
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 181
CHLORINATED PARAFFINS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr K. Kenne and Professor U.G. Ahlborg,
Institute of Environmental Medicine, Karolinska Institute, Stockholm,
Sweden
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the sound Management of Chemicals.
World Health Organization
Geneva, 1996
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme (UNEP), the
International Labour Organisation (ILO), and the World Health
Organization (WHO). The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally comparable
results, and the development of manpower in the field of toxicology.
Other activities carried out by the IPCS include the development of
know-how for coping with chemical accidents, coordination of
laboratory testing and epidemiological studies, and promotion of
research on the mechanisms of the biological action of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Chlorinated paraffins.
(Environmental health criteria ; 181)
1.Paraffin - adverse effects 2.Paraffin - toxicity
3.Environmental exposure I.Series
ISBN 92 4 157181 0 (NLM Classification: QV 800)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CHLORINATED PARAFFINS
Preamble
1. SUMMARY
1.1. Properties, uses and analytical methods
1.2. Sources of human and environmental exposure
1.3. Environmental distribution and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on laboratory mammals and in vitro
test systems
1.7. Effects on humans
1.8. Effects on other organisms in the laboratory
and field
1.9. Evaluation of human health risks and effects on the
environment
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.1.1. Relative molecular mass
2.1.2. Common names
2.1.2.1 CAS registry number and names
2.1.2.2 Synonyms
2.1.3. Technical products
2.2. Chemical and physical properties
2.3. Analysis
2.3.1. Sampling
2.3.2. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
3.2.3. Loss into the environment
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Transformation
4.2.1. Abiotic transformation
4.2.2. Biodegradation
4.2.2.1 Short chain length chlorinated
paraffins
4.2.2.2 Long chain length chlorinated
paraffins
4.2.2.3 Comparative studies
4.3. Bioaccumulation and biomagnification
4.3.1. Summary
4.3.2. Aquatic vertebrates
4.3.2.1 Short chain length chlorinated
paraffins
4.3.2.2 Intermediate chain length chlorinated
paraffins
4.3.2.3 Long chain length chlorinated
paraffins
4.3.3. Aquatic invertebrates
4.3.3.1 Short chain length chlorinated
paraffins
4.3.3.2 Intermediate chain length chlorinated
paraffins
4.3.3.3 Long chain length chlorinated
paraffins
4.3.3.4 Comparative studies
4.3.4. Aquatic plants
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water and sediment
5.1.3. Soil
5.1.4. Aquatic and terrestrial organisms
5.1.5. Food and beverages
5.2. General population exposure
5.2.1. Concentrations in human tissues
5.3. Occupational exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
6.1. Absorption
6.1.1. Oral exposure
6.1.2. Dermal exposure
6.1.3. Inhalation exposure
6.2. Distribution
6.2.1. Short chain length chlorinated paraffins
6.2.1.1 Mouse
6.2.1.2 Rat
6.2.2. Intermediate chain length chlorinated paraffins
6.2.2.1 Rat
6.2.2.2 Mouse
6.2.2.3 Bird
6.2.2.4 Fish
6.2.3. Long chain length chlorinated paraffins
6.2.3.1 Rat
6.2.3.2 Fish
6.2.3.3 Mussel
6.2.4. Comparative studies
6.3. Metabolic transformation
6.3.1. Short chain length chlorinated paraffins
6.3.2. Intermediate chain length chlorinated paraffins
6.4. Elimination and excretion
6.4.1. Short chain length chlorinated paraffins
6.4.2. Intermediate chain length chlorinated paraffins
6.4.2.1 Rat
6.4.2.2 Mouse
6.4.2.3 Bird
6.4.3. Long chain length chlorinated paraffins
6.4.4. Comparative studies
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Acute exposure
7.1.1. Lethal doses
7.1.2. Non-lethal doses
7.1.2.1 Oral route
7.1.2.2 Inhalation route
7.1.2.3 Intraperitoneal route
7.1.3. Skin and eye irritation
7.1.3.1 Short chain length chlorinated
paraffins
7.1.3.2 Intermediate and long chain length
chlorinated paraffins
7.1.4. Skin sensitization
7.2. Repeated exposure
7.2.1. Oral route
7.2.1.1 Short chain length chlorinated
paraffins
7.2.1.2 Intermediate chain length chlorinated
paraffins
7.2.1.3 Long chain length chlorinated
paraffins
7.2.1.4 Comparative studies
7.2.2. Intraperitoneal route
7.2.2.1 Short chain length chlorinated
paraffins
7.2.2.2 Intermediate chain length chlorinated
paraffins
7.2.2.3 Comparative studies
7.3. Neurotoxicity
7.3.1. Short chain length chlorinated paraffins
7.3.2. Intermediate chain length chlorinated paraffins
7.4. Reproductive toxicity, embryotoxicity and
teratogenicity
7.4.1. Reproduction
7.4.2. Embryotoxicity and teratogenicity
7.4.2.1 Short chain length chlorinated
paraffins
7.4.2.2 Intermediate chain length chlorinated
paraffins
7.4.2.3 Long chain length chlorinated
paraffins
7.5. Mutagenicity and related end-points
7.5.1. Prokaryotes
7.5.2. Mammalian cells
7.5.2.1 In vitro studies
7.5.2.2 In vivo studies
7.5.2.3 Cell transformation
7.6. Long-term exposure and carcinogenicity
7.6.1. Oral route
7.6.1.1 Short chain length chlorinated
paraffins
7.6.1.2 Long chain length chlorinated
paraffins
8. EFFECTS ON HUMANS
8.1. General population exposure
8.1.1. Controlled human studies
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.2. Aquatic organisms
9.1.2.1 Aquatic plants
9.1.2.2 Invertebrates
9.1.2.3 Fish
9.1.3. Terrestrial organisms
9.2. Field observations
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure levels
10.1.2. Toxic effects
10.1.3. Risk evaluation
10.1.3.1 Short chain compounds
10.1.3.2 Intermediate chain compounds
10.1.3.3 Long chain compounds
10.2. Evaluation of effects on the environment
10.2.1. Exposure levels
10.2.2. Toxic effects
10.2.3. Risk evaluation
11. RECOMMENDATIONS FOR PROTECTION OF THE ENVIRONMENT
12. FUTURE RESEARCH
13. PREVIOUS EVALUATION BY INTERNATIONAL ORGANIZATIONS
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
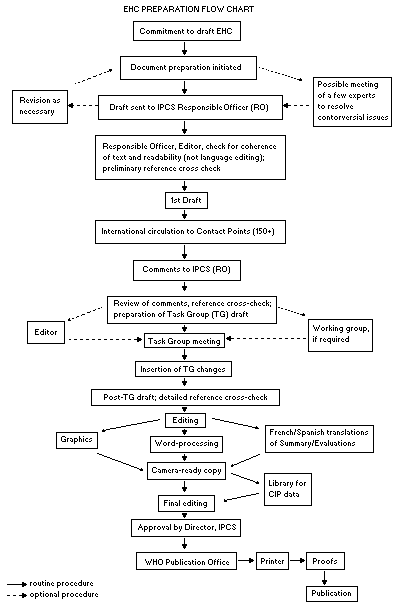 The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the
sole responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLORINATED
PARAFFINS
Members
Professor U.G. Ahlborg, Institute of Environmental Medicine,
Karolinska Institute, Stockholm, Sweden (Vice-Chairman)
Dr D. Anderson, British Industry Biological Research Association
(BIBRA) Toxicology International, Carshalton, Surrey, United
Kingdom
Dr T. Beulshausen, Federal Environment Agency, Berlin, Germany
Dr R.S. Chhabra, Environmental Toxicology Program, National Institute
of Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Dr N. Gregg, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, Cambridgeshire, United Kingdom (Joint Rapporteur)
Dr B. Jansson, Institute of Applied Environmental Research, Stockholm
University, Solna, Sweden
Dr K. Kenne, Institute of Environmental Medicine, Karolinska
Institute, Stockholm, Sweden (Joint Rapporteur)
Dr M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Chairman)
Representatives of other Organizations
Dr P. Montuschi, Institute of Pharmacology, Faculty of Medicine and
Surgery, Catholic University of the Sacred Heart, Rome, Italy
(Representing the International Union of Pharmacology)
Mr D. Farrar, Occupational Health, ICI Chemicals and Polymers Limited,
Runcorn, Cheshire, United Kingdom
(Representing the European Centre for Ecotoxicology and Toxicology
of Chemicals)
Secretariat
Dr E.M. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr J.D. Wilbourn, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR CHLORINATED PARAFFINS
A WHO Task Group on Environmental Health Criteria for Chlorinated
Paraffins met at the World Health Organization, Geneva, from 20 to 24
March 1995. Dr E.M. Smith, IPCS, welcomed the participants on behalf
of Dr M. Mercier, Director of the IPCS, and on behalf the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft and made an evaluation of the risks for human health
and the environment from exposure to chlorinated paraffins.
The first draft was prepared at the Institute of Environmental
Medicine, Karolinska Institute, Stockholm, Sweden, by Dr K. Kenne and
Professor U.G. Ahlborg. The second draft, incorporating comments
received following circulation of the first drafts to the IPCS contact
points for Environmental Health Criteria monographs, was also prepared
by Dr Kenne and Professor Ahlborg.
Dr E.M. Smith and Dr P. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific aspects of the monograph and for
the technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
APDM aminopyrine demethylase
BCF bioconcentration factor
CD coulometric detection
CP chlorinated paraffins
CP-LH chlorinated paraffin with long chain length and high degree
of chlorination
CP-LL chlorinated paraffin with long chain length and low degree of
chlorination
CP-MH chlorinated paraffin with medium chain length and high degree
of chlorination
CP-ML chlorinated paraffin with medium chain length and low degree
of chlorination
CP-SH chlorinated paraffin with short chain length and high degree
of chlorination
CP-SL chlorinated paraffin with short chain length and low degree
of chlorination
EC50 median effective concentration
ECD electron capture detection
GC gas chromatography
LC50 median lethal concentration
LOAEL lowest-observed-adverse-effect level
LOEC lowest-observed-effect concentration
LOEL lowest-observed-effect level
LT50 median lethal time
MS mass spectrometry
NCI negative ion chemical ionization
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
PCB polychlorinated biphenyl
PVC polyvinyl chloride
TDI tolerable daily intake
TLC thin-layer chromatography
TSH thyroid stimulating hormone
UDP uridine diphosphate
1. SUMMARY
1.1 Properties, uses and analytical methods
Chlorinated paraffins (CPs) are produced by chlorination of
straight-chained paraffin fractions. The carbon chain length of
commercial chlorinated paraffins is usually between 10 and 30 carbon
atoms, and the chlorine content is usually between 40 and 70% by
weight. Chlorinated paraffins are viscous colourless or yellowish
dense oils with low vapour pressures, except for those of long carbon
chain length with high chlorine content (70%), which are solid.
Chlorinated paraffins are practically insoluble in water, lower
alcohols, glycerol and glycols, but are soluble in chlorinated
solvents, aromatic hydrocarbons, ketones, esters, ethers, mineral oils
and some cutting oils. They are moderately soluble in unchlorinated
aliphatic hydrocarbons.
Chlorinated paraffins consist of extremely complex mixtures,
owing to the many possible positions for the chlorine atoms. The
products can be subdivided into six groups depending on chain length
(short C10-13, intermediate C14-17 and long C18-30) and degree of
chlorination (low (< 50%) and high (> 50%)).
Chlorinated paraffins are used worldwide in widespread
applications such as plasticizers in plastics (e.g., PVC), extreme
pressure additives in metal working fluids, flame retardants and
additives in paints. Technical grade chlorinated paraffins may be
contaminated by isoparaffins, aromatic compounds and metals, and
normally contain stabilizers, which are added to inhibit
decomposition.
The analysis of chlorinated paraffins is difficult due to the
extreme complexity of these mixtures. In environmental samples, this
is further complicated by interference from other compounds. Analyses
often require extensive clean-up of the samples and the use of
specific detection methods. Early methods were based on thin-layer
chromatography for the clean-up and an unspecific argentation
detection method on the plates. Methods based on different column
liquid chromatography are currently used for the clean-up, although it
is difficult to isolate the chlorinated paraffins due to their wide
range of physical properties. Specific detection methods are
therefore used; gas chromatography combined with mass spectrometry is
now the most common technique. The use of negative ions makes the
detection even more specific. Although use of these sophisticated
techniques has improved the ability to analyse chlorinated paraffins,
it is still impossible to determine exact concentrations. Reported
results should be regarded only as estimates of the true values.
1.2 Sources of human and environmental exposure
Chlorinated paraffins are not known to occur naturally.
Chlorinated paraffins are produced by reacting liquid paraffin
fractions with pure chlorine gas. The reaction may require the use of
a solvent, and often ultraviolet light is used as a catalyst. In
1985, the estimated world production of chlorinated paraffins was
300 000 tonnes.
The widespread uses of chlorinated paraffins probably provide the
major source of environmental contamination. Chlorinated paraffins
may be released into the environment from improperly disposed
metal-working fluids containing chlorinated paraffins or from polymers
containing chlorinated paraffins. Loss of chlorinated paraffins by
leaching from paints and coatings may also contribute to environmental
contamination. The potential for loss during production and transport
is expected to be less than that during product use and disposal.
Owing to their thermal instability, chlorinated paraffins are
expected to be degraded by incineration and thus would not be expected
to volatilize in exhaust gases from incinerators. However, it has
been demonstrated that chlorinated aromatic compounds such as
polychlorinated biphenyls, naphthalenes and benzenes are formed by
pyrolysis of chlorinated paraffins under certain conditions.
1.3 Environmental distribution and transformation
Chlorinated paraffins adsorb strongly to sediment. In water they
are probably transported adsorbed on suspended particles, and in the
atmosphere adsorbed to airborne particulates (and possibly in the
vapour phase). The half-lives for chlorinated paraffins in air have
been estimated to range from 0.85 to 7.2 days, a period sufficiently
long that the possibility of long-range transport cannot be excluded.
Chlorinated paraffins are not readily biodegradable. Short
carbon chain length chlorinated paraffins with a chlorine content of
less than 50% appear to be degradable under aerobic conditions with
acclimated microorganisms, whereas the degradation appears inhibited
at a chlorine content above 58%. Intermediate and long chain length
chlorinated paraffins are degraded more slowly.
Chlorinated paraffins are bioaccumulated in aquatic organisms,
and the reported bioconcentration factors (BCFs) are in the range of 7
to 7155 for fish and 223 to 138 000 for mussels. In fish, chlorinated
paraffins of short chain length are accumulated to a higher degree
than intermediate and long chain length chlorinated paraffins.
Radioactivity has been found mainly in bile, intestine, liver, fat and
gills after administration of radiolabelled chlorinated paraffins. The
uptake of chlorinated paraffins seems to be more efficient for short
chlorinated paraffins with low chlorine content; the elimination rate
is slowest for short chlorinated paraffins with high chlorine content.
The retention in fat-rich tissues appears to increase with increasing
degree of chlorination.
1.4 Environmental levels and human exposure
Few data on levels of chlorinated paraffins in the environment
are available. Chlorinated paraffins have been detected in marine
water samples in the United Kingdom at levels below 4 µg/litre. In
non-marine waters, levels below 6 µg/litre in the United Kingdom have
been reported; in Germany, concentrations determined in 1994 were in
the range of 0.08-0.28 µg/litre. In water in the USA, concentrations
were generally less than 0.03 µg/litre, although levels were above 1.0
µg/litre in a small proportion (1.2%) of samples. In marine sediments,
levels up to 600 µg/kg wet weight have been reported, and in
non-marine sediments in the United Kingdom concentrations were up to
15 000 µg/kg in industrialized regions and 1000 µg/kg in areas
remote from industry. In sediments in an impoundment lagoon from a
chlorinated paraffin manufacturing plant in the USA, concentrations as
high as 170 000 µg/kg dry weight of long chain length chlorinated
paraffins, 50 000 µg/kg of intermediate chain length chlorinated
paraffins and 40 000 µg/kg of short chain length chlorinated paraffins
were reported. In Germany, levels up to 83 µg/kg dry weight of C10-13
and up to 370 µg/kg dry weight of C14-17 were reported in sediments in
1994. In Japan, levels in sediment ranged up to 8500 µg/kg.
Chlorinated paraffins have been detected in various organisms.
Chlorinated paraffins are present in terrestrial mammals in Sweden at
concentrations in the range of 32-88 µg/kg tissue (140-4400 µg/kg
lipid). However, chlorinated paraffins were not detected in sheep
which were grazed remote from production of chlorinated paraffins in
the United Kingdom. In birds in the United Kingdom, concentrations
ranged up to 1500 µg/kg and in fish in Sweden and the United Kingdom,
levels ranged up to 200 µg/kg. In mussels collected in the USA and
United Kingdom, concentrations up to 400 µg/kg were reported.
However, levels of C10-20 in mussels collected close to a chlorinated
paraffin plant effluent discharge ranged up to 12 000 µg/kg.
Chlorinated paraffins have also been detected in post mortem human
tissues, i.e. in adipose tissue (median level of 100-190 µg/kg),
kidney (median level below 90 µg/kg) and liver (median level below 90
µg/kg). In one limited survey, chlorinated paraffins, mostly C10-20,
were present at levels of up to 500 µg/kg in approximately 70% of the
samples of various food products.
Information on occupational exposure to chlorinated paraffins is
limited. Very low levels of exposure to aerosols of short chain
chlorinated paraffins (0.003-1.2 mg/m3) have been found to be
associated with their use as metal-working fluids, although there is
no information available on the proportion that is inhalable. On the
basis of mathematical modelling of exposure without any control
measures, high levels of dermal contact (5-15 mg/cm2 per day) were
estimated for speciality metal-working fluids which contain very high
levels of short chain chlorinated paraffins, although absorption would
be expected to be low. Control measures would reduce dermal exposure.
1.5 Kinetics and metabolism
The toxicokinetics of chlorinated paraffins have been studied
in experimental animals. Adequate information for humans is not
available. Possible differences in toxicokinetics as a result of
different chain lengths have not been sufficiently investigated.
Although the extent of absorption of chlorinated paraffins after oral
administration is unknown, it appears to decrease with increasing
chain length and degree of chlorination. Percutaneous absorption may
also occur depending on chain length, but would be limited (less than
1% of a topical C18 dose). No data on absorption via the lung is
available.
Distribution of chlorinated paraffins occurs mainly in the liver,
kidney, intestine, bone marrow, adipose tissue and ovary. Information
on retention is insufficient but a low degree of chlorination may
enhance retention time due to slower redistribution. Chlorinated
paraffins or their metabolites are present in the central nervous
system up to 30 days after administration. They may cross the
blood-placental barrier. There is no adequate information on the
pathways of metabolism of chlorinated paraffins, although in
radiolabelling studies CO2 has been identified as an end-product.
Chlorinated paraffins may be excreted via the renal, biliary and
the pulmonary routes (as CO2). The relative extent of excretion
via the different routes is difficult to establish due to the
wide variability in different studies. The total elimination of
chlorinated paraffins decreases as the chlorine content increases, and
compounds with high degrees of chlorination are mainly excreted (more
than 50%) as CO2. Chlorinated paraffins may be excreted in milk.
1.6 Effects on laboratory mammals and in vitro test systems
The acute oral toxicity of chlorinated paraffins of various chain
lengths is low. Toxic effects such as muscular incoordination and
piloerection were most evident following single exposure to short
chain length chlorinated paraffins. On the basis of very limited
data, the acute toxicity by the inhalation and dermal routes also
appears to be low. Mild skin and eye irritation has been observed
after application of short and intermediate (skin irritation)
chain length chlorinated paraffins. Results of several studies
indicate that short chain chlorinated paraffins do not induce skin
sensitization.
In repeated dose toxicity studies by the oral route, the liver,
kidney and thyroid are the primary target organs for the toxicity of
the chlorinated paraffins. For the short chain compounds, increases
in liver weight have been observed at lowest doses (lowest-observed-
effect level is 50 to 100 mg/kg body weight per day and no-observed-
effect level is 10 mg/kg body weight per day in rats). At higher
doses, increases in the activity of hepatic enzymes, proliferation
of smooth endoplasmic reticulum and peroxisomes, replicative DNA
synthesis, hypertrophy, hyperplasia and necrosis of the liver have
also been observed. Decreases in body weight gain (125 mg/kg body
weight per day in mice), increases in kidney weight (100 mg/kg body
weight per day in rats), replicative DNA synthesis in renal cells
(313 mg/kg body weight per day) and nephrosis (625 mg/kg body weight
per day in rats) have also been observed. Increases in thyroid
weight, and hypertrophy and hyperplasia of the thyroid (LOEL of
100 mg/kg body weight per day in rats) and replicative DNA synthesis
in thyroid follicular cells (LOEL of 313 mg/kg body weight per day)
have been reported. At higher doses (1000 mg/kg body weight per day),
thyroid function is affected, as determined by free and total levels
of plasma thyroxine and increased plasma thyroid-stimulating hormone
in rats.
For the intermediate chain compounds, effects observed at lowest
doses are generally increases in liver and kidney weight (LOEL in rats
of 100 mg/kg body weight per day; NOAEL in rats of 10 mg/kg body
weight per day). Increases in serum cholesterol and "mild, adaptive"
histological changes in the thyroid have been reported at similar
doses in female rats (NOAEL of 4 mg/kg body weight per day).
For the long chain compounds, effects observed at lowest doses
are multifocal granulomatous hepatitis and increased liver weights in
female rats (LOAEL of 100 mg/kg body weight per day).
In the only identified reproduction study, no adverse
reproductive effects were reported following exposure of rats to an
intermediate chain length chlorinated paraffin with 52% chlorine.
However, survival and body weights of the exposed pups were reduced
(LOEL for non-significant decrease in body weight of 5.7-7.2 mg/kg
body weight per day; LOAEL for decreased survival of 60-70 mg/kg body
weight per day). In a limited number of studies of the developmental
effects of the short, medium and long chain chlorinated paraffins,
adverse effects in the offspring were observed for the short chain
compounds only, at maternally toxic doses in rats (2000 mg/kg body
weight per day). For the medium and long chain compounds, no effects
on the offspring were observed even at very high doses (1000 to
5000 mg/kg body weight per day).
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells, there is a suggestion of a
weak clastogenic potential in vitro but not in vivo. Chlorinated
paraffins are also reported to induce cell transformation in vitro.
Long term carcinogenicity studies by oral gavage in rats and mice
have been conducted on a short chain chlorinated paraffin (C12;
58% Cl) and a long chain chlorinated paraffin (C23; 43% Cl). For
the short chain compound in B6C3F1 mice, there were increases in
the incidence of hepatic tumours in males and females and tumours of
the thyroid gland in females. In Fischer-344 rats exposed to the short
chain compound, there were increases in hepatic tumours in males and
females, renal tumours (adenomas or adenocarcinomas) in males, tumours
of the thyroid in females and mononuclear cell leukaemias in males.
For the long chain chlorinated paraffin, the incidences of malignant
lymphomas in male mice and tumours of the adrenal gland in female rats
were increased.
1.7 Effects on humans
In spite of the widespread use of chlorinated paraffins, there
are no case reports of skin irritation or sensitization. This is
supported by results of a limited number of studies in volunteers in
which chlorinated paraffins have induced minimal irritancy in the
skin, but not sensitization.
Data on other effects of chlorinated paraffins in humans have not
been identified.
1.8 Effects on other organisms in the laboratory and field
Chlorinated paraffins of short chain length have been shown to
be acutely toxic to freshwater and saltwater invertebrates, with
LC50-EC50 values ranging from 14 to 530 µg/litre. Most of the acute
toxicity tests on aquatic invertebrates for intermediate and long
chain chlorinated paraffins exceed the water solubility. However, a
study on an intermediate chlorinated paraffin product shows acute
toxicity to daphnids at an EC50 of 37 µg/litre. Short, intermediate
and long chain chlorinated paraffins appear to be of low acute
toxicity to fish, with LC50 values well in excess of the water
solubility.
Short chain length chlorinated paraffins show long-term toxicity
to algae, aquatic invertebrates and fish at concentrations as low
as 19.6, 8.9 and 3.1 µg/litre, respectively; no-observed-effect
concentrations appear to be in the range of 2 to 5 µg/litre for the
most sensitive species tested. An intermediate and a long chain
product showed chronic effects on daphnids at concentrations of 20 to
35 µg/litre and < 1.2 to 8 µg/litre, respectively. Long-term
toxicity to fish seems to be low. No data are available on algae.
On the basis of limited available data, the acute toxicity of
chlorinated paraffins in birds is low.
1.9 Evaluation of human health risks and effects on the environment
It is likely that the principal source of exposure of the general
population is food. On the basis of limited data on concentrations
present in foodstuffs, worst case estimates of daily intake in dairy
products and mussels, respectively, are 4 and 25 µg/kg body weight
per day. In general, the calculated daily intakes of chlorinated
paraffins are below the tolerable intakes for non-neoplastic effects
or recommended values for neoplastic effects (short chain compounds).
Provided that proper personal hygiene and safety procedures are
followed, the risk to health for workers exposed to chlorinated
paraffins is expected to be minimal.
Available data indicate that chlorinated paraffins are
bioaccumulative and persistent. The data on environmental levels of
short chain chlorinated paraffins indicate that in areas close to
release sources there is a risk to both freshwater and estuarine
organisms. There is also a potential risk to aquatic invertebrates
from intermediate and long chain chlorinated paraffin products.
The enrichment of chlorinated paraffins in sediments, their
resorption behaviour and aquatic toxicity indicate a potential risk
for sediment-dwelling organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chlorinated paraffins (CPs) are produced by chlorination of
normal paraffin fractions (straight-chain hydrocarbons, at least 98%
linear), and have the general formula CxH(2x-y+2)Cly. The length of
the carbon chains is usually between 10 and 30 carbon atoms, and the
chlorine content is between 20 and 70% by weight, although the
commercial products normally fall within the 40-70% Cl range
(Schenker, 1979). In this monograph the different isomers will be
referred to as Cx;y% Cl, i.e., a chlorinated paraffin with a carbon
chain length of 12 and a chlorination degree of 60% will be referred
to as C12;60% Cl.
Commercial chlorinated paraffins, of which there are over 200,
are very complex mixtures of n-alkanes characterized by an average
carbon chain length and chlorination degree. Each grade varies in the
range of carbon chain length, but also in the distribution and degree
of chlorination. The different technical grades have therefore
specific physical and chemical properties which render them useful in
such widespread applications as plasticizers in plastics such as
polyvinyl chloride, extreme pressure additives, flame retardants and
paints.
The number of theoretically possible structures within the ranges
C10-C30 and 40-70% Cl is enormous. Taking C12 and 60% Cl as an
example, there are numerous possibilities, depending on the position
of the chlorine atoms. In just one of these structures (Fig. 1),
there are 25=32 different diastereomers, owing to the five optical
sites (indicated by an asterisk).
The raw materials most frequently used for the production of
chlorinated paraffins are normal paraffin feedstocks, which fall into
three main categories:
1) a liquid fraction including C10-C13 with an average of C12;
2) a liquid fraction including C14-C17 with an average of C15; and
3) a wax fraction including C20-C28 with an average of C24 (Strack,
1986).
A wax fraction including C18-C20 is also used. Depending on
the feedstock and the degree of chlorination, long chain length
chlorinated paraffins (C18-30) range from being mobile to very viscous
liquids, with the exception of the C20-30;70% Cl type, which is a
solid.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the
sole responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLORINATED
PARAFFINS
Members
Professor U.G. Ahlborg, Institute of Environmental Medicine,
Karolinska Institute, Stockholm, Sweden (Vice-Chairman)
Dr D. Anderson, British Industry Biological Research Association
(BIBRA) Toxicology International, Carshalton, Surrey, United
Kingdom
Dr T. Beulshausen, Federal Environment Agency, Berlin, Germany
Dr R.S. Chhabra, Environmental Toxicology Program, National Institute
of Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Dr N. Gregg, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, Cambridgeshire, United Kingdom (Joint Rapporteur)
Dr B. Jansson, Institute of Applied Environmental Research, Stockholm
University, Solna, Sweden
Dr K. Kenne, Institute of Environmental Medicine, Karolinska
Institute, Stockholm, Sweden (Joint Rapporteur)
Dr M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Chairman)
Representatives of other Organizations
Dr P. Montuschi, Institute of Pharmacology, Faculty of Medicine and
Surgery, Catholic University of the Sacred Heart, Rome, Italy
(Representing the International Union of Pharmacology)
Mr D. Farrar, Occupational Health, ICI Chemicals and Polymers Limited,
Runcorn, Cheshire, United Kingdom
(Representing the European Centre for Ecotoxicology and Toxicology
of Chemicals)
Secretariat
Dr E.M. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr J.D. Wilbourn, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR CHLORINATED PARAFFINS
A WHO Task Group on Environmental Health Criteria for Chlorinated
Paraffins met at the World Health Organization, Geneva, from 20 to 24
March 1995. Dr E.M. Smith, IPCS, welcomed the participants on behalf
of Dr M. Mercier, Director of the IPCS, and on behalf the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft and made an evaluation of the risks for human health
and the environment from exposure to chlorinated paraffins.
The first draft was prepared at the Institute of Environmental
Medicine, Karolinska Institute, Stockholm, Sweden, by Dr K. Kenne and
Professor U.G. Ahlborg. The second draft, incorporating comments
received following circulation of the first drafts to the IPCS contact
points for Environmental Health Criteria monographs, was also prepared
by Dr Kenne and Professor Ahlborg.
Dr E.M. Smith and Dr P. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific aspects of the monograph and for
the technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
APDM aminopyrine demethylase
BCF bioconcentration factor
CD coulometric detection
CP chlorinated paraffins
CP-LH chlorinated paraffin with long chain length and high degree
of chlorination
CP-LL chlorinated paraffin with long chain length and low degree of
chlorination
CP-MH chlorinated paraffin with medium chain length and high degree
of chlorination
CP-ML chlorinated paraffin with medium chain length and low degree
of chlorination
CP-SH chlorinated paraffin with short chain length and high degree
of chlorination
CP-SL chlorinated paraffin with short chain length and low degree
of chlorination
EC50 median effective concentration
ECD electron capture detection
GC gas chromatography
LC50 median lethal concentration
LOAEL lowest-observed-adverse-effect level
LOEC lowest-observed-effect concentration
LOEL lowest-observed-effect level
LT50 median lethal time
MS mass spectrometry
NCI negative ion chemical ionization
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
PCB polychlorinated biphenyl
PVC polyvinyl chloride
TDI tolerable daily intake
TLC thin-layer chromatography
TSH thyroid stimulating hormone
UDP uridine diphosphate
1. SUMMARY
1.1 Properties, uses and analytical methods
Chlorinated paraffins (CPs) are produced by chlorination of
straight-chained paraffin fractions. The carbon chain length of
commercial chlorinated paraffins is usually between 10 and 30 carbon
atoms, and the chlorine content is usually between 40 and 70% by
weight. Chlorinated paraffins are viscous colourless or yellowish
dense oils with low vapour pressures, except for those of long carbon
chain length with high chlorine content (70%), which are solid.
Chlorinated paraffins are practically insoluble in water, lower
alcohols, glycerol and glycols, but are soluble in chlorinated
solvents, aromatic hydrocarbons, ketones, esters, ethers, mineral oils
and some cutting oils. They are moderately soluble in unchlorinated
aliphatic hydrocarbons.
Chlorinated paraffins consist of extremely complex mixtures,
owing to the many possible positions for the chlorine atoms. The
products can be subdivided into six groups depending on chain length
(short C10-13, intermediate C14-17 and long C18-30) and degree of
chlorination (low (< 50%) and high (> 50%)).
Chlorinated paraffins are used worldwide in widespread
applications such as plasticizers in plastics (e.g., PVC), extreme
pressure additives in metal working fluids, flame retardants and
additives in paints. Technical grade chlorinated paraffins may be
contaminated by isoparaffins, aromatic compounds and metals, and
normally contain stabilizers, which are added to inhibit
decomposition.
The analysis of chlorinated paraffins is difficult due to the
extreme complexity of these mixtures. In environmental samples, this
is further complicated by interference from other compounds. Analyses
often require extensive clean-up of the samples and the use of
specific detection methods. Early methods were based on thin-layer
chromatography for the clean-up and an unspecific argentation
detection method on the plates. Methods based on different column
liquid chromatography are currently used for the clean-up, although it
is difficult to isolate the chlorinated paraffins due to their wide
range of physical properties. Specific detection methods are
therefore used; gas chromatography combined with mass spectrometry is
now the most common technique. The use of negative ions makes the
detection even more specific. Although use of these sophisticated
techniques has improved the ability to analyse chlorinated paraffins,
it is still impossible to determine exact concentrations. Reported
results should be regarded only as estimates of the true values.
1.2 Sources of human and environmental exposure
Chlorinated paraffins are not known to occur naturally.
Chlorinated paraffins are produced by reacting liquid paraffin
fractions with pure chlorine gas. The reaction may require the use of
a solvent, and often ultraviolet light is used as a catalyst. In
1985, the estimated world production of chlorinated paraffins was
300 000 tonnes.
The widespread uses of chlorinated paraffins probably provide the
major source of environmental contamination. Chlorinated paraffins
may be released into the environment from improperly disposed
metal-working fluids containing chlorinated paraffins or from polymers
containing chlorinated paraffins. Loss of chlorinated paraffins by
leaching from paints and coatings may also contribute to environmental
contamination. The potential for loss during production and transport
is expected to be less than that during product use and disposal.
Owing to their thermal instability, chlorinated paraffins are
expected to be degraded by incineration and thus would not be expected
to volatilize in exhaust gases from incinerators. However, it has
been demonstrated that chlorinated aromatic compounds such as
polychlorinated biphenyls, naphthalenes and benzenes are formed by
pyrolysis of chlorinated paraffins under certain conditions.
1.3 Environmental distribution and transformation
Chlorinated paraffins adsorb strongly to sediment. In water they
are probably transported adsorbed on suspended particles, and in the
atmosphere adsorbed to airborne particulates (and possibly in the
vapour phase). The half-lives for chlorinated paraffins in air have
been estimated to range from 0.85 to 7.2 days, a period sufficiently
long that the possibility of long-range transport cannot be excluded.
Chlorinated paraffins are not readily biodegradable. Short
carbon chain length chlorinated paraffins with a chlorine content of
less than 50% appear to be degradable under aerobic conditions with
acclimated microorganisms, whereas the degradation appears inhibited
at a chlorine content above 58%. Intermediate and long chain length
chlorinated paraffins are degraded more slowly.
Chlorinated paraffins are bioaccumulated in aquatic organisms,
and the reported bioconcentration factors (BCFs) are in the range of 7
to 7155 for fish and 223 to 138 000 for mussels. In fish, chlorinated
paraffins of short chain length are accumulated to a higher degree
than intermediate and long chain length chlorinated paraffins.
Radioactivity has been found mainly in bile, intestine, liver, fat and
gills after administration of radiolabelled chlorinated paraffins. The
uptake of chlorinated paraffins seems to be more efficient for short
chlorinated paraffins with low chlorine content; the elimination rate
is slowest for short chlorinated paraffins with high chlorine content.
The retention in fat-rich tissues appears to increase with increasing
degree of chlorination.
1.4 Environmental levels and human exposure
Few data on levels of chlorinated paraffins in the environment
are available. Chlorinated paraffins have been detected in marine
water samples in the United Kingdom at levels below 4 µg/litre. In
non-marine waters, levels below 6 µg/litre in the United Kingdom have
been reported; in Germany, concentrations determined in 1994 were in
the range of 0.08-0.28 µg/litre. In water in the USA, concentrations
were generally less than 0.03 µg/litre, although levels were above 1.0
µg/litre in a small proportion (1.2%) of samples. In marine sediments,
levels up to 600 µg/kg wet weight have been reported, and in
non-marine sediments in the United Kingdom concentrations were up to
15 000 µg/kg in industrialized regions and 1000 µg/kg in areas
remote from industry. In sediments in an impoundment lagoon from a
chlorinated paraffin manufacturing plant in the USA, concentrations as
high as 170 000 µg/kg dry weight of long chain length chlorinated
paraffins, 50 000 µg/kg of intermediate chain length chlorinated
paraffins and 40 000 µg/kg of short chain length chlorinated paraffins
were reported. In Germany, levels up to 83 µg/kg dry weight of C10-13
and up to 370 µg/kg dry weight of C14-17 were reported in sediments in
1994. In Japan, levels in sediment ranged up to 8500 µg/kg.
Chlorinated paraffins have been detected in various organisms.
Chlorinated paraffins are present in terrestrial mammals in Sweden at
concentrations in the range of 32-88 µg/kg tissue (140-4400 µg/kg
lipid). However, chlorinated paraffins were not detected in sheep
which were grazed remote from production of chlorinated paraffins in
the United Kingdom. In birds in the United Kingdom, concentrations
ranged up to 1500 µg/kg and in fish in Sweden and the United Kingdom,
levels ranged up to 200 µg/kg. In mussels collected in the USA and
United Kingdom, concentrations up to 400 µg/kg were reported.
However, levels of C10-20 in mussels collected close to a chlorinated
paraffin plant effluent discharge ranged up to 12 000 µg/kg.
Chlorinated paraffins have also been detected in post mortem human
tissues, i.e. in adipose tissue (median level of 100-190 µg/kg),
kidney (median level below 90 µg/kg) and liver (median level below 90
µg/kg). In one limited survey, chlorinated paraffins, mostly C10-20,
were present at levels of up to 500 µg/kg in approximately 70% of the
samples of various food products.
Information on occupational exposure to chlorinated paraffins is
limited. Very low levels of exposure to aerosols of short chain
chlorinated paraffins (0.003-1.2 mg/m3) have been found to be
associated with their use as metal-working fluids, although there is
no information available on the proportion that is inhalable. On the
basis of mathematical modelling of exposure without any control
measures, high levels of dermal contact (5-15 mg/cm2 per day) were
estimated for speciality metal-working fluids which contain very high
levels of short chain chlorinated paraffins, although absorption would
be expected to be low. Control measures would reduce dermal exposure.
1.5 Kinetics and metabolism
The toxicokinetics of chlorinated paraffins have been studied
in experimental animals. Adequate information for humans is not
available. Possible differences in toxicokinetics as a result of
different chain lengths have not been sufficiently investigated.
Although the extent of absorption of chlorinated paraffins after oral
administration is unknown, it appears to decrease with increasing
chain length and degree of chlorination. Percutaneous absorption may
also occur depending on chain length, but would be limited (less than
1% of a topical C18 dose). No data on absorption via the lung is
available.
Distribution of chlorinated paraffins occurs mainly in the liver,
kidney, intestine, bone marrow, adipose tissue and ovary. Information
on retention is insufficient but a low degree of chlorination may
enhance retention time due to slower redistribution. Chlorinated
paraffins or their metabolites are present in the central nervous
system up to 30 days after administration. They may cross the
blood-placental barrier. There is no adequate information on the
pathways of metabolism of chlorinated paraffins, although in
radiolabelling studies CO2 has been identified as an end-product.
Chlorinated paraffins may be excreted via the renal, biliary and
the pulmonary routes (as CO2). The relative extent of excretion
via the different routes is difficult to establish due to the
wide variability in different studies. The total elimination of
chlorinated paraffins decreases as the chlorine content increases, and
compounds with high degrees of chlorination are mainly excreted (more
than 50%) as CO2. Chlorinated paraffins may be excreted in milk.
1.6 Effects on laboratory mammals and in vitro test systems
The acute oral toxicity of chlorinated paraffins of various chain
lengths is low. Toxic effects such as muscular incoordination and
piloerection were most evident following single exposure to short
chain length chlorinated paraffins. On the basis of very limited
data, the acute toxicity by the inhalation and dermal routes also
appears to be low. Mild skin and eye irritation has been observed
after application of short and intermediate (skin irritation)
chain length chlorinated paraffins. Results of several studies
indicate that short chain chlorinated paraffins do not induce skin
sensitization.
In repeated dose toxicity studies by the oral route, the liver,
kidney and thyroid are the primary target organs for the toxicity of
the chlorinated paraffins. For the short chain compounds, increases
in liver weight have been observed at lowest doses (lowest-observed-
effect level is 50 to 100 mg/kg body weight per day and no-observed-
effect level is 10 mg/kg body weight per day in rats). At higher
doses, increases in the activity of hepatic enzymes, proliferation
of smooth endoplasmic reticulum and peroxisomes, replicative DNA
synthesis, hypertrophy, hyperplasia and necrosis of the liver have
also been observed. Decreases in body weight gain (125 mg/kg body
weight per day in mice), increases in kidney weight (100 mg/kg body
weight per day in rats), replicative DNA synthesis in renal cells
(313 mg/kg body weight per day) and nephrosis (625 mg/kg body weight
per day in rats) have also been observed. Increases in thyroid
weight, and hypertrophy and hyperplasia of the thyroid (LOEL of
100 mg/kg body weight per day in rats) and replicative DNA synthesis
in thyroid follicular cells (LOEL of 313 mg/kg body weight per day)
have been reported. At higher doses (1000 mg/kg body weight per day),
thyroid function is affected, as determined by free and total levels
of plasma thyroxine and increased plasma thyroid-stimulating hormone
in rats.
For the intermediate chain compounds, effects observed at lowest
doses are generally increases in liver and kidney weight (LOEL in rats
of 100 mg/kg body weight per day; NOAEL in rats of 10 mg/kg body
weight per day). Increases in serum cholesterol and "mild, adaptive"
histological changes in the thyroid have been reported at similar
doses in female rats (NOAEL of 4 mg/kg body weight per day).
For the long chain compounds, effects observed at lowest doses
are multifocal granulomatous hepatitis and increased liver weights in
female rats (LOAEL of 100 mg/kg body weight per day).
In the only identified reproduction study, no adverse
reproductive effects were reported following exposure of rats to an
intermediate chain length chlorinated paraffin with 52% chlorine.
However, survival and body weights of the exposed pups were reduced
(LOEL for non-significant decrease in body weight of 5.7-7.2 mg/kg
body weight per day; LOAEL for decreased survival of 60-70 mg/kg body
weight per day). In a limited number of studies of the developmental
effects of the short, medium and long chain chlorinated paraffins,
adverse effects in the offspring were observed for the short chain
compounds only, at maternally toxic doses in rats (2000 mg/kg body
weight per day). For the medium and long chain compounds, no effects
on the offspring were observed even at very high doses (1000 to
5000 mg/kg body weight per day).
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells, there is a suggestion of a
weak clastogenic potential in vitro but not in vivo. Chlorinated
paraffins are also reported to induce cell transformation in vitro.
Long term carcinogenicity studies by oral gavage in rats and mice
have been conducted on a short chain chlorinated paraffin (C12;
58% Cl) and a long chain chlorinated paraffin (C23; 43% Cl). For
the short chain compound in B6C3F1 mice, there were increases in
the incidence of hepatic tumours in males and females and tumours of
the thyroid gland in females. In Fischer-344 rats exposed to the short
chain compound, there were increases in hepatic tumours in males and
females, renal tumours (adenomas or adenocarcinomas) in males, tumours
of the thyroid in females and mononuclear cell leukaemias in males.
For the long chain chlorinated paraffin, the incidences of malignant
lymphomas in male mice and tumours of the adrenal gland in female rats
were increased.
1.7 Effects on humans
In spite of the widespread use of chlorinated paraffins, there
are no case reports of skin irritation or sensitization. This is
supported by results of a limited number of studies in volunteers in
which chlorinated paraffins have induced minimal irritancy in the
skin, but not sensitization.
Data on other effects of chlorinated paraffins in humans have not
been identified.
1.8 Effects on other organisms in the laboratory and field
Chlorinated paraffins of short chain length have been shown to
be acutely toxic to freshwater and saltwater invertebrates, with
LC50-EC50 values ranging from 14 to 530 µg/litre. Most of the acute
toxicity tests on aquatic invertebrates for intermediate and long
chain chlorinated paraffins exceed the water solubility. However, a
study on an intermediate chlorinated paraffin product shows acute
toxicity to daphnids at an EC50 of 37 µg/litre. Short, intermediate
and long chain chlorinated paraffins appear to be of low acute
toxicity to fish, with LC50 values well in excess of the water
solubility.
Short chain length chlorinated paraffins show long-term toxicity
to algae, aquatic invertebrates and fish at concentrations as low
as 19.6, 8.9 and 3.1 µg/litre, respectively; no-observed-effect
concentrations appear to be in the range of 2 to 5 µg/litre for the
most sensitive species tested. An intermediate and a long chain
product showed chronic effects on daphnids at concentrations of 20 to
35 µg/litre and < 1.2 to 8 µg/litre, respectively. Long-term
toxicity to fish seems to be low. No data are available on algae.
On the basis of limited available data, the acute toxicity of
chlorinated paraffins in birds is low.
1.9 Evaluation of human health risks and effects on the environment
It is likely that the principal source of exposure of the general
population is food. On the basis of limited data on concentrations
present in foodstuffs, worst case estimates of daily intake in dairy
products and mussels, respectively, are 4 and 25 µg/kg body weight
per day. In general, the calculated daily intakes of chlorinated
paraffins are below the tolerable intakes for non-neoplastic effects
or recommended values for neoplastic effects (short chain compounds).
Provided that proper personal hygiene and safety procedures are
followed, the risk to health for workers exposed to chlorinated
paraffins is expected to be minimal.
Available data indicate that chlorinated paraffins are
bioaccumulative and persistent. The data on environmental levels of
short chain chlorinated paraffins indicate that in areas close to
release sources there is a risk to both freshwater and estuarine
organisms. There is also a potential risk to aquatic invertebrates
from intermediate and long chain chlorinated paraffin products.
The enrichment of chlorinated paraffins in sediments, their
resorption behaviour and aquatic toxicity indicate a potential risk
for sediment-dwelling organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chlorinated paraffins (CPs) are produced by chlorination of
normal paraffin fractions (straight-chain hydrocarbons, at least 98%
linear), and have the general formula CxH(2x-y+2)Cly. The length of
the carbon chains is usually between 10 and 30 carbon atoms, and the
chlorine content is between 20 and 70% by weight, although the
commercial products normally fall within the 40-70% Cl range
(Schenker, 1979). In this monograph the different isomers will be
referred to as Cx;y% Cl, i.e., a chlorinated paraffin with a carbon
chain length of 12 and a chlorination degree of 60% will be referred
to as C12;60% Cl.
Commercial chlorinated paraffins, of which there are over 200,
are very complex mixtures of n-alkanes characterized by an average
carbon chain length and chlorination degree. Each grade varies in the
range of carbon chain length, but also in the distribution and degree
of chlorination. The different technical grades have therefore
specific physical and chemical properties which render them useful in
such widespread applications as plasticizers in plastics such as
polyvinyl chloride, extreme pressure additives, flame retardants and
paints.
The number of theoretically possible structures within the ranges
C10-C30 and 40-70% Cl is enormous. Taking C12 and 60% Cl as an
example, there are numerous possibilities, depending on the position
of the chlorine atoms. In just one of these structures (Fig. 1),
there are 25=32 different diastereomers, owing to the five optical
sites (indicated by an asterisk).
The raw materials most frequently used for the production of
chlorinated paraffins are normal paraffin feedstocks, which fall into
three main categories:
1) a liquid fraction including C10-C13 with an average of C12;
2) a liquid fraction including C14-C17 with an average of C15; and
3) a wax fraction including C20-C28 with an average of C24 (Strack,
1986).
A wax fraction including C18-C20 is also used. Depending on
the feedstock and the degree of chlorination, long chain length
chlorinated paraffins (C18-30) range from being mobile to very viscous
liquids, with the exception of the C20-30;70% Cl type, which is a
solid.
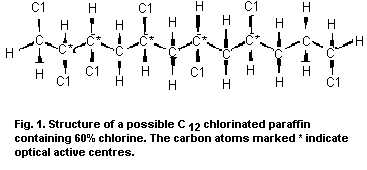 In general chlorinated paraffins are classified as short chain
(C10-13), intermediate chain (C14-17) and long chain (C18-30). These
groups are further divided into two classes according to chlorine
content: < 50% and > 50% chlorine. A suggested classification of
the different chlorinated paraffin isomers is shown in Table 1. The
suggested acronyms are used in this monograph.
2.1.1 Relative molecular mass
The relative molecular mass depends on the carbon chain length
and the degree of chlorination. The chlorinated paraffin C10;50.6%
Cl has a relative molecular mass of 280.1, whereas that of C25;69% Cl
is 1075.
2.1.2 Common names
2.1.2.1 CAS registry number and names
63449-39-8 Paraffin waxes and hydrocarbon waxes, chloro
85422-92-0 Paraffin oils and hydrocarbon oils, chloro
61788-76-9 Alkanes, chloro
68920-70-7 Alkanes, C6-18, chloro
71011-12-6 Alkanes, C12-13, chloro
84082-38-2 Alkanes, C10-21, chloro
84776-06-7 Alkanes, C10-32, chloro
84776-07-8 Alkanes, C16-27, chloro
85049-26-9 Alkanes, C16-35, chloro
85535-84-8 Alkanes, C10-13, chloro
85535-85-9 Alkanes, C14-17, chloro
85535-86-0 Alkanes, C18-28, chloro
85536-22-7 Alkanes, C12-14, chloro
85681-73-8 Alkanes, C10-14, chloro
97659-46-6 Alkanes, C10-26, chloro
97553-43-0 Paraffins (petroleum), normal C > 10, chloro
106232-85-3 Alkanes, C18-20, chloro
106232-86-4 Alkanes, C22-40, chloro
108171-26-2 Alkanes, C10-12, chloro
108171-27-3 Alkanes, C22-26, chloro
In general chlorinated paraffins are classified as short chain
(C10-13), intermediate chain (C14-17) and long chain (C18-30). These
groups are further divided into two classes according to chlorine
content: < 50% and > 50% chlorine. A suggested classification of
the different chlorinated paraffin isomers is shown in Table 1. The
suggested acronyms are used in this monograph.
2.1.1 Relative molecular mass
The relative molecular mass depends on the carbon chain length
and the degree of chlorination. The chlorinated paraffin C10;50.6%
Cl has a relative molecular mass of 280.1, whereas that of C25;69% Cl
is 1075.
2.1.2 Common names
2.1.2.1 CAS registry number and names
63449-39-8 Paraffin waxes and hydrocarbon waxes, chloro
85422-92-0 Paraffin oils and hydrocarbon oils, chloro
61788-76-9 Alkanes, chloro
68920-70-7 Alkanes, C6-18, chloro
71011-12-6 Alkanes, C12-13, chloro
84082-38-2 Alkanes, C10-21, chloro
84776-06-7 Alkanes, C10-32, chloro
84776-07-8 Alkanes, C16-27, chloro
85049-26-9 Alkanes, C16-35, chloro
85535-84-8 Alkanes, C10-13, chloro
85535-85-9 Alkanes, C14-17, chloro
85535-86-0 Alkanes, C18-28, chloro
85536-22-7 Alkanes, C12-14, chloro
85681-73-8 Alkanes, C10-14, chloro
97659-46-6 Alkanes, C10-26, chloro
97553-43-0 Paraffins (petroleum), normal C > 10, chloro
106232-85-3 Alkanes, C18-20, chloro
106232-86-4 Alkanes, C22-40, chloro
108171-26-2 Alkanes, C10-12, chloro
108171-27-3 Alkanes, C22-26, chloro
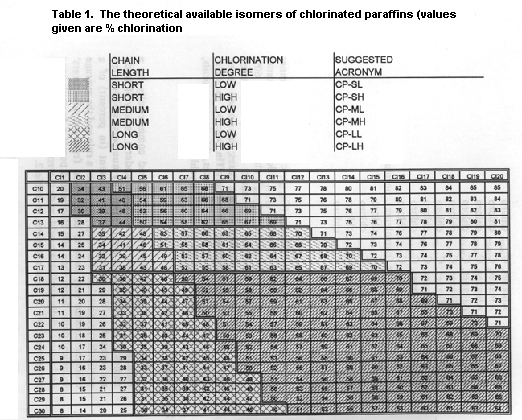 2.1.2.2 Synonyms
Alkanes, chlorinated; alkanes (C10-12), chloro (60%); alkanes (C10-13),
chloro (50-70%); alkanes (C14-17), chloro (40-52%); alkanes (C18-28),
chloro (20-50%); alkanes (C22-26), chloro (43%); C12, 60% chlorine;
C23, 43% chlorine; chlorinated alkanes; chlorinated hydrocarbon
waxes; chlorinated paraffin waxes; chlorinated waxes; chloroalkanes;
chlorocarbons; chloroparaffin waxes; paraffin, chlorinated; paraffins,
chloro; paraffin waxes, chlorinated; paroils, chlorinated; poly-
chlorinated alkanes; polychloro alkanes.
2.1.3 Technical products
Chlorinated paraffins are manufactured commercially by a number
of companies and are marketed under a variety of trade names. The
trade names are followed by numbers, which often are related to the
average chlorine content (in percent) of a particular preparation.
However, this is not a rule and the average chlorine content may have
to be obtained from manufacturers' technical data. More than 200
chlorinated paraffin formulations are commercially available
world-wide (Serrone et al., 1987), and some examples of these are
given in Table 2.
The carbon chain length of the chlorinated paraffins in a
commercial mixture is variable, and the average chain length is
usually specified by the manufacturer. The composition of paraffins
of different chain length in some commercial formulations is shown in
Table 3. The paraffin feedstocks are randomly chlorinated and the
resulting chlorine contents are given as average values.
Commercial chlorinated paraffins may be contaminated by
isoparaffins (usually less than 1%), aromatic compounds (usually less
than 0.1% (1000 ppm)) and metals (Schenker, 1979).
Chlorinated paraffins normally contain stabilizers, which are
added to inhibit decomposition. Common stabilizers include epoxidized
compounds such as epoxidized esters and soya bean oils (indicated in
section 7.1.3 to be present at up to 3%), pentaerythritol, thymol,
urea, glycidyl ethers, acetonitriles and organic phosphites (Schenker,
1979; Strack, 1986; Houghton, 1993). The concentration of stabilizers
is usually below 0.05% w/w (Campbell & McConnell, 1980).
Table 2. Partial list of commercial chlorinated paraffinsa
Average molecular formula C12H15Cl11 C12H19Cl7 C15H26Cl6 C24H29Cl21 C24H42Cl8 C24H44Cl6
Chlorine content (% w/w) 70 60-65 50-52 70 48-54 40-42
Manufacturers:
Oxychem, USA Chlorowax 70L Chlorowax 500C Chlorowax 70 Chlorowax 50 Chlorowax 40
Keil Chemical Div., USA CW-200-70 CW-85-60 CW-52 CW-220-50 CW-170
Dover Chemical Corp., Paroil 170HV Paroil 160 Paroil 152 Chlorez 700 Paroil 150S Paroil 140
USA Paroil 1048
Plastifax, Inc., USAb Plastichlor P-70 Plastichlor P-59 Plastichlor 50-220 Plastichlor
P-65 42-170
ICI, Australia; Canada; Cereclor 70L Cereclor 60L Cereclor S52 Cereclor 70 Cereclor 48 Cereclor 42
UK; France
Neville Chemical Co., Unichlor 60L-60 Unichlor 50L-65 Unichlor 50-450 Unichlor 40-170
USAb Unichlor 40-150
Pearsall Chemical Co., FLX-0012 FLX-0008 CPF-0020 CPF-0004
USA CPF-0003 CPF-0001
Hüls AG, Germanyb Chlorparaffin Chlorparaffin Chlorparaffin Chlorparaffin
70C 60C 52G 40N
Dynamit Nobel, Germanyb Witaclor 171 Witaclor 160 - Witaclor 350 Witaclor 549 Witaclor 540
Witaclor 163 Witaclor 352
Caffaro, Italy Cloparin D70 Cloparin 1059 Cloparin 50 Cloparin S70 Cloparin P42
Table 2. (Cont'd)
Average molecular formula C12H15Cl11 C12H19Cl7 C15H26Cl6 C24H29Cl21 C24H42Cl8 C24H44Cl6
Chlorine content (% w/w) 70 60-65 50-52 70 48-54 40-42
Hoechst AG, Germany Chlorparaffin Hordaflex LC60 Chlorparaffin Chlorparaffin Chlorparaffin
Hoechst 70 Hoechst 52fl Hoechst 70fest Hoechst 40fl
Rhône-Poulenc, France Alaiflex 67B2 Ribeclor 60B2 Alaiflex 50A3 Alaiflex 40A8
a Other producers include Bann Quimica (Brazil), Excel Industry (India), Ajinomoto (Japan), Tosoh (Japan), Asahi Denka (Japan),
Plasticlor (Mexico), NCP (South Africa)
b These companies have ceased production of chlorinated paraffins.
Table 3. Composition of paraffins obtained by dechlorination of
different chlorinated paraffin preparations (Zitko, 1974b)
Chlorinated Percentage of each paraffin
paraffin
C21 C22 C23 C24 C25 C26 C27 C28
Chloroparaffin, 4.5 10.0 15.7 19.3 18.5 15.3 9.8 6.7
40%
Clorafin 40 3.7 8.2 14.0 17.5 19.2 17.4 12.4 7.6
CP 40 3.9 9.1 14.9 19.2 19.8 18.0 15.1 -
Cereclor 42 3.6 8.8 14.7 18.6 19.5 17.2 11.5 6.0
Chloroparaffin, 7.4 14.9 20.7 23.1 19.9 14.0 - -
50%
2.2 Chemical and physical properties
Chlorinated paraffins are viscous, colourless or yellowish, dense
oils, except for the chlorinated paraffins of long carbon chain length
(C20-C30) with high chlorine content (70%), which are solid.
Chlorinated paraffins have a characteristic slight and not unpleasant
odour (Hardie, 1964). The odour is probably due to small quantities
of products of lower relative molecular mass with small but measurable
vapour pressures (Howard et al., 1975). Chlorinated paraffins
themselves have very low vapour pressures. The medium chain length
C14-17;52% Cl has a vapour pressure of approximately 2 × 10-4 Pa at
20°C (1-2 × 10-6 mmHg) (Campbell & McConnell, 1980), and the long
chain length C23;42-54% Cl approximately 3 × 10-3 Pa when measured at
65°C (2 × 10-5 mmHg) (Hardie, 1964). The chemical and physical
properties of chlorinated paraffins are determined by the carbon chain
length of the paraffin and the chlorine content. Increases in the
carbon chain length and chlorination degree of a particular paraffin
increase the viscosity and density but reduce the volatility.
Chlorinated paraffins are practically insoluble in water, but
many products can be emulsified with water (approximately 70/30
chlorinated paraffin to water). The water solubility of 14C-labelled
polychloroundecane (C11;59% Cl) is reported to be 150-470 µg/litre,
polychloropentadecane (C15;51% Cl) 5-27 µg/litre and the poly-
chloropentacosanes (C25;43% Cl) < 5-6.4 µg/litre and (C25;70%
Cl) < 5-5.9 µg/litre, depending on analytical method (Madeley &
Gillings, 1983). Campbell & McConnell (1980) reported the solubility
of C16;52% Cl to be 10 µg/litre in freshwater and 4 µg/litre in
seawater. The solubility of C25;42% Cl was reported to be 3 µg/litre
in seawater. Chlorinated paraffins are also practically insoluble in
lower alcohols, glycerol and glycols, but are soluble in chlorinated
solvents, aromatic hydrocarbons, ketones, esters, ethers, mineral oil
and some cutting oils. They are moderately soluble in unchlorinated
aliphatic hydrocarbons (Houghton, 1993). Some physical properties of
typical commercial chlorinated paraffins are summarized in Table 4.
Assuming a water solubility of 5 µg/litre and a vapour pressure
of 2 × 10-4 Pa as typical of a 52% chlorinated intermediate chain
length paraffin, a Henry's Law constant of 10.9 may be calculated
(Willis et al., 1994).
A key property of chlorinated paraffins, particularly the high
chlorine grades, is their nonflammability. This is due to the ability
of chlorinated paraffins to release hydrochloric acid at elevated
temperatures, and the hydrochloric acid inhibits the radical reaction
in a flame. This property is considerably enhanced by the addition of
antimony trioxide (Houghton, 1993) or other additives. Chlorinated
paraffins are generally unreactive and stable in normal temperatures,
but decompose significantly at temperatures above 300°C with the
release of hydrochloric acid (Strack, 1986). Prolonged exposure
to light can also cause dehydrochlorination. Degradation by
dehydrochlorination can be accelerated at elevated temperatures in
the presence of aluminium, zinc, and iron oxide or chloride (Howard
et al., 1975; Houghton, 1993). Dehydrochlorination leads to darkening
of the material.
2.3 Analysis
The analysis of chlorinated paraffins is very difficult owing to
the many congeners present in the products. The properties of these
congeners cover wide ranges, which makes it difficult to separate the
chlorinated paraffins from other compounds that may interfere in the
analysis.
Table 4. Physical properties of selected commercial chlorinated paraffinsa
Paraffin Chlorine Colour hazen Viscosityb Densityb Thermal stabilityc Volatilityd Refractive Log Powe
feedstock content (APHA) (Pa.s) (g/ml) (% w/w HCl) (% w/w) index
% (w/w)
C10-C13 50 100 0.08 1.19 0.15 16.0 1.493 4.39-6.93
56 100 0.8 1.30 0.15 7.0 1.508 NRg
60 125 3.5 1.36 0.15 4.4 1.516 4.48-7.38
63 125 11.0 1.41 0.15 3.2 1.522 5.47-7.30
65 150 30.0 1.44 0.20 2.5 1.525 NR
70 200 8.0f 1.50 0.20 0.5 1.537 5.68-8.01h
C14-C17 40 80 0.07 1.10 0.2 4.2 1.488 NR
45 80 0.2 1.16 0.2 2.8 1.498 5.52-8.21
52 100 1.6 1.25 0.2 1.4 1.508 5.47-8.01
58 150 40.0 1.36 0.2 0.7 1.522 NR
Wax C18-C20 47 150 1.7 1.21 0.2 0.8 1.506 NR
50 250 18.0 1.27 0.2 0.7 1.512 NR
Wax (C> 20) 42 250 2.5 1.16 0.2 0.4 1.506 9.29->12.83h
48 300 28.0 1.26 0.2 0.3 1.516 8.69-12.83
70 100i j 1.63 0.2 NR - NR
a Data from Houghton (1993) f At 50°C
b At 25°C unless otherwise noted g NR = not reported
c Measured in a standard test for 4 h at 175°C h Data from Cereclor 42
d Measured in a standard test for 4 h at 180°C i 10 g in 100 ml toluene solvent
e Octanol:water partition coefficients. From: Renberg et al (1980) j Solid, softening point = 95-100°C
2.3.1 Sampling
To prevent contamination by trace amounts of chlorinated
paraffins, samples or their extracts must not be allowed to come into
contact with any plastic (especially PVC) container, stopper, cap
liner or tubing, because these may contain chlorinated paraffins
(Hollies et al., 1979). All solvents should be rigorously tested
before use, and it is recommended that glass distilled solvents are
used. All glassware should be decontaminated before use by heating at
250°C for 24 h. Water and sediment samples should be stored at
ambient temperatures, and should be analysed within a month of
sampling.
Treatment of samples for the extraction of chlorinated paraffins
is described in Table 5.
2.3.2 Analytical methods
Methods used for detection of chlorinated paraffins in various
samples are shown in Table 5.
Hollies et al. (1979) determined C13-17 and C20-30 chlorinated
paraffin after clean-up of the samples on aluminium oxide columns.
The chlorinated paraffin fraction was then applied on a silica gel
thin-layer chromatography (TLC) plate. After forward elution with
n-hexane and subsequently with toluene, and backward elution with
n-hexane, chloride from the chlorinated aliphatics was transferred
to an aluminium oxide plate at 240°C and developed with silver
nitrate. The resulting spots were quantified by visual comparison
with spots of known amounts of reference materials. Although the
procedure is complicated and involves several evaporations to dryness,
good recoveries were reported. Possible interference from a number of
other chlorinated compounds was investigated and found to be
negligible, but the method must still be regarded as fairly
non-specific.
Gas chromatographic analysis of chlorinated paraffin, using
microcoulometric detection, has been described by Zitko (1973). This
method gives badly resolved chromatograms and there is a considerable
risk of interference from other halogenated compounds. Owing to high
temperatures in the gas chromatographic system there is also a risk of
dehydrochlorination of the chlorinated paraffin congeners. In a later
study (Zitko & Arsenault, 1977), interference from other compounds was
avoided by a solvent partitioning clean-up procedure.
Table 5. Analytical methods for the determination of chlorinated paraffins in various samplesa
Sample Preparation method Analytical Sample detection Recovery Reference
matrix methodb limitb
Water Extract with petroleum spirit; concentrate; purify by TLC 500 ng/litre 90% Hollies et
aluminium oxide chromatography, elute with toluene; al. (1979)
dry; dissolve in petroleum spirit
Water Extract with hexane; purify by aluminium oxide GC/ECD 3 ng/litre NR Kraemer &
chromatography; elute with hexane/dichloromethane Ballschmiter
(4%); purify by silica gel chromatography, elute with (1987)
hexane:dichloromethane (19:1); dissolve in isooctane.
Water Extraction with hexane (particle phase Soxhlet GC/ approx. 92-120% Steele et
extracted), silica gel and aluminium oxide column MS-NCI 1 µg/litre al. (1988)
chromatography
Biological Homogenize; extract with petroleum spirit:acetone TLC 50 µg/kg 80-90% Hollies et
material (2:1); dry; dissolve in petroleum spirit; extract with al. (1979)
dimethylformamide; wash; back-extract with Na2SO3
solution and petroleum spirit; purify by silica gel
chromatography, elute with CCl4; dry; dissolve in
acetone; extract with petroleum spirit:acetone (1:4)
Cod muscle Homogenize in n-hexane:acetone (1:2.5, v:v); extract GC/MS NR 98-114% at Jansson et
tissue with 10% diethyl ether in n-hexane; evaporate; dissolve 0.465 µg/sample al. (1991)
in dichloromethane:n-hexane (1:1, v/v); purify by gel and 89-92% at
permeation chromatography; concentrate; extract with 2.33 µg/sample
sulfuric acid; concentrated in organic phase
Adipose Homogenize in dichloromethane; percolate through GC/MS 5 ng 80% Schmid &
tissue anhydrous Na2SO4; remove solvent; dissolve residue in Müller (1985)
pentane; wash, dry and concentrate; purify by alumina
chromatography
Table 5. (Cont'd)
Sample Preparation method Analytical Sample detection Recovery Reference
matrix methodb limitb
Mineral oil Extract fish in cyclohexane; introduce extract or MS-NCI NR NR Gjos &
and fish mineral oil sample directly into mass spectrometer Gustavsen
extract (1982)
Fish fillets Homogenize in petroleum ether; clean-up by irradiating GC/CD NR > 90% Friedman &
extracts with high-intensity UV light (90 min, < 20°C) Lombardo
in petroleum ether (1975)
Sewage Homogenize in acetone; extract with pentane; wash, dry GC/MS 5 ng NR Schmid &
sludge and concentrate; purify by alumina chromatography Müller (1985)
Sediment Dry at 70°C; extract with petroleum spirit; TLC 50 µg/kg 80% Hollies et
concentrate; purify by aluminium oxide al. (1979)
chromatography, elute with toluene; dry; dissolve
in petroleum spirit
Sediment Extract with acetone:hexane (1:1, v:v); wash, dry GC/MS 5 ng NR Schmid &
and concentrate; purify by alumina chromatography Müller (1985)
Sediment Soxhlet extraction with hexane, silica gel and GC/ approx. 52-64% Steele et
aluminium oxide column chromatography MS-NCI 1 µg/litre al. (1988)
a Modified from IARC (1990)
b GC/MS = gas chromatography/mass spectrometry; GC/CD = gas chromatography/coulometric detection;
GC/ECD = gas chromatography/electron capture detection; MS-NCI = negative-ion chemical ionization mass spectrometry;
TLC = thin-layer chromatography; NR = not reported
Attempts have been made to reduce the complexity of chlorinated
paraffin mixtures by reductive dechlorination (Cooke & Roberts, 1980;
Roberts et al., 1981; Sistovaris & Donges, 1987). This method gives
information on the "carbon skeleton" of the chlorinated paraffin
compounds but no information on the chlorine content, and it is
difficult to separate the response from that of unchlorinated
hydrocarbons.
Negative ion chemical ionization mass spectrometry (MS-NCI) was
used by Gjös & Gustavsen (1982). In this method the chlorinated
paraffin fractions are introduced directly into the ion source of the
mass spectrometer. As the whole sample is analysed in a very short
time, the concentration in the ion source is high and the sensitivity
can therefore be high. A serious disadvantage is that all other
compounds in the sample come into the mass spectrometer at the same
time, the risk of interference is high and an extensive clean-up of
the samples is needed.
Gas chromatography utilizing MS-NCI for the detection was used by
Schmid & Müller (1985). A fairly simple clean-up based on adsorption
chromatography on aluminium oxide was used, but unfortunately this
has been impossible to reproduce (Jansson, personal communication).
GC/MS-NCI was also used by Steele et al. (1988) to determine
chlorinated paraffins after clean-up of samples on silica gel and
aluminium oxide columns. They used low inlet temperatures in the
gas chromatograph to avoid thermolysis of the analysed compounds.
The use of low temperatures and short capillary columns further
decreases the risk of temperature-related break-down of chlorinated
paraffins during gas chromatographic analysis (Jansson et al., 1991).
In this method a gel permeation column was also used to avoid
interference from other chlorinated compounds, and the Cl2- and
HCl2- ions were used to detect aliphatic chlorinated compounds
selectively.
Developments in chlorinated paraffin analysis have improved
both selectivity and sensitivity. However, although the reliability
of results is now better, these are only estimates of the real
concentrations as it is impossible to detect the individual substances.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Chlorinated paraffins are not known to occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Liquid chlorinated paraffins were first used in large amounts
during the period 1914-1918 as solvents for Dichloramine T in
antiseptic nasal and throat sprays (Howard et al., 1975). The
commercial production of chlorinated paraffins for use as extreme
pressure additives in lubricants started around 1930 (Schenker, 1979).
Estimated data on the production of chlorinated paraffins are
shown in Table 6. Chlorinated paraffins are produced in Australia,
Brazil, Bulgaria, Canada, China, Germany, France, India, Italy, Japan,
Mexico, Poland, Romania, Spain, Slovakia, South Africa, China
(Province of Taiwan), Thailand, the United Kingdom, the USA, and the
former USSR. However, this may not be a complete list of producer
countries. It is believed that 50% of the chlorinated paraffins
produced in the world have carbon chain lengths of between 14 and 17
and a chlorine content of between 45 and 52%. In the United Kingdom
approximately 80% of the total production of chlorinated paraffins is
concentrated on the C14-17 chain length. About 15% of the European
consumption of chlorinated paraffins is estimated to be C10-13, 70%
C14-17 and 15% C20-30 (Willis et al., 1994).
Chlorinated paraffins are produced by reacting liquid paraffin
fractions obtained from petroleum distillation with pure chlorine gas
by a reaction mechanism involving free radicals (Schenker, 1979;
Houghton, 1993). The reaction is exothermic. At a chlorine content
above approximately 54% further chlorination is slow and difficult.
In the production of resinous chlorinated paraffins containing
> 70% Cl, a solvent is usually added to decrease the viscosity
(Howard et al., 1975). Carbon tetrachloride has been the most
commonly used solvent, and may be present in trace amounts in the
final product, although alternative production methods are being
developed because of the phase-out of carbon tetrachloride under the
Montreal Protocol (D. Farrer, personal communication to the IPCS,
1995).
The substituted chlorine atoms are probably randomly distributed,
and at a chlorination of 72% all of the carbon atoms are singly
chlorinated. Further chlorination is difficult since the first
chlorine substitution decreases the reactivity of the other hydrogens
on a particular carbon atom (Hardie, 1964).
Table 6. Estimated production of chlorinated paraffins
Production References
(tonnes/year)
Canada - 1990 2900 Camford Information
Services (1991)
Germany - 1990/1991 20 000-30 000 BUA (1992)
United Kingdom - 1992 50 000 Willis et al. (1994)
USA - 1977 37 000 Schenker (1979)
USA - 1983 45 000 NTP (1986a)
USA - 1987 45 000 IARC (1990)
USA - 1990 26 000 US EPA (1993)
North America - 1978 60 000 Zitko (1980)
Western Europe - 1978 105 000 Zitko (1980)
Western Europe - 1985 95 000 IARC (1990)
World, excluding 230 000 Campbell &
Eastern Europe - 1977 McConnell (1980)
World - 1985a 300 000 Strack (1986)
a Excluding the former Soviet Union and the People's Republic of China.
Depending on producer and paraffin feedstock, the temperature of
the chlorination reaction is usually kept at 80-100°C to decrease the
viscosity but at a temperature where the decomposition of the product
is not extensive (Schenker, 1979; Houghton, 1993). Since the reaction
is exothermic heat removal is important in the process. Ultraviolet
light is often used as a catalyst (Schenker, 1979; Houghton, 1993).
Metal catalysts are avoided since they may promote dechlorination of
the chlorinated paraffins. Since for each tonne of chlorinated
paraffin produced, approximately half a tonne of hydrochloric acid is
generated, the linings of the reactor vessels must be chemically inert
to avoid the formation of metal chlorides, which cause darkening of
the product by decomposition (Strack, 1986; Houghton, 1993).
Additional procedures used in the production of the C20-30;70% Cl solid
grade are stripping of the solvent and grinding of solid products
(Schenker, 1979).
3.2.2 Uses
Chlorinated paraffins are used as secondary plasticizers for
polyvinyl chloride (PVC) and can partially replace primary
plasticizers such as phthalates and phosphate esters (Houghton, 1993).
The use of chlorinated paraffins has the advantage in comparison with
conventional plasticizers of both increasing the flexibility of
the material as well as increasing its flame retardancy and
low-temperature strength (Howard et al., 1975). Chlorinated paraffins
are also used as extreme pressure additives in metal-machining fluids
or as metal-working lubricants or cutting oils because of their
viscous nature, compatibility with oils, and property of releasing
hydrochloric acid at elevated temperatures. The hydrochloric acid
reacts with metal surfaces to form a thin but strong solid film of
metal chloride lubricant. In Sweden, the use of chlorinated paraffins
in metal-working fluids has been reduced from 680 tonnes (1986) to
139 tonnes (1993) as a part of a risk reduction programme (Swedish
Environmental Protection Agency, 1994). They are added to paints,
coatings and sealants to improve resistance to water and chemicals,
which is most suitable when they are used in marine paints, as
coatings for industrial flooring, vessels and swimming pools (e.g.,
rubber and chlorinated rubber coatings), and as road marking paints.
The flame-retarding properties of highly chlorinated paraffins make
them important as additives in plastics, fabrics, paints and coatings.
The most effective fire-retardant action is obtained with a high
degree of chlorination.
By the late 1970s approximately 50% of chlorinated paraffins in
the USA was used as extreme pressure lubricant additives in the
metal-working industry; 25% was used in plastics and fire-retardant
and water-repellant fabric treatments, and the rest was used in paint,
rubber, caulks and sealants (Schenker, 1979). In the United Kingdom,
65-70% of the consumed chlorinated paraffins is used as a secondary
plasticizer in PVC, about 10% in paint, about 10% in metal-cutting
lubricants and about 10% in flame retardants and sealants (Willis et
al., 1994). In Canada approximately 55% of the chlorinated paraffins
is used as plasticizers and 35-40% as high-pressure lubricant
additives (Camford Information Services, 1991). Some examples of
applications for chlorinated paraffins of different chain-lengths are
shown in Table 7.
3.2.3 Loss into the environment
Since chlorinated paraffins are produced without contact with
water, the possibility of leakage into the environment by direct water
discharge is low. After chlorination the solvent is removed and
residual amounts of chlorine gas and hydrogen chloride are removed by
blowing air or other gases through the product. This could possibly
lead to some loss into the air, but since the chlorine gas and
hydrochloric acid are recovered and the volatility of chlorinated
paraffins is very low, the loss is likely to be very low (Howard et
al., 1975). Emission into the atmosphere during manufacture in
Germany in 1990 was estimated to be about 250 kg/year (BUA, 1992). It
is possible that chlorinated paraffins may be a by-product during
chlorination of other hydrocarbon feedstocks if paraffins are present
as contaminants. This could lead to possible environmental
contamination.
Table 7. Uses of various chlorinated paraffins
Paraffin Chlorination (%) Application
feedstock
C10-13 plasticizer for PVC or plastics
C10-13 metal-working fluids; sealants
C10-13 approx. 70 flame retardants for rubber and soft plastics
C14-17 40-60 extreme pressure additives to metal-machining
fluids, pastes, emulsions and lubricants
C14-17 45-52 the chlorinated paraffin most frequently used as a
(40-50) plasticizer for plastics; also used for sealants
C18-30 approx. 70 flame retardants for rigid plastics such
as polyesters and polystyrene
C> 20 plasticizer for PVC or plastics
Some loss into the environment could be expected during transport
and storage. If the drums which are used for the transport of
chlorinated paraffins are cleaned for further use environmental
release might occur. Soil could be contaminated if empty drums are
dumped at landfills. Spills may occur, but clean-up using an
adsorbent material is easy. The adsorbent material would probably
be deposited in a landfill, which in turn could lead to possible
environmental contamination.
The uses of chlorinated paraffins probably provide the major
source of environmental contamination. When chlorinated paraffins are
used as plasticizers or additives in coatings, they are effectively
dissolved in the polymers and will therefore leak into the environment
only very slowly. However, polymers containing chlorinated paraffins
will act as sources of chlorinated paraffins for centuries after
disposal. A more likely route of leakage of chlorinated paraffins
into the environment would be the improper disposal of oils containing
chlorinated paraffins (Campbell & McConnell, 1980) or disposal of
chlorinated paraffins of low quality (Howard et al., 1975). Loss of
chlorinated paraffins by removal from paints and coatings may also
contribute to environmental contamination.
It is estimated that a maximum of 55% of the cutting and
lubricating oils sold to the engineering industry in Sweden becomes
waste. The rest is consumed or released into the air and water (KEMI,
1991). The largest consumer of chlorinated paraffins in Sweden
(1400 tonnes/year) has estimated its emission of chlorinated paraffins
to be 90 kg/year (0.06 g emission/kg chlorinated paraffin produced)
(KEMI, 1991).
Disposal of wastes containing chlorinated paraffins occurs
through resource recovery, destructive incineration or landfill,
usually on disposal sites for special wastes and in compliance with
local regulations. Owing to their thermal instability, chlorinated
paraffins are expected to be degraded by incineration at low
temperatures and thus would not be expected to volatilize in exhaust
gases from an incinerator. However, in a study by Bergman et al.
(1984), chlorinated aromatic compounds such as PCBs, naphthalenes and
benzenes were formed by pyrolysis of chlorinated paraffins (see
section 4.2.1) although the conditions used were not identical to the
operation conditions of waste incineration plants. Chlorinated
paraffins are not expected to be formed de novo. The disposal of
chlorinated paraffins in landfills may give rise to leaching into
water, but owing to the low water solubility and strong adsorption
onto solids the amounts reaching water are likely to be low.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Considering the low vapour pressure (2 × 10-4 Pa at 20°C for
C14-17;52% Cl to 3 × 10-3 Pa at 65°C for C23;42-54% Cl), low water
solubility (3 to 470 µg/litre) and highly lipophilic nature of
chlorinated paraffins (log Pow values range from 4.39 to > 12.83),
it is likely that they will distribute mainly to the soil/sediment
phase with very little volatilization occurring. Chlorinated
paraffins are likely to be transported in water as suspended
particles, and in air as dust particles and possibly in the vapour
phase. However, no experimental data on this subject have been
reported.
4.2 Transformation
4.2.1 Abiotic transformation
No experimental data on the chemical stability of chlorinated
paraffins under simulated environmental conditions have been reported.
However, their chemical reactivities suggest that they do not
hydrolyse, oxidize or react by other mechanisms at significant rates
under normal temperatures and neutral conditions (Howard et al.,
1975). Dehydrochlorination of chlorinated paraffins may possibly
occur naturally in the presence of metal ion contamination.
Because of the high adsorption tendency of chlorinated paraffins,
gas phase reactions are assumed to contribute only little to
degradation in the atmosphere (BUA, 1992). However, calculated
half-lives for chlorinated paraffins in air are reported to range from
0.85 to 7.2 days (Slooff et al., 1992). The theoretical values are
shown in Table 8.
The thermal degradation by pyrolysis of chlorinated paraffins at
various temperatures (300, 500, 700°C) and times (10 sec to 20 min)
was studied by Bergman et al. (1984). The chlorinated paraffins were
totally degraded, and, depending upon degree of chlorination of the
chlorinated paraffin, several aliphatic and aromatic degradation
products, such as polychlorinated biphenyls (PCBs), naphthalenes and
benzenes, were detected. As much as 10 g of PCBs per kg chlorinated
paraffin could be found after thermal degradation of C12;70% Cl
(temperature not specified). Smaller amounts of compounds were formed
at lower temperatures. Considering these results, processes where
chlorinated paraffins are subjected to temperatures above 300°C could
lead to environmental contamination and exposure to more persistent
and toxic substances than the original chlorinated paraffins.
Table 8. Photochemical degradation of chlorinated paraffins in the
atmosphere (From: Slooff et al., 1992)
Carbon chain length Koh (cm3/mol per sec) Half-life (days)
C10-C13 9.0-14.9 × 10-12 1.2-1.8
C14-C17 14.9-18.9 × 10-12 0.85-0.8
C15-C30 20.2-31.1 × 10-12 0.5-0.8
not specified 2.2-18.8 × 10-12 0.85-7.2
4.2.2 Biodegradation
Chlorinated paraffins are generally stable in the natural
environment.
4.2.2.1 Short chain length chlorinated paraffins
A short chain length paraffin (C10-12) with 58% chlorination
(CP-SH) was not readily biodegraded by activated sludge, under either
aerobic or anaerobic conditions, over a 28-day period in an inherent
biodegradability (modified Zahn-Wellens) test (Street & Madeley,
1983a,b) or a 51-day period in a coupled units test (Mather et al.,
1983).
4.2.2.2 Long chain length chlorinated paraffins
Zitko & Arsenault (1977) studied sediment spiked with 596 mg/kg
dry weight of Cereclor 42 C24;42% Cl, CP-LL) or 357 mg/kg dry weight
of Chlorez 700 (C24;70% Cl, CP-LH), which are both long chain length
chlorinated paraffins but have different chlorine contents. There was
no clear trend in the results but they indicated that after 4 weeks
the highly chlorinated Chlorez 700 was degraded to a greater extent
than Cereclor 42. The rate of degradation seems to have been higher
under anaerobic conditions.
4.2.2.3 Comparative studies
In another study, the microbial degradation of several short,
intermediate and long chain length chlorinated paraffins of different
chlorination degree at concentrations of 2, 10 and 20 mg/litre was
examined in a 25-day biochemical oxygen demand (BOD) test (Madeley &
Birtley, 1980). The degradation rate appeared to decrease with
increasing carbon chain length and chlorination degree, and the short
chain chlorinated paraffins with less than 50% Cl were degraded most
rapidly and completely. It can be concluded from the results that
chlorinated paraffins with low chlorine contents (< 50% wt Cl) and,
especially, short chain chlorinated paraffins, biodegrade slowly in
the environment, particularly in the presence of adapted microbial
populations, but that chlorinated paraffins with higher chlorine
contents are unlikely to biodegrade under aerobic conditions.
Anaerobic microorganisms did not degrade Cereclor 42 (C24;42% Cl) in
30 days when readily biodegradable alternative carbon sources were
available.
Omori et al. (1987) found that bacterial strains isolated from
the soil degraded various chlorinated paraffins by dechlorination in
the presence of n-hexadecane. In a mixed culture of four strains,
more than 50% of the chlorine was removed from the shorter paraffins
with lower chlorine content (C14;43% Cl, CP-ML and C15;50% Cl, CP-MH)
within 36 h. Lower amounts of chlorine were removed from the
chlorinated paraffins with longer chain lengths. Activated sludge
from a sewage treatment plant in Tokyo acclimatized to n-hexadecane
for 60 days showed only a limited amount (2%) of dechlorination of the
chlorinated paraffins. The bacterial dechlorination concerned the
terminal chlorine, which produced 2- or 3-chlorinated fatty acids via
ß-oxidation.
4.3 Bioaccumulation and biomagnification
4.3.1 Summary
Chlorinated paraffins of short chain length accumulate in mussels
and fish to a higher degree than intermediate and long chain length
chlorinated paraffins.
Data on bioaccumulation of chlorinated paraffins by aquatic
organisms are summarized in Table 9. Bioconcentration factors (BCFs)
may be uncertain since the applied doses exceeded the water solubility
in several experiments.
Table 9. Bioconcentration factors for some chlorinated paraffins
Species Chlorinated paraffina Exposure Bioconcentration Reference
Concentration Duration factor
(µg/litre) (days) (whole animal)b
Marine diatom C10-12;58% Cl (CP-SH) 1.4 10 < 1 Thompson & Madeley (1983b)
(Skeletonema costatum) 17.8 10 3.5
Freshwater green alga C10-12;58% Cl (CP-SH) 35 10 1.5 Thompson & Madeley (1983d)
(Selenastrum capricornutum) 140 10 7.6
150 10 4.1
Mussel C10-12;58% Cl (CP-SH) 2.3 147 40 900e Madeley et al. (1983a)
(Mytilus edulis) 10 91 24 800e
C10-12;58% Cl (CP-SH) 13 60 25 292e Madeley & Thompson (1983a)
130 60 12 177e
C12;69% Cl (CP-SH) 0.13 28 138 000e Renberg et al. (1986)
C16;34% Cl (CP-ML) 0.13 28 6920e
C14-17;52% Cl (CP-MH) 220 60 2856e Madeley & Thompson (1983b)
3800c 60 429e
C22-26;43% Cl (CP-LL) 120 60 1158e Madeley & Thompson (1983c)
2180c 60 261e
C22-26;70% Cl (CP-LH) 460 60 341e Madeley & Thompson (1983d)
1330c 60 223e
Table 9. (Cont'd)
Species Chlorinated paraffina Exposure Bioconcentration Reference
Concentration Duration factor
(µg/litre) (days) (whole animal)b
Rainbow trout C10-12;58% Cl (CP-SH) 3.1 168 3550e Madeley & Maddock (1983b)
(Oncorhynchus mykiss) 14 168 5260e
C10-12;58% Cl (CP-SH) 33 60 7155e Madeley & Maddock (1983c)
3050c 60 1173e
C14-17;52% Cl (CP-MH) 1050c 60 45e Madeley & Maddock (1983c)
4500c 60 67e
C22-26;43% Cl (CP-LL) 970 60 18e Madeley & Maddock (1983c)
4000c 60 38e
C20-30;70% Cl (CP-LH) 840 60 54e Madeley & Maddock (1983c)
3800c 60 32e
Bleaks C10-13;49% Cl (CP-SL) 125 14 770d,f Bengtsson et al. (1979)
(Alburnus alburnus) 59% Cl (CP-SH) 125 14 740d,f Bengtsson et al. (1979)
71% Cl (CP-SH) 125 14 140d,f Bengtsson et al. (1979)
C14-17;50% Cl (CP-MH) 125 14 30d,f Bengtsson et al. (1979)
C18-26;49% Cl (CP-LL) 125 14 7d,f Bengtsson et al. (1979)
a The classification of chlorinated paraffins is given in Table 1
b Ratio of the concentration of the chemical in the organism to the concentration of the chemical in the environment or food
c May exceed water solubility
d BCFs calculated by Zitko (1980)
e BCFs based on radioactivity (14C)
f BCFs based on parent compounds
4.3.2 Aquatic vertebrates
4.3.2.1 Short chain length chlorinated paraffins
In a study by Lombardo et al. (1975), fingerling rainbow trout
(Oncorhynchus mykiss) were fed a diet containing 10 mg/kg Chlorowax
500C (C12;60% Cl, CP-SH) for 82 days. Samples of 20 exposed and 10
control fish were collected at approximately 2-week intervals during
the time-period and analysed for chlorinated paraffin content by
microcoulometric gas chromatography (Friedman & Lombardo, 1975). The
tissue level of chlorinated paraffins rose during the treatment period
to 1.1 mg/kg (on tissue basis) or 18 mg/kg (on fat basis) after 82
days (detection level: 0.5 mg/kg). The experiment had to be
terminated owing to the failure of the water supply, and it was not
possible to determine whether a steady-state level had been reached.
In studies (Madeley & Maddock, 1983b) on rainbow trout
(Oncorhynchus mykiss) exposed to measured concentrations of 3.1 and
14.3 µg/litre of 14C-labelled C10-12; 58% Cl (CP-SH) for a period of
168 days, BCF value of 1300 (low dose) and 1600 (high dose) were
observed in the flesh, 2800 (low dose) and 16 000 (high dose) in the
liver, and 11 700 (low dose) and 15 500 (high dose) in the viscera;
all values were determined from radioactivity measurements. The BCF
for the whole body was 3350 (low dose) and 5260 (high dose)
(calculated values). Half-lives for elimination in different organs
were calculated to be the following: liver 9.9 (low dose) and 11.6
days (high dose), viscera 23.1 (low dose) and 23.9 days (high dose),
and flesh 16.5 (low dose) and 17.3 days (high dose).
Rainbow trout (Oncorhynchus mykiss) were exposed to measured
concentrations ranging from 33 to 3050 µg/litre of C10-12;58% Cl
(CP-SH) for 60 days (Madeley & Maddock, 1983c). BCFs, which were
determined in whole fish samples at the end of the test, were 7155
(low dose) and 1173 (high dose) based on radioactivity measurements,
and 7273 (low dose) and 574 (high dose) based on parent compound
analysis. Parent compound analysis was performed using a modification
of the method of Hollies et al. (1979) (see section 2.3.2).
4.3.2.2 Intermediate chain length chlorinated paraffins
After 60 days exposure of rainbow trout (Oncorhynchus mykiss)
to measured concentrations of 1050 and 4500 µg/litre of intermediate
length (C14-17) chlorinated paraffins with 52% Cl (CP-MH), whole body
BCFs of 45 (low dose) and 67 (high dose) based on radioactivity
measurements, and of 32 (low dose) and 42 (high dose) based on parent
compound analysis were determined (Madeley & Maddock, 1983c). The
BCFs were determined at the end of the exposure period. Parent
compound analysis was performed using a modification of the method of
Hollies et al. (1979) (see section 3.2.3).
4.3.2.3 Long chain length chlorinated paraffins
Juvenile Atlantic salmon (Salmo salar) were exposed to Cereclor
42 (C24;42% Cl, CP-LL) or Chlorez 700 (C24;70% Cl, CP-LH) by uptake
from suspended solids or from food (Zitko, 1974a). The fish were
treated with either 1000 µg/litre of contaminated suspended solids for
48 h and 144 h, or to 10 mg/kg or 100 mg/kg of contaminated food for
181 days with a subsequent elimination period of 74 days. No or very
low accumulation of the chlorinated paraffins was observed. However,
the analytical method used determined the amount of chlorine and not
of chlorinated paraffin; this method has been considered as nonspecific
and of low sensitivity.
After 60 days exposure of rainbow trout (Oncorhynchus mykiss)
to long chain chlorinated paraffins with 43% Cl (CP-LL) (970 or
4000 µg/litre) or 70% Cl (CP-LH) (840-3800 µg/litre), whole body BCFs,
based on measured exposure concentrations, of 17.9 (low dose, 43% Cl),
37.6 (high dose, 43% Cl) and 53.8 (low dose, 70% Cl) and 32.5 (high
dose, 70% Cl) were determined at the end of the study when measured as
radioactivity. BCF values of 3.6 (low dose, 43% Cl), 9.0 (high dose,
43% Cl), 42.8 (low dose, 70% Cl) and 31.6 (high dose, 70% Cl), based
on parent compound analysis, were determined (Madeley & Maddock,
1983c).
4.3.3 Aquatic invertebrates
4.3.3.1 Short chain length chlorinated paraffins
After 60 days exposure of mussels (Mytilus edulis) to a short
chain length paraffin with 58% Cl (CP-SH) at measured concentrations
of 13 and 130 µg/litre, whole body BCFs were 25 292 and 12 177,
respectively, based on radioactivity measurements, and 20 000 and
7923 when measured as parent compound (Madeley & Thompson, 1983a).
After exposure of mussels (Mytilus edulis) for 147 days to
14C-labelled short chain length chlorinated paraffin with 58% Cl
(CP-SH) followed by a depuration period of 98 days (measured exposure
dose: 2.3 µg/litre), or for 91 days followed by 84 days of depuration
(measured exposure dose: 10.1 µg/litre), plateau levels of the
chlorinated paraffin in tissues were reached. Bioconcentration
factors (BCFs) at the plateau levels were 40 900 for whole mussel
tissue after exposure to 2.35 µg/litre and 24 800 after exposure to
10.1 µg/litre based on wet tissue basis. Of the different organs the
digestive glands had the highest BCF values of 226 000 (low exposure)
and 104 000 (high exposure). Half-lives for the chlorinated paraffin
in whole mussel tissue were 9.2-9.9 days (10.1 µg/litre) and 13.1-19.8
days (2.35 µg/litre) (Madeley et al., 1983a).
4.3.3.2 Intermediate chain length chlorinated paraffins
After 60-day exposures of mussels (Mytilus edulis) to
intermediate chain length paraffin with 52% Cl (CP-MH) at measured
concentrations of 220 and 3800 µg/litre (which was above the limit
of solubility in water), whole body BCFs were 429-2856 based on
radioactivity measurements and 339-2182 based on parent compound
analysis (Madeley & Thompson, 1983b).
4.3.3.3 Long chain length chlorinated paraffins
In mussels exposed to measured concentrations of 120-2180
µg/litre of long chain length chlorinated paraffin with 43% Cl (CP-LL)
and 460-1330 µg/litre of long chain length chlorinated paraffin with
70% Cl for 60 days, whole body BCFs of 1158-261 (43% Cl) and 341-223
(70% Cl), respectively, were observed when measured as radioactivity,
and 87.2-1000 (43% Cl) and 105-167 (70% Cl) when based on parent
compound analysis (Madeley & Thompson, 1983c,d). However, the high
doses exceeded the water solubility of the chlorinated paraffins.
4.3.3.4 Comparative studies
The accumulation during four weeks of two 14C-labelled
chlorinated paraffins, polychloro[1-14C]hexadecane (C16;34% Cl,
CP-ML) and 1-chloropolychloro[1-14C]dodecane (C12;69% Cl, CP-SH), was
studied in the mussel (Mytilus edulis) by Renberg et al. (1986).
Both compounds showed rapid uptake when added at concentrations of
0.13 µg/litre (C16;34% Cl) and 0.0029 µg/litre or 0.13 µg/litre
(C12;69% Cl) in water for 28 days. Steady-state levels were reached
within 14 days after exposure to 0.13 µg/litre. The authors
calculated the BCF values to be 6920 for C16;34% Cl and 138 000 for
C12;69% Cl, based on fresh weight. The mussels exposed to C12;69% Cl
were studied for an additional 28 days without exposure. The
elimination rate for this chlorinated paraffin was slow, and 33% of
the radioactivity remained in the tissues after 28 days.
4.3.4 Aquatic plants
The BCF after 10 days exposure to short chain chlorinated
paraffin with 58% chlorination (CP-SH) has been estimated to be 3.5
for the diatom Sceletonema costatum (17.8 µg/litre) and 1.5 for the
green alga Selenastrum capricornutum (35 µg/litre) (Thompson &
Madeley, 1983a,b).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Techniques for the analysis of chlorinated paraffin are described
in section 2.3.2. The major problem connected with the analysis of
environmental samples is interference from other compounds. In
earlier studies, when pre-separation techniques were not as well
developed, the concentrations may have been overestimated. Another
problem is that the chlorinated paraffin composition in the
environment may be different from that of the original products,
and the quantitative analysis has to be based on comparisons with
the original products. These difficulties make it clear that
analytical results have to be regarded more as estimates than exact
concentrations.
5.1.1 Air
No information on levels in the atmosphere has been found in the
literature.
5.1.2 Water and sediment
Levels of chlorinated paraffins in water and sediment in the
United Kingdom are summarized in Table 10. Chlorinated paraffins have
been detected in United Kingdom sea water at levels in the range of
0.5-4.0 µg/litre for C10-20 and less than 2.0 µg/litre for C20-30
(Campbell & McConnell, 1980). The levels in sediments from the same
areas were analysed; chlorinated paraffins were detected only in a few
samples at levels up to 500 µg/kg wet weight for C10-20 and 600 µg/kg
for C20-30. The levels of chlorinated paraffins are low in water from
rivers and reservoirs not receiving industrial/domestic effluents
(C10-20, 1 µg/litre or below; C20-30, 2 µg/litre or below) and for
waterways in industrialized regions (C10-20, up to 6.0 µg/litre; C20-30,
0.5 µg/litre or below). The level of C10-20 in the latter regions was
higher than for C20-30. Chlorinated paraffins were not detected in
five drinking-water reservoirs either in the water (detection limit:
0.5 µg/litre) or the sediment (detection limit: 250 µg/kg) (Campbell &
McConnell, 1980). The levels of C10-20 in sediment from non-marine
water remote from industry were in the range up to 1000 µg/kg, except
for a sewage sludge sample from the Liverpool area, which had levels
of 4000-10 000 µg/kg. C20-30 was detected only in one sample at
50 µg/kg. The levels of chlorinated paraffins in sediments close to
industrial plants were found to be higher (C10-20 up to 15 000 µg/kg;
C20-30 up to 3200 µg/kg wet weight). The levels in sediment from these
industrial areas were 1000 times higher than in the overlaying water
columns, indicating the ability of chlorinated paraffins to adsorb on
suspended solids.
Table 10. Levels of chlorinated paraffins in United Kingdom water (µg/litre) and sediment (µg/kg)
(From: Campbell & McConnell, 1980)a
C10-20 C20-30
Range Median No. of samples Range Median No. of samples
level below detection level below detection
limit limit
Sea water
water ND-4.0 0.5 7/15 ND-2.0 ND 13/18
sediment ND-500 ND 14/18 ND-600 ND 15/18
Fresh water remote from industry
water ND-1.0 0.5 7/13 ND-2.0 ND 7/11
sediment ND-1000 ND 4/6 ND-< 250 ND 4/5
Fresh water close to industry
water ND-6.0 1-2 4/25 ND-0.5 ND 8/10
sediment ND-15 000 1800 2/21 ND-3200 50 3/9
a ND = not detected (detection limit in water = 0.5 µg/litre; in sediment = 50 µg/kg)
Levels of chlorinated paraffins in water and sediment in Germany
are summarized in Tables 11 and 12. In 1987, chlorinated paraffins
were detected at concentrations of about 1 µg/litre for C10-13 and
20 µg/litre for C14-18 in the Danube, downstream of the confluence
with the River Lech (BUA, 1992). The corresponding contents in the
sediment were 300 and 1800 µg/kg dry weight, respectively. In 1994,
chlorinated paraffin concentrations in the Danube and River Lech were
in the range of 0.05 to 0.12 µg/litre for C10-C13 and < 0.05 to
0.19 µg/litre for C14-C17. Also in 1994, the chlorinated paraffin
concentrations (C10-C13) in sediment varied from 6-10 µg/kg dry
weight in the lake of Constance to maximum concentrations of 76 µg/kg
dry weight in the River Lech and 83 µg/kg dry weight in the Rhine
(BUA, 1992).
Table 11. Concentrations (µg/litre) of short and intermediate chain length
chlorinated paraffins in surface water in Germany (From: BUA, 1992)a
Location 1987 1994
C10-13 C14-18 C10-13 C14-17
River Lech at Augsburg 0.05 < 0.05
River Lech at Gersthofen 0.50 4.5 0.08 0.09
(upstream from a chlorinated
paraffin production plant)
River Lech at Langweid 0.60 4.0 0.10 0.19
(downstream from a chlorinated
paraffin production plant)
River Lech at Rain 0.12 0.17
River Danube at Marxheim 1.2 4.0 0.06 < 0.06
(downstream from the mouth of
the River Lech)
River Danube at Marxheim (upstream 1.2 20 0.06 0.07
from the mouth of the River Lech)
a The data from 1994 were produced with a more specific method than those from 1987.
The two data sets are therefore difficult to compare.
Table 12. Concentrations (µg/kg dry weight) of short and intermediate chain length
chlorinated paraffins in sediments from Germany (From: BUA, 1992)a
Location 1987 1994
C10-13 C14-18 C10-13 C14-17
Bodensee (middle)
0-5 cm depth 9-10 70
5-12 cm depth 6-9 < 10
River Rhine (141 km) at Rheinfelden 33-38 60
River Rhine (152 km) at Rheinfelden,
upper layer 53-60 140
lower layer 26-32 85
River Rhine, near German-Dutch
border (2 sites) 60-83 145-205
River Main (3 sites) 24-55 160-260
Outer Alster, Hamburg 35-36 370
River Elbe at Hamburg (2 sites) 16-25 130-230
River Lech, upstream from 400 2200 < 5-7 < 10
chlorinated paraffin production
plant
River Lech, downstream from 700 1700 70-76 325
chlorinated paraffin production
plant
Danube downstream from mouth 300 1800
of the River Lech
a The data from 1994 were produced with a more specific method than those from 1987.
The two data sets are therefore difficult to compare.
Chlorinated paraffins were detected in water samples collected
in the Bermuda Islands (Kraemer & Ballschmiter, 1987). Water samples
down to a depth of 1200 m were analysed, and the highest concentration,
about 50 µg/litre, was found in the surface film. Chlorinated paraffins
were not detected in water from the Maldives (detection limit:
3 ng/litre).
In surface sediment from Lake Zürich, 5 µg/kg of a chlorinated
paraffin mixture with a carbon chain length of C14-18 and 52% chlorine
(CP-MH) was measured (Schmid & Müller, 1985). In the same study it
was reported that sewage sludge from a sewage treatment plant in an
urban industrialized region with known contamination of heavy metals
and organochlorine compounds contained chlorinated paraffins at a
concentration of 30 000 µg/kg.
In a field study performed by the US Environmental Protection
Agency (Murray et al., 1988), samples from two watersheds were
analysed by the method of Schmid & Müller (1985). Both were close to
a chlorinated paraffin manufacturing plant (Sugar Creek, Ohio) or
industry using lubricating oils (Tinkers Creek, Ohio). Chlorinated
paraffins were detected in most samples from Sugar Creek in the low
ppb range (< 8 µg/litre) near drainage and downstream from the plant.
Only a few samples upstream from the plant contained detectable
levels of chlorinated paraffins (< 0.3 µg/litre) (detection limit:
0.15 µg/litre). Of the three chlorinated paraffin fractions studied, the
fraction containing the long carbon chain length C20-30; 40-50% Cl
(CP-LL) was generally present at highest concentration. Sediments
contained higher concentrations. As much as 170 000 µg/kg dry weight
of C20-30 was detected in sediment in an impoundment lagoon, whereas
50 000 µg/kg of C14-17;50-60% Cl (CP-MH) and 40 000 µg/kg C10-12;
50-60% Cl (CP-SH) were measured in the same sample. In mussels
(family Unionidae) collected downstream from the plant, there were
detectable levels of chlorinated paraffins (280 µg/kg C10-12, 170 µg/kg
C14-17, 180 µg/kg C20-30). The report concluded that the observed
levels were most likely due to the manufacturing plant. The samples
collected in Tinkers Creek did not contain detectable amounts of
chlorinated paraffins. Most of the samples contained organic
compounds, largely halogenated aromatics, at levels high enough to
interfere and mask the presence of chlorinated paraffins. Chlorinated
paraffins were detected in samples collected from the process wastes
stream inside the chlorinated paraffin plant. The levels in these
samples were: C10-12, 8.1 µg/kg; C14-17, 1.3 µg/kg; C20-30, 2.2 µg/kg.
In 1979, 51 water samples and 51 bottom sediment samples were
collected at 17 sites in Japan and analysed for the presence of
chlorinated paraffins (C8-32). Chlorinated paraffins were not
detected in any of the water samples, while 24 bottom sediment samples
from 11 sites contained chlorinated paraffins at concentrations of 600
to 10 000 µg/kg (wet or dry weight not specified) (Environment Agency,
Japan, 1981). In 1980, 120 water samples and 120 bottom sediment
samples were collected at 40 sites in Japan and were analysed for the
presence of chlorinated paraffins (C8-32). Chlorinated paraffins
were not detected in any of the water samples but 31 bottom sediment
samples from 13 sites contained chlorinated paraffins at concentrations
of 500 to 8500 µg/kg. However, the analytical methods were not
specified and the detection limits were high (the detection limit was
10 µg/litre for water and 500 µg/kg for bottom sediment) (Environment
Agency, Japan, 1983).
5.1.3 Soil
No data on the occurrence of chlorinated paraffins in soil have
been reported.
5.1.4 Aquatic and terrestrial organisms
The levels of chlorinated paraffins in organisms from various
ecosystems in Sweden, determined using the method of Jansson et al.
(1991), are shown in Table 13. Chlorinated paraffins were found in
all samples in the range of 130-4400 ng/g lipid (6.6-210 µg/kg tissue)
(Jansson et al., 1993). The terrestrial animals rabbit and moose had
higher chlorinated paraffin concentration in their fat than any of the
aquatic animals. The chlorinated paraffin levels in fish-eaters were
approximately the same as in the fish, compared to the dioxin and PCB
levels, which were several times higher in fish-eaters. The levels
in seal and herring indicated no or only low biomagnification of
chlorinated paraffins in their food chains.
In a study by Campbell & McConnell (1980), chlorinated paraffins
were detected in mussels, fish, seals and seabirds, as well as in
seabird eggs, using the analytical method of Hollies et al. (1979)
(Table 14). The levels of C10-20 were higher than those of C20-30 in
most organisms. Mussels collected close to a chlorinated paraffin
plant effluent discharge had levels of C10-20 up to 12 000 µg/kg. The
measured levels in the organisms were close to the levels in the
sediment near the organisms, but 100 to 1000 times higher than those
of water, thus indicating bioaccumulation. The authors also detected
trace amounts of chlorinated paraffins (C10-20) in the tissues of sheep
grazing near a plant producing chlorinated paraffins. The tissues
containing the highest levels were liver (200 µg/kg), fat and kidney
(50 µg/kg). The fleeces of the sheep contained higher levels
(350 µg/kg), and the authors suggest that this might have been due to
aerial transport. No chlorinated paraffin could be detected in sheep
grazing far from plants producing chlorinated paraffins (detection
limit = 50 µg/kg).
Table 13. The levels of chlorinated paraffins in various terrestrial and aquatic
organisms in Swedena
Organism Samplesc Tissue Extracted CP (µg/kg CP (µg/kg
lipid (%) lipid) tissue)d
Rabbit 15 muscle 1.1 2900 31.9
Moose 13 muscle 2.0 4400 88
Reindeer 31 fat 56 140 78
Whitefish 35 muscle 0.66 1000 6.6
Arctic char 15 muscle 5.3 570 30.2
Herring (Bothnian Sea) 100 muscle 5.4 1400 75.6
Herring (Baltic Proper) 60 muscle 4.4 1500 66
Herring (Skagerrack) 100 muscle 3.2 1600 51.2
Ringed sealb 7 blubber 88 130 114.4
Grey seal 8 blubber 74 280 207
Osprey 35 muscle 4.0 530 21.2
a Data from Jansson et al. (1993)
b The ringed seal was from Kongsfjorden, Svalbard, Sweden
c Pooled samples
d Calculated from the reported data
In a study by Campbell & McConnell (1980), chlorinated paraffins
were detected in mussels, fish, seals and seabirds, as well as in
seabird eggs, using the analytical method of Hollies et al. (1979)
(Table 14). The levels of C10-20 were higher than those of C20-30 in
most organisms. Mussels collected close to a chlorinated paraffin
plant effluent discharge had levels of C10-20 up to 12 000 µg/kg. The
measured levels in the organisms were close to the levels in the
sediment near the organisms, but 100 to 1000 times higher than those
of water, thus indicating bioaccumulation. The authors also detected
trace amounts of chlorinated paraffins (C10-20) in the tissues of sheep
grazing near a plant producing chlorinated paraffins. The tissues
containing the highest levels were liver (200 µg/kg), fat and kidney
(50 µg/kg). The fleeces of the sheep contained higher levels
(350 µg/kg), and the authors suggest that this might have been due to
aerial transport. No chlorinated paraffin could be detected in sheep
grazing far from plants producing chlorinated paraffins (detection
limit = 50 µg/kg).
Table 14. Chlorinated paraffins in organisms in the United Kingdom
(Campbell & McConnell, 1980)
Species Tissuea No. of Carbon chain-length and
specimens concentration in tissues
analysedb (means and ranges)c
C10-20 (µg/kg) C20-30 (µg/kg)
mean range mean range
Aquatic organisms
Plaice NS 6 30 ND-200 30 ND-200
(Pleuronectes platessa)
Pouting NS 4 100 ND-200 ND ND
(Trisopterus luscus)
Mussel NS 9 3250 100-12 000 10 NDe-100
(Mytilus edulis)
Pike NS 2 25 ND-50 25 ND-50
(Esox lucius)
Grey seal Liver 4 75 40-100 ND ND
(Halichoerus grypus) and
blubber
Table 14. Cont'd
Species Tissuea No. of Carbon chain-length and
specimens concentration in tissues
analysedb (means and ranges)c
C10-20 (µg/kg) C20-30 (µg/kg)
mean range mean range
Birds
Heron Liver NR NR 500-1200 NR NDe
(Ardea cinerea) Liver NR NR 100-1000 NR 100-1500
Guillemot Liver NR NR 100-1100 NR NDe
(Uria aalge)
Herring gull Liver NR NR 200-900 NR 100-500
(Larus argentatus)
Seabird eggsd 23 220 ND-2000 20 ND-100
a NS = Not specified
b NR = Not reported
c ND = Not detected (detection limit = 50 µg/kg, except where stated otherwise)
d 9 species
e Detection limit = 100 µg/kg
In 1980, 108 fish samples were collected at 28 places in Japan
and were analysed for the presence of chlorinated paraffins.
Chlorinated paraffins were not detected in any of the samples. The
detection limit was 500 µg/kg (Environment Agency, Japan, 1983).
5.1.5 Food and beverages
Chlorinated paraffins, mostly C10-20, were detected in various
food products in a limited study (Table 15) using the analytical
method of Hollies et al. (1979). C20-30 chlorinated paraffins were
detected only in a few samples. C10-20 chlorinated paraffins were
found at levels up to 500 µg/kg in approximately 70% of the samples
(Campbell & McConnell, 1980).
Chlorinated paraffin residues have been detected in fish and
sheep (see section 5.1.4).
Table 15. Chlorinated paraffins (C10-20) in human foodstuffs
(Campbell & McConnell, 1980)
Foodstuff class No. of foodstuff Concentration
samples tested (µg/kg)
Dairy products 13 300
Vegetable oils and derivatives 6 150
Fruit and vegetables 16 25
Beverages 6 NDa
a Not detected (detection limit = 50 µg/kg)
5.2 General population exposure
Chlorinated paraffins have been detected on human hands (Campbell
& McConnell, 1980). The hands of eight human volunteers were swabbed
with toluene, and all had chlorinated paraffin levels of 0.8-4.0 µg
C10-20 (per two hands). C20-30 chlorinated paraffins (2 µg)
were detected only on one person. The authors suggested that the
chlorinated paraffin content in foodstuffs, as well as direct transfer
from manufactured articles via the hands, could contribute to human
exposure.
Owing to the high octanol-water partition coefficient, it is
likely that the principle source of exposure of the general population
to chlorinated paraffins is food. However, due to lack of data,
exposure to chlorinated paraffins via other routes cannot be ruled
out.
5.2.1 Concentrations in human tissues
Chlorinated paraffins have been detected in postmortem tissue
samples. Campbell & McConnell (1980) measured the amount in 24 tissue
samples and found up to 600 µg/kg of C10-20 in adipose tissue (median
level: 100-190 µg/kg), up to 500 µg/kg in kidney (median level below
90 µg/kg) and up to 1500 µg/kg in liver (median level below 90 µg/kg).
C20-30 chlorinated paraffins were detected only in a few samples and
at a low level. Schmid & Müller (1985) also detected chlorinated
paraffins in adipose tissue at a concentration of 200 µg/kg.
5.3 Occupational exposure
Occupational exposure to chlorinated paraffins is likely among
workers in production plants or in industries using chlorinated
paraffins. The US National Occupational Exposure Survey (1981-1983)
indicated that 573 000 workers, including 38 000 women, were
potentially exposed to chlorinated paraffins (NIOSH, 1990). In
Denmark, it was reported that 60 000 workers in 175 companies have
been potentially exposed to chlorinated paraffins since 1964 (the
amount produced and imported annually is 5000 tonnes) (Hansen et al.,
1992).
Campbell & McConnell (1980) detected chlorinated paraffins on
human hands using the method of Hollies et al. (1979) (see section
2.3.2). When the hands of a worker in a chlorinated paraffin
laboratory were swabbed with toluene, 800 µg of C10-20 and 400 µg
of C20-30 were detected.
Historical exposure data from machine shops, reported as
reflecting worst-case situations, indicated exposures to chlorinated
paraffins of 0.003-1.15 mg/m3 for operation such as milling, cutting
and grinding (HSE, 1992). Other exposure data suggested exposures to
chlorinated paraffins ranging from 0.003 to 0.21 mg/m3. However,
it is not clear if these levels of exposure were intermittent or
time-weighted averages. There is no information on whether or not
chlorinated paraffin aerosols are in the inhalable size range.
Occupational exposure to short chain chlorinated paraffins has
been estimated by the United Kingdom Health and Safety Executive by
mathematical modelling using the "Estimation and Assessment of
Substance Exposure" (EASE), developed as part of the guidance on new
and existing substances by the Commission of the European Union (CEU)
(HSE, 1992). This model takes into account physico-chemical data such
as vapour pressure and process details such as temperatures and the
use of local exhaust ventilation. It does not take into account the
attenuating effect of decontamination of equipment or use of personal
protective equipment.
During the production of short chain chlorinated paraffins in
closed systems, exposure is likely to be intermittent, and the model
predicts that inhalation exposure to a substrate with a vapour
pressure of less than 0.001 kPa is negligible (0 to 0.1 ppm).
Assuming a non-dispersive pattern of use and intermittent skin
contact, the model predicts that exposures of the hand and forearm
will be in the range of 0.1-1 mg/cm2 per day. During formulation of
short chain chlorinated paraffins (in closed systems) the model
predicts negligible (0-0.1 ppm) inhalation exposure with formulation
process temperatures of 40-50°C. Skin exposure to the hands and
forearms during formulation is predicted to be 0.1-1 mg/cm2 per day.
Occupational exposure to short chain chlorinated paraffins during
their use as metal-working fluids is extensive and the model predicts
exposure of 0.1-1.5 mg/cm2 per day, assuming a chlorinated paraffin
content in the fluid of 2-10%, or 5-15 mg/cm2 per day for speciality
fluids which may contain more than 80% chlorinated paraffin.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
There is a lack of systematic investigation of the influence of
carbon chain length and degree of chlorination in studies on the
kinetics of chlorinated paraffins. Studies have almost exclusively
concerned short and intermediate chain length chlorinated paraffins.
6.1 Absorption
6.1.1 Oral exposure
Chlorinated paraffins are absorbed after oral administration.
From the data presented in section 6.5 it appears that short chain
length compounds are more readily absorbed than longer chain length
compounds. Absorption decreases with increasing carbon chain length
and degree of chlorination.
6.1.2 Dermal exposure
Chlorinated paraffins are slowly absorbed by the dermal route
in Sprague-Dawley rats (Yang et al., 1987). Two 14C-labelled
chlorinated paraffins, C18;50-53% Cl (CP-LH) and C28;47% Cl (CP-LL),
were applied to rat skin (5-7 animals of each sex) at a concentration
of 66 mg/cm2, approximately equivalent to 2000 mg/kg body weight.
Only 0.7% (males) and 0.6% (females) of the C18 dose was absorbed
after 96 h. Only 0.02% of the C28 dose was absorbed in males whereas
in females the level was not detectable. This indicates that
increasing chain length leads to decreased permeability. Of the
absorbed C18 dose, 40% was exhaled as 14C-labelled CO2, and 20% was
excreted in urine and 20% in faeces.
The absorption of two chlorinated paraffins through human skin
has been studied in vitro (Scott, 1989). There was no absorption of
Cereclor S52 (C14-19;52% Cl, CP-MH) following a 54-h application to
the surface of the epidermal membranes using five different receptor
media. Similarly, using Cereclor 56L (C10-13; 56% Cl, CP-SH; 18.5% w/w
solution in a typical cutting oil) no absorption was detected for 7 h,
but after 23 h a slow but steady rate of absorption was detected
(e.g., 0.05 ± 0.01 µg/cm2 per h ± SEM; n = 6; receptor medium PEG-20
oleyl ether in saline), which was maintained for the duration of the
experiment (56 h). Owing to the anticipated low rate of absorption,
the chlorinated paraffin samples were spiked with [14C] n-pentadecane
and [14C] n-undecane for Cereclor S52 and 56L, respectively, in
order to facilitate detection of the absorbed material. Measurement of
the 14C-alkanes was taken as a surrogate for the chlorinated
paraffins, on the assumption that their rates of absorption were
similar.
6.1.3 Inhalation exposure
No data on retention of chlorinated paraffins by inhalation have
been reported.
6.2 Distribution
6.2.1 Short chain length chlorinated paraffins
6.2.1.1 Mouse
Female C57Bl mice were administered 12.5 MBq/kg body weight
(340 µCi) (for autoradiography) or 1.25 MBq/kg body weight (34 µCi)
(for determination of radioactivity) of 14C-labelled chlorododecanes
(C12) with different chlorine contents (17.5% [CP-SL], 55.9% [CP-SH]
and 68.5% [CP-SH]) either by gavage or intravenous injection (Darnerud
et al., 1982). Uptake of radioactivity was found by autoradiography to
be highest in tissues with high cell turnover/high metabolic activity,
e.g., intestinal mucosa, bone marrow, salivary glands, thymus and
liver. The highest radioactivity was achieved with the chlorinated
paraffin that had the lowest chlorine content. It was found that the
long period of retention of heptane-soluble radioactivity, which
indicated unmetabolized substance, in liver and fat after oral dosing
increased with degree of chlorination. In this study it was also
found that 30 to 60 days after injection of C12;17.5% Cl and
C12;55.9% Cl a considerable retention of radioactivity was seen in
the central nervous system. Exposure of late gestation mice showed a
transplacental passage of radioactivity, and 14C-labelling was
primarily noted in the liver, brown fat and intestine of the fetuses.
6.2.1.2 Rat
Radioactivity was found in the liver, kidneys, adipose tissue and
ovaries of Fischer-344 rats following administration of an unspecified
single dose by gavage of 14C-labelled chlorinated paraffin (C10-13;58%
chlorination, CP-SH) at the end of a 90-day dosing by gavage with the
same chlorinated paraffin (IRDC, 1984c).
6.2.2 Intermediate chain length chlorinated paraffins
6.2.2.1 Rat
Radioactivity was found initially in the liver and kidneys and
later in adipose tissue and the ovaries of Fischer-344 rats following
a single dose by gavage of 14C-labelled C14-17;52% Cl (CP-MH) at the
end of a 90-day dosing in the diet with the same chlorinated paraffin
(IRDC, 1984b).
In male Wistar rats fed with a diet containing 0.4 or 40 mg/kg
[36Cl]Cereclor S52 (C14-17;52% Cl, CP-MH) for 8 (40 mg/kg) or 10 weeks
(0.4 mg/kg), equilibrium levels of radioactivity were established in
liver and abdominal fat within 1 and 7 weeks, respectively (Birtley et
al., 1980). The equilibrium concentrations were 7000 µg/kg in liver
and 30 000-40 000 µg/kg in fat after high exposure. No radioactivity
was detected in the brain or adrenal glands.
6.2.2.2 Mouse
The distribution of orally administered 14C-labelled
polychlorohexadecane (C16;69% Cl, CP-MH) in female C57Bl mice was
examined by whole body autoradiography (Biessmann et al., 1983).
A high level of radioactivity was observed in the liver, brown
fat, intestine, gall bladder, adrenal cortex and kidney of mice
administered approximately 15 µmol/kg. A high uptake of radioactivity
was also observed in corpora lutea on days 1-4, and was still present
30 days after administration. A high level of radioactivity was also
observed in the adrenal cortex at shorter post-injection times, and in
brown and white fat and in the liver, which were still labelled after
30 days.
14C-Labelled [1-14C]polychlorohexadecane (C16;34.1% Cl, CP-ML)
was given to C57Bl mice either by gavage (females) or intravenously
(both sexes) at a radioactivity level of 370 kBq/animal (10 µCi)
(corresponding to 0.44 µmol of the chlorinated paraffin) (Darnerud &
Brandt, 1982). No difference in the distribution patterns was found
between the oral and intravenous administration routes. After
analysis by autoradiography a high level of radioactivity was found in
tissues with a high cell turnover rate and/or high metabolic activity,
and lower levels could be seen in the white fat depots. High levels
of radioactivity were observed in the liver, kidneys, spleen, bone
marrow, brown fat, intestinal mucosa, pancreas, salivary gland and the
Harderian gland 24 h after intravenous injection. After 12 days high
levels of radioactivity were seen in the adrenal cortex, abdominal
fat and in the bile. Later after injection (30 days), prominent
radiolabelling of the brain was found which was as high as in the
liver. The chlorinated paraffin was also administered intravenously
to pregnant mice, and uptake of radioactivity in the fetuses was
observed. When the mice were administered on day 10 of pregnancy no
tissue-specific localization was found, but after administration in
late pregnancy (day 17) the distribution pattern after 6 h was similar
to that of adult mice when examined 24 h after administration.
The distribution of radioactivity in the brain and liver has been
studied after gavage administration of 14C-labelled polychloro-
hexadecane (C16;69% Cl, CP-MH) (1.48 MBq/kg body weight or 40 µCi,
corresponding to 1.1 mg/kg body weight) to pre-weaning NMRI mice
(Eriksson & Darnerud, 1985). The chlorinated paraffin was
administered by gavage at the age of 3, 10 and 20 days, and the
animals were killed 24 h and 7 days later. The radioactivity in the
brain declined more rapidly in the 3-day-old mice compared to the
10- and 20-day-old mice. In the 10-day-old mice approximately 0.02% of
the total administered dose was detected in the brain 24 h after
administration. Approximately 80% of the radioactivity in the brain
was still present after 7 days. In the liver the radioactivity
disappeared more rapidly both in younger and older animals. The
radioactivity in the brain was found primarily in the white matter
of the cerebellum, in the space between the neocortex and the
mesencephalon and thalamus, the corpus callosum, the pons and the
outer part of medulla spinalis. The radioactivity was higher in the
parts of the brain which also stained for myelin, and the levels at
7 days were almost the same as those at 24 h. After whole body
autoradiography, high levels of radioactivity were found in the liver,
intestinal contents, adipose tissue and adrenals. A differential
labelling of the liver was observed in the 3-day-old mice, where only
certain parts of the liver lobules were labelled.
6.2.2.3 Bird
The distribution of 14C-labelled polychlorohexadecane (C16;69%
Cl, CP-MH) in female Japanese quail (Coturnix coturnix japonica) was
examined by whole body autoradiography (Biessmann et al., 1983). Four
hours after a single dose by gavage of approximately 4.8 µmol/kg to
quail, high levels of radioactivity were observed in the liver,
intestine, gall bladder, egg yolk, kidney, ovary, blood, hypophysis
and retina. Twelve days after administration, radioactivity was
observed only in the uropygial gland, white fat, liver and egg yolk.
A study of the distribution after oral administration by gavage
of 0.74 MBq (20 µCi) (approximately 20 Ci/mol) of either 14C-labelled
polychlorohexadecane (C16;34% Cl, CP-ML) or (1-14C)-labelled
polychlorododecane (C12;56% Cl, CP-SH) in female Japanese quail
(Coturnix coturnix japonica) was performed by Biessmann et al.
(1982). The distribution patterns for the two chlorinated paraffins
were similar, and high levels of radioactivity were initially (up to
1 day) found in the liver, intestinal mucosa, spleen, bone marrow,
oviduct, gall bladder and kidney. After 4 and 12 days, high
radiolabelling was observed in fat, the yolk of the follicles and
the contents of the uropygial glands.
The uptake of Cereclor S52 (C14-17;52% Cl, CP-MH) in mallard ducks
(Anas platyrynchos) or ring-necked pheasants (Phasianus colchicus)
was studied by Madeley & Birtley (1980). After a single oral dose of
Cereclor S52 (10 g/kg) in duck, the highest levels of chlorinated
paraffins were detected in fat (67 mg/kg wet weight), gut (15 mg/kg)
and heart (7 mg/kg). The pheasants were exposed to 1000 mg/kg in the
diet for 5 days. Only low levels were found in the heart (3.1 mg/kg)
and gut (1.4 mg/kg). No visible fat was available in the pheasants
due to immaturity. The levels in other organs in both species were
low. The method of analysis was thin layer chromatography (Hollies
et al., 1979).
6.2.2.4 Fish
The distribution of polychloro-[1-14C]hexadecane (C16;34% Cl,
CP-ML) has been studied in carp (Cyprinus carpio) and bleak
(Alburnus alburnus) (Darnerud et al., 1983). After a single
intra-arterial injection of 60-80 µg in carp, about 6% of the dose was
excreted as 14CO2 in 96 h. Radioactivity was observed, in the
intra-arterially injected carps or in bleak exposed to contaminated
water (125 µg/litre) for 14 days, in the bile, intestine, kidney,
liver, gills and, particularly in bleak, in the nasal cavity, lens and
skin.
6.2.3 Long chain length chlorinated paraffins
6.2.3.1 Rat
After oral administration of 14C-labelled C22-26;70% Cl (CP-LH)
to Fischer-344 rats at the end of a 90-day exposure period, a small
part of the dose was absorbed (Serrone et al., 1987). The highest
level of radioactivity was found in the liver. Retention of
radioactivity in adipose tissue, which was eliminated slowly, was also
observed. In an identical study, C20-30;43% Cl (CP-LL) gave the
highest levels in the liver and ovary (Serrone et al., 1987).
6.2.3.2 Fish
Rainbow trout (Oncorhynchus mykiss) were fed diets containing 47 or
385 mg/kg (dry weight) of Cereclor 42 (C20-30;Cl 42%, CP-LL) containing
a 14C-labelled pentacosane (C25) with 42% Cl for 35 days and
a control diet for the following 49 days (Madeley & Birtley, 1980).
The chlorinated paraffin accumulated in the fish during the exposure
period, mostly in the liver and gut. The radioactivity decreased
during the elimination period, more rapidly in the gut and liver
than in the flesh, but was still detectable at the end of the
experiment. When the chlorinated paraffin was determined by thin-layer
chromatography a lower level was noted as compared to determination
of 14C-labelled molecules, suggesting metabolism in the fish. Up to
70% of the assimilated 14C in tissues was not associated with
chlorinated paraffin after feeding for 13 days.
6.2.3.3 Mussel
Mussels (Mytilus edulis) were fed suspended yeast cells dosed
with 524 mg/kg (dry weight) Cereclor 42 (C20-30;42% Cl, CP-LL)
containing a 14C-labelled pentacosane (C25) with 42% chlorination for
47 days, and were then fed with untreated yeast for a further 56 days
(Madeley & Birtley, 1980). The uptake reached a plateau level after
26 days of exposure, and the tissue concentration was always below
11 mg/kg. The highest level of chlorinated paraffin was detected in
the digestive glands. The chlorinated paraffin was eliminated rapidly,
and less than 10% remained at the end of the experiment. There was no
evidence of metabolism since the expelled radioactivity was in the
form of the parent compound.
6.2.4 Comparative studies
The uptake of three 14C-labelled chlorinated paraffins, C16;23%
Cl (CP-ML), C16;51% Cl (CP-MH) and C12;68% Cl (CP-SH), was studied in
rainbow trout (Oncorhynchus mykiss) by Darnerud et al. (1989). After
exposure to 3.64 µmol (C16) or 1.74 µmol (C12) in water for 7
days and to uncontaminated water for up to 21 days, radiolabelling was
observed in the bile, eye lens, brain and fat for C16;23% Cl, in the
bile, intestine, fat and liver for C16;51% Cl, and in fat, intestine,
liver and bile for C12;68% Cl. The three different chlorinated
paraffins showed an initially high uptake in the olfactory organs and
gills. The retention of radioactivity in the olfactory organs and
gills was relatively higher for C16;23% Cl than for the more highly
chlorinated paraffins. The long-time retention of radioactivity in
fat-rich tissues increased with the degree of chlorination of the
chlorinated paraffin preparation.
The short-term uptake and elimination of chlorinated paraffins in
bleak (Alburnus alburnus) were studied by Bengtsson et al. (1979).
Groups of bleak (15 in each) were exposed for 14 days to five
different Witaclor mixtures (Table 16), which were added to natural
Baltic Sea water (salinity 0.7%) giving a concentration of 125
µg/litre of water. Five fish were analysed to determine the chlorine
content (Lunde & Steinnes, 1975) at the end of exposure, and the rest
were analysed 1 and 7 days after treatment. It was found that the
uptake was more effective for those Witaclor mixtures with short
carbon chain length and low degree of chlorination, i.e. Witaclor 149
and 159. The elimination rates were slow, and 75 to 90% was detected
7 days after exposure.
In an extended study, bleak were exposed to Witaclor 149
(C10-13;49% Cl, CP-SL), Witaclor 171P (C10-13;71% Cl, CP-SH) and
Witaclor 549 (C18-26;49% Cl, CP-LL) via contaminated food for 91 days.
This was followed by an elimination period of 316 days (Bengtsson &
Baumann Ofstad, 1982). The concentrations of chlorinated paraffins in
the food were 590, 2500 and 5800 mg/kg of C10-13;49% Cl, 3180 mg/kg of
C10-13;71% Cl and 3400 mg/kg of C18-26;49% Cl. Three fish from
each exposure group were analysed at different time-points during
accumulation or elimination periods. The most effective uptake was
observed for C10-13;49% Cl, whereas the slowest uptake was detected for
C18-26;49% Cl. Efficiencies of uptake were 12% for C10-13;49% Cl, 6%
for C10-13;71% Cl and 2% for C18-26;49% Cl. The highest retention was
observed for C10-13;71% Cl, which remained in the tissue at a
steady-state level during the whole elimination period. The uptake of
C18-26;49% Cl was inefficient, and during the elimination period 50%
was lost within 4 to 5 weeks. The remaining amount appeared to be
constant for the rest of the experiment. The rate of elimination was
slowest for the Witaclor mixture with the shortest carbon chain length
and highest degree of chlorination. After 625 days of depuration the
fish still had detectable levels of C10-13;71% Cl, as determined by the
analytical method of Gjos & Gustavsen (1982) (Renberg et al., 1986).
Table 16. Structures of the chlorinated paraffins used in a study on
bleak (Alburnus alburnus) (From: Bengtsson et al., 1979)
Tested Carbon Chlorine Acronyma Accumulation Half-life
formulation chain content coefficientb (days)b
length (%)
Witaclor 149 C10-C13 49 CP-SL 770 13
Witaclor 159 C10-C13 59 CP-SH 740 34
Witaclor 171P C10-C13 71 CP-SH 140 7
Witaclor 350 C14-C17 50 CP-MH 40 30
Witaclor 549 C18-C26 49 CP-LL 10 7
a The classification is given in Table 1
b Calculated by Zitko (1980)
6.3 Metabolic transformation
6.3.1 Short chain length chlorinated paraffins
Darnerud (1984) demonstrated that inducers and inhibitors of
cytochrome P-450 (CYP) affect the rate of degradation of 14C-labelled
polychlorinated dodecanes (C12) containing 68.5% (CP-SH), 55.9%
(CP-SH) and 17.4% Cl (CP-SL) to 14CO2 in exposed C57Bl mice.
Pretreatment with the inhibitor piperonyl butoxide decreased the
amount of 14CO2 formed, and the decrease was more pronounced with
increasing degree of chlorination. The inhibitor metyrapone decreased
the exhalation of 14CO2 but was only investigated in mice exposed
to C12;68.5% Cl. The cytochrome P-450 (CYP2B1; CYP2B2) inducer,
phenobarbital, moderately increased the rate of 14CO2 formation from
chlorinated paraffin with 68% Cl, whereas the P-448 (CYP1A1; CYP1A2)
inducer, 3-methylcholanthrene, did not affect the degradation rate,
indicating a cytochrome P-450-dependent metabolism of chlorinated
dodecanes yielding 14CO2.
6.3.2 Intermediate chain length chlorinated paraffins
Female Sprague-Dawley rats in groups of four were exposed
intravenously to 5-6 mg/kg body weight of 14C-labelled poly-
chlorinated hexadecane (C16;65% Cl, CP-MH) (Åhlman et al., 1986).
Less than 3% of the radioactivity in the bile was due to unchanged
parent compound. The metabolites in the bile appeared to be conjugates
of N-acetylcysteine (mercapturic acid) and glutathione.
6.4 Elimination and excretion
6.4.1 Short chain length chlorinated paraffins
The exhalation of 14CO2 was compared after single gavage or
intravenous administration to female C57Bl mice of 1.25 MBq/kg body
weight (34 µCi) of three chlorododecanes (C12) with different
chlorine contents (17.5% [CP-SL], 55.9% [CP-SH] and 68.5% [CP-SH])
(Darnerud et al., 1982). Of the administered radioactive dose 52% of
C12;17.5% Cl, 32% of C12;56% Cl and 8% of C12;68% Cl were exhaled as
14CO2 within 12 h after dosing by either route. The major excretion
route for C12;56% Cl was by urine (intravenous: 21%; oral: 29%) and
for C12;68% Cl was by faeces (intravenous: 8.6%; oral: 21%). The
total elimination decreased as the chlorine content increased.
6.4.2 Intermediate chain length chlorinated paraffins
6.4.2.1 Rat
The half-time for removal of radioactivity from abdominal fat was
estimated during and after dietary administration for 8 or 10 weeks of
0.4 and 40 mg/kg feed of [36Cl]Cereclor S52 (C14-17;52% Cl, CP-MH) in
male Wistar rats (Birtley et al., 1980). Equilibrium in liver and
abdominal fat was reached at 1 and 7 weeks, respectively. The
half-time for removal was about 8 weeks for abdominal fat, and it was
observed that the level of radioactivity in the liver declined below
the detection limit within one week.
Female Sprague-Dawley rats in groups of four were exposed
intravenously to 5-6 mg/kg body weight of 14C-labelled polychlorinated
hexadecane (C16;65% Cl, CP-MH) (Åhlman et al., 1986). Approximately
10% of the administered dose was excreted in the bile after 24 h,
whereas excretion in the urine and faeces was less than 0.5% after
48 h.
6.4.2.2 Mouse
The elimination of radioactivity was studied in female C57Bl mice
after gavage or intravenous administration of a uniformly 14C-labelled
polychlorohexadecane (C16;69% Cl, CP-MH) (1.6 µmol/kg) (Biessmann
et al., 1983). After 8 h, only about 1% of the dose was exhaled
as 14CO2 for both administration routes. Most of the
radioactivity was excreted in faeces when administrated by gavage, and
after 8 h 22% was recovered in faeces and 1.2% in urine. After 96 h,
66% was recovered in faeces and 2.9% in urine. After intravenous
administration, 2.1% was excreted in faeces and 1% in urine after 8 h,
and 43% was excreted in faeces and 3% in urine after 96 h.
The excretion of [1-14C]polychlorohexadecane (C16;34.1% Cl,
CP-ML) (59 kBq/animal, or 1.5 µCi) after intravenous or gavage
administration to C57Bl mice was studied by Darnerud & Brandt (1982).
Twelve hours after intravenous injection, 12% of the radioactive dose
was recovered in urine, 44% in expired air (as 14CO2) and 4%
in faeces. Twelve hours after gavage administration, 6% of the
radioactive dose was recovered in urine, 33% in the expired air and
14% in faeces.
6.4.2.3 Bird
The elimination of radioactivity was studied in female Japanese
quail (Coturnix coturnix japonica) after gavage or intravenous
administration of a uniformly 14C-labelled polychlorohexadecane
(C16;69% Cl, CP-MH) (0.48 µmol/kg) (Biessmann et al., 1983). Of the
administered dose, 1.6% was exhaled as 14CO2 after gavage
administration and 0.9% after intravenous administration. Of the
administered dose 16% was excreted in faeces/urine after 8 h and 58%
after 96 h.
6.4.3 Long chain length chlorinated paraffins
A 14C-labelled chlorinated paraffin, C18; 50-53% Cl (CP-LH), was
administered by gavage as a single dose of 500 mg/kg to three female
Sprague-Dawley rats (Yang et al., 1987). After 24 h, 1% of the
radioactive dose was recovered in the urine, 1.5% in the expired air
and 22% in the faeces. After 96 h, 1.9% of the radioactive dose was
recovered in the urine, 3.3% in the expired air, 5% in body tissue and
76% in the faeces.
6.4.4 Comparative studies
In a study by Beissmann et al. (1982), 148 kBq (4 µCi)
(approximately 20 Ci/mol) of either 14C-labelled polychlorohexadecane
(C16;34% Cl, CP-ML) or 14C-labelled polychlorododecane (C12;56% Cl,
CP-SH) was administered by gavage to Japanese quail (Coturnix
coturnix japonica). A considerably higher level of radioactivity
was found in the gall bladder and kidney after C12 administration
compared with C16. On the other hand, the radioactivity in the yolk
of the first ten eggs was higher after C16 gavage than after C12
gavage. After 8 h, 39% of the C16 dose and 22% of the C12 dose was
exhaled as 14CO2, indicating that the rate of metabolism is
influenced by the chain length and/or the degree of chlorination.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute exposure
7.1.1 Lethal doses
The acute oral toxicity of chlorinated paraffins has been studied
in rats and mice (Table 17). In all studies, the LD50 was reported
to be greater than the highest administered dose (i.e. always
> 4 g/kg body weight). After inhalation of Chlorowax 500C (C12;59% Cl,
CP-SH), an LC50 was not established in the one reported study (LC50
> 3300 mg/m3). The LD50 for dermal exposure of rabbits to
Chlorowax 500C (C12;59% Cl, CP-SH) was in excess of approximately
13 g/kg body weight (Howard et al., 1975).
Table 17. Acute toxicity of chlorinated paraffins
Chlorinated Compounda Species Test Exposure Reference
paraffin concentration
C12;60% Cl (CP-SH) rat oral LD50 > 13.6 g/kg Bucher et
al. (1987)
C12;59% Cl Chlorowax rat oral LD50 > 21.5 g/kg Howard et
500C (CP-SH) al. (1975)
C12;59% Cl Chlorowax rabbit dermal > 13 g/kg Howard et
500C (CP-SH) LD50 al. (1974)
C12;59% Cl Chlorowax rat inhalation > 3300 Howard et
500C (CP-SH) LC50 mg/m3 al. (1975)
C12;60% Cl (CP-SH) mouse oral LD50 > 27.2 g/kg Bucher et
al. (1987)
C10-13;41-70% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C14-17;51-60% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C24;40% Cl Chlorowax 40 rat oral LD50 > 17.7 g/kg Howard et
(CP-LL) al. (1975)
C23;43% Cl (CP-LL) rat oral LD50 > 13.6 g/kg Bucher et
al. (1987)
Table 17. (Cont'd)
Chlorinated Compounda Species Test Exposure Reference
paraffin concentration
C23;43% Cl (CP-LL) mouse oral LD50 > 27.2 g/kg Bucher et
al. (1987)
C20-30;41-70% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C24;70% Cl Chlorowax 70 rat oral LD50 > 50 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorez 700 rat oral LD50 > 50 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorowax 70 guinea-pig oral LD50 > 25 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorez 700 guinea-pig oral LD50 > 25 g/kg Howard et
(CP-LH) al. (1975)
a The classification is given in Table 1
7.1.2 Non-lethal doses
7.1.2.1 Oral route
In a single-administration experiment, F-344/N rats and B6C3F1
mice were dosed by gavage up to 13.6 g/kg body weight (rats) and
27.2 g/kg body weight (mice) of C12;60% Cl (CP-SH) or C23;43% Cl
(CP-LL) dissolved in corn oil. No deaths or compound-related toxic
effects were noted during the 14-day observation period. However, the
animals were inactive with diarrhoea for 2-6 days after dosing, which
was attributed to the large volumes of material administered (NTP,
1986a,b; Bucher et al., 1987).
Female or male Wistar rats were administered by gavage a single
oral dose of different chlorinated paraffins (C10-13; 41-51%, 51-61% or
61-70% Cl), with a range of maximum doses of 4-13 g/kg body weight,
and were observed for 7 days (ICI, 1965, 1966, 1968, 1969, 1971, 1973,
1974a,b; Birtley et al., 1980). Clinical signs of toxicity, such as
piloerection, muscular incoordination and faecal and urinary
incontinence, were observed in rats that received doses of 2 g/kg body
weight or more (generally independent of the chlorine content).
Recovery was usually complete by day 7. There were no deaths except
for one rat treated with 13 g/kg body weight of C10-13;63% Cl.
Similar findings were reported for C14-17;51-60% Cl and
C20-30;41-51%, 51-61% or 61-70% Cl (Birtley et al., 1980). The
toxicity of these chlorinated paraffins was reported to be lower than
that of the short chain length chlorinated paraffins.
7.1.2.2 Inhalation route
No toxic response was observed in rats exposed to a concentration of
3300 mg/m3 of Chlorowax 500C (C12;59% Cl, CP-SH) for one hour (Howard
et al., 1975)
7.1.2.3 Intraperitoneal route
Three different chlorinated paraffin preparations, Chlorez 700
(C20;70% Cl, CP-LH), Paroil 170-HV (C11;70% Cl, CP-SH) and
Chloroparaffin 40 (chain-length not given, 40% Cl), were administered
intraperitoneally as single doses (Chlorez and Chloroparaffin
100 mg/kg body weight; Paroil 52 mg/kg body weight) to male Wistar rats
(Ahotupa et al., 1982). The activities of various drug-metabolizing
enzymes from liver, kidney and small intestinal mucosa were determined
after 24 h, 7 days or 21 days. Minor but significant changes in the
intestinal activities of aryl hydrocarbon hydroxylase (increase),
UDP-glucuronosyltransferase (decrease) and epoxide hydrolase
(increase) were induced by Paroil 170-HV, and in the kidney activity
of aryl hydrocarbon hydroxylase by Chlorez 700, when compared to
polychlorinated biphenyls and naphthalenes.
7.1.3 Skin and eye irritation
7.1.3.1 Short chain length chlorinated paraffins
In a study by Hoechst (1986b), 0.5 ml of undiluted C10-13;50%
Cl(CP-SH) was applied under a semi-occlusive dressing to the shaven
skin of three rabbits for 4 h. The skin was examined for signs of
irritation for up to 72 h after the chlorinated paraffin had been
removed, but none were seen during the test.
When 0.5 ml of C10-13;70% Cl(CP-SH) was applied under a
semi-occlusive dressing to the shaven skin of three rabbits for 4 h,
one rabbit showed clearly defined erythema (grade 2 on a 0-4 scale) at
48 and 72 h. The other two animals showed "slightly noticeable"
erythema (grade 1). Very slight oedema (grade 1) was noted in two
animals for up to 24 h. By day 7, all signs of irritation were
completely resolved (Hoechst, 1983).
Two studies investigated C10-13;70% Cl(CP-SH). In one study the
chlorinated paraffin contained 1 or 2% of an epoxidised vegetable oil
stabilizer with and without additives (0.1% oxalic acid or 0.05%
benzotriazole) (ICI, 1965). Very mild to mild desquamation was only
noted following the applications of chlorinated paraffins containing
additives. The reactions were described as occasional, transient and
inconsistent. It was not stated how many applications were made
before these reactions were seen. In another study, no signs of
irritation were noted following repeated application of a chlorinated
paraffin containing 0.1 or 2% benzoyl peroxide initiator (ICI, 1974a).
Two studies investigated the effects of three C10-13;63%
chlorinated paraffins (CP-SH), containing up to 3% epoxy soya oil
stabilizers or other unspecified additives (ICI, 1973, 1974a). For
all three paraffins, erythema was usually noted following two to four
applications, although on one occasion erythema was noted in 1/3
animals after only one application. The severity of the reactions was
not described. Desquamation was also noted following three or four
applications and increased in severity with further treatments. In
one study (with 0.7% epoxy carboxylate stabilizer) the desquamation
was described as severe following the fourth application when the
study was terminated (ICI, 1973).
Studies have been conducted using C10-13 chlorinated paraffins
which were 48(CP-SL), 50, 52 or 55% chlorinated (CP-SH) (ICI,
1967, 1968, 1969, 1971, 1974a,b). In most of these studies the
chloroparaffins contained 0.2 or 2% epoxy stabilisers. In one study
with 48 or 55% chlorinated paraffins, containing 0.2% epoxy octyl
stearate stabilizer, no signs of irritation were noted (ICI, 1969).
In the other studies there was mild or slight erythema, and mild
desquamation was usually noted following the second or third
application. In one study, testing C10-13;52% with 2% epoxidised octyl
oleate stabilizer, erythema was noted following the first application
(ICI, 1968). It was observed in 4/5 of the studies that the reactions
did not worsen following further applications, although in one study
(testing a 52% chlorinated paraffin with unspecified additives),
slight erythema, noted after the second application, worsened to
severe erythema with slight necrosis after the third application, when
the study was terminated (ICI, 1971).
An unspecified volume of C10-13;40% Cl(CP-SL), containing 1% epoxy
soya oil stabilizer, produced slight desquamation following the second
application and mild erythema after the third (ICI, 1966). This
condition persisted throughout the remaining applications until the
end of the study when small scattered ulcers developed.
Two studies in rats were conducted to investigate the potential
for skin irritation of two short chain length chlorinated paraffins
(C10-11) which were 49% (CP-SL) and 60% chlorinated (CP-SH) (ICI, 1980,
1982c). Repeated and single application tests were conducted. No
signs of irritation were noted following a single application of the
more chlorinated paraffin, although slight desquamation was noted in
2/6 rats, 3-6 h after the treatment with the less chlorinated
paraffin. Both chlorinated paraffins produced slight erythema and/or
slight desquamation with repeated applications.
Rats were treated with 0.1 ml of C14-17;51-60% Cl(CP-MH), or
C20-30;41-51%(CP-LL), 51-61%(CP-LH) or 61-70% Cl(CP-SH), for up to
six 24-h periods. The treatment periods were separated by 24-h
treatment-free periods. In some of the studies the chlorinated
paraffins contained epoxy stabilizers. Mild irritation was seen with
C14-17 chlorinated paraffins, but it is not clear if the response was
due to the stabilizer. No signs of irritation were seen with C20-30
(Birtley et al., 1980).
A C10-13;61% chlorinated paraffin (Cereclor 60HS) and a 50%
chlorinated paraffin (Cereclor 50 HS) of unidentified carbon chain
length produced mild or moderate skin irritation following a single
occlusive application to intact or abraded skin of rabbits. It was
stated that varying degrees of erythema persisted for 72 h (ICI
1975a,b).
In other studies (BUA, 1992) different short and long chain
length chlorinated paraffins were applied to the skin and eyes of
rabbits (skin: C10-13;58% Cl, C19;44% Cl, C20-30;70% Cl; eyes: C12;59%
Cl, C20-30;70% Cl). Only a weak or no irritating effect was observed,
which decreased with increasing chain length.
The eye irritation potential of three different chlorinated
paraffins, C10-13;65% Cl(CP-SH), which contained either 2.5 or 2% of
two different additives or 0.7% of an epoxy stabilizer, was tested in
two studies (ICI, 1971, 1974a). Either 0.1 ml or "one drop" of the
chloroparaffin was instilled into one conjunctival sac of groups
of three rabbits. Similar results were reported for all three
formulations: practically no initial pain (2 on a 6-point scale) was
noted. Slight irritation (3 on a 8-point scale), shown by redness
and chemosis (only noted in the formulation containing the epoxy
stabilizer) of the conjunctiva with some discharge, lasted for 24 h.
One drop of 52% or 40% chlorinated paraffins, containing unspecified
additives or 1% epoxy stabilizer, was also tested (ICI, 1966, 1971).
With the 52% chlorinated paraffin, slight immediate irritation was
followed by slight redness of the conjunctiva which lasted for 24 h.
With the 40% chlorinated paraffin, mild congestion was noted at 1 h
but no effects were seen at 24 h.
7.1.3.2 Intermediate and long chain length chlorinated paraffins
Intermediate and long chain chlorinated paraffins were tested
in eye irritation studies with a single application of 0.1 ml of
C14-17;51-60% Cl(CP-MH), C20-30;41-50%(CP-LL), 51-60% or 61-70%
Cl(CP-LH). No signs of eye irritation were seen (Birtley et al.,
1980).
7.1.4 Skin sensitization
The maximization method was used to assess the skin sensitization
potential of a chlorinated paraffin (C10-13;56% Cl, CP-SH) with 1%
epoxide stabilizer (Edenol D81) and 1% tris-nonylphenyl phosphite
(TNPP) (Hoechst, 1983b). When challenged with undiluted chlorinated
paraffin, 1/20 test animals showed hardly perceptible erythema 24 h
after challenge, and 1/20 test and 1/10 control animals showed clearly
defined erythema or slight oedema at 72 h. The chlorinated paraffin
tested did not induce skin sensitization in this study.
The same chlorinated paraffin (C10-13;56% Cl, CP-SH), with 1% of a
different epoxide stabilizer (Rutapox CY 160) and 1% TNPP, was tested
using the same method (Hoechst, 1984). When challenged with undiluted
chlorinated paraffin, 5/20 test animals showed clearly defined erythema
and another two showed hardly perceptible erythema. None of the
control animals showed any evidence of a skin reaction. A second
challenge was performed 2 weeks after the first. On this occasion
4/20 test animals showed clearly defined erythema and another four
showed hardly perceptible erythema or slight oedema. The authors
concluded that the substance tested was a sensitizer. However, as
less than 30% of the test group showed a clear reaction and it is
possible that the epoxide stabilizer was responsible for producing the
sensitization reactions, this study is not considered to provide
conclusive evidence that C10-13;56% Cl is a skin sensitizer.
An undiluted chlorinated paraffin (C10-13;52% Cl, CP-SH) was
applied to the ears of six guinea-pigs on three successive days (ICI,
1971). Slight erythema was noted when, 4 days later, undiluted
chloroparaffin was applied to the animals' flanks, but it was not
stated how many animals showed a reaction. Four control animals also
showed slight erythema at challenge. It does not appear that this
chlorinated paraffin elicited a sensitization response in this study.
7.2 Repeated exposure
Studies involving repeated exposure have demonstrated that the
liver, kidneys and thyroid are the target organs for the toxicity of
chlorinated paraffins.
7.2.1 Oral route
7.2.1.1 Short chain length chlorinated paraffins
a) Rat, 14-day studies
In a range-finding study, a short chain chlorinated paraffin
(C10-13;58% Cl) (CP-SH) was administered to Fischer-344 rats in
the diet for 14 days at concentrations of 0, 900, 2700, 9100 and
27 300 mg/kg feed, equivalent to approximately to 0, 100, 300, 1000
and 3000 mg/kg body weight per day (IRDC, 1983c). There were five male
and five female rats per group. No deaths occurred during the study. A
marked reduction in body weight and food consumption was seen in the
highest dose group, which was attributed to reduced palatability of
the diet caused by the chlorinated paraffin. The relative liver
weight was increased (20-240%) in all dose groups compared to
controls. The activity of liver aminopyrine demethylase (APDM) was
increased in females, and cytochrome P-450 values increased in both
sexes in all dosed groups. Liver enlargement was observed in some
rats in the groups fed 2700 to 27 300 mg/kg, and a dose-related
increase in the incidence of hepatocellular hypertrophy was present in
all treated groups. Myocardial atrophy was observed at the two
highest dose levels although the relationship to treatment was
unclear. The lowest-observed-effect level (LOEL) in this study was
100 mg/kg body weight per day.
A short chain length chlorinated paraffin with 58% Cl (CP-SH) was
administered by gavage in corn oil to Fischer-344 rats (five/dose of
each sex) for 14 days at dose levels of 0, 30, 100, 300, 1000 and
3000 mg/kg body weight per day (IRDC, 1981a). A significant decrease
in body weight gain in females in the high dose group was noted. A
dose-related increase in APDM activity was observed in females fed
300-3000 mg/kg, whereas in males there was an increase only in the
group treated with 1000 mg/kg. Cytochrome P-450 levels were
significantly increased in females treated with 1000 mg/kg, and
microsomal protein concentration increased in females dosed with
3000 mg/kg. Liver enlargement occurred in both sexes in the 300, 1000
and 3000 mg/kg groups, and mild hepatocellular hypertrophy in all
animals of the 1000 and 3000 mg/kg groups. The absolute and relative
liver weight was increased (20-150%) at doses of 100 mg/kg or more.
Statistically significant reduction of thymus and ovary weight was
observed at 3000 mg/kg. The no-observed-effect level (NOEL) in this
study was considered to be 30 mg/kg body weight per day and the LOEL
100 mg/kg body weight per day, based on liver weight increases.
In a 16-day study on F-344/N rats (groups of five), the animals
were administered a short chain length chlorinated paraffin (C12;60%
chlorination) (CP-SH) by gavage in corn oil daily (5 days per week) at
doses of 0, 469, 938, 1875, 3750 and 7500 mg/kg body weight per day
for 16 days (NTP, 1986a; Bucher et al., 1987). At the highest dose
level there was reduced body weight gain (22% in males and 16% in
females), and 1/5 male rats and 2/5 females died before the end of the
study. Enlarged livers were observed in every dose group except the
females fed 469 mg/kg. No histopathology was performed. The LOEL in
this study was considered to be 469 mg/kg body weight per day.
b) Rat, 90-day studies
Fischer-344 rats (groups of 10 males and 10 females) were given a
short chain chlorinated paraffin (C12;60% chlorination) (CP-SH) in
corn oil by gavage on 5 days a week for 13 weeks at doses of 0, 313,
625, 1250, 2500 and 5000 mg/kg body weight per day (NTP, 1986a; Bucher
et al., 1987). Body weight gain was reduced by approximately 10% in
males at the two highest dose levels. A dose-related statistically
significant increase in relative liver weight (17-100% for males;
30-100% for females) was observed in all treated rats. Hepatocellular
hypertrophy was noted in all rats in the highest dose group, and
nephrosis was more frequent in this group (10/10 males; 3/10 females)
compared to controls (8/10 males; 0/10 females). Rats in other dose
groups were not examined microscopically. On the basis of an increase
in liver weight, the LOEL in this study was 313 mg/kg body weight per
day.
In a 13-week study Fischer-344 rats were administered a short
chain length chlorinated paraffin (C10-13;58% Cl, CP-SH) in corn oil by
gavage at doses of 0, 10, 100 and 625 mg/kg body weight per day in
groups of 15 animals of each sex (IRDC, 1984a). In the groups treated
with 100 mg/kg or more, increased weights of the liver (30-110%) and
the kidneys (20-100%) were observed. At the highest dose level
thyroid weights were increased. Hepatocellular hypertrophy was
observed at 100 and 625 mg/kg. In these groups hypertrophy and
hyperplasia of the thyroid were also observed. There was trace-to-mild
chronic nephrosis in the kidney of males treated with 625 mg/kg, and in
females in the high-dose group, in which pigmentation of the renal
tubular epithelia also occurred. The NOEL was considered to be 10 mg/kg
body weight per day on the basis that no treatment-related microscopic
changes were found in any tissue at this dose. The LOEL was 100 mg/kg
body weight per day based on increased liver and kidney weights and
hypertrophy in liver and thyroid. When the same doses were
administered in the diet essentially identical results were obtained
(IRDC, 1984c).
Male and female Fischer-344 rats (5 or 10 per group) were
administered Chlorowax 500C (C12;58% Cl, CP-SH) in corn oil by gavage
at doses of 0, 313 and 625 mg/kg body weight per day for up to 90 days
(Elcombe et al., 1994). The relative liver weight was increased at
both doses (50 and 75%). Hepatic peroxisomal ß-oxidation (palmitoyl
CoA oxidation) was statistically significantly increased in a
dose-related manner from day 15. The activity of thyroxine-UDPG-
glucuronosyltransferase was significantly increased (at least 150%) at
both dose levels from day 15 onwards. Thyroid follicular cell
hypertrophy was observed at all time points, and hyperplasia at days
56 and 91. Replicative DNA synthesis was increased in thyroid
follicular cells at day 91. Renal tubular eosinophilia was observed
in males from day 15. In renal tubular cells replicative DNA
synthesis was increased in males. When a higher dose was administered
(1000 mg/kg body weight per day) marked decreases in the levels of
plasma thyroxine and increased plasma thyroid stimulating hormone were
observed. None of these effects were observed in male Dunkin Hartley
guinea-pigs, which were administered the chlorinated paraffin at doses
of 500 and 1000 mg/kg body weight per day. The LOEL in rats was 313
mg/kg body weight per day based on increased relative liver weights,
hepatic peroxisomal ß-oxidation and thyroxine-UDPG-glucuronosyl-
transferase activity.
c) Mouse, 14-day studies
B6C3F1 mice in groups of five were administered C10;60% Cl
(CP-SH) in corn oil by gavage daily for 16 days (NTP, 1986a; Bucher et
al., 1987). The doses were 938, 1875, 3750, 7500 and 15 000 mg/kg
body weight per day. All mice receiving 3750 mg/kg or more, and 4/5
males and 2/5 females receiving 1875 mg/kg died before the end of the
study. Diarrhoea was observed in all dosed groups except females
receiving 938 mg/kg. Enlarged livers were found in all treated
surviving mice. No histopathological examinations were conducted.
d) Mouse, 90-day studies
B6C3F1 mice (10 of each sex per dose) were exposed to C12;60%
Cl (CP-SH) by gavage five days per week for 13 weeks (NTP, 1986a;
Bucher et al., 1987). The doses were 125, 250, 500, 1000 or
2000 mg/kg body weight per day. No clinical signs of toxicity were
observed. In the males the body weight gain was reduced by 13% at the
highest dose level. The relative liver weights showed a dose-related
increase (17-160%) and were statistically significant from 250 mg/kg
in females and from 500 mg/kg in males. Hepatocellular hypertrophy
was observed in animals of both sexes treated with 250 mg/kg or more.
Focal hepatic necrosis was related to dosing at 500, 1000 and
2000 mg/kg in males and at 2000 mg/kg in females. The NOEL was
125 mg/kg body weight per day, and the LOEL was 250 mg/kg body weight
per day, based on hepatocellular hypertrophy.
7.2.1.2 Intermediate chain length chlorinated paraffins
a) Rat, 14-day studies
A chlorinated paraffin of intermediate chain length (C14-17) and
52% chlorination (CP-MH) was administered to Fischer-344 rats in the
diet at dosage levels of 150, 500, 1500, 5000 and 15 000 mg/kg feed,
which was reported to correspond to an average compound intake of
17.7, 57.7, 177, 562 and 1412 mg/kg body weight per day (IRDC, 1981b).
Five male and five female rats in each dose group were exposed daily
for 14 days. No mortality occurred among the treated animals.
Hepatic APDM activity was statistically significantly increased in
males receiving 562 mg/kg body weight per day (5000 mg/kg feed) and in
females receiving 1412 mg/kg body weight per day (15 000 mg/kg feed).
Slight increase in cytochrome P-450 values in male rats given
177 mg/kg body weight per day (1500 mg/kg feed) was observed but
appeared not to be related to dosing. Increased relative liver weight
was observed in the highest two dosage groups. Microscopic examination
of the liver revealed mild diffuse hepatocellular hypertrophy in all
animals receiving 562 and 1412 mg/kg body weight per day (5000 and
15 000 mg/kg feed). The NOEL was reported to be 57.7 mg/kg body weight
per day. The LOEL was 177 mg/kg body weight per day in males based on
increased cytochrome P-450 values and 562 mg/kg body weight per day in
females based on increased liver weight and hepatocellular hypertrophy.
b) Rat, 90-day studies
Male and female weanling Sprague-Dawley rats in groups of ten
were fed diets containing 0, 5, 50, 500 or 5000 mg/kg of C14-17;52%
Cl(CP-MH) for 13 weeks, yielding an average intake of 0, 0.4, 3.6, 36
and 360 mg/kg body weight per day for males and 0, 0.4, 4.2, 43 and
420 mg/kg body weight per day for females (Poon et al., in press).
There were no clinical signs of toxicity and no differences in body
weight gain. Relative liver weight was increased at 43 and 420 mg/kg
body weight per day in females and at 360 mg/kg in males. Relative
kidney weight was increased at the highest dose level in both sexes.
Serum cholesterol was increased in females from 4.2 mg/kg in a
dose-related manner. In the highest dose group of both sexes elevated
hepatic UDP-glucuronosyltransferase activity was observed, but only
females at this dose level showed increased APDM activity. Decreased
hepatic vitamin A levels were detected in females at 43 mg/kg and in
both sexes at the highest dose level. Mild, adaptive histopathological
changes were detected in the liver of both sexes at the two highest
dose levels, and in the thyroid of males from 36 mg/kg and females
from 4.2 mg/kg (reduced follicle sizes, collapsed angularity,
increased height, cytoplasmic vacuolation and nuclear vesiculation).
In the kidney, minimal changes were noted in the proximal tubules of
males at 360 mg/kg, and in the inner medulla tubules of females at 43
and 420 mg/kg. The NOEL in this study was 4 mg/kg body weight per day.
The LOEL was 36 mg/kg body weight per day (males) and 43 mg/kg body
weight per day (females).
In a 90-day feeding study, Wistar rats (groups of 24 males and 24
females) were fed diets containing 0, 250, 500, 2500 and 5000 mg/kg
feed of Cereclor S52 (C14-17;52% Cl, CP-MH) containing stabilizer (0.2%
epoxidized vegetable oil) (Birtley et al., 1980). A dose-related
decrease in body weight gain in males fed 500 mg/kg feed or more was
observed, which was accompanied by a reduction in food intake.
Significant increases in the relative liver weights in females were
observed at 500 mg/kg feed or more and in males at 2500 mg/kg feed.
Significant increases in relative kidney weights were observed at
5000 mg/kg feed in both sexes. Microscopic examination of the liver
showed evidence of a dose-related proliferation of the smooth
endoplasmic reticulum in the hepatic cells from 500 mg/kg feed.
Haematological investigation showed no abnormalities attributable to
the test compound. A tendency towards congestion of the kidney with
increasing concentration of Cereclor S52 in the diet was also observed.
The NOEL was 250 mg/kg feed (12.5 mg/kg body weight per day based on
food consumption data) and the LOEL was 500 mg/kg feed (25 mg/kg
body weight per day) based on increased relative liver weights and
proliferation of smooth endoplasmic reticulum.
A medium chain length chlorinated paraffin (C14-17;52% Cl, CP-MH)
was evaluated for subchronic toxicity in Fischer-344 rats (IRDC,
1984b). The chlorinated paraffin was administered to the rats (15 of
each sex per dose group) in the diet to provide dosage levels of 0,
10, 100 and 625 mg/kg body weight per day for 13 weeks. The
treatment did not induce signs of toxicity, alter survival or cause
ophthalmological changes. A slight reduction of body weight gain
(< 5%) was observed in both sexes at the highest dose, and was
associated with reduced food consumption. Traces of hepatocyte
hypertrophy (at 625 mg/kg) and increased absolute and relative liver
and kidney weights (at 100 and 625 mg/kg) were noted in both sexes.
Males in the high-dose group had an increased incidence of nephritis.
In addition, increased thyroid weight and thyroid hypertrophy and
hyperplasia were observed in males at 625 mg/kg. The NOEL was
considered in the report to be 10 mg/kg body weight per day. The LOEL
was 100 mg/kg body weight per day based on increased liver and kidney
weights.
c) Dog, 90-day studies
The effects of Cereclor S52 (C14-17;52% Cl, CP-MH) (containing
0.2% epoxidized vegetable oil as stabilizer) were studied in Beagle
dogs (Birtley et al., 1980). Four male and four female animals in
each group were fed a diet corresponding to 0, 10, 30 or 100 mg/kg
body weight daily for 90 days. No effects were found, except for
significantly increased serum alkaline phosphatase activity and
relative liver weight in males exposed to 100 mg/kg and an increase in
the smooth endoplasmic reticulum of hepatocytes from 30 mg/kg. The
NOEL was 10 mg/kg body weight per day. The LOEL was 30 mg/kg body
weight per day based on an increase of hepatic smooth endoplasmic
reticulum.
7.2.1.3 Long chain length chlorinated paraffins
a) Rat, 14-16 day studies
Fischer-344 rats (groups of five) were exposed to C22-26;43% Cl
(CP-LL) daily by gavage for 16 days at doses of 235, 469, 938, 1875 or
3750 mg/kg body weight per day. No compound-related clinical signs
of toxicity or mortality were observed. There were no changes
in body weight gain and no gross lesions were observed at necropsy.
Histopathological examinations were not conducted (NTP, 1986b; Bucher
et al, 1987).
Fischer-344 rats, in groups of five of each sex, were fed long
chain length paraffins of 70% chlorination for 14 days. The dietary
concentrations of the C22-26;70% Cl (CP-LH) were 0, 150, 500, 1500,
5000 and 15 000 mg/kg feed, corresponding to an average compound
intake of 0, 17.1, 55, 169, 565 and 1715 mg/kg body weight per day
(IRDC, 1982b). Tissues from liver, kidneys, spleen, lungs and
mesenteric lymph nodes were examined microscopically. Hepatic
microsomal Lowry protein, APDM activity and cytochrome P-450 values
were determined. No significant toxic effects were noted, and no
compound-related effects were found after microscopical examinations.
The NOEL was 1715 mg/kg body weight per day.
A chlorinated paraffin, C22-26;43% Cl (CP-LL), was administered by
gavage to Charles River 344 rats at doses of 0, 30, 100, 300, 1000 and
3000 mg/kg body weight per day (IRDC, 1982c). During the 14-day test
period no signs of toxicity were observed. Following sacrifice,
tissues from the liver, spleen, kidneys, pancreas, thymus and eyes
were examined microscopically; the only observation was a possible
increase in kidney nephrolithiasis in females exposed to the highest
dose level. Hepatic microsomal Lowry protein, APDM activity and
cytochrome P-450 values were determined and there were no alterations.
The NOEL was considered to be 3000 mg/kg body weight per day.
b) Rat, 90-day studies
A long chain length chlorinated paraffin, C23;43% Cl (CP-LL),
was administered to Fischer-344 rats in groups of 10 (each sex) by
gavage for 13 weeks at doses of 235, 469, 938, 1875 or 3750 mg/kg body
weight per day (NTP, 1986b; Bucher et al., 1987). No effects on body
or organ weights and no clinical signs of toxicity were observed. A
dose-related increased incidence of granulomatous inflammation was
noted in the livers of all exposed female rats but not in males. The
NOEL was 3750 mg/kg body weight per day for males. For females the
LOEL was 235 mg/kg body weight per day based on increased incidence of
granulomatous inflammation in the liver.
A long chain chlorinated paraffin (C20-30) with 43% Cl (CP-LL) was
administered in corn oil by gavage for 90 days to Fischer-344 rats at
three doses (100, 900 or 3750 mg/kg body weight per day) (IRDC,
1984f). Increases in liver weights and a multifocal granulomatous
hepatitis characterized by inflammatory changes and necrosis were
observed in all exposed females but not in males. In female rats
mineralization in the kidneys at the highest dose level was observed.
In addition to these observations, mild nephrosis was observed in the
males in the highest dose group. In males, the NOEL was 900 mg/kg
body weight per day and the LOEL was 3750 mg/kg body weight per day
(based on the occurrence of mild nephrosis). In females the LOEL was
100 mg/kg body weight per day based on liver effects.
The chlorinated paraffin C22-26;70% Cl (CP-LH) was administered to
Fischer-344 rats in the diet for 90 days at doses of 100, 900 and
3750 mg/kg body weight per day (IRDC, 1984g). Increased liver weight,
hepatocellular hypertrophy and cytoplasmic fat vacuolation were noted
at the highest dose level. The alanine aminotransferase (ALT)
activity was increased in both sexes at the highest dose level, and
aspartate aminotransferase (AST) activity was also increased in
females of this group. The NOEL was considered to be 900 mg/kg body
weight per day. The LOEL was 3750 mg/kg body weight per day based on
liver effects.
c) Mouse, 14-16 day studies
B6C3F1 mice in groups of five were given C22-26;43% Cl (CP-LL) by
gavage daily for 16 days at doses of 469, 938, 1875, 3750 or
7500 mg/kg body weight per day (NTP, 1986b; Bucher et al., 1987). No
compound-related clinical sign of toxicity or mortality was observed,
there were no changes in body weight gain, and no gross lesions were
observed at necropsy. Histopathological examinations were not
conducted.
d) Mouse, 90-day studies
The long chain length chlorinated paraffin C23;43% Cl (CP-LL)
was administered to B6C3F1 mice (in groups of 10 of each sex) by
gavage for 13 weeks at dose levels of 469, 938, 1875, 3750 or
7500 mg/kg body weight per day (NTP, 1986b; Bucher et al., 1987). No
effects on body or organ weights, no clinical signs of toxicity and no
histopathological effects were observed. The NOEL was 7500 mg/kg body
weight per day.
7.2.1.4 Comparative studies
The effects of representative chlorinated paraffins on liver
function and thyroid hormone function has been studied in male rats
(Alpk:APFSD) and male mice (Alpk:APFCD-1) (Wyatt et al., 1993).
Groups of five male rats or five male mice received 0, 10, 50, 100,
250, 500 or 1000 mg/kg body weight per day by gavage in corn oil, once
daily for 14 days. The chlorinated paraffins studied were Chlorowax
500C (C10-13;58%Cl, CP-SH), Cereclor 56L (C10-13;56%, CP-SH) and
Chlorparaffin 40C (C14-17;40% Cl, CP-ML). Effects on liver function
were assessed by changes in liver weight (expressed both as absolute
weights and liver:body weight ratio) and peroxisome proliferation
(expressed as the activity of palmitoyl co-enzyme A (CoA) oxidase).
All three chlorinated paraffins caused increases in liver weight and
palmitoyl CoA oxidation, indicative of peroxisomal proliferation. In
general, the rat was more sensitive to the effects of the chlorinated
paraffins on liver weight than the mouse. The doses of chlorinated
paraffin required to cause peroxisomal proliferation were, in general,
greater than those causing effects on liver weight, although there
appeared to be less of a difference in inter-species sensitivity.
However, the magnitude of the increase in palmitoyl CoA oxidation
caused by the short chain chlorinated paraffins (approximately 10-fold
increase as a maximal change) was greater than for the intermediate
chain grade (approximately 4-fold increase as maximal change) (Table
18). The effect of the chlorinated paraffins on thyroid function was
studied in the male rats receiving 1000 mg/kg body weight per day for
14 days by measuring the plasma levels of triiodothyronine (T3) and
thyroxine (T4) (free and total) and thyroid stimulating hormone
(TSH), and also the activity of hepatic microsomal UDP glucuronosyl-
transferase activity. All three chlorinated paraffins (at 1000 mg/kg
body weight per day) caused a reduction in plasma T4 levels (both
free and total) and an increase in plasma TSH levels. No effect was
observed on plasma T3 levels. All three chlorinated paraffins also
caused an increase (two-fold) in the rate of glucuronidation of T4
by hepatic microsomal UDP glucuronosyltransferase activity, suggesting
that the impact on plasma T4 and TSH levels is due to increased
clearance of T4 by hepatic metabolism.
Table 18. Effects of chlorinated paraffins on liver function in male rats and male mice
(From: Wyatt et al., 1993)
Increase in relative liver weight Increase in palmitoyl CoA
(% liver:body weight ratio) oxidase activity
Rat Mouse Rat Mouse
NOELa LOELb NOELa LOELb NOELa LOELb NOELa LOELb
C10-13;58% Cl 74 100 215 250 184 250 180 250
C10-13;56% Cl 51 50 70 100 600 1000 120 250
C14-17;40% Cl 31 100 426 1000 473 500 252 500
a Calculated using a three-parameter logit model (mg/kg body weight per day)
b Observed (mg/kg body weight per day)
The hepatic effects of representative chlorinated paraffins have
been studied in male and female F-344 rats, male and female B6C3F1
mice and male Alpk:Dunkin Hartley guinea-pigs (Elcombe et al., in
press). Their effects were compared with a range of known inducers of
hepatic enzymes. Groups of 4-5 animals received 1000 to 2000 mg/kg
body weight per day of each chlorinated paraffin (by gavage in corn
oil) for 14 consecutive days. The chlorinated paraffins studied were
Chlorowax 500C (C10-13;58% Cl, CP-SH), Cereclor 56L (C10-13;56% Cl,
CP-SH), Chlorparaffin 40G (C14-17;40% Cl, CP-ML) and Chlorowax 40
(C20-30;43% Cl, CP-LL). The short and intermediate chain length
chlorinated paraffins increased liver:body weight ratios (approximately
1.5 times) and elicited hepatocellular hypertrophy, peroxisome
proliferation (assessed as increases in peroxisomal volume and
palmitoyl CoA oxidase activity) and proliferation of hepatic cell
smooth endoplasmic reticulum in both rats and mice. These effects
were not seen in rats or mice receiving the long chain chlorinated
paraffin. The short and intermediate chain length chlorinated
paraffins also caused induction of cytochrome P-450 IV A1 (assessed by
increases in lauric acid hydroxylation) and P-450 II B1/IIB2 (assessed
by increases in ethoxycoumarin- O-diethylation) in the rat liver, but
only P-450 IV A1 in the mouse liver. The induction of the specific
cytochrome P-450 isoenzymes was confirmed using SOS-polyacrylamide gel
electrophoresis (SPS-PAGE) and Western immunoblotting of microsomes.
The administration of the short or intermediate chain length
chlorinated paraffins to guinea-pigs (1000 mg/kg body weight per day
for 14 days) had a similar effect on liver:body weight ratios (1.5-fold
increase) but had no effect on any of the hepatic ultrastructural or
biochemical parameters measured.
7.2.2 Intraperitoneal route
7.2.2.1 Short chain length chlorinated paraffins
Effects on both hepatic cytosolic and microsomal epoxide
hydrolases have been observed in male C57Bl/6 mice after 5 daily
intraperitoneal injection of 400 mg Cereclor 70L (C12;70% Cl, CP-SH)
(Meijer & DePierre, 1987). The hepatic cytosolic epoxide hydrolase
activity was increased to 130% and the microsomal activity to 250% of
the control. In addition, the amount of microsomal cytochrome P-450
was increased by 50%, and cytosolic DT-diaphorase activity was
increased 2- to 3-fold. There was also liver enlargement.
7.2.2.2 Intermediate chain length chlorinated paraffins
Lundberg (1980) reported a significant, dose-related increase in
total cytochrome P-450 in mice (strain not reported) injected
intraperitoneally with different amounts of Cereclor S52 (C14-17;52%
Cl, CP-MH) (from 0.6 mg to 63.4 mg) on 3 consecutive days. The
N-demethylation of ethylmorphine, a cytochrome P-450-dependent
reaction, decreased at low concentrations but increased at higher
concentrations.
7.2.2.3 Comparative studies
Male Sprague-Dawley rats were given intraperitoneal injections of
1000 mg/kg of Witaclor 149, 159 or 171P (C10-13 with 49% [CP-SL], 59%
[CP-SH] and 71% [CP-SH] chlorination, respectively), Witachlor 350
(C14-17 with 49% chlorination [CP-ML] or Witachlor 549 (C18-26 with 49%
chlorination [CP-LL]), each containing small amounts of epoxidated
soy-bean oil as stabilizer, daily for 4 days (Nilsen et al., 1980,
1981; Nilsen & Toftgård, 1981). Treatment with the C10-13 chlorinated
paraffins, but not those with longer chain lengths, caused increases
in liver weight and an induction of various forms of microsomal
cytochrome P-450. The activity of O-deethylation of 7-ethoxyresorufin
was decreased by the C10-13 chlorinated paraffins with higher
chlorine content, Witaclor 159 and 171P. The metabolism of
benzo (a)pyrene was induced by each of the chlorinated paraffins.
Witaclor 149 (C10-13;49% Cl) caused a significant proliferation of the
smooth endoplasmic reticulum (two-fold) whereas C18-26;49% Cl caused a
smaller increase. All three C10-13 chlorinated paraffins gave rise to
increased occurrence and size of cytoplasmic lipid droplets. Witachlor
149 also caused an increase in the number and size of mitochondria and
peroxisomes. These latter effects were also observed to a lesser
degree with both Witachlor 350 and Witachlor 549.
Effects on microsomal enzymes, after intraperitoneal injection of
Cereclor 42 (C22-26;42% Cl, CP-LL), Cereclor S58 (C14-17;58% Cl, CP-MH),
Cereclor 70 (C23;70% Cl, CP-LH) and Cereclor 70L (C10-13;70% Cl) (1000
mg/kg, once daily for 5 days) in liver from male Sprague-Dawley rats,
have been observed (Meijer et al., 1981). Microsomal epoxide hydrolase
activity was increased by all Cereclors except C22-26;42% Cl. In
addition, the activity of glutathione- S-transferase was increased
except in the case of C22-26;42% Cl, which decreased the activity
slightly. The amount of cytochrome P-450 was not changed.
7.3 Neurotoxicity
7.3.1 Short chain length chlorinated paraffins
The motor capacity, measured as the capacity of adult male NMRI
mice to remain on an accelerating rotarod, was determined after single
intravenous injections of 0, 30, 97.5, 165, 232.5 and 300 mg/kg in
groups of five mice of either Cereclor 50 LV (C10-13;49% Cl, CP-SL) or
Cereclor 70L (C10-13;70% Cl, CP-SH) (Eriksson & Kihlström, 1985). A
statistically significant decrease in the motor capacity and rectal
temperature was observed in mice receiving the highest dose of either
compound.
7.3.2 Intermediate chain length chlorinated paraffins
Immature 10-day-old NMRI mice (groups of at least 6) were given a
single peroral dose of 1 mg/kg body weight of polychlorohexadecane
(C16; chlorination degree not specified) dissolved in a fat emulsion
of egg lecithin and peanut oil (Eriksson & Nordberg, 1986). A
significantly decreased sodium-dependent choline uptake in the
cerebral cortex, 65% of Vmax in controls, was measured 7 days after
treatment indicating a pre-synaptic effect of this chlorinated
paraffin. No significant alteration in high- and low-affinity
muscarinic binding in the cerebral cortex in the brains could be
observed.
7.4 Reproductive toxicity, embryotoxicity and teratogenicity
7.4.1 Reproduction
An intermediate chain length chlorinated paraffin (C14-17) with
52% chlorination (CP-MH) was given in the diet to Charles River rats
at dose levels of 0, 100, 1000 and 6250 mg/kg feed (equivalent to 0,
6, 62 and 384 mg/kg body weight per day for the males and 0, 8, 74 or
463 mg/kg body weight per day for the females based on food
consumption data) (IRDC, 1985). The diet was fed both males and
females for 28 days before mating, during mating, and for females up
to postnatal day 21. Pups were given the same diet from weaning
until 70 days of age. No differences were observed in appearance,
fertility, body-weight gain, food consumption or reproductive
performance in the F0 generation. Among the offspring, no adverse
effects were observed prior to lactation day 7. However, significantly
decreased pup survival was observed in the high-dose group on lactation
day 10. None of the pups in this group survived to weaning. Survival
in pups from the mid-dose group was decreased by lactation day 21.
Necropsy findings in animals that died included pale liver, kidneys and
lungs, and blood in the cranial cavity, brain, stomach and intestines.
The pup weights were lower in the low-dose group (not statistically
significant) and mid-dose group than in the control group on lactation
day 21. In females, the reduced weight continued after weaning but
became less pronounced in males. Other observations in the pups of
the mid- and high-dose groups included bruised areas, decreased
activity, laboured breathing, pale discoloration and/or blood around
orifices. Reduced erythrocyte count, haemoglobin and haematocrit were
noted in the pups in the high-dose group on lactation day 6 relative to
the control values obtained on lactation day 7. The observations in
this study could indicate a high exposure of the pups to chlorinated
paraffins via the milk. This is supported by preliminary results of
a cross-fostering study showing a greater mortality in pups exposed
via milk than in pups exposed only in utero (Serrone et al., 1987).
The LOEL was 5.7 mg/kg body weight per day (males) or 7.2 mg/kg body
weight per day (females) in the F1 generation based on decreased
pup weight.
7.4.2 Embryotoxicity and teratogenicity
Teratology studies are summarized in Table 19.
Table 19. Oral teratology studies with chlorinated paraffins
Chlorinated paraffina Species LOEL NOEL Effects and remarks References
(mg/kg (mg/kg
body weight body weight
per day) per day)
CP-SH: C10-13;58% Cl rat 2000 500 maternal toxicity at 500 and 2000 mg/kg body weight IRDC (1982a)
per day; embryo-fetotoxicity and digital
malformations at 2000 mg/kg body weight per day
C10-13;58% Cl rabbit - 100 IRDC (1982d)
CP-MH: C14-17;52% Cl rat - 5000 slight maternal toxicity at 2000 and 5000 mg/kg IRDC (1984d)
body weight per day
C14-17;52% Cl rabbit - 100 mean maternal body weight losses were seen during IRDC (1983f)
treatment at the high-dose level (80, 100, 160 mg/kg
body weight per day) in a range-finding study
C14-17;70% Cl mouse - 100b Darnerud &
Lundkvist
(1987)
Table 19 (Cont'd)
Chlorinated paraffina Species LOEL NOEL Effects and remarks References
(mg/kg (mg/kg
body weight body weight
per day) per day)
CP-LL: C20-30;43% Cl rat - 5000 IRDC (1983d)
C20-30;43% Cl rabbit - 2000 slight increase in mean implantation loss and IRDC (1982e)
decreased number of viable fetuses at 5000 mg/kg
body weight per day, which were not statistically
significant
CP-LH: C22-26;70% Cl rat - 5000 IRDC (1984e)
C22-26,70% Cl rabbit - 1000 possible maternal toxicity (non-dosage-related IRDC (1983b)
congestion of lungs). In preliminary rabbit studies
an increase in post-implantation loss was observed
at > 1000 mg/kg body weight per day, but this effect
was not observed in the main teratology study
a The classification is given in Table 1
b Single intraperitoneal injection on day 1
7.4.2.1 Short chain length chlorinated paraffins
The teratogenic potential of a short chain length paraffin
(C10-13) with 58% Cl (CP-SH) was studied in pregnant Charles River COBS
CD rats (IRDC, 1982a). The rats, in groups of 25, were administered
0, 100, 500 and 2000 mg/kg body weight per day in corn oil orally by
gavage once daily from days 6 to 19 of gestation, and were examined on
gestation day 20. In the dams, the high dose treatment increased the
frequency of mortality (32%) and decreased body weight gain, and the
mid- and high-dose treatments resulted in dose-related adverse
clinical signs, such as yellow or brown matting and staining of the
anogenital fur, soft stool, red or brown matter (or staining) in the
nasal region, decreased activity, oily fur and excessive salivation.
Treatment at the highest dose level, which was a maternally toxic
dose, resulted in the appearance of fetal malformations such
as adactyly and/or shortened digits, increased incidences of
postimplantation loss and decreased numbers of viable fetuses. Fetal
body weight and incidence of delayed bone ossification were not
affected by the treatment. The NOEL for teratogenic effects was
500 mg/kg body weight per day, which was also a slightly maternally
toxic dose.
Female Dutch Belted rabbits (groups of 16) were treated by gavage
with a short chain length chlorinated paraffin (C10-13) with 58%
chlorination (CP-SH) (IRDC, 1982d). The rabbits were treated at dose
levels of 0, 10, 30 and 100 mg/kg body weight per day on gestation
days 6-27 and examined on day 28. There were no adverse effects on
survival, body weight gain, clinical signs or postmortem observations
in dams. The highest-dose group had an increased incidence of whole
litter resorption (two dams), and in the group exposed to 30 mg/kg
slight increases in the incidence of whole litter resorption (one dam)
and early and late resorptions were observed. Whole litter
resorptions did not occur in the low-dose or control animals, but
occurred in historical controls at an incidence of 13/277. This
indicated that the appearance of one or two dams with whole litter
resorptions could occur by chance. The NOAEL in this study was
100 mg/kg body weight per day.
7.4.2.2 Intermediate chain length chlorinated paraffins
Female Charles River COBS CD rats were treated by gavage with an
intermediate chain length chlorinated paraffin (C14-17;52% Cl) (CP-MH)
(IRDC, 1984d). The administered dose levels (0, 500, 2000 and
5000 mg/kg body weight per day) were given to groups of 25 animals on
gestation days 6-19, and this was followed by examination on day 20.
The end-points studied were weight of the uterus, number and location
of viable fetuses, early and late resorptions, the number of total
implantations and corpora lutea, and the incidence of fetal
malformations. The treatment had no adverse effect on mortality, body
weight gain or uterine weight of dams, but signs of toxicity, such as
wet, matted and yellow-stained hair in the anogenital area and/or soft
stools, were observed in the mid- and high-dose dams. No treatment-
related adverse effects were observed in the fetuses and there was no
evidence of developmental effects.
Female Dutch Belted rabbits were treated by gavage with an
intermediate chain length chlorinated paraffin (C14-17;52% Cl) (CP-MH)
in groups of 16 (IRDC, 1983f). The dose levels were 0, 10, 30 and
100 mg/kg body weight per day and were administered on gestation days
6-27, followed by examination on day 28. The end-points studied were
weight of the uterus, number and location of viable fetuses, early and
late resorptions, the number of total implantations and corpora lutea,
and the incidence of fetal malformations. In the dams, congestion of
the lobes of the lung was noted in all treated groups at necropsy, but
did not occur in a dose-related pattern. No significant adverse
effects were observed in fetuses and there were no developmental
effects.
In a study of NMRI mice, the animals were given a single
intraperitoneal injection of 100 mg/kg body weight of polychloro-
hexadecane (C16;70% Cl) (CP-MH) on the day the vaginal plug was
observed (day 1) (Darnerud & Lundkvist, 1987). The mice were killed
on day 14 of pregnancy, and the number and weight of embryos and
resorptions were determined. No effects on implantation or embryonic
survival were observed.
7.4.2.3 Long chain length chlorinated paraffins
Groups of 25 pregnant Charles River COBS CD rats were administered
(500, 2000 and 5000 mg/kg body weight per day) a long chain chlorinated
paraffin (C22-26;43% Cl) (CP-LL) by gavage from days 6 to 19 of
gestation (IRDC, 1983d). The end-points studied were weight of the
uterus, number and location of viable fetuses, early and late
resorptions, the number of total implantations and corpora lutea,
and the incidence of fetal malformations. In the dams, there were no
adverse effects on appearance, mean body weight gain or mortality
rate, and necropsy findings were normal. No signs of developmental
effects were noted in the pups.
Female Charles River COBS CD rats (groups of 25 animals) were
treated by gavage with a long chain length chlorinated paraffin
(C22-26;70% Cl) (CP-LH) (IRDC, 1984e). The dose levels were 0, 500,
2000 and 5000 mg/kg body weight per day on gestation days 6-19. The
rats were examined on day 20. The end-points studied were the same as
in the previous study. No treatment-related adverse effects were
observed in the dams or pups.
Pregnant Dutch Belted rabbits in groups of 16 were exposed orally
to long chain chlorinated paraffin (C22-26;43% Cl) (CP-LL) at doses of
500, 2000 and 5000 mg/kg body weight per day in corn oil by gavage
once daily from days 6 to 27 of gestation (IRDC, 1982e). The
end-points studied were the same as in the previous studies in this
section. No effects on maternal survival or body weight gain
occurred. In the highest-dose group there was a slight increase in
mean implantation loss and a slight decrease in the mean number of
viable fetuses when compared to the control group. However, these
alterations were not statistically significant. In the fetuses no
alterations related to the treatment were observed, although the
number in the highest-dose group was limited. Treatment of the
rabbits at a dose level of 2000 mg/kg body weight per day or less did
not produce a teratogenic response. No developmental effects were
noted. The NOEL in this study was 2000 mg/kg body weight per day.
Female Dutch Belted rabbits (groups of 16 animals) were given by
gavage a long chain length chlorinated paraffin (C22-26;70% Cl) (CP-LH)
(IRDC, 1983b). Doses of 0, 100, 300 and 1000 mg/kg body weight per
day were administered on gestation days 6-27, followed by examination
on day 28. The end-points studied were the same as in the earlier
studies in this section. The appearance, behaviour and body weight
gain were normal in the treated dams, although at necropsy a
non-dose-related increase in the occurrence of congested lungs was
noted. No adverse effects on the fetuses were observed and no
developmental effects were noted.
7.5 Mutagenicity and related end-points
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells there is a suggestion of a weak
clastogenic potential in vitro but not, according to several reports,
in vivo. Chlorinated paraffins were also reported to induce cell
transformation in vitro.
Table 20. Mutagenicity of chlorinated paraffins in bacterial tests
Chlorinated paraffina Dose (µg/plate) S9 Bacterial strainsb Effects Reference
Cereclor 50LV C10-13;50% Cl (CP-SH) 2500 ± TA1535 - Birtley et al. (1980)
TA1538 -
TA100 -
TA98 -
Hordalub 80 (with 1% C10-13;50% Cl (CP-SH) 10 000 ± TA98 + Hoechst (1986a)
epoxy stabilizer) TA100 -
TA1535 -
TA1537 -
TA1538 -
WP2 uvrAc -
Chloroparaffin 56 C12;57% Cl (CP-SH) 5000 ± TA98 - Hoechst (1988)
(unstabilized) TA100 -
TA1535 -
TA1537 -
TA1538 -
WP2 uvrAc -
Cereclor 70L C10-13;70% Cl (CP-SH) 2300 ± TA98 - Meijer et al. (1981)
TA100 -
TA1537 -
Cereclor S52 C14-17;52% Cl (CP-MH) 2500 ± TA1535 - Birtley et al. (1980)
- stabilizer TA1538 -
TA100 -
TA98 -
+ stabilizer -
Table 20. Cont'd
Chlorinated paraffina Dose (µg/plate) S9 Bacterial strainsb Effects Reference
Solvocaffaro C1642 C14-17;42% Cl (CP-ML) 1, 10, ± TA1535 - Conz & Fumero (1988a)
100, TA1537 -
1000, TA1538 -
5000 TA98 -
TA199 -
Meflex DC 029 C14-17;45% Cl (CP-ML) 1.6, 8, ± TA1535 - Elliott (1989a)
40, TA1537 -
200, TA1538 -
1000, TA98 -
5000 TA1000 -
Cereclor 42 C20-30;42% Cl (CP-LL) 2500 ± TA1535 - Birtley et al. (1980)
TA1538 -
TA100 -
TA98 -
Chorowax 40 C23;43% Cl (CP-LL) 10 000 ± TA97 - NTP (1986b)
TA98 -
TA100 -
TA1535 -
a The classification is given in Table 1
b Unless indicated otherwise, all strains refer to Salmonella typhimurium
c Strain of Escherichia coli
7.5.1 Prokaryotes
Most of the data demonstrate no mutagenic effects in four
Salmonella typhimurium strains after treatment with short,
intermediate or long chain length chlorinated paraffins at doses up to
10 mg/plate (Table 20). A very low but significant effect was
detected in strain TA98 after treatment with the highly chlorinated
short chain length chlorinated paraffin, Cereclor 70L (C10-12;70% Cl,
CP-SH) (Meijer et al., 1981). However, the observation is uncertain
since no dose response was observed, the increase in revertants was
low (less than 2-fold), and the increase was only found in the
presence of metabolic fraction (S9) derived from Aroclor-1254-induced
rat liver. The results from this study were considered to be
negative. Another study apparently demonstrated positive results.
However, the increase in the number of revertants with TA100 in the
presence of S9 was just less than two-fold, and in TA98, in the
absence of S9, the increase only just reached two-fold (Hoechst,
1986a). Furthermore, the possibility that the epoxy stabilizer was
responsible for the increase can not be discounted.
7.5.2 Mammalian cells
The results obtained from mammalian cell systems are summarized
in Tables 21, 22 and 23.
7.5.2.1 In vitro studies
C12;60% Cl (CP-SH) was mutagenic in L5178Y mouse lymphoma cells
at concentrations of 48 and 60 µg/ml in the absence of S9 mix (Myhr et
al., 1990).
When tested up to cytotoxic concentrations, C10-13;56% Cl(CP-SH)
did not induce a significant, reproducible increase in the number of
mutant colonies in Chinese hamster V79 cells (HPRT locus), either in
the presence or absence of S9.
Chlorowax 500C (C23;43% Cl, CP-LL) induced chromosome
aberrations in the absence of S9 mix at a concentration of 5000 µg/ml
and sister chromatid exchange (SCE) with and without S9 at 5, 500,
1700 and 5000 µg/ml in Chinese hamster ovary (CHO) cells in vitro
(Anderson et al., 1990).
7.5.2.2 In vivo studies
a) Short chain length chlorinated paraffins
When C12;60% Cl (CP-SH) (0, 500, 1000 and 2000 mg/kg body weight
was administrated in corn oil by gavage to Alpk:AP male rats (groups
of 4), no effects on unscheduled DNA synthesis (UDS) in hepatocytes
could be detected after exposure for 2 or 12 h (Ashby et al., 1990).
However, a moderate dose- and time-related induction of cell
proliferation, measured as S-phase cells, in the hepatocytes was
detected in animals exposed to 1000 and 2000 mg/kg for 12 h.
Table 21. Genotoxicity in mammalian cells in vitro
Chlorinated paraffin Cell line End-point Exposure Effect References
CP-SH: C10-13;56% Cl Chinese hamster mutations 5, 10, 15, 20, 30, 50, 75 µg/ml - Hoechst (1987)
V79 cells (±S9)
C12;60% Cl L5178Y mouse mutations 12, 24, 36, 48, 60 and 72 µg/ml + (from Myhr et al.
lymphoma cells (-S9) 60 µg/ml) (1990)
CP-LL: C23;43% Cl Chinese hamster chromosome 1250-5000 µg/ml (±S9) + (at 5000 Anderson et al.
ovary cells aberrations µg/ml + S9) (1990)
C23;43% Cl Chinese hamster sister chromatid 5, 500, 1700 and 5000 µg/ml +a Anderson et al.
ovary cells exchange (±S9) (1990)
a The effect was observed at all concentrations
Table 22. Cell transformation in in vitro mammalian cells
Grade Cell line Exposure Effect References
CP-SH: C10-13;50% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
C10-13;58% Cl Baby hamster kidney cells 44 µg/ml (-S9), 58 µg/ml (+S9) + ICI (1982a)
C10-13;58% Cl Baby hamster kidney cells 33 µg/ml (-S9), 88 µg/ml (+S9) + Richold et al. (1982a)
CP-MH: C14-17;52% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
CP-LL: C20-30;42% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
CP-LH: C20-26;70% Cl Baby hamster kidney cells 10, 50, 100, 500 and 1000 µg/ml (±S9) +a Richold et al. (1982b)
C22-26;70% Cl Baby hamster kidney cells 10 µg/ml (+S9), 294 µg/ml (-S9) + ICI (1982b)
a The effect was observed at all concentrations
Table 23. Genotoxicity data from in vivo mammalian cells
Grade Species and End-point Exposure No. of Effect References
strain animals
CP-SH: Fischer-344 Chromosome aberrations 250, 750 and 2500 mg/kg body weight 8 - IRDC (1983h)
C10-12,58% Cl rats in bone marrow cells per day for 5 days by gavage
C10-13,58% Cl Charles River Dominant lethal 250, 750 and 2500 mg/kg body weight 15 - IRDC (1983a)
COBS CD rats mutations per day for 5 days by gavage
C10-13,58% Cl NMRI mice Micronucleus assay 50, 5000 mg/kg body weight (single 10 - Muller (1989)
in bone marrow cells dose by gavage)
C10-13,58% Cl Hoe:NMRKF Micronucleus assay 50, 5000 mg/kg body weight (single 10 - Muller (1989)
SPF 71 mice in bone marrow cells dose by gavage)
C12;60% Cl Alpk: AP rats DNA repair in 500, 1000 and 2000 mg/kg body weight 4 - Ashby et
hepatocytes by gavage (single dose by gavage) al. (1990)
for 2 or 12 h
CP-ML: Crd: CD-1 Micronucleus assay 5000 mg/kg body weight (single dose 10 - Conz &
C14-17;42% Cl (ICR) Br mice in bone marrow cells by gavage) Fumero (1988b)
C14-17;45% Cl C57B1/6JFCD-1/ Micronucleus assay 3125, 5000 mg/kg body weight (single 10 - Elliott
Alpk mice in bone marrow cells dose by gavage) (1989b)
Table 23. (Cont'd)
Grade Species and End-point Exposure No. of Effect References
strain animals
CP-MH: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983g)
C14-17;52% Cl rats in bone marrow cells per day for 5 days by gavage
CP-LL: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983i)
C22-26;43% Cl rats in bone marrow cells per day for 5 days by gavage
CP-LH: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983e)
C20-30;70% Cl rats in bone marrow cells per day for 5 days by gavage
Sexually mature male Fischer-344 rats in groups of eight were
dosed by gavage once daily for 5 days with a short chain length
chlorinated paraffin (C10-12;58% Cl, CP-SH) at doses of 0, 250, 750 or
2500 mg/kg body weight per day (IRDC, 1983h). Metaphase spreads of
rat bone marrow cells were examined for chromosome aberrations. In
the group treated with 2500 mg/kg, all the rats except one died during
the study. Signs of overt toxicity were observed in most of these
rats. The rats receiving up to 750 mg/kg body weight per day did not
show an increased mortality or frequency of chromosome or chromatid
abnormalities, neither did the surviving rat from the highest dose
group. These observations indicate that toxic doses were administered
to the rats. Cytotoxicity was not assessed. However, the information
on distribution of short chain chlorinated paraffins following oral
absorption (section 6.3.1) indicates that there would have been
distribution to the bone marrow. This chlorinated paraffin was not
considered clastogenic in this test system.
Sexually mature NMRI (Hoe, NMRKF [SPF71]) mice (groups of five
males and five females) were given single doses of 50 and 5000 mg/kg
body weight Chlorowax 500C (C10-13;58% Cl, CP-SH) by gavage in sesame
oil (Hoechst, 1989). Responses at the high dose level were examined
at 24, 48 and 72 h sampling times and the low dose level at a 24 h
sampling time only. There were no differences from control values
either in polychromatic cells with micronuclei or in the ratio of
polychromatic erythrocytes to normocytes.
The dominant lethal mutation potential of a chlorinated paraffin
of short chain length and 58% chlorination was examined in Charles
River COBS CD rats (IRDC, 1983a). Groups of 15 males were treated
with 0, 250, 750 and 2000 mg/kg body weight per day in corn oil orally
by gavage for five consecutive days. Each male was then mated with 20
untreated females. There was no evidence of a mutagenic effect on the
post-meiotic stage of spermatogenesis at any dose level, as shown by
the absence of effect on the mean number of viable embryos during the
first four weeks of mating.
b) Intermediate chain length chlorinated paraffins
Sexually mature male Fischer-344 rats in groups of eight were
given unstabilized chlorinated paraffin (C14-17;52% Cl, CP-MH) in corn
oil by gavage once daily for 5 days at doses of 0, 500, 1500 and 5000
mg/kg body weight per day (IRDC, 1983g). Metaphase spreads of rat
bone marrow cells were examined for chromosome aberrations. No signs
of toxicity were observed during the study, and the treatments did not
produce any increase in the frequency of chromosome abnormalities,
compared to the controls.
Two studies yielding negative results in the mouse bone marrow
micronucleus assay have been reported. Sexually mature mice, of
strains CRI: CD-1 (ICR) BR (Conz & Fumero, 1988b) and C57BL/6JF
CD-1/ALpK (Elliott, 1989b) were used. Conz & Fumero (1988b) studied
Solvocaffaro C1642 (C14-17;42% Cl (CP-MH)) and Elliott (1989b) studied
Melex DC 029 (C14-17;45% Cl). In both studies groups of 10 animals:
(five males and five females) were given the limit dose of 5000 mg/kg
body weight by gavage in corn oil. Elliott (1989b) also used a lower
dose of 3125 mg/kg body weight. Responses at 5000 mg/kg were examined
at three sampling times (18, 43 and 66 h (Conz & Fumero, 1988b) or 24,
48 and 72 h (Elliott, 1989b)). Responses at the lower dose level
(Elliott, 1989b) were examined at 24 h. In both studies there were no
differences from negative control values in either polychromatic cells
with micronuclei or in the ratio of polychromatic erythrocytes to
normocytes. The positive control (mitomycin C [8 mg/kg body weight,
Conz & Fumero, 1988b] or cyclophosphamide [65 mg/kg body weight,
Elliott, 1989b]) produced the anticipated positive responses, thus
verifying the sensitivity of the test systems.
c) Long chain length chlorinated paraffins
When a long chain length paraffin with 70% chlorination (CP-LH)
(in 1% carboxymethylcellulose) was administered by gavage to
Fischer-344 rats (groups of 8) at doses of 500, 1500 and 5000 mg/kg
body weight daily for 5 days, no increased frequency of chromosome
abnormalities in bone marrow cells was observed indicating a lack of
clastogenic activity under the experimental conditions (IRDC, 1983e).
Body weight gain was decreased in the high-dose group.
Sexually mature male Fischer-344 rats in groups of eight were
given (gavage once daily for 5 days) a long chain length chlorinated
paraffin (C22-26;43% Cl, CP-LL) at doses of 500, 1500 and 5000 mg/kg
body weight per day (IRDC, 1983i). Metaphase spreads of rat bone
marrow cells were examined for chromosome aberrations. No signs of
toxicity was observed during the study, and the treatment did not
produce any increase in the frequency of chromosome abnormalities,
compared to the controls.
7.5.2.3 Cell transformation
Chlorowax 500C (C10-13;58% Cl, CP-SH), was examined in a cell
culture transformation test using baby hamster kidney (BHK) cells in
soft agar (Richold et al., 1982a). The cells were treated with doses
from 3.125 µg/ml to 500 µg/ml (-S9) and from 6.25 µg/ml to 1000 µg/ml
(+S9). Treatment with LC50 doses of 33 µg/ml (-S9) and 88 µg/ml (+S9)
increased the transformation frequency 52 times in the absence of S9
(the control was negative at 50% simulated survival) and 500 times in
the presence of S9.
Cereclor 50LV (C10-13;50% Cl, CP-SH) was not active at dose levels
up to 2500 µg/ml in a cell transformation assay using BHK cells
(Birtley et al., 1980).
Cereclor S52 (C14-17;52% Cl, CP-MH) with or without stabilizer
did not induce transformation of BHK cells at doses up to 2500 µg/ml
(Birtley et al., 1980).
After treatment of BHK cells with a long chain length paraffin
Electrofine S-70 (C20-26;70% chlorination, CP-LH) in doses from
10 µg/ml to 1000 µg/ml (+S9), a dose-related increased frequency of
transformed colonies was demonstrated at all dose levels (Richold et
al., 1982b).
Cereclor 42 (C20-30;42% Cl, CP-LL) was not active at dose levels
up to 2500 µg/ml in a cell transformation assay in BHK cells (Birtley
et al., 1980).
7.6 Long-term exposure and carcinogenicity
7.6.1 Oral route
7.6.1.1 Short chain length chlorinated paraffins
In a 2-year gavage study using Fischer-344 rats, groups of 50
males and 50 females were given C12;60% Cl, CP-SH at doses of 312 or
625 mg/kg per day, 5 days/week, for 104 weeks) (NTP, 1986a; Bucher et
al., 1987). After week 37, the body weights of the high-dose males
were reduced by 10-23% compared with controls. Survival of both
low- and high-dose males and of low-dose females was significantly
less than that of controls (at termination 27 male controls, 6
low-dose and 3 high-dose males and 34 control females, 23 low-dose and
29 high-dose females survived). Additional groups of 20 male and 20
female rats were added to each dose group for concurrent 6-month and
12-month studies. The spleen, liver, thymus, adrenal glands, brain,
kidney and heart were weighed at necropsy in the 6- and 12-month
studies. Biochemical and haematological effects were not examined.
The incidence of tumours, which was significantly increased, is
presented in Table 24. Liver neoplastic nodules and hepatocellular
carcinomas combined in males and females occurred with a positive
trend. The incidence of kidney tubular cell adenomas and the combined
incidence of adenomas and adenocarcinomas were significantly increased
in low-dose male rats. In low-dose females an increased incidence of
thyroid follicular cell adenomas was observed, and in addition,
adenomas and carcinomas combined showed an increased incidence in
high-dose female rats. Increased incidences of mononuclear cell
leukaemia, pancreatic acinar cell adenomas and endometrial stromal
polyps of the uterus (low-dose female rats) were observed. The
increased incidence of mononuclear cell leukaemia was dose-related in
males but not in females. Although the incidence of endometrial
stromal polyps in the low-dose female rats was greater than in vehicle
controls, these tumours were probably not treatment-related since
there were no increases at higher doses. The incidence of pancreatic
acinar tumours was also increased in the low-dose group of males,
although, owing to the higher incidence in concurrent compared to
historical controls and the absence of a dose-response relationship,
the increase was not considered to be treatment-related.
In the same study some chronic non-neoplastic lesions were
reported. Hepatocellular hypertrophy was observed in 74% of the
low-dose group and in nearly all of the high-dose rats, but in none
of the control group. Necrosis and angiectasis were observed in the
livers in all dosed rats. In addition to increased kidney weight at 6
and 12 months in both sexes and dose levels, the incidence and
severity of nephropathy was increased in dosed females, as was the
severity of nephropathy in males in the concurrent 12-month study.
Erosion, inflammation and ulceration of the glandular stomach and the
forestomach were seen in both groups of the dosed males. Hyperplasia
of the parathyroid was observed in both groups of exposed males and in
high-dose females.
Groups of 50 male and 50 female B6C3F1 mice were given C12;60%
Cl, CP-SH by gavage at doses of 125 and 250 mg/kg 5 days a week for
103 weeks (NTP, 1986a; Bucher et al., 1987). The body weights of
treated females were about 10% lower than those of controls during the
second year. The survival of treated males was not significantly
different from that of the controls, but in the high-dose group of
females fewer animals were still alive after week 100 in comparison
with the control group. The tumour incidences are shown in Table 25.
Increased incidences of liver adenomas and liver adenomas and
carcinomas in combination were observed. The incidences of thyroid
follicular cell adenomas and carcinomas combined were increased in
exposed female mice. The incidences of alveolar/bronchiolar
carcinomas were increased significantly in the high-dose group of male
mice, and the trend with dose was also significant. However, the
incidences of alveolar/bronchiolar carcinomas and adenomas (combined)
in males were not significantly greater than those in vehicle
controls. Among the non-neoplastic lesions, the incidence of
nephrosis was increased in the high-dose group of females, but was
decreased in dosed male mice compared to controls. Biochemical and
haematological effects were not examined.
Table 24. Incidence of tumours in rats administered the chlorinated paraffin C12;60% chlorine
(From: NTP, 1986a; Bucher et al., 1987)
Dose Hepatocellular Hepatocellular Hepatocellular Follicular cell Mononuclear Adenomas or
(mg/kg neoplastic carcinomas neoplastic nodules adenomas and cell leukaemia adeno-carcinomas
body weight) nodules and carcinomas carcinomas of of the kidney
the thyroid
Males
Control 0/50 0/50 0/50 3/50 7/50 0/50
312 10/50a 3/50a 13/50a 3/50 12/50b 9/50b
625 16/48a 2/48 16/48a 3/50 14/50b 3/49
Females
Control 0/50 0/50 0/50 0/50 11/50 0/50
312 4/50 1/50 5/50a 6/50a 22/50b 0/50
625 7/50a 1/50 7/50a 6/50a 16/50 0/50
a Incidental tumour test for trend, p < 0.05, increase relative to control
b Life table analysis, p < 0.05, increase relative to control
Table 25. Incidence of tumours in mice administered the chlorinated paraffin
C12;60% chlorine (From: NTP, 1986a; Bucher et al., 1987)
Dose Hepatocellular Hepatocellular Hepatocellular Follicular cell
(mg/kg adenomas carcinomas adenomas and adenomas and
body carcinomas carcinomas of
weight) the thyroid
Males
Control 11/50 11/50 20/50 3/49
125 20/50a 15/50 34/50a 4/50
250 29/50a 17/50 38/50a 3/49
Females
Control 0/50 3/50 3/50 8/50
125 18/50a 4/50 22/50a 12/49a
250 22/50a 9/50b 28/50a 15/49a
a Incidental tumour test for trend, p < 0.05, increase relative to control
b Life table analysis, p < 0.05, increase relative to control
7.6.1.2 Long chain length chlorinated paraffins
Groups of 50 male and 50 female Fischer-344 rats were treated by
gavage with 1875 and 3750 mg/kg body weight (male rats) and 100, 300
and 900 mg/kg body weight (female rats) of C23;43% Cl, CP-LL
dissolved in corn oil on 5 days a week for 103 weeks (NTP, 1986b;
Bucher et al., 1987). The treatment did not affect body weight or
survival, and no signs of clinical toxicity were observed. Additional
groups of 20 male and 20 female rats were exposed concurrently to the
same doses for 6 or 12 months for analyses of the weights of the
spleen, liver, thymus, adrenal glands, brain, kidneys and heart, serum
hepatic enzymes, including sorbitol dehydrogenase, AST and ALT, and
haematological parameters.
In female rats that were administered the chlorinated paraffin,
an increased incidence of adrenal gland medullary phaeochromocytomas
was observed (1/50; 4/50; 6/50; 7/50, high dose statistically
significant, significant positive trend). In addition, an increased
incidence of endometrial stromal polyps in the uterus was found in the
low-dose group of the females, but this increase was not dose-related
(9/50; 17/50; 10/50; 10/50, low dose statistically significant) (Table
26). In males, acinar cell tumours of the pancreas occurred with a
negative trend. The incidence of benign hepatocellular neoplasia was
not increased in dosed rats.
Table 26. Incidence of tumours in rats administered C23;43% Cl
(From: NTP, 1986b; Bucher et al., 1987)
Dose (mg/kg body Adrenal medulla, Uterus, endometrial
weight) phaeochromocytomas stromal polyps
Females
0 1/50 9/50
100 4/50 17/50a
300 6/50 10/50
900 7/50a 10/50
a Incidental tumour test for trend, p < 0.05, increased relative
to control
Several non-neoplastic lesions were related to the administration
of C23;43% Cl. Relative liver weights were increased in exposed
males at 12 months and in exposed females at 6 and 12 months. The
observed increases were dose-related. Activities of several serum
enzymes were also slightly elevated at both 6 and 12 months. There
were also variations in haematological parameters at 6 and 12 months,
but only in females. The primary non-neoplastic lesion related to
administration of this chlorinated paraffin included a diffuse
lymphohistiocytic inflammation in the liver and in the pancreatic and
mesenteric lymph nodes in male and female rats in all exposed groups.
Splenic congestion was a secondary effect.
Groups of 50 male and 50 female B6C3F1 mice were given, by
gavage, C23;43% Cl, CP-LL dissolved in corn oil at doses of 2500 and
5000 mg/kg on 5 days a week for 103 weeks (NTP, 1986b; Bucher et al.,
1987). The survival of the mice was not significantly different in
treated groups compared to controls, and there were no clinical signs
of toxicity. However, in the female groups a Klebsiella infection
affected the animals after week 65, and 60 to 70% of the early deaths
in each group were attributed to the infection. Low-dose males and
females had lower weight gains than control or high-dose groups. The
incidence of malignant lymphomas was significantly increased in males
of the high-dose group, and occurred with a positive trend (Table 27).
The incidences of hepatocellular carcinomas in females occurred with a
positive trend, but the increase was not significant. The incidences
of adenomas and carcinomas of the liver (combined) were marginally
increased in females. Follicular cell carcinomas in males occurred
with a positive trend (0/49; 0/48; 3/49) in the thyroid gland.
However, the incidence of follicular cell adenomas or carcinomas
(combined) was not significantly greater than that in vehicle controls
(1/49, 3/48; 5/49) and was within the range of historical controls for
the test laboratory. No significant increases in non-neoplastic
lesions were attributed to administration in mice.
Table 27. Incidence of tumours in mice administered C23;43% Cl
(From: NTP, 1986b; Bucher et al., 1987)
Dose (mg/kg Lymphoma Hepatocellular Hepatocellular
body weight) carcinomas adenomas and
carcinomas
Males
0 6/50 9/50 18/50
2500 12/50 12/50 21/50
5000 16/50a,b 12/50 23/50
Females
0 15/50 1/50 4/50
2500 12/49 1/49 3/49
5000 20/50 6/50 10/50
a Incidental tumour test for trend, p < 0.05, increased relative
to controls
b Life table test, p < 0.05, increased relative to controls
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Controlled human studies
Chlorinated paraffins C10-13;50 and 63% Cl(CP-SH) were applied,
under occlusive dressings, to the upper arm of 26 volunteers
(INVERESK, 1975). After 24 h the applications were removed and one
hour later skin reactions were examined by two independent assessors.
A second application was made and reactions were assessed after a
further 24 h contact. Mild erythema and dryness (average scores, read
at the 24 and 50 h time points, of less than 2 and 1, respectively, on
a 4-point scale) were recorded, which were comparable to scores in a
liquid paraffin control group.
Paroil 142 (C20-30;40-41% Cl, CP-LL) and Chlorez 700 (C24;70% Cl,
CP-LH) were applied to the skin of 200 male and female volunteers for
a 5-day period, then reapplied for 2 days beginning 3 weeks after the
initial exposure. The dose level was not reported. No primary local
irritation, allergic response or other toxic responses were observed.
In a similar study, Chlorowax 70 (C24;70% Cl, CP-LH), Chlorowax 500C
(C12;59% Cl, CP-SH) and Chlorowax 40 (C24;43% Cl, CP-LL) were applied
to the skin of 200 males and female volunteers. The exposure time
period and amount of chlorinated paraffins used were not reported.
The treatments did not produce local irritation or allergic responses
(Howard et al., 1975).
8.2 Occupational exposure
In a study on cutting fluid coolants, 134 non-exposed employees
and 75 exposed employees were patch tested with various constituents
of the cutting fluids including chlorinated paraffins (Menter
et al., 1975). No positive reactions were obtained with any of the
constituents, although the authors themselves suggested that the tests
were not sufficiently stringent, as some positive reactions were
anticipated for some of the constituents tested.
Positive skin reactions to chlorinated paraffin constituents were
obtained in patch tests conducted on four employees suffering from
scaly eczema, who had been exposed occupationally to cutting oils
(English et al., 1986). However, the authors concluded that the
reaction was due to additives in the cutting oil, which showed
positive reactions when tested alone, rather than to the chlorinated
paraffin.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
The inhibition of gas production in an anaerobic sewage sludge
digestion process by C10-12;58% Cl (CP-SH) was studied by Madeley et
al. (1983b). A significant (> 10%) inhibition of gas production
occurred at chlorinated paraffin concentrations of 3.2, 5.6 and 10%
(w/w with respect to digester volatile suspended solids) during the
first 3-4 days of the experiment, but the gas production recovered
during the rest of the experiment until termination at day 10. It
was concluded in the report that the chlorinated paraffin induced a
transient partial inhibition and no longer-term effects.
9.1.2 Aquatic organisms
Acute toxicity data for aquatic invertebrates and fish are
summarized in Table 28. Chlorinated paraffins of short-chain length
have been shown to be acutely toxic to both freshwater and saltwater
invertebrates. Most of the acute toxicity tests for intermediate
and long chain chlorinated paraffins on aquatic invertebrates
exceed the water solubility. However, a study on intermediate
chlorinated paraffins suggests that these may be acutely toxic.
Short, intermediate and long chain chlorinated paraffins appear to
be of low acute toxicity to fish, the LC50 values being well
in excess of the water solubility.
9.1.2.1 Aquatic plants
Exposure of the freshwater alga Selenastrum capricornutum for
10 days to C10-12;58% Cl (CP-SH) at dose levels of 110, 220, 390, 570,
900 and 1200 µg/litre resulted in a significant inhibition of growth
at 570 µg/litre. The calculated EC50 values for cell density over 4,
7 and 10 days were 3690, 1550 and 1310 µg/litre, respectively, which
all exceeded the highest tested concentration (Thompson & Madeley,
1983d).
The marine alga Skeletonema costatum was exposed to C10-12;58%
Cl (CP-SH) for 10 days at concentrations of 0, 4.5, 6.7, 12.1, 19.6,
43.1 and 69.8 µg/litre (Thompson & Madeley, 1983b). Significant
inhibition of growth was observed on the first 2 days from
19.6 µg/litre. The EC50 for cell density after 4 days was
42.3-55.6 µg/litre, and EC50 for growth rate after 2 days was
31.6 µg/litre. No significant reduction in cell density was observed
after 10 days, indicating an effect on duration of lag phase prior to
exponential growth or a drop in chlorinated paraffin concentration after
10 days.
Table 28. Acute toxicity of chlorinated paraffins to aquatic organisms
Species Chlorinated Parameter Concentration Reference
paraffin (µg/litre)
Water flea C10-12;58% Cl 48-h EC50b 530 Thompson &
Daphnia magna C10-12;58% Cl 72-h EC50b 24 Madeley
(freshwater) C10-12;58% Cl 96-h EC50b 18 (1983c)
C10-12;58% Cl 120-h EC50b 14
C14-17;52% Cl 48-h EC50b 37 Frank &
Steinhäuser
(in press)
Mysid shrimp C10-12;58% Cl 96-h LC50 14.1-15.5 Thompson &
Mysidopsis bahia Madeley
(estuarine) (1983a)
Nitocra spinipes C10-13;49% Cl 96-h LC50 100 Tarkpea
(marine C10-13;70% Cl 96-h LC50 < 300 et al.
crustacean) C14-17;45% Cl 96-h LC50 9000 (1981)
C14-17;52% Cl 96-h LC50 > 10 × 106
C22-26;42% Cl 96-h LC50 > 1 × 106
C22-26;49% Cl 96-h LC50 > 10 × 106
Bleak C10-13;49%, 96-h LC50 > 5 × 106 Lindén et
Alburnus 63%, 71% Cla al. (1979)
alburnus C10-13;56%, Cl 96-h LC50 > 10 × 106
(estuarine) C11.5;70% Cl 96-h LC50 > 10 × 106
C15.5;40% Cl 96-h LC50 > 5 × 106
C14-17;50%, 96-h LC50 > 5 × 106
52% Cla
C22-26;42% Cl 96-h LC50 > 5 × 106
C18-26;49% Cl 96-h LC50 > 5 × 106
Rainbow trout C20-30;42% Cl 96-h LC50 770 × 103 Madeley &
Oncorhynchus Birtley
mykiss (1980)
(freshwater)
a Consecutive percentage chlorinations refer to different tests
b EC50 based on immobilization
9.1.2.2 Invertebrates
Short-term toxicity data for aquatic invertebrates are summarized
in Table 29 and chronic toxicity data in Table 30.
The freshwater crustacean Daphnia magna was exposed to a short
chain length paraffin with 58% Cl (CP-SH) (Thompson & Madeley, 1983c).
The chlorinated paraffin caused the organisms to float at or near the
surface of the water at 75 µg/litre or more. All water fleas died at
16.3 µg/litre after 6 days in a continuous-flow experiment. The
following LC50 values were calculated: 3 days, 24 µg/litre; 4 days,
18 µg/litre; 5 days, 14 µg/litre; 6 to 21 days, 12 µg/litre. No dead
parent Daphnia were observed at 8.9 µg/litre after 21 days of
exposure, but 37% of the offspring were dead as compared to 6% and 9%
in the controls. No increased mortality in the offspring was observed
at 5.0 µg/litre. The number of offspring per female was reduced at
2.7 µg/litre.
Daphnia magna was studied in a 48-h test with C14-17;52% Cl and
C18-20;52% Cl. Using the water-soluble fraction of a loading
concentration of 100 mg/litre, an EC50 of 37 µg/litre for the
intermediate chain length chlorinated paraffin and an EC0 of
> 26 µg/litre for the long chain length chlorinated paraffin were
observed. In a 21-day reproduction test, daphnids were exposed to the
water-soluble fraction of both chlorinated paraffins. With a loading
of 100 mg/litre, a no-observed-effect concentration of 4.4-8.8 µg/litre
was found for reproduction rate and parent mortality (LOEC =
19.9-35.6 µg/litre) for the intermediate chain length chlorinated
paraffin. For the long chain length chlorinated paraffin, a LOEC of
< 1.2 µg/litre was found for the same two parameters. In these
studies it was observed that a higher loading concentration of
10 g/litre caused an increase in the effect concentrations (Frank &
Steinhäuser, in press).
In studies of the marine shrimp Mysidopsis bahia, the 96-h
LC50 was between 14.1 and 15.5 µg/litre after exposure to a short
chain length chlorinated paraffin with 58% Cl (CP-SH) (Thompson &
Madeley, 1983a). After 28 days of exposure to 0.6, 1.2, 2.4, 3.8 and
7.3 µg/litre, increased mortality was observed but this was not
treatment-related. The increased mortality was significantly
different from the control (at 1.2 and 2.4 µg/litre) but not from the
solvent control, and was therefore considered as not related to the
chlorinated paraffin concentration. No treatment-related effects of
this chlorinated paraffin on reproductive rate or growth over this
time period were observed.
Table 29. Short-term toxicity data for aquatic invertebrates
Chlorinated paraffin Organism LC50 Exposure Other effects LOEC References
period
C10-13;49% Cl (CP-SL) Nitocra spinipes 100 µg/litre 96 h Tarkpea et al.
(1981)
C10-12;58% Cl (CP-SH) Dapnia magna 24 µg/litre 3 days Increased mortality in 8.9 µg/litre Thompson & Madeley
offspring after 21 days (1983c)
C10-12;58% Cl (CP-SH) Mysidopsis bahia 14.1-15.5 96 h No effects after 28 days Thompson & Madeley
µg/litre of exposure up to (1983a)
7.3 µg/litre
C10-12;58% Cl (CP-SH) Midge larvae, 48 h No adverse effects EG & G Bionomics
Chironomus tentans after exposure to (1983)
18-162 µg/litre
C10-13;70% Cl (CP-SH) Nitocra spinipes < 300 µg/litre 96 h Tarkpea et al.
(1981)
C14-17;45% Cl (CP-ML) Nitocra spinipes 9000 µg/litre 96 h Tarkpea et al.
(1981)
C14-17;52% Cl (CP-MH) Nitocra spinipes > 10 × 106 96 h Tarkpea et al.
µg/litrea (1981)
Table 29. (Cont'd)
Chlorinated paraffin Organism LC50 Exposure Other effects LOEC References
period
C22-26;42% Cl (CP-LL) Nitocra spinipes > 1 × 106 96 h Tarkpea et al.
µg/litrea (1981)
C22-26;49% Cl (CP-LL) Nitocra spinipes > 10 × 106 96 h Tarkpea et al.
µg/litrea (1981)
a Exceeding the water solubility
Table 30. Chronic toxicity of chlorinated paraffins to aquatic invertebrates
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C10-12;58% Cl Water flea, 6-12 days LC50 12 Thompson & Madeley (1983c)
Daphnia magna
21 days Increased mortality in 8.9
offspring after 21 days
C10-12;58% Cl Mysid shrimp, 28 days No treatment-related 7.3 Thompson & Madeley (1983a)
Mysidopsis bahia effects
C10-12;58% Cl Midge larvae, 49 days Halting adult 121 EG & G Bionomics (1983)
Chironomus tentans emergence (100%)
C10-12;58% Cl Mussel, 60 days LC50 74 Madeley & Thompson (1983a)
Mytilus edulis
84 days Growth reduction 9.3 Thompson & Shillabeer (1983)
91 days 23% mortality during 10.1 Madeley et al. (1983a)
exposure and 10%
mortality during
depuration
C10-12;58% Cl (CP-SH) Alga, Selenastrum 10 days Inhibition of growth 570 Thompson & Madeley (1983d)
capricornutum
C10-12;58% Cl (CP-SH) Alga, Skeletonema 10 days Inhibition of growth 19.6 Thompson & Madeley (1983b)
costatum
C14-17;52% Cl (CP-MH) Mussel 60 days Decreased filtration 3800 Madeley & Thompson (1983b)
Mytilus edulis activity
Table 30. (Cont'd)
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C14-17;52% Cl Water flea, 21 days Reproduction & parent 19.9-35.6 Frank & Steinhäuser (1994)
Daphnia magna mortality (LOEC)
C18-20;52% Cl Water flea, 21 days Reproduction & parent < 1.2 Frank & Steinhäuser (1994)
Daphnia magna mortality (LOEC)
C22-26;43% Cl (CP-LL) Mussel, 60 days Decreased filtration 2.180 Madeley & Thompson (1983c)
Mytilus edulis activity
C20-30;70% Cl (CP-LH) Mussel, 60 days Decreased filtration 1330 Madeley & Thompson (1983d)
Mytilus edulis activity
Larvae of the midge Chironomus tentans (second instar) were
exposed to C10-12;58% Cl (CP-SH) concentrations from 18 to 162 µg/litre
for 48 h (EG & G Bionomics, 1983). No adverse effects could be
detected. Exposure to 61-394 µg/litre for the 49 days life cycle
gave no effects except for halting adult emergence (100%) at 121 and
394 µg/litre.
The mussel Mytilus edulis was exposed to 2.3 and 10.1 µg/litre
of C10-12;58% Cl (CP-SH) for 147 days followed by 98 days of
depuration (2.3 µg/litre) or 91 days followed by 84 days depuration
(10.1 µg/litre) (Madeley et al. 1983a). A third of the mussels in the
high-dose group died during the exposure (23%) or depuration periods
(10%), and 7% of those in the low dose group, but this did not differ
significantly from the number of deaths in the acetone control.
When the mussel Mytilus edulis was studied over 60 days after
exposure to 13, 44, 71, 130 and 930 µg/litre of C10-12;58% Cl (CP-SH),
there was significant mortality at the three highest dose levels, the
median lethal time (LT50) values being 59.3, 39.7 or 26.7 days,
respectively (Madeley & Thompson, 1983a). The highest dose exceeded
the maximal solubility of the chlorinated paraffin. The LC50 for
this 60-day period was 74 µg/litre.
Mussels (Mytilus edulis) were exposed to 2.3 and 9.3 µg/litre
of a short chain length paraffin (58% Cl) (CP-SH) in sea water for 12
weeks (Thompson & Shillabeer, 1983). There were no mortalities at
either concentration, but at 9.3 µg/litre a reduction in growth rate
was observed, measured as shell length and tissue weight.
Treatment of mussels (Mytilus edulis) for 60 days with
C14-17;52% Cl (CP-MH) at concentration of 220 and 3800 µg/litre
gave no significant mortality, but there was an observed decrease
(non-quantitative visual observation) in filtration activity at the
higher concentration (Madeley & Thompson, 1983b). The highest dose
exceeded the maximal water solubility of the chlorinated paraffin.
Treatment of mussels (Mytilus edulis) with C22-26;43% Cl (CP-LL)
at 120 or 2180 µg/litre or with C20-30;70% Cl (CP-LH) at 460 and
1330 µg/litre for 60 days did not cause mortality, but visual
observation suggested that the filtration activity was reduced at the
higher dose levels (Madeley & Thompson, 1983c,d). The highest doses
exceeded the maximal solubility of the chlorinated paraffins.
9.1.2.3 Fish
Acute toxicity data are shown in Table 28 and chronic toxicity
data for fish are summarized in Table 31.
Table 31. Chronic toxicity of chlorinated paraffins to fish
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C10-13;49%, 59%, Bleak, 14 days Behavioural effects 125 (single Bengtsson & Baumann Ofstad
71% Cl Alburnus alburnus concentration) (1982)
C10-12;58% Cl Sheepshead minnow, 32 days Significantly reduced 279.7 Hill & Maddock (1983)
Cyprinodon variegatus size of larvae (LOEC)
C10-12;58% Cl Rainbow trout 60 days LC50 340 Madeley & Maddock (1983c)
Oncorhynchus mykiss
60 days Behavioural abnormalities 33
168 days after 60-70 days of Madeley & Maddock (1983b)
depuration
50% mortality 3.1
100% mortality 14.3
C14-17;50% Cl Bleak, 14 days No observed effect 125 (single Bengtsson et al. (1979)
Alburnus alburnus concentration)
C14-17;52% Cl Rainbow trout, 60 days No observed effect 1050 Madeley & Maddock (1983c)
Oncorhynchus mykiss
C18-26;49% Cl Bleak, 14 days No observed effect 125 (single Bengtsson et al. (1979)
Alburnus alburnus concentration)
C20-30;43%, 70% Cl Rainbow trout, 60 days No observed effect 3800 Madeley & Maddock (1983c)
Oncorhynchus mykiss
In a 96-h study on rainbow trout (Oncorhynchus mykiss), no
toxic effects or effects on behaviour were observed after exposure
of the fish to an emulsion containing a mean concentration of
770 000 µg/litre of Cereclor 42 (C20-30;42% Cl, CP-LL) (Madeley
& Birtley, 1980).
In bleak (Alburnus alburnus) which were exposed to Witaclor 149
(C10-13;49% Cl, CP-SL), Witaclor 159 (C10-13;59% Cl, CP-SH) and Witaclor
171P (C10-13;71% Cl, CP-SH) (125 µg/litre of water) for 14 days, some
effects on behaviour, such as sluggish movements, absence of shoaling
behaviour and abnormal vertical postures were observed after 7 days
(Bengtsson et al., 1979). The effects disappeared after the fish had
been kept in clean water for 2 days. No effects on behaviour were
observed after exposure to chlorinated paraffins with intermediate or
long chain length chlorinated paraffins, Witaclor 350 (C14-17;50% Cl,
CP-MH) or Witaclor 549 (C18-26;49% Cl, CP-LL), suggesting that
behavioural toxicity is related to the carbon chain length of the
chlorinated paraffin. When bleak were given food contaminated with
590, 2500 or 5800 mg/kg Witaclor 149 or 3180 mg/kg Witaclor 171P for
91 days, effects on behaviour were noted (Bengtsson & Baumann Ofstad,
1982). After 5 weeks of exposure to the high dose of Witaclor 149,
after 7 weeks of exposure to the medium dose and after 12 weeks of
exposure to Witaclor 171P, the fish swam sluggishly and closer to the
bottom than usual. In addition, folded dorsal fins and minor balance
problems were observed. These effects gradually disappeared within 2
weeks after exposure finished.
Flounders (Platichthys flesus) of both sexes were fed Witachlor
149 (C12;49% Cl, CP-SL) or Chlorparaffin Hüls 70C (C12;70% Cl, CP-SH)
on days 1 and 4 with a total exposure of 1000 mg/kg body weight (Haux
et al., 1982). The experiment was performed in both brackish and
seawater. The fish were examined 13 and 27 days after the first
administration. The male flounders did not appear to be affected by
the two chlorinated paraffins. C12;70% Cl did not induce any
haematological responses, whereas C12;49% Cl seemed to affect the
erythrocyte balance of female fish. C12;49% Cl resulted in
hypoglycaemia in marine female fish, whereas C12;70% Cl caused
hyperglycaemia in female brackish water fish. In females exposed to
C12;49% Cl, a significant increase in benzo [a]pyrene hydroxylase
activity was observed after 27 days in brackish water. A decrease in
6ß-hydroxylase activity in marine female fish and an increase in 5
alpha,ß-reductase activity in brackish water female fish were induced
by C12;70% Cl after 13 days.
The hatchability of embryos and survival of larvae of the
sheepshead minnow (Cyprinodon variegatus) was unaffected by a 28-day
exposure to short chain length paraffin with 58% Cl (CP-SH) (2.4, 4.1,
6.4, 22.1 and 54.8 µg/litre (Hill & Maddock, 1983). The treated
minnows showed an increased larval growth compared to the acetone
control. When the larvae were exposed to 36.2, 71, 161.8, 279.7 and
620.5 µg/litre for 32 days they were significantly smaller in the two
highest exposure groups, but in the lower exposure groups (36.2 and
71 µg/litre) they were significantly larger than the controls. No
effect was seen on survival of larvae and hatchability of embryos.
In a study by Madeley & Maddock (1983c) four chlorinated paraffins
were examined for their toxicity to rainbow trout (Oncorhynchus mykiss)
after exposure for 60 days. C10-12;58% Cl was used at mean
concentration of 33, 100, 350, 1070 and 3050 µg/litre. Significant
mortality was observed with the highest three concentrations. LT50
values (mean lethal times) for these three concentrations were
calculated as 44.7, 31.0 and 30.4 days, respectively. The calculated
60-day LC50 was 340 µg/litre. Behavioural abnormalities, which
were dose-related, were also observed.
Rainbow trout (Oncorhynchus mykiss) were exposed to 3.1 and
14.3 µg/litre of C10-12;58% Cl (CP-SH) for 168 days followed by a
depuration period of up to 105 days. No deaths occurred during the
exposure period, but during days 63 to 70 of the depuration period
all of the trout exposed to 14.3 µg/litre died and there was a
significantly increased mortality (50%) among those exposed to
3.1 µg/litre (Madeley & Maddock, 1983b). The relationship to
chlorinated paraffin exposure is unknown, since the surviving fish
recovered after day 70.
Rainbow trout (Oncorhynchus mykiss) were exposed to a short
chain length paraffin (58% Cl) (CP-SH) for 168 days (Madeley &
Maddock, 1983a) at 3.4 and 17.2 µg/litre. The treatment did not cause
any significant mortality or differences in growth, but small changes
in behaviour such as increased food intake were observed (compared to
controls).
Madeley & Maddock (1983c) exposed rainbow trout (Oncorhynchus
mykiss) to 4500 and 1050 µg/litre of a chlorinated paraffin (C14-17;
52% Cl, CP-MH) for 60 days. No toxic effects were found.
In other studies rainbow trout (Oncorhynchus mykiss) were
exposed to C22-26;43% Cl (CP-LL) for 60 days at concentrations of 900
and 4000 µg/litre (Madeley & Maddock, 1983c) or to C20-30;70% Cl
(CP-LH) at 3800, 1900 and 840 µg/litre (Madely & Maddock, 1983d). No
toxic effects were found.
9.1.3 Terrestrial organisms
A one-generation reproduction study was performed with Chlorowax
500C (C10-13;58% Cl, CP-SH) on mallard ducks (Anas platyrhynchos)
(Shults et al., 1984). The ducks were fed 0, 28, 166 and 1000 mg/kg
diet for 22 weeks in groups of 20 pairs. During the treatment the
ducks were mated. The treatment had no effect on the survival,
physical condition, body weight or food consumption of the adult
ducks. Decreased egg-shell thickness and a 10% loss of 14-day embryo
viability were observed in the highest exposure group.
The acute toxicity of orally administered Cereclor S52 (C14-17;52%
Cl, CP-MH) was studied in ring-necked pheasants (Phasianus
colchicus) and mallard ducks (Anas platyrhynchos) (Madeley &
Birtley, 1980). However, no abnormal clinical signs, mortality or
effects on body weight gain were observed 14 days after a single oral
dose by gavage of up to 24 606 mg/kg body weight (pheasant) and
10 280 mg/kg body weight (duck) (five male and five female birds per
group). When the birds were fed with 1000 or 24 063 mg/kg diet of
Cereclor S52 for 5 days followed by 3 days on normal food (five males
and five females per group), no abnormalities were found other than
inferior food intake in ducks from the high-dose group.
The toxicity of Cereclor 42 (C22-26;42% Cl, CP-LL), Cereclor 50LV
(C10-13;49% Cl, CP-SL) and Cereclor 70L (C10-13;70% Cl, CP-SH) in chick
embryos was studied by Brunström (1983). The chlorinated paraffins
were injected into the yolks of eggs incubated for 4 days at
concentrations of 100 and 200 mg/kg egg (based on mean egg weights
before incubation). None of the three mixtures affected the hatching
rate, incubation time, hatching weight, weight gain after hatching or
the liver weights of the chicks.
In an extension of this study the eggs were injected with
300 mg/kg egg weight of the same Cereclors after 4 days of incubation
(Brunström, 1985). An increased liver weight was observed after
treatment with C10-13;49% Cl and C10-13;70% Cl. The microsomal
concentration of cytochrome P-450 in chick embryos was increased by
all three Cereclors. The highest concentration was observed with
C10-13;70% Cl. This Cereclor was also found to increase the microsomal
activity of APMD. The other short-chain chlorinated paraffin,
C10-13;49% Cl, produced decreased activities of aryl hydrocarbon
hydroxylase (AHH) and 7-ethoxycoumarin O-deethylase (ECOD). The
long chain length chlorinated paraffin in this study, C22-26;42% Cl,
caused decreased activities of APDM and ECOD. The greatest effects
were observed after treatment with the most highly chlorinated
short-chain paraffin, C10-13;70% Cl.
9.2 Field observations
No data concerning the effects of chlorinated paraffins in the
field have been reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
Information on occupational exposure to chlorinated paraffins is
very limited. The principal route of exposure is likely to be dermal,
particularly during their use as metal-working fluids. There is also
potential for the formation of inhalable aerosols during this use,
though available information is inadequate to assess exposure via this
route.
Owing to the high octanol-water partition coefficient, it is
likely that the principal source of exposure of the general population
is food, although, owing to lack of data, exposure via other routes
cannot be ruled out. It should be noted, however, that the
composition of chlorinated paraffins to which the population is
exposed in the general environment may be considerably different from
that of the commercial products, although it is not possible currently
to distinguish the chemical composition of chlorinated paraffins with
available methods of analysis. In the only survey of foodstuffs
identified, which was limited in scope, the highest concentration of
chlorinated paraffins (average level of C10-20: 300 µg/kg) was present
in dairy products (Table 15). If the daily consumption of dairy
products is assumed to be 1 kg per person, the daily intake of short
and intermediate chain length chlorinated paraffins from this source
would be 300 µg (4.3 µg/kg body weight, assuming an average body
weight of 70 kg). This is likely to be a worst-case estimate based on
the lack of specificity of the analytical method.
Exposure to chlorinated paraffins via food is also possible
through the consumption of contaminated mussels. Assuming a weekly
consumption of 1 kg of mussels, as a worst case, this would correspond
to a weekly intake of short and intermediate chain length chlorinated
paraffins of 3250 µg, based on chlorinated paraffin levels found in
mussels collected at various sites in the United Kingdom (Table 13)
(6.7 µg/kg body weight per day, assuming an average body weight of
70 kg). If the mussels are collected in highly contaminated water
(section 5.1.4) the weekly intake would be 12 000 µg, which
corresponds to 25 µg/kg body weight per day. The analytical method
used was non-specific.
10.1.2 Toxic effects
In spite of the widespread use of the chlorinated paraffins,
there have been no case reports of skin irritation or sensitization
in humans. Results from a limited number of studies on volunteers
show that chlorinated paraffins can induce minimal irritancy in
the skin but not sensitization. No other data concerning effects of
chlorinated paraffins on humans have been reported.
The acute oral toxicity of chlorinated paraffins of various chain
lengths has been well studied in experimental animals and is low.
Toxic effects such as muscular incoordination and piloerection
were most evident following single exposure to short chain length
chlorinated paraffins. On the basis of very limited data, the acute
toxicity by the inhalation and dermal routes also appears to be low.
Mild skin and eye irritation have been observed after application of
short and intermediate (skin irritation) chain length chlorinated
paraffins. Results of several studies indicate that short chain
chlorinated paraffins do not induce skin sensitization.
In repeated dose toxicity studies by the oral route, the liver,
kidney and thyroid have been shown to be the primary target organs for
the toxicity of chlorinated paraffins (Table 32). For the short chain
compounds, increases in liver and kidney weight and hypertrophy of the
liver and thyroid have been observed at lowest doses (LOEL = 100 mg/kg
body weight per day; NOEL = 10 mg/kg body weight per day; rats).
For the intermediate chain compounds, effects observed at lowest
doses are generally increases in liver and kidney weight (LOEL in rats
= 100 mg/kg body weight per day, respectively; NOAEL in rats =
10 mg/kg body weight per day). Increases in serum cholesterol and
"mild, adaptive" histological changes in the thyroid have been reported
at similar doses in female rats (NOAEL = 4 mg/kg body weight per day).
For the long chain compounds, effects observed at lowest doses
are multifocal granulomatous hepatitis and increased liver weight in
female rats (LOAEL = 100 mg/kg body weight per day).
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells, there is a suggestion of a
weak in vitro clastogenic potential but not in several in vivo
studies. Chlorinated paraffins are also reported to induce in vitro
cell transformation.
Table 32. Effect levels (non-carcinogenic) in repeated dose toxicity tests
Short chain length chlorinated paraffins
Rats
C10-13;58% Cl, 14-day dietary study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in relative liver weights and activities of
hepatic APDM and cytochrome P-450)
C10-13;58% Cl, 14-day gavage study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in relative liver weights);
NOEL = 30 mg/kg body weight per day
C10-13;58% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 100 mg/kg body weight per day (increase in liver/body weight ratio);
NOEL = 50 mg/kg body weight per day
C10-13;56% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 50 mg/kg body weight per day (increase in liver/body weight ratio);
NOEL = 10 mg/kg body weight per day
C12;58% Cl, 90-day gavage study in Fischer-344 rats
LOEL = 313 mg/kg body weight per day (increase in relative liver weight, hepatic
peroxisomal ß-oxidation and thyroxine-UdPG-glucuronosyltransferase; thyroid follicular
cell hypertrophy and hyperplasia and increase in replicative DNA synthesis; renal tubular
eosinophilia and increase in renal replicative DNA synthesis)
No effects in Dunkin Hartley guinea-pigs
NOEL = 1000 mg/kg body weight per day
C10-13;58% Cl, 13-week gavage study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in liver and kidney weights and hypertrophy
of liver and thyroid);
NOEL = 10 mg/kg body weight per day
C12;60% Cl, 90-day gavage study in Fischer-344 rats,
LOEL = 313 mg/kg body weight per day (increase in liver weight)
C12;60% Cl, 2-year gavage study in Fischer-344 rats,
LOAEL = 312 mg/kg body weight per day (increase in liver and kidney weights, hepatic
hypertrophy, necrosis and angiectasis, nephropathy (females), erosion, inflammation and
ulceration of the glandular stomach, hyperplasia of the parathyroid)
Table 32. (Cont'd)
Short chain length chlorinated paraffins (cont'd)
Mice
C10-13;58% Cl, 14-day gavage study in Alpk:APfCD mice,
LOEL = 250 mg/kg body weight per day (induction of peroxisomal fatty acid ß-oxidation and
increase in liver weight);
NOEL = 100 mg/kg body weight per day
C10-13;56% Cl, 14-day gavage study in Alpk:APfCD mice
LOEL = 100 mg/kg body weight per day (increase in liver weight);
NOEL = 50 mg/kg body weight per day
C12;60% Cl, 90-day gavage study in B6C3F1 mice,
LOEL = 250 mg/kg body weight per day (hepatocellular hypertrophy);
NOEL = 125 mg/kg body weight per day
C12;60% Cl, 2-year day gavage study in B6C3F1 mice,
LOEL = 125 mg/kg body weight per day (decrease in body weight gain (females))
Intermediate chain length chlorinated paraffins
Rats
C14-17;52% Cl, 14-day dietary study in Fischer-344 rats,
LOEL = 177 mg/kg body weight per day (slight increase in cytochrome P-450);
NOEL = 57.7 mg/kg body weight per day
C14-17;40% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 10 mg/kg body weight per day (increase in liver weight; not dose-related)
C14-17;52% Cl, 13-week study in Sprague-Dawley rats,
NOAEL (females) = 4.2 mg/kg body weight per day (increase in serum cholesterol; "mild,
adaptive" histopathological changes in the thyroid - reduced follicle sizes and collapsed
angularity; increased height, cytoplasmic vacuolation and nuclear vesiculation);
NOEL = 0.4 mg/kg body weight per day
C14-17;52% Cl, 90-day dietary study in Wistar rats,
LOEL (females) = 25 mg/kg body weight per day (increases in liver and kidney weights and
proliferation of smooth endoplastic reticulum);
NOEL = 12.5 mg/kg body weight per day
C14-17;52% Cl, 90-day dietary study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increased liver and kidney weights);
NOEL = 10 mg/kg body weight per day
Table 32. (Cont'd)
Intermediate chain length chlorinated paraffins (cont'd)
Mice
C14-17;40% Cl, 14-day gavage study in Alpk:APfCD-1 mice,
LOEL = 500 mg/kg body weight (induction of peroxisomal fatty acid ß-oxidation);
NOEL = 250 mg/kg body weight per day
Dogs
C14-17;52% Cl, 90-day dietary study in Beagle dogs,
LOEL (males) = 30 mg/kg body weight per day (increased liver and kidney weights)
NOEL = 10 mg/kg body weight per day (increased smooth endoplasmic reticulum)
Long chain length chlorinated paraffins
Rats
C22-26;70% Cl, 14-day dietary study in Fischer-344 rats,
NOEL = 1715 mg/kg body weight per day (no effects at any dose level)
C22-26;43% Cl, 14-day dietary study in Charles River rats,
NOEL = 3000 mg/kg body weight per day (no effects at any dose; observation of
nephrolithiasis in females exposed to 3000 mg/kg body weight per day)
C23;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL (females) = 235 mg/kg body weight per day (granulomatous inflammation in the liver)
C20-30;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL (females) = 100 mg/kg body weight per day (multifocal granulomatous hepatitis and
increased liver weight)
C22-26;70% CL, 90-day dietary study in Fischer-344 rats,
LOAEL = 3750 mg/kg body weight per day (increased liver weight, hepatocellular hypertrophy
and cytoplasmic fat vacuolation; slight increase of chronic nephritis (males); increase in
ALT and AST (females));
NOEL = 900 mg/kg body weight per day
C23;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL = 100 mg/kg body weight per day (increase in relative liver weights; increased
activities of serum enzymes, variations in haematological parameters (in females); diffuse
lymphohistiocytic inflammation in the livers, pancreatic and mesenteric lymph nodes)
Table 32. (Cont'd)
Long chain length chlorinated paraffins (cont'd)
Mice
C23;43% Cl, 90-day gavage,
NOEL = 7500 mg/kg body weight per day (no effects at any dose)
C23;43% Cl, 90-day gavage,
NOEL = 5000 mg/kg body weight per day (no effects at any dose)
Two-year toxicity and carcinogenicity studies have been conducted
for a short (C12;60% Cl) and a long chain chlorinated paraffin
(C23;43% Cl) in both rats and mice. For the short chain chlorinated
paraffin, the incidences of liver and thyroid tumours were increased
in mice at 125 and 250 mg/kg body weight per day. In rats, the
incidences of liver tumours in both sexes, thyroid tumours in females
and renal cell tumours and leukaemias in males were increased at doses
of 312 and 625 mg/kg body weight per day. For the long chain
chlorinated paraffin, the incidences of malignant lymphomas in male
mice (2500-5000 mg/kg body weight per day) and phaeochromocytomas in
female rats (900 mg/kg body weight per day) were increased. On the
basis of available data, therefore, the short chain chlorinated
paraffin was carcinogenic in rats and mice. No data are available on
intermediate chlorinated paraffins. For the long chain chlorinated
paraffin, the evidence of carcinogenicity is limited, increased
incidences of commonly occurring tumours having been observed at only
one site in one sex of each species.
Some possible mechanisms for the induction of tumours of the
thyroid, liver and kidney have been suggested. On the basis of 14-day
mouse, rat and guinea-pig studies (Wyatt et al., 1993; Elcombe et al.,
in press), with similar short chain and long chain chlorinated
paraffins as used in the carcinogenesis bioassays, it was suggested
that the liver tumours in mice and rats could be correlated with the
degree of peroxisomal proliferation which occurs in the liver of these
species at similar dose levels (250 mg/kg body weight per day). No
peroxisome proliferation was observed in the livers of guinea-pigs
even at doses up to 1 to 2 g/kg body weight per day, although a
similar increase in liver weight to that seen in mice and rats was
observed. No study on peroxisome proliferation in human hepatoctyes
treated with a short chain chlorinated paraffin is available.
Increased DNA synthesis has been demonstrated in hepatocytes and
thyroid follicular cells in rats of both sexes and in proximal kidney
tubular cells in males. Perturbation of thyroid homeostasis and
increased TSH secretion has been observed in the thyroid of rats.
These effects may be related to the development of tumours.
Taking into consideration the available data, it appears that
chlorinated paraffins do not mediate carcinogenic effects via direct
interaction with DNA.
In a reproduction study, no adverse reproductive effects were
reported following exposure of rats to an intermediate chain length
chlorinated paraffin with 52% chlorine. However, survival and body
weight of the exposed pups were reduced (LOEL for non-significant
decrease in body weight = 5.7 to 7.2 mg/kg body weight per day; LOAEL
for decreased survival = 58.7 to 70 mg/kg body weight per day). In a
limited number of studies on the developmental effects of the short,
medium and long chain chlorinated paraffins, adverse effects in
offspring were observed, for the short chain compounds only, at
maternally toxic doses in rats.
10.1.3 Risk evaluation
Available data indicate that absorption of chlorinated paraffins
through the skin (the likely principal route of exposure in the
occupational environment) is minimal. Provided that proper personal
hygiene and safety procedures are followed, the risk to the health of
workers exposed to chlorinated paraffins is expected to be minimal.
10.1.3.1 Short chain compounds
On the basis of available data on repeated dose toxicity, a
Tolerable Daily Intake (TDI) for non-neoplastic effects of short chain
chlorinated paraffins for the general population can be developed:
10 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
100
where 10 mg/kg body weight per day is the lowest reported
no-observed-effect level (increases in liver and kidney weights
and hypertrophy of the liver and thyroid at the next highest dose
in a 13-week study on rats) (IRDC, 1984a); and 100 is the
uncertainty factor (× 10 for interspecies variation; × 10 for
intraspecies variation).
On the basis of multistage modelling of the tumours with highest
incidence (hepatocellular adenomas or carcinomas (combined) in male
mice) in the carcinogenesis bioassay with short chain chlorinated
paraffins, the estimated dose associated with a 5% increase in tumour
incidence is 11 mg/kg body weight per day (amortized for period of
administration). After dividing this value by 1000 (uncertainty
factor for a non-genotoxic carcinogen), it can be recommended that
daily doses of short chain chlorinated paraffins for the general
population should not exceed 11 µg/kg body weight, on the basis of
neoplastic effects.
10.1.3.2 Intermediate chain compounds
On the basis of available data on repeated dose toxicity, a TDI
for non-neoplastic effects of intermediate chain chlorinated paraffins
can be developed:
10 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
100
where 10 mg/kg body weight per day is the no-observed-adverse-effect
level in both sexes (increases in liver and kidney weights at the next
highest dose) (IRDC, 1984b); increases in serum cholesterol and "mild,
adaptive" histological changes in the thyroid have been reported at
similar doses in female rats (NOAEL = 4 mg/kg body weight per day)
(Poon et al., in press); and 100 is the uncertainty factor (× 10 for
interspecies variation; × 10 for intraspecies variation).
10.1.3.3 Long chain compounds
On the basis of available data on repeated dose toxicity, a TDI
for non-neoplastic effects of long chain chlorinated paraffins can be
developed:
100 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
1000
where 100 mg/kg = the lowest-observed-adverse-effect levels in
long-term studies (effects at this dose were multifocal granulomatous
hepatitis and increased liver weight in female rats (NTP, 1986b;
Serrone et al., 1987; Bucher et al., 1987); and 1000 = uncertainty
factor (× 10 for intraspecies variation, × 10 for interspecies
variation, × 10 for LOAEL rather than NOEL).
In general, the calculated daily intake of chlorinated paraffins
based on highly unlikely worst-case scenarios are below the TDIs for
non-neoplastic effects or recommended values for neoplastic effects
(short chain compounds) developed above.
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
Data on chlorinated paraffin levels in the environment are
limited, but the studies reported indicate widespread contamination,
although the highest levels are found close to industries that
manufacture or use chlorinated paraffins.
Chlorinated paraffins bioaccumulate in aquatic organisms, and
bioconcentration factors (BCFs) in the range of 7 to 7155 for fish and
223 to 138 000 for mussels have been reported. In fish, chlorinated
paraffins of short chain length are accumulated to a higher degree
than those of intermediate and long chain length.
In the environment, chlorinated paraffins are persistent.
However, short-chain chlorinated paraffins with a low chlorine content
appear to be degraded by acclimated microorganisms.
10.2.2 Toxic effects
Short chain length chlorinated paraffins are acutely toxic to
freshwater and saltwater invertebrates at LC/EC50 concentrations
ranging from 14 to 530 µg/litre. The acute toxicity of short chain
chlorinated paraffins to fish is low. In long-term studies, the
lowest-observed-effect concentrations for algae, daphnids and fish
ranged from 3 to 20 µg/litre; NOECs appear to range from 2 to
5 µg/litre for the most sensitive species tested. The acute and
long-term toxicity of intermediate and long chain length chlorinated
paraffins to fish appears to be low. However, in daphnids, chronic
effects of an intermediate and a long-chain product have been observed
at similar concentrations to those reported for short chain compounds.
No studies on plants or terrestrial invertebrates have been
reported. The acute toxicity to birds is low. Data from studies on
laboratory mammals suggest a low risk for terrestrial mammals.
10.2.3 Risk evaluation
The evaluation of the environmental risks of chlorinated
paraffins is complicated by the limited quality and quantity of
information regarding environmental levels. Available data indicate
that chlorinated paraffins are bioaccumulative and persistent.
The data on environmental levels of short-chain chlorinated
paraffins indicate that in areas local to release sources, there is a
risk to both freshwater and estuarine organisms. Recent data indicate
that there is also a potential risk to aquatic invertebrates from
intermediate and long chain chlorinated paraffin products.
The enrichment of chlorinated paraffins in sediments, their
resorption behaviour and aquatic toxicity indicate a potential risk
for sediment-dwelling organisms.
The data regarding chlorinated paraffins in the terrestrial
environment are insufficient to estimate the risk to soil-dwelling
organisms.
11. RECOMMENDATIONS FOR PROTECTION OF THE ENVIRONMENT
Since chlorinated paraffins are bioaccumulative and toxic to
environmental organisms and owing to difficulties in monitoring
environmental levels, it is recommended that use and disposal of these
compounds should be controlled to avoid release to the environment.
12. FUTURE RESEARCH
The following studies need to be undertaken:
a) development of more selective and sensitive methods of analysis
in order to provide more reliable data on present and future
levels of chlorinated paraffins in the occupational environment,
soil, water, sediments, foodstuffs and human tissues;
b) further investigation of the influence of the chain lengths and
degrees of chlorination on toxicodynamics and toxicokinetics of
chlorinated paraffins, with particular regard to the relative
extent and rate of absorption and excretion through different
routes; studies to ascertain the metabolic pathways of
chlorinated paraffins should also be performed;
c) investigation of the toxicokinetics and half-lives of chlorinated
paraffins in mammals;
d) studies on perinatal toxicity;
e) further studies to examine effects on sediment-dwelling
organisms.
13. PREVIOUS EVALUATION BY INTERNATIONAL ORGANIZATIONS
Chlorinated paraffins have been evaluated by the International
Agency for Research on Cancer (IARC, 1990). It was concluded that
there is sufficient evidence for the carcinogenicity of a commercial
chlorinated paraffin product of average carbon chain length C12 and
average degree of chlorination of 60% in experimental animals, and
limited evidence for the carcinogenicity of a commercial chlorinated
paraffin product of average carbon chain length C23 and average
degree of chlorination of 43% in experimental animals. The overall
evaluation was that chlorinated paraffins of average carbon chain
length C12 and average degree of chlorination of approximately 60%
are possibly carcinogenic to humans (Group 2B).
REFERENCES
Åhlman M, Bergman Å, Darnerud PO, Egestad B, & Sjövall J (1986)
Chlorinated paraffins: formation of sulphur-containing metabolites of
polychlorohexadecane in rats. Xenobiotica, 16: 225-232.
Ahotupa M, Hietanen E, Mantyla E, & Vainio H (1982) Effects of
polychlorinated paraffins on hepatic, renal and intestinal
biotransformation rates in comparison to the effects of poly-
chlorinated biphenyls and naphthalenes. J Appl Toxicol, 2: 47-53.
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL, &
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environ Mol Mutagen, 16(Suppl 18):
55-137.
Ashby J, Lefevre PA, & Elcombe CR (1990) Cell replication and
unscheduled DNA synthesis (UDS) activity of low molecular weight
chlorinated paraffins in the rat liver in vivo. Mutagenesis,
5: 515-518.
Bengtsson BE & Baumann Ofstad E (1982) Long-term studies on uptake and
elimination of some chlorinated paraffins in the bleak, Alburnus
alburnus. Ambio, 11: 38-40.
Bengtsson BE, Svanberg O, Lindén E, Lunde G, & Baumann Ofstad E (1979)
Structure related uptake of chlorinated paraffins in bleaks ( Alburnus
alburnus L). Ambio, 8: 121-122.
Bergman Å, Hagman A, Jacobsson S, Jansson B, & Åhlman M (1984) Thermal
degradation of polychlorinated alkanes. Chemosphere, 13: 237-250.
Biessmann A, Brandt I, & Darnerud PO (1982) Comparative distribution
and metabolism of two carbon-14 labeled chlorinated paraffins in
Japanese quail (Coturnix coturnix japonica). Environ Pollut,
A28: 109-120.
Biessmann A, Darnerud PO, & Brandt I (1983) Chlorinated paraffins:
disposition of a highly chlorinated polychlorohexadecane in mice and
quail. Arch Toxicol, 53: 79-86.
Birtley RDN, Conning DM, Daniel JW, Ferguson DM, Longstaff E, & Swan
AAB (1980) The toxicological effects of chlorinated paraffins in
mammals. Toxicol Appl Pharmacol, 54: 514-525.
Brunström B (1983) Toxicity in chick embryos of three commercial
mixtures of chlorinated paraffins and of toxaphene injected into eggs.
Arch Toxicol, 54: 353-357.
Brunström B (1985) Effects of chlorinated paraffins on liver weight,
cytochrome P-450 concentration and microsomal enzyme activities in
chick embryos. Arch Toxicol, 57: 69-71.
BUA (German Chemical Society Advisory Committee on Existing Chemicals
of Environmental Relevance) (1992) [Chlorinated paraffins.] Weinheim,
VCH Verlagsgesellschaft, 227 pp (BUA Report 93) (in German).
Bucher JR, Alison RH, Montgomery CA, Huff J, Haseman JK, Farnell
D, Thompson R, & Prejean JD (1987) Comparative toxicity and
carcinogenicity of two chlorinated paraffins in F344/N rats and
B6C3F1 mice. Fundam Appl Toxicol, 9: 454-468.
Camford Information Services (1991) CPI product profiles: chlorinated
paraffins. Don Mills, Ontario, Camford Information Services, 3 pp.
Campbell I & McConnell G (1980) Chlorinated paraffins and the
environment. 1. Environmental occurrence. Environ Sci Technol,
14: 1209-1214.
Conz A & Fumero S (1988a) Study of the capacity of Solvocaffaro C1642
to induce gene mutations in strains of Salmonella typhimurium.
Turin, Istituto di Recerche Biomediche "Antoine Marxer", 37 pp (RBM
Exp. No. 880367).
Conz A & Fumero S (1988b) Micronucleus induction in bone marrow cells
of mice treated by oral route with the test article Solvocaffaro
C1642. Turin, Istituto di Recerche Biomediche "Antoine Marxer", 20 pp
(RBM Exp. No. 880368).
Cooke M & Roberts DJ (1980) Carbon skeleton capillary gas
chromatography. J Chromatogr, 193: 437-443.
Darnerud PO (1984) Chlorinated paraffins: Effect of some microsomal
enzyme inducers and inhibitors of the degradation of 1-14C-chloro-
dodecanes to 14CO2 in mice. Acta Pharmacol Toxicol,
55: 110-115.
Darnerud PO & Brandt I (1982) Studies on the distribution and
metabolism of a 14C-labelled chlorinated alkane in mice. Environ
Pollut, A27: 45-56.
Darnerud PO & Lundkvist U (1987) Studies on implantation and embryonic
development in mice given a highly chlorinated hexadecane. Pharmacol
Toxicol, 60: 239-240.
Darnerud PO, Biessmann A, & Brandt I (1982) Metabolic fate of
chlorinated paraffins: degree of chlorination of [1-14C]-chloro-
dodecanes in relation to degradation and excretion in mice. Arch
Toxicol, 50: 217-226.
Darnerud PO, Bengtsson BE, Bergman A, & Brandt I (1983) Chlorinated
paraffins: disposition of a polychloro-[1-14C]-hexadecane in carp
(Cyprinus carpio) and bleak (Alburnus alburnus). Toxicol Lett,
19: 345-351.
Darnerud PO, Bergman Å, Lund BO, & Brandt I (1989) Selective
accumulation of chlorinated paraffins (C12 and C16) in the olfactory
organ of rainbow trout. Chemosphere, 18: 1821-1827.
EG & G Bionomics (1983) The acute and chronic toxicity of a chlorinated
paraffin (58% chlorination of short chain length n-paraffins) to
midges (Chironomus tentans). Wareham, Massachusetts, EG & G Bionomics,
39 pp (Report No. BW 83-6-1426).
Elcombe CR, Watson SC, Wyatt I, & Foster JR (1994) Chlorinated
paraffins (CP): Mechanisms of carcinogenesis. Toxicologist, 14: 276.
Elcombe CR, Bars RG, Watson SC, & Foster JR (in press) Hepatic effects
of chlorinated paraffins in mice, rats and guinea pigs: Species
differences and implications for hepatocarcinogenicity. Xenobiotica.
Elliott BM (1989a) Meflex DC029 (fully formulated) - Ames test.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 1 p (Report
No. CTL/L/2668).
Elliott BM (1989b) Meflex DC029 (fully formulated) - Mouse
micronucleus test. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 1 p (Report No. CTL/L/2693).
English JSC, Foulds I, White IR, & Rycroft JG (1986) Allergic contact
sensitization to the glycidyl ester of hexahydrophthalic acid in a
cutting oil. Contact Dermatitis, 15: 66-69.
Environment Agency, Japan (1981) Environmental monitoring of chemicals:
Annual report. Tokyo, Environment Agency, p 23.
Environment Agency, Japan (1983) Environmental monitoring of chemicals:
Annual report. Tokyo, Environment Agency, p 75.
Eriksson P & Darnerud PO (1985) Distribution and retention of some
chlorinated hydrocarbons and a phthalate in the mouse brain during the
pre-weaning period. Toxicology, 37: 189-203.
Eriksson P & Kihlström J (1985) Disturbance of motor performance and
thermoregulation in mice given two commercial chlorinated paraffins.
Bull Environ Contam Toxicol, 34: 205-209.
Eriksson P & Nordberg A (1986) The effects of DDT, DDOH-palmitic
acid, and a chlorinated paraffin on muscarinic receptors and the
sodium-dependent choline uptake in the central nervous system of
immature mice. Toxicol Appl Pharmacol, 85: 121-127.
Frank U & Steinhäuser KG (in press) [Ecotoxicity of poorly soluble
substances tested by Daphnia toxicity of chlorinated paraffins.] Vom
Wasser, 83 (in German).
Friedman D & Lombardo P (1975) Photochemical technique for the
elimination of chlorinated aromatic interferences in the gas-liquid
chromatographic analysis for chlorinated paraffins. J Assoc Off Anal
Chem, 58: 703-706.
Gjos N & Gustavsen K (1982) Determination of chlorinated paraffins by
negative ion chemical ionization mass spectrometry. Anal Chem,
54: 1316-1318.
Hansen J, Schneider T, Olsen JH, & Laursen B (1992) Availability of
data on humans potentially exposed to suspected carcinogens in the
Danish working environment. Pharmacol Toxicol, 72(Suppl 1): S77-S85.
Hardie DWF (1964) Chlorocarbons and chlorohydrocarbons: Chlorinated
paraffins. In: Kirk-Othmer encyclopedia of chemical technology. New
York, John Wiley and Sons, vol 5, pp 231-240.
Haux C, Larsson Å, Lidman U, Förlin L, Hansson T, & Johansson-Sjöbeck
M-L (1982) Sublethal physiological effects of chlorinated paraffins on
the flounder, Platichtys flesus L. Ecotoxicol Environ Saf, 6: 49-59.
Hill RW & Maddock BG (1983) Effect of a chlorinated paraffin (58%
chlorination of short chain length n-paraffins) on embryos and
larvae of the sheepshead minnow (Cyprinodon variegatus)-(study one).
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 57 pp
(Report No. BL/B/2326).
Hoechst (1983a) [Chloroparaffin 70 liquid NV - Acute dermal
irritation/corrosion in rabbits.] Frankfurt/Main, Hoechst AG, 11 pp
(Report No. 83.0444) (in German).
Hoechst (1983b) [Hordalub 80 + TNPP - Magnusson and Kligman's test for
sensitising properties on Pirbright-White Guinea Pigs.] Frankfurt/Main,
Hoechst AG, 17 pp (Report No. 83.0573) (in German).
Hoechst (1984) [Hordalub 80 HT - Magnusson and Kligman's test for
sensitising properties on Pirbright-White Guinea Pigs.] Frankfurt/Main,
Hoechst AG, 18 pp (Report No. 84.0458) (in German).
Hoechst (1986a) Hordalub 80 - Study of the mutagenic potential in
strains of Salmonella typhimurium (Ames Test) and Escherichia coli.
Frankfurt/Main, Hoechst AG, 28 pp (Report No. 86.1078).
Hoechst (1986b) [Acute dermal irritation/corrosion in rabbits.]
Frankfurt/Main, Hoechst AG, 12 pp (Report No. 86.1105) (in German).
Hoechst (1987) Chloroparaffin 56 liquid - Detection of gene mutations
in somatic cells in culture. HGPRT-test with V79 cells. Frankfurt/Main,
Hoechst AG, 23 pp (Report No. 87.1719).
Hoechst (1988) Chloroparaffin 56 liquid (unstabilized) - Study of the
mutagenic potential in strains of Salmonella typhimurium (Ames Test)
and Escherichia coli. Frankfurt/Main, Hoechst AG, 30 pp (Report No.
88.0099).
Hoechst (1989) Chlorowax 500C micronucleus test in male and female
NMRI mice after oral administration. Frankfurt/Main, Hoechst AG, 24 pp
(Report No. 89.0253).
Hollies JI, Pinnington DF, Handley AJ, Baldwin MK, & Bennett D (1979)
The determination of chlorinated long-chain paraffins in water,
sediment and biological samples. Anal Chim Acta, 111: 201-213.
Houghton KL (1993) Chlorinated paraffins. In: In: Kirk-Othmer
encyclopedia of chemical technology. New York, John Wiley and Sons,
vol 6, pp 78-87.
Howard PH, Santodonato J, & Saxena J (1975) Investigations of selected
potential environmental contaminants: chlorinated paraffins. Syracuse,
New York, Syracuse University Research Corporation, 107 pp (Report No.
68-01-3101).
HSE (1992) Chlorinated paraffins. London, Health and Safety Executive,
44 pp (Unpublished document).
IARC (1990) Chlorinated paraffins. In: Some flame retardants and
textile chemicals, and exposures in the textile manufacturing
industry. Lyon, International Agency for Research on Cancer, pp 55-72
(IARC Monographs on the Evaluation of Carcinogenic Risks to Humans,
Volume 48).
ICI (1965) Toxicological report: chlorinated hydrocarbons with added
stabilizers: "Cereclor" P70 and 70L. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 15 pp (Report No. CTL/TR/464).
ICI (1966) Toxicological report: 40% chlorinate of C10-13 normal
paraffin. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
4 pp (Report No. CTL/TR/524).
ICI (1967) Toxicological report: "Cereclor" 50LV (MD.115) - 50%
chlorinate of C10-13 normal paraffin. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 4 pp (Report No. CTL/TR/618).
ICI (1968) Toxicological report: fire-resistant hydraulic fluid
10B/1067. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
7 pp (Report No. CTL/TR/635).
ICI (1969) Toxicological report: skin irritancy and oral toxicity of
chlorinated paraffins. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 11 pp (Report No. CTL/TR/691).
ICI (1971) Toxicological report: fire-resistant hydraulic fluid
45D-3271. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
8 pp (Report No. CTL/T/831).
ICI (1973) "Cereclor" 63L: local irritancy and acute oral toxicity.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 7 pp (Report
No. CTL/T/938).
ICI (1974a) "Cereclors" and "Hordolub": local irritancy and acute
toxicity. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
32 pp (Report No. CTL/T/962).
ICI (1974b) "Cereclor" 50 HS: summary of local irritancy, skin
sensitization and acute toxicity. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 3 pp (Report No. CTL/Z/0548).
ICI (1975a) "Cereclor" 60 HS: primary skin irritation in rabbits.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 3 pp (Report
No. CTL/Z/0813).
ICI (1975b) "Cereclor" 50 HS: primary skin irritation in rabbits.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 3 pp (Report
No. CTL/Z/0812).
ICI (1980) Cloparin 1049 and "Meflex" DC029: a comparison of skin
irritation potential. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 11 pp (Report No. CTL/T/1431).
ICI (1982a) Cell transformation test for potential carcinogenicity of
chlorinated paraffin (58% chlorination of short chain length
n-paraffins). Macclesfield, Cheshire, Imperial Chemical Industries
Ltd.
ICI (1982b) Cell transformation test for potential carcinogenicity
of chlorinated paraffin (70% chlorination of short chain length
n-paraffins). Macclesfield, Cheshire, Imperial Chemical Industries
Ltd.
ICI (1982c) Cloparin 59: skin irritation study. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, 14 pp (Report No.
CTL/T/1737).
Inveresk (1975) Patch testing of textile lubricants. Musselburgh,
Inveresk Research International, 14 pp (Report No. 318).
IRDC (1981a) 14-Day oral toxicity study in rats. Chlorinated paraffin:
58% chlorination of short chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 110 pp
(Report No. 438-006).
IRDC (1981b) 14-Day oral toxicity study in rats. Chlorinated paraffin:
52% chlorination of long chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 104 pp
(Report No. 438-005).
IRDC (1982a) Teratology study in rats. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 100 pp (Report
No. 438-016).
IRDC (1982b) 14-Day oral toxicity study in rats. Chlorinated paraffin:
70% chlorination of long chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 103 pp
(Report No. 438/004).
IRDC (1982c) 14-Day oral (gavage) toxicity study in rats. Chlorinated
paraffin: 43% chlorination of long chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
110 pp (Report No. 438/005).
IRDC (1982d) Teratology study in rabbits. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 95 pp (Report
438-031).
IRDC (1982e) Teratology study in rabbits. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 102 pp (Report
438-030).
IRDC (1983a) Dominant lethal study in rats. Chlorinated paraffin: 58%
chlorination of short chain n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 54 pp (Report
No. 438-011).
IRDC (1983b) Teratology study in rabbits. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 245 pp (Report
No. 438-045).
IRDC (1983c) 14-Day dietary range-finding study in rats. Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
136 pp (Report No. 438-002).
IRDC (1983d) Teratology study in rats. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 197 pp (Report
No. 438-015).
IRDC (1983e) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 70% chlorination of long chain
length paraffins. Mattawan, Michigan, International Research and
Development Corporation, 70 pp (Report No. 438-016).
IRDC (1983f) Teratology study in rabbits. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 195 pp
(Report No. 438-032).
IRDC (1983g) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 52% chlorination of intermediate
chain length n-paraffins. Mattawan, Michigan, International Research
and Development Corporation, 62 pp (Report No. 438-014).
IRDC (1983h) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffins: 58% chlorination of short chain
length n-paraffins. Mattawan, Michigan, International Research and
Development Corporation, 58 pp (Report No. 438-013).
IRDC (1983i) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 43% chlorination of long chain
length n-paraffins. Mattawan, Michigan, International Research and
Development Corporation, 56 pp (Report No. 438-012).
IRDC (1984a) 13-week oral (gavage) toxicity study in rats with
combined excretion, tissue level and elimination studies: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 58% chlorination of short
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 350 pp (Report
No. 438-029/022).
IRDC (1984b) 13-week oral (dietary) toxicity study in rats with
combined excretion, tissue level and elimination studies: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 52% chlorination of
intermediate chain length n-paraffins, 14C labeled CP. Mattawan,
Michigan, International Research and Development Corporation, 328 pp
(Report No. 438-023/026).
IRDC (1984c) 13-week oral (dietary) toxicity study in rats with
combined excretion, tissue level and elimination study: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 58% chlorination of
short chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 435 pp (Report
No. 438-035/022).
IRDC (1984d) Teratology study in rats. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 105 pp
(Report No. 438-017).
IRDC (1984e) Teratology study in rats. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 99 pp (Report
No. 438/045).
IRDC (1984f) 13-week oral (gavage) toxicity study in rats with
combined excretion, tissue level and elimination study: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 43% chlorination of long
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 275 pp (Report
No. 438-028/021).
IRDC (1984g) 13-week dietary toxicity study in rats with combined
excretion, tissue level and elimination studies/determination of
excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 70% chlorination of long
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 316 pp (Report
No. 438-027/024).
IRDC (1985) Reproduction range-finding study in rats. Chlorinated
paraffin: 52% chlorination of intermediate chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
179 pp (Report No. 438-049).
Jansson B, Andersson R, Asplund L, Bergman Å, Litze'n K, Nylund K,
Reuthergårdh L, Sellström U, Uvemo U-B, Wahlberg C, & Wideqvist U
(1991) Multiresidue method for the gas-chromatographic analysis of
some polychlorinated and polybrominated pollutants in biological
samples. Fresenius J Anal Chem, 340: 439-445.
Jansson B, Andersson R, Asplund L, Litze'n K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Environ Toxicol Chem, 12: 1163-1174.
KEMI (1991) Chlorinated paraffins. In: Freij L ed. Risk reduction of
chemicals: A Government Commission Report. Solna, The Swedish National
Chemicals Inspectorate, pp 167-187 (KEMI Report 1/91).
Kraemer W & Ballschmiter K (1987) Detection of a new class of
organochlorine compounds in the marine environment: the chlorinated
paraffins. Fresenius Z Anal Chem, 327: 47-48.
Lindén E, Bengtsson BE, Svanberg O, & Sundström G (1979) The acute
toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
Lombardo P, Dennison JL, & Johnson WW (1975) Bioaccumulation of
chlorinated paraffin residues in fish fed Chlorowax 500C. J Assoc Off
Anal Chem, 58: 707-710.
Lundberg P (1980) Effects of some flame retardants on the liver
microsomal enzyme systems. In: Cohn MJ ed. Microsomes, drug
oxidations, and chemical carcinogenesis, 1979. New York, Academic
Press, pp 853-856.
Lunde G & Steinnes E (1975) Presence of lipid-soluble chlorinated
hydrocarbons in marine oils. Environ Sci Technol, 9: 155-157.
Madeley J & Birtley R (1980) Chlorinated paraffins and the
environment. 2. Aquatic and avian toxicology. Environ Sci Technol,
14: 1215-1221.
Madeley JR & Gillings E (1983) Determination of the solubility of four
chlorinated paraffins in water. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 23 pp (Report No. BL/B/2301).
Madeley JR & Maddock BG (1983a) Effects of a chlorinated paraffin on
the growth of rainbow trout. Chlorinated paraffin: 58% chlorination of
short chain length paraffins. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 67 pp (Report No. BL/B/2309).
Madeley JR & Maddock BG (1983b) The bioconcentration of a chlorinated
paraffin in the tissues and organs of rainbow trout (Salmo gairdneri).
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 39 pp
(Report No. BL/B/2310).
Madeley JR & Maddock BG (1983c) Toxicity of a chlorinated paraffin to
rainbow trout over 60 days. Chlorinated paraffin: 52% chlorination of
intermediate chain length n-paraffins. Brixham, Imperial Chemical
Industries Ltd, Brixham Laboratory, 69 pp (Report No. BL/B/2202).
Madeley JR & Maddock BG (1983d) Toxicity of a chlorinated paraffin to
rainbow trout over 60 days. Chlorinated paraffin: 43% chlorination of
long chain length n-paraffins. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 71 pp (Report No. BL/B/2201).
Madeley JR & Thompson RS (1983a) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 71 pp (Report No.
BL/B/2291).
Madeley JR & Thompson RS (1983b) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Brixham,
Imperial Chemical Industries Ltd, Brixham Laboratory, 53 pp (Report
No. BL/B/2289).
Madeley JR & Thompson RS (1983c) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 59 pp (Report No.
BL/B/2288).
Madeley JR & Thompson RS (1983d) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 64 pp (Report No.
BL/B/2290).
Madeley JR, Thompson RS, & Brown D (1983a) The bioconcentration of a
chlorinated paraffin by common mussel (Mytilus edulis). Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 76 pp
(Report BL/B/2351).
Madeley JR, Windeatt AJ, & Street JR (1983b) Assessment of the
toxicity of a chlorinated paraffin to the anaerobic sludge digestion
product. Chlorinated paraffin: 58% chlorination of short chain length
n-paraffins. Brixham, Imperial Chemical Industries Ltd, Brixham
Laboratory, 25 pp (Report No. BL/B/2253).
Mather JI, Street JR, & Madeley JR (1983) Assessment of the inherent
biodegradability of a chlorinated paraffin, under aerobic conditions,
by a method developed from OECD Test Guideline 302B. Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 56 pp
(Report No. BL/B/2298).
Meijer J & DePierre JW (1987) Hepatic levels of cytosolic, microsomal
and "mitochondrial" epoxide hydrolases and other drug-metabolizing
enzymes after treatment of mice with various xenobiotics and endogenous
compounds. Chem-Biol Interact, 62: 249-269.
Meijer J, Rundgren M, Åström A, DePierre JW, Sundvall A, & Rannug U
(1981) Effects of chlorinated paraffins on some drug-metabolizing
enzymes in rat liver and in the Ames test. Adv Exp Med Biol,
A136: 821-828.
Menter P, Harrison W, & Woodin WG (1975) Patch testing of coolant
fractions. J Occup Med, 17(9): 565-568.
Muller W (1989) Chlorowax 500C - Micronucleus test in male and female
NMRI mice after oral administration. Frankfurt/Main, Hoechst AG,
Pharma Research Toxicology and Pathology, 24 pp (Report No. 89.0253).
Murray T, Frankenberry M, Steele DH, & Heath RG (1988) Chlorinated
paraffins: A report on the findings from two field studies, Sugar
Creek, Ohio, Tinkers Creek, Ohio. Volume 1: Technical report.
Washington, DC, US Environmental Protection Agency, 150 pp (EPA-560/
5-87/012).
Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D,
Martin R, & Caspary WJ (1990) L5178Y mouse lymphoma cell mutation
assay results with 41 compounds. Environ Mol Mutagen, 16(Suppl 18):
138-167.
NIOSH (1990) National occupational exposure survey (1980-1983).
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
Nilsen O & Toftgård R (1981) Effect of polychlorinated terphenyls and
paraffins on rat liver microsomal cytochrome P-450 and in vitro
metabolic activities. Arch Toxicol, 47: 1-11.
Nilsen O, Toftgård R, & Glaumann H (1980) Changes in rat liver
morphology and metabolic activities after exposure to chlorinated
paraffins. Dev Toxicol Environ Sci, 8: 525-528.
Nilsen OG, Toftgård R, & Glaumann H (1981) Effects of chlorinated
paraffins on rat liver microsomal activities and morphology.
Importance of the length and the degree of chlorination of the carbon
chain. Arch Toxicol, 49: 1-13.
NTP (1986a) Toxicology and carcinogenesis studies of chlorinated
paraffins (C12, 60% chlorine) (CAS No. 63449-39-8) in F344/N rats and
B6C3F1 mice (gavage studies). Research Triangle Park, North Carolina,
US Department of Health and Human Services, National Toxicology
Program, 67 pp (Technical Report Series No. 308).
NTP (1986b) Toxicology and carcinogenesis studies of chlorinated
paraffins (C23, 43% chlorine) (CAS No. 63449-39-8) in F344/N rats and
B6C3F1 mice (gavage studies). Research Triangle Park, North Carolina,
US Department of Health and Human Services, National Toxicology
Program, 66 pp (Technical Report Series No. 305).
Omori T, Kimura T, & Kodama T (1987) Bacterial cometabolic degradation
of chlorinated paraffins. Appl Microbiol Biotechnol, 25: 553-557.
Poon R, LeCavalier P, Chan P, Viau C, Håkansson H, Chu I, & Valli VE
(in press) Subchronic toxicity of a medium-chain chlorinated paraffin
in the rat. J Appl Toxicol.
Renberg L, Sundström G, & Sundh-Nygård K (1980) Partition coefficients
of organic chemicals derived from reversed-phase thin-layer
chromatography. Evaluation of methods and application on phosphate
esters, polychlorinated paraffins and some PCB-substitutes.
Chemosphere, 9: 683-691.
Renberg L, Tarkpea M, & Sundström G (1986) The use of the bivalve
Mytilus edulis as a test organism for bioconcentration studies. II.
The bioconcentration of two 14C-labeled chlorinated paraffins.
Ecotoxicol Environ Saf, 11: 361-372.
Richold M, Allen JA, Williams A, & Ransome SJ (1982a) Cell
transformation test for potential carcinogenicity of chlorinated
paraffin (58% chlorination of short chain length n-paraffins).
Huntingdon, Huntingdon Research Centre, 40 pp (Report No. ICI
399C/81468).
Richold M, Allen JA, Williams A, & Ransome SJ (1982b) Cell
transformation test for potential carcinogenicity of chlorinated
paraffin (70% chlorination of long chain length n-paraffins).
Huntingdon, Huntingdon Research Centre, 30 pp (Report No. ICI
399A/415/82313).
Roberts DJ, Cooke M, & Nickless G (1981) Determination of poly-
chlorinated alkanes via carbon skeleton capillary gas chromatography.
J Chromatogr, 213: 73-81.
Schenker BA (1979) Chlorinated paraffins. In: Mark HF, Othmer DF,
Overberger CG, Seaborg GT, & Grayson M ed. Kirk-Othmer encyclopedia of
chemical technology. New York, John Wiley and Sons, vol 5, pp 786-791.
Schmid PP & Müller MD (1985) Trace level detection of chlorinated
paraffins in biological and environmental samples, using gas
chromatography/mass spectrometry with negative-ion chemical ionization.
J Assoc Off Anal Chem, 68: 427-430.
Scott RC (1989) In vitro absorption of some chlorinated paraffins
through human skin. Arch Toxicol, 63: 425-426.
Serrone DM, Birtley RDN, Weigand W, & Millischer R (1987) Toxicology
of chlorinated paraffins. Food Chem Toxicol, 25: 553-562.
Shults SK, Serrone DM, Killeen JC, & Ignatoski JA (1984) A one-
generation reproduction study in mallard ducks with chlorinated
paraffins. Painesville, Ohio, SDS Biotech Corporation, 273 pp (Report
No. 558-1IT-83-0032-003).
Sistovaris N & Donges V (1987) Gas chromatographic determination of
total polychlorinated aromates and chloro-paraffins following
catalytic reduction in the injection port. Fresenius Z Anal Chem,
326: 751-753.
Slooff W, Bont PFH, Janus JA, & Annema JA (1992) Exploratory report on
chlorinated paraffins. Bilthoven, The Netherlands, National Institute
of Public Health and Environmental Protection, 47 pp (Report No.
710401016).
Steele DH, Sack TM, Moody LA, Murray TM, Glatz JA, & Breen JJ (1988) A
negative chemical ionization GC/MS method for the determination of
chlorinated paraffins in environmental samples. Presented at the 36th
ASMS Conference on Mass Spectrometry and Allied Topics, San Francisco,
5-10 June 1988. New York, American Society for Mass Spectrometry,
2 pp.
Strack H (1986) Chlorinated paraffins. In: Ullmann's encyclopedia of
industrial chemistry. Weinheim, VCH Verlagsgesellschaft, vol A6,
pp 323-330.
Street JR & Madeley JR (1983a) Summary of experiments conducted in an
attempt to determine the biodegradability of a chlorinated paraffin
under anaerobic conditions by EPA test guide line CG2050. Brixham,
Imperial Chemical Industries Ltd, Brixham Laboratory, 23 pp (Report
BL/B/2363).
Street JR & Madeley JR (1983b) Assessment of the fate of a chlorinated
paraffin during aerobic sewage treatment by a modification of OECD
test guideline 303A. Brixham, Imperial Chemical Industries Ltd,
Brixham Laboratory, 70 pp (Report No. BL/B/2308).
Swedish Environment Protection Agency (1994) Chlorinated paraffins in
metal working. Solna, Swedish Environment Protection Agency, 10 pp
(SEPA Report No. 4372).
Tarkpea M, Lindén E, Bengtsson BE, Larsson Å, & Svanberg O (1981)
[Products control studies at the Brackish Water Toxicology Laboratory,
1979-80.] Nyköping, Swedish Environmental Protection Agency, 22 pp
(NBL Report 1981-03-23) (in Swedish).
Thompson RS & Madeley JR (1983a) The acute and chronic toxicity of a
chlorinated paraffin (58% chlorination of short chain length
n-paraffins) to the mysid shrimp Mysidopsis bahia. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 55 pp (Report No.
BL/B/2373).
Thompson RS & Madeley JR (1983b) Toxicity of a chlorinated paraffin
(58% chlorination of short chain length n-paraffins) to the marine
alga Skeletonema costatum. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 57 pp (Report No. BL/B/2325).
Thompson RS & Madeley JR (1983c) The acute and chronic toxicity of a
chlorinated paraffin (58% chlorination of short chain length
n-paraffins) to Daphnia magna. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 70 pp (Report No. BL/B/2358).
Thompson RS & Madeley JR (1983d) Toxicity of a chlorinated paraffin
(58% chlorination of short chain length n-paraffins) to the green
alga Selenastrum capricornutum. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 59 pp (Report No. BL/B/2325).
Thompson RS & Shillabeer N (1983) Effect of a chlorinated paraffin
(58% chlorination of short chain length n-paraffins) on the growth
of mussels (Mytilus edulis). Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 54 pp (Report No. BL/B/2331).
US EPA (1993) RM2 exit briefing on chlorinated paraffins and olefins.
Washington, DC, US Environmental Protection Agency, 42 pp.
Willis B, Diment J, Dobson S, & Crookes M (1994) Environmental hazard
assessment: Chlorinated paraffins. Garston, Watford, Building Research
Establishment, 47 pp (Report No. TSD/19).
Wyatt I, Coutts CT, & Elcombe CR (1993) The effect of chlorinated
paraffins on hepatic enzymes and thyroid hormones. Toxicology,
77: 81-90.
Yang JJ, Roy TA, Neil W, Kreuger AJ, & Mackerer CR (1987) Percutaneous
and oral absorption of chlorinated paraffins in the rat. Toxicol Ind
Health, 3: 405-412.
Zitko V (1973) Chromatography of chlorinated paraffins on alumina and
silica columns. J Chromatogr, 81: 152-155.
Zitko V (1974a) Uptake of chlorinated paraffins and PCB from suspended
solids and food by juvenile atlantic salmon. Bull Environ Contam
Toxicol, 12: 406-412.
Zitko V (1974b) Confirmation of chlorinated paraffins by dechlor-
ination. J Assoc Off Anal Chem, 57: 1253-1259.
Zitko V (1980) Chlorinated paraffins. In: Hutzinger O ed. Handbook of
environmental chemistry. Volume 3, Part A: Anthropogenic compounds.
Berlin, Heidelberg, New York, Springer Verlag, pp 149-156.
Zitko V & Arsenault E (1977) Fate of high molecular weight-chlorinated
paraffins in the aquatic environment. Adv Environ Sci Technol, 8:
409-418.
RESUME
1. Propriétés, usages et méthodes d'analyse
Les paraffines chlorées s'obtiennent par chloration des fractions
paraffiniques à chaîne droite. La chaîne des paraffines du commerce
comporte habituellement 10 à 30 atomes de carbone et leur teneur en
chlore est généralement comprise entre 40 et 70% en poids. Ce sont
des huiles visqueuses et denses, incolores ou jaunâtres, à faible
tension de vapeur; toutefois, lorsque la chaîne carbonée est
suffisamment longue et que la teneur en chlore est élevée (70%), on a
affaire à des solides. Les paraffines chlorées sont pratiquement
insolubles dans l'eau, les alcools inférieurs, le glycérol et les
glycols, mais solubles dans les solvants chlorés, les hydrocarbures
aromatiques, les cétones, les esters, les éthers,les huiles minérales
et certaines huiles de coupe. Elles sont modérément solubles dans les
hydrocarbures aliphatiques non chlorés.
En raison du nombre de positions possibles pour les atomes de
chlore, les paraffines chlorées sont des mélanges ex trêmement
complexes. Selon la longueur de la chaîne (courte C10-13,
intermédiaire C14-17, longue C18-30) et le degré de chloration (faible
< 50%; élevé > 50%), on peut diviser ces produits en six groupes.
Les paraffines chlorées sont très largement utilisées dans le
monde entier pour diverses applications: plastifiants (par ex. Pour le
PVC), additifs pour lubrifiants de pièces métalliques travaillant à
très haute pression, retardateurs de flammes et additifs pour
peintures. Les produits de qualité technique peuvent contenir
diverses impuretés: isoparaffines, métaux, composés aromatiques et en
principe, ils sont additionnés de stabilisants destinés à en prévenir
la décomposition.
En raison de la très grande complexité des mélanges, l'analyse
des paraffines chlorées est difficile. Lorsqu'il s'agit de travailler
sur des prélèvements effectués dans l'environnement, il s'y ajoute
encore les interférences dues à la présence d'autres composés.
L'analyse proprement dite doit donc souvent être précédée d'une
purification poussée des échantillons et faire appel à des moyens de
détection spécifiques. Au début, on purifiait le mélange par
chromatographie en couche mince et on effectuait ensuite la révélation
sur la plaque par une méthode non spécifique. Actuellement, on
utilise différentes techniques de chromatographie sur colonne pour la
purification des échantillons, mais il est difficile d'isoler les
paraffines chlorées en raison de la grande diversité de leurs
propriétés physiques. Dans ces conditions, il faut utiliser des
méthodes de détection spécifiques; à l'heure actuelle, la plus
utilisée est la chromatographie en phase gazeuse couplée à la
spectrométrie de masse. L'utilisation d'ions négatifs améliore encore
la spécificité. Toutefois, même si ces techniques élaborées
facilitent l'analyse des paraffines chlorées, il encore impossible de
déterminer les concentrations avec exactitude. Les résultats publiés
ne sont donc que des estimations.
2. Sources d'exposition humaine et environnementale
On ne connaît pas de paraffines chlorées d'origine naturelle.
Ces produits s'obtiennent par réaction du chlore gazeux sur des
fractions paraffiniques liquides. Il peut être nécessaire d'utiliser
un solvant et la lumière ultraviolette sert souvent de catalyseur.
Pour 1985, la production mondiale de paraffines chlorées a été estimée
à 300 000 tonnes.
La pollution de l'environnement par les paraffines chlorées
provient sans doute essentiellement du fait qu'elles sont d'un usage
très répandu. Elles peuvent être déversées dans l'environnement
lorsque des lubrifiants pour métaux ou des polymères qui en
contiennent viennent à être dispersés sans précautions dans la nature.
Il peut également y avoir pollution si des paraffines chlorées passent
dans l'environnement par lessivage de peintures ou de revêtements
divers. On pense que davantage de paraffines chlorées disparaissent
dans la nature pendant la production et le transport que lors de
l'utilisation des produits et de leur élimination.
En raison de leur instabilité thermique, les paraffines chlorées
doivent en principe être décomposées par l'incinération et donc ne pas
réapparaître dans les gaz émis par les incinérateurs. On a cependant
montré que lors de la pyrolyse de ces produits, des dérivés chlorés
d'hydrocarbures aromatiques-biphényle, naphtalène ou benzène
polychlorés - peuvent se former dans certaines conditions.
3. Distribution et transformation dans l'environnement
Les paraffines chlorées sont fortement adsorbées par les
sédiments. Dans l'eau, elles sont probablement transportées par
les particules sur lesquelles elles sont adsorbées; dans l'air
l'adsorption a vraisemblablement lieu sur les particules aéroportées
(et peut être aussi dans la phase vapeur). On estime que dans l'air,
la demi-vie des paraffines chlorées est de 0,85 à 7,2 jours, cette
durée étant suffisamment longue pour qu'on ne puisse exclure un
transport sur de longues distances.
Les paraffines chlorées ne sont pas facilement biodégradables.
En fait, celles qui ont une chaîne courte et une teneur en chlore
de moins de 50% se révèlent biodégradables en aérobiose par des
microorganismes acclimatés, la dégradation paraissant inhibée lorsque
la teneur en chlore dépasse 58%. La dégradation des paraffines
chlorées à chaîne moyenne ou longue est plus lente.
Les paraffines chlorées s'accumulent dans les organismes
aquatiques et les facteurs de bioconcentration publiés vont de 7 à
7155 pour les poissons et de 223 à 138 000 pour les moules. Les
poissons accumulent davantage les paraffines chlorées à courte chaîne
que les composés à chaîne moyenne ou longue. Après administration de
produits radiomarqués, on a retrouvé la radioactivité principalement
dans la bile, les intestins, le foie, les graisses et les branchies.
La fixation de ces composés semble donc facilitée par une chaîne
courte et une faible teneur en chlore, les composés à chaîne longue,
quant à eux, étant éliminés le plus lentement. La rétention dans les
tissus à forte adiposité augmente avec la teneur en chlore.
4. Concentrations dans l'environnement et exposition humaine
On ne possède guère de données sur la concentration des
paraffines chlorées dans l'environnement. On en a décelé la présence
au Royaume-Uni dans des échantillons d'eau de mer à des concentrations
inférieures à 4 µg/litre. Dans des eaux n'appartenant pas au domaine
marin, on a mesuré dans ce même pays des concentrations inférieures à
6 µg/litre; en Allemagne, les teneurs relevées en 1994 se situaient
dans les limites de 0,08 à 0,28 µg/litre. Aux Etats-Unis, la teneur
des eaux en paraffines chlorées est en général inférieure à
0,03 µg/litre, mais il est arrivé qu'on ait des concentrations
supérieures à 1,0 µg/litre dans une faible proportion des échantillons
(1,2%). Dans les sédiments marins, on a fait état de concentrations
allant jusqu'à 600 µg/kg de poids frais, cette teneur pouvant aller, au
Royaume-Uni, jusqu'à 15 000 µg/kg de poids frais pour des sédiments
non marins provenant de régions industrialisées et atteindre encore
1000 µg/kg de poids frais dans des zones à l'écart de toute industrie.
Aux Etats-Unis, on a trouvé, dans les eaux d'une retenue qui
provenaient d'une usine produisant des paraffines chlorées, des
sédiments dont la teneur atteignait, en poids sec, 170 000 µg/kg de
dérivés à longue chaîne, 50 000 µg/kg de dérivés à chaîne moyenne et
40 000 µg/kg de dérivés à chaîne courte. En Allemagne, on a trouvé en
1994 dans des sédiments les concentrations suivantes: jusqu'à 83 µg/kg
de dérivés en C10-13 et jusqu'à 370 µg/kg de dérivés en C14-17. Au
Japon, la teneur des sédiments allait jusqu'à 8 500 µg/kg.
La présence de paraffines chlorées a été mise en évidence dans un
certain nombre d'organismes. En Suède, on en a découvert chez des
mammifères terrestres à des concentrations de 32 à 88 µg/kg de tissus
(140-4400 µg/kg de lipides). Cependant au Royaume-Uni, on n'a pas
trouvé de paraffines chlorées chez des moutons qui paissaient à
distance des lieux de production. Dans ce même pays, la concentration
allait jusqu'à 1500 µg/kg chez des oiseaux, et, en ce qui concerne les
poissons, les teneurs pouvaient atteindre 200 µg/kg, valeur également
relevée en Suède. Dans des moules récoltées aux Etats-unis et au
Royaume-Uni, on a signalé des concentrations pouvant atteindre
400 µg/kg. Il est vrai qu'à proximité de la décharge d'une usine de
paraffines chlorées, les moules en contenaient jusqu'à 12 000 µg/kg.
Ces produits ont également été décelés lors d'autopsies dans des
tissus humains, notamment dans les tissus adipeux (teneur médiane
100-190 µg/kg), les reins (teneur médiane inférieure à 90 µg/kg) ainsi
que dans le foie (teneur médiane inférieure à 90 µg/kg). Lors d'une
enquête de portée limitée, on a constaté que des paraffines chlorées,
principalement des dérivés en C10-20, étaient présentes à des teneurs
pouvant atteindre 500 µg/kg dans environ 70% des échantillons de
denrées alimentaires.
Les données concernant l'exposition professionnelle aux
paraffines chlorées sont très limitées. On a constaté l'existence
d'une très faible exposition à des aérosols de paraffines chlorées à
chaîne courte (0,003-1,2 mg/m3), lors de l'utilisation de ces
produits comme lubrifiants de pièces métalliques, mais on ne sait pas
dans quelle proportion ils sont respirables. A partir d'un modèle
mathématique de l'exposition et en l'absence de toute mesure de
protection, on estime que ces lubrifiants à très forte teneur en
paraffines chlorées à chaîne courte doivent très largement entrer en
contact avec la peau (5-15 mg/cm2 par jour), même si l'absorption est
vraisemblablement faibles. Des mesures de protection devraient
permettre de réduire l'exposition cutanée.
5. Cinétique et métabolisme
La toxicocinétique des paraffines chlorées a été étudiée sur des
animaux de laboratoire. En ce qui concerne l'homme, les données sont
insuffisantes. On n'a pas suffisamment étudié les différences d'ordre
toxicocinétique pouvant résulter des différences de longueur de
chaîne. On ignore le degré d'absorption des paraffines chlorées
après administration orale, mais il semble qu'il diminue à mesure
qu'augmentent la longueur de la chaîne et la teneur en chlore. Selon
la longueur de la chaîne, l'absorption cutanée peut également être
plus ou moins importante, mais elle devrait rester limitée (moins de
1% d'une dose de C18 en application topique). On ne dispose d'aucune
donnée sur l'absorption au niveau pulmonaire.
Les paraffines chlorées se répartissent principalement dans le
foie, les reins, les intestins, la moelle osseuse, les tissus adipeux
et les ovaires. On ne dispose pas de données suffisantes sur la
rétention de ces dérivés dans l'organisme mais semble qu'elle est plus
longue lorsque ces produits ont une faible teneur en chlore, du fait
d'une redistribution plus lente. On les retrouve, accompagnées de
leurs métabolites, dans le système nerveux central jusqu'à 30 jours
après l'administration. Il est possible qu'elles traversent la
barrière foeto-placentaire. On ne dispose pas d'informations
suffisantes sur les voies métaboliques des paraffines chlorées, encore
que des études à l'aide de molécules radiomarquées aient montré que le
produit final en est le CO2.
Les paraffines chlorées sont excrétées par la voie rénale,
biliaire ou pulmonaire (sous la forme de CO2). Etant donné les
importantes variations d'une étude à l'autre, il est difficile
d'établir la part relative de chacune de ces voies d'excrétion.
L'élimination totale diminue lorsque le degré de chloration augmente
et les composés fortement chlorés sont principalement excrétés (à plus
de 50%) sous la forme de CO2. Il peut également y avoir excrétion
dans le lait.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Quelle que soit la longueur de la chaîne, les paraffines chlorées
ont une faible toxicité aiguë par voie orale. Après administration
d'une dose unique de produits à chaîne courte, les effets toxiques les
plus évidents consistaient en une perte de la coordination musculaire
et un hérissement des poils. En s'appuyant sur le peu de données dont
on dispose, on peut également dire que la toxicité aiguë par la voie
respiratoire et la voie cutanée semble faible. Après application ou
instillation de paraffines chlorées à chaîne courte ou moyenne, on a
observé une légère irritation de la peau (produits à chaîne moyenne)
et des yeux. Selon certaines études, les produits à courte chaîne ne
provoquent pas de sensibilisation cutanée.
Les études toxicologiques basées sur l'administration de doses
répétées par voie orale ont montré que que le foie, les reins et la
thyroïde sont les principales cibles des paraffines chlorées. Dans le
cas des composés à chaîne courte, on a constaté une augmentation du
poids du foie au doses les plus faibles (la dose effective la plus
faible est de 50 à 100 mg/kg de poids corporel sur une journée et la
dose sans effet observable est de 10 mg/kg de poids corporel par
jour). A doses plus élevées, on a également observé une augmentation
de l'activité des enzymes hépatiques, une prolifération du réticulum
endoplasmique agranulaire et des peroxysomes, un accroissement de la
synthèse réplicative de l'ADN, ainsi qu'une hypertrophie, une
hyperplasie et une nécrose du foie. D'autres effets ont été notés:
diminution du poids corporel (125 mg/kg de poids corporel par jour
chez la souris), augmentation du poids des reins (100 mg/kg de poids
corporel par jour chez le rat), augmentation de la synthèse
réplicative de l'ADN dans les cellules rénales (313 mg/kg de poids
corporel par jour), et néphrose (625 mg/kg de poids corporel par jour
chez le rat). Par ailleurs, on a signalé une augmentation du poids de
la thyroïde ainsi qu'une hypertrophie et une hyperplasie de cette
glande (plus faible dose effective: 100 mg/kg de poids corporel par
jour chez le rat) avec également un accroissement de la synthèse
réplicative de l'ADN dans les cellules folliculaires (plus faible dose
effective: 313 mg/kg de poids corporel par jour). A doses plus
élevées (1000 mg/kg de poids corporel par jour), la fonction
thyroïdienne est affectée, comme en témoignent les taux de thyroxine
(libre et totale) plasmatiques et l'augmentation de la thyréostimuline
plasmatique chez le rat.
En ce qui concerne les composés à chaîne moyenne, les effets
observés aux doses les plus faibles sont généralement une augmentation
du poids du foie et des reins (dose effective la plus faible chez
le rat: 100 mg/kg de poids corporel par jour et dose sans effet
observable chez le même animal: 10 mg/kg de poids corporel par jour).
A des doses analogues (dose sans effets observables de 4 mg/kg de
poids corporel par jour) on a noté un accroissement du cholestérol
sérique et des effets bénins "adaptatifs" consistant en modifications
histologiques au niveau de la thyroïde.
Dans le cas des composés à longue chaîne, les effets observés
aux doses les plus faibles consistaient en une hépatite granulomateuse
multifocale et et un accroissement du poids du foie chez les femelles
(dose effective la plus faible de 100 mg/kg de poids corporel par
jour).
Dans la seule étude de reproduction dont on dispose, on n'a pas
constaté d'effets nocifs chez des rats exposés à des paraffines à
chaîne moyenne contenant 52% de chlore. On a toutefois constaté une
réduction de la survie et du poids corporel chez les ratons (dose la
plus faible à laquelle on constatait une réduction non significative
du poids corporel: 5,7-7,2 mg/kg de poids corporel par jour; dose la
plus faible pour laquelle on constatait une réduction de la survie:
60-70 mg/kg de poids corporel par jour). Dans un petit nombre
d'études consacrées aux effets, sur le développement, des paraffines
chlorées à chaîne courte, moyenne ou longue, les effets observés sur
la progéniture étaient imputables uniquement aux composés à chaîne
courte, à des doses toxiques pour les mères (2000 mg/kg de poids
corporel par jour). Les composés à chaîne longue ou moyenne n'ont eu
aucun effet de ce genre, même à dose très élevée (1000 à 5000 mg/kg de
poids corporel par jour).
Les paraffines chlorées ne semblent pas provoquer de mutations
chez les bactéries. Cependant, il pourrait y avoir un faible effet
clastogène dans des cultures de cellules mammaliennes in vitro (mais
pas in vivo). Les paraffines chlorées provoqueraient également une
transformation cellulaire in vitro.
Des études de cancérogénicité à long terme ont été effectuées sur
des rats et des souris qui ont été gavées respectivement avec un
composé à chaîne courte (C12; 58% Cl) et un composé à chaîne longue
(C23; 43% Cl). Chez les souris B6C3F1 ayant reçu le composé
à chaîne courte, on a observé un accroissement de l'incidence de
certaines tumeurs: hépatiques parmi les mâles et les femelles,
thyroïdiennes parmi les femelles. Chez les rats Fischer-344 exposés
au composé à chaîne courte, il y avait augmentation des tumeurs
hépatiques parmi les mâles et les femelles, des tumeurs rénales
(adénomes et adénocarcinomes) parmi les mâles, des tumeurs
thyroïdiennes parmi les femelles et des leucémies monocytaires parmi
les mâles. Dans le cas du composé à longue chaîne, on constaté une
augmentation de l'incidence des lymphomes malins chez les souris mâles
et de celle des tumeurs surrénaliennes chez les rattes.
7. Effets sur l'homme
Malgré la très large utilisation qui est faite des paraffines
chlorées, on ne connaît aucun cas d'irritation ou de sensibilisation
cutanée. Cette observation est corroborée par les résultats d'un
petit nombre d'études sur des volontaires au cours desquelles on a
observé une irritation cutanée minime, mais pas de sensibilisation.
On n'a pas pu obtenir de données concernant d'autres effets des
paraffines chlorées sur l'homme.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
On a montré que les paraffines chlorées à courte chaîne pouvaient
provoquer des intoxications aiguës chez les invertébrés marins et les
invertébrés d'eau douce, la valeur de la CL50 et de la CE50 allant de
14 à 530 µg/litre. Dans la plupart des épreuves de toxicité aiguë
effectuées sur des invertébrés aquatiques avec des paraffines chlorées
à chaîne moyenne ou longue, les concentrations utilisées étaient
supérieures à la solubilité des composés dans l'eau. Toutefois, selon
une étude, une paraffine chlorée à chaîne moyenne serait toxique pour
les daphnies, avec une CE50 de 37 µg/litre. La toxicité aiguë des
paraffines chlorées pour les poissons est faible, qu'il s'agisse de
composés à chaîne courte, moyenne ou longue, la valeur de la CL50
étant largement supérieure à la solubilité dans l'eau.
Les paraffines chlorées à chaîne courte présentent une toxicité à
long terme pour les algues, les invertébrés aquatiques et les poissons
à des concentrations ne dépasssant pas 19,6, 8,9 et 3,1 µg/litre,
respectivement; la concentration sans effet observable se situe entre
2 et 5 µg/litre pour l'espèce la plus sensible étudiée. Des produits
à chaîne moyenne et à chaîne longue ont eu des effets chroniques
sur des daphnies à des concentrations respectivement égales à
20-35 µg/litre et à < 1,2-8 µg/litre. Il semble que la toxicité à
long terme soit faible pour les poissons. On ne dispose d'aucune
donnée concernant les algues.
D'après les données limitées dont on dispose, on pense que la
toxicité aiguë est faible pour les oiseaux.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
Il est probable que la nourriture soit la principale source
d'exposition de la population générale. D'après les données limitées
dont on dispose au sujet des concentrations dans les denrées
alimentaires, les estimation les plus pessimistes concernant les
produits laitiers et les moules donnent respectivement des apports
journaliers de 4 et 25 µg/kg de poids corporel. En général, les doses
journalières calculées de paraffines chlorées se situent en dessous
des valeurs tolérables relatives aux effets non néoplasiques ou des
valeurs recommandées relatives aux effets néoplasiques (composés à
chaîne courte).
Dans la mesure où ils respectent les règles d'hygiène personnelle
et les consignes de sécurité, les travailleurs exposés à des
paraffines chlorées n'encourent qu'un risque minime.
Les données disponibles montrent que les paraffines chlorées
s'accumulent dans les tissus biologiques et sont persistantes.
D'après les données relatives à la concentration des dérivés à chaîne
courte dans l'environnement, il y a un risque pour la faune
dulçaquicole et estuarielle dans les zones proches des points de
décharge. Les dérivés à chaîne longue et moyenne représentent aussi
un danger pour les invertébrés aquatiques.
L'enrichissement des sédiments en paraffines chlorées, de même
que les modalités de résorption et la toxicité de ces produits pour la
faune aquatique sont l'indication d'un risque pour les organismes qui
peuplent la vase.
RESUMEN
1. Propiedades, usos y métodos analíticos
Las parafinas cloradas (PC) se producen por la cloración de
fracciones de parafina de cadena recta. Por lo general, la cadena
carbonada de las parafinas cloradas comerciales contiene de 10 a 30
átomos de carbono, y su contenido de cloro oscila generalmente entre
el 40% y el 70% por peso. Las parafinas cloradas son aceites densos
viscosos incoloros o amarillentos con bajas presiones de vapor, a
excepción de las de cadena carbonada larga con elevado contenido
de cloro (70%), que son sólidas. Las parafinas cloradas son
prácticamente insolubles en agua, alcoholes inferiores, glicerol y
glicoles, pero son solubles en solventes clorados, hidrocarburos
aromáticos, cetonas, ésteres, éteres, aceites minerales y algunos
lubricantes para cuchillas. Son medianamente solubles en
hidrocarburos alifáticos no clorados.
Las parafinas cloradas están formadas por mezclas sumamente
complejas, lo que obedece a las múltiples posiciones posibles de los
átomos de cloro. Los productos pueden subdividirse en seis grupos,
atendiendo a la longitud de la cadena (corta C10-13, media C14-17 y
larga C18-30) y al grado de cloración (bajo (< 50%) y elevado
(> 50%).
Las parafinas cloradas se utilizan en todo el mundo en múltiples
aplicaciones; se emplean como plastificantes en la fabricación de
plásticos (tales como el policloruro de vinilo), como aditivos en
fluidos para el laboreo de metales a presiones extremas, y como
pirorretardantes y aditivos en la producción de pinturas. Las
parafinas cloradas de calidad técnica pueden estar contaminadas por
isoparafinas, compuestos aromáticos y metales; por lo general
contienen estabilizadores, añadidos para impedir la descomposición.
El análisis de las parafinas cloradas resulta difícil debido a la
enorme complejidad de esas mezclas. En las muestras tomadas del medio
ambiente, ello se ve complicado además por la interferencia de otros
compuestos. En muchos casos, los análisis requieren un considerable
grado de descontaminación de las muestras y el empleo de métodos de
detección específicos. En el pasado, se empleaba como método de
descontaminación la cromatografía en capa fina y un método no
específico de detección por argentación en las placas. En la
actualidad se emplean métodos de descontaminación basados en la
cromatografía líquida en diferentes columnas, aunque resulta difícil
aislar las parafinas cloradas debido al gran número de sus propiedades
físicas. Por lo tanto, se emplean métodos de detección específicos;
en la actualidad, la técnica más corriente es la cromatografía por gas
combinada con la espectrometría de masas. El uso de iones negativos
permite una detección incluso más específica. Si bien el empleo de
esas técnicas avanzadas ha aumentado la capacidad de análisis de
las parafinas cloradas, continúa siendo imposible determinar
las concentraciones exactas. Los resultados comunicados deben
considerarse solamente estimaciones de los valores reales.
2. Fuentes de exposición del ser humano y del medio ambiente
No se tiene conocimiento de la presencia en estado natural de las
parafinas cloradas.
Las parafinas cloradas son producto de la reacción de fracciones
de parafina líquida con gas cloro puro. La reacción puede requerir el
empleo de un solvente, empleándose frecuentemente luz ultravioleta
como catalizador. Se estima que en 1985 la producción mundial de
parafinas cloradas se elevó a 300 000 toneladas.
Los usos muy difundidos de las parafinas cloradas son
probablemente la principal fuente de contaminación ambiental. Las PC
podrían escapar al medio ambiente debido a la eliminación incorrecta
de fluidos para el laboreo de metales que contienen parafinas cloradas
o de polímeros que contienen parafinas cloradas. La pérdida de
parafinas cloradas como resultado de la lixiviación de pinturas y
revestimientos podría ser también fuente de contaminación ambiental.
Cabe prever que las posibles pérdidas durante la producción y el
transporte sean inferiores a las que ocurren durante el uso y
eliminación de los productos.
Debido a su inestabilidad térmica, es de suponer que las
parafinas cloradas se degradan durante la incineración; por lo tanto,
no es de esperar que se volatilicen en los gases de escape de los
incineradores. Sin embargo, se ha demostrado la formación de
compuestos aromáticos clorados como, por ejemplo, bifenilos
policlorados, naftalenas y benzinas, por pirólisis de las parafinas
cloradas en ciertas condiciones.
3. Distribución y transformación en el medio ambiente
Las parafinas cloradas experimentan una adsorción pronunciada en
el sedimento. En el agua son transportadas probablemente adsorbidas
en partículas en suspensión, y en la atmósfera están adsorbidas en
partículas transportadas por el aire (y posiblemente en la fase de
vapor). Se ha estimado que la semivida de las parafinas cloradas en
el aire oscila entre 0,85 y 7,2 días; debido a la duración de ese
período, no puede excluirse la posibilidad de su transporte a larga
distancia.
Las parafinas cloradas no son fácilmente biodegradables. Las de
cadena carbonada corta con un contenido de cloro inferior al 50%
parecen ser degradables en condiciones aeróbicas con microorganismos
aclimatados, mientras que la degradación parece estar inhibida cuando
el contenido de cloro es superior al 58%. Las de cadenas carbonadas
media y larga se degradan más lentamente.
Las parafinas cloradas se bioacumulan en los organismos
acuáticos, habiéndose comunicado factores de bioconcentración que
oscilan entre 7 y 7155 en el caso de peces y entre 223 y 138 000 en el
de mejillones. En los peces, las de cadena corta experimentan mayor
acumulación que las de cadenas media y larga. Se ha observado
radioactividad principalmente en la bilis, el intestino, el hígado, la
grasa y las agallas después de la administración de parafinas cloradas
marcadas con radioisótopos. La absorción de las parafinas cloradas
parece ser más eficiente en el caso de las que tienen menor contenido
de cloro; la tasa de eliminación es más lenta en el caso de aquellas
que tienen un elevado contenido de cloro. La retención en los tejidos
ricos en grasa parece aumentar a medida que aumenta el grado de
cloración.
4. Concentración en el medio ambiente y exposición del ser humano
Se cuenta con poca información sobre la concentración de las
parafinas cloradas en el medio ambiente. Se han detectado
concentraciones inferiores a 4 µg/litro en muestras de agua de
mar en el Reino Unido. En aguas no marítimas, se ha informado de
concentraciones de 6 µg/litro en el Reino Unido; en Alemania, las
concentraciones determinadas en 1994 oscilaban entre 0,08 y
0,28 µg/litro. En los Estados Unidos, si bien las concentraciones en
el agua eran generalmente inferiores a 0,03 µg/litro, se observaron
concentraciones superiores a 1,0 µg/litro en una pequeña proporción
(1,2%) de las muestras. En los sedimentos marinos, se tiene noticias
de concentraciones de hasta 600 µg/kg de peso húmedo, y en sedimentos
no marinos en el Reino Unido las concentraciones han alcanzado hasta
15 000 µg/kg en regiones industrializadas y 1000 µg/kg en zonas
alejadas de la industria. En los sedimentos en un embalse de
confinamiento de una planta de fabricación de parafinas cloradas en
los Estados Unidos, las concentraciones registradas llegaron a
alcanzar 170 000 µg/kg peso seco de PC de cadena larga, 50 000 µg/kg
de PC de cadena media y 40 000 µg/kg de PC de cadena corta. En
Alemania, se han comunicado en 1994 concentraciones de hasta 83 µg/kg
de peso seco de C10-13 y de hasta 370 µg/kg de peso seco de C14-17 en
sedimentos. En el Japón, las concentraciones en el sedimento han
llegado a alcanzar 8500 µg/kg.
Se han detectado parafinas cloradas en diferentes organismos.
Se han encontrado en los mamíferos terrestres en Suecia en
concentraciones que oscilan entre 32 y 88 µg/kg de tejido (140 a
4400 µg/kg de lípidos). Sin embargo, no se detectaron en ovejas que
pastaban en zonas alejadas de la producción de parafinas cloradas en
el Reino Unido. Por lo que respecta a las aves, en el Reino Unido se
observaron concentraciones que llegaron a alcanzar los 1500 µg/kg; en
cuanto a los peces, se han comunicado concentraciones de hasta
200 µg/kg en Suecia y el Reino Unido. En los mejillones recogidos
en los Estados Unidos y el Reino Unido, se tiene noticias de
concentraciones que se han elevado a 400 µg/kg. Con todo, en
mejillones capturados en las cercanías de un punto de descarga de
efluentes de una fábrica de parafinas cloradas se han registrado
concentraciones de C10-20 que han alcanzado 12 000 µg/kg. En
estudios post mortem se han detectado también PC en los tejidos
humanos: en el tejido adiposo (concentración media de 100 a 190 µg/kg),
los riñones (concentración media inferior a 90 µg/kg) y el hígado
(concentración media inferior a 90 µg/kg). En un estudio limitado, se
detectaron concentraciones de hasta 500 µg/kg de parafinas cloradas,
principalmente C10-20, en un 70% de las muestras de diversos
productos alimenticios.
Se dispone de escasa información sobre la exposición ocupacional
a las parafinas cloradas. Se han observado niveles muy bajos de
exposición a los aerosoles de PC de cadena corta (0,003 a 1,2 mg/m3)
asociados con su uso en fluidos para el laboreo de metales, aunque no
existe información disponible sobre la proporción que se puede
inhalar. Sobre la base de modelos matemáticos de la exposición sin
medidas de control, se estimaron niveles elevados de contactos
dérmicos (5 a 15 mg/cm2 al día) por lo que respecta a los fluidos
especiales para labrado de metales que contienen concentraciones muy
elevadas de PC de cadena corta, aunque cabe esperar que la absorción
sea baja. Las medidas de control permitirían reducir la exposición
dérmica.
5. Cinética y metabolismo
Se ha estudiado la toxicocinética de las parafinas cloradas en
animales experimentales. No se cuenta con información adecuada por lo
que respecta a los seres humanos. No se han realizado suficientes
investigaciones sobre las posibles diferencias en materia de
toxicocinética como consecuencia de las diferencias en la longitud de
las cadenas. Si bien se desconoce el grado de absorción de las PC
después de su administración oral, éste parece disminuir a medida que
aumenta la longitud de la cadena y el grado de cloración. Según cuál
sea la longitud de la cadena, puede producirse también absorción
percutánea, aunque en un grado limitado (inferior al 1% de una dosis
tópica de C18). No se cuenta con datos sobre la absorción en el
pulmón.
La distribución de las parafinas cloradas ocurre principalmente
en el hígado, los riñones, el intestino, la médula espinal, el tejido
adiposo y los ovarios. Aunque no existe suficiente información en lo
que respecta a la retención, un grado bajo de cloración podría
aumentar el tiempo de retención al ser más lenta la redistribución.
Se ha observado la presencia de PC, o de sus metabolitos, en el
sistema nervioso central hasta 30 días después de su administración.
Podrían cruzar la barrera hemato-placentaria. Si bien no se cuenta
con información adecuada sobre las vías del metabolismo de las PC, en
los estudios con radioisótopos se ha identificado el CO2 como
producto final.
Las parafinas cloradas pueden excretarse por vía renal, biliar y
pulmonar (como CO2). Resulta difícil establecer el grado relativo de
excreción por las diferentes rutas debido a la gran variabilidad de
los diferentes estudios. La total eliminación de esas sustancias
disminuye a medida que aumenta el contenido de cloro, y los compuestos
con elevado grado de cloración se excretan principalmente (más del
50%) en forma de CO2. Las PC pueden ser excretadas en la leche.
6. Efectos en mamíferos de laboratorio y sistemas de pruebas
in vitro
Las parafinas cloradas de cadenas de diferentes longitudes
presentan reducida toxicidad oral aguda. Los efectos tóxicos como,
por ejemplo, incoordinación muscular y piloerección resultaban más
ostensibles después de una exposición aislada a parafinas cloradas de
cadena corta. Sobre la base de información muy limitada, la toxicidad
aguda por inhalación y por contacto cutáneo parece ser también baja.
Se ha observado ligera irritación de la piel y de los ojos después de
la aplicación de PC de cadena media (irritación cutánea). Los
resultados de varios estudios indican que las PC de cadena corta no
provocan sensibilización cutánea.
En estudios de toxicidad con dosis repetidas por vía oral, los
órganos en que se manifiesta principalmente la toxicidad de las PC son
el hígado, los riñones y la tiroides. Por lo que respecta a los
compuestos de cadena corta, se han registrado aumentos en el peso del
hígado con las dosis más reducidas (en las ratas, el nivel más bajo de
efecto observado es de 50 a 100 mg/kg peso corporal por día, y la
concentración sin efectos observados es de 10 mg/kg de peso corporal
al día). Con posologías superiores, se han registrado también
aumentos en la actividad de las enzimas hepáticas, proliferación
del retículo endoplasmático liso y peroxisomas, síntesis de ADN
replicante, hipertrofia, hiperplasia y necrosis del hígado. Asimismo,
se han observado disminución en el aumento del peso corporal
(125 mg/kg de peso corporal por día en los ratones), aumentos del peso
de los riñones (100 mg/kg de peso corporal por día en las ratas),
síntesis de ADN replicante en las células renales (313 mg/kg de peso
corporal por día) y nefrosis (625 mg/kg de peso corporal por día en
las ratas). Se han comunicado aumentos en el peso de la tiroides, e
hipertrofia e hiperplasia de la tiroides (nivel más bajo de efecto
observado de 100 mg/kg de peso corporal por día en las ratas) y
síntesis de ADN replicante en las células foliculares de la tiroides
(nivel más bajo de efecto observado de 313 mg/kg de peso corporal por
día). Con posologías superiores (1000 mg/kg de peso corporal por
día), la función tiroidea se ve afectada, lo que se puede establecer
por las concentraciones de tiroxina plasmática libre y total y la
mayor concentración plasmática de la hormona tirotrófica en las ratas.
En cuanto a los compuestos de cadena media, el efecto observado
con las dosis más bajas es generalmente el aumento del peso del hígado
y los riñones (nivel más bajo de efecto observado en las ratas de
100 mg/kg de peso corporal al día; nivel sin efecto nocivo observado en
las ratas de 10 mg/kg de peso corporal al día). En estudios con ratas
hembras, se han señalado aumentos en el colesterol sérico y cambios
histológicos "ligeros, adaptativos" en la tiroides cuando se emplean
posologías similares (nivel sin efecto nocivo observado de 4 mg/kg de
peso corporal al día).
Por lo que respecta a los compuestos de cadena larga, los efectos
observados en ratas hembras con las posologías más bajas son hepatitis
granulomatosa multifocal y aumento del peso del hígado (nivel más bajo
de efecto nocivo observado de 100 mg/kg de peso corporal al día).
En el único estudio sobre reproducción descrito, no se informó de
efectos reproductivos adversos después de la exposición de las ratas a
una PC de cadena media con 52% de cloro. Con todo, la supervivencia y
los pesos corporales de los hijuelos expuestos se redujo (el nivel más
bajo de efecto observado para una disminución no significativa del
peso corporal fue de 5,7 a 7,2 mg/kg de peso corporal por día; el
nivel más bajo de efecto nocivo observado para menor supervivencia
estuvo entre 60 y 70 mg/kg de peso corporal por día). En un número
limitado de estudios sobre los efectos de las PC de cadena corta,
media y larga sobre el desarrollo de las camadas de las ratas, se
registraron efectos adversos sólo en el caso de los compuestos de
cadena corta, con dosis tóxicas para las madres (2000 mg/kg de peso
corporal por día). En cuanto a los compuestos de cadena media y
larga, no se observaron efectos en la prole, incluso con posologías
muy elevadas (1000 a 5000 mg/kg de peso corporal por día).
Las parafinas cloradas no parecen inducir mutaciones en las
bacterias. Sin embargo, en las células de los mamíferos hay indicios
de un bajo potencial clastógeno in vitro pero no in vivo. También
se tiene noticia de que las PC provocan transformación celular in
vitro.
Se han llevado a cabo estudios de carcinogenicidad a largo plazo
con ratas y ratones alimentados por sonda empleando una PC de cadena
corta (C12; 58% de Cl) y una PC de cadena larga (C23; 43% de Cl). En
el caso de los ratones B6C3F1 expuestos al compuesto de cadena corta,
se registraron aumentos en la incidencia de tumores hepáticos en los
machos y las hembras, así como tumores de la glándula tiroides en las
hembras. En las ratas Fischer-344 expuestas al compuesto de cadena
corta, se observó un mayor número de tumores hepáticos en los machos y
las hembras, tumores renales (adenomas o adenocarcinomas) en los
machos, tumores de la tiroides en las hembras y leucemias de las
células mononucleares en los machos. Por lo que respecta a las PC de
cadena larga, se vio aumentada la incidencia de linfomas malignos en
ratones machos y de tumores de la glándula adrenal en las ratas
hembra.
7. Efectos en el ser humano
A pesar del uso muy difundido de las parafinas cloradas, no hay
informes de casos de irritación o de sensibilización dérmica. Esto se
ve corroborado por los resultados de un número limitado de estudios
realizados con voluntarios en los que las PC provocaron mínima
irritación dérmica, pero no sensibilización.
No se dispone de datos sobre otros efectos de las PC en el ser
humano.
8. Efectos en otros organismos en laboratorio y sobre el terreno
Se ha demostrado que las parafinas cloradas de cadena corta son
sumamente tóxicas para los invertebrados acuáticos, tanto de agua
dulce como de mar, oscilando los valores de CL50-CE50 entre 14 y
530 µg/litro. La mayoría de las pruebas de toxicidad aguda para los
invertebrados acuáticos con parafinas cloradas de cadena media y larga
son superiores a la solubilidad en agua. Sin embargo, en un estudio
realizado con crustáceos Daphnia con una PC de cadena media se
observó toxicidad aguda con una CE50 de 37 µg/litro. En el caso de
los peces, las PC de cadena corta, media y larga parecen tener poca
toxicidad aguda, con valores de CL50 muy superiores a la solubilidad
en agua.
Las parafinas cloradas de cadena corta presentan toxicidad a
largo plazo a las algas, los invertebrados acuáticos y los peces
con concentraciones tan bajas como 19,6, 8,9 y 3,1 µg/litro,
respectivamente; en cuanto a las especies más sensibles estudiadas,
las concentraciones sin efecto observado parecen oscilar entre 2 y
5 µg/litro. Un producto de cadena media y otro de cadena larga
tuvieron efectos crónicos en crustáceos Daphnia con concentraciones
de 20 a 35 µg/litro y de < 1,2 a 8 µg/litro, respectivamente. La
toxicidad a largo plazo parece ser baja en el caso de los peces. No
se cuenta con datos sobre las algas.
Atendiendo a los limitados datos disponibles, la toxicidad aguda
de las PC en las aves es baja.
9. Evaluación de los riesgos para la salud de los seres humanos y de
los efectos sobre el medio ambiente
Los alimentos son probablemente la fuente principal de exposición
de la población general. Sobre la base de los datos limitados sobre
las concentraciones presentes en los alimentos, las estimaciones más
desfavorables del consumo diario en los productos lácteos y mejillones
son de 4 y 25 µg/kg peso corporal por día, respectivamente. En
general, se calcula que la cantidad de PC ingeridas diariamente es
inferior al límite tolerable por lo que respecta a los efectos no
neoplásicos, o a los valores recomendados en cuanto a los efectos
neoplásicos (compuestos de cadena corta).
Siempre que se sigan procedimientos adecuados de higiene y
seguridad personal, cabe esperar que el riesgo para la salud de los
trabajadores expuestos a las PC sea mínimo.
La información disponible indica que las parafinas cloradas
son bioacumulativas y persistentes. Los datos sobre los niveles
ambientales de las de cadena corta indican que en áreas cercanas a las
fuentes de descarga existe riesgo para los organismos, tanto de agua
dulce como estuarinos. Asimismo, las de cadena media y larga pueden
presentar riesgos para los invertebrados acuáticos.
El enriquecimiento de las parafinas cloradas en los sedimentos,
sus posibilidades de reabsorción y su toxicidad en medio acuático son
indicios de posible riesgo para los organismos que habitan en los
sedimentos.
2.1.2.2 Synonyms
Alkanes, chlorinated; alkanes (C10-12), chloro (60%); alkanes (C10-13),
chloro (50-70%); alkanes (C14-17), chloro (40-52%); alkanes (C18-28),
chloro (20-50%); alkanes (C22-26), chloro (43%); C12, 60% chlorine;
C23, 43% chlorine; chlorinated alkanes; chlorinated hydrocarbon
waxes; chlorinated paraffin waxes; chlorinated waxes; chloroalkanes;
chlorocarbons; chloroparaffin waxes; paraffin, chlorinated; paraffins,
chloro; paraffin waxes, chlorinated; paroils, chlorinated; poly-
chlorinated alkanes; polychloro alkanes.
2.1.3 Technical products
Chlorinated paraffins are manufactured commercially by a number
of companies and are marketed under a variety of trade names. The
trade names are followed by numbers, which often are related to the
average chlorine content (in percent) of a particular preparation.
However, this is not a rule and the average chlorine content may have
to be obtained from manufacturers' technical data. More than 200
chlorinated paraffin formulations are commercially available
world-wide (Serrone et al., 1987), and some examples of these are
given in Table 2.
The carbon chain length of the chlorinated paraffins in a
commercial mixture is variable, and the average chain length is
usually specified by the manufacturer. The composition of paraffins
of different chain length in some commercial formulations is shown in
Table 3. The paraffin feedstocks are randomly chlorinated and the
resulting chlorine contents are given as average values.
Commercial chlorinated paraffins may be contaminated by
isoparaffins (usually less than 1%), aromatic compounds (usually less
than 0.1% (1000 ppm)) and metals (Schenker, 1979).
Chlorinated paraffins normally contain stabilizers, which are
added to inhibit decomposition. Common stabilizers include epoxidized
compounds such as epoxidized esters and soya bean oils (indicated in
section 7.1.3 to be present at up to 3%), pentaerythritol, thymol,
urea, glycidyl ethers, acetonitriles and organic phosphites (Schenker,
1979; Strack, 1986; Houghton, 1993). The concentration of stabilizers
is usually below 0.05% w/w (Campbell & McConnell, 1980).
Table 2. Partial list of commercial chlorinated paraffinsa
Average molecular formula C12H15Cl11 C12H19Cl7 C15H26Cl6 C24H29Cl21 C24H42Cl8 C24H44Cl6
Chlorine content (% w/w) 70 60-65 50-52 70 48-54 40-42
Manufacturers:
Oxychem, USA Chlorowax 70L Chlorowax 500C Chlorowax 70 Chlorowax 50 Chlorowax 40
Keil Chemical Div., USA CW-200-70 CW-85-60 CW-52 CW-220-50 CW-170
Dover Chemical Corp., Paroil 170HV Paroil 160 Paroil 152 Chlorez 700 Paroil 150S Paroil 140
USA Paroil 1048
Plastifax, Inc., USAb Plastichlor P-70 Plastichlor P-59 Plastichlor 50-220 Plastichlor
P-65 42-170
ICI, Australia; Canada; Cereclor 70L Cereclor 60L Cereclor S52 Cereclor 70 Cereclor 48 Cereclor 42
UK; France
Neville Chemical Co., Unichlor 60L-60 Unichlor 50L-65 Unichlor 50-450 Unichlor 40-170
USAb Unichlor 40-150
Pearsall Chemical Co., FLX-0012 FLX-0008 CPF-0020 CPF-0004
USA CPF-0003 CPF-0001
Hüls AG, Germanyb Chlorparaffin Chlorparaffin Chlorparaffin Chlorparaffin
70C 60C 52G 40N
Dynamit Nobel, Germanyb Witaclor 171 Witaclor 160 - Witaclor 350 Witaclor 549 Witaclor 540
Witaclor 163 Witaclor 352
Caffaro, Italy Cloparin D70 Cloparin 1059 Cloparin 50 Cloparin S70 Cloparin P42
Table 2. (Cont'd)
Average molecular formula C12H15Cl11 C12H19Cl7 C15H26Cl6 C24H29Cl21 C24H42Cl8 C24H44Cl6
Chlorine content (% w/w) 70 60-65 50-52 70 48-54 40-42
Hoechst AG, Germany Chlorparaffin Hordaflex LC60 Chlorparaffin Chlorparaffin Chlorparaffin
Hoechst 70 Hoechst 52fl Hoechst 70fest Hoechst 40fl
Rhône-Poulenc, France Alaiflex 67B2 Ribeclor 60B2 Alaiflex 50A3 Alaiflex 40A8
a Other producers include Bann Quimica (Brazil), Excel Industry (India), Ajinomoto (Japan), Tosoh (Japan), Asahi Denka (Japan),
Plasticlor (Mexico), NCP (South Africa)
b These companies have ceased production of chlorinated paraffins.
Table 3. Composition of paraffins obtained by dechlorination of
different chlorinated paraffin preparations (Zitko, 1974b)
Chlorinated Percentage of each paraffin
paraffin
C21 C22 C23 C24 C25 C26 C27 C28
Chloroparaffin, 4.5 10.0 15.7 19.3 18.5 15.3 9.8 6.7
40%
Clorafin 40 3.7 8.2 14.0 17.5 19.2 17.4 12.4 7.6
CP 40 3.9 9.1 14.9 19.2 19.8 18.0 15.1 -
Cereclor 42 3.6 8.8 14.7 18.6 19.5 17.2 11.5 6.0
Chloroparaffin, 7.4 14.9 20.7 23.1 19.9 14.0 - -
50%
2.2 Chemical and physical properties
Chlorinated paraffins are viscous, colourless or yellowish, dense
oils, except for the chlorinated paraffins of long carbon chain length
(C20-C30) with high chlorine content (70%), which are solid.
Chlorinated paraffins have a characteristic slight and not unpleasant
odour (Hardie, 1964). The odour is probably due to small quantities
of products of lower relative molecular mass with small but measurable
vapour pressures (Howard et al., 1975). Chlorinated paraffins
themselves have very low vapour pressures. The medium chain length
C14-17;52% Cl has a vapour pressure of approximately 2 × 10-4 Pa at
20°C (1-2 × 10-6 mmHg) (Campbell & McConnell, 1980), and the long
chain length C23;42-54% Cl approximately 3 × 10-3 Pa when measured at
65°C (2 × 10-5 mmHg) (Hardie, 1964). The chemical and physical
properties of chlorinated paraffins are determined by the carbon chain
length of the paraffin and the chlorine content. Increases in the
carbon chain length and chlorination degree of a particular paraffin
increase the viscosity and density but reduce the volatility.
Chlorinated paraffins are practically insoluble in water, but
many products can be emulsified with water (approximately 70/30
chlorinated paraffin to water). The water solubility of 14C-labelled
polychloroundecane (C11;59% Cl) is reported to be 150-470 µg/litre,
polychloropentadecane (C15;51% Cl) 5-27 µg/litre and the poly-
chloropentacosanes (C25;43% Cl) < 5-6.4 µg/litre and (C25;70%
Cl) < 5-5.9 µg/litre, depending on analytical method (Madeley &
Gillings, 1983). Campbell & McConnell (1980) reported the solubility
of C16;52% Cl to be 10 µg/litre in freshwater and 4 µg/litre in
seawater. The solubility of C25;42% Cl was reported to be 3 µg/litre
in seawater. Chlorinated paraffins are also practically insoluble in
lower alcohols, glycerol and glycols, but are soluble in chlorinated
solvents, aromatic hydrocarbons, ketones, esters, ethers, mineral oil
and some cutting oils. They are moderately soluble in unchlorinated
aliphatic hydrocarbons (Houghton, 1993). Some physical properties of
typical commercial chlorinated paraffins are summarized in Table 4.
Assuming a water solubility of 5 µg/litre and a vapour pressure
of 2 × 10-4 Pa as typical of a 52% chlorinated intermediate chain
length paraffin, a Henry's Law constant of 10.9 may be calculated
(Willis et al., 1994).
A key property of chlorinated paraffins, particularly the high
chlorine grades, is their nonflammability. This is due to the ability
of chlorinated paraffins to release hydrochloric acid at elevated
temperatures, and the hydrochloric acid inhibits the radical reaction
in a flame. This property is considerably enhanced by the addition of
antimony trioxide (Houghton, 1993) or other additives. Chlorinated
paraffins are generally unreactive and stable in normal temperatures,
but decompose significantly at temperatures above 300°C with the
release of hydrochloric acid (Strack, 1986). Prolonged exposure
to light can also cause dehydrochlorination. Degradation by
dehydrochlorination can be accelerated at elevated temperatures in
the presence of aluminium, zinc, and iron oxide or chloride (Howard
et al., 1975; Houghton, 1993). Dehydrochlorination leads to darkening
of the material.
2.3 Analysis
The analysis of chlorinated paraffins is very difficult owing to
the many congeners present in the products. The properties of these
congeners cover wide ranges, which makes it difficult to separate the
chlorinated paraffins from other compounds that may interfere in the
analysis.
Table 4. Physical properties of selected commercial chlorinated paraffinsa
Paraffin Chlorine Colour hazen Viscosityb Densityb Thermal stabilityc Volatilityd Refractive Log Powe
feedstock content (APHA) (Pa.s) (g/ml) (% w/w HCl) (% w/w) index
% (w/w)
C10-C13 50 100 0.08 1.19 0.15 16.0 1.493 4.39-6.93
56 100 0.8 1.30 0.15 7.0 1.508 NRg
60 125 3.5 1.36 0.15 4.4 1.516 4.48-7.38
63 125 11.0 1.41 0.15 3.2 1.522 5.47-7.30
65 150 30.0 1.44 0.20 2.5 1.525 NR
70 200 8.0f 1.50 0.20 0.5 1.537 5.68-8.01h
C14-C17 40 80 0.07 1.10 0.2 4.2 1.488 NR
45 80 0.2 1.16 0.2 2.8 1.498 5.52-8.21
52 100 1.6 1.25 0.2 1.4 1.508 5.47-8.01
58 150 40.0 1.36 0.2 0.7 1.522 NR
Wax C18-C20 47 150 1.7 1.21 0.2 0.8 1.506 NR
50 250 18.0 1.27 0.2 0.7 1.512 NR
Wax (C> 20) 42 250 2.5 1.16 0.2 0.4 1.506 9.29->12.83h
48 300 28.0 1.26 0.2 0.3 1.516 8.69-12.83
70 100i j 1.63 0.2 NR - NR
a Data from Houghton (1993) f At 50°C
b At 25°C unless otherwise noted g NR = not reported
c Measured in a standard test for 4 h at 175°C h Data from Cereclor 42
d Measured in a standard test for 4 h at 180°C i 10 g in 100 ml toluene solvent
e Octanol:water partition coefficients. From: Renberg et al (1980) j Solid, softening point = 95-100°C
2.3.1 Sampling
To prevent contamination by trace amounts of chlorinated
paraffins, samples or their extracts must not be allowed to come into
contact with any plastic (especially PVC) container, stopper, cap
liner or tubing, because these may contain chlorinated paraffins
(Hollies et al., 1979). All solvents should be rigorously tested
before use, and it is recommended that glass distilled solvents are
used. All glassware should be decontaminated before use by heating at
250°C for 24 h. Water and sediment samples should be stored at
ambient temperatures, and should be analysed within a month of
sampling.
Treatment of samples for the extraction of chlorinated paraffins
is described in Table 5.
2.3.2 Analytical methods
Methods used for detection of chlorinated paraffins in various
samples are shown in Table 5.
Hollies et al. (1979) determined C13-17 and C20-30 chlorinated
paraffin after clean-up of the samples on aluminium oxide columns.
The chlorinated paraffin fraction was then applied on a silica gel
thin-layer chromatography (TLC) plate. After forward elution with
n-hexane and subsequently with toluene, and backward elution with
n-hexane, chloride from the chlorinated aliphatics was transferred
to an aluminium oxide plate at 240°C and developed with silver
nitrate. The resulting spots were quantified by visual comparison
with spots of known amounts of reference materials. Although the
procedure is complicated and involves several evaporations to dryness,
good recoveries were reported. Possible interference from a number of
other chlorinated compounds was investigated and found to be
negligible, but the method must still be regarded as fairly
non-specific.
Gas chromatographic analysis of chlorinated paraffin, using
microcoulometric detection, has been described by Zitko (1973). This
method gives badly resolved chromatograms and there is a considerable
risk of interference from other halogenated compounds. Owing to high
temperatures in the gas chromatographic system there is also a risk of
dehydrochlorination of the chlorinated paraffin congeners. In a later
study (Zitko & Arsenault, 1977), interference from other compounds was
avoided by a solvent partitioning clean-up procedure.
Table 5. Analytical methods for the determination of chlorinated paraffins in various samplesa
Sample Preparation method Analytical Sample detection Recovery Reference
matrix methodb limitb
Water Extract with petroleum spirit; concentrate; purify by TLC 500 ng/litre 90% Hollies et
aluminium oxide chromatography, elute with toluene; al. (1979)
dry; dissolve in petroleum spirit
Water Extract with hexane; purify by aluminium oxide GC/ECD 3 ng/litre NR Kraemer &
chromatography; elute with hexane/dichloromethane Ballschmiter
(4%); purify by silica gel chromatography, elute with (1987)
hexane:dichloromethane (19:1); dissolve in isooctane.
Water Extraction with hexane (particle phase Soxhlet GC/ approx. 92-120% Steele et
extracted), silica gel and aluminium oxide column MS-NCI 1 µg/litre al. (1988)
chromatography
Biological Homogenize; extract with petroleum spirit:acetone TLC 50 µg/kg 80-90% Hollies et
material (2:1); dry; dissolve in petroleum spirit; extract with al. (1979)
dimethylformamide; wash; back-extract with Na2SO3
solution and petroleum spirit; purify by silica gel
chromatography, elute with CCl4; dry; dissolve in
acetone; extract with petroleum spirit:acetone (1:4)
Cod muscle Homogenize in n-hexane:acetone (1:2.5, v:v); extract GC/MS NR 98-114% at Jansson et
tissue with 10% diethyl ether in n-hexane; evaporate; dissolve 0.465 µg/sample al. (1991)
in dichloromethane:n-hexane (1:1, v/v); purify by gel and 89-92% at
permeation chromatography; concentrate; extract with 2.33 µg/sample
sulfuric acid; concentrated in organic phase
Adipose Homogenize in dichloromethane; percolate through GC/MS 5 ng 80% Schmid &
tissue anhydrous Na2SO4; remove solvent; dissolve residue in Müller (1985)
pentane; wash, dry and concentrate; purify by alumina
chromatography
Table 5. (Cont'd)
Sample Preparation method Analytical Sample detection Recovery Reference
matrix methodb limitb
Mineral oil Extract fish in cyclohexane; introduce extract or MS-NCI NR NR Gjos &
and fish mineral oil sample directly into mass spectrometer Gustavsen
extract (1982)
Fish fillets Homogenize in petroleum ether; clean-up by irradiating GC/CD NR > 90% Friedman &
extracts with high-intensity UV light (90 min, < 20°C) Lombardo
in petroleum ether (1975)
Sewage Homogenize in acetone; extract with pentane; wash, dry GC/MS 5 ng NR Schmid &
sludge and concentrate; purify by alumina chromatography Müller (1985)
Sediment Dry at 70°C; extract with petroleum spirit; TLC 50 µg/kg 80% Hollies et
concentrate; purify by aluminium oxide al. (1979)
chromatography, elute with toluene; dry; dissolve
in petroleum spirit
Sediment Extract with acetone:hexane (1:1, v:v); wash, dry GC/MS 5 ng NR Schmid &
and concentrate; purify by alumina chromatography Müller (1985)
Sediment Soxhlet extraction with hexane, silica gel and GC/ approx. 52-64% Steele et
aluminium oxide column chromatography MS-NCI 1 µg/litre al. (1988)
a Modified from IARC (1990)
b GC/MS = gas chromatography/mass spectrometry; GC/CD = gas chromatography/coulometric detection;
GC/ECD = gas chromatography/electron capture detection; MS-NCI = negative-ion chemical ionization mass spectrometry;
TLC = thin-layer chromatography; NR = not reported
Attempts have been made to reduce the complexity of chlorinated
paraffin mixtures by reductive dechlorination (Cooke & Roberts, 1980;
Roberts et al., 1981; Sistovaris & Donges, 1987). This method gives
information on the "carbon skeleton" of the chlorinated paraffin
compounds but no information on the chlorine content, and it is
difficult to separate the response from that of unchlorinated
hydrocarbons.
Negative ion chemical ionization mass spectrometry (MS-NCI) was
used by Gjös & Gustavsen (1982). In this method the chlorinated
paraffin fractions are introduced directly into the ion source of the
mass spectrometer. As the whole sample is analysed in a very short
time, the concentration in the ion source is high and the sensitivity
can therefore be high. A serious disadvantage is that all other
compounds in the sample come into the mass spectrometer at the same
time, the risk of interference is high and an extensive clean-up of
the samples is needed.
Gas chromatography utilizing MS-NCI for the detection was used by
Schmid & Müller (1985). A fairly simple clean-up based on adsorption
chromatography on aluminium oxide was used, but unfortunately this
has been impossible to reproduce (Jansson, personal communication).
GC/MS-NCI was also used by Steele et al. (1988) to determine
chlorinated paraffins after clean-up of samples on silica gel and
aluminium oxide columns. They used low inlet temperatures in the
gas chromatograph to avoid thermolysis of the analysed compounds.
The use of low temperatures and short capillary columns further
decreases the risk of temperature-related break-down of chlorinated
paraffins during gas chromatographic analysis (Jansson et al., 1991).
In this method a gel permeation column was also used to avoid
interference from other chlorinated compounds, and the Cl2- and
HCl2- ions were used to detect aliphatic chlorinated compounds
selectively.
Developments in chlorinated paraffin analysis have improved
both selectivity and sensitivity. However, although the reliability
of results is now better, these are only estimates of the real
concentrations as it is impossible to detect the individual substances.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Chlorinated paraffins are not known to occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Liquid chlorinated paraffins were first used in large amounts
during the period 1914-1918 as solvents for Dichloramine T in
antiseptic nasal and throat sprays (Howard et al., 1975). The
commercial production of chlorinated paraffins for use as extreme
pressure additives in lubricants started around 1930 (Schenker, 1979).
Estimated data on the production of chlorinated paraffins are
shown in Table 6. Chlorinated paraffins are produced in Australia,
Brazil, Bulgaria, Canada, China, Germany, France, India, Italy, Japan,
Mexico, Poland, Romania, Spain, Slovakia, South Africa, China
(Province of Taiwan), Thailand, the United Kingdom, the USA, and the
former USSR. However, this may not be a complete list of producer
countries. It is believed that 50% of the chlorinated paraffins
produced in the world have carbon chain lengths of between 14 and 17
and a chlorine content of between 45 and 52%. In the United Kingdom
approximately 80% of the total production of chlorinated paraffins is
concentrated on the C14-17 chain length. About 15% of the European
consumption of chlorinated paraffins is estimated to be C10-13, 70%
C14-17 and 15% C20-30 (Willis et al., 1994).
Chlorinated paraffins are produced by reacting liquid paraffin
fractions obtained from petroleum distillation with pure chlorine gas
by a reaction mechanism involving free radicals (Schenker, 1979;
Houghton, 1993). The reaction is exothermic. At a chlorine content
above approximately 54% further chlorination is slow and difficult.
In the production of resinous chlorinated paraffins containing
> 70% Cl, a solvent is usually added to decrease the viscosity
(Howard et al., 1975). Carbon tetrachloride has been the most
commonly used solvent, and may be present in trace amounts in the
final product, although alternative production methods are being
developed because of the phase-out of carbon tetrachloride under the
Montreal Protocol (D. Farrer, personal communication to the IPCS,
1995).
The substituted chlorine atoms are probably randomly distributed,
and at a chlorination of 72% all of the carbon atoms are singly
chlorinated. Further chlorination is difficult since the first
chlorine substitution decreases the reactivity of the other hydrogens
on a particular carbon atom (Hardie, 1964).
Table 6. Estimated production of chlorinated paraffins
Production References
(tonnes/year)
Canada - 1990 2900 Camford Information
Services (1991)
Germany - 1990/1991 20 000-30 000 BUA (1992)
United Kingdom - 1992 50 000 Willis et al. (1994)
USA - 1977 37 000 Schenker (1979)
USA - 1983 45 000 NTP (1986a)
USA - 1987 45 000 IARC (1990)
USA - 1990 26 000 US EPA (1993)
North America - 1978 60 000 Zitko (1980)
Western Europe - 1978 105 000 Zitko (1980)
Western Europe - 1985 95 000 IARC (1990)
World, excluding 230 000 Campbell &
Eastern Europe - 1977 McConnell (1980)
World - 1985a 300 000 Strack (1986)
a Excluding the former Soviet Union and the People's Republic of China.
Depending on producer and paraffin feedstock, the temperature of
the chlorination reaction is usually kept at 80-100°C to decrease the
viscosity but at a temperature where the decomposition of the product
is not extensive (Schenker, 1979; Houghton, 1993). Since the reaction
is exothermic heat removal is important in the process. Ultraviolet
light is often used as a catalyst (Schenker, 1979; Houghton, 1993).
Metal catalysts are avoided since they may promote dechlorination of
the chlorinated paraffins. Since for each tonne of chlorinated
paraffin produced, approximately half a tonne of hydrochloric acid is
generated, the linings of the reactor vessels must be chemically inert
to avoid the formation of metal chlorides, which cause darkening of
the product by decomposition (Strack, 1986; Houghton, 1993).
Additional procedures used in the production of the C20-30;70% Cl solid
grade are stripping of the solvent and grinding of solid products
(Schenker, 1979).
3.2.2 Uses
Chlorinated paraffins are used as secondary plasticizers for
polyvinyl chloride (PVC) and can partially replace primary
plasticizers such as phthalates and phosphate esters (Houghton, 1993).
The use of chlorinated paraffins has the advantage in comparison with
conventional plasticizers of both increasing the flexibility of
the material as well as increasing its flame retardancy and
low-temperature strength (Howard et al., 1975). Chlorinated paraffins
are also used as extreme pressure additives in metal-machining fluids
or as metal-working lubricants or cutting oils because of their
viscous nature, compatibility with oils, and property of releasing
hydrochloric acid at elevated temperatures. The hydrochloric acid
reacts with metal surfaces to form a thin but strong solid film of
metal chloride lubricant. In Sweden, the use of chlorinated paraffins
in metal-working fluids has been reduced from 680 tonnes (1986) to
139 tonnes (1993) as a part of a risk reduction programme (Swedish
Environmental Protection Agency, 1994). They are added to paints,
coatings and sealants to improve resistance to water and chemicals,
which is most suitable when they are used in marine paints, as
coatings for industrial flooring, vessels and swimming pools (e.g.,
rubber and chlorinated rubber coatings), and as road marking paints.
The flame-retarding properties of highly chlorinated paraffins make
them important as additives in plastics, fabrics, paints and coatings.
The most effective fire-retardant action is obtained with a high
degree of chlorination.
By the late 1970s approximately 50% of chlorinated paraffins in
the USA was used as extreme pressure lubricant additives in the
metal-working industry; 25% was used in plastics and fire-retardant
and water-repellant fabric treatments, and the rest was used in paint,
rubber, caulks and sealants (Schenker, 1979). In the United Kingdom,
65-70% of the consumed chlorinated paraffins is used as a secondary
plasticizer in PVC, about 10% in paint, about 10% in metal-cutting
lubricants and about 10% in flame retardants and sealants (Willis et
al., 1994). In Canada approximately 55% of the chlorinated paraffins
is used as plasticizers and 35-40% as high-pressure lubricant
additives (Camford Information Services, 1991). Some examples of
applications for chlorinated paraffins of different chain-lengths are
shown in Table 7.
3.2.3 Loss into the environment
Since chlorinated paraffins are produced without contact with
water, the possibility of leakage into the environment by direct water
discharge is low. After chlorination the solvent is removed and
residual amounts of chlorine gas and hydrogen chloride are removed by
blowing air or other gases through the product. This could possibly
lead to some loss into the air, but since the chlorine gas and
hydrochloric acid are recovered and the volatility of chlorinated
paraffins is very low, the loss is likely to be very low (Howard et
al., 1975). Emission into the atmosphere during manufacture in
Germany in 1990 was estimated to be about 250 kg/year (BUA, 1992). It
is possible that chlorinated paraffins may be a by-product during
chlorination of other hydrocarbon feedstocks if paraffins are present
as contaminants. This could lead to possible environmental
contamination.
Table 7. Uses of various chlorinated paraffins
Paraffin Chlorination (%) Application
feedstock
C10-13 plasticizer for PVC or plastics
C10-13 metal-working fluids; sealants
C10-13 approx. 70 flame retardants for rubber and soft plastics
C14-17 40-60 extreme pressure additives to metal-machining
fluids, pastes, emulsions and lubricants
C14-17 45-52 the chlorinated paraffin most frequently used as a
(40-50) plasticizer for plastics; also used for sealants
C18-30 approx. 70 flame retardants for rigid plastics such
as polyesters and polystyrene
C> 20 plasticizer for PVC or plastics
Some loss into the environment could be expected during transport
and storage. If the drums which are used for the transport of
chlorinated paraffins are cleaned for further use environmental
release might occur. Soil could be contaminated if empty drums are
dumped at landfills. Spills may occur, but clean-up using an
adsorbent material is easy. The adsorbent material would probably
be deposited in a landfill, which in turn could lead to possible
environmental contamination.
The uses of chlorinated paraffins probably provide the major
source of environmental contamination. When chlorinated paraffins are
used as plasticizers or additives in coatings, they are effectively
dissolved in the polymers and will therefore leak into the environment
only very slowly. However, polymers containing chlorinated paraffins
will act as sources of chlorinated paraffins for centuries after
disposal. A more likely route of leakage of chlorinated paraffins
into the environment would be the improper disposal of oils containing
chlorinated paraffins (Campbell & McConnell, 1980) or disposal of
chlorinated paraffins of low quality (Howard et al., 1975). Loss of
chlorinated paraffins by removal from paints and coatings may also
contribute to environmental contamination.
It is estimated that a maximum of 55% of the cutting and
lubricating oils sold to the engineering industry in Sweden becomes
waste. The rest is consumed or released into the air and water (KEMI,
1991). The largest consumer of chlorinated paraffins in Sweden
(1400 tonnes/year) has estimated its emission of chlorinated paraffins
to be 90 kg/year (0.06 g emission/kg chlorinated paraffin produced)
(KEMI, 1991).
Disposal of wastes containing chlorinated paraffins occurs
through resource recovery, destructive incineration or landfill,
usually on disposal sites for special wastes and in compliance with
local regulations. Owing to their thermal instability, chlorinated
paraffins are expected to be degraded by incineration at low
temperatures and thus would not be expected to volatilize in exhaust
gases from an incinerator. However, in a study by Bergman et al.
(1984), chlorinated aromatic compounds such as PCBs, naphthalenes and
benzenes were formed by pyrolysis of chlorinated paraffins (see
section 4.2.1) although the conditions used were not identical to the
operation conditions of waste incineration plants. Chlorinated
paraffins are not expected to be formed de novo. The disposal of
chlorinated paraffins in landfills may give rise to leaching into
water, but owing to the low water solubility and strong adsorption
onto solids the amounts reaching water are likely to be low.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Considering the low vapour pressure (2 × 10-4 Pa at 20°C for
C14-17;52% Cl to 3 × 10-3 Pa at 65°C for C23;42-54% Cl), low water
solubility (3 to 470 µg/litre) and highly lipophilic nature of
chlorinated paraffins (log Pow values range from 4.39 to > 12.83),
it is likely that they will distribute mainly to the soil/sediment
phase with very little volatilization occurring. Chlorinated
paraffins are likely to be transported in water as suspended
particles, and in air as dust particles and possibly in the vapour
phase. However, no experimental data on this subject have been
reported.
4.2 Transformation
4.2.1 Abiotic transformation
No experimental data on the chemical stability of chlorinated
paraffins under simulated environmental conditions have been reported.
However, their chemical reactivities suggest that they do not
hydrolyse, oxidize or react by other mechanisms at significant rates
under normal temperatures and neutral conditions (Howard et al.,
1975). Dehydrochlorination of chlorinated paraffins may possibly
occur naturally in the presence of metal ion contamination.
Because of the high adsorption tendency of chlorinated paraffins,
gas phase reactions are assumed to contribute only little to
degradation in the atmosphere (BUA, 1992). However, calculated
half-lives for chlorinated paraffins in air are reported to range from
0.85 to 7.2 days (Slooff et al., 1992). The theoretical values are
shown in Table 8.
The thermal degradation by pyrolysis of chlorinated paraffins at
various temperatures (300, 500, 700°C) and times (10 sec to 20 min)
was studied by Bergman et al. (1984). The chlorinated paraffins were
totally degraded, and, depending upon degree of chlorination of the
chlorinated paraffin, several aliphatic and aromatic degradation
products, such as polychlorinated biphenyls (PCBs), naphthalenes and
benzenes, were detected. As much as 10 g of PCBs per kg chlorinated
paraffin could be found after thermal degradation of C12;70% Cl
(temperature not specified). Smaller amounts of compounds were formed
at lower temperatures. Considering these results, processes where
chlorinated paraffins are subjected to temperatures above 300°C could
lead to environmental contamination and exposure to more persistent
and toxic substances than the original chlorinated paraffins.
Table 8. Photochemical degradation of chlorinated paraffins in the
atmosphere (From: Slooff et al., 1992)
Carbon chain length Koh (cm3/mol per sec) Half-life (days)
C10-C13 9.0-14.9 × 10-12 1.2-1.8
C14-C17 14.9-18.9 × 10-12 0.85-0.8
C15-C30 20.2-31.1 × 10-12 0.5-0.8
not specified 2.2-18.8 × 10-12 0.85-7.2
4.2.2 Biodegradation
Chlorinated paraffins are generally stable in the natural
environment.
4.2.2.1 Short chain length chlorinated paraffins
A short chain length paraffin (C10-12) with 58% chlorination
(CP-SH) was not readily biodegraded by activated sludge, under either
aerobic or anaerobic conditions, over a 28-day period in an inherent
biodegradability (modified Zahn-Wellens) test (Street & Madeley,
1983a,b) or a 51-day period in a coupled units test (Mather et al.,
1983).
4.2.2.2 Long chain length chlorinated paraffins
Zitko & Arsenault (1977) studied sediment spiked with 596 mg/kg
dry weight of Cereclor 42 C24;42% Cl, CP-LL) or 357 mg/kg dry weight
of Chlorez 700 (C24;70% Cl, CP-LH), which are both long chain length
chlorinated paraffins but have different chlorine contents. There was
no clear trend in the results but they indicated that after 4 weeks
the highly chlorinated Chlorez 700 was degraded to a greater extent
than Cereclor 42. The rate of degradation seems to have been higher
under anaerobic conditions.
4.2.2.3 Comparative studies
In another study, the microbial degradation of several short,
intermediate and long chain length chlorinated paraffins of different
chlorination degree at concentrations of 2, 10 and 20 mg/litre was
examined in a 25-day biochemical oxygen demand (BOD) test (Madeley &
Birtley, 1980). The degradation rate appeared to decrease with
increasing carbon chain length and chlorination degree, and the short
chain chlorinated paraffins with less than 50% Cl were degraded most
rapidly and completely. It can be concluded from the results that
chlorinated paraffins with low chlorine contents (< 50% wt Cl) and,
especially, short chain chlorinated paraffins, biodegrade slowly in
the environment, particularly in the presence of adapted microbial
populations, but that chlorinated paraffins with higher chlorine
contents are unlikely to biodegrade under aerobic conditions.
Anaerobic microorganisms did not degrade Cereclor 42 (C24;42% Cl) in
30 days when readily biodegradable alternative carbon sources were
available.
Omori et al. (1987) found that bacterial strains isolated from
the soil degraded various chlorinated paraffins by dechlorination in
the presence of n-hexadecane. In a mixed culture of four strains,
more than 50% of the chlorine was removed from the shorter paraffins
with lower chlorine content (C14;43% Cl, CP-ML and C15;50% Cl, CP-MH)
within 36 h. Lower amounts of chlorine were removed from the
chlorinated paraffins with longer chain lengths. Activated sludge
from a sewage treatment plant in Tokyo acclimatized to n-hexadecane
for 60 days showed only a limited amount (2%) of dechlorination of the
chlorinated paraffins. The bacterial dechlorination concerned the
terminal chlorine, which produced 2- or 3-chlorinated fatty acids via
ß-oxidation.
4.3 Bioaccumulation and biomagnification
4.3.1 Summary
Chlorinated paraffins of short chain length accumulate in mussels
and fish to a higher degree than intermediate and long chain length
chlorinated paraffins.
Data on bioaccumulation of chlorinated paraffins by aquatic
organisms are summarized in Table 9. Bioconcentration factors (BCFs)
may be uncertain since the applied doses exceeded the water solubility
in several experiments.
Table 9. Bioconcentration factors for some chlorinated paraffins
Species Chlorinated paraffina Exposure Bioconcentration Reference
Concentration Duration factor
(µg/litre) (days) (whole animal)b
Marine diatom C10-12;58% Cl (CP-SH) 1.4 10 < 1 Thompson & Madeley (1983b)
(Skeletonema costatum) 17.8 10 3.5
Freshwater green alga C10-12;58% Cl (CP-SH) 35 10 1.5 Thompson & Madeley (1983d)
(Selenastrum capricornutum) 140 10 7.6
150 10 4.1
Mussel C10-12;58% Cl (CP-SH) 2.3 147 40 900e Madeley et al. (1983a)
(Mytilus edulis) 10 91 24 800e
C10-12;58% Cl (CP-SH) 13 60 25 292e Madeley & Thompson (1983a)
130 60 12 177e
C12;69% Cl (CP-SH) 0.13 28 138 000e Renberg et al. (1986)
C16;34% Cl (CP-ML) 0.13 28 6920e
C14-17;52% Cl (CP-MH) 220 60 2856e Madeley & Thompson (1983b)
3800c 60 429e
C22-26;43% Cl (CP-LL) 120 60 1158e Madeley & Thompson (1983c)
2180c 60 261e
C22-26;70% Cl (CP-LH) 460 60 341e Madeley & Thompson (1983d)
1330c 60 223e
Table 9. (Cont'd)
Species Chlorinated paraffina Exposure Bioconcentration Reference
Concentration Duration factor
(µg/litre) (days) (whole animal)b
Rainbow trout C10-12;58% Cl (CP-SH) 3.1 168 3550e Madeley & Maddock (1983b)
(Oncorhynchus mykiss) 14 168 5260e
C10-12;58% Cl (CP-SH) 33 60 7155e Madeley & Maddock (1983c)
3050c 60 1173e
C14-17;52% Cl (CP-MH) 1050c 60 45e Madeley & Maddock (1983c)
4500c 60 67e
C22-26;43% Cl (CP-LL) 970 60 18e Madeley & Maddock (1983c)
4000c 60 38e
C20-30;70% Cl (CP-LH) 840 60 54e Madeley & Maddock (1983c)
3800c 60 32e
Bleaks C10-13;49% Cl (CP-SL) 125 14 770d,f Bengtsson et al. (1979)
(Alburnus alburnus) 59% Cl (CP-SH) 125 14 740d,f Bengtsson et al. (1979)
71% Cl (CP-SH) 125 14 140d,f Bengtsson et al. (1979)
C14-17;50% Cl (CP-MH) 125 14 30d,f Bengtsson et al. (1979)
C18-26;49% Cl (CP-LL) 125 14 7d,f Bengtsson et al. (1979)
a The classification of chlorinated paraffins is given in Table 1
b Ratio of the concentration of the chemical in the organism to the concentration of the chemical in the environment or food
c May exceed water solubility
d BCFs calculated by Zitko (1980)
e BCFs based on radioactivity (14C)
f BCFs based on parent compounds
4.3.2 Aquatic vertebrates
4.3.2.1 Short chain length chlorinated paraffins
In a study by Lombardo et al. (1975), fingerling rainbow trout
(Oncorhynchus mykiss) were fed a diet containing 10 mg/kg Chlorowax
500C (C12;60% Cl, CP-SH) for 82 days. Samples of 20 exposed and 10
control fish were collected at approximately 2-week intervals during
the time-period and analysed for chlorinated paraffin content by
microcoulometric gas chromatography (Friedman & Lombardo, 1975). The
tissue level of chlorinated paraffins rose during the treatment period
to 1.1 mg/kg (on tissue basis) or 18 mg/kg (on fat basis) after 82
days (detection level: 0.5 mg/kg). The experiment had to be
terminated owing to the failure of the water supply, and it was not
possible to determine whether a steady-state level had been reached.
In studies (Madeley & Maddock, 1983b) on rainbow trout
(Oncorhynchus mykiss) exposed to measured concentrations of 3.1 and
14.3 µg/litre of 14C-labelled C10-12; 58% Cl (CP-SH) for a period of
168 days, BCF value of 1300 (low dose) and 1600 (high dose) were
observed in the flesh, 2800 (low dose) and 16 000 (high dose) in the
liver, and 11 700 (low dose) and 15 500 (high dose) in the viscera;
all values were determined from radioactivity measurements. The BCF
for the whole body was 3350 (low dose) and 5260 (high dose)
(calculated values). Half-lives for elimination in different organs
were calculated to be the following: liver 9.9 (low dose) and 11.6
days (high dose), viscera 23.1 (low dose) and 23.9 days (high dose),
and flesh 16.5 (low dose) and 17.3 days (high dose).
Rainbow trout (Oncorhynchus mykiss) were exposed to measured
concentrations ranging from 33 to 3050 µg/litre of C10-12;58% Cl
(CP-SH) for 60 days (Madeley & Maddock, 1983c). BCFs, which were
determined in whole fish samples at the end of the test, were 7155
(low dose) and 1173 (high dose) based on radioactivity measurements,
and 7273 (low dose) and 574 (high dose) based on parent compound
analysis. Parent compound analysis was performed using a modification
of the method of Hollies et al. (1979) (see section 2.3.2).
4.3.2.2 Intermediate chain length chlorinated paraffins
After 60 days exposure of rainbow trout (Oncorhynchus mykiss)
to measured concentrations of 1050 and 4500 µg/litre of intermediate
length (C14-17) chlorinated paraffins with 52% Cl (CP-MH), whole body
BCFs of 45 (low dose) and 67 (high dose) based on radioactivity
measurements, and of 32 (low dose) and 42 (high dose) based on parent
compound analysis were determined (Madeley & Maddock, 1983c). The
BCFs were determined at the end of the exposure period. Parent
compound analysis was performed using a modification of the method of
Hollies et al. (1979) (see section 3.2.3).
4.3.2.3 Long chain length chlorinated paraffins
Juvenile Atlantic salmon (Salmo salar) were exposed to Cereclor
42 (C24;42% Cl, CP-LL) or Chlorez 700 (C24;70% Cl, CP-LH) by uptake
from suspended solids or from food (Zitko, 1974a). The fish were
treated with either 1000 µg/litre of contaminated suspended solids for
48 h and 144 h, or to 10 mg/kg or 100 mg/kg of contaminated food for
181 days with a subsequent elimination period of 74 days. No or very
low accumulation of the chlorinated paraffins was observed. However,
the analytical method used determined the amount of chlorine and not
of chlorinated paraffin; this method has been considered as nonspecific
and of low sensitivity.
After 60 days exposure of rainbow trout (Oncorhynchus mykiss)
to long chain chlorinated paraffins with 43% Cl (CP-LL) (970 or
4000 µg/litre) or 70% Cl (CP-LH) (840-3800 µg/litre), whole body BCFs,
based on measured exposure concentrations, of 17.9 (low dose, 43% Cl),
37.6 (high dose, 43% Cl) and 53.8 (low dose, 70% Cl) and 32.5 (high
dose, 70% Cl) were determined at the end of the study when measured as
radioactivity. BCF values of 3.6 (low dose, 43% Cl), 9.0 (high dose,
43% Cl), 42.8 (low dose, 70% Cl) and 31.6 (high dose, 70% Cl), based
on parent compound analysis, were determined (Madeley & Maddock,
1983c).
4.3.3 Aquatic invertebrates
4.3.3.1 Short chain length chlorinated paraffins
After 60 days exposure of mussels (Mytilus edulis) to a short
chain length paraffin with 58% Cl (CP-SH) at measured concentrations
of 13 and 130 µg/litre, whole body BCFs were 25 292 and 12 177,
respectively, based on radioactivity measurements, and 20 000 and
7923 when measured as parent compound (Madeley & Thompson, 1983a).
After exposure of mussels (Mytilus edulis) for 147 days to
14C-labelled short chain length chlorinated paraffin with 58% Cl
(CP-SH) followed by a depuration period of 98 days (measured exposure
dose: 2.3 µg/litre), or for 91 days followed by 84 days of depuration
(measured exposure dose: 10.1 µg/litre), plateau levels of the
chlorinated paraffin in tissues were reached. Bioconcentration
factors (BCFs) at the plateau levels were 40 900 for whole mussel
tissue after exposure to 2.35 µg/litre and 24 800 after exposure to
10.1 µg/litre based on wet tissue basis. Of the different organs the
digestive glands had the highest BCF values of 226 000 (low exposure)
and 104 000 (high exposure). Half-lives for the chlorinated paraffin
in whole mussel tissue were 9.2-9.9 days (10.1 µg/litre) and 13.1-19.8
days (2.35 µg/litre) (Madeley et al., 1983a).
4.3.3.2 Intermediate chain length chlorinated paraffins
After 60-day exposures of mussels (Mytilus edulis) to
intermediate chain length paraffin with 52% Cl (CP-MH) at measured
concentrations of 220 and 3800 µg/litre (which was above the limit
of solubility in water), whole body BCFs were 429-2856 based on
radioactivity measurements and 339-2182 based on parent compound
analysis (Madeley & Thompson, 1983b).
4.3.3.3 Long chain length chlorinated paraffins
In mussels exposed to measured concentrations of 120-2180
µg/litre of long chain length chlorinated paraffin with 43% Cl (CP-LL)
and 460-1330 µg/litre of long chain length chlorinated paraffin with
70% Cl for 60 days, whole body BCFs of 1158-261 (43% Cl) and 341-223
(70% Cl), respectively, were observed when measured as radioactivity,
and 87.2-1000 (43% Cl) and 105-167 (70% Cl) when based on parent
compound analysis (Madeley & Thompson, 1983c,d). However, the high
doses exceeded the water solubility of the chlorinated paraffins.
4.3.3.4 Comparative studies
The accumulation during four weeks of two 14C-labelled
chlorinated paraffins, polychloro[1-14C]hexadecane (C16;34% Cl,
CP-ML) and 1-chloropolychloro[1-14C]dodecane (C12;69% Cl, CP-SH), was
studied in the mussel (Mytilus edulis) by Renberg et al. (1986).
Both compounds showed rapid uptake when added at concentrations of
0.13 µg/litre (C16;34% Cl) and 0.0029 µg/litre or 0.13 µg/litre
(C12;69% Cl) in water for 28 days. Steady-state levels were reached
within 14 days after exposure to 0.13 µg/litre. The authors
calculated the BCF values to be 6920 for C16;34% Cl and 138 000 for
C12;69% Cl, based on fresh weight. The mussels exposed to C12;69% Cl
were studied for an additional 28 days without exposure. The
elimination rate for this chlorinated paraffin was slow, and 33% of
the radioactivity remained in the tissues after 28 days.
4.3.4 Aquatic plants
The BCF after 10 days exposure to short chain chlorinated
paraffin with 58% chlorination (CP-SH) has been estimated to be 3.5
for the diatom Sceletonema costatum (17.8 µg/litre) and 1.5 for the
green alga Selenastrum capricornutum (35 µg/litre) (Thompson &
Madeley, 1983a,b).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Techniques for the analysis of chlorinated paraffin are described
in section 2.3.2. The major problem connected with the analysis of
environmental samples is interference from other compounds. In
earlier studies, when pre-separation techniques were not as well
developed, the concentrations may have been overestimated. Another
problem is that the chlorinated paraffin composition in the
environment may be different from that of the original products,
and the quantitative analysis has to be based on comparisons with
the original products. These difficulties make it clear that
analytical results have to be regarded more as estimates than exact
concentrations.
5.1.1 Air
No information on levels in the atmosphere has been found in the
literature.
5.1.2 Water and sediment
Levels of chlorinated paraffins in water and sediment in the
United Kingdom are summarized in Table 10. Chlorinated paraffins have
been detected in United Kingdom sea water at levels in the range of
0.5-4.0 µg/litre for C10-20 and less than 2.0 µg/litre for C20-30
(Campbell & McConnell, 1980). The levels in sediments from the same
areas were analysed; chlorinated paraffins were detected only in a few
samples at levels up to 500 µg/kg wet weight for C10-20 and 600 µg/kg
for C20-30. The levels of chlorinated paraffins are low in water from
rivers and reservoirs not receiving industrial/domestic effluents
(C10-20, 1 µg/litre or below; C20-30, 2 µg/litre or below) and for
waterways in industrialized regions (C10-20, up to 6.0 µg/litre; C20-30,
0.5 µg/litre or below). The level of C10-20 in the latter regions was
higher than for C20-30. Chlorinated paraffins were not detected in
five drinking-water reservoirs either in the water (detection limit:
0.5 µg/litre) or the sediment (detection limit: 250 µg/kg) (Campbell &
McConnell, 1980). The levels of C10-20 in sediment from non-marine
water remote from industry were in the range up to 1000 µg/kg, except
for a sewage sludge sample from the Liverpool area, which had levels
of 4000-10 000 µg/kg. C20-30 was detected only in one sample at
50 µg/kg. The levels of chlorinated paraffins in sediments close to
industrial plants were found to be higher (C10-20 up to 15 000 µg/kg;
C20-30 up to 3200 µg/kg wet weight). The levels in sediment from these
industrial areas were 1000 times higher than in the overlaying water
columns, indicating the ability of chlorinated paraffins to adsorb on
suspended solids.
Table 10. Levels of chlorinated paraffins in United Kingdom water (µg/litre) and sediment (µg/kg)
(From: Campbell & McConnell, 1980)a
C10-20 C20-30
Range Median No. of samples Range Median No. of samples
level below detection level below detection
limit limit
Sea water
water ND-4.0 0.5 7/15 ND-2.0 ND 13/18
sediment ND-500 ND 14/18 ND-600 ND 15/18
Fresh water remote from industry
water ND-1.0 0.5 7/13 ND-2.0 ND 7/11
sediment ND-1000 ND 4/6 ND-< 250 ND 4/5
Fresh water close to industry
water ND-6.0 1-2 4/25 ND-0.5 ND 8/10
sediment ND-15 000 1800 2/21 ND-3200 50 3/9
a ND = not detected (detection limit in water = 0.5 µg/litre; in sediment = 50 µg/kg)
Levels of chlorinated paraffins in water and sediment in Germany
are summarized in Tables 11 and 12. In 1987, chlorinated paraffins
were detected at concentrations of about 1 µg/litre for C10-13 and
20 µg/litre for C14-18 in the Danube, downstream of the confluence
with the River Lech (BUA, 1992). The corresponding contents in the
sediment were 300 and 1800 µg/kg dry weight, respectively. In 1994,
chlorinated paraffin concentrations in the Danube and River Lech were
in the range of 0.05 to 0.12 µg/litre for C10-C13 and < 0.05 to
0.19 µg/litre for C14-C17. Also in 1994, the chlorinated paraffin
concentrations (C10-C13) in sediment varied from 6-10 µg/kg dry
weight in the lake of Constance to maximum concentrations of 76 µg/kg
dry weight in the River Lech and 83 µg/kg dry weight in the Rhine
(BUA, 1992).
Table 11. Concentrations (µg/litre) of short and intermediate chain length
chlorinated paraffins in surface water in Germany (From: BUA, 1992)a
Location 1987 1994
C10-13 C14-18 C10-13 C14-17
River Lech at Augsburg 0.05 < 0.05
River Lech at Gersthofen 0.50 4.5 0.08 0.09
(upstream from a chlorinated
paraffin production plant)
River Lech at Langweid 0.60 4.0 0.10 0.19
(downstream from a chlorinated
paraffin production plant)
River Lech at Rain 0.12 0.17
River Danube at Marxheim 1.2 4.0 0.06 < 0.06
(downstream from the mouth of
the River Lech)
River Danube at Marxheim (upstream 1.2 20 0.06 0.07
from the mouth of the River Lech)
a The data from 1994 were produced with a more specific method than those from 1987.
The two data sets are therefore difficult to compare.
Table 12. Concentrations (µg/kg dry weight) of short and intermediate chain length
chlorinated paraffins in sediments from Germany (From: BUA, 1992)a
Location 1987 1994
C10-13 C14-18 C10-13 C14-17
Bodensee (middle)
0-5 cm depth 9-10 70
5-12 cm depth 6-9 < 10
River Rhine (141 km) at Rheinfelden 33-38 60
River Rhine (152 km) at Rheinfelden,
upper layer 53-60 140
lower layer 26-32 85
River Rhine, near German-Dutch
border (2 sites) 60-83 145-205
River Main (3 sites) 24-55 160-260
Outer Alster, Hamburg 35-36 370
River Elbe at Hamburg (2 sites) 16-25 130-230
River Lech, upstream from 400 2200 < 5-7 < 10
chlorinated paraffin production
plant
River Lech, downstream from 700 1700 70-76 325
chlorinated paraffin production
plant
Danube downstream from mouth 300 1800
of the River Lech
a The data from 1994 were produced with a more specific method than those from 1987.
The two data sets are therefore difficult to compare.
Chlorinated paraffins were detected in water samples collected
in the Bermuda Islands (Kraemer & Ballschmiter, 1987). Water samples
down to a depth of 1200 m were analysed, and the highest concentration,
about 50 µg/litre, was found in the surface film. Chlorinated paraffins
were not detected in water from the Maldives (detection limit:
3 ng/litre).
In surface sediment from Lake Zürich, 5 µg/kg of a chlorinated
paraffin mixture with a carbon chain length of C14-18 and 52% chlorine
(CP-MH) was measured (Schmid & Müller, 1985). In the same study it
was reported that sewage sludge from a sewage treatment plant in an
urban industrialized region with known contamination of heavy metals
and organochlorine compounds contained chlorinated paraffins at a
concentration of 30 000 µg/kg.
In a field study performed by the US Environmental Protection
Agency (Murray et al., 1988), samples from two watersheds were
analysed by the method of Schmid & Müller (1985). Both were close to
a chlorinated paraffin manufacturing plant (Sugar Creek, Ohio) or
industry using lubricating oils (Tinkers Creek, Ohio). Chlorinated
paraffins were detected in most samples from Sugar Creek in the low
ppb range (< 8 µg/litre) near drainage and downstream from the plant.
Only a few samples upstream from the plant contained detectable
levels of chlorinated paraffins (< 0.3 µg/litre) (detection limit:
0.15 µg/litre). Of the three chlorinated paraffin fractions studied, the
fraction containing the long carbon chain length C20-30; 40-50% Cl
(CP-LL) was generally present at highest concentration. Sediments
contained higher concentrations. As much as 170 000 µg/kg dry weight
of C20-30 was detected in sediment in an impoundment lagoon, whereas
50 000 µg/kg of C14-17;50-60% Cl (CP-MH) and 40 000 µg/kg C10-12;
50-60% Cl (CP-SH) were measured in the same sample. In mussels
(family Unionidae) collected downstream from the plant, there were
detectable levels of chlorinated paraffins (280 µg/kg C10-12, 170 µg/kg
C14-17, 180 µg/kg C20-30). The report concluded that the observed
levels were most likely due to the manufacturing plant. The samples
collected in Tinkers Creek did not contain detectable amounts of
chlorinated paraffins. Most of the samples contained organic
compounds, largely halogenated aromatics, at levels high enough to
interfere and mask the presence of chlorinated paraffins. Chlorinated
paraffins were detected in samples collected from the process wastes
stream inside the chlorinated paraffin plant. The levels in these
samples were: C10-12, 8.1 µg/kg; C14-17, 1.3 µg/kg; C20-30, 2.2 µg/kg.
In 1979, 51 water samples and 51 bottom sediment samples were
collected at 17 sites in Japan and analysed for the presence of
chlorinated paraffins (C8-32). Chlorinated paraffins were not
detected in any of the water samples, while 24 bottom sediment samples
from 11 sites contained chlorinated paraffins at concentrations of 600
to 10 000 µg/kg (wet or dry weight not specified) (Environment Agency,
Japan, 1981). In 1980, 120 water samples and 120 bottom sediment
samples were collected at 40 sites in Japan and were analysed for the
presence of chlorinated paraffins (C8-32). Chlorinated paraffins
were not detected in any of the water samples but 31 bottom sediment
samples from 13 sites contained chlorinated paraffins at concentrations
of 500 to 8500 µg/kg. However, the analytical methods were not
specified and the detection limits were high (the detection limit was
10 µg/litre for water and 500 µg/kg for bottom sediment) (Environment
Agency, Japan, 1983).
5.1.3 Soil
No data on the occurrence of chlorinated paraffins in soil have
been reported.
5.1.4 Aquatic and terrestrial organisms
The levels of chlorinated paraffins in organisms from various
ecosystems in Sweden, determined using the method of Jansson et al.
(1991), are shown in Table 13. Chlorinated paraffins were found in
all samples in the range of 130-4400 ng/g lipid (6.6-210 µg/kg tissue)
(Jansson et al., 1993). The terrestrial animals rabbit and moose had
higher chlorinated paraffin concentration in their fat than any of the
aquatic animals. The chlorinated paraffin levels in fish-eaters were
approximately the same as in the fish, compared to the dioxin and PCB
levels, which were several times higher in fish-eaters. The levels
in seal and herring indicated no or only low biomagnification of
chlorinated paraffins in their food chains.
In a study by Campbell & McConnell (1980), chlorinated paraffins
were detected in mussels, fish, seals and seabirds, as well as in
seabird eggs, using the analytical method of Hollies et al. (1979)
(Table 14). The levels of C10-20 were higher than those of C20-30 in
most organisms. Mussels collected close to a chlorinated paraffin
plant effluent discharge had levels of C10-20 up to 12 000 µg/kg. The
measured levels in the organisms were close to the levels in the
sediment near the organisms, but 100 to 1000 times higher than those
of water, thus indicating bioaccumulation. The authors also detected
trace amounts of chlorinated paraffins (C10-20) in the tissues of sheep
grazing near a plant producing chlorinated paraffins. The tissues
containing the highest levels were liver (200 µg/kg), fat and kidney
(50 µg/kg). The fleeces of the sheep contained higher levels
(350 µg/kg), and the authors suggest that this might have been due to
aerial transport. No chlorinated paraffin could be detected in sheep
grazing far from plants producing chlorinated paraffins (detection
limit = 50 µg/kg).
Table 13. The levels of chlorinated paraffins in various terrestrial and aquatic
organisms in Swedena
Organism Samplesc Tissue Extracted CP (µg/kg CP (µg/kg
lipid (%) lipid) tissue)d
Rabbit 15 muscle 1.1 2900 31.9
Moose 13 muscle 2.0 4400 88
Reindeer 31 fat 56 140 78
Whitefish 35 muscle 0.66 1000 6.6
Arctic char 15 muscle 5.3 570 30.2
Herring (Bothnian Sea) 100 muscle 5.4 1400 75.6
Herring (Baltic Proper) 60 muscle 4.4 1500 66
Herring (Skagerrack) 100 muscle 3.2 1600 51.2
Ringed sealb 7 blubber 88 130 114.4
Grey seal 8 blubber 74 280 207
Osprey 35 muscle 4.0 530 21.2
a Data from Jansson et al. (1993)
b The ringed seal was from Kongsfjorden, Svalbard, Sweden
c Pooled samples
d Calculated from the reported data
In a study by Campbell & McConnell (1980), chlorinated paraffins
were detected in mussels, fish, seals and seabirds, as well as in
seabird eggs, using the analytical method of Hollies et al. (1979)
(Table 14). The levels of C10-20 were higher than those of C20-30 in
most organisms. Mussels collected close to a chlorinated paraffin
plant effluent discharge had levels of C10-20 up to 12 000 µg/kg. The
measured levels in the organisms were close to the levels in the
sediment near the organisms, but 100 to 1000 times higher than those
of water, thus indicating bioaccumulation. The authors also detected
trace amounts of chlorinated paraffins (C10-20) in the tissues of sheep
grazing near a plant producing chlorinated paraffins. The tissues
containing the highest levels were liver (200 µg/kg), fat and kidney
(50 µg/kg). The fleeces of the sheep contained higher levels
(350 µg/kg), and the authors suggest that this might have been due to
aerial transport. No chlorinated paraffin could be detected in sheep
grazing far from plants producing chlorinated paraffins (detection
limit = 50 µg/kg).
Table 14. Chlorinated paraffins in organisms in the United Kingdom
(Campbell & McConnell, 1980)
Species Tissuea No. of Carbon chain-length and
specimens concentration in tissues
analysedb (means and ranges)c
C10-20 (µg/kg) C20-30 (µg/kg)
mean range mean range
Aquatic organisms
Plaice NS 6 30 ND-200 30 ND-200
(Pleuronectes platessa)
Pouting NS 4 100 ND-200 ND ND
(Trisopterus luscus)
Mussel NS 9 3250 100-12 000 10 NDe-100
(Mytilus edulis)
Pike NS 2 25 ND-50 25 ND-50
(Esox lucius)
Grey seal Liver 4 75 40-100 ND ND
(Halichoerus grypus) and
blubber
Table 14. Cont'd
Species Tissuea No. of Carbon chain-length and
specimens concentration in tissues
analysedb (means and ranges)c
C10-20 (µg/kg) C20-30 (µg/kg)
mean range mean range
Birds
Heron Liver NR NR 500-1200 NR NDe
(Ardea cinerea) Liver NR NR 100-1000 NR 100-1500
Guillemot Liver NR NR 100-1100 NR NDe
(Uria aalge)
Herring gull Liver NR NR 200-900 NR 100-500
(Larus argentatus)
Seabird eggsd 23 220 ND-2000 20 ND-100
a NS = Not specified
b NR = Not reported
c ND = Not detected (detection limit = 50 µg/kg, except where stated otherwise)
d 9 species
e Detection limit = 100 µg/kg
In 1980, 108 fish samples were collected at 28 places in Japan
and were analysed for the presence of chlorinated paraffins.
Chlorinated paraffins were not detected in any of the samples. The
detection limit was 500 µg/kg (Environment Agency, Japan, 1983).
5.1.5 Food and beverages
Chlorinated paraffins, mostly C10-20, were detected in various
food products in a limited study (Table 15) using the analytical
method of Hollies et al. (1979). C20-30 chlorinated paraffins were
detected only in a few samples. C10-20 chlorinated paraffins were
found at levels up to 500 µg/kg in approximately 70% of the samples
(Campbell & McConnell, 1980).
Chlorinated paraffin residues have been detected in fish and
sheep (see section 5.1.4).
Table 15. Chlorinated paraffins (C10-20) in human foodstuffs
(Campbell & McConnell, 1980)
Foodstuff class No. of foodstuff Concentration
samples tested (µg/kg)
Dairy products 13 300
Vegetable oils and derivatives 6 150
Fruit and vegetables 16 25
Beverages 6 NDa
a Not detected (detection limit = 50 µg/kg)
5.2 General population exposure
Chlorinated paraffins have been detected on human hands (Campbell
& McConnell, 1980). The hands of eight human volunteers were swabbed
with toluene, and all had chlorinated paraffin levels of 0.8-4.0 µg
C10-20 (per two hands). C20-30 chlorinated paraffins (2 µg)
were detected only on one person. The authors suggested that the
chlorinated paraffin content in foodstuffs, as well as direct transfer
from manufactured articles via the hands, could contribute to human
exposure.
Owing to the high octanol-water partition coefficient, it is
likely that the principle source of exposure of the general population
to chlorinated paraffins is food. However, due to lack of data,
exposure to chlorinated paraffins via other routes cannot be ruled
out.
5.2.1 Concentrations in human tissues
Chlorinated paraffins have been detected in postmortem tissue
samples. Campbell & McConnell (1980) measured the amount in 24 tissue
samples and found up to 600 µg/kg of C10-20 in adipose tissue (median
level: 100-190 µg/kg), up to 500 µg/kg in kidney (median level below
90 µg/kg) and up to 1500 µg/kg in liver (median level below 90 µg/kg).
C20-30 chlorinated paraffins were detected only in a few samples and
at a low level. Schmid & Müller (1985) also detected chlorinated
paraffins in adipose tissue at a concentration of 200 µg/kg.
5.3 Occupational exposure
Occupational exposure to chlorinated paraffins is likely among
workers in production plants or in industries using chlorinated
paraffins. The US National Occupational Exposure Survey (1981-1983)
indicated that 573 000 workers, including 38 000 women, were
potentially exposed to chlorinated paraffins (NIOSH, 1990). In
Denmark, it was reported that 60 000 workers in 175 companies have
been potentially exposed to chlorinated paraffins since 1964 (the
amount produced and imported annually is 5000 tonnes) (Hansen et al.,
1992).
Campbell & McConnell (1980) detected chlorinated paraffins on
human hands using the method of Hollies et al. (1979) (see section
2.3.2). When the hands of a worker in a chlorinated paraffin
laboratory were swabbed with toluene, 800 µg of C10-20 and 400 µg
of C20-30 were detected.
Historical exposure data from machine shops, reported as
reflecting worst-case situations, indicated exposures to chlorinated
paraffins of 0.003-1.15 mg/m3 for operation such as milling, cutting
and grinding (HSE, 1992). Other exposure data suggested exposures to
chlorinated paraffins ranging from 0.003 to 0.21 mg/m3. However,
it is not clear if these levels of exposure were intermittent or
time-weighted averages. There is no information on whether or not
chlorinated paraffin aerosols are in the inhalable size range.
Occupational exposure to short chain chlorinated paraffins has
been estimated by the United Kingdom Health and Safety Executive by
mathematical modelling using the "Estimation and Assessment of
Substance Exposure" (EASE), developed as part of the guidance on new
and existing substances by the Commission of the European Union (CEU)
(HSE, 1992). This model takes into account physico-chemical data such
as vapour pressure and process details such as temperatures and the
use of local exhaust ventilation. It does not take into account the
attenuating effect of decontamination of equipment or use of personal
protective equipment.
During the production of short chain chlorinated paraffins in
closed systems, exposure is likely to be intermittent, and the model
predicts that inhalation exposure to a substrate with a vapour
pressure of less than 0.001 kPa is negligible (0 to 0.1 ppm).
Assuming a non-dispersive pattern of use and intermittent skin
contact, the model predicts that exposures of the hand and forearm
will be in the range of 0.1-1 mg/cm2 per day. During formulation of
short chain chlorinated paraffins (in closed systems) the model
predicts negligible (0-0.1 ppm) inhalation exposure with formulation
process temperatures of 40-50°C. Skin exposure to the hands and
forearms during formulation is predicted to be 0.1-1 mg/cm2 per day.
Occupational exposure to short chain chlorinated paraffins during
their use as metal-working fluids is extensive and the model predicts
exposure of 0.1-1.5 mg/cm2 per day, assuming a chlorinated paraffin
content in the fluid of 2-10%, or 5-15 mg/cm2 per day for speciality
fluids which may contain more than 80% chlorinated paraffin.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
There is a lack of systematic investigation of the influence of
carbon chain length and degree of chlorination in studies on the
kinetics of chlorinated paraffins. Studies have almost exclusively
concerned short and intermediate chain length chlorinated paraffins.
6.1 Absorption
6.1.1 Oral exposure
Chlorinated paraffins are absorbed after oral administration.
From the data presented in section 6.5 it appears that short chain
length compounds are more readily absorbed than longer chain length
compounds. Absorption decreases with increasing carbon chain length
and degree of chlorination.
6.1.2 Dermal exposure
Chlorinated paraffins are slowly absorbed by the dermal route
in Sprague-Dawley rats (Yang et al., 1987). Two 14C-labelled
chlorinated paraffins, C18;50-53% Cl (CP-LH) and C28;47% Cl (CP-LL),
were applied to rat skin (5-7 animals of each sex) at a concentration
of 66 mg/cm2, approximately equivalent to 2000 mg/kg body weight.
Only 0.7% (males) and 0.6% (females) of the C18 dose was absorbed
after 96 h. Only 0.02% of the C28 dose was absorbed in males whereas
in females the level was not detectable. This indicates that
increasing chain length leads to decreased permeability. Of the
absorbed C18 dose, 40% was exhaled as 14C-labelled CO2, and 20% was
excreted in urine and 20% in faeces.
The absorption of two chlorinated paraffins through human skin
has been studied in vitro (Scott, 1989). There was no absorption of
Cereclor S52 (C14-19;52% Cl, CP-MH) following a 54-h application to
the surface of the epidermal membranes using five different receptor
media. Similarly, using Cereclor 56L (C10-13; 56% Cl, CP-SH; 18.5% w/w
solution in a typical cutting oil) no absorption was detected for 7 h,
but after 23 h a slow but steady rate of absorption was detected
(e.g., 0.05 ± 0.01 µg/cm2 per h ± SEM; n = 6; receptor medium PEG-20
oleyl ether in saline), which was maintained for the duration of the
experiment (56 h). Owing to the anticipated low rate of absorption,
the chlorinated paraffin samples were spiked with [14C] n-pentadecane
and [14C] n-undecane for Cereclor S52 and 56L, respectively, in
order to facilitate detection of the absorbed material. Measurement of
the 14C-alkanes was taken as a surrogate for the chlorinated
paraffins, on the assumption that their rates of absorption were
similar.
6.1.3 Inhalation exposure
No data on retention of chlorinated paraffins by inhalation have
been reported.
6.2 Distribution
6.2.1 Short chain length chlorinated paraffins
6.2.1.1 Mouse
Female C57Bl mice were administered 12.5 MBq/kg body weight
(340 µCi) (for autoradiography) or 1.25 MBq/kg body weight (34 µCi)
(for determination of radioactivity) of 14C-labelled chlorododecanes
(C12) with different chlorine contents (17.5% [CP-SL], 55.9% [CP-SH]
and 68.5% [CP-SH]) either by gavage or intravenous injection (Darnerud
et al., 1982). Uptake of radioactivity was found by autoradiography to
be highest in tissues with high cell turnover/high metabolic activity,
e.g., intestinal mucosa, bone marrow, salivary glands, thymus and
liver. The highest radioactivity was achieved with the chlorinated
paraffin that had the lowest chlorine content. It was found that the
long period of retention of heptane-soluble radioactivity, which
indicated unmetabolized substance, in liver and fat after oral dosing
increased with degree of chlorination. In this study it was also
found that 30 to 60 days after injection of C12;17.5% Cl and
C12;55.9% Cl a considerable retention of radioactivity was seen in
the central nervous system. Exposure of late gestation mice showed a
transplacental passage of radioactivity, and 14C-labelling was
primarily noted in the liver, brown fat and intestine of the fetuses.
6.2.1.2 Rat
Radioactivity was found in the liver, kidneys, adipose tissue and
ovaries of Fischer-344 rats following administration of an unspecified
single dose by gavage of 14C-labelled chlorinated paraffin (C10-13;58%
chlorination, CP-SH) at the end of a 90-day dosing by gavage with the
same chlorinated paraffin (IRDC, 1984c).
6.2.2 Intermediate chain length chlorinated paraffins
6.2.2.1 Rat
Radioactivity was found initially in the liver and kidneys and
later in adipose tissue and the ovaries of Fischer-344 rats following
a single dose by gavage of 14C-labelled C14-17;52% Cl (CP-MH) at the
end of a 90-day dosing in the diet with the same chlorinated paraffin
(IRDC, 1984b).
In male Wistar rats fed with a diet containing 0.4 or 40 mg/kg
[36Cl]Cereclor S52 (C14-17;52% Cl, CP-MH) for 8 (40 mg/kg) or 10 weeks
(0.4 mg/kg), equilibrium levels of radioactivity were established in
liver and abdominal fat within 1 and 7 weeks, respectively (Birtley et
al., 1980). The equilibrium concentrations were 7000 µg/kg in liver
and 30 000-40 000 µg/kg in fat after high exposure. No radioactivity
was detected in the brain or adrenal glands.
6.2.2.2 Mouse
The distribution of orally administered 14C-labelled
polychlorohexadecane (C16;69% Cl, CP-MH) in female C57Bl mice was
examined by whole body autoradiography (Biessmann et al., 1983).
A high level of radioactivity was observed in the liver, brown
fat, intestine, gall bladder, adrenal cortex and kidney of mice
administered approximately 15 µmol/kg. A high uptake of radioactivity
was also observed in corpora lutea on days 1-4, and was still present
30 days after administration. A high level of radioactivity was also
observed in the adrenal cortex at shorter post-injection times, and in
brown and white fat and in the liver, which were still labelled after
30 days.
14C-Labelled [1-14C]polychlorohexadecane (C16;34.1% Cl, CP-ML)
was given to C57Bl mice either by gavage (females) or intravenously
(both sexes) at a radioactivity level of 370 kBq/animal (10 µCi)
(corresponding to 0.44 µmol of the chlorinated paraffin) (Darnerud &
Brandt, 1982). No difference in the distribution patterns was found
between the oral and intravenous administration routes. After
analysis by autoradiography a high level of radioactivity was found in
tissues with a high cell turnover rate and/or high metabolic activity,
and lower levels could be seen in the white fat depots. High levels
of radioactivity were observed in the liver, kidneys, spleen, bone
marrow, brown fat, intestinal mucosa, pancreas, salivary gland and the
Harderian gland 24 h after intravenous injection. After 12 days high
levels of radioactivity were seen in the adrenal cortex, abdominal
fat and in the bile. Later after injection (30 days), prominent
radiolabelling of the brain was found which was as high as in the
liver. The chlorinated paraffin was also administered intravenously
to pregnant mice, and uptake of radioactivity in the fetuses was
observed. When the mice were administered on day 10 of pregnancy no
tissue-specific localization was found, but after administration in
late pregnancy (day 17) the distribution pattern after 6 h was similar
to that of adult mice when examined 24 h after administration.
The distribution of radioactivity in the brain and liver has been
studied after gavage administration of 14C-labelled polychloro-
hexadecane (C16;69% Cl, CP-MH) (1.48 MBq/kg body weight or 40 µCi,
corresponding to 1.1 mg/kg body weight) to pre-weaning NMRI mice
(Eriksson & Darnerud, 1985). The chlorinated paraffin was
administered by gavage at the age of 3, 10 and 20 days, and the
animals were killed 24 h and 7 days later. The radioactivity in the
brain declined more rapidly in the 3-day-old mice compared to the
10- and 20-day-old mice. In the 10-day-old mice approximately 0.02% of
the total administered dose was detected in the brain 24 h after
administration. Approximately 80% of the radioactivity in the brain
was still present after 7 days. In the liver the radioactivity
disappeared more rapidly both in younger and older animals. The
radioactivity in the brain was found primarily in the white matter
of the cerebellum, in the space between the neocortex and the
mesencephalon and thalamus, the corpus callosum, the pons and the
outer part of medulla spinalis. The radioactivity was higher in the
parts of the brain which also stained for myelin, and the levels at
7 days were almost the same as those at 24 h. After whole body
autoradiography, high levels of radioactivity were found in the liver,
intestinal contents, adipose tissue and adrenals. A differential
labelling of the liver was observed in the 3-day-old mice, where only
certain parts of the liver lobules were labelled.
6.2.2.3 Bird
The distribution of 14C-labelled polychlorohexadecane (C16;69%
Cl, CP-MH) in female Japanese quail (Coturnix coturnix japonica) was
examined by whole body autoradiography (Biessmann et al., 1983). Four
hours after a single dose by gavage of approximately 4.8 µmol/kg to
quail, high levels of radioactivity were observed in the liver,
intestine, gall bladder, egg yolk, kidney, ovary, blood, hypophysis
and retina. Twelve days after administration, radioactivity was
observed only in the uropygial gland, white fat, liver and egg yolk.
A study of the distribution after oral administration by gavage
of 0.74 MBq (20 µCi) (approximately 20 Ci/mol) of either 14C-labelled
polychlorohexadecane (C16;34% Cl, CP-ML) or (1-14C)-labelled
polychlorododecane (C12;56% Cl, CP-SH) in female Japanese quail
(Coturnix coturnix japonica) was performed by Biessmann et al.
(1982). The distribution patterns for the two chlorinated paraffins
were similar, and high levels of radioactivity were initially (up to
1 day) found in the liver, intestinal mucosa, spleen, bone marrow,
oviduct, gall bladder and kidney. After 4 and 12 days, high
radiolabelling was observed in fat, the yolk of the follicles and
the contents of the uropygial glands.
The uptake of Cereclor S52 (C14-17;52% Cl, CP-MH) in mallard ducks
(Anas platyrynchos) or ring-necked pheasants (Phasianus colchicus)
was studied by Madeley & Birtley (1980). After a single oral dose of
Cereclor S52 (10 g/kg) in duck, the highest levels of chlorinated
paraffins were detected in fat (67 mg/kg wet weight), gut (15 mg/kg)
and heart (7 mg/kg). The pheasants were exposed to 1000 mg/kg in the
diet for 5 days. Only low levels were found in the heart (3.1 mg/kg)
and gut (1.4 mg/kg). No visible fat was available in the pheasants
due to immaturity. The levels in other organs in both species were
low. The method of analysis was thin layer chromatography (Hollies
et al., 1979).
6.2.2.4 Fish
The distribution of polychloro-[1-14C]hexadecane (C16;34% Cl,
CP-ML) has been studied in carp (Cyprinus carpio) and bleak
(Alburnus alburnus) (Darnerud et al., 1983). After a single
intra-arterial injection of 60-80 µg in carp, about 6% of the dose was
excreted as 14CO2 in 96 h. Radioactivity was observed, in the
intra-arterially injected carps or in bleak exposed to contaminated
water (125 µg/litre) for 14 days, in the bile, intestine, kidney,
liver, gills and, particularly in bleak, in the nasal cavity, lens and
skin.
6.2.3 Long chain length chlorinated paraffins
6.2.3.1 Rat
After oral administration of 14C-labelled C22-26;70% Cl (CP-LH)
to Fischer-344 rats at the end of a 90-day exposure period, a small
part of the dose was absorbed (Serrone et al., 1987). The highest
level of radioactivity was found in the liver. Retention of
radioactivity in adipose tissue, which was eliminated slowly, was also
observed. In an identical study, C20-30;43% Cl (CP-LL) gave the
highest levels in the liver and ovary (Serrone et al., 1987).
6.2.3.2 Fish
Rainbow trout (Oncorhynchus mykiss) were fed diets containing 47 or
385 mg/kg (dry weight) of Cereclor 42 (C20-30;Cl 42%, CP-LL) containing
a 14C-labelled pentacosane (C25) with 42% Cl for 35 days and
a control diet for the following 49 days (Madeley & Birtley, 1980).
The chlorinated paraffin accumulated in the fish during the exposure
period, mostly in the liver and gut. The radioactivity decreased
during the elimination period, more rapidly in the gut and liver
than in the flesh, but was still detectable at the end of the
experiment. When the chlorinated paraffin was determined by thin-layer
chromatography a lower level was noted as compared to determination
of 14C-labelled molecules, suggesting metabolism in the fish. Up to
70% of the assimilated 14C in tissues was not associated with
chlorinated paraffin after feeding for 13 days.
6.2.3.3 Mussel
Mussels (Mytilus edulis) were fed suspended yeast cells dosed
with 524 mg/kg (dry weight) Cereclor 42 (C20-30;42% Cl, CP-LL)
containing a 14C-labelled pentacosane (C25) with 42% chlorination for
47 days, and were then fed with untreated yeast for a further 56 days
(Madeley & Birtley, 1980). The uptake reached a plateau level after
26 days of exposure, and the tissue concentration was always below
11 mg/kg. The highest level of chlorinated paraffin was detected in
the digestive glands. The chlorinated paraffin was eliminated rapidly,
and less than 10% remained at the end of the experiment. There was no
evidence of metabolism since the expelled radioactivity was in the
form of the parent compound.
6.2.4 Comparative studies
The uptake of three 14C-labelled chlorinated paraffins, C16;23%
Cl (CP-ML), C16;51% Cl (CP-MH) and C12;68% Cl (CP-SH), was studied in
rainbow trout (Oncorhynchus mykiss) by Darnerud et al. (1989). After
exposure to 3.64 µmol (C16) or 1.74 µmol (C12) in water for 7
days and to uncontaminated water for up to 21 days, radiolabelling was
observed in the bile, eye lens, brain and fat for C16;23% Cl, in the
bile, intestine, fat and liver for C16;51% Cl, and in fat, intestine,
liver and bile for C12;68% Cl. The three different chlorinated
paraffins showed an initially high uptake in the olfactory organs and
gills. The retention of radioactivity in the olfactory organs and
gills was relatively higher for C16;23% Cl than for the more highly
chlorinated paraffins. The long-time retention of radioactivity in
fat-rich tissues increased with the degree of chlorination of the
chlorinated paraffin preparation.
The short-term uptake and elimination of chlorinated paraffins in
bleak (Alburnus alburnus) were studied by Bengtsson et al. (1979).
Groups of bleak (15 in each) were exposed for 14 days to five
different Witaclor mixtures (Table 16), which were added to natural
Baltic Sea water (salinity 0.7%) giving a concentration of 125
µg/litre of water. Five fish were analysed to determine the chlorine
content (Lunde & Steinnes, 1975) at the end of exposure, and the rest
were analysed 1 and 7 days after treatment. It was found that the
uptake was more effective for those Witaclor mixtures with short
carbon chain length and low degree of chlorination, i.e. Witaclor 149
and 159. The elimination rates were slow, and 75 to 90% was detected
7 days after exposure.
In an extended study, bleak were exposed to Witaclor 149
(C10-13;49% Cl, CP-SL), Witaclor 171P (C10-13;71% Cl, CP-SH) and
Witaclor 549 (C18-26;49% Cl, CP-LL) via contaminated food for 91 days.
This was followed by an elimination period of 316 days (Bengtsson &
Baumann Ofstad, 1982). The concentrations of chlorinated paraffins in
the food were 590, 2500 and 5800 mg/kg of C10-13;49% Cl, 3180 mg/kg of
C10-13;71% Cl and 3400 mg/kg of C18-26;49% Cl. Three fish from
each exposure group were analysed at different time-points during
accumulation or elimination periods. The most effective uptake was
observed for C10-13;49% Cl, whereas the slowest uptake was detected for
C18-26;49% Cl. Efficiencies of uptake were 12% for C10-13;49% Cl, 6%
for C10-13;71% Cl and 2% for C18-26;49% Cl. The highest retention was
observed for C10-13;71% Cl, which remained in the tissue at a
steady-state level during the whole elimination period. The uptake of
C18-26;49% Cl was inefficient, and during the elimination period 50%
was lost within 4 to 5 weeks. The remaining amount appeared to be
constant for the rest of the experiment. The rate of elimination was
slowest for the Witaclor mixture with the shortest carbon chain length
and highest degree of chlorination. After 625 days of depuration the
fish still had detectable levels of C10-13;71% Cl, as determined by the
analytical method of Gjos & Gustavsen (1982) (Renberg et al., 1986).
Table 16. Structures of the chlorinated paraffins used in a study on
bleak (Alburnus alburnus) (From: Bengtsson et al., 1979)
Tested Carbon Chlorine Acronyma Accumulation Half-life
formulation chain content coefficientb (days)b
length (%)
Witaclor 149 C10-C13 49 CP-SL 770 13
Witaclor 159 C10-C13 59 CP-SH 740 34
Witaclor 171P C10-C13 71 CP-SH 140 7
Witaclor 350 C14-C17 50 CP-MH 40 30
Witaclor 549 C18-C26 49 CP-LL 10 7
a The classification is given in Table 1
b Calculated by Zitko (1980)
6.3 Metabolic transformation
6.3.1 Short chain length chlorinated paraffins
Darnerud (1984) demonstrated that inducers and inhibitors of
cytochrome P-450 (CYP) affect the rate of degradation of 14C-labelled
polychlorinated dodecanes (C12) containing 68.5% (CP-SH), 55.9%
(CP-SH) and 17.4% Cl (CP-SL) to 14CO2 in exposed C57Bl mice.
Pretreatment with the inhibitor piperonyl butoxide decreased the
amount of 14CO2 formed, and the decrease was more pronounced with
increasing degree of chlorination. The inhibitor metyrapone decreased
the exhalation of 14CO2 but was only investigated in mice exposed
to C12;68.5% Cl. The cytochrome P-450 (CYP2B1; CYP2B2) inducer,
phenobarbital, moderately increased the rate of 14CO2 formation from
chlorinated paraffin with 68% Cl, whereas the P-448 (CYP1A1; CYP1A2)
inducer, 3-methylcholanthrene, did not affect the degradation rate,
indicating a cytochrome P-450-dependent metabolism of chlorinated
dodecanes yielding 14CO2.
6.3.2 Intermediate chain length chlorinated paraffins
Female Sprague-Dawley rats in groups of four were exposed
intravenously to 5-6 mg/kg body weight of 14C-labelled poly-
chlorinated hexadecane (C16;65% Cl, CP-MH) (Åhlman et al., 1986).
Less than 3% of the radioactivity in the bile was due to unchanged
parent compound. The metabolites in the bile appeared to be conjugates
of N-acetylcysteine (mercapturic acid) and glutathione.
6.4 Elimination and excretion
6.4.1 Short chain length chlorinated paraffins
The exhalation of 14CO2 was compared after single gavage or
intravenous administration to female C57Bl mice of 1.25 MBq/kg body
weight (34 µCi) of three chlorododecanes (C12) with different
chlorine contents (17.5% [CP-SL], 55.9% [CP-SH] and 68.5% [CP-SH])
(Darnerud et al., 1982). Of the administered radioactive dose 52% of
C12;17.5% Cl, 32% of C12;56% Cl and 8% of C12;68% Cl were exhaled as
14CO2 within 12 h after dosing by either route. The major excretion
route for C12;56% Cl was by urine (intravenous: 21%; oral: 29%) and
for C12;68% Cl was by faeces (intravenous: 8.6%; oral: 21%). The
total elimination decreased as the chlorine content increased.
6.4.2 Intermediate chain length chlorinated paraffins
6.4.2.1 Rat
The half-time for removal of radioactivity from abdominal fat was
estimated during and after dietary administration for 8 or 10 weeks of
0.4 and 40 mg/kg feed of [36Cl]Cereclor S52 (C14-17;52% Cl, CP-MH) in
male Wistar rats (Birtley et al., 1980). Equilibrium in liver and
abdominal fat was reached at 1 and 7 weeks, respectively. The
half-time for removal was about 8 weeks for abdominal fat, and it was
observed that the level of radioactivity in the liver declined below
the detection limit within one week.
Female Sprague-Dawley rats in groups of four were exposed
intravenously to 5-6 mg/kg body weight of 14C-labelled polychlorinated
hexadecane (C16;65% Cl, CP-MH) (Åhlman et al., 1986). Approximately
10% of the administered dose was excreted in the bile after 24 h,
whereas excretion in the urine and faeces was less than 0.5% after
48 h.
6.4.2.2 Mouse
The elimination of radioactivity was studied in female C57Bl mice
after gavage or intravenous administration of a uniformly 14C-labelled
polychlorohexadecane (C16;69% Cl, CP-MH) (1.6 µmol/kg) (Biessmann
et al., 1983). After 8 h, only about 1% of the dose was exhaled
as 14CO2 for both administration routes. Most of the
radioactivity was excreted in faeces when administrated by gavage, and
after 8 h 22% was recovered in faeces and 1.2% in urine. After 96 h,
66% was recovered in faeces and 2.9% in urine. After intravenous
administration, 2.1% was excreted in faeces and 1% in urine after 8 h,
and 43% was excreted in faeces and 3% in urine after 96 h.
The excretion of [1-14C]polychlorohexadecane (C16;34.1% Cl,
CP-ML) (59 kBq/animal, or 1.5 µCi) after intravenous or gavage
administration to C57Bl mice was studied by Darnerud & Brandt (1982).
Twelve hours after intravenous injection, 12% of the radioactive dose
was recovered in urine, 44% in expired air (as 14CO2) and 4%
in faeces. Twelve hours after gavage administration, 6% of the
radioactive dose was recovered in urine, 33% in the expired air and
14% in faeces.
6.4.2.3 Bird
The elimination of radioactivity was studied in female Japanese
quail (Coturnix coturnix japonica) after gavage or intravenous
administration of a uniformly 14C-labelled polychlorohexadecane
(C16;69% Cl, CP-MH) (0.48 µmol/kg) (Biessmann et al., 1983). Of the
administered dose, 1.6% was exhaled as 14CO2 after gavage
administration and 0.9% after intravenous administration. Of the
administered dose 16% was excreted in faeces/urine after 8 h and 58%
after 96 h.
6.4.3 Long chain length chlorinated paraffins
A 14C-labelled chlorinated paraffin, C18; 50-53% Cl (CP-LH), was
administered by gavage as a single dose of 500 mg/kg to three female
Sprague-Dawley rats (Yang et al., 1987). After 24 h, 1% of the
radioactive dose was recovered in the urine, 1.5% in the expired air
and 22% in the faeces. After 96 h, 1.9% of the radioactive dose was
recovered in the urine, 3.3% in the expired air, 5% in body tissue and
76% in the faeces.
6.4.4 Comparative studies
In a study by Beissmann et al. (1982), 148 kBq (4 µCi)
(approximately 20 Ci/mol) of either 14C-labelled polychlorohexadecane
(C16;34% Cl, CP-ML) or 14C-labelled polychlorododecane (C12;56% Cl,
CP-SH) was administered by gavage to Japanese quail (Coturnix
coturnix japonica). A considerably higher level of radioactivity
was found in the gall bladder and kidney after C12 administration
compared with C16. On the other hand, the radioactivity in the yolk
of the first ten eggs was higher after C16 gavage than after C12
gavage. After 8 h, 39% of the C16 dose and 22% of the C12 dose was
exhaled as 14CO2, indicating that the rate of metabolism is
influenced by the chain length and/or the degree of chlorination.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute exposure
7.1.1 Lethal doses
The acute oral toxicity of chlorinated paraffins has been studied
in rats and mice (Table 17). In all studies, the LD50 was reported
to be greater than the highest administered dose (i.e. always
> 4 g/kg body weight). After inhalation of Chlorowax 500C (C12;59% Cl,
CP-SH), an LC50 was not established in the one reported study (LC50
> 3300 mg/m3). The LD50 for dermal exposure of rabbits to
Chlorowax 500C (C12;59% Cl, CP-SH) was in excess of approximately
13 g/kg body weight (Howard et al., 1975).
Table 17. Acute toxicity of chlorinated paraffins
Chlorinated Compounda Species Test Exposure Reference
paraffin concentration
C12;60% Cl (CP-SH) rat oral LD50 > 13.6 g/kg Bucher et
al. (1987)
C12;59% Cl Chlorowax rat oral LD50 > 21.5 g/kg Howard et
500C (CP-SH) al. (1975)
C12;59% Cl Chlorowax rabbit dermal > 13 g/kg Howard et
500C (CP-SH) LD50 al. (1974)
C12;59% Cl Chlorowax rat inhalation > 3300 Howard et
500C (CP-SH) LC50 mg/m3 al. (1975)
C12;60% Cl (CP-SH) mouse oral LD50 > 27.2 g/kg Bucher et
al. (1987)
C10-13;41-70% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C14-17;51-60% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C24;40% Cl Chlorowax 40 rat oral LD50 > 17.7 g/kg Howard et
(CP-LL) al. (1975)
C23;43% Cl (CP-LL) rat oral LD50 > 13.6 g/kg Bucher et
al. (1987)
Table 17. (Cont'd)
Chlorinated Compounda Species Test Exposure Reference
paraffin concentration
C23;43% Cl (CP-LL) mouse oral LD50 > 27.2 g/kg Bucher et
al. (1987)
C20-30;41-70% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C24;70% Cl Chlorowax 70 rat oral LD50 > 50 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorez 700 rat oral LD50 > 50 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorowax 70 guinea-pig oral LD50 > 25 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorez 700 guinea-pig oral LD50 > 25 g/kg Howard et
(CP-LH) al. (1975)
a The classification is given in Table 1
7.1.2 Non-lethal doses
7.1.2.1 Oral route
In a single-administration experiment, F-344/N rats and B6C3F1
mice were dosed by gavage up to 13.6 g/kg body weight (rats) and
27.2 g/kg body weight (mice) of C12;60% Cl (CP-SH) or C23;43% Cl
(CP-LL) dissolved in corn oil. No deaths or compound-related toxic
effects were noted during the 14-day observation period. However, the
animals were inactive with diarrhoea for 2-6 days after dosing, which
was attributed to the large volumes of material administered (NTP,
1986a,b; Bucher et al., 1987).
Female or male Wistar rats were administered by gavage a single
oral dose of different chlorinated paraffins (C10-13; 41-51%, 51-61% or
61-70% Cl), with a range of maximum doses of 4-13 g/kg body weight,
and were observed for 7 days (ICI, 1965, 1966, 1968, 1969, 1971, 1973,
1974a,b; Birtley et al., 1980). Clinical signs of toxicity, such as
piloerection, muscular incoordination and faecal and urinary
incontinence, were observed in rats that received doses of 2 g/kg body
weight or more (generally independent of the chlorine content).
Recovery was usually complete by day 7. There were no deaths except
for one rat treated with 13 g/kg body weight of C10-13;63% Cl.
Similar findings were reported for C14-17;51-60% Cl and
C20-30;41-51%, 51-61% or 61-70% Cl (Birtley et al., 1980). The
toxicity of these chlorinated paraffins was reported to be lower than
that of the short chain length chlorinated paraffins.
7.1.2.2 Inhalation route
No toxic response was observed in rats exposed to a concentration of
3300 mg/m3 of Chlorowax 500C (C12;59% Cl, CP-SH) for one hour (Howard
et al., 1975)
7.1.2.3 Intraperitoneal route
Three different chlorinated paraffin preparations, Chlorez 700
(C20;70% Cl, CP-LH), Paroil 170-HV (C11;70% Cl, CP-SH) and
Chloroparaffin 40 (chain-length not given, 40% Cl), were administered
intraperitoneally as single doses (Chlorez and Chloroparaffin
100 mg/kg body weight; Paroil 52 mg/kg body weight) to male Wistar rats
(Ahotupa et al., 1982). The activities of various drug-metabolizing
enzymes from liver, kidney and small intestinal mucosa were determined
after 24 h, 7 days or 21 days. Minor but significant changes in the
intestinal activities of aryl hydrocarbon hydroxylase (increase),
UDP-glucuronosyltransferase (decrease) and epoxide hydrolase
(increase) were induced by Paroil 170-HV, and in the kidney activity
of aryl hydrocarbon hydroxylase by Chlorez 700, when compared to
polychlorinated biphenyls and naphthalenes.
7.1.3 Skin and eye irritation
7.1.3.1 Short chain length chlorinated paraffins
In a study by Hoechst (1986b), 0.5 ml of undiluted C10-13;50%
Cl(CP-SH) was applied under a semi-occlusive dressing to the shaven
skin of three rabbits for 4 h. The skin was examined for signs of
irritation for up to 72 h after the chlorinated paraffin had been
removed, but none were seen during the test.
When 0.5 ml of C10-13;70% Cl(CP-SH) was applied under a
semi-occlusive dressing to the shaven skin of three rabbits for 4 h,
one rabbit showed clearly defined erythema (grade 2 on a 0-4 scale) at
48 and 72 h. The other two animals showed "slightly noticeable"
erythema (grade 1). Very slight oedema (grade 1) was noted in two
animals for up to 24 h. By day 7, all signs of irritation were
completely resolved (Hoechst, 1983).
Two studies investigated C10-13;70% Cl(CP-SH). In one study the
chlorinated paraffin contained 1 or 2% of an epoxidised vegetable oil
stabilizer with and without additives (0.1% oxalic acid or 0.05%
benzotriazole) (ICI, 1965). Very mild to mild desquamation was only
noted following the applications of chlorinated paraffins containing
additives. The reactions were described as occasional, transient and
inconsistent. It was not stated how many applications were made
before these reactions were seen. In another study, no signs of
irritation were noted following repeated application of a chlorinated
paraffin containing 0.1 or 2% benzoyl peroxide initiator (ICI, 1974a).
Two studies investigated the effects of three C10-13;63%
chlorinated paraffins (CP-SH), containing up to 3% epoxy soya oil
stabilizers or other unspecified additives (ICI, 1973, 1974a). For
all three paraffins, erythema was usually noted following two to four
applications, although on one occasion erythema was noted in 1/3
animals after only one application. The severity of the reactions was
not described. Desquamation was also noted following three or four
applications and increased in severity with further treatments. In
one study (with 0.7% epoxy carboxylate stabilizer) the desquamation
was described as severe following the fourth application when the
study was terminated (ICI, 1973).
Studies have been conducted using C10-13 chlorinated paraffins
which were 48(CP-SL), 50, 52 or 55% chlorinated (CP-SH) (ICI,
1967, 1968, 1969, 1971, 1974a,b). In most of these studies the
chloroparaffins contained 0.2 or 2% epoxy stabilisers. In one study
with 48 or 55% chlorinated paraffins, containing 0.2% epoxy octyl
stearate stabilizer, no signs of irritation were noted (ICI, 1969).
In the other studies there was mild or slight erythema, and mild
desquamation was usually noted following the second or third
application. In one study, testing C10-13;52% with 2% epoxidised octyl
oleate stabilizer, erythema was noted following the first application
(ICI, 1968). It was observed in 4/5 of the studies that the reactions
did not worsen following further applications, although in one study
(testing a 52% chlorinated paraffin with unspecified additives),
slight erythema, noted after the second application, worsened to
severe erythema with slight necrosis after the third application, when
the study was terminated (ICI, 1971).
An unspecified volume of C10-13;40% Cl(CP-SL), containing 1% epoxy
soya oil stabilizer, produced slight desquamation following the second
application and mild erythema after the third (ICI, 1966). This
condition persisted throughout the remaining applications until the
end of the study when small scattered ulcers developed.
Two studies in rats were conducted to investigate the potential
for skin irritation of two short chain length chlorinated paraffins
(C10-11) which were 49% (CP-SL) and 60% chlorinated (CP-SH) (ICI, 1980,
1982c). Repeated and single application tests were conducted. No
signs of irritation were noted following a single application of the
more chlorinated paraffin, although slight desquamation was noted in
2/6 rats, 3-6 h after the treatment with the less chlorinated
paraffin. Both chlorinated paraffins produced slight erythema and/or
slight desquamation with repeated applications.
Rats were treated with 0.1 ml of C14-17;51-60% Cl(CP-MH), or
C20-30;41-51%(CP-LL), 51-61%(CP-LH) or 61-70% Cl(CP-SH), for up to
six 24-h periods. The treatment periods were separated by 24-h
treatment-free periods. In some of the studies the chlorinated
paraffins contained epoxy stabilizers. Mild irritation was seen with
C14-17 chlorinated paraffins, but it is not clear if the response was
due to the stabilizer. No signs of irritation were seen with C20-30
(Birtley et al., 1980).
A C10-13;61% chlorinated paraffin (Cereclor 60HS) and a 50%
chlorinated paraffin (Cereclor 50 HS) of unidentified carbon chain
length produced mild or moderate skin irritation following a single
occlusive application to intact or abraded skin of rabbits. It was
stated that varying degrees of erythema persisted for 72 h (ICI
1975a,b).
In other studies (BUA, 1992) different short and long chain
length chlorinated paraffins were applied to the skin and eyes of
rabbits (skin: C10-13;58% Cl, C19;44% Cl, C20-30;70% Cl; eyes: C12;59%
Cl, C20-30;70% Cl). Only a weak or no irritating effect was observed,
which decreased with increasing chain length.
The eye irritation potential of three different chlorinated
paraffins, C10-13;65% Cl(CP-SH), which contained either 2.5 or 2% of
two different additives or 0.7% of an epoxy stabilizer, was tested in
two studies (ICI, 1971, 1974a). Either 0.1 ml or "one drop" of the
chloroparaffin was instilled into one conjunctival sac of groups
of three rabbits. Similar results were reported for all three
formulations: practically no initial pain (2 on a 6-point scale) was
noted. Slight irritation (3 on a 8-point scale), shown by redness
and chemosis (only noted in the formulation containing the epoxy
stabilizer) of the conjunctiva with some discharge, lasted for 24 h.
One drop of 52% or 40% chlorinated paraffins, containing unspecified
additives or 1% epoxy stabilizer, was also tested (ICI, 1966, 1971).
With the 52% chlorinated paraffin, slight immediate irritation was
followed by slight redness of the conjunctiva which lasted for 24 h.
With the 40% chlorinated paraffin, mild congestion was noted at 1 h
but no effects were seen at 24 h.
7.1.3.2 Intermediate and long chain length chlorinated paraffins
Intermediate and long chain chlorinated paraffins were tested
in eye irritation studies with a single application of 0.1 ml of
C14-17;51-60% Cl(CP-MH), C20-30;41-50%(CP-LL), 51-60% or 61-70%
Cl(CP-LH). No signs of eye irritation were seen (Birtley et al.,
1980).
7.1.4 Skin sensitization
The maximization method was used to assess the skin sensitization
potential of a chlorinated paraffin (C10-13;56% Cl, CP-SH) with 1%
epoxide stabilizer (Edenol D81) and 1% tris-nonylphenyl phosphite
(TNPP) (Hoechst, 1983b). When challenged with undiluted chlorinated
paraffin, 1/20 test animals showed hardly perceptible erythema 24 h
after challenge, and 1/20 test and 1/10 control animals showed clearly
defined erythema or slight oedema at 72 h. The chlorinated paraffin
tested did not induce skin sensitization in this study.
The same chlorinated paraffin (C10-13;56% Cl, CP-SH), with 1% of a
different epoxide stabilizer (Rutapox CY 160) and 1% TNPP, was tested
using the same method (Hoechst, 1984). When challenged with undiluted
chlorinated paraffin, 5/20 test animals showed clearly defined erythema
and another two showed hardly perceptible erythema. None of the
control animals showed any evidence of a skin reaction. A second
challenge was performed 2 weeks after the first. On this occasion
4/20 test animals showed clearly defined erythema and another four
showed hardly perceptible erythema or slight oedema. The authors
concluded that the substance tested was a sensitizer. However, as
less than 30% of the test group showed a clear reaction and it is
possible that the epoxide stabilizer was responsible for producing the
sensitization reactions, this study is not considered to provide
conclusive evidence that C10-13;56% Cl is a skin sensitizer.
An undiluted chlorinated paraffin (C10-13;52% Cl, CP-SH) was
applied to the ears of six guinea-pigs on three successive days (ICI,
1971). Slight erythema was noted when, 4 days later, undiluted
chloroparaffin was applied to the animals' flanks, but it was not
stated how many animals showed a reaction. Four control animals also
showed slight erythema at challenge. It does not appear that this
chlorinated paraffin elicited a sensitization response in this study.
7.2 Repeated exposure
Studies involving repeated exposure have demonstrated that the
liver, kidneys and thyroid are the target organs for the toxicity of
chlorinated paraffins.
7.2.1 Oral route
7.2.1.1 Short chain length chlorinated paraffins
a) Rat, 14-day studies
In a range-finding study, a short chain chlorinated paraffin
(C10-13;58% Cl) (CP-SH) was administered to Fischer-344 rats in
the diet for 14 days at concentrations of 0, 900, 2700, 9100 and
27 300 mg/kg feed, equivalent to approximately to 0, 100, 300, 1000
and 3000 mg/kg body weight per day (IRDC, 1983c). There were five male
and five female rats per group. No deaths occurred during the study. A
marked reduction in body weight and food consumption was seen in the
highest dose group, which was attributed to reduced palatability of
the diet caused by the chlorinated paraffin. The relative liver
weight was increased (20-240%) in all dose groups compared to
controls. The activity of liver aminopyrine demethylase (APDM) was
increased in females, and cytochrome P-450 values increased in both
sexes in all dosed groups. Liver enlargement was observed in some
rats in the groups fed 2700 to 27 300 mg/kg, and a dose-related
increase in the incidence of hepatocellular hypertrophy was present in
all treated groups. Myocardial atrophy was observed at the two
highest dose levels although the relationship to treatment was
unclear. The lowest-observed-effect level (LOEL) in this study was
100 mg/kg body weight per day.
A short chain length chlorinated paraffin with 58% Cl (CP-SH) was
administered by gavage in corn oil to Fischer-344 rats (five/dose of
each sex) for 14 days at dose levels of 0, 30, 100, 300, 1000 and
3000 mg/kg body weight per day (IRDC, 1981a). A significant decrease
in body weight gain in females in the high dose group was noted. A
dose-related increase in APDM activity was observed in females fed
300-3000 mg/kg, whereas in males there was an increase only in the
group treated with 1000 mg/kg. Cytochrome P-450 levels were
significantly increased in females treated with 1000 mg/kg, and
microsomal protein concentration increased in females dosed with
3000 mg/kg. Liver enlargement occurred in both sexes in the 300, 1000
and 3000 mg/kg groups, and mild hepatocellular hypertrophy in all
animals of the 1000 and 3000 mg/kg groups. The absolute and relative
liver weight was increased (20-150%) at doses of 100 mg/kg or more.
Statistically significant reduction of thymus and ovary weight was
observed at 3000 mg/kg. The no-observed-effect level (NOEL) in this
study was considered to be 30 mg/kg body weight per day and the LOEL
100 mg/kg body weight per day, based on liver weight increases.
In a 16-day study on F-344/N rats (groups of five), the animals
were administered a short chain length chlorinated paraffin (C12;60%
chlorination) (CP-SH) by gavage in corn oil daily (5 days per week) at
doses of 0, 469, 938, 1875, 3750 and 7500 mg/kg body weight per day
for 16 days (NTP, 1986a; Bucher et al., 1987). At the highest dose
level there was reduced body weight gain (22% in males and 16% in
females), and 1/5 male rats and 2/5 females died before the end of the
study. Enlarged livers were observed in every dose group except the
females fed 469 mg/kg. No histopathology was performed. The LOEL in
this study was considered to be 469 mg/kg body weight per day.
b) Rat, 90-day studies
Fischer-344 rats (groups of 10 males and 10 females) were given a
short chain chlorinated paraffin (C12;60% chlorination) (CP-SH) in
corn oil by gavage on 5 days a week for 13 weeks at doses of 0, 313,
625, 1250, 2500 and 5000 mg/kg body weight per day (NTP, 1986a; Bucher
et al., 1987). Body weight gain was reduced by approximately 10% in
males at the two highest dose levels. A dose-related statistically
significant increase in relative liver weight (17-100% for males;
30-100% for females) was observed in all treated rats. Hepatocellular
hypertrophy was noted in all rats in the highest dose group, and
nephrosis was more frequent in this group (10/10 males; 3/10 females)
compared to controls (8/10 males; 0/10 females). Rats in other dose
groups were not examined microscopically. On the basis of an increase
in liver weight, the LOEL in this study was 313 mg/kg body weight per
day.
In a 13-week study Fischer-344 rats were administered a short
chain length chlorinated paraffin (C10-13;58% Cl, CP-SH) in corn oil by
gavage at doses of 0, 10, 100 and 625 mg/kg body weight per day in
groups of 15 animals of each sex (IRDC, 1984a). In the groups treated
with 100 mg/kg or more, increased weights of the liver (30-110%) and
the kidneys (20-100%) were observed. At the highest dose level
thyroid weights were increased. Hepatocellular hypertrophy was
observed at 100 and 625 mg/kg. In these groups hypertrophy and
hyperplasia of the thyroid were also observed. There was trace-to-mild
chronic nephrosis in the kidney of males treated with 625 mg/kg, and in
females in the high-dose group, in which pigmentation of the renal
tubular epithelia also occurred. The NOEL was considered to be 10 mg/kg
body weight per day on the basis that no treatment-related microscopic
changes were found in any tissue at this dose. The LOEL was 100 mg/kg
body weight per day based on increased liver and kidney weights and
hypertrophy in liver and thyroid. When the same doses were
administered in the diet essentially identical results were obtained
(IRDC, 1984c).
Male and female Fischer-344 rats (5 or 10 per group) were
administered Chlorowax 500C (C12;58% Cl, CP-SH) in corn oil by gavage
at doses of 0, 313 and 625 mg/kg body weight per day for up to 90 days
(Elcombe et al., 1994). The relative liver weight was increased at
both doses (50 and 75%). Hepatic peroxisomal ß-oxidation (palmitoyl
CoA oxidation) was statistically significantly increased in a
dose-related manner from day 15. The activity of thyroxine-UDPG-
glucuronosyltransferase was significantly increased (at least 150%) at
both dose levels from day 15 onwards. Thyroid follicular cell
hypertrophy was observed at all time points, and hyperplasia at days
56 and 91. Replicative DNA synthesis was increased in thyroid
follicular cells at day 91. Renal tubular eosinophilia was observed
in males from day 15. In renal tubular cells replicative DNA
synthesis was increased in males. When a higher dose was administered
(1000 mg/kg body weight per day) marked decreases in the levels of
plasma thyroxine and increased plasma thyroid stimulating hormone were
observed. None of these effects were observed in male Dunkin Hartley
guinea-pigs, which were administered the chlorinated paraffin at doses
of 500 and 1000 mg/kg body weight per day. The LOEL in rats was 313
mg/kg body weight per day based on increased relative liver weights,
hepatic peroxisomal ß-oxidation and thyroxine-UDPG-glucuronosyl-
transferase activity.
c) Mouse, 14-day studies
B6C3F1 mice in groups of five were administered C10;60% Cl
(CP-SH) in corn oil by gavage daily for 16 days (NTP, 1986a; Bucher et
al., 1987). The doses were 938, 1875, 3750, 7500 and 15 000 mg/kg
body weight per day. All mice receiving 3750 mg/kg or more, and 4/5
males and 2/5 females receiving 1875 mg/kg died before the end of the
study. Diarrhoea was observed in all dosed groups except females
receiving 938 mg/kg. Enlarged livers were found in all treated
surviving mice. No histopathological examinations were conducted.
d) Mouse, 90-day studies
B6C3F1 mice (10 of each sex per dose) were exposed to C12;60%
Cl (CP-SH) by gavage five days per week for 13 weeks (NTP, 1986a;
Bucher et al., 1987). The doses were 125, 250, 500, 1000 or
2000 mg/kg body weight per day. No clinical signs of toxicity were
observed. In the males the body weight gain was reduced by 13% at the
highest dose level. The relative liver weights showed a dose-related
increase (17-160%) and were statistically significant from 250 mg/kg
in females and from 500 mg/kg in males. Hepatocellular hypertrophy
was observed in animals of both sexes treated with 250 mg/kg or more.
Focal hepatic necrosis was related to dosing at 500, 1000 and
2000 mg/kg in males and at 2000 mg/kg in females. The NOEL was
125 mg/kg body weight per day, and the LOEL was 250 mg/kg body weight
per day, based on hepatocellular hypertrophy.
7.2.1.2 Intermediate chain length chlorinated paraffins
a) Rat, 14-day studies
A chlorinated paraffin of intermediate chain length (C14-17) and
52% chlorination (CP-MH) was administered to Fischer-344 rats in the
diet at dosage levels of 150, 500, 1500, 5000 and 15 000 mg/kg feed,
which was reported to correspond to an average compound intake of
17.7, 57.7, 177, 562 and 1412 mg/kg body weight per day (IRDC, 1981b).
Five male and five female rats in each dose group were exposed daily
for 14 days. No mortality occurred among the treated animals.
Hepatic APDM activity was statistically significantly increased in
males receiving 562 mg/kg body weight per day (5000 mg/kg feed) and in
females receiving 1412 mg/kg body weight per day (15 000 mg/kg feed).
Slight increase in cytochrome P-450 values in male rats given
177 mg/kg body weight per day (1500 mg/kg feed) was observed but
appeared not to be related to dosing. Increased relative liver weight
was observed in the highest two dosage groups. Microscopic examination
of the liver revealed mild diffuse hepatocellular hypertrophy in all
animals receiving 562 and 1412 mg/kg body weight per day (5000 and
15 000 mg/kg feed). The NOEL was reported to be 57.7 mg/kg body weight
per day. The LOEL was 177 mg/kg body weight per day in males based on
increased cytochrome P-450 values and 562 mg/kg body weight per day in
females based on increased liver weight and hepatocellular hypertrophy.
b) Rat, 90-day studies
Male and female weanling Sprague-Dawley rats in groups of ten
were fed diets containing 0, 5, 50, 500 or 5000 mg/kg of C14-17;52%
Cl(CP-MH) for 13 weeks, yielding an average intake of 0, 0.4, 3.6, 36
and 360 mg/kg body weight per day for males and 0, 0.4, 4.2, 43 and
420 mg/kg body weight per day for females (Poon et al., in press).
There were no clinical signs of toxicity and no differences in body
weight gain. Relative liver weight was increased at 43 and 420 mg/kg
body weight per day in females and at 360 mg/kg in males. Relative
kidney weight was increased at the highest dose level in both sexes.
Serum cholesterol was increased in females from 4.2 mg/kg in a
dose-related manner. In the highest dose group of both sexes elevated
hepatic UDP-glucuronosyltransferase activity was observed, but only
females at this dose level showed increased APDM activity. Decreased
hepatic vitamin A levels were detected in females at 43 mg/kg and in
both sexes at the highest dose level. Mild, adaptive histopathological
changes were detected in the liver of both sexes at the two highest
dose levels, and in the thyroid of males from 36 mg/kg and females
from 4.2 mg/kg (reduced follicle sizes, collapsed angularity,
increased height, cytoplasmic vacuolation and nuclear vesiculation).
In the kidney, minimal changes were noted in the proximal tubules of
males at 360 mg/kg, and in the inner medulla tubules of females at 43
and 420 mg/kg. The NOEL in this study was 4 mg/kg body weight per day.
The LOEL was 36 mg/kg body weight per day (males) and 43 mg/kg body
weight per day (females).
In a 90-day feeding study, Wistar rats (groups of 24 males and 24
females) were fed diets containing 0, 250, 500, 2500 and 5000 mg/kg
feed of Cereclor S52 (C14-17;52% Cl, CP-MH) containing stabilizer (0.2%
epoxidized vegetable oil) (Birtley et al., 1980). A dose-related
decrease in body weight gain in males fed 500 mg/kg feed or more was
observed, which was accompanied by a reduction in food intake.
Significant increases in the relative liver weights in females were
observed at 500 mg/kg feed or more and in males at 2500 mg/kg feed.
Significant increases in relative kidney weights were observed at
5000 mg/kg feed in both sexes. Microscopic examination of the liver
showed evidence of a dose-related proliferation of the smooth
endoplasmic reticulum in the hepatic cells from 500 mg/kg feed.
Haematological investigation showed no abnormalities attributable to
the test compound. A tendency towards congestion of the kidney with
increasing concentration of Cereclor S52 in the diet was also observed.
The NOEL was 250 mg/kg feed (12.5 mg/kg body weight per day based on
food consumption data) and the LOEL was 500 mg/kg feed (25 mg/kg
body weight per day) based on increased relative liver weights and
proliferation of smooth endoplasmic reticulum.
A medium chain length chlorinated paraffin (C14-17;52% Cl, CP-MH)
was evaluated for subchronic toxicity in Fischer-344 rats (IRDC,
1984b). The chlorinated paraffin was administered to the rats (15 of
each sex per dose group) in the diet to provide dosage levels of 0,
10, 100 and 625 mg/kg body weight per day for 13 weeks. The
treatment did not induce signs of toxicity, alter survival or cause
ophthalmological changes. A slight reduction of body weight gain
(< 5%) was observed in both sexes at the highest dose, and was
associated with reduced food consumption. Traces of hepatocyte
hypertrophy (at 625 mg/kg) and increased absolute and relative liver
and kidney weights (at 100 and 625 mg/kg) were noted in both sexes.
Males in the high-dose group had an increased incidence of nephritis.
In addition, increased thyroid weight and thyroid hypertrophy and
hyperplasia were observed in males at 625 mg/kg. The NOEL was
considered in the report to be 10 mg/kg body weight per day. The LOEL
was 100 mg/kg body weight per day based on increased liver and kidney
weights.
c) Dog, 90-day studies
The effects of Cereclor S52 (C14-17;52% Cl, CP-MH) (containing
0.2% epoxidized vegetable oil as stabilizer) were studied in Beagle
dogs (Birtley et al., 1980). Four male and four female animals in
each group were fed a diet corresponding to 0, 10, 30 or 100 mg/kg
body weight daily for 90 days. No effects were found, except for
significantly increased serum alkaline phosphatase activity and
relative liver weight in males exposed to 100 mg/kg and an increase in
the smooth endoplasmic reticulum of hepatocytes from 30 mg/kg. The
NOEL was 10 mg/kg body weight per day. The LOEL was 30 mg/kg body
weight per day based on an increase of hepatic smooth endoplasmic
reticulum.
7.2.1.3 Long chain length chlorinated paraffins
a) Rat, 14-16 day studies
Fischer-344 rats (groups of five) were exposed to C22-26;43% Cl
(CP-LL) daily by gavage for 16 days at doses of 235, 469, 938, 1875 or
3750 mg/kg body weight per day. No compound-related clinical signs
of toxicity or mortality were observed. There were no changes
in body weight gain and no gross lesions were observed at necropsy.
Histopathological examinations were not conducted (NTP, 1986b; Bucher
et al, 1987).
Fischer-344 rats, in groups of five of each sex, were fed long
chain length paraffins of 70% chlorination for 14 days. The dietary
concentrations of the C22-26;70% Cl (CP-LH) were 0, 150, 500, 1500,
5000 and 15 000 mg/kg feed, corresponding to an average compound
intake of 0, 17.1, 55, 169, 565 and 1715 mg/kg body weight per day
(IRDC, 1982b). Tissues from liver, kidneys, spleen, lungs and
mesenteric lymph nodes were examined microscopically. Hepatic
microsomal Lowry protein, APDM activity and cytochrome P-450 values
were determined. No significant toxic effects were noted, and no
compound-related effects were found after microscopical examinations.
The NOEL was 1715 mg/kg body weight per day.
A chlorinated paraffin, C22-26;43% Cl (CP-LL), was administered by
gavage to Charles River 344 rats at doses of 0, 30, 100, 300, 1000 and
3000 mg/kg body weight per day (IRDC, 1982c). During the 14-day test
period no signs of toxicity were observed. Following sacrifice,
tissues from the liver, spleen, kidneys, pancreas, thymus and eyes
were examined microscopically; the only observation was a possible
increase in kidney nephrolithiasis in females exposed to the highest
dose level. Hepatic microsomal Lowry protein, APDM activity and
cytochrome P-450 values were determined and there were no alterations.
The NOEL was considered to be 3000 mg/kg body weight per day.
b) Rat, 90-day studies
A long chain length chlorinated paraffin, C23;43% Cl (CP-LL),
was administered to Fischer-344 rats in groups of 10 (each sex) by
gavage for 13 weeks at doses of 235, 469, 938, 1875 or 3750 mg/kg body
weight per day (NTP, 1986b; Bucher et al., 1987). No effects on body
or organ weights and no clinical signs of toxicity were observed. A
dose-related increased incidence of granulomatous inflammation was
noted in the livers of all exposed female rats but not in males. The
NOEL was 3750 mg/kg body weight per day for males. For females the
LOEL was 235 mg/kg body weight per day based on increased incidence of
granulomatous inflammation in the liver.
A long chain chlorinated paraffin (C20-30) with 43% Cl (CP-LL) was
administered in corn oil by gavage for 90 days to Fischer-344 rats at
three doses (100, 900 or 3750 mg/kg body weight per day) (IRDC,
1984f). Increases in liver weights and a multifocal granulomatous
hepatitis characterized by inflammatory changes and necrosis were
observed in all exposed females but not in males. In female rats
mineralization in the kidneys at the highest dose level was observed.
In addition to these observations, mild nephrosis was observed in the
males in the highest dose group. In males, the NOEL was 900 mg/kg
body weight per day and the LOEL was 3750 mg/kg body weight per day
(based on the occurrence of mild nephrosis). In females the LOEL was
100 mg/kg body weight per day based on liver effects.
The chlorinated paraffin C22-26;70% Cl (CP-LH) was administered to
Fischer-344 rats in the diet for 90 days at doses of 100, 900 and
3750 mg/kg body weight per day (IRDC, 1984g). Increased liver weight,
hepatocellular hypertrophy and cytoplasmic fat vacuolation were noted
at the highest dose level. The alanine aminotransferase (ALT)
activity was increased in both sexes at the highest dose level, and
aspartate aminotransferase (AST) activity was also increased in
females of this group. The NOEL was considered to be 900 mg/kg body
weight per day. The LOEL was 3750 mg/kg body weight per day based on
liver effects.
c) Mouse, 14-16 day studies
B6C3F1 mice in groups of five were given C22-26;43% Cl (CP-LL) by
gavage daily for 16 days at doses of 469, 938, 1875, 3750 or
7500 mg/kg body weight per day (NTP, 1986b; Bucher et al., 1987). No
compound-related clinical sign of toxicity or mortality was observed,
there were no changes in body weight gain, and no gross lesions were
observed at necropsy. Histopathological examinations were not
conducted.
d) Mouse, 90-day studies
The long chain length chlorinated paraffin C23;43% Cl (CP-LL)
was administered to B6C3F1 mice (in groups of 10 of each sex) by
gavage for 13 weeks at dose levels of 469, 938, 1875, 3750 or
7500 mg/kg body weight per day (NTP, 1986b; Bucher et al., 1987). No
effects on body or organ weights, no clinical signs of toxicity and no
histopathological effects were observed. The NOEL was 7500 mg/kg body
weight per day.
7.2.1.4 Comparative studies
The effects of representative chlorinated paraffins on liver
function and thyroid hormone function has been studied in male rats
(Alpk:APFSD) and male mice (Alpk:APFCD-1) (Wyatt et al., 1993).
Groups of five male rats or five male mice received 0, 10, 50, 100,
250, 500 or 1000 mg/kg body weight per day by gavage in corn oil, once
daily for 14 days. The chlorinated paraffins studied were Chlorowax
500C (C10-13;58%Cl, CP-SH), Cereclor 56L (C10-13;56%, CP-SH) and
Chlorparaffin 40C (C14-17;40% Cl, CP-ML). Effects on liver function
were assessed by changes in liver weight (expressed both as absolute
weights and liver:body weight ratio) and peroxisome proliferation
(expressed as the activity of palmitoyl co-enzyme A (CoA) oxidase).
All three chlorinated paraffins caused increases in liver weight and
palmitoyl CoA oxidation, indicative of peroxisomal proliferation. In
general, the rat was more sensitive to the effects of the chlorinated
paraffins on liver weight than the mouse. The doses of chlorinated
paraffin required to cause peroxisomal proliferation were, in general,
greater than those causing effects on liver weight, although there
appeared to be less of a difference in inter-species sensitivity.
However, the magnitude of the increase in palmitoyl CoA oxidation
caused by the short chain chlorinated paraffins (approximately 10-fold
increase as a maximal change) was greater than for the intermediate
chain grade (approximately 4-fold increase as maximal change) (Table
18). The effect of the chlorinated paraffins on thyroid function was
studied in the male rats receiving 1000 mg/kg body weight per day for
14 days by measuring the plasma levels of triiodothyronine (T3) and
thyroxine (T4) (free and total) and thyroid stimulating hormone
(TSH), and also the activity of hepatic microsomal UDP glucuronosyl-
transferase activity. All three chlorinated paraffins (at 1000 mg/kg
body weight per day) caused a reduction in plasma T4 levels (both
free and total) and an increase in plasma TSH levels. No effect was
observed on plasma T3 levels. All three chlorinated paraffins also
caused an increase (two-fold) in the rate of glucuronidation of T4
by hepatic microsomal UDP glucuronosyltransferase activity, suggesting
that the impact on plasma T4 and TSH levels is due to increased
clearance of T4 by hepatic metabolism.
Table 18. Effects of chlorinated paraffins on liver function in male rats and male mice
(From: Wyatt et al., 1993)
Increase in relative liver weight Increase in palmitoyl CoA
(% liver:body weight ratio) oxidase activity
Rat Mouse Rat Mouse
NOELa LOELb NOELa LOELb NOELa LOELb NOELa LOELb
C10-13;58% Cl 74 100 215 250 184 250 180 250
C10-13;56% Cl 51 50 70 100 600 1000 120 250
C14-17;40% Cl 31 100 426 1000 473 500 252 500
a Calculated using a three-parameter logit model (mg/kg body weight per day)
b Observed (mg/kg body weight per day)
The hepatic effects of representative chlorinated paraffins have
been studied in male and female F-344 rats, male and female B6C3F1
mice and male Alpk:Dunkin Hartley guinea-pigs (Elcombe et al., in
press). Their effects were compared with a range of known inducers of
hepatic enzymes. Groups of 4-5 animals received 1000 to 2000 mg/kg
body weight per day of each chlorinated paraffin (by gavage in corn
oil) for 14 consecutive days. The chlorinated paraffins studied were
Chlorowax 500C (C10-13;58% Cl, CP-SH), Cereclor 56L (C10-13;56% Cl,
CP-SH), Chlorparaffin 40G (C14-17;40% Cl, CP-ML) and Chlorowax 40
(C20-30;43% Cl, CP-LL). The short and intermediate chain length
chlorinated paraffins increased liver:body weight ratios (approximately
1.5 times) and elicited hepatocellular hypertrophy, peroxisome
proliferation (assessed as increases in peroxisomal volume and
palmitoyl CoA oxidase activity) and proliferation of hepatic cell
smooth endoplasmic reticulum in both rats and mice. These effects
were not seen in rats or mice receiving the long chain chlorinated
paraffin. The short and intermediate chain length chlorinated
paraffins also caused induction of cytochrome P-450 IV A1 (assessed by
increases in lauric acid hydroxylation) and P-450 II B1/IIB2 (assessed
by increases in ethoxycoumarin- O-diethylation) in the rat liver, but
only P-450 IV A1 in the mouse liver. The induction of the specific
cytochrome P-450 isoenzymes was confirmed using SOS-polyacrylamide gel
electrophoresis (SPS-PAGE) and Western immunoblotting of microsomes.
The administration of the short or intermediate chain length
chlorinated paraffins to guinea-pigs (1000 mg/kg body weight per day
for 14 days) had a similar effect on liver:body weight ratios (1.5-fold
increase) but had no effect on any of the hepatic ultrastructural or
biochemical parameters measured.
7.2.2 Intraperitoneal route
7.2.2.1 Short chain length chlorinated paraffins
Effects on both hepatic cytosolic and microsomal epoxide
hydrolases have been observed in male C57Bl/6 mice after 5 daily
intraperitoneal injection of 400 mg Cereclor 70L (C12;70% Cl, CP-SH)
(Meijer & DePierre, 1987). The hepatic cytosolic epoxide hydrolase
activity was increased to 130% and the microsomal activity to 250% of
the control. In addition, the amount of microsomal cytochrome P-450
was increased by 50%, and cytosolic DT-diaphorase activity was
increased 2- to 3-fold. There was also liver enlargement.
7.2.2.2 Intermediate chain length chlorinated paraffins
Lundberg (1980) reported a significant, dose-related increase in
total cytochrome P-450 in mice (strain not reported) injected
intraperitoneally with different amounts of Cereclor S52 (C14-17;52%
Cl, CP-MH) (from 0.6 mg to 63.4 mg) on 3 consecutive days. The
N-demethylation of ethylmorphine, a cytochrome P-450-dependent
reaction, decreased at low concentrations but increased at higher
concentrations.
7.2.2.3 Comparative studies
Male Sprague-Dawley rats were given intraperitoneal injections of
1000 mg/kg of Witaclor 149, 159 or 171P (C10-13 with 49% [CP-SL], 59%
[CP-SH] and 71% [CP-SH] chlorination, respectively), Witachlor 350
(C14-17 with 49% chlorination [CP-ML] or Witachlor 549 (C18-26 with 49%
chlorination [CP-LL]), each containing small amounts of epoxidated
soy-bean oil as stabilizer, daily for 4 days (Nilsen et al., 1980,
1981; Nilsen & Toftgård, 1981). Treatment with the C10-13 chlorinated
paraffins, but not those with longer chain lengths, caused increases
in liver weight and an induction of various forms of microsomal
cytochrome P-450. The activity of O-deethylation of 7-ethoxyresorufin
was decreased by the C10-13 chlorinated paraffins with higher
chlorine content, Witaclor 159 and 171P. The metabolism of
benzo (a)pyrene was induced by each of the chlorinated paraffins.
Witaclor 149 (C10-13;49% Cl) caused a significant proliferation of the
smooth endoplasmic reticulum (two-fold) whereas C18-26;49% Cl caused a
smaller increase. All three C10-13 chlorinated paraffins gave rise to
increased occurrence and size of cytoplasmic lipid droplets. Witachlor
149 also caused an increase in the number and size of mitochondria and
peroxisomes. These latter effects were also observed to a lesser
degree with both Witachlor 350 and Witachlor 549.
Effects on microsomal enzymes, after intraperitoneal injection of
Cereclor 42 (C22-26;42% Cl, CP-LL), Cereclor S58 (C14-17;58% Cl, CP-MH),
Cereclor 70 (C23;70% Cl, CP-LH) and Cereclor 70L (C10-13;70% Cl) (1000
mg/kg, once daily for 5 days) in liver from male Sprague-Dawley rats,
have been observed (Meijer et al., 1981). Microsomal epoxide hydrolase
activity was increased by all Cereclors except C22-26;42% Cl. In
addition, the activity of glutathione- S-transferase was increased
except in the case of C22-26;42% Cl, which decreased the activity
slightly. The amount of cytochrome P-450 was not changed.
7.3 Neurotoxicity
7.3.1 Short chain length chlorinated paraffins
The motor capacity, measured as the capacity of adult male NMRI
mice to remain on an accelerating rotarod, was determined after single
intravenous injections of 0, 30, 97.5, 165, 232.5 and 300 mg/kg in
groups of five mice of either Cereclor 50 LV (C10-13;49% Cl, CP-SL) or
Cereclor 70L (C10-13;70% Cl, CP-SH) (Eriksson & Kihlström, 1985). A
statistically significant decrease in the motor capacity and rectal
temperature was observed in mice receiving the highest dose of either
compound.
7.3.2 Intermediate chain length chlorinated paraffins
Immature 10-day-old NMRI mice (groups of at least 6) were given a
single peroral dose of 1 mg/kg body weight of polychlorohexadecane
(C16; chlorination degree not specified) dissolved in a fat emulsion
of egg lecithin and peanut oil (Eriksson & Nordberg, 1986). A
significantly decreased sodium-dependent choline uptake in the
cerebral cortex, 65% of Vmax in controls, was measured 7 days after
treatment indicating a pre-synaptic effect of this chlorinated
paraffin. No significant alteration in high- and low-affinity
muscarinic binding in the cerebral cortex in the brains could be
observed.
7.4 Reproductive toxicity, embryotoxicity and teratogenicity
7.4.1 Reproduction
An intermediate chain length chlorinated paraffin (C14-17) with
52% chlorination (CP-MH) was given in the diet to Charles River rats
at dose levels of 0, 100, 1000 and 6250 mg/kg feed (equivalent to 0,
6, 62 and 384 mg/kg body weight per day for the males and 0, 8, 74 or
463 mg/kg body weight per day for the females based on food
consumption data) (IRDC, 1985). The diet was fed both males and
females for 28 days before mating, during mating, and for females up
to postnatal day 21. Pups were given the same diet from weaning
until 70 days of age. No differences were observed in appearance,
fertility, body-weight gain, food consumption or reproductive
performance in the F0 generation. Among the offspring, no adverse
effects were observed prior to lactation day 7. However, significantly
decreased pup survival was observed in the high-dose group on lactation
day 10. None of the pups in this group survived to weaning. Survival
in pups from the mid-dose group was decreased by lactation day 21.
Necropsy findings in animals that died included pale liver, kidneys and
lungs, and blood in the cranial cavity, brain, stomach and intestines.
The pup weights were lower in the low-dose group (not statistically
significant) and mid-dose group than in the control group on lactation
day 21. In females, the reduced weight continued after weaning but
became less pronounced in males. Other observations in the pups of
the mid- and high-dose groups included bruised areas, decreased
activity, laboured breathing, pale discoloration and/or blood around
orifices. Reduced erythrocyte count, haemoglobin and haematocrit were
noted in the pups in the high-dose group on lactation day 6 relative to
the control values obtained on lactation day 7. The observations in
this study could indicate a high exposure of the pups to chlorinated
paraffins via the milk. This is supported by preliminary results of
a cross-fostering study showing a greater mortality in pups exposed
via milk than in pups exposed only in utero (Serrone et al., 1987).
The LOEL was 5.7 mg/kg body weight per day (males) or 7.2 mg/kg body
weight per day (females) in the F1 generation based on decreased
pup weight.
7.4.2 Embryotoxicity and teratogenicity
Teratology studies are summarized in Table 19.
Table 19. Oral teratology studies with chlorinated paraffins
Chlorinated paraffina Species LOEL NOEL Effects and remarks References
(mg/kg (mg/kg
body weight body weight
per day) per day)
CP-SH: C10-13;58% Cl rat 2000 500 maternal toxicity at 500 and 2000 mg/kg body weight IRDC (1982a)
per day; embryo-fetotoxicity and digital
malformations at 2000 mg/kg body weight per day
C10-13;58% Cl rabbit - 100 IRDC (1982d)
CP-MH: C14-17;52% Cl rat - 5000 slight maternal toxicity at 2000 and 5000 mg/kg IRDC (1984d)
body weight per day
C14-17;52% Cl rabbit - 100 mean maternal body weight losses were seen during IRDC (1983f)
treatment at the high-dose level (80, 100, 160 mg/kg
body weight per day) in a range-finding study
C14-17;70% Cl mouse - 100b Darnerud &
Lundkvist
(1987)
Table 19 (Cont'd)
Chlorinated paraffina Species LOEL NOEL Effects and remarks References
(mg/kg (mg/kg
body weight body weight
per day) per day)
CP-LL: C20-30;43% Cl rat - 5000 IRDC (1983d)
C20-30;43% Cl rabbit - 2000 slight increase in mean implantation loss and IRDC (1982e)
decreased number of viable fetuses at 5000 mg/kg
body weight per day, which were not statistically
significant
CP-LH: C22-26;70% Cl rat - 5000 IRDC (1984e)
C22-26,70% Cl rabbit - 1000 possible maternal toxicity (non-dosage-related IRDC (1983b)
congestion of lungs). In preliminary rabbit studies
an increase in post-implantation loss was observed
at > 1000 mg/kg body weight per day, but this effect
was not observed in the main teratology study
a The classification is given in Table 1
b Single intraperitoneal injection on day 1
7.4.2.1 Short chain length chlorinated paraffins
The teratogenic potential of a short chain length paraffin
(C10-13) with 58% Cl (CP-SH) was studied in pregnant Charles River COBS
CD rats (IRDC, 1982a). The rats, in groups of 25, were administered
0, 100, 500 and 2000 mg/kg body weight per day in corn oil orally by
gavage once daily from days 6 to 19 of gestation, and were examined on
gestation day 20. In the dams, the high dose treatment increased the
frequency of mortality (32%) and decreased body weight gain, and the
mid- and high-dose treatments resulted in dose-related adverse
clinical signs, such as yellow or brown matting and staining of the
anogenital fur, soft stool, red or brown matter (or staining) in the
nasal region, decreased activity, oily fur and excessive salivation.
Treatment at the highest dose level, which was a maternally toxic
dose, resulted in the appearance of fetal malformations such
as adactyly and/or shortened digits, increased incidences of
postimplantation loss and decreased numbers of viable fetuses. Fetal
body weight and incidence of delayed bone ossification were not
affected by the treatment. The NOEL for teratogenic effects was
500 mg/kg body weight per day, which was also a slightly maternally
toxic dose.
Female Dutch Belted rabbits (groups of 16) were treated by gavage
with a short chain length chlorinated paraffin (C10-13) with 58%
chlorination (CP-SH) (IRDC, 1982d). The rabbits were treated at dose
levels of 0, 10, 30 and 100 mg/kg body weight per day on gestation
days 6-27 and examined on day 28. There were no adverse effects on
survival, body weight gain, clinical signs or postmortem observations
in dams. The highest-dose group had an increased incidence of whole
litter resorption (two dams), and in the group exposed to 30 mg/kg
slight increases in the incidence of whole litter resorption (one dam)
and early and late resorptions were observed. Whole litter
resorptions did not occur in the low-dose or control animals, but
occurred in historical controls at an incidence of 13/277. This
indicated that the appearance of one or two dams with whole litter
resorptions could occur by chance. The NOAEL in this study was
100 mg/kg body weight per day.
7.4.2.2 Intermediate chain length chlorinated paraffins
Female Charles River COBS CD rats were treated by gavage with an
intermediate chain length chlorinated paraffin (C14-17;52% Cl) (CP-MH)
(IRDC, 1984d). The administered dose levels (0, 500, 2000 and
5000 mg/kg body weight per day) were given to groups of 25 animals on
gestation days 6-19, and this was followed by examination on day 20.
The end-points studied were weight of the uterus, number and location
of viable fetuses, early and late resorptions, the number of total
implantations and corpora lutea, and the incidence of fetal
malformations. The treatment had no adverse effect on mortality, body
weight gain or uterine weight of dams, but signs of toxicity, such as
wet, matted and yellow-stained hair in the anogenital area and/or soft
stools, were observed in the mid- and high-dose dams. No treatment-
related adverse effects were observed in the fetuses and there was no
evidence of developmental effects.
Female Dutch Belted rabbits were treated by gavage with an
intermediate chain length chlorinated paraffin (C14-17;52% Cl) (CP-MH)
in groups of 16 (IRDC, 1983f). The dose levels were 0, 10, 30 and
100 mg/kg body weight per day and were administered on gestation days
6-27, followed by examination on day 28. The end-points studied were
weight of the uterus, number and location of viable fetuses, early and
late resorptions, the number of total implantations and corpora lutea,
and the incidence of fetal malformations. In the dams, congestion of
the lobes of the lung was noted in all treated groups at necropsy, but
did not occur in a dose-related pattern. No significant adverse
effects were observed in fetuses and there were no developmental
effects.
In a study of NMRI mice, the animals were given a single
intraperitoneal injection of 100 mg/kg body weight of polychloro-
hexadecane (C16;70% Cl) (CP-MH) on the day the vaginal plug was
observed (day 1) (Darnerud & Lundkvist, 1987). The mice were killed
on day 14 of pregnancy, and the number and weight of embryos and
resorptions were determined. No effects on implantation or embryonic
survival were observed.
7.4.2.3 Long chain length chlorinated paraffins
Groups of 25 pregnant Charles River COBS CD rats were administered
(500, 2000 and 5000 mg/kg body weight per day) a long chain chlorinated
paraffin (C22-26;43% Cl) (CP-LL) by gavage from days 6 to 19 of
gestation (IRDC, 1983d). The end-points studied were weight of the
uterus, number and location of viable fetuses, early and late
resorptions, the number of total implantations and corpora lutea,
and the incidence of fetal malformations. In the dams, there were no
adverse effects on appearance, mean body weight gain or mortality
rate, and necropsy findings were normal. No signs of developmental
effects were noted in the pups.
Female Charles River COBS CD rats (groups of 25 animals) were
treated by gavage with a long chain length chlorinated paraffin
(C22-26;70% Cl) (CP-LH) (IRDC, 1984e). The dose levels were 0, 500,
2000 and 5000 mg/kg body weight per day on gestation days 6-19. The
rats were examined on day 20. The end-points studied were the same as
in the previous study. No treatment-related adverse effects were
observed in the dams or pups.
Pregnant Dutch Belted rabbits in groups of 16 were exposed orally
to long chain chlorinated paraffin (C22-26;43% Cl) (CP-LL) at doses of
500, 2000 and 5000 mg/kg body weight per day in corn oil by gavage
once daily from days 6 to 27 of gestation (IRDC, 1982e). The
end-points studied were the same as in the previous studies in this
section. No effects on maternal survival or body weight gain
occurred. In the highest-dose group there was a slight increase in
mean implantation loss and a slight decrease in the mean number of
viable fetuses when compared to the control group. However, these
alterations were not statistically significant. In the fetuses no
alterations related to the treatment were observed, although the
number in the highest-dose group was limited. Treatment of the
rabbits at a dose level of 2000 mg/kg body weight per day or less did
not produce a teratogenic response. No developmental effects were
noted. The NOEL in this study was 2000 mg/kg body weight per day.
Female Dutch Belted rabbits (groups of 16 animals) were given by
gavage a long chain length chlorinated paraffin (C22-26;70% Cl) (CP-LH)
(IRDC, 1983b). Doses of 0, 100, 300 and 1000 mg/kg body weight per
day were administered on gestation days 6-27, followed by examination
on day 28. The end-points studied were the same as in the earlier
studies in this section. The appearance, behaviour and body weight
gain were normal in the treated dams, although at necropsy a
non-dose-related increase in the occurrence of congested lungs was
noted. No adverse effects on the fetuses were observed and no
developmental effects were noted.
7.5 Mutagenicity and related end-points
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells there is a suggestion of a weak
clastogenic potential in vitro but not, according to several reports,
in vivo. Chlorinated paraffins were also reported to induce cell
transformation in vitro.
Table 20. Mutagenicity of chlorinated paraffins in bacterial tests
Chlorinated paraffina Dose (µg/plate) S9 Bacterial strainsb Effects Reference
Cereclor 50LV C10-13;50% Cl (CP-SH) 2500 ± TA1535 - Birtley et al. (1980)
TA1538 -
TA100 -
TA98 -
Hordalub 80 (with 1% C10-13;50% Cl (CP-SH) 10 000 ± TA98 + Hoechst (1986a)
epoxy stabilizer) TA100 -
TA1535 -
TA1537 -
TA1538 -
WP2 uvrAc -
Chloroparaffin 56 C12;57% Cl (CP-SH) 5000 ± TA98 - Hoechst (1988)
(unstabilized) TA100 -
TA1535 -
TA1537 -
TA1538 -
WP2 uvrAc -
Cereclor 70L C10-13;70% Cl (CP-SH) 2300 ± TA98 - Meijer et al. (1981)
TA100 -
TA1537 -
Cereclor S52 C14-17;52% Cl (CP-MH) 2500 ± TA1535 - Birtley et al. (1980)
- stabilizer TA1538 -
TA100 -
TA98 -
+ stabilizer -
Table 20. Cont'd
Chlorinated paraffina Dose (µg/plate) S9 Bacterial strainsb Effects Reference
Solvocaffaro C1642 C14-17;42% Cl (CP-ML) 1, 10, ± TA1535 - Conz & Fumero (1988a)
100, TA1537 -
1000, TA1538 -
5000 TA98 -
TA199 -
Meflex DC 029 C14-17;45% Cl (CP-ML) 1.6, 8, ± TA1535 - Elliott (1989a)
40, TA1537 -
200, TA1538 -
1000, TA98 -
5000 TA1000 -
Cereclor 42 C20-30;42% Cl (CP-LL) 2500 ± TA1535 - Birtley et al. (1980)
TA1538 -
TA100 -
TA98 -
Chorowax 40 C23;43% Cl (CP-LL) 10 000 ± TA97 - NTP (1986b)
TA98 -
TA100 -
TA1535 -
a The classification is given in Table 1
b Unless indicated otherwise, all strains refer to Salmonella typhimurium
c Strain of Escherichia coli
7.5.1 Prokaryotes
Most of the data demonstrate no mutagenic effects in four
Salmonella typhimurium strains after treatment with short,
intermediate or long chain length chlorinated paraffins at doses up to
10 mg/plate (Table 20). A very low but significant effect was
detected in strain TA98 after treatment with the highly chlorinated
short chain length chlorinated paraffin, Cereclor 70L (C10-12;70% Cl,
CP-SH) (Meijer et al., 1981). However, the observation is uncertain
since no dose response was observed, the increase in revertants was
low (less than 2-fold), and the increase was only found in the
presence of metabolic fraction (S9) derived from Aroclor-1254-induced
rat liver. The results from this study were considered to be
negative. Another study apparently demonstrated positive results.
However, the increase in the number of revertants with TA100 in the
presence of S9 was just less than two-fold, and in TA98, in the
absence of S9, the increase only just reached two-fold (Hoechst,
1986a). Furthermore, the possibility that the epoxy stabilizer was
responsible for the increase can not be discounted.
7.5.2 Mammalian cells
The results obtained from mammalian cell systems are summarized
in Tables 21, 22 and 23.
7.5.2.1 In vitro studies
C12;60% Cl (CP-SH) was mutagenic in L5178Y mouse lymphoma cells
at concentrations of 48 and 60 µg/ml in the absence of S9 mix (Myhr et
al., 1990).
When tested up to cytotoxic concentrations, C10-13;56% Cl(CP-SH)
did not induce a significant, reproducible increase in the number of
mutant colonies in Chinese hamster V79 cells (HPRT locus), either in
the presence or absence of S9.
Chlorowax 500C (C23;43% Cl, CP-LL) induced chromosome
aberrations in the absence of S9 mix at a concentration of 5000 µg/ml
and sister chromatid exchange (SCE) with and without S9 at 5, 500,
1700 and 5000 µg/ml in Chinese hamster ovary (CHO) cells in vitro
(Anderson et al., 1990).
7.5.2.2 In vivo studies
a) Short chain length chlorinated paraffins
When C12;60% Cl (CP-SH) (0, 500, 1000 and 2000 mg/kg body weight
was administrated in corn oil by gavage to Alpk:AP male rats (groups
of 4), no effects on unscheduled DNA synthesis (UDS) in hepatocytes
could be detected after exposure for 2 or 12 h (Ashby et al., 1990).
However, a moderate dose- and time-related induction of cell
proliferation, measured as S-phase cells, in the hepatocytes was
detected in animals exposed to 1000 and 2000 mg/kg for 12 h.
Table 21. Genotoxicity in mammalian cells in vitro
Chlorinated paraffin Cell line End-point Exposure Effect References
CP-SH: C10-13;56% Cl Chinese hamster mutations 5, 10, 15, 20, 30, 50, 75 µg/ml - Hoechst (1987)
V79 cells (±S9)
C12;60% Cl L5178Y mouse mutations 12, 24, 36, 48, 60 and 72 µg/ml + (from Myhr et al.
lymphoma cells (-S9) 60 µg/ml) (1990)
CP-LL: C23;43% Cl Chinese hamster chromosome 1250-5000 µg/ml (±S9) + (at 5000 Anderson et al.
ovary cells aberrations µg/ml + S9) (1990)
C23;43% Cl Chinese hamster sister chromatid 5, 500, 1700 and 5000 µg/ml +a Anderson et al.
ovary cells exchange (±S9) (1990)
a The effect was observed at all concentrations
Table 22. Cell transformation in in vitro mammalian cells
Grade Cell line Exposure Effect References
CP-SH: C10-13;50% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
C10-13;58% Cl Baby hamster kidney cells 44 µg/ml (-S9), 58 µg/ml (+S9) + ICI (1982a)
C10-13;58% Cl Baby hamster kidney cells 33 µg/ml (-S9), 88 µg/ml (+S9) + Richold et al. (1982a)
CP-MH: C14-17;52% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
CP-LL: C20-30;42% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
CP-LH: C20-26;70% Cl Baby hamster kidney cells 10, 50, 100, 500 and 1000 µg/ml (±S9) +a Richold et al. (1982b)
C22-26;70% Cl Baby hamster kidney cells 10 µg/ml (+S9), 294 µg/ml (-S9) + ICI (1982b)
a The effect was observed at all concentrations
Table 23. Genotoxicity data from in vivo mammalian cells
Grade Species and End-point Exposure No. of Effect References
strain animals
CP-SH: Fischer-344 Chromosome aberrations 250, 750 and 2500 mg/kg body weight 8 - IRDC (1983h)
C10-12,58% Cl rats in bone marrow cells per day for 5 days by gavage
C10-13,58% Cl Charles River Dominant lethal 250, 750 and 2500 mg/kg body weight 15 - IRDC (1983a)
COBS CD rats mutations per day for 5 days by gavage
C10-13,58% Cl NMRI mice Micronucleus assay 50, 5000 mg/kg body weight (single 10 - Muller (1989)
in bone marrow cells dose by gavage)
C10-13,58% Cl Hoe:NMRKF Micronucleus assay 50, 5000 mg/kg body weight (single 10 - Muller (1989)
SPF 71 mice in bone marrow cells dose by gavage)
C12;60% Cl Alpk: AP rats DNA repair in 500, 1000 and 2000 mg/kg body weight 4 - Ashby et
hepatocytes by gavage (single dose by gavage) al. (1990)
for 2 or 12 h
CP-ML: Crd: CD-1 Micronucleus assay 5000 mg/kg body weight (single dose 10 - Conz &
C14-17;42% Cl (ICR) Br mice in bone marrow cells by gavage) Fumero (1988b)
C14-17;45% Cl C57B1/6JFCD-1/ Micronucleus assay 3125, 5000 mg/kg body weight (single 10 - Elliott
Alpk mice in bone marrow cells dose by gavage) (1989b)
Table 23. (Cont'd)
Grade Species and End-point Exposure No. of Effect References
strain animals
CP-MH: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983g)
C14-17;52% Cl rats in bone marrow cells per day for 5 days by gavage
CP-LL: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983i)
C22-26;43% Cl rats in bone marrow cells per day for 5 days by gavage
CP-LH: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983e)
C20-30;70% Cl rats in bone marrow cells per day for 5 days by gavage
Sexually mature male Fischer-344 rats in groups of eight were
dosed by gavage once daily for 5 days with a short chain length
chlorinated paraffin (C10-12;58% Cl, CP-SH) at doses of 0, 250, 750 or
2500 mg/kg body weight per day (IRDC, 1983h). Metaphase spreads of
rat bone marrow cells were examined for chromosome aberrations. In
the group treated with 2500 mg/kg, all the rats except one died during
the study. Signs of overt toxicity were observed in most of these
rats. The rats receiving up to 750 mg/kg body weight per day did not
show an increased mortality or frequency of chromosome or chromatid
abnormalities, neither did the surviving rat from the highest dose
group. These observations indicate that toxic doses were administered
to the rats. Cytotoxicity was not assessed. However, the information
on distribution of short chain chlorinated paraffins following oral
absorption (section 6.3.1) indicates that there would have been
distribution to the bone marrow. This chlorinated paraffin was not
considered clastogenic in this test system.
Sexually mature NMRI (Hoe, NMRKF [SPF71]) mice (groups of five
males and five females) were given single doses of 50 and 5000 mg/kg
body weight Chlorowax 500C (C10-13;58% Cl, CP-SH) by gavage in sesame
oil (Hoechst, 1989). Responses at the high dose level were examined
at 24, 48 and 72 h sampling times and the low dose level at a 24 h
sampling time only. There were no differences from control values
either in polychromatic cells with micronuclei or in the ratio of
polychromatic erythrocytes to normocytes.
The dominant lethal mutation potential of a chlorinated paraffin
of short chain length and 58% chlorination was examined in Charles
River COBS CD rats (IRDC, 1983a). Groups of 15 males were treated
with 0, 250, 750 and 2000 mg/kg body weight per day in corn oil orally
by gavage for five consecutive days. Each male was then mated with 20
untreated females. There was no evidence of a mutagenic effect on the
post-meiotic stage of spermatogenesis at any dose level, as shown by
the absence of effect on the mean number of viable embryos during the
first four weeks of mating.
b) Intermediate chain length chlorinated paraffins
Sexually mature male Fischer-344 rats in groups of eight were
given unstabilized chlorinated paraffin (C14-17;52% Cl, CP-MH) in corn
oil by gavage once daily for 5 days at doses of 0, 500, 1500 and 5000
mg/kg body weight per day (IRDC, 1983g). Metaphase spreads of rat
bone marrow cells were examined for chromosome aberrations. No signs
of toxicity were observed during the study, and the treatments did not
produce any increase in the frequency of chromosome abnormalities,
compared to the controls.
Two studies yielding negative results in the mouse bone marrow
micronucleus assay have been reported. Sexually mature mice, of
strains CRI: CD-1 (ICR) BR (Conz & Fumero, 1988b) and C57BL/6JF
CD-1/ALpK (Elliott, 1989b) were used. Conz & Fumero (1988b) studied
Solvocaffaro C1642 (C14-17;42% Cl (CP-MH)) and Elliott (1989b) studied
Melex DC 029 (C14-17;45% Cl). In both studies groups of 10 animals:
(five males and five females) were given the limit dose of 5000 mg/kg
body weight by gavage in corn oil. Elliott (1989b) also used a lower
dose of 3125 mg/kg body weight. Responses at 5000 mg/kg were examined
at three sampling times (18, 43 and 66 h (Conz & Fumero, 1988b) or 24,
48 and 72 h (Elliott, 1989b)). Responses at the lower dose level
(Elliott, 1989b) were examined at 24 h. In both studies there were no
differences from negative control values in either polychromatic cells
with micronuclei or in the ratio of polychromatic erythrocytes to
normocytes. The positive control (mitomycin C [8 mg/kg body weight,
Conz & Fumero, 1988b] or cyclophosphamide [65 mg/kg body weight,
Elliott, 1989b]) produced the anticipated positive responses, thus
verifying the sensitivity of the test systems.
c) Long chain length chlorinated paraffins
When a long chain length paraffin with 70% chlorination (CP-LH)
(in 1% carboxymethylcellulose) was administered by gavage to
Fischer-344 rats (groups of 8) at doses of 500, 1500 and 5000 mg/kg
body weight daily for 5 days, no increased frequency of chromosome
abnormalities in bone marrow cells was observed indicating a lack of
clastogenic activity under the experimental conditions (IRDC, 1983e).
Body weight gain was decreased in the high-dose group.
Sexually mature male Fischer-344 rats in groups of eight were
given (gavage once daily for 5 days) a long chain length chlorinated
paraffin (C22-26;43% Cl, CP-LL) at doses of 500, 1500 and 5000 mg/kg
body weight per day (IRDC, 1983i). Metaphase spreads of rat bone
marrow cells were examined for chromosome aberrations. No signs of
toxicity was observed during the study, and the treatment did not
produce any increase in the frequency of chromosome abnormalities,
compared to the controls.
7.5.2.3 Cell transformation
Chlorowax 500C (C10-13;58% Cl, CP-SH), was examined in a cell
culture transformation test using baby hamster kidney (BHK) cells in
soft agar (Richold et al., 1982a). The cells were treated with doses
from 3.125 µg/ml to 500 µg/ml (-S9) and from 6.25 µg/ml to 1000 µg/ml
(+S9). Treatment with LC50 doses of 33 µg/ml (-S9) and 88 µg/ml (+S9)
increased the transformation frequency 52 times in the absence of S9
(the control was negative at 50% simulated survival) and 500 times in
the presence of S9.
Cereclor 50LV (C10-13;50% Cl, CP-SH) was not active at dose levels
up to 2500 µg/ml in a cell transformation assay using BHK cells
(Birtley et al., 1980).
Cereclor S52 (C14-17;52% Cl, CP-MH) with or without stabilizer
did not induce transformation of BHK cells at doses up to 2500 µg/ml
(Birtley et al., 1980).
After treatment of BHK cells with a long chain length paraffin
Electrofine S-70 (C20-26;70% chlorination, CP-LH) in doses from
10 µg/ml to 1000 µg/ml (+S9), a dose-related increased frequency of
transformed colonies was demonstrated at all dose levels (Richold et
al., 1982b).
Cereclor 42 (C20-30;42% Cl, CP-LL) was not active at dose levels
up to 2500 µg/ml in a cell transformation assay in BHK cells (Birtley
et al., 1980).
7.6 Long-term exposure and carcinogenicity
7.6.1 Oral route
7.6.1.1 Short chain length chlorinated paraffins
In a 2-year gavage study using Fischer-344 rats, groups of 50
males and 50 females were given C12;60% Cl, CP-SH at doses of 312 or
625 mg/kg per day, 5 days/week, for 104 weeks) (NTP, 1986a; Bucher et
al., 1987). After week 37, the body weights of the high-dose males
were reduced by 10-23% compared with controls. Survival of both
low- and high-dose males and of low-dose females was significantly
less than that of controls (at termination 27 male controls, 6
low-dose and 3 high-dose males and 34 control females, 23 low-dose and
29 high-dose females survived). Additional groups of 20 male and 20
female rats were added to each dose group for concurrent 6-month and
12-month studies. The spleen, liver, thymus, adrenal glands, brain,
kidney and heart were weighed at necropsy in the 6- and 12-month
studies. Biochemical and haematological effects were not examined.
The incidence of tumours, which was significantly increased, is
presented in Table 24. Liver neoplastic nodules and hepatocellular
carcinomas combined in males and females occurred with a positive
trend. The incidence of kidney tubular cell adenomas and the combined
incidence of adenomas and adenocarcinomas were significantly increased
in low-dose male rats. In low-dose females an increased incidence of
thyroid follicular cell adenomas was observed, and in addition,
adenomas and carcinomas combined showed an increased incidence in
high-dose female rats. Increased incidences of mononuclear cell
leukaemia, pancreatic acinar cell adenomas and endometrial stromal
polyps of the uterus (low-dose female rats) were observed. The
increased incidence of mononuclear cell leukaemia was dose-related in
males but not in females. Although the incidence of endometrial
stromal polyps in the low-dose female rats was greater than in vehicle
controls, these tumours were probably not treatment-related since
there were no increases at higher doses. The incidence of pancreatic
acinar tumours was also increased in the low-dose group of males,
although, owing to the higher incidence in concurrent compared to
historical controls and the absence of a dose-response relationship,
the increase was not considered to be treatment-related.
In the same study some chronic non-neoplastic lesions were
reported. Hepatocellular hypertrophy was observed in 74% of the
low-dose group and in nearly all of the high-dose rats, but in none
of the control group. Necrosis and angiectasis were observed in the
livers in all dosed rats. In addition to increased kidney weight at 6
and 12 months in both sexes and dose levels, the incidence and
severity of nephropathy was increased in dosed females, as was the
severity of nephropathy in males in the concurrent 12-month study.
Erosion, inflammation and ulceration of the glandular stomach and the
forestomach were seen in both groups of the dosed males. Hyperplasia
of the parathyroid was observed in both groups of exposed males and in
high-dose females.
Groups of 50 male and 50 female B6C3F1 mice were given C12;60%
Cl, CP-SH by gavage at doses of 125 and 250 mg/kg 5 days a week for
103 weeks (NTP, 1986a; Bucher et al., 1987). The body weights of
treated females were about 10% lower than those of controls during the
second year. The survival of treated males was not significantly
different from that of the controls, but in the high-dose group of
females fewer animals were still alive after week 100 in comparison
with the control group. The tumour incidences are shown in Table 25.
Increased incidences of liver adenomas and liver adenomas and
carcinomas in combination were observed. The incidences of thyroid
follicular cell adenomas and carcinomas combined were increased in
exposed female mice. The incidences of alveolar/bronchiolar
carcinomas were increased significantly in the high-dose group of male
mice, and the trend with dose was also significant. However, the
incidences of alveolar/bronchiolar carcinomas and adenomas (combined)
in males were not significantly greater than those in vehicle
controls. Among the non-neoplastic lesions, the incidence of
nephrosis was increased in the high-dose group of females, but was
decreased in dosed male mice compared to controls. Biochemical and
haematological effects were not examined.
Table 24. Incidence of tumours in rats administered the chlorinated paraffin C12;60% chlorine
(From: NTP, 1986a; Bucher et al., 1987)
Dose Hepatocellular Hepatocellular Hepatocellular Follicular cell Mononuclear Adenomas or
(mg/kg neoplastic carcinomas neoplastic nodules adenomas and cell leukaemia adeno-carcinomas
body weight) nodules and carcinomas carcinomas of of the kidney
the thyroid
Males
Control 0/50 0/50 0/50 3/50 7/50 0/50
312 10/50a 3/50a 13/50a 3/50 12/50b 9/50b
625 16/48a 2/48 16/48a 3/50 14/50b 3/49
Females
Control 0/50 0/50 0/50 0/50 11/50 0/50
312 4/50 1/50 5/50a 6/50a 22/50b 0/50
625 7/50a 1/50 7/50a 6/50a 16/50 0/50
a Incidental tumour test for trend, p < 0.05, increase relative to control
b Life table analysis, p < 0.05, increase relative to control
Table 25. Incidence of tumours in mice administered the chlorinated paraffin
C12;60% chlorine (From: NTP, 1986a; Bucher et al., 1987)
Dose Hepatocellular Hepatocellular Hepatocellular Follicular cell
(mg/kg adenomas carcinomas adenomas and adenomas and
body carcinomas carcinomas of
weight) the thyroid
Males
Control 11/50 11/50 20/50 3/49
125 20/50a 15/50 34/50a 4/50
250 29/50a 17/50 38/50a 3/49
Females
Control 0/50 3/50 3/50 8/50
125 18/50a 4/50 22/50a 12/49a
250 22/50a 9/50b 28/50a 15/49a
a Incidental tumour test for trend, p < 0.05, increase relative to control
b Life table analysis, p < 0.05, increase relative to control
7.6.1.2 Long chain length chlorinated paraffins
Groups of 50 male and 50 female Fischer-344 rats were treated by
gavage with 1875 and 3750 mg/kg body weight (male rats) and 100, 300
and 900 mg/kg body weight (female rats) of C23;43% Cl, CP-LL
dissolved in corn oil on 5 days a week for 103 weeks (NTP, 1986b;
Bucher et al., 1987). The treatment did not affect body weight or
survival, and no signs of clinical toxicity were observed. Additional
groups of 20 male and 20 female rats were exposed concurrently to the
same doses for 6 or 12 months for analyses of the weights of the
spleen, liver, thymus, adrenal glands, brain, kidneys and heart, serum
hepatic enzymes, including sorbitol dehydrogenase, AST and ALT, and
haematological parameters.
In female rats that were administered the chlorinated paraffin,
an increased incidence of adrenal gland medullary phaeochromocytomas
was observed (1/50; 4/50; 6/50; 7/50, high dose statistically
significant, significant positive trend). In addition, an increased
incidence of endometrial stromal polyps in the uterus was found in the
low-dose group of the females, but this increase was not dose-related
(9/50; 17/50; 10/50; 10/50, low dose statistically significant) (Table
26). In males, acinar cell tumours of the pancreas occurred with a
negative trend. The incidence of benign hepatocellular neoplasia was
not increased in dosed rats.
Table 26. Incidence of tumours in rats administered C23;43% Cl
(From: NTP, 1986b; Bucher et al., 1987)
Dose (mg/kg body Adrenal medulla, Uterus, endometrial
weight) phaeochromocytomas stromal polyps
Females
0 1/50 9/50
100 4/50 17/50a
300 6/50 10/50
900 7/50a 10/50
a Incidental tumour test for trend, p < 0.05, increased relative
to control
Several non-neoplastic lesions were related to the administration
of C23;43% Cl. Relative liver weights were increased in exposed
males at 12 months and in exposed females at 6 and 12 months. The
observed increases were dose-related. Activities of several serum
enzymes were also slightly elevated at both 6 and 12 months. There
were also variations in haematological parameters at 6 and 12 months,
but only in females. The primary non-neoplastic lesion related to
administration of this chlorinated paraffin included a diffuse
lymphohistiocytic inflammation in the liver and in the pancreatic and
mesenteric lymph nodes in male and female rats in all exposed groups.
Splenic congestion was a secondary effect.
Groups of 50 male and 50 female B6C3F1 mice were given, by
gavage, C23;43% Cl, CP-LL dissolved in corn oil at doses of 2500 and
5000 mg/kg on 5 days a week for 103 weeks (NTP, 1986b; Bucher et al.,
1987). The survival of the mice was not significantly different in
treated groups compared to controls, and there were no clinical signs
of toxicity. However, in the female groups a Klebsiella infection
affected the animals after week 65, and 60 to 70% of the early deaths
in each group were attributed to the infection. Low-dose males and
females had lower weight gains than control or high-dose groups. The
incidence of malignant lymphomas was significantly increased in males
of the high-dose group, and occurred with a positive trend (Table 27).
The incidences of hepatocellular carcinomas in females occurred with a
positive trend, but the increase was not significant. The incidences
of adenomas and carcinomas of the liver (combined) were marginally
increased in females. Follicular cell carcinomas in males occurred
with a positive trend (0/49; 0/48; 3/49) in the thyroid gland.
However, the incidence of follicular cell adenomas or carcinomas
(combined) was not significantly greater than that in vehicle controls
(1/49, 3/48; 5/49) and was within the range of historical controls for
the test laboratory. No significant increases in non-neoplastic
lesions were attributed to administration in mice.
Table 27. Incidence of tumours in mice administered C23;43% Cl
(From: NTP, 1986b; Bucher et al., 1987)
Dose (mg/kg Lymphoma Hepatocellular Hepatocellular
body weight) carcinomas adenomas and
carcinomas
Males
0 6/50 9/50 18/50
2500 12/50 12/50 21/50
5000 16/50a,b 12/50 23/50
Females
0 15/50 1/50 4/50
2500 12/49 1/49 3/49
5000 20/50 6/50 10/50
a Incidental tumour test for trend, p < 0.05, increased relative
to controls
b Life table test, p < 0.05, increased relative to controls
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Controlled human studies
Chlorinated paraffins C10-13;50 and 63% Cl(CP-SH) were applied,
under occlusive dressings, to the upper arm of 26 volunteers
(INVERESK, 1975). After 24 h the applications were removed and one
hour later skin reactions were examined by two independent assessors.
A second application was made and reactions were assessed after a
further 24 h contact. Mild erythema and dryness (average scores, read
at the 24 and 50 h time points, of less than 2 and 1, respectively, on
a 4-point scale) were recorded, which were comparable to scores in a
liquid paraffin control group.
Paroil 142 (C20-30;40-41% Cl, CP-LL) and Chlorez 700 (C24;70% Cl,
CP-LH) were applied to the skin of 200 male and female volunteers for
a 5-day period, then reapplied for 2 days beginning 3 weeks after the
initial exposure. The dose level was not reported. No primary local
irritation, allergic response or other toxic responses were observed.
In a similar study, Chlorowax 70 (C24;70% Cl, CP-LH), Chlorowax 500C
(C12;59% Cl, CP-SH) and Chlorowax 40 (C24;43% Cl, CP-LL) were applied
to the skin of 200 males and female volunteers. The exposure time
period and amount of chlorinated paraffins used were not reported.
The treatments did not produce local irritation or allergic responses
(Howard et al., 1975).
8.2 Occupational exposure
In a study on cutting fluid coolants, 134 non-exposed employees
and 75 exposed employees were patch tested with various constituents
of the cutting fluids including chlorinated paraffins (Menter
et al., 1975). No positive reactions were obtained with any of the
constituents, although the authors themselves suggested that the tests
were not sufficiently stringent, as some positive reactions were
anticipated for some of the constituents tested.
Positive skin reactions to chlorinated paraffin constituents were
obtained in patch tests conducted on four employees suffering from
scaly eczema, who had been exposed occupationally to cutting oils
(English et al., 1986). However, the authors concluded that the
reaction was due to additives in the cutting oil, which showed
positive reactions when tested alone, rather than to the chlorinated
paraffin.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
The inhibition of gas production in an anaerobic sewage sludge
digestion process by C10-12;58% Cl (CP-SH) was studied by Madeley et
al. (1983b). A significant (> 10%) inhibition of gas production
occurred at chlorinated paraffin concentrations of 3.2, 5.6 and 10%
(w/w with respect to digester volatile suspended solids) during the
first 3-4 days of the experiment, but the gas production recovered
during the rest of the experiment until termination at day 10. It
was concluded in the report that the chlorinated paraffin induced a
transient partial inhibition and no longer-term effects.
9.1.2 Aquatic organisms
Acute toxicity data for aquatic invertebrates and fish are
summarized in Table 28. Chlorinated paraffins of short-chain length
have been shown to be acutely toxic to both freshwater and saltwater
invertebrates. Most of the acute toxicity tests for intermediate
and long chain chlorinated paraffins on aquatic invertebrates
exceed the water solubility. However, a study on intermediate
chlorinated paraffins suggests that these may be acutely toxic.
Short, intermediate and long chain chlorinated paraffins appear to
be of low acute toxicity to fish, the LC50 values being well
in excess of the water solubility.
9.1.2.1 Aquatic plants
Exposure of the freshwater alga Selenastrum capricornutum for
10 days to C10-12;58% Cl (CP-SH) at dose levels of 110, 220, 390, 570,
900 and 1200 µg/litre resulted in a significant inhibition of growth
at 570 µg/litre. The calculated EC50 values for cell density over 4,
7 and 10 days were 3690, 1550 and 1310 µg/litre, respectively, which
all exceeded the highest tested concentration (Thompson & Madeley,
1983d).
The marine alga Skeletonema costatum was exposed to C10-12;58%
Cl (CP-SH) for 10 days at concentrations of 0, 4.5, 6.7, 12.1, 19.6,
43.1 and 69.8 µg/litre (Thompson & Madeley, 1983b). Significant
inhibition of growth was observed on the first 2 days from
19.6 µg/litre. The EC50 for cell density after 4 days was
42.3-55.6 µg/litre, and EC50 for growth rate after 2 days was
31.6 µg/litre. No significant reduction in cell density was observed
after 10 days, indicating an effect on duration of lag phase prior to
exponential growth or a drop in chlorinated paraffin concentration after
10 days.
Table 28. Acute toxicity of chlorinated paraffins to aquatic organisms
Species Chlorinated Parameter Concentration Reference
paraffin (µg/litre)
Water flea C10-12;58% Cl 48-h EC50b 530 Thompson &
Daphnia magna C10-12;58% Cl 72-h EC50b 24 Madeley
(freshwater) C10-12;58% Cl 96-h EC50b 18 (1983c)
C10-12;58% Cl 120-h EC50b 14
C14-17;52% Cl 48-h EC50b 37 Frank &
Steinhäuser
(in press)
Mysid shrimp C10-12;58% Cl 96-h LC50 14.1-15.5 Thompson &
Mysidopsis bahia Madeley
(estuarine) (1983a)
Nitocra spinipes C10-13;49% Cl 96-h LC50 100 Tarkpea
(marine C10-13;70% Cl 96-h LC50 < 300 et al.
crustacean) C14-17;45% Cl 96-h LC50 9000 (1981)
C14-17;52% Cl 96-h LC50 > 10 × 106
C22-26;42% Cl 96-h LC50 > 1 × 106
C22-26;49% Cl 96-h LC50 > 10 × 106
Bleak C10-13;49%, 96-h LC50 > 5 × 106 Lindén et
Alburnus 63%, 71% Cla al. (1979)
alburnus C10-13;56%, Cl 96-h LC50 > 10 × 106
(estuarine) C11.5;70% Cl 96-h LC50 > 10 × 106
C15.5;40% Cl 96-h LC50 > 5 × 106
C14-17;50%, 96-h LC50 > 5 × 106
52% Cla
C22-26;42% Cl 96-h LC50 > 5 × 106
C18-26;49% Cl 96-h LC50 > 5 × 106
Rainbow trout C20-30;42% Cl 96-h LC50 770 × 103 Madeley &
Oncorhynchus Birtley
mykiss (1980)
(freshwater)
a Consecutive percentage chlorinations refer to different tests
b EC50 based on immobilization
9.1.2.2 Invertebrates
Short-term toxicity data for aquatic invertebrates are summarized
in Table 29 and chronic toxicity data in Table 30.
The freshwater crustacean Daphnia magna was exposed to a short
chain length paraffin with 58% Cl (CP-SH) (Thompson & Madeley, 1983c).
The chlorinated paraffin caused the organisms to float at or near the
surface of the water at 75 µg/litre or more. All water fleas died at
16.3 µg/litre after 6 days in a continuous-flow experiment. The
following LC50 values were calculated: 3 days, 24 µg/litre; 4 days,
18 µg/litre; 5 days, 14 µg/litre; 6 to 21 days, 12 µg/litre. No dead
parent Daphnia were observed at 8.9 µg/litre after 21 days of
exposure, but 37% of the offspring were dead as compared to 6% and 9%
in the controls. No increased mortality in the offspring was observed
at 5.0 µg/litre. The number of offspring per female was reduced at
2.7 µg/litre.
Daphnia magna was studied in a 48-h test with C14-17;52% Cl and
C18-20;52% Cl. Using the water-soluble fraction of a loading
concentration of 100 mg/litre, an EC50 of 37 µg/litre for the
intermediate chain length chlorinated paraffin and an EC0 of
> 26 µg/litre for the long chain length chlorinated paraffin were
observed. In a 21-day reproduction test, daphnids were exposed to the
water-soluble fraction of both chlorinated paraffins. With a loading
of 100 mg/litre, a no-observed-effect concentration of 4.4-8.8 µg/litre
was found for reproduction rate and parent mortality (LOEC =
19.9-35.6 µg/litre) for the intermediate chain length chlorinated
paraffin. For the long chain length chlorinated paraffin, a LOEC of
< 1.2 µg/litre was found for the same two parameters. In these
studies it was observed that a higher loading concentration of
10 g/litre caused an increase in the effect concentrations (Frank &
Steinhäuser, in press).
In studies of the marine shrimp Mysidopsis bahia, the 96-h
LC50 was between 14.1 and 15.5 µg/litre after exposure to a short
chain length chlorinated paraffin with 58% Cl (CP-SH) (Thompson &
Madeley, 1983a). After 28 days of exposure to 0.6, 1.2, 2.4, 3.8 and
7.3 µg/litre, increased mortality was observed but this was not
treatment-related. The increased mortality was significantly
different from the control (at 1.2 and 2.4 µg/litre) but not from the
solvent control, and was therefore considered as not related to the
chlorinated paraffin concentration. No treatment-related effects of
this chlorinated paraffin on reproductive rate or growth over this
time period were observed.
Table 29. Short-term toxicity data for aquatic invertebrates
Chlorinated paraffin Organism LC50 Exposure Other effects LOEC References
period
C10-13;49% Cl (CP-SL) Nitocra spinipes 100 µg/litre 96 h Tarkpea et al.
(1981)
C10-12;58% Cl (CP-SH) Dapnia magna 24 µg/litre 3 days Increased mortality in 8.9 µg/litre Thompson & Madeley
offspring after 21 days (1983c)
C10-12;58% Cl (CP-SH) Mysidopsis bahia 14.1-15.5 96 h No effects after 28 days Thompson & Madeley
µg/litre of exposure up to (1983a)
7.3 µg/litre
C10-12;58% Cl (CP-SH) Midge larvae, 48 h No adverse effects EG & G Bionomics
Chironomus tentans after exposure to (1983)
18-162 µg/litre
C10-13;70% Cl (CP-SH) Nitocra spinipes < 300 µg/litre 96 h Tarkpea et al.
(1981)
C14-17;45% Cl (CP-ML) Nitocra spinipes 9000 µg/litre 96 h Tarkpea et al.
(1981)
C14-17;52% Cl (CP-MH) Nitocra spinipes > 10 × 106 96 h Tarkpea et al.
µg/litrea (1981)
Table 29. (Cont'd)
Chlorinated paraffin Organism LC50 Exposure Other effects LOEC References
period
C22-26;42% Cl (CP-LL) Nitocra spinipes > 1 × 106 96 h Tarkpea et al.
µg/litrea (1981)
C22-26;49% Cl (CP-LL) Nitocra spinipes > 10 × 106 96 h Tarkpea et al.
µg/litrea (1981)
a Exceeding the water solubility
Table 30. Chronic toxicity of chlorinated paraffins to aquatic invertebrates
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C10-12;58% Cl Water flea, 6-12 days LC50 12 Thompson & Madeley (1983c)
Daphnia magna
21 days Increased mortality in 8.9
offspring after 21 days
C10-12;58% Cl Mysid shrimp, 28 days No treatment-related 7.3 Thompson & Madeley (1983a)
Mysidopsis bahia effects
C10-12;58% Cl Midge larvae, 49 days Halting adult 121 EG & G Bionomics (1983)
Chironomus tentans emergence (100%)
C10-12;58% Cl Mussel, 60 days LC50 74 Madeley & Thompson (1983a)
Mytilus edulis
84 days Growth reduction 9.3 Thompson & Shillabeer (1983)
91 days 23% mortality during 10.1 Madeley et al. (1983a)
exposure and 10%
mortality during
depuration
C10-12;58% Cl (CP-SH) Alga, Selenastrum 10 days Inhibition of growth 570 Thompson & Madeley (1983d)
capricornutum
C10-12;58% Cl (CP-SH) Alga, Skeletonema 10 days Inhibition of growth 19.6 Thompson & Madeley (1983b)
costatum
C14-17;52% Cl (CP-MH) Mussel 60 days Decreased filtration 3800 Madeley & Thompson (1983b)
Mytilus edulis activity
Table 30. (Cont'd)
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C14-17;52% Cl Water flea, 21 days Reproduction & parent 19.9-35.6 Frank & Steinhäuser (1994)
Daphnia magna mortality (LOEC)
C18-20;52% Cl Water flea, 21 days Reproduction & parent < 1.2 Frank & Steinhäuser (1994)
Daphnia magna mortality (LOEC)
C22-26;43% Cl (CP-LL) Mussel, 60 days Decreased filtration 2.180 Madeley & Thompson (1983c)
Mytilus edulis activity
C20-30;70% Cl (CP-LH) Mussel, 60 days Decreased filtration 1330 Madeley & Thompson (1983d)
Mytilus edulis activity
Larvae of the midge Chironomus tentans (second instar) were
exposed to C10-12;58% Cl (CP-SH) concentrations from 18 to 162 µg/litre
for 48 h (EG & G Bionomics, 1983). No adverse effects could be
detected. Exposure to 61-394 µg/litre for the 49 days life cycle
gave no effects except for halting adult emergence (100%) at 121 and
394 µg/litre.
The mussel Mytilus edulis was exposed to 2.3 and 10.1 µg/litre
of C10-12;58% Cl (CP-SH) for 147 days followed by 98 days of
depuration (2.3 µg/litre) or 91 days followed by 84 days depuration
(10.1 µg/litre) (Madeley et al. 1983a). A third of the mussels in the
high-dose group died during the exposure (23%) or depuration periods
(10%), and 7% of those in the low dose group, but this did not differ
significantly from the number of deaths in the acetone control.
When the mussel Mytilus edulis was studied over 60 days after
exposure to 13, 44, 71, 130 and 930 µg/litre of C10-12;58% Cl (CP-SH),
there was significant mortality at the three highest dose levels, the
median lethal time (LT50) values being 59.3, 39.7 or 26.7 days,
respectively (Madeley & Thompson, 1983a). The highest dose exceeded
the maximal solubility of the chlorinated paraffin. The LC50 for
this 60-day period was 74 µg/litre.
Mussels (Mytilus edulis) were exposed to 2.3 and 9.3 µg/litre
of a short chain length paraffin (58% Cl) (CP-SH) in sea water for 12
weeks (Thompson & Shillabeer, 1983). There were no mortalities at
either concentration, but at 9.3 µg/litre a reduction in growth rate
was observed, measured as shell length and tissue weight.
Treatment of mussels (Mytilus edulis) for 60 days with
C14-17;52% Cl (CP-MH) at concentration of 220 and 3800 µg/litre
gave no significant mortality, but there was an observed decrease
(non-quantitative visual observation) in filtration activity at the
higher concentration (Madeley & Thompson, 1983b). The highest dose
exceeded the maximal water solubility of the chlorinated paraffin.
Treatment of mussels (Mytilus edulis) with C22-26;43% Cl (CP-LL)
at 120 or 2180 µg/litre or with C20-30;70% Cl (CP-LH) at 460 and
1330 µg/litre for 60 days did not cause mortality, but visual
observation suggested that the filtration activity was reduced at the
higher dose levels (Madeley & Thompson, 1983c,d). The highest doses
exceeded the maximal solubility of the chlorinated paraffins.
9.1.2.3 Fish
Acute toxicity data are shown in Table 28 and chronic toxicity
data for fish are summarized in Table 31.
Table 31. Chronic toxicity of chlorinated paraffins to fish
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C10-13;49%, 59%, Bleak, 14 days Behavioural effects 125 (single Bengtsson & Baumann Ofstad
71% Cl Alburnus alburnus concentration) (1982)
C10-12;58% Cl Sheepshead minnow, 32 days Significantly reduced 279.7 Hill & Maddock (1983)
Cyprinodon variegatus size of larvae (LOEC)
C10-12;58% Cl Rainbow trout 60 days LC50 340 Madeley & Maddock (1983c)
Oncorhynchus mykiss
60 days Behavioural abnormalities 33
168 days after 60-70 days of Madeley & Maddock (1983b)
depuration
50% mortality 3.1
100% mortality 14.3
C14-17;50% Cl Bleak, 14 days No observed effect 125 (single Bengtsson et al. (1979)
Alburnus alburnus concentration)
C14-17;52% Cl Rainbow trout, 60 days No observed effect 1050 Madeley & Maddock (1983c)
Oncorhynchus mykiss
C18-26;49% Cl Bleak, 14 days No observed effect 125 (single Bengtsson et al. (1979)
Alburnus alburnus concentration)
C20-30;43%, 70% Cl Rainbow trout, 60 days No observed effect 3800 Madeley & Maddock (1983c)
Oncorhynchus mykiss
In a 96-h study on rainbow trout (Oncorhynchus mykiss), no
toxic effects or effects on behaviour were observed after exposure
of the fish to an emulsion containing a mean concentration of
770 000 µg/litre of Cereclor 42 (C20-30;42% Cl, CP-LL) (Madeley
& Birtley, 1980).
In bleak (Alburnus alburnus) which were exposed to Witaclor 149
(C10-13;49% Cl, CP-SL), Witaclor 159 (C10-13;59% Cl, CP-SH) and Witaclor
171P (C10-13;71% Cl, CP-SH) (125 µg/litre of water) for 14 days, some
effects on behaviour, such as sluggish movements, absence of shoaling
behaviour and abnormal vertical postures were observed after 7 days
(Bengtsson et al., 1979). The effects disappeared after the fish had
been kept in clean water for 2 days. No effects on behaviour were
observed after exposure to chlorinated paraffins with intermediate or
long chain length chlorinated paraffins, Witaclor 350 (C14-17;50% Cl,
CP-MH) or Witaclor 549 (C18-26;49% Cl, CP-LL), suggesting that
behavioural toxicity is related to the carbon chain length of the
chlorinated paraffin. When bleak were given food contaminated with
590, 2500 or 5800 mg/kg Witaclor 149 or 3180 mg/kg Witaclor 171P for
91 days, effects on behaviour were noted (Bengtsson & Baumann Ofstad,
1982). After 5 weeks of exposure to the high dose of Witaclor 149,
after 7 weeks of exposure to the medium dose and after 12 weeks of
exposure to Witaclor 171P, the fish swam sluggishly and closer to the
bottom than usual. In addition, folded dorsal fins and minor balance
problems were observed. These effects gradually disappeared within 2
weeks after exposure finished.
Flounders (Platichthys flesus) of both sexes were fed Witachlor
149 (C12;49% Cl, CP-SL) or Chlorparaffin Hüls 70C (C12;70% Cl, CP-SH)
on days 1 and 4 with a total exposure of 1000 mg/kg body weight (Haux
et al., 1982). The experiment was performed in both brackish and
seawater. The fish were examined 13 and 27 days after the first
administration. The male flounders did not appear to be affected by
the two chlorinated paraffins. C12;70% Cl did not induce any
haematological responses, whereas C12;49% Cl seemed to affect the
erythrocyte balance of female fish. C12;49% Cl resulted in
hypoglycaemia in marine female fish, whereas C12;70% Cl caused
hyperglycaemia in female brackish water fish. In females exposed to
C12;49% Cl, a significant increase in benzo [a]pyrene hydroxylase
activity was observed after 27 days in brackish water. A decrease in
6ß-hydroxylase activity in marine female fish and an increase in 5
alpha,ß-reductase activity in brackish water female fish were induced
by C12;70% Cl after 13 days.
The hatchability of embryos and survival of larvae of the
sheepshead minnow (Cyprinodon variegatus) was unaffected by a 28-day
exposure to short chain length paraffin with 58% Cl (CP-SH) (2.4, 4.1,
6.4, 22.1 and 54.8 µg/litre (Hill & Maddock, 1983). The treated
minnows showed an increased larval growth compared to the acetone
control. When the larvae were exposed to 36.2, 71, 161.8, 279.7 and
620.5 µg/litre for 32 days they were significantly smaller in the two
highest exposure groups, but in the lower exposure groups (36.2 and
71 µg/litre) they were significantly larger than the controls. No
effect was seen on survival of larvae and hatchability of embryos.
In a study by Madeley & Maddock (1983c) four chlorinated paraffins
were examined for their toxicity to rainbow trout (Oncorhynchus mykiss)
after exposure for 60 days. C10-12;58% Cl was used at mean
concentration of 33, 100, 350, 1070 and 3050 µg/litre. Significant
mortality was observed with the highest three concentrations. LT50
values (mean lethal times) for these three concentrations were
calculated as 44.7, 31.0 and 30.4 days, respectively. The calculated
60-day LC50 was 340 µg/litre. Behavioural abnormalities, which
were dose-related, were also observed.
Rainbow trout (Oncorhynchus mykiss) were exposed to 3.1 and
14.3 µg/litre of C10-12;58% Cl (CP-SH) for 168 days followed by a
depuration period of up to 105 days. No deaths occurred during the
exposure period, but during days 63 to 70 of the depuration period
all of the trout exposed to 14.3 µg/litre died and there was a
significantly increased mortality (50%) among those exposed to
3.1 µg/litre (Madeley & Maddock, 1983b). The relationship to
chlorinated paraffin exposure is unknown, since the surviving fish
recovered after day 70.
Rainbow trout (Oncorhynchus mykiss) were exposed to a short
chain length paraffin (58% Cl) (CP-SH) for 168 days (Madeley &
Maddock, 1983a) at 3.4 and 17.2 µg/litre. The treatment did not cause
any significant mortality or differences in growth, but small changes
in behaviour such as increased food intake were observed (compared to
controls).
Madeley & Maddock (1983c) exposed rainbow trout (Oncorhynchus
mykiss) to 4500 and 1050 µg/litre of a chlorinated paraffin (C14-17;
52% Cl, CP-MH) for 60 days. No toxic effects were found.
In other studies rainbow trout (Oncorhynchus mykiss) were
exposed to C22-26;43% Cl (CP-LL) for 60 days at concentrations of 900
and 4000 µg/litre (Madeley & Maddock, 1983c) or to C20-30;70% Cl
(CP-LH) at 3800, 1900 and 840 µg/litre (Madely & Maddock, 1983d). No
toxic effects were found.
9.1.3 Terrestrial organisms
A one-generation reproduction study was performed with Chlorowax
500C (C10-13;58% Cl, CP-SH) on mallard ducks (Anas platyrhynchos)
(Shults et al., 1984). The ducks were fed 0, 28, 166 and 1000 mg/kg
diet for 22 weeks in groups of 20 pairs. During the treatment the
ducks were mated. The treatment had no effect on the survival,
physical condition, body weight or food consumption of the adult
ducks. Decreased egg-shell thickness and a 10% loss of 14-day embryo
viability were observed in the highest exposure group.
The acute toxicity of orally administered Cereclor S52 (C14-17;52%
Cl, CP-MH) was studied in ring-necked pheasants (Phasianus
colchicus) and mallard ducks (Anas platyrhynchos) (Madeley &
Birtley, 1980). However, no abnormal clinical signs, mortality or
effects on body weight gain were observed 14 days after a single oral
dose by gavage of up to 24 606 mg/kg body weight (pheasant) and
10 280 mg/kg body weight (duck) (five male and five female birds per
group). When the birds were fed with 1000 or 24 063 mg/kg diet of
Cereclor S52 for 5 days followed by 3 days on normal food (five males
and five females per group), no abnormalities were found other than
inferior food intake in ducks from the high-dose group.
The toxicity of Cereclor 42 (C22-26;42% Cl, CP-LL), Cereclor 50LV
(C10-13;49% Cl, CP-SL) and Cereclor 70L (C10-13;70% Cl, CP-SH) in chick
embryos was studied by Brunström (1983). The chlorinated paraffins
were injected into the yolks of eggs incubated for 4 days at
concentrations of 100 and 200 mg/kg egg (based on mean egg weights
before incubation). None of the three mixtures affected the hatching
rate, incubation time, hatching weight, weight gain after hatching or
the liver weights of the chicks.
In an extension of this study the eggs were injected with
300 mg/kg egg weight of the same Cereclors after 4 days of incubation
(Brunström, 1985). An increased liver weight was observed after
treatment with C10-13;49% Cl and C10-13;70% Cl. The microsomal
concentration of cytochrome P-450 in chick embryos was increased by
all three Cereclors. The highest concentration was observed with
C10-13;70% Cl. This Cereclor was also found to increase the microsomal
activity of APMD. The other short-chain chlorinated paraffin,
C10-13;49% Cl, produced decreased activities of aryl hydrocarbon
hydroxylase (AHH) and 7-ethoxycoumarin O-deethylase (ECOD). The
long chain length chlorinated paraffin in this study, C22-26;42% Cl,
caused decreased activities of APDM and ECOD. The greatest effects
were observed after treatment with the most highly chlorinated
short-chain paraffin, C10-13;70% Cl.
9.2 Field observations
No data concerning the effects of chlorinated paraffins in the
field have been reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
Information on occupational exposure to chlorinated paraffins is
very limited. The principal route of exposure is likely to be dermal,
particularly during their use as metal-working fluids. There is also
potential for the formation of inhalable aerosols during this use,
though available information is inadequate to assess exposure via this
route.
Owing to the high octanol-water partition coefficient, it is
likely that the principal source of exposure of the general population
is food, although, owing to lack of data, exposure via other routes
cannot be ruled out. It should be noted, however, that the
composition of chlorinated paraffins to which the population is
exposed in the general environment may be considerably different from
that of the commercial products, although it is not possible currently
to distinguish the chemical composition of chlorinated paraffins with
available methods of analysis. In the only survey of foodstuffs
identified, which was limited in scope, the highest concentration of
chlorinated paraffins (average level of C10-20: 300 µg/kg) was present
in dairy products (Table 15). If the daily consumption of dairy
products is assumed to be 1 kg per person, the daily intake of short
and intermediate chain length chlorinated paraffins from this source
would be 300 µg (4.3 µg/kg body weight, assuming an average body
weight of 70 kg). This is likely to be a worst-case estimate based on
the lack of specificity of the analytical method.
Exposure to chlorinated paraffins via food is also possible
through the consumption of contaminated mussels. Assuming a weekly
consumption of 1 kg of mussels, as a worst case, this would correspond
to a weekly intake of short and intermediate chain length chlorinated
paraffins of 3250 µg, based on chlorinated paraffin levels found in
mussels collected at various sites in the United Kingdom (Table 13)
(6.7 µg/kg body weight per day, assuming an average body weight of
70 kg). If the mussels are collected in highly contaminated water
(section 5.1.4) the weekly intake would be 12 000 µg, which
corresponds to 25 µg/kg body weight per day. The analytical method
used was non-specific.
10.1.2 Toxic effects
In spite of the widespread use of the chlorinated paraffins,
there have been no case reports of skin irritation or sensitization
in humans. Results from a limited number of studies on volunteers
show that chlorinated paraffins can induce minimal irritancy in
the skin but not sensitization. No other data concerning effects of
chlorinated paraffins on humans have been reported.
The acute oral toxicity of chlorinated paraffins of various chain
lengths has been well studied in experimental animals and is low.
Toxic effects such as muscular incoordination and piloerection
were most evident following single exposure to short chain length
chlorinated paraffins. On the basis of very limited data, the acute
toxicity by the inhalation and dermal routes also appears to be low.
Mild skin and eye irritation have been observed after application of
short and intermediate (skin irritation) chain length chlorinated
paraffins. Results of several studies indicate that short chain
chlorinated paraffins do not induce skin sensitization.
In repeated dose toxicity studies by the oral route, the liver,
kidney and thyroid have been shown to be the primary target organs for
the toxicity of chlorinated paraffins (Table 32). For the short chain
compounds, increases in liver and kidney weight and hypertrophy of the
liver and thyroid have been observed at lowest doses (LOEL = 100 mg/kg
body weight per day; NOEL = 10 mg/kg body weight per day; rats).
For the intermediate chain compounds, effects observed at lowest
doses are generally increases in liver and kidney weight (LOEL in rats
= 100 mg/kg body weight per day, respectively; NOAEL in rats =
10 mg/kg body weight per day). Increases in serum cholesterol and
"mild, adaptive" histological changes in the thyroid have been reported
at similar doses in female rats (NOAEL = 4 mg/kg body weight per day).
For the long chain compounds, effects observed at lowest doses
are multifocal granulomatous hepatitis and increased liver weight in
female rats (LOAEL = 100 mg/kg body weight per day).
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells, there is a suggestion of a
weak in vitro clastogenic potential but not in several in vivo
studies. Chlorinated paraffins are also reported to induce in vitro
cell transformation.
Table 32. Effect levels (non-carcinogenic) in repeated dose toxicity tests
Short chain length chlorinated paraffins
Rats
C10-13;58% Cl, 14-day dietary study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in relative liver weights and activities of
hepatic APDM and cytochrome P-450)
C10-13;58% Cl, 14-day gavage study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in relative liver weights);
NOEL = 30 mg/kg body weight per day
C10-13;58% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 100 mg/kg body weight per day (increase in liver/body weight ratio);
NOEL = 50 mg/kg body weight per day
C10-13;56% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 50 mg/kg body weight per day (increase in liver/body weight ratio);
NOEL = 10 mg/kg body weight per day
C12;58% Cl, 90-day gavage study in Fischer-344 rats
LOEL = 313 mg/kg body weight per day (increase in relative liver weight, hepatic
peroxisomal ß-oxidation and thyroxine-UdPG-glucuronosyltransferase; thyroid follicular
cell hypertrophy and hyperplasia and increase in replicative DNA synthesis; renal tubular
eosinophilia and increase in renal replicative DNA synthesis)
No effects in Dunkin Hartley guinea-pigs
NOEL = 1000 mg/kg body weight per day
C10-13;58% Cl, 13-week gavage study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in liver and kidney weights and hypertrophy
of liver and thyroid);
NOEL = 10 mg/kg body weight per day
C12;60% Cl, 90-day gavage study in Fischer-344 rats,
LOEL = 313 mg/kg body weight per day (increase in liver weight)
C12;60% Cl, 2-year gavage study in Fischer-344 rats,
LOAEL = 312 mg/kg body weight per day (increase in liver and kidney weights, hepatic
hypertrophy, necrosis and angiectasis, nephropathy (females), erosion, inflammation and
ulceration of the glandular stomach, hyperplasia of the parathyroid)
Table 32. (Cont'd)
Short chain length chlorinated paraffins (cont'd)
Mice
C10-13;58% Cl, 14-day gavage study in Alpk:APfCD mice,
LOEL = 250 mg/kg body weight per day (induction of peroxisomal fatty acid ß-oxidation and
increase in liver weight);
NOEL = 100 mg/kg body weight per day
C10-13;56% Cl, 14-day gavage study in Alpk:APfCD mice
LOEL = 100 mg/kg body weight per day (increase in liver weight);
NOEL = 50 mg/kg body weight per day
C12;60% Cl, 90-day gavage study in B6C3F1 mice,
LOEL = 250 mg/kg body weight per day (hepatocellular hypertrophy);
NOEL = 125 mg/kg body weight per day
C12;60% Cl, 2-year day gavage study in B6C3F1 mice,
LOEL = 125 mg/kg body weight per day (decrease in body weight gain (females))
Intermediate chain length chlorinated paraffins
Rats
C14-17;52% Cl, 14-day dietary study in Fischer-344 rats,
LOEL = 177 mg/kg body weight per day (slight increase in cytochrome P-450);
NOEL = 57.7 mg/kg body weight per day
C14-17;40% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 10 mg/kg body weight per day (increase in liver weight; not dose-related)
C14-17;52% Cl, 13-week study in Sprague-Dawley rats,
NOAEL (females) = 4.2 mg/kg body weight per day (increase in serum cholesterol; "mild,
adaptive" histopathological changes in the thyroid - reduced follicle sizes and collapsed
angularity; increased height, cytoplasmic vacuolation and nuclear vesiculation);
NOEL = 0.4 mg/kg body weight per day
C14-17;52% Cl, 90-day dietary study in Wistar rats,
LOEL (females) = 25 mg/kg body weight per day (increases in liver and kidney weights and
proliferation of smooth endoplastic reticulum);
NOEL = 12.5 mg/kg body weight per day
C14-17;52% Cl, 90-day dietary study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increased liver and kidney weights);
NOEL = 10 mg/kg body weight per day
Table 32. (Cont'd)
Intermediate chain length chlorinated paraffins (cont'd)
Mice
C14-17;40% Cl, 14-day gavage study in Alpk:APfCD-1 mice,
LOEL = 500 mg/kg body weight (induction of peroxisomal fatty acid ß-oxidation);
NOEL = 250 mg/kg body weight per day
Dogs
C14-17;52% Cl, 90-day dietary study in Beagle dogs,
LOEL (males) = 30 mg/kg body weight per day (increased liver and kidney weights)
NOEL = 10 mg/kg body weight per day (increased smooth endoplasmic reticulum)
Long chain length chlorinated paraffins
Rats
C22-26;70% Cl, 14-day dietary study in Fischer-344 rats,
NOEL = 1715 mg/kg body weight per day (no effects at any dose level)
C22-26;43% Cl, 14-day dietary study in Charles River rats,
NOEL = 3000 mg/kg body weight per day (no effects at any dose; observation of
nephrolithiasis in females exposed to 3000 mg/kg body weight per day)
C23;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL (females) = 235 mg/kg body weight per day (granulomatous inflammation in the liver)
C20-30;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL (females) = 100 mg/kg body weight per day (multifocal granulomatous hepatitis and
increased liver weight)
C22-26;70% CL, 90-day dietary study in Fischer-344 rats,
LOAEL = 3750 mg/kg body weight per day (increased liver weight, hepatocellular hypertrophy
and cytoplasmic fat vacuolation; slight increase of chronic nephritis (males); increase in
ALT and AST (females));
NOEL = 900 mg/kg body weight per day
C23;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL = 100 mg/kg body weight per day (increase in relative liver weights; increased
activities of serum enzymes, variations in haematological parameters (in females); diffuse
lymphohistiocytic inflammation in the livers, pancreatic and mesenteric lymph nodes)
Table 32. (Cont'd)
Long chain length chlorinated paraffins (cont'd)
Mice
C23;43% Cl, 90-day gavage,
NOEL = 7500 mg/kg body weight per day (no effects at any dose)
C23;43% Cl, 90-day gavage,
NOEL = 5000 mg/kg body weight per day (no effects at any dose)
Two-year toxicity and carcinogenicity studies have been conducted
for a short (C12;60% Cl) and a long chain chlorinated paraffin
(C23;43% Cl) in both rats and mice. For the short chain chlorinated
paraffin, the incidences of liver and thyroid tumours were increased
in mice at 125 and 250 mg/kg body weight per day. In rats, the
incidences of liver tumours in both sexes, thyroid tumours in females
and renal cell tumours and leukaemias in males were increased at doses
of 312 and 625 mg/kg body weight per day. For the long chain
chlorinated paraffin, the incidences of malignant lymphomas in male
mice (2500-5000 mg/kg body weight per day) and phaeochromocytomas in
female rats (900 mg/kg body weight per day) were increased. On the
basis of available data, therefore, the short chain chlorinated
paraffin was carcinogenic in rats and mice. No data are available on
intermediate chlorinated paraffins. For the long chain chlorinated
paraffin, the evidence of carcinogenicity is limited, increased
incidences of commonly occurring tumours having been observed at only
one site in one sex of each species.
Some possible mechanisms for the induction of tumours of the
thyroid, liver and kidney have been suggested. On the basis of 14-day
mouse, rat and guinea-pig studies (Wyatt et al., 1993; Elcombe et al.,
in press), with similar short chain and long chain chlorinated
paraffins as used in the carcinogenesis bioassays, it was suggested
that the liver tumours in mice and rats could be correlated with the
degree of peroxisomal proliferation which occurs in the liver of these
species at similar dose levels (250 mg/kg body weight per day). No
peroxisome proliferation was observed in the livers of guinea-pigs
even at doses up to 1 to 2 g/kg body weight per day, although a
similar increase in liver weight to that seen in mice and rats was
observed. No study on peroxisome proliferation in human hepatoctyes
treated with a short chain chlorinated paraffin is available.
Increased DNA synthesis has been demonstrated in hepatocytes and
thyroid follicular cells in rats of both sexes and in proximal kidney
tubular cells in males. Perturbation of thyroid homeostasis and
increased TSH secretion has been observed in the thyroid of rats.
These effects may be related to the development of tumours.
Taking into consideration the available data, it appears that
chlorinated paraffins do not mediate carcinogenic effects via direct
interaction with DNA.
In a reproduction study, no adverse reproductive effects were
reported following exposure of rats to an intermediate chain length
chlorinated paraffin with 52% chlorine. However, survival and body
weight of the exposed pups were reduced (LOEL for non-significant
decrease in body weight = 5.7 to 7.2 mg/kg body weight per day; LOAEL
for decreased survival = 58.7 to 70 mg/kg body weight per day). In a
limited number of studies on the developmental effects of the short,
medium and long chain chlorinated paraffins, adverse effects in
offspring were observed, for the short chain compounds only, at
maternally toxic doses in rats.
10.1.3 Risk evaluation
Available data indicate that absorption of chlorinated paraffins
through the skin (the likely principal route of exposure in the
occupational environment) is minimal. Provided that proper personal
hygiene and safety procedures are followed, the risk to the health of
workers exposed to chlorinated paraffins is expected to be minimal.
10.1.3.1 Short chain compounds
On the basis of available data on repeated dose toxicity, a
Tolerable Daily Intake (TDI) for non-neoplastic effects of short chain
chlorinated paraffins for the general population can be developed:
10 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
100
where 10 mg/kg body weight per day is the lowest reported
no-observed-effect level (increases in liver and kidney weights
and hypertrophy of the liver and thyroid at the next highest dose
in a 13-week study on rats) (IRDC, 1984a); and 100 is the
uncertainty factor (× 10 for interspecies variation; × 10 for
intraspecies variation).
On the basis of multistage modelling of the tumours with highest
incidence (hepatocellular adenomas or carcinomas (combined) in male
mice) in the carcinogenesis bioassay with short chain chlorinated
paraffins, the estimated dose associated with a 5% increase in tumour
incidence is 11 mg/kg body weight per day (amortized for period of
administration). After dividing this value by 1000 (uncertainty
factor for a non-genotoxic carcinogen), it can be recommended that
daily doses of short chain chlorinated paraffins for the general
population should not exceed 11 µg/kg body weight, on the basis of
neoplastic effects.
10.1.3.2 Intermediate chain compounds
On the basis of available data on repeated dose toxicity, a TDI
for non-neoplastic effects of intermediate chain chlorinated paraffins
can be developed:
10 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
100
where 10 mg/kg body weight per day is the no-observed-adverse-effect
level in both sexes (increases in liver and kidney weights at the next
highest dose) (IRDC, 1984b); increases in serum cholesterol and "mild,
adaptive" histological changes in the thyroid have been reported at
similar doses in female rats (NOAEL = 4 mg/kg body weight per day)
(Poon et al., in press); and 100 is the uncertainty factor (× 10 for
interspecies variation; × 10 for intraspecies variation).
10.1.3.3 Long chain compounds
On the basis of available data on repeated dose toxicity, a TDI
for non-neoplastic effects of long chain chlorinated paraffins can be
developed:
100 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
1000
where 100 mg/kg = the lowest-observed-adverse-effect levels in
long-term studies (effects at this dose were multifocal granulomatous
hepatitis and increased liver weight in female rats (NTP, 1986b;
Serrone et al., 1987; Bucher et al., 1987); and 1000 = uncertainty
factor (× 10 for intraspecies variation, × 10 for interspecies
variation, × 10 for LOAEL rather than NOEL).
In general, the calculated daily intake of chlorinated paraffins
based on highly unlikely worst-case scenarios are below the TDIs for
non-neoplastic effects or recommended values for neoplastic effects
(short chain compounds) developed above.
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
Data on chlorinated paraffin levels in the environment are
limited, but the studies reported indicate widespread contamination,
although the highest levels are found close to industries that
manufacture or use chlorinated paraffins.
Chlorinated paraffins bioaccumulate in aquatic organisms, and
bioconcentration factors (BCFs) in the range of 7 to 7155 for fish and
223 to 138 000 for mussels have been reported. In fish, chlorinated
paraffins of short chain length are accumulated to a higher degree
than those of intermediate and long chain length.
In the environment, chlorinated paraffins are persistent.
However, short-chain chlorinated paraffins with a low chlorine content
appear to be degraded by acclimated microorganisms.
10.2.2 Toxic effects
Short chain length chlorinated paraffins are acutely toxic to
freshwater and saltwater invertebrates at LC/EC50 concentrations
ranging from 14 to 530 µg/litre. The acute toxicity of short chain
chlorinated paraffins to fish is low. In long-term studies, the
lowest-observed-effect concentrations for algae, daphnids and fish
ranged from 3 to 20 µg/litre; NOECs appear to range from 2 to
5 µg/litre for the most sensitive species tested. The acute and
long-term toxicity of intermediate and long chain length chlorinated
paraffins to fish appears to be low. However, in daphnids, chronic
effects of an intermediate and a long-chain product have been observed
at similar concentrations to those reported for short chain compounds.
No studies on plants or terrestrial invertebrates have been
reported. The acute toxicity to birds is low. Data from studies on
laboratory mammals suggest a low risk for terrestrial mammals.
10.2.3 Risk evaluation
The evaluation of the environmental risks of chlorinated
paraffins is complicated by the limited quality and quantity of
information regarding environmental levels. Available data indicate
that chlorinated paraffins are bioaccumulative and persistent.
The data on environmental levels of short-chain chlorinated
paraffins indicate that in areas local to release sources, there is a
risk to both freshwater and estuarine organisms. Recent data indicate
that there is also a potential risk to aquatic invertebrates from
intermediate and long chain chlorinated paraffin products.
The enrichment of chlorinated paraffins in sediments, their
resorption behaviour and aquatic toxicity indicate a potential risk
for sediment-dwelling organisms.
The data regarding chlorinated paraffins in the terrestrial
environment are insufficient to estimate the risk to soil-dwelling
organisms.
11. RECOMMENDATIONS FOR PROTECTION OF THE ENVIRONMENT
Since chlorinated paraffins are bioaccumulative and toxic to
environmental organisms and owing to difficulties in monitoring
environmental levels, it is recommended that use and disposal of these
compounds should be controlled to avoid release to the environment.
12. FUTURE RESEARCH
The following studies need to be undertaken:
a) development of more selective and sensitive methods of analysis
in order to provide more reliable data on present and future
levels of chlorinated paraffins in the occupational environment,
soil, water, sediments, foodstuffs and human tissues;
b) further investigation of the influence of the chain lengths and
degrees of chlorination on toxicodynamics and toxicokinetics of
chlorinated paraffins, with particular regard to the relative
extent and rate of absorption and excretion through different
routes; studies to ascertain the metabolic pathways of
chlorinated paraffins should also be performed;
c) investigation of the toxicokinetics and half-lives of chlorinated
paraffins in mammals;
d) studies on perinatal toxicity;
e) further studies to examine effects on sediment-dwelling
organisms.
13. PREVIOUS EVALUATION BY INTERNATIONAL ORGANIZATIONS
Chlorinated paraffins have been evaluated by the International
Agency for Research on Cancer (IARC, 1990). It was concluded that
there is sufficient evidence for the carcinogenicity of a commercial
chlorinated paraffin product of average carbon chain length C12 and
average degree of chlorination of 60% in experimental animals, and
limited evidence for the carcinogenicity of a commercial chlorinated
paraffin product of average carbon chain length C23 and average
degree of chlorination of 43% in experimental animals. The overall
evaluation was that chlorinated paraffins of average carbon chain
length C12 and average degree of chlorination of approximately 60%
are possibly carcinogenic to humans (Group 2B).
REFERENCES
Åhlman M, Bergman Å, Darnerud PO, Egestad B, & Sjövall J (1986)
Chlorinated paraffins: formation of sulphur-containing metabolites of
polychlorohexadecane in rats. Xenobiotica, 16: 225-232.
Ahotupa M, Hietanen E, Mantyla E, & Vainio H (1982) Effects of
polychlorinated paraffins on hepatic, renal and intestinal
biotransformation rates in comparison to the effects of poly-
chlorinated biphenyls and naphthalenes. J Appl Toxicol, 2: 47-53.
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL, &
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environ Mol Mutagen, 16(Suppl 18):
55-137.
Ashby J, Lefevre PA, & Elcombe CR (1990) Cell replication and
unscheduled DNA synthesis (UDS) activity of low molecular weight
chlorinated paraffins in the rat liver in vivo. Mutagenesis,
5: 515-518.
Bengtsson BE & Baumann Ofstad E (1982) Long-term studies on uptake and
elimination of some chlorinated paraffins in the bleak, Alburnus
alburnus. Ambio, 11: 38-40.
Bengtsson BE, Svanberg O, Lindén E, Lunde G, & Baumann Ofstad E (1979)
Structure related uptake of chlorinated paraffins in bleaks ( Alburnus
alburnus L). Ambio, 8: 121-122.
Bergman Å, Hagman A, Jacobsson S, Jansson B, & Åhlman M (1984) Thermal
degradation of polychlorinated alkanes. Chemosphere, 13: 237-250.
Biessmann A, Brandt I, & Darnerud PO (1982) Comparative distribution
and metabolism of two carbon-14 labeled chlorinated paraffins in
Japanese quail (Coturnix coturnix japonica). Environ Pollut,
A28: 109-120.
Biessmann A, Darnerud PO, & Brandt I (1983) Chlorinated paraffins:
disposition of a highly chlorinated polychlorohexadecane in mice and
quail. Arch Toxicol, 53: 79-86.
Birtley RDN, Conning DM, Daniel JW, Ferguson DM, Longstaff E, & Swan
AAB (1980) The toxicological effects of chlorinated paraffins in
mammals. Toxicol Appl Pharmacol, 54: 514-525.
Brunström B (1983) Toxicity in chick embryos of three commercial
mixtures of chlorinated paraffins and of toxaphene injected into eggs.
Arch Toxicol, 54: 353-357.
Brunström B (1985) Effects of chlorinated paraffins on liver weight,
cytochrome P-450 concentration and microsomal enzyme activities in
chick embryos. Arch Toxicol, 57: 69-71.
BUA (German Chemical Society Advisory Committee on Existing Chemicals
of Environmental Relevance) (1992) [Chlorinated paraffins.] Weinheim,
VCH Verlagsgesellschaft, 227 pp (BUA Report 93) (in German).
Bucher JR, Alison RH, Montgomery CA, Huff J, Haseman JK, Farnell
D, Thompson R, & Prejean JD (1987) Comparative toxicity and
carcinogenicity of two chlorinated paraffins in F344/N rats and
B6C3F1 mice. Fundam Appl Toxicol, 9: 454-468.
Camford Information Services (1991) CPI product profiles: chlorinated
paraffins. Don Mills, Ontario, Camford Information Services, 3 pp.
Campbell I & McConnell G (1980) Chlorinated paraffins and the
environment. 1. Environmental occurrence. Environ Sci Technol,
14: 1209-1214.
Conz A & Fumero S (1988a) Study of the capacity of Solvocaffaro C1642
to induce gene mutations in strains of Salmonella typhimurium.
Turin, Istituto di Recerche Biomediche "Antoine Marxer", 37 pp (RBM
Exp. No. 880367).
Conz A & Fumero S (1988b) Micronucleus induction in bone marrow cells
of mice treated by oral route with the test article Solvocaffaro
C1642. Turin, Istituto di Recerche Biomediche "Antoine Marxer", 20 pp
(RBM Exp. No. 880368).
Cooke M & Roberts DJ (1980) Carbon skeleton capillary gas
chromatography. J Chromatogr, 193: 437-443.
Darnerud PO (1984) Chlorinated paraffins: Effect of some microsomal
enzyme inducers and inhibitors of the degradation of 1-14C-chloro-
dodecanes to 14CO2 in mice. Acta Pharmacol Toxicol,
55: 110-115.
Darnerud PO & Brandt I (1982) Studies on the distribution and
metabolism of a 14C-labelled chlorinated alkane in mice. Environ
Pollut, A27: 45-56.
Darnerud PO & Lundkvist U (1987) Studies on implantation and embryonic
development in mice given a highly chlorinated hexadecane. Pharmacol
Toxicol, 60: 239-240.
Darnerud PO, Biessmann A, & Brandt I (1982) Metabolic fate of
chlorinated paraffins: degree of chlorination of [1-14C]-chloro-
dodecanes in relation to degradation and excretion in mice. Arch
Toxicol, 50: 217-226.
Darnerud PO, Bengtsson BE, Bergman A, & Brandt I (1983) Chlorinated
paraffins: disposition of a polychloro-[1-14C]-hexadecane in carp
(Cyprinus carpio) and bleak (Alburnus alburnus). Toxicol Lett,
19: 345-351.
Darnerud PO, Bergman Å, Lund BO, & Brandt I (1989) Selective
accumulation of chlorinated paraffins (C12 and C16) in the olfactory
organ of rainbow trout. Chemosphere, 18: 1821-1827.
EG & G Bionomics (1983) The acute and chronic toxicity of a chlorinated
paraffin (58% chlorination of short chain length n-paraffins) to
midges (Chironomus tentans). Wareham, Massachusetts, EG & G Bionomics,
39 pp (Report No. BW 83-6-1426).
Elcombe CR, Watson SC, Wyatt I, & Foster JR (1994) Chlorinated
paraffins (CP): Mechanisms of carcinogenesis. Toxicologist, 14: 276.
Elcombe CR, Bars RG, Watson SC, & Foster JR (in press) Hepatic effects
of chlorinated paraffins in mice, rats and guinea pigs: Species
differences and implications for hepatocarcinogenicity. Xenobiotica.
Elliott BM (1989a) Meflex DC029 (fully formulated) - Ames test.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 1 p (Report
No. CTL/L/2668).
Elliott BM (1989b) Meflex DC029 (fully formulated) - Mouse
micronucleus test. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 1 p (Report No. CTL/L/2693).
English JSC, Foulds I, White IR, & Rycroft JG (1986) Allergic contact
sensitization to the glycidyl ester of hexahydrophthalic acid in a
cutting oil. Contact Dermatitis, 15: 66-69.
Environment Agency, Japan (1981) Environmental monitoring of chemicals:
Annual report. Tokyo, Environment Agency, p 23.
Environment Agency, Japan (1983) Environmental monitoring of chemicals:
Annual report. Tokyo, Environment Agency, p 75.
Eriksson P & Darnerud PO (1985) Distribution and retention of some
chlorinated hydrocarbons and a phthalate in the mouse brain during the
pre-weaning period. Toxicology, 37: 189-203.
Eriksson P & Kihlström J (1985) Disturbance of motor performance and
thermoregulation in mice given two commercial chlorinated paraffins.
Bull Environ Contam Toxicol, 34: 205-209.
Eriksson P & Nordberg A (1986) The effects of DDT, DDOH-palmitic
acid, and a chlorinated paraffin on muscarinic receptors and the
sodium-dependent choline uptake in the central nervous system of
immature mice. Toxicol Appl Pharmacol, 85: 121-127.
Frank U & Steinhäuser KG (in press) [Ecotoxicity of poorly soluble
substances tested by Daphnia toxicity of chlorinated paraffins.] Vom
Wasser, 83 (in German).
Friedman D & Lombardo P (1975) Photochemical technique for the
elimination of chlorinated aromatic interferences in the gas-liquid
chromatographic analysis for chlorinated paraffins. J Assoc Off Anal
Chem, 58: 703-706.
Gjos N & Gustavsen K (1982) Determination of chlorinated paraffins by
negative ion chemical ionization mass spectrometry. Anal Chem,
54: 1316-1318.
Hansen J, Schneider T, Olsen JH, & Laursen B (1992) Availability of
data on humans potentially exposed to suspected carcinogens in the
Danish working environment. Pharmacol Toxicol, 72(Suppl 1): S77-S85.
Hardie DWF (1964) Chlorocarbons and chlorohydrocarbons: Chlorinated
paraffins. In: Kirk-Othmer encyclopedia of chemical technology. New
York, John Wiley and Sons, vol 5, pp 231-240.
Haux C, Larsson Å, Lidman U, Förlin L, Hansson T, & Johansson-Sjöbeck
M-L (1982) Sublethal physiological effects of chlorinated paraffins on
the flounder, Platichtys flesus L. Ecotoxicol Environ Saf, 6: 49-59.
Hill RW & Maddock BG (1983) Effect of a chlorinated paraffin (58%
chlorination of short chain length n-paraffins) on embryos and
larvae of the sheepshead minnow (Cyprinodon variegatus)-(study one).
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 57 pp
(Report No. BL/B/2326).
Hoechst (1983a) [Chloroparaffin 70 liquid NV - Acute dermal
irritation/corrosion in rabbits.] Frankfurt/Main, Hoechst AG, 11 pp
(Report No. 83.0444) (in German).
Hoechst (1983b) [Hordalub 80 + TNPP - Magnusson and Kligman's test for
sensitising properties on Pirbright-White Guinea Pigs.] Frankfurt/Main,
Hoechst AG, 17 pp (Report No. 83.0573) (in German).
Hoechst (1984) [Hordalub 80 HT - Magnusson and Kligman's test for
sensitising properties on Pirbright-White Guinea Pigs.] Frankfurt/Main,
Hoechst AG, 18 pp (Report No. 84.0458) (in German).
Hoechst (1986a) Hordalub 80 - Study of the mutagenic potential in
strains of Salmonella typhimurium (Ames Test) and Escherichia coli.
Frankfurt/Main, Hoechst AG, 28 pp (Report No. 86.1078).
Hoechst (1986b) [Acute dermal irritation/corrosion in rabbits.]
Frankfurt/Main, Hoechst AG, 12 pp (Report No. 86.1105) (in German).
Hoechst (1987) Chloroparaffin 56 liquid - Detection of gene mutations
in somatic cells in culture. HGPRT-test with V79 cells. Frankfurt/Main,
Hoechst AG, 23 pp (Report No. 87.1719).
Hoechst (1988) Chloroparaffin 56 liquid (unstabilized) - Study of the
mutagenic potential in strains of Salmonella typhimurium (Ames Test)
and Escherichia coli. Frankfurt/Main, Hoechst AG, 30 pp (Report No.
88.0099).
Hoechst (1989) Chlorowax 500C micronucleus test in male and female
NMRI mice after oral administration. Frankfurt/Main, Hoechst AG, 24 pp
(Report No. 89.0253).
Hollies JI, Pinnington DF, Handley AJ, Baldwin MK, & Bennett D (1979)
The determination of chlorinated long-chain paraffins in water,
sediment and biological samples. Anal Chim Acta, 111: 201-213.
Houghton KL (1993) Chlorinated paraffins. In: In: Kirk-Othmer
encyclopedia of chemical technology. New York, John Wiley and Sons,
vol 6, pp 78-87.
Howard PH, Santodonato J, & Saxena J (1975) Investigations of selected
potential environmental contaminants: chlorinated paraffins. Syracuse,
New York, Syracuse University Research Corporation, 107 pp (Report No.
68-01-3101).
HSE (1992) Chlorinated paraffins. London, Health and Safety Executive,
44 pp (Unpublished document).
IARC (1990) Chlorinated paraffins. In: Some flame retardants and
textile chemicals, and exposures in the textile manufacturing
industry. Lyon, International Agency for Research on Cancer, pp 55-72
(IARC Monographs on the Evaluation of Carcinogenic Risks to Humans,
Volume 48).
ICI (1965) Toxicological report: chlorinated hydrocarbons with added
stabilizers: "Cereclor" P70 and 70L. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 15 pp (Report No. CTL/TR/464).
ICI (1966) Toxicological report: 40% chlorinate of C10-13 normal
paraffin. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
4 pp (Report No. CTL/TR/524).
ICI (1967) Toxicological report: "Cereclor" 50LV (MD.115) - 50%
chlorinate of C10-13 normal paraffin. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 4 pp (Report No. CTL/TR/618).
ICI (1968) Toxicological report: fire-resistant hydraulic fluid
10B/1067. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
7 pp (Report No. CTL/TR/635).
ICI (1969) Toxicological report: skin irritancy and oral toxicity of
chlorinated paraffins. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 11 pp (Report No. CTL/TR/691).
ICI (1971) Toxicological report: fire-resistant hydraulic fluid
45D-3271. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
8 pp (Report No. CTL/T/831).
ICI (1973) "Cereclor" 63L: local irritancy and acute oral toxicity.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 7 pp (Report
No. CTL/T/938).
ICI (1974a) "Cereclors" and "Hordolub": local irritancy and acute
toxicity. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
32 pp (Report No. CTL/T/962).
ICI (1974b) "Cereclor" 50 HS: summary of local irritancy, skin
sensitization and acute toxicity. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 3 pp (Report No. CTL/Z/0548).
ICI (1975a) "Cereclor" 60 HS: primary skin irritation in rabbits.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 3 pp (Report
No. CTL/Z/0813).
ICI (1975b) "Cereclor" 50 HS: primary skin irritation in rabbits.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 3 pp (Report
No. CTL/Z/0812).
ICI (1980) Cloparin 1049 and "Meflex" DC029: a comparison of skin
irritation potential. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 11 pp (Report No. CTL/T/1431).
ICI (1982a) Cell transformation test for potential carcinogenicity of
chlorinated paraffin (58% chlorination of short chain length
n-paraffins). Macclesfield, Cheshire, Imperial Chemical Industries
Ltd.
ICI (1982b) Cell transformation test for potential carcinogenicity
of chlorinated paraffin (70% chlorination of short chain length
n-paraffins). Macclesfield, Cheshire, Imperial Chemical Industries
Ltd.
ICI (1982c) Cloparin 59: skin irritation study. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, 14 pp (Report No.
CTL/T/1737).
Inveresk (1975) Patch testing of textile lubricants. Musselburgh,
Inveresk Research International, 14 pp (Report No. 318).
IRDC (1981a) 14-Day oral toxicity study in rats. Chlorinated paraffin:
58% chlorination of short chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 110 pp
(Report No. 438-006).
IRDC (1981b) 14-Day oral toxicity study in rats. Chlorinated paraffin:
52% chlorination of long chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 104 pp
(Report No. 438-005).
IRDC (1982a) Teratology study in rats. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 100 pp (Report
No. 438-016).
IRDC (1982b) 14-Day oral toxicity study in rats. Chlorinated paraffin:
70% chlorination of long chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 103 pp
(Report No. 438/004).
IRDC (1982c) 14-Day oral (gavage) toxicity study in rats. Chlorinated
paraffin: 43% chlorination of long chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
110 pp (Report No. 438/005).
IRDC (1982d) Teratology study in rabbits. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 95 pp (Report
438-031).
IRDC (1982e) Teratology study in rabbits. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 102 pp (Report
438-030).
IRDC (1983a) Dominant lethal study in rats. Chlorinated paraffin: 58%
chlorination of short chain n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 54 pp (Report
No. 438-011).
IRDC (1983b) Teratology study in rabbits. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 245 pp (Report
No. 438-045).
IRDC (1983c) 14-Day dietary range-finding study in rats. Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
136 pp (Report No. 438-002).
IRDC (1983d) Teratology study in rats. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 197 pp (Report
No. 438-015).
IRDC (1983e) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 70% chlorination of long chain
length paraffins. Mattawan, Michigan, International Research and
Development Corporation, 70 pp (Report No. 438-016).
IRDC (1983f) Teratology study in rabbits. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 195 pp
(Report No. 438-032).
IRDC (1983g) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 52% chlorination of intermediate
chain length n-paraffins. Mattawan, Michigan, International Research
and Development Corporation, 62 pp (Report No. 438-014).
IRDC (1983h) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffins: 58% chlorination of short chain
length n-paraffins. Mattawan, Michigan, International Research and
Development Corporation, 58 pp (Report No. 438-013).
IRDC (1983i) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 43% chlorination of long chain
length n-paraffins. Mattawan, Michigan, International Research and
Development Corporation, 56 pp (Report No. 438-012).
IRDC (1984a) 13-week oral (gavage) toxicity study in rats with
combined excretion, tissue level and elimination studies: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 58% chlorination of short
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 350 pp (Report
No. 438-029/022).
IRDC (1984b) 13-week oral (dietary) toxicity study in rats with
combined excretion, tissue level and elimination studies: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 52% chlorination of
intermediate chain length n-paraffins, 14C labeled CP. Mattawan,
Michigan, International Research and Development Corporation, 328 pp
(Report No. 438-023/026).
IRDC (1984c) 13-week oral (dietary) toxicity study in rats with
combined excretion, tissue level and elimination study: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 58% chlorination of
short chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 435 pp (Report
No. 438-035/022).
IRDC (1984d) Teratology study in rats. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 105 pp
(Report No. 438-017).
IRDC (1984e) Teratology study in rats. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 99 pp (Report
No. 438/045).
IRDC (1984f) 13-week oral (gavage) toxicity study in rats with
combined excretion, tissue level and elimination study: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 43% chlorination of long
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 275 pp (Report
No. 438-028/021).
IRDC (1984g) 13-week dietary toxicity study in rats with combined
excretion, tissue level and elimination studies/determination of
excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 70% chlorination of long
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 316 pp (Report
No. 438-027/024).
IRDC (1985) Reproduction range-finding study in rats. Chlorinated
paraffin: 52% chlorination of intermediate chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
179 pp (Report No. 438-049).
Jansson B, Andersson R, Asplund L, Bergman Å, Litze'n K, Nylund K,
Reuthergårdh L, Sellström U, Uvemo U-B, Wahlberg C, & Wideqvist U
(1991) Multiresidue method for the gas-chromatographic analysis of
some polychlorinated and polybrominated pollutants in biological
samples. Fresenius J Anal Chem, 340: 439-445.
Jansson B, Andersson R, Asplund L, Litze'n K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Environ Toxicol Chem, 12: 1163-1174.
KEMI (1991) Chlorinated paraffins. In: Freij L ed. Risk reduction of
chemicals: A Government Commission Report. Solna, The Swedish National
Chemicals Inspectorate, pp 167-187 (KEMI Report 1/91).
Kraemer W & Ballschmiter K (1987) Detection of a new class of
organochlorine compounds in the marine environment: the chlorinated
paraffins. Fresenius Z Anal Chem, 327: 47-48.
Lindén E, Bengtsson BE, Svanberg O, & Sundström G (1979) The acute
toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
Lombardo P, Dennison JL, & Johnson WW (1975) Bioaccumulation of
chlorinated paraffin residues in fish fed Chlorowax 500C. J Assoc Off
Anal Chem, 58: 707-710.
Lundberg P (1980) Effects of some flame retardants on the liver
microsomal enzyme systems. In: Cohn MJ ed. Microsomes, drug
oxidations, and chemical carcinogenesis, 1979. New York, Academic
Press, pp 853-856.
Lunde G & Steinnes E (1975) Presence of lipid-soluble chlorinated
hydrocarbons in marine oils. Environ Sci Technol, 9: 155-157.
Madeley J & Birtley R (1980) Chlorinated paraffins and the
environment. 2. Aquatic and avian toxicology. Environ Sci Technol,
14: 1215-1221.
Madeley JR & Gillings E (1983) Determination of the solubility of four
chlorinated paraffins in water. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 23 pp (Report No. BL/B/2301).
Madeley JR & Maddock BG (1983a) Effects of a chlorinated paraffin on
the growth of rainbow trout. Chlorinated paraffin: 58% chlorination of
short chain length paraffins. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 67 pp (Report No. BL/B/2309).
Madeley JR & Maddock BG (1983b) The bioconcentration of a chlorinated
paraffin in the tissues and organs of rainbow trout (Salmo gairdneri).
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 39 pp
(Report No. BL/B/2310).
Madeley JR & Maddock BG (1983c) Toxicity of a chlorinated paraffin to
rainbow trout over 60 days. Chlorinated paraffin: 52% chlorination of
intermediate chain length n-paraffins. Brixham, Imperial Chemical
Industries Ltd, Brixham Laboratory, 69 pp (Report No. BL/B/2202).
Madeley JR & Maddock BG (1983d) Toxicity of a chlorinated paraffin to
rainbow trout over 60 days. Chlorinated paraffin: 43% chlorination of
long chain length n-paraffins. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 71 pp (Report No. BL/B/2201).
Madeley JR & Thompson RS (1983a) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 71 pp (Report No.
BL/B/2291).
Madeley JR & Thompson RS (1983b) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Brixham,
Imperial Chemical Industries Ltd, Brixham Laboratory, 53 pp (Report
No. BL/B/2289).
Madeley JR & Thompson RS (1983c) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 59 pp (Report No.
BL/B/2288).
Madeley JR & Thompson RS (1983d) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 64 pp (Report No.
BL/B/2290).
Madeley JR, Thompson RS, & Brown D (1983a) The bioconcentration of a
chlorinated paraffin by common mussel (Mytilus edulis). Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 76 pp
(Report BL/B/2351).
Madeley JR, Windeatt AJ, & Street JR (1983b) Assessment of the
toxicity of a chlorinated paraffin to the anaerobic sludge digestion
product. Chlorinated paraffin: 58% chlorination of short chain length
n-paraffins. Brixham, Imperial Chemical Industries Ltd, Brixham
Laboratory, 25 pp (Report No. BL/B/2253).
Mather JI, Street JR, & Madeley JR (1983) Assessment of the inherent
biodegradability of a chlorinated paraffin, under aerobic conditions,
by a method developed from OECD Test Guideline 302B. Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 56 pp
(Report No. BL/B/2298).
Meijer J & DePierre JW (1987) Hepatic levels of cytosolic, microsomal
and "mitochondrial" epoxide hydrolases and other drug-metabolizing
enzymes after treatment of mice with various xenobiotics and endogenous
compounds. Chem-Biol Interact, 62: 249-269.
Meijer J, Rundgren M, Åström A, DePierre JW, Sundvall A, & Rannug U
(1981) Effects of chlorinated paraffins on some drug-metabolizing
enzymes in rat liver and in the Ames test. Adv Exp Med Biol,
A136: 821-828.
Menter P, Harrison W, & Woodin WG (1975) Patch testing of coolant
fractions. J Occup Med, 17(9): 565-568.
Muller W (1989) Chlorowax 500C - Micronucleus test in male and female
NMRI mice after oral administration. Frankfurt/Main, Hoechst AG,
Pharma Research Toxicology and Pathology, 24 pp (Report No. 89.0253).
Murray T, Frankenberry M, Steele DH, & Heath RG (1988) Chlorinated
paraffins: A report on the findings from two field studies, Sugar
Creek, Ohio, Tinkers Creek, Ohio. Volume 1: Technical report.
Washington, DC, US Environmental Protection Agency, 150 pp (EPA-560/
5-87/012).
Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D,
Martin R, & Caspary WJ (1990) L5178Y mouse lymphoma cell mutation
assay results with 41 compounds. Environ Mol Mutagen, 16(Suppl 18):
138-167.
NIOSH (1990) National occupational exposure survey (1980-1983).
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
Nilsen O & Toftgård R (1981) Effect of polychlorinated terphenyls and
paraffins on rat liver microsomal cytochrome P-450 and in vitro
metabolic activities. Arch Toxicol, 47: 1-11.
Nilsen O, Toftgård R, & Glaumann H (1980) Changes in rat liver
morphology and metabolic activities after exposure to chlorinated
paraffins. Dev Toxicol Environ Sci, 8: 525-528.
Nilsen OG, Toftgård R, & Glaumann H (1981) Effects of chlorinated
paraffins on rat liver microsomal activities and morphology.
Importance of the length and the degree of chlorination of the carbon
chain. Arch Toxicol, 49: 1-13.
NTP (1986a) Toxicology and carcinogenesis studies of chlorinated
paraffins (C12, 60% chlorine) (CAS No. 63449-39-8) in F344/N rats and
B6C3F1 mice (gavage studies). Research Triangle Park, North Carolina,
US Department of Health and Human Services, National Toxicology
Program, 67 pp (Technical Report Series No. 308).
NTP (1986b) Toxicology and carcinogenesis studies of chlorinated
paraffins (C23, 43% chlorine) (CAS No. 63449-39-8) in F344/N rats and
B6C3F1 mice (gavage studies). Research Triangle Park, North Carolina,
US Department of Health and Human Services, National Toxicology
Program, 66 pp (Technical Report Series No. 305).
Omori T, Kimura T, & Kodama T (1987) Bacterial cometabolic degradation
of chlorinated paraffins. Appl Microbiol Biotechnol, 25: 553-557.
Poon R, LeCavalier P, Chan P, Viau C, Håkansson H, Chu I, & Valli VE
(in press) Subchronic toxicity of a medium-chain chlorinated paraffin
in the rat. J Appl Toxicol.
Renberg L, Sundström G, & Sundh-Nygård K (1980) Partition coefficients
of organic chemicals derived from reversed-phase thin-layer
chromatography. Evaluation of methods and application on phosphate
esters, polychlorinated paraffins and some PCB-substitutes.
Chemosphere, 9: 683-691.
Renberg L, Tarkpea M, & Sundström G (1986) The use of the bivalve
Mytilus edulis as a test organism for bioconcentration studies. II.
The bioconcentration of two 14C-labeled chlorinated paraffins.
Ecotoxicol Environ Saf, 11: 361-372.
Richold M, Allen JA, Williams A, & Ransome SJ (1982a) Cell
transformation test for potential carcinogenicity of chlorinated
paraffin (58% chlorination of short chain length n-paraffins).
Huntingdon, Huntingdon Research Centre, 40 pp (Report No. ICI
399C/81468).
Richold M, Allen JA, Williams A, & Ransome SJ (1982b) Cell
transformation test for potential carcinogenicity of chlorinated
paraffin (70% chlorination of long chain length n-paraffins).
Huntingdon, Huntingdon Research Centre, 30 pp (Report No. ICI
399A/415/82313).
Roberts DJ, Cooke M, & Nickless G (1981) Determination of poly-
chlorinated alkanes via carbon skeleton capillary gas chromatography.
J Chromatogr, 213: 73-81.
Schenker BA (1979) Chlorinated paraffins. In: Mark HF, Othmer DF,
Overberger CG, Seaborg GT, & Grayson M ed. Kirk-Othmer encyclopedia of
chemical technology. New York, John Wiley and Sons, vol 5, pp 786-791.
Schmid PP & Müller MD (1985) Trace level detection of chlorinated
paraffins in biological and environmental samples, using gas
chromatography/mass spectrometry with negative-ion chemical ionization.
J Assoc Off Anal Chem, 68: 427-430.
Scott RC (1989) In vitro absorption of some chlorinated paraffins
through human skin. Arch Toxicol, 63: 425-426.
Serrone DM, Birtley RDN, Weigand W, & Millischer R (1987) Toxicology
of chlorinated paraffins. Food Chem Toxicol, 25: 553-562.
Shults SK, Serrone DM, Killeen JC, & Ignatoski JA (1984) A one-
generation reproduction study in mallard ducks with chlorinated
paraffins. Painesville, Ohio, SDS Biotech Corporation, 273 pp (Report
No. 558-1IT-83-0032-003).
Sistovaris N & Donges V (1987) Gas chromatographic determination of
total polychlorinated aromates and chloro-paraffins following
catalytic reduction in the injection port. Fresenius Z Anal Chem,
326: 751-753.
Slooff W, Bont PFH, Janus JA, & Annema JA (1992) Exploratory report on
chlorinated paraffins. Bilthoven, The Netherlands, National Institute
of Public Health and Environmental Protection, 47 pp (Report No.
710401016).
Steele DH, Sack TM, Moody LA, Murray TM, Glatz JA, & Breen JJ (1988) A
negative chemical ionization GC/MS method for the determination of
chlorinated paraffins in environmental samples. Presented at the 36th
ASMS Conference on Mass Spectrometry and Allied Topics, San Francisco,
5-10 June 1988. New York, American Society for Mass Spectrometry,
2 pp.
Strack H (1986) Chlorinated paraffins. In: Ullmann's encyclopedia of
industrial chemistry. Weinheim, VCH Verlagsgesellschaft, vol A6,
pp 323-330.
Street JR & Madeley JR (1983a) Summary of experiments conducted in an
attempt to determine the biodegradability of a chlorinated paraffin
under anaerobic conditions by EPA test guide line CG2050. Brixham,
Imperial Chemical Industries Ltd, Brixham Laboratory, 23 pp (Report
BL/B/2363).
Street JR & Madeley JR (1983b) Assessment of the fate of a chlorinated
paraffin during aerobic sewage treatment by a modification of OECD
test guideline 303A. Brixham, Imperial Chemical Industries Ltd,
Brixham Laboratory, 70 pp (Report No. BL/B/2308).
Swedish Environment Protection Agency (1994) Chlorinated paraffins in
metal working. Solna, Swedish Environment Protection Agency, 10 pp
(SEPA Report No. 4372).
Tarkpea M, Lindén E, Bengtsson BE, Larsson Å, & Svanberg O (1981)
[Products control studies at the Brackish Water Toxicology Laboratory,
1979-80.] Nyköping, Swedish Environmental Protection Agency, 22 pp
(NBL Report 1981-03-23) (in Swedish).
Thompson RS & Madeley JR (1983a) The acute and chronic toxicity of a
chlorinated paraffin (58% chlorination of short chain length
n-paraffins) to the mysid shrimp Mysidopsis bahia. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 55 pp (Report No.
BL/B/2373).
Thompson RS & Madeley JR (1983b) Toxicity of a chlorinated paraffin
(58% chlorination of short chain length n-paraffins) to the marine
alga Skeletonema costatum. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 57 pp (Report No. BL/B/2325).
Thompson RS & Madeley JR (1983c) The acute and chronic toxicity of a
chlorinated paraffin (58% chlorination of short chain length
n-paraffins) to Daphnia magna. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 70 pp (Report No. BL/B/2358).
Thompson RS & Madeley JR (1983d) Toxicity of a chlorinated paraffin
(58% chlorination of short chain length n-paraffins) to the green
alga Selenastrum capricornutum. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 59 pp (Report No. BL/B/2325).
Thompson RS & Shillabeer N (1983) Effect of a chlorinated paraffin
(58% chlorination of short chain length n-paraffins) on the growth
of mussels (Mytilus edulis). Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 54 pp (Report No. BL/B/2331).
US EPA (1993) RM2 exit briefing on chlorinated paraffins and olefins.
Washington, DC, US Environmental Protection Agency, 42 pp.
Willis B, Diment J, Dobson S, & Crookes M (1994) Environmental hazard
assessment: Chlorinated paraffins. Garston, Watford, Building Research
Establishment, 47 pp (Report No. TSD/19).
Wyatt I, Coutts CT, & Elcombe CR (1993) The effect of chlorinated
paraffins on hepatic enzymes and thyroid hormones. Toxicology,
77: 81-90.
Yang JJ, Roy TA, Neil W, Kreuger AJ, & Mackerer CR (1987) Percutaneous
and oral absorption of chlorinated paraffins in the rat. Toxicol Ind
Health, 3: 405-412.
Zitko V (1973) Chromatography of chlorinated paraffins on alumina and
silica columns. J Chromatogr, 81: 152-155.
Zitko V (1974a) Uptake of chlorinated paraffins and PCB from suspended
solids and food by juvenile atlantic salmon. Bull Environ Contam
Toxicol, 12: 406-412.
Zitko V (1974b) Confirmation of chlorinated paraffins by dechlor-
ination. J Assoc Off Anal Chem, 57: 1253-1259.
Zitko V (1980) Chlorinated paraffins. In: Hutzinger O ed. Handbook of
environmental chemistry. Volume 3, Part A: Anthropogenic compounds.
Berlin, Heidelberg, New York, Springer Verlag, pp 149-156.
Zitko V & Arsenault E (1977) Fate of high molecular weight-chlorinated
paraffins in the aquatic environment. Adv Environ Sci Technol, 8:
409-418.
RESUME
1. Propriétés, usages et méthodes d'analyse
Les paraffines chlorées s'obtiennent par chloration des fractions
paraffiniques à chaîne droite. La chaîne des paraffines du commerce
comporte habituellement 10 à 30 atomes de carbone et leur teneur en
chlore est généralement comprise entre 40 et 70% en poids. Ce sont
des huiles visqueuses et denses, incolores ou jaunâtres, à faible
tension de vapeur; toutefois, lorsque la chaîne carbonée est
suffisamment longue et que la teneur en chlore est élevée (70%), on a
affaire à des solides. Les paraffines chlorées sont pratiquement
insolubles dans l'eau, les alcools inférieurs, le glycérol et les
glycols, mais solubles dans les solvants chlorés, les hydrocarbures
aromatiques, les cétones, les esters, les éthers,les huiles minérales
et certaines huiles de coupe. Elles sont modérément solubles dans les
hydrocarbures aliphatiques non chlorés.
En raison du nombre de positions possibles pour les atomes de
chlore, les paraffines chlorées sont des mélanges ex trêmement
complexes. Selon la longueur de la chaîne (courte C10-13,
intermédiaire C14-17, longue C18-30) et le degré de chloration (faible
< 50%; élevé > 50%), on peut diviser ces produits en six groupes.
Les paraffines chlorées sont très largement utilisées dans le
monde entier pour diverses applications: plastifiants (par ex. Pour le
PVC), additifs pour lubrifiants de pièces métalliques travaillant à
très haute pression, retardateurs de flammes et additifs pour
peintures. Les produits de qualité technique peuvent contenir
diverses impuretés: isoparaffines, métaux, composés aromatiques et en
principe, ils sont additionnés de stabilisants destinés à en prévenir
la décomposition.
En raison de la très grande complexité des mélanges, l'analyse
des paraffines chlorées est difficile. Lorsqu'il s'agit de travailler
sur des prélèvements effectués dans l'environnement, il s'y ajoute
encore les interférences dues à la présence d'autres composés.
L'analyse proprement dite doit donc souvent être précédée d'une
purification poussée des échantillons et faire appel à des moyens de
détection spécifiques. Au début, on purifiait le mélange par
chromatographie en couche mince et on effectuait ensuite la révélation
sur la plaque par une méthode non spécifique. Actuellement, on
utilise différentes techniques de chromatographie sur colonne pour la
purification des échantillons, mais il est difficile d'isoler les
paraffines chlorées en raison de la grande diversité de leurs
propriétés physiques. Dans ces conditions, il faut utiliser des
méthodes de détection spécifiques; à l'heure actuelle, la plus
utilisée est la chromatographie en phase gazeuse couplée à la
spectrométrie de masse. L'utilisation d'ions négatifs améliore encore
la spécificité. Toutefois, même si ces techniques élaborées
facilitent l'analyse des paraffines chlorées, il encore impossible de
déterminer les concentrations avec exactitude. Les résultats publiés
ne sont donc que des estimations.
2. Sources d'exposition humaine et environnementale
On ne connaît pas de paraffines chlorées d'origine naturelle.
Ces produits s'obtiennent par réaction du chlore gazeux sur des
fractions paraffiniques liquides. Il peut être nécessaire d'utiliser
un solvant et la lumière ultraviolette sert souvent de catalyseur.
Pour 1985, la production mondiale de paraffines chlorées a été estimée
à 300 000 tonnes.
La pollution de l'environnement par les paraffines chlorées
provient sans doute essentiellement du fait qu'elles sont d'un usage
très répandu. Elles peuvent être déversées dans l'environnement
lorsque des lubrifiants pour métaux ou des polymères qui en
contiennent viennent à être dispersés sans précautions dans la nature.
Il peut également y avoir pollution si des paraffines chlorées passent
dans l'environnement par lessivage de peintures ou de revêtements
divers. On pense que davantage de paraffines chlorées disparaissent
dans la nature pendant la production et le transport que lors de
l'utilisation des produits et de leur élimination.
En raison de leur instabilité thermique, les paraffines chlorées
doivent en principe être décomposées par l'incinération et donc ne pas
réapparaître dans les gaz émis par les incinérateurs. On a cependant
montré que lors de la pyrolyse de ces produits, des dérivés chlorés
d'hydrocarbures aromatiques-biphényle, naphtalène ou benzène
polychlorés - peuvent se former dans certaines conditions.
3. Distribution et transformation dans l'environnement
Les paraffines chlorées sont fortement adsorbées par les
sédiments. Dans l'eau, elles sont probablement transportées par
les particules sur lesquelles elles sont adsorbées; dans l'air
l'adsorption a vraisemblablement lieu sur les particules aéroportées
(et peut être aussi dans la phase vapeur). On estime que dans l'air,
la demi-vie des paraffines chlorées est de 0,85 à 7,2 jours, cette
durée étant suffisamment longue pour qu'on ne puisse exclure un
transport sur de longues distances.
Les paraffines chlorées ne sont pas facilement biodégradables.
En fait, celles qui ont une chaîne courte et une teneur en chlore
de moins de 50% se révèlent biodégradables en aérobiose par des
microorganismes acclimatés, la dégradation paraissant inhibée lorsque
la teneur en chlore dépasse 58%. La dégradation des paraffines
chlorées à chaîne moyenne ou longue est plus lente.
Les paraffines chlorées s'accumulent dans les organismes
aquatiques et les facteurs de bioconcentration publiés vont de 7 à
7155 pour les poissons et de 223 à 138 000 pour les moules. Les
poissons accumulent davantage les paraffines chlorées à courte chaîne
que les composés à chaîne moyenne ou longue. Après administration de
produits radiomarqués, on a retrouvé la radioactivité principalement
dans la bile, les intestins, le foie, les graisses et les branchies.
La fixation de ces composés semble donc facilitée par une chaîne
courte et une faible teneur en chlore, les composés à chaîne longue,
quant à eux, étant éliminés le plus lentement. La rétention dans les
tissus à forte adiposité augmente avec la teneur en chlore.
4. Concentrations dans l'environnement et exposition humaine
On ne possède guère de données sur la concentration des
paraffines chlorées dans l'environnement. On en a décelé la présence
au Royaume-Uni dans des échantillons d'eau de mer à des concentrations
inférieures à 4 µg/litre. Dans des eaux n'appartenant pas au domaine
marin, on a mesuré dans ce même pays des concentrations inférieures à
6 µg/litre; en Allemagne, les teneurs relevées en 1994 se situaient
dans les limites de 0,08 à 0,28 µg/litre. Aux Etats-Unis, la teneur
des eaux en paraffines chlorées est en général inférieure à
0,03 µg/litre, mais il est arrivé qu'on ait des concentrations
supérieures à 1,0 µg/litre dans une faible proportion des échantillons
(1,2%). Dans les sédiments marins, on a fait état de concentrations
allant jusqu'à 600 µg/kg de poids frais, cette teneur pouvant aller, au
Royaume-Uni, jusqu'à 15 000 µg/kg de poids frais pour des sédiments
non marins provenant de régions industrialisées et atteindre encore
1000 µg/kg de poids frais dans des zones à l'écart de toute industrie.
Aux Etats-Unis, on a trouvé, dans les eaux d'une retenue qui
provenaient d'une usine produisant des paraffines chlorées, des
sédiments dont la teneur atteignait, en poids sec, 170 000 µg/kg de
dérivés à longue chaîne, 50 000 µg/kg de dérivés à chaîne moyenne et
40 000 µg/kg de dérivés à chaîne courte. En Allemagne, on a trouvé en
1994 dans des sédiments les concentrations suivantes: jusqu'à 83 µg/kg
de dérivés en C10-13 et jusqu'à 370 µg/kg de dérivés en C14-17. Au
Japon, la teneur des sédiments allait jusqu'à 8 500 µg/kg.
La présence de paraffines chlorées a été mise en évidence dans un
certain nombre d'organismes. En Suède, on en a découvert chez des
mammifères terrestres à des concentrations de 32 à 88 µg/kg de tissus
(140-4400 µg/kg de lipides). Cependant au Royaume-Uni, on n'a pas
trouvé de paraffines chlorées chez des moutons qui paissaient à
distance des lieux de production. Dans ce même pays, la concentration
allait jusqu'à 1500 µg/kg chez des oiseaux, et, en ce qui concerne les
poissons, les teneurs pouvaient atteindre 200 µg/kg, valeur également
relevée en Suède. Dans des moules récoltées aux Etats-unis et au
Royaume-Uni, on a signalé des concentrations pouvant atteindre
400 µg/kg. Il est vrai qu'à proximité de la décharge d'une usine de
paraffines chlorées, les moules en contenaient jusqu'à 12 000 µg/kg.
Ces produits ont également été décelés lors d'autopsies dans des
tissus humains, notamment dans les tissus adipeux (teneur médiane
100-190 µg/kg), les reins (teneur médiane inférieure à 90 µg/kg) ainsi
que dans le foie (teneur médiane inférieure à 90 µg/kg). Lors d'une
enquête de portée limitée, on a constaté que des paraffines chlorées,
principalement des dérivés en C10-20, étaient présentes à des teneurs
pouvant atteindre 500 µg/kg dans environ 70% des échantillons de
denrées alimentaires.
Les données concernant l'exposition professionnelle aux
paraffines chlorées sont très limitées. On a constaté l'existence
d'une très faible exposition à des aérosols de paraffines chlorées à
chaîne courte (0,003-1,2 mg/m3), lors de l'utilisation de ces
produits comme lubrifiants de pièces métalliques, mais on ne sait pas
dans quelle proportion ils sont respirables. A partir d'un modèle
mathématique de l'exposition et en l'absence de toute mesure de
protection, on estime que ces lubrifiants à très forte teneur en
paraffines chlorées à chaîne courte doivent très largement entrer en
contact avec la peau (5-15 mg/cm2 par jour), même si l'absorption est
vraisemblablement faibles. Des mesures de protection devraient
permettre de réduire l'exposition cutanée.
5. Cinétique et métabolisme
La toxicocinétique des paraffines chlorées a été étudiée sur des
animaux de laboratoire. En ce qui concerne l'homme, les données sont
insuffisantes. On n'a pas suffisamment étudié les différences d'ordre
toxicocinétique pouvant résulter des différences de longueur de
chaîne. On ignore le degré d'absorption des paraffines chlorées
après administration orale, mais il semble qu'il diminue à mesure
qu'augmentent la longueur de la chaîne et la teneur en chlore. Selon
la longueur de la chaîne, l'absorption cutanée peut également être
plus ou moins importante, mais elle devrait rester limitée (moins de
1% d'une dose de C18 en application topique). On ne dispose d'aucune
donnée sur l'absorption au niveau pulmonaire.
Les paraffines chlorées se répartissent principalement dans le
foie, les reins, les intestins, la moelle osseuse, les tissus adipeux
et les ovaires. On ne dispose pas de données suffisantes sur la
rétention de ces dérivés dans l'organisme mais semble qu'elle est plus
longue lorsque ces produits ont une faible teneur en chlore, du fait
d'une redistribution plus lente. On les retrouve, accompagnées de
leurs métabolites, dans le système nerveux central jusqu'à 30 jours
après l'administration. Il est possible qu'elles traversent la
barrière foeto-placentaire. On ne dispose pas d'informations
suffisantes sur les voies métaboliques des paraffines chlorées, encore
que des études à l'aide de molécules radiomarquées aient montré que le
produit final en est le CO2.
Les paraffines chlorées sont excrétées par la voie rénale,
biliaire ou pulmonaire (sous la forme de CO2). Etant donné les
importantes variations d'une étude à l'autre, il est difficile
d'établir la part relative de chacune de ces voies d'excrétion.
L'élimination totale diminue lorsque le degré de chloration augmente
et les composés fortement chlorés sont principalement excrétés (à plus
de 50%) sous la forme de CO2. Il peut également y avoir excrétion
dans le lait.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Quelle que soit la longueur de la chaîne, les paraffines chlorées
ont une faible toxicité aiguë par voie orale. Après administration
d'une dose unique de produits à chaîne courte, les effets toxiques les
plus évidents consistaient en une perte de la coordination musculaire
et un hérissement des poils. En s'appuyant sur le peu de données dont
on dispose, on peut également dire que la toxicité aiguë par la voie
respiratoire et la voie cutanée semble faible. Après application ou
instillation de paraffines chlorées à chaîne courte ou moyenne, on a
observé une légère irritation de la peau (produits à chaîne moyenne)
et des yeux. Selon certaines études, les produits à courte chaîne ne
provoquent pas de sensibilisation cutanée.
Les études toxicologiques basées sur l'administration de doses
répétées par voie orale ont montré que que le foie, les reins et la
thyroïde sont les principales cibles des paraffines chlorées. Dans le
cas des composés à chaîne courte, on a constaté une augmentation du
poids du foie au doses les plus faibles (la dose effective la plus
faible est de 50 à 100 mg/kg de poids corporel sur une journée et la
dose sans effet observable est de 10 mg/kg de poids corporel par
jour). A doses plus élevées, on a également observé une augmentation
de l'activité des enzymes hépatiques, une prolifération du réticulum
endoplasmique agranulaire et des peroxysomes, un accroissement de la
synthèse réplicative de l'ADN, ainsi qu'une hypertrophie, une
hyperplasie et une nécrose du foie. D'autres effets ont été notés:
diminution du poids corporel (125 mg/kg de poids corporel par jour
chez la souris), augmentation du poids des reins (100 mg/kg de poids
corporel par jour chez le rat), augmentation de la synthèse
réplicative de l'ADN dans les cellules rénales (313 mg/kg de poids
corporel par jour), et néphrose (625 mg/kg de poids corporel par jour
chez le rat). Par ailleurs, on a signalé une augmentation du poids de
la thyroïde ainsi qu'une hypertrophie et une hyperplasie de cette
glande (plus faible dose effective: 100 mg/kg de poids corporel par
jour chez le rat) avec également un accroissement de la synthèse
réplicative de l'ADN dans les cellules folliculaires (plus faible dose
effective: 313 mg/kg de poids corporel par jour). A doses plus
élevées (1000 mg/kg de poids corporel par jour), la fonction
thyroïdienne est affectée, comme en témoignent les taux de thyroxine
(libre et totale) plasmatiques et l'augmentation de la thyréostimuline
plasmatique chez le rat.
En ce qui concerne les composés à chaîne moyenne, les effets
observés aux doses les plus faibles sont généralement une augmentation
du poids du foie et des reins (dose effective la plus faible chez
le rat: 100 mg/kg de poids corporel par jour et dose sans effet
observable chez le même animal: 10 mg/kg de poids corporel par jour).
A des doses analogues (dose sans effets observables de 4 mg/kg de
poids corporel par jour) on a noté un accroissement du cholestérol
sérique et des effets bénins "adaptatifs" consistant en modifications
histologiques au niveau de la thyroïde.
Dans le cas des composés à longue chaîne, les effets observés
aux doses les plus faibles consistaient en une hépatite granulomateuse
multifocale et et un accroissement du poids du foie chez les femelles
(dose effective la plus faible de 100 mg/kg de poids corporel par
jour).
Dans la seule étude de reproduction dont on dispose, on n'a pas
constaté d'effets nocifs chez des rats exposés à des paraffines à
chaîne moyenne contenant 52% de chlore. On a toutefois constaté une
réduction de la survie et du poids corporel chez les ratons (dose la
plus faible à laquelle on constatait une réduction non significative
du poids corporel: 5,7-7,2 mg/kg de poids corporel par jour; dose la
plus faible pour laquelle on constatait une réduction de la survie:
60-70 mg/kg de poids corporel par jour). Dans un petit nombre
d'études consacrées aux effets, sur le développement, des paraffines
chlorées à chaîne courte, moyenne ou longue, les effets observés sur
la progéniture étaient imputables uniquement aux composés à chaîne
courte, à des doses toxiques pour les mères (2000 mg/kg de poids
corporel par jour). Les composés à chaîne longue ou moyenne n'ont eu
aucun effet de ce genre, même à dose très élevée (1000 à 5000 mg/kg de
poids corporel par jour).
Les paraffines chlorées ne semblent pas provoquer de mutations
chez les bactéries. Cependant, il pourrait y avoir un faible effet
clastogène dans des cultures de cellules mammaliennes in vitro (mais
pas in vivo). Les paraffines chlorées provoqueraient également une
transformation cellulaire in vitro.
Des études de cancérogénicité à long terme ont été effectuées sur
des rats et des souris qui ont été gavées respectivement avec un
composé à chaîne courte (C12; 58% Cl) et un composé à chaîne longue
(C23; 43% Cl). Chez les souris B6C3F1 ayant reçu le composé
à chaîne courte, on a observé un accroissement de l'incidence de
certaines tumeurs: hépatiques parmi les mâles et les femelles,
thyroïdiennes parmi les femelles. Chez les rats Fischer-344 exposés
au composé à chaîne courte, il y avait augmentation des tumeurs
hépatiques parmi les mâles et les femelles, des tumeurs rénales
(adénomes et adénocarcinomes) parmi les mâles, des tumeurs
thyroïdiennes parmi les femelles et des leucémies monocytaires parmi
les mâles. Dans le cas du composé à longue chaîne, on constaté une
augmentation de l'incidence des lymphomes malins chez les souris mâles
et de celle des tumeurs surrénaliennes chez les rattes.
7. Effets sur l'homme
Malgré la très large utilisation qui est faite des paraffines
chlorées, on ne connaît aucun cas d'irritation ou de sensibilisation
cutanée. Cette observation est corroborée par les résultats d'un
petit nombre d'études sur des volontaires au cours desquelles on a
observé une irritation cutanée minime, mais pas de sensibilisation.
On n'a pas pu obtenir de données concernant d'autres effets des
paraffines chlorées sur l'homme.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
On a montré que les paraffines chlorées à courte chaîne pouvaient
provoquer des intoxications aiguës chez les invertébrés marins et les
invertébrés d'eau douce, la valeur de la CL50 et de la CE50 allant de
14 à 530 µg/litre. Dans la plupart des épreuves de toxicité aiguë
effectuées sur des invertébrés aquatiques avec des paraffines chlorées
à chaîne moyenne ou longue, les concentrations utilisées étaient
supérieures à la solubilité des composés dans l'eau. Toutefois, selon
une étude, une paraffine chlorée à chaîne moyenne serait toxique pour
les daphnies, avec une CE50 de 37 µg/litre. La toxicité aiguë des
paraffines chlorées pour les poissons est faible, qu'il s'agisse de
composés à chaîne courte, moyenne ou longue, la valeur de la CL50
étant largement supérieure à la solubilité dans l'eau.
Les paraffines chlorées à chaîne courte présentent une toxicité à
long terme pour les algues, les invertébrés aquatiques et les poissons
à des concentrations ne dépasssant pas 19,6, 8,9 et 3,1 µg/litre,
respectivement; la concentration sans effet observable se situe entre
2 et 5 µg/litre pour l'espèce la plus sensible étudiée. Des produits
à chaîne moyenne et à chaîne longue ont eu des effets chroniques
sur des daphnies à des concentrations respectivement égales à
20-35 µg/litre et à < 1,2-8 µg/litre. Il semble que la toxicité à
long terme soit faible pour les poissons. On ne dispose d'aucune
donnée concernant les algues.
D'après les données limitées dont on dispose, on pense que la
toxicité aiguë est faible pour les oiseaux.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
Il est probable que la nourriture soit la principale source
d'exposition de la population générale. D'après les données limitées
dont on dispose au sujet des concentrations dans les denrées
alimentaires, les estimation les plus pessimistes concernant les
produits laitiers et les moules donnent respectivement des apports
journaliers de 4 et 25 µg/kg de poids corporel. En général, les doses
journalières calculées de paraffines chlorées se situent en dessous
des valeurs tolérables relatives aux effets non néoplasiques ou des
valeurs recommandées relatives aux effets néoplasiques (composés à
chaîne courte).
Dans la mesure où ils respectent les règles d'hygiène personnelle
et les consignes de sécurité, les travailleurs exposés à des
paraffines chlorées n'encourent qu'un risque minime.
Les données disponibles montrent que les paraffines chlorées
s'accumulent dans les tissus biologiques et sont persistantes.
D'après les données relatives à la concentration des dérivés à chaîne
courte dans l'environnement, il y a un risque pour la faune
dulçaquicole et estuarielle dans les zones proches des points de
décharge. Les dérivés à chaîne longue et moyenne représentent aussi
un danger pour les invertébrés aquatiques.
L'enrichissement des sédiments en paraffines chlorées, de même
que les modalités de résorption et la toxicité de ces produits pour la
faune aquatique sont l'indication d'un risque pour les organismes qui
peuplent la vase.
RESUMEN
1. Propiedades, usos y métodos analíticos
Las parafinas cloradas (PC) se producen por la cloración de
fracciones de parafina de cadena recta. Por lo general, la cadena
carbonada de las parafinas cloradas comerciales contiene de 10 a 30
átomos de carbono, y su contenido de cloro oscila generalmente entre
el 40% y el 70% por peso. Las parafinas cloradas son aceites densos
viscosos incoloros o amarillentos con bajas presiones de vapor, a
excepción de las de cadena carbonada larga con elevado contenido
de cloro (70%), que son sólidas. Las parafinas cloradas son
prácticamente insolubles en agua, alcoholes inferiores, glicerol y
glicoles, pero son solubles en solventes clorados, hidrocarburos
aromáticos, cetonas, ésteres, éteres, aceites minerales y algunos
lubricantes para cuchillas. Son medianamente solubles en
hidrocarburos alifáticos no clorados.
Las parafinas cloradas están formadas por mezclas sumamente
complejas, lo que obedece a las múltiples posiciones posibles de los
átomos de cloro. Los productos pueden subdividirse en seis grupos,
atendiendo a la longitud de la cadena (corta C10-13, media C14-17 y
larga C18-30) y al grado de cloración (bajo (< 50%) y elevado
(> 50%).
Las parafinas cloradas se utilizan en todo el mundo en múltiples
aplicaciones; se emplean como plastificantes en la fabricación de
plásticos (tales como el policloruro de vinilo), como aditivos en
fluidos para el laboreo de metales a presiones extremas, y como
pirorretardantes y aditivos en la producción de pinturas. Las
parafinas cloradas de calidad técnica pueden estar contaminadas por
isoparafinas, compuestos aromáticos y metales; por lo general
contienen estabilizadores, añadidos para impedir la descomposición.
El análisis de las parafinas cloradas resulta difícil debido a la
enorme complejidad de esas mezclas. En las muestras tomadas del medio
ambiente, ello se ve complicado además por la interferencia de otros
compuestos. En muchos casos, los análisis requieren un considerable
grado de descontaminación de las muestras y el empleo de métodos de
detección específicos. En el pasado, se empleaba como método de
descontaminación la cromatografía en capa fina y un método no
específico de detección por argentación en las placas. En la
actualidad se emplean métodos de descontaminación basados en la
cromatografía líquida en diferentes columnas, aunque resulta difícil
aislar las parafinas cloradas debido al gran número de sus propiedades
físicas. Por lo tanto, se emplean métodos de detección específicos;
en la actualidad, la técnica más corriente es la cromatografía por gas
combinada con la espectrometría de masas. El uso de iones negativos
permite una detección incluso más específica. Si bien el empleo de
esas técnicas avanzadas ha aumentado la capacidad de análisis de
las parafinas cloradas, continúa siendo imposible determinar
las concentraciones exactas. Los resultados comunicados deben
considerarse solamente estimaciones de los valores reales.
2. Fuentes de exposición del ser humano y del medio ambiente
No se tiene conocimiento de la presencia en estado natural de las
parafinas cloradas.
Las parafinas cloradas son producto de la reacción de fracciones
de parafina líquida con gas cloro puro. La reacción puede requerir el
empleo de un solvente, empleándose frecuentemente luz ultravioleta
como catalizador. Se estima que en 1985 la producción mundial de
parafinas cloradas se elevó a 300 000 toneladas.
Los usos muy difundidos de las parafinas cloradas son
probablemente la principal fuente de contaminación ambiental. Las PC
podrían escapar al medio ambiente debido a la eliminación incorrecta
de fluidos para el laboreo de metales que contienen parafinas cloradas
o de polímeros que contienen parafinas cloradas. La pérdida de
parafinas cloradas como resultado de la lixiviación de pinturas y
revestimientos podría ser también fuente de contaminación ambiental.
Cabe prever que las posibles pérdidas durante la producción y el
transporte sean inferiores a las que ocurren durante el uso y
eliminación de los productos.
Debido a su inestabilidad térmica, es de suponer que las
parafinas cloradas se degradan durante la incineración; por lo tanto,
no es de esperar que se volatilicen en los gases de escape de los
incineradores. Sin embargo, se ha demostrado la formación de
compuestos aromáticos clorados como, por ejemplo, bifenilos
policlorados, naftalenas y benzinas, por pirólisis de las parafinas
cloradas en ciertas condiciones.
3. Distribución y transformación en el medio ambiente
Las parafinas cloradas experimentan una adsorción pronunciada en
el sedimento. En el agua son transportadas probablemente adsorbidas
en partículas en suspensión, y en la atmósfera están adsorbidas en
partículas transportadas por el aire (y posiblemente en la fase de
vapor). Se ha estimado que la semivida de las parafinas cloradas en
el aire oscila entre 0,85 y 7,2 días; debido a la duración de ese
período, no puede excluirse la posibilidad de su transporte a larga
distancia.
Las parafinas cloradas no son fácilmente biodegradables. Las de
cadena carbonada corta con un contenido de cloro inferior al 50%
parecen ser degradables en condiciones aeróbicas con microorganismos
aclimatados, mientras que la degradación parece estar inhibida cuando
el contenido de cloro es superior al 58%. Las de cadenas carbonadas
media y larga se degradan más lentamente.
Las parafinas cloradas se bioacumulan en los organismos
acuáticos, habiéndose comunicado factores de bioconcentración que
oscilan entre 7 y 7155 en el caso de peces y entre 223 y 138 000 en el
de mejillones. En los peces, las de cadena corta experimentan mayor
acumulación que las de cadenas media y larga. Se ha observado
radioactividad principalmente en la bilis, el intestino, el hígado, la
grasa y las agallas después de la administración de parafinas cloradas
marcadas con radioisótopos. La absorción de las parafinas cloradas
parece ser más eficiente en el caso de las que tienen menor contenido
de cloro; la tasa de eliminación es más lenta en el caso de aquellas
que tienen un elevado contenido de cloro. La retención en los tejidos
ricos en grasa parece aumentar a medida que aumenta el grado de
cloración.
4. Concentración en el medio ambiente y exposición del ser humano
Se cuenta con poca información sobre la concentración de las
parafinas cloradas en el medio ambiente. Se han detectado
concentraciones inferiores a 4 µg/litro en muestras de agua de
mar en el Reino Unido. En aguas no marítimas, se ha informado de
concentraciones de 6 µg/litro en el Reino Unido; en Alemania, las
concentraciones determinadas en 1994 oscilaban entre 0,08 y
0,28 µg/litro. En los Estados Unidos, si bien las concentraciones en
el agua eran generalmente inferiores a 0,03 µg/litro, se observaron
concentraciones superiores a 1,0 µg/litro en una pequeña proporción
(1,2%) de las muestras. En los sedimentos marinos, se tiene noticias
de concentraciones de hasta 600 µg/kg de peso húmedo, y en sedimentos
no marinos en el Reino Unido las concentraciones han alcanzado hasta
15 000 µg/kg en regiones industrializadas y 1000 µg/kg en zonas
alejadas de la industria. En los sedimentos en un embalse de
confinamiento de una planta de fabricación de parafinas cloradas en
los Estados Unidos, las concentraciones registradas llegaron a
alcanzar 170 000 µg/kg peso seco de PC de cadena larga, 50 000 µg/kg
de PC de cadena media y 40 000 µg/kg de PC de cadena corta. En
Alemania, se han comunicado en 1994 concentraciones de hasta 83 µg/kg
de peso seco de C10-13 y de hasta 370 µg/kg de peso seco de C14-17 en
sedimentos. En el Japón, las concentraciones en el sedimento han
llegado a alcanzar 8500 µg/kg.
Se han detectado parafinas cloradas en diferentes organismos.
Se han encontrado en los mamíferos terrestres en Suecia en
concentraciones que oscilan entre 32 y 88 µg/kg de tejido (140 a
4400 µg/kg de lípidos). Sin embargo, no se detectaron en ovejas que
pastaban en zonas alejadas de la producción de parafinas cloradas en
el Reino Unido. Por lo que respecta a las aves, en el Reino Unido se
observaron concentraciones que llegaron a alcanzar los 1500 µg/kg; en
cuanto a los peces, se han comunicado concentraciones de hasta
200 µg/kg en Suecia y el Reino Unido. En los mejillones recogidos
en los Estados Unidos y el Reino Unido, se tiene noticias de
concentraciones que se han elevado a 400 µg/kg. Con todo, en
mejillones capturados en las cercanías de un punto de descarga de
efluentes de una fábrica de parafinas cloradas se han registrado
concentraciones de C10-20 que han alcanzado 12 000 µg/kg. En
estudios post mortem se han detectado también PC en los tejidos
humanos: en el tejido adiposo (concentración media de 100 a 190 µg/kg),
los riñones (concentración media inferior a 90 µg/kg) y el hígado
(concentración media inferior a 90 µg/kg). En un estudio limitado, se
detectaron concentraciones de hasta 500 µg/kg de parafinas cloradas,
principalmente C10-20, en un 70% de las muestras de diversos
productos alimenticios.
Se dispone de escasa información sobre la exposición ocupacional
a las parafinas cloradas. Se han observado niveles muy bajos de
exposición a los aerosoles de PC de cadena corta (0,003 a 1,2 mg/m3)
asociados con su uso en fluidos para el laboreo de metales, aunque no
existe información disponible sobre la proporción que se puede
inhalar. Sobre la base de modelos matemáticos de la exposición sin
medidas de control, se estimaron niveles elevados de contactos
dérmicos (5 a 15 mg/cm2 al día) por lo que respecta a los fluidos
especiales para labrado de metales que contienen concentraciones muy
elevadas de PC de cadena corta, aunque cabe esperar que la absorción
sea baja. Las medidas de control permitirían reducir la exposición
dérmica.
5. Cinética y metabolismo
Se ha estudiado la toxicocinética de las parafinas cloradas en
animales experimentales. No se cuenta con información adecuada por lo
que respecta a los seres humanos. No se han realizado suficientes
investigaciones sobre las posibles diferencias en materia de
toxicocinética como consecuencia de las diferencias en la longitud de
las cadenas. Si bien se desconoce el grado de absorción de las PC
después de su administración oral, éste parece disminuir a medida que
aumenta la longitud de la cadena y el grado de cloración. Según cuál
sea la longitud de la cadena, puede producirse también absorción
percutánea, aunque en un grado limitado (inferior al 1% de una dosis
tópica de C18). No se cuenta con datos sobre la absorción en el
pulmón.
La distribución de las parafinas cloradas ocurre principalmente
en el hígado, los riñones, el intestino, la médula espinal, el tejido
adiposo y los ovarios. Aunque no existe suficiente información en lo
que respecta a la retención, un grado bajo de cloración podría
aumentar el tiempo de retención al ser más lenta la redistribución.
Se ha observado la presencia de PC, o de sus metabolitos, en el
sistema nervioso central hasta 30 días después de su administración.
Podrían cruzar la barrera hemato-placentaria. Si bien no se cuenta
con información adecuada sobre las vías del metabolismo de las PC, en
los estudios con radioisótopos se ha identificado el CO2 como
producto final.
Las parafinas cloradas pueden excretarse por vía renal, biliar y
pulmonar (como CO2). Resulta difícil establecer el grado relativo de
excreción por las diferentes rutas debido a la gran variabilidad de
los diferentes estudios. La total eliminación de esas sustancias
disminuye a medida que aumenta el contenido de cloro, y los compuestos
con elevado grado de cloración se excretan principalmente (más del
50%) en forma de CO2. Las PC pueden ser excretadas en la leche.
6. Efectos en mamíferos de laboratorio y sistemas de pruebas
in vitro
Las parafinas cloradas de cadenas de diferentes longitudes
presentan reducida toxicidad oral aguda. Los efectos tóxicos como,
por ejemplo, incoordinación muscular y piloerección resultaban más
ostensibles después de una exposición aislada a parafinas cloradas de
cadena corta. Sobre la base de información muy limitada, la toxicidad
aguda por inhalación y por contacto cutáneo parece ser también baja.
Se ha observado ligera irritación de la piel y de los ojos después de
la aplicación de PC de cadena media (irritación cutánea). Los
resultados de varios estudios indican que las PC de cadena corta no
provocan sensibilización cutánea.
En estudios de toxicidad con dosis repetidas por vía oral, los
órganos en que se manifiesta principalmente la toxicidad de las PC son
el hígado, los riñones y la tiroides. Por lo que respecta a los
compuestos de cadena corta, se han registrado aumentos en el peso del
hígado con las dosis más reducidas (en las ratas, el nivel más bajo de
efecto observado es de 50 a 100 mg/kg peso corporal por día, y la
concentración sin efectos observados es de 10 mg/kg de peso corporal
al día). Con posologías superiores, se han registrado también
aumentos en la actividad de las enzimas hepáticas, proliferación
del retículo endoplasmático liso y peroxisomas, síntesis de ADN
replicante, hipertrofia, hiperplasia y necrosis del hígado. Asimismo,
se han observado disminución en el aumento del peso corporal
(125 mg/kg de peso corporal por día en los ratones), aumentos del peso
de los riñones (100 mg/kg de peso corporal por día en las ratas),
síntesis de ADN replicante en las células renales (313 mg/kg de peso
corporal por día) y nefrosis (625 mg/kg de peso corporal por día en
las ratas). Se han comunicado aumentos en el peso de la tiroides, e
hipertrofia e hiperplasia de la tiroides (nivel más bajo de efecto
observado de 100 mg/kg de peso corporal por día en las ratas) y
síntesis de ADN replicante en las células foliculares de la tiroides
(nivel más bajo de efecto observado de 313 mg/kg de peso corporal por
día). Con posologías superiores (1000 mg/kg de peso corporal por
día), la función tiroidea se ve afectada, lo que se puede establecer
por las concentraciones de tiroxina plasmática libre y total y la
mayor concentración plasmática de la hormona tirotrófica en las ratas.
En cuanto a los compuestos de cadena media, el efecto observado
con las dosis más bajas es generalmente el aumento del peso del hígado
y los riñones (nivel más bajo de efecto observado en las ratas de
100 mg/kg de peso corporal al día; nivel sin efecto nocivo observado en
las ratas de 10 mg/kg de peso corporal al día). En estudios con ratas
hembras, se han señalado aumentos en el colesterol sérico y cambios
histológicos "ligeros, adaptativos" en la tiroides cuando se emplean
posologías similares (nivel sin efecto nocivo observado de 4 mg/kg de
peso corporal al día).
Por lo que respecta a los compuestos de cadena larga, los efectos
observados en ratas hembras con las posologías más bajas son hepatitis
granulomatosa multifocal y aumento del peso del hígado (nivel más bajo
de efecto nocivo observado de 100 mg/kg de peso corporal al día).
En el único estudio sobre reproducción descrito, no se informó de
efectos reproductivos adversos después de la exposición de las ratas a
una PC de cadena media con 52% de cloro. Con todo, la supervivencia y
los pesos corporales de los hijuelos expuestos se redujo (el nivel más
bajo de efecto observado para una disminución no significativa del
peso corporal fue de 5,7 a 7,2 mg/kg de peso corporal por día; el
nivel más bajo de efecto nocivo observado para menor supervivencia
estuvo entre 60 y 70 mg/kg de peso corporal por día). En un número
limitado de estudios sobre los efectos de las PC de cadena corta,
media y larga sobre el desarrollo de las camadas de las ratas, se
registraron efectos adversos sólo en el caso de los compuestos de
cadena corta, con dosis tóxicas para las madres (2000 mg/kg de peso
corporal por día). En cuanto a los compuestos de cadena media y
larga, no se observaron efectos en la prole, incluso con posologías
muy elevadas (1000 a 5000 mg/kg de peso corporal por día).
Las parafinas cloradas no parecen inducir mutaciones en las
bacterias. Sin embargo, en las células de los mamíferos hay indicios
de un bajo potencial clastógeno in vitro pero no in vivo. También
se tiene noticia de que las PC provocan transformación celular in
vitro.
Se han llevado a cabo estudios de carcinogenicidad a largo plazo
con ratas y ratones alimentados por sonda empleando una PC de cadena
corta (C12; 58% de Cl) y una PC de cadena larga (C23; 43% de Cl). En
el caso de los ratones B6C3F1 expuestos al compuesto de cadena corta,
se registraron aumentos en la incidencia de tumores hepáticos en los
machos y las hembras, así como tumores de la glándula tiroides en las
hembras. En las ratas Fischer-344 expuestas al compuesto de cadena
corta, se observó un mayor número de tumores hepáticos en los machos y
las hembras, tumores renales (adenomas o adenocarcinomas) en los
machos, tumores de la tiroides en las hembras y leucemias de las
células mononucleares en los machos. Por lo que respecta a las PC de
cadena larga, se vio aumentada la incidencia de linfomas malignos en
ratones machos y de tumores de la glándula adrenal en las ratas
hembra.
7. Efectos en el ser humano
A pesar del uso muy difundido de las parafinas cloradas, no hay
informes de casos de irritación o de sensibilización dérmica. Esto se
ve corroborado por los resultados de un número limitado de estudios
realizados con voluntarios en los que las PC provocaron mínima
irritación dérmica, pero no sensibilización.
No se dispone de datos sobre otros efectos de las PC en el ser
humano.
8. Efectos en otros organismos en laboratorio y sobre el terreno
Se ha demostrado que las parafinas cloradas de cadena corta son
sumamente tóxicas para los invertebrados acuáticos, tanto de agua
dulce como de mar, oscilando los valores de CL50-CE50 entre 14 y
530 µg/litro. La mayoría de las pruebas de toxicidad aguda para los
invertebrados acuáticos con parafinas cloradas de cadena media y larga
son superiores a la solubilidad en agua. Sin embargo, en un estudio
realizado con crustáceos Daphnia con una PC de cadena media se
observó toxicidad aguda con una CE50 de 37 µg/litro. En el caso de
los peces, las PC de cadena corta, media y larga parecen tener poca
toxicidad aguda, con valores de CL50 muy superiores a la solubilidad
en agua.
Las parafinas cloradas de cadena corta presentan toxicidad a
largo plazo a las algas, los invertebrados acuáticos y los peces
con concentraciones tan bajas como 19,6, 8,9 y 3,1 µg/litro,
respectivamente; en cuanto a las especies más sensibles estudiadas,
las concentraciones sin efecto observado parecen oscilar entre 2 y
5 µg/litro. Un producto de cadena media y otro de cadena larga
tuvieron efectos crónicos en crustáceos Daphnia con concentraciones
de 20 a 35 µg/litro y de < 1,2 a 8 µg/litro, respectivamente. La
toxicidad a largo plazo parece ser baja en el caso de los peces. No
se cuenta con datos sobre las algas.
Atendiendo a los limitados datos disponibles, la toxicidad aguda
de las PC en las aves es baja.
9. Evaluación de los riesgos para la salud de los seres humanos y de
los efectos sobre el medio ambiente
Los alimentos son probablemente la fuente principal de exposición
de la población general. Sobre la base de los datos limitados sobre
las concentraciones presentes en los alimentos, las estimaciones más
desfavorables del consumo diario en los productos lácteos y mejillones
son de 4 y 25 µg/kg peso corporal por día, respectivamente. En
general, se calcula que la cantidad de PC ingeridas diariamente es
inferior al límite tolerable por lo que respecta a los efectos no
neoplásicos, o a los valores recomendados en cuanto a los efectos
neoplásicos (compuestos de cadena corta).
Siempre que se sigan procedimientos adecuados de higiene y
seguridad personal, cabe esperar que el riesgo para la salud de los
trabajadores expuestos a las PC sea mínimo.
La información disponible indica que las parafinas cloradas
son bioacumulativas y persistentes. Los datos sobre los niveles
ambientales de las de cadena corta indican que en áreas cercanas a las
fuentes de descarga existe riesgo para los organismos, tanto de agua
dulce como estuarinos. Asimismo, las de cadena media y larga pueden
presentar riesgos para los invertebrados acuáticos.
El enriquecimiento de las parafinas cloradas en los sedimentos,
sus posibilidades de reabsorción y su toxicidad en medio acuático son
indicios de posible riesgo para los organismos que habitan en los
sedimentos.
 The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the
sole responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLORINATED
PARAFFINS
Members
Professor U.G. Ahlborg, Institute of Environmental Medicine,
Karolinska Institute, Stockholm, Sweden (Vice-Chairman)
Dr D. Anderson, British Industry Biological Research Association
(BIBRA) Toxicology International, Carshalton, Surrey, United
Kingdom
Dr T. Beulshausen, Federal Environment Agency, Berlin, Germany
Dr R.S. Chhabra, Environmental Toxicology Program, National Institute
of Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Dr N. Gregg, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, Cambridgeshire, United Kingdom (Joint Rapporteur)
Dr B. Jansson, Institute of Applied Environmental Research, Stockholm
University, Solna, Sweden
Dr K. Kenne, Institute of Environmental Medicine, Karolinska
Institute, Stockholm, Sweden (Joint Rapporteur)
Dr M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Chairman)
Representatives of other Organizations
Dr P. Montuschi, Institute of Pharmacology, Faculty of Medicine and
Surgery, Catholic University of the Sacred Heart, Rome, Italy
(Representing the International Union of Pharmacology)
Mr D. Farrar, Occupational Health, ICI Chemicals and Polymers Limited,
Runcorn, Cheshire, United Kingdom
(Representing the European Centre for Ecotoxicology and Toxicology
of Chemicals)
Secretariat
Dr E.M. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr J.D. Wilbourn, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR CHLORINATED PARAFFINS
A WHO Task Group on Environmental Health Criteria for Chlorinated
Paraffins met at the World Health Organization, Geneva, from 20 to 24
March 1995. Dr E.M. Smith, IPCS, welcomed the participants on behalf
of Dr M. Mercier, Director of the IPCS, and on behalf the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft and made an evaluation of the risks for human health
and the environment from exposure to chlorinated paraffins.
The first draft was prepared at the Institute of Environmental
Medicine, Karolinska Institute, Stockholm, Sweden, by Dr K. Kenne and
Professor U.G. Ahlborg. The second draft, incorporating comments
received following circulation of the first drafts to the IPCS contact
points for Environmental Health Criteria monographs, was also prepared
by Dr Kenne and Professor Ahlborg.
Dr E.M. Smith and Dr P. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific aspects of the monograph and for
the technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
APDM aminopyrine demethylase
BCF bioconcentration factor
CD coulometric detection
CP chlorinated paraffins
CP-LH chlorinated paraffin with long chain length and high degree
of chlorination
CP-LL chlorinated paraffin with long chain length and low degree of
chlorination
CP-MH chlorinated paraffin with medium chain length and high degree
of chlorination
CP-ML chlorinated paraffin with medium chain length and low degree
of chlorination
CP-SH chlorinated paraffin with short chain length and high degree
of chlorination
CP-SL chlorinated paraffin with short chain length and low degree
of chlorination
EC50 median effective concentration
ECD electron capture detection
GC gas chromatography
LC50 median lethal concentration
LOAEL lowest-observed-adverse-effect level
LOEC lowest-observed-effect concentration
LOEL lowest-observed-effect level
LT50 median lethal time
MS mass spectrometry
NCI negative ion chemical ionization
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
PCB polychlorinated biphenyl
PVC polyvinyl chloride
TDI tolerable daily intake
TLC thin-layer chromatography
TSH thyroid stimulating hormone
UDP uridine diphosphate
1. SUMMARY
1.1 Properties, uses and analytical methods
Chlorinated paraffins (CPs) are produced by chlorination of
straight-chained paraffin fractions. The carbon chain length of
commercial chlorinated paraffins is usually between 10 and 30 carbon
atoms, and the chlorine content is usually between 40 and 70% by
weight. Chlorinated paraffins are viscous colourless or yellowish
dense oils with low vapour pressures, except for those of long carbon
chain length with high chlorine content (70%), which are solid.
Chlorinated paraffins are practically insoluble in water, lower
alcohols, glycerol and glycols, but are soluble in chlorinated
solvents, aromatic hydrocarbons, ketones, esters, ethers, mineral oils
and some cutting oils. They are moderately soluble in unchlorinated
aliphatic hydrocarbons.
Chlorinated paraffins consist of extremely complex mixtures,
owing to the many possible positions for the chlorine atoms. The
products can be subdivided into six groups depending on chain length
(short C10-13, intermediate C14-17 and long C18-30) and degree of
chlorination (low (< 50%) and high (> 50%)).
Chlorinated paraffins are used worldwide in widespread
applications such as plasticizers in plastics (e.g., PVC), extreme
pressure additives in metal working fluids, flame retardants and
additives in paints. Technical grade chlorinated paraffins may be
contaminated by isoparaffins, aromatic compounds and metals, and
normally contain stabilizers, which are added to inhibit
decomposition.
The analysis of chlorinated paraffins is difficult due to the
extreme complexity of these mixtures. In environmental samples, this
is further complicated by interference from other compounds. Analyses
often require extensive clean-up of the samples and the use of
specific detection methods. Early methods were based on thin-layer
chromatography for the clean-up and an unspecific argentation
detection method on the plates. Methods based on different column
liquid chromatography are currently used for the clean-up, although it
is difficult to isolate the chlorinated paraffins due to their wide
range of physical properties. Specific detection methods are
therefore used; gas chromatography combined with mass spectrometry is
now the most common technique. The use of negative ions makes the
detection even more specific. Although use of these sophisticated
techniques has improved the ability to analyse chlorinated paraffins,
it is still impossible to determine exact concentrations. Reported
results should be regarded only as estimates of the true values.
1.2 Sources of human and environmental exposure
Chlorinated paraffins are not known to occur naturally.
Chlorinated paraffins are produced by reacting liquid paraffin
fractions with pure chlorine gas. The reaction may require the use of
a solvent, and often ultraviolet light is used as a catalyst. In
1985, the estimated world production of chlorinated paraffins was
300 000 tonnes.
The widespread uses of chlorinated paraffins probably provide the
major source of environmental contamination. Chlorinated paraffins
may be released into the environment from improperly disposed
metal-working fluids containing chlorinated paraffins or from polymers
containing chlorinated paraffins. Loss of chlorinated paraffins by
leaching from paints and coatings may also contribute to environmental
contamination. The potential for loss during production and transport
is expected to be less than that during product use and disposal.
Owing to their thermal instability, chlorinated paraffins are
expected to be degraded by incineration and thus would not be expected
to volatilize in exhaust gases from incinerators. However, it has
been demonstrated that chlorinated aromatic compounds such as
polychlorinated biphenyls, naphthalenes and benzenes are formed by
pyrolysis of chlorinated paraffins under certain conditions.
1.3 Environmental distribution and transformation
Chlorinated paraffins adsorb strongly to sediment. In water they
are probably transported adsorbed on suspended particles, and in the
atmosphere adsorbed to airborne particulates (and possibly in the
vapour phase). The half-lives for chlorinated paraffins in air have
been estimated to range from 0.85 to 7.2 days, a period sufficiently
long that the possibility of long-range transport cannot be excluded.
Chlorinated paraffins are not readily biodegradable. Short
carbon chain length chlorinated paraffins with a chlorine content of
less than 50% appear to be degradable under aerobic conditions with
acclimated microorganisms, whereas the degradation appears inhibited
at a chlorine content above 58%. Intermediate and long chain length
chlorinated paraffins are degraded more slowly.
Chlorinated paraffins are bioaccumulated in aquatic organisms,
and the reported bioconcentration factors (BCFs) are in the range of 7
to 7155 for fish and 223 to 138 000 for mussels. In fish, chlorinated
paraffins of short chain length are accumulated to a higher degree
than intermediate and long chain length chlorinated paraffins.
Radioactivity has been found mainly in bile, intestine, liver, fat and
gills after administration of radiolabelled chlorinated paraffins. The
uptake of chlorinated paraffins seems to be more efficient for short
chlorinated paraffins with low chlorine content; the elimination rate
is slowest for short chlorinated paraffins with high chlorine content.
The retention in fat-rich tissues appears to increase with increasing
degree of chlorination.
1.4 Environmental levels and human exposure
Few data on levels of chlorinated paraffins in the environment
are available. Chlorinated paraffins have been detected in marine
water samples in the United Kingdom at levels below 4 µg/litre. In
non-marine waters, levels below 6 µg/litre in the United Kingdom have
been reported; in Germany, concentrations determined in 1994 were in
the range of 0.08-0.28 µg/litre. In water in the USA, concentrations
were generally less than 0.03 µg/litre, although levels were above 1.0
µg/litre in a small proportion (1.2%) of samples. In marine sediments,
levels up to 600 µg/kg wet weight have been reported, and in
non-marine sediments in the United Kingdom concentrations were up to
15 000 µg/kg in industrialized regions and 1000 µg/kg in areas
remote from industry. In sediments in an impoundment lagoon from a
chlorinated paraffin manufacturing plant in the USA, concentrations as
high as 170 000 µg/kg dry weight of long chain length chlorinated
paraffins, 50 000 µg/kg of intermediate chain length chlorinated
paraffins and 40 000 µg/kg of short chain length chlorinated paraffins
were reported. In Germany, levels up to 83 µg/kg dry weight of C10-13
and up to 370 µg/kg dry weight of C14-17 were reported in sediments in
1994. In Japan, levels in sediment ranged up to 8500 µg/kg.
Chlorinated paraffins have been detected in various organisms.
Chlorinated paraffins are present in terrestrial mammals in Sweden at
concentrations in the range of 32-88 µg/kg tissue (140-4400 µg/kg
lipid). However, chlorinated paraffins were not detected in sheep
which were grazed remote from production of chlorinated paraffins in
the United Kingdom. In birds in the United Kingdom, concentrations
ranged up to 1500 µg/kg and in fish in Sweden and the United Kingdom,
levels ranged up to 200 µg/kg. In mussels collected in the USA and
United Kingdom, concentrations up to 400 µg/kg were reported.
However, levels of C10-20 in mussels collected close to a chlorinated
paraffin plant effluent discharge ranged up to 12 000 µg/kg.
Chlorinated paraffins have also been detected in post mortem human
tissues, i.e. in adipose tissue (median level of 100-190 µg/kg),
kidney (median level below 90 µg/kg) and liver (median level below 90
µg/kg). In one limited survey, chlorinated paraffins, mostly C10-20,
were present at levels of up to 500 µg/kg in approximately 70% of the
samples of various food products.
Information on occupational exposure to chlorinated paraffins is
limited. Very low levels of exposure to aerosols of short chain
chlorinated paraffins (0.003-1.2 mg/m3) have been found to be
associated with their use as metal-working fluids, although there is
no information available on the proportion that is inhalable. On the
basis of mathematical modelling of exposure without any control
measures, high levels of dermal contact (5-15 mg/cm2 per day) were
estimated for speciality metal-working fluids which contain very high
levels of short chain chlorinated paraffins, although absorption would
be expected to be low. Control measures would reduce dermal exposure.
1.5 Kinetics and metabolism
The toxicokinetics of chlorinated paraffins have been studied
in experimental animals. Adequate information for humans is not
available. Possible differences in toxicokinetics as a result of
different chain lengths have not been sufficiently investigated.
Although the extent of absorption of chlorinated paraffins after oral
administration is unknown, it appears to decrease with increasing
chain length and degree of chlorination. Percutaneous absorption may
also occur depending on chain length, but would be limited (less than
1% of a topical C18 dose). No data on absorption via the lung is
available.
Distribution of chlorinated paraffins occurs mainly in the liver,
kidney, intestine, bone marrow, adipose tissue and ovary. Information
on retention is insufficient but a low degree of chlorination may
enhance retention time due to slower redistribution. Chlorinated
paraffins or their metabolites are present in the central nervous
system up to 30 days after administration. They may cross the
blood-placental barrier. There is no adequate information on the
pathways of metabolism of chlorinated paraffins, although in
radiolabelling studies CO2 has been identified as an end-product.
Chlorinated paraffins may be excreted via the renal, biliary and
the pulmonary routes (as CO2). The relative extent of excretion
via the different routes is difficult to establish due to the
wide variability in different studies. The total elimination of
chlorinated paraffins decreases as the chlorine content increases, and
compounds with high degrees of chlorination are mainly excreted (more
than 50%) as CO2. Chlorinated paraffins may be excreted in milk.
1.6 Effects on laboratory mammals and in vitro test systems
The acute oral toxicity of chlorinated paraffins of various chain
lengths is low. Toxic effects such as muscular incoordination and
piloerection were most evident following single exposure to short
chain length chlorinated paraffins. On the basis of very limited
data, the acute toxicity by the inhalation and dermal routes also
appears to be low. Mild skin and eye irritation has been observed
after application of short and intermediate (skin irritation)
chain length chlorinated paraffins. Results of several studies
indicate that short chain chlorinated paraffins do not induce skin
sensitization.
In repeated dose toxicity studies by the oral route, the liver,
kidney and thyroid are the primary target organs for the toxicity of
the chlorinated paraffins. For the short chain compounds, increases
in liver weight have been observed at lowest doses (lowest-observed-
effect level is 50 to 100 mg/kg body weight per day and no-observed-
effect level is 10 mg/kg body weight per day in rats). At higher
doses, increases in the activity of hepatic enzymes, proliferation
of smooth endoplasmic reticulum and peroxisomes, replicative DNA
synthesis, hypertrophy, hyperplasia and necrosis of the liver have
also been observed. Decreases in body weight gain (125 mg/kg body
weight per day in mice), increases in kidney weight (100 mg/kg body
weight per day in rats), replicative DNA synthesis in renal cells
(313 mg/kg body weight per day) and nephrosis (625 mg/kg body weight
per day in rats) have also been observed. Increases in thyroid
weight, and hypertrophy and hyperplasia of the thyroid (LOEL of
100 mg/kg body weight per day in rats) and replicative DNA synthesis
in thyroid follicular cells (LOEL of 313 mg/kg body weight per day)
have been reported. At higher doses (1000 mg/kg body weight per day),
thyroid function is affected, as determined by free and total levels
of plasma thyroxine and increased plasma thyroid-stimulating hormone
in rats.
For the intermediate chain compounds, effects observed at lowest
doses are generally increases in liver and kidney weight (LOEL in rats
of 100 mg/kg body weight per day; NOAEL in rats of 10 mg/kg body
weight per day). Increases in serum cholesterol and "mild, adaptive"
histological changes in the thyroid have been reported at similar
doses in female rats (NOAEL of 4 mg/kg body weight per day).
For the long chain compounds, effects observed at lowest doses
are multifocal granulomatous hepatitis and increased liver weights in
female rats (LOAEL of 100 mg/kg body weight per day).
In the only identified reproduction study, no adverse
reproductive effects were reported following exposure of rats to an
intermediate chain length chlorinated paraffin with 52% chlorine.
However, survival and body weights of the exposed pups were reduced
(LOEL for non-significant decrease in body weight of 5.7-7.2 mg/kg
body weight per day; LOAEL for decreased survival of 60-70 mg/kg body
weight per day). In a limited number of studies of the developmental
effects of the short, medium and long chain chlorinated paraffins,
adverse effects in the offspring were observed for the short chain
compounds only, at maternally toxic doses in rats (2000 mg/kg body
weight per day). For the medium and long chain compounds, no effects
on the offspring were observed even at very high doses (1000 to
5000 mg/kg body weight per day).
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells, there is a suggestion of a
weak clastogenic potential in vitro but not in vivo. Chlorinated
paraffins are also reported to induce cell transformation in vitro.
Long term carcinogenicity studies by oral gavage in rats and mice
have been conducted on a short chain chlorinated paraffin (C12;
58% Cl) and a long chain chlorinated paraffin (C23; 43% Cl). For
the short chain compound in B6C3F1 mice, there were increases in
the incidence of hepatic tumours in males and females and tumours of
the thyroid gland in females. In Fischer-344 rats exposed to the short
chain compound, there were increases in hepatic tumours in males and
females, renal tumours (adenomas or adenocarcinomas) in males, tumours
of the thyroid in females and mononuclear cell leukaemias in males.
For the long chain chlorinated paraffin, the incidences of malignant
lymphomas in male mice and tumours of the adrenal gland in female rats
were increased.
1.7 Effects on humans
In spite of the widespread use of chlorinated paraffins, there
are no case reports of skin irritation or sensitization. This is
supported by results of a limited number of studies in volunteers in
which chlorinated paraffins have induced minimal irritancy in the
skin, but not sensitization.
Data on other effects of chlorinated paraffins in humans have not
been identified.
1.8 Effects on other organisms in the laboratory and field
Chlorinated paraffins of short chain length have been shown to
be acutely toxic to freshwater and saltwater invertebrates, with
LC50-EC50 values ranging from 14 to 530 µg/litre. Most of the acute
toxicity tests on aquatic invertebrates for intermediate and long
chain chlorinated paraffins exceed the water solubility. However, a
study on an intermediate chlorinated paraffin product shows acute
toxicity to daphnids at an EC50 of 37 µg/litre. Short, intermediate
and long chain chlorinated paraffins appear to be of low acute
toxicity to fish, with LC50 values well in excess of the water
solubility.
Short chain length chlorinated paraffins show long-term toxicity
to algae, aquatic invertebrates and fish at concentrations as low
as 19.6, 8.9 and 3.1 µg/litre, respectively; no-observed-effect
concentrations appear to be in the range of 2 to 5 µg/litre for the
most sensitive species tested. An intermediate and a long chain
product showed chronic effects on daphnids at concentrations of 20 to
35 µg/litre and < 1.2 to 8 µg/litre, respectively. Long-term
toxicity to fish seems to be low. No data are available on algae.
On the basis of limited available data, the acute toxicity of
chlorinated paraffins in birds is low.
1.9 Evaluation of human health risks and effects on the environment
It is likely that the principal source of exposure of the general
population is food. On the basis of limited data on concentrations
present in foodstuffs, worst case estimates of daily intake in dairy
products and mussels, respectively, are 4 and 25 µg/kg body weight
per day. In general, the calculated daily intakes of chlorinated
paraffins are below the tolerable intakes for non-neoplastic effects
or recommended values for neoplastic effects (short chain compounds).
Provided that proper personal hygiene and safety procedures are
followed, the risk to health for workers exposed to chlorinated
paraffins is expected to be minimal.
Available data indicate that chlorinated paraffins are
bioaccumulative and persistent. The data on environmental levels of
short chain chlorinated paraffins indicate that in areas close to
release sources there is a risk to both freshwater and estuarine
organisms. There is also a potential risk to aquatic invertebrates
from intermediate and long chain chlorinated paraffin products.
The enrichment of chlorinated paraffins in sediments, their
resorption behaviour and aquatic toxicity indicate a potential risk
for sediment-dwelling organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chlorinated paraffins (CPs) are produced by chlorination of
normal paraffin fractions (straight-chain hydrocarbons, at least 98%
linear), and have the general formula CxH(2x-y+2)Cly. The length of
the carbon chains is usually between 10 and 30 carbon atoms, and the
chlorine content is between 20 and 70% by weight, although the
commercial products normally fall within the 40-70% Cl range
(Schenker, 1979). In this monograph the different isomers will be
referred to as Cx;y% Cl, i.e., a chlorinated paraffin with a carbon
chain length of 12 and a chlorination degree of 60% will be referred
to as C12;60% Cl.
Commercial chlorinated paraffins, of which there are over 200,
are very complex mixtures of n-alkanes characterized by an average
carbon chain length and chlorination degree. Each grade varies in the
range of carbon chain length, but also in the distribution and degree
of chlorination. The different technical grades have therefore
specific physical and chemical properties which render them useful in
such widespread applications as plasticizers in plastics such as
polyvinyl chloride, extreme pressure additives, flame retardants and
paints.
The number of theoretically possible structures within the ranges
C10-C30 and 40-70% Cl is enormous. Taking C12 and 60% Cl as an
example, there are numerous possibilities, depending on the position
of the chlorine atoms. In just one of these structures (Fig. 1),
there are 25=32 different diastereomers, owing to the five optical
sites (indicated by an asterisk).
The raw materials most frequently used for the production of
chlorinated paraffins are normal paraffin feedstocks, which fall into
three main categories:
1) a liquid fraction including C10-C13 with an average of C12;
2) a liquid fraction including C14-C17 with an average of C15; and
3) a wax fraction including C20-C28 with an average of C24 (Strack,
1986).
A wax fraction including C18-C20 is also used. Depending on
the feedstock and the degree of chlorination, long chain length
chlorinated paraffins (C18-30) range from being mobile to very viscous
liquids, with the exception of the C20-30;70% Cl type, which is a
solid.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the
sole responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLORINATED
PARAFFINS
Members
Professor U.G. Ahlborg, Institute of Environmental Medicine,
Karolinska Institute, Stockholm, Sweden (Vice-Chairman)
Dr D. Anderson, British Industry Biological Research Association
(BIBRA) Toxicology International, Carshalton, Surrey, United
Kingdom
Dr T. Beulshausen, Federal Environment Agency, Berlin, Germany
Dr R.S. Chhabra, Environmental Toxicology Program, National Institute
of Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Dr N. Gregg, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, Cambridgeshire, United Kingdom (Joint Rapporteur)
Dr B. Jansson, Institute of Applied Environmental Research, Stockholm
University, Solna, Sweden
Dr K. Kenne, Institute of Environmental Medicine, Karolinska
Institute, Stockholm, Sweden (Joint Rapporteur)
Dr M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Chairman)
Representatives of other Organizations
Dr P. Montuschi, Institute of Pharmacology, Faculty of Medicine and
Surgery, Catholic University of the Sacred Heart, Rome, Italy
(Representing the International Union of Pharmacology)
Mr D. Farrar, Occupational Health, ICI Chemicals and Polymers Limited,
Runcorn, Cheshire, United Kingdom
(Representing the European Centre for Ecotoxicology and Toxicology
of Chemicals)
Secretariat
Dr E.M. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr J.D. Wilbourn, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR CHLORINATED PARAFFINS
A WHO Task Group on Environmental Health Criteria for Chlorinated
Paraffins met at the World Health Organization, Geneva, from 20 to 24
March 1995. Dr E.M. Smith, IPCS, welcomed the participants on behalf
of Dr M. Mercier, Director of the IPCS, and on behalf the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft and made an evaluation of the risks for human health
and the environment from exposure to chlorinated paraffins.
The first draft was prepared at the Institute of Environmental
Medicine, Karolinska Institute, Stockholm, Sweden, by Dr K. Kenne and
Professor U.G. Ahlborg. The second draft, incorporating comments
received following circulation of the first drafts to the IPCS contact
points for Environmental Health Criteria monographs, was also prepared
by Dr Kenne and Professor Ahlborg.
Dr E.M. Smith and Dr P. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific aspects of the monograph and for
the technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
APDM aminopyrine demethylase
BCF bioconcentration factor
CD coulometric detection
CP chlorinated paraffins
CP-LH chlorinated paraffin with long chain length and high degree
of chlorination
CP-LL chlorinated paraffin with long chain length and low degree of
chlorination
CP-MH chlorinated paraffin with medium chain length and high degree
of chlorination
CP-ML chlorinated paraffin with medium chain length and low degree
of chlorination
CP-SH chlorinated paraffin with short chain length and high degree
of chlorination
CP-SL chlorinated paraffin with short chain length and low degree
of chlorination
EC50 median effective concentration
ECD electron capture detection
GC gas chromatography
LC50 median lethal concentration
LOAEL lowest-observed-adverse-effect level
LOEC lowest-observed-effect concentration
LOEL lowest-observed-effect level
LT50 median lethal time
MS mass spectrometry
NCI negative ion chemical ionization
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
PCB polychlorinated biphenyl
PVC polyvinyl chloride
TDI tolerable daily intake
TLC thin-layer chromatography
TSH thyroid stimulating hormone
UDP uridine diphosphate
1. SUMMARY
1.1 Properties, uses and analytical methods
Chlorinated paraffins (CPs) are produced by chlorination of
straight-chained paraffin fractions. The carbon chain length of
commercial chlorinated paraffins is usually between 10 and 30 carbon
atoms, and the chlorine content is usually between 40 and 70% by
weight. Chlorinated paraffins are viscous colourless or yellowish
dense oils with low vapour pressures, except for those of long carbon
chain length with high chlorine content (70%), which are solid.
Chlorinated paraffins are practically insoluble in water, lower
alcohols, glycerol and glycols, but are soluble in chlorinated
solvents, aromatic hydrocarbons, ketones, esters, ethers, mineral oils
and some cutting oils. They are moderately soluble in unchlorinated
aliphatic hydrocarbons.
Chlorinated paraffins consist of extremely complex mixtures,
owing to the many possible positions for the chlorine atoms. The
products can be subdivided into six groups depending on chain length
(short C10-13, intermediate C14-17 and long C18-30) and degree of
chlorination (low (< 50%) and high (> 50%)).
Chlorinated paraffins are used worldwide in widespread
applications such as plasticizers in plastics (e.g., PVC), extreme
pressure additives in metal working fluids, flame retardants and
additives in paints. Technical grade chlorinated paraffins may be
contaminated by isoparaffins, aromatic compounds and metals, and
normally contain stabilizers, which are added to inhibit
decomposition.
The analysis of chlorinated paraffins is difficult due to the
extreme complexity of these mixtures. In environmental samples, this
is further complicated by interference from other compounds. Analyses
often require extensive clean-up of the samples and the use of
specific detection methods. Early methods were based on thin-layer
chromatography for the clean-up and an unspecific argentation
detection method on the plates. Methods based on different column
liquid chromatography are currently used for the clean-up, although it
is difficult to isolate the chlorinated paraffins due to their wide
range of physical properties. Specific detection methods are
therefore used; gas chromatography combined with mass spectrometry is
now the most common technique. The use of negative ions makes the
detection even more specific. Although use of these sophisticated
techniques has improved the ability to analyse chlorinated paraffins,
it is still impossible to determine exact concentrations. Reported
results should be regarded only as estimates of the true values.
1.2 Sources of human and environmental exposure
Chlorinated paraffins are not known to occur naturally.
Chlorinated paraffins are produced by reacting liquid paraffin
fractions with pure chlorine gas. The reaction may require the use of
a solvent, and often ultraviolet light is used as a catalyst. In
1985, the estimated world production of chlorinated paraffins was
300 000 tonnes.
The widespread uses of chlorinated paraffins probably provide the
major source of environmental contamination. Chlorinated paraffins
may be released into the environment from improperly disposed
metal-working fluids containing chlorinated paraffins or from polymers
containing chlorinated paraffins. Loss of chlorinated paraffins by
leaching from paints and coatings may also contribute to environmental
contamination. The potential for loss during production and transport
is expected to be less than that during product use and disposal.
Owing to their thermal instability, chlorinated paraffins are
expected to be degraded by incineration and thus would not be expected
to volatilize in exhaust gases from incinerators. However, it has
been demonstrated that chlorinated aromatic compounds such as
polychlorinated biphenyls, naphthalenes and benzenes are formed by
pyrolysis of chlorinated paraffins under certain conditions.
1.3 Environmental distribution and transformation
Chlorinated paraffins adsorb strongly to sediment. In water they
are probably transported adsorbed on suspended particles, and in the
atmosphere adsorbed to airborne particulates (and possibly in the
vapour phase). The half-lives for chlorinated paraffins in air have
been estimated to range from 0.85 to 7.2 days, a period sufficiently
long that the possibility of long-range transport cannot be excluded.
Chlorinated paraffins are not readily biodegradable. Short
carbon chain length chlorinated paraffins with a chlorine content of
less than 50% appear to be degradable under aerobic conditions with
acclimated microorganisms, whereas the degradation appears inhibited
at a chlorine content above 58%. Intermediate and long chain length
chlorinated paraffins are degraded more slowly.
Chlorinated paraffins are bioaccumulated in aquatic organisms,
and the reported bioconcentration factors (BCFs) are in the range of 7
to 7155 for fish and 223 to 138 000 for mussels. In fish, chlorinated
paraffins of short chain length are accumulated to a higher degree
than intermediate and long chain length chlorinated paraffins.
Radioactivity has been found mainly in bile, intestine, liver, fat and
gills after administration of radiolabelled chlorinated paraffins. The
uptake of chlorinated paraffins seems to be more efficient for short
chlorinated paraffins with low chlorine content; the elimination rate
is slowest for short chlorinated paraffins with high chlorine content.
The retention in fat-rich tissues appears to increase with increasing
degree of chlorination.
1.4 Environmental levels and human exposure
Few data on levels of chlorinated paraffins in the environment
are available. Chlorinated paraffins have been detected in marine
water samples in the United Kingdom at levels below 4 µg/litre. In
non-marine waters, levels below 6 µg/litre in the United Kingdom have
been reported; in Germany, concentrations determined in 1994 were in
the range of 0.08-0.28 µg/litre. In water in the USA, concentrations
were generally less than 0.03 µg/litre, although levels were above 1.0
µg/litre in a small proportion (1.2%) of samples. In marine sediments,
levels up to 600 µg/kg wet weight have been reported, and in
non-marine sediments in the United Kingdom concentrations were up to
15 000 µg/kg in industrialized regions and 1000 µg/kg in areas
remote from industry. In sediments in an impoundment lagoon from a
chlorinated paraffin manufacturing plant in the USA, concentrations as
high as 170 000 µg/kg dry weight of long chain length chlorinated
paraffins, 50 000 µg/kg of intermediate chain length chlorinated
paraffins and 40 000 µg/kg of short chain length chlorinated paraffins
were reported. In Germany, levels up to 83 µg/kg dry weight of C10-13
and up to 370 µg/kg dry weight of C14-17 were reported in sediments in
1994. In Japan, levels in sediment ranged up to 8500 µg/kg.
Chlorinated paraffins have been detected in various organisms.
Chlorinated paraffins are present in terrestrial mammals in Sweden at
concentrations in the range of 32-88 µg/kg tissue (140-4400 µg/kg
lipid). However, chlorinated paraffins were not detected in sheep
which were grazed remote from production of chlorinated paraffins in
the United Kingdom. In birds in the United Kingdom, concentrations
ranged up to 1500 µg/kg and in fish in Sweden and the United Kingdom,
levels ranged up to 200 µg/kg. In mussels collected in the USA and
United Kingdom, concentrations up to 400 µg/kg were reported.
However, levels of C10-20 in mussels collected close to a chlorinated
paraffin plant effluent discharge ranged up to 12 000 µg/kg.
Chlorinated paraffins have also been detected in post mortem human
tissues, i.e. in adipose tissue (median level of 100-190 µg/kg),
kidney (median level below 90 µg/kg) and liver (median level below 90
µg/kg). In one limited survey, chlorinated paraffins, mostly C10-20,
were present at levels of up to 500 µg/kg in approximately 70% of the
samples of various food products.
Information on occupational exposure to chlorinated paraffins is
limited. Very low levels of exposure to aerosols of short chain
chlorinated paraffins (0.003-1.2 mg/m3) have been found to be
associated with their use as metal-working fluids, although there is
no information available on the proportion that is inhalable. On the
basis of mathematical modelling of exposure without any control
measures, high levels of dermal contact (5-15 mg/cm2 per day) were
estimated for speciality metal-working fluids which contain very high
levels of short chain chlorinated paraffins, although absorption would
be expected to be low. Control measures would reduce dermal exposure.
1.5 Kinetics and metabolism
The toxicokinetics of chlorinated paraffins have been studied
in experimental animals. Adequate information for humans is not
available. Possible differences in toxicokinetics as a result of
different chain lengths have not been sufficiently investigated.
Although the extent of absorption of chlorinated paraffins after oral
administration is unknown, it appears to decrease with increasing
chain length and degree of chlorination. Percutaneous absorption may
also occur depending on chain length, but would be limited (less than
1% of a topical C18 dose). No data on absorption via the lung is
available.
Distribution of chlorinated paraffins occurs mainly in the liver,
kidney, intestine, bone marrow, adipose tissue and ovary. Information
on retention is insufficient but a low degree of chlorination may
enhance retention time due to slower redistribution. Chlorinated
paraffins or their metabolites are present in the central nervous
system up to 30 days after administration. They may cross the
blood-placental barrier. There is no adequate information on the
pathways of metabolism of chlorinated paraffins, although in
radiolabelling studies CO2 has been identified as an end-product.
Chlorinated paraffins may be excreted via the renal, biliary and
the pulmonary routes (as CO2). The relative extent of excretion
via the different routes is difficult to establish due to the
wide variability in different studies. The total elimination of
chlorinated paraffins decreases as the chlorine content increases, and
compounds with high degrees of chlorination are mainly excreted (more
than 50%) as CO2. Chlorinated paraffins may be excreted in milk.
1.6 Effects on laboratory mammals and in vitro test systems
The acute oral toxicity of chlorinated paraffins of various chain
lengths is low. Toxic effects such as muscular incoordination and
piloerection were most evident following single exposure to short
chain length chlorinated paraffins. On the basis of very limited
data, the acute toxicity by the inhalation and dermal routes also
appears to be low. Mild skin and eye irritation has been observed
after application of short and intermediate (skin irritation)
chain length chlorinated paraffins. Results of several studies
indicate that short chain chlorinated paraffins do not induce skin
sensitization.
In repeated dose toxicity studies by the oral route, the liver,
kidney and thyroid are the primary target organs for the toxicity of
the chlorinated paraffins. For the short chain compounds, increases
in liver weight have been observed at lowest doses (lowest-observed-
effect level is 50 to 100 mg/kg body weight per day and no-observed-
effect level is 10 mg/kg body weight per day in rats). At higher
doses, increases in the activity of hepatic enzymes, proliferation
of smooth endoplasmic reticulum and peroxisomes, replicative DNA
synthesis, hypertrophy, hyperplasia and necrosis of the liver have
also been observed. Decreases in body weight gain (125 mg/kg body
weight per day in mice), increases in kidney weight (100 mg/kg body
weight per day in rats), replicative DNA synthesis in renal cells
(313 mg/kg body weight per day) and nephrosis (625 mg/kg body weight
per day in rats) have also been observed. Increases in thyroid
weight, and hypertrophy and hyperplasia of the thyroid (LOEL of
100 mg/kg body weight per day in rats) and replicative DNA synthesis
in thyroid follicular cells (LOEL of 313 mg/kg body weight per day)
have been reported. At higher doses (1000 mg/kg body weight per day),
thyroid function is affected, as determined by free and total levels
of plasma thyroxine and increased plasma thyroid-stimulating hormone
in rats.
For the intermediate chain compounds, effects observed at lowest
doses are generally increases in liver and kidney weight (LOEL in rats
of 100 mg/kg body weight per day; NOAEL in rats of 10 mg/kg body
weight per day). Increases in serum cholesterol and "mild, adaptive"
histological changes in the thyroid have been reported at similar
doses in female rats (NOAEL of 4 mg/kg body weight per day).
For the long chain compounds, effects observed at lowest doses
are multifocal granulomatous hepatitis and increased liver weights in
female rats (LOAEL of 100 mg/kg body weight per day).
In the only identified reproduction study, no adverse
reproductive effects were reported following exposure of rats to an
intermediate chain length chlorinated paraffin with 52% chlorine.
However, survival and body weights of the exposed pups were reduced
(LOEL for non-significant decrease in body weight of 5.7-7.2 mg/kg
body weight per day; LOAEL for decreased survival of 60-70 mg/kg body
weight per day). In a limited number of studies of the developmental
effects of the short, medium and long chain chlorinated paraffins,
adverse effects in the offspring were observed for the short chain
compounds only, at maternally toxic doses in rats (2000 mg/kg body
weight per day). For the medium and long chain compounds, no effects
on the offspring were observed even at very high doses (1000 to
5000 mg/kg body weight per day).
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells, there is a suggestion of a
weak clastogenic potential in vitro but not in vivo. Chlorinated
paraffins are also reported to induce cell transformation in vitro.
Long term carcinogenicity studies by oral gavage in rats and mice
have been conducted on a short chain chlorinated paraffin (C12;
58% Cl) and a long chain chlorinated paraffin (C23; 43% Cl). For
the short chain compound in B6C3F1 mice, there were increases in
the incidence of hepatic tumours in males and females and tumours of
the thyroid gland in females. In Fischer-344 rats exposed to the short
chain compound, there were increases in hepatic tumours in males and
females, renal tumours (adenomas or adenocarcinomas) in males, tumours
of the thyroid in females and mononuclear cell leukaemias in males.
For the long chain chlorinated paraffin, the incidences of malignant
lymphomas in male mice and tumours of the adrenal gland in female rats
were increased.
1.7 Effects on humans
In spite of the widespread use of chlorinated paraffins, there
are no case reports of skin irritation or sensitization. This is
supported by results of a limited number of studies in volunteers in
which chlorinated paraffins have induced minimal irritancy in the
skin, but not sensitization.
Data on other effects of chlorinated paraffins in humans have not
been identified.
1.8 Effects on other organisms in the laboratory and field
Chlorinated paraffins of short chain length have been shown to
be acutely toxic to freshwater and saltwater invertebrates, with
LC50-EC50 values ranging from 14 to 530 µg/litre. Most of the acute
toxicity tests on aquatic invertebrates for intermediate and long
chain chlorinated paraffins exceed the water solubility. However, a
study on an intermediate chlorinated paraffin product shows acute
toxicity to daphnids at an EC50 of 37 µg/litre. Short, intermediate
and long chain chlorinated paraffins appear to be of low acute
toxicity to fish, with LC50 values well in excess of the water
solubility.
Short chain length chlorinated paraffins show long-term toxicity
to algae, aquatic invertebrates and fish at concentrations as low
as 19.6, 8.9 and 3.1 µg/litre, respectively; no-observed-effect
concentrations appear to be in the range of 2 to 5 µg/litre for the
most sensitive species tested. An intermediate and a long chain
product showed chronic effects on daphnids at concentrations of 20 to
35 µg/litre and < 1.2 to 8 µg/litre, respectively. Long-term
toxicity to fish seems to be low. No data are available on algae.
On the basis of limited available data, the acute toxicity of
chlorinated paraffins in birds is low.
1.9 Evaluation of human health risks and effects on the environment
It is likely that the principal source of exposure of the general
population is food. On the basis of limited data on concentrations
present in foodstuffs, worst case estimates of daily intake in dairy
products and mussels, respectively, are 4 and 25 µg/kg body weight
per day. In general, the calculated daily intakes of chlorinated
paraffins are below the tolerable intakes for non-neoplastic effects
or recommended values for neoplastic effects (short chain compounds).
Provided that proper personal hygiene and safety procedures are
followed, the risk to health for workers exposed to chlorinated
paraffins is expected to be minimal.
Available data indicate that chlorinated paraffins are
bioaccumulative and persistent. The data on environmental levels of
short chain chlorinated paraffins indicate that in areas close to
release sources there is a risk to both freshwater and estuarine
organisms. There is also a potential risk to aquatic invertebrates
from intermediate and long chain chlorinated paraffin products.
The enrichment of chlorinated paraffins in sediments, their
resorption behaviour and aquatic toxicity indicate a potential risk
for sediment-dwelling organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chlorinated paraffins (CPs) are produced by chlorination of
normal paraffin fractions (straight-chain hydrocarbons, at least 98%
linear), and have the general formula CxH(2x-y+2)Cly. The length of
the carbon chains is usually between 10 and 30 carbon atoms, and the
chlorine content is between 20 and 70% by weight, although the
commercial products normally fall within the 40-70% Cl range
(Schenker, 1979). In this monograph the different isomers will be
referred to as Cx;y% Cl, i.e., a chlorinated paraffin with a carbon
chain length of 12 and a chlorination degree of 60% will be referred
to as C12;60% Cl.
Commercial chlorinated paraffins, of which there are over 200,
are very complex mixtures of n-alkanes characterized by an average
carbon chain length and chlorination degree. Each grade varies in the
range of carbon chain length, but also in the distribution and degree
of chlorination. The different technical grades have therefore
specific physical and chemical properties which render them useful in
such widespread applications as plasticizers in plastics such as
polyvinyl chloride, extreme pressure additives, flame retardants and
paints.
The number of theoretically possible structures within the ranges
C10-C30 and 40-70% Cl is enormous. Taking C12 and 60% Cl as an
example, there are numerous possibilities, depending on the position
of the chlorine atoms. In just one of these structures (Fig. 1),
there are 25=32 different diastereomers, owing to the five optical
sites (indicated by an asterisk).
The raw materials most frequently used for the production of
chlorinated paraffins are normal paraffin feedstocks, which fall into
three main categories:
1) a liquid fraction including C10-C13 with an average of C12;
2) a liquid fraction including C14-C17 with an average of C15; and
3) a wax fraction including C20-C28 with an average of C24 (Strack,
1986).
A wax fraction including C18-C20 is also used. Depending on
the feedstock and the degree of chlorination, long chain length
chlorinated paraffins (C18-30) range from being mobile to very viscous
liquids, with the exception of the C20-30;70% Cl type, which is a
solid.
 In general chlorinated paraffins are classified as short chain
(C10-13), intermediate chain (C14-17) and long chain (C18-30). These
groups are further divided into two classes according to chlorine
content: < 50% and > 50% chlorine. A suggested classification of
the different chlorinated paraffin isomers is shown in Table 1. The
suggested acronyms are used in this monograph.
2.1.1 Relative molecular mass
The relative molecular mass depends on the carbon chain length
and the degree of chlorination. The chlorinated paraffin C10;50.6%
Cl has a relative molecular mass of 280.1, whereas that of C25;69% Cl
is 1075.
2.1.2 Common names
2.1.2.1 CAS registry number and names
In general chlorinated paraffins are classified as short chain
(C10-13), intermediate chain (C14-17) and long chain (C18-30). These
groups are further divided into two classes according to chlorine
content: < 50% and > 50% chlorine. A suggested classification of
the different chlorinated paraffin isomers is shown in Table 1. The
suggested acronyms are used in this monograph.
2.1.1 Relative molecular mass
The relative molecular mass depends on the carbon chain length
and the degree of chlorination. The chlorinated paraffin C10;50.6%
Cl has a relative molecular mass of 280.1, whereas that of C25;69% Cl
is 1075.
2.1.2 Common names
2.1.2.1 CAS registry number and names
 2.1.2.2 Synonyms
Alkanes, chlorinated; alkanes (C10-12), chloro (60%); alkanes (C10-13),
chloro (50-70%); alkanes (C14-17), chloro (40-52%); alkanes (C18-28),
chloro (20-50%); alkanes (C22-26), chloro (43%); C12, 60% chlorine;
C23, 43% chlorine; chlorinated alkanes; chlorinated hydrocarbon
waxes; chlorinated paraffin waxes; chlorinated waxes; chloroalkanes;
chlorocarbons; chloroparaffin waxes; paraffin, chlorinated; paraffins,
chloro; paraffin waxes, chlorinated; paroils, chlorinated; poly-
chlorinated alkanes; polychloro alkanes.
2.1.3 Technical products
Chlorinated paraffins are manufactured commercially by a number
of companies and are marketed under a variety of trade names. The
trade names are followed by numbers, which often are related to the
average chlorine content (in percent) of a particular preparation.
However, this is not a rule and the average chlorine content may have
to be obtained from manufacturers' technical data. More than 200
chlorinated paraffin formulations are commercially available
world-wide (Serrone et al., 1987), and some examples of these are
given in Table 2.
The carbon chain length of the chlorinated paraffins in a
commercial mixture is variable, and the average chain length is
usually specified by the manufacturer. The composition of paraffins
of different chain length in some commercial formulations is shown in
Table 3. The paraffin feedstocks are randomly chlorinated and the
resulting chlorine contents are given as average values.
Commercial chlorinated paraffins may be contaminated by
isoparaffins (usually less than 1%), aromatic compounds (usually less
than 0.1% (1000 ppm)) and metals (Schenker, 1979).
Chlorinated paraffins normally contain stabilizers, which are
added to inhibit decomposition. Common stabilizers include epoxidized
compounds such as epoxidized esters and soya bean oils (indicated in
section 7.1.3 to be present at up to 3%), pentaerythritol, thymol,
urea, glycidyl ethers, acetonitriles and organic phosphites (Schenker,
1979; Strack, 1986; Houghton, 1993). The concentration of stabilizers
is usually below 0.05% w/w (Campbell & McConnell, 1980).
Table 2. Partial list of commercial chlorinated paraffinsa
Average molecular formula C12H15Cl11 C12H19Cl7 C15H26Cl6 C24H29Cl21 C24H42Cl8 C24H44Cl6
Chlorine content (% w/w) 70 60-65 50-52 70 48-54 40-42
Manufacturers:
Oxychem, USA Chlorowax 70L Chlorowax 500C Chlorowax 70 Chlorowax 50 Chlorowax 40
Keil Chemical Div., USA CW-200-70 CW-85-60 CW-52 CW-220-50 CW-170
Dover Chemical Corp., Paroil 170HV Paroil 160 Paroil 152 Chlorez 700 Paroil 150S Paroil 140
USA Paroil 1048
Plastifax, Inc., USAb Plastichlor P-70 Plastichlor P-59 Plastichlor 50-220 Plastichlor
P-65 42-170
ICI, Australia; Canada; Cereclor 70L Cereclor 60L Cereclor S52 Cereclor 70 Cereclor 48 Cereclor 42
UK; France
Neville Chemical Co., Unichlor 60L-60 Unichlor 50L-65 Unichlor 50-450 Unichlor 40-170
USAb Unichlor 40-150
Pearsall Chemical Co., FLX-0012 FLX-0008 CPF-0020 CPF-0004
USA CPF-0003 CPF-0001
Hüls AG, Germanyb Chlorparaffin Chlorparaffin Chlorparaffin Chlorparaffin
70C 60C 52G 40N
Dynamit Nobel, Germanyb Witaclor 171 Witaclor 160 - Witaclor 350 Witaclor 549 Witaclor 540
Witaclor 163 Witaclor 352
Caffaro, Italy Cloparin D70 Cloparin 1059 Cloparin 50 Cloparin S70 Cloparin P42
Table 2. (Cont'd)
Average molecular formula C12H15Cl11 C12H19Cl7 C15H26Cl6 C24H29Cl21 C24H42Cl8 C24H44Cl6
Chlorine content (% w/w) 70 60-65 50-52 70 48-54 40-42
Hoechst AG, Germany Chlorparaffin Hordaflex LC60 Chlorparaffin Chlorparaffin Chlorparaffin
Hoechst 70 Hoechst 52fl Hoechst 70fest Hoechst 40fl
Rhône-Poulenc, France Alaiflex 67B2 Ribeclor 60B2 Alaiflex 50A3 Alaiflex 40A8
a Other producers include Bann Quimica (Brazil), Excel Industry (India), Ajinomoto (Japan), Tosoh (Japan), Asahi Denka (Japan),
Plasticlor (Mexico), NCP (South Africa)
b These companies have ceased production of chlorinated paraffins.
Table 3. Composition of paraffins obtained by dechlorination of
different chlorinated paraffin preparations (Zitko, 1974b)
Chlorinated Percentage of each paraffin
paraffin
C21 C22 C23 C24 C25 C26 C27 C28
Chloroparaffin, 4.5 10.0 15.7 19.3 18.5 15.3 9.8 6.7
40%
Clorafin 40 3.7 8.2 14.0 17.5 19.2 17.4 12.4 7.6
CP 40 3.9 9.1 14.9 19.2 19.8 18.0 15.1 -
Cereclor 42 3.6 8.8 14.7 18.6 19.5 17.2 11.5 6.0
Chloroparaffin, 7.4 14.9 20.7 23.1 19.9 14.0 - -
50%
2.2 Chemical and physical properties
Chlorinated paraffins are viscous, colourless or yellowish, dense
oils, except for the chlorinated paraffins of long carbon chain length
(C20-C30) with high chlorine content (70%), which are solid.
Chlorinated paraffins have a characteristic slight and not unpleasant
odour (Hardie, 1964). The odour is probably due to small quantities
of products of lower relative molecular mass with small but measurable
vapour pressures (Howard et al., 1975). Chlorinated paraffins
themselves have very low vapour pressures. The medium chain length
C14-17;52% Cl has a vapour pressure of approximately 2 × 10-4 Pa at
20°C (1-2 × 10-6 mmHg) (Campbell & McConnell, 1980), and the long
chain length C23;42-54% Cl approximately 3 × 10-3 Pa when measured at
65°C (2 × 10-5 mmHg) (Hardie, 1964). The chemical and physical
properties of chlorinated paraffins are determined by the carbon chain
length of the paraffin and the chlorine content. Increases in the
carbon chain length and chlorination degree of a particular paraffin
increase the viscosity and density but reduce the volatility.
Chlorinated paraffins are practically insoluble in water, but
many products can be emulsified with water (approximately 70/30
chlorinated paraffin to water). The water solubility of 14C-labelled
polychloroundecane (C11;59% Cl) is reported to be 150-470 µg/litre,
polychloropentadecane (C15;51% Cl) 5-27 µg/litre and the poly-
chloropentacosanes (C25;43% Cl) < 5-6.4 µg/litre and (C25;70%
Cl) < 5-5.9 µg/litre, depending on analytical method (Madeley &
Gillings, 1983). Campbell & McConnell (1980) reported the solubility
of C16;52% Cl to be 10 µg/litre in freshwater and 4 µg/litre in
seawater. The solubility of C25;42% Cl was reported to be 3 µg/litre
in seawater. Chlorinated paraffins are also practically insoluble in
lower alcohols, glycerol and glycols, but are soluble in chlorinated
solvents, aromatic hydrocarbons, ketones, esters, ethers, mineral oil
and some cutting oils. They are moderately soluble in unchlorinated
aliphatic hydrocarbons (Houghton, 1993). Some physical properties of
typical commercial chlorinated paraffins are summarized in Table 4.
Assuming a water solubility of 5 µg/litre and a vapour pressure
of 2 × 10-4 Pa as typical of a 52% chlorinated intermediate chain
length paraffin, a Henry's Law constant of 10.9 may be calculated
(Willis et al., 1994).
A key property of chlorinated paraffins, particularly the high
chlorine grades, is their nonflammability. This is due to the ability
of chlorinated paraffins to release hydrochloric acid at elevated
temperatures, and the hydrochloric acid inhibits the radical reaction
in a flame. This property is considerably enhanced by the addition of
antimony trioxide (Houghton, 1993) or other additives. Chlorinated
paraffins are generally unreactive and stable in normal temperatures,
but decompose significantly at temperatures above 300°C with the
release of hydrochloric acid (Strack, 1986). Prolonged exposure
to light can also cause dehydrochlorination. Degradation by
dehydrochlorination can be accelerated at elevated temperatures in
the presence of aluminium, zinc, and iron oxide or chloride (Howard
et al., 1975; Houghton, 1993). Dehydrochlorination leads to darkening
of the material.
2.3 Analysis
The analysis of chlorinated paraffins is very difficult owing to
the many congeners present in the products. The properties of these
congeners cover wide ranges, which makes it difficult to separate the
chlorinated paraffins from other compounds that may interfere in the
analysis.
Table 4. Physical properties of selected commercial chlorinated paraffinsa
Paraffin Chlorine Colour hazen Viscosityb Densityb Thermal stabilityc Volatilityd Refractive Log Powe
feedstock content (APHA) (Pa.s) (g/ml) (% w/w HCl) (% w/w) index
% (w/w)
C10-C13 50 100 0.08 1.19 0.15 16.0 1.493 4.39-6.93
56 100 0.8 1.30 0.15 7.0 1.508 NRg
60 125 3.5 1.36 0.15 4.4 1.516 4.48-7.38
63 125 11.0 1.41 0.15 3.2 1.522 5.47-7.30
65 150 30.0 1.44 0.20 2.5 1.525 NR
70 200 8.0f 1.50 0.20 0.5 1.537 5.68-8.01h
C14-C17 40 80 0.07 1.10 0.2 4.2 1.488 NR
45 80 0.2 1.16 0.2 2.8 1.498 5.52-8.21
52 100 1.6 1.25 0.2 1.4 1.508 5.47-8.01
58 150 40.0 1.36 0.2 0.7 1.522 NR
Wax C18-C20 47 150 1.7 1.21 0.2 0.8 1.506 NR
50 250 18.0 1.27 0.2 0.7 1.512 NR
Wax (C> 20) 42 250 2.5 1.16 0.2 0.4 1.506 9.29->12.83h
48 300 28.0 1.26 0.2 0.3 1.516 8.69-12.83
70 100i j 1.63 0.2 NR - NR
a Data from Houghton (1993) f At 50°C
b At 25°C unless otherwise noted g NR = not reported
c Measured in a standard test for 4 h at 175°C h Data from Cereclor 42
d Measured in a standard test for 4 h at 180°C i 10 g in 100 ml toluene solvent
e Octanol:water partition coefficients. From: Renberg et al (1980) j Solid, softening point = 95-100°C
2.3.1 Sampling
To prevent contamination by trace amounts of chlorinated
paraffins, samples or their extracts must not be allowed to come into
contact with any plastic (especially PVC) container, stopper, cap
liner or tubing, because these may contain chlorinated paraffins
(Hollies et al., 1979). All solvents should be rigorously tested
before use, and it is recommended that glass distilled solvents are
used. All glassware should be decontaminated before use by heating at
250°C for 24 h. Water and sediment samples should be stored at
ambient temperatures, and should be analysed within a month of
sampling.
Treatment of samples for the extraction of chlorinated paraffins
is described in Table 5.
2.3.2 Analytical methods
Methods used for detection of chlorinated paraffins in various
samples are shown in Table 5.
Hollies et al. (1979) determined C13-17 and C20-30 chlorinated
paraffin after clean-up of the samples on aluminium oxide columns.
The chlorinated paraffin fraction was then applied on a silica gel
thin-layer chromatography (TLC) plate. After forward elution with
n-hexane and subsequently with toluene, and backward elution with
n-hexane, chloride from the chlorinated aliphatics was transferred
to an aluminium oxide plate at 240°C and developed with silver
nitrate. The resulting spots were quantified by visual comparison
with spots of known amounts of reference materials. Although the
procedure is complicated and involves several evaporations to dryness,
good recoveries were reported. Possible interference from a number of
other chlorinated compounds was investigated and found to be
negligible, but the method must still be regarded as fairly
non-specific.
Gas chromatographic analysis of chlorinated paraffin, using
microcoulometric detection, has been described by Zitko (1973). This
method gives badly resolved chromatograms and there is a considerable
risk of interference from other halogenated compounds. Owing to high
temperatures in the gas chromatographic system there is also a risk of
dehydrochlorination of the chlorinated paraffin congeners. In a later
study (Zitko & Arsenault, 1977), interference from other compounds was
avoided by a solvent partitioning clean-up procedure.
Table 5. Analytical methods for the determination of chlorinated paraffins in various samplesa
Sample Preparation method Analytical Sample detection Recovery Reference
matrix methodb limitb
Water Extract with petroleum spirit; concentrate; purify by TLC 500 ng/litre 90% Hollies et
aluminium oxide chromatography, elute with toluene; al. (1979)
dry; dissolve in petroleum spirit
Water Extract with hexane; purify by aluminium oxide GC/ECD 3 ng/litre NR Kraemer &
chromatography; elute with hexane/dichloromethane Ballschmiter
(4%); purify by silica gel chromatography, elute with (1987)
hexane:dichloromethane (19:1); dissolve in isooctane.
Water Extraction with hexane (particle phase Soxhlet GC/ approx. 92-120% Steele et
extracted), silica gel and aluminium oxide column MS-NCI 1 µg/litre al. (1988)
chromatography
Biological Homogenize; extract with petroleum spirit:acetone TLC 50 µg/kg 80-90% Hollies et
material (2:1); dry; dissolve in petroleum spirit; extract with al. (1979)
dimethylformamide; wash; back-extract with Na2SO3
solution and petroleum spirit; purify by silica gel
chromatography, elute with CCl4; dry; dissolve in
acetone; extract with petroleum spirit:acetone (1:4)
Cod muscle Homogenize in n-hexane:acetone (1:2.5, v:v); extract GC/MS NR 98-114% at Jansson et
tissue with 10% diethyl ether in n-hexane; evaporate; dissolve 0.465 µg/sample al. (1991)
in dichloromethane:n-hexane (1:1, v/v); purify by gel and 89-92% at
permeation chromatography; concentrate; extract with 2.33 µg/sample
sulfuric acid; concentrated in organic phase
Adipose Homogenize in dichloromethane; percolate through GC/MS 5 ng 80% Schmid &
tissue anhydrous Na2SO4; remove solvent; dissolve residue in Müller (1985)
pentane; wash, dry and concentrate; purify by alumina
chromatography
Table 5. (Cont'd)
Sample Preparation method Analytical Sample detection Recovery Reference
matrix methodb limitb
Mineral oil Extract fish in cyclohexane; introduce extract or MS-NCI NR NR Gjos &
and fish mineral oil sample directly into mass spectrometer Gustavsen
extract (1982)
Fish fillets Homogenize in petroleum ether; clean-up by irradiating GC/CD NR > 90% Friedman &
extracts with high-intensity UV light (90 min, < 20°C) Lombardo
in petroleum ether (1975)
Sewage Homogenize in acetone; extract with pentane; wash, dry GC/MS 5 ng NR Schmid &
sludge and concentrate; purify by alumina chromatography Müller (1985)
Sediment Dry at 70°C; extract with petroleum spirit; TLC 50 µg/kg 80% Hollies et
concentrate; purify by aluminium oxide al. (1979)
chromatography, elute with toluene; dry; dissolve
in petroleum spirit
Sediment Extract with acetone:hexane (1:1, v:v); wash, dry GC/MS 5 ng NR Schmid &
and concentrate; purify by alumina chromatography Müller (1985)
Sediment Soxhlet extraction with hexane, silica gel and GC/ approx. 52-64% Steele et
aluminium oxide column chromatography MS-NCI 1 µg/litre al. (1988)
a Modified from IARC (1990)
b GC/MS = gas chromatography/mass spectrometry; GC/CD = gas chromatography/coulometric detection;
GC/ECD = gas chromatography/electron capture detection; MS-NCI = negative-ion chemical ionization mass spectrometry;
TLC = thin-layer chromatography; NR = not reported
Attempts have been made to reduce the complexity of chlorinated
paraffin mixtures by reductive dechlorination (Cooke & Roberts, 1980;
Roberts et al., 1981; Sistovaris & Donges, 1987). This method gives
information on the "carbon skeleton" of the chlorinated paraffin
compounds but no information on the chlorine content, and it is
difficult to separate the response from that of unchlorinated
hydrocarbons.
Negative ion chemical ionization mass spectrometry (MS-NCI) was
used by Gjös & Gustavsen (1982). In this method the chlorinated
paraffin fractions are introduced directly into the ion source of the
mass spectrometer. As the whole sample is analysed in a very short
time, the concentration in the ion source is high and the sensitivity
can therefore be high. A serious disadvantage is that all other
compounds in the sample come into the mass spectrometer at the same
time, the risk of interference is high and an extensive clean-up of
the samples is needed.
Gas chromatography utilizing MS-NCI for the detection was used by
Schmid & Müller (1985). A fairly simple clean-up based on adsorption
chromatography on aluminium oxide was used, but unfortunately this
has been impossible to reproduce (Jansson, personal communication).
GC/MS-NCI was also used by Steele et al. (1988) to determine
chlorinated paraffins after clean-up of samples on silica gel and
aluminium oxide columns. They used low inlet temperatures in the
gas chromatograph to avoid thermolysis of the analysed compounds.
The use of low temperatures and short capillary columns further
decreases the risk of temperature-related break-down of chlorinated
paraffins during gas chromatographic analysis (Jansson et al., 1991).
In this method a gel permeation column was also used to avoid
interference from other chlorinated compounds, and the Cl2- and
HCl2- ions were used to detect aliphatic chlorinated compounds
selectively.
Developments in chlorinated paraffin analysis have improved
both selectivity and sensitivity. However, although the reliability
of results is now better, these are only estimates of the real
concentrations as it is impossible to detect the individual substances.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Chlorinated paraffins are not known to occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Liquid chlorinated paraffins were first used in large amounts
during the period 1914-1918 as solvents for Dichloramine T in
antiseptic nasal and throat sprays (Howard et al., 1975). The
commercial production of chlorinated paraffins for use as extreme
pressure additives in lubricants started around 1930 (Schenker, 1979).
Estimated data on the production of chlorinated paraffins are
shown in Table 6. Chlorinated paraffins are produced in Australia,
Brazil, Bulgaria, Canada, China, Germany, France, India, Italy, Japan,
Mexico, Poland, Romania, Spain, Slovakia, South Africa, China
(Province of Taiwan), Thailand, the United Kingdom, the USA, and the
former USSR. However, this may not be a complete list of producer
countries. It is believed that 50% of the chlorinated paraffins
produced in the world have carbon chain lengths of between 14 and 17
and a chlorine content of between 45 and 52%. In the United Kingdom
approximately 80% of the total production of chlorinated paraffins is
concentrated on the C14-17 chain length. About 15% of the European
consumption of chlorinated paraffins is estimated to be C10-13, 70%
C14-17 and 15% C20-30 (Willis et al., 1994).
Chlorinated paraffins are produced by reacting liquid paraffin
fractions obtained from petroleum distillation with pure chlorine gas
by a reaction mechanism involving free radicals (Schenker, 1979;
Houghton, 1993). The reaction is exothermic. At a chlorine content
above approximately 54% further chlorination is slow and difficult.
In the production of resinous chlorinated paraffins containing
> 70% Cl, a solvent is usually added to decrease the viscosity
(Howard et al., 1975). Carbon tetrachloride has been the most
commonly used solvent, and may be present in trace amounts in the
final product, although alternative production methods are being
developed because of the phase-out of carbon tetrachloride under the
Montreal Protocol (D. Farrer, personal communication to the IPCS,
1995).
The substituted chlorine atoms are probably randomly distributed,
and at a chlorination of 72% all of the carbon atoms are singly
chlorinated. Further chlorination is difficult since the first
chlorine substitution decreases the reactivity of the other hydrogens
on a particular carbon atom (Hardie, 1964).
Table 6. Estimated production of chlorinated paraffins
Production References
(tonnes/year)
Canada - 1990 2900 Camford Information
Services (1991)
Germany - 1990/1991 20 000-30 000 BUA (1992)
United Kingdom - 1992 50 000 Willis et al. (1994)
USA - 1977 37 000 Schenker (1979)
USA - 1983 45 000 NTP (1986a)
USA - 1987 45 000 IARC (1990)
USA - 1990 26 000 US EPA (1993)
North America - 1978 60 000 Zitko (1980)
Western Europe - 1978 105 000 Zitko (1980)
Western Europe - 1985 95 000 IARC (1990)
World, excluding 230 000 Campbell &
Eastern Europe - 1977 McConnell (1980)
World - 1985a 300 000 Strack (1986)
a Excluding the former Soviet Union and the People's Republic of China.
Depending on producer and paraffin feedstock, the temperature of
the chlorination reaction is usually kept at 80-100°C to decrease the
viscosity but at a temperature where the decomposition of the product
is not extensive (Schenker, 1979; Houghton, 1993). Since the reaction
is exothermic heat removal is important in the process. Ultraviolet
light is often used as a catalyst (Schenker, 1979; Houghton, 1993).
Metal catalysts are avoided since they may promote dechlorination of
the chlorinated paraffins. Since for each tonne of chlorinated
paraffin produced, approximately half a tonne of hydrochloric acid is
generated, the linings of the reactor vessels must be chemically inert
to avoid the formation of metal chlorides, which cause darkening of
the product by decomposition (Strack, 1986; Houghton, 1993).
Additional procedures used in the production of the C20-30;70% Cl solid
grade are stripping of the solvent and grinding of solid products
(Schenker, 1979).
3.2.2 Uses
Chlorinated paraffins are used as secondary plasticizers for
polyvinyl chloride (PVC) and can partially replace primary
plasticizers such as phthalates and phosphate esters (Houghton, 1993).
The use of chlorinated paraffins has the advantage in comparison with
conventional plasticizers of both increasing the flexibility of
the material as well as increasing its flame retardancy and
low-temperature strength (Howard et al., 1975). Chlorinated paraffins
are also used as extreme pressure additives in metal-machining fluids
or as metal-working lubricants or cutting oils because of their
viscous nature, compatibility with oils, and property of releasing
hydrochloric acid at elevated temperatures. The hydrochloric acid
reacts with metal surfaces to form a thin but strong solid film of
metal chloride lubricant. In Sweden, the use of chlorinated paraffins
in metal-working fluids has been reduced from 680 tonnes (1986) to
139 tonnes (1993) as a part of a risk reduction programme (Swedish
Environmental Protection Agency, 1994). They are added to paints,
coatings and sealants to improve resistance to water and chemicals,
which is most suitable when they are used in marine paints, as
coatings for industrial flooring, vessels and swimming pools (e.g.,
rubber and chlorinated rubber coatings), and as road marking paints.
The flame-retarding properties of highly chlorinated paraffins make
them important as additives in plastics, fabrics, paints and coatings.
The most effective fire-retardant action is obtained with a high
degree of chlorination.
By the late 1970s approximately 50% of chlorinated paraffins in
the USA was used as extreme pressure lubricant additives in the
metal-working industry; 25% was used in plastics and fire-retardant
and water-repellant fabric treatments, and the rest was used in paint,
rubber, caulks and sealants (Schenker, 1979). In the United Kingdom,
65-70% of the consumed chlorinated paraffins is used as a secondary
plasticizer in PVC, about 10% in paint, about 10% in metal-cutting
lubricants and about 10% in flame retardants and sealants (Willis et
al., 1994). In Canada approximately 55% of the chlorinated paraffins
is used as plasticizers and 35-40% as high-pressure lubricant
additives (Camford Information Services, 1991). Some examples of
applications for chlorinated paraffins of different chain-lengths are
shown in Table 7.
3.2.3 Loss into the environment
Since chlorinated paraffins are produced without contact with
water, the possibility of leakage into the environment by direct water
discharge is low. After chlorination the solvent is removed and
residual amounts of chlorine gas and hydrogen chloride are removed by
blowing air or other gases through the product. This could possibly
lead to some loss into the air, but since the chlorine gas and
hydrochloric acid are recovered and the volatility of chlorinated
paraffins is very low, the loss is likely to be very low (Howard et
al., 1975). Emission into the atmosphere during manufacture in
Germany in 1990 was estimated to be about 250 kg/year (BUA, 1992). It
is possible that chlorinated paraffins may be a by-product during
chlorination of other hydrocarbon feedstocks if paraffins are present
as contaminants. This could lead to possible environmental
contamination.
Table 7. Uses of various chlorinated paraffins
Paraffin Chlorination (%) Application
feedstock
C10-13 plasticizer for PVC or plastics
C10-13 metal-working fluids; sealants
C10-13 approx. 70 flame retardants for rubber and soft plastics
C14-17 40-60 extreme pressure additives to metal-machining
fluids, pastes, emulsions and lubricants
C14-17 45-52 the chlorinated paraffin most frequently used as a
(40-50) plasticizer for plastics; also used for sealants
C18-30 approx. 70 flame retardants for rigid plastics such
as polyesters and polystyrene
C> 20 plasticizer for PVC or plastics
Some loss into the environment could be expected during transport
and storage. If the drums which are used for the transport of
chlorinated paraffins are cleaned for further use environmental
release might occur. Soil could be contaminated if empty drums are
dumped at landfills. Spills may occur, but clean-up using an
adsorbent material is easy. The adsorbent material would probably
be deposited in a landfill, which in turn could lead to possible
environmental contamination.
The uses of chlorinated paraffins probably provide the major
source of environmental contamination. When chlorinated paraffins are
used as plasticizers or additives in coatings, they are effectively
dissolved in the polymers and will therefore leak into the environment
only very slowly. However, polymers containing chlorinated paraffins
will act as sources of chlorinated paraffins for centuries after
disposal. A more likely route of leakage of chlorinated paraffins
into the environment would be the improper disposal of oils containing
chlorinated paraffins (Campbell & McConnell, 1980) or disposal of
chlorinated paraffins of low quality (Howard et al., 1975). Loss of
chlorinated paraffins by removal from paints and coatings may also
contribute to environmental contamination.
It is estimated that a maximum of 55% of the cutting and
lubricating oils sold to the engineering industry in Sweden becomes
waste. The rest is consumed or released into the air and water (KEMI,
1991). The largest consumer of chlorinated paraffins in Sweden
(1400 tonnes/year) has estimated its emission of chlorinated paraffins
to be 90 kg/year (0.06 g emission/kg chlorinated paraffin produced)
(KEMI, 1991).
Disposal of wastes containing chlorinated paraffins occurs
through resource recovery, destructive incineration or landfill,
usually on disposal sites for special wastes and in compliance with
local regulations. Owing to their thermal instability, chlorinated
paraffins are expected to be degraded by incineration at low
temperatures and thus would not be expected to volatilize in exhaust
gases from an incinerator. However, in a study by Bergman et al.
(1984), chlorinated aromatic compounds such as PCBs, naphthalenes and
benzenes were formed by pyrolysis of chlorinated paraffins (see
section 4.2.1) although the conditions used were not identical to the
operation conditions of waste incineration plants. Chlorinated
paraffins are not expected to be formed de novo. The disposal of
chlorinated paraffins in landfills may give rise to leaching into
water, but owing to the low water solubility and strong adsorption
onto solids the amounts reaching water are likely to be low.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Considering the low vapour pressure (2 × 10-4 Pa at 20°C for
C14-17;52% Cl to 3 × 10-3 Pa at 65°C for C23;42-54% Cl), low water
solubility (3 to 470 µg/litre) and highly lipophilic nature of
chlorinated paraffins (log Pow values range from 4.39 to > 12.83),
it is likely that they will distribute mainly to the soil/sediment
phase with very little volatilization occurring. Chlorinated
paraffins are likely to be transported in water as suspended
particles, and in air as dust particles and possibly in the vapour
phase. However, no experimental data on this subject have been
reported.
4.2 Transformation
4.2.1 Abiotic transformation
No experimental data on the chemical stability of chlorinated
paraffins under simulated environmental conditions have been reported.
However, their chemical reactivities suggest that they do not
hydrolyse, oxidize or react by other mechanisms at significant rates
under normal temperatures and neutral conditions (Howard et al.,
1975). Dehydrochlorination of chlorinated paraffins may possibly
occur naturally in the presence of metal ion contamination.
Because of the high adsorption tendency of chlorinated paraffins,
gas phase reactions are assumed to contribute only little to
degradation in the atmosphere (BUA, 1992). However, calculated
half-lives for chlorinated paraffins in air are reported to range from
0.85 to 7.2 days (Slooff et al., 1992). The theoretical values are
shown in Table 8.
The thermal degradation by pyrolysis of chlorinated paraffins at
various temperatures (300, 500, 700°C) and times (10 sec to 20 min)
was studied by Bergman et al. (1984). The chlorinated paraffins were
totally degraded, and, depending upon degree of chlorination of the
chlorinated paraffin, several aliphatic and aromatic degradation
products, such as polychlorinated biphenyls (PCBs), naphthalenes and
benzenes, were detected. As much as 10 g of PCBs per kg chlorinated
paraffin could be found after thermal degradation of C12;70% Cl
(temperature not specified). Smaller amounts of compounds were formed
at lower temperatures. Considering these results, processes where
chlorinated paraffins are subjected to temperatures above 300°C could
lead to environmental contamination and exposure to more persistent
and toxic substances than the original chlorinated paraffins.
Table 8. Photochemical degradation of chlorinated paraffins in the
atmosphere (From: Slooff et al., 1992)
Carbon chain length Koh (cm3/mol per sec) Half-life (days)
C10-C13 9.0-14.9 × 10-12 1.2-1.8
C14-C17 14.9-18.9 × 10-12 0.85-0.8
C15-C30 20.2-31.1 × 10-12 0.5-0.8
not specified 2.2-18.8 × 10-12 0.85-7.2
4.2.2 Biodegradation
Chlorinated paraffins are generally stable in the natural
environment.
4.2.2.1 Short chain length chlorinated paraffins
A short chain length paraffin (C10-12) with 58% chlorination
(CP-SH) was not readily biodegraded by activated sludge, under either
aerobic or anaerobic conditions, over a 28-day period in an inherent
biodegradability (modified Zahn-Wellens) test (Street & Madeley,
1983a,b) or a 51-day period in a coupled units test (Mather et al.,
1983).
4.2.2.2 Long chain length chlorinated paraffins
Zitko & Arsenault (1977) studied sediment spiked with 596 mg/kg
dry weight of Cereclor 42 C24;42% Cl, CP-LL) or 357 mg/kg dry weight
of Chlorez 700 (C24;70% Cl, CP-LH), which are both long chain length
chlorinated paraffins but have different chlorine contents. There was
no clear trend in the results but they indicated that after 4 weeks
the highly chlorinated Chlorez 700 was degraded to a greater extent
than Cereclor 42. The rate of degradation seems to have been higher
under anaerobic conditions.
4.2.2.3 Comparative studies
In another study, the microbial degradation of several short,
intermediate and long chain length chlorinated paraffins of different
chlorination degree at concentrations of 2, 10 and 20 mg/litre was
examined in a 25-day biochemical oxygen demand (BOD) test (Madeley &
Birtley, 1980). The degradation rate appeared to decrease with
increasing carbon chain length and chlorination degree, and the short
chain chlorinated paraffins with less than 50% Cl were degraded most
rapidly and completely. It can be concluded from the results that
chlorinated paraffins with low chlorine contents (< 50% wt Cl) and,
especially, short chain chlorinated paraffins, biodegrade slowly in
the environment, particularly in the presence of adapted microbial
populations, but that chlorinated paraffins with higher chlorine
contents are unlikely to biodegrade under aerobic conditions.
Anaerobic microorganisms did not degrade Cereclor 42 (C24;42% Cl) in
30 days when readily biodegradable alternative carbon sources were
available.
Omori et al. (1987) found that bacterial strains isolated from
the soil degraded various chlorinated paraffins by dechlorination in
the presence of n-hexadecane. In a mixed culture of four strains,
more than 50% of the chlorine was removed from the shorter paraffins
with lower chlorine content (C14;43% Cl, CP-ML and C15;50% Cl, CP-MH)
within 36 h. Lower amounts of chlorine were removed from the
chlorinated paraffins with longer chain lengths. Activated sludge
from a sewage treatment plant in Tokyo acclimatized to n-hexadecane
for 60 days showed only a limited amount (2%) of dechlorination of the
chlorinated paraffins. The bacterial dechlorination concerned the
terminal chlorine, which produced 2- or 3-chlorinated fatty acids via
ß-oxidation.
4.3 Bioaccumulation and biomagnification
4.3.1 Summary
Chlorinated paraffins of short chain length accumulate in mussels
and fish to a higher degree than intermediate and long chain length
chlorinated paraffins.
Data on bioaccumulation of chlorinated paraffins by aquatic
organisms are summarized in Table 9. Bioconcentration factors (BCFs)
may be uncertain since the applied doses exceeded the water solubility
in several experiments.
Table 9. Bioconcentration factors for some chlorinated paraffins
Species Chlorinated paraffina Exposure Bioconcentration Reference
Concentration Duration factor
(µg/litre) (days) (whole animal)b
Marine diatom C10-12;58% Cl (CP-SH) 1.4 10 < 1 Thompson & Madeley (1983b)
(Skeletonema costatum) 17.8 10 3.5
Freshwater green alga C10-12;58% Cl (CP-SH) 35 10 1.5 Thompson & Madeley (1983d)
(Selenastrum capricornutum) 140 10 7.6
150 10 4.1
Mussel C10-12;58% Cl (CP-SH) 2.3 147 40 900e Madeley et al. (1983a)
(Mytilus edulis) 10 91 24 800e
C10-12;58% Cl (CP-SH) 13 60 25 292e Madeley & Thompson (1983a)
130 60 12 177e
C12;69% Cl (CP-SH) 0.13 28 138 000e Renberg et al. (1986)
C16;34% Cl (CP-ML) 0.13 28 6920e
C14-17;52% Cl (CP-MH) 220 60 2856e Madeley & Thompson (1983b)
3800c 60 429e
C22-26;43% Cl (CP-LL) 120 60 1158e Madeley & Thompson (1983c)
2180c 60 261e
C22-26;70% Cl (CP-LH) 460 60 341e Madeley & Thompson (1983d)
1330c 60 223e
Table 9. (Cont'd)
Species Chlorinated paraffina Exposure Bioconcentration Reference
Concentration Duration factor
(µg/litre) (days) (whole animal)b
Rainbow trout C10-12;58% Cl (CP-SH) 3.1 168 3550e Madeley & Maddock (1983b)
(Oncorhynchus mykiss) 14 168 5260e
C10-12;58% Cl (CP-SH) 33 60 7155e Madeley & Maddock (1983c)
3050c 60 1173e
C14-17;52% Cl (CP-MH) 1050c 60 45e Madeley & Maddock (1983c)
4500c 60 67e
C22-26;43% Cl (CP-LL) 970 60 18e Madeley & Maddock (1983c)
4000c 60 38e
C20-30;70% Cl (CP-LH) 840 60 54e Madeley & Maddock (1983c)
3800c 60 32e
Bleaks C10-13;49% Cl (CP-SL) 125 14 770d,f Bengtsson et al. (1979)
(Alburnus alburnus) 59% Cl (CP-SH) 125 14 740d,f Bengtsson et al. (1979)
71% Cl (CP-SH) 125 14 140d,f Bengtsson et al. (1979)
C14-17;50% Cl (CP-MH) 125 14 30d,f Bengtsson et al. (1979)
C18-26;49% Cl (CP-LL) 125 14 7d,f Bengtsson et al. (1979)
a The classification of chlorinated paraffins is given in Table 1
b Ratio of the concentration of the chemical in the organism to the concentration of the chemical in the environment or food
c May exceed water solubility
d BCFs calculated by Zitko (1980)
e BCFs based on radioactivity (14C)
f BCFs based on parent compounds
4.3.2 Aquatic vertebrates
4.3.2.1 Short chain length chlorinated paraffins
In a study by Lombardo et al. (1975), fingerling rainbow trout
(Oncorhynchus mykiss) were fed a diet containing 10 mg/kg Chlorowax
500C (C12;60% Cl, CP-SH) for 82 days. Samples of 20 exposed and 10
control fish were collected at approximately 2-week intervals during
the time-period and analysed for chlorinated paraffin content by
microcoulometric gas chromatography (Friedman & Lombardo, 1975). The
tissue level of chlorinated paraffins rose during the treatment period
to 1.1 mg/kg (on tissue basis) or 18 mg/kg (on fat basis) after 82
days (detection level: 0.5 mg/kg). The experiment had to be
terminated owing to the failure of the water supply, and it was not
possible to determine whether a steady-state level had been reached.
In studies (Madeley & Maddock, 1983b) on rainbow trout
(Oncorhynchus mykiss) exposed to measured concentrations of 3.1 and
14.3 µg/litre of 14C-labelled C10-12; 58% Cl (CP-SH) for a period of
168 days, BCF value of 1300 (low dose) and 1600 (high dose) were
observed in the flesh, 2800 (low dose) and 16 000 (high dose) in the
liver, and 11 700 (low dose) and 15 500 (high dose) in the viscera;
all values were determined from radioactivity measurements. The BCF
for the whole body was 3350 (low dose) and 5260 (high dose)
(calculated values). Half-lives for elimination in different organs
were calculated to be the following: liver 9.9 (low dose) and 11.6
days (high dose), viscera 23.1 (low dose) and 23.9 days (high dose),
and flesh 16.5 (low dose) and 17.3 days (high dose).
Rainbow trout (Oncorhynchus mykiss) were exposed to measured
concentrations ranging from 33 to 3050 µg/litre of C10-12;58% Cl
(CP-SH) for 60 days (Madeley & Maddock, 1983c). BCFs, which were
determined in whole fish samples at the end of the test, were 7155
(low dose) and 1173 (high dose) based on radioactivity measurements,
and 7273 (low dose) and 574 (high dose) based on parent compound
analysis. Parent compound analysis was performed using a modification
of the method of Hollies et al. (1979) (see section 2.3.2).
4.3.2.2 Intermediate chain length chlorinated paraffins
After 60 days exposure of rainbow trout (Oncorhynchus mykiss)
to measured concentrations of 1050 and 4500 µg/litre of intermediate
length (C14-17) chlorinated paraffins with 52% Cl (CP-MH), whole body
BCFs of 45 (low dose) and 67 (high dose) based on radioactivity
measurements, and of 32 (low dose) and 42 (high dose) based on parent
compound analysis were determined (Madeley & Maddock, 1983c). The
BCFs were determined at the end of the exposure period. Parent
compound analysis was performed using a modification of the method of
Hollies et al. (1979) (see section 3.2.3).
4.3.2.3 Long chain length chlorinated paraffins
Juvenile Atlantic salmon (Salmo salar) were exposed to Cereclor
42 (C24;42% Cl, CP-LL) or Chlorez 700 (C24;70% Cl, CP-LH) by uptake
from suspended solids or from food (Zitko, 1974a). The fish were
treated with either 1000 µg/litre of contaminated suspended solids for
48 h and 144 h, or to 10 mg/kg or 100 mg/kg of contaminated food for
181 days with a subsequent elimination period of 74 days. No or very
low accumulation of the chlorinated paraffins was observed. However,
the analytical method used determined the amount of chlorine and not
of chlorinated paraffin; this method has been considered as nonspecific
and of low sensitivity.
After 60 days exposure of rainbow trout (Oncorhynchus mykiss)
to long chain chlorinated paraffins with 43% Cl (CP-LL) (970 or
4000 µg/litre) or 70% Cl (CP-LH) (840-3800 µg/litre), whole body BCFs,
based on measured exposure concentrations, of 17.9 (low dose, 43% Cl),
37.6 (high dose, 43% Cl) and 53.8 (low dose, 70% Cl) and 32.5 (high
dose, 70% Cl) were determined at the end of the study when measured as
radioactivity. BCF values of 3.6 (low dose, 43% Cl), 9.0 (high dose,
43% Cl), 42.8 (low dose, 70% Cl) and 31.6 (high dose, 70% Cl), based
on parent compound analysis, were determined (Madeley & Maddock,
1983c).
4.3.3 Aquatic invertebrates
4.3.3.1 Short chain length chlorinated paraffins
After 60 days exposure of mussels (Mytilus edulis) to a short
chain length paraffin with 58% Cl (CP-SH) at measured concentrations
of 13 and 130 µg/litre, whole body BCFs were 25 292 and 12 177,
respectively, based on radioactivity measurements, and 20 000 and
7923 when measured as parent compound (Madeley & Thompson, 1983a).
After exposure of mussels (Mytilus edulis) for 147 days to
14C-labelled short chain length chlorinated paraffin with 58% Cl
(CP-SH) followed by a depuration period of 98 days (measured exposure
dose: 2.3 µg/litre), or for 91 days followed by 84 days of depuration
(measured exposure dose: 10.1 µg/litre), plateau levels of the
chlorinated paraffin in tissues were reached. Bioconcentration
factors (BCFs) at the plateau levels were 40 900 for whole mussel
tissue after exposure to 2.35 µg/litre and 24 800 after exposure to
10.1 µg/litre based on wet tissue basis. Of the different organs the
digestive glands had the highest BCF values of 226 000 (low exposure)
and 104 000 (high exposure). Half-lives for the chlorinated paraffin
in whole mussel tissue were 9.2-9.9 days (10.1 µg/litre) and 13.1-19.8
days (2.35 µg/litre) (Madeley et al., 1983a).
4.3.3.2 Intermediate chain length chlorinated paraffins
After 60-day exposures of mussels (Mytilus edulis) to
intermediate chain length paraffin with 52% Cl (CP-MH) at measured
concentrations of 220 and 3800 µg/litre (which was above the limit
of solubility in water), whole body BCFs were 429-2856 based on
radioactivity measurements and 339-2182 based on parent compound
analysis (Madeley & Thompson, 1983b).
4.3.3.3 Long chain length chlorinated paraffins
In mussels exposed to measured concentrations of 120-2180
µg/litre of long chain length chlorinated paraffin with 43% Cl (CP-LL)
and 460-1330 µg/litre of long chain length chlorinated paraffin with
70% Cl for 60 days, whole body BCFs of 1158-261 (43% Cl) and 341-223
(70% Cl), respectively, were observed when measured as radioactivity,
and 87.2-1000 (43% Cl) and 105-167 (70% Cl) when based on parent
compound analysis (Madeley & Thompson, 1983c,d). However, the high
doses exceeded the water solubility of the chlorinated paraffins.
4.3.3.4 Comparative studies
The accumulation during four weeks of two 14C-labelled
chlorinated paraffins, polychloro[1-14C]hexadecane (C16;34% Cl,
CP-ML) and 1-chloropolychloro[1-14C]dodecane (C12;69% Cl, CP-SH), was
studied in the mussel (Mytilus edulis) by Renberg et al. (1986).
Both compounds showed rapid uptake when added at concentrations of
0.13 µg/litre (C16;34% Cl) and 0.0029 µg/litre or 0.13 µg/litre
(C12;69% Cl) in water for 28 days. Steady-state levels were reached
within 14 days after exposure to 0.13 µg/litre. The authors
calculated the BCF values to be 6920 for C16;34% Cl and 138 000 for
C12;69% Cl, based on fresh weight. The mussels exposed to C12;69% Cl
were studied for an additional 28 days without exposure. The
elimination rate for this chlorinated paraffin was slow, and 33% of
the radioactivity remained in the tissues after 28 days.
4.3.4 Aquatic plants
The BCF after 10 days exposure to short chain chlorinated
paraffin with 58% chlorination (CP-SH) has been estimated to be 3.5
for the diatom Sceletonema costatum (17.8 µg/litre) and 1.5 for the
green alga Selenastrum capricornutum (35 µg/litre) (Thompson &
Madeley, 1983a,b).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Techniques for the analysis of chlorinated paraffin are described
in section 2.3.2. The major problem connected with the analysis of
environmental samples is interference from other compounds. In
earlier studies, when pre-separation techniques were not as well
developed, the concentrations may have been overestimated. Another
problem is that the chlorinated paraffin composition in the
environment may be different from that of the original products,
and the quantitative analysis has to be based on comparisons with
the original products. These difficulties make it clear that
analytical results have to be regarded more as estimates than exact
concentrations.
5.1.1 Air
No information on levels in the atmosphere has been found in the
literature.
5.1.2 Water and sediment
Levels of chlorinated paraffins in water and sediment in the
United Kingdom are summarized in Table 10. Chlorinated paraffins have
been detected in United Kingdom sea water at levels in the range of
0.5-4.0 µg/litre for C10-20 and less than 2.0 µg/litre for C20-30
(Campbell & McConnell, 1980). The levels in sediments from the same
areas were analysed; chlorinated paraffins were detected only in a few
samples at levels up to 500 µg/kg wet weight for C10-20 and 600 µg/kg
for C20-30. The levels of chlorinated paraffins are low in water from
rivers and reservoirs not receiving industrial/domestic effluents
(C10-20, 1 µg/litre or below; C20-30, 2 µg/litre or below) and for
waterways in industrialized regions (C10-20, up to 6.0 µg/litre; C20-30,
0.5 µg/litre or below). The level of C10-20 in the latter regions was
higher than for C20-30. Chlorinated paraffins were not detected in
five drinking-water reservoirs either in the water (detection limit:
0.5 µg/litre) or the sediment (detection limit: 250 µg/kg) (Campbell &
McConnell, 1980). The levels of C10-20 in sediment from non-marine
water remote from industry were in the range up to 1000 µg/kg, except
for a sewage sludge sample from the Liverpool area, which had levels
of 4000-10 000 µg/kg. C20-30 was detected only in one sample at
50 µg/kg. The levels of chlorinated paraffins in sediments close to
industrial plants were found to be higher (C10-20 up to 15 000 µg/kg;
C20-30 up to 3200 µg/kg wet weight). The levels in sediment from these
industrial areas were 1000 times higher than in the overlaying water
columns, indicating the ability of chlorinated paraffins to adsorb on
suspended solids.
Table 10. Levels of chlorinated paraffins in United Kingdom water (µg/litre) and sediment (µg/kg)
(From: Campbell & McConnell, 1980)a
C10-20 C20-30
Range Median No. of samples Range Median No. of samples
level below detection level below detection
limit limit
Sea water
water ND-4.0 0.5 7/15 ND-2.0 ND 13/18
sediment ND-500 ND 14/18 ND-600 ND 15/18
Fresh water remote from industry
water ND-1.0 0.5 7/13 ND-2.0 ND 7/11
sediment ND-1000 ND 4/6 ND-< 250 ND 4/5
Fresh water close to industry
water ND-6.0 1-2 4/25 ND-0.5 ND 8/10
sediment ND-15 000 1800 2/21 ND-3200 50 3/9
a ND = not detected (detection limit in water = 0.5 µg/litre; in sediment = 50 µg/kg)
Levels of chlorinated paraffins in water and sediment in Germany
are summarized in Tables 11 and 12. In 1987, chlorinated paraffins
were detected at concentrations of about 1 µg/litre for C10-13 and
20 µg/litre for C14-18 in the Danube, downstream of the confluence
with the River Lech (BUA, 1992). The corresponding contents in the
sediment were 300 and 1800 µg/kg dry weight, respectively. In 1994,
chlorinated paraffin concentrations in the Danube and River Lech were
in the range of 0.05 to 0.12 µg/litre for C10-C13 and < 0.05 to
0.19 µg/litre for C14-C17. Also in 1994, the chlorinated paraffin
concentrations (C10-C13) in sediment varied from 6-10 µg/kg dry
weight in the lake of Constance to maximum concentrations of 76 µg/kg
dry weight in the River Lech and 83 µg/kg dry weight in the Rhine
(BUA, 1992).
Table 11. Concentrations (µg/litre) of short and intermediate chain length
chlorinated paraffins in surface water in Germany (From: BUA, 1992)a
Location 1987 1994
C10-13 C14-18 C10-13 C14-17
River Lech at Augsburg 0.05 < 0.05
River Lech at Gersthofen 0.50 4.5 0.08 0.09
(upstream from a chlorinated
paraffin production plant)
River Lech at Langweid 0.60 4.0 0.10 0.19
(downstream from a chlorinated
paraffin production plant)
River Lech at Rain 0.12 0.17
River Danube at Marxheim 1.2 4.0 0.06 < 0.06
(downstream from the mouth of
the River Lech)
River Danube at Marxheim (upstream 1.2 20 0.06 0.07
from the mouth of the River Lech)
a The data from 1994 were produced with a more specific method than those from 1987.
The two data sets are therefore difficult to compare.
Table 12. Concentrations (µg/kg dry weight) of short and intermediate chain length
chlorinated paraffins in sediments from Germany (From: BUA, 1992)a
Location 1987 1994
C10-13 C14-18 C10-13 C14-17
Bodensee (middle)
0-5 cm depth 9-10 70
5-12 cm depth 6-9 < 10
River Rhine (141 km) at Rheinfelden 33-38 60
River Rhine (152 km) at Rheinfelden,
upper layer 53-60 140
lower layer 26-32 85
River Rhine, near German-Dutch
border (2 sites) 60-83 145-205
River Main (3 sites) 24-55 160-260
Outer Alster, Hamburg 35-36 370
River Elbe at Hamburg (2 sites) 16-25 130-230
River Lech, upstream from 400 2200 < 5-7 < 10
chlorinated paraffin production
plant
River Lech, downstream from 700 1700 70-76 325
chlorinated paraffin production
plant
Danube downstream from mouth 300 1800
of the River Lech
a The data from 1994 were produced with a more specific method than those from 1987.
The two data sets are therefore difficult to compare.
Chlorinated paraffins were detected in water samples collected
in the Bermuda Islands (Kraemer & Ballschmiter, 1987). Water samples
down to a depth of 1200 m were analysed, and the highest concentration,
about 50 µg/litre, was found in the surface film. Chlorinated paraffins
were not detected in water from the Maldives (detection limit:
3 ng/litre).
In surface sediment from Lake Zürich, 5 µg/kg of a chlorinated
paraffin mixture with a carbon chain length of C14-18 and 52% chlorine
(CP-MH) was measured (Schmid & Müller, 1985). In the same study it
was reported that sewage sludge from a sewage treatment plant in an
urban industrialized region with known contamination of heavy metals
and organochlorine compounds contained chlorinated paraffins at a
concentration of 30 000 µg/kg.
In a field study performed by the US Environmental Protection
Agency (Murray et al., 1988), samples from two watersheds were
analysed by the method of Schmid & Müller (1985). Both were close to
a chlorinated paraffin manufacturing plant (Sugar Creek, Ohio) or
industry using lubricating oils (Tinkers Creek, Ohio). Chlorinated
paraffins were detected in most samples from Sugar Creek in the low
ppb range (< 8 µg/litre) near drainage and downstream from the plant.
Only a few samples upstream from the plant contained detectable
levels of chlorinated paraffins (< 0.3 µg/litre) (detection limit:
0.15 µg/litre). Of the three chlorinated paraffin fractions studied, the
fraction containing the long carbon chain length C20-30; 40-50% Cl
(CP-LL) was generally present at highest concentration. Sediments
contained higher concentrations. As much as 170 000 µg/kg dry weight
of C20-30 was detected in sediment in an impoundment lagoon, whereas
50 000 µg/kg of C14-17;50-60% Cl (CP-MH) and 40 000 µg/kg C10-12;
50-60% Cl (CP-SH) were measured in the same sample. In mussels
(family Unionidae) collected downstream from the plant, there were
detectable levels of chlorinated paraffins (280 µg/kg C10-12, 170 µg/kg
C14-17, 180 µg/kg C20-30). The report concluded that the observed
levels were most likely due to the manufacturing plant. The samples
collected in Tinkers Creek did not contain detectable amounts of
chlorinated paraffins. Most of the samples contained organic
compounds, largely halogenated aromatics, at levels high enough to
interfere and mask the presence of chlorinated paraffins. Chlorinated
paraffins were detected in samples collected from the process wastes
stream inside the chlorinated paraffin plant. The levels in these
samples were: C10-12, 8.1 µg/kg; C14-17, 1.3 µg/kg; C20-30, 2.2 µg/kg.
In 1979, 51 water samples and 51 bottom sediment samples were
collected at 17 sites in Japan and analysed for the presence of
chlorinated paraffins (C8-32). Chlorinated paraffins were not
detected in any of the water samples, while 24 bottom sediment samples
from 11 sites contained chlorinated paraffins at concentrations of 600
to 10 000 µg/kg (wet or dry weight not specified) (Environment Agency,
Japan, 1981). In 1980, 120 water samples and 120 bottom sediment
samples were collected at 40 sites in Japan and were analysed for the
presence of chlorinated paraffins (C8-32). Chlorinated paraffins
were not detected in any of the water samples but 31 bottom sediment
samples from 13 sites contained chlorinated paraffins at concentrations
of 500 to 8500 µg/kg. However, the analytical methods were not
specified and the detection limits were high (the detection limit was
10 µg/litre for water and 500 µg/kg for bottom sediment) (Environment
Agency, Japan, 1983).
5.1.3 Soil
No data on the occurrence of chlorinated paraffins in soil have
been reported.
5.1.4 Aquatic and terrestrial organisms
The levels of chlorinated paraffins in organisms from various
ecosystems in Sweden, determined using the method of Jansson et al.
(1991), are shown in Table 13. Chlorinated paraffins were found in
all samples in the range of 130-4400 ng/g lipid (6.6-210 µg/kg tissue)
(Jansson et al., 1993). The terrestrial animals rabbit and moose had
higher chlorinated paraffin concentration in their fat than any of the
aquatic animals. The chlorinated paraffin levels in fish-eaters were
approximately the same as in the fish, compared to the dioxin and PCB
levels, which were several times higher in fish-eaters. The levels
in seal and herring indicated no or only low biomagnification of
chlorinated paraffins in their food chains.
In a study by Campbell & McConnell (1980), chlorinated paraffins
were detected in mussels, fish, seals and seabirds, as well as in
seabird eggs, using the analytical method of Hollies et al. (1979)
(Table 14). The levels of C10-20 were higher than those of C20-30 in
most organisms. Mussels collected close to a chlorinated paraffin
plant effluent discharge had levels of C10-20 up to 12 000 µg/kg. The
measured levels in the organisms were close to the levels in the
sediment near the organisms, but 100 to 1000 times higher than those
of water, thus indicating bioaccumulation. The authors also detected
trace amounts of chlorinated paraffins (C10-20) in the tissues of sheep
grazing near a plant producing chlorinated paraffins. The tissues
containing the highest levels were liver (200 µg/kg), fat and kidney
(50 µg/kg). The fleeces of the sheep contained higher levels
(350 µg/kg), and the authors suggest that this might have been due to
aerial transport. No chlorinated paraffin could be detected in sheep
grazing far from plants producing chlorinated paraffins (detection
limit = 50 µg/kg).
Table 13. The levels of chlorinated paraffins in various terrestrial and aquatic
organisms in Swedena
Organism Samplesc Tissue Extracted CP (µg/kg CP (µg/kg
lipid (%) lipid) tissue)d
Rabbit 15 muscle 1.1 2900 31.9
Moose 13 muscle 2.0 4400 88
Reindeer 31 fat 56 140 78
Whitefish 35 muscle 0.66 1000 6.6
Arctic char 15 muscle 5.3 570 30.2
Herring (Bothnian Sea) 100 muscle 5.4 1400 75.6
Herring (Baltic Proper) 60 muscle 4.4 1500 66
Herring (Skagerrack) 100 muscle 3.2 1600 51.2
Ringed sealb 7 blubber 88 130 114.4
Grey seal 8 blubber 74 280 207
Osprey 35 muscle 4.0 530 21.2
a Data from Jansson et al. (1993)
b The ringed seal was from Kongsfjorden, Svalbard, Sweden
c Pooled samples
d Calculated from the reported data
In a study by Campbell & McConnell (1980), chlorinated paraffins
were detected in mussels, fish, seals and seabirds, as well as in
seabird eggs, using the analytical method of Hollies et al. (1979)
(Table 14). The levels of C10-20 were higher than those of C20-30 in
most organisms. Mussels collected close to a chlorinated paraffin
plant effluent discharge had levels of C10-20 up to 12 000 µg/kg. The
measured levels in the organisms were close to the levels in the
sediment near the organisms, but 100 to 1000 times higher than those
of water, thus indicating bioaccumulation. The authors also detected
trace amounts of chlorinated paraffins (C10-20) in the tissues of sheep
grazing near a plant producing chlorinated paraffins. The tissues
containing the highest levels were liver (200 µg/kg), fat and kidney
(50 µg/kg). The fleeces of the sheep contained higher levels
(350 µg/kg), and the authors suggest that this might have been due to
aerial transport. No chlorinated paraffin could be detected in sheep
grazing far from plants producing chlorinated paraffins (detection
limit = 50 µg/kg).
Table 14. Chlorinated paraffins in organisms in the United Kingdom
(Campbell & McConnell, 1980)
Species Tissuea No. of Carbon chain-length and
specimens concentration in tissues
analysedb (means and ranges)c
C10-20 (µg/kg) C20-30 (µg/kg)
mean range mean range
Aquatic organisms
Plaice NS 6 30 ND-200 30 ND-200
(Pleuronectes platessa)
Pouting NS 4 100 ND-200 ND ND
(Trisopterus luscus)
Mussel NS 9 3250 100-12 000 10 NDe-100
(Mytilus edulis)
Pike NS 2 25 ND-50 25 ND-50
(Esox lucius)
Grey seal Liver 4 75 40-100 ND ND
(Halichoerus grypus) and
blubber
Table 14. Cont'd
Species Tissuea No. of Carbon chain-length and
specimens concentration in tissues
analysedb (means and ranges)c
C10-20 (µg/kg) C20-30 (µg/kg)
mean range mean range
Birds
Heron Liver NR NR 500-1200 NR NDe
(Ardea cinerea) Liver NR NR 100-1000 NR 100-1500
Guillemot Liver NR NR 100-1100 NR NDe
(Uria aalge)
Herring gull Liver NR NR 200-900 NR 100-500
(Larus argentatus)
Seabird eggsd 23 220 ND-2000 20 ND-100
a NS = Not specified
b NR = Not reported
c ND = Not detected (detection limit = 50 µg/kg, except where stated otherwise)
d 9 species
e Detection limit = 100 µg/kg
In 1980, 108 fish samples were collected at 28 places in Japan
and were analysed for the presence of chlorinated paraffins.
Chlorinated paraffins were not detected in any of the samples. The
detection limit was 500 µg/kg (Environment Agency, Japan, 1983).
5.1.5 Food and beverages
Chlorinated paraffins, mostly C10-20, were detected in various
food products in a limited study (Table 15) using the analytical
method of Hollies et al. (1979). C20-30 chlorinated paraffins were
detected only in a few samples. C10-20 chlorinated paraffins were
found at levels up to 500 µg/kg in approximately 70% of the samples
(Campbell & McConnell, 1980).
Chlorinated paraffin residues have been detected in fish and
sheep (see section 5.1.4).
Table 15. Chlorinated paraffins (C10-20) in human foodstuffs
(Campbell & McConnell, 1980)
Foodstuff class No. of foodstuff Concentration
samples tested (µg/kg)
Dairy products 13 300
Vegetable oils and derivatives 6 150
Fruit and vegetables 16 25
Beverages 6 NDa
a Not detected (detection limit = 50 µg/kg)
5.2 General population exposure
Chlorinated paraffins have been detected on human hands (Campbell
& McConnell, 1980). The hands of eight human volunteers were swabbed
with toluene, and all had chlorinated paraffin levels of 0.8-4.0 µg
C10-20 (per two hands). C20-30 chlorinated paraffins (2 µg)
were detected only on one person. The authors suggested that the
chlorinated paraffin content in foodstuffs, as well as direct transfer
from manufactured articles via the hands, could contribute to human
exposure.
Owing to the high octanol-water partition coefficient, it is
likely that the principle source of exposure of the general population
to chlorinated paraffins is food. However, due to lack of data,
exposure to chlorinated paraffins via other routes cannot be ruled
out.
5.2.1 Concentrations in human tissues
Chlorinated paraffins have been detected in postmortem tissue
samples. Campbell & McConnell (1980) measured the amount in 24 tissue
samples and found up to 600 µg/kg of C10-20 in adipose tissue (median
level: 100-190 µg/kg), up to 500 µg/kg in kidney (median level below
90 µg/kg) and up to 1500 µg/kg in liver (median level below 90 µg/kg).
C20-30 chlorinated paraffins were detected only in a few samples and
at a low level. Schmid & Müller (1985) also detected chlorinated
paraffins in adipose tissue at a concentration of 200 µg/kg.
5.3 Occupational exposure
Occupational exposure to chlorinated paraffins is likely among
workers in production plants or in industries using chlorinated
paraffins. The US National Occupational Exposure Survey (1981-1983)
indicated that 573 000 workers, including 38 000 women, were
potentially exposed to chlorinated paraffins (NIOSH, 1990). In
Denmark, it was reported that 60 000 workers in 175 companies have
been potentially exposed to chlorinated paraffins since 1964 (the
amount produced and imported annually is 5000 tonnes) (Hansen et al.,
1992).
Campbell & McConnell (1980) detected chlorinated paraffins on
human hands using the method of Hollies et al. (1979) (see section
2.3.2). When the hands of a worker in a chlorinated paraffin
laboratory were swabbed with toluene, 800 µg of C10-20 and 400 µg
of C20-30 were detected.
Historical exposure data from machine shops, reported as
reflecting worst-case situations, indicated exposures to chlorinated
paraffins of 0.003-1.15 mg/m3 for operation such as milling, cutting
and grinding (HSE, 1992). Other exposure data suggested exposures to
chlorinated paraffins ranging from 0.003 to 0.21 mg/m3. However,
it is not clear if these levels of exposure were intermittent or
time-weighted averages. There is no information on whether or not
chlorinated paraffin aerosols are in the inhalable size range.
Occupational exposure to short chain chlorinated paraffins has
been estimated by the United Kingdom Health and Safety Executive by
mathematical modelling using the "Estimation and Assessment of
Substance Exposure" (EASE), developed as part of the guidance on new
and existing substances by the Commission of the European Union (CEU)
(HSE, 1992). This model takes into account physico-chemical data such
as vapour pressure and process details such as temperatures and the
use of local exhaust ventilation. It does not take into account the
attenuating effect of decontamination of equipment or use of personal
protective equipment.
During the production of short chain chlorinated paraffins in
closed systems, exposure is likely to be intermittent, and the model
predicts that inhalation exposure to a substrate with a vapour
pressure of less than 0.001 kPa is negligible (0 to 0.1 ppm).
Assuming a non-dispersive pattern of use and intermittent skin
contact, the model predicts that exposures of the hand and forearm
will be in the range of 0.1-1 mg/cm2 per day. During formulation of
short chain chlorinated paraffins (in closed systems) the model
predicts negligible (0-0.1 ppm) inhalation exposure with formulation
process temperatures of 40-50°C. Skin exposure to the hands and
forearms during formulation is predicted to be 0.1-1 mg/cm2 per day.
Occupational exposure to short chain chlorinated paraffins during
their use as metal-working fluids is extensive and the model predicts
exposure of 0.1-1.5 mg/cm2 per day, assuming a chlorinated paraffin
content in the fluid of 2-10%, or 5-15 mg/cm2 per day for speciality
fluids which may contain more than 80% chlorinated paraffin.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
There is a lack of systematic investigation of the influence of
carbon chain length and degree of chlorination in studies on the
kinetics of chlorinated paraffins. Studies have almost exclusively
concerned short and intermediate chain length chlorinated paraffins.
6.1 Absorption
6.1.1 Oral exposure
Chlorinated paraffins are absorbed after oral administration.
From the data presented in section 6.5 it appears that short chain
length compounds are more readily absorbed than longer chain length
compounds. Absorption decreases with increasing carbon chain length
and degree of chlorination.
6.1.2 Dermal exposure
Chlorinated paraffins are slowly absorbed by the dermal route
in Sprague-Dawley rats (Yang et al., 1987). Two 14C-labelled
chlorinated paraffins, C18;50-53% Cl (CP-LH) and C28;47% Cl (CP-LL),
were applied to rat skin (5-7 animals of each sex) at a concentration
of 66 mg/cm2, approximately equivalent to 2000 mg/kg body weight.
Only 0.7% (males) and 0.6% (females) of the C18 dose was absorbed
after 96 h. Only 0.02% of the C28 dose was absorbed in males whereas
in females the level was not detectable. This indicates that
increasing chain length leads to decreased permeability. Of the
absorbed C18 dose, 40% was exhaled as 14C-labelled CO2, and 20% was
excreted in urine and 20% in faeces.
The absorption of two chlorinated paraffins through human skin
has been studied in vitro (Scott, 1989). There was no absorption of
Cereclor S52 (C14-19;52% Cl, CP-MH) following a 54-h application to
the surface of the epidermal membranes using five different receptor
media. Similarly, using Cereclor 56L (C10-13; 56% Cl, CP-SH; 18.5% w/w
solution in a typical cutting oil) no absorption was detected for 7 h,
but after 23 h a slow but steady rate of absorption was detected
(e.g., 0.05 ± 0.01 µg/cm2 per h ± SEM; n = 6; receptor medium PEG-20
oleyl ether in saline), which was maintained for the duration of the
experiment (56 h). Owing to the anticipated low rate of absorption,
the chlorinated paraffin samples were spiked with [14C] n-pentadecane
and [14C] n-undecane for Cereclor S52 and 56L, respectively, in
order to facilitate detection of the absorbed material. Measurement of
the 14C-alkanes was taken as a surrogate for the chlorinated
paraffins, on the assumption that their rates of absorption were
similar.
6.1.3 Inhalation exposure
No data on retention of chlorinated paraffins by inhalation have
been reported.
6.2 Distribution
6.2.1 Short chain length chlorinated paraffins
6.2.1.1 Mouse
Female C57Bl mice were administered 12.5 MBq/kg body weight
(340 µCi) (for autoradiography) or 1.25 MBq/kg body weight (34 µCi)
(for determination of radioactivity) of 14C-labelled chlorododecanes
(C12) with different chlorine contents (17.5% [CP-SL], 55.9% [CP-SH]
and 68.5% [CP-SH]) either by gavage or intravenous injection (Darnerud
et al., 1982). Uptake of radioactivity was found by autoradiography to
be highest in tissues with high cell turnover/high metabolic activity,
e.g., intestinal mucosa, bone marrow, salivary glands, thymus and
liver. The highest radioactivity was achieved with the chlorinated
paraffin that had the lowest chlorine content. It was found that the
long period of retention of heptane-soluble radioactivity, which
indicated unmetabolized substance, in liver and fat after oral dosing
increased with degree of chlorination. In this study it was also
found that 30 to 60 days after injection of C12;17.5% Cl and
C12;55.9% Cl a considerable retention of radioactivity was seen in
the central nervous system. Exposure of late gestation mice showed a
transplacental passage of radioactivity, and 14C-labelling was
primarily noted in the liver, brown fat and intestine of the fetuses.
6.2.1.2 Rat
Radioactivity was found in the liver, kidneys, adipose tissue and
ovaries of Fischer-344 rats following administration of an unspecified
single dose by gavage of 14C-labelled chlorinated paraffin (C10-13;58%
chlorination, CP-SH) at the end of a 90-day dosing by gavage with the
same chlorinated paraffin (IRDC, 1984c).
6.2.2 Intermediate chain length chlorinated paraffins
6.2.2.1 Rat
Radioactivity was found initially in the liver and kidneys and
later in adipose tissue and the ovaries of Fischer-344 rats following
a single dose by gavage of 14C-labelled C14-17;52% Cl (CP-MH) at the
end of a 90-day dosing in the diet with the same chlorinated paraffin
(IRDC, 1984b).
In male Wistar rats fed with a diet containing 0.4 or 40 mg/kg
[36Cl]Cereclor S52 (C14-17;52% Cl, CP-MH) for 8 (40 mg/kg) or 10 weeks
(0.4 mg/kg), equilibrium levels of radioactivity were established in
liver and abdominal fat within 1 and 7 weeks, respectively (Birtley et
al., 1980). The equilibrium concentrations were 7000 µg/kg in liver
and 30 000-40 000 µg/kg in fat after high exposure. No radioactivity
was detected in the brain or adrenal glands.
6.2.2.2 Mouse
The distribution of orally administered 14C-labelled
polychlorohexadecane (C16;69% Cl, CP-MH) in female C57Bl mice was
examined by whole body autoradiography (Biessmann et al., 1983).
A high level of radioactivity was observed in the liver, brown
fat, intestine, gall bladder, adrenal cortex and kidney of mice
administered approximately 15 µmol/kg. A high uptake of radioactivity
was also observed in corpora lutea on days 1-4, and was still present
30 days after administration. A high level of radioactivity was also
observed in the adrenal cortex at shorter post-injection times, and in
brown and white fat and in the liver, which were still labelled after
30 days.
14C-Labelled [1-14C]polychlorohexadecane (C16;34.1% Cl, CP-ML)
was given to C57Bl mice either by gavage (females) or intravenously
(both sexes) at a radioactivity level of 370 kBq/animal (10 µCi)
(corresponding to 0.44 µmol of the chlorinated paraffin) (Darnerud &
Brandt, 1982). No difference in the distribution patterns was found
between the oral and intravenous administration routes. After
analysis by autoradiography a high level of radioactivity was found in
tissues with a high cell turnover rate and/or high metabolic activity,
and lower levels could be seen in the white fat depots. High levels
of radioactivity were observed in the liver, kidneys, spleen, bone
marrow, brown fat, intestinal mucosa, pancreas, salivary gland and the
Harderian gland 24 h after intravenous injection. After 12 days high
levels of radioactivity were seen in the adrenal cortex, abdominal
fat and in the bile. Later after injection (30 days), prominent
radiolabelling of the brain was found which was as high as in the
liver. The chlorinated paraffin was also administered intravenously
to pregnant mice, and uptake of radioactivity in the fetuses was
observed. When the mice were administered on day 10 of pregnancy no
tissue-specific localization was found, but after administration in
late pregnancy (day 17) the distribution pattern after 6 h was similar
to that of adult mice when examined 24 h after administration.
The distribution of radioactivity in the brain and liver has been
studied after gavage administration of 14C-labelled polychloro-
hexadecane (C16;69% Cl, CP-MH) (1.48 MBq/kg body weight or 40 µCi,
corresponding to 1.1 mg/kg body weight) to pre-weaning NMRI mice
(Eriksson & Darnerud, 1985). The chlorinated paraffin was
administered by gavage at the age of 3, 10 and 20 days, and the
animals were killed 24 h and 7 days later. The radioactivity in the
brain declined more rapidly in the 3-day-old mice compared to the
10- and 20-day-old mice. In the 10-day-old mice approximately 0.02% of
the total administered dose was detected in the brain 24 h after
administration. Approximately 80% of the radioactivity in the brain
was still present after 7 days. In the liver the radioactivity
disappeared more rapidly both in younger and older animals. The
radioactivity in the brain was found primarily in the white matter
of the cerebellum, in the space between the neocortex and the
mesencephalon and thalamus, the corpus callosum, the pons and the
outer part of medulla spinalis. The radioactivity was higher in the
parts of the brain which also stained for myelin, and the levels at
7 days were almost the same as those at 24 h. After whole body
autoradiography, high levels of radioactivity were found in the liver,
intestinal contents, adipose tissue and adrenals. A differential
labelling of the liver was observed in the 3-day-old mice, where only
certain parts of the liver lobules were labelled.
6.2.2.3 Bird
The distribution of 14C-labelled polychlorohexadecane (C16;69%
Cl, CP-MH) in female Japanese quail (Coturnix coturnix japonica) was
examined by whole body autoradiography (Biessmann et al., 1983). Four
hours after a single dose by gavage of approximately 4.8 µmol/kg to
quail, high levels of radioactivity were observed in the liver,
intestine, gall bladder, egg yolk, kidney, ovary, blood, hypophysis
and retina. Twelve days after administration, radioactivity was
observed only in the uropygial gland, white fat, liver and egg yolk.
A study of the distribution after oral administration by gavage
of 0.74 MBq (20 µCi) (approximately 20 Ci/mol) of either 14C-labelled
polychlorohexadecane (C16;34% Cl, CP-ML) or (1-14C)-labelled
polychlorododecane (C12;56% Cl, CP-SH) in female Japanese quail
(Coturnix coturnix japonica) was performed by Biessmann et al.
(1982). The distribution patterns for the two chlorinated paraffins
were similar, and high levels of radioactivity were initially (up to
1 day) found in the liver, intestinal mucosa, spleen, bone marrow,
oviduct, gall bladder and kidney. After 4 and 12 days, high
radiolabelling was observed in fat, the yolk of the follicles and
the contents of the uropygial glands.
The uptake of Cereclor S52 (C14-17;52% Cl, CP-MH) in mallard ducks
(Anas platyrynchos) or ring-necked pheasants (Phasianus colchicus)
was studied by Madeley & Birtley (1980). After a single oral dose of
Cereclor S52 (10 g/kg) in duck, the highest levels of chlorinated
paraffins were detected in fat (67 mg/kg wet weight), gut (15 mg/kg)
and heart (7 mg/kg). The pheasants were exposed to 1000 mg/kg in the
diet for 5 days. Only low levels were found in the heart (3.1 mg/kg)
and gut (1.4 mg/kg). No visible fat was available in the pheasants
due to immaturity. The levels in other organs in both species were
low. The method of analysis was thin layer chromatography (Hollies
et al., 1979).
6.2.2.4 Fish
The distribution of polychloro-[1-14C]hexadecane (C16;34% Cl,
CP-ML) has been studied in carp (Cyprinus carpio) and bleak
(Alburnus alburnus) (Darnerud et al., 1983). After a single
intra-arterial injection of 60-80 µg in carp, about 6% of the dose was
excreted as 14CO2 in 96 h. Radioactivity was observed, in the
intra-arterially injected carps or in bleak exposed to contaminated
water (125 µg/litre) for 14 days, in the bile, intestine, kidney,
liver, gills and, particularly in bleak, in the nasal cavity, lens and
skin.
6.2.3 Long chain length chlorinated paraffins
6.2.3.1 Rat
After oral administration of 14C-labelled C22-26;70% Cl (CP-LH)
to Fischer-344 rats at the end of a 90-day exposure period, a small
part of the dose was absorbed (Serrone et al., 1987). The highest
level of radioactivity was found in the liver. Retention of
radioactivity in adipose tissue, which was eliminated slowly, was also
observed. In an identical study, C20-30;43% Cl (CP-LL) gave the
highest levels in the liver and ovary (Serrone et al., 1987).
6.2.3.2 Fish
Rainbow trout (Oncorhynchus mykiss) were fed diets containing 47 or
385 mg/kg (dry weight) of Cereclor 42 (C20-30;Cl 42%, CP-LL) containing
a 14C-labelled pentacosane (C25) with 42% Cl for 35 days and
a control diet for the following 49 days (Madeley & Birtley, 1980).
The chlorinated paraffin accumulated in the fish during the exposure
period, mostly in the liver and gut. The radioactivity decreased
during the elimination period, more rapidly in the gut and liver
than in the flesh, but was still detectable at the end of the
experiment. When the chlorinated paraffin was determined by thin-layer
chromatography a lower level was noted as compared to determination
of 14C-labelled molecules, suggesting metabolism in the fish. Up to
70% of the assimilated 14C in tissues was not associated with
chlorinated paraffin after feeding for 13 days.
6.2.3.3 Mussel
Mussels (Mytilus edulis) were fed suspended yeast cells dosed
with 524 mg/kg (dry weight) Cereclor 42 (C20-30;42% Cl, CP-LL)
containing a 14C-labelled pentacosane (C25) with 42% chlorination for
47 days, and were then fed with untreated yeast for a further 56 days
(Madeley & Birtley, 1980). The uptake reached a plateau level after
26 days of exposure, and the tissue concentration was always below
11 mg/kg. The highest level of chlorinated paraffin was detected in
the digestive glands. The chlorinated paraffin was eliminated rapidly,
and less than 10% remained at the end of the experiment. There was no
evidence of metabolism since the expelled radioactivity was in the
form of the parent compound.
6.2.4 Comparative studies
The uptake of three 14C-labelled chlorinated paraffins, C16;23%
Cl (CP-ML), C16;51% Cl (CP-MH) and C12;68% Cl (CP-SH), was studied in
rainbow trout (Oncorhynchus mykiss) by Darnerud et al. (1989). After
exposure to 3.64 µmol (C16) or 1.74 µmol (C12) in water for 7
days and to uncontaminated water for up to 21 days, radiolabelling was
observed in the bile, eye lens, brain and fat for C16;23% Cl, in the
bile, intestine, fat and liver for C16;51% Cl, and in fat, intestine,
liver and bile for C12;68% Cl. The three different chlorinated
paraffins showed an initially high uptake in the olfactory organs and
gills. The retention of radioactivity in the olfactory organs and
gills was relatively higher for C16;23% Cl than for the more highly
chlorinated paraffins. The long-time retention of radioactivity in
fat-rich tissues increased with the degree of chlorination of the
chlorinated paraffin preparation.
The short-term uptake and elimination of chlorinated paraffins in
bleak (Alburnus alburnus) were studied by Bengtsson et al. (1979).
Groups of bleak (15 in each) were exposed for 14 days to five
different Witaclor mixtures (Table 16), which were added to natural
Baltic Sea water (salinity 0.7%) giving a concentration of 125
µg/litre of water. Five fish were analysed to determine the chlorine
content (Lunde & Steinnes, 1975) at the end of exposure, and the rest
were analysed 1 and 7 days after treatment. It was found that the
uptake was more effective for those Witaclor mixtures with short
carbon chain length and low degree of chlorination, i.e. Witaclor 149
and 159. The elimination rates were slow, and 75 to 90% was detected
7 days after exposure.
In an extended study, bleak were exposed to Witaclor 149
(C10-13;49% Cl, CP-SL), Witaclor 171P (C10-13;71% Cl, CP-SH) and
Witaclor 549 (C18-26;49% Cl, CP-LL) via contaminated food for 91 days.
This was followed by an elimination period of 316 days (Bengtsson &
Baumann Ofstad, 1982). The concentrations of chlorinated paraffins in
the food were 590, 2500 and 5800 mg/kg of C10-13;49% Cl, 3180 mg/kg of
C10-13;71% Cl and 3400 mg/kg of C18-26;49% Cl. Three fish from
each exposure group were analysed at different time-points during
accumulation or elimination periods. The most effective uptake was
observed for C10-13;49% Cl, whereas the slowest uptake was detected for
C18-26;49% Cl. Efficiencies of uptake were 12% for C10-13;49% Cl, 6%
for C10-13;71% Cl and 2% for C18-26;49% Cl. The highest retention was
observed for C10-13;71% Cl, which remained in the tissue at a
steady-state level during the whole elimination period. The uptake of
C18-26;49% Cl was inefficient, and during the elimination period 50%
was lost within 4 to 5 weeks. The remaining amount appeared to be
constant for the rest of the experiment. The rate of elimination was
slowest for the Witaclor mixture with the shortest carbon chain length
and highest degree of chlorination. After 625 days of depuration the
fish still had detectable levels of C10-13;71% Cl, as determined by the
analytical method of Gjos & Gustavsen (1982) (Renberg et al., 1986).
Table 16. Structures of the chlorinated paraffins used in a study on
bleak (Alburnus alburnus) (From: Bengtsson et al., 1979)
Tested Carbon Chlorine Acronyma Accumulation Half-life
formulation chain content coefficientb (days)b
length (%)
Witaclor 149 C10-C13 49 CP-SL 770 13
Witaclor 159 C10-C13 59 CP-SH 740 34
Witaclor 171P C10-C13 71 CP-SH 140 7
Witaclor 350 C14-C17 50 CP-MH 40 30
Witaclor 549 C18-C26 49 CP-LL 10 7
a The classification is given in Table 1
b Calculated by Zitko (1980)
6.3 Metabolic transformation
6.3.1 Short chain length chlorinated paraffins
Darnerud (1984) demonstrated that inducers and inhibitors of
cytochrome P-450 (CYP) affect the rate of degradation of 14C-labelled
polychlorinated dodecanes (C12) containing 68.5% (CP-SH), 55.9%
(CP-SH) and 17.4% Cl (CP-SL) to 14CO2 in exposed C57Bl mice.
Pretreatment with the inhibitor piperonyl butoxide decreased the
amount of 14CO2 formed, and the decrease was more pronounced with
increasing degree of chlorination. The inhibitor metyrapone decreased
the exhalation of 14CO2 but was only investigated in mice exposed
to C12;68.5% Cl. The cytochrome P-450 (CYP2B1; CYP2B2) inducer,
phenobarbital, moderately increased the rate of 14CO2 formation from
chlorinated paraffin with 68% Cl, whereas the P-448 (CYP1A1; CYP1A2)
inducer, 3-methylcholanthrene, did not affect the degradation rate,
indicating a cytochrome P-450-dependent metabolism of chlorinated
dodecanes yielding 14CO2.
6.3.2 Intermediate chain length chlorinated paraffins
Female Sprague-Dawley rats in groups of four were exposed
intravenously to 5-6 mg/kg body weight of 14C-labelled poly-
chlorinated hexadecane (C16;65% Cl, CP-MH) (Åhlman et al., 1986).
Less than 3% of the radioactivity in the bile was due to unchanged
parent compound. The metabolites in the bile appeared to be conjugates
of N-acetylcysteine (mercapturic acid) and glutathione.
6.4 Elimination and excretion
6.4.1 Short chain length chlorinated paraffins
The exhalation of 14CO2 was compared after single gavage or
intravenous administration to female C57Bl mice of 1.25 MBq/kg body
weight (34 µCi) of three chlorododecanes (C12) with different
chlorine contents (17.5% [CP-SL], 55.9% [CP-SH] and 68.5% [CP-SH])
(Darnerud et al., 1982). Of the administered radioactive dose 52% of
C12;17.5% Cl, 32% of C12;56% Cl and 8% of C12;68% Cl were exhaled as
14CO2 within 12 h after dosing by either route. The major excretion
route for C12;56% Cl was by urine (intravenous: 21%; oral: 29%) and
for C12;68% Cl was by faeces (intravenous: 8.6%; oral: 21%). The
total elimination decreased as the chlorine content increased.
6.4.2 Intermediate chain length chlorinated paraffins
6.4.2.1 Rat
The half-time for removal of radioactivity from abdominal fat was
estimated during and after dietary administration for 8 or 10 weeks of
0.4 and 40 mg/kg feed of [36Cl]Cereclor S52 (C14-17;52% Cl, CP-MH) in
male Wistar rats (Birtley et al., 1980). Equilibrium in liver and
abdominal fat was reached at 1 and 7 weeks, respectively. The
half-time for removal was about 8 weeks for abdominal fat, and it was
observed that the level of radioactivity in the liver declined below
the detection limit within one week.
Female Sprague-Dawley rats in groups of four were exposed
intravenously to 5-6 mg/kg body weight of 14C-labelled polychlorinated
hexadecane (C16;65% Cl, CP-MH) (Åhlman et al., 1986). Approximately
10% of the administered dose was excreted in the bile after 24 h,
whereas excretion in the urine and faeces was less than 0.5% after
48 h.
6.4.2.2 Mouse
The elimination of radioactivity was studied in female C57Bl mice
after gavage or intravenous administration of a uniformly 14C-labelled
polychlorohexadecane (C16;69% Cl, CP-MH) (1.6 µmol/kg) (Biessmann
et al., 1983). After 8 h, only about 1% of the dose was exhaled
as 14CO2 for both administration routes. Most of the
radioactivity was excreted in faeces when administrated by gavage, and
after 8 h 22% was recovered in faeces and 1.2% in urine. After 96 h,
66% was recovered in faeces and 2.9% in urine. After intravenous
administration, 2.1% was excreted in faeces and 1% in urine after 8 h,
and 43% was excreted in faeces and 3% in urine after 96 h.
The excretion of [1-14C]polychlorohexadecane (C16;34.1% Cl,
CP-ML) (59 kBq/animal, or 1.5 µCi) after intravenous or gavage
administration to C57Bl mice was studied by Darnerud & Brandt (1982).
Twelve hours after intravenous injection, 12% of the radioactive dose
was recovered in urine, 44% in expired air (as 14CO2) and 4%
in faeces. Twelve hours after gavage administration, 6% of the
radioactive dose was recovered in urine, 33% in the expired air and
14% in faeces.
6.4.2.3 Bird
The elimination of radioactivity was studied in female Japanese
quail (Coturnix coturnix japonica) after gavage or intravenous
administration of a uniformly 14C-labelled polychlorohexadecane
(C16;69% Cl, CP-MH) (0.48 µmol/kg) (Biessmann et al., 1983). Of the
administered dose, 1.6% was exhaled as 14CO2 after gavage
administration and 0.9% after intravenous administration. Of the
administered dose 16% was excreted in faeces/urine after 8 h and 58%
after 96 h.
6.4.3 Long chain length chlorinated paraffins
A 14C-labelled chlorinated paraffin, C18; 50-53% Cl (CP-LH), was
administered by gavage as a single dose of 500 mg/kg to three female
Sprague-Dawley rats (Yang et al., 1987). After 24 h, 1% of the
radioactive dose was recovered in the urine, 1.5% in the expired air
and 22% in the faeces. After 96 h, 1.9% of the radioactive dose was
recovered in the urine, 3.3% in the expired air, 5% in body tissue and
76% in the faeces.
6.4.4 Comparative studies
In a study by Beissmann et al. (1982), 148 kBq (4 µCi)
(approximately 20 Ci/mol) of either 14C-labelled polychlorohexadecane
(C16;34% Cl, CP-ML) or 14C-labelled polychlorododecane (C12;56% Cl,
CP-SH) was administered by gavage to Japanese quail (Coturnix
coturnix japonica). A considerably higher level of radioactivity
was found in the gall bladder and kidney after C12 administration
compared with C16. On the other hand, the radioactivity in the yolk
of the first ten eggs was higher after C16 gavage than after C12
gavage. After 8 h, 39% of the C16 dose and 22% of the C12 dose was
exhaled as 14CO2, indicating that the rate of metabolism is
influenced by the chain length and/or the degree of chlorination.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute exposure
7.1.1 Lethal doses
The acute oral toxicity of chlorinated paraffins has been studied
in rats and mice (Table 17). In all studies, the LD50 was reported
to be greater than the highest administered dose (i.e. always
> 4 g/kg body weight). After inhalation of Chlorowax 500C (C12;59% Cl,
CP-SH), an LC50 was not established in the one reported study (LC50
> 3300 mg/m3). The LD50 for dermal exposure of rabbits to
Chlorowax 500C (C12;59% Cl, CP-SH) was in excess of approximately
13 g/kg body weight (Howard et al., 1975).
Table 17. Acute toxicity of chlorinated paraffins
Chlorinated Compounda Species Test Exposure Reference
paraffin concentration
C12;60% Cl (CP-SH) rat oral LD50 > 13.6 g/kg Bucher et
al. (1987)
C12;59% Cl Chlorowax rat oral LD50 > 21.5 g/kg Howard et
500C (CP-SH) al. (1975)
C12;59% Cl Chlorowax rabbit dermal > 13 g/kg Howard et
500C (CP-SH) LD50 al. (1974)
C12;59% Cl Chlorowax rat inhalation > 3300 Howard et
500C (CP-SH) LC50 mg/m3 al. (1975)
C12;60% Cl (CP-SH) mouse oral LD50 > 27.2 g/kg Bucher et
al. (1987)
C10-13;41-70% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C14-17;51-60% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C24;40% Cl Chlorowax 40 rat oral LD50 > 17.7 g/kg Howard et
(CP-LL) al. (1975)
C23;43% Cl (CP-LL) rat oral LD50 > 13.6 g/kg Bucher et
al. (1987)
Table 17. (Cont'd)
Chlorinated Compounda Species Test Exposure Reference
paraffin concentration
C23;43% Cl (CP-LL) mouse oral LD50 > 27.2 g/kg Bucher et
al. (1987)
C20-30;41-70% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C24;70% Cl Chlorowax 70 rat oral LD50 > 50 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorez 700 rat oral LD50 > 50 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorowax 70 guinea-pig oral LD50 > 25 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorez 700 guinea-pig oral LD50 > 25 g/kg Howard et
(CP-LH) al. (1975)
a The classification is given in Table 1
7.1.2 Non-lethal doses
7.1.2.1 Oral route
In a single-administration experiment, F-344/N rats and B6C3F1
mice were dosed by gavage up to 13.6 g/kg body weight (rats) and
27.2 g/kg body weight (mice) of C12;60% Cl (CP-SH) or C23;43% Cl
(CP-LL) dissolved in corn oil. No deaths or compound-related toxic
effects were noted during the 14-day observation period. However, the
animals were inactive with diarrhoea for 2-6 days after dosing, which
was attributed to the large volumes of material administered (NTP,
1986a,b; Bucher et al., 1987).
Female or male Wistar rats were administered by gavage a single
oral dose of different chlorinated paraffins (C10-13; 41-51%, 51-61% or
61-70% Cl), with a range of maximum doses of 4-13 g/kg body weight,
and were observed for 7 days (ICI, 1965, 1966, 1968, 1969, 1971, 1973,
1974a,b; Birtley et al., 1980). Clinical signs of toxicity, such as
piloerection, muscular incoordination and faecal and urinary
incontinence, were observed in rats that received doses of 2 g/kg body
weight or more (generally independent of the chlorine content).
Recovery was usually complete by day 7. There were no deaths except
for one rat treated with 13 g/kg body weight of C10-13;63% Cl.
Similar findings were reported for C14-17;51-60% Cl and
C20-30;41-51%, 51-61% or 61-70% Cl (Birtley et al., 1980). The
toxicity of these chlorinated paraffins was reported to be lower than
that of the short chain length chlorinated paraffins.
7.1.2.2 Inhalation route
No toxic response was observed in rats exposed to a concentration of
3300 mg/m3 of Chlorowax 500C (C12;59% Cl, CP-SH) for one hour (Howard
et al., 1975)
7.1.2.3 Intraperitoneal route
Three different chlorinated paraffin preparations, Chlorez 700
(C20;70% Cl, CP-LH), Paroil 170-HV (C11;70% Cl, CP-SH) and
Chloroparaffin 40 (chain-length not given, 40% Cl), were administered
intraperitoneally as single doses (Chlorez and Chloroparaffin
100 mg/kg body weight; Paroil 52 mg/kg body weight) to male Wistar rats
(Ahotupa et al., 1982). The activities of various drug-metabolizing
enzymes from liver, kidney and small intestinal mucosa were determined
after 24 h, 7 days or 21 days. Minor but significant changes in the
intestinal activities of aryl hydrocarbon hydroxylase (increase),
UDP-glucuronosyltransferase (decrease) and epoxide hydrolase
(increase) were induced by Paroil 170-HV, and in the kidney activity
of aryl hydrocarbon hydroxylase by Chlorez 700, when compared to
polychlorinated biphenyls and naphthalenes.
7.1.3 Skin and eye irritation
7.1.3.1 Short chain length chlorinated paraffins
In a study by Hoechst (1986b), 0.5 ml of undiluted C10-13;50%
Cl(CP-SH) was applied under a semi-occlusive dressing to the shaven
skin of three rabbits for 4 h. The skin was examined for signs of
irritation for up to 72 h after the chlorinated paraffin had been
removed, but none were seen during the test.
When 0.5 ml of C10-13;70% Cl(CP-SH) was applied under a
semi-occlusive dressing to the shaven skin of three rabbits for 4 h,
one rabbit showed clearly defined erythema (grade 2 on a 0-4 scale) at
48 and 72 h. The other two animals showed "slightly noticeable"
erythema (grade 1). Very slight oedema (grade 1) was noted in two
animals for up to 24 h. By day 7, all signs of irritation were
completely resolved (Hoechst, 1983).
Two studies investigated C10-13;70% Cl(CP-SH). In one study the
chlorinated paraffin contained 1 or 2% of an epoxidised vegetable oil
stabilizer with and without additives (0.1% oxalic acid or 0.05%
benzotriazole) (ICI, 1965). Very mild to mild desquamation was only
noted following the applications of chlorinated paraffins containing
additives. The reactions were described as occasional, transient and
inconsistent. It was not stated how many applications were made
before these reactions were seen. In another study, no signs of
irritation were noted following repeated application of a chlorinated
paraffin containing 0.1 or 2% benzoyl peroxide initiator (ICI, 1974a).
Two studies investigated the effects of three C10-13;63%
chlorinated paraffins (CP-SH), containing up to 3% epoxy soya oil
stabilizers or other unspecified additives (ICI, 1973, 1974a). For
all three paraffins, erythema was usually noted following two to four
applications, although on one occasion erythema was noted in 1/3
animals after only one application. The severity of the reactions was
not described. Desquamation was also noted following three or four
applications and increased in severity with further treatments. In
one study (with 0.7% epoxy carboxylate stabilizer) the desquamation
was described as severe following the fourth application when the
study was terminated (ICI, 1973).
Studies have been conducted using C10-13 chlorinated paraffins
which were 48(CP-SL), 50, 52 or 55% chlorinated (CP-SH) (ICI,
1967, 1968, 1969, 1971, 1974a,b). In most of these studies the
chloroparaffins contained 0.2 or 2% epoxy stabilisers. In one study
with 48 or 55% chlorinated paraffins, containing 0.2% epoxy octyl
stearate stabilizer, no signs of irritation were noted (ICI, 1969).
In the other studies there was mild or slight erythema, and mild
desquamation was usually noted following the second or third
application. In one study, testing C10-13;52% with 2% epoxidised octyl
oleate stabilizer, erythema was noted following the first application
(ICI, 1968). It was observed in 4/5 of the studies that the reactions
did not worsen following further applications, although in one study
(testing a 52% chlorinated paraffin with unspecified additives),
slight erythema, noted after the second application, worsened to
severe erythema with slight necrosis after the third application, when
the study was terminated (ICI, 1971).
An unspecified volume of C10-13;40% Cl(CP-SL), containing 1% epoxy
soya oil stabilizer, produced slight desquamation following the second
application and mild erythema after the third (ICI, 1966). This
condition persisted throughout the remaining applications until the
end of the study when small scattered ulcers developed.
Two studies in rats were conducted to investigate the potential
for skin irritation of two short chain length chlorinated paraffins
(C10-11) which were 49% (CP-SL) and 60% chlorinated (CP-SH) (ICI, 1980,
1982c). Repeated and single application tests were conducted. No
signs of irritation were noted following a single application of the
more chlorinated paraffin, although slight desquamation was noted in
2/6 rats, 3-6 h after the treatment with the less chlorinated
paraffin. Both chlorinated paraffins produced slight erythema and/or
slight desquamation with repeated applications.
Rats were treated with 0.1 ml of C14-17;51-60% Cl(CP-MH), or
C20-30;41-51%(CP-LL), 51-61%(CP-LH) or 61-70% Cl(CP-SH), for up to
six 24-h periods. The treatment periods were separated by 24-h
treatment-free periods. In some of the studies the chlorinated
paraffins contained epoxy stabilizers. Mild irritation was seen with
C14-17 chlorinated paraffins, but it is not clear if the response was
due to the stabilizer. No signs of irritation were seen with C20-30
(Birtley et al., 1980).
A C10-13;61% chlorinated paraffin (Cereclor 60HS) and a 50%
chlorinated paraffin (Cereclor 50 HS) of unidentified carbon chain
length produced mild or moderate skin irritation following a single
occlusive application to intact or abraded skin of rabbits. It was
stated that varying degrees of erythema persisted for 72 h (ICI
1975a,b).
In other studies (BUA, 1992) different short and long chain
length chlorinated paraffins were applied to the skin and eyes of
rabbits (skin: C10-13;58% Cl, C19;44% Cl, C20-30;70% Cl; eyes: C12;59%
Cl, C20-30;70% Cl). Only a weak or no irritating effect was observed,
which decreased with increasing chain length.
The eye irritation potential of three different chlorinated
paraffins, C10-13;65% Cl(CP-SH), which contained either 2.5 or 2% of
two different additives or 0.7% of an epoxy stabilizer, was tested in
two studies (ICI, 1971, 1974a). Either 0.1 ml or "one drop" of the
chloroparaffin was instilled into one conjunctival sac of groups
of three rabbits. Similar results were reported for all three
formulations: practically no initial pain (2 on a 6-point scale) was
noted. Slight irritation (3 on a 8-point scale), shown by redness
and chemosis (only noted in the formulation containing the epoxy
stabilizer) of the conjunctiva with some discharge, lasted for 24 h.
One drop of 52% or 40% chlorinated paraffins, containing unspecified
additives or 1% epoxy stabilizer, was also tested (ICI, 1966, 1971).
With the 52% chlorinated paraffin, slight immediate irritation was
followed by slight redness of the conjunctiva which lasted for 24 h.
With the 40% chlorinated paraffin, mild congestion was noted at 1 h
but no effects were seen at 24 h.
7.1.3.2 Intermediate and long chain length chlorinated paraffins
Intermediate and long chain chlorinated paraffins were tested
in eye irritation studies with a single application of 0.1 ml of
C14-17;51-60% Cl(CP-MH), C20-30;41-50%(CP-LL), 51-60% or 61-70%
Cl(CP-LH). No signs of eye irritation were seen (Birtley et al.,
1980).
7.1.4 Skin sensitization
The maximization method was used to assess the skin sensitization
potential of a chlorinated paraffin (C10-13;56% Cl, CP-SH) with 1%
epoxide stabilizer (Edenol D81) and 1% tris-nonylphenyl phosphite
(TNPP) (Hoechst, 1983b). When challenged with undiluted chlorinated
paraffin, 1/20 test animals showed hardly perceptible erythema 24 h
after challenge, and 1/20 test and 1/10 control animals showed clearly
defined erythema or slight oedema at 72 h. The chlorinated paraffin
tested did not induce skin sensitization in this study.
The same chlorinated paraffin (C10-13;56% Cl, CP-SH), with 1% of a
different epoxide stabilizer (Rutapox CY 160) and 1% TNPP, was tested
using the same method (Hoechst, 1984). When challenged with undiluted
chlorinated paraffin, 5/20 test animals showed clearly defined erythema
and another two showed hardly perceptible erythema. None of the
control animals showed any evidence of a skin reaction. A second
challenge was performed 2 weeks after the first. On this occasion
4/20 test animals showed clearly defined erythema and another four
showed hardly perceptible erythema or slight oedema. The authors
concluded that the substance tested was a sensitizer. However, as
less than 30% of the test group showed a clear reaction and it is
possible that the epoxide stabilizer was responsible for producing the
sensitization reactions, this study is not considered to provide
conclusive evidence that C10-13;56% Cl is a skin sensitizer.
An undiluted chlorinated paraffin (C10-13;52% Cl, CP-SH) was
applied to the ears of six guinea-pigs on three successive days (ICI,
1971). Slight erythema was noted when, 4 days later, undiluted
chloroparaffin was applied to the animals' flanks, but it was not
stated how many animals showed a reaction. Four control animals also
showed slight erythema at challenge. It does not appear that this
chlorinated paraffin elicited a sensitization response in this study.
7.2 Repeated exposure
Studies involving repeated exposure have demonstrated that the
liver, kidneys and thyroid are the target organs for the toxicity of
chlorinated paraffins.
7.2.1 Oral route
7.2.1.1 Short chain length chlorinated paraffins
a) Rat, 14-day studies
In a range-finding study, a short chain chlorinated paraffin
(C10-13;58% Cl) (CP-SH) was administered to Fischer-344 rats in
the diet for 14 days at concentrations of 0, 900, 2700, 9100 and
27 300 mg/kg feed, equivalent to approximately to 0, 100, 300, 1000
and 3000 mg/kg body weight per day (IRDC, 1983c). There were five male
and five female rats per group. No deaths occurred during the study. A
marked reduction in body weight and food consumption was seen in the
highest dose group, which was attributed to reduced palatability of
the diet caused by the chlorinated paraffin. The relative liver
weight was increased (20-240%) in all dose groups compared to
controls. The activity of liver aminopyrine demethylase (APDM) was
increased in females, and cytochrome P-450 values increased in both
sexes in all dosed groups. Liver enlargement was observed in some
rats in the groups fed 2700 to 27 300 mg/kg, and a dose-related
increase in the incidence of hepatocellular hypertrophy was present in
all treated groups. Myocardial atrophy was observed at the two
highest dose levels although the relationship to treatment was
unclear. The lowest-observed-effect level (LOEL) in this study was
100 mg/kg body weight per day.
A short chain length chlorinated paraffin with 58% Cl (CP-SH) was
administered by gavage in corn oil to Fischer-344 rats (five/dose of
each sex) for 14 days at dose levels of 0, 30, 100, 300, 1000 and
3000 mg/kg body weight per day (IRDC, 1981a). A significant decrease
in body weight gain in females in the high dose group was noted. A
dose-related increase in APDM activity was observed in females fed
300-3000 mg/kg, whereas in males there was an increase only in the
group treated with 1000 mg/kg. Cytochrome P-450 levels were
significantly increased in females treated with 1000 mg/kg, and
microsomal protein concentration increased in females dosed with
3000 mg/kg. Liver enlargement occurred in both sexes in the 300, 1000
and 3000 mg/kg groups, and mild hepatocellular hypertrophy in all
animals of the 1000 and 3000 mg/kg groups. The absolute and relative
liver weight was increased (20-150%) at doses of 100 mg/kg or more.
Statistically significant reduction of thymus and ovary weight was
observed at 3000 mg/kg. The no-observed-effect level (NOEL) in this
study was considered to be 30 mg/kg body weight per day and the LOEL
100 mg/kg body weight per day, based on liver weight increases.
In a 16-day study on F-344/N rats (groups of five), the animals
were administered a short chain length chlorinated paraffin (C12;60%
chlorination) (CP-SH) by gavage in corn oil daily (5 days per week) at
doses of 0, 469, 938, 1875, 3750 and 7500 mg/kg body weight per day
for 16 days (NTP, 1986a; Bucher et al., 1987). At the highest dose
level there was reduced body weight gain (22% in males and 16% in
females), and 1/5 male rats and 2/5 females died before the end of the
study. Enlarged livers were observed in every dose group except the
females fed 469 mg/kg. No histopathology was performed. The LOEL in
this study was considered to be 469 mg/kg body weight per day.
b) Rat, 90-day studies
Fischer-344 rats (groups of 10 males and 10 females) were given a
short chain chlorinated paraffin (C12;60% chlorination) (CP-SH) in
corn oil by gavage on 5 days a week for 13 weeks at doses of 0, 313,
625, 1250, 2500 and 5000 mg/kg body weight per day (NTP, 1986a; Bucher
et al., 1987). Body weight gain was reduced by approximately 10% in
males at the two highest dose levels. A dose-related statistically
significant increase in relative liver weight (17-100% for males;
30-100% for females) was observed in all treated rats. Hepatocellular
hypertrophy was noted in all rats in the highest dose group, and
nephrosis was more frequent in this group (10/10 males; 3/10 females)
compared to controls (8/10 males; 0/10 females). Rats in other dose
groups were not examined microscopically. On the basis of an increase
in liver weight, the LOEL in this study was 313 mg/kg body weight per
day.
In a 13-week study Fischer-344 rats were administered a short
chain length chlorinated paraffin (C10-13;58% Cl, CP-SH) in corn oil by
gavage at doses of 0, 10, 100 and 625 mg/kg body weight per day in
groups of 15 animals of each sex (IRDC, 1984a). In the groups treated
with 100 mg/kg or more, increased weights of the liver (30-110%) and
the kidneys (20-100%) were observed. At the highest dose level
thyroid weights were increased. Hepatocellular hypertrophy was
observed at 100 and 625 mg/kg. In these groups hypertrophy and
hyperplasia of the thyroid were also observed. There was trace-to-mild
chronic nephrosis in the kidney of males treated with 625 mg/kg, and in
females in the high-dose group, in which pigmentation of the renal
tubular epithelia also occurred. The NOEL was considered to be 10 mg/kg
body weight per day on the basis that no treatment-related microscopic
changes were found in any tissue at this dose. The LOEL was 100 mg/kg
body weight per day based on increased liver and kidney weights and
hypertrophy in liver and thyroid. When the same doses were
administered in the diet essentially identical results were obtained
(IRDC, 1984c).
Male and female Fischer-344 rats (5 or 10 per group) were
administered Chlorowax 500C (C12;58% Cl, CP-SH) in corn oil by gavage
at doses of 0, 313 and 625 mg/kg body weight per day for up to 90 days
(Elcombe et al., 1994). The relative liver weight was increased at
both doses (50 and 75%). Hepatic peroxisomal ß-oxidation (palmitoyl
CoA oxidation) was statistically significantly increased in a
dose-related manner from day 15. The activity of thyroxine-UDPG-
glucuronosyltransferase was significantly increased (at least 150%) at
both dose levels from day 15 onwards. Thyroid follicular cell
hypertrophy was observed at all time points, and hyperplasia at days
56 and 91. Replicative DNA synthesis was increased in thyroid
follicular cells at day 91. Renal tubular eosinophilia was observed
in males from day 15. In renal tubular cells replicative DNA
synthesis was increased in males. When a higher dose was administered
(1000 mg/kg body weight per day) marked decreases in the levels of
plasma thyroxine and increased plasma thyroid stimulating hormone were
observed. None of these effects were observed in male Dunkin Hartley
guinea-pigs, which were administered the chlorinated paraffin at doses
of 500 and 1000 mg/kg body weight per day. The LOEL in rats was 313
mg/kg body weight per day based on increased relative liver weights,
hepatic peroxisomal ß-oxidation and thyroxine-UDPG-glucuronosyl-
transferase activity.
c) Mouse, 14-day studies
B6C3F1 mice in groups of five were administered C10;60% Cl
(CP-SH) in corn oil by gavage daily for 16 days (NTP, 1986a; Bucher et
al., 1987). The doses were 938, 1875, 3750, 7500 and 15 000 mg/kg
body weight per day. All mice receiving 3750 mg/kg or more, and 4/5
males and 2/5 females receiving 1875 mg/kg died before the end of the
study. Diarrhoea was observed in all dosed groups except females
receiving 938 mg/kg. Enlarged livers were found in all treated
surviving mice. No histopathological examinations were conducted.
d) Mouse, 90-day studies
B6C3F1 mice (10 of each sex per dose) were exposed to C12;60%
Cl (CP-SH) by gavage five days per week for 13 weeks (NTP, 1986a;
Bucher et al., 1987). The doses were 125, 250, 500, 1000 or
2000 mg/kg body weight per day. No clinical signs of toxicity were
observed. In the males the body weight gain was reduced by 13% at the
highest dose level. The relative liver weights showed a dose-related
increase (17-160%) and were statistically significant from 250 mg/kg
in females and from 500 mg/kg in males. Hepatocellular hypertrophy
was observed in animals of both sexes treated with 250 mg/kg or more.
Focal hepatic necrosis was related to dosing at 500, 1000 and
2000 mg/kg in males and at 2000 mg/kg in females. The NOEL was
125 mg/kg body weight per day, and the LOEL was 250 mg/kg body weight
per day, based on hepatocellular hypertrophy.
7.2.1.2 Intermediate chain length chlorinated paraffins
a) Rat, 14-day studies
A chlorinated paraffin of intermediate chain length (C14-17) and
52% chlorination (CP-MH) was administered to Fischer-344 rats in the
diet at dosage levels of 150, 500, 1500, 5000 and 15 000 mg/kg feed,
which was reported to correspond to an average compound intake of
17.7, 57.7, 177, 562 and 1412 mg/kg body weight per day (IRDC, 1981b).
Five male and five female rats in each dose group were exposed daily
for 14 days. No mortality occurred among the treated animals.
Hepatic APDM activity was statistically significantly increased in
males receiving 562 mg/kg body weight per day (5000 mg/kg feed) and in
females receiving 1412 mg/kg body weight per day (15 000 mg/kg feed).
Slight increase in cytochrome P-450 values in male rats given
177 mg/kg body weight per day (1500 mg/kg feed) was observed but
appeared not to be related to dosing. Increased relative liver weight
was observed in the highest two dosage groups. Microscopic examination
of the liver revealed mild diffuse hepatocellular hypertrophy in all
animals receiving 562 and 1412 mg/kg body weight per day (5000 and
15 000 mg/kg feed). The NOEL was reported to be 57.7 mg/kg body weight
per day. The LOEL was 177 mg/kg body weight per day in males based on
increased cytochrome P-450 values and 562 mg/kg body weight per day in
females based on increased liver weight and hepatocellular hypertrophy.
b) Rat, 90-day studies
Male and female weanling Sprague-Dawley rats in groups of ten
were fed diets containing 0, 5, 50, 500 or 5000 mg/kg of C14-17;52%
Cl(CP-MH) for 13 weeks, yielding an average intake of 0, 0.4, 3.6, 36
and 360 mg/kg body weight per day for males and 0, 0.4, 4.2, 43 and
420 mg/kg body weight per day for females (Poon et al., in press).
There were no clinical signs of toxicity and no differences in body
weight gain. Relative liver weight was increased at 43 and 420 mg/kg
body weight per day in females and at 360 mg/kg in males. Relative
kidney weight was increased at the highest dose level in both sexes.
Serum cholesterol was increased in females from 4.2 mg/kg in a
dose-related manner. In the highest dose group of both sexes elevated
hepatic UDP-glucuronosyltransferase activity was observed, but only
females at this dose level showed increased APDM activity. Decreased
hepatic vitamin A levels were detected in females at 43 mg/kg and in
both sexes at the highest dose level. Mild, adaptive histopathological
changes were detected in the liver of both sexes at the two highest
dose levels, and in the thyroid of males from 36 mg/kg and females
from 4.2 mg/kg (reduced follicle sizes, collapsed angularity,
increased height, cytoplasmic vacuolation and nuclear vesiculation).
In the kidney, minimal changes were noted in the proximal tubules of
males at 360 mg/kg, and in the inner medulla tubules of females at 43
and 420 mg/kg. The NOEL in this study was 4 mg/kg body weight per day.
The LOEL was 36 mg/kg body weight per day (males) and 43 mg/kg body
weight per day (females).
In a 90-day feeding study, Wistar rats (groups of 24 males and 24
females) were fed diets containing 0, 250, 500, 2500 and 5000 mg/kg
feed of Cereclor S52 (C14-17;52% Cl, CP-MH) containing stabilizer (0.2%
epoxidized vegetable oil) (Birtley et al., 1980). A dose-related
decrease in body weight gain in males fed 500 mg/kg feed or more was
observed, which was accompanied by a reduction in food intake.
Significant increases in the relative liver weights in females were
observed at 500 mg/kg feed or more and in males at 2500 mg/kg feed.
Significant increases in relative kidney weights were observed at
5000 mg/kg feed in both sexes. Microscopic examination of the liver
showed evidence of a dose-related proliferation of the smooth
endoplasmic reticulum in the hepatic cells from 500 mg/kg feed.
Haematological investigation showed no abnormalities attributable to
the test compound. A tendency towards congestion of the kidney with
increasing concentration of Cereclor S52 in the diet was also observed.
The NOEL was 250 mg/kg feed (12.5 mg/kg body weight per day based on
food consumption data) and the LOEL was 500 mg/kg feed (25 mg/kg
body weight per day) based on increased relative liver weights and
proliferation of smooth endoplasmic reticulum.
A medium chain length chlorinated paraffin (C14-17;52% Cl, CP-MH)
was evaluated for subchronic toxicity in Fischer-344 rats (IRDC,
1984b). The chlorinated paraffin was administered to the rats (15 of
each sex per dose group) in the diet to provide dosage levels of 0,
10, 100 and 625 mg/kg body weight per day for 13 weeks. The
treatment did not induce signs of toxicity, alter survival or cause
ophthalmological changes. A slight reduction of body weight gain
(< 5%) was observed in both sexes at the highest dose, and was
associated with reduced food consumption. Traces of hepatocyte
hypertrophy (at 625 mg/kg) and increased absolute and relative liver
and kidney weights (at 100 and 625 mg/kg) were noted in both sexes.
Males in the high-dose group had an increased incidence of nephritis.
In addition, increased thyroid weight and thyroid hypertrophy and
hyperplasia were observed in males at 625 mg/kg. The NOEL was
considered in the report to be 10 mg/kg body weight per day. The LOEL
was 100 mg/kg body weight per day based on increased liver and kidney
weights.
c) Dog, 90-day studies
The effects of Cereclor S52 (C14-17;52% Cl, CP-MH) (containing
0.2% epoxidized vegetable oil as stabilizer) were studied in Beagle
dogs (Birtley et al., 1980). Four male and four female animals in
each group were fed a diet corresponding to 0, 10, 30 or 100 mg/kg
body weight daily for 90 days. No effects were found, except for
significantly increased serum alkaline phosphatase activity and
relative liver weight in males exposed to 100 mg/kg and an increase in
the smooth endoplasmic reticulum of hepatocytes from 30 mg/kg. The
NOEL was 10 mg/kg body weight per day. The LOEL was 30 mg/kg body
weight per day based on an increase of hepatic smooth endoplasmic
reticulum.
7.2.1.3 Long chain length chlorinated paraffins
a) Rat, 14-16 day studies
Fischer-344 rats (groups of five) were exposed to C22-26;43% Cl
(CP-LL) daily by gavage for 16 days at doses of 235, 469, 938, 1875 or
3750 mg/kg body weight per day. No compound-related clinical signs
of toxicity or mortality were observed. There were no changes
in body weight gain and no gross lesions were observed at necropsy.
Histopathological examinations were not conducted (NTP, 1986b; Bucher
et al, 1987).
Fischer-344 rats, in groups of five of each sex, were fed long
chain length paraffins of 70% chlorination for 14 days. The dietary
concentrations of the C22-26;70% Cl (CP-LH) were 0, 150, 500, 1500,
5000 and 15 000 mg/kg feed, corresponding to an average compound
intake of 0, 17.1, 55, 169, 565 and 1715 mg/kg body weight per day
(IRDC, 1982b). Tissues from liver, kidneys, spleen, lungs and
mesenteric lymph nodes were examined microscopically. Hepatic
microsomal Lowry protein, APDM activity and cytochrome P-450 values
were determined. No significant toxic effects were noted, and no
compound-related effects were found after microscopical examinations.
The NOEL was 1715 mg/kg body weight per day.
A chlorinated paraffin, C22-26;43% Cl (CP-LL), was administered by
gavage to Charles River 344 rats at doses of 0, 30, 100, 300, 1000 and
3000 mg/kg body weight per day (IRDC, 1982c). During the 14-day test
period no signs of toxicity were observed. Following sacrifice,
tissues from the liver, spleen, kidneys, pancreas, thymus and eyes
were examined microscopically; the only observation was a possible
increase in kidney nephrolithiasis in females exposed to the highest
dose level. Hepatic microsomal Lowry protein, APDM activity and
cytochrome P-450 values were determined and there were no alterations.
The NOEL was considered to be 3000 mg/kg body weight per day.
b) Rat, 90-day studies
A long chain length chlorinated paraffin, C23;43% Cl (CP-LL),
was administered to Fischer-344 rats in groups of 10 (each sex) by
gavage for 13 weeks at doses of 235, 469, 938, 1875 or 3750 mg/kg body
weight per day (NTP, 1986b; Bucher et al., 1987). No effects on body
or organ weights and no clinical signs of toxicity were observed. A
dose-related increased incidence of granulomatous inflammation was
noted in the livers of all exposed female rats but not in males. The
NOEL was 3750 mg/kg body weight per day for males. For females the
LOEL was 235 mg/kg body weight per day based on increased incidence of
granulomatous inflammation in the liver.
A long chain chlorinated paraffin (C20-30) with 43% Cl (CP-LL) was
administered in corn oil by gavage for 90 days to Fischer-344 rats at
three doses (100, 900 or 3750 mg/kg body weight per day) (IRDC,
1984f). Increases in liver weights and a multifocal granulomatous
hepatitis characterized by inflammatory changes and necrosis were
observed in all exposed females but not in males. In female rats
mineralization in the kidneys at the highest dose level was observed.
In addition to these observations, mild nephrosis was observed in the
males in the highest dose group. In males, the NOEL was 900 mg/kg
body weight per day and the LOEL was 3750 mg/kg body weight per day
(based on the occurrence of mild nephrosis). In females the LOEL was
100 mg/kg body weight per day based on liver effects.
The chlorinated paraffin C22-26;70% Cl (CP-LH) was administered to
Fischer-344 rats in the diet for 90 days at doses of 100, 900 and
3750 mg/kg body weight per day (IRDC, 1984g). Increased liver weight,
hepatocellular hypertrophy and cytoplasmic fat vacuolation were noted
at the highest dose level. The alanine aminotransferase (ALT)
activity was increased in both sexes at the highest dose level, and
aspartate aminotransferase (AST) activity was also increased in
females of this group. The NOEL was considered to be 900 mg/kg body
weight per day. The LOEL was 3750 mg/kg body weight per day based on
liver effects.
c) Mouse, 14-16 day studies
B6C3F1 mice in groups of five were given C22-26;43% Cl (CP-LL) by
gavage daily for 16 days at doses of 469, 938, 1875, 3750 or
7500 mg/kg body weight per day (NTP, 1986b; Bucher et al., 1987). No
compound-related clinical sign of toxicity or mortality was observed,
there were no changes in body weight gain, and no gross lesions were
observed at necropsy. Histopathological examinations were not
conducted.
d) Mouse, 90-day studies
The long chain length chlorinated paraffin C23;43% Cl (CP-LL)
was administered to B6C3F1 mice (in groups of 10 of each sex) by
gavage for 13 weeks at dose levels of 469, 938, 1875, 3750 or
7500 mg/kg body weight per day (NTP, 1986b; Bucher et al., 1987). No
effects on body or organ weights, no clinical signs of toxicity and no
histopathological effects were observed. The NOEL was 7500 mg/kg body
weight per day.
7.2.1.4 Comparative studies
The effects of representative chlorinated paraffins on liver
function and thyroid hormone function has been studied in male rats
(Alpk:APFSD) and male mice (Alpk:APFCD-1) (Wyatt et al., 1993).
Groups of five male rats or five male mice received 0, 10, 50, 100,
250, 500 or 1000 mg/kg body weight per day by gavage in corn oil, once
daily for 14 days. The chlorinated paraffins studied were Chlorowax
500C (C10-13;58%Cl, CP-SH), Cereclor 56L (C10-13;56%, CP-SH) and
Chlorparaffin 40C (C14-17;40% Cl, CP-ML). Effects on liver function
were assessed by changes in liver weight (expressed both as absolute
weights and liver:body weight ratio) and peroxisome proliferation
(expressed as the activity of palmitoyl co-enzyme A (CoA) oxidase).
All three chlorinated paraffins caused increases in liver weight and
palmitoyl CoA oxidation, indicative of peroxisomal proliferation. In
general, the rat was more sensitive to the effects of the chlorinated
paraffins on liver weight than the mouse. The doses of chlorinated
paraffin required to cause peroxisomal proliferation were, in general,
greater than those causing effects on liver weight, although there
appeared to be less of a difference in inter-species sensitivity.
However, the magnitude of the increase in palmitoyl CoA oxidation
caused by the short chain chlorinated paraffins (approximately 10-fold
increase as a maximal change) was greater than for the intermediate
chain grade (approximately 4-fold increase as maximal change) (Table
18). The effect of the chlorinated paraffins on thyroid function was
studied in the male rats receiving 1000 mg/kg body weight per day for
14 days by measuring the plasma levels of triiodothyronine (T3) and
thyroxine (T4) (free and total) and thyroid stimulating hormone
(TSH), and also the activity of hepatic microsomal UDP glucuronosyl-
transferase activity. All three chlorinated paraffins (at 1000 mg/kg
body weight per day) caused a reduction in plasma T4 levels (both
free and total) and an increase in plasma TSH levels. No effect was
observed on plasma T3 levels. All three chlorinated paraffins also
caused an increase (two-fold) in the rate of glucuronidation of T4
by hepatic microsomal UDP glucuronosyltransferase activity, suggesting
that the impact on plasma T4 and TSH levels is due to increased
clearance of T4 by hepatic metabolism.
Table 18. Effects of chlorinated paraffins on liver function in male rats and male mice
(From: Wyatt et al., 1993)
Increase in relative liver weight Increase in palmitoyl CoA
(% liver:body weight ratio) oxidase activity
Rat Mouse Rat Mouse
NOELa LOELb NOELa LOELb NOELa LOELb NOELa LOELb
C10-13;58% Cl 74 100 215 250 184 250 180 250
C10-13;56% Cl 51 50 70 100 600 1000 120 250
C14-17;40% Cl 31 100 426 1000 473 500 252 500
a Calculated using a three-parameter logit model (mg/kg body weight per day)
b Observed (mg/kg body weight per day)
The hepatic effects of representative chlorinated paraffins have
been studied in male and female F-344 rats, male and female B6C3F1
mice and male Alpk:Dunkin Hartley guinea-pigs (Elcombe et al., in
press). Their effects were compared with a range of known inducers of
hepatic enzymes. Groups of 4-5 animals received 1000 to 2000 mg/kg
body weight per day of each chlorinated paraffin (by gavage in corn
oil) for 14 consecutive days. The chlorinated paraffins studied were
Chlorowax 500C (C10-13;58% Cl, CP-SH), Cereclor 56L (C10-13;56% Cl,
CP-SH), Chlorparaffin 40G (C14-17;40% Cl, CP-ML) and Chlorowax 40
(C20-30;43% Cl, CP-LL). The short and intermediate chain length
chlorinated paraffins increased liver:body weight ratios (approximately
1.5 times) and elicited hepatocellular hypertrophy, peroxisome
proliferation (assessed as increases in peroxisomal volume and
palmitoyl CoA oxidase activity) and proliferation of hepatic cell
smooth endoplasmic reticulum in both rats and mice. These effects
were not seen in rats or mice receiving the long chain chlorinated
paraffin. The short and intermediate chain length chlorinated
paraffins also caused induction of cytochrome P-450 IV A1 (assessed by
increases in lauric acid hydroxylation) and P-450 II B1/IIB2 (assessed
by increases in ethoxycoumarin- O-diethylation) in the rat liver, but
only P-450 IV A1 in the mouse liver. The induction of the specific
cytochrome P-450 isoenzymes was confirmed using SOS-polyacrylamide gel
electrophoresis (SPS-PAGE) and Western immunoblotting of microsomes.
The administration of the short or intermediate chain length
chlorinated paraffins to guinea-pigs (1000 mg/kg body weight per day
for 14 days) had a similar effect on liver:body weight ratios (1.5-fold
increase) but had no effect on any of the hepatic ultrastructural or
biochemical parameters measured.
7.2.2 Intraperitoneal route
7.2.2.1 Short chain length chlorinated paraffins
Effects on both hepatic cytosolic and microsomal epoxide
hydrolases have been observed in male C57Bl/6 mice after 5 daily
intraperitoneal injection of 400 mg Cereclor 70L (C12;70% Cl, CP-SH)
(Meijer & DePierre, 1987). The hepatic cytosolic epoxide hydrolase
activity was increased to 130% and the microsomal activity to 250% of
the control. In addition, the amount of microsomal cytochrome P-450
was increased by 50%, and cytosolic DT-diaphorase activity was
increased 2- to 3-fold. There was also liver enlargement.
7.2.2.2 Intermediate chain length chlorinated paraffins
Lundberg (1980) reported a significant, dose-related increase in
total cytochrome P-450 in mice (strain not reported) injected
intraperitoneally with different amounts of Cereclor S52 (C14-17;52%
Cl, CP-MH) (from 0.6 mg to 63.4 mg) on 3 consecutive days. The
N-demethylation of ethylmorphine, a cytochrome P-450-dependent
reaction, decreased at low concentrations but increased at higher
concentrations.
7.2.2.3 Comparative studies
Male Sprague-Dawley rats were given intraperitoneal injections of
1000 mg/kg of Witaclor 149, 159 or 171P (C10-13 with 49% [CP-SL], 59%
[CP-SH] and 71% [CP-SH] chlorination, respectively), Witachlor 350
(C14-17 with 49% chlorination [CP-ML] or Witachlor 549 (C18-26 with 49%
chlorination [CP-LL]), each containing small amounts of epoxidated
soy-bean oil as stabilizer, daily for 4 days (Nilsen et al., 1980,
1981; Nilsen & Toftgård, 1981). Treatment with the C10-13 chlorinated
paraffins, but not those with longer chain lengths, caused increases
in liver weight and an induction of various forms of microsomal
cytochrome P-450. The activity of O-deethylation of 7-ethoxyresorufin
was decreased by the C10-13 chlorinated paraffins with higher
chlorine content, Witaclor 159 and 171P. The metabolism of
benzo (a)pyrene was induced by each of the chlorinated paraffins.
Witaclor 149 (C10-13;49% Cl) caused a significant proliferation of the
smooth endoplasmic reticulum (two-fold) whereas C18-26;49% Cl caused a
smaller increase. All three C10-13 chlorinated paraffins gave rise to
increased occurrence and size of cytoplasmic lipid droplets. Witachlor
149 also caused an increase in the number and size of mitochondria and
peroxisomes. These latter effects were also observed to a lesser
degree with both Witachlor 350 and Witachlor 549.
Effects on microsomal enzymes, after intraperitoneal injection of
Cereclor 42 (C22-26;42% Cl, CP-LL), Cereclor S58 (C14-17;58% Cl, CP-MH),
Cereclor 70 (C23;70% Cl, CP-LH) and Cereclor 70L (C10-13;70% Cl) (1000
mg/kg, once daily for 5 days) in liver from male Sprague-Dawley rats,
have been observed (Meijer et al., 1981). Microsomal epoxide hydrolase
activity was increased by all Cereclors except C22-26;42% Cl. In
addition, the activity of glutathione- S-transferase was increased
except in the case of C22-26;42% Cl, which decreased the activity
slightly. The amount of cytochrome P-450 was not changed.
7.3 Neurotoxicity
7.3.1 Short chain length chlorinated paraffins
The motor capacity, measured as the capacity of adult male NMRI
mice to remain on an accelerating rotarod, was determined after single
intravenous injections of 0, 30, 97.5, 165, 232.5 and 300 mg/kg in
groups of five mice of either Cereclor 50 LV (C10-13;49% Cl, CP-SL) or
Cereclor 70L (C10-13;70% Cl, CP-SH) (Eriksson & Kihlström, 1985). A
statistically significant decrease in the motor capacity and rectal
temperature was observed in mice receiving the highest dose of either
compound.
7.3.2 Intermediate chain length chlorinated paraffins
Immature 10-day-old NMRI mice (groups of at least 6) were given a
single peroral dose of 1 mg/kg body weight of polychlorohexadecane
(C16; chlorination degree not specified) dissolved in a fat emulsion
of egg lecithin and peanut oil (Eriksson & Nordberg, 1986). A
significantly decreased sodium-dependent choline uptake in the
cerebral cortex, 65% of Vmax in controls, was measured 7 days after
treatment indicating a pre-synaptic effect of this chlorinated
paraffin. No significant alteration in high- and low-affinity
muscarinic binding in the cerebral cortex in the brains could be
observed.
7.4 Reproductive toxicity, embryotoxicity and teratogenicity
7.4.1 Reproduction
An intermediate chain length chlorinated paraffin (C14-17) with
52% chlorination (CP-MH) was given in the diet to Charles River rats
at dose levels of 0, 100, 1000 and 6250 mg/kg feed (equivalent to 0,
6, 62 and 384 mg/kg body weight per day for the males and 0, 8, 74 or
463 mg/kg body weight per day for the females based on food
consumption data) (IRDC, 1985). The diet was fed both males and
females for 28 days before mating, during mating, and for females up
to postnatal day 21. Pups were given the same diet from weaning
until 70 days of age. No differences were observed in appearance,
fertility, body-weight gain, food consumption or reproductive
performance in the F0 generation. Among the offspring, no adverse
effects were observed prior to lactation day 7. However, significantly
decreased pup survival was observed in the high-dose group on lactation
day 10. None of the pups in this group survived to weaning. Survival
in pups from the mid-dose group was decreased by lactation day 21.
Necropsy findings in animals that died included pale liver, kidneys and
lungs, and blood in the cranial cavity, brain, stomach and intestines.
The pup weights were lower in the low-dose group (not statistically
significant) and mid-dose group than in the control group on lactation
day 21. In females, the reduced weight continued after weaning but
became less pronounced in males. Other observations in the pups of
the mid- and high-dose groups included bruised areas, decreased
activity, laboured breathing, pale discoloration and/or blood around
orifices. Reduced erythrocyte count, haemoglobin and haematocrit were
noted in the pups in the high-dose group on lactation day 6 relative to
the control values obtained on lactation day 7. The observations in
this study could indicate a high exposure of the pups to chlorinated
paraffins via the milk. This is supported by preliminary results of
a cross-fostering study showing a greater mortality in pups exposed
via milk than in pups exposed only in utero (Serrone et al., 1987).
The LOEL was 5.7 mg/kg body weight per day (males) or 7.2 mg/kg body
weight per day (females) in the F1 generation based on decreased
pup weight.
7.4.2 Embryotoxicity and teratogenicity
Teratology studies are summarized in Table 19.
Table 19. Oral teratology studies with chlorinated paraffins
Chlorinated paraffina Species LOEL NOEL Effects and remarks References
(mg/kg (mg/kg
body weight body weight
per day) per day)
CP-SH: C10-13;58% Cl rat 2000 500 maternal toxicity at 500 and 2000 mg/kg body weight IRDC (1982a)
per day; embryo-fetotoxicity and digital
malformations at 2000 mg/kg body weight per day
C10-13;58% Cl rabbit - 100 IRDC (1982d)
CP-MH: C14-17;52% Cl rat - 5000 slight maternal toxicity at 2000 and 5000 mg/kg IRDC (1984d)
body weight per day
C14-17;52% Cl rabbit - 100 mean maternal body weight losses were seen during IRDC (1983f)
treatment at the high-dose level (80, 100, 160 mg/kg
body weight per day) in a range-finding study
C14-17;70% Cl mouse - 100b Darnerud &
Lundkvist
(1987)
Table 19 (Cont'd)
Chlorinated paraffina Species LOEL NOEL Effects and remarks References
(mg/kg (mg/kg
body weight body weight
per day) per day)
CP-LL: C20-30;43% Cl rat - 5000 IRDC (1983d)
C20-30;43% Cl rabbit - 2000 slight increase in mean implantation loss and IRDC (1982e)
decreased number of viable fetuses at 5000 mg/kg
body weight per day, which were not statistically
significant
CP-LH: C22-26;70% Cl rat - 5000 IRDC (1984e)
C22-26,70% Cl rabbit - 1000 possible maternal toxicity (non-dosage-related IRDC (1983b)
congestion of lungs). In preliminary rabbit studies
an increase in post-implantation loss was observed
at > 1000 mg/kg body weight per day, but this effect
was not observed in the main teratology study
a The classification is given in Table 1
b Single intraperitoneal injection on day 1
7.4.2.1 Short chain length chlorinated paraffins
The teratogenic potential of a short chain length paraffin
(C10-13) with 58% Cl (CP-SH) was studied in pregnant Charles River COBS
CD rats (IRDC, 1982a). The rats, in groups of 25, were administered
0, 100, 500 and 2000 mg/kg body weight per day in corn oil orally by
gavage once daily from days 6 to 19 of gestation, and were examined on
gestation day 20. In the dams, the high dose treatment increased the
frequency of mortality (32%) and decreased body weight gain, and the
mid- and high-dose treatments resulted in dose-related adverse
clinical signs, such as yellow or brown matting and staining of the
anogenital fur, soft stool, red or brown matter (or staining) in the
nasal region, decreased activity, oily fur and excessive salivation.
Treatment at the highest dose level, which was a maternally toxic
dose, resulted in the appearance of fetal malformations such
as adactyly and/or shortened digits, increased incidences of
postimplantation loss and decreased numbers of viable fetuses. Fetal
body weight and incidence of delayed bone ossification were not
affected by the treatment. The NOEL for teratogenic effects was
500 mg/kg body weight per day, which was also a slightly maternally
toxic dose.
Female Dutch Belted rabbits (groups of 16) were treated by gavage
with a short chain length chlorinated paraffin (C10-13) with 58%
chlorination (CP-SH) (IRDC, 1982d). The rabbits were treated at dose
levels of 0, 10, 30 and 100 mg/kg body weight per day on gestation
days 6-27 and examined on day 28. There were no adverse effects on
survival, body weight gain, clinical signs or postmortem observations
in dams. The highest-dose group had an increased incidence of whole
litter resorption (two dams), and in the group exposed to 30 mg/kg
slight increases in the incidence of whole litter resorption (one dam)
and early and late resorptions were observed. Whole litter
resorptions did not occur in the low-dose or control animals, but
occurred in historical controls at an incidence of 13/277. This
indicated that the appearance of one or two dams with whole litter
resorptions could occur by chance. The NOAEL in this study was
100 mg/kg body weight per day.
7.4.2.2 Intermediate chain length chlorinated paraffins
Female Charles River COBS CD rats were treated by gavage with an
intermediate chain length chlorinated paraffin (C14-17;52% Cl) (CP-MH)
(IRDC, 1984d). The administered dose levels (0, 500, 2000 and
5000 mg/kg body weight per day) were given to groups of 25 animals on
gestation days 6-19, and this was followed by examination on day 20.
The end-points studied were weight of the uterus, number and location
of viable fetuses, early and late resorptions, the number of total
implantations and corpora lutea, and the incidence of fetal
malformations. The treatment had no adverse effect on mortality, body
weight gain or uterine weight of dams, but signs of toxicity, such as
wet, matted and yellow-stained hair in the anogenital area and/or soft
stools, were observed in the mid- and high-dose dams. No treatment-
related adverse effects were observed in the fetuses and there was no
evidence of developmental effects.
Female Dutch Belted rabbits were treated by gavage with an
intermediate chain length chlorinated paraffin (C14-17;52% Cl) (CP-MH)
in groups of 16 (IRDC, 1983f). The dose levels were 0, 10, 30 and
100 mg/kg body weight per day and were administered on gestation days
6-27, followed by examination on day 28. The end-points studied were
weight of the uterus, number and location of viable fetuses, early and
late resorptions, the number of total implantations and corpora lutea,
and the incidence of fetal malformations. In the dams, congestion of
the lobes of the lung was noted in all treated groups at necropsy, but
did not occur in a dose-related pattern. No significant adverse
effects were observed in fetuses and there were no developmental
effects.
In a study of NMRI mice, the animals were given a single
intraperitoneal injection of 100 mg/kg body weight of polychloro-
hexadecane (C16;70% Cl) (CP-MH) on the day the vaginal plug was
observed (day 1) (Darnerud & Lundkvist, 1987). The mice were killed
on day 14 of pregnancy, and the number and weight of embryos and
resorptions were determined. No effects on implantation or embryonic
survival were observed.
7.4.2.3 Long chain length chlorinated paraffins
Groups of 25 pregnant Charles River COBS CD rats were administered
(500, 2000 and 5000 mg/kg body weight per day) a long chain chlorinated
paraffin (C22-26;43% Cl) (CP-LL) by gavage from days 6 to 19 of
gestation (IRDC, 1983d). The end-points studied were weight of the
uterus, number and location of viable fetuses, early and late
resorptions, the number of total implantations and corpora lutea,
and the incidence of fetal malformations. In the dams, there were no
adverse effects on appearance, mean body weight gain or mortality
rate, and necropsy findings were normal. No signs of developmental
effects were noted in the pups.
Female Charles River COBS CD rats (groups of 25 animals) were
treated by gavage with a long chain length chlorinated paraffin
(C22-26;70% Cl) (CP-LH) (IRDC, 1984e). The dose levels were 0, 500,
2000 and 5000 mg/kg body weight per day on gestation days 6-19. The
rats were examined on day 20. The end-points studied were the same as
in the previous study. No treatment-related adverse effects were
observed in the dams or pups.
Pregnant Dutch Belted rabbits in groups of 16 were exposed orally
to long chain chlorinated paraffin (C22-26;43% Cl) (CP-LL) at doses of
500, 2000 and 5000 mg/kg body weight per day in corn oil by gavage
once daily from days 6 to 27 of gestation (IRDC, 1982e). The
end-points studied were the same as in the previous studies in this
section. No effects on maternal survival or body weight gain
occurred. In the highest-dose group there was a slight increase in
mean implantation loss and a slight decrease in the mean number of
viable fetuses when compared to the control group. However, these
alterations were not statistically significant. In the fetuses no
alterations related to the treatment were observed, although the
number in the highest-dose group was limited. Treatment of the
rabbits at a dose level of 2000 mg/kg body weight per day or less did
not produce a teratogenic response. No developmental effects were
noted. The NOEL in this study was 2000 mg/kg body weight per day.
Female Dutch Belted rabbits (groups of 16 animals) were given by
gavage a long chain length chlorinated paraffin (C22-26;70% Cl) (CP-LH)
(IRDC, 1983b). Doses of 0, 100, 300 and 1000 mg/kg body weight per
day were administered on gestation days 6-27, followed by examination
on day 28. The end-points studied were the same as in the earlier
studies in this section. The appearance, behaviour and body weight
gain were normal in the treated dams, although at necropsy a
non-dose-related increase in the occurrence of congested lungs was
noted. No adverse effects on the fetuses were observed and no
developmental effects were noted.
7.5 Mutagenicity and related end-points
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells there is a suggestion of a weak
clastogenic potential in vitro but not, according to several reports,
in vivo. Chlorinated paraffins were also reported to induce cell
transformation in vitro.
Table 20. Mutagenicity of chlorinated paraffins in bacterial tests
Chlorinated paraffina Dose (µg/plate) S9 Bacterial strainsb Effects Reference
Cereclor 50LV C10-13;50% Cl (CP-SH) 2500 ± TA1535 - Birtley et al. (1980)
TA1538 -
TA100 -
TA98 -
Hordalub 80 (with 1% C10-13;50% Cl (CP-SH) 10 000 ± TA98 + Hoechst (1986a)
epoxy stabilizer) TA100 -
TA1535 -
TA1537 -
TA1538 -
WP2 uvrAc -
Chloroparaffin 56 C12;57% Cl (CP-SH) 5000 ± TA98 - Hoechst (1988)
(unstabilized) TA100 -
TA1535 -
TA1537 -
TA1538 -
WP2 uvrAc -
Cereclor 70L C10-13;70% Cl (CP-SH) 2300 ± TA98 - Meijer et al. (1981)
TA100 -
TA1537 -
Cereclor S52 C14-17;52% Cl (CP-MH) 2500 ± TA1535 - Birtley et al. (1980)
- stabilizer TA1538 -
TA100 -
TA98 -
+ stabilizer -
Table 20. Cont'd
Chlorinated paraffina Dose (µg/plate) S9 Bacterial strainsb Effects Reference
Solvocaffaro C1642 C14-17;42% Cl (CP-ML) 1, 10, ± TA1535 - Conz & Fumero (1988a)
100, TA1537 -
1000, TA1538 -
5000 TA98 -
TA199 -
Meflex DC 029 C14-17;45% Cl (CP-ML) 1.6, 8, ± TA1535 - Elliott (1989a)
40, TA1537 -
200, TA1538 -
1000, TA98 -
5000 TA1000 -
Cereclor 42 C20-30;42% Cl (CP-LL) 2500 ± TA1535 - Birtley et al. (1980)
TA1538 -
TA100 -
TA98 -
Chorowax 40 C23;43% Cl (CP-LL) 10 000 ± TA97 - NTP (1986b)
TA98 -
TA100 -
TA1535 -
a The classification is given in Table 1
b Unless indicated otherwise, all strains refer to Salmonella typhimurium
c Strain of Escherichia coli
7.5.1 Prokaryotes
Most of the data demonstrate no mutagenic effects in four
Salmonella typhimurium strains after treatment with short,
intermediate or long chain length chlorinated paraffins at doses up to
10 mg/plate (Table 20). A very low but significant effect was
detected in strain TA98 after treatment with the highly chlorinated
short chain length chlorinated paraffin, Cereclor 70L (C10-12;70% Cl,
CP-SH) (Meijer et al., 1981). However, the observation is uncertain
since no dose response was observed, the increase in revertants was
low (less than 2-fold), and the increase was only found in the
presence of metabolic fraction (S9) derived from Aroclor-1254-induced
rat liver. The results from this study were considered to be
negative. Another study apparently demonstrated positive results.
However, the increase in the number of revertants with TA100 in the
presence of S9 was just less than two-fold, and in TA98, in the
absence of S9, the increase only just reached two-fold (Hoechst,
1986a). Furthermore, the possibility that the epoxy stabilizer was
responsible for the increase can not be discounted.
7.5.2 Mammalian cells
The results obtained from mammalian cell systems are summarized
in Tables 21, 22 and 23.
7.5.2.1 In vitro studies
C12;60% Cl (CP-SH) was mutagenic in L5178Y mouse lymphoma cells
at concentrations of 48 and 60 µg/ml in the absence of S9 mix (Myhr et
al., 1990).
When tested up to cytotoxic concentrations, C10-13;56% Cl(CP-SH)
did not induce a significant, reproducible increase in the number of
mutant colonies in Chinese hamster V79 cells (HPRT locus), either in
the presence or absence of S9.
Chlorowax 500C (C23;43% Cl, CP-LL) induced chromosome
aberrations in the absence of S9 mix at a concentration of 5000 µg/ml
and sister chromatid exchange (SCE) with and without S9 at 5, 500,
1700 and 5000 µg/ml in Chinese hamster ovary (CHO) cells in vitro
(Anderson et al., 1990).
7.5.2.2 In vivo studies
a) Short chain length chlorinated paraffins
When C12;60% Cl (CP-SH) (0, 500, 1000 and 2000 mg/kg body weight
was administrated in corn oil by gavage to Alpk:AP male rats (groups
of 4), no effects on unscheduled DNA synthesis (UDS) in hepatocytes
could be detected after exposure for 2 or 12 h (Ashby et al., 1990).
However, a moderate dose- and time-related induction of cell
proliferation, measured as S-phase cells, in the hepatocytes was
detected in animals exposed to 1000 and 2000 mg/kg for 12 h.
Table 21. Genotoxicity in mammalian cells in vitro
Chlorinated paraffin Cell line End-point Exposure Effect References
CP-SH: C10-13;56% Cl Chinese hamster mutations 5, 10, 15, 20, 30, 50, 75 µg/ml - Hoechst (1987)
V79 cells (±S9)
C12;60% Cl L5178Y mouse mutations 12, 24, 36, 48, 60 and 72 µg/ml + (from Myhr et al.
lymphoma cells (-S9) 60 µg/ml) (1990)
CP-LL: C23;43% Cl Chinese hamster chromosome 1250-5000 µg/ml (±S9) + (at 5000 Anderson et al.
ovary cells aberrations µg/ml + S9) (1990)
C23;43% Cl Chinese hamster sister chromatid 5, 500, 1700 and 5000 µg/ml +a Anderson et al.
ovary cells exchange (±S9) (1990)
a The effect was observed at all concentrations
Table 22. Cell transformation in in vitro mammalian cells
Grade Cell line Exposure Effect References
CP-SH: C10-13;50% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
C10-13;58% Cl Baby hamster kidney cells 44 µg/ml (-S9), 58 µg/ml (+S9) + ICI (1982a)
C10-13;58% Cl Baby hamster kidney cells 33 µg/ml (-S9), 88 µg/ml (+S9) + Richold et al. (1982a)
CP-MH: C14-17;52% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
CP-LL: C20-30;42% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
CP-LH: C20-26;70% Cl Baby hamster kidney cells 10, 50, 100, 500 and 1000 µg/ml (±S9) +a Richold et al. (1982b)
C22-26;70% Cl Baby hamster kidney cells 10 µg/ml (+S9), 294 µg/ml (-S9) + ICI (1982b)
a The effect was observed at all concentrations
Table 23. Genotoxicity data from in vivo mammalian cells
Grade Species and End-point Exposure No. of Effect References
strain animals
CP-SH: Fischer-344 Chromosome aberrations 250, 750 and 2500 mg/kg body weight 8 - IRDC (1983h)
C10-12,58% Cl rats in bone marrow cells per day for 5 days by gavage
C10-13,58% Cl Charles River Dominant lethal 250, 750 and 2500 mg/kg body weight 15 - IRDC (1983a)
COBS CD rats mutations per day for 5 days by gavage
C10-13,58% Cl NMRI mice Micronucleus assay 50, 5000 mg/kg body weight (single 10 - Muller (1989)
in bone marrow cells dose by gavage)
C10-13,58% Cl Hoe:NMRKF Micronucleus assay 50, 5000 mg/kg body weight (single 10 - Muller (1989)
SPF 71 mice in bone marrow cells dose by gavage)
C12;60% Cl Alpk: AP rats DNA repair in 500, 1000 and 2000 mg/kg body weight 4 - Ashby et
hepatocytes by gavage (single dose by gavage) al. (1990)
for 2 or 12 h
CP-ML: Crd: CD-1 Micronucleus assay 5000 mg/kg body weight (single dose 10 - Conz &
C14-17;42% Cl (ICR) Br mice in bone marrow cells by gavage) Fumero (1988b)
C14-17;45% Cl C57B1/6JFCD-1/ Micronucleus assay 3125, 5000 mg/kg body weight (single 10 - Elliott
Alpk mice in bone marrow cells dose by gavage) (1989b)
Table 23. (Cont'd)
Grade Species and End-point Exposure No. of Effect References
strain animals
CP-MH: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983g)
C14-17;52% Cl rats in bone marrow cells per day for 5 days by gavage
CP-LL: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983i)
C22-26;43% Cl rats in bone marrow cells per day for 5 days by gavage
CP-LH: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983e)
C20-30;70% Cl rats in bone marrow cells per day for 5 days by gavage
Sexually mature male Fischer-344 rats in groups of eight were
dosed by gavage once daily for 5 days with a short chain length
chlorinated paraffin (C10-12;58% Cl, CP-SH) at doses of 0, 250, 750 or
2500 mg/kg body weight per day (IRDC, 1983h). Metaphase spreads of
rat bone marrow cells were examined for chromosome aberrations. In
the group treated with 2500 mg/kg, all the rats except one died during
the study. Signs of overt toxicity were observed in most of these
rats. The rats receiving up to 750 mg/kg body weight per day did not
show an increased mortality or frequency of chromosome or chromatid
abnormalities, neither did the surviving rat from the highest dose
group. These observations indicate that toxic doses were administered
to the rats. Cytotoxicity was not assessed. However, the information
on distribution of short chain chlorinated paraffins following oral
absorption (section 6.3.1) indicates that there would have been
distribution to the bone marrow. This chlorinated paraffin was not
considered clastogenic in this test system.
Sexually mature NMRI (Hoe, NMRKF [SPF71]) mice (groups of five
males and five females) were given single doses of 50 and 5000 mg/kg
body weight Chlorowax 500C (C10-13;58% Cl, CP-SH) by gavage in sesame
oil (Hoechst, 1989). Responses at the high dose level were examined
at 24, 48 and 72 h sampling times and the low dose level at a 24 h
sampling time only. There were no differences from control values
either in polychromatic cells with micronuclei or in the ratio of
polychromatic erythrocytes to normocytes.
The dominant lethal mutation potential of a chlorinated paraffin
of short chain length and 58% chlorination was examined in Charles
River COBS CD rats (IRDC, 1983a). Groups of 15 males were treated
with 0, 250, 750 and 2000 mg/kg body weight per day in corn oil orally
by gavage for five consecutive days. Each male was then mated with 20
untreated females. There was no evidence of a mutagenic effect on the
post-meiotic stage of spermatogenesis at any dose level, as shown by
the absence of effect on the mean number of viable embryos during the
first four weeks of mating.
b) Intermediate chain length chlorinated paraffins
Sexually mature male Fischer-344 rats in groups of eight were
given unstabilized chlorinated paraffin (C14-17;52% Cl, CP-MH) in corn
oil by gavage once daily for 5 days at doses of 0, 500, 1500 and 5000
mg/kg body weight per day (IRDC, 1983g). Metaphase spreads of rat
bone marrow cells were examined for chromosome aberrations. No signs
of toxicity were observed during the study, and the treatments did not
produce any increase in the frequency of chromosome abnormalities,
compared to the controls.
Two studies yielding negative results in the mouse bone marrow
micronucleus assay have been reported. Sexually mature mice, of
strains CRI: CD-1 (ICR) BR (Conz & Fumero, 1988b) and C57BL/6JF
CD-1/ALpK (Elliott, 1989b) were used. Conz & Fumero (1988b) studied
Solvocaffaro C1642 (C14-17;42% Cl (CP-MH)) and Elliott (1989b) studied
Melex DC 029 (C14-17;45% Cl). In both studies groups of 10 animals:
(five males and five females) were given the limit dose of 5000 mg/kg
body weight by gavage in corn oil. Elliott (1989b) also used a lower
dose of 3125 mg/kg body weight. Responses at 5000 mg/kg were examined
at three sampling times (18, 43 and 66 h (Conz & Fumero, 1988b) or 24,
48 and 72 h (Elliott, 1989b)). Responses at the lower dose level
(Elliott, 1989b) were examined at 24 h. In both studies there were no
differences from negative control values in either polychromatic cells
with micronuclei or in the ratio of polychromatic erythrocytes to
normocytes. The positive control (mitomycin C [8 mg/kg body weight,
Conz & Fumero, 1988b] or cyclophosphamide [65 mg/kg body weight,
Elliott, 1989b]) produced the anticipated positive responses, thus
verifying the sensitivity of the test systems.
c) Long chain length chlorinated paraffins
When a long chain length paraffin with 70% chlorination (CP-LH)
(in 1% carboxymethylcellulose) was administered by gavage to
Fischer-344 rats (groups of 8) at doses of 500, 1500 and 5000 mg/kg
body weight daily for 5 days, no increased frequency of chromosome
abnormalities in bone marrow cells was observed indicating a lack of
clastogenic activity under the experimental conditions (IRDC, 1983e).
Body weight gain was decreased in the high-dose group.
Sexually mature male Fischer-344 rats in groups of eight were
given (gavage once daily for 5 days) a long chain length chlorinated
paraffin (C22-26;43% Cl, CP-LL) at doses of 500, 1500 and 5000 mg/kg
body weight per day (IRDC, 1983i). Metaphase spreads of rat bone
marrow cells were examined for chromosome aberrations. No signs of
toxicity was observed during the study, and the treatment did not
produce any increase in the frequency of chromosome abnormalities,
compared to the controls.
7.5.2.3 Cell transformation
Chlorowax 500C (C10-13;58% Cl, CP-SH), was examined in a cell
culture transformation test using baby hamster kidney (BHK) cells in
soft agar (Richold et al., 1982a). The cells were treated with doses
from 3.125 µg/ml to 500 µg/ml (-S9) and from 6.25 µg/ml to 1000 µg/ml
(+S9). Treatment with LC50 doses of 33 µg/ml (-S9) and 88 µg/ml (+S9)
increased the transformation frequency 52 times in the absence of S9
(the control was negative at 50% simulated survival) and 500 times in
the presence of S9.
Cereclor 50LV (C10-13;50% Cl, CP-SH) was not active at dose levels
up to 2500 µg/ml in a cell transformation assay using BHK cells
(Birtley et al., 1980).
Cereclor S52 (C14-17;52% Cl, CP-MH) with or without stabilizer
did not induce transformation of BHK cells at doses up to 2500 µg/ml
(Birtley et al., 1980).
After treatment of BHK cells with a long chain length paraffin
Electrofine S-70 (C20-26;70% chlorination, CP-LH) in doses from
10 µg/ml to 1000 µg/ml (+S9), a dose-related increased frequency of
transformed colonies was demonstrated at all dose levels (Richold et
al., 1982b).
Cereclor 42 (C20-30;42% Cl, CP-LL) was not active at dose levels
up to 2500 µg/ml in a cell transformation assay in BHK cells (Birtley
et al., 1980).
7.6 Long-term exposure and carcinogenicity
7.6.1 Oral route
7.6.1.1 Short chain length chlorinated paraffins
In a 2-year gavage study using Fischer-344 rats, groups of 50
males and 50 females were given C12;60% Cl, CP-SH at doses of 312 or
625 mg/kg per day, 5 days/week, for 104 weeks) (NTP, 1986a; Bucher et
al., 1987). After week 37, the body weights of the high-dose males
were reduced by 10-23% compared with controls. Survival of both
low- and high-dose males and of low-dose females was significantly
less than that of controls (at termination 27 male controls, 6
low-dose and 3 high-dose males and 34 control females, 23 low-dose and
29 high-dose females survived). Additional groups of 20 male and 20
female rats were added to each dose group for concurrent 6-month and
12-month studies. The spleen, liver, thymus, adrenal glands, brain,
kidney and heart were weighed at necropsy in the 6- and 12-month
studies. Biochemical and haematological effects were not examined.
The incidence of tumours, which was significantly increased, is
presented in Table 24. Liver neoplastic nodules and hepatocellular
carcinomas combined in males and females occurred with a positive
trend. The incidence of kidney tubular cell adenomas and the combined
incidence of adenomas and adenocarcinomas were significantly increased
in low-dose male rats. In low-dose females an increased incidence of
thyroid follicular cell adenomas was observed, and in addition,
adenomas and carcinomas combined showed an increased incidence in
high-dose female rats. Increased incidences of mononuclear cell
leukaemia, pancreatic acinar cell adenomas and endometrial stromal
polyps of the uterus (low-dose female rats) were observed. The
increased incidence of mononuclear cell leukaemia was dose-related in
males but not in females. Although the incidence of endometrial
stromal polyps in the low-dose female rats was greater than in vehicle
controls, these tumours were probably not treatment-related since
there were no increases at higher doses. The incidence of pancreatic
acinar tumours was also increased in the low-dose group of males,
although, owing to the higher incidence in concurrent compared to
historical controls and the absence of a dose-response relationship,
the increase was not considered to be treatment-related.
In the same study some chronic non-neoplastic lesions were
reported. Hepatocellular hypertrophy was observed in 74% of the
low-dose group and in nearly all of the high-dose rats, but in none
of the control group. Necrosis and angiectasis were observed in the
livers in all dosed rats. In addition to increased kidney weight at 6
and 12 months in both sexes and dose levels, the incidence and
severity of nephropathy was increased in dosed females, as was the
severity of nephropathy in males in the concurrent 12-month study.
Erosion, inflammation and ulceration of the glandular stomach and the
forestomach were seen in both groups of the dosed males. Hyperplasia
of the parathyroid was observed in both groups of exposed males and in
high-dose females.
Groups of 50 male and 50 female B6C3F1 mice were given C12;60%
Cl, CP-SH by gavage at doses of 125 and 250 mg/kg 5 days a week for
103 weeks (NTP, 1986a; Bucher et al., 1987). The body weights of
treated females were about 10% lower than those of controls during the
second year. The survival of treated males was not significantly
different from that of the controls, but in the high-dose group of
females fewer animals were still alive after week 100 in comparison
with the control group. The tumour incidences are shown in Table 25.
Increased incidences of liver adenomas and liver adenomas and
carcinomas in combination were observed. The incidences of thyroid
follicular cell adenomas and carcinomas combined were increased in
exposed female mice. The incidences of alveolar/bronchiolar
carcinomas were increased significantly in the high-dose group of male
mice, and the trend with dose was also significant. However, the
incidences of alveolar/bronchiolar carcinomas and adenomas (combined)
in males were not significantly greater than those in vehicle
controls. Among the non-neoplastic lesions, the incidence of
nephrosis was increased in the high-dose group of females, but was
decreased in dosed male mice compared to controls. Biochemical and
haematological effects were not examined.
Table 24. Incidence of tumours in rats administered the chlorinated paraffin C12;60% chlorine
(From: NTP, 1986a; Bucher et al., 1987)
Dose Hepatocellular Hepatocellular Hepatocellular Follicular cell Mononuclear Adenomas or
(mg/kg neoplastic carcinomas neoplastic nodules adenomas and cell leukaemia adeno-carcinomas
body weight) nodules and carcinomas carcinomas of of the kidney
the thyroid
Males
Control 0/50 0/50 0/50 3/50 7/50 0/50
312 10/50a 3/50a 13/50a 3/50 12/50b 9/50b
625 16/48a 2/48 16/48a 3/50 14/50b 3/49
Females
Control 0/50 0/50 0/50 0/50 11/50 0/50
312 4/50 1/50 5/50a 6/50a 22/50b 0/50
625 7/50a 1/50 7/50a 6/50a 16/50 0/50
a Incidental tumour test for trend, p < 0.05, increase relative to control
b Life table analysis, p < 0.05, increase relative to control
Table 25. Incidence of tumours in mice administered the chlorinated paraffin
C12;60% chlorine (From: NTP, 1986a; Bucher et al., 1987)
Dose Hepatocellular Hepatocellular Hepatocellular Follicular cell
(mg/kg adenomas carcinomas adenomas and adenomas and
body carcinomas carcinomas of
weight) the thyroid
Males
Control 11/50 11/50 20/50 3/49
125 20/50a 15/50 34/50a 4/50
250 29/50a 17/50 38/50a 3/49
Females
Control 0/50 3/50 3/50 8/50
125 18/50a 4/50 22/50a 12/49a
250 22/50a 9/50b 28/50a 15/49a
a Incidental tumour test for trend, p < 0.05, increase relative to control
b Life table analysis, p < 0.05, increase relative to control
7.6.1.2 Long chain length chlorinated paraffins
Groups of 50 male and 50 female Fischer-344 rats were treated by
gavage with 1875 and 3750 mg/kg body weight (male rats) and 100, 300
and 900 mg/kg body weight (female rats) of C23;43% Cl, CP-LL
dissolved in corn oil on 5 days a week for 103 weeks (NTP, 1986b;
Bucher et al., 1987). The treatment did not affect body weight or
survival, and no signs of clinical toxicity were observed. Additional
groups of 20 male and 20 female rats were exposed concurrently to the
same doses for 6 or 12 months for analyses of the weights of the
spleen, liver, thymus, adrenal glands, brain, kidneys and heart, serum
hepatic enzymes, including sorbitol dehydrogenase, AST and ALT, and
haematological parameters.
In female rats that were administered the chlorinated paraffin,
an increased incidence of adrenal gland medullary phaeochromocytomas
was observed (1/50; 4/50; 6/50; 7/50, high dose statistically
significant, significant positive trend). In addition, an increased
incidence of endometrial stromal polyps in the uterus was found in the
low-dose group of the females, but this increase was not dose-related
(9/50; 17/50; 10/50; 10/50, low dose statistically significant) (Table
26). In males, acinar cell tumours of the pancreas occurred with a
negative trend. The incidence of benign hepatocellular neoplasia was
not increased in dosed rats.
Table 26. Incidence of tumours in rats administered C23;43% Cl
(From: NTP, 1986b; Bucher et al., 1987)
Dose (mg/kg body Adrenal medulla, Uterus, endometrial
weight) phaeochromocytomas stromal polyps
Females
0 1/50 9/50
100 4/50 17/50a
300 6/50 10/50
900 7/50a 10/50
a Incidental tumour test for trend, p < 0.05, increased relative
to control
Several non-neoplastic lesions were related to the administration
of C23;43% Cl. Relative liver weights were increased in exposed
males at 12 months and in exposed females at 6 and 12 months. The
observed increases were dose-related. Activities of several serum
enzymes were also slightly elevated at both 6 and 12 months. There
were also variations in haematological parameters at 6 and 12 months,
but only in females. The primary non-neoplastic lesion related to
administration of this chlorinated paraffin included a diffuse
lymphohistiocytic inflammation in the liver and in the pancreatic and
mesenteric lymph nodes in male and female rats in all exposed groups.
Splenic congestion was a secondary effect.
Groups of 50 male and 50 female B6C3F1 mice were given, by
gavage, C23;43% Cl, CP-LL dissolved in corn oil at doses of 2500 and
5000 mg/kg on 5 days a week for 103 weeks (NTP, 1986b; Bucher et al.,
1987). The survival of the mice was not significantly different in
treated groups compared to controls, and there were no clinical signs
of toxicity. However, in the female groups a Klebsiella infection
affected the animals after week 65, and 60 to 70% of the early deaths
in each group were attributed to the infection. Low-dose males and
females had lower weight gains than control or high-dose groups. The
incidence of malignant lymphomas was significantly increased in males
of the high-dose group, and occurred with a positive trend (Table 27).
The incidences of hepatocellular carcinomas in females occurred with a
positive trend, but the increase was not significant. The incidences
of adenomas and carcinomas of the liver (combined) were marginally
increased in females. Follicular cell carcinomas in males occurred
with a positive trend (0/49; 0/48; 3/49) in the thyroid gland.
However, the incidence of follicular cell adenomas or carcinomas
(combined) was not significantly greater than that in vehicle controls
(1/49, 3/48; 5/49) and was within the range of historical controls for
the test laboratory. No significant increases in non-neoplastic
lesions were attributed to administration in mice.
Table 27. Incidence of tumours in mice administered C23;43% Cl
(From: NTP, 1986b; Bucher et al., 1987)
Dose (mg/kg Lymphoma Hepatocellular Hepatocellular
body weight) carcinomas adenomas and
carcinomas
Males
0 6/50 9/50 18/50
2500 12/50 12/50 21/50
5000 16/50a,b 12/50 23/50
Females
0 15/50 1/50 4/50
2500 12/49 1/49 3/49
5000 20/50 6/50 10/50
a Incidental tumour test for trend, p < 0.05, increased relative
to controls
b Life table test, p < 0.05, increased relative to controls
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Controlled human studies
Chlorinated paraffins C10-13;50 and 63% Cl(CP-SH) were applied,
under occlusive dressings, to the upper arm of 26 volunteers
(INVERESK, 1975). After 24 h the applications were removed and one
hour later skin reactions were examined by two independent assessors.
A second application was made and reactions were assessed after a
further 24 h contact. Mild erythema and dryness (average scores, read
at the 24 and 50 h time points, of less than 2 and 1, respectively, on
a 4-point scale) were recorded, which were comparable to scores in a
liquid paraffin control group.
Paroil 142 (C20-30;40-41% Cl, CP-LL) and Chlorez 700 (C24;70% Cl,
CP-LH) were applied to the skin of 200 male and female volunteers for
a 5-day period, then reapplied for 2 days beginning 3 weeks after the
initial exposure. The dose level was not reported. No primary local
irritation, allergic response or other toxic responses were observed.
In a similar study, Chlorowax 70 (C24;70% Cl, CP-LH), Chlorowax 500C
(C12;59% Cl, CP-SH) and Chlorowax 40 (C24;43% Cl, CP-LL) were applied
to the skin of 200 males and female volunteers. The exposure time
period and amount of chlorinated paraffins used were not reported.
The treatments did not produce local irritation or allergic responses
(Howard et al., 1975).
8.2 Occupational exposure
In a study on cutting fluid coolants, 134 non-exposed employees
and 75 exposed employees were patch tested with various constituents
of the cutting fluids including chlorinated paraffins (Menter
et al., 1975). No positive reactions were obtained with any of the
constituents, although the authors themselves suggested that the tests
were not sufficiently stringent, as some positive reactions were
anticipated for some of the constituents tested.
Positive skin reactions to chlorinated paraffin constituents were
obtained in patch tests conducted on four employees suffering from
scaly eczema, who had been exposed occupationally to cutting oils
(English et al., 1986). However, the authors concluded that the
reaction was due to additives in the cutting oil, which showed
positive reactions when tested alone, rather than to the chlorinated
paraffin.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
The inhibition of gas production in an anaerobic sewage sludge
digestion process by C10-12;58% Cl (CP-SH) was studied by Madeley et
al. (1983b). A significant (> 10%) inhibition of gas production
occurred at chlorinated paraffin concentrations of 3.2, 5.6 and 10%
(w/w with respect to digester volatile suspended solids) during the
first 3-4 days of the experiment, but the gas production recovered
during the rest of the experiment until termination at day 10. It
was concluded in the report that the chlorinated paraffin induced a
transient partial inhibition and no longer-term effects.
9.1.2 Aquatic organisms
Acute toxicity data for aquatic invertebrates and fish are
summarized in Table 28. Chlorinated paraffins of short-chain length
have been shown to be acutely toxic to both freshwater and saltwater
invertebrates. Most of the acute toxicity tests for intermediate
and long chain chlorinated paraffins on aquatic invertebrates
exceed the water solubility. However, a study on intermediate
chlorinated paraffins suggests that these may be acutely toxic.
Short, intermediate and long chain chlorinated paraffins appear to
be of low acute toxicity to fish, the LC50 values being well
in excess of the water solubility.
9.1.2.1 Aquatic plants
Exposure of the freshwater alga Selenastrum capricornutum for
10 days to C10-12;58% Cl (CP-SH) at dose levels of 110, 220, 390, 570,
900 and 1200 µg/litre resulted in a significant inhibition of growth
at 570 µg/litre. The calculated EC50 values for cell density over 4,
7 and 10 days were 3690, 1550 and 1310 µg/litre, respectively, which
all exceeded the highest tested concentration (Thompson & Madeley,
1983d).
The marine alga Skeletonema costatum was exposed to C10-12;58%
Cl (CP-SH) for 10 days at concentrations of 0, 4.5, 6.7, 12.1, 19.6,
43.1 and 69.8 µg/litre (Thompson & Madeley, 1983b). Significant
inhibition of growth was observed on the first 2 days from
19.6 µg/litre. The EC50 for cell density after 4 days was
42.3-55.6 µg/litre, and EC50 for growth rate after 2 days was
31.6 µg/litre. No significant reduction in cell density was observed
after 10 days, indicating an effect on duration of lag phase prior to
exponential growth or a drop in chlorinated paraffin concentration after
10 days.
Table 28. Acute toxicity of chlorinated paraffins to aquatic organisms
Species Chlorinated Parameter Concentration Reference
paraffin (µg/litre)
Water flea C10-12;58% Cl 48-h EC50b 530 Thompson &
Daphnia magna C10-12;58% Cl 72-h EC50b 24 Madeley
(freshwater) C10-12;58% Cl 96-h EC50b 18 (1983c)
C10-12;58% Cl 120-h EC50b 14
C14-17;52% Cl 48-h EC50b 37 Frank &
Steinhäuser
(in press)
Mysid shrimp C10-12;58% Cl 96-h LC50 14.1-15.5 Thompson &
Mysidopsis bahia Madeley
(estuarine) (1983a)
Nitocra spinipes C10-13;49% Cl 96-h LC50 100 Tarkpea
(marine C10-13;70% Cl 96-h LC50 < 300 et al.
crustacean) C14-17;45% Cl 96-h LC50 9000 (1981)
C14-17;52% Cl 96-h LC50 > 10 × 106
C22-26;42% Cl 96-h LC50 > 1 × 106
C22-26;49% Cl 96-h LC50 > 10 × 106
Bleak C10-13;49%, 96-h LC50 > 5 × 106 Lindén et
Alburnus 63%, 71% Cla al. (1979)
alburnus C10-13;56%, Cl 96-h LC50 > 10 × 106
(estuarine) C11.5;70% Cl 96-h LC50 > 10 × 106
C15.5;40% Cl 96-h LC50 > 5 × 106
C14-17;50%, 96-h LC50 > 5 × 106
52% Cla
C22-26;42% Cl 96-h LC50 > 5 × 106
C18-26;49% Cl 96-h LC50 > 5 × 106
Rainbow trout C20-30;42% Cl 96-h LC50 770 × 103 Madeley &
Oncorhynchus Birtley
mykiss (1980)
(freshwater)
a Consecutive percentage chlorinations refer to different tests
b EC50 based on immobilization
9.1.2.2 Invertebrates
Short-term toxicity data for aquatic invertebrates are summarized
in Table 29 and chronic toxicity data in Table 30.
The freshwater crustacean Daphnia magna was exposed to a short
chain length paraffin with 58% Cl (CP-SH) (Thompson & Madeley, 1983c).
The chlorinated paraffin caused the organisms to float at or near the
surface of the water at 75 µg/litre or more. All water fleas died at
16.3 µg/litre after 6 days in a continuous-flow experiment. The
following LC50 values were calculated: 3 days, 24 µg/litre; 4 days,
18 µg/litre; 5 days, 14 µg/litre; 6 to 21 days, 12 µg/litre. No dead
parent Daphnia were observed at 8.9 µg/litre after 21 days of
exposure, but 37% of the offspring were dead as compared to 6% and 9%
in the controls. No increased mortality in the offspring was observed
at 5.0 µg/litre. The number of offspring per female was reduced at
2.7 µg/litre.
Daphnia magna was studied in a 48-h test with C14-17;52% Cl and
C18-20;52% Cl. Using the water-soluble fraction of a loading
concentration of 100 mg/litre, an EC50 of 37 µg/litre for the
intermediate chain length chlorinated paraffin and an EC0 of
> 26 µg/litre for the long chain length chlorinated paraffin were
observed. In a 21-day reproduction test, daphnids were exposed to the
water-soluble fraction of both chlorinated paraffins. With a loading
of 100 mg/litre, a no-observed-effect concentration of 4.4-8.8 µg/litre
was found for reproduction rate and parent mortality (LOEC =
19.9-35.6 µg/litre) for the intermediate chain length chlorinated
paraffin. For the long chain length chlorinated paraffin, a LOEC of
< 1.2 µg/litre was found for the same two parameters. In these
studies it was observed that a higher loading concentration of
10 g/litre caused an increase in the effect concentrations (Frank &
Steinhäuser, in press).
In studies of the marine shrimp Mysidopsis bahia, the 96-h
LC50 was between 14.1 and 15.5 µg/litre after exposure to a short
chain length chlorinated paraffin with 58% Cl (CP-SH) (Thompson &
Madeley, 1983a). After 28 days of exposure to 0.6, 1.2, 2.4, 3.8 and
7.3 µg/litre, increased mortality was observed but this was not
treatment-related. The increased mortality was significantly
different from the control (at 1.2 and 2.4 µg/litre) but not from the
solvent control, and was therefore considered as not related to the
chlorinated paraffin concentration. No treatment-related effects of
this chlorinated paraffin on reproductive rate or growth over this
time period were observed.
Table 29. Short-term toxicity data for aquatic invertebrates
Chlorinated paraffin Organism LC50 Exposure Other effects LOEC References
period
C10-13;49% Cl (CP-SL) Nitocra spinipes 100 µg/litre 96 h Tarkpea et al.
(1981)
C10-12;58% Cl (CP-SH) Dapnia magna 24 µg/litre 3 days Increased mortality in 8.9 µg/litre Thompson & Madeley
offspring after 21 days (1983c)
C10-12;58% Cl (CP-SH) Mysidopsis bahia 14.1-15.5 96 h No effects after 28 days Thompson & Madeley
µg/litre of exposure up to (1983a)
7.3 µg/litre
C10-12;58% Cl (CP-SH) Midge larvae, 48 h No adverse effects EG & G Bionomics
Chironomus tentans after exposure to (1983)
18-162 µg/litre
C10-13;70% Cl (CP-SH) Nitocra spinipes < 300 µg/litre 96 h Tarkpea et al.
(1981)
C14-17;45% Cl (CP-ML) Nitocra spinipes 9000 µg/litre 96 h Tarkpea et al.
(1981)
C14-17;52% Cl (CP-MH) Nitocra spinipes > 10 × 106 96 h Tarkpea et al.
µg/litrea (1981)
Table 29. (Cont'd)
Chlorinated paraffin Organism LC50 Exposure Other effects LOEC References
period
C22-26;42% Cl (CP-LL) Nitocra spinipes > 1 × 106 96 h Tarkpea et al.
µg/litrea (1981)
C22-26;49% Cl (CP-LL) Nitocra spinipes > 10 × 106 96 h Tarkpea et al.
µg/litrea (1981)
a Exceeding the water solubility
Table 30. Chronic toxicity of chlorinated paraffins to aquatic invertebrates
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C10-12;58% Cl Water flea, 6-12 days LC50 12 Thompson & Madeley (1983c)
Daphnia magna
21 days Increased mortality in 8.9
offspring after 21 days
C10-12;58% Cl Mysid shrimp, 28 days No treatment-related 7.3 Thompson & Madeley (1983a)
Mysidopsis bahia effects
C10-12;58% Cl Midge larvae, 49 days Halting adult 121 EG & G Bionomics (1983)
Chironomus tentans emergence (100%)
C10-12;58% Cl Mussel, 60 days LC50 74 Madeley & Thompson (1983a)
Mytilus edulis
84 days Growth reduction 9.3 Thompson & Shillabeer (1983)
91 days 23% mortality during 10.1 Madeley et al. (1983a)
exposure and 10%
mortality during
depuration
C10-12;58% Cl (CP-SH) Alga, Selenastrum 10 days Inhibition of growth 570 Thompson & Madeley (1983d)
capricornutum
C10-12;58% Cl (CP-SH) Alga, Skeletonema 10 days Inhibition of growth 19.6 Thompson & Madeley (1983b)
costatum
C14-17;52% Cl (CP-MH) Mussel 60 days Decreased filtration 3800 Madeley & Thompson (1983b)
Mytilus edulis activity
Table 30. (Cont'd)
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C14-17;52% Cl Water flea, 21 days Reproduction & parent 19.9-35.6 Frank & Steinhäuser (1994)
Daphnia magna mortality (LOEC)
C18-20;52% Cl Water flea, 21 days Reproduction & parent < 1.2 Frank & Steinhäuser (1994)
Daphnia magna mortality (LOEC)
C22-26;43% Cl (CP-LL) Mussel, 60 days Decreased filtration 2.180 Madeley & Thompson (1983c)
Mytilus edulis activity
C20-30;70% Cl (CP-LH) Mussel, 60 days Decreased filtration 1330 Madeley & Thompson (1983d)
Mytilus edulis activity
Larvae of the midge Chironomus tentans (second instar) were
exposed to C10-12;58% Cl (CP-SH) concentrations from 18 to 162 µg/litre
for 48 h (EG & G Bionomics, 1983). No adverse effects could be
detected. Exposure to 61-394 µg/litre for the 49 days life cycle
gave no effects except for halting adult emergence (100%) at 121 and
394 µg/litre.
The mussel Mytilus edulis was exposed to 2.3 and 10.1 µg/litre
of C10-12;58% Cl (CP-SH) for 147 days followed by 98 days of
depuration (2.3 µg/litre) or 91 days followed by 84 days depuration
(10.1 µg/litre) (Madeley et al. 1983a). A third of the mussels in the
high-dose group died during the exposure (23%) or depuration periods
(10%), and 7% of those in the low dose group, but this did not differ
significantly from the number of deaths in the acetone control.
When the mussel Mytilus edulis was studied over 60 days after
exposure to 13, 44, 71, 130 and 930 µg/litre of C10-12;58% Cl (CP-SH),
there was significant mortality at the three highest dose levels, the
median lethal time (LT50) values being 59.3, 39.7 or 26.7 days,
respectively (Madeley & Thompson, 1983a). The highest dose exceeded
the maximal solubility of the chlorinated paraffin. The LC50 for
this 60-day period was 74 µg/litre.
Mussels (Mytilus edulis) were exposed to 2.3 and 9.3 µg/litre
of a short chain length paraffin (58% Cl) (CP-SH) in sea water for 12
weeks (Thompson & Shillabeer, 1983). There were no mortalities at
either concentration, but at 9.3 µg/litre a reduction in growth rate
was observed, measured as shell length and tissue weight.
Treatment of mussels (Mytilus edulis) for 60 days with
C14-17;52% Cl (CP-MH) at concentration of 220 and 3800 µg/litre
gave no significant mortality, but there was an observed decrease
(non-quantitative visual observation) in filtration activity at the
higher concentration (Madeley & Thompson, 1983b). The highest dose
exceeded the maximal water solubility of the chlorinated paraffin.
Treatment of mussels (Mytilus edulis) with C22-26;43% Cl (CP-LL)
at 120 or 2180 µg/litre or with C20-30;70% Cl (CP-LH) at 460 and
1330 µg/litre for 60 days did not cause mortality, but visual
observation suggested that the filtration activity was reduced at the
higher dose levels (Madeley & Thompson, 1983c,d). The highest doses
exceeded the maximal solubility of the chlorinated paraffins.
9.1.2.3 Fish
Acute toxicity data are shown in Table 28 and chronic toxicity
data for fish are summarized in Table 31.
Table 31. Chronic toxicity of chlorinated paraffins to fish
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C10-13;49%, 59%, Bleak, 14 days Behavioural effects 125 (single Bengtsson & Baumann Ofstad
71% Cl Alburnus alburnus concentration) (1982)
C10-12;58% Cl Sheepshead minnow, 32 days Significantly reduced 279.7 Hill & Maddock (1983)
Cyprinodon variegatus size of larvae (LOEC)
C10-12;58% Cl Rainbow trout 60 days LC50 340 Madeley & Maddock (1983c)
Oncorhynchus mykiss
60 days Behavioural abnormalities 33
168 days after 60-70 days of Madeley & Maddock (1983b)
depuration
50% mortality 3.1
100% mortality 14.3
C14-17;50% Cl Bleak, 14 days No observed effect 125 (single Bengtsson et al. (1979)
Alburnus alburnus concentration)
C14-17;52% Cl Rainbow trout, 60 days No observed effect 1050 Madeley & Maddock (1983c)
Oncorhynchus mykiss
C18-26;49% Cl Bleak, 14 days No observed effect 125 (single Bengtsson et al. (1979)
Alburnus alburnus concentration)
C20-30;43%, 70% Cl Rainbow trout, 60 days No observed effect 3800 Madeley & Maddock (1983c)
Oncorhynchus mykiss
In a 96-h study on rainbow trout (Oncorhynchus mykiss), no
toxic effects or effects on behaviour were observed after exposure
of the fish to an emulsion containing a mean concentration of
770 000 µg/litre of Cereclor 42 (C20-30;42% Cl, CP-LL) (Madeley
& Birtley, 1980).
In bleak (Alburnus alburnus) which were exposed to Witaclor 149
(C10-13;49% Cl, CP-SL), Witaclor 159 (C10-13;59% Cl, CP-SH) and Witaclor
171P (C10-13;71% Cl, CP-SH) (125 µg/litre of water) for 14 days, some
effects on behaviour, such as sluggish movements, absence of shoaling
behaviour and abnormal vertical postures were observed after 7 days
(Bengtsson et al., 1979). The effects disappeared after the fish had
been kept in clean water for 2 days. No effects on behaviour were
observed after exposure to chlorinated paraffins with intermediate or
long chain length chlorinated paraffins, Witaclor 350 (C14-17;50% Cl,
CP-MH) or Witaclor 549 (C18-26;49% Cl, CP-LL), suggesting that
behavioural toxicity is related to the carbon chain length of the
chlorinated paraffin. When bleak were given food contaminated with
590, 2500 or 5800 mg/kg Witaclor 149 or 3180 mg/kg Witaclor 171P for
91 days, effects on behaviour were noted (Bengtsson & Baumann Ofstad,
1982). After 5 weeks of exposure to the high dose of Witaclor 149,
after 7 weeks of exposure to the medium dose and after 12 weeks of
exposure to Witaclor 171P, the fish swam sluggishly and closer to the
bottom than usual. In addition, folded dorsal fins and minor balance
problems were observed. These effects gradually disappeared within 2
weeks after exposure finished.
Flounders (Platichthys flesus) of both sexes were fed Witachlor
149 (C12;49% Cl, CP-SL) or Chlorparaffin Hüls 70C (C12;70% Cl, CP-SH)
on days 1 and 4 with a total exposure of 1000 mg/kg body weight (Haux
et al., 1982). The experiment was performed in both brackish and
seawater. The fish were examined 13 and 27 days after the first
administration. The male flounders did not appear to be affected by
the two chlorinated paraffins. C12;70% Cl did not induce any
haematological responses, whereas C12;49% Cl seemed to affect the
erythrocyte balance of female fish. C12;49% Cl resulted in
hypoglycaemia in marine female fish, whereas C12;70% Cl caused
hyperglycaemia in female brackish water fish. In females exposed to
C12;49% Cl, a significant increase in benzo [a]pyrene hydroxylase
activity was observed after 27 days in brackish water. A decrease in
6ß-hydroxylase activity in marine female fish and an increase in 5
alpha,ß-reductase activity in brackish water female fish were induced
by C12;70% Cl after 13 days.
The hatchability of embryos and survival of larvae of the
sheepshead minnow (Cyprinodon variegatus) was unaffected by a 28-day
exposure to short chain length paraffin with 58% Cl (CP-SH) (2.4, 4.1,
6.4, 22.1 and 54.8 µg/litre (Hill & Maddock, 1983). The treated
minnows showed an increased larval growth compared to the acetone
control. When the larvae were exposed to 36.2, 71, 161.8, 279.7 and
620.5 µg/litre for 32 days they were significantly smaller in the two
highest exposure groups, but in the lower exposure groups (36.2 and
71 µg/litre) they were significantly larger than the controls. No
effect was seen on survival of larvae and hatchability of embryos.
In a study by Madeley & Maddock (1983c) four chlorinated paraffins
were examined for their toxicity to rainbow trout (Oncorhynchus mykiss)
after exposure for 60 days. C10-12;58% Cl was used at mean
concentration of 33, 100, 350, 1070 and 3050 µg/litre. Significant
mortality was observed with the highest three concentrations. LT50
values (mean lethal times) for these three concentrations were
calculated as 44.7, 31.0 and 30.4 days, respectively. The calculated
60-day LC50 was 340 µg/litre. Behavioural abnormalities, which
were dose-related, were also observed.
Rainbow trout (Oncorhynchus mykiss) were exposed to 3.1 and
14.3 µg/litre of C10-12;58% Cl (CP-SH) for 168 days followed by a
depuration period of up to 105 days. No deaths occurred during the
exposure period, but during days 63 to 70 of the depuration period
all of the trout exposed to 14.3 µg/litre died and there was a
significantly increased mortality (50%) among those exposed to
3.1 µg/litre (Madeley & Maddock, 1983b). The relationship to
chlorinated paraffin exposure is unknown, since the surviving fish
recovered after day 70.
Rainbow trout (Oncorhynchus mykiss) were exposed to a short
chain length paraffin (58% Cl) (CP-SH) for 168 days (Madeley &
Maddock, 1983a) at 3.4 and 17.2 µg/litre. The treatment did not cause
any significant mortality or differences in growth, but small changes
in behaviour such as increased food intake were observed (compared to
controls).
Madeley & Maddock (1983c) exposed rainbow trout (Oncorhynchus
mykiss) to 4500 and 1050 µg/litre of a chlorinated paraffin (C14-17;
52% Cl, CP-MH) for 60 days. No toxic effects were found.
In other studies rainbow trout (Oncorhynchus mykiss) were
exposed to C22-26;43% Cl (CP-LL) for 60 days at concentrations of 900
and 4000 µg/litre (Madeley & Maddock, 1983c) or to C20-30;70% Cl
(CP-LH) at 3800, 1900 and 840 µg/litre (Madely & Maddock, 1983d). No
toxic effects were found.
9.1.3 Terrestrial organisms
A one-generation reproduction study was performed with Chlorowax
500C (C10-13;58% Cl, CP-SH) on mallard ducks (Anas platyrhynchos)
(Shults et al., 1984). The ducks were fed 0, 28, 166 and 1000 mg/kg
diet for 22 weeks in groups of 20 pairs. During the treatment the
ducks were mated. The treatment had no effect on the survival,
physical condition, body weight or food consumption of the adult
ducks. Decreased egg-shell thickness and a 10% loss of 14-day embryo
viability were observed in the highest exposure group.
The acute toxicity of orally administered Cereclor S52 (C14-17;52%
Cl, CP-MH) was studied in ring-necked pheasants (Phasianus
colchicus) and mallard ducks (Anas platyrhynchos) (Madeley &
Birtley, 1980). However, no abnormal clinical signs, mortality or
effects on body weight gain were observed 14 days after a single oral
dose by gavage of up to 24 606 mg/kg body weight (pheasant) and
10 280 mg/kg body weight (duck) (five male and five female birds per
group). When the birds were fed with 1000 or 24 063 mg/kg diet of
Cereclor S52 for 5 days followed by 3 days on normal food (five males
and five females per group), no abnormalities were found other than
inferior food intake in ducks from the high-dose group.
The toxicity of Cereclor 42 (C22-26;42% Cl, CP-LL), Cereclor 50LV
(C10-13;49% Cl, CP-SL) and Cereclor 70L (C10-13;70% Cl, CP-SH) in chick
embryos was studied by Brunström (1983). The chlorinated paraffins
were injected into the yolks of eggs incubated for 4 days at
concentrations of 100 and 200 mg/kg egg (based on mean egg weights
before incubation). None of the three mixtures affected the hatching
rate, incubation time, hatching weight, weight gain after hatching or
the liver weights of the chicks.
In an extension of this study the eggs were injected with
300 mg/kg egg weight of the same Cereclors after 4 days of incubation
(Brunström, 1985). An increased liver weight was observed after
treatment with C10-13;49% Cl and C10-13;70% Cl. The microsomal
concentration of cytochrome P-450 in chick embryos was increased by
all three Cereclors. The highest concentration was observed with
C10-13;70% Cl. This Cereclor was also found to increase the microsomal
activity of APMD. The other short-chain chlorinated paraffin,
C10-13;49% Cl, produced decreased activities of aryl hydrocarbon
hydroxylase (AHH) and 7-ethoxycoumarin O-deethylase (ECOD). The
long chain length chlorinated paraffin in this study, C22-26;42% Cl,
caused decreased activities of APDM and ECOD. The greatest effects
were observed after treatment with the most highly chlorinated
short-chain paraffin, C10-13;70% Cl.
9.2 Field observations
No data concerning the effects of chlorinated paraffins in the
field have been reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
Information on occupational exposure to chlorinated paraffins is
very limited. The principal route of exposure is likely to be dermal,
particularly during their use as metal-working fluids. There is also
potential for the formation of inhalable aerosols during this use,
though available information is inadequate to assess exposure via this
route.
Owing to the high octanol-water partition coefficient, it is
likely that the principal source of exposure of the general population
is food, although, owing to lack of data, exposure via other routes
cannot be ruled out. It should be noted, however, that the
composition of chlorinated paraffins to which the population is
exposed in the general environment may be considerably different from
that of the commercial products, although it is not possible currently
to distinguish the chemical composition of chlorinated paraffins with
available methods of analysis. In the only survey of foodstuffs
identified, which was limited in scope, the highest concentration of
chlorinated paraffins (average level of C10-20: 300 µg/kg) was present
in dairy products (Table 15). If the daily consumption of dairy
products is assumed to be 1 kg per person, the daily intake of short
and intermediate chain length chlorinated paraffins from this source
would be 300 µg (4.3 µg/kg body weight, assuming an average body
weight of 70 kg). This is likely to be a worst-case estimate based on
the lack of specificity of the analytical method.
Exposure to chlorinated paraffins via food is also possible
through the consumption of contaminated mussels. Assuming a weekly
consumption of 1 kg of mussels, as a worst case, this would correspond
to a weekly intake of short and intermediate chain length chlorinated
paraffins of 3250 µg, based on chlorinated paraffin levels found in
mussels collected at various sites in the United Kingdom (Table 13)
(6.7 µg/kg body weight per day, assuming an average body weight of
70 kg). If the mussels are collected in highly contaminated water
(section 5.1.4) the weekly intake would be 12 000 µg, which
corresponds to 25 µg/kg body weight per day. The analytical method
used was non-specific.
10.1.2 Toxic effects
In spite of the widespread use of the chlorinated paraffins,
there have been no case reports of skin irritation or sensitization
in humans. Results from a limited number of studies on volunteers
show that chlorinated paraffins can induce minimal irritancy in
the skin but not sensitization. No other data concerning effects of
chlorinated paraffins on humans have been reported.
The acute oral toxicity of chlorinated paraffins of various chain
lengths has been well studied in experimental animals and is low.
Toxic effects such as muscular incoordination and piloerection
were most evident following single exposure to short chain length
chlorinated paraffins. On the basis of very limited data, the acute
toxicity by the inhalation and dermal routes also appears to be low.
Mild skin and eye irritation have been observed after application of
short and intermediate (skin irritation) chain length chlorinated
paraffins. Results of several studies indicate that short chain
chlorinated paraffins do not induce skin sensitization.
In repeated dose toxicity studies by the oral route, the liver,
kidney and thyroid have been shown to be the primary target organs for
the toxicity of chlorinated paraffins (Table 32). For the short chain
compounds, increases in liver and kidney weight and hypertrophy of the
liver and thyroid have been observed at lowest doses (LOEL = 100 mg/kg
body weight per day; NOEL = 10 mg/kg body weight per day; rats).
For the intermediate chain compounds, effects observed at lowest
doses are generally increases in liver and kidney weight (LOEL in rats
= 100 mg/kg body weight per day, respectively; NOAEL in rats =
10 mg/kg body weight per day). Increases in serum cholesterol and
"mild, adaptive" histological changes in the thyroid have been reported
at similar doses in female rats (NOAEL = 4 mg/kg body weight per day).
For the long chain compounds, effects observed at lowest doses
are multifocal granulomatous hepatitis and increased liver weight in
female rats (LOAEL = 100 mg/kg body weight per day).
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells, there is a suggestion of a
weak in vitro clastogenic potential but not in several in vivo
studies. Chlorinated paraffins are also reported to induce in vitro
cell transformation.
Table 32. Effect levels (non-carcinogenic) in repeated dose toxicity tests
Short chain length chlorinated paraffins
Rats
C10-13;58% Cl, 14-day dietary study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in relative liver weights and activities of
hepatic APDM and cytochrome P-450)
C10-13;58% Cl, 14-day gavage study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in relative liver weights);
NOEL = 30 mg/kg body weight per day
C10-13;58% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 100 mg/kg body weight per day (increase in liver/body weight ratio);
NOEL = 50 mg/kg body weight per day
C10-13;56% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 50 mg/kg body weight per day (increase in liver/body weight ratio);
NOEL = 10 mg/kg body weight per day
C12;58% Cl, 90-day gavage study in Fischer-344 rats
LOEL = 313 mg/kg body weight per day (increase in relative liver weight, hepatic
peroxisomal ß-oxidation and thyroxine-UdPG-glucuronosyltransferase; thyroid follicular
cell hypertrophy and hyperplasia and increase in replicative DNA synthesis; renal tubular
eosinophilia and increase in renal replicative DNA synthesis)
No effects in Dunkin Hartley guinea-pigs
NOEL = 1000 mg/kg body weight per day
C10-13;58% Cl, 13-week gavage study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in liver and kidney weights and hypertrophy
of liver and thyroid);
NOEL = 10 mg/kg body weight per day
C12;60% Cl, 90-day gavage study in Fischer-344 rats,
LOEL = 313 mg/kg body weight per day (increase in liver weight)
C12;60% Cl, 2-year gavage study in Fischer-344 rats,
LOAEL = 312 mg/kg body weight per day (increase in liver and kidney weights, hepatic
hypertrophy, necrosis and angiectasis, nephropathy (females), erosion, inflammation and
ulceration of the glandular stomach, hyperplasia of the parathyroid)
Table 32. (Cont'd)
Short chain length chlorinated paraffins (cont'd)
Mice
C10-13;58% Cl, 14-day gavage study in Alpk:APfCD mice,
LOEL = 250 mg/kg body weight per day (induction of peroxisomal fatty acid ß-oxidation and
increase in liver weight);
NOEL = 100 mg/kg body weight per day
C10-13;56% Cl, 14-day gavage study in Alpk:APfCD mice
LOEL = 100 mg/kg body weight per day (increase in liver weight);
NOEL = 50 mg/kg body weight per day
C12;60% Cl, 90-day gavage study in B6C3F1 mice,
LOEL = 250 mg/kg body weight per day (hepatocellular hypertrophy);
NOEL = 125 mg/kg body weight per day
C12;60% Cl, 2-year day gavage study in B6C3F1 mice,
LOEL = 125 mg/kg body weight per day (decrease in body weight gain (females))
Intermediate chain length chlorinated paraffins
Rats
C14-17;52% Cl, 14-day dietary study in Fischer-344 rats,
LOEL = 177 mg/kg body weight per day (slight increase in cytochrome P-450);
NOEL = 57.7 mg/kg body weight per day
C14-17;40% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 10 mg/kg body weight per day (increase in liver weight; not dose-related)
C14-17;52% Cl, 13-week study in Sprague-Dawley rats,
NOAEL (females) = 4.2 mg/kg body weight per day (increase in serum cholesterol; "mild,
adaptive" histopathological changes in the thyroid - reduced follicle sizes and collapsed
angularity; increased height, cytoplasmic vacuolation and nuclear vesiculation);
NOEL = 0.4 mg/kg body weight per day
C14-17;52% Cl, 90-day dietary study in Wistar rats,
LOEL (females) = 25 mg/kg body weight per day (increases in liver and kidney weights and
proliferation of smooth endoplastic reticulum);
NOEL = 12.5 mg/kg body weight per day
C14-17;52% Cl, 90-day dietary study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increased liver and kidney weights);
NOEL = 10 mg/kg body weight per day
Table 32. (Cont'd)
Intermediate chain length chlorinated paraffins (cont'd)
Mice
C14-17;40% Cl, 14-day gavage study in Alpk:APfCD-1 mice,
LOEL = 500 mg/kg body weight (induction of peroxisomal fatty acid ß-oxidation);
NOEL = 250 mg/kg body weight per day
Dogs
C14-17;52% Cl, 90-day dietary study in Beagle dogs,
LOEL (males) = 30 mg/kg body weight per day (increased liver and kidney weights)
NOEL = 10 mg/kg body weight per day (increased smooth endoplasmic reticulum)
Long chain length chlorinated paraffins
Rats
C22-26;70% Cl, 14-day dietary study in Fischer-344 rats,
NOEL = 1715 mg/kg body weight per day (no effects at any dose level)
C22-26;43% Cl, 14-day dietary study in Charles River rats,
NOEL = 3000 mg/kg body weight per day (no effects at any dose; observation of
nephrolithiasis in females exposed to 3000 mg/kg body weight per day)
C23;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL (females) = 235 mg/kg body weight per day (granulomatous inflammation in the liver)
C20-30;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL (females) = 100 mg/kg body weight per day (multifocal granulomatous hepatitis and
increased liver weight)
C22-26;70% CL, 90-day dietary study in Fischer-344 rats,
LOAEL = 3750 mg/kg body weight per day (increased liver weight, hepatocellular hypertrophy
and cytoplasmic fat vacuolation; slight increase of chronic nephritis (males); increase in
ALT and AST (females));
NOEL = 900 mg/kg body weight per day
C23;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL = 100 mg/kg body weight per day (increase in relative liver weights; increased
activities of serum enzymes, variations in haematological parameters (in females); diffuse
lymphohistiocytic inflammation in the livers, pancreatic and mesenteric lymph nodes)
Table 32. (Cont'd)
Long chain length chlorinated paraffins (cont'd)
Mice
C23;43% Cl, 90-day gavage,
NOEL = 7500 mg/kg body weight per day (no effects at any dose)
C23;43% Cl, 90-day gavage,
NOEL = 5000 mg/kg body weight per day (no effects at any dose)
Two-year toxicity and carcinogenicity studies have been conducted
for a short (C12;60% Cl) and a long chain chlorinated paraffin
(C23;43% Cl) in both rats and mice. For the short chain chlorinated
paraffin, the incidences of liver and thyroid tumours were increased
in mice at 125 and 250 mg/kg body weight per day. In rats, the
incidences of liver tumours in both sexes, thyroid tumours in females
and renal cell tumours and leukaemias in males were increased at doses
of 312 and 625 mg/kg body weight per day. For the long chain
chlorinated paraffin, the incidences of malignant lymphomas in male
mice (2500-5000 mg/kg body weight per day) and phaeochromocytomas in
female rats (900 mg/kg body weight per day) were increased. On the
basis of available data, therefore, the short chain chlorinated
paraffin was carcinogenic in rats and mice. No data are available on
intermediate chlorinated paraffins. For the long chain chlorinated
paraffin, the evidence of carcinogenicity is limited, increased
incidences of commonly occurring tumours having been observed at only
one site in one sex of each species.
Some possible mechanisms for the induction of tumours of the
thyroid, liver and kidney have been suggested. On the basis of 14-day
mouse, rat and guinea-pig studies (Wyatt et al., 1993; Elcombe et al.,
in press), with similar short chain and long chain chlorinated
paraffins as used in the carcinogenesis bioassays, it was suggested
that the liver tumours in mice and rats could be correlated with the
degree of peroxisomal proliferation which occurs in the liver of these
species at similar dose levels (250 mg/kg body weight per day). No
peroxisome proliferation was observed in the livers of guinea-pigs
even at doses up to 1 to 2 g/kg body weight per day, although a
similar increase in liver weight to that seen in mice and rats was
observed. No study on peroxisome proliferation in human hepatoctyes
treated with a short chain chlorinated paraffin is available.
Increased DNA synthesis has been demonstrated in hepatocytes and
thyroid follicular cells in rats of both sexes and in proximal kidney
tubular cells in males. Perturbation of thyroid homeostasis and
increased TSH secretion has been observed in the thyroid of rats.
These effects may be related to the development of tumours.
Taking into consideration the available data, it appears that
chlorinated paraffins do not mediate carcinogenic effects via direct
interaction with DNA.
In a reproduction study, no adverse reproductive effects were
reported following exposure of rats to an intermediate chain length
chlorinated paraffin with 52% chlorine. However, survival and body
weight of the exposed pups were reduced (LOEL for non-significant
decrease in body weight = 5.7 to 7.2 mg/kg body weight per day; LOAEL
for decreased survival = 58.7 to 70 mg/kg body weight per day). In a
limited number of studies on the developmental effects of the short,
medium and long chain chlorinated paraffins, adverse effects in
offspring were observed, for the short chain compounds only, at
maternally toxic doses in rats.
10.1.3 Risk evaluation
Available data indicate that absorption of chlorinated paraffins
through the skin (the likely principal route of exposure in the
occupational environment) is minimal. Provided that proper personal
hygiene and safety procedures are followed, the risk to the health of
workers exposed to chlorinated paraffins is expected to be minimal.
10.1.3.1 Short chain compounds
On the basis of available data on repeated dose toxicity, a
Tolerable Daily Intake (TDI) for non-neoplastic effects of short chain
chlorinated paraffins for the general population can be developed:
10 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
100
where 10 mg/kg body weight per day is the lowest reported
no-observed-effect level (increases in liver and kidney weights
and hypertrophy of the liver and thyroid at the next highest dose
in a 13-week study on rats) (IRDC, 1984a); and 100 is the
uncertainty factor (× 10 for interspecies variation; × 10 for
intraspecies variation).
On the basis of multistage modelling of the tumours with highest
incidence (hepatocellular adenomas or carcinomas (combined) in male
mice) in the carcinogenesis bioassay with short chain chlorinated
paraffins, the estimated dose associated with a 5% increase in tumour
incidence is 11 mg/kg body weight per day (amortized for period of
administration). After dividing this value by 1000 (uncertainty
factor for a non-genotoxic carcinogen), it can be recommended that
daily doses of short chain chlorinated paraffins for the general
population should not exceed 11 µg/kg body weight, on the basis of
neoplastic effects.
10.1.3.2 Intermediate chain compounds
On the basis of available data on repeated dose toxicity, a TDI
for non-neoplastic effects of intermediate chain chlorinated paraffins
can be developed:
10 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
100
where 10 mg/kg body weight per day is the no-observed-adverse-effect
level in both sexes (increases in liver and kidney weights at the next
highest dose) (IRDC, 1984b); increases in serum cholesterol and "mild,
adaptive" histological changes in the thyroid have been reported at
similar doses in female rats (NOAEL = 4 mg/kg body weight per day)
(Poon et al., in press); and 100 is the uncertainty factor (× 10 for
interspecies variation; × 10 for intraspecies variation).
10.1.3.3 Long chain compounds
On the basis of available data on repeated dose toxicity, a TDI
for non-neoplastic effects of long chain chlorinated paraffins can be
developed:
100 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
1000
where 100 mg/kg = the lowest-observed-adverse-effect levels in
long-term studies (effects at this dose were multifocal granulomatous
hepatitis and increased liver weight in female rats (NTP, 1986b;
Serrone et al., 1987; Bucher et al., 1987); and 1000 = uncertainty
factor (× 10 for intraspecies variation, × 10 for interspecies
variation, × 10 for LOAEL rather than NOEL).
In general, the calculated daily intake of chlorinated paraffins
based on highly unlikely worst-case scenarios are below the TDIs for
non-neoplastic effects or recommended values for neoplastic effects
(short chain compounds) developed above.
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
Data on chlorinated paraffin levels in the environment are
limited, but the studies reported indicate widespread contamination,
although the highest levels are found close to industries that
manufacture or use chlorinated paraffins.
Chlorinated paraffins bioaccumulate in aquatic organisms, and
bioconcentration factors (BCFs) in the range of 7 to 7155 for fish and
223 to 138 000 for mussels have been reported. In fish, chlorinated
paraffins of short chain length are accumulated to a higher degree
than those of intermediate and long chain length.
In the environment, chlorinated paraffins are persistent.
However, short-chain chlorinated paraffins with a low chlorine content
appear to be degraded by acclimated microorganisms.
10.2.2 Toxic effects
Short chain length chlorinated paraffins are acutely toxic to
freshwater and saltwater invertebrates at LC/EC50 concentrations
ranging from 14 to 530 µg/litre. The acute toxicity of short chain
chlorinated paraffins to fish is low. In long-term studies, the
lowest-observed-effect concentrations for algae, daphnids and fish
ranged from 3 to 20 µg/litre; NOECs appear to range from 2 to
5 µg/litre for the most sensitive species tested. The acute and
long-term toxicity of intermediate and long chain length chlorinated
paraffins to fish appears to be low. However, in daphnids, chronic
effects of an intermediate and a long-chain product have been observed
at similar concentrations to those reported for short chain compounds.
No studies on plants or terrestrial invertebrates have been
reported. The acute toxicity to birds is low. Data from studies on
laboratory mammals suggest a low risk for terrestrial mammals.
10.2.3 Risk evaluation
The evaluation of the environmental risks of chlorinated
paraffins is complicated by the limited quality and quantity of
information regarding environmental levels. Available data indicate
that chlorinated paraffins are bioaccumulative and persistent.
The data on environmental levels of short-chain chlorinated
paraffins indicate that in areas local to release sources, there is a
risk to both freshwater and estuarine organisms. Recent data indicate
that there is also a potential risk to aquatic invertebrates from
intermediate and long chain chlorinated paraffin products.
The enrichment of chlorinated paraffins in sediments, their
resorption behaviour and aquatic toxicity indicate a potential risk
for sediment-dwelling organisms.
The data regarding chlorinated paraffins in the terrestrial
environment are insufficient to estimate the risk to soil-dwelling
organisms.
11. RECOMMENDATIONS FOR PROTECTION OF THE ENVIRONMENT
Since chlorinated paraffins are bioaccumulative and toxic to
environmental organisms and owing to difficulties in monitoring
environmental levels, it is recommended that use and disposal of these
compounds should be controlled to avoid release to the environment.
12. FUTURE RESEARCH
The following studies need to be undertaken:
a) development of more selective and sensitive methods of analysis
in order to provide more reliable data on present and future
levels of chlorinated paraffins in the occupational environment,
soil, water, sediments, foodstuffs and human tissues;
b) further investigation of the influence of the chain lengths and
degrees of chlorination on toxicodynamics and toxicokinetics of
chlorinated paraffins, with particular regard to the relative
extent and rate of absorption and excretion through different
routes; studies to ascertain the metabolic pathways of
chlorinated paraffins should also be performed;
c) investigation of the toxicokinetics and half-lives of chlorinated
paraffins in mammals;
d) studies on perinatal toxicity;
e) further studies to examine effects on sediment-dwelling
organisms.
13. PREVIOUS EVALUATION BY INTERNATIONAL ORGANIZATIONS
Chlorinated paraffins have been evaluated by the International
Agency for Research on Cancer (IARC, 1990). It was concluded that
there is sufficient evidence for the carcinogenicity of a commercial
chlorinated paraffin product of average carbon chain length C12 and
average degree of chlorination of 60% in experimental animals, and
limited evidence for the carcinogenicity of a commercial chlorinated
paraffin product of average carbon chain length C23 and average
degree of chlorination of 43% in experimental animals. The overall
evaluation was that chlorinated paraffins of average carbon chain
length C12 and average degree of chlorination of approximately 60%
are possibly carcinogenic to humans (Group 2B).
REFERENCES
Åhlman M, Bergman Å, Darnerud PO, Egestad B, & Sjövall J (1986)
Chlorinated paraffins: formation of sulphur-containing metabolites of
polychlorohexadecane in rats. Xenobiotica, 16: 225-232.
Ahotupa M, Hietanen E, Mantyla E, & Vainio H (1982) Effects of
polychlorinated paraffins on hepatic, renal and intestinal
biotransformation rates in comparison to the effects of poly-
chlorinated biphenyls and naphthalenes. J Appl Toxicol, 2: 47-53.
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL, &
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environ Mol Mutagen, 16(Suppl 18):
55-137.
Ashby J, Lefevre PA, & Elcombe CR (1990) Cell replication and
unscheduled DNA synthesis (UDS) activity of low molecular weight
chlorinated paraffins in the rat liver in vivo. Mutagenesis,
5: 515-518.
Bengtsson BE & Baumann Ofstad E (1982) Long-term studies on uptake and
elimination of some chlorinated paraffins in the bleak, Alburnus
alburnus. Ambio, 11: 38-40.
Bengtsson BE, Svanberg O, Lindén E, Lunde G, & Baumann Ofstad E (1979)
Structure related uptake of chlorinated paraffins in bleaks ( Alburnus
alburnus L). Ambio, 8: 121-122.
Bergman Å, Hagman A, Jacobsson S, Jansson B, & Åhlman M (1984) Thermal
degradation of polychlorinated alkanes. Chemosphere, 13: 237-250.
Biessmann A, Brandt I, & Darnerud PO (1982) Comparative distribution
and metabolism of two carbon-14 labeled chlorinated paraffins in
Japanese quail (Coturnix coturnix japonica). Environ Pollut,
A28: 109-120.
Biessmann A, Darnerud PO, & Brandt I (1983) Chlorinated paraffins:
disposition of a highly chlorinated polychlorohexadecane in mice and
quail. Arch Toxicol, 53: 79-86.
Birtley RDN, Conning DM, Daniel JW, Ferguson DM, Longstaff E, & Swan
AAB (1980) The toxicological effects of chlorinated paraffins in
mammals. Toxicol Appl Pharmacol, 54: 514-525.
Brunström B (1983) Toxicity in chick embryos of three commercial
mixtures of chlorinated paraffins and of toxaphene injected into eggs.
Arch Toxicol, 54: 353-357.
Brunström B (1985) Effects of chlorinated paraffins on liver weight,
cytochrome P-450 concentration and microsomal enzyme activities in
chick embryos. Arch Toxicol, 57: 69-71.
BUA (German Chemical Society Advisory Committee on Existing Chemicals
of Environmental Relevance) (1992) [Chlorinated paraffins.] Weinheim,
VCH Verlagsgesellschaft, 227 pp (BUA Report 93) (in German).
Bucher JR, Alison RH, Montgomery CA, Huff J, Haseman JK, Farnell
D, Thompson R, & Prejean JD (1987) Comparative toxicity and
carcinogenicity of two chlorinated paraffins in F344/N rats and
B6C3F1 mice. Fundam Appl Toxicol, 9: 454-468.
Camford Information Services (1991) CPI product profiles: chlorinated
paraffins. Don Mills, Ontario, Camford Information Services, 3 pp.
Campbell I & McConnell G (1980) Chlorinated paraffins and the
environment. 1. Environmental occurrence. Environ Sci Technol,
14: 1209-1214.
Conz A & Fumero S (1988a) Study of the capacity of Solvocaffaro C1642
to induce gene mutations in strains of Salmonella typhimurium.
Turin, Istituto di Recerche Biomediche "Antoine Marxer", 37 pp (RBM
Exp. No. 880367).
Conz A & Fumero S (1988b) Micronucleus induction in bone marrow cells
of mice treated by oral route with the test article Solvocaffaro
C1642. Turin, Istituto di Recerche Biomediche "Antoine Marxer", 20 pp
(RBM Exp. No. 880368).
Cooke M & Roberts DJ (1980) Carbon skeleton capillary gas
chromatography. J Chromatogr, 193: 437-443.
Darnerud PO (1984) Chlorinated paraffins: Effect of some microsomal
enzyme inducers and inhibitors of the degradation of 1-14C-chloro-
dodecanes to 14CO2 in mice. Acta Pharmacol Toxicol,
55: 110-115.
Darnerud PO & Brandt I (1982) Studies on the distribution and
metabolism of a 14C-labelled chlorinated alkane in mice. Environ
Pollut, A27: 45-56.
Darnerud PO & Lundkvist U (1987) Studies on implantation and embryonic
development in mice given a highly chlorinated hexadecane. Pharmacol
Toxicol, 60: 239-240.
Darnerud PO, Biessmann A, & Brandt I (1982) Metabolic fate of
chlorinated paraffins: degree of chlorination of [1-14C]-chloro-
dodecanes in relation to degradation and excretion in mice. Arch
Toxicol, 50: 217-226.
Darnerud PO, Bengtsson BE, Bergman A, & Brandt I (1983) Chlorinated
paraffins: disposition of a polychloro-[1-14C]-hexadecane in carp
(Cyprinus carpio) and bleak (Alburnus alburnus). Toxicol Lett,
19: 345-351.
Darnerud PO, Bergman Å, Lund BO, & Brandt I (1989) Selective
accumulation of chlorinated paraffins (C12 and C16) in the olfactory
organ of rainbow trout. Chemosphere, 18: 1821-1827.
EG & G Bionomics (1983) The acute and chronic toxicity of a chlorinated
paraffin (58% chlorination of short chain length n-paraffins) to
midges (Chironomus tentans). Wareham, Massachusetts, EG & G Bionomics,
39 pp (Report No. BW 83-6-1426).
Elcombe CR, Watson SC, Wyatt I, & Foster JR (1994) Chlorinated
paraffins (CP): Mechanisms of carcinogenesis. Toxicologist, 14: 276.
Elcombe CR, Bars RG, Watson SC, & Foster JR (in press) Hepatic effects
of chlorinated paraffins in mice, rats and guinea pigs: Species
differences and implications for hepatocarcinogenicity. Xenobiotica.
Elliott BM (1989a) Meflex DC029 (fully formulated) - Ames test.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 1 p (Report
No. CTL/L/2668).
Elliott BM (1989b) Meflex DC029 (fully formulated) - Mouse
micronucleus test. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 1 p (Report No. CTL/L/2693).
English JSC, Foulds I, White IR, & Rycroft JG (1986) Allergic contact
sensitization to the glycidyl ester of hexahydrophthalic acid in a
cutting oil. Contact Dermatitis, 15: 66-69.
Environment Agency, Japan (1981) Environmental monitoring of chemicals:
Annual report. Tokyo, Environment Agency, p 23.
Environment Agency, Japan (1983) Environmental monitoring of chemicals:
Annual report. Tokyo, Environment Agency, p 75.
Eriksson P & Darnerud PO (1985) Distribution and retention of some
chlorinated hydrocarbons and a phthalate in the mouse brain during the
pre-weaning period. Toxicology, 37: 189-203.
Eriksson P & Kihlström J (1985) Disturbance of motor performance and
thermoregulation in mice given two commercial chlorinated paraffins.
Bull Environ Contam Toxicol, 34: 205-209.
Eriksson P & Nordberg A (1986) The effects of DDT, DDOH-palmitic
acid, and a chlorinated paraffin on muscarinic receptors and the
sodium-dependent choline uptake in the central nervous system of
immature mice. Toxicol Appl Pharmacol, 85: 121-127.
Frank U & Steinhäuser KG (in press) [Ecotoxicity of poorly soluble
substances tested by Daphnia toxicity of chlorinated paraffins.] Vom
Wasser, 83 (in German).
Friedman D & Lombardo P (1975) Photochemical technique for the
elimination of chlorinated aromatic interferences in the gas-liquid
chromatographic analysis for chlorinated paraffins. J Assoc Off Anal
Chem, 58: 703-706.
Gjos N & Gustavsen K (1982) Determination of chlorinated paraffins by
negative ion chemical ionization mass spectrometry. Anal Chem,
54: 1316-1318.
Hansen J, Schneider T, Olsen JH, & Laursen B (1992) Availability of
data on humans potentially exposed to suspected carcinogens in the
Danish working environment. Pharmacol Toxicol, 72(Suppl 1): S77-S85.
Hardie DWF (1964) Chlorocarbons and chlorohydrocarbons: Chlorinated
paraffins. In: Kirk-Othmer encyclopedia of chemical technology. New
York, John Wiley and Sons, vol 5, pp 231-240.
Haux C, Larsson Å, Lidman U, Förlin L, Hansson T, & Johansson-Sjöbeck
M-L (1982) Sublethal physiological effects of chlorinated paraffins on
the flounder, Platichtys flesus L. Ecotoxicol Environ Saf, 6: 49-59.
Hill RW & Maddock BG (1983) Effect of a chlorinated paraffin (58%
chlorination of short chain length n-paraffins) on embryos and
larvae of the sheepshead minnow (Cyprinodon variegatus)-(study one).
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 57 pp
(Report No. BL/B/2326).
Hoechst (1983a) [Chloroparaffin 70 liquid NV - Acute dermal
irritation/corrosion in rabbits.] Frankfurt/Main, Hoechst AG, 11 pp
(Report No. 83.0444) (in German).
Hoechst (1983b) [Hordalub 80 + TNPP - Magnusson and Kligman's test for
sensitising properties on Pirbright-White Guinea Pigs.] Frankfurt/Main,
Hoechst AG, 17 pp (Report No. 83.0573) (in German).
Hoechst (1984) [Hordalub 80 HT - Magnusson and Kligman's test for
sensitising properties on Pirbright-White Guinea Pigs.] Frankfurt/Main,
Hoechst AG, 18 pp (Report No. 84.0458) (in German).
Hoechst (1986a) Hordalub 80 - Study of the mutagenic potential in
strains of Salmonella typhimurium (Ames Test) and Escherichia coli.
Frankfurt/Main, Hoechst AG, 28 pp (Report No. 86.1078).
Hoechst (1986b) [Acute dermal irritation/corrosion in rabbits.]
Frankfurt/Main, Hoechst AG, 12 pp (Report No. 86.1105) (in German).
Hoechst (1987) Chloroparaffin 56 liquid - Detection of gene mutations
in somatic cells in culture. HGPRT-test with V79 cells. Frankfurt/Main,
Hoechst AG, 23 pp (Report No. 87.1719).
Hoechst (1988) Chloroparaffin 56 liquid (unstabilized) - Study of the
mutagenic potential in strains of Salmonella typhimurium (Ames Test)
and Escherichia coli. Frankfurt/Main, Hoechst AG, 30 pp (Report No.
88.0099).
Hoechst (1989) Chlorowax 500C micronucleus test in male and female
NMRI mice after oral administration. Frankfurt/Main, Hoechst AG, 24 pp
(Report No. 89.0253).
Hollies JI, Pinnington DF, Handley AJ, Baldwin MK, & Bennett D (1979)
The determination of chlorinated long-chain paraffins in water,
sediment and biological samples. Anal Chim Acta, 111: 201-213.
Houghton KL (1993) Chlorinated paraffins. In: In: Kirk-Othmer
encyclopedia of chemical technology. New York, John Wiley and Sons,
vol 6, pp 78-87.
Howard PH, Santodonato J, & Saxena J (1975) Investigations of selected
potential environmental contaminants: chlorinated paraffins. Syracuse,
New York, Syracuse University Research Corporation, 107 pp (Report No.
68-01-3101).
HSE (1992) Chlorinated paraffins. London, Health and Safety Executive,
44 pp (Unpublished document).
IARC (1990) Chlorinated paraffins. In: Some flame retardants and
textile chemicals, and exposures in the textile manufacturing
industry. Lyon, International Agency for Research on Cancer, pp 55-72
(IARC Monographs on the Evaluation of Carcinogenic Risks to Humans,
Volume 48).
ICI (1965) Toxicological report: chlorinated hydrocarbons with added
stabilizers: "Cereclor" P70 and 70L. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 15 pp (Report No. CTL/TR/464).
ICI (1966) Toxicological report: 40% chlorinate of C10-13 normal
paraffin. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
4 pp (Report No. CTL/TR/524).
ICI (1967) Toxicological report: "Cereclor" 50LV (MD.115) - 50%
chlorinate of C10-13 normal paraffin. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 4 pp (Report No. CTL/TR/618).
ICI (1968) Toxicological report: fire-resistant hydraulic fluid
10B/1067. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
7 pp (Report No. CTL/TR/635).
ICI (1969) Toxicological report: skin irritancy and oral toxicity of
chlorinated paraffins. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 11 pp (Report No. CTL/TR/691).
ICI (1971) Toxicological report: fire-resistant hydraulic fluid
45D-3271. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
8 pp (Report No. CTL/T/831).
ICI (1973) "Cereclor" 63L: local irritancy and acute oral toxicity.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 7 pp (Report
No. CTL/T/938).
ICI (1974a) "Cereclors" and "Hordolub": local irritancy and acute
toxicity. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
32 pp (Report No. CTL/T/962).
ICI (1974b) "Cereclor" 50 HS: summary of local irritancy, skin
sensitization and acute toxicity. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 3 pp (Report No. CTL/Z/0548).
ICI (1975a) "Cereclor" 60 HS: primary skin irritation in rabbits.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 3 pp (Report
No. CTL/Z/0813).
ICI (1975b) "Cereclor" 50 HS: primary skin irritation in rabbits.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 3 pp (Report
No. CTL/Z/0812).
ICI (1980) Cloparin 1049 and "Meflex" DC029: a comparison of skin
irritation potential. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 11 pp (Report No. CTL/T/1431).
ICI (1982a) Cell transformation test for potential carcinogenicity of
chlorinated paraffin (58% chlorination of short chain length
n-paraffins). Macclesfield, Cheshire, Imperial Chemical Industries
Ltd.
ICI (1982b) Cell transformation test for potential carcinogenicity
of chlorinated paraffin (70% chlorination of short chain length
n-paraffins). Macclesfield, Cheshire, Imperial Chemical Industries
Ltd.
ICI (1982c) Cloparin 59: skin irritation study. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, 14 pp (Report No.
CTL/T/1737).
Inveresk (1975) Patch testing of textile lubricants. Musselburgh,
Inveresk Research International, 14 pp (Report No. 318).
IRDC (1981a) 14-Day oral toxicity study in rats. Chlorinated paraffin:
58% chlorination of short chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 110 pp
(Report No. 438-006).
IRDC (1981b) 14-Day oral toxicity study in rats. Chlorinated paraffin:
52% chlorination of long chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 104 pp
(Report No. 438-005).
IRDC (1982a) Teratology study in rats. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 100 pp (Report
No. 438-016).
IRDC (1982b) 14-Day oral toxicity study in rats. Chlorinated paraffin:
70% chlorination of long chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 103 pp
(Report No. 438/004).
IRDC (1982c) 14-Day oral (gavage) toxicity study in rats. Chlorinated
paraffin: 43% chlorination of long chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
110 pp (Report No. 438/005).
IRDC (1982d) Teratology study in rabbits. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 95 pp (Report
438-031).
IRDC (1982e) Teratology study in rabbits. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 102 pp (Report
438-030).
IRDC (1983a) Dominant lethal study in rats. Chlorinated paraffin: 58%
chlorination of short chain n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 54 pp (Report
No. 438-011).
IRDC (1983b) Teratology study in rabbits. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 245 pp (Report
No. 438-045).
IRDC (1983c) 14-Day dietary range-finding study in rats. Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
136 pp (Report No. 438-002).
IRDC (1983d) Teratology study in rats. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 197 pp (Report
No. 438-015).
IRDC (1983e) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 70% chlorination of long chain
length paraffins. Mattawan, Michigan, International Research and
Development Corporation, 70 pp (Report No. 438-016).
IRDC (1983f) Teratology study in rabbits. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 195 pp
(Report No. 438-032).
IRDC (1983g) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 52% chlorination of intermediate
chain length n-paraffins. Mattawan, Michigan, International Research
and Development Corporation, 62 pp (Report No. 438-014).
IRDC (1983h) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffins: 58% chlorination of short chain
length n-paraffins. Mattawan, Michigan, International Research and
Development Corporation, 58 pp (Report No. 438-013).
IRDC (1983i) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 43% chlorination of long chain
length n-paraffins. Mattawan, Michigan, International Research and
Development Corporation, 56 pp (Report No. 438-012).
IRDC (1984a) 13-week oral (gavage) toxicity study in rats with
combined excretion, tissue level and elimination studies: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 58% chlorination of short
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 350 pp (Report
No. 438-029/022).
IRDC (1984b) 13-week oral (dietary) toxicity study in rats with
combined excretion, tissue level and elimination studies: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 52% chlorination of
intermediate chain length n-paraffins, 14C labeled CP. Mattawan,
Michigan, International Research and Development Corporation, 328 pp
(Report No. 438-023/026).
IRDC (1984c) 13-week oral (dietary) toxicity study in rats with
combined excretion, tissue level and elimination study: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 58% chlorination of
short chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 435 pp (Report
No. 438-035/022).
IRDC (1984d) Teratology study in rats. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 105 pp
(Report No. 438-017).
IRDC (1984e) Teratology study in rats. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 99 pp (Report
No. 438/045).
IRDC (1984f) 13-week oral (gavage) toxicity study in rats with
combined excretion, tissue level and elimination study: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 43% chlorination of long
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 275 pp (Report
No. 438-028/021).
IRDC (1984g) 13-week dietary toxicity study in rats with combined
excretion, tissue level and elimination studies/determination of
excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 70% chlorination of long
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 316 pp (Report
No. 438-027/024).
IRDC (1985) Reproduction range-finding study in rats. Chlorinated
paraffin: 52% chlorination of intermediate chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
179 pp (Report No. 438-049).
Jansson B, Andersson R, Asplund L, Bergman Å, Litze'n K, Nylund K,
Reuthergårdh L, Sellström U, Uvemo U-B, Wahlberg C, & Wideqvist U
(1991) Multiresidue method for the gas-chromatographic analysis of
some polychlorinated and polybrominated pollutants in biological
samples. Fresenius J Anal Chem, 340: 439-445.
Jansson B, Andersson R, Asplund L, Litze'n K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Environ Toxicol Chem, 12: 1163-1174.
KEMI (1991) Chlorinated paraffins. In: Freij L ed. Risk reduction of
chemicals: A Government Commission Report. Solna, The Swedish National
Chemicals Inspectorate, pp 167-187 (KEMI Report 1/91).
Kraemer W & Ballschmiter K (1987) Detection of a new class of
organochlorine compounds in the marine environment: the chlorinated
paraffins. Fresenius Z Anal Chem, 327: 47-48.
Lindén E, Bengtsson BE, Svanberg O, & Sundström G (1979) The acute
toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
Lombardo P, Dennison JL, & Johnson WW (1975) Bioaccumulation of
chlorinated paraffin residues in fish fed Chlorowax 500C. J Assoc Off
Anal Chem, 58: 707-710.
Lundberg P (1980) Effects of some flame retardants on the liver
microsomal enzyme systems. In: Cohn MJ ed. Microsomes, drug
oxidations, and chemical carcinogenesis, 1979. New York, Academic
Press, pp 853-856.
Lunde G & Steinnes E (1975) Presence of lipid-soluble chlorinated
hydrocarbons in marine oils. Environ Sci Technol, 9: 155-157.
Madeley J & Birtley R (1980) Chlorinated paraffins and the
environment. 2. Aquatic and avian toxicology. Environ Sci Technol,
14: 1215-1221.
Madeley JR & Gillings E (1983) Determination of the solubility of four
chlorinated paraffins in water. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 23 pp (Report No. BL/B/2301).
Madeley JR & Maddock BG (1983a) Effects of a chlorinated paraffin on
the growth of rainbow trout. Chlorinated paraffin: 58% chlorination of
short chain length paraffins. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 67 pp (Report No. BL/B/2309).
Madeley JR & Maddock BG (1983b) The bioconcentration of a chlorinated
paraffin in the tissues and organs of rainbow trout (Salmo gairdneri).
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 39 pp
(Report No. BL/B/2310).
Madeley JR & Maddock BG (1983c) Toxicity of a chlorinated paraffin to
rainbow trout over 60 days. Chlorinated paraffin: 52% chlorination of
intermediate chain length n-paraffins. Brixham, Imperial Chemical
Industries Ltd, Brixham Laboratory, 69 pp (Report No. BL/B/2202).
Madeley JR & Maddock BG (1983d) Toxicity of a chlorinated paraffin to
rainbow trout over 60 days. Chlorinated paraffin: 43% chlorination of
long chain length n-paraffins. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 71 pp (Report No. BL/B/2201).
Madeley JR & Thompson RS (1983a) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 71 pp (Report No.
BL/B/2291).
Madeley JR & Thompson RS (1983b) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Brixham,
Imperial Chemical Industries Ltd, Brixham Laboratory, 53 pp (Report
No. BL/B/2289).
Madeley JR & Thompson RS (1983c) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 59 pp (Report No.
BL/B/2288).
Madeley JR & Thompson RS (1983d) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 64 pp (Report No.
BL/B/2290).
Madeley JR, Thompson RS, & Brown D (1983a) The bioconcentration of a
chlorinated paraffin by common mussel (Mytilus edulis). Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 76 pp
(Report BL/B/2351).
Madeley JR, Windeatt AJ, & Street JR (1983b) Assessment of the
toxicity of a chlorinated paraffin to the anaerobic sludge digestion
product. Chlorinated paraffin: 58% chlorination of short chain length
n-paraffins. Brixham, Imperial Chemical Industries Ltd, Brixham
Laboratory, 25 pp (Report No. BL/B/2253).
Mather JI, Street JR, & Madeley JR (1983) Assessment of the inherent
biodegradability of a chlorinated paraffin, under aerobic conditions,
by a method developed from OECD Test Guideline 302B. Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 56 pp
(Report No. BL/B/2298).
Meijer J & DePierre JW (1987) Hepatic levels of cytosolic, microsomal
and "mitochondrial" epoxide hydrolases and other drug-metabolizing
enzymes after treatment of mice with various xenobiotics and endogenous
compounds. Chem-Biol Interact, 62: 249-269.
Meijer J, Rundgren M, Åström A, DePierre JW, Sundvall A, & Rannug U
(1981) Effects of chlorinated paraffins on some drug-metabolizing
enzymes in rat liver and in the Ames test. Adv Exp Med Biol,
A136: 821-828.
Menter P, Harrison W, & Woodin WG (1975) Patch testing of coolant
fractions. J Occup Med, 17(9): 565-568.
Muller W (1989) Chlorowax 500C - Micronucleus test in male and female
NMRI mice after oral administration. Frankfurt/Main, Hoechst AG,
Pharma Research Toxicology and Pathology, 24 pp (Report No. 89.0253).
Murray T, Frankenberry M, Steele DH, & Heath RG (1988) Chlorinated
paraffins: A report on the findings from two field studies, Sugar
Creek, Ohio, Tinkers Creek, Ohio. Volume 1: Technical report.
Washington, DC, US Environmental Protection Agency, 150 pp (EPA-560/
5-87/012).
Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D,
Martin R, & Caspary WJ (1990) L5178Y mouse lymphoma cell mutation
assay results with 41 compounds. Environ Mol Mutagen, 16(Suppl 18):
138-167.
NIOSH (1990) National occupational exposure survey (1980-1983).
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
Nilsen O & Toftgård R (1981) Effect of polychlorinated terphenyls and
paraffins on rat liver microsomal cytochrome P-450 and in vitro
metabolic activities. Arch Toxicol, 47: 1-11.
Nilsen O, Toftgård R, & Glaumann H (1980) Changes in rat liver
morphology and metabolic activities after exposure to chlorinated
paraffins. Dev Toxicol Environ Sci, 8: 525-528.
Nilsen OG, Toftgård R, & Glaumann H (1981) Effects of chlorinated
paraffins on rat liver microsomal activities and morphology.
Importance of the length and the degree of chlorination of the carbon
chain. Arch Toxicol, 49: 1-13.
NTP (1986a) Toxicology and carcinogenesis studies of chlorinated
paraffins (C12, 60% chlorine) (CAS No.
2.1.2.2 Synonyms
Alkanes, chlorinated; alkanes (C10-12), chloro (60%); alkanes (C10-13),
chloro (50-70%); alkanes (C14-17), chloro (40-52%); alkanes (C18-28),
chloro (20-50%); alkanes (C22-26), chloro (43%); C12, 60% chlorine;
C23, 43% chlorine; chlorinated alkanes; chlorinated hydrocarbon
waxes; chlorinated paraffin waxes; chlorinated waxes; chloroalkanes;
chlorocarbons; chloroparaffin waxes; paraffin, chlorinated; paraffins,
chloro; paraffin waxes, chlorinated; paroils, chlorinated; poly-
chlorinated alkanes; polychloro alkanes.
2.1.3 Technical products
Chlorinated paraffins are manufactured commercially by a number
of companies and are marketed under a variety of trade names. The
trade names are followed by numbers, which often are related to the
average chlorine content (in percent) of a particular preparation.
However, this is not a rule and the average chlorine content may have
to be obtained from manufacturers' technical data. More than 200
chlorinated paraffin formulations are commercially available
world-wide (Serrone et al., 1987), and some examples of these are
given in Table 2.
The carbon chain length of the chlorinated paraffins in a
commercial mixture is variable, and the average chain length is
usually specified by the manufacturer. The composition of paraffins
of different chain length in some commercial formulations is shown in
Table 3. The paraffin feedstocks are randomly chlorinated and the
resulting chlorine contents are given as average values.
Commercial chlorinated paraffins may be contaminated by
isoparaffins (usually less than 1%), aromatic compounds (usually less
than 0.1% (1000 ppm)) and metals (Schenker, 1979).
Chlorinated paraffins normally contain stabilizers, which are
added to inhibit decomposition. Common stabilizers include epoxidized
compounds such as epoxidized esters and soya bean oils (indicated in
section 7.1.3 to be present at up to 3%), pentaerythritol, thymol,
urea, glycidyl ethers, acetonitriles and organic phosphites (Schenker,
1979; Strack, 1986; Houghton, 1993). The concentration of stabilizers
is usually below 0.05% w/w (Campbell & McConnell, 1980).
Table 2. Partial list of commercial chlorinated paraffinsa
Average molecular formula C12H15Cl11 C12H19Cl7 C15H26Cl6 C24H29Cl21 C24H42Cl8 C24H44Cl6
Chlorine content (% w/w) 70 60-65 50-52 70 48-54 40-42
Manufacturers:
Oxychem, USA Chlorowax 70L Chlorowax 500C Chlorowax 70 Chlorowax 50 Chlorowax 40
Keil Chemical Div., USA CW-200-70 CW-85-60 CW-52 CW-220-50 CW-170
Dover Chemical Corp., Paroil 170HV Paroil 160 Paroil 152 Chlorez 700 Paroil 150S Paroil 140
USA Paroil 1048
Plastifax, Inc., USAb Plastichlor P-70 Plastichlor P-59 Plastichlor 50-220 Plastichlor
P-65 42-170
ICI, Australia; Canada; Cereclor 70L Cereclor 60L Cereclor S52 Cereclor 70 Cereclor 48 Cereclor 42
UK; France
Neville Chemical Co., Unichlor 60L-60 Unichlor 50L-65 Unichlor 50-450 Unichlor 40-170
USAb Unichlor 40-150
Pearsall Chemical Co., FLX-0012 FLX-0008 CPF-0020 CPF-0004
USA CPF-0003 CPF-0001
Hüls AG, Germanyb Chlorparaffin Chlorparaffin Chlorparaffin Chlorparaffin
70C 60C 52G 40N
Dynamit Nobel, Germanyb Witaclor 171 Witaclor 160 - Witaclor 350 Witaclor 549 Witaclor 540
Witaclor 163 Witaclor 352
Caffaro, Italy Cloparin D70 Cloparin 1059 Cloparin 50 Cloparin S70 Cloparin P42
Table 2. (Cont'd)
Average molecular formula C12H15Cl11 C12H19Cl7 C15H26Cl6 C24H29Cl21 C24H42Cl8 C24H44Cl6
Chlorine content (% w/w) 70 60-65 50-52 70 48-54 40-42
Hoechst AG, Germany Chlorparaffin Hordaflex LC60 Chlorparaffin Chlorparaffin Chlorparaffin
Hoechst 70 Hoechst 52fl Hoechst 70fest Hoechst 40fl
Rhône-Poulenc, France Alaiflex 67B2 Ribeclor 60B2 Alaiflex 50A3 Alaiflex 40A8
a Other producers include Bann Quimica (Brazil), Excel Industry (India), Ajinomoto (Japan), Tosoh (Japan), Asahi Denka (Japan),
Plasticlor (Mexico), NCP (South Africa)
b These companies have ceased production of chlorinated paraffins.
Table 3. Composition of paraffins obtained by dechlorination of
different chlorinated paraffin preparations (Zitko, 1974b)
Chlorinated Percentage of each paraffin
paraffin
C21 C22 C23 C24 C25 C26 C27 C28
Chloroparaffin, 4.5 10.0 15.7 19.3 18.5 15.3 9.8 6.7
40%
Clorafin 40 3.7 8.2 14.0 17.5 19.2 17.4 12.4 7.6
CP 40 3.9 9.1 14.9 19.2 19.8 18.0 15.1 -
Cereclor 42 3.6 8.8 14.7 18.6 19.5 17.2 11.5 6.0
Chloroparaffin, 7.4 14.9 20.7 23.1 19.9 14.0 - -
50%
2.2 Chemical and physical properties
Chlorinated paraffins are viscous, colourless or yellowish, dense
oils, except for the chlorinated paraffins of long carbon chain length
(C20-C30) with high chlorine content (70%), which are solid.
Chlorinated paraffins have a characteristic slight and not unpleasant
odour (Hardie, 1964). The odour is probably due to small quantities
of products of lower relative molecular mass with small but measurable
vapour pressures (Howard et al., 1975). Chlorinated paraffins
themselves have very low vapour pressures. The medium chain length
C14-17;52% Cl has a vapour pressure of approximately 2 × 10-4 Pa at
20°C (1-2 × 10-6 mmHg) (Campbell & McConnell, 1980), and the long
chain length C23;42-54% Cl approximately 3 × 10-3 Pa when measured at
65°C (2 × 10-5 mmHg) (Hardie, 1964). The chemical and physical
properties of chlorinated paraffins are determined by the carbon chain
length of the paraffin and the chlorine content. Increases in the
carbon chain length and chlorination degree of a particular paraffin
increase the viscosity and density but reduce the volatility.
Chlorinated paraffins are practically insoluble in water, but
many products can be emulsified with water (approximately 70/30
chlorinated paraffin to water). The water solubility of 14C-labelled
polychloroundecane (C11;59% Cl) is reported to be 150-470 µg/litre,
polychloropentadecane (C15;51% Cl) 5-27 µg/litre and the poly-
chloropentacosanes (C25;43% Cl) < 5-6.4 µg/litre and (C25;70%
Cl) < 5-5.9 µg/litre, depending on analytical method (Madeley &
Gillings, 1983). Campbell & McConnell (1980) reported the solubility
of C16;52% Cl to be 10 µg/litre in freshwater and 4 µg/litre in
seawater. The solubility of C25;42% Cl was reported to be 3 µg/litre
in seawater. Chlorinated paraffins are also practically insoluble in
lower alcohols, glycerol and glycols, but are soluble in chlorinated
solvents, aromatic hydrocarbons, ketones, esters, ethers, mineral oil
and some cutting oils. They are moderately soluble in unchlorinated
aliphatic hydrocarbons (Houghton, 1993). Some physical properties of
typical commercial chlorinated paraffins are summarized in Table 4.
Assuming a water solubility of 5 µg/litre and a vapour pressure
of 2 × 10-4 Pa as typical of a 52% chlorinated intermediate chain
length paraffin, a Henry's Law constant of 10.9 may be calculated
(Willis et al., 1994).
A key property of chlorinated paraffins, particularly the high
chlorine grades, is their nonflammability. This is due to the ability
of chlorinated paraffins to release hydrochloric acid at elevated
temperatures, and the hydrochloric acid inhibits the radical reaction
in a flame. This property is considerably enhanced by the addition of
antimony trioxide (Houghton, 1993) or other additives. Chlorinated
paraffins are generally unreactive and stable in normal temperatures,
but decompose significantly at temperatures above 300°C with the
release of hydrochloric acid (Strack, 1986). Prolonged exposure
to light can also cause dehydrochlorination. Degradation by
dehydrochlorination can be accelerated at elevated temperatures in
the presence of aluminium, zinc, and iron oxide or chloride (Howard
et al., 1975; Houghton, 1993). Dehydrochlorination leads to darkening
of the material.
2.3 Analysis
The analysis of chlorinated paraffins is very difficult owing to
the many congeners present in the products. The properties of these
congeners cover wide ranges, which makes it difficult to separate the
chlorinated paraffins from other compounds that may interfere in the
analysis.
Table 4. Physical properties of selected commercial chlorinated paraffinsa
Paraffin Chlorine Colour hazen Viscosityb Densityb Thermal stabilityc Volatilityd Refractive Log Powe
feedstock content (APHA) (Pa.s) (g/ml) (% w/w HCl) (% w/w) index
% (w/w)
C10-C13 50 100 0.08 1.19 0.15 16.0 1.493 4.39-6.93
56 100 0.8 1.30 0.15 7.0 1.508 NRg
60 125 3.5 1.36 0.15 4.4 1.516 4.48-7.38
63 125 11.0 1.41 0.15 3.2 1.522 5.47-7.30
65 150 30.0 1.44 0.20 2.5 1.525 NR
70 200 8.0f 1.50 0.20 0.5 1.537 5.68-8.01h
C14-C17 40 80 0.07 1.10 0.2 4.2 1.488 NR
45 80 0.2 1.16 0.2 2.8 1.498 5.52-8.21
52 100 1.6 1.25 0.2 1.4 1.508 5.47-8.01
58 150 40.0 1.36 0.2 0.7 1.522 NR
Wax C18-C20 47 150 1.7 1.21 0.2 0.8 1.506 NR
50 250 18.0 1.27 0.2 0.7 1.512 NR
Wax (C> 20) 42 250 2.5 1.16 0.2 0.4 1.506 9.29->12.83h
48 300 28.0 1.26 0.2 0.3 1.516 8.69-12.83
70 100i j 1.63 0.2 NR - NR
a Data from Houghton (1993) f At 50°C
b At 25°C unless otherwise noted g NR = not reported
c Measured in a standard test for 4 h at 175°C h Data from Cereclor 42
d Measured in a standard test for 4 h at 180°C i 10 g in 100 ml toluene solvent
e Octanol:water partition coefficients. From: Renberg et al (1980) j Solid, softening point = 95-100°C
2.3.1 Sampling
To prevent contamination by trace amounts of chlorinated
paraffins, samples or their extracts must not be allowed to come into
contact with any plastic (especially PVC) container, stopper, cap
liner or tubing, because these may contain chlorinated paraffins
(Hollies et al., 1979). All solvents should be rigorously tested
before use, and it is recommended that glass distilled solvents are
used. All glassware should be decontaminated before use by heating at
250°C for 24 h. Water and sediment samples should be stored at
ambient temperatures, and should be analysed within a month of
sampling.
Treatment of samples for the extraction of chlorinated paraffins
is described in Table 5.
2.3.2 Analytical methods
Methods used for detection of chlorinated paraffins in various
samples are shown in Table 5.
Hollies et al. (1979) determined C13-17 and C20-30 chlorinated
paraffin after clean-up of the samples on aluminium oxide columns.
The chlorinated paraffin fraction was then applied on a silica gel
thin-layer chromatography (TLC) plate. After forward elution with
n-hexane and subsequently with toluene, and backward elution with
n-hexane, chloride from the chlorinated aliphatics was transferred
to an aluminium oxide plate at 240°C and developed with silver
nitrate. The resulting spots were quantified by visual comparison
with spots of known amounts of reference materials. Although the
procedure is complicated and involves several evaporations to dryness,
good recoveries were reported. Possible interference from a number of
other chlorinated compounds was investigated and found to be
negligible, but the method must still be regarded as fairly
non-specific.
Gas chromatographic analysis of chlorinated paraffin, using
microcoulometric detection, has been described by Zitko (1973). This
method gives badly resolved chromatograms and there is a considerable
risk of interference from other halogenated compounds. Owing to high
temperatures in the gas chromatographic system there is also a risk of
dehydrochlorination of the chlorinated paraffin congeners. In a later
study (Zitko & Arsenault, 1977), interference from other compounds was
avoided by a solvent partitioning clean-up procedure.
Table 5. Analytical methods for the determination of chlorinated paraffins in various samplesa
Sample Preparation method Analytical Sample detection Recovery Reference
matrix methodb limitb
Water Extract with petroleum spirit; concentrate; purify by TLC 500 ng/litre 90% Hollies et
aluminium oxide chromatography, elute with toluene; al. (1979)
dry; dissolve in petroleum spirit
Water Extract with hexane; purify by aluminium oxide GC/ECD 3 ng/litre NR Kraemer &
chromatography; elute with hexane/dichloromethane Ballschmiter
(4%); purify by silica gel chromatography, elute with (1987)
hexane:dichloromethane (19:1); dissolve in isooctane.
Water Extraction with hexane (particle phase Soxhlet GC/ approx. 92-120% Steele et
extracted), silica gel and aluminium oxide column MS-NCI 1 µg/litre al. (1988)
chromatography
Biological Homogenize; extract with petroleum spirit:acetone TLC 50 µg/kg 80-90% Hollies et
material (2:1); dry; dissolve in petroleum spirit; extract with al. (1979)
dimethylformamide; wash; back-extract with Na2SO3
solution and petroleum spirit; purify by silica gel
chromatography, elute with CCl4; dry; dissolve in
acetone; extract with petroleum spirit:acetone (1:4)
Cod muscle Homogenize in n-hexane:acetone (1:2.5, v:v); extract GC/MS NR 98-114% at Jansson et
tissue with 10% diethyl ether in n-hexane; evaporate; dissolve 0.465 µg/sample al. (1991)
in dichloromethane:n-hexane (1:1, v/v); purify by gel and 89-92% at
permeation chromatography; concentrate; extract with 2.33 µg/sample
sulfuric acid; concentrated in organic phase
Adipose Homogenize in dichloromethane; percolate through GC/MS 5 ng 80% Schmid &
tissue anhydrous Na2SO4; remove solvent; dissolve residue in Müller (1985)
pentane; wash, dry and concentrate; purify by alumina
chromatography
Table 5. (Cont'd)
Sample Preparation method Analytical Sample detection Recovery Reference
matrix methodb limitb
Mineral oil Extract fish in cyclohexane; introduce extract or MS-NCI NR NR Gjos &
and fish mineral oil sample directly into mass spectrometer Gustavsen
extract (1982)
Fish fillets Homogenize in petroleum ether; clean-up by irradiating GC/CD NR > 90% Friedman &
extracts with high-intensity UV light (90 min, < 20°C) Lombardo
in petroleum ether (1975)
Sewage Homogenize in acetone; extract with pentane; wash, dry GC/MS 5 ng NR Schmid &
sludge and concentrate; purify by alumina chromatography Müller (1985)
Sediment Dry at 70°C; extract with petroleum spirit; TLC 50 µg/kg 80% Hollies et
concentrate; purify by aluminium oxide al. (1979)
chromatography, elute with toluene; dry; dissolve
in petroleum spirit
Sediment Extract with acetone:hexane (1:1, v:v); wash, dry GC/MS 5 ng NR Schmid &
and concentrate; purify by alumina chromatography Müller (1985)
Sediment Soxhlet extraction with hexane, silica gel and GC/ approx. 52-64% Steele et
aluminium oxide column chromatography MS-NCI 1 µg/litre al. (1988)
a Modified from IARC (1990)
b GC/MS = gas chromatography/mass spectrometry; GC/CD = gas chromatography/coulometric detection;
GC/ECD = gas chromatography/electron capture detection; MS-NCI = negative-ion chemical ionization mass spectrometry;
TLC = thin-layer chromatography; NR = not reported
Attempts have been made to reduce the complexity of chlorinated
paraffin mixtures by reductive dechlorination (Cooke & Roberts, 1980;
Roberts et al., 1981; Sistovaris & Donges, 1987). This method gives
information on the "carbon skeleton" of the chlorinated paraffin
compounds but no information on the chlorine content, and it is
difficult to separate the response from that of unchlorinated
hydrocarbons.
Negative ion chemical ionization mass spectrometry (MS-NCI) was
used by Gjös & Gustavsen (1982). In this method the chlorinated
paraffin fractions are introduced directly into the ion source of the
mass spectrometer. As the whole sample is analysed in a very short
time, the concentration in the ion source is high and the sensitivity
can therefore be high. A serious disadvantage is that all other
compounds in the sample come into the mass spectrometer at the same
time, the risk of interference is high and an extensive clean-up of
the samples is needed.
Gas chromatography utilizing MS-NCI for the detection was used by
Schmid & Müller (1985). A fairly simple clean-up based on adsorption
chromatography on aluminium oxide was used, but unfortunately this
has been impossible to reproduce (Jansson, personal communication).
GC/MS-NCI was also used by Steele et al. (1988) to determine
chlorinated paraffins after clean-up of samples on silica gel and
aluminium oxide columns. They used low inlet temperatures in the
gas chromatograph to avoid thermolysis of the analysed compounds.
The use of low temperatures and short capillary columns further
decreases the risk of temperature-related break-down of chlorinated
paraffins during gas chromatographic analysis (Jansson et al., 1991).
In this method a gel permeation column was also used to avoid
interference from other chlorinated compounds, and the Cl2- and
HCl2- ions were used to detect aliphatic chlorinated compounds
selectively.
Developments in chlorinated paraffin analysis have improved
both selectivity and sensitivity. However, although the reliability
of results is now better, these are only estimates of the real
concentrations as it is impossible to detect the individual substances.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Chlorinated paraffins are not known to occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Liquid chlorinated paraffins were first used in large amounts
during the period 1914-1918 as solvents for Dichloramine T in
antiseptic nasal and throat sprays (Howard et al., 1975). The
commercial production of chlorinated paraffins for use as extreme
pressure additives in lubricants started around 1930 (Schenker, 1979).
Estimated data on the production of chlorinated paraffins are
shown in Table 6. Chlorinated paraffins are produced in Australia,
Brazil, Bulgaria, Canada, China, Germany, France, India, Italy, Japan,
Mexico, Poland, Romania, Spain, Slovakia, South Africa, China
(Province of Taiwan), Thailand, the United Kingdom, the USA, and the
former USSR. However, this may not be a complete list of producer
countries. It is believed that 50% of the chlorinated paraffins
produced in the world have carbon chain lengths of between 14 and 17
and a chlorine content of between 45 and 52%. In the United Kingdom
approximately 80% of the total production of chlorinated paraffins is
concentrated on the C14-17 chain length. About 15% of the European
consumption of chlorinated paraffins is estimated to be C10-13, 70%
C14-17 and 15% C20-30 (Willis et al., 1994).
Chlorinated paraffins are produced by reacting liquid paraffin
fractions obtained from petroleum distillation with pure chlorine gas
by a reaction mechanism involving free radicals (Schenker, 1979;
Houghton, 1993). The reaction is exothermic. At a chlorine content
above approximately 54% further chlorination is slow and difficult.
In the production of resinous chlorinated paraffins containing
> 70% Cl, a solvent is usually added to decrease the viscosity
(Howard et al., 1975). Carbon tetrachloride has been the most
commonly used solvent, and may be present in trace amounts in the
final product, although alternative production methods are being
developed because of the phase-out of carbon tetrachloride under the
Montreal Protocol (D. Farrer, personal communication to the IPCS,
1995).
The substituted chlorine atoms are probably randomly distributed,
and at a chlorination of 72% all of the carbon atoms are singly
chlorinated. Further chlorination is difficult since the first
chlorine substitution decreases the reactivity of the other hydrogens
on a particular carbon atom (Hardie, 1964).
Table 6. Estimated production of chlorinated paraffins
Production References
(tonnes/year)
Canada - 1990 2900 Camford Information
Services (1991)
Germany - 1990/1991 20 000-30 000 BUA (1992)
United Kingdom - 1992 50 000 Willis et al. (1994)
USA - 1977 37 000 Schenker (1979)
USA - 1983 45 000 NTP (1986a)
USA - 1987 45 000 IARC (1990)
USA - 1990 26 000 US EPA (1993)
North America - 1978 60 000 Zitko (1980)
Western Europe - 1978 105 000 Zitko (1980)
Western Europe - 1985 95 000 IARC (1990)
World, excluding 230 000 Campbell &
Eastern Europe - 1977 McConnell (1980)
World - 1985a 300 000 Strack (1986)
a Excluding the former Soviet Union and the People's Republic of China.
Depending on producer and paraffin feedstock, the temperature of
the chlorination reaction is usually kept at 80-100°C to decrease the
viscosity but at a temperature where the decomposition of the product
is not extensive (Schenker, 1979; Houghton, 1993). Since the reaction
is exothermic heat removal is important in the process. Ultraviolet
light is often used as a catalyst (Schenker, 1979; Houghton, 1993).
Metal catalysts are avoided since they may promote dechlorination of
the chlorinated paraffins. Since for each tonne of chlorinated
paraffin produced, approximately half a tonne of hydrochloric acid is
generated, the linings of the reactor vessels must be chemically inert
to avoid the formation of metal chlorides, which cause darkening of
the product by decomposition (Strack, 1986; Houghton, 1993).
Additional procedures used in the production of the C20-30;70% Cl solid
grade are stripping of the solvent and grinding of solid products
(Schenker, 1979).
3.2.2 Uses
Chlorinated paraffins are used as secondary plasticizers for
polyvinyl chloride (PVC) and can partially replace primary
plasticizers such as phthalates and phosphate esters (Houghton, 1993).
The use of chlorinated paraffins has the advantage in comparison with
conventional plasticizers of both increasing the flexibility of
the material as well as increasing its flame retardancy and
low-temperature strength (Howard et al., 1975). Chlorinated paraffins
are also used as extreme pressure additives in metal-machining fluids
or as metal-working lubricants or cutting oils because of their
viscous nature, compatibility with oils, and property of releasing
hydrochloric acid at elevated temperatures. The hydrochloric acid
reacts with metal surfaces to form a thin but strong solid film of
metal chloride lubricant. In Sweden, the use of chlorinated paraffins
in metal-working fluids has been reduced from 680 tonnes (1986) to
139 tonnes (1993) as a part of a risk reduction programme (Swedish
Environmental Protection Agency, 1994). They are added to paints,
coatings and sealants to improve resistance to water and chemicals,
which is most suitable when they are used in marine paints, as
coatings for industrial flooring, vessels and swimming pools (e.g.,
rubber and chlorinated rubber coatings), and as road marking paints.
The flame-retarding properties of highly chlorinated paraffins make
them important as additives in plastics, fabrics, paints and coatings.
The most effective fire-retardant action is obtained with a high
degree of chlorination.
By the late 1970s approximately 50% of chlorinated paraffins in
the USA was used as extreme pressure lubricant additives in the
metal-working industry; 25% was used in plastics and fire-retardant
and water-repellant fabric treatments, and the rest was used in paint,
rubber, caulks and sealants (Schenker, 1979). In the United Kingdom,
65-70% of the consumed chlorinated paraffins is used as a secondary
plasticizer in PVC, about 10% in paint, about 10% in metal-cutting
lubricants and about 10% in flame retardants and sealants (Willis et
al., 1994). In Canada approximately 55% of the chlorinated paraffins
is used as plasticizers and 35-40% as high-pressure lubricant
additives (Camford Information Services, 1991). Some examples of
applications for chlorinated paraffins of different chain-lengths are
shown in Table 7.
3.2.3 Loss into the environment
Since chlorinated paraffins are produced without contact with
water, the possibility of leakage into the environment by direct water
discharge is low. After chlorination the solvent is removed and
residual amounts of chlorine gas and hydrogen chloride are removed by
blowing air or other gases through the product. This could possibly
lead to some loss into the air, but since the chlorine gas and
hydrochloric acid are recovered and the volatility of chlorinated
paraffins is very low, the loss is likely to be very low (Howard et
al., 1975). Emission into the atmosphere during manufacture in
Germany in 1990 was estimated to be about 250 kg/year (BUA, 1992). It
is possible that chlorinated paraffins may be a by-product during
chlorination of other hydrocarbon feedstocks if paraffins are present
as contaminants. This could lead to possible environmental
contamination.
Table 7. Uses of various chlorinated paraffins
Paraffin Chlorination (%) Application
feedstock
C10-13 plasticizer for PVC or plastics
C10-13 metal-working fluids; sealants
C10-13 approx. 70 flame retardants for rubber and soft plastics
C14-17 40-60 extreme pressure additives to metal-machining
fluids, pastes, emulsions and lubricants
C14-17 45-52 the chlorinated paraffin most frequently used as a
(40-50) plasticizer for plastics; also used for sealants
C18-30 approx. 70 flame retardants for rigid plastics such
as polyesters and polystyrene
C> 20 plasticizer for PVC or plastics
Some loss into the environment could be expected during transport
and storage. If the drums which are used for the transport of
chlorinated paraffins are cleaned for further use environmental
release might occur. Soil could be contaminated if empty drums are
dumped at landfills. Spills may occur, but clean-up using an
adsorbent material is easy. The adsorbent material would probably
be deposited in a landfill, which in turn could lead to possible
environmental contamination.
The uses of chlorinated paraffins probably provide the major
source of environmental contamination. When chlorinated paraffins are
used as plasticizers or additives in coatings, they are effectively
dissolved in the polymers and will therefore leak into the environment
only very slowly. However, polymers containing chlorinated paraffins
will act as sources of chlorinated paraffins for centuries after
disposal. A more likely route of leakage of chlorinated paraffins
into the environment would be the improper disposal of oils containing
chlorinated paraffins (Campbell & McConnell, 1980) or disposal of
chlorinated paraffins of low quality (Howard et al., 1975). Loss of
chlorinated paraffins by removal from paints and coatings may also
contribute to environmental contamination.
It is estimated that a maximum of 55% of the cutting and
lubricating oils sold to the engineering industry in Sweden becomes
waste. The rest is consumed or released into the air and water (KEMI,
1991). The largest consumer of chlorinated paraffins in Sweden
(1400 tonnes/year) has estimated its emission of chlorinated paraffins
to be 90 kg/year (0.06 g emission/kg chlorinated paraffin produced)
(KEMI, 1991).
Disposal of wastes containing chlorinated paraffins occurs
through resource recovery, destructive incineration or landfill,
usually on disposal sites for special wastes and in compliance with
local regulations. Owing to their thermal instability, chlorinated
paraffins are expected to be degraded by incineration at low
temperatures and thus would not be expected to volatilize in exhaust
gases from an incinerator. However, in a study by Bergman et al.
(1984), chlorinated aromatic compounds such as PCBs, naphthalenes and
benzenes were formed by pyrolysis of chlorinated paraffins (see
section 4.2.1) although the conditions used were not identical to the
operation conditions of waste incineration plants. Chlorinated
paraffins are not expected to be formed de novo. The disposal of
chlorinated paraffins in landfills may give rise to leaching into
water, but owing to the low water solubility and strong adsorption
onto solids the amounts reaching water are likely to be low.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Considering the low vapour pressure (2 × 10-4 Pa at 20°C for
C14-17;52% Cl to 3 × 10-3 Pa at 65°C for C23;42-54% Cl), low water
solubility (3 to 470 µg/litre) and highly lipophilic nature of
chlorinated paraffins (log Pow values range from 4.39 to > 12.83),
it is likely that they will distribute mainly to the soil/sediment
phase with very little volatilization occurring. Chlorinated
paraffins are likely to be transported in water as suspended
particles, and in air as dust particles and possibly in the vapour
phase. However, no experimental data on this subject have been
reported.
4.2 Transformation
4.2.1 Abiotic transformation
No experimental data on the chemical stability of chlorinated
paraffins under simulated environmental conditions have been reported.
However, their chemical reactivities suggest that they do not
hydrolyse, oxidize or react by other mechanisms at significant rates
under normal temperatures and neutral conditions (Howard et al.,
1975). Dehydrochlorination of chlorinated paraffins may possibly
occur naturally in the presence of metal ion contamination.
Because of the high adsorption tendency of chlorinated paraffins,
gas phase reactions are assumed to contribute only little to
degradation in the atmosphere (BUA, 1992). However, calculated
half-lives for chlorinated paraffins in air are reported to range from
0.85 to 7.2 days (Slooff et al., 1992). The theoretical values are
shown in Table 8.
The thermal degradation by pyrolysis of chlorinated paraffins at
various temperatures (300, 500, 700°C) and times (10 sec to 20 min)
was studied by Bergman et al. (1984). The chlorinated paraffins were
totally degraded, and, depending upon degree of chlorination of the
chlorinated paraffin, several aliphatic and aromatic degradation
products, such as polychlorinated biphenyls (PCBs), naphthalenes and
benzenes, were detected. As much as 10 g of PCBs per kg chlorinated
paraffin could be found after thermal degradation of C12;70% Cl
(temperature not specified). Smaller amounts of compounds were formed
at lower temperatures. Considering these results, processes where
chlorinated paraffins are subjected to temperatures above 300°C could
lead to environmental contamination and exposure to more persistent
and toxic substances than the original chlorinated paraffins.
Table 8. Photochemical degradation of chlorinated paraffins in the
atmosphere (From: Slooff et al., 1992)
Carbon chain length Koh (cm3/mol per sec) Half-life (days)
C10-C13 9.0-14.9 × 10-12 1.2-1.8
C14-C17 14.9-18.9 × 10-12 0.85-0.8
C15-C30 20.2-31.1 × 10-12 0.5-0.8
not specified 2.2-18.8 × 10-12 0.85-7.2
4.2.2 Biodegradation
Chlorinated paraffins are generally stable in the natural
environment.
4.2.2.1 Short chain length chlorinated paraffins
A short chain length paraffin (C10-12) with 58% chlorination
(CP-SH) was not readily biodegraded by activated sludge, under either
aerobic or anaerobic conditions, over a 28-day period in an inherent
biodegradability (modified Zahn-Wellens) test (Street & Madeley,
1983a,b) or a 51-day period in a coupled units test (Mather et al.,
1983).
4.2.2.2 Long chain length chlorinated paraffins
Zitko & Arsenault (1977) studied sediment spiked with 596 mg/kg
dry weight of Cereclor 42 C24;42% Cl, CP-LL) or 357 mg/kg dry weight
of Chlorez 700 (C24;70% Cl, CP-LH), which are both long chain length
chlorinated paraffins but have different chlorine contents. There was
no clear trend in the results but they indicated that after 4 weeks
the highly chlorinated Chlorez 700 was degraded to a greater extent
than Cereclor 42. The rate of degradation seems to have been higher
under anaerobic conditions.
4.2.2.3 Comparative studies
In another study, the microbial degradation of several short,
intermediate and long chain length chlorinated paraffins of different
chlorination degree at concentrations of 2, 10 and 20 mg/litre was
examined in a 25-day biochemical oxygen demand (BOD) test (Madeley &
Birtley, 1980). The degradation rate appeared to decrease with
increasing carbon chain length and chlorination degree, and the short
chain chlorinated paraffins with less than 50% Cl were degraded most
rapidly and completely. It can be concluded from the results that
chlorinated paraffins with low chlorine contents (< 50% wt Cl) and,
especially, short chain chlorinated paraffins, biodegrade slowly in
the environment, particularly in the presence of adapted microbial
populations, but that chlorinated paraffins with higher chlorine
contents are unlikely to biodegrade under aerobic conditions.
Anaerobic microorganisms did not degrade Cereclor 42 (C24;42% Cl) in
30 days when readily biodegradable alternative carbon sources were
available.
Omori et al. (1987) found that bacterial strains isolated from
the soil degraded various chlorinated paraffins by dechlorination in
the presence of n-hexadecane. In a mixed culture of four strains,
more than 50% of the chlorine was removed from the shorter paraffins
with lower chlorine content (C14;43% Cl, CP-ML and C15;50% Cl, CP-MH)
within 36 h. Lower amounts of chlorine were removed from the
chlorinated paraffins with longer chain lengths. Activated sludge
from a sewage treatment plant in Tokyo acclimatized to n-hexadecane
for 60 days showed only a limited amount (2%) of dechlorination of the
chlorinated paraffins. The bacterial dechlorination concerned the
terminal chlorine, which produced 2- or 3-chlorinated fatty acids via
ß-oxidation.
4.3 Bioaccumulation and biomagnification
4.3.1 Summary
Chlorinated paraffins of short chain length accumulate in mussels
and fish to a higher degree than intermediate and long chain length
chlorinated paraffins.
Data on bioaccumulation of chlorinated paraffins by aquatic
organisms are summarized in Table 9. Bioconcentration factors (BCFs)
may be uncertain since the applied doses exceeded the water solubility
in several experiments.
Table 9. Bioconcentration factors for some chlorinated paraffins
Species Chlorinated paraffina Exposure Bioconcentration Reference
Concentration Duration factor
(µg/litre) (days) (whole animal)b
Marine diatom C10-12;58% Cl (CP-SH) 1.4 10 < 1 Thompson & Madeley (1983b)
(Skeletonema costatum) 17.8 10 3.5
Freshwater green alga C10-12;58% Cl (CP-SH) 35 10 1.5 Thompson & Madeley (1983d)
(Selenastrum capricornutum) 140 10 7.6
150 10 4.1
Mussel C10-12;58% Cl (CP-SH) 2.3 147 40 900e Madeley et al. (1983a)
(Mytilus edulis) 10 91 24 800e
C10-12;58% Cl (CP-SH) 13 60 25 292e Madeley & Thompson (1983a)
130 60 12 177e
C12;69% Cl (CP-SH) 0.13 28 138 000e Renberg et al. (1986)
C16;34% Cl (CP-ML) 0.13 28 6920e
C14-17;52% Cl (CP-MH) 220 60 2856e Madeley & Thompson (1983b)
3800c 60 429e
C22-26;43% Cl (CP-LL) 120 60 1158e Madeley & Thompson (1983c)
2180c 60 261e
C22-26;70% Cl (CP-LH) 460 60 341e Madeley & Thompson (1983d)
1330c 60 223e
Table 9. (Cont'd)
Species Chlorinated paraffina Exposure Bioconcentration Reference
Concentration Duration factor
(µg/litre) (days) (whole animal)b
Rainbow trout C10-12;58% Cl (CP-SH) 3.1 168 3550e Madeley & Maddock (1983b)
(Oncorhynchus mykiss) 14 168 5260e
C10-12;58% Cl (CP-SH) 33 60 7155e Madeley & Maddock (1983c)
3050c 60 1173e
C14-17;52% Cl (CP-MH) 1050c 60 45e Madeley & Maddock (1983c)
4500c 60 67e
C22-26;43% Cl (CP-LL) 970 60 18e Madeley & Maddock (1983c)
4000c 60 38e
C20-30;70% Cl (CP-LH) 840 60 54e Madeley & Maddock (1983c)
3800c 60 32e
Bleaks C10-13;49% Cl (CP-SL) 125 14 770d,f Bengtsson et al. (1979)
(Alburnus alburnus) 59% Cl (CP-SH) 125 14 740d,f Bengtsson et al. (1979)
71% Cl (CP-SH) 125 14 140d,f Bengtsson et al. (1979)
C14-17;50% Cl (CP-MH) 125 14 30d,f Bengtsson et al. (1979)
C18-26;49% Cl (CP-LL) 125 14 7d,f Bengtsson et al. (1979)
a The classification of chlorinated paraffins is given in Table 1
b Ratio of the concentration of the chemical in the organism to the concentration of the chemical in the environment or food
c May exceed water solubility
d BCFs calculated by Zitko (1980)
e BCFs based on radioactivity (14C)
f BCFs based on parent compounds
4.3.2 Aquatic vertebrates
4.3.2.1 Short chain length chlorinated paraffins
In a study by Lombardo et al. (1975), fingerling rainbow trout
(Oncorhynchus mykiss) were fed a diet containing 10 mg/kg Chlorowax
500C (C12;60% Cl, CP-SH) for 82 days. Samples of 20 exposed and 10
control fish were collected at approximately 2-week intervals during
the time-period and analysed for chlorinated paraffin content by
microcoulometric gas chromatography (Friedman & Lombardo, 1975). The
tissue level of chlorinated paraffins rose during the treatment period
to 1.1 mg/kg (on tissue basis) or 18 mg/kg (on fat basis) after 82
days (detection level: 0.5 mg/kg). The experiment had to be
terminated owing to the failure of the water supply, and it was not
possible to determine whether a steady-state level had been reached.
In studies (Madeley & Maddock, 1983b) on rainbow trout
(Oncorhynchus mykiss) exposed to measured concentrations of 3.1 and
14.3 µg/litre of 14C-labelled C10-12; 58% Cl (CP-SH) for a period of
168 days, BCF value of 1300 (low dose) and 1600 (high dose) were
observed in the flesh, 2800 (low dose) and 16 000 (high dose) in the
liver, and 11 700 (low dose) and 15 500 (high dose) in the viscera;
all values were determined from radioactivity measurements. The BCF
for the whole body was 3350 (low dose) and 5260 (high dose)
(calculated values). Half-lives for elimination in different organs
were calculated to be the following: liver 9.9 (low dose) and 11.6
days (high dose), viscera 23.1 (low dose) and 23.9 days (high dose),
and flesh 16.5 (low dose) and 17.3 days (high dose).
Rainbow trout (Oncorhynchus mykiss) were exposed to measured
concentrations ranging from 33 to 3050 µg/litre of C10-12;58% Cl
(CP-SH) for 60 days (Madeley & Maddock, 1983c). BCFs, which were
determined in whole fish samples at the end of the test, were 7155
(low dose) and 1173 (high dose) based on radioactivity measurements,
and 7273 (low dose) and 574 (high dose) based on parent compound
analysis. Parent compound analysis was performed using a modification
of the method of Hollies et al. (1979) (see section 2.3.2).
4.3.2.2 Intermediate chain length chlorinated paraffins
After 60 days exposure of rainbow trout (Oncorhynchus mykiss)
to measured concentrations of 1050 and 4500 µg/litre of intermediate
length (C14-17) chlorinated paraffins with 52% Cl (CP-MH), whole body
BCFs of 45 (low dose) and 67 (high dose) based on radioactivity
measurements, and of 32 (low dose) and 42 (high dose) based on parent
compound analysis were determined (Madeley & Maddock, 1983c). The
BCFs were determined at the end of the exposure period. Parent
compound analysis was performed using a modification of the method of
Hollies et al. (1979) (see section 3.2.3).
4.3.2.3 Long chain length chlorinated paraffins
Juvenile Atlantic salmon (Salmo salar) were exposed to Cereclor
42 (C24;42% Cl, CP-LL) or Chlorez 700 (C24;70% Cl, CP-LH) by uptake
from suspended solids or from food (Zitko, 1974a). The fish were
treated with either 1000 µg/litre of contaminated suspended solids for
48 h and 144 h, or to 10 mg/kg or 100 mg/kg of contaminated food for
181 days with a subsequent elimination period of 74 days. No or very
low accumulation of the chlorinated paraffins was observed. However,
the analytical method used determined the amount of chlorine and not
of chlorinated paraffin; this method has been considered as nonspecific
and of low sensitivity.
After 60 days exposure of rainbow trout (Oncorhynchus mykiss)
to long chain chlorinated paraffins with 43% Cl (CP-LL) (970 or
4000 µg/litre) or 70% Cl (CP-LH) (840-3800 µg/litre), whole body BCFs,
based on measured exposure concentrations, of 17.9 (low dose, 43% Cl),
37.6 (high dose, 43% Cl) and 53.8 (low dose, 70% Cl) and 32.5 (high
dose, 70% Cl) were determined at the end of the study when measured as
radioactivity. BCF values of 3.6 (low dose, 43% Cl), 9.0 (high dose,
43% Cl), 42.8 (low dose, 70% Cl) and 31.6 (high dose, 70% Cl), based
on parent compound analysis, were determined (Madeley & Maddock,
1983c).
4.3.3 Aquatic invertebrates
4.3.3.1 Short chain length chlorinated paraffins
After 60 days exposure of mussels (Mytilus edulis) to a short
chain length paraffin with 58% Cl (CP-SH) at measured concentrations
of 13 and 130 µg/litre, whole body BCFs were 25 292 and 12 177,
respectively, based on radioactivity measurements, and 20 000 and
7923 when measured as parent compound (Madeley & Thompson, 1983a).
After exposure of mussels (Mytilus edulis) for 147 days to
14C-labelled short chain length chlorinated paraffin with 58% Cl
(CP-SH) followed by a depuration period of 98 days (measured exposure
dose: 2.3 µg/litre), or for 91 days followed by 84 days of depuration
(measured exposure dose: 10.1 µg/litre), plateau levels of the
chlorinated paraffin in tissues were reached. Bioconcentration
factors (BCFs) at the plateau levels were 40 900 for whole mussel
tissue after exposure to 2.35 µg/litre and 24 800 after exposure to
10.1 µg/litre based on wet tissue basis. Of the different organs the
digestive glands had the highest BCF values of 226 000 (low exposure)
and 104 000 (high exposure). Half-lives for the chlorinated paraffin
in whole mussel tissue were 9.2-9.9 days (10.1 µg/litre) and 13.1-19.8
days (2.35 µg/litre) (Madeley et al., 1983a).
4.3.3.2 Intermediate chain length chlorinated paraffins
After 60-day exposures of mussels (Mytilus edulis) to
intermediate chain length paraffin with 52% Cl (CP-MH) at measured
concentrations of 220 and 3800 µg/litre (which was above the limit
of solubility in water), whole body BCFs were 429-2856 based on
radioactivity measurements and 339-2182 based on parent compound
analysis (Madeley & Thompson, 1983b).
4.3.3.3 Long chain length chlorinated paraffins
In mussels exposed to measured concentrations of 120-2180
µg/litre of long chain length chlorinated paraffin with 43% Cl (CP-LL)
and 460-1330 µg/litre of long chain length chlorinated paraffin with
70% Cl for 60 days, whole body BCFs of 1158-261 (43% Cl) and 341-223
(70% Cl), respectively, were observed when measured as radioactivity,
and 87.2-1000 (43% Cl) and 105-167 (70% Cl) when based on parent
compound analysis (Madeley & Thompson, 1983c,d). However, the high
doses exceeded the water solubility of the chlorinated paraffins.
4.3.3.4 Comparative studies
The accumulation during four weeks of two 14C-labelled
chlorinated paraffins, polychloro[1-14C]hexadecane (C16;34% Cl,
CP-ML) and 1-chloropolychloro[1-14C]dodecane (C12;69% Cl, CP-SH), was
studied in the mussel (Mytilus edulis) by Renberg et al. (1986).
Both compounds showed rapid uptake when added at concentrations of
0.13 µg/litre (C16;34% Cl) and 0.0029 µg/litre or 0.13 µg/litre
(C12;69% Cl) in water for 28 days. Steady-state levels were reached
within 14 days after exposure to 0.13 µg/litre. The authors
calculated the BCF values to be 6920 for C16;34% Cl and 138 000 for
C12;69% Cl, based on fresh weight. The mussels exposed to C12;69% Cl
were studied for an additional 28 days without exposure. The
elimination rate for this chlorinated paraffin was slow, and 33% of
the radioactivity remained in the tissues after 28 days.
4.3.4 Aquatic plants
The BCF after 10 days exposure to short chain chlorinated
paraffin with 58% chlorination (CP-SH) has been estimated to be 3.5
for the diatom Sceletonema costatum (17.8 µg/litre) and 1.5 for the
green alga Selenastrum capricornutum (35 µg/litre) (Thompson &
Madeley, 1983a,b).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Techniques for the analysis of chlorinated paraffin are described
in section 2.3.2. The major problem connected with the analysis of
environmental samples is interference from other compounds. In
earlier studies, when pre-separation techniques were not as well
developed, the concentrations may have been overestimated. Another
problem is that the chlorinated paraffin composition in the
environment may be different from that of the original products,
and the quantitative analysis has to be based on comparisons with
the original products. These difficulties make it clear that
analytical results have to be regarded more as estimates than exact
concentrations.
5.1.1 Air
No information on levels in the atmosphere has been found in the
literature.
5.1.2 Water and sediment
Levels of chlorinated paraffins in water and sediment in the
United Kingdom are summarized in Table 10. Chlorinated paraffins have
been detected in United Kingdom sea water at levels in the range of
0.5-4.0 µg/litre for C10-20 and less than 2.0 µg/litre for C20-30
(Campbell & McConnell, 1980). The levels in sediments from the same
areas were analysed; chlorinated paraffins were detected only in a few
samples at levels up to 500 µg/kg wet weight for C10-20 and 600 µg/kg
for C20-30. The levels of chlorinated paraffins are low in water from
rivers and reservoirs not receiving industrial/domestic effluents
(C10-20, 1 µg/litre or below; C20-30, 2 µg/litre or below) and for
waterways in industrialized regions (C10-20, up to 6.0 µg/litre; C20-30,
0.5 µg/litre or below). The level of C10-20 in the latter regions was
higher than for C20-30. Chlorinated paraffins were not detected in
five drinking-water reservoirs either in the water (detection limit:
0.5 µg/litre) or the sediment (detection limit: 250 µg/kg) (Campbell &
McConnell, 1980). The levels of C10-20 in sediment from non-marine
water remote from industry were in the range up to 1000 µg/kg, except
for a sewage sludge sample from the Liverpool area, which had levels
of 4000-10 000 µg/kg. C20-30 was detected only in one sample at
50 µg/kg. The levels of chlorinated paraffins in sediments close to
industrial plants were found to be higher (C10-20 up to 15 000 µg/kg;
C20-30 up to 3200 µg/kg wet weight). The levels in sediment from these
industrial areas were 1000 times higher than in the overlaying water
columns, indicating the ability of chlorinated paraffins to adsorb on
suspended solids.
Table 10. Levels of chlorinated paraffins in United Kingdom water (µg/litre) and sediment (µg/kg)
(From: Campbell & McConnell, 1980)a
C10-20 C20-30
Range Median No. of samples Range Median No. of samples
level below detection level below detection
limit limit
Sea water
water ND-4.0 0.5 7/15 ND-2.0 ND 13/18
sediment ND-500 ND 14/18 ND-600 ND 15/18
Fresh water remote from industry
water ND-1.0 0.5 7/13 ND-2.0 ND 7/11
sediment ND-1000 ND 4/6 ND-< 250 ND 4/5
Fresh water close to industry
water ND-6.0 1-2 4/25 ND-0.5 ND 8/10
sediment ND-15 000 1800 2/21 ND-3200 50 3/9
a ND = not detected (detection limit in water = 0.5 µg/litre; in sediment = 50 µg/kg)
Levels of chlorinated paraffins in water and sediment in Germany
are summarized in Tables 11 and 12. In 1987, chlorinated paraffins
were detected at concentrations of about 1 µg/litre for C10-13 and
20 µg/litre for C14-18 in the Danube, downstream of the confluence
with the River Lech (BUA, 1992). The corresponding contents in the
sediment were 300 and 1800 µg/kg dry weight, respectively. In 1994,
chlorinated paraffin concentrations in the Danube and River Lech were
in the range of 0.05 to 0.12 µg/litre for C10-C13 and < 0.05 to
0.19 µg/litre for C14-C17. Also in 1994, the chlorinated paraffin
concentrations (C10-C13) in sediment varied from 6-10 µg/kg dry
weight in the lake of Constance to maximum concentrations of 76 µg/kg
dry weight in the River Lech and 83 µg/kg dry weight in the Rhine
(BUA, 1992).
Table 11. Concentrations (µg/litre) of short and intermediate chain length
chlorinated paraffins in surface water in Germany (From: BUA, 1992)a
Location 1987 1994
C10-13 C14-18 C10-13 C14-17
River Lech at Augsburg 0.05 < 0.05
River Lech at Gersthofen 0.50 4.5 0.08 0.09
(upstream from a chlorinated
paraffin production plant)
River Lech at Langweid 0.60 4.0 0.10 0.19
(downstream from a chlorinated
paraffin production plant)
River Lech at Rain 0.12 0.17
River Danube at Marxheim 1.2 4.0 0.06 < 0.06
(downstream from the mouth of
the River Lech)
River Danube at Marxheim (upstream 1.2 20 0.06 0.07
from the mouth of the River Lech)
a The data from 1994 were produced with a more specific method than those from 1987.
The two data sets are therefore difficult to compare.
Table 12. Concentrations (µg/kg dry weight) of short and intermediate chain length
chlorinated paraffins in sediments from Germany (From: BUA, 1992)a
Location 1987 1994
C10-13 C14-18 C10-13 C14-17
Bodensee (middle)
0-5 cm depth 9-10 70
5-12 cm depth 6-9 < 10
River Rhine (141 km) at Rheinfelden 33-38 60
River Rhine (152 km) at Rheinfelden,
upper layer 53-60 140
lower layer 26-32 85
River Rhine, near German-Dutch
border (2 sites) 60-83 145-205
River Main (3 sites) 24-55 160-260
Outer Alster, Hamburg 35-36 370
River Elbe at Hamburg (2 sites) 16-25 130-230
River Lech, upstream from 400 2200 < 5-7 < 10
chlorinated paraffin production
plant
River Lech, downstream from 700 1700 70-76 325
chlorinated paraffin production
plant
Danube downstream from mouth 300 1800
of the River Lech
a The data from 1994 were produced with a more specific method than those from 1987.
The two data sets are therefore difficult to compare.
Chlorinated paraffins were detected in water samples collected
in the Bermuda Islands (Kraemer & Ballschmiter, 1987). Water samples
down to a depth of 1200 m were analysed, and the highest concentration,
about 50 µg/litre, was found in the surface film. Chlorinated paraffins
were not detected in water from the Maldives (detection limit:
3 ng/litre).
In surface sediment from Lake Zürich, 5 µg/kg of a chlorinated
paraffin mixture with a carbon chain length of C14-18 and 52% chlorine
(CP-MH) was measured (Schmid & Müller, 1985). In the same study it
was reported that sewage sludge from a sewage treatment plant in an
urban industrialized region with known contamination of heavy metals
and organochlorine compounds contained chlorinated paraffins at a
concentration of 30 000 µg/kg.
In a field study performed by the US Environmental Protection
Agency (Murray et al., 1988), samples from two watersheds were
analysed by the method of Schmid & Müller (1985). Both were close to
a chlorinated paraffin manufacturing plant (Sugar Creek, Ohio) or
industry using lubricating oils (Tinkers Creek, Ohio). Chlorinated
paraffins were detected in most samples from Sugar Creek in the low
ppb range (< 8 µg/litre) near drainage and downstream from the plant.
Only a few samples upstream from the plant contained detectable
levels of chlorinated paraffins (< 0.3 µg/litre) (detection limit:
0.15 µg/litre). Of the three chlorinated paraffin fractions studied, the
fraction containing the long carbon chain length C20-30; 40-50% Cl
(CP-LL) was generally present at highest concentration. Sediments
contained higher concentrations. As much as 170 000 µg/kg dry weight
of C20-30 was detected in sediment in an impoundment lagoon, whereas
50 000 µg/kg of C14-17;50-60% Cl (CP-MH) and 40 000 µg/kg C10-12;
50-60% Cl (CP-SH) were measured in the same sample. In mussels
(family Unionidae) collected downstream from the plant, there were
detectable levels of chlorinated paraffins (280 µg/kg C10-12, 170 µg/kg
C14-17, 180 µg/kg C20-30). The report concluded that the observed
levels were most likely due to the manufacturing plant. The samples
collected in Tinkers Creek did not contain detectable amounts of
chlorinated paraffins. Most of the samples contained organic
compounds, largely halogenated aromatics, at levels high enough to
interfere and mask the presence of chlorinated paraffins. Chlorinated
paraffins were detected in samples collected from the process wastes
stream inside the chlorinated paraffin plant. The levels in these
samples were: C10-12, 8.1 µg/kg; C14-17, 1.3 µg/kg; C20-30, 2.2 µg/kg.
In 1979, 51 water samples and 51 bottom sediment samples were
collected at 17 sites in Japan and analysed for the presence of
chlorinated paraffins (C8-32). Chlorinated paraffins were not
detected in any of the water samples, while 24 bottom sediment samples
from 11 sites contained chlorinated paraffins at concentrations of 600
to 10 000 µg/kg (wet or dry weight not specified) (Environment Agency,
Japan, 1981). In 1980, 120 water samples and 120 bottom sediment
samples were collected at 40 sites in Japan and were analysed for the
presence of chlorinated paraffins (C8-32). Chlorinated paraffins
were not detected in any of the water samples but 31 bottom sediment
samples from 13 sites contained chlorinated paraffins at concentrations
of 500 to 8500 µg/kg. However, the analytical methods were not
specified and the detection limits were high (the detection limit was
10 µg/litre for water and 500 µg/kg for bottom sediment) (Environment
Agency, Japan, 1983).
5.1.3 Soil
No data on the occurrence of chlorinated paraffins in soil have
been reported.
5.1.4 Aquatic and terrestrial organisms
The levels of chlorinated paraffins in organisms from various
ecosystems in Sweden, determined using the method of Jansson et al.
(1991), are shown in Table 13. Chlorinated paraffins were found in
all samples in the range of 130-4400 ng/g lipid (6.6-210 µg/kg tissue)
(Jansson et al., 1993). The terrestrial animals rabbit and moose had
higher chlorinated paraffin concentration in their fat than any of the
aquatic animals. The chlorinated paraffin levels in fish-eaters were
approximately the same as in the fish, compared to the dioxin and PCB
levels, which were several times higher in fish-eaters. The levels
in seal and herring indicated no or only low biomagnification of
chlorinated paraffins in their food chains.
In a study by Campbell & McConnell (1980), chlorinated paraffins
were detected in mussels, fish, seals and seabirds, as well as in
seabird eggs, using the analytical method of Hollies et al. (1979)
(Table 14). The levels of C10-20 were higher than those of C20-30 in
most organisms. Mussels collected close to a chlorinated paraffin
plant effluent discharge had levels of C10-20 up to 12 000 µg/kg. The
measured levels in the organisms were close to the levels in the
sediment near the organisms, but 100 to 1000 times higher than those
of water, thus indicating bioaccumulation. The authors also detected
trace amounts of chlorinated paraffins (C10-20) in the tissues of sheep
grazing near a plant producing chlorinated paraffins. The tissues
containing the highest levels were liver (200 µg/kg), fat and kidney
(50 µg/kg). The fleeces of the sheep contained higher levels
(350 µg/kg), and the authors suggest that this might have been due to
aerial transport. No chlorinated paraffin could be detected in sheep
grazing far from plants producing chlorinated paraffins (detection
limit = 50 µg/kg).
Table 13. The levels of chlorinated paraffins in various terrestrial and aquatic
organisms in Swedena
Organism Samplesc Tissue Extracted CP (µg/kg CP (µg/kg
lipid (%) lipid) tissue)d
Rabbit 15 muscle 1.1 2900 31.9
Moose 13 muscle 2.0 4400 88
Reindeer 31 fat 56 140 78
Whitefish 35 muscle 0.66 1000 6.6
Arctic char 15 muscle 5.3 570 30.2
Herring (Bothnian Sea) 100 muscle 5.4 1400 75.6
Herring (Baltic Proper) 60 muscle 4.4 1500 66
Herring (Skagerrack) 100 muscle 3.2 1600 51.2
Ringed sealb 7 blubber 88 130 114.4
Grey seal 8 blubber 74 280 207
Osprey 35 muscle 4.0 530 21.2
a Data from Jansson et al. (1993)
b The ringed seal was from Kongsfjorden, Svalbard, Sweden
c Pooled samples
d Calculated from the reported data
In a study by Campbell & McConnell (1980), chlorinated paraffins
were detected in mussels, fish, seals and seabirds, as well as in
seabird eggs, using the analytical method of Hollies et al. (1979)
(Table 14). The levels of C10-20 were higher than those of C20-30 in
most organisms. Mussels collected close to a chlorinated paraffin
plant effluent discharge had levels of C10-20 up to 12 000 µg/kg. The
measured levels in the organisms were close to the levels in the
sediment near the organisms, but 100 to 1000 times higher than those
of water, thus indicating bioaccumulation. The authors also detected
trace amounts of chlorinated paraffins (C10-20) in the tissues of sheep
grazing near a plant producing chlorinated paraffins. The tissues
containing the highest levels were liver (200 µg/kg), fat and kidney
(50 µg/kg). The fleeces of the sheep contained higher levels
(350 µg/kg), and the authors suggest that this might have been due to
aerial transport. No chlorinated paraffin could be detected in sheep
grazing far from plants producing chlorinated paraffins (detection
limit = 50 µg/kg).
Table 14. Chlorinated paraffins in organisms in the United Kingdom
(Campbell & McConnell, 1980)
Species Tissuea No. of Carbon chain-length and
specimens concentration in tissues
analysedb (means and ranges)c
C10-20 (µg/kg) C20-30 (µg/kg)
mean range mean range
Aquatic organisms
Plaice NS 6 30 ND-200 30 ND-200
(Pleuronectes platessa)
Pouting NS 4 100 ND-200 ND ND
(Trisopterus luscus)
Mussel NS 9 3250 100-12 000 10 NDe-100
(Mytilus edulis)
Pike NS 2 25 ND-50 25 ND-50
(Esox lucius)
Grey seal Liver 4 75 40-100 ND ND
(Halichoerus grypus) and
blubber
Table 14. Cont'd
Species Tissuea No. of Carbon chain-length and
specimens concentration in tissues
analysedb (means and ranges)c
C10-20 (µg/kg) C20-30 (µg/kg)
mean range mean range
Birds
Heron Liver NR NR 500-1200 NR NDe
(Ardea cinerea) Liver NR NR 100-1000 NR 100-1500
Guillemot Liver NR NR 100-1100 NR NDe
(Uria aalge)
Herring gull Liver NR NR 200-900 NR 100-500
(Larus argentatus)
Seabird eggsd 23 220 ND-2000 20 ND-100
a NS = Not specified
b NR = Not reported
c ND = Not detected (detection limit = 50 µg/kg, except where stated otherwise)
d 9 species
e Detection limit = 100 µg/kg
In 1980, 108 fish samples were collected at 28 places in Japan
and were analysed for the presence of chlorinated paraffins.
Chlorinated paraffins were not detected in any of the samples. The
detection limit was 500 µg/kg (Environment Agency, Japan, 1983).
5.1.5 Food and beverages
Chlorinated paraffins, mostly C10-20, were detected in various
food products in a limited study (Table 15) using the analytical
method of Hollies et al. (1979). C20-30 chlorinated paraffins were
detected only in a few samples. C10-20 chlorinated paraffins were
found at levels up to 500 µg/kg in approximately 70% of the samples
(Campbell & McConnell, 1980).
Chlorinated paraffin residues have been detected in fish and
sheep (see section 5.1.4).
Table 15. Chlorinated paraffins (C10-20) in human foodstuffs
(Campbell & McConnell, 1980)
Foodstuff class No. of foodstuff Concentration
samples tested (µg/kg)
Dairy products 13 300
Vegetable oils and derivatives 6 150
Fruit and vegetables 16 25
Beverages 6 NDa
a Not detected (detection limit = 50 µg/kg)
5.2 General population exposure
Chlorinated paraffins have been detected on human hands (Campbell
& McConnell, 1980). The hands of eight human volunteers were swabbed
with toluene, and all had chlorinated paraffin levels of 0.8-4.0 µg
C10-20 (per two hands). C20-30 chlorinated paraffins (2 µg)
were detected only on one person. The authors suggested that the
chlorinated paraffin content in foodstuffs, as well as direct transfer
from manufactured articles via the hands, could contribute to human
exposure.
Owing to the high octanol-water partition coefficient, it is
likely that the principle source of exposure of the general population
to chlorinated paraffins is food. However, due to lack of data,
exposure to chlorinated paraffins via other routes cannot be ruled
out.
5.2.1 Concentrations in human tissues
Chlorinated paraffins have been detected in postmortem tissue
samples. Campbell & McConnell (1980) measured the amount in 24 tissue
samples and found up to 600 µg/kg of C10-20 in adipose tissue (median
level: 100-190 µg/kg), up to 500 µg/kg in kidney (median level below
90 µg/kg) and up to 1500 µg/kg in liver (median level below 90 µg/kg).
C20-30 chlorinated paraffins were detected only in a few samples and
at a low level. Schmid & Müller (1985) also detected chlorinated
paraffins in adipose tissue at a concentration of 200 µg/kg.
5.3 Occupational exposure
Occupational exposure to chlorinated paraffins is likely among
workers in production plants or in industries using chlorinated
paraffins. The US National Occupational Exposure Survey (1981-1983)
indicated that 573 000 workers, including 38 000 women, were
potentially exposed to chlorinated paraffins (NIOSH, 1990). In
Denmark, it was reported that 60 000 workers in 175 companies have
been potentially exposed to chlorinated paraffins since 1964 (the
amount produced and imported annually is 5000 tonnes) (Hansen et al.,
1992).
Campbell & McConnell (1980) detected chlorinated paraffins on
human hands using the method of Hollies et al. (1979) (see section
2.3.2). When the hands of a worker in a chlorinated paraffin
laboratory were swabbed with toluene, 800 µg of C10-20 and 400 µg
of C20-30 were detected.
Historical exposure data from machine shops, reported as
reflecting worst-case situations, indicated exposures to chlorinated
paraffins of 0.003-1.15 mg/m3 for operation such as milling, cutting
and grinding (HSE, 1992). Other exposure data suggested exposures to
chlorinated paraffins ranging from 0.003 to 0.21 mg/m3. However,
it is not clear if these levels of exposure were intermittent or
time-weighted averages. There is no information on whether or not
chlorinated paraffin aerosols are in the inhalable size range.
Occupational exposure to short chain chlorinated paraffins has
been estimated by the United Kingdom Health and Safety Executive by
mathematical modelling using the "Estimation and Assessment of
Substance Exposure" (EASE), developed as part of the guidance on new
and existing substances by the Commission of the European Union (CEU)
(HSE, 1992). This model takes into account physico-chemical data such
as vapour pressure and process details such as temperatures and the
use of local exhaust ventilation. It does not take into account the
attenuating effect of decontamination of equipment or use of personal
protective equipment.
During the production of short chain chlorinated paraffins in
closed systems, exposure is likely to be intermittent, and the model
predicts that inhalation exposure to a substrate with a vapour
pressure of less than 0.001 kPa is negligible (0 to 0.1 ppm).
Assuming a non-dispersive pattern of use and intermittent skin
contact, the model predicts that exposures of the hand and forearm
will be in the range of 0.1-1 mg/cm2 per day. During formulation of
short chain chlorinated paraffins (in closed systems) the model
predicts negligible (0-0.1 ppm) inhalation exposure with formulation
process temperatures of 40-50°C. Skin exposure to the hands and
forearms during formulation is predicted to be 0.1-1 mg/cm2 per day.
Occupational exposure to short chain chlorinated paraffins during
their use as metal-working fluids is extensive and the model predicts
exposure of 0.1-1.5 mg/cm2 per day, assuming a chlorinated paraffin
content in the fluid of 2-10%, or 5-15 mg/cm2 per day for speciality
fluids which may contain more than 80% chlorinated paraffin.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
There is a lack of systematic investigation of the influence of
carbon chain length and degree of chlorination in studies on the
kinetics of chlorinated paraffins. Studies have almost exclusively
concerned short and intermediate chain length chlorinated paraffins.
6.1 Absorption
6.1.1 Oral exposure
Chlorinated paraffins are absorbed after oral administration.
From the data presented in section 6.5 it appears that short chain
length compounds are more readily absorbed than longer chain length
compounds. Absorption decreases with increasing carbon chain length
and degree of chlorination.
6.1.2 Dermal exposure
Chlorinated paraffins are slowly absorbed by the dermal route
in Sprague-Dawley rats (Yang et al., 1987). Two 14C-labelled
chlorinated paraffins, C18;50-53% Cl (CP-LH) and C28;47% Cl (CP-LL),
were applied to rat skin (5-7 animals of each sex) at a concentration
of 66 mg/cm2, approximately equivalent to 2000 mg/kg body weight.
Only 0.7% (males) and 0.6% (females) of the C18 dose was absorbed
after 96 h. Only 0.02% of the C28 dose was absorbed in males whereas
in females the level was not detectable. This indicates that
increasing chain length leads to decreased permeability. Of the
absorbed C18 dose, 40% was exhaled as 14C-labelled CO2, and 20% was
excreted in urine and 20% in faeces.
The absorption of two chlorinated paraffins through human skin
has been studied in vitro (Scott, 1989). There was no absorption of
Cereclor S52 (C14-19;52% Cl, CP-MH) following a 54-h application to
the surface of the epidermal membranes using five different receptor
media. Similarly, using Cereclor 56L (C10-13; 56% Cl, CP-SH; 18.5% w/w
solution in a typical cutting oil) no absorption was detected for 7 h,
but after 23 h a slow but steady rate of absorption was detected
(e.g., 0.05 ± 0.01 µg/cm2 per h ± SEM; n = 6; receptor medium PEG-20
oleyl ether in saline), which was maintained for the duration of the
experiment (56 h). Owing to the anticipated low rate of absorption,
the chlorinated paraffin samples were spiked with [14C] n-pentadecane
and [14C] n-undecane for Cereclor S52 and 56L, respectively, in
order to facilitate detection of the absorbed material. Measurement of
the 14C-alkanes was taken as a surrogate for the chlorinated
paraffins, on the assumption that their rates of absorption were
similar.
6.1.3 Inhalation exposure
No data on retention of chlorinated paraffins by inhalation have
been reported.
6.2 Distribution
6.2.1 Short chain length chlorinated paraffins
6.2.1.1 Mouse
Female C57Bl mice were administered 12.5 MBq/kg body weight
(340 µCi) (for autoradiography) or 1.25 MBq/kg body weight (34 µCi)
(for determination of radioactivity) of 14C-labelled chlorododecanes
(C12) with different chlorine contents (17.5% [CP-SL], 55.9% [CP-SH]
and 68.5% [CP-SH]) either by gavage or intravenous injection (Darnerud
et al., 1982). Uptake of radioactivity was found by autoradiography to
be highest in tissues with high cell turnover/high metabolic activity,
e.g., intestinal mucosa, bone marrow, salivary glands, thymus and
liver. The highest radioactivity was achieved with the chlorinated
paraffin that had the lowest chlorine content. It was found that the
long period of retention of heptane-soluble radioactivity, which
indicated unmetabolized substance, in liver and fat after oral dosing
increased with degree of chlorination. In this study it was also
found that 30 to 60 days after injection of C12;17.5% Cl and
C12;55.9% Cl a considerable retention of radioactivity was seen in
the central nervous system. Exposure of late gestation mice showed a
transplacental passage of radioactivity, and 14C-labelling was
primarily noted in the liver, brown fat and intestine of the fetuses.
6.2.1.2 Rat
Radioactivity was found in the liver, kidneys, adipose tissue and
ovaries of Fischer-344 rats following administration of an unspecified
single dose by gavage of 14C-labelled chlorinated paraffin (C10-13;58%
chlorination, CP-SH) at the end of a 90-day dosing by gavage with the
same chlorinated paraffin (IRDC, 1984c).
6.2.2 Intermediate chain length chlorinated paraffins
6.2.2.1 Rat
Radioactivity was found initially in the liver and kidneys and
later in adipose tissue and the ovaries of Fischer-344 rats following
a single dose by gavage of 14C-labelled C14-17;52% Cl (CP-MH) at the
end of a 90-day dosing in the diet with the same chlorinated paraffin
(IRDC, 1984b).
In male Wistar rats fed with a diet containing 0.4 or 40 mg/kg
[36Cl]Cereclor S52 (C14-17;52% Cl, CP-MH) for 8 (40 mg/kg) or 10 weeks
(0.4 mg/kg), equilibrium levels of radioactivity were established in
liver and abdominal fat within 1 and 7 weeks, respectively (Birtley et
al., 1980). The equilibrium concentrations were 7000 µg/kg in liver
and 30 000-40 000 µg/kg in fat after high exposure. No radioactivity
was detected in the brain or adrenal glands.
6.2.2.2 Mouse
The distribution of orally administered 14C-labelled
polychlorohexadecane (C16;69% Cl, CP-MH) in female C57Bl mice was
examined by whole body autoradiography (Biessmann et al., 1983).
A high level of radioactivity was observed in the liver, brown
fat, intestine, gall bladder, adrenal cortex and kidney of mice
administered approximately 15 µmol/kg. A high uptake of radioactivity
was also observed in corpora lutea on days 1-4, and was still present
30 days after administration. A high level of radioactivity was also
observed in the adrenal cortex at shorter post-injection times, and in
brown and white fat and in the liver, which were still labelled after
30 days.
14C-Labelled [1-14C]polychlorohexadecane (C16;34.1% Cl, CP-ML)
was given to C57Bl mice either by gavage (females) or intravenously
(both sexes) at a radioactivity level of 370 kBq/animal (10 µCi)
(corresponding to 0.44 µmol of the chlorinated paraffin) (Darnerud &
Brandt, 1982). No difference in the distribution patterns was found
between the oral and intravenous administration routes. After
analysis by autoradiography a high level of radioactivity was found in
tissues with a high cell turnover rate and/or high metabolic activity,
and lower levels could be seen in the white fat depots. High levels
of radioactivity were observed in the liver, kidneys, spleen, bone
marrow, brown fat, intestinal mucosa, pancreas, salivary gland and the
Harderian gland 24 h after intravenous injection. After 12 days high
levels of radioactivity were seen in the adrenal cortex, abdominal
fat and in the bile. Later after injection (30 days), prominent
radiolabelling of the brain was found which was as high as in the
liver. The chlorinated paraffin was also administered intravenously
to pregnant mice, and uptake of radioactivity in the fetuses was
observed. When the mice were administered on day 10 of pregnancy no
tissue-specific localization was found, but after administration in
late pregnancy (day 17) the distribution pattern after 6 h was similar
to that of adult mice when examined 24 h after administration.
The distribution of radioactivity in the brain and liver has been
studied after gavage administration of 14C-labelled polychloro-
hexadecane (C16;69% Cl, CP-MH) (1.48 MBq/kg body weight or 40 µCi,
corresponding to 1.1 mg/kg body weight) to pre-weaning NMRI mice
(Eriksson & Darnerud, 1985). The chlorinated paraffin was
administered by gavage at the age of 3, 10 and 20 days, and the
animals were killed 24 h and 7 days later. The radioactivity in the
brain declined more rapidly in the 3-day-old mice compared to the
10- and 20-day-old mice. In the 10-day-old mice approximately 0.02% of
the total administered dose was detected in the brain 24 h after
administration. Approximately 80% of the radioactivity in the brain
was still present after 7 days. In the liver the radioactivity
disappeared more rapidly both in younger and older animals. The
radioactivity in the brain was found primarily in the white matter
of the cerebellum, in the space between the neocortex and the
mesencephalon and thalamus, the corpus callosum, the pons and the
outer part of medulla spinalis. The radioactivity was higher in the
parts of the brain which also stained for myelin, and the levels at
7 days were almost the same as those at 24 h. After whole body
autoradiography, high levels of radioactivity were found in the liver,
intestinal contents, adipose tissue and adrenals. A differential
labelling of the liver was observed in the 3-day-old mice, where only
certain parts of the liver lobules were labelled.
6.2.2.3 Bird
The distribution of 14C-labelled polychlorohexadecane (C16;69%
Cl, CP-MH) in female Japanese quail (Coturnix coturnix japonica) was
examined by whole body autoradiography (Biessmann et al., 1983). Four
hours after a single dose by gavage of approximately 4.8 µmol/kg to
quail, high levels of radioactivity were observed in the liver,
intestine, gall bladder, egg yolk, kidney, ovary, blood, hypophysis
and retina. Twelve days after administration, radioactivity was
observed only in the uropygial gland, white fat, liver and egg yolk.
A study of the distribution after oral administration by gavage
of 0.74 MBq (20 µCi) (approximately 20 Ci/mol) of either 14C-labelled
polychlorohexadecane (C16;34% Cl, CP-ML) or (1-14C)-labelled
polychlorododecane (C12;56% Cl, CP-SH) in female Japanese quail
(Coturnix coturnix japonica) was performed by Biessmann et al.
(1982). The distribution patterns for the two chlorinated paraffins
were similar, and high levels of radioactivity were initially (up to
1 day) found in the liver, intestinal mucosa, spleen, bone marrow,
oviduct, gall bladder and kidney. After 4 and 12 days, high
radiolabelling was observed in fat, the yolk of the follicles and
the contents of the uropygial glands.
The uptake of Cereclor S52 (C14-17;52% Cl, CP-MH) in mallard ducks
(Anas platyrynchos) or ring-necked pheasants (Phasianus colchicus)
was studied by Madeley & Birtley (1980). After a single oral dose of
Cereclor S52 (10 g/kg) in duck, the highest levels of chlorinated
paraffins were detected in fat (67 mg/kg wet weight), gut (15 mg/kg)
and heart (7 mg/kg). The pheasants were exposed to 1000 mg/kg in the
diet for 5 days. Only low levels were found in the heart (3.1 mg/kg)
and gut (1.4 mg/kg). No visible fat was available in the pheasants
due to immaturity. The levels in other organs in both species were
low. The method of analysis was thin layer chromatography (Hollies
et al., 1979).
6.2.2.4 Fish
The distribution of polychloro-[1-14C]hexadecane (C16;34% Cl,
CP-ML) has been studied in carp (Cyprinus carpio) and bleak
(Alburnus alburnus) (Darnerud et al., 1983). After a single
intra-arterial injection of 60-80 µg in carp, about 6% of the dose was
excreted as 14CO2 in 96 h. Radioactivity was observed, in the
intra-arterially injected carps or in bleak exposed to contaminated
water (125 µg/litre) for 14 days, in the bile, intestine, kidney,
liver, gills and, particularly in bleak, in the nasal cavity, lens and
skin.
6.2.3 Long chain length chlorinated paraffins
6.2.3.1 Rat
After oral administration of 14C-labelled C22-26;70% Cl (CP-LH)
to Fischer-344 rats at the end of a 90-day exposure period, a small
part of the dose was absorbed (Serrone et al., 1987). The highest
level of radioactivity was found in the liver. Retention of
radioactivity in adipose tissue, which was eliminated slowly, was also
observed. In an identical study, C20-30;43% Cl (CP-LL) gave the
highest levels in the liver and ovary (Serrone et al., 1987).
6.2.3.2 Fish
Rainbow trout (Oncorhynchus mykiss) were fed diets containing 47 or
385 mg/kg (dry weight) of Cereclor 42 (C20-30;Cl 42%, CP-LL) containing
a 14C-labelled pentacosane (C25) with 42% Cl for 35 days and
a control diet for the following 49 days (Madeley & Birtley, 1980).
The chlorinated paraffin accumulated in the fish during the exposure
period, mostly in the liver and gut. The radioactivity decreased
during the elimination period, more rapidly in the gut and liver
than in the flesh, but was still detectable at the end of the
experiment. When the chlorinated paraffin was determined by thin-layer
chromatography a lower level was noted as compared to determination
of 14C-labelled molecules, suggesting metabolism in the fish. Up to
70% of the assimilated 14C in tissues was not associated with
chlorinated paraffin after feeding for 13 days.
6.2.3.3 Mussel
Mussels (Mytilus edulis) were fed suspended yeast cells dosed
with 524 mg/kg (dry weight) Cereclor 42 (C20-30;42% Cl, CP-LL)
containing a 14C-labelled pentacosane (C25) with 42% chlorination for
47 days, and were then fed with untreated yeast for a further 56 days
(Madeley & Birtley, 1980). The uptake reached a plateau level after
26 days of exposure, and the tissue concentration was always below
11 mg/kg. The highest level of chlorinated paraffin was detected in
the digestive glands. The chlorinated paraffin was eliminated rapidly,
and less than 10% remained at the end of the experiment. There was no
evidence of metabolism since the expelled radioactivity was in the
form of the parent compound.
6.2.4 Comparative studies
The uptake of three 14C-labelled chlorinated paraffins, C16;23%
Cl (CP-ML), C16;51% Cl (CP-MH) and C12;68% Cl (CP-SH), was studied in
rainbow trout (Oncorhynchus mykiss) by Darnerud et al. (1989). After
exposure to 3.64 µmol (C16) or 1.74 µmol (C12) in water for 7
days and to uncontaminated water for up to 21 days, radiolabelling was
observed in the bile, eye lens, brain and fat for C16;23% Cl, in the
bile, intestine, fat and liver for C16;51% Cl, and in fat, intestine,
liver and bile for C12;68% Cl. The three different chlorinated
paraffins showed an initially high uptake in the olfactory organs and
gills. The retention of radioactivity in the olfactory organs and
gills was relatively higher for C16;23% Cl than for the more highly
chlorinated paraffins. The long-time retention of radioactivity in
fat-rich tissues increased with the degree of chlorination of the
chlorinated paraffin preparation.
The short-term uptake and elimination of chlorinated paraffins in
bleak (Alburnus alburnus) were studied by Bengtsson et al. (1979).
Groups of bleak (15 in each) were exposed for 14 days to five
different Witaclor mixtures (Table 16), which were added to natural
Baltic Sea water (salinity 0.7%) giving a concentration of 125
µg/litre of water. Five fish were analysed to determine the chlorine
content (Lunde & Steinnes, 1975) at the end of exposure, and the rest
were analysed 1 and 7 days after treatment. It was found that the
uptake was more effective for those Witaclor mixtures with short
carbon chain length and low degree of chlorination, i.e. Witaclor 149
and 159. The elimination rates were slow, and 75 to 90% was detected
7 days after exposure.
In an extended study, bleak were exposed to Witaclor 149
(C10-13;49% Cl, CP-SL), Witaclor 171P (C10-13;71% Cl, CP-SH) and
Witaclor 549 (C18-26;49% Cl, CP-LL) via contaminated food for 91 days.
This was followed by an elimination period of 316 days (Bengtsson &
Baumann Ofstad, 1982). The concentrations of chlorinated paraffins in
the food were 590, 2500 and 5800 mg/kg of C10-13;49% Cl, 3180 mg/kg of
C10-13;71% Cl and 3400 mg/kg of C18-26;49% Cl. Three fish from
each exposure group were analysed at different time-points during
accumulation or elimination periods. The most effective uptake was
observed for C10-13;49% Cl, whereas the slowest uptake was detected for
C18-26;49% Cl. Efficiencies of uptake were 12% for C10-13;49% Cl, 6%
for C10-13;71% Cl and 2% for C18-26;49% Cl. The highest retention was
observed for C10-13;71% Cl, which remained in the tissue at a
steady-state level during the whole elimination period. The uptake of
C18-26;49% Cl was inefficient, and during the elimination period 50%
was lost within 4 to 5 weeks. The remaining amount appeared to be
constant for the rest of the experiment. The rate of elimination was
slowest for the Witaclor mixture with the shortest carbon chain length
and highest degree of chlorination. After 625 days of depuration the
fish still had detectable levels of C10-13;71% Cl, as determined by the
analytical method of Gjos & Gustavsen (1982) (Renberg et al., 1986).
Table 16. Structures of the chlorinated paraffins used in a study on
bleak (Alburnus alburnus) (From: Bengtsson et al., 1979)
Tested Carbon Chlorine Acronyma Accumulation Half-life
formulation chain content coefficientb (days)b
length (%)
Witaclor 149 C10-C13 49 CP-SL 770 13
Witaclor 159 C10-C13 59 CP-SH 740 34
Witaclor 171P C10-C13 71 CP-SH 140 7
Witaclor 350 C14-C17 50 CP-MH 40 30
Witaclor 549 C18-C26 49 CP-LL 10 7
a The classification is given in Table 1
b Calculated by Zitko (1980)
6.3 Metabolic transformation
6.3.1 Short chain length chlorinated paraffins
Darnerud (1984) demonstrated that inducers and inhibitors of
cytochrome P-450 (CYP) affect the rate of degradation of 14C-labelled
polychlorinated dodecanes (C12) containing 68.5% (CP-SH), 55.9%
(CP-SH) and 17.4% Cl (CP-SL) to 14CO2 in exposed C57Bl mice.
Pretreatment with the inhibitor piperonyl butoxide decreased the
amount of 14CO2 formed, and the decrease was more pronounced with
increasing degree of chlorination. The inhibitor metyrapone decreased
the exhalation of 14CO2 but was only investigated in mice exposed
to C12;68.5% Cl. The cytochrome P-450 (CYP2B1; CYP2B2) inducer,
phenobarbital, moderately increased the rate of 14CO2 formation from
chlorinated paraffin with 68% Cl, whereas the P-448 (CYP1A1; CYP1A2)
inducer, 3-methylcholanthrene, did not affect the degradation rate,
indicating a cytochrome P-450-dependent metabolism of chlorinated
dodecanes yielding 14CO2.
6.3.2 Intermediate chain length chlorinated paraffins
Female Sprague-Dawley rats in groups of four were exposed
intravenously to 5-6 mg/kg body weight of 14C-labelled poly-
chlorinated hexadecane (C16;65% Cl, CP-MH) (Åhlman et al., 1986).
Less than 3% of the radioactivity in the bile was due to unchanged
parent compound. The metabolites in the bile appeared to be conjugates
of N-acetylcysteine (mercapturic acid) and glutathione.
6.4 Elimination and excretion
6.4.1 Short chain length chlorinated paraffins
The exhalation of 14CO2 was compared after single gavage or
intravenous administration to female C57Bl mice of 1.25 MBq/kg body
weight (34 µCi) of three chlorododecanes (C12) with different
chlorine contents (17.5% [CP-SL], 55.9% [CP-SH] and 68.5% [CP-SH])
(Darnerud et al., 1982). Of the administered radioactive dose 52% of
C12;17.5% Cl, 32% of C12;56% Cl and 8% of C12;68% Cl were exhaled as
14CO2 within 12 h after dosing by either route. The major excretion
route for C12;56% Cl was by urine (intravenous: 21%; oral: 29%) and
for C12;68% Cl was by faeces (intravenous: 8.6%; oral: 21%). The
total elimination decreased as the chlorine content increased.
6.4.2 Intermediate chain length chlorinated paraffins
6.4.2.1 Rat
The half-time for removal of radioactivity from abdominal fat was
estimated during and after dietary administration for 8 or 10 weeks of
0.4 and 40 mg/kg feed of [36Cl]Cereclor S52 (C14-17;52% Cl, CP-MH) in
male Wistar rats (Birtley et al., 1980). Equilibrium in liver and
abdominal fat was reached at 1 and 7 weeks, respectively. The
half-time for removal was about 8 weeks for abdominal fat, and it was
observed that the level of radioactivity in the liver declined below
the detection limit within one week.
Female Sprague-Dawley rats in groups of four were exposed
intravenously to 5-6 mg/kg body weight of 14C-labelled polychlorinated
hexadecane (C16;65% Cl, CP-MH) (Åhlman et al., 1986). Approximately
10% of the administered dose was excreted in the bile after 24 h,
whereas excretion in the urine and faeces was less than 0.5% after
48 h.
6.4.2.2 Mouse
The elimination of radioactivity was studied in female C57Bl mice
after gavage or intravenous administration of a uniformly 14C-labelled
polychlorohexadecane (C16;69% Cl, CP-MH) (1.6 µmol/kg) (Biessmann
et al., 1983). After 8 h, only about 1% of the dose was exhaled
as 14CO2 for both administration routes. Most of the
radioactivity was excreted in faeces when administrated by gavage, and
after 8 h 22% was recovered in faeces and 1.2% in urine. After 96 h,
66% was recovered in faeces and 2.9% in urine. After intravenous
administration, 2.1% was excreted in faeces and 1% in urine after 8 h,
and 43% was excreted in faeces and 3% in urine after 96 h.
The excretion of [1-14C]polychlorohexadecane (C16;34.1% Cl,
CP-ML) (59 kBq/animal, or 1.5 µCi) after intravenous or gavage
administration to C57Bl mice was studied by Darnerud & Brandt (1982).
Twelve hours after intravenous injection, 12% of the radioactive dose
was recovered in urine, 44% in expired air (as 14CO2) and 4%
in faeces. Twelve hours after gavage administration, 6% of the
radioactive dose was recovered in urine, 33% in the expired air and
14% in faeces.
6.4.2.3 Bird
The elimination of radioactivity was studied in female Japanese
quail (Coturnix coturnix japonica) after gavage or intravenous
administration of a uniformly 14C-labelled polychlorohexadecane
(C16;69% Cl, CP-MH) (0.48 µmol/kg) (Biessmann et al., 1983). Of the
administered dose, 1.6% was exhaled as 14CO2 after gavage
administration and 0.9% after intravenous administration. Of the
administered dose 16% was excreted in faeces/urine after 8 h and 58%
after 96 h.
6.4.3 Long chain length chlorinated paraffins
A 14C-labelled chlorinated paraffin, C18; 50-53% Cl (CP-LH), was
administered by gavage as a single dose of 500 mg/kg to three female
Sprague-Dawley rats (Yang et al., 1987). After 24 h, 1% of the
radioactive dose was recovered in the urine, 1.5% in the expired air
and 22% in the faeces. After 96 h, 1.9% of the radioactive dose was
recovered in the urine, 3.3% in the expired air, 5% in body tissue and
76% in the faeces.
6.4.4 Comparative studies
In a study by Beissmann et al. (1982), 148 kBq (4 µCi)
(approximately 20 Ci/mol) of either 14C-labelled polychlorohexadecane
(C16;34% Cl, CP-ML) or 14C-labelled polychlorododecane (C12;56% Cl,
CP-SH) was administered by gavage to Japanese quail (Coturnix
coturnix japonica). A considerably higher level of radioactivity
was found in the gall bladder and kidney after C12 administration
compared with C16. On the other hand, the radioactivity in the yolk
of the first ten eggs was higher after C16 gavage than after C12
gavage. After 8 h, 39% of the C16 dose and 22% of the C12 dose was
exhaled as 14CO2, indicating that the rate of metabolism is
influenced by the chain length and/or the degree of chlorination.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute exposure
7.1.1 Lethal doses
The acute oral toxicity of chlorinated paraffins has been studied
in rats and mice (Table 17). In all studies, the LD50 was reported
to be greater than the highest administered dose (i.e. always
> 4 g/kg body weight). After inhalation of Chlorowax 500C (C12;59% Cl,
CP-SH), an LC50 was not established in the one reported study (LC50
> 3300 mg/m3). The LD50 for dermal exposure of rabbits to
Chlorowax 500C (C12;59% Cl, CP-SH) was in excess of approximately
13 g/kg body weight (Howard et al., 1975).
Table 17. Acute toxicity of chlorinated paraffins
Chlorinated Compounda Species Test Exposure Reference
paraffin concentration
C12;60% Cl (CP-SH) rat oral LD50 > 13.6 g/kg Bucher et
al. (1987)
C12;59% Cl Chlorowax rat oral LD50 > 21.5 g/kg Howard et
500C (CP-SH) al. (1975)
C12;59% Cl Chlorowax rabbit dermal > 13 g/kg Howard et
500C (CP-SH) LD50 al. (1974)
C12;59% Cl Chlorowax rat inhalation > 3300 Howard et
500C (CP-SH) LC50 mg/m3 al. (1975)
C12;60% Cl (CP-SH) mouse oral LD50 > 27.2 g/kg Bucher et
al. (1987)
C10-13;41-70% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C14-17;51-60% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C24;40% Cl Chlorowax 40 rat oral LD50 > 17.7 g/kg Howard et
(CP-LL) al. (1975)
C23;43% Cl (CP-LL) rat oral LD50 > 13.6 g/kg Bucher et
al. (1987)
Table 17. (Cont'd)
Chlorinated Compounda Species Test Exposure Reference
paraffin concentration
C23;43% Cl (CP-LL) mouse oral LD50 > 27.2 g/kg Bucher et
al. (1987)
C20-30;41-70% Cl rat oral LD50 > 4 g/kg Birtley et
al. (1980)
C24;70% Cl Chlorowax 70 rat oral LD50 > 50 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorez 700 rat oral LD50 > 50 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorowax 70 guinea-pig oral LD50 > 25 g/kg Howard et
(CP-LH) al. (1975)
C24;70% Cl Chlorez 700 guinea-pig oral LD50 > 25 g/kg Howard et
(CP-LH) al. (1975)
a The classification is given in Table 1
7.1.2 Non-lethal doses
7.1.2.1 Oral route
In a single-administration experiment, F-344/N rats and B6C3F1
mice were dosed by gavage up to 13.6 g/kg body weight (rats) and
27.2 g/kg body weight (mice) of C12;60% Cl (CP-SH) or C23;43% Cl
(CP-LL) dissolved in corn oil. No deaths or compound-related toxic
effects were noted during the 14-day observation period. However, the
animals were inactive with diarrhoea for 2-6 days after dosing, which
was attributed to the large volumes of material administered (NTP,
1986a,b; Bucher et al., 1987).
Female or male Wistar rats were administered by gavage a single
oral dose of different chlorinated paraffins (C10-13; 41-51%, 51-61% or
61-70% Cl), with a range of maximum doses of 4-13 g/kg body weight,
and were observed for 7 days (ICI, 1965, 1966, 1968, 1969, 1971, 1973,
1974a,b; Birtley et al., 1980). Clinical signs of toxicity, such as
piloerection, muscular incoordination and faecal and urinary
incontinence, were observed in rats that received doses of 2 g/kg body
weight or more (generally independent of the chlorine content).
Recovery was usually complete by day 7. There were no deaths except
for one rat treated with 13 g/kg body weight of C10-13;63% Cl.
Similar findings were reported for C14-17;51-60% Cl and
C20-30;41-51%, 51-61% or 61-70% Cl (Birtley et al., 1980). The
toxicity of these chlorinated paraffins was reported to be lower than
that of the short chain length chlorinated paraffins.
7.1.2.2 Inhalation route
No toxic response was observed in rats exposed to a concentration of
3300 mg/m3 of Chlorowax 500C (C12;59% Cl, CP-SH) for one hour (Howard
et al., 1975)
7.1.2.3 Intraperitoneal route
Three different chlorinated paraffin preparations, Chlorez 700
(C20;70% Cl, CP-LH), Paroil 170-HV (C11;70% Cl, CP-SH) and
Chloroparaffin 40 (chain-length not given, 40% Cl), were administered
intraperitoneally as single doses (Chlorez and Chloroparaffin
100 mg/kg body weight; Paroil 52 mg/kg body weight) to male Wistar rats
(Ahotupa et al., 1982). The activities of various drug-metabolizing
enzymes from liver, kidney and small intestinal mucosa were determined
after 24 h, 7 days or 21 days. Minor but significant changes in the
intestinal activities of aryl hydrocarbon hydroxylase (increase),
UDP-glucuronosyltransferase (decrease) and epoxide hydrolase
(increase) were induced by Paroil 170-HV, and in the kidney activity
of aryl hydrocarbon hydroxylase by Chlorez 700, when compared to
polychlorinated biphenyls and naphthalenes.
7.1.3 Skin and eye irritation
7.1.3.1 Short chain length chlorinated paraffins
In a study by Hoechst (1986b), 0.5 ml of undiluted C10-13;50%
Cl(CP-SH) was applied under a semi-occlusive dressing to the shaven
skin of three rabbits for 4 h. The skin was examined for signs of
irritation for up to 72 h after the chlorinated paraffin had been
removed, but none were seen during the test.
When 0.5 ml of C10-13;70% Cl(CP-SH) was applied under a
semi-occlusive dressing to the shaven skin of three rabbits for 4 h,
one rabbit showed clearly defined erythema (grade 2 on a 0-4 scale) at
48 and 72 h. The other two animals showed "slightly noticeable"
erythema (grade 1). Very slight oedema (grade 1) was noted in two
animals for up to 24 h. By day 7, all signs of irritation were
completely resolved (Hoechst, 1983).
Two studies investigated C10-13;70% Cl(CP-SH). In one study the
chlorinated paraffin contained 1 or 2% of an epoxidised vegetable oil
stabilizer with and without additives (0.1% oxalic acid or 0.05%
benzotriazole) (ICI, 1965). Very mild to mild desquamation was only
noted following the applications of chlorinated paraffins containing
additives. The reactions were described as occasional, transient and
inconsistent. It was not stated how many applications were made
before these reactions were seen. In another study, no signs of
irritation were noted following repeated application of a chlorinated
paraffin containing 0.1 or 2% benzoyl peroxide initiator (ICI, 1974a).
Two studies investigated the effects of three C10-13;63%
chlorinated paraffins (CP-SH), containing up to 3% epoxy soya oil
stabilizers or other unspecified additives (ICI, 1973, 1974a). For
all three paraffins, erythema was usually noted following two to four
applications, although on one occasion erythema was noted in 1/3
animals after only one application. The severity of the reactions was
not described. Desquamation was also noted following three or four
applications and increased in severity with further treatments. In
one study (with 0.7% epoxy carboxylate stabilizer) the desquamation
was described as severe following the fourth application when the
study was terminated (ICI, 1973).
Studies have been conducted using C10-13 chlorinated paraffins
which were 48(CP-SL), 50, 52 or 55% chlorinated (CP-SH) (ICI,
1967, 1968, 1969, 1971, 1974a,b). In most of these studies the
chloroparaffins contained 0.2 or 2% epoxy stabilisers. In one study
with 48 or 55% chlorinated paraffins, containing 0.2% epoxy octyl
stearate stabilizer, no signs of irritation were noted (ICI, 1969).
In the other studies there was mild or slight erythema, and mild
desquamation was usually noted following the second or third
application. In one study, testing C10-13;52% with 2% epoxidised octyl
oleate stabilizer, erythema was noted following the first application
(ICI, 1968). It was observed in 4/5 of the studies that the reactions
did not worsen following further applications, although in one study
(testing a 52% chlorinated paraffin with unspecified additives),
slight erythema, noted after the second application, worsened to
severe erythema with slight necrosis after the third application, when
the study was terminated (ICI, 1971).
An unspecified volume of C10-13;40% Cl(CP-SL), containing 1% epoxy
soya oil stabilizer, produced slight desquamation following the second
application and mild erythema after the third (ICI, 1966). This
condition persisted throughout the remaining applications until the
end of the study when small scattered ulcers developed.
Two studies in rats were conducted to investigate the potential
for skin irritation of two short chain length chlorinated paraffins
(C10-11) which were 49% (CP-SL) and 60% chlorinated (CP-SH) (ICI, 1980,
1982c). Repeated and single application tests were conducted. No
signs of irritation were noted following a single application of the
more chlorinated paraffin, although slight desquamation was noted in
2/6 rats, 3-6 h after the treatment with the less chlorinated
paraffin. Both chlorinated paraffins produced slight erythema and/or
slight desquamation with repeated applications.
Rats were treated with 0.1 ml of C14-17;51-60% Cl(CP-MH), or
C20-30;41-51%(CP-LL), 51-61%(CP-LH) or 61-70% Cl(CP-SH), for up to
six 24-h periods. The treatment periods were separated by 24-h
treatment-free periods. In some of the studies the chlorinated
paraffins contained epoxy stabilizers. Mild irritation was seen with
C14-17 chlorinated paraffins, but it is not clear if the response was
due to the stabilizer. No signs of irritation were seen with C20-30
(Birtley et al., 1980).
A C10-13;61% chlorinated paraffin (Cereclor 60HS) and a 50%
chlorinated paraffin (Cereclor 50 HS) of unidentified carbon chain
length produced mild or moderate skin irritation following a single
occlusive application to intact or abraded skin of rabbits. It was
stated that varying degrees of erythema persisted for 72 h (ICI
1975a,b).
In other studies (BUA, 1992) different short and long chain
length chlorinated paraffins were applied to the skin and eyes of
rabbits (skin: C10-13;58% Cl, C19;44% Cl, C20-30;70% Cl; eyes: C12;59%
Cl, C20-30;70% Cl). Only a weak or no irritating effect was observed,
which decreased with increasing chain length.
The eye irritation potential of three different chlorinated
paraffins, C10-13;65% Cl(CP-SH), which contained either 2.5 or 2% of
two different additives or 0.7% of an epoxy stabilizer, was tested in
two studies (ICI, 1971, 1974a). Either 0.1 ml or "one drop" of the
chloroparaffin was instilled into one conjunctival sac of groups
of three rabbits. Similar results were reported for all three
formulations: practically no initial pain (2 on a 6-point scale) was
noted. Slight irritation (3 on a 8-point scale), shown by redness
and chemosis (only noted in the formulation containing the epoxy
stabilizer) of the conjunctiva with some discharge, lasted for 24 h.
One drop of 52% or 40% chlorinated paraffins, containing unspecified
additives or 1% epoxy stabilizer, was also tested (ICI, 1966, 1971).
With the 52% chlorinated paraffin, slight immediate irritation was
followed by slight redness of the conjunctiva which lasted for 24 h.
With the 40% chlorinated paraffin, mild congestion was noted at 1 h
but no effects were seen at 24 h.
7.1.3.2 Intermediate and long chain length chlorinated paraffins
Intermediate and long chain chlorinated paraffins were tested
in eye irritation studies with a single application of 0.1 ml of
C14-17;51-60% Cl(CP-MH), C20-30;41-50%(CP-LL), 51-60% or 61-70%
Cl(CP-LH). No signs of eye irritation were seen (Birtley et al.,
1980).
7.1.4 Skin sensitization
The maximization method was used to assess the skin sensitization
potential of a chlorinated paraffin (C10-13;56% Cl, CP-SH) with 1%
epoxide stabilizer (Edenol D81) and 1% tris-nonylphenyl phosphite
(TNPP) (Hoechst, 1983b). When challenged with undiluted chlorinated
paraffin, 1/20 test animals showed hardly perceptible erythema 24 h
after challenge, and 1/20 test and 1/10 control animals showed clearly
defined erythema or slight oedema at 72 h. The chlorinated paraffin
tested did not induce skin sensitization in this study.
The same chlorinated paraffin (C10-13;56% Cl, CP-SH), with 1% of a
different epoxide stabilizer (Rutapox CY 160) and 1% TNPP, was tested
using the same method (Hoechst, 1984). When challenged with undiluted
chlorinated paraffin, 5/20 test animals showed clearly defined erythema
and another two showed hardly perceptible erythema. None of the
control animals showed any evidence of a skin reaction. A second
challenge was performed 2 weeks after the first. On this occasion
4/20 test animals showed clearly defined erythema and another four
showed hardly perceptible erythema or slight oedema. The authors
concluded that the substance tested was a sensitizer. However, as
less than 30% of the test group showed a clear reaction and it is
possible that the epoxide stabilizer was responsible for producing the
sensitization reactions, this study is not considered to provide
conclusive evidence that C10-13;56% Cl is a skin sensitizer.
An undiluted chlorinated paraffin (C10-13;52% Cl, CP-SH) was
applied to the ears of six guinea-pigs on three successive days (ICI,
1971). Slight erythema was noted when, 4 days later, undiluted
chloroparaffin was applied to the animals' flanks, but it was not
stated how many animals showed a reaction. Four control animals also
showed slight erythema at challenge. It does not appear that this
chlorinated paraffin elicited a sensitization response in this study.
7.2 Repeated exposure
Studies involving repeated exposure have demonstrated that the
liver, kidneys and thyroid are the target organs for the toxicity of
chlorinated paraffins.
7.2.1 Oral route
7.2.1.1 Short chain length chlorinated paraffins
a) Rat, 14-day studies
In a range-finding study, a short chain chlorinated paraffin
(C10-13;58% Cl) (CP-SH) was administered to Fischer-344 rats in
the diet for 14 days at concentrations of 0, 900, 2700, 9100 and
27 300 mg/kg feed, equivalent to approximately to 0, 100, 300, 1000
and 3000 mg/kg body weight per day (IRDC, 1983c). There were five male
and five female rats per group. No deaths occurred during the study. A
marked reduction in body weight and food consumption was seen in the
highest dose group, which was attributed to reduced palatability of
the diet caused by the chlorinated paraffin. The relative liver
weight was increased (20-240%) in all dose groups compared to
controls. The activity of liver aminopyrine demethylase (APDM) was
increased in females, and cytochrome P-450 values increased in both
sexes in all dosed groups. Liver enlargement was observed in some
rats in the groups fed 2700 to 27 300 mg/kg, and a dose-related
increase in the incidence of hepatocellular hypertrophy was present in
all treated groups. Myocardial atrophy was observed at the two
highest dose levels although the relationship to treatment was
unclear. The lowest-observed-effect level (LOEL) in this study was
100 mg/kg body weight per day.
A short chain length chlorinated paraffin with 58% Cl (CP-SH) was
administered by gavage in corn oil to Fischer-344 rats (five/dose of
each sex) for 14 days at dose levels of 0, 30, 100, 300, 1000 and
3000 mg/kg body weight per day (IRDC, 1981a). A significant decrease
in body weight gain in females in the high dose group was noted. A
dose-related increase in APDM activity was observed in females fed
300-3000 mg/kg, whereas in males there was an increase only in the
group treated with 1000 mg/kg. Cytochrome P-450 levels were
significantly increased in females treated with 1000 mg/kg, and
microsomal protein concentration increased in females dosed with
3000 mg/kg. Liver enlargement occurred in both sexes in the 300, 1000
and 3000 mg/kg groups, and mild hepatocellular hypertrophy in all
animals of the 1000 and 3000 mg/kg groups. The absolute and relative
liver weight was increased (20-150%) at doses of 100 mg/kg or more.
Statistically significant reduction of thymus and ovary weight was
observed at 3000 mg/kg. The no-observed-effect level (NOEL) in this
study was considered to be 30 mg/kg body weight per day and the LOEL
100 mg/kg body weight per day, based on liver weight increases.
In a 16-day study on F-344/N rats (groups of five), the animals
were administered a short chain length chlorinated paraffin (C12;60%
chlorination) (CP-SH) by gavage in corn oil daily (5 days per week) at
doses of 0, 469, 938, 1875, 3750 and 7500 mg/kg body weight per day
for 16 days (NTP, 1986a; Bucher et al., 1987). At the highest dose
level there was reduced body weight gain (22% in males and 16% in
females), and 1/5 male rats and 2/5 females died before the end of the
study. Enlarged livers were observed in every dose group except the
females fed 469 mg/kg. No histopathology was performed. The LOEL in
this study was considered to be 469 mg/kg body weight per day.
b) Rat, 90-day studies
Fischer-344 rats (groups of 10 males and 10 females) were given a
short chain chlorinated paraffin (C12;60% chlorination) (CP-SH) in
corn oil by gavage on 5 days a week for 13 weeks at doses of 0, 313,
625, 1250, 2500 and 5000 mg/kg body weight per day (NTP, 1986a; Bucher
et al., 1987). Body weight gain was reduced by approximately 10% in
males at the two highest dose levels. A dose-related statistically
significant increase in relative liver weight (17-100% for males;
30-100% for females) was observed in all treated rats. Hepatocellular
hypertrophy was noted in all rats in the highest dose group, and
nephrosis was more frequent in this group (10/10 males; 3/10 females)
compared to controls (8/10 males; 0/10 females). Rats in other dose
groups were not examined microscopically. On the basis of an increase
in liver weight, the LOEL in this study was 313 mg/kg body weight per
day.
In a 13-week study Fischer-344 rats were administered a short
chain length chlorinated paraffin (C10-13;58% Cl, CP-SH) in corn oil by
gavage at doses of 0, 10, 100 and 625 mg/kg body weight per day in
groups of 15 animals of each sex (IRDC, 1984a). In the groups treated
with 100 mg/kg or more, increased weights of the liver (30-110%) and
the kidneys (20-100%) were observed. At the highest dose level
thyroid weights were increased. Hepatocellular hypertrophy was
observed at 100 and 625 mg/kg. In these groups hypertrophy and
hyperplasia of the thyroid were also observed. There was trace-to-mild
chronic nephrosis in the kidney of males treated with 625 mg/kg, and in
females in the high-dose group, in which pigmentation of the renal
tubular epithelia also occurred. The NOEL was considered to be 10 mg/kg
body weight per day on the basis that no treatment-related microscopic
changes were found in any tissue at this dose. The LOEL was 100 mg/kg
body weight per day based on increased liver and kidney weights and
hypertrophy in liver and thyroid. When the same doses were
administered in the diet essentially identical results were obtained
(IRDC, 1984c).
Male and female Fischer-344 rats (5 or 10 per group) were
administered Chlorowax 500C (C12;58% Cl, CP-SH) in corn oil by gavage
at doses of 0, 313 and 625 mg/kg body weight per day for up to 90 days
(Elcombe et al., 1994). The relative liver weight was increased at
both doses (50 and 75%). Hepatic peroxisomal ß-oxidation (palmitoyl
CoA oxidation) was statistically significantly increased in a
dose-related manner from day 15. The activity of thyroxine-UDPG-
glucuronosyltransferase was significantly increased (at least 150%) at
both dose levels from day 15 onwards. Thyroid follicular cell
hypertrophy was observed at all time points, and hyperplasia at days
56 and 91. Replicative DNA synthesis was increased in thyroid
follicular cells at day 91. Renal tubular eosinophilia was observed
in males from day 15. In renal tubular cells replicative DNA
synthesis was increased in males. When a higher dose was administered
(1000 mg/kg body weight per day) marked decreases in the levels of
plasma thyroxine and increased plasma thyroid stimulating hormone were
observed. None of these effects were observed in male Dunkin Hartley
guinea-pigs, which were administered the chlorinated paraffin at doses
of 500 and 1000 mg/kg body weight per day. The LOEL in rats was 313
mg/kg body weight per day based on increased relative liver weights,
hepatic peroxisomal ß-oxidation and thyroxine-UDPG-glucuronosyl-
transferase activity.
c) Mouse, 14-day studies
B6C3F1 mice in groups of five were administered C10;60% Cl
(CP-SH) in corn oil by gavage daily for 16 days (NTP, 1986a; Bucher et
al., 1987). The doses were 938, 1875, 3750, 7500 and 15 000 mg/kg
body weight per day. All mice receiving 3750 mg/kg or more, and 4/5
males and 2/5 females receiving 1875 mg/kg died before the end of the
study. Diarrhoea was observed in all dosed groups except females
receiving 938 mg/kg. Enlarged livers were found in all treated
surviving mice. No histopathological examinations were conducted.
d) Mouse, 90-day studies
B6C3F1 mice (10 of each sex per dose) were exposed to C12;60%
Cl (CP-SH) by gavage five days per week for 13 weeks (NTP, 1986a;
Bucher et al., 1987). The doses were 125, 250, 500, 1000 or
2000 mg/kg body weight per day. No clinical signs of toxicity were
observed. In the males the body weight gain was reduced by 13% at the
highest dose level. The relative liver weights showed a dose-related
increase (17-160%) and were statistically significant from 250 mg/kg
in females and from 500 mg/kg in males. Hepatocellular hypertrophy
was observed in animals of both sexes treated with 250 mg/kg or more.
Focal hepatic necrosis was related to dosing at 500, 1000 and
2000 mg/kg in males and at 2000 mg/kg in females. The NOEL was
125 mg/kg body weight per day, and the LOEL was 250 mg/kg body weight
per day, based on hepatocellular hypertrophy.
7.2.1.2 Intermediate chain length chlorinated paraffins
a) Rat, 14-day studies
A chlorinated paraffin of intermediate chain length (C14-17) and
52% chlorination (CP-MH) was administered to Fischer-344 rats in the
diet at dosage levels of 150, 500, 1500, 5000 and 15 000 mg/kg feed,
which was reported to correspond to an average compound intake of
17.7, 57.7, 177, 562 and 1412 mg/kg body weight per day (IRDC, 1981b).
Five male and five female rats in each dose group were exposed daily
for 14 days. No mortality occurred among the treated animals.
Hepatic APDM activity was statistically significantly increased in
males receiving 562 mg/kg body weight per day (5000 mg/kg feed) and in
females receiving 1412 mg/kg body weight per day (15 000 mg/kg feed).
Slight increase in cytochrome P-450 values in male rats given
177 mg/kg body weight per day (1500 mg/kg feed) was observed but
appeared not to be related to dosing. Increased relative liver weight
was observed in the highest two dosage groups. Microscopic examination
of the liver revealed mild diffuse hepatocellular hypertrophy in all
animals receiving 562 and 1412 mg/kg body weight per day (5000 and
15 000 mg/kg feed). The NOEL was reported to be 57.7 mg/kg body weight
per day. The LOEL was 177 mg/kg body weight per day in males based on
increased cytochrome P-450 values and 562 mg/kg body weight per day in
females based on increased liver weight and hepatocellular hypertrophy.
b) Rat, 90-day studies
Male and female weanling Sprague-Dawley rats in groups of ten
were fed diets containing 0, 5, 50, 500 or 5000 mg/kg of C14-17;52%
Cl(CP-MH) for 13 weeks, yielding an average intake of 0, 0.4, 3.6, 36
and 360 mg/kg body weight per day for males and 0, 0.4, 4.2, 43 and
420 mg/kg body weight per day for females (Poon et al., in press).
There were no clinical signs of toxicity and no differences in body
weight gain. Relative liver weight was increased at 43 and 420 mg/kg
body weight per day in females and at 360 mg/kg in males. Relative
kidney weight was increased at the highest dose level in both sexes.
Serum cholesterol was increased in females from 4.2 mg/kg in a
dose-related manner. In the highest dose group of both sexes elevated
hepatic UDP-glucuronosyltransferase activity was observed, but only
females at this dose level showed increased APDM activity. Decreased
hepatic vitamin A levels were detected in females at 43 mg/kg and in
both sexes at the highest dose level. Mild, adaptive histopathological
changes were detected in the liver of both sexes at the two highest
dose levels, and in the thyroid of males from 36 mg/kg and females
from 4.2 mg/kg (reduced follicle sizes, collapsed angularity,
increased height, cytoplasmic vacuolation and nuclear vesiculation).
In the kidney, minimal changes were noted in the proximal tubules of
males at 360 mg/kg, and in the inner medulla tubules of females at 43
and 420 mg/kg. The NOEL in this study was 4 mg/kg body weight per day.
The LOEL was 36 mg/kg body weight per day (males) and 43 mg/kg body
weight per day (females).
In a 90-day feeding study, Wistar rats (groups of 24 males and 24
females) were fed diets containing 0, 250, 500, 2500 and 5000 mg/kg
feed of Cereclor S52 (C14-17;52% Cl, CP-MH) containing stabilizer (0.2%
epoxidized vegetable oil) (Birtley et al., 1980). A dose-related
decrease in body weight gain in males fed 500 mg/kg feed or more was
observed, which was accompanied by a reduction in food intake.
Significant increases in the relative liver weights in females were
observed at 500 mg/kg feed or more and in males at 2500 mg/kg feed.
Significant increases in relative kidney weights were observed at
5000 mg/kg feed in both sexes. Microscopic examination of the liver
showed evidence of a dose-related proliferation of the smooth
endoplasmic reticulum in the hepatic cells from 500 mg/kg feed.
Haematological investigation showed no abnormalities attributable to
the test compound. A tendency towards congestion of the kidney with
increasing concentration of Cereclor S52 in the diet was also observed.
The NOEL was 250 mg/kg feed (12.5 mg/kg body weight per day based on
food consumption data) and the LOEL was 500 mg/kg feed (25 mg/kg
body weight per day) based on increased relative liver weights and
proliferation of smooth endoplasmic reticulum.
A medium chain length chlorinated paraffin (C14-17;52% Cl, CP-MH)
was evaluated for subchronic toxicity in Fischer-344 rats (IRDC,
1984b). The chlorinated paraffin was administered to the rats (15 of
each sex per dose group) in the diet to provide dosage levels of 0,
10, 100 and 625 mg/kg body weight per day for 13 weeks. The
treatment did not induce signs of toxicity, alter survival or cause
ophthalmological changes. A slight reduction of body weight gain
(< 5%) was observed in both sexes at the highest dose, and was
associated with reduced food consumption. Traces of hepatocyte
hypertrophy (at 625 mg/kg) and increased absolute and relative liver
and kidney weights (at 100 and 625 mg/kg) were noted in both sexes.
Males in the high-dose group had an increased incidence of nephritis.
In addition, increased thyroid weight and thyroid hypertrophy and
hyperplasia were observed in males at 625 mg/kg. The NOEL was
considered in the report to be 10 mg/kg body weight per day. The LOEL
was 100 mg/kg body weight per day based on increased liver and kidney
weights.
c) Dog, 90-day studies
The effects of Cereclor S52 (C14-17;52% Cl, CP-MH) (containing
0.2% epoxidized vegetable oil as stabilizer) were studied in Beagle
dogs (Birtley et al., 1980). Four male and four female animals in
each group were fed a diet corresponding to 0, 10, 30 or 100 mg/kg
body weight daily for 90 days. No effects were found, except for
significantly increased serum alkaline phosphatase activity and
relative liver weight in males exposed to 100 mg/kg and an increase in
the smooth endoplasmic reticulum of hepatocytes from 30 mg/kg. The
NOEL was 10 mg/kg body weight per day. The LOEL was 30 mg/kg body
weight per day based on an increase of hepatic smooth endoplasmic
reticulum.
7.2.1.3 Long chain length chlorinated paraffins
a) Rat, 14-16 day studies
Fischer-344 rats (groups of five) were exposed to C22-26;43% Cl
(CP-LL) daily by gavage for 16 days at doses of 235, 469, 938, 1875 or
3750 mg/kg body weight per day. No compound-related clinical signs
of toxicity or mortality were observed. There were no changes
in body weight gain and no gross lesions were observed at necropsy.
Histopathological examinations were not conducted (NTP, 1986b; Bucher
et al, 1987).
Fischer-344 rats, in groups of five of each sex, were fed long
chain length paraffins of 70% chlorination for 14 days. The dietary
concentrations of the C22-26;70% Cl (CP-LH) were 0, 150, 500, 1500,
5000 and 15 000 mg/kg feed, corresponding to an average compound
intake of 0, 17.1, 55, 169, 565 and 1715 mg/kg body weight per day
(IRDC, 1982b). Tissues from liver, kidneys, spleen, lungs and
mesenteric lymph nodes were examined microscopically. Hepatic
microsomal Lowry protein, APDM activity and cytochrome P-450 values
were determined. No significant toxic effects were noted, and no
compound-related effects were found after microscopical examinations.
The NOEL was 1715 mg/kg body weight per day.
A chlorinated paraffin, C22-26;43% Cl (CP-LL), was administered by
gavage to Charles River 344 rats at doses of 0, 30, 100, 300, 1000 and
3000 mg/kg body weight per day (IRDC, 1982c). During the 14-day test
period no signs of toxicity were observed. Following sacrifice,
tissues from the liver, spleen, kidneys, pancreas, thymus and eyes
were examined microscopically; the only observation was a possible
increase in kidney nephrolithiasis in females exposed to the highest
dose level. Hepatic microsomal Lowry protein, APDM activity and
cytochrome P-450 values were determined and there were no alterations.
The NOEL was considered to be 3000 mg/kg body weight per day.
b) Rat, 90-day studies
A long chain length chlorinated paraffin, C23;43% Cl (CP-LL),
was administered to Fischer-344 rats in groups of 10 (each sex) by
gavage for 13 weeks at doses of 235, 469, 938, 1875 or 3750 mg/kg body
weight per day (NTP, 1986b; Bucher et al., 1987). No effects on body
or organ weights and no clinical signs of toxicity were observed. A
dose-related increased incidence of granulomatous inflammation was
noted in the livers of all exposed female rats but not in males. The
NOEL was 3750 mg/kg body weight per day for males. For females the
LOEL was 235 mg/kg body weight per day based on increased incidence of
granulomatous inflammation in the liver.
A long chain chlorinated paraffin (C20-30) with 43% Cl (CP-LL) was
administered in corn oil by gavage for 90 days to Fischer-344 rats at
three doses (100, 900 or 3750 mg/kg body weight per day) (IRDC,
1984f). Increases in liver weights and a multifocal granulomatous
hepatitis characterized by inflammatory changes and necrosis were
observed in all exposed females but not in males. In female rats
mineralization in the kidneys at the highest dose level was observed.
In addition to these observations, mild nephrosis was observed in the
males in the highest dose group. In males, the NOEL was 900 mg/kg
body weight per day and the LOEL was 3750 mg/kg body weight per day
(based on the occurrence of mild nephrosis). In females the LOEL was
100 mg/kg body weight per day based on liver effects.
The chlorinated paraffin C22-26;70% Cl (CP-LH) was administered to
Fischer-344 rats in the diet for 90 days at doses of 100, 900 and
3750 mg/kg body weight per day (IRDC, 1984g). Increased liver weight,
hepatocellular hypertrophy and cytoplasmic fat vacuolation were noted
at the highest dose level. The alanine aminotransferase (ALT)
activity was increased in both sexes at the highest dose level, and
aspartate aminotransferase (AST) activity was also increased in
females of this group. The NOEL was considered to be 900 mg/kg body
weight per day. The LOEL was 3750 mg/kg body weight per day based on
liver effects.
c) Mouse, 14-16 day studies
B6C3F1 mice in groups of five were given C22-26;43% Cl (CP-LL) by
gavage daily for 16 days at doses of 469, 938, 1875, 3750 or
7500 mg/kg body weight per day (NTP, 1986b; Bucher et al., 1987). No
compound-related clinical sign of toxicity or mortality was observed,
there were no changes in body weight gain, and no gross lesions were
observed at necropsy. Histopathological examinations were not
conducted.
d) Mouse, 90-day studies
The long chain length chlorinated paraffin C23;43% Cl (CP-LL)
was administered to B6C3F1 mice (in groups of 10 of each sex) by
gavage for 13 weeks at dose levels of 469, 938, 1875, 3750 or
7500 mg/kg body weight per day (NTP, 1986b; Bucher et al., 1987). No
effects on body or organ weights, no clinical signs of toxicity and no
histopathological effects were observed. The NOEL was 7500 mg/kg body
weight per day.
7.2.1.4 Comparative studies
The effects of representative chlorinated paraffins on liver
function and thyroid hormone function has been studied in male rats
(Alpk:APFSD) and male mice (Alpk:APFCD-1) (Wyatt et al., 1993).
Groups of five male rats or five male mice received 0, 10, 50, 100,
250, 500 or 1000 mg/kg body weight per day by gavage in corn oil, once
daily for 14 days. The chlorinated paraffins studied were Chlorowax
500C (C10-13;58%Cl, CP-SH), Cereclor 56L (C10-13;56%, CP-SH) and
Chlorparaffin 40C (C14-17;40% Cl, CP-ML). Effects on liver function
were assessed by changes in liver weight (expressed both as absolute
weights and liver:body weight ratio) and peroxisome proliferation
(expressed as the activity of palmitoyl co-enzyme A (CoA) oxidase).
All three chlorinated paraffins caused increases in liver weight and
palmitoyl CoA oxidation, indicative of peroxisomal proliferation. In
general, the rat was more sensitive to the effects of the chlorinated
paraffins on liver weight than the mouse. The doses of chlorinated
paraffin required to cause peroxisomal proliferation were, in general,
greater than those causing effects on liver weight, although there
appeared to be less of a difference in inter-species sensitivity.
However, the magnitude of the increase in palmitoyl CoA oxidation
caused by the short chain chlorinated paraffins (approximately 10-fold
increase as a maximal change) was greater than for the intermediate
chain grade (approximately 4-fold increase as maximal change) (Table
18). The effect of the chlorinated paraffins on thyroid function was
studied in the male rats receiving 1000 mg/kg body weight per day for
14 days by measuring the plasma levels of triiodothyronine (T3) and
thyroxine (T4) (free and total) and thyroid stimulating hormone
(TSH), and also the activity of hepatic microsomal UDP glucuronosyl-
transferase activity. All three chlorinated paraffins (at 1000 mg/kg
body weight per day) caused a reduction in plasma T4 levels (both
free and total) and an increase in plasma TSH levels. No effect was
observed on plasma T3 levels. All three chlorinated paraffins also
caused an increase (two-fold) in the rate of glucuronidation of T4
by hepatic microsomal UDP glucuronosyltransferase activity, suggesting
that the impact on plasma T4 and TSH levels is due to increased
clearance of T4 by hepatic metabolism.
Table 18. Effects of chlorinated paraffins on liver function in male rats and male mice
(From: Wyatt et al., 1993)
Increase in relative liver weight Increase in palmitoyl CoA
(% liver:body weight ratio) oxidase activity
Rat Mouse Rat Mouse
NOELa LOELb NOELa LOELb NOELa LOELb NOELa LOELb
C10-13;58% Cl 74 100 215 250 184 250 180 250
C10-13;56% Cl 51 50 70 100 600 1000 120 250
C14-17;40% Cl 31 100 426 1000 473 500 252 500
a Calculated using a three-parameter logit model (mg/kg body weight per day)
b Observed (mg/kg body weight per day)
The hepatic effects of representative chlorinated paraffins have
been studied in male and female F-344 rats, male and female B6C3F1
mice and male Alpk:Dunkin Hartley guinea-pigs (Elcombe et al., in
press). Their effects were compared with a range of known inducers of
hepatic enzymes. Groups of 4-5 animals received 1000 to 2000 mg/kg
body weight per day of each chlorinated paraffin (by gavage in corn
oil) for 14 consecutive days. The chlorinated paraffins studied were
Chlorowax 500C (C10-13;58% Cl, CP-SH), Cereclor 56L (C10-13;56% Cl,
CP-SH), Chlorparaffin 40G (C14-17;40% Cl, CP-ML) and Chlorowax 40
(C20-30;43% Cl, CP-LL). The short and intermediate chain length
chlorinated paraffins increased liver:body weight ratios (approximately
1.5 times) and elicited hepatocellular hypertrophy, peroxisome
proliferation (assessed as increases in peroxisomal volume and
palmitoyl CoA oxidase activity) and proliferation of hepatic cell
smooth endoplasmic reticulum in both rats and mice. These effects
were not seen in rats or mice receiving the long chain chlorinated
paraffin. The short and intermediate chain length chlorinated
paraffins also caused induction of cytochrome P-450 IV A1 (assessed by
increases in lauric acid hydroxylation) and P-450 II B1/IIB2 (assessed
by increases in ethoxycoumarin- O-diethylation) in the rat liver, but
only P-450 IV A1 in the mouse liver. The induction of the specific
cytochrome P-450 isoenzymes was confirmed using SOS-polyacrylamide gel
electrophoresis (SPS-PAGE) and Western immunoblotting of microsomes.
The administration of the short or intermediate chain length
chlorinated paraffins to guinea-pigs (1000 mg/kg body weight per day
for 14 days) had a similar effect on liver:body weight ratios (1.5-fold
increase) but had no effect on any of the hepatic ultrastructural or
biochemical parameters measured.
7.2.2 Intraperitoneal route
7.2.2.1 Short chain length chlorinated paraffins
Effects on both hepatic cytosolic and microsomal epoxide
hydrolases have been observed in male C57Bl/6 mice after 5 daily
intraperitoneal injection of 400 mg Cereclor 70L (C12;70% Cl, CP-SH)
(Meijer & DePierre, 1987). The hepatic cytosolic epoxide hydrolase
activity was increased to 130% and the microsomal activity to 250% of
the control. In addition, the amount of microsomal cytochrome P-450
was increased by 50%, and cytosolic DT-diaphorase activity was
increased 2- to 3-fold. There was also liver enlargement.
7.2.2.2 Intermediate chain length chlorinated paraffins
Lundberg (1980) reported a significant, dose-related increase in
total cytochrome P-450 in mice (strain not reported) injected
intraperitoneally with different amounts of Cereclor S52 (C14-17;52%
Cl, CP-MH) (from 0.6 mg to 63.4 mg) on 3 consecutive days. The
N-demethylation of ethylmorphine, a cytochrome P-450-dependent
reaction, decreased at low concentrations but increased at higher
concentrations.
7.2.2.3 Comparative studies
Male Sprague-Dawley rats were given intraperitoneal injections of
1000 mg/kg of Witaclor 149, 159 or 171P (C10-13 with 49% [CP-SL], 59%
[CP-SH] and 71% [CP-SH] chlorination, respectively), Witachlor 350
(C14-17 with 49% chlorination [CP-ML] or Witachlor 549 (C18-26 with 49%
chlorination [CP-LL]), each containing small amounts of epoxidated
soy-bean oil as stabilizer, daily for 4 days (Nilsen et al., 1980,
1981; Nilsen & Toftgård, 1981). Treatment with the C10-13 chlorinated
paraffins, but not those with longer chain lengths, caused increases
in liver weight and an induction of various forms of microsomal
cytochrome P-450. The activity of O-deethylation of 7-ethoxyresorufin
was decreased by the C10-13 chlorinated paraffins with higher
chlorine content, Witaclor 159 and 171P. The metabolism of
benzo (a)pyrene was induced by each of the chlorinated paraffins.
Witaclor 149 (C10-13;49% Cl) caused a significant proliferation of the
smooth endoplasmic reticulum (two-fold) whereas C18-26;49% Cl caused a
smaller increase. All three C10-13 chlorinated paraffins gave rise to
increased occurrence and size of cytoplasmic lipid droplets. Witachlor
149 also caused an increase in the number and size of mitochondria and
peroxisomes. These latter effects were also observed to a lesser
degree with both Witachlor 350 and Witachlor 549.
Effects on microsomal enzymes, after intraperitoneal injection of
Cereclor 42 (C22-26;42% Cl, CP-LL), Cereclor S58 (C14-17;58% Cl, CP-MH),
Cereclor 70 (C23;70% Cl, CP-LH) and Cereclor 70L (C10-13;70% Cl) (1000
mg/kg, once daily for 5 days) in liver from male Sprague-Dawley rats,
have been observed (Meijer et al., 1981). Microsomal epoxide hydrolase
activity was increased by all Cereclors except C22-26;42% Cl. In
addition, the activity of glutathione- S-transferase was increased
except in the case of C22-26;42% Cl, which decreased the activity
slightly. The amount of cytochrome P-450 was not changed.
7.3 Neurotoxicity
7.3.1 Short chain length chlorinated paraffins
The motor capacity, measured as the capacity of adult male NMRI
mice to remain on an accelerating rotarod, was determined after single
intravenous injections of 0, 30, 97.5, 165, 232.5 and 300 mg/kg in
groups of five mice of either Cereclor 50 LV (C10-13;49% Cl, CP-SL) or
Cereclor 70L (C10-13;70% Cl, CP-SH) (Eriksson & Kihlström, 1985). A
statistically significant decrease in the motor capacity and rectal
temperature was observed in mice receiving the highest dose of either
compound.
7.3.2 Intermediate chain length chlorinated paraffins
Immature 10-day-old NMRI mice (groups of at least 6) were given a
single peroral dose of 1 mg/kg body weight of polychlorohexadecane
(C16; chlorination degree not specified) dissolved in a fat emulsion
of egg lecithin and peanut oil (Eriksson & Nordberg, 1986). A
significantly decreased sodium-dependent choline uptake in the
cerebral cortex, 65% of Vmax in controls, was measured 7 days after
treatment indicating a pre-synaptic effect of this chlorinated
paraffin. No significant alteration in high- and low-affinity
muscarinic binding in the cerebral cortex in the brains could be
observed.
7.4 Reproductive toxicity, embryotoxicity and teratogenicity
7.4.1 Reproduction
An intermediate chain length chlorinated paraffin (C14-17) with
52% chlorination (CP-MH) was given in the diet to Charles River rats
at dose levels of 0, 100, 1000 and 6250 mg/kg feed (equivalent to 0,
6, 62 and 384 mg/kg body weight per day for the males and 0, 8, 74 or
463 mg/kg body weight per day for the females based on food
consumption data) (IRDC, 1985). The diet was fed both males and
females for 28 days before mating, during mating, and for females up
to postnatal day 21. Pups were given the same diet from weaning
until 70 days of age. No differences were observed in appearance,
fertility, body-weight gain, food consumption or reproductive
performance in the F0 generation. Among the offspring, no adverse
effects were observed prior to lactation day 7. However, significantly
decreased pup survival was observed in the high-dose group on lactation
day 10. None of the pups in this group survived to weaning. Survival
in pups from the mid-dose group was decreased by lactation day 21.
Necropsy findings in animals that died included pale liver, kidneys and
lungs, and blood in the cranial cavity, brain, stomach and intestines.
The pup weights were lower in the low-dose group (not statistically
significant) and mid-dose group than in the control group on lactation
day 21. In females, the reduced weight continued after weaning but
became less pronounced in males. Other observations in the pups of
the mid- and high-dose groups included bruised areas, decreased
activity, laboured breathing, pale discoloration and/or blood around
orifices. Reduced erythrocyte count, haemoglobin and haematocrit were
noted in the pups in the high-dose group on lactation day 6 relative to
the control values obtained on lactation day 7. The observations in
this study could indicate a high exposure of the pups to chlorinated
paraffins via the milk. This is supported by preliminary results of
a cross-fostering study showing a greater mortality in pups exposed
via milk than in pups exposed only in utero (Serrone et al., 1987).
The LOEL was 5.7 mg/kg body weight per day (males) or 7.2 mg/kg body
weight per day (females) in the F1 generation based on decreased
pup weight.
7.4.2 Embryotoxicity and teratogenicity
Teratology studies are summarized in Table 19.
Table 19. Oral teratology studies with chlorinated paraffins
Chlorinated paraffina Species LOEL NOEL Effects and remarks References
(mg/kg (mg/kg
body weight body weight
per day) per day)
CP-SH: C10-13;58% Cl rat 2000 500 maternal toxicity at 500 and 2000 mg/kg body weight IRDC (1982a)
per day; embryo-fetotoxicity and digital
malformations at 2000 mg/kg body weight per day
C10-13;58% Cl rabbit - 100 IRDC (1982d)
CP-MH: C14-17;52% Cl rat - 5000 slight maternal toxicity at 2000 and 5000 mg/kg IRDC (1984d)
body weight per day
C14-17;52% Cl rabbit - 100 mean maternal body weight losses were seen during IRDC (1983f)
treatment at the high-dose level (80, 100, 160 mg/kg
body weight per day) in a range-finding study
C14-17;70% Cl mouse - 100b Darnerud &
Lundkvist
(1987)
Table 19 (Cont'd)
Chlorinated paraffina Species LOEL NOEL Effects and remarks References
(mg/kg (mg/kg
body weight body weight
per day) per day)
CP-LL: C20-30;43% Cl rat - 5000 IRDC (1983d)
C20-30;43% Cl rabbit - 2000 slight increase in mean implantation loss and IRDC (1982e)
decreased number of viable fetuses at 5000 mg/kg
body weight per day, which were not statistically
significant
CP-LH: C22-26;70% Cl rat - 5000 IRDC (1984e)
C22-26,70% Cl rabbit - 1000 possible maternal toxicity (non-dosage-related IRDC (1983b)
congestion of lungs). In preliminary rabbit studies
an increase in post-implantation loss was observed
at > 1000 mg/kg body weight per day, but this effect
was not observed in the main teratology study
a The classification is given in Table 1
b Single intraperitoneal injection on day 1
7.4.2.1 Short chain length chlorinated paraffins
The teratogenic potential of a short chain length paraffin
(C10-13) with 58% Cl (CP-SH) was studied in pregnant Charles River COBS
CD rats (IRDC, 1982a). The rats, in groups of 25, were administered
0, 100, 500 and 2000 mg/kg body weight per day in corn oil orally by
gavage once daily from days 6 to 19 of gestation, and were examined on
gestation day 20. In the dams, the high dose treatment increased the
frequency of mortality (32%) and decreased body weight gain, and the
mid- and high-dose treatments resulted in dose-related adverse
clinical signs, such as yellow or brown matting and staining of the
anogenital fur, soft stool, red or brown matter (or staining) in the
nasal region, decreased activity, oily fur and excessive salivation.
Treatment at the highest dose level, which was a maternally toxic
dose, resulted in the appearance of fetal malformations such
as adactyly and/or shortened digits, increased incidences of
postimplantation loss and decreased numbers of viable fetuses. Fetal
body weight and incidence of delayed bone ossification were not
affected by the treatment. The NOEL for teratogenic effects was
500 mg/kg body weight per day, which was also a slightly maternally
toxic dose.
Female Dutch Belted rabbits (groups of 16) were treated by gavage
with a short chain length chlorinated paraffin (C10-13) with 58%
chlorination (CP-SH) (IRDC, 1982d). The rabbits were treated at dose
levels of 0, 10, 30 and 100 mg/kg body weight per day on gestation
days 6-27 and examined on day 28. There were no adverse effects on
survival, body weight gain, clinical signs or postmortem observations
in dams. The highest-dose group had an increased incidence of whole
litter resorption (two dams), and in the group exposed to 30 mg/kg
slight increases in the incidence of whole litter resorption (one dam)
and early and late resorptions were observed. Whole litter
resorptions did not occur in the low-dose or control animals, but
occurred in historical controls at an incidence of 13/277. This
indicated that the appearance of one or two dams with whole litter
resorptions could occur by chance. The NOAEL in this study was
100 mg/kg body weight per day.
7.4.2.2 Intermediate chain length chlorinated paraffins
Female Charles River COBS CD rats were treated by gavage with an
intermediate chain length chlorinated paraffin (C14-17;52% Cl) (CP-MH)
(IRDC, 1984d). The administered dose levels (0, 500, 2000 and
5000 mg/kg body weight per day) were given to groups of 25 animals on
gestation days 6-19, and this was followed by examination on day 20.
The end-points studied were weight of the uterus, number and location
of viable fetuses, early and late resorptions, the number of total
implantations and corpora lutea, and the incidence of fetal
malformations. The treatment had no adverse effect on mortality, body
weight gain or uterine weight of dams, but signs of toxicity, such as
wet, matted and yellow-stained hair in the anogenital area and/or soft
stools, were observed in the mid- and high-dose dams. No treatment-
related adverse effects were observed in the fetuses and there was no
evidence of developmental effects.
Female Dutch Belted rabbits were treated by gavage with an
intermediate chain length chlorinated paraffin (C14-17;52% Cl) (CP-MH)
in groups of 16 (IRDC, 1983f). The dose levels were 0, 10, 30 and
100 mg/kg body weight per day and were administered on gestation days
6-27, followed by examination on day 28. The end-points studied were
weight of the uterus, number and location of viable fetuses, early and
late resorptions, the number of total implantations and corpora lutea,
and the incidence of fetal malformations. In the dams, congestion of
the lobes of the lung was noted in all treated groups at necropsy, but
did not occur in a dose-related pattern. No significant adverse
effects were observed in fetuses and there were no developmental
effects.
In a study of NMRI mice, the animals were given a single
intraperitoneal injection of 100 mg/kg body weight of polychloro-
hexadecane (C16;70% Cl) (CP-MH) on the day the vaginal plug was
observed (day 1) (Darnerud & Lundkvist, 1987). The mice were killed
on day 14 of pregnancy, and the number and weight of embryos and
resorptions were determined. No effects on implantation or embryonic
survival were observed.
7.4.2.3 Long chain length chlorinated paraffins
Groups of 25 pregnant Charles River COBS CD rats were administered
(500, 2000 and 5000 mg/kg body weight per day) a long chain chlorinated
paraffin (C22-26;43% Cl) (CP-LL) by gavage from days 6 to 19 of
gestation (IRDC, 1983d). The end-points studied were weight of the
uterus, number and location of viable fetuses, early and late
resorptions, the number of total implantations and corpora lutea,
and the incidence of fetal malformations. In the dams, there were no
adverse effects on appearance, mean body weight gain or mortality
rate, and necropsy findings were normal. No signs of developmental
effects were noted in the pups.
Female Charles River COBS CD rats (groups of 25 animals) were
treated by gavage with a long chain length chlorinated paraffin
(C22-26;70% Cl) (CP-LH) (IRDC, 1984e). The dose levels were 0, 500,
2000 and 5000 mg/kg body weight per day on gestation days 6-19. The
rats were examined on day 20. The end-points studied were the same as
in the previous study. No treatment-related adverse effects were
observed in the dams or pups.
Pregnant Dutch Belted rabbits in groups of 16 were exposed orally
to long chain chlorinated paraffin (C22-26;43% Cl) (CP-LL) at doses of
500, 2000 and 5000 mg/kg body weight per day in corn oil by gavage
once daily from days 6 to 27 of gestation (IRDC, 1982e). The
end-points studied were the same as in the previous studies in this
section. No effects on maternal survival or body weight gain
occurred. In the highest-dose group there was a slight increase in
mean implantation loss and a slight decrease in the mean number of
viable fetuses when compared to the control group. However, these
alterations were not statistically significant. In the fetuses no
alterations related to the treatment were observed, although the
number in the highest-dose group was limited. Treatment of the
rabbits at a dose level of 2000 mg/kg body weight per day or less did
not produce a teratogenic response. No developmental effects were
noted. The NOEL in this study was 2000 mg/kg body weight per day.
Female Dutch Belted rabbits (groups of 16 animals) were given by
gavage a long chain length chlorinated paraffin (C22-26;70% Cl) (CP-LH)
(IRDC, 1983b). Doses of 0, 100, 300 and 1000 mg/kg body weight per
day were administered on gestation days 6-27, followed by examination
on day 28. The end-points studied were the same as in the earlier
studies in this section. The appearance, behaviour and body weight
gain were normal in the treated dams, although at necropsy a
non-dose-related increase in the occurrence of congested lungs was
noted. No adverse effects on the fetuses were observed and no
developmental effects were noted.
7.5 Mutagenicity and related end-points
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells there is a suggestion of a weak
clastogenic potential in vitro but not, according to several reports,
in vivo. Chlorinated paraffins were also reported to induce cell
transformation in vitro.
Table 20. Mutagenicity of chlorinated paraffins in bacterial tests
Chlorinated paraffina Dose (µg/plate) S9 Bacterial strainsb Effects Reference
Cereclor 50LV C10-13;50% Cl (CP-SH) 2500 ± TA1535 - Birtley et al. (1980)
TA1538 -
TA100 -
TA98 -
Hordalub 80 (with 1% C10-13;50% Cl (CP-SH) 10 000 ± TA98 + Hoechst (1986a)
epoxy stabilizer) TA100 -
TA1535 -
TA1537 -
TA1538 -
WP2 uvrAc -
Chloroparaffin 56 C12;57% Cl (CP-SH) 5000 ± TA98 - Hoechst (1988)
(unstabilized) TA100 -
TA1535 -
TA1537 -
TA1538 -
WP2 uvrAc -
Cereclor 70L C10-13;70% Cl (CP-SH) 2300 ± TA98 - Meijer et al. (1981)
TA100 -
TA1537 -
Cereclor S52 C14-17;52% Cl (CP-MH) 2500 ± TA1535 - Birtley et al. (1980)
- stabilizer TA1538 -
TA100 -
TA98 -
+ stabilizer -
Table 20. Cont'd
Chlorinated paraffina Dose (µg/plate) S9 Bacterial strainsb Effects Reference
Solvocaffaro C1642 C14-17;42% Cl (CP-ML) 1, 10, ± TA1535 - Conz & Fumero (1988a)
100, TA1537 -
1000, TA1538 -
5000 TA98 -
TA199 -
Meflex DC 029 C14-17;45% Cl (CP-ML) 1.6, 8, ± TA1535 - Elliott (1989a)
40, TA1537 -
200, TA1538 -
1000, TA98 -
5000 TA1000 -
Cereclor 42 C20-30;42% Cl (CP-LL) 2500 ± TA1535 - Birtley et al. (1980)
TA1538 -
TA100 -
TA98 -
Chorowax 40 C23;43% Cl (CP-LL) 10 000 ± TA97 - NTP (1986b)
TA98 -
TA100 -
TA1535 -
a The classification is given in Table 1
b Unless indicated otherwise, all strains refer to Salmonella typhimurium
c Strain of Escherichia coli
7.5.1 Prokaryotes
Most of the data demonstrate no mutagenic effects in four
Salmonella typhimurium strains after treatment with short,
intermediate or long chain length chlorinated paraffins at doses up to
10 mg/plate (Table 20). A very low but significant effect was
detected in strain TA98 after treatment with the highly chlorinated
short chain length chlorinated paraffin, Cereclor 70L (C10-12;70% Cl,
CP-SH) (Meijer et al., 1981). However, the observation is uncertain
since no dose response was observed, the increase in revertants was
low (less than 2-fold), and the increase was only found in the
presence of metabolic fraction (S9) derived from Aroclor-1254-induced
rat liver. The results from this study were considered to be
negative. Another study apparently demonstrated positive results.
However, the increase in the number of revertants with TA100 in the
presence of S9 was just less than two-fold, and in TA98, in the
absence of S9, the increase only just reached two-fold (Hoechst,
1986a). Furthermore, the possibility that the epoxy stabilizer was
responsible for the increase can not be discounted.
7.5.2 Mammalian cells
The results obtained from mammalian cell systems are summarized
in Tables 21, 22 and 23.
7.5.2.1 In vitro studies
C12;60% Cl (CP-SH) was mutagenic in L5178Y mouse lymphoma cells
at concentrations of 48 and 60 µg/ml in the absence of S9 mix (Myhr et
al., 1990).
When tested up to cytotoxic concentrations, C10-13;56% Cl(CP-SH)
did not induce a significant, reproducible increase in the number of
mutant colonies in Chinese hamster V79 cells (HPRT locus), either in
the presence or absence of S9.
Chlorowax 500C (C23;43% Cl, CP-LL) induced chromosome
aberrations in the absence of S9 mix at a concentration of 5000 µg/ml
and sister chromatid exchange (SCE) with and without S9 at 5, 500,
1700 and 5000 µg/ml in Chinese hamster ovary (CHO) cells in vitro
(Anderson et al., 1990).
7.5.2.2 In vivo studies
a) Short chain length chlorinated paraffins
When C12;60% Cl (CP-SH) (0, 500, 1000 and 2000 mg/kg body weight
was administrated in corn oil by gavage to Alpk:AP male rats (groups
of 4), no effects on unscheduled DNA synthesis (UDS) in hepatocytes
could be detected after exposure for 2 or 12 h (Ashby et al., 1990).
However, a moderate dose- and time-related induction of cell
proliferation, measured as S-phase cells, in the hepatocytes was
detected in animals exposed to 1000 and 2000 mg/kg for 12 h.
Table 21. Genotoxicity in mammalian cells in vitro
Chlorinated paraffin Cell line End-point Exposure Effect References
CP-SH: C10-13;56% Cl Chinese hamster mutations 5, 10, 15, 20, 30, 50, 75 µg/ml - Hoechst (1987)
V79 cells (±S9)
C12;60% Cl L5178Y mouse mutations 12, 24, 36, 48, 60 and 72 µg/ml + (from Myhr et al.
lymphoma cells (-S9) 60 µg/ml) (1990)
CP-LL: C23;43% Cl Chinese hamster chromosome 1250-5000 µg/ml (±S9) + (at 5000 Anderson et al.
ovary cells aberrations µg/ml + S9) (1990)
C23;43% Cl Chinese hamster sister chromatid 5, 500, 1700 and 5000 µg/ml +a Anderson et al.
ovary cells exchange (±S9) (1990)
a The effect was observed at all concentrations
Table 22. Cell transformation in in vitro mammalian cells
Grade Cell line Exposure Effect References
CP-SH: C10-13;50% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
C10-13;58% Cl Baby hamster kidney cells 44 µg/ml (-S9), 58 µg/ml (+S9) + ICI (1982a)
C10-13;58% Cl Baby hamster kidney cells 33 µg/ml (-S9), 88 µg/ml (+S9) + Richold et al. (1982a)
CP-MH: C14-17;52% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
CP-LL: C20-30;42% Cl Baby hamster kidney cells 0.25, 2.5, 25, 250 and 2500 µg/ml (-S9) - Birtley et al. (1980)
CP-LH: C20-26;70% Cl Baby hamster kidney cells 10, 50, 100, 500 and 1000 µg/ml (±S9) +a Richold et al. (1982b)
C22-26;70% Cl Baby hamster kidney cells 10 µg/ml (+S9), 294 µg/ml (-S9) + ICI (1982b)
a The effect was observed at all concentrations
Table 23. Genotoxicity data from in vivo mammalian cells
Grade Species and End-point Exposure No. of Effect References
strain animals
CP-SH: Fischer-344 Chromosome aberrations 250, 750 and 2500 mg/kg body weight 8 - IRDC (1983h)
C10-12,58% Cl rats in bone marrow cells per day for 5 days by gavage
C10-13,58% Cl Charles River Dominant lethal 250, 750 and 2500 mg/kg body weight 15 - IRDC (1983a)
COBS CD rats mutations per day for 5 days by gavage
C10-13,58% Cl NMRI mice Micronucleus assay 50, 5000 mg/kg body weight (single 10 - Muller (1989)
in bone marrow cells dose by gavage)
C10-13,58% Cl Hoe:NMRKF Micronucleus assay 50, 5000 mg/kg body weight (single 10 - Muller (1989)
SPF 71 mice in bone marrow cells dose by gavage)
C12;60% Cl Alpk: AP rats DNA repair in 500, 1000 and 2000 mg/kg body weight 4 - Ashby et
hepatocytes by gavage (single dose by gavage) al. (1990)
for 2 or 12 h
CP-ML: Crd: CD-1 Micronucleus assay 5000 mg/kg body weight (single dose 10 - Conz &
C14-17;42% Cl (ICR) Br mice in bone marrow cells by gavage) Fumero (1988b)
C14-17;45% Cl C57B1/6JFCD-1/ Micronucleus assay 3125, 5000 mg/kg body weight (single 10 - Elliott
Alpk mice in bone marrow cells dose by gavage) (1989b)
Table 23. (Cont'd)
Grade Species and End-point Exposure No. of Effect References
strain animals
CP-MH: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983g)
C14-17;52% Cl rats in bone marrow cells per day for 5 days by gavage
CP-LL: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983i)
C22-26;43% Cl rats in bone marrow cells per day for 5 days by gavage
CP-LH: Fischer-344 Chromosome aberrations 500, 1500 and 5000 mg/kg body weight 8 - IRDC (1983e)
C20-30;70% Cl rats in bone marrow cells per day for 5 days by gavage
Sexually mature male Fischer-344 rats in groups of eight were
dosed by gavage once daily for 5 days with a short chain length
chlorinated paraffin (C10-12;58% Cl, CP-SH) at doses of 0, 250, 750 or
2500 mg/kg body weight per day (IRDC, 1983h). Metaphase spreads of
rat bone marrow cells were examined for chromosome aberrations. In
the group treated with 2500 mg/kg, all the rats except one died during
the study. Signs of overt toxicity were observed in most of these
rats. The rats receiving up to 750 mg/kg body weight per day did not
show an increased mortality or frequency of chromosome or chromatid
abnormalities, neither did the surviving rat from the highest dose
group. These observations indicate that toxic doses were administered
to the rats. Cytotoxicity was not assessed. However, the information
on distribution of short chain chlorinated paraffins following oral
absorption (section 6.3.1) indicates that there would have been
distribution to the bone marrow. This chlorinated paraffin was not
considered clastogenic in this test system.
Sexually mature NMRI (Hoe, NMRKF [SPF71]) mice (groups of five
males and five females) were given single doses of 50 and 5000 mg/kg
body weight Chlorowax 500C (C10-13;58% Cl, CP-SH) by gavage in sesame
oil (Hoechst, 1989). Responses at the high dose level were examined
at 24, 48 and 72 h sampling times and the low dose level at a 24 h
sampling time only. There were no differences from control values
either in polychromatic cells with micronuclei or in the ratio of
polychromatic erythrocytes to normocytes.
The dominant lethal mutation potential of a chlorinated paraffin
of short chain length and 58% chlorination was examined in Charles
River COBS CD rats (IRDC, 1983a). Groups of 15 males were treated
with 0, 250, 750 and 2000 mg/kg body weight per day in corn oil orally
by gavage for five consecutive days. Each male was then mated with 20
untreated females. There was no evidence of a mutagenic effect on the
post-meiotic stage of spermatogenesis at any dose level, as shown by
the absence of effect on the mean number of viable embryos during the
first four weeks of mating.
b) Intermediate chain length chlorinated paraffins
Sexually mature male Fischer-344 rats in groups of eight were
given unstabilized chlorinated paraffin (C14-17;52% Cl, CP-MH) in corn
oil by gavage once daily for 5 days at doses of 0, 500, 1500 and 5000
mg/kg body weight per day (IRDC, 1983g). Metaphase spreads of rat
bone marrow cells were examined for chromosome aberrations. No signs
of toxicity were observed during the study, and the treatments did not
produce any increase in the frequency of chromosome abnormalities,
compared to the controls.
Two studies yielding negative results in the mouse bone marrow
micronucleus assay have been reported. Sexually mature mice, of
strains CRI: CD-1 (ICR) BR (Conz & Fumero, 1988b) and C57BL/6JF
CD-1/ALpK (Elliott, 1989b) were used. Conz & Fumero (1988b) studied
Solvocaffaro C1642 (C14-17;42% Cl (CP-MH)) and Elliott (1989b) studied
Melex DC 029 (C14-17;45% Cl). In both studies groups of 10 animals:
(five males and five females) were given the limit dose of 5000 mg/kg
body weight by gavage in corn oil. Elliott (1989b) also used a lower
dose of 3125 mg/kg body weight. Responses at 5000 mg/kg were examined
at three sampling times (18, 43 and 66 h (Conz & Fumero, 1988b) or 24,
48 and 72 h (Elliott, 1989b)). Responses at the lower dose level
(Elliott, 1989b) were examined at 24 h. In both studies there were no
differences from negative control values in either polychromatic cells
with micronuclei or in the ratio of polychromatic erythrocytes to
normocytes. The positive control (mitomycin C [8 mg/kg body weight,
Conz & Fumero, 1988b] or cyclophosphamide [65 mg/kg body weight,
Elliott, 1989b]) produced the anticipated positive responses, thus
verifying the sensitivity of the test systems.
c) Long chain length chlorinated paraffins
When a long chain length paraffin with 70% chlorination (CP-LH)
(in 1% carboxymethylcellulose) was administered by gavage to
Fischer-344 rats (groups of 8) at doses of 500, 1500 and 5000 mg/kg
body weight daily for 5 days, no increased frequency of chromosome
abnormalities in bone marrow cells was observed indicating a lack of
clastogenic activity under the experimental conditions (IRDC, 1983e).
Body weight gain was decreased in the high-dose group.
Sexually mature male Fischer-344 rats in groups of eight were
given (gavage once daily for 5 days) a long chain length chlorinated
paraffin (C22-26;43% Cl, CP-LL) at doses of 500, 1500 and 5000 mg/kg
body weight per day (IRDC, 1983i). Metaphase spreads of rat bone
marrow cells were examined for chromosome aberrations. No signs of
toxicity was observed during the study, and the treatment did not
produce any increase in the frequency of chromosome abnormalities,
compared to the controls.
7.5.2.3 Cell transformation
Chlorowax 500C (C10-13;58% Cl, CP-SH), was examined in a cell
culture transformation test using baby hamster kidney (BHK) cells in
soft agar (Richold et al., 1982a). The cells were treated with doses
from 3.125 µg/ml to 500 µg/ml (-S9) and from 6.25 µg/ml to 1000 µg/ml
(+S9). Treatment with LC50 doses of 33 µg/ml (-S9) and 88 µg/ml (+S9)
increased the transformation frequency 52 times in the absence of S9
(the control was negative at 50% simulated survival) and 500 times in
the presence of S9.
Cereclor 50LV (C10-13;50% Cl, CP-SH) was not active at dose levels
up to 2500 µg/ml in a cell transformation assay using BHK cells
(Birtley et al., 1980).
Cereclor S52 (C14-17;52% Cl, CP-MH) with or without stabilizer
did not induce transformation of BHK cells at doses up to 2500 µg/ml
(Birtley et al., 1980).
After treatment of BHK cells with a long chain length paraffin
Electrofine S-70 (C20-26;70% chlorination, CP-LH) in doses from
10 µg/ml to 1000 µg/ml (+S9), a dose-related increased frequency of
transformed colonies was demonstrated at all dose levels (Richold et
al., 1982b).
Cereclor 42 (C20-30;42% Cl, CP-LL) was not active at dose levels
up to 2500 µg/ml in a cell transformation assay in BHK cells (Birtley
et al., 1980).
7.6 Long-term exposure and carcinogenicity
7.6.1 Oral route
7.6.1.1 Short chain length chlorinated paraffins
In a 2-year gavage study using Fischer-344 rats, groups of 50
males and 50 females were given C12;60% Cl, CP-SH at doses of 312 or
625 mg/kg per day, 5 days/week, for 104 weeks) (NTP, 1986a; Bucher et
al., 1987). After week 37, the body weights of the high-dose males
were reduced by 10-23% compared with controls. Survival of both
low- and high-dose males and of low-dose females was significantly
less than that of controls (at termination 27 male controls, 6
low-dose and 3 high-dose males and 34 control females, 23 low-dose and
29 high-dose females survived). Additional groups of 20 male and 20
female rats were added to each dose group for concurrent 6-month and
12-month studies. The spleen, liver, thymus, adrenal glands, brain,
kidney and heart were weighed at necropsy in the 6- and 12-month
studies. Biochemical and haematological effects were not examined.
The incidence of tumours, which was significantly increased, is
presented in Table 24. Liver neoplastic nodules and hepatocellular
carcinomas combined in males and females occurred with a positive
trend. The incidence of kidney tubular cell adenomas and the combined
incidence of adenomas and adenocarcinomas were significantly increased
in low-dose male rats. In low-dose females an increased incidence of
thyroid follicular cell adenomas was observed, and in addition,
adenomas and carcinomas combined showed an increased incidence in
high-dose female rats. Increased incidences of mononuclear cell
leukaemia, pancreatic acinar cell adenomas and endometrial stromal
polyps of the uterus (low-dose female rats) were observed. The
increased incidence of mononuclear cell leukaemia was dose-related in
males but not in females. Although the incidence of endometrial
stromal polyps in the low-dose female rats was greater than in vehicle
controls, these tumours were probably not treatment-related since
there were no increases at higher doses. The incidence of pancreatic
acinar tumours was also increased in the low-dose group of males,
although, owing to the higher incidence in concurrent compared to
historical controls and the absence of a dose-response relationship,
the increase was not considered to be treatment-related.
In the same study some chronic non-neoplastic lesions were
reported. Hepatocellular hypertrophy was observed in 74% of the
low-dose group and in nearly all of the high-dose rats, but in none
of the control group. Necrosis and angiectasis were observed in the
livers in all dosed rats. In addition to increased kidney weight at 6
and 12 months in both sexes and dose levels, the incidence and
severity of nephropathy was increased in dosed females, as was the
severity of nephropathy in males in the concurrent 12-month study.
Erosion, inflammation and ulceration of the glandular stomach and the
forestomach were seen in both groups of the dosed males. Hyperplasia
of the parathyroid was observed in both groups of exposed males and in
high-dose females.
Groups of 50 male and 50 female B6C3F1 mice were given C12;60%
Cl, CP-SH by gavage at doses of 125 and 250 mg/kg 5 days a week for
103 weeks (NTP, 1986a; Bucher et al., 1987). The body weights of
treated females were about 10% lower than those of controls during the
second year. The survival of treated males was not significantly
different from that of the controls, but in the high-dose group of
females fewer animals were still alive after week 100 in comparison
with the control group. The tumour incidences are shown in Table 25.
Increased incidences of liver adenomas and liver adenomas and
carcinomas in combination were observed. The incidences of thyroid
follicular cell adenomas and carcinomas combined were increased in
exposed female mice. The incidences of alveolar/bronchiolar
carcinomas were increased significantly in the high-dose group of male
mice, and the trend with dose was also significant. However, the
incidences of alveolar/bronchiolar carcinomas and adenomas (combined)
in males were not significantly greater than those in vehicle
controls. Among the non-neoplastic lesions, the incidence of
nephrosis was increased in the high-dose group of females, but was
decreased in dosed male mice compared to controls. Biochemical and
haematological effects were not examined.
Table 24. Incidence of tumours in rats administered the chlorinated paraffin C12;60% chlorine
(From: NTP, 1986a; Bucher et al., 1987)
Dose Hepatocellular Hepatocellular Hepatocellular Follicular cell Mononuclear Adenomas or
(mg/kg neoplastic carcinomas neoplastic nodules adenomas and cell leukaemia adeno-carcinomas
body weight) nodules and carcinomas carcinomas of of the kidney
the thyroid
Males
Control 0/50 0/50 0/50 3/50 7/50 0/50
312 10/50a 3/50a 13/50a 3/50 12/50b 9/50b
625 16/48a 2/48 16/48a 3/50 14/50b 3/49
Females
Control 0/50 0/50 0/50 0/50 11/50 0/50
312 4/50 1/50 5/50a 6/50a 22/50b 0/50
625 7/50a 1/50 7/50a 6/50a 16/50 0/50
a Incidental tumour test for trend, p < 0.05, increase relative to control
b Life table analysis, p < 0.05, increase relative to control
Table 25. Incidence of tumours in mice administered the chlorinated paraffin
C12;60% chlorine (From: NTP, 1986a; Bucher et al., 1987)
Dose Hepatocellular Hepatocellular Hepatocellular Follicular cell
(mg/kg adenomas carcinomas adenomas and adenomas and
body carcinomas carcinomas of
weight) the thyroid
Males
Control 11/50 11/50 20/50 3/49
125 20/50a 15/50 34/50a 4/50
250 29/50a 17/50 38/50a 3/49
Females
Control 0/50 3/50 3/50 8/50
125 18/50a 4/50 22/50a 12/49a
250 22/50a 9/50b 28/50a 15/49a
a Incidental tumour test for trend, p < 0.05, increase relative to control
b Life table analysis, p < 0.05, increase relative to control
7.6.1.2 Long chain length chlorinated paraffins
Groups of 50 male and 50 female Fischer-344 rats were treated by
gavage with 1875 and 3750 mg/kg body weight (male rats) and 100, 300
and 900 mg/kg body weight (female rats) of C23;43% Cl, CP-LL
dissolved in corn oil on 5 days a week for 103 weeks (NTP, 1986b;
Bucher et al., 1987). The treatment did not affect body weight or
survival, and no signs of clinical toxicity were observed. Additional
groups of 20 male and 20 female rats were exposed concurrently to the
same doses for 6 or 12 months for analyses of the weights of the
spleen, liver, thymus, adrenal glands, brain, kidneys and heart, serum
hepatic enzymes, including sorbitol dehydrogenase, AST and ALT, and
haematological parameters.
In female rats that were administered the chlorinated paraffin,
an increased incidence of adrenal gland medullary phaeochromocytomas
was observed (1/50; 4/50; 6/50; 7/50, high dose statistically
significant, significant positive trend). In addition, an increased
incidence of endometrial stromal polyps in the uterus was found in the
low-dose group of the females, but this increase was not dose-related
(9/50; 17/50; 10/50; 10/50, low dose statistically significant) (Table
26). In males, acinar cell tumours of the pancreas occurred with a
negative trend. The incidence of benign hepatocellular neoplasia was
not increased in dosed rats.
Table 26. Incidence of tumours in rats administered C23;43% Cl
(From: NTP, 1986b; Bucher et al., 1987)
Dose (mg/kg body Adrenal medulla, Uterus, endometrial
weight) phaeochromocytomas stromal polyps
Females
0 1/50 9/50
100 4/50 17/50a
300 6/50 10/50
900 7/50a 10/50
a Incidental tumour test for trend, p < 0.05, increased relative
to control
Several non-neoplastic lesions were related to the administration
of C23;43% Cl. Relative liver weights were increased in exposed
males at 12 months and in exposed females at 6 and 12 months. The
observed increases were dose-related. Activities of several serum
enzymes were also slightly elevated at both 6 and 12 months. There
were also variations in haematological parameters at 6 and 12 months,
but only in females. The primary non-neoplastic lesion related to
administration of this chlorinated paraffin included a diffuse
lymphohistiocytic inflammation in the liver and in the pancreatic and
mesenteric lymph nodes in male and female rats in all exposed groups.
Splenic congestion was a secondary effect.
Groups of 50 male and 50 female B6C3F1 mice were given, by
gavage, C23;43% Cl, CP-LL dissolved in corn oil at doses of 2500 and
5000 mg/kg on 5 days a week for 103 weeks (NTP, 1986b; Bucher et al.,
1987). The survival of the mice was not significantly different in
treated groups compared to controls, and there were no clinical signs
of toxicity. However, in the female groups a Klebsiella infection
affected the animals after week 65, and 60 to 70% of the early deaths
in each group were attributed to the infection. Low-dose males and
females had lower weight gains than control or high-dose groups. The
incidence of malignant lymphomas was significantly increased in males
of the high-dose group, and occurred with a positive trend (Table 27).
The incidences of hepatocellular carcinomas in females occurred with a
positive trend, but the increase was not significant. The incidences
of adenomas and carcinomas of the liver (combined) were marginally
increased in females. Follicular cell carcinomas in males occurred
with a positive trend (0/49; 0/48; 3/49) in the thyroid gland.
However, the incidence of follicular cell adenomas or carcinomas
(combined) was not significantly greater than that in vehicle controls
(1/49, 3/48; 5/49) and was within the range of historical controls for
the test laboratory. No significant increases in non-neoplastic
lesions were attributed to administration in mice.
Table 27. Incidence of tumours in mice administered C23;43% Cl
(From: NTP, 1986b; Bucher et al., 1987)
Dose (mg/kg Lymphoma Hepatocellular Hepatocellular
body weight) carcinomas adenomas and
carcinomas
Males
0 6/50 9/50 18/50
2500 12/50 12/50 21/50
5000 16/50a,b 12/50 23/50
Females
0 15/50 1/50 4/50
2500 12/49 1/49 3/49
5000 20/50 6/50 10/50
a Incidental tumour test for trend, p < 0.05, increased relative
to controls
b Life table test, p < 0.05, increased relative to controls
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Controlled human studies
Chlorinated paraffins C10-13;50 and 63% Cl(CP-SH) were applied,
under occlusive dressings, to the upper arm of 26 volunteers
(INVERESK, 1975). After 24 h the applications were removed and one
hour later skin reactions were examined by two independent assessors.
A second application was made and reactions were assessed after a
further 24 h contact. Mild erythema and dryness (average scores, read
at the 24 and 50 h time points, of less than 2 and 1, respectively, on
a 4-point scale) were recorded, which were comparable to scores in a
liquid paraffin control group.
Paroil 142 (C20-30;40-41% Cl, CP-LL) and Chlorez 700 (C24;70% Cl,
CP-LH) were applied to the skin of 200 male and female volunteers for
a 5-day period, then reapplied for 2 days beginning 3 weeks after the
initial exposure. The dose level was not reported. No primary local
irritation, allergic response or other toxic responses were observed.
In a similar study, Chlorowax 70 (C24;70% Cl, CP-LH), Chlorowax 500C
(C12;59% Cl, CP-SH) and Chlorowax 40 (C24;43% Cl, CP-LL) were applied
to the skin of 200 males and female volunteers. The exposure time
period and amount of chlorinated paraffins used were not reported.
The treatments did not produce local irritation or allergic responses
(Howard et al., 1975).
8.2 Occupational exposure
In a study on cutting fluid coolants, 134 non-exposed employees
and 75 exposed employees were patch tested with various constituents
of the cutting fluids including chlorinated paraffins (Menter
et al., 1975). No positive reactions were obtained with any of the
constituents, although the authors themselves suggested that the tests
were not sufficiently stringent, as some positive reactions were
anticipated for some of the constituents tested.
Positive skin reactions to chlorinated paraffin constituents were
obtained in patch tests conducted on four employees suffering from
scaly eczema, who had been exposed occupationally to cutting oils
(English et al., 1986). However, the authors concluded that the
reaction was due to additives in the cutting oil, which showed
positive reactions when tested alone, rather than to the chlorinated
paraffin.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
The inhibition of gas production in an anaerobic sewage sludge
digestion process by C10-12;58% Cl (CP-SH) was studied by Madeley et
al. (1983b). A significant (> 10%) inhibition of gas production
occurred at chlorinated paraffin concentrations of 3.2, 5.6 and 10%
(w/w with respect to digester volatile suspended solids) during the
first 3-4 days of the experiment, but the gas production recovered
during the rest of the experiment until termination at day 10. It
was concluded in the report that the chlorinated paraffin induced a
transient partial inhibition and no longer-term effects.
9.1.2 Aquatic organisms
Acute toxicity data for aquatic invertebrates and fish are
summarized in Table 28. Chlorinated paraffins of short-chain length
have been shown to be acutely toxic to both freshwater and saltwater
invertebrates. Most of the acute toxicity tests for intermediate
and long chain chlorinated paraffins on aquatic invertebrates
exceed the water solubility. However, a study on intermediate
chlorinated paraffins suggests that these may be acutely toxic.
Short, intermediate and long chain chlorinated paraffins appear to
be of low acute toxicity to fish, the LC50 values being well
in excess of the water solubility.
9.1.2.1 Aquatic plants
Exposure of the freshwater alga Selenastrum capricornutum for
10 days to C10-12;58% Cl (CP-SH) at dose levels of 110, 220, 390, 570,
900 and 1200 µg/litre resulted in a significant inhibition of growth
at 570 µg/litre. The calculated EC50 values for cell density over 4,
7 and 10 days were 3690, 1550 and 1310 µg/litre, respectively, which
all exceeded the highest tested concentration (Thompson & Madeley,
1983d).
The marine alga Skeletonema costatum was exposed to C10-12;58%
Cl (CP-SH) for 10 days at concentrations of 0, 4.5, 6.7, 12.1, 19.6,
43.1 and 69.8 µg/litre (Thompson & Madeley, 1983b). Significant
inhibition of growth was observed on the first 2 days from
19.6 µg/litre. The EC50 for cell density after 4 days was
42.3-55.6 µg/litre, and EC50 for growth rate after 2 days was
31.6 µg/litre. No significant reduction in cell density was observed
after 10 days, indicating an effect on duration of lag phase prior to
exponential growth or a drop in chlorinated paraffin concentration after
10 days.
Table 28. Acute toxicity of chlorinated paraffins to aquatic organisms
Species Chlorinated Parameter Concentration Reference
paraffin (µg/litre)
Water flea C10-12;58% Cl 48-h EC50b 530 Thompson &
Daphnia magna C10-12;58% Cl 72-h EC50b 24 Madeley
(freshwater) C10-12;58% Cl 96-h EC50b 18 (1983c)
C10-12;58% Cl 120-h EC50b 14
C14-17;52% Cl 48-h EC50b 37 Frank &
Steinhäuser
(in press)
Mysid shrimp C10-12;58% Cl 96-h LC50 14.1-15.5 Thompson &
Mysidopsis bahia Madeley
(estuarine) (1983a)
Nitocra spinipes C10-13;49% Cl 96-h LC50 100 Tarkpea
(marine C10-13;70% Cl 96-h LC50 < 300 et al.
crustacean) C14-17;45% Cl 96-h LC50 9000 (1981)
C14-17;52% Cl 96-h LC50 > 10 × 106
C22-26;42% Cl 96-h LC50 > 1 × 106
C22-26;49% Cl 96-h LC50 > 10 × 106
Bleak C10-13;49%, 96-h LC50 > 5 × 106 Lindén et
Alburnus 63%, 71% Cla al. (1979)
alburnus C10-13;56%, Cl 96-h LC50 > 10 × 106
(estuarine) C11.5;70% Cl 96-h LC50 > 10 × 106
C15.5;40% Cl 96-h LC50 > 5 × 106
C14-17;50%, 96-h LC50 > 5 × 106
52% Cla
C22-26;42% Cl 96-h LC50 > 5 × 106
C18-26;49% Cl 96-h LC50 > 5 × 106
Rainbow trout C20-30;42% Cl 96-h LC50 770 × 103 Madeley &
Oncorhynchus Birtley
mykiss (1980)
(freshwater)
a Consecutive percentage chlorinations refer to different tests
b EC50 based on immobilization
9.1.2.2 Invertebrates
Short-term toxicity data for aquatic invertebrates are summarized
in Table 29 and chronic toxicity data in Table 30.
The freshwater crustacean Daphnia magna was exposed to a short
chain length paraffin with 58% Cl (CP-SH) (Thompson & Madeley, 1983c).
The chlorinated paraffin caused the organisms to float at or near the
surface of the water at 75 µg/litre or more. All water fleas died at
16.3 µg/litre after 6 days in a continuous-flow experiment. The
following LC50 values were calculated: 3 days, 24 µg/litre; 4 days,
18 µg/litre; 5 days, 14 µg/litre; 6 to 21 days, 12 µg/litre. No dead
parent Daphnia were observed at 8.9 µg/litre after 21 days of
exposure, but 37% of the offspring were dead as compared to 6% and 9%
in the controls. No increased mortality in the offspring was observed
at 5.0 µg/litre. The number of offspring per female was reduced at
2.7 µg/litre.
Daphnia magna was studied in a 48-h test with C14-17;52% Cl and
C18-20;52% Cl. Using the water-soluble fraction of a loading
concentration of 100 mg/litre, an EC50 of 37 µg/litre for the
intermediate chain length chlorinated paraffin and an EC0 of
> 26 µg/litre for the long chain length chlorinated paraffin were
observed. In a 21-day reproduction test, daphnids were exposed to the
water-soluble fraction of both chlorinated paraffins. With a loading
of 100 mg/litre, a no-observed-effect concentration of 4.4-8.8 µg/litre
was found for reproduction rate and parent mortality (LOEC =
19.9-35.6 µg/litre) for the intermediate chain length chlorinated
paraffin. For the long chain length chlorinated paraffin, a LOEC of
< 1.2 µg/litre was found for the same two parameters. In these
studies it was observed that a higher loading concentration of
10 g/litre caused an increase in the effect concentrations (Frank &
Steinhäuser, in press).
In studies of the marine shrimp Mysidopsis bahia, the 96-h
LC50 was between 14.1 and 15.5 µg/litre after exposure to a short
chain length chlorinated paraffin with 58% Cl (CP-SH) (Thompson &
Madeley, 1983a). After 28 days of exposure to 0.6, 1.2, 2.4, 3.8 and
7.3 µg/litre, increased mortality was observed but this was not
treatment-related. The increased mortality was significantly
different from the control (at 1.2 and 2.4 µg/litre) but not from the
solvent control, and was therefore considered as not related to the
chlorinated paraffin concentration. No treatment-related effects of
this chlorinated paraffin on reproductive rate or growth over this
time period were observed.
Table 29. Short-term toxicity data for aquatic invertebrates
Chlorinated paraffin Organism LC50 Exposure Other effects LOEC References
period
C10-13;49% Cl (CP-SL) Nitocra spinipes 100 µg/litre 96 h Tarkpea et al.
(1981)
C10-12;58% Cl (CP-SH) Dapnia magna 24 µg/litre 3 days Increased mortality in 8.9 µg/litre Thompson & Madeley
offspring after 21 days (1983c)
C10-12;58% Cl (CP-SH) Mysidopsis bahia 14.1-15.5 96 h No effects after 28 days Thompson & Madeley
µg/litre of exposure up to (1983a)
7.3 µg/litre
C10-12;58% Cl (CP-SH) Midge larvae, 48 h No adverse effects EG & G Bionomics
Chironomus tentans after exposure to (1983)
18-162 µg/litre
C10-13;70% Cl (CP-SH) Nitocra spinipes < 300 µg/litre 96 h Tarkpea et al.
(1981)
C14-17;45% Cl (CP-ML) Nitocra spinipes 9000 µg/litre 96 h Tarkpea et al.
(1981)
C14-17;52% Cl (CP-MH) Nitocra spinipes > 10 × 106 96 h Tarkpea et al.
µg/litrea (1981)
Table 29. (Cont'd)
Chlorinated paraffin Organism LC50 Exposure Other effects LOEC References
period
C22-26;42% Cl (CP-LL) Nitocra spinipes > 1 × 106 96 h Tarkpea et al.
µg/litrea (1981)
C22-26;49% Cl (CP-LL) Nitocra spinipes > 10 × 106 96 h Tarkpea et al.
µg/litrea (1981)
a Exceeding the water solubility
Table 30. Chronic toxicity of chlorinated paraffins to aquatic invertebrates
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C10-12;58% Cl Water flea, 6-12 days LC50 12 Thompson & Madeley (1983c)
Daphnia magna
21 days Increased mortality in 8.9
offspring after 21 days
C10-12;58% Cl Mysid shrimp, 28 days No treatment-related 7.3 Thompson & Madeley (1983a)
Mysidopsis bahia effects
C10-12;58% Cl Midge larvae, 49 days Halting adult 121 EG & G Bionomics (1983)
Chironomus tentans emergence (100%)
C10-12;58% Cl Mussel, 60 days LC50 74 Madeley & Thompson (1983a)
Mytilus edulis
84 days Growth reduction 9.3 Thompson & Shillabeer (1983)
91 days 23% mortality during 10.1 Madeley et al. (1983a)
exposure and 10%
mortality during
depuration
C10-12;58% Cl (CP-SH) Alga, Selenastrum 10 days Inhibition of growth 570 Thompson & Madeley (1983d)
capricornutum
C10-12;58% Cl (CP-SH) Alga, Skeletonema 10 days Inhibition of growth 19.6 Thompson & Madeley (1983b)
costatum
C14-17;52% Cl (CP-MH) Mussel 60 days Decreased filtration 3800 Madeley & Thompson (1983b)
Mytilus edulis activity
Table 30. (Cont'd)
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C14-17;52% Cl Water flea, 21 days Reproduction & parent 19.9-35.6 Frank & Steinhäuser (1994)
Daphnia magna mortality (LOEC)
C18-20;52% Cl Water flea, 21 days Reproduction & parent < 1.2 Frank & Steinhäuser (1994)
Daphnia magna mortality (LOEC)
C22-26;43% Cl (CP-LL) Mussel, 60 days Decreased filtration 2.180 Madeley & Thompson (1983c)
Mytilus edulis activity
C20-30;70% Cl (CP-LH) Mussel, 60 days Decreased filtration 1330 Madeley & Thompson (1983d)
Mytilus edulis activity
Larvae of the midge Chironomus tentans (second instar) were
exposed to C10-12;58% Cl (CP-SH) concentrations from 18 to 162 µg/litre
for 48 h (EG & G Bionomics, 1983). No adverse effects could be
detected. Exposure to 61-394 µg/litre for the 49 days life cycle
gave no effects except for halting adult emergence (100%) at 121 and
394 µg/litre.
The mussel Mytilus edulis was exposed to 2.3 and 10.1 µg/litre
of C10-12;58% Cl (CP-SH) for 147 days followed by 98 days of
depuration (2.3 µg/litre) or 91 days followed by 84 days depuration
(10.1 µg/litre) (Madeley et al. 1983a). A third of the mussels in the
high-dose group died during the exposure (23%) or depuration periods
(10%), and 7% of those in the low dose group, but this did not differ
significantly from the number of deaths in the acetone control.
When the mussel Mytilus edulis was studied over 60 days after
exposure to 13, 44, 71, 130 and 930 µg/litre of C10-12;58% Cl (CP-SH),
there was significant mortality at the three highest dose levels, the
median lethal time (LT50) values being 59.3, 39.7 or 26.7 days,
respectively (Madeley & Thompson, 1983a). The highest dose exceeded
the maximal solubility of the chlorinated paraffin. The LC50 for
this 60-day period was 74 µg/litre.
Mussels (Mytilus edulis) were exposed to 2.3 and 9.3 µg/litre
of a short chain length paraffin (58% Cl) (CP-SH) in sea water for 12
weeks (Thompson & Shillabeer, 1983). There were no mortalities at
either concentration, but at 9.3 µg/litre a reduction in growth rate
was observed, measured as shell length and tissue weight.
Treatment of mussels (Mytilus edulis) for 60 days with
C14-17;52% Cl (CP-MH) at concentration of 220 and 3800 µg/litre
gave no significant mortality, but there was an observed decrease
(non-quantitative visual observation) in filtration activity at the
higher concentration (Madeley & Thompson, 1983b). The highest dose
exceeded the maximal water solubility of the chlorinated paraffin.
Treatment of mussels (Mytilus edulis) with C22-26;43% Cl (CP-LL)
at 120 or 2180 µg/litre or with C20-30;70% Cl (CP-LH) at 460 and
1330 µg/litre for 60 days did not cause mortality, but visual
observation suggested that the filtration activity was reduced at the
higher dose levels (Madeley & Thompson, 1983c,d). The highest doses
exceeded the maximal solubility of the chlorinated paraffins.
9.1.2.3 Fish
Acute toxicity data are shown in Table 28 and chronic toxicity
data for fish are summarized in Table 31.
Table 31. Chronic toxicity of chlorinated paraffins to fish
Chlorinated paraffin Organism Exposure Effect Concentration References
period (µg/litre)
C10-13;49%, 59%, Bleak, 14 days Behavioural effects 125 (single Bengtsson & Baumann Ofstad
71% Cl Alburnus alburnus concentration) (1982)
C10-12;58% Cl Sheepshead minnow, 32 days Significantly reduced 279.7 Hill & Maddock (1983)
Cyprinodon variegatus size of larvae (LOEC)
C10-12;58% Cl Rainbow trout 60 days LC50 340 Madeley & Maddock (1983c)
Oncorhynchus mykiss
60 days Behavioural abnormalities 33
168 days after 60-70 days of Madeley & Maddock (1983b)
depuration
50% mortality 3.1
100% mortality 14.3
C14-17;50% Cl Bleak, 14 days No observed effect 125 (single Bengtsson et al. (1979)
Alburnus alburnus concentration)
C14-17;52% Cl Rainbow trout, 60 days No observed effect 1050 Madeley & Maddock (1983c)
Oncorhynchus mykiss
C18-26;49% Cl Bleak, 14 days No observed effect 125 (single Bengtsson et al. (1979)
Alburnus alburnus concentration)
C20-30;43%, 70% Cl Rainbow trout, 60 days No observed effect 3800 Madeley & Maddock (1983c)
Oncorhynchus mykiss
In a 96-h study on rainbow trout (Oncorhynchus mykiss), no
toxic effects or effects on behaviour were observed after exposure
of the fish to an emulsion containing a mean concentration of
770 000 µg/litre of Cereclor 42 (C20-30;42% Cl, CP-LL) (Madeley
& Birtley, 1980).
In bleak (Alburnus alburnus) which were exposed to Witaclor 149
(C10-13;49% Cl, CP-SL), Witaclor 159 (C10-13;59% Cl, CP-SH) and Witaclor
171P (C10-13;71% Cl, CP-SH) (125 µg/litre of water) for 14 days, some
effects on behaviour, such as sluggish movements, absence of shoaling
behaviour and abnormal vertical postures were observed after 7 days
(Bengtsson et al., 1979). The effects disappeared after the fish had
been kept in clean water for 2 days. No effects on behaviour were
observed after exposure to chlorinated paraffins with intermediate or
long chain length chlorinated paraffins, Witaclor 350 (C14-17;50% Cl,
CP-MH) or Witaclor 549 (C18-26;49% Cl, CP-LL), suggesting that
behavioural toxicity is related to the carbon chain length of the
chlorinated paraffin. When bleak were given food contaminated with
590, 2500 or 5800 mg/kg Witaclor 149 or 3180 mg/kg Witaclor 171P for
91 days, effects on behaviour were noted (Bengtsson & Baumann Ofstad,
1982). After 5 weeks of exposure to the high dose of Witaclor 149,
after 7 weeks of exposure to the medium dose and after 12 weeks of
exposure to Witaclor 171P, the fish swam sluggishly and closer to the
bottom than usual. In addition, folded dorsal fins and minor balance
problems were observed. These effects gradually disappeared within 2
weeks after exposure finished.
Flounders (Platichthys flesus) of both sexes were fed Witachlor
149 (C12;49% Cl, CP-SL) or Chlorparaffin Hüls 70C (C12;70% Cl, CP-SH)
on days 1 and 4 with a total exposure of 1000 mg/kg body weight (Haux
et al., 1982). The experiment was performed in both brackish and
seawater. The fish were examined 13 and 27 days after the first
administration. The male flounders did not appear to be affected by
the two chlorinated paraffins. C12;70% Cl did not induce any
haematological responses, whereas C12;49% Cl seemed to affect the
erythrocyte balance of female fish. C12;49% Cl resulted in
hypoglycaemia in marine female fish, whereas C12;70% Cl caused
hyperglycaemia in female brackish water fish. In females exposed to
C12;49% Cl, a significant increase in benzo [a]pyrene hydroxylase
activity was observed after 27 days in brackish water. A decrease in
6ß-hydroxylase activity in marine female fish and an increase in 5
alpha,ß-reductase activity in brackish water female fish were induced
by C12;70% Cl after 13 days.
The hatchability of embryos and survival of larvae of the
sheepshead minnow (Cyprinodon variegatus) was unaffected by a 28-day
exposure to short chain length paraffin with 58% Cl (CP-SH) (2.4, 4.1,
6.4, 22.1 and 54.8 µg/litre (Hill & Maddock, 1983). The treated
minnows showed an increased larval growth compared to the acetone
control. When the larvae were exposed to 36.2, 71, 161.8, 279.7 and
620.5 µg/litre for 32 days they were significantly smaller in the two
highest exposure groups, but in the lower exposure groups (36.2 and
71 µg/litre) they were significantly larger than the controls. No
effect was seen on survival of larvae and hatchability of embryos.
In a study by Madeley & Maddock (1983c) four chlorinated paraffins
were examined for their toxicity to rainbow trout (Oncorhynchus mykiss)
after exposure for 60 days. C10-12;58% Cl was used at mean
concentration of 33, 100, 350, 1070 and 3050 µg/litre. Significant
mortality was observed with the highest three concentrations. LT50
values (mean lethal times) for these three concentrations were
calculated as 44.7, 31.0 and 30.4 days, respectively. The calculated
60-day LC50 was 340 µg/litre. Behavioural abnormalities, which
were dose-related, were also observed.
Rainbow trout (Oncorhynchus mykiss) were exposed to 3.1 and
14.3 µg/litre of C10-12;58% Cl (CP-SH) for 168 days followed by a
depuration period of up to 105 days. No deaths occurred during the
exposure period, but during days 63 to 70 of the depuration period
all of the trout exposed to 14.3 µg/litre died and there was a
significantly increased mortality (50%) among those exposed to
3.1 µg/litre (Madeley & Maddock, 1983b). The relationship to
chlorinated paraffin exposure is unknown, since the surviving fish
recovered after day 70.
Rainbow trout (Oncorhynchus mykiss) were exposed to a short
chain length paraffin (58% Cl) (CP-SH) for 168 days (Madeley &
Maddock, 1983a) at 3.4 and 17.2 µg/litre. The treatment did not cause
any significant mortality or differences in growth, but small changes
in behaviour such as increased food intake were observed (compared to
controls).
Madeley & Maddock (1983c) exposed rainbow trout (Oncorhynchus
mykiss) to 4500 and 1050 µg/litre of a chlorinated paraffin (C14-17;
52% Cl, CP-MH) for 60 days. No toxic effects were found.
In other studies rainbow trout (Oncorhynchus mykiss) were
exposed to C22-26;43% Cl (CP-LL) for 60 days at concentrations of 900
and 4000 µg/litre (Madeley & Maddock, 1983c) or to C20-30;70% Cl
(CP-LH) at 3800, 1900 and 840 µg/litre (Madely & Maddock, 1983d). No
toxic effects were found.
9.1.3 Terrestrial organisms
A one-generation reproduction study was performed with Chlorowax
500C (C10-13;58% Cl, CP-SH) on mallard ducks (Anas platyrhynchos)
(Shults et al., 1984). The ducks were fed 0, 28, 166 and 1000 mg/kg
diet for 22 weeks in groups of 20 pairs. During the treatment the
ducks were mated. The treatment had no effect on the survival,
physical condition, body weight or food consumption of the adult
ducks. Decreased egg-shell thickness and a 10% loss of 14-day embryo
viability were observed in the highest exposure group.
The acute toxicity of orally administered Cereclor S52 (C14-17;52%
Cl, CP-MH) was studied in ring-necked pheasants (Phasianus
colchicus) and mallard ducks (Anas platyrhynchos) (Madeley &
Birtley, 1980). However, no abnormal clinical signs, mortality or
effects on body weight gain were observed 14 days after a single oral
dose by gavage of up to 24 606 mg/kg body weight (pheasant) and
10 280 mg/kg body weight (duck) (five male and five female birds per
group). When the birds were fed with 1000 or 24 063 mg/kg diet of
Cereclor S52 for 5 days followed by 3 days on normal food (five males
and five females per group), no abnormalities were found other than
inferior food intake in ducks from the high-dose group.
The toxicity of Cereclor 42 (C22-26;42% Cl, CP-LL), Cereclor 50LV
(C10-13;49% Cl, CP-SL) and Cereclor 70L (C10-13;70% Cl, CP-SH) in chick
embryos was studied by Brunström (1983). The chlorinated paraffins
were injected into the yolks of eggs incubated for 4 days at
concentrations of 100 and 200 mg/kg egg (based on mean egg weights
before incubation). None of the three mixtures affected the hatching
rate, incubation time, hatching weight, weight gain after hatching or
the liver weights of the chicks.
In an extension of this study the eggs were injected with
300 mg/kg egg weight of the same Cereclors after 4 days of incubation
(Brunström, 1985). An increased liver weight was observed after
treatment with C10-13;49% Cl and C10-13;70% Cl. The microsomal
concentration of cytochrome P-450 in chick embryos was increased by
all three Cereclors. The highest concentration was observed with
C10-13;70% Cl. This Cereclor was also found to increase the microsomal
activity of APMD. The other short-chain chlorinated paraffin,
C10-13;49% Cl, produced decreased activities of aryl hydrocarbon
hydroxylase (AHH) and 7-ethoxycoumarin O-deethylase (ECOD). The
long chain length chlorinated paraffin in this study, C22-26;42% Cl,
caused decreased activities of APDM and ECOD. The greatest effects
were observed after treatment with the most highly chlorinated
short-chain paraffin, C10-13;70% Cl.
9.2 Field observations
No data concerning the effects of chlorinated paraffins in the
field have been reported.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
Information on occupational exposure to chlorinated paraffins is
very limited. The principal route of exposure is likely to be dermal,
particularly during their use as metal-working fluids. There is also
potential for the formation of inhalable aerosols during this use,
though available information is inadequate to assess exposure via this
route.
Owing to the high octanol-water partition coefficient, it is
likely that the principal source of exposure of the general population
is food, although, owing to lack of data, exposure via other routes
cannot be ruled out. It should be noted, however, that the
composition of chlorinated paraffins to which the population is
exposed in the general environment may be considerably different from
that of the commercial products, although it is not possible currently
to distinguish the chemical composition of chlorinated paraffins with
available methods of analysis. In the only survey of foodstuffs
identified, which was limited in scope, the highest concentration of
chlorinated paraffins (average level of C10-20: 300 µg/kg) was present
in dairy products (Table 15). If the daily consumption of dairy
products is assumed to be 1 kg per person, the daily intake of short
and intermediate chain length chlorinated paraffins from this source
would be 300 µg (4.3 µg/kg body weight, assuming an average body
weight of 70 kg). This is likely to be a worst-case estimate based on
the lack of specificity of the analytical method.
Exposure to chlorinated paraffins via food is also possible
through the consumption of contaminated mussels. Assuming a weekly
consumption of 1 kg of mussels, as a worst case, this would correspond
to a weekly intake of short and intermediate chain length chlorinated
paraffins of 3250 µg, based on chlorinated paraffin levels found in
mussels collected at various sites in the United Kingdom (Table 13)
(6.7 µg/kg body weight per day, assuming an average body weight of
70 kg). If the mussels are collected in highly contaminated water
(section 5.1.4) the weekly intake would be 12 000 µg, which
corresponds to 25 µg/kg body weight per day. The analytical method
used was non-specific.
10.1.2 Toxic effects
In spite of the widespread use of the chlorinated paraffins,
there have been no case reports of skin irritation or sensitization
in humans. Results from a limited number of studies on volunteers
show that chlorinated paraffins can induce minimal irritancy in
the skin but not sensitization. No other data concerning effects of
chlorinated paraffins on humans have been reported.
The acute oral toxicity of chlorinated paraffins of various chain
lengths has been well studied in experimental animals and is low.
Toxic effects such as muscular incoordination and piloerection
were most evident following single exposure to short chain length
chlorinated paraffins. On the basis of very limited data, the acute
toxicity by the inhalation and dermal routes also appears to be low.
Mild skin and eye irritation have been observed after application of
short and intermediate (skin irritation) chain length chlorinated
paraffins. Results of several studies indicate that short chain
chlorinated paraffins do not induce skin sensitization.
In repeated dose toxicity studies by the oral route, the liver,
kidney and thyroid have been shown to be the primary target organs for
the toxicity of chlorinated paraffins (Table 32). For the short chain
compounds, increases in liver and kidney weight and hypertrophy of the
liver and thyroid have been observed at lowest doses (LOEL = 100 mg/kg
body weight per day; NOEL = 10 mg/kg body weight per day; rats).
For the intermediate chain compounds, effects observed at lowest
doses are generally increases in liver and kidney weight (LOEL in rats
= 100 mg/kg body weight per day, respectively; NOAEL in rats =
10 mg/kg body weight per day). Increases in serum cholesterol and
"mild, adaptive" histological changes in the thyroid have been reported
at similar doses in female rats (NOAEL = 4 mg/kg body weight per day).
For the long chain compounds, effects observed at lowest doses
are multifocal granulomatous hepatitis and increased liver weight in
female rats (LOAEL = 100 mg/kg body weight per day).
Chlorinated paraffins do not appear to induce mutations in
bacteria. However, in mammalian cells, there is a suggestion of a
weak in vitro clastogenic potential but not in several in vivo
studies. Chlorinated paraffins are also reported to induce in vitro
cell transformation.
Table 32. Effect levels (non-carcinogenic) in repeated dose toxicity tests
Short chain length chlorinated paraffins
Rats
C10-13;58% Cl, 14-day dietary study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in relative liver weights and activities of
hepatic APDM and cytochrome P-450)
C10-13;58% Cl, 14-day gavage study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in relative liver weights);
NOEL = 30 mg/kg body weight per day
C10-13;58% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 100 mg/kg body weight per day (increase in liver/body weight ratio);
NOEL = 50 mg/kg body weight per day
C10-13;56% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 50 mg/kg body weight per day (increase in liver/body weight ratio);
NOEL = 10 mg/kg body weight per day
C12;58% Cl, 90-day gavage study in Fischer-344 rats
LOEL = 313 mg/kg body weight per day (increase in relative liver weight, hepatic
peroxisomal ß-oxidation and thyroxine-UdPG-glucuronosyltransferase; thyroid follicular
cell hypertrophy and hyperplasia and increase in replicative DNA synthesis; renal tubular
eosinophilia and increase in renal replicative DNA synthesis)
No effects in Dunkin Hartley guinea-pigs
NOEL = 1000 mg/kg body weight per day
C10-13;58% Cl, 13-week gavage study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increase in liver and kidney weights and hypertrophy
of liver and thyroid);
NOEL = 10 mg/kg body weight per day
C12;60% Cl, 90-day gavage study in Fischer-344 rats,
LOEL = 313 mg/kg body weight per day (increase in liver weight)
C12;60% Cl, 2-year gavage study in Fischer-344 rats,
LOAEL = 312 mg/kg body weight per day (increase in liver and kidney weights, hepatic
hypertrophy, necrosis and angiectasis, nephropathy (females), erosion, inflammation and
ulceration of the glandular stomach, hyperplasia of the parathyroid)
Table 32. (Cont'd)
Short chain length chlorinated paraffins (cont'd)
Mice
C10-13;58% Cl, 14-day gavage study in Alpk:APfCD mice,
LOEL = 250 mg/kg body weight per day (induction of peroxisomal fatty acid ß-oxidation and
increase in liver weight);
NOEL = 100 mg/kg body weight per day
C10-13;56% Cl, 14-day gavage study in Alpk:APfCD mice
LOEL = 100 mg/kg body weight per day (increase in liver weight);
NOEL = 50 mg/kg body weight per day
C12;60% Cl, 90-day gavage study in B6C3F1 mice,
LOEL = 250 mg/kg body weight per day (hepatocellular hypertrophy);
NOEL = 125 mg/kg body weight per day
C12;60% Cl, 2-year day gavage study in B6C3F1 mice,
LOEL = 125 mg/kg body weight per day (decrease in body weight gain (females))
Intermediate chain length chlorinated paraffins
Rats
C14-17;52% Cl, 14-day dietary study in Fischer-344 rats,
LOEL = 177 mg/kg body weight per day (slight increase in cytochrome P-450);
NOEL = 57.7 mg/kg body weight per day
C14-17;40% Cl, 14-day gavage study in Alpk:APfSD rats,
LOEL = 10 mg/kg body weight per day (increase in liver weight; not dose-related)
C14-17;52% Cl, 13-week study in Sprague-Dawley rats,
NOAEL (females) = 4.2 mg/kg body weight per day (increase in serum cholesterol; "mild,
adaptive" histopathological changes in the thyroid - reduced follicle sizes and collapsed
angularity; increased height, cytoplasmic vacuolation and nuclear vesiculation);
NOEL = 0.4 mg/kg body weight per day
C14-17;52% Cl, 90-day dietary study in Wistar rats,
LOEL (females) = 25 mg/kg body weight per day (increases in liver and kidney weights and
proliferation of smooth endoplastic reticulum);
NOEL = 12.5 mg/kg body weight per day
C14-17;52% Cl, 90-day dietary study in Fischer-344 rats,
LOEL = 100 mg/kg body weight per day (increased liver and kidney weights);
NOEL = 10 mg/kg body weight per day
Table 32. (Cont'd)
Intermediate chain length chlorinated paraffins (cont'd)
Mice
C14-17;40% Cl, 14-day gavage study in Alpk:APfCD-1 mice,
LOEL = 500 mg/kg body weight (induction of peroxisomal fatty acid ß-oxidation);
NOEL = 250 mg/kg body weight per day
Dogs
C14-17;52% Cl, 90-day dietary study in Beagle dogs,
LOEL (males) = 30 mg/kg body weight per day (increased liver and kidney weights)
NOEL = 10 mg/kg body weight per day (increased smooth endoplasmic reticulum)
Long chain length chlorinated paraffins
Rats
C22-26;70% Cl, 14-day dietary study in Fischer-344 rats,
NOEL = 1715 mg/kg body weight per day (no effects at any dose level)
C22-26;43% Cl, 14-day dietary study in Charles River rats,
NOEL = 3000 mg/kg body weight per day (no effects at any dose; observation of
nephrolithiasis in females exposed to 3000 mg/kg body weight per day)
C23;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL (females) = 235 mg/kg body weight per day (granulomatous inflammation in the liver)
C20-30;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL (females) = 100 mg/kg body weight per day (multifocal granulomatous hepatitis and
increased liver weight)
C22-26;70% CL, 90-day dietary study in Fischer-344 rats,
LOAEL = 3750 mg/kg body weight per day (increased liver weight, hepatocellular hypertrophy
and cytoplasmic fat vacuolation; slight increase of chronic nephritis (males); increase in
ALT and AST (females));
NOEL = 900 mg/kg body weight per day
C23;43% Cl, 90-day gavage study in Fischer-344 rats,
LOAEL = 100 mg/kg body weight per day (increase in relative liver weights; increased
activities of serum enzymes, variations in haematological parameters (in females); diffuse
lymphohistiocytic inflammation in the livers, pancreatic and mesenteric lymph nodes)
Table 32. (Cont'd)
Long chain length chlorinated paraffins (cont'd)
Mice
C23;43% Cl, 90-day gavage,
NOEL = 7500 mg/kg body weight per day (no effects at any dose)
C23;43% Cl, 90-day gavage,
NOEL = 5000 mg/kg body weight per day (no effects at any dose)
Two-year toxicity and carcinogenicity studies have been conducted
for a short (C12;60% Cl) and a long chain chlorinated paraffin
(C23;43% Cl) in both rats and mice. For the short chain chlorinated
paraffin, the incidences of liver and thyroid tumours were increased
in mice at 125 and 250 mg/kg body weight per day. In rats, the
incidences of liver tumours in both sexes, thyroid tumours in females
and renal cell tumours and leukaemias in males were increased at doses
of 312 and 625 mg/kg body weight per day. For the long chain
chlorinated paraffin, the incidences of malignant lymphomas in male
mice (2500-5000 mg/kg body weight per day) and phaeochromocytomas in
female rats (900 mg/kg body weight per day) were increased. On the
basis of available data, therefore, the short chain chlorinated
paraffin was carcinogenic in rats and mice. No data are available on
intermediate chlorinated paraffins. For the long chain chlorinated
paraffin, the evidence of carcinogenicity is limited, increased
incidences of commonly occurring tumours having been observed at only
one site in one sex of each species.
Some possible mechanisms for the induction of tumours of the
thyroid, liver and kidney have been suggested. On the basis of 14-day
mouse, rat and guinea-pig studies (Wyatt et al., 1993; Elcombe et al.,
in press), with similar short chain and long chain chlorinated
paraffins as used in the carcinogenesis bioassays, it was suggested
that the liver tumours in mice and rats could be correlated with the
degree of peroxisomal proliferation which occurs in the liver of these
species at similar dose levels (250 mg/kg body weight per day). No
peroxisome proliferation was observed in the livers of guinea-pigs
even at doses up to 1 to 2 g/kg body weight per day, although a
similar increase in liver weight to that seen in mice and rats was
observed. No study on peroxisome proliferation in human hepatoctyes
treated with a short chain chlorinated paraffin is available.
Increased DNA synthesis has been demonstrated in hepatocytes and
thyroid follicular cells in rats of both sexes and in proximal kidney
tubular cells in males. Perturbation of thyroid homeostasis and
increased TSH secretion has been observed in the thyroid of rats.
These effects may be related to the development of tumours.
Taking into consideration the available data, it appears that
chlorinated paraffins do not mediate carcinogenic effects via direct
interaction with DNA.
In a reproduction study, no adverse reproductive effects were
reported following exposure of rats to an intermediate chain length
chlorinated paraffin with 52% chlorine. However, survival and body
weight of the exposed pups were reduced (LOEL for non-significant
decrease in body weight = 5.7 to 7.2 mg/kg body weight per day; LOAEL
for decreased survival = 58.7 to 70 mg/kg body weight per day). In a
limited number of studies on the developmental effects of the short,
medium and long chain chlorinated paraffins, adverse effects in
offspring were observed, for the short chain compounds only, at
maternally toxic doses in rats.
10.1.3 Risk evaluation
Available data indicate that absorption of chlorinated paraffins
through the skin (the likely principal route of exposure in the
occupational environment) is minimal. Provided that proper personal
hygiene and safety procedures are followed, the risk to the health of
workers exposed to chlorinated paraffins is expected to be minimal.
10.1.3.1 Short chain compounds
On the basis of available data on repeated dose toxicity, a
Tolerable Daily Intake (TDI) for non-neoplastic effects of short chain
chlorinated paraffins for the general population can be developed:
10 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
100
where 10 mg/kg body weight per day is the lowest reported
no-observed-effect level (increases in liver and kidney weights
and hypertrophy of the liver and thyroid at the next highest dose
in a 13-week study on rats) (IRDC, 1984a); and 100 is the
uncertainty factor (× 10 for interspecies variation; × 10 for
intraspecies variation).
On the basis of multistage modelling of the tumours with highest
incidence (hepatocellular adenomas or carcinomas (combined) in male
mice) in the carcinogenesis bioassay with short chain chlorinated
paraffins, the estimated dose associated with a 5% increase in tumour
incidence is 11 mg/kg body weight per day (amortized for period of
administration). After dividing this value by 1000 (uncertainty
factor for a non-genotoxic carcinogen), it can be recommended that
daily doses of short chain chlorinated paraffins for the general
population should not exceed 11 µg/kg body weight, on the basis of
neoplastic effects.
10.1.3.2 Intermediate chain compounds
On the basis of available data on repeated dose toxicity, a TDI
for non-neoplastic effects of intermediate chain chlorinated paraffins
can be developed:
10 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
100
where 10 mg/kg body weight per day is the no-observed-adverse-effect
level in both sexes (increases in liver and kidney weights at the next
highest dose) (IRDC, 1984b); increases in serum cholesterol and "mild,
adaptive" histological changes in the thyroid have been reported at
similar doses in female rats (NOAEL = 4 mg/kg body weight per day)
(Poon et al., in press); and 100 is the uncertainty factor (× 10 for
interspecies variation; × 10 for intraspecies variation).
10.1.3.3 Long chain compounds
On the basis of available data on repeated dose toxicity, a TDI
for non-neoplastic effects of long chain chlorinated paraffins can be
developed:
100 mg/kg body
weight per day
TDI = = 100 µg/kg body weight per day
1000
where 100 mg/kg = the lowest-observed-adverse-effect levels in
long-term studies (effects at this dose were multifocal granulomatous
hepatitis and increased liver weight in female rats (NTP, 1986b;
Serrone et al., 1987; Bucher et al., 1987); and 1000 = uncertainty
factor (× 10 for intraspecies variation, × 10 for interspecies
variation, × 10 for LOAEL rather than NOEL).
In general, the calculated daily intake of chlorinated paraffins
based on highly unlikely worst-case scenarios are below the TDIs for
non-neoplastic effects or recommended values for neoplastic effects
(short chain compounds) developed above.
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
Data on chlorinated paraffin levels in the environment are
limited, but the studies reported indicate widespread contamination,
although the highest levels are found close to industries that
manufacture or use chlorinated paraffins.
Chlorinated paraffins bioaccumulate in aquatic organisms, and
bioconcentration factors (BCFs) in the range of 7 to 7155 for fish and
223 to 138 000 for mussels have been reported. In fish, chlorinated
paraffins of short chain length are accumulated to a higher degree
than those of intermediate and long chain length.
In the environment, chlorinated paraffins are persistent.
However, short-chain chlorinated paraffins with a low chlorine content
appear to be degraded by acclimated microorganisms.
10.2.2 Toxic effects
Short chain length chlorinated paraffins are acutely toxic to
freshwater and saltwater invertebrates at LC/EC50 concentrations
ranging from 14 to 530 µg/litre. The acute toxicity of short chain
chlorinated paraffins to fish is low. In long-term studies, the
lowest-observed-effect concentrations for algae, daphnids and fish
ranged from 3 to 20 µg/litre; NOECs appear to range from 2 to
5 µg/litre for the most sensitive species tested. The acute and
long-term toxicity of intermediate and long chain length chlorinated
paraffins to fish appears to be low. However, in daphnids, chronic
effects of an intermediate and a long-chain product have been observed
at similar concentrations to those reported for short chain compounds.
No studies on plants or terrestrial invertebrates have been
reported. The acute toxicity to birds is low. Data from studies on
laboratory mammals suggest a low risk for terrestrial mammals.
10.2.3 Risk evaluation
The evaluation of the environmental risks of chlorinated
paraffins is complicated by the limited quality and quantity of
information regarding environmental levels. Available data indicate
that chlorinated paraffins are bioaccumulative and persistent.
The data on environmental levels of short-chain chlorinated
paraffins indicate that in areas local to release sources, there is a
risk to both freshwater and estuarine organisms. Recent data indicate
that there is also a potential risk to aquatic invertebrates from
intermediate and long chain chlorinated paraffin products.
The enrichment of chlorinated paraffins in sediments, their
resorption behaviour and aquatic toxicity indicate a potential risk
for sediment-dwelling organisms.
The data regarding chlorinated paraffins in the terrestrial
environment are insufficient to estimate the risk to soil-dwelling
organisms.
11. RECOMMENDATIONS FOR PROTECTION OF THE ENVIRONMENT
Since chlorinated paraffins are bioaccumulative and toxic to
environmental organisms and owing to difficulties in monitoring
environmental levels, it is recommended that use and disposal of these
compounds should be controlled to avoid release to the environment.
12. FUTURE RESEARCH
The following studies need to be undertaken:
a) development of more selective and sensitive methods of analysis
in order to provide more reliable data on present and future
levels of chlorinated paraffins in the occupational environment,
soil, water, sediments, foodstuffs and human tissues;
b) further investigation of the influence of the chain lengths and
degrees of chlorination on toxicodynamics and toxicokinetics of
chlorinated paraffins, with particular regard to the relative
extent and rate of absorption and excretion through different
routes; studies to ascertain the metabolic pathways of
chlorinated paraffins should also be performed;
c) investigation of the toxicokinetics and half-lives of chlorinated
paraffins in mammals;
d) studies on perinatal toxicity;
e) further studies to examine effects on sediment-dwelling
organisms.
13. PREVIOUS EVALUATION BY INTERNATIONAL ORGANIZATIONS
Chlorinated paraffins have been evaluated by the International
Agency for Research on Cancer (IARC, 1990). It was concluded that
there is sufficient evidence for the carcinogenicity of a commercial
chlorinated paraffin product of average carbon chain length C12 and
average degree of chlorination of 60% in experimental animals, and
limited evidence for the carcinogenicity of a commercial chlorinated
paraffin product of average carbon chain length C23 and average
degree of chlorination of 43% in experimental animals. The overall
evaluation was that chlorinated paraffins of average carbon chain
length C12 and average degree of chlorination of approximately 60%
are possibly carcinogenic to humans (Group 2B).
REFERENCES
Åhlman M, Bergman Å, Darnerud PO, Egestad B, & Sjövall J (1986)
Chlorinated paraffins: formation of sulphur-containing metabolites of
polychlorohexadecane in rats. Xenobiotica, 16: 225-232.
Ahotupa M, Hietanen E, Mantyla E, & Vainio H (1982) Effects of
polychlorinated paraffins on hepatic, renal and intestinal
biotransformation rates in comparison to the effects of poly-
chlorinated biphenyls and naphthalenes. J Appl Toxicol, 2: 47-53.
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL, &
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environ Mol Mutagen, 16(Suppl 18):
55-137.
Ashby J, Lefevre PA, & Elcombe CR (1990) Cell replication and
unscheduled DNA synthesis (UDS) activity of low molecular weight
chlorinated paraffins in the rat liver in vivo. Mutagenesis,
5: 515-518.
Bengtsson BE & Baumann Ofstad E (1982) Long-term studies on uptake and
elimination of some chlorinated paraffins in the bleak, Alburnus
alburnus. Ambio, 11: 38-40.
Bengtsson BE, Svanberg O, Lindén E, Lunde G, & Baumann Ofstad E (1979)
Structure related uptake of chlorinated paraffins in bleaks ( Alburnus
alburnus L). Ambio, 8: 121-122.
Bergman Å, Hagman A, Jacobsson S, Jansson B, & Åhlman M (1984) Thermal
degradation of polychlorinated alkanes. Chemosphere, 13: 237-250.
Biessmann A, Brandt I, & Darnerud PO (1982) Comparative distribution
and metabolism of two carbon-14 labeled chlorinated paraffins in
Japanese quail (Coturnix coturnix japonica). Environ Pollut,
A28: 109-120.
Biessmann A, Darnerud PO, & Brandt I (1983) Chlorinated paraffins:
disposition of a highly chlorinated polychlorohexadecane in mice and
quail. Arch Toxicol, 53: 79-86.
Birtley RDN, Conning DM, Daniel JW, Ferguson DM, Longstaff E, & Swan
AAB (1980) The toxicological effects of chlorinated paraffins in
mammals. Toxicol Appl Pharmacol, 54: 514-525.
Brunström B (1983) Toxicity in chick embryos of three commercial
mixtures of chlorinated paraffins and of toxaphene injected into eggs.
Arch Toxicol, 54: 353-357.
Brunström B (1985) Effects of chlorinated paraffins on liver weight,
cytochrome P-450 concentration and microsomal enzyme activities in
chick embryos. Arch Toxicol, 57: 69-71.
BUA (German Chemical Society Advisory Committee on Existing Chemicals
of Environmental Relevance) (1992) [Chlorinated paraffins.] Weinheim,
VCH Verlagsgesellschaft, 227 pp (BUA Report 93) (in German).
Bucher JR, Alison RH, Montgomery CA, Huff J, Haseman JK, Farnell
D, Thompson R, & Prejean JD (1987) Comparative toxicity and
carcinogenicity of two chlorinated paraffins in F344/N rats and
B6C3F1 mice. Fundam Appl Toxicol, 9: 454-468.
Camford Information Services (1991) CPI product profiles: chlorinated
paraffins. Don Mills, Ontario, Camford Information Services, 3 pp.
Campbell I & McConnell G (1980) Chlorinated paraffins and the
environment. 1. Environmental occurrence. Environ Sci Technol,
14: 1209-1214.
Conz A & Fumero S (1988a) Study of the capacity of Solvocaffaro C1642
to induce gene mutations in strains of Salmonella typhimurium.
Turin, Istituto di Recerche Biomediche "Antoine Marxer", 37 pp (RBM
Exp. No. 880367).
Conz A & Fumero S (1988b) Micronucleus induction in bone marrow cells
of mice treated by oral route with the test article Solvocaffaro
C1642. Turin, Istituto di Recerche Biomediche "Antoine Marxer", 20 pp
(RBM Exp. No. 880368).
Cooke M & Roberts DJ (1980) Carbon skeleton capillary gas
chromatography. J Chromatogr, 193: 437-443.
Darnerud PO (1984) Chlorinated paraffins: Effect of some microsomal
enzyme inducers and inhibitors of the degradation of 1-14C-chloro-
dodecanes to 14CO2 in mice. Acta Pharmacol Toxicol,
55: 110-115.
Darnerud PO & Brandt I (1982) Studies on the distribution and
metabolism of a 14C-labelled chlorinated alkane in mice. Environ
Pollut, A27: 45-56.
Darnerud PO & Lundkvist U (1987) Studies on implantation and embryonic
development in mice given a highly chlorinated hexadecane. Pharmacol
Toxicol, 60: 239-240.
Darnerud PO, Biessmann A, & Brandt I (1982) Metabolic fate of
chlorinated paraffins: degree of chlorination of [1-14C]-chloro-
dodecanes in relation to degradation and excretion in mice. Arch
Toxicol, 50: 217-226.
Darnerud PO, Bengtsson BE, Bergman A, & Brandt I (1983) Chlorinated
paraffins: disposition of a polychloro-[1-14C]-hexadecane in carp
(Cyprinus carpio) and bleak (Alburnus alburnus). Toxicol Lett,
19: 345-351.
Darnerud PO, Bergman Å, Lund BO, & Brandt I (1989) Selective
accumulation of chlorinated paraffins (C12 and C16) in the olfactory
organ of rainbow trout. Chemosphere, 18: 1821-1827.
EG & G Bionomics (1983) The acute and chronic toxicity of a chlorinated
paraffin (58% chlorination of short chain length n-paraffins) to
midges (Chironomus tentans). Wareham, Massachusetts, EG & G Bionomics,
39 pp (Report No. BW 83-6-1426).
Elcombe CR, Watson SC, Wyatt I, & Foster JR (1994) Chlorinated
paraffins (CP): Mechanisms of carcinogenesis. Toxicologist, 14: 276.
Elcombe CR, Bars RG, Watson SC, & Foster JR (in press) Hepatic effects
of chlorinated paraffins in mice, rats and guinea pigs: Species
differences and implications for hepatocarcinogenicity. Xenobiotica.
Elliott BM (1989a) Meflex DC029 (fully formulated) - Ames test.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 1 p (Report
No. CTL/L/2668).
Elliott BM (1989b) Meflex DC029 (fully formulated) - Mouse
micronucleus test. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 1 p (Report No. CTL/L/2693).
English JSC, Foulds I, White IR, & Rycroft JG (1986) Allergic contact
sensitization to the glycidyl ester of hexahydrophthalic acid in a
cutting oil. Contact Dermatitis, 15: 66-69.
Environment Agency, Japan (1981) Environmental monitoring of chemicals:
Annual report. Tokyo, Environment Agency, p 23.
Environment Agency, Japan (1983) Environmental monitoring of chemicals:
Annual report. Tokyo, Environment Agency, p 75.
Eriksson P & Darnerud PO (1985) Distribution and retention of some
chlorinated hydrocarbons and a phthalate in the mouse brain during the
pre-weaning period. Toxicology, 37: 189-203.
Eriksson P & Kihlström J (1985) Disturbance of motor performance and
thermoregulation in mice given two commercial chlorinated paraffins.
Bull Environ Contam Toxicol, 34: 205-209.
Eriksson P & Nordberg A (1986) The effects of DDT, DDOH-palmitic
acid, and a chlorinated paraffin on muscarinic receptors and the
sodium-dependent choline uptake in the central nervous system of
immature mice. Toxicol Appl Pharmacol, 85: 121-127.
Frank U & Steinhäuser KG (in press) [Ecotoxicity of poorly soluble
substances tested by Daphnia toxicity of chlorinated paraffins.] Vom
Wasser, 83 (in German).
Friedman D & Lombardo P (1975) Photochemical technique for the
elimination of chlorinated aromatic interferences in the gas-liquid
chromatographic analysis for chlorinated paraffins. J Assoc Off Anal
Chem, 58: 703-706.
Gjos N & Gustavsen K (1982) Determination of chlorinated paraffins by
negative ion chemical ionization mass spectrometry. Anal Chem,
54: 1316-1318.
Hansen J, Schneider T, Olsen JH, & Laursen B (1992) Availability of
data on humans potentially exposed to suspected carcinogens in the
Danish working environment. Pharmacol Toxicol, 72(Suppl 1): S77-S85.
Hardie DWF (1964) Chlorocarbons and chlorohydrocarbons: Chlorinated
paraffins. In: Kirk-Othmer encyclopedia of chemical technology. New
York, John Wiley and Sons, vol 5, pp 231-240.
Haux C, Larsson Å, Lidman U, Förlin L, Hansson T, & Johansson-Sjöbeck
M-L (1982) Sublethal physiological effects of chlorinated paraffins on
the flounder, Platichtys flesus L. Ecotoxicol Environ Saf, 6: 49-59.
Hill RW & Maddock BG (1983) Effect of a chlorinated paraffin (58%
chlorination of short chain length n-paraffins) on embryos and
larvae of the sheepshead minnow (Cyprinodon variegatus)-(study one).
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 57 pp
(Report No. BL/B/2326).
Hoechst (1983a) [Chloroparaffin 70 liquid NV - Acute dermal
irritation/corrosion in rabbits.] Frankfurt/Main, Hoechst AG, 11 pp
(Report No. 83.0444) (in German).
Hoechst (1983b) [Hordalub 80 + TNPP - Magnusson and Kligman's test for
sensitising properties on Pirbright-White Guinea Pigs.] Frankfurt/Main,
Hoechst AG, 17 pp (Report No. 83.0573) (in German).
Hoechst (1984) [Hordalub 80 HT - Magnusson and Kligman's test for
sensitising properties on Pirbright-White Guinea Pigs.] Frankfurt/Main,
Hoechst AG, 18 pp (Report No. 84.0458) (in German).
Hoechst (1986a) Hordalub 80 - Study of the mutagenic potential in
strains of Salmonella typhimurium (Ames Test) and Escherichia coli.
Frankfurt/Main, Hoechst AG, 28 pp (Report No. 86.1078).
Hoechst (1986b) [Acute dermal irritation/corrosion in rabbits.]
Frankfurt/Main, Hoechst AG, 12 pp (Report No. 86.1105) (in German).
Hoechst (1987) Chloroparaffin 56 liquid - Detection of gene mutations
in somatic cells in culture. HGPRT-test with V79 cells. Frankfurt/Main,
Hoechst AG, 23 pp (Report No. 87.1719).
Hoechst (1988) Chloroparaffin 56 liquid (unstabilized) - Study of the
mutagenic potential in strains of Salmonella typhimurium (Ames Test)
and Escherichia coli. Frankfurt/Main, Hoechst AG, 30 pp (Report No.
88.0099).
Hoechst (1989) Chlorowax 500C micronucleus test in male and female
NMRI mice after oral administration. Frankfurt/Main, Hoechst AG, 24 pp
(Report No. 89.0253).
Hollies JI, Pinnington DF, Handley AJ, Baldwin MK, & Bennett D (1979)
The determination of chlorinated long-chain paraffins in water,
sediment and biological samples. Anal Chim Acta, 111: 201-213.
Houghton KL (1993) Chlorinated paraffins. In: In: Kirk-Othmer
encyclopedia of chemical technology. New York, John Wiley and Sons,
vol 6, pp 78-87.
Howard PH, Santodonato J, & Saxena J (1975) Investigations of selected
potential environmental contaminants: chlorinated paraffins. Syracuse,
New York, Syracuse University Research Corporation, 107 pp (Report No.
68-01-3101).
HSE (1992) Chlorinated paraffins. London, Health and Safety Executive,
44 pp (Unpublished document).
IARC (1990) Chlorinated paraffins. In: Some flame retardants and
textile chemicals, and exposures in the textile manufacturing
industry. Lyon, International Agency for Research on Cancer, pp 55-72
(IARC Monographs on the Evaluation of Carcinogenic Risks to Humans,
Volume 48).
ICI (1965) Toxicological report: chlorinated hydrocarbons with added
stabilizers: "Cereclor" P70 and 70L. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 15 pp (Report No. CTL/TR/464).
ICI (1966) Toxicological report: 40% chlorinate of C10-13 normal
paraffin. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
4 pp (Report No. CTL/TR/524).
ICI (1967) Toxicological report: "Cereclor" 50LV (MD.115) - 50%
chlorinate of C10-13 normal paraffin. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 4 pp (Report No. CTL/TR/618).
ICI (1968) Toxicological report: fire-resistant hydraulic fluid
10B/1067. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
7 pp (Report No. CTL/TR/635).
ICI (1969) Toxicological report: skin irritancy and oral toxicity of
chlorinated paraffins. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 11 pp (Report No. CTL/TR/691).
ICI (1971) Toxicological report: fire-resistant hydraulic fluid
45D-3271. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
8 pp (Report No. CTL/T/831).
ICI (1973) "Cereclor" 63L: local irritancy and acute oral toxicity.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 7 pp (Report
No. CTL/T/938).
ICI (1974a) "Cereclors" and "Hordolub": local irritancy and acute
toxicity. Macclesfield, Cheshire, Imperial Chemical Industries Ltd,
32 pp (Report No. CTL/T/962).
ICI (1974b) "Cereclor" 50 HS: summary of local irritancy, skin
sensitization and acute toxicity. Macclesfield, Cheshire, Imperial
Chemical Industries Ltd, 3 pp (Report No. CTL/Z/0548).
ICI (1975a) "Cereclor" 60 HS: primary skin irritation in rabbits.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 3 pp (Report
No. CTL/Z/0813).
ICI (1975b) "Cereclor" 50 HS: primary skin irritation in rabbits.
Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 3 pp (Report
No. CTL/Z/0812).
ICI (1980) Cloparin 1049 and "Meflex" DC029: a comparison of skin
irritation potential. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd, 11 pp (Report No. CTL/T/1431).
ICI (1982a) Cell transformation test for potential carcinogenicity of
chlorinated paraffin (58% chlorination of short chain length
n-paraffins). Macclesfield, Cheshire, Imperial Chemical Industries
Ltd.
ICI (1982b) Cell transformation test for potential carcinogenicity
of chlorinated paraffin (70% chlorination of short chain length
n-paraffins). Macclesfield, Cheshire, Imperial Chemical Industries
Ltd.
ICI (1982c) Cloparin 59: skin irritation study. Macclesfield,
Cheshire, Imperial Chemical Industries Ltd, 14 pp (Report No.
CTL/T/1737).
Inveresk (1975) Patch testing of textile lubricants. Musselburgh,
Inveresk Research International, 14 pp (Report No. 318).
IRDC (1981a) 14-Day oral toxicity study in rats. Chlorinated paraffin:
58% chlorination of short chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 110 pp
(Report No. 438-006).
IRDC (1981b) 14-Day oral toxicity study in rats. Chlorinated paraffin:
52% chlorination of long chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 104 pp
(Report No. 438-005).
IRDC (1982a) Teratology study in rats. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 100 pp (Report
No. 438-016).
IRDC (1982b) 14-Day oral toxicity study in rats. Chlorinated paraffin:
70% chlorination of long chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 103 pp
(Report No. 438/004).
IRDC (1982c) 14-Day oral (gavage) toxicity study in rats. Chlorinated
paraffin: 43% chlorination of long chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
110 pp (Report No. 438/005).
IRDC (1982d) Teratology study in rabbits. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 95 pp (Report
438-031).
IRDC (1982e) Teratology study in rabbits. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 102 pp (Report
438-030).
IRDC (1983a) Dominant lethal study in rats. Chlorinated paraffin: 58%
chlorination of short chain n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 54 pp (Report
No. 438-011).
IRDC (1983b) Teratology study in rabbits. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 245 pp (Report
No. 438-045).
IRDC (1983c) 14-Day dietary range-finding study in rats. Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
136 pp (Report No. 438-002).
IRDC (1983d) Teratology study in rats. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 197 pp (Report
No. 438-015).
IRDC (1983e) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 70% chlorination of long chain
length paraffins. Mattawan, Michigan, International Research and
Development Corporation, 70 pp (Report No. 438-016).
IRDC (1983f) Teratology study in rabbits. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 195 pp
(Report No. 438-032).
IRDC (1983g) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 52% chlorination of intermediate
chain length n-paraffins. Mattawan, Michigan, International Research
and Development Corporation, 62 pp (Report No. 438-014).
IRDC (1983h) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffins: 58% chlorination of short chain
length n-paraffins. Mattawan, Michigan, International Research and
Development Corporation, 58 pp (Report No. 438-013).
IRDC (1983i) In vivo cytogenetic evaluation by analysis of rat bone
marrow cells. Chlorinated paraffin: 43% chlorination of long chain
length n-paraffins. Mattawan, Michigan, International Research and
Development Corporation, 56 pp (Report No. 438-012).
IRDC (1984a) 13-week oral (gavage) toxicity study in rats with
combined excretion, tissue level and elimination studies: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 58% chlorination of short
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 350 pp (Report
No. 438-029/022).
IRDC (1984b) 13-week oral (dietary) toxicity study in rats with
combined excretion, tissue level and elimination studies: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 52% chlorination of
intermediate chain length n-paraffins, 14C labeled CP. Mattawan,
Michigan, International Research and Development Corporation, 328 pp
(Report No. 438-023/026).
IRDC (1984c) 13-week oral (dietary) toxicity study in rats with
combined excretion, tissue level and elimination study: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 58% chlorination of
short chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 435 pp (Report
No. 438-035/022).
IRDC (1984d) Teratology study in rats. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Mattawan,
Michigan, International Research and Development Corporation, 105 pp
(Report No. 438-017).
IRDC (1984e) Teratology study in rats. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Mattawan, Michigan,
International Research and Development Corporation, 99 pp (Report
No. 438/045).
IRDC (1984f) 13-week oral (gavage) toxicity study in rats with
combined excretion, tissue level and elimination study: determination
of excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 43% chlorination of long
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 275 pp (Report
No. 438-028/021).
IRDC (1984g) 13-week dietary toxicity study in rats with combined
excretion, tissue level and elimination studies/determination of
excretion, tissue level and elimination after single oral (gavage)
administration to rats. Chlorinated paraffin: 70% chlorination of long
chain length n-paraffins; 14C labeled CP. Mattawan, Michigan,
International Research and Development Corporation, 316 pp (Report
No. 438-027/024).
IRDC (1985) Reproduction range-finding study in rats. Chlorinated
paraffin: 52% chlorination of intermediate chain length n-paraffins.
Mattawan, Michigan, International Research and Development Corporation,
179 pp (Report No. 438-049).
Jansson B, Andersson R, Asplund L, Bergman Å, Litze'n K, Nylund K,
Reuthergårdh L, Sellström U, Uvemo U-B, Wahlberg C, & Wideqvist U
(1991) Multiresidue method for the gas-chromatographic analysis of
some polychlorinated and polybrominated pollutants in biological
samples. Fresenius J Anal Chem, 340: 439-445.
Jansson B, Andersson R, Asplund L, Litze'n K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Environ Toxicol Chem, 12: 1163-1174.
KEMI (1991) Chlorinated paraffins. In: Freij L ed. Risk reduction of
chemicals: A Government Commission Report. Solna, The Swedish National
Chemicals Inspectorate, pp 167-187 (KEMI Report 1/91).
Kraemer W & Ballschmiter K (1987) Detection of a new class of
organochlorine compounds in the marine environment: the chlorinated
paraffins. Fresenius Z Anal Chem, 327: 47-48.
Lindén E, Bengtsson BE, Svanberg O, & Sundström G (1979) The acute
toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
Lombardo P, Dennison JL, & Johnson WW (1975) Bioaccumulation of
chlorinated paraffin residues in fish fed Chlorowax 500C. J Assoc Off
Anal Chem, 58: 707-710.
Lundberg P (1980) Effects of some flame retardants on the liver
microsomal enzyme systems. In: Cohn MJ ed. Microsomes, drug
oxidations, and chemical carcinogenesis, 1979. New York, Academic
Press, pp 853-856.
Lunde G & Steinnes E (1975) Presence of lipid-soluble chlorinated
hydrocarbons in marine oils. Environ Sci Technol, 9: 155-157.
Madeley J & Birtley R (1980) Chlorinated paraffins and the
environment. 2. Aquatic and avian toxicology. Environ Sci Technol,
14: 1215-1221.
Madeley JR & Gillings E (1983) Determination of the solubility of four
chlorinated paraffins in water. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 23 pp (Report No. BL/B/2301).
Madeley JR & Maddock BG (1983a) Effects of a chlorinated paraffin on
the growth of rainbow trout. Chlorinated paraffin: 58% chlorination of
short chain length paraffins. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 67 pp (Report No. BL/B/2309).
Madeley JR & Maddock BG (1983b) The bioconcentration of a chlorinated
paraffin in the tissues and organs of rainbow trout (Salmo gairdneri).
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 39 pp
(Report No. BL/B/2310).
Madeley JR & Maddock BG (1983c) Toxicity of a chlorinated paraffin to
rainbow trout over 60 days. Chlorinated paraffin: 52% chlorination of
intermediate chain length n-paraffins. Brixham, Imperial Chemical
Industries Ltd, Brixham Laboratory, 69 pp (Report No. BL/B/2202).
Madeley JR & Maddock BG (1983d) Toxicity of a chlorinated paraffin to
rainbow trout over 60 days. Chlorinated paraffin: 43% chlorination of
long chain length n-paraffins. Brixham, Imperial Chemical Industries
Ltd, Brixham Laboratory, 71 pp (Report No. BL/B/2201).
Madeley JR & Thompson RS (1983a) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 58%
chlorination of short chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 71 pp (Report No.
BL/B/2291).
Madeley JR & Thompson RS (1983b) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 52%
chlorination of intermediate chain length n-paraffins. Brixham,
Imperial Chemical Industries Ltd, Brixham Laboratory, 53 pp (Report
No. BL/B/2289).
Madeley JR & Thompson RS (1983c) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 43%
chlorination of long chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 59 pp (Report No.
BL/B/2288).
Madeley JR & Thompson RS (1983d) Toxicity of chlorinated paraffins to
mussels (Mytilus edulis) over 60 days. Chlorinated paraffin: 70%
chlorination of long chain length n-paraffins. Brixham, Imperial
Chemical Industries Ltd, Brixham Laboratory, 64 pp (Report No.
BL/B/2290).
Madeley JR, Thompson RS, & Brown D (1983a) The bioconcentration of a
chlorinated paraffin by common mussel (Mytilus edulis). Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 76 pp
(Report BL/B/2351).
Madeley JR, Windeatt AJ, & Street JR (1983b) Assessment of the
toxicity of a chlorinated paraffin to the anaerobic sludge digestion
product. Chlorinated paraffin: 58% chlorination of short chain length
n-paraffins. Brixham, Imperial Chemical Industries Ltd, Brixham
Laboratory, 25 pp (Report No. BL/B/2253).
Mather JI, Street JR, & Madeley JR (1983) Assessment of the inherent
biodegradability of a chlorinated paraffin, under aerobic conditions,
by a method developed from OECD Test Guideline 302B. Chlorinated
paraffin: 58% chlorination of short chain length n-paraffins.
Brixham, Imperial Chemical Industries Ltd, Brixham Laboratory, 56 pp
(Report No. BL/B/2298).
Meijer J & DePierre JW (1987) Hepatic levels of cytosolic, microsomal
and "mitochondrial" epoxide hydrolases and other drug-metabolizing
enzymes after treatment of mice with various xenobiotics and endogenous
compounds. Chem-Biol Interact, 62: 249-269.
Meijer J, Rundgren M, Åström A, DePierre JW, Sundvall A, & Rannug U
(1981) Effects of chlorinated paraffins on some drug-metabolizing
enzymes in rat liver and in the Ames test. Adv Exp Med Biol,
A136: 821-828.
Menter P, Harrison W, & Woodin WG (1975) Patch testing of coolant
fractions. J Occup Med, 17(9): 565-568.
Muller W (1989) Chlorowax 500C - Micronucleus test in male and female
NMRI mice after oral administration. Frankfurt/Main, Hoechst AG,
Pharma Research Toxicology and Pathology, 24 pp (Report No. 89.0253).
Murray T, Frankenberry M, Steele DH, & Heath RG (1988) Chlorinated
paraffins: A report on the findings from two field studies, Sugar
Creek, Ohio, Tinkers Creek, Ohio. Volume 1: Technical report.
Washington, DC, US Environmental Protection Agency, 150 pp (EPA-560/
5-87/012).
Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D,
Martin R, & Caspary WJ (1990) L5178Y mouse lymphoma cell mutation
assay results with 41 compounds. Environ Mol Mutagen, 16(Suppl 18):
138-167.
NIOSH (1990) National occupational exposure survey (1980-1983).
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
Nilsen O & Toftgård R (1981) Effect of polychlorinated terphenyls and
paraffins on rat liver microsomal cytochrome P-450 and in vitro
metabolic activities. Arch Toxicol, 47: 1-11.
Nilsen O, Toftgård R, & Glaumann H (1980) Changes in rat liver
morphology and metabolic activities after exposure to chlorinated
paraffins. Dev Toxicol Environ Sci, 8: 525-528.
Nilsen OG, Toftgård R, & Glaumann H (1981) Effects of chlorinated
paraffins on rat liver microsomal activities and morphology.
Importance of the length and the degree of chlorination of the carbon
chain. Arch Toxicol, 49: 1-13.
NTP (1986a) Toxicology and carcinogenesis studies of chlorinated
paraffins (C12, 60% chlorine) (CAS No. 