci, 55(2): 522-524.
Dutkiewicz T & Tyras H (1967) A study of the skin absorption of
ethylbenzene in man. Br J Ind Med, 24: 330-332.
EAJ (1989) Chemicals in the environment. Tokyo, Environment Agency of
Japan.
ECETOC (1986) Joint assessment of commodity chemicals No.7:
Ethylbenzene. Brussels, European Chemical Industry Ecology and
Toxicology Centre.
El Masry AM, Smith JN, & Williams RT (1956) Studies in detoxication.
69. The metabolism of alkylbenzenes: n-propylbenzene and
n-butylbenzene with further observations on ethylbenzene. Biochem J,
64: 50-56.
Elovaara E, Engström K, Nickels J, Aitio A, & Vainio H (1985)
Biochemical and morphological effects of long-term inhalation exposure
of rats to ethylbenzene. Xenobiotica, 15: 299-308.
Engström KM (1984a) Urinalysis of minor metabolites of ethylbenzene
and m-xylene. Scand J Work Environ Health, 10: 75-81.
Engström KM (1984b) Metabolism of inhaled ethylbenzene in rats. Scand
J Work Environ Health, 10: 83-87.
Engström J & Bjurström R (1978) Exposure to xylene and ethylbenzene.
II. Concentration in subcutaneous adipose tissue. Scand J Work Environ
Health, 4: 195-203.
Engström KM & Rantanen J (1974) A new gas chromatographic method for
determination of mandelic acid in urine. Int Arch Arbeitsmed,
33: 163-167.
Engström KM, Riihimäki V, & Laine A (1984) Urinary disposition of
ethylbenzene and m-xylene in man following separate and combined
exposure. Int Arch Occup Environ Health, 54: 355-363.
Engström KM, Elovaara E, & Aitio A (1985) Metabolism of ethylbenzene
in the rat during long-term intermittent inhalation exposure.
Xenobiotica, 15: 281-286.
Feiler HD, Vernick AS, & Storch PJ (1979) Fate of priority pollutants
in POTW's. In: Proceedings of the 8th National Conference on Municipal
Sludge Management, pp 72-81.
Fellin P & Otson R (1993) Seasonal trends of volatile organic
compounds (VOCs) in Canadian homes. In: Proceedings of the 6th
Conference on Indoor Air Quality and Climate, Helsinki, 1993, vol 2,
pp 117-122.
Filipovic D, Paulsen MD, Loida PJ, Sligar SG, & Ornstein RL (1992)
Ethylbenzene hydroxylation by cytochrome P450cam. Biochem Biophys Res
Commun, 189: 488-495.
Fishbein L (1985) An overview of environmental and toxicological
aspects of aromatic hydrocarbons. IV. Ethylbenzene. Sci Total Environ,
44: 269-287.
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15: 219-232.
Först C, Stieglitz L, Roth W, & Kuhnmünch S (1984) Quantitative
analysis of volatile organic compounds in landfill leachates. Int J
Environ Anal Chem, 37: 287-293.
Frank H, Senf L, & Welsch T (1990) Some application of wide bore
capillary gas chromatography in occupational medicine. Z Gesamte Hyg
Grenzgeb, 36(12): 668-671.
Gamberale F, Annwall G, & Hultengren M (1978) Exposure to xylene and
ethylbenzene. III. Effects on central nervous functions. Scand J Work
Environ Health, 4: 204-211.
Galassi S, Mingazzini M, Vigano L, Cesareo D, & Tosato ML (1988)
Approaches to modelling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicol Environ Saf, 16: 158-169.
Garcia JP, Beyne-Masclet S, Mouvier G, & Masclet P (1992) Emissions of
volatile organic compounds by coal-fire power stations. Atmos Environ,
A26(9): 1589-1597.
Gentry SJ & Walsh PT (1987) Eight-hour TWA personal monitoring using a
diffusive sampler and short-term stain tube. Am Ind Hyg Assoc J,
48: 287-292.
Gibson DT, Gschwendt B, Yeh WK, & Kobal VM (1973) Initial reactions in
the oxidation of ethylbenzene by Pseudomonas putida. Biochemistry,
12: 1520-1528.
Gomez-Belinchon JI, Grimalt JO, & Abaigčs J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water Res, 25: 577-589.
Goodenkauf O & Atkinson JC (1986) Occurrence of volatile organic
chemicals in Nebraska ground water. Ground Water, 24: 231-233.
Gossett RW, Brown DA, & Young DR (1983) Predicting the bioaccumulation
of organic compounds in marine organisms using octanol/water partition
coefficients. Mar Pollut Bull, 14: 387-392.
Grob K (1973) Organic substances in potable water and in its
precursor. Part 1. Methods for their determination by gas-liquid
chromatography. J Chromatogr, 84: 255-273.
Grob K & Grob G (1971) Gas-liquid chromatographic-mass spectrometric
investigation of C6-C20 organic compounds in an urban atmosphere
- An application of ultra trace analysis on capillary columns. J
Chromatogr, 62: 1-13.
Gromiec JP & & Piotrowski JK (1984) Urinary mandelic acid as an
exposure test for ethylbenzene. Int Arch Occup Environ Health,
55: 61-72.
Gschwend PM, Zafiriou OC, Mantoura RFC, Schwarzenbach RP, & Gagosian
RB (1982) Volatile organic compounds at a coastal site. I. Seasonal
variations. Environ Sci Technol, 16: 31-38.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gut I, Terelius Y, Frantik E, Linhart I, Soucek P, Filipcová B, &
Klucková H (1993) Exposure to various benzene derivatives
differentially induces cytochromes P450 2B1 and P450 2E1 in rat liver.
Arch Toxicol, 67: 237-243.
Hajimiragha H, Ewers U, Brockhaus A, & Boettger A (1989) Levels of
benzene and other volatile aromatic compounds in the blood of
non-smokers and smokers. Int Arch Occup Environ Health, 61: 513-518.
Hampton CV, Pierson WR, Schuetzle D, & Harvey TM (1983) Hydrocarbon
gases emitted from vehicles on the road. 2. Determination of emission
rates from diesel and spark-ignition vehicles. Environ Sci Technol,
17: 699-708.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(Suppl 4): 66-75.
Harkov R, Kebbekus B, Bozzelli JW, & Lioy PJ (1983) Measurement of
selected volatile organic compounds at three locations in New Jersey
during the summer season. J Air Pollut Control Assoc, 33: 1177-1183.
Harper M & Purnell CJ (1990) Alkylammonium montmorillonites as
adsorbents for organic vapors from air. Environ Sci Technol,
24: 55-62.
Heilbron I, Bunbury HM, & Jones WE ed. (1946) Dictionary of organic
compounds. London, vol II, p 20.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Herman DC, Inniss WE, & Mayfield CI (1990) Impact of volatile aromatic
hydrocarbon, alone or in combination, on growth of the freshwater alga
Selenastrum capriocornutum. Aquat Toxicol, 18(2): 87-100.
Hester NE & Meyer RA (1979) A sensitive technique for measurement of
benzene and alkylbenzenes in air. Environ Sci Technol, 13: 107-109.
Hiatt MH (1981) Analysis of fish and sediment for volatile priority
pollutants. Anal Chem, 53: 1541-1543.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography-mass spectrometry. Anal Chem, 55: 506-516.
Hodgson AT, Daisey JM, & Grot RA (1991) Sources and source strengths
of volatile organic compounds in a new office building. J Air Waste
Manage Assoc, 41(11): 1461-1468.
Holm S, Norbäck D, Frenning B, & Göthe CJ (1987) Hydrocarbon exposure
from handling jet fuel of some Swedish aircraft units. Scand J Work
Environ Health, 13: 438-444.
Holmberg B & Malmfors T (1974) The cytotoxicity of some organic
solvents. Environ Res, 7: 183-192.
Howard PH (1989) Handbook of environmental fate and exposure data for
organic chemicals. Volume 1: Large production and priority pollutants.
Chelsea, Michigan, Lewis Publishers, Inc.
Howard PH (1991) Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers, Inc., pp 340-341.
Hunt WF Jr, Faoro RB, & Freas W (1986) Report of the interim data base
for state and local air toxic volatile organic chemical measurements.
Washington, DC, US Environmental Protection Agency (EPA 450/4-86-012).
Hutchins SR, Sewell GW, Kovacs DA, & Smith GA (1991a) Biodegradation
of aromatic hydrocarbons by aquifer microorganisms under denitrifying
conditions. Environ Sci Technol, 25: 68-76.
Hutchins SR, Downs WC, Wilson JT, Smith GB, Kovacs DA, Fine DD,
Douglass RH, & Hendrix DJ (1991b) Effect of nitrate addition on
biotransformation of fuel-contaminated aquifer: Field demonstration.
Ground Water, 29(4): 571-580.
Inoue O, Seiji K, Kudo S, Jin C, Cai SX, Liu SJ, Watanabe T, Nakatsuka
H, & Ikeda M (1995) Urinary phenylglyoxylic acid excretion after
exposure to ethylbenzene among exposed Chinese workers. Int J Occup
Environ Health, 1: 1-8.
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: Assessing human health risks of chemicals:
Derivation of guidance values for health-based exposure limits.
Geneva, World Health Organization, 73 pp.
Jayanty RKM (1989) Evaluation of sampling and analytical methods for
monitoring toxic organics in air. Atmos Environ, 23: 777-782.
Jaynes WF & Boyd SA (1991) Clay mineral type and organic compound
sorption by hexadecyltrine-thylammonium-exchanged clays. Soil Sci Soc
Am J, 55(1): 43-48.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and in vehicle emissions. Environ Int, 11: 383-392.
Jüttner F (1988) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in forest air of the Southern Black
Forest. Chemosphere, 17: 309-317.
Kappeler T & Wuhrmann K (1978a) Microbial degradation of the
water-soluble fraction of gas oil, I. Water Res, 12: 327-333.
Kappeler T & Wuhrmann K (1978b) Microbial degradation of the
water-soluble fraction of gas oil. II. Bioassays with pure strains.
Water Res, 12: 335-342.
Kawai T, Yasugi T, Mizunuma K, Horiguchi S. Iguchi H, Uchida Y, Iwami
O, & Ikeda M (1992) Comparative evaluation of urinalysis and blood
analysis as means of detecting exposure to organic solvents at low
concentrations. Int Arch Occup Environ Health, 64(4): 223-234.
Kawamura K & Kaplan IR (1983) Organic compounds in the rainwater of
Los Angeles. Environ Sci Technol, 17: 497-501.
Kawata K & Fujeda Y (1993) Volatile aromatic hydrocarbons in ambient
air. Jpn J Toxicol Environ Health, 39(1): 37-43.
Kennicutt MC II, Brooks JM, & McDonald TJ (1984) Volatile organic
inputs from an ocean outfall near Barceloneta, Puerto Rico.
Chemosphere, 13: 535-548.
Kenrick MAP, Clark L, Baxter KM, Fleet M, Jones HA, Gibson TM, &
Turrell MB (1985) Trace organics in British aquifers - a baseline
survey. Water Research Centre, Environment (TR No. 223).
Keymeulen R, Van-Langenhove H, & Schamp N (1991) Determination of
monocyclic aromatic hydrocarbons in plant cuticles by gas
chromatography-mass spectrometry. J Chromatogr, 541(1-2): 83-88.
Kiese M & Lenk W (1974) Hydroxyacetophenones: urinary metabolites of
ethylbenzene and acetophenone in the rabbit. Xenobiotica, 4: 337-343.
Kleopfer RD & Fairless BJ (1972) Characterization of organic
components in a municipal water supply. Environ Sci Technol,
6: 1036-1037.
Koop DR & Laethem CL (1992) Inhibition of rabbit microsomal cytochrome
P-450 2E1-dependent p-nitrophenol hydroxylation by substituted benzene
derivatives. Drug Metab Dispos, 20: 775-777.
Korn M, Gfrörer W, Herz R, Wodarz I, & Wodarz R (1992)
Stereometabolism of ethylbenzene in man: gas chromatographic
determination of urinary excreted mandelic acid enantiomers and
phenylglyoxylic acid and their relation to the height of occupational
exposure. Int Arch Occup Environ Health, 64: 75-78.
Korte F & Boedefeld E (1978) Ecotoxicological review of the global
impact of the petroleum industry and its products. Ecotoxicol Environ
Saf, 2: 55-103.
Kroneld R (1989) Volatile pollutants in suburban and industrial air.
Bull Environ Contam Toxicol, 42: 868-872.
Kuhn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Labows J, Preti G, Hoelzle E, Leyden J, & Klingman A (1979) Analysis
of human axillary volatiles: Compounds of exogenous origin. J
Chromatogr, 163: 294-299.
Lanzerstorfer CH & Puxbaum H (1990) Volatile hydrocarbons in and
around Vienna, Austria. Water Air Soil Pollut, 51: 345-355.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lee JF, Crum JR, & Boyd SA (1989) Enhanced retention of organic
contaminants by soils exchanged with organic cations. Environ Sci
Technol, 23: 1365-1372.
LeGore RS (1974) The effect of Alaskan crude oil and selected
hydrocarbon compounds on embryonic development of the pacific oyster,
Crassostrea gigas. Doctoral Thesis, University of Washington. Diss
Abstr, B35: 3168.
Lesage S, Jackson RE, Priddle MW, & Riemann PG (1990) Occurrence and
fate of organic solvent residues in anoxic groundwater at the
Gloucester Landfill, Canada. Environ Sci Technol, 24: 559-566.
Levesque P & Dao LH (1989) Alkylation of benzene using an aqueous
solution of ethanol. Appl Catal, 53(2-3): 157-167.
Lewis PJ, Hagopian C, & Koch P (1983) Styrene. In: Kirk RE & Othmer DF
ed. Encyclopedia of chemical technology, 3rd ed. New York, Wiley
Interscience, vol 21, pp 770-801.
Lockhart WL, Wagemann R, Tracey B, Sutherland D, & Thomas DJ (1992)
Presence and implications of chemical contaminants in the freshwaters
of the Canadian Arctic. Sci Total Environ, 122(1/2): 165-243
Louw CW, Richards JF, & Faure PK (1977) Determination of volatile
organic compounds in city air by gas chromatography combined with
standard addition, selective subtraction, infra red spectrometry and
mass spectrometry. Atmos Environ, 11: 703-717.
Lovegren NV, Fisher GS, Legendre MG, & Schuller WH (1979) Volatile
constituents of dried legumes. J Agric Food Chem, 27: 851-853.
Lu BQ & Zhen ZH (1989) [Health standards for ethylbenzene in the air
of workplaces.] Beijing, Chinese Academy of Preventive Medicine,
Institute of Occupational Medicine (in Chinese).
Mabey W, Smith JH, Podoll, RT, Johnson HL, Mill T, Chou TW, Gate J,
Waight-Partridge I, Jaber H, & Vandenberg D (1982) Aquatic fate
process for organic priority pollutants. Washington, DC, US
Environmental Protection Agency (EPA 440/481-14).
McFall JA, Antoine SR, & DeLeon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14: 1253-1265.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma
cell forward mutation assay: III. 72 coded chemicals. Environ Mol
Mutagen, 12: 85-154.
Mackay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to atmosphere. Environ Sci Technol,
9: 1178-1180.
Mackay D, Shiu WY, & Sutherland RP (1979) Determination of air-water
Henry's law constants for hydrophobic pollutants. Environ Sci Technol,
13: 333-337.
Mackay D, Shiu WY, & Ma KC (1992) The illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Volume 1. Monoaromatic hydrocarbons, chlorobenzenes and
polychlorinated biphenyls. Chelsea, Michigan, Lewis Publishers, Inc.
MacLeod G & Ames JM (1987) Effect of water on the production of cooked
beef aroma compounds. J Food Sci, 52: 42-45.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
Current results and ongoing research. Am J Ind Med, 7: 415-446.
Masten LW, Boeri RL & Walker JD (1994) Strategies employed to
determine the acute aquatic toxicity of ethyl benzene, a
highly-volatile, poorly water-soluble chemical. Ecotoxicol Environ
Saf, 27: 335-348.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, p 460 (Resource Publication No. 160).
Michael LC, Pellizzari ED, & Norwood DL (1991) Application of the
master analytical scheme to the determination of volatile organics in
wastewater influent and effluents. Environ Sci Technol, 25: 150-155
Molnár J, Paksy KA, & Náray M (1986) Changes in the rat's motor
behaviour during 4-hour inhalation exposure to prenarcotic
concentrations of benzene and its derivatives. Acta Physiol Hung,
67: 349-354.
Morgan DL, Cooper SW, Carlock DL, Sykora JJ, Sutton B, Mattie DR, &
McDougal JN (1991) Dermal absorption of neat and aqueous volatile
organic chemicals in the Fischer 344 rat. Environ Res, 55: 51-63.
Mott JG & Romanow S (1991/1992) Sludge characterization, removal and
dewatering. J Hazard Mater, 29(1): 127-140.
Murray DAJ & Lockhart WL (1981) Microextraction and gas chromatographic
analysis of selected petroleum hydrocarbons in water and fish tissue.
J Chromatogr, 212: 305-311.
Namkung E & Rittmann BE (1987) Estimating volatile organic compound
emissions from publicly owned treatment works. J Water Pollut Control
Fed, 59: 670-678.
Nelson PF & Quigley SM (1982) Non-methane hydrocarbons in the
atmosphere of Sydney, Australia. Environ Sci Technol, 16: 650-655.
Neuhauser EF, Loehr RC, & Malecki MR (1986) Contact and artificial
soil tests using earthworms to evaluate the impact of wastes in soil.
In: Petros JK, Lacy WJ, & Conway RA end. Hazardous and industrial
solid waste testing: Fourth symposium. Philadelphia, Pennsylvania,
American Society for Testing and Materials, pp 192-203 (ASTM STP 886).
NIOSH (1984) Method 1501. In: Manual of analytical methods, 3rd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 2.
Noleau I & Toulemonde B (1988) Volatile components of roasted guinea
hen. Lebensm.Wiss Technol, 21: 195-197.
Norppa H & Vainio H (1983) Induction of sister-chromatid exchanges by
styrene analogues in cultured human lymphocytes. Mutat Res,
116: 379-387.
NTP (1992) Toxicity studies of ethylbenzene in F344/N rats and B6C3F1
mice (inhalation studies). Research Triangle Park, North Carolina, US
Department of Health and Human Services, National Toxicology Program
(NIH Publication No. 92-3129).
Nunes P & Benville PE Jr (1979) Uptake and depuration of petroleum
hydrocarbons in the Manila clam, Tapes semidecussata Reeve. Bull
Environ Contam Toxicol, 21:719-726.
Ogata M, Sugihara R, & Kira S (1977) Quantitative determination of
urinary hippuric acid and m- or p-methylhippuric acid as indices
of toluene and m- or p-xylene exposure by high performance liquid
chromatography. Int Arch Occup Environ Health, 39: 199-206.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities. J
Assoc Off Anal Chem, 65: 1370-1374.
Pankow JF, Isabelle LM, & Asher WE (1984) Trace organic compounds in
rain. I. Sampler design and analysis by adsorption/thermal desorption
(ATD). Environ Sci Technol, 18: 310-318.
Pellizzari ED, Hartwell TD, Harris BSH, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pellizzari ED, Sheldon L, Sparacino C, Bursey J, Wallace L, & Bromberg
S (1984) Volatile organic levels in indoor air. In: Berglund B,
Lindvall T, & Sundell J ed. Indoor air, chemical characterization and
personal exposure. Stockholm, Swedish Council for Building Research,
vol 4, pp 21-26.
Pellizzari ED, Wallace LA, & Gordon SM (1992) Elimination kinetics of
volatile organics in humans using breath measurements. J Expo Anal
Environ Epidemiol, 2: 341-355.
Perry DL, Chuang CC, Jungclaus GA, & Warner JS (1979) Identification
of organic compounds in industrial effluent discharges. Washington,
DC, US Environmental Protection Agency (EPA 600/4-79-016).
Petersson G (1982) Ambient hydrocarbons from motor-car assembly plants
in Scandinavia. Environ Pollut, B4: 207-217.
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38: 1419-1429.
Pitter P & Chudoba J (1990) Biodegradability of organic substances in
the aquatic environment. Boca Raton, Florida, CRC Press, Inc., p 269.
Potera FT (1975) The effects of benzene, toluene and ethylbenzene on
several important members of the estuarine ecosystem. Lehigh
University (Ph.D. dissertation).
Pussemier L, Szabó G, & Bulman RA (1990) Prediction of the soil
adsorption coefficient Koc for aromatic pollutants. Chemosphere
21: 1199-1212.
Pyykkö K, Paavilainen S, Metsä-Ketelä T, & Laustiola K (1987) The
increasing and decreasing effects of aromatic hydrocarbon solvents on
pulmonary and hepatic cytochrome P-450 in the rat. Pharmacol Toxicol,
60: 288-293.
Radzikowska-Kintzi H & Jakubowski M (1981) Internal standardization in
the head space analysis of organic solvents in blood. Int Arch Occup
Environ Health, 49: 115-123.
Rao PSC, Hornsby AG, & Jessup RE (1985) Indices for ranking the
potential for pesticide contamination of groundwater. Soil Crop Sci
Soc Proc, 44: 1-8.
Rappaport SM, Selvin S, & Waters MA (1987) Exposures to hydrocarbon
components of gasoline in the petroleum industry. Appl Ind Hyg,
2: 148-154.
Reinhard M, Goodman NL, & Barker JF (1984) Occurrence and distribution
of organic chemicals in two landfill leachate plumes. Environ Sci
Technol, 18: 953-961.
Rhue RD, Pennell KD, Rao PS, & Reve WH (1989) Competitive adsorption
of alkylbenzene and water vapors on predominantly mineral surfaces.
Chemosphere, 18(9-10): 1971-1986.
Romanelli A, Falzoi M, Mutti A, Bergamaschi E, & Franchini I (1986)
Effects of some monocyclic aromatic solvents and their metabolites on
brain dopamine in rabbits. J Appl Toxicol, 6: 431-435.
Römer KG, Federsel RJ, & Freundt KJ (1986) Rise of inhaled toluene,
ethyl benzene, m-xylene, or mesitylene in rat blood after treatment
with ethanol. Bull Environ Contam Toxicol, 37: 874-876.
Rosen AA, Skeel RJ, & Ettinger MB (1963) Relationship of river water
odour to specific organic contaminants. J Water Pollut Control Fed,
35: 777-782.
Roy WR & Griffin RA (1985) Mobility of organic solvents in
water-saturated soil material. Environ Geol Water Sci, 7: 241-247.
SAC (1989) Analysis of potentially dangerous substances in UK
waters. Bedford, United Kingdom, SAC Scientific (DOE Reference
No. PECD-7/7/306).
Sato A & Nakajima T (1987) Pharmacokinetics of organic solvent vapors
in relation to their toxicity. Scand J Work Environ Health, 13: 81-93.
Sauer TC Jr (1981) Volatile liquid hydrocarbon characterization of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environ Sci Technol, 15: 917-923.
Sax NI (1979) Dangerous properties of industrial chemicals, 5th ed.
New York, Van Nostrand Reinhold Co.
Seader JD (1982) Separation systems synthesis. In: Kirk RE & Othmer DF
ed. Encyclopedia of chemical technology, 3rd ed. New York, Wiley
Interscience, vol 20, pp 710-711.
Sequeira DJ, Eyer CS, Cawley GF, Nick TG, & Backes WL (1992)
Ethylbenzene-mediated induction of cytochrome P450 isozymes in male
and female rats. Biochem Pharmacol, 44: 1171-1182.
Sexton K & Westberg H (1980) Ambient hydrocarbon and ozone
measurements downwind of a large automotive painting plant. Environ
Sci Technol, 14: 329-332.
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environ Sci Technol, 16: 872-880.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1983) Measurements of
hazardous organic chemicals in the ambient atmosphere. Washington, DC,
US Environmental Protection Agency (EPA 600/3-83-002).
Singh HB, Ferek RJ, Salas LJ, & Nitz KC (1986) Toxic chemicals in the
environment: A program of field measurements. Washington, DC, US
Environmental Protection Agency (EPA 600/3-86/047).
Smith MR & Ratledge C (1989) Catabolism of alkylbenzenes by
Pseudomonas sp. NCIB 10643. Appl Microbiol Biotechnol, 32: 68-75.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: List VI. Am Ind Hyg Assoc J, 23: 95-107.
Sollenberg J (1991) Analytical isotachophoresis in biological
monitoring of exposure to industrial chemicals. J Chromatogr,
545: 369-374.
Sollenberg J, Smallwood AW, & Lowry LK (1985) Determination of
mandelic and phenylglyoxylic acids in rat urine by high-performance
liquid chromatography and by isotachophoresis. J Chromatogr,
343: 175-178.
Staples CA, Werner AF, & Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environ Toxicol Chem, 4(2): 131-142.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near to an underground coal gasification site in
North-Eastern Wyoming. Environ Sci Technol, 16: 582-587.
Susten AS, Niemeier RW, & Simon SD (1990) In vivo percutaneous
absorption studies of volatile organic solvents in hairless mice. II.
Toluene, ethylbenzene and aniline. J Appl Toxicol, 10: 217-225.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tester DJ & Harker RJ (1981) Groundwater pollution investigations in
the Great Ouse basin. Water Pollut Control, 80: 614-631.
Thomann RV (1989) Bioaccumulation model of organic chemical
distribution in aquatic food chains. Environ Sci Technol,
23(6): 699-707.
Thomas JM, Gordy VR, Fiorenza S, & Ward CH (1990) Biodegradation of
BTEX in subsurface materials contaminated with gasoline: Granger,
Indiana. Water Sci Technol, 22: 53-62.
Thomas RG (1982) Volatilization. In: Lyman WJ, Reehl WF, & Rosenblatt
DH ed. Handbook of chemical properties: Estimation methods. New York,
McGraw-Hill Book Co.
Thorburn S & Colenutt BA (1979) A gas chromatographic comparison of
volatile organic compounds in urban and rural atmospheres. Int J
Environ Stud, 13: 265-271.
Triebig G, Claus D, Csuzda I, Druschky KF, Holler P, Kinzel W, Lehrl
S, Reichwein P, Weidenhammer W, Weitbrecht WU, Weltle D, Schaller KH,
& Valentin H (1988) Cross-sectional epidemiological study on
neurotoxicity of solvents in paints and lacquers. Int Arch Occup
Environ Health, 60: 233-241.
Tsuruta H (1982) Percutaneous absorption of organic solvents. III. On
the penetration rates of hydrophobic solvents through the excised rat
skin. Ind Health, 20: 335-345.
Ullman (1983) [Encyclopaedia of chemical technology.] Weinheim, Verlag
Chemie, vol 24, pp 525-544 (in German).
Ungváry G & Tátrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats, and rabbits. Arch Toxicol,
8(suppl): 425-430.
US Chemical Industry (1994) Facts and figures for the chemical
industry. Chem Eng News, July 4: 28-33.
US EPA (1978) In-depth studies on health and environmental impacts of
selected water pollutants. Washington, DC, US Environmental Protection
Agency (Contract No. 68-01-4646).
US ITC (1987) Synthetic organic chemicals: United States production
and sales, 1986. Washington, DC, US International Trade Commission.
Utkin IB, Yakimov MM, Matveeva LN, Kozlyak EI, Rogozhin IS, Solomon
ZG, & Bezborodov AM (1991) Degradation of styrene and ethylbenzene by
Pseudomonas species Y2. Microbiol Lett, 77: 237-242.
Van Duijvenbooden W & Kooper WF (1981) Effects on groundwater flow and
groundwater quality of a waste disposal site in Noordwijk, The
Netherlands. Sci Total Environ, 21: 85-92.
Verhoeff AP, Suk J, & Van Wijnen JH (1988) Residential indoor air
contamination by screen printing plants. Int Arch Occup Environ
Health, 60: 201-209.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., pp 628-630.
Viganň L (1993) Reproductive strategy of Daphnia magna and toxicity
of organic compounds. Water Res, 27: 903-909.
Vincent R, Poirot P, Sabra I, Rieger B, & Cicolella A (1994)
Occupational exposure to organic solvents during paint stripping and
painting operations in the aeronautical industry. Int Arch Occup
Environ Health, 65(6): 377-380.
Volskay VT Jr & Grady CPL Jr (1990) Respiration inhibition kinetic
analysis. Water Res, 24: 863-874.
Vowles PD & Mantoura RFC (1987) Sediment-water partition coefficients
and HPLC retention factors of aromatic hydrocarbons. Chemosphere,
16: 109-116.
Waggott A (1981) Trace organic substances in the River Lee. In:
Chemistry in water reuse. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., vol 2, pp 55-99.
Wakeham SG, Davis AC, & Karas JL (1983) Mesocosm experiments to
determine the fate and persistence of volatile organic compounds in
coastal seawater. Environ Sci Technol, 17: 611-617.
Wallace LA, Pellizzari E, Hartwell TD, Perritt R, & Ziegenfus R
(1987a) Exposures to benzene and other volatile compounds from active
and passive smoking. Arch Environ Health, 42(5): 272-279.
Wallace LA, Pellizzari E, Hartwell TD, Sparacino C, Withmore R,
Sheldon L, Zelon H, & Perritt R (1987b) The TEAM study: Personal
exposures to toxic substances in air, drinking water, and breath of
400 residents of New Jersey, North Carolina, and North Dakota. Environ
Res, 43: 290-307.
Wallace LA, Pellizzari E, Leaderer B, Zelon H, & Sheldon L (1987c)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Wallace LA, Pellizzari ED, Hartwell TD, Davis V, Michael LC, &
Whitmore RW (1989) The influence of personal activities on exposure to
volatile organic compounds. Environ Res, 50(1): 37-55.
Warner JM & Beasley RK (1984) Purge and trap chromatographic method
for the determination of acrylonitrile, chlorobenzene,
1,2-dichloroethane and ethylbenzene in aqueous samples. Anal Chem,
56: 1953-1956.
Water Pollution Control (1989) Fate of organics on land-applied
sludges. Water Pollut Control, 127: 12.
Weast RC ed (1988) Handbook of chemistry and physics, 69th ed. Boca
Raton, Florida, CRC Press, Inc.
Webber D (1984) Top 50 chemical products. Chem Eng News, May 7: 8-10.
Westrick JJ, Mello JW, & Thomas RF (1984) The groundwater supply
survey. J Am Water Works Assoc, 76: 52-59.
Whitby FJ, Harland BJ, & Comber MHI (1982) Aromatic hydrocarbons in
the Tees Estuary. London, Department of Health (DOE Contract
PECD-7/7/23).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization.
Wiesenburg DA, Bodennec G, & Brooks JM (1981) Volatile liquid
hydrocarbons around a production platform in the northwest Gulf of
Mexico. Bull Environ Contam Toxicol, 27: 167-174.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformation of selected
alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic
aquifer material: A microcosm study. Environ Sci Technol, 20:
997-1002.
Windholz M ed (1983) The Merck index: An encyclopedia of chemicals,
drugs and biologicals, 10th ed. Rahway, New Jersey, Merck & Co., Inc.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene. Arch
Ind Health, 14: 387-398.
Wolff MS (1976) Evidence for existence in human tissues of monomers
for plastics and rubber manufacture. Environ Health Perspect,
17: 183-187.
Wolff MS, Daum SM, Lorimer WV, Selikoff IJ, & Aubrey BB (1977) Styrene
and related hydrocarbons in subcutaneous fat from polymerization
workers. J Toxicol Environ Health, 2: 997-1005.
Yamamoto RK & Cook WA (1968) Determination of ethyl benzene and
styrene in air by ultraviolet spectrophotometry. Am Ind Hyg Assoc J,
29: 238-241.
Zhang Z & Pawliszyn J (1993) Headspace solid-phase microextraction.
Anal Chem, 65(14): 1843-1852.
Zlatkis A & Jiao J (1991) Evaluation of new organoclay stationary
phases for the separation of ethylbenzene and xylene isomers.
Chromatographia, 31(9-10): 457-464.
1. RESUME
L'éthylbenzčne est un hydrocarbure aromatique qui s'obtient par
une réaction d'alkylation mettant en jeu le benzčne et l'éthylčne.
Aux Etats-Unis, on estime sa production annuelle ŕ environ 5 millions
de tonnes. En 1983, l'Europe occidentale en a produit environ 3
millions de tonnes. Il se présente sous la forme d'un liquide
incolore dégageant une odeur douceâtre qui rappelle l'essence. On
l'utilise principalement pour la production de styrčne. Ajouté ŕ du
xylčne technique, il sert également de solvant pour les peintures et
les vernis et on l'emploie aussi dans l'industrie chimique et dans
celle du caoutchouc. Il est présent dans le pétrole brut, les
produits raffinés dérivés du pétrole et dans leurs produits de
combustion.
L'éthylbenzčne n'est pas persistant car il se décompose dans
l'environnement, principalement par photo-oxydation et par dégradation
biologique. Il est possible que la photo-oxydation de l'éthylbenzčne
dans l'atmosphčre contribue ŕ la formation du smog photochimique.
Le logarithme du coéfficient de partage entre l'octanol et l'eau
est égal ŕ 3,13, ce qui indique que le composé se pręte ŕ une
bioaccumulation. Cependant, les données limitées dont on dispose
montrent que le facteur de bioaccumulation de l'éthylbenzčne est
faible pour les mollusques et les poissons. Apparemment, il est vite
éliminé par les organismes aquatiques.
A la campagne, la concentration d'éthylbenzčne dans l'air est
généralement inférieure ŕ 2 µg/m3. Sur des sites urbains, on a
trouvé des teneurs moyennes allant de 0,74 ŕ 100 µg/m3. La
concentration d'éthylbenzčne présente dans les eaux superficielles
est généralement inférieure ŕ 0,1 µg/litre dans les zones non
industrialisées. En revanche, dans les zones urbaines ou
industrialisées, la concentration peut atteindre 15 µg/litre. Dans
les sédiments, la concentration est en général inférieure ŕ
0,5 µg/litre, encore que l'on ait signalé des valeurs comprises entre
1 et 5 µg/litre dans des sédiments provenant de régions fortement
industrialisées. Dans les eaux souterraines non contaminées, la
concentration est habituellement inférieure ŕ 0,1 µg/litre, mais elle
est beaucoup plus élevée dans les eaux contaminées.
L'éthylbenzčne présente une toxicité aiguë modérée pour les
algues, les invertébrés aquatiques et les poissons. La valeur de la
CE50 ŕ 72 h est de 4,6 mg/litre pour l'algue Selenastrum
capricornutum, la CL50 ŕ 48 h est de 1,8 mg/litre pour Daphnia
magna, et on a une CL50 ŕ 96 h de 4,2 mg/litre pour la truite
arc-en-ciel. On ne possčde aucune donnée sur l'exposition chronique
des organismes aquatiques ŕ l'éthylbenzčne.
En ce qui concerne les bactéries et les lombrics, les données
toxicologiques sont limitées. Il n'en n'existe aucune sur les
végétaux terrestres, les oiseaux ou les mammifčres sauvages.
Chez l'homme, l'exposition ŕ l'éthylbenzčne se produit
principalement par inhalation; 40 ŕ 60% du composé sont retenus dans
les poumons. L'éthylbenzčne est fortement métabolisé, principalement
en acides mandélique et phénylglyoxylique. On peut utiliser les
métabolites présents dans les urines pour surveiller l'exposition
humaine.
Qu'elle soit aiguë ou chronique, la toxicité de l'éthylbenzčne
est faible pour l'homme et les animaux. Il exerce des effets toxiques
sur le systčme nerveux central et il est irritant pour les muqueuses
et les yeux. Le seuil de concentration pour ces effets chez l'homme a
été estimé ŕ environ 430-860 mg/m3 (100-200 ppm) lors d'une seule
exposition de courte durée.
Des rats et des souris ŕ qui on avait fait inhaler pendant 13
semaines de l'éthylbenzčne ŕ des concentrations allant jusqu'ŕ 4300
mg/m3, n'ont présenté aucune lésion histopathologique. La dose sans
effet observable (critčre retenu: l'augmentation du poids du foie) a
été estimée ŕ 2150 mg/m3 (500 ppm).
L'éthylbenzčne stimule les enzymes des microsomes hépatiques. Il
n'est ni mutagčne ni tératogčne pour le rat ou le lapin. On ne dispose
d'aucune donnée au sujet de ses effets toxiques éventuels sur
l'appareil reproducteur ni sur son pouvoir cancérogčne.
Une valeur-guide de 22 mg/litre (5 ppm) a été calculée ŕ partir
des résultats fournis par les études sur l'animal. On estime que la
population générale est exposée ŕ des concentrations inférieures ŕ
cette valeur, męme dans les cas les plus graves. On a constaté qu'une
exposition de longue durée en milieu professionnel, ŕ des concentration
de cet ordre, n'avait aucun effet nocif sur la santé des travailleurs
exposés.
RESUMEN
El etilbenceno es un hidrocarburo aromático que se obtiene por
alkilación del benceno y del etileno. La producción estimada en los
Estados Unidos de América es de unos cinco millones de toneladas por
ańo, y en Europa occidental fue de aproximadamente tres millones de
toneladas en 1983. El etilbenceno es un líquido incoloro de olor
dulce semejante al de la gasolina. Se utiliza principalmente para la
producción de estireno. También se utiliza en el xileno técnico como
disolvente de pinturas y lacas, así como en la industria del caucho y
en la fabricación de sustancias químicas. Se encuentra en el petróleo
crudo, en los productos de petróleo refinados y en productos de
combustión.
El etilbenceno es una sustancia química no persistente, que se
degrada principalmente por fotooxidación y biodegradación. Su
volatilización en la atmósfera es rápida. La reacción de foto-
oxidación del etilbenceno en la atmósfera puede contribuir a la
formación de niebla fotoquímica.
El logaritmo del coeficiente de reparto octanol-agua es 3,13, lo
que indica posibilidad de bioacumulación. Sin embargo, los limitados
indicios disponibles muestran que los factores de bioconcentración del
etilbenceno son bajos para peces y moluscos. La eliminación por los
organismos acuáticos parece ser rápida.
Los niveles de etilbenceno en el aire en puntos rurales son
generalmente inferiores a 2 µg/m3. En puntos urbanos se han
registrado niveles medios de etilbenceno que oscilan entre 0,74 y 100
µg/m3. Los niveles de etilbenceno detectados en las aguas
superficiales son generalmente inferiores a 0,1 µg/litro en zonas no
industriales. Se han comunicado concentraciones de etilbenceno de
hasta 15 µg/litro en zonas industriales y urbanas. Los niveles de
etilbenceno en sedimentos son generalmente inferiores a 0,5 µg/kg,
aunque en sedimentos de zonas muy industrializadas se han encontrado
niveles de 1 a 5 µg/kg. Las concentraciones en aguas subterráneas no
contaminadas son generalmente inferiores a 0,1 µg/litro, pero son
mucho más elevadas en aguas subterráneas contaminadas.
La toxicidad aguda del etilbenceno para las algas, los
invertebrados acuáticos y los peces es moderada. Los valores de
toxicidad aguda más bajos son de 4,6 mg/litro para el alga
Selenastrum capricornutum (CE50 a las 72 horas, sobre la base de la
inhibición del crecimiento), 1,8 mg/litro para Daphnia magna (CL50
a las 48 horas) y 4,2 mg/litro para la trucha irisada (CL50 a las 96
horas). No se dispone de información sobre la exposición crónica de
los organismos acuáticos al etilbenceno.
Hay información limitada sobre la toxicidad del etilbenceno para
las bacterias y para las lombrices. No hay datos relativos a las
plantas terrestres, las aves y los mamíferos silvestres.
La exposición humana al etilbenceno se produce principalmente por
inhalación; el 40-60% del etilbenceno inhalado se retiene en los
pulmones. El etilbenceno se metaboliza extensamente, transformándose
sobre todo en ácidos mandélico y fenilglioxílico. Estos metabolitos
urinarios pueden utilizarse para vigilar la exposición humana.
El etilbenceno tiene una toxicidad aguda y crónica baja tanto
para los animales como para el hombre. Es tóxico para el sistema
nervioso central e irrita las mucosas y los ojos. El umbral para esos
efectos en el ser humano después de exposiciones únicas breves se
estimó en aproximadamente 430-860 mg/m3 (100-200 ppm).
La inhalación de etilbenceno por ratas y ratones durante 13
semanas en concentraciones de hasta 4300 mg/m3 (1000 ppm) no dio
lugar a lesiones histopatológicas. El nivel sin efectos observados,
sobre la base de un aumento del peso del hígado en las ratas, fue de
2150 mg/m3 (500 ppm).
El etilbenceno es un inductor de las enzimas microsómicas
hepáticas. No es mutagénico ni teratogénico en ratas y conejos. No
se dispone de información sobre la toxicidad reproductiva ni la
carcinogenicidad del etilbenceno.
Se ha calculado un valor de orientación de 22 mg/m3 (5 ppm) a
partir de estudios realizados en animales. La exposición estimada de
la población general (incluso en la peor de las situaciones) es
inferior a ese valor de orientación. La exposición ocupacional a
largo plazo a concentraciones de etilbenceno estimadas en este orden
de magnitud no ocasionaron efectos adversos en la salud de los
trabajadores.
 governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by
the range of expertise required for the subject of the meeting and by
the need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLBENZENE
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton, Surrey,
United Kingdom
Dr A. Bobra, Environment Canada, Orleans, Ontario, Canada
Dr K. Hatfield, Division of Standards Development and Technology
Transfer, National Institute for Occupational Safety and Health,
Cincinnati, Ohio, USA
Mr L. Heiskanen, Environmental Health Assessment and Criteria Section,
Chemical Safety Unit, Department of Human Services and Health,
Canberra, Australia
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Co-rapporteur)
Dr You-xin Liang, Department of Occupational Health, Shanghai Medical
University, Shanghai, China
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Dr P. Lundberg, Department of Toxicology, National Institute of
Occupational Health, Solna, Sweden (Co-rapporteur)
Dr Vesa Riihimaki, Institute of Occupational Health, Helsinki, Finland
Dr Leif Simonsen, National Institute of Occupational Health,
Copenhagen, Denmark
Representatives of other Organizations
Dr P. Montuschi, Institute of Pharmacology, Faculty of Medicine and
Surgery, Catholic University of the Sacred Heart, Rome, Italy
(representing the International Union of Pharmacology)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLBENZENE
A WHO Task Group on Environmental Health Criteria for
Ethylbenzene met at the British Industrial Biological Research
Association (BIBRA) Toxicology International, Carshalton, Surrey,
United Kingdom, from 27 February to 2 March 1995. Dr D. Anderson
opened the meeting and welcomed the participants on behalf of the host
institute. Dr B.H. Chen, IPCS, welcomed the participants on behalf of
the Director, IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
criteria monograph and made an evaluation of the risks for human
health and the environment from exposure to ethylbenzene.
Dr P. Lundberg, National Institute of Occupational Health,
Sweden, Dr M. Crookes, Building Research Establishment, United Kingdom
and Dr S. Dobson and Mr P. Howe, Institute of Terrestrial Ecology,
Monks Wood, United Kingdom, prepared the first draft of this
monograph. The second draft was prepared by Dr Lundberg and Mr Howe,
incorporating comments received following the circulation of the first
draft to the IPCS Contact Points for Environmental Health Criteria
monographs. Dr S. Soliman, College of Agriculture & Veterinary
Medicine, Saudi Arabia, contributed to the final text of the document.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contributions to the IPCS.
ABBREVIATIONS
FID flame ionization detection
GC gas chromatography
MS mass spectrometry
PID photoionization detection
VOC volatile organic compound
1. SUMMARY
Ethylbenzene is an aromatic hydrocarbon manufactured by
alkylation from benzene and ethylene. The estimated yearly production
in the USA is about 5 million tonnes, and in 1983 it was approximately
3 million tonnes in western Europe. Ethylbenzene is a colourless
liquid with a sweet gasoline-like odour. It is mainly used for the
production of styrene. It is also used in technical xylene as a
solvent in paints and lacquers and in the rubber and chemical
manufacturing industries. It is found in crude oils, refined
petroleum products and combustion products.
Ethylbenzene is a non-persistent chemical, being degraded
primarily by photo-oxidation and biodegradation. Volatilization to
the atmosphere is rapid. The photo-oxidation reaction of ethylbenzene
in the atmosphere may contribute to photochemical smog formation.
The log octanol-water partition coefficient is 3.13, indicating a
potential for bioaccumulation. However, the limited evidence
available shows that ethylbenzene bioconcentration factors are low for
fish and molluscs. Elimination from aquatic organisms appears to be
rapid.
Ethylbenzene levels in air at rural sites are generally less than
2 µg/m3. Mean levels of ethylbenzene ranging from 0.74 to 100 µg/m3
have been measured at urban sites. The levels of ethylbenzene found
in surface waters are generally less than 0.1 µg/litre in
non-industrial areas. In industrial and urban areas ethylbenzene
concentrations of up to 15 µg/litre have been reported. Ethylbenzene
levels in sediments are generally less than 0.5 µg/kg, although levels
between 1 and 5 µg/kg have been found in sediments from heavily
industrialized areas. Concentrations in uncontaminated groundwater are
generally less than 0.1 µg/litre, but are much higher in contaminated
groundwater.
The acute toxicity of ethylbenzene to algae, aquatic
invertebrates and fish is moderate. The lowest acute toxicity values
are 4.6 mg/litre for the alga Selenastrum capricornutum (72-h EC50
based on growth inhibition), 1.8 mg/litre for Daphnia magna (48-h
LC50) and 4.2 mg/litre for rainbow trout (96-h LC50). No information
is available regarding chronic exposure of aquatic organisms to
ethylbenzene.
There is limited information regarding the toxicity of
ethylbenzene to bacteria and earthworms. There are no data for
terrestrial plants, birds or wild mammals.
Human exposure to ethylbenzene occurs mainly by inhalation;
40-60% of inhaled ethylbenzene is retained in the lung. Ethylbenzene is
extensively metabolized, mainly to mandelic and phenylglyoxylic acids.
These urinary metabolites can be used to monitor human exposures.
Ethylbenzene has low acute and chronic toxicity for both animals
and humans. It is toxic to the central nervous system and is an
irritant of mucous membranes and the eyes. The threshold for these
effects in humans after short single exposures was estimated to be
about 430-860 mg/m3 (100-200 ppm).
Inhalation of ethylbenzene for 13 weeks by rats and mice at
concentrations up to 4300 mg/m3 (1000 ppm) did not lead to
histopathological lesions. The no-observed-effect level, based on
increased liver weight in rats, was 2150 mg/m3 (500 ppm).
Ethylbenzene is an inducer of liver microsomal enzymes. It is
not mutagenic or teratogenic in rats and rabbits. No information is
available on reproductive toxicity or carcinogenicity of ethylbenzene.
A guidance value of 22 mg/m3 (5 ppm) has been calculated from
animal studies. The estimated exposure of the general population
(even in the worst case situation) is below this guidance value.
Long-term occupational exposure to ethylbenzene concentrations
estimated to be of this order of magnitude did not cause adverse
health effects in workers.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Empirical formula: C8H10
Chemical structure:
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by
the range of expertise required for the subject of the meeting and by
the need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLBENZENE
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton, Surrey,
United Kingdom
Dr A. Bobra, Environment Canada, Orleans, Ontario, Canada
Dr K. Hatfield, Division of Standards Development and Technology
Transfer, National Institute for Occupational Safety and Health,
Cincinnati, Ohio, USA
Mr L. Heiskanen, Environmental Health Assessment and Criteria Section,
Chemical Safety Unit, Department of Human Services and Health,
Canberra, Australia
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Co-rapporteur)
Dr You-xin Liang, Department of Occupational Health, Shanghai Medical
University, Shanghai, China
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Dr P. Lundberg, Department of Toxicology, National Institute of
Occupational Health, Solna, Sweden (Co-rapporteur)
Dr Vesa Riihimaki, Institute of Occupational Health, Helsinki, Finland
Dr Leif Simonsen, National Institute of Occupational Health,
Copenhagen, Denmark
Representatives of other Organizations
Dr P. Montuschi, Institute of Pharmacology, Faculty of Medicine and
Surgery, Catholic University of the Sacred Heart, Rome, Italy
(representing the International Union of Pharmacology)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLBENZENE
A WHO Task Group on Environmental Health Criteria for
Ethylbenzene met at the British Industrial Biological Research
Association (BIBRA) Toxicology International, Carshalton, Surrey,
United Kingdom, from 27 February to 2 March 1995. Dr D. Anderson
opened the meeting and welcomed the participants on behalf of the host
institute. Dr B.H. Chen, IPCS, welcomed the participants on behalf of
the Director, IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
criteria monograph and made an evaluation of the risks for human
health and the environment from exposure to ethylbenzene.
Dr P. Lundberg, National Institute of Occupational Health,
Sweden, Dr M. Crookes, Building Research Establishment, United Kingdom
and Dr S. Dobson and Mr P. Howe, Institute of Terrestrial Ecology,
Monks Wood, United Kingdom, prepared the first draft of this
monograph. The second draft was prepared by Dr Lundberg and Mr Howe,
incorporating comments received following the circulation of the first
draft to the IPCS Contact Points for Environmental Health Criteria
monographs. Dr S. Soliman, College of Agriculture & Veterinary
Medicine, Saudi Arabia, contributed to the final text of the document.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contributions to the IPCS.
ABBREVIATIONS
FID flame ionization detection
GC gas chromatography
MS mass spectrometry
PID photoionization detection
VOC volatile organic compound
1. SUMMARY
Ethylbenzene is an aromatic hydrocarbon manufactured by
alkylation from benzene and ethylene. The estimated yearly production
in the USA is about 5 million tonnes, and in 1983 it was approximately
3 million tonnes in western Europe. Ethylbenzene is a colourless
liquid with a sweet gasoline-like odour. It is mainly used for the
production of styrene. It is also used in technical xylene as a
solvent in paints and lacquers and in the rubber and chemical
manufacturing industries. It is found in crude oils, refined
petroleum products and combustion products.
Ethylbenzene is a non-persistent chemical, being degraded
primarily by photo-oxidation and biodegradation. Volatilization to
the atmosphere is rapid. The photo-oxidation reaction of ethylbenzene
in the atmosphere may contribute to photochemical smog formation.
The log octanol-water partition coefficient is 3.13, indicating a
potential for bioaccumulation. However, the limited evidence
available shows that ethylbenzene bioconcentration factors are low for
fish and molluscs. Elimination from aquatic organisms appears to be
rapid.
Ethylbenzene levels in air at rural sites are generally less than
2 µg/m3. Mean levels of ethylbenzene ranging from 0.74 to 100 µg/m3
have been measured at urban sites. The levels of ethylbenzene found
in surface waters are generally less than 0.1 µg/litre in
non-industrial areas. In industrial and urban areas ethylbenzene
concentrations of up to 15 µg/litre have been reported. Ethylbenzene
levels in sediments are generally less than 0.5 µg/kg, although levels
between 1 and 5 µg/kg have been found in sediments from heavily
industrialized areas. Concentrations in uncontaminated groundwater are
generally less than 0.1 µg/litre, but are much higher in contaminated
groundwater.
The acute toxicity of ethylbenzene to algae, aquatic
invertebrates and fish is moderate. The lowest acute toxicity values
are 4.6 mg/litre for the alga Selenastrum capricornutum (72-h EC50
based on growth inhibition), 1.8 mg/litre for Daphnia magna (48-h
LC50) and 4.2 mg/litre for rainbow trout (96-h LC50). No information
is available regarding chronic exposure of aquatic organisms to
ethylbenzene.
There is limited information regarding the toxicity of
ethylbenzene to bacteria and earthworms. There are no data for
terrestrial plants, birds or wild mammals.
Human exposure to ethylbenzene occurs mainly by inhalation;
40-60% of inhaled ethylbenzene is retained in the lung. Ethylbenzene is
extensively metabolized, mainly to mandelic and phenylglyoxylic acids.
These urinary metabolites can be used to monitor human exposures.
Ethylbenzene has low acute and chronic toxicity for both animals
and humans. It is toxic to the central nervous system and is an
irritant of mucous membranes and the eyes. The threshold for these
effects in humans after short single exposures was estimated to be
about 430-860 mg/m3 (100-200 ppm).
Inhalation of ethylbenzene for 13 weeks by rats and mice at
concentrations up to 4300 mg/m3 (1000 ppm) did not lead to
histopathological lesions. The no-observed-effect level, based on
increased liver weight in rats, was 2150 mg/m3 (500 ppm).
Ethylbenzene is an inducer of liver microsomal enzymes. It is
not mutagenic or teratogenic in rats and rabbits. No information is
available on reproductive toxicity or carcinogenicity of ethylbenzene.
A guidance value of 22 mg/m3 (5 ppm) has been calculated from
animal studies. The estimated exposure of the general population
(even in the worst case situation) is below this guidance value.
Long-term occupational exposure to ethylbenzene concentrations
estimated to be of this order of magnitude did not cause adverse
health effects in workers.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Empirical formula: C8H10
Chemical structure:
 Chemical name: Ethylbenzene
Synonyms: Phenylethane, EB, Ethylbenzol
Relative molecular mass: 106.16
CAS registry number:
Chemical name: Ethylbenzene
Synonyms: Phenylethane, EB, Ethylbenzol
Relative molecular mass: 106.16
CAS registry number: 
 Ethylbenzene is metabolized by the microsomal cytochrome P-450
enzyme system. Although specific isozymes have not been unequivocally
identified, enzyme induction studies suggest that CYP2B1/2, CYP1A1/2
and CYP2E1 may be involved (see section 7.8).
Four male volunteers were exposed to 655 mg/m3 (150 ppm)
ethylbenzene for 4 h and urine was collected for 24 h. Mandelic acid
(71.5%) and phenylglyoxylic acid (19.1%) were the main metabolites,
but smaller amounts of 1-phenylethanol, p-hydroxyacetophenone,
m-hydroxyacetophenone, 1-phenyl-1,2-ethandiol, 4-ethylphenol,
omega-hydroxyacetophenone and acetophenone were also found. Ring
oxidation accounted for 4.0%. Simultaneous exposure to 150 ppm
m-xylene did not alter the urinary metabolite pattern, but it
delayed excretion and decreased the amounts of metabolites excreted
(Engström et al., 1984). Mandelic acid (64%) and phenylglyoxylic aid
(25%) were found to be the main urinary excretion products in
volunteers after an 8-h exposure to 99-365 mg/m3 (23-85 ppm)
ethylbenzene (Bardodej & Bardodejová, 1970).
When two volunteers were exposed by inhalation to 430 mg/m3 (100
ppm) ethylbenzene for 4 h, most of the mandelic acid was excreted as
the R-enantiomer (Drummond et al., 1989).
In a study by Korn et al. (1992), urinary samples from workers
exposed to ethylbenzene, toluene and xylenes were analysed. The
average urinary concentration of phenylglyoxylic acid was 50.1
mg/litre. The concentration of mandelic acids was 135.2 mg/litre, of
which 127.8 mg/litre was R-mandelic acid and 7.3 mg/litre was
S-mandelic acid. The R/S ratio was independent of the air
concentration of ethylbenzene, which varied between 6.4 and 142 mg/m3
(1.5 and 33 ppm).
Four female histology laboratory assistants were exposed to a
mixture of xylenes (75%) and ethylbenzene (25%). The air concentration
of the solvent was 160-179 mg/m3, and the concentration of
ethylbenzene in blood collected at the end of the working day was
reported to be 0.5 to 0.8 mg/litre. The 24-h excretion of
2-ethylphenol in urine varied between 4.4 and 6.0 mg corresponding to
1 to 1.5% of the retained ethylbenzene (Angerer & Lehnert, 1979). The
findings by Angerer & Lehnert (1979) that ethylbenzene is metabolized
to 2-ethylphenol could not be verified by Engström et al. (1984).
Male Wistar rats (6 per group) were exposed for 6 h to
ethylbenzene at 1290 or 2580 mg/m3 (300 or 600 ppm). The urine was
collected for 48 h from the onset of exposure. Altogether, 14
different metabolites from ethylbenzene were identified. The main
metabolites were 1-phenylethanol, mandelic acid and benzoic acid, each
of which accounted for about 25%. Only 13% (low dose) and 6% (high
dose) of the estimated absorbed doses were eliminated during the 6-h
exposure. Over the 48-h period, the corresponding values were 83% and
59%, respectively. The metabolic pattern was similar, irrespective of
the exposure level (Engström, 1984b).
In another study, male Wistar rats (20 per group) were exposed to
215, 1290 or 2580 mg ethylbenzene/m3 (50, 300 or 600 ppm) for 6
h/day, 5 days/week, for up to 16 weeks. Urinary excretion of some of
the metabolites was measured in weeks 2, 5 and 9. A significant
dose-related percentage decrease of phenylglyoxylic acid and hippuric
acid plus benzoic acid was found. A corresponding increase of
1-phenylethanol and omega-hydroxyacetophenone excretion was also
noted. The total amount of metabolites in urine collected during the
24 h after onset of exposure remained, however, constant at each
exposure level throughout the study (Engström et al., 1985).
When a single oral dose of 318 mg ethylbenzene/kg body weight was
administered to rabbits, the main urinary metabolites found were
hippuric acid and methylphenylglucuronic acid, which together
represented 60-70% of the dose, while mandelic acid and phenaceturic
acid were minor metabolites (El Masry et al., 1956).
It has been established in in vivo studies that experimental
animals convert ethylbenzene mainly to the R-enantiomer of mandelic
acid (Drummond et al., 1990). In an in vitro study it was found
that ethylbenzene is hydroxylated by cytochrome P-450cam (from
Escherichera coli) almost exclusively at the secondary ethyl carbon
with about a 2:1 ratio of R:S products (Filipovic et al., 1992).
The rates of metabolism of ethylbenzene have been studied in
vitro in rabbit liver and lung. Organs from five female animals
were used, but details of the experimental procedures were not
reported. For the liver (mean weight 76 g) the rate of metabolism was
453 nmol/g tissue per 10 min (34.4 µmol per liver per 10 min or 11.7
nmol per nmol cytochrome P-450 per 10 min). The corresponding figures
for lung (mean weight 7.7 g), were 680 nmol, 5.3µmol and 200.1 nmol,
respectively. Thus, lung tissue may significantly contribute to the
body clearance of ethylbenzene in rabbits (Sato & Nakajima, 1987).
6.4 Elimination and excretion
In humans, ethylbenzene is mainly excreted in the urine as
mandelic and phenylglyoxylic acids (Bardodej & Bardodejová, 1970;
Ĺstrand et al., 1978; Engström et al., 1984; Gromiec & Piotrowski,
1984). Only up to 5% of retained ethylbenzene is estimated to be
exhaled without transformation (Ĺstrand et al., 1978). The
elimination half-lives of ethylbenzene in exhaled air and urine have
been estimated to be 0.5-3 h and 8 h, respectively (Wolff, 1976). In
human volunteers exposed to 100 or 200 ppm "industrial xylene" for
2 h, there was no decline in the concentration of xylenes plus
ethylbenzene in the gluteal subcutaneous adipose tissue between 30 min
and 22 h after exposure (Engström & Bjurström, 1978). The elimination
of mandelic acid has been found to be biphasic, with half-lives of 3.1
and 24.5 h (Gromiec & Piotrowski, 1984).
The elimination kinetics for 10 volatile organic compounds,
including ethylbenzene, has been studied in human volunteers exposed
to a variety of consumer products. Breath samples were collected
post-exposure and analysed by GC/MS. The half-lives for the 10
chemicals varied from a few hours to 1-2 days. The authors concluded
that volatile organic compounds exhibit relatively short residence
times in the body (Pellizzari et al., 1992).
Male Harlan-Wistar rats exposed to 14C-ring-labelled
ethylbenzene (1000 mg/m3) for 6 h excreted 82% of the radioactivity
in the urine, 8.2% in expired air (0.03% as CO2) and 0.7% in faeces.
After 42 h, 0.2% remained in the tissues. The remaining 8.3% could not
be accounted for (Chin et al., 1980a).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Single high exposures to ethylbenzene cause irritation of the
mucous membranes and central nervous system effects. The results from
single exposure in vivo studies are summarized in Table 5.
Table 5. Single exposure of animals to ethylbenzenea
Species Route Dose Parameter Reference
Rat oral 3.5 g/kg LD50 Wolf et al. (1956)
Rat oral 4.7 g/kg LD50 Smyth et al. (1962)
Rat inhalation 9.37 g/m3 (2180 ppm) Minimum Molnár et al. (1986)
narcotic conc.
Rat inhalation 17.2 g/m3 (4000 ppm) 1 h LC10 Smyth et al. (1962)
Rat inhalation 17.2 g/m3 (4000 ppm) 4 h LC50 Smyth et al. (1962)
Rat inhalation 34.4 g/m3 (8000 ppm) 1 h LC100 Smyth et al. (1962)
Rabbit dermal 77.4 g/kg LD50 Smyth et al. (1962)
a Additional information is given in the following reviews: DFG (1985), ECETOC (1986).
7.2 Short-term exposure
In a short-term study, six male rats (Sprague Dawley) were
exposed for 6 h/day during 3 consecutive days to 8.6 g/m3 (2000 ppm)
ethylbenzene. The animals were killed 16-18 h after the last
exposure. Small increases in dopamine and noradrenaline levels and
turnover in various parts of the hypothalamus and the median eminence
were reported. Ethylbenzene was also found to produce selective
reduction in prolactin and corticosterone secretion and selective
increase in dopamine turnover within the dopamine-cholecystokinin-8-
immuno-reactive nerve terminals of the nucleus accumbens (posterior
part) (Andersson et al., 1981).
When eight male rabbits (New Zealand) were exposed 12 h daily for
7 days to 3.22 g/m3 (750 ppm) ethylbenzene, there was a marked
(p<0.05) depletion of striatal and tuberoinfundibular dopamine. Such
an effect was also caused by intraperitoneal dosing of rabbits (eight
per group) with mandelic or phenylglyoxylic acid (4 mmol/kg per day
for 3 days) in saline (Romanelli et al., 1986).
In a 4-week inhalation study Fischer-344 rats (five of each sex
per group) were exposed to ethylbenzene for 6 h/day, 5 days per week,
at exposure levels of 0, 426, 1643 or 3363 mg/m3 (0, 99, 382 or 782
ppm). At the two highest exposure levels, sporadic lacrimation and
salivation, as well as significantly (p<0.05) increased liver
weights, were seen. At the highest exposure level, there was a small
increase in leukocyte counts and, in males, a marginal increase in
platelet counts (Cragg et al., 1989).
In the same study, mice (B6C3F1) of both sexes were similarly
exposed. At 1643 and 3363 mg ethylbenzene/m3, females showed
significantly (p<0.01) increased absolute and relative liver weights.
In males a significantly (p<0.05) increased relative liver-to-brain
weight ratio was seen. Male and female rabbits (New Zealand White)
were also used in this study. The exposure levels were 0, 1643, 3363
and 6923 mg/m3 (0, 382, 782 and 1610 ppm). At the highest exposure
level females gained weight more slowly than controls but neither sex
exhibited gross or microscopic organ changes (Cragg et al., 1989).
No changes in mortality pattern were seen in the three species.
There were no changes in clinical chemistry parameters in rats or
rabbits. Mice were not subjected to clinical chemistry or
haematological examinations due to the small volume of blood that
could be collected. For similar reasons, urinalyses were performed
for rats (no change) but not for mice. Rabbits were excluded from
urinalysis for logistical reasons. No changes in gross or microscopic
pathology were noted in any of over 30 tissues from each of the three
species when the animals were exposed at the highest concentration
(Cragg et al., 1989).
7.3 Long-term exposure
7.3.1 Oral exposure
Matched groups of 10 Wistar female rats were given daily
ethylbenzene doses of 0, 13.6, 136, 408 or 680 mg/kg by stomach tube
5 days a week for 6 months. The two highest dosages induced slight
increases in liver and kidney weights and slight cloudy swelling of
parenchymal liver cells and of the tubular epithelium in the kidney
(Wolf et al., 1956).
7.3.2 Inhalation exposure
In a 13-week National Toxicology Program study, groups of 10 rats
(F-344/N) and 10 mice (B6C3F1) of each sex were exposed for 6 h (plus
10 min to reach 90% of the target chamber concentration) per day,
5 days per week for 92 (female rats), 93 (male rats), 97 (female mice)
or 98 (male mice) days, at ethylbenzene concen-trations of 0, 430,
1075, 2150, 3225 or 4300 mg/m3 (0, 100, 250, 500, 750 or 1000 ppm).
Blood for clinical chemistry and haematological examination was
collected on study days 4 and 23 and again at week 13 from both male
and female rats. Dose-related increases in absolute liver weight were
seen in both sexes of mice exposed to the two highest dose levels, and
the relative kidney weight of female mice exposed to 4300 mg/m3 was
greater than that of the controls. Increased absolute and relative
liver and kidney weights were seen in male rats exposed to the two
highest dose levels. Increased absolute liver and kidney weights were
seen in female rats exposed to the three highest dose levels, but no
increased relative liver and kidney weights were seen. No chemically
related histopathological changes were observed in any rat or mouse
tissues. Clinical chemistry results were negative (NTP, 1992).
In another study, groups of five male rats (Wistar) were exposed
for 6 h/day, 5 days/week to ethylbenzene concentrations of 0, 215,
1290 or 2580 mg/m3 (0, 50, 300 or 600 ppm) and sacrificed after 2, 5,
9 or 16 weeks of exposure. At 2580 mg/m3 liver cells showed a slight
proliferation of smooth endoplasmic reticulum, slight degranulation
and splitting of rough endoplasmic reticulum, and enlarged
mitochondria. At the same dose level, liver microsomal protein, but
not cytochrome P-450, concentration was slightly increased. There was
also an increase in NADPH-cytochrome c reductase, 7-ethoxycoumarin-
O-deethylase and UDPG-transferase activities in the liver. In the
kidney only the two latter enzymes showed dose-related increases.
Urinary excretion of thioethers was measured to ascertain the
generation of electrophilic intermediates during ethylbenzene
metabolism. Excretion of thioethers increased in a dose-dependent
manner, with some fluctuation over the course of 7 weeks, reaching
about eight times the control level at 2580 mg ethylbenzene/m3.
However, there was no decrease in hepatic or renal levels of
glutathione (GSH), indicating that the cells were able to maintain the
intra-cellular homeostasis of GSH during exposure (Elovaara et al.,
1985).
In inhalation experiments, matched groups of 10-25 male and
female Wistar rats, 5-10 guinea-pigs, 1-2 rabbits and 1-2 rhesus
monkeys of either sex or both sexes were all exposed 7 h/day, 5
days/week, for up to 6 months. The exposure levels were 0, 1720 and
2580 mg/m3 (0, 400 and 600 ppm) for 186 days, 5375 mg/m3 (1250 ppm)
for 214 days (no monkeys) or 9460 mg/m3 (2200 ppm) for 144 days (rats
only). Slight effects were seen in rats: increased liver and kidney
weights at 1720 mg/m3; increased liver and kidney weights at 2580
mg/m3; and small histopathological changes (cloudy swelling) in liver
and kidney at 5375 and 9460 mg/m3 (Wolf et al. 1956).
In guinea-pigs and monkeys slightly increased liver weights were
noted in the 2580 mg/m3 group only. At the same exposure level,
small histopathological effects in the testes, described as
degeneration of the germinal epithelium, were seen in rabbits and
monkeys. At 5375 mg/m3 a slight growth depression was noted in
guinea-pigs. The no-observed-effect level (all four species) was
considered to be about 860 mg/m3 (200 ppm) (Wolf et al., 1956). It
should be noted, however, that Cragg et al. (1989) found no
histopathological effects in the testes of rats and rabbits exposed to
up to 3363 mg/m3 (782 ppm) for 4 weeks, and the lack of toxicity was
confirmed by NTP (1992).
7.4 Skin and eye irritation, sensitization
Inhalation for 3 min of ethylbenzene at a concentration of 4300
mg/m3 caused slight nasal irritation in guinea-pigs, and an 8-min
exposure caused eye irritation as well. At 8600 mg/m3 a 1-min
exposure was enough to cause both effects (Cavender, 1993).
Two drops of undiluted ethylbenzene placed in the eyes of rabbits
resulted in slight conjunctival irritation but no effects on the
cornea (Wolf et al., 1956). A slight conjunctival irritation with
some reversible corneal injury was reported in rabbits in a study by
Smyth et al. 1962.
Undiluted ethylbenzene has been shown to produce moderate
irritation when applied to the uncovered skin of rabbits (Smyth et
al., 1962). The application of undiluted ethylbenzene to the ear and
to the shaved abdomen of rabbits up to 20 times during a 4-week period
resulted in moderate irritation. There was erythema and oedema with
superficial necrosis and exfoliation of large patches of skin (Wolf et
al., 1956).
No animal sensitization studies have been reported.
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
In an inhalation study, rats (Wistar or Sprague-Dawley) and
rabbits (New Zealand White) were exposed to 430 or 4300 mg/m3 (100 or
1000 ppm) ethylbenzene for 6 to 7 h/day on gestation days 1 to 19
(rats) or 1 to 24 (rabbits). All pregnant animals were sacrificed on
the day before term (day 21 for rats, day 30 for rabbits). The
rabbits had a significantly (p<0.05) reduced number of live pups per
litter at both exposure levels, but the number of implantations per
litter and the number of dead or resorbed fetuses per litter did not
differ from those of the controls. Maternal toxicity in rats exposed
to 4300 mg/m3 was reflected in increased liver, kidney and spleen
weights. There was a significant (p<0.05) increase in the incidence
of extra ribs in both of the exposed rat groups (Hardin et al., 1981).
In a further study, rats (CFY) were exposed to ethylbenzene
concentrations of 600, 1200 or 2400 mg/m3 continuously (24 h/day)
from day 7 to day 15 of pregnancy. They were then killed on day 21.
Mice (CFLP) were exposed for three periods of 4 h per day to 500
mg/m3 on days 6-15 of pregnancy and killed on day 18. Rabbits (New
Zealand White) were exposed continuously on days 7-20 of gestation to
500 or 1000 mg/m3 and were killed at day 30. The maternal toxic
effects (not specified) in mice and rats were moderate and
dose-dependent. In both species ethylbenzene caused skeletal growth
retardation, extra ribs and reduced fetal growth rate at the highest
concentration. In rabbits, the highest dose concentration caused mild
maternal toxic effects (decreased weight gain) and reduction in the
number of fetuses due to abortion (Ungváry & Tátrai, 1985).
When rats (F-344/N) and mice (B6C3F1) were exposed to
ethylbenzene at concentrations of 0, 430, 2150 and 4300 mg/m3 (0,
100, 500 and 1000 ppm), 6 h per day, 5 days per week, for 13 weeks,
there were no changes in sperm or vaginal cytology (NTP, 1992).
Rat embryos were explanted on day 9 of gestation and cultured in
rat serum with added xylene (containing 18% ethylbenzene) at
concentrations up to 1.0 ml/litre serum. Dose-dependent retardation
of growth and development was seen but there were no observable
teratogenic effects (Brown-Woodman et al., 1991).
No multigeneration and reproductive studies on ethylbenzene have
been reported.
7.6 Mutagenicity and related end-points
In a National Toxicology Program study, ethylbenzene was not
mutagenic in Salmonella tests and did not induce chromosomal
aberrations or sister chromatid exchange in Chinese hamster ovary
(CHO) cells in vitro, although it did induce trifluorothymidine
resistance in mouse lymphoma cells at the highest concentration tested
(80 mg/litre). There was no increase of micronuclei in the peripheral
blood of mice exposed to ethylbenzene (NTP, 1992).
In several other studies ethylbenzene did not induce point
mutations (with or without added metabolic activation system). In
addition, it did not cause an increase in the spontaneous
recessive-lethal frequency in the Drosophila recessive-lethal test,
nor had it any chromosomal effects in vitro (Donner et al., 1980;
Florin et al., 1980; Dean et al., 1985). Ethylbenzene had a marginal
effect on sister chromatid exchange in human lymphocytes in vitro
when a high (10 mmol/litre) concentration was used (Norppa & Vainio,
1983). In addition, in the TK+/- test in mouse lymphoma cells there
was a slight effect at a high concentration (80 mg/litre) (McGregor et
al., 1988). This study is obviously the same as the one subsequently
reported by NTP (1992).
No excess of chromosomal aberrations in bone marrow cells was
seen in rats after up to 18 weeks of exposure (6 h/day, 5 days/week)
to 300 ppm of a xylene mixture containing 18.3% ethylbenzene (Donner
et al., 1980).
7.7 Carcinogenicity
In a carcinogenicity study, rats (Sprague-Dawley) were exposed to
one of several aromatic hydrocarbons, including ethylbenzene. Groups
of rats (40 of each sex) were exposed to 500 mg ethylbenzene (in olive
oil) per kg body weight by gavage, 4 or 5 days per week for 104 weeks.
Results were determined after 141 weeks. The first malignant tumour,
a nephroblastoma, was observed after 33 weeks. The total number of
malignant tumours was 31 in the 77 animals of the exposed group alive
at 33 weeks compared with an incidence of 23 of 94 animals in the
control group. The authors concluded that ethylbenzene caused an
increase in the incidence of total malignant tumours, although there
was no increase in the incidence of any specific type of tumour
(Maltoni et al., 1985). It is difficult to draw any firm conclusion
from this study because of inadequate reporting.
7.8 Other special studies
Ethylbenzene was found to have low acute cytotoxicity in vitro
on Ehrlich ascites cells (Holmberg & Malmfors, 1974).
The effects of ethylbenzene have been studied in four in vitro
test systems: decreased cell growth in Ascites sarcoma BP 8 cells;
decreased oxidative metabolism in hamster brown fat cells; cell
membrane damage of human embryonic lung fibroblasts; and inhibition of
ciliary activity in chicken embryo trachea. On a 0-9 point scale
(equivalent to 0-100%) the activity of these four systems scored 4, 6,
8 and 8, respectively (Curvall et al., 1984).
The activity of cytochrome CYP2E1 can be monitored in microsomal
preparations by p-nitrophenol hydroxylation. When rabbit (white
male New Zealand) liver microsomes were treated with up to 0.25 mM
ethylbenzene an inhibition of p-nitrophenol hydroxylation was seen
(Koop & Laethem, 1992).
In a study by Pyykkö et al. (1987), Sprague-Dawley male rats were
given ethylbenzene dissolved in corn oil intraperitoneally in a single
dose of 5 mmol/kg (530 mg/kg body weight). The rats were killed 24 h
later and the livers and lungs removed. In the liver 7-ethoxycoumarin-
0-deethylase (a marker of CYP2B1/2) was induced 4-fold; no induction
was found in the lung. Ethylbenzene also induced 7-ethoxyresorufin-
0-deethylase (a marker of CYP1A1/2) both in the liver (4-fold)
and the lung (2.5 fold). These findings are common to many aromatic
solvents.
Alterations in the levels of specific cytochrome P-450 isozymes
were measured by Western immunoblotting techniques using rabbit
anti-rat polyclonal antibodies for cytochrome CYP1A1, CYP2B1 and
CYP2E1 on rat liver microsomes. The rats, male and female Holtzman
rats, were given 10 mmoles ethylbenzene per kg body weight for 3 days
and killed 24 h after the last injection. Ethylbenzene was shown to
induce CYP2B1/2B2 to a greater extent in male rats, while cytochrome
CYP2E1 was only induced in female rats. The level of cytochrome
CYP1A1 was not affected by ethylbenzene (Sequeira et al., 1992).
In another study (Gut et al., 1993), Wistar male rats were
exposed in a dynamic inhalation apparatus to 4 mg ethylbenzene/litre
air, 20 h per day, for 4 days. The rats were then killed and liver
microsomes prepared. By use of Western immunoblotting techniques it
was shown that cytochrome CYP2B1 was induced and the cytochrome CYP2E1
levels were decreased.
In a sensory irritation test, groups of four male mice
(Swiss-Webster) were exposed for 30 min to ethylbenzene at
concentrations of 1.76, 3.7, 8.06, 17.07 or 41.45 g/m3 (410, 860,
1875, 3970 or 9640 ppm). The RD50 value, i.e. the concentration
necessary to depress the respiratory rate by 50%, was calculated to be
17.46 g/m3 (4060 ppm) (95% confidence interval 10.6-28.6 g/m3;
2480-6660 ppm). The respiratory rate decreased due to sensory
irritation of the upper respiratory tract (Damgĺrd Nielsen & Alarie,
1982). In a similar test male mice (Swiss F1) were exposed for about
5 min to different concentrations of ethylbenzene. At least four
different concentrations were used and there were six mice for each
concentration. In this study the RD50 value was calculated to be
6.16 g/m3 (1432 ppm) (De Ceaurriz et al., 1981).
7.9 Factors modifying toxicity
Rats (Sprague-Dawley) were exposed for 2 h to 774 mg/m3 (180
ppm) ethylbenzene. One of two corresponding animals received 20 mmol
ethanol/kg body weight in physiological saline intraperitoneally
before exposure to ethylbenzene. Ethanol enhanced significantly (1.4
fold) the blood levels of inhaled ethylbenzene (Römer et al., 1986).
8. EFFECTS ON HUMANS
8.1 Volunteer studies
A dermal maximization test conducted on 25 volunteers at a
concentration of 10% ethylbenzene in petrolatum produced no skin
sensitization reaction (ECETOC, 1986).
In a review of studies from the 1930s, it was stated that
exposure to 21.5 g/m3 (5000 ppm) ethylbenzene for a few seconds gives
intolerable irritation of nose, eyes and throat. A few seconds of
exposure to 4.3 g/m3 (1000 ppm) initially gives eye irritation which
diminishes after a few minutes of exposure (Damgĺrd Nielsen & Alarie,
1982).
In a study on ethylbenzene metabolism in man it was incidentally
reported that, when the exposure was above the occupational limit
value (8 h; 430 mg/m3,100 ppm), complaints of fatigue, sleepiness,
headache and irritation of the eyes and respiratory tract were
reported (Bardodej & Bardodejová, 1970).
Healthy male subjects were exposed to technical xylene
(containing 20.7% ethylbenzene) for 2 h with or without a 100-watt
workload on an ergometer cycle. The air concentration of technical
xylene was 435 or 1300 mg/m3. During work at the higher exposure
level, evidence of performance decrement was observed in three of the
five performance tests: reaction time addition test (p<0.05),
short-term memory (p<0.05) and choice reaction time (p<0.10)
(Gamberale et al., 1978).
8.2 Occupational exposure
A number of epidemiological studies have been carried out on
groups occupationally exposed to mixtures of solvents, including
ethylbenzene. In these studies it is difficult to attribute the
effects to ethylbenzene or any other single chemical present.
Ethylbenzene occurs in mixed xylenes (at up to 30%) and effects of
occupational exposure to mixed xylenes are usually presented as
effects of xylenes and not of ethylbenzene.
A cross-sectional epidemiological study showed no strong evidence
of adverse neurobehavioural effects in 105 house painters when
compared with 53 workers from various professions (non-painters). The
concentration of ethylbenzene at the workplace was up to 12.9 mg/m3
(3 ppm). Other solvents present were ethyl acetate, toluene, butyl
acetate, methyl isobutyl ketone and xylene. In two neurobehavioural
tests significant differences were found between painters and controls
(the tests were "change of personality" and "short-term memory
capacity"). In a subgroup of painters with repeated prenarcotic
symptoms at the workplace, the differences were more pronounced
(Triebig et al., 1988).
In a study involving 35 spray painters, employed for between 2
and 26 years, erythrocyte and haemoglobin levels were slightly (but
not statistically significantly) lower than those of the controls.
The concentration of ethylbenzene was 17.2 mg/m3 (4 ppm) (Angerer &
Wulf, 1985).
In a medical surveillance report of some 200 ethylbenzene-
production workers, mandelic acid concentrations in urine were
measured twice a year for 20 years. Mandelic acid concentration
in the samples never exceeded 3.25 mmol/litre (=497 mg/litre), and the
mean value was 0.20-0.30 mmol/litre. According to the authors, a
post-shift urine mandelic acid concentration of 6.25 mmol/litre is
equivalent to an air concentration of 200 mg/m3. Therefore, the
measured maximum and mean mandelic acid values were considered to be
equivalent to air ethylbenzene concentrations of about 86 and 8.6
mg/m3 (20 and 2 ppm), respectively. None of the workers examined
over the last 10 years showed any effects on the levels of
haemoglobin, leucocytes or platelets, nor did they have a changed
haematocrit or alanine aminotransferase activity (Bardodej & Círek,
1988).
Minor changes in evoked potential and nerve conduction velocity
were found in 22 workers exposed to ethylbenzene at concentrations of
0.43-17.2 mg/m3 (0.1-4 ppm) for 4-20 years. They were also exposed
to styrene (about 1.5 ppm) (Lu & Zhen, 1989).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Ethylbenzene has been shown to inhibit the respiration of sewage
sludge utilizing biogenic substrates. Two screening tests were used,
RIKA and OECD 209. The concentration of ethylbenzene used was at the
limit of solubility in the medium (approximately 150 mg/litre), and
inhibitions of the respiration rate of 30% (ACCEDE 209) and 100%
(RIKA) were observed (Volskay & Grady, 1990).
Bringmann & Kühn (1980) studied the effect of ethylbenzene on
bacteria. A toxicity threshold of 12 mg/litre for Pseudomonas putida
was obtained in a cell multiplication inhibition test.
9.2 Aquatic organisms
Numerous acute toxicity tests have been carried out on
ethylbenzene. Organisms that have been studied include protozoans
(Bringmann & Kühn, 1980), algae (US EPA, 1978; Bringmann & Kühn, 1980;
Galassi et al., 1988; Masten et al. 1994), water fleas (LeBlanc, 1980;
Bringmann & Kühn, 1982; Abernethy et al., 1986; Galassi et al., 1988;
Vigano, 1993), diatoms (US EPA, 1978; Masten et al., 1994), copepods,
grass shrimp (Potera, 1975), bay shrimps (Benville & Korn, 1977),
mysid shrimps (US EPA, 1978; Masten et al., 1994), Dungeness crabs
(Caldwell et al., 1976) and Pacific oysters (LeGore, 1974). Fish that
have been studied include rainbow trout (Mayer & Ellersieck, 1986;
Galassi et al., 1988), guppy (Pickering & Henderson, 1966; Galassi et
al., 1988), bluegill (Pickering & Henderson, 1966; Buccafusco et al.,
1981), fathead minnow, goldfish (Pickering & Henderson, 1966), channel
catfish (Mayer & Ellersieck, 1986), Atlantic silverside (Masten et
al., 1994), striped bass (Benville & Korn, 1977) and sheepshead minnow
(US EPA, 1978; Heitmuller et al., 1981).
Many of the test results are not comparable, owing to
inconsistent exposure conditions, resulting from emulsions, open
static systems and systems with large air spaces. Aquatic toxicity
results from consistent exposure conditions, which are comparable, are
shown in Table 6.
No information regarding chronic exposure of aquatic organisms to
ethylbenzene has been reported.
Table 6. Toxicity of ethylbenzene to aquatic organisms
Species Age/size Stat/flowa Temperature Salinity pH Parameterc Concentration Reference
(°C) (0/00) (mg/litre)d
Alga stat 72-h EC50 4.6 Galassi et al. (1988)
(Selenastrum stat 19-21 48-h EC50 7.2 (3.4-15.1) Masten et al. (1994)
capricornutum) stat 19-21 96-h EC50 3.6 (1.7-7.6) Masten et al. (1994)
Water flea < 24 h statb 24-h LC50 2.2 Galassi et al. (1988)
(Daphnia magna) statb 21-25 48-h LC50 2.1e Abernethy et al. (1986)
< 24 h statb 48-h LC50 1.81-2.38 Viganň (1993)
Diatom
(Skeletonema statb 19-21 48-h EC50 7.5 (5.0-11.2) Masten et al. (1994)
costatum) statb 19-21 72-h EC50 4.9 (2.4-9.8) Masten et al. (1994)
statb 19-21 96-h EC50 7.7 (5.9-10.0) Masten et al. (1994)
Mysid shrimp < 24 h flow 24-26 20 8.0 48-h LC50 >5.2 Masten et al. (1994)
(Mysidopsis < 24 h flow 24-26 20 8.0 96-h LC50 2.6 (2.0-3.3) Masten et al. (1994)
bahia)
Rainbow trout stat 96-h LC50 4.2 Galassi et al. (1988)
(Oncorhynchus
mykiss)
Guppy stat 96-h LC50 9.2 Galassi et al. (1988)
(Poecilia
reticulata)
Table 6. (Cont'd)
Species Age/size Stat/flowa Temperature Salinity pH Parameterc Concentration Reference
(°C) (0/00) (mg/litre)d
Atlantic silverside 3-15 mg flow 21-23 20 48-h LC50 6.4 (5.8-7.5) Masten et al. (1994)
(Menidia menidia) 3-15 mg flow 21-23 20 96-h LC50 5.1 (4.4-5.7) Masten et al. (1994)
a in all cases a closed system was used; stat = static conditions (water unchanged for the duration of the test);
flow = flow-through conditions (ethylbenzene concentration in water continously maintained)
b air space was eliminated
c the EC50 for algae was based on growth inhibition
d test concentrations were measured, unless stated otherwise
e nominal test concentration;
9.3 Terrestrial organisms
An LC50 value of 47 µg per cm2 of contact area was obtained for
earthworms exposed to ethylbenzene adsorbed on filter paper in glass
vials (Neuhauser et al., 1986). Callahan et al. (1994) reported a
2-day LC50 value of 4.93 µg/kg for Eisenia foetida in a contact
toxicity test.
No toxicity data on plants, birds and wild mammals have been
reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
The acute and chronic toxicities of ethylbenzene are low. The
toxic effects in humans and animals relate to depression of the
central nervous system (CNS) and to irritation of the mucous membranes
and eyes. No data concerning carcinogenic or reproductive effects
have been reported. Ethylbenzene does not have significant mutagenic
properties or teratogenic effects.
Exposure to more than 430 mg/m3 (100 ppm) causes symptoms of CNS
depression and irritation in humans.
A 20-year medical surveillance study of 200 workers showed no
indications of effects in routine blood tests. The maximum exposure,
estimated from the urinary concentration of mandelic acid, was less
than about 86 mg/m3 (20 ppm) and the mean value about 8.6 mg/m3
(2 ppm).
In a 13-week animal study, increased liver weight was the only
dose-related biological finding in male rats. This was seen at a
concentration of 3225 mg/m3 (750 ppm) or more.
The definition and aim of the use of a guidance value for the
general population have been described by IPCS (1994).
On the basis of biological significance criteria cited above, a
no-observed-effect level (NOEL) of 2150 mg/m3 (500 ppm) was defined.
The no-observed-adverse-effect level would be higher than 4300 mg/m3
(1000 ppm) (highest concentration used), since the increase in liver
weight was not associated with any histopathological findings. A NOEL
of 2150 mg/m3 (500 ppm) was used as the basis for determining the
guidance value. The following uncertainty factors were used: 10 for
interspecies variability; 5 for intraspecies variability (effect seen
in males only); and 2 for lack of chronic toxicity data. This gives a
guidance value of 22 mg/m3 (5 ppm).
From the medical surveillance study the NOEL could be estimated
to be between 8.6 mg/m3 (2 ppm) (mean value) and 86 mg/m3 (20 ppm)
(maximum value). However, since no dose-response relationship was
derived, this study is not suitable for the estimation of a guidance
value. Furthermore, no effects would be expected at this exposure
level, which is almost the same as the guidance value.
Humans are exposed to ethylbenzene principally by inhalation,
where the substance is rapidly absorbed into the body. Exposure by
skin absorption or ingestion may also occur. Ethylbenzene is not
considered to bioaccumulate.
Table 7 gives the estimated weekly ethylbenzene dose resulting
from different types of exposure. A guidance value of 22 mg/m3
(5 ppm) ethylbenzene is approximately equivalent to a weekly ethyl-
benzene dose of 2000 mg. This is 100 times higher than the worst
exposure situation of the general population.
Table 7. Estimated weekly ethylbenzene dose from different types of exposures
Type of exposure Reported concentration Weekly total amount Dose mg/week
in media inhaled, ingested
or smoked
General population
Inhalation (means)b 0.74-100 µg/m3 140 m3 0.07-10c
Ingestion (food) 13 µg/kgd 7 kg 0.1
Drinking- or groundwaterb 0.07-1.1 µg/litree 14 litres 0.01
30.0 µg/litref 0.42
Occupational
Inhalation (mean)g 10 mg/m3 300c
Inhalation (max)g 100 mg/m3 50 m3 3000c
Inhalationh 430 mg/m3 12700c
Smokers 8 µg/cigarettei 140 cigarettes/week 1.1
a Based on a breathing rate of 20 m3/day
b Values from Tables 2 and 3
c Retention 60%
d Lovegren et al. (1979) (highest reported value)
e Highest reported values of uncontaminated groundwater
f Lowest reported value of contaminated groundwater
g Bardodej & Círek (1988)
h Corresponds to occupational exposure limits in several countries
i Wallace et al. (1987a)
A 2-year carcinogenicity study has been performed by the National
Toxicology Program (USA) but the results are not yet available.
10.2 Evaluation of effects on the environment
Ethylbenzene is found in air, water, soil, sediment, biota and
groundwater. It is released primarily into air and water from various
natural and anthropogenic sources. The atmosphere is the major sink
for ethylbenzene. Ethylbenzene is rapidly photo-oxidized in the
atmosphere and this may contribute to photo-chemical smog formation.
In water, the key processes determining overall fate are
volatilization and biodegradation.
The log octanol/water partition coefficient is 3.13, indicating a
potential for bioaccumulation. However, the limited evidence
available shows that ethylbenzene bioconcentration factors are low for
fish and molluscs. Elimination from aquatic organisms appears to be
rapid. Biomagnification through the food chain is unlikely.
Mean levels of ethylbenzene in air ranging from 0.74 to 100
µg/m3 have been measured at urban sites. Industrial releases and
vehicle emissions are the principal sources of ethylbenzene. Levels
found at rural sites are generally <2 µg/m3. The levels of
ethylbenzene in surface water are generally less than 0.1 µg/litre in
non-industrial areas. In industrial and urban areas ethylbenzene
concentrations of up to 15 µg/litre have been reported. Urban
run-off, effluent and landfill leachate are sources of local
contamination. Ethylbenzene levels in sediment are generally < 0.5
µg/kg. Levels of ethylbenzene between 1 and 5 µg/kg have been found
in sediments from heavily industrialized areas. Ethylbenzene levels
in uncontaminated groundwater are generally < 0.1 µg/litre. However,
much higher levels have been reported for groundwater contaminated via
waste disposal, fuel spillage and industrial facilities.
Acute toxicity studies on aquatic organisms show ethylbenzene to
be of moderate toxicity. The lowest acute toxicity values are 4.6
mg/litre for algae (72-h EC50), 1.8 mg/litre for daphnids (48-h LC50)
and 4.2 mg/litre for fish (96-h LC50). There are no chronic toxicity
studies on aquatic organisms.
There is limited information regarding the toxicity of
ethylbenzene to bacteria and earthworms. There are no data for
terrestrial plants, birds or wild mammals.
On the basis of available data, it is concluded that ethylbenzene
is unlikely to be found at levels in the environment that will cause
adverse effects on aquatic and terrestrial ecosystems, except in cases
of spills or point-source emissions.
11. CONCLUSIONS
Ethylbenzene has low toxicity. Its vapour irritates the mucous
membranes to a limited extent and causes prenarcotic effects on the
central nervous system.
With respect to the general population, a tentative guidance
value of 22 mg/m3 (5 ppm) for ethylbenzene in inhaled air has been
derived, although information on certain important toxicity end-points
are unavailable. This value would correspond to a weekly absorbed
dose (daily ventilation of 20 m3 with 60% retention) of about 2000
mg. It is at least 200 times higher than the dose received in the
most polluted living environment reported. Hence, no harmful effects
on the general population would be expected. However, it should be
noted that the guidance value of 22 mg/m3 (5 ppm) is about 10 times
higher than the odour threshold (about 2.2 mg/m3; 0.5 ppm), and so
exposure at that level may cause annoyance. On the other hand, odour
detection may be considered to be a safeguard against excessive
exposure.
Ethylbenzene is a non-persistent chemical and is degraded
primarily by photooxidation and biodegradation. Volatilization to the
atmosphere is rapid. Photooxidation reactions of ethylbenzene may
contribute to photochemical smog formation.
Limited evidence suggests that bioaccumulation is low in aquatic
organisms. Ethylbenzene is unlikely to cause adverse effects in
aquatic or terrestrial ecosystems except in cases of spills or
point-source emissions.
12. FURTHER RESEARCH
a) To fill the data gaps that currently limit the toxicological
evaluation of ethylbenzene, an appropriate rodent carcinogenicity
study and a reproductive toxicity study are needed. (The former
study has been conducted but the study report has not yet been
published.)
b) There is little information on the long-term effects of
ethylbenzene in humans and, in particular, no dose-response or
dose-effect data are at hand. Epidemiological studies of
populations occupationally exposed to ethylbenzene should be
encouraged. In this context, the use of ethylbenzene metabolites
in urine as a marker of exposure can be of special value because
the method determines the internal doses that individuals receive
via all routes of exposure. Because ethylbenzene is a central
nervous system depressant, and because some studies suggest that
high doses of the substance or its metabolites may affect the
metabolism of some neurotransmitters in the brain, epidemiological
studies should address the central nervous system as a potential
target organ. Moreover, since ethylbenzene is almost invariably
only one of the components in solvent mixtures at the workplace,
study designs that address possible interactions between
ethylbenzene and other solvents are desirable.
c) Further mechanistic studies are needed.
13. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
A guideline value of 300 µg/litre for ethylbenzene in
drinking-water was recommended by WHO in 1993.
REFERENCES
Abernethy S, Bobra AM, Shiu WY, Wells PG, & Mackay D (1986) Acute
lethal toxicity of hydrocarbons and chlorinated hydrocarbons to two
planktonic crustaceans: The key role of organism-water partitioning.
Aquat Toxicol, 8: 163-174.
ACGIH (1985-1986) Threshold limit values and biological exposure
indices. Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
Aitio A, Riihimäki V, Liesivuori J, Järvisalo J, & Hernberg S (1994)
Biological monitoring. In: Zenz C, Dickerson OB, & Horvath EP Jr ed.
Occupational medicine, 3rd ed. Saint Louis, Missouri, C.V. Mosby &
Co., pp 132-158.
Andersson K, Fuxe K, Nilsen OG, Toftgĺrd R, Eneroth P, & Gustafsson JĹ
(1981) Production of discrete changes in dopamine and noradrenaline
levels and turnover in various parts of the rat brain following
exposure to xylene, ortho-, meta-, and para-xylene, and
ethylbenzene. Toxicol Appl Pharmacol, 60: 535-548.
Angerer J & Lehnert G (1979) Occupational chronic exposure to organic
solvents. VIII. Phenolic compounds-metabolites of alkylbenzenes in
man. Simultaneous exposure to ethylbenzene and xylenes. Int Arch Occup
Environ Health, 43: 145-150.
Angerer J & Wulf H (1985) Occupational chronic exposure to organic
solvents. XI. Alkylbenzene exposure of varnish workers: effects on
hematopoietic system. Int Arch Occup Environ Health, 56: 307-321.
Annable MD, Wallace RB, Hayden NJ, & Voice TC (1993) Reduction of
gasoline component leaching potential by soil venting. J Contam
Hydrol, 12(1-2): 151-170.
Anon (1987) Chemical review: ethylbenzene. Danger Prop Ind Mater Rep,
7: 13-35.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Holler JS, Needham LL, &
Patterson DG Jr (1992) Determining volatile organic compounds in human
blood from a large sample production by using purge and trap gas
chromatography and mass spectrometry. Anal Chem, 64(9): 1021-1029.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, & Wooten JV (1994) Blood
concentrations of volatile organic compounds in a non occupationally
exposed US population and in groups with suspected exposure. Clin
Chem, 40(7): 1401-1404.
Ĺstrand I, Engström J, & Övrum P (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man. Scand J
Work Environ Health, 4: 185-194.
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions
of the hydroxyl radical with organic compounds under atmospheric
conditions. Chem Rev, 85: 69-201.
ATSDR (1990) Toxicological profile for ethylbenzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry (ATSDR/TP/-90-15).
Barber ED, Teetsel NM, Kolberg KF, & Guest D (1992) A comparative
study of the rates of in vitro percutaneous absorption of eight
chemicals using rat and human skin. Fundam Appl Toxicol, 19: 493-497.
Bardodej Z & Bardodejová E (1970) Biotransformation of ethylbenzene,
styrene, and alpha-methylstyrene in man. Am Ind Hyg Assoc J, 31:
206-209.
Bardodej Z & Círek A (1988) Long-term study on workers occupationally
exposed to ethylbenzene. J Hyg Epidemiol Microbiol Immunol, 32: 1-5.
Benville PE Jr & Korn S (1977) The acute toxicity of six monocyclic
aromatic crude oil components to striped bass (Morone saxatilis) and
bay shrimp (Crago franciscorum). Calif Fish Game, 63: 204-209.
Bestetti G & Galli E (1984) Plasmid-coded degradation of ethylbenzene
and 1-phenylethanol in Pseudomonas fluorescens. FEMS Microbiol Lett,
21: 165-168.
Bevan MAJ, Proctor CJ, Baker-Rogers J, & Warren ND (1991) Exposure to
carbon monoxide, respirable suspended particulates and volatile
organic compounds while commuting by bicycle. Environ Sci Technol,
25: 788-791.
Bos R, Guicherit R, & Hoogeveen A (1977) Distribution of some
hydrocarbons in ambient air near Delft and the influence on the
formation of secondary air pollutants. Sci Total Environ, 7: 269-281.
Boyd SA, Xiangcan J, & Lee JF (1990) Sorption of nonionic organic
compounds by corn residues from a no-tillage field. J Environ Qual,
19(4): 734-738.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1982) [Findings concerning the harmful effect
of water pollutants on Daphnia magna in a further developed
standardized test procedure.] Z Wasser/Abwasser Forsch, 15(1): 1 (in
German).
Brown-Woodman PDC, Webster WS, Picher K, & Ritchie HE (1991)
Embryotoxicity of xylene and toluene: an in vitro study. Ind Health,
29: 139-152.
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other trace
compounds in urban air. Chemosphere, 17: 2363-2380.
Buccafusco RJ, Ells SJ, & LeBlanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Burghardt E & Jeltes R (1975) Gas chromatographic determination of
aromatic hydrocarbons in air using a semi-automatic preconcentration
method. Atmos Environ, 9: 935-940.
Burnham AK, Calder GV, Fritz JS, Junk GA, Svec HJ, & Willis R (1972)
Identification and estimation of neutral organic contaminants in
potable water. Anal Chem, 44: 139-142.
Bysshe SE (1982) Bioconcentration factors in aquatic organisms. In:
Lyan WJ, Reehl WF, & Rosenblatt DH ed. Handbook of chemical property
estimation methods - Environmental behaviour of organic compounds. New
York, McGraw-Hill Book Company, pp 5/1-5/30.
Caldwell RS, Caldarone EM, & Mallon MH (1976) Effects of a seawater-
soluble fraction of Cook Inlet crude oil and its major aromatic
components on larval stages of the Dungeness crab, Cancer
magister dana. In: Fate and effects of petroleum hydrocarbon in
marine organisms and ecosystems. Oxford, New York, Pergamon Press, pp
210-220.
Callahan CA, Shirazi MA, & Neuhauser EF (1994) Comparative toxicity of
chemicals to earthworms. Environ Toxicol Chem, 13(2): 291-298.
Callahan MA (1979) Water related environmental fate of 129 priority
pollutants. Washington, DC, US Environmental Protection Agency (EPA
440/4-79-029b).
Cavender F (1993) Aromatic hydrocarbons. In: Clayton GD & Clayton FE
ed. Patty's industrial hygiene and toxicology, 4th revis ed. New
York, John Wiley and Sons, vol 2B, pp 1267-1442.
Chan CC, Özkaynak H, Spengler JD, & Sheldon L (1991a) Driver exposure
to volatile organic compounds, CO, ozone and NO2 under different
driving conditions. Environ Sci Technol, 25: 964-972.
Chan CC, Spengler JD, Özkaynak H, & Lefkopoulou M (1991b) Commuter
exposures to VOCs in Boston, Massachusetts. J Air Waste Manage Assoc,
41(12): 1594-1600.
Chin BH, McKelvey JA, Tyler TR, Calisti LJ, Kozbelt SJ, & Sullivan LJ
(1980a) Absorption, distribution, and excretion of ethylbenzene,
ethylcyclohexane and methylethylbenzene isomers in rats. Bull Environ
Contam Toxicol, 24: 477-483.
Chin BH, McKelvey JA, Calisti LJ, Kozbelt SJ, & Sullivan LJ (1980b) A
comparison of in vivo and in vitro (tissue explant) techniques:
metabolic profile of ethylbenzene in the rat and the dog. Bull Environ
Contam Toxicol, 25: 241-245.
Chiou CT, Porter PE, & Schmedding DW (1983) Partition equilibria of
non-ionic organic compounds between soil organic matter and water.
Environ Sci Technol, 17: 227-231.
Chou WL, Speece RE, & Siddiqi RH (1978) Acclimation and degradation of
petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
CITI (1992) Biodegradation and bioaccumulation data on existing
chemicals based on the CSCL Japan. Tokyo, Chemicals Inspection and
Testing Institute, pp 3-9.
Clapp LW, Talarczyk MR, Park JK, & Boyle WC (1994) Performance
comparison between activated sludge and fixed-film processes for
priority pollutant removals. Water Environ Res, 66(2): 153-160.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984a) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural and motorway locations.
Environ Pollut, B7: 141-158.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984b) Ambient air
measurements of aromatic and halogenated hydrocarbons at urban, rural
and motorway locations. Sci Total Environ, 39: 265-279.
Climie IJG, Hutson DH, & Stoydin G (1983) The metabolism of
ethylbenzene hydroperoxide in the rat. Xenobiotica, 13: 611-618.
Cline PV & Viste DR (1985) Migration and degradation patterns of
volatile organic compounds. Waste Manage Res, 3: 351-360.
Cole RH, Frederick RE, Healy RP, & Rolan RG (1984) Preliminary
findings of the priority pollutant monitoring project of the
nationwide urban runoff program. J Water Pollut Control Fed,
56: 898-908.
Coleman WE, Munch JW, Streicher RP, Ringhand HP, & Kopfler FC (1984)
Identification and measurement of components in gasoline, kerosene and
No.2 fuel oil that partition into the aqueous phase after mixing. Arch
Environ Contam Toxicol, 13: 171-178.
Cotruvo JA (1985) Organic micropollutants in drinking water: An
overview. Sci Total Environ, 47: 7-26.
Cox DP & Goldsmith CD (1979) Microbial conversion of ethylbenzene to
1-phenylethanol and acetophenone by Nocardia tartaricans ATCC 31190.
Appl Environ Microbiol, 38: 514-520.
Cozzarelli IM, Eganhouse RP, & Baedecker MJ (1990) Transformation of
monoaromatic hydrocarbons to organic acids in anoxic groundwater
environment. Environ Geol Water Sci, 16: 135-141.
Cragg ST, Clarke EA, Daly IW, Miller RR, Terrill JB, & Ouellette RE
(1989) Subchronic inhalation toxicity of ethylbenzene in mice, rats,
and rabbits. Fundam Appl Toxicol, 13: 399-408.
Crookes MJ & Howe PD (1992) Environmental hazard assessment:
Ethylbenzene. Garston, United Kingdom, Department of the Environment,
Directorate for Air, Climate and Toxic Substances, Toxic Substances
Division, Public Building Research Establishment, 40 pp (TSD/7).
Curvall M, Enzell CR, & Pettersson B (1984) An evaluation of the
utility of four in vitro short term tests for predicting the
cytotoxicity of individual compounds derived from tobacco smoke. Cell
Biol Toxicol, 1: 173-193.
Damgĺrd Nielsen GD & Alarie Y (1982) Sensory irritation, pulmonary
irritation and respiratory stimulation by airborne benzene and
alkylbenzenes: Prediction of safe industrial exposure levels and
correlation with their thermodynamic properties. Toxicol Appl
Pharmacol, 65: 459-477.
Dannecker W, Schröder B, & Stechmann H (1990) Organic and inorganic
substances in highway tunnel exhaust air. Sci Total Environ, 93:
293-300.
Dean BJ, Brooks TM, Hodson-Walker G, & Hutson DH (1985) Genetic
toxicology testing of 41 industrial chemicals. Mutat Res, 153: 57-77.
De Bortoli M, Knöppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1984) Integrating 'real life' measurements of
organic pollution in indoor and outdoor air of homes in Northern
Italy. In: Proceedings of the 3rd International Conference on Indoor
Air Quality and Climate. Stockholm, Swedish Council for Building
Research, vol 4, pp 21-26.
DEC (1992) Health-based recommended occupational exposure limit for
ethylbenzene. The Hague, Directorate-General of Labour.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
DFG (German Research Council) (1985) [Materials hazardous to health.
Justification of MAC values in terms of toxicology and occupational
medicine], 11th ed. Weinheim, VCH Publishers (in German).
Dills RL, Kent SD, Checkoway H, & Kalman DA (1991) Quantification of
volatile solvents in blood by static headspace analysis. Talanta,
38: 365-374.
Donner M, Mäki-Paakkanen J, Norppa H, Sorsa M, & Vainio H (1980)
Genetic toxicology of xylenes. Mutat Res, 74: 171-172.
Dreisch FA & Munson TO (1983) Purge-and-trap analysis using fused
silica capillary column GC/MS. J Chromatogr Sci, 21: 111-118.
Drummond L, Caldwell J, & Wilson HK (1989) The metabolism of
ethylbenzene and styrene to mandelic acid: stereo chemical
considerations. Xenobiotica, 19: 199-207.
Drummond L, Caldwell J, & Wilson HK (1990) The stereo selectivity of
1,2-phenylethanediol and mandelic acid metabolism and disposition in
the rat. Xenobiotica, 20: 159-168.
Durst GL & Laperle EA (1990) Styrene monomer migration as monitored by
purge and trap gas chromatography and sensory analysis for polystyrene
containers. J Food Sci, 55(2): 522-524.
Dutkiewicz T & Tyras H (1967) A study of the skin absorption of
ethylbenzene in man. Br J Ind Med, 24: 330-332.
EAJ (1989) Chemicals in the environment. Tokyo, Environment Agency of
Japan.
ECETOC (1986) Joint assessment of commodity chemicals No.7:
Ethylbenzene. Brussels, European Chemical Industry Ecology and
Toxicology Centre.
El Masry AM, Smith JN, & Williams RT (1956) Studies in detoxication.
69. The metabolism of alkylbenzenes: n-propylbenzene and
n-butylbenzene with further observations on ethylbenzene. Biochem J,
64: 50-56.
Elovaara E, Engström K, Nickels J, Aitio A, & Vainio H (1985)
Biochemical and morphological effects of long-term inhalation exposure
of rats to ethylbenzene. Xenobiotica, 15: 299-308.
Engström KM (1984a) Urinalysis of minor metabolites of ethylbenzene
and m-xylene. Scand J Work Environ Health, 10: 75-81.
Engström KM (1984b) Metabolism of inhaled ethylbenzene in rats. Scand
J Work Environ Health, 10: 83-87.
Engström J & Bjurström R (1978) Exposure to xylene and ethylbenzene.
II. Concentration in subcutaneous adipose tissue. Scand J Work Environ
Health, 4: 195-203.
Engström KM & Rantanen J (1974) A new gas chromatographic method for
determination of mandelic acid in urine. Int Arch Arbeitsmed,
33: 163-167.
Engström KM, Riihimäki V, & Laine A (1984) Urinary disposition of
ethylbenzene and m-xylene in man following separate and combined
exposure. Int Arch Occup Environ Health, 54: 355-363.
Engström KM, Elovaara E, & Aitio A (1985) Metabolism of ethylbenzene
in the rat during long-term intermittent inhalation exposure.
Xenobiotica, 15: 281-286.
Feiler HD, Vernick AS, & Storch PJ (1979) Fate of priority pollutants
in POTW's. In: Proceedings of the 8th National Conference on Municipal
Sludge Management, pp 72-81.
Fellin P & Otson R (1993) Seasonal trends of volatile organic
compounds (VOCs) in Canadian homes. In: Proceedings of the 6th
Conference on Indoor Air Quality and Climate, Helsinki, 1993, vol 2,
pp 117-122.
Filipovic D, Paulsen MD, Loida PJ, Sligar SG, & Ornstein RL (1992)
Ethylbenzene hydroxylation by cytochrome P450cam. Biochem Biophys Res
Commun, 189: 488-495.
Fishbein L (1985) An overview of environmental and toxicological
aspects of aromatic hydrocarbons. IV. Ethylbenzene. Sci Total Environ,
44: 269-287.
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15: 219-232.
Först C, Stieglitz L, Roth W, & Kuhnmünch S (1984) Quantitative
analysis of volatile organic compounds in landfill leachates. Int J
Environ Anal Chem, 37: 287-293.
Frank H, Senf L, & Welsch T (1990) Some application of wide bore
capillary gas chromatography in occupational medicine. Z Gesamte Hyg
Grenzgeb, 36(12): 668-671.
Gamberale F, Annwall G, & Hultengren M (1978) Exposure to xylene and
ethylbenzene. III. Effects on central nervous functions. Scand J Work
Environ Health, 4: 204-211.
Galassi S, Mingazzini M, Vigano L, Cesareo D, & Tosato ML (1988)
Approaches to modelling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicol Environ Saf, 16: 158-169.
Garcia JP, Beyne-Masclet S, Mouvier G, & Masclet P (1992) Emissions of
volatile organic compounds by coal-fire power stations. Atmos Environ,
A26(9): 1589-1597.
Gentry SJ & Walsh PT (1987) Eight-hour TWA personal monitoring using a
diffusive sampler and short-term stain tube. Am Ind Hyg Assoc J,
48: 287-292.
Gibson DT, Gschwendt B, Yeh WK, & Kobal VM (1973) Initial reactions in
the oxidation of ethylbenzene by Pseudomonas putida. Biochemistry,
12: 1520-1528.
Gomez-Belinchon JI, Grimalt JO, & Abaigčs J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water Res, 25: 577-589.
Goodenkauf O & Atkinson JC (1986) Occurrence of volatile organic
chemicals in Nebraska ground water. Ground Water, 24: 231-233.
Gossett RW, Brown DA, & Young DR (1983) Predicting the bioaccumulation
of organic compounds in marine organisms using octanol/water partition
coefficients. Mar Pollut Bull, 14: 387-392.
Grob K (1973) Organic substances in potable water and in its
precursor. Part 1. Methods for their determination by gas-liquid
chromatography. J Chromatogr, 84: 255-273.
Grob K & Grob G (1971) Gas-liquid chromatographic-mass spectrometric
investigation of C6-C20 organic compounds in an urban atmosphere
- An application of ultra trace analysis on capillary columns. J
Chromatogr, 62: 1-13.
Gromiec JP & & Piotrowski JK (1984) Urinary mandelic acid as an
exposure test for ethylbenzene. Int Arch Occup Environ Health,
55: 61-72.
Gschwend PM, Zafiriou OC, Mantoura RFC, Schwarzenbach RP, & Gagosian
RB (1982) Volatile organic compounds at a coastal site. I. Seasonal
variations. Environ Sci Technol, 16: 31-38.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gut I, Terelius Y, Frantik E, Linhart I, Soucek P, Filipcová B, &
Klucková H (1993) Exposure to various benzene derivatives
differentially induces cytochromes P450 2B1 and P450 2E1 in rat liver.
Arch Toxicol, 67: 237-243.
Hajimiragha H, Ewers U, Brockhaus A, & Boettger A (1989) Levels of
benzene and other volatile aromatic compounds in the blood of
non-smokers and smokers. Int Arch Occup Environ Health, 61: 513-518.
Hampton CV, Pierson WR, Schuetzle D, & Harvey TM (1983) Hydrocarbon
gases emitted from vehicles on the road. 2. Determination of emission
rates from diesel and spark-ignition vehicles. Environ Sci Technol,
17: 699-708.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(Suppl 4): 66-75.
Harkov R, Kebbekus B, Bozzelli JW, & Lioy PJ (1983) Measurement of
selected volatile organic compounds at three locations in New Jersey
during the summer season. J Air Pollut Control Assoc, 33: 1177-1183.
Harper M & Purnell CJ (1990) Alkylammonium montmorillonites as
adsorbents for organic vapors from air. Environ Sci Technol,
24: 55-62.
Heilbron I, Bunbury HM, & Jones WE ed. (1946) Dictionary of organic
compounds. London, vol II, p 20.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Herman DC, Inniss WE, & Mayfield CI (1990) Impact of volatile aromatic
hydrocarbon, alone or in combination, on growth of the freshwater alga
Selenastrum capriocornutum. Aquat Toxicol, 18(2): 87-100.
Hester NE & Meyer RA (1979) A sensitive technique for measurement of
benzene and alkylbenzenes in air. Environ Sci Technol, 13: 107-109.
Hiatt MH (1981) Analysis of fish and sediment for volatile priority
pollutants. Anal Chem, 53: 1541-1543.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography-mass spectrometry. Anal Chem, 55: 506-516.
Hodgson AT, Daisey JM, & Grot RA (1991) Sources and source strengths
of volatile organic compounds in a new office building. J Air Waste
Manage Assoc, 41(11): 1461-1468.
Holm S, Norbäck D, Frenning B, & Göthe CJ (1987) Hydrocarbon exposure
from handling jet fuel of some Swedish aircraft units. Scand J Work
Environ Health, 13: 438-444.
Holmberg B & Malmfors T (1974) The cytotoxicity of some organic
solvents. Environ Res, 7: 183-192.
Howard PH (1989) Handbook of environmental fate and exposure data for
organic chemicals. Volume 1: Large production and priority pollutants.
Chelsea, Michigan, Lewis Publishers, Inc.
Howard PH (1991) Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers, Inc., pp 340-341.
Hunt WF Jr, Faoro RB, & Freas W (1986) Report of the interim data base
for state and local air toxic volatile organic chemical measurements.
Washington, DC, US Environmental Protection Agency (EPA 450/4-86-012).
Hutchins SR, Sewell GW, Kovacs DA, & Smith GA (1991a) Biodegradation
of aromatic hydrocarbons by aquifer microorganisms under denitrifying
conditions. Environ Sci Technol, 25: 68-76.
Hutchins SR, Downs WC, Wilson JT, Smith GB, Kovacs DA, Fine DD,
Douglass RH, & Hendrix DJ (1991b) Effect of nitrate addition on
biotransformation of fuel-contaminated aquifer: Field demonstration.
Ground Water, 29(4): 571-580.
Inoue O, Seiji K, Kudo S, Jin C, Cai SX, Liu SJ, Watanabe T, Nakatsuka
H, & Ikeda M (1995) Urinary phenylglyoxylic acid excretion after
exposure to ethylbenzene among exposed Chinese workers. Int J Occup
Environ Health, 1: 1-8.
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: Assessing human health risks of chemicals:
Derivation of guidance values for health-based exposure limits.
Geneva, World Health Organization, 73 pp.
Jayanty RKM (1989) Evaluation of sampling and analytical methods for
monitoring toxic organics in air. Atmos Environ, 23: 777-782.
Jaynes WF & Boyd SA (1991) Clay mineral type and organic compound
sorption by hexadecyltrine-thylammonium-exchanged clays. Soil Sci Soc
Am J, 55(1): 43-48.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and in vehicle emissions. Environ Int, 11: 383-392.
Jüttner F (1988) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in forest air of the Southern Black
Forest. Chemosphere, 17: 309-317.
Kappeler T & Wuhrmann K (1978a) Microbial degradation of the
water-soluble fraction of gas oil, I. Water Res, 12: 327-333.
Kappeler T & Wuhrmann K (1978b) Microbial degradation of the
water-soluble fraction of gas oil. II. Bioassays with pure strains.
Water Res, 12: 335-342.
Kawai T, Yasugi T, Mizunuma K, Horiguchi S. Iguchi H, Uchida Y, Iwami
O, & Ikeda M (1992) Comparative evaluation of urinalysis and blood
analysis as means of detecting exposure to organic solvents at low
concentrations. Int Arch Occup Environ Health, 64(4): 223-234.
Kawamura K & Kaplan IR (1983) Organic compounds in the rainwater of
Los Angeles. Environ Sci Technol, 17: 497-501.
Kawata K & Fujeda Y (1993) Volatile aromatic hydrocarbons in ambient
air. Jpn J Toxicol Environ Health, 39(1): 37-43.
Kennicutt MC II, Brooks JM, & McDonald TJ (1984) Volatile organic
inputs from an ocean outfall near Barceloneta, Puerto Rico.
Chemosphere, 13: 535-548.
Kenrick MAP, Clark L, Baxter KM, Fleet M, Jones HA, Gibson TM, &
Turrell MB (1985) Trace organics in British aquifers - a baseline
survey. Water Research Centre, Environment (TR No. 223).
Keymeulen R, Van-Langenhove H, & Schamp N (1991) Determination of
monocyclic aromatic hydrocarbons in plant cuticles by gas
chromatography-mass spectrometry. J Chromatogr, 541(1-2): 83-88.
Kiese M & Lenk W (1974) Hydroxyacetophenones: urinary metabolites of
ethylbenzene and acetophenone in the rabbit. Xenobiotica, 4: 337-343.
Kleopfer RD & Fairless BJ (1972) Characterization of organic
components in a municipal water supply. Environ Sci Technol,
6: 1036-1037.
Koop DR & Laethem CL (1992) Inhibition of rabbit microsomal cytochrome
P-450 2E1-dependent p-nitrophenol hydroxylation by substituted benzene
derivatives. Drug Metab Dispos, 20: 775-777.
Korn M, Gfrörer W, Herz R, Wodarz I, & Wodarz R (1992)
Stereometabolism of ethylbenzene in man: gas chromatographic
determination of urinary excreted mandelic acid enantiomers and
phenylglyoxylic acid and their relation to the height of occupational
exposure. Int Arch Occup Environ Health, 64: 75-78.
Korte F & Boedefeld E (1978) Ecotoxicological review of the global
impact of the petroleum industry and its products. Ecotoxicol Environ
Saf, 2: 55-103.
Kroneld R (1989) Volatile pollutants in suburban and industrial air.
Bull Environ Contam Toxicol, 42: 868-872.
Kuhn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Labows J, Preti G, Hoelzle E, Leyden J, & Klingman A (1979) Analysis
of human axillary volatiles: Compounds of exogenous origin. J
Chromatogr, 163: 294-299.
Lanzerstorfer CH & Puxbaum H (1990) Volatile hydrocarbons in and
around Vienna, Austria. Water Air Soil Pollut, 51: 345-355.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lee JF, Crum JR, & Boyd SA (1989) Enhanced retention of organic
contaminants by soils exchanged with organic cations. Environ Sci
Technol, 23: 1365-1372.
LeGore RS (1974) The effect of Alaskan crude oil and selected
hydrocarbon compounds on embryonic development of the pacific oyster,
Crassostrea gigas. Doctoral Thesis, University of Washington. Diss
Abstr, B35: 3168.
Lesage S, Jackson RE, Priddle MW, & Riemann PG (1990) Occurrence and
fate of organic solvent residues in anoxic groundwater at the
Gloucester Landfill, Canada. Environ Sci Technol, 24: 559-566.
Levesque P & Dao LH (1989) Alkylation of benzene using an aqueous
solution of ethanol. Appl Catal, 53(2-3): 157-167.
Lewis PJ, Hagopian C, & Koch P (1983) Styrene. In: Kirk RE & Othmer DF
ed. Encyclopedia of chemical technology, 3rd ed. New York, Wiley
Interscience, vol 21, pp 770-801.
Lockhart WL, Wagemann R, Tracey B, Sutherland D, & Thomas DJ (1992)
Presence and implications of chemical contaminants in the freshwaters
of the Canadian Arctic. Sci Total Environ, 122(1/2): 165-243
Louw CW, Richards JF, & Faure PK (1977) Determination of volatile
organic compounds in city air by gas chromatography combined with
standard addition, selective subtraction, infra red spectrometry and
mass spectrometry. Atmos Environ, 11: 703-717.
Lovegren NV, Fisher GS, Legendre MG, & Schuller WH (1979) Volatile
constituents of dried legumes. J Agric Food Chem, 27: 851-853.
Lu BQ & Zhen ZH (1989) [Health standards for ethylbenzene in the air
of workplaces.] Beijing, Chinese Academy of Preventive Medicine,
Institute of Occupational Medicine (in Chinese).
Mabey W, Smith JH, Podoll, RT, Johnson HL, Mill T, Chou TW, Gate J,
Waight-Partridge I, Jaber H, & Vandenberg D (1982) Aquatic fate
process for organic priority pollutants. Washington, DC, US
Environmental Protection Agency (EPA 440/481-14).
McFall JA, Antoine SR, & DeLeon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14: 1253-1265.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma
cell forward mutation assay: III. 72 coded chemicals. Environ Mol
Mutagen, 12: 85-154.
Mackay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to atmosphere. Environ Sci Technol,
9: 1178-1180.
Mackay D, Shiu WY, & Sutherland RP (1979) Determination of air-water
Henry's law constants for hydrophobic pollutants. Environ Sci Technol,
13: 333-337.
Mackay D, Shiu WY, & Ma KC (1992) The illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Volume 1. Monoaromatic hydrocarbons, chlorobenzenes and
polychlorinated biphenyls. Chelsea, Michigan, Lewis Publishers, Inc.
MacLeod G & Ames JM (1987) Effect of water on the production of cooked
beef aroma compounds. J Food Sci, 52: 42-45.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
Current results and ongoing research. Am J Ind Med, 7: 415-446.
Masten LW, Boeri RL & Walker JD (1994) Strategies employed to
determine the acute aquatic toxicity of ethyl benzene, a
highly-volatile, poorly water-soluble chemical. Ecotoxicol Environ
Saf, 27: 335-348.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, p 460 (Resource Publication No. 160).
Michael LC, Pellizzari ED, & Norwood DL (1991) Application of the
master analytical scheme to the determination of volatile organics in
wastewater influent and effluents. Environ Sci Technol, 25: 150-155
Molnár J, Paksy KA, & Náray M (1986) Changes in the rat's motor
behaviour during 4-hour inhalation exposure to prenarcotic
concentrations of benzene and its derivatives. Acta Physiol Hung,
67: 349-354.
Morgan DL, Cooper SW, Carlock DL, Sykora JJ, Sutton B, Mattie DR, &
McDougal JN (1991) Dermal absorption of neat and aqueous volatile
organic chemicals in the Fischer 344 rat. Environ Res, 55: 51-63.
Mott JG & Romanow S (1991/1992) Sludge characterization, removal and
dewatering. J Hazard Mater, 29(1): 127-140.
Murray DAJ & Lockhart WL (1981) Microextraction and gas chromatographic
analysis of selected petroleum hydrocarbons in water and fish tissue.
J Chromatogr, 212: 305-311.
Namkung E & Rittmann BE (1987) Estimating volatile organic compound
emissions from publicly owned treatment works. J Water Pollut Control
Fed, 59: 670-678.
Nelson PF & Quigley SM (1982) Non-methane hydrocarbons in the
atmosphere of Sydney, Australia. Environ Sci Technol, 16: 650-655.
Neuhauser EF, Loehr RC, & Malecki MR (1986) Contact and artificial
soil tests using earthworms to evaluate the impact of wastes in soil.
In: Petros JK, Lacy WJ, & Conway RA end. Hazardous and industrial
solid waste testing: Fourth symposium. Philadelphia, Pennsylvania,
American Society for Testing and Materials, pp 192-203 (ASTM STP 886).
NIOSH (1984) Method 1501. In: Manual of analytical methods, 3rd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 2.
Noleau I & Toulemonde B (1988) Volatile components of roasted guinea
hen. Lebensm.Wiss Technol, 21: 195-197.
Norppa H & Vainio H (1983) Induction of sister-chromatid exchanges by
styrene analogues in cultured human lymphocytes. Mutat Res,
116: 379-387.
NTP (1992) Toxicity studies of ethylbenzene in F344/N rats and B6C3F1
mice (inhalation studies). Research Triangle Park, North Carolina, US
Department of Health and Human Services, National Toxicology Program
(NIH Publication No. 92-3129).
Nunes P & Benville PE Jr (1979) Uptake and depuration of petroleum
hydrocarbons in the Manila clam, Tapes semidecussata Reeve. Bull
Environ Contam Toxicol, 21:719-726.
Ogata M, Sugihara R, & Kira S (1977) Quantitative determination of
urinary hippuric acid and m- or p-methylhippuric acid as indices
of toluene and m- or p-xylene exposure by high performance liquid
chromatography. Int Arch Occup Environ Health, 39: 199-206.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities. J
Assoc Off Anal Chem, 65: 1370-1374.
Pankow JF, Isabelle LM, & Asher WE (1984) Trace organic compounds in
rain. I. Sampler design and analysis by adsorption/thermal desorption
(ATD). Environ Sci Technol, 18: 310-318.
Pellizzari ED, Hartwell TD, Harris BSH, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pellizzari ED, Sheldon L, Sparacino C, Bursey J, Wallace L, & Bromberg
S (1984) Volatile organic levels in indoor air. In: Berglund B,
Lindvall T, & Sundell J ed. Indoor air, chemical characterization and
personal exposure. Stockholm, Swedish Council for Building Research,
vol 4, pp 21-26.
Pellizzari ED, Wallace LA, & Gordon SM (1992) Elimination kinetics of
volatile organics in humans using breath measurements. J Expo Anal
Environ Epidemiol, 2: 341-355.
Perry DL, Chuang CC, Jungclaus GA, & Warner JS (1979) Identification
of organic compounds in industrial effluent discharges. Washington,
DC, US Environmental Protection Agency (EPA 600/4-79-016).
Petersson G (1982) Ambient hydrocarbons from motor-car assembly plants
in Scandinavia. Environ Pollut, B4: 207-217.
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38: 1419-1429.
Pitter P & Chudoba J (1990) Biodegradability of organic substances in
the aquatic environment. Boca Raton, Florida, CRC Press, Inc., p 269.
Potera FT (1975) The effects of benzene, toluene and ethylbenzene on
several important members of the estuarine ecosystem. Lehigh
University (Ph.D. dissertation).
Pussemier L, Szabó G, & Bulman RA (1990) Prediction of the soil
adsorption coefficient Koc for aromatic pollutants. Chemosphere
21: 1199-1212.
Pyykkö K, Paavilainen S, Metsä-Ketelä T, & Laustiola K (1987) The
increasing and decreasing effects of aromatic hydrocarbon solvents on
pulmonary and hepatic cytochrome P-450 in the rat. Pharmacol Toxicol,
60: 288-293.
Radzikowska-Kintzi H & Jakubowski M (1981) Internal standardization in
the head space analysis of organic solvents in blood. Int Arch Occup
Environ Health, 49: 115-123.
Rao PSC, Hornsby AG, & Jessup RE (1985) Indices for ranking the
potential for pesticide contamination of groundwater. Soil Crop Sci
Soc Proc, 44: 1-8.
Rappaport SM, Selvin S, & Waters MA (1987) Exposures to hydrocarbon
components of gasoline in the petroleum industry. Appl Ind Hyg,
2: 148-154.
Reinhard M, Goodman NL, & Barker JF (1984) Occurrence and distribution
of organic chemicals in two landfill leachate plumes. Environ Sci
Technol, 18: 953-961.
Rhue RD, Pennell KD, Rao PS, & Reve WH (1989) Competitive adsorption
of alkylbenzene and water vapors on predominantly mineral surfaces.
Chemosphere, 18(9-10): 1971-1986.
Romanelli A, Falzoi M, Mutti A, Bergamaschi E, & Franchini I (1986)
Effects of some monocyclic aromatic solvents and their metabolites on
brain dopamine in rabbits. J Appl Toxicol, 6: 431-435.
Römer KG, Federsel RJ, & Freundt KJ (1986) Rise of inhaled toluene,
ethyl benzene, m-xylene, or mesitylene in rat blood after treatment
with ethanol. Bull Environ Contam Toxicol, 37: 874-876.
Rosen AA, Skeel RJ, & Ettinger MB (1963) Relationship of river water
odour to specific organic contaminants. J Water Pollut Control Fed,
35: 777-782.
Roy WR & Griffin RA (1985) Mobility of organic solvents in
water-saturated soil material. Environ Geol Water Sci, 7: 241-247.
SAC (1989) Analysis of potentially dangerous substances in UK
waters. Bedford, United Kingdom, SAC Scientific (DOE Reference
No. PECD-7/7/306).
Sato A & Nakajima T (1987) Pharmacokinetics of organic solvent vapors
in relation to their toxicity. Scand J Work Environ Health, 13: 81-93.
Sauer TC Jr (1981) Volatile liquid hydrocarbon characterization of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environ Sci Technol, 15: 917-923.
Sax NI (1979) Dangerous properties of industrial chemicals, 5th ed.
New York, Van Nostrand Reinhold Co.
Seader JD (1982) Separation systems synthesis. In: Kirk RE & Othmer DF
ed. Encyclopedia of chemical technology, 3rd ed. New York, Wiley
Interscience, vol 20, pp 710-711.
Sequeira DJ, Eyer CS, Cawley GF, Nick TG, & Backes WL (1992)
Ethylbenzene-mediated induction of cytochrome P450 isozymes in male
and female rats. Biochem Pharmacol, 44: 1171-1182.
Sexton K & Westberg H (1980) Ambient hydrocarbon and ozone
measurements downwind of a large automotive painting plant. Environ
Sci Technol, 14: 329-332.
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environ Sci Technol, 16: 872-880.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1983) Measurements of
hazardous organic chemicals in the ambient atmosphere. Washington, DC,
US Environmental Protection Agency (EPA 600/3-83-002).
Singh HB, Ferek RJ, Salas LJ, & Nitz KC (1986) Toxic chemicals in the
environment: A program of field measurements. Washington, DC, US
Environmental Protection Agency (EPA 600/3-86/047).
Smith MR & Ratledge C (1989) Catabolism of alkylbenzenes by
Pseudomonas sp. NCIB 10643. Appl Microbiol Biotechnol, 32: 68-75.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: List VI. Am Ind Hyg Assoc J, 23: 95-107.
Sollenberg J (1991) Analytical isotachophoresis in biological
monitoring of exposure to industrial chemicals. J Chromatogr,
545: 369-374.
Sollenberg J, Smallwood AW, & Lowry LK (1985) Determination of
mandelic and phenylglyoxylic acids in rat urine by high-performance
liquid chromatography and by isotachophoresis. J Chromatogr,
343: 175-178.
Staples CA, Werner AF, & Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environ Toxicol Chem, 4(2): 131-142.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near to an underground coal gasification site in
North-Eastern Wyoming. Environ Sci Technol, 16: 582-587.
Susten AS, Niemeier RW, & Simon SD (1990) In vivo percutaneous
absorption studies of volatile organic solvents in hairless mice. II.
Toluene, ethylbenzene and aniline. J Appl Toxicol, 10: 217-225.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tester DJ & Harker RJ (1981) Groundwater pollution investigations in
the Great Ouse basin. Water Pollut Control, 80: 614-631.
Thomann RV (1989) Bioaccumulation model of organic chemical
distribution in aquatic food chains. Environ Sci Technol,
23(6): 699-707.
Thomas JM, Gordy VR, Fiorenza S, & Ward CH (1990) Biodegradation of
BTEX in subsurface materials contaminated with gasoline: Granger,
Indiana. Water Sci Technol, 22: 53-62.
Thomas RG (1982) Volatilization. In: Lyman WJ, Reehl WF, & Rosenblatt
DH ed. Handbook of chemical properties: Estimation methods. New York,
McGraw-Hill Book Co.
Thorburn S & Colenutt BA (1979) A gas chromatographic comparison of
volatile organic compounds in urban and rural atmospheres. Int J
Environ Stud, 13: 265-271.
Triebig G, Claus D, Csuzda I, Druschky KF, Holler P, Kinzel W, Lehrl
S, Reichwein P, Weidenhammer W, Weitbrecht WU, Weltle D, Schaller KH,
& Valentin H (1988) Cross-sectional epidemiological study on
neurotoxicity of solvents in paints and lacquers. Int Arch Occup
Environ Health, 60: 233-241.
Tsuruta H (1982) Percutaneous absorption of organic solvents. III. On
the penetration rates of hydrophobic solvents through the excised rat
skin. Ind Health, 20: 335-345.
Ullman (1983) [Encyclopaedia of chemical technology.] Weinheim, Verlag
Chemie, vol 24, pp 525-544 (in German).
Ungváry G & Tátrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats, and rabbits. Arch Toxicol,
8(suppl): 425-430.
US Chemical Industry (1994) Facts and figures for the chemical
industry. Chem Eng News, July 4: 28-33.
US EPA (1978) In-depth studies on health and environmental impacts of
selected water pollutants. Washington, DC, US Environmental Protection
Agency (Contract No. 68-01-4646).
US ITC (1987) Synthetic organic chemicals: United States production
and sales, 1986. Washington, DC, US International Trade Commission.
Utkin IB, Yakimov MM, Matveeva LN, Kozlyak EI, Rogozhin IS, Solomon
ZG, & Bezborodov AM (1991) Degradation of styrene and ethylbenzene by
Pseudomonas species Y2. Microbiol Lett, 77: 237-242.
Van Duijvenbooden W & Kooper WF (1981) Effects on groundwater flow and
groundwater quality of a waste disposal site in Noordwijk, The
Netherlands. Sci Total Environ, 21: 85-92.
Verhoeff AP, Suk J, & Van Wijnen JH (1988) Residential indoor air
contamination by screen printing plants. Int Arch Occup Environ
Health, 60: 201-209.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., pp 628-630.
Viganň L (1993) Reproductive strategy of Daphnia magna and toxicity
of organic compounds. Water Res, 27: 903-909.
Vincent R, Poirot P, Sabra I, Rieger B, & Cicolella A (1994)
Occupational exposure to organic solvents during paint stripping and
painting operations in the aeronautical industry. Int Arch Occup
Environ Health, 65(6): 377-380.
Volskay VT Jr & Grady CPL Jr (1990) Respiration inhibition kinetic
analysis. Water Res, 24: 863-874.
Vowles PD & Mantoura RFC (1987) Sediment-water partition coefficients
and HPLC retention factors of aromatic hydrocarbons. Chemosphere,
16: 109-116.
Waggott A (1981) Trace organic substances in the River Lee. In:
Chemistry in water reuse. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., vol 2, pp 55-99.
Wakeham SG, Davis AC, & Karas JL (1983) Mesocosm experiments to
determine the fate and persistence of volatile organic compounds in
coastal seawater. Environ Sci Technol, 17: 611-617.
Wallace LA, Pellizzari E, Hartwell TD, Perritt R, & Ziegenfus R
(1987a) Exposures to benzene and other volatile compounds from active
and passive smoking. Arch Environ Health, 42(5): 272-279.
Wallace LA, Pellizzari E, Hartwell TD, Sparacino C, Withmore R,
Sheldon L, Zelon H, & Perritt R (1987b) The TEAM study: Personal
exposures to toxic substances in air, drinking water, and breath of
400 residents of New Jersey, North Carolina, and North Dakota. Environ
Res, 43: 290-307.
Wallace LA, Pellizzari E, Leaderer B, Zelon H, & Sheldon L (1987c)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Wallace LA, Pellizzari ED, Hartwell TD, Davis V, Michael LC, &
Whitmore RW (1989) The influence of personal activities on exposure to
volatile organic compounds. Environ Res, 50(1): 37-55.
Warner JM & Beasley RK (1984) Purge and trap chromatographic method
for the determination of acrylonitrile, chlorobenzene,
1,2-dichloroethane and ethylbenzene in aqueous samples. Anal Chem,
56: 1953-1956.
Water Pollution Control (1989) Fate of organics on land-applied
sludges. Water Pollut Control, 127: 12.
Weast RC ed (1988) Handbook of chemistry and physics, 69th ed. Boca
Raton, Florida, CRC Press, Inc.
Webber D (1984) Top 50 chemical products. Chem Eng News, May 7: 8-10.
Westrick JJ, Mello JW, & Thomas RF (1984) The groundwater supply
survey. J Am Water Works Assoc, 76: 52-59.
Whitby FJ, Harland BJ, & Comber MHI (1982) Aromatic hydrocarbons in
the Tees Estuary. London, Department of Health (DOE Contract
PECD-7/7/23).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization.
Wiesenburg DA, Bodennec G, & Brooks JM (1981) Volatile liquid
hydrocarbons around a production platform in the northwest Gulf of
Mexico. Bull Environ Contam Toxicol, 27: 167-174.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformation of selected
alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic
aquifer material: A microcosm study. Environ Sci Technol, 20:
997-1002.
Windholz M ed (1983) The Merck index: An encyclopedia of chemicals,
drugs and biologicals, 10th ed. Rahway, New Jersey, Merck & Co., Inc.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene. Arch
Ind Health, 14: 387-398.
Wolff MS (1976) Evidence for existence in human tissues of monomers
for plastics and rubber manufacture. Environ Health Perspect,
17: 183-187.
Wolff MS, Daum SM, Lorimer WV, Selikoff IJ, & Aubrey BB (1977) Styrene
and related hydrocarbons in subcutaneous fat from polymerization
workers. J Toxicol Environ Health, 2: 997-1005.
Yamamoto RK & Cook WA (1968) Determination of ethyl benzene and
styrene in air by ultraviolet spectrophotometry. Am Ind Hyg Assoc J,
29: 238-241.
Zhang Z & Pawliszyn J (1993) Headspace solid-phase microextraction.
Anal Chem, 65(14): 1843-1852.
Zlatkis A & Jiao J (1991) Evaluation of new organoclay stationary
phases for the separation of ethylbenzene and xylene isomers.
Chromatographia, 31(9-10): 457-464.
1. RESUME
L'éthylbenzčne est un hydrocarbure aromatique qui s'obtient par
une réaction d'alkylation mettant en jeu le benzčne et l'éthylčne.
Aux Etats-Unis, on estime sa production annuelle ŕ environ 5 millions
de tonnes. En 1983, l'Europe occidentale en a produit environ 3
millions de tonnes. Il se présente sous la forme d'un liquide
incolore dégageant une odeur douceâtre qui rappelle l'essence. On
l'utilise principalement pour la production de styrčne. Ajouté ŕ du
xylčne technique, il sert également de solvant pour les peintures et
les vernis et on l'emploie aussi dans l'industrie chimique et dans
celle du caoutchouc. Il est présent dans le pétrole brut, les
produits raffinés dérivés du pétrole et dans leurs produits de
combustion.
L'éthylbenzčne n'est pas persistant car il se décompose dans
l'environnement, principalement par photo-oxydation et par dégradation
biologique. Il est possible que la photo-oxydation de l'éthylbenzčne
dans l'atmosphčre contribue ŕ la formation du smog photochimique.
Le logarithme du coéfficient de partage entre l'octanol et l'eau
est égal ŕ 3,13, ce qui indique que le composé se pręte ŕ une
bioaccumulation. Cependant, les données limitées dont on dispose
montrent que le facteur de bioaccumulation de l'éthylbenzčne est
faible pour les mollusques et les poissons. Apparemment, il est vite
éliminé par les organismes aquatiques.
A la campagne, la concentration d'éthylbenzčne dans l'air est
généralement inférieure ŕ 2 µg/m3. Sur des sites urbains, on a
trouvé des teneurs moyennes allant de 0,74 ŕ 100 µg/m3. La
concentration d'éthylbenzčne présente dans les eaux superficielles
est généralement inférieure ŕ 0,1 µg/litre dans les zones non
industrialisées. En revanche, dans les zones urbaines ou
industrialisées, la concentration peut atteindre 15 µg/litre. Dans
les sédiments, la concentration est en général inférieure ŕ
0,5 µg/litre, encore que l'on ait signalé des valeurs comprises entre
1 et 5 µg/litre dans des sédiments provenant de régions fortement
industrialisées. Dans les eaux souterraines non contaminées, la
concentration est habituellement inférieure ŕ 0,1 µg/litre, mais elle
est beaucoup plus élevée dans les eaux contaminées.
L'éthylbenzčne présente une toxicité aiguë modérée pour les
algues, les invertébrés aquatiques et les poissons. La valeur de la
CE50 ŕ 72 h est de 4,6 mg/litre pour l'algue Selenastrum
capricornutum, la CL50 ŕ 48 h est de 1,8 mg/litre pour Daphnia
magna, et on a une CL50 ŕ 96 h de 4,2 mg/litre pour la truite
arc-en-ciel. On ne possčde aucune donnée sur l'exposition chronique
des organismes aquatiques ŕ l'éthylbenzčne.
En ce qui concerne les bactéries et les lombrics, les données
toxicologiques sont limitées. Il n'en n'existe aucune sur les
végétaux terrestres, les oiseaux ou les mammifčres sauvages.
Chez l'homme, l'exposition ŕ l'éthylbenzčne se produit
principalement par inhalation; 40 ŕ 60% du composé sont retenus dans
les poumons. L'éthylbenzčne est fortement métabolisé, principalement
en acides mandélique et phénylglyoxylique. On peut utiliser les
métabolites présents dans les urines pour surveiller l'exposition
humaine.
Qu'elle soit aiguë ou chronique, la toxicité de l'éthylbenzčne
est faible pour l'homme et les animaux. Il exerce des effets toxiques
sur le systčme nerveux central et il est irritant pour les muqueuses
et les yeux. Le seuil de concentration pour ces effets chez l'homme a
été estimé ŕ environ 430-860 mg/m3 (100-200 ppm) lors d'une seule
exposition de courte durée.
Des rats et des souris ŕ qui on avait fait inhaler pendant 13
semaines de l'éthylbenzčne ŕ des concentrations allant jusqu'ŕ 4300
mg/m3, n'ont présenté aucune lésion histopathologique. La dose sans
effet observable (critčre retenu: l'augmentation du poids du foie) a
été estimée ŕ 2150 mg/m3 (500 ppm).
L'éthylbenzčne stimule les enzymes des microsomes hépatiques. Il
n'est ni mutagčne ni tératogčne pour le rat ou le lapin. On ne dispose
d'aucune donnée au sujet de ses effets toxiques éventuels sur
l'appareil reproducteur ni sur son pouvoir cancérogčne.
Une valeur-guide de 22 mg/litre (5 ppm) a été calculée ŕ partir
des résultats fournis par les études sur l'animal. On estime que la
population générale est exposée ŕ des concentrations inférieures ŕ
cette valeur, męme dans les cas les plus graves. On a constaté qu'une
exposition de longue durée en milieu professionnel, ŕ des concentration
de cet ordre, n'avait aucun effet nocif sur la santé des travailleurs
exposés.
RESUMEN
El etilbenceno es un hidrocarburo aromático que se obtiene por
alkilación del benceno y del etileno. La producción estimada en los
Estados Unidos de América es de unos cinco millones de toneladas por
ańo, y en Europa occidental fue de aproximadamente tres millones de
toneladas en 1983. El etilbenceno es un líquido incoloro de olor
dulce semejante al de la gasolina. Se utiliza principalmente para la
producción de estireno. También se utiliza en el xileno técnico como
disolvente de pinturas y lacas, así como en la industria del caucho y
en la fabricación de sustancias químicas. Se encuentra en el petróleo
crudo, en los productos de petróleo refinados y en productos de
combustión.
El etilbenceno es una sustancia química no persistente, que se
degrada principalmente por fotooxidación y biodegradación. Su
volatilización en la atmósfera es rápida. La reacción de foto-
oxidación del etilbenceno en la atmósfera puede contribuir a la
formación de niebla fotoquímica.
El logaritmo del coeficiente de reparto octanol-agua es 3,13, lo
que indica posibilidad de bioacumulación. Sin embargo, los limitados
indicios disponibles muestran que los factores de bioconcentración del
etilbenceno son bajos para peces y moluscos. La eliminación por los
organismos acuáticos parece ser rápida.
Los niveles de etilbenceno en el aire en puntos rurales son
generalmente inferiores a 2 µg/m3. En puntos urbanos se han
registrado niveles medios de etilbenceno que oscilan entre 0,74 y 100
µg/m3. Los niveles de etilbenceno detectados en las aguas
superficiales son generalmente inferiores a 0,1 µg/litro en zonas no
industriales. Se han comunicado concentraciones de etilbenceno de
hasta 15 µg/litro en zonas industriales y urbanas. Los niveles de
etilbenceno en sedimentos son generalmente inferiores a 0,5 µg/kg,
aunque en sedimentos de zonas muy industrializadas se han encontrado
niveles de 1 a 5 µg/kg. Las concentraciones en aguas subterráneas no
contaminadas son generalmente inferiores a 0,1 µg/litro, pero son
mucho más elevadas en aguas subterráneas contaminadas.
La toxicidad aguda del etilbenceno para las algas, los
invertebrados acuáticos y los peces es moderada. Los valores de
toxicidad aguda más bajos son de 4,6 mg/litro para el alga
Selenastrum capricornutum (CE50 a las 72 horas, sobre la base de la
inhibición del crecimiento), 1,8 mg/litro para Daphnia magna (CL50
a las 48 horas) y 4,2 mg/litro para la trucha irisada (CL50 a las 96
horas). No se dispone de información sobre la exposición crónica de
los organismos acuáticos al etilbenceno.
Hay información limitada sobre la toxicidad del etilbenceno para
las bacterias y para las lombrices. No hay datos relativos a las
plantas terrestres, las aves y los mamíferos silvestres.
La exposición humana al etilbenceno se produce principalmente por
inhalación; el 40-60% del etilbenceno inhalado se retiene en los
pulmones. El etilbenceno se metaboliza extensamente, transformándose
sobre todo en ácidos mandélico y fenilglioxílico. Estos metabolitos
urinarios pueden utilizarse para vigilar la exposición humana.
El etilbenceno tiene una toxicidad aguda y crónica baja tanto
para los animales como para el hombre. Es tóxico para el sistema
nervioso central e irrita las mucosas y los ojos. El umbral para esos
efectos en el ser humano después de exposiciones únicas breves se
estimó en aproximadamente 430-860 mg/m3 (100-200 ppm).
La inhalación de etilbenceno por ratas y ratones durante 13
semanas en concentraciones de hasta 4300 mg/m3 (1000 ppm) no dio
lugar a lesiones histopatológicas. El nivel sin efectos observados,
sobre la base de un aumento del peso del hígado en las ratas, fue de
2150 mg/m3 (500 ppm).
El etilbenceno es un inductor de las enzimas microsómicas
hepáticas. No es mutagénico ni teratogénico en ratas y conejos. No
se dispone de información sobre la toxicidad reproductiva ni la
carcinogenicidad del etilbenceno.
Se ha calculado un valor de orientación de 22 mg/m3 (5 ppm) a
partir de estudios realizados en animales. La exposición estimada de
la población general (incluso en la peor de las situaciones) es
inferior a ese valor de orientación. La exposición ocupacional a
largo plazo a concentraciones de etilbenceno estimadas en este orden
de magnitud no ocasionaron efectos adversos en la salud de los
trabajadores.
Ethylbenzene is metabolized by the microsomal cytochrome P-450
enzyme system. Although specific isozymes have not been unequivocally
identified, enzyme induction studies suggest that CYP2B1/2, CYP1A1/2
and CYP2E1 may be involved (see section 7.8).
Four male volunteers were exposed to 655 mg/m3 (150 ppm)
ethylbenzene for 4 h and urine was collected for 24 h. Mandelic acid
(71.5%) and phenylglyoxylic acid (19.1%) were the main metabolites,
but smaller amounts of 1-phenylethanol, p-hydroxyacetophenone,
m-hydroxyacetophenone, 1-phenyl-1,2-ethandiol, 4-ethylphenol,
omega-hydroxyacetophenone and acetophenone were also found. Ring
oxidation accounted for 4.0%. Simultaneous exposure to 150 ppm
m-xylene did not alter the urinary metabolite pattern, but it
delayed excretion and decreased the amounts of metabolites excreted
(Engström et al., 1984). Mandelic acid (64%) and phenylglyoxylic aid
(25%) were found to be the main urinary excretion products in
volunteers after an 8-h exposure to 99-365 mg/m3 (23-85 ppm)
ethylbenzene (Bardodej & Bardodejová, 1970).
When two volunteers were exposed by inhalation to 430 mg/m3 (100
ppm) ethylbenzene for 4 h, most of the mandelic acid was excreted as
the R-enantiomer (Drummond et al., 1989).
In a study by Korn et al. (1992), urinary samples from workers
exposed to ethylbenzene, toluene and xylenes were analysed. The
average urinary concentration of phenylglyoxylic acid was 50.1
mg/litre. The concentration of mandelic acids was 135.2 mg/litre, of
which 127.8 mg/litre was R-mandelic acid and 7.3 mg/litre was
S-mandelic acid. The R/S ratio was independent of the air
concentration of ethylbenzene, which varied between 6.4 and 142 mg/m3
(1.5 and 33 ppm).
Four female histology laboratory assistants were exposed to a
mixture of xylenes (75%) and ethylbenzene (25%). The air concentration
of the solvent was 160-179 mg/m3, and the concentration of
ethylbenzene in blood collected at the end of the working day was
reported to be 0.5 to 0.8 mg/litre. The 24-h excretion of
2-ethylphenol in urine varied between 4.4 and 6.0 mg corresponding to
1 to 1.5% of the retained ethylbenzene (Angerer & Lehnert, 1979). The
findings by Angerer & Lehnert (1979) that ethylbenzene is metabolized
to 2-ethylphenol could not be verified by Engström et al. (1984).
Male Wistar rats (6 per group) were exposed for 6 h to
ethylbenzene at 1290 or 2580 mg/m3 (300 or 600 ppm). The urine was
collected for 48 h from the onset of exposure. Altogether, 14
different metabolites from ethylbenzene were identified. The main
metabolites were 1-phenylethanol, mandelic acid and benzoic acid, each
of which accounted for about 25%. Only 13% (low dose) and 6% (high
dose) of the estimated absorbed doses were eliminated during the 6-h
exposure. Over the 48-h period, the corresponding values were 83% and
59%, respectively. The metabolic pattern was similar, irrespective of
the exposure level (Engström, 1984b).
In another study, male Wistar rats (20 per group) were exposed to
215, 1290 or 2580 mg ethylbenzene/m3 (50, 300 or 600 ppm) for 6
h/day, 5 days/week, for up to 16 weeks. Urinary excretion of some of
the metabolites was measured in weeks 2, 5 and 9. A significant
dose-related percentage decrease of phenylglyoxylic acid and hippuric
acid plus benzoic acid was found. A corresponding increase of
1-phenylethanol and omega-hydroxyacetophenone excretion was also
noted. The total amount of metabolites in urine collected during the
24 h after onset of exposure remained, however, constant at each
exposure level throughout the study (Engström et al., 1985).
When a single oral dose of 318 mg ethylbenzene/kg body weight was
administered to rabbits, the main urinary metabolites found were
hippuric acid and methylphenylglucuronic acid, which together
represented 60-70% of the dose, while mandelic acid and phenaceturic
acid were minor metabolites (El Masry et al., 1956).
It has been established in in vivo studies that experimental
animals convert ethylbenzene mainly to the R-enantiomer of mandelic
acid (Drummond et al., 1990). In an in vitro study it was found
that ethylbenzene is hydroxylated by cytochrome P-450cam (from
Escherichera coli) almost exclusively at the secondary ethyl carbon
with about a 2:1 ratio of R:S products (Filipovic et al., 1992).
The rates of metabolism of ethylbenzene have been studied in
vitro in rabbit liver and lung. Organs from five female animals
were used, but details of the experimental procedures were not
reported. For the liver (mean weight 76 g) the rate of metabolism was
453 nmol/g tissue per 10 min (34.4 µmol per liver per 10 min or 11.7
nmol per nmol cytochrome P-450 per 10 min). The corresponding figures
for lung (mean weight 7.7 g), were 680 nmol, 5.3µmol and 200.1 nmol,
respectively. Thus, lung tissue may significantly contribute to the
body clearance of ethylbenzene in rabbits (Sato & Nakajima, 1987).
6.4 Elimination and excretion
In humans, ethylbenzene is mainly excreted in the urine as
mandelic and phenylglyoxylic acids (Bardodej & Bardodejová, 1970;
Ĺstrand et al., 1978; Engström et al., 1984; Gromiec & Piotrowski,
1984). Only up to 5% of retained ethylbenzene is estimated to be
exhaled without transformation (Ĺstrand et al., 1978). The
elimination half-lives of ethylbenzene in exhaled air and urine have
been estimated to be 0.5-3 h and 8 h, respectively (Wolff, 1976). In
human volunteers exposed to 100 or 200 ppm "industrial xylene" for
2 h, there was no decline in the concentration of xylenes plus
ethylbenzene in the gluteal subcutaneous adipose tissue between 30 min
and 22 h after exposure (Engström & Bjurström, 1978). The elimination
of mandelic acid has been found to be biphasic, with half-lives of 3.1
and 24.5 h (Gromiec & Piotrowski, 1984).
The elimination kinetics for 10 volatile organic compounds,
including ethylbenzene, has been studied in human volunteers exposed
to a variety of consumer products. Breath samples were collected
post-exposure and analysed by GC/MS. The half-lives for the 10
chemicals varied from a few hours to 1-2 days. The authors concluded
that volatile organic compounds exhibit relatively short residence
times in the body (Pellizzari et al., 1992).
Male Harlan-Wistar rats exposed to 14C-ring-labelled
ethylbenzene (1000 mg/m3) for 6 h excreted 82% of the radioactivity
in the urine, 8.2% in expired air (0.03% as CO2) and 0.7% in faeces.
After 42 h, 0.2% remained in the tissues. The remaining 8.3% could not
be accounted for (Chin et al., 1980a).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Single high exposures to ethylbenzene cause irritation of the
mucous membranes and central nervous system effects. The results from
single exposure in vivo studies are summarized in Table 5.
Table 5. Single exposure of animals to ethylbenzenea
Species Route Dose Parameter Reference
Rat oral 3.5 g/kg LD50 Wolf et al. (1956)
Rat oral 4.7 g/kg LD50 Smyth et al. (1962)
Rat inhalation 9.37 g/m3 (2180 ppm) Minimum Molnár et al. (1986)
narcotic conc.
Rat inhalation 17.2 g/m3 (4000 ppm) 1 h LC10 Smyth et al. (1962)
Rat inhalation 17.2 g/m3 (4000 ppm) 4 h LC50 Smyth et al. (1962)
Rat inhalation 34.4 g/m3 (8000 ppm) 1 h LC100 Smyth et al. (1962)
Rabbit dermal 77.4 g/kg LD50 Smyth et al. (1962)
a Additional information is given in the following reviews: DFG (1985), ECETOC (1986).
7.2 Short-term exposure
In a short-term study, six male rats (Sprague Dawley) were
exposed for 6 h/day during 3 consecutive days to 8.6 g/m3 (2000 ppm)
ethylbenzene. The animals were killed 16-18 h after the last
exposure. Small increases in dopamine and noradrenaline levels and
turnover in various parts of the hypothalamus and the median eminence
were reported. Ethylbenzene was also found to produce selective
reduction in prolactin and corticosterone secretion and selective
increase in dopamine turnover within the dopamine-cholecystokinin-8-
immuno-reactive nerve terminals of the nucleus accumbens (posterior
part) (Andersson et al., 1981).
When eight male rabbits (New Zealand) were exposed 12 h daily for
7 days to 3.22 g/m3 (750 ppm) ethylbenzene, there was a marked
(p<0.05) depletion of striatal and tuberoinfundibular dopamine. Such
an effect was also caused by intraperitoneal dosing of rabbits (eight
per group) with mandelic or phenylglyoxylic acid (4 mmol/kg per day
for 3 days) in saline (Romanelli et al., 1986).
In a 4-week inhalation study Fischer-344 rats (five of each sex
per group) were exposed to ethylbenzene for 6 h/day, 5 days per week,
at exposure levels of 0, 426, 1643 or 3363 mg/m3 (0, 99, 382 or 782
ppm). At the two highest exposure levels, sporadic lacrimation and
salivation, as well as significantly (p<0.05) increased liver
weights, were seen. At the highest exposure level, there was a small
increase in leukocyte counts and, in males, a marginal increase in
platelet counts (Cragg et al., 1989).
In the same study, mice (B6C3F1) of both sexes were similarly
exposed. At 1643 and 3363 mg ethylbenzene/m3, females showed
significantly (p<0.01) increased absolute and relative liver weights.
In males a significantly (p<0.05) increased relative liver-to-brain
weight ratio was seen. Male and female rabbits (New Zealand White)
were also used in this study. The exposure levels were 0, 1643, 3363
and 6923 mg/m3 (0, 382, 782 and 1610 ppm). At the highest exposure
level females gained weight more slowly than controls but neither sex
exhibited gross or microscopic organ changes (Cragg et al., 1989).
No changes in mortality pattern were seen in the three species.
There were no changes in clinical chemistry parameters in rats or
rabbits. Mice were not subjected to clinical chemistry or
haematological examinations due to the small volume of blood that
could be collected. For similar reasons, urinalyses were performed
for rats (no change) but not for mice. Rabbits were excluded from
urinalysis for logistical reasons. No changes in gross or microscopic
pathology were noted in any of over 30 tissues from each of the three
species when the animals were exposed at the highest concentration
(Cragg et al., 1989).
7.3 Long-term exposure
7.3.1 Oral exposure
Matched groups of 10 Wistar female rats were given daily
ethylbenzene doses of 0, 13.6, 136, 408 or 680 mg/kg by stomach tube
5 days a week for 6 months. The two highest dosages induced slight
increases in liver and kidney weights and slight cloudy swelling of
parenchymal liver cells and of the tubular epithelium in the kidney
(Wolf et al., 1956).
7.3.2 Inhalation exposure
In a 13-week National Toxicology Program study, groups of 10 rats
(F-344/N) and 10 mice (B6C3F1) of each sex were exposed for 6 h (plus
10 min to reach 90% of the target chamber concentration) per day,
5 days per week for 92 (female rats), 93 (male rats), 97 (female mice)
or 98 (male mice) days, at ethylbenzene concen-trations of 0, 430,
1075, 2150, 3225 or 4300 mg/m3 (0, 100, 250, 500, 750 or 1000 ppm).
Blood for clinical chemistry and haematological examination was
collected on study days 4 and 23 and again at week 13 from both male
and female rats. Dose-related increases in absolute liver weight were
seen in both sexes of mice exposed to the two highest dose levels, and
the relative kidney weight of female mice exposed to 4300 mg/m3 was
greater than that of the controls. Increased absolute and relative
liver and kidney weights were seen in male rats exposed to the two
highest dose levels. Increased absolute liver and kidney weights were
seen in female rats exposed to the three highest dose levels, but no
increased relative liver and kidney weights were seen. No chemically
related histopathological changes were observed in any rat or mouse
tissues. Clinical chemistry results were negative (NTP, 1992).
In another study, groups of five male rats (Wistar) were exposed
for 6 h/day, 5 days/week to ethylbenzene concentrations of 0, 215,
1290 or 2580 mg/m3 (0, 50, 300 or 600 ppm) and sacrificed after 2, 5,
9 or 16 weeks of exposure. At 2580 mg/m3 liver cells showed a slight
proliferation of smooth endoplasmic reticulum, slight degranulation
and splitting of rough endoplasmic reticulum, and enlarged
mitochondria. At the same dose level, liver microsomal protein, but
not cytochrome P-450, concentration was slightly increased. There was
also an increase in NADPH-cytochrome c reductase, 7-ethoxycoumarin-
O-deethylase and UDPG-transferase activities in the liver. In the
kidney only the two latter enzymes showed dose-related increases.
Urinary excretion of thioethers was measured to ascertain the
generation of electrophilic intermediates during ethylbenzene
metabolism. Excretion of thioethers increased in a dose-dependent
manner, with some fluctuation over the course of 7 weeks, reaching
about eight times the control level at 2580 mg ethylbenzene/m3.
However, there was no decrease in hepatic or renal levels of
glutathione (GSH), indicating that the cells were able to maintain the
intra-cellular homeostasis of GSH during exposure (Elovaara et al.,
1985).
In inhalation experiments, matched groups of 10-25 male and
female Wistar rats, 5-10 guinea-pigs, 1-2 rabbits and 1-2 rhesus
monkeys of either sex or both sexes were all exposed 7 h/day, 5
days/week, for up to 6 months. The exposure levels were 0, 1720 and
2580 mg/m3 (0, 400 and 600 ppm) for 186 days, 5375 mg/m3 (1250 ppm)
for 214 days (no monkeys) or 9460 mg/m3 (2200 ppm) for 144 days (rats
only). Slight effects were seen in rats: increased liver and kidney
weights at 1720 mg/m3; increased liver and kidney weights at 2580
mg/m3; and small histopathological changes (cloudy swelling) in liver
and kidney at 5375 and 9460 mg/m3 (Wolf et al. 1956).
In guinea-pigs and monkeys slightly increased liver weights were
noted in the 2580 mg/m3 group only. At the same exposure level,
small histopathological effects in the testes, described as
degeneration of the germinal epithelium, were seen in rabbits and
monkeys. At 5375 mg/m3 a slight growth depression was noted in
guinea-pigs. The no-observed-effect level (all four species) was
considered to be about 860 mg/m3 (200 ppm) (Wolf et al., 1956). It
should be noted, however, that Cragg et al. (1989) found no
histopathological effects in the testes of rats and rabbits exposed to
up to 3363 mg/m3 (782 ppm) for 4 weeks, and the lack of toxicity was
confirmed by NTP (1992).
7.4 Skin and eye irritation, sensitization
Inhalation for 3 min of ethylbenzene at a concentration of 4300
mg/m3 caused slight nasal irritation in guinea-pigs, and an 8-min
exposure caused eye irritation as well. At 8600 mg/m3 a 1-min
exposure was enough to cause both effects (Cavender, 1993).
Two drops of undiluted ethylbenzene placed in the eyes of rabbits
resulted in slight conjunctival irritation but no effects on the
cornea (Wolf et al., 1956). A slight conjunctival irritation with
some reversible corneal injury was reported in rabbits in a study by
Smyth et al. 1962.
Undiluted ethylbenzene has been shown to produce moderate
irritation when applied to the uncovered skin of rabbits (Smyth et
al., 1962). The application of undiluted ethylbenzene to the ear and
to the shaved abdomen of rabbits up to 20 times during a 4-week period
resulted in moderate irritation. There was erythema and oedema with
superficial necrosis and exfoliation of large patches of skin (Wolf et
al., 1956).
No animal sensitization studies have been reported.
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
In an inhalation study, rats (Wistar or Sprague-Dawley) and
rabbits (New Zealand White) were exposed to 430 or 4300 mg/m3 (100 or
1000 ppm) ethylbenzene for 6 to 7 h/day on gestation days 1 to 19
(rats) or 1 to 24 (rabbits). All pregnant animals were sacrificed on
the day before term (day 21 for rats, day 30 for rabbits). The
rabbits had a significantly (p<0.05) reduced number of live pups per
litter at both exposure levels, but the number of implantations per
litter and the number of dead or resorbed fetuses per litter did not
differ from those of the controls. Maternal toxicity in rats exposed
to 4300 mg/m3 was reflected in increased liver, kidney and spleen
weights. There was a significant (p<0.05) increase in the incidence
of extra ribs in both of the exposed rat groups (Hardin et al., 1981).
In a further study, rats (CFY) were exposed to ethylbenzene
concentrations of 600, 1200 or 2400 mg/m3 continuously (24 h/day)
from day 7 to day 15 of pregnancy. They were then killed on day 21.
Mice (CFLP) were exposed for three periods of 4 h per day to 500
mg/m3 on days 6-15 of pregnancy and killed on day 18. Rabbits (New
Zealand White) were exposed continuously on days 7-20 of gestation to
500 or 1000 mg/m3 and were killed at day 30. The maternal toxic
effects (not specified) in mice and rats were moderate and
dose-dependent. In both species ethylbenzene caused skeletal growth
retardation, extra ribs and reduced fetal growth rate at the highest
concentration. In rabbits, the highest dose concentration caused mild
maternal toxic effects (decreased weight gain) and reduction in the
number of fetuses due to abortion (Ungváry & Tátrai, 1985).
When rats (F-344/N) and mice (B6C3F1) were exposed to
ethylbenzene at concentrations of 0, 430, 2150 and 4300 mg/m3 (0,
100, 500 and 1000 ppm), 6 h per day, 5 days per week, for 13 weeks,
there were no changes in sperm or vaginal cytology (NTP, 1992).
Rat embryos were explanted on day 9 of gestation and cultured in
rat serum with added xylene (containing 18% ethylbenzene) at
concentrations up to 1.0 ml/litre serum. Dose-dependent retardation
of growth and development was seen but there were no observable
teratogenic effects (Brown-Woodman et al., 1991).
No multigeneration and reproductive studies on ethylbenzene have
been reported.
7.6 Mutagenicity and related end-points
In a National Toxicology Program study, ethylbenzene was not
mutagenic in Salmonella tests and did not induce chromosomal
aberrations or sister chromatid exchange in Chinese hamster ovary
(CHO) cells in vitro, although it did induce trifluorothymidine
resistance in mouse lymphoma cells at the highest concentration tested
(80 mg/litre). There was no increase of micronuclei in the peripheral
blood of mice exposed to ethylbenzene (NTP, 1992).
In several other studies ethylbenzene did not induce point
mutations (with or without added metabolic activation system). In
addition, it did not cause an increase in the spontaneous
recessive-lethal frequency in the Drosophila recessive-lethal test,
nor had it any chromosomal effects in vitro (Donner et al., 1980;
Florin et al., 1980; Dean et al., 1985). Ethylbenzene had a marginal
effect on sister chromatid exchange in human lymphocytes in vitro
when a high (10 mmol/litre) concentration was used (Norppa & Vainio,
1983). In addition, in the TK+/- test in mouse lymphoma cells there
was a slight effect at a high concentration (80 mg/litre) (McGregor et
al., 1988). This study is obviously the same as the one subsequently
reported by NTP (1992).
No excess of chromosomal aberrations in bone marrow cells was
seen in rats after up to 18 weeks of exposure (6 h/day, 5 days/week)
to 300 ppm of a xylene mixture containing 18.3% ethylbenzene (Donner
et al., 1980).
7.7 Carcinogenicity
In a carcinogenicity study, rats (Sprague-Dawley) were exposed to
one of several aromatic hydrocarbons, including ethylbenzene. Groups
of rats (40 of each sex) were exposed to 500 mg ethylbenzene (in olive
oil) per kg body weight by gavage, 4 or 5 days per week for 104 weeks.
Results were determined after 141 weeks. The first malignant tumour,
a nephroblastoma, was observed after 33 weeks. The total number of
malignant tumours was 31 in the 77 animals of the exposed group alive
at 33 weeks compared with an incidence of 23 of 94 animals in the
control group. The authors concluded that ethylbenzene caused an
increase in the incidence of total malignant tumours, although there
was no increase in the incidence of any specific type of tumour
(Maltoni et al., 1985). It is difficult to draw any firm conclusion
from this study because of inadequate reporting.
7.8 Other special studies
Ethylbenzene was found to have low acute cytotoxicity in vitro
on Ehrlich ascites cells (Holmberg & Malmfors, 1974).
The effects of ethylbenzene have been studied in four in vitro
test systems: decreased cell growth in Ascites sarcoma BP 8 cells;
decreased oxidative metabolism in hamster brown fat cells; cell
membrane damage of human embryonic lung fibroblasts; and inhibition of
ciliary activity in chicken embryo trachea. On a 0-9 point scale
(equivalent to 0-100%) the activity of these four systems scored 4, 6,
8 and 8, respectively (Curvall et al., 1984).
The activity of cytochrome CYP2E1 can be monitored in microsomal
preparations by p-nitrophenol hydroxylation. When rabbit (white
male New Zealand) liver microsomes were treated with up to 0.25 mM
ethylbenzene an inhibition of p-nitrophenol hydroxylation was seen
(Koop & Laethem, 1992).
In a study by Pyykkö et al. (1987), Sprague-Dawley male rats were
given ethylbenzene dissolved in corn oil intraperitoneally in a single
dose of 5 mmol/kg (530 mg/kg body weight). The rats were killed 24 h
later and the livers and lungs removed. In the liver 7-ethoxycoumarin-
0-deethylase (a marker of CYP2B1/2) was induced 4-fold; no induction
was found in the lung. Ethylbenzene also induced 7-ethoxyresorufin-
0-deethylase (a marker of CYP1A1/2) both in the liver (4-fold)
and the lung (2.5 fold). These findings are common to many aromatic
solvents.
Alterations in the levels of specific cytochrome P-450 isozymes
were measured by Western immunoblotting techniques using rabbit
anti-rat polyclonal antibodies for cytochrome CYP1A1, CYP2B1 and
CYP2E1 on rat liver microsomes. The rats, male and female Holtzman
rats, were given 10 mmoles ethylbenzene per kg body weight for 3 days
and killed 24 h after the last injection. Ethylbenzene was shown to
induce CYP2B1/2B2 to a greater extent in male rats, while cytochrome
CYP2E1 was only induced in female rats. The level of cytochrome
CYP1A1 was not affected by ethylbenzene (Sequeira et al., 1992).
In another study (Gut et al., 1993), Wistar male rats were
exposed in a dynamic inhalation apparatus to 4 mg ethylbenzene/litre
air, 20 h per day, for 4 days. The rats were then killed and liver
microsomes prepared. By use of Western immunoblotting techniques it
was shown that cytochrome CYP2B1 was induced and the cytochrome CYP2E1
levels were decreased.
In a sensory irritation test, groups of four male mice
(Swiss-Webster) were exposed for 30 min to ethylbenzene at
concentrations of 1.76, 3.7, 8.06, 17.07 or 41.45 g/m3 (410, 860,
1875, 3970 or 9640 ppm). The RD50 value, i.e. the concentration
necessary to depress the respiratory rate by 50%, was calculated to be
17.46 g/m3 (4060 ppm) (95% confidence interval 10.6-28.6 g/m3;
2480-6660 ppm). The respiratory rate decreased due to sensory
irritation of the upper respiratory tract (Damgĺrd Nielsen & Alarie,
1982). In a similar test male mice (Swiss F1) were exposed for about
5 min to different concentrations of ethylbenzene. At least four
different concentrations were used and there were six mice for each
concentration. In this study the RD50 value was calculated to be
6.16 g/m3 (1432 ppm) (De Ceaurriz et al., 1981).
7.9 Factors modifying toxicity
Rats (Sprague-Dawley) were exposed for 2 h to 774 mg/m3 (180
ppm) ethylbenzene. One of two corresponding animals received 20 mmol
ethanol/kg body weight in physiological saline intraperitoneally
before exposure to ethylbenzene. Ethanol enhanced significantly (1.4
fold) the blood levels of inhaled ethylbenzene (Römer et al., 1986).
8. EFFECTS ON HUMANS
8.1 Volunteer studies
A dermal maximization test conducted on 25 volunteers at a
concentration of 10% ethylbenzene in petrolatum produced no skin
sensitization reaction (ECETOC, 1986).
In a review of studies from the 1930s, it was stated that
exposure to 21.5 g/m3 (5000 ppm) ethylbenzene for a few seconds gives
intolerable irritation of nose, eyes and throat. A few seconds of
exposure to 4.3 g/m3 (1000 ppm) initially gives eye irritation which
diminishes after a few minutes of exposure (Damgĺrd Nielsen & Alarie,
1982).
In a study on ethylbenzene metabolism in man it was incidentally
reported that, when the exposure was above the occupational limit
value (8 h; 430 mg/m3,100 ppm), complaints of fatigue, sleepiness,
headache and irritation of the eyes and respiratory tract were
reported (Bardodej & Bardodejová, 1970).
Healthy male subjects were exposed to technical xylene
(containing 20.7% ethylbenzene) for 2 h with or without a 100-watt
workload on an ergometer cycle. The air concentration of technical
xylene was 435 or 1300 mg/m3. During work at the higher exposure
level, evidence of performance decrement was observed in three of the
five performance tests: reaction time addition test (p<0.05),
short-term memory (p<0.05) and choice reaction time (p<0.10)
(Gamberale et al., 1978).
8.2 Occupational exposure
A number of epidemiological studies have been carried out on
groups occupationally exposed to mixtures of solvents, including
ethylbenzene. In these studies it is difficult to attribute the
effects to ethylbenzene or any other single chemical present.
Ethylbenzene occurs in mixed xylenes (at up to 30%) and effects of
occupational exposure to mixed xylenes are usually presented as
effects of xylenes and not of ethylbenzene.
A cross-sectional epidemiological study showed no strong evidence
of adverse neurobehavioural effects in 105 house painters when
compared with 53 workers from various professions (non-painters). The
concentration of ethylbenzene at the workplace was up to 12.9 mg/m3
(3 ppm). Other solvents present were ethyl acetate, toluene, butyl
acetate, methyl isobutyl ketone and xylene. In two neurobehavioural
tests significant differences were found between painters and controls
(the tests were "change of personality" and "short-term memory
capacity"). In a subgroup of painters with repeated prenarcotic
symptoms at the workplace, the differences were more pronounced
(Triebig et al., 1988).
In a study involving 35 spray painters, employed for between 2
and 26 years, erythrocyte and haemoglobin levels were slightly (but
not statistically significantly) lower than those of the controls.
The concentration of ethylbenzene was 17.2 mg/m3 (4 ppm) (Angerer &
Wulf, 1985).
In a medical surveillance report of some 200 ethylbenzene-
production workers, mandelic acid concentrations in urine were
measured twice a year for 20 years. Mandelic acid concentration
in the samples never exceeded 3.25 mmol/litre (=497 mg/litre), and the
mean value was 0.20-0.30 mmol/litre. According to the authors, a
post-shift urine mandelic acid concentration of 6.25 mmol/litre is
equivalent to an air concentration of 200 mg/m3. Therefore, the
measured maximum and mean mandelic acid values were considered to be
equivalent to air ethylbenzene concentrations of about 86 and 8.6
mg/m3 (20 and 2 ppm), respectively. None of the workers examined
over the last 10 years showed any effects on the levels of
haemoglobin, leucocytes or platelets, nor did they have a changed
haematocrit or alanine aminotransferase activity (Bardodej & Círek,
1988).
Minor changes in evoked potential and nerve conduction velocity
were found in 22 workers exposed to ethylbenzene at concentrations of
0.43-17.2 mg/m3 (0.1-4 ppm) for 4-20 years. They were also exposed
to styrene (about 1.5 ppm) (Lu & Zhen, 1989).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Ethylbenzene has been shown to inhibit the respiration of sewage
sludge utilizing biogenic substrates. Two screening tests were used,
RIKA and OECD 209. The concentration of ethylbenzene used was at the
limit of solubility in the medium (approximately 150 mg/litre), and
inhibitions of the respiration rate of 30% (ACCEDE 209) and 100%
(RIKA) were observed (Volskay & Grady, 1990).
Bringmann & Kühn (1980) studied the effect of ethylbenzene on
bacteria. A toxicity threshold of 12 mg/litre for Pseudomonas putida
was obtained in a cell multiplication inhibition test.
9.2 Aquatic organisms
Numerous acute toxicity tests have been carried out on
ethylbenzene. Organisms that have been studied include protozoans
(Bringmann & Kühn, 1980), algae (US EPA, 1978; Bringmann & Kühn, 1980;
Galassi et al., 1988; Masten et al. 1994), water fleas (LeBlanc, 1980;
Bringmann & Kühn, 1982; Abernethy et al., 1986; Galassi et al., 1988;
Vigano, 1993), diatoms (US EPA, 1978; Masten et al., 1994), copepods,
grass shrimp (Potera, 1975), bay shrimps (Benville & Korn, 1977),
mysid shrimps (US EPA, 1978; Masten et al., 1994), Dungeness crabs
(Caldwell et al., 1976) and Pacific oysters (LeGore, 1974). Fish that
have been studied include rainbow trout (Mayer & Ellersieck, 1986;
Galassi et al., 1988), guppy (Pickering & Henderson, 1966; Galassi et
al., 1988), bluegill (Pickering & Henderson, 1966; Buccafusco et al.,
1981), fathead minnow, goldfish (Pickering & Henderson, 1966), channel
catfish (Mayer & Ellersieck, 1986), Atlantic silverside (Masten et
al., 1994), striped bass (Benville & Korn, 1977) and sheepshead minnow
(US EPA, 1978; Heitmuller et al., 1981).
Many of the test results are not comparable, owing to
inconsistent exposure conditions, resulting from emulsions, open
static systems and systems with large air spaces. Aquatic toxicity
results from consistent exposure conditions, which are comparable, are
shown in Table 6.
No information regarding chronic exposure of aquatic organisms to
ethylbenzene has been reported.
Table 6. Toxicity of ethylbenzene to aquatic organisms
Species Age/size Stat/flowa Temperature Salinity pH Parameterc Concentration Reference
(°C) (0/00) (mg/litre)d
Alga stat 72-h EC50 4.6 Galassi et al. (1988)
(Selenastrum stat 19-21 48-h EC50 7.2 (3.4-15.1) Masten et al. (1994)
capricornutum) stat 19-21 96-h EC50 3.6 (1.7-7.6) Masten et al. (1994)
Water flea < 24 h statb 24-h LC50 2.2 Galassi et al. (1988)
(Daphnia magna) statb 21-25 48-h LC50 2.1e Abernethy et al. (1986)
< 24 h statb 48-h LC50 1.81-2.38 Viganň (1993)
Diatom
(Skeletonema statb 19-21 48-h EC50 7.5 (5.0-11.2) Masten et al. (1994)
costatum) statb 19-21 72-h EC50 4.9 (2.4-9.8) Masten et al. (1994)
statb 19-21 96-h EC50 7.7 (5.9-10.0) Masten et al. (1994)
Mysid shrimp < 24 h flow 24-26 20 8.0 48-h LC50 >5.2 Masten et al. (1994)
(Mysidopsis < 24 h flow 24-26 20 8.0 96-h LC50 2.6 (2.0-3.3) Masten et al. (1994)
bahia)
Rainbow trout stat 96-h LC50 4.2 Galassi et al. (1988)
(Oncorhynchus
mykiss)
Guppy stat 96-h LC50 9.2 Galassi et al. (1988)
(Poecilia
reticulata)
Table 6. (Cont'd)
Species Age/size Stat/flowa Temperature Salinity pH Parameterc Concentration Reference
(°C) (0/00) (mg/litre)d
Atlantic silverside 3-15 mg flow 21-23 20 48-h LC50 6.4 (5.8-7.5) Masten et al. (1994)
(Menidia menidia) 3-15 mg flow 21-23 20 96-h LC50 5.1 (4.4-5.7) Masten et al. (1994)
a in all cases a closed system was used; stat = static conditions (water unchanged for the duration of the test);
flow = flow-through conditions (ethylbenzene concentration in water continously maintained)
b air space was eliminated
c the EC50 for algae was based on growth inhibition
d test concentrations were measured, unless stated otherwise
e nominal test concentration;
9.3 Terrestrial organisms
An LC50 value of 47 µg per cm2 of contact area was obtained for
earthworms exposed to ethylbenzene adsorbed on filter paper in glass
vials (Neuhauser et al., 1986). Callahan et al. (1994) reported a
2-day LC50 value of 4.93 µg/kg for Eisenia foetida in a contact
toxicity test.
No toxicity data on plants, birds and wild mammals have been
reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
The acute and chronic toxicities of ethylbenzene are low. The
toxic effects in humans and animals relate to depression of the
central nervous system (CNS) and to irritation of the mucous membranes
and eyes. No data concerning carcinogenic or reproductive effects
have been reported. Ethylbenzene does not have significant mutagenic
properties or teratogenic effects.
Exposure to more than 430 mg/m3 (100 ppm) causes symptoms of CNS
depression and irritation in humans.
A 20-year medical surveillance study of 200 workers showed no
indications of effects in routine blood tests. The maximum exposure,
estimated from the urinary concentration of mandelic acid, was less
than about 86 mg/m3 (20 ppm) and the mean value about 8.6 mg/m3
(2 ppm).
In a 13-week animal study, increased liver weight was the only
dose-related biological finding in male rats. This was seen at a
concentration of 3225 mg/m3 (750 ppm) or more.
The definition and aim of the use of a guidance value for the
general population have been described by IPCS (1994).
On the basis of biological significance criteria cited above, a
no-observed-effect level (NOEL) of 2150 mg/m3 (500 ppm) was defined.
The no-observed-adverse-effect level would be higher than 4300 mg/m3
(1000 ppm) (highest concentration used), since the increase in liver
weight was not associated with any histopathological findings. A NOEL
of 2150 mg/m3 (500 ppm) was used as the basis for determining the
guidance value. The following uncertainty factors were used: 10 for
interspecies variability; 5 for intraspecies variability (effect seen
in males only); and 2 for lack of chronic toxicity data. This gives a
guidance value of 22 mg/m3 (5 ppm).
From the medical surveillance study the NOEL could be estimated
to be between 8.6 mg/m3 (2 ppm) (mean value) and 86 mg/m3 (20 ppm)
(maximum value). However, since no dose-response relationship was
derived, this study is not suitable for the estimation of a guidance
value. Furthermore, no effects would be expected at this exposure
level, which is almost the same as the guidance value.
Humans are exposed to ethylbenzene principally by inhalation,
where the substance is rapidly absorbed into the body. Exposure by
skin absorption or ingestion may also occur. Ethylbenzene is not
considered to bioaccumulate.
Table 7 gives the estimated weekly ethylbenzene dose resulting
from different types of exposure. A guidance value of 22 mg/m3
(5 ppm) ethylbenzene is approximately equivalent to a weekly ethyl-
benzene dose of 2000 mg. This is 100 times higher than the worst
exposure situation of the general population.
Table 7. Estimated weekly ethylbenzene dose from different types of exposures
Type of exposure Reported concentration Weekly total amount Dose mg/week
in media inhaled, ingested
or smoked
General population
Inhalation (means)b 0.74-100 µg/m3 140 m3 0.07-10c
Ingestion (food) 13 µg/kgd 7 kg 0.1
Drinking- or groundwaterb 0.07-1.1 µg/litree 14 litres 0.01
30.0 µg/litref 0.42
Occupational
Inhalation (mean)g 10 mg/m3 300c
Inhalation (max)g 100 mg/m3 50 m3 3000c
Inhalationh 430 mg/m3 12700c
Smokers 8 µg/cigarettei 140 cigarettes/week 1.1
a Based on a breathing rate of 20 m3/day
b Values from Tables 2 and 3
c Retention 60%
d Lovegren et al. (1979) (highest reported value)
e Highest reported values of uncontaminated groundwater
f Lowest reported value of contaminated groundwater
g Bardodej & Círek (1988)
h Corresponds to occupational exposure limits in several countries
i Wallace et al. (1987a)
A 2-year carcinogenicity study has been performed by the National
Toxicology Program (USA) but the results are not yet available.
10.2 Evaluation of effects on the environment
Ethylbenzene is found in air, water, soil, sediment, biota and
groundwater. It is released primarily into air and water from various
natural and anthropogenic sources. The atmosphere is the major sink
for ethylbenzene. Ethylbenzene is rapidly photo-oxidized in the
atmosphere and this may contribute to photo-chemical smog formation.
In water, the key processes determining overall fate are
volatilization and biodegradation.
The log octanol/water partition coefficient is 3.13, indicating a
potential for bioaccumulation. However, the limited evidence
available shows that ethylbenzene bioconcentration factors are low for
fish and molluscs. Elimination from aquatic organisms appears to be
rapid. Biomagnification through the food chain is unlikely.
Mean levels of ethylbenzene in air ranging from 0.74 to 100
µg/m3 have been measured at urban sites. Industrial releases and
vehicle emissions are the principal sources of ethylbenzene. Levels
found at rural sites are generally <2 µg/m3. The levels of
ethylbenzene in surface water are generally less than 0.1 µg/litre in
non-industrial areas. In industrial and urban areas ethylbenzene
concentrations of up to 15 µg/litre have been reported. Urban
run-off, effluent and landfill leachate are sources of local
contamination. Ethylbenzene levels in sediment are generally < 0.5
µg/kg. Levels of ethylbenzene between 1 and 5 µg/kg have been found
in sediments from heavily industrialized areas. Ethylbenzene levels
in uncontaminated groundwater are generally < 0.1 µg/litre. However,
much higher levels have been reported for groundwater contaminated via
waste disposal, fuel spillage and industrial facilities.
Acute toxicity studies on aquatic organisms show ethylbenzene to
be of moderate toxicity. The lowest acute toxicity values are 4.6
mg/litre for algae (72-h EC50), 1.8 mg/litre for daphnids (48-h LC50)
and 4.2 mg/litre for fish (96-h LC50). There are no chronic toxicity
studies on aquatic organisms.
There is limited information regarding the toxicity of
ethylbenzene to bacteria and earthworms. There are no data for
terrestrial plants, birds or wild mammals.
On the basis of available data, it is concluded that ethylbenzene
is unlikely to be found at levels in the environment that will cause
adverse effects on aquatic and terrestrial ecosystems, except in cases
of spills or point-source emissions.
11. CONCLUSIONS
Ethylbenzene has low toxicity. Its vapour irritates the mucous
membranes to a limited extent and causes prenarcotic effects on the
central nervous system.
With respect to the general population, a tentative guidance
value of 22 mg/m3 (5 ppm) for ethylbenzene in inhaled air has been
derived, although information on certain important toxicity end-points
are unavailable. This value would correspond to a weekly absorbed
dose (daily ventilation of 20 m3 with 60% retention) of about 2000
mg. It is at least 200 times higher than the dose received in the
most polluted living environment reported. Hence, no harmful effects
on the general population would be expected. However, it should be
noted that the guidance value of 22 mg/m3 (5 ppm) is about 10 times
higher than the odour threshold (about 2.2 mg/m3; 0.5 ppm), and so
exposure at that level may cause annoyance. On the other hand, odour
detection may be considered to be a safeguard against excessive
exposure.
Ethylbenzene is a non-persistent chemical and is degraded
primarily by photooxidation and biodegradation. Volatilization to the
atmosphere is rapid. Photooxidation reactions of ethylbenzene may
contribute to photochemical smog formation.
Limited evidence suggests that bioaccumulation is low in aquatic
organisms. Ethylbenzene is unlikely to cause adverse effects in
aquatic or terrestrial ecosystems except in cases of spills or
point-source emissions.
12. FURTHER RESEARCH
a) To fill the data gaps that currently limit the toxicological
evaluation of ethylbenzene, an appropriate rodent carcinogenicity
study and a reproductive toxicity study are needed. (The former
study has been conducted but the study report has not yet been
published.)
b) There is little information on the long-term effects of
ethylbenzene in humans and, in particular, no dose-response or
dose-effect data are at hand. Epidemiological studies of
populations occupationally exposed to ethylbenzene should be
encouraged. In this context, the use of ethylbenzene metabolites
in urine as a marker of exposure can be of special value because
the method determines the internal doses that individuals receive
via all routes of exposure. Because ethylbenzene is a central
nervous system depressant, and because some studies suggest that
high doses of the substance or its metabolites may affect the
metabolism of some neurotransmitters in the brain, epidemiological
studies should address the central nervous system as a potential
target organ. Moreover, since ethylbenzene is almost invariably
only one of the components in solvent mixtures at the workplace,
study designs that address possible interactions between
ethylbenzene and other solvents are desirable.
c) Further mechanistic studies are needed.
13. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
A guideline value of 300 µg/litre for ethylbenzene in
drinking-water was recommended by WHO in 1993.
REFERENCES
Abernethy S, Bobra AM, Shiu WY, Wells PG, & Mackay D (1986) Acute
lethal toxicity of hydrocarbons and chlorinated hydrocarbons to two
planktonic crustaceans: The key role of organism-water partitioning.
Aquat Toxicol, 8: 163-174.
ACGIH (1985-1986) Threshold limit values and biological exposure
indices. Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
Aitio A, Riihimäki V, Liesivuori J, Järvisalo J, & Hernberg S (1994)
Biological monitoring. In: Zenz C, Dickerson OB, & Horvath EP Jr ed.
Occupational medicine, 3rd ed. Saint Louis, Missouri, C.V. Mosby &
Co., pp 132-158.
Andersson K, Fuxe K, Nilsen OG, Toftgĺrd R, Eneroth P, & Gustafsson JĹ
(1981) Production of discrete changes in dopamine and noradrenaline
levels and turnover in various parts of the rat brain following
exposure to xylene, ortho-, meta-, and para-xylene, and
ethylbenzene. Toxicol Appl Pharmacol, 60: 535-548.
Angerer J & Lehnert G (1979) Occupational chronic exposure to organic
solvents. VIII. Phenolic compounds-metabolites of alkylbenzenes in
man. Simultaneous exposure to ethylbenzene and xylenes. Int Arch Occup
Environ Health, 43: 145-150.
Angerer J & Wulf H (1985) Occupational chronic exposure to organic
solvents. XI. Alkylbenzene exposure of varnish workers: effects on
hematopoietic system. Int Arch Occup Environ Health, 56: 307-321.
Annable MD, Wallace RB, Hayden NJ, & Voice TC (1993) Reduction of
gasoline component leaching potential by soil venting. J Contam
Hydrol, 12(1-2): 151-170.
Anon (1987) Chemical review: ethylbenzene. Danger Prop Ind Mater Rep,
7: 13-35.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Holler JS, Needham LL, &
Patterson DG Jr (1992) Determining volatile organic compounds in human
blood from a large sample production by using purge and trap gas
chromatography and mass spectrometry. Anal Chem, 64(9): 1021-1029.
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, & Wooten JV (1994) Blood
concentrations of volatile organic compounds in a non occupationally
exposed US population and in groups with suspected exposure. Clin
Chem, 40(7): 1401-1404.
Ĺstrand I, Engström J, & Övrum P (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man. Scand J
Work Environ Health, 4: 185-194.
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions
of the hydroxyl radical with organic compounds under atmospheric
conditions. Chem Rev, 85: 69-201.
ATSDR (1990) Toxicological profile for ethylbenzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry (ATSDR/TP/-90-15).
Barber ED, Teetsel NM, Kolberg KF, & Guest D (1992) A comparative
study of the rates of in vitro percutaneous absorption of eight
chemicals using rat and human skin. Fundam Appl Toxicol, 19: 493-497.
Bardodej Z & Bardodejová E (1970) Biotransformation of ethylbenzene,
styrene, and alpha-methylstyrene in man. Am Ind Hyg Assoc J, 31:
206-209.
Bardodej Z & Círek A (1988) Long-term study on workers occupationally
exposed to ethylbenzene. J Hyg Epidemiol Microbiol Immunol, 32: 1-5.
Benville PE Jr & Korn S (1977) The acute toxicity of six monocyclic
aromatic crude oil components to striped bass (Morone saxatilis) and
bay shrimp (Crago franciscorum). Calif Fish Game, 63: 204-209.
Bestetti G & Galli E (1984) Plasmid-coded degradation of ethylbenzene
and 1-phenylethanol in Pseudomonas fluorescens. FEMS Microbiol Lett,
21: 165-168.
Bevan MAJ, Proctor CJ, Baker-Rogers J, & Warren ND (1991) Exposure to
carbon monoxide, respirable suspended particulates and volatile
organic compounds while commuting by bicycle. Environ Sci Technol,
25: 788-791.
Bos R, Guicherit R, & Hoogeveen A (1977) Distribution of some
hydrocarbons in ambient air near Delft and the influence on the
formation of secondary air pollutants. Sci Total Environ, 7: 269-281.
Boyd SA, Xiangcan J, & Lee JF (1990) Sorption of nonionic organic
compounds by corn residues from a no-tillage field. J Environ Qual,
19(4): 734-738.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1982) [Findings concerning the harmful effect
of water pollutants on Daphnia magna in a further developed
standardized test procedure.] Z Wasser/Abwasser Forsch, 15(1): 1 (in
German).
Brown-Woodman PDC, Webster WS, Picher K, & Ritchie HE (1991)
Embryotoxicity of xylene and toluene: an in vitro study. Ind Health,
29: 139-152.
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other trace
compounds in urban air. Chemosphere, 17: 2363-2380.
Buccafusco RJ, Ells SJ, & LeBlanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Burghardt E & Jeltes R (1975) Gas chromatographic determination of
aromatic hydrocarbons in air using a semi-automatic preconcentration
method. Atmos Environ, 9: 935-940.
Burnham AK, Calder GV, Fritz JS, Junk GA, Svec HJ, & Willis R (1972)
Identification and estimation of neutral organic contaminants in
potable water. Anal Chem, 44: 139-142.
Bysshe SE (1982) Bioconcentration factors in aquatic organisms. In:
Lyan WJ, Reehl WF, & Rosenblatt DH ed. Handbook of chemical property
estimation methods - Environmental behaviour of organic compounds. New
York, McGraw-Hill Book Company, pp 5/1-5/30.
Caldwell RS, Caldarone EM, & Mallon MH (1976) Effects of a seawater-
soluble fraction of Cook Inlet crude oil and its major aromatic
components on larval stages of the Dungeness crab, Cancer
magister dana. In: Fate and effects of petroleum hydrocarbon in
marine organisms and ecosystems. Oxford, New York, Pergamon Press, pp
210-220.
Callahan CA, Shirazi MA, & Neuhauser EF (1994) Comparative toxicity of
chemicals to earthworms. Environ Toxicol Chem, 13(2): 291-298.
Callahan MA (1979) Water related environmental fate of 129 priority
pollutants. Washington, DC, US Environmental Protection Agency (EPA
440/4-79-029b).
Cavender F (1993) Aromatic hydrocarbons. In: Clayton GD & Clayton FE
ed. Patty's industrial hygiene and toxicology, 4th revis ed. New
York, John Wiley and Sons, vol 2B, pp 1267-1442.
Chan CC, Özkaynak H, Spengler JD, & Sheldon L (1991a) Driver exposure
to volatile organic compounds, CO, ozone and NO2 under different
driving conditions. Environ Sci Technol, 25: 964-972.
Chan CC, Spengler JD, Özkaynak H, & Lefkopoulou M (1991b) Commuter
exposures to VOCs in Boston, Massachusetts. J Air Waste Manage Assoc,
41(12): 1594-1600.
Chin BH, McKelvey JA, Tyler TR, Calisti LJ, Kozbelt SJ, & Sullivan LJ
(1980a) Absorption, distribution, and excretion of ethylbenzene,
ethylcyclohexane and methylethylbenzene isomers in rats. Bull Environ
Contam Toxicol, 24: 477-483.
Chin BH, McKelvey JA, Calisti LJ, Kozbelt SJ, & Sullivan LJ (1980b) A
comparison of in vivo and in vitro (tissue explant) techniques:
metabolic profile of ethylbenzene in the rat and the dog. Bull Environ
Contam Toxicol, 25: 241-245.
Chiou CT, Porter PE, & Schmedding DW (1983) Partition equilibria of
non-ionic organic compounds between soil organic matter and water.
Environ Sci Technol, 17: 227-231.
Chou WL, Speece RE, & Siddiqi RH (1978) Acclimation and degradation of
petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
CITI (1992) Biodegradation and bioaccumulation data on existing
chemicals based on the CSCL Japan. Tokyo, Chemicals Inspection and
Testing Institute, pp 3-9.
Clapp LW, Talarczyk MR, Park JK, & Boyle WC (1994) Performance
comparison between activated sludge and fixed-film processes for
priority pollutant removals. Water Environ Res, 66(2): 153-160.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984a) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural and motorway locations.
Environ Pollut, B7: 141-158.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984b) Ambient air
measurements of aromatic and halogenated hydrocarbons at urban, rural
and motorway locations. Sci Total Environ, 39: 265-279.
Climie IJG, Hutson DH, & Stoydin G (1983) The metabolism of
ethylbenzene hydroperoxide in the rat. Xenobiotica, 13: 611-618.
Cline PV & Viste DR (1985) Migration and degradation patterns of
volatile organic compounds. Waste Manage Res, 3: 351-360.
Cole RH, Frederick RE, Healy RP, & Rolan RG (1984) Preliminary
findings of the priority pollutant monitoring project of the
nationwide urban runoff program. J Water Pollut Control Fed,
56: 898-908.
Coleman WE, Munch JW, Streicher RP, Ringhand HP, & Kopfler FC (1984)
Identification and measurement of components in gasoline, kerosene and
No.2 fuel oil that partition into the aqueous phase after mixing. Arch
Environ Contam Toxicol, 13: 171-178.
Cotruvo JA (1985) Organic micropollutants in drinking water: An
overview. Sci Total Environ, 47: 7-26.
Cox DP & Goldsmith CD (1979) Microbial conversion of ethylbenzene to
1-phenylethanol and acetophenone by Nocardia tartaricans ATCC 31190.
Appl Environ Microbiol, 38: 514-520.
Cozzarelli IM, Eganhouse RP, & Baedecker MJ (1990) Transformation of
monoaromatic hydrocarbons to organic acids in anoxic groundwater
environment. Environ Geol Water Sci, 16: 135-141.
Cragg ST, Clarke EA, Daly IW, Miller RR, Terrill JB, & Ouellette RE
(1989) Subchronic inhalation toxicity of ethylbenzene in mice, rats,
and rabbits. Fundam Appl Toxicol, 13: 399-408.
Crookes MJ & Howe PD (1992) Environmental hazard assessment:
Ethylbenzene. Garston, United Kingdom, Department of the Environment,
Directorate for Air, Climate and Toxic Substances, Toxic Substances
Division, Public Building Research Establishment, 40 pp (TSD/7).
Curvall M, Enzell CR, & Pettersson B (1984) An evaluation of the
utility of four in vitro short term tests for predicting the
cytotoxicity of individual compounds derived from tobacco smoke. Cell
Biol Toxicol, 1: 173-193.
Damgĺrd Nielsen GD & Alarie Y (1982) Sensory irritation, pulmonary
irritation and respiratory stimulation by airborne benzene and
alkylbenzenes: Prediction of safe industrial exposure levels and
correlation with their thermodynamic properties. Toxicol Appl
Pharmacol, 65: 459-477.
Dannecker W, Schröder B, & Stechmann H (1990) Organic and inorganic
substances in highway tunnel exhaust air. Sci Total Environ, 93:
293-300.
Dean BJ, Brooks TM, Hodson-Walker G, & Hutson DH (1985) Genetic
toxicology testing of 41 industrial chemicals. Mutat Res, 153: 57-77.
De Bortoli M, Knöppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1984) Integrating 'real life' measurements of
organic pollution in indoor and outdoor air of homes in Northern
Italy. In: Proceedings of the 3rd International Conference on Indoor
Air Quality and Climate. Stockholm, Swedish Council for Building
Research, vol 4, pp 21-26.
DEC (1992) Health-based recommended occupational exposure limit for
ethylbenzene. The Hague, Directorate-General of Labour.
De Ceaurriz JC, Micillino JC, Bonnet P, & Guenier JP (1981) Sensory
irritation caused by various industrial airborne chemicals. Toxicol
Lett, 9: 137-143.
DFG (German Research Council) (1985) [Materials hazardous to health.
Justification of MAC values in terms of toxicology and occupational
medicine], 11th ed. Weinheim, VCH Publishers (in German).
Dills RL, Kent SD, Checkoway H, & Kalman DA (1991) Quantification of
volatile solvents in blood by static headspace analysis. Talanta,
38: 365-374.
Donner M, Mäki-Paakkanen J, Norppa H, Sorsa M, & Vainio H (1980)
Genetic toxicology of xylenes. Mutat Res, 74: 171-172.
Dreisch FA & Munson TO (1983) Purge-and-trap analysis using fused
silica capillary column GC/MS. J Chromatogr Sci, 21: 111-118.
Drummond L, Caldwell J, & Wilson HK (1989) The metabolism of
ethylbenzene and styrene to mandelic acid: stereo chemical
considerations. Xenobiotica, 19: 199-207.
Drummond L, Caldwell J, & Wilson HK (1990) The stereo selectivity of
1,2-phenylethanediol and mandelic acid metabolism and disposition in
the rat. Xenobiotica, 20: 159-168.
Durst GL & Laperle EA (1990) Styrene monomer migration as monitored by
purge and trap gas chromatography and sensory analysis for polystyrene
containers. J Food Sci, 55(2): 522-524.
Dutkiewicz T & Tyras H (1967) A study of the skin absorption of
ethylbenzene in man. Br J Ind Med, 24: 330-332.
EAJ (1989) Chemicals in the environment. Tokyo, Environment Agency of
Japan.
ECETOC (1986) Joint assessment of commodity chemicals No.7:
Ethylbenzene. Brussels, European Chemical Industry Ecology and
Toxicology Centre.
El Masry AM, Smith JN, & Williams RT (1956) Studies in detoxication.
69. The metabolism of alkylbenzenes: n-propylbenzene and
n-butylbenzene with further observations on ethylbenzene. Biochem J,
64: 50-56.
Elovaara E, Engström K, Nickels J, Aitio A, & Vainio H (1985)
Biochemical and morphological effects of long-term inhalation exposure
of rats to ethylbenzene. Xenobiotica, 15: 299-308.
Engström KM (1984a) Urinalysis of minor metabolites of ethylbenzene
and m-xylene. Scand J Work Environ Health, 10: 75-81.
Engström KM (1984b) Metabolism of inhaled ethylbenzene in rats. Scand
J Work Environ Health, 10: 83-87.
Engström J & Bjurström R (1978) Exposure to xylene and ethylbenzene.
II. Concentration in subcutaneous adipose tissue. Scand J Work Environ
Health, 4: 195-203.
Engström KM & Rantanen J (1974) A new gas chromatographic method for
determination of mandelic acid in urine. Int Arch Arbeitsmed,
33: 163-167.
Engström KM, Riihimäki V, & Laine A (1984) Urinary disposition of
ethylbenzene and m-xylene in man following separate and combined
exposure. Int Arch Occup Environ Health, 54: 355-363.
Engström KM, Elovaara E, & Aitio A (1985) Metabolism of ethylbenzene
in the rat during long-term intermittent inhalation exposure.
Xenobiotica, 15: 281-286.
Feiler HD, Vernick AS, & Storch PJ (1979) Fate of priority pollutants
in POTW's. In: Proceedings of the 8th National Conference on Municipal
Sludge Management, pp 72-81.
Fellin P & Otson R (1993) Seasonal trends of volatile organic
compounds (VOCs) in Canadian homes. In: Proceedings of the 6th
Conference on Indoor Air Quality and Climate, Helsinki, 1993, vol 2,
pp 117-122.
Filipovic D, Paulsen MD, Loida PJ, Sligar SG, & Ornstein RL (1992)
Ethylbenzene hydroxylation by cytochrome P450cam. Biochem Biophys Res
Commun, 189: 488-495.
Fishbein L (1985) An overview of environmental and toxicological
aspects of aromatic hydrocarbons. IV. Ethylbenzene. Sci Total Environ,
44: 269-287.
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15: 219-232.
Först C, Stieglitz L, Roth W, & Kuhnmünch S (1984) Quantitative
analysis of volatile organic compounds in landfill leachates. Int J
Environ Anal Chem, 37: 287-293.
Frank H, Senf L, & Welsch T (1990) Some application of wide bore
capillary gas chromatography in occupational medicine. Z Gesamte Hyg
Grenzgeb, 36(12): 668-671.
Gamberale F, Annwall G, & Hultengren M (1978) Exposure to xylene and
ethylbenzene. III. Effects on central nervous functions. Scand J Work
Environ Health, 4: 204-211.
Galassi S, Mingazzini M, Vigano L, Cesareo D, & Tosato ML (1988)
Approaches to modelling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicol Environ Saf, 16: 158-169.
Garcia JP, Beyne-Masclet S, Mouvier G, & Masclet P (1992) Emissions of
volatile organic compounds by coal-fire power stations. Atmos Environ,
A26(9): 1589-1597.
Gentry SJ & Walsh PT (1987) Eight-hour TWA personal monitoring using a
diffusive sampler and short-term stain tube. Am Ind Hyg Assoc J,
48: 287-292.
Gibson DT, Gschwendt B, Yeh WK, & Kobal VM (1973) Initial reactions in
the oxidation of ethylbenzene by Pseudomonas putida. Biochemistry,
12: 1520-1528.
Gomez-Belinchon JI, Grimalt JO, & Abaigčs J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water Res, 25: 577-589.
Goodenkauf O & Atkinson JC (1986) Occurrence of volatile organic
chemicals in Nebraska ground water. Ground Water, 24: 231-233.
Gossett RW, Brown DA, & Young DR (1983) Predicting the bioaccumulation
of organic compounds in marine organisms using octanol/water partition
coefficients. Mar Pollut Bull, 14: 387-392.
Grob K (1973) Organic substances in potable water and in its
precursor. Part 1. Methods for their determination by gas-liquid
chromatography. J Chromatogr, 84: 255-273.
Grob K & Grob G (1971) Gas-liquid chromatographic-mass spectrometric
investigation of C6-C20 organic compounds in an urban atmosphere
- An application of ultra trace analysis on capillary columns. J
Chromatogr, 62: 1-13.
Gromiec JP & & Piotrowski JK (1984) Urinary mandelic acid as an
exposure test for ethylbenzene. Int Arch Occup Environ Health,
55: 61-72.
Gschwend PM, Zafiriou OC, Mantoura RFC, Schwarzenbach RP, & Gagosian
RB (1982) Volatile organic compounds at a coastal site. I. Seasonal
variations. Environ Sci Technol, 16: 31-38.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gut I, Terelius Y, Frantik E, Linhart I, Soucek P, Filipcová B, &
Klucková H (1993) Exposure to various benzene derivatives
differentially induces cytochromes P450 2B1 and P450 2E1 in rat liver.
Arch Toxicol, 67: 237-243.
Hajimiragha H, Ewers U, Brockhaus A, & Boettger A (1989) Levels of
benzene and other volatile aromatic compounds in the blood of
non-smokers and smokers. Int Arch Occup Environ Health, 61: 513-518.
Hampton CV, Pierson WR, Schuetzle D, & Harvey TM (1983) Hydrocarbon
gases emitted from vehicles on the road. 2. Determination of emission
rates from diesel and spark-ignition vehicles. Environ Sci Technol,
17: 699-708.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(Suppl 4): 66-75.
Harkov R, Kebbekus B, Bozzelli JW, & Lioy PJ (1983) Measurement of
selected volatile organic compounds at three locations in New Jersey
during the summer season. J Air Pollut Control Assoc, 33: 1177-1183.
Harper M & Purnell CJ (1990) Alkylammonium montmorillonites as
adsorbents for organic vapors from air. Environ Sci Technol,
24: 55-62.
Heilbron I, Bunbury HM, & Jones WE ed. (1946) Dictionary of organic
compounds. London, vol II, p 20.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Herman DC, Inniss WE, & Mayfield CI (1990) Impact of volatile aromatic
hydrocarbon, alone or in combination, on growth of the freshwater alga
Selenastrum capriocornutum. Aquat Toxicol, 18(2): 87-100.
Hester NE & Meyer RA (1979) A sensitive technique for measurement of
benzene and alkylbenzenes in air. Environ Sci Technol, 13: 107-109.
Hiatt MH (1981) Analysis of fish and sediment for volatile priority
pollutants. Anal Chem, 53: 1541-1543.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography-mass spectrometry. Anal Chem, 55: 506-516.
Hodgson AT, Daisey JM, & Grot RA (1991) Sources and source strengths
of volatile organic compounds in a new office building. J Air Waste
Manage Assoc, 41(11): 1461-1468.
Holm S, Norbäck D, Frenning B, & Göthe CJ (1987) Hydrocarbon exposure
from handling jet fuel of some Swedish aircraft units. Scand J Work
Environ Health, 13: 438-444.
Holmberg B & Malmfors T (1974) The cytotoxicity of some organic
solvents. Environ Res, 7: 183-192.
Howard PH (1989) Handbook of environmental fate and exposure data for
organic chemicals. Volume 1: Large production and priority pollutants.
Chelsea, Michigan, Lewis Publishers, Inc.
Howard PH (1991) Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers, Inc., pp 340-341.
Hunt WF Jr, Faoro RB, & Freas W (1986) Report of the interim data base
for state and local air toxic volatile organic chemical measurements.
Washington, DC, US Environmental Protection Agency (EPA 450/4-86-012).
Hutchins SR, Sewell GW, Kovacs DA, & Smith GA (1991a) Biodegradation
of aromatic hydrocarbons by aquifer microorganisms under denitrifying
conditions. Environ Sci Technol, 25: 68-76.
Hutchins SR, Downs WC, Wilson JT, Smith GB, Kovacs DA, Fine DD,
Douglass RH, & Hendrix DJ (1991b) Effect of nitrate addition on
biotransformation of fuel-contaminated aquifer: Field demonstration.
Ground Water, 29(4): 571-580.
Inoue O, Seiji K, Kudo S, Jin C, Cai SX, Liu SJ, Watanabe T, Nakatsuka
H, & Ikeda M (1995) Urinary phenylglyoxylic acid excretion after
exposure to ethylbenzene among exposed Chinese workers. Int J Occup
Environ Health, 1: 1-8.
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: Assessing human health risks of chemicals:
Derivation of guidance values for health-based exposure limits.
Geneva, World Health Organization, 73 pp.
Jayanty RKM (1989) Evaluation of sampling and analytical methods for
monitoring toxic organics in air. Atmos Environ, 23: 777-782.
Jaynes WF & Boyd SA (1991) Clay mineral type and organic compound
sorption by hexadecyltrine-thylammonium-exchanged clays. Soil Sci Soc
Am J, 55(1): 43-48.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and in vehicle emissions. Environ Int, 11: 383-392.
Jüttner F (1988) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in forest air of the Southern Black
Forest. Chemosphere, 17: 309-317.
Kappeler T & Wuhrmann K (1978a) Microbial degradation of the
water-soluble fraction of gas oil, I. Water Res, 12: 327-333.
Kappeler T & Wuhrmann K (1978b) Microbial degradation of the
water-soluble fraction of gas oil. II. Bioassays with pure strains.
Water Res, 12: 335-342.
Kawai T, Yasugi T, Mizunuma K, Horiguchi S. Iguchi H, Uchida Y, Iwami
O, & Ikeda M (1992) Comparative evaluation of urinalysis and blood
analysis as means of detecting exposure to organic solvents at low
concentrations. Int Arch Occup Environ Health, 64(4): 223-234.
Kawamura K & Kaplan IR (1983) Organic compounds in the rainwater of
Los Angeles. Environ Sci Technol, 17: 497-501.
Kawata K & Fujeda Y (1993) Volatile aromatic hydrocarbons in ambient
air. Jpn J Toxicol Environ Health, 39(1): 37-43.
Kennicutt MC II, Brooks JM, & McDonald TJ (1984) Volatile organic
inputs from an ocean outfall near Barceloneta, Puerto Rico.
Chemosphere, 13: 535-548.
Kenrick MAP, Clark L, Baxter KM, Fleet M, Jones HA, Gibson TM, &
Turrell MB (1985) Trace organics in British aquifers - a baseline
survey. Water Research Centre, Environment (TR No. 223).
Keymeulen R, Van-Langenhove H, & Schamp N (1991) Determination of
monocyclic aromatic hydrocarbons in plant cuticles by gas
chromatography-mass spectrometry. J Chromatogr, 541(1-2): 83-88.
Kiese M & Lenk W (1974) Hydroxyacetophenones: urinary metabolites of
ethylbenzene and acetophenone in the rabbit. Xenobiotica, 4: 337-343.
Kleopfer RD & Fairless BJ (1972) Characterization of organic
components in a municipal water supply. Environ Sci Technol,
6: 1036-1037.
Koop DR & Laethem CL (1992) Inhibition of rabbit microsomal cytochrome
P-450 2E1-dependent p-nitrophenol hydroxylation by substituted benzene
derivatives. Drug Metab Dispos, 20: 775-777.
Korn M, Gfrörer W, Herz R, Wodarz I, & Wodarz R (1992)
Stereometabolism of ethylbenzene in man: gas chromatographic
determination of urinary excreted mandelic acid enantiomers and
phenylglyoxylic acid and their relation to the height of occupational
exposure. Int Arch Occup Environ Health, 64: 75-78.
Korte F & Boedefeld E (1978) Ecotoxicological review of the global
impact of the petroleum industry and its products. Ecotoxicol Environ
Saf, 2: 55-103.
Kroneld R (1989) Volatile pollutants in suburban and industrial air.
Bull Environ Contam Toxicol, 42: 868-872.
Kuhn EP, Zeyer J, Eicher P, & Schwarzenbach RP (1988) Anaerobic
degradation of alkylated benzenes in denitrifying laboratory aquifer
columns. Appl Environ Microbiol, 54: 490-496.
Labows J, Preti G, Hoelzle E, Leyden J, & Klingman A (1979) Analysis
of human axillary volatiles: Compounds of exogenous origin. J
Chromatogr, 163: 294-299.
Lanzerstorfer CH & Puxbaum H (1990) Volatile hydrocarbons in and
around Vienna, Austria. Water Air Soil Pollut, 51: 345-355.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lee JF, Crum JR, & Boyd SA (1989) Enhanced retention of organic
contaminants by soils exchanged with organic cations. Environ Sci
Technol, 23: 1365-1372.
LeGore RS (1974) The effect of Alaskan crude oil and selected
hydrocarbon compounds on embryonic development of the pacific oyster,
Crassostrea gigas. Doctoral Thesis, University of Washington. Diss
Abstr, B35: 3168.
Lesage S, Jackson RE, Priddle MW, & Riemann PG (1990) Occurrence and
fate of organic solvent residues in anoxic groundwater at the
Gloucester Landfill, Canada. Environ Sci Technol, 24: 559-566.
Levesque P & Dao LH (1989) Alkylation of benzene using an aqueous
solution of ethanol. Appl Catal, 53(2-3): 157-167.
Lewis PJ, Hagopian C, & Koch P (1983) Styrene. In: Kirk RE & Othmer DF
ed. Encyclopedia of chemical technology, 3rd ed. New York, Wiley
Interscience, vol 21, pp 770-801.
Lockhart WL, Wagemann R, Tracey B, Sutherland D, & Thomas DJ (1992)
Presence and implications of chemical contaminants in the freshwaters
of the Canadian Arctic. Sci Total Environ, 122(1/2): 165-243
Louw CW, Richards JF, & Faure PK (1977) Determination of volatile
organic compounds in city air by gas chromatography combined with
standard addition, selective subtraction, infra red spectrometry and
mass spectrometry. Atmos Environ, 11: 703-717.
Lovegren NV, Fisher GS, Legendre MG, & Schuller WH (1979) Volatile
constituents of dried legumes. J Agric Food Chem, 27: 851-853.
Lu BQ & Zhen ZH (1989) [Health standards for ethylbenzene in the air
of workplaces.] Beijing, Chinese Academy of Preventive Medicine,
Institute of Occupational Medicine (in Chinese).
Mabey W, Smith JH, Podoll, RT, Johnson HL, Mill T, Chou TW, Gate J,
Waight-Partridge I, Jaber H, & Vandenberg D (1982) Aquatic fate
process for organic priority pollutants. Washington, DC, US
Environmental Protection Agency (EPA 440/481-14).
McFall JA, Antoine SR, & DeLeon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14: 1253-1265.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma
cell forward mutation assay: III. 72 coded chemicals. Environ Mol
Mutagen, 12: 85-154.
Mackay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to atmosphere. Environ Sci Technol,
9: 1178-1180.
Mackay D, Shiu WY, & Sutherland RP (1979) Determination of air-water
Henry's law constants for hydrophobic pollutants. Environ Sci Technol,
13: 333-337.
Mackay D, Shiu WY, & Ma KC (1992) The illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Volume 1. Monoaromatic hydrocarbons, chlorobenzenes and
polychlorinated biphenyls. Chelsea, Michigan, Lewis Publishers, Inc.
MacLeod G & Ames JM (1987) Effect of water on the production of cooked
beef aroma compounds. J Food Sci, 52: 42-45.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
Current results and ongoing research. Am J Ind Med, 7: 415-446.
Masten LW, Boeri RL & Walker JD (1994) Strategies employed to
determine the acute aquatic toxicity of ethyl benzene, a
highly-volatile, poorly water-soluble chemical. Ecotoxicol Environ
Saf, 27: 335-348.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, p 460 (Resource Publication No. 160).
Michael LC, Pellizzari ED, & Norwood DL (1991) Application of the
master analytical scheme to the determination of volatile organics in
wastewater influent and effluents. Environ Sci Technol, 25: 150-155
Molnár J, Paksy KA, & Náray M (1986) Changes in the rat's motor
behaviour during 4-hour inhalation exposure to prenarcotic
concentrations of benzene and its derivatives. Acta Physiol Hung,
67: 349-354.
Morgan DL, Cooper SW, Carlock DL, Sykora JJ, Sutton B, Mattie DR, &
McDougal JN (1991) Dermal absorption of neat and aqueous volatile
organic chemicals in the Fischer 344 rat. Environ Res, 55: 51-63.
Mott JG & Romanow S (1991/1992) Sludge characterization, removal and
dewatering. J Hazard Mater, 29(1): 127-140.
Murray DAJ & Lockhart WL (1981) Microextraction and gas chromatographic
analysis of selected petroleum hydrocarbons in water and fish tissue.
J Chromatogr, 212: 305-311.
Namkung E & Rittmann BE (1987) Estimating volatile organic compound
emissions from publicly owned treatment works. J Water Pollut Control
Fed, 59: 670-678.
Nelson PF & Quigley SM (1982) Non-methane hydrocarbons in the
atmosphere of Sydney, Australia. Environ Sci Technol, 16: 650-655.
Neuhauser EF, Loehr RC, & Malecki MR (1986) Contact and artificial
soil tests using earthworms to evaluate the impact of wastes in soil.
In: Petros JK, Lacy WJ, & Conway RA end. Hazardous and industrial
solid waste testing: Fourth symposium. Philadelphia, Pennsylvania,
American Society for Testing and Materials, pp 192-203 (ASTM STP 886).
NIOSH (1984) Method 1501. In: Manual of analytical methods, 3rd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 2.
Noleau I & Toulemonde B (1988) Volatile components of roasted guinea
hen. Lebensm.Wiss Technol, 21: 195-197.
Norppa H & Vainio H (1983) Induction of sister-chromatid exchanges by
styrene analogues in cultured human lymphocytes. Mutat Res,
116: 379-387.
NTP (1992) Toxicity studies of ethylbenzene in F344/N rats and B6C3F1
mice (inhalation studies). Research Triangle Park, North Carolina, US
Department of Health and Human Services, National Toxicology Program
(NIH Publication No. 92-3129).
Nunes P & Benville PE Jr (1979) Uptake and depuration of petroleum
hydrocarbons in the Manila clam, Tapes semidecussata Reeve. Bull
Environ Contam Toxicol, 21:719-726.
Ogata M, Sugihara R, & Kira S (1977) Quantitative determination of
urinary hippuric acid and m- or p-methylhippuric acid as indices
of toluene and m- or p-xylene exposure by high performance liquid
chromatography. Int Arch Occup Environ Health, 39: 199-206.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities. J
Assoc Off Anal Chem, 65: 1370-1374.
Pankow JF, Isabelle LM, & Asher WE (1984) Trace organic compounds in
rain. I. Sampler design and analysis by adsorption/thermal desorption
(ATD). Environ Sci Technol, 18: 310-318.
Pellizzari ED, Hartwell TD, Harris BSH, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pellizzari ED, Sheldon L, Sparacino C, Bursey J, Wallace L, & Bromberg
S (1984) Volatile organic levels in indoor air. In: Berglund B,
Lindvall T, & Sundell J ed. Indoor air, chemical characterization and
personal exposure. Stockholm, Swedish Council for Building Research,
vol 4, pp 21-26.
Pellizzari ED, Wallace LA, & Gordon SM (1992) Elimination kinetics of
volatile organics in humans using breath measurements. J Expo Anal
Environ Epidemiol, 2: 341-355.
Perry DL, Chuang CC, Jungclaus GA, & Warner JS (1979) Identification
of organic compounds in industrial effluent discharges. Washington,
DC, US Environmental Protection Agency (EPA 600/4-79-016).
Petersson G (1982) Ambient hydrocarbons from motor-car assembly plants
in Scandinavia. Environ Pollut, B4: 207-217.
Pickering QH & Henderson C (1966) Acute toxicity of some important
petrochemicals to fish. J Water Pollut Control Fed, 38: 1419-1429.
Pitter P & Chudoba J (1990) Biodegradability of organic substances in
the aquatic environment. Boca Raton, Florida, CRC Press, Inc., p 269.
Potera FT (1975) The effects of benzene, toluene and ethylbenzene on
several important members of the estuarine ecosystem. Lehigh
University (Ph.D. dissertation).
Pussemier L, Szabó G, & Bulman RA (1990) Prediction of the soil
adsorption coefficient Koc for aromatic pollutants. Chemosphere
21: 1199-1212.
Pyykkö K, Paavilainen S, Metsä-Ketelä T, & Laustiola K (1987) The
increasing and decreasing effects of aromatic hydrocarbon solvents on
pulmonary and hepatic cytochrome P-450 in the rat. Pharmacol Toxicol,
60: 288-293.
Radzikowska-Kintzi H & Jakubowski M (1981) Internal standardization in
the head space analysis of organic solvents in blood. Int Arch Occup
Environ Health, 49: 115-123.
Rao PSC, Hornsby AG, & Jessup RE (1985) Indices for ranking the
potential for pesticide contamination of groundwater. Soil Crop Sci
Soc Proc, 44: 1-8.
Rappaport SM, Selvin S, & Waters MA (1987) Exposures to hydrocarbon
components of gasoline in the petroleum industry. Appl Ind Hyg,
2: 148-154.
Reinhard M, Goodman NL, & Barker JF (1984) Occurrence and distribution
of organic chemicals in two landfill leachate plumes. Environ Sci
Technol, 18: 953-961.
Rhue RD, Pennell KD, Rao PS, & Reve WH (1989) Competitive adsorption
of alkylbenzene and water vapors on predominantly mineral surfaces.
Chemosphere, 18(9-10): 1971-1986.
Romanelli A, Falzoi M, Mutti A, Bergamaschi E, & Franchini I (1986)
Effects of some monocyclic aromatic solvents and their metabolites on
brain dopamine in rabbits. J Appl Toxicol, 6: 431-435.
Römer KG, Federsel RJ, & Freundt KJ (1986) Rise of inhaled toluene,
ethyl benzene, m-xylene, or mesitylene in rat blood after treatment
with ethanol. Bull Environ Contam Toxicol, 37: 874-876.
Rosen AA, Skeel RJ, & Ettinger MB (1963) Relationship of river water
odour to specific organic contaminants. J Water Pollut Control Fed,
35: 777-782.
Roy WR & Griffin RA (1985) Mobility of organic solvents in
water-saturated soil material. Environ Geol Water Sci, 7: 241-247.
SAC (1989) Analysis of potentially dangerous substances in UK
waters. Bedford, United Kingdom, SAC Scientific (DOE Reference
No. PECD-7/7/306).
Sato A & Nakajima T (1987) Pharmacokinetics of organic solvent vapors
in relation to their toxicity. Scand J Work Environ Health, 13: 81-93.
Sauer TC Jr (1981) Volatile liquid hydrocarbon characterization of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environ Sci Technol, 15: 917-923.
Sax NI (1979) Dangerous properties of industrial chemicals, 5th ed.
New York, Van Nostrand Reinhold Co.
Seader JD (1982) Separation systems synthesis. In: Kirk RE & Othmer DF
ed. Encyclopedia of chemical technology, 3rd ed. New York, Wiley
Interscience, vol 20, pp 710-711.
Sequeira DJ, Eyer CS, Cawley GF, Nick TG, & Backes WL (1992)
Ethylbenzene-mediated induction of cytochrome P450 isozymes in male
and female rats. Biochem Pharmacol, 44: 1171-1182.
Sexton K & Westberg H (1980) Ambient hydrocarbon and ozone
measurements downwind of a large automotive painting plant. Environ
Sci Technol, 14: 329-332.
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environ Sci Technol, 16: 872-880.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1983) Measurements of
hazardous organic chemicals in the ambient atmosphere. Washington, DC,
US Environmental Protection Agency (EPA 600/3-83-002).
Singh HB, Ferek RJ, Salas LJ, & Nitz KC (1986) Toxic chemicals in the
environment: A program of field measurements. Washington, DC, US
Environmental Protection Agency (EPA 600/3-86/047).
Smith MR & Ratledge C (1989) Catabolism of alkylbenzenes by
Pseudomonas sp. NCIB 10643. Appl Microbiol Biotechnol, 32: 68-75.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: List VI. Am Ind Hyg Assoc J, 23: 95-107.
Sollenberg J (1991) Analytical isotachophoresis in biological
monitoring of exposure to industrial chemicals. J Chromatogr,
545: 369-374.
Sollenberg J, Smallwood AW, & Lowry LK (1985) Determination of
mandelic and phenylglyoxylic acids in rat urine by high-performance
liquid chromatography and by isotachophoresis. J Chromatogr,
343: 175-178.
Staples CA, Werner AF, & Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environ Toxicol Chem, 4(2): 131-142.
Stuermer DH, Ng DJ, & Morris CJ (1982) Organic contaminants in
groundwater near to an underground coal gasification site in
North-Eastern Wyoming. Environ Sci Technol, 16: 582-587.
Susten AS, Niemeier RW, & Simon SD (1990) In vivo percutaneous
absorption studies of volatile organic solvents in hairless mice. II.
Toluene, ethylbenzene and aniline. J Appl Toxicol, 10: 217-225.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tester DJ & Harker RJ (1981) Groundwater pollution investigations in
the Great Ouse basin. Water Pollut Control, 80: 614-631.
Thomann RV (1989) Bioaccumulation model of organic chemical
distribution in aquatic food chains. Environ Sci Technol,
23(6): 699-707.
Thomas JM, Gordy VR, Fiorenza S, & Ward CH (1990) Biodegradation of
BTEX in subsurface materials contaminated with gasoline: Granger,
Indiana. Water Sci Technol, 22: 53-62.
Thomas RG (1982) Volatilization. In: Lyman WJ, Reehl WF, & Rosenblatt
DH ed. Handbook of chemical properties: Estimation methods. New York,
McGraw-Hill Book Co.
Thorburn S & Colenutt BA (1979) A gas chromatographic comparison of
volatile organic compounds in urban and rural atmospheres. Int J
Environ Stud, 13: 265-271.
Triebig G, Claus D, Csuzda I, Druschky KF, Holler P, Kinzel W, Lehrl
S, Reichwein P, Weidenhammer W, Weitbrecht WU, Weltle D, Schaller KH,
& Valentin H (1988) Cross-sectional epidemiological study on
neurotoxicity of solvents in paints and lacquers. Int Arch Occup
Environ Health, 60: 233-241.
Tsuruta H (1982) Percutaneous absorption of organic solvents. III. On
the penetration rates of hydrophobic solvents through the excised rat
skin. Ind Health, 20: 335-345.
Ullman (1983) [Encyclopaedia of chemical technology.] Weinheim, Verlag
Chemie, vol 24, pp 525-544 (in German).
Ungváry G & Tátrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats, and rabbits. Arch Toxicol,
8(suppl): 425-430.
US Chemical Industry (1994) Facts and figures for the chemical
industry. Chem Eng News, July 4: 28-33.
US EPA (1978) In-depth studies on health and environmental impacts of
selected water pollutants. Washington, DC, US Environmental Protection
Agency (Contract No. 68-01-4646).
US ITC (1987) Synthetic organic chemicals: United States production
and sales, 1986. Washington, DC, US International Trade Commission.
Utkin IB, Yakimov MM, Matveeva LN, Kozlyak EI, Rogozhin IS, Solomon
ZG, & Bezborodov AM (1991) Degradation of styrene and ethylbenzene by
Pseudomonas species Y2. Microbiol Lett, 77: 237-242.
Van Duijvenbooden W & Kooper WF (1981) Effects on groundwater flow and
groundwater quality of a waste disposal site in Noordwijk, The
Netherlands. Sci Total Environ, 21: 85-92.
Verhoeff AP, Suk J, & Van Wijnen JH (1988) Residential indoor air
contamination by screen printing plants. Int Arch Occup Environ
Health, 60: 201-209.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., pp 628-630.
Viganň L (1993) Reproductive strategy of Daphnia magna and toxicity
of organic compounds. Water Res, 27: 903-909.
Vincent R, Poirot P, Sabra I, Rieger B, & Cicolella A (1994)
Occupational exposure to organic solvents during paint stripping and
painting operations in the aeronautical industry. Int Arch Occup
Environ Health, 65(6): 377-380.
Volskay VT Jr & Grady CPL Jr (1990) Respiration inhibition kinetic
analysis. Water Res, 24: 863-874.
Vowles PD & Mantoura RFC (1987) Sediment-water partition coefficients
and HPLC retention factors of aromatic hydrocarbons. Chemosphere,
16: 109-116.
Waggott A (1981) Trace organic substances in the River Lee. In:
Chemistry in water reuse. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., vol 2, pp 55-99.
Wakeham SG, Davis AC, & Karas JL (1983) Mesocosm experiments to
determine the fate and persistence of volatile organic compounds in
coastal seawater. Environ Sci Technol, 17: 611-617.
Wallace LA, Pellizzari E, Hartwell TD, Perritt R, & Ziegenfus R
(1987a) Exposures to benzene and other volatile compounds from active
and passive smoking. Arch Environ Health, 42(5): 272-279.
Wallace LA, Pellizzari E, Hartwell TD, Sparacino C, Withmore R,
Sheldon L, Zelon H, & Perritt R (1987b) The TEAM study: Personal
exposures to toxic substances in air, drinking water, and breath of
400 residents of New Jersey, North Carolina, and North Dakota. Environ
Res, 43: 290-307.
Wallace LA, Pellizzari E, Leaderer B, Zelon H, & Sheldon L (1987c)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Wallace LA, Pellizzari ED, Hartwell TD, Davis V, Michael LC, &
Whitmore RW (1989) The influence of personal activities on exposure to
volatile organic compounds. Environ Res, 50(1): 37-55.
Warner JM & Beasley RK (1984) Purge and trap chromatographic method
for the determination of acrylonitrile, chlorobenzene,
1,2-dichloroethane and ethylbenzene in aqueous samples. Anal Chem,
56: 1953-1956.
Water Pollution Control (1989) Fate of organics on land-applied
sludges. Water Pollut Control, 127: 12.
Weast RC ed (1988) Handbook of chemistry and physics, 69th ed. Boca
Raton, Florida, CRC Press, Inc.
Webber D (1984) Top 50 chemical products. Chem Eng News, May 7: 8-10.
Westrick JJ, Mello JW, & Thomas RF (1984) The groundwater supply
survey. J Am Water Works Assoc, 76: 52-59.
Whitby FJ, Harland BJ, & Comber MHI (1982) Aromatic hydrocarbons in
the Tees Estuary. London, Department of Health (DOE Contract
PECD-7/7/23).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization.
Wiesenburg DA, Bodennec G, & Brooks JM (1981) Volatile liquid
hydrocarbons around a production platform in the northwest Gulf of
Mexico. Bull Environ Contam Toxicol, 27: 167-174.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformation of selected
alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic
aquifer material: A microcosm study. Environ Sci Technol, 20:
997-1002.
Windholz M ed (1983) The Merck index: An encyclopedia of chemicals,
drugs and biologicals, 10th ed. Rahway, New Jersey, Merck & Co., Inc.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene. Arch
Ind Health, 14: 387-398.
Wolff MS (1976) Evidence for existence in human tissues of monomers
for plastics and rubber manufacture. Environ Health Perspect,
17: 183-187.
Wolff MS, Daum SM, Lorimer WV, Selikoff IJ, & Aubrey BB (1977) Styrene
and related hydrocarbons in subcutaneous fat from polymerization
workers. J Toxicol Environ Health, 2: 997-1005.
Yamamoto RK & Cook WA (1968) Determination of ethyl benzene and
styrene in air by ultraviolet spectrophotometry. Am Ind Hyg Assoc J,
29: 238-241.
Zhang Z & Pawliszyn J (1993) Headspace solid-phase microextraction.
Anal Chem, 65(14): 1843-1852.
Zlatkis A & Jiao J (1991) Evaluation of new organoclay stationary
phases for the separation of ethylbenzene and xylene isomers.
Chromatographia, 31(9-10): 457-464.
1. RESUME
L'éthylbenzčne est un hydrocarbure aromatique qui s'obtient par
une réaction d'alkylation mettant en jeu le benzčne et l'éthylčne.
Aux Etats-Unis, on estime sa production annuelle ŕ environ 5 millions
de tonnes. En 1983, l'Europe occidentale en a produit environ 3
millions de tonnes. Il se présente sous la forme d'un liquide
incolore dégageant une odeur douceâtre qui rappelle l'essence. On
l'utilise principalement pour la production de styrčne. Ajouté ŕ du
xylčne technique, il sert également de solvant pour les peintures et
les vernis et on l'emploie aussi dans l'industrie chimique et dans
celle du caoutchouc. Il est présent dans le pétrole brut, les
produits raffinés dérivés du pétrole et dans leurs produits de
combustion.
L'éthylbenzčne n'est pas persistant car il se décompose dans
l'environnement, principalement par photo-oxydation et par dégradation
biologique. Il est possible que la photo-oxydation de l'éthylbenzčne
dans l'atmosphčre contribue ŕ la formation du smog photochimique.
Le logarithme du coéfficient de partage entre l'octanol et l'eau
est égal ŕ 3,13, ce qui indique que le composé se pręte ŕ une
bioaccumulation. Cependant, les données limitées dont on dispose
montrent que le facteur de bioaccumulation de l'éthylbenzčne est
faible pour les mollusques et les poissons. Apparemment, il est vite
éliminé par les organismes aquatiques.
A la campagne, la concentration d'éthylbenzčne dans l'air est
généralement inférieure ŕ 2 µg/m3. Sur des sites urbains, on a
trouvé des teneurs moyennes allant de 0,74 ŕ 100 µg/m3. La
concentration d'éthylbenzčne présente dans les eaux superficielles
est généralement inférieure ŕ 0,1 µg/litre dans les zones non
industrialisées. En revanche, dans les zones urbaines ou
industrialisées, la concentration peut atteindre 15 µg/litre. Dans
les sédiments, la concentration est en général inférieure ŕ
0,5 µg/litre, encore que l'on ait signalé des valeurs comprises entre
1 et 5 µg/litre dans des sédiments provenant de régions fortement
industrialisées. Dans les eaux souterraines non contaminées, la
concentration est habituellement inférieure ŕ 0,1 µg/litre, mais elle
est beaucoup plus élevée dans les eaux contaminées.
L'éthylbenzčne présente une toxicité aiguë modérée pour les
algues, les invertébrés aquatiques et les poissons. La valeur de la
CE50 ŕ 72 h est de 4,6 mg/litre pour l'algue Selenastrum
capricornutum, la CL50 ŕ 48 h est de 1,8 mg/litre pour Daphnia
magna, et on a une CL50 ŕ 96 h de 4,2 mg/litre pour la truite
arc-en-ciel. On ne possčde aucune donnée sur l'exposition chronique
des organismes aquatiques ŕ l'éthylbenzčne.
En ce qui concerne les bactéries et les lombrics, les données
toxicologiques sont limitées. Il n'en n'existe aucune sur les
végétaux terrestres, les oiseaux ou les mammifčres sauvages.
Chez l'homme, l'exposition ŕ l'éthylbenzčne se produit
principalement par inhalation; 40 ŕ 60% du composé sont retenus dans
les poumons. L'éthylbenzčne est fortement métabolisé, principalement
en acides mandélique et phénylglyoxylique. On peut utiliser les
métabolites présents dans les urines pour surveiller l'exposition
humaine.
Qu'elle soit aiguë ou chronique, la toxicité de l'éthylbenzčne
est faible pour l'homme et les animaux. Il exerce des effets toxiques
sur le systčme nerveux central et il est irritant pour les muqueuses
et les yeux. Le seuil de concentration pour ces effets chez l'homme a
été estimé ŕ environ 430-860 mg/m3 (100-200 ppm) lors d'une seule
exposition de courte durée.
Des rats et des souris ŕ qui on avait fait inhaler pendant 13
semaines de l'éthylbenzčne ŕ des concentrations allant jusqu'ŕ 4300
mg/m3, n'ont présenté aucune lésion histopathologique. La dose sans
effet observable (critčre retenu: l'augmentation du poids du foie) a
été estimée ŕ 2150 mg/m3 (500 ppm).
L'éthylbenzčne stimule les enzymes des microsomes hépatiques. Il
n'est ni mutagčne ni tératogčne pour le rat ou le lapin. On ne dispose
d'aucune donnée au sujet de ses effets toxiques éventuels sur
l'appareil reproducteur ni sur son pouvoir cancérogčne.
Une valeur-guide de 22 mg/litre (5 ppm) a été calculée ŕ partir
des résultats fournis par les études sur l'animal. On estime que la
population générale est exposée ŕ des concentrations inférieures ŕ
cette valeur, męme dans les cas les plus graves. On a constaté qu'une
exposition de longue durée en milieu professionnel, ŕ des concentration
de cet ordre, n'avait aucun effet nocif sur la santé des travailleurs
exposés.
RESUMEN
El etilbenceno es un hidrocarburo aromático que se obtiene por
alkilación del benceno y del etileno. La producción estimada en los
Estados Unidos de América es de unos cinco millones de toneladas por
ańo, y en Europa occidental fue de aproximadamente tres millones de
toneladas en 1983. El etilbenceno es un líquido incoloro de olor
dulce semejante al de la gasolina. Se utiliza principalmente para la
producción de estireno. También se utiliza en el xileno técnico como
disolvente de pinturas y lacas, así como en la industria del caucho y
en la fabricación de sustancias químicas. Se encuentra en el petróleo
crudo, en los productos de petróleo refinados y en productos de
combustión.
El etilbenceno es una sustancia química no persistente, que se
degrada principalmente por fotooxidación y biodegradación. Su
volatilización en la atmósfera es rápida. La reacción de foto-
oxidación del etilbenceno en la atmósfera puede contribuir a la
formación de niebla fotoquímica.
El logaritmo del coeficiente de reparto octanol-agua es 3,13, lo
que indica posibilidad de bioacumulación. Sin embargo, los limitados
indicios disponibles muestran que los factores de bioconcentración del
etilbenceno son bajos para peces y moluscos. La eliminación por los
organismos acuáticos parece ser rápida.
Los niveles de etilbenceno en el aire en puntos rurales son
generalmente inferiores a 2 µg/m3. En puntos urbanos se han
registrado niveles medios de etilbenceno que oscilan entre 0,74 y 100
µg/m3. Los niveles de etilbenceno detectados en las aguas
superficiales son generalmente inferiores a 0,1 µg/litro en zonas no
industriales. Se han comunicado concentraciones de etilbenceno de
hasta 15 µg/litro en zonas industriales y urbanas. Los niveles de
etilbenceno en sedimentos son generalmente inferiores a 0,5 µg/kg,
aunque en sedimentos de zonas muy industrializadas se han encontrado
niveles de 1 a 5 µg/kg. Las concentraciones en aguas subterráneas no
contaminadas son generalmente inferiores a 0,1 µg/litro, pero son
mucho más elevadas en aguas subterráneas contaminadas.
La toxicidad aguda del etilbenceno para las algas, los
invertebrados acuáticos y los peces es moderada. Los valores de
toxicidad aguda más bajos son de 4,6 mg/litro para el alga
Selenastrum capricornutum (CE50 a las 72 horas, sobre la base de la
inhibición del crecimiento), 1,8 mg/litro para Daphnia magna (CL50
a las 48 horas) y 4,2 mg/litro para la trucha irisada (CL50 a las 96
horas). No se dispone de información sobre la exposición crónica de
los organismos acuáticos al etilbenceno.
Hay información limitada sobre la toxicidad del etilbenceno para
las bacterias y para las lombrices. No hay datos relativos a las
plantas terrestres, las aves y los mamíferos silvestres.
La exposición humana al etilbenceno se produce principalmente por
inhalación; el 40-60% del etilbenceno inhalado se retiene en los
pulmones. El etilbenceno se metaboliza extensamente, transformándose
sobre todo en ácidos mandélico y fenilglioxílico. Estos metabolitos
urinarios pueden utilizarse para vigilar la exposición humana.
El etilbenceno tiene una toxicidad aguda y crónica baja tanto
para los animales como para el hombre. Es tóxico para el sistema
nervioso central e irrita las mucosas y los ojos. El umbral para esos
efectos en el ser humano después de exposiciones únicas breves se
estimó en aproximadamente 430-860 mg/m3 (100-200 ppm).
La inhalación de etilbenceno por ratas y ratones durante 13
semanas en concentraciones de hasta 4300 mg/m3 (1000 ppm) no dio
lugar a lesiones histopatológicas. El nivel sin efectos observados,
sobre la base de un aumento del peso del hígado en las ratas, fue de
2150 mg/m3 (500 ppm).
El etilbenceno es un inductor de las enzimas microsómicas
hepáticas. No es mutagénico ni teratogénico en ratas y conejos. No
se dispone de información sobre la toxicidad reproductiva ni la
carcinogenicidad del etilbenceno.
Se ha calculado un valor de orientación de 22 mg/m3 (5 ppm) a
partir de estudios realizados en animales. La exposición estimada de
la población general (incluso en la peor de las situaciones) es
inferior a ese valor de orientación. La exposición ocupacional a
largo plazo a concentraciones de etilbenceno estimadas en este orden
de magnitud no ocasionaron efectos adversos en la salud de los
trabajadores.
