
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 170
ASSESSING HUMAN HEALTH RISKS OF CHEMICALS: DERIVATION
OF GUIDANCE VALUES FOR HEALTH-BASED EXPOSURE LIMITS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared at the National Institute of Health Sciences,
Tokyo, Japan, and the Institute of Terrestrial Ecology, Monk's Wood,
United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1994
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Assessing human health risks of chemicals: derivation of guidance
values for health-based exposure limits.
(Environmental health criteria ; 170)
1.Hazardous substances - toxicity 2.Environmental exposure
3.Guidelines I.Series
ISBN 92 4 157170 5 (NLM Classification: WA 465)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1994
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR GUIDANCE VALUES FOR
HUMAN EXPOSURE LIMITS
SUMMARY
1. INTRODUCTION
1.1. Scope and purpose
1.2. Guidance value
1.3. Quality of data
1.4. Clarity and transparency of presentations
2. GUIDANCE VALUES
2.1. General considerations
2.1.1. Precision of a guidance value
2.2. Derivation of guidance values
2.3. Interpretation and use of guidance values
2.4. Terminology
3. APPLICATION OF THE TOXICITY DATA BASE TO DETERMINE
TOLERABLE INTAKES
3.1. Approaches to risk assessment
3.1.1. Non-threshold effects
3.1.2. Threshold effects
3.1.2.1 Uncertainty factors
3.1.2.2 Relevant toxicokinetic and
toxicodynamic data
3.1.2.3 Uncertainty factors for occupational
exposure
4. PROCEDURE FOR EXTRAPOLATION FROM A TOXICITY DATA BASE TO
A TOLERABLE INTAKE
4.1. Overall procedure
4.2. Selection of pivotal study and critical effect(s)
4.3. Adequacy of the pivotal study
4.4. Interspecies extrapolation
4.5. Inter-individual variability in humans
4.6. Other considerations
4.6.1. Adequacy of the overall data base
4.6.2. Nature of toxicity
4.7. Final review of the total uncertainty factor
4.8. Precision of the tolerable intake
4.9. Alternative approaches
5. ALLOCATION OF TOLERABLE INTAKES TO DERIVE GUIDANCE VALUES
5.1. General considerations
5.2. General approach
5.3. Detailed approach
5.3.1. Biomarkers of exposure
5.3.2. Critical effects which are not route specific
5.3.3. Difference in magnitude of effect by
route of exposure
5.3.4. Route-specific effect variation at portals
of entry (due to local bioactivation or
local effects)
5.3.5. Limited data base
6. EXAMPLES OF THE DERIVATION OF GUIDANCE VALUES
REFERENCES
APPENDIX 1: EXAMPLES - DEVELOPMENT OF GUIDANCE VALUES
APPENDIX 2: GRAPHICAL APPROACHES
APPENDIX 3: ALTERNATIVE APPROACHES
APPENDIX 4: BODY WEIGHT AND VOLUMES OF INTAKE FOR REFERENCE MAN
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR GUIDANCE
VALUES FOR HUMAN EXPOSURE LIMITS
Members
Dr D. Andersonc, British Industrial and Biological Research
Association (BIBRA), Carshalton, Surrey, United Kingdom
Professor B. Baranskib, Nofer's Institute of Occupational Medicine,
Lodz, Poland
Dr V. Benesb, Toxicology and Reference Laboratory, Institute of
Hygiene and Epidemiology, Prague, Czech Republic
Dr T. DeRosaa, Division of Toxicology, Agency for Toxic Substances
and Disease Registry, Atlanta, Georgia, USA
Dr M. Doursona, b, c II, Systemic Toxicants Assessment Branch,
Environmental Criteria and Assessments Office, Office of Research and
Development, US Environmental Protection Agency, Cincinnati, Ohio, USA
Dr J. Duffusc, Department of Biological Sciences, The Edinburgh
Centre for Toxicology, Herriot-Watt University, Edinburgh, United
Kingdom
Dr E. Dybinga I, Department of Environmental Medicine, National
Institute of Public Health, Oslo, Norway
Dr R.J. Fielderc, Department of Health, Elephant and Castle,
London, United Kingdom
Dr T. Harveyb, c, Environmental Criteria and Assessment Office, US
Environmental Protection Agency, Cincinnati, Ohio, USA
Dr R. Hasegawac, Division of Toxicology Safety, National Institute
of Health Sciences, Setagaya-ku, Tokyo, Japan
Dr E. Kamataa, National Institute of Health Sciences, Setagaya-ku,
Tokyo, Japan
Dr A.G.A.C. Knaapa, b, c, Toxicology Advisory Centre, National
Institute of Public Health and Environmental Protection, Bilthoven,
The Netherlands
Dr M.E. Meeka II, b II, c I, Environmental Health Directorate,
Health Protection Branch, Health and Welfare, Ottawa, Ontario, Canada
Dr E. Poulsenb, Humlebaek, Denmark
Dr A.G. Renwicka II, b II, c II, Clinical Pharmacology Group,
University of Southampton, Medical and Biological Sciences Building,
Southampton, United Kingdom
Dr A.E. Robinsona, Toronto, Ontario, Canada
Professor J.A. Sokalb, Institute of Occupational Medicine and
Environmental Health, Sosnowiec, Poland
Dr R. Türcka, b I, c, Federal Ministry of Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Professor F. Valicb, c, Department of Occupational Health, Andrija
tampar School of Public Health, Zagreb University, Zagreb, Croatia
Dr G.A. Zapponib, Laboratory of Environmental Health, Istituto
Superiore di Sanità, Rome, Italy
Representatives of other Organizations
Mr S. Araia, b, Organisation for Economic Co-operation and
Development, Paris, France
Dr P. Boffettaa, International Agency for Research on Cancer,
Lyon, France
Dr J. Furlongb, Directorate-General XI A.2, Environment, Nuclear
Safety and Civil Protection, Commission of the European Communities,
Brussels, Belgium
Mr S. Machidaa, Occupational Safety and Health Branch, Working
Conditions and Environment Department, International Labour Office,
Geneva, Switzerland
Dr H. Mollerc, International Agency for Research on Cancer, Lyon,
France
Dr V. Morgenrothc, Organisation for Economic Co-operation and
Development, Paris, France
Ms F. Ouanea, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme, Geneva, Switzerland
Ms A. Sundena, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva, Switzerland
Observers
Dr G.P. Dastonc, Procter & Gamble, Miami Valley Laboratories,
Cincinnati, Ohio, USA
Dr He Fengshengc, Division of Health Protection and Promotion, World
Health Organization, Geneva, Switzerland
Dr C. Lallyc, Procter & Gamble, European Technical Centre,
Stombeek-Bever, Belgium
Dr D. Magea, c, Division of Environment Health, Prevention of
Environmental Pollution, World Health Organization, Geneva,
Switzerland
Dr M.I. Mikheeva, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
Professor G. Di Renzoc, Department of Human Communication Science,
Faculty of Medicine, Frederico II University, Naples, Italy
Dr J.A. Stobera, Division of Environment Health, World Health
Organization, Geneva, Switzerland
Dr G. Würtzenb, c, Coca-Cola International, Glostrup Centre,
Glostrup, Denmark
Secretariat
Dr G. Beckinga, c, International Programme on Chemical Safety,
Interregional Research Unit, World Health Organization, Research
Triangle Park, North Carolina, USA
Dr H. Galal-Gorcheva, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr J. Herrmana, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr D. Kellob, World Health Organization, Regional Office for Europe,
Copenhagen, Denmark
Mr D. Schutza, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smitha, b, c, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Mr K. Tanakaa, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Younesb, c, European Centre for Environment and Health, World
Health Organization, Bilthoven, The Netherlands
a Participated in IPCS Discussions on Methodology for Establishing
Guidance Values (GV) for Various Exposure Situations, Geneva,
Switzerland, 14-17 January 1992
b Participated in WHO EURO/IPCS Consultation on Guiding Principles
and Methodology for Quantitative Risk Assessment in Setting
Exposure Limits, Langen, Germany, 19-22 January 1993
c Participated in WHO Task Group Meeting on Risk Assessment
Methodology (Guidance Values), Geneva, Switzerland, 14-18 June
1993
I Chairman of meeting
II Joint Rapporteur
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
ENVIRONMENTAL HEALTH CRITERIA FOR GUIDANCE VALUES FOR HUMAN EXPOSURE
LIMITS
This Environmental Health Criteria monograph was developed in the
course of three meetings, i) a Discussion Group, World Health
Organization, Geneva, Switzerland, 14-17 January 1992, opened by
Dr E. Smith, IPCS, ii) a Consultation, Langen, Germany, 19-22 January
1993, opened by Dr D. Kello, World Health Organization, Regional
Office for Europe, and iii) the final Task Group, World Health
Organization, Geneva, 14-18 June 1993, opened by Dr E. Smith, IPCS.
Dr E. Smith and Dr P.G. Jenkins, both members of the IPCS Central
Unit, were responsible for the overall scientific content and
technical editing, respectively.
The WHO Regional Office for Europe collaborated with the
International Programme on Chemical Safety in the development of the
Guidance Value concept.
The German Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety provided funding support for the
Consultation in Langen, Germany.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ADI acceptable daily intake
AUC area under the curve
EPI exposure/potency index
LO(A)EL lowest-observed-(adverse)-effect level
NO(A)EL no-observed-(adverse)-effect level
SAR structure-activity relationship
TI tolerable intake
UF uncertainty factor
SUMMARY
Guidance values for exposure to chemicals in environmental media
should be developed in IPCS Environmental Health Criteria (EHC)
monographs and can be modified by national and local authorities in
their development of limits and standards for environmental media.
For any chemical, the steps involved are:
1. Evaluate and summarize the information on toxicity in animals and
humans and exposure in humans which is most relevant to derivation of
guidance values. The most appropriate format for presentation of the
data relevant to derivation of guidance values is a written narrative
summarizing the critical data complemented by graphical presentation.
2. Such data can be used to derive a Tolerable Intake (TI) for
various routes of exposure for effects considered to have a threshold.
This will involve application of uncertainty factors, generally to the
no-observed-adverse-effect level (NOAEL) for critical effects in the
most relevant study. For non-threshold effects, the dose-response
relationship will be characterized to the extent possible.
3. Estimate the proportion of total intake that originates from
various media (e.g., indoor and ambient air, food and water), based on
exposure estimates for a consistent set of assumed volumes of intake
(using the International Commission on Radiological Protection (ICRP)
reference man) and representative concentrations in the general
environment, for a given situation. In the absence of adequate data
on concentrations in various media, mathematical models may be used to
estimate the distribution through the various media.
4. Allocate a proportion of the TI to various media of exposure
(based on the exposure estimate described in step 3 above) to
determine the intake or exposure in each medium.
5. Develop guidance values from intakes assigned to each medium,
taking into account (if necessary) body weight, volume of intake and
absorption efficiency (the relative absorption efficiency in
situations where the guidance value is derived on the basis of a TI by
another route of exposure). In EHC monographs, development of
guidance values would be undertaken for a clearly defined exposure
scenario, based on the data for ICRP reference man, and not
necessarily representative of national or local exposure conditions.
Guidance values would commonly be derived for a representative general
population with representative exposure conditions. The guidance
values should be adapted at national and local levels as appropriate
for local circumstances.
6. The basis for the derivation of both the TI and the guidance
values should be described clearly in EHC monographs (see level of
detail in examples in Appendix 1).
1. INTRODUCTION
1.1 Scope and purpose
The objective of IPCS Environmental Health Criteria (EHC)
monographs is to provide evaluated information, including guidance for
exposure limits, for the protection of human health and the
maintenance of environmental integrity against the possible
deleterious effects of chemical and/or physical agents. EHC
monographs contain a comprehensive review and evaluation of available
information on the biological effects of selected chemicals and
physical agents that can influence human health and the environment.
The evaluation typically contains information on the relative
contribution of concentrations in various media to a total dose for
human or environmental targets, data on dose-effect and dose-response
relationships and numerical values, such as Tolerable Intake (TI) and
advisory Guidance Value (GV) to enable regulatory authorities to set
their own exposure limits whenever necessary.
Though effects on environmental organisms are not addressed in
this report, a holistic approach is implicit in the protection of
human health and environmental integrity. Such approaches have been
developed by some national institutions for the protection of human
health (see, for example, Health and Welfare Canada, 1992 and US EPA,
1993). A more integrated approach aimed at the protection of both man
and the ecosystem has been developed in the Netherlands (USES, 1994)
and is incorporated in some national legislation (Canada, 1988).
Evaluation for human health protection in EHC monographs entails
consideration of the general and occupationally exposed populations
and susceptible subgroups. The approach described herein relates
primarily to long-term exposure of the general population in the
ambient environment (i.e. principally ambient air, food, water and,
occasionally, other media). Some degree of human variability is taken
into account in the uncertainty factors applied in the derivation of
the TI (see section 4.5). Where a uniquely sensitive group forms a
significant proportion of the population then the TI would be
developed based on that group. In cases where the exposure profiles
of this subgroup and the general population are similar, the guidance
values should be based on the TI for the sensitive subgroup. If the
exposure profiles differ, guidance values should be calculated
separately for the subgroup and general population based on their
respective TIs and exposure profiles, and the more conservative values
adopted. Idiosyncratic hypersusceptibility (excessive reaction
following exposure to a given dose of a substance compared with the
large majority of those exposed to the same dose) in a few individuals
would not be the basis for the derivation of the TI in EHC monographs.
Though the basic methodology would be similar, development of
guidance values relating to intermittent, short-term (e.g.,
accidental) and occupational exposures are not addressed in detail
herein, since this would entail consideration of additional relevant
factors. (See, for example, discussion in section 3 concerning
development of TIs for occupational exposure).
1.2 Guidance value
The term guidance value is considered appropriate for the type of
advice provided by the IPCS in its EHC and other documents because it
does not carry connotations of formal standards and regulatory limits.
In addition, its derivation is consistent with the process of health
risk assessment and risk characterization for risk management. In
this context guidance values are defined as:--
values, such as concentrations in air or water, which are
derived after appropriate allocation of the TI among the
different possible media of exposure. Combined exposures from
all media at the guidance values over a lifetime would be
expected to be without appreciable health risk. The aim of a
guidance value is to provide quantitative information from risk
assessment for risk managers to enable them to make decisions
concerning the protection of human health.
1.3 Quality of data
Review and evaluation of data for inclusion in EHC monographs
necessarily involves a critical approach to the selection and quality
of data sources. Draft documents are prepared by various
institutes/authors and assessed by various expert groups each with a
different membership. Consequently, there can be a lack of
consistency in the selection of data sources and variation on the part
of different authors and assessors in the interpretation and
extrapolation of data. The formulation of criteria for determining
the quality of data is a current IPCS activity and considered to be
critical to the derivation of sound guidance values in EHCs.
Many toxicological studies are directed mainly to hazard
identification. The available data may not always contain sufficient
information on the dose-response relationship for risk assessment and
for the derivation of TIs for guidance values. Reports and
publications in which no-observed-effect level (NOEL) or NOAEL values
are presented should include sufficient information on all possible
effects investigated and those observed or not observed to allow an
assessment of the validity of the derived values.
1.4 Clarity and transparency of presentations
Data on the dose-response relationship for the critical effect
which served as the basis for the derivation of the guidance values
(GVs) should be characterized in EHC monographs to the extent possible
(including graphical presentation, similar to that illustrated in
Appendix 3 for benchmark doses). It is recognized that in many cases,
the data base will be insufficient for provision of such information
and that it may only be possible to develop single guidance values in
individual media with little additional risk characterization.
Similarly, the basis for the uncertainty factors by which the NOAEL or
lowest-observable-adverse-effect level (LOAEL) have been divided to
obtain the TI should be clearly specified. The conversion of the TI
into media-specific GVs should be presented in sufficient detail to
allow the values to be adapted to national or local circumstances (see
examples in Appendix 1 for relevant level of detail).
2. GUIDANCE VALUES
2.1 General considerations
A consistent methodology should be used in the derivation of
quantitative guidance values for human exposures to chemical
substances present in food, drinking-water, air and other media by
ad hoc IPCS Task Groups (of varying membership) reviewing and
evaluating data and finalizing EHC monographs on various chemicals.
This approach embodies the concept that, to the extent possible,
guidance values for the protection of human health should reflect
consideration of total exposure to the substance whether present in
air, water, soil, food or other media. Guidance values should be
derived for a clearly defined exposure scenario, based on the data for
the ICRP reference man (Appendix 4), and therefore might not represent
national or local circumstances.
2.1.1 Precision of a guidance value
The precision of the guidance values is dependent upon the
validity and reliability of the available data. Frequently, there are
sources of uncertainty in the derivation of TIs (see section 4.8) and
in their allocation as a basis for GVs, so that the resulting values
represent a best estimate based on the available data at the time. A
description of the derivation of guidance value should clearly
indicate the nature and sources of uncertainty and the manner in which
they have been taken into account in the derivation. The numerical
value of GVs should reflect the precision present in their derivation;
usually GVs should be given to only one
significant figure.
2.2 Derivation of guidance values
Establishing TIs is central to the determination of guidance
values. A TI is defined as:--
an estimate of the intake of a substance over a lifetime that is
considered to be without appreciable health risk. It may have
different units depending upon the route of administration upon
which it is based and is generally expressed on a daily or weekly
basis. Though not strictly an "intake", TIs for inhalation are
generally expressed as airborne concentrations (i.e. µg or mg per
m3). The TI is similar in definition and intent to terms such
as reference dose (RfD) (Barnes & Dourson, 1988), reference
concentration (RfC) (Jarabek et al., 1990) and acceptable daily
intake (ADI).
This monograph addresses two areas that are critical in the
methodology for the derivation of guidance values for human exposures
to chemical substances in the environment:
* Development of a tolerable intake on the basis of interpretation
of the available data on toxicity. For practical purposes,
toxic effects are considered to be of two types, threshold and
non-threshold. For substances where the critical effect is
considered to have a threshold (including non-genotoxic carcino-
genesis for which there is adequate mechanistic data), a TI is
developed usually on the basis of a NOAEL. Development of guidance
values in EHC monographs for non-threshold effects (e.g.,
genotoxic carcinogenesis and germ cell mutations) is discussed in
section 3.1.1.
* Allocation of the proportions of the tolerable intake to various media.
Depending on available information, the development of guidance
values for compounds present in more than one environmental medium
will require the allocation of proportions of the TI to various
media (for example, air, food and water). For the derivation of
guidance values, the allocation will be based on information on
relative exposure via different routes.
2.3 Interpretation and use of guidance values
Media exposure allocations of TIs for the derivation of guidance
values in EHC monographs are based on relative exposure by different
routes for a given scenario. Though this is suggested as a practical
approach, the use of allocations based on exposure in different media
does not preclude the development of more stringent limits. It is
also important to recognize that the proportions of total intake from
various media may vary, based on circumstances. Site- or
context-specific guidance values better suited to local circumstances
and conditions could be developed from TIs presented in the EHC in
situations where relevant data on exposure are available, and
particularly where there are other significant sources of exposure to
a chemical substance (e.g., in the vicinity of a waste site).
Regulatory authorities may also take other factors into account, such
as cost, ease and effectiveness of control, to develop risk management
strategies appropriate for local circumstances, although the ultimate
objective of control should be reduction of exposure from all sources
to less than the TIs. In addition, where data on organoleptic
thresholds are included in EHC monographs, these can also be
considered by relevant authorities in the development of limits.
The basis for derivation of guidance values in EHC monographs
must be clearly specified in sufficient detail to enable, where
appropriate, step-by-step development of exposure limits for national
or local conditions by appropriate regulatory or other authorities
(Appendix 1).
2.4 Terminology
Adverse effect: change in morphology, physiology, growth,
development or life span of an organism which results in impairment of
functional capacity or impairment of capacity to compensate for
additional stress or increase in susceptibility to the harmful effects
of other environmental influences. Decisions on whether or not any
effect is adverse require expert judgement.
Critical effect(s): the adverse effect(s) judged to be most
appropriate for determining the TI.
No-observed-adverse-effect level (NOAEL): greatest concentration or
amount of a substance, found by experiment or observation, which
causes no detectable adverse alteration of morphology, functional
capacity, growth, development or life span of the target organism
under defined conditions of exposure. Alterations of morphology,
functional capacity, growth, development or life span of the target
may be detected which are judged not to be adverse.
No-observed-effect level (NOEL): greatest concentration or amount
of a substance, found by experiment or observation, that causes no
alterations of morphology, functional capacity, growth, development or
life span of target organisms distinguishable from those observed in
normal (control) organisms of the same species and strain under the
same defined conditions of exposure.
Lowest-observed-adverse-effect level (LOAEL): lowest concentration
or amount of a substance, found by experiment or observation, which
causes an adverse alteration of morphology, functional capacity,
growth, development or life span of the target organism
distinguishable from normal (control) organisms of the same species
and strain under the same defined conditions of exposure.
Benchmark dose: the lower confidence limit of the dose calculated
to be associated with a given incidence (e.g., 5 or 10% incidence) of
effect estimated from all toxicity data on that effect within that
study (Crump, 1984).
Uncertainty factor (UF): a product of several single factors by
which the NOAEL or LOAEL of the critical effect is divided to derive a
TI. These factors account for adequacy of the pivotal study,
interspecies extrapolation, inter-individual variability in humans,
adequacy of the overall data base, and nature of toxicity. The term
uncertainty factor was considered to be a more appropriate expression
than safety factor since it avoids the notion of absolute safety and
because the size of this factor is proportional to the magnitude of
uncertainty rather than safety. The choice of UF should be based on
the available scientific evidence.
Toxicodynamics: the process of interaction of chemical substances
with target sites and the subsequent reactions leading to adverse
effects.
Toxicokinetics: the process of the uptake of potentially toxic
substances by the body, the biotransformation they undergo, the
distribution of the substances and their metabolites in the tissues,
and the elimination of the substances and their metabolites from the
body. Both the amounts and the concentrations of the substances and
their metabolites are studied. The term has essentially the same
meaning as pharmacokinetics, but the latter term should be restricted
to the study of pharmaceutical substances.
Tolerable intake (TI): an estimate of the intake of a substance
which can occur over a lifetime without appreciable health risk. It
may have different units depending upon the route of administration.
Though not strictly an "intake", TIs for inhalation are generally
expressed as airborne concentrations (i.e., µg or mg per m3).
Default value: pragmatic, fixed or standard value used in the
absence of relevant data.
Guidance values (GVs): values, such as concentrations in air or
water, which are derived after appropriate allocation of the TI among
the different possible media of exposure. Combined exposures from all
media at the guidance values over a lifetime would be expected to be
without appreciable health risk. The aim of the guidance value is to
provide quantitative information from risk assessment for risk
managers to enable them to make decisions concerning the protection of
human health.
3. APPLICATION OF THE TOXICITY DATA BASE TO DETERMINE TOLERABLE
INTAKES
3.1 Approaches to risk assessment
A review of the data base on a chemical should be undertaken to
determine the critical effect(s), which can be considered to be of two
types: those considered to have a threshold and those for which there
is considered to be some risk at any level (non-threshold: genotoxic
carcinogens and germ cell mutagens). Data available for risk
assessments include studies in humans and animals, structure-activity
relationships (SAR) and in vitro investigations. Risk assessments
should be based on all available data at the time of review, but it is
appreciated that recognition of additional hazards or risk may emerge
which will require subsequent re-evaluation. Wherever possible,
appropriate human data should be used as the basis for the risk
assessment.
For threshold effects, where data in humans are used as the basis
for development of TIs, uncertainty factors should be applied to
observed effect levels to allow for the magnitude of any effect seen
in the exposed group and their sensitivity compared with the general
population or target group. The incidence of effects detected in
humans in vivo will be the result of inter-individual differences in
both toxicokinetic and toxicodynamic aspects. The extent of any
possible human variability not present within the exposed population
groups should be considered in the development of uncertainty factors.
Information on the NOAEL (or LOAEL) by different routes is
sometimes available. In cases where information exists on only one
route, e.g., inhalation, the bioequivalence for exposure from other
routes should be estimated if suitable information and models are
available. The aim of the risk assessment is to estimate an overall
tolerable intake derived from data on toxicity using appropriate
routes of administration. Guidance values can then be developed
through allocation of the TI to the various media of human exposure,
based on considerations of relevant exposure profiles.
3.1.1 Non-threshold effects
There is no clear consensus on appropriate methodology for the
risk assessment of chemicals for which the critical effect may not
have a threshold, such as genotoxic carcinogens and germ cell
mutagens. A number of approaches based largely on characterization of
dose response have been adopted for assessment of such effects.
However, these approaches are not amenable to the development of
guidance values in EHC monographs because they require socio-political
judgements of acceptable health risk. Those preparing EHC and other
documents for the IPCS should evaluate the relevant available data and
characterize the dose-response relationship for such effects to the
extent possible, based on one or more methods as considered
appropriate (some approaches are described below). This should enable
the development of guidance values or limits by appropriate
authorities on the basis of information on such effects included in
EHC monographs.
Approaches have included:
* quantitative extrapolation by mathematical modelling of the
dose-response curve to estimate the risk at likely human intakes
or exposures (low-dose risk extrapolation)
* relative ranking of potencies in the experimental range
* division of effect levels by an uncertainty factor.
Low-dose risk extrapolation has been accomplished by the use of
mathematical models such as the Armitage-Doll multi-stage model. In
more recently developed biological models, the different stages in the
process of carcinogenesis have been incorporated and time to tumour
has been taken into account (Moolgavkar et al., 1988). In some cases
where data permit, the dose delivered to the target tissue has been
incorporated into the dose-response analysis (physiologically based
pharmacokinetic or PBPK modelling). It should be noted that crude
expression of risk in terms of excess incidence or numbers of cancers
per unit of the population at doses or concentrations much less than
those on which the estimates are based may be inappropriate, owing to
the uncertainties of quantitative extrapolation over several orders of
magnitude. Estimated risks are believed to represent only the
plausible upper bounds and vary depending upon the assumptions on
which they are based.
Comparison of human exposure to the carcinogenic potency in the
experimental range can also be used to indicate the magnitude of risk
as a basis of derivation of guidance values. One such measure which
provides a practical way to prioritize substances on the basis of
their carcinogenic potency in a range close to the observed
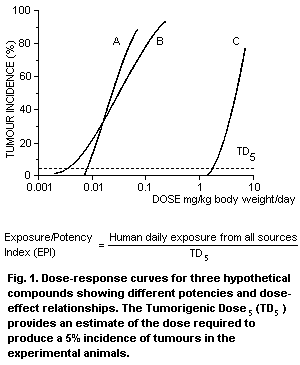
dose-response is the Exposure/Potency Index (EPI) (Health and Welfare
Canada, 1992). The EPI is defined as the estimated daily human intake
or exposure divided by the intake or exposure associated with a 5%
incidence of tumours in experimental studies in animals or
epidemiological studies in human populations (Tumorigenic Dose5;
TD5) (Fig. 1). A calculated EPI of 10-6 represents a one million
fold difference between human exposure and the intake which is at the
lower end of the dose-response curve. Wherever possible, relevant
toxicokinetic and mechanistic data are taken into account in the
development of the EPIs.
An alternative approach is to divide the highest dose at which
there is no observed increase in tumour incidence in comparison with
controls by a large composite uncertainty factor (for example 5000;
Weil, 1972). The magnitude of the factor could be a function of the
weight of evidence (e.g., numbers of species in which the tumours have
been observed or nature of the tumours). This approach is sometimes
used when data on dose-response are limited.
A risk management approach which has been adopted for compounds
for which the critical effect is considered not to have a threshold
involves eliminating or reducing exposure as far as is practicable or
to the lowest level technologically possible. Characterization of the
dose-response as indicated in the procedures described above can be
used in conjunction with this approach to assess the need to improve
technology to reduce exposure.
3.1.2 Threshold effects
For compounds with critical effects for which there is a
threshold, a primary objective of a review of data is to consider
the comparability of experimental species and humans, and determine
the highest doses or exposures that can be administered experimentally
to animals or taken up by humans without producing the critical effect
(see Environmental Health Criteria 70: Principles for the Safety
Assessment of Food Additives and Contaminants in Food, section 5.5.1)
(WHO, 1987). In studies in experimental animals, the value of the
NOAEL is an observed value that is dependent on the protocol and
design of the study from which it was derived. There are several
"study-dependent" factors that influence the magnitude of the value
observed, including:
* the species, sex, age, strain and developmental status of the
animals studied
* the group size
* the sensitivity of the methods used to measure the response
* the duration of exposure
* the selection of dose levels, which are frequently widely spaced,
so that the observed value of the NOAEL can be in some cases
considerably less than the true no-adverse-effect level.
3.1.2.1 Uncertainty factors
There is enormous variability in the extent and nature of
different data bases for risk assessment. For example, in some cases,
the evaluation must be based on limited data in experimental animals;
in other cases detailed information on the mechanism of toxicity
and/or toxicokinetics may be available, while in some cases the risk
evaluation can be based on data on effects in exposed human
populations. Consequently, for the general population, the range of
uncertainty factors applied in the derivation of TIs has been wide
(1-10 000), although a value of 100 has been used most often. For
example, the historic use of a factor of 100 based on animal studies
in the absence of specific data to suggest a more appropriate value
was first proposed by Lehman & Fitzhugh (1954) and later used in the
derivation of ADIs for food additives by WHO (WHO, 1987; Lu, 1988).
 More recently, additional uncertainty factors have been incorporated
to account for, for example, deficiencies in the data base, such as
the absence of a NOAEL (US EPA, 1985a,b) or the absence of chronic
data (NAS, 1977).
If data from well-conducted studies in human populations are the
basis for the safety evaluation, a factor of 10 has been considered
appropriate, as a default value (WHO, 1987). Thus the value of 100
has been regarded as comprising two factors of 10 each to allow for
interspecies and inter-individual (intraspecies) variations. A scheme
has been proposed which retains the two 10-fold factors as the
cornerstone for extrapolating from animals to man but which allows
subdivision of each to incorporate appropriate data on toxicodynamics
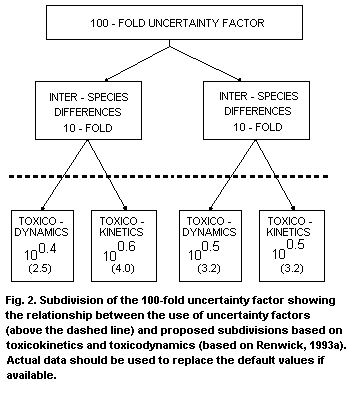
or toxicokinetics where these exist (Renwick, 1993a) (see Fig. 2).
This approach improves the extrapolation process, and where
appropriate data can be introduced, it has the effect of replacing
"uncertainty" factors with "correction" factors. Data on differences
in dynamics and kinetics between humans and common laboratory animals,
such as rats, mice and dogs, indicated that there was greater
potential for differences in kinetics than in dynamics so that an
equal split of the 10-fold factor was inappropriate. The usual
10-fold factor (log 1) should be split into default values of 2.5
(100.4) for dynamics and 4 (100.6) for kinetics (Renwick, 1993a).
A similar split was proposed for interindividual differences between
humans in toxicokinetics (pharmacokinetics) and toxicodynamics (using
pharmacokinetic-pharmacodynamic modelling). However, it was
considered that the variability for both aspects was similar and it
was concluded that the 10-fold factor should be split evenly between
both aspects, i.e. 3.2 (100.5) for kinetics and 3.2 (100.5) for
dynamics. The commonly applied 100-fold uncertainty factor should be
split as indicated in Fig. 2.
Precise default values for kinetics and dynamics cannot be
expected on the basis of subdivision of the imprecise 10-fold
composite factor. The values above are reasonable since they provide
a positive value > 2 for both aspects and are compatible with the
species differences in physiological parameters such as renal and
hepatic blood flow. Since the data base examined was limited, it is
proposed that the values for subdivision of inter-species and
inter-individual variation presented in Fig. 2 be adopted on an
interim basis. Adoption of the approach should encourage the
development and generation of appropriate data, which could then
contribute to any future revision of the default values, and further
improve the scientific basis of the use of uncertainty factors.
More recently, additional uncertainty factors have been incorporated
to account for, for example, deficiencies in the data base, such as
the absence of a NOAEL (US EPA, 1985a,b) or the absence of chronic
data (NAS, 1977).
If data from well-conducted studies in human populations are the
basis for the safety evaluation, a factor of 10 has been considered
appropriate, as a default value (WHO, 1987). Thus the value of 100
has been regarded as comprising two factors of 10 each to allow for
interspecies and inter-individual (intraspecies) variations. A scheme
has been proposed which retains the two 10-fold factors as the
cornerstone for extrapolating from animals to man but which allows
subdivision of each to incorporate appropriate data on toxicodynamics
or toxicokinetics where these exist (Renwick, 1993a) (see Fig. 2).
This approach improves the extrapolation process, and where
appropriate data can be introduced, it has the effect of replacing
"uncertainty" factors with "correction" factors. Data on differences
in dynamics and kinetics between humans and common laboratory animals,
such as rats, mice and dogs, indicated that there was greater
potential for differences in kinetics than in dynamics so that an
equal split of the 10-fold factor was inappropriate. The usual
10-fold factor (log 1) should be split into default values of 2.5
(100.4) for dynamics and 4 (100.6) for kinetics (Renwick, 1993a).
A similar split was proposed for interindividual differences between
humans in toxicokinetics (pharmacokinetics) and toxicodynamics (using
pharmacokinetic-pharmacodynamic modelling). However, it was
considered that the variability for both aspects was similar and it
was concluded that the 10-fold factor should be split evenly between
both aspects, i.e. 3.2 (100.5) for kinetics and 3.2 (100.5) for
dynamics. The commonly applied 100-fold uncertainty factor should be
split as indicated in Fig. 2.
Precise default values for kinetics and dynamics cannot be
expected on the basis of subdivision of the imprecise 10-fold
composite factor. The values above are reasonable since they provide
a positive value > 2 for both aspects and are compatible with the
species differences in physiological parameters such as renal and
hepatic blood flow. Since the data base examined was limited, it is
proposed that the values for subdivision of inter-species and
inter-individual variation presented in Fig. 2 be adopted on an
interim basis. Adoption of the approach should encourage the
development and generation of appropriate data, which could then
contribute to any future revision of the default values, and further
improve the scientific basis of the use of uncertainty factors.
 It was recognised that appropriate toxicokinetic and
toxicodynamic data are rarely available for the same compound and that
to incorporate data in one area only would require the normal
composite factor of 10 to be subdivided. For example, if the
mechanism of action for the critical effects and differences in
sensitivity between the test species and man based on in vitro
studies were known, then these data could contribute quantitatively to
the risk assessment by replacement of the default factor for
interspecies differences in toxicodynamics, or differences in
sensitivity (the value of 2.5 in Fig. 2) by the value indicated by the
actual data. However, there could still be differences in
toxicokinetics between the test species and humans so that a portion
of the normal 10-fold factor would need to be retained (the value of 4
in Fig. 2).
3.1.2.2 Relevant toxicokinetic and toxicodynamic data
Toxicokinetics includes data on the rate and extent of absorption
(bioavailability), pattern of distribution, rate and pathway of any
bioactivation, and rate, route and extent of elimination. Factors
such as peak plasma concentration (Cmax), and area under the plasma
concentration-time curve (AUC) of the toxic entity are particularly
important since they are usually indicative of the extent and duration
of exposure of the target organ (Renwick, 1993a). Dosimetric
adjustments of administered animal dose to equivalent human dose are
also possible (Jarabek et al., 1990). However it is important to
define which parameter is relevant to the toxicity since some are
dependent on the Cmax and not AUC (e.g., the teratogenicity of
valproic acid; Nau, 1986) while for long-term bioassays, the AUC may
be of greater importance. Appropriate toxicodynamic factors include
the identification of the toxic entity (i.e. parent compound or a
metabolite), the nature of the molecular target, the presence and
activity of protective and repair mechanisms and the in vitro
sensitivity of the target tissue (see Renwick, 1993a for details and
examples). These toxicokinetic and toxicodynamic parameters should be
compared between the test species and humans for derivation of
interspecies factors where this is possible. Modification of the
10-fold factor for inter-individual variability in humans would
require data on toxicokinetics and toxicodynamics in a wide and fully
representative sample of the general or exposed population, including
an assessment of neonates if appropriate.
It is emphasised that in the absence of reliable information on
toxicokinetics and toxicodynamics, the default values for these
factors become the commonly used composite value of 100 (i.e., 10 for
inter-individual variability and 10 for interspecies variation).
3.1.2.3 Uncertainty factors for occupational exposure
The consideration of uncertainty factors given above relates
primarily to exposure of the general population. However, the general
principles for derivation of TIs for occupational exposure would be
somewhat similar (see, for example, Zielhuis & van der Kreek, 1979a,b;
Hallenbeck & Cunningham, 1986) although they have not been widely
adopted for this purpose. However, although the components of the
uncertainty factor relating to the nature and severity of the toxic
effect, the adequacy of the data base and interspecies variability
would be similar for the development of guidance values for
occupational exposure, the nature of the population exposed differs.
The more vulnerable members of the human population (i.e. the very
young, the sick and the elderly) do not form part of the exposed
occupational population, whereas for the development of TIs for the
general population, these groups must be considered. Furthermore,
workplace levels and patterns of exposure can be controlled and the
exposed population protected or monitored on an individual or group
basis. For these reasons, it is often appropriate to use
significantly lower uncertainty factors when deriving health-based
limits for occupational exposure compared with those used for the
development of TIs for the general population.
4. PROCEDURE FOR EXTRAPOLATION FROM A TOXICITY DATA BASE TO A
TOLERABLE INTAKE
4.1 Overall procedure
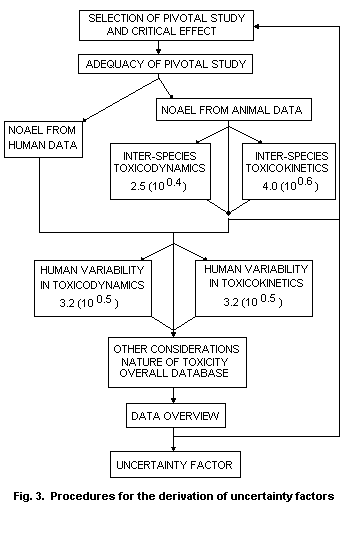
The procedure, which is presented in Fig. 3, is designed to be
applicable to widely differing data bases on toxicity. The procedure
is also suitable for the incorporation of human data, under which
circumstances some of the uncertainty factors will not be required.
The scheme is presented as a series of steps, but it is important that
the full data base continue to be reviewed to ensure that the final
decision is appropriate. A TI for a reversible toxic effect in an
animal species, for which there is complete toxicological data but
without appropriate toxicokinetic or toxicodynamic data, is based on
the commonly used and appropriate factor of 100. The scheme
incorporates those aspects which would normally be considered in the
conversion of a NOAEL (or LOAEL or equivalent) from an animal study
into a TI in such a way that appropriate mechanistic or toxicokinetic
data can contribute numerically to the uncertainty factor and hence to
the TI.
The procedure suggested here and discussed more fully in Renwick
(1993a) is based, in part, on discussions occurring over a number of
years regarding the basis of uncertainty factors (see, for example,
Zielhuis & van der Kreek, 1979a,b; Dourson & Stara, 1983; Lewis et
al., 1990; Rubery et al., 1990). To some extent, the principles
outlined here have been adopted in approaches of various national
agencies (e.g., Jarabek et al., 1990; Health and Welfare Canada, 1992;
US EPA, 1993).
4.2 Selection of pivotal study and critical effect(s)
Determination of the NOAEL, LOAEL or equivalent (possible use of
benchmark dose approach) is the first step in derivation of the TI.
This requires a thorough evaluation of available data on toxicity.
Sophisticated detection methods may be of such sensitivity that
effects can be detected at lower doses than by normal techniques; the
adversity of these effects requires very careful evaluation in the
determination of the NOAEL. For some chemicals, a review of the data
base may reveal that two (or possibly more) adverse effects occur at
low doses with NOAELs within one order of magnitude. Under such
circumstances and providing: a) that the data on which the NOAELs are
based are of sufficient quality to be used for risk evaluation; and b)
that the NOAELs may require different uncertainty factors based on,
for example, data on mechanisms or nature of toxicity (see below),
then each effect should be considered in the following scheme and the
one with the lower resulting TI used for development of guidance
values. Available LOAELs within the same order of magnitude as the
lowest reported NOAELs need also to be considered in this exercise
since they could lead to the development of more conservative TIs.
It was recognised that appropriate toxicokinetic and
toxicodynamic data are rarely available for the same compound and that
to incorporate data in one area only would require the normal
composite factor of 10 to be subdivided. For example, if the
mechanism of action for the critical effects and differences in
sensitivity between the test species and man based on in vitro
studies were known, then these data could contribute quantitatively to
the risk assessment by replacement of the default factor for
interspecies differences in toxicodynamics, or differences in
sensitivity (the value of 2.5 in Fig. 2) by the value indicated by the
actual data. However, there could still be differences in
toxicokinetics between the test species and humans so that a portion
of the normal 10-fold factor would need to be retained (the value of 4
in Fig. 2).
3.1.2.2 Relevant toxicokinetic and toxicodynamic data
Toxicokinetics includes data on the rate and extent of absorption
(bioavailability), pattern of distribution, rate and pathway of any
bioactivation, and rate, route and extent of elimination. Factors
such as peak plasma concentration (Cmax), and area under the plasma
concentration-time curve (AUC) of the toxic entity are particularly
important since they are usually indicative of the extent and duration
of exposure of the target organ (Renwick, 1993a). Dosimetric
adjustments of administered animal dose to equivalent human dose are
also possible (Jarabek et al., 1990). However it is important to
define which parameter is relevant to the toxicity since some are
dependent on the Cmax and not AUC (e.g., the teratogenicity of
valproic acid; Nau, 1986) while for long-term bioassays, the AUC may
be of greater importance. Appropriate toxicodynamic factors include
the identification of the toxic entity (i.e. parent compound or a
metabolite), the nature of the molecular target, the presence and
activity of protective and repair mechanisms and the in vitro
sensitivity of the target tissue (see Renwick, 1993a for details and
examples). These toxicokinetic and toxicodynamic parameters should be
compared between the test species and humans for derivation of
interspecies factors where this is possible. Modification of the
10-fold factor for inter-individual variability in humans would
require data on toxicokinetics and toxicodynamics in a wide and fully
representative sample of the general or exposed population, including
an assessment of neonates if appropriate.
It is emphasised that in the absence of reliable information on
toxicokinetics and toxicodynamics, the default values for these
factors become the commonly used composite value of 100 (i.e., 10 for
inter-individual variability and 10 for interspecies variation).
3.1.2.3 Uncertainty factors for occupational exposure
The consideration of uncertainty factors given above relates
primarily to exposure of the general population. However, the general
principles for derivation of TIs for occupational exposure would be
somewhat similar (see, for example, Zielhuis & van der Kreek, 1979a,b;
Hallenbeck & Cunningham, 1986) although they have not been widely
adopted for this purpose. However, although the components of the
uncertainty factor relating to the nature and severity of the toxic
effect, the adequacy of the data base and interspecies variability
would be similar for the development of guidance values for
occupational exposure, the nature of the population exposed differs.
The more vulnerable members of the human population (i.e. the very
young, the sick and the elderly) do not form part of the exposed
occupational population, whereas for the development of TIs for the
general population, these groups must be considered. Furthermore,
workplace levels and patterns of exposure can be controlled and the
exposed population protected or monitored on an individual or group
basis. For these reasons, it is often appropriate to use
significantly lower uncertainty factors when deriving health-based
limits for occupational exposure compared with those used for the
development of TIs for the general population.
4. PROCEDURE FOR EXTRAPOLATION FROM A TOXICITY DATA BASE TO A
TOLERABLE INTAKE
4.1 Overall procedure
The procedure, which is presented in Fig. 3, is designed to be
applicable to widely differing data bases on toxicity. The procedure
is also suitable for the incorporation of human data, under which
circumstances some of the uncertainty factors will not be required.
The scheme is presented as a series of steps, but it is important that
the full data base continue to be reviewed to ensure that the final
decision is appropriate. A TI for a reversible toxic effect in an
animal species, for which there is complete toxicological data but
without appropriate toxicokinetic or toxicodynamic data, is based on
the commonly used and appropriate factor of 100. The scheme
incorporates those aspects which would normally be considered in the
conversion of a NOAEL (or LOAEL or equivalent) from an animal study
into a TI in such a way that appropriate mechanistic or toxicokinetic
data can contribute numerically to the uncertainty factor and hence to
the TI.
The procedure suggested here and discussed more fully in Renwick
(1993a) is based, in part, on discussions occurring over a number of
years regarding the basis of uncertainty factors (see, for example,
Zielhuis & van der Kreek, 1979a,b; Dourson & Stara, 1983; Lewis et
al., 1990; Rubery et al., 1990). To some extent, the principles
outlined here have been adopted in approaches of various national
agencies (e.g., Jarabek et al., 1990; Health and Welfare Canada, 1992;
US EPA, 1993).
4.2 Selection of pivotal study and critical effect(s)
Determination of the NOAEL, LOAEL or equivalent (possible use of
benchmark dose approach) is the first step in derivation of the TI.
This requires a thorough evaluation of available data on toxicity.
Sophisticated detection methods may be of such sensitivity that
effects can be detected at lower doses than by normal techniques; the
adversity of these effects requires very careful evaluation in the
determination of the NOAEL. For some chemicals, a review of the data
base may reveal that two (or possibly more) adverse effects occur at
low doses with NOAELs within one order of magnitude. Under such
circumstances and providing: a) that the data on which the NOAELs are
based are of sufficient quality to be used for risk evaluation; and b)
that the NOAELs may require different uncertainty factors based on,
for example, data on mechanisms or nature of toxicity (see below),
then each effect should be considered in the following scheme and the
one with the lower resulting TI used for development of guidance
values. Available LOAELs within the same order of magnitude as the
lowest reported NOAELs need also to be considered in this exercise
since they could lead to the development of more conservative TIs.
 Graphical presentation of available data can facilitate
identification of effect levels relevant to development of TIs.
Although the form of graphical presentation is necessarily dependent
upon the size of the data base, a dose-duration graph in which NOELs,
NOAELs and LOAELs are presented as a function of duration of exposure
is considered to be helpful and is more fully described in Appendix 2.
4.3 Adequacy of the pivotal study
In situations where a NOAEL has not been achieved but the data
on effects are of sufficient quality to be the basis of the risk
assessment, then a no-adverse-effect level should be developed by the
application of an appropriate uncertainty factor to the LOAEL.
Uncertainty factors of 3, 5 or 10 have been used previously to
extrapolate from a LOAEL to a NOAEL depending on the nature of the
effect(s) and dose-response relationship (see, for example, US EPA,
1993). Alternatively, a benchmark dose may be developed by
mathematical modelling of the dose-response data as an alternative to
the uncertainty factor in extrapolating to the NOAEL (see Appendix 3).
The pivotal study may also be considered inadequate for other reasons
(e.g., duration of study, numbers of animals per group and sensitivity
of the analyses of effect), and an additional uncertainty factor
applied.
4.4 Interspecies extrapolation
In situations where appropriate toxicokinetic and/or
toxicodynamic data exist for a particular compound, then the relevant
uncertainty factor in Fig. 3 should be replaced by the data-derived
factor. Data on PBPK and/or data on target organ exposure should be
included when they are available. Subdivision of the 10-fold
uncertainty factor has been used in the development of a reference
concentration for 1,2-epoxybutane (US EPA, 1993). Chemicals for which
the approach described here has been applied include saccharin
(Renwick, 1993b), erythrosine (Poulsen, 1993), butylated
hydroxyanisole (BHA) (Wurtzen, 1993) and diethylhexyl phthalate (DEHP)
(Morgenroth, 1993).
If a data-derived factor is introduced then the commonly used
10-fold factor would be replaced by the product of that data-derived
factor and the remaining default factor. For some classes of
compounds a data-derived factor for one member of the class may be
applicable to all members, thereby producing a group-based
data-derived factor (see Calabrese, 1992). The interspecies
uncertainty factor is not necessary if the NOAEL or LOAEL is based on
human data.
4.5 Inter-individual variability in humans
A factor of 10 is normally used to allow for differences in
sensitivity in vivo between the population mean and highly sensitive
subjects. In cases where there are appropriate data on the
inter-individual variability in toxicokinetics or toxicodynamics for a
particular compound in humans, then the relevant uncertainty factor
should be replaced by the data-derived factor. Data on PBPK may also
be able to contribute to this assessment. If a data-derived factor is
introduced, then the commonly used 10-fold factor would be replaced by
the product of the data-derived factor and the remaining default
factor. (For additional discussion, see Calabrese, 1985; Hattis et
al., 1987).
For some compounds, it may be known that a subset of the
population would be particularly sensitive, for example due to
deficiencies in detoxication processes. Many of the enzymes involved
in xenobiotic biotransformation are polymorphically distributed in the
human population. Such polymorphism should be taken into account
where the enzymatic differences result either in a marked change in
bioavailability or clearance of the parent compound or in a major
change in the extent of formation of the toxic entity. In cases where
the default factor will not adequately cover this additional
variability, then the default should be modified appropriately.
Alternatively, these groups may require special strategies for health
protection. In cases where the risk assessment is based on in vivo
data in the sensitive subgroup, then the composite factor (10) should
be reduced to a much lower value. A value of 1 could be used if there
is an extensive data base in humans and the data base adequately
addresses any identified sensitive subgroups. For example, the US EPA
estimated an oral reference dose for fluoride based on the absence of
dental mottling in children 12 to 14 years of age. Since this group
was considered to be a sensitive subpopulation, a factor of 1 for
inter-individual variation was considered to be appropriate (US EPA,
1993).
4.6 Other considerations
4.6.1 Adequacy of the overall data base
Major deficiencies in a toxicity data base (other than those
related to the pivotal study) which increase the uncertainty of the
extrapolation process should be recognized by the use of an additional
uncertainty factor. Since the quality and/or completeness of
different data bases vary, the additional uncertainty factor will also
vary. For example, a value of 1 would be applied to a data base that
was considered complete for the evaluation of the compound under
consideration, but a factor of 1-100 might be necessary for limited
data bases. If minor deficiencies in the data exist with respect to
quality, quantity or omission, then an extra factor of 3 or 5 would be
appropriate. An extra factor of 10 would be appropriate where major
deficiencies in the data exist with respect to quality, quantity or
omission, such as a lack of chronic toxicity studies and reproductive
toxicity studies (for additional discussion see Dourson et al., 1992).
It should be appreciated that when very large uncertainty factors
are incorporated, the derived TI should be considered as an very
imprecise temporary estimate pending the generation of a better data
base. It should be recognized that inadequacies of the pivotal study
(section 4.3) could also be considered as a subset of inadequacies of
the data base; the total factor for limitations of the pivotal study
plus adequacy of the overall data base should not exceed 100 since
such a data base is generally not acceptable for development of a TI.
4.6.2 Nature of toxicity
The nature of toxicity, i.e. whether the effect is adverse or
not, is considered in the determination of NOAEL and LOAEL. For
example, a concentration or dose which induces a transient increase in
organ weight without accompanying biochemical or histopathological
effects might be considered to be a NOAEL. If there are accompanying
adverse histopathological effects in the target organ, the lowest
concentration or dose at which these effects occur would be considered
a LOAEL. The sensitivity of analyses of effects should also be taken
into account in establishing the NOAEL or LOAEL (see discussion in
section 4.2).
In addition, a number of bodies, including the WHO and FAO Joint
Expert Committee on Food Additives (JECFA) and the Joint Meeting on
Pesticide Residues (JMPR) have incorporated an additional "safety
factor" of up to 10 (corresponding to an uncertainty factor in the
current discussion) in cases where the NOAEL is derived for a critical
effect which is a severe and irreversible phenomenon, such as
teratogenicity or non-genotoxic carcinogenicity, especially if
associated with a shallow dose-response relationship (Weil, 1972; WHO,
1987, 1990). Provision for the application of additional safety
factors is included in the sequence shown in Fig. 3.
4.7 Final review of the total uncertainty factor
It is important that there is a final review of the total
uncertainty factor applied, particularly in cases where a low value
has been used, based on toxicokinetic or toxicodynamic data, to
replace one of the default values. Under such circumstances, a TI
derived on the basis of the appropriate overall uncertainty factor for
that toxic effect might be greater than that which would be produced
by an alternative, well-defined toxic end-point observed at slightly
higher intakes or exposures. For this reason, there are arrows shown
in Fig. 3 leading back to the data base.
4.8 Precision of the tolerable intake
The TI is calculated by dividing the NOAEL for the critical
effect by the derived total uncertainty factor. The precision of the
estimate depends in large part on the magnitude of the overall
uncertainty factor used in the calculation. The precision is probably
to one significant figure at best, and more usually to one order of
magnitude, and for uncertainty factors of 1000 or more the precision
becomes even less. Because of the imprecision of the default factors
and in order to maintain credibility of the risk assessment process,
the total default uncertainty factor should not exceed 10 000. If the
risk assessment leads to a higher factor then the resulting TI would
be so imprecise as to lack meaning. Such a situation indicates an
urgent need for additional data.
4.9 Alternative approaches
Approaches being developed to characterize quantitatively the
dose-response relationship for non-threshold effects (including the
benchmark dose and categorical regression) are described in
Appendix 3.
5. ALLOCATION OF TOLERABLE INTAKES TO DERIVE GUIDANCE VALUES
5.1 General considerations
Allocations of the TIs to various media for the development of
guidance values are based on relative proportion of total exposure
from each of the media. This necessitates the presentation of
consistent and detailed estimates of exposure for as many media as
possible in draft EHCs prior to review and evaluation. Wherever
possible, estimation of exposure should be based on concentrations in
environmental media including (but not necessarily limited to) air,
food, drinking-water, soil and consumer products. With respect to
soil, wherever possible, estimated exposure should take into account
both ingestion and dermal contact. Since the bioavailability of
contaminants in soil from both ingestion and dermal contact may be
limited, this should be taken into account in assessing the
contribution that soil makes to total intake from all media.
It is recommended that unless there are other age groups which
are more sensitive or have widely differing exposure profiles, intake
from each of the media (generally expressed as µg/kg body weight per
day) should be estimated for adults, based on ICRP reference values
for body weights and ingestion volumes (ICRP, 1974; Appendix 4).
Wherever possible, estimation of exposure should be based on ranges of
mean concentrations in environmen-tal media on a global basis. Where
data are more limited, ranges of individual values could be used.
Estimates of exposure as a basis for derivation of guidance values are
presented in the examples in Appendix 1.
Where the data on concentrations of a substance in environmental
media are inconsistent or inadequate, exposure can be estimated based
on models which incorporate as much data as possible on, for example,
production, use patterns and physical and chemical properties. Models
to predict distribution in environmental media and estimation of
proportion of total exposure by various routes from consumer products
are available (Mackay, 1991; USES, 1994). For estimation of
proportions of exposure from various environmental media for
development of guidance values in EHCs, it is recommended that the
latest version of the Mackay level III model be used (Mackay et al.,
1992). It is important that all assumptions concerning releases and
physico-chemical properties and limitations of the estimated
proportions be clearly specified. In some cases, it may also be
possible to estimate the contribution of each medium to total exposure
on the basis simply of data on physical and chemical properties (e.g.,
for substances which are likely to be present primarily in one
environmental medium).
When available, toxicokinetic data should be used to the extent
possible in extrapolating across routes in the approaches to
allocation described below. Dermal exposure and absorption should
also be taken into account in the derivation of guidance values,
although relevant data are often not available. It is also recognized
that a source in one medium (e.g., potable water) may lead to
additional intake from other routes (e.g., dermal and inhalation) and
that, where possible, such intake should be considered in the
derivation of guidance values.
In addition, total allocations of less than 100% of the TI are
encouraged to account for, for example, those media for which exposure
has not been characterized and cross-route exposure. The magnitude of
the proportion of total intake which is not allocated should vary as a
function of the adequacy of characterization of total exposure from
all media.
In cases where the proportion of total exposure from a specific
medium is small (less than a few percent), allocation for derivation
of guidance values is not recommended since this would result in
direction of risk management strategies to media which are
inconsequential in contributing to total exposure.
5.2 General approach
The steps subsequent to development of a TI in deriving guidance
values for a general population are as follows:
1. If necessary, conversion of TIs for systemic effects for different
routes of exposure to a common unit for comparison based on
consideration of volumes and rates of inhalation and ingestion and
relevant toxicokinetic data, such as bioavailability, if available.
2. Allocation of TI to various routes and media based on estimated
exposure developed on the basis of available data on measured
concentrations or predicted proportions (i.e., model-derived values)
to which humans are exposed. Default values can be used in the
absence of data on measured concentrations or predicted proportions of
total exposure in various media.
3. Development of guidance values from intake assigned to each
medium, taking into account, for instance, body weight, volume of
intake and (relative) absorption efficiency ( relative where guidance
value is derived on the basis of a TI by another route of exposure).
Guidance values for drinking-water are generally expressed in µg/litre
or mg/litre, those in food as µg/g or mg/kg, those in air as µg/m3
or mg/m3, and those for dermal exposure as µg/m2 surface area.
5.3 Detailed approach
In the following section, an approach to the allocation of
tolerable intakes for development of guidance values (general
population) is provided by way of example for most of the scenarios
which may arise based on evaluations presented in EHCs.
The five most likely scenarios are considered to be:
5.3.1 Biomarkers of exposure
There is a common biomarker related to the critical effect which
integrates exposure from all sources. For example, Choudhury et al.
(1992) describe a model which predicts blood lead concentrations as a
function of concentrations in various media.
* The contributions from the various media are determined based on
a quantitative biomarker. Following allocation to various media
based on an exposure scenario, guidance values are developed
through incorporation of adjustment of body weight and volume of
intake for each medium.
5.3.2 Critical effects which are not route specific
TIs have been derived for each route, e.g., TI for oral exposure
(TIo) and TI for inhalation (TIi), and are based either on the
same or on different critical effects which are not at the portal of
entry. The TIs for the two routes are similar within one order of
magnitude since such variation is consistent with that inherent in
deriving TIs, as discussed in section 4, e.g., developmental toxicity
of 2-methoxyethanol (Doe et al., 1983; Wickramaratne, 1986). This
reflects the assumption that, in the absence of data to the contrary,
exposure via each route is considered to contribute to a combined dose
at the target site(s), i.e., additivity of dose at the target site(s).
* Allocate one TI to various media based on an exposure scenario to
determine the intake in each medium on which guidance values
should be based. Selection of the TIo or the TIi for this
purpose should be based on either:
a) if there is one major route of exposure then the TI for that
route should be used (if there is confidence in the data
base on which the exposure estimates are based); or
b) the more conservative TI (if there is uncertainty about the
relative contribution of various routes or media to total
exposure).
5.3.3 Difference in magnitude of effect by route of exposure
TIo and TIi for similar effects vary by 1 to 2 orders of
magnitude (exact magnitude of the difference for which this approach
is appropriate will be dependent upon availability of additional data;
e.g., manganese is more potent by inhalation than by ingestion).
* Derive the guidance values independently for each route (for
example, the oral and inhalation routes, based on the TIo and
TIi, respectively), but allocate the proportion of the TI for
each route to the appropriate medium or media based on an
exposure scenario.
5.3.4 Route-specific effect variation at portals of entry
(due to local bioactivation or local effects)
TIo and TIi for route-specific effects at the site of entry
vary by 1 to 2 orders of magnitude (exact magnitude of the difference
for which this approach is appropriate will be dependent upon
knowledge of additional data; e.g., nasal toxicity following
inhalation of acrylic acid).
* Derive the guidance values independently for each route (for
example, the oral and inhalation routes, based on the TIo and
TIi, respectively), using the full TI for each route to the
appropriate medium or media based on an exposure scenario.
5.3.5 Limited data base
In this scenario, the data base is limited such that only either
a TIo or a TIi can be developed.
* Allocate the available TI to various media based on an exposure
scenario to determine the intake in each medium on which guidance
values should be based, if the effects are qualitatively similar,
if toxicokinetic data are consistent with this approach and if
there are no effects at the site of entry. If any one of these
criteria is not met, do not derive guidance values for the
alternate route. If a TI is available for a route of exposure
which does not make an important contribution to total intake, do
not derive guidance values for that route.
6. EXAMPLES OF THE DERIVATION OF GUIDANCE VALUES
Example 1
The principal route of exposure is oral. Based on estimated
exposure for a scenario in the general environment, 50% of total
intake comes from food, 20% from water and 30% from air.
Data are adequate to establish both a TIo and a TIi. The
TIo and the TIi are based on similar effects and are similar
(within one order of magnitude).
Allocate 50% of TIo to food to derive a guidance value for food
* multiply TIo by 0.5
Allocate 20% of TIo to water to derive a guidance value for
drinking-water
* multiply TIo by 0.2
Allocate 30% of TIo to air to derive a guidance value for air
* multiply TIo by 0.3
Example 2
Based on an exposure scenario, 70% of total intake comes from
air, 20% from water and 10% from food. The compound is also present
in some consumer products but quantification of exposure is not
possible. There are no data on concentrations in soil but due to its
physicochemical properties, concentrations in this medium are likely
to be low.
Data are sufficient to establish a TIi and a TIo. The TIo
and the TIi are based on similar effects and are similar to within
an order of magnitude.
Convert TIi so that the values for the TIs for different routes
are expressed in the same units for comparison (generally mg/kg body
weight per day). This requires incorporation of information on
inhalation volumes, body weight and toxicokinetic data, if available.
Use TI for principal route of exposure to derive guidance values:
Allocate 63% of TIi to air to derive a guidance value for air.
* multiply TIi by 0.63
Allocate 18% of TIi to water to derive a guidance value for
drinking-water.
* multiply TIi by 0.18
Allocate 9% of TIi to food to derive a guidance value for food.
* multiply TIi by 0.09
Reserve 10% for exposure from consumer products and soil.
(Wherever possible, there should be an attempt to quantitatively
estimate the proportion of total intake from these sources).
Develop a guidance value for each medium by (if necessary)
adjustment for body weight, volume of intake and relative absorption.
Example 3
The principal route of exposure is oral. Based on estimated
exposure, 50% of total intake comes from food, 20% from water, 20%
from air and 10% from soil (after taking bioavailability into account
from the oral and dermal routes). The compound is believed not to be
present in consumer products.
Data are adequate to establish both a TIo and a TIi. The
TIo and the TIi are based on the same effects but the compound is
much more toxic by the oral route (e.g., TIo is less than the TIi
by more than two orders of magnitude).
Allocate 50% of TIo to food to derive a guidance value for food
* multiply TIo by 0.5
Allocate 20% of TIo to water to derive a guidance value for
drinking-water
* multiply TIo by 0.2
Allocate 10% of TIo to soil to derive a guidance value for soil
* multiply TIo by 0.1
Allocate 20% of TIi to air to derive a guidance value for air
* multiply TIi by 0.2
Example 4
The principal route of exposure is oral. Based on estimated
exposure, 50% of total intake comes from food, 20% from water and 30%
from air. There are no data indicating exposure from soil and
consumer products.
Data are adequate to establish both a TIo and a TIi for
route-specific effects. The TIo and the TIi are based on
route-specific effects and the compound is much more toxic by the oral
route (e.g., TIo is less than the TIi by more than two orders of
magnitude).
Because the effects are route specific and the TIs are different
by two orders of magnitude, each TI can be allocated in full to
appropriate media.
Allocate 50/70 (71%) of TIo to food to derive a guidance value
for food
* multiply TIo by 0.71
Allocate 20/70 (29)% of TIo to water to derive a guidance value
for drinking-water
* multiply TIo by 0.29
Allocate 100% of TIi to air to derive a guidance value for air
* multiply TIi by 1
Example 5
The principal route of exposure is inhalation.
Data are inadequate to establish a TIi.
Data are sufficient to establish a TIo.
Available toxicokinetic data are inadequate for or inconsistent
with extrapolation across routes.
Do not establish guidance values.
REFERENCES
Allen BC, Van Landingham C, Howe RB, Kavlock RJ, Kimmel CA, & Faustman
EM (1992) Dose-response modeling for developmental toxicity.
Toxicologist, 12(1): 300.
Allen B, Kavlock RJ, Kimmel CA, & Faustman EM (1993) Comparison of
quantitative dose response modeling approaches for evaluating fetal
weight changes in segment II developmental toxicity studies.
Teratology, 47(5): 41.
ATSDR (1989) Toxicological profile for aldrin/dieldrin. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (Publication
ATSDR/TP-88/01).
Barnes DG & Dourson M (1988) Reference dose (RfD): Description and use
in health risk assessment. Regul Toxicol Pharmacol, 8: 471-486.
Calabrese EJ (1985) Uncertainty factors and interindividual
variation. Regul Toxicol Pharmacol, 5: 190-196.
Calabrese EJ (1992) Does the animal to human uncertainty factor
incorporate interspecies differences in surface area? Regul Toxicol
Pharmacol, 15(2): 172-179.
Canada (1988) Government of Canada: Canadian Environmental Protection
Act, Ottawa. Canada Gaz, II(4): 415-512.
Choudhury H, Peirano WB, Marcus A, Elias R, Griffin S, & Derosa CT
(1992) Utilization of uptake biokinetic (UBK) lead model to assess
risk in contaminated sites. In: Superfund risk assessment in soil
contamination studies. Philadelphia, Pennsylvania, American Society of
Testing and Materials, pp 193-204.
Crump KS (1984) A new method for determining allowable daily intakes.
Fundam Appl Toxicol, 4: 854-871.
Doe JE, Samuels DM, Tinston DJ, & Wickramaratne GA de S (1983)
Comparative aspects of the reproductive toxicology by inhalation in
rats of ethylene glycol monomethyl ether and propylene glycol
monomethyl ether. Toxicol Appl Pharmacol, 69: 43-47.
Dourson ML & Stara J (1983) Regulatory history and experimental
support of uncertainty (safety) factors. Regul Toxicol Pharmacol, 3:
224-238.
Dourson ML, Hertzberg R, Hartung R, & Blackburn K (1985) Novel methods
for the estimation of acceptable daily intake. Toxicol Ind Health,
1(4): 23-33.
Dourson ML, Knauf L, & Swartout J (1992) On reference dose (RfD) and
its underlying toxicity data base. Toxicol Ind Health, 8(3): 171-189.
Farland W & Dourson ML (1992) Noncancer health endpoints: approaches
to quantitative risk assessment. In: Cothern R ed. Comparative
environmental risk assessment. Boca Raton, Florida, Lewis Publishers,
pp 87-106.
Gaylor DW (1989) Quantitative risk analysis for quantal reproductive
and developmental effects. Environ Health Perspect, 79: 243-246.
Gaylor DW (1992) Incidence of developmental defects at the No
Observed Effect Level (NOEL). Regul Toxicol Pharmacol, 15: 151-160.
Guth DJ, Jarabek AM, Wymer L, & Hertzberg RC (1991) Evaluation of risk
assessment methods for short-term inhalation exposure. Presentation
at the 84th Annual Meeting of the Air and Waste Management
Association, Vancouver, British Columbia, 16-21 June 1991.
Hallenbeck WH & Cunningham KM (1986) Quantitative risk assessment for
environmental and occupational health. Chelsea, Michigan, Lewis
Publishers, Inc., p 199.
Hattis D, Erdreich L, & Ballew M (1987) Human variability in
susceptibility to toxic chemicals -A preliminary analysis of
pharmacokinetic data from normal volunteers. Risk Anal, 7(4):
415-426.
Hartung R (1986) Ranking the severity of toxic effects. In: Trace
substances in environmental health. Columbia, Missouri, University of
Missouri, pp 204-211.
Hartung R & Durkin PR (1986) Ranking the severity of toxic effects:
Potential applications to risk assessment. Comments Toxicol, 1: 49-63.
Health and Welfare Canada (1992) Determination of "toxic" under
paragraph 11c) of the Canadian Environmental Protection Act, 1st ed.
Ottawa, Ontario, Bureau of Chemical Hazards, Environmental Health
Directorate.
Hertzberg RC (1989) Fitting a model to categorical response data with
application to special extrapolation to toxicity. Health Phys,
57(Suppl 1): 404-409.
Hertzberg RC & Miller M (1985) A statistical model for species
extrapolation using categorical response data. Toxicol Ind Health, 1:
43-57.
ICRP (1974) International Commission on Radiological Protection:
Report of the Task Group on Reference Man. Oxford, Pergamon Press
(ICRP Publication No. 23).
Jarabek AM, Menache MG, Overton JH Jr, Dourson ML, & Miller FJ (1990)
The US Environmental Protection Agency's inhalation RfD methodology:
Risk assessment for air toxics. Toxicol Ind Health, 6(5): 279-301.
Kavlock RJ, Greene JA, Kimmel GL, Morrissey RE, Owens E, Rogers JM,
Sadler TW, Stack F, Waters MD, & Welsch F (1991) Activity profiles of
developmental toxicity: design considerations and pilot
implementation. Teratology, 43: 159-185.
Kimmel CA & Gaylor DW (1988) Issues in qualitative and quantitative
risk analysis for developmental toxicology. Risk Anal, 8: 15-20.
Lehman AJ & Fitzhugh OG (1954) 100-fold margin of safety. Assoc Food
Drug Off US Q Bull, 18: 33-35.
Lewis SC, Lynch JR, & Nikiforov AI (1990) A new approach to deriving
community exposure guidelines from no-observed-adverse-effect levels.
Regul Toxicol Pharmacol, 11: 314-330.
Lu F (1988) Acceptable daily intake: inception, evolution and
application. Regul Toxicol Pharmacol, 8(1): 45-60.
Mackay D (1991) Multimedia environmental models. The fugacity
approach. Chelsea, Michigan, Lewis Publishers, Inc., 260 pp.
Mackay D, Paterson S, & Shiu WY (1992) Generic models for evaluating
the regional fate of chemicals. Chemosphere, 24: 695-717.
Moolgavkar SH, Dewanji A, & Venzon DJ (1988) A stochastic two-stage
model for cancer risk assessment. I: The hazard function and the
probability of tumor. Risk Anal, 8: 383-392.
Morgenroth V (1993) Scientific evaluation of the data-derived safety
factors for the acceptable daily intake. Case study:
diethylhexylphthalate. Food Addit Contam, 10(3): 363-373.
NAS (1977) Drinking water and health. Washington, DC, National
Research Council, US National Academy of Sciences, 939 pp.
Nau H (1986) Species differences in pharmacokinetics and drug
teratogenesis. Environ Health Perspect, 70: 113-129.
Poulsen E (1993) Case study: erythrosine. Food Addit Contam, 10:
315-323.
Renwick AG (1993a) Data-derived safety factors for the evaluation of
food additives and environmental contaminants. Food Addit Contam, 10:
275-305.
Renwick AG (1993b) A data-derived safety (uncertainty) factor for the
intense sweetener, saccharin. Food Addit Contam, 10: 337-350.
Rubery ED, Barlow SM, & Steadman JH (1990) Criteria for setting
quantitative estimates of acceptable intakes of chemicals in food in
the U.K. Food Addit Contam, 7: 287-302.
Shoaf CR (1994) US EPA inhalation testing guidelines. In: Jenkins PG,
Kayser D, Muhle H, Rosner G, & Smith EM ed. Respiratory toxicology and
risk assessment. Proceedings of an International Symposium. Stuttgart,
Wissenschaftliche Verlagsgesellschaft mbH, pp 11-25 (IPCS Joint Series
No. 18).
US EPA (1985a) National primary drinking water regulations; volatile
synthetic organic chemicals; final rule and proposed rule. Fed Reg,
50: 46830-46901.
US EPA (1985b) National primary drinking water regulations, synthetic
organic chemicals, inorganic chemicals and microorganisms; proposed
rule. Fed Reg, 50: 46936-47025.
US EPA (1993) The Integrated Risk Information System (IRIS).
Cincinnati, Ohio. Office of Health and Environmental Assessment,
Environmental Criteria and Assessment Office.
USES (1994) Uniform system for the evaluation of substances (USES),
version 1.0. Bilthoven, The Netherlands, National Institute for
Public Health and Environmental Protection, 332 pp.
Waters MD, Stack HF, Brady AL, Lohman PHM, Haroun L, & Vainio H (1988)
Use of computerized data listings and activity profiles of genetic and
related effects in the review of 195 compounds. Mutat Res, 205:
295-312.
Weil CS (1972) Statistics versus safety factors and scientific
judgement in the evaluation for man. Toxicol Appl Pharmacol, 21:
454-463.
WHO (1987) IPCS Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food. Geneva,
World Health Organization, 174 pp.
WHO (1990) IPCS Environmental Health Criteria 104: Principles for the
toxicological assessment of pesticide residues in food. Geneva, World
Health Organization. 117 pp.
WHO (1993) Guidelines for drinking-water quality, Second edition.
Volume 1: Recommendations. Geneva, World Health Organization, 198 pp.
Wickramaratne GA de S (1986) The teratogenic potential and
dose-response of dermally administered ethylene glycol monomethyl
ether (EGME) estimated in rats with the Chernoff-Kavlock assay. J Appl
Toxicol, 6(3): 165-166.
Wurtzen G (1993) Scientific evaluation of the safety factor for the
acceptable daily intake (ADI). Case study: butylated hydroxy anisole
(BHA). Food Addit Contam, 10: 307-314.
Zielhuis RL & van der Kreek FW (1979a) The use of a safety factor in
setting health based permissible levels for occupational exposure. Int
Arch Occup Environ Health, 42: 191-201.
Zielhuis RL & van der Kreek FW (1979b) Calculation of a safety factor
in setting health based permissible levels for occupational exposure.
Int Arch Occup Environ Health, 42: 203-215.
APPENDIX 1
EXAMPLES - DEVELOPMENT OF GUIDANCE VALUES
The following practical examples are provided to illustrate the
manner in which tolerable intakes (TIs) may be developed and allocated
for the derivation of guidance values for a general population (on the
basis of calculated proportions of exposure from various media). In
the calculation of guidance values, TIs may be rounded up to 1 or 2
significant figures depending on the quality of the data base and the
extent of uncertainties involved in deriving the TI. The level of
detail shown is that which is considered necessary for EHCs and should
be sufficient for adaptation at national and local levels.
Compound A
Chlorinated hydrocarbon
Estimates of exposure
Estimated daily intakes of Compound A for adults (µg/kg
body weight per day)1 in the general population are as follows:
Ambient air2 < 0.03
Drinking-water3 0.00007-< 0.0004
Food4 0.004
Soil no data
Consumer products no data
Total Intake 0.03
1 Assumed to weigh 64 kg, breathe 22 m3 of air per day and drink
1.4 litres of water Per day (ICRP, 1974) and to consume 125 g per day
of a meat composite (the compound was not detected in other dietary
composites).
2 Based on a mean concentration of Compound A reported in a survey
of ambient Air from 22 sites (< 0.10 µg/m3); concentrations in
indoor air were similar to those in ambient air.
3 Based on a range of mean concentrations of Compound A in
drinking-water of 0.003 µg/litre to < 0.02 µg/litre.
4 Based on a concentration of 0.0018 µg/g of Compound A detected in
a representative daily diet.
On the basis of these estimates, it is considered that the
percentage of total exposure from various media for the general
population (midpoints of estimated intakes) is as follows:
outdoor/indoor air = < 0.03/0.03 = 85.9% (86%)
(< 0.03 considered to be 0.03 minus
intake from other media)
drinking-water = 0.000245/0.03 = 0.82% (0.8%)
food = 0.004/0.03 = 13.3% (13%)
soil = no data
consumer products = no data
Development of TI
The only data identified on long-term toxicity following
inhalation are the results of a single subchronic study for which no
effects were observed at any concentration. Available data are
considered inadequate, therefore, to establish a TI on the basis of
the results of studies in which Compound A has been administered by
inhalation. Moreover although the general population appears to be
exposed to Compound A principally in air, based on limited available
data on concentrations in food, the estimated intake in food is within
the range of that estimated for air for some age groups. In addition,
the principal route of intake of the most exposed age group (i.e.
suckling infants) is ingestion (of mothers' milk). Owing to the lack
of adequate long-term toxicity studies by the inhalation route and the
possible relatively important contribution that food makes to total
exposure to Compound A, a TI is derived on the basis of a long-term
ingestion bioassay, as follows:
60 mg/kg body
weight per day × 5
TI = approx. 0.43 mg/kg (430 µg/kg)
body weight per day
100 × 7
where:
* 60 mg/kg body weight per day is the NOAEL, determined in a
well-conducted and documented long-term (chronic and
carcinogenesis) bioassay, with renal tubular degeneration
observed at higher doses
* 5/7 is the conversion of five days per week of dosing to seven
days per week
* 100 is the uncertainty factor (×10 for inter-individual
variation; ×10 for interspecies variation; available data on
toxicokinetics and toxicodynamics were inadequate to modify the
10 × 10-fold uncertainty factor)
Derivation of Guidance Values
Outdoor/indoor air
The proportion of TI allocated to outdoor air based on exposure
estimates = 86%
86% × TI (430 µg/kg body
weight per day) = 370 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
mean body weight of adults = 64 kg
Guidance value for 370 µg/kg × 64 kg
outdoor/indoor air =
22 m3
= 1100 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 0.8% (too small to permit development of
meaningful guidance values since it contributes negligibly to total
intake)
Food
The proportion of TI allocated to food based on exposure
estimates = 13%
13% × TI (430 µg/kg
body weight per day) = 57 µg/kg body weight per day
(tolerances in various foodstuffs can be developed on the basis
of the amounts ingested.)
Soil
Owing to lack of relevant data, it is not possible to allocate a
proportion of the TI to this source.
Compound B
Chlorinated hydrocarbon solvent
Estimates of Exposure
Estimated daily intakes of Compound B for adults (µg/kg body
weight per day)1 in the general population are as follows:
Ambient air2 0.01-0.27
Indoor air3 1.4
Drinking-water4 0.002-0.02
Food5 0.12
Soil no data
Consumer products no data
Total Intake 1.5-1.8
On the basis of these estimates, it is considered that the
percentage of total exposure from various media for the general
population (based on midpoints of estimated intakes) is as follows:
outdoor air = 0.14/1.67 = 8.3%
indoor air = 1.4/1.67 = 83.8% (84%)
drinking-water = 0.011/1.67 = 0.65%
food = 0.12/1.67 = 7.1%
soil = no data
consumer products = no data
1 Assumed to weigh 64 kg, breathe 22 m3 air and drink 1.4 litres
of water per day (ICRP, 1974).
2 Assumed to spend 4 h/day outdoors and based on a range of mean
concentrations of Compound B (0.2 to 5.0 µg/m3) from a survey.
3 Assumed to spend 20 h/day indoors and based on the mean
concentration of Compound B of approximately 5.1 µg/m3 in the
indoor air of 757 randomly selected homes examined in a survey.
4 Based on a range of mean concentrations of Compound B (0.1 to
0.9 µg/litre) in drinking-water from a number of surveys.
5 Based on the average levels of Compound B in the various
composite food groups in a study on the daily intake of these
food groups.
Development of TIs
A Tolerable Intake for Compound B can be derived as follows:
[(678 mg/m3) × (0.043 m3/day) × (6/24) × (5/7)]
TI =
(0.0305 kg) × 1000
= 170 µg/kg body weight per day
where:
* 678 mg/m3 is the lowest-observed-adverse-effect level (LOAEL)
overall in mice determined in an adequate long-term inhalation
study and based on reduced survival and hepato-toxicity in males,
and lung congestion and nephrotoxicity in males and females.
* 0.043 m3/day is the assumed volume of air inhaled by mice
* 6/24 and 5/7 is the conversion of 6 h/day, 5 days/week to
continuous exposure.
* 0.0305 kg is the average body weight of the mice in the critical
study.
* 1000 is the uncertainty factor (×10 for inter-individual
variation, ×10 for interspecies variation since available data on
toxicokinetics and toxicodynamics were inadequate for
modification of these factors, ×10 for use of a LOAEL rather than
a NOAEL).
In order to ensure that the TI derived on the basis of an
inhalation study is sufficiently protective, another TI can be derived
on the basis of studies in which Compound B was administered by
ingestion. With the exception of one investigation in which
reversible erythropoietic damage was reported at low concentrations
(50 µg/kg body weight per day) but not confirmed in other studies, the
lowest NOAEL in the longest-term (90-day) available study in which
Compound B was administered orally in drinking-water to rats is
14 mg/kg body weight per day, based on effects on body weight gain,
the ratio of liver or kidney weight to body weight, and serum
5'-nucleotidase activity at the next highest dose. A LOEL of 20 mg/kg
body weight per day based on a slight increase in liver weight was
reported in a 6-week study on mice. Values for the TI derived on the
basis of the results of these two studies are within the same order of
magnitude as the TI calculated from the inhalation study.
Derivation of Guidance Values
Since the TIs derived on the basis of studies by inhalation and
ingestion are within the same range and inhalation is the most
important route of exposure of the general population, the TI
developed for the inhalation route will be used as the basis for
derivation of guidance values.
Outdoor air
The proportion of TI allocated to outdoor air based on exposure
estimates = 8.3%
8.3% × TI (170 µg/kg body
weight per day) = 14 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
proportion of the day
spent outdoors = 4/24
volume of outdoor air
inhaled daily = 22 m3 × 4/24 = 3.7 m3
mean body weight of adults = 64 kg
Guidance value for 14 µg/kg × 64 kg
outdoor air =
3.7 m3
= 242 µg/m3
Indoor air
The proportion of TI allocated to indoor air based on exposure
estimates = 84%
84% × TI (170 µg/kg body
weight per day) = 140 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
proportion of the day
spent indoors = 20/24
volume of indoor air
inhaled daily = 22 m3 × 20/24 = 18 m3
mean body weight of adults = 64 kg
Guidance value for 140 µg/kg × 64 kg
indoor air =
18 m3
= 498 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 0.65% (too small to permit development of
meaningful guidance values since it contributes negligibly to total
intake)
Food
The proportion of TI allocated to food based on exposure
estimates = 7.1%
7.1% × TI (170 µg/kg body
weight per day) = 12 µg/kg body weight per day or
10 µg/kg body weight per day to
one significant figure
(tolerances in various foodstuffs could then be developed on the
basis of amounts ingested.)
Soil
Owing to lack of relevant data, it is not possible to allocate a
proportion of the TI to this source.
Compound C
Naturally occurring inorganic chemical
Estimates of Exposure
The percentage of total exposure from various media for adults in
the general population in country 1 is as follows:
outdoor/indoor air = 0.02%
drinking-water = 6.9%
food = 80%
soil = 0.11%
consumer products = 12.8%
In contrast, the percentage of total exposure from various media
for adults in the general population in one area in country 2 is as
follows:
outdoor/indoor air = 35%
drinking-water = 11%
food = 55%
Development of TIs
It is concluded, on the basis of data from several studies in
human populations, that the TI is 200 µg/kg body weight per day.
Derivation of Guidance Values - Country 1
Outdoor/indoor air
The proportion of TI allocated to air based on exposure estimates
= 0.02% (too small to permit development of meaningful guidance
values)
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 6.9%
6.9% × TI (200 µg/kg body
weight per day) = 13.8 µg/kg body weight per day
daily volume of ingestion of
drinking-water for adults
in Country 1 = 1.5 litres
mean body weight of adults
in Country 1 = 70 kg
Guidance value for 13.8 µg/kg × 70 kg
drinking-water =
1.5
= 644 µg/litre
Food
The proportion of TI allocated to food based on exposure
estimates = 80%
80% × TI (200 µg/kg body 160 µg/kg body weight per day
weight per day) = or 200 µg/kg body weight per
day to one significant figure
(tolerances in various foodstuffs can then be developed on the basis
of amounts ingested.)
Soil
The proportion of TI allocated to air based on exposure estimates
= 0.11%
(too small to permit development of meaningful guidance values)
Consumer products
The proportion of TI allocated to consumer products based on
exposure estimates = 12.8%
12.8% × TI (200 µg/kg body
weight per day) = 26 µg/kg body weight per day
(limits in consumer products can be developed on the basis of
patterns of use.)
Derivation of Guidance Values - Country 2
Outdoor/indoor air
The proportion of TI allocated to air based on exposure
estimates = 35%
35% × TI (200 µg/kg body
weight per day) = 70 µg/kg body weight per day
daily inhalation volume for
adults in Country 2 = 20 m3
mean body weight of
adults in Country 2 = 60 kg
Guidance value for 70 µg/kg × 60 kg
outdoor/indoor air =
20 m3
= 210 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 11%
11% × TI (200 µg/kg body
weight per day) = 22 µg/kg body weight per day
daily volume of ingestion of
drinking-water for
adults in Country 2 = 1.5 litres
mean body weight of
adults in Country 2= 60 kg
Guidance value for 22 µg/kg × 60 kg
drinking-water =
1.5
= 880 µg/litre
Food
The proportion of TI allocated to food based on exposure
estimates = 55%
55% × TI (200 µg/kg body
weight per day) = 110 µg/kg body weight per day
(tolerances in various foodstuffs can be developed on the basis of
amounts ingested.)
Soil
No data are available.
Consumer products
No data are available.
APPENDIX 2
GRAHICAL APPROACHES
The use of graphs of dose-effect and dose-response toxicity data
to complement the text discussion in the development of TIs and
guidance values is considered valuable. Such graphs can display an
overview of the full range of dose-response information. Graphs can
range from simple "thermometer" presentations as employed by the US
Agency for Toxic Substances and Disease Registry (ATSDR, 1989), to
dose-effect and dose-response graphs for specific toxic effects such
as genotoxicity (Waters et al., 1988) or developmental toxicity
(Kavlock et al., 1991), or to dose-duration graphs described by
Hartung (1986), Hartung & Durkin (1986), and Dourson et al. (1985).
Fig. 4 is an example of a dose-duration graph and presents data
for methoxychlor adapted from Dourson et al. (1985). This figure
summarizes the available frank-effect levels (FEL), adverse-effect
levels (AEL), no-observed-adverse-effect levels (NOAEL), and
no-observed-effect levels (NOEL). Adverse-effect levels are presented
as a function of both dose in mg/day and exposure as a fraction of
lifespan.
Each point in the graph represents one dose group from one study.
The size of the point is a rough indication of its usefulness for
determining tolerable intakes, where larger points indicate more
useful information. Other information includes target organs. These
data can also be used to estimate a best fitting line for NOAEL across
duration.
Graphical presentation of available data can facilitate
identification of effect levels relevant to development of TIs.
Although the form of graphical presentation is necessarily dependent
upon the size of the data base, a dose-duration graph in which NOELs,
NOAELs and LOAELs are presented as a function of duration of exposure
is considered to be helpful and is more fully described in Appendix 2.
4.3 Adequacy of the pivotal study
In situations where a NOAEL has not been achieved but the data
on effects are of sufficient quality to be the basis of the risk
assessment, then a no-adverse-effect level should be developed by the
application of an appropriate uncertainty factor to the LOAEL.
Uncertainty factors of 3, 5 or 10 have been used previously to
extrapolate from a LOAEL to a NOAEL depending on the nature of the
effect(s) and dose-response relationship (see, for example, US EPA,
1993). Alternatively, a benchmark dose may be developed by
mathematical modelling of the dose-response data as an alternative to
the uncertainty factor in extrapolating to the NOAEL (see Appendix 3).
The pivotal study may also be considered inadequate for other reasons
(e.g., duration of study, numbers of animals per group and sensitivity
of the analyses of effect), and an additional uncertainty factor
applied.
4.4 Interspecies extrapolation
In situations where appropriate toxicokinetic and/or
toxicodynamic data exist for a particular compound, then the relevant
uncertainty factor in Fig. 3 should be replaced by the data-derived
factor. Data on PBPK and/or data on target organ exposure should be
included when they are available. Subdivision of the 10-fold
uncertainty factor has been used in the development of a reference
concentration for 1,2-epoxybutane (US EPA, 1993). Chemicals for which
the approach described here has been applied include saccharin
(Renwick, 1993b), erythrosine (Poulsen, 1993), butylated
hydroxyanisole (BHA) (Wurtzen, 1993) and diethylhexyl phthalate (DEHP)
(Morgenroth, 1993).
If a data-derived factor is introduced then the commonly used
10-fold factor would be replaced by the product of that data-derived
factor and the remaining default factor. For some classes of
compounds a data-derived factor for one member of the class may be
applicable to all members, thereby producing a group-based
data-derived factor (see Calabrese, 1992). The interspecies
uncertainty factor is not necessary if the NOAEL or LOAEL is based on
human data.
4.5 Inter-individual variability in humans
A factor of 10 is normally used to allow for differences in
sensitivity in vivo between the population mean and highly sensitive
subjects. In cases where there are appropriate data on the
inter-individual variability in toxicokinetics or toxicodynamics for a
particular compound in humans, then the relevant uncertainty factor
should be replaced by the data-derived factor. Data on PBPK may also
be able to contribute to this assessment. If a data-derived factor is
introduced, then the commonly used 10-fold factor would be replaced by
the product of the data-derived factor and the remaining default
factor. (For additional discussion, see Calabrese, 1985; Hattis et
al., 1987).
For some compounds, it may be known that a subset of the
population would be particularly sensitive, for example due to
deficiencies in detoxication processes. Many of the enzymes involved
in xenobiotic biotransformation are polymorphically distributed in the
human population. Such polymorphism should be taken into account
where the enzymatic differences result either in a marked change in
bioavailability or clearance of the parent compound or in a major
change in the extent of formation of the toxic entity. In cases where
the default factor will not adequately cover this additional
variability, then the default should be modified appropriately.
Alternatively, these groups may require special strategies for health
protection. In cases where the risk assessment is based on in vivo
data in the sensitive subgroup, then the composite factor (10) should
be reduced to a much lower value. A value of 1 could be used if there
is an extensive data base in humans and the data base adequately
addresses any identified sensitive subgroups. For example, the US EPA
estimated an oral reference dose for fluoride based on the absence of
dental mottling in children 12 to 14 years of age. Since this group
was considered to be a sensitive subpopulation, a factor of 1 for
inter-individual variation was considered to be appropriate (US EPA,
1993).
4.6 Other considerations
4.6.1 Adequacy of the overall data base
Major deficiencies in a toxicity data base (other than those
related to the pivotal study) which increase the uncertainty of the
extrapolation process should be recognized by the use of an additional
uncertainty factor. Since the quality and/or completeness of
different data bases vary, the additional uncertainty factor will also
vary. For example, a value of 1 would be applied to a data base that
was considered complete for the evaluation of the compound under
consideration, but a factor of 1-100 might be necessary for limited
data bases. If minor deficiencies in the data exist with respect to
quality, quantity or omission, then an extra factor of 3 or 5 would be
appropriate. An extra factor of 10 would be appropriate where major
deficiencies in the data exist with respect to quality, quantity or
omission, such as a lack of chronic toxicity studies and reproductive
toxicity studies (for additional discussion see Dourson et al., 1992).
It should be appreciated that when very large uncertainty factors
are incorporated, the derived TI should be considered as an very
imprecise temporary estimate pending the generation of a better data
base. It should be recognized that inadequacies of the pivotal study
(section 4.3) could also be considered as a subset of inadequacies of
the data base; the total factor for limitations of the pivotal study
plus adequacy of the overall data base should not exceed 100 since
such a data base is generally not acceptable for development of a TI.
4.6.2 Nature of toxicity
The nature of toxicity, i.e. whether the effect is adverse or
not, is considered in the determination of NOAEL and LOAEL. For
example, a concentration or dose which induces a transient increase in
organ weight without accompanying biochemical or histopathological
effects might be considered to be a NOAEL. If there are accompanying
adverse histopathological effects in the target organ, the lowest
concentration or dose at which these effects occur would be considered
a LOAEL. The sensitivity of analyses of effects should also be taken
into account in establishing the NOAEL or LOAEL (see discussion in
section 4.2).
In addition, a number of bodies, including the WHO and FAO Joint
Expert Committee on Food Additives (JECFA) and the Joint Meeting on
Pesticide Residues (JMPR) have incorporated an additional "safety
factor" of up to 10 (corresponding to an uncertainty factor in the
current discussion) in cases where the NOAEL is derived for a critical
effect which is a severe and irreversible phenomenon, such as
teratogenicity or non-genotoxic carcinogenicity, especially if
associated with a shallow dose-response relationship (Weil, 1972; WHO,
1987, 1990). Provision for the application of additional safety
factors is included in the sequence shown in Fig. 3.
4.7 Final review of the total uncertainty factor
It is important that there is a final review of the total
uncertainty factor applied, particularly in cases where a low value
has been used, based on toxicokinetic or toxicodynamic data, to
replace one of the default values. Under such circumstances, a TI
derived on the basis of the appropriate overall uncertainty factor for
that toxic effect might be greater than that which would be produced
by an alternative, well-defined toxic end-point observed at slightly
higher intakes or exposures. For this reason, there are arrows shown
in Fig. 3 leading back to the data base.
4.8 Precision of the tolerable intake
The TI is calculated by dividing the NOAEL for the critical
effect by the derived total uncertainty factor. The precision of the
estimate depends in large part on the magnitude of the overall
uncertainty factor used in the calculation. The precision is probably
to one significant figure at best, and more usually to one order of
magnitude, and for uncertainty factors of 1000 or more the precision
becomes even less. Because of the imprecision of the default factors
and in order to maintain credibility of the risk assessment process,
the total default uncertainty factor should not exceed 10 000. If the
risk assessment leads to a higher factor then the resulting TI would
be so imprecise as to lack meaning. Such a situation indicates an
urgent need for additional data.
4.9 Alternative approaches
Approaches being developed to characterize quantitatively the
dose-response relationship for non-threshold effects (including the
benchmark dose and categorical regression) are described in
Appendix 3.
5. ALLOCATION OF TOLERABLE INTAKES TO DERIVE GUIDANCE VALUES
5.1 General considerations
Allocations of the TIs to various media for the development of
guidance values are based on relative proportion of total exposure
from each of the media. This necessitates the presentation of
consistent and detailed estimates of exposure for as many media as
possible in draft EHCs prior to review and evaluation. Wherever
possible, estimation of exposure should be based on concentrations in
environmental media including (but not necessarily limited to) air,
food, drinking-water, soil and consumer products. With respect to
soil, wherever possible, estimated exposure should take into account
both ingestion and dermal contact. Since the bioavailability of
contaminants in soil from both ingestion and dermal contact may be
limited, this should be taken into account in assessing the
contribution that soil makes to total intake from all media.
It is recommended that unless there are other age groups which
are more sensitive or have widely differing exposure profiles, intake
from each of the media (generally expressed as µg/kg body weight per
day) should be estimated for adults, based on ICRP reference values
for body weights and ingestion volumes (ICRP, 1974; Appendix 4).
Wherever possible, estimation of exposure should be based on ranges of
mean concentrations in environmen-tal media on a global basis. Where
data are more limited, ranges of individual values could be used.
Estimates of exposure as a basis for derivation of guidance values are
presented in the examples in Appendix 1.
Where the data on concentrations of a substance in environmental
media are inconsistent or inadequate, exposure can be estimated based
on models which incorporate as much data as possible on, for example,
production, use patterns and physical and chemical properties. Models
to predict distribution in environmental media and estimation of
proportion of total exposure by various routes from consumer products
are available (Mackay, 1991; USES, 1994). For estimation of
proportions of exposure from various environmental media for
development of guidance values in EHCs, it is recommended that the
latest version of the Mackay level III model be used (Mackay et al.,
1992). It is important that all assumptions concerning releases and
physico-chemical properties and limitations of the estimated
proportions be clearly specified. In some cases, it may also be
possible to estimate the contribution of each medium to total exposure
on the basis simply of data on physical and chemical properties (e.g.,
for substances which are likely to be present primarily in one
environmental medium).
When available, toxicokinetic data should be used to the extent
possible in extrapolating across routes in the approaches to
allocation described below. Dermal exposure and absorption should
also be taken into account in the derivation of guidance values,
although relevant data are often not available. It is also recognized
that a source in one medium (e.g., potable water) may lead to
additional intake from other routes (e.g., dermal and inhalation) and
that, where possible, such intake should be considered in the
derivation of guidance values.
In addition, total allocations of less than 100% of the TI are
encouraged to account for, for example, those media for which exposure
has not been characterized and cross-route exposure. The magnitude of
the proportion of total intake which is not allocated should vary as a
function of the adequacy of characterization of total exposure from
all media.
In cases where the proportion of total exposure from a specific
medium is small (less than a few percent), allocation for derivation
of guidance values is not recommended since this would result in
direction of risk management strategies to media which are
inconsequential in contributing to total exposure.
5.2 General approach
The steps subsequent to development of a TI in deriving guidance
values for a general population are as follows:
1. If necessary, conversion of TIs for systemic effects for different
routes of exposure to a common unit for comparison based on
consideration of volumes and rates of inhalation and ingestion and
relevant toxicokinetic data, such as bioavailability, if available.
2. Allocation of TI to various routes and media based on estimated
exposure developed on the basis of available data on measured
concentrations or predicted proportions (i.e., model-derived values)
to which humans are exposed. Default values can be used in the
absence of data on measured concentrations or predicted proportions of
total exposure in various media.
3. Development of guidance values from intake assigned to each
medium, taking into account, for instance, body weight, volume of
intake and (relative) absorption efficiency ( relative where guidance
value is derived on the basis of a TI by another route of exposure).
Guidance values for drinking-water are generally expressed in µg/litre
or mg/litre, those in food as µg/g or mg/kg, those in air as µg/m3
or mg/m3, and those for dermal exposure as µg/m2 surface area.
5.3 Detailed approach
In the following section, an approach to the allocation of
tolerable intakes for development of guidance values (general
population) is provided by way of example for most of the scenarios
which may arise based on evaluations presented in EHCs.
The five most likely scenarios are considered to be:
5.3.1 Biomarkers of exposure
There is a common biomarker related to the critical effect which
integrates exposure from all sources. For example, Choudhury et al.
(1992) describe a model which predicts blood lead concentrations as a
function of concentrations in various media.
* The contributions from the various media are determined based on
a quantitative biomarker. Following allocation to various media
based on an exposure scenario, guidance values are developed
through incorporation of adjustment of body weight and volume of
intake for each medium.
5.3.2 Critical effects which are not route specific
TIs have been derived for each route, e.g., TI for oral exposure
(TIo) and TI for inhalation (TIi), and are based either on the
same or on different critical effects which are not at the portal of
entry. The TIs for the two routes are similar within one order of
magnitude since such variation is consistent with that inherent in
deriving TIs, as discussed in section 4, e.g., developmental toxicity
of 2-methoxyethanol (Doe et al., 1983; Wickramaratne, 1986). This
reflects the assumption that, in the absence of data to the contrary,
exposure via each route is considered to contribute to a combined dose
at the target site(s), i.e., additivity of dose at the target site(s).
* Allocate one TI to various media based on an exposure scenario to
determine the intake in each medium on which guidance values
should be based. Selection of the TIo or the TIi for this
purpose should be based on either:
a) if there is one major route of exposure then the TI for that
route should be used (if there is confidence in the data
base on which the exposure estimates are based); or
b) the more conservative TI (if there is uncertainty about the
relative contribution of various routes or media to total
exposure).
5.3.3 Difference in magnitude of effect by route of exposure
TIo and TIi for similar effects vary by 1 to 2 orders of
magnitude (exact magnitude of the difference for which this approach
is appropriate will be dependent upon availability of additional data;
e.g., manganese is more potent by inhalation than by ingestion).
* Derive the guidance values independently for each route (for
example, the oral and inhalation routes, based on the TIo and
TIi, respectively), but allocate the proportion of the TI for
each route to the appropriate medium or media based on an
exposure scenario.
5.3.4 Route-specific effect variation at portals of entry
(due to local bioactivation or local effects)
TIo and TIi for route-specific effects at the site of entry
vary by 1 to 2 orders of magnitude (exact magnitude of the difference
for which this approach is appropriate will be dependent upon
knowledge of additional data; e.g., nasal toxicity following
inhalation of acrylic acid).
* Derive the guidance values independently for each route (for
example, the oral and inhalation routes, based on the TIo and
TIi, respectively), using the full TI for each route to the
appropriate medium or media based on an exposure scenario.
5.3.5 Limited data base
In this scenario, the data base is limited such that only either
a TIo or a TIi can be developed.
* Allocate the available TI to various media based on an exposure
scenario to determine the intake in each medium on which guidance
values should be based, if the effects are qualitatively similar,
if toxicokinetic data are consistent with this approach and if
there are no effects at the site of entry. If any one of these
criteria is not met, do not derive guidance values for the
alternate route. If a TI is available for a route of exposure
which does not make an important contribution to total intake, do
not derive guidance values for that route.
6. EXAMPLES OF THE DERIVATION OF GUIDANCE VALUES
Example 1
The principal route of exposure is oral. Based on estimated
exposure for a scenario in the general environment, 50% of total
intake comes from food, 20% from water and 30% from air.
Data are adequate to establish both a TIo and a TIi. The
TIo and the TIi are based on similar effects and are similar
(within one order of magnitude).
Allocate 50% of TIo to food to derive a guidance value for food
* multiply TIo by 0.5
Allocate 20% of TIo to water to derive a guidance value for
drinking-water
* multiply TIo by 0.2
Allocate 30% of TIo to air to derive a guidance value for air
* multiply TIo by 0.3
Example 2
Based on an exposure scenario, 70% of total intake comes from
air, 20% from water and 10% from food. The compound is also present
in some consumer products but quantification of exposure is not
possible. There are no data on concentrations in soil but due to its
physicochemical properties, concentrations in this medium are likely
to be low.
Data are sufficient to establish a TIi and a TIo. The TIo
and the TIi are based on similar effects and are similar to within
an order of magnitude.
Convert TIi so that the values for the TIs for different routes
are expressed in the same units for comparison (generally mg/kg body
weight per day). This requires incorporation of information on
inhalation volumes, body weight and toxicokinetic data, if available.
Use TI for principal route of exposure to derive guidance values:
Allocate 63% of TIi to air to derive a guidance value for air.
* multiply TIi by 0.63
Allocate 18% of TIi to water to derive a guidance value for
drinking-water.
* multiply TIi by 0.18
Allocate 9% of TIi to food to derive a guidance value for food.
* multiply TIi by 0.09
Reserve 10% for exposure from consumer products and soil.
(Wherever possible, there should be an attempt to quantitatively
estimate the proportion of total intake from these sources).
Develop a guidance value for each medium by (if necessary)
adjustment for body weight, volume of intake and relative absorption.
Example 3
The principal route of exposure is oral. Based on estimated
exposure, 50% of total intake comes from food, 20% from water, 20%
from air and 10% from soil (after taking bioavailability into account
from the oral and dermal routes). The compound is believed not to be
present in consumer products.
Data are adequate to establish both a TIo and a TIi. The
TIo and the TIi are based on the same effects but the compound is
much more toxic by the oral route (e.g., TIo is less than the TIi
by more than two orders of magnitude).
Allocate 50% of TIo to food to derive a guidance value for food
* multiply TIo by 0.5
Allocate 20% of TIo to water to derive a guidance value for
drinking-water
* multiply TIo by 0.2
Allocate 10% of TIo to soil to derive a guidance value for soil
* multiply TIo by 0.1
Allocate 20% of TIi to air to derive a guidance value for air
* multiply TIi by 0.2
Example 4
The principal route of exposure is oral. Based on estimated
exposure, 50% of total intake comes from food, 20% from water and 30%
from air. There are no data indicating exposure from soil and
consumer products.
Data are adequate to establish both a TIo and a TIi for
route-specific effects. The TIo and the TIi are based on
route-specific effects and the compound is much more toxic by the oral
route (e.g., TIo is less than the TIi by more than two orders of
magnitude).
Because the effects are route specific and the TIs are different
by two orders of magnitude, each TI can be allocated in full to
appropriate media.
Allocate 50/70 (71%) of TIo to food to derive a guidance value
for food
* multiply TIo by 0.71
Allocate 20/70 (29)% of TIo to water to derive a guidance value
for drinking-water
* multiply TIo by 0.29
Allocate 100% of TIi to air to derive a guidance value for air
* multiply TIi by 1
Example 5
The principal route of exposure is inhalation.
Data are inadequate to establish a TIi.
Data are sufficient to establish a TIo.
Available toxicokinetic data are inadequate for or inconsistent
with extrapolation across routes.
Do not establish guidance values.
REFERENCES
Allen BC, Van Landingham C, Howe RB, Kavlock RJ, Kimmel CA, & Faustman
EM (1992) Dose-response modeling for developmental toxicity.
Toxicologist, 12(1): 300.
Allen B, Kavlock RJ, Kimmel CA, & Faustman EM (1993) Comparison of
quantitative dose response modeling approaches for evaluating fetal
weight changes in segment II developmental toxicity studies.
Teratology, 47(5): 41.
ATSDR (1989) Toxicological profile for aldrin/dieldrin. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (Publication
ATSDR/TP-88/01).
Barnes DG & Dourson M (1988) Reference dose (RfD): Description and use
in health risk assessment. Regul Toxicol Pharmacol, 8: 471-486.
Calabrese EJ (1985) Uncertainty factors and interindividual
variation. Regul Toxicol Pharmacol, 5: 190-196.
Calabrese EJ (1992) Does the animal to human uncertainty factor
incorporate interspecies differences in surface area? Regul Toxicol
Pharmacol, 15(2): 172-179.
Canada (1988) Government of Canada: Canadian Environmental Protection
Act, Ottawa. Canada Gaz, II(4): 415-512.
Choudhury H, Peirano WB, Marcus A, Elias R, Griffin S, & Derosa CT
(1992) Utilization of uptake biokinetic (UBK) lead model to assess
risk in contaminated sites. In: Superfund risk assessment in soil
contamination studies. Philadelphia, Pennsylvania, American Society of
Testing and Materials, pp 193-204.
Crump KS (1984) A new method for determining allowable daily intakes.
Fundam Appl Toxicol, 4: 854-871.
Doe JE, Samuels DM, Tinston DJ, & Wickramaratne GA de S (1983)
Comparative aspects of the reproductive toxicology by inhalation in
rats of ethylene glycol monomethyl ether and propylene glycol
monomethyl ether. Toxicol Appl Pharmacol, 69: 43-47.
Dourson ML & Stara J (1983) Regulatory history and experimental
support of uncertainty (safety) factors. Regul Toxicol Pharmacol, 3:
224-238.
Dourson ML, Hertzberg R, Hartung R, & Blackburn K (1985) Novel methods
for the estimation of acceptable daily intake. Toxicol Ind Health,
1(4): 23-33.
Dourson ML, Knauf L, & Swartout J (1992) On reference dose (RfD) and
its underlying toxicity data base. Toxicol Ind Health, 8(3): 171-189.
Farland W & Dourson ML (1992) Noncancer health endpoints: approaches
to quantitative risk assessment. In: Cothern R ed. Comparative
environmental risk assessment. Boca Raton, Florida, Lewis Publishers,
pp 87-106.
Gaylor DW (1989) Quantitative risk analysis for quantal reproductive
and developmental effects. Environ Health Perspect, 79: 243-246.
Gaylor DW (1992) Incidence of developmental defects at the No
Observed Effect Level (NOEL). Regul Toxicol Pharmacol, 15: 151-160.
Guth DJ, Jarabek AM, Wymer L, & Hertzberg RC (1991) Evaluation of risk
assessment methods for short-term inhalation exposure. Presentation
at the 84th Annual Meeting of the Air and Waste Management
Association, Vancouver, British Columbia, 16-21 June 1991.
Hallenbeck WH & Cunningham KM (1986) Quantitative risk assessment for
environmental and occupational health. Chelsea, Michigan, Lewis
Publishers, Inc., p 199.
Hattis D, Erdreich L, & Ballew M (1987) Human variability in
susceptibility to toxic chemicals -A preliminary analysis of
pharmacokinetic data from normal volunteers. Risk Anal, 7(4):
415-426.
Hartung R (1986) Ranking the severity of toxic effects. In: Trace
substances in environmental health. Columbia, Missouri, University of
Missouri, pp 204-211.
Hartung R & Durkin PR (1986) Ranking the severity of toxic effects:
Potential applications to risk assessment. Comments Toxicol, 1: 49-63.
Health and Welfare Canada (1992) Determination of "toxic" under
paragraph 11c) of the Canadian Environmental Protection Act, 1st ed.
Ottawa, Ontario, Bureau of Chemical Hazards, Environmental Health
Directorate.
Hertzberg RC (1989) Fitting a model to categorical response data with
application to special extrapolation to toxicity. Health Phys,
57(Suppl 1): 404-409.
Hertzberg RC & Miller M (1985) A statistical model for species
extrapolation using categorical response data. Toxicol Ind Health, 1:
43-57.
ICRP (1974) International Commission on Radiological Protection:
Report of the Task Group on Reference Man. Oxford, Pergamon Press
(ICRP Publication No. 23).
Jarabek AM, Menache MG, Overton JH Jr, Dourson ML, & Miller FJ (1990)
The US Environmental Protection Agency's inhalation RfD methodology:
Risk assessment for air toxics. Toxicol Ind Health, 6(5): 279-301.
Kavlock RJ, Greene JA, Kimmel GL, Morrissey RE, Owens E, Rogers JM,
Sadler TW, Stack F, Waters MD, & Welsch F (1991) Activity profiles of
developmental toxicity: design considerations and pilot
implementation. Teratology, 43: 159-185.
Kimmel CA & Gaylor DW (1988) Issues in qualitative and quantitative
risk analysis for developmental toxicology. Risk Anal, 8: 15-20.
Lehman AJ & Fitzhugh OG (1954) 100-fold margin of safety. Assoc Food
Drug Off US Q Bull, 18: 33-35.
Lewis SC, Lynch JR, & Nikiforov AI (1990) A new approach to deriving
community exposure guidelines from no-observed-adverse-effect levels.
Regul Toxicol Pharmacol, 11: 314-330.
Lu F (1988) Acceptable daily intake: inception, evolution and
application. Regul Toxicol Pharmacol, 8(1): 45-60.
Mackay D (1991) Multimedia environmental models. The fugacity
approach. Chelsea, Michigan, Lewis Publishers, Inc., 260 pp.
Mackay D, Paterson S, & Shiu WY (1992) Generic models for evaluating
the regional fate of chemicals. Chemosphere, 24: 695-717.
Moolgavkar SH, Dewanji A, & Venzon DJ (1988) A stochastic two-stage
model for cancer risk assessment. I: The hazard function and the
probability of tumor. Risk Anal, 8: 383-392.
Morgenroth V (1993) Scientific evaluation of the data-derived safety
factors for the acceptable daily intake. Case study:
diethylhexylphthalate. Food Addit Contam, 10(3): 363-373.
NAS (1977) Drinking water and health. Washington, DC, National
Research Council, US National Academy of Sciences, 939 pp.
Nau H (1986) Species differences in pharmacokinetics and drug
teratogenesis. Environ Health Perspect, 70: 113-129.
Poulsen E (1993) Case study: erythrosine. Food Addit Contam, 10:
315-323.
Renwick AG (1993a) Data-derived safety factors for the evaluation of
food additives and environmental contaminants. Food Addit Contam, 10:
275-305.
Renwick AG (1993b) A data-derived safety (uncertainty) factor for the
intense sweetener, saccharin. Food Addit Contam, 10: 337-350.
Rubery ED, Barlow SM, & Steadman JH (1990) Criteria for setting
quantitative estimates of acceptable intakes of chemicals in food in
the U.K. Food Addit Contam, 7: 287-302.
Shoaf CR (1994) US EPA inhalation testing guidelines. In: Jenkins PG,
Kayser D, Muhle H, Rosner G, & Smith EM ed. Respiratory toxicology and
risk assessment. Proceedings of an International Symposium. Stuttgart,
Wissenschaftliche Verlagsgesellschaft mbH, pp 11-25 (IPCS Joint Series
No. 18).
US EPA (1985a) National primary drinking water regulations; volatile
synthetic organic chemicals; final rule and proposed rule. Fed Reg,
50: 46830-46901.
US EPA (1985b) National primary drinking water regulations, synthetic
organic chemicals, inorganic chemicals and microorganisms; proposed
rule. Fed Reg, 50: 46936-47025.
US EPA (1993) The Integrated Risk Information System (IRIS).
Cincinnati, Ohio. Office of Health and Environmental Assessment,
Environmental Criteria and Assessment Office.
USES (1994) Uniform system for the evaluation of substances (USES),
version 1.0. Bilthoven, The Netherlands, National Institute for
Public Health and Environmental Protection, 332 pp.
Waters MD, Stack HF, Brady AL, Lohman PHM, Haroun L, & Vainio H (1988)
Use of computerized data listings and activity profiles of genetic and
related effects in the review of 195 compounds. Mutat Res, 205:
295-312.
Weil CS (1972) Statistics versus safety factors and scientific
judgement in the evaluation for man. Toxicol Appl Pharmacol, 21:
454-463.
WHO (1987) IPCS Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food. Geneva,
World Health Organization, 174 pp.
WHO (1990) IPCS Environmental Health Criteria 104: Principles for the
toxicological assessment of pesticide residues in food. Geneva, World
Health Organization. 117 pp.
WHO (1993) Guidelines for drinking-water quality, Second edition.
Volume 1: Recommendations. Geneva, World Health Organization, 198 pp.
Wickramaratne GA de S (1986) The teratogenic potential and
dose-response of dermally administered ethylene glycol monomethyl
ether (EGME) estimated in rats with the Chernoff-Kavlock assay. J Appl
Toxicol, 6(3): 165-166.
Wurtzen G (1993) Scientific evaluation of the safety factor for the
acceptable daily intake (ADI). Case study: butylated hydroxy anisole
(BHA). Food Addit Contam, 10: 307-314.
Zielhuis RL & van der Kreek FW (1979a) The use of a safety factor in
setting health based permissible levels for occupational exposure. Int
Arch Occup Environ Health, 42: 191-201.
Zielhuis RL & van der Kreek FW (1979b) Calculation of a safety factor
in setting health based permissible levels for occupational exposure.
Int Arch Occup Environ Health, 42: 203-215.
APPENDIX 1
EXAMPLES - DEVELOPMENT OF GUIDANCE VALUES
The following practical examples are provided to illustrate the
manner in which tolerable intakes (TIs) may be developed and allocated
for the derivation of guidance values for a general population (on the
basis of calculated proportions of exposure from various media). In
the calculation of guidance values, TIs may be rounded up to 1 or 2
significant figures depending on the quality of the data base and the
extent of uncertainties involved in deriving the TI. The level of
detail shown is that which is considered necessary for EHCs and should
be sufficient for adaptation at national and local levels.
Compound A
Chlorinated hydrocarbon
Estimates of exposure
Estimated daily intakes of Compound A for adults (µg/kg
body weight per day)1 in the general population are as follows:
Ambient air2 < 0.03
Drinking-water3 0.00007-< 0.0004
Food4 0.004
Soil no data
Consumer products no data
Total Intake 0.03
1 Assumed to weigh 64 kg, breathe 22 m3 of air per day and drink
1.4 litres of water Per day (ICRP, 1974) and to consume 125 g per day
of a meat composite (the compound was not detected in other dietary
composites).
2 Based on a mean concentration of Compound A reported in a survey
of ambient Air from 22 sites (< 0.10 µg/m3); concentrations in
indoor air were similar to those in ambient air.
3 Based on a range of mean concentrations of Compound A in
drinking-water of 0.003 µg/litre to < 0.02 µg/litre.
4 Based on a concentration of 0.0018 µg/g of Compound A detected in
a representative daily diet.
On the basis of these estimates, it is considered that the
percentage of total exposure from various media for the general
population (midpoints of estimated intakes) is as follows:
outdoor/indoor air = < 0.03/0.03 = 85.9% (86%)
(< 0.03 considered to be 0.03 minus
intake from other media)
drinking-water = 0.000245/0.03 = 0.82% (0.8%)
food = 0.004/0.03 = 13.3% (13%)
soil = no data
consumer products = no data
Development of TI
The only data identified on long-term toxicity following
inhalation are the results of a single subchronic study for which no
effects were observed at any concentration. Available data are
considered inadequate, therefore, to establish a TI on the basis of
the results of studies in which Compound A has been administered by
inhalation. Moreover although the general population appears to be
exposed to Compound A principally in air, based on limited available
data on concentrations in food, the estimated intake in food is within
the range of that estimated for air for some age groups. In addition,
the principal route of intake of the most exposed age group (i.e.
suckling infants) is ingestion (of mothers' milk). Owing to the lack
of adequate long-term toxicity studies by the inhalation route and the
possible relatively important contribution that food makes to total
exposure to Compound A, a TI is derived on the basis of a long-term
ingestion bioassay, as follows:
60 mg/kg body
weight per day × 5
TI = approx. 0.43 mg/kg (430 µg/kg)
body weight per day
100 × 7
where:
* 60 mg/kg body weight per day is the NOAEL, determined in a
well-conducted and documented long-term (chronic and
carcinogenesis) bioassay, with renal tubular degeneration
observed at higher doses
* 5/7 is the conversion of five days per week of dosing to seven
days per week
* 100 is the uncertainty factor (×10 for inter-individual
variation; ×10 for interspecies variation; available data on
toxicokinetics and toxicodynamics were inadequate to modify the
10 × 10-fold uncertainty factor)
Derivation of Guidance Values
Outdoor/indoor air
The proportion of TI allocated to outdoor air based on exposure
estimates = 86%
86% × TI (430 µg/kg body
weight per day) = 370 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
mean body weight of adults = 64 kg
Guidance value for 370 µg/kg × 64 kg
outdoor/indoor air =
22 m3
= 1100 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 0.8% (too small to permit development of
meaningful guidance values since it contributes negligibly to total
intake)
Food
The proportion of TI allocated to food based on exposure
estimates = 13%
13% × TI (430 µg/kg
body weight per day) = 57 µg/kg body weight per day
(tolerances in various foodstuffs can be developed on the basis
of the amounts ingested.)
Soil
Owing to lack of relevant data, it is not possible to allocate a
proportion of the TI to this source.
Compound B
Chlorinated hydrocarbon solvent
Estimates of Exposure
Estimated daily intakes of Compound B for adults (µg/kg body
weight per day)1 in the general population are as follows:
Ambient air2 0.01-0.27
Indoor air3 1.4
Drinking-water4 0.002-0.02
Food5 0.12
Soil no data
Consumer products no data
Total Intake 1.5-1.8
On the basis of these estimates, it is considered that the
percentage of total exposure from various media for the general
population (based on midpoints of estimated intakes) is as follows:
outdoor air = 0.14/1.67 = 8.3%
indoor air = 1.4/1.67 = 83.8% (84%)
drinking-water = 0.011/1.67 = 0.65%
food = 0.12/1.67 = 7.1%
soil = no data
consumer products = no data
1 Assumed to weigh 64 kg, breathe 22 m3 air and drink 1.4 litres
of water per day (ICRP, 1974).
2 Assumed to spend 4 h/day outdoors and based on a range of mean
concentrations of Compound B (0.2 to 5.0 µg/m3) from a survey.
3 Assumed to spend 20 h/day indoors and based on the mean
concentration of Compound B of approximately 5.1 µg/m3 in the
indoor air of 757 randomly selected homes examined in a survey.
4 Based on a range of mean concentrations of Compound B (0.1 to
0.9 µg/litre) in drinking-water from a number of surveys.
5 Based on the average levels of Compound B in the various
composite food groups in a study on the daily intake of these
food groups.
Development of TIs
A Tolerable Intake for Compound B can be derived as follows:
[(678 mg/m3) × (0.043 m3/day) × (6/24) × (5/7)]
TI =
(0.0305 kg) × 1000
= 170 µg/kg body weight per day
where:
* 678 mg/m3 is the lowest-observed-adverse-effect level (LOAEL)
overall in mice determined in an adequate long-term inhalation
study and based on reduced survival and hepato-toxicity in males,
and lung congestion and nephrotoxicity in males and females.
* 0.043 m3/day is the assumed volume of air inhaled by mice
* 6/24 and 5/7 is the conversion of 6 h/day, 5 days/week to
continuous exposure.
* 0.0305 kg is the average body weight of the mice in the critical
study.
* 1000 is the uncertainty factor (×10 for inter-individual
variation, ×10 for interspecies variation since available data on
toxicokinetics and toxicodynamics were inadequate for
modification of these factors, ×10 for use of a LOAEL rather than
a NOAEL).
In order to ensure that the TI derived on the basis of an
inhalation study is sufficiently protective, another TI can be derived
on the basis of studies in which Compound B was administered by
ingestion. With the exception of one investigation in which
reversible erythropoietic damage was reported at low concentrations
(50 µg/kg body weight per day) but not confirmed in other studies, the
lowest NOAEL in the longest-term (90-day) available study in which
Compound B was administered orally in drinking-water to rats is
14 mg/kg body weight per day, based on effects on body weight gain,
the ratio of liver or kidney weight to body weight, and serum
5'-nucleotidase activity at the next highest dose. A LOEL of 20 mg/kg
body weight per day based on a slight increase in liver weight was
reported in a 6-week study on mice. Values for the TI derived on the
basis of the results of these two studies are within the same order of
magnitude as the TI calculated from the inhalation study.
Derivation of Guidance Values
Since the TIs derived on the basis of studies by inhalation and
ingestion are within the same range and inhalation is the most
important route of exposure of the general population, the TI
developed for the inhalation route will be used as the basis for
derivation of guidance values.
Outdoor air
The proportion of TI allocated to outdoor air based on exposure
estimates = 8.3%
8.3% × TI (170 µg/kg body
weight per day) = 14 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
proportion of the day
spent outdoors = 4/24
volume of outdoor air
inhaled daily = 22 m3 × 4/24 = 3.7 m3
mean body weight of adults = 64 kg
Guidance value for 14 µg/kg × 64 kg
outdoor air =
3.7 m3
= 242 µg/m3
Indoor air
The proportion of TI allocated to indoor air based on exposure
estimates = 84%
84% × TI (170 µg/kg body
weight per day) = 140 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
proportion of the day
spent indoors = 20/24
volume of indoor air
inhaled daily = 22 m3 × 20/24 = 18 m3
mean body weight of adults = 64 kg
Guidance value for 140 µg/kg × 64 kg
indoor air =
18 m3
= 498 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 0.65% (too small to permit development of
meaningful guidance values since it contributes negligibly to total
intake)
Food
The proportion of TI allocated to food based on exposure
estimates = 7.1%
7.1% × TI (170 µg/kg body
weight per day) = 12 µg/kg body weight per day or
10 µg/kg body weight per day to
one significant figure
(tolerances in various foodstuffs could then be developed on the
basis of amounts ingested.)
Soil
Owing to lack of relevant data, it is not possible to allocate a
proportion of the TI to this source.
Compound C
Naturally occurring inorganic chemical
Estimates of Exposure
The percentage of total exposure from various media for adults in
the general population in country 1 is as follows:
outdoor/indoor air = 0.02%
drinking-water = 6.9%
food = 80%
soil = 0.11%
consumer products = 12.8%
In contrast, the percentage of total exposure from various media
for adults in the general population in one area in country 2 is as
follows:
outdoor/indoor air = 35%
drinking-water = 11%
food = 55%
Development of TIs
It is concluded, on the basis of data from several studies in
human populations, that the TI is 200 µg/kg body weight per day.
Derivation of Guidance Values - Country 1
Outdoor/indoor air
The proportion of TI allocated to air based on exposure estimates
= 0.02% (too small to permit development of meaningful guidance
values)
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 6.9%
6.9% × TI (200 µg/kg body
weight per day) = 13.8 µg/kg body weight per day
daily volume of ingestion of
drinking-water for adults
in Country 1 = 1.5 litres
mean body weight of adults
in Country 1 = 70 kg
Guidance value for 13.8 µg/kg × 70 kg
drinking-water =
1.5
= 644 µg/litre
Food
The proportion of TI allocated to food based on exposure
estimates = 80%
80% × TI (200 µg/kg body 160 µg/kg body weight per day
weight per day) = or 200 µg/kg body weight per
day to one significant figure
(tolerances in various foodstuffs can then be developed on the basis
of amounts ingested.)
Soil
The proportion of TI allocated to air based on exposure estimates
= 0.11%
(too small to permit development of meaningful guidance values)
Consumer products
The proportion of TI allocated to consumer products based on
exposure estimates = 12.8%
12.8% × TI (200 µg/kg body
weight per day) = 26 µg/kg body weight per day
(limits in consumer products can be developed on the basis of
patterns of use.)
Derivation of Guidance Values - Country 2
Outdoor/indoor air
The proportion of TI allocated to air based on exposure
estimates = 35%
35% × TI (200 µg/kg body
weight per day) = 70 µg/kg body weight per day
daily inhalation volume for
adults in Country 2 = 20 m3
mean body weight of
adults in Country 2 = 60 kg
Guidance value for 70 µg/kg × 60 kg
outdoor/indoor air =
20 m3
= 210 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 11%
11% × TI (200 µg/kg body
weight per day) = 22 µg/kg body weight per day
daily volume of ingestion of
drinking-water for
adults in Country 2 = 1.5 litres
mean body weight of
adults in Country 2= 60 kg
Guidance value for 22 µg/kg × 60 kg
drinking-water =
1.5
= 880 µg/litre
Food
The proportion of TI allocated to food based on exposure
estimates = 55%
55% × TI (200 µg/kg body
weight per day) = 110 µg/kg body weight per day
(tolerances in various foodstuffs can be developed on the basis of
amounts ingested.)
Soil
No data are available.
Consumer products
No data are available.
APPENDIX 2
GRAHICAL APPROACHES
The use of graphs of dose-effect and dose-response toxicity data
to complement the text discussion in the development of TIs and
guidance values is considered valuable. Such graphs can display an
overview of the full range of dose-response information. Graphs can
range from simple "thermometer" presentations as employed by the US
Agency for Toxic Substances and Disease Registry (ATSDR, 1989), to
dose-effect and dose-response graphs for specific toxic effects such
as genotoxicity (Waters et al., 1988) or developmental toxicity
(Kavlock et al., 1991), or to dose-duration graphs described by
Hartung (1986), Hartung & Durkin (1986), and Dourson et al. (1985).
Fig. 4 is an example of a dose-duration graph and presents data
for methoxychlor adapted from Dourson et al. (1985). This figure
summarizes the available frank-effect levels (FEL), adverse-effect
levels (AEL), no-observed-adverse-effect levels (NOAEL), and
no-observed-effect levels (NOEL). Adverse-effect levels are presented
as a function of both dose in mg/day and exposure as a fraction of
lifespan.
Each point in the graph represents one dose group from one study.
The size of the point is a rough indication of its usefulness for
determining tolerable intakes, where larger points indicate more
useful information. Other information includes target organs. These
data can also be used to estimate a best fitting line for NOAEL across
duration.
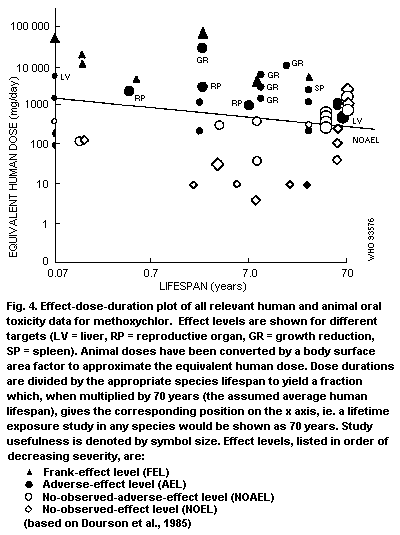 APPENDIX 3
ALTERNATIVE APPROACHES
Alternative approaches being considered in the derivation of TIs
for threshold effects include the benchmark dose and
categorical regression.
Benchmark dose
The benchmark dose (BD) is the lower confidence limit (LCL) of
the dose that produces a small increase in the level of adverse
effects (e.g., 5 or 10%; Crump, 1984) to which uncertainty factors
(UF) can be applied to develop a tolerable intake (see Fig. 5, adapted
from Kimmel & Gaylor, 1988).
APPENDIX 3
ALTERNATIVE APPROACHES
Alternative approaches being considered in the derivation of TIs
for threshold effects include the benchmark dose and
categorical regression.
Benchmark dose
The benchmark dose (BD) is the lower confidence limit (LCL) of
the dose that produces a small increase in the level of adverse
effects (e.g., 5 or 10%; Crump, 1984) to which uncertainty factors
(UF) can be applied to develop a tolerable intake (see Fig. 5, adapted
from Kimmel & Gaylor, 1988).
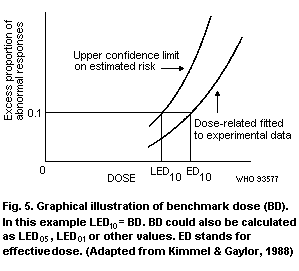 The BD has a number of advantages over the NOAEL. Firstly, it is
derived on the basis of data from the entire dose-response curve for
the critical effect rather than that from the single dose group at the
NOAEL (i.e. one of the few (usually three) preselected dose levels).
Use of the BD also facilitates comparison of studies on the same agent
or the potencies of different agents. The BD can also be calculated
from data sets in which a NOAEL was not determined, eliminating the
need for an additional uncertainty factor to be applied to the LOAEL.
Lastly, definition of the BD as a lower confidence limit accounts for
the statistical power and quality of the data. That is, the
confidence intervals around the dose-response curve for studies with
small numbers of animals and, therefore, lower statistical power would
be wide; similarly, confidence intervals in studies of poor quality
with highly variable responses would also be wide. In either case,
the wider confidence interval would lead to a lower BD, reflecting the
greater uncertainty of the data base. On the other hand, narrow
confidence limits (reflecting better studies) would result in higher
BDs.
One of the chief disadvantages of this approach is that it is not
possible to determine a BD for many types of data on toxicity (e.g.,
histopathological data).
Several methods have been published for determining both the
dose-response curve from which the BD is derived and appropriate
uncertainty factors to estimate the TI (e.g., Crump, 1984; Dourson et
al., 1985; Kimmel & Gaylor, 1988; Gaylor, 1989; Allen et al., 1992).
However, there is as yet, no consensus on the incidence of effect to
be used as a basis for the BD, although it should be comparable to the
level of effect typically associated with the NOAEL. For data bases
on developmental toxicity, it has been estimated that this level of
effect is in the range of 1-10% (Crump, 1984; Gaylor, 1989, 1992);
this range is similar for other toxic end-points (Farland & Dourson,
1992; Shoaf, 1994). Allen et al. (1992, 1993) have estimated that a
BD calculated from the LCL at 5% is, on average, comparable to the
NOAEL, whereas choosing a BD from the LCL at 10% is more
representative of a LOAEL (Farland & Dourson, 1992). Choosing a BD
that is comparable to the NOAEL has two advantages: (i) it is within
the experimental dose-range, eliminating the need to interpolate the
dose-response curve to low levels; and (ii) justification of the
application of similar UFs as are currently applied to the NOAEL for
interspecies and inter-individual variation.
Categorical Regression
The theory and application of categorical regression has been
addressed by Hertzberg & Miller (1985), Hertzberg, (1989), Guth et al.
(1991) (inhalation exposure to methylisocyanate), and Farland &
Dourson (1992) (oral exposure to arsenic). Data on toxicity are
classified into one of several categories, such as NOEL, NOAEL, AEL or
FEL, or others, as appropriate. These categories are then regressed
on the basis of dose and, if required, duration of exposure. The
result is a graph of probability of a given category of effect with
dose or concentration, which is useful in the analysis of potential
risks above the TI, especially for comparisons amongst chemicals.
Depending on the extent of the available data on toxicity,
additional estimations regarding the percentage of individuals with
specific adverse effects are possible. Such estimations require,
however, an understanding of the mechanisms of toxicity of the
critical effect, knowledge of the extrapolation between the
experimental animal and man, and/or incidences of specific effects in
humans.
Similar to the BD, categorical regression utilizes information
from the entire dose-response curve, resulting in more precise
estimates of risk when compared to the current approach (NOAEL-based
TIs). However, categorical regression requires more information than
the current TI method, and the interpretation of the probability scale
can be problematic.
APPENDIX 4
BODY WEIGHT AND VOLUMES OF INTAKE FOR REFERENCE MAN
(based on ICRP, 1974, unless otherwise indicated)
Body weight, kg
Adult male = 70
Adult female = 58
Average = 64a
Daily fluid intake (milk, tap water, other beverages), ml/day
Normal conditions:
Adults = 1000-2400, representative
figure = 1900b (excluding
milk: 1400c)
Adult male = 1950
Adult female = 1400
Child (10 years) = 1400
High average temperature (32°C):
Adults = 2840-3410
Moderate activity:
Adults = 3700
Respiratory volumes
8-h respiratory volume, litres
resting: Adult man = 3600
Adult woman = 2900
Child (10 years) = 2300
light/non-occupational
activity: Adult man = 9600
Adult woman = 9100
Child (10 years) = 6240
Daily inhalation volume, m3
(8-h resting, 16-h light/non-occupational activity)
Adult male = 23
Adult female = 21
Child (10 years) = 15
Average adult = 22
Proportion of time
spent indoorsc = 20 h/day
Amount of soil ingestedc = 20 mg/day
Dietary intaked
Cereals = 323 g/day (flour and milled rice)
Starchy roots = 225 g/day (sweet potatoes, cassava
and other)
Sugar = 72 g/day (includes raw sugar,
excludes syrups and honey)
Pulses and nuts = 33 g/day (includes cocoa beans)
Vegetables and fruits = 325 g/day (fresh equivalent)
Meat = 125 g/day (includes offal, poultry
and game in terms of carcass weight,
excluding slaughter fats)
Eggs = 19 g/day (fresh equivalent)
Fish = 23 g/day (landed weight)
Milk = 360 g/day (excludes butter; includes
milk products as fresh milk
equivalent)
Fats and oils = 31 g/day (pure fat content)
a WHO uses 60 kg for calculation of acceptable daily intakes and
water quality guidelines (WHO, 1987, 1993).
b WHO uses a daily per capita drinking-water consumption of 2 litres
in calculating water quality guidelines (WHO, 1993)
c From Health and Welfare Canada (1992)
d Based on average of estimates for 7 geographical regions
(ICRP, 1974)
RESUME
Des valeurs guides devraient être établies dans les Critères
d'hygiène de l'environnement (CHE) de l'IPCS pour l'exposition aux
produits chimiques présents dans l'environnement. Ces valeurs guides
pourront être modifiées par les autorités nationales et locales
lorsque celles-ci fixeront leurs normes et limites pour les différents
milieux. L'élaboration des valeurs guides pour les produits chimiques
comporte les étapes suivantes:
1. Evaluer et résumer les données relatives à la toxicité pour
l'homme et l'animal et à l'exposition humaine qui offrent un intérêt
particulier pour le calcul des valeurs guides. Ces données devraient
de préférence être présentées sous la forme d'un texte explicatif
résumant les points cruciaux, complété par des graphiques.
2. Ces données pourront servir à calculer une dose tolérable (DT)
pour les différentes voies d'exposition dans le cas des effets pour
lesquels on considère qu'il existe un seuil. Le calcul consiste
généralement à appliquer des facteurs d'incertitude aux doses sans
effet indésirable observé (DSEIO) établies par l'étude la plus
pertinente pour les effets critiques. En ce qui concerne les effets
pour lesquels il n'existe pas de seuil, la relation dose-réponse devra
être caractérisée aussi complètement que possible.
3. Estimer la proportion de la dose totale provenant des différents
milieux (atmosphère à l'intérieur des locaux, air ambiant, nourriture,
eau, etc.) dans une situation donnée, en prenant comme base de calcul
un ensemble cohérent de données sur les volumes théoriques absorbés
par l'"homme de référence" de la Commission internationale de
protection contre les radiations (CIPR) et des concentrations
représentatives de l'environnement général. En l'absence de données
adéquates sur les concentrations dans les différents milieux, on
pourra utiliser des modèles mathématiques pour estimer la répartition
entre ces milieux.
4. Attribuer une proportion de la DT aux différents milieux (d'après
les résultats de l'estimation décrite à l'étape 3 ci-dessus) de façon
à déterminer la dose ou l'exposition attribuable à chaque milieu.
5. Etablir des valeurs guides pour les doses attribuées à chaque
milieu en tenant compte éventuellement du poids corporel, du volume
absorbé et de l'efficacité de l'absorption (efficacité d'absorption
relative lorsque la valeur guide est calculée à partir de la DT
établie pour une autre voie d'exposition). Dans les monographies de
la série CHE, les valeurs guides devraient être établies pour un
scénario d'exposition clairement défini, fondé sur les données
applicables à l'homme de référence de la CIPR, données qui ne sont pas
nécessairement représentatives des conditions nationales ou locales
d'exposition. Normalement, les valeurs guides seront calculées pour
une population générale représentative, soumise à des conditions
d'exposition également représentatives. Elles devront être adaptées
au niveau national et local en fonction des circonstances.
6. La base de calcul des DT et des valeurs guides devrait être
clairement expliquée dans les monographies de la série CHE (pour le
niveau de détail exigé, voir les exemples de l'appendice 1).
RESUMEN
En los monografías de la serie Criterios de Salud Ambiental (EHC)
del IPCS deben formularse valores orientativos para la exposición a
sustancias químicas presentes en el medio ambiente, valores que las
autoridades nacionales y locales pueden modificar al determinar sus
límites y normas aplicables al medio. Los pasos previstos para
cualquier sustancia química son los siguientes:
1. Evaluar y resumir la información referente a la toxicidad en los
animales y el hombre y la exposición en el hombre, seleccionando la
más pertinente para el cálculo de los valores orientativos. El
esquema más apropiado para presentar los datos pertinentes con miras
al cálculo de los valores orientativos es un texto que describa
sucintamente los datos críticos, complementado con los gráficos
oportunos.
2. Calcular a partir de esos datos una Ingesta Tolerable (IT) para
las diversas vías de exposición y para los distintos efectos que se
considere que tienen un umbral. Ello entraña el uso de factores de
incertidumbre, aplicados por lo general al nivel sin efectos adversos
observados (NOAEL) para los efectos críticos referidos en el estudio
más pertinente. En el caso de los efectos sin umbral, se
caracterizará en la medida de lo posible la relación dosis-respuesta.
3. Estimar la proporción de ingesta total que tiene su origen en los
diversos medios (p. ej., aire de espacios interiores y ambiental,
alimentos y agua), sobre la base de las exposiciones calculadas para
un conjunto coherente de volúmenes supuestos de ingesta (utilizando el
hombre de referencia de la Comisión Internacional de Protección contra
las Radiaciones (CIPR)) y de concentraciones representativas en el
medio ambiente general para una determinada situación. Si no se
dispone de datos suficientes sobre las concentraciones en diversos
medios, pueden emplearse modelos matemáticos para estimar la
distribución por esos medios.
4. Asignar una proporción de la IT a diversos medios de exposición
(basándose en la exposición estimada conforme a lo explicado en el
paso 3 precedente) para determinar la ingesta o exposición en cada
medio.
5. Formular valores orientativos a partir de las ingestas asignadas a
cada medio, teniendo en cuenta (si es necesario) el peso corporal, el
volumen de ingesta y la eficiencia de absorción (la eficiencia de
absorción relativa cuando para calcular el valor orientativo se
utilice la IT correspondiente a otra vía de exposición). En los
monografías de la serie EHC se formularían valores orientativos para
unas condiciones de exposición claramente definidas, basadas en los
datos del hombre de referencia de la CIPR y no necesariamente
representativas de las condiciones de exposición nacionales o locales.
Se calcularán comúnmente valores orientativos para una población
general representativa y unas condiciones de exposición
representativas. Los valores orientativos se deberán adaptar a nivel
nacional y local según proceda en función de las circunstancias
locales.
6. En los monografías de la serie EHC se deberán detallar claramente
los fundamentos del cálculo tanto de la IT como de los valores
orientativos (respecto al grado de detalle, véanse los ejemplos
presentados en el apéndice 1).
The BD has a number of advantages over the NOAEL. Firstly, it is
derived on the basis of data from the entire dose-response curve for
the critical effect rather than that from the single dose group at the
NOAEL (i.e. one of the few (usually three) preselected dose levels).
Use of the BD also facilitates comparison of studies on the same agent
or the potencies of different agents. The BD can also be calculated
from data sets in which a NOAEL was not determined, eliminating the
need for an additional uncertainty factor to be applied to the LOAEL.
Lastly, definition of the BD as a lower confidence limit accounts for
the statistical power and quality of the data. That is, the
confidence intervals around the dose-response curve for studies with
small numbers of animals and, therefore, lower statistical power would
be wide; similarly, confidence intervals in studies of poor quality
with highly variable responses would also be wide. In either case,
the wider confidence interval would lead to a lower BD, reflecting the
greater uncertainty of the data base. On the other hand, narrow
confidence limits (reflecting better studies) would result in higher
BDs.
One of the chief disadvantages of this approach is that it is not
possible to determine a BD for many types of data on toxicity (e.g.,
histopathological data).
Several methods have been published for determining both the
dose-response curve from which the BD is derived and appropriate
uncertainty factors to estimate the TI (e.g., Crump, 1984; Dourson et
al., 1985; Kimmel & Gaylor, 1988; Gaylor, 1989; Allen et al., 1992).
However, there is as yet, no consensus on the incidence of effect to
be used as a basis for the BD, although it should be comparable to the
level of effect typically associated with the NOAEL. For data bases
on developmental toxicity, it has been estimated that this level of
effect is in the range of 1-10% (Crump, 1984; Gaylor, 1989, 1992);
this range is similar for other toxic end-points (Farland & Dourson,
1992; Shoaf, 1994). Allen et al. (1992, 1993) have estimated that a
BD calculated from the LCL at 5% is, on average, comparable to the
NOAEL, whereas choosing a BD from the LCL at 10% is more
representative of a LOAEL (Farland & Dourson, 1992). Choosing a BD
that is comparable to the NOAEL has two advantages: (i) it is within
the experimental dose-range, eliminating the need to interpolate the
dose-response curve to low levels; and (ii) justification of the
application of similar UFs as are currently applied to the NOAEL for
interspecies and inter-individual variation.
Categorical Regression
The theory and application of categorical regression has been
addressed by Hertzberg & Miller (1985), Hertzberg, (1989), Guth et al.
(1991) (inhalation exposure to methylisocyanate), and Farland &
Dourson (1992) (oral exposure to arsenic). Data on toxicity are
classified into one of several categories, such as NOEL, NOAEL, AEL or
FEL, or others, as appropriate. These categories are then regressed
on the basis of dose and, if required, duration of exposure. The
result is a graph of probability of a given category of effect with
dose or concentration, which is useful in the analysis of potential
risks above the TI, especially for comparisons amongst chemicals.
Depending on the extent of the available data on toxicity,
additional estimations regarding the percentage of individuals with
specific adverse effects are possible. Such estimations require,
however, an understanding of the mechanisms of toxicity of the
critical effect, knowledge of the extrapolation between the
experimental animal and man, and/or incidences of specific effects in
humans.
Similar to the BD, categorical regression utilizes information
from the entire dose-response curve, resulting in more precise
estimates of risk when compared to the current approach (NOAEL-based
TIs). However, categorical regression requires more information than
the current TI method, and the interpretation of the probability scale
can be problematic.
APPENDIX 4
BODY WEIGHT AND VOLUMES OF INTAKE FOR REFERENCE MAN
(based on ICRP, 1974, unless otherwise indicated)
Body weight, kg
Adult male = 70
Adult female = 58
Average = 64a
Daily fluid intake (milk, tap water, other beverages), ml/day
Normal conditions:
Adults = 1000-2400, representative
figure = 1900b (excluding
milk: 1400c)
Adult male = 1950
Adult female = 1400
Child (10 years) = 1400
High average temperature (32°C):
Adults = 2840-3410
Moderate activity:
Adults = 3700
Respiratory volumes
8-h respiratory volume, litres
resting: Adult man = 3600
Adult woman = 2900
Child (10 years) = 2300
light/non-occupational
activity: Adult man = 9600
Adult woman = 9100
Child (10 years) = 6240
Daily inhalation volume, m3
(8-h resting, 16-h light/non-occupational activity)
Adult male = 23
Adult female = 21
Child (10 years) = 15
Average adult = 22
Proportion of time
spent indoorsc = 20 h/day
Amount of soil ingestedc = 20 mg/day
Dietary intaked
Cereals = 323 g/day (flour and milled rice)
Starchy roots = 225 g/day (sweet potatoes, cassava
and other)
Sugar = 72 g/day (includes raw sugar,
excludes syrups and honey)
Pulses and nuts = 33 g/day (includes cocoa beans)
Vegetables and fruits = 325 g/day (fresh equivalent)
Meat = 125 g/day (includes offal, poultry
and game in terms of carcass weight,
excluding slaughter fats)
Eggs = 19 g/day (fresh equivalent)
Fish = 23 g/day (landed weight)
Milk = 360 g/day (excludes butter; includes
milk products as fresh milk
equivalent)
Fats and oils = 31 g/day (pure fat content)
a WHO uses 60 kg for calculation of acceptable daily intakes and
water quality guidelines (WHO, 1987, 1993).
b WHO uses a daily per capita drinking-water consumption of 2 litres
in calculating water quality guidelines (WHO, 1993)
c From Health and Welfare Canada (1992)
d Based on average of estimates for 7 geographical regions
(ICRP, 1974)
RESUME
Des valeurs guides devraient être établies dans les Critères
d'hygiène de l'environnement (CHE) de l'IPCS pour l'exposition aux
produits chimiques présents dans l'environnement. Ces valeurs guides
pourront être modifiées par les autorités nationales et locales
lorsque celles-ci fixeront leurs normes et limites pour les différents
milieux. L'élaboration des valeurs guides pour les produits chimiques
comporte les étapes suivantes:
1. Evaluer et résumer les données relatives à la toxicité pour
l'homme et l'animal et à l'exposition humaine qui offrent un intérêt
particulier pour le calcul des valeurs guides. Ces données devraient
de préférence être présentées sous la forme d'un texte explicatif
résumant les points cruciaux, complété par des graphiques.
2. Ces données pourront servir à calculer une dose tolérable (DT)
pour les différentes voies d'exposition dans le cas des effets pour
lesquels on considère qu'il existe un seuil. Le calcul consiste
généralement à appliquer des facteurs d'incertitude aux doses sans
effet indésirable observé (DSEIO) établies par l'étude la plus
pertinente pour les effets critiques. En ce qui concerne les effets
pour lesquels il n'existe pas de seuil, la relation dose-réponse devra
être caractérisée aussi complètement que possible.
3. Estimer la proportion de la dose totale provenant des différents
milieux (atmosphère à l'intérieur des locaux, air ambiant, nourriture,
eau, etc.) dans une situation donnée, en prenant comme base de calcul
un ensemble cohérent de données sur les volumes théoriques absorbés
par l'"homme de référence" de la Commission internationale de
protection contre les radiations (CIPR) et des concentrations
représentatives de l'environnement général. En l'absence de données
adéquates sur les concentrations dans les différents milieux, on
pourra utiliser des modèles mathématiques pour estimer la répartition
entre ces milieux.
4. Attribuer une proportion de la DT aux différents milieux (d'après
les résultats de l'estimation décrite à l'étape 3 ci-dessus) de façon
à déterminer la dose ou l'exposition attribuable à chaque milieu.
5. Etablir des valeurs guides pour les doses attribuées à chaque
milieu en tenant compte éventuellement du poids corporel, du volume
absorbé et de l'efficacité de l'absorption (efficacité d'absorption
relative lorsque la valeur guide est calculée à partir de la DT
établie pour une autre voie d'exposition). Dans les monographies de
la série CHE, les valeurs guides devraient être établies pour un
scénario d'exposition clairement défini, fondé sur les données
applicables à l'homme de référence de la CIPR, données qui ne sont pas
nécessairement représentatives des conditions nationales ou locales
d'exposition. Normalement, les valeurs guides seront calculées pour
une population générale représentative, soumise à des conditions
d'exposition également représentatives. Elles devront être adaptées
au niveau national et local en fonction des circonstances.
6. La base de calcul des DT et des valeurs guides devrait être
clairement expliquée dans les monographies de la série CHE (pour le
niveau de détail exigé, voir les exemples de l'appendice 1).
RESUMEN
En los monografías de la serie Criterios de Salud Ambiental (EHC)
del IPCS deben formularse valores orientativos para la exposición a
sustancias químicas presentes en el medio ambiente, valores que las
autoridades nacionales y locales pueden modificar al determinar sus
límites y normas aplicables al medio. Los pasos previstos para
cualquier sustancia química son los siguientes:
1. Evaluar y resumir la información referente a la toxicidad en los
animales y el hombre y la exposición en el hombre, seleccionando la
más pertinente para el cálculo de los valores orientativos. El
esquema más apropiado para presentar los datos pertinentes con miras
al cálculo de los valores orientativos es un texto que describa
sucintamente los datos críticos, complementado con los gráficos
oportunos.
2. Calcular a partir de esos datos una Ingesta Tolerable (IT) para
las diversas vías de exposición y para los distintos efectos que se
considere que tienen un umbral. Ello entraña el uso de factores de
incertidumbre, aplicados por lo general al nivel sin efectos adversos
observados (NOAEL) para los efectos críticos referidos en el estudio
más pertinente. En el caso de los efectos sin umbral, se
caracterizará en la medida de lo posible la relación dosis-respuesta.
3. Estimar la proporción de ingesta total que tiene su origen en los
diversos medios (p. ej., aire de espacios interiores y ambiental,
alimentos y agua), sobre la base de las exposiciones calculadas para
un conjunto coherente de volúmenes supuestos de ingesta (utilizando el
hombre de referencia de la Comisión Internacional de Protección contra
las Radiaciones (CIPR)) y de concentraciones representativas en el
medio ambiente general para una determinada situación. Si no se
dispone de datos suficientes sobre las concentraciones en diversos
medios, pueden emplearse modelos matemáticos para estimar la
distribución por esos medios.
4. Asignar una proporción de la IT a diversos medios de exposición
(basándose en la exposición estimada conforme a lo explicado en el
paso 3 precedente) para determinar la ingesta o exposición en cada
medio.
5. Formular valores orientativos a partir de las ingestas asignadas a
cada medio, teniendo en cuenta (si es necesario) el peso corporal, el
volumen de ingesta y la eficiencia de absorción (la eficiencia de
absorción relativa cuando para calcular el valor orientativo se
utilice la IT correspondiente a otra vía de exposición). En los
monografías de la serie EHC se formularían valores orientativos para
unas condiciones de exposición claramente definidas, basadas en los
datos del hombre de referencia de la CIPR y no necesariamente
representativas de las condiciones de exposición nacionales o locales.
Se calcularán comúnmente valores orientativos para una población
general representativa y unas condiciones de exposición
representativas. Los valores orientativos se deberán adaptar a nivel
nacional y local según proceda en función de las circunstancias
locales.
6. En los monografías de la serie EHC se deberán detallar claramente
los fundamentos del cálculo tanto de la IT como de los valores
orientativos (respecto al grado de detalle, véanse los ejemplos
presentados en el apéndice 1).
 More recently, additional uncertainty factors have been incorporated
to account for, for example, deficiencies in the data base, such as
the absence of a NOAEL (US EPA, 1985a,b) or the absence of chronic
data (NAS, 1977).
If data from well-conducted studies in human populations are the
basis for the safety evaluation, a factor of 10 has been considered
appropriate, as a default value (WHO, 1987). Thus the value of 100
has been regarded as comprising two factors of 10 each to allow for
interspecies and inter-individual (intraspecies) variations. A scheme
has been proposed which retains the two 10-fold factors as the
cornerstone for extrapolating from animals to man but which allows
subdivision of each to incorporate appropriate data on toxicodynamics
or toxicokinetics where these exist (Renwick, 1993a) (see Fig. 2).
This approach improves the extrapolation process, and where
appropriate data can be introduced, it has the effect of replacing
"uncertainty" factors with "correction" factors. Data on differences
in dynamics and kinetics between humans and common laboratory animals,
such as rats, mice and dogs, indicated that there was greater
potential for differences in kinetics than in dynamics so that an
equal split of the 10-fold factor was inappropriate. The usual
10-fold factor (log 1) should be split into default values of 2.5
(100.4) for dynamics and 4 (100.6) for kinetics (Renwick, 1993a).
A similar split was proposed for interindividual differences between
humans in toxicokinetics (pharmacokinetics) and toxicodynamics (using
pharmacokinetic-pharmacodynamic modelling). However, it was
considered that the variability for both aspects was similar and it
was concluded that the 10-fold factor should be split evenly between
both aspects, i.e. 3.2 (100.5) for kinetics and 3.2 (100.5) for
dynamics. The commonly applied 100-fold uncertainty factor should be
split as indicated in Fig. 2.
Precise default values for kinetics and dynamics cannot be
expected on the basis of subdivision of the imprecise 10-fold
composite factor. The values above are reasonable since they provide
a positive value > 2 for both aspects and are compatible with the
species differences in physiological parameters such as renal and
hepatic blood flow. Since the data base examined was limited, it is
proposed that the values for subdivision of inter-species and
inter-individual variation presented in Fig. 2 be adopted on an
interim basis. Adoption of the approach should encourage the
development and generation of appropriate data, which could then
contribute to any future revision of the default values, and further
improve the scientific basis of the use of uncertainty factors.
More recently, additional uncertainty factors have been incorporated
to account for, for example, deficiencies in the data base, such as
the absence of a NOAEL (US EPA, 1985a,b) or the absence of chronic
data (NAS, 1977).
If data from well-conducted studies in human populations are the
basis for the safety evaluation, a factor of 10 has been considered
appropriate, as a default value (WHO, 1987). Thus the value of 100
has been regarded as comprising two factors of 10 each to allow for
interspecies and inter-individual (intraspecies) variations. A scheme
has been proposed which retains the two 10-fold factors as the
cornerstone for extrapolating from animals to man but which allows
subdivision of each to incorporate appropriate data on toxicodynamics
or toxicokinetics where these exist (Renwick, 1993a) (see Fig. 2).
This approach improves the extrapolation process, and where
appropriate data can be introduced, it has the effect of replacing
"uncertainty" factors with "correction" factors. Data on differences
in dynamics and kinetics between humans and common laboratory animals,
such as rats, mice and dogs, indicated that there was greater
potential for differences in kinetics than in dynamics so that an
equal split of the 10-fold factor was inappropriate. The usual
10-fold factor (log 1) should be split into default values of 2.5
(100.4) for dynamics and 4 (100.6) for kinetics (Renwick, 1993a).
A similar split was proposed for interindividual differences between
humans in toxicokinetics (pharmacokinetics) and toxicodynamics (using
pharmacokinetic-pharmacodynamic modelling). However, it was
considered that the variability for both aspects was similar and it
was concluded that the 10-fold factor should be split evenly between
both aspects, i.e. 3.2 (100.5) for kinetics and 3.2 (100.5) for
dynamics. The commonly applied 100-fold uncertainty factor should be
split as indicated in Fig. 2.
Precise default values for kinetics and dynamics cannot be
expected on the basis of subdivision of the imprecise 10-fold
composite factor. The values above are reasonable since they provide
a positive value > 2 for both aspects and are compatible with the
species differences in physiological parameters such as renal and
hepatic blood flow. Since the data base examined was limited, it is
proposed that the values for subdivision of inter-species and
inter-individual variation presented in Fig. 2 be adopted on an
interim basis. Adoption of the approach should encourage the
development and generation of appropriate data, which could then
contribute to any future revision of the default values, and further
improve the scientific basis of the use of uncertainty factors.
 It was recognised that appropriate toxicokinetic and
toxicodynamic data are rarely available for the same compound and that
to incorporate data in one area only would require the normal
composite factor of 10 to be subdivided. For example, if the
mechanism of action for the critical effects and differences in
sensitivity between the test species and man based on in vitro
studies were known, then these data could contribute quantitatively to
the risk assessment by replacement of the default factor for
interspecies differences in toxicodynamics, or differences in
sensitivity (the value of 2.5 in Fig. 2) by the value indicated by the
actual data. However, there could still be differences in
toxicokinetics between the test species and humans so that a portion
of the normal 10-fold factor would need to be retained (the value of 4
in Fig. 2).
3.1.2.2 Relevant toxicokinetic and toxicodynamic data
Toxicokinetics includes data on the rate and extent of absorption
(bioavailability), pattern of distribution, rate and pathway of any
bioactivation, and rate, route and extent of elimination. Factors
such as peak plasma concentration (Cmax), and area under the plasma
concentration-time curve (AUC) of the toxic entity are particularly
important since they are usually indicative of the extent and duration
of exposure of the target organ (Renwick, 1993a). Dosimetric
adjustments of administered animal dose to equivalent human dose are
also possible (Jarabek et al., 1990). However it is important to
define which parameter is relevant to the toxicity since some are
dependent on the Cmax and not AUC (e.g., the teratogenicity of
valproic acid; Nau, 1986) while for long-term bioassays, the AUC may
be of greater importance. Appropriate toxicodynamic factors include
the identification of the toxic entity (i.e. parent compound or a
metabolite), the nature of the molecular target, the presence and
activity of protective and repair mechanisms and the in vitro
sensitivity of the target tissue (see Renwick, 1993a for details and
examples). These toxicokinetic and toxicodynamic parameters should be
compared between the test species and humans for derivation of
interspecies factors where this is possible. Modification of the
10-fold factor for inter-individual variability in humans would
require data on toxicokinetics and toxicodynamics in a wide and fully
representative sample of the general or exposed population, including
an assessment of neonates if appropriate.
It is emphasised that in the absence of reliable information on
toxicokinetics and toxicodynamics, the default values for these
factors become the commonly used composite value of 100 (i.e., 10 for
inter-individual variability and 10 for interspecies variation).
3.1.2.3 Uncertainty factors for occupational exposure
The consideration of uncertainty factors given above relates
primarily to exposure of the general population. However, the general
principles for derivation of TIs for occupational exposure would be
somewhat similar (see, for example, Zielhuis & van der Kreek, 1979a,b;
Hallenbeck & Cunningham, 1986) although they have not been widely
adopted for this purpose. However, although the components of the
uncertainty factor relating to the nature and severity of the toxic
effect, the adequacy of the data base and interspecies variability
would be similar for the development of guidance values for
occupational exposure, the nature of the population exposed differs.
The more vulnerable members of the human population (i.e. the very
young, the sick and the elderly) do not form part of the exposed
occupational population, whereas for the development of TIs for the
general population, these groups must be considered. Furthermore,
workplace levels and patterns of exposure can be controlled and the
exposed population protected or monitored on an individual or group
basis. For these reasons, it is often appropriate to use
significantly lower uncertainty factors when deriving health-based
limits for occupational exposure compared with those used for the
development of TIs for the general population.
4. PROCEDURE FOR EXTRAPOLATION FROM A TOXICITY DATA BASE TO A
TOLERABLE INTAKE
4.1 Overall procedure
The procedure, which is presented in Fig. 3, is designed to be
applicable to widely differing data bases on toxicity. The procedure
is also suitable for the incorporation of human data, under which
circumstances some of the uncertainty factors will not be required.
The scheme is presented as a series of steps, but it is important that
the full data base continue to be reviewed to ensure that the final
decision is appropriate. A TI for a reversible toxic effect in an
animal species, for which there is complete toxicological data but
without appropriate toxicokinetic or toxicodynamic data, is based on
the commonly used and appropriate factor of 100. The scheme
incorporates those aspects which would normally be considered in the
conversion of a NOAEL (or LOAEL or equivalent) from an animal study
into a TI in such a way that appropriate mechanistic or toxicokinetic
data can contribute numerically to the uncertainty factor and hence to
the TI.
The procedure suggested here and discussed more fully in Renwick
(1993a) is based, in part, on discussions occurring over a number of
years regarding the basis of uncertainty factors (see, for example,
Zielhuis & van der Kreek, 1979a,b; Dourson & Stara, 1983; Lewis et
al., 1990; Rubery et al., 1990). To some extent, the principles
outlined here have been adopted in approaches of various national
agencies (e.g., Jarabek et al., 1990; Health and Welfare Canada, 1992;
US EPA, 1993).
4.2 Selection of pivotal study and critical effect(s)
Determination of the NOAEL, LOAEL or equivalent (possible use of
benchmark dose approach) is the first step in derivation of the TI.
This requires a thorough evaluation of available data on toxicity.
Sophisticated detection methods may be of such sensitivity that
effects can be detected at lower doses than by normal techniques; the
adversity of these effects requires very careful evaluation in the
determination of the NOAEL. For some chemicals, a review of the data
base may reveal that two (or possibly more) adverse effects occur at
low doses with NOAELs within one order of magnitude. Under such
circumstances and providing: a) that the data on which the NOAELs are
based are of sufficient quality to be used for risk evaluation; and b)
that the NOAELs may require different uncertainty factors based on,
for example, data on mechanisms or nature of toxicity (see below),
then each effect should be considered in the following scheme and the
one with the lower resulting TI used for development of guidance
values. Available LOAELs within the same order of magnitude as the
lowest reported NOAELs need also to be considered in this exercise
since they could lead to the development of more conservative TIs.
It was recognised that appropriate toxicokinetic and
toxicodynamic data are rarely available for the same compound and that
to incorporate data in one area only would require the normal
composite factor of 10 to be subdivided. For example, if the
mechanism of action for the critical effects and differences in
sensitivity between the test species and man based on in vitro
studies were known, then these data could contribute quantitatively to
the risk assessment by replacement of the default factor for
interspecies differences in toxicodynamics, or differences in
sensitivity (the value of 2.5 in Fig. 2) by the value indicated by the
actual data. However, there could still be differences in
toxicokinetics between the test species and humans so that a portion
of the normal 10-fold factor would need to be retained (the value of 4
in Fig. 2).
3.1.2.2 Relevant toxicokinetic and toxicodynamic data
Toxicokinetics includes data on the rate and extent of absorption
(bioavailability), pattern of distribution, rate and pathway of any
bioactivation, and rate, route and extent of elimination. Factors
such as peak plasma concentration (Cmax), and area under the plasma
concentration-time curve (AUC) of the toxic entity are particularly
important since they are usually indicative of the extent and duration
of exposure of the target organ (Renwick, 1993a). Dosimetric
adjustments of administered animal dose to equivalent human dose are
also possible (Jarabek et al., 1990). However it is important to
define which parameter is relevant to the toxicity since some are
dependent on the Cmax and not AUC (e.g., the teratogenicity of
valproic acid; Nau, 1986) while for long-term bioassays, the AUC may
be of greater importance. Appropriate toxicodynamic factors include
the identification of the toxic entity (i.e. parent compound or a
metabolite), the nature of the molecular target, the presence and
activity of protective and repair mechanisms and the in vitro
sensitivity of the target tissue (see Renwick, 1993a for details and
examples). These toxicokinetic and toxicodynamic parameters should be
compared between the test species and humans for derivation of
interspecies factors where this is possible. Modification of the
10-fold factor for inter-individual variability in humans would
require data on toxicokinetics and toxicodynamics in a wide and fully
representative sample of the general or exposed population, including
an assessment of neonates if appropriate.
It is emphasised that in the absence of reliable information on
toxicokinetics and toxicodynamics, the default values for these
factors become the commonly used composite value of 100 (i.e., 10 for
inter-individual variability and 10 for interspecies variation).
3.1.2.3 Uncertainty factors for occupational exposure
The consideration of uncertainty factors given above relates
primarily to exposure of the general population. However, the general
principles for derivation of TIs for occupational exposure would be
somewhat similar (see, for example, Zielhuis & van der Kreek, 1979a,b;
Hallenbeck & Cunningham, 1986) although they have not been widely
adopted for this purpose. However, although the components of the
uncertainty factor relating to the nature and severity of the toxic
effect, the adequacy of the data base and interspecies variability
would be similar for the development of guidance values for
occupational exposure, the nature of the population exposed differs.
The more vulnerable members of the human population (i.e. the very
young, the sick and the elderly) do not form part of the exposed
occupational population, whereas for the development of TIs for the
general population, these groups must be considered. Furthermore,
workplace levels and patterns of exposure can be controlled and the
exposed population protected or monitored on an individual or group
basis. For these reasons, it is often appropriate to use
significantly lower uncertainty factors when deriving health-based
limits for occupational exposure compared with those used for the
development of TIs for the general population.
4. PROCEDURE FOR EXTRAPOLATION FROM A TOXICITY DATA BASE TO A
TOLERABLE INTAKE
4.1 Overall procedure
The procedure, which is presented in Fig. 3, is designed to be
applicable to widely differing data bases on toxicity. The procedure
is also suitable for the incorporation of human data, under which
circumstances some of the uncertainty factors will not be required.
The scheme is presented as a series of steps, but it is important that
the full data base continue to be reviewed to ensure that the final
decision is appropriate. A TI for a reversible toxic effect in an
animal species, for which there is complete toxicological data but
without appropriate toxicokinetic or toxicodynamic data, is based on
the commonly used and appropriate factor of 100. The scheme
incorporates those aspects which would normally be considered in the
conversion of a NOAEL (or LOAEL or equivalent) from an animal study
into a TI in such a way that appropriate mechanistic or toxicokinetic
data can contribute numerically to the uncertainty factor and hence to
the TI.
The procedure suggested here and discussed more fully in Renwick
(1993a) is based, in part, on discussions occurring over a number of
years regarding the basis of uncertainty factors (see, for example,
Zielhuis & van der Kreek, 1979a,b; Dourson & Stara, 1983; Lewis et
al., 1990; Rubery et al., 1990). To some extent, the principles
outlined here have been adopted in approaches of various national
agencies (e.g., Jarabek et al., 1990; Health and Welfare Canada, 1992;
US EPA, 1993).
4.2 Selection of pivotal study and critical effect(s)
Determination of the NOAEL, LOAEL or equivalent (possible use of
benchmark dose approach) is the first step in derivation of the TI.
This requires a thorough evaluation of available data on toxicity.
Sophisticated detection methods may be of such sensitivity that
effects can be detected at lower doses than by normal techniques; the
adversity of these effects requires very careful evaluation in the
determination of the NOAEL. For some chemicals, a review of the data
base may reveal that two (or possibly more) adverse effects occur at
low doses with NOAELs within one order of magnitude. Under such
circumstances and providing: a) that the data on which the NOAELs are
based are of sufficient quality to be used for risk evaluation; and b)
that the NOAELs may require different uncertainty factors based on,
for example, data on mechanisms or nature of toxicity (see below),
then each effect should be considered in the following scheme and the
one with the lower resulting TI used for development of guidance
values. Available LOAELs within the same order of magnitude as the
lowest reported NOAELs need also to be considered in this exercise
since they could lead to the development of more conservative TIs.
 Graphical presentation of available data can facilitate
identification of effect levels relevant to development of TIs.
Although the form of graphical presentation is necessarily dependent
upon the size of the data base, a dose-duration graph in which NOELs,
NOAELs and LOAELs are presented as a function of duration of exposure
is considered to be helpful and is more fully described in Appendix 2.
4.3 Adequacy of the pivotal study
In situations where a NOAEL has not been achieved but the data
on effects are of sufficient quality to be the basis of the risk
assessment, then a no-adverse-effect level should be developed by the
application of an appropriate uncertainty factor to the LOAEL.
Uncertainty factors of 3, 5 or 10 have been used previously to
extrapolate from a LOAEL to a NOAEL depending on the nature of the
effect(s) and dose-response relationship (see, for example, US EPA,
1993). Alternatively, a benchmark dose may be developed by
mathematical modelling of the dose-response data as an alternative to
the uncertainty factor in extrapolating to the NOAEL (see Appendix 3).
The pivotal study may also be considered inadequate for other reasons
(e.g., duration of study, numbers of animals per group and sensitivity
of the analyses of effect), and an additional uncertainty factor
applied.
4.4 Interspecies extrapolation
In situations where appropriate toxicokinetic and/or
toxicodynamic data exist for a particular compound, then the relevant
uncertainty factor in Fig. 3 should be replaced by the data-derived
factor. Data on PBPK and/or data on target organ exposure should be
included when they are available. Subdivision of the 10-fold
uncertainty factor has been used in the development of a reference
concentration for 1,2-epoxybutane (US EPA, 1993). Chemicals for which
the approach described here has been applied include saccharin
(Renwick, 1993b), erythrosine (Poulsen, 1993), butylated
hydroxyanisole (BHA) (Wurtzen, 1993) and diethylhexyl phthalate (DEHP)
(Morgenroth, 1993).
If a data-derived factor is introduced then the commonly used
10-fold factor would be replaced by the product of that data-derived
factor and the remaining default factor. For some classes of
compounds a data-derived factor for one member of the class may be
applicable to all members, thereby producing a group-based
data-derived factor (see Calabrese, 1992). The interspecies
uncertainty factor is not necessary if the NOAEL or LOAEL is based on
human data.
4.5 Inter-individual variability in humans
A factor of 10 is normally used to allow for differences in
sensitivity in vivo between the population mean and highly sensitive
subjects. In cases where there are appropriate data on the
inter-individual variability in toxicokinetics or toxicodynamics for a
particular compound in humans, then the relevant uncertainty factor
should be replaced by the data-derived factor. Data on PBPK may also
be able to contribute to this assessment. If a data-derived factor is
introduced, then the commonly used 10-fold factor would be replaced by
the product of the data-derived factor and the remaining default
factor. (For additional discussion, see Calabrese, 1985; Hattis et
al., 1987).
For some compounds, it may be known that a subset of the
population would be particularly sensitive, for example due to
deficiencies in detoxication processes. Many of the enzymes involved
in xenobiotic biotransformation are polymorphically distributed in the
human population. Such polymorphism should be taken into account
where the enzymatic differences result either in a marked change in
bioavailability or clearance of the parent compound or in a major
change in the extent of formation of the toxic entity. In cases where
the default factor will not adequately cover this additional
variability, then the default should be modified appropriately.
Alternatively, these groups may require special strategies for health
protection. In cases where the risk assessment is based on in vivo
data in the sensitive subgroup, then the composite factor (10) should
be reduced to a much lower value. A value of 1 could be used if there
is an extensive data base in humans and the data base adequately
addresses any identified sensitive subgroups. For example, the US EPA
estimated an oral reference dose for fluoride based on the absence of
dental mottling in children 12 to 14 years of age. Since this group
was considered to be a sensitive subpopulation, a factor of 1 for
inter-individual variation was considered to be appropriate (US EPA,
1993).
4.6 Other considerations
4.6.1 Adequacy of the overall data base
Major deficiencies in a toxicity data base (other than those
related to the pivotal study) which increase the uncertainty of the
extrapolation process should be recognized by the use of an additional
uncertainty factor. Since the quality and/or completeness of
different data bases vary, the additional uncertainty factor will also
vary. For example, a value of 1 would be applied to a data base that
was considered complete for the evaluation of the compound under
consideration, but a factor of 1-100 might be necessary for limited
data bases. If minor deficiencies in the data exist with respect to
quality, quantity or omission, then an extra factor of 3 or 5 would be
appropriate. An extra factor of 10 would be appropriate where major
deficiencies in the data exist with respect to quality, quantity or
omission, such as a lack of chronic toxicity studies and reproductive
toxicity studies (for additional discussion see Dourson et al., 1992).
It should be appreciated that when very large uncertainty factors
are incorporated, the derived TI should be considered as an very
imprecise temporary estimate pending the generation of a better data
base. It should be recognized that inadequacies of the pivotal study
(section 4.3) could also be considered as a subset of inadequacies of
the data base; the total factor for limitations of the pivotal study
plus adequacy of the overall data base should not exceed 100 since
such a data base is generally not acceptable for development of a TI.
4.6.2 Nature of toxicity
The nature of toxicity, i.e. whether the effect is adverse or
not, is considered in the determination of NOAEL and LOAEL. For
example, a concentration or dose which induces a transient increase in
organ weight without accompanying biochemical or histopathological
effects might be considered to be a NOAEL. If there are accompanying
adverse histopathological effects in the target organ, the lowest
concentration or dose at which these effects occur would be considered
a LOAEL. The sensitivity of analyses of effects should also be taken
into account in establishing the NOAEL or LOAEL (see discussion in
section 4.2).
In addition, a number of bodies, including the WHO and FAO Joint
Expert Committee on Food Additives (JECFA) and the Joint Meeting on
Pesticide Residues (JMPR) have incorporated an additional "safety
factor" of up to 10 (corresponding to an uncertainty factor in the
current discussion) in cases where the NOAEL is derived for a critical
effect which is a severe and irreversible phenomenon, such as
teratogenicity or non-genotoxic carcinogenicity, especially if
associated with a shallow dose-response relationship (Weil, 1972; WHO,
1987, 1990). Provision for the application of additional safety
factors is included in the sequence shown in Fig. 3.
4.7 Final review of the total uncertainty factor
It is important that there is a final review of the total
uncertainty factor applied, particularly in cases where a low value
has been used, based on toxicokinetic or toxicodynamic data, to
replace one of the default values. Under such circumstances, a TI
derived on the basis of the appropriate overall uncertainty factor for
that toxic effect might be greater than that which would be produced
by an alternative, well-defined toxic end-point observed at slightly
higher intakes or exposures. For this reason, there are arrows shown
in Fig. 3 leading back to the data base.
4.8 Precision of the tolerable intake
The TI is calculated by dividing the NOAEL for the critical
effect by the derived total uncertainty factor. The precision of the
estimate depends in large part on the magnitude of the overall
uncertainty factor used in the calculation. The precision is probably
to one significant figure at best, and more usually to one order of
magnitude, and for uncertainty factors of 1000 or more the precision
becomes even less. Because of the imprecision of the default factors
and in order to maintain credibility of the risk assessment process,
the total default uncertainty factor should not exceed 10 000. If the
risk assessment leads to a higher factor then the resulting TI would
be so imprecise as to lack meaning. Such a situation indicates an
urgent need for additional data.
4.9 Alternative approaches
Approaches being developed to characterize quantitatively the
dose-response relationship for non-threshold effects (including the
benchmark dose and categorical regression) are described in
Appendix 3.
5. ALLOCATION OF TOLERABLE INTAKES TO DERIVE GUIDANCE VALUES
5.1 General considerations
Allocations of the TIs to various media for the development of
guidance values are based on relative proportion of total exposure
from each of the media. This necessitates the presentation of
consistent and detailed estimates of exposure for as many media as
possible in draft EHCs prior to review and evaluation. Wherever
possible, estimation of exposure should be based on concentrations in
environmental media including (but not necessarily limited to) air,
food, drinking-water, soil and consumer products. With respect to
soil, wherever possible, estimated exposure should take into account
both ingestion and dermal contact. Since the bioavailability of
contaminants in soil from both ingestion and dermal contact may be
limited, this should be taken into account in assessing the
contribution that soil makes to total intake from all media.
It is recommended that unless there are other age groups which
are more sensitive or have widely differing exposure profiles, intake
from each of the media (generally expressed as µg/kg body weight per
day) should be estimated for adults, based on ICRP reference values
for body weights and ingestion volumes (ICRP, 1974; Appendix 4).
Wherever possible, estimation of exposure should be based on ranges of
mean concentrations in environmen-tal media on a global basis. Where
data are more limited, ranges of individual values could be used.
Estimates of exposure as a basis for derivation of guidance values are
presented in the examples in Appendix 1.
Where the data on concentrations of a substance in environmental
media are inconsistent or inadequate, exposure can be estimated based
on models which incorporate as much data as possible on, for example,
production, use patterns and physical and chemical properties. Models
to predict distribution in environmental media and estimation of
proportion of total exposure by various routes from consumer products
are available (Mackay, 1991; USES, 1994). For estimation of
proportions of exposure from various environmental media for
development of guidance values in EHCs, it is recommended that the
latest version of the Mackay level III model be used (Mackay et al.,
1992). It is important that all assumptions concerning releases and
physico-chemical properties and limitations of the estimated
proportions be clearly specified. In some cases, it may also be
possible to estimate the contribution of each medium to total exposure
on the basis simply of data on physical and chemical properties (e.g.,
for substances which are likely to be present primarily in one
environmental medium).
When available, toxicokinetic data should be used to the extent
possible in extrapolating across routes in the approaches to
allocation described below. Dermal exposure and absorption should
also be taken into account in the derivation of guidance values,
although relevant data are often not available. It is also recognized
that a source in one medium (e.g., potable water) may lead to
additional intake from other routes (e.g., dermal and inhalation) and
that, where possible, such intake should be considered in the
derivation of guidance values.
In addition, total allocations of less than 100% of the TI are
encouraged to account for, for example, those media for which exposure
has not been characterized and cross-route exposure. The magnitude of
the proportion of total intake which is not allocated should vary as a
function of the adequacy of characterization of total exposure from
all media.
In cases where the proportion of total exposure from a specific
medium is small (less than a few percent), allocation for derivation
of guidance values is not recommended since this would result in
direction of risk management strategies to media which are
inconsequential in contributing to total exposure.
5.2 General approach
The steps subsequent to development of a TI in deriving guidance
values for a general population are as follows:
1. If necessary, conversion of TIs for systemic effects for different
routes of exposure to a common unit for comparison based on
consideration of volumes and rates of inhalation and ingestion and
relevant toxicokinetic data, such as bioavailability, if available.
2. Allocation of TI to various routes and media based on estimated
exposure developed on the basis of available data on measured
concentrations or predicted proportions (i.e., model-derived values)
to which humans are exposed. Default values can be used in the
absence of data on measured concentrations or predicted proportions of
total exposure in various media.
3. Development of guidance values from intake assigned to each
medium, taking into account, for instance, body weight, volume of
intake and (relative) absorption efficiency ( relative where guidance
value is derived on the basis of a TI by another route of exposure).
Guidance values for drinking-water are generally expressed in µg/litre
or mg/litre, those in food as µg/g or mg/kg, those in air as µg/m3
or mg/m3, and those for dermal exposure as µg/m2 surface area.
5.3 Detailed approach
In the following section, an approach to the allocation of
tolerable intakes for development of guidance values (general
population) is provided by way of example for most of the scenarios
which may arise based on evaluations presented in EHCs.
The five most likely scenarios are considered to be:
5.3.1 Biomarkers of exposure
There is a common biomarker related to the critical effect which
integrates exposure from all sources. For example, Choudhury et al.
(1992) describe a model which predicts blood lead concentrations as a
function of concentrations in various media.
* The contributions from the various media are determined based on
a quantitative biomarker. Following allocation to various media
based on an exposure scenario, guidance values are developed
through incorporation of adjustment of body weight and volume of
intake for each medium.
5.3.2 Critical effects which are not route specific
TIs have been derived for each route, e.g., TI for oral exposure
(TIo) and TI for inhalation (TIi), and are based either on the
same or on different critical effects which are not at the portal of
entry. The TIs for the two routes are similar within one order of
magnitude since such variation is consistent with that inherent in
deriving TIs, as discussed in section 4, e.g., developmental toxicity
of 2-methoxyethanol (Doe et al., 1983; Wickramaratne, 1986). This
reflects the assumption that, in the absence of data to the contrary,
exposure via each route is considered to contribute to a combined dose
at the target site(s), i.e., additivity of dose at the target site(s).
* Allocate one TI to various media based on an exposure scenario to
determine the intake in each medium on which guidance values
should be based. Selection of the TIo or the TIi for this
purpose should be based on either:
a) if there is one major route of exposure then the TI for that
route should be used (if there is confidence in the data
base on which the exposure estimates are based); or
b) the more conservative TI (if there is uncertainty about the
relative contribution of various routes or media to total
exposure).
5.3.3 Difference in magnitude of effect by route of exposure
TIo and TIi for similar effects vary by 1 to 2 orders of
magnitude (exact magnitude of the difference for which this approach
is appropriate will be dependent upon availability of additional data;
e.g., manganese is more potent by inhalation than by ingestion).
* Derive the guidance values independently for each route (for
example, the oral and inhalation routes, based on the TIo and
TIi, respectively), but allocate the proportion of the TI for
each route to the appropriate medium or media based on an
exposure scenario.
5.3.4 Route-specific effect variation at portals of entry
(due to local bioactivation or local effects)
TIo and TIi for route-specific effects at the site of entry
vary by 1 to 2 orders of magnitude (exact magnitude of the difference
for which this approach is appropriate will be dependent upon
knowledge of additional data; e.g., nasal toxicity following
inhalation of acrylic acid).
* Derive the guidance values independently for each route (for
example, the oral and inhalation routes, based on the TIo and
TIi, respectively), using the full TI for each route to the
appropriate medium or media based on an exposure scenario.
5.3.5 Limited data base
In this scenario, the data base is limited such that only either
a TIo or a TIi can be developed.
* Allocate the available TI to various media based on an exposure
scenario to determine the intake in each medium on which guidance
values should be based, if the effects are qualitatively similar,
if toxicokinetic data are consistent with this approach and if
there are no effects at the site of entry. If any one of these
criteria is not met, do not derive guidance values for the
alternate route. If a TI is available for a route of exposure
which does not make an important contribution to total intake, do
not derive guidance values for that route.
6. EXAMPLES OF THE DERIVATION OF GUIDANCE VALUES
Example 1
The principal route of exposure is oral. Based on estimated
exposure for a scenario in the general environment, 50% of total
intake comes from food, 20% from water and 30% from air.
Data are adequate to establish both a TIo and a TIi. The
TIo and the TIi are based on similar effects and are similar
(within one order of magnitude).
Allocate 50% of TIo to food to derive a guidance value for food
* multiply TIo by 0.5
Allocate 20% of TIo to water to derive a guidance value for
drinking-water
* multiply TIo by 0.2
Allocate 30% of TIo to air to derive a guidance value for air
* multiply TIo by 0.3
Example 2
Based on an exposure scenario, 70% of total intake comes from
air, 20% from water and 10% from food. The compound is also present
in some consumer products but quantification of exposure is not
possible. There are no data on concentrations in soil but due to its
physicochemical properties, concentrations in this medium are likely
to be low.
Data are sufficient to establish a TIi and a TIo. The TIo
and the TIi are based on similar effects and are similar to within
an order of magnitude.
Convert TIi so that the values for the TIs for different routes
are expressed in the same units for comparison (generally mg/kg body
weight per day). This requires incorporation of information on
inhalation volumes, body weight and toxicokinetic data, if available.
Use TI for principal route of exposure to derive guidance values:
Allocate 63% of TIi to air to derive a guidance value for air.
* multiply TIi by 0.63
Allocate 18% of TIi to water to derive a guidance value for
drinking-water.
* multiply TIi by 0.18
Allocate 9% of TIi to food to derive a guidance value for food.
* multiply TIi by 0.09
Reserve 10% for exposure from consumer products and soil.
(Wherever possible, there should be an attempt to quantitatively
estimate the proportion of total intake from these sources).
Develop a guidance value for each medium by (if necessary)
adjustment for body weight, volume of intake and relative absorption.
Example 3
The principal route of exposure is oral. Based on estimated
exposure, 50% of total intake comes from food, 20% from water, 20%
from air and 10% from soil (after taking bioavailability into account
from the oral and dermal routes). The compound is believed not to be
present in consumer products.
Data are adequate to establish both a TIo and a TIi. The
TIo and the TIi are based on the same effects but the compound is
much more toxic by the oral route (e.g., TIo is less than the TIi
by more than two orders of magnitude).
Allocate 50% of TIo to food to derive a guidance value for food
* multiply TIo by 0.5
Allocate 20% of TIo to water to derive a guidance value for
drinking-water
* multiply TIo by 0.2
Allocate 10% of TIo to soil to derive a guidance value for soil
* multiply TIo by 0.1
Allocate 20% of TIi to air to derive a guidance value for air
* multiply TIi by 0.2
Example 4
The principal route of exposure is oral. Based on estimated
exposure, 50% of total intake comes from food, 20% from water and 30%
from air. There are no data indicating exposure from soil and
consumer products.
Data are adequate to establish both a TIo and a TIi for
route-specific effects. The TIo and the TIi are based on
route-specific effects and the compound is much more toxic by the oral
route (e.g., TIo is less than the TIi by more than two orders of
magnitude).
Because the effects are route specific and the TIs are different
by two orders of magnitude, each TI can be allocated in full to
appropriate media.
Allocate 50/70 (71%) of TIo to food to derive a guidance value
for food
* multiply TIo by 0.71
Allocate 20/70 (29)% of TIo to water to derive a guidance value
for drinking-water
* multiply TIo by 0.29
Allocate 100% of TIi to air to derive a guidance value for air
* multiply TIi by 1
Example 5
The principal route of exposure is inhalation.
Data are inadequate to establish a TIi.
Data are sufficient to establish a TIo.
Available toxicokinetic data are inadequate for or inconsistent
with extrapolation across routes.
Do not establish guidance values.
REFERENCES
Allen BC, Van Landingham C, Howe RB, Kavlock RJ, Kimmel CA, & Faustman
EM (1992) Dose-response modeling for developmental toxicity.
Toxicologist, 12(1): 300.
Allen B, Kavlock RJ, Kimmel CA, & Faustman EM (1993) Comparison of
quantitative dose response modeling approaches for evaluating fetal
weight changes in segment II developmental toxicity studies.
Teratology, 47(5): 41.
ATSDR (1989) Toxicological profile for aldrin/dieldrin. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (Publication
ATSDR/TP-88/01).
Barnes DG & Dourson M (1988) Reference dose (RfD): Description and use
in health risk assessment. Regul Toxicol Pharmacol, 8: 471-486.
Calabrese EJ (1985) Uncertainty factors and interindividual
variation. Regul Toxicol Pharmacol, 5: 190-196.
Calabrese EJ (1992) Does the animal to human uncertainty factor
incorporate interspecies differences in surface area? Regul Toxicol
Pharmacol, 15(2): 172-179.
Canada (1988) Government of Canada: Canadian Environmental Protection
Act, Ottawa. Canada Gaz, II(4): 415-512.
Choudhury H, Peirano WB, Marcus A, Elias R, Griffin S, & Derosa CT
(1992) Utilization of uptake biokinetic (UBK) lead model to assess
risk in contaminated sites. In: Superfund risk assessment in soil
contamination studies. Philadelphia, Pennsylvania, American Society of
Testing and Materials, pp 193-204.
Crump KS (1984) A new method for determining allowable daily intakes.
Fundam Appl Toxicol, 4: 854-871.
Doe JE, Samuels DM, Tinston DJ, & Wickramaratne GA de S (1983)
Comparative aspects of the reproductive toxicology by inhalation in
rats of ethylene glycol monomethyl ether and propylene glycol
monomethyl ether. Toxicol Appl Pharmacol, 69: 43-47.
Dourson ML & Stara J (1983) Regulatory history and experimental
support of uncertainty (safety) factors. Regul Toxicol Pharmacol, 3:
224-238.
Dourson ML, Hertzberg R, Hartung R, & Blackburn K (1985) Novel methods
for the estimation of acceptable daily intake. Toxicol Ind Health,
1(4): 23-33.
Dourson ML, Knauf L, & Swartout J (1992) On reference dose (RfD) and
its underlying toxicity data base. Toxicol Ind Health, 8(3): 171-189.
Farland W & Dourson ML (1992) Noncancer health endpoints: approaches
to quantitative risk assessment. In: Cothern R ed. Comparative
environmental risk assessment. Boca Raton, Florida, Lewis Publishers,
pp 87-106.
Gaylor DW (1989) Quantitative risk analysis for quantal reproductive
and developmental effects. Environ Health Perspect, 79: 243-246.
Gaylor DW (1992) Incidence of developmental defects at the No
Observed Effect Level (NOEL). Regul Toxicol Pharmacol, 15: 151-160.
Guth DJ, Jarabek AM, Wymer L, & Hertzberg RC (1991) Evaluation of risk
assessment methods for short-term inhalation exposure. Presentation
at the 84th Annual Meeting of the Air and Waste Management
Association, Vancouver, British Columbia, 16-21 June 1991.
Hallenbeck WH & Cunningham KM (1986) Quantitative risk assessment for
environmental and occupational health. Chelsea, Michigan, Lewis
Publishers, Inc., p 199.
Hattis D, Erdreich L, & Ballew M (1987) Human variability in
susceptibility to toxic chemicals -A preliminary analysis of
pharmacokinetic data from normal volunteers. Risk Anal, 7(4):
415-426.
Hartung R (1986) Ranking the severity of toxic effects. In: Trace
substances in environmental health. Columbia, Missouri, University of
Missouri, pp 204-211.
Hartung R & Durkin PR (1986) Ranking the severity of toxic effects:
Potential applications to risk assessment. Comments Toxicol, 1: 49-63.
Health and Welfare Canada (1992) Determination of "toxic" under
paragraph 11c) of the Canadian Environmental Protection Act, 1st ed.
Ottawa, Ontario, Bureau of Chemical Hazards, Environmental Health
Directorate.
Hertzberg RC (1989) Fitting a model to categorical response data with
application to special extrapolation to toxicity. Health Phys,
57(Suppl 1): 404-409.
Hertzberg RC & Miller M (1985) A statistical model for species
extrapolation using categorical response data. Toxicol Ind Health, 1:
43-57.
ICRP (1974) International Commission on Radiological Protection:
Report of the Task Group on Reference Man. Oxford, Pergamon Press
(ICRP Publication No. 23).
Jarabek AM, Menache MG, Overton JH Jr, Dourson ML, & Miller FJ (1990)
The US Environmental Protection Agency's inhalation RfD methodology:
Risk assessment for air toxics. Toxicol Ind Health, 6(5): 279-301.
Kavlock RJ, Greene JA, Kimmel GL, Morrissey RE, Owens E, Rogers JM,
Sadler TW, Stack F, Waters MD, & Welsch F (1991) Activity profiles of
developmental toxicity: design considerations and pilot
implementation. Teratology, 43: 159-185.
Kimmel CA & Gaylor DW (1988) Issues in qualitative and quantitative
risk analysis for developmental toxicology. Risk Anal, 8: 15-20.
Lehman AJ & Fitzhugh OG (1954) 100-fold margin of safety. Assoc Food
Drug Off US Q Bull, 18: 33-35.
Lewis SC, Lynch JR, & Nikiforov AI (1990) A new approach to deriving
community exposure guidelines from no-observed-adverse-effect levels.
Regul Toxicol Pharmacol, 11: 314-330.
Lu F (1988) Acceptable daily intake: inception, evolution and
application. Regul Toxicol Pharmacol, 8(1): 45-60.
Mackay D (1991) Multimedia environmental models. The fugacity
approach. Chelsea, Michigan, Lewis Publishers, Inc., 260 pp.
Mackay D, Paterson S, & Shiu WY (1992) Generic models for evaluating
the regional fate of chemicals. Chemosphere, 24: 695-717.
Moolgavkar SH, Dewanji A, & Venzon DJ (1988) A stochastic two-stage
model for cancer risk assessment. I: The hazard function and the
probability of tumor. Risk Anal, 8: 383-392.
Morgenroth V (1993) Scientific evaluation of the data-derived safety
factors for the acceptable daily intake. Case study:
diethylhexylphthalate. Food Addit Contam, 10(3): 363-373.
NAS (1977) Drinking water and health. Washington, DC, National
Research Council, US National Academy of Sciences, 939 pp.
Nau H (1986) Species differences in pharmacokinetics and drug
teratogenesis. Environ Health Perspect, 70: 113-129.
Poulsen E (1993) Case study: erythrosine. Food Addit Contam, 10:
315-323.
Renwick AG (1993a) Data-derived safety factors for the evaluation of
food additives and environmental contaminants. Food Addit Contam, 10:
275-305.
Renwick AG (1993b) A data-derived safety (uncertainty) factor for the
intense sweetener, saccharin. Food Addit Contam, 10: 337-350.
Rubery ED, Barlow SM, & Steadman JH (1990) Criteria for setting
quantitative estimates of acceptable intakes of chemicals in food in
the U.K. Food Addit Contam, 7: 287-302.
Shoaf CR (1994) US EPA inhalation testing guidelines. In: Jenkins PG,
Kayser D, Muhle H, Rosner G, & Smith EM ed. Respiratory toxicology and
risk assessment. Proceedings of an International Symposium. Stuttgart,
Wissenschaftliche Verlagsgesellschaft mbH, pp 11-25 (IPCS Joint Series
No. 18).
US EPA (1985a) National primary drinking water regulations; volatile
synthetic organic chemicals; final rule and proposed rule. Fed Reg,
50: 46830-46901.
US EPA (1985b) National primary drinking water regulations, synthetic
organic chemicals, inorganic chemicals and microorganisms; proposed
rule. Fed Reg, 50: 46936-47025.
US EPA (1993) The Integrated Risk Information System (IRIS).
Cincinnati, Ohio. Office of Health and Environmental Assessment,
Environmental Criteria and Assessment Office.
USES (1994) Uniform system for the evaluation of substances (USES),
version 1.0. Bilthoven, The Netherlands, National Institute for
Public Health and Environmental Protection, 332 pp.
Waters MD, Stack HF, Brady AL, Lohman PHM, Haroun L, & Vainio H (1988)
Use of computerized data listings and activity profiles of genetic and
related effects in the review of 195 compounds. Mutat Res, 205:
295-312.
Weil CS (1972) Statistics versus safety factors and scientific
judgement in the evaluation for man. Toxicol Appl Pharmacol, 21:
454-463.
WHO (1987) IPCS Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food. Geneva,
World Health Organization, 174 pp.
WHO (1990) IPCS Environmental Health Criteria 104: Principles for the
toxicological assessment of pesticide residues in food. Geneva, World
Health Organization. 117 pp.
WHO (1993) Guidelines for drinking-water quality, Second edition.
Volume 1: Recommendations. Geneva, World Health Organization, 198 pp.
Wickramaratne GA de S (1986) The teratogenic potential and
dose-response of dermally administered ethylene glycol monomethyl
ether (EGME) estimated in rats with the Chernoff-Kavlock assay. J Appl
Toxicol, 6(3): 165-166.
Wurtzen G (1993) Scientific evaluation of the safety factor for the
acceptable daily intake (ADI). Case study: butylated hydroxy anisole
(BHA). Food Addit Contam, 10: 307-314.
Zielhuis RL & van der Kreek FW (1979a) The use of a safety factor in
setting health based permissible levels for occupational exposure. Int
Arch Occup Environ Health, 42: 191-201.
Zielhuis RL & van der Kreek FW (1979b) Calculation of a safety factor
in setting health based permissible levels for occupational exposure.
Int Arch Occup Environ Health, 42: 203-215.
APPENDIX 1
EXAMPLES - DEVELOPMENT OF GUIDANCE VALUES
The following practical examples are provided to illustrate the
manner in which tolerable intakes (TIs) may be developed and allocated
for the derivation of guidance values for a general population (on the
basis of calculated proportions of exposure from various media). In
the calculation of guidance values, TIs may be rounded up to 1 or 2
significant figures depending on the quality of the data base and the
extent of uncertainties involved in deriving the TI. The level of
detail shown is that which is considered necessary for EHCs and should
be sufficient for adaptation at national and local levels.
Compound A
Chlorinated hydrocarbon
Estimates of exposure
Estimated daily intakes of Compound A for adults (µg/kg
body weight per day)1 in the general population are as follows:
Ambient air2 < 0.03
Drinking-water3 0.00007-< 0.0004
Food4 0.004
Soil no data
Consumer products no data
Total Intake 0.03
1 Assumed to weigh 64 kg, breathe 22 m3 of air per day and drink
1.4 litres of water Per day (ICRP, 1974) and to consume 125 g per day
of a meat composite (the compound was not detected in other dietary
composites).
2 Based on a mean concentration of Compound A reported in a survey
of ambient Air from 22 sites (< 0.10 µg/m3); concentrations in
indoor air were similar to those in ambient air.
3 Based on a range of mean concentrations of Compound A in
drinking-water of 0.003 µg/litre to < 0.02 µg/litre.
4 Based on a concentration of 0.0018 µg/g of Compound A detected in
a representative daily diet.
On the basis of these estimates, it is considered that the
percentage of total exposure from various media for the general
population (midpoints of estimated intakes) is as follows:
outdoor/indoor air = < 0.03/0.03 = 85.9% (86%)
(< 0.03 considered to be 0.03 minus
intake from other media)
drinking-water = 0.000245/0.03 = 0.82% (0.8%)
food = 0.004/0.03 = 13.3% (13%)
soil = no data
consumer products = no data
Development of TI
The only data identified on long-term toxicity following
inhalation are the results of a single subchronic study for which no
effects were observed at any concentration. Available data are
considered inadequate, therefore, to establish a TI on the basis of
the results of studies in which Compound A has been administered by
inhalation. Moreover although the general population appears to be
exposed to Compound A principally in air, based on limited available
data on concentrations in food, the estimated intake in food is within
the range of that estimated for air for some age groups. In addition,
the principal route of intake of the most exposed age group (i.e.
suckling infants) is ingestion (of mothers' milk). Owing to the lack
of adequate long-term toxicity studies by the inhalation route and the
possible relatively important contribution that food makes to total
exposure to Compound A, a TI is derived on the basis of a long-term
ingestion bioassay, as follows:
60 mg/kg body
weight per day × 5
TI = approx. 0.43 mg/kg (430 µg/kg)
body weight per day
100 × 7
where:
* 60 mg/kg body weight per day is the NOAEL, determined in a
well-conducted and documented long-term (chronic and
carcinogenesis) bioassay, with renal tubular degeneration
observed at higher doses
* 5/7 is the conversion of five days per week of dosing to seven
days per week
* 100 is the uncertainty factor (×10 for inter-individual
variation; ×10 for interspecies variation; available data on
toxicokinetics and toxicodynamics were inadequate to modify the
10 × 10-fold uncertainty factor)
Derivation of Guidance Values
Outdoor/indoor air
The proportion of TI allocated to outdoor air based on exposure
estimates = 86%
86% × TI (430 µg/kg body
weight per day) = 370 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
mean body weight of adults = 64 kg
Guidance value for 370 µg/kg × 64 kg
outdoor/indoor air =
22 m3
= 1100 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 0.8% (too small to permit development of
meaningful guidance values since it contributes negligibly to total
intake)
Food
The proportion of TI allocated to food based on exposure
estimates = 13%
13% × TI (430 µg/kg
body weight per day) = 57 µg/kg body weight per day
(tolerances in various foodstuffs can be developed on the basis
of the amounts ingested.)
Soil
Owing to lack of relevant data, it is not possible to allocate a
proportion of the TI to this source.
Compound B
Chlorinated hydrocarbon solvent
Estimates of Exposure
Estimated daily intakes of Compound B for adults (µg/kg body
weight per day)1 in the general population are as follows:
Ambient air2 0.01-0.27
Indoor air3 1.4
Drinking-water4 0.002-0.02
Food5 0.12
Soil no data
Consumer products no data
Total Intake 1.5-1.8
On the basis of these estimates, it is considered that the
percentage of total exposure from various media for the general
population (based on midpoints of estimated intakes) is as follows:
outdoor air = 0.14/1.67 = 8.3%
indoor air = 1.4/1.67 = 83.8% (84%)
drinking-water = 0.011/1.67 = 0.65%
food = 0.12/1.67 = 7.1%
soil = no data
consumer products = no data
1 Assumed to weigh 64 kg, breathe 22 m3 air and drink 1.4 litres
of water per day (ICRP, 1974).
2 Assumed to spend 4 h/day outdoors and based on a range of mean
concentrations of Compound B (0.2 to 5.0 µg/m3) from a survey.
3 Assumed to spend 20 h/day indoors and based on the mean
concentration of Compound B of approximately 5.1 µg/m3 in the
indoor air of 757 randomly selected homes examined in a survey.
4 Based on a range of mean concentrations of Compound B (0.1 to
0.9 µg/litre) in drinking-water from a number of surveys.
5 Based on the average levels of Compound B in the various
composite food groups in a study on the daily intake of these
food groups.
Development of TIs
A Tolerable Intake for Compound B can be derived as follows:
[(678 mg/m3) × (0.043 m3/day) × (6/24) × (5/7)]
TI =
(0.0305 kg) × 1000
= 170 µg/kg body weight per day
where:
* 678 mg/m3 is the lowest-observed-adverse-effect level (LOAEL)
overall in mice determined in an adequate long-term inhalation
study and based on reduced survival and hepato-toxicity in males,
and lung congestion and nephrotoxicity in males and females.
* 0.043 m3/day is the assumed volume of air inhaled by mice
* 6/24 and 5/7 is the conversion of 6 h/day, 5 days/week to
continuous exposure.
* 0.0305 kg is the average body weight of the mice in the critical
study.
* 1000 is the uncertainty factor (×10 for inter-individual
variation, ×10 for interspecies variation since available data on
toxicokinetics and toxicodynamics were inadequate for
modification of these factors, ×10 for use of a LOAEL rather than
a NOAEL).
In order to ensure that the TI derived on the basis of an
inhalation study is sufficiently protective, another TI can be derived
on the basis of studies in which Compound B was administered by
ingestion. With the exception of one investigation in which
reversible erythropoietic damage was reported at low concentrations
(50 µg/kg body weight per day) but not confirmed in other studies, the
lowest NOAEL in the longest-term (90-day) available study in which
Compound B was administered orally in drinking-water to rats is
14 mg/kg body weight per day, based on effects on body weight gain,
the ratio of liver or kidney weight to body weight, and serum
5'-nucleotidase activity at the next highest dose. A LOEL of 20 mg/kg
body weight per day based on a slight increase in liver weight was
reported in a 6-week study on mice. Values for the TI derived on the
basis of the results of these two studies are within the same order of
magnitude as the TI calculated from the inhalation study.
Derivation of Guidance Values
Since the TIs derived on the basis of studies by inhalation and
ingestion are within the same range and inhalation is the most
important route of exposure of the general population, the TI
developed for the inhalation route will be used as the basis for
derivation of guidance values.
Outdoor air
The proportion of TI allocated to outdoor air based on exposure
estimates = 8.3%
8.3% × TI (170 µg/kg body
weight per day) = 14 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
proportion of the day
spent outdoors = 4/24
volume of outdoor air
inhaled daily = 22 m3 × 4/24 = 3.7 m3
mean body weight of adults = 64 kg
Guidance value for 14 µg/kg × 64 kg
outdoor air =
3.7 m3
= 242 µg/m3
Indoor air
The proportion of TI allocated to indoor air based on exposure
estimates = 84%
84% × TI (170 µg/kg body
weight per day) = 140 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
proportion of the day
spent indoors = 20/24
volume of indoor air
inhaled daily = 22 m3 × 20/24 = 18 m3
mean body weight of adults = 64 kg
Guidance value for 140 µg/kg × 64 kg
indoor air =
18 m3
= 498 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 0.65% (too small to permit development of
meaningful guidance values since it contributes negligibly to total
intake)
Food
The proportion of TI allocated to food based on exposure
estimates = 7.1%
7.1% × TI (170 µg/kg body
weight per day) = 12 µg/kg body weight per day or
10 µg/kg body weight per day to
one significant figure
(tolerances in various foodstuffs could then be developed on the
basis of amounts ingested.)
Soil
Owing to lack of relevant data, it is not possible to allocate a
proportion of the TI to this source.
Compound C
Naturally occurring inorganic chemical
Estimates of Exposure
The percentage of total exposure from various media for adults in
the general population in country 1 is as follows:
outdoor/indoor air = 0.02%
drinking-water = 6.9%
food = 80%
soil = 0.11%
consumer products = 12.8%
In contrast, the percentage of total exposure from various media
for adults in the general population in one area in country 2 is as
follows:
outdoor/indoor air = 35%
drinking-water = 11%
food = 55%
Development of TIs
It is concluded, on the basis of data from several studies in
human populations, that the TI is 200 µg/kg body weight per day.
Derivation of Guidance Values - Country 1
Outdoor/indoor air
The proportion of TI allocated to air based on exposure estimates
= 0.02% (too small to permit development of meaningful guidance
values)
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 6.9%
6.9% × TI (200 µg/kg body
weight per day) = 13.8 µg/kg body weight per day
daily volume of ingestion of
drinking-water for adults
in Country 1 = 1.5 litres
mean body weight of adults
in Country 1 = 70 kg
Guidance value for 13.8 µg/kg × 70 kg
drinking-water =
1.5
= 644 µg/litre
Food
The proportion of TI allocated to food based on exposure
estimates = 80%
80% × TI (200 µg/kg body 160 µg/kg body weight per day
weight per day) = or 200 µg/kg body weight per
day to one significant figure
(tolerances in various foodstuffs can then be developed on the basis
of amounts ingested.)
Soil
The proportion of TI allocated to air based on exposure estimates
= 0.11%
(too small to permit development of meaningful guidance values)
Consumer products
The proportion of TI allocated to consumer products based on
exposure estimates = 12.8%
12.8% × TI (200 µg/kg body
weight per day) = 26 µg/kg body weight per day
(limits in consumer products can be developed on the basis of
patterns of use.)
Derivation of Guidance Values - Country 2
Outdoor/indoor air
The proportion of TI allocated to air based on exposure
estimates = 35%
35% × TI (200 µg/kg body
weight per day) = 70 µg/kg body weight per day
daily inhalation volume for
adults in Country 2 = 20 m3
mean body weight of
adults in Country 2 = 60 kg
Guidance value for 70 µg/kg × 60 kg
outdoor/indoor air =
20 m3
= 210 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 11%
11% × TI (200 µg/kg body
weight per day) = 22 µg/kg body weight per day
daily volume of ingestion of
drinking-water for
adults in Country 2 = 1.5 litres
mean body weight of
adults in Country 2= 60 kg
Guidance value for 22 µg/kg × 60 kg
drinking-water =
1.5
= 880 µg/litre
Food
The proportion of TI allocated to food based on exposure
estimates = 55%
55% × TI (200 µg/kg body
weight per day) = 110 µg/kg body weight per day
(tolerances in various foodstuffs can be developed on the basis of
amounts ingested.)
Soil
No data are available.
Consumer products
No data are available.
APPENDIX 2
GRAHICAL APPROACHES
The use of graphs of dose-effect and dose-response toxicity data
to complement the text discussion in the development of TIs and
guidance values is considered valuable. Such graphs can display an
overview of the full range of dose-response information. Graphs can
range from simple "thermometer" presentations as employed by the US
Agency for Toxic Substances and Disease Registry (ATSDR, 1989), to
dose-effect and dose-response graphs for specific toxic effects such
as genotoxicity (Waters et al., 1988) or developmental toxicity
(Kavlock et al., 1991), or to dose-duration graphs described by
Hartung (1986), Hartung & Durkin (1986), and Dourson et al. (1985).
Fig. 4 is an example of a dose-duration graph and presents data
for methoxychlor adapted from Dourson et al. (1985). This figure
summarizes the available frank-effect levels (FEL), adverse-effect
levels (AEL), no-observed-adverse-effect levels (NOAEL), and
no-observed-effect levels (NOEL). Adverse-effect levels are presented
as a function of both dose in mg/day and exposure as a fraction of
lifespan.
Each point in the graph represents one dose group from one study.
The size of the point is a rough indication of its usefulness for
determining tolerable intakes, where larger points indicate more
useful information. Other information includes target organs. These
data can also be used to estimate a best fitting line for NOAEL across
duration.
Graphical presentation of available data can facilitate
identification of effect levels relevant to development of TIs.
Although the form of graphical presentation is necessarily dependent
upon the size of the data base, a dose-duration graph in which NOELs,
NOAELs and LOAELs are presented as a function of duration of exposure
is considered to be helpful and is more fully described in Appendix 2.
4.3 Adequacy of the pivotal study
In situations where a NOAEL has not been achieved but the data
on effects are of sufficient quality to be the basis of the risk
assessment, then a no-adverse-effect level should be developed by the
application of an appropriate uncertainty factor to the LOAEL.
Uncertainty factors of 3, 5 or 10 have been used previously to
extrapolate from a LOAEL to a NOAEL depending on the nature of the
effect(s) and dose-response relationship (see, for example, US EPA,
1993). Alternatively, a benchmark dose may be developed by
mathematical modelling of the dose-response data as an alternative to
the uncertainty factor in extrapolating to the NOAEL (see Appendix 3).
The pivotal study may also be considered inadequate for other reasons
(e.g., duration of study, numbers of animals per group and sensitivity
of the analyses of effect), and an additional uncertainty factor
applied.
4.4 Interspecies extrapolation
In situations where appropriate toxicokinetic and/or
toxicodynamic data exist for a particular compound, then the relevant
uncertainty factor in Fig. 3 should be replaced by the data-derived
factor. Data on PBPK and/or data on target organ exposure should be
included when they are available. Subdivision of the 10-fold
uncertainty factor has been used in the development of a reference
concentration for 1,2-epoxybutane (US EPA, 1993). Chemicals for which
the approach described here has been applied include saccharin
(Renwick, 1993b), erythrosine (Poulsen, 1993), butylated
hydroxyanisole (BHA) (Wurtzen, 1993) and diethylhexyl phthalate (DEHP)
(Morgenroth, 1993).
If a data-derived factor is introduced then the commonly used
10-fold factor would be replaced by the product of that data-derived
factor and the remaining default factor. For some classes of
compounds a data-derived factor for one member of the class may be
applicable to all members, thereby producing a group-based
data-derived factor (see Calabrese, 1992). The interspecies
uncertainty factor is not necessary if the NOAEL or LOAEL is based on
human data.
4.5 Inter-individual variability in humans
A factor of 10 is normally used to allow for differences in
sensitivity in vivo between the population mean and highly sensitive
subjects. In cases where there are appropriate data on the
inter-individual variability in toxicokinetics or toxicodynamics for a
particular compound in humans, then the relevant uncertainty factor
should be replaced by the data-derived factor. Data on PBPK may also
be able to contribute to this assessment. If a data-derived factor is
introduced, then the commonly used 10-fold factor would be replaced by
the product of the data-derived factor and the remaining default
factor. (For additional discussion, see Calabrese, 1985; Hattis et
al., 1987).
For some compounds, it may be known that a subset of the
population would be particularly sensitive, for example due to
deficiencies in detoxication processes. Many of the enzymes involved
in xenobiotic biotransformation are polymorphically distributed in the
human population. Such polymorphism should be taken into account
where the enzymatic differences result either in a marked change in
bioavailability or clearance of the parent compound or in a major
change in the extent of formation of the toxic entity. In cases where
the default factor will not adequately cover this additional
variability, then the default should be modified appropriately.
Alternatively, these groups may require special strategies for health
protection. In cases where the risk assessment is based on in vivo
data in the sensitive subgroup, then the composite factor (10) should
be reduced to a much lower value. A value of 1 could be used if there
is an extensive data base in humans and the data base adequately
addresses any identified sensitive subgroups. For example, the US EPA
estimated an oral reference dose for fluoride based on the absence of
dental mottling in children 12 to 14 years of age. Since this group
was considered to be a sensitive subpopulation, a factor of 1 for
inter-individual variation was considered to be appropriate (US EPA,
1993).
4.6 Other considerations
4.6.1 Adequacy of the overall data base
Major deficiencies in a toxicity data base (other than those
related to the pivotal study) which increase the uncertainty of the
extrapolation process should be recognized by the use of an additional
uncertainty factor. Since the quality and/or completeness of
different data bases vary, the additional uncertainty factor will also
vary. For example, a value of 1 would be applied to a data base that
was considered complete for the evaluation of the compound under
consideration, but a factor of 1-100 might be necessary for limited
data bases. If minor deficiencies in the data exist with respect to
quality, quantity or omission, then an extra factor of 3 or 5 would be
appropriate. An extra factor of 10 would be appropriate where major
deficiencies in the data exist with respect to quality, quantity or
omission, such as a lack of chronic toxicity studies and reproductive
toxicity studies (for additional discussion see Dourson et al., 1992).
It should be appreciated that when very large uncertainty factors
are incorporated, the derived TI should be considered as an very
imprecise temporary estimate pending the generation of a better data
base. It should be recognized that inadequacies of the pivotal study
(section 4.3) could also be considered as a subset of inadequacies of
the data base; the total factor for limitations of the pivotal study
plus adequacy of the overall data base should not exceed 100 since
such a data base is generally not acceptable for development of a TI.
4.6.2 Nature of toxicity
The nature of toxicity, i.e. whether the effect is adverse or
not, is considered in the determination of NOAEL and LOAEL. For
example, a concentration or dose which induces a transient increase in
organ weight without accompanying biochemical or histopathological
effects might be considered to be a NOAEL. If there are accompanying
adverse histopathological effects in the target organ, the lowest
concentration or dose at which these effects occur would be considered
a LOAEL. The sensitivity of analyses of effects should also be taken
into account in establishing the NOAEL or LOAEL (see discussion in
section 4.2).
In addition, a number of bodies, including the WHO and FAO Joint
Expert Committee on Food Additives (JECFA) and the Joint Meeting on
Pesticide Residues (JMPR) have incorporated an additional "safety
factor" of up to 10 (corresponding to an uncertainty factor in the
current discussion) in cases where the NOAEL is derived for a critical
effect which is a severe and irreversible phenomenon, such as
teratogenicity or non-genotoxic carcinogenicity, especially if
associated with a shallow dose-response relationship (Weil, 1972; WHO,
1987, 1990). Provision for the application of additional safety
factors is included in the sequence shown in Fig. 3.
4.7 Final review of the total uncertainty factor
It is important that there is a final review of the total
uncertainty factor applied, particularly in cases where a low value
has been used, based on toxicokinetic or toxicodynamic data, to
replace one of the default values. Under such circumstances, a TI
derived on the basis of the appropriate overall uncertainty factor for
that toxic effect might be greater than that which would be produced
by an alternative, well-defined toxic end-point observed at slightly
higher intakes or exposures. For this reason, there are arrows shown
in Fig. 3 leading back to the data base.
4.8 Precision of the tolerable intake
The TI is calculated by dividing the NOAEL for the critical
effect by the derived total uncertainty factor. The precision of the
estimate depends in large part on the magnitude of the overall
uncertainty factor used in the calculation. The precision is probably
to one significant figure at best, and more usually to one order of
magnitude, and for uncertainty factors of 1000 or more the precision
becomes even less. Because of the imprecision of the default factors
and in order to maintain credibility of the risk assessment process,
the total default uncertainty factor should not exceed 10 000. If the
risk assessment leads to a higher factor then the resulting TI would
be so imprecise as to lack meaning. Such a situation indicates an
urgent need for additional data.
4.9 Alternative approaches
Approaches being developed to characterize quantitatively the
dose-response relationship for non-threshold effects (including the
benchmark dose and categorical regression) are described in
Appendix 3.
5. ALLOCATION OF TOLERABLE INTAKES TO DERIVE GUIDANCE VALUES
5.1 General considerations
Allocations of the TIs to various media for the development of
guidance values are based on relative proportion of total exposure
from each of the media. This necessitates the presentation of
consistent and detailed estimates of exposure for as many media as
possible in draft EHCs prior to review and evaluation. Wherever
possible, estimation of exposure should be based on concentrations in
environmental media including (but not necessarily limited to) air,
food, drinking-water, soil and consumer products. With respect to
soil, wherever possible, estimated exposure should take into account
both ingestion and dermal contact. Since the bioavailability of
contaminants in soil from both ingestion and dermal contact may be
limited, this should be taken into account in assessing the
contribution that soil makes to total intake from all media.
It is recommended that unless there are other age groups which
are more sensitive or have widely differing exposure profiles, intake
from each of the media (generally expressed as µg/kg body weight per
day) should be estimated for adults, based on ICRP reference values
for body weights and ingestion volumes (ICRP, 1974; Appendix 4).
Wherever possible, estimation of exposure should be based on ranges of
mean concentrations in environmen-tal media on a global basis. Where
data are more limited, ranges of individual values could be used.
Estimates of exposure as a basis for derivation of guidance values are
presented in the examples in Appendix 1.
Where the data on concentrations of a substance in environmental
media are inconsistent or inadequate, exposure can be estimated based
on models which incorporate as much data as possible on, for example,
production, use patterns and physical and chemical properties. Models
to predict distribution in environmental media and estimation of
proportion of total exposure by various routes from consumer products
are available (Mackay, 1991; USES, 1994). For estimation of
proportions of exposure from various environmental media for
development of guidance values in EHCs, it is recommended that the
latest version of the Mackay level III model be used (Mackay et al.,
1992). It is important that all assumptions concerning releases and
physico-chemical properties and limitations of the estimated
proportions be clearly specified. In some cases, it may also be
possible to estimate the contribution of each medium to total exposure
on the basis simply of data on physical and chemical properties (e.g.,
for substances which are likely to be present primarily in one
environmental medium).
When available, toxicokinetic data should be used to the extent
possible in extrapolating across routes in the approaches to
allocation described below. Dermal exposure and absorption should
also be taken into account in the derivation of guidance values,
although relevant data are often not available. It is also recognized
that a source in one medium (e.g., potable water) may lead to
additional intake from other routes (e.g., dermal and inhalation) and
that, where possible, such intake should be considered in the
derivation of guidance values.
In addition, total allocations of less than 100% of the TI are
encouraged to account for, for example, those media for which exposure
has not been characterized and cross-route exposure. The magnitude of
the proportion of total intake which is not allocated should vary as a
function of the adequacy of characterization of total exposure from
all media.
In cases where the proportion of total exposure from a specific
medium is small (less than a few percent), allocation for derivation
of guidance values is not recommended since this would result in
direction of risk management strategies to media which are
inconsequential in contributing to total exposure.
5.2 General approach
The steps subsequent to development of a TI in deriving guidance
values for a general population are as follows:
1. If necessary, conversion of TIs for systemic effects for different
routes of exposure to a common unit for comparison based on
consideration of volumes and rates of inhalation and ingestion and
relevant toxicokinetic data, such as bioavailability, if available.
2. Allocation of TI to various routes and media based on estimated
exposure developed on the basis of available data on measured
concentrations or predicted proportions (i.e., model-derived values)
to which humans are exposed. Default values can be used in the
absence of data on measured concentrations or predicted proportions of
total exposure in various media.
3. Development of guidance values from intake assigned to each
medium, taking into account, for instance, body weight, volume of
intake and (relative) absorption efficiency ( relative where guidance
value is derived on the basis of a TI by another route of exposure).
Guidance values for drinking-water are generally expressed in µg/litre
or mg/litre, those in food as µg/g or mg/kg, those in air as µg/m3
or mg/m3, and those for dermal exposure as µg/m2 surface area.
5.3 Detailed approach
In the following section, an approach to the allocation of
tolerable intakes for development of guidance values (general
population) is provided by way of example for most of the scenarios
which may arise based on evaluations presented in EHCs.
The five most likely scenarios are considered to be:
5.3.1 Biomarkers of exposure
There is a common biomarker related to the critical effect which
integrates exposure from all sources. For example, Choudhury et al.
(1992) describe a model which predicts blood lead concentrations as a
function of concentrations in various media.
* The contributions from the various media are determined based on
a quantitative biomarker. Following allocation to various media
based on an exposure scenario, guidance values are developed
through incorporation of adjustment of body weight and volume of
intake for each medium.
5.3.2 Critical effects which are not route specific
TIs have been derived for each route, e.g., TI for oral exposure
(TIo) and TI for inhalation (TIi), and are based either on the
same or on different critical effects which are not at the portal of
entry. The TIs for the two routes are similar within one order of
magnitude since such variation is consistent with that inherent in
deriving TIs, as discussed in section 4, e.g., developmental toxicity
of 2-methoxyethanol (Doe et al., 1983; Wickramaratne, 1986). This
reflects the assumption that, in the absence of data to the contrary,
exposure via each route is considered to contribute to a combined dose
at the target site(s), i.e., additivity of dose at the target site(s).
* Allocate one TI to various media based on an exposure scenario to
determine the intake in each medium on which guidance values
should be based. Selection of the TIo or the TIi for this
purpose should be based on either:
a) if there is one major route of exposure then the TI for that
route should be used (if there is confidence in the data
base on which the exposure estimates are based); or
b) the more conservative TI (if there is uncertainty about the
relative contribution of various routes or media to total
exposure).
5.3.3 Difference in magnitude of effect by route of exposure
TIo and TIi for similar effects vary by 1 to 2 orders of
magnitude (exact magnitude of the difference for which this approach
is appropriate will be dependent upon availability of additional data;
e.g., manganese is more potent by inhalation than by ingestion).
* Derive the guidance values independently for each route (for
example, the oral and inhalation routes, based on the TIo and
TIi, respectively), but allocate the proportion of the TI for
each route to the appropriate medium or media based on an
exposure scenario.
5.3.4 Route-specific effect variation at portals of entry
(due to local bioactivation or local effects)
TIo and TIi for route-specific effects at the site of entry
vary by 1 to 2 orders of magnitude (exact magnitude of the difference
for which this approach is appropriate will be dependent upon
knowledge of additional data; e.g., nasal toxicity following
inhalation of acrylic acid).
* Derive the guidance values independently for each route (for
example, the oral and inhalation routes, based on the TIo and
TIi, respectively), using the full TI for each route to the
appropriate medium or media based on an exposure scenario.
5.3.5 Limited data base
In this scenario, the data base is limited such that only either
a TIo or a TIi can be developed.
* Allocate the available TI to various media based on an exposure
scenario to determine the intake in each medium on which guidance
values should be based, if the effects are qualitatively similar,
if toxicokinetic data are consistent with this approach and if
there are no effects at the site of entry. If any one of these
criteria is not met, do not derive guidance values for the
alternate route. If a TI is available for a route of exposure
which does not make an important contribution to total intake, do
not derive guidance values for that route.
6. EXAMPLES OF THE DERIVATION OF GUIDANCE VALUES
Example 1
The principal route of exposure is oral. Based on estimated
exposure for a scenario in the general environment, 50% of total
intake comes from food, 20% from water and 30% from air.
Data are adequate to establish both a TIo and a TIi. The
TIo and the TIi are based on similar effects and are similar
(within one order of magnitude).
Allocate 50% of TIo to food to derive a guidance value for food
* multiply TIo by 0.5
Allocate 20% of TIo to water to derive a guidance value for
drinking-water
* multiply TIo by 0.2
Allocate 30% of TIo to air to derive a guidance value for air
* multiply TIo by 0.3
Example 2
Based on an exposure scenario, 70% of total intake comes from
air, 20% from water and 10% from food. The compound is also present
in some consumer products but quantification of exposure is not
possible. There are no data on concentrations in soil but due to its
physicochemical properties, concentrations in this medium are likely
to be low.
Data are sufficient to establish a TIi and a TIo. The TIo
and the TIi are based on similar effects and are similar to within
an order of magnitude.
Convert TIi so that the values for the TIs for different routes
are expressed in the same units for comparison (generally mg/kg body
weight per day). This requires incorporation of information on
inhalation volumes, body weight and toxicokinetic data, if available.
Use TI for principal route of exposure to derive guidance values:
Allocate 63% of TIi to air to derive a guidance value for air.
* multiply TIi by 0.63
Allocate 18% of TIi to water to derive a guidance value for
drinking-water.
* multiply TIi by 0.18
Allocate 9% of TIi to food to derive a guidance value for food.
* multiply TIi by 0.09
Reserve 10% for exposure from consumer products and soil.
(Wherever possible, there should be an attempt to quantitatively
estimate the proportion of total intake from these sources).
Develop a guidance value for each medium by (if necessary)
adjustment for body weight, volume of intake and relative absorption.
Example 3
The principal route of exposure is oral. Based on estimated
exposure, 50% of total intake comes from food, 20% from water, 20%
from air and 10% from soil (after taking bioavailability into account
from the oral and dermal routes). The compound is believed not to be
present in consumer products.
Data are adequate to establish both a TIo and a TIi. The
TIo and the TIi are based on the same effects but the compound is
much more toxic by the oral route (e.g., TIo is less than the TIi
by more than two orders of magnitude).
Allocate 50% of TIo to food to derive a guidance value for food
* multiply TIo by 0.5
Allocate 20% of TIo to water to derive a guidance value for
drinking-water
* multiply TIo by 0.2
Allocate 10% of TIo to soil to derive a guidance value for soil
* multiply TIo by 0.1
Allocate 20% of TIi to air to derive a guidance value for air
* multiply TIi by 0.2
Example 4
The principal route of exposure is oral. Based on estimated
exposure, 50% of total intake comes from food, 20% from water and 30%
from air. There are no data indicating exposure from soil and
consumer products.
Data are adequate to establish both a TIo and a TIi for
route-specific effects. The TIo and the TIi are based on
route-specific effects and the compound is much more toxic by the oral
route (e.g., TIo is less than the TIi by more than two orders of
magnitude).
Because the effects are route specific and the TIs are different
by two orders of magnitude, each TI can be allocated in full to
appropriate media.
Allocate 50/70 (71%) of TIo to food to derive a guidance value
for food
* multiply TIo by 0.71
Allocate 20/70 (29)% of TIo to water to derive a guidance value
for drinking-water
* multiply TIo by 0.29
Allocate 100% of TIi to air to derive a guidance value for air
* multiply TIi by 1
Example 5
The principal route of exposure is inhalation.
Data are inadequate to establish a TIi.
Data are sufficient to establish a TIo.
Available toxicokinetic data are inadequate for or inconsistent
with extrapolation across routes.
Do not establish guidance values.
REFERENCES
Allen BC, Van Landingham C, Howe RB, Kavlock RJ, Kimmel CA, & Faustman
EM (1992) Dose-response modeling for developmental toxicity.
Toxicologist, 12(1): 300.
Allen B, Kavlock RJ, Kimmel CA, & Faustman EM (1993) Comparison of
quantitative dose response modeling approaches for evaluating fetal
weight changes in segment II developmental toxicity studies.
Teratology, 47(5): 41.
ATSDR (1989) Toxicological profile for aldrin/dieldrin. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (Publication
ATSDR/TP-88/01).
Barnes DG & Dourson M (1988) Reference dose (RfD): Description and use
in health risk assessment. Regul Toxicol Pharmacol, 8: 471-486.
Calabrese EJ (1985) Uncertainty factors and interindividual
variation. Regul Toxicol Pharmacol, 5: 190-196.
Calabrese EJ (1992) Does the animal to human uncertainty factor
incorporate interspecies differences in surface area? Regul Toxicol
Pharmacol, 15(2): 172-179.
Canada (1988) Government of Canada: Canadian Environmental Protection
Act, Ottawa. Canada Gaz, II(4): 415-512.
Choudhury H, Peirano WB, Marcus A, Elias R, Griffin S, & Derosa CT
(1992) Utilization of uptake biokinetic (UBK) lead model to assess
risk in contaminated sites. In: Superfund risk assessment in soil
contamination studies. Philadelphia, Pennsylvania, American Society of
Testing and Materials, pp 193-204.
Crump KS (1984) A new method for determining allowable daily intakes.
Fundam Appl Toxicol, 4: 854-871.
Doe JE, Samuels DM, Tinston DJ, & Wickramaratne GA de S (1983)
Comparative aspects of the reproductive toxicology by inhalation in
rats of ethylene glycol monomethyl ether and propylene glycol
monomethyl ether. Toxicol Appl Pharmacol, 69: 43-47.
Dourson ML & Stara J (1983) Regulatory history and experimental
support of uncertainty (safety) factors. Regul Toxicol Pharmacol, 3:
224-238.
Dourson ML, Hertzberg R, Hartung R, & Blackburn K (1985) Novel methods
for the estimation of acceptable daily intake. Toxicol Ind Health,
1(4): 23-33.
Dourson ML, Knauf L, & Swartout J (1992) On reference dose (RfD) and
its underlying toxicity data base. Toxicol Ind Health, 8(3): 171-189.
Farland W & Dourson ML (1992) Noncancer health endpoints: approaches
to quantitative risk assessment. In: Cothern R ed. Comparative
environmental risk assessment. Boca Raton, Florida, Lewis Publishers,
pp 87-106.
Gaylor DW (1989) Quantitative risk analysis for quantal reproductive
and developmental effects. Environ Health Perspect, 79: 243-246.
Gaylor DW (1992) Incidence of developmental defects at the No
Observed Effect Level (NOEL). Regul Toxicol Pharmacol, 15: 151-160.
Guth DJ, Jarabek AM, Wymer L, & Hertzberg RC (1991) Evaluation of risk
assessment methods for short-term inhalation exposure. Presentation
at the 84th Annual Meeting of the Air and Waste Management
Association, Vancouver, British Columbia, 16-21 June 1991.
Hallenbeck WH & Cunningham KM (1986) Quantitative risk assessment for
environmental and occupational health. Chelsea, Michigan, Lewis
Publishers, Inc., p 199.
Hattis D, Erdreich L, & Ballew M (1987) Human variability in
susceptibility to toxic chemicals -A preliminary analysis of
pharmacokinetic data from normal volunteers. Risk Anal, 7(4):
415-426.
Hartung R (1986) Ranking the severity of toxic effects. In: Trace
substances in environmental health. Columbia, Missouri, University of
Missouri, pp 204-211.
Hartung R & Durkin PR (1986) Ranking the severity of toxic effects:
Potential applications to risk assessment. Comments Toxicol, 1: 49-63.
Health and Welfare Canada (1992) Determination of "toxic" under
paragraph 11c) of the Canadian Environmental Protection Act, 1st ed.
Ottawa, Ontario, Bureau of Chemical Hazards, Environmental Health
Directorate.
Hertzberg RC (1989) Fitting a model to categorical response data with
application to special extrapolation to toxicity. Health Phys,
57(Suppl 1): 404-409.
Hertzberg RC & Miller M (1985) A statistical model for species
extrapolation using categorical response data. Toxicol Ind Health, 1:
43-57.
ICRP (1974) International Commission on Radiological Protection:
Report of the Task Group on Reference Man. Oxford, Pergamon Press
(ICRP Publication No. 23).
Jarabek AM, Menache MG, Overton JH Jr, Dourson ML, & Miller FJ (1990)
The US Environmental Protection Agency's inhalation RfD methodology:
Risk assessment for air toxics. Toxicol Ind Health, 6(5): 279-301.
Kavlock RJ, Greene JA, Kimmel GL, Morrissey RE, Owens E, Rogers JM,
Sadler TW, Stack F, Waters MD, & Welsch F (1991) Activity profiles of
developmental toxicity: design considerations and pilot
implementation. Teratology, 43: 159-185.
Kimmel CA & Gaylor DW (1988) Issues in qualitative and quantitative
risk analysis for developmental toxicology. Risk Anal, 8: 15-20.
Lehman AJ & Fitzhugh OG (1954) 100-fold margin of safety. Assoc Food
Drug Off US Q Bull, 18: 33-35.
Lewis SC, Lynch JR, & Nikiforov AI (1990) A new approach to deriving
community exposure guidelines from no-observed-adverse-effect levels.
Regul Toxicol Pharmacol, 11: 314-330.
Lu F (1988) Acceptable daily intake: inception, evolution and
application. Regul Toxicol Pharmacol, 8(1): 45-60.
Mackay D (1991) Multimedia environmental models. The fugacity
approach. Chelsea, Michigan, Lewis Publishers, Inc., 260 pp.
Mackay D, Paterson S, & Shiu WY (1992) Generic models for evaluating
the regional fate of chemicals. Chemosphere, 24: 695-717.
Moolgavkar SH, Dewanji A, & Venzon DJ (1988) A stochastic two-stage
model for cancer risk assessment. I: The hazard function and the
probability of tumor. Risk Anal, 8: 383-392.
Morgenroth V (1993) Scientific evaluation of the data-derived safety
factors for the acceptable daily intake. Case study:
diethylhexylphthalate. Food Addit Contam, 10(3): 363-373.
NAS (1977) Drinking water and health. Washington, DC, National
Research Council, US National Academy of Sciences, 939 pp.
Nau H (1986) Species differences in pharmacokinetics and drug
teratogenesis. Environ Health Perspect, 70: 113-129.
Poulsen E (1993) Case study: erythrosine. Food Addit Contam, 10:
315-323.
Renwick AG (1993a) Data-derived safety factors for the evaluation of
food additives and environmental contaminants. Food Addit Contam, 10:
275-305.
Renwick AG (1993b) A data-derived safety (uncertainty) factor for the
intense sweetener, saccharin. Food Addit Contam, 10: 337-350.
Rubery ED, Barlow SM, & Steadman JH (1990) Criteria for setting
quantitative estimates of acceptable intakes of chemicals in food in
the U.K. Food Addit Contam, 7: 287-302.
Shoaf CR (1994) US EPA inhalation testing guidelines. In: Jenkins PG,
Kayser D, Muhle H, Rosner G, & Smith EM ed. Respiratory toxicology and
risk assessment. Proceedings of an International Symposium. Stuttgart,
Wissenschaftliche Verlagsgesellschaft mbH, pp 11-25 (IPCS Joint Series
No. 18).
US EPA (1985a) National primary drinking water regulations; volatile
synthetic organic chemicals; final rule and proposed rule. Fed Reg,
50: 46830-46901.
US EPA (1985b) National primary drinking water regulations, synthetic
organic chemicals, inorganic chemicals and microorganisms; proposed
rule. Fed Reg, 50: 46936-47025.
US EPA (1993) The Integrated Risk Information System (IRIS).
Cincinnati, Ohio. Office of Health and Environmental Assessment,
Environmental Criteria and Assessment Office.
USES (1994) Uniform system for the evaluation of substances (USES),
version 1.0. Bilthoven, The Netherlands, National Institute for
Public Health and Environmental Protection, 332 pp.
Waters MD, Stack HF, Brady AL, Lohman PHM, Haroun L, & Vainio H (1988)
Use of computerized data listings and activity profiles of genetic and
related effects in the review of 195 compounds. Mutat Res, 205:
295-312.
Weil CS (1972) Statistics versus safety factors and scientific
judgement in the evaluation for man. Toxicol Appl Pharmacol, 21:
454-463.
WHO (1987) IPCS Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food. Geneva,
World Health Organization, 174 pp.
WHO (1990) IPCS Environmental Health Criteria 104: Principles for the
toxicological assessment of pesticide residues in food. Geneva, World
Health Organization. 117 pp.
WHO (1993) Guidelines for drinking-water quality, Second edition.
Volume 1: Recommendations. Geneva, World Health Organization, 198 pp.
Wickramaratne GA de S (1986) The teratogenic potential and
dose-response of dermally administered ethylene glycol monomethyl
ether (EGME) estimated in rats with the Chernoff-Kavlock assay. J Appl
Toxicol, 6(3): 165-166.
Wurtzen G (1993) Scientific evaluation of the safety factor for the
acceptable daily intake (ADI). Case study: butylated hydroxy anisole
(BHA). Food Addit Contam, 10: 307-314.
Zielhuis RL & van der Kreek FW (1979a) The use of a safety factor in
setting health based permissible levels for occupational exposure. Int
Arch Occup Environ Health, 42: 191-201.
Zielhuis RL & van der Kreek FW (1979b) Calculation of a safety factor
in setting health based permissible levels for occupational exposure.
Int Arch Occup Environ Health, 42: 203-215.
APPENDIX 1
EXAMPLES - DEVELOPMENT OF GUIDANCE VALUES
The following practical examples are provided to illustrate the
manner in which tolerable intakes (TIs) may be developed and allocated
for the derivation of guidance values for a general population (on the
basis of calculated proportions of exposure from various media). In
the calculation of guidance values, TIs may be rounded up to 1 or 2
significant figures depending on the quality of the data base and the
extent of uncertainties involved in deriving the TI. The level of
detail shown is that which is considered necessary for EHCs and should
be sufficient for adaptation at national and local levels.
Compound A
Chlorinated hydrocarbon
Estimates of exposure
Estimated daily intakes of Compound A for adults (µg/kg
body weight per day)1 in the general population are as follows:
Ambient air2 < 0.03
Drinking-water3 0.00007-< 0.0004
Food4 0.004
Soil no data
Consumer products no data
Total Intake 0.03
1 Assumed to weigh 64 kg, breathe 22 m3 of air per day and drink
1.4 litres of water Per day (ICRP, 1974) and to consume 125 g per day
of a meat composite (the compound was not detected in other dietary
composites).
2 Based on a mean concentration of Compound A reported in a survey
of ambient Air from 22 sites (< 0.10 µg/m3); concentrations in
indoor air were similar to those in ambient air.
3 Based on a range of mean concentrations of Compound A in
drinking-water of 0.003 µg/litre to < 0.02 µg/litre.
4 Based on a concentration of 0.0018 µg/g of Compound A detected in
a representative daily diet.
On the basis of these estimates, it is considered that the
percentage of total exposure from various media for the general
population (midpoints of estimated intakes) is as follows:
outdoor/indoor air = < 0.03/0.03 = 85.9% (86%)
(< 0.03 considered to be 0.03 minus
intake from other media)
drinking-water = 0.000245/0.03 = 0.82% (0.8%)
food = 0.004/0.03 = 13.3% (13%)
soil = no data
consumer products = no data
Development of TI
The only data identified on long-term toxicity following
inhalation are the results of a single subchronic study for which no
effects were observed at any concentration. Available data are
considered inadequate, therefore, to establish a TI on the basis of
the results of studies in which Compound A has been administered by
inhalation. Moreover although the general population appears to be
exposed to Compound A principally in air, based on limited available
data on concentrations in food, the estimated intake in food is within
the range of that estimated for air for some age groups. In addition,
the principal route of intake of the most exposed age group (i.e.
suckling infants) is ingestion (of mothers' milk). Owing to the lack
of adequate long-term toxicity studies by the inhalation route and the
possible relatively important contribution that food makes to total
exposure to Compound A, a TI is derived on the basis of a long-term
ingestion bioassay, as follows:
60 mg/kg body
weight per day × 5
TI = approx. 0.43 mg/kg (430 µg/kg)
body weight per day
100 × 7
where:
* 60 mg/kg body weight per day is the NOAEL, determined in a
well-conducted and documented long-term (chronic and
carcinogenesis) bioassay, with renal tubular degeneration
observed at higher doses
* 5/7 is the conversion of five days per week of dosing to seven
days per week
* 100 is the uncertainty factor (×10 for inter-individual
variation; ×10 for interspecies variation; available data on
toxicokinetics and toxicodynamics were inadequate to modify the
10 × 10-fold uncertainty factor)
Derivation of Guidance Values
Outdoor/indoor air
The proportion of TI allocated to outdoor air based on exposure
estimates = 86%
86% × TI (430 µg/kg body
weight per day) = 370 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
mean body weight of adults = 64 kg
Guidance value for 370 µg/kg × 64 kg
outdoor/indoor air =
22 m3
= 1100 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 0.8% (too small to permit development of
meaningful guidance values since it contributes negligibly to total
intake)
Food
The proportion of TI allocated to food based on exposure
estimates = 13%
13% × TI (430 µg/kg
body weight per day) = 57 µg/kg body weight per day
(tolerances in various foodstuffs can be developed on the basis
of the amounts ingested.)
Soil
Owing to lack of relevant data, it is not possible to allocate a
proportion of the TI to this source.
Compound B
Chlorinated hydrocarbon solvent
Estimates of Exposure
Estimated daily intakes of Compound B for adults (µg/kg body
weight per day)1 in the general population are as follows:
Ambient air2 0.01-0.27
Indoor air3 1.4
Drinking-water4 0.002-0.02
Food5 0.12
Soil no data
Consumer products no data
Total Intake 1.5-1.8
On the basis of these estimates, it is considered that the
percentage of total exposure from various media for the general
population (based on midpoints of estimated intakes) is as follows:
outdoor air = 0.14/1.67 = 8.3%
indoor air = 1.4/1.67 = 83.8% (84%)
drinking-water = 0.011/1.67 = 0.65%
food = 0.12/1.67 = 7.1%
soil = no data
consumer products = no data
1 Assumed to weigh 64 kg, breathe 22 m3 air and drink 1.4 litres
of water per day (ICRP, 1974).
2 Assumed to spend 4 h/day outdoors and based on a range of mean
concentrations of Compound B (0.2 to 5.0 µg/m3) from a survey.
3 Assumed to spend 20 h/day indoors and based on the mean
concentration of Compound B of approximately 5.1 µg/m3 in the
indoor air of 757 randomly selected homes examined in a survey.
4 Based on a range of mean concentrations of Compound B (0.1 to
0.9 µg/litre) in drinking-water from a number of surveys.
5 Based on the average levels of Compound B in the various
composite food groups in a study on the daily intake of these
food groups.
Development of TIs
A Tolerable Intake for Compound B can be derived as follows:
[(678 mg/m3) × (0.043 m3/day) × (6/24) × (5/7)]
TI =
(0.0305 kg) × 1000
= 170 µg/kg body weight per day
where:
* 678 mg/m3 is the lowest-observed-adverse-effect level (LOAEL)
overall in mice determined in an adequate long-term inhalation
study and based on reduced survival and hepato-toxicity in males,
and lung congestion and nephrotoxicity in males and females.
* 0.043 m3/day is the assumed volume of air inhaled by mice
* 6/24 and 5/7 is the conversion of 6 h/day, 5 days/week to
continuous exposure.
* 0.0305 kg is the average body weight of the mice in the critical
study.
* 1000 is the uncertainty factor (×10 for inter-individual
variation, ×10 for interspecies variation since available data on
toxicokinetics and toxicodynamics were inadequate for
modification of these factors, ×10 for use of a LOAEL rather than
a NOAEL).
In order to ensure that the TI derived on the basis of an
inhalation study is sufficiently protective, another TI can be derived
on the basis of studies in which Compound B was administered by
ingestion. With the exception of one investigation in which
reversible erythropoietic damage was reported at low concentrations
(50 µg/kg body weight per day) but not confirmed in other studies, the
lowest NOAEL in the longest-term (90-day) available study in which
Compound B was administered orally in drinking-water to rats is
14 mg/kg body weight per day, based on effects on body weight gain,
the ratio of liver or kidney weight to body weight, and serum
5'-nucleotidase activity at the next highest dose. A LOEL of 20 mg/kg
body weight per day based on a slight increase in liver weight was
reported in a 6-week study on mice. Values for the TI derived on the
basis of the results of these two studies are within the same order of
magnitude as the TI calculated from the inhalation study.
Derivation of Guidance Values
Since the TIs derived on the basis of studies by inhalation and
ingestion are within the same range and inhalation is the most
important route of exposure of the general population, the TI
developed for the inhalation route will be used as the basis for
derivation of guidance values.
Outdoor air
The proportion of TI allocated to outdoor air based on exposure
estimates = 8.3%
8.3% × TI (170 µg/kg body
weight per day) = 14 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
proportion of the day
spent outdoors = 4/24
volume of outdoor air
inhaled daily = 22 m3 × 4/24 = 3.7 m3
mean body weight of adults = 64 kg
Guidance value for 14 µg/kg × 64 kg
outdoor air =
3.7 m3
= 242 µg/m3
Indoor air
The proportion of TI allocated to indoor air based on exposure
estimates = 84%
84% × TI (170 µg/kg body
weight per day) = 140 µg/kg body weight per day
daily inhalation volume
for adults = 22 m3
proportion of the day
spent indoors = 20/24
volume of indoor air
inhaled daily = 22 m3 × 20/24 = 18 m3
mean body weight of adults = 64 kg
Guidance value for 140 µg/kg × 64 kg
indoor air =
18 m3
= 498 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 0.65% (too small to permit development of
meaningful guidance values since it contributes negligibly to total
intake)
Food
The proportion of TI allocated to food based on exposure
estimates = 7.1%
7.1% × TI (170 µg/kg body
weight per day) = 12 µg/kg body weight per day or
10 µg/kg body weight per day to
one significant figure
(tolerances in various foodstuffs could then be developed on the
basis of amounts ingested.)
Soil
Owing to lack of relevant data, it is not possible to allocate a
proportion of the TI to this source.
Compound C
Naturally occurring inorganic chemical
Estimates of Exposure
The percentage of total exposure from various media for adults in
the general population in country 1 is as follows:
outdoor/indoor air = 0.02%
drinking-water = 6.9%
food = 80%
soil = 0.11%
consumer products = 12.8%
In contrast, the percentage of total exposure from various media
for adults in the general population in one area in country 2 is as
follows:
outdoor/indoor air = 35%
drinking-water = 11%
food = 55%
Development of TIs
It is concluded, on the basis of data from several studies in
human populations, that the TI is 200 µg/kg body weight per day.
Derivation of Guidance Values - Country 1
Outdoor/indoor air
The proportion of TI allocated to air based on exposure estimates
= 0.02% (too small to permit development of meaningful guidance
values)
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 6.9%
6.9% × TI (200 µg/kg body
weight per day) = 13.8 µg/kg body weight per day
daily volume of ingestion of
drinking-water for adults
in Country 1 = 1.5 litres
mean body weight of adults
in Country 1 = 70 kg
Guidance value for 13.8 µg/kg × 70 kg
drinking-water =
1.5
= 644 µg/litre
Food
The proportion of TI allocated to food based on exposure
estimates = 80%
80% × TI (200 µg/kg body 160 µg/kg body weight per day
weight per day) = or 200 µg/kg body weight per
day to one significant figure
(tolerances in various foodstuffs can then be developed on the basis
of amounts ingested.)
Soil
The proportion of TI allocated to air based on exposure estimates
= 0.11%
(too small to permit development of meaningful guidance values)
Consumer products
The proportion of TI allocated to consumer products based on
exposure estimates = 12.8%
12.8% × TI (200 µg/kg body
weight per day) = 26 µg/kg body weight per day
(limits in consumer products can be developed on the basis of
patterns of use.)
Derivation of Guidance Values - Country 2
Outdoor/indoor air
The proportion of TI allocated to air based on exposure
estimates = 35%
35% × TI (200 µg/kg body
weight per day) = 70 µg/kg body weight per day
daily inhalation volume for
adults in Country 2 = 20 m3
mean body weight of
adults in Country 2 = 60 kg
Guidance value for 70 µg/kg × 60 kg
outdoor/indoor air =
20 m3
= 210 µg/m3
Drinking-water
The proportion of TI allocated to drinking-water based on
exposure estimates = 11%
11% × TI (200 µg/kg body
weight per day) = 22 µg/kg body weight per day
daily volume of ingestion of
drinking-water for
adults in Country 2 = 1.5 litres
mean body weight of
adults in Country 2= 60 kg
Guidance value for 22 µg/kg × 60 kg
drinking-water =
1.5
= 880 µg/litre
Food
The proportion of TI allocated to food based on exposure
estimates = 55%
55% × TI (200 µg/kg body
weight per day) = 110 µg/kg body weight per day
(tolerances in various foodstuffs can be developed on the basis of
amounts ingested.)
Soil
No data are available.
Consumer products
No data are available.
APPENDIX 2
GRAHICAL APPROACHES
The use of graphs of dose-effect and dose-response toxicity data
to complement the text discussion in the development of TIs and
guidance values is considered valuable. Such graphs can display an
overview of the full range of dose-response information. Graphs can
range from simple "thermometer" presentations as employed by the US
Agency for Toxic Substances and Disease Registry (ATSDR, 1989), to
dose-effect and dose-response graphs for specific toxic effects such
as genotoxicity (Waters et al., 1988) or developmental toxicity
(Kavlock et al., 1991), or to dose-duration graphs described by
Hartung (1986), Hartung & Durkin (1986), and Dourson et al. (1985).
Fig. 4 is an example of a dose-duration graph and presents data
for methoxychlor adapted from Dourson et al. (1985). This figure
summarizes the available frank-effect levels (FEL), adverse-effect
levels (AEL), no-observed-adverse-effect levels (NOAEL), and
no-observed-effect levels (NOEL). Adverse-effect levels are presented
as a function of both dose in mg/day and exposure as a fraction of
lifespan.
Each point in the graph represents one dose group from one study.
The size of the point is a rough indication of its usefulness for
determining tolerable intakes, where larger points indicate more
useful information. Other information includes target organs. These
data can also be used to estimate a best fitting line for NOAEL across
duration.
 APPENDIX 3
ALTERNATIVE APPROACHES
Alternative approaches being considered in the derivation of TIs
for threshold effects include the benchmark dose and
categorical regression.
Benchmark dose
The benchmark dose (BD) is the lower confidence limit (LCL) of
the dose that produces a small increase in the level of adverse
effects (e.g., 5 or 10%; Crump, 1984) to which uncertainty factors
(UF) can be applied to develop a tolerable intake (see Fig. 5, adapted
from Kimmel & Gaylor, 1988).
APPENDIX 3
ALTERNATIVE APPROACHES
Alternative approaches being considered in the derivation of TIs
for threshold effects include the benchmark dose and
categorical regression.
Benchmark dose
The benchmark dose (BD) is the lower confidence limit (LCL) of
the dose that produces a small increase in the level of adverse
effects (e.g., 5 or 10%; Crump, 1984) to which uncertainty factors
(UF) can be applied to develop a tolerable intake (see Fig. 5, adapted
from Kimmel & Gaylor, 1988).
 The BD has a number of advantages over the NOAEL. Firstly, it is
derived on the basis of data from the entire dose-response curve for
the critical effect rather than that from the single dose group at the
NOAEL (i.e. one of the few (usually three) preselected dose levels).
Use of the BD also facilitates comparison of studies on the same agent
or the potencies of different agents. The BD can also be calculated
from data sets in which a NOAEL was not determined, eliminating the
need for an additional uncertainty factor to be applied to the LOAEL.
Lastly, definition of the BD as a lower confidence limit accounts for
the statistical power and quality of the data. That is, the
confidence intervals around the dose-response curve for studies with
small numbers of animals and, therefore, lower statistical power would
be wide; similarly, confidence intervals in studies of poor quality
with highly variable responses would also be wide. In either case,
the wider confidence interval would lead to a lower BD, reflecting the
greater uncertainty of the data base. On the other hand, narrow
confidence limits (reflecting better studies) would result in higher
BDs.
One of the chief disadvantages of this approach is that it is not
possible to determine a BD for many types of data on toxicity (e.g.,
histopathological data).
Several methods have been published for determining both the
dose-response curve from which the BD is derived and appropriate
uncertainty factors to estimate the TI (e.g., Crump, 1984; Dourson et
al., 1985; Kimmel & Gaylor, 1988; Gaylor, 1989; Allen et al., 1992).
However, there is as yet, no consensus on the incidence of effect to
be used as a basis for the BD, although it should be comparable to the
level of effect typically associated with the NOAEL. For data bases
on developmental toxicity, it has been estimated that this level of
effect is in the range of 1-10% (Crump, 1984; Gaylor, 1989, 1992);
this range is similar for other toxic end-points (Farland & Dourson,
1992; Shoaf, 1994). Allen et al. (1992, 1993) have estimated that a
BD calculated from the LCL at 5% is, on average, comparable to the
NOAEL, whereas choosing a BD from the LCL at 10% is more
representative of a LOAEL (Farland & Dourson, 1992). Choosing a BD
that is comparable to the NOAEL has two advantages: (i) it is within
the experimental dose-range, eliminating the need to interpolate the
dose-response curve to low levels; and (ii) justification of the
application of similar UFs as are currently applied to the NOAEL for
interspecies and inter-individual variation.
Categorical Regression
The theory and application of categorical regression has been
addressed by Hertzberg & Miller (1985), Hertzberg, (1989), Guth et al.
(1991) (inhalation exposure to methylisocyanate), and Farland &
Dourson (1992) (oral exposure to arsenic). Data on toxicity are
classified into one of several categories, such as NOEL, NOAEL, AEL or
FEL, or others, as appropriate. These categories are then regressed
on the basis of dose and, if required, duration of exposure. The
result is a graph of probability of a given category of effect with
dose or concentration, which is useful in the analysis of potential
risks above the TI, especially for comparisons amongst chemicals.
Depending on the extent of the available data on toxicity,
additional estimations regarding the percentage of individuals with
specific adverse effects are possible. Such estimations require,
however, an understanding of the mechanisms of toxicity of the
critical effect, knowledge of the extrapolation between the
experimental animal and man, and/or incidences of specific effects in
humans.
Similar to the BD, categorical regression utilizes information
from the entire dose-response curve, resulting in more precise
estimates of risk when compared to the current approach (NOAEL-based
TIs). However, categorical regression requires more information than
the current TI method, and the interpretation of the probability scale
can be problematic.
APPENDIX 4
BODY WEIGHT AND VOLUMES OF INTAKE FOR REFERENCE MAN
(based on ICRP, 1974, unless otherwise indicated)
Body weight, kg
Adult male = 70
Adult female = 58
Average = 64a
Daily fluid intake (milk, tap water, other beverages), ml/day
Normal conditions:
Adults = 1000-2400, representative
figure = 1900b (excluding
milk: 1400c)
Adult male = 1950
Adult female = 1400
Child (10 years) = 1400
High average temperature (32°C):
Adults = 2840-3410
Moderate activity:
Adults = 3700
Respiratory volumes
8-h respiratory volume, litres
resting: Adult man = 3600
Adult woman = 2900
Child (10 years) = 2300
light/non-occupational
activity: Adult man = 9600
Adult woman = 9100
Child (10 years) = 6240
Daily inhalation volume, m3
(8-h resting, 16-h light/non-occupational activity)
Adult male = 23
Adult female = 21
Child (10 years) = 15
Average adult = 22
Proportion of time
spent indoorsc = 20 h/day
Amount of soil ingestedc = 20 mg/day
Dietary intaked
Cereals = 323 g/day (flour and milled rice)
Starchy roots = 225 g/day (sweet potatoes, cassava
and other)
Sugar = 72 g/day (includes raw sugar,
excludes syrups and honey)
Pulses and nuts = 33 g/day (includes cocoa beans)
Vegetables and fruits = 325 g/day (fresh equivalent)
Meat = 125 g/day (includes offal, poultry
and game in terms of carcass weight,
excluding slaughter fats)
Eggs = 19 g/day (fresh equivalent)
Fish = 23 g/day (landed weight)
Milk = 360 g/day (excludes butter; includes
milk products as fresh milk
equivalent)
Fats and oils = 31 g/day (pure fat content)
a WHO uses 60 kg for calculation of acceptable daily intakes and
water quality guidelines (WHO, 1987, 1993).
b WHO uses a daily per capita drinking-water consumption of 2 litres
in calculating water quality guidelines (WHO, 1993)
c From Health and Welfare Canada (1992)
d Based on average of estimates for 7 geographical regions
(ICRP, 1974)
RESUME
Des valeurs guides devraient être établies dans les Critères
d'hygiène de l'environnement (CHE) de l'IPCS pour l'exposition aux
produits chimiques présents dans l'environnement. Ces valeurs guides
pourront être modifiées par les autorités nationales et locales
lorsque celles-ci fixeront leurs normes et limites pour les différents
milieux. L'élaboration des valeurs guides pour les produits chimiques
comporte les étapes suivantes:
1. Evaluer et résumer les données relatives à la toxicité pour
l'homme et l'animal et à l'exposition humaine qui offrent un intérêt
particulier pour le calcul des valeurs guides. Ces données devraient
de préférence être présentées sous la forme d'un texte explicatif
résumant les points cruciaux, complété par des graphiques.
2. Ces données pourront servir à calculer une dose tolérable (DT)
pour les différentes voies d'exposition dans le cas des effets pour
lesquels on considère qu'il existe un seuil. Le calcul consiste
généralement à appliquer des facteurs d'incertitude aux doses sans
effet indésirable observé (DSEIO) établies par l'étude la plus
pertinente pour les effets critiques. En ce qui concerne les effets
pour lesquels il n'existe pas de seuil, la relation dose-réponse devra
être caractérisée aussi complètement que possible.
3. Estimer la proportion de la dose totale provenant des différents
milieux (atmosphère à l'intérieur des locaux, air ambiant, nourriture,
eau, etc.) dans une situation donnée, en prenant comme base de calcul
un ensemble cohérent de données sur les volumes théoriques absorbés
par l'"homme de référence" de la Commission internationale de
protection contre les radiations (CIPR) et des concentrations
représentatives de l'environnement général. En l'absence de données
adéquates sur les concentrations dans les différents milieux, on
pourra utiliser des modèles mathématiques pour estimer la répartition
entre ces milieux.
4. Attribuer une proportion de la DT aux différents milieux (d'après
les résultats de l'estimation décrite à l'étape 3 ci-dessus) de façon
à déterminer la dose ou l'exposition attribuable à chaque milieu.
5. Etablir des valeurs guides pour les doses attribuées à chaque
milieu en tenant compte éventuellement du poids corporel, du volume
absorbé et de l'efficacité de l'absorption (efficacité d'absorption
relative lorsque la valeur guide est calculée à partir de la DT
établie pour une autre voie d'exposition). Dans les monographies de
la série CHE, les valeurs guides devraient être établies pour un
scénario d'exposition clairement défini, fondé sur les données
applicables à l'homme de référence de la CIPR, données qui ne sont pas
nécessairement représentatives des conditions nationales ou locales
d'exposition. Normalement, les valeurs guides seront calculées pour
une population générale représentative, soumise à des conditions
d'exposition également représentatives. Elles devront être adaptées
au niveau national et local en fonction des circonstances.
6. La base de calcul des DT et des valeurs guides devrait être
clairement expliquée dans les monographies de la série CHE (pour le
niveau de détail exigé, voir les exemples de l'appendice 1).
RESUMEN
En los monografías de la serie Criterios de Salud Ambiental (EHC)
del IPCS deben formularse valores orientativos para la exposición a
sustancias químicas presentes en el medio ambiente, valores que las
autoridades nacionales y locales pueden modificar al determinar sus
límites y normas aplicables al medio. Los pasos previstos para
cualquier sustancia química son los siguientes:
1. Evaluar y resumir la información referente a la toxicidad en los
animales y el hombre y la exposición en el hombre, seleccionando la
más pertinente para el cálculo de los valores orientativos. El
esquema más apropiado para presentar los datos pertinentes con miras
al cálculo de los valores orientativos es un texto que describa
sucintamente los datos críticos, complementado con los gráficos
oportunos.
2. Calcular a partir de esos datos una Ingesta Tolerable (IT) para
las diversas vías de exposición y para los distintos efectos que se
considere que tienen un umbral. Ello entraña el uso de factores de
incertidumbre, aplicados por lo general al nivel sin efectos adversos
observados (NOAEL) para los efectos críticos referidos en el estudio
más pertinente. En el caso de los efectos sin umbral, se
caracterizará en la medida de lo posible la relación dosis-respuesta.
3. Estimar la proporción de ingesta total que tiene su origen en los
diversos medios (p. ej., aire de espacios interiores y ambiental,
alimentos y agua), sobre la base de las exposiciones calculadas para
un conjunto coherente de volúmenes supuestos de ingesta (utilizando el
hombre de referencia de la Comisión Internacional de Protección contra
las Radiaciones (CIPR)) y de concentraciones representativas en el
medio ambiente general para una determinada situación. Si no se
dispone de datos suficientes sobre las concentraciones en diversos
medios, pueden emplearse modelos matemáticos para estimar la
distribución por esos medios.
4. Asignar una proporción de la IT a diversos medios de exposición
(basándose en la exposición estimada conforme a lo explicado en el
paso 3 precedente) para determinar la ingesta o exposición en cada
medio.
5. Formular valores orientativos a partir de las ingestas asignadas a
cada medio, teniendo en cuenta (si es necesario) el peso corporal, el
volumen de ingesta y la eficiencia de absorción (la eficiencia de
absorción relativa cuando para calcular el valor orientativo se
utilice la IT correspondiente a otra vía de exposición). En los
monografías de la serie EHC se formularían valores orientativos para
unas condiciones de exposición claramente definidas, basadas en los
datos del hombre de referencia de la CIPR y no necesariamente
representativas de las condiciones de exposición nacionales o locales.
Se calcularán comúnmente valores orientativos para una población
general representativa y unas condiciones de exposición
representativas. Los valores orientativos se deberán adaptar a nivel
nacional y local según proceda en función de las circunstancias
locales.
6. En los monografías de la serie EHC se deberán detallar claramente
los fundamentos del cálculo tanto de la IT como de los valores
orientativos (respecto al grado de detalle, véanse los ejemplos
presentados en el apéndice 1).
The BD has a number of advantages over the NOAEL. Firstly, it is
derived on the basis of data from the entire dose-response curve for
the critical effect rather than that from the single dose group at the
NOAEL (i.e. one of the few (usually three) preselected dose levels).
Use of the BD also facilitates comparison of studies on the same agent
or the potencies of different agents. The BD can also be calculated
from data sets in which a NOAEL was not determined, eliminating the
need for an additional uncertainty factor to be applied to the LOAEL.
Lastly, definition of the BD as a lower confidence limit accounts for
the statistical power and quality of the data. That is, the
confidence intervals around the dose-response curve for studies with
small numbers of animals and, therefore, lower statistical power would
be wide; similarly, confidence intervals in studies of poor quality
with highly variable responses would also be wide. In either case,
the wider confidence interval would lead to a lower BD, reflecting the
greater uncertainty of the data base. On the other hand, narrow
confidence limits (reflecting better studies) would result in higher
BDs.
One of the chief disadvantages of this approach is that it is not
possible to determine a BD for many types of data on toxicity (e.g.,
histopathological data).
Several methods have been published for determining both the
dose-response curve from which the BD is derived and appropriate
uncertainty factors to estimate the TI (e.g., Crump, 1984; Dourson et
al., 1985; Kimmel & Gaylor, 1988; Gaylor, 1989; Allen et al., 1992).
However, there is as yet, no consensus on the incidence of effect to
be used as a basis for the BD, although it should be comparable to the
level of effect typically associated with the NOAEL. For data bases
on developmental toxicity, it has been estimated that this level of
effect is in the range of 1-10% (Crump, 1984; Gaylor, 1989, 1992);
this range is similar for other toxic end-points (Farland & Dourson,
1992; Shoaf, 1994). Allen et al. (1992, 1993) have estimated that a
BD calculated from the LCL at 5% is, on average, comparable to the
NOAEL, whereas choosing a BD from the LCL at 10% is more
representative of a LOAEL (Farland & Dourson, 1992). Choosing a BD
that is comparable to the NOAEL has two advantages: (i) it is within
the experimental dose-range, eliminating the need to interpolate the
dose-response curve to low levels; and (ii) justification of the
application of similar UFs as are currently applied to the NOAEL for
interspecies and inter-individual variation.
Categorical Regression
The theory and application of categorical regression has been
addressed by Hertzberg & Miller (1985), Hertzberg, (1989), Guth et al.
(1991) (inhalation exposure to methylisocyanate), and Farland &
Dourson (1992) (oral exposure to arsenic). Data on toxicity are
classified into one of several categories, such as NOEL, NOAEL, AEL or
FEL, or others, as appropriate. These categories are then regressed
on the basis of dose and, if required, duration of exposure. The
result is a graph of probability of a given category of effect with
dose or concentration, which is useful in the analysis of potential
risks above the TI, especially for comparisons amongst chemicals.
Depending on the extent of the available data on toxicity,
additional estimations regarding the percentage of individuals with
specific adverse effects are possible. Such estimations require,
however, an understanding of the mechanisms of toxicity of the
critical effect, knowledge of the extrapolation between the
experimental animal and man, and/or incidences of specific effects in
humans.
Similar to the BD, categorical regression utilizes information
from the entire dose-response curve, resulting in more precise
estimates of risk when compared to the current approach (NOAEL-based
TIs). However, categorical regression requires more information than
the current TI method, and the interpretation of the probability scale
can be problematic.
APPENDIX 4
BODY WEIGHT AND VOLUMES OF INTAKE FOR REFERENCE MAN
(based on ICRP, 1974, unless otherwise indicated)
Body weight, kg
Adult male = 70
Adult female = 58
Average = 64a
Daily fluid intake (milk, tap water, other beverages), ml/day
Normal conditions:
Adults = 1000-2400, representative
figure = 1900b (excluding
milk: 1400c)
Adult male = 1950
Adult female = 1400
Child (10 years) = 1400
High average temperature (32°C):
Adults = 2840-3410
Moderate activity:
Adults = 3700
Respiratory volumes
8-h respiratory volume, litres
resting: Adult man = 3600
Adult woman = 2900
Child (10 years) = 2300
light/non-occupational
activity: Adult man = 9600
Adult woman = 9100
Child (10 years) = 6240
Daily inhalation volume, m3
(8-h resting, 16-h light/non-occupational activity)
Adult male = 23
Adult female = 21
Child (10 years) = 15
Average adult = 22
Proportion of time
spent indoorsc = 20 h/day
Amount of soil ingestedc = 20 mg/day
Dietary intaked
Cereals = 323 g/day (flour and milled rice)
Starchy roots = 225 g/day (sweet potatoes, cassava
and other)
Sugar = 72 g/day (includes raw sugar,
excludes syrups and honey)
Pulses and nuts = 33 g/day (includes cocoa beans)
Vegetables and fruits = 325 g/day (fresh equivalent)
Meat = 125 g/day (includes offal, poultry
and game in terms of carcass weight,
excluding slaughter fats)
Eggs = 19 g/day (fresh equivalent)
Fish = 23 g/day (landed weight)
Milk = 360 g/day (excludes butter; includes
milk products as fresh milk
equivalent)
Fats and oils = 31 g/day (pure fat content)
a WHO uses 60 kg for calculation of acceptable daily intakes and
water quality guidelines (WHO, 1987, 1993).
b WHO uses a daily per capita drinking-water consumption of 2 litres
in calculating water quality guidelines (WHO, 1993)
c From Health and Welfare Canada (1992)
d Based on average of estimates for 7 geographical regions
(ICRP, 1974)
RESUME
Des valeurs guides devraient être établies dans les Critères
d'hygiène de l'environnement (CHE) de l'IPCS pour l'exposition aux
produits chimiques présents dans l'environnement. Ces valeurs guides
pourront être modifiées par les autorités nationales et locales
lorsque celles-ci fixeront leurs normes et limites pour les différents
milieux. L'élaboration des valeurs guides pour les produits chimiques
comporte les étapes suivantes:
1. Evaluer et résumer les données relatives à la toxicité pour
l'homme et l'animal et à l'exposition humaine qui offrent un intérêt
particulier pour le calcul des valeurs guides. Ces données devraient
de préférence être présentées sous la forme d'un texte explicatif
résumant les points cruciaux, complété par des graphiques.
2. Ces données pourront servir à calculer une dose tolérable (DT)
pour les différentes voies d'exposition dans le cas des effets pour
lesquels on considère qu'il existe un seuil. Le calcul consiste
généralement à appliquer des facteurs d'incertitude aux doses sans
effet indésirable observé (DSEIO) établies par l'étude la plus
pertinente pour les effets critiques. En ce qui concerne les effets
pour lesquels il n'existe pas de seuil, la relation dose-réponse devra
être caractérisée aussi complètement que possible.
3. Estimer la proportion de la dose totale provenant des différents
milieux (atmosphère à l'intérieur des locaux, air ambiant, nourriture,
eau, etc.) dans une situation donnée, en prenant comme base de calcul
un ensemble cohérent de données sur les volumes théoriques absorbés
par l'"homme de référence" de la Commission internationale de
protection contre les radiations (CIPR) et des concentrations
représentatives de l'environnement général. En l'absence de données
adéquates sur les concentrations dans les différents milieux, on
pourra utiliser des modèles mathématiques pour estimer la répartition
entre ces milieux.
4. Attribuer une proportion de la DT aux différents milieux (d'après
les résultats de l'estimation décrite à l'étape 3 ci-dessus) de façon
à déterminer la dose ou l'exposition attribuable à chaque milieu.
5. Etablir des valeurs guides pour les doses attribuées à chaque
milieu en tenant compte éventuellement du poids corporel, du volume
absorbé et de l'efficacité de l'absorption (efficacité d'absorption
relative lorsque la valeur guide est calculée à partir de la DT
établie pour une autre voie d'exposition). Dans les monographies de
la série CHE, les valeurs guides devraient être établies pour un
scénario d'exposition clairement défini, fondé sur les données
applicables à l'homme de référence de la CIPR, données qui ne sont pas
nécessairement représentatives des conditions nationales ou locales
d'exposition. Normalement, les valeurs guides seront calculées pour
une population générale représentative, soumise à des conditions
d'exposition également représentatives. Elles devront être adaptées
au niveau national et local en fonction des circonstances.
6. La base de calcul des DT et des valeurs guides devrait être
clairement expliquée dans les monographies de la série CHE (pour le
niveau de détail exigé, voir les exemples de l'appendice 1).
RESUMEN
En los monografías de la serie Criterios de Salud Ambiental (EHC)
del IPCS deben formularse valores orientativos para la exposición a
sustancias químicas presentes en el medio ambiente, valores que las
autoridades nacionales y locales pueden modificar al determinar sus
límites y normas aplicables al medio. Los pasos previstos para
cualquier sustancia química son los siguientes:
1. Evaluar y resumir la información referente a la toxicidad en los
animales y el hombre y la exposición en el hombre, seleccionando la
más pertinente para el cálculo de los valores orientativos. El
esquema más apropiado para presentar los datos pertinentes con miras
al cálculo de los valores orientativos es un texto que describa
sucintamente los datos críticos, complementado con los gráficos
oportunos.
2. Calcular a partir de esos datos una Ingesta Tolerable (IT) para
las diversas vías de exposición y para los distintos efectos que se
considere que tienen un umbral. Ello entraña el uso de factores de
incertidumbre, aplicados por lo general al nivel sin efectos adversos
observados (NOAEL) para los efectos críticos referidos en el estudio
más pertinente. En el caso de los efectos sin umbral, se
caracterizará en la medida de lo posible la relación dosis-respuesta.
3. Estimar la proporción de ingesta total que tiene su origen en los
diversos medios (p. ej., aire de espacios interiores y ambiental,
alimentos y agua), sobre la base de las exposiciones calculadas para
un conjunto coherente de volúmenes supuestos de ingesta (utilizando el
hombre de referencia de la Comisión Internacional de Protección contra
las Radiaciones (CIPR)) y de concentraciones representativas en el
medio ambiente general para una determinada situación. Si no se
dispone de datos suficientes sobre las concentraciones en diversos
medios, pueden emplearse modelos matemáticos para estimar la
distribución por esos medios.
4. Asignar una proporción de la IT a diversos medios de exposición
(basándose en la exposición estimada conforme a lo explicado en el
paso 3 precedente) para determinar la ingesta o exposición en cada
medio.
5. Formular valores orientativos a partir de las ingestas asignadas a
cada medio, teniendo en cuenta (si es necesario) el peso corporal, el
volumen de ingesta y la eficiencia de absorción (la eficiencia de
absorción relativa cuando para calcular el valor orientativo se
utilice la IT correspondiente a otra vía de exposición). En los
monografías de la serie EHC se formularían valores orientativos para
unas condiciones de exposición claramente definidas, basadas en los
datos del hombre de referencia de la CIPR y no necesariamente
representativas de las condiciones de exposición nacionales o locales.
Se calcularán comúnmente valores orientativos para una población
general representativa y unas condiciones de exposición
representativas. Los valores orientativos se deberán adaptar a nivel
nacional y local según proceda en función de las circunstancias
locales.
6. En los monografías de la serie EHC se deberán detallar claramente
los fundamentos del cálculo tanto de la IT como de los valores
orientativos (respecto al grado de detalle, véanse los ejemplos
presentados en el apéndice 1).
